User login
Stopping the ooze
Many of us will do anything or use any product available to stop oozing from suture needle holes. After all, waiting for bleeding to stop is usually not something most vascular surgeons enjoy. Most hemostatic agents are quite expensive and some don’t work very well at all.
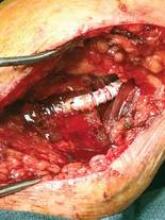
Our group has found a cheap alternative that is freely available in every OR and, although not perfect, works well enough in most cases – the standard ultrasonic transmission Doppler gel that you use to listen to arteries in the operative field. We usually have these available in sterile packets (Fig. 1). We cut off one end and squeeze the contents as a large glob onto the patch or anastomosis (Fig. 2). Presumably the weight of the material is enough to stop the needle-hole bleeds.
Active bleeding will usually only occur between stitches and is evidence that another stitch would be prudent. Since the gel is clear, any bleeding is easily seen. We routinely also use protamine reversal for our carotid artery endarterectomies and bypasses and so we leave the jelly on until all the protamine has been given. By that time, the bleeding has almost always stopped.
The jelly can be sucked away (it makes a great sounding noise in the suction!) or just diluted out with saline. I do note on the package insert that Doppler gel is not for internal use, and it is not FDA approved for this indication, but I believe we all use it anyway?
Dr. Samson is a clinical associate professor of surgery (vascular), Florida State University Medical School and a member of Sarasota Vascular Specialists, Sarasota, Fl., and the Medical Editor of Vascular Specialist.
[Editor’s Note: Please submit your own helpful tips and tricks for inclusion in this column to [email protected].]
Many of us will do anything or use any product available to stop oozing from suture needle holes. After all, waiting for bleeding to stop is usually not something most vascular surgeons enjoy. Most hemostatic agents are quite expensive and some don’t work very well at all.
Our group has found a cheap alternative that is freely available in every OR and, although not perfect, works well enough in most cases – the standard ultrasonic transmission Doppler gel that you use to listen to arteries in the operative field. We usually have these available in sterile packets (Fig. 1). We cut off one end and squeeze the contents as a large glob onto the patch or anastomosis (Fig. 2). Presumably the weight of the material is enough to stop the needle-hole bleeds.
Active bleeding will usually only occur between stitches and is evidence that another stitch would be prudent. Since the gel is clear, any bleeding is easily seen. We routinely also use protamine reversal for our carotid artery endarterectomies and bypasses and so we leave the jelly on until all the protamine has been given. By that time, the bleeding has almost always stopped.
The jelly can be sucked away (it makes a great sounding noise in the suction!) or just diluted out with saline. I do note on the package insert that Doppler gel is not for internal use, and it is not FDA approved for this indication, but I believe we all use it anyway?
Dr. Samson is a clinical associate professor of surgery (vascular), Florida State University Medical School and a member of Sarasota Vascular Specialists, Sarasota, Fl., and the Medical Editor of Vascular Specialist.
[Editor’s Note: Please submit your own helpful tips and tricks for inclusion in this column to [email protected].]
Many of us will do anything or use any product available to stop oozing from suture needle holes. After all, waiting for bleeding to stop is usually not something most vascular surgeons enjoy. Most hemostatic agents are quite expensive and some don’t work very well at all.
Our group has found a cheap alternative that is freely available in every OR and, although not perfect, works well enough in most cases – the standard ultrasonic transmission Doppler gel that you use to listen to arteries in the operative field. We usually have these available in sterile packets (Fig. 1). We cut off one end and squeeze the contents as a large glob onto the patch or anastomosis (Fig. 2). Presumably the weight of the material is enough to stop the needle-hole bleeds.
Active bleeding will usually only occur between stitches and is evidence that another stitch would be prudent. Since the gel is clear, any bleeding is easily seen. We routinely also use protamine reversal for our carotid artery endarterectomies and bypasses and so we leave the jelly on until all the protamine has been given. By that time, the bleeding has almost always stopped.
The jelly can be sucked away (it makes a great sounding noise in the suction!) or just diluted out with saline. I do note on the package insert that Doppler gel is not for internal use, and it is not FDA approved for this indication, but I believe we all use it anyway?
Dr. Samson is a clinical associate professor of surgery (vascular), Florida State University Medical School and a member of Sarasota Vascular Specialists, Sarasota, Fl., and the Medical Editor of Vascular Specialist.
[Editor’s Note: Please submit your own helpful tips and tricks for inclusion in this column to [email protected].]
Premature baby is severely handicapped: $21M verdict
AT 31 2/7 WEEKS' GESTATION, a woman was admitted to the hospital for hypertension. A maternal-fetal medicine specialist determined that a vaginal delivery was reasonable as long as the mother and fetus remained clinically stable; a cesarean delivery would be required if the status changed. An ObGyn and nurse midwife took over the mother’s care. Before dinoprostone and oxytocin were administered the next morning, a second ObGyn conducted a vaginal exam and found the mother’s cervix to be 4-cm dilated. After noon, the fetal heart rate became nonreassuring, with late and prolonged variable decelerations. The baby was born shortly after 5:00 pm with the umbilical cord wrapped around his neck. He was pale, lifeless, and had Apgar scores of 4 and 7 at 1 and 5 minutes, respectively. He required initial positive pressure ventilation due to bradycardia and poor respiratory effort.
The boy has cerebral palsy; although not cognitively impaired, he is severely physically handicapped. He has had several operations because one leg is shorter than the other. He has 65% function of his arms, making it impossible for him to complete normal, daily tasks by himself.
PARENTS' CLAIM A cesarean delivery should have been performed 3 hours earlier.
DEFENDANT' DEFENSE Fetal heart-rate monitoring was reassuring during the last 40 minutes of labor. An Apgar score of 7 at 5 minutes is normal. Blood gases taken at birth were normal (7.3 pH). Ultrasonography of the baby’s head at age 3 days showed normal findings. Problems were not evident on the head ultrasound until the child was 2 weeks of age, showing that the injury occurred after birth and was due to prematurity. Defendants included both ObGyns, the midwife, and the hospital.
VERDICT A $21 million Maryland verdict was returned, including $1 million in noneconomic damages that was reduced to $650,000 under the state cap.
PHYSICIAN APOLOGIZED: DIDN'T READ BIOPSY REPORT BEFORE SURGERY
A 34-YEAR-OLD WOMAN with a family history of breast cancer found a lump in her left breast. After fine-needle aspiration, a general surgeon diagnosed cancer and performed a double mastectomy.
At the first postoperative visit, the surgeon told the patient that she did not have breast cancer, and that the fine-needle aspiration results were negative. The surgeon apologized for never looking at the biopsy report prior to surgery, and admitted that is she had seen the report, she would have cancelled surgery.
PATIENT'S CLAIM The surgeon was negligent in performing bilateral mastectomies without first reading biopsy results.
PHYSICIAN'S DEFENSE The case was settled before trial.
VERDICT Michigan case evaluation delivered an award of $542,000, which both parties accepted.
CYSTOSCOPY BLAMED FOR URETERAL OBSTRUCTION, POOR KIDNEY FUNCTION
WHEN A 59-YEAR-OLD WOMAN underwent gynecologic surgery that included a cystoscopy, her uterers were functioning normally. During the following month, the ObGyn performed several follow-up examinations. A year later, the patient's right ureter was completely obstructed. The obstruction was repaired, but the patient lost function in her right kidney. She must take a drug to improve kidney function for the rest of her life.
PATIENT'S CLAIM The obstruction was caused by ligation that occurred during cystoscopy. The ObGyn should have diagnosed the obstruction during the weeks following surgery.
PHYSICIAN'S DEFENSE The cystoscopy was properly performed. The patient had not reported any symptoms after the procedure that suggested the presence of an obstruction. The obstruction gradually developed and could not have been diagnosed earlier.
VERDICT A New York defense verdict was returned.
INFERIOR VENA CAVA DAMAGED DURING ROBOTIC HYSTERECTOMY
A HYSTERECTOMY AND SALPINGO-OOPHORECTOMY were performed on a 64-year-old woman using the da Vinci Surgical System. The gynecologist also removed a cancerous endometrial mass and dissected the periaortic lymph nodes. When the gynecologist used the robot to lift a lymph fat pad, the inferior vena cava was injured and the patient lost 3 L of blood. After converting the laparotomy, a vascular surgeon implanted an artificial graft to repair the inferior vena cava. The patient fully recovered.
PATIENT'S CLAIM The gynecologist did not perform robotic surgery properly, and the patient was not told of all of the risks associated with robotic surgery. Due to the uncertainty regarding the graft's effectiveness, the patient developed posttraumatic stress disorder.
PHYSICIAN'S DEFENSE The vascular injury was a known risk associated with the procedure. The vena cava was not lacerated or transected: perforator veins that joined the lymph fat pad were unintentionally pulled out. The injury was most likely due to the application of pressure, not laceration by the surgical instrument.
VERDICT A $300,000 New York settlement was reached.
READ: The robot is gaining ground in gynecologic surgery. Should you be using it? A roundtable discussion with Arnold P. Advincula, MD; Cheryl B. Iglesia, MD; Rosanne M. Kho, MD; Jamal Mourad, DO; Marie Fidela R. Paraiso, MD; and Jason D. Wright, MD (April 2013)
FETAL DISTRESS CAUSED BRAIN INJURY: $13.9M
DURING THE LAST 2 HOURS OF LABOR, the mother was febrile, the baby's heart rate rose to over 160 bpm, and fetal monitoring indicated fetal distress. Oxytocin was administered to hasten delivery, but the mother's uterus became hyperstimulated. After nearly 17 hours of labor, the child was born without respirations. A video of the vaginal birth shows that the child was blue and unresponsive. The baby was resuscitated, and was subsequently found to have cerebral palsy, epilepsy, and mental retardation. At the time of trial, the 10-year-old had the mental capacity of a 3-year-old.
PARENTS' CLAIM The child suffered brain injury due to hypoxic ischemic encephalopathy. A cesarean delivery should have been performed as soon as fetal distress was evident. The doctors and nurses misread the baseline heart rate, and did not react when the baby did not recover well from the mother's contractions. Brain imaging did not show damage caused by infection or meningitis.
PHYSICIAN'S DEFENSE The girl's condition was caused by an infection or meningitis.
VERDICT A confidential settlement was reached with the midwife before the trial. The ObGyn was dismissed because he was never alerted to any problem by the labor and delivery team. A $13.9 million Georgia verdict was returned against the hospital system.
UTERINE ARTERY INJURED DURING CESAREAN DELIVERY
AFTER A SCHEDULED CESAREAN delivery, the 29-year-old mother had low blood pressure and an altered state of consciousness When she returned to the OR several hours later, her ObGyn found a uterine artery hematoma and laceration. After the laceration was clamped and sutured, uterine atony was noted and an emergency hysterectomy was performed
PATIENT'S CLAIM The mother was no longer able to bear children. The ObGyn was negligent in lacerating the uterine artery, failing to recognize the laceration during cesarean surgery, failing to properly monitor the patient after surgery, and failing to repair the artery in a timely manner. The patient's low blood pressure and altered state of consciousness should have been an indication that she had severe blood loss. The hospital's nursing staff failed to properly check her vital signs after surgery, and failed to report the abnormalities in blood pressure and consciousness to the ObGyn.
DEFENDANTS' DEFENSE The ObGyn claimed that a uterine laceration is a known risk of cesarean delivery; it can occur in the absence of negligence. The hospital also denied negligence.
VERDICT A Texas defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.versictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
AT 31 2/7 WEEKS' GESTATION, a woman was admitted to the hospital for hypertension. A maternal-fetal medicine specialist determined that a vaginal delivery was reasonable as long as the mother and fetus remained clinically stable; a cesarean delivery would be required if the status changed. An ObGyn and nurse midwife took over the mother’s care. Before dinoprostone and oxytocin were administered the next morning, a second ObGyn conducted a vaginal exam and found the mother’s cervix to be 4-cm dilated. After noon, the fetal heart rate became nonreassuring, with late and prolonged variable decelerations. The baby was born shortly after 5:00 pm with the umbilical cord wrapped around his neck. He was pale, lifeless, and had Apgar scores of 4 and 7 at 1 and 5 minutes, respectively. He required initial positive pressure ventilation due to bradycardia and poor respiratory effort.
The boy has cerebral palsy; although not cognitively impaired, he is severely physically handicapped. He has had several operations because one leg is shorter than the other. He has 65% function of his arms, making it impossible for him to complete normal, daily tasks by himself.
PARENTS' CLAIM A cesarean delivery should have been performed 3 hours earlier.
DEFENDANT' DEFENSE Fetal heart-rate monitoring was reassuring during the last 40 minutes of labor. An Apgar score of 7 at 5 minutes is normal. Blood gases taken at birth were normal (7.3 pH). Ultrasonography of the baby’s head at age 3 days showed normal findings. Problems were not evident on the head ultrasound until the child was 2 weeks of age, showing that the injury occurred after birth and was due to prematurity. Defendants included both ObGyns, the midwife, and the hospital.
VERDICT A $21 million Maryland verdict was returned, including $1 million in noneconomic damages that was reduced to $650,000 under the state cap.
PHYSICIAN APOLOGIZED: DIDN'T READ BIOPSY REPORT BEFORE SURGERY
A 34-YEAR-OLD WOMAN with a family history of breast cancer found a lump in her left breast. After fine-needle aspiration, a general surgeon diagnosed cancer and performed a double mastectomy.
At the first postoperative visit, the surgeon told the patient that she did not have breast cancer, and that the fine-needle aspiration results were negative. The surgeon apologized for never looking at the biopsy report prior to surgery, and admitted that is she had seen the report, she would have cancelled surgery.
PATIENT'S CLAIM The surgeon was negligent in performing bilateral mastectomies without first reading biopsy results.
PHYSICIAN'S DEFENSE The case was settled before trial.
VERDICT Michigan case evaluation delivered an award of $542,000, which both parties accepted.
CYSTOSCOPY BLAMED FOR URETERAL OBSTRUCTION, POOR KIDNEY FUNCTION
WHEN A 59-YEAR-OLD WOMAN underwent gynecologic surgery that included a cystoscopy, her uterers were functioning normally. During the following month, the ObGyn performed several follow-up examinations. A year later, the patient's right ureter was completely obstructed. The obstruction was repaired, but the patient lost function in her right kidney. She must take a drug to improve kidney function for the rest of her life.
PATIENT'S CLAIM The obstruction was caused by ligation that occurred during cystoscopy. The ObGyn should have diagnosed the obstruction during the weeks following surgery.
PHYSICIAN'S DEFENSE The cystoscopy was properly performed. The patient had not reported any symptoms after the procedure that suggested the presence of an obstruction. The obstruction gradually developed and could not have been diagnosed earlier.
VERDICT A New York defense verdict was returned.
INFERIOR VENA CAVA DAMAGED DURING ROBOTIC HYSTERECTOMY
A HYSTERECTOMY AND SALPINGO-OOPHORECTOMY were performed on a 64-year-old woman using the da Vinci Surgical System. The gynecologist also removed a cancerous endometrial mass and dissected the periaortic lymph nodes. When the gynecologist used the robot to lift a lymph fat pad, the inferior vena cava was injured and the patient lost 3 L of blood. After converting the laparotomy, a vascular surgeon implanted an artificial graft to repair the inferior vena cava. The patient fully recovered.
PATIENT'S CLAIM The gynecologist did not perform robotic surgery properly, and the patient was not told of all of the risks associated with robotic surgery. Due to the uncertainty regarding the graft's effectiveness, the patient developed posttraumatic stress disorder.
PHYSICIAN'S DEFENSE The vascular injury was a known risk associated with the procedure. The vena cava was not lacerated or transected: perforator veins that joined the lymph fat pad were unintentionally pulled out. The injury was most likely due to the application of pressure, not laceration by the surgical instrument.
VERDICT A $300,000 New York settlement was reached.
READ: The robot is gaining ground in gynecologic surgery. Should you be using it? A roundtable discussion with Arnold P. Advincula, MD; Cheryl B. Iglesia, MD; Rosanne M. Kho, MD; Jamal Mourad, DO; Marie Fidela R. Paraiso, MD; and Jason D. Wright, MD (April 2013)
FETAL DISTRESS CAUSED BRAIN INJURY: $13.9M
DURING THE LAST 2 HOURS OF LABOR, the mother was febrile, the baby's heart rate rose to over 160 bpm, and fetal monitoring indicated fetal distress. Oxytocin was administered to hasten delivery, but the mother's uterus became hyperstimulated. After nearly 17 hours of labor, the child was born without respirations. A video of the vaginal birth shows that the child was blue and unresponsive. The baby was resuscitated, and was subsequently found to have cerebral palsy, epilepsy, and mental retardation. At the time of trial, the 10-year-old had the mental capacity of a 3-year-old.
PARENTS' CLAIM The child suffered brain injury due to hypoxic ischemic encephalopathy. A cesarean delivery should have been performed as soon as fetal distress was evident. The doctors and nurses misread the baseline heart rate, and did not react when the baby did not recover well from the mother's contractions. Brain imaging did not show damage caused by infection or meningitis.
PHYSICIAN'S DEFENSE The girl's condition was caused by an infection or meningitis.
VERDICT A confidential settlement was reached with the midwife before the trial. The ObGyn was dismissed because he was never alerted to any problem by the labor and delivery team. A $13.9 million Georgia verdict was returned against the hospital system.
UTERINE ARTERY INJURED DURING CESAREAN DELIVERY
AFTER A SCHEDULED CESAREAN delivery, the 29-year-old mother had low blood pressure and an altered state of consciousness When she returned to the OR several hours later, her ObGyn found a uterine artery hematoma and laceration. After the laceration was clamped and sutured, uterine atony was noted and an emergency hysterectomy was performed
PATIENT'S CLAIM The mother was no longer able to bear children. The ObGyn was negligent in lacerating the uterine artery, failing to recognize the laceration during cesarean surgery, failing to properly monitor the patient after surgery, and failing to repair the artery in a timely manner. The patient's low blood pressure and altered state of consciousness should have been an indication that she had severe blood loss. The hospital's nursing staff failed to properly check her vital signs after surgery, and failed to report the abnormalities in blood pressure and consciousness to the ObGyn.
DEFENDANTS' DEFENSE The ObGyn claimed that a uterine laceration is a known risk of cesarean delivery; it can occur in the absence of negligence. The hospital also denied negligence.
VERDICT A Texas defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.versictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
AT 31 2/7 WEEKS' GESTATION, a woman was admitted to the hospital for hypertension. A maternal-fetal medicine specialist determined that a vaginal delivery was reasonable as long as the mother and fetus remained clinically stable; a cesarean delivery would be required if the status changed. An ObGyn and nurse midwife took over the mother’s care. Before dinoprostone and oxytocin were administered the next morning, a second ObGyn conducted a vaginal exam and found the mother’s cervix to be 4-cm dilated. After noon, the fetal heart rate became nonreassuring, with late and prolonged variable decelerations. The baby was born shortly after 5:00 pm with the umbilical cord wrapped around his neck. He was pale, lifeless, and had Apgar scores of 4 and 7 at 1 and 5 minutes, respectively. He required initial positive pressure ventilation due to bradycardia and poor respiratory effort.
The boy has cerebral palsy; although not cognitively impaired, he is severely physically handicapped. He has had several operations because one leg is shorter than the other. He has 65% function of his arms, making it impossible for him to complete normal, daily tasks by himself.
PARENTS' CLAIM A cesarean delivery should have been performed 3 hours earlier.
DEFENDANT' DEFENSE Fetal heart-rate monitoring was reassuring during the last 40 minutes of labor. An Apgar score of 7 at 5 minutes is normal. Blood gases taken at birth were normal (7.3 pH). Ultrasonography of the baby’s head at age 3 days showed normal findings. Problems were not evident on the head ultrasound until the child was 2 weeks of age, showing that the injury occurred after birth and was due to prematurity. Defendants included both ObGyns, the midwife, and the hospital.
VERDICT A $21 million Maryland verdict was returned, including $1 million in noneconomic damages that was reduced to $650,000 under the state cap.
PHYSICIAN APOLOGIZED: DIDN'T READ BIOPSY REPORT BEFORE SURGERY
A 34-YEAR-OLD WOMAN with a family history of breast cancer found a lump in her left breast. After fine-needle aspiration, a general surgeon diagnosed cancer and performed a double mastectomy.
At the first postoperative visit, the surgeon told the patient that she did not have breast cancer, and that the fine-needle aspiration results were negative. The surgeon apologized for never looking at the biopsy report prior to surgery, and admitted that is she had seen the report, she would have cancelled surgery.
PATIENT'S CLAIM The surgeon was negligent in performing bilateral mastectomies without first reading biopsy results.
PHYSICIAN'S DEFENSE The case was settled before trial.
VERDICT Michigan case evaluation delivered an award of $542,000, which both parties accepted.
CYSTOSCOPY BLAMED FOR URETERAL OBSTRUCTION, POOR KIDNEY FUNCTION
WHEN A 59-YEAR-OLD WOMAN underwent gynecologic surgery that included a cystoscopy, her uterers were functioning normally. During the following month, the ObGyn performed several follow-up examinations. A year later, the patient's right ureter was completely obstructed. The obstruction was repaired, but the patient lost function in her right kidney. She must take a drug to improve kidney function for the rest of her life.
PATIENT'S CLAIM The obstruction was caused by ligation that occurred during cystoscopy. The ObGyn should have diagnosed the obstruction during the weeks following surgery.
PHYSICIAN'S DEFENSE The cystoscopy was properly performed. The patient had not reported any symptoms after the procedure that suggested the presence of an obstruction. The obstruction gradually developed and could not have been diagnosed earlier.
VERDICT A New York defense verdict was returned.
INFERIOR VENA CAVA DAMAGED DURING ROBOTIC HYSTERECTOMY
A HYSTERECTOMY AND SALPINGO-OOPHORECTOMY were performed on a 64-year-old woman using the da Vinci Surgical System. The gynecologist also removed a cancerous endometrial mass and dissected the periaortic lymph nodes. When the gynecologist used the robot to lift a lymph fat pad, the inferior vena cava was injured and the patient lost 3 L of blood. After converting the laparotomy, a vascular surgeon implanted an artificial graft to repair the inferior vena cava. The patient fully recovered.
PATIENT'S CLAIM The gynecologist did not perform robotic surgery properly, and the patient was not told of all of the risks associated with robotic surgery. Due to the uncertainty regarding the graft's effectiveness, the patient developed posttraumatic stress disorder.
PHYSICIAN'S DEFENSE The vascular injury was a known risk associated with the procedure. The vena cava was not lacerated or transected: perforator veins that joined the lymph fat pad were unintentionally pulled out. The injury was most likely due to the application of pressure, not laceration by the surgical instrument.
VERDICT A $300,000 New York settlement was reached.
READ: The robot is gaining ground in gynecologic surgery. Should you be using it? A roundtable discussion with Arnold P. Advincula, MD; Cheryl B. Iglesia, MD; Rosanne M. Kho, MD; Jamal Mourad, DO; Marie Fidela R. Paraiso, MD; and Jason D. Wright, MD (April 2013)
FETAL DISTRESS CAUSED BRAIN INJURY: $13.9M
DURING THE LAST 2 HOURS OF LABOR, the mother was febrile, the baby's heart rate rose to over 160 bpm, and fetal monitoring indicated fetal distress. Oxytocin was administered to hasten delivery, but the mother's uterus became hyperstimulated. After nearly 17 hours of labor, the child was born without respirations. A video of the vaginal birth shows that the child was blue and unresponsive. The baby was resuscitated, and was subsequently found to have cerebral palsy, epilepsy, and mental retardation. At the time of trial, the 10-year-old had the mental capacity of a 3-year-old.
PARENTS' CLAIM The child suffered brain injury due to hypoxic ischemic encephalopathy. A cesarean delivery should have been performed as soon as fetal distress was evident. The doctors and nurses misread the baseline heart rate, and did not react when the baby did not recover well from the mother's contractions. Brain imaging did not show damage caused by infection or meningitis.
PHYSICIAN'S DEFENSE The girl's condition was caused by an infection or meningitis.
VERDICT A confidential settlement was reached with the midwife before the trial. The ObGyn was dismissed because he was never alerted to any problem by the labor and delivery team. A $13.9 million Georgia verdict was returned against the hospital system.
UTERINE ARTERY INJURED DURING CESAREAN DELIVERY
AFTER A SCHEDULED CESAREAN delivery, the 29-year-old mother had low blood pressure and an altered state of consciousness When she returned to the OR several hours later, her ObGyn found a uterine artery hematoma and laceration. After the laceration was clamped and sutured, uterine atony was noted and an emergency hysterectomy was performed
PATIENT'S CLAIM The mother was no longer able to bear children. The ObGyn was negligent in lacerating the uterine artery, failing to recognize the laceration during cesarean surgery, failing to properly monitor the patient after surgery, and failing to repair the artery in a timely manner. The patient's low blood pressure and altered state of consciousness should have been an indication that she had severe blood loss. The hospital's nursing staff failed to properly check her vital signs after surgery, and failed to report the abnormalities in blood pressure and consciousness to the ObGyn.
DEFENDANTS' DEFENSE The ObGyn claimed that a uterine laceration is a known risk of cesarean delivery; it can occur in the absence of negligence. The hospital also denied negligence.
VERDICT A Texas defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.versictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
SVS Resident Research Prize given to AAA study
Dr. Nathan D. Airhart, Washington University School of Medicine, St. Louis, was the recipient of this year’s SVS Foundation Resident Research Prize Paper, which was presented at the Vascular Annual Meeting as part of the William J. von Liebig Forum, which features the best in resident research.
