User login
The Numerators: Treating Noncompliant, Medically Complicated Hospital Patients
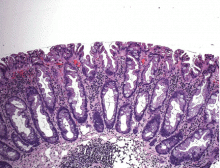
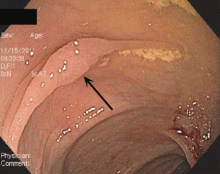
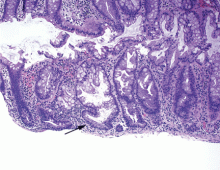
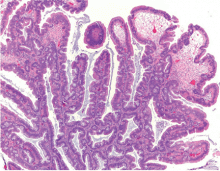
We hospitalists are scientifically minded. We understand basic statistics, including percentages, percentiles, numerators, denominators (see Figure 1, right). In healthcare, we see a lot of patients we call denominators; these denominators are generally the types of patients to whom not much happens. They come in “pre-” and they leave “post-.” They generally pass through our walls, and our lives, according to plan, without leaving an impenetrable memory of who they were or what they experienced.
The numerators, on the other hand, do have something happen to them—something unexpected, untoward, unanticipated, unlikely. Sometimes we describe numerators as “noncompliant” or “medically complicated” or “refractory to treatment.” We often find ways to rationalize and explain how the patient turned from a denominator into a numerator—something they did, or didn’t do, to nudge them above the line. They smoked, they ate too much, they didn’t take their medications “as prescribed.” Often there is a less robust discussion about what we could have done to reduce the nudge: understand their background, their literacy, their finances, their physical/cognitive limitations, their understanding of risks and benefits.
I read a powerful piece about “numerators” written by Kerry O’Connell. In this piece, she describes what it was like to cross over the line into being a numerator after acquiring a hospital-acquired infection:
Five years ago this summer while under deep anesthesia for arm surgery number 3, I drifted above the line and joined the group called Numerators. … Numerators have lost a lot to join this group; many have lost organs, and some have lost all their limbs, all have many kinds of scars from their journey. It was not our choice to leave the world of Denominators … and many will struggle the rest of their lives to understand why...
There are lots of silly rules for not counting some infected souls, as if by not counting us we might not exist. Numerators that are identified are then divided by the Denominators to create a nameless, faceless, mysteriously small number called infection rates. “Rates,” like their cousin “odds,” claim to portray hope while predicting doom for some of us. Denominators are in love with rates, for no matter how many Numerators they have sired, someone else has sired more. Rates soothe the Denominator conscious and allow them to sleep peacefully at night ...
Numerators don’t ask for much from the world. We ask that Denominators look behind the numbers to see the people, to love us, count us, respect our suffering, and help keep us out of bankruptcy, for once we were Denominators just like you. Our greatest dream is that you find the daily strength to truly care. To care enough to follow the checklists, to care enough to wash your hands, to care enough to only use virgin needles, for the saddest day for all Numerators is when another unsuspecting Denominator rises above the line to join our group.1
CB’s Story
Now think of all the numerators you have met. I am going to repeat that phrase. Think of all the numerators you have met. I have met quite a few. Now I am going to tell you about my most memorable numerator.
CB was a 36-year-old white female admitted to the hospital with a recent diagnosis of ulcerative colitis. She had a protracted hospital course on various immunosuppressant drugs, none of which relieved her symptoms. During her hospital stay, her family, including her 2-year-old twins, visited every single day. After several weeks with no improvement, the decision was made to proceed to a colectomy. The surgical procedure itself was uncomplicated, a true denominator.
Then, on post-op Day 5, the day of her anticipated discharge, a pulmonary embolus thrust her into the numerator position. A preventable, eventually fatal numerator—a numerator who “just would not keep her compression devices on” and whom the staff tried to get out of bed, “but she just wouldn’t do it.” A numerator who just so happened to be my sister.
Every year on April 2, when I call my niece and nephew to wish them a happy birthday, I think about numerators. And I think about how incredibly different life would be for those 10-year-old twins, had their mom just stayed a denominator. And every day, when I sit in conference rooms and hear from countless people about how difficult it is to prevent this and reduce that, and how zero is not feasible, I think about numerators. I don’t look at their bar chart, or their run chart, or their red line, or their blue line, or whether their line is within the control limits, or what their P-value is. I think about who represents that black dot, and about how we are going to actually convince ourselves to “First, do no harm.”
When I find myself amongst a crowd quibbling about finances, lunch breaks, workflows, accountability, and about who is going to check the box or fill out the form, I think about the numerators, and how we are truly wasting their time, their livelihood, and their ability to stay below the line.
And someday, when my niece and nephew are old enough to understand, I will try to help them tolerate and accept the fact that “preventable” and “prevented” are not interchangeable. At least not in the medical industry. At least not yet.
In memory of Colleen Conlin Bowen, May 14, 2004
Dr. Scheurer is a hospitalist and chief quality officer at the Medical University of South Carolina in Charleston. She is physician editor of The Hospitalist. Email her at [email protected].
Reference
We hospitalists are scientifically minded. We understand basic statistics, including percentages, percentiles, numerators, denominators (see Figure 1, right). In healthcare, we see a lot of patients we call denominators; these denominators are generally the types of patients to whom not much happens. They come in “pre-” and they leave “post-.” They generally pass through our walls, and our lives, according to plan, without leaving an impenetrable memory of who they were or what they experienced.
The numerators, on the other hand, do have something happen to them—something unexpected, untoward, unanticipated, unlikely. Sometimes we describe numerators as “noncompliant” or “medically complicated” or “refractory to treatment.” We often find ways to rationalize and explain how the patient turned from a denominator into a numerator—something they did, or didn’t do, to nudge them above the line. They smoked, they ate too much, they didn’t take their medications “as prescribed.” Often there is a less robust discussion about what we could have done to reduce the nudge: understand their background, their literacy, their finances, their physical/cognitive limitations, their understanding of risks and benefits.
I read a powerful piece about “numerators” written by Kerry O’Connell. In this piece, she describes what it was like to cross over the line into being a numerator after acquiring a hospital-acquired infection:
Five years ago this summer while under deep anesthesia for arm surgery number 3, I drifted above the line and joined the group called Numerators. … Numerators have lost a lot to join this group; many have lost organs, and some have lost all their limbs, all have many kinds of scars from their journey. It was not our choice to leave the world of Denominators … and many will struggle the rest of their lives to understand why...
There are lots of silly rules for not counting some infected souls, as if by not counting us we might not exist. Numerators that are identified are then divided by the Denominators to create a nameless, faceless, mysteriously small number called infection rates. “Rates,” like their cousin “odds,” claim to portray hope while predicting doom for some of us. Denominators are in love with rates, for no matter how many Numerators they have sired, someone else has sired more. Rates soothe the Denominator conscious and allow them to sleep peacefully at night ...
Numerators don’t ask for much from the world. We ask that Denominators look behind the numbers to see the people, to love us, count us, respect our suffering, and help keep us out of bankruptcy, for once we were Denominators just like you. Our greatest dream is that you find the daily strength to truly care. To care enough to follow the checklists, to care enough to wash your hands, to care enough to only use virgin needles, for the saddest day for all Numerators is when another unsuspecting Denominator rises above the line to join our group.1
CB’s Story
Now think of all the numerators you have met. I am going to repeat that phrase. Think of all the numerators you have met. I have met quite a few. Now I am going to tell you about my most memorable numerator.
CB was a 36-year-old white female admitted to the hospital with a recent diagnosis of ulcerative colitis. She had a protracted hospital course on various immunosuppressant drugs, none of which relieved her symptoms. During her hospital stay, her family, including her 2-year-old twins, visited every single day. After several weeks with no improvement, the decision was made to proceed to a colectomy. The surgical procedure itself was uncomplicated, a true denominator.
Then, on post-op Day 5, the day of her anticipated discharge, a pulmonary embolus thrust her into the numerator position. A preventable, eventually fatal numerator—a numerator who “just would not keep her compression devices on” and whom the staff tried to get out of bed, “but she just wouldn’t do it.” A numerator who just so happened to be my sister.
Every year on April 2, when I call my niece and nephew to wish them a happy birthday, I think about numerators. And I think about how incredibly different life would be for those 10-year-old twins, had their mom just stayed a denominator. And every day, when I sit in conference rooms and hear from countless people about how difficult it is to prevent this and reduce that, and how zero is not feasible, I think about numerators. I don’t look at their bar chart, or their run chart, or their red line, or their blue line, or whether their line is within the control limits, or what their P-value is. I think about who represents that black dot, and about how we are going to actually convince ourselves to “First, do no harm.”
When I find myself amongst a crowd quibbling about finances, lunch breaks, workflows, accountability, and about who is going to check the box or fill out the form, I think about the numerators, and how we are truly wasting their time, their livelihood, and their ability to stay below the line.
And someday, when my niece and nephew are old enough to understand, I will try to help them tolerate and accept the fact that “preventable” and “prevented” are not interchangeable. At least not in the medical industry. At least not yet.
In memory of Colleen Conlin Bowen, May 14, 2004
Dr. Scheurer is a hospitalist and chief quality officer at the Medical University of South Carolina in Charleston. She is physician editor of The Hospitalist. Email her at [email protected].
Reference
We hospitalists are scientifically minded. We understand basic statistics, including percentages, percentiles, numerators, denominators (see Figure 1, right). In healthcare, we see a lot of patients we call denominators; these denominators are generally the types of patients to whom not much happens. They come in “pre-” and they leave “post-.” They generally pass through our walls, and our lives, according to plan, without leaving an impenetrable memory of who they were or what they experienced.
The numerators, on the other hand, do have something happen to them—something unexpected, untoward, unanticipated, unlikely. Sometimes we describe numerators as “noncompliant” or “medically complicated” or “refractory to treatment.” We often find ways to rationalize and explain how the patient turned from a denominator into a numerator—something they did, or didn’t do, to nudge them above the line. They smoked, they ate too much, they didn’t take their medications “as prescribed.” Often there is a less robust discussion about what we could have done to reduce the nudge: understand their background, their literacy, their finances, their physical/cognitive limitations, their understanding of risks and benefits.
I read a powerful piece about “numerators” written by Kerry O’Connell. In this piece, she describes what it was like to cross over the line into being a numerator after acquiring a hospital-acquired infection:
Five years ago this summer while under deep anesthesia for arm surgery number 3, I drifted above the line and joined the group called Numerators. … Numerators have lost a lot to join this group; many have lost organs, and some have lost all their limbs, all have many kinds of scars from their journey. It was not our choice to leave the world of Denominators … and many will struggle the rest of their lives to understand why...
There are lots of silly rules for not counting some infected souls, as if by not counting us we might not exist. Numerators that are identified are then divided by the Denominators to create a nameless, faceless, mysteriously small number called infection rates. “Rates,” like their cousin “odds,” claim to portray hope while predicting doom for some of us. Denominators are in love with rates, for no matter how many Numerators they have sired, someone else has sired more. Rates soothe the Denominator conscious and allow them to sleep peacefully at night ...
Numerators don’t ask for much from the world. We ask that Denominators look behind the numbers to see the people, to love us, count us, respect our suffering, and help keep us out of bankruptcy, for once we were Denominators just like you. Our greatest dream is that you find the daily strength to truly care. To care enough to follow the checklists, to care enough to wash your hands, to care enough to only use virgin needles, for the saddest day for all Numerators is when another unsuspecting Denominator rises above the line to join our group.1
CB’s Story
Now think of all the numerators you have met. I am going to repeat that phrase. Think of all the numerators you have met. I have met quite a few. Now I am going to tell you about my most memorable numerator.
CB was a 36-year-old white female admitted to the hospital with a recent diagnosis of ulcerative colitis. She had a protracted hospital course on various immunosuppressant drugs, none of which relieved her symptoms. During her hospital stay, her family, including her 2-year-old twins, visited every single day. After several weeks with no improvement, the decision was made to proceed to a colectomy. The surgical procedure itself was uncomplicated, a true denominator.
Then, on post-op Day 5, the day of her anticipated discharge, a pulmonary embolus thrust her into the numerator position. A preventable, eventually fatal numerator—a numerator who “just would not keep her compression devices on” and whom the staff tried to get out of bed, “but she just wouldn’t do it.” A numerator who just so happened to be my sister.
Every year on April 2, when I call my niece and nephew to wish them a happy birthday, I think about numerators. And I think about how incredibly different life would be for those 10-year-old twins, had their mom just stayed a denominator. And every day, when I sit in conference rooms and hear from countless people about how difficult it is to prevent this and reduce that, and how zero is not feasible, I think about numerators. I don’t look at their bar chart, or their run chart, or their red line, or their blue line, or whether their line is within the control limits, or what their P-value is. I think about who represents that black dot, and about how we are going to actually convince ourselves to “First, do no harm.”
When I find myself amongst a crowd quibbling about finances, lunch breaks, workflows, accountability, and about who is going to check the box or fill out the form, I think about the numerators, and how we are truly wasting their time, their livelihood, and their ability to stay below the line.
And someday, when my niece and nephew are old enough to understand, I will try to help them tolerate and accept the fact that “preventable” and “prevented” are not interchangeable. At least not in the medical industry. At least not yet.
In memory of Colleen Conlin Bowen, May 14, 2004
Dr. Scheurer is a hospitalist and chief quality officer at the Medical University of South Carolina in Charleston. She is physician editor of The Hospitalist. Email her at [email protected].
Reference
Interfacility Transfers to Pediatric Academic EDs Often Discharged or Admitted Briefly
Clinical question: What are the characteristics of interfacility transfers to pediatric academic EDs?
Background: The majority of pediatric ED visits (89%) and hospital admissions (69%) occur via general hospital EDs, not freestanding academic children's hospitals. Pediatric hospitalists often provide consultation services in these community hospital settings and might be the primary admitting team in either setting (community hospital or children's hospital). Questions concerning the quality of pediatric ED care in community hospitals have been raised, with acknowledged improvements in post-transfer care for critically ill patients. The characteristics of less acutely ill transfers are unknown and could provide insight into opportunities for improvement.
Study design: Cross-sectional, retrospective database review.
Setting: Twenty-nine tertiary-care pediatric hospitals.
Synopsis: The Pediatric Health Information System (PHIS) database of the Child Health Corporation of America was reviewed; over a one-year period, 24,905 interfacility transfers were identified from 29 hospitals. Fifty-eight percent of patients were admitted for more than 24 hours with common respiratory illnesses (pneumonia, bronchiolitis, asthma) and surgical conditions representing the most common diagnostic categories. Among the remaining patients, 24.7% were discharged directly from the academic pediatric EDs; 17% were admitted for less than 24 hours. Among those discharged or briefly admitted, common nonsurgical diagnostic categories included abdominal pain, viral gastroenteritis/dehydration, and other gastrointestinal conditions.
The authors attempted to define areas for improvement in pediatric care in community hospital EDs. Limitations of their analysis include the use of a database without validated code for source of admission, as well as an inability to drill down further into the specifics of what additional expertise was provided at the pediatric EDs. However, this study provides a platform by which pediatric hospitalists can view and subsequently improve the value of their regional care systems.
Bottom line: Interfacility transfers to pediatric academic EDs might offer an opportunity for improved pediatric care in community hospital EDs.
Citation: Li J, Monuteaux MC, Bachur RG. Interfacility transfers of noncritically ill children to academic pediatric emergency departments. Pediatrics. 2012;130:83-92.
Reviewed by Pediatric Editor Mark Shen, MD, SFHM, medical director of hospital medicine at Dell Children's Medical Center, Austin, Texas.
Clinical question: What are the characteristics of interfacility transfers to pediatric academic EDs?
Background: The majority of pediatric ED visits (89%) and hospital admissions (69%) occur via general hospital EDs, not freestanding academic children's hospitals. Pediatric hospitalists often provide consultation services in these community hospital settings and might be the primary admitting team in either setting (community hospital or children's hospital). Questions concerning the quality of pediatric ED care in community hospitals have been raised, with acknowledged improvements in post-transfer care for critically ill patients. The characteristics of less acutely ill transfers are unknown and could provide insight into opportunities for improvement.
Study design: Cross-sectional, retrospective database review.
Setting: Twenty-nine tertiary-care pediatric hospitals.
Synopsis: The Pediatric Health Information System (PHIS) database of the Child Health Corporation of America was reviewed; over a one-year period, 24,905 interfacility transfers were identified from 29 hospitals. Fifty-eight percent of patients were admitted for more than 24 hours with common respiratory illnesses (pneumonia, bronchiolitis, asthma) and surgical conditions representing the most common diagnostic categories. Among the remaining patients, 24.7% were discharged directly from the academic pediatric EDs; 17% were admitted for less than 24 hours. Among those discharged or briefly admitted, common nonsurgical diagnostic categories included abdominal pain, viral gastroenteritis/dehydration, and other gastrointestinal conditions.
The authors attempted to define areas for improvement in pediatric care in community hospital EDs. Limitations of their analysis include the use of a database without validated code for source of admission, as well as an inability to drill down further into the specifics of what additional expertise was provided at the pediatric EDs. However, this study provides a platform by which pediatric hospitalists can view and subsequently improve the value of their regional care systems.
Bottom line: Interfacility transfers to pediatric academic EDs might offer an opportunity for improved pediatric care in community hospital EDs.
Citation: Li J, Monuteaux MC, Bachur RG. Interfacility transfers of noncritically ill children to academic pediatric emergency departments. Pediatrics. 2012;130:83-92.
Reviewed by Pediatric Editor Mark Shen, MD, SFHM, medical director of hospital medicine at Dell Children's Medical Center, Austin, Texas.
Clinical question: What are the characteristics of interfacility transfers to pediatric academic EDs?
Background: The majority of pediatric ED visits (89%) and hospital admissions (69%) occur via general hospital EDs, not freestanding academic children's hospitals. Pediatric hospitalists often provide consultation services in these community hospital settings and might be the primary admitting team in either setting (community hospital or children's hospital). Questions concerning the quality of pediatric ED care in community hospitals have been raised, with acknowledged improvements in post-transfer care for critically ill patients. The characteristics of less acutely ill transfers are unknown and could provide insight into opportunities for improvement.
Study design: Cross-sectional, retrospective database review.
Setting: Twenty-nine tertiary-care pediatric hospitals.
Synopsis: The Pediatric Health Information System (PHIS) database of the Child Health Corporation of America was reviewed; over a one-year period, 24,905 interfacility transfers were identified from 29 hospitals. Fifty-eight percent of patients were admitted for more than 24 hours with common respiratory illnesses (pneumonia, bronchiolitis, asthma) and surgical conditions representing the most common diagnostic categories. Among the remaining patients, 24.7% were discharged directly from the academic pediatric EDs; 17% were admitted for less than 24 hours. Among those discharged or briefly admitted, common nonsurgical diagnostic categories included abdominal pain, viral gastroenteritis/dehydration, and other gastrointestinal conditions.
The authors attempted to define areas for improvement in pediatric care in community hospital EDs. Limitations of their analysis include the use of a database without validated code for source of admission, as well as an inability to drill down further into the specifics of what additional expertise was provided at the pediatric EDs. However, this study provides a platform by which pediatric hospitalists can view and subsequently improve the value of their regional care systems.
Bottom line: Interfacility transfers to pediatric academic EDs might offer an opportunity for improved pediatric care in community hospital EDs.
Citation: Li J, Monuteaux MC, Bachur RG. Interfacility transfers of noncritically ill children to academic pediatric emergency departments. Pediatrics. 2012;130:83-92.
Reviewed by Pediatric Editor Mark Shen, MD, SFHM, medical director of hospital medicine at Dell Children's Medical Center, Austin, Texas.
Recommendations for Antithrombotic and Thrombolytic Therapy
Background
Each year, 1 million people are hospitalized with a diagnosis of stroke; it was the fourth-leading cause of death in the U.S. in 2009 and 2010.1 The majority of strokes (80%) are caused by focal cerebral ischemia, and the remainder are caused by hemorrhage.1 In 2008, the direct medical costs of stroke were approximately $18.8 billion, with almost half of this amount directed toward hospitalization.1 Although stroke inpatients make up only 3% of total hospitalizations, the mortality rate is more than twice that of other patients’.1
Over the past several decades, much has been learned about the pathophysiology and treatment for ischemic stroke. The mainstays of therapies include restoring perfusion in a timely manner and targeting both clot formation and hemostasis. These therapies improve patient outcomes and reduce the risk of recurrence in appropriately selected populations.
Guideline Update
In February, the American College of Chest Physicians (ACCP) published new practice guidelines for medical patients regarding antithrombotic and thrombolytic therapy in acute ischemic stroke.2 These evidence-based guidelines are the result of new clinical trial data and a review of previous studies. They address three aspects of management decisions for stroke, including acute treatment, VTE prevention, and secondary prevention, as well as specifically address the treatment of cerebral venous sinus thrombosis.
In the management of acute ischemic stroke, several recommendations were made. In terms of IV recombinant tissue plasminogen activator (r-tPA) administration, the guidelines were expanded and allow for a less restrictive time threshold for administration. Previous recommendations limited the usage of IV r-tPA to within three hours of symptom onset in acute ischemic stroke. A science advisory from the American Heart Association/American Stroke Association (AHA/ASA) from 2009 extended that window to 4.5 hours. The 2012 ACCP guidelines have followed suit to extend this time to 4.5 hours from symptom onset as well.
In addition, intrarterial r-tPA can be given in patients not eligible for IV r-tPA within six hours of presentation of acute ischemic stroke due to proximal cerebral artery occlusion.
These updated acute stroke guidelines recommend against the use of mechanical thrombectomy based mostly on lack of data rather than lack of benefit.2
The new guidelines continue to recommend early aspirin therapy at a dosage of 160 mg to 325 mg within the first 48 hours of acute ischemic stroke. Therapeutic parenteral anticoagulation with heparin or related drugs was not recommended in patients with noncardioembolic stroke due to atrial fibrillation (afib) or in patients with stroke due to large artery stenosis or arterial dissection. In this updated analysis, there was no benefit of anticoagulation compared with antiplatelet therapy, and the risk for extracranial hemorrhage was increased. No specific recommendation regarding anticoagulation was made in patients with mechanical heart valves or intracardiac thrombus.
Updates have been made for VTE prophylaxis in patients hospitalized for acute stroke. In stroke patients with restricted mobility, prophylactic unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH) and/or intermittent pneumatic compression devices should be initiated as early as possible. The panel is no longer recommending elastic compression stockings as VTE prevention given the risk of skin damage and no clear benefit in symptomatic VTE prevention. For patients with hemorrhagic stroke and restricted mobility, similar recommendations were made for VTE prevention, except to start pharmacologic treatment between days 2 and 4 of the hospital stay. However, if there is a bleeding concern, intermittent pneumatic compression devices are favored over pharmacologic prophylaxis. In all patients for whom pharmacologic prevention is utilized, prophylactic LMWH is preferred over UFH.
Secondary stroke prevention is addressed, with 2012 guidelines outlining a preference for clopidogrel or aspirin/extended-release dipyridamole rather than aspirin or cilostazol in patients with a history of noncardioembolic ischemic stroke or TIA. Oral anticoagulation is preferred in patients with a history of stroke or TIA with afib over aspirin alone, aspirin plus clopidogrel, or no antithrombotic therapy. Of the available anticoagulants, the panel recommended dabigatran 150 mg twice daily over adjusted-dose warfarin.2 This recommendation is based on results from the RE-LY trial, which showed dabigatran as noninferior to warfarin in patients with nonvalvular afib without severe renal failure or advanced liver disease.3
For patients who have contraindications or choose not to initiate anticoagulation, the combination of aspirin (ASA) and clopidogrel is a reasonable alternative. Timing of the initiation of oral anticoagulation should be between one and two weeks after the stroke. Patients with extensive infarction or hemorrhagic transformation should delay starting oral anticoagulation, with no exact timeline. Long-term antithrombotic therapy is contraindicated in patients with history of a symptomatic primary intracerebral hemorrhage.2 New guidelines also recommend full anticoagulation for patients with symptomatic cerebral venous sinus thrombosis.
The panel did not make any recommendations regarding statin usage. In several studies, findings showed that statins reduced infarct size and had improved outcome in all stroke types.4
Analysis
Prior to the 2012 update, the last guideline for antithrombotic and thrombolytic therapy for ischemic stroke was published by the ACCP in the June 2008 issue of Chest.5 Dating back to 2001, medications included r-tPA administration within three hours of stroke symptom onset, and aspirin, clopidogrel, or a com bination of aspirin and extended-release dipyridamole for stroke prophylaxis.
The management of stroke continues to focus on early intervention and secondary prevention. Thrombolytic therapy is an effective treatment of acute ischemic stroke if given within the narrow window from onset of stroke symptoms up to 4.5 hours, with the goal of treatment within a three-hour window. Beyond this time constraint, the risk outweighs the benefit of using r-tPA except in the case of intra-arterial r-tPA administration for proximal cerebral artery occlusion.
In 2010, a meta-analysis supported this by showing that the risk of death increased significantly in patients receiving r-tPA beyond 4.5 hours. Therefore, antiplatelet therapy is the best alternative for patients ineligible for thrombolytic therapy.6 Even so, that study offered little data for patients with mechanical heart valves or intracardiac thrombi. Thus, the choice for acute anticoagulation therapy is variable and uncertain. If the hemorrhagic risk is low, anticoagulation can be considered in this subgroup, but no specific guideline endorsement was made.
In 2011, the AHA/ASA published an updated treatment guideline for patients with stroke or TIA. This was an update to 2007 guidelines that outlined the early management of ischemic stroke and affirmed the benefit of IV r-tPA at 4.5 hours for the treatment of stroke.7 Of note, IV r-tPA is only FDA-approved for treatment of acute ischemic stroke within the previously recommended three-hour period from symptom onset.
Aspirin has been found to be effective in both early treatment of acute ischemic stroke and secondary prevention. The CAST trial showed a statistically significant rate of reduction of nonfatal strokes with the use of aspirin. Other antiplatelet agents, including clopidogrel and dipyridamole, can be used. The FASTER trial compared aspirin alone versus aspirin plus clopidogrel, with no difference in outcome measures, although the MATCH trial found a larger risk of hemorrhagic and bleeding complications in the acetylsalicylic acid (ASA)-plus-clopidogrel group.6,7
In TIA or stroke patients, clopidogrel is not superior to ASA in preventing recurrent stroke. However, patients who have peripheral artery disease (PAD), previous coronary artery bypass grafting (CABG), insulin dependent diabetes mellitus (IDDM), or recurrent vascular events show a benefit of transitioning from ASA to clopidogrel for secondary long-term prevention. Clopidogrel or aspirin/extended-release dipyridamole is preferred over aspirin alone or cilostazol for long-term treatment in patients with a history of noncardioembolic ischemic stroke or TIA based on the PROFESS trial.2,7
HM Takeaways
The 2012 guidelines are a resource available to hospitalists for treating acute ischemic stroke either alone or with neurology consultation. These guidelines further define the timing of r-tPA and the use of both anticoagulation and antiplatelet therapy in the proper clinical settings.
In terms of VTE prevention, the guidelines recommend using LMWH preferentially over UH, except in patients at risk for rebleeding. The clinician should be aware of the treatment considerations for secondary prevention, noting the primary role of aspirin therapy in ischemic stroke with consideration of other agents (i.e. clopidogrel) in select populations.
Drs. Barr and Schumacher are hospitalists and assistant professors in the division of hospital medicine at The Ohio State University College of Medicine in Columbus.
References
Available at the-hospitalist.org.
Background
Each year, 1 million people are hospitalized with a diagnosis of stroke; it was the fourth-leading cause of death in the U.S. in 2009 and 2010.1 The majority of strokes (80%) are caused by focal cerebral ischemia, and the remainder are caused by hemorrhage.1 In 2008, the direct medical costs of stroke were approximately $18.8 billion, with almost half of this amount directed toward hospitalization.1 Although stroke inpatients make up only 3% of total hospitalizations, the mortality rate is more than twice that of other patients’.1
Over the past several decades, much has been learned about the pathophysiology and treatment for ischemic stroke. The mainstays of therapies include restoring perfusion in a timely manner and targeting both clot formation and hemostasis. These therapies improve patient outcomes and reduce the risk of recurrence in appropriately selected populations.
Guideline Update
In February, the American College of Chest Physicians (ACCP) published new practice guidelines for medical patients regarding antithrombotic and thrombolytic therapy in acute ischemic stroke.2 These evidence-based guidelines are the result of new clinical trial data and a review of previous studies. They address three aspects of management decisions for stroke, including acute treatment, VTE prevention, and secondary prevention, as well as specifically address the treatment of cerebral venous sinus thrombosis.
In the management of acute ischemic stroke, several recommendations were made. In terms of IV recombinant tissue plasminogen activator (r-tPA) administration, the guidelines were expanded and allow for a less restrictive time threshold for administration. Previous recommendations limited the usage of IV r-tPA to within three hours of symptom onset in acute ischemic stroke. A science advisory from the American Heart Association/American Stroke Association (AHA/ASA) from 2009 extended that window to 4.5 hours. The 2012 ACCP guidelines have followed suit to extend this time to 4.5 hours from symptom onset as well.
In addition, intrarterial r-tPA can be given in patients not eligible for IV r-tPA within six hours of presentation of acute ischemic stroke due to proximal cerebral artery occlusion.
These updated acute stroke guidelines recommend against the use of mechanical thrombectomy based mostly on lack of data rather than lack of benefit.2
The new guidelines continue to recommend early aspirin therapy at a dosage of 160 mg to 325 mg within the first 48 hours of acute ischemic stroke. Therapeutic parenteral anticoagulation with heparin or related drugs was not recommended in patients with noncardioembolic stroke due to atrial fibrillation (afib) or in patients with stroke due to large artery stenosis or arterial dissection. In this updated analysis, there was no benefit of anticoagulation compared with antiplatelet therapy, and the risk for extracranial hemorrhage was increased. No specific recommendation regarding anticoagulation was made in patients with mechanical heart valves or intracardiac thrombus.
Updates have been made for VTE prophylaxis in patients hospitalized for acute stroke. In stroke patients with restricted mobility, prophylactic unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH) and/or intermittent pneumatic compression devices should be initiated as early as possible. The panel is no longer recommending elastic compression stockings as VTE prevention given the risk of skin damage and no clear benefit in symptomatic VTE prevention. For patients with hemorrhagic stroke and restricted mobility, similar recommendations were made for VTE prevention, except to start pharmacologic treatment between days 2 and 4 of the hospital stay. However, if there is a bleeding concern, intermittent pneumatic compression devices are favored over pharmacologic prophylaxis. In all patients for whom pharmacologic prevention is utilized, prophylactic LMWH is preferred over UFH.
Secondary stroke prevention is addressed, with 2012 guidelines outlining a preference for clopidogrel or aspirin/extended-release dipyridamole rather than aspirin or cilostazol in patients with a history of noncardioembolic ischemic stroke or TIA. Oral anticoagulation is preferred in patients with a history of stroke or TIA with afib over aspirin alone, aspirin plus clopidogrel, or no antithrombotic therapy. Of the available anticoagulants, the panel recommended dabigatran 150 mg twice daily over adjusted-dose warfarin.2 This recommendation is based on results from the RE-LY trial, which showed dabigatran as noninferior to warfarin in patients with nonvalvular afib without severe renal failure or advanced liver disease.3
For patients who have contraindications or choose not to initiate anticoagulation, the combination of aspirin (ASA) and clopidogrel is a reasonable alternative. Timing of the initiation of oral anticoagulation should be between one and two weeks after the stroke. Patients with extensive infarction or hemorrhagic transformation should delay starting oral anticoagulation, with no exact timeline. Long-term antithrombotic therapy is contraindicated in patients with history of a symptomatic primary intracerebral hemorrhage.2 New guidelines also recommend full anticoagulation for patients with symptomatic cerebral venous sinus thrombosis.
The panel did not make any recommendations regarding statin usage. In several studies, findings showed that statins reduced infarct size and had improved outcome in all stroke types.4
Analysis
Prior to the 2012 update, the last guideline for antithrombotic and thrombolytic therapy for ischemic stroke was published by the ACCP in the June 2008 issue of Chest.5 Dating back to 2001, medications included r-tPA administration within three hours of stroke symptom onset, and aspirin, clopidogrel, or a com bination of aspirin and extended-release dipyridamole for stroke prophylaxis.
The management of stroke continues to focus on early intervention and secondary prevention. Thrombolytic therapy is an effective treatment of acute ischemic stroke if given within the narrow window from onset of stroke symptoms up to 4.5 hours, with the goal of treatment within a three-hour window. Beyond this time constraint, the risk outweighs the benefit of using r-tPA except in the case of intra-arterial r-tPA administration for proximal cerebral artery occlusion.
