User login
Relationship Between Red Blood Cells and Protein Levels in Cerebrospinal Fluid in Young Infants Defined
Clinical question: What is the association between cerebrospinal fluid (CSF) red blood cell (RBC) counts and protein concentrations in infants younger than 57 days of age?
Background: Lumbar puncture (LP) is commonly performed in young infants to evaluate for meningitis in the clinical scenario of fever without source. Traumatic LP is common in children, and higher RBC counts are associated with increased CSF protein concentrations. The dynamic nature of CSF composition in young infants makes determination of the exact relationship between RBC counts and protein concentration challenging, which then complicates interpretation of CSF.
Study design: Retrospective, cross-sectional study.
Setting: Tertiary-care children's hospital.
Synopsis: Over a four-year period, 1,241 infants younger than 57 days of age that underwent LP were studied, excluding infants with conditions known to increase CSF protein concentrations: ventricular shunt, serious bacterial infection, congenital infection, herpes simplex virus or enterovirus positive PCR in CSF, seizure, or elevated serum bilirubin. Grossly bloody specimens with RBC counts >150,000 cells/mm3 were also excluded. Linear regression was used to determine relationship between CSF RBCs and protein, with protein increasing at a rate of 1.9 mg/dL per 1,000 CSF RBCs.
This ratio is different from a more traditional correction factor of approximately 1 mg/dL CSF protein increase per 1,000 CSF RBCs, which is derived from older populations of children.
However, this study is limited by the exclusion of grossly bloody specimens, which if included would have resulted in a ratio similar to the more traditional values. Additionally, application of this specific correction factor to prediction rules for bacterial meningitis has not been studied. Nonetheless, this study provides a baseline by which clinicians may interpret protein concentrations in traumatically bloody CSF specimens in young infants.
Bottom line: CSF protein concentrations increase at roughly 2 mg/dL per 1,000 CSF RBCs.
Citation: Hines BA, Nigrovic LE, Neuman MI, Shah SS. Adjustment of cerebrospinal fluid protein for red blood cells in neonates and young infants. J Hosp Med. 2012;7:325-328.
Reviewed by Pediatric Editor Mark Shen, MD, SFHM, medical director of hospital medicine at Dell Children's Medical Center, Austin, Texas.
Clinical question: What is the association between cerebrospinal fluid (CSF) red blood cell (RBC) counts and protein concentrations in infants younger than 57 days of age?
Background: Lumbar puncture (LP) is commonly performed in young infants to evaluate for meningitis in the clinical scenario of fever without source. Traumatic LP is common in children, and higher RBC counts are associated with increased CSF protein concentrations. The dynamic nature of CSF composition in young infants makes determination of the exact relationship between RBC counts and protein concentration challenging, which then complicates interpretation of CSF.
Study design: Retrospective, cross-sectional study.
Setting: Tertiary-care children's hospital.
Synopsis: Over a four-year period, 1,241 infants younger than 57 days of age that underwent LP were studied, excluding infants with conditions known to increase CSF protein concentrations: ventricular shunt, serious bacterial infection, congenital infection, herpes simplex virus or enterovirus positive PCR in CSF, seizure, or elevated serum bilirubin. Grossly bloody specimens with RBC counts >150,000 cells/mm3 were also excluded. Linear regression was used to determine relationship between CSF RBCs and protein, with protein increasing at a rate of 1.9 mg/dL per 1,000 CSF RBCs.
This ratio is different from a more traditional correction factor of approximately 1 mg/dL CSF protein increase per 1,000 CSF RBCs, which is derived from older populations of children.
However, this study is limited by the exclusion of grossly bloody specimens, which if included would have resulted in a ratio similar to the more traditional values. Additionally, application of this specific correction factor to prediction rules for bacterial meningitis has not been studied. Nonetheless, this study provides a baseline by which clinicians may interpret protein concentrations in traumatically bloody CSF specimens in young infants.
Bottom line: CSF protein concentrations increase at roughly 2 mg/dL per 1,000 CSF RBCs.
Citation: Hines BA, Nigrovic LE, Neuman MI, Shah SS. Adjustment of cerebrospinal fluid protein for red blood cells in neonates and young infants. J Hosp Med. 2012;7:325-328.
Reviewed by Pediatric Editor Mark Shen, MD, SFHM, medical director of hospital medicine at Dell Children's Medical Center, Austin, Texas.
Clinical question: What is the association between cerebrospinal fluid (CSF) red blood cell (RBC) counts and protein concentrations in infants younger than 57 days of age?
Background: Lumbar puncture (LP) is commonly performed in young infants to evaluate for meningitis in the clinical scenario of fever without source. Traumatic LP is common in children, and higher RBC counts are associated with increased CSF protein concentrations. The dynamic nature of CSF composition in young infants makes determination of the exact relationship between RBC counts and protein concentration challenging, which then complicates interpretation of CSF.
Study design: Retrospective, cross-sectional study.
Setting: Tertiary-care children's hospital.
Synopsis: Over a four-year period, 1,241 infants younger than 57 days of age that underwent LP were studied, excluding infants with conditions known to increase CSF protein concentrations: ventricular shunt, serious bacterial infection, congenital infection, herpes simplex virus or enterovirus positive PCR in CSF, seizure, or elevated serum bilirubin. Grossly bloody specimens with RBC counts >150,000 cells/mm3 were also excluded. Linear regression was used to determine relationship between CSF RBCs and protein, with protein increasing at a rate of 1.9 mg/dL per 1,000 CSF RBCs.
This ratio is different from a more traditional correction factor of approximately 1 mg/dL CSF protein increase per 1,000 CSF RBCs, which is derived from older populations of children.
However, this study is limited by the exclusion of grossly bloody specimens, which if included would have resulted in a ratio similar to the more traditional values. Additionally, application of this specific correction factor to prediction rules for bacterial meningitis has not been studied. Nonetheless, this study provides a baseline by which clinicians may interpret protein concentrations in traumatically bloody CSF specimens in young infants.
Bottom line: CSF protein concentrations increase at roughly 2 mg/dL per 1,000 CSF RBCs.
Citation: Hines BA, Nigrovic LE, Neuman MI, Shah SS. Adjustment of cerebrospinal fluid protein for red blood cells in neonates and young infants. J Hosp Med. 2012;7:325-328.
Reviewed by Pediatric Editor Mark Shen, MD, SFHM, medical director of hospital medicine at Dell Children's Medical Center, Austin, Texas.
How is Acute Pericarditis Diagnosed and Treated?
Case
A 32-year-old female with no significant past medical history is evaluated for sharp, left-sided chest pain for five days. Her pain is intermittent, worse with deep inspiration and in the supine position. She denies any shortness of breath. Her temperature is 100.8ºF, but otherwise her vital signs are normal. The physical exam and chest radiograph are unremarkable, but an electrocardiogram shows diffuse ST-segment elevations. The initial troponin is mildly elevated at 0.35 ng/ml.
Could this patient have acute pericarditis? If so, how should she be managed?
Background
Pericarditis is the most common pericardial disease encountered by hospitalists. As many as 5% of chest pain cases unattributable to myocardial infarction (MI) are diagnosed with pericarditis.1 In immunocompetent individuals, as many as 90% of acute pericarditis cases are viral or idiopathic in etiology.1,2 Human immunodeficiency virus (HIV) and tuberculosis are common culprits in developing countries and immunocompromised hosts.3 Other specific etiologies of acute pericarditis include autoimmune diseases, neoplasms, chest irradiation, trauma, and metabolic disturbances (e.g. uremia). An etiologic classification of acute pericarditis is shown in Table 2 (p. 16).
Pericarditis primarily is a clinical diagnosis. Most patients present with chest pain.4 A pericardial friction rub may or may not be heard (sensitivity 16% to 85%), but when present is nearly 100% specific for pericarditis.2,5 Diffuse ST-segment elevation on electrocardiogram (EKG) is present in 60% to 90% of cases, but it can be difficult to differentiate from ST-segment elevations in acute MI.4,6
Uncomplicated acute pericarditis often is treated successfully as an outpatient.4 However, patients with high-risk features (see Table 1, right) should be hospitalized for identification and treatment of specific underlying etiology and for monitoring of complications, such as tamponade.7
Our patient has features consistent with pericarditis. In the following sections, we will review the diagnosis and treatment of acute pericarditis.
Review of the Data
How is acute pericarditis diagnosed?
Acute pericarditis is a clinical diagnosis supported by EKG and echocardiogram. At least two of the following four criteria must be present for the diagnosis: pleuritic chest pain, pericardial rub, diffuse ST-segment elevation on EKG, and pericardial effusion.8
History. Patients may report fever (46% in one small study of 69 patients) or a recent history of respiratory or gastrointestinal infection (40%).5 Most patients will report pleuritic chest pain. Typically, the pain is improved when sitting up and leaning forward, and gets worse when lying supine.4 Pain might radiate to the trapezius muscle ridge due to the common phrenic nerve innervation of pericardium and trapezius.9 However, pain might be minimal or absent in patients with uremic, neoplastic, tuberculous, or post-irradiation pericarditis.
Physical exam. A pericardial friction rub is nearly 100% specific for a pericarditis diagnosis, but sensitivity can vary (16% to 85%) depending on the frequency of auscultation and underlying etiology.2,5 It is thought to be caused by friction between the parietal and visceral layers of inflamed pericardium. A pericardial rub classically is described as a superficial, high-pitched, scratchy, or squeaking sound best heard with the diaphragm of the stethoscope at the lower left sternal border with the patient leaning forward.
Laboratory data. A complete blood count, metabolic panel, and cardiac enzymes should be checked in all patients with suspected acute pericarditis. Troponin values are elevated in up to one-third of patients, indicating cardiac muscle injury or myopericarditis, but have not been shown to adversely impact hospital length of stay, readmission, or complication rates.5,10 Markers of inflammation (e.g. erythrocyte sedimentation rate or C-reactive protein) are frequently elevated but do not point to a specific underlying etiology. Routine viral cultures and antibody titers are not useful.11
Most cases of pericarditis are presumed idiopathic (viral); however, finding a specific etiology should be considered in patients who do not respond after one week of therapy. Anti-nuclear antibody, complement levels, and rheumatoid factor can serve as screening tests for autoimmune disease. Purified protein derivative or quantiferon testing and HIV testing might be indicated in patients with appropriate risk factors. In cases of suspected tuberculous or neoplastic pericarditis, pericardial fluid analysis and biopsy could be warranted.
Electrocardiography. The EKG is the most useful test in diagnosing acute pericarditis. EKG changes in acute pericarditis can progress over four stages:
- Stage 1: diffuse ST elevations with or without PR depressions, initially;
- Stage 2: normalization of ST and PR segments, typically after several days;
- Stage 3: diffuse T-wave inversions; and
- Stage 4: normalization of T-waves, typically after weeks or months.
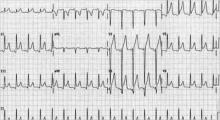
While all four stages are unlikely to be present in a given case, 80% of patients with pericarditis will demonstrate diffuse ST-segment elevations and PR-segment depression (see Figure 2, above).12
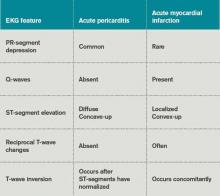
Table 3 lists EKG features helpful in differentiating acute pericarditis from acute myocardial infarction.
Chest radiography. Because a pericardial effusion often accompanies pericarditis, a chest radiograph (CXR) should be performed in all suspected cases. The CXR might show enlargement of the cardiac silhouette if more than 250 ml of pericardial fluid is present.3 A CXR also is helpful to diagnose concomitant pulmonary infection, pleural effusion, or mediastinal mass—all findings that could point to an underlying specific etiology of pericarditis and/or pericardial effusion.
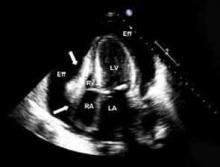
Echocardiography. An echocardiogram should be performed in all patients with suspected pericarditis to detect effusion, associated myocardial, or paracardial disease.13 The echocardiogram frequently is normal but could show an effusion in 60%, and tamponade (see Figure 1, p. 15) in 5%, of cases.4
Computed tomography (CT) and cardiac magnetic resonance imaging (CMR).CT or CMR are the imaging modalities of choice when an echocardiogram is inconclusive or in cases of pericarditis complicated by a hemorrhagic or localized effusion, pericardial thickening, or pericardial mass.14 They also help in precise imaging of neighboring structures, such as lungs or mediastinum.
Pericardial fluid analysis and pericardial biopsy. In cases of refractory pericarditis with effusion, pericardial fluid analysis might provide clues to the underlying etiology. Routine chemistry, cell count, gram and acid fast staining, culture, and cytology should be sent. In addition, acid-fast bacillus staining and culture, adenosine deaminase, and interferon-gamma testing should be ordered when tuberculous pericarditis is suspected. A pericardial biopsy may show granulomas or neoplastic cells. Overall, pericardial fluid analysis and biopsy reveal a diagnosis in roughly 20% of cases.11
How is acute pericarditis treated?
Most cases of uncomplicated acute pericarditis are viral and respond well to NSAID plus colchicine therapy.2,4 Failure to respond to NSAIDs plus colchicine—evidenced by persistent fever, pericardial chest pain, new pericardial effusion, or worsening of general illness—within a week of treatment should prompt a search for an underlying systemic illness. If found, treatment should be aimed at the causative illness.
Bacterial pericarditis usually requires surgical drainage in addition to treatment with appropriate antibiotics.11 Tuberculous pericarditis is treated with multidrug therapy; when underlying HIV is present, patients should receive highly active anti-retroviral therapy as well. Steroids and immunosuppressants should be considered in addition to NSAIDs and colchicine in autoimmune pericarditis.10 Neoplastic pericarditis may resolve with chemotherapy but it has a high recurrence rate.13 Uremic pericarditis requires intensified dialysis.
Treatment options for uncomplicated idiopathic or viral pericarditis include:
NSAIDs. It is important to adequately dose NSAIDs when treating acute pericarditis. Initial treatment options include ibuprofen (1,600 to 3,200 mg daily), indomethacin (75 to 150 mg daily) or aspirin (2 to 4 gm daily) for one week.11,15 Aspirin is preferred in patients with ischemic heart disease. For patients with symptoms that persist longer than a week, NSAIDS may be continued, but investigation for an underlying etiology is indicated. Concomitant proton-pump-inhibitor therapy should be considered in patients at high risk for peptic ulcer disease to minimize gastric side effects.
Colchicine. Colchicine has a favorable risk-benefit profile as an adjunct treatment for acute and recurrent pericarditis. Patients experience better symptom relief when treated with both colchicine and an NSAID, compared with NSAIDs alone (88% versus 63%). Recurrence rates are lower with combined therapy (11% versus 32%).16 Colchicine treatment (0.6 mg twice daily after a loading dose of up to 2 mg) is recommended for several months to greater than one year.13,16,17
Glucocorticoids. Routine glucocorticoid use should be avoided in the treatment of acute pericarditis, as it has been associated with an increased risk for recurrence (OR 4.3).16,18 Glucocorticoid use should be considered in cases of pericarditis refractory to NSAIDs and colchicine, cases in which NSAIDs and or colchicine are contraindicated, and in autoimmune or connective-tissue-disease-related pericarditis. Prednisone should be dosed up to 1 mg/kg/day for at least one month, depending on symptom resolution, then tapered after either NSAIDs or colchicine have been started.13 Smaller prednisone doses of up to 0.5 mg/kg/day could be as effective, with the added benefit of reduced side effects and recurrences.19
Invasive treatment. Pericardiocentesis and/or pericardiectomy should be considered when pericarditis is complicated by a large effusion or tamponade, constrictive physiology, or recurrent effusion.11 Pericardiocentesis is the least invasive option and helps provide immediate relief in cases of tamponade or large symptomatic effusions. It is the preferred modality for obtaining pericardial fluid for diagnostic analysis. However, effusions can recur and in those cases pericardial window is preferred, as it provides continued outflow of pericardial fluid. Pericardiectomy is recommended in cases of symptomatic constrictive pericarditis unresponsive to medical therapy.15
Back to the Case
The patient’s presentation—prodrome followed by fever and pleuritic chest pain—is characteristic of acute idiopathic pericarditis. No pericardial rub was heard, but EKG findings were typical. Troponin I elevation suggested underlying myopericarditis. An echocardiogram was unremarkable. Given the likely viral or idiopathic etiology, no further diagnostic tests were ordered to explore the possibility of an underlying systemic illness.
