User login
Doctors (and Health Care) Without Borders?
I had a thought experiment based on two truths about myself: I am all for universal health care, and I would not have strong objections to receiving a fixed salary.
But I know a lot of doctors who oppose Obamacare. I can see how having to see more patients and accept lower reimbursement rates, all while filling out disability paperwork for people you believe actually do have the capacity to work might leave a bad taste in any doctor’s mouth.
So here is the thought experiment: In this increasingly interdependent world, what if we could open up licensing regulations for physicians that would allow those of us who favor universal health care to practice in Canada or other parts of the United Kingdom, for example, and those who believe in traditional insurance-based health care to practice in the United States?
Wouldn’t that be ideal? Win-win.
I thought about the lung cancer patient in Wales whose surgery, radiation, and chemotherapy were all paid for by the National Health Service. Were he to develop metastatic disease, the NHS would not pay for Tarceva (erlotinib), because the National Institute for Health and Clinical Excellence (NICE) has decided that the cost of the drug is not worth the 8 extra weeks that the patient gets.
To some, this is the very definition of fairness. Health care is universal and is paid for by taxes. What is deemed reasonable is given to you completely free, and what is not, well, isn’t. It seems to be a more thoughtful, compassionate, and just way to practice medicine, and perhaps more practical as well. Everyone gets standard of care.
For others, the above scenario is inherently unfair. Why should a government agency decide what will be good for the individual? Why should government decide that an extra 8 weeks for any individual is futile and not worth taxpayer money? People should be able to buy what they can afford, regardless of outcome. It is their money after all.
And then I realized that it is in such societies where capitalism is unbridled that pharmaceuticals thrive. Capitalism provides pharmaceutical companies with the incentives to invest in research and development. And it is precisely that R&D that leads to the kind of information that NICE needs to make decisions about drug utilization.
Said another way, the information and innovation that come from cutthroat capitalist societies is precisely what allows the nationalized health care systems to succeed.
So while I may dislike the unabashed concern for profit that I imagine motivates health insurance agencies and pharmaceutical companies (who among us, left or right leaning, didn’t find the increase in colchicine pricing offensive?), I recognize their necessity and am ultimately grateful that what they do allows societies that do provide universal health care to do so.
Bonus: Interestingly enough, I found a paper from the economics department at MIT that says basically the same thing, not just about health care, but about welfare in general.
Dr. Chan practices rheumatology in Pawtucket, R.I.
I had a thought experiment based on two truths about myself: I am all for universal health care, and I would not have strong objections to receiving a fixed salary.
But I know a lot of doctors who oppose Obamacare. I can see how having to see more patients and accept lower reimbursement rates, all while filling out disability paperwork for people you believe actually do have the capacity to work might leave a bad taste in any doctor’s mouth.
So here is the thought experiment: In this increasingly interdependent world, what if we could open up licensing regulations for physicians that would allow those of us who favor universal health care to practice in Canada or other parts of the United Kingdom, for example, and those who believe in traditional insurance-based health care to practice in the United States?
Wouldn’t that be ideal? Win-win.
I thought about the lung cancer patient in Wales whose surgery, radiation, and chemotherapy were all paid for by the National Health Service. Were he to develop metastatic disease, the NHS would not pay for Tarceva (erlotinib), because the National Institute for Health and Clinical Excellence (NICE) has decided that the cost of the drug is not worth the 8 extra weeks that the patient gets.
To some, this is the very definition of fairness. Health care is universal and is paid for by taxes. What is deemed reasonable is given to you completely free, and what is not, well, isn’t. It seems to be a more thoughtful, compassionate, and just way to practice medicine, and perhaps more practical as well. Everyone gets standard of care.
For others, the above scenario is inherently unfair. Why should a government agency decide what will be good for the individual? Why should government decide that an extra 8 weeks for any individual is futile and not worth taxpayer money? People should be able to buy what they can afford, regardless of outcome. It is their money after all.
And then I realized that it is in such societies where capitalism is unbridled that pharmaceuticals thrive. Capitalism provides pharmaceutical companies with the incentives to invest in research and development. And it is precisely that R&D that leads to the kind of information that NICE needs to make decisions about drug utilization.
Said another way, the information and innovation that come from cutthroat capitalist societies is precisely what allows the nationalized health care systems to succeed.
So while I may dislike the unabashed concern for profit that I imagine motivates health insurance agencies and pharmaceutical companies (who among us, left or right leaning, didn’t find the increase in colchicine pricing offensive?), I recognize their necessity and am ultimately grateful that what they do allows societies that do provide universal health care to do so.
Bonus: Interestingly enough, I found a paper from the economics department at MIT that says basically the same thing, not just about health care, but about welfare in general.
Dr. Chan practices rheumatology in Pawtucket, R.I.
I had a thought experiment based on two truths about myself: I am all for universal health care, and I would not have strong objections to receiving a fixed salary.
But I know a lot of doctors who oppose Obamacare. I can see how having to see more patients and accept lower reimbursement rates, all while filling out disability paperwork for people you believe actually do have the capacity to work might leave a bad taste in any doctor’s mouth.
So here is the thought experiment: In this increasingly interdependent world, what if we could open up licensing regulations for physicians that would allow those of us who favor universal health care to practice in Canada or other parts of the United Kingdom, for example, and those who believe in traditional insurance-based health care to practice in the United States?
Wouldn’t that be ideal? Win-win.
I thought about the lung cancer patient in Wales whose surgery, radiation, and chemotherapy were all paid for by the National Health Service. Were he to develop metastatic disease, the NHS would not pay for Tarceva (erlotinib), because the National Institute for Health and Clinical Excellence (NICE) has decided that the cost of the drug is not worth the 8 extra weeks that the patient gets.
To some, this is the very definition of fairness. Health care is universal and is paid for by taxes. What is deemed reasonable is given to you completely free, and what is not, well, isn’t. It seems to be a more thoughtful, compassionate, and just way to practice medicine, and perhaps more practical as well. Everyone gets standard of care.
For others, the above scenario is inherently unfair. Why should a government agency decide what will be good for the individual? Why should government decide that an extra 8 weeks for any individual is futile and not worth taxpayer money? People should be able to buy what they can afford, regardless of outcome. It is their money after all.
And then I realized that it is in such societies where capitalism is unbridled that pharmaceuticals thrive. Capitalism provides pharmaceutical companies with the incentives to invest in research and development. And it is precisely that R&D that leads to the kind of information that NICE needs to make decisions about drug utilization.
Said another way, the information and innovation that come from cutthroat capitalist societies is precisely what allows the nationalized health care systems to succeed.
So while I may dislike the unabashed concern for profit that I imagine motivates health insurance agencies and pharmaceutical companies (who among us, left or right leaning, didn’t find the increase in colchicine pricing offensive?), I recognize their necessity and am ultimately grateful that what they do allows societies that do provide universal health care to do so.
Bonus: Interestingly enough, I found a paper from the economics department at MIT that says basically the same thing, not just about health care, but about welfare in general.
Dr. Chan practices rheumatology in Pawtucket, R.I.
Panel Advises Approving Short Bowel Syndrome Drug
SILVER SPRING, MD. – A Food and Drug Administration advisory panel unanimously supported the approval of teduglutide, a recombinant analogue of human glucagon-like peptide-2 (GLP-2), as a treatment to improve the intestinal absorption of fluid and nutrients in adults with short bowel syndrome, with recommendations to follow the drug’s long-term safety, at a meeting on Oct. 16.
At a meeting in September, the FDA’s Gastrointestinal Drugs Advisory Committee voted 12 to 0 that the benefits of teduglutide outweighed the potential risks in patients with short bowel syndrome (SBS). In phase III studies of 169 adults with SBS, whose estimated small bowel length was a mean of 72 cm and who had been on parenteral nutrition and IV therapy for a mean of 7 years, teduglutide significantly reduced the volume of parenteral nutrition and IV fluids they needed after 6 months of treatment, compared with placebo.
While the panelists unanimously agreed that the results represented a clinically meaningful benefit for these patients and generally felt comfortable with the drug’s safety profile, they recommended that more safety data are needed, including determining whether the risk of colorectal cancer is increased with treatment.
Produced in the distal small intestine and proximal large intestine, GLP-2 is "an intestinotrophic peptide that stimulates mucosal epithelium," increasing absorption of fluids and nutrients, according to the manufacturer, NPS Pharmaceuticals. In studies, patients treated with teduglutide, administered subcutaneously once a week, had evidence of increased villus height after 21 days of treatment, according to the company.
In the main phase III study of 86 adults with SBS, 63% of those treated with 0.05 mg/kg daily had at least a 20% reduction in the volume of parenteral nutrition and IV fluids (the primary end point) required after 24 weeks of treatment, compared with 30% of those on placebo; this was a statistically significant difference. The volume of parenteral nutrition and IV fluids from baseline to the 24th week of treatment was reduced by a median of 4.4 L/week among patients on teduglutide, compared with 2.3 L/week for patients on placebo. Teduglutide treatment also led to a higher percentage of patients with at least one day less on therapy than did placebo (54% vs. 23%). An extension study indicated that this effect was maintained through 1 year of treatment.
While the FDA reviewers agreed that the drug had clinically meaningful effects in the studies, they raised some safety issues, mainly potential tumor-promoting effects, as well as potential GI side effects that included biliary and pancreatic disease and GI stenosis and obstruction. To date, there have been no reports of small bowel malignancies in treated patients; the three malignancies reported in treated patients have been a metastatic adenocarcinoma in a patient who had been on treatment for almost a year and lung cancer in two patients who had a history of smoking. Adenomas were seen in the bile duct and jejunum of rats at 700 times the human dose, findings that are "consistent with the pharmacological effects of the drug," according to the FDA.
There was also a low incidence of intestinal obstruction and stenosis, cholecystitis, and pancreatic disease in treated patients, compared with no cases among those on placebo.
NPS has proposed a registry of treated patients to evaluate the long-term safety and effectiveness of teduglutide, and a risk evaluation and mitigation strategy (REMS) aimed at educating prescribers about the potential and known risks of treatment. The REMS would include a letter to health care professionals and professional societies, including the American Gastroenterological Association and the American College of Gastroenterology; and drug labeling that includes contraindications in patients with a history of malignancy or with active or currently suspected malignancy, as well as warnings and precautions about the possible acceleration of neoplastic growth, colorectal polyps, and small bowel neoplasia.
The panel voted 10 to 1, with one abstention, that the company’s plans were adequate for addressing the safety concerns, although they pointed out that the number of patients in the studies was small and recommended close follow-up of treated patients.
"It seems like it’s a fairly safe drug," but follow-up is short term and fewer than 200 patients have been treated with teduglutide, said panelist Dr. Kevin Kelly, director of the division of solid tumor oncology at Thomas Jefferson University, Philadelphia. He and others recommended that patients be followed for up to 10 years.
"My gut feeling is that this is probably a very safe drug" that does not increase the risk of carcinogenesis, said another panelist, Dr. Ronald Fogel of the Digestive Health Center of Michigan in Chesterfield. He recommended more aggressive follow-up of treated patients than was proposed by the company, which he said could include serial colonoscopies at 2-year intervals with multiple biopsies at areas of dysplasia.
If teduglutide is approved, the company will market it as Gattex. About 10,000-15,000 adults in the United States with SBS are dependent on parenteral nutrition and IV fluids for fluid and nutrient replacement, according to NPS. Teduglutide was recently approved in Europe for the same indication. The FDA is expected to make a decision on approval by Dec. 30; if the drug is approved, the company plans to pursue studies in pediatric patients with SBS.
The two drugs currently approved by the FDA for people with SBS who are dependent on parenteral nutrition are somatropin rhGH (Zorbtive), a growth hormone approved in 2003, and L-glutamine powder for oral solution (Nutrestore), an adjunctive treatment approved in 2004.
The FDA usually follows the recommendations of its advisory panels, which are not binding. Panelists have been cleared of potential conflicts of interest related to the topic of the meeting. Occasionally, a panelist may be given a waiver, but not at this meeting.
SILVER SPRING, MD. – A Food and Drug Administration advisory panel unanimously supported the approval of teduglutide, a recombinant analogue of human glucagon-like peptide-2 (GLP-2), as a treatment to improve the intestinal absorption of fluid and nutrients in adults with short bowel syndrome, with recommendations to follow the drug’s long-term safety, at a meeting on Oct. 16.
At a meeting in September, the FDA’s Gastrointestinal Drugs Advisory Committee voted 12 to 0 that the benefits of teduglutide outweighed the potential risks in patients with short bowel syndrome (SBS). In phase III studies of 169 adults with SBS, whose estimated small bowel length was a mean of 72 cm and who had been on parenteral nutrition and IV therapy for a mean of 7 years, teduglutide significantly reduced the volume of parenteral nutrition and IV fluids they needed after 6 months of treatment, compared with placebo.
While the panelists unanimously agreed that the results represented a clinically meaningful benefit for these patients and generally felt comfortable with the drug’s safety profile, they recommended that more safety data are needed, including determining whether the risk of colorectal cancer is increased with treatment.
Produced in the distal small intestine and proximal large intestine, GLP-2 is "an intestinotrophic peptide that stimulates mucosal epithelium," increasing absorption of fluids and nutrients, according to the manufacturer, NPS Pharmaceuticals. In studies, patients treated with teduglutide, administered subcutaneously once a week, had evidence of increased villus height after 21 days of treatment, according to the company.
In the main phase III study of 86 adults with SBS, 63% of those treated with 0.05 mg/kg daily had at least a 20% reduction in the volume of parenteral nutrition and IV fluids (the primary end point) required after 24 weeks of treatment, compared with 30% of those on placebo; this was a statistically significant difference. The volume of parenteral nutrition and IV fluids from baseline to the 24th week of treatment was reduced by a median of 4.4 L/week among patients on teduglutide, compared with 2.3 L/week for patients on placebo. Teduglutide treatment also led to a higher percentage of patients with at least one day less on therapy than did placebo (54% vs. 23%). An extension study indicated that this effect was maintained through 1 year of treatment.
While the FDA reviewers agreed that the drug had clinically meaningful effects in the studies, they raised some safety issues, mainly potential tumor-promoting effects, as well as potential GI side effects that included biliary and pancreatic disease and GI stenosis and obstruction. To date, there have been no reports of small bowel malignancies in treated patients; the three malignancies reported in treated patients have been a metastatic adenocarcinoma in a patient who had been on treatment for almost a year and lung cancer in two patients who had a history of smoking. Adenomas were seen in the bile duct and jejunum of rats at 700 times the human dose, findings that are "consistent with the pharmacological effects of the drug," according to the FDA.
There was also a low incidence of intestinal obstruction and stenosis, cholecystitis, and pancreatic disease in treated patients, compared with no cases among those on placebo.
NPS has proposed a registry of treated patients to evaluate the long-term safety and effectiveness of teduglutide, and a risk evaluation and mitigation strategy (REMS) aimed at educating prescribers about the potential and known risks of treatment. The REMS would include a letter to health care professionals and professional societies, including the American Gastroenterological Association and the American College of Gastroenterology; and drug labeling that includes contraindications in patients with a history of malignancy or with active or currently suspected malignancy, as well as warnings and precautions about the possible acceleration of neoplastic growth, colorectal polyps, and small bowel neoplasia.
The panel voted 10 to 1, with one abstention, that the company’s plans were adequate for addressing the safety concerns, although they pointed out that the number of patients in the studies was small and recommended close follow-up of treated patients.
"It seems like it’s a fairly safe drug," but follow-up is short term and fewer than 200 patients have been treated with teduglutide, said panelist Dr. Kevin Kelly, director of the division of solid tumor oncology at Thomas Jefferson University, Philadelphia. He and others recommended that patients be followed for up to 10 years.
"My gut feeling is that this is probably a very safe drug" that does not increase the risk of carcinogenesis, said another panelist, Dr. Ronald Fogel of the Digestive Health Center of Michigan in Chesterfield. He recommended more aggressive follow-up of treated patients than was proposed by the company, which he said could include serial colonoscopies at 2-year intervals with multiple biopsies at areas of dysplasia.
If teduglutide is approved, the company will market it as Gattex. About 10,000-15,000 adults in the United States with SBS are dependent on parenteral nutrition and IV fluids for fluid and nutrient replacement, according to NPS. Teduglutide was recently approved in Europe for the same indication. The FDA is expected to make a decision on approval by Dec. 30; if the drug is approved, the company plans to pursue studies in pediatric patients with SBS.
The two drugs currently approved by the FDA for people with SBS who are dependent on parenteral nutrition are somatropin rhGH (Zorbtive), a growth hormone approved in 2003, and L-glutamine powder for oral solution (Nutrestore), an adjunctive treatment approved in 2004.
The FDA usually follows the recommendations of its advisory panels, which are not binding. Panelists have been cleared of potential conflicts of interest related to the topic of the meeting. Occasionally, a panelist may be given a waiver, but not at this meeting.
SILVER SPRING, MD. – A Food and Drug Administration advisory panel unanimously supported the approval of teduglutide, a recombinant analogue of human glucagon-like peptide-2 (GLP-2), as a treatment to improve the intestinal absorption of fluid and nutrients in adults with short bowel syndrome, with recommendations to follow the drug’s long-term safety, at a meeting on Oct. 16.
At a meeting in September, the FDA’s Gastrointestinal Drugs Advisory Committee voted 12 to 0 that the benefits of teduglutide outweighed the potential risks in patients with short bowel syndrome (SBS). In phase III studies of 169 adults with SBS, whose estimated small bowel length was a mean of 72 cm and who had been on parenteral nutrition and IV therapy for a mean of 7 years, teduglutide significantly reduced the volume of parenteral nutrition and IV fluids they needed after 6 months of treatment, compared with placebo.
While the panelists unanimously agreed that the results represented a clinically meaningful benefit for these patients and generally felt comfortable with the drug’s safety profile, they recommended that more safety data are needed, including determining whether the risk of colorectal cancer is increased with treatment.
Produced in the distal small intestine and proximal large intestine, GLP-2 is "an intestinotrophic peptide that stimulates mucosal epithelium," increasing absorption of fluids and nutrients, according to the manufacturer, NPS Pharmaceuticals. In studies, patients treated with teduglutide, administered subcutaneously once a week, had evidence of increased villus height after 21 days of treatment, according to the company.
In the main phase III study of 86 adults with SBS, 63% of those treated with 0.05 mg/kg daily had at least a 20% reduction in the volume of parenteral nutrition and IV fluids (the primary end point) required after 24 weeks of treatment, compared with 30% of those on placebo; this was a statistically significant difference. The volume of parenteral nutrition and IV fluids from baseline to the 24th week of treatment was reduced by a median of 4.4 L/week among patients on teduglutide, compared with 2.3 L/week for patients on placebo. Teduglutide treatment also led to a higher percentage of patients with at least one day less on therapy than did placebo (54% vs. 23%). An extension study indicated that this effect was maintained through 1 year of treatment.
While the FDA reviewers agreed that the drug had clinically meaningful effects in the studies, they raised some safety issues, mainly potential tumor-promoting effects, as well as potential GI side effects that included biliary and pancreatic disease and GI stenosis and obstruction. To date, there have been no reports of small bowel malignancies in treated patients; the three malignancies reported in treated patients have been a metastatic adenocarcinoma in a patient who had been on treatment for almost a year and lung cancer in two patients who had a history of smoking. Adenomas were seen in the bile duct and jejunum of rats at 700 times the human dose, findings that are "consistent with the pharmacological effects of the drug," according to the FDA.
There was also a low incidence of intestinal obstruction and stenosis, cholecystitis, and pancreatic disease in treated patients, compared with no cases among those on placebo.
NPS has proposed a registry of treated patients to evaluate the long-term safety and effectiveness of teduglutide, and a risk evaluation and mitigation strategy (REMS) aimed at educating prescribers about the potential and known risks of treatment. The REMS would include a letter to health care professionals and professional societies, including the American Gastroenterological Association and the American College of Gastroenterology; and drug labeling that includes contraindications in patients with a history of malignancy or with active or currently suspected malignancy, as well as warnings and precautions about the possible acceleration of neoplastic growth, colorectal polyps, and small bowel neoplasia.
The panel voted 10 to 1, with one abstention, that the company’s plans were adequate for addressing the safety concerns, although they pointed out that the number of patients in the studies was small and recommended close follow-up of treated patients.
"It seems like it’s a fairly safe drug," but follow-up is short term and fewer than 200 patients have been treated with teduglutide, said panelist Dr. Kevin Kelly, director of the division of solid tumor oncology at Thomas Jefferson University, Philadelphia. He and others recommended that patients be followed for up to 10 years.
"My gut feeling is that this is probably a very safe drug" that does not increase the risk of carcinogenesis, said another panelist, Dr. Ronald Fogel of the Digestive Health Center of Michigan in Chesterfield. He recommended more aggressive follow-up of treated patients than was proposed by the company, which he said could include serial colonoscopies at 2-year intervals with multiple biopsies at areas of dysplasia.
If teduglutide is approved, the company will market it as Gattex. About 10,000-15,000 adults in the United States with SBS are dependent on parenteral nutrition and IV fluids for fluid and nutrient replacement, according to NPS. Teduglutide was recently approved in Europe for the same indication. The FDA is expected to make a decision on approval by Dec. 30; if the drug is approved, the company plans to pursue studies in pediatric patients with SBS.
The two drugs currently approved by the FDA for people with SBS who are dependent on parenteral nutrition are somatropin rhGH (Zorbtive), a growth hormone approved in 2003, and L-glutamine powder for oral solution (Nutrestore), an adjunctive treatment approved in 2004.
The FDA usually follows the recommendations of its advisory panels, which are not binding. Panelists have been cleared of potential conflicts of interest related to the topic of the meeting. Occasionally, a panelist may be given a waiver, but not at this meeting.
Cheerleader Injury Rates Prompt AAP Statement
NEW ORLEANS – The increasing rate of injuries, especially catastrophic ones, among cheerleaders has prompted the American Academy of Pediatrics to publish its first policy statement on the competitive, year-round activity.
Although the overall risk of injury in cheerleading is lower than for other sports, it accounted for 65% of all catastrophic injuries to high school female athletes and almost 71% of those in college during a 30-year period ending in 2009.
"The academy feels that cheerleading is a valuable activity," said report author Dr. Cynthia LaBella at the annual meeting of the American Academy of Pediatrics. "We just want to make sure that [the cheerleaders] have the same safety precautions in place as those participating in other sports do," noted Dr. LaBella, medical director of the Institute for Sports Medicine at Ann & Robert H. Lurie Children’s Hospital of Chicago.
The American Academy of Pediatrics (AAP) has made 12 recommendations to help reduce cheerleading injuries, including a call for cheerleading to be designated as a sport "so that it is subject to rules and regulations set forth by sports governing bodies ... and school athletic departments," according to the report (Pediatrics 2012;130:966-971).
Cheerleading has become increasingly popular, with approximately 400,000 participants in high school cheerleading alone, according to 2009 data from the National Federation of State High School Associations. Yet only 29 state high school athletic associations recognize cheerleading as a sport, according to the AAP report.
Being classified as a sport, the academy points out, brings safety resources and regulations such as qualified coaches as well as access to athletic trainers and team physicians.
The academy also recommends that cheerleaders undergo a preparticipation physical examination and have access to appropriate strength and conditioning programs.
The catastrophic injury rate among female high school cheerleaders was between 0.5 and 1.62, compared with 0.44 for gymnastics. The rate was 0.3 or lower for female athletes participating in sports such as soccer, basketball, and softball. Catastrophic injuries included closed-head injury, skull fractures, cervical spine injuries, paralysis, and death.
Dr. LaBella said the lower rate of catastrophic injury among gymnasts could be due to the level of supervision, better conditioning programs, and more protective equipment, including flooring.
The AAP also recommends that cheerleaders be supervised by qualified coaches who have been properly trained and certified, and avoid performing certain technical skills on hard, wet, or uneven surfaces. "No cheer events should take place on dirt, vinyl floors, concrete, or asphalt," wrote Dr. LaBella and coauthor Dr. Jeffrey Mjaanes, of the departments of orthopedic surgery and pediatrics at Rush University Medical Center, Chicago.
In addition, the AAP recommends that any cheerleader who shows signs of a head injury "should be removed from practice or competition and not be allowed to return until he or she has received written clearance from a physician or a qualified health care provider."
