User login
Painful nail with longitudinal erythronychia
A 46-year-old Caucasian woman was referred to our dermatology clinic with a one year history of progressively increasing pain radiating from the proximal nail fold of her right middle finger. She denied any history of trauma and noted that the pain was worse when her finger was exposed to cold.
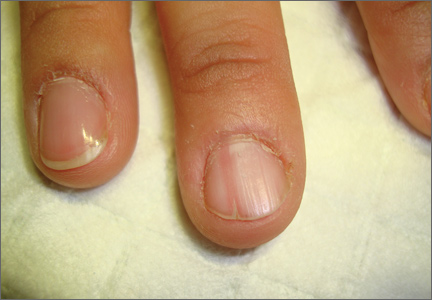
On examination, we noted that there was a red line that extended the length of the nail, beginning at the area of pain and ending distally, where the nail split (FIGURE).
FIGURE
Red line extends from area of pain to area of nail splitting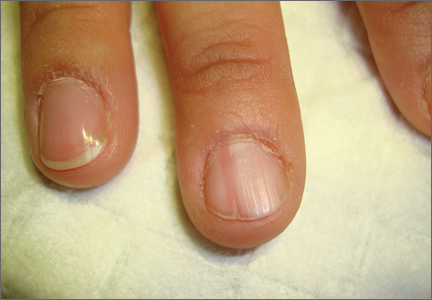
What is your diagnosis?
How would you treat this patient?
Diagnosis: Subungual glomus tumor
Glomus tumor is a rare vascular neoplasm derived from the cells of the glomus body, a specialized arteriovenous shunt involved in temperature regulation. Glomus bodies are most abundant in the extremities, and 75% of glomus tumors are found in the hand.1 The most common location is the subungual region, where glomus bodies are highly concentrated.
These lesions are typically benign, although a malignant variant has been reported in 1% of cases.1,2 Glomus tumors are most common in adults 30 to 50 years of age, with subungual tumors occurring more often in women.3 The majority of glomus tumors are solitary and less than 1 cm in size.2,4 Multiple tumors may be familial and tend to occur in children.2,4
In an analysis of 43 patients with glomus tumors, only 19% of referring practitioners and 49% of hospital practitioners made the correct diagnosis.Patients with subungual glomus tumors present with intense pain that they may describe as shooting or pulsating in nature.
The pain may be spontaneous or triggered by mild trauma or changes in temperature—especially warm to cold. The classic triad of symptoms includes pinpoint tenderness, paroxysmal pain, and cold hypersensitivity. 3,4 The glomus tumor may appear as a focal bluish to erythematous discoloration visible through the nail plate, and in some cases the tumor may form a palpable nodule. Nail deformities such as ridging and distal fissuring occur in approximately one-third of patients.4
Longitudinal erythronychia, as seen in our patient, results when the glomus tumor exerts pressure on the distal nail matrix. This force leads to a thinning of the nail plate and the formation of a groove on the ventral surface of the nail. Swelling of the underlying nail bed with engorgement of vessels produces the red streak that is seen through the thinned nail.5 And, because the affected portion of the nail is fragile, it tends to split distally.
Longitudinal erythronychia with nail dystrophy involving multiple nails is also seen in inflammatory diseases, such as lichen planus and Darier disease, due to multifocal loss of nail matrix function.5
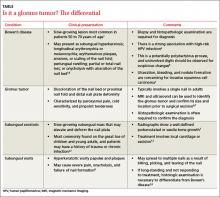
Differential Dx includes subungual warts, Bowen’s disease
Clinical mimics of glomus tumors include neuromas, melanomas, Bowen’s disease, arthritis, gout, paronychia, causalgia, subungual exostosis, osteochondroma, and subungual warts. (The TABLE1,6-8 describes some of the more common mimics.)
In an analysis of 43 patients with glomus tumors, only 19% of referring practitioners and 49% of hospital-based practitioners correctly made the diagnosis.3
Suspect a glomus tumor? Perform these tests
Three clinical tests can aid in evaluating for glomus tumors.
- Love’s test involves applying pressure to the affected fingertip using the head of a pin or the end of a paperclip. The point of maximal tenderness locates the tumor.
- In Hildreth’s test, the physician applies a tourniquet to the digit and repeats the Love’s test. The test is considered suggestive of glomus tumor if the patient no longer experiences tenderness with pressure.
- The cold sensitivity test requires that the physician expose the finger to cold by, say, placing the finger in an ice bath. This exposure will elicit increased pain in a patient who has a glomus tumor.
The sensitivity and specificity of these tests, according to one study involving 18 patients, is as follows: Love’s test (100%, 78%); Hildreth’s test (77.4%, 100%); and the cold sensitivity test (100%, 100%).9 Clinical suspicion must be confirmed by histopathologic examination and the patient must be alerted to the risks of biopsy, which include permanent nail deformity.
In addition, imaging studies may aid in the diagnosis as well as determine the preoperative size and location of the tumor. Radiography may show bone erosion in certain cases, and it is useful in differentiating a glomus tumor from subungual exostosis.10 Magnetic resonance imaging and ultrasound imaging have also been used to identify glomus tumors and to aid in determining the method of excision.10,11
Surgical excision is the preferred approach
While there are reports of successful treatment with laser and sclerotherapy, surgical excision remains the accepted intervention to relieve pain and minimize recurrence.12,13 The optimal surgical approach, which depends on the location of the tumor,13,14 will minimize the risk of postsurgical nail deformity while allowing for complete tumor removal.
A biopsy for our patient
While the intent of our biopsy was diagnostic, it also proved to be therapeutic as our patient experienced complete resolution of her pain immediately after the procedure. Six months later, she remained asymptomatic and reported no nail deformity. We counseled her on the possibility that her symptoms might return and encouraged her to come back in for further care as needed.
Correspondence
Thomas M. Beachkofsky, MD, Wilford Hall Medical Center, Department of Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; [email protected]
1. Baran R, Richert B. Common nail tumors. Dermatol Clin. 206;24:297-311.
2. Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132:1448-1452.
3. Heys SD, Brittenden J, Atkinson P, et al. Glomus tumour: an analysis of 43 patients and review of the literature. Br J Surg. 1992;79:345-347.
4. McDermott EM, Weiss AP. Glomus tumors. J Hand Surg Am. 2006;31:1397-1400.
5. De Berker DA, Perrin C, Baran R. Localized longitudinal erythronychia: diagnostic significance and physical explanation. Arch Dermatol. 2004;140:1253-1257.
6. Grundmeier N, Hamm H, Weissbrich B, et al. High-risk human papillomavirus infection in Bowen’s disease of the nail unit: report of three cases and review of the literature. Dermatology. 2011;223:293-300.
7. Bach DQ, McQueen AA, Lio PA. A refractory wart? Subungual exostosis. Ann Emerg Med. 2011;58:e3-e4.
8. Garman ME, Orengo IF, Netscher D, et al. On glomus tumors, warts, and razors. Dermatol Surg. 2003;29:192-194.
9. Bhaskaranand K, Navadgi BC. Glomus tumour of the hand. J Hand Surg Br. 2002;27:229-231.
10. Takemura N, Fujii N, Tanaka T. Subungual glomus tumor diagnosis based on imaging. J Dermatol. 2006;33:389-393.
11. Matsunaga A, Ochiai T, Abe I, et al. Subungual glomus tumour: evaluation of ultrasound imaging in preoperative assessment. Eur J Dermatol. 2007;17:67-69.
12. Vergilis-Kalner IJ, Friedman PM, Goldberg LH. Long-pulse 595-nm pulsed dye laser for the treatment of a glomus tumor. Dermatol Surg. 2010;36:1463-1465.
13. Netscher DT, Aburto J, Koepplinger M. Subungual glomus tumor. J Hand Surg Am. 2012;37:821-823.
14. Takata H, Ikuta Y, Ishida O, et al. Treatment of subungual glomus tumour. Hand Surg. 2001;6:25-27.
A 46-year-old Caucasian woman was referred to our dermatology clinic with a one year history of progressively increasing pain radiating from the proximal nail fold of her right middle finger. She denied any history of trauma and noted that the pain was worse when her finger was exposed to cold.
On examination, we noted that there was a red line that extended the length of the nail, beginning at the area of pain and ending distally, where the nail split (FIGURE).
FIGURE
Red line extends from area of pain to area of nail splitting
What is your diagnosis?
How would you treat this patient?
Diagnosis: Subungual glomus tumor
Glomus tumor is a rare vascular neoplasm derived from the cells of the glomus body, a specialized arteriovenous shunt involved in temperature regulation. Glomus bodies are most abundant in the extremities, and 75% of glomus tumors are found in the hand.1 The most common location is the subungual region, where glomus bodies are highly concentrated.
These lesions are typically benign, although a malignant variant has been reported in 1% of cases.1,2 Glomus tumors are most common in adults 30 to 50 years of age, with subungual tumors occurring more often in women.3 The majority of glomus tumors are solitary and less than 1 cm in size.2,4 Multiple tumors may be familial and tend to occur in children.2,4
In an analysis of 43 patients with glomus tumors, only 19% of referring practitioners and 49% of hospital practitioners made the correct diagnosis.Patients with subungual glomus tumors present with intense pain that they may describe as shooting or pulsating in nature.
The pain may be spontaneous or triggered by mild trauma or changes in temperature—especially warm to cold. The classic triad of symptoms includes pinpoint tenderness, paroxysmal pain, and cold hypersensitivity. 3,4 The glomus tumor may appear as a focal bluish to erythematous discoloration visible through the nail plate, and in some cases the tumor may form a palpable nodule. Nail deformities such as ridging and distal fissuring occur in approximately one-third of patients.4
Longitudinal erythronychia, as seen in our patient, results when the glomus tumor exerts pressure on the distal nail matrix. This force leads to a thinning of the nail plate and the formation of a groove on the ventral surface of the nail. Swelling of the underlying nail bed with engorgement of vessels produces the red streak that is seen through the thinned nail.5 And, because the affected portion of the nail is fragile, it tends to split distally.
Longitudinal erythronychia with nail dystrophy involving multiple nails is also seen in inflammatory diseases, such as lichen planus and Darier disease, due to multifocal loss of nail matrix function.5
Differential Dx includes subungual warts, Bowen’s disease
Clinical mimics of glomus tumors include neuromas, melanomas, Bowen’s disease, arthritis, gout, paronychia, causalgia, subungual exostosis, osteochondroma, and subungual warts. (The TABLE1,6-8 describes some of the more common mimics.)
In an analysis of 43 patients with glomus tumors, only 19% of referring practitioners and 49% of hospital-based practitioners correctly made the diagnosis.3
Suspect a glomus tumor? Perform these tests
Three clinical tests can aid in evaluating for glomus tumors.
- Love’s test involves applying pressure to the affected fingertip using the head of a pin or the end of a paperclip. The point of maximal tenderness locates the tumor.
- In Hildreth’s test, the physician applies a tourniquet to the digit and repeats the Love’s test. The test is considered suggestive of glomus tumor if the patient no longer experiences tenderness with pressure.
- The cold sensitivity test requires that the physician expose the finger to cold by, say, placing the finger in an ice bath. This exposure will elicit increased pain in a patient who has a glomus tumor.
The sensitivity and specificity of these tests, according to one study involving 18 patients, is as follows: Love’s test (100%, 78%); Hildreth’s test (77.4%, 100%); and the cold sensitivity test (100%, 100%).9 Clinical suspicion must be confirmed by histopathologic examination and the patient must be alerted to the risks of biopsy, which include permanent nail deformity.
In addition, imaging studies may aid in the diagnosis as well as determine the preoperative size and location of the tumor. Radiography may show bone erosion in certain cases, and it is useful in differentiating a glomus tumor from subungual exostosis.10 Magnetic resonance imaging and ultrasound imaging have also been used to identify glomus tumors and to aid in determining the method of excision.10,11
Surgical excision is the preferred approach
While there are reports of successful treatment with laser and sclerotherapy, surgical excision remains the accepted intervention to relieve pain and minimize recurrence.12,13 The optimal surgical approach, which depends on the location of the tumor,13,14 will minimize the risk of postsurgical nail deformity while allowing for complete tumor removal.
A biopsy for our patient
While the intent of our biopsy was diagnostic, it also proved to be therapeutic as our patient experienced complete resolution of her pain immediately after the procedure. Six months later, she remained asymptomatic and reported no nail deformity. We counseled her on the possibility that her symptoms might return and encouraged her to come back in for further care as needed.
Correspondence
Thomas M. Beachkofsky, MD, Wilford Hall Medical Center, Department of Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; [email protected]
A 46-year-old Caucasian woman was referred to our dermatology clinic with a one year history of progressively increasing pain radiating from the proximal nail fold of her right middle finger. She denied any history of trauma and noted that the pain was worse when her finger was exposed to cold.
On examination, we noted that there was a red line that extended the length of the nail, beginning at the area of pain and ending distally, where the nail split (FIGURE).
FIGURE
Red line extends from area of pain to area of nail splitting
What is your diagnosis?
How would you treat this patient?
Diagnosis: Subungual glomus tumor
Glomus tumor is a rare vascular neoplasm derived from the cells of the glomus body, a specialized arteriovenous shunt involved in temperature regulation. Glomus bodies are most abundant in the extremities, and 75% of glomus tumors are found in the hand.1 The most common location is the subungual region, where glomus bodies are highly concentrated.
These lesions are typically benign, although a malignant variant has been reported in 1% of cases.1,2 Glomus tumors are most common in adults 30 to 50 years of age, with subungual tumors occurring more often in women.3 The majority of glomus tumors are solitary and less than 1 cm in size.2,4 Multiple tumors may be familial and tend to occur in children.2,4
In an analysis of 43 patients with glomus tumors, only 19% of referring practitioners and 49% of hospital practitioners made the correct diagnosis.Patients with subungual glomus tumors present with intense pain that they may describe as shooting or pulsating in nature.
The pain may be spontaneous or triggered by mild trauma or changes in temperature—especially warm to cold. The classic triad of symptoms includes pinpoint tenderness, paroxysmal pain, and cold hypersensitivity. 3,4 The glomus tumor may appear as a focal bluish to erythematous discoloration visible through the nail plate, and in some cases the tumor may form a palpable nodule. Nail deformities such as ridging and distal fissuring occur in approximately one-third of patients.4
Longitudinal erythronychia, as seen in our patient, results when the glomus tumor exerts pressure on the distal nail matrix. This force leads to a thinning of the nail plate and the formation of a groove on the ventral surface of the nail. Swelling of the underlying nail bed with engorgement of vessels produces the red streak that is seen through the thinned nail.5 And, because the affected portion of the nail is fragile, it tends to split distally.
Longitudinal erythronychia with nail dystrophy involving multiple nails is also seen in inflammatory diseases, such as lichen planus and Darier disease, due to multifocal loss of nail matrix function.5
Differential Dx includes subungual warts, Bowen’s disease
Clinical mimics of glomus tumors include neuromas, melanomas, Bowen’s disease, arthritis, gout, paronychia, causalgia, subungual exostosis, osteochondroma, and subungual warts. (The TABLE1,6-8 describes some of the more common mimics.)
In an analysis of 43 patients with glomus tumors, only 19% of referring practitioners and 49% of hospital-based practitioners correctly made the diagnosis.3
Suspect a glomus tumor? Perform these tests
Three clinical tests can aid in evaluating for glomus tumors.
- Love’s test involves applying pressure to the affected fingertip using the head of a pin or the end of a paperclip. The point of maximal tenderness locates the tumor.
- In Hildreth’s test, the physician applies a tourniquet to the digit and repeats the Love’s test. The test is considered suggestive of glomus tumor if the patient no longer experiences tenderness with pressure.
- The cold sensitivity test requires that the physician expose the finger to cold by, say, placing the finger in an ice bath. This exposure will elicit increased pain in a patient who has a glomus tumor.
The sensitivity and specificity of these tests, according to one study involving 18 patients, is as follows: Love’s test (100%, 78%); Hildreth’s test (77.4%, 100%); and the cold sensitivity test (100%, 100%).9 Clinical suspicion must be confirmed by histopathologic examination and the patient must be alerted to the risks of biopsy, which include permanent nail deformity.
In addition, imaging studies may aid in the diagnosis as well as determine the preoperative size and location of the tumor. Radiography may show bone erosion in certain cases, and it is useful in differentiating a glomus tumor from subungual exostosis.10 Magnetic resonance imaging and ultrasound imaging have also been used to identify glomus tumors and to aid in determining the method of excision.10,11
Surgical excision is the preferred approach
While there are reports of successful treatment with laser and sclerotherapy, surgical excision remains the accepted intervention to relieve pain and minimize recurrence.12,13 The optimal surgical approach, which depends on the location of the tumor,13,14 will minimize the risk of postsurgical nail deformity while allowing for complete tumor removal.
A biopsy for our patient
While the intent of our biopsy was diagnostic, it also proved to be therapeutic as our patient experienced complete resolution of her pain immediately after the procedure. Six months later, she remained asymptomatic and reported no nail deformity. We counseled her on the possibility that her symptoms might return and encouraged her to come back in for further care as needed.
Correspondence
Thomas M. Beachkofsky, MD, Wilford Hall Medical Center, Department of Dermatology, 2200 Bergquist Drive, Suite 1, Lackland AFB, TX 78236-9908; [email protected]
1. Baran R, Richert B. Common nail tumors. Dermatol Clin. 206;24:297-311.
2. Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132:1448-1452.
3. Heys SD, Brittenden J, Atkinson P, et al. Glomus tumour: an analysis of 43 patients and review of the literature. Br J Surg. 1992;79:345-347.
4. McDermott EM, Weiss AP. Glomus tumors. J Hand Surg Am. 2006;31:1397-1400.
5. De Berker DA, Perrin C, Baran R. Localized longitudinal erythronychia: diagnostic significance and physical explanation. Arch Dermatol. 2004;140:1253-1257.
6. Grundmeier N, Hamm H, Weissbrich B, et al. High-risk human papillomavirus infection in Bowen’s disease of the nail unit: report of three cases and review of the literature. Dermatology. 2011;223:293-300.
7. Bach DQ, McQueen AA, Lio PA. A refractory wart? Subungual exostosis. Ann Emerg Med. 2011;58:e3-e4.
8. Garman ME, Orengo IF, Netscher D, et al. On glomus tumors, warts, and razors. Dermatol Surg. 2003;29:192-194.
9. Bhaskaranand K, Navadgi BC. Glomus tumour of the hand. J Hand Surg Br. 2002;27:229-231.
10. Takemura N, Fujii N, Tanaka T. Subungual glomus tumor diagnosis based on imaging. J Dermatol. 2006;33:389-393.
11. Matsunaga A, Ochiai T, Abe I, et al. Subungual glomus tumour: evaluation of ultrasound imaging in preoperative assessment. Eur J Dermatol. 2007;17:67-69.
12. Vergilis-Kalner IJ, Friedman PM, Goldberg LH. Long-pulse 595-nm pulsed dye laser for the treatment of a glomus tumor. Dermatol Surg. 2010;36:1463-1465.
13. Netscher DT, Aburto J, Koepplinger M. Subungual glomus tumor. J Hand Surg Am. 2012;37:821-823.
14. Takata H, Ikuta Y, Ishida O, et al. Treatment of subungual glomus tumour. Hand Surg. 2001;6:25-27.
1. Baran R, Richert B. Common nail tumors. Dermatol Clin. 206;24:297-311.
2. Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132:1448-1452.
3. Heys SD, Brittenden J, Atkinson P, et al. Glomus tumour: an analysis of 43 patients and review of the literature. Br J Surg. 1992;79:345-347.
4. McDermott EM, Weiss AP. Glomus tumors. J Hand Surg Am. 2006;31:1397-1400.
5. De Berker DA, Perrin C, Baran R. Localized longitudinal erythronychia: diagnostic significance and physical explanation. Arch Dermatol. 2004;140:1253-1257.
6. Grundmeier N, Hamm H, Weissbrich B, et al. High-risk human papillomavirus infection in Bowen’s disease of the nail unit: report of three cases and review of the literature. Dermatology. 2011;223:293-300.
7. Bach DQ, McQueen AA, Lio PA. A refractory wart? Subungual exostosis. Ann Emerg Med. 2011;58:e3-e4.
8. Garman ME, Orengo IF, Netscher D, et al. On glomus tumors, warts, and razors. Dermatol Surg. 2003;29:192-194.
9. Bhaskaranand K, Navadgi BC. Glomus tumour of the hand. J Hand Surg Br. 2002;27:229-231.
10. Takemura N, Fujii N, Tanaka T. Subungual glomus tumor diagnosis based on imaging. J Dermatol. 2006;33:389-393.
11. Matsunaga A, Ochiai T, Abe I, et al. Subungual glomus tumour: evaluation of ultrasound imaging in preoperative assessment. Eur J Dermatol. 2007;17:67-69.
12. Vergilis-Kalner IJ, Friedman PM, Goldberg LH. Long-pulse 595-nm pulsed dye laser for the treatment of a glomus tumor. Dermatol Surg. 2010;36:1463-1465.
13. Netscher DT, Aburto J, Koepplinger M. Subungual glomus tumor. J Hand Surg Am. 2012;37:821-823.
14. Takata H, Ikuta Y, Ishida O, et al. Treatment of subungual glomus tumour. Hand Surg. 2001;6:25-27.
When war follows combat veterans home
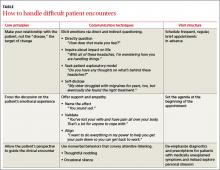
› Ask, “Have you or a loved one ever served in the military?” as a way to uncover service-related concerns. C
› Conduct a thorough neurological evaluation with suspected mild traumatic brain injury, including vestibular, vision, postural, and neuro-cognitive assessments. C
› Use the Post-Traumatic Checklist–Military to assess individuals with possible post-traumatic stress disorder. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A 37-year-old white woman presents for an employment physical. Your nurse reports that she also has a complaint of headaches, that she scored an 8 on the Alcohol Use Disorders identification Test-consumption (AUDiT-c), and that the result on her patient health Questionnaire (phQ-2) suggests a depressive disorder. You ask the patient whether she has served in the military and discover that, in the last 4 years, she served 2 year-long tours in Afghanistan with her Army reserve unit, returning home 6 months ago. Since her return, she has lost her job due to chronic tardiness (sleeping through her alarm, she says) and admits she has “started drinking again.” Her visit with you this day is only to undergo the physical exam required by her new employer. What are your next steps with this patient? What resources can you use to help her?
As long as human beings have engaged in combat, there have often been extraordinarily damaging psychiatric1 injuries among those who survive. Combat survivability today is 84% to 90%, the highest in the history of armed conflict,2,3 thanks to improvements in personal protective gear, vehicle armor, rapid casualty evacuation, and surgical resuscitation and stabilization that is “far forward” on the battlefield. These survivors are subsequently at high risk for a host of other medical conditions, which commonly include traumatic brain injury (TBI), post-traumatic stress disorder (PTSD), depression, suicide, and substance abuse.4-8
Family physicians—both civilian and uniformed—may be the first to encounter these individuals. Of the more than 2.4 million US service members who have been deployed to Afghanistan or Iraq in support of Operation Enduring Freedom (OEF) or Operation Iraqi Freedom (OIF), nearly 60% are no longer on active duty.
Among this group, only half receive care from the US Department of Veterans Affairs (VA).9 Despite a concerted effort on the part of the Department of Defense (DoD) and the VA to develop and distribute effective, evidenced-based treatment protocols for veterans with combat-related conditions, major gaps remain in the care provided to combat veterans.10
This article seeks to help fill that gap by providing the information you need to recognize and treat common combat-related illness, as well as resources to help improve the quality of life for veterans and their families (TABLE 1).
Initial roadblocks to care
One of the biggest challenges in treating veterans with behavioral health issues is the fact that only 23% to 40% of those with mental illness seek care.11 Among the reasons veterans have offered for avoiding behavioral health care are a fear of the stigma associated with mental illness, concern that treatment will negatively affect their career, lack of comfort with mental health professionals, and the perception that mental health treatment is a “last resort.”12 Unfortunately, efforts by the DoD leadership to overcome these inherent biases have been largely unsuccessful13 and much work is still required to see that service members get the care they need.
Due to low rates of self-reporting, effective screening is essential. With this in mind, the DoD has implemented the deployment health assessment program (DHAP), which requires service members to be screened for common conditions within 60 days of deployment, within 30 days of returning, and again at 90 to 180 days after their return.
While the long-term effects of this program are yet to be determined, results to date are promising. Since the DHAP was implemented, there has been a significant decrease in occupationally impairing mental health problems and suicidal ideation requiring medical evacuation from a combat theater.14
FPs should begin with a simple question. Many of the 20+ million veterans living in the United States will not be wearing a uniform when they enter your office. Simply asking all of your patients, “Have you or a loved one ever served in the military?” may help you discover service-related questions or concerns.15,16 Underscoring the importance of such screening is the recent decision by the American Academy of Family Physicians to partner with First Lady Michelle Obama and Dr. Jill Biden in a new campaign called “Joining Forces,” which aims to support veterans and their families.16
Mild traumatic brain injury: Common—though overlooked
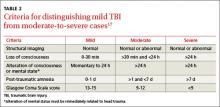
A TBI is any temporary or permanent neurologic dysfunction after a blow to the head.10,17 TBI is classified based on severity and mechanism (direct blow to the head or exposure to blast waves). Mild TBI (mTBI) is commonly referred to as a concussion and usually is not associated with loss of consciousness or altered mental status. Brain imaging results are also normal with mTBI. Severe TBI, on the other hand, is associated with prolonged loss of consciousness, altered mental status, and abnormal brain imaging results (TABLE 2).17
A unique obstacle to accurate evaluation in the field. It is important to emphasize that mTBI is a clinical diagnosis, and its detection requires honest patient communication. This can be problematic with motivated soldiers who are anxious to continue the mission and fear that any admission of symptoms might delay a return to their unit. As with a concussed athlete eager to return to the field of play, the clinical diagnosis of mTBI requires a high index of clinical suspicion and constant vigilance by the health care provider. Despite being the most common combat- related injury, mTBI is often overlooked due to the absence of obvious physical injuries.4 Recent data suggest that 28% to 60% of ser- vice members evacuated from combat have a TBI. Most of these injuries (77%) are mTBI.18-20 Improved personal protective equipment (including Kevlar helmets and body armor) and the high number of blast-related injuries are likely responsible for the high incidence of mTBI among OEF/OIF veterans.8,21,22 The prevalence of mTBI among service members not evacuated is estimated to be 20% to 30%.20
Symptoms can persist. Most patients with mTBI completely recover within 30 days of the injury. Unfortunately, 10% to 15% of mTBI patients develop chronic problems lasting months to years.4 Residual symptoms most commonly include headache, irritability, depression, sleep disturbance, impaired reasoning, memory problems, and difficulty concentrating. These symptoms are not unique to mTBI and overlap with comorbid combat diagnoses like PTSD, depression, and sleep deprivation.10 The following tools can help physicians determine whether mTBI is present.
Checking for possible mtBi. In the field, patients with possible mTBI can be screened rapidly using the Military Acute Concussion Evaluation (MACE, found at www.dvbic.org), a modification of the validated and widely used Sideline Assessment of Concussion (SAC) tool. More challenging is evaluating potential mTBI patients who present weeks or months after a traumatic event, for which there are no simple confirmatory tests. In this event, conduct a thorough neurological evaluation that includes vestibular, vision, postural, and neurocognitive assessments. For patients with persistent symptoms or possible anatomic brain abnormalities, magnetic resonance imaging (MRI) is the imaging modality of choice. Patients with complications or a questionable diagnosis are best managed in consultation with a neurologist.
Initial treatment of mtBi is symptom-based. When practical, try nonpharmacologic interventions first (TABLE 3).10 In particular, have the patient avoid further high-risk exposures that could lead to second impact syndrome (an issue increasingly recognized in contact sports). Also critical are physical and cognitive rest and the restoration of sleep until the patient is completely asymptomatic.