The patient was started on ibuprofen 600 mg every eight hours. She had significant relief of her symptoms within two days. A routine fever workup was negative. She was discharged the following day.
The patient was readmitted three months later with recurrent pleuritic chest pain, which did not improve with resumption of NSAID therapy. Initial troponin I was 0.22 ng/ml, electrocardiogram was unchanged, and an echocardiogram showed small effusion. She was started on ibuprofen 800 mg every eight hours, as well as colchicine 0.6 mg twice daily. Her symptoms resolved the next day and she was discharged with prescriptions for ibuprofen and colchicine. She was instructed to follow up with a primary-care doctor in one week.
At the clinic visit, ibuprofen was tapered but colchicine was continued for another six months. She remained asymptomatic at her six-month clinic follow-up.
Bottom Line
Acute pericarditis is a clinical diagnosis supported by EKG findings. Most cases are idiopathic or viral, and can be treated successfully with NSAIDs and colchicine. For cases that do not respond to initial therapy, or cases that present with high-risk features, a specific etiology should be sought.
Dr. Southern is chief of the division of hospital medicine at Montefiore Medical Center in Bronx, N.Y. Dr. Galhorta is an instructor and Drs. Martin, Korcak, and Stehlihova are assistant professors in the department of medicine at Albert Einstein.
References
- Lange RA, Hillis LD. Clinical practice. Acute pericarditis. N Engl J Med. 2004;351:2195-2202.
- Zayas R, Anguita M, Torres F, et al. Incidence of specific etiology and role of methods for specific etiologic diagnosis of primary acute pericarditis. Am J Cardiol. 1995;75:378-382.
- Troughton RW, Asher CR, Klein AL. Pericarditis. Lancet. 2004;363:717-727.
- Imazio M, Demichelis B, Parrini I, et al. Day-hospital treatment of acute pericarditis: a management program for outpatient therapy. J Am Coll Cardiol. 2004;43:1042-1046.
- Bonnefoy E, Godon P, Kirkorian G, et al. Serum cardiac troponin I and ST-segment elevation in patients with acute pericarditis. Eur Heart J. 2000;21:832-836.
- Salisbury AC, Olalla-Gomez C, Rihal CS, et al. Frequency and predictors of urgent coronary angiography in patients with acute pericarditis. Mayo Clin Proc. 2009;84(1):11-15.
- Imazio M, Cecchi E, Demichelis B, et al. Indicators of poor prognosis of acute pericarditis. Circulation. 2007;115:2739-2744.
- Imazio M, Spodick DH, Brucato A, et al. Diagnostic issues in the clinical management of pericarditis. Int J Clin Pract. 2010;64(10):1384-1392.
- Spodick DH. Acute pericarditis: current concepts and practice. JAMA. 2003;289:1150-1153.
- Imazio M, Demichelis B, Cecchi E. Cardiac troponin I in acute pericarditis. J Am Coll Cardiol. 2003;42(12):2144-2148.
- Sagristà Sauleda J, Permanyer Miralda G, Soler Soler J. Diagnosis and management of pericardial syndromes. Rev Esp Cardiol. 2005;58(7):830-841.
- Bruce MA, Spodick DH. Atypical electrocardiogram in acute pericarditis: characteristics and prevalence. J Electrocardiol. 1980;13:61-66.
- Maisch B, Seferovic PM, Ristic AD, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; the task force on the diagnosis and management of pericardial diseases of the European Society of Cardiology. Eur Heart J. 2004; 25(7):587-610.
- Verhaert D, Gabriel RS, Johnston D, et al. The role of multimodality imaging in the management of pericardial disease. Circ Cardiovasc Imaging. 2010;3:333-343.
- Imazio M, Spodick DH, Brucato A, et al. Controversial issues in the management of pericardial diseases. Circulation. 2010;121:916-928.
- Imazio M, Bobbio M, Cecchi E, et al. Colchicine in addition to conventional therapy for acute pericarditis: results of the colchicine for acute pericarditis (COPE) trial. Circulation. 2005;112(13):2012-2016.
- Adler Y, Finkelstein Y, Guindo J, et al. Colchicine treatment for recurrent pericarditis: a decade of experience. Circulation. 1998;97:2183-185.
- Imazio M, Bobbio M, Cecchi E, et al. Colchicine as first-choice therapy for recurrent pericarditis: results of the colchicine for recurrent pericarditis (CORE) trial. Arch Intern Med. 2005;165:1987-1991.
- Imazio M, Brucato A, Cumetti D, et al. Corticosteroids for recurrent pericarditis: high versus low doses: a nonrandomized observation. Circulation. 2008;118:667-771.
Case
A 32-year-old female with no significant past medical history is evaluated for sharp, left-sided chest pain for five days. Her pain is intermittent, worse with deep inspiration and in the supine position. She denies any shortness of breath. Her temperature is 100.8ºF, but otherwise her vital signs are normal. The physical exam and chest radiograph are unremarkable, but an electrocardiogram shows diffuse ST-segment elevations. The initial troponin is mildly elevated at 0.35 ng/ml.
Could this patient have acute pericarditis? If so, how should she be managed?
Background
Pericarditis is the most common pericardial disease encountered by hospitalists. As many as 5% of chest pain cases unattributable to myocardial infarction (MI) are diagnosed with pericarditis.1 In immunocompetent individuals, as many as 90% of acute pericarditis cases are viral or idiopathic in etiology.1,2 Human immunodeficiency virus (HIV) and tuberculosis are common culprits in developing countries and immunocompromised hosts.3 Other specific etiologies of acute pericarditis include autoimmune diseases, neoplasms, chest irradiation, trauma, and metabolic disturbances (e.g. uremia). An etiologic classification of acute pericarditis is shown in Table 2 (p. 16).
Pericarditis primarily is a clinical diagnosis. Most patients present with chest pain.4 A pericardial friction rub may or may not be heard (sensitivity 16% to 85%), but when present is nearly 100% specific for pericarditis.2,5 Diffuse ST-segment elevation on electrocardiogram (EKG) is present in 60% to 90% of cases, but it can be difficult to differentiate from ST-segment elevations in acute MI.4,6
Uncomplicated acute pericarditis often is treated successfully as an outpatient.4 However, patients with high-risk features (see Table 1, right) should be hospitalized for identification and treatment of specific underlying etiology and for monitoring of complications, such as tamponade.7
Our patient has features consistent with pericarditis. In the following sections, we will review the diagnosis and treatment of acute pericarditis.
Review of the Data
How is acute pericarditis diagnosed?
Acute pericarditis is a clinical diagnosis supported by EKG and echocardiogram. At least two of the following four criteria must be present for the diagnosis: pleuritic chest pain, pericardial rub, diffuse ST-segment elevation on EKG, and pericardial effusion.8
History. Patients may report fever (46% in one small study of 69 patients) or a recent history of respiratory or gastrointestinal infection (40%).5 Most patients will report pleuritic chest pain. Typically, the pain is improved when sitting up and leaning forward, and gets worse when lying supine.4 Pain might radiate to the trapezius muscle ridge due to the common phrenic nerve innervation of pericardium and trapezius.9 However, pain might be minimal or absent in patients with uremic, neoplastic, tuberculous, or post-irradiation pericarditis.
Physical exam. A pericardial friction rub is nearly 100% specific for a pericarditis diagnosis, but sensitivity can vary (16% to 85%) depending on the frequency of auscultation and underlying etiology.2,5 It is thought to be caused by friction between the parietal and visceral layers of inflamed pericardium. A pericardial rub classically is described as a superficial, high-pitched, scratchy, or squeaking sound best heard with the diaphragm of the stethoscope at the lower left sternal border with the patient leaning forward.
Laboratory data. A complete blood count, metabolic panel, and cardiac enzymes should be checked in all patients with suspected acute pericarditis. Troponin values are elevated in up to one-third of patients, indicating cardiac muscle injury or myopericarditis, but have not been shown to adversely impact hospital length of stay, readmission, or complication rates.5,10 Markers of inflammation (e.g. erythrocyte sedimentation rate or C-reactive protein) are frequently elevated but do not point to a specific underlying etiology. Routine viral cultures and antibody titers are not useful.11
Most cases of pericarditis are presumed idiopathic (viral); however, finding a specific etiology should be considered in patients who do not respond after one week of therapy. Anti-nuclear antibody, complement levels, and rheumatoid factor can serve as screening tests for autoimmune disease. Purified protein derivative or quantiferon testing and HIV testing might be indicated in patients with appropriate risk factors. In cases of suspected tuberculous or neoplastic pericarditis, pericardial fluid analysis and biopsy could be warranted.
Electrocardiography. The EKG is the most useful test in diagnosing acute pericarditis. EKG changes in acute pericarditis can progress over four stages:
- Stage 1: diffuse ST elevations with or without PR depressions, initially;
- Stage 2: normalization of ST and PR segments, typically after several days;
- Stage 3: diffuse T-wave inversions; and
- Stage 4: normalization of T-waves, typically after weeks or months.
While all four stages are unlikely to be present in a given case, 80% of patients with pericarditis will demonstrate diffuse ST-segment elevations and PR-segment depression (see Figure 2, above).12
Table 3 lists EKG features helpful in differentiating acute pericarditis from acute myocardial infarction.
Chest radiography. Because a pericardial effusion often accompanies pericarditis, a chest radiograph (CXR) should be performed in all suspected cases. The CXR might show enlargement of the cardiac silhouette if more than 250 ml of pericardial fluid is present.3 A CXR also is helpful to diagnose concomitant pulmonary infection, pleural effusion, or mediastinal mass—all findings that could point to an underlying specific etiology of pericarditis and/or pericardial effusion.
Echocardiography. An echocardiogram should be performed in all patients with suspected pericarditis to detect effusion, associated myocardial, or paracardial disease.13 The echocardiogram frequently is normal but could show an effusion in 60%, and tamponade (see Figure 1, p. 15) in 5%, of cases.4
Computed tomography (CT) and cardiac magnetic resonance imaging (CMR).CT or CMR are the imaging modalities of choice when an echocardiogram is inconclusive or in cases of pericarditis complicated by a hemorrhagic or localized effusion, pericardial thickening, or pericardial mass.14 They also help in precise imaging of neighboring structures, such as lungs or mediastinum.
Pericardial fluid analysis and pericardial biopsy. In cases of refractory pericarditis with effusion, pericardial fluid analysis might provide clues to the underlying etiology. Routine chemistry, cell count, gram and acid fast staining, culture, and cytology should be sent. In addition, acid-fast bacillus staining and culture, adenosine deaminase, and interferon-gamma testing should be ordered when tuberculous pericarditis is suspected. A pericardial biopsy may show granulomas or neoplastic cells. Overall, pericardial fluid analysis and biopsy reveal a diagnosis in roughly 20% of cases.11
How is acute pericarditis treated?
Most cases of uncomplicated acute pericarditis are viral and respond well to NSAID plus colchicine therapy.2,4 Failure to respond to NSAIDs plus colchicine—evidenced by persistent fever, pericardial chest pain, new pericardial effusion, or worsening of general illness—within a week of treatment should prompt a search for an underlying systemic illness. If found, treatment should be aimed at the causative illness.
Bacterial pericarditis usually requires surgical drainage in addition to treatment with appropriate antibiotics.11 Tuberculous pericarditis is treated with multidrug therapy; when underlying HIV is present, patients should receive highly active anti-retroviral therapy as well. Steroids and immunosuppressants should be considered in addition to NSAIDs and colchicine in autoimmune pericarditis.10 Neoplastic pericarditis may resolve with chemotherapy but it has a high recurrence rate.13 Uremic pericarditis requires intensified dialysis.
Treatment options for uncomplicated idiopathic or viral pericarditis include:
NSAIDs. It is important to adequately dose NSAIDs when treating acute pericarditis. Initial treatment options include ibuprofen (1,600 to 3,200 mg daily), indomethacin (75 to 150 mg daily) or aspirin (2 to 4 gm daily) for one week.11,15 Aspirin is preferred in patients with ischemic heart disease. For patients with symptoms that persist longer than a week, NSAIDS may be continued, but investigation for an underlying etiology is indicated. Concomitant proton-pump-inhibitor therapy should be considered in patients at high risk for peptic ulcer disease to minimize gastric side effects.
Colchicine. Colchicine has a favorable risk-benefit profile as an adjunct treatment for acute and recurrent pericarditis. Patients experience better symptom relief when treated with both colchicine and an NSAID, compared with NSAIDs alone (88% versus 63%). Recurrence rates are lower with combined therapy (11% versus 32%).16 Colchicine treatment (0.6 mg twice daily after a loading dose of up to 2 mg) is recommended for several months to greater than one year.13,16,17
Glucocorticoids. Routine glucocorticoid use should be avoided in the treatment of acute pericarditis, as it has been associated with an increased risk for recurrence (OR 4.3).16,18 Glucocorticoid use should be considered in cases of pericarditis refractory to NSAIDs and colchicine, cases in which NSAIDs and or colchicine are contraindicated, and in autoimmune or connective-tissue-disease-related pericarditis. Prednisone should be dosed up to 1 mg/kg/day for at least one month, depending on symptom resolution, then tapered after either NSAIDs or colchicine have been started.13 Smaller prednisone doses of up to 0.5 mg/kg/day could be as effective, with the added benefit of reduced side effects and recurrences.19
Invasive treatment. Pericardiocentesis and/or pericardiectomy should be considered when pericarditis is complicated by a large effusion or tamponade, constrictive physiology, or recurrent effusion.11 Pericardiocentesis is the least invasive option and helps provide immediate relief in cases of tamponade or large symptomatic effusions. It is the preferred modality for obtaining pericardial fluid for diagnostic analysis. However, effusions can recur and in those cases pericardial window is preferred, as it provides continued outflow of pericardial fluid. Pericardiectomy is recommended in cases of symptomatic constrictive pericarditis unresponsive to medical therapy.15
Back to the Case
The patient’s presentation—prodrome followed by fever and pleuritic chest pain—is characteristic of acute idiopathic pericarditis. No pericardial rub was heard, but EKG findings were typical. Troponin I elevation suggested underlying myopericarditis. An echocardiogram was unremarkable. Given the likely viral or idiopathic etiology, no further diagnostic tests were ordered to explore the possibility of an underlying systemic illness.
The patient was started on ibuprofen 600 mg every eight hours. She had significant relief of her symptoms within two days. A routine fever workup was negative. She was discharged the following day.
The patient was readmitted three months later with recurrent pleuritic chest pain, which did not improve with resumption of NSAID therapy. Initial troponin I was 0.22 ng/ml, electrocardiogram was unchanged, and an echocardiogram showed small effusion. She was started on ibuprofen 800 mg every eight hours, as well as colchicine 0.6 mg twice daily. Her symptoms resolved the next day and she was discharged with prescriptions for ibuprofen and colchicine. She was instructed to follow up with a primary-care doctor in one week.
At the clinic visit, ibuprofen was tapered but colchicine was continued for another six months. She remained asymptomatic at her six-month clinic follow-up.
Bottom Line
Acute pericarditis is a clinical diagnosis supported by EKG findings. Most cases are idiopathic or viral, and can be treated successfully with NSAIDs and colchicine. For cases that do not respond to initial therapy, or cases that present with high-risk features, a specific etiology should be sought.
Dr. Southern is chief of the division of hospital medicine at Montefiore Medical Center in Bronx, N.Y. Dr. Galhorta is an instructor and Drs. Martin, Korcak, and Stehlihova are assistant professors in the department of medicine at Albert Einstein.
References
- Lange RA, Hillis LD. Clinical practice. Acute pericarditis. N Engl J Med. 2004;351:2195-2202.
- Zayas R, Anguita M, Torres F, et al. Incidence of specific etiology and role of methods for specific etiologic diagnosis of primary acute pericarditis. Am J Cardiol. 1995;75:378-382.
- Troughton RW, Asher CR, Klein AL. Pericarditis. Lancet. 2004;363:717-727.