The number of U.S. cheerleaders 6 years and older increased from 3.0 million in 1990 to 3.6 million in 2003, and girls represented 96% of the participants, according to the report.
Middle and high school cheerleaders have lower overall injury rates than collegiate-level cheerleaders, partly because older cheerleaders are better skilled and tend to perform more complex gymnastics and height-based stunts, the authors noted.
Meanwhile, the number of hospital emergency department visits for cheerleading injuries rose from roughly 5,000 in 1980 to 27,000 in 2007, a 400% increase. Of these injuries, 98% were treated and released.
Lower-extremity injuries are the most common injuries (30%-37%), followed by upper-extremities injuries (16%-19%) and trunk injuries (7%-17%).
Concussions and other closed-head injuries account for 4%-6% of all cheerleading injuries, according to the AAP report. Meanwhile, head and neck injuries account for roughly 15% of all cheerleading injuries seen in emergency departments.
Although the concussion rate in cheerleading is low (0.06 per 1,000 exposures) compared with other girls’ sports such as soccer (0.36), the rate increased by 26% annually from 1998 to 2008.
"Our goal is to make people more aware of the potential injury risk," said Dr. LaBella, who is also an associate professor of pediatrics at Northwestern University in Chicago.
She encouraged cheerleaders and those who supervise them to report the injuries. "That knowledge of what's going on with injuries will help with making further recommendations for the future."
Dr. LaBella had no disclosures.
NEW ORLEANS – The increasing rate of injuries, especially catastrophic ones, among cheerleaders has prompted the American Academy of Pediatrics to publish its first policy statement on the competitive, year-round activity.
Although the overall risk of injury in cheerleading is lower than for other sports, it accounted for 65% of all catastrophic injuries to high school female athletes and almost 71% of those in college during a 30-year period ending in 2009.
"The academy feels that cheerleading is a valuable activity," said report author Dr. Cynthia LaBella at the annual meeting of the American Academy of Pediatrics. "We just want to make sure that [the cheerleaders] have the same safety precautions in place as those participating in other sports do," noted Dr. LaBella, medical director of the Institute for Sports Medicine at Ann & Robert H. Lurie Children’s Hospital of Chicago.
The American Academy of Pediatrics (AAP) has made 12 recommendations to help reduce cheerleading injuries, including a call for cheerleading to be designated as a sport "so that it is subject to rules and regulations set forth by sports governing bodies ... and school athletic departments," according to the report (Pediatrics 2012;130:966-971).
Cheerleading has become increasingly popular, with approximately 400,000 participants in high school cheerleading alone, according to 2009 data from the National Federation of State High School Associations. Yet only 29 state high school athletic associations recognize cheerleading as a sport, according to the AAP report.
Being classified as a sport, the academy points out, brings safety resources and regulations such as qualified coaches as well as access to athletic trainers and team physicians.
The academy also recommends that cheerleaders undergo a preparticipation physical examination and have access to appropriate strength and conditioning programs.
The catastrophic injury rate among female high school cheerleaders was between 0.5 and 1.62, compared with 0.44 for gymnastics. The rate was 0.3 or lower for female athletes participating in sports such as soccer, basketball, and softball. Catastrophic injuries included closed-head injury, skull fractures, cervical spine injuries, paralysis, and death.
Dr. LaBella said the lower rate of catastrophic injury among gymnasts could be due to the level of supervision, better conditioning programs, and more protective equipment, including flooring.
The AAP also recommends that cheerleaders be supervised by qualified coaches who have been properly trained and certified, and avoid performing certain technical skills on hard, wet, or uneven surfaces. "No cheer events should take place on dirt, vinyl floors, concrete, or asphalt," wrote Dr. LaBella and coauthor Dr. Jeffrey Mjaanes, of the departments of orthopedic surgery and pediatrics at Rush University Medical Center, Chicago.
In addition, the AAP recommends that any cheerleader who shows signs of a head injury "should be removed from practice or competition and not be allowed to return until he or she has received written clearance from a physician or a qualified health care provider."
The number of U.S. cheerleaders 6 years and older increased from 3.0 million in 1990 to 3.6 million in 2003, and girls represented 96% of the participants, according to the report.
Middle and high school cheerleaders have lower overall injury rates than collegiate-level cheerleaders, partly because older cheerleaders are better skilled and tend to perform more complex gymnastics and height-based stunts, the authors noted.
Meanwhile, the number of hospital emergency department visits for cheerleading injuries rose from roughly 5,000 in 1980 to 27,000 in 2007, a 400% increase. Of these injuries, 98% were treated and released.
Lower-extremity injuries are the most common injuries (30%-37%), followed by upper-extremities injuries (16%-19%) and trunk injuries (7%-17%).
Concussions and other closed-head injuries account for 4%-6% of all cheerleading injuries, according to the AAP report. Meanwhile, head and neck injuries account for roughly 15% of all cheerleading injuries seen in emergency departments.
Although the concussion rate in cheerleading is low (0.06 per 1,000 exposures) compared with other girls’ sports such as soccer (0.36), the rate increased by 26% annually from 1998 to 2008.
"Our goal is to make people more aware of the potential injury risk," said Dr. LaBella, who is also an associate professor of pediatrics at Northwestern University in Chicago.
She encouraged cheerleaders and those who supervise them to report the injuries. "That knowledge of what's going on with injuries will help with making further recommendations for the future."
Dr. LaBella had no disclosures.
NEW ORLEANS – The increasing rate of injuries, especially catastrophic ones, among cheerleaders has prompted the American Academy of Pediatrics to publish its first policy statement on the competitive, year-round activity.
Although the overall risk of injury in cheerleading is lower than for other sports, it accounted for 65% of all catastrophic injuries to high school female athletes and almost 71% of those in college during a 30-year period ending in 2009.
"The academy feels that cheerleading is a valuable activity," said report author Dr. Cynthia LaBella at the annual meeting of the American Academy of Pediatrics. "We just want to make sure that [the cheerleaders] have the same safety precautions in place as those participating in other sports do," noted Dr. LaBella, medical director of the Institute for Sports Medicine at Ann & Robert H. Lurie Children’s Hospital of Chicago.
The American Academy of Pediatrics (AAP) has made 12 recommendations to help reduce cheerleading injuries, including a call for cheerleading to be designated as a sport "so that it is subject to rules and regulations set forth by sports governing bodies ... and school athletic departments," according to the report (Pediatrics 2012;130:966-971).
Cheerleading has become increasingly popular, with approximately 400,000 participants in high school cheerleading alone, according to 2009 data from the National Federation of State High School Associations. Yet only 29 state high school athletic associations recognize cheerleading as a sport, according to the AAP report.
Being classified as a sport, the academy points out, brings safety resources and regulations such as qualified coaches as well as access to athletic trainers and team physicians.
The academy also recommends that cheerleaders undergo a preparticipation physical examination and have access to appropriate strength and conditioning programs.
The catastrophic injury rate among female high school cheerleaders was between 0.5 and 1.62, compared with 0.44 for gymnastics. The rate was 0.3 or lower for female athletes participating in sports such as soccer, basketball, and softball. Catastrophic injuries included closed-head injury, skull fractures, cervical spine injuries, paralysis, and death.
Dr. LaBella said the lower rate of catastrophic injury among gymnasts could be due to the level of supervision, better conditioning programs, and more protective equipment, including flooring.
The AAP also recommends that cheerleaders be supervised by qualified coaches who have been properly trained and certified, and avoid performing certain technical skills on hard, wet, or uneven surfaces. "No cheer events should take place on dirt, vinyl floors, concrete, or asphalt," wrote Dr. LaBella and coauthor Dr. Jeffrey Mjaanes, of the departments of orthopedic surgery and pediatrics at Rush University Medical Center, Chicago.
In addition, the AAP recommends that any cheerleader who shows signs of a head injury "should be removed from practice or competition and not be allowed to return until he or she has received written clearance from a physician or a qualified health care provider."
The number of U.S. cheerleaders 6 years and older increased from 3.0 million in 1990 to 3.6 million in 2003, and girls represented 96% of the participants, according to the report.
Middle and high school cheerleaders have lower overall injury rates than collegiate-level cheerleaders, partly because older cheerleaders are better skilled and tend to perform more complex gymnastics and height-based stunts, the authors noted.
Meanwhile, the number of hospital emergency department visits for cheerleading injuries rose from roughly 5,000 in 1980 to 27,000 in 2007, a 400% increase. Of these injuries, 98% were treated and released.
Lower-extremity injuries are the most common injuries (30%-37%), followed by upper-extremities injuries (16%-19%) and trunk injuries (7%-17%).
Concussions and other closed-head injuries account for 4%-6% of all cheerleading injuries, according to the AAP report. Meanwhile, head and neck injuries account for roughly 15% of all cheerleading injuries seen in emergency departments.
Although the concussion rate in cheerleading is low (0.06 per 1,000 exposures) compared with other girls’ sports such as soccer (0.36), the rate increased by 26% annually from 1998 to 2008.
"Our goal is to make people more aware of the potential injury risk," said Dr. LaBella, who is also an associate professor of pediatrics at Northwestern University in Chicago.
She encouraged cheerleaders and those who supervise them to report the injuries. "That knowledge of what's going on with injuries will help with making further recommendations for the future."
Dr. LaBella had no disclosures.
AT THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF PEDIATRICS
Beta-Blockers and Acute Myocardial Infarction
From the time that propranolol significantly lowered mortality after an acute myocardial infarction in the Beta-Blocker Heart Attack Trial in 1981, it took nearly 20 years for beta-blocker therapy to take hold as standard practice in AMI patients. Now, results of a recent trial may cause many to question the established therapy.
The Nov. 6, 1981, issue of JAMA announced that the National Heart, Lung, and Blood Institute had "taken the unusual step of curtailing" the Beta-Blocker Heart Attack Trial (BHAT) on the basis of findings that treatment of patients with the beta-adrenergic blocking agent, propranolol, resulted in a 26% decrease in all-cause mortality and a 23% decrease in sudden death (JAMA 1982;247:1707-14).
The study included 3,837 patients treated within 5-21 days of an acute myocardial infarction (AMI) and randomized to either propranolol 160-240 mg/day or placebo. Two-thirds of the patients had a ST-elevation MI; the remaining patients had symptoms compatible with an AMI with electrocardiographic changes accompanied by an increase in serum enzymatic elevations (serum glutamic oxaloacetic transaminase or creatine phosphokinase). This followed the report of similar results in Europe with the beta-blocker timolol in a similar group of patients. Since those early reports of randomized clinical trials, based on a subgroup analysis of BHAT, confirmed the benefit of beta-blocker therapy for both ischemic and nonischemic systolic heart failure. As steering committee chair of BHAT, I was excited about the result of our study and anticipated that beta-blocker therapy would rapidly become part of the treatment of AMI.
This was not to be. It took almost 20 years before beta-blocker therapy was incorporated into the standard treatment of AMI patients. In the interval, thousands of patients who could have benefited with this therapy died. As late as 1998, fewer than 50% of AMI patients without contraindication to therapy received that class of drug.
Why did it take so long? At the time of the BHAT results, many of the leading academic cardiologists were enamored with the use of calcium entry blocking agents for AMI, for which there were little data but a lot of encouragement by pharmaceutical companies. When propranolol went off patent and became available as a generic, there was little industrial support to publicize its benefit. Furthermore, there was little interest at the National Heart, Lung, and Blood Institute to educate physicians about the importance of BHAT. In 1996, beta-blocker therapy post-AMI was established as a quality standard by the National Committee for Quality Assurance (NCQA). At about the same time, it was incorporated in the American College of Cardiology guidelines. Not until 2000, 19 years after the initial report of beta-blocker therapy, did its usage reach 90% at discharge. A recent study from the NCQA indicated that 6 months after discharge only 71% of patients were taking the medication.
In the intervening 2 decades, the definition of an AMI has dramatically changed as a result of more sensitive, if less specific, enzyme measurements. In 1981, most of the patients in BHAT had a STEMI, whereas contemporary clinical trials include less than one-third STEMI patients. Therapy certainly has changed: first with the use of thrombolytic therapy and subsequently with the wide use of interventional angioplasty technology, particularly in the STEMI population. Aspirin, statins, and ACE inhibitors have also been added to the therapeutic mix.
Now, an observational study of almost 7,000 patients with a history of an AMI collected in 2004 and followed for 43 months, suggests that beta-blocker therapy is no longer necessary. Using a composite end point including cardiovascular death, nonfatal MI, or stroke, patients receiving propranolol had an event rate of 16.9%, compared with 18.6% not taking beta-blocker (hazard ratio, .90; P less than .14) (JAMA 2012;308:1340-9). It should be noted, however, that 74% of the patients in the study had a history of hypertension, 44% angina, and 22% heart failure, all clinical problems for which beta-blockers have been proven to be effective.
The most recent American College of Cardiology/American Heart Association guidelines suggest that beta-blocker therapy "is greatest (benefit) among patients with recent myocardial infarction [of up to 3 years prior] and/or left ventricular systolic dysfunction [left ventricular ejection fraction of 40% or less]. For those patient without these class I indication, [beta]-blocker therapy is optional (class IIa or IIb) (Circulation 2011;124:2458-73). I suppose that if you can find an AMI patient without hypertension, angina, or heart failure, discontinuing beta-blocker therapy could be justified. Until that rare patient appears in my office, I plan to maintain beta-blockers in my post-AMI patients.
Dr. Goldstein, medical editor of Cardiology News, is a professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
This column, "Heart of the Matter," appears regularly in Cardiology News.
From the time that propranolol significantly lowered mortality after an acute myocardial infarction in the Beta-Blocker Heart Attack Trial in 1981, it took nearly 20 years for beta-blocker therapy to take hold as standard practice in AMI patients. Now, results of a recent trial may cause many to question the established therapy.
The Nov. 6, 1981, issue of JAMA announced that the National Heart, Lung, and Blood Institute had "taken the unusual step of curtailing" the Beta-Blocker Heart Attack Trial (BHAT) on the basis of findings that treatment of patients with the beta-adrenergic blocking agent, propranolol, resulted in a 26% decrease in all-cause mortality and a 23% decrease in sudden death (JAMA 1982;247:1707-14).
The study included 3,837 patients treated within 5-21 days of an acute myocardial infarction (AMI) and randomized to either propranolol 160-240 mg/day or placebo. Two-thirds of the patients had a ST-elevation MI; the remaining patients had symptoms compatible with an AMI with electrocardiographic changes accompanied by an increase in serum enzymatic elevations (serum glutamic oxaloacetic transaminase or creatine phosphokinase). This followed the report of similar results in Europe with the beta-blocker timolol in a similar group of patients. Since those early reports of randomized clinical trials, based on a subgroup analysis of BHAT, confirmed the benefit of beta-blocker therapy for both ischemic and nonischemic systolic heart failure. As steering committee chair of BHAT, I was excited about the result of our study and anticipated that beta-blocker therapy would rapidly become part of the treatment of AMI.
This was not to be. It took almost 20 years before beta-blocker therapy was incorporated into the standard treatment of AMI patients. In the interval, thousands of patients who could have benefited with this therapy died. As late as 1998, fewer than 50% of AMI patients without contraindication to therapy received that class of drug.
Why did it take so long? At the time of the BHAT results, many of the leading academic cardiologists were enamored with the use of calcium entry blocking agents for AMI, for which there were little data but a lot of encouragement by pharmaceutical companies. When propranolol went off patent and became available as a generic, there was little industrial support to publicize its benefit. Furthermore, there was little interest at the National Heart, Lung, and Blood Institute to educate physicians about the importance of BHAT. In 1996, beta-blocker therapy post-AMI was established as a quality standard by the National Committee for Quality Assurance (NCQA). At about the same time, it was incorporated in the American College of Cardiology guidelines. Not until 2000, 19 years after the initial report of beta-blocker therapy, did its usage reach 90% at discharge. A recent study from the NCQA indicated that 6 months after discharge only 71% of patients were taking the medication.
In the intervening 2 decades, the definition of an AMI has dramatically changed as a result of more sensitive, if less specific, enzyme measurements. In 1981, most of the patients in BHAT had a STEMI, whereas contemporary clinical trials include less than one-third STEMI patients. Therapy certainly has changed: first with the use of thrombolytic therapy and subsequently with the wide use of interventional angioplasty technology, particularly in the STEMI population. Aspirin, statins, and ACE inhibitors have also been added to the therapeutic mix.
Now, an observational study of almost 7,000 patients with a history of an AMI collected in 2004 and followed for 43 months, suggests that beta-blocker therapy is no longer necessary. Using a composite end point including cardiovascular death, nonfatal MI, or stroke, patients receiving propranolol had an event rate of 16.9%, compared with 18.6% not taking beta-blocker (hazard ratio, .90; P less than .14) (JAMA 2012;308:1340-9). It should be noted, however, that 74% of the patients in the study had a history of hypertension, 44% angina, and 22% heart failure, all clinical problems for which beta-blockers have been proven to be effective.
The most recent American College of Cardiology/American Heart Association guidelines suggest that beta-blocker therapy "is greatest (benefit) among patients with recent myocardial infarction [of up to 3 years prior] and/or left ventricular systolic dysfunction [left ventricular ejection fraction of 40% or less]. For those patient without these class I indication, [beta]-blocker therapy is optional (class IIa or IIb) (Circulation 2011;124:2458-73). I suppose that if you can find an AMI patient without hypertension, angina, or heart failure, discontinuing beta-blocker therapy could be justified. Until that rare patient appears in my office, I plan to maintain beta-blockers in my post-AMI patients.
Dr. Goldstein, medical editor of Cardiology News, is a professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
This column, "Heart of the Matter," appears regularly in Cardiology News.
From the time that propranolol significantly lowered mortality after an acute myocardial infarction in the Beta-Blocker Heart Attack Trial in 1981, it took nearly 20 years for beta-blocker therapy to take hold as standard practice in AMI patients. Now, results of a recent trial may cause many to question the established therapy.
The Nov. 6, 1981, issue of JAMA announced that the National Heart, Lung, and Blood Institute had "taken the unusual step of curtailing" the Beta-Blocker Heart Attack Trial (BHAT) on the basis of findings that treatment of patients with the beta-adrenergic blocking agent, propranolol, resulted in a 26% decrease in all-cause mortality and a 23% decrease in sudden death (JAMA 1982;247:1707-14).
The study included 3,837 patients treated within 5-21 days of an acute myocardial infarction (AMI) and randomized to either propranolol 160-240 mg/day or placebo. Two-thirds of the patients had a ST-elevation MI; the remaining patients had symptoms compatible with an AMI with electrocardiographic changes accompanied by an increase in serum enzymatic elevations (serum glutamic oxaloacetic transaminase or creatine phosphokinase). This followed the report of similar results in Europe with the beta-blocker timolol in a similar group of patients. Since those early reports of randomized clinical trials, based on a subgroup analysis of BHAT, confirmed the benefit of beta-blocker therapy for both ischemic and nonischemic systolic heart failure. As steering committee chair of BHAT, I was excited about the result of our study and anticipated that beta-blocker therapy would rapidly become part of the treatment of AMI.
This was not to be. It took almost 20 years before beta-blocker therapy was incorporated into the standard treatment of AMI patients. In the interval, thousands of patients who could have benefited with this therapy died. As late as 1998, fewer than 50% of AMI patients without contraindication to therapy received that class of drug.
Why did it take so long? At the time of the BHAT results, many of the leading academic cardiologists were enamored with the use of calcium entry blocking agents for AMI, for which there were little data but a lot of encouragement by pharmaceutical companies. When propranolol went off patent and became available as a generic, there was little industrial support to publicize its benefit. Furthermore, there was little interest at the National Heart, Lung, and Blood Institute to educate physicians about the importance of BHAT. In 1996, beta-blocker therapy post-AMI was established as a quality standard by the National Committee for Quality Assurance (NCQA). At about the same time, it was incorporated in the American College of Cardiology guidelines. Not until 2000, 19 years after the initial report of beta-blocker therapy, did its usage reach 90% at discharge. A recent study from the NCQA indicated that 6 months after discharge only 71% of patients were taking the medication.
In the intervening 2 decades, the definition of an AMI has dramatically changed as a result of more sensitive, if less specific, enzyme measurements. In 1981, most of the patients in BHAT had a STEMI, whereas contemporary clinical trials include less than one-third STEMI patients. Therapy certainly has changed: first with the use of thrombolytic therapy and subsequently with the wide use of interventional angioplasty technology, particularly in the STEMI population. Aspirin, statins, and ACE inhibitors have also been added to the therapeutic mix.
Now, an observational study of almost 7,000 patients with a history of an AMI collected in 2004 and followed for 43 months, suggests that beta-blocker therapy is no longer necessary. Using a composite end point including cardiovascular death, nonfatal MI, or stroke, patients receiving propranolol had an event rate of 16.9%, compared with 18.6% not taking beta-blocker (hazard ratio, .90; P less than .14) (JAMA 2012;308:1340-9). It should be noted, however, that 74% of the patients in the study had a history of hypertension, 44% angina, and 22% heart failure, all clinical problems for which beta-blockers have been proven to be effective.
The most recent American College of Cardiology/American Heart Association guidelines suggest that beta-blocker therapy "is greatest (benefit) among patients with recent myocardial infarction [of up to 3 years prior] and/or left ventricular systolic dysfunction [left ventricular ejection fraction of 40% or less]. For those patient without these class I indication, [beta]-blocker therapy is optional (class IIa or IIb) (Circulation 2011;124:2458-73). I suppose that if you can find an AMI patient without hypertension, angina, or heart failure, discontinuing beta-blocker therapy could be justified. Until that rare patient appears in my office, I plan to maintain beta-blockers in my post-AMI patients.
Dr. Goldstein, medical editor of Cardiology News, is a professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
This column, "Heart of the Matter," appears regularly in Cardiology News.
Infant Meningococcal Vaccine: Why Not?
Haemophilus influenzae type B-Neisseria meningitidis serogroups C (MenC) and Y (MenY)-tetanus toxoid (Hib-MenCY-TT, MenHibrix) vaccine has been approved by the Food and Drug Administration for an infant indication to be administered according to the standard 2-, 4-, 6-, and 12 months vaccine schedule in the United States as endorsed by the American Academy of Pediatrics (AAP), the American Academy of Family Physicians (AAFP), and the Centers for Disease Control and Prevention (CDC).
According to the presentation given at the CDC meeting Oct. 14, 2011, by Dr. Ismael Ortega-Sanchez, giving the vaccine could prevent 130 of the projected 377 (34.5%) cases of meningococcal infection in the 4-million-child birth cohort of the United States cumulatively to age 10 years. Also infant vaccination would prevent one death per 642,000 infants (seven deaths/year). The vaccine could be given along with a DTaP/inactivated polio vaccine/hepatitis b vaccine (DTaP/IPV/HepB, Pediarix) and the 13-valent pneumococcal conjugate vaccine (PCV13, Prevnar13) without increasing the total number of shots in a visit. The vaccine has been proven safe and effective.
Yet at the October meeting of the CDC Advisory Committee on Immunization Practices (ACIP), Hib-MenCY-TT (MenHibrix) vaccine was not recommended for universal use. Instead, it was recommended for high risk children as previously defined as complement deficient and asplenic. Why the restricted recommendation?*
• It isn’t the right time. When the ACIP/AAP/AAFP endorsed meningococcal vaccination for 11- to 12-year-olds 7 years ago, the annual incidence of meningococcal disease was fivefold higher than it is now. The drop in incidence cannot be fully attributed to the initiation of our national vaccination campaign. It was known before the meningococcal conjugate vaccine was recommended that meningococcal disease had a cyclical pattern with high and low years of incidence. But, had the incidence been as low as it is now, then one might speculate that the vote to recommend universal vaccination might have been different. The passionate pleas of concerned parents and the desire by all of us in health care to protect every single adolescent against the devastation of meningococcal infections carried the day, even though the cost for prevention of those cases and deaths was the highest ever seen up to that time. So, if the incidence of meningococcal infections is now at an all time low, the calculations of cost to prevent cases and deaths would be a multiple of what it was 7 years ago.
