User login
Inattention to history dooms patient to repeat it ... Persistent breast lumps but no biopsy ... more
When an atypical presentation is missed
A 50-YEAR-OLD MORBIDLY OBESE MAN went to his family physician with complaints of back pain radiating to the chest, episodic shortness of breath, and diaphoresis. He had a history of uncontrolled high cholesterol. An electrocardiogram showed a Q wave in an inferior lead, which the physician attributed to an old infarct. The doctor didn’t order cardiac enzymes because his office couldn’t do the test.
The physician discharged the patient with a diagnosis of chest pain and a prescription for acetaminophen and hydrocodone. He was scheduled to see a cardiologist in 10 days, but no further cardiology workup was done.
The man died an hour later.
PLAINTIFF’S CLAIM The doctor was negligent in failing to recognize acute coronary syndrome resulting from obstructive coronary artery disease.
THE DEFENSE The patient was discharged in stable condition; cardiac arrest so soon after discharge increased the likelihood that the patient would have suffered sudden cardiac death even if he’d received emergency treatment.
VERDICT $825,000 Virginia settlement.
COMMENT Common, serious problems can present in atypical ways. A high index of suspicion for coronary artery disease in high-risk patients with thoracic pain and shortness of breath—as well as a rapid, thorough evaluation—should keep you out of court (and your patients alive).
Treatment delayed while infection spins out of control
VOMITING, DIARRHEA, AND PAIN AND SWELLING IN THE RIGHT HAND led to an ambulance trip to the emergency department (ED) for a 31-year-old woman. The ED physician diagnosed cellulitis and sepsis. Later that day, the patient was admitted to the intensive care unit, where the admitting physician noted lethargy and confusion, tachycardia, and blueness of the middle and ring fingers on the woman’s right hand. Her medical record suggested that she might have been bitten by a spider.
The patient spent the next 3 days in the ICU in deteriorating condition. She was then transferred to another hospital for treatment of necrotizing fasciitis. She underwent a number of surgeries, including amputation of her right middle and ring fingers, which resulted in significant scarring and deformity of her right hand and forearm.
PLAINTIFF’S CLAIM The defendants were negligent in failing to diagnose necrotizing fasciitis promptly.
THE DEFENSE The defendants who didn’t settle denied any negligence.
VERDICT $80,000 Indiana settlement with the defendant hospital and 1 physician; Indiana defense verdict for the other defendants.
COMMENT When serious infections don’t resolve in a timely manner, expert consultation is imperative.
Inattention to history dooms patient to repeat it
HEADACHES, FEVER, CHILLS, AND JOINT AND MUSCLE PAIN prompted a 42-year-old man to visit his medical group. He told the nurse practitioner (NP) who examined him that his mother had died of a ruptured cerebral aneurysm. The NP diagnosed a viral syndrome, ordered blood tests, and sent the patient home with prescriptions for antibiotics and pain medication. The patient didn’t undergo a neurologic examination.
About 2 weeks later, while continuing to suffer from headaches, the man collapsed and was found unresponsive. A computed tomography scan of his brain showed a subarachnoid hemorrhage and intercerebral hematoma. Further tests revealed a ruptured complex aneurysm, the cause of the hemorrhage. Despite aggressive treatment, the patient fell into a coma and died 3 months later.
PLAINTIFF’S CLAIM The NP should have realized that the patient was at high risk of an aneurysm.
THE DEFENSE No information about the defense is available.
VERDICT $1.5 million New Jersey settlement.
COMMENT I provided expert opinion in a similar case a couple of years ago. The lesson: Pay attention to the family history!
Persistent breast lumps, but no biopsy
ABOUT 3 YEARS AFTER GIVING BIRTH, a 38-year-old woman, who was still breastfeeding, went to her primary care physician complaining of pain, a dime-sized lump in her breast, and discharge from the nipple. The patient’s 2-year-old breast implants limited examination by the nurse practitioner (NP) who saw her. Galactorrhea was diagnosed and the woman was told to stop breastfeeding, apply ice packs, and come back in 2 weeks.
When the patient returned, her only remaining complaint was the lump, which the primary care physician attributed to mastitis. At a routine check-up 5 months later, the patient continued to complain of breast lumps. No breast exam was done, but the woman was referred to a gynecologist. An appointment for a breast ultrasound was scheduled for later in the month, but the patient said she didn’t receive notification of the date.
Metastatic breast cancer was subsequently diagnosed, and the woman died about 3 years later.
PLAINTIFF’S CLAIM The NP and primary care physician should have recommended a biopsy sooner.
THE DEFENSE The care given was proper; an earlier diagnosis wouldn’t have changed the outcome.
VERDICT $750,000 Massachusetts settlement.
COMMENT Failure to recommend biopsy of breast lumps is a leading cause of malpractice cases against family physicians. All persistent breast lumps require referral for biopsy— regardless of the patient’s age.
A red flag that was ignored for too long
A MAN IN HIS EARLY 30S consulted an orthopedist for mid-back pain. The doctor took radiographs of the man’s lower back and reported that he saw nothing amiss. When the man returned 3 months later complaining of the same kind of pain, the orthopedist examined him, prescribed a muscle relaxant, and sent him for physical therapy. The physician did not take any radiographs.
Four months later, the patient came back with pain in his mid-back and ribs. The orthopedist ordered radiographs of the ribs, which were read as normal.
After 18 months, the patient consulted another orthopedist, who ordered a magnetic resonance imaging scan and diagnosed a spinal plasmacytoma at levels T9 to T11. The tumor had destroyed some vertebrae and was compressing the spinal cord.
The patient underwent surgery to remove the tumor and insert screws from T6 to L2 to stabilize the spine. He wore a brace around his torso for months and had a bone marrow transplant. The patient couldn’t return to work.
PLAINTIFF’S CLAIM The tumor was clearly visible on the radiographs taken at the patient’s third visit to the first orthopedist; thoracic spine radiographs should have been taken at the previous 2 visits.
THE DEFENSE No information about the defense is available.
VERDICT $875,000 New Jersey settlement.
COMMENT Current guidelines recommend a red flags approach to imaging. This patient had a red flag—unremitting pain. When back pain persists unabated, it’s time for a thorough evaluation.
When an atypical presentation is missed
A 50-YEAR-OLD MORBIDLY OBESE MAN went to his family physician with complaints of back pain radiating to the chest, episodic shortness of breath, and diaphoresis. He had a history of uncontrolled high cholesterol. An electrocardiogram showed a Q wave in an inferior lead, which the physician attributed to an old infarct. The doctor didn’t order cardiac enzymes because his office couldn’t do the test.
The physician discharged the patient with a diagnosis of chest pain and a prescription for acetaminophen and hydrocodone. He was scheduled to see a cardiologist in 10 days, but no further cardiology workup was done.
The man died an hour later.
PLAINTIFF’S CLAIM The doctor was negligent in failing to recognize acute coronary syndrome resulting from obstructive coronary artery disease.
THE DEFENSE The patient was discharged in stable condition; cardiac arrest so soon after discharge increased the likelihood that the patient would have suffered sudden cardiac death even if he’d received emergency treatment.
VERDICT $825,000 Virginia settlement.
COMMENT Common, serious problems can present in atypical ways. A high index of suspicion for coronary artery disease in high-risk patients with thoracic pain and shortness of breath—as well as a rapid, thorough evaluation—should keep you out of court (and your patients alive).
Treatment delayed while infection spins out of control
VOMITING, DIARRHEA, AND PAIN AND SWELLING IN THE RIGHT HAND led to an ambulance trip to the emergency department (ED) for a 31-year-old woman. The ED physician diagnosed cellulitis and sepsis. Later that day, the patient was admitted to the intensive care unit, where the admitting physician noted lethargy and confusion, tachycardia, and blueness of the middle and ring fingers on the woman’s right hand. Her medical record suggested that she might have been bitten by a spider.
The patient spent the next 3 days in the ICU in deteriorating condition. She was then transferred to another hospital for treatment of necrotizing fasciitis. She underwent a number of surgeries, including amputation of her right middle and ring fingers, which resulted in significant scarring and deformity of her right hand and forearm.
PLAINTIFF’S CLAIM The defendants were negligent in failing to diagnose necrotizing fasciitis promptly.
THE DEFENSE The defendants who didn’t settle denied any negligence.
VERDICT $80,000 Indiana settlement with the defendant hospital and 1 physician; Indiana defense verdict for the other defendants.
COMMENT When serious infections don’t resolve in a timely manner, expert consultation is imperative.
Inattention to history dooms patient to repeat it
HEADACHES, FEVER, CHILLS, AND JOINT AND MUSCLE PAIN prompted a 42-year-old man to visit his medical group. He told the nurse practitioner (NP) who examined him that his mother had died of a ruptured cerebral aneurysm. The NP diagnosed a viral syndrome, ordered blood tests, and sent the patient home with prescriptions for antibiotics and pain medication. The patient didn’t undergo a neurologic examination.
About 2 weeks later, while continuing to suffer from headaches, the man collapsed and was found unresponsive. A computed tomography scan of his brain showed a subarachnoid hemorrhage and intercerebral hematoma. Further tests revealed a ruptured complex aneurysm, the cause of the hemorrhage. Despite aggressive treatment, the patient fell into a coma and died 3 months later.
PLAINTIFF’S CLAIM The NP should have realized that the patient was at high risk of an aneurysm.
THE DEFENSE No information about the defense is available.
VERDICT $1.5 million New Jersey settlement.
COMMENT I provided expert opinion in a similar case a couple of years ago. The lesson: Pay attention to the family history!
Persistent breast lumps, but no biopsy
ABOUT 3 YEARS AFTER GIVING BIRTH, a 38-year-old woman, who was still breastfeeding, went to her primary care physician complaining of pain, a dime-sized lump in her breast, and discharge from the nipple. The patient’s 2-year-old breast implants limited examination by the nurse practitioner (NP) who saw her. Galactorrhea was diagnosed and the woman was told to stop breastfeeding, apply ice packs, and come back in 2 weeks.
When the patient returned, her only remaining complaint was the lump, which the primary care physician attributed to mastitis. At a routine check-up 5 months later, the patient continued to complain of breast lumps. No breast exam was done, but the woman was referred to a gynecologist. An appointment for a breast ultrasound was scheduled for later in the month, but the patient said she didn’t receive notification of the date.
Metastatic breast cancer was subsequently diagnosed, and the woman died about 3 years later.
PLAINTIFF’S CLAIM The NP and primary care physician should have recommended a biopsy sooner.
THE DEFENSE The care given was proper; an earlier diagnosis wouldn’t have changed the outcome.
VERDICT $750,000 Massachusetts settlement.
COMMENT Failure to recommend biopsy of breast lumps is a leading cause of malpractice cases against family physicians. All persistent breast lumps require referral for biopsy— regardless of the patient’s age.
A red flag that was ignored for too long
A MAN IN HIS EARLY 30S consulted an orthopedist for mid-back pain. The doctor took radiographs of the man’s lower back and reported that he saw nothing amiss. When the man returned 3 months later complaining of the same kind of pain, the orthopedist examined him, prescribed a muscle relaxant, and sent him for physical therapy. The physician did not take any radiographs.
Four months later, the patient came back with pain in his mid-back and ribs. The orthopedist ordered radiographs of the ribs, which were read as normal.
After 18 months, the patient consulted another orthopedist, who ordered a magnetic resonance imaging scan and diagnosed a spinal plasmacytoma at levels T9 to T11. The tumor had destroyed some vertebrae and was compressing the spinal cord.
The patient underwent surgery to remove the tumor and insert screws from T6 to L2 to stabilize the spine. He wore a brace around his torso for months and had a bone marrow transplant. The patient couldn’t return to work.
PLAINTIFF’S CLAIM The tumor was clearly visible on the radiographs taken at the patient’s third visit to the first orthopedist; thoracic spine radiographs should have been taken at the previous 2 visits.
THE DEFENSE No information about the defense is available.
VERDICT $875,000 New Jersey settlement.
COMMENT Current guidelines recommend a red flags approach to imaging. This patient had a red flag—unremitting pain. When back pain persists unabated, it’s time for a thorough evaluation.
When an atypical presentation is missed
A 50-YEAR-OLD MORBIDLY OBESE MAN went to his family physician with complaints of back pain radiating to the chest, episodic shortness of breath, and diaphoresis. He had a history of uncontrolled high cholesterol. An electrocardiogram showed a Q wave in an inferior lead, which the physician attributed to an old infarct. The doctor didn’t order cardiac enzymes because his office couldn’t do the test.
The physician discharged the patient with a diagnosis of chest pain and a prescription for acetaminophen and hydrocodone. He was scheduled to see a cardiologist in 10 days, but no further cardiology workup was done.
The man died an hour later.
PLAINTIFF’S CLAIM The doctor was negligent in failing to recognize acute coronary syndrome resulting from obstructive coronary artery disease.
THE DEFENSE The patient was discharged in stable condition; cardiac arrest so soon after discharge increased the likelihood that the patient would have suffered sudden cardiac death even if he’d received emergency treatment.
VERDICT $825,000 Virginia settlement.
COMMENT Common, serious problems can present in atypical ways. A high index of suspicion for coronary artery disease in high-risk patients with thoracic pain and shortness of breath—as well as a rapid, thorough evaluation—should keep you out of court (and your patients alive).
Treatment delayed while infection spins out of control
VOMITING, DIARRHEA, AND PAIN AND SWELLING IN THE RIGHT HAND led to an ambulance trip to the emergency department (ED) for a 31-year-old woman. The ED physician diagnosed cellulitis and sepsis. Later that day, the patient was admitted to the intensive care unit, where the admitting physician noted lethargy and confusion, tachycardia, and blueness of the middle and ring fingers on the woman’s right hand. Her medical record suggested that she might have been bitten by a spider.
The patient spent the next 3 days in the ICU in deteriorating condition. She was then transferred to another hospital for treatment of necrotizing fasciitis. She underwent a number of surgeries, including amputation of her right middle and ring fingers, which resulted in significant scarring and deformity of her right hand and forearm.
PLAINTIFF’S CLAIM The defendants were negligent in failing to diagnose necrotizing fasciitis promptly.
THE DEFENSE The defendants who didn’t settle denied any negligence.
VERDICT $80,000 Indiana settlement with the defendant hospital and 1 physician; Indiana defense verdict for the other defendants.
COMMENT When serious infections don’t resolve in a timely manner, expert consultation is imperative.
Inattention to history dooms patient to repeat it
HEADACHES, FEVER, CHILLS, AND JOINT AND MUSCLE PAIN prompted a 42-year-old man to visit his medical group. He told the nurse practitioner (NP) who examined him that his mother had died of a ruptured cerebral aneurysm. The NP diagnosed a viral syndrome, ordered blood tests, and sent the patient home with prescriptions for antibiotics and pain medication. The patient didn’t undergo a neurologic examination.
About 2 weeks later, while continuing to suffer from headaches, the man collapsed and was found unresponsive. A computed tomography scan of his brain showed a subarachnoid hemorrhage and intercerebral hematoma. Further tests revealed a ruptured complex aneurysm, the cause of the hemorrhage. Despite aggressive treatment, the patient fell into a coma and died 3 months later.
PLAINTIFF’S CLAIM The NP should have realized that the patient was at high risk of an aneurysm.
THE DEFENSE No information about the defense is available.
VERDICT $1.5 million New Jersey settlement.
COMMENT I provided expert opinion in a similar case a couple of years ago. The lesson: Pay attention to the family history!
Persistent breast lumps, but no biopsy
ABOUT 3 YEARS AFTER GIVING BIRTH, a 38-year-old woman, who was still breastfeeding, went to her primary care physician complaining of pain, a dime-sized lump in her breast, and discharge from the nipple. The patient’s 2-year-old breast implants limited examination by the nurse practitioner (NP) who saw her. Galactorrhea was diagnosed and the woman was told to stop breastfeeding, apply ice packs, and come back in 2 weeks.
When the patient returned, her only remaining complaint was the lump, which the primary care physician attributed to mastitis. At a routine check-up 5 months later, the patient continued to complain of breast lumps. No breast exam was done, but the woman was referred to a gynecologist. An appointment for a breast ultrasound was scheduled for later in the month, but the patient said she didn’t receive notification of the date.
Metastatic breast cancer was subsequently diagnosed, and the woman died about 3 years later.
PLAINTIFF’S CLAIM The NP and primary care physician should have recommended a biopsy sooner.
THE DEFENSE The care given was proper; an earlier diagnosis wouldn’t have changed the outcome.
VERDICT $750,000 Massachusetts settlement.
COMMENT Failure to recommend biopsy of breast lumps is a leading cause of malpractice cases against family physicians. All persistent breast lumps require referral for biopsy— regardless of the patient’s age.
A red flag that was ignored for too long
A MAN IN HIS EARLY 30S consulted an orthopedist for mid-back pain. The doctor took radiographs of the man’s lower back and reported that he saw nothing amiss. When the man returned 3 months later complaining of the same kind of pain, the orthopedist examined him, prescribed a muscle relaxant, and sent him for physical therapy. The physician did not take any radiographs.
Four months later, the patient came back with pain in his mid-back and ribs. The orthopedist ordered radiographs of the ribs, which were read as normal.
After 18 months, the patient consulted another orthopedist, who ordered a magnetic resonance imaging scan and diagnosed a spinal plasmacytoma at levels T9 to T11. The tumor had destroyed some vertebrae and was compressing the spinal cord.
The patient underwent surgery to remove the tumor and insert screws from T6 to L2 to stabilize the spine. He wore a brace around his torso for months and had a bone marrow transplant. The patient couldn’t return to work.
PLAINTIFF’S CLAIM The tumor was clearly visible on the radiographs taken at the patient’s third visit to the first orthopedist; thoracic spine radiographs should have been taken at the previous 2 visits.
THE DEFENSE No information about the defense is available.
VERDICT $875,000 New Jersey settlement.
COMMENT Current guidelines recommend a red flags approach to imaging. This patient had a red flag—unremitting pain. When back pain persists unabated, it’s time for a thorough evaluation.
Patient overusing antianxiety meds? Say so (in a letter)
Express your concern about long-term use of benzodiazepines in a letter—a simple intervention that patients often respond to by reducing or eliminating their use of the drug.1
STRENGTH OF RECOMMENDATION
A: Based on a well-done meta-analysis with few clinical trials.
Mugunthan K, McGuire T, Glasziou P. Minimal interventions to decrease long-term use of benzodiazepines in primary care: a systematic review and meta-analysis. Br J Gen Pract. 2011;61:e573-e578.
ILLUSTRATIVE CASE
A 65-year-old patient has been taking lorazepam for insomnia for more than a year. You are concerned about her ongoing use of the benzodiazepine and want to wean her from the medication. What strategies can you use to decrease, or eliminate, her use of the drug?
Benzodiazepines are commonly used medications, with an estimated 12-month prevalence of use of 8.6% in the United States.2 While short-term use of these antianxiety medications can be effective, long-term use (defined as regular use for >3 months) is associated with significant risk.
Abuse linked to chronic use
Prescription drug abuse has recently become the nation’s leading cause of accidental death, overtaking motor vehicle accidents.3 And tranquilizers, including benzodiazepines, are the second most abused prescription medication, after pain relievers.4 In addition to dependence and withdrawal, chronic use of benzodiazepines is associated with daytime somnolence, blunted reflexes, memory loss, cognitive impairment, and an increased risk of falls and fractures—particularly in older patients.5
Reducing long-term use of benzodiazepines in a primary care setting is important but challenging. Until recently, most of the successful strategies reported were resource intensive and required multiple office visits.6
STUDY SUMMARY: Brief interventions are often effective
This study was a meta-analysis of randomized controlled trials in which “minimal interventions” were compared with usual care for their effectiveness in reducing or eliminating benzodiazepine use in primary care patients. A minimal intervention was defined as a letter, self-help information, or short consultation with a primary care provider. In each case, the message to the patient included (a) an expression of concern about the patient’s long-term use of the medication, (b) information about the potential adverse effects of the medication, and (c) advice on how to gradually reduce or stop using it.
Three studies met the inclusion criteria for randomization, blinding, and analysis by intention-to-treat.7-9 All 3 (n=615) had a 6-month follow-up period, a higher proportion of women (>60%), and participants with a mean age >60 years. Few patients were lost to follow-up; withdrawal rates were low and similar in all 3 studies. Each study compared a letter with usual care; 2 of the 3 had a third arm that included both a letter and a short consultation.
Pooled results from the studies showed twice the reduction in benzodiazepine use in the intervention groups compared with the control groups (risk ratio [RR]=2.04; 95% confidence interval [CI], 1.5-2.8; P< .001). The RR for cessation of benzodiazepine use was 2.3 (95% CI, 1.3-4.2; P= .003). The number needed to treat for a reduction or cessation of use was 12. The studies reported benzodiazepine reduction rates of 20% to 35% in the intervention groups vs 6% to 15% in the usual care groups.7-9 There appeared to be no additional benefit to adding the brief consultation compared with the letter alone.
WHAT’S NEW?: This strategy is easy to implement
While many methods to reduce benzodiazepine use have been studied, most involved levels of skill and resources that are not feasible for widespread use. This study found that a letter, stating the risks of continued use of the medication and providing a weaning schedule and tips for handling withdrawal, can be effective in reducing chronic use in a small but significant part of the population.
CAVEATS: Effects of withdrawal went unaddressed
The study did not adequately address the adverse effects of withdrawal from benzodiazepines, with one of the studies reporting significantly worse qualitative (but not quantitative) withdrawal symptoms at 6 months.7 This is of particular concern, as withdrawal symptoms are associated with the potential for relapse and concomitant abuse of other drugs and alcohol. We recommend that primary care physicians screen for substance abuse prior to the intervention and arrange for adequate follow-up.
All 3 studies in the meta-analysis lasted 6 months; no longer-term outcomes were reported. In addition, the study did not yield enough information to identify patients who would be most likely to respond to this brief intervention.
CHALLENGES TO IMPLEMENTATION: Determining which patients to target
Identifying patients who are appropriate candidates for this brief intervention and providing adequate monitoring for adverse effects of withdrawal are the main challenges of this practice changer. Nonetheless, chronic benzodiazepine use is of considerable concern, and we believe that this is a useful, and manageable intervention.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Mugunthan K, McGuire T, Glasziou P. Minimal interventions to decrease long-term use of benzodiazepines in primary care: a systematic review and meta-analysis. Br J Gen Pract. 2011;61:e573-e578.
2. Tyrer PJ. Benzodiazepines on trial. Br Med J. 1984;288:1101-1102.
3. Centers for Disease Control and Prevention. Deaths: Leading causes for 2008. June 6, 2012. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_06.pdf. Accessed October 10, 2012.
4. National Institute on Drug Abuse. Topics in brief: Prescription drug abuse. Available at: http://www.drugabuse.gov/publications/topics-in-brief/prescription-drug-abuse. Accessed October 11, 2012.
5. Morin CM, Bastien C, Guay B, et al. Randomized clinical trail of supervised tapering and cognitive behavior therapy to facilitate benzodiazepine discontinuation in older adults with chronic insomnia. Am J Psychiatry. 2004;161:332-342.
6. Oude Voshaar RC, Couvee JE, van Balkorn AJ, et al. Strategies for discontinuing long-term benzodiazepine use-meta-analysis. Br J Psychiatr. 2006;189:213-220.
7. Bashir K, King M, Ashworth M. Controlled evaluation of brief intervention by general practitioners to reduce chronic use of benzodiazepines. Br J Gen Pract. 1994;44:408-412.
8. Cormack MA, Sweeney KG, Hughes-Jones H, et al. Evaluation of an easy, cost-effective strategy to cut benzodiazepine use in general practice. Br J Gen Pract. 1994;44:5-8
9. Heather NA, Bowie A, Ashton H, et al. Randomized controlled trial of two brief interventions against long-term benzodiazepine use: outcome of intervention. Addict Res Theory. 2004;12:141-145.
Express your concern about long-term use of benzodiazepines in a letter—a simple intervention that patients often respond to by reducing or eliminating their use of the drug.1
STRENGTH OF RECOMMENDATION
A: Based on a well-done meta-analysis with few clinical trials.
Mugunthan K, McGuire T, Glasziou P. Minimal interventions to decrease long-term use of benzodiazepines in primary care: a systematic review and meta-analysis. Br J Gen Pract. 2011;61:e573-e578.
ILLUSTRATIVE CASE
A 65-year-old patient has been taking lorazepam for insomnia for more than a year. You are concerned about her ongoing use of the benzodiazepine and want to wean her from the medication. What strategies can you use to decrease, or eliminate, her use of the drug?
Benzodiazepines are commonly used medications, with an estimated 12-month prevalence of use of 8.6% in the United States.2 While short-term use of these antianxiety medications can be effective, long-term use (defined as regular use for >3 months) is associated with significant risk.
Abuse linked to chronic use
Prescription drug abuse has recently become the nation’s leading cause of accidental death, overtaking motor vehicle accidents.3 And tranquilizers, including benzodiazepines, are the second most abused prescription medication, after pain relievers.4 In addition to dependence and withdrawal, chronic use of benzodiazepines is associated with daytime somnolence, blunted reflexes, memory loss, cognitive impairment, and an increased risk of falls and fractures—particularly in older patients.5
Reducing long-term use of benzodiazepines in a primary care setting is important but challenging. Until recently, most of the successful strategies reported were resource intensive and required multiple office visits.6
STUDY SUMMARY: Brief interventions are often effective
This study was a meta-analysis of randomized controlled trials in which “minimal interventions” were compared with usual care for their effectiveness in reducing or eliminating benzodiazepine use in primary care patients. A minimal intervention was defined as a letter, self-help information, or short consultation with a primary care provider. In each case, the message to the patient included (a) an expression of concern about the patient’s long-term use of the medication, (b) information about the potential adverse effects of the medication, and (c) advice on how to gradually reduce or stop using it.
Three studies met the inclusion criteria for randomization, blinding, and analysis by intention-to-treat.7-9 All 3 (n=615) had a 6-month follow-up period, a higher proportion of women (>60%), and participants with a mean age >60 years. Few patients were lost to follow-up; withdrawal rates were low and similar in all 3 studies. Each study compared a letter with usual care; 2 of the 3 had a third arm that included both a letter and a short consultation.
Pooled results from the studies showed twice the reduction in benzodiazepine use in the intervention groups compared with the control groups (risk ratio [RR]=2.04; 95% confidence interval [CI], 1.5-2.8; P< .001). The RR for cessation of benzodiazepine use was 2.3 (95% CI, 1.3-4.2; P= .003). The number needed to treat for a reduction or cessation of use was 12. The studies reported benzodiazepine reduction rates of 20% to 35% in the intervention groups vs 6% to 15% in the usual care groups.7-9 There appeared to be no additional benefit to adding the brief consultation compared with the letter alone.
WHAT’S NEW?: This strategy is easy to implement
While many methods to reduce benzodiazepine use have been studied, most involved levels of skill and resources that are not feasible for widespread use. This study found that a letter, stating the risks of continued use of the medication and providing a weaning schedule and tips for handling withdrawal, can be effective in reducing chronic use in a small but significant part of the population.
CAVEATS: Effects of withdrawal went unaddressed
The study did not adequately address the adverse effects of withdrawal from benzodiazepines, with one of the studies reporting significantly worse qualitative (but not quantitative) withdrawal symptoms at 6 months.7 This is of particular concern, as withdrawal symptoms are associated with the potential for relapse and concomitant abuse of other drugs and alcohol. We recommend that primary care physicians screen for substance abuse prior to the intervention and arrange for adequate follow-up.
All 3 studies in the meta-analysis lasted 6 months; no longer-term outcomes were reported. In addition, the study did not yield enough information to identify patients who would be most likely to respond to this brief intervention.
CHALLENGES TO IMPLEMENTATION: Determining which patients to target
Identifying patients who are appropriate candidates for this brief intervention and providing adequate monitoring for adverse effects of withdrawal are the main challenges of this practice changer. Nonetheless, chronic benzodiazepine use is of considerable concern, and we believe that this is a useful, and manageable intervention.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Express your concern about long-term use of benzodiazepines in a letter—a simple intervention that patients often respond to by reducing or eliminating their use of the drug.1
STRENGTH OF RECOMMENDATION
A: Based on a well-done meta-analysis with few clinical trials.
Mugunthan K, McGuire T, Glasziou P. Minimal interventions to decrease long-term use of benzodiazepines in primary care: a systematic review and meta-analysis. Br J Gen Pract. 2011;61:e573-e578.
ILLUSTRATIVE CASE
A 65-year-old patient has been taking lorazepam for insomnia for more than a year. You are concerned about her ongoing use of the benzodiazepine and want to wean her from the medication. What strategies can you use to decrease, or eliminate, her use of the drug?
Benzodiazepines are commonly used medications, with an estimated 12-month prevalence of use of 8.6% in the United States.2 While short-term use of these antianxiety medications can be effective, long-term use (defined as regular use for >3 months) is associated with significant risk.
Abuse linked to chronic use
Prescription drug abuse has recently become the nation’s leading cause of accidental death, overtaking motor vehicle accidents.3 And tranquilizers, including benzodiazepines, are the second most abused prescription medication, after pain relievers.4 In addition to dependence and withdrawal, chronic use of benzodiazepines is associated with daytime somnolence, blunted reflexes, memory loss, cognitive impairment, and an increased risk of falls and fractures—particularly in older patients.5
Reducing long-term use of benzodiazepines in a primary care setting is important but challenging. Until recently, most of the successful strategies reported were resource intensive and required multiple office visits.6
STUDY SUMMARY: Brief interventions are often effective
This study was a meta-analysis of randomized controlled trials in which “minimal interventions” were compared with usual care for their effectiveness in reducing or eliminating benzodiazepine use in primary care patients. A minimal intervention was defined as a letter, self-help information, or short consultation with a primary care provider. In each case, the message to the patient included (a) an expression of concern about the patient’s long-term use of the medication, (b) information about the potential adverse effects of the medication, and (c) advice on how to gradually reduce or stop using it.
Three studies met the inclusion criteria for randomization, blinding, and analysis by intention-to-treat.7-9 All 3 (n=615) had a 6-month follow-up period, a higher proportion of women (>60%), and participants with a mean age >60 years. Few patients were lost to follow-up; withdrawal rates were low and similar in all 3 studies. Each study compared a letter with usual care; 2 of the 3 had a third arm that included both a letter and a short consultation.
Pooled results from the studies showed twice the reduction in benzodiazepine use in the intervention groups compared with the control groups (risk ratio [RR]=2.04; 95% confidence interval [CI], 1.5-2.8; P< .001). The RR for cessation of benzodiazepine use was 2.3 (95% CI, 1.3-4.2; P= .003). The number needed to treat for a reduction or cessation of use was 12. The studies reported benzodiazepine reduction rates of 20% to 35% in the intervention groups vs 6% to 15% in the usual care groups.7-9 There appeared to be no additional benefit to adding the brief consultation compared with the letter alone.
WHAT’S NEW?: This strategy is easy to implement
While many methods to reduce benzodiazepine use have been studied, most involved levels of skill and resources that are not feasible for widespread use. This study found that a letter, stating the risks of continued use of the medication and providing a weaning schedule and tips for handling withdrawal, can be effective in reducing chronic use in a small but significant part of the population.
CAVEATS: Effects of withdrawal went unaddressed
The study did not adequately address the adverse effects of withdrawal from benzodiazepines, with one of the studies reporting significantly worse qualitative (but not quantitative) withdrawal symptoms at 6 months.7 This is of particular concern, as withdrawal symptoms are associated with the potential for relapse and concomitant abuse of other drugs and alcohol. We recommend that primary care physicians screen for substance abuse prior to the intervention and arrange for adequate follow-up.
All 3 studies in the meta-analysis lasted 6 months; no longer-term outcomes were reported. In addition, the study did not yield enough information to identify patients who would be most likely to respond to this brief intervention.
CHALLENGES TO IMPLEMENTATION: Determining which patients to target
Identifying patients who are appropriate candidates for this brief intervention and providing adequate monitoring for adverse effects of withdrawal are the main challenges of this practice changer. Nonetheless, chronic benzodiazepine use is of considerable concern, and we believe that this is a useful, and manageable intervention.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Mugunthan K, McGuire T, Glasziou P. Minimal interventions to decrease long-term use of benzodiazepines in primary care: a systematic review and meta-analysis. Br J Gen Pract. 2011;61:e573-e578.
2. Tyrer PJ. Benzodiazepines on trial. Br Med J. 1984;288:1101-1102.
3. Centers for Disease Control and Prevention. Deaths: Leading causes for 2008. June 6, 2012. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_06.pdf. Accessed October 10, 2012.
4. National Institute on Drug Abuse. Topics in brief: Prescription drug abuse. Available at: http://www.drugabuse.gov/publications/topics-in-brief/prescription-drug-abuse. Accessed October 11, 2012.
5. Morin CM, Bastien C, Guay B, et al. Randomized clinical trail of supervised tapering and cognitive behavior therapy to facilitate benzodiazepine discontinuation in older adults with chronic insomnia. Am J Psychiatry. 2004;161:332-342.
6. Oude Voshaar RC, Couvee JE, van Balkorn AJ, et al. Strategies for discontinuing long-term benzodiazepine use-meta-analysis. Br J Psychiatr. 2006;189:213-220.
7. Bashir K, King M, Ashworth M. Controlled evaluation of brief intervention by general practitioners to reduce chronic use of benzodiazepines. Br J Gen Pract. 1994;44:408-412.
8. Cormack MA, Sweeney KG, Hughes-Jones H, et al. Evaluation of an easy, cost-effective strategy to cut benzodiazepine use in general practice. Br J Gen Pract. 1994;44:5-8
9. Heather NA, Bowie A, Ashton H, et al. Randomized controlled trial of two brief interventions against long-term benzodiazepine use: outcome of intervention. Addict Res Theory. 2004;12:141-145.
1. Mugunthan K, McGuire T, Glasziou P. Minimal interventions to decrease long-term use of benzodiazepines in primary care: a systematic review and meta-analysis. Br J Gen Pract. 2011;61:e573-e578.
2. Tyrer PJ. Benzodiazepines on trial. Br Med J. 1984;288:1101-1102.
3. Centers for Disease Control and Prevention. Deaths: Leading causes for 2008. June 6, 2012. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_06.pdf. Accessed October 10, 2012.
4. National Institute on Drug Abuse. Topics in brief: Prescription drug abuse. Available at: http://www.drugabuse.gov/publications/topics-in-brief/prescription-drug-abuse. Accessed October 11, 2012.
5. Morin CM, Bastien C, Guay B, et al. Randomized clinical trail of supervised tapering and cognitive behavior therapy to facilitate benzodiazepine discontinuation in older adults with chronic insomnia. Am J Psychiatry. 2004;161:332-342.
6. Oude Voshaar RC, Couvee JE, van Balkorn AJ, et al. Strategies for discontinuing long-term benzodiazepine use-meta-analysis. Br J Psychiatr. 2006;189:213-220.
7. Bashir K, King M, Ashworth M. Controlled evaluation of brief intervention by general practitioners to reduce chronic use of benzodiazepines. Br J Gen Pract. 1994;44:408-412.
8. Cormack MA, Sweeney KG, Hughes-Jones H, et al. Evaluation of an easy, cost-effective strategy to cut benzodiazepine use in general practice. Br J Gen Pract. 1994;44:5-8
9. Heather NA, Bowie A, Ashton H, et al. Randomized controlled trial of two brief interventions against long-term benzodiazepine use: outcome of intervention. Addict Res Theory. 2004;12:141-145.
Copyright © 2012 The Family Physicians Inquiries Network. All rights reserved.
Let’s put a stop to the prescribing cascade
I am delighted by the commonsense approach Drs. Weiss and Lee have taken in advising us to be wary of prescribing—or continuing—too many medications for our older patients (“Is your patient taking too many pills?”). Frankly, this advice applies to all patients, regardless of their age, and to virtually all family physicians. We all have stories about medication overuse. I’d like to tell you 2 of mine.
When Mrs. S, a 68-year-old patient, came to see me for the first time, I scanned her medication list. It included a nasal steroid for allergic rhinitis, a PPI for reflux, and 2 asthma inhalers—albuterol and an inhaled corticosteroid.
I asked her if she had hay fever. She didn’t think so. Heartburn? She said No. A history of asthma? No. So why was she taking these medications? To treat a chronic cough, the patient said. Was the cough better? No.
In the past 12 months, Mrs. S had seen an allergist, a gastroenterologist, and an otolaryngologist. The result? All 3 specialists added their favorite medication. I scanned the patient’s medication list again and noticed that she was taking amitriptyline 25 mg as a sleep aid. Because of the drug’s anticholinergic adverse effects, I had a hunch, and asked her to go one week without the amitriptyline. She agreed.
You can guess the happy ending. Mrs. S’s cough vanished, along with 4 medications she never needed in the first place. She was a victim of the prescribing cascade.
The other story is even more dramatic.
A friend who’s both an FP and a geriatrician became medical director of a local nursing home. To his chagrin, the average number of prescription drugs per resident when he took over was 9.6. Systematically, he went about reevaluating what residents really required. After a year and a half, the average had fallen to 5.4. The residents were no more depressed or agitated, and were generally more alert.
But here’s the catch: I checked back at the nursing home a couple of years after my friend left, and the average number of meds was back up to 10. It takes constant attention to not overprescribe. In fact, I now spend about as much time stopping meds as starting them.
Our health care system is the land of excess. It is up to family physicians—indeed, to all primary care clinicians—to ensure that we only prescribe or continue prescriptions when it’s the right patient, the right medication, at the right time.
Now it’s your turn. Send me your favorite, or most dramatic, medication overtreatment stories for our Letters column. We’ll continue the dialogue there.
I am delighted by the commonsense approach Drs. Weiss and Lee have taken in advising us to be wary of prescribing—or continuing—too many medications for our older patients (“Is your patient taking too many pills?”). Frankly, this advice applies to all patients, regardless of their age, and to virtually all family physicians. We all have stories about medication overuse. I’d like to tell you 2 of mine.
When Mrs. S, a 68-year-old patient, came to see me for the first time, I scanned her medication list. It included a nasal steroid for allergic rhinitis, a PPI for reflux, and 2 asthma inhalers—albuterol and an inhaled corticosteroid.
I asked her if she had hay fever. She didn’t think so. Heartburn? She said No. A history of asthma? No. So why was she taking these medications? To treat a chronic cough, the patient said. Was the cough better? No.
In the past 12 months, Mrs. S had seen an allergist, a gastroenterologist, and an otolaryngologist. The result? All 3 specialists added their favorite medication. I scanned the patient’s medication list again and noticed that she was taking amitriptyline 25 mg as a sleep aid. Because of the drug’s anticholinergic adverse effects, I had a hunch, and asked her to go one week without the amitriptyline. She agreed.
You can guess the happy ending. Mrs. S’s cough vanished, along with 4 medications she never needed in the first place. She was a victim of the prescribing cascade.
The other story is even more dramatic.
A friend who’s both an FP and a geriatrician became medical director of a local nursing home. To his chagrin, the average number of prescription drugs per resident when he took over was 9.6. Systematically, he went about reevaluating what residents really required. After a year and a half, the average had fallen to 5.4. The residents were no more depressed or agitated, and were generally more alert.
But here’s the catch: I checked back at the nursing home a couple of years after my friend left, and the average number of meds was back up to 10. It takes constant attention to not overprescribe. In fact, I now spend about as much time stopping meds as starting them.
Our health care system is the land of excess. It is up to family physicians—indeed, to all primary care clinicians—to ensure that we only prescribe or continue prescriptions when it’s the right patient, the right medication, at the right time.
Now it’s your turn. Send me your favorite, or most dramatic, medication overtreatment stories for our Letters column. We’ll continue the dialogue there.
I am delighted by the commonsense approach Drs. Weiss and Lee have taken in advising us to be wary of prescribing—or continuing—too many medications for our older patients (“Is your patient taking too many pills?”). Frankly, this advice applies to all patients, regardless of their age, and to virtually all family physicians. We all have stories about medication overuse. I’d like to tell you 2 of mine.
When Mrs. S, a 68-year-old patient, came to see me for the first time, I scanned her medication list. It included a nasal steroid for allergic rhinitis, a PPI for reflux, and 2 asthma inhalers—albuterol and an inhaled corticosteroid.
I asked her if she had hay fever. She didn’t think so. Heartburn? She said No. A history of asthma? No. So why was she taking these medications? To treat a chronic cough, the patient said. Was the cough better? No.
In the past 12 months, Mrs. S had seen an allergist, a gastroenterologist, and an otolaryngologist. The result? All 3 specialists added their favorite medication. I scanned the patient’s medication list again and noticed that she was taking amitriptyline 25 mg as a sleep aid. Because of the drug’s anticholinergic adverse effects, I had a hunch, and asked her to go one week without the amitriptyline. She agreed.
You can guess the happy ending. Mrs. S’s cough vanished, along with 4 medications she never needed in the first place. She was a victim of the prescribing cascade.
The other story is even more dramatic.
A friend who’s both an FP and a geriatrician became medical director of a local nursing home. To his chagrin, the average number of prescription drugs per resident when he took over was 9.6. Systematically, he went about reevaluating what residents really required. After a year and a half, the average had fallen to 5.4. The residents were no more depressed or agitated, and were generally more alert.
But here’s the catch: I checked back at the nursing home a couple of years after my friend left, and the average number of meds was back up to 10. It takes constant attention to not overprescribe. In fact, I now spend about as much time stopping meds as starting them.
Our health care system is the land of excess. It is up to family physicians—indeed, to all primary care clinicians—to ensure that we only prescribe or continue prescriptions when it’s the right patient, the right medication, at the right time.
Now it’s your turn. Send me your favorite, or most dramatic, medication overtreatment stories for our Letters column. We’ll continue the dialogue there.
AGING: Are these 4 pain myths complicating care?
Beliefs about aging itself can also have dramatic consequences, both positive and negative. In one longitudinal study, those who had positive self-perceptions of aging when they were 50 had better health during 2 decades of follow-up and lived, on average, 7½ years longer than those who had negative self-perceptions at the age of 50.4
Although little research has focused specifically on pain-related stereotypes held by older adults, their importance has long been recognized.
Twenty years ago, a review found that the failure to incorporate older patients’ beliefs about pain could have a negative effect on pain management.5 And in 2011, an Institute of Medicine report found a critical need for public education to counter the myths, misunderstandings, stereotypes, and stigma that hinder pain management in patients across the lifespan.6
We set out to identify widely held stereotypes that older adults and physicians have about pain—and to report on primary studies that support or refute them. We focused on noncancer pain. In the pages that follow, we identify 4 key stereotypes that misrepresent the experience of older adults with regard to pain, and present evidence to debunk them.
Stereotype #1: Pain is a natural part of getting older
Chronic pain is often perceived as an age-related condition. In in-depth interviews, older adults with osteoarthritis reported pain as a normal, even essential, part of life. As one patient put it, “That’s how you know you’re alive … you ache.”7
Among primary care patients with osteoarthritis, those older than 70 years were more likely than younger patients to believe that people should expect to live with pain as they get older.8 And more than half of older adults who responded to a community-based survey considered arthritis to be a natural part of getting old.9
Physicians, too, often view pain as an inevitable part of the aging process, giving patients feedback such as “What do you expect? You’re just getting older.”10
Are they right?
Is pain inevitable? No
In fact, chronic pain is common in older adults, occurring in more than half of those assessed, according to some studies.11 In addition, some epidemiological studies have found an age-related increase in the prevalence of pain,12-14 with older age predicting a more likely onset of, and failure to recover from, persistent pain.15 But numerous studies have failed to find a direct relationship between pain and age.
A National Center for Health Statistics report found that 29% of adults between the ages of 45 and 64 years vs 21% of those 65 or older reported pain lasting >24 hours in the month before the survey.16 And a meta-analysis comparing age-related differences in pain perception found that the highest prevalence of chronic pain occurred at about age 65; a slight decline with advancing age followed, even beyond the age of 85.17
Chronic pain disorders are less frequent. In fact, many chronic pain disorders occur less frequently with advancing age. Population-based studies have found a lower prevalence of low back, neck, and face pain among older adults compared with their younger counterparts;16 evidence has also found lower rates of headache and abdominal pain.18 Other epidemiological studies suggest that the prevalence of musculoskeletal pain generally declines with advancing age,19 and a study of patients in their last 2 years of life found pain to be inversely correlated with age.20 These findings refute the stereotype that advancing age inexorably involves pain, and challenge the notion that pain in later life is normal and expected, and unworthy of treatment.
Stereotype #2: Pain worsens
over time
Some patients and physicians expect that as people age, their pain will increase in intensity. In one study of community-dwelling older adults, 87% of those surveyed rated the belief that more aches and pains are an accepted part of aging as definitely or somewhat true.21 Indeed, patients of all ages have expressed the belief that older age confers greater susceptibility to, and suffering from, painful conditions like arthritis.22 Many common causes of pain in older adults, especially osteoarthritis, are seen as resulting from degenerative changes, which worsen over time.23
Does pain intensify? Not necessarily
Some studies have linked older age to a worse prognosis for patients with musculoskeletal pain, but a greater number have found that aging has no effect on it.24
Pain does not always progress. In a large cohort of patients with peripheral joint osteoarthritis, radiographic joint space narrowing worsened over 3 years, but this did not correlate consistently with worsening pain.25 When the same cohort was assessed after 8 years, there was significant variability in pain, with no clear progression.26
In another study involving older patients with restrictive back pain, the pain was frequently short-lived and episodic and did not increase with age.27 And in a population sample in Norway, the mean number of pain sites decreased slightly over 14 years in those older than 60 years, while increasing in those aged 44 to 60.28 Another study of patients with knee osteoarthritis identified factors that were protective against a decline in pain-related function: These included good mental health, self-efficacy, social support, and greater activity—but not younger age.29 The enormous heterogeneity in both the experience and the course of pain suggests that age-related pain progression is neither universal nor expected—and contradicts a purely biological paradigm in which pain inevitably worsens over time.
Stereotype #3: Stoicism leads to pain tolerance
Some patients believe that the inability to deal with pain is a sign of being soft or weak, and that a “tough it out” approach makes pain easier to tolerate.7 In one survey, older adults were more likely than their younger counterparts to express such stoicism, frequently agreeing with statements like, “I maintain my pride and keep a stiff upper lip when in pain,” “I go on as if nothing had happened …,” and “Pain is something that should be ignored.” 30
Unfortunately, some physicians reinforce such attitudes, telling older patients, in effect, that they’d better “get used to it.”10 And family and friends may make it worse. Patients taking opioids reported that it wasn’t unusual for those close to them to view their use of these analgesics as a sign of weakness.31
Does stoicism help? Probably not
Older adults seem less likely than younger adults to label a sensation as painful, suggesting a more stoic approach in general.30 While some research has found that nociception—the perception of pain in response to painful stimuli—decreases with advancing age,32 other studies have found the opposite.33 And population-based studies focusing on the consequences of pain indicate that it continues to have powerful negative effects, especially depression and insomnia, in older patients.
The degree of pain experienced is more strongly associated with depression in older patients compared with younger adults,34 and greater pain reduces the likelihood that depression will improve with treatment.35 Pain also continues to interfere with sleep. In one national sample, 25% of those with arthritis said they suffered from insomnia, roughly twice the prevalence of insomnia found in those without arthritis.36 In another study, individuals with arthritis were 3 times more likely to have sleep problems compared with individuals without arthritis37—an association independent of age. Being stoic about pain, it appears, does not diminish its consequences over time or help patients better tolerate it.
Stereotype #4: Prescription analgesics are highly addictive
Patients often think that prescription analgesics, especially opioids, are highly addictive or harmful—and older adults may refuse to take them for fear of becoming addicted.7 The stereotype is often shared by family and friends, as well as clinicians.
In one study, one-third of physicians said they hesitated to prescribe opioid medications to older adults because of the risk of addiction (a concern that no clinician with training in geriatrics shared).38 What’s more, 16% of the physicians estimated that about one in 4 older patients receiving chronic opioid therapy becomes addicted. The actual risk is far lower. (More on that below.) News reports of an epidemic of prescription opioid addictions and fatalities,39 including the assertion that opioids are replacing heroin as the primary drug of choice on the street,40 may reinforce such stereotypes.
How great is the risk of addiction? For older adults, it’s very low
While rates of aberrant opioid use vary widely depending on the context, one consistent theme is that older age is associated with decreased risk.41 In one retrospective cohort study of older patients who had recently been started on an opioid medication for the treatment of chronic pain, only 3% showed evidence of behaviors associated with abuse or misuse.42
What’s more, long-term opioid use among older patients with painful conditions is relatively uncommon, and prescription patterns suggest that most older adults discontinue opioids after one or 2 prescriptions.42-44 Decades of research have found that, although opioid medications can cause physiological dependence, addiction is rare in patients treated with them.45,46 (To learn more, see “Diagnosing and treating opioid dependence,” J Fam Pract. 2012;61: 588-597.)
Debunking myths: Implications for practice
Our findings—that pain is not a natural part of aging and often improves or remains stable over time, stoicism does not lead to acclimation, and pain medications are not highly addictive in older adults—make it clear that the stereotypes we identified are misconceptions of pain in later life. Debunking these stereotypes has several implications for clinical practice. We recommend the following:
Identify and counter these stereotypes. Avoid reinforcing stereotypes; counter them by summarizing these evidence-based findings for older patients. We believe patients would be receptive.
In one study, more than 80% of patients with osteoarthritis said they wanted prognostic information about the course of the disease, but only about one-third had received it.47 Presenting the research findings would challenge patients’ stereotypes and help them reframe their expectations.
Elicit patients’ perspectives. Ask patients about age- and pain-related stereotypes and their expectations and perspectives of what constitutes successful treatment. Research shows that patients often wish to discuss lifestyle changes and nonmedical approaches to pain, for example, but that clinicians typically focus on medications instead.48
Emphasize the positive. Frame discussions of pain and aging in a positive light, offering encouragement rather than supporting stoicism or resignation. Attention to protective factors, including good mental health, self-efficacy, social support, and greater activity, may enable older patients to adapt better to any pain they experience.
CORRESPONDENCE
Stephen Thielke, MD, MSPH, MA, University of Washington, Psychiatry and Behavioral Sciences, Box 356560, Seattle, WA 98195; [email protected]
1. Herr K. Pain in the older adult: an imperative across all health care settings. Pain Manag Nurs. 2010;11(2 suppl):S1-S10.
2. Pitkala KH, Strandberg TE, Tilvis RS. Management of nonmalignant pain in home-dwelling older people: a population-based survey. J Am Geriatr Soc. 2002;50:1861-1865.
3. Levy B. Stereotype embodiment: a psychosocial approach to aging. Curr Dir Psychol Sci. 2009;18:332-336.
4. Levy BR, Slade MD, Kasl SV. Longitudinal benefit of positive self-perceptions of aging on functional health. J Gerontol B Psychol Sci Soc Sci. 2002;57:409-417.
5. Hofland SL. Elder beliefs: blocks to pain management. J Gerontol Nurs. 1992;18:19-23.
6. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011.
7. Sale J, Gignac M, Hawker G. How “bad” does the pain have to be? A qualitative study examining adherence to pain medication in older adults with osteoarthritis. Arthritis Rheum. 2006;55:272-278.
8. Appelt CJ, Burant BC, Siminoff LA, et al. Health beliefs related to aging among older male patients with knee and/or hip osteoarthritis. J Gerontol A Biol Sci Med Sci. 2007;62:184-190.
9. Goodwin JS, Black SA, Satish S. Aging versus disease: the opinions of older black, Hispanic, and non-Hispanic white Americans about the causes and treatment of common medical conditions. J Am Geriatr Soc. 1999;47:973-979.
10. Gignac M, Davis A, Hawker G, et al. “What do you expect? You’re just getting older”: a comparison of perceived osteoarthritis-related and aging-related health experiences in middle- and older-age adults. Arthritis Rheum. 2006;55:905-912.
11. Helme RD, Gibson SJ. Pain in the elderly. In: Jensen TS, Turner JA, Weisenfeld-Hallin Z, eds. Progress in Pain Research and Management. Proceedings of the 8th World Congress on Pain. Vol 8. Seattle, Wash: IASP Press; 1997:919–944.
12. Badley EM, Tennant A. Changing profile of joint disorders with age: findings from a postal survey of the population of Calderdale, West Yorkshire, United Kingdom. Ann Rheumatic Dis. 1992;51:366-371.
13. Brattberg G, Parker MG, Thorslund M. A longitudinal study of pain: reported pain from middle age to old age. Clin J Pain. 1997;13:144-149.
14. Crook J, Rideout E, Browne G. The prevalence of pain complaints in a general population. Pain. 1984;18:299-314.
15. Gureje O, Simon GE, Von Korff M. A cross-national study of the course of persistent pain in primary care. Pain. 2001;92:195-200.
16. National Center for Health Statistics. Special feature: pain. In: Health, United States, 2006 with Chartbook on Trends in the Health of Americans. Hyattsville, Md: Centers for Disease Control and Prevention; 2006:68–87. Available at: http://www.cdc.gov/nchs/data/hus/hus06.pdf. Accessed October 16, 2012.
17. Gibson SJ, Helme RD. Age differences in pain perception and report: a review of physiological, psychological, laboratory and clinical studies. Pain Rev. 1995;2:111-137.
18. Gallagher RM, Verma S, Mossey J. Chronic pain. Sources of late-life pain and risk factors for disability. Geriatrics. 2000;55:40-44, 47.
19. Picavet HS, Schouten JS. Musculoskeletal pain in the Netherlands: prevalences, consequences and risk groups, the DMC(3)-study. Pain. 2003;102:167-178.
20. Smith AK, Cenzer IS, Knight SJ, et al. The epidemiology of pain during the last 2 years of life. Ann Intern Med. 2010;153:563-569.
21. Sarkisian CA, Hays RD, Mangione CM. Do older adults expect to age successfully? The association between expectations regarding aging and beliefs regarding healthcare seeking among older adults. J Am Geriatr Soc. 2002;50:1837-1843.
22. Keller ML, Leventhal H, Prohaska TR, et al. Beliefs about aging and illness in a community sample. Res Nurs Health. 1989;12:247-255.
23. Dougados M, Gueguen A, Nguyen M, et al. Longitudinal radiologic evaluation of osteoarthritis of the knee. J Rheumatology. 1992;19:378-384.
24. Mallen CD, Peat G, Thomas E, et al. Prognostic factors for musculoskeletal pain in primary care: a systematic review. Br J Gen Pract. 2007;57:655-661.
25. Dieppe PA, Cushnaghan J, Shepstone L. The Bristol ‘OA500’ study: progression of osteoarthritis (OA) over 3 years and the relationship between clinical and radiographic changes at the knee joint. Osteoarthritis Cartilage. 1997;5:87-97.
26. Dieppe P, Cushnaghan J, Tucker M, et al. The Bristol ‘OA500 study’: progression and impact of the disease after 8 years. Osteoarthritis Cartilage. 2000;8:63-68.
27. Makris UE, Fraenkel L, Han L, et al. Epidemiology of restricting back pain in community-living older persons. J Am Geriatr Soc. 2011;59:610-614.
28. Kamaleri Y, Natvig B, Ihlebaek CM, et al. Change in the number of musculoskeletal pain sites: a 14-year prospective study. Pain. 2009;141:25-30.
29. Sharma L, Cahue S, Song J, et al. Physical functioning over three years in knee osteoarthritis: role of psychosocial, local mechanical, and neuromuscular factors. Arthritis Rheum. 2003;48:3359-3370.
30. Yong HH, Gibson SJ, Horne DJ, et al. Development of a pin attitudes questionnaire to assess stoicism and cautiousness for possible age differences. J Gerontol B Psychol Sci Soc Sci. 2001;56:279-284.
31. Vallerand A, Nowak L. Chronic opioid therapy for nonmalignant pain: the patient’s perspective. Part II—barriers to chronic opioid therapy. Pain manag nurs. 2010;11:126-131.
32. Gibson SJ, Farrell M. A review of age differences in the neurophysiology of nociception and the perceptual experience of pain. Clin J Pain. 2004;20:227-239.
33. Woodrow KM, Friedman GD, Siegelaub AB, et al. Pain tolerance: differences according to age, sex and race. Psychosom Med. 1972;34:548-556.
34. Turk DC, Okifuji A, Scharff L. Chronic pain and depression: role of perceived impact and perceived control in different age cohorts. Pain. 1995;61:93-101.
35. Thielke SM, Fan MY, Sullivan M, et al. Pain limits the effectiveness of collaborative care for depression. Am J Geriatr Psychiatry. 2007;15:699-707.
36. Power JD, Perruccio AV, Badley EM. Pain as a mediator of sleep problems in arthritis and other chronic conditions. Arthritis Rheum. 2005;53:911-919.
37. Louie GH, Tektonidou MG, Caban-Martizen AJ, et al. Sleep disturbances in adults with arthritis: prevalence, mediators, and subgroups at greatest risk. Arthritis Care Res. 2011;63:247-260.
38. Lin JJ, Alfandre D, Moore C. Physician attitudes toward opioid prescribing for patients with persistent noncancer pain. Clin J Pain. 2007;23:799-803.
39. Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613-2620.
40. Fischer B, Gittins J, Kendall P, et al. Thinking the unthinkable: could the increasing misuse of prescription opioids among street drug users offer benefits for public health? Public Health. 2009;123:145-146.
41. Fleming MF, Davis J, Passik SD. Reported lifetime aberrant drug-taking behaviors are predictive of current substance use and mental health problems in primary care patients. Pain Med. 2008;9:1098-1106.
42. Reid MC, Henderson CR, Jr, Papaleontiou M, et al. Characteristics of older adults receiving opioids in primary care: treatment duration and outcomes. Pain Med. 2010;11:1063-1071.
43. Solomon DH, Rassen JA, Glynn RJ, et al. The comparative safety of opioids for nonmalignant pain in older adults. Arch Intern Med. 2010;170:1979-1986.
44. Thielke SM, Simoni-Wastila L, Edlund MJ, et al. Age and sex trends in long-term opioid use in two large American health systems between 2000 and 2005. Pain Med. 2010;11:248-256.
45. Soden K, Ali S, Alloway L, et al. How do nurses assess and manage breakthrough pain in specialist palliative care inpatient units? A multicentre study. Palliat Med. 2010;24:294-298.
46. Papaleontiou M, Henderson CR, Jr, Turner BJ, et al. Outcomes associated with opioid use in the treatment of chronic noncancer pain in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2010;58:1353-1369.
47. Mallen CD, Peat G. Discussing prognosis with older people with musculoskeletal pain: a cross-sectional study in general practice. BMC Fam Pract. 2009;10:50.-
48. Rosemann T, Wensing M, Joest K, et al. Problems and needs for improving primary care of osteoarthritis patients: the views of patients, general practitioners and practice nurses. BMC Musculoskelet Disord. 2006;7:48.
Beliefs about aging itself can also have dramatic consequences, both positive and negative. In one longitudinal study, those who had positive self-perceptions of aging when they were 50 had better health during 2 decades of follow-up and lived, on average, 7½ years longer than those who had negative self-perceptions at the age of 50.4
Although little research has focused specifically on pain-related stereotypes held by older adults, their importance has long been recognized.
Twenty years ago, a review found that the failure to incorporate older patients’ beliefs about pain could have a negative effect on pain management.5 And in 2011, an Institute of Medicine report found a critical need for public education to counter the myths, misunderstandings, stereotypes, and stigma that hinder pain management in patients across the lifespan.6
We set out to identify widely held stereotypes that older adults and physicians have about pain—and to report on primary studies that support or refute them. We focused on noncancer pain. In the pages that follow, we identify 4 key stereotypes that misrepresent the experience of older adults with regard to pain, and present evidence to debunk them.
Stereotype #1: Pain is a natural part of getting older
Chronic pain is often perceived as an age-related condition. In in-depth interviews, older adults with osteoarthritis reported pain as a normal, even essential, part of life. As one patient put it, “That’s how you know you’re alive … you ache.”7
Among primary care patients with osteoarthritis, those older than 70 years were more likely than younger patients to believe that people should expect to live with pain as they get older.8 And more than half of older adults who responded to a community-based survey considered arthritis to be a natural part of getting old.9
Physicians, too, often view pain as an inevitable part of the aging process, giving patients feedback such as “What do you expect? You’re just getting older.”10
Are they right?
Is pain inevitable? No
In fact, chronic pain is common in older adults, occurring in more than half of those assessed, according to some studies.11 In addition, some epidemiological studies have found an age-related increase in the prevalence of pain,12-14 with older age predicting a more likely onset of, and failure to recover from, persistent pain.15 But numerous studies have failed to find a direct relationship between pain and age.
A National Center for Health Statistics report found that 29% of adults between the ages of 45 and 64 years vs 21% of those 65 or older reported pain lasting >24 hours in the month before the survey.16 And a meta-analysis comparing age-related differences in pain perception found that the highest prevalence of chronic pain occurred at about age 65; a slight decline with advancing age followed, even beyond the age of 85.17
Chronic pain disorders are less frequent. In fact, many chronic pain disorders occur less frequently with advancing age. Population-based studies have found a lower prevalence of low back, neck, and face pain among older adults compared with their younger counterparts;16 evidence has also found lower rates of headache and abdominal pain.18 Other epidemiological studies suggest that the prevalence of musculoskeletal pain generally declines with advancing age,19 and a study of patients in their last 2 years of life found pain to be inversely correlated with age.20 These findings refute the stereotype that advancing age inexorably involves pain, and challenge the notion that pain in later life is normal and expected, and unworthy of treatment.
Stereotype #2: Pain worsens
over time
Some patients and physicians expect that as people age, their pain will increase in intensity. In one study of community-dwelling older adults, 87% of those surveyed rated the belief that more aches and pains are an accepted part of aging as definitely or somewhat true.21 Indeed, patients of all ages have expressed the belief that older age confers greater susceptibility to, and suffering from, painful conditions like arthritis.22 Many common causes of pain in older adults, especially osteoarthritis, are seen as resulting from degenerative changes, which worsen over time.23
Does pain intensify? Not necessarily
Some studies have linked older age to a worse prognosis for patients with musculoskeletal pain, but a greater number have found that aging has no effect on it.24
Pain does not always progress. In a large cohort of patients with peripheral joint osteoarthritis, radiographic joint space narrowing worsened over 3 years, but this did not correlate consistently with worsening pain.25 When the same cohort was assessed after 8 years, there was significant variability in pain, with no clear progression.26
In another study involving older patients with restrictive back pain, the pain was frequently short-lived and episodic and did not increase with age.27 And in a population sample in Norway, the mean number of pain sites decreased slightly over 14 years in those older than 60 years, while increasing in those aged 44 to 60.28 Another study of patients with knee osteoarthritis identified factors that were protective against a decline in pain-related function: These included good mental health, self-efficacy, social support, and greater activity—but not younger age.29 The enormous heterogeneity in both the experience and the course of pain suggests that age-related pain progression is neither universal nor expected—and contradicts a purely biological paradigm in which pain inevitably worsens over time.
Stereotype #3: Stoicism leads to pain tolerance
Some patients believe that the inability to deal with pain is a sign of being soft or weak, and that a “tough it out” approach makes pain easier to tolerate.7 In one survey, older adults were more likely than their younger counterparts to express such stoicism, frequently agreeing with statements like, “I maintain my pride and keep a stiff upper lip when in pain,” “I go on as if nothing had happened …,” and “Pain is something that should be ignored.” 30
Unfortunately, some physicians reinforce such attitudes, telling older patients, in effect, that they’d better “get used to it.”10 And family and friends may make it worse. Patients taking opioids reported that it wasn’t unusual for those close to them to view their use of these analgesics as a sign of weakness.31
Does stoicism help? Probably not
Older adults seem less likely than younger adults to label a sensation as painful, suggesting a more stoic approach in general.30 While some research has found that nociception—the perception of pain in response to painful stimuli—decreases with advancing age,32 other studies have found the opposite.33 And population-based studies focusing on the consequences of pain indicate that it continues to have powerful negative effects, especially depression and insomnia, in older patients.
The degree of pain experienced is more strongly associated with depression in older patients compared with younger adults,34 and greater pain reduces the likelihood that depression will improve with treatment.35 Pain also continues to interfere with sleep. In one national sample, 25% of those with arthritis said they suffered from insomnia, roughly twice the prevalence of insomnia found in those without arthritis.36 In another study, individuals with arthritis were 3 times more likely to have sleep problems compared with individuals without arthritis37—an association independent of age. Being stoic about pain, it appears, does not diminish its consequences over time or help patients better tolerate it.
Stereotype #4: Prescription analgesics are highly addictive
Patients often think that prescription analgesics, especially opioids, are highly addictive or harmful—and older adults may refuse to take them for fear of becoming addicted.7 The stereotype is often shared by family and friends, as well as clinicians.
In one study, one-third of physicians said they hesitated to prescribe opioid medications to older adults because of the risk of addiction (a concern that no clinician with training in geriatrics shared).38 What’s more, 16% of the physicians estimated that about one in 4 older patients receiving chronic opioid therapy becomes addicted. The actual risk is far lower. (More on that below.) News reports of an epidemic of prescription opioid addictions and fatalities,39 including the assertion that opioids are replacing heroin as the primary drug of choice on the street,40 may reinforce such stereotypes.
How great is the risk of addiction? For older adults, it’s very low
While rates of aberrant opioid use vary widely depending on the context, one consistent theme is that older age is associated with decreased risk.41 In one retrospective cohort study of older patients who had recently been started on an opioid medication for the treatment of chronic pain, only 3% showed evidence of behaviors associated with abuse or misuse.42
What’s more, long-term opioid use among older patients with painful conditions is relatively uncommon, and prescription patterns suggest that most older adults discontinue opioids after one or 2 prescriptions.42-44 Decades of research have found that, although opioid medications can cause physiological dependence, addiction is rare in patients treated with them.45,46 (To learn more, see “Diagnosing and treating opioid dependence,” J Fam Pract. 2012;61: 588-597.)
Debunking myths: Implications for practice
Our findings—that pain is not a natural part of aging and often improves or remains stable over time, stoicism does not lead to acclimation, and pain medications are not highly addictive in older adults—make it clear that the stereotypes we identified are misconceptions of pain in later life. Debunking these stereotypes has several implications for clinical practice. We recommend the following:
Identify and counter these stereotypes. Avoid reinforcing stereotypes; counter them by summarizing these evidence-based findings for older patients. We believe patients would be receptive.
In one study, more than 80% of patients with osteoarthritis said they wanted prognostic information about the course of the disease, but only about one-third had received it.47 Presenting the research findings would challenge patients’ stereotypes and help them reframe their expectations.
Elicit patients’ perspectives. Ask patients about age- and pain-related stereotypes and their expectations and perspectives of what constitutes successful treatment. Research shows that patients often wish to discuss lifestyle changes and nonmedical approaches to pain, for example, but that clinicians typically focus on medications instead.48
Emphasize the positive. Frame discussions of pain and aging in a positive light, offering encouragement rather than supporting stoicism or resignation. Attention to protective factors, including good mental health, self-efficacy, social support, and greater activity, may enable older patients to adapt better to any pain they experience.
CORRESPONDENCE
Stephen Thielke, MD, MSPH, MA, University of Washington, Psychiatry and Behavioral Sciences, Box 356560, Seattle, WA 98195; [email protected]
Beliefs about aging itself can also have dramatic consequences, both positive and negative. In one longitudinal study, those who had positive self-perceptions of aging when they were 50 had better health during 2 decades of follow-up and lived, on average, 7½ years longer than those who had negative self-perceptions at the age of 50.4
Although little research has focused specifically on pain-related stereotypes held by older adults, their importance has long been recognized.
Twenty years ago, a review found that the failure to incorporate older patients’ beliefs about pain could have a negative effect on pain management.5 And in 2011, an Institute of Medicine report found a critical need for public education to counter the myths, misunderstandings, stereotypes, and stigma that hinder pain management in patients across the lifespan.6
We set out to identify widely held stereotypes that older adults and physicians have about pain—and to report on primary studies that support or refute them. We focused on noncancer pain. In the pages that follow, we identify 4 key stereotypes that misrepresent the experience of older adults with regard to pain, and present evidence to debunk them.
Stereotype #1: Pain is a natural part of getting older
Chronic pain is often perceived as an age-related condition. In in-depth interviews, older adults with osteoarthritis reported pain as a normal, even essential, part of life. As one patient put it, “That’s how you know you’re alive … you ache.”7
Among primary care patients with osteoarthritis, those older than 70 years were more likely than younger patients to believe that people should expect to live with pain as they get older.8 And more than half of older adults who responded to a community-based survey considered arthritis to be a natural part of getting old.9
Physicians, too, often view pain as an inevitable part of the aging process, giving patients feedback such as “What do you expect? You’re just getting older.”10
Are they right?
Is pain inevitable? No
In fact, chronic pain is common in older adults, occurring in more than half of those assessed, according to some studies.11 In addition, some epidemiological studies have found an age-related increase in the prevalence of pain,12-14 with older age predicting a more likely onset of, and failure to recover from, persistent pain.15 But numerous studies have failed to find a direct relationship between pain and age.
A National Center for Health Statistics report found that 29% of adults between the ages of 45 and 64 years vs 21% of those 65 or older reported pain lasting >24 hours in the month before the survey.16 And a meta-analysis comparing age-related differences in pain perception found that the highest prevalence of chronic pain occurred at about age 65; a slight decline with advancing age followed, even beyond the age of 85.17
Chronic pain disorders are less frequent. In fact, many chronic pain disorders occur less frequently with advancing age. Population-based studies have found a lower prevalence of low back, neck, and face pain among older adults compared with their younger counterparts;16 evidence has also found lower rates of headache and abdominal pain.18 Other epidemiological studies suggest that the prevalence of musculoskeletal pain generally declines with advancing age,19 and a study of patients in their last 2 years of life found pain to be inversely correlated with age.20 These findings refute the stereotype that advancing age inexorably involves pain, and challenge the notion that pain in later life is normal and expected, and unworthy of treatment.
Stereotype #2: Pain worsens
over time
Some patients and physicians expect that as people age, their pain will increase in intensity. In one study of community-dwelling older adults, 87% of those surveyed rated the belief that more aches and pains are an accepted part of aging as definitely or somewhat true.21 Indeed, patients of all ages have expressed the belief that older age confers greater susceptibility to, and suffering from, painful conditions like arthritis.22 Many common causes of pain in older adults, especially osteoarthritis, are seen as resulting from degenerative changes, which worsen over time.23
Does pain intensify? Not necessarily
Some studies have linked older age to a worse prognosis for patients with musculoskeletal pain, but a greater number have found that aging has no effect on it.24
Pain does not always progress. In a large cohort of patients with peripheral joint osteoarthritis, radiographic joint space narrowing worsened over 3 years, but this did not correlate consistently with worsening pain.25 When the same cohort was assessed after 8 years, there was significant variability in pain, with no clear progression.26
In another study involving older patients with restrictive back pain, the pain was frequently short-lived and episodic and did not increase with age.27 And in a population sample in Norway, the mean number of pain sites decreased slightly over 14 years in those older than 60 years, while increasing in those aged 44 to 60.28 Another study of patients with knee osteoarthritis identified factors that were protective against a decline in pain-related function: These included good mental health, self-efficacy, social support, and greater activity—but not younger age.29 The enormous heterogeneity in both the experience and the course of pain suggests that age-related pain progression is neither universal nor expected—and contradicts a purely biological paradigm in which pain inevitably worsens over time.
Stereotype #3: Stoicism leads to pain tolerance
Some patients believe that the inability to deal with pain is a sign of being soft or weak, and that a “tough it out” approach makes pain easier to tolerate.7 In one survey, older adults were more likely than their younger counterparts to express such stoicism, frequently agreeing with statements like, “I maintain my pride and keep a stiff upper lip when in pain,” “I go on as if nothing had happened …,” and “Pain is something that should be ignored.” 30
Unfortunately, some physicians reinforce such attitudes, telling older patients, in effect, that they’d better “get used to it.”10 And family and friends may make it worse. Patients taking opioids reported that it wasn’t unusual for those close to them to view their use of these analgesics as a sign of weakness.31
Does stoicism help? Probably not
Older adults seem less likely than younger adults to label a sensation as painful, suggesting a more stoic approach in general.30 While some research has found that nociception—the perception of pain in response to painful stimuli—decreases with advancing age,32 other studies have found the opposite.33 And population-based studies focusing on the consequences of pain indicate that it continues to have powerful negative effects, especially depression and insomnia, in older patients.
The degree of pain experienced is more strongly associated with depression in older patients compared with younger adults,34 and greater pain reduces the likelihood that depression will improve with treatment.35 Pain also continues to interfere with sleep. In one national sample, 25% of those with arthritis said they suffered from insomnia, roughly twice the prevalence of insomnia found in those without arthritis.36 In another study, individuals with arthritis were 3 times more likely to have sleep problems compared with individuals without arthritis37—an association independent of age. Being stoic about pain, it appears, does not diminish its consequences over time or help patients better tolerate it.
Stereotype #4: Prescription analgesics are highly addictive
Patients often think that prescription analgesics, especially opioids, are highly addictive or harmful—and older adults may refuse to take them for fear of becoming addicted.7 The stereotype is often shared by family and friends, as well as clinicians.
In one study, one-third of physicians said they hesitated to prescribe opioid medications to older adults because of the risk of addiction (a concern that no clinician with training in geriatrics shared).38 What’s more, 16% of the physicians estimated that about one in 4 older patients receiving chronic opioid therapy becomes addicted. The actual risk is far lower. (More on that below.) News reports of an epidemic of prescription opioid addictions and fatalities,39 including the assertion that opioids are replacing heroin as the primary drug of choice on the street,40 may reinforce such stereotypes.
How great is the risk of addiction? For older adults, it’s very low
While rates of aberrant opioid use vary widely depending on the context, one consistent theme is that older age is associated with decreased risk.41 In one retrospective cohort study of older patients who had recently been started on an opioid medication for the treatment of chronic pain, only 3% showed evidence of behaviors associated with abuse or misuse.42
What’s more, long-term opioid use among older patients with painful conditions is relatively uncommon, and prescription patterns suggest that most older adults discontinue opioids after one or 2 prescriptions.42-44 Decades of research have found that, although opioid medications can cause physiological dependence, addiction is rare in patients treated with them.45,46 (To learn more, see “Diagnosing and treating opioid dependence,” J Fam Pract. 2012;61: 588-597.)
Debunking myths: Implications for practice
Our findings—that pain is not a natural part of aging and often improves or remains stable over time, stoicism does not lead to acclimation, and pain medications are not highly addictive in older adults—make it clear that the stereotypes we identified are misconceptions of pain in later life. Debunking these stereotypes has several implications for clinical practice. We recommend the following:
Identify and counter these stereotypes. Avoid reinforcing stereotypes; counter them by summarizing these evidence-based findings for older patients. We believe patients would be receptive.
In one study, more than 80% of patients with osteoarthritis said they wanted prognostic information about the course of the disease, but only about one-third had received it.47 Presenting the research findings would challenge patients’ stereotypes and help them reframe their expectations.
Elicit patients’ perspectives. Ask patients about age- and pain-related stereotypes and their expectations and perspectives of what constitutes successful treatment. Research shows that patients often wish to discuss lifestyle changes and nonmedical approaches to pain, for example, but that clinicians typically focus on medications instead.48
Emphasize the positive. Frame discussions of pain and aging in a positive light, offering encouragement rather than supporting stoicism or resignation. Attention to protective factors, including good mental health, self-efficacy, social support, and greater activity, may enable older patients to adapt better to any pain they experience.
CORRESPONDENCE
Stephen Thielke, MD, MSPH, MA, University of Washington, Psychiatry and Behavioral Sciences, Box 356560, Seattle, WA 98195; [email protected]
1. Herr K. Pain in the older adult: an imperative across all health care settings. Pain Manag Nurs. 2010;11(2 suppl):S1-S10.
2. Pitkala KH, Strandberg TE, Tilvis RS. Management of nonmalignant pain in home-dwelling older people: a population-based survey. J Am Geriatr Soc. 2002;50:1861-1865.
3. Levy B. Stereotype embodiment: a psychosocial approach to aging. Curr Dir Psychol Sci. 2009;18:332-336.
4. Levy BR, Slade MD, Kasl SV. Longitudinal benefit of positive self-perceptions of aging on functional health. J Gerontol B Psychol Sci Soc Sci. 2002;57:409-417.
5. Hofland SL. Elder beliefs: blocks to pain management. J Gerontol Nurs. 1992;18:19-23.
6. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011.
7. Sale J, Gignac M, Hawker G. How “bad” does the pain have to be? A qualitative study examining adherence to pain medication in older adults with osteoarthritis. Arthritis Rheum. 2006;55:272-278.
8. Appelt CJ, Burant BC, Siminoff LA, et al. Health beliefs related to aging among older male patients with knee and/or hip osteoarthritis. J Gerontol A Biol Sci Med Sci. 2007;62:184-190.
9. Goodwin JS, Black SA, Satish S. Aging versus disease: the opinions of older black, Hispanic, and non-Hispanic white Americans about the causes and treatment of common medical conditions. J Am Geriatr Soc. 1999;47:973-979.
10. Gignac M, Davis A, Hawker G, et al. “What do you expect? You’re just getting older”: a comparison of perceived osteoarthritis-related and aging-related health experiences in middle- and older-age adults. Arthritis Rheum. 2006;55:905-912.
11. Helme RD, Gibson SJ. Pain in the elderly. In: Jensen TS, Turner JA, Weisenfeld-Hallin Z, eds. Progress in Pain Research and Management. Proceedings of the 8th World Congress on Pain. Vol 8. Seattle, Wash: IASP Press; 1997:919–944.
12. Badley EM, Tennant A. Changing profile of joint disorders with age: findings from a postal survey of the population of Calderdale, West Yorkshire, United Kingdom. Ann Rheumatic Dis. 1992;51:366-371.
13. Brattberg G, Parker MG, Thorslund M. A longitudinal study of pain: reported pain from middle age to old age. Clin J Pain. 1997;13:144-149.
14. Crook J, Rideout E, Browne G. The prevalence of pain complaints in a general population. Pain. 1984;18:299-314.
15. Gureje O, Simon GE, Von Korff M. A cross-national study of the course of persistent pain in primary care. Pain. 2001;92:195-200.
16. National Center for Health Statistics. Special feature: pain. In: Health, United States, 2006 with Chartbook on Trends in the Health of Americans. Hyattsville, Md: Centers for Disease Control and Prevention; 2006:68–87. Available at: http://www.cdc.gov/nchs/data/hus/hus06.pdf. Accessed October 16, 2012.
17. Gibson SJ, Helme RD. Age differences in pain perception and report: a review of physiological, psychological, laboratory and clinical studies. Pain Rev. 1995;2:111-137.
18. Gallagher RM, Verma S, Mossey J. Chronic pain. Sources of late-life pain and risk factors for disability. Geriatrics. 2000;55:40-44, 47.
19. Picavet HS, Schouten JS. Musculoskeletal pain in the Netherlands: prevalences, consequences and risk groups, the DMC(3)-study. Pain. 2003;102:167-178.
20. Smith AK, Cenzer IS, Knight SJ, et al. The epidemiology of pain during the last 2 years of life. Ann Intern Med. 2010;153:563-569.
21. Sarkisian CA, Hays RD, Mangione CM. Do older adults expect to age successfully? The association between expectations regarding aging and beliefs regarding healthcare seeking among older adults. J Am Geriatr Soc. 2002;50:1837-1843.
22. Keller ML, Leventhal H, Prohaska TR, et al. Beliefs about aging and illness in a community sample. Res Nurs Health. 1989;12:247-255.
23. Dougados M, Gueguen A, Nguyen M, et al. Longitudinal radiologic evaluation of osteoarthritis of the knee. J Rheumatology. 1992;19:378-384.
24. Mallen CD, Peat G, Thomas E, et al. Prognostic factors for musculoskeletal pain in primary care: a systematic review. Br J Gen Pract. 2007;57:655-661.
25. Dieppe PA, Cushnaghan J, Shepstone L. The Bristol ‘OA500’ study: progression of osteoarthritis (OA) over 3 years and the relationship between clinical and radiographic changes at the knee joint. Osteoarthritis Cartilage. 1997;5:87-97.
26. Dieppe P, Cushnaghan J, Tucker M, et al. The Bristol ‘OA500 study’: progression and impact of the disease after 8 years. Osteoarthritis Cartilage. 2000;8:63-68.
27. Makris UE, Fraenkel L, Han L, et al. Epidemiology of restricting back pain in community-living older persons. J Am Geriatr Soc. 2011;59:610-614.
28. Kamaleri Y, Natvig B, Ihlebaek CM, et al. Change in the number of musculoskeletal pain sites: a 14-year prospective study. Pain. 2009;141:25-30.
29. Sharma L, Cahue S, Song J, et al. Physical functioning over three years in knee osteoarthritis: role of psychosocial, local mechanical, and neuromuscular factors. Arthritis Rheum. 2003;48:3359-3370.
30. Yong HH, Gibson SJ, Horne DJ, et al. Development of a pin attitudes questionnaire to assess stoicism and cautiousness for possible age differences. J Gerontol B Psychol Sci Soc Sci. 2001;56:279-284.
31. Vallerand A, Nowak L. Chronic opioid therapy for nonmalignant pain: the patient’s perspective. Part II—barriers to chronic opioid therapy. Pain manag nurs. 2010;11:126-131.
32. Gibson SJ, Farrell M. A review of age differences in the neurophysiology of nociception and the perceptual experience of pain. Clin J Pain. 2004;20:227-239.
33. Woodrow KM, Friedman GD, Siegelaub AB, et al. Pain tolerance: differences according to age, sex and race. Psychosom Med. 1972;34:548-556.
34. Turk DC, Okifuji A, Scharff L. Chronic pain and depression: role of perceived impact and perceived control in different age cohorts. Pain. 1995;61:93-101.
35. Thielke SM, Fan MY, Sullivan M, et al. Pain limits the effectiveness of collaborative care for depression. Am J Geriatr Psychiatry. 2007;15:699-707.
36. Power JD, Perruccio AV, Badley EM. Pain as a mediator of sleep problems in arthritis and other chronic conditions. Arthritis Rheum. 2005;53:911-919.
37. Louie GH, Tektonidou MG, Caban-Martizen AJ, et al. Sleep disturbances in adults with arthritis: prevalence, mediators, and subgroups at greatest risk. Arthritis Care Res. 2011;63:247-260.
38. Lin JJ, Alfandre D, Moore C. Physician attitudes toward opioid prescribing for patients with persistent noncancer pain. Clin J Pain. 2007;23:799-803.
39. Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613-2620.
40. Fischer B, Gittins J, Kendall P, et al. Thinking the unthinkable: could the increasing misuse of prescription opioids among street drug users offer benefits for public health? Public Health. 2009;123:145-146.
41. Fleming MF, Davis J, Passik SD. Reported lifetime aberrant drug-taking behaviors are predictive of current substance use and mental health problems in primary care patients. Pain Med. 2008;9:1098-1106.
42. Reid MC, Henderson CR, Jr, Papaleontiou M, et al. Characteristics of older adults receiving opioids in primary care: treatment duration and outcomes. Pain Med. 2010;11:1063-1071.
43. Solomon DH, Rassen JA, Glynn RJ, et al. The comparative safety of opioids for nonmalignant pain in older adults. Arch Intern Med. 2010;170:1979-1986.
44. Thielke SM, Simoni-Wastila L, Edlund MJ, et al. Age and sex trends in long-term opioid use in two large American health systems between 2000 and 2005. Pain Med. 2010;11:248-256.
45. Soden K, Ali S, Alloway L, et al. How do nurses assess and manage breakthrough pain in specialist palliative care inpatient units? A multicentre study. Palliat Med. 2010;24:294-298.
46. Papaleontiou M, Henderson CR, Jr, Turner BJ, et al. Outcomes associated with opioid use in the treatment of chronic noncancer pain in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2010;58:1353-1369.
47. Mallen CD, Peat G. Discussing prognosis with older people with musculoskeletal pain: a cross-sectional study in general practice. BMC Fam Pract. 2009;10:50.-
48. Rosemann T, Wensing M, Joest K, et al. Problems and needs for improving primary care of osteoarthritis patients: the views of patients, general practitioners and practice nurses. BMC Musculoskelet Disord. 2006;7:48.
1. Herr K. Pain in the older adult: an imperative across all health care settings. Pain Manag Nurs. 2010;11(2 suppl):S1-S10.
2. Pitkala KH, Strandberg TE, Tilvis RS. Management of nonmalignant pain in home-dwelling older people: a population-based survey. J Am Geriatr Soc. 2002;50:1861-1865.
3. Levy B. Stereotype embodiment: a psychosocial approach to aging. Curr Dir Psychol Sci. 2009;18:332-336.
4. Levy BR, Slade MD, Kasl SV. Longitudinal benefit of positive self-perceptions of aging on functional health. J Gerontol B Psychol Sci Soc Sci. 2002;57:409-417.
5. Hofland SL. Elder beliefs: blocks to pain management. J Gerontol Nurs. 1992;18:19-23.
6. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011.
7. Sale J, Gignac M, Hawker G. How “bad” does the pain have to be? A qualitative study examining adherence to pain medication in older adults with osteoarthritis. Arthritis Rheum. 2006;55:272-278.
8. Appelt CJ, Burant BC, Siminoff LA, et al. Health beliefs related to aging among older male patients with knee and/or hip osteoarthritis. J Gerontol A Biol Sci Med Sci. 2007;62:184-190.
9. Goodwin JS, Black SA, Satish S. Aging versus disease: the opinions of older black, Hispanic, and non-Hispanic white Americans about the causes and treatment of common medical conditions. J Am Geriatr Soc. 1999;47:973-979.
10. Gignac M, Davis A, Hawker G, et al. “What do you expect? You’re just getting older”: a comparison of perceived osteoarthritis-related and aging-related health experiences in middle- and older-age adults. Arthritis Rheum. 2006;55:905-912.
11. Helme RD, Gibson SJ. Pain in the elderly. In: Jensen TS, Turner JA, Weisenfeld-Hallin Z, eds. Progress in Pain Research and Management. Proceedings of the 8th World Congress on Pain. Vol 8. Seattle, Wash: IASP Press; 1997:919–944.
12. Badley EM, Tennant A. Changing profile of joint disorders with age: findings from a postal survey of the population of Calderdale, West Yorkshire, United Kingdom. Ann Rheumatic Dis. 1992;51:366-371.
13. Brattberg G, Parker MG, Thorslund M. A longitudinal study of pain: reported pain from middle age to old age. Clin J Pain. 1997;13:144-149.
14. Crook J, Rideout E, Browne G. The prevalence of pain complaints in a general population. Pain. 1984;18:299-314.
15. Gureje O, Simon GE, Von Korff M. A cross-national study of the course of persistent pain in primary care. Pain. 2001;92:195-200.
16. National Center for Health Statistics. Special feature: pain. In: Health, United States, 2006 with Chartbook on Trends in the Health of Americans. Hyattsville, Md: Centers for Disease Control and Prevention; 2006:68–87. Available at: http://www.cdc.gov/nchs/data/hus/hus06.pdf. Accessed October 16, 2012.
17. Gibson SJ, Helme RD. Age differences in pain perception and report: a review of physiological, psychological, laboratory and clinical studies. Pain Rev. 1995;2:111-137.
18. Gallagher RM, Verma S, Mossey J. Chronic pain. Sources of late-life pain and risk factors for disability. Geriatrics. 2000;55:40-44, 47.
19. Picavet HS, Schouten JS. Musculoskeletal pain in the Netherlands: prevalences, consequences and risk groups, the DMC(3)-study. Pain. 2003;102:167-178.
20. Smith AK, Cenzer IS, Knight SJ, et al. The epidemiology of pain during the last 2 years of life. Ann Intern Med. 2010;153:563-569.
21. Sarkisian CA, Hays RD, Mangione CM. Do older adults expect to age successfully? The association between expectations regarding aging and beliefs regarding healthcare seeking among older adults. J Am Geriatr Soc. 2002;50:1837-1843.
22. Keller ML, Leventhal H, Prohaska TR, et al. Beliefs about aging and illness in a community sample. Res Nurs Health. 1989;12:247-255.
23. Dougados M, Gueguen A, Nguyen M, et al. Longitudinal radiologic evaluation of osteoarthritis of the knee. J Rheumatology. 1992;19:378-384.
24. Mallen CD, Peat G, Thomas E, et al. Prognostic factors for musculoskeletal pain in primary care: a systematic review. Br J Gen Pract. 2007;57:655-661.
25. Dieppe PA, Cushnaghan J, Shepstone L. The Bristol ‘OA500’ study: progression of osteoarthritis (OA) over 3 years and the relationship between clinical and radiographic changes at the knee joint. Osteoarthritis Cartilage. 1997;5:87-97.
26. Dieppe P, Cushnaghan J, Tucker M, et al. The Bristol ‘OA500 study’: progression and impact of the disease after 8 years. Osteoarthritis Cartilage. 2000;8:63-68.
27. Makris UE, Fraenkel L, Han L, et al. Epidemiology of restricting back pain in community-living older persons. J Am Geriatr Soc. 2011;59:610-614.
28. Kamaleri Y, Natvig B, Ihlebaek CM, et al. Change in the number of musculoskeletal pain sites: a 14-year prospective study. Pain. 2009;141:25-30.
29. Sharma L, Cahue S, Song J, et al. Physical functioning over three years in knee osteoarthritis: role of psychosocial, local mechanical, and neuromuscular factors. Arthritis Rheum. 2003;48:3359-3370.
30. Yong HH, Gibson SJ, Horne DJ, et al. Development of a pin attitudes questionnaire to assess stoicism and cautiousness for possible age differences. J Gerontol B Psychol Sci Soc Sci. 2001;56:279-284.
31. Vallerand A, Nowak L. Chronic opioid therapy for nonmalignant pain: the patient’s perspective. Part II—barriers to chronic opioid therapy. Pain manag nurs. 2010;11:126-131.
32. Gibson SJ, Farrell M. A review of age differences in the neurophysiology of nociception and the perceptual experience of pain. Clin J Pain. 2004;20:227-239.
33. Woodrow KM, Friedman GD, Siegelaub AB, et al. Pain tolerance: differences according to age, sex and race. Psychosom Med. 1972;34:548-556.
34. Turk DC, Okifuji A, Scharff L. Chronic pain and depression: role of perceived impact and perceived control in different age cohorts. Pain. 1995;61:93-101.
35. Thielke SM, Fan MY, Sullivan M, et al. Pain limits the effectiveness of collaborative care for depression. Am J Geriatr Psychiatry. 2007;15:699-707.
36. Power JD, Perruccio AV, Badley EM. Pain as a mediator of sleep problems in arthritis and other chronic conditions. Arthritis Rheum. 2005;53:911-919.
37. Louie GH, Tektonidou MG, Caban-Martizen AJ, et al. Sleep disturbances in adults with arthritis: prevalence, mediators, and subgroups at greatest risk. Arthritis Care Res. 2011;63:247-260.
38. Lin JJ, Alfandre D, Moore C. Physician attitudes toward opioid prescribing for patients with persistent noncancer pain. Clin J Pain. 2007;23:799-803.
39. Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613-2620.
40. Fischer B, Gittins J, Kendall P, et al. Thinking the unthinkable: could the increasing misuse of prescription opioids among street drug users offer benefits for public health? Public Health. 2009;123:145-146.
41. Fleming MF, Davis J, Passik SD. Reported lifetime aberrant drug-taking behaviors are predictive of current substance use and mental health problems in primary care patients. Pain Med. 2008;9:1098-1106.
42. Reid MC, Henderson CR, Jr, Papaleontiou M, et al. Characteristics of older adults receiving opioids in primary care: treatment duration and outcomes. Pain Med. 2010;11:1063-1071.
43. Solomon DH, Rassen JA, Glynn RJ, et al. The comparative safety of opioids for nonmalignant pain in older adults. Arch Intern Med. 2010;170:1979-1986.
44. Thielke SM, Simoni-Wastila L, Edlund MJ, et al. Age and sex trends in long-term opioid use in two large American health systems between 2000 and 2005. Pain Med. 2010;11:248-256.
45. Soden K, Ali S, Alloway L, et al. How do nurses assess and manage breakthrough pain in specialist palliative care inpatient units? A multicentre study. Palliat Med. 2010;24:294-298.
46. Papaleontiou M, Henderson CR, Jr, Turner BJ, et al. Outcomes associated with opioid use in the treatment of chronic noncancer pain in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2010;58:1353-1369.
47. Mallen CD, Peat G. Discussing prognosis with older people with musculoskeletal pain: a cross-sectional study in general practice. BMC Fam Pract. 2009;10:50.-
48. Rosemann T, Wensing M, Joest K, et al. Problems and needs for improving primary care of osteoarthritis patients: the views of patients, general practitioners and practice nurses. BMC Musculoskelet Disord. 2006;7:48.
AGING: Is your patient taking too many pills?
• Consider the possibility that an adverse drug effect—rather than a new condition—is at play when a patient taking multiple medications develops a new symptom. C
• Use an online interaction checker, which can be accessed via a smart phone or tablet, to check for potential drug-drug interactions in patients on multiple medications. C
• Cross-check patients’ medications with a list of their medical problems, with the goal of discontinuing any drug that duplicates the action of another or is age-inappropriate, ineffective, or not indicated for the condition for which it was prescribed. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Older adults are taking more medications than ever before. Nearly 9 out of 10 US residents who are 60 years of age or older take at least one prescription drug, more than a third take 5 to 9 medications, and 12% take 10 or more.1
The increase is largely driven by newer medications to effectively treat a variety of medical conditions, and by practice guidelines that often recommend multidrug regimens.2
As a result, the term “polypharmacy,” which once referred to a specific number of medications, is now used more broadly to mean “a large number” of drugs.
From a safety standpoint, the number of medications a patient takes matters. The risk of adverse drug effects and dangerous drug-drug interactions increases significantly when an individual takes ≥5 medications.3
More than 4.5 million adverse drug effects occur each year in the United States, and nearly three quarters of them are initially evaluated in outpatient settings.4 Research suggests that about 80% of the time, these adverse effects are not recognized as such by the patient’s physician. So instead of discontinuing the offending medication, physicians treat the drug-related symptoms by adding yet another medication—a phenomenon known as “the prescribing cascade.”5
This review can help you safeguard older patients taking multiple medications by recognizing and responding to drug-related problems, identifying drugs that can be safely eliminated (or, in some cases, drugs that should be added), and checking regularly to ensure that the medication regimen is appropriate and up to date.
CASE Mrs. R, a 79-year-old woman who recently moved to town, is brought to your office by her daughter and son-in-law. The patient has a hard time reporting her medical history, but her daughter tells you her mother has chronic obstructive pulmonary disease (COPD), heart failure, type 2 diabetes, and mild urinary incontinence, and was recently diagnosed with early dementia.
Mrs. R’s daughter has brought in a bagful of medications, but she’s not sure which ones her mother takes regularly. The medications are an albuterol inhaler, alprazolam, digoxin, diphenhydramine, donepezil, furosemide, glargine insulin, guaifenesin, levothyroxine, metformin, extended-release metoprolol, naproxen, omeprazole, simvastatin, tolterodine, and zolpidem—a total of 16 different drugs.
If Mrs. R were your patient, how would you manage her multidrug regimen?
Start with a medication review
The first step in evaluating a patient’s medication regimen is to find out whether the drugs in the patient’s possession and/or in the medical record are the ones he or she is actually taking. Ask older patients who haven’t brought in their medications, or the caregiver of a confused patient, to bring them to the next visit.
The next step: Determine whether the medication regimen is right for the patient.
Polypharmacy may be indicated
Despite the risks associated with polypharmacy, do not assume that it is inappropriate. For some conditions, multiple medications are routinely recommended. Patients with heart failure, for example, have been shown to have better outcomes when they take 3 to 5 medications, including beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, and diuretics.2
Some treatment guidelines also call for multiple medications. Achieving the more stringent blood pressure goals recommended in the Seventh Report of the Joint National Committee on Prevention, for instance, often requires 2 or more antihypertensive agents.6 In many cases, however, patients end up taking more drugs than necessary.
Is the patient taking the right drugs?
Medication reconciliation (determining whether the treatment regimen is appropriate for the patient’s diagnoses) is the way to find out.
The most widely recommended approach to medication reconciliation is to create a table and do a systematic review.7 List all the patient’s medical conditions in the first column and all current medications in the second column. Use the third column to note whether each medication is one the patient should be on, based not only on his or her medical conditions and other drugs being taken but also on current renal and hepatic function and body size, and contraindications.
A medication may be inappropriate if it duplicates, cancels out the action of, or otherwise interacts with another drug the patient is taking; is contraindicated in older patients; or is ineffective for the condition for which it was prescribed. In one key study of nearly 200 patients 65 years and older who took 5 or more medications, more than half had been prescribed at least one drug that was ineffective for the patient’s condition or that duplicated the action of another medication.8
In addition to finding drugs that the patient should not be taking, medication reconciliation may also reveal that the patient is not receiving optimal therapy and that one or more drugs should be added to his or her treatment regimen.
Check meds after transitions. A move from home to hospital, from emergency department to home, or any other transition relating to patient care should prompt a medication reconciliation. Medications are often added or inadvertently discontinued at such times,9,10 and instructions relating to medication are often misunderstood.11 In one study of 384 frail elderly patients being discharged from a hospital, for example, 44% were found to have been given at least one unnecessary prescription—most commonly for a medication that was neither indicated nor effective for any of the patient’s medical problems.12 It was also common for patients to be given drugs that duplicated the action of others they were already taking.
Even in the absence of such transitions, medication reconciliation should occur at regular intervals. Many physicians do a medication reconciliation at every visit to ensure that the medical record is accurate and the patient’s medication regimen is optimal.
Managing polypharmacy: These resources can help
Numerous tools are available to help you evaluate and monitor patients’ medication regimens, including some that were developed specifically for older patients.
START (Screening Tool to Alert doctors to Right Treatment) identifies drugs and drug classes that are underused with older patients.13 START criteria (TABLE 1)13-17 focus on medications that should be used yet are often omitted in older patients who have the appropriate indications.
TABLE 1
START criteria: Drug therapy that should be given to older patients13-17
Cardiovascular
|
Endocrine
|
Gastrointestinal
|
Musculoskeletal
|
Nervous system
|
Respiratory
|
| ACE, angiotensin-converting enzyme; BP, blood pressure; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; FEV1, forced expiratory volume in 1 second; GI, gastrointestinal; MI, myocardial infarction; PPI, proton-pump inhibitor; START, Screening Tool to alert doctors to right Treatment. |
In using START or any other drug-related tool, it is important to keep in mind that therapy should be individualized. Not all the medications in the START criteria are appropriate for every patient, and a medication that is indicated for a given medical condition may or may not provide real benefit for a particular patient. That would depend on the individual’s overall health and life expectancy, the goals of treatment, and how long it would take for the patient to realize any benefit from the drug in question.18 A vigorous 79-year-old might benefit from statin therapy for prevention of cardiovascular events, for instance, while a patient like Mrs. R, who is also 79 but has dementia and multiple other medical problems, would be unlikely to live long enough to realize such a benefit.
”Age” assessment tool. One criterion in deciding whether medication(s) are appropriate for an older patient is his or her “physiologic age”—calculated on the basis of the individual’s chronological age and self-reported health status (TABLE 2).19
TABLE 2
Calculating your patient’s “real” age19
| Actual age (y) | Physiologic age (y) | |||||||
|---|---|---|---|---|---|---|---|---|
| Self-reported health | ||||||||
| Excellent | Good | Fair | Poor | |||||
| Male | Female | Male | Female | Male | Female | Male | Female | |
| 65 | 58 | 60 | 64 | 64 | 68 | 66 | 73 | 72 |
| 70 | 62 | 65 | 69 | 69 | 73 | 71 | 78 | 77 |
| 75 | 67 | 70 | 74 | 74 | 78 | 76 | 83 | 82 |
| 80 | 72 | 75 | 79 | 79 | 83 | 81 | 85+ | 85+ |
Flagging drugs that may be inappropriate
Several tools have been developed to aid clinicians in identifying medications that are potentially inappropriate for older adults, although here, too, decisions about their use must be individualized. Two of the most widely used tools are the Beers criteria and STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions).
Beers criteria were developed by Mark Beers et al in 199120 and have been updated at regular intervals, most recently by the American Geriatrics Society in 2012.21 The drugs and drug classes included in the Beers criteria should not be prescribed for older patients in most cases, either because the risk of using them outweighs the benefit or because safer alternatives are available. Key components are listed in TABLE 3.21
TABLE 3
Beers criteria:* Drug classes that may be inappropriate for older adults21
| Drug class | Concern |
|---|---|
| Alpha-blockers with peripheral activity | Orthostatic hypotension |
| Anticholinergics | Cognitive impairment, urinary retention |
| Antipsychotics | Increased death rate when used for behavior control in patients with dementia |
| NSAIDs | Renal dysfunction, GI bleeding, fluid retention, exacerbation of heart failure |
| Sedative hypnotics | Cognitive impairment, delirium |
| Tricyclic antidepressants | Cognitive impairment, delirium, urinary retention |
| GI, gastrointestinal; NSAIDs, nonsteroidal anti-inflammatory drugs. *The full Beers criteria contains 53 drugs and drug classes that are generally inappropriate for older adults. The full list is available from the American Geriatrics Society at: www.americangeriatrics.org/files/documents/beers/2012BeersCriteria_JAGS.pdf. | |
One limitation of the Beers criteria has been its all-or-nothing approach, with many of the medications on the list deemed inappropriate for all older adults regardless of their circumstances. The 2012 update does a better job of individualizing recommendations: Medications are now categorized as those that should be avoided in older patients regardless of their diseases or conditions, those that should be avoided only in patients with certain diseases or conditions, and those that may be used for this patient population but require caution.21
STOPP is similar to the Beers criteria, but uses a different approach: Most medications on this list are considered in the context of specific medical problems.22 While the Beers criteria classify digoxin >0.125 mg/d as generally inappropriate for older adults, for example, STOPP criteria state that long-term dosing at that level is inappropriate only for those with impaired renal function.22 A list of medications identified by STOPP as contributing to hospitalization due to adverse drug effects is available at http://ageing.oxfordjournals.org/content/37/6/673.
Both tools address this drug category. Cumulative anticholinergic burden is a concept applied to the use of anticholinergic medications, which are included in both the Beers and STOPP criteria. Although isolated short-term exposure to a drug with anticholinergic properties may be tolerated by a healthy and cognitively intact older patient, repetitive exposure to such drugs, even if separated in time, has negative effects. One study evaluated more than 500 community-dwelling older adults and found that the more exposure an individual had to anticholinergic medications over the course of a year, the greater the impairment in short-term memory and activities of daily living.23 Another study, this one involving more than 13,000 community-dwelling and institutionalized patients, showed that the longer an older patient takes an anticholinergic medication, the more likely there is to be a measurable decline in performance on the Mini-Mental State Examination.24
Programs that flag potential interactions
Drug-drug interactions are a key concern of polypharmacy, and electronic medical records and prescribing systems that flag potential drug-drug interactions when a new medication is ordered are designed to help physicians avoid them. Unfortunately, clinicians only react to 3% to 9% of such notifications, overriding them because computerized systems often fail to distinguish between important and unimportant interactions.25-27 Thus, clinicians often must decide whether to react to or override warnings, an often difficult decision with patient safety and medicolegal implications. The best advice we can offer is to carefully evaluate drug interaction warnings using common sense, and seek consultation with a clinical pharmacist when uncertainty exists. This approach should prevent prescribing medications that have potentially harmful interactions with drugs the patient is already taking.
For physicians who do not have access to an electronic prescribing system that provides such notification, several online resources are available, some by subscription (eg, Lexicomp, www.lexi.com; Micromedex, www.micromedex.com/index.html; and Pepid, www.pepid.com) and others with free access (eg, AARP, healthtools.aarp.org/drug-interactions; Drugs.com (www.drugs.com/drug_interactions.php; and HealthLine, www.healthline.com/druginteractions).
CASE After doing a medication reconciliation for Mrs. R, you find that she is taking tolterodine, an anticholinergic medication for urge urinary incontinence, and donepezil, a procholinergic medication for dementia. This type of drug-drug interaction, in which the action of one drug effectively cancels out the effect of another, should not be ignored.
Overall, you identify 8 of her medications that could be discontinued: The list includes guaifenesin (a nonessential medication of questionable efficacy); naproxen (inappropriate per Beers criteria; inappropriate in patients with heart failure, according to STOPP); alprazolam, zolpidem, and diphenhydramine (duplicate medications that are all on the Beers criteria as inappropriate for chronic use and ill-advised in patients with cognitive impairment); and omeprazole and levothyroxine (for which nothing in the patient’s history suggests a need), as well as tolterodine. Depending on dose, digoxin is yet another candidate for discontinuation.
Discontinuing medications: Proceed carefully
Physicians are often reluctant to discontinue chronic medications in older patients—even in those with advanced disease who are not likely to benefit from treatment. Focus groups have identified a number of reasons for their hesitation, including:
- the assumption that patients have no problem taking large numbers of drugs
- the fear that patients may misinterpret a plan to discontinue medications as evidence that the physician is giving up on them
- the belief that physicians must comply with practice guidelines that recommend multiple drug treatments
- concern that proposing discontinuation of medications often leads to a discussion of life expectancy and end-of-life care.28
Physicians may also fear that discontinuation of certain drugs will increase the risk of adverse outcomes. More than 30 studies have evaluated discontinuation of chronic medications in older adults, however, and found that drugs as diverse as antihypertensives, antipsychotics, benzodiazepines, and selective serotonin reuptake inhibitors (SSRIs) can often be discontinued without adverse outcomes. In many cases, improvement in patient function results.29 Medications that present the most difficulty are those that patients often become physically or psychologically dependent on, such as benzodiazepines, guaifenesin, proton-pump inhibitors, nonsteroidal anti-inflammatory drugs, and SSRIs. Some (eg, benzodiazepines, SSRIs) require a gradual reduction; for others, no taper is required
(TABLE 4).30-37
TABLE 4
Recommendations for discontinuing hard-to-stop drugs
| Medication or drug class | Discontinuation regimen | Comments |
|---|---|---|
| Benzodiazepines30 | Taper dose by 25% q 2 wk | No withdrawal symptoms reported with this taper regimen. Subtle cognitive improvement noted over a period of months |
| Guaifenesin31 | Can be discontinued without tapering if not combined with opioids or other medications. Elimination half-life is approximately 1 hour | Guaifenesin is often marketed as a combination product with opioids; such combination products require tapering |
| PPIs32-34 | Decrease dose by 50% q 2 wk; supplement with H2 blocker if needed, but tapering of H2 blocker may be required | Abrupt discontinuation after long-term use causes rebound gastric acid hypersecretion and lowers rate of success. Higher success rates with taper regimen and in patients who do not have documented GERD |
| NSAIDs35 | No taper required | Short-term use (<3 mo) acceptable for patients with no contraindications |
| SSRIs36,37 | Gradual reduction in dose over 6-8 wk | Highest rate of success in patients without a clear diagnosis of depression |
| GERD, gastroesophageal reflux disease; NSAIDs, nonsteroidal anti-inflammatory drugs; PPIs, proton-pump inhibitors; SSRIs, selective serotonin reuptake inhibitors. | ||
CASE You trim down Mrs. R’s regimen by discontinuing each of the 8 drugs, one at a time, and carefully monitor the patient during the withdrawal period. Because she had been taking alprazolam daily, the dose is tapered slowly to avoid withdrawal. Omeprazole also requires a gradual taper to avoid rebound hyperacidity.3
After confirming that Mrs. R has heart failure and COPD, you identify 2 medications that should be added to her drug regimen—an ACE inhibitor for heart failure and an inhaled anticholinergic for COPD.
Going from 16 medications to 10 saves money, decreases the likelihood of adverse events and drug-drug interactions, and helps with adherence. Mrs. R’s new drug regimen is expected to lead to improvements in memory and overall quality of life, as well.
CORRESPONDENCE
Barry D. Weiss, MD, Department of Family and Community Medicine, University of Arizona College of Medicine, Tucson, AZ 85724; [email protected]
1. Gu Q, Dillon CF, Burt V. Prescription drug use continued to increase: US prescription drug data for 2007-2008. CDC/NCHS Data Brief. 2010;42:1-2.
2. Jessup K, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;119:1977-2016.
3. Johnell K, Klarin I. The relationship between number of drugs and potential drug-drug interactions in the elderly: a study of over 600,000 elderly patients from the Swedish Prescribed Drug Register. Drug Saf. 2007;30:911-918.
4. Sarkar U, Lopez A, Maselli JH, et al. Adverse drug events in US adult ambulatory medical care. Health Services Res. 2011;46:1517-1533.
5. Rollason V, Vogt N. Reduction of polypharmacy in the elderly. A systematic review of the role of the pharmacist. Drugs Aging. 2003;20:817-832.
6. National Heart, Lung, and Blood Institute. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Available at: www.nhlbi.nih.gov/guidelines/hypertension/jnc7full.pdf. Accessed October 11, 2012.
7. Steinman MA, Hanlon JT. Managing medications in clinically complex elders. JAMA. 2010;304:1592-1601.
8. Steinman MA, Landefeld CS, Rosenthal GE, et al. Polypharmacy and prescribing quality in older people. J Am Geriatr Soc. 2006;54:1516-23.
9. Bell CM, Brener SS, Gunraj N, et al. Association of ICU or hospital admission with unintentional discontinuations of medications for chronic disease. JAMA. 2011;306:840-847.
10. Moore C, Wisnivesky J, Williams S, et al. Medical errors related to discontinuity of care from an inpatient to an outpatient setting. J Gen Intern Med. 2003;18:646-651.
11. Ziaeian B, Arauho KL, Van Ness PH, et al. Medication reconciliation accuracy and patient understanding of intended medication changes on hospital discharge. J Gen Intern Med. 2012 July 12. ePub ahead of print.
12. Hajjar ER, Hanlon JT, Sloane RJ, et al. Unnecessary drug use in frail older people at hospital discharge. J Am Geriatr Soc. 2005;53:1518-1523.
13. O’Mahony D, Gallagher P, Ryan C, et al. STOPP & START criteria: a new approach to detecting potentially inappropriate prescribing in old age. Eur Geriatr Med. 2010;1:45-51.
14. Denneboom W, Dautzenberg KGH, Grol R, et al. Analysis of polypharmacy in older patients in primary acre using a multidisciplinary expert panel. Br J Gen Pract. 2006;56:504-510.
15. Ko DT, Mamdani M, Alter DA. Lipid-lowering therapy with statins in high-risk elderly patients. JAMA. 2004;291:1864-70.
16. Wright RM, Sloane R, Pieper CF, et al. Underuse of indicated medications among physically frail older US veterans at the time of hospital discharge: results of a cross-sectional analysis of data from the Geriatric Evaluation and Management Drug Study. Am J Geriatr Pharmacother. 2009;7:271-280.
17. Garwood CL. Use of anticoagulation in elderly patients with atrial fibrillation who are risk for falls. Ann Pharmacother. 2008;42:523-532.
18. Holmes HM, Hayley DC, Alexander GC, et al. Reconsidering medication appropriateness for patients late in life. Arch Intern Med. 2006;166:605-609.
19. Simplified Methods for Estimating Life Expectancy. Available at: http://painconsortium.nih.gov/symptomresearch/chapter_14/Part_3/sec4/chspt3s4pg1.htm. Accessed October 9, 2012.
20. Beers MH, Ouslander JG, Rollingher I, et al. Explicit criteria for determining inappropriate medication use in nursing home residents. Arch Intern Med. 1991;151:1825-1832.
21. The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society Update Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616-631.
22. Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers’ criteria. Age Aging. 2008;37:673-379.
23. Han L, Agostini JV, Allore HG. Cumulative anticholinergic exposure is associated with poor memory and executive function in older men. J Am Geriatr Soc. 2008;56:2203-2210.
24. Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59:1477-1483.
25. Knight A, Falade O, Maygers J, et al. Factors associated with medication warning acceptance [abstract]. J Hosp Med. 2012;7(suppl 2):515.-
26. Isaac T, Weissman JS, Davis RB, et al. Overrides of medication alerts in ambulatory care. Arch Intern Med. 2009;169:305-311.
27. Van Der Sijs H, Aarts J, Vulto A, et al. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc. 2006;12:138-147.
28. Schuling J, Gebben H, Veehof LJG, et al. Deprescribing medication in very elderly patients with multimorbidity: the view of Dutch GPs. A qualitative study. BMC Family Practice. 2012;13:56. http://www.biomedcentral.com/1471-2296/13/56.
29. Iyer S, Naganathan V, McLachlan AJ, et al. Medication withdrawal trials in people aged 65 years and older. A systematic review. Drugs Aging. 2008;25:1021-1031.
30. Curran HV, Collins R, Fletcher S, et al. Older adults and withdrawal from benzodiazepine hypnotics in general practice: effects on cognitive function, sleep, mood and quality of life. Psychol Med. 2003;33:1223-1237.
31. Krinsky DL, Berardi RR, Ferreris SP, et al. Handbook of Nonprescription Drugs: An Interactive Approach to Self-Care. Washington, DC: American Pharmacists Association; 2012:209.
32. Bjornsson E, Abrahamsson H, Simren M, et al. Discontinuation of proton pump inhibitors in patients on long-term therapy: a double-blind, placebo-controlled trial. Aliment Pharmacol Ther. 2006;24:945-954.
33. Inadomi JM, Jamai R, Murata GH, et al. Step-down management of gastroesophageal reflux disease. Gastroenterology. 2001;131:1095-1100.
34. Hester SA. Proton pump inhibitors and rebound acid hypersecretion. Pharm Lett. 2009;25:250920.-
35. Taylor R, Jr, Lemtouni S, Weiss K, et al. Pain management in the elderly: an FDA safe use initiative expert panel’s view on preventable harm associated with NSAID therapy. Curr Gerontol Geriatr Res. 2012;196159.-
36. Ulfvarson J, Adami J, Wredling R, et al. Controlled withdrawal of selective serotonin reuptake inhibitor drugs in elderly patients in nursing homes with no indication of depression. Eur J Clin Pharmacol. 2003;59:735-740.
37. Lindstrom K, Ekedahl A, Carlsten A, et al. Can selective serotonin inhibitor drugs in elderly patients in nursing homes be reduced? Scand J Prim Health Care. 2007;25:3-8.
• Consider the possibility that an adverse drug effect—rather than a new condition—is at play when a patient taking multiple medications develops a new symptom. C
• Use an online interaction checker, which can be accessed via a smart phone or tablet, to check for potential drug-drug interactions in patients on multiple medications. C
• Cross-check patients’ medications with a list of their medical problems, with the goal of discontinuing any drug that duplicates the action of another or is age-inappropriate, ineffective, or not indicated for the condition for which it was prescribed. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Older adults are taking more medications than ever before. Nearly 9 out of 10 US residents who are 60 years of age or older take at least one prescription drug, more than a third take 5 to 9 medications, and 12% take 10 or more.1
The increase is largely driven by newer medications to effectively treat a variety of medical conditions, and by practice guidelines that often recommend multidrug regimens.2
As a result, the term “polypharmacy,” which once referred to a specific number of medications, is now used more broadly to mean “a large number” of drugs.
From a safety standpoint, the number of medications a patient takes matters. The risk of adverse drug effects and dangerous drug-drug interactions increases significantly when an individual takes ≥5 medications.3
More than 4.5 million adverse drug effects occur each year in the United States, and nearly three quarters of them are initially evaluated in outpatient settings.4 Research suggests that about 80% of the time, these adverse effects are not recognized as such by the patient’s physician. So instead of discontinuing the offending medication, physicians treat the drug-related symptoms by adding yet another medication—a phenomenon known as “the prescribing cascade.”5
This review can help you safeguard older patients taking multiple medications by recognizing and responding to drug-related problems, identifying drugs that can be safely eliminated (or, in some cases, drugs that should be added), and checking regularly to ensure that the medication regimen is appropriate and up to date.
CASE Mrs. R, a 79-year-old woman who recently moved to town, is brought to your office by her daughter and son-in-law. The patient has a hard time reporting her medical history, but her daughter tells you her mother has chronic obstructive pulmonary disease (COPD), heart failure, type 2 diabetes, and mild urinary incontinence, and was recently diagnosed with early dementia.
Mrs. R’s daughter has brought in a bagful of medications, but she’s not sure which ones her mother takes regularly. The medications are an albuterol inhaler, alprazolam, digoxin, diphenhydramine, donepezil, furosemide, glargine insulin, guaifenesin, levothyroxine, metformin, extended-release metoprolol, naproxen, omeprazole, simvastatin, tolterodine, and zolpidem—a total of 16 different drugs.
If Mrs. R were your patient, how would you manage her multidrug regimen?
Start with a medication review
The first step in evaluating a patient’s medication regimen is to find out whether the drugs in the patient’s possession and/or in the medical record are the ones he or she is actually taking. Ask older patients who haven’t brought in their medications, or the caregiver of a confused patient, to bring them to the next visit.
The next step: Determine whether the medication regimen is right for the patient.
Polypharmacy may be indicated
Despite the risks associated with polypharmacy, do not assume that it is inappropriate. For some conditions, multiple medications are routinely recommended. Patients with heart failure, for example, have been shown to have better outcomes when they take 3 to 5 medications, including beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, and diuretics.2
Some treatment guidelines also call for multiple medications. Achieving the more stringent blood pressure goals recommended in the Seventh Report of the Joint National Committee on Prevention, for instance, often requires 2 or more antihypertensive agents.6 In many cases, however, patients end up taking more drugs than necessary.
Is the patient taking the right drugs?
Medication reconciliation (determining whether the treatment regimen is appropriate for the patient’s diagnoses) is the way to find out.
The most widely recommended approach to medication reconciliation is to create a table and do a systematic review.7 List all the patient’s medical conditions in the first column and all current medications in the second column. Use the third column to note whether each medication is one the patient should be on, based not only on his or her medical conditions and other drugs being taken but also on current renal and hepatic function and body size, and contraindications.
A medication may be inappropriate if it duplicates, cancels out the action of, or otherwise interacts with another drug the patient is taking; is contraindicated in older patients; or is ineffective for the condition for which it was prescribed. In one key study of nearly 200 patients 65 years and older who took 5 or more medications, more than half had been prescribed at least one drug that was ineffective for the patient’s condition or that duplicated the action of another medication.8
In addition to finding drugs that the patient should not be taking, medication reconciliation may also reveal that the patient is not receiving optimal therapy and that one or more drugs should be added to his or her treatment regimen.
Check meds after transitions. A move from home to hospital, from emergency department to home, or any other transition relating to patient care should prompt a medication reconciliation. Medications are often added or inadvertently discontinued at such times,9,10 and instructions relating to medication are often misunderstood.11 In one study of 384 frail elderly patients being discharged from a hospital, for example, 44% were found to have been given at least one unnecessary prescription—most commonly for a medication that was neither indicated nor effective for any of the patient’s medical problems.12 It was also common for patients to be given drugs that duplicated the action of others they were already taking.
Even in the absence of such transitions, medication reconciliation should occur at regular intervals. Many physicians do a medication reconciliation at every visit to ensure that the medical record is accurate and the patient’s medication regimen is optimal.
Managing polypharmacy: These resources can help
Numerous tools are available to help you evaluate and monitor patients’ medication regimens, including some that were developed specifically for older patients.
START (Screening Tool to Alert doctors to Right Treatment) identifies drugs and drug classes that are underused with older patients.13 START criteria (TABLE 1)13-17 focus on medications that should be used yet are often omitted in older patients who have the appropriate indications.
TABLE 1
START criteria: Drug therapy that should be given to older patients13-17
Cardiovascular
|
Endocrine
|
Gastrointestinal
|
Musculoskeletal
|
Nervous system
|
Respiratory
|
| ACE, angiotensin-converting enzyme; BP, blood pressure; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; FEV1, forced expiratory volume in 1 second; GI, gastrointestinal; MI, myocardial infarction; PPI, proton-pump inhibitor; START, Screening Tool to alert doctors to right Treatment. |
In using START or any other drug-related tool, it is important to keep in mind that therapy should be individualized. Not all the medications in the START criteria are appropriate for every patient, and a medication that is indicated for a given medical condition may or may not provide real benefit for a particular patient. That would depend on the individual’s overall health and life expectancy, the goals of treatment, and how long it would take for the patient to realize any benefit from the drug in question.18 A vigorous 79-year-old might benefit from statin therapy for prevention of cardiovascular events, for instance, while a patient like Mrs. R, who is also 79 but has dementia and multiple other medical problems, would be unlikely to live long enough to realize such a benefit.
”Age” assessment tool. One criterion in deciding whether medication(s) are appropriate for an older patient is his or her “physiologic age”—calculated on the basis of the individual’s chronological age and self-reported health status (TABLE 2).19
TABLE 2
Calculating your patient’s “real” age19
| Actual age (y) | Physiologic age (y) | |||||||
|---|---|---|---|---|---|---|---|---|
| Self-reported health | ||||||||
| Excellent | Good | Fair | Poor | |||||
| Male | Female | Male | Female | Male | Female | Male | Female | |
| 65 | 58 | 60 | 64 | 64 | 68 | 66 | 73 | 72 |
| 70 | 62 | 65 | 69 | 69 | 73 | 71 | 78 | 77 |
| 75 | 67 | 70 | 74 | 74 | 78 | 76 | 83 | 82 |
| 80 | 72 | 75 | 79 | 79 | 83 | 81 | 85+ | 85+ |
Flagging drugs that may be inappropriate
Several tools have been developed to aid clinicians in identifying medications that are potentially inappropriate for older adults, although here, too, decisions about their use must be individualized. Two of the most widely used tools are the Beers criteria and STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions).
Beers criteria were developed by Mark Beers et al in 199120 and have been updated at regular intervals, most recently by the American Geriatrics Society in 2012.21 The drugs and drug classes included in the Beers criteria should not be prescribed for older patients in most cases, either because the risk of using them outweighs the benefit or because safer alternatives are available. Key components are listed in TABLE 3.21
TABLE 3
Beers criteria:* Drug classes that may be inappropriate for older adults21
| Drug class | Concern |
|---|---|
| Alpha-blockers with peripheral activity | Orthostatic hypotension |
| Anticholinergics | Cognitive impairment, urinary retention |
| Antipsychotics | Increased death rate when used for behavior control in patients with dementia |
| NSAIDs | Renal dysfunction, GI bleeding, fluid retention, exacerbation of heart failure |
| Sedative hypnotics | Cognitive impairment, delirium |
| Tricyclic antidepressants | Cognitive impairment, delirium, urinary retention |
| GI, gastrointestinal; NSAIDs, nonsteroidal anti-inflammatory drugs. *The full Beers criteria contains 53 drugs and drug classes that are generally inappropriate for older adults. The full list is available from the American Geriatrics Society at: www.americangeriatrics.org/files/documents/beers/2012BeersCriteria_JAGS.pdf. | |
One limitation of the Beers criteria has been its all-or-nothing approach, with many of the medications on the list deemed inappropriate for all older adults regardless of their circumstances. The 2012 update does a better job of individualizing recommendations: Medications are now categorized as those that should be avoided in older patients regardless of their diseases or conditions, those that should be avoided only in patients with certain diseases or conditions, and those that may be used for this patient population but require caution.21
STOPP is similar to the Beers criteria, but uses a different approach: Most medications on this list are considered in the context of specific medical problems.22 While the Beers criteria classify digoxin >0.125 mg/d as generally inappropriate for older adults, for example, STOPP criteria state that long-term dosing at that level is inappropriate only for those with impaired renal function.22 A list of medications identified by STOPP as contributing to hospitalization due to adverse drug effects is available at http://ageing.oxfordjournals.org/content/37/6/673.
Both tools address this drug category. Cumulative anticholinergic burden is a concept applied to the use of anticholinergic medications, which are included in both the Beers and STOPP criteria. Although isolated short-term exposure to a drug with anticholinergic properties may be tolerated by a healthy and cognitively intact older patient, repetitive exposure to such drugs, even if separated in time, has negative effects. One study evaluated more than 500 community-dwelling older adults and found that the more exposure an individual had to anticholinergic medications over the course of a year, the greater the impairment in short-term memory and activities of daily living.23 Another study, this one involving more than 13,000 community-dwelling and institutionalized patients, showed that the longer an older patient takes an anticholinergic medication, the more likely there is to be a measurable decline in performance on the Mini-Mental State Examination.24
Programs that flag potential interactions
Drug-drug interactions are a key concern of polypharmacy, and electronic medical records and prescribing systems that flag potential drug-drug interactions when a new medication is ordered are designed to help physicians avoid them. Unfortunately, clinicians only react to 3% to 9% of such notifications, overriding them because computerized systems often fail to distinguish between important and unimportant interactions.25-27 Thus, clinicians often must decide whether to react to or override warnings, an often difficult decision with patient safety and medicolegal implications. The best advice we can offer is to carefully evaluate drug interaction warnings using common sense, and seek consultation with a clinical pharmacist when uncertainty exists. This approach should prevent prescribing medications that have potentially harmful interactions with drugs the patient is already taking.
For physicians who do not have access to an electronic prescribing system that provides such notification, several online resources are available, some by subscription (eg, Lexicomp, www.lexi.com; Micromedex, www.micromedex.com/index.html; and Pepid, www.pepid.com) and others with free access (eg, AARP, healthtools.aarp.org/drug-interactions; Drugs.com (www.drugs.com/drug_interactions.php; and HealthLine, www.healthline.com/druginteractions).
CASE After doing a medication reconciliation for Mrs. R, you find that she is taking tolterodine, an anticholinergic medication for urge urinary incontinence, and donepezil, a procholinergic medication for dementia. This type of drug-drug interaction, in which the action of one drug effectively cancels out the effect of another, should not be ignored.
Overall, you identify 8 of her medications that could be discontinued: The list includes guaifenesin (a nonessential medication of questionable efficacy); naproxen (inappropriate per Beers criteria; inappropriate in patients with heart failure, according to STOPP); alprazolam, zolpidem, and diphenhydramine (duplicate medications that are all on the Beers criteria as inappropriate for chronic use and ill-advised in patients with cognitive impairment); and omeprazole and levothyroxine (for which nothing in the patient’s history suggests a need), as well as tolterodine. Depending on dose, digoxin is yet another candidate for discontinuation.
Discontinuing medications: Proceed carefully
Physicians are often reluctant to discontinue chronic medications in older patients—even in those with advanced disease who are not likely to benefit from treatment. Focus groups have identified a number of reasons for their hesitation, including:
- the assumption that patients have no problem taking large numbers of drugs
- the fear that patients may misinterpret a plan to discontinue medications as evidence that the physician is giving up on them
- the belief that physicians must comply with practice guidelines that recommend multiple drug treatments
- concern that proposing discontinuation of medications often leads to a discussion of life expectancy and end-of-life care.28
Physicians may also fear that discontinuation of certain drugs will increase the risk of adverse outcomes. More than 30 studies have evaluated discontinuation of chronic medications in older adults, however, and found that drugs as diverse as antihypertensives, antipsychotics, benzodiazepines, and selective serotonin reuptake inhibitors (SSRIs) can often be discontinued without adverse outcomes. In many cases, improvement in patient function results.29 Medications that present the most difficulty are those that patients often become physically or psychologically dependent on, such as benzodiazepines, guaifenesin, proton-pump inhibitors, nonsteroidal anti-inflammatory drugs, and SSRIs. Some (eg, benzodiazepines, SSRIs) require a gradual reduction; for others, no taper is required
(TABLE 4).30-37
TABLE 4
Recommendations for discontinuing hard-to-stop drugs
| Medication or drug class | Discontinuation regimen | Comments |
|---|---|---|
| Benzodiazepines30 | Taper dose by 25% q 2 wk | No withdrawal symptoms reported with this taper regimen. Subtle cognitive improvement noted over a period of months |
| Guaifenesin31 | Can be discontinued without tapering if not combined with opioids or other medications. Elimination half-life is approximately 1 hour | Guaifenesin is often marketed as a combination product with opioids; such combination products require tapering |
| PPIs32-34 | Decrease dose by 50% q 2 wk; supplement with H2 blocker if needed, but tapering of H2 blocker may be required | Abrupt discontinuation after long-term use causes rebound gastric acid hypersecretion and lowers rate of success. Higher success rates with taper regimen and in patients who do not have documented GERD |
| NSAIDs35 | No taper required | Short-term use (<3 mo) acceptable for patients with no contraindications |
| SSRIs36,37 | Gradual reduction in dose over 6-8 wk | Highest rate of success in patients without a clear diagnosis of depression |
| GERD, gastroesophageal reflux disease; NSAIDs, nonsteroidal anti-inflammatory drugs; PPIs, proton-pump inhibitors; SSRIs, selective serotonin reuptake inhibitors. | ||
CASE You trim down Mrs. R’s regimen by discontinuing each of the 8 drugs, one at a time, and carefully monitor the patient during the withdrawal period. Because she had been taking alprazolam daily, the dose is tapered slowly to avoid withdrawal. Omeprazole also requires a gradual taper to avoid rebound hyperacidity.3
After confirming that Mrs. R has heart failure and COPD, you identify 2 medications that should be added to her drug regimen—an ACE inhibitor for heart failure and an inhaled anticholinergic for COPD.
Going from 16 medications to 10 saves money, decreases the likelihood of adverse events and drug-drug interactions, and helps with adherence. Mrs. R’s new drug regimen is expected to lead to improvements in memory and overall quality of life, as well.
CORRESPONDENCE
Barry D. Weiss, MD, Department of Family and Community Medicine, University of Arizona College of Medicine, Tucson, AZ 85724; [email protected]
• Consider the possibility that an adverse drug effect—rather than a new condition—is at play when a patient taking multiple medications develops a new symptom. C
• Use an online interaction checker, which can be accessed via a smart phone or tablet, to check for potential drug-drug interactions in patients on multiple medications. C
• Cross-check patients’ medications with a list of their medical problems, with the goal of discontinuing any drug that duplicates the action of another or is age-inappropriate, ineffective, or not indicated for the condition for which it was prescribed. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Older adults are taking more medications than ever before. Nearly 9 out of 10 US residents who are 60 years of age or older take at least one prescription drug, more than a third take 5 to 9 medications, and 12% take 10 or more.1
The increase is largely driven by newer medications to effectively treat a variety of medical conditions, and by practice guidelines that often recommend multidrug regimens.2
As a result, the term “polypharmacy,” which once referred to a specific number of medications, is now used more broadly to mean “a large number” of drugs.
From a safety standpoint, the number of medications a patient takes matters. The risk of adverse drug effects and dangerous drug-drug interactions increases significantly when an individual takes ≥5 medications.3
More than 4.5 million adverse drug effects occur each year in the United States, and nearly three quarters of them are initially evaluated in outpatient settings.4 Research suggests that about 80% of the time, these adverse effects are not recognized as such by the patient’s physician. So instead of discontinuing the offending medication, physicians treat the drug-related symptoms by adding yet another medication—a phenomenon known as “the prescribing cascade.”5
This review can help you safeguard older patients taking multiple medications by recognizing and responding to drug-related problems, identifying drugs that can be safely eliminated (or, in some cases, drugs that should be added), and checking regularly to ensure that the medication regimen is appropriate and up to date.
CASE Mrs. R, a 79-year-old woman who recently moved to town, is brought to your office by her daughter and son-in-law. The patient has a hard time reporting her medical history, but her daughter tells you her mother has chronic obstructive pulmonary disease (COPD), heart failure, type 2 diabetes, and mild urinary incontinence, and was recently diagnosed with early dementia.
Mrs. R’s daughter has brought in a bagful of medications, but she’s not sure which ones her mother takes regularly. The medications are an albuterol inhaler, alprazolam, digoxin, diphenhydramine, donepezil, furosemide, glargine insulin, guaifenesin, levothyroxine, metformin, extended-release metoprolol, naproxen, omeprazole, simvastatin, tolterodine, and zolpidem—a total of 16 different drugs.
If Mrs. R were your patient, how would you manage her multidrug regimen?
Start with a medication review
The first step in evaluating a patient’s medication regimen is to find out whether the drugs in the patient’s possession and/or in the medical record are the ones he or she is actually taking. Ask older patients who haven’t brought in their medications, or the caregiver of a confused patient, to bring them to the next visit.
The next step: Determine whether the medication regimen is right for the patient.
Polypharmacy may be indicated
Despite the risks associated with polypharmacy, do not assume that it is inappropriate. For some conditions, multiple medications are routinely recommended. Patients with heart failure, for example, have been shown to have better outcomes when they take 3 to 5 medications, including beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, and diuretics.2
Some treatment guidelines also call for multiple medications. Achieving the more stringent blood pressure goals recommended in the Seventh Report of the Joint National Committee on Prevention, for instance, often requires 2 or more antihypertensive agents.6 In many cases, however, patients end up taking more drugs than necessary.
Is the patient taking the right drugs?
Medication reconciliation (determining whether the treatment regimen is appropriate for the patient’s diagnoses) is the way to find out.
The most widely recommended approach to medication reconciliation is to create a table and do a systematic review.7 List all the patient’s medical conditions in the first column and all current medications in the second column. Use the third column to note whether each medication is one the patient should be on, based not only on his or her medical conditions and other drugs being taken but also on current renal and hepatic function and body size, and contraindications.
A medication may be inappropriate if it duplicates, cancels out the action of, or otherwise interacts with another drug the patient is taking; is contraindicated in older patients; or is ineffective for the condition for which it was prescribed. In one key study of nearly 200 patients 65 years and older who took 5 or more medications, more than half had been prescribed at least one drug that was ineffective for the patient’s condition or that duplicated the action of another medication.8
In addition to finding drugs that the patient should not be taking, medication reconciliation may also reveal that the patient is not receiving optimal therapy and that one or more drugs should be added to his or her treatment regimen.
Check meds after transitions. A move from home to hospital, from emergency department to home, or any other transition relating to patient care should prompt a medication reconciliation. Medications are often added or inadvertently discontinued at such times,9,10 and instructions relating to medication are often misunderstood.11 In one study of 384 frail elderly patients being discharged from a hospital, for example, 44% were found to have been given at least one unnecessary prescription—most commonly for a medication that was neither indicated nor effective for any of the patient’s medical problems.12 It was also common for patients to be given drugs that duplicated the action of others they were already taking.
Even in the absence of such transitions, medication reconciliation should occur at regular intervals. Many physicians do a medication reconciliation at every visit to ensure that the medical record is accurate and the patient’s medication regimen is optimal.
Managing polypharmacy: These resources can help
Numerous tools are available to help you evaluate and monitor patients’ medication regimens, including some that were developed specifically for older patients.
START (Screening Tool to Alert doctors to Right Treatment) identifies drugs and drug classes that are underused with older patients.13 START criteria (TABLE 1)13-17 focus on medications that should be used yet are often omitted in older patients who have the appropriate indications.
TABLE 1
START criteria: Drug therapy that should be given to older patients13-17
Cardiovascular
|
Endocrine
|
Gastrointestinal
|
Musculoskeletal
|
Nervous system
|
Respiratory
|
| ACE, angiotensin-converting enzyme; BP, blood pressure; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; FEV1, forced expiratory volume in 1 second; GI, gastrointestinal; MI, myocardial infarction; PPI, proton-pump inhibitor; START, Screening Tool to alert doctors to right Treatment. |
In using START or any other drug-related tool, it is important to keep in mind that therapy should be individualized. Not all the medications in the START criteria are appropriate for every patient, and a medication that is indicated for a given medical condition may or may not provide real benefit for a particular patient. That would depend on the individual’s overall health and life expectancy, the goals of treatment, and how long it would take for the patient to realize any benefit from the drug in question.18 A vigorous 79-year-old might benefit from statin therapy for prevention of cardiovascular events, for instance, while a patient like Mrs. R, who is also 79 but has dementia and multiple other medical problems, would be unlikely to live long enough to realize such a benefit.
”Age” assessment tool. One criterion in deciding whether medication(s) are appropriate for an older patient is his or her “physiologic age”—calculated on the basis of the individual’s chronological age and self-reported health status (TABLE 2).19
TABLE 2
Calculating your patient’s “real” age19
| Actual age (y) | Physiologic age (y) | |||||||
|---|---|---|---|---|---|---|---|---|
| Self-reported health | ||||||||
| Excellent | Good | Fair | Poor | |||||
| Male | Female | Male | Female | Male | Female | Male | Female | |
| 65 | 58 | 60 | 64 | 64 | 68 | 66 | 73 | 72 |
| 70 | 62 | 65 | 69 | 69 | 73 | 71 | 78 | 77 |
| 75 | 67 | 70 | 74 | 74 | 78 | 76 | 83 | 82 |
| 80 | 72 | 75 | 79 | 79 | 83 | 81 | 85+ | 85+ |
Flagging drugs that may be inappropriate
Several tools have been developed to aid clinicians in identifying medications that are potentially inappropriate for older adults, although here, too, decisions about their use must be individualized. Two of the most widely used tools are the Beers criteria and STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions).
Beers criteria were developed by Mark Beers et al in 199120 and have been updated at regular intervals, most recently by the American Geriatrics Society in 2012.21 The drugs and drug classes included in the Beers criteria should not be prescribed for older patients in most cases, either because the risk of using them outweighs the benefit or because safer alternatives are available. Key components are listed in TABLE 3.21
TABLE 3
Beers criteria:* Drug classes that may be inappropriate for older adults21
| Drug class | Concern |
|---|---|
| Alpha-blockers with peripheral activity | Orthostatic hypotension |
| Anticholinergics | Cognitive impairment, urinary retention |
| Antipsychotics | Increased death rate when used for behavior control in patients with dementia |
| NSAIDs | Renal dysfunction, GI bleeding, fluid retention, exacerbation of heart failure |
| Sedative hypnotics | Cognitive impairment, delirium |
| Tricyclic antidepressants | Cognitive impairment, delirium, urinary retention |
| GI, gastrointestinal; NSAIDs, nonsteroidal anti-inflammatory drugs. *The full Beers criteria contains 53 drugs and drug classes that are generally inappropriate for older adults. The full list is available from the American Geriatrics Society at: www.americangeriatrics.org/files/documents/beers/2012BeersCriteria_JAGS.pdf. | |
One limitation of the Beers criteria has been its all-or-nothing approach, with many of the medications on the list deemed inappropriate for all older adults regardless of their circumstances. The 2012 update does a better job of individualizing recommendations: Medications are now categorized as those that should be avoided in older patients regardless of their diseases or conditions, those that should be avoided only in patients with certain diseases or conditions, and those that may be used for this patient population but require caution.21
STOPP is similar to the Beers criteria, but uses a different approach: Most medications on this list are considered in the context of specific medical problems.22 While the Beers criteria classify digoxin >0.125 mg/d as generally inappropriate for older adults, for example, STOPP criteria state that long-term dosing at that level is inappropriate only for those with impaired renal function.22 A list of medications identified by STOPP as contributing to hospitalization due to adverse drug effects is available at http://ageing.oxfordjournals.org/content/37/6/673.
Both tools address this drug category. Cumulative anticholinergic burden is a concept applied to the use of anticholinergic medications, which are included in both the Beers and STOPP criteria. Although isolated short-term exposure to a drug with anticholinergic properties may be tolerated by a healthy and cognitively intact older patient, repetitive exposure to such drugs, even if separated in time, has negative effects. One study evaluated more than 500 community-dwelling older adults and found that the more exposure an individual had to anticholinergic medications over the course of a year, the greater the impairment in short-term memory and activities of daily living.23 Another study, this one involving more than 13,000 community-dwelling and institutionalized patients, showed that the longer an older patient takes an anticholinergic medication, the more likely there is to be a measurable decline in performance on the Mini-Mental State Examination.24
Programs that flag potential interactions
Drug-drug interactions are a key concern of polypharmacy, and electronic medical records and prescribing systems that flag potential drug-drug interactions when a new medication is ordered are designed to help physicians avoid them. Unfortunately, clinicians only react to 3% to 9% of such notifications, overriding them because computerized systems often fail to distinguish between important and unimportant interactions.25-27 Thus, clinicians often must decide whether to react to or override warnings, an often difficult decision with patient safety and medicolegal implications. The best advice we can offer is to carefully evaluate drug interaction warnings using common sense, and seek consultation with a clinical pharmacist when uncertainty exists. This approach should prevent prescribing medications that have potentially harmful interactions with drugs the patient is already taking.
For physicians who do not have access to an electronic prescribing system that provides such notification, several online resources are available, some by subscription (eg, Lexicomp, www.lexi.com; Micromedex, www.micromedex.com/index.html; and Pepid, www.pepid.com) and others with free access (eg, AARP, healthtools.aarp.org/drug-interactions; Drugs.com (www.drugs.com/drug_interactions.php; and HealthLine, www.healthline.com/druginteractions).
CASE After doing a medication reconciliation for Mrs. R, you find that she is taking tolterodine, an anticholinergic medication for urge urinary incontinence, and donepezil, a procholinergic medication for dementia. This type of drug-drug interaction, in which the action of one drug effectively cancels out the effect of another, should not be ignored.
Overall, you identify 8 of her medications that could be discontinued: The list includes guaifenesin (a nonessential medication of questionable efficacy); naproxen (inappropriate per Beers criteria; inappropriate in patients with heart failure, according to STOPP); alprazolam, zolpidem, and diphenhydramine (duplicate medications that are all on the Beers criteria as inappropriate for chronic use and ill-advised in patients with cognitive impairment); and omeprazole and levothyroxine (for which nothing in the patient’s history suggests a need), as well as tolterodine. Depending on dose, digoxin is yet another candidate for discontinuation.
Discontinuing medications: Proceed carefully
Physicians are often reluctant to discontinue chronic medications in older patients—even in those with advanced disease who are not likely to benefit from treatment. Focus groups have identified a number of reasons for their hesitation, including:
- the assumption that patients have no problem taking large numbers of drugs
- the fear that patients may misinterpret a plan to discontinue medications as evidence that the physician is giving up on them
- the belief that physicians must comply with practice guidelines that recommend multiple drug treatments
- concern that proposing discontinuation of medications often leads to a discussion of life expectancy and end-of-life care.28
Physicians may also fear that discontinuation of certain drugs will increase the risk of adverse outcomes. More than 30 studies have evaluated discontinuation of chronic medications in older adults, however, and found that drugs as diverse as antihypertensives, antipsychotics, benzodiazepines, and selective serotonin reuptake inhibitors (SSRIs) can often be discontinued without adverse outcomes. In many cases, improvement in patient function results.29 Medications that present the most difficulty are those that patients often become physically or psychologically dependent on, such as benzodiazepines, guaifenesin, proton-pump inhibitors, nonsteroidal anti-inflammatory drugs, and SSRIs. Some (eg, benzodiazepines, SSRIs) require a gradual reduction; for others, no taper is required
(TABLE 4).30-37
TABLE 4
Recommendations for discontinuing hard-to-stop drugs
| Medication or drug class | Discontinuation regimen | Comments |
|---|---|---|
| Benzodiazepines30 | Taper dose by 25% q 2 wk | No withdrawal symptoms reported with this taper regimen. Subtle cognitive improvement noted over a period of months |
| Guaifenesin31 | Can be discontinued without tapering if not combined with opioids or other medications. Elimination half-life is approximately 1 hour | Guaifenesin is often marketed as a combination product with opioids; such combination products require tapering |
| PPIs32-34 | Decrease dose by 50% q 2 wk; supplement with H2 blocker if needed, but tapering of H2 blocker may be required | Abrupt discontinuation after long-term use causes rebound gastric acid hypersecretion and lowers rate of success. Higher success rates with taper regimen and in patients who do not have documented GERD |
| NSAIDs35 | No taper required | Short-term use (<3 mo) acceptable for patients with no contraindications |
| SSRIs36,37 | Gradual reduction in dose over 6-8 wk | Highest rate of success in patients without a clear diagnosis of depression |
| GERD, gastroesophageal reflux disease; NSAIDs, nonsteroidal anti-inflammatory drugs; PPIs, proton-pump inhibitors; SSRIs, selective serotonin reuptake inhibitors. | ||
CASE You trim down Mrs. R’s regimen by discontinuing each of the 8 drugs, one at a time, and carefully monitor the patient during the withdrawal period. Because she had been taking alprazolam daily, the dose is tapered slowly to avoid withdrawal. Omeprazole also requires a gradual taper to avoid rebound hyperacidity.3
After confirming that Mrs. R has heart failure and COPD, you identify 2 medications that should be added to her drug regimen—an ACE inhibitor for heart failure and an inhaled anticholinergic for COPD.
Going from 16 medications to 10 saves money, decreases the likelihood of adverse events and drug-drug interactions, and helps with adherence. Mrs. R’s new drug regimen is expected to lead to improvements in memory and overall quality of life, as well.
CORRESPONDENCE
Barry D. Weiss, MD, Department of Family and Community Medicine, University of Arizona College of Medicine, Tucson, AZ 85724; [email protected]
1. Gu Q, Dillon CF, Burt V. Prescription drug use continued to increase: US prescription drug data for 2007-2008. CDC/NCHS Data Brief. 2010;42:1-2.
2. Jessup K, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;119:1977-2016.
3. Johnell K, Klarin I. The relationship between number of drugs and potential drug-drug interactions in the elderly: a study of over 600,000 elderly patients from the Swedish Prescribed Drug Register. Drug Saf. 2007;30:911-918.
4. Sarkar U, Lopez A, Maselli JH, et al. Adverse drug events in US adult ambulatory medical care. Health Services Res. 2011;46:1517-1533.
5. Rollason V, Vogt N. Reduction of polypharmacy in the elderly. A systematic review of the role of the pharmacist. Drugs Aging. 2003;20:817-832.
6. National Heart, Lung, and Blood Institute. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Available at: www.nhlbi.nih.gov/guidelines/hypertension/jnc7full.pdf. Accessed October 11, 2012.
7. Steinman MA, Hanlon JT. Managing medications in clinically complex elders. JAMA. 2010;304:1592-1601.
8. Steinman MA, Landefeld CS, Rosenthal GE, et al. Polypharmacy and prescribing quality in older people. J Am Geriatr Soc. 2006;54:1516-23.
9. Bell CM, Brener SS, Gunraj N, et al. Association of ICU or hospital admission with unintentional discontinuations of medications for chronic disease. JAMA. 2011;306:840-847.
10. Moore C, Wisnivesky J, Williams S, et al. Medical errors related to discontinuity of care from an inpatient to an outpatient setting. J Gen Intern Med. 2003;18:646-651.
11. Ziaeian B, Arauho KL, Van Ness PH, et al. Medication reconciliation accuracy and patient understanding of intended medication changes on hospital discharge. J Gen Intern Med. 2012 July 12. ePub ahead of print.
12. Hajjar ER, Hanlon JT, Sloane RJ, et al. Unnecessary drug use in frail older people at hospital discharge. J Am Geriatr Soc. 2005;53:1518-1523.
13. O’Mahony D, Gallagher P, Ryan C, et al. STOPP & START criteria: a new approach to detecting potentially inappropriate prescribing in old age. Eur Geriatr Med. 2010;1:45-51.
14. Denneboom W, Dautzenberg KGH, Grol R, et al. Analysis of polypharmacy in older patients in primary acre using a multidisciplinary expert panel. Br J Gen Pract. 2006;56:504-510.
15. Ko DT, Mamdani M, Alter DA. Lipid-lowering therapy with statins in high-risk elderly patients. JAMA. 2004;291:1864-70.
16. Wright RM, Sloane R, Pieper CF, et al. Underuse of indicated medications among physically frail older US veterans at the time of hospital discharge: results of a cross-sectional analysis of data from the Geriatric Evaluation and Management Drug Study. Am J Geriatr Pharmacother. 2009;7:271-280.
17. Garwood CL. Use of anticoagulation in elderly patients with atrial fibrillation who are risk for falls. Ann Pharmacother. 2008;42:523-532.
18. Holmes HM, Hayley DC, Alexander GC, et al. Reconsidering medication appropriateness for patients late in life. Arch Intern Med. 2006;166:605-609.
19. Simplified Methods for Estimating Life Expectancy. Available at: http://painconsortium.nih.gov/symptomresearch/chapter_14/Part_3/sec4/chspt3s4pg1.htm. Accessed October 9, 2012.
20. Beers MH, Ouslander JG, Rollingher I, et al. Explicit criteria for determining inappropriate medication use in nursing home residents. Arch Intern Med. 1991;151:1825-1832.
21. The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society Update Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616-631.
22. Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers’ criteria. Age Aging. 2008;37:673-379.
23. Han L, Agostini JV, Allore HG. Cumulative anticholinergic exposure is associated with poor memory and executive function in older men. J Am Geriatr Soc. 2008;56:2203-2210.
24. Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59:1477-1483.
25. Knight A, Falade O, Maygers J, et al. Factors associated with medication warning acceptance [abstract]. J Hosp Med. 2012;7(suppl 2):515.-
26. Isaac T, Weissman JS, Davis RB, et al. Overrides of medication alerts in ambulatory care. Arch Intern Med. 2009;169:305-311.
27. Van Der Sijs H, Aarts J, Vulto A, et al. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc. 2006;12:138-147.
28. Schuling J, Gebben H, Veehof LJG, et al. Deprescribing medication in very elderly patients with multimorbidity: the view of Dutch GPs. A qualitative study. BMC Family Practice. 2012;13:56. http://www.biomedcentral.com/1471-2296/13/56.
29. Iyer S, Naganathan V, McLachlan AJ, et al. Medication withdrawal trials in people aged 65 years and older. A systematic review. Drugs Aging. 2008;25:1021-1031.
30. Curran HV, Collins R, Fletcher S, et al. Older adults and withdrawal from benzodiazepine hypnotics in general practice: effects on cognitive function, sleep, mood and quality of life. Psychol Med. 2003;33:1223-1237.
31. Krinsky DL, Berardi RR, Ferreris SP, et al. Handbook of Nonprescription Drugs: An Interactive Approach to Self-Care. Washington, DC: American Pharmacists Association; 2012:209.
32. Bjornsson E, Abrahamsson H, Simren M, et al. Discontinuation of proton pump inhibitors in patients on long-term therapy: a double-blind, placebo-controlled trial. Aliment Pharmacol Ther. 2006;24:945-954.
33. Inadomi JM, Jamai R, Murata GH, et al. Step-down management of gastroesophageal reflux disease. Gastroenterology. 2001;131:1095-1100.
34. Hester SA. Proton pump inhibitors and rebound acid hypersecretion. Pharm Lett. 2009;25:250920.-
35. Taylor R, Jr, Lemtouni S, Weiss K, et al. Pain management in the elderly: an FDA safe use initiative expert panel’s view on preventable harm associated with NSAID therapy. Curr Gerontol Geriatr Res. 2012;196159.-
36. Ulfvarson J, Adami J, Wredling R, et al. Controlled withdrawal of selective serotonin reuptake inhibitor drugs in elderly patients in nursing homes with no indication of depression. Eur J Clin Pharmacol. 2003;59:735-740.
37. Lindstrom K, Ekedahl A, Carlsten A, et al. Can selective serotonin inhibitor drugs in elderly patients in nursing homes be reduced? Scand J Prim Health Care. 2007;25:3-8.
1. Gu Q, Dillon CF, Burt V. Prescription drug use continued to increase: US prescription drug data for 2007-2008. CDC/NCHS Data Brief. 2010;42:1-2.
2. Jessup K, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;119:1977-2016.
3. Johnell K, Klarin I. The relationship between number of drugs and potential drug-drug interactions in the elderly: a study of over 600,000 elderly patients from the Swedish Prescribed Drug Register. Drug Saf. 2007;30:911-918.
4. Sarkar U, Lopez A, Maselli JH, et al. Adverse drug events in US adult ambulatory medical care. Health Services Res. 2011;46:1517-1533.
5. Rollason V, Vogt N. Reduction of polypharmacy in the elderly. A systematic review of the role of the pharmacist. Drugs Aging. 2003;20:817-832.
6. National Heart, Lung, and Blood Institute. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Available at: www.nhlbi.nih.gov/guidelines/hypertension/jnc7full.pdf. Accessed October 11, 2012.
7. Steinman MA, Hanlon JT. Managing medications in clinically complex elders. JAMA. 2010;304:1592-1601.
8. Steinman MA, Landefeld CS, Rosenthal GE, et al. Polypharmacy and prescribing quality in older people. J Am Geriatr Soc. 2006;54:1516-23.
9. Bell CM, Brener SS, Gunraj N, et al. Association of ICU or hospital admission with unintentional discontinuations of medications for chronic disease. JAMA. 2011;306:840-847.
10. Moore C, Wisnivesky J, Williams S, et al. Medical errors related to discontinuity of care from an inpatient to an outpatient setting. J Gen Intern Med. 2003;18:646-651.
11. Ziaeian B, Arauho KL, Van Ness PH, et al. Medication reconciliation accuracy and patient understanding of intended medication changes on hospital discharge. J Gen Intern Med. 2012 July 12. ePub ahead of print.
12. Hajjar ER, Hanlon JT, Sloane RJ, et al. Unnecessary drug use in frail older people at hospital discharge. J Am Geriatr Soc. 2005;53:1518-1523.
13. O’Mahony D, Gallagher P, Ryan C, et al. STOPP & START criteria: a new approach to detecting potentially inappropriate prescribing in old age. Eur Geriatr Med. 2010;1:45-51.
14. Denneboom W, Dautzenberg KGH, Grol R, et al. Analysis of polypharmacy in older patients in primary acre using a multidisciplinary expert panel. Br J Gen Pract. 2006;56:504-510.
15. Ko DT, Mamdani M, Alter DA. Lipid-lowering therapy with statins in high-risk elderly patients. JAMA. 2004;291:1864-70.
16. Wright RM, Sloane R, Pieper CF, et al. Underuse of indicated medications among physically frail older US veterans at the time of hospital discharge: results of a cross-sectional analysis of data from the Geriatric Evaluation and Management Drug Study. Am J Geriatr Pharmacother. 2009;7:271-280.
17. Garwood CL. Use of anticoagulation in elderly patients with atrial fibrillation who are risk for falls. Ann Pharmacother. 2008;42:523-532.
18. Holmes HM, Hayley DC, Alexander GC, et al. Reconsidering medication appropriateness for patients late in life. Arch Intern Med. 2006;166:605-609.
19. Simplified Methods for Estimating Life Expectancy. Available at: http://painconsortium.nih.gov/symptomresearch/chapter_14/Part_3/sec4/chspt3s4pg1.htm. Accessed October 9, 2012.
20. Beers MH, Ouslander JG, Rollingher I, et al. Explicit criteria for determining inappropriate medication use in nursing home residents. Arch Intern Med. 1991;151:1825-1832.
21. The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society Update Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616-631.
22. Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers’ criteria. Age Aging. 2008;37:673-379.
23. Han L, Agostini JV, Allore HG. Cumulative anticholinergic exposure is associated with poor memory and executive function in older men. J Am Geriatr Soc. 2008;56:2203-2210.
24. Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59:1477-1483.
25. Knight A, Falade O, Maygers J, et al. Factors associated with medication warning acceptance [abstract]. J Hosp Med. 2012;7(suppl 2):515.-
26. Isaac T, Weissman JS, Davis RB, et al. Overrides of medication alerts in ambulatory care. Arch Intern Med. 2009;169:305-311.
27. Van Der Sijs H, Aarts J, Vulto A, et al. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc. 2006;12:138-147.
28. Schuling J, Gebben H, Veehof LJG, et al. Deprescribing medication in very elderly patients with multimorbidity: the view of Dutch GPs. A qualitative study. BMC Family Practice. 2012;13:56. http://www.biomedcentral.com/1471-2296/13/56.
29. Iyer S, Naganathan V, McLachlan AJ, et al. Medication withdrawal trials in people aged 65 years and older. A systematic review. Drugs Aging. 2008;25:1021-1031.
30. Curran HV, Collins R, Fletcher S, et al. Older adults and withdrawal from benzodiazepine hypnotics in general practice: effects on cognitive function, sleep, mood and quality of life. Psychol Med. 2003;33:1223-1237.
31. Krinsky DL, Berardi RR, Ferreris SP, et al. Handbook of Nonprescription Drugs: An Interactive Approach to Self-Care. Washington, DC: American Pharmacists Association; 2012:209.
32. Bjornsson E, Abrahamsson H, Simren M, et al. Discontinuation of proton pump inhibitors in patients on long-term therapy: a double-blind, placebo-controlled trial. Aliment Pharmacol Ther. 2006;24:945-954.
33. Inadomi JM, Jamai R, Murata GH, et al. Step-down management of gastroesophageal reflux disease. Gastroenterology. 2001;131:1095-1100.
34. Hester SA. Proton pump inhibitors and rebound acid hypersecretion. Pharm Lett. 2009;25:250920.-
35. Taylor R, Jr, Lemtouni S, Weiss K, et al. Pain management in the elderly: an FDA safe use initiative expert panel’s view on preventable harm associated with NSAID therapy. Curr Gerontol Geriatr Res. 2012;196159.-
36. Ulfvarson J, Adami J, Wredling R, et al. Controlled withdrawal of selective serotonin reuptake inhibitor drugs in elderly patients in nursing homes with no indication of depression. Eur J Clin Pharmacol. 2003;59:735-740.
37. Lindstrom K, Ekedahl A, Carlsten A, et al. Can selective serotonin inhibitor drugs in elderly patients in nursing homes be reduced? Scand J Prim Health Care. 2007;25:3-8.
Infant’s brain damage blamed on delayed delivery … and more
DURING DELIVERY, THE MOTHER’S PERINATOLOGIST recognized a severe shoulder dystocia. The perinatologist abandoned vaginal delivery and ordered an emergency cesarean delivery. The mother was transferred to an operating room (OR) with the baby’s head out between her legs. In the OR, the perinatologist pushed the baby’s head back into the uterus and performed a cesarean extraction. Nineteen minutes elapsed from when the vaginal delivery was abandoned and the baby was delivered.
The child was unresponsive at birth with no spontaneous movement or respiration. She was intubated and transferred to the NICU, where she was resuscitated. MRI confirmed that the child had hypoxic ischemia and severe, permanent brain damage from acute birth asphyxia. The child is blind, deaf, hypertensive, and has diffuse spasticity. She has a tracheostomy, a gastrostomy tube, and requires 24-hour care.
PARENTS’ CLAIM The perinatologist was negligent for abandoning vaginal delivery when delivery was progressing appropriately and there was no fetal distress. If the perinatologist had rotated the baby’s shoulder to the oblique position and/or used suprapubic pressure, the shoulder would have become disimpacted and the baby would have been safely delivered within seconds. Delay in delivery allowed for 19 minutes of umbilical cord compression, resulting in brain damage.
PHYSICIAN’S DEFENSE Cesarean delivery was appropriate; the baby did not suffer cord compression. Injury to the brain occurred days before delivery, based on prenatal ultrasonography.
VERDICT A $5.5 million California settlement was reached.
Failure to diagnose breast cancer: death
A 38-YEAR-OLD WOMAN went to her primary care physician (PCP) 3 years after giving birth. She reported breast pain, nipple discharge, and a dime-sized lump. The woman was still breastfeeding. An exam by the nurse practitioner (NP) was limited because the patient had breast implants. The NP suspected a galactocele and advised the patient to stop breastfeeding and apply ice packs. When the patient returned in 2 weeks, only the lump remained. The PCP determined that she had mastitis.
Five months later, she returned with additional lumps in both breasts, and was referred to a gynecologist. Ultrasonography (US) was ordered, but the patient never followed up. A year later, the patient was found to have metastatic breast cancer and died after 3 years of treatment.
ESTATE’S CLAIM The PCP and NP were negligent for not referring her for a breast biopsy when a lump was first detected.
DEFENDANTS’ DEFENSE Proper care was given. An earlier diagnosis would not have changed the outcome.
VERDICT A $750,000 Massachusetts settlement was reached.
What caused this child’s autism?
AFTER 33 HOURS OF LABOR, a baby was delivered vaginally by an ObGyn, nurse, and midwife. The child was diagnosed with autism several years later. His development is delayed, and he suffers cognitive impairment.
PARENTS’ CLAIM The child’s autism is due to a prolonged hypoxic event during labor. Fetal heart-rate monitoring demonstrated fetal distress, with a bradycardia. A cesarean delivery should have been performed.
PHYSICIAN’S DEFENSE The child has genetic autism unrelated to the birth process.
VERDICT A $1.35 million New York settlement was reached.
Was oxytocin the culprit?
DURING AN EXTENDED LABOR, the ObGyn continued to give the mother oxytocin, although there were signs of fetal distress. The child was born with brain damage, cannot walk, talk, or see, and requires 24-hour care.
PATIENT’S CLAIM The use of oxytocin was inappropriate given the signs of fetal distress. Oxytocin caused a lack of oxygen to the child, resulting in brain damage. A cesarean delivery should have been performed when fetal distress was identified.
DEFENDANTS’ DEFENSE The case was settled before trial.
VERDICT A $12 million Illinois settlement was reached: $11 million from the hospital and $1 million from the ObGyn.
DURING LEFT OOPHORECTOMY, the ObGyn encountered adhesions. Five days later, the 41-year-old patient reported severe pain. A second procedure revealed sepsis and perforation of the large bowel. A colostomy was performed. The patient underwent additional corrective operations.
PATIENT’S CLAIM The ObGyn was negligent for causing tissue damage to the colon that perforated and escalated into sepsis. A surgeon should have been consulted when the ObGyn found the adhesions, so the bowel could be properly inspected before the abdomen was closed. The physician was also negligent for not recognizing symptoms of sepsis earlier.
PHYSICIAN’S DEFENSE Bowel injury is a known complication of oophorectomy. The patient appeared to be making a fairly good recovery until infection became evident; she was immediately treated.
VERDICT A $6.3 million New Jersey verdict was returned, including $300,000 for the husband’s loss of consortium.
Traumatic delivery causes seizures
DURING CESAREAN DELIVERY, the ObGyn rotated the baby from a transverse to a cephalic lie, and used a vacuum extractor to deliver the head through the hysterotomy incision.
When the child was 25 hours old, he suffered a seizure that lasted 6 minutes. Focal seizure activity involving the left side of his body and a skull fracture were identified. He was transferred to another hospital, where radiologic studies indicated a middle right cerebral artery infarct. The child developed an ongoing seizure disorder, speech and language delays, and mild, left-sided weakness.
PARENTS’ CLAIM The baby’s head was not properly delivered through the cesarean incision nor should the ObGyn have used vacuum extraction. The combination of the rotation and use of vacuum caused trauma to the infant’s head. In addition, the baby was placed in the well-baby nursery, which was inappropriate because he was born through thick meconium, resuscitated by a neonatal nurse, and had a depressed skull fracture.
PHYSICIAN’S DEFENSE Delivery was not traumatic; all treatment was appropriate.
VERDICT A $4.6 million New York settlement was reached with the hospital and ObGyn’s insurer.
Mother gets severe headache during birth
A 35-YEAR-OLD WOMAN began having a severe headache during delivery that continued after birth. She was discharged from the hospital and collapsed at home a day later. She was returned to the ED, where she was left in a hallway for 6 hours. She lost consciousness while in the hallway. Imaging and neurologic evaluation determined that she suffered a hypoxic brain injury from intracranial bleeding. She has slow response time, difficulty with all aspects of everyday life, and requires full-time attendant care.
PATIENT’S CLAIM Although she complained of a headache, no testing was done prior to her hospital discharge. Treatment was extremely delayed in the ED; an earlier diagnosis could have prevented brain damage.
PHYSICIAN’S DEFENSE Nothing could have prevented the brain damage.
VERDICT A $3.5 million California settlement was mediated.
Shoulder dystocia; brachial plexus injury
WHEN SHOULDER DYSTOCIA was encountered during delivery, the ObGyn applied gentle pressure to deliver the head. He was assisted by an ObGyn resident. The child was born with a brachial plexus injury, causing left-arm paralysis. She underwent surgery that increased her range of motion, but she will need years of physical therapy.
PATIENT’S CLAIM The ObGyn applied excessive traction and the resident improperly applied fundal pressure.
DEFENDANTS’ DEFENSE Only gentle traction was used. The resident did not apply fundal pressure.
VERDICT A New York jury found the ObGyn at fault and awarded the patient $3.5 million. The resident was vindicated.