In 2010, a meta-analysis supported this by showing that the risk of death increased significantly in patients receiving r-tPA beyond 4.5 hours. Therefore, antiplatelet therapy is the best alternative for patients ineligible for thrombolytic therapy.6 Even so, that study offered little data for patients with mechanical heart valves or intracardiac thrombi. Thus, the choice for acute anticoagulation therapy is variable and uncertain. If the hemorrhagic risk is low, anticoagulation can be considered in this subgroup, but no specific guideline endorsement was made.
In 2011, the AHA/ASA published an updated treatment guideline for patients with stroke or TIA. This was an update to 2007 guidelines that outlined the early management of ischemic stroke and affirmed the benefit of IV r-tPA at 4.5 hours for the treatment of stroke.7 Of note, IV r-tPA is only FDA-approved for treatment of acute ischemic stroke within the previously recommended three-hour period from symptom onset.
Aspirin has been found to be effective in both early treatment of acute ischemic stroke and secondary prevention. The CAST trial showed a statistically significant rate of reduction of nonfatal strokes with the use of aspirin. Other antiplatelet agents, including clopidogrel and dipyridamole, can be used. The FASTER trial compared aspirin alone versus aspirin plus clopidogrel, with no difference in outcome measures, although the MATCH trial found a larger risk of hemorrhagic and bleeding complications in the acetylsalicylic acid (ASA)-plus-clopidogrel group.6,7
In TIA or stroke patients, clopidogrel is not superior to ASA in preventing recurrent stroke. However, patients who have peripheral artery disease (PAD), previous coronary artery bypass grafting (CABG), insulin dependent diabetes mellitus (IDDM), or recurrent vascular events show a benefit of transitioning from ASA to clopidogrel for secondary long-term prevention. Clopidogrel or aspirin/extended-release dipyridamole is preferred over aspirin alone or cilostazol for long-term treatment in patients with a history of noncardioembolic ischemic stroke or TIA based on the PROFESS trial.2,7
HM Takeaways
The 2012 guidelines are a resource available to hospitalists for treating acute ischemic stroke either alone or with neurology consultation. These guidelines further define the timing of r-tPA and the use of both anticoagulation and antiplatelet therapy in the proper clinical settings.
In terms of VTE prevention, the guidelines recommend using LMWH preferentially over UH, except in patients at risk for rebleeding. The clinician should be aware of the treatment considerations for secondary prevention, noting the primary role of aspirin therapy in ischemic stroke with consideration of other agents (i.e. clopidogrel) in select populations.
Drs. Barr and Schumacher are hospitalists and assistant professors in the division of hospital medicine at The Ohio State University College of Medicine in Columbus.
References
Available at the-hospitalist.org.
Background
Each year, 1 million people are hospitalized with a diagnosis of stroke; it was the fourth-leading cause of death in the U.S. in 2009 and 2010.1 The majority of strokes (80%) are caused by focal cerebral ischemia, and the remainder are caused by hemorrhage.1 In 2008, the direct medical costs of stroke were approximately $18.8 billion, with almost half of this amount directed toward hospitalization.1 Although stroke inpatients make up only 3% of total hospitalizations, the mortality rate is more than twice that of other patients’.1
Over the past several decades, much has been learned about the pathophysiology and treatment for ischemic stroke. The mainstays of therapies include restoring perfusion in a timely manner and targeting both clot formation and hemostasis. These therapies improve patient outcomes and reduce the risk of recurrence in appropriately selected populations.
Guideline Update
In February, the American College of Chest Physicians (ACCP) published new practice guidelines for medical patients regarding antithrombotic and thrombolytic therapy in acute ischemic stroke.2 These evidence-based guidelines are the result of new clinical trial data and a review of previous studies. They address three aspects of management decisions for stroke, including acute treatment, VTE prevention, and secondary prevention, as well as specifically address the treatment of cerebral venous sinus thrombosis.
In the management of acute ischemic stroke, several recommendations were made. In terms of IV recombinant tissue plasminogen activator (r-tPA) administration, the guidelines were expanded and allow for a less restrictive time threshold for administration. Previous recommendations limited the usage of IV r-tPA to within three hours of symptom onset in acute ischemic stroke. A science advisory from the American Heart Association/American Stroke Association (AHA/ASA) from 2009 extended that window to 4.5 hours. The 2012 ACCP guidelines have followed suit to extend this time to 4.5 hours from symptom onset as well.
In addition, intrarterial r-tPA can be given in patients not eligible for IV r-tPA within six hours of presentation of acute ischemic stroke due to proximal cerebral artery occlusion.
These updated acute stroke guidelines recommend against the use of mechanical thrombectomy based mostly on lack of data rather than lack of benefit.2
The new guidelines continue to recommend early aspirin therapy at a dosage of 160 mg to 325 mg within the first 48 hours of acute ischemic stroke. Therapeutic parenteral anticoagulation with heparin or related drugs was not recommended in patients with noncardioembolic stroke due to atrial fibrillation (afib) or in patients with stroke due to large artery stenosis or arterial dissection. In this updated analysis, there was no benefit of anticoagulation compared with antiplatelet therapy, and the risk for extracranial hemorrhage was increased. No specific recommendation regarding anticoagulation was made in patients with mechanical heart valves or intracardiac thrombus.
Updates have been made for VTE prophylaxis in patients hospitalized for acute stroke. In stroke patients with restricted mobility, prophylactic unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH) and/or intermittent pneumatic compression devices should be initiated as early as possible. The panel is no longer recommending elastic compression stockings as VTE prevention given the risk of skin damage and no clear benefit in symptomatic VTE prevention. For patients with hemorrhagic stroke and restricted mobility, similar recommendations were made for VTE prevention, except to start pharmacologic treatment between days 2 and 4 of the hospital stay. However, if there is a bleeding concern, intermittent pneumatic compression devices are favored over pharmacologic prophylaxis. In all patients for whom pharmacologic prevention is utilized, prophylactic LMWH is preferred over UFH.
Secondary stroke prevention is addressed, with 2012 guidelines outlining a preference for clopidogrel or aspirin/extended-release dipyridamole rather than aspirin or cilostazol in patients with a history of noncardioembolic ischemic stroke or TIA. Oral anticoagulation is preferred in patients with a history of stroke or TIA with afib over aspirin alone, aspirin plus clopidogrel, or no antithrombotic therapy. Of the available anticoagulants, the panel recommended dabigatran 150 mg twice daily over adjusted-dose warfarin.2 This recommendation is based on results from the RE-LY trial, which showed dabigatran as noninferior to warfarin in patients with nonvalvular afib without severe renal failure or advanced liver disease.3
For patients who have contraindications or choose not to initiate anticoagulation, the combination of aspirin (ASA) and clopidogrel is a reasonable alternative. Timing of the initiation of oral anticoagulation should be between one and two weeks after the stroke. Patients with extensive infarction or hemorrhagic transformation should delay starting oral anticoagulation, with no exact timeline. Long-term antithrombotic therapy is contraindicated in patients with history of a symptomatic primary intracerebral hemorrhage.2 New guidelines also recommend full anticoagulation for patients with symptomatic cerebral venous sinus thrombosis.
The panel did not make any recommendations regarding statin usage. In several studies, findings showed that statins reduced infarct size and had improved outcome in all stroke types.4
Analysis
Prior to the 2012 update, the last guideline for antithrombotic and thrombolytic therapy for ischemic stroke was published by the ACCP in the June 2008 issue of Chest.5 Dating back to 2001, medications included r-tPA administration within three hours of stroke symptom onset, and aspirin, clopidogrel, or a com bination of aspirin and extended-release dipyridamole for stroke prophylaxis.
The management of stroke continues to focus on early intervention and secondary prevention. Thrombolytic therapy is an effective treatment of acute ischemic stroke if given within the narrow window from onset of stroke symptoms up to 4.5 hours, with the goal of treatment within a three-hour window. Beyond this time constraint, the risk outweighs the benefit of using r-tPA except in the case of intra-arterial r-tPA administration for proximal cerebral artery occlusion.
In 2010, a meta-analysis supported this by showing that the risk of death increased significantly in patients receiving r-tPA beyond 4.5 hours. Therefore, antiplatelet therapy is the best alternative for patients ineligible for thrombolytic therapy.6 Even so, that study offered little data for patients with mechanical heart valves or intracardiac thrombi. Thus, the choice for acute anticoagulation therapy is variable and uncertain. If the hemorrhagic risk is low, anticoagulation can be considered in this subgroup, but no specific guideline endorsement was made.
In 2011, the AHA/ASA published an updated treatment guideline for patients with stroke or TIA. This was an update to 2007 guidelines that outlined the early management of ischemic stroke and affirmed the benefit of IV r-tPA at 4.5 hours for the treatment of stroke.7 Of note, IV r-tPA is only FDA-approved for treatment of acute ischemic stroke within the previously recommended three-hour period from symptom onset.
Aspirin has been found to be effective in both early treatment of acute ischemic stroke and secondary prevention. The CAST trial showed a statistically significant rate of reduction of nonfatal strokes with the use of aspirin. Other antiplatelet agents, including clopidogrel and dipyridamole, can be used. The FASTER trial compared aspirin alone versus aspirin plus clopidogrel, with no difference in outcome measures, although the MATCH trial found a larger risk of hemorrhagic and bleeding complications in the acetylsalicylic acid (ASA)-plus-clopidogrel group.6,7
In TIA or stroke patients, clopidogrel is not superior to ASA in preventing recurrent stroke. However, patients who have peripheral artery disease (PAD), previous coronary artery bypass grafting (CABG), insulin dependent diabetes mellitus (IDDM), or recurrent vascular events show a benefit of transitioning from ASA to clopidogrel for secondary long-term prevention. Clopidogrel or aspirin/extended-release dipyridamole is preferred over aspirin alone or cilostazol for long-term treatment in patients with a history of noncardioembolic ischemic stroke or TIA based on the PROFESS trial.2,7
HM Takeaways
The 2012 guidelines are a resource available to hospitalists for treating acute ischemic stroke either alone or with neurology consultation. These guidelines further define the timing of r-tPA and the use of both anticoagulation and antiplatelet therapy in the proper clinical settings.
In terms of VTE prevention, the guidelines recommend using LMWH preferentially over UH, except in patients at risk for rebleeding. The clinician should be aware of the treatment considerations for secondary prevention, noting the primary role of aspirin therapy in ischemic stroke with consideration of other agents (i.e. clopidogrel) in select populations.
Drs. Barr and Schumacher are hospitalists and assistant professors in the division of hospital medicine at The Ohio State University College of Medicine in Columbus.
References
Available at the-hospitalist.org.
John Nelson: Peformance Key to Federal Value-Based Payment Modifier Plan
For years, your hospital was paid additional money by Medicare to report its performance on such things as core measures. Medicare then shared that information with the public via www.hospitalcompare.hhs.gov. Even if the hospital never gave Pneumovax when indicated, it was paid more simply for reporting that fact. (Fortunately, there were lots of reasons hospitals wanted to perform well.)
The days of hospitals being paid more simply for reporting ended a long time ago. Now performance, e.g., how often Pneumovax was given when indicated, influences payment. That is, things have transitioned from pay-for-reporting to a pay-for-performance program called hospital value-based purchasing (VBP).
I hope that at least one member of your hospitalist group is keeping up with hospital VBP. It got a lot of attention in the fall because it was the first time Medicare Part A payments to hospitals were adjusted based on performance on some core measures and patient satisfaction domains, as well as readmission rates for congestive heart failure (CHF), acute myocardial infarction (AMI), and pneumonia patients. The dollars at stake and performance metrics change will change every year, so plan to pay attention to hospital VBP on an ongoing basis.
Physicians’ Turn
Medicare payment to physicians is evolving along the same trajectory as hospitals. For several years, doctors have had the option to voluntarily participate in the Physician Quality Reporting System (PQRS). As long as a doctor reported quality performance on a sufficient portion of certain patient types, Medicare would provide a “bonus” at the end of the year. From 2012 through 2014, the “bonus” is 0.5% of that doctor’s total allowable Medicare charges. For example, if that doctor generated $150,000 of Medicare allowable charges over the calendar year, the additional payment for successful reporting PQRS would be $750 (0.5% of $150,000).
Although $750 is only a tiny fraction of collections, the right charge-capture system can make it pretty easy to achieve. And an extra payment of $750 sure is better than the 1.5% penalty for not participating; that program starts in 2015 and increases to a 2% penalty in 2016. If you are still not participating successfully in PQRS in 2015, the reimbursement for that $150,000 in charges will be reduced by $2,250 (1.5% of $150,000). So I strongly recommend that you begin reporting in 2013 so that you have time to work out the kinks well ahead of 2015. Don’t delay, but don’t panic, either, because you can still succeed in 2013 even if you don’t start capturing or reporting PQRS data until late winter or early spring.
At some point in the next year or so, data from as early as January 2013 for doctors reporting through PQRS will be made public on the Centers for Medicare & Medicaid’s (CMS) physician compare website: www.medicare.gov/find-a-doctor/provider-search.aspx. For example, should you choose to report the portion of stroke patients for whom you prescribed DVT prophylaxis, the public will be able to see your data.
The Next Wave of Physician Pay for Performance
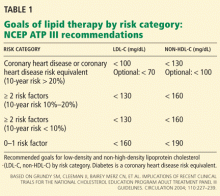
As the name implies, PQRS is a program based on reporting. Now CMS is adding the Value-Based Payment Modifier (VBPM) program, in which performance determines payments (see Table 1). It incorporates quality measures from PQRS, but is for now a separate program. It is very similar in name and structure to the hospital VBP program mentioned above, but incorporates cost of care data as well as quality performance. So it is really about value and not just quality performance (hence the name).
For providers in groups of more than 100 that bill under the same tax ID number (they don’t have to be in the same specialty), VBPM will first influence Part B Medicare reimbursement for physician services in 2015. It will expand to include all providers in 2017.
But don’t think you have until 2015 or 2017 to learn about all of this. There is a two-year lag, so payments in 2015 are based on performance in 2013 and 2017 payments presumably will be based on 2015 performance. In the fall of 2013, CMS plans to provide group-level (not individual) performance reports to all doctors in groups of 100 or more under the same tax ID number. These performance reports are known as quality resource use reports (QRURs). QRURs were trialed on physicians in a few states who received reports in 2012 based on 2011 performance, but in 2013, reports based on 2012 performance will be distributed to all doctors who practice in groups of 100 or more.
The calculation to determine whether a doctor is due additional payment for good performance (more accurately, good value) is awfully complicated. But providers have a choice to make. They can choose to:
- Not report data and accept a 1% penalty (likely to increase in successive years and in addition to the penalty for not reporting PQRS data, for a total penalty of 2.5%);
- Report data but not compete for financial upside or downside; or
- Compete for additional payments (amount to be determined) and risk a penalty of 0.5% or 1% for poor performance.
Look for more details about the VBPM program in future columns and other articles in The Hospitalist. There are a number of good online resources, including a CMS presentation titled “CMS Proposals for the Physician Value-Based Payment Modifier under the Medicare Physician Fee Schedule.” Type “Value-Based Payment Modifier” and “CMS” into any search engine to locate the video.
Parting Recommendations
Just about every hospitalist group should:
- Designate someone in your group to keep up with evolving pay-for-performance programs. It doesn’t have to be an MD, but you do need someone local that can guide your group through it. Consider becoming the most expert physician at your hospital on this topic.
- Start reporting through PQRS in 2013 if you haven’t already.
- Support SHM’s efforts to provide feedback to CMS to ensure that the metrics are meaningful for the type of care we provide.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is course co-director for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. Write to him at [email protected].
Author’s note: For helping to explain all this pay-for-performance stuff, I once again owe thanks to Dr. Pat Torcson, a hospitalist in Covington, La., and member of SHM’s Public Policy Committee. He does an amazing job of keeping up with the evolving pay-for-performance programs, advocating on behalf of hospitalists and the patients we serve, and graciously answers my tedious questions with thoughtful and informative replies. He is a really pleasant guy and a terrific asset to SHM and hospital medicine.
For years, your hospital was paid additional money by Medicare to report its performance on such things as core measures. Medicare then shared that information with the public via www.hospitalcompare.hhs.gov. Even if the hospital never gave Pneumovax when indicated, it was paid more simply for reporting that fact. (Fortunately, there were lots of reasons hospitals wanted to perform well.)
The days of hospitals being paid more simply for reporting ended a long time ago. Now performance, e.g., how often Pneumovax was given when indicated, influences payment. That is, things have transitioned from pay-for-reporting to a pay-for-performance program called hospital value-based purchasing (VBP).
I hope that at least one member of your hospitalist group is keeping up with hospital VBP. It got a lot of attention in the fall because it was the first time Medicare Part A payments to hospitals were adjusted based on performance on some core measures and patient satisfaction domains, as well as readmission rates for congestive heart failure (CHF), acute myocardial infarction (AMI), and pneumonia patients. The dollars at stake and performance metrics change will change every year, so plan to pay attention to hospital VBP on an ongoing basis.
Physicians’ Turn
Medicare payment to physicians is evolving along the same trajectory as hospitals. For several years, doctors have had the option to voluntarily participate in the Physician Quality Reporting System (PQRS). As long as a doctor reported quality performance on a sufficient portion of certain patient types, Medicare would provide a “bonus” at the end of the year. From 2012 through 2014, the “bonus” is 0.5% of that doctor’s total allowable Medicare charges. For example, if that doctor generated $150,000 of Medicare allowable charges over the calendar year, the additional payment for successful reporting PQRS would be $750 (0.5% of $150,000).
Although $750 is only a tiny fraction of collections, the right charge-capture system can make it pretty easy to achieve. And an extra payment of $750 sure is better than the 1.5% penalty for not participating; that program starts in 2015 and increases to a 2% penalty in 2016. If you are still not participating successfully in PQRS in 2015, the reimbursement for that $150,000 in charges will be reduced by $2,250 (1.5% of $150,000). So I strongly recommend that you begin reporting in 2013 so that you have time to work out the kinks well ahead of 2015. Don’t delay, but don’t panic, either, because you can still succeed in 2013 even if you don’t start capturing or reporting PQRS data until late winter or early spring.
At some point in the next year or so, data from as early as January 2013 for doctors reporting through PQRS will be made public on the Centers for Medicare & Medicaid’s (CMS) physician compare website: www.medicare.gov/find-a-doctor/provider-search.aspx. For example, should you choose to report the portion of stroke patients for whom you prescribed DVT prophylaxis, the public will be able to see your data.
The Next Wave of Physician Pay for Performance
As the name implies, PQRS is a program based on reporting. Now CMS is adding the Value-Based Payment Modifier (VBPM) program, in which performance determines payments (see Table 1). It incorporates quality measures from PQRS, but is for now a separate program. It is very similar in name and structure to the hospital VBP program mentioned above, but incorporates cost of care data as well as quality performance. So it is really about value and not just quality performance (hence the name).
For providers in groups of more than 100 that bill under the same tax ID number (they don’t have to be in the same specialty), VBPM will first influence Part B Medicare reimbursement for physician services in 2015. It will expand to include all providers in 2017.
But don’t think you have until 2015 or 2017 to learn about all of this. There is a two-year lag, so payments in 2015 are based on performance in 2013 and 2017 payments presumably will be based on 2015 performance. In the fall of 2013, CMS plans to provide group-level (not individual) performance reports to all doctors in groups of 100 or more under the same tax ID number. These performance reports are known as quality resource use reports (QRURs). QRURs were trialed on physicians in a few states who received reports in 2012 based on 2011 performance, but in 2013, reports based on 2012 performance will be distributed to all doctors who practice in groups of 100 or more.
The calculation to determine whether a doctor is due additional payment for good performance (more accurately, good value) is awfully complicated. But providers have a choice to make. They can choose to:
- Not report data and accept a 1% penalty (likely to increase in successive years and in addition to the penalty for not reporting PQRS data, for a total penalty of 2.5%);
- Report data but not compete for financial upside or downside; or
- Compete for additional payments (amount to be determined) and risk a penalty of 0.5% or 1% for poor performance.
Look for more details about the VBPM program in future columns and other articles in The Hospitalist. There are a number of good online resources, including a CMS presentation titled “CMS Proposals for the Physician Value-Based Payment Modifier under the Medicare Physician Fee Schedule.” Type “Value-Based Payment Modifier” and “CMS” into any search engine to locate the video.
Parting Recommendations
Just about every hospitalist group should:
- Designate someone in your group to keep up with evolving pay-for-performance programs. It doesn’t have to be an MD, but you do need someone local that can guide your group through it. Consider becoming the most expert physician at your hospital on this topic.
- Start reporting through PQRS in 2013 if you haven’t already.
- Support SHM’s efforts to provide feedback to CMS to ensure that the metrics are meaningful for the type of care we provide.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is course co-director for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. Write to him at [email protected].
Author’s note: For helping to explain all this pay-for-performance stuff, I once again owe thanks to Dr. Pat Torcson, a hospitalist in Covington, La., and member of SHM’s Public Policy Committee. He does an amazing job of keeping up with the evolving pay-for-performance programs, advocating on behalf of hospitalists and the patients we serve, and graciously answers my tedious questions with thoughtful and informative replies. He is a really pleasant guy and a terrific asset to SHM and hospital medicine.
For years, your hospital was paid additional money by Medicare to report its performance on such things as core measures. Medicare then shared that information with the public via www.hospitalcompare.hhs.gov. Even if the hospital never gave Pneumovax when indicated, it was paid more simply for reporting that fact. (Fortunately, there were lots of reasons hospitals wanted to perform well.)
The days of hospitals being paid more simply for reporting ended a long time ago. Now performance, e.g., how often Pneumovax was given when indicated, influences payment. That is, things have transitioned from pay-for-reporting to a pay-for-performance program called hospital value-based purchasing (VBP).
I hope that at least one member of your hospitalist group is keeping up with hospital VBP. It got a lot of attention in the fall because it was the first time Medicare Part A payments to hospitals were adjusted based on performance on some core measures and patient satisfaction domains, as well as readmission rates for congestive heart failure (CHF), acute myocardial infarction (AMI), and pneumonia patients. The dollars at stake and performance metrics change will change every year, so plan to pay attention to hospital VBP on an ongoing basis.
Physicians’ Turn
Medicare payment to physicians is evolving along the same trajectory as hospitals. For several years, doctors have had the option to voluntarily participate in the Physician Quality Reporting System (PQRS). As long as a doctor reported quality performance on a sufficient portion of certain patient types, Medicare would provide a “bonus” at the end of the year. From 2012 through 2014, the “bonus” is 0.5% of that doctor’s total allowable Medicare charges. For example, if that doctor generated $150,000 of Medicare allowable charges over the calendar year, the additional payment for successful reporting PQRS would be $750 (0.5% of $150,000).
Although $750 is only a tiny fraction of collections, the right charge-capture system can make it pretty easy to achieve. And an extra payment of $750 sure is better than the 1.5% penalty for not participating; that program starts in 2015 and increases to a 2% penalty in 2016. If you are still not participating successfully in PQRS in 2015, the reimbursement for that $150,000 in charges will be reduced by $2,250 (1.5% of $150,000). So I strongly recommend that you begin reporting in 2013 so that you have time to work out the kinks well ahead of 2015. Don’t delay, but don’t panic, either, because you can still succeed in 2013 even if you don’t start capturing or reporting PQRS data until late winter or early spring.
At some point in the next year or so, data from as early as January 2013 for doctors reporting through PQRS will be made public on the Centers for Medicare & Medicaid’s (CMS) physician compare website: www.medicare.gov/find-a-doctor/provider-search.aspx. For example, should you choose to report the portion of stroke patients for whom you prescribed DVT prophylaxis, the public will be able to see your data.
The Next Wave of Physician Pay for Performance
As the name implies, PQRS is a program based on reporting. Now CMS is adding the Value-Based Payment Modifier (VBPM) program, in which performance determines payments (see Table 1). It incorporates quality measures from PQRS, but is for now a separate program. It is very similar in name and structure to the hospital VBP program mentioned above, but incorporates cost of care data as well as quality performance. So it is really about value and not just quality performance (hence the name).
For providers in groups of more than 100 that bill under the same tax ID number (they don’t have to be in the same specialty), VBPM will first influence Part B Medicare reimbursement for physician services in 2015. It will expand to include all providers in 2017.
But don’t think you have until 2015 or 2017 to learn about all of this. There is a two-year lag, so payments in 2015 are based on performance in 2013 and 2017 payments presumably will be based on 2015 performance. In the fall of 2013, CMS plans to provide group-level (not individual) performance reports to all doctors in groups of 100 or more under the same tax ID number. These performance reports are known as quality resource use reports (QRURs). QRURs were trialed on physicians in a few states who received reports in 2012 based on 2011 performance, but in 2013, reports based on 2012 performance will be distributed to all doctors who practice in groups of 100 or more.
The calculation to determine whether a doctor is due additional payment for good performance (more accurately, good value) is awfully complicated. But providers have a choice to make. They can choose to:
- Not report data and accept a 1% penalty (likely to increase in successive years and in addition to the penalty for not reporting PQRS data, for a total penalty of 2.5%);
- Report data but not compete for financial upside or downside; or
- Compete for additional payments (amount to be determined) and risk a penalty of 0.5% or 1% for poor performance.
Look for more details about the VBPM program in future columns and other articles in The Hospitalist. There are a number of good online resources, including a CMS presentation titled “CMS Proposals for the Physician Value-Based Payment Modifier under the Medicare Physician Fee Schedule.” Type “Value-Based Payment Modifier” and “CMS” into any search engine to locate the video.
Parting Recommendations
Just about every hospitalist group should:
- Designate someone in your group to keep up with evolving pay-for-performance programs. It doesn’t have to be an MD, but you do need someone local that can guide your group through it. Consider becoming the most expert physician at your hospital on this topic.
- Start reporting through PQRS in 2013 if you haven’t already.
- Support SHM’s efforts to provide feedback to CMS to ensure that the metrics are meaningful for the type of care we provide.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is course co-director for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. Write to him at [email protected].
Author’s note: For helping to explain all this pay-for-performance stuff, I once again owe thanks to Dr. Pat Torcson, a hospitalist in Covington, La., and member of SHM’s Public Policy Committee. He does an amazing job of keeping up with the evolving pay-for-performance programs, advocating on behalf of hospitalists and the patients we serve, and graciously answers my tedious questions with thoughtful and informative replies. He is a really pleasant guy and a terrific asset to SHM and hospital medicine.
Consider Patient Safety, Outcomes Risk before Prescribing Off-Label Drugs
Consider Patient Safety, Outcomes Risk before Prescribing “Off-Label”
What is the story with off-label drug use? I have seen some other physicians in my group use dabigatran for VTE prophylaxis, which I know it is not an approved indication. Am I taking on risk by continuing this treatment?
—Fabian Harris, Tuscaloosa, Ala.
Dr. Hospitalist responds:
Our friends at the FDA are in the business of approving drugs for use, but they do not regulate medical practice. So the short answer to your question is that off-label drug use is perfectly acceptable. Once a drug has been approved for use, if, in your clinical judgment, there are other indications for which it could be beneficial, then you are well within your rights to prescribe it. The FDA does not dictate how you practice medicine.
However, you will still be held to the community standard when it comes to your medical practice. As an example, gabapentin is used all the time for neuropathic pain syndromes, though technically it is only approved for seizures and post-herpetic neuralgia. Although the FDA won’t restrict your prescribing, it does prohibit pharmaceutical companies from marketing their drugs for anything other than their approved indications. In fact, Pfizer settled a case in 2004 on this very drug due to the promotion of prescribing it for nonapproved indications. I think at this point it’s fairly well accepted that lots of physicians use gabapentin for neuropathic pain, so you would not be too far out on a limb in prescribing it yourself in this manner.
For newer drugs, I might proceed with a little more caution. Anyone out there remember trovofloxacin (Trovan)? It was a new antibiotic approved in the late 1990s, with a coverage spectrum similar to levofloxacin, but with even more weight toward the gram positives. A wonder drug! Oral! As a result, it got prescribed like water, but not for the serious infections it was designed for: It got prescribed “off label” for common URIs and sinusitis. Unfortunately, it also caused a fair amount of liver failure and was summarily pulled from the market.
Does this mean dabigatran is a bad drug? No, but we don’t have much history with it, either. So while it might seem to be an innocuous extension to prescribe it for VTE prevention when it has already been approved for stroke prevention in afib, I think you carry some risk by doing this. In addition, some insurers will not cover a drug being prescribed in this manner, so you might be exposing your patient to added costs as well. Additionally, there’s nothing about off-label prescribing that says you have to tell the patient that’s what you’re doing. However, if you put together the factors of not informing a patient about an off-label use, and a patient having to pay out of pocket for that medicine, with an adverse outcome ... well, let’s just say that might not end too well.
Ultimately, I think you will need to consider the safety profile of the drug, the risk for an adverse outcome, your own risk tolerance, and the current state of medical practice before you consistently agree to use a drug “off label.” Given the slow-moving jungle of FDA approval, I can understand the desire to use a newer drug in an off-label manner, but it’s probably best to stop and think about the alternatives before proceeding. If you’re practicing in a group, then it’s just as important to come to a consensus with your partners about which drugs you will comfortably use off-label and which ones you won’t, especially as newer drugs come into the marketplace.
Consider Patient Safety, Outcomes Risk before Prescribing “Off-Label”
What is the story with off-label drug use? I have seen some other physicians in my group use dabigatran for VTE prophylaxis, which I know it is not an approved indication. Am I taking on risk by continuing this treatment?
—Fabian Harris, Tuscaloosa, Ala.
Dr. Hospitalist responds:
Our friends at the FDA are in the business of approving drugs for use, but they do not regulate medical practice. So the short answer to your question is that off-label drug use is perfectly acceptable. Once a drug has been approved for use, if, in your clinical judgment, there are other indications for which it could be beneficial, then you are well within your rights to prescribe it. The FDA does not dictate how you practice medicine.
However, you will still be held to the community standard when it comes to your medical practice. As an example, gabapentin is used all the time for neuropathic pain syndromes, though technically it is only approved for seizures and post-herpetic neuralgia. Although the FDA won’t restrict your prescribing, it does prohibit pharmaceutical companies from marketing their drugs for anything other than their approved indications. In fact, Pfizer settled a case in 2004 on this very drug due to the promotion of prescribing it for nonapproved indications. I think at this point it’s fairly well accepted that lots of physicians use gabapentin for neuropathic pain, so you would not be too far out on a limb in prescribing it yourself in this manner.
For newer drugs, I might proceed with a little more caution. Anyone out there remember trovofloxacin (Trovan)? It was a new antibiotic approved in the late 1990s, with a coverage spectrum similar to levofloxacin, but with even more weight toward the gram positives. A wonder drug! Oral! As a result, it got prescribed like water, but not for the serious infections it was designed for: It got prescribed “off label” for common URIs and sinusitis. Unfortunately, it also caused a fair amount of liver failure and was summarily pulled from the market.
Does this mean dabigatran is a bad drug? No, but we don’t have much history with it, either. So while it might seem to be an innocuous extension to prescribe it for VTE prevention when it has already been approved for stroke prevention in afib, I think you carry some risk by doing this. In addition, some insurers will not cover a drug being prescribed in this manner, so you might be exposing your patient to added costs as well. Additionally, there’s nothing about off-label prescribing that says you have to tell the patient that’s what you’re doing. However, if you put together the factors of not informing a patient about an off-label use, and a patient having to pay out of pocket for that medicine, with an adverse outcome ... well, let’s just say that might not end too well.
Ultimately, I think you will need to consider the safety profile of the drug, the risk for an adverse outcome, your own risk tolerance, and the current state of medical practice before you consistently agree to use a drug “off label.” Given the slow-moving jungle of FDA approval, I can understand the desire to use a newer drug in an off-label manner, but it’s probably best to stop and think about the alternatives before proceeding. If you’re practicing in a group, then it’s just as important to come to a consensus with your partners about which drugs you will comfortably use off-label and which ones you won’t, especially as newer drugs come into the marketplace.
Consider Patient Safety, Outcomes Risk before Prescribing “Off-Label”
What is the story with off-label drug use? I have seen some other physicians in my group use dabigatran for VTE prophylaxis, which I know it is not an approved indication. Am I taking on risk by continuing this treatment?
—Fabian Harris, Tuscaloosa, Ala.
Dr. Hospitalist responds:
Our friends at the FDA are in the business of approving drugs for use, but they do not regulate medical practice. So the short answer to your question is that off-label drug use is perfectly acceptable. Once a drug has been approved for use, if, in your clinical judgment, there are other indications for which it could be beneficial, then you are well within your rights to prescribe it. The FDA does not dictate how you practice medicine.