If the patient exhibits irritability and depression, selective serotonin reuptake inhibitors (SSRIs) are first-line treatment. Avoid narcotics and sedative-hypnotic sleep medications if treating comorbidities such as pain and sleep deprivation. The VA/DoD guideline on managing concussion and mTBI provides additional detailed, evidence-based treatment recommendations.17
Reliving the horror again and again: PTSD
PTSD is a persistent and, at times, debilitating clinical syndrome that develops after exposure to a psychologically traumatic event. It’s the second most common illness among OEF/OIF combat veterans, with an estimated prevalence of 3% to 20%, a finding consistent with prior wars.6,23-25 In the case of combat veterans, the inciting event usually involves an actual or perceived risk of death or serious injury. The individual’s response to the event involves intense fear, helplessness, or horror. The traumatic event is persistently re-experienced through intrusive and disturbing recollections or dreams that cause intense psychological distress. This, in turn, leads to a state of persistent sympathetic arousal. As symptoms are often triggered by specific cues, individuals with PTSD actively seek to avoid thoughts, situations, or stimuli associated with the event.23,26
Symptoms commonly associated with PTSD include difficulty falling or staying asleep, recurrent nightmares, hypervigilance, and an exaggerated startle response. Individuals with PTSD also have a poorer sense of well-being, a higher rate of work absenteeism, and significantly more somatic complaints than age-matched peers.27 For symptoms to be attributable to PTSD, their onset must follow a recent inciting event and must also cause clinically significant distress or impairment in social, occupational, or other areas of daily living. Common comorbid illnesses include mTBI, depression, and substance abuse. As with mTBI, the presence of multiple comorbidities in patients with PTSD can complicate evaluation, diagnosis, and treatment.
Diagnosis. PTSD is subdivided into acute (symptoms lasting more than one month but less than 3 months after the traumatic event) and chronic (symptoms lasting longer than 3 months after the traumatic event).28 The distinction of acute or chronic does not affect treatment, but it is useful information for the patient to have regarding prognosis and eventual outcome. Like mTBI, PTSD is a clinical diagnosis made only after a thorough, structured diagnostic interview. The use of a validated, self-administered checklist, such as the Post-Traumatic Checklist-Military (PCL-M), allows for an efficient review of a patient’s symptoms and a reliable way to track treatment progress (http://www.ptsd.va.gov/professional/ pages/assessments/ptsd-checklist.asp).
Treatment Options. Effective evidence-based treatments for PTSD are cognitive behavioral therapy, eye movement desensitization and reprocessing (EMDR), and pharmacotherapy. SSRIs and serotonin- norepinephrine reuptake inhibitors (SNRIs) have the strongest evidence for pharmacologic benefit in the treatment of PTSD.28,29 Other helpful medications are prazosin for nightmares and trazodone for sleep. Family physicians can use these medications as part of a patient-centered collaboration with the rest of the integrated care team, to offer the best chance for treatment success.10,28,30
Depression: Vets are reluctant to self-report
Combat experience is a significant risk factor for major depression. Estimates of the lifetime prevalence of depression in the general US population vary from 9% to 25% in women and 5% to 12% in men. By contrast, the prevalence of depression in OIF/OEF veterans ranges from 2% to 37%.24,31,32
Screening can yield false negatives. Many combat veterans are reluctant to self-report behavioral conditions, including depression. Screening, therefore, is important to identify potential depression and allow for intervention. Validated screening tools for depression include the PHQ-2 and PHQ-9, which are easy to use in the office setting. (See http://www.cqaimh.org/pdf/ tool_phq2.pdf [PHQ-2] and http://www. integration.samhsa.gov/images/res/ PHQ%20-%20Questions.pdf [PHQ-9]). Importantly, some veterans will have a negative depression screen on return from deployment, and then test positive 6 to 12 months later.24 Explanations for the early false-negative results include the excitement of being home and patients intentionally answering questions inaccurately to avoid excessive screening at their home base.11
Treatment is most effective with a combination approach. As with most cases of depression, combining psychotherapy and psychopharmacology appears to be most effective for treating depression related to combat experience.33,34 While SSRIs and SNRIs are typical first-line pharmacologic agents, combat veterans often have comorbid mTBI, PTSD, or substance abuse issues that may influence the initial choice of therapy35 (TABLE 3).10
Suicide is on the rise in the military
Historically, the incidence of suicide has been 25% lower in military personnel than in civilian peers.36 However, between 2005 and 2009, the incidence of suicide in the Marine Corps and Army almost doubled.37 While the exact reasons remain unknown, it is likely due to prolonged and repeated deployments to a combat environment.12 While the incidence of suicide has been particularly high in the Army (22 per 100,000 active-duty and reserve personnel per year), all services have been affected. In fact, since 2009, the number of suicides among active duty service members exceeds those killed in action.37
Consider all veterans to be at risk for suicide, and screen accordingly. An effective screening tool is the Columbia-Suicide Severity Rating Scale (C-SSRS), which is able to predict those most at risk for an impending suicide attempt.34 Service members identified as high risk for suicide require unhindered access to care. The VA has worked to improve access to care and provide evidence-based point-of-care treatment strategies.38 Available resources can be found in TABLE 1.
Unfortunately, even with effective screening and treatment, not all suicides can be prevented. Studies have demonstrated that approximately 65% of service members who commit suicide had no known history of communicating their suicidal intent. Sadly, 25% of service members who committed suicide had seen a mental health provider within the previous 30 days.39
Alcohol abuse is common; opioids present a unique risk
Excessive use of alcohol and recreational and prescription drugs is common among OEF/ OIF veterans, especially those with comorbid mental health disorders. Retrospective cross-sectional studies show that 11% to 20% of OEF/OIF veterans met DSM-IV-TR diagnostic criteria for substance use disorders.40-42 At highest risk are single enlisted men under the age of 24 in the Army or Marine Corps who serve in a combat-specific capacity. Interestingly, the prevalence of substance use disorders among OEF/OIF veterans closely mirrors that reported in epidemiologic studies of Vietnam veterans (11%-14%).41 This similarity, combined with the 39% lifetime prevalence of substance use disorders among Vietnam veterans, may foreshadow a similar lifetime prevalence of substance use disorders among OEF/OIF veterans.41
Most-abused substances. Alcohol is the most commonly abused substance among OEF/OIF veterans (10%-20%).40,41,43-45 Other abused substances include opioids (prescribed or illicitly obtained), synthetic marijuana (“Spice” and “K2”), and “bath salts” (synthetic stimulants) (W.M. Sauve, MD, personal communication, August 27, 2012).
OEF/OIF veterans seem to be at particular risk for developing problems related to opioid use. A 2012 retrospective cohort study showed that veterans with non–cancer- related pain diagnoses treated with opioid analgesics had an increased risk for adverse clinical outcomes compared with those not treated with opioid analgesics (9.5% vs 4.1%; relative risk [RR]=2.33; 95% confidence interval [CI], 2.20-2.46). These outcomes included traumatic accidents, overdoses, self-inflicted injuries, and injuries related to violence. This study also demonstrated that, compared with veterans without mental illness, veterans with mental illness (particularly PTSD) and non–cancer-related pain were significantly more likely to receive opioids to treat their pain and had a higher risk of adverse clinical outcomes, including overdose.46,47
Recreational use of synthetic marijuana and “bath salts” has increased in recent years. These substances are commonly labeled “not for human consumption,” which allows them to remain outside US Food and Drug Administration (FDA) regulation and be sold legally in the United States. Efforts to prohibit the sale or possession of these drugs, including the Federal Synthetic Bath Salt Ban in 2012, have fallen short, often due to creative product ”re-engineering.”33 Synthetic marijuana and stimulants are inexpensive, readily available, and perceived by users to be safe. Health care providers are often unaware that their patients are using these products. Adverse health outcomes associated with the use of these synthetic drugs include memory loss, depression, and psychosis.
These alcohol and drug screens can help
One efficient screening tool to identify veterans at risk for alcohol abuse is the AUDIT-C, developed by the World Health Organization. This brief 3-question test identifies past-year hazardous drinking and alcohol abuse or dependence with >79% sensitivity and >56% specificity in male veterans, and >66% sensitivity and >87% specificity in female veterans. These numbers are similar to those provided by the full 10-question AUDIT.48,49 The Drug Abuse Screen Test-10 (DAST-10) provides a similar screening instrument for other substances. Condensed from the original DAST-28 instrument, the DAST-10 identifies high-risk substance abuse with 74% to 94% sensitivity and 68% to 88% specificity.3
Screen for comorbidities. When you see veterans with a diagnosis of substance abuse, also evaluate for comorbid disease. Most veterans with substance use disorders (82%-93%) have at least one other mental health diagnosis (a 45% greater risk than that of civilians with substance abuse disorders),50 most commonly PTSD, depression, anxiety, and adjustment disorders.41,44,45 A number of hypotheses exist to explain the association between substance use disorders and other mental health diagnoses (“dual diagnoses”). The prevailing theory, in both veteran and civilian populations, is that substance abuse is an attempt to self-treat mental illness. Other evidence suggests that substance abuse promotes the development of mental illness, either by leading to a higher risk for traumatic experiences (increasing the chance of developing PTSD) or through a direct biochemical mechanism. Finally, con- current substance use disorder and mental illness may be due to an undefined genetic or biological vulnerability.38,44 This complicated relationship between substance abuse and behavioral health reinforces the need for screening, early diagnosis, and a comprehensive, multidisciplinary approach to treatment.
Treatment options. Office-based treatment options for narcotic and alcohol abuse and dependency are available to family physicians. Methadone has been used since the 1950s to treat opioid addiction and remains one of the mainstays of outpatient treatment.47,51 Originally, methadone was restricted to detoxification and maintenance treatment in narcotic addiction treatment programs approved by the FDA. In 1976, this restriction was lifted, and all physicians registered with the Drug Enforcement Agency (DEA) were permitted to prescribe methadone for analgesia.
In 2002, the FDA approved buprenorphine monotherapy and the combination product buprenorphine/naloxone for the treatment of opioid addiction. The prescribing of buprenorphine products requires physicians to undergo extra training, declare to the DEA their intent to prescribe buprenorphine, and obtain a special DEA identification number.52,53 Physicians interested in finding out more about buprenorphine treatment and prescribing requirements can go to the Substance Abuse and Mental Health Services Administration (SAMHSA) Web page at http://samhsa.gov.
Naltrexone is an opioid receptor agonist that is used primarily to treat alcohol dependency, and is thought to work by reducing the craving for alcohol. Multiple studies have proven the efficacy of naltrexone in an outpatient setting when used alone or in combination with psychotherapy.54,55 If you are uncomfortable or unfamiliar with the use or prescribing of these medications, referral to a substance abuse clinic specializing in dual-diagnosis treatment (TABLE 1) may offer optimal outcomes for patients with substance abuse disorders and other mental illness.
Cognitive behavioral therapy—including coping skills training, relapse prevention, contingency management, and behavioral couples’ therapy—and 12-step treatment programs are evidence-based options for the treatment of substance abuse disorders. Behavioral counseling interventions in the primary care setting (typically lasting 5-15 minutes) result in decreases in alcohol consumption, heavy drinking episodes, drinking above recommended amounts, and the number of days spent in the hospital, but have not been demonstrated to affect mortality, alcohol-related liver problems, outpatient visits, legal problems, or quality of life.56 Resources can be found at www.niaaa.nih.gov. For patients with dual diagnoses, it is not yet known whether sequential therapy (in which substance abuse is treated first, followed by treatment of the comorbid mental illness) or concurrent therapy results in better outcomes.57
CASE Your patient’s history of recent combat service, acknowledgement of employment and behavioral difficulties, and initial screening results lead you to diagnose alcoholism and depression. Additionally, she denies any suicidal ideation, but admits to experimenting with synthetic marijuana. After some discussion, she agrees to see your clinic’s social worker, and you start her on an SSri with scheduled follow-up.
CORRESPONDENCE
Shawn Kane, MD, USASoc, Attn: Surgeon (AomD), 2929 Desert Storm Drive, Ft. Bragg, NC 28310, or PO Box 3639 Pinehurst, NC 28374; [email protected]
1. Wessely S. Risk, psychiatry and the military. Br J Psychiatry. 2005;186:459-466.
2. Gawande A. Casualties of war—military care for the wounded from Iraq and Afghanistan. N Engl J Med. 2004;351:2471-2475.
3. Kotwal RS, Montgomery HR, Kotwal BM, et al. Eliminating pre- ventable death on the battlefield. Arch Surg. 2011;146:1350-1358.
4. Belanger HG, Uomoto JM, Vanderploeg RD. The Veterans Health Administration’s (VHA’s) Polytrauma System of Care for mild traumatic brain injury: costs, benefits, and controversies. J Head Trauma Rehabil. 2009;24:4-13.
5. Galarneau MR, Woodruff SI, Dye JL, et al. Traumatic brain in- jury during Operation Iraqi Freedom: findings from the United States Navy-Marine Corps Combat Trauma Registry. J Neurosurg. 2008;108:950-957.
6. Hermann BA, Shiner B, Friedman MJ. Epidemiology and preven- tion of combat-related post-traumatic stress in OEF/OIF/OND service members. Mil Med. 2012;177:1-6.
7. Uomoto JM. Best practices in veteran traumatic brain injury care. J Head Trauma Rehabil. 2012;27:241-243.
8. Warden D. Military TBI during the Iraq and Afghanistan wars. J Head Trauma Rehabil. 2006;21:398-402.
9. Taylor BC, Hagel EM, Carlson KF, et al. Prevalence and costs of co-occurring traumatic brain injury with and without psychiatric disturbance and pain among Afghanistan and Iraq War Veteran V.A. users. Med Care. 2012;50:342-346.
10. Quinlan JD, Guaron MR, Deschere BR, et al. Care of the returning veteran. Am Fam Physician. 2010;82:43-49.
11. Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351:13-22.
12. Hoge CW, Castro CA. Preventing suicides in US service mem- bers and veterans: concerns after a decade of war. JAMA. 2012;308:671-672.
13. Jaffe G. New name for PTSD could mean less stigma. The Washington Post. May 5, 2012. Available at: http://articles. washingtonpost.com/2012-05-05/world/35454931_1_ptsd-post- traumatic-stress-psychiatrists. Accessed June 19, 2013.
14. Warner CH, Appenzeller GN, Parker JR, et al. Effectiveness of mental health screening and coordination of in-theater care prior to deployment to Iraq: a cohort study. Am J Psychiatry. 2011;168:378-385.
15. United States Census Bureau. Sex by age by veteran sta- tus for civilian population 18 years and over. 2010 American community survey 1-year estimates. Available at: https:// d3gqux9sl0z33u.cloudfront.net/AA/AT/gambillingonjustice- com/downloads/206273/ACS_10_1YR_B21001A.pdf. Accessed June 19, 2013.
16. American Academy of Family Physicians. Joining forces. Avail- able at: http://www.aafp.org/online/en/home/membership/ initiatives/joiningforces.html. Accessed June 19, 2013.
17. Department of Veterans Affairs and Department of Defense. Clinical Practice Guideline for Management of Concussion/Mild Traumatic Brain Injury. April 2009. Available at: http://www. healthquality.va.gov/mtbi/concussion_mtbi_full_1_0.pdf. Accessed June 19, 2013.
18. Lew HL, Poole JH, Alvarez S, et al. Soldiers with occult traumatic brain injury. Am J Phys Med Rehabil. 2005;84:393-398.
19. Marshall KR, Holland SL, Meyer KS, et al. Mild traumatic brain injury screening, diagnosis, and treatment. Mil Med. 2012;177:67- 75.
20. Terrio H, Brenner LA, Ivins BJ, et al. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J Head Trauma Rehabil. 2009;24:14-23.
21. Mossadegh S, Tai N, Midwinter M, et al. Improvised explosive de- vice related pelvi-perineal trauma: anatomic injuries and surgical management. J Trauma Acute Care Surg. 2012;73:S24-S31.
22. Okie S. Traumatic brain injury in the war zone. N Engl J Med. 2005;352:2043-2047.
23. Espinoza JM. Posttraumatic stress disorder and the perceived consequences of seeking therapy among US Army special forces operators exposed to combat. J Psychol Issues Organ Culture. 2010;1:6-28.
24. Grieger TA, Cozza SJ, Ursano RJ, et al. Posttraumatic stress dis- order and depression in battle-injured soldiers. Am J Psychiatry. 2006;163:1777-1783.
25. Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA. 2006;295:1023-1032.
26. Adler AB, Wright KM, Bliese PD, et al. A2 diagnostic criterion for combat-related posttraumatic stress disorder. J Trauma Stress. 2008;21:301-308.
27. Hoge CW, Terhakopian A, Castro CA, et al. Association of post- traumatic stress disorder with somatic symptoms, health care vis- its, and absenteeism among Iraq war veterans. Am J Psychiatry. 2007;164:150-153.
28. Department of Veterans Affairs and Department of Defense. Clin- ical Practice Guideline for Management of Post-Traumatic Stress. October 2010. Available at: http://www.healthquality.va.gov/ ptsd/cpg_PTSD-FULL-201011612.pdf. Accessed June 19, 2013.
29. Alexander W. Pharmacotherapy for post-traumatic stress disor- der in combat veterans: focus on antidepressants and atypical antipsychotic agents. P T. 2012;37:32-38.
30. Wisco BE, Marx BP, Keane TM. Screening, diagnosis, and treat- ment of post-traumatic stress disorder. Mil Med. 2012;177:7-13.
31. Gadermann AM, Engel CC, Naifeh JA, et al. Prevalence of DSM-IV major depression among U.S. military personnel: meta-analysis and simulation. Mil Med. 2012;177:47-59.
32. Seal KH, Shi Y, Cohen G, et al. Association of mental health dis- orders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307:940-947.
33. Perrone M. Many drugs remain legal after ‘bath salts’ ban. Boston. com. July 25, 2012. Available at: http://articles.boston.com/2012- 07-25/lifestyle/32850962_1_bath-salts-mdpv-synthetic-drugs. Accessed June 19, 2013.
34. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Se- verity Rating Scale: initial validity and internal consistency find- ings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266-1277.
35. Greenberg J, Tesfazion AA, Robinson CS. Screening, diagnosis, and treatment of depression. Mil Med. 2012;177:60-66.
36. Eaton KM, Messer SC, Garvey Wilson AL, et al. Strengthening the validity of population-based suicide rate comparisons: an il- lustration using U.S. military and civilian data. Suicide Life Threat Behav. 2006;36:182-191.
37. Miller M, Azrael D, Barber C, et al. A call to link data to answer pressing questions about suicide risk among veterans. Am J Pub Health. 2012;102(suppl 1):S20-S22.
38. Department of Veterans Affairs. Report of the Blue Ribbon Work Group on suicide prevention in the veteran population. June 2008. Available at: http://www.mentalhealth.va.gov/suicide_ prevention/Blue_Ribbon_Report-FINAL_June-30-08.pdf. Accessed July 18, 2013.
39. Kinn JT, Luxton DD, Reger MA, et al. Department of Defense sui- cide event report: calendar year 2010 annual report. September 2011. Available at: http://t2health.org/sites/default/files/dodser/ DoDSER_2010_Annual_Report.pdf. Accessed June 19, 2013.
40. Fontana A, Rosenheck R. Treatment-seeking veterans of Iraq and Afghanistan: comparison with veterans of previous wars. J Nerv Ment Dis. 2008;196:513-521.
41. Seal KH, Cohen G, Waldrop A, et al. Substance use disorders in Iraq and Afghanistan veterans in VA healthcare, 2001-2010: implications for screening, diagnosis and treatment. Drug Alcohol Depend. 2011;116:93-101.
42. Mirza RA, Eick-Cost A, Otto JL. The risk of mental health disor- ders among U.S. military personnel infected with human immu- nodeficiency virus, active component, U.S. Armed Forces, 2000- 2011. MSMR. 2012;19:10-13.
43. Bohnert AS, Ilgen MA, Bossarte RM, et al. Veteran status and alco- hol use in men in the United States. Mil Med. 2012;177:198-203.
44. Erbes CR, Kaler ME, Schult T, et al. Mental health diagnosis and occupational functioning in National Guard/Reserve veterans re- turning from Iraq. J Rehabil Res Dev. 2011;48:1159-1170.
45. Stecker T, Fortney J, Owen R, et al. Co-occurring medical, psychi- atric, and alcohol-related disorders among veterans returning from Iraq and Afghanistan. Psychosomatics. 2010;51:503-507.
46. Seal KH, Shi Y, Cohen G, et al. Association of mental health dis- orders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307:940-947.
47. Praveen KT, Law F, O’Shea J, et al. Opioid dependence. Am Fam Physician. 2012;86:565-566.
48. Bradley KA, Bush KR, Epler AJ, et al. Two brief alcohol-screening tests from the Alcohol Use Disorders Identification Test (AUDIT): validation in a female Veterans Affairs patient population. Arch Intern Med. 2003;163:821-829.
49. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-1795.
50. Farrell M, Howes S, Taylor C, et al. Substance misuse and psychi- atric comorbidity: an overview of the OPCS National Psychiatric Morbidity Survey. Addict Behav. 1998;23:909-918.
51. Toombs JD, Kral LA. Methadone treatment for pain states. Am Fam Physician. 2005;71:1353-1358.
52. Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Treatment Improvement Protocol (TIP) series 40. DHHS pub- lication (SMA) 04-3939. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004. Available at: http:// buprenorphine.samhsa.gov/Bup_Guidelines.pdf. Accessed June 19, 2013.
53. U.S.DepartmentofHealthandHumanServices,SubstanceAbuse and Mental Health Services Administration Web site. About buprenorphine therapy. Available at: http://buprenorphine. samhsa.gov/about.html. Accessed June 19, 2013.
54. Volpicelli JR, Alterman AI, Hayashida M, et al. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876-880.
55. O’Brien CP, Volpicelli LA, Volpicelli JR. Naltrexone in the treat- ment of alcoholism: a clinical review. Alcohol. 1996;13:35-39.
56. Jonas DE, Garbutt JC, Amick HR, et al. Behavioral counseling after screening for alcohol misuse in primary care: a systematic review and meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2012;157:645-654.
57. van Dam D, Vedel E, Ehring T, et al. Psychological treatments for concurrent posttraumatic stress disorder and substance use dis- order: a systematic review. Clin Psychol Rev. 2012;32:202-214.
› Ask, “Have you or a loved one ever served in the military?” as a way to uncover service-related concerns. C
› Conduct a thorough neurological evaluation with suspected mild traumatic brain injury, including vestibular, vision, postural, and neuro-cognitive assessments. C
› Use the Post-Traumatic Checklist–Military to assess individuals with possible post-traumatic stress disorder. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A 37-year-old white woman presents for an employment physical. Your nurse reports that she also has a complaint of headaches, that she scored an 8 on the Alcohol Use Disorders identification Test-consumption (AUDiT-c), and that the result on her patient health Questionnaire (phQ-2) suggests a depressive disorder. You ask the patient whether she has served in the military and discover that, in the last 4 years, she served 2 year-long tours in Afghanistan with her Army reserve unit, returning home 6 months ago. Since her return, she has lost her job due to chronic tardiness (sleeping through her alarm, she says) and admits she has “started drinking again.” Her visit with you this day is only to undergo the physical exam required by her new employer. What are your next steps with this patient? What resources can you use to help her?
As long as human beings have engaged in combat, there have often been extraordinarily damaging psychiatric1 injuries among those who survive. Combat survivability today is 84% to 90%, the highest in the history of armed conflict,2,3 thanks to improvements in personal protective gear, vehicle armor, rapid casualty evacuation, and surgical resuscitation and stabilization that is “far forward” on the battlefield. These survivors are subsequently at high risk for a host of other medical conditions, which commonly include traumatic brain injury (TBI), post-traumatic stress disorder (PTSD), depression, suicide, and substance abuse.4-8
Family physicians—both civilian and uniformed—may be the first to encounter these individuals. Of the more than 2.4 million US service members who have been deployed to Afghanistan or Iraq in support of Operation Enduring Freedom (OEF) or Operation Iraqi Freedom (OIF), nearly 60% are no longer on active duty.
Among this group, only half receive care from the US Department of Veterans Affairs (VA).9 Despite a concerted effort on the part of the Department of Defense (DoD) and the VA to develop and distribute effective, evidenced-based treatment protocols for veterans with combat-related conditions, major gaps remain in the care provided to combat veterans.10
This article seeks to help fill that gap by providing the information you need to recognize and treat common combat-related illness, as well as resources to help improve the quality of life for veterans and their families (TABLE 1).
Initial roadblocks to care
One of the biggest challenges in treating veterans with behavioral health issues is the fact that only 23% to 40% of those with mental illness seek care.11 Among the reasons veterans have offered for avoiding behavioral health care are a fear of the stigma associated with mental illness, concern that treatment will negatively affect their career, lack of comfort with mental health professionals, and the perception that mental health treatment is a “last resort.”12 Unfortunately, efforts by the DoD leadership to overcome these inherent biases have been largely unsuccessful13 and much work is still required to see that service members get the care they need.
Due to low rates of self-reporting, effective screening is essential. With this in mind, the DoD has implemented the deployment health assessment program (DHAP), which requires service members to be screened for common conditions within 60 days of deployment, within 30 days of returning, and again at 90 to 180 days after their return.
While the long-term effects of this program are yet to be determined, results to date are promising. Since the DHAP was implemented, there has been a significant decrease in occupationally impairing mental health problems and suicidal ideation requiring medical evacuation from a combat theater.14
FPs should begin with a simple question. Many of the 20+ million veterans living in the United States will not be wearing a uniform when they enter your office. Simply asking all of your patients, “Have you or a loved one ever served in the military?” may help you discover service-related questions or concerns.15,16 Underscoring the importance of such screening is the recent decision by the American Academy of Family Physicians to partner with First Lady Michelle Obama and Dr. Jill Biden in a new campaign called “Joining Forces,” which aims to support veterans and their families.16
Mild traumatic brain injury: Common—though overlooked
A TBI is any temporary or permanent neurologic dysfunction after a blow to the head.10,17 TBI is classified based on severity and mechanism (direct blow to the head or exposure to blast waves). Mild TBI (mTBI) is commonly referred to as a concussion and usually is not associated with loss of consciousness or altered mental status. Brain imaging results are also normal with mTBI. Severe TBI, on the other hand, is associated with prolonged loss of consciousness, altered mental status, and abnormal brain imaging results (TABLE 2).17
A unique obstacle to accurate evaluation in the field. It is important to emphasize that mTBI is a clinical diagnosis, and its detection requires honest patient communication. This can be problematic with motivated soldiers who are anxious to continue the mission and fear that any admission of symptoms might delay a return to their unit. As with a concussed athlete eager to return to the field of play, the clinical diagnosis of mTBI requires a high index of clinical suspicion and constant vigilance by the health care provider. Despite being the most common combat- related injury, mTBI is often overlooked due to the absence of obvious physical injuries.4 Recent data suggest that 28% to 60% of ser- vice members evacuated from combat have a TBI. Most of these injuries (77%) are mTBI.18-20 Improved personal protective equipment (including Kevlar helmets and body armor) and the high number of blast-related injuries are likely responsible for the high incidence of mTBI among OEF/OIF veterans.8,21,22 The prevalence of mTBI among service members not evacuated is estimated to be 20% to 30%.20
Symptoms can persist. Most patients with mTBI completely recover within 30 days of the injury. Unfortunately, 10% to 15% of mTBI patients develop chronic problems lasting months to years.4 Residual symptoms most commonly include headache, irritability, depression, sleep disturbance, impaired reasoning, memory problems, and difficulty concentrating. These symptoms are not unique to mTBI and overlap with comorbid combat diagnoses like PTSD, depression, and sleep deprivation.10 The following tools can help physicians determine whether mTBI is present.
Checking for possible mtBi. In the field, patients with possible mTBI can be screened rapidly using the Military Acute Concussion Evaluation (MACE, found at www.dvbic.org), a modification of the validated and widely used Sideline Assessment of Concussion (SAC) tool. More challenging is evaluating potential mTBI patients who present weeks or months after a traumatic event, for which there are no simple confirmatory tests. In this event, conduct a thorough neurological evaluation that includes vestibular, vision, postural, and neurocognitive assessments. For patients with persistent symptoms or possible anatomic brain abnormalities, magnetic resonance imaging (MRI) is the imaging modality of choice. Patients with complications or a questionable diagnosis are best managed in consultation with a neurologist.
Initial treatment of mtBi is symptom-based. When practical, try nonpharmacologic interventions first (TABLE 3).10 In particular, have the patient avoid further high-risk exposures that could lead to second impact syndrome (an issue increasingly recognized in contact sports). Also critical are physical and cognitive rest and the restoration of sleep until the patient is completely asymptomatic.