AN EXPECTANT MOTHER MISCARRIED AT HOME at 6 months’ gestation, and an ambulance was called. After the EMTs helped the mother to the ambulance, they retrieved the fetus. When the baby was seen moving its head, the EMTs requested assistance from the advanced life support (ALS) team. ALS personnel visually assessed the fetus, determined it was nonviable, and placed the baby in a small container. The mother and baby arrived at the hospital 17 minutes after the ambulance was called.
At the hospital, a nurse noticed that the fetus was warm and had a heartbeat. The baby was taken to a special-care nursery for resuscitation and then transferred to another hospital’s NICU. The baby died after 46 days from severe brain damage due to lack of oxygen.
PARENTS’ CLAIM The EMTs and ALS team should have provided better evaluation and treatment for the infant; they were not trained to determine an infant’s viability. Placing the infant inside a plastic bag inside a box with a lid further deprived the baby of oxygen.
DEFENDANTS’ DEFENSE The case was settled before trial.
VERDICT A $1 million Massachusetts settlement was reached.
Were records altered because of a delayed diagnosis?
A WOMAN FOUND A LUMP in her left breast. A gynecologist ordered mammography. In January 2006, the radiologist requested ultrasonography (US), and reported that it conclusively indicated that the mass was a cyst. The gynecologist told the patient the tests were normal; further action was unnecessary. The patient saw the gynecologist four more times before being referred to a breast surgeon. In June 2006, she underwent surgical resection and chemotherapy for a malignant breast tumor.
PATIENT’S CLAIM The gynecologist was negligent for not referring the patient to a surgeon earlier. The gynecologist altered records: excerpts from the mammogram and US reports had been scanned in with a notation that the gynecologist had told the patient to follow up with a surgeon. When the gynecologist faxed the same reports to the surgeon, the annotations were absent. The gynecologist also changed the December 2005 chart, which referred to an US she never ordered.
PHYSICIAN’S DEFENSE The gynecologist stated that she regularly “merged” two reports into one document in her practice.
VERDICT A $700,000 Pennsylvania verdict was returned.
Excessive force or standard of care?
SHOULDER DYSTOCIA occurred during labor. The child sustained left brachial plexus palsy. At age 6, his left arm is paralyzed and smaller than the right arm. He has trouble performing normal daily tasks.
PATIENT’S CLAIM The ObGyn used excessive force by pulling on the baby’s head to complete the delivery. Standard of care required the ObGyn to take a more gentle approach to achieve delivery.
PHYSICIAN’S DEFENSE Delivery was performed appropriately, and did not deviate from standard of care.
VERDICT A $20.881 million Maryland verdict was returned, including $20 million for pain and suffering. The total award was reduced to $1,531,082 when the pain and suffering award was cut to $650,000 under the state’s statutory cap.
Preterm birth from an asymptomatic UTI?
A BABY WAS BORN AT 31 WEEKS’ gestation. The child has cerebral palsy, spastic quadriplegia, and requires assistance in all aspects of life.
PARENTS’ CLAIM Chorioamnionitis from a urinary tract infection (UTI) caused preterm birth. Urinalysis performed 7 weeks earlier indicated an infection, but the second-year resident caring for the mother failed to treat the UTI. The resident should have obtained a confirming urine culture, prescribed antibiotics, and monitored the mother more closely. The resident was poorly supervised.
DEFENDANTS’ DEFENSE Chorioamnionitis developed just before birth and could not be detected or prevented. A UTI cannot remain asymptomatic for 7 weeks and still cause premature birth. The mother was at increased risk of premature delivery because she had given birth to an anencephalic infant a year earlier. She began prenatal care in the middle of her pregnancy and ignored a referral to a high-risk maternal fetal specialist.
VERDICT A New York defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
We want to hear from you! Tell us what you think.