• The vaccine doesn’t include all the serotypes. Hib-MenCY-TT has meningococcal serotypes C and Y. The vaccine does not include serotype A or serotype W-135 (because these serotypes are virtually absent and uncommon, respectively, in the United States and other developed countries. So, a concern could be that serotype replacement might occur over time as we have seen with Prevnar7, now replaced in the United States with Prevnar13 because of serotype replacement. But more importantly is the absence of serotype B in the vaccine. Serotype B meningococci cause 60%-65% of meningococcal disease in the United States in infants. That is why the number of cases projected to be prevented with Hib-MenCY-TT is about one-third of all cases among infants.
• The total number of shots goes up per visit unless GlaxoSmithKline vaccines are preferentially used. Hib-MenCY-TT was developed and is licensed by GlaxoSmithKline, a world leader in pediatric vaccines, and they are building a portfolio of vaccines that can fit together well. There is nothing wrong with that – it is good marketing. Sanofi Pasteur Vaccines is doing the same thing and, as more products are forthcoming from Pfizer Vaccines, Novartis Vaccines, and others, we can expect the same strategy. However, the CDC, AAP, and AAFP do not want to endorse products that limit choices and/or provide any single company with a competitive advantage. So, to endorse Hib-MenCY-TT that clearly fits best with only GlaxoSmithKline vaccine products may be an unspoken concern.
• The National Immunization Program cannot afford it. Going back to the presentation to ACIP at the CDC in October 2011, a key aspect was the cost of vaccination calculated against cases prevented and lives saved. The calculation for Quality-Adjusted Life Year (QALY) saved for a two-dose schedule among adolescents came out to $157,000/case. For the infant vaccination, the numbers were pretty staggering at $3.6 million/case, based on the current incidence of meningococcal infections in the United States (see sidebar). Even if the incidence of meningococcal infections were currently as high as they were back in 1997-1999, the cost would be $0.5 million/case. For those thinking about the option of toddler vaccination with the quadrivalent meningococcal conjugate vaccine (Menactra), the calculations for QALYs concluded that such a strategy prevented half as many cases at half the cost.
So where do we go from here? The lack of an endorsement by ACIP/AAP/AAFP for universal use* normally means that the vaccine will not be available within the Vaccines for Children free program, and it will not be covered by commercial health insurance plans except for the specific indications endorsed by the recommending bodies.* So these are huge barriers to use. Nevertheless, it is a licensed vaccine and it is safe and effective, just not perfect and not cost-effective for widespread public use at government expense. How much is a child’s life worth? If it is your child, then the life is priceless. But in public health there are limits to what can be afforded. We will see more of these types of issues in the future. Another unspoken concern of the ACIP/AAP/AAFP is that a two-tiered vaccine access situation develops. In other words, for those who can afford to pay, Hib-MenCY-TT is available and, if they can pay for it out-of-pocket, then they can buy it to protect their child.
The following are cost-effectiveness analysis conclusions that were presented to the CDC:
• Vaccinating infants or toddlers with meningococcal vaccine has a high cost per case prevented – even at a low vaccine price.
• Cost estimates are much higher than prior analyses because of declining incidence and shorter duration of protection.
• Infant vaccination prevents twice as many cases as toddler vaccination but at twice the cost – cost per QALY saved is similar for both strategies.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Hospital Research Institute. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero disclosed that in the past 3 years, he has served as a consultant to Sanofi Pasteur, Pfizer, Novartis, and Crucell for their vaccines currently licensed and in development. Dr. Pichichero also disclosed that in the past 3 years, his academic institution has received research grants to support vaccine work from GlaxoSmithKline, Sanofi Pasteur, Pfizer, Novartis, and Crucell, including studies involving Hib-MenCY-TT produced by GlaxoSmithKline and Quadrivalent meningococcal vaccine by Sanofi Pasteur and Novartis.
* This article was updated on 10/26/12.
Haemophilus influenzae type B-Neisseria meningitidis serogroups C (MenC) and Y (MenY)-tetanus toxoid (Hib-MenCY-TT, MenHibrix) vaccine has been approved by the Food and Drug Administration for an infant indication to be administered according to the standard 2-, 4-, 6-, and 12 months vaccine schedule in the United States as endorsed by the American Academy of Pediatrics (AAP), the American Academy of Family Physicians (AAFP), and the Centers for Disease Control and Prevention (CDC).
According to the presentation given at the CDC meeting Oct. 14, 2011, by Dr. Ismael Ortega-Sanchez, giving the vaccine could prevent 130 of the projected 377 (34.5%) cases of meningococcal infection in the 4-million-child birth cohort of the United States cumulatively to age 10 years. Also infant vaccination would prevent one death per 642,000 infants (seven deaths/year). The vaccine could be given along with a DTaP/inactivated polio vaccine/hepatitis b vaccine (DTaP/IPV/HepB, Pediarix) and the 13-valent pneumococcal conjugate vaccine (PCV13, Prevnar13) without increasing the total number of shots in a visit. The vaccine has been proven safe and effective.
Yet at the October meeting of the CDC Advisory Committee on Immunization Practices (ACIP), Hib-MenCY-TT (MenHibrix) vaccine was not recommended for universal use. Instead, it was recommended for high risk children as previously defined as complement deficient and asplenic. Why the restricted recommendation?*
• It isn’t the right time. When the ACIP/AAP/AAFP endorsed meningococcal vaccination for 11- to 12-year-olds 7 years ago, the annual incidence of meningococcal disease was fivefold higher than it is now. The drop in incidence cannot be fully attributed to the initiation of our national vaccination campaign. It was known before the meningococcal conjugate vaccine was recommended that meningococcal disease had a cyclical pattern with high and low years of incidence. But, had the incidence been as low as it is now, then one might speculate that the vote to recommend universal vaccination might have been different. The passionate pleas of concerned parents and the desire by all of us in health care to protect every single adolescent against the devastation of meningococcal infections carried the day, even though the cost for prevention of those cases and deaths was the highest ever seen up to that time. So, if the incidence of meningococcal infections is now at an all time low, the calculations of cost to prevent cases and deaths would be a multiple of what it was 7 years ago.
• The vaccine doesn’t include all the serotypes. Hib-MenCY-TT has meningococcal serotypes C and Y. The vaccine does not include serotype A or serotype W-135 (because these serotypes are virtually absent and uncommon, respectively, in the United States and other developed countries. So, a concern could be that serotype replacement might occur over time as we have seen with Prevnar7, now replaced in the United States with Prevnar13 because of serotype replacement. But more importantly is the absence of serotype B in the vaccine. Serotype B meningococci cause 60%-65% of meningococcal disease in the United States in infants. That is why the number of cases projected to be prevented with Hib-MenCY-TT is about one-third of all cases among infants.
• The total number of shots goes up per visit unless GlaxoSmithKline vaccines are preferentially used. Hib-MenCY-TT was developed and is licensed by GlaxoSmithKline, a world leader in pediatric vaccines, and they are building a portfolio of vaccines that can fit together well. There is nothing wrong with that – it is good marketing. Sanofi Pasteur Vaccines is doing the same thing and, as more products are forthcoming from Pfizer Vaccines, Novartis Vaccines, and others, we can expect the same strategy. However, the CDC, AAP, and AAFP do not want to endorse products that limit choices and/or provide any single company with a competitive advantage. So, to endorse Hib-MenCY-TT that clearly fits best with only GlaxoSmithKline vaccine products may be an unspoken concern.
• The National Immunization Program cannot afford it. Going back to the presentation to ACIP at the CDC in October 2011, a key aspect was the cost of vaccination calculated against cases prevented and lives saved. The calculation for Quality-Adjusted Life Year (QALY) saved for a two-dose schedule among adolescents came out to $157,000/case. For the infant vaccination, the numbers were pretty staggering at $3.6 million/case, based on the current incidence of meningococcal infections in the United States (see sidebar). Even if the incidence of meningococcal infections were currently as high as they were back in 1997-1999, the cost would be $0.5 million/case. For those thinking about the option of toddler vaccination with the quadrivalent meningococcal conjugate vaccine (Menactra), the calculations for QALYs concluded that such a strategy prevented half as many cases at half the cost.
So where do we go from here? The lack of an endorsement by ACIP/AAP/AAFP for universal use* normally means that the vaccine will not be available within the Vaccines for Children free program, and it will not be covered by commercial health insurance plans except for the specific indications endorsed by the recommending bodies.* So these are huge barriers to use. Nevertheless, it is a licensed vaccine and it is safe and effective, just not perfect and not cost-effective for widespread public use at government expense. How much is a child’s life worth? If it is your child, then the life is priceless. But in public health there are limits to what can be afforded. We will see more of these types of issues in the future. Another unspoken concern of the ACIP/AAP/AAFP is that a two-tiered vaccine access situation develops. In other words, for those who can afford to pay, Hib-MenCY-TT is available and, if they can pay for it out-of-pocket, then they can buy it to protect their child.
The following are cost-effectiveness analysis conclusions that were presented to the CDC:
• Vaccinating infants or toddlers with meningococcal vaccine has a high cost per case prevented – even at a low vaccine price.
• Cost estimates are much higher than prior analyses because of declining incidence and shorter duration of protection.
• Infant vaccination prevents twice as many cases as toddler vaccination but at twice the cost – cost per QALY saved is similar for both strategies.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Hospital Research Institute. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero disclosed that in the past 3 years, he has served as a consultant to Sanofi Pasteur, Pfizer, Novartis, and Crucell for their vaccines currently licensed and in development. Dr. Pichichero also disclosed that in the past 3 years, his academic institution has received research grants to support vaccine work from GlaxoSmithKline, Sanofi Pasteur, Pfizer, Novartis, and Crucell, including studies involving Hib-MenCY-TT produced by GlaxoSmithKline and Quadrivalent meningococcal vaccine by Sanofi Pasteur and Novartis.
* This article was updated on 10/26/12.
Haemophilus influenzae type B-Neisseria meningitidis serogroups C (MenC) and Y (MenY)-tetanus toxoid (Hib-MenCY-TT, MenHibrix) vaccine has been approved by the Food and Drug Administration for an infant indication to be administered according to the standard 2-, 4-, 6-, and 12 months vaccine schedule in the United States as endorsed by the American Academy of Pediatrics (AAP), the American Academy of Family Physicians (AAFP), and the Centers for Disease Control and Prevention (CDC).
According to the presentation given at the CDC meeting Oct. 14, 2011, by Dr. Ismael Ortega-Sanchez, giving the vaccine could prevent 130 of the projected 377 (34.5%) cases of meningococcal infection in the 4-million-child birth cohort of the United States cumulatively to age 10 years. Also infant vaccination would prevent one death per 642,000 infants (seven deaths/year). The vaccine could be given along with a DTaP/inactivated polio vaccine/hepatitis b vaccine (DTaP/IPV/HepB, Pediarix) and the 13-valent pneumococcal conjugate vaccine (PCV13, Prevnar13) without increasing the total number of shots in a visit. The vaccine has been proven safe and effective.
Yet at the October meeting of the CDC Advisory Committee on Immunization Practices (ACIP), Hib-MenCY-TT (MenHibrix) vaccine was not recommended for universal use. Instead, it was recommended for high risk children as previously defined as complement deficient and asplenic. Why the restricted recommendation?*
• It isn’t the right time. When the ACIP/AAP/AAFP endorsed meningococcal vaccination for 11- to 12-year-olds 7 years ago, the annual incidence of meningococcal disease was fivefold higher than it is now. The drop in incidence cannot be fully attributed to the initiation of our national vaccination campaign. It was known before the meningococcal conjugate vaccine was recommended that meningococcal disease had a cyclical pattern with high and low years of incidence. But, had the incidence been as low as it is now, then one might speculate that the vote to recommend universal vaccination might have been different. The passionate pleas of concerned parents and the desire by all of us in health care to protect every single adolescent against the devastation of meningococcal infections carried the day, even though the cost for prevention of those cases and deaths was the highest ever seen up to that time. So, if the incidence of meningococcal infections is now at an all time low, the calculations of cost to prevent cases and deaths would be a multiple of what it was 7 years ago.
• The vaccine doesn’t include all the serotypes. Hib-MenCY-TT has meningococcal serotypes C and Y. The vaccine does not include serotype A or serotype W-135 (because these serotypes are virtually absent and uncommon, respectively, in the United States and other developed countries. So, a concern could be that serotype replacement might occur over time as we have seen with Prevnar7, now replaced in the United States with Prevnar13 because of serotype replacement. But more importantly is the absence of serotype B in the vaccine. Serotype B meningococci cause 60%-65% of meningococcal disease in the United States in infants. That is why the number of cases projected to be prevented with Hib-MenCY-TT is about one-third of all cases among infants.
• The total number of shots goes up per visit unless GlaxoSmithKline vaccines are preferentially used. Hib-MenCY-TT was developed and is licensed by GlaxoSmithKline, a world leader in pediatric vaccines, and they are building a portfolio of vaccines that can fit together well. There is nothing wrong with that – it is good marketing. Sanofi Pasteur Vaccines is doing the same thing and, as more products are forthcoming from Pfizer Vaccines, Novartis Vaccines, and others, we can expect the same strategy. However, the CDC, AAP, and AAFP do not want to endorse products that limit choices and/or provide any single company with a competitive advantage. So, to endorse Hib-MenCY-TT that clearly fits best with only GlaxoSmithKline vaccine products may be an unspoken concern.
• The National Immunization Program cannot afford it. Going back to the presentation to ACIP at the CDC in October 2011, a key aspect was the cost of vaccination calculated against cases prevented and lives saved. The calculation for Quality-Adjusted Life Year (QALY) saved for a two-dose schedule among adolescents came out to $157,000/case. For the infant vaccination, the numbers were pretty staggering at $3.6 million/case, based on the current incidence of meningococcal infections in the United States (see sidebar). Even if the incidence of meningococcal infections were currently as high as they were back in 1997-1999, the cost would be $0.5 million/case. For those thinking about the option of toddler vaccination with the quadrivalent meningococcal conjugate vaccine (Menactra), the calculations for QALYs concluded that such a strategy prevented half as many cases at half the cost.
So where do we go from here? The lack of an endorsement by ACIP/AAP/AAFP for universal use* normally means that the vaccine will not be available within the Vaccines for Children free program, and it will not be covered by commercial health insurance plans except for the specific indications endorsed by the recommending bodies.* So these are huge barriers to use. Nevertheless, it is a licensed vaccine and it is safe and effective, just not perfect and not cost-effective for widespread public use at government expense. How much is a child’s life worth? If it is your child, then the life is priceless. But in public health there are limits to what can be afforded. We will see more of these types of issues in the future. Another unspoken concern of the ACIP/AAP/AAFP is that a two-tiered vaccine access situation develops. In other words, for those who can afford to pay, Hib-MenCY-TT is available and, if they can pay for it out-of-pocket, then they can buy it to protect their child.
The following are cost-effectiveness analysis conclusions that were presented to the CDC:
• Vaccinating infants or toddlers with meningococcal vaccine has a high cost per case prevented – even at a low vaccine price.
• Cost estimates are much higher than prior analyses because of declining incidence and shorter duration of protection.
• Infant vaccination prevents twice as many cases as toddler vaccination but at twice the cost – cost per QALY saved is similar for both strategies.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Rochester (N.Y.) General Hospital Research Institute. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero disclosed that in the past 3 years, he has served as a consultant to Sanofi Pasteur, Pfizer, Novartis, and Crucell for their vaccines currently licensed and in development. Dr. Pichichero also disclosed that in the past 3 years, his academic institution has received research grants to support vaccine work from GlaxoSmithKline, Sanofi Pasteur, Pfizer, Novartis, and Crucell, including studies involving Hib-MenCY-TT produced by GlaxoSmithKline and Quadrivalent meningococcal vaccine by Sanofi Pasteur and Novartis.
* This article was updated on 10/26/12.
Relational Diagnoses and the DSM
In our understanding of family systems, we have long known that dysfunctional relational patterns often lie at the root of our patients’ problems. But to what extent should family diagnoses be incorporated into the DSM?
I sat down with Dr. Marianne Z. Wamboldt to explore these issues. Dr. Wamboldt, a child psychiatrist, is chair of the board of the Family Process Institute. She also holds the Leslie and William Vollbracht Family Chair in Stress and Anxiety Disorders at the University of Colorado, Denver, and serves as professor and vice chair in the department of psychiatry in the medical school.
Marianne Wamboldt: So why exactly do you oppose the inclusion of family diagnoses in the DSM?
Alison Heru: I don’t like the labeling of families. I like helping families understand their strengths and helping them work out their problems. I don’t see family problems as psychiatric problems.
MW: I think it is important that we have a way of measuring and classifying what we see, so that we know what we are talking about and that we can measure whether or not we have success in what we are doing.
AH: I agree that these goals are important. However, I think we can use tools like the GARF (Global Assessment of Relational Functioning) or other measuring devices, to do this. I don’t think we need to go as far as including measurements of family functioning in the DSM.
MW: Did you know the GARF is included in DSM-IV-TR already? What is the difference between the GARF and the DSM?
AH: The GARF is good and useful, like the GAF (Global Assessment Scale) in that it gives you a way to describe functioning on a range from healthy to unhealthy, without defining a pathological state. When you put something in the DSM, you are saying it is a disease. You are saying something about etiology.
MW: Not necessarily. In the DSM-IV, ADHD is a description of behavior; there is no attempt to talk about causality. We had hoped that the DSM-5 could start talking about causality, but most of the research is not yet ready. In the meantime, having a clear definition of what we are treating is useful for researchers as well as clinicians. Having a universal description is helpful for everyone.
AH: I agree that a universal description is good, but I still think that more harm than good comes from including family diagnoses in the DSM. I just don’t see families needing to be labeled as pathological. I understand your point, but I think that the repercussions of having a family diagnosis in the DSM outweigh the benefits. The DSM is used in all kinds of ways. In the court system, it is described as "The Psychiatrist’s Bible." If a family diagnosis is in the DSM, then it becomes an "illness" with all the repercussions that come from that label.
MW: However, if it is not in the DSM, many insurance companies won’t pay for the treatment, and persons in the family get labeled with some other diagnosis in order to get therapy. Moreover, if you label something and talk about it, stigma is reduced. Think about cancer and how we used to think about it. It used to be feared, and people with cancer were isolated. That is not what we want for psychiatry and family problems.
AH: I understand that view, but I think that the analogy with cancer is not accurate. Cancer research is well funded, and cancer in many instances can be cured. Mental illnesses have not seen this kind of support, funding, or understanding. In fact, funding has been drastically cut, and the prisons are full of people with mental illness. People now think about mental illness and crime together.
MW: When cancer research started, it was first to try to find commonalities among the many illnesses people with cancer had. The first treatment efforts improved conditions only a very little, but it was by using each effort and tweaking treatment again and again that the field moved forward to where it is today.
AH: I also heard (from Dr. Carl C. Bell of the University of Illinois at Chicago) about a study that showed that stigma was actually increased after the community was educated that psychiatric illnesses are biological illnesses. Before the educational intervention, people with mental illness were seen in the community as odd or quirky and accepted as "different." After the study, these same people were shunned by the community as having an immutable biological disease.
MW: What about child mistreatment? Don’t you think that would be good to include in the DSM? What about domestic violence? And there is a big push to include parental alienation syndrome. What do you think about that?
AH: I don’t see these as psychiatric illnesses, [they are] more social or criminal problems. I don’t think couples with IPV [interpersonal violence] would come for treatment if they knew that they would be labeled by the insurance companies or doctors. I think the parental alienation syndrome is also not a good thing to put in the DSM, whatever the science (or not) behind it. It is a social problem that parents do bad things to their children. I do not see that as a psychiatric issue.
MW: But we treat these people. They come to us for help, and we try to help them. I think it is better to have clear definition that is well thought out and scientifically based, rather than just vague and impressionistic. Relational problems have been written about for years. There is a huge literature on this topic. I think that the evidence for many relational processes that lead to morbidity is at least as good, if not better, than many diagnoses in the DSM-IV.
AH: I agree. However, my conclusion is that we should take things OUT of the DSM that don’t belong there and prevent social or criminal diagnoses from going into the DSM.
MW: How are we going to measure and be scientific about our work?
AH: I think we can do that without using the DSM. Why is the DSM so important? I also don’t think we should let insurance companies dictate how we think about what we do.
MW: Insurance companies pay for treating persons with some diagnoses, but not other diagnoses. For example, in some states, they do not pay for treatment for ADHD, which is extensively researched to be a primarily heritable illness, responsive to medications more than even very intensive psychotherapeutic interventions, and quite disabling for some youth. The DSM has to be used for more than merely what insurance companies decide what to do with it. It originally began as a method of reliably describing patients that psychiatrists were treating, so as to share knowledge about the illnesses, what worked, and what did not work. The [people behind the] ICD-9, 10, and now 11, also think that relational disorders are important to include. We don’t want to be a country left behind!
AH: A good question is, "What is the DSM for?"
MW: To provide a framework for us to diagnose and treat mental illness.
AH: Should it not just focus on biological disease? That is my preference of a system that calls itself a disease manual.
MW: But that is not what the DSM is. It reflects the biopsychosocial model and includes behaviors and clusters of symptoms that are a focus of treatment.
AH: I am not a biological psychiatrist; (I’m) more of a social and family psychiatrist, but I see the DSM as being a biological manual. I think it is hard to categorize social and family behaviors that can be pathological in one situation or culture and not in another. Take expressed emotion (EE). Research in Japan has found that two components of high EE – high criticism and high overinvolvement – need to be parsed out and that families benefit from specific treatments, depending on which component of high EE is present. I don’t think that relational diagnoses are fixed enough. This is another argument.
MW: So how do you see us communicating and working on this problem without a good system of description?
AH: Well, I think we can continue as we have, using these constructs but not embedding them in the DSM. I don’t think that is necessary. I think it might be better to get diagnoses that are subjective or significantly influenced by the prevailing culture out of the DSM.
MW: I would argue that many illnesses, let alone psychiatric disorders in the DSM, are significantly influenced by the prevailing culture. Read "The Spirit Catches You and You Fall Down" (New York: Farrar, Straus and Giroux, 2012), which illustrates the different cultural beliefs of the Hmong (who think the spirit moves a child) from Western medicine (which diagnoses epilepsy). If relational diagnoses are not in the DSM, many therapists will not think about relationships and will not have them as a focus for change.
AH: I think that is an assumption that is not proven.
MW: If relational disorders are in the DSM, then practitioners are more likely to think of them, and faculty are more likely to teach them to new clinicians.
AH: Yes, but think of them as a pathological entity and then we are back where we were 50 years ago – pathologizing families.
MW: I disagree with that. With education and knowledge about effective family interventions, stigma can be reduced, and people can access the treatments they need!
AH: This is a good argument that our field needs to engage in. Better measurement and reduced stigma are the benefits that you see from including relational problems in the DSM.
MW: Yes, and you see this as unnecessary labeling that might be used against families.
AH: Yes. I also think that relational diagnoses are more fluid than biological diagnoses and that we are not ready, and may never be ready, to carve these out as definitive immutable constructs.
Dr. Heru is with the department of psychiatry at the University of Colorado at Denver. She has been a member of the Association of Family Psychiatrists since 2002 and currently serves as the organization’s treasurer. In addition, she is the coauthor of two books on working with families and is the author of numerous articles on this topic.
In our understanding of family systems, we have long known that dysfunctional relational patterns often lie at the root of our patients’ problems. But to what extent should family diagnoses be incorporated into the DSM?
I sat down with Dr. Marianne Z. Wamboldt to explore these issues. Dr. Wamboldt, a child psychiatrist, is chair of the board of the Family Process Institute. She also holds the Leslie and William Vollbracht Family Chair in Stress and Anxiety Disorders at the University of Colorado, Denver, and serves as professor and vice chair in the department of psychiatry in the medical school.
Marianne Wamboldt: So why exactly do you oppose the inclusion of family diagnoses in the DSM?
Alison Heru: I don’t like the labeling of families. I like helping families understand their strengths and helping them work out their problems. I don’t see family problems as psychiatric problems.
MW: I think it is important that we have a way of measuring and classifying what we see, so that we know what we are talking about and that we can measure whether or not we have success in what we are doing.
AH: I agree that these goals are important. However, I think we can use tools like the GARF (Global Assessment of Relational Functioning) or other measuring devices, to do this. I don’t think we need to go as far as including measurements of family functioning in the DSM.
MW: Did you know the GARF is included in DSM-IV-TR already? What is the difference between the GARF and the DSM?