If the patient exhibits irritability and depression, selective serotonin reuptake inhibitors (SSRIs) are first-line treatment. Avoid narcotics and sedative-hypnotic sleep medications if treating comorbidities such as pain and sleep deprivation. The VA/DoD guideline on managing concussion and mTBI provides additional detailed, evidence-based treatment recommendations.17
Reliving the horror again and again: PTSD
PTSD is a persistent and, at times, debilitating clinical syndrome that develops after exposure to a psychologically traumatic event. It’s the second most common illness among OEF/OIF combat veterans, with an estimated prevalence of 3% to 20%, a finding consistent with prior wars.6,23-25 In the case of combat veterans, the inciting event usually involves an actual or perceived risk of death or serious injury. The individual’s response to the event involves intense fear, helplessness, or horror. The traumatic event is persistently re-experienced through intrusive and disturbing recollections or dreams that cause intense psychological distress. This, in turn, leads to a state of persistent sympathetic arousal. As symptoms are often triggered by specific cues, individuals with PTSD actively seek to avoid thoughts, situations, or stimuli associated with the event.23,26
Symptoms commonly associated with PTSD include difficulty falling or staying asleep, recurrent nightmares, hypervigilance, and an exaggerated startle response. Individuals with PTSD also have a poorer sense of well-being, a higher rate of work absenteeism, and significantly more somatic complaints than age-matched peers.27 For symptoms to be attributable to PTSD, their onset must follow a recent inciting event and must also cause clinically significant distress or impairment in social, occupational, or other areas of daily living. Common comorbid illnesses include mTBI, depression, and substance abuse. As with mTBI, the presence of multiple comorbidities in patients with PTSD can complicate evaluation, diagnosis, and treatment.
Diagnosis. PTSD is subdivided into acute (symptoms lasting more than one month but less than 3 months after the traumatic event) and chronic (symptoms lasting longer than 3 months after the traumatic event).28 The distinction of acute or chronic does not affect treatment, but it is useful information for the patient to have regarding prognosis and eventual outcome. Like mTBI, PTSD is a clinical diagnosis made only after a thorough, structured diagnostic interview. The use of a validated, self-administered checklist, such as the Post-Traumatic Checklist-Military (PCL-M), allows for an efficient review of a patient’s symptoms and a reliable way to track treatment progress (http://www.ptsd.va.gov/professional/ pages/assessments/ptsd-checklist.asp).
Treatment Options. Effective evidence-based treatments for PTSD are cognitive behavioral therapy, eye movement desensitization and reprocessing (EMDR), and pharmacotherapy. SSRIs and serotonin- norepinephrine reuptake inhibitors (SNRIs) have the strongest evidence for pharmacologic benefit in the treatment of PTSD.28,29 Other helpful medications are prazosin for nightmares and trazodone for sleep. Family physicians can use these medications as part of a patient-centered collaboration with the rest of the integrated care team, to offer the best chance for treatment success.10,28,30
Depression: Vets are reluctant to self-report
Combat experience is a significant risk factor for major depression. Estimates of the lifetime prevalence of depression in the general US population vary from 9% to 25% in women and 5% to 12% in men. By contrast, the prevalence of depression in OIF/OEF veterans ranges from 2% to 37%.24,31,32
Screening can yield false negatives. Many combat veterans are reluctant to self-report behavioral conditions, including depression. Screening, therefore, is important to identify potential depression and allow for intervention. Validated screening tools for depression include the PHQ-2 and PHQ-9, which are easy to use in the office setting. (See http://www.cqaimh.org/pdf/ tool_phq2.pdf [PHQ-2] and http://www. integration.samhsa.gov/images/res/ PHQ%20-%20Questions.pdf [PHQ-9]). Importantly, some veterans will have a negative depression screen on return from deployment, and then test positive 6 to 12 months later.24 Explanations for the early false-negative results include the excitement of being home and patients intentionally answering questions inaccurately to avoid excessive screening at their home base.11
Treatment is most effective with a combination approach. As with most cases of depression, combining psychotherapy and psychopharmacology appears to be most effective for treating depression related to combat experience.33,34 While SSRIs and SNRIs are typical first-line pharmacologic agents, combat veterans often have comorbid mTBI, PTSD, or substance abuse issues that may influence the initial choice of therapy35 (TABLE 3).10
Suicide is on the rise in the military
Historically, the incidence of suicide has been 25% lower in military personnel than in civilian peers.36 However, between 2005 and 2009, the incidence of suicide in the Marine Corps and Army almost doubled.37 While the exact reasons remain unknown, it is likely due to prolonged and repeated deployments to a combat environment.12 While the incidence of suicide has been particularly high in the Army (22 per 100,000 active-duty and reserve personnel per year), all services have been affected. In fact, since 2009, the number of suicides among active duty service members exceeds those killed in action.37
Consider all veterans to be at risk for suicide, and screen accordingly. An effective screening tool is the Columbia-Suicide Severity Rating Scale (C-SSRS), which is able to predict those most at risk for an impending suicide attempt.34 Service members identified as high risk for suicide require unhindered access to care. The VA has worked to improve access to care and provide evidence-based point-of-care treatment strategies.38 Available resources can be found in TABLE 1.
Unfortunately, even with effective screening and treatment, not all suicides can be prevented. Studies have demonstrated that approximately 65% of service members who commit suicide had no known history of communicating their suicidal intent. Sadly, 25% of service members who committed suicide had seen a mental health provider within the previous 30 days.39
Alcohol abuse is common; opioids present a unique risk
Excessive use of alcohol and recreational and prescription drugs is common among OEF/ OIF veterans, especially those with comorbid mental health disorders. Retrospective cross-sectional studies show that 11% to 20% of OEF/OIF veterans met DSM-IV-TR diagnostic criteria for substance use disorders.40-42 At highest risk are single enlisted men under the age of 24 in the Army or Marine Corps who serve in a combat-specific capacity. Interestingly, the prevalence of substance use disorders among OEF/OIF veterans closely mirrors that reported in epidemiologic studies of Vietnam veterans (11%-14%).41 This similarity, combined with the 39% lifetime prevalence of substance use disorders among Vietnam veterans, may foreshadow a similar lifetime prevalence of substance use disorders among OEF/OIF veterans.41
Most-abused substances. Alcohol is the most commonly abused substance among OEF/OIF veterans (10%-20%).40,41,43-45 Other abused substances include opioids (prescribed or illicitly obtained), synthetic marijuana (“Spice” and “K2”), and “bath salts” (synthetic stimulants) (W.M. Sauve, MD, personal communication, August 27, 2012).
OEF/OIF veterans seem to be at particular risk for developing problems related to opioid use. A 2012 retrospective cohort study showed that veterans with non–cancer- related pain diagnoses treated with opioid analgesics had an increased risk for adverse clinical outcomes compared with those not treated with opioid analgesics (9.5% vs 4.1%; relative risk [RR]=2.33; 95% confidence interval [CI], 2.20-2.46). These outcomes included traumatic accidents, overdoses, self-inflicted injuries, and injuries related to violence. This study also demonstrated that, compared with veterans without mental illness, veterans with mental illness (particularly PTSD) and non–cancer-related pain were significantly more likely to receive opioids to treat their pain and had a higher risk of adverse clinical outcomes, including overdose.46,47
Recreational use of synthetic marijuana and “bath salts” has increased in recent years. These substances are commonly labeled “not for human consumption,” which allows them to remain outside US Food and Drug Administration (FDA) regulation and be sold legally in the United States. Efforts to prohibit the sale or possession of these drugs, including the Federal Synthetic Bath Salt Ban in 2012, have fallen short, often due to creative product ”re-engineering.”33 Synthetic marijuana and stimulants are inexpensive, readily available, and perceived by users to be safe. Health care providers are often unaware that their patients are using these products. Adverse health outcomes associated with the use of these synthetic drugs include memory loss, depression, and psychosis.
These alcohol and drug screens can help
One efficient screening tool to identify veterans at risk for alcohol abuse is the AUDIT-C, developed by the World Health Organization. This brief 3-question test identifies past-year hazardous drinking and alcohol abuse or dependence with >79% sensitivity and >56% specificity in male veterans, and >66% sensitivity and >87% specificity in female veterans. These numbers are similar to those provided by the full 10-question AUDIT.48,49 The Drug Abuse Screen Test-10 (DAST-10) provides a similar screening instrument for other substances. Condensed from the original DAST-28 instrument, the DAST-10 identifies high-risk substance abuse with 74% to 94% sensitivity and 68% to 88% specificity.3
Screen for comorbidities. When you see veterans with a diagnosis of substance abuse, also evaluate for comorbid disease. Most veterans with substance use disorders (82%-93%) have at least one other mental health diagnosis (a 45% greater risk than that of civilians with substance abuse disorders),50 most commonly PTSD, depression, anxiety, and adjustment disorders.41,44,45 A number of hypotheses exist to explain the association between substance use disorders and other mental health diagnoses (“dual diagnoses”). The prevailing theory, in both veteran and civilian populations, is that substance abuse is an attempt to self-treat mental illness. Other evidence suggests that substance abuse promotes the development of mental illness, either by leading to a higher risk for traumatic experiences (increasing the chance of developing PTSD) or through a direct biochemical mechanism. Finally, con- current substance use disorder and mental illness may be due to an undefined genetic or biological vulnerability.38,44 This complicated relationship between substance abuse and behavioral health reinforces the need for screening, early diagnosis, and a comprehensive, multidisciplinary approach to treatment.
Treatment options. Office-based treatment options for narcotic and alcohol abuse and dependency are available to family physicians. Methadone has been used since the 1950s to treat opioid addiction and remains one of the mainstays of outpatient treatment.47,51 Originally, methadone was restricted to detoxification and maintenance treatment in narcotic addiction treatment programs approved by the FDA. In 1976, this restriction was lifted, and all physicians registered with the Drug Enforcement Agency (DEA) were permitted to prescribe methadone for analgesia.
In 2002, the FDA approved buprenorphine monotherapy and the combination product buprenorphine/naloxone for the treatment of opioid addiction. The prescribing of buprenorphine products requires physicians to undergo extra training, declare to the DEA their intent to prescribe buprenorphine, and obtain a special DEA identification number.52,53 Physicians interested in finding out more about buprenorphine treatment and prescribing requirements can go to the Substance Abuse and Mental Health Services Administration (SAMHSA) Web page at http://samhsa.gov.
Naltrexone is an opioid receptor agonist that is used primarily to treat alcohol dependency, and is thought to work by reducing the craving for alcohol. Multiple studies have proven the efficacy of naltrexone in an outpatient setting when used alone or in combination with psychotherapy.54,55 If you are uncomfortable or unfamiliar with the use or prescribing of these medications, referral to a substance abuse clinic specializing in dual-diagnosis treatment (TABLE 1) may offer optimal outcomes for patients with substance abuse disorders and other mental illness.
Cognitive behavioral therapy—including coping skills training, relapse prevention, contingency management, and behavioral couples’ therapy—and 12-step treatment programs are evidence-based options for the treatment of substance abuse disorders. Behavioral counseling interventions in the primary care setting (typically lasting 5-15 minutes) result in decreases in alcohol consumption, heavy drinking episodes, drinking above recommended amounts, and the number of days spent in the hospital, but have not been demonstrated to affect mortality, alcohol-related liver problems, outpatient visits, legal problems, or quality of life.56 Resources can be found at www.niaaa.nih.gov. For patients with dual diagnoses, it is not yet known whether sequential therapy (in which substance abuse is treated first, followed by treatment of the comorbid mental illness) or concurrent therapy results in better outcomes.57
CASE Your patient’s history of recent combat service, acknowledgement of employment and behavioral difficulties, and initial screening results lead you to diagnose alcoholism and depression. Additionally, she denies any suicidal ideation, but admits to experimenting with synthetic marijuana. After some discussion, she agrees to see your clinic’s social worker, and you start her on an SSri with scheduled follow-up.
CORRESPONDENCE
Shawn Kane, MD, USASoc, Attn: Surgeon (AomD), 2929 Desert Storm Drive, Ft. Bragg, NC 28310, or PO Box 3639 Pinehurst, NC 28374; [email protected]
› Ask, “Have you or a loved one ever served in the military?” as a way to uncover service-related concerns. C
› Conduct a thorough neurological evaluation with suspected mild traumatic brain injury, including vestibular, vision, postural, and neuro-cognitive assessments. C
› Use the Post-Traumatic Checklist–Military to assess individuals with possible post-traumatic stress disorder. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A 37-year-old white woman presents for an employment physical. Your nurse reports that she also has a complaint of headaches, that she scored an 8 on the Alcohol Use Disorders identification Test-consumption (AUDiT-c), and that the result on her patient health Questionnaire (phQ-2) suggests a depressive disorder. You ask the patient whether she has served in the military and discover that, in the last 4 years, she served 2 year-long tours in Afghanistan with her Army reserve unit, returning home 6 months ago. Since her return, she has lost her job due to chronic tardiness (sleeping through her alarm, she says) and admits she has “started drinking again.” Her visit with you this day is only to undergo the physical exam required by her new employer. What are your next steps with this patient? What resources can you use to help her?
As long as human beings have engaged in combat, there have often been extraordinarily damaging psychiatric1 injuries among those who survive. Combat survivability today is 84% to 90%, the highest in the history of armed conflict,2,3 thanks to improvements in personal protective gear, vehicle armor, rapid casualty evacuation, and surgical resuscitation and stabilization that is “far forward” on the battlefield. These survivors are subsequently at high risk for a host of other medical conditions, which commonly include traumatic brain injury (TBI), post-traumatic stress disorder (PTSD), depression, suicide, and substance abuse.4-8
Family physicians—both civilian and uniformed—may be the first to encounter these individuals. Of the more than 2.4 million US service members who have been deployed to Afghanistan or Iraq in support of Operation Enduring Freedom (OEF) or Operation Iraqi Freedom (OIF), nearly 60% are no longer on active duty.
Among this group, only half receive care from the US Department of Veterans Affairs (VA).9 Despite a concerted effort on the part of the Department of Defense (DoD) and the VA to develop and distribute effective, evidenced-based treatment protocols for veterans with combat-related conditions, major gaps remain in the care provided to combat veterans.10
This article seeks to help fill that gap by providing the information you need to recognize and treat common combat-related illness, as well as resources to help improve the quality of life for veterans and their families (TABLE 1).
Initial roadblocks to care
One of the biggest challenges in treating veterans with behavioral health issues is the fact that only 23% to 40% of those with mental illness seek care.11 Among the reasons veterans have offered for avoiding behavioral health care are a fear of the stigma associated with mental illness, concern that treatment will negatively affect their career, lack of comfort with mental health professionals, and the perception that mental health treatment is a “last resort.”12 Unfortunately, efforts by the DoD leadership to overcome these inherent biases have been largely unsuccessful13 and much work is still required to see that service members get the care they need.
Due to low rates of self-reporting, effective screening is essential. With this in mind, the DoD has implemented the deployment health assessment program (DHAP), which requires service members to be screened for common conditions within 60 days of deployment, within 30 days of returning, and again at 90 to 180 days after their return.
While the long-term effects of this program are yet to be determined, results to date are promising. Since the DHAP was implemented, there has been a significant decrease in occupationally impairing mental health problems and suicidal ideation requiring medical evacuation from a combat theater.14
FPs should begin with a simple question. Many of the 20+ million veterans living in the United States will not be wearing a uniform when they enter your office. Simply asking all of your patients, “Have you or a loved one ever served in the military?” may help you discover service-related questions or concerns.15,16 Underscoring the importance of such screening is the recent decision by the American Academy of Family Physicians to partner with First Lady Michelle Obama and Dr. Jill Biden in a new campaign called “Joining Forces,” which aims to support veterans and their families.16
Mild traumatic brain injury: Common—though overlooked
A TBI is any temporary or permanent neurologic dysfunction after a blow to the head.10,17 TBI is classified based on severity and mechanism (direct blow to the head or exposure to blast waves). Mild TBI (mTBI) is commonly referred to as a concussion and usually is not associated with loss of consciousness or altered mental status. Brain imaging results are also normal with mTBI. Severe TBI, on the other hand, is associated with prolonged loss of consciousness, altered mental status, and abnormal brain imaging results (TABLE 2).17
A unique obstacle to accurate evaluation in the field. It is important to emphasize that mTBI is a clinical diagnosis, and its detection requires honest patient communication. This can be problematic with motivated soldiers who are anxious to continue the mission and fear that any admission of symptoms might delay a return to their unit. As with a concussed athlete eager to return to the field of play, the clinical diagnosis of mTBI requires a high index of clinical suspicion and constant vigilance by the health care provider. Despite being the most common combat- related injury, mTBI is often overlooked due to the absence of obvious physical injuries.4 Recent data suggest that 28% to 60% of ser- vice members evacuated from combat have a TBI. Most of these injuries (77%) are mTBI.18-20 Improved personal protective equipment (including Kevlar helmets and body armor) and the high number of blast-related injuries are likely responsible for the high incidence of mTBI among OEF/OIF veterans.8,21,22 The prevalence of mTBI among service members not evacuated is estimated to be 20% to 30%.20
Symptoms can persist. Most patients with mTBI completely recover within 30 days of the injury. Unfortunately, 10% to 15% of mTBI patients develop chronic problems lasting months to years.4 Residual symptoms most commonly include headache, irritability, depression, sleep disturbance, impaired reasoning, memory problems, and difficulty concentrating. These symptoms are not unique to mTBI and overlap with comorbid combat diagnoses like PTSD, depression, and sleep deprivation.10 The following tools can help physicians determine whether mTBI is present.
Checking for possible mtBi. In the field, patients with possible mTBI can be screened rapidly using the Military Acute Concussion Evaluation (MACE, found at www.dvbic.org), a modification of the validated and widely used Sideline Assessment of Concussion (SAC) tool. More challenging is evaluating potential mTBI patients who present weeks or months after a traumatic event, for which there are no simple confirmatory tests. In this event, conduct a thorough neurological evaluation that includes vestibular, vision, postural, and neurocognitive assessments. For patients with persistent symptoms or possible anatomic brain abnormalities, magnetic resonance imaging (MRI) is the imaging modality of choice. Patients with complications or a questionable diagnosis are best managed in consultation with a neurologist.
Initial treatment of mtBi is symptom-based. When practical, try nonpharmacologic interventions first (TABLE 3).10 In particular, have the patient avoid further high-risk exposures that could lead to second impact syndrome (an issue increasingly recognized in contact sports). Also critical are physical and cognitive rest and the restoration of sleep until the patient is completely asymptomatic.
If the patient exhibits irritability and depression, selective serotonin reuptake inhibitors (SSRIs) are first-line treatment. Avoid narcotics and sedative-hypnotic sleep medications if treating comorbidities such as pain and sleep deprivation. The VA/DoD guideline on managing concussion and mTBI provides additional detailed, evidence-based treatment recommendations.17
Reliving the horror again and again: PTSD
PTSD is a persistent and, at times, debilitating clinical syndrome that develops after exposure to a psychologically traumatic event. It’s the second most common illness among OEF/OIF combat veterans, with an estimated prevalence of 3% to 20%, a finding consistent with prior wars.6,23-25 In the case of combat veterans, the inciting event usually involves an actual or perceived risk of death or serious injury. The individual’s response to the event involves intense fear, helplessness, or horror. The traumatic event is persistently re-experienced through intrusive and disturbing recollections or dreams that cause intense psychological distress. This, in turn, leads to a state of persistent sympathetic arousal. As symptoms are often triggered by specific cues, individuals with PTSD actively seek to avoid thoughts, situations, or stimuli associated with the event.23,26
Symptoms commonly associated with PTSD include difficulty falling or staying asleep, recurrent nightmares, hypervigilance, and an exaggerated startle response. Individuals with PTSD also have a poorer sense of well-being, a higher rate of work absenteeism, and significantly more somatic complaints than age-matched peers.27 For symptoms to be attributable to PTSD, their onset must follow a recent inciting event and must also cause clinically significant distress or impairment in social, occupational, or other areas of daily living. Common comorbid illnesses include mTBI, depression, and substance abuse. As with mTBI, the presence of multiple comorbidities in patients with PTSD can complicate evaluation, diagnosis, and treatment.
Diagnosis. PTSD is subdivided into acute (symptoms lasting more than one month but less than 3 months after the traumatic event) and chronic (symptoms lasting longer than 3 months after the traumatic event).28 The distinction of acute or chronic does not affect treatment, but it is useful information for the patient to have regarding prognosis and eventual outcome. Like mTBI, PTSD is a clinical diagnosis made only after a thorough, structured diagnostic interview. The use of a validated, self-administered checklist, such as the Post-Traumatic Checklist-Military (PCL-M), allows for an efficient review of a patient’s symptoms and a reliable way to track treatment progress (http://www.ptsd.va.gov/professional/ pages/assessments/ptsd-checklist.asp).
Treatment Options. Effective evidence-based treatments for PTSD are cognitive behavioral therapy, eye movement desensitization and reprocessing (EMDR), and pharmacotherapy. SSRIs and serotonin- norepinephrine reuptake inhibitors (SNRIs) have the strongest evidence for pharmacologic benefit in the treatment of PTSD.28,29 Other helpful medications are prazosin for nightmares and trazodone for sleep. Family physicians can use these medications as part of a patient-centered collaboration with the rest of the integrated care team, to offer the best chance for treatment success.10,28,30
Depression: Vets are reluctant to self-report
Combat experience is a significant risk factor for major depression. Estimates of the lifetime prevalence of depression in the general US population vary from 9% to 25% in women and 5% to 12% in men. By contrast, the prevalence of depression in OIF/OEF veterans ranges from 2% to 37%.24,31,32
Screening can yield false negatives. Many combat veterans are reluctant to self-report behavioral conditions, including depression. Screening, therefore, is important to identify potential depression and allow for intervention. Validated screening tools for depression include the PHQ-2 and PHQ-9, which are easy to use in the office setting. (See http://www.cqaimh.org/pdf/ tool_phq2.pdf [PHQ-2] and http://www. integration.samhsa.gov/images/res/ PHQ%20-%20Questions.pdf [PHQ-9]). Importantly, some veterans will have a negative depression screen on return from deployment, and then test positive 6 to 12 months later.24 Explanations for the early false-negative results include the excitement of being home and patients intentionally answering questions inaccurately to avoid excessive screening at their home base.11
Treatment is most effective with a combination approach. As with most cases of depression, combining psychotherapy and psychopharmacology appears to be most effective for treating depression related to combat experience.33,34 While SSRIs and SNRIs are typical first-line pharmacologic agents, combat veterans often have comorbid mTBI, PTSD, or substance abuse issues that may influence the initial choice of therapy35 (TABLE 3).10
Suicide is on the rise in the military
Historically, the incidence of suicide has been 25% lower in military personnel than in civilian peers.36 However, between 2005 and 2009, the incidence of suicide in the Marine Corps and Army almost doubled.37 While the exact reasons remain unknown, it is likely due to prolonged and repeated deployments to a combat environment.12 While the incidence of suicide has been particularly high in the Army (22 per 100,000 active-duty and reserve personnel per year), all services have been affected. In fact, since 2009, the number of suicides among active duty service members exceeds those killed in action.37
Consider all veterans to be at risk for suicide, and screen accordingly. An effective screening tool is the Columbia-Suicide Severity Rating Scale (C-SSRS), which is able to predict those most at risk for an impending suicide attempt.34 Service members identified as high risk for suicide require unhindered access to care. The VA has worked to improve access to care and provide evidence-based point-of-care treatment strategies.38 Available resources can be found in TABLE 1.
Unfortunately, even with effective screening and treatment, not all suicides can be prevented. Studies have demonstrated that approximately 65% of service members who commit suicide had no known history of communicating their suicidal intent. Sadly, 25% of service members who committed suicide had seen a mental health provider within the previous 30 days.39
Alcohol abuse is common; opioids present a unique risk
Excessive use of alcohol and recreational and prescription drugs is common among OEF/ OIF veterans, especially those with comorbid mental health disorders. Retrospective cross-sectional studies show that 11% to 20% of OEF/OIF veterans met DSM-IV-TR diagnostic criteria for substance use disorders.40-42 At highest risk are single enlisted men under the age of 24 in the Army or Marine Corps who serve in a combat-specific capacity. Interestingly, the prevalence of substance use disorders among OEF/OIF veterans closely mirrors that reported in epidemiologic studies of Vietnam veterans (11%-14%).41 This similarity, combined with the 39% lifetime prevalence of substance use disorders among Vietnam veterans, may foreshadow a similar lifetime prevalence of substance use disorders among OEF/OIF veterans.41
Most-abused substances. Alcohol is the most commonly abused substance among OEF/OIF veterans (10%-20%).40,41,43-45 Other abused substances include opioids (prescribed or illicitly obtained), synthetic marijuana (“Spice” and “K2”), and “bath salts” (synthetic stimulants) (W.M. Sauve, MD, personal communication, August 27, 2012).
OEF/OIF veterans seem to be at particular risk for developing problems related to opioid use. A 2012 retrospective cohort study showed that veterans with non–cancer- related pain diagnoses treated with opioid analgesics had an increased risk for adverse clinical outcomes compared with those not treated with opioid analgesics (9.5% vs 4.1%; relative risk [RR]=2.33; 95% confidence interval [CI], 2.20-2.46). These outcomes included traumatic accidents, overdoses, self-inflicted injuries, and injuries related to violence. This study also demonstrated that, compared with veterans without mental illness, veterans with mental illness (particularly PTSD) and non–cancer-related pain were significantly more likely to receive opioids to treat their pain and had a higher risk of adverse clinical outcomes, including overdose.46,47
Recreational use of synthetic marijuana and “bath salts” has increased in recent years. These substances are commonly labeled “not for human consumption,” which allows them to remain outside US Food and Drug Administration (FDA) regulation and be sold legally in the United States. Efforts to prohibit the sale or possession of these drugs, including the Federal Synthetic Bath Salt Ban in 2012, have fallen short, often due to creative product ”re-engineering.”33 Synthetic marijuana and stimulants are inexpensive, readily available, and perceived by users to be safe. Health care providers are often unaware that their patients are using these products. Adverse health outcomes associated with the use of these synthetic drugs include memory loss, depression, and psychosis.
These alcohol and drug screens can help
One efficient screening tool to identify veterans at risk for alcohol abuse is the AUDIT-C, developed by the World Health Organization. This brief 3-question test identifies past-year hazardous drinking and alcohol abuse or dependence with >79% sensitivity and >56% specificity in male veterans, and >66% sensitivity and >87% specificity in female veterans. These numbers are similar to those provided by the full 10-question AUDIT.48,49 The Drug Abuse Screen Test-10 (DAST-10) provides a similar screening instrument for other substances. Condensed from the original DAST-28 instrument, the DAST-10 identifies high-risk substance abuse with 74% to 94% sensitivity and 68% to 88% specificity.3
Screen for comorbidities. When you see veterans with a diagnosis of substance abuse, also evaluate for comorbid disease. Most veterans with substance use disorders (82%-93%) have at least one other mental health diagnosis (a 45% greater risk than that of civilians with substance abuse disorders),50 most commonly PTSD, depression, anxiety, and adjustment disorders.41,44,45 A number of hypotheses exist to explain the association between substance use disorders and other mental health diagnoses (“dual diagnoses”). The prevailing theory, in both veteran and civilian populations, is that substance abuse is an attempt to self-treat mental illness. Other evidence suggests that substance abuse promotes the development of mental illness, either by leading to a higher risk for traumatic experiences (increasing the chance of developing PTSD) or through a direct biochemical mechanism. Finally, con- current substance use disorder and mental illness may be due to an undefined genetic or biological vulnerability.38,44 This complicated relationship between substance abuse and behavioral health reinforces the need for screening, early diagnosis, and a comprehensive, multidisciplinary approach to treatment.
Treatment options. Office-based treatment options for narcotic and alcohol abuse and dependency are available to family physicians. Methadone has been used since the 1950s to treat opioid addiction and remains one of the mainstays of outpatient treatment.47,51 Originally, methadone was restricted to detoxification and maintenance treatment in narcotic addiction treatment programs approved by the FDA. In 1976, this restriction was lifted, and all physicians registered with the Drug Enforcement Agency (DEA) were permitted to prescribe methadone for analgesia.
In 2002, the FDA approved buprenorphine monotherapy and the combination product buprenorphine/naloxone for the treatment of opioid addiction. The prescribing of buprenorphine products requires physicians to undergo extra training, declare to the DEA their intent to prescribe buprenorphine, and obtain a special DEA identification number.52,53 Physicians interested in finding out more about buprenorphine treatment and prescribing requirements can go to the Substance Abuse and Mental Health Services Administration (SAMHSA) Web page at http://samhsa.gov.