DURING DELIVERY, THE MOTHER’S PERINATOLOGIST recognized a severe shoulder dystocia. The perinatologist abandoned vaginal delivery and ordered an emergency cesarean delivery. The mother was transferred to an operating room (OR) with the baby’s head out between her legs. In the OR, the perinatologist pushed the baby’s head back into the uterus and performed a cesarean extraction. Nineteen minutes elapsed from when the vaginal delivery was abandoned and the baby was delivered.
The child was unresponsive at birth with no spontaneous movement or respiration. She was intubated and transferred to the NICU, where she was resuscitated. MRI confirmed that the child had hypoxic ischemia and severe, permanent brain damage from acute birth asphyxia. The child is blind, deaf, hypertensive, and has diffuse spasticity. She has a tracheostomy, a gastrostomy tube, and requires 24-hour care.
PARENTS’ CLAIM The perinatologist was negligent for abandoning vaginal delivery when delivery was progressing appropriately and there was no fetal distress. If the perinatologist had rotated the baby’s shoulder to the oblique position and/or used suprapubic pressure, the shoulder would have become disimpacted and the baby would have been safely delivered within seconds. Delay in delivery allowed for 19 minutes of umbilical cord compression, resulting in brain damage.
PHYSICIAN’S DEFENSE Cesarean delivery was appropriate; the baby did not suffer cord compression. Injury to the brain occurred days before delivery, based on prenatal ultrasonography.
VERDICT A $5.5 million California settlement was reached.
Failure to diagnose breast cancer: death
A 38-YEAR-OLD WOMAN went to her primary care physician (PCP) 3 years after giving birth. She reported breast pain, nipple discharge, and a dime-sized lump. The woman was still breastfeeding. An exam by the nurse practitioner (NP) was limited because the patient had breast implants. The NP suspected a galactocele and advised the patient to stop breastfeeding and apply ice packs. When the patient returned in 2 weeks, only the lump remained. The PCP determined that she had mastitis.
Five months later, she returned with additional lumps in both breasts, and was referred to a gynecologist. Ultrasonography (US) was ordered, but the patient never followed up. A year later, the patient was found to have metastatic breast cancer and died after 3 years of treatment.
ESTATE’S CLAIM The PCP and NP were negligent for not referring her for a breast biopsy when a lump was first detected.
DEFENDANTS’ DEFENSE Proper care was given. An earlier diagnosis would not have changed the outcome.
VERDICT A $750,000 Massachusetts settlement was reached.
What caused this child’s autism?
AFTER 33 HOURS OF LABOR, a baby was delivered vaginally by an ObGyn, nurse, and midwife. The child was diagnosed with autism several years later. His development is delayed, and he suffers cognitive impairment.
PARENTS’ CLAIM The child’s autism is due to a prolonged hypoxic event during labor. Fetal heart-rate monitoring demonstrated fetal distress, with a bradycardia. A cesarean delivery should have been performed.
PHYSICIAN’S DEFENSE The child has genetic autism unrelated to the birth process.
VERDICT A $1.35 million New York settlement was reached.
Was oxytocin the culprit?
DURING AN EXTENDED LABOR, the ObGyn continued to give the mother oxytocin, although there were signs of fetal distress. The child was born with brain damage, cannot walk, talk, or see, and requires 24-hour care.
PATIENT’S CLAIM The use of oxytocin was inappropriate given the signs of fetal distress. Oxytocin caused a lack of oxygen to the child, resulting in brain damage. A cesarean delivery should have been performed when fetal distress was identified.
DEFENDANTS’ DEFENSE The case was settled before trial.
VERDICT A $12 million Illinois settlement was reached: $11 million from the hospital and $1 million from the ObGyn.