- Imazio M, Demichelis B, Parrini I, et al. Day-hospital treatment of acute pericarditis: a management program for outpatient therapy. J Am Coll Cardiol. 2004;43:1042-1046.
- Bonnefoy E, Godon P, Kirkorian G, et al. Serum cardiac troponin I and ST-segment elevation in patients with acute pericarditis. Eur Heart J. 2000;21:832-836.
- Salisbury AC, Olalla-Gomez C, Rihal CS, et al. Frequency and predictors of urgent coronary angiography in patients with acute pericarditis. Mayo Clin Proc. 2009;84(1):11-15.
- Imazio M, Cecchi E, Demichelis B, et al. Indicators of poor prognosis of acute pericarditis. Circulation. 2007;115:2739-2744.
- Imazio M, Spodick DH, Brucato A, et al. Diagnostic issues in the clinical management of pericarditis. Int J Clin Pract. 2010;64(10):1384-1392.
- Spodick DH. Acute pericarditis: current concepts and practice. JAMA. 2003;289:1150-1153.
- Imazio M, Demichelis B, Cecchi E. Cardiac troponin I in acute pericarditis. J Am Coll Cardiol. 2003;42(12):2144-2148.
- Sagristà Sauleda J, Permanyer Miralda G, Soler Soler J. Diagnosis and management of pericardial syndromes. Rev Esp Cardiol. 2005;58(7):830-841.
- Bruce MA, Spodick DH. Atypical electrocardiogram in acute pericarditis: characteristics and prevalence. J Electrocardiol. 1980;13:61-66.
- Maisch B, Seferovic PM, Ristic AD, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; the task force on the diagnosis and management of pericardial diseases of the European Society of Cardiology. Eur Heart J. 2004; 25(7):587-610.
- Verhaert D, Gabriel RS, Johnston D, et al. The role of multimodality imaging in the management of pericardial disease. Circ Cardiovasc Imaging. 2010;3:333-343.
- Imazio M, Spodick DH, Brucato A, et al. Controversial issues in the management of pericardial diseases. Circulation. 2010;121:916-928.
- Imazio M, Bobbio M, Cecchi E, et al. Colchicine in addition to conventional therapy for acute pericarditis: results of the colchicine for acute pericarditis (COPE) trial. Circulation. 2005;112(13):2012-2016.
- Adler Y, Finkelstein Y, Guindo J, et al. Colchicine treatment for recurrent pericarditis: a decade of experience. Circulation. 1998;97:2183-185.
- Imazio M, Bobbio M, Cecchi E, et al. Colchicine as first-choice therapy for recurrent pericarditis: results of the colchicine for recurrent pericarditis (CORE) trial. Arch Intern Med. 2005;165:1987-1991.
- Imazio M, Brucato A, Cumetti D, et al. Corticosteroids for recurrent pericarditis: high versus low doses: a nonrandomized observation. Circulation. 2008;118:667-771.
Case
A 32-year-old female with no significant past medical history is evaluated for sharp, left-sided chest pain for five days. Her pain is intermittent, worse with deep inspiration and in the supine position. She denies any shortness of breath. Her temperature is 100.8ºF, but otherwise her vital signs are normal. The physical exam and chest radiograph are unremarkable, but an electrocardiogram shows diffuse ST-segment elevations. The initial troponin is mildly elevated at 0.35 ng/ml.
Could this patient have acute pericarditis? If so, how should she be managed?
Background
Pericarditis is the most common pericardial disease encountered by hospitalists. As many as 5% of chest pain cases unattributable to myocardial infarction (MI) are diagnosed with pericarditis.1 In immunocompetent individuals, as many as 90% of acute pericarditis cases are viral or idiopathic in etiology.1,2 Human immunodeficiency virus (HIV) and tuberculosis are common culprits in developing countries and immunocompromised hosts.3 Other specific etiologies of acute pericarditis include autoimmune diseases, neoplasms, chest irradiation, trauma, and metabolic disturbances (e.g. uremia). An etiologic classification of acute pericarditis is shown in Table 2 (p. 16).
Pericarditis primarily is a clinical diagnosis. Most patients present with chest pain.4 A pericardial friction rub may or may not be heard (sensitivity 16% to 85%), but when present is nearly 100% specific for pericarditis.2,5 Diffuse ST-segment elevation on electrocardiogram (EKG) is present in 60% to 90% of cases, but it can be difficult to differentiate from ST-segment elevations in acute MI.4,6
Uncomplicated acute pericarditis often is treated successfully as an outpatient.4 However, patients with high-risk features (see Table 1, right) should be hospitalized for identification and treatment of specific underlying etiology and for monitoring of complications, such as tamponade.7
Our patient has features consistent with pericarditis. In the following sections, we will review the diagnosis and treatment of acute pericarditis.
Review of the Data
How is acute pericarditis diagnosed?
Acute pericarditis is a clinical diagnosis supported by EKG and echocardiogram. At least two of the following four criteria must be present for the diagnosis: pleuritic chest pain, pericardial rub, diffuse ST-segment elevation on EKG, and pericardial effusion.8
History. Patients may report fever (46% in one small study of 69 patients) or a recent history of respiratory or gastrointestinal infection (40%).5 Most patients will report pleuritic chest pain. Typically, the pain is improved when sitting up and leaning forward, and gets worse when lying supine.4 Pain might radiate to the trapezius muscle ridge due to the common phrenic nerve innervation of pericardium and trapezius.9 However, pain might be minimal or absent in patients with uremic, neoplastic, tuberculous, or post-irradiation pericarditis.
Physical exam. A pericardial friction rub is nearly 100% specific for a pericarditis diagnosis, but sensitivity can vary (16% to 85%) depending on the frequency of auscultation and underlying etiology.2,5 It is thought to be caused by friction between the parietal and visceral layers of inflamed pericardium. A pericardial rub classically is described as a superficial, high-pitched, scratchy, or squeaking sound best heard with the diaphragm of the stethoscope at the lower left sternal border with the patient leaning forward.
Laboratory data. A complete blood count, metabolic panel, and cardiac enzymes should be checked in all patients with suspected acute pericarditis. Troponin values are elevated in up to one-third of patients, indicating cardiac muscle injury or myopericarditis, but have not been shown to adversely impact hospital length of stay, readmission, or complication rates.5,10 Markers of inflammation (e.g. erythrocyte sedimentation rate or C-reactive protein) are frequently elevated but do not point to a specific underlying etiology. Routine viral cultures and antibody titers are not useful.11
Most cases of pericarditis are presumed idiopathic (viral); however, finding a specific etiology should be considered in patients who do not respond after one week of therapy. Anti-nuclear antibody, complement levels, and rheumatoid factor can serve as screening tests for autoimmune disease. Purified protein derivative or quantiferon testing and HIV testing might be indicated in patients with appropriate risk factors. In cases of suspected tuberculous or neoplastic pericarditis, pericardial fluid analysis and biopsy could be warranted.
Electrocardiography. The EKG is the most useful test in diagnosing acute pericarditis. EKG changes in acute pericarditis can progress over four stages:
- Stage 1: diffuse ST elevations with or without PR depressions, initially;
- Stage 2: normalization of ST and PR segments, typically after several days;
- Stage 3: diffuse T-wave inversions; and
- Stage 4: normalization of T-waves, typically after weeks or months.
While all four stages are unlikely to be present in a given case, 80% of patients with pericarditis will demonstrate diffuse ST-segment elevations and PR-segment depression (see Figure 2, above).12
Table 3 lists EKG features helpful in differentiating acute pericarditis from acute myocardial infarction.
Chest radiography. Because a pericardial effusion often accompanies pericarditis, a chest radiograph (CXR) should be performed in all suspected cases. The CXR might show enlargement of the cardiac silhouette if more than 250 ml of pericardial fluid is present.3 A CXR also is helpful to diagnose concomitant pulmonary infection, pleural effusion, or mediastinal mass—all findings that could point to an underlying specific etiology of pericarditis and/or pericardial effusion.
Echocardiography. An echocardiogram should be performed in all patients with suspected pericarditis to detect effusion, associated myocardial, or paracardial disease.13 The echocardiogram frequently is normal but could show an effusion in 60%, and tamponade (see Figure 1, p. 15) in 5%, of cases.4
Computed tomography (CT) and cardiac magnetic resonance imaging (CMR).CT or CMR are the imaging modalities of choice when an echocardiogram is inconclusive or in cases of pericarditis complicated by a hemorrhagic or localized effusion, pericardial thickening, or pericardial mass.14 They also help in precise imaging of neighboring structures, such as lungs or mediastinum.
Pericardial fluid analysis and pericardial biopsy. In cases of refractory pericarditis with effusion, pericardial fluid analysis might provide clues to the underlying etiology. Routine chemistry, cell count, gram and acid fast staining, culture, and cytology should be sent. In addition, acid-fast bacillus staining and culture, adenosine deaminase, and interferon-gamma testing should be ordered when tuberculous pericarditis is suspected. A pericardial biopsy may show granulomas or neoplastic cells. Overall, pericardial fluid analysis and biopsy reveal a diagnosis in roughly 20% of cases.11
How is acute pericarditis treated?
Most cases of uncomplicated acute pericarditis are viral and respond well to NSAID plus colchicine therapy.2,4 Failure to respond to NSAIDs plus colchicine—evidenced by persistent fever, pericardial chest pain, new pericardial effusion, or worsening of general illness—within a week of treatment should prompt a search for an underlying systemic illness. If found, treatment should be aimed at the causative illness.
Bacterial pericarditis usually requires surgical drainage in addition to treatment with appropriate antibiotics.11 Tuberculous pericarditis is treated with multidrug therapy; when underlying HIV is present, patients should receive highly active anti-retroviral therapy as well. Steroids and immunosuppressants should be considered in addition to NSAIDs and colchicine in autoimmune pericarditis.10 Neoplastic pericarditis may resolve with chemotherapy but it has a high recurrence rate.13 Uremic pericarditis requires intensified dialysis.
Treatment options for uncomplicated idiopathic or viral pericarditis include:
NSAIDs. It is important to adequately dose NSAIDs when treating acute pericarditis. Initial treatment options include ibuprofen (1,600 to 3,200 mg daily), indomethacin (75 to 150 mg daily) or aspirin (2 to 4 gm daily) for one week.11,15 Aspirin is preferred in patients with ischemic heart disease. For patients with symptoms that persist longer than a week, NSAIDS may be continued, but investigation for an underlying etiology is indicated. Concomitant proton-pump-inhibitor therapy should be considered in patients at high risk for peptic ulcer disease to minimize gastric side effects.
Colchicine. Colchicine has a favorable risk-benefit profile as an adjunct treatment for acute and recurrent pericarditis. Patients experience better symptom relief when treated with both colchicine and an NSAID, compared with NSAIDs alone (88% versus 63%). Recurrence rates are lower with combined therapy (11% versus 32%).16 Colchicine treatment (0.6 mg twice daily after a loading dose of up to 2 mg) is recommended for several months to greater than one year.13,16,17
Glucocorticoids. Routine glucocorticoid use should be avoided in the treatment of acute pericarditis, as it has been associated with an increased risk for recurrence (OR 4.3).16,18 Glucocorticoid use should be considered in cases of pericarditis refractory to NSAIDs and colchicine, cases in which NSAIDs and or colchicine are contraindicated, and in autoimmune or connective-tissue-disease-related pericarditis. Prednisone should be dosed up to 1 mg/kg/day for at least one month, depending on symptom resolution, then tapered after either NSAIDs or colchicine have been started.13 Smaller prednisone doses of up to 0.5 mg/kg/day could be as effective, with the added benefit of reduced side effects and recurrences.19
Invasive treatment. Pericardiocentesis and/or pericardiectomy should be considered when pericarditis is complicated by a large effusion or tamponade, constrictive physiology, or recurrent effusion.11 Pericardiocentesis is the least invasive option and helps provide immediate relief in cases of tamponade or large symptomatic effusions. It is the preferred modality for obtaining pericardial fluid for diagnostic analysis. However, effusions can recur and in those cases pericardial window is preferred, as it provides continued outflow of pericardial fluid. Pericardiectomy is recommended in cases of symptomatic constrictive pericarditis unresponsive to medical therapy.15
Back to the Case
The patient’s presentation—prodrome followed by fever and pleuritic chest pain—is characteristic of acute idiopathic pericarditis. No pericardial rub was heard, but EKG findings were typical. Troponin I elevation suggested underlying myopericarditis. An echocardiogram was unremarkable. Given the likely viral or idiopathic etiology, no further diagnostic tests were ordered to explore the possibility of an underlying systemic illness.
The patient was started on ibuprofen 600 mg every eight hours. She had significant relief of her symptoms within two days. A routine fever workup was negative. She was discharged the following day.
The patient was readmitted three months later with recurrent pleuritic chest pain, which did not improve with resumption of NSAID therapy. Initial troponin I was 0.22 ng/ml, electrocardiogram was unchanged, and an echocardiogram showed small effusion. She was started on ibuprofen 800 mg every eight hours, as well as colchicine 0.6 mg twice daily. Her symptoms resolved the next day and she was discharged with prescriptions for ibuprofen and colchicine. She was instructed to follow up with a primary-care doctor in one week.
At the clinic visit, ibuprofen was tapered but colchicine was continued for another six months. She remained asymptomatic at her six-month clinic follow-up.
Bottom Line
Acute pericarditis is a clinical diagnosis supported by EKG findings. Most cases are idiopathic or viral, and can be treated successfully with NSAIDs and colchicine. For cases that do not respond to initial therapy, or cases that present with high-risk features, a specific etiology should be sought.
Dr. Southern is chief of the division of hospital medicine at Montefiore Medical Center in Bronx, N.Y. Dr. Galhorta is an instructor and Drs. Martin, Korcak, and Stehlihova are assistant professors in the department of medicine at Albert Einstein.
References
- Lange RA, Hillis LD. Clinical practice. Acute pericarditis. N Engl J Med. 2004;351:2195-2202.
- Zayas R, Anguita M, Torres F, et al. Incidence of specific etiology and role of methods for specific etiologic diagnosis of primary acute pericarditis. Am J Cardiol. 1995;75:378-382.
- Troughton RW, Asher CR, Klein AL. Pericarditis. Lancet. 2004;363:717-727.
- Imazio M, Demichelis B, Parrini I, et al. Day-hospital treatment of acute pericarditis: a management program for outpatient therapy. J Am Coll Cardiol. 2004;43:1042-1046.
- Bonnefoy E, Godon P, Kirkorian G, et al. Serum cardiac troponin I and ST-segment elevation in patients with acute pericarditis. Eur Heart J. 2000;21:832-836.
- Salisbury AC, Olalla-Gomez C, Rihal CS, et al. Frequency and predictors of urgent coronary angiography in patients with acute pericarditis. Mayo Clin Proc. 2009;84(1):11-15.
- Imazio M, Cecchi E, Demichelis B, et al. Indicators of poor prognosis of acute pericarditis. Circulation. 2007;115:2739-2744.
- Imazio M, Spodick DH, Brucato A, et al. Diagnostic issues in the clinical management of pericarditis. Int J Clin Pract. 2010;64(10):1384-1392.
- Spodick DH. Acute pericarditis: current concepts and practice. JAMA. 2003;289:1150-1153.
- Imazio M, Demichelis B, Cecchi E. Cardiac troponin I in acute pericarditis. J Am Coll Cardiol. 2003;42(12):2144-2148.
- Sagristà Sauleda J, Permanyer Miralda G, Soler Soler J. Diagnosis and management of pericardial syndromes. Rev Esp Cardiol. 2005;58(7):830-841.
- Bruce MA, Spodick DH. Atypical electrocardiogram in acute pericarditis: characteristics and prevalence. J Electrocardiol. 1980;13:61-66.
- Maisch B, Seferovic PM, Ristic AD, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; the task force on the diagnosis and management of pericardial diseases of the European Society of Cardiology. Eur Heart J. 2004; 25(7):587-610.
- Verhaert D, Gabriel RS, Johnston D, et al. The role of multimodality imaging in the management of pericardial disease. Circ Cardiovasc Imaging. 2010;3:333-343.