However, you will still be held to the community standard when it comes to your medical practice. As an example, gabapentin is used all the time for neuropathic pain syndromes, though technically it is only approved for seizures and post-herpetic neuralgia. Although the FDA won’t restrict your prescribing, it does prohibit pharmaceutical companies from marketing their drugs for anything other than their approved indications. In fact, Pfizer settled a case in 2004 on this very drug due to the promotion of prescribing it for nonapproved indications. I think at this point it’s fairly well accepted that lots of physicians use gabapentin for neuropathic pain, so you would not be too far out on a limb in prescribing it yourself in this manner.
For newer drugs, I might proceed with a little more caution. Anyone out there remember trovofloxacin (Trovan)? It was a new antibiotic approved in the late 1990s, with a coverage spectrum similar to levofloxacin, but with even more weight toward the gram positives. A wonder drug! Oral! As a result, it got prescribed like water, but not for the serious infections it was designed for: It got prescribed “off label” for common URIs and sinusitis. Unfortunately, it also caused a fair amount of liver failure and was summarily pulled from the market.
Does this mean dabigatran is a bad drug? No, but we don’t have much history with it, either. So while it might seem to be an innocuous extension to prescribe it for VTE prevention when it has already been approved for stroke prevention in afib, I think you carry some risk by doing this. In addition, some insurers will not cover a drug being prescribed in this manner, so you might be exposing your patient to added costs as well. Additionally, there’s nothing about off-label prescribing that says you have to tell the patient that’s what you’re doing. However, if you put together the factors of not informing a patient about an off-label use, and a patient having to pay out of pocket for that medicine, with an adverse outcome ... well, let’s just say that might not end too well.
Ultimately, I think you will need to consider the safety profile of the drug, the risk for an adverse outcome, your own risk tolerance, and the current state of medical practice before you consistently agree to use a drug “off label.” Given the slow-moving jungle of FDA approval, I can understand the desire to use a newer drug in an off-label manner, but it’s probably best to stop and think about the alternatives before proceeding. If you’re practicing in a group, then it’s just as important to come to a consensus with your partners about which drugs you will comfortably use off-label and which ones you won’t, especially as newer drugs come into the marketplace.
Defining a Safe Workload for Pediatric Hospitalists
As I write this column, I am on the second leg of an overnight flight back home to Austin, Texas. I think it actually went pretty well, considering my 2-year-old daughter was wide awake after sleeping for the first three hours of this 14-hour odyssey. The remainder of the trip is a blur of awkward sleep positions interspersed with brief periods of semilucidity. Those of you with first-hand knowledge of what this experience is like might be feeling sorry for me, but you shouldn’t. I am returning from a “why don’t I live here” kind of vacation week in Hawaii. The rest of you are probably wondering how anyone could write a coherent column at this point, which is fair, but to which I would reply: Aren’t all hospitalists expected to function at high levels during periods of sleep deprivation?
While the issue of resident duty-hours has been discussed endlessly and studied increasingly, in terms of effects on outcomes, I am surprised there has not been more discussion surrounding the concept of attending duty-hours. The subject might not always be phrased to include the term “duty-hours,” but it seems that when it comes to scheduling, strong opinions come out in my group when the duration of, frequency of, or time off between night shifts are brought up. And when it comes to safety, I am certain sleep deprivation and sleep inertia (that period of haziness immediately after being awakened in the middle of the night) have led to questionable decisions on my part.
Why? Well...
So why do pediatric hospitalists avoid the issue of sleep hygiene, work schedules, and clinical impact? I think the reasons are multifactorial.
First, there are definitely individual variations in how all of us tolerate this work, and I suspect some of this is based on such traits as age and general ability to adapt to uncomfortable circadian flip-flops. I will admit that every time I wake up achy after a call night, I begin to wonder if I will be able to handle this in 10 to 15 years.
Second, I think pediatric HM as a field has not yet explored this topic fully because we are young both in terms of chronological age as well as nocturnal work-years. The work has not yet aged us to the point of making this a critical issue. We’re also somewhat behind our adult-hospitalist colleagues in terms of the volume of nocturnal work. Adult HM groups have long explored different shift schedules (seven-on/seven-off, day/evening/overnight distribution, etc.) because they routinely cover large services of more than 100 patients in large hospitals with more than 500 beds. In pediatrics, most of us operate in small community hospital settings or large academic centers where the nightly in-house quantity of work is relatively low, mitigated by the smaller size of most community programs and the presence of residents in most large children’s hospitals.
But I see this as an important issue for us to define: the imperative to define safe, round-the-clock clinical care and sustainable careers. Although we will need to learn from other fields, HM is somewhat different from other types of 24/7 medicine in that we require more continuity in our daytime work, which also carries over to night shifts both in terms of how the schedule is made as well as the benefit on the clinical side. The need for continuity adds an extra degree of difficulty in creating and studying different schedules that try to optimize nocturnal functioning.
Clarity, Please
Unfortunately, those looking for evidence-based, or even consensus-based, solutions might have to wait. A recent article in the Journal of Hospital Medicine does a nice job of synthesizing the literature and highlights the lack of clear answers for what kind of shift schedules work best.1
In the absence of scientific solutions, it might be too easy to say that we need “more research,” because what doesn’t need more research? (OK, we don’t need more research on interventions for bronchiolitis.) But in the same manner in which pediatric hospitalists have taken the lead in defining a night curriculum for residents (congratulations, Becky Blankenburg, on winning the Ray E. Helfer award in pediatric education), I believe there is an opportunity to improve circadian functioning for all hospital-based physicians, but more specifically attendings. This is even more important as residents work less and a 24/7 attending presence becomes the norm in teaching facilities. While the link between safety and fatigue may have been seen as a nonissue in past decades, I think that in our current era, this is something that we own and/or will be asked to define in the near future.
In the meantime, I think we’re left to our own schedules. And in defense of all schedulers like me out there, I will say that there are no proven solutions, so local culture will predominate. Different groups with different personalities and family makeups will have varying preferences. Smaller groups will tend to have longer shift times with less flexibility in “swing”-type midday or evening shifts, while larger groups might have increased flexibility in defining different shifts at the expense of added complexity in terms of creating a schedule with no gaps.
As we come up with more rules about night shifts, such as “clockwise” scheduling of day-evening-overnight shifts, single versus clustered nights based on frequency, and days off after night shifts, the more complex and awkward our Tetris-like schedule will become. I predict that this is something hospitalists will begin to think about more, with a necessary push for safe and sustainable schedules. In the short-term, allowing for financial and structural wiggle room in the scheduling process (i.e. adjusting shift patterns and differential pay for night work) might be the most balanced approach for the immediate future.
Dr. Shen is pediatric editor of The Hospitalist. Write to him at [email protected].
Reference
As I write this column, I am on the second leg of an overnight flight back home to Austin, Texas. I think it actually went pretty well, considering my 2-year-old daughter was wide awake after sleeping for the first three hours of this 14-hour odyssey. The remainder of the trip is a blur of awkward sleep positions interspersed with brief periods of semilucidity. Those of you with first-hand knowledge of what this experience is like might be feeling sorry for me, but you shouldn’t. I am returning from a “why don’t I live here” kind of vacation week in Hawaii. The rest of you are probably wondering how anyone could write a coherent column at this point, which is fair, but to which I would reply: Aren’t all hospitalists expected to function at high levels during periods of sleep deprivation?
While the issue of resident duty-hours has been discussed endlessly and studied increasingly, in terms of effects on outcomes, I am surprised there has not been more discussion surrounding the concept of attending duty-hours. The subject might not always be phrased to include the term “duty-hours,” but it seems that when it comes to scheduling, strong opinions come out in my group when the duration of, frequency of, or time off between night shifts are brought up. And when it comes to safety, I am certain sleep deprivation and sleep inertia (that period of haziness immediately after being awakened in the middle of the night) have led to questionable decisions on my part.
Why? Well...
So why do pediatric hospitalists avoid the issue of sleep hygiene, work schedules, and clinical impact? I think the reasons are multifactorial.
First, there are definitely individual variations in how all of us tolerate this work, and I suspect some of this is based on such traits as age and general ability to adapt to uncomfortable circadian flip-flops. I will admit that every time I wake up achy after a call night, I begin to wonder if I will be able to handle this in 10 to 15 years.
Second, I think pediatric HM as a field has not yet explored this topic fully because we are young both in terms of chronological age as well as nocturnal work-years. The work has not yet aged us to the point of making this a critical issue. We’re also somewhat behind our adult-hospitalist colleagues in terms of the volume of nocturnal work. Adult HM groups have long explored different shift schedules (seven-on/seven-off, day/evening/overnight distribution, etc.) because they routinely cover large services of more than 100 patients in large hospitals with more than 500 beds. In pediatrics, most of us operate in small community hospital settings or large academic centers where the nightly in-house quantity of work is relatively low, mitigated by the smaller size of most community programs and the presence of residents in most large children’s hospitals.
But I see this as an important issue for us to define: the imperative to define safe, round-the-clock clinical care and sustainable careers. Although we will need to learn from other fields, HM is somewhat different from other types of 24/7 medicine in that we require more continuity in our daytime work, which also carries over to night shifts both in terms of how the schedule is made as well as the benefit on the clinical side. The need for continuity adds an extra degree of difficulty in creating and studying different schedules that try to optimize nocturnal functioning.
Clarity, Please
Unfortunately, those looking for evidence-based, or even consensus-based, solutions might have to wait. A recent article in the Journal of Hospital Medicine does a nice job of synthesizing the literature and highlights the lack of clear answers for what kind of shift schedules work best.1
In the absence of scientific solutions, it might be too easy to say that we need “more research,” because what doesn’t need more research? (OK, we don’t need more research on interventions for bronchiolitis.) But in the same manner in which pediatric hospitalists have taken the lead in defining a night curriculum for residents (congratulations, Becky Blankenburg, on winning the Ray E. Helfer award in pediatric education), I believe there is an opportunity to improve circadian functioning for all hospital-based physicians, but more specifically attendings. This is even more important as residents work less and a 24/7 attending presence becomes the norm in teaching facilities. While the link between safety and fatigue may have been seen as a nonissue in past decades, I think that in our current era, this is something that we own and/or will be asked to define in the near future.
In the meantime, I think we’re left to our own schedules. And in defense of all schedulers like me out there, I will say that there are no proven solutions, so local culture will predominate. Different groups with different personalities and family makeups will have varying preferences. Smaller groups will tend to have longer shift times with less flexibility in “swing”-type midday or evening shifts, while larger groups might have increased flexibility in defining different shifts at the expense of added complexity in terms of creating a schedule with no gaps.
As we come up with more rules about night shifts, such as “clockwise” scheduling of day-evening-overnight shifts, single versus clustered nights based on frequency, and days off after night shifts, the more complex and awkward our Tetris-like schedule will become. I predict that this is something hospitalists will begin to think about more, with a necessary push for safe and sustainable schedules. In the short-term, allowing for financial and structural wiggle room in the scheduling process (i.e. adjusting shift patterns and differential pay for night work) might be the most balanced approach for the immediate future.
Dr. Shen is pediatric editor of The Hospitalist. Write to him at [email protected].
Reference
As I write this column, I am on the second leg of an overnight flight back home to Austin, Texas. I think it actually went pretty well, considering my 2-year-old daughter was wide awake after sleeping for the first three hours of this 14-hour odyssey. The remainder of the trip is a blur of awkward sleep positions interspersed with brief periods of semilucidity. Those of you with first-hand knowledge of what this experience is like might be feeling sorry for me, but you shouldn’t. I am returning from a “why don’t I live here” kind of vacation week in Hawaii. The rest of you are probably wondering how anyone could write a coherent column at this point, which is fair, but to which I would reply: Aren’t all hospitalists expected to function at high levels during periods of sleep deprivation?
While the issue of resident duty-hours has been discussed endlessly and studied increasingly, in terms of effects on outcomes, I am surprised there has not been more discussion surrounding the concept of attending duty-hours. The subject might not always be phrased to include the term “duty-hours,” but it seems that when it comes to scheduling, strong opinions come out in my group when the duration of, frequency of, or time off between night shifts are brought up. And when it comes to safety, I am certain sleep deprivation and sleep inertia (that period of haziness immediately after being awakened in the middle of the night) have led to questionable decisions on my part.
Why? Well...
So why do pediatric hospitalists avoid the issue of sleep hygiene, work schedules, and clinical impact? I think the reasons are multifactorial.
First, there are definitely individual variations in how all of us tolerate this work, and I suspect some of this is based on such traits as age and general ability to adapt to uncomfortable circadian flip-flops. I will admit that every time I wake up achy after a call night, I begin to wonder if I will be able to handle this in 10 to 15 years.
Second, I think pediatric HM as a field has not yet explored this topic fully because we are young both in terms of chronological age as well as nocturnal work-years. The work has not yet aged us to the point of making this a critical issue. We’re also somewhat behind our adult-hospitalist colleagues in terms of the volume of nocturnal work. Adult HM groups have long explored different shift schedules (seven-on/seven-off, day/evening/overnight distribution, etc.) because they routinely cover large services of more than 100 patients in large hospitals with more than 500 beds. In pediatrics, most of us operate in small community hospital settings or large academic centers where the nightly in-house quantity of work is relatively low, mitigated by the smaller size of most community programs and the presence of residents in most large children’s hospitals.
But I see this as an important issue for us to define: the imperative to define safe, round-the-clock clinical care and sustainable careers. Although we will need to learn from other fields, HM is somewhat different from other types of 24/7 medicine in that we require more continuity in our daytime work, which also carries over to night shifts both in terms of how the schedule is made as well as the benefit on the clinical side. The need for continuity adds an extra degree of difficulty in creating and studying different schedules that try to optimize nocturnal functioning.
Clarity, Please
Unfortunately, those looking for evidence-based, or even consensus-based, solutions might have to wait. A recent article in the Journal of Hospital Medicine does a nice job of synthesizing the literature and highlights the lack of clear answers for what kind of shift schedules work best.1
In the absence of scientific solutions, it might be too easy to say that we need “more research,” because what doesn’t need more research? (OK, we don’t need more research on interventions for bronchiolitis.) But in the same manner in which pediatric hospitalists have taken the lead in defining a night curriculum for residents (congratulations, Becky Blankenburg, on winning the Ray E. Helfer award in pediatric education), I believe there is an opportunity to improve circadian functioning for all hospital-based physicians, but more specifically attendings. This is even more important as residents work less and a 24/7 attending presence becomes the norm in teaching facilities. While the link between safety and fatigue may have been seen as a nonissue in past decades, I think that in our current era, this is something that we own and/or will be asked to define in the near future.
In the meantime, I think we’re left to our own schedules. And in defense of all schedulers like me out there, I will say that there are no proven solutions, so local culture will predominate. Different groups with different personalities and family makeups will have varying preferences. Smaller groups will tend to have longer shift times with less flexibility in “swing”-type midday or evening shifts, while larger groups might have increased flexibility in defining different shifts at the expense of added complexity in terms of creating a schedule with no gaps.
As we come up with more rules about night shifts, such as “clockwise” scheduling of day-evening-overnight shifts, single versus clustered nights based on frequency, and days off after night shifts, the more complex and awkward our Tetris-like schedule will become. I predict that this is something hospitalists will begin to think about more, with a necessary push for safe and sustainable schedules. In the short-term, allowing for financial and structural wiggle room in the scheduling process (i.e. adjusting shift patterns and differential pay for night work) might be the most balanced approach for the immediate future.
Dr. Shen is pediatric editor of The Hospitalist. Write to him at [email protected].
Reference
Teduglutide Trims Parenteral Support in Short Bowel Syndrome
Teduglutide significantly reduced the need for parenteral support in patients with short bowel syndrome and intestinal failure, based on data from 85 adults in a randomized, controlled multicenter trial. The findings were published in the December issue of Gastroenterology.
Patients with short bowel syndrome and intestinal failure (SBS-IF) have inadequate intestinal absorption and require parenteral support (PS) to maintain fluids, electrolytes, trace elements, vitamins, and nutrient balances, said Dr. Palle Bekker Jeppesen of Rigshospitalet in Copenhagen and colleagues.
Source: American Gastroenterological Association
Data from previous open-label studies suggest an association between teduglutide and clinically meaningful reductions in wet weight and energy, which may reduce the need for PS in these patients, the investigators noted.
The researchers randomized 86 adults with SBS-IF to either 0.05 mg/kg per day of teduglutide or a placebo. One patient was randomized in error; complete data were available for 42 teduglutide patients and 43 placebo patients.
Significantly more patients in the teduglutide group responded to treatment, compared with the placebo group (63% vs. 30%). This response was defined as sustaining a 20%-100% reduction from baseline in weekly PS volume during weeks 20-24. "Small bowel length did not appear to be a predictor of response," the researchers noted.
The high placebo response may be explained by examining the fluid composite effect, a measure of the combined effects of teduglutide on PS volume reduction as well as the ability to reduce oral fluid intake and increase urine output volume, the researchers noted.
"In the current study, where protocol modifications encouraged earlier and more aggressive PS reductions, significantly larger PS reductions were also achieved in patients receiving placebo, but subsequently these patients had to increase their oral fluid intake significantly to maintain urine production and hydration constant," they said.
After 24 weeks, overall PS volume was reduced by 32% from baseline in teduglutide patients, compared with 21% in placebo patients. Although no patients in either group were completely weaned from parenteral support at 24 weeks, the difference in PS volume reduction was significantly greater in the teduglutide group.
The average weekly PS volume in teduglutide patients decreased significantly from 12.5 L/wk at baseline to 8.1 L/wk at week 24. The placebo patients also had a significant decrease in average weekly PS volume, from 13.4 L/wk at baseline to 11.1 L/wk at week 24.
Treatment-ending adverse events were similar between the two groups; 5% of teduglutide patients and 7% of placebo patients discontinued treatment because of such events during the study period. The most frequently reported treatment-emergent adverse events included abdominal pain, abdominal distension, nausea, and gastrointestinal stoma complications.
Although the study did not specifically assess quality of life measures, significantly more teduglutide patients had at least 1 day off PS, compared with placebo patients, which could help to "liberate considerable time for unhindered daytime activities or undisturbed sleep," the researchers said.
The study did not address the possible benefit of teduglutide therapy earlier in the course of SBS, or the duration of effect after patients discontinued teduglutide, the researchers added.
However, the findings indicate that teduglutide was safe and well tolerated, and "could positively add to the limited treatment armamentarium" for patients with SBS-IF.
Dr. Jeppesen and several coauthors have served on the advisory board of and as consultants to NPS Pharmaceuticals, the company that funded the study. One author is an employee of NPS Pharmaceuticals.
Patients with short bowel syndrome whose absorption is insufficient to maintain nutritional or fluid autonomy have intestinal failure. These patients, particularly those with proximal jejunostomies, who may actually secrete more fluid than they ingest, are among the most complex and challenging to manage of patients with any gastrointestinal disease. Patients with short bowel syndrome and intestinal failure are dependent on parenteral nutrition and/or fluid support (PS) to maintain life. This therapy has substantial implications for employment, activities, sleep, and finances. Numerous, often life-threatening, complications develop.

|
|
A myriad of growth factors may be involved in the process of postresection intestinal adaptation, including glucagonlike peptide-2 (GLP-2), wherein intestinal epithelial growth is promoted. Teduglutide is a long-acting analog of native GLP-2 and is somewhat more resistant to enzymatic degradation in the enterocyte than is the native enzyme. Dr. Jeppesen and colleagues reported a sustained 20%-100% decrease in PS volume requirements during weeks 20-24 of treatment in 63% of patients who received teduglutide, compared with 30% of placebo-treated patients. The mean drop in weekly PS volume from baseline to week 24 totaled 4.4 L in patients who received teduglutide, which equates to a decrease of 1-2 nights of infusion weekly, a very profound improvement for individual patients. The PS weaning protocol used was similar to that used in most centers experienced in the care of these patients.
As would be expected in the SBS-IF patient population, there were many adverse events, although these were equally distributed across teduglutide and placebo groups. Stomal changes, primarily related to enlargement, were evident in a significant minority of patients in the teduglutide group, as would be expected given the hyperplastic effect of the medication on intestinal epithelial tissue.Concern has been raised about GLP-2’s potential to stimulate the development of colonic adenomas in rodent models. Although the risk for malignancy is hypothetical in humans, colonoscopy should be considered at baseline for those patients with residual colons and perhaps as frequently as annually while the patients are on therapy until more long-term safety data are available.
Is teduglutide a "game changer"? The only patients who will be able to discontinue PS completely will be those who are on the borderline between nutritional autonomy and PS dependence. It is important to realize that teduglutide should be used to augment, not replace conventional management. What happens when teduglutide is stopped? Preliminary evidence suggests the effects on adaptation may be persistent, although earlier study noted that histologic changes trended toward baseline within 4 weeks of discontinuation. Perhaps longer treatment or maintenance will be required. The real future is an artificially grown and harvested intestine; even intestinal transplantation represents a bridge at best.
Dr. Alan L. Buchman is a former professor of medicine and surgery at the Feinberg School of Medicine at Northwestern University, Chicago. Within the past 12 months he has consulted for Takeda Pharmaceuticals and NPS Pharmaceuticals.
Patients with short bowel syndrome whose absorption is insufficient to maintain nutritional or fluid autonomy have intestinal failure. These patients, particularly those with proximal jejunostomies, who may actually secrete more fluid than they ingest, are among the most complex and challenging to manage of patients with any gastrointestinal disease. Patients with short bowel syndrome and intestinal failure are dependent on parenteral nutrition and/or fluid support (PS) to maintain life. This therapy has substantial implications for employment, activities, sleep, and finances. Numerous, often life-threatening, complications develop.

|
|
A myriad of growth factors may be involved in the process of postresection intestinal adaptation, including glucagonlike peptide-2 (GLP-2), wherein intestinal epithelial growth is promoted. Teduglutide is a long-acting analog of native GLP-2 and is somewhat more resistant to enzymatic degradation in the enterocyte than is the native enzyme. Dr. Jeppesen and colleagues reported a sustained 20%-100% decrease in PS volume requirements during weeks 20-24 of treatment in 63% of patients who received teduglutide, compared with 30% of placebo-treated patients. The mean drop in weekly PS volume from baseline to week 24 totaled 4.4 L in patients who received teduglutide, which equates to a decrease of 1-2 nights of infusion weekly, a very profound improvement for individual patients. The PS weaning protocol used was similar to that used in most centers experienced in the care of these patients.
As would be expected in the SBS-IF patient population, there were many adverse events, although these were equally distributed across teduglutide and placebo groups. Stomal changes, primarily related to enlargement, were evident in a significant minority of patients in the teduglutide group, as would be expected given the hyperplastic effect of the medication on intestinal epithelial tissue.Concern has been raised about GLP-2’s potential to stimulate the development of colonic adenomas in rodent models. Although the risk for malignancy is hypothetical in humans, colonoscopy should be considered at baseline for those patients with residual colons and perhaps as frequently as annually while the patients are on therapy until more long-term safety data are available.
Is teduglutide a "game changer"? The only patients who will be able to discontinue PS completely will be those who are on the borderline between nutritional autonomy and PS dependence. It is important to realize that teduglutide should be used to augment, not replace conventional management. What happens when teduglutide is stopped? Preliminary evidence suggests the effects on adaptation may be persistent, although earlier study noted that histologic changes trended toward baseline within 4 weeks of discontinuation. Perhaps longer treatment or maintenance will be required. The real future is an artificially grown and harvested intestine; even intestinal transplantation represents a bridge at best.
Dr. Alan L. Buchman is a former professor of medicine and surgery at the Feinberg School of Medicine at Northwestern University, Chicago. Within the past 12 months he has consulted for Takeda Pharmaceuticals and NPS Pharmaceuticals.
Patients with short bowel syndrome whose absorption is insufficient to maintain nutritional or fluid autonomy have intestinal failure. These patients, particularly those with proximal jejunostomies, who may actually secrete more fluid than they ingest, are among the most complex and challenging to manage of patients with any gastrointestinal disease. Patients with short bowel syndrome and intestinal failure are dependent on parenteral nutrition and/or fluid support (PS) to maintain life. This therapy has substantial implications for employment, activities, sleep, and finances. Numerous, often life-threatening, complications develop.

|
|
A myriad of growth factors may be involved in the process of postresection intestinal adaptation, including glucagonlike peptide-2 (GLP-2), wherein intestinal epithelial growth is promoted. Teduglutide is a long-acting analog of native GLP-2 and is somewhat more resistant to enzymatic degradation in the enterocyte than is the native enzyme. Dr. Jeppesen and colleagues reported a sustained 20%-100% decrease in PS volume requirements during weeks 20-24 of treatment in 63% of patients who received teduglutide, compared with 30% of placebo-treated patients. The mean drop in weekly PS volume from baseline to week 24 totaled 4.4 L in patients who received teduglutide, which equates to a decrease of 1-2 nights of infusion weekly, a very profound improvement for individual patients. The PS weaning protocol used was similar to that used in most centers experienced in the care of these patients.
As would be expected in the SBS-IF patient population, there were many adverse events, although these were equally distributed across teduglutide and placebo groups. Stomal changes, primarily related to enlargement, were evident in a significant minority of patients in the teduglutide group, as would be expected given the hyperplastic effect of the medication on intestinal epithelial tissue.Concern has been raised about GLP-2’s potential to stimulate the development of colonic adenomas in rodent models. Although the risk for malignancy is hypothetical in humans, colonoscopy should be considered at baseline for those patients with residual colons and perhaps as frequently as annually while the patients are on therapy until more long-term safety data are available.
Is teduglutide a "game changer"? The only patients who will be able to discontinue PS completely will be those who are on the borderline between nutritional autonomy and PS dependence. It is important to realize that teduglutide should be used to augment, not replace conventional management. What happens when teduglutide is stopped? Preliminary evidence suggests the effects on adaptation may be persistent, although earlier study noted that histologic changes trended toward baseline within 4 weeks of discontinuation. Perhaps longer treatment or maintenance will be required. The real future is an artificially grown and harvested intestine; even intestinal transplantation represents a bridge at best.
Dr. Alan L. Buchman is a former professor of medicine and surgery at the Feinberg School of Medicine at Northwestern University, Chicago. Within the past 12 months he has consulted for Takeda Pharmaceuticals and NPS Pharmaceuticals.
Teduglutide significantly reduced the need for parenteral support in patients with short bowel syndrome and intestinal failure, based on data from 85 adults in a randomized, controlled multicenter trial. The findings were published in the December issue of Gastroenterology.
Patients with short bowel syndrome and intestinal failure (SBS-IF) have inadequate intestinal absorption and require parenteral support (PS) to maintain fluids, electrolytes, trace elements, vitamins, and nutrient balances, said Dr. Palle Bekker Jeppesen of Rigshospitalet in Copenhagen and colleagues.
Source: American Gastroenterological Association
Data from previous open-label studies suggest an association between teduglutide and clinically meaningful reductions in wet weight and energy, which may reduce the need for PS in these patients, the investigators noted.
The researchers randomized 86 adults with SBS-IF to either 0.05 mg/kg per day of teduglutide or a placebo. One patient was randomized in error; complete data were available for 42 teduglutide patients and 43 placebo patients.
Significantly more patients in the teduglutide group responded to treatment, compared with the placebo group (63% vs. 30%). This response was defined as sustaining a 20%-100% reduction from baseline in weekly PS volume during weeks 20-24. "Small bowel length did not appear to be a predictor of response," the researchers noted.
The high placebo response may be explained by examining the fluid composite effect, a measure of the combined effects of teduglutide on PS volume reduction as well as the ability to reduce oral fluid intake and increase urine output volume, the researchers noted.
"In the current study, where protocol modifications encouraged earlier and more aggressive PS reductions, significantly larger PS reductions were also achieved in patients receiving placebo, but subsequently these patients had to increase their oral fluid intake significantly to maintain urine production and hydration constant," they said.
After 24 weeks, overall PS volume was reduced by 32% from baseline in teduglutide patients, compared with 21% in placebo patients. Although no patients in either group were completely weaned from parenteral support at 24 weeks, the difference in PS volume reduction was significantly greater in the teduglutide group.
The average weekly PS volume in teduglutide patients decreased significantly from 12.5 L/wk at baseline to 8.1 L/wk at week 24. The placebo patients also had a significant decrease in average weekly PS volume, from 13.4 L/wk at baseline to 11.1 L/wk at week 24.
Treatment-ending adverse events were similar between the two groups; 5% of teduglutide patients and 7% of placebo patients discontinued treatment because of such events during the study period. The most frequently reported treatment-emergent adverse events included abdominal pain, abdominal distension, nausea, and gastrointestinal stoma complications.
Although the study did not specifically assess quality of life measures, significantly more teduglutide patients had at least 1 day off PS, compared with placebo patients, which could help to "liberate considerable time for unhindered daytime activities or undisturbed sleep," the researchers said.
The study did not address the possible benefit of teduglutide therapy earlier in the course of SBS, or the duration of effect after patients discontinued teduglutide, the researchers added.
However, the findings indicate that teduglutide was safe and well tolerated, and "could positively add to the limited treatment armamentarium" for patients with SBS-IF.
Dr. Jeppesen and several coauthors have served on the advisory board of and as consultants to NPS Pharmaceuticals, the company that funded the study. One author is an employee of NPS Pharmaceuticals.
Teduglutide significantly reduced the need for parenteral support in patients with short bowel syndrome and intestinal failure, based on data from 85 adults in a randomized, controlled multicenter trial. The findings were published in the December issue of Gastroenterology.
Patients with short bowel syndrome and intestinal failure (SBS-IF) have inadequate intestinal absorption and require parenteral support (PS) to maintain fluids, electrolytes, trace elements, vitamins, and nutrient balances, said Dr. Palle Bekker Jeppesen of Rigshospitalet in Copenhagen and colleagues.
Source: American Gastroenterological Association
Data from previous open-label studies suggest an association between teduglutide and clinically meaningful reductions in wet weight and energy, which may reduce the need for PS in these patients, the investigators noted.
The researchers randomized 86 adults with SBS-IF to either 0.05 mg/kg per day of teduglutide or a placebo. One patient was randomized in error; complete data were available for 42 teduglutide patients and 43 placebo patients.
Significantly more patients in the teduglutide group responded to treatment, compared with the placebo group (63% vs. 30%). This response was defined as sustaining a 20%-100% reduction from baseline in weekly PS volume during weeks 20-24. "Small bowel length did not appear to be a predictor of response," the researchers noted.
The high placebo response may be explained by examining the fluid composite effect, a measure of the combined effects of teduglutide on PS volume reduction as well as the ability to reduce oral fluid intake and increase urine output volume, the researchers noted.
"In the current study, where protocol modifications encouraged earlier and more aggressive PS reductions, significantly larger PS reductions were also achieved in patients receiving placebo, but subsequently these patients had to increase their oral fluid intake significantly to maintain urine production and hydration constant," they said.
After 24 weeks, overall PS volume was reduced by 32% from baseline in teduglutide patients, compared with 21% in placebo patients. Although no patients in either group were completely weaned from parenteral support at 24 weeks, the difference in PS volume reduction was significantly greater in the teduglutide group.
The average weekly PS volume in teduglutide patients decreased significantly from 12.5 L/wk at baseline to 8.1 L/wk at week 24. The placebo patients also had a significant decrease in average weekly PS volume, from 13.4 L/wk at baseline to 11.1 L/wk at week 24.
Treatment-ending adverse events were similar between the two groups; 5% of teduglutide patients and 7% of placebo patients discontinued treatment because of such events during the study period. The most frequently reported treatment-emergent adverse events included abdominal pain, abdominal distension, nausea, and gastrointestinal stoma complications.
Although the study did not specifically assess quality of life measures, significantly more teduglutide patients had at least 1 day off PS, compared with placebo patients, which could help to "liberate considerable time for unhindered daytime activities or undisturbed sleep," the researchers said.
The study did not address the possible benefit of teduglutide therapy earlier in the course of SBS, or the duration of effect after patients discontinued teduglutide, the researchers added.
However, the findings indicate that teduglutide was safe and well tolerated, and "could positively add to the limited treatment armamentarium" for patients with SBS-IF.
Dr. Jeppesen and several coauthors have served on the advisory board of and as consultants to NPS Pharmaceuticals, the company that funded the study. One author is an employee of NPS Pharmaceuticals.
FROM GASTROENTEROLOGY
Major Finding: Significantly more patients who received 0.05 mg/kg per day of teduglutide had a sustained response to treatment during weeks 20-24, compared with the placebo group (63% vs. 30%).