Dr. Airhart, his mentor, Dr. John A. Curci, and his colleagues studied the specific contribution of the vascular smooth muscle cells (SMCs) to the destruction of the elastic proteins that are uniquely absent in the walls of abdominal aortic aneurysms (AAAs). "Although the SMC is the dominant cell type in the aortic wall, our understanding of the role of these cells in aneurysms has been very limited," said Dr. Airhart.
To directly study the function of these cells, Dr. Airhart and his colleagues embarked on an ambitious project to isolate live SMCs from AAAs, normal abdominal aorta (NAA), and plaque from carotid endarterectomy (CEA) procedures. The group profiled the mRNA produced by these cultured cells by microarray and clearly demonstrated a unique pattern of expression of the AAA-SMC.
"The mRNA profiles confirmed that the AAA cells were likely interacting with the matrix differently than the other SMCs, but it did not necessarily tell us how they were influencing aneurysm development," said Dr. Airhart. To better understand the role of these cells, the investigators evaluated the ability of these cells to break down elastic fibers in culture.
Under standard culture conditions, AAA-SMCs were able to degrade three times more elastin than the NAA-SMCs. "Even more remarkable was the finding that co-culture with activated macrophages – a cell type always found in the wall of aneurysms – resulted in a further doubling of the elastic fiber damage by the AAA-SMCs. Co-culture of macrophages with NAA-SMCs had no effect on the elastin degraded," said Dr. Airhart.
Further experiments suggested that the enzymes principally responsible for the elastolytic activity of these cells are the matrix metalloproteinases (MMPs). Increases in the production and/or activation of MMP-2 and/or MMP-9 were prominently found in cultures of AAA-SMCs.
"These studies present the strongest evidence that AAA-SMCs exhibit a disease-specific gene expression pattern and can very potently damage the elastic fiber matrix in the aortic wall. The unique and remarkable synergy with activated inflammatory cells might help explain the characteristic elastin loss of aortic aneurysms. Future studies will allow us to understand and alter the cellular mechanisms which lead to increased production and activation of elastolytic MMPs by these cells," Dr. Curci concluded.
The prestigious Resident Research Prize is intended to motivate new physicians to pursue vascular research. The prize recipient is invited to present his or her research results at the Society for Vascular Surgery’s Vascular Annual Meeting and the prize includes a 1-year subscription to the Journal of Vascular Surgery.
Dr. Nathan D. Airhart, Washington University School of Medicine, St. Louis, was the recipient of this year’s SVS Foundation Resident Research Prize Paper, which was presented at the Vascular Annual Meeting as part of the William J. von Liebig Forum, which features the best in resident research.
Dr. Airhart, his mentor, Dr. John A. Curci, and his colleagues studied the specific contribution of the vascular smooth muscle cells (SMCs) to the destruction of the elastic proteins that are uniquely absent in the walls of abdominal aortic aneurysms (AAAs). "Although the SMC is the dominant cell type in the aortic wall, our understanding of the role of these cells in aneurysms has been very limited," said Dr. Airhart.
To directly study the function of these cells, Dr. Airhart and his colleagues embarked on an ambitious project to isolate live SMCs from AAAs, normal abdominal aorta (NAA), and plaque from carotid endarterectomy (CEA) procedures. The group profiled the mRNA produced by these cultured cells by microarray and clearly demonstrated a unique pattern of expression of the AAA-SMC.
"The mRNA profiles confirmed that the AAA cells were likely interacting with the matrix differently than the other SMCs, but it did not necessarily tell us how they were influencing aneurysm development," said Dr. Airhart. To better understand the role of these cells, the investigators evaluated the ability of these cells to break down elastic fibers in culture.
Under standard culture conditions, AAA-SMCs were able to degrade three times more elastin than the NAA-SMCs. "Even more remarkable was the finding that co-culture with activated macrophages – a cell type always found in the wall of aneurysms – resulted in a further doubling of the elastic fiber damage by the AAA-SMCs. Co-culture of macrophages with NAA-SMCs had no effect on the elastin degraded," said Dr. Airhart.
Further experiments suggested that the enzymes principally responsible for the elastolytic activity of these cells are the matrix metalloproteinases (MMPs). Increases in the production and/or activation of MMP-2 and/or MMP-9 were prominently found in cultures of AAA-SMCs.
"These studies present the strongest evidence that AAA-SMCs exhibit a disease-specific gene expression pattern and can very potently damage the elastic fiber matrix in the aortic wall. The unique and remarkable synergy with activated inflammatory cells might help explain the characteristic elastin loss of aortic aneurysms. Future studies will allow us to understand and alter the cellular mechanisms which lead to increased production and activation of elastolytic MMPs by these cells," Dr. Curci concluded.
The prestigious Resident Research Prize is intended to motivate new physicians to pursue vascular research. The prize recipient is invited to present his or her research results at the Society for Vascular Surgery’s Vascular Annual Meeting and the prize includes a 1-year subscription to the Journal of Vascular Surgery.
Dr. Nathan D. Airhart, Washington University School of Medicine, St. Louis, was the recipient of this year’s SVS Foundation Resident Research Prize Paper, which was presented at the Vascular Annual Meeting as part of the William J. von Liebig Forum, which features the best in resident research.
Dr. Airhart, his mentor, Dr. John A. Curci, and his colleagues studied the specific contribution of the vascular smooth muscle cells (SMCs) to the destruction of the elastic proteins that are uniquely absent in the walls of abdominal aortic aneurysms (AAAs). "Although the SMC is the dominant cell type in the aortic wall, our understanding of the role of these cells in aneurysms has been very limited," said Dr. Airhart.
To directly study the function of these cells, Dr. Airhart and his colleagues embarked on an ambitious project to isolate live SMCs from AAAs, normal abdominal aorta (NAA), and plaque from carotid endarterectomy (CEA) procedures. The group profiled the mRNA produced by these cultured cells by microarray and clearly demonstrated a unique pattern of expression of the AAA-SMC.
"The mRNA profiles confirmed that the AAA cells were likely interacting with the matrix differently than the other SMCs, but it did not necessarily tell us how they were influencing aneurysm development," said Dr. Airhart. To better understand the role of these cells, the investigators evaluated the ability of these cells to break down elastic fibers in culture.
Under standard culture conditions, AAA-SMCs were able to degrade three times more elastin than the NAA-SMCs. "Even more remarkable was the finding that co-culture with activated macrophages – a cell type always found in the wall of aneurysms – resulted in a further doubling of the elastic fiber damage by the AAA-SMCs. Co-culture of macrophages with NAA-SMCs had no effect on the elastin degraded," said Dr. Airhart.
Further experiments suggested that the enzymes principally responsible for the elastolytic activity of these cells are the matrix metalloproteinases (MMPs). Increases in the production and/or activation of MMP-2 and/or MMP-9 were prominently found in cultures of AAA-SMCs.
"These studies present the strongest evidence that AAA-SMCs exhibit a disease-specific gene expression pattern and can very potently damage the elastic fiber matrix in the aortic wall. The unique and remarkable synergy with activated inflammatory cells might help explain the characteristic elastin loss of aortic aneurysms. Future studies will allow us to understand and alter the cellular mechanisms which lead to increased production and activation of elastolytic MMPs by these cells," Dr. Curci concluded.
The prestigious Resident Research Prize is intended to motivate new physicians to pursue vascular research. The prize recipient is invited to present his or her research results at the Society for Vascular Surgery’s Vascular Annual Meeting and the prize includes a 1-year subscription to the Journal of Vascular Surgery.
Veith's Views: Second opinions are overrated
A middle aged man goes to his primary care physician for his annual check-up. Because of an abnormal physical finding or laboratory test, he is referred to a specialist, who, after additional tests, recommends an operation with considerable risks. Before agreeing to the procedure, the man decides to seek a "second opinion." This sequence of events occurs routinely as the second opinion is generally accepted as one of the sacred cows of American medical care.
Let’s examine this sacred cow to see if it is a good thing or an overrated practice that serves little useful purpose. First the potential advantages. If the second specialist agrees with the first opinion, it can be reassuring to the patient and his family, but it really is unnecessary.
On the other hand, if the original specialist is less than optimal or motivated by the financial rewards of performing his recommended procedure, the second opinion can possibly benefit the patient by saving him from an unnecessary, wrong or possibly harmful operation. However, why not solicit the opinion of the second, better specialist first.
Now the downside. If the second specialist disagrees with the first, the patient faces a dilemma. He has to pick between the two specialists. How does he do this? Does he follow the advice of the more articulate and likeable specialist? Does he pick the opinion he likes despite being a non-expert? Does he solicit a third opinion – a tie breaker? Taking a vote on a medical or scientific question does not ensure arriving at the correct answer – especially if the vote is 2:1 and especially if one of the specialists is self-appointed or a full-fledged phony. So disagreement between the first and second specialist does not ensure better care. It can lead to confusion and uncertainty. It may lead to the wrong course of action. Our second opinion process may therefore be unnecessary or misleading, and is in reality not worth much.
What should replace this flawed sacred cow? In principle it is simple, in practice not so simple. The first specialist referral should be to an exemplary medical practitioner, one whose knowledge, judgment, skill level, and motivation can be trusted. Finding such an individual is complex. Referral patterns can be flawed and based on proximity, personality or economic considerations.
Examining a "top doctors list" can also be misleading since inclusion in some of these listings can be based on flawed criteria or even payment of a fee. Similar considerations may apply to some listings of top hospitals. Moreover, not every specialist in top hospitals is expert in all aspects of his or her specialty.
The key to finding an initial exemplary specialist whose first opinion can be trusted is to have that specialist identified by another knowledgeable physician who represents the patient’s interests. Such a "physician-trustee" can be a primary care physician with whom the patient has a solid relationship.
Alternatively, it can be a physician who is a friend, relative or acquaintance. In either case the physician-trustee has to take the time and make the effort to identify specialists he knows in the field in which the patient needs care. The physician-trustee must then make the additional effort to use these contacts to identify a first-rate specialist in the field and to explore the qualities, reputation, and results of this specialist by speaking to those who have worked with him directly and know him first hand.
Making such an effort is not a casual business in today’s complex medical environment. Yet it is one for which there is no other substitute. I have done it for friends and family on a number of occasions – often for patients who live in other cities and countries. It may take a number of phone calls to individuals in my own and other specialties within my own and other institutions. It does, however, produce positive results and solve the problem.
Unfortunately many who require expert specialty care in the United States do not have access to a dependable primary care giver or a trusted physician friend or relative who can serve as a physician-trustee.
Moreover, many insurance plans discourage specialist referrals or will only cover the costs of their selected, less than optimal in-network specialists. Finally, in the U.S. health care system even under the Affordable Care Act, no financial compensation is provided for physician-trustee services and the time and effort involved.
This deficiency must be corrected since physician-trustees can provide a uniquely valuable service. They can eliminate unnecessary financially motivated procedures; they facilitate identification of genuinely superior care-givers; and they enable patients to obtain referral to a specialist whose first opinion can be counted on to be dependable and who will deliver exemplary care. They also obviate the need for flawed and unnecessary second opinions.
Dr. Veith is Professor of Surgery at New York University Medical Center and the Cleveland Clinic. He is an associate medical editor for Vascular Specialist.
The ideas and opinions expressed in Vascular Specialist do not necessarily reflect those of the Society or Publisher.
A middle aged man goes to his primary care physician for his annual check-up. Because of an abnormal physical finding or laboratory test, he is referred to a specialist, who, after additional tests, recommends an operation with considerable risks. Before agreeing to the procedure, the man decides to seek a "second opinion." This sequence of events occurs routinely as the second opinion is generally accepted as one of the sacred cows of American medical care.
Let’s examine this sacred cow to see if it is a good thing or an overrated practice that serves little useful purpose. First the potential advantages. If the second specialist agrees with the first opinion, it can be reassuring to the patient and his family, but it really is unnecessary.
On the other hand, if the original specialist is less than optimal or motivated by the financial rewards of performing his recommended procedure, the second opinion can possibly benefit the patient by saving him from an unnecessary, wrong or possibly harmful operation. However, why not solicit the opinion of the second, better specialist first.
Now the downside. If the second specialist disagrees with the first, the patient faces a dilemma. He has to pick between the two specialists. How does he do this? Does he follow the advice of the more articulate and likeable specialist? Does he pick the opinion he likes despite being a non-expert? Does he solicit a third opinion – a tie breaker? Taking a vote on a medical or scientific question does not ensure arriving at the correct answer – especially if the vote is 2:1 and especially if one of the specialists is self-appointed or a full-fledged phony. So disagreement between the first and second specialist does not ensure better care. It can lead to confusion and uncertainty. It may lead to the wrong course of action. Our second opinion process may therefore be unnecessary or misleading, and is in reality not worth much.
What should replace this flawed sacred cow? In principle it is simple, in practice not so simple. The first specialist referral should be to an exemplary medical practitioner, one whose knowledge, judgment, skill level, and motivation can be trusted. Finding such an individual is complex. Referral patterns can be flawed and based on proximity, personality or economic considerations.
Examining a "top doctors list" can also be misleading since inclusion in some of these listings can be based on flawed criteria or even payment of a fee. Similar considerations may apply to some listings of top hospitals. Moreover, not every specialist in top hospitals is expert in all aspects of his or her specialty.
The key to finding an initial exemplary specialist whose first opinion can be trusted is to have that specialist identified by another knowledgeable physician who represents the patient’s interests. Such a "physician-trustee" can be a primary care physician with whom the patient has a solid relationship.
Alternatively, it can be a physician who is a friend, relative or acquaintance. In either case the physician-trustee has to take the time and make the effort to identify specialists he knows in the field in which the patient needs care. The physician-trustee must then make the additional effort to use these contacts to identify a first-rate specialist in the field and to explore the qualities, reputation, and results of this specialist by speaking to those who have worked with him directly and know him first hand.
Making such an effort is not a casual business in today’s complex medical environment. Yet it is one for which there is no other substitute. I have done it for friends and family on a number of occasions – often for patients who live in other cities and countries. It may take a number of phone calls to individuals in my own and other specialties within my own and other institutions. It does, however, produce positive results and solve the problem.
Unfortunately many who require expert specialty care in the United States do not have access to a dependable primary care giver or a trusted physician friend or relative who can serve as a physician-trustee.
Moreover, many insurance plans discourage specialist referrals or will only cover the costs of their selected, less than optimal in-network specialists. Finally, in the U.S. health care system even under the Affordable Care Act, no financial compensation is provided for physician-trustee services and the time and effort involved.
This deficiency must be corrected since physician-trustees can provide a uniquely valuable service. They can eliminate unnecessary financially motivated procedures; they facilitate identification of genuinely superior care-givers; and they enable patients to obtain referral to a specialist whose first opinion can be counted on to be dependable and who will deliver exemplary care. They also obviate the need for flawed and unnecessary second opinions.
Dr. Veith is Professor of Surgery at New York University Medical Center and the Cleveland Clinic. He is an associate medical editor for Vascular Specialist.
The ideas and opinions expressed in Vascular Specialist do not necessarily reflect those of the Society or Publisher.
A middle aged man goes to his primary care physician for his annual check-up. Because of an abnormal physical finding or laboratory test, he is referred to a specialist, who, after additional tests, recommends an operation with considerable risks. Before agreeing to the procedure, the man decides to seek a "second opinion." This sequence of events occurs routinely as the second opinion is generally accepted as one of the sacred cows of American medical care.
Let’s examine this sacred cow to see if it is a good thing or an overrated practice that serves little useful purpose. First the potential advantages. If the second specialist agrees with the first opinion, it can be reassuring to the patient and his family, but it really is unnecessary.
On the other hand, if the original specialist is less than optimal or motivated by the financial rewards of performing his recommended procedure, the second opinion can possibly benefit the patient by saving him from an unnecessary, wrong or possibly harmful operation. However, why not solicit the opinion of the second, better specialist first.
Now the downside. If the second specialist disagrees with the first, the patient faces a dilemma. He has to pick between the two specialists. How does he do this? Does he follow the advice of the more articulate and likeable specialist? Does he pick the opinion he likes despite being a non-expert? Does he solicit a third opinion – a tie breaker? Taking a vote on a medical or scientific question does not ensure arriving at the correct answer – especially if the vote is 2:1 and especially if one of the specialists is self-appointed or a full-fledged phony. So disagreement between the first and second specialist does not ensure better care. It can lead to confusion and uncertainty. It may lead to the wrong course of action. Our second opinion process may therefore be unnecessary or misleading, and is in reality not worth much.
What should replace this flawed sacred cow? In principle it is simple, in practice not so simple. The first specialist referral should be to an exemplary medical practitioner, one whose knowledge, judgment, skill level, and motivation can be trusted. Finding such an individual is complex. Referral patterns can be flawed and based on proximity, personality or economic considerations.
Examining a "top doctors list" can also be misleading since inclusion in some of these listings can be based on flawed criteria or even payment of a fee. Similar considerations may apply to some listings of top hospitals. Moreover, not every specialist in top hospitals is expert in all aspects of his or her specialty.
The key to finding an initial exemplary specialist whose first opinion can be trusted is to have that specialist identified by another knowledgeable physician who represents the patient’s interests. Such a "physician-trustee" can be a primary care physician with whom the patient has a solid relationship.
Alternatively, it can be a physician who is a friend, relative or acquaintance. In either case the physician-trustee has to take the time and make the effort to identify specialists he knows in the field in which the patient needs care. The physician-trustee must then make the additional effort to use these contacts to identify a first-rate specialist in the field and to explore the qualities, reputation, and results of this specialist by speaking to those who have worked with him directly and know him first hand.
Making such an effort is not a casual business in today’s complex medical environment. Yet it is one for which there is no other substitute. I have done it for friends and family on a number of occasions – often for patients who live in other cities and countries. It may take a number of phone calls to individuals in my own and other specialties within my own and other institutions. It does, however, produce positive results and solve the problem.
Unfortunately many who require expert specialty care in the United States do not have access to a dependable primary care giver or a trusted physician friend or relative who can serve as a physician-trustee.
Moreover, many insurance plans discourage specialist referrals or will only cover the costs of their selected, less than optimal in-network specialists. Finally, in the U.S. health care system even under the Affordable Care Act, no financial compensation is provided for physician-trustee services and the time and effort involved.
This deficiency must be corrected since physician-trustees can provide a uniquely valuable service. They can eliminate unnecessary financially motivated procedures; they facilitate identification of genuinely superior care-givers; and they enable patients to obtain referral to a specialist whose first opinion can be counted on to be dependable and who will deliver exemplary care. They also obviate the need for flawed and unnecessary second opinions.
Dr. Veith is Professor of Surgery at New York University Medical Center and the Cleveland Clinic. He is an associate medical editor for Vascular Specialist.
The ideas and opinions expressed in Vascular Specialist do not necessarily reflect those of the Society or Publisher.
Tips for talking to teens about drug use
Oxygen debt key in multiple organ dysfunction
SAN FRANCISCO – Multiple organ dysfunction syndrome is "underappreciated" by many of today’s clinicians, as optimal ways to treat it remain elusive, said Dr. Larry H. Hollier.
At the Vascular Annual Meeting, Dr. Hollier, professor of surgery and chancellor of the Louisiana State University Health Sciences Center, New Orleans, defined multiple organ dysfunction syndrome (MODS) as altered organ functions in an acutely ill patient requiring intervention to achieve homeostasis. "That’s a pretty broad definition, but it’s one of the most common causes of death in surgical intensive care units," he said. "Numerous precipitating factors classically described in multiple organ dysfunction syndrome include sepsis, trauma, cardiac arrest, visceral ischemia, burns, pancreatitis, shock, and major surgery with postoperative instability."
The pathophysiology of MODS "is fairly straightforward," he continued. "Some events result in ischemia and tissue hypoxia. Reperfusion occurs with the activation of cytokines, and an exaggerated inflammatory response generates oxygen free radicals, tissue damage, and then organ dysfunction." said Dr. Hollier, the invited speaker for the John Homans Lectureship of the SVS.
The major underlying issue in MODS stems from uncorrected oxygen debt in tissues. In fact, the level of perioperative tissue debt has a direct relationship on postoperative morbidity and mortality. According to Dr. Hollier, the predicted outcome by acutely accumulated oxygen debt in the first 4 hours post injury works like this: 8 L/m2 leads to a severe flulike syndrome (mild SIRS); 26 L/m2 leads to multiple organ dysfunction syndrome; and 33 L/m2 or more leads to death. "The uncorrected oxygen debt in tissues that is initiated is not the end of it," he said. "There’s an accumulating oxygen debt that amasses to keep biomass viable during low oxygen delivery. After resuscitation, there’s increased oxygen required above the basal rate, because explosive oxygen needs occur in order to fuel the inflammation of reperfusion injury."
Conventional therapies for MODS include volume resuscitation, ionotropic agents to improve cardiac performance and increase oxygen delivery, and ventilator support to improve oxygen input. Multiple experimental therapies have also been used, but no universal treatment has been found that reverses MODS, he said. "Early diagnosis and prompt treatment of organ hypoperfusion and hypoxia are of utmost importance. The major goal is to increase oxygen delivery as soon as possible."
Vascular surgeons are most likely to encounter MODS in cases of extensive blunt trauma, aortic transection/dissection, crush injury, severe ischemia following acute aortic occlusion, mesenteric infarction, and thoracoabdominal aortic surgery, both with extensive direct repair and with hybrid repair. The "hypoxia cascade" can occur without progression to the full multiple organ dysfunction syndrome. "The cascade can occur in refractory hypotension following repair of ruptured aortic aneurysm or other major vascular procedure, during brain ischemia, visceral ischemia, delayed onset paraplegia following repair of thoracoabdominal aortic aneurysms, and during the compartment syndrome."
Recommendations for intraoperative management of thoracoabdominal aortic aneurysms include maintaining visceral perfusion with a pump or a bypass or using visceral perfusion catheters, and perioperative CSF drainage "to allow reduction in the pressure around the spinal cord," he said.
Dr. Hollier said that management of serious injury in the commercial diver in the field has afforded two observations. First, high-dose hyperbaric oxygen, used very early in acute resuscitation of the severely injured, "effectively reduces oxygen debt." Second, the quick reduction of the oxygen debt by high-dose hyperbaric oxygen leverages chances of recovery. "What we do know is that there is only one variable that consistently predicts both mortality and multiple organ dysfunction syndrome following traumatic shock. That is oxygen debt."
Dr. Hollier had no disclosures.
SAN FRANCISCO – Multiple organ dysfunction syndrome is "underappreciated" by many of today’s clinicians, as optimal ways to treat it remain elusive, said Dr. Larry H. Hollier.
At the Vascular Annual Meeting, Dr. Hollier, professor of surgery and chancellor of the Louisiana State University Health Sciences Center, New Orleans, defined multiple organ dysfunction syndrome (MODS) as altered organ functions in an acutely ill patient requiring intervention to achieve homeostasis. "That’s a pretty broad definition, but it’s one of the most common causes of death in surgical intensive care units," he said. "Numerous precipitating factors classically described in multiple organ dysfunction syndrome include sepsis, trauma, cardiac arrest, visceral ischemia, burns, pancreatitis, shock, and major surgery with postoperative instability."
The pathophysiology of MODS "is fairly straightforward," he continued. "Some events result in ischemia and tissue hypoxia. Reperfusion occurs with the activation of cytokines, and an exaggerated inflammatory response generates oxygen free radicals, tissue damage, and then organ dysfunction." said Dr. Hollier, the invited speaker for the John Homans Lectureship of the SVS.
The major underlying issue in MODS stems from uncorrected oxygen debt in tissues. In fact, the level of perioperative tissue debt has a direct relationship on postoperative morbidity and mortality. According to Dr. Hollier, the predicted outcome by acutely accumulated oxygen debt in the first 4 hours post injury works like this: 8 L/m2 leads to a severe flulike syndrome (mild SIRS); 26 L/m2 leads to multiple organ dysfunction syndrome; and 33 L/m2 or more leads to death. "The uncorrected oxygen debt in tissues that is initiated is not the end of it," he said. "There’s an accumulating oxygen debt that amasses to keep biomass viable during low oxygen delivery. After resuscitation, there’s increased oxygen required above the basal rate, because explosive oxygen needs occur in order to fuel the inflammation of reperfusion injury."
Conventional therapies for MODS include volume resuscitation, ionotropic agents to improve cardiac performance and increase oxygen delivery, and ventilator support to improve oxygen input. Multiple experimental therapies have also been used, but no universal treatment has been found that reverses MODS, he said. "Early diagnosis and prompt treatment of organ hypoperfusion and hypoxia are of utmost importance. The major goal is to increase oxygen delivery as soon as possible."
Vascular surgeons are most likely to encounter MODS in cases of extensive blunt trauma, aortic transection/dissection, crush injury, severe ischemia following acute aortic occlusion, mesenteric infarction, and thoracoabdominal aortic surgery, both with extensive direct repair and with hybrid repair. The "hypoxia cascade" can occur without progression to the full multiple organ dysfunction syndrome. "The cascade can occur in refractory hypotension following repair of ruptured aortic aneurysm or other major vascular procedure, during brain ischemia, visceral ischemia, delayed onset paraplegia following repair of thoracoabdominal aortic aneurysms, and during the compartment syndrome."
Recommendations for intraoperative management of thoracoabdominal aortic aneurysms include maintaining visceral perfusion with a pump or a bypass or using visceral perfusion catheters, and perioperative CSF drainage "to allow reduction in the pressure around the spinal cord," he said.