AH: The GARF is good and useful, like the GAF (Global Assessment Scale) in that it gives you a way to describe functioning on a range from healthy to unhealthy, without defining a pathological state. When you put something in the DSM, you are saying it is a disease. You are saying something about etiology.
MW: Not necessarily. In the DSM-IV, ADHD is a description of behavior; there is no attempt to talk about causality. We had hoped that the DSM-5 could start talking about causality, but most of the research is not yet ready. In the meantime, having a clear definition of what we are treating is useful for researchers as well as clinicians. Having a universal description is helpful for everyone.
AH: I agree that a universal description is good, but I still think that more harm than good comes from including family diagnoses in the DSM. I just don’t see families needing to be labeled as pathological. I understand your point, but I think that the repercussions of having a family diagnosis in the DSM outweigh the benefits. The DSM is used in all kinds of ways. In the court system, it is described as "The Psychiatrist’s Bible." If a family diagnosis is in the DSM, then it becomes an "illness" with all the repercussions that come from that label.
MW: However, if it is not in the DSM, many insurance companies won’t pay for the treatment, and persons in the family get labeled with some other diagnosis in order to get therapy. Moreover, if you label something and talk about it, stigma is reduced. Think about cancer and how we used to think about it. It used to be feared, and people with cancer were isolated. That is not what we want for psychiatry and family problems.
AH: I understand that view, but I think that the analogy with cancer is not accurate. Cancer research is well funded, and cancer in many instances can be cured. Mental illnesses have not seen this kind of support, funding, or understanding. In fact, funding has been drastically cut, and the prisons are full of people with mental illness. People now think about mental illness and crime together.
MW: When cancer research started, it was first to try to find commonalities among the many illnesses people with cancer had. The first treatment efforts improved conditions only a very little, but it was by using each effort and tweaking treatment again and again that the field moved forward to where it is today.
AH: I also heard (from Dr. Carl C. Bell of the University of Illinois at Chicago) about a study that showed that stigma was actually increased after the community was educated that psychiatric illnesses are biological illnesses. Before the educational intervention, people with mental illness were seen in the community as odd or quirky and accepted as "different." After the study, these same people were shunned by the community as having an immutable biological disease.
MW: What about child mistreatment? Don’t you think that would be good to include in the DSM? What about domestic violence? And there is a big push to include parental alienation syndrome. What do you think about that?
AH: I don’t see these as psychiatric illnesses, [they are] more social or criminal problems. I don’t think couples with IPV [interpersonal violence] would come for treatment if they knew that they would be labeled by the insurance companies or doctors. I think the parental alienation syndrome is also not a good thing to put in the DSM, whatever the science (or not) behind it. It is a social problem that parents do bad things to their children. I do not see that as a psychiatric issue.
MW: But we treat these people. They come to us for help, and we try to help them. I think it is better to have clear definition that is well thought out and scientifically based, rather than just vague and impressionistic. Relational problems have been written about for years. There is a huge literature on this topic. I think that the evidence for many relational processes that lead to morbidity is at least as good, if not better, than many diagnoses in the DSM-IV.
AH: I agree. However, my conclusion is that we should take things OUT of the DSM that don’t belong there and prevent social or criminal diagnoses from going into the DSM.
MW: How are we going to measure and be scientific about our work?
AH: I think we can do that without using the DSM. Why is the DSM so important? I also don’t think we should let insurance companies dictate how we think about what we do.
MW: Insurance companies pay for treating persons with some diagnoses, but not other diagnoses. For example, in some states, they do not pay for treatment for ADHD, which is extensively researched to be a primarily heritable illness, responsive to medications more than even very intensive psychotherapeutic interventions, and quite disabling for some youth. The DSM has to be used for more than merely what insurance companies decide what to do with it. It originally began as a method of reliably describing patients that psychiatrists were treating, so as to share knowledge about the illnesses, what worked, and what did not work. The [people behind the] ICD-9, 10, and now 11, also think that relational disorders are important to include. We don’t want to be a country left behind!
AH: A good question is, "What is the DSM for?"
MW: To provide a framework for us to diagnose and treat mental illness.
AH: Should it not just focus on biological disease? That is my preference of a system that calls itself a disease manual.
MW: But that is not what the DSM is. It reflects the biopsychosocial model and includes behaviors and clusters of symptoms that are a focus of treatment.
AH: I am not a biological psychiatrist; (I’m) more of a social and family psychiatrist, but I see the DSM as being a biological manual. I think it is hard to categorize social and family behaviors that can be pathological in one situation or culture and not in another. Take expressed emotion (EE). Research in Japan has found that two components of high EE – high criticism and high overinvolvement – need to be parsed out and that families benefit from specific treatments, depending on which component of high EE is present. I don’t think that relational diagnoses are fixed enough. This is another argument.
MW: So how do you see us communicating and working on this problem without a good system of description?
AH: Well, I think we can continue as we have, using these constructs but not embedding them in the DSM. I don’t think that is necessary. I think it might be better to get diagnoses that are subjective or significantly influenced by the prevailing culture out of the DSM.
MW: I would argue that many illnesses, let alone psychiatric disorders in the DSM, are significantly influenced by the prevailing culture. Read "The Spirit Catches You and You Fall Down" (New York: Farrar, Straus and Giroux, 2012), which illustrates the different cultural beliefs of the Hmong (who think the spirit moves a child) from Western medicine (which diagnoses epilepsy). If relational diagnoses are not in the DSM, many therapists will not think about relationships and will not have them as a focus for change.
AH: I think that is an assumption that is not proven.
MW: If relational disorders are in the DSM, then practitioners are more likely to think of them, and faculty are more likely to teach them to new clinicians.
AH: Yes, but think of them as a pathological entity and then we are back where we were 50 years ago – pathologizing families.
MW: I disagree with that. With education and knowledge about effective family interventions, stigma can be reduced, and people can access the treatments they need!
AH: This is a good argument that our field needs to engage in. Better measurement and reduced stigma are the benefits that you see from including relational problems in the DSM.
MW: Yes, and you see this as unnecessary labeling that might be used against families.
AH: Yes. I also think that relational diagnoses are more fluid than biological diagnoses and that we are not ready, and may never be ready, to carve these out as definitive immutable constructs.
Dr. Heru is with the department of psychiatry at the University of Colorado at Denver. She has been a member of the Association of Family Psychiatrists since 2002 and currently serves as the organization’s treasurer. In addition, she is the coauthor of two books on working with families and is the author of numerous articles on this topic.
In our understanding of family systems, we have long known that dysfunctional relational patterns often lie at the root of our patients’ problems. But to what extent should family diagnoses be incorporated into the DSM?
I sat down with Dr. Marianne Z. Wamboldt to explore these issues. Dr. Wamboldt, a child psychiatrist, is chair of the board of the Family Process Institute. She also holds the Leslie and William Vollbracht Family Chair in Stress and Anxiety Disorders at the University of Colorado, Denver, and serves as professor and vice chair in the department of psychiatry in the medical school.
Marianne Wamboldt: So why exactly do you oppose the inclusion of family diagnoses in the DSM?
Alison Heru: I don’t like the labeling of families. I like helping families understand their strengths and helping them work out their problems. I don’t see family problems as psychiatric problems.
MW: I think it is important that we have a way of measuring and classifying what we see, so that we know what we are talking about and that we can measure whether or not we have success in what we are doing.
AH: I agree that these goals are important. However, I think we can use tools like the GARF (Global Assessment of Relational Functioning) or other measuring devices, to do this. I don’t think we need to go as far as including measurements of family functioning in the DSM.
MW: Did you know the GARF is included in DSM-IV-TR already? What is the difference between the GARF and the DSM?
AH: The GARF is good and useful, like the GAF (Global Assessment Scale) in that it gives you a way to describe functioning on a range from healthy to unhealthy, without defining a pathological state. When you put something in the DSM, you are saying it is a disease. You are saying something about etiology.
MW: Not necessarily. In the DSM-IV, ADHD is a description of behavior; there is no attempt to talk about causality. We had hoped that the DSM-5 could start talking about causality, but most of the research is not yet ready. In the meantime, having a clear definition of what we are treating is useful for researchers as well as clinicians. Having a universal description is helpful for everyone.
AH: I agree that a universal description is good, but I still think that more harm than good comes from including family diagnoses in the DSM. I just don’t see families needing to be labeled as pathological. I understand your point, but I think that the repercussions of having a family diagnosis in the DSM outweigh the benefits. The DSM is used in all kinds of ways. In the court system, it is described as "The Psychiatrist’s Bible." If a family diagnosis is in the DSM, then it becomes an "illness" with all the repercussions that come from that label.
MW: However, if it is not in the DSM, many insurance companies won’t pay for the treatment, and persons in the family get labeled with some other diagnosis in order to get therapy. Moreover, if you label something and talk about it, stigma is reduced. Think about cancer and how we used to think about it. It used to be feared, and people with cancer were isolated. That is not what we want for psychiatry and family problems.
AH: I understand that view, but I think that the analogy with cancer is not accurate. Cancer research is well funded, and cancer in many instances can be cured. Mental illnesses have not seen this kind of support, funding, or understanding. In fact, funding has been drastically cut, and the prisons are full of people with mental illness. People now think about mental illness and crime together.
MW: When cancer research started, it was first to try to find commonalities among the many illnesses people with cancer had. The first treatment efforts improved conditions only a very little, but it was by using each effort and tweaking treatment again and again that the field moved forward to where it is today.
AH: I also heard (from Dr. Carl C. Bell of the University of Illinois at Chicago) about a study that showed that stigma was actually increased after the community was educated that psychiatric illnesses are biological illnesses. Before the educational intervention, people with mental illness were seen in the community as odd or quirky and accepted as "different." After the study, these same people were shunned by the community as having an immutable biological disease.
MW: What about child mistreatment? Don’t you think that would be good to include in the DSM? What about domestic violence? And there is a big push to include parental alienation syndrome. What do you think about that?
AH: I don’t see these as psychiatric illnesses, [they are] more social or criminal problems. I don’t think couples with IPV [interpersonal violence] would come for treatment if they knew that they would be labeled by the insurance companies or doctors. I think the parental alienation syndrome is also not a good thing to put in the DSM, whatever the science (or not) behind it. It is a social problem that parents do bad things to their children. I do not see that as a psychiatric issue.
MW: But we treat these people. They come to us for help, and we try to help them. I think it is better to have clear definition that is well thought out and scientifically based, rather than just vague and impressionistic. Relational problems have been written about for years. There is a huge literature on this topic. I think that the evidence for many relational processes that lead to morbidity is at least as good, if not better, than many diagnoses in the DSM-IV.
AH: I agree. However, my conclusion is that we should take things OUT of the DSM that don’t belong there and prevent social or criminal diagnoses from going into the DSM.
MW: How are we going to measure and be scientific about our work?
AH: I think we can do that without using the DSM. Why is the DSM so important? I also don’t think we should let insurance companies dictate how we think about what we do.
MW: Insurance companies pay for treating persons with some diagnoses, but not other diagnoses. For example, in some states, they do not pay for treatment for ADHD, which is extensively researched to be a primarily heritable illness, responsive to medications more than even very intensive psychotherapeutic interventions, and quite disabling for some youth. The DSM has to be used for more than merely what insurance companies decide what to do with it. It originally began as a method of reliably describing patients that psychiatrists were treating, so as to share knowledge about the illnesses, what worked, and what did not work. The [people behind the] ICD-9, 10, and now 11, also think that relational disorders are important to include. We don’t want to be a country left behind!
AH: A good question is, "What is the DSM for?"
MW: To provide a framework for us to diagnose and treat mental illness.
AH: Should it not just focus on biological disease? That is my preference of a system that calls itself a disease manual.
MW: But that is not what the DSM is. It reflects the biopsychosocial model and includes behaviors and clusters of symptoms that are a focus of treatment.
AH: I am not a biological psychiatrist; (I’m) more of a social and family psychiatrist, but I see the DSM as being a biological manual. I think it is hard to categorize social and family behaviors that can be pathological in one situation or culture and not in another. Take expressed emotion (EE). Research in Japan has found that two components of high EE – high criticism and high overinvolvement – need to be parsed out and that families benefit from specific treatments, depending on which component of high EE is present. I don’t think that relational diagnoses are fixed enough. This is another argument.
MW: So how do you see us communicating and working on this problem without a good system of description?
AH: Well, I think we can continue as we have, using these constructs but not embedding them in the DSM. I don’t think that is necessary. I think it might be better to get diagnoses that are subjective or significantly influenced by the prevailing culture out of the DSM.
MW: I would argue that many illnesses, let alone psychiatric disorders in the DSM, are significantly influenced by the prevailing culture. Read "The Spirit Catches You and You Fall Down" (New York: Farrar, Straus and Giroux, 2012), which illustrates the different cultural beliefs of the Hmong (who think the spirit moves a child) from Western medicine (which diagnoses epilepsy). If relational diagnoses are not in the DSM, many therapists will not think about relationships and will not have them as a focus for change.
AH: I think that is an assumption that is not proven.
MW: If relational disorders are in the DSM, then practitioners are more likely to think of them, and faculty are more likely to teach them to new clinicians.
AH: Yes, but think of them as a pathological entity and then we are back where we were 50 years ago – pathologizing families.
MW: I disagree with that. With education and knowledge about effective family interventions, stigma can be reduced, and people can access the treatments they need!
AH: This is a good argument that our field needs to engage in. Better measurement and reduced stigma are the benefits that you see from including relational problems in the DSM.
MW: Yes, and you see this as unnecessary labeling that might be used against families.
AH: Yes. I also think that relational diagnoses are more fluid than biological diagnoses and that we are not ready, and may never be ready, to carve these out as definitive immutable constructs.
Dr. Heru is with the department of psychiatry at the University of Colorado at Denver. She has been a member of the Association of Family Psychiatrists since 2002 and currently serves as the organization’s treasurer. In addition, she is the coauthor of two books on working with families and is the author of numerous articles on this topic.
Procalcitonin and Findings
Pneumonia is a common reason for hospitalization and major rationale for administration of antibiotics in the United States.[1] Management guidelines for patients hospitalized with community‐acquired pneumonia recommend early antibiotic therapy. Quality measures adopted by the Centers for Medicare and Medicaid Services include antibiotic administration within 6 hours of presentation, based on a relationship between early administration and improved survival.[2, 3, 4, 5] However, this imperative has been associated with an increase in incorrect diagnoses of pneumonia.[6, 7] While pneumonia diagnosis would seem straightforward, clinical findings frequently do not differentiate pneumonia from other respiratory illnesses. Thus, an infiltrate on chest radiographs (CXR) is commonly used to assign an admitting diagnosis of pneumonia.[8, 9, 10] However, radiographic reports are often inconclusive and frequently fail to discriminate pneumonic infiltrates from atelectasis, edema, small pleural effusions, or chronic abnormalities. In cases of uncertainty, physicians invariably initiate antibiotics, even when the illness is more consistent with viral bronchitis or asthma, resulting in unnecessary antibiotic use.[6] Previously considered relatively harmless, antibiotic complications can be lethal, and excessive use promotes antimicrobial resistance.[11, 12]
Procalcitonin (ProCT), a calcitonin precursor normally produced in the thyroid and lungs, is secreted by cells throughout the body in response to bacterial infections.[13, 14] Elevated ProCT is used to screen patients with suspected bacterial infection and predict mortality in critically ill patients.[15, 16] Recently, European investigators have used ProCT to guide more selective antibiotic use in patients with symptoms of lower respiratory tract infection (LRTI), including pneumonia.[17, 18, 19] However, few reports describe the relationship of ProCT with radiographic features in hospitalized patients with LRTI, and none focus on patients with indeterminate radiographic readings.[18, 20, 21]
We sought to determine if elevated serum ProCT in adults hospitalized with LRTI symptoms correlates with the clinical diagnosis of pneumonia and definitive infiltrates on CXRs. We specifically assessed ProCT as a diagnostic marker of pneumonia to augment clinical judgment in patients when radiographic findings are indeterminate.
METHODS
Subjects
The study was performed in a 520‐bed, general medicalsurgical hospital, using subjects participating in a study of the relationship between biomarkers and microbiologic diagnoses (to be described in a separate publication). Adults 21 years of age admitted with a diagnosis compatible with respiratory tract infection (pneumonia, acute exacerbations of chronic obstructive pulmonary disease [AECOPD], acute bronchitis, asthma, upper respiratory infection, viral syndrome, respiratory failure, and congestive heart failure [CHF] with signs of infection) were recruited during winters of 20082009 and 20092010. Patients were screened within 24 hours of admission; immunosupression, lung abscess, witnessed aspiration, or previous antibiotic use were exclusion criteria. Blood for ProCT measurement was collected in the emergency room prior to antibiotics. Subjects or legal guardians provided written informed consent, and institutional review boards approved the study.
Illness Evaluation
At enrollment, demographic, clinical, and laboratory data were collected. To provide consistency of data, 1 investigator (a clinical pulmonary specialist), after interviewing, examining each subject, and considering the radiographic findings, assigned a primary and secondary clinical admitting diagnosis: pneumonia, AECOPD, asthma exacerbation, CHF, acute bronchitis, viral syndrome or influenza, other respiratory illness, or non‐respiratory illness. These diagnoses were used for the analysis but were not available to treating physicians. Discharge diagnoses, based on International Classification of Diseases, Ninth Revision (ICD‐9) codes, and official results of follow‐up chest radiographs were also recorded.
Radiographic Interpretations
All official admission chest radiographic reports were reviewed by one of the investigators. Radiographic descriptions were noted with attention to the following comments: clear or no acute change from prior radiographs, infiltrates without consolidation, consolidation, edema, atelectasis, and pleural effusion. For our analysis, chest radiographs were categorized into 4 groups: (1) No acute disease (NAD) (ie, clear or no change from prior radiographs); (2) Other definitive radiographic abnormality (atelectasis, edema, or pleural effusion); (3) Indeterminate (findings consistent with pneumonia, but also other processes including atelectasis, edema, scarring but with no favored interpretation [often including the phrase pneumonia cannot be excluded]); and (4) infiltrate (ie, an infiltrate or consolidation most consistent with pneumonia). The pulmonary specialist independently categorized CXRs and provided a definitive radiographic interpretation whenever possible.
Microbiology
Blood cultures, sputum Gram stain and culture were performed by the clinical laboratory. Pneumococcal‐specific urinary antigen and serology were performed as previously described.[22]
Procalcitonin Measurement
Serum ProCT was measured using resolved amplified cryptate emission technology (Kryptor PCT, Brahms, Henningsdorf, Germany). Functional sensitivity is 0.06 ng/mL (normal serum levels are 0.033 0.003 ng/mL).[23, 24, 25] ProCT results were not available to investigators at the time of clinical or radiographic assessments.
Statistical Analysis
Categorical and continuous distributions were compared using Fisher's exact and Wilcoxon tests, respectively, and summarized by proportions and medians, and means and standard deviations. Log‐axis boxplots were used to graphically display the skewed distributions of ProCT. Stacked bar charts were used to depict discrete distributions of diagnoses by ProCT group. Receiver operating characteristic (ROC) curves relating ProCT to chest radiographs were computed, and sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated for ProCT thresholds. Logistic regression was used to estimate odds ratios (OR), and associated P values and confidence intervals, to quantify associations between infiltrate/non‐infiltrate and dichotomized or log‐transformed ProCT. SAS 9.2 (SAS Institute, Inc, Cary, NC) was used for analyses, using 2‐sided 0.05 level tests.
RESULTS
Study Population and Clinical Diagnoses
During 2 winters, 532 subjects admitted for 556 respiratory illnesses were recruited. Two did not have chest radiographs, 16 illnesses were non‐pulmonary, and 10 did not have admission ProCT levels, leaving 528 illnesses for analysis (Table 1). Subjects averaged 65 years of age, predominately lived at home, and a high percentage had underlying diseases. The leading primary admission diagnoses assigned by the pulmonologist were pneumonia and AECOPD at 31% and 27%, respectively. Of the 163 illnesses assigned a primary or secondary admitting diagnosis of pneumonia, infiltrates were identified on the admission radiograph by the pulmonologist in 156 (96%).
| Illnesses N = 528 (%) | |
|---|---|
| |
| Age (mean SD) | 65 17 |
| Female | 278 (53) |
| Race | |
| White | 409 (77) |
| African American | 119 (23) |
| Hispanic | 53 (10) |
| Residence | |
| Community | 488 (92) |
| Assisted living | 27 (5) |
| Nursing home | 13 (2) |
| Medical conditions | |
| Diabetes | 188 (36) |
| CHF | 153 (29) |
| COPD | 216 (41) |
| Chronic renal failure | 21 (4) |
| Past or active smoker | 395 (75) |
| Current influenza vaccine | 371 (70) |
| Prior pneumococcal vaccine | 432 (82) |
| Oral steroids | 69 (13) |
| Inhaled steroids | 201 (38) |
| Home oxygen | 129 (24) |
| Symptoms, No. (%) | |
| Upper respiratory | 350 (66) |
| Cough | 489 (93) |
| Sputum production | 390 (74) |
| Dyspnea | 496 (94) |
| Wheezing | 345 (65) |
| Constitutional | 271 (51) |
| Feverish | 293 (55) |
| Rigors | 118 (22) |
| Physical exam | |
| Wheezing | 279 (53) |
| Rales | 211 (40) |
| Rhonchi | 144 (27) |
| Temperature (mean SD) | 37.2 1.0 |
| Respiratory rate (mean SD) | 25 7 |
| Laboratory data | |
| Oxygen saturation (mean SD) | 92.3 5.5 |
| WBC 103/ml (mean SD) | 9.4 4.8 |
| Clinical diagnosis | |
| Pneumonia | 163 (31) |
| Asthma | 76 (14) |
| CHF | 45 (9) |
| Bronchitis | 83 (16) |
| AECOPD | 142 (27) |
| Other pulmonary | 19 (4) |
| Therapy | |
| Antibacterials | 463 (88) |
| Antivirals | 38 (7) |
| Outcome | |
| Intensive care | 43 (8) |
| Respiratory failure | 29 (6) |
| Death | 6 (1) |
| Length of stay (mean SD) | 5.9 18.7 |
Radiographic Classifications
Based on radiology reports, 213 (40%) were classified as NAD, 76 (14%) as other definitive findings, 75 (14%) as infiltrates, and 164 (31%) as indeterminate. The pulmonologist concurred with the radiology report for most NAD (199/213) and infiltrate (67/75) classifications, but only 4 as indeterminate, assigning approximately half the radiologist's indeterminate CXRs to infiltrate and one‐quarter each to NAD and other categories.
Relationship of ProCT With Clinical and Radiographic Features
The relationship between clinical admitting diagnosis and ProCT is shown in Figure 1A. Subjects with pneumonia had a median ProCT of 0.27 ng/ml (interquartile range [IQR] 1.3), significantly greater than subjects with AECOPD, asthma, acute bronchitis, and viral/emnfluenza. Subjects assigned to the other diagnoses category (ie, skin or urinary infections, empyema) generally had higher ProCT values (median 0.70 ng/ml, IQR 4.6).