Data Source: The data come from a randomized, controlled multicenter trial of 85 adults with short bowel syndrome and intestinal failure.
Disclosures: Dr. Jeppesen and several coauthors have served on the advisory board of and as consultants to NPS Pharmaceuticals, the company that funded the study. One author is an employee of NPS Pharmaceuticals.
Statins and diabetes risk: Fact, fiction, and clinical implications
On february 28, 2012, the US Food and Drug Administration (FDA) updated its labeling requirements for statins. In addition to revising its recommendations for monitoring liver function and its alerts about reports of memory loss, the FDA also warned of the possibility of new-onset diabetes mellitus and worse glycemic control in patients taking statin drugs.1
This change stoked an ongoing debate about the risk of diabetes with statin use and the implications of such an effect. To understand the clinical consequences of this alert and its effect on treatment decisions, we need to consider the degree to which statins lower the risk of cardiovascular disease in patients at high risk (including diabetic patients), the magnitude of the risk of developing new diabetes while on statin therapy, and the ratio of risk to benefit in treated populations.
This review will discuss the evidence for this possible adverse effect and the implications for clinical practice.
DO STATINS CAUSE DIABETES?
Individual controlled trials dating back more than a decade have had conflicting results about new diabetes and poorer diabetic control in patients taking statins.
The West of Scotland Coronary Prevention Study (WOSCOPS)2 suggested that the incidence of diabetes was 30% lower in patients taking pravastatin (Pravachol) 40 mg/day than with placebo. However, this was not observed with atorvastatin (Lipitor) 10 mg/day in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid-Lowering Arm (ASCOT-LLA)3 in hypertensive patients or in the Collaborative Atorvastatin Diabetes Study (CARDS)4 in diabetic patients,4 nor was it noted with simvastatin (Zocor) 40 mg/day in the Heart Protection Study (HPS).5
The Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER),6 using the more potent agent rosuvastatin (Crestor) 20 mg/day in patients with elevated levels of C-reactive protein (CRP), was stopped early when an interim analysis found a 44% lower incidence of the primary end point. However, the trial also reported a 26% higher incidence of diabetes in follow-up of less than 2 years.
In the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER),7 with a mean age at entry of 75, there was a 32% higher incidence of diabetes with pravastatin therapy.7
Results of meta-analyses
Several meta-analyses have addressed these differences.
Rajpathak et al8 performed a meta-analysis, published in 2009, of six trials—WOSCOPS,2 ASCOT-LLA,3 JUPITER,6 HPS,5 the Long-term Intervention With Pravastatin in Ischaemic Disease (LIPID) study,9 and the Controlled Rosuvastatin Multinational Study in Heart Failure (CORONA),10 with a total of 57,593 patients. They calculated that the incidence of diabetes was 13% higher (an absolute difference of 0.5%) in statin recipients, which was statistically significant. In their initial analysis, the authors excluded WOSCOPS, describing it as hypothesis-generating. The relative increase in risk was less—6%—and was not statistically significant when WOSCOPS was included.
Sattar et al,11 in a larger meta-analysis published in 2010, included 91,140 participants in 13 major statin trials conducted between 1994 and 2009; each trial had more than 1,000 patients and more than 1 year of follow-up.2,3,5–7,9,10,12–17 New diabetes was defined as physician reporting of new diabetes, new diabetic medication use, or a fasting glucose greater than 7 mmol/L (126 mg/dL).
New diabetes occurred in 2,226 (4.89%) of the statin recipients and in 2,052 (4.5%) of the placebo recipients, an absolute difference of 0.39%, or 9% more (odds ratio [OR] 1.09; 95% confidence interval [CI] 1.02–1.17) (Figure 1).
The incidence of diabetes varied substantially among the 13 trials, with only JUPITER6 and PROSPER7 finding statistically significant increases in rates (26% and 32%, respectively). Of the other 11 trials, 4 had nonsignificant trends toward lower incidence,2,9,13,17 while the 7 others had nonsignificant trends toward higher incidence.
Does the specific statin make a difference?
Questions have been raised as to whether the type of statin used, the intensity of therapy, or the population studied contributed to these differences. Various studies suggest that factors such as using hydrophilic vs lipophilic statins (hydrophilic statins include pravastatin and rosuvastatin; lipophilic statins include atorvastatin, lovastatin, and simvastatin), the dose, the extent of lowering of low-density lipoprotein cholesterol (LDL-C), and the age or clinical characteristics of the population studied may influence this relationship.18–20
Yamakawa et al18 examined the effect of atorvastatin 10 mg/day, pravastatin 10 mg/day, and pitavastatin (Livalo) 2 mg/day on glycemic control over 3 months in a retrospective analysis. Random blood glucose and hemoglobin A1c levels were increased in the atorvastatin group but not in the other two.18
A prospective comparison of atorvastatin 20 mg vs pitavastatin 4 mg in patients with type 2 diabetes, presented at the American College of Cardiology’s 2011 annual meeting, reported a significant increase in fasting glucose levels with atorvastatin, particularly in women, but not with pitavastatin.19
In the Compare the Effect of Rosuvastatin With Atorvastatin on Apo B/Apo A-1 Ratio in Patients With Type 2 Diabetes Mellitus and Dyslipidaemia (CORALL) study,20 both high-dose rosuvastatin (40 mg) and high-dose atorvastatin (80 mg) were associated with significant increases in hemoglobin A1c, although the mean fasting glucose levels were not significantly different at 18 weeks of therapy.
A meta-analysis by Sattar et al11 did not find a clear difference between lipophilic statins (OR 1.10 vs placebo) and hydrophilic statins (OR 1.08). In analysis by statin type, the combined rosuvastatin trials were statistically significant in favor of a higher diabetes risk (OR 1.18, 95% CI 1.04–1.44). Nonsignificant trends were noted for atorvastatin trials (OR 1.14) and simvastatin trials (OR 1.11) and less so for pravastatin (OR 1.03); the OR for lovastatin was 0.98. This may suggest that there is a stronger effect with more potent statins or with greater lowering of LDL-C.
Meta-regression analysis in this study demonstrated that diabetes risk with statins was higher in older patients but was not influenced by body mass index or by the extent that LDL-C was lowered.
Statin dose as a risk factor
Intensive-dose statin therapy has been shown to reduce cardiovascular risk more than low-dose or moderate-dose therapy, thus supporting more aggressive treatment of LDL-C in higher-risk patients. However, some controlled studies comparing more-potent with less-potent statin regimens suggest that there may also be a higher risk of incident diabetes at higher doses.21–24
In a post hoc analysis of the Pravastatin or Atorvastatin Evaluation and Infection Therapy– Thrombolysis in Myocardial Infarction 22 (PROVE-IT TIMI 22) trial,21 patients who had experienced an acute coronary syndrome had a greater increase in hemoglobin A1c if treated with atorvastatin 80 mg/day than with pravastatin 40 mg/day.
Waters et al23 reported a higher risk of new diabetes with atorvastatin 80 mg than with placebo and a trend toward a higher risk with atorvastatin 80 mg than with atorvastatin 10 mg or simvastatin 20 mg.
In contrast, a review by Yousef et al24 of the data from the Enhanced Feedback for Effective Cardiac Treatment (EFFECT) study did not find a higher diabetes risk with more intensive statin therapy based on the magnitude of LDL-C reduction. A propensity-matched examination of deaths, recurrent acute ischemic events, or new diabetes in patients previously hospitalized with myocardial infarction found no differences in these end points each year out to 5 years. The risk of diabetes was in fact lower (but the difference was not statistically significant) in the high-dose groups out to 5 years. The risk of myocardial infarction or death was numerically different in the high-dose groups, but the difference was not statistically significant.
Preiss et al25 in 2011 performed a meta-analysis of the impact of intensity of statin therapy on diabetes risk. They examined data from 32,752 participants without diabetes at baseline in five randomized controlled trials with more than 1,000 participants and more than 1 year of follow-up, comparing high-dose therapy against moderate-dose statin therapy.21,22,26–28 New diabetes was considered present if there was an adverse event report of diabetes, if glucose-lowering drugs were started, or if two fasting plasma glucose measurements were higher than 7 mmol/L (126 mg/dL).
Diabetes developed in 1,449 (8.8%) of the intensive-therapy group and 1,300 (8.0%) of the moderate-therapy group (OR 1.12, 95% CI 1.04–1.22). In contrast, incident cardiovascular disease occurred in 3,134 (19.1%) of the intensive-therapy group and 3,550 (21.7%) of the moderate-therapy group (OR 0.84, 95% CI 0.75–0.94). Therefore, there was an 0.8% absolute increase in diabetes cases on high-dose statins and a 2.6% absolute reduction in adverse cardiovascular events.
CAUTION IN INTERPRETING THESE DATA
There are many reasons for caution in interpreting these studies.
The trials were not designed to look for diabetes
The data supporting the relationship between statin therapy and higher risk of diabetes are primarily from observational studies. These studies were not prospectively designed to address this question, and we therefore need to view this as association and not as causation.
The definition of diabetes varied between trials, and new-onset diabetes was often not rigorously screened for. In many trials the outcome of diabetes was at least partially based on nonstandardized, nonadjudicated physician reporting.
Consequently, if statins reduce the risk of diabetes, the results from WOSCOPS may overstate the reduction, since this study used a non-standard definition of incident diabetes (fasting plasma glucose > 126 mg/dL plus a > 36 mg/dL increase from baseline). When Sattar et al11 reanalyzed WOSCOPS data using a more standard definition, they found a smaller effect.
On the other hand, nonstandardized physician reporting may overstate an adverse effect. Sattar et al11 also found that when fasting plasma glucose levels alone were used as the definition for diabetes, the overall risk was attenuated and was no longer statistically significant (OR 1.07, 95% CI 0.97–1.17).
Perhaps statin therapy uncovers diabetes only in people at risk of diabetes
Perhaps statin therapy uncovers diabetes only in people at higher baseline risk of developing diabetes. Therefore, this adverse effect may be restricted to certain groups and not applicable to the general population.
In JUPITER, one of the two trials in which, on independent analysis, statin use was associated with new diabetes, 77% of patients in the rosuvastatin group who developed diabetes had impaired fasting glucose at entry and therefore were at higher risk of developing diabetes.6
Possibly, the relationship is driven by preexisting metabolic syndrome or other risk factors for diabetes. In the two studies that reported a statistically significantly higher incidence of new diabetes, more than 40% of patients in JUPITER met the criteria for metabolic syndrome, and metabolic syndrome, which increases in prevalence with age, was likely more prominent in the elderly population in PROSPER.
Waters et al23 grouped patients according to whether they had risk factors for diabetes (impaired fasting glucose, obesity, elevated triglycerides, and hypertension) and found that those who had none or one of these risk factors had no difference in the rate of new-onset diabetes with either moderate or intensive statin therapy, but the risk was pronounced in those who had three or four risk factors.
Ridker et al29 reanalyzed the JUPITER data from patients who did not have cardiovascular disease at baseline. Overall, for every 54 new cases of diabetes in follow-up, 134 cardiovascular events or deaths were prevented. In subgroup analysis, those who had one or more risk factors for diabetes at baseline (metabolic syndrome, impaired fasting glucose, obesity, or hemoglobin A1c > 6%) had a 39% reduction in the primary end point and a 28% increase in new diabetes. Those who had none of these risk factors had a 52% lower rate of cardiovascular events but no increase in diabetes.
Other confounding factors
Bias and confounding factors are difficult to control for in studies without prospectively defined, recognized, and analyzed outcomes.
Although it may be a bit of a stretch, residual confounding factors such as myalgia side effects while on statins may reduce exercise in the statin-treatment groups. Perhaps a change to a healthier lifestyle after cardiovascular events may be more common in placebo groups. Improved survival with statins may allow more people at risk of diabetes to live longer and present with the diagnosis.30
POSSIBLE EXPLANATIONS, BUT NO UNIFYING MECHANISM
If mechanisms could be identified to explain the association between statins and diabetes, this would strengthen the argument that it is a cause-and-effect relationship. Many explanations have been proposed as to how statins may influence glucose metabolism and insulin sensitivity.31–34 These are possible explanations based on other observations.
In theory, statins may improve insulin sensitivity via their anti-inflammatory effect, since inflammatory markers and proinflammatory cytokines have been linked with insulin resistance. However, other effects of statins may adversely affect glycemic control.
In vivo analysis has shown that some but not all statins increase insulin levels and decrease insulin sensitivity in a dose-dependent fashion. Some statins decrease adiponectin and may worsen glycemic control through loss of adiponectin’s proposed protective anti-proliferative and antiangiogenic properties. In vitro studies and animal studies have demonstrated a decrease in expression of insulin-responsive glucose transporter 4 (GLUT4) with atorvastatin, and an increase in GLUT1. It has been hypothesized that reduction in isoprenoid biosynthesis or decreased insulin signaling may explain these effects and that changes in glucose transport in adipocytes may cause insulin resistance. Other studies suggest that dysregulation of cellular cholesterol may attenuate beta-cell function. Impaired biosynthesis of ubiquinones may result in delayed production of adenosine triphosphate and consequently diminish insulin release.
But different effects have been reported for atorvastatin, simvastatin, and pravastatin, arguing against a unifying explanation or, alternatively, suggesting that differences in lipophilicity and potency among statins are important. Hydrophilic statins may be less likely to be taken up by extrahepatic cells such as pancreatic cells and adipocytes, possibly lessening these effects. However, the strong association between rosuvastatin (which is hydrophilic) and new diabetes would not support this hypothesis.
Despite these speculations, lack of conformity in response to different statins and discrepancies in the clinical outcomes noted in trials fail to clearly identify a common causative mechanism.
OTHER COMMON THERAPIES MAY INFLUENCE GLYCEMIC CONTROL
Statins are not the first drugs for reducing cardiovascular risk that have been shown to affect glucose levels during treatment.
Niacin
Niacin has been known to increase glucose levels but has long been used as a treatment for dyslipidemia despite this caution. Reduced glycemic control during niacin treatment in diabetic patients does not seem to alter the beneficial effects of treatment.35–37
In a post hoc analysis of the Coronary Drug Project (CDP), in patient subgroups defined by baseline fasting plasma glucose and compared with placebo, niacin reduced the 6-year risk of recurrent myocardial infarction and the combined end point of coronary heart disease death or nonfatal myocardial infarction similarly (interactive P value nonsignificant) across all levels of baseline fasting plasma glucose, including levels of 126 mg/dL or higher at study entry.36
In another post hoc analysis of CDP patient subgroups defined by the change in glycemic status from baseline to 1 year, niacin reduced the 6-year risk of the same end points similarly (interactive P value nonsignificant) across all levels of change in fasting plasma glucose from baseline to year 1, whether baseline fasting plasma glucose levels decreased, stayed the same, or increased to 10 mg/dL or higher on niacin therapy.36
Therefore, the beneficial effect of niacin of reducing the rate of recurrent nonfatal myocardial infarction and coronary heart disease events was not significantly diminished when impaired fasting glucose or diabetes was present when therapy was started or by on-therapy increases from baseline fasting plasma glucose.
In addition, on-therapy changes in glycemic control may be dose-related and minimized by surveillance and therapy adjustments. The Assessment of Diabetes Control and Evaluation of the Efficacy of Niaspan Trial (ADVENT)38 found that changes in glycemic control were minimal as measured by fasting glucose and hemoglobin A1c; were associated with a higher niacin dose (1.5 g/day vs 1 g/day); and, when present, were successfully managed by adjusting the diabetes treatment regimen.
Antihypertensive drugs
Diuretics as well as beta-blockers have been reported to increase the incidence of diabetes in patients with hypertension.15,38–40
A retrospective longitudinal cohort study40 in 2009 examined the development of new-onset diabetes (defined as a new ICD-9 code for diabetes or initiation of diabetes treatment) in 24,688 treated hypertensive patients without diabetes at baseline; 4,385 (17.8%) of the patients developed diabetes. After adjusting for sex and age, the risk of new diabetes was significant in users of diuretics (OR 1.10), beta-blockers (OR 1.12), and calcium channel blockers (OR 1.10) compared with users of angiotensin-converting enzyme inhibitors, (OR 0.92), angiotensin receptor blockers (OR 0.90), or alpha-blockers (OR 0.88).
However, the increase in blood glucose does not seem to attenuate the beneficial effects of reducing cardiovascular events. In the Antihypertensive and Lipid-lowering Treatment to Prevent Heart Attack Trial (ALLHAT),15 a long-term follow-up of those developing new-onset diabetes while taking chlorthalidone (Hygroton) found no difference in the risk of death from cardiovascular disease or from any cause (hazard ratio = 1.04).15
CLINICAL IMPLICATIONS
Balancing the benefits and risks of statins
It is important to examine how the 0.4% increase in absolute risk of new-onset diabetes as calculated in meta-analyses compares with the benefits of statin treatment in terms of cardiovascular risk reduction.
Using data from the Cholesterol Treatment Trialists (CTT) meta-analysis of statin trials in 71,370 participants, Sattar et al11 estimated that statin treatment is associated with 5.4 fewer deaths from coronary heart disease and cases of nonfatal myocardial infarction per 255 patients treated over 4 years for each 1-mmol/L (39 mg/dL) reduction in LDL-C compared with controls. The benefit would be even greater if stroke, revascularization, and hospitalization are included as end points. This benefit is contrasted with the risk of developing 1 additional case of diabetes for every 255 patients treated with statins over the same period.
Preiss et al25 calculated that there were 2 more cases of diabetes per 1,000 patient-years in patients receiving intensive doses than in those receiving moderate doses (18.9 vs 16.9), corresponding to 1 additional case of diabetes for every 498 patients treated per year. However, there were 6.5 fewer first major cardiovascular events per 1,000 patient-years (44.5 vs 51.0), corresponding to a number needed to treat per year to prevent 1 cardiovascular event of 155. Most of the benefit was due to fewer revascularizations, followed by nonfatal myocardial infarctions. The 12% increase in new diabetes with high-dose therapy contrasted with a 16% reduction in new cardiovascular disease combined events (OR 0.84, 95% CI 0.75–0.94).
As previously noted, in the JUPITER trial, the benefits of preventing cardiovascular events with statin therapy outweighed the risk of new diabetes in people both with and without baseline risk factors for diabetes.29 Similar to the observations with niacin and some antihypertensive drugs, the increase in blood glucose with statins does not appear to reduce the benefits of cardiovascular risk reduction in these patients at moderate to high risk, even when used at high doses.
People with diabetes need aggressive lipid-lowering—with statins
Diabetes is a coronary heart disease risk equivalent and is associated with high risk of cardiovascular events.41–46 Overall, the risk for these adverse events is two to four times greater in people with diabetes than without. Atherosclerosis-related events account for approximately 65% to 75% of all deaths in people with diabetes, and 75% of these events are coronary. Lipid abnormalities are strongly correlated with the risk of cardiovascular disease in people with diabetes, and aggressive treatment of risk factors, particularly lipid abnormalities, has been shown to reduce this risk.47–49 And data from multiple clinical trials support the use of statins to lower LDL-C as the first-line therapy for dyslipidemia in people with diabetes, just as it is in the general population.3–7,9,13,23,50–61
Analyses of diabetic subgroups encompassing 18,000 to 20,000 patients in the large statin trials have clearly demonstrated the benefits of statin therapy. A recent metaanalysis of 10 placebo-controlled trials that included approximately 16,000 patients with diabetes and 54,000 without diabetes demonstrated a 30% reduction in coronary heart disease, a 19% reduction in strokes, and a 12% reduction in mortality.54 Furthermore, in another meta-analysis of 14 trials, a similar 22% reduction in coronary heart disease was noted in people with diabetes whether or not they had a history of cardiovascular disease.55
Therefore, aggressive treatment of lipid abnormalities with statins as primary treatment has generally been adopted as a standard of care in diabetic patients, particularly those with clinical cardiovascular disease or one or more risk factors. The Adult Treatment Panel III guidelines recommend a minimum LDL-C goal of less than 100 mg/dL and a goal of less than 70 mg/dL as an option for patients with diabetes (Table 1).41,62 Similar recommendations have been issued by the American Diabetes Association together with the American College of Cardiology (Table 2),30 the American Diabetes Association by itself,63 and the American Academy of Pediatrics.6
Is new-onset diabetes as dangerous as established diabetes?
In studies to date, there did not appear to be more events in those who developed new-onset diabetes.
Waters et al,24 evaluating three trials of high-dose atorvastatin therapy, found that major cardiovascular events occurred in 11.3% of those with new-onset diabetes, 10.8% of those without new-onset diabetes (HR 1.02, 95% CI 0.77–1.35), and 17.5% of those who had diabetes at baseline.
Therefore, it may not be appropriate to extrapolate the glucose changes seen on statin therapy to an equivalent increase in adverse cardiovascular events as seen in other diabetic patients. The beneficial reduction in cardiovascular events does not appear to be diminished in those developing diabetes. It is not clear that the increase in glucose on statins has the same implications of a new diagnosis of diabetes. Does this elevation in glucose represent true diabetes or some downstream effect? For example, thiazide diuretics have been known to increase blood glucose levels, but the levels drop when these drugs are discontinued, even after many years of treatment.
On the other hand, it is possible that follow-up of 5 years or less in clinical trials has not allowed sufficient time to examine the influence of the increase in new-onset diabetes on future cardiovascular events. In addition, because of the widespread use of statins across a broad range of cardiovascular risk, even if the effect is small in absolute terms, the potential adverse effects are magnified, particularly in a low-risk population in which the cardiovascular benefits are smaller.
The association is real, but questions remain
In view of the evidence, it is difficult to refute that an association exits between statin use and new-onset diabetes, at least in some subgroups. The dose response noted in some studies further reinforces the conclusion that the association is real. However, many questions remain unanswered regarding mechanism of effect, whether there are differences depending on the particular statin or dose used, or differential effects in the populations treated (such as patients with metabolic syndrome or the elderly).
Until the contradictory observations can be resolved and plausible mechanisms of action elucidated, causality cannot be established. From a clinical standpoint there is no current evidence suggesting that the elevations in blood glucose seen while on lipid-lowering or blood-pressure-lowering therapy are associated with an increased risk of cardiovascular events or that they attenuate the beneficial effects of the therapy.
Statins should continue to be used in patients at high risk
Until further studies are done, statins should continue to be used, after assessing the risks and the benefits.
Primary prevention patients at moderate to high risk and secondary prevention patients stand to gain from statin therapy, and it should not be denied or doses reduced on the basis of concerns about the development of new-onset diabetes. The recognized modest risk of developing diabetes does not appear to blunt the cardioprotective effects of statin therapy in these moderate-to high-risk groups.
Rather than stop statins in patients at risk of diabetes such as the elderly or those with prediabetes, insulin resistance, or metabolic syndrome who are on therapy for appropriate reasons, it is reasonable to continue these drugs, monitoring glucose more closely and emphasizing the importance of weight reduction, diet, and aerobic exercise for preventing diabetes. The Diabetes Prevention Program Research Group, for example, reduced the incidence of diabetes by 58% over 2.8 years of follow-up with intensive lifestyle interventions (a low-calorie, low-fat diet plus moderate physical activity 150 minutes per week) vs usual care in at-risk populations.65
Should statins be used more cautiously in patients at lower risk?
The most recent Cholesterol Treatment Trialists meta-analysis of 27 randomized clinical trials (22 placebo-controlled, 134,537 people; 5 high-dose vs low-dose, 39,612 people) reported that reducing LDL-C with statins lowered cardiovascular risk even in low-risk patients.66 Overall, there were 21% fewer major cardiovascular events (coronary heart disease, stroke, or coronary revascularization) for every 1-mmol/L reduction in LDL-C.
The proportional reduction in events was at least as large in the two lowest-risk groups (estimated 5-year risk of < 5% and 5% to < 10%, 53,152 people) as in the higher-risk groups. This was reflected mainly in fewer nonfatal myocardial infarctions and coronary revascularizations. In these groups, the absolute reduction in risk for each 1-mmol/L reduction in LDL-C was 11 per 1,000 patients over 5 years. Even in this low-risk population, the reduction in cardiovascular risk seems to compare favorably with the small estimated increase risk of diabetes.
However, even in the lowest-risk group studied, the average baseline LDL-C level was greater than 130 mg/dL.
Therefore, in groups in which the benefits of statins on cardiovascular risk reduction are less robust (eg, low-risk primary prevention groups without significant elevations in LDLC, particularly the elderly), it would not be difficult to justify the case for more cautious use of statin therapy. If statins are used in these low-risk groups, restricting their use to those with at least moderate LDL-C elevation, using less aggressive LDL-C-lowering targets, and regular monitoring of fasting glucose seem reasonable until further information is available.
- US Food and Drug Administration. Statin drugs—drug safety communication: class labeling change. February 28, 2012. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm293670.htm.
- Freeman DJ, Norrie J, Sattar N, et al. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation 2001; 103:357–362.
- Sever PS, Dahlof B, Poulter NR, et al; ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003; 361:1149–1158.
- Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–696.
- Collins R, Armitage J, Parish S, Sleigh P, Peto R; for the Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 2003; 361:2005–2016.
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER Study Group. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002; 360:1623–1630.
- Rajpathak SN, Kumbhani DJ, Crandall J, Barzilai N, Alderman M, Ridker PM. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care 2009; 32:1924–1929.
- Keech A, Colquhoun D, Best J, et al. Secondary prevention of cardiovascular events with long-term pravastatin in patients with diabetes or impaired fasting glucose—results from the LIPID trial. Diabetes Care 2003; 26:2713–2721.
- Kjekshus J, Apetrei E, Barrios V, et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med 2007; 357:2248–2261.
- Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 2010; 375:735–742.
- Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet 2006; 368:1155–1163.
- Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998; 279:1615–1622.
- Scandinavian Simvastatin Survival Study study group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- Barzilay JI, Davis BR, Pressel SL, et al; ALLHAT Collaborative Research Group. Long-term effects of incident diabetes mellitus on cardiovascular outcomes in people treated for hypertension: the ALLHAT Diabetes Extension Study. Circ Cardiovasc Qual Outcomes 2012; 5:153–162.
- Tavazzi L, Maggioni AP, Marchioli R, et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 2008; 372:1231–1239.
- GISSI Prevenzione Investigators (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico). Results of the low-dose (20 mg) pravastatin GISSI Prevenzione trial in 4271 patients with recent myocardial infarction: do stopped trials contribute to overall knowledge? Ital Heart J 2000; 1:810–820.
- Yamakawa T, Takano T, Tanaka S, Kadonosono K, Terauchi Y. Influence of pitavastatin on glucose tolerance in patients with type 2 diabetes mellitus. J Atheroscler Thromb 2008; 15:269–275.
- Kryzhanovski V, Gumprecht J, Zhu B, Yu CY, Hounslow N, Sponseller CA. Atorvastatin but not pitavastatin significantly increases fasting plasma glucose in patients with type 2 diabetes and combined dyslipidemia (abstract). J Am Coll Cardiol 2011; 57:E575.
- Simsek S, Schalkwijk CG, Wolffenbuttel BH. Effects of rosuvastatin and atorvastatin on glycaemic control in type 2 diabetes—the CORALL study. Diabet Med 2012; 29:628–631.
- Sabatine MS, Morrow DA, Giugliano RP, et al. Implications of upstream glycoprotein IIb/IIIa inhibition and coronary artery stenting in the invasive management of unstable angina/non-ST-elevation myocardial infarction: a comparison of the Thrombolysis In Myocardial Infarction (TIMI) IIIB trial and the Treat angina with Aggrastat and determine Cost of Therapy with Invasive or Conservative Strategy (TACTICS)-TIMI 18 trial. Circulation 2004; 110(suppl III):834–880.
- Shepherd J, Barter P, Carmena R, et al. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the Treating to New Targets (TNT) study. Diabetes Care 2006; 29:1220–1226.
- Waters DD, Ho JE, DeMicco DA, et al. Predictors of new-onset diabetes in patients treated with atorvastatin: results from 3 large randomized clinical trials. J Am Coll Cardiol 2011; 57:1535–1545.
- Yousef A, Tu JV, Wang J, Donovan L, Ko DT. The association of intensive statin therapy on long-term risks of cardiovascular events and diabetes following acute myocardial infarction (abstract). Circulation 2012; 125:e859.
- Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a metaanalysis. JAMA 2011; 305:2556–2564.
- de Lemos JA, Blazing MA, Wiviott SD, et al; A to Z Investigators. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA 2004; 292:1307–1316.
- Pedersen TR, Faegeman O, Kastelein JJ, et al; Incremental Decrease in End Points Through Aggressive Lipid Lowering (IDEAL) Study Group. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005; 294:2437–2445.
- Armitage J, Bowman L, Wallendszus K, et al; Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double-blind randomised trial. Lancet 2010; 37:1658–1669.
- Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet 2012; 380:565–571.
- Brunzell JD, Davidson M, Furberg CD, et al; American Diabetes Association; American College of Cardiology Foundation. Lipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care 2008; 31:811–822.
- Koh KK, Quon MJ, Han SH, Lee Y, Kim SJ, Shin EK. Atorvastatin causes insulin resistance and increases ambient glycemia in hypercholesterolemic patients. J Am Coll Cardiol 2010; 55:1209–1216.
- Koh KK, Quon MJ, Han SH, et al. Differential metabolic effects of pravastatin and simvastatin in hypercholesterolemic patients. Atherosclerosis 2009; 204:483–490.
- Nakata M, Nagasaka S, Kusaka I, Matsuoka H, Ishibashi S, Yada T. Effects of statins on the adipocyte maturation and expression of glucose transporter 4 (SLC2A4): implications in glycaemic control. Diabetologia 2006; 49:1881–1892.
- Yada T, Nakata M, Shiraishi T, Kakei M. Inhibition by simvastatin, but not pravastatin, of glucose-induced cytosolic Ca2+ signalling and insulin secretion due to blockade of L-type Ca2+ channels in rat islet beta-cells. Br J Pharmacol 1999; 126:1205–1213.
- Guyton JR, Fazio S, Adewale AJ, et al. Effect of extended-release niacin on new-onset diabetes among hyperlipidemic patients treated with ezetimibe/simvastatin in a randomized controlled trial. Diabetes Care 2012; 35:857–860.
- Canner PL, Furberg CD, Terrin ML, McGovern ME. Benefits of niacin by glycemic status in patients with healed myocardial infarction (from the Coronary Drug Project). Am J Cardiol 2005; 95:254–257.
- Grundy SM, Vega GL, McGovern ME, et al; Diabetes Multicenter Research Group. Efficacy, safety, and tolerability of once-daily niacin for the treatment of dyslipidemia associated with type 2 diabetes: results of the Assessment of Diabetes Control and Evaluation of the Efficacy of Niaspan Trial. Arch Intern Med 2002; 162:1568–1576.
- Gupta AK, Dahlof B, Dobson J, Sever PS, Wedel H, Poulter NRAnglo-Scandinavian Cardiac Outcomes Trial Investigators. Determinants of new-onset diabetes among 19,257 hypertensive patients randomized in the Anglo-Scandinavian Cardiac Outcomes Trial—Blood Pressure Lowering Arm and the relative influence of antihypertensive medication. Diabetes Care 2008; 31:982–988.
- Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet 2007; 369:201–207.
- Jong JP, Chang MH, Tien L, et al. Antihypertensive drugs and new-onset diabetes: a retrospective longitudinal cohort study. Cardiovasc Ther 2009; 27:159–163.
- Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421.
- Norhammar A, Tenerz A, Nilsson G, et al. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study Lancet 2002; 359:2140–2144.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Sprafka JM, Burke GL, Folsom AR, McGovbern PG, Hahn LP. Trends in prevalence of diabetes mellitus in patients with myocardial infarction and effect of diabetes on survival. The Minnesota Heart Survey. Diabetes Care 1991; 14:537–543.
- Geiss LS, Herman WH, Smith PJ. Mortality in non-insulin-dependent diabetes. In:Harris MI, Cowie CC, Stern MP, et al, editors. Diabetes in America. 2nd ed. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 1995:233–257.
- Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 1993; 16:434–444.
- Turner RC, Millns H, Neil HA, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ 1998; 316:823–828.
- Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003; 348:383–393.
- Gaede P, Pederson O. Intensive integrated therapy of type 2 diabetes: implications for long-term prognosis. Diabetes 2004; 53:S39–S47.
- Goldberg RB, Mellies MJ, Sacks FM, et al. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the cholesterol and recurrent events (CARE) trial. The Care Investigators. Circulation 1998; 98:2513–2519.
- Pyðrälä K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care 1997; 20:614–620.
- Vijan S, Hayward RA; American College of Physicians. Pharmacologic lipid-lowering therapy in type 2 diabetes mellitus: background paper for the American College of Physicians. Ann Intern Med 2004; 140:650–658.