DURING LEFT OOPHORECTOMY, the ObGyn encountered adhesions. Five days later, the 41-year-old patient reported severe pain. A second procedure revealed sepsis and perforation of the large bowel. A colostomy was performed. The patient underwent additional corrective operations.
PATIENT’S CLAIM The ObGyn was negligent for causing tissue damage to the colon that perforated and escalated into sepsis. A surgeon should have been consulted when the ObGyn found the adhesions, so the bowel could be properly inspected before the abdomen was closed. The physician was also negligent for not recognizing symptoms of sepsis earlier.
PHYSICIAN’S DEFENSE Bowel injury is a known complication of oophorectomy. The patient appeared to be making a fairly good recovery until infection became evident; she was immediately treated.
VERDICT A $6.3 million New Jersey verdict was returned, including $300,000 for the husband’s loss of consortium.
Traumatic delivery causes seizures
DURING CESAREAN DELIVERY, the ObGyn rotated the baby from a transverse to a cephalic lie, and used a vacuum extractor to deliver the head through the hysterotomy incision.
When the child was 25 hours old, he suffered a seizure that lasted 6 minutes. Focal seizure activity involving the left side of his body and a skull fracture were identified. He was transferred to another hospital, where radiologic studies indicated a middle right cerebral artery infarct. The child developed an ongoing seizure disorder, speech and language delays, and mild, left-sided weakness.
PARENTS’ CLAIM The baby’s head was not properly delivered through the cesarean incision nor should the ObGyn have used vacuum extraction. The combination of the rotation and use of vacuum caused trauma to the infant’s head. In addition, the baby was placed in the well-baby nursery, which was inappropriate because he was born through thick meconium, resuscitated by a neonatal nurse, and had a depressed skull fracture.
PHYSICIAN’S DEFENSE Delivery was not traumatic; all treatment was appropriate.
VERDICT A $4.6 million New York settlement was reached with the hospital and ObGyn’s insurer.
Mother gets severe headache during birth
A 35-YEAR-OLD WOMAN began having a severe headache during delivery that continued after birth. She was discharged from the hospital and collapsed at home a day later. She was returned to the ED, where she was left in a hallway for 6 hours. She lost consciousness while in the hallway. Imaging and neurologic evaluation determined that she suffered a hypoxic brain injury from intracranial bleeding. She has slow response time, difficulty with all aspects of everyday life, and requires full-time attendant care.
PATIENT’S CLAIM Although she complained of a headache, no testing was done prior to her hospital discharge. Treatment was extremely delayed in the ED; an earlier diagnosis could have prevented brain damage.
PHYSICIAN’S DEFENSE Nothing could have prevented the brain damage.
VERDICT A $3.5 million California settlement was mediated.
Shoulder dystocia; brachial plexus injury
WHEN SHOULDER DYSTOCIA was encountered during delivery, the ObGyn applied gentle pressure to deliver the head. He was assisted by an ObGyn resident. The child was born with a brachial plexus injury, causing left-arm paralysis. She underwent surgery that increased her range of motion, but she will need years of physical therapy.
PATIENT’S CLAIM The ObGyn applied excessive traction and the resident improperly applied fundal pressure.
DEFENDANTS’ DEFENSE Only gentle traction was used. The resident did not apply fundal pressure.
VERDICT A New York jury found the ObGyn at fault and awarded the patient $3.5 million. The resident was vindicated.