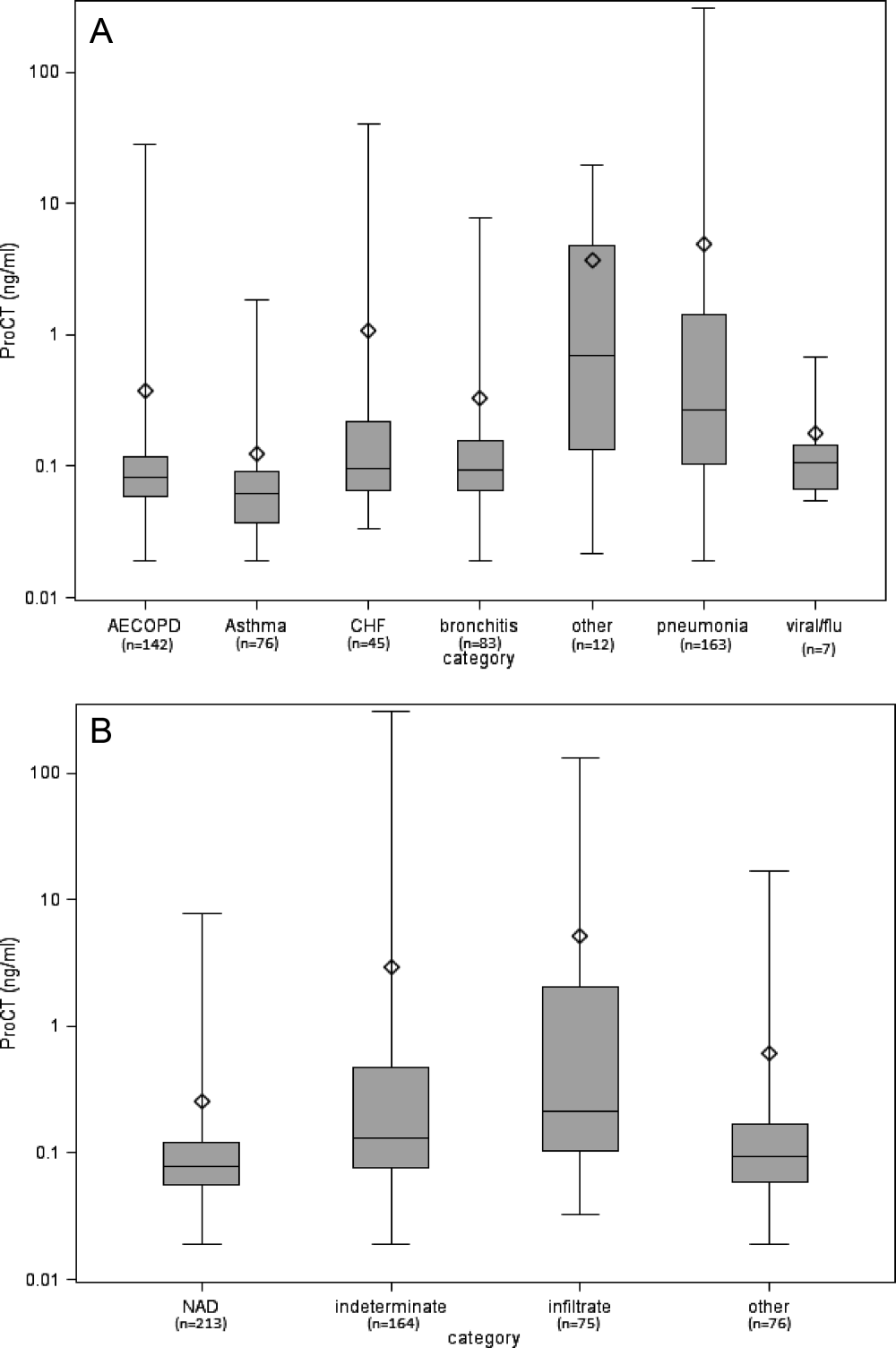
The relationship between the radiologist's classification and ProCT is shown in Figure 1B. Median ProCT levels were significantly lower in subjects with radiographs classified as NAD compared to those showing infiltrates (P < 0.0001). Those classified as indeterminate in radiology reports had a median ProCT value (0.13 ng/ml) midway between those with NAD (0.08 ng/ml) and infiltrates (0.21 ng/ml). Similarly, illnesses with radiographs classified by the pulmonologist as infiltrates also had significantly higher median ProCT levels (0.33 ng/ml, IQR 1.33) than those classified as NAD (0.08 ng/ml, IQR 0.06; P < 0.0001), and other definitive findings (0.11 ng/ml, IQR 0.17). Notably, duration of symptoms prior to evaluation did not affect the relationship between ProCT level and radiographic classification (data not shown). All subjects with infiltrates according to the radiologist's report received early antibiotics, as did 97% and 80% with indeterminate radiographs and NAD, respectively.
Predictive Value of ProCT for Interpreting Indeterminate Radiographs
We were specifically interested to learn if ProCT could help clinicians interpret the large number of indeterminate radiographs. As a first step, we evaluated the diagnostic accuracy of ProCT for radiographic infiltrates by calculating the ROC curve (Figure 2A), using cases in which radiologist and pulmonologist concurred on the classification as infiltrate (n = 67) or no infiltrate (ie, NAD or other definitive finding; n = 273). For these cases, the ROC had an area under curve (AUC) of 0.80 (P < 0.0001), indicating moderate predictive accuracy of ProCT for the presence of an infiltrate. The OR for an infiltrate increased steadily with higher ProCT thresholds, quadrupling from 3.9 to 15.6 as the threshold increased from 0.05 to 0.50 ng/ml (Table 2). Using the commonly defined 0.25 ng/ml ProCT threshold for which antibiotics have been recommended for respiratory infections, the PPV for an infiltrate was 61% and the NPV 89%.
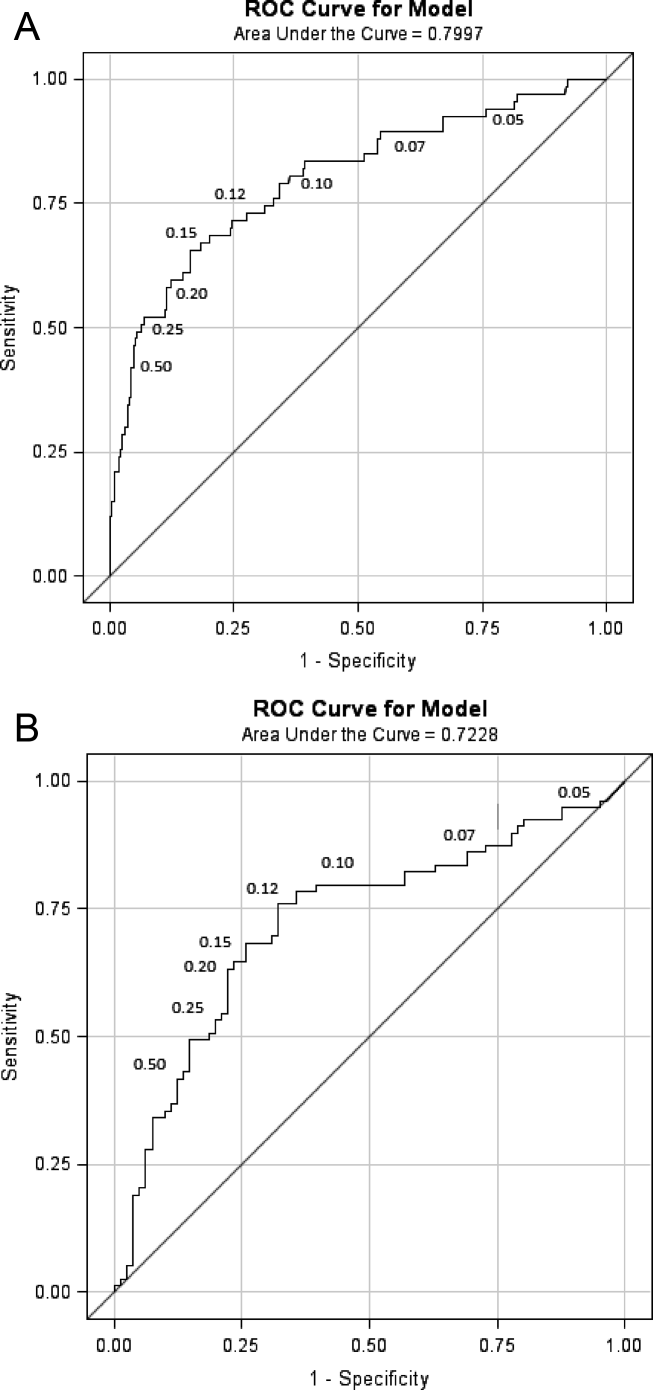
| ProCT Threshold (ng/ml) | Sensitivity (%) | Specificity (%) | OR | P Value |
|---|---|---|---|---|
| ||||
| 0.05 | 94 | 20 | 3.9 | 0.012 |
| 0.07 | 90 | 42 | 6.1 | <0.0001 |
| 0.10 | 79 | 65 | 7.1 | <0.0001 |
| 0.12 | 73 | 72 | 7.1 | <0.0001 |
| 0.15* | 67 | 82 | 9.1 | <0.0001 |
| 0.20 | 58 | 88 | 10.5 | <0.0001 |
| 0.25 | 52 | 92 | 12.5 | <0.0001 |
| 0.50 | 42 | 96 | 15.6 | <0.0001 |
Next, we analyzed the 164 illnesses with indeterminate radiographs. Of these, the pulmonologist classified 79 as infiltrate (median ProCT 0.29 ng/ml), 40 as NAD (median ProCT 0.08 ng/ml), 41 as other definitive findings (median ProCT 0.10 ng/ml), and 4 as indeterminate. The admitting diagnosis and corresponding median ProCT for these subjects was pneumonia in 78 (0.28 ng/ml), AECOPD in 26 (0.09 ng/ml), acute bronchitis in 30 (0.09 ng/ml), asthma in 11 (0.08 ng/ml), and CHF in 14 (0.10 ng/ml). The ROC AUC for these cases (excluding 4 considered indeterminate by the pulmonologist) was slightly lower (0.72, P < 0.0001) than the prior analysis (Figure 2B), but ProCT retained moderate predictive value for the presence of infiltrates as diagnosed by the pulmonologist on indeterminate radiographs (Table 3).
| ProCT Threshold | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | OR | P Value | Predicted Probability (%) |
|---|---|---|---|---|---|---|---|
| |||||||
| 0.05 | 95 | 7 | 50 | 60 | 1.5 | 0.54 | 32 |
| 0.07 | 86 | 27 | 54 | 67 | 2.3 | 0.04 | 35 |
| 0.10 | 80 | 59 | 66 | 75 | 5.7 | <0.0001 | 40 |
| 0.12* | 76 | 67 | 69 | 74 | 6.3 | <0.0001 | 42 |
| 0.15 | 67 | 74 | 72 | 70 | 5.8 | <0.0001 | 45 |
| 0.20 | 63 | 78 | 74 | 69 | 6.0 | <0.0001 | 49 |
| 0.25 | 53 | 80 | 72 | 64 | 4.6 | <0.0001 | 52 |
| 0.50 | 37 | 88 | 74 | 59 | 4.1 | 0.0006 | 60 |
Since clinical determinations by a single pulmonologist may be subjective, we sought to objectively assess the accuracy of assigned admission diagnoses by analyzing additional data, including bacterial tests, follow‐up radiographs, and final discharge diagnoses. As a surrogate of invasive bacterial infection, we used identification of S. pneumoniae as the outcome, since multiple complementary diagnostic assays were used, thus minimizing the uncertainty of sputum cultures. Overall, we identified 58 pneumococcal infections (Table 4). The pulmonologist's admitting diagnosis of pneumonia captured a higher proportion (52% for any test positive) of S. pneumoniae cases than identification of a radiographic infiltrate by the radiologist (26%), and equivalent to the pulmonologist's radiographic reading (57%) or elevated ProCT alone (57%). There was an absolute 14% increase (27% relative increase) in the detection of S. pneumoniae infections by addition of ProCT to the pulmonologist's diagnosis. A similar analysis limited to the 164 subjects with indeterminate radiographs found that 13 of 19 (68%) of S. pneumoniae diagnoses had been assigned a clinical diagnosis of pneumonia by the pulmonologist.
| S. pneumonia Confirmed by Diagnostic Assay No. (%) | |||||
|---|---|---|---|---|---|
| Criteria | Blood Culture + (n = 7) | Urine Ag + (n = 27) | Serology + (n = 28) | Sputum + (n = 20) | Any Test + (n = 58) |
| |||||
| Radiologist CXR read as infiltrate | 3 (43) | 9 (33) | 10 (36) | 3 (15) | 15 (26) |
| Pulmonologist CXR read as infiltrate | 6 (86) | 17 (62) | 18 (64) | 10 (50) | 33 (57) |
| Pulmonologist clinical diagnosis of pneumonia | 6 (86) | 16 (60) | 16 (57) | 8 (40) | 30 (52) |
| Pulmonologist clinical diagnosis of AECOPD | 0 (0) | 4 (15) | 5 (18) | 5 (25) | 8 (14) |
| Procalcitonin 0.25 ng/ml | 6 (86) | 18 (67) | 17 (61) | 9 (45) | 33 (57) |
| Pulmonologist diagnosis of pneumonia and/or ProCT 0.25 ng/ml | 7 (100) | 19 (70) | 20 (71) | 10 (50) | 38 (66) |
We also determined how often subsequent radiographs showed evolution to a definitive infiltrate in indeterminate cases that the pulmonologist had classified as either NAD or other finding. Only 3/20 with repeat films showed a definitive infiltrate, none of whom had evidence of bacterial infection, and all 3 had ProCT <0.25 ng/ml. In contrast, 10 of 21 subjects with indeterminate CXRs, that the pulmonologist had classified as an infiltrate, developed definitive infiltrates on follow‐up studies (P = 0.04), and 8 remained indeterminate.
Finally, 93% of those assigned an admitting pneumonia diagnosis by the pulmonologist had primary (71%) or secondary (22%) discharge diagnosis of pneumonia. Of the additional 28 subjects with a discharge diagnosis of pneumonia, for whom the pulmonologist assigned non‐pneumonia admitting diagnoses, none had positive blood cultures or evidence of S. pneumoniae infection, and only one had a follow‐up radiograph showing a definitive infiltrate.
DISCUSSION
Pneumonia can be a serious illness and, if left untreated, result in death. Hence, infectious disease and pulmonary societies advocate timely administration of antibiotics. Unfortunately, clinical diagnosis of pneumonia is difficult, as clinical and radiographic features are often ambiguous. These factors result in liberal use of broad‐spectrum antibiotics, often in patients with viral infection, in whom antibiotic side effects outweigh benefit.[26] Although pneumonia guidelines recommend early antibiotic therapy for patients with infiltrates on CXR, we found that early administration of antibiotics for possible pneumonia is nearly universal.[2, 27] Thus, an objective laboratory test indicative of pneumonia would be a useful adjunct when making treatment decisions.
In an attempt to curb excessive antibiotic use, European investigators have used ProCT to guide therapy for respiratory tract infection, including pneumonia, without increased adverse outcomes.[17, 19, 28, 29] Antibiotics are recommended when ProCT levels are 0.25 ng/ml, rather than based on radiographic or microbiological results. However, ProCT‐guided antibiotic management has not been adopted in the United States, and radiographic findings are a major determinant for diagnosis of pneumonia and antibiotic administration.
Thus, we were interested in correlating ProCT levels with the presence of radiographic infiltrates, and especially how ProCT could assist in interpreting the vexing problem of indeterminate CXRs. We found a direct relationship between an increased ProCT level and definitive infiltrates on CXR, similar to results reported by others.[18, 20, 21] However, these studies did not address illnesses with indeterminate radiographs, which accounted for approximately one‐third of the radiologist's readings in our study. Our situation is likely not unique. In a post hoc analysis of CXRs from subjects with pneumonia in the PORT study, independent radiologists interpreted a similar proportion of CXRs as either no acute disease or possible infiltrates.[30] Since indeterminate readings include the possibility of an infiltrate, it is not surprising that 97% of our subjects received early antibiotic treatment.
Given current pneumonia treatment guidelines, ProCT testing for those with definitive infiltrates would not add to patient management, as all should receive early antibiotics. However, ProCT measurement could have significant impact on management in those with indeterminate films. We found ProCT was moderately predictive for infiltrates when utilizing the pulmonologist's interpretation of indeterminate CXRs. This is concordant with a recent study reporting a similar ROC (0.72) for ProCT for diagnosing pneumonia in patients with dyspnea, and more predictive of pneumonia than any clinical variable.[31]
The major limitation of our study is the lack of a gold standard for diagnosis of pneumonia. We chose to use a pulmonary specialist to provide admission diagnoses, and independently interpret radiographs in the context of clinical findings. Although imperfect, we feel this approach best reflects good medical practice by an experienced clinician evaluating the CXR in context of a patient's history and physical exam. Importantly, analysis of the concurrent microbiology data, follow‐up radiographs, and discharge diagnoses supports the accuracy of the admitting diagnoses of pneumonia and radiographic interpretations by the pulmonologist. Secondly, our findings may not be applicable to immunocompromised or previously treated patients.
How should a busy physician respond to a patient with dyspnea and fever when faced with an indeterminate radiographic report, since no method for the diagnosis of pneumonia is infallible?[8, 32] Clearly, a pulmonary consultation on all such patients is impractical. Our data confirms previous results that high ProCT values are correlated with an infiltrate on CXR and low values have a NPV of nearly 90%. Furthermore, low ProCT values predict low mortality across all levels of standard severity scoring indexes.[33] Thus, a low ProCT may help clinicians feel more confident to delay or stop antibiotics in a patient in whom clinical suspicion for bacterial pneumonia is low, yet the radiographic report states pneumonia cannot be ruled out.[34] Such strategies may assist in reducing unnecessary antibiotic use in the United States.[35]
Disclosures
Funding for this study was provided by NIAID‐1R01AI079446‐01. This work was presented in part at the Infectious Diseases Society of America, Boston, MA, October 2011. The authors report no conflicts of interest.
- , , , et al. The burden of community‐acquired pneumonia in seniors: results of a population‐based study. Clin Infect Dis. 2004;39:1642–1650.
- , , , et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72.
- Centers for Medicare and Medicaid Services. The Medicare national pneumonia quality improvement project. Available at: http://www.cms.gov/HospitalQualityInits/. Accessed September 8, 2011.
- , , , , . Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community‐acquired pneumonia. Arch Intern Med. 2004;164:637–644.
- , , , et al. Quality of care, process, and outcomes in elderly patients with pneumonia. JAMA. 1997;278:2080–2084.
- , , , . Misdiagnosis of community‐acquired pneumonia and inappropriate utilization of antibiotics: side effects of the 4‐h antibiotic administration rule. Chest. 2007;131:1865–1869.
- , , . Antibiotic timing and errors in diagnosing pneumonia. Arch Intern Med. 2008;168:351–356.
- , , , . Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med. 2004;117:305–311.
- , , , , . Reliability of radiographic findings and the relation to etiologic agents in community‐acquired pneumonia. Respir Med. 2006;100:926–932.
- , , , . Admission chest radiograph lacks sensitivity in the diagnosis of community‐acquired pneumonia. Am J Med Sci. 2009;337:236–240.
- , . Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis. 2011;52:1232–1240.
- , , , et al. The epidemic of antibiotic‐resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:155–164.
- , , , , . Clinical review 167: procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J Clin Endocrinol Metab. 2004;89:1512–1525.
- , , . Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations. Crit Care Med. 2008;36:941–952.
- , , . Procalcitonin in sepsis and systemic inflammation: a harmful biomarker and a therapeutic target. Br J Pharmacol. 2010;159:253–264.
- , , , , . Pneumonitis‐associated hyperprocalcitoninemia. Am J Med Sci. 1996;312:12–18.
- , , , et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin‐guidance with standard therapy. Chest. 2007;131:9–19.
- , , , et al. Procalcitonin versus C‐reactive protein for predicting pneumonia in adults with lower respiratory tract infection in primary care. Br J Gen Pract. 2007;57:555–560.
- , , , et al. Effect of procalcitonin‐based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302:1059–1066.
- , , , et al. Procalcitonin and C‐reactive protein in hospitalized adult patients with community‐acquired pneumonia or exacerbation of asthma or COPD. Chest. 2011;139:1410–1418.
- , , , et al. Diagnostic and prognostic accuracy of clinical and laboratory parameters in community‐acquired pneumonia. BMC Infect Dis. 2008;7:10.
- , , , et al. Utility of serum procalcitonin values in patients with acute exacerbations of chronic obstructive pulmonary disease: a cautionary note. Int J Chron Obstruct Pulmon Dis. 2112;7:127–135.
- , , . Procalcitonin and its component peptides in systemic inflammation: immunochemical characterization. J Investig Med. 1997;45:552–560.
- , , , . The future diagnostic role of procalcitonin levels: the need for improved sensitivity. Clin Infect Dis. 2003;36:823–824.
- , , , et al. Calcitonin precursor levels in human medullary thyroid carcinoma. Thyroid 2003;13:819–822.
- , , , . Emergency department visits for antibiotic‐associated adverse events. Clin Infect Dis. 2008;47:735–743.
- , , , et al. Guideline tyranny: a response to the article by Baum and Kaltsas. Clin Infect Dis. 2008;47:1117–1118.
- , , , et al. Procalcitonin guidance of antibiotic therapy in community‐acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174:84–93.
- , , , et al. Effect of procalcitonin‐guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster‐randomised, single‐blinded intervention trial. Lancet. 2004;363:600–607.
- , , , et al. Interobserver reliability of the chest radiograph in community‐acquired pneumonia. PORT investigators. Chest. 1996;110:343–350.
- , , , et al. Use of procalcitonin for the diagnosis of pneumonia in patients presenting with a chief complaint of dyspnea: results from the BACH (Biomarkers in Acute Heart Failure) trial. Eur J Heart Fail. 2012;14:278–286.
- , , , , . A multicenter analysis of the ED diagnosis of pneumonia. Am J Emerg Med. 2010;28:862–865.
- , , , et al. Procalcitonin predicts patients at low risk of death from community‐acquired pneumonia across all CRB‐65 classes. Eur Respir J. 2008;31:349–355.
- , , , , , . Antibiotic timing and diagnostic uncertainty in Medicare patients with pneumonia: is it reasonable to expect all patients to receive antibiotics within 4 hours? Chest. 2006;130:16–21.
- . Procalcitonin for triage of patients with respiratory tract symptoms: a case study in the trial design process for approval of a new diagnostic test for lower respiratory tract infection. Clin Infect Dis. 2011;52(S4):S351–S356.
Pneumonia is a common reason for hospitalization and major rationale for administration of antibiotics in the United States.[1] Management guidelines for patients hospitalized with community‐acquired pneumonia recommend early antibiotic therapy. Quality measures adopted by the Centers for Medicare and Medicaid Services include antibiotic administration within 6 hours of presentation, based on a relationship between early administration and improved survival.[2, 3, 4, 5] However, this imperative has been associated with an increase in incorrect diagnoses of pneumonia.[6, 7] While pneumonia diagnosis would seem straightforward, clinical findings frequently do not differentiate pneumonia from other respiratory illnesses. Thus, an infiltrate on chest radiographs (CXR) is commonly used to assign an admitting diagnosis of pneumonia.[8, 9, 10] However, radiographic reports are often inconclusive and frequently fail to discriminate pneumonic infiltrates from atelectasis, edema, small pleural effusions, or chronic abnormalities. In cases of uncertainty, physicians invariably initiate antibiotics, even when the illness is more consistent with viral bronchitis or asthma, resulting in unnecessary antibiotic use.[6] Previously considered relatively harmless, antibiotic complications can be lethal, and excessive use promotes antimicrobial resistance.[11, 12]
Procalcitonin (ProCT), a calcitonin precursor normally produced in the thyroid and lungs, is secreted by cells throughout the body in response to bacterial infections.[13, 14] Elevated ProCT is used to screen patients with suspected bacterial infection and predict mortality in critically ill patients.[15, 16] Recently, European investigators have used ProCT to guide more selective antibiotic use in patients with symptoms of lower respiratory tract infection (LRTI), including pneumonia.[17, 18, 19] However, few reports describe the relationship of ProCT with radiographic features in hospitalized patients with LRTI, and none focus on patients with indeterminate radiographic readings.[18, 20, 21]
We sought to determine if elevated serum ProCT in adults hospitalized with LRTI symptoms correlates with the clinical diagnosis of pneumonia and definitive infiltrates on CXRs. We specifically assessed ProCT as a diagnostic marker of pneumonia to augment clinical judgment in patients when radiographic findings are indeterminate.
METHODS
Subjects
The study was performed in a 520‐bed, general medicalsurgical hospital, using subjects participating in a study of the relationship between biomarkers and microbiologic diagnoses (to be described in a separate publication). Adults 21 years of age admitted with a diagnosis compatible with respiratory tract infection (pneumonia, acute exacerbations of chronic obstructive pulmonary disease [AECOPD], acute bronchitis, asthma, upper respiratory infection, viral syndrome, respiratory failure, and congestive heart failure [CHF] with signs of infection) were recruited during winters of 20082009 and 20092010. Patients were screened within 24 hours of admission; immunosupression, lung abscess, witnessed aspiration, or previous antibiotic use were exclusion criteria. Blood for ProCT measurement was collected in the emergency room prior to antibiotics. Subjects or legal guardians provided written informed consent, and institutional review boards approved the study.