- Baigent C, Keech A, Kearney PM, et al; Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278. Errata in Lancet 2008; 371:2084, Lancet 2005; 366:1358.
- Brugts JJ, Yetgin T, Hoeks SE, et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ 2009; 338:b2376.
- Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Baigent C; Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008; 371:117–125.
- Nissen SE, Nicholls SJ, Sipahi I, et al; ASTEROID Investigators. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006; 295:1556–1565.
- Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes et al. N Engl J Med 2004; 350:1495–1504.
- LaRosa JC, Grundy SM, Waters DD, et al; Treating to New Targets (TNT) Investigators. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005; 352:1425–1435.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010; 376:1670–1681.
- LIPID Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med 1998; 339:1349–1357.
- Grundy SM, Cleeman JI, Bairey Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110:227–239.
- American Diabetes Association. Executive summary: standards of medical care in diabetes—2012. Diabetes Care 2012; 35(suppl 1):S5–S10.
- Daniels SR, Greer FR; Committee on Nutrition. Lipid screening and cardiovascular health in childhood. Pediatrics 2008; 122:198–208.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346:393–403.
- Cholesterol Treatment Trialists’ (CTT) Collaborators; Mihaylova B, Emberson J, Blackwell L, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012; 380:581–590.
On february 28, 2012, the US Food and Drug Administration (FDA) updated its labeling requirements for statins. In addition to revising its recommendations for monitoring liver function and its alerts about reports of memory loss, the FDA also warned of the possibility of new-onset diabetes mellitus and worse glycemic control in patients taking statin drugs.1
This change stoked an ongoing debate about the risk of diabetes with statin use and the implications of such an effect. To understand the clinical consequences of this alert and its effect on treatment decisions, we need to consider the degree to which statins lower the risk of cardiovascular disease in patients at high risk (including diabetic patients), the magnitude of the risk of developing new diabetes while on statin therapy, and the ratio of risk to benefit in treated populations.
This review will discuss the evidence for this possible adverse effect and the implications for clinical practice.
DO STATINS CAUSE DIABETES?
Individual controlled trials dating back more than a decade have had conflicting results about new diabetes and poorer diabetic control in patients taking statins.
The West of Scotland Coronary Prevention Study (WOSCOPS)2 suggested that the incidence of diabetes was 30% lower in patients taking pravastatin (Pravachol) 40 mg/day than with placebo. However, this was not observed with atorvastatin (Lipitor) 10 mg/day in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid-Lowering Arm (ASCOT-LLA)3 in hypertensive patients or in the Collaborative Atorvastatin Diabetes Study (CARDS)4 in diabetic patients,4 nor was it noted with simvastatin (Zocor) 40 mg/day in the Heart Protection Study (HPS).5
The Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER),6 using the more potent agent rosuvastatin (Crestor) 20 mg/day in patients with elevated levels of C-reactive protein (CRP), was stopped early when an interim analysis found a 44% lower incidence of the primary end point. However, the trial also reported a 26% higher incidence of diabetes in follow-up of less than 2 years.
In the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER),7 with a mean age at entry of 75, there was a 32% higher incidence of diabetes with pravastatin therapy.7
Results of meta-analyses
Several meta-analyses have addressed these differences.
Rajpathak et al8 performed a meta-analysis, published in 2009, of six trials—WOSCOPS,2 ASCOT-LLA,3 JUPITER,6 HPS,5 the Long-term Intervention With Pravastatin in Ischaemic Disease (LIPID) study,9 and the Controlled Rosuvastatin Multinational Study in Heart Failure (CORONA),10 with a total of 57,593 patients. They calculated that the incidence of diabetes was 13% higher (an absolute difference of 0.5%) in statin recipients, which was statistically significant. In their initial analysis, the authors excluded WOSCOPS, describing it as hypothesis-generating. The relative increase in risk was less—6%—and was not statistically significant when WOSCOPS was included.
Sattar et al,11 in a larger meta-analysis published in 2010, included 91,140 participants in 13 major statin trials conducted between 1994 and 2009; each trial had more than 1,000 patients and more than 1 year of follow-up.2,3,5–7,9,10,12–17 New diabetes was defined as physician reporting of new diabetes, new diabetic medication use, or a fasting glucose greater than 7 mmol/L (126 mg/dL).
New diabetes occurred in 2,226 (4.89%) of the statin recipients and in 2,052 (4.5%) of the placebo recipients, an absolute difference of 0.39%, or 9% more (odds ratio [OR] 1.09; 95% confidence interval [CI] 1.02–1.17) (Figure 1).
The incidence of diabetes varied substantially among the 13 trials, with only JUPITER6 and PROSPER7 finding statistically significant increases in rates (26% and 32%, respectively). Of the other 11 trials, 4 had nonsignificant trends toward lower incidence,2,9,13,17 while the 7 others had nonsignificant trends toward higher incidence.
Does the specific statin make a difference?
Questions have been raised as to whether the type of statin used, the intensity of therapy, or the population studied contributed to these differences. Various studies suggest that factors such as using hydrophilic vs lipophilic statins (hydrophilic statins include pravastatin and rosuvastatin; lipophilic statins include atorvastatin, lovastatin, and simvastatin), the dose, the extent of lowering of low-density lipoprotein cholesterol (LDL-C), and the age or clinical characteristics of the population studied may influence this relationship.18–20
Yamakawa et al18 examined the effect of atorvastatin 10 mg/day, pravastatin 10 mg/day, and pitavastatin (Livalo) 2 mg/day on glycemic control over 3 months in a retrospective analysis. Random blood glucose and hemoglobin A1c levels were increased in the atorvastatin group but not in the other two.18
A prospective comparison of atorvastatin 20 mg vs pitavastatin 4 mg in patients with type 2 diabetes, presented at the American College of Cardiology’s 2011 annual meeting, reported a significant increase in fasting glucose levels with atorvastatin, particularly in women, but not with pitavastatin.19
In the Compare the Effect of Rosuvastatin With Atorvastatin on Apo B/Apo A-1 Ratio in Patients With Type 2 Diabetes Mellitus and Dyslipidaemia (CORALL) study,20 both high-dose rosuvastatin (40 mg) and high-dose atorvastatin (80 mg) were associated with significant increases in hemoglobin A1c, although the mean fasting glucose levels were not significantly different at 18 weeks of therapy.
A meta-analysis by Sattar et al11 did not find a clear difference between lipophilic statins (OR 1.10 vs placebo) and hydrophilic statins (OR 1.08). In analysis by statin type, the combined rosuvastatin trials were statistically significant in favor of a higher diabetes risk (OR 1.18, 95% CI 1.04–1.44). Nonsignificant trends were noted for atorvastatin trials (OR 1.14) and simvastatin trials (OR 1.11) and less so for pravastatin (OR 1.03); the OR for lovastatin was 0.98. This may suggest that there is a stronger effect with more potent statins or with greater lowering of LDL-C.
Meta-regression analysis in this study demonstrated that diabetes risk with statins was higher in older patients but was not influenced by body mass index or by the extent that LDL-C was lowered.
Statin dose as a risk factor
Intensive-dose statin therapy has been shown to reduce cardiovascular risk more than low-dose or moderate-dose therapy, thus supporting more aggressive treatment of LDL-C in higher-risk patients. However, some controlled studies comparing more-potent with less-potent statin regimens suggest that there may also be a higher risk of incident diabetes at higher doses.21–24
In a post hoc analysis of the Pravastatin or Atorvastatin Evaluation and Infection Therapy– Thrombolysis in Myocardial Infarction 22 (PROVE-IT TIMI 22) trial,21 patients who had experienced an acute coronary syndrome had a greater increase in hemoglobin A1c if treated with atorvastatin 80 mg/day than with pravastatin 40 mg/day.
Waters et al23 reported a higher risk of new diabetes with atorvastatin 80 mg than with placebo and a trend toward a higher risk with atorvastatin 80 mg than with atorvastatin 10 mg or simvastatin 20 mg.
In contrast, a review by Yousef et al24 of the data from the Enhanced Feedback for Effective Cardiac Treatment (EFFECT) study did not find a higher diabetes risk with more intensive statin therapy based on the magnitude of LDL-C reduction. A propensity-matched examination of deaths, recurrent acute ischemic events, or new diabetes in patients previously hospitalized with myocardial infarction found no differences in these end points each year out to 5 years. The risk of diabetes was in fact lower (but the difference was not statistically significant) in the high-dose groups out to 5 years. The risk of myocardial infarction or death was numerically different in the high-dose groups, but the difference was not statistically significant.
Preiss et al25 in 2011 performed a meta-analysis of the impact of intensity of statin therapy on diabetes risk. They examined data from 32,752 participants without diabetes at baseline in five randomized controlled trials with more than 1,000 participants and more than 1 year of follow-up, comparing high-dose therapy against moderate-dose statin therapy.21,22,26–28 New diabetes was considered present if there was an adverse event report of diabetes, if glucose-lowering drugs were started, or if two fasting plasma glucose measurements were higher than 7 mmol/L (126 mg/dL).
Diabetes developed in 1,449 (8.8%) of the intensive-therapy group and 1,300 (8.0%) of the moderate-therapy group (OR 1.12, 95% CI 1.04–1.22). In contrast, incident cardiovascular disease occurred in 3,134 (19.1%) of the intensive-therapy group and 3,550 (21.7%) of the moderate-therapy group (OR 0.84, 95% CI 0.75–0.94). Therefore, there was an 0.8% absolute increase in diabetes cases on high-dose statins and a 2.6% absolute reduction in adverse cardiovascular events.
CAUTION IN INTERPRETING THESE DATA
There are many reasons for caution in interpreting these studies.
The trials were not designed to look for diabetes
The data supporting the relationship between statin therapy and higher risk of diabetes are primarily from observational studies. These studies were not prospectively designed to address this question, and we therefore need to view this as association and not as causation.
The definition of diabetes varied between trials, and new-onset diabetes was often not rigorously screened for. In many trials the outcome of diabetes was at least partially based on nonstandardized, nonadjudicated physician reporting.
Consequently, if statins reduce the risk of diabetes, the results from WOSCOPS may overstate the reduction, since this study used a non-standard definition of incident diabetes (fasting plasma glucose > 126 mg/dL plus a > 36 mg/dL increase from baseline). When Sattar et al11 reanalyzed WOSCOPS data using a more standard definition, they found a smaller effect.
On the other hand, nonstandardized physician reporting may overstate an adverse effect. Sattar et al11 also found that when fasting plasma glucose levels alone were used as the definition for diabetes, the overall risk was attenuated and was no longer statistically significant (OR 1.07, 95% CI 0.97–1.17).
Perhaps statin therapy uncovers diabetes only in people at risk of diabetes
Perhaps statin therapy uncovers diabetes only in people at higher baseline risk of developing diabetes. Therefore, this adverse effect may be restricted to certain groups and not applicable to the general population.
In JUPITER, one of the two trials in which, on independent analysis, statin use was associated with new diabetes, 77% of patients in the rosuvastatin group who developed diabetes had impaired fasting glucose at entry and therefore were at higher risk of developing diabetes.6
Possibly, the relationship is driven by preexisting metabolic syndrome or other risk factors for diabetes. In the two studies that reported a statistically significantly higher incidence of new diabetes, more than 40% of patients in JUPITER met the criteria for metabolic syndrome, and metabolic syndrome, which increases in prevalence with age, was likely more prominent in the elderly population in PROSPER.
Waters et al23 grouped patients according to whether they had risk factors for diabetes (impaired fasting glucose, obesity, elevated triglycerides, and hypertension) and found that those who had none or one of these risk factors had no difference in the rate of new-onset diabetes with either moderate or intensive statin therapy, but the risk was pronounced in those who had three or four risk factors.
Ridker et al29 reanalyzed the JUPITER data from patients who did not have cardiovascular disease at baseline. Overall, for every 54 new cases of diabetes in follow-up, 134 cardiovascular events or deaths were prevented. In subgroup analysis, those who had one or more risk factors for diabetes at baseline (metabolic syndrome, impaired fasting glucose, obesity, or hemoglobin A1c > 6%) had a 39% reduction in the primary end point and a 28% increase in new diabetes. Those who had none of these risk factors had a 52% lower rate of cardiovascular events but no increase in diabetes.
Other confounding factors
Bias and confounding factors are difficult to control for in studies without prospectively defined, recognized, and analyzed outcomes.
Although it may be a bit of a stretch, residual confounding factors such as myalgia side effects while on statins may reduce exercise in the statin-treatment groups. Perhaps a change to a healthier lifestyle after cardiovascular events may be more common in placebo groups. Improved survival with statins may allow more people at risk of diabetes to live longer and present with the diagnosis.30
POSSIBLE EXPLANATIONS, BUT NO UNIFYING MECHANISM
If mechanisms could be identified to explain the association between statins and diabetes, this would strengthen the argument that it is a cause-and-effect relationship. Many explanations have been proposed as to how statins may influence glucose metabolism and insulin sensitivity.31–34 These are possible explanations based on other observations.
In theory, statins may improve insulin sensitivity via their anti-inflammatory effect, since inflammatory markers and proinflammatory cytokines have been linked with insulin resistance. However, other effects of statins may adversely affect glycemic control.
In vivo analysis has shown that some but not all statins increase insulin levels and decrease insulin sensitivity in a dose-dependent fashion. Some statins decrease adiponectin and may worsen glycemic control through loss of adiponectin’s proposed protective anti-proliferative and antiangiogenic properties. In vitro studies and animal studies have demonstrated a decrease in expression of insulin-responsive glucose transporter 4 (GLUT4) with atorvastatin, and an increase in GLUT1. It has been hypothesized that reduction in isoprenoid biosynthesis or decreased insulin signaling may explain these effects and that changes in glucose transport in adipocytes may cause insulin resistance. Other studies suggest that dysregulation of cellular cholesterol may attenuate beta-cell function. Impaired biosynthesis of ubiquinones may result in delayed production of adenosine triphosphate and consequently diminish insulin release.
But different effects have been reported for atorvastatin, simvastatin, and pravastatin, arguing against a unifying explanation or, alternatively, suggesting that differences in lipophilicity and potency among statins are important. Hydrophilic statins may be less likely to be taken up by extrahepatic cells such as pancreatic cells and adipocytes, possibly lessening these effects. However, the strong association between rosuvastatin (which is hydrophilic) and new diabetes would not support this hypothesis.
Despite these speculations, lack of conformity in response to different statins and discrepancies in the clinical outcomes noted in trials fail to clearly identify a common causative mechanism.
OTHER COMMON THERAPIES MAY INFLUENCE GLYCEMIC CONTROL
Statins are not the first drugs for reducing cardiovascular risk that have been shown to affect glucose levels during treatment.
Niacin
Niacin has been known to increase glucose levels but has long been used as a treatment for dyslipidemia despite this caution. Reduced glycemic control during niacin treatment in diabetic patients does not seem to alter the beneficial effects of treatment.35–37
In a post hoc analysis of the Coronary Drug Project (CDP), in patient subgroups defined by baseline fasting plasma glucose and compared with placebo, niacin reduced the 6-year risk of recurrent myocardial infarction and the combined end point of coronary heart disease death or nonfatal myocardial infarction similarly (interactive P value nonsignificant) across all levels of baseline fasting plasma glucose, including levels of 126 mg/dL or higher at study entry.36
In another post hoc analysis of CDP patient subgroups defined by the change in glycemic status from baseline to 1 year, niacin reduced the 6-year risk of the same end points similarly (interactive P value nonsignificant) across all levels of change in fasting plasma glucose from baseline to year 1, whether baseline fasting plasma glucose levels decreased, stayed the same, or increased to 10 mg/dL or higher on niacin therapy.36
Therefore, the beneficial effect of niacin of reducing the rate of recurrent nonfatal myocardial infarction and coronary heart disease events was not significantly diminished when impaired fasting glucose or diabetes was present when therapy was started or by on-therapy increases from baseline fasting plasma glucose.
In addition, on-therapy changes in glycemic control may be dose-related and minimized by surveillance and therapy adjustments. The Assessment of Diabetes Control and Evaluation of the Efficacy of Niaspan Trial (ADVENT)38 found that changes in glycemic control were minimal as measured by fasting glucose and hemoglobin A1c; were associated with a higher niacin dose (1.5 g/day vs 1 g/day); and, when present, were successfully managed by adjusting the diabetes treatment regimen.
Antihypertensive drugs
Diuretics as well as beta-blockers have been reported to increase the incidence of diabetes in patients with hypertension.15,38–40
A retrospective longitudinal cohort study40 in 2009 examined the development of new-onset diabetes (defined as a new ICD-9 code for diabetes or initiation of diabetes treatment) in 24,688 treated hypertensive patients without diabetes at baseline; 4,385 (17.8%) of the patients developed diabetes. After adjusting for sex and age, the risk of new diabetes was significant in users of diuretics (OR 1.10), beta-blockers (OR 1.12), and calcium channel blockers (OR 1.10) compared with users of angiotensin-converting enzyme inhibitors, (OR 0.92), angiotensin receptor blockers (OR 0.90), or alpha-blockers (OR 0.88).
However, the increase in blood glucose does not seem to attenuate the beneficial effects of reducing cardiovascular events. In the Antihypertensive and Lipid-lowering Treatment to Prevent Heart Attack Trial (ALLHAT),15 a long-term follow-up of those developing new-onset diabetes while taking chlorthalidone (Hygroton) found no difference in the risk of death from cardiovascular disease or from any cause (hazard ratio = 1.04).15
CLINICAL IMPLICATIONS
Balancing the benefits and risks of statins
It is important to examine how the 0.4% increase in absolute risk of new-onset diabetes as calculated in meta-analyses compares with the benefits of statin treatment in terms of cardiovascular risk reduction.
Using data from the Cholesterol Treatment Trialists (CTT) meta-analysis of statin trials in 71,370 participants, Sattar et al11 estimated that statin treatment is associated with 5.4 fewer deaths from coronary heart disease and cases of nonfatal myocardial infarction per 255 patients treated over 4 years for each 1-mmol/L (39 mg/dL) reduction in LDL-C compared with controls. The benefit would be even greater if stroke, revascularization, and hospitalization are included as end points. This benefit is contrasted with the risk of developing 1 additional case of diabetes for every 255 patients treated with statins over the same period.
Preiss et al25 calculated that there were 2 more cases of diabetes per 1,000 patient-years in patients receiving intensive doses than in those receiving moderate doses (18.9 vs 16.9), corresponding to 1 additional case of diabetes for every 498 patients treated per year. However, there were 6.5 fewer first major cardiovascular events per 1,000 patient-years (44.5 vs 51.0), corresponding to a number needed to treat per year to prevent 1 cardiovascular event of 155. Most of the benefit was due to fewer revascularizations, followed by nonfatal myocardial infarctions. The 12% increase in new diabetes with high-dose therapy contrasted with a 16% reduction in new cardiovascular disease combined events (OR 0.84, 95% CI 0.75–0.94).
As previously noted, in the JUPITER trial, the benefits of preventing cardiovascular events with statin therapy outweighed the risk of new diabetes in people both with and without baseline risk factors for diabetes.29 Similar to the observations with niacin and some antihypertensive drugs, the increase in blood glucose with statins does not appear to reduce the benefits of cardiovascular risk reduction in these patients at moderate to high risk, even when used at high doses.
People with diabetes need aggressive lipid-lowering—with statins
Diabetes is a coronary heart disease risk equivalent and is associated with high risk of cardiovascular events.41–46 Overall, the risk for these adverse events is two to four times greater in people with diabetes than without. Atherosclerosis-related events account for approximately 65% to 75% of all deaths in people with diabetes, and 75% of these events are coronary. Lipid abnormalities are strongly correlated with the risk of cardiovascular disease in people with diabetes, and aggressive treatment of risk factors, particularly lipid abnormalities, has been shown to reduce this risk.47–49 And data from multiple clinical trials support the use of statins to lower LDL-C as the first-line therapy for dyslipidemia in people with diabetes, just as it is in the general population.3–7,9,13,23,50–61
Analyses of diabetic subgroups encompassing 18,000 to 20,000 patients in the large statin trials have clearly demonstrated the benefits of statin therapy. A recent metaanalysis of 10 placebo-controlled trials that included approximately 16,000 patients with diabetes and 54,000 without diabetes demonstrated a 30% reduction in coronary heart disease, a 19% reduction in strokes, and a 12% reduction in mortality.54 Furthermore, in another meta-analysis of 14 trials, a similar 22% reduction in coronary heart disease was noted in people with diabetes whether or not they had a history of cardiovascular disease.55
Therefore, aggressive treatment of lipid abnormalities with statins as primary treatment has generally been adopted as a standard of care in diabetic patients, particularly those with clinical cardiovascular disease or one or more risk factors. The Adult Treatment Panel III guidelines recommend a minimum LDL-C goal of less than 100 mg/dL and a goal of less than 70 mg/dL as an option for patients with diabetes (Table 1).41,62 Similar recommendations have been issued by the American Diabetes Association together with the American College of Cardiology (Table 2),30 the American Diabetes Association by itself,63 and the American Academy of Pediatrics.6
Is new-onset diabetes as dangerous as established diabetes?
In studies to date, there did not appear to be more events in those who developed new-onset diabetes.
Waters et al,24 evaluating three trials of high-dose atorvastatin therapy, found that major cardiovascular events occurred in 11.3% of those with new-onset diabetes, 10.8% of those without new-onset diabetes (HR 1.02, 95% CI 0.77–1.35), and 17.5% of those who had diabetes at baseline.
Therefore, it may not be appropriate to extrapolate the glucose changes seen on statin therapy to an equivalent increase in adverse cardiovascular events as seen in other diabetic patients. The beneficial reduction in cardiovascular events does not appear to be diminished in those developing diabetes. It is not clear that the increase in glucose on statins has the same implications of a new diagnosis of diabetes. Does this elevation in glucose represent true diabetes or some downstream effect? For example, thiazide diuretics have been known to increase blood glucose levels, but the levels drop when these drugs are discontinued, even after many years of treatment.
On the other hand, it is possible that follow-up of 5 years or less in clinical trials has not allowed sufficient time to examine the influence of the increase in new-onset diabetes on future cardiovascular events. In addition, because of the widespread use of statins across a broad range of cardiovascular risk, even if the effect is small in absolute terms, the potential adverse effects are magnified, particularly in a low-risk population in which the cardiovascular benefits are smaller.
The association is real, but questions remain
In view of the evidence, it is difficult to refute that an association exits between statin use and new-onset diabetes, at least in some subgroups. The dose response noted in some studies further reinforces the conclusion that the association is real. However, many questions remain unanswered regarding mechanism of effect, whether there are differences depending on the particular statin or dose used, or differential effects in the populations treated (such as patients with metabolic syndrome or the elderly).
Until the contradictory observations can be resolved and plausible mechanisms of action elucidated, causality cannot be established. From a clinical standpoint there is no current evidence suggesting that the elevations in blood glucose seen while on lipid-lowering or blood-pressure-lowering therapy are associated with an increased risk of cardiovascular events or that they attenuate the beneficial effects of the therapy.
Statins should continue to be used in patients at high risk
Until further studies are done, statins should continue to be used, after assessing the risks and the benefits.
Primary prevention patients at moderate to high risk and secondary prevention patients stand to gain from statin therapy, and it should not be denied or doses reduced on the basis of concerns about the development of new-onset diabetes. The recognized modest risk of developing diabetes does not appear to blunt the cardioprotective effects of statin therapy in these moderate-to high-risk groups.
Rather than stop statins in patients at risk of diabetes such as the elderly or those with prediabetes, insulin resistance, or metabolic syndrome who are on therapy for appropriate reasons, it is reasonable to continue these drugs, monitoring glucose more closely and emphasizing the importance of weight reduction, diet, and aerobic exercise for preventing diabetes. The Diabetes Prevention Program Research Group, for example, reduced the incidence of diabetes by 58% over 2.8 years of follow-up with intensive lifestyle interventions (a low-calorie, low-fat diet plus moderate physical activity 150 minutes per week) vs usual care in at-risk populations.65
Should statins be used more cautiously in patients at lower risk?
The most recent Cholesterol Treatment Trialists meta-analysis of 27 randomized clinical trials (22 placebo-controlled, 134,537 people; 5 high-dose vs low-dose, 39,612 people) reported that reducing LDL-C with statins lowered cardiovascular risk even in low-risk patients.66 Overall, there were 21% fewer major cardiovascular events (coronary heart disease, stroke, or coronary revascularization) for every 1-mmol/L reduction in LDL-C.
The proportional reduction in events was at least as large in the two lowest-risk groups (estimated 5-year risk of < 5% and 5% to < 10%, 53,152 people) as in the higher-risk groups. This was reflected mainly in fewer nonfatal myocardial infarctions and coronary revascularizations. In these groups, the absolute reduction in risk for each 1-mmol/L reduction in LDL-C was 11 per 1,000 patients over 5 years. Even in this low-risk population, the reduction in cardiovascular risk seems to compare favorably with the small estimated increase risk of diabetes.
However, even in the lowest-risk group studied, the average baseline LDL-C level was greater than 130 mg/dL.
Therefore, in groups in which the benefits of statins on cardiovascular risk reduction are less robust (eg, low-risk primary prevention groups without significant elevations in LDLC, particularly the elderly), it would not be difficult to justify the case for more cautious use of statin therapy. If statins are used in these low-risk groups, restricting their use to those with at least moderate LDL-C elevation, using less aggressive LDL-C-lowering targets, and regular monitoring of fasting glucose seem reasonable until further information is available.
On february 28, 2012, the US Food and Drug Administration (FDA) updated its labeling requirements for statins. In addition to revising its recommendations for monitoring liver function and its alerts about reports of memory loss, the FDA also warned of the possibility of new-onset diabetes mellitus and worse glycemic control in patients taking statin drugs.1
This change stoked an ongoing debate about the risk of diabetes with statin use and the implications of such an effect. To understand the clinical consequences of this alert and its effect on treatment decisions, we need to consider the degree to which statins lower the risk of cardiovascular disease in patients at high risk (including diabetic patients), the magnitude of the risk of developing new diabetes while on statin therapy, and the ratio of risk to benefit in treated populations.
This review will discuss the evidence for this possible adverse effect and the implications for clinical practice.
DO STATINS CAUSE DIABETES?
Individual controlled trials dating back more than a decade have had conflicting results about new diabetes and poorer diabetic control in patients taking statins.
The West of Scotland Coronary Prevention Study (WOSCOPS)2 suggested that the incidence of diabetes was 30% lower in patients taking pravastatin (Pravachol) 40 mg/day than with placebo. However, this was not observed with atorvastatin (Lipitor) 10 mg/day in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid-Lowering Arm (ASCOT-LLA)3 in hypertensive patients or in the Collaborative Atorvastatin Diabetes Study (CARDS)4 in diabetic patients,4 nor was it noted with simvastatin (Zocor) 40 mg/day in the Heart Protection Study (HPS).5
The Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER),6 using the more potent agent rosuvastatin (Crestor) 20 mg/day in patients with elevated levels of C-reactive protein (CRP), was stopped early when an interim analysis found a 44% lower incidence of the primary end point. However, the trial also reported a 26% higher incidence of diabetes in follow-up of less than 2 years.
In the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER),7 with a mean age at entry of 75, there was a 32% higher incidence of diabetes with pravastatin therapy.7
Results of meta-analyses
Several meta-analyses have addressed these differences.
Rajpathak et al8 performed a meta-analysis, published in 2009, of six trials—WOSCOPS,2 ASCOT-LLA,3 JUPITER,6 HPS,5 the Long-term Intervention With Pravastatin in Ischaemic Disease (LIPID) study,9 and the Controlled Rosuvastatin Multinational Study in Heart Failure (CORONA),10 with a total of 57,593 patients. They calculated that the incidence of diabetes was 13% higher (an absolute difference of 0.5%) in statin recipients, which was statistically significant. In their initial analysis, the authors excluded WOSCOPS, describing it as hypothesis-generating. The relative increase in risk was less—6%—and was not statistically significant when WOSCOPS was included.
Sattar et al,11 in a larger meta-analysis published in 2010, included 91,140 participants in 13 major statin trials conducted between 1994 and 2009; each trial had more than 1,000 patients and more than 1 year of follow-up.2,3,5–7,9,10,12–17 New diabetes was defined as physician reporting of new diabetes, new diabetic medication use, or a fasting glucose greater than 7 mmol/L (126 mg/dL).
New diabetes occurred in 2,226 (4.89%) of the statin recipients and in 2,052 (4.5%) of the placebo recipients, an absolute difference of 0.39%, or 9% more (odds ratio [OR] 1.09; 95% confidence interval [CI] 1.02–1.17) (Figure 1).
The incidence of diabetes varied substantially among the 13 trials, with only JUPITER6 and PROSPER7 finding statistically significant increases in rates (26% and 32%, respectively). Of the other 11 trials, 4 had nonsignificant trends toward lower incidence,2,9,13,17 while the 7 others had nonsignificant trends toward higher incidence.
Does the specific statin make a difference?
Questions have been raised as to whether the type of statin used, the intensity of therapy, or the population studied contributed to these differences. Various studies suggest that factors such as using hydrophilic vs lipophilic statins (hydrophilic statins include pravastatin and rosuvastatin; lipophilic statins include atorvastatin, lovastatin, and simvastatin), the dose, the extent of lowering of low-density lipoprotein cholesterol (LDL-C), and the age or clinical characteristics of the population studied may influence this relationship.18–20
Yamakawa et al18 examined the effect of atorvastatin 10 mg/day, pravastatin 10 mg/day, and pitavastatin (Livalo) 2 mg/day on glycemic control over 3 months in a retrospective analysis. Random blood glucose and hemoglobin A1c levels were increased in the atorvastatin group but not in the other two.18
A prospective comparison of atorvastatin 20 mg vs pitavastatin 4 mg in patients with type 2 diabetes, presented at the American College of Cardiology’s 2011 annual meeting, reported a significant increase in fasting glucose levels with atorvastatin, particularly in women, but not with pitavastatin.19
In the Compare the Effect of Rosuvastatin With Atorvastatin on Apo B/Apo A-1 Ratio in Patients With Type 2 Diabetes Mellitus and Dyslipidaemia (CORALL) study,20 both high-dose rosuvastatin (40 mg) and high-dose atorvastatin (80 mg) were associated with significant increases in hemoglobin A1c, although the mean fasting glucose levels were not significantly different at 18 weeks of therapy.
A meta-analysis by Sattar et al11 did not find a clear difference between lipophilic statins (OR 1.10 vs placebo) and hydrophilic statins (OR 1.08). In analysis by statin type, the combined rosuvastatin trials were statistically significant in favor of a higher diabetes risk (OR 1.18, 95% CI 1.04–1.44). Nonsignificant trends were noted for atorvastatin trials (OR 1.14) and simvastatin trials (OR 1.11) and less so for pravastatin (OR 1.03); the OR for lovastatin was 0.98. This may suggest that there is a stronger effect with more potent statins or with greater lowering of LDL-C.
Meta-regression analysis in this study demonstrated that diabetes risk with statins was higher in older patients but was not influenced by body mass index or by the extent that LDL-C was lowered.
Statin dose as a risk factor
Intensive-dose statin therapy has been shown to reduce cardiovascular risk more than low-dose or moderate-dose therapy, thus supporting more aggressive treatment of LDL-C in higher-risk patients. However, some controlled studies comparing more-potent with less-potent statin regimens suggest that there may also be a higher risk of incident diabetes at higher doses.21–24
In a post hoc analysis of the Pravastatin or Atorvastatin Evaluation and Infection Therapy– Thrombolysis in Myocardial Infarction 22 (PROVE-IT TIMI 22) trial,21 patients who had experienced an acute coronary syndrome had a greater increase in hemoglobin A1c if treated with atorvastatin 80 mg/day than with pravastatin 40 mg/day.
Waters et al23 reported a higher risk of new diabetes with atorvastatin 80 mg than with placebo and a trend toward a higher risk with atorvastatin 80 mg than with atorvastatin 10 mg or simvastatin 20 mg.
In contrast, a review by Yousef et al24 of the data from the Enhanced Feedback for Effective Cardiac Treatment (EFFECT) study did not find a higher diabetes risk with more intensive statin therapy based on the magnitude of LDL-C reduction. A propensity-matched examination of deaths, recurrent acute ischemic events, or new diabetes in patients previously hospitalized with myocardial infarction found no differences in these end points each year out to 5 years. The risk of diabetes was in fact lower (but the difference was not statistically significant) in the high-dose groups out to 5 years. The risk of myocardial infarction or death was numerically different in the high-dose groups, but the difference was not statistically significant.