Dr. Hollier said that management of serious injury in the commercial diver in the field has afforded two observations. First, high-dose hyperbaric oxygen, used very early in acute resuscitation of the severely injured, "effectively reduces oxygen debt." Second, the quick reduction of the oxygen debt by high-dose hyperbaric oxygen leverages chances of recovery. "What we do know is that there is only one variable that consistently predicts both mortality and multiple organ dysfunction syndrome following traumatic shock. That is oxygen debt."
Dr. Hollier had no disclosures.
SAN FRANCISCO – Multiple organ dysfunction syndrome is "underappreciated" by many of today’s clinicians, as optimal ways to treat it remain elusive, said Dr. Larry H. Hollier.
At the Vascular Annual Meeting, Dr. Hollier, professor of surgery and chancellor of the Louisiana State University Health Sciences Center, New Orleans, defined multiple organ dysfunction syndrome (MODS) as altered organ functions in an acutely ill patient requiring intervention to achieve homeostasis. "That’s a pretty broad definition, but it’s one of the most common causes of death in surgical intensive care units," he said. "Numerous precipitating factors classically described in multiple organ dysfunction syndrome include sepsis, trauma, cardiac arrest, visceral ischemia, burns, pancreatitis, shock, and major surgery with postoperative instability."
The pathophysiology of MODS "is fairly straightforward," he continued. "Some events result in ischemia and tissue hypoxia. Reperfusion occurs with the activation of cytokines, and an exaggerated inflammatory response generates oxygen free radicals, tissue damage, and then organ dysfunction." said Dr. Hollier, the invited speaker for the John Homans Lectureship of the SVS.
The major underlying issue in MODS stems from uncorrected oxygen debt in tissues. In fact, the level of perioperative tissue debt has a direct relationship on postoperative morbidity and mortality. According to Dr. Hollier, the predicted outcome by acutely accumulated oxygen debt in the first 4 hours post injury works like this: 8 L/m2 leads to a severe flulike syndrome (mild SIRS); 26 L/m2 leads to multiple organ dysfunction syndrome; and 33 L/m2 or more leads to death. "The uncorrected oxygen debt in tissues that is initiated is not the end of it," he said. "There’s an accumulating oxygen debt that amasses to keep biomass viable during low oxygen delivery. After resuscitation, there’s increased oxygen required above the basal rate, because explosive oxygen needs occur in order to fuel the inflammation of reperfusion injury."
Conventional therapies for MODS include volume resuscitation, ionotropic agents to improve cardiac performance and increase oxygen delivery, and ventilator support to improve oxygen input. Multiple experimental therapies have also been used, but no universal treatment has been found that reverses MODS, he said. "Early diagnosis and prompt treatment of organ hypoperfusion and hypoxia are of utmost importance. The major goal is to increase oxygen delivery as soon as possible."
Vascular surgeons are most likely to encounter MODS in cases of extensive blunt trauma, aortic transection/dissection, crush injury, severe ischemia following acute aortic occlusion, mesenteric infarction, and thoracoabdominal aortic surgery, both with extensive direct repair and with hybrid repair. The "hypoxia cascade" can occur without progression to the full multiple organ dysfunction syndrome. "The cascade can occur in refractory hypotension following repair of ruptured aortic aneurysm or other major vascular procedure, during brain ischemia, visceral ischemia, delayed onset paraplegia following repair of thoracoabdominal aortic aneurysms, and during the compartment syndrome."
Recommendations for intraoperative management of thoracoabdominal aortic aneurysms include maintaining visceral perfusion with a pump or a bypass or using visceral perfusion catheters, and perioperative CSF drainage "to allow reduction in the pressure around the spinal cord," he said.
Dr. Hollier said that management of serious injury in the commercial diver in the field has afforded two observations. First, high-dose hyperbaric oxygen, used very early in acute resuscitation of the severely injured, "effectively reduces oxygen debt." Second, the quick reduction of the oxygen debt by high-dose hyperbaric oxygen leverages chances of recovery. "What we do know is that there is only one variable that consistently predicts both mortality and multiple organ dysfunction syndrome following traumatic shock. That is oxygen debt."
Dr. Hollier had no disclosures.
EXPERT ANALYSIS FROM THE VASCULAR ANNUAL MEETING
Project BOOST
Enactment of federal legislation imposing hospital reimbursement penalties for excess rates of rehospitalizations among Medicare fee for service beneficiaries markedly increased interest in hospital quality improvement (QI) efforts to reduce the observed 30‐day rehospitalization of 19.6% in this elderly population.[1, 2] The Congressional Budget Office estimated that reimbursement penalties to hospitals for high readmission rates are expected to save the Medicare program approximately $7 billion between 2010 and 2019.[3] These penalties are complemented by resources from the Center for Medicare and Medicaid Innovation aiming to reduce hospital readmissions by 20% by the end of 2013 through the Partnership for Patients campaign.[4] Although potential financial penalties and provision of resources for QI intensified efforts to enhance the quality of the hospital discharge transition, patient safety risks associated with hospital discharge are well documented.[5, 6] Approximately 20% of patients discharged from the hospital may suffer adverse events,[7, 8] of which up to three‐quarters (72%) are medication related,[9] and over one‐third of required follow‐up testing after discharge is not completed.[10] Such findings indicate opportunities for improvement in the discharge process.[11]
Numerous publications describe studies aiming to improve the hospital discharge process and mitigate these hazards, though a systematic review of interventions to reduce 30‐day rehospitalization indicated that the existing evidence base for the effectiveness of transition interventions demonstrates irregular effectiveness and limitations to generalizability.[12] Most studies showing effectiveness are confined to single academic medical centers. Existing evidence supports multifaceted interventions implemented in both the pre‐ and postdischarge periods and focused on risk assessment and tailored, patient‐centered application of interventions to mitigate risk. For example Project RED (Re‐Engineered Discharge) applied a bundled intervention consisting of intensified patient education and discharge planning, improved medication reconciliation and discharge instructions, and longitudinal patient contact with follow‐up phone calls and a dedicated discharge advocate.[13] However, the mean age of patients participating in the study was 50 years, and it excluded patients admitted from or discharged to skilled nursing facilities, making generalizability to the geriatric population uncertain.
An integral aspect of QI projects is the contribution of local context to translation of best practices to disparate settings.[14, 15, 16] Most available reports of successful interventions to reduce rehospitalization have not fully described the specifics of either the intervention context or design. Moreover, the available evidence base for common interventions to reduce rehospitalization was developed in the academic setting. Validation of single academic center studies in a broader healthcare context is necessary.
Project BOOST (Better Outcomes for Older adults through Safe Transitions) recruited a diverse national cohort of both academic and nonacademic hospitals to participate in a QI effort to implement best practices for hospital discharge care transitions using a national collaborative approach facilitated by external expert mentorship. This study aimed to determine the effectiveness of BOOST in lowering hospital readmission rates and impact on length of stay.
METHODS
The study of Project BOOST was undertaken in accordance with the SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines.[17]
Participants
The unit of observation for the prospective cohort study was the clinical acute‐care unit within hospitals. Sites were instructed to designate a pilot unit for the intervention that cared for medical or mixed medicalsurgical patient populations. Sites were also asked to provide outcome data for a clinically and organizationally similar non‐BOOST unit to provide a site‐matched control. Control units were matched by local site leadership based on comparable patient demographics, clinical mix, and extent of housestaff presence. An initial cohort of 6 hospitals in 2008 was followed by a second cohort of 24 hospitals initiated in 2009. All hospitals were invited to participate in the national effectiveness analysis, which required submission of readmission and length of stay data for both a BOOST intervention unit and a clinically matched control unit.
Description of the Intervention
The BOOST intervention consisted of 2 major sequential processes, planning and implementation, both facilitated by external site mentorsphysicians expert in QI and care transitionsfor a period of 12 months. Extensive background on the planning and implementation components is available at
| Enrollment Sites, n=30 | Sites Reporting Outcome Data, n=11 | Sites Not Reporting Outcome Data, n=19 | P Value for Comparison of Outcome Data Sites Compared to Othersa | |
|---|---|---|---|---|
| ||||
| Region, n (%) | 0.194 | |||
| Northeast | 8 (26.7) | 2 (18.2) | 6 (31.6) | |
| West | 7 (23.4) | 2 (18.2) | 5 (26.3) | |
| South | 7 (23.4) | 3 (27.3) | 4 (21.1) | |
| Midwest | 8 (26.7) | 4 (36.4) | 4 (21.1) | |
| Urban location, n (%) | 25 (83.3) | 11 (100) | 15 (78.9) | 0.035 |
| Teaching status, n (%) | 0.036 | |||
| Academic medical center | 10 (33.4) | 5 (45.5) | 5 (26.3) | |
| Community teaching | 8 (26.7) | 3 (27.3) | 5 (26.3) | |
| Community nonteaching | 12 (40) | 3 (27.3) | 9 (47.4) | |
| Beds number, mean (SD) | 426.6 (220.6) | 559.2 (187.8) | 349.79 (204.48) | 0.003 |
| Number of tools implemented, n (%) | 0.194 | |||
| 0 | 2 (6.7) | 0 | 2 (10.5) | |
| 1 | 2 (6.7) | 0 | 2 (10.5) | |
| 2 | 4 (13.3) | 2 (18.2) | 2 (10.5) | |
| 3 | 12 (40.0) | 3 (27.3) | 8 (42.1) | |
| 4 | 9 (30.0) | 5 (45.5) | 4 (21.1) | |
| 5 | 1 (3.3) | 1 (9.1) | 1 (5.3) | |
Mentor engagement with sites consisted of a 2‐day kickoff training on the BOOST tools, where site teams met their mentor and initiated development of structured action plans, followed by 5 to 6 scheduled phone calls in the subsequent 12 months. During these conference calls, mentors gauged progress and sought to help troubleshoot barriers to implementation. Some mentors also conducted a site visit with participant sites. Project BOOST provided sites with several collaborative activities including online webinars and an online listserv. Sites also received a quarterly newsletter.
Outcome Measures
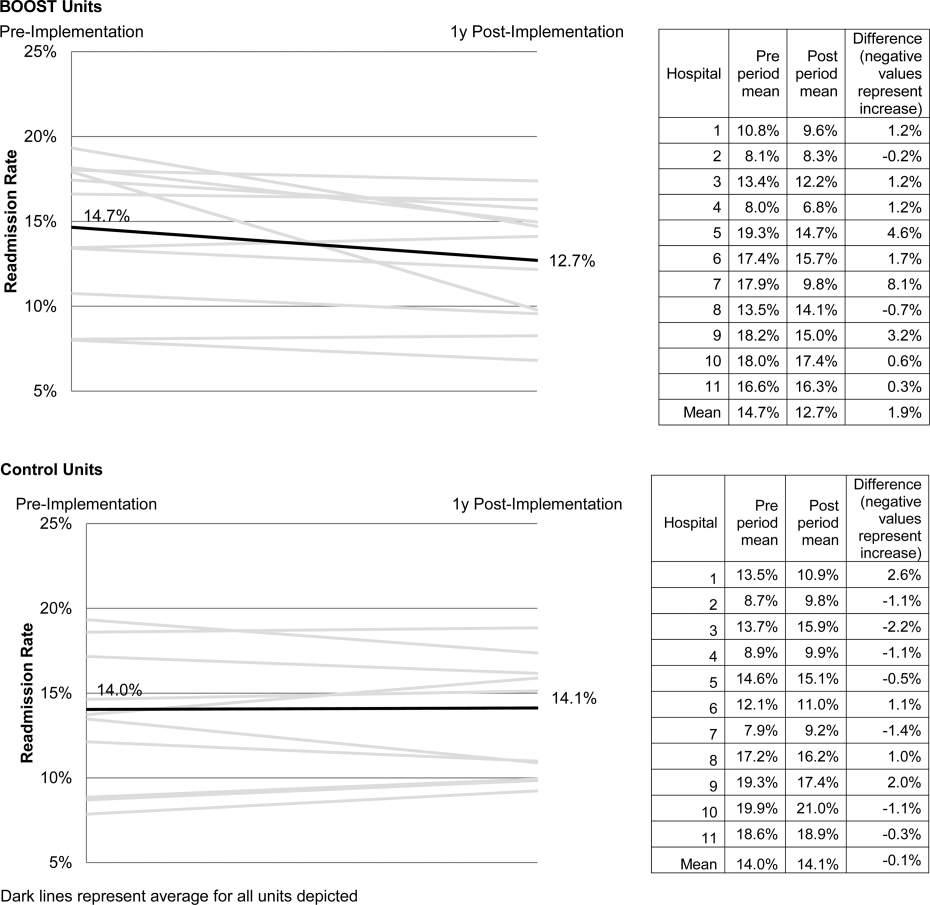
The primary outcome was 30‐day rehospitalization defined as same hospital, all‐cause rehospitalization. Home discharges as well as discharges or transfers to other healthcare facilities were included in the discharge calculation. Elective or scheduled rehospitalizations as well as multiple rehospitalizations in the same 30‐day window were considered individual rehospitalization events. Rehospitalization was reported as a ratio of 30‐day rehospitalizations divided by live discharges in a calendar month. Length of stay was reported as the mean length of stay among live discharges in a calendar month. Outcomes were calculated at the participant site and then uploaded as overall monthly unit outcomes to a Web‐based research database.
To account for seasonal trends as well as marked variation in month‐to‐month rehospitalization rates identified in longitudinal data, we elected to compare 3‐month year‐over‐year averages to determine relative changes in readmission rates from the period prior to BOOST implementation to the period after BOOST implementation. We calculated averages for rehospitalization and length of stay in the 3‐month period preceding the sites' first reported month of front‐line implementation and in the corresponding 3‐month period in the subsequent calendar year. For example, if a site reported implementing its first tool in April 2010, the average readmission rate in the unit for January 2011 through March 2011 was subtracted from the average readmission rate for January 2010 through March 2010.
Sites were surveyed regarding tool implementation rates 6 months and 24 months after the 2009 kickoff training session. Surveys were electronically completed by site leaders in consultation with site team members. The survey identified new tool implementation as well as modification of existing care processes using the BOOST tools (admission risk assessment, discharge readiness checklist, teach back use, mandate regarding discharge summary completion, follow‐up phone calls to >80% of discharges). Use of a sixth tool, creation of individualized written discharge instructions, was not measured. We credited sites with tool implementation if they reported either de novo tool use or alteration of previous care processes influenced by BOOST tools.
Clinical outcome reporting was voluntary, and sites did not receive compensation and were not subject to penalty for the degree of implementation or outcome reporting. No patient‐level information was collected for the analysis, which was approved by the Northwestern University institutional review board.
Data Sources and Methods
Readmission and length of stay data, including the unit level readmission rate, as collected from administrative sources at each hospital, were collected using templated spreadsheet software between December 2008 and June 2010, after which data were loaded directly to a Web‐based data‐tracking platform. Sites were asked to load data as they became available. Sites were asked to report the number of study unit discharges as well as the number of those discharges readmitted within 30 days; however, reporting of the number of patient discharges was inconsistent across sites. Serial outreach consisting of monthly phone calls or email messaging to site leaders was conducted throughout 2011 to increase site participation in the project analysis.
Implementation date information was collected from 2 sources. The first was through online surveys distributed in November 2009 and April 2011. The second was through fields in the Web‐based data tracking platform to which sites uploaded data. In cases where disagreement was found between these 2 sources, the site leader was contacted for clarification.
Practice setting (community teaching, community nonteaching, academic medical center) was determined by site‐leader report within the Web‐based data tracking platform. Data for hospital characteristics (number of licensed beds and geographic region) were obtained from the American Hospital Association's Annual Survey of Hospitals.[18] Hospital region was characterized as West, South, Midwest, or Northeast.
Analysis
The null hypothesis was that no prepost difference existed in readmission rates within BOOST units, and no difference existed in the prepost change in readmission rates in BOOST units when compared to site‐matched control units. The Wilcoxon rank sum test was used to test whether observed changes described above were significantly different from 0, supporting rejection of the null hypotheses. We performed similar tests to determine the significance of observed changes in length of stay. We performed our analysis using SAS 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Eleven hospitals provided rehospitalization and length‐of‐stay outcome data for both a BOOST and control unit for the pre‐ and postimplementation periods. Compared to the 19 sites that did not participate in the analysis, these 11 sites were significantly larger (559188 beds vs 350205 beds, P=0.003), more likely to be located in an urban area (100.0% [n=11] vs 78.9% [n=15], P=0.035), and more likely to be academic medical centers (45.5% [n=5] vs 26.3% [n=5], P=0.036) (Table 1).
The mean number of tools implemented by sites participating in the analysis was 3.50.9. All sites implemented at least 2 tools. The duration between attendance at the BOOST kickoff event and first tool implementation ranged from 3 months (first tool implemented prior to attending the kickoff) and 9 months (mean duration, 3.34.3 months) (Table 2).
| Hospital | Region | Hospital Type | No. Licensed Beds | Kickoff Implementationa | Risk Assessment | Discharge Checklist | Teach Back | Discharge Summary Completion | Follow‐up Phone Call | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
| 1 | Midwest | Community teaching | <300 | 8 | 3 | |||||
| 2 | West | Community teaching | >600 | 0 | 4 | |||||
| 3 | Northeast | Academic medical center | >600 | 2 | 4 | |||||
| 4 | Northeast | Community nonteaching | <300 | 9 | 2 | |||||
| 5 | South | Community nonteaching | >600 | 6 | 3 | |||||
| 6 | South | Community nonteaching | >600 | 3 | 4 | |||||
| 7 | Midwest | Community teaching | 300600 | 1 | 5 | |||||
| 8 | West | Academic medical center | 300600 | 1 | 4 | |||||
| 9 | South | Academic medical center | >600 | 4 | 4 | |||||
| 10 | Midwest | Academic medical center | 300600 | 3 | 3 | |||||
| 11 | Midwest | Academic medical center | >600 | 9 | 2 | |||||
The average rate of 30‐day rehospitalization among BOOST units was 14.7% in the preimplementation period and 12.7% during the postimplementation period (P=0.010) (Figure 1). Rehospitalization rates for matched control units were 14.0% in the preintervention period and 14.1% in the postintervention period (P=0.831). The mean absolute reduction in readmission rates over the 1‐year study period in BOOST units compared to control units was 2.0%, or a relative reduction of 13.6% (P=0.054 for signed rank test comparing differences in readmission rate reduction in BOOST units compared to site‐matched control units). Length of stay in BOOST and control units decreased an average of 0.5 days and 0.3 days, respectively. There was no difference in length of stay change between BOOST units and control units (P=0.966).
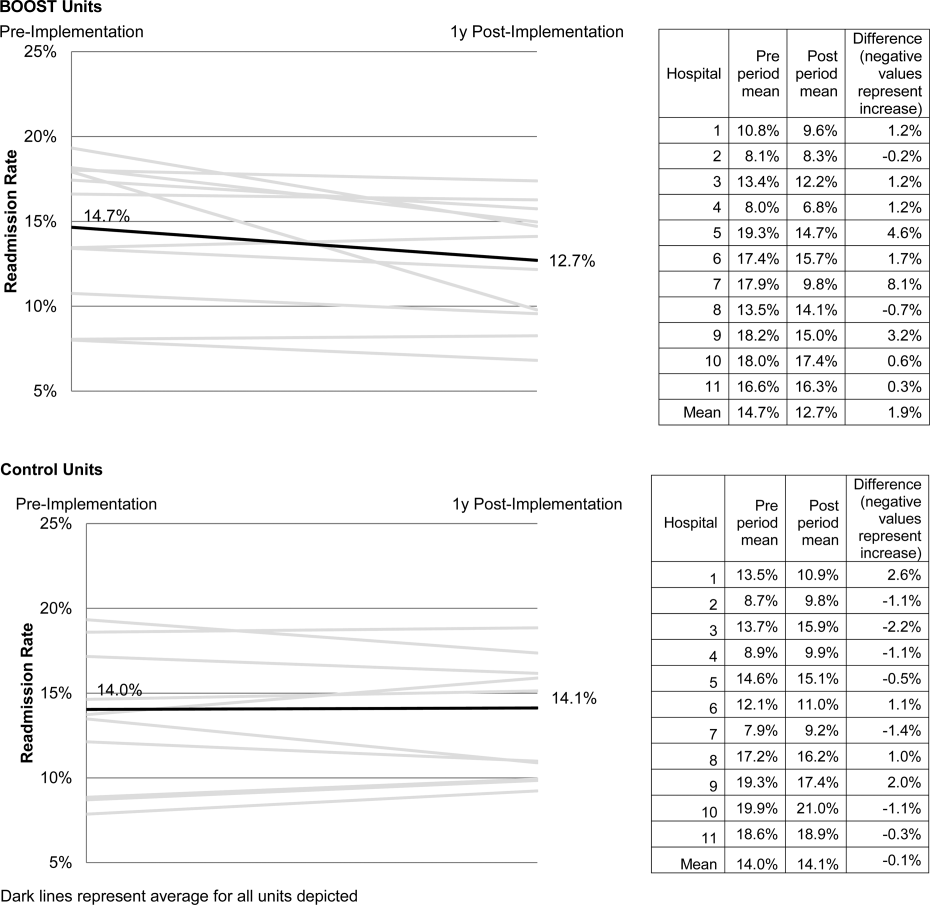
DISCUSSION
As hospitals strive to reduce their readmission rates to avoid Centers for Medicare and Medicaid Services penalties, Project BOOST may be a viable QI approach to achieve their goals. This initial evaluation of participation in Project BOOST by 11 hospitals of varying sizes across the United States showed an associated reduction in rehospitalization rates (absolute=2.0% and relative=13.6%, P=0.054). We did not find any significant change in length of stay among these hospitals implementing BOOST tools.
The tools provided to participating hospitals were developed from evidence found in peer‐reviewed literature established through experimental methods in well‐controlled academic settings. Further tool development was informed by recommendations of an advisory board consisting of expert representatives and advocates involved in the hospital discharge process: patients, caregivers, physicians, nurses, case managers, social workers, insurers, and regulatory and research agencies.[19] The toolkit components address multiple aspects of hospital discharge and follow‐up with the goal of improving health by optimizing the safety of care transitions. Our observation that readmission rates appeared to improve in a diverse hospital sample including nonacademic and community hospitals engaged in Project BOOST is reassuring that the benefits seen in existing research literature, developed in distinctly academic settings, can be replicated in diverse acute‐care settings.
The effect size observed in our study was modest but consistent with several studies identified in a recent review of trials measuring interventions to reduce rehospitalization, where 7 of 16 studies showing a significant improvement registered change in the 0% to 5% absolute range.[12] Impact of this project may have been tempered by the need to translate external QI content to the local setting. Additionally, in contrast to experimental studies that are limited in scope and timing and often scaled to a research budget, BOOST sites were encouraged to implement Project BOOST in the clinical setting even if no new funds were available to support the effort.[12]
The recruitment of a national sample of both academic and nonacademic hospital participants imposed several limitations on our study and analysis. We recognize that intervention units selected by hospitals may have had unmeasured unit and patient characteristics that facilitated successful change and contributed to the observed improvements. However, because external pressure to reduce readmission is present across all hospitals independent of the BOOST intervention, we felt site‐matched controls were essential to understanding effects attributable to the BOOST tools. Differences between units would be expected to be stable over the course of the study period, and comparison of outcome differences between 2 different time periods would be reasonable. Additionally, we could not collect data on readmissions to other hospitals. Theoretically, patients discharged from BOOST units might be more likely to have been rehospitalized elsewhere, but the fraction of rehospitalizations occurring at alternate facilities would also be expected to be similar on the matched control unit.
We report findings from a voluntary cohort willing and capable of designating a comparison clinical unit and contributing the requested data outcomes. Pilot sites that did not report outcomes were not analyzed, but comparison of hospital characteristics shows that participating hospitals were more likely to be large, urban, academic medical centers. Although barriers to data submission were not formally analyzed, reports from nonparticipating sites describe data submission limited by local implementation design (no geographic rollout or simultaneous rollout on all appropriate clinical units), site specific inability to generate unit level outcome statistics, and competing organizational priorities for data analyst time (electronic medical record deployment, alternative QI initiatives). The external validity of our results may be limited to organizations capable of analytics at the level of the individual clinical unit as well as those with sufficient QI resources to support reporting to a national database in the absence of a payer mandate. It is possible that additional financial support for on‐site data collection would have bolstered participation, making the example of participation rates we present potentially informative to organizations hoping to widely disseminate a QI agenda.
Nonetheless, the effectiveness demonstrated in the 11 sites that did participate is encouraging, and ongoing collaboration with subsequent BOOST cohorts has been designed to further facilitate data collection. Among the insights gained from this pilot experience, and incorporated into ongoing BOOST cohorts, is the importance of intensive mentor engagement to foster accountability among participant sites, assist with implementation troubleshooting, and offer expertise that is often particularly effective in gaining local support. We now encourage sites to have 2 mentor site visits to further these roles and more frequent conference calls. Further research to understand the marginal benefit of the mentored implementation approach is ongoing.
The limitations in data submission we experienced with the pilot cohort likely reflect resource constraints not uncommon at many hospitals. Increasing pressure placed on hospitals as a result of the Readmission Reduction Program within the Affordable Care Act as well as increasing interest from private and Medicaid payors to incorporate similar readmission‐based penalties provide encouragement for hospitals to enhance their data and analytic skills. National incentives for implementation of electronic health records (EHR) should also foster such capabilities, though we often saw EHRs as a barrier to QI, especially rapid cycle trials. Fortunately, hospitals are increasingly being afforded access to comprehensive claims databases to assist in tracking readmission rates to other facilities, and these data are becoming available in a more timely fashion. This more robust data collection, facilitated by private payors, state QI organizations, and state hospital associations, will support additional analytic methods such as multivariate regression models and interrupted time series designs to appreciate the experience of current BOOST participants.