Illness Evaluation
At enrollment, demographic, clinical, and laboratory data were collected. To provide consistency of data, 1 investigator (a clinical pulmonary specialist), after interviewing, examining each subject, and considering the radiographic findings, assigned a primary and secondary clinical admitting diagnosis: pneumonia, AECOPD, asthma exacerbation, CHF, acute bronchitis, viral syndrome or influenza, other respiratory illness, or non‐respiratory illness. These diagnoses were used for the analysis but were not available to treating physicians. Discharge diagnoses, based on International Classification of Diseases, Ninth Revision (ICD‐9) codes, and official results of follow‐up chest radiographs were also recorded.
Radiographic Interpretations
All official admission chest radiographic reports were reviewed by one of the investigators. Radiographic descriptions were noted with attention to the following comments: clear or no acute change from prior radiographs, infiltrates without consolidation, consolidation, edema, atelectasis, and pleural effusion. For our analysis, chest radiographs were categorized into 4 groups: (1) No acute disease (NAD) (ie, clear or no change from prior radiographs); (2) Other definitive radiographic abnormality (atelectasis, edema, or pleural effusion); (3) Indeterminate (findings consistent with pneumonia, but also other processes including atelectasis, edema, scarring but with no favored interpretation [often including the phrase pneumonia cannot be excluded]); and (4) infiltrate (ie, an infiltrate or consolidation most consistent with pneumonia). The pulmonary specialist independently categorized CXRs and provided a definitive radiographic interpretation whenever possible.
Microbiology
Blood cultures, sputum Gram stain and culture were performed by the clinical laboratory. Pneumococcal‐specific urinary antigen and serology were performed as previously described.[22]
Procalcitonin Measurement
Serum ProCT was measured using resolved amplified cryptate emission technology (Kryptor PCT, Brahms, Henningsdorf, Germany). Functional sensitivity is 0.06 ng/mL (normal serum levels are 0.033 0.003 ng/mL).[23, 24, 25] ProCT results were not available to investigators at the time of clinical or radiographic assessments.
Statistical Analysis
Categorical and continuous distributions were compared using Fisher's exact and Wilcoxon tests, respectively, and summarized by proportions and medians, and means and standard deviations. Log‐axis boxplots were used to graphically display the skewed distributions of ProCT. Stacked bar charts were used to depict discrete distributions of diagnoses by ProCT group. Receiver operating characteristic (ROC) curves relating ProCT to chest radiographs were computed, and sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated for ProCT thresholds. Logistic regression was used to estimate odds ratios (OR), and associated P values and confidence intervals, to quantify associations between infiltrate/non‐infiltrate and dichotomized or log‐transformed ProCT. SAS 9.2 (SAS Institute, Inc, Cary, NC) was used for analyses, using 2‐sided 0.05 level tests.
RESULTS
Study Population and Clinical Diagnoses
During 2 winters, 532 subjects admitted for 556 respiratory illnesses were recruited. Two did not have chest radiographs, 16 illnesses were non‐pulmonary, and 10 did not have admission ProCT levels, leaving 528 illnesses for analysis (Table 1). Subjects averaged 65 years of age, predominately lived at home, and a high percentage had underlying diseases. The leading primary admission diagnoses assigned by the pulmonologist were pneumonia and AECOPD at 31% and 27%, respectively. Of the 163 illnesses assigned a primary or secondary admitting diagnosis of pneumonia, infiltrates were identified on the admission radiograph by the pulmonologist in 156 (96%).
| Illnesses N = 528 (%) | |
|---|---|
| |
| Age (mean SD) | 65 17 |
| Female | 278 (53) |
| Race | |
| White | 409 (77) |
| African American | 119 (23) |
| Hispanic | 53 (10) |
| Residence | |
| Community | 488 (92) |
| Assisted living | 27 (5) |
| Nursing home | 13 (2) |
| Medical conditions | |
| Diabetes | 188 (36) |
| CHF | 153 (29) |
| COPD | 216 (41) |
| Chronic renal failure | 21 (4) |
| Past or active smoker | 395 (75) |
| Current influenza vaccine | 371 (70) |
| Prior pneumococcal vaccine | 432 (82) |
| Oral steroids | 69 (13) |
| Inhaled steroids | 201 (38) |
| Home oxygen | 129 (24) |
| Symptoms, No. (%) | |
| Upper respiratory | 350 (66) |
| Cough | 489 (93) |
| Sputum production | 390 (74) |
| Dyspnea | 496 (94) |
| Wheezing | 345 (65) |
| Constitutional | 271 (51) |
| Feverish | 293 (55) |
| Rigors | 118 (22) |
| Physical exam | |
| Wheezing | 279 (53) |
| Rales | 211 (40) |
| Rhonchi | 144 (27) |
| Temperature (mean SD) | 37.2 1.0 |
| Respiratory rate (mean SD) | 25 7 |
| Laboratory data | |
| Oxygen saturation (mean SD) | 92.3 5.5 |
| WBC 103/ml (mean SD) | 9.4 4.8 |
| Clinical diagnosis | |
| Pneumonia | 163 (31) |
| Asthma | 76 (14) |
| CHF | 45 (9) |
| Bronchitis | 83 (16) |
| AECOPD | 142 (27) |
| Other pulmonary | 19 (4) |
| Therapy | |
| Antibacterials | 463 (88) |
| Antivirals | 38 (7) |
| Outcome | |
| Intensive care | 43 (8) |
| Respiratory failure | 29 (6) |
| Death | 6 (1) |
| Length of stay (mean SD) | 5.9 18.7 |
Radiographic Classifications
Based on radiology reports, 213 (40%) were classified as NAD, 76 (14%) as other definitive findings, 75 (14%) as infiltrates, and 164 (31%) as indeterminate. The pulmonologist concurred with the radiology report for most NAD (199/213) and infiltrate (67/75) classifications, but only 4 as indeterminate, assigning approximately half the radiologist's indeterminate CXRs to infiltrate and one‐quarter each to NAD and other categories.
Relationship of ProCT With Clinical and Radiographic Features
The relationship between clinical admitting diagnosis and ProCT is shown in Figure 1A. Subjects with pneumonia had a median ProCT of 0.27 ng/ml (interquartile range [IQR] 1.3), significantly greater than subjects with AECOPD, asthma, acute bronchitis, and viral/emnfluenza. Subjects assigned to the other diagnoses category (ie, skin or urinary infections, empyema) generally had higher ProCT values (median 0.70 ng/ml, IQR 4.6).

The relationship between the radiologist's classification and ProCT is shown in Figure 1B. Median ProCT levels were significantly lower in subjects with radiographs classified as NAD compared to those showing infiltrates (P < 0.0001). Those classified as indeterminate in radiology reports had a median ProCT value (0.13 ng/ml) midway between those with NAD (0.08 ng/ml) and infiltrates (0.21 ng/ml). Similarly, illnesses with radiographs classified by the pulmonologist as infiltrates also had significantly higher median ProCT levels (0.33 ng/ml, IQR 1.33) than those classified as NAD (0.08 ng/ml, IQR 0.06; P < 0.0001), and other definitive findings (0.11 ng/ml, IQR 0.17). Notably, duration of symptoms prior to evaluation did not affect the relationship between ProCT level and radiographic classification (data not shown). All subjects with infiltrates according to the radiologist's report received early antibiotics, as did 97% and 80% with indeterminate radiographs and NAD, respectively.
Predictive Value of ProCT for Interpreting Indeterminate Radiographs
We were specifically interested to learn if ProCT could help clinicians interpret the large number of indeterminate radiographs. As a first step, we evaluated the diagnostic accuracy of ProCT for radiographic infiltrates by calculating the ROC curve (Figure 2A), using cases in which radiologist and pulmonologist concurred on the classification as infiltrate (n = 67) or no infiltrate (ie, NAD or other definitive finding; n = 273). For these cases, the ROC had an area under curve (AUC) of 0.80 (P < 0.0001), indicating moderate predictive accuracy of ProCT for the presence of an infiltrate. The OR for an infiltrate increased steadily with higher ProCT thresholds, quadrupling from 3.9 to 15.6 as the threshold increased from 0.05 to 0.50 ng/ml (Table 2). Using the commonly defined 0.25 ng/ml ProCT threshold for which antibiotics have been recommended for respiratory infections, the PPV for an infiltrate was 61% and the NPV 89%.

| ProCT Threshold (ng/ml) | Sensitivity (%) | Specificity (%) | OR | P Value |
|---|---|---|---|---|
| ||||
| 0.05 | 94 | 20 | 3.9 | 0.012 |
| 0.07 | 90 | 42 | 6.1 | <0.0001 |
| 0.10 | 79 | 65 | 7.1 | <0.0001 |
| 0.12 | 73 | 72 | 7.1 | <0.0001 |
| 0.15* | 67 | 82 | 9.1 | <0.0001 |
| 0.20 | 58 | 88 | 10.5 | <0.0001 |
| 0.25 | 52 | 92 | 12.5 | <0.0001 |
| 0.50 | 42 | 96 | 15.6 | <0.0001 |
Next, we analyzed the 164 illnesses with indeterminate radiographs. Of these, the pulmonologist classified 79 as infiltrate (median ProCT 0.29 ng/ml), 40 as NAD (median ProCT 0.08 ng/ml), 41 as other definitive findings (median ProCT 0.10 ng/ml), and 4 as indeterminate. The admitting diagnosis and corresponding median ProCT for these subjects was pneumonia in 78 (0.28 ng/ml), AECOPD in 26 (0.09 ng/ml), acute bronchitis in 30 (0.09 ng/ml), asthma in 11 (0.08 ng/ml), and CHF in 14 (0.10 ng/ml). The ROC AUC for these cases (excluding 4 considered indeterminate by the pulmonologist) was slightly lower (0.72, P < 0.0001) than the prior analysis (Figure 2B), but ProCT retained moderate predictive value for the presence of infiltrates as diagnosed by the pulmonologist on indeterminate radiographs (Table 3).
| ProCT Threshold | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | OR | P Value | Predicted Probability (%) |
|---|---|---|---|---|---|---|---|
| |||||||
| 0.05 | 95 | 7 | 50 | 60 | 1.5 | 0.54 | 32 |
| 0.07 | 86 | 27 | 54 | 67 | 2.3 | 0.04 | 35 |
| 0.10 | 80 | 59 | 66 | 75 | 5.7 | <0.0001 | 40 |
| 0.12* | 76 | 67 | 69 | 74 | 6.3 | <0.0001 | 42 |
| 0.15 | 67 | 74 | 72 | 70 | 5.8 | <0.0001 | 45 |
| 0.20 | 63 | 78 | 74 | 69 | 6.0 | <0.0001 | 49 |
| 0.25 | 53 | 80 | 72 | 64 | 4.6 | <0.0001 | 52 |
| 0.50 | 37 | 88 | 74 | 59 | 4.1 | 0.0006 | 60 |
Since clinical determinations by a single pulmonologist may be subjective, we sought to objectively assess the accuracy of assigned admission diagnoses by analyzing additional data, including bacterial tests, follow‐up radiographs, and final discharge diagnoses. As a surrogate of invasive bacterial infection, we used identification of S. pneumoniae as the outcome, since multiple complementary diagnostic assays were used, thus minimizing the uncertainty of sputum cultures. Overall, we identified 58 pneumococcal infections (Table 4). The pulmonologist's admitting diagnosis of pneumonia captured a higher proportion (52% for any test positive) of S. pneumoniae cases than identification of a radiographic infiltrate by the radiologist (26%), and equivalent to the pulmonologist's radiographic reading (57%) or elevated ProCT alone (57%). There was an absolute 14% increase (27% relative increase) in the detection of S. pneumoniae infections by addition of ProCT to the pulmonologist's diagnosis. A similar analysis limited to the 164 subjects with indeterminate radiographs found that 13 of 19 (68%) of S. pneumoniae diagnoses had been assigned a clinical diagnosis of pneumonia by the pulmonologist.
| S. pneumonia Confirmed by Diagnostic Assay No. (%) | |||||
|---|---|---|---|---|---|
| Criteria | Blood Culture + (n = 7) | Urine Ag + (n = 27) | Serology + (n = 28) | Sputum + (n = 20) | Any Test + (n = 58) |
| |||||
| Radiologist CXR read as infiltrate | 3 (43) | 9 (33) | 10 (36) | 3 (15) | 15 (26) |
| Pulmonologist CXR read as infiltrate | 6 (86) | 17 (62) | 18 (64) | 10 (50) | 33 (57) |
| Pulmonologist clinical diagnosis of pneumonia | 6 (86) | 16 (60) | 16 (57) | 8 (40) | 30 (52) |
| Pulmonologist clinical diagnosis of AECOPD | 0 (0) | 4 (15) | 5 (18) | 5 (25) | 8 (14) |
| Procalcitonin 0.25 ng/ml | 6 (86) | 18 (67) | 17 (61) | 9 (45) | 33 (57) |
| Pulmonologist diagnosis of pneumonia and/or ProCT 0.25 ng/ml | 7 (100) | 19 (70) | 20 (71) | 10 (50) | 38 (66) |
We also determined how often subsequent radiographs showed evolution to a definitive infiltrate in indeterminate cases that the pulmonologist had classified as either NAD or other finding. Only 3/20 with repeat films showed a definitive infiltrate, none of whom had evidence of bacterial infection, and all 3 had ProCT <0.25 ng/ml. In contrast, 10 of 21 subjects with indeterminate CXRs, that the pulmonologist had classified as an infiltrate, developed definitive infiltrates on follow‐up studies (P = 0.04), and 8 remained indeterminate.
Finally, 93% of those assigned an admitting pneumonia diagnosis by the pulmonologist had primary (71%) or secondary (22%) discharge diagnosis of pneumonia. Of the additional 28 subjects with a discharge diagnosis of pneumonia, for whom the pulmonologist assigned non‐pneumonia admitting diagnoses, none had positive blood cultures or evidence of S. pneumoniae infection, and only one had a follow‐up radiograph showing a definitive infiltrate.
DISCUSSION
Pneumonia can be a serious illness and, if left untreated, result in death. Hence, infectious disease and pulmonary societies advocate timely administration of antibiotics. Unfortunately, clinical diagnosis of pneumonia is difficult, as clinical and radiographic features are often ambiguous. These factors result in liberal use of broad‐spectrum antibiotics, often in patients with viral infection, in whom antibiotic side effects outweigh benefit.[26] Although pneumonia guidelines recommend early antibiotic therapy for patients with infiltrates on CXR, we found that early administration of antibiotics for possible pneumonia is nearly universal.[2, 27] Thus, an objective laboratory test indicative of pneumonia would be a useful adjunct when making treatment decisions.
In an attempt to curb excessive antibiotic use, European investigators have used ProCT to guide therapy for respiratory tract infection, including pneumonia, without increased adverse outcomes.[17, 19, 28, 29] Antibiotics are recommended when ProCT levels are 0.25 ng/ml, rather than based on radiographic or microbiological results. However, ProCT‐guided antibiotic management has not been adopted in the United States, and radiographic findings are a major determinant for diagnosis of pneumonia and antibiotic administration.
Thus, we were interested in correlating ProCT levels with the presence of radiographic infiltrates, and especially how ProCT could assist in interpreting the vexing problem of indeterminate CXRs. We found a direct relationship between an increased ProCT level and definitive infiltrates on CXR, similar to results reported by others.[18, 20, 21] However, these studies did not address illnesses with indeterminate radiographs, which accounted for approximately one‐third of the radiologist's readings in our study. Our situation is likely not unique. In a post hoc analysis of CXRs from subjects with pneumonia in the PORT study, independent radiologists interpreted a similar proportion of CXRs as either no acute disease or possible infiltrates.[30] Since indeterminate readings include the possibility of an infiltrate, it is not surprising that 97% of our subjects received early antibiotic treatment.
Given current pneumonia treatment guidelines, ProCT testing for those with definitive infiltrates would not add to patient management, as all should receive early antibiotics. However, ProCT measurement could have significant impact on management in those with indeterminate films. We found ProCT was moderately predictive for infiltrates when utilizing the pulmonologist's interpretation of indeterminate CXRs. This is concordant with a recent study reporting a similar ROC (0.72) for ProCT for diagnosing pneumonia in patients with dyspnea, and more predictive of pneumonia than any clinical variable.[31]
The major limitation of our study is the lack of a gold standard for diagnosis of pneumonia. We chose to use a pulmonary specialist to provide admission diagnoses, and independently interpret radiographs in the context of clinical findings. Although imperfect, we feel this approach best reflects good medical practice by an experienced clinician evaluating the CXR in context of a patient's history and physical exam. Importantly, analysis of the concurrent microbiology data, follow‐up radiographs, and discharge diagnoses supports the accuracy of the admitting diagnoses of pneumonia and radiographic interpretations by the pulmonologist. Secondly, our findings may not be applicable to immunocompromised or previously treated patients.
How should a busy physician respond to a patient with dyspnea and fever when faced with an indeterminate radiographic report, since no method for the diagnosis of pneumonia is infallible?[8, 32] Clearly, a pulmonary consultation on all such patients is impractical. Our data confirms previous results that high ProCT values are correlated with an infiltrate on CXR and low values have a NPV of nearly 90%. Furthermore, low ProCT values predict low mortality across all levels of standard severity scoring indexes.[33] Thus, a low ProCT may help clinicians feel more confident to delay or stop antibiotics in a patient in whom clinical suspicion for bacterial pneumonia is low, yet the radiographic report states pneumonia cannot be ruled out.[34] Such strategies may assist in reducing unnecessary antibiotic use in the United States.[35]
Disclosures
Funding for this study was provided by NIAID‐1R01AI079446‐01. This work was presented in part at the Infectious Diseases Society of America, Boston, MA, October 2011. The authors report no conflicts of interest.
Pneumonia is a common reason for hospitalization and major rationale for administration of antibiotics in the United States.[1] Management guidelines for patients hospitalized with community‐acquired pneumonia recommend early antibiotic therapy. Quality measures adopted by the Centers for Medicare and Medicaid Services include antibiotic administration within 6 hours of presentation, based on a relationship between early administration and improved survival.[2, 3, 4, 5] However, this imperative has been associated with an increase in incorrect diagnoses of pneumonia.[6, 7] While pneumonia diagnosis would seem straightforward, clinical findings frequently do not differentiate pneumonia from other respiratory illnesses. Thus, an infiltrate on chest radiographs (CXR) is commonly used to assign an admitting diagnosis of pneumonia.[8, 9, 10] However, radiographic reports are often inconclusive and frequently fail to discriminate pneumonic infiltrates from atelectasis, edema, small pleural effusions, or chronic abnormalities. In cases of uncertainty, physicians invariably initiate antibiotics, even when the illness is more consistent with viral bronchitis or asthma, resulting in unnecessary antibiotic use.[6] Previously considered relatively harmless, antibiotic complications can be lethal, and excessive use promotes antimicrobial resistance.[11, 12]
Procalcitonin (ProCT), a calcitonin precursor normally produced in the thyroid and lungs, is secreted by cells throughout the body in response to bacterial infections.[13, 14] Elevated ProCT is used to screen patients with suspected bacterial infection and predict mortality in critically ill patients.[15, 16] Recently, European investigators have used ProCT to guide more selective antibiotic use in patients with symptoms of lower respiratory tract infection (LRTI), including pneumonia.[17, 18, 19] However, few reports describe the relationship of ProCT with radiographic features in hospitalized patients with LRTI, and none focus on patients with indeterminate radiographic readings.[18, 20, 21]
We sought to determine if elevated serum ProCT in adults hospitalized with LRTI symptoms correlates with the clinical diagnosis of pneumonia and definitive infiltrates on CXRs. We specifically assessed ProCT as a diagnostic marker of pneumonia to augment clinical judgment in patients when radiographic findings are indeterminate.
METHODS
Subjects
The study was performed in a 520‐bed, general medicalsurgical hospital, using subjects participating in a study of the relationship between biomarkers and microbiologic diagnoses (to be described in a separate publication). Adults 21 years of age admitted with a diagnosis compatible with respiratory tract infection (pneumonia, acute exacerbations of chronic obstructive pulmonary disease [AECOPD], acute bronchitis, asthma, upper respiratory infection, viral syndrome, respiratory failure, and congestive heart failure [CHF] with signs of infection) were recruited during winters of 20082009 and 20092010. Patients were screened within 24 hours of admission; immunosupression, lung abscess, witnessed aspiration, or previous antibiotic use were exclusion criteria. Blood for ProCT measurement was collected in the emergency room prior to antibiotics. Subjects or legal guardians provided written informed consent, and institutional review boards approved the study.
Illness Evaluation
At enrollment, demographic, clinical, and laboratory data were collected. To provide consistency of data, 1 investigator (a clinical pulmonary specialist), after interviewing, examining each subject, and considering the radiographic findings, assigned a primary and secondary clinical admitting diagnosis: pneumonia, AECOPD, asthma exacerbation, CHF, acute bronchitis, viral syndrome or influenza, other respiratory illness, or non‐respiratory illness. These diagnoses were used for the analysis but were not available to treating physicians. Discharge diagnoses, based on International Classification of Diseases, Ninth Revision (ICD‐9) codes, and official results of follow‐up chest radiographs were also recorded.
Radiographic Interpretations
All official admission chest radiographic reports were reviewed by one of the investigators. Radiographic descriptions were noted with attention to the following comments: clear or no acute change from prior radiographs, infiltrates without consolidation, consolidation, edema, atelectasis, and pleural effusion. For our analysis, chest radiographs were categorized into 4 groups: (1) No acute disease (NAD) (ie, clear or no change from prior radiographs); (2) Other definitive radiographic abnormality (atelectasis, edema, or pleural effusion); (3) Indeterminate (findings consistent with pneumonia, but also other processes including atelectasis, edema, scarring but with no favored interpretation [often including the phrase pneumonia cannot be excluded]); and (4) infiltrate (ie, an infiltrate or consolidation most consistent with pneumonia). The pulmonary specialist independently categorized CXRs and provided a definitive radiographic interpretation whenever possible.
Microbiology
Blood cultures, sputum Gram stain and culture were performed by the clinical laboratory. Pneumococcal‐specific urinary antigen and serology were performed as previously described.[22]
Procalcitonin Measurement
Serum ProCT was measured using resolved amplified cryptate emission technology (Kryptor PCT, Brahms, Henningsdorf, Germany). Functional sensitivity is 0.06 ng/mL (normal serum levels are 0.033 0.003 ng/mL).[23, 24, 25] ProCT results were not available to investigators at the time of clinical or radiographic assessments.
Statistical Analysis
Categorical and continuous distributions were compared using Fisher's exact and Wilcoxon tests, respectively, and summarized by proportions and medians, and means and standard deviations. Log‐axis boxplots were used to graphically display the skewed distributions of ProCT. Stacked bar charts were used to depict discrete distributions of diagnoses by ProCT group. Receiver operating characteristic (ROC) curves relating ProCT to chest radiographs were computed, and sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated for ProCT thresholds. Logistic regression was used to estimate odds ratios (OR), and associated P values and confidence intervals, to quantify associations between infiltrate/non‐infiltrate and dichotomized or log‐transformed ProCT. SAS 9.2 (SAS Institute, Inc, Cary, NC) was used for analyses, using 2‐sided 0.05 level tests.