AN EXPECTANT MOTHER MISCARRIED AT HOME at 6 months’ gestation, and an ambulance was called. After the EMTs helped the mother to the ambulance, they retrieved the fetus. When the baby was seen moving its head, the EMTs requested assistance from the advanced life support (ALS) team. ALS personnel visually assessed the fetus, determined it was nonviable, and placed the baby in a small container. The mother and baby arrived at the hospital 17 minutes after the ambulance was called.
At the hospital, a nurse noticed that the fetus was warm and had a heartbeat. The baby was taken to a special-care nursery for resuscitation and then transferred to another hospital’s NICU. The baby died after 46 days from severe brain damage due to lack of oxygen.
PARENTS’ CLAIM The EMTs and ALS team should have provided better evaluation and treatment for the infant; they were not trained to determine an infant’s viability. Placing the infant inside a plastic bag inside a box with a lid further deprived the baby of oxygen.
DEFENDANTS’ DEFENSE The case was settled before trial.
VERDICT A $1 million Massachusetts settlement was reached.
Were records altered because of a delayed diagnosis?
A WOMAN FOUND A LUMP in her left breast. A gynecologist ordered mammography. In January 2006, the radiologist requested ultrasonography (US), and reported that it conclusively indicated that the mass was a cyst. The gynecologist told the patient the tests were normal; further action was unnecessary. The patient saw the gynecologist four more times before being referred to a breast surgeon. In June 2006, she underwent surgical resection and chemotherapy for a malignant breast tumor.
PATIENT’S CLAIM The gynecologist was negligent for not referring the patient to a surgeon earlier. The gynecologist altered records: excerpts from the mammogram and US reports had been scanned in with a notation that the gynecologist had told the patient to follow up with a surgeon. When the gynecologist faxed the same reports to the surgeon, the annotations were absent. The gynecologist also changed the December 2005 chart, which referred to an US she never ordered.
PHYSICIAN’S DEFENSE The gynecologist stated that she regularly “merged” two reports into one document in her practice.
VERDICT A $700,000 Pennsylvania verdict was returned.
Excessive force or standard of care?
SHOULDER DYSTOCIA occurred during labor. The child sustained left brachial plexus palsy. At age 6, his left arm is paralyzed and smaller than the right arm. He has trouble performing normal daily tasks.
PATIENT’S CLAIM The ObGyn used excessive force by pulling on the baby’s head to complete the delivery. Standard of care required the ObGyn to take a more gentle approach to achieve delivery.
PHYSICIAN’S DEFENSE Delivery was performed appropriately, and did not deviate from standard of care.
VERDICT A $20.881 million Maryland verdict was returned, including $20 million for pain and suffering. The total award was reduced to $1,531,082 when the pain and suffering award was cut to $650,000 under the state’s statutory cap.
Preterm birth from an asymptomatic UTI?
A BABY WAS BORN AT 31 WEEKS’ gestation. The child has cerebral palsy, spastic quadriplegia, and requires assistance in all aspects of life.
PARENTS’ CLAIM Chorioamnionitis from a urinary tract infection (UTI) caused preterm birth. Urinalysis performed 7 weeks earlier indicated an infection, but the second-year resident caring for the mother failed to treat the UTI. The resident should have obtained a confirming urine culture, prescribed antibiotics, and monitored the mother more closely. The resident was poorly supervised.
DEFENDANTS’ DEFENSE Chorioamnionitis developed just before birth and could not be detected or prevented. A UTI cannot remain asymptomatic for 7 weeks and still cause premature birth. The mother was at increased risk of premature delivery because she had given birth to an anencephalic infant a year earlier. She began prenatal care in the middle of her pregnancy and ignored a referral to a high-risk maternal fetal specialist.
VERDICT A New York defense verdict was returned.

DURING DELIVERY, THE MOTHER’S PERINATOLOGIST recognized a severe shoulder dystocia. The perinatologist abandoned vaginal delivery and ordered an emergency cesarean delivery. The mother was transferred to an operating room (OR) with the baby’s head out between her legs. In the OR, the perinatologist pushed the baby’s head back into the uterus and performed a cesarean extraction. Nineteen minutes elapsed from when the vaginal delivery was abandoned and the baby was delivered.
The child was unresponsive at birth with no spontaneous movement or respiration. She was intubated and transferred to the NICU, where she was resuscitated. MRI confirmed that the child had hypoxic ischemia and severe, permanent brain damage from acute birth asphyxia. The child is blind, deaf, hypertensive, and has diffuse spasticity. She has a tracheostomy, a gastrostomy tube, and requires 24-hour care.
PARENTS’ CLAIM The perinatologist was negligent for abandoning vaginal delivery when delivery was progressing appropriately and there was no fetal distress. If the perinatologist had rotated the baby’s shoulder to the oblique position and/or used suprapubic pressure, the shoulder would have become disimpacted and the baby would have been safely delivered within seconds. Delay in delivery allowed for 19 minutes of umbilical cord compression, resulting in brain damage.
PHYSICIAN’S DEFENSE Cesarean delivery was appropriate; the baby did not suffer cord compression. Injury to the brain occurred days before delivery, based on prenatal ultrasonography.
VERDICT A $5.5 million California settlement was reached.
Failure to diagnose breast cancer: death
A 38-YEAR-OLD WOMAN went to her primary care physician (PCP) 3 years after giving birth. She reported breast pain, nipple discharge, and a dime-sized lump. The woman was still breastfeeding. An exam by the nurse practitioner (NP) was limited because the patient had breast implants. The NP suspected a galactocele and advised the patient to stop breastfeeding and apply ice packs. When the patient returned in 2 weeks, only the lump remained. The PCP determined that she had mastitis.
Five months later, she returned with additional lumps in both breasts, and was referred to a gynecologist. Ultrasonography (US) was ordered, but the patient never followed up. A year later, the patient was found to have metastatic breast cancer and died after 3 years of treatment.
ESTATE’S CLAIM The PCP and NP were negligent for not referring her for a breast biopsy when a lump was first detected.
DEFENDANTS’ DEFENSE Proper care was given. An earlier diagnosis would not have changed the outcome.
VERDICT A $750,000 Massachusetts settlement was reached.
What caused this child’s autism?
AFTER 33 HOURS OF LABOR, a baby was delivered vaginally by an ObGyn, nurse, and midwife. The child was diagnosed with autism several years later. His development is delayed, and he suffers cognitive impairment.
PARENTS’ CLAIM The child’s autism is due to a prolonged hypoxic event during labor. Fetal heart-rate monitoring demonstrated fetal distress, with a bradycardia. A cesarean delivery should have been performed.
PHYSICIAN’S DEFENSE The child has genetic autism unrelated to the birth process.
VERDICT A $1.35 million New York settlement was reached.
Was oxytocin the culprit?
DURING AN EXTENDED LABOR, the ObGyn continued to give the mother oxytocin, although there were signs of fetal distress. The child was born with brain damage, cannot walk, talk, or see, and requires 24-hour care.
PATIENT’S CLAIM The use of oxytocin was inappropriate given the signs of fetal distress. Oxytocin caused a lack of oxygen to the child, resulting in brain damage. A cesarean delivery should have been performed when fetal distress was identified.
DEFENDANTS’ DEFENSE The case was settled before trial.
VERDICT A $12 million Illinois settlement was reached: $11 million from the hospital and $1 million from the ObGyn.

DURING LEFT OOPHORECTOMY, the ObGyn encountered adhesions. Five days later, the 41-year-old patient reported severe pain. A second procedure revealed sepsis and perforation of the large bowel. A colostomy was performed. The patient underwent additional corrective operations.
PATIENT’S CLAIM The ObGyn was negligent for causing tissue damage to the colon that perforated and escalated into sepsis. A surgeon should have been consulted when the ObGyn found the adhesions, so the bowel could be properly inspected before the abdomen was closed. The physician was also negligent for not recognizing symptoms of sepsis earlier.
PHYSICIAN’S DEFENSE Bowel injury is a known complication of oophorectomy. The patient appeared to be making a fairly good recovery until infection became evident; she was immediately treated.
VERDICT A $6.3 million New Jersey verdict was returned, including $300,000 for the husband’s loss of consortium.
Traumatic delivery causes seizures
DURING CESAREAN DELIVERY, the ObGyn rotated the baby from a transverse to a cephalic lie, and used a vacuum extractor to deliver the head through the hysterotomy incision.
When the child was 25 hours old, he suffered a seizure that lasted 6 minutes. Focal seizure activity involving the left side of his body and a skull fracture were identified. He was transferred to another hospital, where radiologic studies indicated a middle right cerebral artery infarct. The child developed an ongoing seizure disorder, speech and language delays, and mild, left-sided weakness.
PARENTS’ CLAIM The baby’s head was not properly delivered through the cesarean incision nor should the ObGyn have used vacuum extraction. The combination of the rotation and use of vacuum caused trauma to the infant’s head. In addition, the baby was placed in the well-baby nursery, which was inappropriate because he was born through thick meconium, resuscitated by a neonatal nurse, and had a depressed skull fracture.
PHYSICIAN’S DEFENSE Delivery was not traumatic; all treatment was appropriate.
VERDICT A $4.6 million New York settlement was reached with the hospital and ObGyn’s insurer.
Mother gets severe headache during birth
A 35-YEAR-OLD WOMAN began having a severe headache during delivery that continued after birth. She was discharged from the hospital and collapsed at home a day later. She was returned to the ED, where she was left in a hallway for 6 hours. She lost consciousness while in the hallway. Imaging and neurologic evaluation determined that she suffered a hypoxic brain injury from intracranial bleeding. She has slow response time, difficulty with all aspects of everyday life, and requires full-time attendant care.
PATIENT’S CLAIM Although she complained of a headache, no testing was done prior to her hospital discharge. Treatment was extremely delayed in the ED; an earlier diagnosis could have prevented brain damage.
PHYSICIAN’S DEFENSE Nothing could have prevented the brain damage.
VERDICT A $3.5 million California settlement was mediated.
Shoulder dystocia; brachial plexus injury
WHEN SHOULDER DYSTOCIA was encountered during delivery, the ObGyn applied gentle pressure to deliver the head. He was assisted by an ObGyn resident. The child was born with a brachial plexus injury, causing left-arm paralysis. She underwent surgery that increased her range of motion, but she will need years of physical therapy.
PATIENT’S CLAIM The ObGyn applied excessive traction and the resident improperly applied fundal pressure.
DEFENDANTS’ DEFENSE Only gentle traction was used. The resident did not apply fundal pressure.
VERDICT A New York jury found the ObGyn at fault and awarded the patient $3.5 million. The resident was vindicated.

AN EXPECTANT MOTHER MISCARRIED AT HOME at 6 months’ gestation, and an ambulance was called. After the EMTs helped the mother to the ambulance, they retrieved the fetus. When the baby was seen moving its head, the EMTs requested assistance from the advanced life support (ALS) team. ALS personnel visually assessed the fetus, determined it was nonviable, and placed the baby in a small container. The mother and baby arrived at the hospital 17 minutes after the ambulance was called.
At the hospital, a nurse noticed that the fetus was warm and had a heartbeat. The baby was taken to a special-care nursery for resuscitation and then transferred to another hospital’s NICU. The baby died after 46 days from severe brain damage due to lack of oxygen.
PARENTS’ CLAIM The EMTs and ALS team should have provided better evaluation and treatment for the infant; they were not trained to determine an infant’s viability. Placing the infant inside a plastic bag inside a box with a lid further deprived the baby of oxygen.
DEFENDANTS’ DEFENSE The case was settled before trial.
VERDICT A $1 million Massachusetts settlement was reached.
Were records altered because of a delayed diagnosis?
A WOMAN FOUND A LUMP in her left breast. A gynecologist ordered mammography. In January 2006, the radiologist requested ultrasonography (US), and reported that it conclusively indicated that the mass was a cyst. The gynecologist told the patient the tests were normal; further action was unnecessary. The patient saw the gynecologist four more times before being referred to a breast surgeon. In June 2006, she underwent surgical resection and chemotherapy for a malignant breast tumor.
PATIENT’S CLAIM The gynecologist was negligent for not referring the patient to a surgeon earlier. The gynecologist altered records: excerpts from the mammogram and US reports had been scanned in with a notation that the gynecologist had told the patient to follow up with a surgeon. When the gynecologist faxed the same reports to the surgeon, the annotations were absent. The gynecologist also changed the December 2005 chart, which referred to an US she never ordered.
PHYSICIAN’S DEFENSE The gynecologist stated that she regularly “merged” two reports into one document in her practice.
VERDICT A $700,000 Pennsylvania verdict was returned.
Excessive force or standard of care?
SHOULDER DYSTOCIA occurred during labor. The child sustained left brachial plexus palsy. At age 6, his left arm is paralyzed and smaller than the right arm. He has trouble performing normal daily tasks.
PATIENT’S CLAIM The ObGyn used excessive force by pulling on the baby’s head to complete the delivery. Standard of care required the ObGyn to take a more gentle approach to achieve delivery.
PHYSICIAN’S DEFENSE Delivery was performed appropriately, and did not deviate from standard of care.
VERDICT A $20.881 million Maryland verdict was returned, including $20 million for pain and suffering. The total award was reduced to $1,531,082 when the pain and suffering award was cut to $650,000 under the state’s statutory cap.
Preterm birth from an asymptomatic UTI?
A BABY WAS BORN AT 31 WEEKS’ gestation. The child has cerebral palsy, spastic quadriplegia, and requires assistance in all aspects of life.
PARENTS’ CLAIM Chorioamnionitis from a urinary tract infection (UTI) caused preterm birth. Urinalysis performed 7 weeks earlier indicated an infection, but the second-year resident caring for the mother failed to treat the UTI. The resident should have obtained a confirming urine culture, prescribed antibiotics, and monitored the mother more closely. The resident was poorly supervised.
DEFENDANTS’ DEFENSE Chorioamnionitis developed just before birth and could not be detected or prevented. A UTI cannot remain asymptomatic for 7 weeks and still cause premature birth. The mother was at increased risk of premature delivery because she had given birth to an anencephalic infant a year earlier. She began prenatal care in the middle of her pregnancy and ignored a referral to a high-risk maternal fetal specialist.
VERDICT A New York defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
We want to hear from you! Tell us what you think.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
We want to hear from you! Tell us what you think.
When and how to place an autologous rectus fascia pubovaginal sling
Watch 2 intraoperative videos
These videos were selected by Mickey Karram, MD, and presented courtesy of
International Academy of Pelvic Surgery
Developed in Partnership with International Academy of Pelvic Surgery
CASE 1: Recurrent SUI and mesh erosion
A 50-year-old woman reports urinary incontinence that is associated with activity and exertion—stress urinary incontinence (SUI)—and says it has worsened over the past year. She mentions that she underwent vaginal hysterectomy, with placement of a tension-free vaginal tape (TVT), about 2 years earlier.
During physical examination, the patient becomes incontinent when abdominal pressure is increased, with some urethral mobility (cotton-swab deflection to 25° from the horizontal). She is also noted to have erosion of the TVT tape into the vaginal lumen.
Urodynamic testing reveals easily demonstrable SUI at a volume of 150 mL when she is in the sitting position, with a Valsalva leak-point pressure of 55 cm H2O. Her bladder remains stable to a capacity of 520 mL. Cystoscopy yields unremarkable findings.
When she is offered surgical correction of her SUI, the patient expresses a preference for the use of her own tissues and says she does not want to have synthetic mesh placed.
Is this patient a candidate for a rectus fascia pubovaginal sling?
As more patients express reservations about the placement of synthetic mesh during sling procedures, the use of autologous rectus fascia pubovaginal slings has risen. The concept of using a patient’s own tissue as a sling to support the urethra dates to the early 20th century, but it was not until late in that century that the procedure gained widespread appreciation and evolved into its current form. Initially, the procedure entailed mobilizing a strip of abdominal muscle (either rectus or pyramidalis), freeing one end of the strip from its attachment, passing that end under the bladder neck, and reaffixing it to the abdominal muscle wall, forming a “U”-shaped sling around the bladder outlet. Subsequently, overlying abdominal fascia was included in the sling, eventually replacing the muscle altogether. The final innovation: An isolated strip of fascia was suspended by free sutures that were tied to the abdominal wall or attached on top of the abdominal rectus sheath.
The autologous pubovaginal sling supports the proximal urethra and bladder neck to achieve continence by providing a direct compressive force on the urethra and bladder outlet, or by reestablishing a reinforcing platform or hammock against which the urethra is compressed during the transmission of increased abdominal pressure.
The sling is suspended on each end by free sutures that are attached directly to the abdominal wall musculature or, more commonly, tied to each other on the anterior surface of the abdominal wall.
Long-term success depends on healing and fibrotic processes, which occur primarily where the sling passes through the endopelvic fascia.
Although the pubovaginal sling procedure was pioneered as a surgical option for intrinsic sphincter deficiency (ISD), its indications have broadened to encompass all types of SUI. Its reliable results and durable outcomes make it one of the main standards of treatment, and the pubovaginal sling has been used extensively as primary therapy for:
- SUI related to ISD or urethral hypermobility
- as a salvage procedure for recurrent SUI
- as an adjunct to urethral and bladder reconstruction
- as a way to functionally close the urethra to abandon urethral access to the bladder.
In our opinion, the autologous pubovaginal sling is appropriate for patients with SUI who decline to have synthetic material implanted because of concerns related to long-term placement of synthetic mesh. Other good candidates are women who experience recurrent incontinence after placement of a synthetic sling or who develop a complication, such as vaginal erosion (VIDEO 1, Rectus fascia pubovaginal sling after an unsuccessful TVT), after placement of a synthetic sling. We also prefer to use an autologous sling in patients who have been radiated or who have sustained urethral injuries, as well as in patients who are undergoing simultaneous repair of urethrovaginal fistula or diverticulum—or those who have already undergone such repair.
What is the optimal sling material?
Rectus abdominis fascia versus fascia lata. The two most commonly used autologous tissues are rectus abdominus fascia and fascia lata. Both of these materials have been studied extensively and proven to be effective and reliable. Most surgeons prefer rectus fascia because it is easier and quicker to harvest.
Allogenic and xenogenic tissues. Allogenic (cadaveric) fascia lata and cadaveric dermis provide reasonable efficacy, but durability remains an issue, as high failure rates have been reported. Bovine and porcine dermis, as well as porcine small-intestine submucosa, are also effective for SUI, although durability remains a concern.
Synthetic materials. Synthetic graft materials of various designs and substances also have been used as sling material. Monofilament, large-pore weave grafts (Type 1 mesh) are recommended for implantation in the vagina. Although good efficacy can be achieved with synthetic mesh, the material also may increase the risk of serious complications, such as infection, vaginal extrusion, and genitourinary erosion, and is not recommended for use beneath the proximal urethra or bladder neck.
The autologous pubovaginal sling supports the proximal urethra and bladder neck to achieve continence by providing a direct compressive force on the urethra and bladder outlet, or by reestablishing a reinforcing platform or hammock against which the urethra is compressed during increased abdominal pressure.
How to harvest rectus fascia and create a sling
1. Choose anesthesia and perioperative antibiotics
Pubovaginal sling procedures are generally carried out under general anesthesia, but spinal or epidural anesthesia also is possible. Full-patient paralysis is not warranted but may facilitate closure of the rectus fascia after fascial harvesting.
Perioperative antibiotics usually are given to ensure appropriate coverage against skin and vaginal flora (for example, a cephalosporin or fluoroquinolone). In fact, perioperative antibiotics have become a mandated quality of care measure in the United States.
2. Position the patient for optimal access
Place the patient in the low lithotomy position with her legs in stirrups. The abdomen and perineum should be sterilely prepared and draped to provide access to the vagina and lower abdomen.
After the bladder is drained with a Foley catheter, place a weighted vaginal speculum and use either lateral labial retraction sutures or a self-retaining retractor system to facilitate vaginal exposure.
3. Make an abdominal incision
Make an 8- to 10-cm Pfannenstiel incision approximately 3 to 5 cm above the pubic bone, carry the dissection down to the level of the rectus fascia using a combination of electrocautery and blunt dissection, and sweep the fat and subcutaneous tissue clear of the rectus tissue (FIGURE 1).
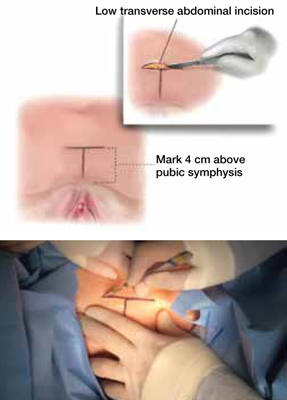
FIGURE 1 Skin incision
Before initiating the operation, delineate the location of the transverse skin incision, which should measure 8 to 10 cm and be situated about 4 cm above the symphysis pubis. A vertical incision is also feasible, although it usually is less aesthetic.
4. Harvest the fascia
The rectus abdominis fascia can be harvested in a transverse or vertical orientation. A fascial segment at least 8 cm in length and 1.5 to 2 cm in width is recommended.
Delineate the fascial segment to be resected using a surgical marking pen or electrocautery, then incise the tissue sharply with a scalpel, scissors, or electrocautery along the drawn lines.
Virgin fascia is preferred, but the presence of fibrotic rectus fascia does not prohibit its use. If you are resecting the fascia close and parallel to the symphysis pubis, leave at least 0.5 to 1.0 cm attached to facilitate closure of the defect created in the fascia. Small Army/Navy retractors permit aggressive retraction of skin edges, making it possible to use a smaller skin incision (FIGURE 2).
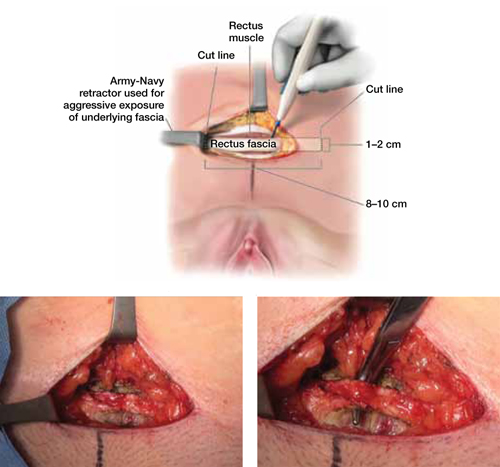
FIGURE 2 Resect the fascial strip
After choosing the optimal location for excision, mark the area using electrocautery or a surgical marking pen. Then resect the strip using a scalpel or electrocautery. The strip should measure 8 to 10 cm in length and 1 to 2 cm in width. If the skin incision is small, Army/Navy retractors may enhance exposure.
5. Close the fascial defect
Use heavy-gauge (#1 or #0) delayed, absorbable suture in a running fashion. It may be necessary to mobilize the rectus abdominis fascial edges to ensure appropriate tension-free approximation. It is important that anesthesia be sufficient to ensure muscular relaxation and paralysis during closure.
6. Prepare the fascial sling
Affix a single #1 permanent (for example, polypropylene or polyester) suture to each end of the fascial segment by passing the needle through the undersurface of the sling and then back through the top of the sling. If necessary, defat the sling (FIGURE 3).
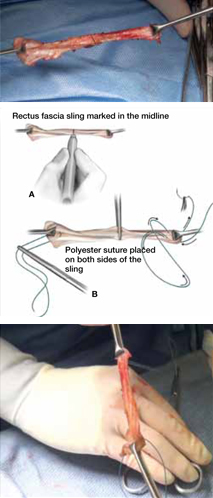
FIGURE 3 Attach suspensory sutures
A. Mark the midline of the fascial sling with a pen and gently grasp it using a hemostat. B. Attach a polyester suture to each end of the fascial sling after stripping it of any adipose tissue. Ensure that the initial entry and exit points of the polyester sutures are on the same side of the strip that originally abutted the rectus muscles.
7. Dissect the vagina
Use injectable-grade saline or a local analgesic, such as 1% lidocaine, to hydrodissect the subepithelial tissues of the distal portion of the anterior vaginal wall. Make a midline or inverted “U” incision into the vagina (FIGURE 4).
Create vaginal flaps that have sufficient mobility to ensure tension-free closure over the sling. Carry out dissection laterally and anteriorly until you encounter the endopelvic fascia, then incise the endopelvic fascia and dissect it from the posterior surface of the pubis to enter the retropubic space.
Although blunt dissection sometimes can be performed, sharp dissection with Mayo scissors is often required, especially in cases that involve recurrent stress incontinence (FIGURE 4).
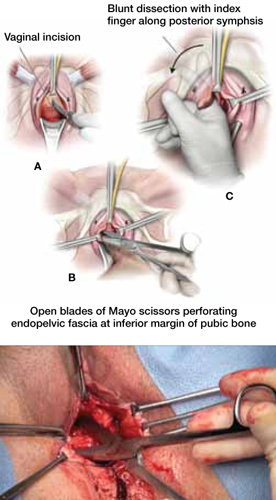
FIGURE 4 Dissect the vagina
A. Use an inverted “U” or vertical incision on the vaginal mucosa overlying the midurethra and bladder. B. Carefully dissect the tissue to the pubic rami bilaterally until the urogenital diaphragm is identified, then sharply penetrate it using Mayo scissors. C. Enlarge the opening by repeating the procedure on the opposite side.
8. Pass retropubic needles
Pass Stamey needles or long clamps through the retropubic space from the open abdominal wound immediately posterior to the pubic bone, approximately 4 cm apart. You can maintain distal control of the needles by direct finger guidance through the vaginal incision. Be careful to advance the tip of the needle adjacent to the posterior surface of the pubic bone to avoid inadvertent bladder injury (FIGURE 5). Proper bladder drainage also helps to minimize injury to the bladder, which may be closely adherent to the pubis, especially if a prior retropubic procedure has been performed, as in Case 1.
FIGURE 5 Place the sling
A. Insert the Stamey needle through the rectus fascia and guide it into the vagina with the index finger placed against the tip of the needle. B. Thread both ends of the polyester suture into the eye of the Stamey needle and then retract the needle carefully until the suture ends are delivered abdominally at the level of the fascia.
9. Rule out bladder injury
Careful cystoscopic examination of the bladder is mandatory after passing the needles to rule out inadvertent injury. Injuries to the bladder typically occur at the 1 o’clock and 11 o’clock positions, so use a 70° lens, and fill the bladder completely to expand any mucosal redundancy. Wiggle the needles or clamps to help localize their position relative to the bladder wall.
10. Deploy the sling
Thread the free ends of the sutures affixed to the sling into the ends of the Stamey needles—or grasp them with clamps—and pull each suture up to the anterior abdominal wall through the retropubic space (FIGURE 5). Keep the sling centered and flat at the area of the bladder neck.
Some surgeons fix the sling in the midline to the underlying periurethral tissue using numerous delayed absorbable sutures. We prefer to leave the sling unattached to the underlying urethra and bladder neck.
11. Tension the sling
Various techniques are applicable. To ensure adequate “looseness,” we tie the sutures across the midline while holding a right-angle clamp between the sling material and the posterior urethral surface. The goal is for the sling to prevent the descent of the proximal urethra during increases in abdominal pressure without creating any outlet obstruction to the normal flow of urine (FIGURE 6).
FIGURE 6 Tension the sling
A. Tie the suspensory sutures abdominally above the fascial closure line. Tie the sutures across the assistant’s index finger to avoid excessive tension. B. Assess the tension using a right-angle clamp placed between the pubovaginal sling and the vagina.
12. Close the incisions
Close the abdominal skin incision using 3-0 and 4-0 absorbable sutures. Use 3-0 absorbable sutures to close the vaginal mucosa. We prefer to close the vagina after completion of the tensioning procedure, but some surgeons complete this step prior to tensioning.
13. Place a catheter, packing material
Place a bladder catheter and vaginal gauze packing. Both the catheter and gauze may be removed after 24 hours. If the patient is unable to void at that time, teach her intermittent self-catheterization, or place an indwelling Foley catheter for 1 week.
Outcomes show good efficacy
Pubovaginal slings are highly effective, with success rates between 50% and 75% after follow-up as long as 10 years.1 In 2011, Blaivas and Chaikin reported 4-year follow-up data, with improvement or cure in 100% of patients with uncomplicated SUI and in as many as 93% of patients in more complicated cases.2 Most failures were due to urge incontinence and occurred within the first 6 postoperative months; 3% of these urge patients were thought to have developed de novo urge incontinence.
Other studies have found de novo urgency and storage symptoms in as many as 23% of patients, with 11% of patients reporting voiding dysfunction and as many as 7.8% requiring long-term self-catheterization.1
Flawed methodology in the few randomized, controlled trials that have compared the pubovaginal sling with the tension-free vaginal tape (TVT) has cast doubt on their findings.3 Basok and colleagues found an increased rate of de novo urgency in the women treated with a pubovaginal sling, compared with those who underwent intravaginal slingplasty,4 whereas Sharifiaghdas and Mortazavi found equal efficacy between pubovaginal and retropubic midurethral synthetic slings.5 The most scientifically valid randomized, controlled trial found equal subjective cure rates and complication rates when a biologic pubovaginal sling was compared with the TVT.6 In that study, the pubovaginal sling was of porcine origin.
In a comparison of autologous and autograft slings, Flynn found equal control of SUI over 2 years, with reduced postoperative discomfort in the allograft group.7
When autologous pubovaginal slings were compared with Burch colposuspension in a randomized, controlled trial, fascial slings were better at controlling incontinence despite an increased morbidity profile.8
A meta-analysis found equal subjective cure rates and overall efficacy between pubovaginal and midurethral synthetic slings.9
Voiding dysfunction is the most common complication
Transient urinary retention may occur in as many as 20% of patients and requires intermittent self-catheterization until resolution (typically 2–4 weeks). Prolonged postoperative voiding dysfunction (lasting more than 4–6 weeks), including de novo urgency, urgency incontinence, and obstructive symptoms, may occur to some degree in as many as 25% of patients. However, fewer than 3% of women require subsequent urethrolysis for treatment of prolonged retention or obstructive voiding symptoms.
Synthetic full-length midurethral slings remain the standard of care
for SUI
Charles W. Nager, MD (November 2012)
Harvest the fascia first. Because substantial bleeding can occur during vaginal dissection, it is advisable to harvest the autologous fascia and prepare the sling by affixing sutures to it before dissecting the vagina. This facilitates timely insertion of the sling and minimal blood loss. Retropubic bleeding from high in the space that occurs during dissection almost always resolves upon placement of the sling. We recommend against prolonged attempts at hemostasis.
In urethral reconstruction, tension the sling after reconstruction. When placing an autologous pubovaginal sling in the setting of urethral reconstruction or as tissue interposition, harvest the fascia and prepare and deploy the sling (with passage of the retropubic sutures) before reconstructing the urethra—but refrain from tensioning until after the reconstruction is completed. Then affix the sling in the appropriate location and tension it. When the sling is placed after reconstruction, it can damage the reconstruction through traction or direct injury.
Don’t worry about surface orientation. During placement of the autologous sling material, surface orientation does not matter. Conventionally, however, the “body-side” or underside of the graft is placed on the body-side of the patient.
Tensioning varies between patients. For most women, sling tensioning can be accomplished by tying the sutures over one or two fingers placed across the fascia. In patients who have undergone multiple procedures and who have a nonmobile urethra, however, tension should be tighter and must be individualized, based on the patient’s anatomy, lower urinary tract function, and willingness to perform intermittent self-catheterization for a prolonged period of time.
CASE 1: Resolved
After you advise the patient of the risks and benefits of the rectus fascia pubovaginal sling, in comparison with a repeat synthetic midurethral sling, she continues to insist on the use of autologous tissue. She undergoes the pubovaginal sling operation with excision of eroded mesh without complication.
A 35-year-old woman reports continuous urinary leakage that is not associated with movement. She was previously told that she had an ectopic ureter implanted into a congenitally short urethra, and she underwent repair of the problem, including reimplantation of the ureter and placement of a cadaveric fascia lata sling. A congenital remnant—observed as a blind pouch via cystoscopy—was left attached to the urethra. Two years have passed since that operation.
Physical findings: A pelvic examination reveals complete loss of the posterior urethra. One possible explanation: The remnant became infected and caused a breakdown of the posterior urethra, with complete disappearance of the cadaveric fascia lata.
Recommended management: Complete urethral reconstruction, with transposition of a martius fat pad and repeat placement of a cadaveric fascia pubovaginal sling.
Technique: See Video 2, Urethral reconstruction.
We want to hear from you! Tell us what you think.
Watch 2 intraoperative videos
These videos were selected by Mickey Karram, MD, and presented courtesy of
International Academy of Pelvic Surgery
Developed in Partnership with International Academy of Pelvic Surgery
CASE 1: Recurrent SUI and mesh erosion
A 50-year-old woman reports urinary incontinence that is associated with activity and exertion—stress urinary incontinence (SUI)—and says it has worsened over the past year. She mentions that she underwent vaginal hysterectomy, with placement of a tension-free vaginal tape (TVT), about 2 years earlier.
During physical examination, the patient becomes incontinent when abdominal pressure is increased, with some urethral mobility (cotton-swab deflection to 25° from the horizontal). She is also noted to have erosion of the TVT tape into the vaginal lumen.
Urodynamic testing reveals easily demonstrable SUI at a volume of 150 mL when she is in the sitting position, with a Valsalva leak-point pressure of 55 cm H2O. Her bladder remains stable to a capacity of 520 mL. Cystoscopy yields unremarkable findings.
When she is offered surgical correction of her SUI, the patient expresses a preference for the use of her own tissues and says she does not want to have synthetic mesh placed.
Is this patient a candidate for a rectus fascia pubovaginal sling?
As more patients express reservations about the placement of synthetic mesh during sling procedures, the use of autologous rectus fascia pubovaginal slings has risen. The concept of using a patient’s own tissue as a sling to support the urethra dates to the early 20th century, but it was not until late in that century that the procedure gained widespread appreciation and evolved into its current form. Initially, the procedure entailed mobilizing a strip of abdominal muscle (either rectus or pyramidalis), freeing one end of the strip from its attachment, passing that end under the bladder neck, and reaffixing it to the abdominal muscle wall, forming a “U”-shaped sling around the bladder outlet. Subsequently, overlying abdominal fascia was included in the sling, eventually replacing the muscle altogether. The final innovation: An isolated strip of fascia was suspended by free sutures that were tied to the abdominal wall or attached on top of the abdominal rectus sheath.
The autologous pubovaginal sling supports the proximal urethra and bladder neck to achieve continence by providing a direct compressive force on the urethra and bladder outlet, or by reestablishing a reinforcing platform or hammock against which the urethra is compressed during the transmission of increased abdominal pressure.
The sling is suspended on each end by free sutures that are attached directly to the abdominal wall musculature or, more commonly, tied to each other on the anterior surface of the abdominal wall.
Long-term success depends on healing and fibrotic processes, which occur primarily where the sling passes through the endopelvic fascia.
Although the pubovaginal sling procedure was pioneered as a surgical option for intrinsic sphincter deficiency (ISD), its indications have broadened to encompass all types of SUI. Its reliable results and durable outcomes make it one of the main standards of treatment, and the pubovaginal sling has been used extensively as primary therapy for:
- SUI related to ISD or urethral hypermobility
- as a salvage procedure for recurrent SUI
- as an adjunct to urethral and bladder reconstruction
- as a way to functionally close the urethra to abandon urethral access to the bladder.
In our opinion, the autologous pubovaginal sling is appropriate for patients with SUI who decline to have synthetic material implanted because of concerns related to long-term placement of synthetic mesh. Other good candidates are women who experience recurrent incontinence after placement of a synthetic sling or who develop a complication, such as vaginal erosion (VIDEO 1, Rectus fascia pubovaginal sling after an unsuccessful TVT), after placement of a synthetic sling. We also prefer to use an autologous sling in patients who have been radiated or who have sustained urethral injuries, as well as in patients who are undergoing simultaneous repair of urethrovaginal fistula or diverticulum—or those who have already undergone such repair.
What is the optimal sling material?
Rectus abdominis fascia versus fascia lata. The two most commonly used autologous tissues are rectus abdominus fascia and fascia lata. Both of these materials have been studied extensively and proven to be effective and reliable. Most surgeons prefer rectus fascia because it is easier and quicker to harvest.
Allogenic and xenogenic tissues. Allogenic (cadaveric) fascia lata and cadaveric dermis provide reasonable efficacy, but durability remains an issue, as high failure rates have been reported. Bovine and porcine dermis, as well as porcine small-intestine submucosa, are also effective for SUI, although durability remains a concern.
Synthetic materials. Synthetic graft materials of various designs and substances also have been used as sling material. Monofilament, large-pore weave grafts (Type 1 mesh) are recommended for implantation in the vagina. Although good efficacy can be achieved with synthetic mesh, the material also may increase the risk of serious complications, such as infection, vaginal extrusion, and genitourinary erosion, and is not recommended for use beneath the proximal urethra or bladder neck.

The autologous pubovaginal sling supports the proximal urethra and bladder neck to achieve continence by providing a direct compressive force on the urethra and bladder outlet, or by reestablishing a reinforcing platform or hammock against which the urethra is compressed during increased abdominal pressure.
How to harvest rectus fascia and create a sling
1. Choose anesthesia and perioperative antibiotics
Pubovaginal sling procedures are generally carried out under general anesthesia, but spinal or epidural anesthesia also is possible. Full-patient paralysis is not warranted but may facilitate closure of the rectus fascia after fascial harvesting.
Perioperative antibiotics usually are given to ensure appropriate coverage against skin and vaginal flora (for example, a cephalosporin or fluoroquinolone). In fact, perioperative antibiotics have become a mandated quality of care measure in the United States.
2. Position the patient for optimal access
Place the patient in the low lithotomy position with her legs in stirrups. The abdomen and perineum should be sterilely prepared and draped to provide access to the vagina and lower abdomen.
After the bladder is drained with a Foley catheter, place a weighted vaginal speculum and use either lateral labial retraction sutures or a self-retaining retractor system to facilitate vaginal exposure.
3. Make an abdominal incision
Make an 8- to 10-cm Pfannenstiel incision approximately 3 to 5 cm above the pubic bone, carry the dissection down to the level of the rectus fascia using a combination of electrocautery and blunt dissection, and sweep the fat and subcutaneous tissue clear of the rectus tissue (FIGURE 1).