- Imazio M, Spodick DH, Brucato A, et al. Controversial issues in the management of pericardial diseases. Circulation. 2010;121:916-928.
- Imazio M, Bobbio M, Cecchi E, et al. Colchicine in addition to conventional therapy for acute pericarditis: results of the colchicine for acute pericarditis (COPE) trial. Circulation. 2005;112(13):2012-2016.
- Adler Y, Finkelstein Y, Guindo J, et al. Colchicine treatment for recurrent pericarditis: a decade of experience. Circulation. 1998;97:2183-185.
- Imazio M, Bobbio M, Cecchi E, et al. Colchicine as first-choice therapy for recurrent pericarditis: results of the colchicine for recurrent pericarditis (CORE) trial. Arch Intern Med. 2005;165:1987-1991.
- Imazio M, Brucato A, Cumetti D, et al. Corticosteroids for recurrent pericarditis: high versus low doses: a nonrandomized observation. Circulation. 2008;118:667-771.
Surprise patients with the truth about pain and aging
Healthcare Quality Accounting Metrics Need Improvement

—Gregg Meyer, MD, MSc, chief clinical officer and executive vice president for population health for the Dartmouth-Hitchcock Health System in Lebanon, N.H.
As healthcare quality reporting continues to evolve in this era of value-based purchasing (VBP), players on both the giving and receiving ends of performance incentives agree on the need to improve the accountability metrics with which providers are measured, ranked, rewarded, and penalized. Many of the measures currently in use—e.g., Centers for Medicare & Medicaid Services’ (CMS) core process measures and patient satisfaction ratings, the gross outcome metrics of mortality, infection, and readmission rates—are blunt instruments in need of refinement.
Entities such as the National Quality Forum (NQF), the American Medical Association’s Physician Consortium for Performance Improvement (PCPI), and the National Quality Measures Clearinghouse (NQMC) recognize the need to develop and endorse more timely, credible, and patient-centered outcome metrics. Largely missing from the current crop of outcome measure sets is a meaningful account of the patient’s perspective.
Enter patient-reported outcomes (PROs), defined as “any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else.”1 PRO tools “measure what patients are able to do and how they feel by asking questions” (see “Types of Patient-Reported Outcomes [PROs],” p. 19).
If successfully adapted for public reporting on a wide scale, PROs could become the next evolutionary step in healthcare quality reporting, integrating health status and patient experience data into outcome metrics that truly matter to patients. They could enable a richer understanding of their clinical experiences and responses to therapy, and help providers target necessary improvements with greater precision.
“As a provider, I care about my patients not developing infections, getting the right medications, and not being readmitted. Patients, however, have a different set of priorities around issues like ‘How quickly will I be able to return to work? When will I be able to chase my grandkids around the yard? How much is this care going to cost me out of pocket?’” says healthcare quality expert Gregg Meyer, MD, MSc, chief clinical officer and executive vice president for population health for the Dartmouth-Hitchcock Health System in Lebanon, N.H. “This next generation of accountability will allow us to move from being provider- and payor-centered to becoming truly patient-centered, and will serve as a key reminder that patients are no longer passive participants. They are key partners, in both the delivery of care and the measurement of that care.”
The idea of PROs is one whose “time has finally arrived,” according to medical outcomes researcher David Cella, PhD, professor and chair of the Department of Medical Social Sciences at Northwestern University Feinberg School of Medicine in Chicago.
“The case for inclusion of outcomes that matter most to patients, like the effect of treatment upon their symptoms, function, and overall well-being, has always been compelling as an ideal to strive toward,” Cella adds. “PROs can and should be considered as true treatment outcome measures, and their ability to capture quality information efficiently make them well-suited for this role.”
The FDA even permits PROs (i.e. pain, anxiety, depression, sleep, and physical and social functioning) to be used as experimental endpoints for clinical trials to support claims in medical product labeling.2
The Patient Voice
The Department Health and Human Services (HHS) is searching for ways to fill current gaps in outcome measures, and has funded a patient outcomes project by the NQF to help ramp up patient-focused measure development activities within the federal government. In a recent report stemming from that project, the NQF states: “The patient’s voice is not readily captured in traditional health records and data systems, yet the beneficiary of healthcare services is often in the best position to evaluate the effectiveness of those services.”3
The NQF also is conducting foundational work to evaluate the most promising and viable PROs for quality measurement use and methodological issues involved in collecting and aggregating PRO data for provider performance assessment, says Helen Burstin, MD, MPH, NQF’s senior vice president for performance measures.
“PROs provide the opportunity to hear about the outcome of a clinician’s intervention directly from the patient—for example, visual improvement after cataract surgery, relief from nausea after chemotherapy, and mobility enhancement and pain relief after a hip or knee replacement,” she says. “The goal is to develop reliable and valid PRO performance measures that are applicable across multiple settings of care and/or multiple conditions, which the NQF can endorse for accountability and quality-improvement purposes.”
Specific NQF recommendations regarding PROs and performance measurement are expected to be available for review and comment this month, with a 30-day public and member comment period.
A wide variety of patient-level instruments to measure PROs have been used for clinical research purposes, many of which have been evaluated and catalogued within a system of assessment tools known as the National Institutes of Health’s (NIH) Patient-Reported Outcome Measurement Information System (PROMIS), Dr. Burstin says. PROMIS questionnaires prompt patients to measure such outcomes as how much difficulty they experience when walking a block on flat ground, getting in and out of bed, or doing strenuous activities, such as bicycling or jogging. NIH-funded studies using PROMIS tools are taking place at 12 sites across the country (http://nihpromis.org/default).
“PROMIS provides two distinct advantages to the PRO performance metric landscape,” argues Cella, who is principal investigator of the Statistical Center for PROMIS. “It has a computerized adaptive testing option, so efficient and accurate assessment is now possible at the individual patient level, with just a few questions per area. It also standardizes its scoring and reporting, such that many other similar measures can be used and their scores reported on a common, PROMIS metric.”
HM Applications
“The voice of the clinician is also needed during this PRO development process,” Dr. Burstin says. “We welcome hospitalists to engage in our projects and weigh in about the most meaningful and actionable patient outcomes that are relevant to their practice.”
“Taking PROs and applying them to hospital medicine is really doable if you take into account the lessons learned from providers who have already used PROs successfully in clinical settings,” says Pat Courneya, MD, medical director for HealthPartners Health Plan in Minnesota.
HealthPartners recently began using PROs in a quality measurement and reward program, offering financial bonuses to physical therapists who achieve a high PRO score relative to resource use (number of PT sessions required). “Having objective PRO measurements allows clinicians to create benchmarks for their patients regarding how much functional improvement they expect to achieve, and how many PT sessions are required to achieve that degree of improvement,” Dr. Courneya says. Using an interactive, Web-based PRO assessment tool, the program has helped tailor care to the expectations of patients while also significantly reducing the overall number of PT visits, especially by medically complex, post-operative patients.
HealthPartners has successfully used PROs as part of an innovative care model for managing patients with depression. At the outset of treatment, patients are administered the PHQ-9, a nine-item patient health questionnaire designed to assess depression symptoms and functional impairment, and derive a severity score. Patients receive care by a team composed of a primary-care physician, a care manager, and a consulting psychiatrist, after which their degree of symptom improvement is again measured. With this program, HealthPartners has achieved significantly more patients with depression into remission by six months compared with typical primary-care treatment, Dr. Courneya says. This model of care has since garnered a CMS Innovation Grant, managed by the HealthPartners Institute for Education and Research and directed by Minnesota’s Institute for Clinical Systems Improvement, aimed at spreading the model to five other states.
“PROs are potentially as useful for hospital medicine as for any other type of medical practice,” says Shaun Frost, MD, SFHM, SHM president and associate medical director of care delivery systems for HealthPartners Health Plan. “There is a big opportunity for hospitalists to incorporate shared decision-making to learn patients’ preferences, such as expectations of when they will be discharged, and understanding of therapeutic options.”
Peri-surgical care is a particularly important opportunity for hospitalists to demonstrate their value by leveraging PROs, according to Dr. Frost. “Patients sometimes come to the table with unrealistic prior expectations that physicians can make pain go away completely. We need to clarify their expectations preoperatively, when we meet them for the very first time, so that they establish a realistic baseline,” he says. “We then need to have a diligent conversation with them immediately after their operation to discuss their pain-management goals, a realistic physical therapy schedule, and post-discharge expectations.”
By clearly understanding patient objectives, hospitalists can “adjust the therapy they’re getting to their expectations, maximizing its effectiveness while minimizing delays in care and transitions to other care settings,” Dr. Frost says.
Chris Guadagnino is a freelance medical writer in Philadelphia.
References
- National Quality Forum. Patient-reported outcomes. National Quality Forum website. Available at: http://www.qualityforum.org/Projects/n-r/Patient-Reported_Outcomes/Patient-Reported_Outcomes.aspx. Accessed Oct. 2, 2012.
- U.S. Food and Drug Administration. The Patient-Reported Outcomes Consortium. U.S. Food and Drug Administration website. Available at: http://www.fda.gov/AboutFDA/PartnershipsCollaborations/PublicPrivatePartnershipProgram/ucm231129.htm. Accessed Oct. 2, 2012.
- National Quality Forum. National voluntary consensus standards for patient outcomes 2009.National Quality Forum website. Available at: http://www.qualityforum.org/Publications/2011/07/National_Voluntary_Consensus_Standards_for_Patient_Outcomes_2009.aspx. Accessed Oct. 2, 2012.

—Gregg Meyer, MD, MSc, chief clinical officer and executive vice president for population health for the Dartmouth-Hitchcock Health System in Lebanon, N.H.
As healthcare quality reporting continues to evolve in this era of value-based purchasing (VBP), players on both the giving and receiving ends of performance incentives agree on the need to improve the accountability metrics with which providers are measured, ranked, rewarded, and penalized. Many of the measures currently in use—e.g., Centers for Medicare & Medicaid Services’ (CMS) core process measures and patient satisfaction ratings, the gross outcome metrics of mortality, infection, and readmission rates—are blunt instruments in need of refinement.
Entities such as the National Quality Forum (NQF), the American Medical Association’s Physician Consortium for Performance Improvement (PCPI), and the National Quality Measures Clearinghouse (NQMC) recognize the need to develop and endorse more timely, credible, and patient-centered outcome metrics. Largely missing from the current crop of outcome measure sets is a meaningful account of the patient’s perspective.
Enter patient-reported outcomes (PROs), defined as “any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else.”1 PRO tools “measure what patients are able to do and how they feel by asking questions” (see “Types of Patient-Reported Outcomes [PROs],” p. 19).
If successfully adapted for public reporting on a wide scale, PROs could become the next evolutionary step in healthcare quality reporting, integrating health status and patient experience data into outcome metrics that truly matter to patients. They could enable a richer understanding of their clinical experiences and responses to therapy, and help providers target necessary improvements with greater precision.
“As a provider, I care about my patients not developing infections, getting the right medications, and not being readmitted. Patients, however, have a different set of priorities around issues like ‘How quickly will I be able to return to work? When will I be able to chase my grandkids around the yard? How much is this care going to cost me out of pocket?’” says healthcare quality expert Gregg Meyer, MD, MSc, chief clinical officer and executive vice president for population health for the Dartmouth-Hitchcock Health System in Lebanon, N.H. “This next generation of accountability will allow us to move from being provider- and payor-centered to becoming truly patient-centered, and will serve as a key reminder that patients are no longer passive participants. They are key partners, in both the delivery of care and the measurement of that care.”
The idea of PROs is one whose “time has finally arrived,” according to medical outcomes researcher David Cella, PhD, professor and chair of the Department of Medical Social Sciences at Northwestern University Feinberg School of Medicine in Chicago.
“The case for inclusion of outcomes that matter most to patients, like the effect of treatment upon their symptoms, function, and overall well-being, has always been compelling as an ideal to strive toward,” Cella adds. “PROs can and should be considered as true treatment outcome measures, and their ability to capture quality information efficiently make them well-suited for this role.”
The FDA even permits PROs (i.e. pain, anxiety, depression, sleep, and physical and social functioning) to be used as experimental endpoints for clinical trials to support claims in medical product labeling.2
The Patient Voice
The Department Health and Human Services (HHS) is searching for ways to fill current gaps in outcome measures, and has funded a patient outcomes project by the NQF to help ramp up patient-focused measure development activities within the federal government. In a recent report stemming from that project, the NQF states: “The patient’s voice is not readily captured in traditional health records and data systems, yet the beneficiary of healthcare services is often in the best position to evaluate the effectiveness of those services.”3
The NQF also is conducting foundational work to evaluate the most promising and viable PROs for quality measurement use and methodological issues involved in collecting and aggregating PRO data for provider performance assessment, says Helen Burstin, MD, MPH, NQF’s senior vice president for performance measures.
“PROs provide the opportunity to hear about the outcome of a clinician’s intervention directly from the patient—for example, visual improvement after cataract surgery, relief from nausea after chemotherapy, and mobility enhancement and pain relief after a hip or knee replacement,” she says. “The goal is to develop reliable and valid PRO performance measures that are applicable across multiple settings of care and/or multiple conditions, which the NQF can endorse for accountability and quality-improvement purposes.”
Specific NQF recommendations regarding PROs and performance measurement are expected to be available for review and comment this month, with a 30-day public and member comment period.
A wide variety of patient-level instruments to measure PROs have been used for clinical research purposes, many of which have been evaluated and catalogued within a system of assessment tools known as the National Institutes of Health’s (NIH) Patient-Reported Outcome Measurement Information System (PROMIS), Dr. Burstin says. PROMIS questionnaires prompt patients to measure such outcomes as how much difficulty they experience when walking a block on flat ground, getting in and out of bed, or doing strenuous activities, such as bicycling or jogging. NIH-funded studies using PROMIS tools are taking place at 12 sites across the country (http://nihpromis.org/default).
“PROMIS provides two distinct advantages to the PRO performance metric landscape,” argues Cella, who is principal investigator of the Statistical Center for PROMIS. “It has a computerized adaptive testing option, so efficient and accurate assessment is now possible at the individual patient level, with just a few questions per area. It also standardizes its scoring and reporting, such that many other similar measures can be used and their scores reported on a common, PROMIS metric.”
HM Applications
“The voice of the clinician is also needed during this PRO development process,” Dr. Burstin says. “We welcome hospitalists to engage in our projects and weigh in about the most meaningful and actionable patient outcomes that are relevant to their practice.”
“Taking PROs and applying them to hospital medicine is really doable if you take into account the lessons learned from providers who have already used PROs successfully in clinical settings,” says Pat Courneya, MD, medical director for HealthPartners Health Plan in Minnesota.
HealthPartners recently began using PROs in a quality measurement and reward program, offering financial bonuses to physical therapists who achieve a high PRO score relative to resource use (number of PT sessions required). “Having objective PRO measurements allows clinicians to create benchmarks for their patients regarding how much functional improvement they expect to achieve, and how many PT sessions are required to achieve that degree of improvement,” Dr. Courneya says. Using an interactive, Web-based PRO assessment tool, the program has helped tailor care to the expectations of patients while also significantly reducing the overall number of PT visits, especially by medically complex, post-operative patients.
HealthPartners has successfully used PROs as part of an innovative care model for managing patients with depression. At the outset of treatment, patients are administered the PHQ-9, a nine-item patient health questionnaire designed to assess depression symptoms and functional impairment, and derive a severity score. Patients receive care by a team composed of a primary-care physician, a care manager, and a consulting psychiatrist, after which their degree of symptom improvement is again measured. With this program, HealthPartners has achieved significantly more patients with depression into remission by six months compared with typical primary-care treatment, Dr. Courneya says. This model of care has since garnered a CMS Innovation Grant, managed by the HealthPartners Institute for Education and Research and directed by Minnesota’s Institute for Clinical Systems Improvement, aimed at spreading the model to five other states.
“PROs are potentially as useful for hospital medicine as for any other type of medical practice,” says Shaun Frost, MD, SFHM, SHM president and associate medical director of care delivery systems for HealthPartners Health Plan. “There is a big opportunity for hospitalists to incorporate shared decision-making to learn patients’ preferences, such as expectations of when they will be discharged, and understanding of therapeutic options.”