RESULTS
Study Population and Clinical Diagnoses
During 2 winters, 532 subjects admitted for 556 respiratory illnesses were recruited. Two did not have chest radiographs, 16 illnesses were non‐pulmonary, and 10 did not have admission ProCT levels, leaving 528 illnesses for analysis (Table 1). Subjects averaged 65 years of age, predominately lived at home, and a high percentage had underlying diseases. The leading primary admission diagnoses assigned by the pulmonologist were pneumonia and AECOPD at 31% and 27%, respectively. Of the 163 illnesses assigned a primary or secondary admitting diagnosis of pneumonia, infiltrates were identified on the admission radiograph by the pulmonologist in 156 (96%).
| Illnesses N = 528 (%) | |
|---|---|
| |
| Age (mean SD) | 65 17 |
| Female | 278 (53) |
| Race | |
| White | 409 (77) |
| African American | 119 (23) |
| Hispanic | 53 (10) |
| Residence | |
| Community | 488 (92) |
| Assisted living | 27 (5) |
| Nursing home | 13 (2) |
| Medical conditions | |
| Diabetes | 188 (36) |
| CHF | 153 (29) |
| COPD | 216 (41) |
| Chronic renal failure | 21 (4) |
| Past or active smoker | 395 (75) |
| Current influenza vaccine | 371 (70) |
| Prior pneumococcal vaccine | 432 (82) |
| Oral steroids | 69 (13) |
| Inhaled steroids | 201 (38) |
| Home oxygen | 129 (24) |
| Symptoms, No. (%) | |
| Upper respiratory | 350 (66) |
| Cough | 489 (93) |
| Sputum production | 390 (74) |
| Dyspnea | 496 (94) |
| Wheezing | 345 (65) |
| Constitutional | 271 (51) |
| Feverish | 293 (55) |
| Rigors | 118 (22) |
| Physical exam | |
| Wheezing | 279 (53) |
| Rales | 211 (40) |
| Rhonchi | 144 (27) |
| Temperature (mean SD) | 37.2 1.0 |
| Respiratory rate (mean SD) | 25 7 |
| Laboratory data | |
| Oxygen saturation (mean SD) | 92.3 5.5 |
| WBC 103/ml (mean SD) | 9.4 4.8 |
| Clinical diagnosis | |
| Pneumonia | 163 (31) |
| Asthma | 76 (14) |
| CHF | 45 (9) |
| Bronchitis | 83 (16) |
| AECOPD | 142 (27) |
| Other pulmonary | 19 (4) |
| Therapy | |
| Antibacterials | 463 (88) |
| Antivirals | 38 (7) |
| Outcome | |
| Intensive care | 43 (8) |
| Respiratory failure | 29 (6) |
| Death | 6 (1) |
| Length of stay (mean SD) | 5.9 18.7 |
Radiographic Classifications
Based on radiology reports, 213 (40%) were classified as NAD, 76 (14%) as other definitive findings, 75 (14%) as infiltrates, and 164 (31%) as indeterminate. The pulmonologist concurred with the radiology report for most NAD (199/213) and infiltrate (67/75) classifications, but only 4 as indeterminate, assigning approximately half the radiologist's indeterminate CXRs to infiltrate and one‐quarter each to NAD and other categories.
Relationship of ProCT With Clinical and Radiographic Features
The relationship between clinical admitting diagnosis and ProCT is shown in Figure 1A. Subjects with pneumonia had a median ProCT of 0.27 ng/ml (interquartile range [IQR] 1.3), significantly greater than subjects with AECOPD, asthma, acute bronchitis, and viral/emnfluenza. Subjects assigned to the other diagnoses category (ie, skin or urinary infections, empyema) generally had higher ProCT values (median 0.70 ng/ml, IQR 4.6).

The relationship between the radiologist's classification and ProCT is shown in Figure 1B. Median ProCT levels were significantly lower in subjects with radiographs classified as NAD compared to those showing infiltrates (P < 0.0001). Those classified as indeterminate in radiology reports had a median ProCT value (0.13 ng/ml) midway between those with NAD (0.08 ng/ml) and infiltrates (0.21 ng/ml). Similarly, illnesses with radiographs classified by the pulmonologist as infiltrates also had significantly higher median ProCT levels (0.33 ng/ml, IQR 1.33) than those classified as NAD (0.08 ng/ml, IQR 0.06; P < 0.0001), and other definitive findings (0.11 ng/ml, IQR 0.17). Notably, duration of symptoms prior to evaluation did not affect the relationship between ProCT level and radiographic classification (data not shown). All subjects with infiltrates according to the radiologist's report received early antibiotics, as did 97% and 80% with indeterminate radiographs and NAD, respectively.
Predictive Value of ProCT for Interpreting Indeterminate Radiographs
We were specifically interested to learn if ProCT could help clinicians interpret the large number of indeterminate radiographs. As a first step, we evaluated the diagnostic accuracy of ProCT for radiographic infiltrates by calculating the ROC curve (Figure 2A), using cases in which radiologist and pulmonologist concurred on the classification as infiltrate (n = 67) or no infiltrate (ie, NAD or other definitive finding; n = 273). For these cases, the ROC had an area under curve (AUC) of 0.80 (P < 0.0001), indicating moderate predictive accuracy of ProCT for the presence of an infiltrate. The OR for an infiltrate increased steadily with higher ProCT thresholds, quadrupling from 3.9 to 15.6 as the threshold increased from 0.05 to 0.50 ng/ml (Table 2). Using the commonly defined 0.25 ng/ml ProCT threshold for which antibiotics have been recommended for respiratory infections, the PPV for an infiltrate was 61% and the NPV 89%.

| ProCT Threshold (ng/ml) | Sensitivity (%) | Specificity (%) | OR | P Value |
|---|---|---|---|---|
| ||||
| 0.05 | 94 | 20 | 3.9 | 0.012 |
| 0.07 | 90 | 42 | 6.1 | <0.0001 |
| 0.10 | 79 | 65 | 7.1 | <0.0001 |
| 0.12 | 73 | 72 | 7.1 | <0.0001 |
| 0.15* | 67 | 82 | 9.1 | <0.0001 |
| 0.20 | 58 | 88 | 10.5 | <0.0001 |
| 0.25 | 52 | 92 | 12.5 | <0.0001 |
| 0.50 | 42 | 96 | 15.6 | <0.0001 |
Next, we analyzed the 164 illnesses with indeterminate radiographs. Of these, the pulmonologist classified 79 as infiltrate (median ProCT 0.29 ng/ml), 40 as NAD (median ProCT 0.08 ng/ml), 41 as other definitive findings (median ProCT 0.10 ng/ml), and 4 as indeterminate. The admitting diagnosis and corresponding median ProCT for these subjects was pneumonia in 78 (0.28 ng/ml), AECOPD in 26 (0.09 ng/ml), acute bronchitis in 30 (0.09 ng/ml), asthma in 11 (0.08 ng/ml), and CHF in 14 (0.10 ng/ml). The ROC AUC for these cases (excluding 4 considered indeterminate by the pulmonologist) was slightly lower (0.72, P < 0.0001) than the prior analysis (Figure 2B), but ProCT retained moderate predictive value for the presence of infiltrates as diagnosed by the pulmonologist on indeterminate radiographs (Table 3).
| ProCT Threshold | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | OR | P Value | Predicted Probability (%) |
|---|---|---|---|---|---|---|---|
| |||||||
| 0.05 | 95 | 7 | 50 | 60 | 1.5 | 0.54 | 32 |
| 0.07 | 86 | 27 | 54 | 67 | 2.3 | 0.04 | 35 |
| 0.10 | 80 | 59 | 66 | 75 | 5.7 | <0.0001 | 40 |
| 0.12* | 76 | 67 | 69 | 74 | 6.3 | <0.0001 | 42 |
| 0.15 | 67 | 74 | 72 | 70 | 5.8 | <0.0001 | 45 |
| 0.20 | 63 | 78 | 74 | 69 | 6.0 | <0.0001 | 49 |
| 0.25 | 53 | 80 | 72 | 64 | 4.6 | <0.0001 | 52 |
| 0.50 | 37 | 88 | 74 | 59 | 4.1 | 0.0006 | 60 |
Since clinical determinations by a single pulmonologist may be subjective, we sought to objectively assess the accuracy of assigned admission diagnoses by analyzing additional data, including bacterial tests, follow‐up radiographs, and final discharge diagnoses. As a surrogate of invasive bacterial infection, we used identification of S. pneumoniae as the outcome, since multiple complementary diagnostic assays were used, thus minimizing the uncertainty of sputum cultures. Overall, we identified 58 pneumococcal infections (Table 4). The pulmonologist's admitting diagnosis of pneumonia captured a higher proportion (52% for any test positive) of S. pneumoniae cases than identification of a radiographic infiltrate by the radiologist (26%), and equivalent to the pulmonologist's radiographic reading (57%) or elevated ProCT alone (57%). There was an absolute 14% increase (27% relative increase) in the detection of S. pneumoniae infections by addition of ProCT to the pulmonologist's diagnosis. A similar analysis limited to the 164 subjects with indeterminate radiographs found that 13 of 19 (68%) of S. pneumoniae diagnoses had been assigned a clinical diagnosis of pneumonia by the pulmonologist.
| S. pneumonia Confirmed by Diagnostic Assay No. (%) | |||||
|---|---|---|---|---|---|
| Criteria | Blood Culture + (n = 7) | Urine Ag + (n = 27) | Serology + (n = 28) | Sputum + (n = 20) | Any Test + (n = 58) |
| |||||
| Radiologist CXR read as infiltrate | 3 (43) | 9 (33) | 10 (36) | 3 (15) | 15 (26) |
| Pulmonologist CXR read as infiltrate | 6 (86) | 17 (62) | 18 (64) | 10 (50) | 33 (57) |
| Pulmonologist clinical diagnosis of pneumonia | 6 (86) | 16 (60) | 16 (57) | 8 (40) | 30 (52) |
| Pulmonologist clinical diagnosis of AECOPD | 0 (0) | 4 (15) | 5 (18) | 5 (25) | 8 (14) |
| Procalcitonin 0.25 ng/ml | 6 (86) | 18 (67) | 17 (61) | 9 (45) | 33 (57) |
| Pulmonologist diagnosis of pneumonia and/or ProCT 0.25 ng/ml | 7 (100) | 19 (70) | 20 (71) | 10 (50) | 38 (66) |
We also determined how often subsequent radiographs showed evolution to a definitive infiltrate in indeterminate cases that the pulmonologist had classified as either NAD or other finding. Only 3/20 with repeat films showed a definitive infiltrate, none of whom had evidence of bacterial infection, and all 3 had ProCT <0.25 ng/ml. In contrast, 10 of 21 subjects with indeterminate CXRs, that the pulmonologist had classified as an infiltrate, developed definitive infiltrates on follow‐up studies (P = 0.04), and 8 remained indeterminate.
Finally, 93% of those assigned an admitting pneumonia diagnosis by the pulmonologist had primary (71%) or secondary (22%) discharge diagnosis of pneumonia. Of the additional 28 subjects with a discharge diagnosis of pneumonia, for whom the pulmonologist assigned non‐pneumonia admitting diagnoses, none had positive blood cultures or evidence of S. pneumoniae infection, and only one had a follow‐up radiograph showing a definitive infiltrate.
DISCUSSION
Pneumonia can be a serious illness and, if left untreated, result in death. Hence, infectious disease and pulmonary societies advocate timely administration of antibiotics. Unfortunately, clinical diagnosis of pneumonia is difficult, as clinical and radiographic features are often ambiguous. These factors result in liberal use of broad‐spectrum antibiotics, often in patients with viral infection, in whom antibiotic side effects outweigh benefit.[26] Although pneumonia guidelines recommend early antibiotic therapy for patients with infiltrates on CXR, we found that early administration of antibiotics for possible pneumonia is nearly universal.[2, 27] Thus, an objective laboratory test indicative of pneumonia would be a useful adjunct when making treatment decisions.
In an attempt to curb excessive antibiotic use, European investigators have used ProCT to guide therapy for respiratory tract infection, including pneumonia, without increased adverse outcomes.[17, 19, 28, 29] Antibiotics are recommended when ProCT levels are 0.25 ng/ml, rather than based on radiographic or microbiological results. However, ProCT‐guided antibiotic management has not been adopted in the United States, and radiographic findings are a major determinant for diagnosis of pneumonia and antibiotic administration.
Thus, we were interested in correlating ProCT levels with the presence of radiographic infiltrates, and especially how ProCT could assist in interpreting the vexing problem of indeterminate CXRs. We found a direct relationship between an increased ProCT level and definitive infiltrates on CXR, similar to results reported by others.[18, 20, 21] However, these studies did not address illnesses with indeterminate radiographs, which accounted for approximately one‐third of the radiologist's readings in our study. Our situation is likely not unique. In a post hoc analysis of CXRs from subjects with pneumonia in the PORT study, independent radiologists interpreted a similar proportion of CXRs as either no acute disease or possible infiltrates.[30] Since indeterminate readings include the possibility of an infiltrate, it is not surprising that 97% of our subjects received early antibiotic treatment.
Given current pneumonia treatment guidelines, ProCT testing for those with definitive infiltrates would not add to patient management, as all should receive early antibiotics. However, ProCT measurement could have significant impact on management in those with indeterminate films. We found ProCT was moderately predictive for infiltrates when utilizing the pulmonologist's interpretation of indeterminate CXRs. This is concordant with a recent study reporting a similar ROC (0.72) for ProCT for diagnosing pneumonia in patients with dyspnea, and more predictive of pneumonia than any clinical variable.[31]
The major limitation of our study is the lack of a gold standard for diagnosis of pneumonia. We chose to use a pulmonary specialist to provide admission diagnoses, and independently interpret radiographs in the context of clinical findings. Although imperfect, we feel this approach best reflects good medical practice by an experienced clinician evaluating the CXR in context of a patient's history and physical exam. Importantly, analysis of the concurrent microbiology data, follow‐up radiographs, and discharge diagnoses supports the accuracy of the admitting diagnoses of pneumonia and radiographic interpretations by the pulmonologist. Secondly, our findings may not be applicable to immunocompromised or previously treated patients.
How should a busy physician respond to a patient with dyspnea and fever when faced with an indeterminate radiographic report, since no method for the diagnosis of pneumonia is infallible?[8, 32] Clearly, a pulmonary consultation on all such patients is impractical. Our data confirms previous results that high ProCT values are correlated with an infiltrate on CXR and low values have a NPV of nearly 90%. Furthermore, low ProCT values predict low mortality across all levels of standard severity scoring indexes.[33] Thus, a low ProCT may help clinicians feel more confident to delay or stop antibiotics in a patient in whom clinical suspicion for bacterial pneumonia is low, yet the radiographic report states pneumonia cannot be ruled out.[34] Such strategies may assist in reducing unnecessary antibiotic use in the United States.[35]
Disclosures
Funding for this study was provided by NIAID‐1R01AI079446‐01. This work was presented in part at the Infectious Diseases Society of America, Boston, MA, October 2011. The authors report no conflicts of interest.
- , , , et al. The burden of community‐acquired pneumonia in seniors: results of a population‐based study. Clin Infect Dis. 2004;39:1642–1650.
- , , , et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72.
- Centers for Medicare and Medicaid Services. The Medicare national pneumonia quality improvement project. Available at: http://www.cms.gov/HospitalQualityInits/. Accessed September 8, 2011.
- , , , , . Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community‐acquired pneumonia. Arch Intern Med. 2004;164:637–644.
- , , , et al. Quality of care, process, and outcomes in elderly patients with pneumonia. JAMA. 1997;278:2080–2084.
- , , , . Misdiagnosis of community‐acquired pneumonia and inappropriate utilization of antibiotics: side effects of the 4‐h antibiotic administration rule. Chest. 2007;131:1865–1869.
- , , . Antibiotic timing and errors in diagnosing pneumonia. Arch Intern Med. 2008;168:351–356.
- , , , . Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med. 2004;117:305–311.
- , , , , . Reliability of radiographic findings and the relation to etiologic agents in community‐acquired pneumonia. Respir Med. 2006;100:926–932.
- , , , . Admission chest radiograph lacks sensitivity in the diagnosis of community‐acquired pneumonia. Am J Med Sci. 2009;337:236–240.
- , . Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis. 2011;52:1232–1240.
- , , , et al. The epidemic of antibiotic‐resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:155–164.
- , , , , . Clinical review 167: procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J Clin Endocrinol Metab. 2004;89:1512–1525.
- , , . Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations. Crit Care Med. 2008;36:941–952.
- , , . Procalcitonin in sepsis and systemic inflammation: a harmful biomarker and a therapeutic target. Br J Pharmacol. 2010;159:253–264.
- , , , , . Pneumonitis‐associated hyperprocalcitoninemia. Am J Med Sci. 1996;312:12–18.
- , , , et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin‐guidance with standard therapy. Chest. 2007;131:9–19.
- , , , et al. Procalcitonin versus C‐reactive protein for predicting pneumonia in adults with lower respiratory tract infection in primary care. Br J Gen Pract. 2007;57:555–560.
- , , , et al. Effect of procalcitonin‐based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302:1059–1066.
- , , , et al. Procalcitonin and C‐reactive protein in hospitalized adult patients with community‐acquired pneumonia or exacerbation of asthma or COPD. Chest. 2011;139:1410–1418.
- , , , et al. Diagnostic and prognostic accuracy of clinical and laboratory parameters in community‐acquired pneumonia. BMC Infect Dis. 2008;7:10.
- , , , et al. Utility of serum procalcitonin values in patients with acute exacerbations of chronic obstructive pulmonary disease: a cautionary note. Int J Chron Obstruct Pulmon Dis. 2112;7:127–135.
- , , . Procalcitonin and its component peptides in systemic inflammation: immunochemical characterization. J Investig Med. 1997;45:552–560.
- , , , . The future diagnostic role of procalcitonin levels: the need for improved sensitivity. Clin Infect Dis. 2003;36:823–824.
- , , , et al. Calcitonin precursor levels in human medullary thyroid carcinoma. Thyroid 2003;13:819–822.
- , , , . Emergency department visits for antibiotic‐associated adverse events. Clin Infect Dis. 2008;47:735–743.
- , , , et al. Guideline tyranny: a response to the article by Baum and Kaltsas. Clin Infect Dis. 2008;47:1117–1118.
- , , , et al. Procalcitonin guidance of antibiotic therapy in community‐acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174:84–93.
- , , , et al. Effect of procalcitonin‐guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster‐randomised, single‐blinded intervention trial. Lancet. 2004;363:600–607.
- , , , et al. Interobserver reliability of the chest radiograph in community‐acquired pneumonia. PORT investigators. Chest. 1996;110:343–350.
- , , , et al. Use of procalcitonin for the diagnosis of pneumonia in patients presenting with a chief complaint of dyspnea: results from the BACH (Biomarkers in Acute Heart Failure) trial. Eur J Heart Fail. 2012;14:278–286.
- , , , , . A multicenter analysis of the ED diagnosis of pneumonia. Am J Emerg Med. 2010;28:862–865.
- , , , et al. Procalcitonin predicts patients at low risk of death from community‐acquired pneumonia across all CRB‐65 classes. Eur Respir J. 2008;31:349–355.
- , , , , , . Antibiotic timing and diagnostic uncertainty in Medicare patients with pneumonia: is it reasonable to expect all patients to receive antibiotics within 4 hours? Chest. 2006;130:16–21.
- . Procalcitonin for triage of patients with respiratory tract symptoms: a case study in the trial design process for approval of a new diagnostic test for lower respiratory tract infection. Clin Infect Dis. 2011;52(S4):S351–S356.
- , , , et al. The burden of community‐acquired pneumonia in seniors: results of a population‐based study. Clin Infect Dis. 2004;39:1642–1650.
- , , , et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72.
- Centers for Medicare and Medicaid Services. The Medicare national pneumonia quality improvement project. Available at: http://www.cms.gov/HospitalQualityInits/. Accessed September 8, 2011.
- , , , , . Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community‐acquired pneumonia. Arch Intern Med. 2004;164:637–644.
- , , , et al. Quality of care, process, and outcomes in elderly patients with pneumonia. JAMA. 1997;278:2080–2084.
- , , , . Misdiagnosis of community‐acquired pneumonia and inappropriate utilization of antibiotics: side effects of the 4‐h antibiotic administration rule. Chest. 2007;131:1865–1869.
- , , . Antibiotic timing and errors in diagnosing pneumonia. Arch Intern Med. 2008;168:351–356.
- , , , . Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med. 2004;117:305–311.
- , , , , . Reliability of radiographic findings and the relation to etiologic agents in community‐acquired pneumonia. Respir Med. 2006;100:926–932.
- , , , . Admission chest radiograph lacks sensitivity in the diagnosis of community‐acquired pneumonia. Am J Med Sci. 2009;337:236–240.
- , . Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis. 2011;52:1232–1240.
- , , , et al. The epidemic of antibiotic‐resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:155–164.
- , , , , . Clinical review 167: procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J Clin Endocrinol Metab. 2004;89:1512–1525.
- , , . Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations. Crit Care Med. 2008;36:941–952.
- , , . Procalcitonin in sepsis and systemic inflammation: a harmful biomarker and a therapeutic target. Br J Pharmacol. 2010;159:253–264.
- , , , , . Pneumonitis‐associated hyperprocalcitoninemia. Am J Med Sci. 1996;312:12–18.
- , , , et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin‐guidance with standard therapy. Chest. 2007;131:9–19.
- , , , et al. Procalcitonin versus C‐reactive protein for predicting pneumonia in adults with lower respiratory tract infection in primary care. Br J Gen Pract. 2007;57:555–560.
- , , , et al. Effect of procalcitonin‐based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302:1059–1066.
- , , , et al. Procalcitonin and C‐reactive protein in hospitalized adult patients with community‐acquired pneumonia or exacerbation of asthma or COPD. Chest. 2011;139:1410–1418.
- , , , et al. Diagnostic and prognostic accuracy of clinical and laboratory parameters in community‐acquired pneumonia. BMC Infect Dis. 2008;7:10.
- , , , et al. Utility of serum procalcitonin values in patients with acute exacerbations of chronic obstructive pulmonary disease: a cautionary note. Int J Chron Obstruct Pulmon Dis. 2112;7:127–135.
- , , . Procalcitonin and its component peptides in systemic inflammation: immunochemical characterization. J Investig Med. 1997;45:552–560.
- , , , . The future diagnostic role of procalcitonin levels: the need for improved sensitivity. Clin Infect Dis. 2003;36:823–824.
- , , , et al. Calcitonin precursor levels in human medullary thyroid carcinoma. Thyroid 2003;13:819–822.
- , , , . Emergency department visits for antibiotic‐associated adverse events. Clin Infect Dis. 2008;47:735–743.
- , , , et al. Guideline tyranny: a response to the article by Baum and Kaltsas. Clin Infect Dis. 2008;47:1117–1118.
- , , , et al. Procalcitonin guidance of antibiotic therapy in community‐acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174:84–93.
- , , , et al. Effect of procalcitonin‐guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster‐randomised, single‐blinded intervention trial. Lancet. 2004;363:600–607.
- , , , et al. Interobserver reliability of the chest radiograph in community‐acquired pneumonia. PORT investigators. Chest. 1996;110:343–350.
- , , , et al. Use of procalcitonin for the diagnosis of pneumonia in patients presenting with a chief complaint of dyspnea: results from the BACH (Biomarkers in Acute Heart Failure) trial. Eur J Heart Fail. 2012;14:278–286.
- , , , , . A multicenter analysis of the ED diagnosis of pneumonia. Am J Emerg Med. 2010;28:862–865.
- , , , et al. Procalcitonin predicts patients at low risk of death from community‐acquired pneumonia across all CRB‐65 classes. Eur Respir J. 2008;31:349–355.
- , , , , , . Antibiotic timing and diagnostic uncertainty in Medicare patients with pneumonia: is it reasonable to expect all patients to receive antibiotics within 4 hours? Chest. 2006;130:16–21.
- . Procalcitonin for triage of patients with respiratory tract symptoms: a case study in the trial design process for approval of a new diagnostic test for lower respiratory tract infection. Clin Infect Dis. 2011;52(S4):S351–S356.
Copyright © 2012 Society of Hospital Medicine
Can Lapatinib Prevent Brain Metastases from Breast Cancer? Data 'Inconclusive'
VIENNA – Mixed results in phase II and III clinical trials leave open the question of whether lapatinib can prevent brain metastases in women with HER2-positive breast cancer.
Lapatinib (Tykerb) did not decrease the development of brain metastases when compared with trastuzumab (Herceptin) – each added to capecitabine (Xeloda) – in the open-label phase III CEREBREL trial. The results were inconclusive, however, for the primary end point of the incidence of central nervous system events as a first sign of relapse.
Favorable findings were reported from the 45-patient phase II LANDSCAPE study. Two-thirds of patients had an objective CNS response to up-front lapatinib plus capecitabine in the single-arm study.
Investigators from both trials reported outcomes at the European Society for Medical Oncology Congress.
‘No Conclusion Can Be Made...’