Additional research is needed to understand the role of organizational context in the effectiveness of Project BOOST. Differences in rates of tool implementation and changes in clinical outcomes are likely dependent on local implementation context at the level of the healthcare organization and individual clinical unit.[20] Progress reports from site mentors and previously described experiences of QI implementation indicate that successful implementation of a multidimensional bundle of interventions may have reflected a higher level of institutional support, more robust team engagement in the work of reducing readmissions, increased clinical staff support for change, the presence of an effective project champion, or a key facilitating role of external mentorship.[21, 22] Ongoing data collection will continue to measure the sustainability of tool use and observed outcome changes to inform strategies to maintain gains associated with implementation. The role of mentored implementation in facilitating gains also requires further study.
Increasing attention to the problem of avoidable rehospitalization is driving hospitals, insurers, and policy makers to pursue QI efforts that favorably impact readmission rates. Our analysis of the BOOST intervention suggests that modest gains can be achieved following evidence‐based hospital process change facilitated by a mentored implementation model. However, realization of the goal of a 20% reduction in rehospitalization proposed by the Center for Medicare and Medicaid Services' Partnership for Patients initiative may be difficult to achieve on a national scale,[23] especially if efforts focus on just the hospital.
Acknowledgments
The authors acknowledge the contributions of Amanda Creden, BA (data collection), Julia Lee (biostatistical support), and the support of Amy Berman, BS, RN, Senior Program Officer at The John A. Hartford Foundation.
Disclosures
Project BOOST was funded by a grant from The John A. Hartford Foundation. Project BOOST is administered by the Society of Hospital Medicine (SHM). The development of the Project BOOST toolkit, recruitment of sites for this study, mentorship of the pilot cohort, project evaluation planning, and collection of pilot data were funded by a grant from The John A. Harford Foundation. Additional funding for continued data collection and analysis was funded by the SHM through funds from hospitals to participate in Project BOOST, specifically with funding support for Dr. Hansen. Dr. Williams has received funding to serve as Principal Investigator for Project BOOST. Since the time of initial cohort participation, approximately 125 additional hospitals have participated in the mentored implementation of Project BOOST. This participation was funded through a combination of site‐based tuition, third‐party payor support from private insurers, foundations, and federal funding through the Center for Medicare and Medicaid Innovation Partnership for Patients program. Drs. Greenwald, Hansen, and Williams are Project BOOST mentors for current Project BOOST sites and receive financial support through the SHM for this work. Dr. Howell has previously received funding as a Project BOOST mentor. Ms. Budnitz is the BOOST Project Director and is Chief Strategy and Development Officer for the HM. Dr. Maynard is the Senior Vice President of the SHM's Center for Hospital Innovation and Improvement.
References
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- United States Congress. House Committee on Education and Labor. Coe on Ways and Means, Committee on Energy and Commerce, Compilation of Patient Protection and Affordable Care Act: as amended through November 1, 2010 including Patient Protection and Affordable Care Act health‐related portions of the Health Care and Education Reconciliation Act of 2010. Washington, DC: US Government Printing Office; 2010.
- Cost estimate for the amendment in the nature of a substitute to H.R. 3590, as proposed in the Senate on November 18, 2009. Washington, DC: Congressional Budget Office; 2009.
- Partnership for Patients, Center for Medicare and Medicaid Innovation. Available at: http://www.innovations.cms.gov/initiatives/Partnership‐for‐Patients/index.html. Accessed December 12, 2012.
- , . Providers have failed to work for continuity. Hospitals. 1979;53(10):79.
- , . Executing high‐quality care transitions: a call to do it right. J Hosp Med. 2007;2(5):287–290.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345–349.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477–485.
- , , . Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167(12):1305.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians. JAMA. 2007;297(8):831–841.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178.
- , , , et al. Advancing the science of patient safety. Ann Intern Med. 2011;154(10):693–696.
- , . From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362(9391):1225–1230.
- , , , et al. Quality improvement projects targeting health care‐associated infections: comparing virtual collaborative and toolkit approaches. J Hosp Med. 2011;6(5):271–278.
- , , , , . Publication guidelines for improvement studies in health care: evolution of the SQUIRE project. Ann Intern Med. 2008;149(9):670–676.
- , , , . Risk stratification and therapeutic decision making in acute coronary syndromes. JAMA. 2000;284(7):876–878.
- , , . Are diagnosis specific outcome indicators based on administrative data useful in assessing quality of hospital care? BMJ. 2004;13(1):32.
- , , , et al. What distinguishes top‐performing hospitals in acute myocardial infarction mortality rates? Ann Intern Med. 2011;154(6):384–390.
- , , , . The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf. 2012;21(1):13–20.
- , . Evidence‐based quality improvement: the state of the science. Health Aff (Millwood). 2005;24(1):138–150.
- Center for Medicare and Medicaid Innovation. Partnership for patients. Available at: http://www.innovations.cms.gov/initiatives/Partnership‐for‐Patients/index.html. Accessed April 2, 2012.
Enactment of federal legislation imposing hospital reimbursement penalties for excess rates of rehospitalizations among Medicare fee for service beneficiaries markedly increased interest in hospital quality improvement (QI) efforts to reduce the observed 30‐day rehospitalization of 19.6% in this elderly population.[1, 2] The Congressional Budget Office estimated that reimbursement penalties to hospitals for high readmission rates are expected to save the Medicare program approximately $7 billion between 2010 and 2019.[3] These penalties are complemented by resources from the Center for Medicare and Medicaid Innovation aiming to reduce hospital readmissions by 20% by the end of 2013 through the Partnership for Patients campaign.[4] Although potential financial penalties and provision of resources for QI intensified efforts to enhance the quality of the hospital discharge transition, patient safety risks associated with hospital discharge are well documented.[5, 6] Approximately 20% of patients discharged from the hospital may suffer adverse events,[7, 8] of which up to three‐quarters (72%) are medication related,[9] and over one‐third of required follow‐up testing after discharge is not completed.[10] Such findings indicate opportunities for improvement in the discharge process.[11]
Numerous publications describe studies aiming to improve the hospital discharge process and mitigate these hazards, though a systematic review of interventions to reduce 30‐day rehospitalization indicated that the existing evidence base for the effectiveness of transition interventions demonstrates irregular effectiveness and limitations to generalizability.[12] Most studies showing effectiveness are confined to single academic medical centers. Existing evidence supports multifaceted interventions implemented in both the pre‐ and postdischarge periods and focused on risk assessment and tailored, patient‐centered application of interventions to mitigate risk. For example Project RED (Re‐Engineered Discharge) applied a bundled intervention consisting of intensified patient education and discharge planning, improved medication reconciliation and discharge instructions, and longitudinal patient contact with follow‐up phone calls and a dedicated discharge advocate.[13] However, the mean age of patients participating in the study was 50 years, and it excluded patients admitted from or discharged to skilled nursing facilities, making generalizability to the geriatric population uncertain.
An integral aspect of QI projects is the contribution of local context to translation of best practices to disparate settings.[14, 15, 16] Most available reports of successful interventions to reduce rehospitalization have not fully described the specifics of either the intervention context or design. Moreover, the available evidence base for common interventions to reduce rehospitalization was developed in the academic setting. Validation of single academic center studies in a broader healthcare context is necessary.
Project BOOST (Better Outcomes for Older adults through Safe Transitions) recruited a diverse national cohort of both academic and nonacademic hospitals to participate in a QI effort to implement best practices for hospital discharge care transitions using a national collaborative approach facilitated by external expert mentorship. This study aimed to determine the effectiveness of BOOST in lowering hospital readmission rates and impact on length of stay.
METHODS
The study of Project BOOST was undertaken in accordance with the SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines.[17]
Participants
The unit of observation for the prospective cohort study was the clinical acute‐care unit within hospitals. Sites were instructed to designate a pilot unit for the intervention that cared for medical or mixed medicalsurgical patient populations. Sites were also asked to provide outcome data for a clinically and organizationally similar non‐BOOST unit to provide a site‐matched control. Control units were matched by local site leadership based on comparable patient demographics, clinical mix, and extent of housestaff presence. An initial cohort of 6 hospitals in 2008 was followed by a second cohort of 24 hospitals initiated in 2009. All hospitals were invited to participate in the national effectiveness analysis, which required submission of readmission and length of stay data for both a BOOST intervention unit and a clinically matched control unit.
Description of the Intervention
The BOOST intervention consisted of 2 major sequential processes, planning and implementation, both facilitated by external site mentorsphysicians expert in QI and care transitionsfor a period of 12 months. Extensive background on the planning and implementation components is available at
| Enrollment Sites, n=30 | Sites Reporting Outcome Data, n=11 | Sites Not Reporting Outcome Data, n=19 | P Value for Comparison of Outcome Data Sites Compared to Othersa | |
|---|---|---|---|---|
| ||||
| Region, n (%) | 0.194 | |||
| Northeast | 8 (26.7) | 2 (18.2) | 6 (31.6) | |
| West | 7 (23.4) | 2 (18.2) | 5 (26.3) | |
| South | 7 (23.4) | 3 (27.3) | 4 (21.1) | |
| Midwest | 8 (26.7) | 4 (36.4) | 4 (21.1) | |
| Urban location, n (%) | 25 (83.3) | 11 (100) | 15 (78.9) | 0.035 |
| Teaching status, n (%) | 0.036 | |||
| Academic medical center | 10 (33.4) | 5 (45.5) | 5 (26.3) | |
| Community teaching | 8 (26.7) | 3 (27.3) | 5 (26.3) | |
| Community nonteaching | 12 (40) | 3 (27.3) | 9 (47.4) | |
| Beds number, mean (SD) | 426.6 (220.6) | 559.2 (187.8) | 349.79 (204.48) | 0.003 |
| Number of tools implemented, n (%) | 0.194 | |||
| 0 | 2 (6.7) | 0 | 2 (10.5) | |
| 1 | 2 (6.7) | 0 | 2 (10.5) | |
| 2 | 4 (13.3) | 2 (18.2) | 2 (10.5) | |
| 3 | 12 (40.0) | 3 (27.3) | 8 (42.1) | |
| 4 | 9 (30.0) | 5 (45.5) | 4 (21.1) | |
| 5 | 1 (3.3) | 1 (9.1) | 1 (5.3) | |
Mentor engagement with sites consisted of a 2‐day kickoff training on the BOOST tools, where site teams met their mentor and initiated development of structured action plans, followed by 5 to 6 scheduled phone calls in the subsequent 12 months. During these conference calls, mentors gauged progress and sought to help troubleshoot barriers to implementation. Some mentors also conducted a site visit with participant sites. Project BOOST provided sites with several collaborative activities including online webinars and an online listserv. Sites also received a quarterly newsletter.
Outcome Measures
The primary outcome was 30‐day rehospitalization defined as same hospital, all‐cause rehospitalization. Home discharges as well as discharges or transfers to other healthcare facilities were included in the discharge calculation. Elective or scheduled rehospitalizations as well as multiple rehospitalizations in the same 30‐day window were considered individual rehospitalization events. Rehospitalization was reported as a ratio of 30‐day rehospitalizations divided by live discharges in a calendar month. Length of stay was reported as the mean length of stay among live discharges in a calendar month. Outcomes were calculated at the participant site and then uploaded as overall monthly unit outcomes to a Web‐based research database.
To account for seasonal trends as well as marked variation in month‐to‐month rehospitalization rates identified in longitudinal data, we elected to compare 3‐month year‐over‐year averages to determine relative changes in readmission rates from the period prior to BOOST implementation to the period after BOOST implementation. We calculated averages for rehospitalization and length of stay in the 3‐month period preceding the sites' first reported month of front‐line implementation and in the corresponding 3‐month period in the subsequent calendar year. For example, if a site reported implementing its first tool in April 2010, the average readmission rate in the unit for January 2011 through March 2011 was subtracted from the average readmission rate for January 2010 through March 2010.
Sites were surveyed regarding tool implementation rates 6 months and 24 months after the 2009 kickoff training session. Surveys were electronically completed by site leaders in consultation with site team members. The survey identified new tool implementation as well as modification of existing care processes using the BOOST tools (admission risk assessment, discharge readiness checklist, teach back use, mandate regarding discharge summary completion, follow‐up phone calls to >80% of discharges). Use of a sixth tool, creation of individualized written discharge instructions, was not measured. We credited sites with tool implementation if they reported either de novo tool use or alteration of previous care processes influenced by BOOST tools.
Clinical outcome reporting was voluntary, and sites did not receive compensation and were not subject to penalty for the degree of implementation or outcome reporting. No patient‐level information was collected for the analysis, which was approved by the Northwestern University institutional review board.
Data Sources and Methods
Readmission and length of stay data, including the unit level readmission rate, as collected from administrative sources at each hospital, were collected using templated spreadsheet software between December 2008 and June 2010, after which data were loaded directly to a Web‐based data‐tracking platform. Sites were asked to load data as they became available. Sites were asked to report the number of study unit discharges as well as the number of those discharges readmitted within 30 days; however, reporting of the number of patient discharges was inconsistent across sites. Serial outreach consisting of monthly phone calls or email messaging to site leaders was conducted throughout 2011 to increase site participation in the project analysis.
Implementation date information was collected from 2 sources. The first was through online surveys distributed in November 2009 and April 2011. The second was through fields in the Web‐based data tracking platform to which sites uploaded data. In cases where disagreement was found between these 2 sources, the site leader was contacted for clarification.
Practice setting (community teaching, community nonteaching, academic medical center) was determined by site‐leader report within the Web‐based data tracking platform. Data for hospital characteristics (number of licensed beds and geographic region) were obtained from the American Hospital Association's Annual Survey of Hospitals.[18] Hospital region was characterized as West, South, Midwest, or Northeast.
Analysis
The null hypothesis was that no prepost difference existed in readmission rates within BOOST units, and no difference existed in the prepost change in readmission rates in BOOST units when compared to site‐matched control units. The Wilcoxon rank sum test was used to test whether observed changes described above were significantly different from 0, supporting rejection of the null hypotheses. We performed similar tests to determine the significance of observed changes in length of stay. We performed our analysis using SAS 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Eleven hospitals provided rehospitalization and length‐of‐stay outcome data for both a BOOST and control unit for the pre‐ and postimplementation periods. Compared to the 19 sites that did not participate in the analysis, these 11 sites were significantly larger (559188 beds vs 350205 beds, P=0.003), more likely to be located in an urban area (100.0% [n=11] vs 78.9% [n=15], P=0.035), and more likely to be academic medical centers (45.5% [n=5] vs 26.3% [n=5], P=0.036) (Table 1).
The mean number of tools implemented by sites participating in the analysis was 3.50.9. All sites implemented at least 2 tools. The duration between attendance at the BOOST kickoff event and first tool implementation ranged from 3 months (first tool implemented prior to attending the kickoff) and 9 months (mean duration, 3.34.3 months) (Table 2).
| Hospital | Region | Hospital Type | No. Licensed Beds | Kickoff Implementationa | Risk Assessment | Discharge Checklist | Teach Back | Discharge Summary Completion | Follow‐up Phone Call | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
| 1 | Midwest | Community teaching | <300 | 8 | 3 | |||||
| 2 | West | Community teaching | >600 | 0 | 4 | |||||
| 3 | Northeast | Academic medical center | >600 | 2 | 4 | |||||
| 4 | Northeast | Community nonteaching | <300 | 9 | 2 | |||||
| 5 | South | Community nonteaching | >600 | 6 | 3 | |||||
| 6 | South | Community nonteaching | >600 | 3 | 4 | |||||
| 7 | Midwest | Community teaching | 300600 | 1 | 5 | |||||
| 8 | West | Academic medical center | 300600 | 1 | 4 | |||||
| 9 | South | Academic medical center | >600 | 4 | 4 | |||||
| 10 | Midwest | Academic medical center | 300600 | 3 | 3 | |||||
| 11 | Midwest | Academic medical center | >600 | 9 | 2 | |||||
The average rate of 30‐day rehospitalization among BOOST units was 14.7% in the preimplementation period and 12.7% during the postimplementation period (P=0.010) (Figure 1). Rehospitalization rates for matched control units were 14.0% in the preintervention period and 14.1% in the postintervention period (P=0.831). The mean absolute reduction in readmission rates over the 1‐year study period in BOOST units compared to control units was 2.0%, or a relative reduction of 13.6% (P=0.054 for signed rank test comparing differences in readmission rate reduction in BOOST units compared to site‐matched control units). Length of stay in BOOST and control units decreased an average of 0.5 days and 0.3 days, respectively. There was no difference in length of stay change between BOOST units and control units (P=0.966).

DISCUSSION
As hospitals strive to reduce their readmission rates to avoid Centers for Medicare and Medicaid Services penalties, Project BOOST may be a viable QI approach to achieve their goals. This initial evaluation of participation in Project BOOST by 11 hospitals of varying sizes across the United States showed an associated reduction in rehospitalization rates (absolute=2.0% and relative=13.6%, P=0.054). We did not find any significant change in length of stay among these hospitals implementing BOOST tools.
The tools provided to participating hospitals were developed from evidence found in peer‐reviewed literature established through experimental methods in well‐controlled academic settings. Further tool development was informed by recommendations of an advisory board consisting of expert representatives and advocates involved in the hospital discharge process: patients, caregivers, physicians, nurses, case managers, social workers, insurers, and regulatory and research agencies.[19] The toolkit components address multiple aspects of hospital discharge and follow‐up with the goal of improving health by optimizing the safety of care transitions. Our observation that readmission rates appeared to improve in a diverse hospital sample including nonacademic and community hospitals engaged in Project BOOST is reassuring that the benefits seen in existing research literature, developed in distinctly academic settings, can be replicated in diverse acute‐care settings.
The effect size observed in our study was modest but consistent with several studies identified in a recent review of trials measuring interventions to reduce rehospitalization, where 7 of 16 studies showing a significant improvement registered change in the 0% to 5% absolute range.[12] Impact of this project may have been tempered by the need to translate external QI content to the local setting. Additionally, in contrast to experimental studies that are limited in scope and timing and often scaled to a research budget, BOOST sites were encouraged to implement Project BOOST in the clinical setting even if no new funds were available to support the effort.[12]
The recruitment of a national sample of both academic and nonacademic hospital participants imposed several limitations on our study and analysis. We recognize that intervention units selected by hospitals may have had unmeasured unit and patient characteristics that facilitated successful change and contributed to the observed improvements. However, because external pressure to reduce readmission is present across all hospitals independent of the BOOST intervention, we felt site‐matched controls were essential to understanding effects attributable to the BOOST tools. Differences between units would be expected to be stable over the course of the study period, and comparison of outcome differences between 2 different time periods would be reasonable. Additionally, we could not collect data on readmissions to other hospitals. Theoretically, patients discharged from BOOST units might be more likely to have been rehospitalized elsewhere, but the fraction of rehospitalizations occurring at alternate facilities would also be expected to be similar on the matched control unit.
We report findings from a voluntary cohort willing and capable of designating a comparison clinical unit and contributing the requested data outcomes. Pilot sites that did not report outcomes were not analyzed, but comparison of hospital characteristics shows that participating hospitals were more likely to be large, urban, academic medical centers. Although barriers to data submission were not formally analyzed, reports from nonparticipating sites describe data submission limited by local implementation design (no geographic rollout or simultaneous rollout on all appropriate clinical units), site specific inability to generate unit level outcome statistics, and competing organizational priorities for data analyst time (electronic medical record deployment, alternative QI initiatives). The external validity of our results may be limited to organizations capable of analytics at the level of the individual clinical unit as well as those with sufficient QI resources to support reporting to a national database in the absence of a payer mandate. It is possible that additional financial support for on‐site data collection would have bolstered participation, making the example of participation rates we present potentially informative to organizations hoping to widely disseminate a QI agenda.
Nonetheless, the effectiveness demonstrated in the 11 sites that did participate is encouraging, and ongoing collaboration with subsequent BOOST cohorts has been designed to further facilitate data collection. Among the insights gained from this pilot experience, and incorporated into ongoing BOOST cohorts, is the importance of intensive mentor engagement to foster accountability among participant sites, assist with implementation troubleshooting, and offer expertise that is often particularly effective in gaining local support. We now encourage sites to have 2 mentor site visits to further these roles and more frequent conference calls. Further research to understand the marginal benefit of the mentored implementation approach is ongoing.
The limitations in data submission we experienced with the pilot cohort likely reflect resource constraints not uncommon at many hospitals. Increasing pressure placed on hospitals as a result of the Readmission Reduction Program within the Affordable Care Act as well as increasing interest from private and Medicaid payors to incorporate similar readmission‐based penalties provide encouragement for hospitals to enhance their data and analytic skills. National incentives for implementation of electronic health records (EHR) should also foster such capabilities, though we often saw EHRs as a barrier to QI, especially rapid cycle trials. Fortunately, hospitals are increasingly being afforded access to comprehensive claims databases to assist in tracking readmission rates to other facilities, and these data are becoming available in a more timely fashion. This more robust data collection, facilitated by private payors, state QI organizations, and state hospital associations, will support additional analytic methods such as multivariate regression models and interrupted time series designs to appreciate the experience of current BOOST participants.
Additional research is needed to understand the role of organizational context in the effectiveness of Project BOOST. Differences in rates of tool implementation and changes in clinical outcomes are likely dependent on local implementation context at the level of the healthcare organization and individual clinical unit.[20] Progress reports from site mentors and previously described experiences of QI implementation indicate that successful implementation of a multidimensional bundle of interventions may have reflected a higher level of institutional support, more robust team engagement in the work of reducing readmissions, increased clinical staff support for change, the presence of an effective project champion, or a key facilitating role of external mentorship.[21, 22] Ongoing data collection will continue to measure the sustainability of tool use and observed outcome changes to inform strategies to maintain gains associated with implementation. The role of mentored implementation in facilitating gains also requires further study.
Increasing attention to the problem of avoidable rehospitalization is driving hospitals, insurers, and policy makers to pursue QI efforts that favorably impact readmission rates. Our analysis of the BOOST intervention suggests that modest gains can be achieved following evidence‐based hospital process change facilitated by a mentored implementation model. However, realization of the goal of a 20% reduction in rehospitalization proposed by the Center for Medicare and Medicaid Services' Partnership for Patients initiative may be difficult to achieve on a national scale,[23] especially if efforts focus on just the hospital.
Acknowledgments
The authors acknowledge the contributions of Amanda Creden, BA (data collection), Julia Lee (biostatistical support), and the support of Amy Berman, BS, RN, Senior Program Officer at The John A. Hartford Foundation.
Disclosures
Project BOOST was funded by a grant from The John A. Hartford Foundation. Project BOOST is administered by the Society of Hospital Medicine (SHM). The development of the Project BOOST toolkit, recruitment of sites for this study, mentorship of the pilot cohort, project evaluation planning, and collection of pilot data were funded by a grant from The John A. Harford Foundation. Additional funding for continued data collection and analysis was funded by the SHM through funds from hospitals to participate in Project BOOST, specifically with funding support for Dr. Hansen. Dr. Williams has received funding to serve as Principal Investigator for Project BOOST. Since the time of initial cohort participation, approximately 125 additional hospitals have participated in the mentored implementation of Project BOOST. This participation was funded through a combination of site‐based tuition, third‐party payor support from private insurers, foundations, and federal funding through the Center for Medicare and Medicaid Innovation Partnership for Patients program. Drs. Greenwald, Hansen, and Williams are Project BOOST mentors for current Project BOOST sites and receive financial support through the SHM for this work. Dr. Howell has previously received funding as a Project BOOST mentor. Ms. Budnitz is the BOOST Project Director and is Chief Strategy and Development Officer for the HM. Dr. Maynard is the Senior Vice President of the SHM's Center for Hospital Innovation and Improvement.
References
Enactment of federal legislation imposing hospital reimbursement penalties for excess rates of rehospitalizations among Medicare fee for service beneficiaries markedly increased interest in hospital quality improvement (QI) efforts to reduce the observed 30‐day rehospitalization of 19.6% in this elderly population.[1, 2] The Congressional Budget Office estimated that reimbursement penalties to hospitals for high readmission rates are expected to save the Medicare program approximately $7 billion between 2010 and 2019.[3] These penalties are complemented by resources from the Center for Medicare and Medicaid Innovation aiming to reduce hospital readmissions by 20% by the end of 2013 through the Partnership for Patients campaign.[4] Although potential financial penalties and provision of resources for QI intensified efforts to enhance the quality of the hospital discharge transition, patient safety risks associated with hospital discharge are well documented.[5, 6] Approximately 20% of patients discharged from the hospital may suffer adverse events,[7, 8] of which up to three‐quarters (72%) are medication related,[9] and over one‐third of required follow‐up testing after discharge is not completed.[10] Such findings indicate opportunities for improvement in the discharge process.[11]
Numerous publications describe studies aiming to improve the hospital discharge process and mitigate these hazards, though a systematic review of interventions to reduce 30‐day rehospitalization indicated that the existing evidence base for the effectiveness of transition interventions demonstrates irregular effectiveness and limitations to generalizability.[12] Most studies showing effectiveness are confined to single academic medical centers. Existing evidence supports multifaceted interventions implemented in both the pre‐ and postdischarge periods and focused on risk assessment and tailored, patient‐centered application of interventions to mitigate risk. For example Project RED (Re‐Engineered Discharge) applied a bundled intervention consisting of intensified patient education and discharge planning, improved medication reconciliation and discharge instructions, and longitudinal patient contact with follow‐up phone calls and a dedicated discharge advocate.[13] However, the mean age of patients participating in the study was 50 years, and it excluded patients admitted from or discharged to skilled nursing facilities, making generalizability to the geriatric population uncertain.