Peri-surgical care is a particularly important opportunity for hospitalists to demonstrate their value by leveraging PROs, according to Dr. Frost. “Patients sometimes come to the table with unrealistic prior expectations that physicians can make pain go away completely. We need to clarify their expectations preoperatively, when we meet them for the very first time, so that they establish a realistic baseline,” he says. “We then need to have a diligent conversation with them immediately after their operation to discuss their pain-management goals, a realistic physical therapy schedule, and post-discharge expectations.”
By clearly understanding patient objectives, hospitalists can “adjust the therapy they’re getting to their expectations, maximizing its effectiveness while minimizing delays in care and transitions to other care settings,” Dr. Frost says.
Chris Guadagnino is a freelance medical writer in Philadelphia.
References
- National Quality Forum. Patient-reported outcomes. National Quality Forum website. Available at: http://www.qualityforum.org/Projects/n-r/Patient-Reported_Outcomes/Patient-Reported_Outcomes.aspx. Accessed Oct. 2, 2012.
- U.S. Food and Drug Administration. The Patient-Reported Outcomes Consortium. U.S. Food and Drug Administration website. Available at: http://www.fda.gov/AboutFDA/PartnershipsCollaborations/PublicPrivatePartnershipProgram/ucm231129.htm. Accessed Oct. 2, 2012.
- National Quality Forum. National voluntary consensus standards for patient outcomes 2009.National Quality Forum website. Available at: http://www.qualityforum.org/Publications/2011/07/National_Voluntary_Consensus_Standards_for_Patient_Outcomes_2009.aspx. Accessed Oct. 2, 2012.

—Gregg Meyer, MD, MSc, chief clinical officer and executive vice president for population health for the Dartmouth-Hitchcock Health System in Lebanon, N.H.
As healthcare quality reporting continues to evolve in this era of value-based purchasing (VBP), players on both the giving and receiving ends of performance incentives agree on the need to improve the accountability metrics with which providers are measured, ranked, rewarded, and penalized. Many of the measures currently in use—e.g., Centers for Medicare & Medicaid Services’ (CMS) core process measures and patient satisfaction ratings, the gross outcome metrics of mortality, infection, and readmission rates—are blunt instruments in need of refinement.
Entities such as the National Quality Forum (NQF), the American Medical Association’s Physician Consortium for Performance Improvement (PCPI), and the National Quality Measures Clearinghouse (NQMC) recognize the need to develop and endorse more timely, credible, and patient-centered outcome metrics. Largely missing from the current crop of outcome measure sets is a meaningful account of the patient’s perspective.
Enter patient-reported outcomes (PROs), defined as “any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else.”1 PRO tools “measure what patients are able to do and how they feel by asking questions” (see “Types of Patient-Reported Outcomes [PROs],” p. 19).
If successfully adapted for public reporting on a wide scale, PROs could become the next evolutionary step in healthcare quality reporting, integrating health status and patient experience data into outcome metrics that truly matter to patients. They could enable a richer understanding of their clinical experiences and responses to therapy, and help providers target necessary improvements with greater precision.
“As a provider, I care about my patients not developing infections, getting the right medications, and not being readmitted. Patients, however, have a different set of priorities around issues like ‘How quickly will I be able to return to work? When will I be able to chase my grandkids around the yard? How much is this care going to cost me out of pocket?’” says healthcare quality expert Gregg Meyer, MD, MSc, chief clinical officer and executive vice president for population health for the Dartmouth-Hitchcock Health System in Lebanon, N.H. “This next generation of accountability will allow us to move from being provider- and payor-centered to becoming truly patient-centered, and will serve as a key reminder that patients are no longer passive participants. They are key partners, in both the delivery of care and the measurement of that care.”
The idea of PROs is one whose “time has finally arrived,” according to medical outcomes researcher David Cella, PhD, professor and chair of the Department of Medical Social Sciences at Northwestern University Feinberg School of Medicine in Chicago.
“The case for inclusion of outcomes that matter most to patients, like the effect of treatment upon their symptoms, function, and overall well-being, has always been compelling as an ideal to strive toward,” Cella adds. “PROs can and should be considered as true treatment outcome measures, and their ability to capture quality information efficiently make them well-suited for this role.”
The FDA even permits PROs (i.e. pain, anxiety, depression, sleep, and physical and social functioning) to be used as experimental endpoints for clinical trials to support claims in medical product labeling.2
The Patient Voice
The Department Health and Human Services (HHS) is searching for ways to fill current gaps in outcome measures, and has funded a patient outcomes project by the NQF to help ramp up patient-focused measure development activities within the federal government. In a recent report stemming from that project, the NQF states: “The patient’s voice is not readily captured in traditional health records and data systems, yet the beneficiary of healthcare services is often in the best position to evaluate the effectiveness of those services.”3
The NQF also is conducting foundational work to evaluate the most promising and viable PROs for quality measurement use and methodological issues involved in collecting and aggregating PRO data for provider performance assessment, says Helen Burstin, MD, MPH, NQF’s senior vice president for performance measures.
“PROs provide the opportunity to hear about the outcome of a clinician’s intervention directly from the patient—for example, visual improvement after cataract surgery, relief from nausea after chemotherapy, and mobility enhancement and pain relief after a hip or knee replacement,” she says. “The goal is to develop reliable and valid PRO performance measures that are applicable across multiple settings of care and/or multiple conditions, which the NQF can endorse for accountability and quality-improvement purposes.”
Specific NQF recommendations regarding PROs and performance measurement are expected to be available for review and comment this month, with a 30-day public and member comment period.
A wide variety of patient-level instruments to measure PROs have been used for clinical research purposes, many of which have been evaluated and catalogued within a system of assessment tools known as the National Institutes of Health’s (NIH) Patient-Reported Outcome Measurement Information System (PROMIS), Dr. Burstin says. PROMIS questionnaires prompt patients to measure such outcomes as how much difficulty they experience when walking a block on flat ground, getting in and out of bed, or doing strenuous activities, such as bicycling or jogging. NIH-funded studies using PROMIS tools are taking place at 12 sites across the country (http://nihpromis.org/default).
“PROMIS provides two distinct advantages to the PRO performance metric landscape,” argues Cella, who is principal investigator of the Statistical Center for PROMIS. “It has a computerized adaptive testing option, so efficient and accurate assessment is now possible at the individual patient level, with just a few questions per area. It also standardizes its scoring and reporting, such that many other similar measures can be used and their scores reported on a common, PROMIS metric.”
HM Applications
“The voice of the clinician is also needed during this PRO development process,” Dr. Burstin says. “We welcome hospitalists to engage in our projects and weigh in about the most meaningful and actionable patient outcomes that are relevant to their practice.”
“Taking PROs and applying them to hospital medicine is really doable if you take into account the lessons learned from providers who have already used PROs successfully in clinical settings,” says Pat Courneya, MD, medical director for HealthPartners Health Plan in Minnesota.
HealthPartners recently began using PROs in a quality measurement and reward program, offering financial bonuses to physical therapists who achieve a high PRO score relative to resource use (number of PT sessions required). “Having objective PRO measurements allows clinicians to create benchmarks for their patients regarding how much functional improvement they expect to achieve, and how many PT sessions are required to achieve that degree of improvement,” Dr. Courneya says. Using an interactive, Web-based PRO assessment tool, the program has helped tailor care to the expectations of patients while also significantly reducing the overall number of PT visits, especially by medically complex, post-operative patients.
HealthPartners has successfully used PROs as part of an innovative care model for managing patients with depression. At the outset of treatment, patients are administered the PHQ-9, a nine-item patient health questionnaire designed to assess depression symptoms and functional impairment, and derive a severity score. Patients receive care by a team composed of a primary-care physician, a care manager, and a consulting psychiatrist, after which their degree of symptom improvement is again measured. With this program, HealthPartners has achieved significantly more patients with depression into remission by six months compared with typical primary-care treatment, Dr. Courneya says. This model of care has since garnered a CMS Innovation Grant, managed by the HealthPartners Institute for Education and Research and directed by Minnesota’s Institute for Clinical Systems Improvement, aimed at spreading the model to five other states.
“PROs are potentially as useful for hospital medicine as for any other type of medical practice,” says Shaun Frost, MD, SFHM, SHM president and associate medical director of care delivery systems for HealthPartners Health Plan. “There is a big opportunity for hospitalists to incorporate shared decision-making to learn patients’ preferences, such as expectations of when they will be discharged, and understanding of therapeutic options.”
Peri-surgical care is a particularly important opportunity for hospitalists to demonstrate their value by leveraging PROs, according to Dr. Frost. “Patients sometimes come to the table with unrealistic prior expectations that physicians can make pain go away completely. We need to clarify their expectations preoperatively, when we meet them for the very first time, so that they establish a realistic baseline,” he says. “We then need to have a diligent conversation with them immediately after their operation to discuss their pain-management goals, a realistic physical therapy schedule, and post-discharge expectations.”
By clearly understanding patient objectives, hospitalists can “adjust the therapy they’re getting to their expectations, maximizing its effectiveness while minimizing delays in care and transitions to other care settings,” Dr. Frost says.
Chris Guadagnino is a freelance medical writer in Philadelphia.
References
- National Quality Forum. Patient-reported outcomes. National Quality Forum website. Available at: http://www.qualityforum.org/Projects/n-r/Patient-Reported_Outcomes/Patient-Reported_Outcomes.aspx. Accessed Oct. 2, 2012.
- U.S. Food and Drug Administration. The Patient-Reported Outcomes Consortium. U.S. Food and Drug Administration website. Available at: http://www.fda.gov/AboutFDA/PartnershipsCollaborations/PublicPrivatePartnershipProgram/ucm231129.htm. Accessed Oct. 2, 2012.
- National Quality Forum. National voluntary consensus standards for patient outcomes 2009.National Quality Forum website. Available at: http://www.qualityforum.org/Publications/2011/07/National_Voluntary_Consensus_Standards_for_Patient_Outcomes_2009.aspx. Accessed Oct. 2, 2012.
Medical Coding: Hospice Care vs. Palliative Care
Hospice care” and “palliative care” are not synonymous terms. Hospice care is defined as a comprehensive set of services (see “Hospice Coverage,” below) identified and coordinated by an interdisciplinary group to provide for the physical, psychosocial, spiritual, and emotional needs of a terminally ill patient and/or family members, as delineated in a specific patient plan of care.1 Palliative care is defined as patient- and family-centered care that optimizes quality of life by anticipating, preventing, and treating suffering. Palliative care throughout the continuum of illness involves addressing physical, intellectual, emotional, social, and spiritual needs, and facilitates patient autonomy, access to information, and choice.1
As an approach, hospice care of terminally ill individuals involves palliative care (relief of pain and uncomfortable symptoms), and emphasizes maintaining the patient at home with family and friends as long as possible. Hospice services can be provided in a home, center, skilled-nursing facility, or hospital setting. In contrast, palliative-care services can be provided during hospice care, or coincide with care that is focused on a cure.
Many hospitalists provide both hospice care and palliative-care services to their patients. Different factors affect how to report these services. These programs can be quite costly, as they involve several team members and a substantial amount of time delivering these services. Capturing services appropriately and obtaining reimbursement to help continue program initiatives are significant issues.
Hospice Care
When a patient enrolls in hospice, all rights to Medicare Part B payments are waived during the benefit period involving professional services related to the treatment and management of the terminal illness. Payment is made through the Part A benefit for the associated costs of daily care and the services provided by the hospice-employed physician. An exception occurs for professional services of an independent attending physician who is not an employee of the designated hospice and does not receive compensation from the hospice for those services. The “attending physician” for hospice services must be an individual who is a doctor of medicine or osteopathy, or a nurse practitioner identified by the individual, at the time they elect hospice coverage, as having the most significant role in the determination and delivery of their medical care.2
Patients often receive hospice in the hospital setting, where the hospitalist manages the patient’s daily care. If the hospitalist is designated as the “attending physician” for hospice services, the visits should be reported to Medicare Part B with modifier GV (e.g. 99232-GV).3 This will allow for separate payment to the hospitalist (the independent attending physician), while the hospice agency maintains its daily-care rate. Reporting services absent this modifier will result in denial.
In some cases, the hospitalist is not identified as the “attending physician” for hospice services but occasionally provides care related to the terminal illness. This situation proves most difficult. Although the hospitalist might be the most accessible physician to the staff and is putting the patient’s needs first, reimbursement is unlikely. Regulations stipulate that patients must not see independent physicians other than their “attending physician” for care related to their terminal illness unless the hospice arranges it. When the service is related to the hospice patient’s terminal illness but was furnished by someone other than the designated “attending physician,” this “other physician” must look to the hospice for payment.3
Nonhospice Palliative Care
Members of the palliative-care team often are called to provide management options to assist in reducing pain and suffering. When the palliative-care specialist is asked to provide opinions or advice, the initial service may qualify as a consultation for those payors that still recognize these codes. However, all of the requirements4 must be met in order to report the service as an inpatient consultation (99251-99255):3
- There must be a written request from a qualified healthcare provider who is involved in the patient’s care (e.g. physician, resident, nurse practitioner); this may be documented as a physician order or in the assessment/plan of the requesting provider’s progress note. Standing orders for consultation are not permitted.
- The requesting provider should clearly and accurately identify the reason for consult request to support the medical necessity of the service.
- The palliative-care physician renders and documents the service.
- The palliative-care physician reports his or her findings to the requesting physician via written communication; because the requesting physician and the consultant share a common inpatient medical record, the consultant’s inpatient progress note satisfies the “written report” requirement.
Consider the nature of the request when reporting a consultation. If the request demonstrates the need for opinions or advice from the palliative-care specialist, the service can be reported as a consultation. If the indication cites “medical management” or “palliative management,” payors are less likely to consider the service as a consultation because the physician is not seeking opinions or advice from the consultant to incorporate into his or her own plan of care for the patient and would rather the consultant just take over that portion of patient care. When consultations do not meet the requirements, subsequent hospital care services should be reported (99231-99233).3
The requesting physician can be in the same or a different provider group as the consultant. The consultant must possess expertise in an area that is beyond that of the requesting provider. Because most hospitalists carry a specialty designation of internal medicine (physician specialty code 11), hospitalists providing palliative-care services can distinguish themselves by their own code (physician specialty code 17, hospice and palliative care).5 Payor concerns arise when physicians of the same designated specialty submit a claim for the same patient on the same date. The payor is likely to pay the first claim received and deny the second claim received pending review of documentation. If this occurs, submit a copy of both progress notes for the date in question to distinguish the services provided. The payor may still require that both encounters be reported as one cumulative service under one physician.
Consultations are not an option for Medicare beneficiaries. Hospitalists providing palliative care can report initial hospital care codes (99221-99223) for their first encounter with the patient.3 This is only acceptable when no other hospitalist from the group has reported initial hospital care during the patient stay, unless the palliative-care hospitalist carries the corresponding designation (i.e. enrolled with Medicare as physician specialty code 17). Without this separate designation, the palliative-care hospitalist can only report subsequent hospital care codes (99231-99233) as the patient was seen previously by a hospitalist in the same group.3
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is also on the faculty of SHM’s inpatient coding course.
References
- U.S. Government Printing Office. Electronic Code of Federal Regulations: Title 42: Public Health, Part 418: Hospice Care, §418.3. June 2012. U.S. Government Printing Office website. Available at: http://ecfr.gpoaccess.gov/cgi/t/text/text-idx?c=ecfr&sid=818258235647b14d2961ad30fa3e68e6&rgn=div5&view=text&node=42:3.0.1.1.5&idno=42#42:3.0.1.1.5.1.3.3. Accessed June 23, 2012.
- Centers for Medicare & Medicaid Services. Medicare Claims Processing Manual: Chapter 11: processing hospice claims. Centers for Medicare & Medicaid Services website. Available at: http://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/clm104c11.pdf. Accessed June 23, 2012.
- Abraham M, Ahlman J, Anderson C, Boudreau A, Connelly J. Current Procedural Terminology 2012 Professional Edition. Chicago: American Medical Association Press; 2011.
- American Medical Association. Consultation services and transfer of care. American Medical Association website. Available at: http://www.ama-assn.org/resources/doc/cpt/cpt-consultation-services.pdf. Accessed June 23, 2012.