Naltrexone is an opioid receptor agonist that is used primarily to treat alcohol dependency, and is thought to work by reducing the craving for alcohol. Multiple studies have proven the efficacy of naltrexone in an outpatient setting when used alone or in combination with psychotherapy.54,55 If you are uncomfortable or unfamiliar with the use or prescribing of these medications, referral to a substance abuse clinic specializing in dual-diagnosis treatment (TABLE 1) may offer optimal outcomes for patients with substance abuse disorders and other mental illness.
Cognitive behavioral therapy—including coping skills training, relapse prevention, contingency management, and behavioral couples’ therapy—and 12-step treatment programs are evidence-based options for the treatment of substance abuse disorders. Behavioral counseling interventions in the primary care setting (typically lasting 5-15 minutes) result in decreases in alcohol consumption, heavy drinking episodes, drinking above recommended amounts, and the number of days spent in the hospital, but have not been demonstrated to affect mortality, alcohol-related liver problems, outpatient visits, legal problems, or quality of life.56 Resources can be found at www.niaaa.nih.gov. For patients with dual diagnoses, it is not yet known whether sequential therapy (in which substance abuse is treated first, followed by treatment of the comorbid mental illness) or concurrent therapy results in better outcomes.57
CASE Your patient’s history of recent combat service, acknowledgement of employment and behavioral difficulties, and initial screening results lead you to diagnose alcoholism and depression. Additionally, she denies any suicidal ideation, but admits to experimenting with synthetic marijuana. After some discussion, she agrees to see your clinic’s social worker, and you start her on an SSri with scheduled follow-up.
CORRESPONDENCE
Shawn Kane, MD, USASoc, Attn: Surgeon (AomD), 2929 Desert Storm Drive, Ft. Bragg, NC 28310, or PO Box 3639 Pinehurst, NC 28374; [email protected]
1. Wessely S. Risk, psychiatry and the military. Br J Psychiatry. 2005;186:459-466.
2. Gawande A. Casualties of war—military care for the wounded from Iraq and Afghanistan. N Engl J Med. 2004;351:2471-2475.
3. Kotwal RS, Montgomery HR, Kotwal BM, et al. Eliminating pre- ventable death on the battlefield. Arch Surg. 2011;146:1350-1358.
4. Belanger HG, Uomoto JM, Vanderploeg RD. The Veterans Health Administration’s (VHA’s) Polytrauma System of Care for mild traumatic brain injury: costs, benefits, and controversies. J Head Trauma Rehabil. 2009;24:4-13.
5. Galarneau MR, Woodruff SI, Dye JL, et al. Traumatic brain in- jury during Operation Iraqi Freedom: findings from the United States Navy-Marine Corps Combat Trauma Registry. J Neurosurg. 2008;108:950-957.
6. Hermann BA, Shiner B, Friedman MJ. Epidemiology and preven- tion of combat-related post-traumatic stress in OEF/OIF/OND service members. Mil Med. 2012;177:1-6.
7. Uomoto JM. Best practices in veteran traumatic brain injury care. J Head Trauma Rehabil. 2012;27:241-243.
8. Warden D. Military TBI during the Iraq and Afghanistan wars. J Head Trauma Rehabil. 2006;21:398-402.
9. Taylor BC, Hagel EM, Carlson KF, et al. Prevalence and costs of co-occurring traumatic brain injury with and without psychiatric disturbance and pain among Afghanistan and Iraq War Veteran V.A. users. Med Care. 2012;50:342-346.
10. Quinlan JD, Guaron MR, Deschere BR, et al. Care of the returning veteran. Am Fam Physician. 2010;82:43-49.
11. Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351:13-22.
12. Hoge CW, Castro CA. Preventing suicides in US service mem- bers and veterans: concerns after a decade of war. JAMA. 2012;308:671-672.
13. Jaffe G. New name for PTSD could mean less stigma. The Washington Post. May 5, 2012. Available at: http://articles. washingtonpost.com/2012-05-05/world/35454931_1_ptsd-post- traumatic-stress-psychiatrists. Accessed June 19, 2013.
14. Warner CH, Appenzeller GN, Parker JR, et al. Effectiveness of mental health screening and coordination of in-theater care prior to deployment to Iraq: a cohort study. Am J Psychiatry. 2011;168:378-385.
15. United States Census Bureau. Sex by age by veteran sta- tus for civilian population 18 years and over. 2010 American community survey 1-year estimates. Available at: https:// d3gqux9sl0z33u.cloudfront.net/AA/AT/gambillingonjustice- com/downloads/206273/ACS_10_1YR_B21001A.pdf. Accessed June 19, 2013.
16. American Academy of Family Physicians. Joining forces. Avail- able at: http://www.aafp.org/online/en/home/membership/ initiatives/joiningforces.html. Accessed June 19, 2013.
17. Department of Veterans Affairs and Department of Defense. Clinical Practice Guideline for Management of Concussion/Mild Traumatic Brain Injury. April 2009. Available at: http://www. healthquality.va.gov/mtbi/concussion_mtbi_full_1_0.pdf. Accessed June 19, 2013.
18. Lew HL, Poole JH, Alvarez S, et al. Soldiers with occult traumatic brain injury. Am J Phys Med Rehabil. 2005;84:393-398.
19. Marshall KR, Holland SL, Meyer KS, et al. Mild traumatic brain injury screening, diagnosis, and treatment. Mil Med. 2012;177:67- 75.
20. Terrio H, Brenner LA, Ivins BJ, et al. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J Head Trauma Rehabil. 2009;24:14-23.
21. Mossadegh S, Tai N, Midwinter M, et al. Improvised explosive de- vice related pelvi-perineal trauma: anatomic injuries and surgical management. J Trauma Acute Care Surg. 2012;73:S24-S31.
22. Okie S. Traumatic brain injury in the war zone. N Engl J Med. 2005;352:2043-2047.
23. Espinoza JM. Posttraumatic stress disorder and the perceived consequences of seeking therapy among US Army special forces operators exposed to combat. J Psychol Issues Organ Culture. 2010;1:6-28.
24. Grieger TA, Cozza SJ, Ursano RJ, et al. Posttraumatic stress dis- order and depression in battle-injured soldiers. Am J Psychiatry. 2006;163:1777-1783.
25. Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA. 2006;295:1023-1032.
26. Adler AB, Wright KM, Bliese PD, et al. A2 diagnostic criterion for combat-related posttraumatic stress disorder. J Trauma Stress. 2008;21:301-308.
27. Hoge CW, Terhakopian A, Castro CA, et al. Association of post- traumatic stress disorder with somatic symptoms, health care vis- its, and absenteeism among Iraq war veterans. Am J Psychiatry. 2007;164:150-153.
28. Department of Veterans Affairs and Department of Defense. Clin- ical Practice Guideline for Management of Post-Traumatic Stress. October 2010. Available at: http://www.healthquality.va.gov/ ptsd/cpg_PTSD-FULL-201011612.pdf. Accessed June 19, 2013.
29. Alexander W. Pharmacotherapy for post-traumatic stress disor- der in combat veterans: focus on antidepressants and atypical antipsychotic agents. P T. 2012;37:32-38.
30. Wisco BE, Marx BP, Keane TM. Screening, diagnosis, and treat- ment of post-traumatic stress disorder. Mil Med. 2012;177:7-13.
31. Gadermann AM, Engel CC, Naifeh JA, et al. Prevalence of DSM-IV major depression among U.S. military personnel: meta-analysis and simulation. Mil Med. 2012;177:47-59.
32. Seal KH, Shi Y, Cohen G, et al. Association of mental health dis- orders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307:940-947.
33. Perrone M. Many drugs remain legal after ‘bath salts’ ban. Boston. com. July 25, 2012. Available at: http://articles.boston.com/2012- 07-25/lifestyle/32850962_1_bath-salts-mdpv-synthetic-drugs. Accessed June 19, 2013.
34. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Se- verity Rating Scale: initial validity and internal consistency find- ings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266-1277.
35. Greenberg J, Tesfazion AA, Robinson CS. Screening, diagnosis, and treatment of depression. Mil Med. 2012;177:60-66.
36. Eaton KM, Messer SC, Garvey Wilson AL, et al. Strengthening the validity of population-based suicide rate comparisons: an il- lustration using U.S. military and civilian data. Suicide Life Threat Behav. 2006;36:182-191.
37. Miller M, Azrael D, Barber C, et al. A call to link data to answer pressing questions about suicide risk among veterans. Am J Pub Health. 2012;102(suppl 1):S20-S22.
38. Department of Veterans Affairs. Report of the Blue Ribbon Work Group on suicide prevention in the veteran population. June 2008. Available at: http://www.mentalhealth.va.gov/suicide_ prevention/Blue_Ribbon_Report-FINAL_June-30-08.pdf. Accessed July 18, 2013.
39. Kinn JT, Luxton DD, Reger MA, et al. Department of Defense sui- cide event report: calendar year 2010 annual report. September 2011. Available at: http://t2health.org/sites/default/files/dodser/ DoDSER_2010_Annual_Report.pdf. Accessed June 19, 2013.
40. Fontana A, Rosenheck R. Treatment-seeking veterans of Iraq and Afghanistan: comparison with veterans of previous wars. J Nerv Ment Dis. 2008;196:513-521.
41. Seal KH, Cohen G, Waldrop A, et al. Substance use disorders in Iraq and Afghanistan veterans in VA healthcare, 2001-2010: implications for screening, diagnosis and treatment. Drug Alcohol Depend. 2011;116:93-101.
42. Mirza RA, Eick-Cost A, Otto JL. The risk of mental health disor- ders among U.S. military personnel infected with human immu- nodeficiency virus, active component, U.S. Armed Forces, 2000- 2011. MSMR. 2012;19:10-13.
43. Bohnert AS, Ilgen MA, Bossarte RM, et al. Veteran status and alco- hol use in men in the United States. Mil Med. 2012;177:198-203.
44. Erbes CR, Kaler ME, Schult T, et al. Mental health diagnosis and occupational functioning in National Guard/Reserve veterans re- turning from Iraq. J Rehabil Res Dev. 2011;48:1159-1170.
45. Stecker T, Fortney J, Owen R, et al. Co-occurring medical, psychi- atric, and alcohol-related disorders among veterans returning from Iraq and Afghanistan. Psychosomatics. 2010;51:503-507.
46. Seal KH, Shi Y, Cohen G, et al. Association of mental health dis- orders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307:940-947.
47. Praveen KT, Law F, O’Shea J, et al. Opioid dependence. Am Fam Physician. 2012;86:565-566.
48. Bradley KA, Bush KR, Epler AJ, et al. Two brief alcohol-screening tests from the Alcohol Use Disorders Identification Test (AUDIT): validation in a female Veterans Affairs patient population. Arch Intern Med. 2003;163:821-829.
49. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-1795.
50. Farrell M, Howes S, Taylor C, et al. Substance misuse and psychi- atric comorbidity: an overview of the OPCS National Psychiatric Morbidity Survey. Addict Behav. 1998;23:909-918.
51. Toombs JD, Kral LA. Methadone treatment for pain states. Am Fam Physician. 2005;71:1353-1358.
52. Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Treatment Improvement Protocol (TIP) series 40. DHHS pub- lication (SMA) 04-3939. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004. Available at: http:// buprenorphine.samhsa.gov/Bup_Guidelines.pdf. Accessed June 19, 2013.
53. U.S.DepartmentofHealthandHumanServices,SubstanceAbuse and Mental Health Services Administration Web site. About buprenorphine therapy. Available at: http://buprenorphine. samhsa.gov/about.html. Accessed June 19, 2013.
54. Volpicelli JR, Alterman AI, Hayashida M, et al. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876-880.
55. O’Brien CP, Volpicelli LA, Volpicelli JR. Naltrexone in the treat- ment of alcoholism: a clinical review. Alcohol. 1996;13:35-39.
56. Jonas DE, Garbutt JC, Amick HR, et al. Behavioral counseling after screening for alcohol misuse in primary care: a systematic review and meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2012;157:645-654.
57. van Dam D, Vedel E, Ehring T, et al. Psychological treatments for concurrent posttraumatic stress disorder and substance use dis- order: a systematic review. Clin Psychol Rev. 2012;32:202-214.
1. Wessely S. Risk, psychiatry and the military. Br J Psychiatry. 2005;186:459-466.
2. Gawande A. Casualties of war—military care for the wounded from Iraq and Afghanistan. N Engl J Med. 2004;351:2471-2475.
3. Kotwal RS, Montgomery HR, Kotwal BM, et al. Eliminating pre- ventable death on the battlefield. Arch Surg. 2011;146:1350-1358.
4. Belanger HG, Uomoto JM, Vanderploeg RD. The Veterans Health Administration’s (VHA’s) Polytrauma System of Care for mild traumatic brain injury: costs, benefits, and controversies. J Head Trauma Rehabil. 2009;24:4-13.
5. Galarneau MR, Woodruff SI, Dye JL, et al. Traumatic brain in- jury during Operation Iraqi Freedom: findings from the United States Navy-Marine Corps Combat Trauma Registry. J Neurosurg. 2008;108:950-957.
6. Hermann BA, Shiner B, Friedman MJ. Epidemiology and preven- tion of combat-related post-traumatic stress in OEF/OIF/OND service members. Mil Med. 2012;177:1-6.
7. Uomoto JM. Best practices in veteran traumatic brain injury care. J Head Trauma Rehabil. 2012;27:241-243.
8. Warden D. Military TBI during the Iraq and Afghanistan wars. J Head Trauma Rehabil. 2006;21:398-402.
9. Taylor BC, Hagel EM, Carlson KF, et al. Prevalence and costs of co-occurring traumatic brain injury with and without psychiatric disturbance and pain among Afghanistan and Iraq War Veteran V.A. users. Med Care. 2012;50:342-346.
10. Quinlan JD, Guaron MR, Deschere BR, et al. Care of the returning veteran. Am Fam Physician. 2010;82:43-49.
11. Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351:13-22.
12. Hoge CW, Castro CA. Preventing suicides in US service mem- bers and veterans: concerns after a decade of war. JAMA. 2012;308:671-672.
13. Jaffe G. New name for PTSD could mean less stigma. The Washington Post. May 5, 2012. Available at: http://articles. washingtonpost.com/2012-05-05/world/35454931_1_ptsd-post- traumatic-stress-psychiatrists. Accessed June 19, 2013.
14. Warner CH, Appenzeller GN, Parker JR, et al. Effectiveness of mental health screening and coordination of in-theater care prior to deployment to Iraq: a cohort study. Am J Psychiatry. 2011;168:378-385.
15. United States Census Bureau. Sex by age by veteran sta- tus for civilian population 18 years and over. 2010 American community survey 1-year estimates. Available at: https:// d3gqux9sl0z33u.cloudfront.net/AA/AT/gambillingonjustice- com/downloads/206273/ACS_10_1YR_B21001A.pdf. Accessed June 19, 2013.
16. American Academy of Family Physicians. Joining forces. Avail- able at: http://www.aafp.org/online/en/home/membership/ initiatives/joiningforces.html. Accessed June 19, 2013.
17. Department of Veterans Affairs and Department of Defense. Clinical Practice Guideline for Management of Concussion/Mild Traumatic Brain Injury. April 2009. Available at: http://www. healthquality.va.gov/mtbi/concussion_mtbi_full_1_0.pdf. Accessed June 19, 2013.
18. Lew HL, Poole JH, Alvarez S, et al. Soldiers with occult traumatic brain injury. Am J Phys Med Rehabil. 2005;84:393-398.
19. Marshall KR, Holland SL, Meyer KS, et al. Mild traumatic brain injury screening, diagnosis, and treatment. Mil Med. 2012;177:67- 75.
20. Terrio H, Brenner LA, Ivins BJ, et al. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J Head Trauma Rehabil. 2009;24:14-23.
21. Mossadegh S, Tai N, Midwinter M, et al. Improvised explosive de- vice related pelvi-perineal trauma: anatomic injuries and surgical management. J Trauma Acute Care Surg. 2012;73:S24-S31.
22. Okie S. Traumatic brain injury in the war zone. N Engl J Med. 2005;352:2043-2047.
23. Espinoza JM. Posttraumatic stress disorder and the perceived consequences of seeking therapy among US Army special forces operators exposed to combat. J Psychol Issues Organ Culture. 2010;1:6-28.
24. Grieger TA, Cozza SJ, Ursano RJ, et al. Posttraumatic stress dis- order and depression in battle-injured soldiers. Am J Psychiatry. 2006;163:1777-1783.
25. Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA. 2006;295:1023-1032.
26. Adler AB, Wright KM, Bliese PD, et al. A2 diagnostic criterion for combat-related posttraumatic stress disorder. J Trauma Stress. 2008;21:301-308.
27. Hoge CW, Terhakopian A, Castro CA, et al. Association of post- traumatic stress disorder with somatic symptoms, health care vis- its, and absenteeism among Iraq war veterans. Am J Psychiatry. 2007;164:150-153.
28. Department of Veterans Affairs and Department of Defense. Clin- ical Practice Guideline for Management of Post-Traumatic Stress. October 2010. Available at: http://www.healthquality.va.gov/ ptsd/cpg_PTSD-FULL-201011612.pdf. Accessed June 19, 2013.
29. Alexander W. Pharmacotherapy for post-traumatic stress disor- der in combat veterans: focus on antidepressants and atypical antipsychotic agents. P T. 2012;37:32-38.
30. Wisco BE, Marx BP, Keane TM. Screening, diagnosis, and treat- ment of post-traumatic stress disorder. Mil Med. 2012;177:7-13.
31. Gadermann AM, Engel CC, Naifeh JA, et al. Prevalence of DSM-IV major depression among U.S. military personnel: meta-analysis and simulation. Mil Med. 2012;177:47-59.
32. Seal KH, Shi Y, Cohen G, et al. Association of mental health dis- orders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307:940-947.
33. Perrone M. Many drugs remain legal after ‘bath salts’ ban. Boston. com. July 25, 2012. Available at: http://articles.boston.com/2012- 07-25/lifestyle/32850962_1_bath-salts-mdpv-synthetic-drugs. Accessed June 19, 2013.
34. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Se- verity Rating Scale: initial validity and internal consistency find- ings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266-1277.
35. Greenberg J, Tesfazion AA, Robinson CS. Screening, diagnosis, and treatment of depression. Mil Med. 2012;177:60-66.
36. Eaton KM, Messer SC, Garvey Wilson AL, et al. Strengthening the validity of population-based suicide rate comparisons: an il- lustration using U.S. military and civilian data. Suicide Life Threat Behav. 2006;36:182-191.
37. Miller M, Azrael D, Barber C, et al. A call to link data to answer pressing questions about suicide risk among veterans. Am J Pub Health. 2012;102(suppl 1):S20-S22.
38. Department of Veterans Affairs. Report of the Blue Ribbon Work Group on suicide prevention in the veteran population. June 2008. Available at: http://www.mentalhealth.va.gov/suicide_ prevention/Blue_Ribbon_Report-FINAL_June-30-08.pdf. Accessed July 18, 2013.
39. Kinn JT, Luxton DD, Reger MA, et al. Department of Defense sui- cide event report: calendar year 2010 annual report. September 2011. Available at: http://t2health.org/sites/default/files/dodser/ DoDSER_2010_Annual_Report.pdf. Accessed June 19, 2013.
40. Fontana A, Rosenheck R. Treatment-seeking veterans of Iraq and Afghanistan: comparison with veterans of previous wars. J Nerv Ment Dis. 2008;196:513-521.
41. Seal KH, Cohen G, Waldrop A, et al. Substance use disorders in Iraq and Afghanistan veterans in VA healthcare, 2001-2010: implications for screening, diagnosis and treatment. Drug Alcohol Depend. 2011;116:93-101.
42. Mirza RA, Eick-Cost A, Otto JL. The risk of mental health disor- ders among U.S. military personnel infected with human immu- nodeficiency virus, active component, U.S. Armed Forces, 2000- 2011. MSMR. 2012;19:10-13.
43. Bohnert AS, Ilgen MA, Bossarte RM, et al. Veteran status and alco- hol use in men in the United States. Mil Med. 2012;177:198-203.
44. Erbes CR, Kaler ME, Schult T, et al. Mental health diagnosis and occupational functioning in National Guard/Reserve veterans re- turning from Iraq. J Rehabil Res Dev. 2011;48:1159-1170.
45. Stecker T, Fortney J, Owen R, et al. Co-occurring medical, psychi- atric, and alcohol-related disorders among veterans returning from Iraq and Afghanistan. Psychosomatics. 2010;51:503-507.
46. Seal KH, Shi Y, Cohen G, et al. Association of mental health dis- orders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307:940-947.
47. Praveen KT, Law F, O’Shea J, et al. Opioid dependence. Am Fam Physician. 2012;86:565-566.
48. Bradley KA, Bush KR, Epler AJ, et al. Two brief alcohol-screening tests from the Alcohol Use Disorders Identification Test (AUDIT): validation in a female Veterans Affairs patient population. Arch Intern Med. 2003;163:821-829.
49. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-1795.
50. Farrell M, Howes S, Taylor C, et al. Substance misuse and psychi- atric comorbidity: an overview of the OPCS National Psychiatric Morbidity Survey. Addict Behav. 1998;23:909-918.
51. Toombs JD, Kral LA. Methadone treatment for pain states. Am Fam Physician. 2005;71:1353-1358.
52. Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Treatment Improvement Protocol (TIP) series 40. DHHS pub- lication (SMA) 04-3939. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004. Available at: http:// buprenorphine.samhsa.gov/Bup_Guidelines.pdf. Accessed June 19, 2013.
53. U.S.DepartmentofHealthandHumanServices,SubstanceAbuse and Mental Health Services Administration Web site. About buprenorphine therapy. Available at: http://buprenorphine. samhsa.gov/about.html. Accessed June 19, 2013.
54. Volpicelli JR, Alterman AI, Hayashida M, et al. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876-880.
55. O’Brien CP, Volpicelli LA, Volpicelli JR. Naltrexone in the treat- ment of alcoholism: a clinical review. Alcohol. 1996;13:35-39.
56. Jonas DE, Garbutt JC, Amick HR, et al. Behavioral counseling after screening for alcohol misuse in primary care: a systematic review and meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2012;157:645-654.
57. van Dam D, Vedel E, Ehring T, et al. Psychological treatments for concurrent posttraumatic stress disorder and substance use dis- order: a systematic review. Clin Psychol Rev. 2012;32:202-214.
How can we better manage difficult patient encounters?
It’s a scenario all office-based physicians are familiar with: Your day has been going well, and all your appointments have been (relatively) on time. But when you scan your afternoon schedule your mood shifts from buoyant to crestfallen. The reason: Three patients whom you find particularly frustrating are slotted for the last appointments of the day.
CASE 1 Mr. E, age 44, a high-powered attorney, has chronic headache that he has complained off and on about for several years. He has no other past medical history. Physical examination strongly suggests that Mr. E suffers from tension-type headaches, but he continues to demand magnetic resonance imaging (MRI) of the brain. “I think there’s something wrong in there,” he has stated repeatedly. “If my last doctor, who is one of the best in the country, ordered an MRI for me, why can’t you?”
CASE 2 Mr. A is a 37-year-old man who lost his home in a hurricane 3 years ago. Although he sustained only minor physical injuries, Mr. A appears to have lost his sense of well-being. He has developed chronic—and debilitating— musculoskeletal pain in his neck and low back in the aftermath of the storm and has been unable to work since then.
At his last 2 visits, Mr. A requested increasing doses of oxycodone, insisting that nothing else alleviates the pain. When you suggested a nonopioid analgesic, he broke down in tears. “Nobody takes my injuries seriously! My insurance doesn’t want to compensate me for my losses. Now you don’t even believe I’m in pain.”
CASE 3 Ms. S is a 65-year-old socially isolated widow who lost her husband several years ago. She has a history of multiple somatic complaints, including fatigue, abdominal pain, back pain, joint pain, and dizziness. As a result, she has undergone numerous diagnostic procedures, including esophagastroduodenoscopy, colonoscopy, and various blood tests, all of which have been negative. Ms. S consistently requires longer than the usual 20-minute visit. When you try to end an appointment, she typically brings up new issues. “Two days ago, i had this pain by my belly button and left shoulder blade. i think that there must be something wrong with me. Can you examine me?” she asked toward the end of her last visit 6 weeks ago.
The burden of difficult patient encounters
Cases such as these are frustrating, not so much for their clinical complexity, but rather because of the elusiveness of satisfying doctor-patient interactions. Besides a litany of physical complaints, such patients typically present with anxiety, depression, and other psychiatric symptoms; express dissatisfaction with the care they are receiving; and repeatedly request tests and interventions that are not medically indicated.1
From a primary care perspective, such cases can be frustrating and time- consuming, significantly contribute to exhaustion and burnout, and result in unnecessary health care expenditures.1 (See “Unexplained complaints in primary care: Evidence of action bias” also in this issue to learn more.) Studies suggest that family physicians see such patients on a daily basis, and rate about one patient in 6 as a “difficult” case.2,3
Physician attitudes, training play a role
Research has established other critical spheres of influence that conspire to create difficult or frustrating patient encounters, including “system” factors (ie, reduced duration of visits and interrupted visits)4 and physician factors. In fact, physicians’ negative attitude toward psychosocial aspects of patient care may be a more potent factor in shaping difficult encounters than any patient characteristic.3,5
Consider the following statements:
- “Talking about psychosocial issues causes more trouble than it is worth.”
- “I do not focus on psychosocial problems until I have ruled out organic disease.”
- “I am too pressed for time to routinely investigate psychosocial issues.”
Such sentiments, which have been associated with difficult encounters, are part of the 32-item Physician Belief Scale, developed 30 years ago and still used to assess beliefs about psychosocial aspects of patient care held by primary care physicians.6
Lack of training is a potential problem, as well. In one survey, more than half of directors of family medicine programs agreed that training in mental health is inadequate.7Thus, family physicians often respond to patients like Mr. E, Mr. A, and Ms. S by becoming irritated or avoiding further interaction. A more appropriate response is for the physician to self-acknowledge his or her emotions, then to engage in an empathic interaction in keeping with patient expectations.4
As mental health treatment becomes more integrated within family medicine,8 pointers for handling difficult patient encounters can be gleaned from the traditional psychiatric approach to difficult or frustrating cases. Indeed, we believe that what is now known as a “patient-centered approach” is rooted in traditions and techniques that psychiatrists and psychologists have used for decades.9
Core principles for handling frustrating cases
A useful approach to the difficult patient encounter, detailed in the TABLE that we created, is based on 3 key principles:
- The doctor-patient relationship should be the target for change.
- The patient’s emotional experience should be an explicit focus of the clinical interaction.
- The patient’s perspective should guide the clinical encounter.
In our experience, when the interaction between patient and doctor shifts from searching for specific pathologies to building a collaborative relationship, previously recalcitrant symptoms often improve.
Use these communication techniques
There are 2 main ways to elicit a conversation about personal issues, including emotions, with patients. One is to directly ask patients to describe the distress they are experiencing and elicit the emotions connected to this distress. The other is to invite a discussion about emotional issues indirectly, by asking patients how the symptoms affect their lives and what they think is causing the problem or by selectively sharing an emotional experience of your own.
Once a patient has shared emotions, you will need to show support and empathy in order to build an alliance. There is more than one way to do this, and methods can be used alone or in combination, depending on the particular situation. (You’ll find examples in the TABLE.)
Name the affect. The simple act of naming the patient’s affect or emotional expression (eg, “You sound sad”) is surprisingly helpful, as it lets patients know they have been “heard.”
Validate. You can also validate patients like Mr. E, Mr. A, and Ms. S by stating that their emotional reactions are legitimate, praising them for how well they have coped with difficult symptoms, and acknowledging the seriousness and complexity of their situations.
Align. Once a patient expresses his or her interests and goals, aligning yourself with them (eg, “I want to do everything in my power to help you reduce your pain...”) will elicit hope and improve patient satisfaction.
And 2 mnemonic devices—detailed in the box 10,11—can help you improve the way you communicate with patients.
Communication can also be nonverbal, such as thoughtful nodding or a timely therapeutic silence. The former is characterized by slow, steady, and purposeful movement accompanied by eye contact; in the latter case, you simply resist the urge to immediately respond after a patient has revealed something emotionally laden, and wait a few seconds to take in what has been said.