An integral aspect of QI projects is the contribution of local context to translation of best practices to disparate settings.[14, 15, 16] Most available reports of successful interventions to reduce rehospitalization have not fully described the specifics of either the intervention context or design. Moreover, the available evidence base for common interventions to reduce rehospitalization was developed in the academic setting. Validation of single academic center studies in a broader healthcare context is necessary.
Project BOOST (Better Outcomes for Older adults through Safe Transitions) recruited a diverse national cohort of both academic and nonacademic hospitals to participate in a QI effort to implement best practices for hospital discharge care transitions using a national collaborative approach facilitated by external expert mentorship. This study aimed to determine the effectiveness of BOOST in lowering hospital readmission rates and impact on length of stay.
METHODS
The study of Project BOOST was undertaken in accordance with the SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines.[17]
Participants
The unit of observation for the prospective cohort study was the clinical acute‐care unit within hospitals. Sites were instructed to designate a pilot unit for the intervention that cared for medical or mixed medicalsurgical patient populations. Sites were also asked to provide outcome data for a clinically and organizationally similar non‐BOOST unit to provide a site‐matched control. Control units were matched by local site leadership based on comparable patient demographics, clinical mix, and extent of housestaff presence. An initial cohort of 6 hospitals in 2008 was followed by a second cohort of 24 hospitals initiated in 2009. All hospitals were invited to participate in the national effectiveness analysis, which required submission of readmission and length of stay data for both a BOOST intervention unit and a clinically matched control unit.
Description of the Intervention
The BOOST intervention consisted of 2 major sequential processes, planning and implementation, both facilitated by external site mentorsphysicians expert in QI and care transitionsfor a period of 12 months. Extensive background on the planning and implementation components is available at
| Enrollment Sites, n=30 | Sites Reporting Outcome Data, n=11 | Sites Not Reporting Outcome Data, n=19 | P Value for Comparison of Outcome Data Sites Compared to Othersa | |
|---|---|---|---|---|
| ||||
| Region, n (%) | 0.194 | |||
| Northeast | 8 (26.7) | 2 (18.2) | 6 (31.6) | |
| West | 7 (23.4) | 2 (18.2) | 5 (26.3) | |
| South | 7 (23.4) | 3 (27.3) | 4 (21.1) | |
| Midwest | 8 (26.7) | 4 (36.4) | 4 (21.1) | |
| Urban location, n (%) | 25 (83.3) | 11 (100) | 15 (78.9) | 0.035 |
| Teaching status, n (%) | 0.036 | |||
| Academic medical center | 10 (33.4) | 5 (45.5) | 5 (26.3) | |
| Community teaching | 8 (26.7) | 3 (27.3) | 5 (26.3) | |
| Community nonteaching | 12 (40) | 3 (27.3) | 9 (47.4) | |
| Beds number, mean (SD) | 426.6 (220.6) | 559.2 (187.8) | 349.79 (204.48) | 0.003 |
| Number of tools implemented, n (%) | 0.194 | |||
| 0 | 2 (6.7) | 0 | 2 (10.5) | |
| 1 | 2 (6.7) | 0 | 2 (10.5) | |
| 2 | 4 (13.3) | 2 (18.2) | 2 (10.5) | |
| 3 | 12 (40.0) | 3 (27.3) | 8 (42.1) | |
| 4 | 9 (30.0) | 5 (45.5) | 4 (21.1) | |
| 5 | 1 (3.3) | 1 (9.1) | 1 (5.3) | |
Mentor engagement with sites consisted of a 2‐day kickoff training on the BOOST tools, where site teams met their mentor and initiated development of structured action plans, followed by 5 to 6 scheduled phone calls in the subsequent 12 months. During these conference calls, mentors gauged progress and sought to help troubleshoot barriers to implementation. Some mentors also conducted a site visit with participant sites. Project BOOST provided sites with several collaborative activities including online webinars and an online listserv. Sites also received a quarterly newsletter.
Outcome Measures
The primary outcome was 30‐day rehospitalization defined as same hospital, all‐cause rehospitalization. Home discharges as well as discharges or transfers to other healthcare facilities were included in the discharge calculation. Elective or scheduled rehospitalizations as well as multiple rehospitalizations in the same 30‐day window were considered individual rehospitalization events. Rehospitalization was reported as a ratio of 30‐day rehospitalizations divided by live discharges in a calendar month. Length of stay was reported as the mean length of stay among live discharges in a calendar month. Outcomes were calculated at the participant site and then uploaded as overall monthly unit outcomes to a Web‐based research database.
To account for seasonal trends as well as marked variation in month‐to‐month rehospitalization rates identified in longitudinal data, we elected to compare 3‐month year‐over‐year averages to determine relative changes in readmission rates from the period prior to BOOST implementation to the period after BOOST implementation. We calculated averages for rehospitalization and length of stay in the 3‐month period preceding the sites' first reported month of front‐line implementation and in the corresponding 3‐month period in the subsequent calendar year. For example, if a site reported implementing its first tool in April 2010, the average readmission rate in the unit for January 2011 through March 2011 was subtracted from the average readmission rate for January 2010 through March 2010.
Sites were surveyed regarding tool implementation rates 6 months and 24 months after the 2009 kickoff training session. Surveys were electronically completed by site leaders in consultation with site team members. The survey identified new tool implementation as well as modification of existing care processes using the BOOST tools (admission risk assessment, discharge readiness checklist, teach back use, mandate regarding discharge summary completion, follow‐up phone calls to >80% of discharges). Use of a sixth tool, creation of individualized written discharge instructions, was not measured. We credited sites with tool implementation if they reported either de novo tool use or alteration of previous care processes influenced by BOOST tools.
Clinical outcome reporting was voluntary, and sites did not receive compensation and were not subject to penalty for the degree of implementation or outcome reporting. No patient‐level information was collected for the analysis, which was approved by the Northwestern University institutional review board.
Data Sources and Methods
Readmission and length of stay data, including the unit level readmission rate, as collected from administrative sources at each hospital, were collected using templated spreadsheet software between December 2008 and June 2010, after which data were loaded directly to a Web‐based data‐tracking platform. Sites were asked to load data as they became available. Sites were asked to report the number of study unit discharges as well as the number of those discharges readmitted within 30 days; however, reporting of the number of patient discharges was inconsistent across sites. Serial outreach consisting of monthly phone calls or email messaging to site leaders was conducted throughout 2011 to increase site participation in the project analysis.
Implementation date information was collected from 2 sources. The first was through online surveys distributed in November 2009 and April 2011. The second was through fields in the Web‐based data tracking platform to which sites uploaded data. In cases where disagreement was found between these 2 sources, the site leader was contacted for clarification.
Practice setting (community teaching, community nonteaching, academic medical center) was determined by site‐leader report within the Web‐based data tracking platform. Data for hospital characteristics (number of licensed beds and geographic region) were obtained from the American Hospital Association's Annual Survey of Hospitals.[18] Hospital region was characterized as West, South, Midwest, or Northeast.
Analysis
The null hypothesis was that no prepost difference existed in readmission rates within BOOST units, and no difference existed in the prepost change in readmission rates in BOOST units when compared to site‐matched control units. The Wilcoxon rank sum test was used to test whether observed changes described above were significantly different from 0, supporting rejection of the null hypotheses. We performed similar tests to determine the significance of observed changes in length of stay. We performed our analysis using SAS 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Eleven hospitals provided rehospitalization and length‐of‐stay outcome data for both a BOOST and control unit for the pre‐ and postimplementation periods. Compared to the 19 sites that did not participate in the analysis, these 11 sites were significantly larger (559188 beds vs 350205 beds, P=0.003), more likely to be located in an urban area (100.0% [n=11] vs 78.9% [n=15], P=0.035), and more likely to be academic medical centers (45.5% [n=5] vs 26.3% [n=5], P=0.036) (Table 1).
The mean number of tools implemented by sites participating in the analysis was 3.50.9. All sites implemented at least 2 tools. The duration between attendance at the BOOST kickoff event and first tool implementation ranged from 3 months (first tool implemented prior to attending the kickoff) and 9 months (mean duration, 3.34.3 months) (Table 2).
| Hospital | Region | Hospital Type | No. Licensed Beds | Kickoff Implementationa | Risk Assessment | Discharge Checklist | Teach Back | Discharge Summary Completion | Follow‐up Phone Call | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
| 1 | Midwest | Community teaching | <300 | 8 | 3 | |||||
| 2 | West | Community teaching | >600 | 0 | 4 | |||||
| 3 | Northeast | Academic medical center | >600 | 2 | 4 | |||||
| 4 | Northeast | Community nonteaching | <300 | 9 | 2 | |||||
| 5 | South | Community nonteaching | >600 | 6 | 3 | |||||
| 6 | South | Community nonteaching | >600 | 3 | 4 | |||||
| 7 | Midwest | Community teaching | 300600 | 1 | 5 | |||||
| 8 | West | Academic medical center | 300600 | 1 | 4 | |||||
| 9 | South | Academic medical center | >600 | 4 | 4 | |||||
| 10 | Midwest | Academic medical center | 300600 | 3 | 3 | |||||
| 11 | Midwest | Academic medical center | >600 | 9 | 2 | |||||
The average rate of 30‐day rehospitalization among BOOST units was 14.7% in the preimplementation period and 12.7% during the postimplementation period (P=0.010) (Figure 1). Rehospitalization rates for matched control units were 14.0% in the preintervention period and 14.1% in the postintervention period (P=0.831). The mean absolute reduction in readmission rates over the 1‐year study period in BOOST units compared to control units was 2.0%, or a relative reduction of 13.6% (P=0.054 for signed rank test comparing differences in readmission rate reduction in BOOST units compared to site‐matched control units). Length of stay in BOOST and control units decreased an average of 0.5 days and 0.3 days, respectively. There was no difference in length of stay change between BOOST units and control units (P=0.966).

DISCUSSION
As hospitals strive to reduce their readmission rates to avoid Centers for Medicare and Medicaid Services penalties, Project BOOST may be a viable QI approach to achieve their goals. This initial evaluation of participation in Project BOOST by 11 hospitals of varying sizes across the United States showed an associated reduction in rehospitalization rates (absolute=2.0% and relative=13.6%, P=0.054). We did not find any significant change in length of stay among these hospitals implementing BOOST tools.
The tools provided to participating hospitals were developed from evidence found in peer‐reviewed literature established through experimental methods in well‐controlled academic settings. Further tool development was informed by recommendations of an advisory board consisting of expert representatives and advocates involved in the hospital discharge process: patients, caregivers, physicians, nurses, case managers, social workers, insurers, and regulatory and research agencies.[19] The toolkit components address multiple aspects of hospital discharge and follow‐up with the goal of improving health by optimizing the safety of care transitions. Our observation that readmission rates appeared to improve in a diverse hospital sample including nonacademic and community hospitals engaged in Project BOOST is reassuring that the benefits seen in existing research literature, developed in distinctly academic settings, can be replicated in diverse acute‐care settings.
The effect size observed in our study was modest but consistent with several studies identified in a recent review of trials measuring interventions to reduce rehospitalization, where 7 of 16 studies showing a significant improvement registered change in the 0% to 5% absolute range.[12] Impact of this project may have been tempered by the need to translate external QI content to the local setting. Additionally, in contrast to experimental studies that are limited in scope and timing and often scaled to a research budget, BOOST sites were encouraged to implement Project BOOST in the clinical setting even if no new funds were available to support the effort.[12]
The recruitment of a national sample of both academic and nonacademic hospital participants imposed several limitations on our study and analysis. We recognize that intervention units selected by hospitals may have had unmeasured unit and patient characteristics that facilitated successful change and contributed to the observed improvements. However, because external pressure to reduce readmission is present across all hospitals independent of the BOOST intervention, we felt site‐matched controls were essential to understanding effects attributable to the BOOST tools. Differences between units would be expected to be stable over the course of the study period, and comparison of outcome differences between 2 different time periods would be reasonable. Additionally, we could not collect data on readmissions to other hospitals. Theoretically, patients discharged from BOOST units might be more likely to have been rehospitalized elsewhere, but the fraction of rehospitalizations occurring at alternate facilities would also be expected to be similar on the matched control unit.
We report findings from a voluntary cohort willing and capable of designating a comparison clinical unit and contributing the requested data outcomes. Pilot sites that did not report outcomes were not analyzed, but comparison of hospital characteristics shows that participating hospitals were more likely to be large, urban, academic medical centers. Although barriers to data submission were not formally analyzed, reports from nonparticipating sites describe data submission limited by local implementation design (no geographic rollout or simultaneous rollout on all appropriate clinical units), site specific inability to generate unit level outcome statistics, and competing organizational priorities for data analyst time (electronic medical record deployment, alternative QI initiatives). The external validity of our results may be limited to organizations capable of analytics at the level of the individual clinical unit as well as those with sufficient QI resources to support reporting to a national database in the absence of a payer mandate. It is possible that additional financial support for on‐site data collection would have bolstered participation, making the example of participation rates we present potentially informative to organizations hoping to widely disseminate a QI agenda.
Nonetheless, the effectiveness demonstrated in the 11 sites that did participate is encouraging, and ongoing collaboration with subsequent BOOST cohorts has been designed to further facilitate data collection. Among the insights gained from this pilot experience, and incorporated into ongoing BOOST cohorts, is the importance of intensive mentor engagement to foster accountability among participant sites, assist with implementation troubleshooting, and offer expertise that is often particularly effective in gaining local support. We now encourage sites to have 2 mentor site visits to further these roles and more frequent conference calls. Further research to understand the marginal benefit of the mentored implementation approach is ongoing.
The limitations in data submission we experienced with the pilot cohort likely reflect resource constraints not uncommon at many hospitals. Increasing pressure placed on hospitals as a result of the Readmission Reduction Program within the Affordable Care Act as well as increasing interest from private and Medicaid payors to incorporate similar readmission‐based penalties provide encouragement for hospitals to enhance their data and analytic skills. National incentives for implementation of electronic health records (EHR) should also foster such capabilities, though we often saw EHRs as a barrier to QI, especially rapid cycle trials. Fortunately, hospitals are increasingly being afforded access to comprehensive claims databases to assist in tracking readmission rates to other facilities, and these data are becoming available in a more timely fashion. This more robust data collection, facilitated by private payors, state QI organizations, and state hospital associations, will support additional analytic methods such as multivariate regression models and interrupted time series designs to appreciate the experience of current BOOST participants.
Additional research is needed to understand the role of organizational context in the effectiveness of Project BOOST. Differences in rates of tool implementation and changes in clinical outcomes are likely dependent on local implementation context at the level of the healthcare organization and individual clinical unit.[20] Progress reports from site mentors and previously described experiences of QI implementation indicate that successful implementation of a multidimensional bundle of interventions may have reflected a higher level of institutional support, more robust team engagement in the work of reducing readmissions, increased clinical staff support for change, the presence of an effective project champion, or a key facilitating role of external mentorship.[21, 22] Ongoing data collection will continue to measure the sustainability of tool use and observed outcome changes to inform strategies to maintain gains associated with implementation. The role of mentored implementation in facilitating gains also requires further study.
Increasing attention to the problem of avoidable rehospitalization is driving hospitals, insurers, and policy makers to pursue QI efforts that favorably impact readmission rates. Our analysis of the BOOST intervention suggests that modest gains can be achieved following evidence‐based hospital process change facilitated by a mentored implementation model. However, realization of the goal of a 20% reduction in rehospitalization proposed by the Center for Medicare and Medicaid Services' Partnership for Patients initiative may be difficult to achieve on a national scale,[23] especially if efforts focus on just the hospital.
Acknowledgments
The authors acknowledge the contributions of Amanda Creden, BA (data collection), Julia Lee (biostatistical support), and the support of Amy Berman, BS, RN, Senior Program Officer at The John A. Hartford Foundation.
Disclosures
Project BOOST was funded by a grant from The John A. Hartford Foundation. Project BOOST is administered by the Society of Hospital Medicine (SHM). The development of the Project BOOST toolkit, recruitment of sites for this study, mentorship of the pilot cohort, project evaluation planning, and collection of pilot data were funded by a grant from The John A. Harford Foundation. Additional funding for continued data collection and analysis was funded by the SHM through funds from hospitals to participate in Project BOOST, specifically with funding support for Dr. Hansen. Dr. Williams has received funding to serve as Principal Investigator for Project BOOST. Since the time of initial cohort participation, approximately 125 additional hospitals have participated in the mentored implementation of Project BOOST. This participation was funded through a combination of site‐based tuition, third‐party payor support from private insurers, foundations, and federal funding through the Center for Medicare and Medicaid Innovation Partnership for Patients program. Drs. Greenwald, Hansen, and Williams are Project BOOST mentors for current Project BOOST sites and receive financial support through the SHM for this work. Dr. Howell has previously received funding as a Project BOOST mentor. Ms. Budnitz is the BOOST Project Director and is Chief Strategy and Development Officer for the HM. Dr. Maynard is the Senior Vice President of the SHM's Center for Hospital Innovation and Improvement.
References
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- United States Congress. House Committee on Education and Labor. Coe on Ways and Means, Committee on Energy and Commerce, Compilation of Patient Protection and Affordable Care Act: as amended through November 1, 2010 including Patient Protection and Affordable Care Act health‐related portions of the Health Care and Education Reconciliation Act of 2010. Washington, DC: US Government Printing Office; 2010.
- Cost estimate for the amendment in the nature of a substitute to H.R. 3590, as proposed in the Senate on November 18, 2009. Washington, DC: Congressional Budget Office; 2009.
- Partnership for Patients, Center for Medicare and Medicaid Innovation. Available at: http://www.innovations.cms.gov/initiatives/Partnership‐for‐Patients/index.html. Accessed December 12, 2012.
- , . Providers have failed to work for continuity. Hospitals. 1979;53(10):79.
- , . Executing high‐quality care transitions: a call to do it right. J Hosp Med. 2007;2(5):287–290.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345–349.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477–485.
- , , . Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167(12):1305.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians. JAMA. 2007;297(8):831–841.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178.
- , , , et al. Advancing the science of patient safety. Ann Intern Med. 2011;154(10):693–696.
- , . From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362(9391):1225–1230.
- , , , et al. Quality improvement projects targeting health care‐associated infections: comparing virtual collaborative and toolkit approaches. J Hosp Med. 2011;6(5):271–278.
- , , , , . Publication guidelines for improvement studies in health care: evolution of the SQUIRE project. Ann Intern Med. 2008;149(9):670–676.
- , , , . Risk stratification and therapeutic decision making in acute coronary syndromes. JAMA. 2000;284(7):876–878.
- , , . Are diagnosis specific outcome indicators based on administrative data useful in assessing quality of hospital care? BMJ. 2004;13(1):32.
- , , , et al. What distinguishes top‐performing hospitals in acute myocardial infarction mortality rates? Ann Intern Med. 2011;154(6):384–390.
- , , , . The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf. 2012;21(1):13–20.
- , . Evidence‐based quality improvement: the state of the science. Health Aff (Millwood). 2005;24(1):138–150.
- Center for Medicare and Medicaid Innovation. Partnership for patients. Available at: http://www.innovations.cms.gov/initiatives/Partnership‐for‐Patients/index.html. Accessed April 2, 2012.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- United States Congress. House Committee on Education and Labor. Coe on Ways and Means, Committee on Energy and Commerce, Compilation of Patient Protection and Affordable Care Act: as amended through November 1, 2010 including Patient Protection and Affordable Care Act health‐related portions of the Health Care and Education Reconciliation Act of 2010. Washington, DC: US Government Printing Office; 2010.
- Cost estimate for the amendment in the nature of a substitute to H.R. 3590, as proposed in the Senate on November 18, 2009. Washington, DC: Congressional Budget Office; 2009.
- Partnership for Patients, Center for Medicare and Medicaid Innovation. Available at: http://www.innovations.cms.gov/initiatives/Partnership‐for‐Patients/index.html. Accessed December 12, 2012.
- , . Providers have failed to work for continuity. Hospitals. 1979;53(10):79.
- , . Executing high‐quality care transitions: a call to do it right. J Hosp Med. 2007;2(5):287–290.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345–349.
- , , , et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;5(8):477–485.
- , , . Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167(12):1305.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians. JAMA. 2007;297(8):831–841.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178.
- , , , et al. Advancing the science of patient safety. Ann Intern Med. 2011;154(10):693–696.
- , . From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362(9391):1225–1230.
- , , , et al. Quality improvement projects targeting health care‐associated infections: comparing virtual collaborative and toolkit approaches. J Hosp Med. 2011;6(5):271–278.
- , , , , . Publication guidelines for improvement studies in health care: evolution of the SQUIRE project. Ann Intern Med. 2008;149(9):670–676.
- , , , . Risk stratification and therapeutic decision making in acute coronary syndromes. JAMA. 2000;284(7):876–878.
- , , . Are diagnosis specific outcome indicators based on administrative data useful in assessing quality of hospital care? BMJ. 2004;13(1):32.
- , , , et al. What distinguishes top‐performing hospitals in acute myocardial infarction mortality rates? Ann Intern Med. 2011;154(6):384–390.
- , , , . The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf. 2012;21(1):13–20.
- , . Evidence‐based quality improvement: the state of the science. Health Aff (Millwood). 2005;24(1):138–150.
- Center for Medicare and Medicaid Innovation. Partnership for patients. Available at: http://www.innovations.cms.gov/initiatives/Partnership‐for‐Patients/index.html. Accessed April 2, 2012.
Copyright © 2013 Society of Hospital Medicine
Evidence Needing a Lift
In this issue of the Journal of Hospital Medicine, Hansen and colleagues provide a first, early look at the effectiveness of the BOOST intervention to reduce 30‐day readmissions among hospitalized patients.[1] BOOST[2] is 1 of a number of care transition improvement methodologies that have been applied to the problem of readmissions, each of which has evidence to support its effectiveness in its initial settings[3, 4] but has proven to be difficult to translate to other sites.[5, 6, 7]
BOOST stands in contrast with other, largely research protocol‐derived, programs in that it allows sites to tailor adoption of recommendations to local contexts and is therefore potentially more feasible to implement. Feasibility and practicality has led BOOST to be adopted in large national settings, even if it has had little evidence to support its effectiveness to date.
Given the nonstandardized and ad hoc nature of most multicenter collaboratives generally, and the flexibility of the BOOST model specifically, the BOOST authors are to be commended for undertaking any evaluation at all. Perhaps, not surprisingly, they encountered many of the problems associated with a multicenter studydropout of sites, problematic data, and limited evidence for adoption of the intervention at participating hospitals. Although these represent real‐world experiences of a quality‐improvement program, as a group they pose a number of problems that limit the study's robustness, and generate important caveats that readers should use to temper their interpretation of the authors' findings.
The first caveat relates to the substantial number of sites that either dropped out of BOOST or failed to submit data after enlisting in the collaborative. Although this may be common in quality improvement collaboratives, similar problems would not be permissible in a trial of a new drug or device. Dropout and selected ability to contribute data suggest that the ability to fully adopt BOOST may not be universal, and raises the possibility of bias, because the least successful sites may have had less interest in remaining engaged and submitting data.
The second caveat relates to how readmission rates were assessed. Because sites provided rates of readmissions at the unit level rather than the actual counts of admissions or readmissions, the authors were unable to conduct statistical analyses typically performed for these interventions, such as time series or difference‐in‐difference analyses. More importantly, one cannot discern whether their results are driven by a small absolute but large relative change in the number of readmissions at small sites. That is, large percentage changes of low statistical significance could have misleadingly affected the overall results. Conversely, we cannot identify large sites where a similar relative reduction could be statistically significant and more broadly interpreted as representing the real effectiveness of BOOST efforts.
The third caveat is in regard to the data describing the sites' performance. The effectiveness of BOOST in this analysis varied greatly among sites, with only 1 site showing a strong reduction in readmission rate, and nearly all others showing no statistical improvements. In fact, it appears that their overall results were almost entirely driven by the improvements at that 1 site.
Variable effectiveness of an intervention can be related to variable adoption or contextual factors (such as availability of personnel to implement the program). Although these authors have data on BOOST programmatic adoption, they do not have qualitative data on local barriers and facilitators to BOOST implementation, which at this stage of evaluation would be particularly valuable in understanding the results. Analyzing site‐level effectiveness is of growing relevance to multicenter quality improvement collaboratives,[8, 9] but this evaluation provides little insight into reasons for variable success across institutions.
Finally, their study design does not allow us to understand a number of key questions. How many patients were involved in the intervention? How many patients received all BOOST‐recommended interventions? Which of these interventions seemed most effective in which patients? To what degree did patient severity of illness, cognitive status, social supports, or access to primary care influence readmission risk? Such information would help frame cost‐effective deployment of BOOST or related tools.
In the end, it seems unlikely that this iteration of the BOOST program produced broad reductions in readmission rates. Having said this, the authors provide the necessary start down the road toward a fuller understanding of real‐world efforts to reduce readmissions. Stated alternately, the nuances and flaws of this study provide ample fodder for others working in the field. BOOST is in good stead with other care transition models that have not translated well from their initial research environment to real‐world practices. The question now is: Do any of these interventions actually work in clinical practice settings, and will we ever know? Even more fundamentally, how important and meaningful are these hospital‐based care transition interventions? Where is the engagement with primary care? Where are the primary care outcomes? Does BOOST truly impact outcomes other than readmission?[10]
Doing high‐quality research in the context of a rapidly evolving quality improvement program is hard. Doing it at more than 1 site is harder. BOOST's flexibility is both a great source of strength and a clear challenge to rigorous evaluation. However, when the costs of care transition programs are so high, and the potential consequences of high readmission rates are so great for patients and for hospitals, the need to address these issues with real data and better evidence is paramount. We look forward to the next phase of BOOST and to the growth and refinement of the evidence base for how to improve care coordination and transitions effectively.