In the CEREBREL trial, 251 women were treated with lapatinib in combination with capecitabine. Eight (3%) exhibited CNS progression as the first site of relapse. In comparison, 12 (5%) of 250 women given trastuzumab plus capecitabine exhibited CNS progression (P = .360).
The incidence of CNS progression at any time (7% vs. 6%, respectively) and the median time to first CNS progression (5.7 vs. 4.4 months) also did not differ significantly.
"No conclusion should be made from these results," said Dr. Xavier Pivot of the Université de Franche-Comté in Besançon, France.
Dr. Pivot noted that a very low rate of CNS events had occurred in the trial because of the stringent accrual process, undermining the conclusions that can be drawn.
Trial Excluded Asymptomatic Brain Metastases
"The CEREBREL study was a front-line study, but the problem was that asymptomatic brain metastases were being screened out of the population," Dr. Stephen Johnston, who was not involved the study, commented in an interview.
"The overall incidence of brain metastases that they found in the study was a lot lower than they were anticipating, so they were never going to meet their end point," added Dr. Johnston, a consultant medical oncologist and director of clinical research and development at the Royal Marsden and the Institute of Cancer Research in London.
CEREBREL enrolled 540 of a planned 650 women with HER2-positive metastatic breast cancer who had received prior treatment with an anthracycline or taxane but who had no CNS metastases. To ensure that no metastases were present, patients had a baseline MRI scan, with 20% of women excluded because they had asymptomatic lesions.
In total, 271 women received lapatinib (1,250 mg/day) plus capecitabine (2,000 mg/m2/day on days 1-14 every 21 days) and 269 trastuzumab (6 mg/kg every 21 days) plus capecitabine (2,500 mg/m2/day on days 1-14 every 21 days).
PFS Longer with Trastuzumab and Capecitabine
The trial results also showed that median progression-free survival (PFS) was longer in patients who received trastuzumab in combination with the chemotherapy than in those who received lapatinib (8 months vs. 6.6 months, hazard ratio 1.3, P = .021).
This effect dissipated, however, when prior treatment with trastuzumab was considered; it had been received by 62% of patients in the lapatinib-containing arm and 59% of patients in the trastuzumab-containing arm.
While there was no difference in PFS among patients who had previously been treated with trastuzumab, those who had never received the drug before the trial appeared to obtain a greater benefit.
Commenting on these data, Dr. Johnston noted that they do seem to suggest that the combination of lapatinib and capecitabine was not equivalent and was actually inferior to trastuzumab plus capecitabine.
Nevertheless, "I think the question about the effects of lapatinib on brain metastases is still relevant," he said.
"We’re doing a trial [LANTERN] of lapatinib-capecitabine versus continuing the trastuzumab and adding in capecitabine, to see if switching the HER2-targeting keeps the brain disease under control for longer," Dr. Johnston explained. "This is where lapatinib may still have a role."
LANDSCAPE Results Favorable
The primary hypothesis of the phase II LANDSCAPE study was that up-front treatment with lapatinib might help prevent brain metastasis in HER2-positive metastatic breast cancer and delay the need for whole-brain radiation and its associated neurotoxicity, said study investigator Dr. Thomas Bachelot, on behalf of the Unicancer Federation Française group.
Dr. Bachelot of INSERM in Lyon said the response and overall survival results compared favorably with published data for whole-brain radiotherapy (WBRT).
Two thirds (66%) of patients treated with lapatinib and capecitabine exhibited a CNS objective response: 46% achieved a reduction in brain metastases of 50%-80%, and 20% exhibited a reduction in brain metastases of 80% or more. The median time to progression was 5.5 months, and the median time to WBRT was 7.8 months. Median overall survival was 17 months.
"This strategy could help delay whole-brain radiotherapy and its associated [neurological] toxicity," Dr. Bachelot concluded, noting that the up-front use of lapatinib and capecitabine warrants further evaluation.
The first analysis of the LANDSCAPE trial was presented at the American Society of Clinical Oncology (ASCO) in 2011.
Both the CEREBREL and LANDSCAPE studies were supported by funding from GlaxoSmithKline. All authors have received research support or consultancy fees from GlaxoSmithKline and Roche. Dr. Bachelot and Dr. Johnston have also received consultancy fees from Novartis.
VIENNA – Mixed results in phase II and III clinical trials leave open the question of whether lapatinib can prevent brain metastases in women with HER2-positive breast cancer.
Lapatinib (Tykerb) did not decrease the development of brain metastases when compared with trastuzumab (Herceptin) – each added to capecitabine (Xeloda) – in the open-label phase III CEREBREL trial. The results were inconclusive, however, for the primary end point of the incidence of central nervous system events as a first sign of relapse.
Favorable findings were reported from the 45-patient phase II LANDSCAPE study. Two-thirds of patients had an objective CNS response to up-front lapatinib plus capecitabine in the single-arm study.
Investigators from both trials reported outcomes at the European Society for Medical Oncology Congress.
‘No Conclusion Can Be Made...’
In the CEREBREL trial, 251 women were treated with lapatinib in combination with capecitabine. Eight (3%) exhibited CNS progression as the first site of relapse. In comparison, 12 (5%) of 250 women given trastuzumab plus capecitabine exhibited CNS progression (P = .360).
The incidence of CNS progression at any time (7% vs. 6%, respectively) and the median time to first CNS progression (5.7 vs. 4.4 months) also did not differ significantly.
"No conclusion should be made from these results," said Dr. Xavier Pivot of the Université de Franche-Comté in Besançon, France.
Dr. Pivot noted that a very low rate of CNS events had occurred in the trial because of the stringent accrual process, undermining the conclusions that can be drawn.
Trial Excluded Asymptomatic Brain Metastases
"The CEREBREL study was a front-line study, but the problem was that asymptomatic brain metastases were being screened out of the population," Dr. Stephen Johnston, who was not involved the study, commented in an interview.
"The overall incidence of brain metastases that they found in the study was a lot lower than they were anticipating, so they were never going to meet their end point," added Dr. Johnston, a consultant medical oncologist and director of clinical research and development at the Royal Marsden and the Institute of Cancer Research in London.
CEREBREL enrolled 540 of a planned 650 women with HER2-positive metastatic breast cancer who had received prior treatment with an anthracycline or taxane but who had no CNS metastases. To ensure that no metastases were present, patients had a baseline MRI scan, with 20% of women excluded because they had asymptomatic lesions.
In total, 271 women received lapatinib (1,250 mg/day) plus capecitabine (2,000 mg/m2/day on days 1-14 every 21 days) and 269 trastuzumab (6 mg/kg every 21 days) plus capecitabine (2,500 mg/m2/day on days 1-14 every 21 days).
PFS Longer with Trastuzumab and Capecitabine
The trial results also showed that median progression-free survival (PFS) was longer in patients who received trastuzumab in combination with the chemotherapy than in those who received lapatinib (8 months vs. 6.6 months, hazard ratio 1.3, P = .021).
This effect dissipated, however, when prior treatment with trastuzumab was considered; it had been received by 62% of patients in the lapatinib-containing arm and 59% of patients in the trastuzumab-containing arm.
While there was no difference in PFS among patients who had previously been treated with trastuzumab, those who had never received the drug before the trial appeared to obtain a greater benefit.
Commenting on these data, Dr. Johnston noted that they do seem to suggest that the combination of lapatinib and capecitabine was not equivalent and was actually inferior to trastuzumab plus capecitabine.
Nevertheless, "I think the question about the effects of lapatinib on brain metastases is still relevant," he said.
"We’re doing a trial [LANTERN] of lapatinib-capecitabine versus continuing the trastuzumab and adding in capecitabine, to see if switching the HER2-targeting keeps the brain disease under control for longer," Dr. Johnston explained. "This is where lapatinib may still have a role."
LANDSCAPE Results Favorable
The primary hypothesis of the phase II LANDSCAPE study was that up-front treatment with lapatinib might help prevent brain metastasis in HER2-positive metastatic breast cancer and delay the need for whole-brain radiation and its associated neurotoxicity, said study investigator Dr. Thomas Bachelot, on behalf of the Unicancer Federation Française group.
Dr. Bachelot of INSERM in Lyon said the response and overall survival results compared favorably with published data for whole-brain radiotherapy (WBRT).
Two thirds (66%) of patients treated with lapatinib and capecitabine exhibited a CNS objective response: 46% achieved a reduction in brain metastases of 50%-80%, and 20% exhibited a reduction in brain metastases of 80% or more. The median time to progression was 5.5 months, and the median time to WBRT was 7.8 months. Median overall survival was 17 months.
"This strategy could help delay whole-brain radiotherapy and its associated [neurological] toxicity," Dr. Bachelot concluded, noting that the up-front use of lapatinib and capecitabine warrants further evaluation.
The first analysis of the LANDSCAPE trial was presented at the American Society of Clinical Oncology (ASCO) in 2011.
Both the CEREBREL and LANDSCAPE studies were supported by funding from GlaxoSmithKline. All authors have received research support or consultancy fees from GlaxoSmithKline and Roche. Dr. Bachelot and Dr. Johnston have also received consultancy fees from Novartis.
VIENNA – Mixed results in phase II and III clinical trials leave open the question of whether lapatinib can prevent brain metastases in women with HER2-positive breast cancer.
Lapatinib (Tykerb) did not decrease the development of brain metastases when compared with trastuzumab (Herceptin) – each added to capecitabine (Xeloda) – in the open-label phase III CEREBREL trial. The results were inconclusive, however, for the primary end point of the incidence of central nervous system events as a first sign of relapse.
Favorable findings were reported from the 45-patient phase II LANDSCAPE study. Two-thirds of patients had an objective CNS response to up-front lapatinib plus capecitabine in the single-arm study.
Investigators from both trials reported outcomes at the European Society for Medical Oncology Congress.
‘No Conclusion Can Be Made...’
In the CEREBREL trial, 251 women were treated with lapatinib in combination with capecitabine. Eight (3%) exhibited CNS progression as the first site of relapse. In comparison, 12 (5%) of 250 women given trastuzumab plus capecitabine exhibited CNS progression (P = .360).
The incidence of CNS progression at any time (7% vs. 6%, respectively) and the median time to first CNS progression (5.7 vs. 4.4 months) also did not differ significantly.
"No conclusion should be made from these results," said Dr. Xavier Pivot of the Université de Franche-Comté in Besançon, France.
Dr. Pivot noted that a very low rate of CNS events had occurred in the trial because of the stringent accrual process, undermining the conclusions that can be drawn.
Trial Excluded Asymptomatic Brain Metastases
"The CEREBREL study was a front-line study, but the problem was that asymptomatic brain metastases were being screened out of the population," Dr. Stephen Johnston, who was not involved the study, commented in an interview.
"The overall incidence of brain metastases that they found in the study was a lot lower than they were anticipating, so they were never going to meet their end point," added Dr. Johnston, a consultant medical oncologist and director of clinical research and development at the Royal Marsden and the Institute of Cancer Research in London.
CEREBREL enrolled 540 of a planned 650 women with HER2-positive metastatic breast cancer who had received prior treatment with an anthracycline or taxane but who had no CNS metastases. To ensure that no metastases were present, patients had a baseline MRI scan, with 20% of women excluded because they had asymptomatic lesions.
In total, 271 women received lapatinib (1,250 mg/day) plus capecitabine (2,000 mg/m2/day on days 1-14 every 21 days) and 269 trastuzumab (6 mg/kg every 21 days) plus capecitabine (2,500 mg/m2/day on days 1-14 every 21 days).
PFS Longer with Trastuzumab and Capecitabine
The trial results also showed that median progression-free survival (PFS) was longer in patients who received trastuzumab in combination with the chemotherapy than in those who received lapatinib (8 months vs. 6.6 months, hazard ratio 1.3, P = .021).
This effect dissipated, however, when prior treatment with trastuzumab was considered; it had been received by 62% of patients in the lapatinib-containing arm and 59% of patients in the trastuzumab-containing arm.
While there was no difference in PFS among patients who had previously been treated with trastuzumab, those who had never received the drug before the trial appeared to obtain a greater benefit.
Commenting on these data, Dr. Johnston noted that they do seem to suggest that the combination of lapatinib and capecitabine was not equivalent and was actually inferior to trastuzumab plus capecitabine.
Nevertheless, "I think the question about the effects of lapatinib on brain metastases is still relevant," he said.
"We’re doing a trial [LANTERN] of lapatinib-capecitabine versus continuing the trastuzumab and adding in capecitabine, to see if switching the HER2-targeting keeps the brain disease under control for longer," Dr. Johnston explained. "This is where lapatinib may still have a role."
LANDSCAPE Results Favorable
The primary hypothesis of the phase II LANDSCAPE study was that up-front treatment with lapatinib might help prevent brain metastasis in HER2-positive metastatic breast cancer and delay the need for whole-brain radiation and its associated neurotoxicity, said study investigator Dr. Thomas Bachelot, on behalf of the Unicancer Federation Française group.
Dr. Bachelot of INSERM in Lyon said the response and overall survival results compared favorably with published data for whole-brain radiotherapy (WBRT).
Two thirds (66%) of patients treated with lapatinib and capecitabine exhibited a CNS objective response: 46% achieved a reduction in brain metastases of 50%-80%, and 20% exhibited a reduction in brain metastases of 80% or more. The median time to progression was 5.5 months, and the median time to WBRT was 7.8 months. Median overall survival was 17 months.
"This strategy could help delay whole-brain radiotherapy and its associated [neurological] toxicity," Dr. Bachelot concluded, noting that the up-front use of lapatinib and capecitabine warrants further evaluation.
The first analysis of the LANDSCAPE trial was presented at the American Society of Clinical Oncology (ASCO) in 2011.
Both the CEREBREL and LANDSCAPE studies were supported by funding from GlaxoSmithKline. All authors have received research support or consultancy fees from GlaxoSmithKline and Roche. Dr. Bachelot and Dr. Johnston have also received consultancy fees from Novartis.
AT THE EUROPEAN SOCIETY FOR MEDICAL ONCOLOGY CONGRESS
Major Findings: The primary end point of CNS as first site of relapse was no different comparing lapatinib-capecitabine (3%) vs. trastuzumab-capecitabine (5%) in the CEREBREL trial. The LANDSCAPE study showed a CNS objective response in 66% of patients treated with up-front lapatinib plus capecitabine.
Data Source: CEREBREL was an open-label phase III study, and LANDSCAPE was a phase II study. Both enrolled women with metastatic breast cancer.
Disclosures: Both studies were supported by funding from GlaxoSmithKline. All authors have received research support or consultancy fees from GlaxoSmithKline and Roche. Dr. Bachelot and Dr. Johnston have also received consultancy fees from Novartis.
Society of Hospital Medicine Joins Fight to Delay Medicare Cuts that Reduce Pay for Hospitalists
SHM has joined scores of medical societies pushing Congress to stop pending cuts to Medicare that would directly impact hospitalists.
Scheduled to go into effect at the start of the New Year, the cuts include sequestration, which would reduce hospitalists' Medicare payments by 2%, and slash funding to the National Institutes of Health (NIH) and the Centers for Disease Control and Prevention (CDC). This is in addition to a 27% cut to Medicare physician payment rates resulting from Medicare's sustainable growth rate (SGR) formula.
In a letter last month to congressional leaders [PDF], and an accompanying note to society members, SHM said hospitalists need to lobby legislators "to find a reasonable and measured solution to deficit reduction that does not include arbitrary across-the-board cuts to Medicare providers."
"Congress needs to know we're not happy," says SHM board member Eric Siegal, MD, SFHM, board liaison SHM's Public Policy Committee. "The only way that we are going to get them to change their behavior is if enough of us mobilize, and make enough noise to make it clear that we are not going to stand for this anymore."
Dr. Siegal says that because Congress has repeatedly delayed draconian cuts, there is a general consensus that another delay is likely. But Dr. Siegal also notes lobbying is still necessary to ensure that will happen. SHM has previously supported a meaningful replacement to the SGR, which has yet to receive significant action in Congress.
"What the entire healthcare community needs to push for is a solution," Dr. Siegal adds. "It's very hard to develop any kind of a strategy for how you're going to deliver care if every X number of months you have to worry [whether] you're going to take a massive cut in your compensation."
SHM has joined scores of medical societies pushing Congress to stop pending cuts to Medicare that would directly impact hospitalists.
Scheduled to go into effect at the start of the New Year, the cuts include sequestration, which would reduce hospitalists' Medicare payments by 2%, and slash funding to the National Institutes of Health (NIH) and the Centers for Disease Control and Prevention (CDC). This is in addition to a 27% cut to Medicare physician payment rates resulting from Medicare's sustainable growth rate (SGR) formula.
In a letter last month to congressional leaders [PDF], and an accompanying note to society members, SHM said hospitalists need to lobby legislators "to find a reasonable and measured solution to deficit reduction that does not include arbitrary across-the-board cuts to Medicare providers."
"Congress needs to know we're not happy," says SHM board member Eric Siegal, MD, SFHM, board liaison SHM's Public Policy Committee. "The only way that we are going to get them to change their behavior is if enough of us mobilize, and make enough noise to make it clear that we are not going to stand for this anymore."
Dr. Siegal says that because Congress has repeatedly delayed draconian cuts, there is a general consensus that another delay is likely. But Dr. Siegal also notes lobbying is still necessary to ensure that will happen. SHM has previously supported a meaningful replacement to the SGR, which has yet to receive significant action in Congress.
"What the entire healthcare community needs to push for is a solution," Dr. Siegal adds. "It's very hard to develop any kind of a strategy for how you're going to deliver care if every X number of months you have to worry [whether] you're going to take a massive cut in your compensation."
SHM has joined scores of medical societies pushing Congress to stop pending cuts to Medicare that would directly impact hospitalists.
Scheduled to go into effect at the start of the New Year, the cuts include sequestration, which would reduce hospitalists' Medicare payments by 2%, and slash funding to the National Institutes of Health (NIH) and the Centers for Disease Control and Prevention (CDC). This is in addition to a 27% cut to Medicare physician payment rates resulting from Medicare's sustainable growth rate (SGR) formula.
In a letter last month to congressional leaders [PDF], and an accompanying note to society members, SHM said hospitalists need to lobby legislators "to find a reasonable and measured solution to deficit reduction that does not include arbitrary across-the-board cuts to Medicare providers."
"Congress needs to know we're not happy," says SHM board member Eric Siegal, MD, SFHM, board liaison SHM's Public Policy Committee. "The only way that we are going to get them to change their behavior is if enough of us mobilize, and make enough noise to make it clear that we are not going to stand for this anymore."
Dr. Siegal says that because Congress has repeatedly delayed draconian cuts, there is a general consensus that another delay is likely. But Dr. Siegal also notes lobbying is still necessary to ensure that will happen. SHM has previously supported a meaningful replacement to the SGR, which has yet to receive significant action in Congress.
"What the entire healthcare community needs to push for is a solution," Dr. Siegal adds. "It's very hard to develop any kind of a strategy for how you're going to deliver care if every X number of months you have to worry [whether] you're going to take a massive cut in your compensation."
In the Literature: Research You Need to Know
Clinical question: What is the impact, and sustainability, of chlorhexidine bathing on central-venous-catheter-associated bloodstream infections?
Background: Chlorhexidine bathing has been associated with reductions in healthcare-associated bloodstream infections, including vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus. No prospective studies have evaluated the impact and sustainability of chlorhexidine bathing.
Study design: Prospective, three-phase study.
Setting: Medical-surgical ICUs and respiratory-care units at five New York hospitals.
Synopsis: In the pre-intervention phase (six to nine months, 1,808 admissions), patients were bathed with soap and water or nonmedicated bathing cloths. In the intervention phase (eight months, 1,832 admissions), patients were bathed with 2% chlorhexidine cloths. In the post-intervention phase (12 months, 2,834 admissions), chlorhexidine bathing was continued without oversight by researchers.
During the intervention phase, there were significantly fewer central-venous-catheter-associated bloodstream infections (2.6/1,000 catheter days vs. 6.4/1,000 pre-intervention). The reductions in bloodstream infections were sustained during the post-intervention period (2.9/1,000 catheter days). Compliance with chlorhexidine bathing was 82% and 88% during the intervention and post-intervention phases, and was well tolerated by the patients.
Limitations of this study include lack of patient-specific data and severity of illness data, as well as lack of randomization and blinding. Although not evaluated in this study, the savings associated with decreased bloodstream infections likely outweigh the cost of chlorhexidine bathing.
Bottom line: Chlorhexidine bathing is a well-tolerated, sustainable intervention that significantly reduces central-venous-catheter-associated bloodstream infections.
Citation: Montecalvo MA, McKenna D, Yarrish R, et al. Chlorhexidine bathing to reduce central venous catheter-associated bloodstream infection: impact and sustainability.Am J Med. 2012;125(5):505-511.
For more physician reviews of recent HM-relevant literature, visit our website.
Clinical question: What is the impact, and sustainability, of chlorhexidine bathing on central-venous-catheter-associated bloodstream infections?
Background: Chlorhexidine bathing has been associated with reductions in healthcare-associated bloodstream infections, including vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus. No prospective studies have evaluated the impact and sustainability of chlorhexidine bathing.
Study design: Prospective, three-phase study.
Setting: Medical-surgical ICUs and respiratory-care units at five New York hospitals.
Synopsis: In the pre-intervention phase (six to nine months, 1,808 admissions), patients were bathed with soap and water or nonmedicated bathing cloths. In the intervention phase (eight months, 1,832 admissions), patients were bathed with 2% chlorhexidine cloths. In the post-intervention phase (12 months, 2,834 admissions), chlorhexidine bathing was continued without oversight by researchers.
During the intervention phase, there were significantly fewer central-venous-catheter-associated bloodstream infections (2.6/1,000 catheter days vs. 6.4/1,000 pre-intervention). The reductions in bloodstream infections were sustained during the post-intervention period (2.9/1,000 catheter days). Compliance with chlorhexidine bathing was 82% and 88% during the intervention and post-intervention phases, and was well tolerated by the patients.
Limitations of this study include lack of patient-specific data and severity of illness data, as well as lack of randomization and blinding. Although not evaluated in this study, the savings associated with decreased bloodstream infections likely outweigh the cost of chlorhexidine bathing.
Bottom line: Chlorhexidine bathing is a well-tolerated, sustainable intervention that significantly reduces central-venous-catheter-associated bloodstream infections.
Citation: Montecalvo MA, McKenna D, Yarrish R, et al. Chlorhexidine bathing to reduce central venous catheter-associated bloodstream infection: impact and sustainability.Am J Med. 2012;125(5):505-511.
For more physician reviews of recent HM-relevant literature, visit our website.
Clinical question: What is the impact, and sustainability, of chlorhexidine bathing on central-venous-catheter-associated bloodstream infections?
Background: Chlorhexidine bathing has been associated with reductions in healthcare-associated bloodstream infections, including vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus. No prospective studies have evaluated the impact and sustainability of chlorhexidine bathing.
Study design: Prospective, three-phase study.
Setting: Medical-surgical ICUs and respiratory-care units at five New York hospitals.
Synopsis: In the pre-intervention phase (six to nine months, 1,808 admissions), patients were bathed with soap and water or nonmedicated bathing cloths. In the intervention phase (eight months, 1,832 admissions), patients were bathed with 2% chlorhexidine cloths. In the post-intervention phase (12 months, 2,834 admissions), chlorhexidine bathing was continued without oversight by researchers.
During the intervention phase, there were significantly fewer central-venous-catheter-associated bloodstream infections (2.6/1,000 catheter days vs. 6.4/1,000 pre-intervention). The reductions in bloodstream infections were sustained during the post-intervention period (2.9/1,000 catheter days). Compliance with chlorhexidine bathing was 82% and 88% during the intervention and post-intervention phases, and was well tolerated by the patients.
Limitations of this study include lack of patient-specific data and severity of illness data, as well as lack of randomization and blinding. Although not evaluated in this study, the savings associated with decreased bloodstream infections likely outweigh the cost of chlorhexidine bathing.
Bottom line: Chlorhexidine bathing is a well-tolerated, sustainable intervention that significantly reduces central-venous-catheter-associated bloodstream infections.
Citation: Montecalvo MA, McKenna D, Yarrish R, et al. Chlorhexidine bathing to reduce central venous catheter-associated bloodstream infection: impact and sustainability.Am J Med. 2012;125(5):505-511.
For more physician reviews of recent HM-relevant literature, visit our website.