NURS is a reminder to:
Name the patient’s emotion (“you say that these constant headaches really get on your nerves.”)
Understand (“i can see why you feel this way.”)
Respect (“you’ve been through a lot and that takes a lot of courage.”)
Support (“i want to help you get better.”)10
Background (“What has been going on in your life?”)
Affect (“how do you feel about that?”)
Trouble (“What troubles you the most about this situation?”)
Handling (“how are you handling this?”)
Empathy (“That must be difficult.”)11
How to provide therapeutic structure
Family physicians can further manage patient behaviors that they find bothersome by implementing changes in the way they organize and conduct patient visits. Studies of patients with complex somatic symptoms offer additional hints for the management of frustrating cases. The following strategies can lead to positive outcomes, including a decrease in disability and health care costs.12
Schedule regular brief visits. Mr. E, Mr. A, and Ms. S should have frequent and regular, but brief, appointments (eg, 15 minutes every 2 weeks for 2 months). Proactively schedule return visits, rather than waiting for the patient to call for an appointment PRN.11 Sharing this kind of plan gives such patients a concrete time line and clear evidence of support. Avoid the temptation to schedule difficult cases for the last time slot of the day, as going over the allotted time can insidiously give some patients the expectation of progressively longer visits.
Set the agenda. To prevent “doorknob questions” like Ms. S’s new symptoms, reported just as you’re about to leave the exam room, the agenda must be set at the outset of the visit. This can be done by asking, “What did you want to discuss today?”, “Is there anything else you want to address today?”, or “What else did you need taken care of?”13 Explicitly inquiring about patient expectations at the start of the visit lets patients like Ms. S know that they are being taken seriously. If the agenda still balloons, you can simply state, “You deserve more than 15 minutes for all these issues. Let’s pick the top 2 for today and tackle others at our next visit in 2 weeks.” To further save time, you can ask the patient to bring a symptom diary or written agenda to the appointment. We’ve found that many anxious patients benefit from this exercise.
Avoid the urge to act. When a patient suffers from unexplained symptoms, effective interventions require physicians to avoid certain “reflex” behaviors—repeatedly performing diagnostic tests, prescribing medications for symptoms of unknown etiology, insinuating that the problem is “in your head,” formulating ambiguous diagnoses, and repeating physical exams.12 Such physician behaviors tend to reinforce the pathology in patients with unexplained symptoms. The time saved avoiding these pitfalls is better invested in exploring personal issues and stressors.
The point is that such patients should be reassured via discussion, rather than with dubious diagnostic labels and potentially dangerous drugs. This approach has been shown to improve patients’ physical functioning while reducing medical expenditures.12
Into action with our 3 cases
CASE 1 Given these principles, how would you handle Mr. E, the patient who is demanding an MRI for a simple tension headache? Although placating him by ordering the test, providing a referral to a specialist, or defending your recommendation through medical reasoning may seem to be more intuitive (or a “quick fix”), these strategies often lead to excessive medical spending, transfer of the burden to a specialist colleague, and ongoing frustration and dissatisfaction on the part of the patient. In this case, validation may be a more useful approach.
“I can totally understand why you’re frustrated that we disagree,” you might say to Mr. E. “But you’re right! you definitely deserve the best care. That’s why I’m recommending against the MRI, as I feel that would be a suboptimal approach.”
Often patients like Mr. E will require repeated validation of their suffering and frustration. The key is to be persistent in validating their feelings without compromising your own principles in providing optimal medical care.
CASE 2 Let’s turn now to Mr. A, who is requesting escalating doses of opioids. Some physicians might write the prescription for the dose he’s insisting on, while others draw a hard boundary by refusing to prescribe above a certain dose or beyond a specific time frame. Both strategies may compromise optimal care or endanger the doctor-patient alliance. Another quick solution would be to provide a referral to a psychiatrist, without further discussion.
In cases like that of Mr. A, however, the patient’s demands are often a sign of more complicated emotions and dynamics below the surface. So you might respond by stating, “I’m sorry to hear that things haven’t been going well. How are you feeling about these things? How does the oxycodone help you? In what way doesn’t it help?”
It is important here to understand how the medication serves the patient—in addition to the ways it hurts him—in order for him to feel understood. Inviting Mr. A to have an open-ended discussion may allow him to reveal what is the real source of his distress—losing his job and his home.
CASE 3 Now let’s turn to Ms. S, who is convinced that she has a physical malady despite negative exams and tests. In truth, she may be depressed or anxious over her husband’s death. One way to address this is to confront the patient directly by suggesting that she has depression triggered by her husband’s death. But this strategy—if used too early—may feel like an accusation, make her angry, and jeopardize your relationship with her.
An alternative approach would be to say, “I think your problems are long-standing and could require a while to treat. Let’s see each other every 2 weeks for the next 2 months so we get adequate time to work on them.” This would be an example of structuring more frequent visits, while also validating the distressing nature of her symptoms.
These strategies are evidence-based
These techniques, while easily adaptable to primary care, are grounded in psychotherapy theory and are evidence-based. A seminal randomized controlled trial conducted more than 30 years ago showed that a patient-centered interview incorporating a number of these techniques bolstered physicians’ knowledge, interviewing skills, attitudes, and ability to manage patients with unexplained complaints.14
A multicenter study analyzed audio recordings of strategies used by primary care physicians to deny patient requests for a particular medication. It revealed that explanations based on patient perspectives were significantly more likely to result in excellent patient satisfaction than biomedical explanations or other explanatory approaches.15 Research has also shown that agenda-setting improves both patient and provider satisfaction.16
Some cases will still be frustrating, and some “difficult” patients will still need a psychiatric referral at some point—ideally, to a psychiatric or psychological consultant who collaborates closely with the primary care clinic.17,18
Family physicians sometimes worry that the communication techniques we’ve outlined cannot be incorporated into an already harried primary care visit. Many may think it’s better not ask at all than risk opening a Pandora’s box. We urge you to reconsider. Although the techniques we’ve outlined certainly require practice, they need not be time-consuming.19 By embracing this management approach, you can improve patient satisfaction while enhancing your own repertoire of doctoring skills.
CORRESPONDENCE
Alan R. Teo, MD, MS, 3710 SW US Veterans Hospital Road, Portland, OR 97239; [email protected]
ACKNOWLEDGEMENT
The authors thank Drs. Michael Fetters and Rod Hayward for their help in the development of the manuscript.
1. Hahn SR, Kroenke K, Spitzer RL, et al. The difficult patient: preva- lence, psychopathology, and functional impairment. J Gen Intern Med. 1996;11:1-8.
2. An PG, Rabatin JS, Manwell LB, et al. Burden of difficult encoun- ters in primary care: data From the minimizing error, maximizing outcomes study. Arch Intern Med. 2009;169:410-414.
3. Hinchey SA, Jackson JL. A cohort study assessing difficult patient encoun- ters in a walk-in primary care clinic, predictors and outcomes. J Gen Intern Med. 2011;26:588-594.
4. Haas LJ, Leiser JP, Magill MK, et al. Management of the difficult patient. Am Fam Physician. 2005;72:2063-2068.
5. Jackson JL, Kroenke K. Difficult patient encounters in the ambulatory clinic: clinical predictors and outcomes. Arch Intern Med. 1999;159:1069- 1075.
6. Ashworth CD, Williamson P, Montano D. A scale to measure physi- cian beliefs about psychosocial aspects of patient care. Soc Sci Med. 1984;19:1235-1238.
7. Leigh H, Stewart D, Mallios R. Mental health and psychiatry training in primary care residency programs. Gen Hosp Psychiatry. 2006;28:189-194.
8. Katon W, Unützer J. Collaborative care models for depression: time to move from evidence to practice. Arch Intern Med. 2006;166:2304-2306.
9. Groves JE. Taking care of the hateful patient. N Engl J Med. 1978;298:883- 887.
10. Fortin AH, Dwamena FC, Frankel RM, et al. Smith’s Patient-Centered Interviewing: An Evidence-Based Method. 3rd ed. New York, NY: McGraw-Hill; 2012.
11. Stuart MR, Lieberman JA. The Fifteen Minute Hour: Therapeutic Talk in Primary Care. 4th ed. Milton Keynes, UK: Radcliffe Publishing; 2008.
12. Smith GR Jr, Rost K, Kashner TM. A trial of the effect of a standardized psychiatric consultation on health outcomes and costs in somatizing patients. Arch Gen Psychiatry. 1995;52:238-243.
13. Baker LH, O’Connell D, Platt FW. “What else?” Setting the agenda for the clinical interview. Ann Intern Med. 2005;143:766 -770.
14. Smith RC, Lyles JS, Mettler J, et al. The effectiveness of intensive training for residents in interviewing. A randomized, controlled study. Ann Intern Med. 1998;128:118-126.
15. Paterniti DA, Fancher TL, Cipri CS, et al. Getting to “no”: strategies pri- mary care physicians use to deny patient requests. Arch Intern Med. 2010;170:381-388.
16. Kroenke K. Unburdening the difficult clinical encounter. Arch Intern Med. 2009;169:333-334.
17. Katon W, Unützer J, Wells K, et al. Collaborative depression care: history, evolution and ways to enhance dissemination and sustainability. Gen Hosp Psychiatry. 2010;32:456-464.
18. Williams M, Angstman K, Johnson I, et al. Implementation of a care man- agement model for depression at two primary care clinics. J Ambul Care Manage. 2011;34:163-173.
19. Lieberman JA III, Stuart MR. The BATHE method: incorporating counsel- ing and psychotherapy into the everyday management of patients. Prim Care Companion J Clin Psychiatry. 1999;1:35-38.
It’s a scenario all office-based physicians are familiar with: Your day has been going well, and all your appointments have been (relatively) on time. But when you scan your afternoon schedule your mood shifts from buoyant to crestfallen. The reason: Three patients whom you find particularly frustrating are slotted for the last appointments of the day.
CASE 1 Mr. E, age 44, a high-powered attorney, has chronic headache that he has complained off and on about for several years. He has no other past medical history. Physical examination strongly suggests that Mr. E suffers from tension-type headaches, but he continues to demand magnetic resonance imaging (MRI) of the brain. “I think there’s something wrong in there,” he has stated repeatedly. “If my last doctor, who is one of the best in the country, ordered an MRI for me, why can’t you?”
CASE 2 Mr. A is a 37-year-old man who lost his home in a hurricane 3 years ago. Although he sustained only minor physical injuries, Mr. A appears to have lost his sense of well-being. He has developed chronic—and debilitating— musculoskeletal pain in his neck and low back in the aftermath of the storm and has been unable to work since then.
At his last 2 visits, Mr. A requested increasing doses of oxycodone, insisting that nothing else alleviates the pain. When you suggested a nonopioid analgesic, he broke down in tears. “Nobody takes my injuries seriously! My insurance doesn’t want to compensate me for my losses. Now you don’t even believe I’m in pain.”
CASE 3 Ms. S is a 65-year-old socially isolated widow who lost her husband several years ago. She has a history of multiple somatic complaints, including fatigue, abdominal pain, back pain, joint pain, and dizziness. As a result, she has undergone numerous diagnostic procedures, including esophagastroduodenoscopy, colonoscopy, and various blood tests, all of which have been negative. Ms. S consistently requires longer than the usual 20-minute visit. When you try to end an appointment, she typically brings up new issues. “Two days ago, i had this pain by my belly button and left shoulder blade. i think that there must be something wrong with me. Can you examine me?” she asked toward the end of her last visit 6 weeks ago.
The burden of difficult patient encounters
Cases such as these are frustrating, not so much for their clinical complexity, but rather because of the elusiveness of satisfying doctor-patient interactions. Besides a litany of physical complaints, such patients typically present with anxiety, depression, and other psychiatric symptoms; express dissatisfaction with the care they are receiving; and repeatedly request tests and interventions that are not medically indicated.1
From a primary care perspective, such cases can be frustrating and time- consuming, significantly contribute to exhaustion and burnout, and result in unnecessary health care expenditures.1 (See “Unexplained complaints in primary care: Evidence of action bias” also in this issue to learn more.) Studies suggest that family physicians see such patients on a daily basis, and rate about one patient in 6 as a “difficult” case.2,3
Physician attitudes, training play a role
Research has established other critical spheres of influence that conspire to create difficult or frustrating patient encounters, including “system” factors (ie, reduced duration of visits and interrupted visits)4 and physician factors. In fact, physicians’ negative attitude toward psychosocial aspects of patient care may be a more potent factor in shaping difficult encounters than any patient characteristic.3,5
Consider the following statements:
- “Talking about psychosocial issues causes more trouble than it is worth.”
- “I do not focus on psychosocial problems until I have ruled out organic disease.”
- “I am too pressed for time to routinely investigate psychosocial issues.”
Such sentiments, which have been associated with difficult encounters, are part of the 32-item Physician Belief Scale, developed 30 years ago and still used to assess beliefs about psychosocial aspects of patient care held by primary care physicians.6
Lack of training is a potential problem, as well. In one survey, more than half of directors of family medicine programs agreed that training in mental health is inadequate.7Thus, family physicians often respond to patients like Mr. E, Mr. A, and Ms. S by becoming irritated or avoiding further interaction. A more appropriate response is for the physician to self-acknowledge his or her emotions, then to engage in an empathic interaction in keeping with patient expectations.4
As mental health treatment becomes more integrated within family medicine,8 pointers for handling difficult patient encounters can be gleaned from the traditional psychiatric approach to difficult or frustrating cases. Indeed, we believe that what is now known as a “patient-centered approach” is rooted in traditions and techniques that psychiatrists and psychologists have used for decades.9
Core principles for handling frustrating cases
A useful approach to the difficult patient encounter, detailed in the TABLE that we created, is based on 3 key principles:
- The doctor-patient relationship should be the target for change.
- The patient’s emotional experience should be an explicit focus of the clinical interaction.
- The patient’s perspective should guide the clinical encounter.
In our experience, when the interaction between patient and doctor shifts from searching for specific pathologies to building a collaborative relationship, previously recalcitrant symptoms often improve.
Use these communication techniques
There are 2 main ways to elicit a conversation about personal issues, including emotions, with patients. One is to directly ask patients to describe the distress they are experiencing and elicit the emotions connected to this distress. The other is to invite a discussion about emotional issues indirectly, by asking patients how the symptoms affect their lives and what they think is causing the problem or by selectively sharing an emotional experience of your own.
Once a patient has shared emotions, you will need to show support and empathy in order to build an alliance. There is more than one way to do this, and methods can be used alone or in combination, depending on the particular situation. (You’ll find examples in the TABLE.)
Name the affect. The simple act of naming the patient’s affect or emotional expression (eg, “You sound sad”) is surprisingly helpful, as it lets patients know they have been “heard.”
Validate. You can also validate patients like Mr. E, Mr. A, and Ms. S by stating that their emotional reactions are legitimate, praising them for how well they have coped with difficult symptoms, and acknowledging the seriousness and complexity of their situations.
Align. Once a patient expresses his or her interests and goals, aligning yourself with them (eg, “I want to do everything in my power to help you reduce your pain...”) will elicit hope and improve patient satisfaction.
And 2 mnemonic devices—detailed in the box 10,11—can help you improve the way you communicate with patients.
Communication can also be nonverbal, such as thoughtful nodding or a timely therapeutic silence. The former is characterized by slow, steady, and purposeful movement accompanied by eye contact; in the latter case, you simply resist the urge to immediately respond after a patient has revealed something emotionally laden, and wait a few seconds to take in what has been said.
NURS is a reminder to:
Name the patient’s emotion (“you say that these constant headaches really get on your nerves.”)
Understand (“i can see why you feel this way.”)
Respect (“you’ve been through a lot and that takes a lot of courage.”)
Support (“i want to help you get better.”)10
Background (“What has been going on in your life?”)
Affect (“how do you feel about that?”)
Trouble (“What troubles you the most about this situation?”)
Handling (“how are you handling this?”)
Empathy (“That must be difficult.”)11
How to provide therapeutic structure
Family physicians can further manage patient behaviors that they find bothersome by implementing changes in the way they organize and conduct patient visits. Studies of patients with complex somatic symptoms offer additional hints for the management of frustrating cases. The following strategies can lead to positive outcomes, including a decrease in disability and health care costs.12
Schedule regular brief visits. Mr. E, Mr. A, and Ms. S should have frequent and regular, but brief, appointments (eg, 15 minutes every 2 weeks for 2 months). Proactively schedule return visits, rather than waiting for the patient to call for an appointment PRN.11 Sharing this kind of plan gives such patients a concrete time line and clear evidence of support. Avoid the temptation to schedule difficult cases for the last time slot of the day, as going over the allotted time can insidiously give some patients the expectation of progressively longer visits.
Set the agenda. To prevent “doorknob questions” like Ms. S’s new symptoms, reported just as you’re about to leave the exam room, the agenda must be set at the outset of the visit. This can be done by asking, “What did you want to discuss today?”, “Is there anything else you want to address today?”, or “What else did you need taken care of?”13 Explicitly inquiring about patient expectations at the start of the visit lets patients like Ms. S know that they are being taken seriously. If the agenda still balloons, you can simply state, “You deserve more than 15 minutes for all these issues. Let’s pick the top 2 for today and tackle others at our next visit in 2 weeks.” To further save time, you can ask the patient to bring a symptom diary or written agenda to the appointment. We’ve found that many anxious patients benefit from this exercise.
Avoid the urge to act. When a patient suffers from unexplained symptoms, effective interventions require physicians to avoid certain “reflex” behaviors—repeatedly performing diagnostic tests, prescribing medications for symptoms of unknown etiology, insinuating that the problem is “in your head,” formulating ambiguous diagnoses, and repeating physical exams.12 Such physician behaviors tend to reinforce the pathology in patients with unexplained symptoms. The time saved avoiding these pitfalls is better invested in exploring personal issues and stressors.
The point is that such patients should be reassured via discussion, rather than with dubious diagnostic labels and potentially dangerous drugs. This approach has been shown to improve patients’ physical functioning while reducing medical expenditures.12
Into action with our 3 cases
CASE 1 Given these principles, how would you handle Mr. E, the patient who is demanding an MRI for a simple tension headache? Although placating him by ordering the test, providing a referral to a specialist, or defending your recommendation through medical reasoning may seem to be more intuitive (or a “quick fix”), these strategies often lead to excessive medical spending, transfer of the burden to a specialist colleague, and ongoing frustration and dissatisfaction on the part of the patient. In this case, validation may be a more useful approach.
“I can totally understand why you’re frustrated that we disagree,” you might say to Mr. E. “But you’re right! you definitely deserve the best care. That’s why I’m recommending against the MRI, as I feel that would be a suboptimal approach.”
Often patients like Mr. E will require repeated validation of their suffering and frustration. The key is to be persistent in validating their feelings without compromising your own principles in providing optimal medical care.
CASE 2 Let’s turn now to Mr. A, who is requesting escalating doses of opioids. Some physicians might write the prescription for the dose he’s insisting on, while others draw a hard boundary by refusing to prescribe above a certain dose or beyond a specific time frame. Both strategies may compromise optimal care or endanger the doctor-patient alliance. Another quick solution would be to provide a referral to a psychiatrist, without further discussion.
In cases like that of Mr. A, however, the patient’s demands are often a sign of more complicated emotions and dynamics below the surface. So you might respond by stating, “I’m sorry to hear that things haven’t been going well. How are you feeling about these things? How does the oxycodone help you? In what way doesn’t it help?”
It is important here to understand how the medication serves the patient—in addition to the ways it hurts him—in order for him to feel understood. Inviting Mr. A to have an open-ended discussion may allow him to reveal what is the real source of his distress—losing his job and his home.
CASE 3 Now let’s turn to Ms. S, who is convinced that she has a physical malady despite negative exams and tests. In truth, she may be depressed or anxious over her husband’s death. One way to address this is to confront the patient directly by suggesting that she has depression triggered by her husband’s death. But this strategy—if used too early—may feel like an accusation, make her angry, and jeopardize your relationship with her.
An alternative approach would be to say, “I think your problems are long-standing and could require a while to treat. Let’s see each other every 2 weeks for the next 2 months so we get adequate time to work on them.” This would be an example of structuring more frequent visits, while also validating the distressing nature of her symptoms.
These strategies are evidence-based
These techniques, while easily adaptable to primary care, are grounded in psychotherapy theory and are evidence-based. A seminal randomized controlled trial conducted more than 30 years ago showed that a patient-centered interview incorporating a number of these techniques bolstered physicians’ knowledge, interviewing skills, attitudes, and ability to manage patients with unexplained complaints.14
A multicenter study analyzed audio recordings of strategies used by primary care physicians to deny patient requests for a particular medication. It revealed that explanations based on patient perspectives were significantly more likely to result in excellent patient satisfaction than biomedical explanations or other explanatory approaches.15 Research has also shown that agenda-setting improves both patient and provider satisfaction.16
Some cases will still be frustrating, and some “difficult” patients will still need a psychiatric referral at some point—ideally, to a psychiatric or psychological consultant who collaborates closely with the primary care clinic.17,18
Family physicians sometimes worry that the communication techniques we’ve outlined cannot be incorporated into an already harried primary care visit. Many may think it’s better not ask at all than risk opening a Pandora’s box. We urge you to reconsider. Although the techniques we’ve outlined certainly require practice, they need not be time-consuming.19 By embracing this management approach, you can improve patient satisfaction while enhancing your own repertoire of doctoring skills.
CORRESPONDENCE
Alan R. Teo, MD, MS, 3710 SW US Veterans Hospital Road, Portland, OR 97239; [email protected]
ACKNOWLEDGEMENT
The authors thank Drs. Michael Fetters and Rod Hayward for their help in the development of the manuscript.
It’s a scenario all office-based physicians are familiar with: Your day has been going well, and all your appointments have been (relatively) on time. But when you scan your afternoon schedule your mood shifts from buoyant to crestfallen. The reason: Three patients whom you find particularly frustrating are slotted for the last appointments of the day.
CASE 1 Mr. E, age 44, a high-powered attorney, has chronic headache that he has complained off and on about for several years. He has no other past medical history. Physical examination strongly suggests that Mr. E suffers from tension-type headaches, but he continues to demand magnetic resonance imaging (MRI) of the brain. “I think there’s something wrong in there,” he has stated repeatedly. “If my last doctor, who is one of the best in the country, ordered an MRI for me, why can’t you?”
CASE 2 Mr. A is a 37-year-old man who lost his home in a hurricane 3 years ago. Although he sustained only minor physical injuries, Mr. A appears to have lost his sense of well-being. He has developed chronic—and debilitating— musculoskeletal pain in his neck and low back in the aftermath of the storm and has been unable to work since then.
At his last 2 visits, Mr. A requested increasing doses of oxycodone, insisting that nothing else alleviates the pain. When you suggested a nonopioid analgesic, he broke down in tears. “Nobody takes my injuries seriously! My insurance doesn’t want to compensate me for my losses. Now you don’t even believe I’m in pain.”
CASE 3 Ms. S is a 65-year-old socially isolated widow who lost her husband several years ago. She has a history of multiple somatic complaints, including fatigue, abdominal pain, back pain, joint pain, and dizziness. As a result, she has undergone numerous diagnostic procedures, including esophagastroduodenoscopy, colonoscopy, and various blood tests, all of which have been negative. Ms. S consistently requires longer than the usual 20-minute visit. When you try to end an appointment, she typically brings up new issues. “Two days ago, i had this pain by my belly button and left shoulder blade. i think that there must be something wrong with me. Can you examine me?” she asked toward the end of her last visit 6 weeks ago.
The burden of difficult patient encounters
Cases such as these are frustrating, not so much for their clinical complexity, but rather because of the elusiveness of satisfying doctor-patient interactions. Besides a litany of physical complaints, such patients typically present with anxiety, depression, and other psychiatric symptoms; express dissatisfaction with the care they are receiving; and repeatedly request tests and interventions that are not medically indicated.1
From a primary care perspective, such cases can be frustrating and time- consuming, significantly contribute to exhaustion and burnout, and result in unnecessary health care expenditures.1 (See “Unexplained complaints in primary care: Evidence of action bias” also in this issue to learn more.) Studies suggest that family physicians see such patients on a daily basis, and rate about one patient in 6 as a “difficult” case.2,3
Physician attitudes, training play a role
Research has established other critical spheres of influence that conspire to create difficult or frustrating patient encounters, including “system” factors (ie, reduced duration of visits and interrupted visits)4 and physician factors. In fact, physicians’ negative attitude toward psychosocial aspects of patient care may be a more potent factor in shaping difficult encounters than any patient characteristic.3,5
Consider the following statements:
- “Talking about psychosocial issues causes more trouble than it is worth.”
- “I do not focus on psychosocial problems until I have ruled out organic disease.”
- “I am too pressed for time to routinely investigate psychosocial issues.”
Such sentiments, which have been associated with difficult encounters, are part of the 32-item Physician Belief Scale, developed 30 years ago and still used to assess beliefs about psychosocial aspects of patient care held by primary care physicians.6
Lack of training is a potential problem, as well. In one survey, more than half of directors of family medicine programs agreed that training in mental health is inadequate.7Thus, family physicians often respond to patients like Mr. E, Mr. A, and Ms. S by becoming irritated or avoiding further interaction. A more appropriate response is for the physician to self-acknowledge his or her emotions, then to engage in an empathic interaction in keeping with patient expectations.4
As mental health treatment becomes more integrated within family medicine,8 pointers for handling difficult patient encounters can be gleaned from the traditional psychiatric approach to difficult or frustrating cases. Indeed, we believe that what is now known as a “patient-centered approach” is rooted in traditions and techniques that psychiatrists and psychologists have used for decades.9
Core principles for handling frustrating cases
A useful approach to the difficult patient encounter, detailed in the TABLE that we created, is based on 3 key principles:
- The doctor-patient relationship should be the target for change.
- The patient’s emotional experience should be an explicit focus of the clinical interaction.
- The patient’s perspective should guide the clinical encounter.
In our experience, when the interaction between patient and doctor shifts from searching for specific pathologies to building a collaborative relationship, previously recalcitrant symptoms often improve.
Use these communication techniques
There are 2 main ways to elicit a conversation about personal issues, including emotions, with patients. One is to directly ask patients to describe the distress they are experiencing and elicit the emotions connected to this distress. The other is to invite a discussion about emotional issues indirectly, by asking patients how the symptoms affect their lives and what they think is causing the problem or by selectively sharing an emotional experience of your own.
Once a patient has shared emotions, you will need to show support and empathy in order to build an alliance. There is more than one way to do this, and methods can be used alone or in combination, depending on the particular situation. (You’ll find examples in the TABLE.)
Name the affect. The simple act of naming the patient’s affect or emotional expression (eg, “You sound sad”) is surprisingly helpful, as it lets patients know they have been “heard.”
Validate. You can also validate patients like Mr. E, Mr. A, and Ms. S by stating that their emotional reactions are legitimate, praising them for how well they have coped with difficult symptoms, and acknowledging the seriousness and complexity of their situations.
Align. Once a patient expresses his or her interests and goals, aligning yourself with them (eg, “I want to do everything in my power to help you reduce your pain...”) will elicit hope and improve patient satisfaction.
And 2 mnemonic devices—detailed in the box 10,11—can help you improve the way you communicate with patients.
Communication can also be nonverbal, such as thoughtful nodding or a timely therapeutic silence. The former is characterized by slow, steady, and purposeful movement accompanied by eye contact; in the latter case, you simply resist the urge to immediately respond after a patient has revealed something emotionally laden, and wait a few seconds to take in what has been said.
NURS is a reminder to:
Name the patient’s emotion (“you say that these constant headaches really get on your nerves.”)
Understand (“i can see why you feel this way.”)
Respect (“you’ve been through a lot and that takes a lot of courage.”)
Support (“i want to help you get better.”)10
Background (“What has been going on in your life?”)
Affect (“how do you feel about that?”)
Trouble (“What troubles you the most about this situation?”)
Handling (“how are you handling this?”)
Empathy (“That must be difficult.”)11
How to provide therapeutic structure
Family physicians can further manage patient behaviors that they find bothersome by implementing changes in the way they organize and conduct patient visits. Studies of patients with complex somatic symptoms offer additional hints for the management of frustrating cases. The following strategies can lead to positive outcomes, including a decrease in disability and health care costs.12
Schedule regular brief visits. Mr. E, Mr. A, and Ms. S should have frequent and regular, but brief, appointments (eg, 15 minutes every 2 weeks for 2 months). Proactively schedule return visits, rather than waiting for the patient to call for an appointment PRN.11 Sharing this kind of plan gives such patients a concrete time line and clear evidence of support. Avoid the temptation to schedule difficult cases for the last time slot of the day, as going over the allotted time can insidiously give some patients the expectation of progressively longer visits.