- Centers for Medicare & Medicaid Services. Medicare Claims Processing Manual: Chapter 26: completing and processing form CMS-1500 data set. Centers for Medicare & Medicaid Services website. Available at: http://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c26.pdf. Accessed June 23, 2012. Department of Health and Human Services.
- Hospice Payment System: payment system fact sheet series. Centers for Medicare & Medicaid Services website. Available at: http://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/hospice_pay_sys_fs.pdf. Accessed June 23, 2012.
Hospice care” and “palliative care” are not synonymous terms. Hospice care is defined as a comprehensive set of services (see “Hospice Coverage,” below) identified and coordinated by an interdisciplinary group to provide for the physical, psychosocial, spiritual, and emotional needs of a terminally ill patient and/or family members, as delineated in a specific patient plan of care.1 Palliative care is defined as patient- and family-centered care that optimizes quality of life by anticipating, preventing, and treating suffering. Palliative care throughout the continuum of illness involves addressing physical, intellectual, emotional, social, and spiritual needs, and facilitates patient autonomy, access to information, and choice.1
As an approach, hospice care of terminally ill individuals involves palliative care (relief of pain and uncomfortable symptoms), and emphasizes maintaining the patient at home with family and friends as long as possible. Hospice services can be provided in a home, center, skilled-nursing facility, or hospital setting. In contrast, palliative-care services can be provided during hospice care, or coincide with care that is focused on a cure.
Many hospitalists provide both hospice care and palliative-care services to their patients. Different factors affect how to report these services. These programs can be quite costly, as they involve several team members and a substantial amount of time delivering these services. Capturing services appropriately and obtaining reimbursement to help continue program initiatives are significant issues.
Hospice Care
When a patient enrolls in hospice, all rights to Medicare Part B payments are waived during the benefit period involving professional services related to the treatment and management of the terminal illness. Payment is made through the Part A benefit for the associated costs of daily care and the services provided by the hospice-employed physician. An exception occurs for professional services of an independent attending physician who is not an employee of the designated hospice and does not receive compensation from the hospice for those services. The “attending physician” for hospice services must be an individual who is a doctor of medicine or osteopathy, or a nurse practitioner identified by the individual, at the time they elect hospice coverage, as having the most significant role in the determination and delivery of their medical care.2
Patients often receive hospice in the hospital setting, where the hospitalist manages the patient’s daily care. If the hospitalist is designated as the “attending physician” for hospice services, the visits should be reported to Medicare Part B with modifier GV (e.g. 99232-GV).3 This will allow for separate payment to the hospitalist (the independent attending physician), while the hospice agency maintains its daily-care rate. Reporting services absent this modifier will result in denial.
In some cases, the hospitalist is not identified as the “attending physician” for hospice services but occasionally provides care related to the terminal illness. This situation proves most difficult. Although the hospitalist might be the most accessible physician to the staff and is putting the patient’s needs first, reimbursement is unlikely. Regulations stipulate that patients must not see independent physicians other than their “attending physician” for care related to their terminal illness unless the hospice arranges it. When the service is related to the hospice patient’s terminal illness but was furnished by someone other than the designated “attending physician,” this “other physician” must look to the hospice for payment.3
Nonhospice Palliative Care
Members of the palliative-care team often are called to provide management options to assist in reducing pain and suffering. When the palliative-care specialist is asked to provide opinions or advice, the initial service may qualify as a consultation for those payors that still recognize these codes. However, all of the requirements4 must be met in order to report the service as an inpatient consultation (99251-99255):3
- There must be a written request from a qualified healthcare provider who is involved in the patient’s care (e.g. physician, resident, nurse practitioner); this may be documented as a physician order or in the assessment/plan of the requesting provider’s progress note. Standing orders for consultation are not permitted.
- The requesting provider should clearly and accurately identify the reason for consult request to support the medical necessity of the service.
- The palliative-care physician renders and documents the service.
- The palliative-care physician reports his or her findings to the requesting physician via written communication; because the requesting physician and the consultant share a common inpatient medical record, the consultant’s inpatient progress note satisfies the “written report” requirement.
Consider the nature of the request when reporting a consultation. If the request demonstrates the need for opinions or advice from the palliative-care specialist, the service can be reported as a consultation. If the indication cites “medical management” or “palliative management,” payors are less likely to consider the service as a consultation because the physician is not seeking opinions or advice from the consultant to incorporate into his or her own plan of care for the patient and would rather the consultant just take over that portion of patient care. When consultations do not meet the requirements, subsequent hospital care services should be reported (99231-99233).3
The requesting physician can be in the same or a different provider group as the consultant. The consultant must possess expertise in an area that is beyond that of the requesting provider. Because most hospitalists carry a specialty designation of internal medicine (physician specialty code 11), hospitalists providing palliative-care services can distinguish themselves by their own code (physician specialty code 17, hospice and palliative care).5 Payor concerns arise when physicians of the same designated specialty submit a claim for the same patient on the same date. The payor is likely to pay the first claim received and deny the second claim received pending review of documentation. If this occurs, submit a copy of both progress notes for the date in question to distinguish the services provided. The payor may still require that both encounters be reported as one cumulative service under one physician.
Consultations are not an option for Medicare beneficiaries. Hospitalists providing palliative care can report initial hospital care codes (99221-99223) for their first encounter with the patient.3 This is only acceptable when no other hospitalist from the group has reported initial hospital care during the patient stay, unless the palliative-care hospitalist carries the corresponding designation (i.e. enrolled with Medicare as physician specialty code 17). Without this separate designation, the palliative-care hospitalist can only report subsequent hospital care codes (99231-99233) as the patient was seen previously by a hospitalist in the same group.3
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is also on the faculty of SHM’s inpatient coding course.
References
- U.S. Government Printing Office. Electronic Code of Federal Regulations: Title 42: Public Health, Part 418: Hospice Care, §418.3. June 2012. U.S. Government Printing Office website. Available at: http://ecfr.gpoaccess.gov/cgi/t/text/text-idx?c=ecfr&sid=818258235647b14d2961ad30fa3e68e6&rgn=div5&view=text&node=42:3.0.1.1.5&idno=42#42:3.0.1.1.5.1.3.3. Accessed June 23, 2012.
- Centers for Medicare & Medicaid Services. Medicare Claims Processing Manual: Chapter 11: processing hospice claims. Centers for Medicare & Medicaid Services website. Available at: http://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/clm104c11.pdf. Accessed June 23, 2012.
- Abraham M, Ahlman J, Anderson C, Boudreau A, Connelly J. Current Procedural Terminology 2012 Professional Edition. Chicago: American Medical Association Press; 2011.
- American Medical Association. Consultation services and transfer of care. American Medical Association website. Available at: http://www.ama-assn.org/resources/doc/cpt/cpt-consultation-services.pdf. Accessed June 23, 2012.
- Centers for Medicare & Medicaid Services. Medicare Claims Processing Manual: Chapter 26: completing and processing form CMS-1500 data set. Centers for Medicare & Medicaid Services website. Available at: http://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c26.pdf. Accessed June 23, 2012. Department of Health and Human Services.
- Hospice Payment System: payment system fact sheet series. Centers for Medicare & Medicaid Services website. Available at: http://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/hospice_pay_sys_fs.pdf. Accessed June 23, 2012.
Hospice care” and “palliative care” are not synonymous terms. Hospice care is defined as a comprehensive set of services (see “Hospice Coverage,” below) identified and coordinated by an interdisciplinary group to provide for the physical, psychosocial, spiritual, and emotional needs of a terminally ill patient and/or family members, as delineated in a specific patient plan of care.1 Palliative care is defined as patient- and family-centered care that optimizes quality of life by anticipating, preventing, and treating suffering. Palliative care throughout the continuum of illness involves addressing physical, intellectual, emotional, social, and spiritual needs, and facilitates patient autonomy, access to information, and choice.1
As an approach, hospice care of terminally ill individuals involves palliative care (relief of pain and uncomfortable symptoms), and emphasizes maintaining the patient at home with family and friends as long as possible. Hospice services can be provided in a home, center, skilled-nursing facility, or hospital setting. In contrast, palliative-care services can be provided during hospice care, or coincide with care that is focused on a cure.
Many hospitalists provide both hospice care and palliative-care services to their patients. Different factors affect how to report these services. These programs can be quite costly, as they involve several team members and a substantial amount of time delivering these services. Capturing services appropriately and obtaining reimbursement to help continue program initiatives are significant issues.
Hospice Care
When a patient enrolls in hospice, all rights to Medicare Part B payments are waived during the benefit period involving professional services related to the treatment and management of the terminal illness. Payment is made through the Part A benefit for the associated costs of daily care and the services provided by the hospice-employed physician. An exception occurs for professional services of an independent attending physician who is not an employee of the designated hospice and does not receive compensation from the hospice for those services. The “attending physician” for hospice services must be an individual who is a doctor of medicine or osteopathy, or a nurse practitioner identified by the individual, at the time they elect hospice coverage, as having the most significant role in the determination and delivery of their medical care.2
Patients often receive hospice in the hospital setting, where the hospitalist manages the patient’s daily care. If the hospitalist is designated as the “attending physician” for hospice services, the visits should be reported to Medicare Part B with modifier GV (e.g. 99232-GV).3 This will allow for separate payment to the hospitalist (the independent attending physician), while the hospice agency maintains its daily-care rate. Reporting services absent this modifier will result in denial.
In some cases, the hospitalist is not identified as the “attending physician” for hospice services but occasionally provides care related to the terminal illness. This situation proves most difficult. Although the hospitalist might be the most accessible physician to the staff and is putting the patient’s needs first, reimbursement is unlikely. Regulations stipulate that patients must not see independent physicians other than their “attending physician” for care related to their terminal illness unless the hospice arranges it. When the service is related to the hospice patient’s terminal illness but was furnished by someone other than the designated “attending physician,” this “other physician” must look to the hospice for payment.3
Nonhospice Palliative Care
Members of the palliative-care team often are called to provide management options to assist in reducing pain and suffering. When the palliative-care specialist is asked to provide opinions or advice, the initial service may qualify as a consultation for those payors that still recognize these codes. However, all of the requirements4 must be met in order to report the service as an inpatient consultation (99251-99255):3
- There must be a written request from a qualified healthcare provider who is involved in the patient’s care (e.g. physician, resident, nurse practitioner); this may be documented as a physician order or in the assessment/plan of the requesting provider’s progress note. Standing orders for consultation are not permitted.
- The requesting provider should clearly and accurately identify the reason for consult request to support the medical necessity of the service.
- The palliative-care physician renders and documents the service.
- The palliative-care physician reports his or her findings to the requesting physician via written communication; because the requesting physician and the consultant share a common inpatient medical record, the consultant’s inpatient progress note satisfies the “written report” requirement.
Consider the nature of the request when reporting a consultation. If the request demonstrates the need for opinions or advice from the palliative-care specialist, the service can be reported as a consultation. If the indication cites “medical management” or “palliative management,” payors are less likely to consider the service as a consultation because the physician is not seeking opinions or advice from the consultant to incorporate into his or her own plan of care for the patient and would rather the consultant just take over that portion of patient care. When consultations do not meet the requirements, subsequent hospital care services should be reported (99231-99233).3
The requesting physician can be in the same or a different provider group as the consultant. The consultant must possess expertise in an area that is beyond that of the requesting provider. Because most hospitalists carry a specialty designation of internal medicine (physician specialty code 11), hospitalists providing palliative-care services can distinguish themselves by their own code (physician specialty code 17, hospice and palliative care).5 Payor concerns arise when physicians of the same designated specialty submit a claim for the same patient on the same date. The payor is likely to pay the first claim received and deny the second claim received pending review of documentation. If this occurs, submit a copy of both progress notes for the date in question to distinguish the services provided. The payor may still require that both encounters be reported as one cumulative service under one physician.
Consultations are not an option for Medicare beneficiaries. Hospitalists providing palliative care can report initial hospital care codes (99221-99223) for their first encounter with the patient.3 This is only acceptable when no other hospitalist from the group has reported initial hospital care during the patient stay, unless the palliative-care hospitalist carries the corresponding designation (i.e. enrolled with Medicare as physician specialty code 17). Without this separate designation, the palliative-care hospitalist can only report subsequent hospital care codes (99231-99233) as the patient was seen previously by a hospitalist in the same group.3
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is also on the faculty of SHM’s inpatient coding course.
References
- U.S. Government Printing Office. Electronic Code of Federal Regulations: Title 42: Public Health, Part 418: Hospice Care, §418.3. June 2012. U.S. Government Printing Office website. Available at: http://ecfr.gpoaccess.gov/cgi/t/text/text-idx?c=ecfr&sid=818258235647b14d2961ad30fa3e68e6&rgn=div5&view=text&node=42:3.0.1.1.5&idno=42#42:3.0.1.1.5.1.3.3. Accessed June 23, 2012.
- Centers for Medicare & Medicaid Services. Medicare Claims Processing Manual: Chapter 11: processing hospice claims. Centers for Medicare & Medicaid Services website. Available at: http://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/clm104c11.pdf. Accessed June 23, 2012.
- Abraham M, Ahlman J, Anderson C, Boudreau A, Connelly J. Current Procedural Terminology 2012 Professional Edition. Chicago: American Medical Association Press; 2011.
- American Medical Association. Consultation services and transfer of care. American Medical Association website. Available at: http://www.ama-assn.org/resources/doc/cpt/cpt-consultation-services.pdf. Accessed June 23, 2012.
- Centers for Medicare & Medicaid Services. Medicare Claims Processing Manual: Chapter 26: completing and processing form CMS-1500 data set. Centers for Medicare & Medicaid Services website. Available at: http://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c26.pdf. Accessed June 23, 2012. Department of Health and Human Services.
- Hospice Payment System: payment system fact sheet series. Centers for Medicare & Medicaid Services website. Available at: http://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/hospice_pay_sys_fs.pdf. Accessed June 23, 2012.
UV Light Beat Bleach for C. difficile Decontamination
SAN FRANCISCO – The M.D. Anderson Cancer Center is abandoning bleach for cleaning hospital rooms exposed to Clostridium difficile in favor of a new machine that kills the organism using ultraviolet light.
The machine reduced C. difficile counts as much as, or more than, bleach cleaning in a preliminary prospective trial in 30 hospital rooms previously occupied by patients infected with C. difficile. The machine is a bit more expensive than bleach at a cost of approximately $82,000 (or $3,000-$4,000 per month to lease), but it avoids damage to materials and the toxic environment for workers caused by the use of bleach or other corrosive chemicals, Dr. Shashank S. Ghantoji said in an interview at a poster presentation at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Bleach treatment reduced the average number of colony-forming units of C. difficile from 2.39 before cleaning to 0.71, a 70% reduction in the contamination level. Treatment with the Pulsed Xenon UV machine (PX-UV) reduced the average number of colony-forming units from 22.97 to 1.10, a 95% reduction.
The postcleaning contamination levels were not statistically different between the bleach and PX-UV rooms, Dr. Ghantoji and his associates found. However, PX-UV decontamination is faster than using bleach, Dr. Ghantoji said. "It takes at least 45 minutes to clean a room with bleach, and it’s not good for the patients or the health care professionals," plus admissions staff usually are clamoring for the room to be ready as soon as possible, he said. Cleaning a room using the PX-UV method takes perhaps 15 minutes.
The PX-UV machine has been available for some time, but its adoption depends on how proactive hospital infection control teams are, he added. He said he is aware of at least two medical centers beyond M.D. Anderson that are also using the machine.
In the study, 298 samples were taken before and after cleaning from high-touch surfaces – the bathroom handrail, the bed control panel, the bed rail, the top of the bedside table, and the IV pole control panel or other equipment control panel – and analyzed for C. difficile endospores. Fifteen rooms were cleaned by the conventional method using a 1:10 solution of sodium hypochlorite (bleach), and 15 underwent a visual, nonbleach cleaning of surfaces followed by 15 minutes of treatment with the PX-UV.
With the PX-UV method, housekeeping workers clean the bathroom and place the remote-operated PX-UV in the bathroom with the door shut while they finish cleaning the rest of the room. Then the machine is placed on each side of the bed for 4 minutes of operation with workers gone. Sensors stop the machine if any movement is detected.