- , , , et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8:421–427.
- , . BOOSTing the hospital discharge. J Hosp Med. 2009;4:209–210.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150:178–187.
- , , , et al. Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281:613–620.
- , , , et al. Effectiveness and cost of a transitional care program for heart failure: a prospective study with concurrent controls. Arch Intern Med. 2011;171:1238–1243.
- . Hospitals question Medicare rules on readmissions. New York Times. March 29, 2013. Available at: http://www.nytimes.com/2013/03/30/business/hospitals‐question‐fairness‐of‐new‐medicare‐rules.html?pagewanted=all
In this issue of the Journal of Hospital Medicine, Hansen and colleagues provide a first, early look at the effectiveness of the BOOST intervention to reduce 30‐day readmissions among hospitalized patients.[1] BOOST[2] is 1 of a number of care transition improvement methodologies that have been applied to the problem of readmissions, each of which has evidence to support its effectiveness in its initial settings[3, 4] but has proven to be difficult to translate to other sites.[5, 6, 7]
BOOST stands in contrast with other, largely research protocol‐derived, programs in that it allows sites to tailor adoption of recommendations to local contexts and is therefore potentially more feasible to implement. Feasibility and practicality has led BOOST to be adopted in large national settings, even if it has had little evidence to support its effectiveness to date.
Given the nonstandardized and ad hoc nature of most multicenter collaboratives generally, and the flexibility of the BOOST model specifically, the BOOST authors are to be commended for undertaking any evaluation at all. Perhaps, not surprisingly, they encountered many of the problems associated with a multicenter studydropout of sites, problematic data, and limited evidence for adoption of the intervention at participating hospitals. Although these represent real‐world experiences of a quality‐improvement program, as a group they pose a number of problems that limit the study's robustness, and generate important caveats that readers should use to temper their interpretation of the authors' findings.
The first caveat relates to the substantial number of sites that either dropped out of BOOST or failed to submit data after enlisting in the collaborative. Although this may be common in quality improvement collaboratives, similar problems would not be permissible in a trial of a new drug or device. Dropout and selected ability to contribute data suggest that the ability to fully adopt BOOST may not be universal, and raises the possibility of bias, because the least successful sites may have had less interest in remaining engaged and submitting data.
The second caveat relates to how readmission rates were assessed. Because sites provided rates of readmissions at the unit level rather than the actual counts of admissions or readmissions, the authors were unable to conduct statistical analyses typically performed for these interventions, such as time series or difference‐in‐difference analyses. More importantly, one cannot discern whether their results are driven by a small absolute but large relative change in the number of readmissions at small sites. That is, large percentage changes of low statistical significance could have misleadingly affected the overall results. Conversely, we cannot identify large sites where a similar relative reduction could be statistically significant and more broadly interpreted as representing the real effectiveness of BOOST efforts.
The third caveat is in regard to the data describing the sites' performance. The effectiveness of BOOST in this analysis varied greatly among sites, with only 1 site showing a strong reduction in readmission rate, and nearly all others showing no statistical improvements. In fact, it appears that their overall results were almost entirely driven by the improvements at that 1 site.
Variable effectiveness of an intervention can be related to variable adoption or contextual factors (such as availability of personnel to implement the program). Although these authors have data on BOOST programmatic adoption, they do not have qualitative data on local barriers and facilitators to BOOST implementation, which at this stage of evaluation would be particularly valuable in understanding the results. Analyzing site‐level effectiveness is of growing relevance to multicenter quality improvement collaboratives,[8, 9] but this evaluation provides little insight into reasons for variable success across institutions.
Finally, their study design does not allow us to understand a number of key questions. How many patients were involved in the intervention? How many patients received all BOOST‐recommended interventions? Which of these interventions seemed most effective in which patients? To what degree did patient severity of illness, cognitive status, social supports, or access to primary care influence readmission risk? Such information would help frame cost‐effective deployment of BOOST or related tools.
In the end, it seems unlikely that this iteration of the BOOST program produced broad reductions in readmission rates. Having said this, the authors provide the necessary start down the road toward a fuller understanding of real‐world efforts to reduce readmissions. Stated alternately, the nuances and flaws of this study provide ample fodder for others working in the field. BOOST is in good stead with other care transition models that have not translated well from their initial research environment to real‐world practices. The question now is: Do any of these interventions actually work in clinical practice settings, and will we ever know? Even more fundamentally, how important and meaningful are these hospital‐based care transition interventions? Where is the engagement with primary care? Where are the primary care outcomes? Does BOOST truly impact outcomes other than readmission?[10]
Doing high‐quality research in the context of a rapidly evolving quality improvement program is hard. Doing it at more than 1 site is harder. BOOST's flexibility is both a great source of strength and a clear challenge to rigorous evaluation. However, when the costs of care transition programs are so high, and the potential consequences of high readmission rates are so great for patients and for hospitals, the need to address these issues with real data and better evidence is paramount. We look forward to the next phase of BOOST and to the growth and refinement of the evidence base for how to improve care coordination and transitions effectively.
In this issue of the Journal of Hospital Medicine, Hansen and colleagues provide a first, early look at the effectiveness of the BOOST intervention to reduce 30‐day readmissions among hospitalized patients.[1] BOOST[2] is 1 of a number of care transition improvement methodologies that have been applied to the problem of readmissions, each of which has evidence to support its effectiveness in its initial settings[3, 4] but has proven to be difficult to translate to other sites.[5, 6, 7]
BOOST stands in contrast with other, largely research protocol‐derived, programs in that it allows sites to tailor adoption of recommendations to local contexts and is therefore potentially more feasible to implement. Feasibility and practicality has led BOOST to be adopted in large national settings, even if it has had little evidence to support its effectiveness to date.
Given the nonstandardized and ad hoc nature of most multicenter collaboratives generally, and the flexibility of the BOOST model specifically, the BOOST authors are to be commended for undertaking any evaluation at all. Perhaps, not surprisingly, they encountered many of the problems associated with a multicenter studydropout of sites, problematic data, and limited evidence for adoption of the intervention at participating hospitals. Although these represent real‐world experiences of a quality‐improvement program, as a group they pose a number of problems that limit the study's robustness, and generate important caveats that readers should use to temper their interpretation of the authors' findings.
The first caveat relates to the substantial number of sites that either dropped out of BOOST or failed to submit data after enlisting in the collaborative. Although this may be common in quality improvement collaboratives, similar problems would not be permissible in a trial of a new drug or device. Dropout and selected ability to contribute data suggest that the ability to fully adopt BOOST may not be universal, and raises the possibility of bias, because the least successful sites may have had less interest in remaining engaged and submitting data.
The second caveat relates to how readmission rates were assessed. Because sites provided rates of readmissions at the unit level rather than the actual counts of admissions or readmissions, the authors were unable to conduct statistical analyses typically performed for these interventions, such as time series or difference‐in‐difference analyses. More importantly, one cannot discern whether their results are driven by a small absolute but large relative change in the number of readmissions at small sites. That is, large percentage changes of low statistical significance could have misleadingly affected the overall results. Conversely, we cannot identify large sites where a similar relative reduction could be statistically significant and more broadly interpreted as representing the real effectiveness of BOOST efforts.
The third caveat is in regard to the data describing the sites' performance. The effectiveness of BOOST in this analysis varied greatly among sites, with only 1 site showing a strong reduction in readmission rate, and nearly all others showing no statistical improvements. In fact, it appears that their overall results were almost entirely driven by the improvements at that 1 site.
Variable effectiveness of an intervention can be related to variable adoption or contextual factors (such as availability of personnel to implement the program). Although these authors have data on BOOST programmatic adoption, they do not have qualitative data on local barriers and facilitators to BOOST implementation, which at this stage of evaluation would be particularly valuable in understanding the results. Analyzing site‐level effectiveness is of growing relevance to multicenter quality improvement collaboratives,[8, 9] but this evaluation provides little insight into reasons for variable success across institutions.
Finally, their study design does not allow us to understand a number of key questions. How many patients were involved in the intervention? How many patients received all BOOST‐recommended interventions? Which of these interventions seemed most effective in which patients? To what degree did patient severity of illness, cognitive status, social supports, or access to primary care influence readmission risk? Such information would help frame cost‐effective deployment of BOOST or related tools.
In the end, it seems unlikely that this iteration of the BOOST program produced broad reductions in readmission rates. Having said this, the authors provide the necessary start down the road toward a fuller understanding of real‐world efforts to reduce readmissions. Stated alternately, the nuances and flaws of this study provide ample fodder for others working in the field. BOOST is in good stead with other care transition models that have not translated well from their initial research environment to real‐world practices. The question now is: Do any of these interventions actually work in clinical practice settings, and will we ever know? Even more fundamentally, how important and meaningful are these hospital‐based care transition interventions? Where is the engagement with primary care? Where are the primary care outcomes? Does BOOST truly impact outcomes other than readmission?[10]
Doing high‐quality research in the context of a rapidly evolving quality improvement program is hard. Doing it at more than 1 site is harder. BOOST's flexibility is both a great source of strength and a clear challenge to rigorous evaluation. However, when the costs of care transition programs are so high, and the potential consequences of high readmission rates are so great for patients and for hospitals, the need to address these issues with real data and better evidence is paramount. We look forward to the next phase of BOOST and to the growth and refinement of the evidence base for how to improve care coordination and transitions effectively.
- , , , et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8:421–427.
- , . BOOSTing the hospital discharge. J Hosp Med. 2009;4:209–210.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150:178–187.
- , , , et al. Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281:613–620.
- , , , et al. Effectiveness and cost of a transitional care program for heart failure: a prospective study with concurrent controls. Arch Intern Med. 2011;171:1238–1243.
- . Hospitals question Medicare rules on readmissions. New York Times. March 29, 2013. Available at: http://www.nytimes.com/2013/03/30/business/hospitals‐question‐fairness‐of‐new‐medicare‐rules.html?pagewanted=all
- , , , et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8:421–427.
- , . BOOSTing the hospital discharge. J Hosp Med. 2009;4:209–210.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150:178–187.
- , , , et al. Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281:613–620.
- , , , et al. Effectiveness and cost of a transitional care program for heart failure: a prospective study with concurrent controls. Arch Intern Med. 2011;171:1238–1243.
- . Hospitals question Medicare rules on readmissions. New York Times. March 29, 2013. Available at: http://www.nytimes.com/2013/03/30/business/hospitals‐question‐fairness‐of‐new‐medicare‐rules.html?pagewanted=all
A Raw Deal
A 39‐year‐old woman presented to the emergency department (ED) with fever and headache. One to two weeks prior to presentation, she developed nightly fevers that gradually increased to as high as 39.4C. She subsequently developed generalized throbbing headaches, malaise, and diffuse body pain. The headache gradually worsened. The day prior to presentation, she developed photophobia, nausea, and vomiting. She also reported right scalp pain while combing her hair, difficulty emptying her bladder, and left buttock pain radiating down the leg. She denied rash, joint pain, visual changes, dysarthria, cough, chest pain, abdominal pain, or diarrhea.
Fever and headache can be explained by meningitis, encephalitis, or brain abscess. The combination is seen far more frequently, however, in patients with common systemic infections such as influenza. For either bacterial meningitis or influenza, a 2‐week course is prolonged and atypical. The progressive nature of the symptoms and photophobia suggest a chronic meningitis, and the development of nausea and vomiting, although nonspecific, is also consistent with elevated intracranial pressure. In a young woman, subacute fever and aches should prompt consideration of an autoimmune disorder such as systemic lupus erythematosus (SLE), although early central nervous system (CNS) involvement is atypical. Migraine headaches are characterized by light sensitivity, nausea, and vomiting and can be precipitated by a viral syndrome, but in this case, the headaches were present at the outset, and 2 weeks is too long for a migraine attack.
Pain while combing hair is not characteristic of the aforementioned syndromes. The scalp should be examined to confirm that there are no skin lesions associated with herpes zoster and no arterial prominence associated with temporal arteritis. She is young for the latter, which would otherwise be a suitable explanation for fever, headache, scalp tenderness, and visual complaints (usually impairment not photophobia).
Incomplete bladder emptying and left buttock pain suggest that there might be a concomitant lumbosacral myelopathy or radiculopathy. Some nonbacterial causes of meningitis such as cytomegalovirus (CMV), syphilis, and cancer simultaneously involve the CNS and peripheral nerve roots. It is also possible that the scalp tenderness associated with combing reflects a cervical sensory radiculopathy.
She had presented to the ED 2 and 4 days before the current (third) ED visit. Both times her main complaint was left buttock pain and left leg paresthesias. Although she had no skin lesions, she was diagnosed with prodromal herpes zoster in the S2 dermatomal distribution and was prescribed valacyclovir (to be started should eruptions develop, which never occurred).
She reported intermittent self‐limited fevers at 3‐ to 4‐week intervals during the prior 6 months; two fever episodes were accompanied by an influenza‐like illness, and one was associated with gastrointestinal symptoms. Her last fever prior to this evaluation was 6 weeks earlier when she was treated with azithromycin for suspected pneumonia at an outside facility.
Her past medical history included hypothyroidism, gastroesophageal reflux disease, diverticulitis, and gluten intolerance. Her medications included porcine (natural) thyroid, fish oil, ibuprofen, and acetaminophen. She lived in Michigan and traveled to the northeast United States (Maine, Cape Cod, New Hampshire, Connecticut, and Vermont) 7 months prior to this evaluation. She was married and had no pets at home. She denied any tobacco, alcohol, or illicit drug use.
Her illness now appears to be chronic, associated with fever, and multisystem (potentially involving the pulmonary and gastrointestinal tract). None of her medical problems would predispose her to subacute meningitis, myelopathy, or radiculopathy. Hypothyroidism raises the possibility of a concomitant autoimmune disorder which causes meningitis, such as SLE or Behet's disease. Sarcoidosis can cause chronic meningitis and neuropathy with concomitant lung and gastrointestinal involvement and rarely fever.
Residency in the upper Midwest increases exposure to chronic infections that rarely cause subacute meningitis such as histoplasmosis, blastomycosis, or human granulocytic anaplasmosis. Travel to the northeast United States 1 month before the onset of her symptoms raises the possibility of other endemic infections like Lyme disease, babesiosis, and tularemia, which may account for her recurrent fevers. Of these, Lyme is most likely to present as chronic meningitis with cranial neuropathy and radiculoneuropathy.
Although the diagnosis of pneumonia was made late in her 6‐month illness, its etiology and treatment may be relevant. If the recent pneumonia was viral, a subsequent viral meningitis may be manifesting now or may have triggered an autoimmune process, such as acute disseminated encephalomyelitis. Bacterial pneumonia is a common precursor to bacterial meningitis, and treatment with azithromycin for the pneumonia may have delayed the meningitis onset or muted its course; this should be taken into account when interpreting cerebrospinal fluid (CSF) culture results.
On physical examination, her temperature was 39.1C, blood pressure was 135/91 mm Hg, with pulse of 87 beats per minute, respiratory rate of 16 breaths per minute, and oxygenation saturation of 97% on room air. She appeared in distress and was covering her eyes. She was alert and oriented. She had photophobia and mild nuchal rigidity. Pupils were equal and reactive to light, but she could not tolerate the eye exam for papilledema. Lung, heart, and abdominal exam were normal. No cranial nerve abnormalities were noted, and muscle strength was 5/5 in all 4 extremities. She had decreased sensation to light touch with allodynia throughout her lower extremities in addition to the lateral portion of the right scalp, which was also tender to palpation. Deep tendon reflexes were 2+ and symmetric in her bilateral upper and lower extremities. She did not have joint swelling, edema, lymphadenopathy, or a rash.
Her fever, headache, nuchal rigidity and photophobia collectively suggest meningitis, which requires evaluation by a lumbar puncture. There is no rash that supports herpes zoster or SLE. She does not have signs of myelopathy that would explain the urinary complaints, but lower motor neuron involvement has not been excluded. The sensory abnormalities in the scalp and leg are consistent with a polyneuroradiculopathy. Anterior lateral scalp tenderness may signal trigeminal nerve involvement, whereas posterior scalp tenderness would localize to the upper cervical cord nerve roots. The contralateral distribution of the scalp and leg sensory deficits suggests a multifocal peripheral nervous system process rather than a single CNS lesion.
Initial laboratory data showed serum white blood cell count (WBC) of 12,000/mm3 (79% polymorphonuclear leukocytes). Hemoglobin was 14.2 g/dL, and platelets were 251,000/mm3. Electrolytes, renal function, and liver function were normal. Thyroid‐stimulating hormone, erythrocyte sedimentation rate, and C‐reactive protein were normal. Urinalysis was negative. Chest x‐ray was normal. Noncontrast head computed tomography (CT) was normal. The patient was unable to void; 500 mL of urine returned when catheterization was performed.
CSF WBC count was 1,280/mm3 (39% neutrophils and 49% lymphocytes). CSF total protein was 175 mg/dL, and glucose was 48 mg/dL; serum glucose was 104 mg/dL. Opening pressure was not recorded. Gram stain was negative. Ceftriaxone, vancomycin, ampicillin, and acyclovir were administered for presumed bacterial or viral meningitis. Magnetic resonance imaging (MRI) of the brain and spine showed diffuse leptomeningeal enhancement (Figure 1).
The urinary retention in the absence of myelopathic findings on exam or MRI suggests a sacral polyradiculoneuropathy. Diffuse leptomeningeal enhancement is consistent with many, if not all, causes of meningitis. The high WBC count, elevated protein, and low glucosecollectively signaling active inflammation in the CNSare highly compatible with bacterial meningitis, although the lymphocytic predominance and other clinical data point to nonbacterial etiologies. The negative Gram stain further lowers the probability of bacterial meningitis, but it has limited sensitivity, may be affected by recent antibiotics, and is typically negative with Listeria. Enterovirus, acute human immunodeficiency virus (HIV), and herpes viruses (eg, CMV or herpes simplex virus [HSV]) are important considerations, with the latter 2 causing associated polyneuroradiculopathy. Patients with genital HSV (not detected here) can have a concomitant sacral radiculitis leading to urinary retention.
Fungal and mycobacterial meningitis is a possibility (especially with the high protein), but the patient does not have the typical multisystem disease or immunosuppression that frequently accompanies those conditions when CNS disease is present. Autoimmune conditions like SLE, Behet's disease, and sarcoidosis remain important conditions, especially with the polyneuroradiculopathy or mononeuritis multiplex, which may reflect multifocal nerve infarction or invasion. Similarly, lymphomatous or carcinomatous meningitis should be considered, although an isolated manifestation in the CNS is unusual. Based on the multifocal neurologic deficits, I favor a viral, spirochete, or malignant etiology of her meningoencephalitis.
Despite ongoing broad spectrum antibiotics and supportive care, she became confused on hospital day 3 and developed anomia, agitation, and worsening headache. A repeat CT of the brain did not show any new abnormalities, but repeat lumbar puncture demonstrated elevated intracranial pressure (opening pressure of 47 cm water) with 427 WBC/mm3. Blood and CSF cultures remained negative.
Detailed questioning of the family revealed that she had been horseback riding 3 weeks prior to admission; there were no other livestock where she rode horses. In addition, the family reported that she and other family members routinely drank raw milk from a cow share program.
HIV antibody test was negative. Herpes simplex, varicella zoster, enteroviruses, and adenovirus CSF polymerase chain reaction (PCR) were negative. Cytomegalovirus and Epstein‐Barr virus PCR were negative in serum and CSF. Arbovirus, lymphocytic choriomeningitis, Coccidioides, Blastomyces, Histoplasma, Brucella, and Lyme serologies were negative. Cryptococcus neoformans antigen was negative in CSF. Serum QuantiFERON‐TB test was negative. Blood and CSF acid‐fast bacilli smears (and eventually mycobacterial cultures) were also negative. Her CSF flow cytometry and cytology were negative for lymphoma.
Unpasteurized milk conveys multiple infectious risks. Listeriosis is a food‐borne illness that can cause meningoencephalitis, but peripheral neuropathies are not characteristic. Brucellosis is usually characterized by severe bone pain, pancytopenia, and hepatosplenomegaly, which are absent. Infection with Mycobacterium bovis mimics Mycobacterium tuberculosis and can cause multisystem disease, typically involving the lung. Campylobacter infection is characterized by gastroenteritis, which has not been prominent.
Rhodococcus equi is a horse‐related pathogen which leads to pulmonary infections in immunocompromised hosts but not meningitis. Rather than focusing on horse exposure alone, however, it may be useful to consider her at risk for vector‐borne pathogens based on her time outdoors, such as Lyme disease (which can cause radiculopathy and encephalopathy), West Nile virus (although motor weakness rather than sensory symptoms is typical), or eastern equine encephalitis.
The absence of weight loss, cytopenias, lymphadenopathy, and organomegaly with the negative CSF cytology and flow cytometry makes lymphomatous meningitis unlikely. The case for an autoimmune disorder is not strong in the absence of joint pains, rash, or autoimmune serologies. In a young woman with unexplained encephalitis, antibodies to the N‐methyl‐D‐aspartate receptor should be assayed.
Although the CSF leukocytosis is declining, the elevated pressure and clinical deterioration signal that the disease process is not controlled. At this point I am uncertain as to the cause of her progressive meningoencephalitis with polyneuroradiculopathy. The latter feature makes me favor a viral or spirochete etiology.
On hospital day 4, Coxiella burnetii serologies were reported as positive (phase II immunoglobulin [Ig] G 1:256; phase II IgM 1:16; phase I IgG 1:16; phase I IgM 1:16) suggesting acute Q fever. Antibiotics were changed to intravenous doxycycline and ciprofloxacin. Her increased intracranial pressure was managed with serial lumbar punctures. The patient was discharged after 12 days of hospitalization taking oral doxycycline and ciprofloxacin. Her symptoms resolved over 10 weeks. No vegetations were seen on transesophageal echocardiogram. She had no evidence of chronic Q fever on repeat serologies.
I was not aware that Q fever causes meningitis or meningoencephalitis. However, I should have considered it in light of her indirect exposure to cows. It is possible that her pneumonia 6 weeks earlier represented acute Q fever, as pneumonia and hepatitis are among the most typical acute manifestations of this infection.
COMMENTARY
Hospitalists are commonly confronted by the combination of fever, headache, and confusion and are familiar with the diagnostic and therapeutic dilemmas related to prompt discrimination between CNS and non‐CNS processes, particularly infections. At the time of this patient's final ED presentation, her illness unambiguously localized to the CNS. As common and emergent conditions such as acute bacterial meningitis were excluded, the greatest challenge was finding the clue that could direct investigations into less common causes of meningoencephalitis.
The Infectious Disease Society of America has developed clinical practice guidelines for the diagnosis and management of encephalitis which highlight the importance of epidemiology and risk factor assessment.[1] This approach requires the clinician to examine potential clues and to go beyond initial associationsfor instance, not simply linking horseback riding to horse‐associated pathogens, but interpreting horseback riding as a proxy for outdoor exposure, which places her at risk for contact with mosquitos, which transmit West Nile virus or eastern equine encephalitis. Similarly, ingestion of raw milk, which is typically linked to Listeria monocytogenes, Brucella, and other pathogens prompted the infectious disease consultant to think more broadly and include livestock (cow)‐associated pathogens including C. burnetii.
Although involvement of the CNS is common in chronic Q fever endocarditis due to septic embolism, neurologic involvement in acute Q fever varies in prevalence (range of 1.7%22%).[2, 3, 4] The 3 major neurological syndromes of acute Q fever are (1) meningoencephalitis or encephalitis, (2) lymphocytic meningitis, and (3) peripheral neuropathy (myelitis, polyradiculoneuritis, or peripheral neuritis). CSF analysis usually shows mild pleocytosis with a predominance of lymphocytic cells; CSF protein elevation is variable, and glucose is usually normal. Neuroradiologic examination is usually normal, and there are no pathognomonic imaging abnormalities for Q fever meningoencephalitis.[2, 3] The mechanism by which C. burnetii causes neurologic injury and dysfunction is unknown.
The diagnosis of Q fever is usually established by serologic testing. In acute Q fever, antibodies to phase II antigen are higher than the phase I antibody titer. Phase II IgM antibodies are the first to appear, but then decline on average after week 8, often reaching undetectable levels 10 to 12 weeks after disease onset.[5] If this patient's pneumonia 6 weeks prior to this presentation was acute Q fever pneumonia, her IgM titers may have been declining by the time her neurologic illness developed. A false negative test result is also possible; immunofluorescence assays are more specific than sensitive in acute Q fever.[5]
Evaluating this case in isolation may raise some doubt as to the accuracy of the diagnosis as she did not have a 4‐fold rise in the phase II IgG titer and did not have a detectable phase II IgM. However, she was part of a cluster of individuals who regularly consumed raw milk from the same dairy and had evidence of C. burnetii infection. This group included her spouse, who had a robust serologic evidence of C. burnetii, characterized by a >4‐fold rise in phase II IgM and IgG titers.[6]
C. burnetii is found primarily in cattle, sheep, and goats and is shed in large quantities by infected periparturient animals in their urine, feces, and milk.[7] Inhalation of contaminated aerosols is the principal route of transmission.[7, 8] Acute Q fever is underdiagnosed because the majority of acute infections are asymptomatic (60%) or present as a nonspecific flu‐like illness.[7] This case represents a rare manifestation of a rare infection acquired through a rare route of transmission, but highlights the importance of epidemiology and risk factor assessment when clinicians are faced with a diagnostic challenge.