FIGURE 1 Skin incision
Before initiating the operation, delineate the location of the transverse skin incision, which should measure 8 to 10 cm and be situated about 4 cm above the symphysis pubis. A vertical incision is also feasible, although it usually is less aesthetic.
4. Harvest the fascia
The rectus abdominis fascia can be harvested in a transverse or vertical orientation. A fascial segment at least 8 cm in length and 1.5 to 2 cm in width is recommended.
Delineate the fascial segment to be resected using a surgical marking pen or electrocautery, then incise the tissue sharply with a scalpel, scissors, or electrocautery along the drawn lines.
Virgin fascia is preferred, but the presence of fibrotic rectus fascia does not prohibit its use. If you are resecting the fascia close and parallel to the symphysis pubis, leave at least 0.5 to 1.0 cm attached to facilitate closure of the defect created in the fascia. Small Army/Navy retractors permit aggressive retraction of skin edges, making it possible to use a smaller skin incision (FIGURE 2).

FIGURE 2 Resect the fascial strip
After choosing the optimal location for excision, mark the area using electrocautery or a surgical marking pen. Then resect the strip using a scalpel or electrocautery. The strip should measure 8 to 10 cm in length and 1 to 2 cm in width. If the skin incision is small, Army/Navy retractors may enhance exposure.
5. Close the fascial defect
Use heavy-gauge (#1 or #0) delayed, absorbable suture in a running fashion. It may be necessary to mobilize the rectus abdominis fascial edges to ensure appropriate tension-free approximation. It is important that anesthesia be sufficient to ensure muscular relaxation and paralysis during closure.
6. Prepare the fascial sling
Affix a single #1 permanent (for example, polypropylene or polyester) suture to each end of the fascial segment by passing the needle through the undersurface of the sling and then back through the top of the sling. If necessary, defat the sling (FIGURE 3).

FIGURE 3 Attach suspensory sutures
A. Mark the midline of the fascial sling with a pen and gently grasp it using a hemostat. B. Attach a polyester suture to each end of the fascial sling after stripping it of any adipose tissue. Ensure that the initial entry and exit points of the polyester sutures are on the same side of the strip that originally abutted the rectus muscles.
7. Dissect the vagina
Use injectable-grade saline or a local analgesic, such as 1% lidocaine, to hydrodissect the subepithelial tissues of the distal portion of the anterior vaginal wall. Make a midline or inverted “U” incision into the vagina (FIGURE 4).
Create vaginal flaps that have sufficient mobility to ensure tension-free closure over the sling. Carry out dissection laterally and anteriorly until you encounter the endopelvic fascia, then incise the endopelvic fascia and dissect it from the posterior surface of the pubis to enter the retropubic space.
Although blunt dissection sometimes can be performed, sharp dissection with Mayo scissors is often required, especially in cases that involve recurrent stress incontinence (FIGURE 4).

FIGURE 4 Dissect the vagina
A. Use an inverted “U” or vertical incision on the vaginal mucosa overlying the midurethra and bladder. B. Carefully dissect the tissue to the pubic rami bilaterally until the urogenital diaphragm is identified, then sharply penetrate it using Mayo scissors. C. Enlarge the opening by repeating the procedure on the opposite side.
8. Pass retropubic needles
Pass Stamey needles or long clamps through the retropubic space from the open abdominal wound immediately posterior to the pubic bone, approximately 4 cm apart. You can maintain distal control of the needles by direct finger guidance through the vaginal incision. Be careful to advance the tip of the needle adjacent to the posterior surface of the pubic bone to avoid inadvertent bladder injury (FIGURE 5). Proper bladder drainage also helps to minimize injury to the bladder, which may be closely adherent to the pubis, especially if a prior retropubic procedure has been performed, as in Case 1.

FIGURE 5 Place the sling
A. Insert the Stamey needle through the rectus fascia and guide it into the vagina with the index finger placed against the tip of the needle. B. Thread both ends of the polyester suture into the eye of the Stamey needle and then retract the needle carefully until the suture ends are delivered abdominally at the level of the fascia.
9. Rule out bladder injury
Careful cystoscopic examination of the bladder is mandatory after passing the needles to rule out inadvertent injury. Injuries to the bladder typically occur at the 1 o’clock and 11 o’clock positions, so use a 70° lens, and fill the bladder completely to expand any mucosal redundancy. Wiggle the needles or clamps to help localize their position relative to the bladder wall.
10. Deploy the sling
Thread the free ends of the sutures affixed to the sling into the ends of the Stamey needles—or grasp them with clamps—and pull each suture up to the anterior abdominal wall through the retropubic space (FIGURE 5). Keep the sling centered and flat at the area of the bladder neck.
Some surgeons fix the sling in the midline to the underlying periurethral tissue using numerous delayed absorbable sutures. We prefer to leave the sling unattached to the underlying urethra and bladder neck.
11. Tension the sling
Various techniques are applicable. To ensure adequate “looseness,” we tie the sutures across the midline while holding a right-angle clamp between the sling material and the posterior urethral surface. The goal is for the sling to prevent the descent of the proximal urethra during increases in abdominal pressure without creating any outlet obstruction to the normal flow of urine (FIGURE 6).

FIGURE 6 Tension the sling
A. Tie the suspensory sutures abdominally above the fascial closure line. Tie the sutures across the assistant’s index finger to avoid excessive tension. B. Assess the tension using a right-angle clamp placed between the pubovaginal sling and the vagina.
12. Close the incisions
Close the abdominal skin incision using 3-0 and 4-0 absorbable sutures. Use 3-0 absorbable sutures to close the vaginal mucosa. We prefer to close the vagina after completion of the tensioning procedure, but some surgeons complete this step prior to tensioning.
13. Place a catheter, packing material
Place a bladder catheter and vaginal gauze packing. Both the catheter and gauze may be removed after 24 hours. If the patient is unable to void at that time, teach her intermittent self-catheterization, or place an indwelling Foley catheter for 1 week.
Outcomes show good efficacy
Pubovaginal slings are highly effective, with success rates between 50% and 75% after follow-up as long as 10 years.1 In 2011, Blaivas and Chaikin reported 4-year follow-up data, with improvement or cure in 100% of patients with uncomplicated SUI and in as many as 93% of patients in more complicated cases.2 Most failures were due to urge incontinence and occurred within the first 6 postoperative months; 3% of these urge patients were thought to have developed de novo urge incontinence.
Other studies have found de novo urgency and storage symptoms in as many as 23% of patients, with 11% of patients reporting voiding dysfunction and as many as 7.8% requiring long-term self-catheterization.1
Flawed methodology in the few randomized, controlled trials that have compared the pubovaginal sling with the tension-free vaginal tape (TVT) has cast doubt on their findings.3 Basok and colleagues found an increased rate of de novo urgency in the women treated with a pubovaginal sling, compared with those who underwent intravaginal slingplasty,4 whereas Sharifiaghdas and Mortazavi found equal efficacy between pubovaginal and retropubic midurethral synthetic slings.5 The most scientifically valid randomized, controlled trial found equal subjective cure rates and complication rates when a biologic pubovaginal sling was compared with the TVT.6 In that study, the pubovaginal sling was of porcine origin.
In a comparison of autologous and autograft slings, Flynn found equal control of SUI over 2 years, with reduced postoperative discomfort in the allograft group.7
When autologous pubovaginal slings were compared with Burch colposuspension in a randomized, controlled trial, fascial slings were better at controlling incontinence despite an increased morbidity profile.8
A meta-analysis found equal subjective cure rates and overall efficacy between pubovaginal and midurethral synthetic slings.9
Voiding dysfunction is the most common complication
Transient urinary retention may occur in as many as 20% of patients and requires intermittent self-catheterization until resolution (typically 2–4 weeks). Prolonged postoperative voiding dysfunction (lasting more than 4–6 weeks), including de novo urgency, urgency incontinence, and obstructive symptoms, may occur to some degree in as many as 25% of patients. However, fewer than 3% of women require subsequent urethrolysis for treatment of prolonged retention or obstructive voiding symptoms.
Synthetic full-length midurethral slings remain the standard of care
for SUI
Charles W. Nager, MD (November 2012)
Harvest the fascia first. Because substantial bleeding can occur during vaginal dissection, it is advisable to harvest the autologous fascia and prepare the sling by affixing sutures to it before dissecting the vagina. This facilitates timely insertion of the sling and minimal blood loss. Retropubic bleeding from high in the space that occurs during dissection almost always resolves upon placement of the sling. We recommend against prolonged attempts at hemostasis.
In urethral reconstruction, tension the sling after reconstruction. When placing an autologous pubovaginal sling in the setting of urethral reconstruction or as tissue interposition, harvest the fascia and prepare and deploy the sling (with passage of the retropubic sutures) before reconstructing the urethra—but refrain from tensioning until after the reconstruction is completed. Then affix the sling in the appropriate location and tension it. When the sling is placed after reconstruction, it can damage the reconstruction through traction or direct injury.
Don’t worry about surface orientation. During placement of the autologous sling material, surface orientation does not matter. Conventionally, however, the “body-side” or underside of the graft is placed on the body-side of the patient.
Tensioning varies between patients. For most women, sling tensioning can be accomplished by tying the sutures over one or two fingers placed across the fascia. In patients who have undergone multiple procedures and who have a nonmobile urethra, however, tension should be tighter and must be individualized, based on the patient’s anatomy, lower urinary tract function, and willingness to perform intermittent self-catheterization for a prolonged period of time.
CASE 1: Resolved
After you advise the patient of the risks and benefits of the rectus fascia pubovaginal sling, in comparison with a repeat synthetic midurethral sling, she continues to insist on the use of autologous tissue. She undergoes the pubovaginal sling operation with excision of eroded mesh without complication.
A 35-year-old woman reports continuous urinary leakage that is not associated with movement. She was previously told that she had an ectopic ureter implanted into a congenitally short urethra, and she underwent repair of the problem, including reimplantation of the ureter and placement of a cadaveric fascia lata sling. A congenital remnant—observed as a blind pouch via cystoscopy—was left attached to the urethra. Two years have passed since that operation.
Physical findings: A pelvic examination reveals complete loss of the posterior urethra. One possible explanation: The remnant became infected and caused a breakdown of the posterior urethra, with complete disappearance of the cadaveric fascia lata.
Recommended management: Complete urethral reconstruction, with transposition of a martius fat pad and repeat placement of a cadaveric fascia pubovaginal sling.
Technique: See Video 2, Urethral reconstruction.
We want to hear from you! Tell us what you think.
Watch 2 intraoperative videos
These videos were selected by Mickey Karram, MD, and presented courtesy of
International Academy of Pelvic Surgery
Developed in Partnership with International Academy of Pelvic Surgery
CASE 1: Recurrent SUI and mesh erosion
A 50-year-old woman reports urinary incontinence that is associated with activity and exertion—stress urinary incontinence (SUI)—and says it has worsened over the past year. She mentions that she underwent vaginal hysterectomy, with placement of a tension-free vaginal tape (TVT), about 2 years earlier.
During physical examination, the patient becomes incontinent when abdominal pressure is increased, with some urethral mobility (cotton-swab deflection to 25° from the horizontal). She is also noted to have erosion of the TVT tape into the vaginal lumen.
Urodynamic testing reveals easily demonstrable SUI at a volume of 150 mL when she is in the sitting position, with a Valsalva leak-point pressure of 55 cm H2O. Her bladder remains stable to a capacity of 520 mL. Cystoscopy yields unremarkable findings.
When she is offered surgical correction of her SUI, the patient expresses a preference for the use of her own tissues and says she does not want to have synthetic mesh placed.
Is this patient a candidate for a rectus fascia pubovaginal sling?
As more patients express reservations about the placement of synthetic mesh during sling procedures, the use of autologous rectus fascia pubovaginal slings has risen. The concept of using a patient’s own tissue as a sling to support the urethra dates to the early 20th century, but it was not until late in that century that the procedure gained widespread appreciation and evolved into its current form. Initially, the procedure entailed mobilizing a strip of abdominal muscle (either rectus or pyramidalis), freeing one end of the strip from its attachment, passing that end under the bladder neck, and reaffixing it to the abdominal muscle wall, forming a “U”-shaped sling around the bladder outlet. Subsequently, overlying abdominal fascia was included in the sling, eventually replacing the muscle altogether. The final innovation: An isolated strip of fascia was suspended by free sutures that were tied to the abdominal wall or attached on top of the abdominal rectus sheath.
The autologous pubovaginal sling supports the proximal urethra and bladder neck to achieve continence by providing a direct compressive force on the urethra and bladder outlet, or by reestablishing a reinforcing platform or hammock against which the urethra is compressed during the transmission of increased abdominal pressure.
The sling is suspended on each end by free sutures that are attached directly to the abdominal wall musculature or, more commonly, tied to each other on the anterior surface of the abdominal wall.
Long-term success depends on healing and fibrotic processes, which occur primarily where the sling passes through the endopelvic fascia.
Although the pubovaginal sling procedure was pioneered as a surgical option for intrinsic sphincter deficiency (ISD), its indications have broadened to encompass all types of SUI. Its reliable results and durable outcomes make it one of the main standards of treatment, and the pubovaginal sling has been used extensively as primary therapy for:
- SUI related to ISD or urethral hypermobility
- as a salvage procedure for recurrent SUI
- as an adjunct to urethral and bladder reconstruction
- as a way to functionally close the urethra to abandon urethral access to the bladder.
In our opinion, the autologous pubovaginal sling is appropriate for patients with SUI who decline to have synthetic material implanted because of concerns related to long-term placement of synthetic mesh. Other good candidates are women who experience recurrent incontinence after placement of a synthetic sling or who develop a complication, such as vaginal erosion (VIDEO 1, Rectus fascia pubovaginal sling after an unsuccessful TVT), after placement of a synthetic sling. We also prefer to use an autologous sling in patients who have been radiated or who have sustained urethral injuries, as well as in patients who are undergoing simultaneous repair of urethrovaginal fistula or diverticulum—or those who have already undergone such repair.
What is the optimal sling material?
Rectus abdominis fascia versus fascia lata. The two most commonly used autologous tissues are rectus abdominus fascia and fascia lata. Both of these materials have been studied extensively and proven to be effective and reliable. Most surgeons prefer rectus fascia because it is easier and quicker to harvest.
Allogenic and xenogenic tissues. Allogenic (cadaveric) fascia lata and cadaveric dermis provide reasonable efficacy, but durability remains an issue, as high failure rates have been reported. Bovine and porcine dermis, as well as porcine small-intestine submucosa, are also effective for SUI, although durability remains a concern.
Synthetic materials. Synthetic graft materials of various designs and substances also have been used as sling material. Monofilament, large-pore weave grafts (Type 1 mesh) are recommended for implantation in the vagina. Although good efficacy can be achieved with synthetic mesh, the material also may increase the risk of serious complications, such as infection, vaginal extrusion, and genitourinary erosion, and is not recommended for use beneath the proximal urethra or bladder neck.

The autologous pubovaginal sling supports the proximal urethra and bladder neck to achieve continence by providing a direct compressive force on the urethra and bladder outlet, or by reestablishing a reinforcing platform or hammock against which the urethra is compressed during increased abdominal pressure.
How to harvest rectus fascia and create a sling
1. Choose anesthesia and perioperative antibiotics
Pubovaginal sling procedures are generally carried out under general anesthesia, but spinal or epidural anesthesia also is possible. Full-patient paralysis is not warranted but may facilitate closure of the rectus fascia after fascial harvesting.
Perioperative antibiotics usually are given to ensure appropriate coverage against skin and vaginal flora (for example, a cephalosporin or fluoroquinolone). In fact, perioperative antibiotics have become a mandated quality of care measure in the United States.
2. Position the patient for optimal access
Place the patient in the low lithotomy position with her legs in stirrups. The abdomen and perineum should be sterilely prepared and draped to provide access to the vagina and lower abdomen.
After the bladder is drained with a Foley catheter, place a weighted vaginal speculum and use either lateral labial retraction sutures or a self-retaining retractor system to facilitate vaginal exposure.
3. Make an abdominal incision
Make an 8- to 10-cm Pfannenstiel incision approximately 3 to 5 cm above the pubic bone, carry the dissection down to the level of the rectus fascia using a combination of electrocautery and blunt dissection, and sweep the fat and subcutaneous tissue clear of the rectus tissue (FIGURE 1).

FIGURE 1 Skin incision
Before initiating the operation, delineate the location of the transverse skin incision, which should measure 8 to 10 cm and be situated about 4 cm above the symphysis pubis. A vertical incision is also feasible, although it usually is less aesthetic.
4. Harvest the fascia
The rectus abdominis fascia can be harvested in a transverse or vertical orientation. A fascial segment at least 8 cm in length and 1.5 to 2 cm in width is recommended.
Delineate the fascial segment to be resected using a surgical marking pen or electrocautery, then incise the tissue sharply with a scalpel, scissors, or electrocautery along the drawn lines.
Virgin fascia is preferred, but the presence of fibrotic rectus fascia does not prohibit its use. If you are resecting the fascia close and parallel to the symphysis pubis, leave at least 0.5 to 1.0 cm attached to facilitate closure of the defect created in the fascia. Small Army/Navy retractors permit aggressive retraction of skin edges, making it possible to use a smaller skin incision (FIGURE 2).

FIGURE 2 Resect the fascial strip
After choosing the optimal location for excision, mark the area using electrocautery or a surgical marking pen. Then resect the strip using a scalpel or electrocautery. The strip should measure 8 to 10 cm in length and 1 to 2 cm in width. If the skin incision is small, Army/Navy retractors may enhance exposure.
5. Close the fascial defect
Use heavy-gauge (#1 or #0) delayed, absorbable suture in a running fashion. It may be necessary to mobilize the rectus abdominis fascial edges to ensure appropriate tension-free approximation. It is important that anesthesia be sufficient to ensure muscular relaxation and paralysis during closure.
6. Prepare the fascial sling
Affix a single #1 permanent (for example, polypropylene or polyester) suture to each end of the fascial segment by passing the needle through the undersurface of the sling and then back through the top of the sling. If necessary, defat the sling (FIGURE 3).

FIGURE 3 Attach suspensory sutures
A. Mark the midline of the fascial sling with a pen and gently grasp it using a hemostat. B. Attach a polyester suture to each end of the fascial sling after stripping it of any adipose tissue. Ensure that the initial entry and exit points of the polyester sutures are on the same side of the strip that originally abutted the rectus muscles.
7. Dissect the vagina
Use injectable-grade saline or a local analgesic, such as 1% lidocaine, to hydrodissect the subepithelial tissues of the distal portion of the anterior vaginal wall. Make a midline or inverted “U” incision into the vagina (FIGURE 4).
Create vaginal flaps that have sufficient mobility to ensure tension-free closure over the sling. Carry out dissection laterally and anteriorly until you encounter the endopelvic fascia, then incise the endopelvic fascia and dissect it from the posterior surface of the pubis to enter the retropubic space.
Although blunt dissection sometimes can be performed, sharp dissection with Mayo scissors is often required, especially in cases that involve recurrent stress incontinence (FIGURE 4).

FIGURE 4 Dissect the vagina
A. Use an inverted “U” or vertical incision on the vaginal mucosa overlying the midurethra and bladder. B. Carefully dissect the tissue to the pubic rami bilaterally until the urogenital diaphragm is identified, then sharply penetrate it using Mayo scissors. C. Enlarge the opening by repeating the procedure on the opposite side.
8. Pass retropubic needles
Pass Stamey needles or long clamps through the retropubic space from the open abdominal wound immediately posterior to the pubic bone, approximately 4 cm apart. You can maintain distal control of the needles by direct finger guidance through the vaginal incision. Be careful to advance the tip of the needle adjacent to the posterior surface of the pubic bone to avoid inadvertent bladder injury (FIGURE 5). Proper bladder drainage also helps to minimize injury to the bladder, which may be closely adherent to the pubis, especially if a prior retropubic procedure has been performed, as in Case 1.

FIGURE 5 Place the sling
A. Insert the Stamey needle through the rectus fascia and guide it into the vagina with the index finger placed against the tip of the needle. B. Thread both ends of the polyester suture into the eye of the Stamey needle and then retract the needle carefully until the suture ends are delivered abdominally at the level of the fascia.
9. Rule out bladder injury
Careful cystoscopic examination of the bladder is mandatory after passing the needles to rule out inadvertent injury. Injuries to the bladder typically occur at the 1 o’clock and 11 o’clock positions, so use a 70° lens, and fill the bladder completely to expand any mucosal redundancy. Wiggle the needles or clamps to help localize their position relative to the bladder wall.
10. Deploy the sling
Thread the free ends of the sutures affixed to the sling into the ends of the Stamey needles—or grasp them with clamps—and pull each suture up to the anterior abdominal wall through the retropubic space (FIGURE 5). Keep the sling centered and flat at the area of the bladder neck.
Some surgeons fix the sling in the midline to the underlying periurethral tissue using numerous delayed absorbable sutures. We prefer to leave the sling unattached to the underlying urethra and bladder neck.
11. Tension the sling
Various techniques are applicable. To ensure adequate “looseness,” we tie the sutures across the midline while holding a right-angle clamp between the sling material and the posterior urethral surface. The goal is for the sling to prevent the descent of the proximal urethra during increases in abdominal pressure without creating any outlet obstruction to the normal flow of urine (FIGURE 6).