Preiss et al25 in 2011 performed a meta-analysis of the impact of intensity of statin therapy on diabetes risk. They examined data from 32,752 participants without diabetes at baseline in five randomized controlled trials with more than 1,000 participants and more than 1 year of follow-up, comparing high-dose therapy against moderate-dose statin therapy.21,22,26–28 New diabetes was considered present if there was an adverse event report of diabetes, if glucose-lowering drugs were started, or if two fasting plasma glucose measurements were higher than 7 mmol/L (126 mg/dL).
Diabetes developed in 1,449 (8.8%) of the intensive-therapy group and 1,300 (8.0%) of the moderate-therapy group (OR 1.12, 95% CI 1.04–1.22). In contrast, incident cardiovascular disease occurred in 3,134 (19.1%) of the intensive-therapy group and 3,550 (21.7%) of the moderate-therapy group (OR 0.84, 95% CI 0.75–0.94). Therefore, there was an 0.8% absolute increase in diabetes cases on high-dose statins and a 2.6% absolute reduction in adverse cardiovascular events.
CAUTION IN INTERPRETING THESE DATA
There are many reasons for caution in interpreting these studies.
The trials were not designed to look for diabetes
The data supporting the relationship between statin therapy and higher risk of diabetes are primarily from observational studies. These studies were not prospectively designed to address this question, and we therefore need to view this as association and not as causation.
The definition of diabetes varied between trials, and new-onset diabetes was often not rigorously screened for. In many trials the outcome of diabetes was at least partially based on nonstandardized, nonadjudicated physician reporting.
Consequently, if statins reduce the risk of diabetes, the results from WOSCOPS may overstate the reduction, since this study used a non-standard definition of incident diabetes (fasting plasma glucose > 126 mg/dL plus a > 36 mg/dL increase from baseline). When Sattar et al11 reanalyzed WOSCOPS data using a more standard definition, they found a smaller effect.
On the other hand, nonstandardized physician reporting may overstate an adverse effect. Sattar et al11 also found that when fasting plasma glucose levels alone were used as the definition for diabetes, the overall risk was attenuated and was no longer statistically significant (OR 1.07, 95% CI 0.97–1.17).
Perhaps statin therapy uncovers diabetes only in people at risk of diabetes
Perhaps statin therapy uncovers diabetes only in people at higher baseline risk of developing diabetes. Therefore, this adverse effect may be restricted to certain groups and not applicable to the general population.
In JUPITER, one of the two trials in which, on independent analysis, statin use was associated with new diabetes, 77% of patients in the rosuvastatin group who developed diabetes had impaired fasting glucose at entry and therefore were at higher risk of developing diabetes.6
Possibly, the relationship is driven by preexisting metabolic syndrome or other risk factors for diabetes. In the two studies that reported a statistically significantly higher incidence of new diabetes, more than 40% of patients in JUPITER met the criteria for metabolic syndrome, and metabolic syndrome, which increases in prevalence with age, was likely more prominent in the elderly population in PROSPER.
Waters et al23 grouped patients according to whether they had risk factors for diabetes (impaired fasting glucose, obesity, elevated triglycerides, and hypertension) and found that those who had none or one of these risk factors had no difference in the rate of new-onset diabetes with either moderate or intensive statin therapy, but the risk was pronounced in those who had three or four risk factors.
Ridker et al29 reanalyzed the JUPITER data from patients who did not have cardiovascular disease at baseline. Overall, for every 54 new cases of diabetes in follow-up, 134 cardiovascular events or deaths were prevented. In subgroup analysis, those who had one or more risk factors for diabetes at baseline (metabolic syndrome, impaired fasting glucose, obesity, or hemoglobin A1c > 6%) had a 39% reduction in the primary end point and a 28% increase in new diabetes. Those who had none of these risk factors had a 52% lower rate of cardiovascular events but no increase in diabetes.
Other confounding factors
Bias and confounding factors are difficult to control for in studies without prospectively defined, recognized, and analyzed outcomes.
Although it may be a bit of a stretch, residual confounding factors such as myalgia side effects while on statins may reduce exercise in the statin-treatment groups. Perhaps a change to a healthier lifestyle after cardiovascular events may be more common in placebo groups. Improved survival with statins may allow more people at risk of diabetes to live longer and present with the diagnosis.30
POSSIBLE EXPLANATIONS, BUT NO UNIFYING MECHANISM
If mechanisms could be identified to explain the association between statins and diabetes, this would strengthen the argument that it is a cause-and-effect relationship. Many explanations have been proposed as to how statins may influence glucose metabolism and insulin sensitivity.31–34 These are possible explanations based on other observations.
In theory, statins may improve insulin sensitivity via their anti-inflammatory effect, since inflammatory markers and proinflammatory cytokines have been linked with insulin resistance. However, other effects of statins may adversely affect glycemic control.
In vivo analysis has shown that some but not all statins increase insulin levels and decrease insulin sensitivity in a dose-dependent fashion. Some statins decrease adiponectin and may worsen glycemic control through loss of adiponectin’s proposed protective anti-proliferative and antiangiogenic properties. In vitro studies and animal studies have demonstrated a decrease in expression of insulin-responsive glucose transporter 4 (GLUT4) with atorvastatin, and an increase in GLUT1. It has been hypothesized that reduction in isoprenoid biosynthesis or decreased insulin signaling may explain these effects and that changes in glucose transport in adipocytes may cause insulin resistance. Other studies suggest that dysregulation of cellular cholesterol may attenuate beta-cell function. Impaired biosynthesis of ubiquinones may result in delayed production of adenosine triphosphate and consequently diminish insulin release.
But different effects have been reported for atorvastatin, simvastatin, and pravastatin, arguing against a unifying explanation or, alternatively, suggesting that differences in lipophilicity and potency among statins are important. Hydrophilic statins may be less likely to be taken up by extrahepatic cells such as pancreatic cells and adipocytes, possibly lessening these effects. However, the strong association between rosuvastatin (which is hydrophilic) and new diabetes would not support this hypothesis.
Despite these speculations, lack of conformity in response to different statins and discrepancies in the clinical outcomes noted in trials fail to clearly identify a common causative mechanism.
OTHER COMMON THERAPIES MAY INFLUENCE GLYCEMIC CONTROL
Statins are not the first drugs for reducing cardiovascular risk that have been shown to affect glucose levels during treatment.
Niacin
Niacin has been known to increase glucose levels but has long been used as a treatment for dyslipidemia despite this caution. Reduced glycemic control during niacin treatment in diabetic patients does not seem to alter the beneficial effects of treatment.35–37
In a post hoc analysis of the Coronary Drug Project (CDP), in patient subgroups defined by baseline fasting plasma glucose and compared with placebo, niacin reduced the 6-year risk of recurrent myocardial infarction and the combined end point of coronary heart disease death or nonfatal myocardial infarction similarly (interactive P value nonsignificant) across all levels of baseline fasting plasma glucose, including levels of 126 mg/dL or higher at study entry.36
In another post hoc analysis of CDP patient subgroups defined by the change in glycemic status from baseline to 1 year, niacin reduced the 6-year risk of the same end points similarly (interactive P value nonsignificant) across all levels of change in fasting plasma glucose from baseline to year 1, whether baseline fasting plasma glucose levels decreased, stayed the same, or increased to 10 mg/dL or higher on niacin therapy.36
Therefore, the beneficial effect of niacin of reducing the rate of recurrent nonfatal myocardial infarction and coronary heart disease events was not significantly diminished when impaired fasting glucose or diabetes was present when therapy was started or by on-therapy increases from baseline fasting plasma glucose.
In addition, on-therapy changes in glycemic control may be dose-related and minimized by surveillance and therapy adjustments. The Assessment of Diabetes Control and Evaluation of the Efficacy of Niaspan Trial (ADVENT)38 found that changes in glycemic control were minimal as measured by fasting glucose and hemoglobin A1c; were associated with a higher niacin dose (1.5 g/day vs 1 g/day); and, when present, were successfully managed by adjusting the diabetes treatment regimen.
Antihypertensive drugs
Diuretics as well as beta-blockers have been reported to increase the incidence of diabetes in patients with hypertension.15,38–40
A retrospective longitudinal cohort study40 in 2009 examined the development of new-onset diabetes (defined as a new ICD-9 code for diabetes or initiation of diabetes treatment) in 24,688 treated hypertensive patients without diabetes at baseline; 4,385 (17.8%) of the patients developed diabetes. After adjusting for sex and age, the risk of new diabetes was significant in users of diuretics (OR 1.10), beta-blockers (OR 1.12), and calcium channel blockers (OR 1.10) compared with users of angiotensin-converting enzyme inhibitors, (OR 0.92), angiotensin receptor blockers (OR 0.90), or alpha-blockers (OR 0.88).
However, the increase in blood glucose does not seem to attenuate the beneficial effects of reducing cardiovascular events. In the Antihypertensive and Lipid-lowering Treatment to Prevent Heart Attack Trial (ALLHAT),15 a long-term follow-up of those developing new-onset diabetes while taking chlorthalidone (Hygroton) found no difference in the risk of death from cardiovascular disease or from any cause (hazard ratio = 1.04).15
CLINICAL IMPLICATIONS
Balancing the benefits and risks of statins
It is important to examine how the 0.4% increase in absolute risk of new-onset diabetes as calculated in meta-analyses compares with the benefits of statin treatment in terms of cardiovascular risk reduction.
Using data from the Cholesterol Treatment Trialists (CTT) meta-analysis of statin trials in 71,370 participants, Sattar et al11 estimated that statin treatment is associated with 5.4 fewer deaths from coronary heart disease and cases of nonfatal myocardial infarction per 255 patients treated over 4 years for each 1-mmol/L (39 mg/dL) reduction in LDL-C compared with controls. The benefit would be even greater if stroke, revascularization, and hospitalization are included as end points. This benefit is contrasted with the risk of developing 1 additional case of diabetes for every 255 patients treated with statins over the same period.
Preiss et al25 calculated that there were 2 more cases of diabetes per 1,000 patient-years in patients receiving intensive doses than in those receiving moderate doses (18.9 vs 16.9), corresponding to 1 additional case of diabetes for every 498 patients treated per year. However, there were 6.5 fewer first major cardiovascular events per 1,000 patient-years (44.5 vs 51.0), corresponding to a number needed to treat per year to prevent 1 cardiovascular event of 155. Most of the benefit was due to fewer revascularizations, followed by nonfatal myocardial infarctions. The 12% increase in new diabetes with high-dose therapy contrasted with a 16% reduction in new cardiovascular disease combined events (OR 0.84, 95% CI 0.75–0.94).
As previously noted, in the JUPITER trial, the benefits of preventing cardiovascular events with statin therapy outweighed the risk of new diabetes in people both with and without baseline risk factors for diabetes.29 Similar to the observations with niacin and some antihypertensive drugs, the increase in blood glucose with statins does not appear to reduce the benefits of cardiovascular risk reduction in these patients at moderate to high risk, even when used at high doses.
People with diabetes need aggressive lipid-lowering—with statins
Diabetes is a coronary heart disease risk equivalent and is associated with high risk of cardiovascular events.41–46 Overall, the risk for these adverse events is two to four times greater in people with diabetes than without. Atherosclerosis-related events account for approximately 65% to 75% of all deaths in people with diabetes, and 75% of these events are coronary. Lipid abnormalities are strongly correlated with the risk of cardiovascular disease in people with diabetes, and aggressive treatment of risk factors, particularly lipid abnormalities, has been shown to reduce this risk.47–49 And data from multiple clinical trials support the use of statins to lower LDL-C as the first-line therapy for dyslipidemia in people with diabetes, just as it is in the general population.3–7,9,13,23,50–61
Analyses of diabetic subgroups encompassing 18,000 to 20,000 patients in the large statin trials have clearly demonstrated the benefits of statin therapy. A recent metaanalysis of 10 placebo-controlled trials that included approximately 16,000 patients with diabetes and 54,000 without diabetes demonstrated a 30% reduction in coronary heart disease, a 19% reduction in strokes, and a 12% reduction in mortality.54 Furthermore, in another meta-analysis of 14 trials, a similar 22% reduction in coronary heart disease was noted in people with diabetes whether or not they had a history of cardiovascular disease.55
Therefore, aggressive treatment of lipid abnormalities with statins as primary treatment has generally been adopted as a standard of care in diabetic patients, particularly those with clinical cardiovascular disease or one or more risk factors. The Adult Treatment Panel III guidelines recommend a minimum LDL-C goal of less than 100 mg/dL and a goal of less than 70 mg/dL as an option for patients with diabetes (Table 1).41,62 Similar recommendations have been issued by the American Diabetes Association together with the American College of Cardiology (Table 2),30 the American Diabetes Association by itself,63 and the American Academy of Pediatrics.6
Is new-onset diabetes as dangerous as established diabetes?
In studies to date, there did not appear to be more events in those who developed new-onset diabetes.
Waters et al,24 evaluating three trials of high-dose atorvastatin therapy, found that major cardiovascular events occurred in 11.3% of those with new-onset diabetes, 10.8% of those without new-onset diabetes (HR 1.02, 95% CI 0.77–1.35), and 17.5% of those who had diabetes at baseline.
Therefore, it may not be appropriate to extrapolate the glucose changes seen on statin therapy to an equivalent increase in adverse cardiovascular events as seen in other diabetic patients. The beneficial reduction in cardiovascular events does not appear to be diminished in those developing diabetes. It is not clear that the increase in glucose on statins has the same implications of a new diagnosis of diabetes. Does this elevation in glucose represent true diabetes or some downstream effect? For example, thiazide diuretics have been known to increase blood glucose levels, but the levels drop when these drugs are discontinued, even after many years of treatment.
On the other hand, it is possible that follow-up of 5 years or less in clinical trials has not allowed sufficient time to examine the influence of the increase in new-onset diabetes on future cardiovascular events. In addition, because of the widespread use of statins across a broad range of cardiovascular risk, even if the effect is small in absolute terms, the potential adverse effects are magnified, particularly in a low-risk population in which the cardiovascular benefits are smaller.
The association is real, but questions remain
In view of the evidence, it is difficult to refute that an association exits between statin use and new-onset diabetes, at least in some subgroups. The dose response noted in some studies further reinforces the conclusion that the association is real. However, many questions remain unanswered regarding mechanism of effect, whether there are differences depending on the particular statin or dose used, or differential effects in the populations treated (such as patients with metabolic syndrome or the elderly).
Until the contradictory observations can be resolved and plausible mechanisms of action elucidated, causality cannot be established. From a clinical standpoint there is no current evidence suggesting that the elevations in blood glucose seen while on lipid-lowering or blood-pressure-lowering therapy are associated with an increased risk of cardiovascular events or that they attenuate the beneficial effects of the therapy.
Statins should continue to be used in patients at high risk
Until further studies are done, statins should continue to be used, after assessing the risks and the benefits.
Primary prevention patients at moderate to high risk and secondary prevention patients stand to gain from statin therapy, and it should not be denied or doses reduced on the basis of concerns about the development of new-onset diabetes. The recognized modest risk of developing diabetes does not appear to blunt the cardioprotective effects of statin therapy in these moderate-to high-risk groups.
Rather than stop statins in patients at risk of diabetes such as the elderly or those with prediabetes, insulin resistance, or metabolic syndrome who are on therapy for appropriate reasons, it is reasonable to continue these drugs, monitoring glucose more closely and emphasizing the importance of weight reduction, diet, and aerobic exercise for preventing diabetes. The Diabetes Prevention Program Research Group, for example, reduced the incidence of diabetes by 58% over 2.8 years of follow-up with intensive lifestyle interventions (a low-calorie, low-fat diet plus moderate physical activity 150 minutes per week) vs usual care in at-risk populations.65
Should statins be used more cautiously in patients at lower risk?
The most recent Cholesterol Treatment Trialists meta-analysis of 27 randomized clinical trials (22 placebo-controlled, 134,537 people; 5 high-dose vs low-dose, 39,612 people) reported that reducing LDL-C with statins lowered cardiovascular risk even in low-risk patients.66 Overall, there were 21% fewer major cardiovascular events (coronary heart disease, stroke, or coronary revascularization) for every 1-mmol/L reduction in LDL-C.
The proportional reduction in events was at least as large in the two lowest-risk groups (estimated 5-year risk of < 5% and 5% to < 10%, 53,152 people) as in the higher-risk groups. This was reflected mainly in fewer nonfatal myocardial infarctions and coronary revascularizations. In these groups, the absolute reduction in risk for each 1-mmol/L reduction in LDL-C was 11 per 1,000 patients over 5 years. Even in this low-risk population, the reduction in cardiovascular risk seems to compare favorably with the small estimated increase risk of diabetes.
However, even in the lowest-risk group studied, the average baseline LDL-C level was greater than 130 mg/dL.
Therefore, in groups in which the benefits of statins on cardiovascular risk reduction are less robust (eg, low-risk primary prevention groups without significant elevations in LDLC, particularly the elderly), it would not be difficult to justify the case for more cautious use of statin therapy. If statins are used in these low-risk groups, restricting their use to those with at least moderate LDL-C elevation, using less aggressive LDL-C-lowering targets, and regular monitoring of fasting glucose seem reasonable until further information is available.
- US Food and Drug Administration. Statin drugs—drug safety communication: class labeling change. February 28, 2012. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm293670.htm.
- Freeman DJ, Norrie J, Sattar N, et al. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation 2001; 103:357–362.
- Sever PS, Dahlof B, Poulter NR, et al; ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003; 361:1149–1158.
- Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–696.
- Collins R, Armitage J, Parish S, Sleigh P, Peto R; for the Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 2003; 361:2005–2016.
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER Study Group. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002; 360:1623–1630.
- Rajpathak SN, Kumbhani DJ, Crandall J, Barzilai N, Alderman M, Ridker PM. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care 2009; 32:1924–1929.
- Keech A, Colquhoun D, Best J, et al. Secondary prevention of cardiovascular events with long-term pravastatin in patients with diabetes or impaired fasting glucose—results from the LIPID trial. Diabetes Care 2003; 26:2713–2721.
- Kjekshus J, Apetrei E, Barrios V, et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med 2007; 357:2248–2261.
- Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 2010; 375:735–742.
- Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet 2006; 368:1155–1163.
- Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998; 279:1615–1622.
- Scandinavian Simvastatin Survival Study study group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- Barzilay JI, Davis BR, Pressel SL, et al; ALLHAT Collaborative Research Group. Long-term effects of incident diabetes mellitus on cardiovascular outcomes in people treated for hypertension: the ALLHAT Diabetes Extension Study. Circ Cardiovasc Qual Outcomes 2012; 5:153–162.
- Tavazzi L, Maggioni AP, Marchioli R, et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 2008; 372:1231–1239.
- GISSI Prevenzione Investigators (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico). Results of the low-dose (20 mg) pravastatin GISSI Prevenzione trial in 4271 patients with recent myocardial infarction: do stopped trials contribute to overall knowledge? Ital Heart J 2000; 1:810–820.
- Yamakawa T, Takano T, Tanaka S, Kadonosono K, Terauchi Y. Influence of pitavastatin on glucose tolerance in patients with type 2 diabetes mellitus. J Atheroscler Thromb 2008; 15:269–275.
- Kryzhanovski V, Gumprecht J, Zhu B, Yu CY, Hounslow N, Sponseller CA. Atorvastatin but not pitavastatin significantly increases fasting plasma glucose in patients with type 2 diabetes and combined dyslipidemia (abstract). J Am Coll Cardiol 2011; 57:E575.
- Simsek S, Schalkwijk CG, Wolffenbuttel BH. Effects of rosuvastatin and atorvastatin on glycaemic control in type 2 diabetes—the CORALL study. Diabet Med 2012; 29:628–631.
- Sabatine MS, Morrow DA, Giugliano RP, et al. Implications of upstream glycoprotein IIb/IIIa inhibition and coronary artery stenting in the invasive management of unstable angina/non-ST-elevation myocardial infarction: a comparison of the Thrombolysis In Myocardial Infarction (TIMI) IIIB trial and the Treat angina with Aggrastat and determine Cost of Therapy with Invasive or Conservative Strategy (TACTICS)-TIMI 18 trial. Circulation 2004; 110(suppl III):834–880.
- Shepherd J, Barter P, Carmena R, et al. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the Treating to New Targets (TNT) study. Diabetes Care 2006; 29:1220–1226.
- Waters DD, Ho JE, DeMicco DA, et al. Predictors of new-onset diabetes in patients treated with atorvastatin: results from 3 large randomized clinical trials. J Am Coll Cardiol 2011; 57:1535–1545.
- Yousef A, Tu JV, Wang J, Donovan L, Ko DT. The association of intensive statin therapy on long-term risks of cardiovascular events and diabetes following acute myocardial infarction (abstract). Circulation 2012; 125:e859.
- Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a metaanalysis. JAMA 2011; 305:2556–2564.
- de Lemos JA, Blazing MA, Wiviott SD, et al; A to Z Investigators. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA 2004; 292:1307–1316.
- Pedersen TR, Faegeman O, Kastelein JJ, et al; Incremental Decrease in End Points Through Aggressive Lipid Lowering (IDEAL) Study Group. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005; 294:2437–2445.
- Armitage J, Bowman L, Wallendszus K, et al; Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double-blind randomised trial. Lancet 2010; 37:1658–1669.
- Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet 2012; 380:565–571.
- Brunzell JD, Davidson M, Furberg CD, et al; American Diabetes Association; American College of Cardiology Foundation. Lipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care 2008; 31:811–822.
- Koh KK, Quon MJ, Han SH, Lee Y, Kim SJ, Shin EK. Atorvastatin causes insulin resistance and increases ambient glycemia in hypercholesterolemic patients. J Am Coll Cardiol 2010; 55:1209–1216.
- Koh KK, Quon MJ, Han SH, et al. Differential metabolic effects of pravastatin and simvastatin in hypercholesterolemic patients. Atherosclerosis 2009; 204:483–490.
- Nakata M, Nagasaka S, Kusaka I, Matsuoka H, Ishibashi S, Yada T. Effects of statins on the adipocyte maturation and expression of glucose transporter 4 (SLC2A4): implications in glycaemic control. Diabetologia 2006; 49:1881–1892.
- Yada T, Nakata M, Shiraishi T, Kakei M. Inhibition by simvastatin, but not pravastatin, of glucose-induced cytosolic Ca2+ signalling and insulin secretion due to blockade of L-type Ca2+ channels in rat islet beta-cells. Br J Pharmacol 1999; 126:1205–1213.
- Guyton JR, Fazio S, Adewale AJ, et al. Effect of extended-release niacin on new-onset diabetes among hyperlipidemic patients treated with ezetimibe/simvastatin in a randomized controlled trial. Diabetes Care 2012; 35:857–860.
- Canner PL, Furberg CD, Terrin ML, McGovern ME. Benefits of niacin by glycemic status in patients with healed myocardial infarction (from the Coronary Drug Project). Am J Cardiol 2005; 95:254–257.
- Grundy SM, Vega GL, McGovern ME, et al; Diabetes Multicenter Research Group. Efficacy, safety, and tolerability of once-daily niacin for the treatment of dyslipidemia associated with type 2 diabetes: results of the Assessment of Diabetes Control and Evaluation of the Efficacy of Niaspan Trial. Arch Intern Med 2002; 162:1568–1576.
- Gupta AK, Dahlof B, Dobson J, Sever PS, Wedel H, Poulter NRAnglo-Scandinavian Cardiac Outcomes Trial Investigators. Determinants of new-onset diabetes among 19,257 hypertensive patients randomized in the Anglo-Scandinavian Cardiac Outcomes Trial—Blood Pressure Lowering Arm and the relative influence of antihypertensive medication. Diabetes Care 2008; 31:982–988.
- Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet 2007; 369:201–207.
- Jong JP, Chang MH, Tien L, et al. Antihypertensive drugs and new-onset diabetes: a retrospective longitudinal cohort study. Cardiovasc Ther 2009; 27:159–163.
- Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421.
- Norhammar A, Tenerz A, Nilsson G, et al. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study Lancet 2002; 359:2140–2144.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Sprafka JM, Burke GL, Folsom AR, McGovbern PG, Hahn LP. Trends in prevalence of diabetes mellitus in patients with myocardial infarction and effect of diabetes on survival. The Minnesota Heart Survey. Diabetes Care 1991; 14:537–543.
- Geiss LS, Herman WH, Smith PJ. Mortality in non-insulin-dependent diabetes. In:Harris MI, Cowie CC, Stern MP, et al, editors. Diabetes in America. 2nd ed. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 1995:233–257.
- Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 1993; 16:434–444.
- Turner RC, Millns H, Neil HA, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ 1998; 316:823–828.
- Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003; 348:383–393.
- Gaede P, Pederson O. Intensive integrated therapy of type 2 diabetes: implications for long-term prognosis. Diabetes 2004; 53:S39–S47.
- Goldberg RB, Mellies MJ, Sacks FM, et al. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the cholesterol and recurrent events (CARE) trial. The Care Investigators. Circulation 1998; 98:2513–2519.
- Pyðrälä K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care 1997; 20:614–620.
- Vijan S, Hayward RA; American College of Physicians. Pharmacologic lipid-lowering therapy in type 2 diabetes mellitus: background paper for the American College of Physicians. Ann Intern Med 2004; 140:650–658.
- Baigent C, Keech A, Kearney PM, et al; Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278. Errata in Lancet 2008; 371:2084, Lancet 2005; 366:1358.
- Brugts JJ, Yetgin T, Hoeks SE, et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ 2009; 338:b2376.
- Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Baigent C; Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008; 371:117–125.
- Nissen SE, Nicholls SJ, Sipahi I, et al; ASTEROID Investigators. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006; 295:1556–1565.
- Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes et al. N Engl J Med 2004; 350:1495–1504.
- LaRosa JC, Grundy SM, Waters DD, et al; Treating to New Targets (TNT) Investigators. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005; 352:1425–1435.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010; 376:1670–1681.
- LIPID Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med 1998; 339:1349–1357.
- Grundy SM, Cleeman JI, Bairey Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110:227–239.
- American Diabetes Association. Executive summary: standards of medical care in diabetes—2012. Diabetes Care 2012; 35(suppl 1):S5–S10.
- Daniels SR, Greer FR; Committee on Nutrition. Lipid screening and cardiovascular health in childhood. Pediatrics 2008; 122:198–208.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346:393–403.
- Cholesterol Treatment Trialists’ (CTT) Collaborators; Mihaylova B, Emberson J, Blackwell L, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012; 380:581–590.
- US Food and Drug Administration. Statin drugs—drug safety communication: class labeling change. February 28, 2012. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm293670.htm.
- Freeman DJ, Norrie J, Sattar N, et al. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation 2001; 103:357–362.
- Sever PS, Dahlof B, Poulter NR, et al; ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003; 361:1149–1158.
- Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–696.
- Collins R, Armitage J, Parish S, Sleigh P, Peto R; for the Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 2003; 361:2005–2016.
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER Study Group. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002; 360:1623–1630.
- Rajpathak SN, Kumbhani DJ, Crandall J, Barzilai N, Alderman M, Ridker PM. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care 2009; 32:1924–1929.
- Keech A, Colquhoun D, Best J, et al. Secondary prevention of cardiovascular events with long-term pravastatin in patients with diabetes or impaired fasting glucose—results from the LIPID trial. Diabetes Care 2003; 26:2713–2721.
- Kjekshus J, Apetrei E, Barrios V, et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med 2007; 357:2248–2261.
- Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 2010; 375:735–742.
- Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet 2006; 368:1155–1163.
- Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998; 279:1615–1622.
- Scandinavian Simvastatin Survival Study study group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- Barzilay JI, Davis BR, Pressel SL, et al; ALLHAT Collaborative Research Group. Long-term effects of incident diabetes mellitus on cardiovascular outcomes in people treated for hypertension: the ALLHAT Diabetes Extension Study. Circ Cardiovasc Qual Outcomes 2012; 5:153–162.
- Tavazzi L, Maggioni AP, Marchioli R, et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 2008; 372:1231–1239.
- GISSI Prevenzione Investigators (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico). Results of the low-dose (20 mg) pravastatin GISSI Prevenzione trial in 4271 patients with recent myocardial infarction: do stopped trials contribute to overall knowledge? Ital Heart J 2000; 1:810–820.
- Yamakawa T, Takano T, Tanaka S, Kadonosono K, Terauchi Y. Influence of pitavastatin on glucose tolerance in patients with type 2 diabetes mellitus. J Atheroscler Thromb 2008; 15:269–275.
- Kryzhanovski V, Gumprecht J, Zhu B, Yu CY, Hounslow N, Sponseller CA. Atorvastatin but not pitavastatin significantly increases fasting plasma glucose in patients with type 2 diabetes and combined dyslipidemia (abstract). J Am Coll Cardiol 2011; 57:E575.
- Simsek S, Schalkwijk CG, Wolffenbuttel BH. Effects of rosuvastatin and atorvastatin on glycaemic control in type 2 diabetes—the CORALL study. Diabet Med 2012; 29:628–631.
- Sabatine MS, Morrow DA, Giugliano RP, et al. Implications of upstream glycoprotein IIb/IIIa inhibition and coronary artery stenting in the invasive management of unstable angina/non-ST-elevation myocardial infarction: a comparison of the Thrombolysis In Myocardial Infarction (TIMI) IIIB trial and the Treat angina with Aggrastat and determine Cost of Therapy with Invasive or Conservative Strategy (TACTICS)-TIMI 18 trial. Circulation 2004; 110(suppl III):834–880.
- Shepherd J, Barter P, Carmena R, et al. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the Treating to New Targets (TNT) study. Diabetes Care 2006; 29:1220–1226.
- Waters DD, Ho JE, DeMicco DA, et al. Predictors of new-onset diabetes in patients treated with atorvastatin: results from 3 large randomized clinical trials. J Am Coll Cardiol 2011; 57:1535–1545.
- Yousef A, Tu JV, Wang J, Donovan L, Ko DT. The association of intensive statin therapy on long-term risks of cardiovascular events and diabetes following acute myocardial infarction (abstract). Circulation 2012; 125:e859.
- Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a metaanalysis. JAMA 2011; 305:2556–2564.
- de Lemos JA, Blazing MA, Wiviott SD, et al; A to Z Investigators. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA 2004; 292:1307–1316.
- Pedersen TR, Faegeman O, Kastelein JJ, et al; Incremental Decrease in End Points Through Aggressive Lipid Lowering (IDEAL) Study Group. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005; 294:2437–2445.
- Armitage J, Bowman L, Wallendszus K, et al; Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double-blind randomised trial. Lancet 2010; 37:1658–1669.
- Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet 2012; 380:565–571.
- Brunzell JD, Davidson M, Furberg CD, et al; American Diabetes Association; American College of Cardiology Foundation. Lipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care 2008; 31:811–822.
- Koh KK, Quon MJ, Han SH, Lee Y, Kim SJ, Shin EK. Atorvastatin causes insulin resistance and increases ambient glycemia in hypercholesterolemic patients. J Am Coll Cardiol 2010; 55:1209–1216.
- Koh KK, Quon MJ, Han SH, et al. Differential metabolic effects of pravastatin and simvastatin in hypercholesterolemic patients. Atherosclerosis 2009; 204:483–490.
- Nakata M, Nagasaka S, Kusaka I, Matsuoka H, Ishibashi S, Yada T. Effects of statins on the adipocyte maturation and expression of glucose transporter 4 (SLC2A4): implications in glycaemic control. Diabetologia 2006; 49:1881–1892.
- Yada T, Nakata M, Shiraishi T, Kakei M. Inhibition by simvastatin, but not pravastatin, of glucose-induced cytosolic Ca2+ signalling and insulin secretion due to blockade of L-type Ca2+ channels in rat islet beta-cells. Br J Pharmacol 1999; 126:1205–1213.
- Guyton JR, Fazio S, Adewale AJ, et al. Effect of extended-release niacin on new-onset diabetes among hyperlipidemic patients treated with ezetimibe/simvastatin in a randomized controlled trial. Diabetes Care 2012; 35:857–860.
- Canner PL, Furberg CD, Terrin ML, McGovern ME. Benefits of niacin by glycemic status in patients with healed myocardial infarction (from the Coronary Drug Project). Am J Cardiol 2005; 95:254–257.
- Grundy SM, Vega GL, McGovern ME, et al; Diabetes Multicenter Research Group. Efficacy, safety, and tolerability of once-daily niacin for the treatment of dyslipidemia associated with type 2 diabetes: results of the Assessment of Diabetes Control and Evaluation of the Efficacy of Niaspan Trial. Arch Intern Med 2002; 162:1568–1576.