TEACHING POINTS
- Exploration of epidemiology and exposure history is central to diagnosing meningoencephalitis with negative bacterial cultures and undetectable HSV PCR, although the etiology of meningoencephalitis can elude identification even after exhaustive investigation.
- Inhalation of contaminated aerosols is the principal route of transmission for C. burnetii, but it can also be transmitted via infected unpasteurized milk.[7, 9]
- Acute presentations of Q fever, which may warrant admission, include pneumonia, hepatitis, or meningoencephalitis.
- Q fever is diagnosed by serologic testing, and doxycycline is the antibiotic of choice.
Disclosures
This case was presented at the 2012 Annual Meeting of the Society of Hospital Medicine. It was subsequently reported in the epidemiologic report of the outbreak.[6] The authors report no conflicts of interest.
- , , , et al. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2008;47:303–327.
- , , , et al. Q fever 1985–1998 clinical and epidemiologic features of 1,383 Infections. Medicine. 2000;79:109–123.
- , , , et al. Neurological involvement in acute Q fever: a report of 29 cases and review of the literature. Arch Intern Med. 2002;162:693–700.
- , , . Q fever in Plymouth 1972–88, a review with particular reference to neurological manifestations. Epidemiol Infect. 1990;105:391–408.
- , , . Diagnosis of Q fever. J Clin Microbiol. 1998;36:1823–1834.
- , , . Q fever cluster among raw milk drinkers in Michigan, (2011). Clin Infect Dis. 2012;55:1387–1389.
- , . Q fever. Clin Micorbial Rev. 1999;12:18–53.
- , , , et al. A large outbreak of Q fever in the West Midlands: windborne spread into a metropolitan area? Commun Dis Public Health. 1998;1:180–187.
- , . A cluster of Coxiella burnetii infections associated with exposure to vaccinated goats and their unpasteurized dairy products. Am J Trop Med Hyg. 1992;47:35–40.
A 39‐year‐old woman presented to the emergency department (ED) with fever and headache. One to two weeks prior to presentation, she developed nightly fevers that gradually increased to as high as 39.4C. She subsequently developed generalized throbbing headaches, malaise, and diffuse body pain. The headache gradually worsened. The day prior to presentation, she developed photophobia, nausea, and vomiting. She also reported right scalp pain while combing her hair, difficulty emptying her bladder, and left buttock pain radiating down the leg. She denied rash, joint pain, visual changes, dysarthria, cough, chest pain, abdominal pain, or diarrhea.
Fever and headache can be explained by meningitis, encephalitis, or brain abscess. The combination is seen far more frequently, however, in patients with common systemic infections such as influenza. For either bacterial meningitis or influenza, a 2‐week course is prolonged and atypical. The progressive nature of the symptoms and photophobia suggest a chronic meningitis, and the development of nausea and vomiting, although nonspecific, is also consistent with elevated intracranial pressure. In a young woman, subacute fever and aches should prompt consideration of an autoimmune disorder such as systemic lupus erythematosus (SLE), although early central nervous system (CNS) involvement is atypical. Migraine headaches are characterized by light sensitivity, nausea, and vomiting and can be precipitated by a viral syndrome, but in this case, the headaches were present at the outset, and 2 weeks is too long for a migraine attack.
Pain while combing hair is not characteristic of the aforementioned syndromes. The scalp should be examined to confirm that there are no skin lesions associated with herpes zoster and no arterial prominence associated with temporal arteritis. She is young for the latter, which would otherwise be a suitable explanation for fever, headache, scalp tenderness, and visual complaints (usually impairment not photophobia).
Incomplete bladder emptying and left buttock pain suggest that there might be a concomitant lumbosacral myelopathy or radiculopathy. Some nonbacterial causes of meningitis such as cytomegalovirus (CMV), syphilis, and cancer simultaneously involve the CNS and peripheral nerve roots. It is also possible that the scalp tenderness associated with combing reflects a cervical sensory radiculopathy.
She had presented to the ED 2 and 4 days before the current (third) ED visit. Both times her main complaint was left buttock pain and left leg paresthesias. Although she had no skin lesions, she was diagnosed with prodromal herpes zoster in the S2 dermatomal distribution and was prescribed valacyclovir (to be started should eruptions develop, which never occurred).
She reported intermittent self‐limited fevers at 3‐ to 4‐week intervals during the prior 6 months; two fever episodes were accompanied by an influenza‐like illness, and one was associated with gastrointestinal symptoms. Her last fever prior to this evaluation was 6 weeks earlier when she was treated with azithromycin for suspected pneumonia at an outside facility.
Her past medical history included hypothyroidism, gastroesophageal reflux disease, diverticulitis, and gluten intolerance. Her medications included porcine (natural) thyroid, fish oil, ibuprofen, and acetaminophen. She lived in Michigan and traveled to the northeast United States (Maine, Cape Cod, New Hampshire, Connecticut, and Vermont) 7 months prior to this evaluation. She was married and had no pets at home. She denied any tobacco, alcohol, or illicit drug use.
Her illness now appears to be chronic, associated with fever, and multisystem (potentially involving the pulmonary and gastrointestinal tract). None of her medical problems would predispose her to subacute meningitis, myelopathy, or radiculopathy. Hypothyroidism raises the possibility of a concomitant autoimmune disorder which causes meningitis, such as SLE or Behet's disease. Sarcoidosis can cause chronic meningitis and neuropathy with concomitant lung and gastrointestinal involvement and rarely fever.
Residency in the upper Midwest increases exposure to chronic infections that rarely cause subacute meningitis such as histoplasmosis, blastomycosis, or human granulocytic anaplasmosis. Travel to the northeast United States 1 month before the onset of her symptoms raises the possibility of other endemic infections like Lyme disease, babesiosis, and tularemia, which may account for her recurrent fevers. Of these, Lyme is most likely to present as chronic meningitis with cranial neuropathy and radiculoneuropathy.
Although the diagnosis of pneumonia was made late in her 6‐month illness, its etiology and treatment may be relevant. If the recent pneumonia was viral, a subsequent viral meningitis may be manifesting now or may have triggered an autoimmune process, such as acute disseminated encephalomyelitis. Bacterial pneumonia is a common precursor to bacterial meningitis, and treatment with azithromycin for the pneumonia may have delayed the meningitis onset or muted its course; this should be taken into account when interpreting cerebrospinal fluid (CSF) culture results.
On physical examination, her temperature was 39.1C, blood pressure was 135/91 mm Hg, with pulse of 87 beats per minute, respiratory rate of 16 breaths per minute, and oxygenation saturation of 97% on room air. She appeared in distress and was covering her eyes. She was alert and oriented. She had photophobia and mild nuchal rigidity. Pupils were equal and reactive to light, but she could not tolerate the eye exam for papilledema. Lung, heart, and abdominal exam were normal. No cranial nerve abnormalities were noted, and muscle strength was 5/5 in all 4 extremities. She had decreased sensation to light touch with allodynia throughout her lower extremities in addition to the lateral portion of the right scalp, which was also tender to palpation. Deep tendon reflexes were 2+ and symmetric in her bilateral upper and lower extremities. She did not have joint swelling, edema, lymphadenopathy, or a rash.
Her fever, headache, nuchal rigidity and photophobia collectively suggest meningitis, which requires evaluation by a lumbar puncture. There is no rash that supports herpes zoster or SLE. She does not have signs of myelopathy that would explain the urinary complaints, but lower motor neuron involvement has not been excluded. The sensory abnormalities in the scalp and leg are consistent with a polyneuroradiculopathy. Anterior lateral scalp tenderness may signal trigeminal nerve involvement, whereas posterior scalp tenderness would localize to the upper cervical cord nerve roots. The contralateral distribution of the scalp and leg sensory deficits suggests a multifocal peripheral nervous system process rather than a single CNS lesion.
Initial laboratory data showed serum white blood cell count (WBC) of 12,000/mm3 (79% polymorphonuclear leukocytes). Hemoglobin was 14.2 g/dL, and platelets were 251,000/mm3. Electrolytes, renal function, and liver function were normal. Thyroid‐stimulating hormone, erythrocyte sedimentation rate, and C‐reactive protein were normal. Urinalysis was negative. Chest x‐ray was normal. Noncontrast head computed tomography (CT) was normal. The patient was unable to void; 500 mL of urine returned when catheterization was performed.
CSF WBC count was 1,280/mm3 (39% neutrophils and 49% lymphocytes). CSF total protein was 175 mg/dL, and glucose was 48 mg/dL; serum glucose was 104 mg/dL. Opening pressure was not recorded. Gram stain was negative. Ceftriaxone, vancomycin, ampicillin, and acyclovir were administered for presumed bacterial or viral meningitis. Magnetic resonance imaging (MRI) of the brain and spine showed diffuse leptomeningeal enhancement (Figure 1).

The urinary retention in the absence of myelopathic findings on exam or MRI suggests a sacral polyradiculoneuropathy. Diffuse leptomeningeal enhancement is consistent with many, if not all, causes of meningitis. The high WBC count, elevated protein, and low glucosecollectively signaling active inflammation in the CNSare highly compatible with bacterial meningitis, although the lymphocytic predominance and other clinical data point to nonbacterial etiologies. The negative Gram stain further lowers the probability of bacterial meningitis, but it has limited sensitivity, may be affected by recent antibiotics, and is typically negative with Listeria. Enterovirus, acute human immunodeficiency virus (HIV), and herpes viruses (eg, CMV or herpes simplex virus [HSV]) are important considerations, with the latter 2 causing associated polyneuroradiculopathy. Patients with genital HSV (not detected here) can have a concomitant sacral radiculitis leading to urinary retention.
Fungal and mycobacterial meningitis is a possibility (especially with the high protein), but the patient does not have the typical multisystem disease or immunosuppression that frequently accompanies those conditions when CNS disease is present. Autoimmune conditions like SLE, Behet's disease, and sarcoidosis remain important conditions, especially with the polyneuroradiculopathy or mononeuritis multiplex, which may reflect multifocal nerve infarction or invasion. Similarly, lymphomatous or carcinomatous meningitis should be considered, although an isolated manifestation in the CNS is unusual. Based on the multifocal neurologic deficits, I favor a viral, spirochete, or malignant etiology of her meningoencephalitis.
Despite ongoing broad spectrum antibiotics and supportive care, she became confused on hospital day 3 and developed anomia, agitation, and worsening headache. A repeat CT of the brain did not show any new abnormalities, but repeat lumbar puncture demonstrated elevated intracranial pressure (opening pressure of 47 cm water) with 427 WBC/mm3. Blood and CSF cultures remained negative.
Detailed questioning of the family revealed that she had been horseback riding 3 weeks prior to admission; there were no other livestock where she rode horses. In addition, the family reported that she and other family members routinely drank raw milk from a cow share program.
HIV antibody test was negative. Herpes simplex, varicella zoster, enteroviruses, and adenovirus CSF polymerase chain reaction (PCR) were negative. Cytomegalovirus and Epstein‐Barr virus PCR were negative in serum and CSF. Arbovirus, lymphocytic choriomeningitis, Coccidioides, Blastomyces, Histoplasma, Brucella, and Lyme serologies were negative. Cryptococcus neoformans antigen was negative in CSF. Serum QuantiFERON‐TB test was negative. Blood and CSF acid‐fast bacilli smears (and eventually mycobacterial cultures) were also negative. Her CSF flow cytometry and cytology were negative for lymphoma.
Unpasteurized milk conveys multiple infectious risks. Listeriosis is a food‐borne illness that can cause meningoencephalitis, but peripheral neuropathies are not characteristic. Brucellosis is usually characterized by severe bone pain, pancytopenia, and hepatosplenomegaly, which are absent. Infection with Mycobacterium bovis mimics Mycobacterium tuberculosis and can cause multisystem disease, typically involving the lung. Campylobacter infection is characterized by gastroenteritis, which has not been prominent.
Rhodococcus equi is a horse‐related pathogen which leads to pulmonary infections in immunocompromised hosts but not meningitis. Rather than focusing on horse exposure alone, however, it may be useful to consider her at risk for vector‐borne pathogens based on her time outdoors, such as Lyme disease (which can cause radiculopathy and encephalopathy), West Nile virus (although motor weakness rather than sensory symptoms is typical), or eastern equine encephalitis.
The absence of weight loss, cytopenias, lymphadenopathy, and organomegaly with the negative CSF cytology and flow cytometry makes lymphomatous meningitis unlikely. The case for an autoimmune disorder is not strong in the absence of joint pains, rash, or autoimmune serologies. In a young woman with unexplained encephalitis, antibodies to the N‐methyl‐D‐aspartate receptor should be assayed.
Although the CSF leukocytosis is declining, the elevated pressure and clinical deterioration signal that the disease process is not controlled. At this point I am uncertain as to the cause of her progressive meningoencephalitis with polyneuroradiculopathy. The latter feature makes me favor a viral or spirochete etiology.
On hospital day 4, Coxiella burnetii serologies were reported as positive (phase II immunoglobulin [Ig] G 1:256; phase II IgM 1:16; phase I IgG 1:16; phase I IgM 1:16) suggesting acute Q fever. Antibiotics were changed to intravenous doxycycline and ciprofloxacin. Her increased intracranial pressure was managed with serial lumbar punctures. The patient was discharged after 12 days of hospitalization taking oral doxycycline and ciprofloxacin. Her symptoms resolved over 10 weeks. No vegetations were seen on transesophageal echocardiogram. She had no evidence of chronic Q fever on repeat serologies.
I was not aware that Q fever causes meningitis or meningoencephalitis. However, I should have considered it in light of her indirect exposure to cows. It is possible that her pneumonia 6 weeks earlier represented acute Q fever, as pneumonia and hepatitis are among the most typical acute manifestations of this infection.
COMMENTARY
Hospitalists are commonly confronted by the combination of fever, headache, and confusion and are familiar with the diagnostic and therapeutic dilemmas related to prompt discrimination between CNS and non‐CNS processes, particularly infections. At the time of this patient's final ED presentation, her illness unambiguously localized to the CNS. As common and emergent conditions such as acute bacterial meningitis were excluded, the greatest challenge was finding the clue that could direct investigations into less common causes of meningoencephalitis.
The Infectious Disease Society of America has developed clinical practice guidelines for the diagnosis and management of encephalitis which highlight the importance of epidemiology and risk factor assessment.[1] This approach requires the clinician to examine potential clues and to go beyond initial associationsfor instance, not simply linking horseback riding to horse‐associated pathogens, but interpreting horseback riding as a proxy for outdoor exposure, which places her at risk for contact with mosquitos, which transmit West Nile virus or eastern equine encephalitis. Similarly, ingestion of raw milk, which is typically linked to Listeria monocytogenes, Brucella, and other pathogens prompted the infectious disease consultant to think more broadly and include livestock (cow)‐associated pathogens including C. burnetii.
Although involvement of the CNS is common in chronic Q fever endocarditis due to septic embolism, neurologic involvement in acute Q fever varies in prevalence (range of 1.7%22%).[2, 3, 4] The 3 major neurological syndromes of acute Q fever are (1) meningoencephalitis or encephalitis, (2) lymphocytic meningitis, and (3) peripheral neuropathy (myelitis, polyradiculoneuritis, or peripheral neuritis). CSF analysis usually shows mild pleocytosis with a predominance of lymphocytic cells; CSF protein elevation is variable, and glucose is usually normal. Neuroradiologic examination is usually normal, and there are no pathognomonic imaging abnormalities for Q fever meningoencephalitis.[2, 3] The mechanism by which C. burnetii causes neurologic injury and dysfunction is unknown.
The diagnosis of Q fever is usually established by serologic testing. In acute Q fever, antibodies to phase II antigen are higher than the phase I antibody titer. Phase II IgM antibodies are the first to appear, but then decline on average after week 8, often reaching undetectable levels 10 to 12 weeks after disease onset.[5] If this patient's pneumonia 6 weeks prior to this presentation was acute Q fever pneumonia, her IgM titers may have been declining by the time her neurologic illness developed. A false negative test result is also possible; immunofluorescence assays are more specific than sensitive in acute Q fever.[5]
Evaluating this case in isolation may raise some doubt as to the accuracy of the diagnosis as she did not have a 4‐fold rise in the phase II IgG titer and did not have a detectable phase II IgM. However, she was part of a cluster of individuals who regularly consumed raw milk from the same dairy and had evidence of C. burnetii infection. This group included her spouse, who had a robust serologic evidence of C. burnetii, characterized by a >4‐fold rise in phase II IgM and IgG titers.[6]
C. burnetii is found primarily in cattle, sheep, and goats and is shed in large quantities by infected periparturient animals in their urine, feces, and milk.[7] Inhalation of contaminated aerosols is the principal route of transmission.[7, 8] Acute Q fever is underdiagnosed because the majority of acute infections are asymptomatic (60%) or present as a nonspecific flu‐like illness.[7] This case represents a rare manifestation of a rare infection acquired through a rare route of transmission, but highlights the importance of epidemiology and risk factor assessment when clinicians are faced with a diagnostic challenge.
TEACHING POINTS
- Exploration of epidemiology and exposure history is central to diagnosing meningoencephalitis with negative bacterial cultures and undetectable HSV PCR, although the etiology of meningoencephalitis can elude identification even after exhaustive investigation.
- Inhalation of contaminated aerosols is the principal route of transmission for C. burnetii, but it can also be transmitted via infected unpasteurized milk.[7, 9]
- Acute presentations of Q fever, which may warrant admission, include pneumonia, hepatitis, or meningoencephalitis.
- Q fever is diagnosed by serologic testing, and doxycycline is the antibiotic of choice.
Disclosures
This case was presented at the 2012 Annual Meeting of the Society of Hospital Medicine. It was subsequently reported in the epidemiologic report of the outbreak.[6] The authors report no conflicts of interest.
A 39‐year‐old woman presented to the emergency department (ED) with fever and headache. One to two weeks prior to presentation, she developed nightly fevers that gradually increased to as high as 39.4C. She subsequently developed generalized throbbing headaches, malaise, and diffuse body pain. The headache gradually worsened. The day prior to presentation, she developed photophobia, nausea, and vomiting. She also reported right scalp pain while combing her hair, difficulty emptying her bladder, and left buttock pain radiating down the leg. She denied rash, joint pain, visual changes, dysarthria, cough, chest pain, abdominal pain, or diarrhea.
Fever and headache can be explained by meningitis, encephalitis, or brain abscess. The combination is seen far more frequently, however, in patients with common systemic infections such as influenza. For either bacterial meningitis or influenza, a 2‐week course is prolonged and atypical. The progressive nature of the symptoms and photophobia suggest a chronic meningitis, and the development of nausea and vomiting, although nonspecific, is also consistent with elevated intracranial pressure. In a young woman, subacute fever and aches should prompt consideration of an autoimmune disorder such as systemic lupus erythematosus (SLE), although early central nervous system (CNS) involvement is atypical. Migraine headaches are characterized by light sensitivity, nausea, and vomiting and can be precipitated by a viral syndrome, but in this case, the headaches were present at the outset, and 2 weeks is too long for a migraine attack.
Pain while combing hair is not characteristic of the aforementioned syndromes. The scalp should be examined to confirm that there are no skin lesions associated with herpes zoster and no arterial prominence associated with temporal arteritis. She is young for the latter, which would otherwise be a suitable explanation for fever, headache, scalp tenderness, and visual complaints (usually impairment not photophobia).
Incomplete bladder emptying and left buttock pain suggest that there might be a concomitant lumbosacral myelopathy or radiculopathy. Some nonbacterial causes of meningitis such as cytomegalovirus (CMV), syphilis, and cancer simultaneously involve the CNS and peripheral nerve roots. It is also possible that the scalp tenderness associated with combing reflects a cervical sensory radiculopathy.
She had presented to the ED 2 and 4 days before the current (third) ED visit. Both times her main complaint was left buttock pain and left leg paresthesias. Although she had no skin lesions, she was diagnosed with prodromal herpes zoster in the S2 dermatomal distribution and was prescribed valacyclovir (to be started should eruptions develop, which never occurred).
She reported intermittent self‐limited fevers at 3‐ to 4‐week intervals during the prior 6 months; two fever episodes were accompanied by an influenza‐like illness, and one was associated with gastrointestinal symptoms. Her last fever prior to this evaluation was 6 weeks earlier when she was treated with azithromycin for suspected pneumonia at an outside facility.
Her past medical history included hypothyroidism, gastroesophageal reflux disease, diverticulitis, and gluten intolerance. Her medications included porcine (natural) thyroid, fish oil, ibuprofen, and acetaminophen. She lived in Michigan and traveled to the northeast United States (Maine, Cape Cod, New Hampshire, Connecticut, and Vermont) 7 months prior to this evaluation. She was married and had no pets at home. She denied any tobacco, alcohol, or illicit drug use.
Her illness now appears to be chronic, associated with fever, and multisystem (potentially involving the pulmonary and gastrointestinal tract). None of her medical problems would predispose her to subacute meningitis, myelopathy, or radiculopathy. Hypothyroidism raises the possibility of a concomitant autoimmune disorder which causes meningitis, such as SLE or Behet's disease. Sarcoidosis can cause chronic meningitis and neuropathy with concomitant lung and gastrointestinal involvement and rarely fever.
Residency in the upper Midwest increases exposure to chronic infections that rarely cause subacute meningitis such as histoplasmosis, blastomycosis, or human granulocytic anaplasmosis. Travel to the northeast United States 1 month before the onset of her symptoms raises the possibility of other endemic infections like Lyme disease, babesiosis, and tularemia, which may account for her recurrent fevers. Of these, Lyme is most likely to present as chronic meningitis with cranial neuropathy and radiculoneuropathy.
Although the diagnosis of pneumonia was made late in her 6‐month illness, its etiology and treatment may be relevant. If the recent pneumonia was viral, a subsequent viral meningitis may be manifesting now or may have triggered an autoimmune process, such as acute disseminated encephalomyelitis. Bacterial pneumonia is a common precursor to bacterial meningitis, and treatment with azithromycin for the pneumonia may have delayed the meningitis onset or muted its course; this should be taken into account when interpreting cerebrospinal fluid (CSF) culture results.
On physical examination, her temperature was 39.1C, blood pressure was 135/91 mm Hg, with pulse of 87 beats per minute, respiratory rate of 16 breaths per minute, and oxygenation saturation of 97% on room air. She appeared in distress and was covering her eyes. She was alert and oriented. She had photophobia and mild nuchal rigidity. Pupils were equal and reactive to light, but she could not tolerate the eye exam for papilledema. Lung, heart, and abdominal exam were normal. No cranial nerve abnormalities were noted, and muscle strength was 5/5 in all 4 extremities. She had decreased sensation to light touch with allodynia throughout her lower extremities in addition to the lateral portion of the right scalp, which was also tender to palpation. Deep tendon reflexes were 2+ and symmetric in her bilateral upper and lower extremities. She did not have joint swelling, edema, lymphadenopathy, or a rash.
Her fever, headache, nuchal rigidity and photophobia collectively suggest meningitis, which requires evaluation by a lumbar puncture. There is no rash that supports herpes zoster or SLE. She does not have signs of myelopathy that would explain the urinary complaints, but lower motor neuron involvement has not been excluded. The sensory abnormalities in the scalp and leg are consistent with a polyneuroradiculopathy. Anterior lateral scalp tenderness may signal trigeminal nerve involvement, whereas posterior scalp tenderness would localize to the upper cervical cord nerve roots. The contralateral distribution of the scalp and leg sensory deficits suggests a multifocal peripheral nervous system process rather than a single CNS lesion.
Initial laboratory data showed serum white blood cell count (WBC) of 12,000/mm3 (79% polymorphonuclear leukocytes). Hemoglobin was 14.2 g/dL, and platelets were 251,000/mm3. Electrolytes, renal function, and liver function were normal. Thyroid‐stimulating hormone, erythrocyte sedimentation rate, and C‐reactive protein were normal. Urinalysis was negative. Chest x‐ray was normal. Noncontrast head computed tomography (CT) was normal. The patient was unable to void; 500 mL of urine returned when catheterization was performed.
CSF WBC count was 1,280/mm3 (39% neutrophils and 49% lymphocytes). CSF total protein was 175 mg/dL, and glucose was 48 mg/dL; serum glucose was 104 mg/dL. Opening pressure was not recorded. Gram stain was negative. Ceftriaxone, vancomycin, ampicillin, and acyclovir were administered for presumed bacterial or viral meningitis. Magnetic resonance imaging (MRI) of the brain and spine showed diffuse leptomeningeal enhancement (Figure 1).

The urinary retention in the absence of myelopathic findings on exam or MRI suggests a sacral polyradiculoneuropathy. Diffuse leptomeningeal enhancement is consistent with many, if not all, causes of meningitis. The high WBC count, elevated protein, and low glucosecollectively signaling active inflammation in the CNSare highly compatible with bacterial meningitis, although the lymphocytic predominance and other clinical data point to nonbacterial etiologies. The negative Gram stain further lowers the probability of bacterial meningitis, but it has limited sensitivity, may be affected by recent antibiotics, and is typically negative with Listeria. Enterovirus, acute human immunodeficiency virus (HIV), and herpes viruses (eg, CMV or herpes simplex virus [HSV]) are important considerations, with the latter 2 causing associated polyneuroradiculopathy. Patients with genital HSV (not detected here) can have a concomitant sacral radiculitis leading to urinary retention.