It works by emitting ultraviolet C light, which kills C. difficile. And here’s a bonus – it also kills vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus, Dr. Ghantoji of M.D. Anderson, Houston, said at the meeting, sponsored by the American Society for Microbiology.
"The PX-UV method may be a promising alternative to the current standard of decontamination, bleach," he said. Future studies should look at whether the PX-UV method decreases not just endospore counts but transmission of C. difficile, he added.
C. difficile causes more than 300,000 health care–associated infections each year in the United States, incurring $2,500-$3,500 in costs per infection aside from any surgical costs, he estimated. Current guidelines recommend that rooms previously occupied by patients infected with C. difficile be cleaned with a disinfectant registered with the Environmental Protection Agency as effective against the organism.
Xenex Healthcare Services, which markets the PX-UV machine, funded the study, and two of the investigators are employees of the company. Dr. Ghantoji reported having no other relevant financial disclosures.
SAN FRANCISCO – The M.D. Anderson Cancer Center is abandoning bleach for cleaning hospital rooms exposed to Clostridium difficile in favor of a new machine that kills the organism using ultraviolet light.
The machine reduced C. difficile counts as much as, or more than, bleach cleaning in a preliminary prospective trial in 30 hospital rooms previously occupied by patients infected with C. difficile. The machine is a bit more expensive than bleach at a cost of approximately $82,000 (or $3,000-$4,000 per month to lease), but it avoids damage to materials and the toxic environment for workers caused by the use of bleach or other corrosive chemicals, Dr. Shashank S. Ghantoji said in an interview at a poster presentation at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Bleach treatment reduced the average number of colony-forming units of C. difficile from 2.39 before cleaning to 0.71, a 70% reduction in the contamination level. Treatment with the Pulsed Xenon UV machine (PX-UV) reduced the average number of colony-forming units from 22.97 to 1.10, a 95% reduction.
The postcleaning contamination levels were not statistically different between the bleach and PX-UV rooms, Dr. Ghantoji and his associates found. However, PX-UV decontamination is faster than using bleach, Dr. Ghantoji said. "It takes at least 45 minutes to clean a room with bleach, and it’s not good for the patients or the health care professionals," plus admissions staff usually are clamoring for the room to be ready as soon as possible, he said. Cleaning a room using the PX-UV method takes perhaps 15 minutes.
The PX-UV machine has been available for some time, but its adoption depends on how proactive hospital infection control teams are, he added. He said he is aware of at least two medical centers beyond M.D. Anderson that are also using the machine.
In the study, 298 samples were taken before and after cleaning from high-touch surfaces – the bathroom handrail, the bed control panel, the bed rail, the top of the bedside table, and the IV pole control panel or other equipment control panel – and analyzed for C. difficile endospores. Fifteen rooms were cleaned by the conventional method using a 1:10 solution of sodium hypochlorite (bleach), and 15 underwent a visual, nonbleach cleaning of surfaces followed by 15 minutes of treatment with the PX-UV.
With the PX-UV method, housekeeping workers clean the bathroom and place the remote-operated PX-UV in the bathroom with the door shut while they finish cleaning the rest of the room. Then the machine is placed on each side of the bed for 4 minutes of operation with workers gone. Sensors stop the machine if any movement is detected.
It works by emitting ultraviolet C light, which kills C. difficile. And here’s a bonus – it also kills vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus, Dr. Ghantoji of M.D. Anderson, Houston, said at the meeting, sponsored by the American Society for Microbiology.
"The PX-UV method may be a promising alternative to the current standard of decontamination, bleach," he said. Future studies should look at whether the PX-UV method decreases not just endospore counts but transmission of C. difficile, he added.
C. difficile causes more than 300,000 health care–associated infections each year in the United States, incurring $2,500-$3,500 in costs per infection aside from any surgical costs, he estimated. Current guidelines recommend that rooms previously occupied by patients infected with C. difficile be cleaned with a disinfectant registered with the Environmental Protection Agency as effective against the organism.
Xenex Healthcare Services, which markets the PX-UV machine, funded the study, and two of the investigators are employees of the company. Dr. Ghantoji reported having no other relevant financial disclosures.
SAN FRANCISCO – The M.D. Anderson Cancer Center is abandoning bleach for cleaning hospital rooms exposed to Clostridium difficile in favor of a new machine that kills the organism using ultraviolet light.
The machine reduced C. difficile counts as much as, or more than, bleach cleaning in a preliminary prospective trial in 30 hospital rooms previously occupied by patients infected with C. difficile. The machine is a bit more expensive than bleach at a cost of approximately $82,000 (or $3,000-$4,000 per month to lease), but it avoids damage to materials and the toxic environment for workers caused by the use of bleach or other corrosive chemicals, Dr. Shashank S. Ghantoji said in an interview at a poster presentation at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Bleach treatment reduced the average number of colony-forming units of C. difficile from 2.39 before cleaning to 0.71, a 70% reduction in the contamination level. Treatment with the Pulsed Xenon UV machine (PX-UV) reduced the average number of colony-forming units from 22.97 to 1.10, a 95% reduction.
The postcleaning contamination levels were not statistically different between the bleach and PX-UV rooms, Dr. Ghantoji and his associates found. However, PX-UV decontamination is faster than using bleach, Dr. Ghantoji said. "It takes at least 45 minutes to clean a room with bleach, and it’s not good for the patients or the health care professionals," plus admissions staff usually are clamoring for the room to be ready as soon as possible, he said. Cleaning a room using the PX-UV method takes perhaps 15 minutes.
The PX-UV machine has been available for some time, but its adoption depends on how proactive hospital infection control teams are, he added. He said he is aware of at least two medical centers beyond M.D. Anderson that are also using the machine.
In the study, 298 samples were taken before and after cleaning from high-touch surfaces – the bathroom handrail, the bed control panel, the bed rail, the top of the bedside table, and the IV pole control panel or other equipment control panel – and analyzed for C. difficile endospores. Fifteen rooms were cleaned by the conventional method using a 1:10 solution of sodium hypochlorite (bleach), and 15 underwent a visual, nonbleach cleaning of surfaces followed by 15 minutes of treatment with the PX-UV.
With the PX-UV method, housekeeping workers clean the bathroom and place the remote-operated PX-UV in the bathroom with the door shut while they finish cleaning the rest of the room. Then the machine is placed on each side of the bed for 4 minutes of operation with workers gone. Sensors stop the machine if any movement is detected.
It works by emitting ultraviolet C light, which kills C. difficile. And here’s a bonus – it also kills vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus, Dr. Ghantoji of M.D. Anderson, Houston, said at the meeting, sponsored by the American Society for Microbiology.
"The PX-UV method may be a promising alternative to the current standard of decontamination, bleach," he said. Future studies should look at whether the PX-UV method decreases not just endospore counts but transmission of C. difficile, he added.
C. difficile causes more than 300,000 health care–associated infections each year in the United States, incurring $2,500-$3,500 in costs per infection aside from any surgical costs, he estimated. Current guidelines recommend that rooms previously occupied by patients infected with C. difficile be cleaned with a disinfectant registered with the Environmental Protection Agency as effective against the organism.
Xenex Healthcare Services, which markets the PX-UV machine, funded the study, and two of the investigators are employees of the company. Dr. Ghantoji reported having no other relevant financial disclosures.
Major Finding: Bleach killed 70% of C. difficile spores in hospital rooms compared with 95% decontamination using nonbleach cleaning plus UV light treatment. The difference between groups was not statistically significant.
Data Source: A prospective comparison was performed of the two cleaning methods in 30 rooms after discharge of patients infected with C. difficile.
Disclosures: Xenex Healthcare Services, which markets the PX-UV machine, funded the study, and two of the investigators are employees of the company. Dr. Ghantoji reported having no other relevant financial disclosures.
UV-C Light Blasts 'Bad Bugs' in Hospital Rooms
SAN DIEGO – A portable device that emits ultraviolet C light destroyed vancomycin-resistant enterococci, Acinetobacter, and Clostridium difficile from hospital rooms where patients infected with those bacteria had been housed, results from a small study demonstrated.
"There is growing evidence that the environment can be a source for acquisition of bad bugs," lead study investigator Dr. Deverick J. Anderson said in an interview prior to IDWeek 2012, where the research was presented during a poster session.
"Our study further strengthens the data that no-touch systems like UV-C light kill important bacteria and can potentially help with current cleaning strategies. While several groups have demonstrated that UV-C light work in experimental conditions we are demonstrating that it works in a real-world hospital environment."
Dr. Anderson of the department of medicine in the division of infectious diseases at Duke University, Durham, N.C., and his associates analyzed 39 rooms at two tertiary care hospitals that had just housed a patient with one of the different bad bugs: vancomycin-resistant enterococci (VRE), Acinetobacter, and C. difficile. After the patient was discharged but prior to the regular cleaning, the investigators obtained 15 or more cultures from several different locations in the hospital rooms, including bed rails, remote controls, and toilets. Then they wheeled in the TRU-D, an automated mobile disinfection system manufactured by Lumalier that is about 6 feet tall and is equipped with 8 sensors and 16 bulbs that emit UV-C light.
"Each room was irradiated between 25 and 45 minutes in order to eradicate both bacteria and bacterial spores," Dr. Anderson explained during a premeeting telephone press conference. "We then went back into the rooms and cultured the environment from the same locations."
After comparing the number of colony-forming units (CFUs) before and after irradiation "we were able to demonstrate that we could achieve well over 90% reduction in each of those three bad bugs after using the UV light," said Dr. Anderson, who also chairs the antimicrobial stewardship and evaluation team at Duke University Medical Center. "This occurred in all locations sampled, in both direct and indirect light."
Specifically, the UV-C irradiation reduced CFUs of VRE by 98%, C. difficile by 93%, and Acinetobacter by 98%.
"Based on these results we came to the conclusion that UV-C light is indeed effective in killing VRE, C. difficile, and Acinetobacter from the real-world hospital environment," Dr. Anderson said during the telephone press conference. "The idea behind achieving bacterial irradiation in shadow is actually taking advantage of the reflective properties of UV light. It literally bounces around the room and ends up hitting areas in shadow. That’s how bacterial reduction occurs."
He acknowledged certain limitations of the study, including the fact that the researchers were able to evaluate onlytwo hospital rooms with Acinetobacter "because of how infrequently this organism causes infections. Regardless, we reduced the amount of Acinetobacter in both of those rooms."
The study was sponsored by the Centers for Disease Control and Prevention. Lumalier donated the machines used in the study but had no role in the trial design or in review of the data. Dr. Anderson said that he had no relevant financial conflicts to disclose.
IDWeek 2012 is the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
SAN DIEGO – A portable device that emits ultraviolet C light destroyed vancomycin-resistant enterococci, Acinetobacter, and Clostridium difficile from hospital rooms where patients infected with those bacteria had been housed, results from a small study demonstrated.
"There is growing evidence that the environment can be a source for acquisition of bad bugs," lead study investigator Dr. Deverick J. Anderson said in an interview prior to IDWeek 2012, where the research was presented during a poster session.
"Our study further strengthens the data that no-touch systems like UV-C light kill important bacteria and can potentially help with current cleaning strategies. While several groups have demonstrated that UV-C light work in experimental conditions we are demonstrating that it works in a real-world hospital environment."
Dr. Anderson of the department of medicine in the division of infectious diseases at Duke University, Durham, N.C., and his associates analyzed 39 rooms at two tertiary care hospitals that had just housed a patient with one of the different bad bugs: vancomycin-resistant enterococci (VRE), Acinetobacter, and C. difficile. After the patient was discharged but prior to the regular cleaning, the investigators obtained 15 or more cultures from several different locations in the hospital rooms, including bed rails, remote controls, and toilets. Then they wheeled in the TRU-D, an automated mobile disinfection system manufactured by Lumalier that is about 6 feet tall and is equipped with 8 sensors and 16 bulbs that emit UV-C light.
"Each room was irradiated between 25 and 45 minutes in order to eradicate both bacteria and bacterial spores," Dr. Anderson explained during a premeeting telephone press conference. "We then went back into the rooms and cultured the environment from the same locations."
After comparing the number of colony-forming units (CFUs) before and after irradiation "we were able to demonstrate that we could achieve well over 90% reduction in each of those three bad bugs after using the UV light," said Dr. Anderson, who also chairs the antimicrobial stewardship and evaluation team at Duke University Medical Center. "This occurred in all locations sampled, in both direct and indirect light."
Specifically, the UV-C irradiation reduced CFUs of VRE by 98%, C. difficile by 93%, and Acinetobacter by 98%.
"Based on these results we came to the conclusion that UV-C light is indeed effective in killing VRE, C. difficile, and Acinetobacter from the real-world hospital environment," Dr. Anderson said during the telephone press conference. "The idea behind achieving bacterial irradiation in shadow is actually taking advantage of the reflective properties of UV light. It literally bounces around the room and ends up hitting areas in shadow. That’s how bacterial reduction occurs."
He acknowledged certain limitations of the study, including the fact that the researchers were able to evaluate onlytwo hospital rooms with Acinetobacter "because of how infrequently this organism causes infections. Regardless, we reduced the amount of Acinetobacter in both of those rooms."
The study was sponsored by the Centers for Disease Control and Prevention. Lumalier donated the machines used in the study but had no role in the trial design or in review of the data. Dr. Anderson said that he had no relevant financial conflicts to disclose.
IDWeek 2012 is the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
SAN DIEGO – A portable device that emits ultraviolet C light destroyed vancomycin-resistant enterococci, Acinetobacter, and Clostridium difficile from hospital rooms where patients infected with those bacteria had been housed, results from a small study demonstrated.
"There is growing evidence that the environment can be a source for acquisition of bad bugs," lead study investigator Dr. Deverick J. Anderson said in an interview prior to IDWeek 2012, where the research was presented during a poster session.
"Our study further strengthens the data that no-touch systems like UV-C light kill important bacteria and can potentially help with current cleaning strategies. While several groups have demonstrated that UV-C light work in experimental conditions we are demonstrating that it works in a real-world hospital environment."
Dr. Anderson of the department of medicine in the division of infectious diseases at Duke University, Durham, N.C., and his associates analyzed 39 rooms at two tertiary care hospitals that had just housed a patient with one of the different bad bugs: vancomycin-resistant enterococci (VRE), Acinetobacter, and C. difficile. After the patient was discharged but prior to the regular cleaning, the investigators obtained 15 or more cultures from several different locations in the hospital rooms, including bed rails, remote controls, and toilets. Then they wheeled in the TRU-D, an automated mobile disinfection system manufactured by Lumalier that is about 6 feet tall and is equipped with 8 sensors and 16 bulbs that emit UV-C light.
"Each room was irradiated between 25 and 45 minutes in order to eradicate both bacteria and bacterial spores," Dr. Anderson explained during a premeeting telephone press conference. "We then went back into the rooms and cultured the environment from the same locations."
After comparing the number of colony-forming units (CFUs) before and after irradiation "we were able to demonstrate that we could achieve well over 90% reduction in each of those three bad bugs after using the UV light," said Dr. Anderson, who also chairs the antimicrobial stewardship and evaluation team at Duke University Medical Center. "This occurred in all locations sampled, in both direct and indirect light."
Specifically, the UV-C irradiation reduced CFUs of VRE by 98%, C. difficile by 93%, and Acinetobacter by 98%.
"Based on these results we came to the conclusion that UV-C light is indeed effective in killing VRE, C. difficile, and Acinetobacter from the real-world hospital environment," Dr. Anderson said during the telephone press conference. "The idea behind achieving bacterial irradiation in shadow is actually taking advantage of the reflective properties of UV light. It literally bounces around the room and ends up hitting areas in shadow. That’s how bacterial reduction occurs."
He acknowledged certain limitations of the study, including the fact that the researchers were able to evaluate onlytwo hospital rooms with Acinetobacter "because of how infrequently this organism causes infections. Regardless, we reduced the amount of Acinetobacter in both of those rooms."
The study was sponsored by the Centers for Disease Control and Prevention. Lumalier donated the machines used in the study but had no role in the trial design or in review of the data. Dr. Anderson said that he had no relevant financial conflicts to disclose.