- Gupta AK, Dahlof B, Dobson J, Sever PS, Wedel H, Poulter NRAnglo-Scandinavian Cardiac Outcomes Trial Investigators. Determinants of new-onset diabetes among 19,257 hypertensive patients randomized in the Anglo-Scandinavian Cardiac Outcomes Trial—Blood Pressure Lowering Arm and the relative influence of antihypertensive medication. Diabetes Care 2008; 31:982–988.
- Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet 2007; 369:201–207.
- Jong JP, Chang MH, Tien L, et al. Antihypertensive drugs and new-onset diabetes: a retrospective longitudinal cohort study. Cardiovasc Ther 2009; 27:159–163.
- Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421.
- Norhammar A, Tenerz A, Nilsson G, et al. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study Lancet 2002; 359:2140–2144.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Sprafka JM, Burke GL, Folsom AR, McGovbern PG, Hahn LP. Trends in prevalence of diabetes mellitus in patients with myocardial infarction and effect of diabetes on survival. The Minnesota Heart Survey. Diabetes Care 1991; 14:537–543.
- Geiss LS, Herman WH, Smith PJ. Mortality in non-insulin-dependent diabetes. In:Harris MI, Cowie CC, Stern MP, et al, editors. Diabetes in America. 2nd ed. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 1995:233–257.
- Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 1993; 16:434–444.
- Turner RC, Millns H, Neil HA, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ 1998; 316:823–828.
- Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003; 348:383–393.
- Gaede P, Pederson O. Intensive integrated therapy of type 2 diabetes: implications for long-term prognosis. Diabetes 2004; 53:S39–S47.
- Goldberg RB, Mellies MJ, Sacks FM, et al. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the cholesterol and recurrent events (CARE) trial. The Care Investigators. Circulation 1998; 98:2513–2519.
- Pyðrälä K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care 1997; 20:614–620.
- Vijan S, Hayward RA; American College of Physicians. Pharmacologic lipid-lowering therapy in type 2 diabetes mellitus: background paper for the American College of Physicians. Ann Intern Med 2004; 140:650–658.
- Baigent C, Keech A, Kearney PM, et al; Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278. Errata in Lancet 2008; 371:2084, Lancet 2005; 366:1358.
- Brugts JJ, Yetgin T, Hoeks SE, et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ 2009; 338:b2376.
- Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Baigent C; Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008; 371:117–125.
- Nissen SE, Nicholls SJ, Sipahi I, et al; ASTEROID Investigators. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006; 295:1556–1565.
- Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes et al. N Engl J Med 2004; 350:1495–1504.
- LaRosa JC, Grundy SM, Waters DD, et al; Treating to New Targets (TNT) Investigators. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005; 352:1425–1435.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010; 376:1670–1681.
- LIPID Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med 1998; 339:1349–1357.
- Grundy SM, Cleeman JI, Bairey Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110:227–239.
- American Diabetes Association. Executive summary: standards of medical care in diabetes—2012. Diabetes Care 2012; 35(suppl 1):S5–S10.
- Daniels SR, Greer FR; Committee on Nutrition. Lipid screening and cardiovascular health in childhood. Pediatrics 2008; 122:198–208.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346:393–403.
- Cholesterol Treatment Trialists’ (CTT) Collaborators; Mihaylova B, Emberson J, Blackwell L, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012; 380:581–590.
KEY POINTS
- The evidence from individual clinical trials is mixed, but meta-analyses indicate that statin therapy is associated with approximately a 9% higher risk of diabetes (an absolute difference of about 0.4%).
- We need to interpret this information cautiously. Many potentially confounding factors are involved, and rigorous prospective trials are needed to examine this issue.
- The benefit of preventing serious cardiovascular events seems to outweigh the higher risks of diabetes and poorer glycemic control, and we should continue to give statins to patients at moderate to high risk, including those with diabetes, with vigilance for these side effects.
Sessile serrated polyps: Cancer risk and appropriate surveillance
Sessile serrated polyps are a type of polyp recently recognized to be a precursor of colorectal cancer. They arise from a pathway of genetic alterations different from the pathway that causes the more common and well-understood conventional adenomas (also called tubular adenomas, tubulovillous adenomas, and villous adenomas).
We do not yet know enough about the lifetime colorectal cancer risk for individuals with sessile serrated polyps, nor do we know the optimal surveillance interval for patients who have these polyps on colonoscopy. It is believed that sessile serrated polyps may be the cause of a substantial number of “interval” colorectal cancers—ie, cancers that occur after colonoscopy but before the next scheduled examination.
Serrated polyps get their name from their jagged appearance on microscopy. In the past, all serrated colorectal lesions were called hyperplastic polyps. But with the advent of molecular and genetic diagnostics and with the ability to recognize the subtle morphologic differences of serrated lesions, they have been reclassified into those without malignant potential (hyperplastic polyps) and those that are neoplastic (sessile serrated polyps and traditional serrated adenomas) (Table 1).
In this article, we discuss the evolving understanding of the different types of serrated polyps, and we offer our thoughts on a reasonable postpolypectomy surveillance plan in patients with these lesions. We focus on sessile serrated polyps, the most common form of serrated polyp with cancerous potential, since it may be one of our greatest challenges in optimal colorectal cancer prevention.
CLINICAL SCENARIO
A 65-year-old woman with no family history of colorectal cancer undergoes screening colonoscopy, during which three polyps are found and removed—a 3-mm tubular adenoma in the sigmoid colon, an 8-mm sessile serrated polyp at the hepatic flexure, and a 2-mm hyperplastic polyp in the rectum. When should she undergo follow-up colonoscopy?
Based on the number, size, and pathologic makeup of the polyps in this patient, we would recommend follow-up surveillance colonoscopy in 5 years.
THE SERRATED POLYP PATHWAY: A DIFFERENT PATH TO COLORECTAL CANCER
Colorectal cancer is the third most common cancer in the United States.1 From 70% to 80% of these cancers arise from adenomatous polyps via the adenoma-carcinoma pathway. This molecular pathway develops through chromosomal instability (CIN) and involves the loss of heterozygosity (the loss of function of one allele). This leads to the progressive accumulation of mutations in tumor-suppressor genes such as adenomatous polyposis coli (APC) and p53, and oncogenes such as KRAS. The result of these mutations is the development of adenomatous polyps that lead to microsatellite-stable colorectal cancers (Figure 1).2
More recently, studies have shown that the other 20% to 30% of colorectal cancers likely arise through a separate pathway, called the serrated polyp pathway or serrated neoplasia pathway. In contrast to CIN, this pathway is characterized by methylation of CpG islands (CIMP–CpG island methylation phenotype, CIMP) in the promoter regions of specific genes.3 Central to the serrated polyp pathway is progressive methylation in colonic mucosa; mutation in the BRAF oncogene, activating cell proliferation leading to a sessile serrated polyp; and epigenetic silencing of the DNA mismatch repair gene hMLH1, which is a key step in the progression to a sessile serrated polyp with dysplasia, which may rapidly become a microsatellite-unstable colorectal cancer.4
Histologically, serrated polyps have a serrated or sawtooth appearance from the folding in of the crypt epithelium, and they include hyperplastic polyps, traditional serrated adenomas, and sessile serrated polyps (sessile serrated adenomas).
Sessile serrated polyps and traditional serrated adenomas (which are rare) are thought to be precancerous, whereas hyperplastic polyps do not have malignant potential.
COMMON, BUT PREVALENCE IS NOT CLEARLY ESTABLISHED
The histologic criteria for sessile serrated polyps and traditional serrated adenomas have been elucidated,4–7 but the epidemiology of these serrated polyps is not clear. Small studies have shown that sessile serrated polyps account for 2% to 9% of all polyps removed at colonoscopy8–10; however, larger studies are needed to determine the prevalence because detection by an endoscopist and pathologic diagnosis of these polyps are both operator-dependent.
Traditional serrated adenomas are the least common type of serrated polyp, with a reported prevalence of 0.3%.7 Hyperplastic polyps are by far the most common, accounting for 20% to 30% of all polyps removed at colonoscopy.9,11 Sessile serrated polyps have a predilection for the proximal colon and are associated with female sex and with smoking, 12,13 but no consistent effect of other factors on their formation has been reported. In contrast, Wallace et al13 found that obesity, cigarette smoking, dietary fat intake, total caloric intake, and the consumption of red meat were associated with an increased risk of distal (but not proximal) serrated polyps, including hyperplastic polyps, sessile serrated polyps, and traditional serrated adenomas.
HYPERPLASTIC POLYPS
Hyperplastic polyps usually occur in the rectosigmoid colon. They appear as slightly elevated, whitish lesions with a diameter less than 5 mm (Figure 2). Microscopically, the serrated architecture is present in the upper half of their crypts (Figure 3). The proliferative zone is more or less normally located in the basal half of the crypt (the nonserrated portion), with nuclei that are small, uniform, and basally located.14 The bases of the crypts have a rounded contour and do not grow laterally along the muscularis mucosae.
SESSILE SERRATED POLYPS
Endoscopically, sessile serrated polyps are often subtle, appear flat or slightly elevated, and can be covered by yellow mucus (Figure 4). They are typically found in the proximal colon and are usually larger than typical adenomas, with 50% being larger than 10 mm.10
Histologically, the serrations are more prominent than those of hyperplastic polyps and involve the entire length of the crypt (Figure 5). The crypt bases are often dilated and display lateral growth along the lamina muscularis mucosae, resembling a letter t or l. The lamina muscularis mucosae is often thinner than normal. Crypts from sessile serrated polyps are occasionally found beneath the muscularis mucosae, a condition called pseudoinvasion.7
TRADITIONAL SERRATED ADENOMAS
Traditional serrated adenomas are usually left-sided. In contrast to the other types of serrated polyps, they are histologically often villiform and are lined by cells with elongated nuclei and abundant eosinophilic cytoplasm (Figure 6). Unlike those in sessile serrated polyps, the crypt bases do not display an abnormal architecture; rather, traditional serrated adenomas have abundant ectopic crypts (“budding crypts”) in the long, slender villi.7
Traditional serrated adenomas also appear to be genetically distinct from sessile serrated polyps. They are most often characterized by a KRAS (or less commonly, BRAF) mutation and commonly have methylation of the DNA repair gene MGMT (O-6-methylguanine-DNA methyltransferase) rather than hMLH1.
CHALLENGES TO EFFECTIVE COLONOSCOPY
Colonoscopic polypectomy of adenomatous polyps reduces the incidence of colorectal cancer and the rate of death from it.15,16 However, recent data show that colonoscopy may not be as effective as once thought. As many as 9% of patients with colorectal cancer have had a “normal” colonoscopic examination in the preceding 3 years.17,18 In addition, the reduction in incidence and mortality rates was less for cancers in the proximal colon than for cancers in the distal colon.19,20
Possible explanations for this discrepancy include the skill of the endoscopist, technical limitations of the examination, incomplete removal of polyps, and inadequate bowel preparation. Several studies have shown that interval colorectal cancers are more likely to be found in the proximal colon and to have the same molecular characteristics as sessile serrated polyps and the serrated colorectal cancer pathway (CIMP-high and MSI-H).21,22 Therefore, it is now thought that sessile serrated polyps may account for a substantial portion of “postcolonoscopy cancers” (ie, interval cancers) that arise in the proximal colon.
Two large studies of screening colonoscopy confirmed that the ability to detect sessile serrated polyps depends greatly on the skill of the endoscopist. Hetzel et al9 studied the differences in the rates of polyp detection among endoscopists performing more than 7,000 colonoscopies. Detection rates varied significantly for adenomas, hyperplastic polyps, and sessile serrated polyps, with the greatest variability noted in the detection of sessile serrated polyps. Significant variability was also noted in the ability of the pathologist to diagnose sessile serrated polyps.9
In the other study, a strong correlation was found between physicians who are “high detectors” of adenomas and their detection rates for proximal serrated polyps.23 There is widespread acceptance that screening colonoscopy in average-risk patients age 50 and older should detect adenomas in more than 25% of men and more than 15% of women. There is no current minimum recommended detection rate for sessile serrated polyps, but some have suggested 1.5%.8
POLYPS AS PREDICTORS OF CANCER RISK
Certain polyp characteristics predict the risk of metachronous, advanced neoplasia. Advanced neoplasms are defined as invasive carcinomas, adenomas 10 mm or larger, or adenomas with any villous histology or high-grade dysplasia. Patients with one or two small tubular adenomas have a much lower risk of metachronous advanced neoplasia than do patients with more than two adenomas or advanced neoplasms.24 Current recommended surveillance intervals vary on that basis (Table 2).25
People who harbor serrated neoplasms are at high risk of synchronous serrated polyps and advanced adenomatous neoplasia. Pai et al26 found that patients with one sessile serrated polyp were four times more likely to have additional serrated polyps at the same time than an unselected population. The authors suggested that this indicates a strong colonic mucosal-field defect in patients with sessile serrated polyps, thereby predisposing them to the development of synchronous serrated polyps.
Li et al27 found that large serrated polyps (ie, > 10 mm) are associated with a risk of synchronous advanced neoplasia that is three times higher than in patients without adenomas. Schreiner et al28 determined that patients with either a proximal or a large serrated polyp were at higher risk of synchronous advanced neoplasia compared with patients who did not have those lesions. Vu et al29 found that patients who have both sessile serrated polyps and conventional adenomas have significantly larger and more numerous lesions of both types.29 In addition, these lesions are more likely to be pathologically advanced when compared with people with only one or the other type.
In the only study of the risk of advanced neoplasia on follow-up colonoscopy,28 patients with advanced neoplasia and proximal serrated polyps at baseline examination were twice as likely to have advanced neoplasia during subsequent surveillance than those with only advanced neoplasia at baseline examination.28
Therefore, it seems clear that the presence of large or proximal serrated polyps or serrated neoplasms predicts the presence of synchronous and likely metachronous advanced neoplasms.
Guidelines for postpolypectomy surveillance for individuals with serrated lesions of the colon have recently been published.25 Patients with large serrated lesions (≥ 10 mm) or an advanced serrated lesion (a sessile serrated polyp with or without cytologic dysplasia or a traditional serrated adenoma) should be followed closely. Patients with small (< 10-mm) rectosigmoid hyperplastic polyps should be followed as average-risk patients. If a patient with a sessile serrated polyp also has adenomas, the surveillance interval should be the shortest interval recommended for either lesion.29
SURVEILLANCE FOR OUR PATIENT
In our patient, given the number, size, and histologic features of the polyps found, surveillance colonoscopy should be considered in 5 years. Although the clinical significance of the serrated pathway to colorectal cancer cannot be argued, further study is required to understand the lifetime risk to patients with serrated neoplasms and the optimal surveillance interval.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29.
- Pino MS, Chung DC. The chromosomal instability pathway in colon cancer. Gastroenterology 2010; 138;2059–2072.
- Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology 2010; 138:2088–2100.
- Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol 2011; 42:1–10.
- O’Brien MJ, Yang S, Mack C, et al. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol 2006; 30:1491–1501.
- Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol 2003; 27:65–81.
- Torlakovic EE, Gomez JD, Driman DK, et al. Sessile serrated adenoma (SSA) vs traditional serrated adenoma (TSA). Am J Surg Pathol 2008; 32:21–29.
- Sanaka MR, Gohel T, Podugu A, et al. Quality indicators to enhance adenoma detection rate: should there be reconsideration of the current standard? Gastrointest Endosc 2011; 73:AB138.
- Hetzel JT, Huang CS, Coukos JA, et al. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol 2010; 105:2656–2664.
- Spring KJ, Zhao ZZ, Karamatic R, et al. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology 2006; 131:1400–1407.
- Higuchi T, Sugihara K, Jass JR. Demographic and pathological characteristics of serrated polyps of colorectum. Histopathology 2005; 47:32–40.
- Lieberman DA, Prindiville S, Weiss DG, Willett W; VA Cooperative Study Group 380. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA 2003; 290:2959–2967.
- Wallace K, Grau MV, Ahnen D, et al. The association of lifestyle and dietary factors with the risk for serrated polyps of the colorectum. Cancer Epidemiol Biomarkers Prev 2009; 18:2310–2317.
- Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol 2012; 107:1315–1329.
- Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993; 329:1977–1981.
- Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012; 366:687–696.
- Sawhney MS, Farrar WD, Gudiseva S, et al. Microsatellite instability in interval colon cancers. Gastroenterology 2006; 131:1700–1705.
- Baxter NN, Sutradhar R, Forbes SS, Paszat lF, Saskin R, Rabeneck l. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology 2011; 140:65–72.
- Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology 2010; 139:1128–1137.
- Baxter NN, Goldwasser MA, Paszat lF, Saskin R, Urbach DR, Rabeneck l. Association of colonoscopy and death from colorectal cancer. Ann Intern Med 2009; 150:1–8.
- Arain MA, Sawhney M, Sheikh S, et al. CIMP status of interval colon cancers: another piece to the puzzle. Am J Gastroenterol 2010; 105:1189–1195.
- Farrar WD, Sawhney MS, Nelson DB, Lederle FA, Bond JH. Colorectal cancers found after a complete colonoscopy. Clin Gastroenterol Hepatol 2006; 4:1259–1264.
- Kahi CJ, Hewett DG, Norton Dl, Eckert GJ, Rex DK. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol 2011; 9:42–46.
- Martínez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology 2009; 136:832–841.
- Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012; 143:844–857.
- Pai RK, Hart J, Noffsinger AE. Sessile serrated adenomas strongly predispose to synchronous serrated polyps in nonsyndromic patients. Histopathology 2010; 56:581–588.
- Li D, Jin C, McCulloch C, et al. Association of large serrated polyps with synchronous advanced colorectal neoplasia. Am J Gastroenterol 2009; 104:695–702.
- Schreiner MA, Weiss DG, Lieberman DA. Proximal and large hyperplastic and nondysplastic serrated polyps detected by colonoscopy are associated with neoplasia. Gastroenterology 2010; 139:1497–1502.
- Vu HT, Lopez R, Bennett A, Burke CA. Individuals with sessile serrated polyps express an aggressive colorectal phenotype. Dis Colon Rectum 2011; 54:1216–1223.
Sessile serrated polyps are a type of polyp recently recognized to be a precursor of colorectal cancer. They arise from a pathway of genetic alterations different from the pathway that causes the more common and well-understood conventional adenomas (also called tubular adenomas, tubulovillous adenomas, and villous adenomas).
We do not yet know enough about the lifetime colorectal cancer risk for individuals with sessile serrated polyps, nor do we know the optimal surveillance interval for patients who have these polyps on colonoscopy. It is believed that sessile serrated polyps may be the cause of a substantial number of “interval” colorectal cancers—ie, cancers that occur after colonoscopy but before the next scheduled examination.
Serrated polyps get their name from their jagged appearance on microscopy. In the past, all serrated colorectal lesions were called hyperplastic polyps. But with the advent of molecular and genetic diagnostics and with the ability to recognize the subtle morphologic differences of serrated lesions, they have been reclassified into those without malignant potential (hyperplastic polyps) and those that are neoplastic (sessile serrated polyps and traditional serrated adenomas) (Table 1).
In this article, we discuss the evolving understanding of the different types of serrated polyps, and we offer our thoughts on a reasonable postpolypectomy surveillance plan in patients with these lesions. We focus on sessile serrated polyps, the most common form of serrated polyp with cancerous potential, since it may be one of our greatest challenges in optimal colorectal cancer prevention.
CLINICAL SCENARIO
A 65-year-old woman with no family history of colorectal cancer undergoes screening colonoscopy, during which three polyps are found and removed—a 3-mm tubular adenoma in the sigmoid colon, an 8-mm sessile serrated polyp at the hepatic flexure, and a 2-mm hyperplastic polyp in the rectum. When should she undergo follow-up colonoscopy?
Based on the number, size, and pathologic makeup of the polyps in this patient, we would recommend follow-up surveillance colonoscopy in 5 years.
THE SERRATED POLYP PATHWAY: A DIFFERENT PATH TO COLORECTAL CANCER
Colorectal cancer is the third most common cancer in the United States.1 From 70% to 80% of these cancers arise from adenomatous polyps via the adenoma-carcinoma pathway. This molecular pathway develops through chromosomal instability (CIN) and involves the loss of heterozygosity (the loss of function of one allele). This leads to the progressive accumulation of mutations in tumor-suppressor genes such as adenomatous polyposis coli (APC) and p53, and oncogenes such as KRAS. The result of these mutations is the development of adenomatous polyps that lead to microsatellite-stable colorectal cancers (Figure 1).2
More recently, studies have shown that the other 20% to 30% of colorectal cancers likely arise through a separate pathway, called the serrated polyp pathway or serrated neoplasia pathway. In contrast to CIN, this pathway is characterized by methylation of CpG islands (CIMP–CpG island methylation phenotype, CIMP) in the promoter regions of specific genes.3 Central to the serrated polyp pathway is progressive methylation in colonic mucosa; mutation in the BRAF oncogene, activating cell proliferation leading to a sessile serrated polyp; and epigenetic silencing of the DNA mismatch repair gene hMLH1, which is a key step in the progression to a sessile serrated polyp with dysplasia, which may rapidly become a microsatellite-unstable colorectal cancer.4
Histologically, serrated polyps have a serrated or sawtooth appearance from the folding in of the crypt epithelium, and they include hyperplastic polyps, traditional serrated adenomas, and sessile serrated polyps (sessile serrated adenomas).
Sessile serrated polyps and traditional serrated adenomas (which are rare) are thought to be precancerous, whereas hyperplastic polyps do not have malignant potential.
COMMON, BUT PREVALENCE IS NOT CLEARLY ESTABLISHED
The histologic criteria for sessile serrated polyps and traditional serrated adenomas have been elucidated,4–7 but the epidemiology of these serrated polyps is not clear. Small studies have shown that sessile serrated polyps account for 2% to 9% of all polyps removed at colonoscopy8–10; however, larger studies are needed to determine the prevalence because detection by an endoscopist and pathologic diagnosis of these polyps are both operator-dependent.
Traditional serrated adenomas are the least common type of serrated polyp, with a reported prevalence of 0.3%.7 Hyperplastic polyps are by far the most common, accounting for 20% to 30% of all polyps removed at colonoscopy.9,11 Sessile serrated polyps have a predilection for the proximal colon and are associated with female sex and with smoking, 12,13 but no consistent effect of other factors on their formation has been reported. In contrast, Wallace et al13 found that obesity, cigarette smoking, dietary fat intake, total caloric intake, and the consumption of red meat were associated with an increased risk of distal (but not proximal) serrated polyps, including hyperplastic polyps, sessile serrated polyps, and traditional serrated adenomas.
HYPERPLASTIC POLYPS
Hyperplastic polyps usually occur in the rectosigmoid colon. They appear as slightly elevated, whitish lesions with a diameter less than 5 mm (Figure 2). Microscopically, the serrated architecture is present in the upper half of their crypts (Figure 3). The proliferative zone is more or less normally located in the basal half of the crypt (the nonserrated portion), with nuclei that are small, uniform, and basally located.14 The bases of the crypts have a rounded contour and do not grow laterally along the muscularis mucosae.
SESSILE SERRATED POLYPS
Endoscopically, sessile serrated polyps are often subtle, appear flat or slightly elevated, and can be covered by yellow mucus (Figure 4). They are typically found in the proximal colon and are usually larger than typical adenomas, with 50% being larger than 10 mm.10
Histologically, the serrations are more prominent than those of hyperplastic polyps and involve the entire length of the crypt (Figure 5). The crypt bases are often dilated and display lateral growth along the lamina muscularis mucosae, resembling a letter t or l. The lamina muscularis mucosae is often thinner than normal. Crypts from sessile serrated polyps are occasionally found beneath the muscularis mucosae, a condition called pseudoinvasion.7
TRADITIONAL SERRATED ADENOMAS
Traditional serrated adenomas are usually left-sided. In contrast to the other types of serrated polyps, they are histologically often villiform and are lined by cells with elongated nuclei and abundant eosinophilic cytoplasm (Figure 6). Unlike those in sessile serrated polyps, the crypt bases do not display an abnormal architecture; rather, traditional serrated adenomas have abundant ectopic crypts (“budding crypts”) in the long, slender villi.7
Traditional serrated adenomas also appear to be genetically distinct from sessile serrated polyps. They are most often characterized by a KRAS (or less commonly, BRAF) mutation and commonly have methylation of the DNA repair gene MGMT (O-6-methylguanine-DNA methyltransferase) rather than hMLH1.
CHALLENGES TO EFFECTIVE COLONOSCOPY
Colonoscopic polypectomy of adenomatous polyps reduces the incidence of colorectal cancer and the rate of death from it.15,16 However, recent data show that colonoscopy may not be as effective as once thought. As many as 9% of patients with colorectal cancer have had a “normal” colonoscopic examination in the preceding 3 years.17,18 In addition, the reduction in incidence and mortality rates was less for cancers in the proximal colon than for cancers in the distal colon.19,20
Possible explanations for this discrepancy include the skill of the endoscopist, technical limitations of the examination, incomplete removal of polyps, and inadequate bowel preparation. Several studies have shown that interval colorectal cancers are more likely to be found in the proximal colon and to have the same molecular characteristics as sessile serrated polyps and the serrated colorectal cancer pathway (CIMP-high and MSI-H).21,22 Therefore, it is now thought that sessile serrated polyps may account for a substantial portion of “postcolonoscopy cancers” (ie, interval cancers) that arise in the proximal colon.
Two large studies of screening colonoscopy confirmed that the ability to detect sessile serrated polyps depends greatly on the skill of the endoscopist. Hetzel et al9 studied the differences in the rates of polyp detection among endoscopists performing more than 7,000 colonoscopies. Detection rates varied significantly for adenomas, hyperplastic polyps, and sessile serrated polyps, with the greatest variability noted in the detection of sessile serrated polyps. Significant variability was also noted in the ability of the pathologist to diagnose sessile serrated polyps.9
In the other study, a strong correlation was found between physicians who are “high detectors” of adenomas and their detection rates for proximal serrated polyps.23 There is widespread acceptance that screening colonoscopy in average-risk patients age 50 and older should detect adenomas in more than 25% of men and more than 15% of women. There is no current minimum recommended detection rate for sessile serrated polyps, but some have suggested 1.5%.8
POLYPS AS PREDICTORS OF CANCER RISK
Certain polyp characteristics predict the risk of metachronous, advanced neoplasia. Advanced neoplasms are defined as invasive carcinomas, adenomas 10 mm or larger, or adenomas with any villous histology or high-grade dysplasia. Patients with one or two small tubular adenomas have a much lower risk of metachronous advanced neoplasia than do patients with more than two adenomas or advanced neoplasms.24 Current recommended surveillance intervals vary on that basis (Table 2).25
People who harbor serrated neoplasms are at high risk of synchronous serrated polyps and advanced adenomatous neoplasia. Pai et al26 found that patients with one sessile serrated polyp were four times more likely to have additional serrated polyps at the same time than an unselected population. The authors suggested that this indicates a strong colonic mucosal-field defect in patients with sessile serrated polyps, thereby predisposing them to the development of synchronous serrated polyps.
Li et al27 found that large serrated polyps (ie, > 10 mm) are associated with a risk of synchronous advanced neoplasia that is three times higher than in patients without adenomas. Schreiner et al28 determined that patients with either a proximal or a large serrated polyp were at higher risk of synchronous advanced neoplasia compared with patients who did not have those lesions. Vu et al29 found that patients who have both sessile serrated polyps and conventional adenomas have significantly larger and more numerous lesions of both types.29 In addition, these lesions are more likely to be pathologically advanced when compared with people with only one or the other type.
In the only study of the risk of advanced neoplasia on follow-up colonoscopy,28 patients with advanced neoplasia and proximal serrated polyps at baseline examination were twice as likely to have advanced neoplasia during subsequent surveillance than those with only advanced neoplasia at baseline examination.28
Therefore, it seems clear that the presence of large or proximal serrated polyps or serrated neoplasms predicts the presence of synchronous and likely metachronous advanced neoplasms.
Guidelines for postpolypectomy surveillance for individuals with serrated lesions of the colon have recently been published.25 Patients with large serrated lesions (≥ 10 mm) or an advanced serrated lesion (a sessile serrated polyp with or without cytologic dysplasia or a traditional serrated adenoma) should be followed closely. Patients with small (< 10-mm) rectosigmoid hyperplastic polyps should be followed as average-risk patients. If a patient with a sessile serrated polyp also has adenomas, the surveillance interval should be the shortest interval recommended for either lesion.29
SURVEILLANCE FOR OUR PATIENT
In our patient, given the number, size, and histologic features of the polyps found, surveillance colonoscopy should be considered in 5 years. Although the clinical significance of the serrated pathway to colorectal cancer cannot be argued, further study is required to understand the lifetime risk to patients with serrated neoplasms and the optimal surveillance interval.
Sessile serrated polyps are a type of polyp recently recognized to be a precursor of colorectal cancer. They arise from a pathway of genetic alterations different from the pathway that causes the more common and well-understood conventional adenomas (also called tubular adenomas, tubulovillous adenomas, and villous adenomas).
We do not yet know enough about the lifetime colorectal cancer risk for individuals with sessile serrated polyps, nor do we know the optimal surveillance interval for patients who have these polyps on colonoscopy. It is believed that sessile serrated polyps may be the cause of a substantial number of “interval” colorectal cancers—ie, cancers that occur after colonoscopy but before the next scheduled examination.
Serrated polyps get their name from their jagged appearance on microscopy. In the past, all serrated colorectal lesions were called hyperplastic polyps. But with the advent of molecular and genetic diagnostics and with the ability to recognize the subtle morphologic differences of serrated lesions, they have been reclassified into those without malignant potential (hyperplastic polyps) and those that are neoplastic (sessile serrated polyps and traditional serrated adenomas) (Table 1).
In this article, we discuss the evolving understanding of the different types of serrated polyps, and we offer our thoughts on a reasonable postpolypectomy surveillance plan in patients with these lesions. We focus on sessile serrated polyps, the most common form of serrated polyp with cancerous potential, since it may be one of our greatest challenges in optimal colorectal cancer prevention.
CLINICAL SCENARIO
A 65-year-old woman with no family history of colorectal cancer undergoes screening colonoscopy, during which three polyps are found and removed—a 3-mm tubular adenoma in the sigmoid colon, an 8-mm sessile serrated polyp at the hepatic flexure, and a 2-mm hyperplastic polyp in the rectum. When should she undergo follow-up colonoscopy?
Based on the number, size, and pathologic makeup of the polyps in this patient, we would recommend follow-up surveillance colonoscopy in 5 years.
THE SERRATED POLYP PATHWAY: A DIFFERENT PATH TO COLORECTAL CANCER
Colorectal cancer is the third most common cancer in the United States.1 From 70% to 80% of these cancers arise from adenomatous polyps via the adenoma-carcinoma pathway. This molecular pathway develops through chromosomal instability (CIN) and involves the loss of heterozygosity (the loss of function of one allele). This leads to the progressive accumulation of mutations in tumor-suppressor genes such as adenomatous polyposis coli (APC) and p53, and oncogenes such as KRAS. The result of these mutations is the development of adenomatous polyps that lead to microsatellite-stable colorectal cancers (Figure 1).2
More recently, studies have shown that the other 20% to 30% of colorectal cancers likely arise through a separate pathway, called the serrated polyp pathway or serrated neoplasia pathway. In contrast to CIN, this pathway is characterized by methylation of CpG islands (CIMP–CpG island methylation phenotype, CIMP) in the promoter regions of specific genes.3 Central to the serrated polyp pathway is progressive methylation in colonic mucosa; mutation in the BRAF oncogene, activating cell proliferation leading to a sessile serrated polyp; and epigenetic silencing of the DNA mismatch repair gene hMLH1, which is a key step in the progression to a sessile serrated polyp with dysplasia, which may rapidly become a microsatellite-unstable colorectal cancer.4
Histologically, serrated polyps have a serrated or sawtooth appearance from the folding in of the crypt epithelium, and they include hyperplastic polyps, traditional serrated adenomas, and sessile serrated polyps (sessile serrated adenomas).
Sessile serrated polyps and traditional serrated adenomas (which are rare) are thought to be precancerous, whereas hyperplastic polyps do not have malignant potential.
COMMON, BUT PREVALENCE IS NOT CLEARLY ESTABLISHED
The histologic criteria for sessile serrated polyps and traditional serrated adenomas have been elucidated,4–7 but the epidemiology of these serrated polyps is not clear. Small studies have shown that sessile serrated polyps account for 2% to 9% of all polyps removed at colonoscopy8–10; however, larger studies are needed to determine the prevalence because detection by an endoscopist and pathologic diagnosis of these polyps are both operator-dependent.