Fungal and mycobacterial meningitis is a possibility (especially with the high protein), but the patient does not have the typical multisystem disease or immunosuppression that frequently accompanies those conditions when CNS disease is present. Autoimmune conditions like SLE, Behet's disease, and sarcoidosis remain important conditions, especially with the polyneuroradiculopathy or mononeuritis multiplex, which may reflect multifocal nerve infarction or invasion. Similarly, lymphomatous or carcinomatous meningitis should be considered, although an isolated manifestation in the CNS is unusual. Based on the multifocal neurologic deficits, I favor a viral, spirochete, or malignant etiology of her meningoencephalitis.
Despite ongoing broad spectrum antibiotics and supportive care, she became confused on hospital day 3 and developed anomia, agitation, and worsening headache. A repeat CT of the brain did not show any new abnormalities, but repeat lumbar puncture demonstrated elevated intracranial pressure (opening pressure of 47 cm water) with 427 WBC/mm3. Blood and CSF cultures remained negative.
Detailed questioning of the family revealed that she had been horseback riding 3 weeks prior to admission; there were no other livestock where she rode horses. In addition, the family reported that she and other family members routinely drank raw milk from a cow share program.
HIV antibody test was negative. Herpes simplex, varicella zoster, enteroviruses, and adenovirus CSF polymerase chain reaction (PCR) were negative. Cytomegalovirus and Epstein‐Barr virus PCR were negative in serum and CSF. Arbovirus, lymphocytic choriomeningitis, Coccidioides, Blastomyces, Histoplasma, Brucella, and Lyme serologies were negative. Cryptococcus neoformans antigen was negative in CSF. Serum QuantiFERON‐TB test was negative. Blood and CSF acid‐fast bacilli smears (and eventually mycobacterial cultures) were also negative. Her CSF flow cytometry and cytology were negative for lymphoma.
Unpasteurized milk conveys multiple infectious risks. Listeriosis is a food‐borne illness that can cause meningoencephalitis, but peripheral neuropathies are not characteristic. Brucellosis is usually characterized by severe bone pain, pancytopenia, and hepatosplenomegaly, which are absent. Infection with Mycobacterium bovis mimics Mycobacterium tuberculosis and can cause multisystem disease, typically involving the lung. Campylobacter infection is characterized by gastroenteritis, which has not been prominent.
Rhodococcus equi is a horse‐related pathogen which leads to pulmonary infections in immunocompromised hosts but not meningitis. Rather than focusing on horse exposure alone, however, it may be useful to consider her at risk for vector‐borne pathogens based on her time outdoors, such as Lyme disease (which can cause radiculopathy and encephalopathy), West Nile virus (although motor weakness rather than sensory symptoms is typical), or eastern equine encephalitis.
The absence of weight loss, cytopenias, lymphadenopathy, and organomegaly with the negative CSF cytology and flow cytometry makes lymphomatous meningitis unlikely. The case for an autoimmune disorder is not strong in the absence of joint pains, rash, or autoimmune serologies. In a young woman with unexplained encephalitis, antibodies to the N‐methyl‐D‐aspartate receptor should be assayed.
Although the CSF leukocytosis is declining, the elevated pressure and clinical deterioration signal that the disease process is not controlled. At this point I am uncertain as to the cause of her progressive meningoencephalitis with polyneuroradiculopathy. The latter feature makes me favor a viral or spirochete etiology.
On hospital day 4, Coxiella burnetii serologies were reported as positive (phase II immunoglobulin [Ig] G 1:256; phase II IgM 1:16; phase I IgG 1:16; phase I IgM 1:16) suggesting acute Q fever. Antibiotics were changed to intravenous doxycycline and ciprofloxacin. Her increased intracranial pressure was managed with serial lumbar punctures. The patient was discharged after 12 days of hospitalization taking oral doxycycline and ciprofloxacin. Her symptoms resolved over 10 weeks. No vegetations were seen on transesophageal echocardiogram. She had no evidence of chronic Q fever on repeat serologies.
I was not aware that Q fever causes meningitis or meningoencephalitis. However, I should have considered it in light of her indirect exposure to cows. It is possible that her pneumonia 6 weeks earlier represented acute Q fever, as pneumonia and hepatitis are among the most typical acute manifestations of this infection.
COMMENTARY
Hospitalists are commonly confronted by the combination of fever, headache, and confusion and are familiar with the diagnostic and therapeutic dilemmas related to prompt discrimination between CNS and non‐CNS processes, particularly infections. At the time of this patient's final ED presentation, her illness unambiguously localized to the CNS. As common and emergent conditions such as acute bacterial meningitis were excluded, the greatest challenge was finding the clue that could direct investigations into less common causes of meningoencephalitis.
The Infectious Disease Society of America has developed clinical practice guidelines for the diagnosis and management of encephalitis which highlight the importance of epidemiology and risk factor assessment.[1] This approach requires the clinician to examine potential clues and to go beyond initial associationsfor instance, not simply linking horseback riding to horse‐associated pathogens, but interpreting horseback riding as a proxy for outdoor exposure, which places her at risk for contact with mosquitos, which transmit West Nile virus or eastern equine encephalitis. Similarly, ingestion of raw milk, which is typically linked to Listeria monocytogenes, Brucella, and other pathogens prompted the infectious disease consultant to think more broadly and include livestock (cow)‐associated pathogens including C. burnetii.
Although involvement of the CNS is common in chronic Q fever endocarditis due to septic embolism, neurologic involvement in acute Q fever varies in prevalence (range of 1.7%22%).[2, 3, 4] The 3 major neurological syndromes of acute Q fever are (1) meningoencephalitis or encephalitis, (2) lymphocytic meningitis, and (3) peripheral neuropathy (myelitis, polyradiculoneuritis, or peripheral neuritis). CSF analysis usually shows mild pleocytosis with a predominance of lymphocytic cells; CSF protein elevation is variable, and glucose is usually normal. Neuroradiologic examination is usually normal, and there are no pathognomonic imaging abnormalities for Q fever meningoencephalitis.[2, 3] The mechanism by which C. burnetii causes neurologic injury and dysfunction is unknown.
The diagnosis of Q fever is usually established by serologic testing. In acute Q fever, antibodies to phase II antigen are higher than the phase I antibody titer. Phase II IgM antibodies are the first to appear, but then decline on average after week 8, often reaching undetectable levels 10 to 12 weeks after disease onset.[5] If this patient's pneumonia 6 weeks prior to this presentation was acute Q fever pneumonia, her IgM titers may have been declining by the time her neurologic illness developed. A false negative test result is also possible; immunofluorescence assays are more specific than sensitive in acute Q fever.[5]
Evaluating this case in isolation may raise some doubt as to the accuracy of the diagnosis as she did not have a 4‐fold rise in the phase II IgG titer and did not have a detectable phase II IgM. However, she was part of a cluster of individuals who regularly consumed raw milk from the same dairy and had evidence of C. burnetii infection. This group included her spouse, who had a robust serologic evidence of C. burnetii, characterized by a >4‐fold rise in phase II IgM and IgG titers.[6]
C. burnetii is found primarily in cattle, sheep, and goats and is shed in large quantities by infected periparturient animals in their urine, feces, and milk.[7] Inhalation of contaminated aerosols is the principal route of transmission.[7, 8] Acute Q fever is underdiagnosed because the majority of acute infections are asymptomatic (60%) or present as a nonspecific flu‐like illness.[7] This case represents a rare manifestation of a rare infection acquired through a rare route of transmission, but highlights the importance of epidemiology and risk factor assessment when clinicians are faced with a diagnostic challenge.
TEACHING POINTS
- Exploration of epidemiology and exposure history is central to diagnosing meningoencephalitis with negative bacterial cultures and undetectable HSV PCR, although the etiology of meningoencephalitis can elude identification even after exhaustive investigation.
- Inhalation of contaminated aerosols is the principal route of transmission for C. burnetii, but it can also be transmitted via infected unpasteurized milk.[7, 9]
- Acute presentations of Q fever, which may warrant admission, include pneumonia, hepatitis, or meningoencephalitis.
- Q fever is diagnosed by serologic testing, and doxycycline is the antibiotic of choice.
Disclosures
This case was presented at the 2012 Annual Meeting of the Society of Hospital Medicine. It was subsequently reported in the epidemiologic report of the outbreak.[6] The authors report no conflicts of interest.
- , , , et al. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2008;47:303–327.
- , , , et al. Q fever 1985–1998 clinical and epidemiologic features of 1,383 Infections. Medicine. 2000;79:109–123.
- , , , et al. Neurological involvement in acute Q fever: a report of 29 cases and review of the literature. Arch Intern Med. 2002;162:693–700.
- , , . Q fever in Plymouth 1972–88, a review with particular reference to neurological manifestations. Epidemiol Infect. 1990;105:391–408.
- , , . Diagnosis of Q fever. J Clin Microbiol. 1998;36:1823–1834.
- , , . Q fever cluster among raw milk drinkers in Michigan, (2011). Clin Infect Dis. 2012;55:1387–1389.
- , . Q fever. Clin Micorbial Rev. 1999;12:18–53.
- , , , et al. A large outbreak of Q fever in the West Midlands: windborne spread into a metropolitan area? Commun Dis Public Health. 1998;1:180–187.
- , . A cluster of Coxiella burnetii infections associated with exposure to vaccinated goats and their unpasteurized dairy products. Am J Trop Med Hyg. 1992;47:35–40.
- , , , et al. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2008;47:303–327.
- , , , et al. Q fever 1985–1998 clinical and epidemiologic features of 1,383 Infections. Medicine. 2000;79:109–123.
- , , , et al. Neurological involvement in acute Q fever: a report of 29 cases and review of the literature. Arch Intern Med. 2002;162:693–700.
- , , . Q fever in Plymouth 1972–88, a review with particular reference to neurological manifestations. Epidemiol Infect. 1990;105:391–408.
- , , . Diagnosis of Q fever. J Clin Microbiol. 1998;36:1823–1834.
- , , . Q fever cluster among raw milk drinkers in Michigan, (2011). Clin Infect Dis. 2012;55:1387–1389.
- , . Q fever. Clin Micorbial Rev. 1999;12:18–53.
- , , , et al. A large outbreak of Q fever in the West Midlands: windborne spread into a metropolitan area? Commun Dis Public Health. 1998;1:180–187.
- , . A cluster of Coxiella burnetii infections associated with exposure to vaccinated goats and their unpasteurized dairy products. Am J Trop Med Hyg. 1992;47:35–40.
BOOST and Readmissions
Although hospital readmissions have been a problem for at least the past 5 decades, they are now receiving more attention than ever before. Starting with the 2007 Medicare Payment Advisory Commission report detailing the vast scope of the problem,[1] readmissions have garnered substantial policy interest, culminating with Congress' inclusion of a penalty for hospitals with excessive readmission rates in the Affordable Care Act. Clinical leaders have become increasingly active in this issue as well, and hospitals around the nation have become engaged in finding ways to reduce the number of times patients return after discharge.
The Hospital Readmissions Reduction Program (HRRP), which is the penalty program put in place by Congress to address readmissions, has been controversial from its inception. Supporters point to the large number of patients whose discharge is fraught with poor communication, ineffective medication management, and inadequate handoffs to the primary care physician. Critics have countered that only a small proportion of readmissions are likely preventable by what hospitals can control,[2] and that patient factors, especially social and economic circumstances,[3] primarily drive readmissions. Despite this debate, we can all agree there is ample opportunity to improve the care of patients at the time of discharge.
In this context, we see important evidence emerging from the Better Outcomes by Optimizing Safe Transitions (BOOST) program. Funded by the Hartford Foundation among others, BOOST is specifically aimed at improving care transitions among older hospitalized adults. BOOST focuses on identifying those at highest risk for readmissions, communicating the discharge plan effectively, and ensuring close follow‐up, both through phone calls after discharge and timely appointments with primary care providers. These are all interventions that seem intuitively like good ideas. In this issue of the Journal of Hospital Medicine, leaders of the BOOST program report on the impact on readmissions rate.[4] However, as the accompanying editorial points out, the data are disappointing.[5] The evidence, seen in the best possible light, suggests a small improvement among a very select group of hospitals. Although the authors should be commended for writing up their findings, the fact that 19 of the 30 hospitals that received substantial training and assistance through the BOOST program chose not to report their data is unconscionable. The decision by those 19 hospitals to withhold data makes the results nearly uninterpretable and jeopardizes the hard work that so many others have engaged in. BOOST should require that hospitals agree to share data as a condition of participation in the program.
The Hansen study,[4] despite its disappointing findings, may signal that it is time for a new approach. First of all, we may need to focus on different metrics. Looking ahead, the most important question may not be Does BOOST lower readmission rates? but rather Does BOOST improve the care for patients at the time of discharge from the hospital? There are several good measures of the quality of a care transition, such as those by Coleman and colleagues,[6] and these could be used to measure the quality of care hospitals deliver at discharge. We could also develop new metrics of transitions of care. For example, hospitals truly committed to improvement could field an ongoing survey of primary care physicians in their community to ensure that care transitions are happening smoothly from the primary care providers' perspective. Patient experience metrics, beyond those captured in the Hospital Consumer Assessment of Healthcare Providers and Systems survey, may be necessary to better assess patient and family perspectives on the transition from the hospital to home. These and other approaches can help hospitals better understand how effectively they manage the handoff as patients leave their doors.
However, we should also recognize that although such approaches may improve care transitions, they are unlikely to substantially reduce readmissions. Instead, hospitals serious about reducing readmissions may need to reconsider their business model.[7] In the days following a discharge, patients are medically and socially vulnerable. Patients without robust social support at home may need more than just the right medications, a phone call, or a follow‐up appointment. They may need help with groceries, having their meals prepared, or getting a ride to the doctor's office. Hospitals that want to reduce readmissions may need to make investments in creating the community and social support that so many patients lack when they leave the hospital. This has never been part of the hospital business model before, but it may be time for a change.
The HRRP, an effort by federal policymakers to drive down readmissions through penalties, has clearly begun to make hospitals think about changing their business models in precisely these ways. Readmission rates are falling, although a concurrent increase in the number of patients being admitted to observation status makes it unclear whether patient care has actually improved. More data and time will tell. Furthermore, the program as currently designed targets hospitals that care for the sickest and poorest patients for penalties.[8] There are plenty of good options for addressing these unintended consequences, such as comparing safety‐net hospitals' performance to other similar institutions, or focusing only on preventable readmissions. However, regardless of its limitations, the HRRP in some form or another is here to stay. Therefore, hospitals will need to find ways to reduce readmissions, and programs like BOOST, even when executed perfectly, will be necessary but likely insufficient. Improving the quality of care transitions is critically important. But to truly get to better outcomes for older Americans, hospitals will need to think beyond their 4 walls.
- Report to the Congress: Promoting Greater Efficiency in Medicare. Washington, DC: Medicare Payment Advisory Commission; 2007.
- , , , , . Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183(7):E391–E402.
- , , . Thirty‐day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011;305(7):675–681.
- , , , , , , , , . Project BOOST: Effectiveness of a Multihospital Effort to Reduce Rehospitalization. J Hosp Med. 2013;8(8):421–427.
- , , , , , . BOOST: Evidence Needing a Lift. J Hosp Med. 2013;8(8):468–469.
- . The Care Transitions Program Web site. Available at: http://www.caretransitions.org/ctm_main.asp. Accessed June 6, 2013.
- , . A path forward on Medicare readmissions. N Engl J Med. 2013;368(13):1175–1177.
- , . Characteristics of hospitals receiving penalties under the Hospital Readmissions Reduction Program. JAMA. 2013;309(4):342–343.
Although hospital readmissions have been a problem for at least the past 5 decades, they are now receiving more attention than ever before. Starting with the 2007 Medicare Payment Advisory Commission report detailing the vast scope of the problem,[1] readmissions have garnered substantial policy interest, culminating with Congress' inclusion of a penalty for hospitals with excessive readmission rates in the Affordable Care Act. Clinical leaders have become increasingly active in this issue as well, and hospitals around the nation have become engaged in finding ways to reduce the number of times patients return after discharge.
The Hospital Readmissions Reduction Program (HRRP), which is the penalty program put in place by Congress to address readmissions, has been controversial from its inception. Supporters point to the large number of patients whose discharge is fraught with poor communication, ineffective medication management, and inadequate handoffs to the primary care physician. Critics have countered that only a small proportion of readmissions are likely preventable by what hospitals can control,[2] and that patient factors, especially social and economic circumstances,[3] primarily drive readmissions. Despite this debate, we can all agree there is ample opportunity to improve the care of patients at the time of discharge.
In this context, we see important evidence emerging from the Better Outcomes by Optimizing Safe Transitions (BOOST) program. Funded by the Hartford Foundation among others, BOOST is specifically aimed at improving care transitions among older hospitalized adults. BOOST focuses on identifying those at highest risk for readmissions, communicating the discharge plan effectively, and ensuring close follow‐up, both through phone calls after discharge and timely appointments with primary care providers. These are all interventions that seem intuitively like good ideas. In this issue of the Journal of Hospital Medicine, leaders of the BOOST program report on the impact on readmissions rate.[4] However, as the accompanying editorial points out, the data are disappointing.[5] The evidence, seen in the best possible light, suggests a small improvement among a very select group of hospitals. Although the authors should be commended for writing up their findings, the fact that 19 of the 30 hospitals that received substantial training and assistance through the BOOST program chose not to report their data is unconscionable. The decision by those 19 hospitals to withhold data makes the results nearly uninterpretable and jeopardizes the hard work that so many others have engaged in. BOOST should require that hospitals agree to share data as a condition of participation in the program.
The Hansen study,[4] despite its disappointing findings, may signal that it is time for a new approach. First of all, we may need to focus on different metrics. Looking ahead, the most important question may not be Does BOOST lower readmission rates? but rather Does BOOST improve the care for patients at the time of discharge from the hospital? There are several good measures of the quality of a care transition, such as those by Coleman and colleagues,[6] and these could be used to measure the quality of care hospitals deliver at discharge. We could also develop new metrics of transitions of care. For example, hospitals truly committed to improvement could field an ongoing survey of primary care physicians in their community to ensure that care transitions are happening smoothly from the primary care providers' perspective. Patient experience metrics, beyond those captured in the Hospital Consumer Assessment of Healthcare Providers and Systems survey, may be necessary to better assess patient and family perspectives on the transition from the hospital to home. These and other approaches can help hospitals better understand how effectively they manage the handoff as patients leave their doors.
However, we should also recognize that although such approaches may improve care transitions, they are unlikely to substantially reduce readmissions. Instead, hospitals serious about reducing readmissions may need to reconsider their business model.[7] In the days following a discharge, patients are medically and socially vulnerable. Patients without robust social support at home may need more than just the right medications, a phone call, or a follow‐up appointment. They may need help with groceries, having their meals prepared, or getting a ride to the doctor's office. Hospitals that want to reduce readmissions may need to make investments in creating the community and social support that so many patients lack when they leave the hospital. This has never been part of the hospital business model before, but it may be time for a change.
The HRRP, an effort by federal policymakers to drive down readmissions through penalties, has clearly begun to make hospitals think about changing their business models in precisely these ways. Readmission rates are falling, although a concurrent increase in the number of patients being admitted to observation status makes it unclear whether patient care has actually improved. More data and time will tell. Furthermore, the program as currently designed targets hospitals that care for the sickest and poorest patients for penalties.[8] There are plenty of good options for addressing these unintended consequences, such as comparing safety‐net hospitals' performance to other similar institutions, or focusing only on preventable readmissions. However, regardless of its limitations, the HRRP in some form or another is here to stay. Therefore, hospitals will need to find ways to reduce readmissions, and programs like BOOST, even when executed perfectly, will be necessary but likely insufficient. Improving the quality of care transitions is critically important. But to truly get to better outcomes for older Americans, hospitals will need to think beyond their 4 walls.
Although hospital readmissions have been a problem for at least the past 5 decades, they are now receiving more attention than ever before. Starting with the 2007 Medicare Payment Advisory Commission report detailing the vast scope of the problem,[1] readmissions have garnered substantial policy interest, culminating with Congress' inclusion of a penalty for hospitals with excessive readmission rates in the Affordable Care Act. Clinical leaders have become increasingly active in this issue as well, and hospitals around the nation have become engaged in finding ways to reduce the number of times patients return after discharge.
The Hospital Readmissions Reduction Program (HRRP), which is the penalty program put in place by Congress to address readmissions, has been controversial from its inception. Supporters point to the large number of patients whose discharge is fraught with poor communication, ineffective medication management, and inadequate handoffs to the primary care physician. Critics have countered that only a small proportion of readmissions are likely preventable by what hospitals can control,[2] and that patient factors, especially social and economic circumstances,[3] primarily drive readmissions. Despite this debate, we can all agree there is ample opportunity to improve the care of patients at the time of discharge.
In this context, we see important evidence emerging from the Better Outcomes by Optimizing Safe Transitions (BOOST) program. Funded by the Hartford Foundation among others, BOOST is specifically aimed at improving care transitions among older hospitalized adults. BOOST focuses on identifying those at highest risk for readmissions, communicating the discharge plan effectively, and ensuring close follow‐up, both through phone calls after discharge and timely appointments with primary care providers. These are all interventions that seem intuitively like good ideas. In this issue of the Journal of Hospital Medicine, leaders of the BOOST program report on the impact on readmissions rate.[4] However, as the accompanying editorial points out, the data are disappointing.[5] The evidence, seen in the best possible light, suggests a small improvement among a very select group of hospitals. Although the authors should be commended for writing up their findings, the fact that 19 of the 30 hospitals that received substantial training and assistance through the BOOST program chose not to report their data is unconscionable. The decision by those 19 hospitals to withhold data makes the results nearly uninterpretable and jeopardizes the hard work that so many others have engaged in. BOOST should require that hospitals agree to share data as a condition of participation in the program.
The Hansen study,[4] despite its disappointing findings, may signal that it is time for a new approach. First of all, we may need to focus on different metrics. Looking ahead, the most important question may not be Does BOOST lower readmission rates? but rather Does BOOST improve the care for patients at the time of discharge from the hospital? There are several good measures of the quality of a care transition, such as those by Coleman and colleagues,[6] and these could be used to measure the quality of care hospitals deliver at discharge. We could also develop new metrics of transitions of care. For example, hospitals truly committed to improvement could field an ongoing survey of primary care physicians in their community to ensure that care transitions are happening smoothly from the primary care providers' perspective. Patient experience metrics, beyond those captured in the Hospital Consumer Assessment of Healthcare Providers and Systems survey, may be necessary to better assess patient and family perspectives on the transition from the hospital to home. These and other approaches can help hospitals better understand how effectively they manage the handoff as patients leave their doors.
However, we should also recognize that although such approaches may improve care transitions, they are unlikely to substantially reduce readmissions. Instead, hospitals serious about reducing readmissions may need to reconsider their business model.[7] In the days following a discharge, patients are medically and socially vulnerable. Patients without robust social support at home may need more than just the right medications, a phone call, or a follow‐up appointment. They may need help with groceries, having their meals prepared, or getting a ride to the doctor's office. Hospitals that want to reduce readmissions may need to make investments in creating the community and social support that so many patients lack when they leave the hospital. This has never been part of the hospital business model before, but it may be time for a change.
The HRRP, an effort by federal policymakers to drive down readmissions through penalties, has clearly begun to make hospitals think about changing their business models in precisely these ways. Readmission rates are falling, although a concurrent increase in the number of patients being admitted to observation status makes it unclear whether patient care has actually improved. More data and time will tell. Furthermore, the program as currently designed targets hospitals that care for the sickest and poorest patients for penalties.[8] There are plenty of good options for addressing these unintended consequences, such as comparing safety‐net hospitals' performance to other similar institutions, or focusing only on preventable readmissions. However, regardless of its limitations, the HRRP in some form or another is here to stay. Therefore, hospitals will need to find ways to reduce readmissions, and programs like BOOST, even when executed perfectly, will be necessary but likely insufficient. Improving the quality of care transitions is critically important. But to truly get to better outcomes for older Americans, hospitals will need to think beyond their 4 walls.
- Report to the Congress: Promoting Greater Efficiency in Medicare. Washington, DC: Medicare Payment Advisory Commission; 2007.
- , , , , . Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183(7):E391–E402.
- , , . Thirty‐day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011;305(7):675–681.
- , , , , , , , , . Project BOOST: Effectiveness of a Multihospital Effort to Reduce Rehospitalization. J Hosp Med. 2013;8(8):421–427.
- , , , , , . BOOST: Evidence Needing a Lift. J Hosp Med. 2013;8(8):468–469.
- . The Care Transitions Program Web site. Available at: http://www.caretransitions.org/ctm_main.asp. Accessed June 6, 2013.
- , . A path forward on Medicare readmissions. N Engl J Med. 2013;368(13):1175–1177.
- , . Characteristics of hospitals receiving penalties under the Hospital Readmissions Reduction Program. JAMA. 2013;309(4):342–343.
- Report to the Congress: Promoting Greater Efficiency in Medicare. Washington, DC: Medicare Payment Advisory Commission; 2007.
- , , , , . Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183(7):E391–E402.
- , , . Thirty‐day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011;305(7):675–681.
- , , , , , , , , . Project BOOST: Effectiveness of a Multihospital Effort to Reduce Rehospitalization. J Hosp Med. 2013;8(8):421–427.
- , , , , , . BOOST: Evidence Needing a Lift. J Hosp Med. 2013;8(8):468–469.
- . The Care Transitions Program Web site. Available at: http://www.caretransitions.org/ctm_main.asp. Accessed June 6, 2013.
- , . A path forward on Medicare readmissions. N Engl J Med. 2013;368(13):1175–1177.
- , . Characteristics of hospitals receiving penalties under the Hospital Readmissions Reduction Program. JAMA. 2013;309(4):342–343.