IDWeek 2012 is the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Major Finding: UV-C irradiation of hospital rooms with a portable disinfection system reduced colony-forming units of vancomycin-resistant enterococci by 98%, C. difficile by 93%, and Acinetobacter by 98%.
Data Source: Results were taken from a study conducted in 39 hospital rooms at two tertiary medical centers.
Disclosures: The study was sponsored by the Centers for Disease Control and Prevention. Lumalier donated the machines used in the study but had no role in the trial design or in review of the data. Dr. Anderson said that he had no relevant financial conflicts to disclose.
Pharmacologic and nonpharmacologic treatment options for panic disorder
Promising C. difficile Antibiotic in Pipeline
SAN FRANCISCO – Those desperate for new treatments for Clostridium difficile infection may want to keep an eye on the experimental oral antibiotic cadazolid, which looked promising in an early-phase trial, according to Daniela Baldoni, Pharm.D.
Cadazolid is in the oxazolidinone class of antibiotics. Its mechanism of action consists mainly of bacterial protein-synthesis inhibition.
Cadazolid produced low systemic exposure with high concentrations at the desired site – the colon – and was well tolerated in 64 healthy men who received up to 3,000 mg b.i.d. for 10 days, she reported in a poster presentation at the conference. Dr. Baldoni is employed by Actelion Pharmaceuticals, the company that is developing cadazolid.
The study randomized nonsmoking men aged 45-60 years and a body mass index of 18-32 kg/m2 to single or multiple doses of cadazolid or placebo.
In the single-dose group, 30 fasting subjects received a single dose of 30, 100, 300, 1,000, or 3,000 mg cadazolid and 10 subjects received matching placebo. After a wash-out period of 8-15 days, the six subjects who had taken 300 mg received a second dose of 300 mg after eating instead of after fasting. In the multiple-dose group, 18 subjects took 300, 1,000, or 3,000 mg of cadazolid twice a day and 6 received matching placebo for 10 days.
Taking cadazolid with food appeared to increase the rate and extent of drug absorption by two- to fivefold. Blood samples showed low systemic exposure after single or multiple doses, with a minor, twofold increase in cadazolid in plasma after 10 days for all doses in the twice-a-day group, Dr. Baldoni reported at the meeting, sponsored by the American Society for Microbiology.
The dose or duration of treatment did not seem to affect the number of adverse events (none of which were serious). They occurred in 27%-39% of cadazolid-treated subjects and in 17%-40% taking placebo and were mostly headache or diarrhea.
All subjects completed the study except one man in the 100-mg single-dose subgroup who withdrew consent for reasons unrelated to adverse events.
SAN FRANCISCO – Those desperate for new treatments for Clostridium difficile infection may want to keep an eye on the experimental oral antibiotic cadazolid, which looked promising in an early-phase trial, according to Daniela Baldoni, Pharm.D.
Cadazolid is in the oxazolidinone class of antibiotics. Its mechanism of action consists mainly of bacterial protein-synthesis inhibition.
Cadazolid produced low systemic exposure with high concentrations at the desired site – the colon – and was well tolerated in 64 healthy men who received up to 3,000 mg b.i.d. for 10 days, she reported in a poster presentation at the conference. Dr. Baldoni is employed by Actelion Pharmaceuticals, the company that is developing cadazolid.
The study randomized nonsmoking men aged 45-60 years and a body mass index of 18-32 kg/m2 to single or multiple doses of cadazolid or placebo.
In the single-dose group, 30 fasting subjects received a single dose of 30, 100, 300, 1,000, or 3,000 mg cadazolid and 10 subjects received matching placebo. After a wash-out period of 8-15 days, the six subjects who had taken 300 mg received a second dose of 300 mg after eating instead of after fasting. In the multiple-dose group, 18 subjects took 300, 1,000, or 3,000 mg of cadazolid twice a day and 6 received matching placebo for 10 days.
Taking cadazolid with food appeared to increase the rate and extent of drug absorption by two- to fivefold. Blood samples showed low systemic exposure after single or multiple doses, with a minor, twofold increase in cadazolid in plasma after 10 days for all doses in the twice-a-day group, Dr. Baldoni reported at the meeting, sponsored by the American Society for Microbiology.
The dose or duration of treatment did not seem to affect the number of adverse events (none of which were serious). They occurred in 27%-39% of cadazolid-treated subjects and in 17%-40% taking placebo and were mostly headache or diarrhea.
All subjects completed the study except one man in the 100-mg single-dose subgroup who withdrew consent for reasons unrelated to adverse events.
SAN FRANCISCO – Those desperate for new treatments for Clostridium difficile infection may want to keep an eye on the experimental oral antibiotic cadazolid, which looked promising in an early-phase trial, according to Daniela Baldoni, Pharm.D.
Cadazolid is in the oxazolidinone class of antibiotics. Its mechanism of action consists mainly of bacterial protein-synthesis inhibition.
Cadazolid produced low systemic exposure with high concentrations at the desired site – the colon – and was well tolerated in 64 healthy men who received up to 3,000 mg b.i.d. for 10 days, she reported in a poster presentation at the conference. Dr. Baldoni is employed by Actelion Pharmaceuticals, the company that is developing cadazolid.
The study randomized nonsmoking men aged 45-60 years and a body mass index of 18-32 kg/m2 to single or multiple doses of cadazolid or placebo.
In the single-dose group, 30 fasting subjects received a single dose of 30, 100, 300, 1,000, or 3,000 mg cadazolid and 10 subjects received matching placebo. After a wash-out period of 8-15 days, the six subjects who had taken 300 mg received a second dose of 300 mg after eating instead of after fasting. In the multiple-dose group, 18 subjects took 300, 1,000, or 3,000 mg of cadazolid twice a day and 6 received matching placebo for 10 days.
Taking cadazolid with food appeared to increase the rate and extent of drug absorption by two- to fivefold. Blood samples showed low systemic exposure after single or multiple doses, with a minor, twofold increase in cadazolid in plasma after 10 days for all doses in the twice-a-day group, Dr. Baldoni reported at the meeting, sponsored by the American Society for Microbiology.
The dose or duration of treatment did not seem to affect the number of adverse events (none of which were serious). They occurred in 27%-39% of cadazolid-treated subjects and in 17%-40% taking placebo and were mostly headache or diarrhea.
All subjects completed the study except one man in the 100-mg single-dose subgroup who withdrew consent for reasons unrelated to adverse events.
Major Finding: The experimental antibiotic cadazolid concentrated in feces with low systemic exposure and few side effects after single doses or twice-a-day dosing for 10 days.
Data Source: Data are from a randomized, placebo-controlled study in 64 healthy, nonsmoking men.
Disclosures: Dr. Baldoni and most of her coinvestigators are employees of Actelion Pharmaceuticals, which funded the study.
Higher Dose for Severe C. difficile Speeds Response
SAN FRANCISCO – Two small studies suggest that treating severe Clostridium difficile infection with a higher initial dose of vancomycin may work better than the recommended dose of 125 mg every 6 hours.
The most recent study, presented in a poster at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy, surprised the investigators.
"Pharmacodynamically, the concentrations in stool of the standard dose of 125 mg are about 500-1,000 times greater than the MIC [minimum inhibitory concentration]," Yleana T. Garcia, Pharm.D., said in an interview. "So, we have enough concentration in the stool. We wanted to see that we have similar outcomes with patients who are treated with standard doses as with higher doses."
Instead, the retrospective review of 62 patients with severe diarrhea who received oral vancomycin for at least 3 days found that symptoms resolved significantly quicker in 19 patients who got 250 mg every 6 hours compared with 43 patients who got 125 mg every 6 hours, she and her associates reported.
Symptoms resolved by day 3 in nine patients (47%) on the high dose and six patients (15%) on the conventional dose, said Dr. Garcia, a palliative care fellow at the James J. Peters Veterans Affairs Medical Center, Bronx, N.Y.
There also were statistically nonsignificant trends toward a higher likelihood of clinical cure, shorter length of stay, and reduced risk of recurrence in patients with the higher dose. An increased death rate in the higher-dose group also was not statistically significant, and might be due to greater severity of illness at baseline in patients who got the higher dose of vancomycin, she said at the meeting, sponsored by the American Society for Microbiology.
The findings support those of a small prospective study that analyzed levels of vancomycin in feces collected from 15 patients with presumed or confirmed C. difficile infection. Drug concentrations were high in patients who got 250 or 500 mg q.i.d. but were inadequate in at least one patient on the first day of treatment with 125 mg q.i.d. (BMC Infect. Dis. 2010;10:363).<< http://www.biomedcentral.com/1471-2334/10/363 >>
"Higher doses like 250-500 mg may be warranted to reach adequate concentrations in the stool in the first 24-48 hours," Dr. Garcia said. "I’m not saying to use 250 for the whole treatment course, but there may be a role for a loading dose of 250 mg q6 for the first 24-48 hours, and then switching to 125 mg q6 for the remainder of the treatment course. We know that 125 does have adequate fecal concentration; it just may not be adequate on day 1."
The 2010 update to clinical practice guidelines for C. difficile infection in adults recommends treating severe C. difficile infection with oral vancomycin 125 mg every 6 hours or using 500 mg every 6 hours for patients with severe disease complicated by ileus, megacolon, or hypotension (Infect. Control Hosp. Epidemiol. 2010;31:431-55).
Severe C. difficile infection generally is defined as the presence of the organism plus leukocytosis with a white blood cell count of 15,000 cells/microL or greater, or a serum creatinine level at least 1.5 times baseline. The study reviewed records of patients who received vancomycin for these indications or hypotension, shock, ileus, megacolon, or evidence of colitis. The study excluded patients who were treated with any other medication besides metronidazole.
The study is continuing in order to increase the number of patients reviewed and the power of the findings.
Dr. Garcia reported having no financial disclosures.
SAN FRANCISCO – Two small studies suggest that treating severe Clostridium difficile infection with a higher initial dose of vancomycin may work better than the recommended dose of 125 mg every 6 hours.
The most recent study, presented in a poster at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy, surprised the investigators.
"Pharmacodynamically, the concentrations in stool of the standard dose of 125 mg are about 500-1,000 times greater than the MIC [minimum inhibitory concentration]," Yleana T. Garcia, Pharm.D., said in an interview. "So, we have enough concentration in the stool. We wanted to see that we have similar outcomes with patients who are treated with standard doses as with higher doses."
Instead, the retrospective review of 62 patients with severe diarrhea who received oral vancomycin for at least 3 days found that symptoms resolved significantly quicker in 19 patients who got 250 mg every 6 hours compared with 43 patients who got 125 mg every 6 hours, she and her associates reported.
Symptoms resolved by day 3 in nine patients (47%) on the high dose and six patients (15%) on the conventional dose, said Dr. Garcia, a palliative care fellow at the James J. Peters Veterans Affairs Medical Center, Bronx, N.Y.
There also were statistically nonsignificant trends toward a higher likelihood of clinical cure, shorter length of stay, and reduced risk of recurrence in patients with the higher dose. An increased death rate in the higher-dose group also was not statistically significant, and might be due to greater severity of illness at baseline in patients who got the higher dose of vancomycin, she said at the meeting, sponsored by the American Society for Microbiology.
The findings support those of a small prospective study that analyzed levels of vancomycin in feces collected from 15 patients with presumed or confirmed C. difficile infection. Drug concentrations were high in patients who got 250 or 500 mg q.i.d. but were inadequate in at least one patient on the first day of treatment with 125 mg q.i.d. (BMC Infect. Dis. 2010;10:363).<< http://www.biomedcentral.com/1471-2334/10/363 >>
"Higher doses like 250-500 mg may be warranted to reach adequate concentrations in the stool in the first 24-48 hours," Dr. Garcia said. "I’m not saying to use 250 for the whole treatment course, but there may be a role for a loading dose of 250 mg q6 for the first 24-48 hours, and then switching to 125 mg q6 for the remainder of the treatment course. We know that 125 does have adequate fecal concentration; it just may not be adequate on day 1."
The 2010 update to clinical practice guidelines for C. difficile infection in adults recommends treating severe C. difficile infection with oral vancomycin 125 mg every 6 hours or using 500 mg every 6 hours for patients with severe disease complicated by ileus, megacolon, or hypotension (Infect. Control Hosp. Epidemiol. 2010;31:431-55).
Severe C. difficile infection generally is defined as the presence of the organism plus leukocytosis with a white blood cell count of 15,000 cells/microL or greater, or a serum creatinine level at least 1.5 times baseline. The study reviewed records of patients who received vancomycin for these indications or hypotension, shock, ileus, megacolon, or evidence of colitis. The study excluded patients who were treated with any other medication besides metronidazole.
The study is continuing in order to increase the number of patients reviewed and the power of the findings.
Dr. Garcia reported having no financial disclosures.
SAN FRANCISCO – Two small studies suggest that treating severe Clostridium difficile infection with a higher initial dose of vancomycin may work better than the recommended dose of 125 mg every 6 hours.
The most recent study, presented in a poster at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy, surprised the investigators.
"Pharmacodynamically, the concentrations in stool of the standard dose of 125 mg are about 500-1,000 times greater than the MIC [minimum inhibitory concentration]," Yleana T. Garcia, Pharm.D., said in an interview. "So, we have enough concentration in the stool. We wanted to see that we have similar outcomes with patients who are treated with standard doses as with higher doses."
Instead, the retrospective review of 62 patients with severe diarrhea who received oral vancomycin for at least 3 days found that symptoms resolved significantly quicker in 19 patients who got 250 mg every 6 hours compared with 43 patients who got 125 mg every 6 hours, she and her associates reported.
Symptoms resolved by day 3 in nine patients (47%) on the high dose and six patients (15%) on the conventional dose, said Dr. Garcia, a palliative care fellow at the James J. Peters Veterans Affairs Medical Center, Bronx, N.Y.
There also were statistically nonsignificant trends toward a higher likelihood of clinical cure, shorter length of stay, and reduced risk of recurrence in patients with the higher dose. An increased death rate in the higher-dose group also was not statistically significant, and might be due to greater severity of illness at baseline in patients who got the higher dose of vancomycin, she said at the meeting, sponsored by the American Society for Microbiology.
The findings support those of a small prospective study that analyzed levels of vancomycin in feces collected from 15 patients with presumed or confirmed C. difficile infection. Drug concentrations were high in patients who got 250 or 500 mg q.i.d. but were inadequate in at least one patient on the first day of treatment with 125 mg q.i.d. (BMC Infect. Dis. 2010;10:363).<< http://www.biomedcentral.com/1471-2334/10/363 >>
"Higher doses like 250-500 mg may be warranted to reach adequate concentrations in the stool in the first 24-48 hours," Dr. Garcia said. "I’m not saying to use 250 for the whole treatment course, but there may be a role for a loading dose of 250 mg q6 for the first 24-48 hours, and then switching to 125 mg q6 for the remainder of the treatment course. We know that 125 does have adequate fecal concentration; it just may not be adequate on day 1."
The 2010 update to clinical practice guidelines for C. difficile infection in adults recommends treating severe C. difficile infection with oral vancomycin 125 mg every 6 hours or using 500 mg every 6 hours for patients with severe disease complicated by ileus, megacolon, or hypotension (Infect. Control Hosp. Epidemiol. 2010;31:431-55).
Severe C. difficile infection generally is defined as the presence of the organism plus leukocytosis with a white blood cell count of 15,000 cells/microL or greater, or a serum creatinine level at least 1.5 times baseline. The study reviewed records of patients who received vancomycin for these indications or hypotension, shock, ileus, megacolon, or evidence of colitis. The study excluded patients who were treated with any other medication besides metronidazole.
The study is continuing in order to increase the number of patients reviewed and the power of the findings.
Dr. Garcia reported having no financial disclosures.
Major Finding: Symptoms of severe C. difficile infection resolved by day 3 of oral vancomycin treatment in 9 of 19 patients treated with 250 mg every 6 hours (47%) compared with 6 of 43 patients on 125 mg every 6 hours (15%).
Data Source: Retrospective review of records on 62 adults at one institution treated for at least 3 days with oral vancomycin for severe C. difficile infection.
Disclosures: Dr. Garcia reported having no financial disclosures.