Set the agenda. To prevent “doorknob questions” like Ms. S’s new symptoms, reported just as you’re about to leave the exam room, the agenda must be set at the outset of the visit. This can be done by asking, “What did you want to discuss today?”, “Is there anything else you want to address today?”, or “What else did you need taken care of?”13 Explicitly inquiring about patient expectations at the start of the visit lets patients like Ms. S know that they are being taken seriously. If the agenda still balloons, you can simply state, “You deserve more than 15 minutes for all these issues. Let’s pick the top 2 for today and tackle others at our next visit in 2 weeks.” To further save time, you can ask the patient to bring a symptom diary or written agenda to the appointment. We’ve found that many anxious patients benefit from this exercise.
Avoid the urge to act. When a patient suffers from unexplained symptoms, effective interventions require physicians to avoid certain “reflex” behaviors—repeatedly performing diagnostic tests, prescribing medications for symptoms of unknown etiology, insinuating that the problem is “in your head,” formulating ambiguous diagnoses, and repeating physical exams.12 Such physician behaviors tend to reinforce the pathology in patients with unexplained symptoms. The time saved avoiding these pitfalls is better invested in exploring personal issues and stressors.
The point is that such patients should be reassured via discussion, rather than with dubious diagnostic labels and potentially dangerous drugs. This approach has been shown to improve patients’ physical functioning while reducing medical expenditures.12
Into action with our 3 cases
CASE 1 Given these principles, how would you handle Mr. E, the patient who is demanding an MRI for a simple tension headache? Although placating him by ordering the test, providing a referral to a specialist, or defending your recommendation through medical reasoning may seem to be more intuitive (or a “quick fix”), these strategies often lead to excessive medical spending, transfer of the burden to a specialist colleague, and ongoing frustration and dissatisfaction on the part of the patient. In this case, validation may be a more useful approach.
“I can totally understand why you’re frustrated that we disagree,” you might say to Mr. E. “But you’re right! you definitely deserve the best care. That’s why I’m recommending against the MRI, as I feel that would be a suboptimal approach.”
Often patients like Mr. E will require repeated validation of their suffering and frustration. The key is to be persistent in validating their feelings without compromising your own principles in providing optimal medical care.
CASE 2 Let’s turn now to Mr. A, who is requesting escalating doses of opioids. Some physicians might write the prescription for the dose he’s insisting on, while others draw a hard boundary by refusing to prescribe above a certain dose or beyond a specific time frame. Both strategies may compromise optimal care or endanger the doctor-patient alliance. Another quick solution would be to provide a referral to a psychiatrist, without further discussion.
In cases like that of Mr. A, however, the patient’s demands are often a sign of more complicated emotions and dynamics below the surface. So you might respond by stating, “I’m sorry to hear that things haven’t been going well. How are you feeling about these things? How does the oxycodone help you? In what way doesn’t it help?”
It is important here to understand how the medication serves the patient—in addition to the ways it hurts him—in order for him to feel understood. Inviting Mr. A to have an open-ended discussion may allow him to reveal what is the real source of his distress—losing his job and his home.
CASE 3 Now let’s turn to Ms. S, who is convinced that she has a physical malady despite negative exams and tests. In truth, she may be depressed or anxious over her husband’s death. One way to address this is to confront the patient directly by suggesting that she has depression triggered by her husband’s death. But this strategy—if used too early—may feel like an accusation, make her angry, and jeopardize your relationship with her.
An alternative approach would be to say, “I think your problems are long-standing and could require a while to treat. Let’s see each other every 2 weeks for the next 2 months so we get adequate time to work on them.” This would be an example of structuring more frequent visits, while also validating the distressing nature of her symptoms.
These strategies are evidence-based
These techniques, while easily adaptable to primary care, are grounded in psychotherapy theory and are evidence-based. A seminal randomized controlled trial conducted more than 30 years ago showed that a patient-centered interview incorporating a number of these techniques bolstered physicians’ knowledge, interviewing skills, attitudes, and ability to manage patients with unexplained complaints.14
A multicenter study analyzed audio recordings of strategies used by primary care physicians to deny patient requests for a particular medication. It revealed that explanations based on patient perspectives were significantly more likely to result in excellent patient satisfaction than biomedical explanations or other explanatory approaches.15 Research has also shown that agenda-setting improves both patient and provider satisfaction.16
Some cases will still be frustrating, and some “difficult” patients will still need a psychiatric referral at some point—ideally, to a psychiatric or psychological consultant who collaborates closely with the primary care clinic.17,18
Family physicians sometimes worry that the communication techniques we’ve outlined cannot be incorporated into an already harried primary care visit. Many may think it’s better not ask at all than risk opening a Pandora’s box. We urge you to reconsider. Although the techniques we’ve outlined certainly require practice, they need not be time-consuming.19 By embracing this management approach, you can improve patient satisfaction while enhancing your own repertoire of doctoring skills.
CORRESPONDENCE
Alan R. Teo, MD, MS, 3710 SW US Veterans Hospital Road, Portland, OR 97239; [email protected]
ACKNOWLEDGEMENT
The authors thank Drs. Michael Fetters and Rod Hayward for their help in the development of the manuscript.
1. Hahn SR, Kroenke K, Spitzer RL, et al. The difficult patient: preva- lence, psychopathology, and functional impairment. J Gen Intern Med. 1996;11:1-8.
2. An PG, Rabatin JS, Manwell LB, et al. Burden of difficult encoun- ters in primary care: data From the minimizing error, maximizing outcomes study. Arch Intern Med. 2009;169:410-414.
3. Hinchey SA, Jackson JL. A cohort study assessing difficult patient encoun- ters in a walk-in primary care clinic, predictors and outcomes. J Gen Intern Med. 2011;26:588-594.
4. Haas LJ, Leiser JP, Magill MK, et al. Management of the difficult patient. Am Fam Physician. 2005;72:2063-2068.
5. Jackson JL, Kroenke K. Difficult patient encounters in the ambulatory clinic: clinical predictors and outcomes. Arch Intern Med. 1999;159:1069- 1075.
6. Ashworth CD, Williamson P, Montano D. A scale to measure physi- cian beliefs about psychosocial aspects of patient care. Soc Sci Med. 1984;19:1235-1238.
7. Leigh H, Stewart D, Mallios R. Mental health and psychiatry training in primary care residency programs. Gen Hosp Psychiatry. 2006;28:189-194.
8. Katon W, Unützer J. Collaborative care models for depression: time to move from evidence to practice. Arch Intern Med. 2006;166:2304-2306.
9. Groves JE. Taking care of the hateful patient. N Engl J Med. 1978;298:883- 887.
10. Fortin AH, Dwamena FC, Frankel RM, et al. Smith’s Patient-Centered Interviewing: An Evidence-Based Method. 3rd ed. New York, NY: McGraw-Hill; 2012.
11. Stuart MR, Lieberman JA. The Fifteen Minute Hour: Therapeutic Talk in Primary Care. 4th ed. Milton Keynes, UK: Radcliffe Publishing; 2008.
12. Smith GR Jr, Rost K, Kashner TM. A trial of the effect of a standardized psychiatric consultation on health outcomes and costs in somatizing patients. Arch Gen Psychiatry. 1995;52:238-243.
13. Baker LH, O’Connell D, Platt FW. “What else?” Setting the agenda for the clinical interview. Ann Intern Med. 2005;143:766 -770.
14. Smith RC, Lyles JS, Mettler J, et al. The effectiveness of intensive training for residents in interviewing. A randomized, controlled study. Ann Intern Med. 1998;128:118-126.
15. Paterniti DA, Fancher TL, Cipri CS, et al. Getting to “no”: strategies pri- mary care physicians use to deny patient requests. Arch Intern Med. 2010;170:381-388.
16. Kroenke K. Unburdening the difficult clinical encounter. Arch Intern Med. 2009;169:333-334.
17. Katon W, Unützer J, Wells K, et al. Collaborative depression care: history, evolution and ways to enhance dissemination and sustainability. Gen Hosp Psychiatry. 2010;32:456-464.
18. Williams M, Angstman K, Johnson I, et al. Implementation of a care man- agement model for depression at two primary care clinics. J Ambul Care Manage. 2011;34:163-173.
19. Lieberman JA III, Stuart MR. The BATHE method: incorporating counsel- ing and psychotherapy into the everyday management of patients. Prim Care Companion J Clin Psychiatry. 1999;1:35-38.
1. Hahn SR, Kroenke K, Spitzer RL, et al. The difficult patient: preva- lence, psychopathology, and functional impairment. J Gen Intern Med. 1996;11:1-8.
2. An PG, Rabatin JS, Manwell LB, et al. Burden of difficult encoun- ters in primary care: data From the minimizing error, maximizing outcomes study. Arch Intern Med. 2009;169:410-414.
3. Hinchey SA, Jackson JL. A cohort study assessing difficult patient encoun- ters in a walk-in primary care clinic, predictors and outcomes. J Gen Intern Med. 2011;26:588-594.
4. Haas LJ, Leiser JP, Magill MK, et al. Management of the difficult patient. Am Fam Physician. 2005;72:2063-2068.
5. Jackson JL, Kroenke K. Difficult patient encounters in the ambulatory clinic: clinical predictors and outcomes. Arch Intern Med. 1999;159:1069- 1075.
6. Ashworth CD, Williamson P, Montano D. A scale to measure physi- cian beliefs about psychosocial aspects of patient care. Soc Sci Med. 1984;19:1235-1238.
7. Leigh H, Stewart D, Mallios R. Mental health and psychiatry training in primary care residency programs. Gen Hosp Psychiatry. 2006;28:189-194.
8. Katon W, Unützer J. Collaborative care models for depression: time to move from evidence to practice. Arch Intern Med. 2006;166:2304-2306.
9. Groves JE. Taking care of the hateful patient. N Engl J Med. 1978;298:883- 887.
10. Fortin AH, Dwamena FC, Frankel RM, et al. Smith’s Patient-Centered Interviewing: An Evidence-Based Method. 3rd ed. New York, NY: McGraw-Hill; 2012.
11. Stuart MR, Lieberman JA. The Fifteen Minute Hour: Therapeutic Talk in Primary Care. 4th ed. Milton Keynes, UK: Radcliffe Publishing; 2008.
12. Smith GR Jr, Rost K, Kashner TM. A trial of the effect of a standardized psychiatric consultation on health outcomes and costs in somatizing patients. Arch Gen Psychiatry. 1995;52:238-243.
13. Baker LH, O’Connell D, Platt FW. “What else?” Setting the agenda for the clinical interview. Ann Intern Med. 2005;143:766 -770.
14. Smith RC, Lyles JS, Mettler J, et al. The effectiveness of intensive training for residents in interviewing. A randomized, controlled study. Ann Intern Med. 1998;128:118-126.
15. Paterniti DA, Fancher TL, Cipri CS, et al. Getting to “no”: strategies pri- mary care physicians use to deny patient requests. Arch Intern Med. 2010;170:381-388.
16. Kroenke K. Unburdening the difficult clinical encounter. Arch Intern Med. 2009;169:333-334.
17. Katon W, Unützer J, Wells K, et al. Collaborative depression care: history, evolution and ways to enhance dissemination and sustainability. Gen Hosp Psychiatry. 2010;32:456-464.
18. Williams M, Angstman K, Johnson I, et al. Implementation of a care man- agement model for depression at two primary care clinics. J Ambul Care Manage. 2011;34:163-173.
19. Lieberman JA III, Stuart MR. The BATHE method: incorporating counsel- ing and psychotherapy into the everyday management of patients. Prim Care Companion J Clin Psychiatry. 1999;1:35-38.
Unexplained complaints in primary care: Evidence of action bias
ABSTRACT
Purpose Primary care physicians sometimes encounter patients with clinical complaints that do not fit into a recognized diagnostic pattern. This study was undertaken to assess the way physicians respond to patients whose symptoms are unusual or unexplained—that is, what approach they take in the absence of a working hypothesis.
Methods We surveyed 130 primary care physicians affiliated with 3 academic centers in Israel, presenting 5 clinical vignettes describing patients who had unusual complaints, no clear diagnosis, and no apparent need for urgent care. We asked physicians to provide the most likely diagnosis for each case and to rate their level of confidence in that diagnosis;respondents were also asked to provide a management strategy for each case and their level of confidence in the chosen approach. Finally, we asked the physicians to estimate how many of their own patients have presentations similar to the individuals in the clinical vignettes.
Results Physicians proposed, on average, 22 diagnoses for each case. Most indicated that they would choose action (testing, consulting, sending the patient to the emergency department, or prescribing) rather than follow-up only (87% vs 13%; P<.01). Respondents’ confidence in the management approach they had chosen for all the cases was higher than their confidence in the diagnoses (5.6 vs 4.3, respectively, on a scale of1-10; P<.001). Physicians estimated that 10% to 20% of the patients they see in their practice have unusual or unexplained symptoms that are difficult to diagnose.
Conclusion Uncertain diagnosis is a regular challenge for primary care physicians. In such cases, we found that physicians prefer a workup to follow-up, an inclination consistent with“action bias.”
Physicians in primary care sometimes encounter patients with clinical complaints that do not fit into a recognized diagnostic pattern.1 There are varying reports of the prevalence of such cases, ranging from ≤10% when stringent definitions of medically unexplained symptoms are used2 to as high as 40% to 60% of visits.3,4 Unexplained complaints, which may or may not be related to psychiatric disorders, can significantly contribute to high consumption of health care resources.5 Uncertain diagnoses are associated with increased testing6 and false-positive results, which often lead to more tests and complications.7
When physicians face medically unexplained symptoms, their behavior often differs from the watchful waiting approach some recommend.6 This behavior has been attributed to various factors, such as fear of litigation, greater concern about omission than commission, and perception of patient expectations.5 A study involving young patients suggested bias toward intervention for common pediatric diagnoses.8 Using a similar design of physician responses to clinical vignettes,we sought to evaluate a potential bias toward action, such as testing or referral, for patients with unexplained medical complaints.
Methods
Over several months, 2 of us (AK, IG) identified 60 patients in our practices who had(1) unusual medical complaints, (2) no clear diagnosis, and (3) no apparent need for urgent care. After careful consideration, our team selected 5 cases that best fit the above criteria and reflected the widest spectrum of clinical presentations encountered in primary care settings. After removing identifying patient information, we wrote each case up as a clinical vignette, then presented all 5 cases to primary care physicians affiliated with 3 major academic centers. For each case, respondents were asked to provide:
- the most likely diagnosis and their level of confidence in that diagnosis (on a scale of 1 [no confidence] to 10 [complete confidence])
- a management strategy (testing, consulting with a specialist, referral to the emergency department [ED], prescribing medication, or follow-up only) and their level of confidence in that choice.
Physicians were asked to estimate the frequency of such cases in their practice, as well.
Preparation of the data (cleaning, sorting, and filtering) was carried out using JMPv9.0 (SAS Institute, Cary, NC), and analyses were conducted with SPSS v19.0 (IBM,Chicago, Ill). We used descriptive statistics to represent the data and chi-square and ANOVA to compare physicians’ decisions(action vs follow-up). Nonparametric tests were used to compare levels of confidence for diagnosis and management.
Results
We surveyed a convenience sample of 130 primary care physicians affiliated with academic medical centers, 100 of whom responded. Most respondents (62%) were female, and 86% were certified in family medicine. The average age was 45 years (range 30-68 years),with a mean time out of medical school of 17 years (range 1-26 years). Respondents were born in 14 different countries and had undergone medical training in Europe, the United States, or Israel. The diagnoses and management approaches selected for each clinical vignette are presented in TABLE 1. For each case, an average of 22 diagnoses (range 18-25) were proposed. Most physicians (87%; P<.01) indicated that they would choose some type of action (testing, consulting, sending the patient to the ED,or prescribing medication) rather than follow-up alone (TABLE 2). Respondents were able to choose multiple management There appears to be a stronger perceived need to “do something” than to engage in watchful waiting and follow-up.strategies. For all 5 cases, the physicians had more confidence in their patient management approach than in their diagnosis (5.6 vs 4.3;P<.001). On average, men had higher levels of confidence than women for both diagnosis and management (P<.05). Other demographic characteristics, including age, experience, certification, and site of training, were not predictive of confidence level. Respondents estimated that 10% to 20% of their own patients present with unusual and unexplained symptoms, like the patients in the clinical vignettes.
Discussion
Patients with undiagnosed signs and/or symptoms present a significant challenge in primary care. In such cases, physicians prefer a work-up to follow-up, with a confidence level in their management strategy that is higher than for their diagnostic hypotheses. There appears to be a stronger perceived need to “do something” than to engage in watchful waiting and follow-up.
Symptoms subside without treatment. Notably, in all the cases that formed the basis for the clinical vignettes used in our survey, the patients’ complaints eventually subsided, with no specific therapy. In some cases of unclear diagnosis, an active work-up may be justified; in others, watchful waiting before testing for unexplained complaints may be preferable.
Action bias. The preference for action over inaction in all the cases presented suggests what has been described as “action bias.”9 The term is derived from sports; in soccer penalty kicks, for example, it applies to goalkeepers who jump before they can see the kick direction and miss.10 According to the norm theory,11 such errors of commission derive from players’ perception that they are expected to act.10 Conversely, in instances in which inaction is the norm, an omission bias prevails, as people tend to judge acts that are harmful as worse than omissions that are even more harmful.10 In medicine, action bias has been found to influence clinical practice and contribute to overuse of both diagnostic testing and procedures.12-14
Gender difference. Gender has been shown to affect self-perception in cognitive bias.15 In a study of confidence levels among undergraduate students, overconfidence was found to be more prevalent among males than females, particularly for incorrect answers.16 This observation may relate to the gender differences in our study in physicians facing diagnostic uncertainty.
Study limitations. Our research was limited by the nature and type of our sample, but because the inclination to act was found in both immigrant and native practitioners, the observation of action bias could be generalizable to all primary care physicians. The clinical vignettes we chose may not be representative of commonly seen cases of medically unexplained symptoms. Also, our questionnaire was not tested beyond at face validity. It is possible, too, that nonresponders would be less inclined to action in the face of uncertainty. With the high (77%) response rate to our survey, however, their inclusion would be unlikely to strikingly alter the results.
Another limitation inherent to the design of our study is that physicians may respond to vignettes in a way that is substantially different than their response in actual practice. In a practice setting, physicians are able to listen to a full narrative and apply various doctor-patient communication tools, which are especially important in the context of unexplained complaints.17 On the other hand, the artificial setting may reduce the fear of litigation. Our observation of greater confidence in the need for action than for the diagnostic hypothesis is consistent with testing overuse in field studies.6 The fact that our survey went only to physicians affiliated with academic centers is another potential limitation, although it is not clear whether these clinicians differ from nonacademic physicians in their approach to unexplained complaints. Finally, the design of this study did not allow us to explore the reasons for action bias, a task that might be addressed in focus groups or interviews.
A closer look at bias. Our findings suggest a need for more in-depth research on potential biases that drive medical overuse, as part of an overall strategy to improve physicians’approach to medically unexplained symptoms.17 Remedies may require training, practice and failure feedback, quality improvement tools, and innovative management strategies.1,18 Uncertain diagnosis appears to be a frequent challenge in primary care settings. Inthe face of uncertainty, weighing the potential harms of overtesting vs follow-up and facilitating an informed decision-making process with the patient may lead to a reduction inaction bias,19 and thus, in the increased testing and higher health care consumption that often result.
CORRESPONDENCE
Mayer Brezis, MD, Center for Clinical Quality & Safety, Hadassah-Hebrew University Medical Center, 91120 Jerusalem, Israel; [email protected]
ACKNOWLEDGEMENT
The authors thank Steven R. Simon, MD, MPH, for his help with the preparation of this manuscript.
1. Nimnuan C, Hotopf M, Wessely S. Medically unexplained symptoms: an epidemiological study in seven specialities. J Psychosom Res. 2001;51:361-367.
2. Swanson LM, Hamilton JC, Feldman MD. Physician-based estimates of medically unexplained symptoms: a comparison of four case definitions. Fam Pract. 2010;27:487-493.
3. Thomas KB. Temporarily dependent patient in general practice. BMJ. 1974;1:625-626.
4. Jones R, Barraclough K, Dowrick C. When no diagnostic label is applied. BMJ. 2010;340:1302-1304.
5. Todd JW. Wasted resources. Investigations. Lancet. 1984;2:1146-1147.
6. van der Weijden T, van Velsen M, Dinant GJ, et al. Unexplained complaints in general practice: prevalence, patients’ expectations, and professionals’ test-ordering behavior. Med Decis Making. 2003;23:226-231.
7. Brody H. From an ethics of rationing to an ethics of waste avoidance. N Engl J Med. 2012;366:1949-1951.
8. Ayanian JZ, Berwick DM. Do physicians have a bias toward action? A classic study revisited. Med Decis Making. 1991;11:154-158.
9. Patt A, Zeckhauser R. Action bias and environmental decisions. J Risk Uncertain. 2000;21:45-72.
10. Bar-Eli M, Azar OH, Ritov I, et al. Action bias among elite soccer goalkeepers: the case of penalty kicks. J Econ Psychol. 2007;28:606-621.
11. Kahneman D, Miller DT. Norm theory: comparing reality to its alternatives. Psychol Rev. 1986;93:136-153.
12. Doust J, Del Mar C. Why do doctors use treatments that do not work? BMJ. 2004;328:474-475.
13. Scott IA. Errors in clinical reasoning: causes and remedial strategies. BMJ. 2009;338:22-25.
14. Cohain JS. Is action bias one of the numerous causes of ‘unnecaesareans’? Midwif Dig. 2009;19:495-499.
15. Beyer S, Bowden EM. Gender differences in self-perceptions: convergent evidence from three measures of accuracy and bias. Pers Soc Psychol Bull. 1997;23:157-172.
16. Lundeberg MA, Fox PW, Punccohar J. Highly confident but wrong: gender differences and similarities in confidence judgments. J Educ Psychol. 1994;86:114-121.
17. Heijmans M, Olde Hartman TC, van Weel-Baumgarten E, et al. Experts’ opinions on the management of medically unexplained symptoms in primary care. A qualitative analysis of narrative reviews and scientific editorials. Fam Pract. 2011;28:444-455.
18. Croskerry P, Norman G. Overconfidence in clinical decision making. Am J Med. 2008;121(5 suppl):S24-S29.
19. Feinstein AR. The ‘chagrin factor’ and qualitative decision analysis. Arch Intern Med. 1985;145:1257-1259.
Mayer Brezis; MD; MPH; action bias; unexplained complaints; management strategies
ABSTRACT
Purpose Primary care physicians sometimes encounter patients with clinical complaints that do not fit into a recognized diagnostic pattern. This study was undertaken to assess the way physicians respond to patients whose symptoms are unusual or unexplained—that is, what approach they take in the absence of a working hypothesis.
Methods We surveyed 130 primary care physicians affiliated with 3 academic centers in Israel, presenting 5 clinical vignettes describing patients who had unusual complaints, no clear diagnosis, and no apparent need for urgent care. We asked physicians to provide the most likely diagnosis for each case and to rate their level of confidence in that diagnosis;respondents were also asked to provide a management strategy for each case and their level of confidence in the chosen approach. Finally, we asked the physicians to estimate how many of their own patients have presentations similar to the individuals in the clinical vignettes.
Results Physicians proposed, on average, 22 diagnoses for each case. Most indicated that they would choose action (testing, consulting, sending the patient to the emergency department, or prescribing) rather than follow-up only (87% vs 13%; P<.01). Respondents’ confidence in the management approach they had chosen for all the cases was higher than their confidence in the diagnoses (5.6 vs 4.3, respectively, on a scale of1-10; P<.001). Physicians estimated that 10% to 20% of the patients they see in their practice have unusual or unexplained symptoms that are difficult to diagnose.
Conclusion Uncertain diagnosis is a regular challenge for primary care physicians. In such cases, we found that physicians prefer a workup to follow-up, an inclination consistent with“action bias.”
Physicians in primary care sometimes encounter patients with clinical complaints that do not fit into a recognized diagnostic pattern.1 There are varying reports of the prevalence of such cases, ranging from ≤10% when stringent definitions of medically unexplained symptoms are used2 to as high as 40% to 60% of visits.3,4 Unexplained complaints, which may or may not be related to psychiatric disorders, can significantly contribute to high consumption of health care resources.5 Uncertain diagnoses are associated with increased testing6 and false-positive results, which often lead to more tests and complications.7
When physicians face medically unexplained symptoms, their behavior often differs from the watchful waiting approach some recommend.6 This behavior has been attributed to various factors, such as fear of litigation, greater concern about omission than commission, and perception of patient expectations.5 A study involving young patients suggested bias toward intervention for common pediatric diagnoses.8 Using a similar design of physician responses to clinical vignettes,we sought to evaluate a potential bias toward action, such as testing or referral, for patients with unexplained medical complaints.
Methods
Over several months, 2 of us (AK, IG) identified 60 patients in our practices who had(1) unusual medical complaints, (2) no clear diagnosis, and (3) no apparent need for urgent care. After careful consideration, our team selected 5 cases that best fit the above criteria and reflected the widest spectrum of clinical presentations encountered in primary care settings. After removing identifying patient information, we wrote each case up as a clinical vignette, then presented all 5 cases to primary care physicians affiliated with 3 major academic centers. For each case, respondents were asked to provide:
- the most likely diagnosis and their level of confidence in that diagnosis (on a scale of 1 [no confidence] to 10 [complete confidence])
- a management strategy (testing, consulting with a specialist, referral to the emergency department [ED], prescribing medication, or follow-up only) and their level of confidence in that choice.
Physicians were asked to estimate the frequency of such cases in their practice, as well.
Preparation of the data (cleaning, sorting, and filtering) was carried out using JMPv9.0 (SAS Institute, Cary, NC), and analyses were conducted with SPSS v19.0 (IBM,Chicago, Ill). We used descriptive statistics to represent the data and chi-square and ANOVA to compare physicians’ decisions(action vs follow-up). Nonparametric tests were used to compare levels of confidence for diagnosis and management.
Results
We surveyed a convenience sample of 130 primary care physicians affiliated with academic medical centers, 100 of whom responded. Most respondents (62%) were female, and 86% were certified in family medicine. The average age was 45 years (range 30-68 years),with a mean time out of medical school of 17 years (range 1-26 years). Respondents were born in 14 different countries and had undergone medical training in Europe, the United States, or Israel. The diagnoses and management approaches selected for each clinical vignette are presented in TABLE 1. For each case, an average of 22 diagnoses (range 18-25) were proposed. Most physicians (87%; P<.01) indicated that they would choose some type of action (testing, consulting, sending the patient to the ED,or prescribing medication) rather than follow-up alone (TABLE 2). Respondents were able to choose multiple management There appears to be a stronger perceived need to “do something” than to engage in watchful waiting and follow-up.strategies. For all 5 cases, the physicians had more confidence in their patient management approach than in their diagnosis (5.6 vs 4.3;P<.001). On average, men had higher levels of confidence than women for both diagnosis and management (P<.05). Other demographic characteristics, including age, experience, certification, and site of training, were not predictive of confidence level. Respondents estimated that 10% to 20% of their own patients present with unusual and unexplained symptoms, like the patients in the clinical vignettes.
Discussion
Patients with undiagnosed signs and/or symptoms present a significant challenge in primary care. In such cases, physicians prefer a work-up to follow-up, with a confidence level in their management strategy that is higher than for their diagnostic hypotheses. There appears to be a stronger perceived need to “do something” than to engage in watchful waiting and follow-up.
Symptoms subside without treatment. Notably, in all the cases that formed the basis for the clinical vignettes used in our survey, the patients’ complaints eventually subsided, with no specific therapy. In some cases of unclear diagnosis, an active work-up may be justified; in others, watchful waiting before testing for unexplained complaints may be preferable.