FIGURE 6 Tension the sling
A. Tie the suspensory sutures abdominally above the fascial closure line. Tie the sutures across the assistant’s index finger to avoid excessive tension. B. Assess the tension using a right-angle clamp placed between the pubovaginal sling and the vagina.
12. Close the incisions
Close the abdominal skin incision using 3-0 and 4-0 absorbable sutures. Use 3-0 absorbable sutures to close the vaginal mucosa. We prefer to close the vagina after completion of the tensioning procedure, but some surgeons complete this step prior to tensioning.
13. Place a catheter, packing material
Place a bladder catheter and vaginal gauze packing. Both the catheter and gauze may be removed after 24 hours. If the patient is unable to void at that time, teach her intermittent self-catheterization, or place an indwelling Foley catheter for 1 week.
Outcomes show good efficacy
Pubovaginal slings are highly effective, with success rates between 50% and 75% after follow-up as long as 10 years.1 In 2011, Blaivas and Chaikin reported 4-year follow-up data, with improvement or cure in 100% of patients with uncomplicated SUI and in as many as 93% of patients in more complicated cases.2 Most failures were due to urge incontinence and occurred within the first 6 postoperative months; 3% of these urge patients were thought to have developed de novo urge incontinence.
Other studies have found de novo urgency and storage symptoms in as many as 23% of patients, with 11% of patients reporting voiding dysfunction and as many as 7.8% requiring long-term self-catheterization.1
Flawed methodology in the few randomized, controlled trials that have compared the pubovaginal sling with the tension-free vaginal tape (TVT) has cast doubt on their findings.3 Basok and colleagues found an increased rate of de novo urgency in the women treated with a pubovaginal sling, compared with those who underwent intravaginal slingplasty,4 whereas Sharifiaghdas and Mortazavi found equal efficacy between pubovaginal and retropubic midurethral synthetic slings.5 The most scientifically valid randomized, controlled trial found equal subjective cure rates and complication rates when a biologic pubovaginal sling was compared with the TVT.6 In that study, the pubovaginal sling was of porcine origin.
In a comparison of autologous and autograft slings, Flynn found equal control of SUI over 2 years, with reduced postoperative discomfort in the allograft group.7
When autologous pubovaginal slings were compared with Burch colposuspension in a randomized, controlled trial, fascial slings were better at controlling incontinence despite an increased morbidity profile.8
A meta-analysis found equal subjective cure rates and overall efficacy between pubovaginal and midurethral synthetic slings.9
Voiding dysfunction is the most common complication
Transient urinary retention may occur in as many as 20% of patients and requires intermittent self-catheterization until resolution (typically 2–4 weeks). Prolonged postoperative voiding dysfunction (lasting more than 4–6 weeks), including de novo urgency, urgency incontinence, and obstructive symptoms, may occur to some degree in as many as 25% of patients. However, fewer than 3% of women require subsequent urethrolysis for treatment of prolonged retention or obstructive voiding symptoms.
Synthetic full-length midurethral slings remain the standard of care
for SUI
Charles W. Nager, MD (November 2012)
Harvest the fascia first. Because substantial bleeding can occur during vaginal dissection, it is advisable to harvest the autologous fascia and prepare the sling by affixing sutures to it before dissecting the vagina. This facilitates timely insertion of the sling and minimal blood loss. Retropubic bleeding from high in the space that occurs during dissection almost always resolves upon placement of the sling. We recommend against prolonged attempts at hemostasis.
In urethral reconstruction, tension the sling after reconstruction. When placing an autologous pubovaginal sling in the setting of urethral reconstruction or as tissue interposition, harvest the fascia and prepare and deploy the sling (with passage of the retropubic sutures) before reconstructing the urethra—but refrain from tensioning until after the reconstruction is completed. Then affix the sling in the appropriate location and tension it. When the sling is placed after reconstruction, it can damage the reconstruction through traction or direct injury.
Don’t worry about surface orientation. During placement of the autologous sling material, surface orientation does not matter. Conventionally, however, the “body-side” or underside of the graft is placed on the body-side of the patient.
Tensioning varies between patients. For most women, sling tensioning can be accomplished by tying the sutures over one or two fingers placed across the fascia. In patients who have undergone multiple procedures and who have a nonmobile urethra, however, tension should be tighter and must be individualized, based on the patient’s anatomy, lower urinary tract function, and willingness to perform intermittent self-catheterization for a prolonged period of time.
CASE 1: Resolved
After you advise the patient of the risks and benefits of the rectus fascia pubovaginal sling, in comparison with a repeat synthetic midurethral sling, she continues to insist on the use of autologous tissue. She undergoes the pubovaginal sling operation with excision of eroded mesh without complication.
A 35-year-old woman reports continuous urinary leakage that is not associated with movement. She was previously told that she had an ectopic ureter implanted into a congenitally short urethra, and she underwent repair of the problem, including reimplantation of the ureter and placement of a cadaveric fascia lata sling. A congenital remnant—observed as a blind pouch via cystoscopy—was left attached to the urethra. Two years have passed since that operation.
Physical findings: A pelvic examination reveals complete loss of the posterior urethra. One possible explanation: The remnant became infected and caused a breakdown of the posterior urethra, with complete disappearance of the cadaveric fascia lata.
Recommended management: Complete urethral reconstruction, with transposition of a martius fat pad and repeat placement of a cadaveric fascia pubovaginal sling.
Technique: See Video 2, Urethral reconstruction.
We want to hear from you! Tell us what you think.
Vitamin D deficiency in older adults
Low vitamin D levels can impact cognitive functioning in older adults.1 As vitamin D levels decrease, cognitive impairment increases.
Vitamin D deficiency can occur because few foods contain this nutrient2 and patients have limited exposure to sunlight—vitamin D is produced when sunlight strikes the skin.2 In addition to rickets, low levels of vitamin D have been linked to slower information processing in middle age and older men, cognitive decline, mood disorders, and altered brain development and function resulting in neurodegenerative diseases and other medical disorders.3
One study suggested that one-half of adults age >60 do not get sufficient vitamin D, with an even higher rate among women with Alzheimer’s disease.4 Patients in dementia units typically are not tested for vitamin D levels. These patients rarely leave the unit, which leaves them deprived of the vitamin D provided by sunlight. Even patients exposed to sunlight may receive minimal vitamin D because they use sunscreen.
The following protocol can help patients who may benefit from vitamin D supplementation and increased sun exposure.
Obtain and assess vitamin D levels. Evaluate your patient’s level in the context of physical or cognitive symptoms and other lab values:
- deficient: <12 ng/mL
- inadequate: 12 to 20 ng/mL
- adequate: ≥20 ng/mL.2
Order dietary assessment to identify foods that may increase vitamin D levels. The best sources are fish—salmon, tuna, and mackerel—fish oils, beef, liver, cheese, and egg yolks.2 Several food products, including milk and orange juice, are fortified with vitamin D.
Suggest a daily vitamin D supplement ranging from 400 IU/d to 1,000 IU/d. The Institute of Medicine suggests 600 IU/d for patients age 60 to 70 and 800 IU/d for those age ≥71. For vitamin D deficient patients, recommend >1,000 IU/d.1
Recommend 15 minutes per day in the sun without sunscreen from spring to autumn; late summer to fall is ideal because vitamin D’s half-life is 30 days. Midday is the best time to produce vitamin D.5
Recheck the patient’s Mini-Mental State Examination score every 4 months. Vitamin D supplementation is correlated with cognitive functioning.6
Disclosure
Dr. LaFerney reports no financial, relationship with any company whose, products are mentioned in this article, or with manufacturers of competing, products.
1. Mayo Clinic. Vitamin D. http://www.mayoclinic.com/health/vitamin-d/NS_patient-vitamind/DSECTION=dosing. Updated October 1 2011. Accessed September 26, 2012.
2. National Institutes of Health. Office of Dietary Supplements. Dietary supplement fact sheet: vitamin D. http://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional. Accessed September 26, 2012.
3. Lee DM, Tajar A, Ulubaev A, et al. Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. J Neurol Neurosurg Psychiatry. 2009;80(7):722-729.
4. Wilkins CH, Sheline YI, Roe CM, et al. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14(12):1032-1040.
5. Webb AR, Engelsen O. Calculated ultraviolet exposure levels for a healthy vitamin D status. Photochem Photobiol. 2006;82(6):1697-1703.
6. Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys. 2007;460(2):202-205.
Low vitamin D levels can impact cognitive functioning in older adults.1 As vitamin D levels decrease, cognitive impairment increases.
Vitamin D deficiency can occur because few foods contain this nutrient2 and patients have limited exposure to sunlight—vitamin D is produced when sunlight strikes the skin.2 In addition to rickets, low levels of vitamin D have been linked to slower information processing in middle age and older men, cognitive decline, mood disorders, and altered brain development and function resulting in neurodegenerative diseases and other medical disorders.3
One study suggested that one-half of adults age >60 do not get sufficient vitamin D, with an even higher rate among women with Alzheimer’s disease.4 Patients in dementia units typically are not tested for vitamin D levels. These patients rarely leave the unit, which leaves them deprived of the vitamin D provided by sunlight. Even patients exposed to sunlight may receive minimal vitamin D because they use sunscreen.
The following protocol can help patients who may benefit from vitamin D supplementation and increased sun exposure.
Obtain and assess vitamin D levels. Evaluate your patient’s level in the context of physical or cognitive symptoms and other lab values:
- deficient: <12 ng/mL
- inadequate: 12 to 20 ng/mL
- adequate: ≥20 ng/mL.2
Order dietary assessment to identify foods that may increase vitamin D levels. The best sources are fish—salmon, tuna, and mackerel—fish oils, beef, liver, cheese, and egg yolks.2 Several food products, including milk and orange juice, are fortified with vitamin D.
Suggest a daily vitamin D supplement ranging from 400 IU/d to 1,000 IU/d. The Institute of Medicine suggests 600 IU/d for patients age 60 to 70 and 800 IU/d for those age ≥71. For vitamin D deficient patients, recommend >1,000 IU/d.1
Recommend 15 minutes per day in the sun without sunscreen from spring to autumn; late summer to fall is ideal because vitamin D’s half-life is 30 days. Midday is the best time to produce vitamin D.5
Recheck the patient’s Mini-Mental State Examination score every 4 months. Vitamin D supplementation is correlated with cognitive functioning.6
Disclosure
Dr. LaFerney reports no financial, relationship with any company whose, products are mentioned in this article, or with manufacturers of competing, products.
Low vitamin D levels can impact cognitive functioning in older adults.1 As vitamin D levels decrease, cognitive impairment increases.
Vitamin D deficiency can occur because few foods contain this nutrient2 and patients have limited exposure to sunlight—vitamin D is produced when sunlight strikes the skin.2 In addition to rickets, low levels of vitamin D have been linked to slower information processing in middle age and older men, cognitive decline, mood disorders, and altered brain development and function resulting in neurodegenerative diseases and other medical disorders.3
One study suggested that one-half of adults age >60 do not get sufficient vitamin D, with an even higher rate among women with Alzheimer’s disease.4 Patients in dementia units typically are not tested for vitamin D levels. These patients rarely leave the unit, which leaves them deprived of the vitamin D provided by sunlight. Even patients exposed to sunlight may receive minimal vitamin D because they use sunscreen.
The following protocol can help patients who may benefit from vitamin D supplementation and increased sun exposure.
Obtain and assess vitamin D levels. Evaluate your patient’s level in the context of physical or cognitive symptoms and other lab values:
- deficient: <12 ng/mL
- inadequate: 12 to 20 ng/mL
- adequate: ≥20 ng/mL.2
Order dietary assessment to identify foods that may increase vitamin D levels. The best sources are fish—salmon, tuna, and mackerel—fish oils, beef, liver, cheese, and egg yolks.2 Several food products, including milk and orange juice, are fortified with vitamin D.
Suggest a daily vitamin D supplement ranging from 400 IU/d to 1,000 IU/d. The Institute of Medicine suggests 600 IU/d for patients age 60 to 70 and 800 IU/d for those age ≥71. For vitamin D deficient patients, recommend >1,000 IU/d.1
Recommend 15 minutes per day in the sun without sunscreen from spring to autumn; late summer to fall is ideal because vitamin D’s half-life is 30 days. Midday is the best time to produce vitamin D.5
Recheck the patient’s Mini-Mental State Examination score every 4 months. Vitamin D supplementation is correlated with cognitive functioning.6
Disclosure
Dr. LaFerney reports no financial, relationship with any company whose, products are mentioned in this article, or with manufacturers of competing, products.
1. Mayo Clinic. Vitamin D. http://www.mayoclinic.com/health/vitamin-d/NS_patient-vitamind/DSECTION=dosing. Updated October 1 2011. Accessed September 26, 2012.
2. National Institutes of Health. Office of Dietary Supplements. Dietary supplement fact sheet: vitamin D. http://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional. Accessed September 26, 2012.
3. Lee DM, Tajar A, Ulubaev A, et al. Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. J Neurol Neurosurg Psychiatry. 2009;80(7):722-729.
4. Wilkins CH, Sheline YI, Roe CM, et al. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14(12):1032-1040.
5. Webb AR, Engelsen O. Calculated ultraviolet exposure levels for a healthy vitamin D status. Photochem Photobiol. 2006;82(6):1697-1703.
6. Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys. 2007;460(2):202-205.
1. Mayo Clinic. Vitamin D. http://www.mayoclinic.com/health/vitamin-d/NS_patient-vitamind/DSECTION=dosing. Updated October 1 2011. Accessed September 26, 2012.
2. National Institutes of Health. Office of Dietary Supplements. Dietary supplement fact sheet: vitamin D. http://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional. Accessed September 26, 2012.
3. Lee DM, Tajar A, Ulubaev A, et al. Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. J Neurol Neurosurg Psychiatry. 2009;80(7):722-729.
4. Wilkins CH, Sheline YI, Roe CM, et al. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14(12):1032-1040.
5. Webb AR, Engelsen O. Calculated ultraviolet exposure levels for a healthy vitamin D status. Photochem Photobiol. 2006;82(6):1697-1703.
6. Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys. 2007;460(2):202-205.
An open-label trial of escitalopram for PPD: Considerations for research
Challenges in recruiting women to postpartum depression (PPD) antidepressant treatment trials, which we encountered when conducting a trial of escitalopram, contribute to the limited body of knowledge about PPD treatment. Here we discuss results from a preliminary trial of escitalopram for PPD, and challenges of research in this area.
Escitalopram, the S-enantiomer of citalopram, is a selective serotonin reuptake inhibitor with high selectivity and potency that is FDA-approved for treating major depressive disorder (MDD) and generalized anxiety disorder. An agent with antidepressant and anxiolytic effects is particularly desirable for PPD because anxiety is more common in postpartum major depressive episodes than non-postpartum MDD.1 Anxiety and depressive disorders commonly are comorbid in postpartum women.2
We conducted an open-label trial of escitalopram for women with PPD and anxiety. We initially attempted to recruit 20 women.
Methods
Patients received 8 weeks of treatment with escitalopram, 10 to 20 mg/d (flexible dose). After completing the initial phone screen, patients had 5 follow-up visits, once every 2 weeks for 8 weeks. The institutional review board at Massachusetts General Hospital approved this study and we obtained written informed consent from all patients at the first visit. Twelve patients completed the phone screen and 7 eligible patients were enrolled in the study over 32 months. Reasons for ineligibility included having a history of psychosis, onset of symptoms >3 months postpartum, or presenting >6 months after onset. Others declined to participate because of concern about the time commitment or because they pursued nonpharmacologic treatments after the evaluation visit. One patient was lost to follow-up. Three patients completed the study. The study was halted because of the slow pace of recruitment.
Patient selection. Patients were screened for a major depressive episode with postpartum onset within 3 months of childbirth; depressive symptoms may have developed during pregnancy and worsened postpartum to meet criteria for MDD. Women were eligible for the study if they:
- were age 18 to 45
- experienced a major depressive episode with symptoms developing within 3 months of childbirth
- presented within 6 months of childbirth
- had a Montgomery-Åsberg Depression Rating Scale (MADRS) score >15
- had a Beck Anxiety Inventory (BAI) score >10.
Patients who were pregnant or breast-feeding were excluded from the study per an agreement with the sponsor. In addition, women were excluded if they had taken any psychotropic medication within 2 weeks of enrollment; had active suicidal ideation, homicidal ideation, or presence of psychotic symptoms; had chronic depression or dysthymia; had chronic or treatment-resistant anxiety disorders; had a history of mania or hypomania; or had active alcohol or substance abuse within the past year.
Treatment. Patients received escitalopram, 10 mg/d, after the baseline visit. At the investigator’s discretion, the dose could be increased to 20 mg/d or lowered to 5 mg/d if side effects occurred.
Measures. At the first visit, patients were assessed with the Mini-International Neuropsychiatric Interview to verify MDD and exclude diagnoses that would determine ineligibility. MADRS and Edinburgh Postnatal Depression Scale (EPDS) were used at each visit to measure depressive symptoms.3,4 The BAI was completed at each visit to measure anxiety symptoms. Obsessions and compulsions were measured with the Yale-Brown Obsessive Compulsive Scale (Y-BOCS)5 at baseline, and at all following visits if the patient scored >8 at baseline. The Clinical Global Impression Scales for severity and improvement were completed at each visit.6
Results
Of 7 patients enrolled, 3 completed the study, 2 were ineligible after the baseline visit, and 2 did not participate after the baseline visit (1 selected to pursue psychotherapy, and 1 was lost to follow-up).
Two of 3 patients responded to escitalopram (≥50% decrease on MADRS), and both were remitters (MADRS score <7). All 3 patients were responders on EPDS and BAI. One patient had Y-BOCS >8 at baseline (Total Y-BOCS score of 9, and final Y-BOCS score of 8) (Table).
Table
Symptom rating scale scores at baseline and study end
| Baseline (Visit 1) | Final (Visit 5) | |||||
|---|---|---|---|---|---|---|
| Patient | MADRS | BAI | EPDS | MADRS | BAI | EPDS |
| Ms. A | 21 | 18 | 22 | 12 | 0 | 0 |
| Ms. B | 28 | 28 | 19 | 4 | 5 | 2 |
| Ms. C | 37 | 6 | 19 | 6 | 2 | 0 |
| BAI: Beck Anxiety Inventory; EPDS: Edinburgh Postnatal Depression Scale; MADRS: Montgomery-Åsberg Depression Rating Scale | ||||||
Discussion
Patients who stayed in treatment improved during the course of this study. Recruitment was difficult; we were able to recruit only 7 patients out of a projected 20 for the screening visit. We solicited feedback from local obstetrics health care providers and social workers on recruitment and attractiveness of the study as part of our routine collaboration with obstetrical services that screen for PPD. Primary reasons patients were not referred were that they were breast-feeding or they stated they would prefer to receive treatment from their primary care doctor. Recruitment difficulty in this study was in stark contrast to other recent studies completed at our center. For example, we have successfully recruited for menopausal depression and premenstrual dysphoric disorder treatment studies, and have completed large naturalistic studies of women with unipolar depression and bipolar disorder across pregnancy and postpartum. We suspect that many patients who were eligible for the study preferred to seek care from an obstetrician or primary care doctor with whom they already had a therapeutic alliance, and we also suspect that many women with PPD do not seek treatment at all, which is consistent with findings from other research groups.
Lessons learned from PPD research include:
- Including women who are breast-feeding is important because many women choose to breast-feed and suffer from PPD. Because antidepressant use during breast-feeding has been closely studied, it is appropriate to include potential research participants who are breast-feeding as long as they receive adequate information and are able to provide informed consent.
- Participants in PPD studies may require accommodations that take into account their role as a new mother, such as on-site childcare, home visits, or other strategies.
- Because of recruitment challenges in postpartum patients, multisite trials may be required to include adequate numbers of participants.
Related Resource
- Freeman MP, Joffe H, Cohen LS. Postpartum depression: Help patients find the right treatment. Current Psychiatry. 2012;11(11):14-21.
Drug Brand Names
- Citalopram • Celexa
- Escitalopram • Lexapro
Disclosures
Dr. Freeman has received grant or research support from Eli Lilly and Company, Forest Laboratories, and GlaxoSmithKline, is on the advisory boards of Otsuka and Takeda/Lundbeck, and is a consultant for PamLab LLC.
Dr. Joffe has received grant or research support from Cephalon/Teva, and is a consultant to Noven and Sunovion.
Dr. Cohen has received research support from AstraZeneca, Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb, Forest Laboratories, GlaxoSmithKline, National Institute of Mental Health, National Institute on Aging, National Institutes of Health, Ortho-McNeil Janssen, and Pfizer and has served on an advisory board for PamLab LLC.
This study was funded as an investigator-initiated trial by Forest Pharmaceuticals.
1. Bernstein IH, Rush AJ, Yonkers K, et al. Symptom features of postpartum depression: are they distinct? Depress Anxiety. 2008;25(1):20-26.
2. Wenzel A, Haugen EN, Jackson LC, et al. Anxiety symptoms and disorders at eight weeks postpartum. J Anxiety Disord. 2005;19(3):295-311.
3. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
4. Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389.
5. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006-1011.
6. Guy W. ECDEU assessment manual for psychopharmacology. Rockville MD: US Department of Health and Human Services; 1976. Department of Health, Education, and Welfare Publication (ADM) 76–338.
Challenges in recruiting women to postpartum depression (PPD) antidepressant treatment trials, which we encountered when conducting a trial of escitalopram, contribute to the limited body of knowledge about PPD treatment. Here we discuss results from a preliminary trial of escitalopram for PPD, and challenges of research in this area.
Escitalopram, the S-enantiomer of citalopram, is a selective serotonin reuptake inhibitor with high selectivity and potency that is FDA-approved for treating major depressive disorder (MDD) and generalized anxiety disorder. An agent with antidepressant and anxiolytic effects is particularly desirable for PPD because anxiety is more common in postpartum major depressive episodes than non-postpartum MDD.1 Anxiety and depressive disorders commonly are comorbid in postpartum women.2
We conducted an open-label trial of escitalopram for women with PPD and anxiety. We initially attempted to recruit 20 women.
Methods
Patients received 8 weeks of treatment with escitalopram, 10 to 20 mg/d (flexible dose). After completing the initial phone screen, patients had 5 follow-up visits, once every 2 weeks for 8 weeks. The institutional review board at Massachusetts General Hospital approved this study and we obtained written informed consent from all patients at the first visit. Twelve patients completed the phone screen and 7 eligible patients were enrolled in the study over 32 months. Reasons for ineligibility included having a history of psychosis, onset of symptoms >3 months postpartum, or presenting >6 months after onset. Others declined to participate because of concern about the time commitment or because they pursued nonpharmacologic treatments after the evaluation visit. One patient was lost to follow-up. Three patients completed the study. The study was halted because of the slow pace of recruitment.
Patient selection. Patients were screened for a major depressive episode with postpartum onset within 3 months of childbirth; depressive symptoms may have developed during pregnancy and worsened postpartum to meet criteria for MDD. Women were eligible for the study if they:
- were age 18 to 45
- experienced a major depressive episode with symptoms developing within 3 months of childbirth
- presented within 6 months of childbirth
- had a Montgomery-Åsberg Depression Rating Scale (MADRS) score >15
- had a Beck Anxiety Inventory (BAI) score >10.
Patients who were pregnant or breast-feeding were excluded from the study per an agreement with the sponsor. In addition, women were excluded if they had taken any psychotropic medication within 2 weeks of enrollment; had active suicidal ideation, homicidal ideation, or presence of psychotic symptoms; had chronic depression or dysthymia; had chronic or treatment-resistant anxiety disorders; had a history of mania or hypomania; or had active alcohol or substance abuse within the past year.
Treatment. Patients received escitalopram, 10 mg/d, after the baseline visit. At the investigator’s discretion, the dose could be increased to 20 mg/d or lowered to 5 mg/d if side effects occurred.
Measures. At the first visit, patients were assessed with the Mini-International Neuropsychiatric Interview to verify MDD and exclude diagnoses that would determine ineligibility. MADRS and Edinburgh Postnatal Depression Scale (EPDS) were used at each visit to measure depressive symptoms.3,4 The BAI was completed at each visit to measure anxiety symptoms. Obsessions and compulsions were measured with the Yale-Brown Obsessive Compulsive Scale (Y-BOCS)5 at baseline, and at all following visits if the patient scored >8 at baseline. The Clinical Global Impression Scales for severity and improvement were completed at each visit.6
Results
Of 7 patients enrolled, 3 completed the study, 2 were ineligible after the baseline visit, and 2 did not participate after the baseline visit (1 selected to pursue psychotherapy, and 1 was lost to follow-up).
Two of 3 patients responded to escitalopram (≥50% decrease on MADRS), and both were remitters (MADRS score <7). All 3 patients were responders on EPDS and BAI. One patient had Y-BOCS >8 at baseline (Total Y-BOCS score of 9, and final Y-BOCS score of 8) (Table).
Table
Symptom rating scale scores at baseline and study end
| Baseline (Visit 1) | Final (Visit 5) | |||||
|---|---|---|---|---|---|---|
| Patient | MADRS | BAI | EPDS | MADRS | BAI | EPDS |
| Ms. A | 21 | 18 | 22 | 12 | 0 | 0 |
| Ms. B | 28 | 28 | 19 | 4 | 5 | 2 |
| Ms. C | 37 | 6 | 19 | 6 | 2 | 0 |
| BAI: Beck Anxiety Inventory; EPDS: Edinburgh Postnatal Depression Scale; MADRS: Montgomery-Åsberg Depression Rating Scale | ||||||
Discussion
Patients who stayed in treatment improved during the course of this study. Recruitment was difficult; we were able to recruit only 7 patients out of a projected 20 for the screening visit. We solicited feedback from local obstetrics health care providers and social workers on recruitment and attractiveness of the study as part of our routine collaboration with obstetrical services that screen for PPD. Primary reasons patients were not referred were that they were breast-feeding or they stated they would prefer to receive treatment from their primary care doctor. Recruitment difficulty in this study was in stark contrast to other recent studies completed at our center. For example, we have successfully recruited for menopausal depression and premenstrual dysphoric disorder treatment studies, and have completed large naturalistic studies of women with unipolar depression and bipolar disorder across pregnancy and postpartum. We suspect that many patients who were eligible for the study preferred to seek care from an obstetrician or primary care doctor with whom they already had a therapeutic alliance, and we also suspect that many women with PPD do not seek treatment at all, which is consistent with findings from other research groups.
Lessons learned from PPD research include:
- Including women who are breast-feeding is important because many women choose to breast-feed and suffer from PPD. Because antidepressant use during breast-feeding has been closely studied, it is appropriate to include potential research participants who are breast-feeding as long as they receive adequate information and are able to provide informed consent.
- Participants in PPD studies may require accommodations that take into account their role as a new mother, such as on-site childcare, home visits, or other strategies.
- Because of recruitment challenges in postpartum patients, multisite trials may be required to include adequate numbers of participants.
Related Resource
- Freeman MP, Joffe H, Cohen LS. Postpartum depression: Help patients find the right treatment. Current Psychiatry. 2012;11(11):14-21.
Drug Brand Names
- Citalopram • Celexa
- Escitalopram • Lexapro
Disclosures
Dr. Freeman has received grant or research support from Eli Lilly and Company, Forest Laboratories, and GlaxoSmithKline, is on the advisory boards of Otsuka and Takeda/Lundbeck, and is a consultant for PamLab LLC.
Dr. Joffe has received grant or research support from Cephalon/Teva, and is a consultant to Noven and Sunovion.
Dr. Cohen has received research support from AstraZeneca, Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb, Forest Laboratories, GlaxoSmithKline, National Institute of Mental Health, National Institute on Aging, National Institutes of Health, Ortho-McNeil Janssen, and Pfizer and has served on an advisory board for PamLab LLC.
This study was funded as an investigator-initiated trial by Forest Pharmaceuticals.
Challenges in recruiting women to postpartum depression (PPD) antidepressant treatment trials, which we encountered when conducting a trial of escitalopram, contribute to the limited body of knowledge about PPD treatment. Here we discuss results from a preliminary trial of escitalopram for PPD, and challenges of research in this area.
Escitalopram, the S-enantiomer of citalopram, is a selective serotonin reuptake inhibitor with high selectivity and potency that is FDA-approved for treating major depressive disorder (MDD) and generalized anxiety disorder. An agent with antidepressant and anxiolytic effects is particularly desirable for PPD because anxiety is more common in postpartum major depressive episodes than non-postpartum MDD.1 Anxiety and depressive disorders commonly are comorbid in postpartum women.2
We conducted an open-label trial of escitalopram for women with PPD and anxiety. We initially attempted to recruit 20 women.
Methods
Patients received 8 weeks of treatment with escitalopram, 10 to 20 mg/d (flexible dose). After completing the initial phone screen, patients had 5 follow-up visits, once every 2 weeks for 8 weeks. The institutional review board at Massachusetts General Hospital approved this study and we obtained written informed consent from all patients at the first visit. Twelve patients completed the phone screen and 7 eligible patients were enrolled in the study over 32 months. Reasons for ineligibility included having a history of psychosis, onset of symptoms >3 months postpartum, or presenting >6 months after onset. Others declined to participate because of concern about the time commitment or because they pursued nonpharmacologic treatments after the evaluation visit. One patient was lost to follow-up. Three patients completed the study. The study was halted because of the slow pace of recruitment.
Patient selection. Patients were screened for a major depressive episode with postpartum onset within 3 months of childbirth; depressive symptoms may have developed during pregnancy and worsened postpartum to meet criteria for MDD. Women were eligible for the study if they:
- were age 18 to 45
- experienced a major depressive episode with symptoms developing within 3 months of childbirth
- presented within 6 months of childbirth
- had a Montgomery-Åsberg Depression Rating Scale (MADRS) score >15
- had a Beck Anxiety Inventory (BAI) score >10.
Patients who were pregnant or breast-feeding were excluded from the study per an agreement with the sponsor. In addition, women were excluded if they had taken any psychotropic medication within 2 weeks of enrollment; had active suicidal ideation, homicidal ideation, or presence of psychotic symptoms; had chronic depression or dysthymia; had chronic or treatment-resistant anxiety disorders; had a history of mania or hypomania; or had active alcohol or substance abuse within the past year.
Treatment. Patients received escitalopram, 10 mg/d, after the baseline visit. At the investigator’s discretion, the dose could be increased to 20 mg/d or lowered to 5 mg/d if side effects occurred.
Measures. At the first visit, patients were assessed with the Mini-International Neuropsychiatric Interview to verify MDD and exclude diagnoses that would determine ineligibility. MADRS and Edinburgh Postnatal Depression Scale (EPDS) were used at each visit to measure depressive symptoms.3,4 The BAI was completed at each visit to measure anxiety symptoms. Obsessions and compulsions were measured with the Yale-Brown Obsessive Compulsive Scale (Y-BOCS)5 at baseline, and at all following visits if the patient scored >8 at baseline. The Clinical Global Impression Scales for severity and improvement were completed at each visit.6
Results
Of 7 patients enrolled, 3 completed the study, 2 were ineligible after the baseline visit, and 2 did not participate after the baseline visit (1 selected to pursue psychotherapy, and 1 was lost to follow-up).
Two of 3 patients responded to escitalopram (≥50% decrease on MADRS), and both were remitters (MADRS score <7). All 3 patients were responders on EPDS and BAI. One patient had Y-BOCS >8 at baseline (Total Y-BOCS score of 9, and final Y-BOCS score of 8) (Table).
Table
Symptom rating scale scores at baseline and study end
| Baseline (Visit 1) | Final (Visit 5) | |||||
|---|---|---|---|---|---|---|
| Patient | MADRS | BAI | EPDS | MADRS | BAI | EPDS |
| Ms. A | 21 | 18 | 22 | 12 | 0 | 0 |
| Ms. B | 28 | 28 | 19 | 4 | 5 | 2 |
| Ms. C | 37 | 6 | 19 | 6 | 2 | 0 |
| BAI: Beck Anxiety Inventory; EPDS: Edinburgh Postnatal Depression Scale; MADRS: Montgomery-Åsberg Depression Rating Scale | ||||||
Discussion
Patients who stayed in treatment improved during the course of this study. Recruitment was difficult; we were able to recruit only 7 patients out of a projected 20 for the screening visit. We solicited feedback from local obstetrics health care providers and social workers on recruitment and attractiveness of the study as part of our routine collaboration with obstetrical services that screen for PPD. Primary reasons patients were not referred were that they were breast-feeding or they stated they would prefer to receive treatment from their primary care doctor. Recruitment difficulty in this study was in stark contrast to other recent studies completed at our center. For example, we have successfully recruited for menopausal depression and premenstrual dysphoric disorder treatment studies, and have completed large naturalistic studies of women with unipolar depression and bipolar disorder across pregnancy and postpartum. We suspect that many patients who were eligible for the study preferred to seek care from an obstetrician or primary care doctor with whom they already had a therapeutic alliance, and we also suspect that many women with PPD do not seek treatment at all, which is consistent with findings from other research groups.
Lessons learned from PPD research include:
- Including women who are breast-feeding is important because many women choose to breast-feed and suffer from PPD. Because antidepressant use during breast-feeding has been closely studied, it is appropriate to include potential research participants who are breast-feeding as long as they receive adequate information and are able to provide informed consent.
- Participants in PPD studies may require accommodations that take into account their role as a new mother, such as on-site childcare, home visits, or other strategies.
- Because of recruitment challenges in postpartum patients, multisite trials may be required to include adequate numbers of participants.
Related Resource
- Freeman MP, Joffe H, Cohen LS. Postpartum depression: Help patients find the right treatment. Current Psychiatry. 2012;11(11):14-21.
Drug Brand Names
- Citalopram • Celexa
- Escitalopram • Lexapro
Disclosures
Dr. Freeman has received grant or research support from Eli Lilly and Company, Forest Laboratories, and GlaxoSmithKline, is on the advisory boards of Otsuka and Takeda/Lundbeck, and is a consultant for PamLab LLC.
Dr. Joffe has received grant or research support from Cephalon/Teva, and is a consultant to Noven and Sunovion.
Dr. Cohen has received research support from AstraZeneca, Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb, Forest Laboratories, GlaxoSmithKline, National Institute of Mental Health, National Institute on Aging, National Institutes of Health, Ortho-McNeil Janssen, and Pfizer and has served on an advisory board for PamLab LLC.
This study was funded as an investigator-initiated trial by Forest Pharmaceuticals.
1. Bernstein IH, Rush AJ, Yonkers K, et al. Symptom features of postpartum depression: are they distinct? Depress Anxiety. 2008;25(1):20-26.
2. Wenzel A, Haugen EN, Jackson LC, et al. Anxiety symptoms and disorders at eight weeks postpartum. J Anxiety Disord. 2005;19(3):295-311.
3. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
4. Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389.
5. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006-1011.
6. Guy W. ECDEU assessment manual for psychopharmacology. Rockville MD: US Department of Health and Human Services; 1976. Department of Health, Education, and Welfare Publication (ADM) 76–338.
1. Bernstein IH, Rush AJ, Yonkers K, et al. Symptom features of postpartum depression: are they distinct? Depress Anxiety. 2008;25(1):20-26.
2. Wenzel A, Haugen EN, Jackson LC, et al. Anxiety symptoms and disorders at eight weeks postpartum. J Anxiety Disord. 2005;19(3):295-311.
3. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
4. Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389.
5. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006-1011.
6. Guy W. ECDEU assessment manual for psychopharmacology. Rockville MD: US Department of Health and Human Services; 1976. Department of Health, Education, and Welfare Publication (ADM) 76–338.
Panic disorder: Break the fear circuit
Ms. K, a 24-year-old waitress who lives with her boyfriend, was referred by her primary care physician for evaluation of panic attacks that began “out of nowhere” at work approximately 6 months ago. The unpredictable attacks occur multiple times per week, causing her to leave work and cancel shifts.
Ms. K reports that before the panic attacks began, she felt happy in her relationship, enjoyed hobbies, and was hopeful about the future. However, she has become concerned that a potentially catastrophic illness is causing her panic attacks. She researches her symptoms on the Internet, and is preoccupied with the possibility of sudden death due to an undiagnosed heart condition. Multiple visits to the emergency room have not identified any physical abnormalities. Her primary care doctor prescribed alprazolam, 0.5 mg as needed for panic attacks, which she reports is helpful, “but only in the moment of the attacks.” Ms. K avoids alcohol and illicit substances and limits her caffeine intake. She is not willing to accept that her life “feels so limited.” Her dream of earning a nursing degree and eventually starting a family now seems unattainable.
Panic disorder (PD) occurs in 3% to 5% of adults, with women affected at roughly twice the rate of men.1 Causing a broad range of distress and varying degrees of impairment, PD commonly occurs with other psychiatric disorders. For most patients, treatment is effective, but those who do not respond to initial approaches require a thoughtful, stepped approach to care. Key considerations include establishing an accurate diagnosis, clarifying comorbid illnesses, ascertaining patient beliefs and expectations, and providing appropriately dosed and maintained treatments.
Panic attacks vs PD
Panic attacks consist of rapid onset of intense anxiety, with prominent somatic symptoms, that peaks within 10 minutes (Figure).2 Attacks in which <4 of the listed symptoms occur are considered limited-symptom panic attacks.
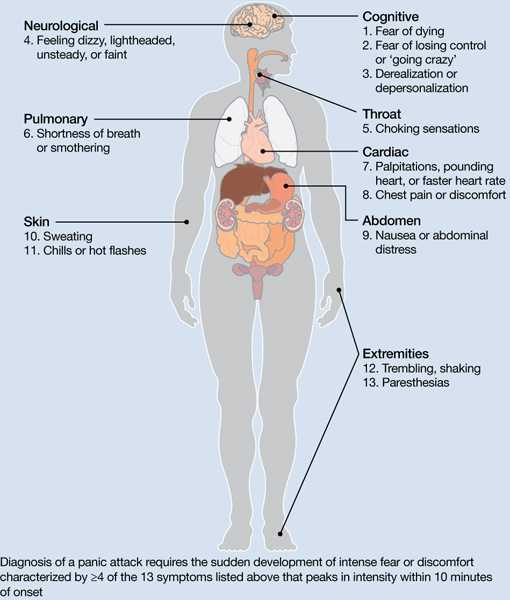
Figure: Body locations of panic attack symptoms
Diagnosis of a panic attack requires the sudden development of intense fear or discomfort characterized by ≥4 of the 13 symptoms listed above that peaks in intensity within 10 minutes of onset
Source: Reference 2
Panic attacks can occur with various disorders, including other anxiety disorders, mood disorders, and substance intoxication or withdrawal. Because serious medical conditions can present with panic-like symptoms, the initial occurrence of such symptoms warrants consideration of physiological causes. For a Box2 that describes the differential diagnosis of panic attacks, see this article at CurrentPsychiatry.com.
To meet diagnostic criteria for panic disorder, panic attacks must initially occur “out of the blue,” meaning no specific object or situation induced the attack. The differential diagnosis of panic attacks includes assessing for other psychiatric disorders that may involve panic attacks. Evaluation requires considering the context in which the panic attacks occur, including their start date, pattern of attacks, instigating situations, and associated thoughts.
Social phobia. Attacks occur only during or immediately before a social interaction in which the patient fears embarrassing himself or herself.
Obsessive-compulsive disorder (OCD). Attacks occur when the patient cannot avoid exposure to an obsessional fear or is prevented from performing a ritual that diffuses obsessional anxiety.
Posttraumatic stress disorder (PTSD). Attacks occur when confronted by a trauma-related memory or trigger.
Specific phobia. Attacks occur only when the patient encounters a specifically feared object, place, or situation, unrelated to social phobia, OCD, or PTSD.
Medical conditions. Conditions to consider include—but are not limited to—hyperthyroidism, pulmonary embolism, myocardial infarction, cardiac dysrhythmias, hypoglycemia, asthma, partial complex seizures, and pheochromocytoma.
Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000
A PD diagnosis requires that repeated panic attacks initially must occur from “out of the blue,” meaning no specific object or situation induced the attack. In addition, the diagnosis requires 1 of 3 types of psychological or behavioral changes as a result of the attacks (Table 1).2 Agoraphobia is diagnosed if 1 of the behavioral changes is avoidance of places or situations from which escape might be embarrassing or difficult should an attack occur. A patient can be diagnosed as having PD with agoraphobia, PD without agoraphobia, or agoraphobia without PD (ie, experiences only limited symptom panic attacks, but avoids situations or stimuli associated with them).
Table 1
Definitions of panic disorder and agoraphobia
| Panic disorder |
|---|
|
| Agoraphobia |
| Anxiety about, or avoidance of, being in places or situations from which escape might be difficult or embarrassing, or in which help may not be available in the event of having an unexpected or situationally predisposed panic attack or panic-like symptoms. Agoraphobic fears typically involve characteristic clusters of situations that include being outside the home alone, being in a crowd, standing in a line, being on a bridge, or traveling in a bus, train, or automobile |
| Source: Reference 2 |
Comorbidities are common in patients with PD and predict greater difficulty achieving remission (Box).1,3-6
The most common psychiatric conditions that co-occur with panic disorder (PD) are other anxiety disorders, mood disorders, personality disorders, and substance use disorders.1 Carefully assess the severity and degree of impairment or distress arising from each condition to prioritize treatment goals. For example, treating panic attacks would be a lower priority in a patient with untreated bipolar disorder.
Assessing comorbid substance abuse is important in selecting PD treatments. Benzodiazepines should almost always be avoided in patients with a history of drug abuse—illicit or prescribed. Although complete abstinence should not be a prerequisite for beginning PD treatment, detoxification and concomitant substance abuse treatment are essential.3
Comorbid mood disorders also affect the course of PD treatment. Antidepressants are effective for treating depression and PD, whereas benzodiazepines are not effective for depression.4 Antidepressants in patients with bipolar disorder are controversial because these medications might induce mixed or elevated mood states or rapid cycling. In these complicated patients, consider antidepressants lower in the treatment algorithm.5
Other conditions to consider before beginning treatment include pregnancy or the possibility of becoming pregnant in the near future and suicidal ideation. PD is associated with increased risk for suicidal ideation and progression to suicide attempts, particularly in patients with a comorbid mood or psychotic disorder.6 In addition, consider the potential impact of medications on comorbid medical conditions.
Treatment begins with education
The goal of treatment is remission of symptoms, ideally including an absence of panic attacks, agoraphobic avoidance, and anticipatory anxiety.1 The Panic Disorder Severity Scale self-report is a validated measure of panic symptoms that may be useful in clinical practice.7
The first step in treatment is educating patients about panic attacks, framing them as an overreactive fear circuit in the brain that produces physical symptoms that are not dangerous. Using a brain model that shows the location of the amygdala, hippocampus, and prefrontal cortex—which play crucial roles in generating and controlling anxiety and fear—can make this discussion more concrete.8 Although highly simplified, such models allow clinicians to demonstrate that excessive reactivity of limbic regions can be reduced by both top-down (cortico-limbic connections via cognitive-behavioral therapy [CBT]) and bottom-up (pharmacotherapy directly acting on limbic structures) approaches. Such discussions lead to treatment recommendations for CBT, pharmacotherapy, or their combination.
No single treatment has emerged as the definitive “best” for PD, and no reliable predictors can guide specific treatment for an individual.3 Combining CBT with pharmacotherapy produces higher short-term response rates than either treatment alone, but in the long term, combination treatment does not appear to be superior to CBT alone.9 Base the initial treatment selection for PD on patient preference, treatment availability and cost, and comorbid medical and psychiatric conditions. For an Algorithm to guide treatment decisions, see this article at CurrentPsychiatry.com.

Algorithm: Treatment for panic disorder: A suggested algorithm
aPoor response to an SSRI should lead to a switch to venlafaxine extended-release, and vice versa
bBenzodiazepines are relatively contraindicated in geriatric patients and patients with a history of substance abuse or dependence
CBT: cognitive-behavioral therapy; MAOI: monoamine oxidase inhibitor; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant; Ven XR: venlafaxine extended-release
First-line treatments
Psychotherapy. CBT is the most efficacious psychotherapy for PD. Twelve to 15 sessions of CBT has demonstrated efficacy for PD, with additional effects on comorbid anxiety and depressive symptoms.10 No large clinical trials of CBT have used cognitive restructuring alone; all have included at least some component of exposure that requires the patient to confront feared physical sensations. Gains during treatment may be steady and gradual or sudden and uneven, with rapid improvement in some but not all symptoms. CBT and pharmacotherapy have demonstrated similar levels of benefit in short-term trials, but CBT has proven superior in most9 but not all11 trials evaluating long-term outcomes, particularly compared with pharmacotherapy that is discontinued during follow-up. Although less studied, group CBT also may be considered if a patient cannot afford individual CBT.
Pharmacotherapy. Evidence supports selective serotonin reuptake inhibitors (SSRIs), venlafaxine extended-release (XR), benzodiazepines, and tricyclic antidepressants (TCAs) as effective treatments for PD.3 No class of medication has demonstrated superiority over others in short-term treatment.3,12 Because of the medical risks associated with benzodiazepines and TCAs, an SSRI or venlafaxine XR should be the first medication option for most patients. Fluoxetine, paroxetine, sertraline, and venlafaxine XR are FDA-approved for PD. Paroxetine is associated with weight gain and may increase the risk for panic recurrence upon discontinuation more than sertraline, making it a less favorable option for many patients.13 Start doses at half the normal starting dose used for treating major depressive disorder and continue for 4 to 7 days, then increase to the minimal effective dose. For a Table3 that lists dosing recommendations for antidepressants to treat PD, see this article at CurrentPsychiatry.com. If there is no improvement by 4 weeks, increase the dose every 2 to 4 weeks until remission is achieved or side effects prevent further dose increases.
Table
Recommended doses for antidepressants used to treat panic disorder
| Medication | Starting dose (mg/d) | Therapeutic range (mg/d) |
|---|---|---|
| SSRIs | ||
| Citalopram | 10 | 20 to 40 |
| Escitalopram | 5 | 10 to 40 |
| Fluoxetine | 5 to 10 | 20 to 80 |
| Fluvoxamine | 25 | 100 to 300 |
| Paroxetine | 10 | 20 to 80 |
| Paroxetine CR | 12.5 | 25 to 50 |
| Sertraline | 25 | 100 to 200 |
| SNRIs | ||
| Duloxetine | 20 to 30 | 60 to 120 |
| Venlafaxine XR | 37.5 | 150 to 225 |
| TCAs | ||
| Clomipramine | 10 to 25 | 100 to 300 |
| Imipramine | 10 | 100 to 300 |
| MAOI | ||
| Phenelzine | 15 | 45 to 90 |
| CR: controlled release; MAOI: monoamine oxidase inhibitor; SNRIs: serotonin-norepinephrine reuptake inhibitors; SSRIs: selective serotonin reuptake inhibitors; TCAs: tricyclic antidepressants; XR: extended release Source: American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder. 2nd ed. Washington, DC: American Psychiatric Association; 2009 | ||
Treatment nonresponse. True non-response needs to be distinguished from poor response caused by inadequate treatment delivery, eg, patients not completing homework assignments in CBT or not adhering to pharmacotherapy. Asking patients about adverse effects or personal and family beliefs about treatment may reveal reasons for nonadherence.
Second-line treatments
Little data are available to guide next-step treatment options in patients who don’t achieve remission from their initial treatment. Patients who benefit from an SSRI, venlafaxine XR, or CBT but still have symptoms should be started on combination treatment. For a patient who experiences complete non-response to the initial treatment, discontinue the first treatment and switch to the other modality. In general, completely ineffective treatments should be discontinued when another treatment is added, but when partial improvement (>30%) occurs, continue the original treatment and augment it with another approach.
For patients pursuing pharmacotherapy, poor response to an adequate SSRI trial usually should lead to a switch to venlafaxine XR, and vice versa. Failure to respond to both of these medication classes should prompt a switch to a benzodiazepine or TCA.
Benzodiazepines are a fast-acting, effective treatment for PD, with efficacy similar to SSRIs in acute and long-term treatment.14 Benzodiazepines may be prescribed with antidepressants at the beginning of treatment to improve response speed.15 Clonazepam and alprazolam are FDA-approved for treating PD. A high-potency, long-acting agent, clonazepam is the preferred initial benzodiazepine, dosed 0.5 to 4 mg/d on a fixed schedule. Although substantial data support using alprazolam for PD, it requires more frequent dosing and has a greater risk of rebound anxiety and abuse potential because of its more rapid onset of action. Compared with immediate-release alprazolam, alprazolam XR has a slower absorption rate and longer steady state in the blood, but this formulation does not have lower abuse potential or greater efficacy. Although not FDA-approved for PD, diazepam and lorazepam also have proven efficacy for PD.3
Benzodiazepines should be considered contraindicated in patients with a history of substance abuse, except in select cases.4 Benzodiazepines generally should be avoided in older patients because of increased risk for falls, cognitive impairment, and motor vehicle accidents. Table 2 lists situations in which benzodiazepines may be used to treat PD.
Table 2
Clinical scenarios in which to consider using benzodiazepines
| Coadministration for 2 to 4 weeks when initiating treatment with an SSRI or venlafaxine XR to achieve more rapid relief and mitigate potential antidepressant-induced anxiety |
| For patients who wish to avoid antidepressants because of concern about sexual dysfunction |
| For patients who need chronic aspirin or an NSAID, which may increase the risk for upper gastrointestinal bleeding when taken in combination with an SSRI |
| For patients with comorbid bipolar disorder or epilepsy |
| Next-step monotherapy or augmentation in patients who respond poorly to an SSRI, venlafaxine XR, TCA, or CBT |
| CBT: cognitive-behavioral therapy; NSAID: nonsteroidal anti-inflammatory drug; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant; XR: extended release |
TCAs are effective as monotherapy for PD. Most support comes from studies of imipramine or clomipramine.12 Similar to SSRIs and venlafaxine XR, use a low initial dose and gradually increase until the patient remits or side effects prevent further increases. SSRI and TCA combinations rarely are used unless the TCA is a relatively specific norepinephrine reuptake inhibitor (eg, desipramine, nortriptyline). Because TCAs are metabolized via the cytochrome P450 2D6 system and some SSRIs—particularly fluoxetine and paroxetine—strongly inhibit 2D6, combinations of TCAs with these agents may lead to dangerously high plasma TCA levels, placing patients at risk for cardiac dysrhythmias and other side effects.16
Monoamine oxidase inhibitors (MAOIs)—particularly phenelzine—are underused for PD. They have the strongest efficacy data for any class of medications outside the first- and second-line agents and have a unique mechanism of action. In patients who can comply with the dietary and medication limitations, an MAOI generally should be the next step after nonresponse to other treatments.3
Alternative treatments
For patients who do not respond to any of the treatments described above, data from uncontrolled studies support mirtazapine, levetiracetam, and the serotonin-norepinephrine reuptake inhibitors duloxetine and milnacipran as monotherapy for PD.17 Pindolol—a beta blocker and 5-HT1A receptor antagonist—proved superior to placebo as an adjunctive agent to SSRIs in treatment-resistant PD in 1 of 2 trials.17 Minimal evidence supports the atypical antipsychotics risperidone and olanzapine in treatment-resistant PD, although a placebo-controlled trial of quetiapine SR coadministered with SSRIs recently was completed (NCT00619892; results pending). Atypical antipsychotics are best reserved for patients with a primary psychotic disorder or bipolar disorder who experience panic attacks.5
Panic-focused psychodynamic psychotherapy, a 12-week (approximately 24 sessions) form of psychotherapy, has demonstrated superiority vs applied relaxation therapy.18 This treatment could be considered for patients who do not respond to standard first-line treatments, but few community therapists are familiar with this method.
For many patients with PD, complementary and alternative medicine (CAM) approaches are appealing. See this article at CurrentPsychiatry.com for a Box that discusses CAM for PD.
Although no complementary and alternative medicine treatments have strong evidence of efficacy as monotherapy for panic disorder (PD), several have data that suggest benefit with little evidence of risk. These include bibliotherapy, yoga, aerobic exercise, and the dietary supplements kava and inositol.a Exercise as a treatment poses a challenge because it can induce symptoms that the patient fears, such as tachycardia and shortness of breath. In addition to any direct physiologic benefit from aerobic exercise, there is also an exposure component that can be harnessed by gradually increasing the exertion level.
Another approach undergoing extensive evaluation is Internet-provided cognitive-behavioral therapy (CBT). Using guided CBT modules with or without therapist support, Internet-provided CBT provides an option for motivated patients unable to complete in-person CBT because of logistical factors.b A helpful resource that reviews Internet self-help and psychotherapy guided programs for PD and other psychiatric conditions is http://beacon.anu.edu.au.
References
a. Antonacci DJ, Davis E, Bloch RM, et al. CAM for your anxious patient: what the evidence says. Current Psychiatry. 2010;9(10):42-52.
b. Johnston L, Titov N, Andrews G, et al. A RCT of a transdiagnostic internet-delivered treatment for three anxiety disorders: examination of support roles and disorder-specific outcomes. PLoS One. 2011;6(11):e28079.
Maintenance treatment
Patients who complete a course of CBT for PD often follow up with several “booster sessions” at monthly or longer intervals that focus on relapse prevention techniques. Few controlled trials have evaluated pharmacotherapy discontinuation in PD. Most guidelines recommend continuing treatment for ≥1 year after achieving remission to minimize the risk of relapse.3 Researchers are focusing on whether medication dosage can be reduced during maintenance without loss of efficacy.
Treatment discontinuation
In the absence of urgent medical need, taper medications for PD gradually over several months. PD patients are highly sensitive to unusual physical sensations, which can occur while discontinuing antidepressants or benzodiazepines. If a benzodiazepine is used in conjunction with an antidepressant, the benzodiazepine should be discontinued first, so that the antidepressant can help ease benzodiazepine-associated discontinuation symptoms. A brief course of CBT during pharmacotherapy discontinuation may increase the likelihood of successful tapering.19
CASE CONTINUED: A successful switch
Ms. K has to discontinue sequential trials of fluoxetine, 40 mg/d, and venlafaxine XR, 225 mg/d because of side effects, and she does not reduce the frequency of her alprazolam use. She agrees to switch from alprazolam to clonazepam, 0.5 mg every morning and 1 mg at bedtime, and to start CBT. Clonazepam reduces her anxiety sufficiently so she can address her symptoms in therapy. Through CBT she becomes motivated to monitor her thoughts and treat them as guesses rather than facts, reviewing the evidence for her thoughts and generating rational responses. She participates in exposure exercises, which she practices between sessions, and grows to tolerate uncomfortable sensations until they no longer signal danger. After 12 CBT sessions, she is panic-free. Despite some trepidation, she agrees to a slow taper off clonazepam, reducing the dose by 0.25 mg every 2 weeks. She continues booster sessions with her therapist to manage any re-emerging anxiety. After an additional 12 weeks, she successfully discontinues clonazepam and remains panic-free.
Related Resources
- American Psychiatric Association. Panic disorder. http://healthyminds.org/Main-Topic/Panic-Disorder.aspx.
- Anxiety and Depression Association of America. Panic disorder & agoraphobia. http://adaa.org/understanding-anxiety/panic-disorder-agoraphobia.
- Mayo Clinic. Panic attacks and panic disorder. www.mayoclinic.com/health/panic-attacks/DS00338.
- National Health Service Self-Help Guides. www.ntw.nhs.uk/pic/selfhelp.
- National Institute of Mental Health. Panic disorder. www.nimh.nih.gov/health/topics/panic-disorder/index.shtml.
Drug Brand Names
- Alprazolam • Xanax
- Alprazolam XR • Xanax XR
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Desipramine • Norpramin
- Diazepam • Valium
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Levetiracetam • Keppra
- Lorazepam • Ativan
- Milnacipran • Savella
- Mirtazapine • Remeron
- Nortriptyline • Aventyl, Pamelor
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Paroxetine CR • Paxil CR
- Phenelzine • Nardil
- Pindolol • Visken
- Quetiapine SR • Seroquel SR
- Risperidone • Risperdal
- Sertraline • Zoloft
- Venlafaxine XR • Effexor XR
Disclosures
Dr. Dunlop receives research support from Bristol-Myers Squibb, GlaxoSmithKline, and the National Institute of Mental Health. He serves as a consultant to MedAvante and Roche.
Ms. Schneider and Dr. Gerardi report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Roy-Byrne PP, Craske MG, Stein MB. Panic disorder. Lancet. 2006;368(9540):1023-1032.
2. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
3. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder. 2nd ed. Washington DC: American Psychiatric Association; 2009.
4. Dunlop BW, Davis PG. Combination treatment with benzodiazepines and SSRIs for comorbid anxiety and depression: a review. Prim Care Companion J Clin Psychiatry. 2008;10(3):222-228.
5. Rakofsky JJ, Dunlop BW. Treating nonspecific anxiety and anxiety disorders in patients with bipolar disorder: a review. J Clin Psychiatry. 2011;72(1):81-90.
6. Sareen J, Cox BJ, Afifi TO, et al. Anxiety disorders and risk for suicidal ideation and suicide attempts: a population-based longitudinal study of adults. Arch Gen Psychiatry. 2005;62(11):1249-1257.
7. Houck PR, Spiegel DA, Shear MK, et al. Reliability of the self-report version of the panic disorder severity scale. Depress Anxiety. 2002;15(4):183-185.
8. Ninan PT, Dunlop BW. Neurobiology and etiology of panic disorder. J Clin Psychiatry. 2005;66(suppl 4):3-7.
9. Furukawa TA, Watanabe N, Churchill R. Psychotherapy plus antidepressant for panic disorder with or without agoraphobia: systematic review. Br J Psychiatry. 2006;188:305-312.
10. Barlow DH, Gorman JM, Shear MK, et al. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: a randomized controlled trial. JAMA. 2000;283(19):2529-2536.
11. van Apeldoorn FJ, Timmerman ME, Mersch PP, et al. A randomized trial of cognitive-behavioral therapy or selective serotonin reuptake inhibitor or both combined for panic disorder with or without agoraphobia: treatment results through 1-year follow-up. J Clin Psychiatry. 2010;71(5):574-586.
12. Bakker A, van Balkom AJ, Spinhoven P. SSRIs vs. TCAs in the treatment of panic disorder: a meta-analysis. Acta Psychiatr Scand. 2002;106(3):163-167.
13. Bandelow B, Behnke K, Lenoir S, et al. Sertraline versus paroxetine in the treatment of panic disorder: an acute, double-blind noninferiority comparison. J Clin Psychiatry. 2004;65(3):405-413.
14. Nardi AE, Freire RC, Mochcovitch MD, et al. A randomized, naturalistic, parallel-group study for the long-term treatment of panic disorder with clonazepam or paroxetine. J Clin Psychopharmacol. 2012;32(1):120-126.
15. Goddard AW, Brouette T, Almai A, et al. Early coadministration of clonazepam with sertraline for panic disorder. Arch Gen Psychiatry. 2001;58(7):681-686.
16. Preskorn SH, Shah R, Neff M, et al. The potential for clinically significant drug-drug interactions involving the CYP 2D6 system: effects with fluoxetine and paroxetine versus sertraline. J Psychiatr Pract. 2007;13(1):5-12.
17. Perna G, Guerriero G, Caldirola D. Emerging drugs for panic disorder. Expert Opin Emerg Drugs. 2011;16(4):631-645.
18. Milrod B, Leon AC, Busch F, et al. A randomized controlled clinical trial of psychoanalytic psychotherapy for panic disorder. Am J Psychiatry. 2007;164(2):265-272.
19. Otto MW, Pollack MH, Sachs GS, et al. Discontinuation of benzodiazepine treatment: efficacy of cognitive-behavioral therapy for patients with panic disorder. Am J Psychiatry. 1993;150(10):1485-1490.
Ms. K, a 24-year-old waitress who lives with her boyfriend, was referred by her primary care physician for evaluation of panic attacks that began “out of nowhere” at work approximately 6 months ago. The unpredictable attacks occur multiple times per week, causing her to leave work and cancel shifts.
Ms. K reports that before the panic attacks began, she felt happy in her relationship, enjoyed hobbies, and was hopeful about the future. However, she has become concerned that a potentially catastrophic illness is causing her panic attacks. She researches her symptoms on the Internet, and is preoccupied with the possibility of sudden death due to an undiagnosed heart condition. Multiple visits to the emergency room have not identified any physical abnormalities. Her primary care doctor prescribed alprazolam, 0.5 mg as needed for panic attacks, which she reports is helpful, “but only in the moment of the attacks.” Ms. K avoids alcohol and illicit substances and limits her caffeine intake. She is not willing to accept that her life “feels so limited.” Her dream of earning a nursing degree and eventually starting a family now seems unattainable.
Panic disorder (PD) occurs in 3% to 5% of adults, with women affected at roughly twice the rate of men.1 Causing a broad range of distress and varying degrees of impairment, PD commonly occurs with other psychiatric disorders. For most patients, treatment is effective, but those who do not respond to initial approaches require a thoughtful, stepped approach to care. Key considerations include establishing an accurate diagnosis, clarifying comorbid illnesses, ascertaining patient beliefs and expectations, and providing appropriately dosed and maintained treatments.
Panic attacks vs PD
Panic attacks consist of rapid onset of intense anxiety, with prominent somatic symptoms, that peaks within 10 minutes (Figure).2 Attacks in which <4 of the listed symptoms occur are considered limited-symptom panic attacks.