Traditional serrated adenomas are the least common type of serrated polyp, with a reported prevalence of 0.3%.7 Hyperplastic polyps are by far the most common, accounting for 20% to 30% of all polyps removed at colonoscopy.9,11 Sessile serrated polyps have a predilection for the proximal colon and are associated with female sex and with smoking, 12,13 but no consistent effect of other factors on their formation has been reported. In contrast, Wallace et al13 found that obesity, cigarette smoking, dietary fat intake, total caloric intake, and the consumption of red meat were associated with an increased risk of distal (but not proximal) serrated polyps, including hyperplastic polyps, sessile serrated polyps, and traditional serrated adenomas.
HYPERPLASTIC POLYPS
Hyperplastic polyps usually occur in the rectosigmoid colon. They appear as slightly elevated, whitish lesions with a diameter less than 5 mm (Figure 2). Microscopically, the serrated architecture is present in the upper half of their crypts (Figure 3). The proliferative zone is more or less normally located in the basal half of the crypt (the nonserrated portion), with nuclei that are small, uniform, and basally located.14 The bases of the crypts have a rounded contour and do not grow laterally along the muscularis mucosae.
SESSILE SERRATED POLYPS
Endoscopically, sessile serrated polyps are often subtle, appear flat or slightly elevated, and can be covered by yellow mucus (Figure 4). They are typically found in the proximal colon and are usually larger than typical adenomas, with 50% being larger than 10 mm.10
Histologically, the serrations are more prominent than those of hyperplastic polyps and involve the entire length of the crypt (Figure 5). The crypt bases are often dilated and display lateral growth along the lamina muscularis mucosae, resembling a letter t or l. The lamina muscularis mucosae is often thinner than normal. Crypts from sessile serrated polyps are occasionally found beneath the muscularis mucosae, a condition called pseudoinvasion.7
TRADITIONAL SERRATED ADENOMAS
Traditional serrated adenomas are usually left-sided. In contrast to the other types of serrated polyps, they are histologically often villiform and are lined by cells with elongated nuclei and abundant eosinophilic cytoplasm (Figure 6). Unlike those in sessile serrated polyps, the crypt bases do not display an abnormal architecture; rather, traditional serrated adenomas have abundant ectopic crypts (“budding crypts”) in the long, slender villi.7
Traditional serrated adenomas also appear to be genetically distinct from sessile serrated polyps. They are most often characterized by a KRAS (or less commonly, BRAF) mutation and commonly have methylation of the DNA repair gene MGMT (O-6-methylguanine-DNA methyltransferase) rather than hMLH1.
CHALLENGES TO EFFECTIVE COLONOSCOPY
Colonoscopic polypectomy of adenomatous polyps reduces the incidence of colorectal cancer and the rate of death from it.15,16 However, recent data show that colonoscopy may not be as effective as once thought. As many as 9% of patients with colorectal cancer have had a “normal” colonoscopic examination in the preceding 3 years.17,18 In addition, the reduction in incidence and mortality rates was less for cancers in the proximal colon than for cancers in the distal colon.19,20
Possible explanations for this discrepancy include the skill of the endoscopist, technical limitations of the examination, incomplete removal of polyps, and inadequate bowel preparation. Several studies have shown that interval colorectal cancers are more likely to be found in the proximal colon and to have the same molecular characteristics as sessile serrated polyps and the serrated colorectal cancer pathway (CIMP-high and MSI-H).21,22 Therefore, it is now thought that sessile serrated polyps may account for a substantial portion of “postcolonoscopy cancers” (ie, interval cancers) that arise in the proximal colon.
Two large studies of screening colonoscopy confirmed that the ability to detect sessile serrated polyps depends greatly on the skill of the endoscopist. Hetzel et al9 studied the differences in the rates of polyp detection among endoscopists performing more than 7,000 colonoscopies. Detection rates varied significantly for adenomas, hyperplastic polyps, and sessile serrated polyps, with the greatest variability noted in the detection of sessile serrated polyps. Significant variability was also noted in the ability of the pathologist to diagnose sessile serrated polyps.9
In the other study, a strong correlation was found between physicians who are “high detectors” of adenomas and their detection rates for proximal serrated polyps.23 There is widespread acceptance that screening colonoscopy in average-risk patients age 50 and older should detect adenomas in more than 25% of men and more than 15% of women. There is no current minimum recommended detection rate for sessile serrated polyps, but some have suggested 1.5%.8
POLYPS AS PREDICTORS OF CANCER RISK
Certain polyp characteristics predict the risk of metachronous, advanced neoplasia. Advanced neoplasms are defined as invasive carcinomas, adenomas 10 mm or larger, or adenomas with any villous histology or high-grade dysplasia. Patients with one or two small tubular adenomas have a much lower risk of metachronous advanced neoplasia than do patients with more than two adenomas or advanced neoplasms.24 Current recommended surveillance intervals vary on that basis (Table 2).25
People who harbor serrated neoplasms are at high risk of synchronous serrated polyps and advanced adenomatous neoplasia. Pai et al26 found that patients with one sessile serrated polyp were four times more likely to have additional serrated polyps at the same time than an unselected population. The authors suggested that this indicates a strong colonic mucosal-field defect in patients with sessile serrated polyps, thereby predisposing them to the development of synchronous serrated polyps.
Li et al27 found that large serrated polyps (ie, > 10 mm) are associated with a risk of synchronous advanced neoplasia that is three times higher than in patients without adenomas. Schreiner et al28 determined that patients with either a proximal or a large serrated polyp were at higher risk of synchronous advanced neoplasia compared with patients who did not have those lesions. Vu et al29 found that patients who have both sessile serrated polyps and conventional adenomas have significantly larger and more numerous lesions of both types.29 In addition, these lesions are more likely to be pathologically advanced when compared with people with only one or the other type.
In the only study of the risk of advanced neoplasia on follow-up colonoscopy,28 patients with advanced neoplasia and proximal serrated polyps at baseline examination were twice as likely to have advanced neoplasia during subsequent surveillance than those with only advanced neoplasia at baseline examination.28
Therefore, it seems clear that the presence of large or proximal serrated polyps or serrated neoplasms predicts the presence of synchronous and likely metachronous advanced neoplasms.
Guidelines for postpolypectomy surveillance for individuals with serrated lesions of the colon have recently been published.25 Patients with large serrated lesions (≥ 10 mm) or an advanced serrated lesion (a sessile serrated polyp with or without cytologic dysplasia or a traditional serrated adenoma) should be followed closely. Patients with small (< 10-mm) rectosigmoid hyperplastic polyps should be followed as average-risk patients. If a patient with a sessile serrated polyp also has adenomas, the surveillance interval should be the shortest interval recommended for either lesion.29
SURVEILLANCE FOR OUR PATIENT
In our patient, given the number, size, and histologic features of the polyps found, surveillance colonoscopy should be considered in 5 years. Although the clinical significance of the serrated pathway to colorectal cancer cannot be argued, further study is required to understand the lifetime risk to patients with serrated neoplasms and the optimal surveillance interval.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29.
- Pino MS, Chung DC. The chromosomal instability pathway in colon cancer. Gastroenterology 2010; 138;2059–2072.
- Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology 2010; 138:2088–2100.
- Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol 2011; 42:1–10.
- O’Brien MJ, Yang S, Mack C, et al. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol 2006; 30:1491–1501.
- Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol 2003; 27:65–81.
- Torlakovic EE, Gomez JD, Driman DK, et al. Sessile serrated adenoma (SSA) vs traditional serrated adenoma (TSA). Am J Surg Pathol 2008; 32:21–29.
- Sanaka MR, Gohel T, Podugu A, et al. Quality indicators to enhance adenoma detection rate: should there be reconsideration of the current standard? Gastrointest Endosc 2011; 73:AB138.
- Hetzel JT, Huang CS, Coukos JA, et al. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol 2010; 105:2656–2664.
- Spring KJ, Zhao ZZ, Karamatic R, et al. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology 2006; 131:1400–1407.
- Higuchi T, Sugihara K, Jass JR. Demographic and pathological characteristics of serrated polyps of colorectum. Histopathology 2005; 47:32–40.
- Lieberman DA, Prindiville S, Weiss DG, Willett W; VA Cooperative Study Group 380. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA 2003; 290:2959–2967.
- Wallace K, Grau MV, Ahnen D, et al. The association of lifestyle and dietary factors with the risk for serrated polyps of the colorectum. Cancer Epidemiol Biomarkers Prev 2009; 18:2310–2317.
- Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol 2012; 107:1315–1329.
- Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993; 329:1977–1981.
- Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012; 366:687–696.
- Sawhney MS, Farrar WD, Gudiseva S, et al. Microsatellite instability in interval colon cancers. Gastroenterology 2006; 131:1700–1705.
- Baxter NN, Sutradhar R, Forbes SS, Paszat lF, Saskin R, Rabeneck l. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology 2011; 140:65–72.
- Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology 2010; 139:1128–1137.
- Baxter NN, Goldwasser MA, Paszat lF, Saskin R, Urbach DR, Rabeneck l. Association of colonoscopy and death from colorectal cancer. Ann Intern Med 2009; 150:1–8.
- Arain MA, Sawhney M, Sheikh S, et al. CIMP status of interval colon cancers: another piece to the puzzle. Am J Gastroenterol 2010; 105:1189–1195.
- Farrar WD, Sawhney MS, Nelson DB, Lederle FA, Bond JH. Colorectal cancers found after a complete colonoscopy. Clin Gastroenterol Hepatol 2006; 4:1259–1264.
- Kahi CJ, Hewett DG, Norton Dl, Eckert GJ, Rex DK. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol 2011; 9:42–46.
- Martínez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology 2009; 136:832–841.
- Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012; 143:844–857.
- Pai RK, Hart J, Noffsinger AE. Sessile serrated adenomas strongly predispose to synchronous serrated polyps in nonsyndromic patients. Histopathology 2010; 56:581–588.
- Li D, Jin C, McCulloch C, et al. Association of large serrated polyps with synchronous advanced colorectal neoplasia. Am J Gastroenterol 2009; 104:695–702.
- Schreiner MA, Weiss DG, Lieberman DA. Proximal and large hyperplastic and nondysplastic serrated polyps detected by colonoscopy are associated with neoplasia. Gastroenterology 2010; 139:1497–1502.
- Vu HT, Lopez R, Bennett A, Burke CA. Individuals with sessile serrated polyps express an aggressive colorectal phenotype. Dis Colon Rectum 2011; 54:1216–1223.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29.
- Pino MS, Chung DC. The chromosomal instability pathway in colon cancer. Gastroenterology 2010; 138;2059–2072.
- Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology 2010; 138:2088–2100.
- Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol 2011; 42:1–10.
- O’Brien MJ, Yang S, Mack C, et al. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol 2006; 30:1491–1501.
- Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol 2003; 27:65–81.
- Torlakovic EE, Gomez JD, Driman DK, et al. Sessile serrated adenoma (SSA) vs traditional serrated adenoma (TSA). Am J Surg Pathol 2008; 32:21–29.
- Sanaka MR, Gohel T, Podugu A, et al. Quality indicators to enhance adenoma detection rate: should there be reconsideration of the current standard? Gastrointest Endosc 2011; 73:AB138.
- Hetzel JT, Huang CS, Coukos JA, et al. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol 2010; 105:2656–2664.
- Spring KJ, Zhao ZZ, Karamatic R, et al. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology 2006; 131:1400–1407.
- Higuchi T, Sugihara K, Jass JR. Demographic and pathological characteristics of serrated polyps of colorectum. Histopathology 2005; 47:32–40.
- Lieberman DA, Prindiville S, Weiss DG, Willett W; VA Cooperative Study Group 380. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA 2003; 290:2959–2967.
- Wallace K, Grau MV, Ahnen D, et al. The association of lifestyle and dietary factors with the risk for serrated polyps of the colorectum. Cancer Epidemiol Biomarkers Prev 2009; 18:2310–2317.
- Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol 2012; 107:1315–1329.
- Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993; 329:1977–1981.
- Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012; 366:687–696.
- Sawhney MS, Farrar WD, Gudiseva S, et al. Microsatellite instability in interval colon cancers. Gastroenterology 2006; 131:1700–1705.
- Baxter NN, Sutradhar R, Forbes SS, Paszat lF, Saskin R, Rabeneck l. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology 2011; 140:65–72.
- Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology 2010; 139:1128–1137.
- Baxter NN, Goldwasser MA, Paszat lF, Saskin R, Urbach DR, Rabeneck l. Association of colonoscopy and death from colorectal cancer. Ann Intern Med 2009; 150:1–8.
- Arain MA, Sawhney M, Sheikh S, et al. CIMP status of interval colon cancers: another piece to the puzzle. Am J Gastroenterol 2010; 105:1189–1195.
- Farrar WD, Sawhney MS, Nelson DB, Lederle FA, Bond JH. Colorectal cancers found after a complete colonoscopy. Clin Gastroenterol Hepatol 2006; 4:1259–1264.
- Kahi CJ, Hewett DG, Norton Dl, Eckert GJ, Rex DK. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol 2011; 9:42–46.
- Martínez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology 2009; 136:832–841.
- Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012; 143:844–857.
- Pai RK, Hart J, Noffsinger AE. Sessile serrated adenomas strongly predispose to synchronous serrated polyps in nonsyndromic patients. Histopathology 2010; 56:581–588.
- Li D, Jin C, McCulloch C, et al. Association of large serrated polyps with synchronous advanced colorectal neoplasia. Am J Gastroenterol 2009; 104:695–702.
- Schreiner MA, Weiss DG, Lieberman DA. Proximal and large hyperplastic and nondysplastic serrated polyps detected by colonoscopy are associated with neoplasia. Gastroenterology 2010; 139:1497–1502.
- Vu HT, Lopez R, Bennett A, Burke CA. Individuals with sessile serrated polyps express an aggressive colorectal phenotype. Dis Colon Rectum 2011; 54:1216–1223.
KEY POINTS
- From 20% to 30% of colorectal cancers arise through the serrated polyp pathway (the serrated neoplasia pathway.)
- Histologically, serrated polyps have a serrated or sawtooth appearance from the folding in of the crypt epithelium. Types of serrated polyps include hyperplastic polyps, traditional serrated adenomas, and sessile serrated polyps (also known as sessile serrated adenomas).
- Guidelines for surveillance after polypectomy of serrated lesions recommend that patients with a large (≥ 10-mm) or a sessile serrated polyp with cytologic dysplasia or a traditional serrated adenoma be followed more closely than patients with a sessile serrated polyp smaller than 10 mm. Patients with small rectosigmoid hyperplastic polyps should be followed the same as people at average risk.
Mild cognitive impairment: Challenges in research and in practice
The integrity of cognitive function is a reliable indicator of healthy aging. But the progression of cognitive changes from normal aging to dementia is often insidious and easily underrecognized. Consequently, mild cognitive impairment (MCI)—the entity that characterizes this transition—has become an area of intense research. Since 1999, the number of research publications related to MCI has exploded, with more than 1,000 peer-reviewed studies in 2010 alone.
Controversy remains over the definition, diagnosis, prognosis, and management of MCI. However, in an evidence-based review of the literature,1 the American Academy of Neurology concluded that MCI is a useful clinical entity and that patients with MCI should be identified and monitored because of the increased risk of progression to dementia.
Early studies appeared to indicate that patients with MCI were at high risk of further cognitive decline and progression to Alzheimer dementia.1 But subsequent research found that not all were, leading to the recognition of two subtypes of MCI: amnestic, which mainly involves memory loss, and nonamnestic, which involves impairment of other cognitive domains. Patients with the amnestic type were determined to be more likely to eventually develop Alzheimer disease.2 The amnestic subtype is being considered for inclusion in the next revision of the Diagnostic and Statistical Manual of Mental Disorders, ie, the fifth edition (DSM-V).3
MCI varies with each person affected. Neither its clinical nor its neuropathologic course follows a predictable, linear path, making its study especially challenging. The pathologic and molecular mechanisms of MCI are not well established. In the amnestic type, the distribution of cortical amyloid deposits appears transitional to the pathologic changes seen in Alzheimer disease.4 But postmortem brain tissues5 and clinical imaging studies6 reveal that some normal controls have a degree of amyloid deposition similar to that in patients with MCI. These findings limit the use of amyloid lesions as a robust pathologic marker for distinguishing normal aging from MCI.
MCI is diagnosed clinically, and clinicians should be able to diagnose most cases of MCI in the office. The first step is cognitive concern (ie, a change from the patient’s baseline cognitive status) raised by the patient, by an informant, or by a clinician. Often, in amnestic MCI, the earliest symptom is memory loss. Once persistent memory loss is documented, the patient is assessed for the ability to perform activities of daily living. To fulfill the criteria for the diagnosis of MCI, patients need to have intact function in the activities of daily living and no features of neurologic and psychiatric diseases that affect cognition. Further office-based cognitive testing helps to determine whether MCI is the amnestic or the nonamnestic type. A brief neuropsychological test such as the Montreal Cognitive Assessment often supports the diagnosis of MCI, although accurate characterization of cognitive dysfunction is enhanced with thorough neuropsychological testing.
MCI remains a clinical diagnosis with an imprecise prognosis. Although the amnestic MCI criteria are reasonably specific, they do not always predict progression to Alzheimer disease. Growing evidence suggests that neuropsychiatric symptoms, including depression, apathy, and anxiety, are clinical predictors of the progression of MCI to Alzheimer disease, and that the added risk can be substantial. For example, in one study, the risk of incident dementia was seven times higher if apathy was present.7 As such, a careful psychiatric evaluation of patients with MCI is strongly recommended and should be part of a comprehensive workup.
The study of MCI touches on almost all aspects of aging and dementia investigation. A great deal of research is focusing on the development of cerebrospinal fluid or imaging biomarkers of amyloid deposition, structural magnetic resonance imaging markers of neuronal loss, and genetic predisposition to detect the earliest signs of the disease in people who may be at risk. The rationale for the intense study of MCI is that the sooner the intervention in a degenerative process is started, the more likely that further cognitive and functional decline can be prevented: early diagnosis is paramount in trying to prevent subsequent disability. Clinical trials are needed to determine whether early detection of MCI or the detection of biomarkers in asymptomatic individuals alters the incidence of dementia or its prognosis.
In this issue of the Cleveland Clinic Journal of Medicine, Patel and Holland8 present a comprehensive overview of MCI and highlight the issues related to its diagnosis and management. The treatment of MCI is another area that is unclear. At this time, prescription of cognition-enhancing medications is not indicated. No pharmacologic agent is approved by the US Food and Drug Administration for treating MCI, although cholinesterase inhibitors have been studied. At the pathologic level, there is no clear consensus on whether presynaptic or postsynaptic (or both) cholinergic receptors are defective in MCI.9 There is some evidence of increased choline acetyltransferase activity in the hippocampus and the superior frontal cortex.10 Selected hippocampal and cortical cholinergic systems may be capable of compensatory responses in MCI. This may help explain why cholinesterase inhibitors are ineffective in preventing dementia in patients with MCI in therapeutic trials.
Patel and Holland recommend a reasonable multidisciplinary approach for managing MCI, although supporting evidence for such recommendations from clinical trials is lacking. Realizing that not all patients with MCI progress to Alzheimer disease and that some cases are reversible is cause for recommending close follow-up and monitoring of neuropsychiatric and cognitive symptoms in older patients.
MCI is now a clinical reality for all physicians dealing with older patients. Thus, MCI is of more than merely research interest to clinicians, who will come to recognize and diagnose this condition frequently in the aging population.
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999; 56:303–308.
- Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004; 256:240–246.
- Petersen RC, O’Brien J. Mild cognitive impairment should be considered for DSM-V. J Geriatr Psychiatry Neurol 2006; 19:147–154.
- Markesbery WR. Neuropathologic alterations in mild cognitive impairment: a review. J Alzheimers Dis 2010; 19:221–228.
- Price JL, McKeel DW, Buckles VD, et al. Neuropathology of nondemented aging: presumptive evidence for pre-clinical Alzheimer disease. Neurobiol Aging 2009; 30:1026–1036.
- Aizenstein HJ, Nebes RD, Saxton JA, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol 2008; 65:1509–1517.
- Palmer K, Di Iulio F, Varsi AE, et al. Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer’s disease: the role of depression and apathy. J Alzheimers Dis 2010; 20:175–183.
- Patel BB, Holland NW. Mild cognitive impairment: hope for stability, plan for progression. Cleve Clin J Med 2012; 79:857–864.
- Mufson EJ, Binder L, Counts SE, et al. Mild cognitive impairment: pathology and mechanisms. Acta Neuropathol 2012; 123:13–30.
- DeKosky ST, Ikonomovic MD, Styren SD, et al. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol 2002; 51:145–155.
The integrity of cognitive function is a reliable indicator of healthy aging. But the progression of cognitive changes from normal aging to dementia is often insidious and easily underrecognized. Consequently, mild cognitive impairment (MCI)—the entity that characterizes this transition—has become an area of intense research. Since 1999, the number of research publications related to MCI has exploded, with more than 1,000 peer-reviewed studies in 2010 alone.
Controversy remains over the definition, diagnosis, prognosis, and management of MCI. However, in an evidence-based review of the literature,1 the American Academy of Neurology concluded that MCI is a useful clinical entity and that patients with MCI should be identified and monitored because of the increased risk of progression to dementia.
Early studies appeared to indicate that patients with MCI were at high risk of further cognitive decline and progression to Alzheimer dementia.1 But subsequent research found that not all were, leading to the recognition of two subtypes of MCI: amnestic, which mainly involves memory loss, and nonamnestic, which involves impairment of other cognitive domains. Patients with the amnestic type were determined to be more likely to eventually develop Alzheimer disease.2 The amnestic subtype is being considered for inclusion in the next revision of the Diagnostic and Statistical Manual of Mental Disorders, ie, the fifth edition (DSM-V).3
MCI varies with each person affected. Neither its clinical nor its neuropathologic course follows a predictable, linear path, making its study especially challenging. The pathologic and molecular mechanisms of MCI are not well established. In the amnestic type, the distribution of cortical amyloid deposits appears transitional to the pathologic changes seen in Alzheimer disease.4 But postmortem brain tissues5 and clinical imaging studies6 reveal that some normal controls have a degree of amyloid deposition similar to that in patients with MCI. These findings limit the use of amyloid lesions as a robust pathologic marker for distinguishing normal aging from MCI.
MCI is diagnosed clinically, and clinicians should be able to diagnose most cases of MCI in the office. The first step is cognitive concern (ie, a change from the patient’s baseline cognitive status) raised by the patient, by an informant, or by a clinician. Often, in amnestic MCI, the earliest symptom is memory loss. Once persistent memory loss is documented, the patient is assessed for the ability to perform activities of daily living. To fulfill the criteria for the diagnosis of MCI, patients need to have intact function in the activities of daily living and no features of neurologic and psychiatric diseases that affect cognition. Further office-based cognitive testing helps to determine whether MCI is the amnestic or the nonamnestic type. A brief neuropsychological test such as the Montreal Cognitive Assessment often supports the diagnosis of MCI, although accurate characterization of cognitive dysfunction is enhanced with thorough neuropsychological testing.
MCI remains a clinical diagnosis with an imprecise prognosis. Although the amnestic MCI criteria are reasonably specific, they do not always predict progression to Alzheimer disease. Growing evidence suggests that neuropsychiatric symptoms, including depression, apathy, and anxiety, are clinical predictors of the progression of MCI to Alzheimer disease, and that the added risk can be substantial. For example, in one study, the risk of incident dementia was seven times higher if apathy was present.7 As such, a careful psychiatric evaluation of patients with MCI is strongly recommended and should be part of a comprehensive workup.
The study of MCI touches on almost all aspects of aging and dementia investigation. A great deal of research is focusing on the development of cerebrospinal fluid or imaging biomarkers of amyloid deposition, structural magnetic resonance imaging markers of neuronal loss, and genetic predisposition to detect the earliest signs of the disease in people who may be at risk. The rationale for the intense study of MCI is that the sooner the intervention in a degenerative process is started, the more likely that further cognitive and functional decline can be prevented: early diagnosis is paramount in trying to prevent subsequent disability. Clinical trials are needed to determine whether early detection of MCI or the detection of biomarkers in asymptomatic individuals alters the incidence of dementia or its prognosis.
In this issue of the Cleveland Clinic Journal of Medicine, Patel and Holland8 present a comprehensive overview of MCI and highlight the issues related to its diagnosis and management. The treatment of MCI is another area that is unclear. At this time, prescription of cognition-enhancing medications is not indicated. No pharmacologic agent is approved by the US Food and Drug Administration for treating MCI, although cholinesterase inhibitors have been studied. At the pathologic level, there is no clear consensus on whether presynaptic or postsynaptic (or both) cholinergic receptors are defective in MCI.9 There is some evidence of increased choline acetyltransferase activity in the hippocampus and the superior frontal cortex.10 Selected hippocampal and cortical cholinergic systems may be capable of compensatory responses in MCI. This may help explain why cholinesterase inhibitors are ineffective in preventing dementia in patients with MCI in therapeutic trials.
Patel and Holland recommend a reasonable multidisciplinary approach for managing MCI, although supporting evidence for such recommendations from clinical trials is lacking. Realizing that not all patients with MCI progress to Alzheimer disease and that some cases are reversible is cause for recommending close follow-up and monitoring of neuropsychiatric and cognitive symptoms in older patients.
MCI is now a clinical reality for all physicians dealing with older patients. Thus, MCI is of more than merely research interest to clinicians, who will come to recognize and diagnose this condition frequently in the aging population.
The integrity of cognitive function is a reliable indicator of healthy aging. But the progression of cognitive changes from normal aging to dementia is often insidious and easily underrecognized. Consequently, mild cognitive impairment (MCI)—the entity that characterizes this transition—has become an area of intense research. Since 1999, the number of research publications related to MCI has exploded, with more than 1,000 peer-reviewed studies in 2010 alone.
Controversy remains over the definition, diagnosis, prognosis, and management of MCI. However, in an evidence-based review of the literature,1 the American Academy of Neurology concluded that MCI is a useful clinical entity and that patients with MCI should be identified and monitored because of the increased risk of progression to dementia.
Early studies appeared to indicate that patients with MCI were at high risk of further cognitive decline and progression to Alzheimer dementia.1 But subsequent research found that not all were, leading to the recognition of two subtypes of MCI: amnestic, which mainly involves memory loss, and nonamnestic, which involves impairment of other cognitive domains. Patients with the amnestic type were determined to be more likely to eventually develop Alzheimer disease.2 The amnestic subtype is being considered for inclusion in the next revision of the Diagnostic and Statistical Manual of Mental Disorders, ie, the fifth edition (DSM-V).3
MCI varies with each person affected. Neither its clinical nor its neuropathologic course follows a predictable, linear path, making its study especially challenging. The pathologic and molecular mechanisms of MCI are not well established. In the amnestic type, the distribution of cortical amyloid deposits appears transitional to the pathologic changes seen in Alzheimer disease.4 But postmortem brain tissues5 and clinical imaging studies6 reveal that some normal controls have a degree of amyloid deposition similar to that in patients with MCI. These findings limit the use of amyloid lesions as a robust pathologic marker for distinguishing normal aging from MCI.
MCI is diagnosed clinically, and clinicians should be able to diagnose most cases of MCI in the office. The first step is cognitive concern (ie, a change from the patient’s baseline cognitive status) raised by the patient, by an informant, or by a clinician. Often, in amnestic MCI, the earliest symptom is memory loss. Once persistent memory loss is documented, the patient is assessed for the ability to perform activities of daily living. To fulfill the criteria for the diagnosis of MCI, patients need to have intact function in the activities of daily living and no features of neurologic and psychiatric diseases that affect cognition. Further office-based cognitive testing helps to determine whether MCI is the amnestic or the nonamnestic type. A brief neuropsychological test such as the Montreal Cognitive Assessment often supports the diagnosis of MCI, although accurate characterization of cognitive dysfunction is enhanced with thorough neuropsychological testing.
MCI remains a clinical diagnosis with an imprecise prognosis. Although the amnestic MCI criteria are reasonably specific, they do not always predict progression to Alzheimer disease. Growing evidence suggests that neuropsychiatric symptoms, including depression, apathy, and anxiety, are clinical predictors of the progression of MCI to Alzheimer disease, and that the added risk can be substantial. For example, in one study, the risk of incident dementia was seven times higher if apathy was present.7 As such, a careful psychiatric evaluation of patients with MCI is strongly recommended and should be part of a comprehensive workup.
The study of MCI touches on almost all aspects of aging and dementia investigation. A great deal of research is focusing on the development of cerebrospinal fluid or imaging biomarkers of amyloid deposition, structural magnetic resonance imaging markers of neuronal loss, and genetic predisposition to detect the earliest signs of the disease in people who may be at risk. The rationale for the intense study of MCI is that the sooner the intervention in a degenerative process is started, the more likely that further cognitive and functional decline can be prevented: early diagnosis is paramount in trying to prevent subsequent disability. Clinical trials are needed to determine whether early detection of MCI or the detection of biomarkers in asymptomatic individuals alters the incidence of dementia or its prognosis.
In this issue of the Cleveland Clinic Journal of Medicine, Patel and Holland8 present a comprehensive overview of MCI and highlight the issues related to its diagnosis and management. The treatment of MCI is another area that is unclear. At this time, prescription of cognition-enhancing medications is not indicated. No pharmacologic agent is approved by the US Food and Drug Administration for treating MCI, although cholinesterase inhibitors have been studied. At the pathologic level, there is no clear consensus on whether presynaptic or postsynaptic (or both) cholinergic receptors are defective in MCI.9 There is some evidence of increased choline acetyltransferase activity in the hippocampus and the superior frontal cortex.10 Selected hippocampal and cortical cholinergic systems may be capable of compensatory responses in MCI. This may help explain why cholinesterase inhibitors are ineffective in preventing dementia in patients with MCI in therapeutic trials.
Patel and Holland recommend a reasonable multidisciplinary approach for managing MCI, although supporting evidence for such recommendations from clinical trials is lacking. Realizing that not all patients with MCI progress to Alzheimer disease and that some cases are reversible is cause for recommending close follow-up and monitoring of neuropsychiatric and cognitive symptoms in older patients.
MCI is now a clinical reality for all physicians dealing with older patients. Thus, MCI is of more than merely research interest to clinicians, who will come to recognize and diagnose this condition frequently in the aging population.
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999; 56:303–308.
- Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004; 256:240–246.
- Petersen RC, O’Brien J. Mild cognitive impairment should be considered for DSM-V. J Geriatr Psychiatry Neurol 2006; 19:147–154.
- Markesbery WR. Neuropathologic alterations in mild cognitive impairment: a review. J Alzheimers Dis 2010; 19:221–228.
- Price JL, McKeel DW, Buckles VD, et al. Neuropathology of nondemented aging: presumptive evidence for pre-clinical Alzheimer disease. Neurobiol Aging 2009; 30:1026–1036.
- Aizenstein HJ, Nebes RD, Saxton JA, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol 2008; 65:1509–1517.
- Palmer K, Di Iulio F, Varsi AE, et al. Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer’s disease: the role of depression and apathy. J Alzheimers Dis 2010; 20:175–183.
- Patel BB, Holland NW. Mild cognitive impairment: hope for stability, plan for progression. Cleve Clin J Med 2012; 79:857–864.
- Mufson EJ, Binder L, Counts SE, et al. Mild cognitive impairment: pathology and mechanisms. Acta Neuropathol 2012; 123:13–30.
- DeKosky ST, Ikonomovic MD, Styren SD, et al. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol 2002; 51:145–155.
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999; 56:303–308.
- Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004; 256:240–246.
- Petersen RC, O’Brien J. Mild cognitive impairment should be considered for DSM-V. J Geriatr Psychiatry Neurol 2006; 19:147–154.
- Markesbery WR. Neuropathologic alterations in mild cognitive impairment: a review. J Alzheimers Dis 2010; 19:221–228.
- Price JL, McKeel DW, Buckles VD, et al. Neuropathology of nondemented aging: presumptive evidence for pre-clinical Alzheimer disease. Neurobiol Aging 2009; 30:1026–1036.
- Aizenstein HJ, Nebes RD, Saxton JA, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol 2008; 65:1509–1517.
- Palmer K, Di Iulio F, Varsi AE, et al. Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer’s disease: the role of depression and apathy. J Alzheimers Dis 2010; 20:175–183.
- Patel BB, Holland NW. Mild cognitive impairment: hope for stability, plan for progression. Cleve Clin J Med 2012; 79:857–864.
- Mufson EJ, Binder L, Counts SE, et al. Mild cognitive impairment: pathology and mechanisms. Acta Neuropathol 2012; 123:13–30.
- DeKosky ST, Ikonomovic MD, Styren SD, et al. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol 2002; 51:145–155.