Action bias. The preference for action over inaction in all the cases presented suggests what has been described as “action bias.”9 The term is derived from sports; in soccer penalty kicks, for example, it applies to goalkeepers who jump before they can see the kick direction and miss.10 According to the norm theory,11 such errors of commission derive from players’ perception that they are expected to act.10 Conversely, in instances in which inaction is the norm, an omission bias prevails, as people tend to judge acts that are harmful as worse than omissions that are even more harmful.10 In medicine, action bias has been found to influence clinical practice and contribute to overuse of both diagnostic testing and procedures.12-14
Gender difference. Gender has been shown to affect self-perception in cognitive bias.15 In a study of confidence levels among undergraduate students, overconfidence was found to be more prevalent among males than females, particularly for incorrect answers.16 This observation may relate to the gender differences in our study in physicians facing diagnostic uncertainty.
Study limitations. Our research was limited by the nature and type of our sample, but because the inclination to act was found in both immigrant and native practitioners, the observation of action bias could be generalizable to all primary care physicians. The clinical vignettes we chose may not be representative of commonly seen cases of medically unexplained symptoms. Also, our questionnaire was not tested beyond at face validity. It is possible, too, that nonresponders would be less inclined to action in the face of uncertainty. With the high (77%) response rate to our survey, however, their inclusion would be unlikely to strikingly alter the results.
Another limitation inherent to the design of our study is that physicians may respond to vignettes in a way that is substantially different than their response in actual practice. In a practice setting, physicians are able to listen to a full narrative and apply various doctor-patient communication tools, which are especially important in the context of unexplained complaints.17 On the other hand, the artificial setting may reduce the fear of litigation. Our observation of greater confidence in the need for action than for the diagnostic hypothesis is consistent with testing overuse in field studies.6 The fact that our survey went only to physicians affiliated with academic centers is another potential limitation, although it is not clear whether these clinicians differ from nonacademic physicians in their approach to unexplained complaints. Finally, the design of this study did not allow us to explore the reasons for action bias, a task that might be addressed in focus groups or interviews.
A closer look at bias. Our findings suggest a need for more in-depth research on potential biases that drive medical overuse, as part of an overall strategy to improve physicians’approach to medically unexplained symptoms.17 Remedies may require training, practice and failure feedback, quality improvement tools, and innovative management strategies.1,18 Uncertain diagnosis appears to be a frequent challenge in primary care settings. Inthe face of uncertainty, weighing the potential harms of overtesting vs follow-up and facilitating an informed decision-making process with the patient may lead to a reduction inaction bias,19 and thus, in the increased testing and higher health care consumption that often result.
CORRESPONDENCE
Mayer Brezis, MD, Center for Clinical Quality & Safety, Hadassah-Hebrew University Medical Center, 91120 Jerusalem, Israel; [email protected]
ACKNOWLEDGEMENT
The authors thank Steven R. Simon, MD, MPH, for his help with the preparation of this manuscript.
ABSTRACT
Purpose Primary care physicians sometimes encounter patients with clinical complaints that do not fit into a recognized diagnostic pattern. This study was undertaken to assess the way physicians respond to patients whose symptoms are unusual or unexplained—that is, what approach they take in the absence of a working hypothesis.
Methods We surveyed 130 primary care physicians affiliated with 3 academic centers in Israel, presenting 5 clinical vignettes describing patients who had unusual complaints, no clear diagnosis, and no apparent need for urgent care. We asked physicians to provide the most likely diagnosis for each case and to rate their level of confidence in that diagnosis;respondents were also asked to provide a management strategy for each case and their level of confidence in the chosen approach. Finally, we asked the physicians to estimate how many of their own patients have presentations similar to the individuals in the clinical vignettes.
Results Physicians proposed, on average, 22 diagnoses for each case. Most indicated that they would choose action (testing, consulting, sending the patient to the emergency department, or prescribing) rather than follow-up only (87% vs 13%; P<.01). Respondents’ confidence in the management approach they had chosen for all the cases was higher than their confidence in the diagnoses (5.6 vs 4.3, respectively, on a scale of1-10; P<.001). Physicians estimated that 10% to 20% of the patients they see in their practice have unusual or unexplained symptoms that are difficult to diagnose.
Conclusion Uncertain diagnosis is a regular challenge for primary care physicians. In such cases, we found that physicians prefer a workup to follow-up, an inclination consistent with“action bias.”
Physicians in primary care sometimes encounter patients with clinical complaints that do not fit into a recognized diagnostic pattern.1 There are varying reports of the prevalence of such cases, ranging from ≤10% when stringent definitions of medically unexplained symptoms are used2 to as high as 40% to 60% of visits.3,4 Unexplained complaints, which may or may not be related to psychiatric disorders, can significantly contribute to high consumption of health care resources.5 Uncertain diagnoses are associated with increased testing6 and false-positive results, which often lead to more tests and complications.7
When physicians face medically unexplained symptoms, their behavior often differs from the watchful waiting approach some recommend.6 This behavior has been attributed to various factors, such as fear of litigation, greater concern about omission than commission, and perception of patient expectations.5 A study involving young patients suggested bias toward intervention for common pediatric diagnoses.8 Using a similar design of physician responses to clinical vignettes,we sought to evaluate a potential bias toward action, such as testing or referral, for patients with unexplained medical complaints.
Methods
Over several months, 2 of us (AK, IG) identified 60 patients in our practices who had(1) unusual medical complaints, (2) no clear diagnosis, and (3) no apparent need for urgent care. After careful consideration, our team selected 5 cases that best fit the above criteria and reflected the widest spectrum of clinical presentations encountered in primary care settings. After removing identifying patient information, we wrote each case up as a clinical vignette, then presented all 5 cases to primary care physicians affiliated with 3 major academic centers. For each case, respondents were asked to provide:
- the most likely diagnosis and their level of confidence in that diagnosis (on a scale of 1 [no confidence] to 10 [complete confidence])
- a management strategy (testing, consulting with a specialist, referral to the emergency department [ED], prescribing medication, or follow-up only) and their level of confidence in that choice.
Physicians were asked to estimate the frequency of such cases in their practice, as well.
Preparation of the data (cleaning, sorting, and filtering) was carried out using JMPv9.0 (SAS Institute, Cary, NC), and analyses were conducted with SPSS v19.0 (IBM,Chicago, Ill). We used descriptive statistics to represent the data and chi-square and ANOVA to compare physicians’ decisions(action vs follow-up). Nonparametric tests were used to compare levels of confidence for diagnosis and management.
Results
We surveyed a convenience sample of 130 primary care physicians affiliated with academic medical centers, 100 of whom responded. Most respondents (62%) were female, and 86% were certified in family medicine. The average age was 45 years (range 30-68 years),with a mean time out of medical school of 17 years (range 1-26 years). Respondents were born in 14 different countries and had undergone medical training in Europe, the United States, or Israel. The diagnoses and management approaches selected for each clinical vignette are presented in TABLE 1. For each case, an average of 22 diagnoses (range 18-25) were proposed. Most physicians (87%; P<.01) indicated that they would choose some type of action (testing, consulting, sending the patient to the ED,or prescribing medication) rather than follow-up alone (TABLE 2). Respondents were able to choose multiple management There appears to be a stronger perceived need to “do something” than to engage in watchful waiting and follow-up.strategies. For all 5 cases, the physicians had more confidence in their patient management approach than in their diagnosis (5.6 vs 4.3;P<.001). On average, men had higher levels of confidence than women for both diagnosis and management (P<.05). Other demographic characteristics, including age, experience, certification, and site of training, were not predictive of confidence level. Respondents estimated that 10% to 20% of their own patients present with unusual and unexplained symptoms, like the patients in the clinical vignettes.
Discussion
Patients with undiagnosed signs and/or symptoms present a significant challenge in primary care. In such cases, physicians prefer a work-up to follow-up, with a confidence level in their management strategy that is higher than for their diagnostic hypotheses. There appears to be a stronger perceived need to “do something” than to engage in watchful waiting and follow-up.
Symptoms subside without treatment. Notably, in all the cases that formed the basis for the clinical vignettes used in our survey, the patients’ complaints eventually subsided, with no specific therapy. In some cases of unclear diagnosis, an active work-up may be justified; in others, watchful waiting before testing for unexplained complaints may be preferable.
Action bias. The preference for action over inaction in all the cases presented suggests what has been described as “action bias.”9 The term is derived from sports; in soccer penalty kicks, for example, it applies to goalkeepers who jump before they can see the kick direction and miss.10 According to the norm theory,11 such errors of commission derive from players’ perception that they are expected to act.10 Conversely, in instances in which inaction is the norm, an omission bias prevails, as people tend to judge acts that are harmful as worse than omissions that are even more harmful.10 In medicine, action bias has been found to influence clinical practice and contribute to overuse of both diagnostic testing and procedures.12-14
Gender difference. Gender has been shown to affect self-perception in cognitive bias.15 In a study of confidence levels among undergraduate students, overconfidence was found to be more prevalent among males than females, particularly for incorrect answers.16 This observation may relate to the gender differences in our study in physicians facing diagnostic uncertainty.
Study limitations. Our research was limited by the nature and type of our sample, but because the inclination to act was found in both immigrant and native practitioners, the observation of action bias could be generalizable to all primary care physicians. The clinical vignettes we chose may not be representative of commonly seen cases of medically unexplained symptoms. Also, our questionnaire was not tested beyond at face validity. It is possible, too, that nonresponders would be less inclined to action in the face of uncertainty. With the high (77%) response rate to our survey, however, their inclusion would be unlikely to strikingly alter the results.
Another limitation inherent to the design of our study is that physicians may respond to vignettes in a way that is substantially different than their response in actual practice. In a practice setting, physicians are able to listen to a full narrative and apply various doctor-patient communication tools, which are especially important in the context of unexplained complaints.17 On the other hand, the artificial setting may reduce the fear of litigation. Our observation of greater confidence in the need for action than for the diagnostic hypothesis is consistent with testing overuse in field studies.6 The fact that our survey went only to physicians affiliated with academic centers is another potential limitation, although it is not clear whether these clinicians differ from nonacademic physicians in their approach to unexplained complaints. Finally, the design of this study did not allow us to explore the reasons for action bias, a task that might be addressed in focus groups or interviews.
A closer look at bias. Our findings suggest a need for more in-depth research on potential biases that drive medical overuse, as part of an overall strategy to improve physicians’approach to medically unexplained symptoms.17 Remedies may require training, practice and failure feedback, quality improvement tools, and innovative management strategies.1,18 Uncertain diagnosis appears to be a frequent challenge in primary care settings. Inthe face of uncertainty, weighing the potential harms of overtesting vs follow-up and facilitating an informed decision-making process with the patient may lead to a reduction inaction bias,19 and thus, in the increased testing and higher health care consumption that often result.
CORRESPONDENCE
Mayer Brezis, MD, Center for Clinical Quality & Safety, Hadassah-Hebrew University Medical Center, 91120 Jerusalem, Israel; [email protected]
ACKNOWLEDGEMENT
The authors thank Steven R. Simon, MD, MPH, for his help with the preparation of this manuscript.
1. Nimnuan C, Hotopf M, Wessely S. Medically unexplained symptoms: an epidemiological study in seven specialities. J Psychosom Res. 2001;51:361-367.
2. Swanson LM, Hamilton JC, Feldman MD. Physician-based estimates of medically unexplained symptoms: a comparison of four case definitions. Fam Pract. 2010;27:487-493.
3. Thomas KB. Temporarily dependent patient in general practice. BMJ. 1974;1:625-626.
4. Jones R, Barraclough K, Dowrick C. When no diagnostic label is applied. BMJ. 2010;340:1302-1304.
5. Todd JW. Wasted resources. Investigations. Lancet. 1984;2:1146-1147.
6. van der Weijden T, van Velsen M, Dinant GJ, et al. Unexplained complaints in general practice: prevalence, patients’ expectations, and professionals’ test-ordering behavior. Med Decis Making. 2003;23:226-231.
7. Brody H. From an ethics of rationing to an ethics of waste avoidance. N Engl J Med. 2012;366:1949-1951.
8. Ayanian JZ, Berwick DM. Do physicians have a bias toward action? A classic study revisited. Med Decis Making. 1991;11:154-158.
9. Patt A, Zeckhauser R. Action bias and environmental decisions. J Risk Uncertain. 2000;21:45-72.
10. Bar-Eli M, Azar OH, Ritov I, et al. Action bias among elite soccer goalkeepers: the case of penalty kicks. J Econ Psychol. 2007;28:606-621.
11. Kahneman D, Miller DT. Norm theory: comparing reality to its alternatives. Psychol Rev. 1986;93:136-153.
12. Doust J, Del Mar C. Why do doctors use treatments that do not work? BMJ. 2004;328:474-475.
13. Scott IA. Errors in clinical reasoning: causes and remedial strategies. BMJ. 2009;338:22-25.
14. Cohain JS. Is action bias one of the numerous causes of ‘unnecaesareans’? Midwif Dig. 2009;19:495-499.
15. Beyer S, Bowden EM. Gender differences in self-perceptions: convergent evidence from three measures of accuracy and bias. Pers Soc Psychol Bull. 1997;23:157-172.
16. Lundeberg MA, Fox PW, Punccohar J. Highly confident but wrong: gender differences and similarities in confidence judgments. J Educ Psychol. 1994;86:114-121.
17. Heijmans M, Olde Hartman TC, van Weel-Baumgarten E, et al. Experts’ opinions on the management of medically unexplained symptoms in primary care. A qualitative analysis of narrative reviews and scientific editorials. Fam Pract. 2011;28:444-455.
18. Croskerry P, Norman G. Overconfidence in clinical decision making. Am J Med. 2008;121(5 suppl):S24-S29.
19. Feinstein AR. The ‘chagrin factor’ and qualitative decision analysis. Arch Intern Med. 1985;145:1257-1259.
1. Nimnuan C, Hotopf M, Wessely S. Medically unexplained symptoms: an epidemiological study in seven specialities. J Psychosom Res. 2001;51:361-367.
2. Swanson LM, Hamilton JC, Feldman MD. Physician-based estimates of medically unexplained symptoms: a comparison of four case definitions. Fam Pract. 2010;27:487-493.
3. Thomas KB. Temporarily dependent patient in general practice. BMJ. 1974;1:625-626.
4. Jones R, Barraclough K, Dowrick C. When no diagnostic label is applied. BMJ. 2010;340:1302-1304.
5. Todd JW. Wasted resources. Investigations. Lancet. 1984;2:1146-1147.
6. van der Weijden T, van Velsen M, Dinant GJ, et al. Unexplained complaints in general practice: prevalence, patients’ expectations, and professionals’ test-ordering behavior. Med Decis Making. 2003;23:226-231.
7. Brody H. From an ethics of rationing to an ethics of waste avoidance. N Engl J Med. 2012;366:1949-1951.
8. Ayanian JZ, Berwick DM. Do physicians have a bias toward action? A classic study revisited. Med Decis Making. 1991;11:154-158.
9. Patt A, Zeckhauser R. Action bias and environmental decisions. J Risk Uncertain. 2000;21:45-72.
10. Bar-Eli M, Azar OH, Ritov I, et al. Action bias among elite soccer goalkeepers: the case of penalty kicks. J Econ Psychol. 2007;28:606-621.
11. Kahneman D, Miller DT. Norm theory: comparing reality to its alternatives. Psychol Rev. 1986;93:136-153.
12. Doust J, Del Mar C. Why do doctors use treatments that do not work? BMJ. 2004;328:474-475.
13. Scott IA. Errors in clinical reasoning: causes and remedial strategies. BMJ. 2009;338:22-25.
14. Cohain JS. Is action bias one of the numerous causes of ‘unnecaesareans’? Midwif Dig. 2009;19:495-499.
15. Beyer S, Bowden EM. Gender differences in self-perceptions: convergent evidence from three measures of accuracy and bias. Pers Soc Psychol Bull. 1997;23:157-172.
16. Lundeberg MA, Fox PW, Punccohar J. Highly confident but wrong: gender differences and similarities in confidence judgments. J Educ Psychol. 1994;86:114-121.
17. Heijmans M, Olde Hartman TC, van Weel-Baumgarten E, et al. Experts’ opinions on the management of medically unexplained symptoms in primary care. A qualitative analysis of narrative reviews and scientific editorials. Fam Pract. 2011;28:444-455.
18. Croskerry P, Norman G. Overconfidence in clinical decision making. Am J Med. 2008;121(5 suppl):S24-S29.
19. Feinstein AR. The ‘chagrin factor’ and qualitative decision analysis. Arch Intern Med. 1985;145:1257-1259.
Mayer Brezis; MD; MPH; action bias; unexplained complaints; management strategies
Mayer Brezis; MD; MPH; action bias; unexplained complaints; management strategies
The Luddite cardiologist vs. the handheld ultrasound
A recent report at the American College of Cardiology annual meeting concluded that handheld ultrasound was superior to a group of trained cardiologists in the determination of cardiac pathology and function (J. Am. Coll. Cardiol. 2013;61:E1442).
This sort of information immediately raised my hackles. I am all for technological advances, but this proclamation struck close to my heart. I have progressed from cardiac catheterization using "red glass" accommodation for fluoroscopy to high-intensity multiplane angiography. I have even participated in the development of biventricular pacemakers for the treatment of heart failure and defibrillation. But the suggestion that a cardiologist can be replaced by a toy activated my Luddite receptors.
For those of you who are unaware of who or what a Luddite is, I refer you to England in the year 1811, when Edward (Ned) Ludd protested the replacement of hand-loom workers with a mechanized knitting process that threw thousands of English weavers out of work. He started a protest movement by Luddites that led to attacks on weaving mills and ultimately the hanging of some of his followers as terrorists in 1817. At about that time, in 1816, Rene Laennec developed the stethoscope by using a "quire" of paper rolled into a cylinder to listen to an obese young lady’s heart rather than his naked ear placed on her chest, which was the practice at that time.
From that paper tube has evolved the stethoscope as we know it today. Occasionally it is used to listen to the heart and lungs of patients, but it is seen mostly as a professional "necktie" in TV dramas. The fact that cardiologists and the stethoscope were to be replaced by the handheld ultrasound just as the loom weavers were replaced by the knitting machine led me to respond to the challenge.
I believe that the author, Dr. Manish Mehta of Oregon Health and Science University, Portland, spoke to an important issue. I would agree that many cardiologists are more comfortable using an echocardiogram than a stethoscope. The value of auscultation skills can be judged by the fact that cardiology board examinations do not include testing of auscultation skills but provide numerous questions on the interpretation of echocardiograms. Of course, there are the economic benefits of performing an echocardiogram compared with auscultation, which does not come up on my charge sheet.
I would grant that a handheld ultrasound can identify whether a pericardial effusion is present, a physical diagnostic challenge that I have frequently failed, particularly in thick-chested individuals. But give me a thin, young guy and I’ll get it every time. But does the presence of a leaking or stenotic valve or an enlarged heart, both easily identified by the handheld ultrasound, indicate heart failure? Give me an S3 gallop or distended neck veins and I can make the diagnosis of heart failure without a B-type natriuretic peptide level. The problem is that no one – well, very few of us – teaches how to examine the heart.
There is also the importance of the physician actually touching the patient as part of the examination. Not only examining the heart; but how about the abdomen? If we followed the path led by the BS echo, we could take the nurse’s recorded chief complaint and send the patient directly to radiology for a CT or an MRI. The fact that this is what the patient really wants does not escape this skeptic. But is this what medicine is really about? Much has been written about physician-patient interaction, but has it come to doctors being only a triage to the radiology department?
I am not going to break up the nearest echo machine with my stethoscope and end up on the hospital director’s "scaffolds" like the 18th century Luddites did. Echocardiography clearly provides a wonderful view of the heart and its valves, and can guide us to the surgical correction of valvular and muscular defects. Some technology, such as Doppler imaging, actually does provide information about physiologic phenomena including myocardial function and ischemia. But if the cardiologists lose in a contest with handheld ultrasound, it is because we have lost our bedside skills as a result of our overreliance on technology and have been blinded to its limitations.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
A recent report at the American College of Cardiology annual meeting concluded that handheld ultrasound was superior to a group of trained cardiologists in the determination of cardiac pathology and function (J. Am. Coll. Cardiol. 2013;61:E1442).
This sort of information immediately raised my hackles. I am all for technological advances, but this proclamation struck close to my heart. I have progressed from cardiac catheterization using "red glass" accommodation for fluoroscopy to high-intensity multiplane angiography. I have even participated in the development of biventricular pacemakers for the treatment of heart failure and defibrillation. But the suggestion that a cardiologist can be replaced by a toy activated my Luddite receptors.
For those of you who are unaware of who or what a Luddite is, I refer you to England in the year 1811, when Edward (Ned) Ludd protested the replacement of hand-loom workers with a mechanized knitting process that threw thousands of English weavers out of work. He started a protest movement by Luddites that led to attacks on weaving mills and ultimately the hanging of some of his followers as terrorists in 1817. At about that time, in 1816, Rene Laennec developed the stethoscope by using a "quire" of paper rolled into a cylinder to listen to an obese young lady’s heart rather than his naked ear placed on her chest, which was the practice at that time.
From that paper tube has evolved the stethoscope as we know it today. Occasionally it is used to listen to the heart and lungs of patients, but it is seen mostly as a professional "necktie" in TV dramas. The fact that cardiologists and the stethoscope were to be replaced by the handheld ultrasound just as the loom weavers were replaced by the knitting machine led me to respond to the challenge.
I believe that the author, Dr. Manish Mehta of Oregon Health and Science University, Portland, spoke to an important issue. I would agree that many cardiologists are more comfortable using an echocardiogram than a stethoscope. The value of auscultation skills can be judged by the fact that cardiology board examinations do not include testing of auscultation skills but provide numerous questions on the interpretation of echocardiograms. Of course, there are the economic benefits of performing an echocardiogram compared with auscultation, which does not come up on my charge sheet.
I would grant that a handheld ultrasound can identify whether a pericardial effusion is present, a physical diagnostic challenge that I have frequently failed, particularly in thick-chested individuals. But give me a thin, young guy and I’ll get it every time. But does the presence of a leaking or stenotic valve or an enlarged heart, both easily identified by the handheld ultrasound, indicate heart failure? Give me an S3 gallop or distended neck veins and I can make the diagnosis of heart failure without a B-type natriuretic peptide level. The problem is that no one – well, very few of us – teaches how to examine the heart.
There is also the importance of the physician actually touching the patient as part of the examination. Not only examining the heart; but how about the abdomen? If we followed the path led by the BS echo, we could take the nurse’s recorded chief complaint and send the patient directly to radiology for a CT or an MRI. The fact that this is what the patient really wants does not escape this skeptic. But is this what medicine is really about? Much has been written about physician-patient interaction, but has it come to doctors being only a triage to the radiology department?
I am not going to break up the nearest echo machine with my stethoscope and end up on the hospital director’s "scaffolds" like the 18th century Luddites did. Echocardiography clearly provides a wonderful view of the heart and its valves, and can guide us to the surgical correction of valvular and muscular defects. Some technology, such as Doppler imaging, actually does provide information about physiologic phenomena including myocardial function and ischemia. But if the cardiologists lose in a contest with handheld ultrasound, it is because we have lost our bedside skills as a result of our overreliance on technology and have been blinded to its limitations.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
A recent report at the American College of Cardiology annual meeting concluded that handheld ultrasound was superior to a group of trained cardiologists in the determination of cardiac pathology and function (J. Am. Coll. Cardiol. 2013;61:E1442).
This sort of information immediately raised my hackles. I am all for technological advances, but this proclamation struck close to my heart. I have progressed from cardiac catheterization using "red glass" accommodation for fluoroscopy to high-intensity multiplane angiography. I have even participated in the development of biventricular pacemakers for the treatment of heart failure and defibrillation. But the suggestion that a cardiologist can be replaced by a toy activated my Luddite receptors.
For those of you who are unaware of who or what a Luddite is, I refer you to England in the year 1811, when Edward (Ned) Ludd protested the replacement of hand-loom workers with a mechanized knitting process that threw thousands of English weavers out of work. He started a protest movement by Luddites that led to attacks on weaving mills and ultimately the hanging of some of his followers as terrorists in 1817. At about that time, in 1816, Rene Laennec developed the stethoscope by using a "quire" of paper rolled into a cylinder to listen to an obese young lady’s heart rather than his naked ear placed on her chest, which was the practice at that time.
From that paper tube has evolved the stethoscope as we know it today. Occasionally it is used to listen to the heart and lungs of patients, but it is seen mostly as a professional "necktie" in TV dramas. The fact that cardiologists and the stethoscope were to be replaced by the handheld ultrasound just as the loom weavers were replaced by the knitting machine led me to respond to the challenge.
I believe that the author, Dr. Manish Mehta of Oregon Health and Science University, Portland, spoke to an important issue. I would agree that many cardiologists are more comfortable using an echocardiogram than a stethoscope. The value of auscultation skills can be judged by the fact that cardiology board examinations do not include testing of auscultation skills but provide numerous questions on the interpretation of echocardiograms. Of course, there are the economic benefits of performing an echocardiogram compared with auscultation, which does not come up on my charge sheet.
I would grant that a handheld ultrasound can identify whether a pericardial effusion is present, a physical diagnostic challenge that I have frequently failed, particularly in thick-chested individuals. But give me a thin, young guy and I’ll get it every time. But does the presence of a leaking or stenotic valve or an enlarged heart, both easily identified by the handheld ultrasound, indicate heart failure? Give me an S3 gallop or distended neck veins and I can make the diagnosis of heart failure without a B-type natriuretic peptide level. The problem is that no one – well, very few of us – teaches how to examine the heart.
There is also the importance of the physician actually touching the patient as part of the examination. Not only examining the heart; but how about the abdomen? If we followed the path led by the BS echo, we could take the nurse’s recorded chief complaint and send the patient directly to radiology for a CT or an MRI. The fact that this is what the patient really wants does not escape this skeptic. But is this what medicine is really about? Much has been written about physician-patient interaction, but has it come to doctors being only a triage to the radiology department?
I am not going to break up the nearest echo machine with my stethoscope and end up on the hospital director’s "scaffolds" like the 18th century Luddites did. Echocardiography clearly provides a wonderful view of the heart and its valves, and can guide us to the surgical correction of valvular and muscular defects. Some technology, such as Doppler imaging, actually does provide information about physiologic phenomena including myocardial function and ischemia. But if the cardiologists lose in a contest with handheld ultrasound, it is because we have lost our bedside skills as a result of our overreliance on technology and have been blinded to its limitations.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Hospital Mortality Measure for COPD
Chronic obstructive pulmonary disease (COPD) affects as many as 24 million individuals in the United States, is responsible for more than 700,000 annual hospital admissions, and is currently the nation's third leading cause of death, accounting for nearly $49.9 billion in medical spending in 2010.[1, 2] Reported in‐hospital mortality rates for patients hospitalized for exacerbations of COPD range from 2% to 5%.[3, 4, 5, 6, 7] Information about 30‐day mortality rates following hospitalization for COPD is more limited; however, international studies suggest that rates range from 3% to 9%,[8, 9] and 90‐day mortality rates exceed 15%.[10]
Despite this significant clinical and economic impact, there have been no large‐scale, sustained efforts to measure the quality or outcomes of hospital care for patients with COPD in the United States. What little is known about the treatment of patients with COPD suggests widespread opportunities to increase adherence to guideline‐recommended therapies, to reduce the use of ineffective treatments and tests, and to address variation in care across institutions.[5, 11, 12]
Public reporting of hospital performance is a key strategy for improving the quality and safety of hospital care, both in the United States and internationally.[13] Since 2007, the Centers for Medicare and Medicaid Services (CMS) has reported hospital mortality rates on the Hospital Compare Web site, and COPD is 1 of the conditions highlighted in the Affordable Care Act for future consideration.[14] Such initiatives rely on validated, risk‐adjusted performance measures for comparisons across institutions and to enable outcomes to be tracked over time. We present the development, validation, and results of a model intended for public reporting of risk‐standardized mortality rates for patients hospitalized with exacerbations of COPD that has been endorsed by the National Quality Forum.[15]
METHODS
Approach to Measure Development
We developed this measure in accordance with guidelines described by the National Quality Forum,[16] CMS' Measure Management System,[17] and the American Heart Association scientific statement, Standards for Statistical Models Used for Public Reporting of Health Outcomes.[18] Throughout the process we obtained expert clinical and stakeholder input through meetings with a clinical advisory group and a national technical expert panel (see Acknowledgments). Last, we presented the proposed measure specifications and a summary of the technical expert panel discussions online and made a widely distributed call for public comments. We took the comments into consideration during the final stages of measure development (available at
Data Sources
We used claims data from Medicare inpatient, outpatient, and carrier (physician) Standard Analytic Files from 2008 to develop and validate the model, and examined model reliability using data from 2007 and 2009. The Medicare enrollment database was used to determine Medicare Fee‐for‐Service enrollment and mortality.