Figure: Body locations of panic attack symptoms
Diagnosis of a panic attack requires the sudden development of intense fear or discomfort characterized by ≥4 of the 13 symptoms listed above that peaks in intensity within 10 minutes of onset
Source: Reference 2
Panic attacks can occur with various disorders, including other anxiety disorders, mood disorders, and substance intoxication or withdrawal. Because serious medical conditions can present with panic-like symptoms, the initial occurrence of such symptoms warrants consideration of physiological causes. For a Box2 that describes the differential diagnosis of panic attacks, see this article at CurrentPsychiatry.com.
To meet diagnostic criteria for panic disorder, panic attacks must initially occur “out of the blue,” meaning no specific object or situation induced the attack. The differential diagnosis of panic attacks includes assessing for other psychiatric disorders that may involve panic attacks. Evaluation requires considering the context in which the panic attacks occur, including their start date, pattern of attacks, instigating situations, and associated thoughts.
Social phobia. Attacks occur only during or immediately before a social interaction in which the patient fears embarrassing himself or herself.
Obsessive-compulsive disorder (OCD). Attacks occur when the patient cannot avoid exposure to an obsessional fear or is prevented from performing a ritual that diffuses obsessional anxiety.
Posttraumatic stress disorder (PTSD). Attacks occur when confronted by a trauma-related memory or trigger.
Specific phobia. Attacks occur only when the patient encounters a specifically feared object, place, or situation, unrelated to social phobia, OCD, or PTSD.
Medical conditions. Conditions to consider include—but are not limited to—hyperthyroidism, pulmonary embolism, myocardial infarction, cardiac dysrhythmias, hypoglycemia, asthma, partial complex seizures, and pheochromocytoma.
Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000
A PD diagnosis requires that repeated panic attacks initially must occur from “out of the blue,” meaning no specific object or situation induced the attack. In addition, the diagnosis requires 1 of 3 types of psychological or behavioral changes as a result of the attacks (Table 1).2 Agoraphobia is diagnosed if 1 of the behavioral changes is avoidance of places or situations from which escape might be embarrassing or difficult should an attack occur. A patient can be diagnosed as having PD with agoraphobia, PD without agoraphobia, or agoraphobia without PD (ie, experiences only limited symptom panic attacks, but avoids situations or stimuli associated with them).
Table 1
Definitions of panic disorder and agoraphobia
| Panic disorder |
|---|
|
| Agoraphobia |
| Anxiety about, or avoidance of, being in places or situations from which escape might be difficult or embarrassing, or in which help may not be available in the event of having an unexpected or situationally predisposed panic attack or panic-like symptoms. Agoraphobic fears typically involve characteristic clusters of situations that include being outside the home alone, being in a crowd, standing in a line, being on a bridge, or traveling in a bus, train, or automobile |
| Source: Reference 2 |
Comorbidities are common in patients with PD and predict greater difficulty achieving remission (Box).1,3-6
The most common psychiatric conditions that co-occur with panic disorder (PD) are other anxiety disorders, mood disorders, personality disorders, and substance use disorders.1 Carefully assess the severity and degree of impairment or distress arising from each condition to prioritize treatment goals. For example, treating panic attacks would be a lower priority in a patient with untreated bipolar disorder.
Assessing comorbid substance abuse is important in selecting PD treatments. Benzodiazepines should almost always be avoided in patients with a history of drug abuse—illicit or prescribed. Although complete abstinence should not be a prerequisite for beginning PD treatment, detoxification and concomitant substance abuse treatment are essential.3
Comorbid mood disorders also affect the course of PD treatment. Antidepressants are effective for treating depression and PD, whereas benzodiazepines are not effective for depression.4 Antidepressants in patients with bipolar disorder are controversial because these medications might induce mixed or elevated mood states or rapid cycling. In these complicated patients, consider antidepressants lower in the treatment algorithm.5
Other conditions to consider before beginning treatment include pregnancy or the possibility of becoming pregnant in the near future and suicidal ideation. PD is associated with increased risk for suicidal ideation and progression to suicide attempts, particularly in patients with a comorbid mood or psychotic disorder.6 In addition, consider the potential impact of medications on comorbid medical conditions.
Treatment begins with education
The goal of treatment is remission of symptoms, ideally including an absence of panic attacks, agoraphobic avoidance, and anticipatory anxiety.1 The Panic Disorder Severity Scale self-report is a validated measure of panic symptoms that may be useful in clinical practice.7
The first step in treatment is educating patients about panic attacks, framing them as an overreactive fear circuit in the brain that produces physical symptoms that are not dangerous. Using a brain model that shows the location of the amygdala, hippocampus, and prefrontal cortex—which play crucial roles in generating and controlling anxiety and fear—can make this discussion more concrete.8 Although highly simplified, such models allow clinicians to demonstrate that excessive reactivity of limbic regions can be reduced by both top-down (cortico-limbic connections via cognitive-behavioral therapy [CBT]) and bottom-up (pharmacotherapy directly acting on limbic structures) approaches. Such discussions lead to treatment recommendations for CBT, pharmacotherapy, or their combination.
No single treatment has emerged as the definitive “best” for PD, and no reliable predictors can guide specific treatment for an individual.3 Combining CBT with pharmacotherapy produces higher short-term response rates than either treatment alone, but in the long term, combination treatment does not appear to be superior to CBT alone.9 Base the initial treatment selection for PD on patient preference, treatment availability and cost, and comorbid medical and psychiatric conditions. For an Algorithm to guide treatment decisions, see this article at CurrentPsychiatry.com.

Algorithm: Treatment for panic disorder: A suggested algorithm
aPoor response to an SSRI should lead to a switch to venlafaxine extended-release, and vice versa
bBenzodiazepines are relatively contraindicated in geriatric patients and patients with a history of substance abuse or dependence
CBT: cognitive-behavioral therapy; MAOI: monoamine oxidase inhibitor; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant; Ven XR: venlafaxine extended-release
First-line treatments
Psychotherapy. CBT is the most efficacious psychotherapy for PD. Twelve to 15 sessions of CBT has demonstrated efficacy for PD, with additional effects on comorbid anxiety and depressive symptoms.10 No large clinical trials of CBT have used cognitive restructuring alone; all have included at least some component of exposure that requires the patient to confront feared physical sensations. Gains during treatment may be steady and gradual or sudden and uneven, with rapid improvement in some but not all symptoms. CBT and pharmacotherapy have demonstrated similar levels of benefit in short-term trials, but CBT has proven superior in most9 but not all11 trials evaluating long-term outcomes, particularly compared with pharmacotherapy that is discontinued during follow-up. Although less studied, group CBT also may be considered if a patient cannot afford individual CBT.
Pharmacotherapy. Evidence supports selective serotonin reuptake inhibitors (SSRIs), venlafaxine extended-release (XR), benzodiazepines, and tricyclic antidepressants (TCAs) as effective treatments for PD.3 No class of medication has demonstrated superiority over others in short-term treatment.3,12 Because of the medical risks associated with benzodiazepines and TCAs, an SSRI or venlafaxine XR should be the first medication option for most patients. Fluoxetine, paroxetine, sertraline, and venlafaxine XR are FDA-approved for PD. Paroxetine is associated with weight gain and may increase the risk for panic recurrence upon discontinuation more than sertraline, making it a less favorable option for many patients.13 Start doses at half the normal starting dose used for treating major depressive disorder and continue for 4 to 7 days, then increase to the minimal effective dose. For a Table3 that lists dosing recommendations for antidepressants to treat PD, see this article at CurrentPsychiatry.com. If there is no improvement by 4 weeks, increase the dose every 2 to 4 weeks until remission is achieved or side effects prevent further dose increases.
Table
Recommended doses for antidepressants used to treat panic disorder
| Medication | Starting dose (mg/d) | Therapeutic range (mg/d) |
|---|---|---|
| SSRIs | ||
| Citalopram | 10 | 20 to 40 |
| Escitalopram | 5 | 10 to 40 |
| Fluoxetine | 5 to 10 | 20 to 80 |
| Fluvoxamine | 25 | 100 to 300 |
| Paroxetine | 10 | 20 to 80 |
| Paroxetine CR | 12.5 | 25 to 50 |
| Sertraline | 25 | 100 to 200 |
| SNRIs | ||
| Duloxetine | 20 to 30 | 60 to 120 |
| Venlafaxine XR | 37.5 | 150 to 225 |
| TCAs | ||
| Clomipramine | 10 to 25 | 100 to 300 |
| Imipramine | 10 | 100 to 300 |
| MAOI | ||
| Phenelzine | 15 | 45 to 90 |
| CR: controlled release; MAOI: monoamine oxidase inhibitor; SNRIs: serotonin-norepinephrine reuptake inhibitors; SSRIs: selective serotonin reuptake inhibitors; TCAs: tricyclic antidepressants; XR: extended release Source: American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder. 2nd ed. Washington, DC: American Psychiatric Association; 2009 | ||
Treatment nonresponse. True non-response needs to be distinguished from poor response caused by inadequate treatment delivery, eg, patients not completing homework assignments in CBT or not adhering to pharmacotherapy. Asking patients about adverse effects or personal and family beliefs about treatment may reveal reasons for nonadherence.
Second-line treatments
Little data are available to guide next-step treatment options in patients who don’t achieve remission from their initial treatment. Patients who benefit from an SSRI, venlafaxine XR, or CBT but still have symptoms should be started on combination treatment. For a patient who experiences complete non-response to the initial treatment, discontinue the first treatment and switch to the other modality. In general, completely ineffective treatments should be discontinued when another treatment is added, but when partial improvement (>30%) occurs, continue the original treatment and augment it with another approach.
For patients pursuing pharmacotherapy, poor response to an adequate SSRI trial usually should lead to a switch to venlafaxine XR, and vice versa. Failure to respond to both of these medication classes should prompt a switch to a benzodiazepine or TCA.
Benzodiazepines are a fast-acting, effective treatment for PD, with efficacy similar to SSRIs in acute and long-term treatment.14 Benzodiazepines may be prescribed with antidepressants at the beginning of treatment to improve response speed.15 Clonazepam and alprazolam are FDA-approved for treating PD. A high-potency, long-acting agent, clonazepam is the preferred initial benzodiazepine, dosed 0.5 to 4 mg/d on a fixed schedule. Although substantial data support using alprazolam for PD, it requires more frequent dosing and has a greater risk of rebound anxiety and abuse potential because of its more rapid onset of action. Compared with immediate-release alprazolam, alprazolam XR has a slower absorption rate and longer steady state in the blood, but this formulation does not have lower abuse potential or greater efficacy. Although not FDA-approved for PD, diazepam and lorazepam also have proven efficacy for PD.3
Benzodiazepines should be considered contraindicated in patients with a history of substance abuse, except in select cases.4 Benzodiazepines generally should be avoided in older patients because of increased risk for falls, cognitive impairment, and motor vehicle accidents. Table 2 lists situations in which benzodiazepines may be used to treat PD.
Table 2
Clinical scenarios in which to consider using benzodiazepines
| Coadministration for 2 to 4 weeks when initiating treatment with an SSRI or venlafaxine XR to achieve more rapid relief and mitigate potential antidepressant-induced anxiety |
| For patients who wish to avoid antidepressants because of concern about sexual dysfunction |
| For patients who need chronic aspirin or an NSAID, which may increase the risk for upper gastrointestinal bleeding when taken in combination with an SSRI |
| For patients with comorbid bipolar disorder or epilepsy |
| Next-step monotherapy or augmentation in patients who respond poorly to an SSRI, venlafaxine XR, TCA, or CBT |
| CBT: cognitive-behavioral therapy; NSAID: nonsteroidal anti-inflammatory drug; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant; XR: extended release |
TCAs are effective as monotherapy for PD. Most support comes from studies of imipramine or clomipramine.12 Similar to SSRIs and venlafaxine XR, use a low initial dose and gradually increase until the patient remits or side effects prevent further increases. SSRI and TCA combinations rarely are used unless the TCA is a relatively specific norepinephrine reuptake inhibitor (eg, desipramine, nortriptyline). Because TCAs are metabolized via the cytochrome P450 2D6 system and some SSRIs—particularly fluoxetine and paroxetine—strongly inhibit 2D6, combinations of TCAs with these agents may lead to dangerously high plasma TCA levels, placing patients at risk for cardiac dysrhythmias and other side effects.16
Monoamine oxidase inhibitors (MAOIs)—particularly phenelzine—are underused for PD. They have the strongest efficacy data for any class of medications outside the first- and second-line agents and have a unique mechanism of action. In patients who can comply with the dietary and medication limitations, an MAOI generally should be the next step after nonresponse to other treatments.3
Alternative treatments
For patients who do not respond to any of the treatments described above, data from uncontrolled studies support mirtazapine, levetiracetam, and the serotonin-norepinephrine reuptake inhibitors duloxetine and milnacipran as monotherapy for PD.17 Pindolol—a beta blocker and 5-HT1A receptor antagonist—proved superior to placebo as an adjunctive agent to SSRIs in treatment-resistant PD in 1 of 2 trials.17 Minimal evidence supports the atypical antipsychotics risperidone and olanzapine in treatment-resistant PD, although a placebo-controlled trial of quetiapine SR coadministered with SSRIs recently was completed (NCT00619892; results pending). Atypical antipsychotics are best reserved for patients with a primary psychotic disorder or bipolar disorder who experience panic attacks.5
Panic-focused psychodynamic psychotherapy, a 12-week (approximately 24 sessions) form of psychotherapy, has demonstrated superiority vs applied relaxation therapy.18 This treatment could be considered for patients who do not respond to standard first-line treatments, but few community therapists are familiar with this method.
For many patients with PD, complementary and alternative medicine (CAM) approaches are appealing. See this article at CurrentPsychiatry.com for a Box that discusses CAM for PD.
Although no complementary and alternative medicine treatments have strong evidence of efficacy as monotherapy for panic disorder (PD), several have data that suggest benefit with little evidence of risk. These include bibliotherapy, yoga, aerobic exercise, and the dietary supplements kava and inositol.a Exercise as a treatment poses a challenge because it can induce symptoms that the patient fears, such as tachycardia and shortness of breath. In addition to any direct physiologic benefit from aerobic exercise, there is also an exposure component that can be harnessed by gradually increasing the exertion level.
Another approach undergoing extensive evaluation is Internet-provided cognitive-behavioral therapy (CBT). Using guided CBT modules with or without therapist support, Internet-provided CBT provides an option for motivated patients unable to complete in-person CBT because of logistical factors.b A helpful resource that reviews Internet self-help and psychotherapy guided programs for PD and other psychiatric conditions is http://beacon.anu.edu.au.
References
a. Antonacci DJ, Davis E, Bloch RM, et al. CAM for your anxious patient: what the evidence says. Current Psychiatry. 2010;9(10):42-52.
b. Johnston L, Titov N, Andrews G, et al. A RCT of a transdiagnostic internet-delivered treatment for three anxiety disorders: examination of support roles and disorder-specific outcomes. PLoS One. 2011;6(11):e28079.
Maintenance treatment
Patients who complete a course of CBT for PD often follow up with several “booster sessions” at monthly or longer intervals that focus on relapse prevention techniques. Few controlled trials have evaluated pharmacotherapy discontinuation in PD. Most guidelines recommend continuing treatment for ≥1 year after achieving remission to minimize the risk of relapse.3 Researchers are focusing on whether medication dosage can be reduced during maintenance without loss of efficacy.
Treatment discontinuation
In the absence of urgent medical need, taper medications for PD gradually over several months. PD patients are highly sensitive to unusual physical sensations, which can occur while discontinuing antidepressants or benzodiazepines. If a benzodiazepine is used in conjunction with an antidepressant, the benzodiazepine should be discontinued first, so that the antidepressant can help ease benzodiazepine-associated discontinuation symptoms. A brief course of CBT during pharmacotherapy discontinuation may increase the likelihood of successful tapering.19
CASE CONTINUED: A successful switch
Ms. K has to discontinue sequential trials of fluoxetine, 40 mg/d, and venlafaxine XR, 225 mg/d because of side effects, and she does not reduce the frequency of her alprazolam use. She agrees to switch from alprazolam to clonazepam, 0.5 mg every morning and 1 mg at bedtime, and to start CBT. Clonazepam reduces her anxiety sufficiently so she can address her symptoms in therapy. Through CBT she becomes motivated to monitor her thoughts and treat them as guesses rather than facts, reviewing the evidence for her thoughts and generating rational responses. She participates in exposure exercises, which she practices between sessions, and grows to tolerate uncomfortable sensations until they no longer signal danger. After 12 CBT sessions, she is panic-free. Despite some trepidation, she agrees to a slow taper off clonazepam, reducing the dose by 0.25 mg every 2 weeks. She continues booster sessions with her therapist to manage any re-emerging anxiety. After an additional 12 weeks, she successfully discontinues clonazepam and remains panic-free.
Related Resources
- American Psychiatric Association. Panic disorder. http://healthyminds.org/Main-Topic/Panic-Disorder.aspx.
- Anxiety and Depression Association of America. Panic disorder & agoraphobia. http://adaa.org/understanding-anxiety/panic-disorder-agoraphobia.
- Mayo Clinic. Panic attacks and panic disorder. www.mayoclinic.com/health/panic-attacks/DS00338.
- National Health Service Self-Help Guides. www.ntw.nhs.uk/pic/selfhelp.
- National Institute of Mental Health. Panic disorder. www.nimh.nih.gov/health/topics/panic-disorder/index.shtml.
Drug Brand Names
- Alprazolam • Xanax
- Alprazolam XR • Xanax XR
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Desipramine • Norpramin
- Diazepam • Valium
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Levetiracetam • Keppra
- Lorazepam • Ativan
- Milnacipran • Savella
- Mirtazapine • Remeron
- Nortriptyline • Aventyl, Pamelor
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Paroxetine CR • Paxil CR
- Phenelzine • Nardil
- Pindolol • Visken
- Quetiapine SR • Seroquel SR
- Risperidone • Risperdal
- Sertraline • Zoloft
- Venlafaxine XR • Effexor XR
Disclosures
Dr. Dunlop receives research support from Bristol-Myers Squibb, GlaxoSmithKline, and the National Institute of Mental Health. He serves as a consultant to MedAvante and Roche.
Ms. Schneider and Dr. Gerardi report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Ms. K, a 24-year-old waitress who lives with her boyfriend, was referred by her primary care physician for evaluation of panic attacks that began “out of nowhere” at work approximately 6 months ago. The unpredictable attacks occur multiple times per week, causing her to leave work and cancel shifts.
Ms. K reports that before the panic attacks began, she felt happy in her relationship, enjoyed hobbies, and was hopeful about the future. However, she has become concerned that a potentially catastrophic illness is causing her panic attacks. She researches her symptoms on the Internet, and is preoccupied with the possibility of sudden death due to an undiagnosed heart condition. Multiple visits to the emergency room have not identified any physical abnormalities. Her primary care doctor prescribed alprazolam, 0.5 mg as needed for panic attacks, which she reports is helpful, “but only in the moment of the attacks.” Ms. K avoids alcohol and illicit substances and limits her caffeine intake. She is not willing to accept that her life “feels so limited.” Her dream of earning a nursing degree and eventually starting a family now seems unattainable.
Panic disorder (PD) occurs in 3% to 5% of adults, with women affected at roughly twice the rate of men.1 Causing a broad range of distress and varying degrees of impairment, PD commonly occurs with other psychiatric disorders. For most patients, treatment is effective, but those who do not respond to initial approaches require a thoughtful, stepped approach to care. Key considerations include establishing an accurate diagnosis, clarifying comorbid illnesses, ascertaining patient beliefs and expectations, and providing appropriately dosed and maintained treatments.
Panic attacks vs PD
Panic attacks consist of rapid onset of intense anxiety, with prominent somatic symptoms, that peaks within 10 minutes (Figure).2 Attacks in which <4 of the listed symptoms occur are considered limited-symptom panic attacks.

Figure: Body locations of panic attack symptoms
Diagnosis of a panic attack requires the sudden development of intense fear or discomfort characterized by ≥4 of the 13 symptoms listed above that peaks in intensity within 10 minutes of onset
Source: Reference 2
Panic attacks can occur with various disorders, including other anxiety disorders, mood disorders, and substance intoxication or withdrawal. Because serious medical conditions can present with panic-like symptoms, the initial occurrence of such symptoms warrants consideration of physiological causes. For a Box2 that describes the differential diagnosis of panic attacks, see this article at CurrentPsychiatry.com.
To meet diagnostic criteria for panic disorder, panic attacks must initially occur “out of the blue,” meaning no specific object or situation induced the attack. The differential diagnosis of panic attacks includes assessing for other psychiatric disorders that may involve panic attacks. Evaluation requires considering the context in which the panic attacks occur, including their start date, pattern of attacks, instigating situations, and associated thoughts.
Social phobia. Attacks occur only during or immediately before a social interaction in which the patient fears embarrassing himself or herself.
Obsessive-compulsive disorder (OCD). Attacks occur when the patient cannot avoid exposure to an obsessional fear or is prevented from performing a ritual that diffuses obsessional anxiety.
Posttraumatic stress disorder (PTSD). Attacks occur when confronted by a trauma-related memory or trigger.
Specific phobia. Attacks occur only when the patient encounters a specifically feared object, place, or situation, unrelated to social phobia, OCD, or PTSD.
Medical conditions. Conditions to consider include—but are not limited to—hyperthyroidism, pulmonary embolism, myocardial infarction, cardiac dysrhythmias, hypoglycemia, asthma, partial complex seizures, and pheochromocytoma.
Source: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000
A PD diagnosis requires that repeated panic attacks initially must occur from “out of the blue,” meaning no specific object or situation induced the attack. In addition, the diagnosis requires 1 of 3 types of psychological or behavioral changes as a result of the attacks (Table 1).2 Agoraphobia is diagnosed if 1 of the behavioral changes is avoidance of places or situations from which escape might be embarrassing or difficult should an attack occur. A patient can be diagnosed as having PD with agoraphobia, PD without agoraphobia, or agoraphobia without PD (ie, experiences only limited symptom panic attacks, but avoids situations or stimuli associated with them).
Table 1
Definitions of panic disorder and agoraphobia
| Panic disorder |
|---|
|
| Agoraphobia |
| Anxiety about, or avoidance of, being in places or situations from which escape might be difficult or embarrassing, or in which help may not be available in the event of having an unexpected or situationally predisposed panic attack or panic-like symptoms. Agoraphobic fears typically involve characteristic clusters of situations that include being outside the home alone, being in a crowd, standing in a line, being on a bridge, or traveling in a bus, train, or automobile |
| Source: Reference 2 |
Comorbidities are common in patients with PD and predict greater difficulty achieving remission (Box).1,3-6
The most common psychiatric conditions that co-occur with panic disorder (PD) are other anxiety disorders, mood disorders, personality disorders, and substance use disorders.1 Carefully assess the severity and degree of impairment or distress arising from each condition to prioritize treatment goals. For example, treating panic attacks would be a lower priority in a patient with untreated bipolar disorder.
Assessing comorbid substance abuse is important in selecting PD treatments. Benzodiazepines should almost always be avoided in patients with a history of drug abuse—illicit or prescribed. Although complete abstinence should not be a prerequisite for beginning PD treatment, detoxification and concomitant substance abuse treatment are essential.3
Comorbid mood disorders also affect the course of PD treatment. Antidepressants are effective for treating depression and PD, whereas benzodiazepines are not effective for depression.4 Antidepressants in patients with bipolar disorder are controversial because these medications might induce mixed or elevated mood states or rapid cycling. In these complicated patients, consider antidepressants lower in the treatment algorithm.5
Other conditions to consider before beginning treatment include pregnancy or the possibility of becoming pregnant in the near future and suicidal ideation. PD is associated with increased risk for suicidal ideation and progression to suicide attempts, particularly in patients with a comorbid mood or psychotic disorder.6 In addition, consider the potential impact of medications on comorbid medical conditions.
Treatment begins with education
The goal of treatment is remission of symptoms, ideally including an absence of panic attacks, agoraphobic avoidance, and anticipatory anxiety.1 The Panic Disorder Severity Scale self-report is a validated measure of panic symptoms that may be useful in clinical practice.7
The first step in treatment is educating patients about panic attacks, framing them as an overreactive fear circuit in the brain that produces physical symptoms that are not dangerous. Using a brain model that shows the location of the amygdala, hippocampus, and prefrontal cortex—which play crucial roles in generating and controlling anxiety and fear—can make this discussion more concrete.8 Although highly simplified, such models allow clinicians to demonstrate that excessive reactivity of limbic regions can be reduced by both top-down (cortico-limbic connections via cognitive-behavioral therapy [CBT]) and bottom-up (pharmacotherapy directly acting on limbic structures) approaches. Such discussions lead to treatment recommendations for CBT, pharmacotherapy, or their combination.
No single treatment has emerged as the definitive “best” for PD, and no reliable predictors can guide specific treatment for an individual.3 Combining CBT with pharmacotherapy produces higher short-term response rates than either treatment alone, but in the long term, combination treatment does not appear to be superior to CBT alone.9 Base the initial treatment selection for PD on patient preference, treatment availability and cost, and comorbid medical and psychiatric conditions. For an Algorithm to guide treatment decisions, see this article at CurrentPsychiatry.com.

Algorithm: Treatment for panic disorder: A suggested algorithm
aPoor response to an SSRI should lead to a switch to venlafaxine extended-release, and vice versa
bBenzodiazepines are relatively contraindicated in geriatric patients and patients with a history of substance abuse or dependence
CBT: cognitive-behavioral therapy; MAOI: monoamine oxidase inhibitor; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant; Ven XR: venlafaxine extended-release
First-line treatments
Psychotherapy. CBT is the most efficacious psychotherapy for PD. Twelve to 15 sessions of CBT has demonstrated efficacy for PD, with additional effects on comorbid anxiety and depressive symptoms.10 No large clinical trials of CBT have used cognitive restructuring alone; all have included at least some component of exposure that requires the patient to confront feared physical sensations. Gains during treatment may be steady and gradual or sudden and uneven, with rapid improvement in some but not all symptoms. CBT and pharmacotherapy have demonstrated similar levels of benefit in short-term trials, but CBT has proven superior in most9 but not all11 trials evaluating long-term outcomes, particularly compared with pharmacotherapy that is discontinued during follow-up. Although less studied, group CBT also may be considered if a patient cannot afford individual CBT.
Pharmacotherapy. Evidence supports selective serotonin reuptake inhibitors (SSRIs), venlafaxine extended-release (XR), benzodiazepines, and tricyclic antidepressants (TCAs) as effective treatments for PD.3 No class of medication has demonstrated superiority over others in short-term treatment.3,12 Because of the medical risks associated with benzodiazepines and TCAs, an SSRI or venlafaxine XR should be the first medication option for most patients. Fluoxetine, paroxetine, sertraline, and venlafaxine XR are FDA-approved for PD. Paroxetine is associated with weight gain and may increase the risk for panic recurrence upon discontinuation more than sertraline, making it a less favorable option for many patients.13 Start doses at half the normal starting dose used for treating major depressive disorder and continue for 4 to 7 days, then increase to the minimal effective dose. For a Table3 that lists dosing recommendations for antidepressants to treat PD, see this article at CurrentPsychiatry.com. If there is no improvement by 4 weeks, increase the dose every 2 to 4 weeks until remission is achieved or side effects prevent further dose increases.
Table
Recommended doses for antidepressants used to treat panic disorder
| Medication | Starting dose (mg/d) | Therapeutic range (mg/d) |
|---|---|---|
| SSRIs | ||
| Citalopram | 10 | 20 to 40 |
| Escitalopram | 5 | 10 to 40 |
| Fluoxetine | 5 to 10 | 20 to 80 |
| Fluvoxamine | 25 | 100 to 300 |
| Paroxetine | 10 | 20 to 80 |
| Paroxetine CR | 12.5 | 25 to 50 |
| Sertraline | 25 | 100 to 200 |
| SNRIs | ||
| Duloxetine | 20 to 30 | 60 to 120 |
| Venlafaxine XR | 37.5 | 150 to 225 |
| TCAs | ||
| Clomipramine | 10 to 25 | 100 to 300 |
| Imipramine | 10 | 100 to 300 |
| MAOI | ||
| Phenelzine | 15 | 45 to 90 |
| CR: controlled release; MAOI: monoamine oxidase inhibitor; SNRIs: serotonin-norepinephrine reuptake inhibitors; SSRIs: selective serotonin reuptake inhibitors; TCAs: tricyclic antidepressants; XR: extended release Source: American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder. 2nd ed. Washington, DC: American Psychiatric Association; 2009 | ||
Treatment nonresponse. True non-response needs to be distinguished from poor response caused by inadequate treatment delivery, eg, patients not completing homework assignments in CBT or not adhering to pharmacotherapy. Asking patients about adverse effects or personal and family beliefs about treatment may reveal reasons for nonadherence.
Second-line treatments
Little data are available to guide next-step treatment options in patients who don’t achieve remission from their initial treatment. Patients who benefit from an SSRI, venlafaxine XR, or CBT but still have symptoms should be started on combination treatment. For a patient who experiences complete non-response to the initial treatment, discontinue the first treatment and switch to the other modality. In general, completely ineffective treatments should be discontinued when another treatment is added, but when partial improvement (>30%) occurs, continue the original treatment and augment it with another approach.
For patients pursuing pharmacotherapy, poor response to an adequate SSRI trial usually should lead to a switch to venlafaxine XR, and vice versa. Failure to respond to both of these medication classes should prompt a switch to a benzodiazepine or TCA.
Benzodiazepines are a fast-acting, effective treatment for PD, with efficacy similar to SSRIs in acute and long-term treatment.14 Benzodiazepines may be prescribed with antidepressants at the beginning of treatment to improve response speed.15 Clonazepam and alprazolam are FDA-approved for treating PD. A high-potency, long-acting agent, clonazepam is the preferred initial benzodiazepine, dosed 0.5 to 4 mg/d on a fixed schedule. Although substantial data support using alprazolam for PD, it requires more frequent dosing and has a greater risk of rebound anxiety and abuse potential because of its more rapid onset of action. Compared with immediate-release alprazolam, alprazolam XR has a slower absorption rate and longer steady state in the blood, but this formulation does not have lower abuse potential or greater efficacy. Although not FDA-approved for PD, diazepam and lorazepam also have proven efficacy for PD.3
Benzodiazepines should be considered contraindicated in patients with a history of substance abuse, except in select cases.4 Benzodiazepines generally should be avoided in older patients because of increased risk for falls, cognitive impairment, and motor vehicle accidents. Table 2 lists situations in which benzodiazepines may be used to treat PD.
Table 2
Clinical scenarios in which to consider using benzodiazepines
| Coadministration for 2 to 4 weeks when initiating treatment with an SSRI or venlafaxine XR to achieve more rapid relief and mitigate potential antidepressant-induced anxiety |
| For patients who wish to avoid antidepressants because of concern about sexual dysfunction |
| For patients who need chronic aspirin or an NSAID, which may increase the risk for upper gastrointestinal bleeding when taken in combination with an SSRI |
| For patients with comorbid bipolar disorder or epilepsy |
| Next-step monotherapy or augmentation in patients who respond poorly to an SSRI, venlafaxine XR, TCA, or CBT |
| CBT: cognitive-behavioral therapy; NSAID: nonsteroidal anti-inflammatory drug; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant; XR: extended release |
TCAs are effective as monotherapy for PD. Most support comes from studies of imipramine or clomipramine.12 Similar to SSRIs and venlafaxine XR, use a low initial dose and gradually increase until the patient remits or side effects prevent further increases. SSRI and TCA combinations rarely are used unless the TCA is a relatively specific norepinephrine reuptake inhibitor (eg, desipramine, nortriptyline). Because TCAs are metabolized via the cytochrome P450 2D6 system and some SSRIs—particularly fluoxetine and paroxetine—strongly inhibit 2D6, combinations of TCAs with these agents may lead to dangerously high plasma TCA levels, placing patients at risk for cardiac dysrhythmias and other side effects.16
Monoamine oxidase inhibitors (MAOIs)—particularly phenelzine—are underused for PD. They have the strongest efficacy data for any class of medications outside the first- and second-line agents and have a unique mechanism of action. In patients who can comply with the dietary and medication limitations, an MAOI generally should be the next step after nonresponse to other treatments.3
Alternative treatments
For patients who do not respond to any of the treatments described above, data from uncontrolled studies support mirtazapine, levetiracetam, and the serotonin-norepinephrine reuptake inhibitors duloxetine and milnacipran as monotherapy for PD.17 Pindolol—a beta blocker and 5-HT1A receptor antagonist—proved superior to placebo as an adjunctive agent to SSRIs in treatment-resistant PD in 1 of 2 trials.17 Minimal evidence supports the atypical antipsychotics risperidone and olanzapine in treatment-resistant PD, although a placebo-controlled trial of quetiapine SR coadministered with SSRIs recently was completed (NCT00619892; results pending). Atypical antipsychotics are best reserved for patients with a primary psychotic disorder or bipolar disorder who experience panic attacks.5
Panic-focused psychodynamic psychotherapy, a 12-week (approximately 24 sessions) form of psychotherapy, has demonstrated superiority vs applied relaxation therapy.18 This treatment could be considered for patients who do not respond to standard first-line treatments, but few community therapists are familiar with this method.
For many patients with PD, complementary and alternative medicine (CAM) approaches are appealing. See this article at CurrentPsychiatry.com for a Box that discusses CAM for PD.
Although no complementary and alternative medicine treatments have strong evidence of efficacy as monotherapy for panic disorder (PD), several have data that suggest benefit with little evidence of risk. These include bibliotherapy, yoga, aerobic exercise, and the dietary supplements kava and inositol.a Exercise as a treatment poses a challenge because it can induce symptoms that the patient fears, such as tachycardia and shortness of breath. In addition to any direct physiologic benefit from aerobic exercise, there is also an exposure component that can be harnessed by gradually increasing the exertion level.
Another approach undergoing extensive evaluation is Internet-provided cognitive-behavioral therapy (CBT). Using guided CBT modules with or without therapist support, Internet-provided CBT provides an option for motivated patients unable to complete in-person CBT because of logistical factors.b A helpful resource that reviews Internet self-help and psychotherapy guided programs for PD and other psychiatric conditions is http://beacon.anu.edu.au.
References
a. Antonacci DJ, Davis E, Bloch RM, et al. CAM for your anxious patient: what the evidence says. Current Psychiatry. 2010;9(10):42-52.
b. Johnston L, Titov N, Andrews G, et al. A RCT of a transdiagnostic internet-delivered treatment for three anxiety disorders: examination of support roles and disorder-specific outcomes. PLoS One. 2011;6(11):e28079.
Maintenance treatment
Patients who complete a course of CBT for PD often follow up with several “booster sessions” at monthly or longer intervals that focus on relapse prevention techniques. Few controlled trials have evaluated pharmacotherapy discontinuation in PD. Most guidelines recommend continuing treatment for ≥1 year after achieving remission to minimize the risk of relapse.3 Researchers are focusing on whether medication dosage can be reduced during maintenance without loss of efficacy.
Treatment discontinuation
In the absence of urgent medical need, taper medications for PD gradually over several months. PD patients are highly sensitive to unusual physical sensations, which can occur while discontinuing antidepressants or benzodiazepines. If a benzodiazepine is used in conjunction with an antidepressant, the benzodiazepine should be discontinued first, so that the antidepressant can help ease benzodiazepine-associated discontinuation symptoms. A brief course of CBT during pharmacotherapy discontinuation may increase the likelihood of successful tapering.19
CASE CONTINUED: A successful switch
Ms. K has to discontinue sequential trials of fluoxetine, 40 mg/d, and venlafaxine XR, 225 mg/d because of side effects, and she does not reduce the frequency of her alprazolam use. She agrees to switch from alprazolam to clonazepam, 0.5 mg every morning and 1 mg at bedtime, and to start CBT. Clonazepam reduces her anxiety sufficiently so she can address her symptoms in therapy. Through CBT she becomes motivated to monitor her thoughts and treat them as guesses rather than facts, reviewing the evidence for her thoughts and generating rational responses. She participates in exposure exercises, which she practices between sessions, and grows to tolerate uncomfortable sensations until they no longer signal danger. After 12 CBT sessions, she is panic-free. Despite some trepidation, she agrees to a slow taper off clonazepam, reducing the dose by 0.25 mg every 2 weeks. She continues booster sessions with her therapist to manage any re-emerging anxiety. After an additional 12 weeks, she successfully discontinues clonazepam and remains panic-free.
Related Resources
- American Psychiatric Association. Panic disorder. http://healthyminds.org/Main-Topic/Panic-Disorder.aspx.
- Anxiety and Depression Association of America. Panic disorder & agoraphobia. http://adaa.org/understanding-anxiety/panic-disorder-agoraphobia.
- Mayo Clinic. Panic attacks and panic disorder. www.mayoclinic.com/health/panic-attacks/DS00338.
- National Health Service Self-Help Guides. www.ntw.nhs.uk/pic/selfhelp.
- National Institute of Mental Health. Panic disorder. www.nimh.nih.gov/health/topics/panic-disorder/index.shtml.
Drug Brand Names
- Alprazolam • Xanax
- Alprazolam XR • Xanax XR
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Desipramine • Norpramin
- Diazepam • Valium
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Levetiracetam • Keppra
- Lorazepam • Ativan
- Milnacipran • Savella
- Mirtazapine • Remeron
- Nortriptyline • Aventyl, Pamelor
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Paroxetine CR • Paxil CR
- Phenelzine • Nardil
- Pindolol • Visken
- Quetiapine SR • Seroquel SR
- Risperidone • Risperdal
- Sertraline • Zoloft
- Venlafaxine XR • Effexor XR
Disclosures
Dr. Dunlop receives research support from Bristol-Myers Squibb, GlaxoSmithKline, and the National Institute of Mental Health. He serves as a consultant to MedAvante and Roche.
Ms. Schneider and Dr. Gerardi report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Roy-Byrne PP, Craske MG, Stein MB. Panic disorder. Lancet. 2006;368(9540):1023-1032.
2. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
3. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder. 2nd ed. Washington DC: American Psychiatric Association; 2009.
4. Dunlop BW, Davis PG. Combination treatment with benzodiazepines and SSRIs for comorbid anxiety and depression: a review. Prim Care Companion J Clin Psychiatry. 2008;10(3):222-228.
5. Rakofsky JJ, Dunlop BW. Treating nonspecific anxiety and anxiety disorders in patients with bipolar disorder: a review. J Clin Psychiatry. 2011;72(1):81-90.
6. Sareen J, Cox BJ, Afifi TO, et al. Anxiety disorders and risk for suicidal ideation and suicide attempts: a population-based longitudinal study of adults. Arch Gen Psychiatry. 2005;62(11):1249-1257.
7. Houck PR, Spiegel DA, Shear MK, et al. Reliability of the self-report version of the panic disorder severity scale. Depress Anxiety. 2002;15(4):183-185.
8. Ninan PT, Dunlop BW. Neurobiology and etiology of panic disorder. J Clin Psychiatry. 2005;66(suppl 4):3-7.
9. Furukawa TA, Watanabe N, Churchill R. Psychotherapy plus antidepressant for panic disorder with or without agoraphobia: systematic review. Br J Psychiatry. 2006;188:305-312.
10. Barlow DH, Gorman JM, Shear MK, et al. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: a randomized controlled trial. JAMA. 2000;283(19):2529-2536.
11. van Apeldoorn FJ, Timmerman ME, Mersch PP, et al. A randomized trial of cognitive-behavioral therapy or selective serotonin reuptake inhibitor or both combined for panic disorder with or without agoraphobia: treatment results through 1-year follow-up. J Clin Psychiatry. 2010;71(5):574-586.
12. Bakker A, van Balkom AJ, Spinhoven P. SSRIs vs. TCAs in the treatment of panic disorder: a meta-analysis. Acta Psychiatr Scand. 2002;106(3):163-167.
13. Bandelow B, Behnke K, Lenoir S, et al. Sertraline versus paroxetine in the treatment of panic disorder: an acute, double-blind noninferiority comparison. J Clin Psychiatry. 2004;65(3):405-413.
14. Nardi AE, Freire RC, Mochcovitch MD, et al. A randomized, naturalistic, parallel-group study for the long-term treatment of panic disorder with clonazepam or paroxetine. J Clin Psychopharmacol. 2012;32(1):120-126.
15. Goddard AW, Brouette T, Almai A, et al. Early coadministration of clonazepam with sertraline for panic disorder. Arch Gen Psychiatry. 2001;58(7):681-686.
16. Preskorn SH, Shah R, Neff M, et al. The potential for clinically significant drug-drug interactions involving the CYP 2D6 system: effects with fluoxetine and paroxetine versus sertraline. J Psychiatr Pract. 2007;13(1):5-12.
17. Perna G, Guerriero G, Caldirola D. Emerging drugs for panic disorder. Expert Opin Emerg Drugs. 2011;16(4):631-645.
18. Milrod B, Leon AC, Busch F, et al. A randomized controlled clinical trial of psychoanalytic psychotherapy for panic disorder. Am J Psychiatry. 2007;164(2):265-272.
19. Otto MW, Pollack MH, Sachs GS, et al. Discontinuation of benzodiazepine treatment: efficacy of cognitive-behavioral therapy for patients with panic disorder. Am J Psychiatry. 1993;150(10):1485-1490.
1. Roy-Byrne PP, Craske MG, Stein MB. Panic disorder. Lancet. 2006;368(9540):1023-1032.
2. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
3. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder. 2nd ed. Washington DC: American Psychiatric Association; 2009.
4. Dunlop BW, Davis PG. Combination treatment with benzodiazepines and SSRIs for comorbid anxiety and depression: a review. Prim Care Companion J Clin Psychiatry. 2008;10(3):222-228.
5. Rakofsky JJ, Dunlop BW. Treating nonspecific anxiety and anxiety disorders in patients with bipolar disorder: a review. J Clin Psychiatry. 2011;72(1):81-90.
6. Sareen J, Cox BJ, Afifi TO, et al. Anxiety disorders and risk for suicidal ideation and suicide attempts: a population-based longitudinal study of adults. Arch Gen Psychiatry. 2005;62(11):1249-1257.
7. Houck PR, Spiegel DA, Shear MK, et al. Reliability of the self-report version of the panic disorder severity scale. Depress Anxiety. 2002;15(4):183-185.
8. Ninan PT, Dunlop BW. Neurobiology and etiology of panic disorder. J Clin Psychiatry. 2005;66(suppl 4):3-7.
9. Furukawa TA, Watanabe N, Churchill R. Psychotherapy plus antidepressant for panic disorder with or without agoraphobia: systematic review. Br J Psychiatry. 2006;188:305-312.
10. Barlow DH, Gorman JM, Shear MK, et al. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: a randomized controlled trial. JAMA. 2000;283(19):2529-2536.
11. van Apeldoorn FJ, Timmerman ME, Mersch PP, et al. A randomized trial of cognitive-behavioral therapy or selective serotonin reuptake inhibitor or both combined for panic disorder with or without agoraphobia: treatment results through 1-year follow-up. J Clin Psychiatry. 2010;71(5):574-586.
12. Bakker A, van Balkom AJ, Spinhoven P. SSRIs vs. TCAs in the treatment of panic disorder: a meta-analysis. Acta Psychiatr Scand. 2002;106(3):163-167.
13. Bandelow B, Behnke K, Lenoir S, et al. Sertraline versus paroxetine in the treatment of panic disorder: an acute, double-blind noninferiority comparison. J Clin Psychiatry. 2004;65(3):405-413.
14. Nardi AE, Freire RC, Mochcovitch MD, et al. A randomized, naturalistic, parallel-group study for the long-term treatment of panic disorder with clonazepam or paroxetine. J Clin Psychopharmacol. 2012;32(1):120-126.
15. Goddard AW, Brouette T, Almai A, et al. Early coadministration of clonazepam with sertraline for panic disorder. Arch Gen Psychiatry. 2001;58(7):681-686.
16. Preskorn SH, Shah R, Neff M, et al. The potential for clinically significant drug-drug interactions involving the CYP 2D6 system: effects with fluoxetine and paroxetine versus sertraline. J Psychiatr Pract. 2007;13(1):5-12.
17. Perna G, Guerriero G, Caldirola D. Emerging drugs for panic disorder. Expert Opin Emerg Drugs. 2011;16(4):631-645.
18. Milrod B, Leon AC, Busch F, et al. A randomized controlled clinical trial of psychoanalytic psychotherapy for panic disorder. Am J Psychiatry. 2007;164(2):265-272.
19. Otto MW, Pollack MH, Sachs GS, et al. Discontinuation of benzodiazepine treatment: efficacy of cognitive-behavioral therapy for patients with panic disorder. Am J Psychiatry. 1993;150(10):1485-1490.