Study Cohort
Admissions were considered eligible for inclusion if the patient was 65 years or older, was admitted to a nonfederal acute care hospital in the United States, and had a principal diagnosis of COPD or a principal diagnosis of acute respiratory failure or respiratory arrest when paired with a secondary diagnosis of COPD with exacerbation (Table 1).
| ICD‐9‐CM | Description |
|---|---|
| |
| 491.21 | Obstructive chronic bronchitis; with (acute) exacerbation; acute exacerbation of COPD, decompensated COPD, decompensated COPD with exacerbation |
| 491.22 | Obstructive chronic bronchitis; with acute bronchitis |
| 491.8 | Other chronic bronchitis; chronic: tracheitis, tracheobronchitis |
| 491.9 | Unspecified chronic bronchitis |
| 492.8 | Other emphysema; emphysema (lung or pulmonary): NOS, centriacinar, centrilobular, obstructive, panacinar, panlobular, unilateral, vesicular; MacLeod's syndrome; Swyer‐James syndrome; unilateral hyperlucent lung |
| 493.20 | Chronic obstructive asthma; asthma with COPD, chronic asthmatic bronchitis, unspecified |
| 493.21 | Chronic obstructive asthma; asthma with COPD, chronic asthmatic bronchitis, with status asthmaticus |
| 493.22 | Chronic obstructive asthma; asthma with COPD, chronic asthmatic bronchitis, with (acute) exacerbation |
| 496 | Chronic: nonspecific lung disease, obstructive lung disease, obstructive pulmonary disease (COPD) NOS. (Note: This code is not to be used with any code from categories 491493.) |
| 518.81a | Other diseases of lung; acute respiratory failure; respiratory failure NOS |
| 518.82a | Other diseases of lung; acute respiratory failure; other pulmonary insufficiency, acute respiratory distress |
| 518.84a | Other diseases of lung; acute respiratory failure; acute and chronic respiratory failure |
| 799.1a | Other ill‐defined and unknown causes of morbidity and mortality; respiratory arrest, cardiorespiratory failure |
If a patient was discharged and readmitted to a second hospital on the same or the next day, we combined the 2 acute care admissions into a single episode of care and assigned the mortality outcome to the first admitting hospital. We excluded admissions for patients who were enrolled in Medicare Hospice in the 12 months prior to or on the first day of the index hospitalization. An index admission was any eligible admission assessed in the measure for the outcome. We also excluded admissions for patients who were discharged against medical advice, those for whom vital status at 30 days was unknown or recorded inconsistently, and patients with unreliable data (eg, age >115 years). For patients with multiple hospitalizations during a single year, we randomly selected 1 admission per patient to avoid survival bias. Finally, to assure adequate risk adjustment we limited the analysis to patients who had continuous enrollment in Medicare Fee‐for‐Service Parts A and B for the 12 months prior to their index admission so that we could identify comorbid conditions coded during all prior encounters.
Outcomes
The outcome of 30‐day mortality was defined as death from any cause within 30 days of the admission date for the index hospitalization. Mortality was assessed at 30 days to standardize the period of outcome ascertainment,[19] and because 30 days is a clinically meaningful time frame, during which differences in the quality of hospital care may be revealed.
Risk‐Adjustment Variables
We randomly selected half of all COPD admissions in 2008 that met the inclusion and exclusion criteria to create a model development sample. Candidate variables for inclusion in the risk‐standardized model were selected by a clinician team from diagnostic groups included in the Hierarchical Condition Category clinical classification system[20] and included age and comorbid conditions. Sleep apnea (International Classification of Diseases, 9th Revision, Clinical Modification [ICD‐9‐CM] condition codes 327.20, 327.21, 327.23, 327.27, 327.29, 780.51, 780.53, and 780.57) and mechanical ventilation (ICD‐9‐CM procedure codes 93.90, 96.70, 96.71, and 96.72) were also included as candidate variables.
We defined a condition as present for a given patient if it was coded in the inpatient, outpatient, or physician claims data sources in the preceding 12 months, including the index admission. Because a subset of the condition category variables can represent a complication of care, we did not consider them to be risk factors if they appeared only as secondary diagnosis codes for the index admission and not in claims submitted during the prior year.
We selected final variables for inclusion in the risk‐standardized model based on clinical considerations and a modified approach to stepwise logistic regression. The final patient‐level risk‐adjustment model included 42 variables (Table 2).
| Variable | Development Sample (150,035 Admissions at 4537 Hospitals) | Validation Sample (149,646 Admissions at 4535 Hospitals) | ||||
|---|---|---|---|---|---|---|
| Frequency, % | OR | 95% CI | Frequency, % | OR | 95% CI | |
| ||||||
| Demographics | ||||||
| Age 65 years (continuous) | 1.03 | 1.03‐1.04 | 1.03 | 1.03‐1.04 | ||
| Cardiovascular/respiratory | ||||||
| Sleep apnea (ICD‐9‐CM: 327.20, 327.21, 327.23, 327.27, 327.29, 780.51, 780.53, 780.57)a | 9.57 | 0.87 | 0.81‐0.94 | 9.72 | 0.84 | 0.78‐0.90 |
| History of mechanical ventilation (ICD‐9‐CM: 93.90, 96.70, 96.71, 96.72)a | 6.00 | 1.19 | 1.11‐1.27 | 6.00 | 1.15 | 1.08‐1.24 |
| Respirator dependence/respiratory failure (CC 7778)a | 1.15 | 0.89 | 0.77‐1.02 | 1.20 | 0.78 | 0.68‐0.91 |
| Cardiorespiratory failure and shock (CC 79) | 26.35 | 1.60 | 1.53‐1.68 | 26.34 | 1.59 | 1.52‐1.66 |
| Congestive heart failure (CC 80) | 41.50 | 1.34 | 1.28‐1.39 | 41.39 | 1.31 | 1.25‐1.36 |
| Chronic atherosclerosis (CC 8384)a | 50.44 | 0.87 | 0.83‐0.90 | 50.12 | 0.91 | 0.87‐0.94 |
| Arrhythmias (CC 9293) | 37.15 | 1.17 | 1.12‐1.22 | 37.06 | 1.15 | 1.10‐1.20 |
| Vascular or circulatory disease (CC 104106) | 38.20 | 1.09 | 1.05‐1.14 | 38.09 | 1.02 | 0.98‐1.06 |
| Fibrosis of lung and other chronic lung disorder (CC 109) | 16.96 | 1.08 | 1.03‐1.13 | 17.08 | 1.11 | 1.06‐1.17 |
| Asthma (CC 110) | 17.05 | 0.67 | 0.63‐0.70 | 16.90 | 0.67 | 0.63‐0.70 |
| Pneumonia (CC 111113) | 49.46 | 1.29 | 1.24‐1.35 | 49.41 | 1.27 | 1.22‐1.33 |
| Pleural effusion/pneumothorax (CC 114) | 11.78 | 1.17 | 1.11‐1.23 | 11.54 | 1.18 | 1.12‐1.25 |
| Other lung disorders (CC 115) | 53.07 | 0.80 | 0.77‐0.83 | 53.17 | 0.83 | 0.80‐0.87 |
| Other comorbid conditions | ||||||
| Metastatic cancer and acute leukemia (CC 7) | 2.76 | 2.34 | 2.14‐2.56 | 2.79 | 2.15 | 1.97‐2.35 |
| Lung, upper digestive tract, and other severe cancers (CC 8)a | 5.98 | 1.80 | 1.68‐1.92 | 6.02 | 1.98 | 1.85‐2.11 |
| Lymphatic, head and neck, brain, and other major cancers; breast, prostate, colorectal and other cancers and tumors; other respiratory and heart neoplasms (CC 911) | 14.13 | 1.03 | 0.97‐1.08 | 14.19 | 1.01 | 0.95‐1.06 |
| Other digestive and urinary neoplasms (CC 12) | 6.91 | 0.91 | 0.84‐0.98 | 7.05 | 0.85 | 0.79‐0.92 |
| Diabetes and DM complications (CC 1520, 119120) | 38.31 | 0.91 | 0.87‐0.94 | 38.29 | 0.91 | 0.87‐0.94 |
| Protein‐calorie malnutrition (CC 21) | 7.40 | 2.18 | 2.07‐2.30 | 7.44 | 2.09 | 1.98‐2.20 |
| Disorders of fluid/electrolyte/acid‐base (CC 2223) | 32.05 | 1.13 | 1.08‐1.18 | 32.16 | 1.24 | 1.19‐1.30 |
| Other endocrine/metabolic/nutritional disorders (CC 24) | 67.99 | 0.75 | 0.72‐0.78 | 67.88 | 0.76 | 0.73‐0.79 |
| Other gastrointestinal disorders (CC 36) | 56.21 | 0.81 | 0.78‐0.84 | 56.18 | 0.78 | 0.75‐0.81 |
| Osteoarthritis of hip or knee (CC 40) | 9.32 | 0.74 | 0.69‐0.79 | 9.33 | 0.80 | 0.74‐0.85 |
| Other musculoskeletal and connective tissue disorders (CC 43) | 64.14 | 0.83 | 0.80‐0.86 | 64.20 | 0.83 | 0.80‐0.87 |
| Iron deficiency and other/unspecified anemias and blood disease (CC 47) | 40.80 | 1.08 | 1.04‐1.12 | 40.72 | 1.08 | 1.04‐1.13 |
| Dementia and senility (CC 4950) | 17.06 | 1.09 | 1.04‐1.14 | 16.97 | 1.09 | 1.04‐1.15 |
| Drug/alcohol abuse, without dependence (CC 53)a | 23.51 | 0.78 | 0.75‐0.82 | 23.38 | 0.76 | 0.72‐0.80 |
| Other psychiatric disorders (CC 60)a | 16.49 | 1.12 | 1.07‐1.18 | 16.43 | 1.12 | 1.06‐1.17 |
| Quadriplegia, paraplegia, functional disability (CC 6769, 100102, 177178) | 4.92 | 1.03 | 0.95‐1.12 | 4.92 | 1.08 | 0.99‐1.17 |
| Mononeuropathy, other neurological conditions/emnjuries (CC 76) | 11.35 | 0.85 | 0.80‐0.91 | 11.28 | 0.88 | 0.83‐0.93 |
| Hypertension and hypertensive disease (CC 9091) | 80.40 | 0.78 | 0.75‐0.82 | 80.35 | 0.79 | 0.75‐0.83 |
| Stroke (CC 9596)a | 6.77 | 1.00 | 0.93‐1.08 | 6.73 | 0.98 | 0.91‐1.05 |
| Retinal disorders, except detachment and vascular retinopathies (CC 121) | 10.79 | 0.87 | 0.82‐0.93 | 10.69 | 0.90 | 0.85‐0.96 |
| Other eye disorders (CC 124)a | 19.05 | 0.90 | 0.86‐0.95 | 19.13 | 0.98 | 0.85‐0.93 |
| Other ear, nose, throat, and mouth disorders (CC 127) | 35.21 | 0.83 | 0.80‐0.87 | 35.02 | 0.80 | 0.77‐0.83 |
| Renal failure (CC 131)a | 17.92 | 1.12 | 1.07‐1.18 | 18.16 | 1.13 | 1.08‐1.19 |
| Decubitus ulcer or chronic skin ulcer (CC 148149) | 7.42 | 1.27 | 1.19‐1.35 | 7.42 | 1.33 | 1.25‐1.42 |
| Other dermatological disorders (CC 153) | 28.46 | 0.90 | 0.87‐0.94 | 28.32 | 0.89 | 0.86‐0.93 |
| Trauma (CC 154156, 158161) | 9.04 | 1.09 | 1.03‐1.16 | 8.99 | 1.15 | 1.08‐1.22 |
| Vertebral fractures (CC 157) | 5.01 | 1.33 | 1.24‐1.44 | 4.97 | 1.29 | 1.20‐1.39 |
| Major complications of medical care and trauma (CC 164) | 5.47 | 0.81 | 0.75‐0.88 | 5.55 | 0.82 | 0.76‐0.89 |
Model Derivation
We used hierarchical logistic regression models to model the log‐odds of mortality as a function of patient‐level clinical characteristics and a random hospital‐level intercept. At the patient level, each model adjusts the log‐odds of mortality for age and the selected clinical covariates. The second level models the hospital‐specific intercepts as arising from a normal distribution. The hospital intercept represents the underlying risk of mortality, after accounting for patient risk. If there were no differences among hospitals, then after adjusting for patient risk, the hospital intercepts should be identical across all hospitals.
Estimation of Hospital Risk‐Standardized Mortality Rate
We calculated a risk‐standardized mortality rate, defined as the ratio of predicted to expected deaths (similar to observed‐to‐expected), multiplied by the national unadjusted mortality rate.[21] The expected number of deaths for each hospital was estimated by applying the estimated regression coefficients to the characteristics of each hospital's patients, adding the average of the hospital‐specific intercepts, transforming the data by using an inverse logit function, and summing the data from all patients in the hospital to obtain the count. The predicted number of deaths was calculated in the same way, substituting the hospital‐specific intercept for the average hospital‐specific intercept.
Model Performance, Validation, and Reliability Testing
We used the remaining admissions in 2008 as the model validation sample. We computed several summary statistics to assess the patient‐level model performance in both the development and validation samples,[22] including over‐fitting indices, predictive ability, area under the receiver operating characteristic (ROC) curve, distribution of residuals, and model 2. In addition, we assessed face validity through a survey of members of the technical expert panel. To assess reliability of the model across data years, we repeated the modeling process using qualifying COPD admissions in both 2007 and 2009. Finally, to assess generalizability we evaluated the model's performance in an all‐payer sample of data from patients admitted to California hospitals in 2006.
Analyses were conducted using SAS version 9.1.3 (SAS Institute Inc., Cary, NC). We estimated the hierarchical models using the GLIMMIX procedure in SAS.
The Human Investigation Committee at the Yale University School of Medicine/Yale New Haven Hospital approved an exemption (HIC#0903004927) for the authors to use CMS claims and enrollment data for research analyses and publication.
RESULTS
Model Derivation
After exclusions were applied, the development sample included 150,035 admissions in 2008 at 4537 US hospitals (Figure 1). Factors that were most strongly associated with the risk of mortality included metastatic cancer (odds ratio [OR] 2.34), protein calorie malnutrition (OR 2.18), nonmetastatic cancers of the lung and upper digestive tract, (OR 1.80) cardiorespiratory failure and shock (OR 1.60), and congestive heart failure (OR 1.34) (Table 2).
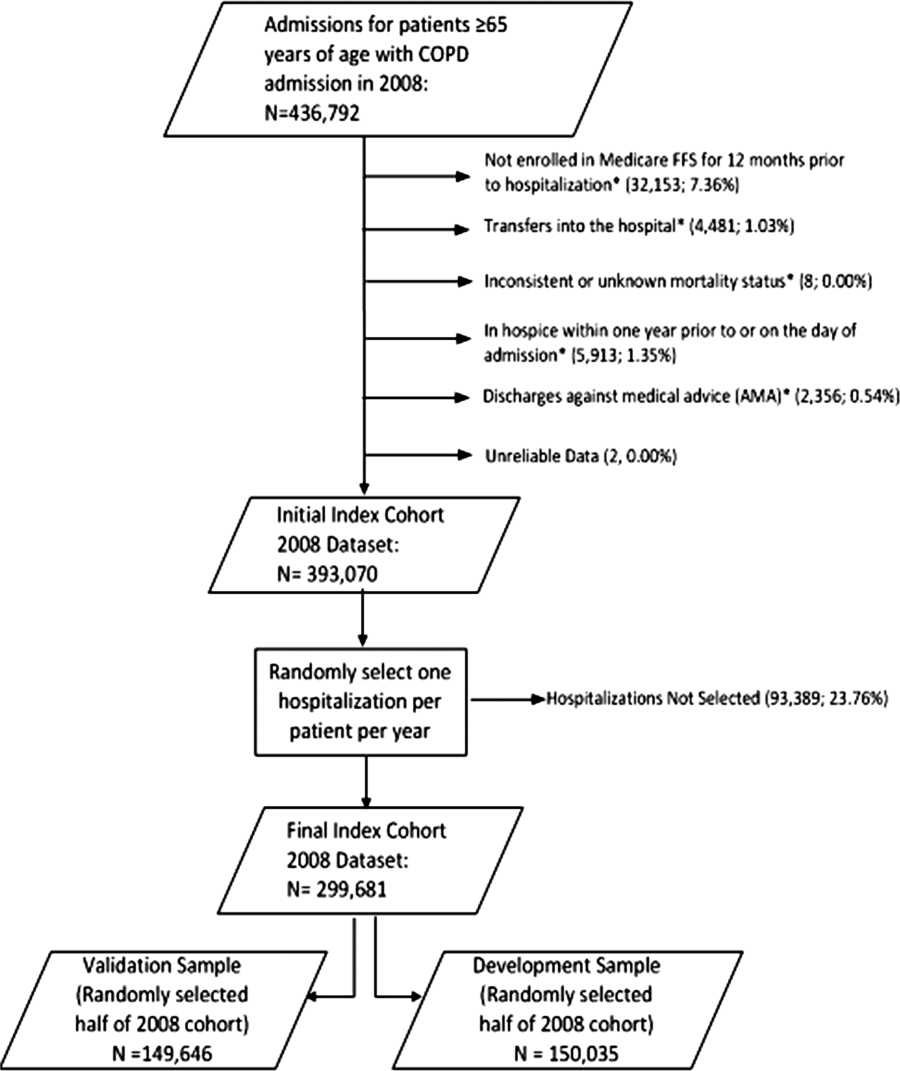
Model Performance, Validation, and Reliability
The model had a C statistic of 0.72, indicating good discrimination, and predicted mortality in the development sample ranged from 1.52% in the lowest decile to 23.74% in the highest. The model validation sample, using the remaining cases from 2008, included 149,646 admissions from 4535 hospitals. Variable frequencies and ORs were similar in both samples (Table 2). Model performance was also similar in the validation samples, with good model discrimination and fit (Table 3). Ten of 12 technical expert panel members responded to the survey, of whom 90% at least somewhat agreed with the statement, the COPD mortality measure provides an accurate reflection of quality. When the model was applied to patients age 18 years and older in the 2006 California Patient Discharge Data, overall discrimination was good (C statistic, 0.74), including in those age 18 to 64 years (C statistic, 0.75; 65 and above C statistic, 0.70).
| Development | Validation | Data Years | ||
|---|---|---|---|---|
| Indices | Sample, 2008 | Sample, 2008 | 2007 | 2009 |
| ||||
| Number of admissions | 150,035 | 149,646 | 259,911 | 279,377 |
| Number of hospitals | 4537 | 4535 | 4636 | 4571 |
| Mean risk‐standardized mortality rate, % (SD) | 8.62 (0.94) | 8.64 (1.07) | 8.97 (1.12) | 8.08 (1.09) |
| Calibration, 0, 1 | 0.034, 0.985 | 0.009, 1.004 | 0.095, 1.022 | 0.120, 0.981 |
| Discriminationpredictive ability, lowest decile %highest decile % | 1.5223.74 | 1.6023.78 | 1.5424.64 | 1.4222.36 |
| Discriminationarea under the ROC curve, C statistic | 0.720 | 0.723 | 0.728 | 0.722 |
| Residuals lack of fit, Pearson residual fall % | ||||
| 2 | 0 | 0 | 0 | 0 |
| 2, 0 | 91.14 | 91.4 | 91.08 | 91.93 |
| 0, 2 | 1.66 | 1.7 | 1.96 | 1.42 |
| 2+ | 6.93 | 6.91 | 6.96 | 6.65 |
| Model Wald 2 (number of covariates) | 6982.11 (42) | 7051.50 (42) | 13042.35 (42) | 12542.15 (42) |
| P value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Between‐hospital variance, (standard error) | 0.067 (0.008) | 0.078 (0.009) | 0.067 (0.006) | 0.072 (0.006) |
Reliability testing demonstrated consistent performance over several years. The frequency and ORs of the variables included in the model showed only minor changes over time. The area under the ROC curve (C statistic) was 0.73 for the model in the 2007 sample and 0.72 for the model using 2009 data (Table 3).
Hospital Risk‐Standardized Mortality Rates
The mean unadjusted hospital 30‐day mortality rate was 8.6% and ranged from 0% to 100% (Figure 2a). Risk‐standardized mortality rates varied across hospitals (Figure 2b). The mean risk‐standardized mortality rate was 8.6% and ranged from 5.9% to 13.5%. The odds of mortality at a hospital 1 standard deviation above average was 1.20 times that of a hospital 1 standard deviation below average.
DISCUSSION
We present a hospital‐level risk‐standardized mortality measure for patients admitted with COPD based on administrative claims data that are intended for public reporting and that have achieved endorsement by the National Quality Forum, a voluntary consensus standards‐setting organization. Across more than 4500 US hospitals, the mean 30‐day risk‐standardized mortality rate in 2008 was 8.6%, and we observed considerable variation across institutions, despite adjustment for case mix, suggesting that improvement by lower‐performing institutions may be an achievable goal.
Although improving the delivery of evidence‐based care processes and outcomes of patients with acute myocardial infarction, heart failure, and pneumonia has been the focus of national quality improvement efforts for more than a decade, COPD has largely been overlooked.[23] Within this context, this analysis represents the first attempt to systematically measure, at the hospital level, 30‐day all‐cause mortality for patients admitted to US hospitals for exacerbation of COPD. The model we have developed and validated is intended to be used to compare the performance of hospitals while controlling for differences in the pretreatment risk of mortality of patients and accounting for the clustering of patients within hospitals, and will facilitate surveillance of hospital‐level risk‐adjusted outcomes over time.
In contrast to process‐based measures of quality, such as the percentage of patients with pneumonia who receive appropriate antibiotic therapy, performance measures based on patient outcomes provide a more comprehensive view of care and are more consistent with patients' goals.[24] Additionally, it is well established that hospital performance on individual and composite process measures explains only a small amount of the observed variation in patient outcomes between institutions.[25] In this regard, outcome measures incorporate important, but difficult to measure aspects of care, such as diagnostic accuracy and timing, communication and teamwork, the recognition and response to complications, care coordination at the time of transfers between levels of care, and care settings. Nevertheless, when used for making inferences about the quality of hospital care, individual measures such as the risk‐standardized hospital mortality rate should be interpreted in the context of other performance measures, including readmission, patient experience, and costs of care.
A number of prior investigators have described the outcomes of care for patients hospitalized with exacerbations of COPD, including identifying risk factors for mortality. Patil et al. carried out an analysis of the 1996 Nationwide Inpatient Sample and described an overall in‐hospital mortality rate of 2.5% among patients with COPD, and reported that a multivariable model containing sociodemographic characteristics about the patient and comorbidities had an area under the ROC curve of 0.70.[3] In contrast, this hospital‐level measure includes patients with a principal diagnosis of respiratory failure and focuses on 30‐day rather than inpatient mortality, accounting for the nearly 3‐fold higher mortality rate we observed. In a more recent study that used clinical from a large multistate database, Tabak et al. developed a prediction model for inpatient mortality for patients with COPD that contained only 4 factors: age, blood urea nitrogen, mental status, and pulse, and achieved an area under the ROC curve of 0.72.[4] The simplicity of such a model and its reliance on clinical measurements makes it particularly well suited for bedside application by clinicians, but less valuable for large‐scale public reporting programs that rely on administrative data. In the only other study identified that focused on the assessment of hospital mortality rates, Agabiti et al. analyzed the outcomes of 12,756 patients hospitalized for exacerbations of COPD, using similar ICD‐9‐CM diagnostic criteria as in this study, at 21 hospitals in Rome, Italy.[26] They reported an average crude 30‐day mortality rate of 3.8% among a group of 5 benchmark hospitals and an average mortality of 7.5% (range, 5.2%17.2%) among the remaining institutions.
To put the variation we observed in mortality rates into a broader context, the relative difference in the risk‐standardized hospital mortality rates across the 10th to 90th percentiles of hospital performance was 25% for acute myocardial infarction and 39% for heart failure, whereas rates varied 30% for COPD, from 7.6% to 9.9%.[27] Model discrimination in COPD (C statistic, 0.72) was also similar to that reported for models used for public reporting of hospital mortality in acute myocardial infarction (C statistic, 0.71) and pneumonia (C statistic, 0.72).
This study has a number of important strengths. First, the model was developed from a large sample of recent Medicare claims, achieved good discrimination, and was validated in samples not limited to Medicare beneficiaries. Second, by including patients with a principal diagnosis of COPD, as well as those with a principal diagnosis of acute respiratory failure when accompanied by a secondary diagnosis of COPD with acute exacerbation, this model can be used to assess hospital performance across the full spectrum of disease severity. This broad set of ICD‐9‐CM codes used to define the cohort also ensures that efforts to measure hospital performance will be less influenced by differences in documentation and coding practices across hospitals relating to the diagnosis or sequencing of acute respiratory failure diagnoses. Moreover, the inclusion of patients with respiratory failure is important because these patients have the greatest risk of mortality, and are those in whom efforts to improve the quality and safety of care may have the greatest impact. Third, rather than relying solely on information documented during the index admission, we used ambulatory and inpatient claims from the full year prior to the index admission to identify comorbidities and to distinguish them from potential complications of care. Finally, we did not include factors such as hospital characteristics (eg, number of beds, teaching status) in the model. Although they might have improved overall predictive ability, the goal of the hospital mortality measure is to enable comparisons of mortality rates among hospitals while controlling for differences in patient characteristics. To the extent that factors such as size or teaching status might be independently associated with hospital outcomes, it would be inappropriate to adjust away their effects, because mortality risk should not be influenced by hospital characteristics other than through their effects on quality.
These results should be viewed in light of several limitations. First, we used ICD‐9‐CM codes derived from claims files to define the patient populations included in the measure rather than collecting clinical or physiologic information prospectively or through manual review of medical records, such as the forced expiratory volume in 1 second or whether the patient required long‐term oxygen therapy. Nevertheless, we included a broad set of potential diagnosis codes to capture the full spectrum of COPD exacerbations and to minimize differences in coding across hospitals. Second, because the risk‐adjustment included diagnoses coded in the year prior to the index admission, it is potentially subject to bias due to regional differences in medical care utilization that are not driven by underlying differences in patient illness.[28] Third, using administrative claims data, we observed some paradoxical associations in the model that are difficult to explain on clinical grounds, such as a protective effect of substance and alcohol abuse or prior episodes of respiratory failure. Fourth, although we excluded patients from the analysis who were enrolled in hospice prior to, or on the day of, the index admission, we did not exclude those who choose to withdraw support, transition to comfort measures only, or enrolled in hospice care during a hospitalization. We do not seek to penalize hospitals for being sensitive to the preferences of patients at the end of life. At the same time, it is equally important that the measure is capable of detecting the outcomes of suboptimal care that may in some instances lead a patient or their family to withdraw support or choose hospice. Finally, we did not have the opportunity to validate the model against a clinical registry of patients with COPD, because such data do not currently exist. Nevertheless, the use of claims as a surrogate for chart data for risk adjustment has been validated for several conditions, including acute myocardial infarction, heart failure, and pneumonia.[29, 30]
CONCLUSIONS
Risk‐standardized 30‐day mortality rates for Medicare beneficiaries with COPD vary across hospitals in the US. Calculating and reporting hospital outcomes using validated performance measures may catalyze quality improvement activities and lead to better outcomes. Additional research would be helpful to confirm that hospitals with lower mortality rates achieve care that meets the goals of patients and their families better than at hospitals with higher mortality rates.
Acknowledgment
The authors thank the following members of the technical expert panel: Darlene Bainbridge, RN, MS, NHA, CPHQ, CPHRM, President/CEO, Darlene D. Bainbridge & Associates, Inc.; Robert A. Balk, MD, Director of Pulmonary and Critical Care Medicine, Rush University Medical Center; Dale Bratzler, DO, MPH, President and CEO, Oklahoma Foundation for Medical Quality; Scott Cerreta, RRT, Director of Education, COPD Foundation; Gerard J. Criner, MD, Director of Temple Lung Center and Divisions of Pulmonary and Critical Care Medicine, Temple University; Guy D'Andrea, MBA, President, Discern Consulting; Jonathan Fine, MD, Director of Pulmonary Fellowship, Research and Medical Education, Norwalk Hospital; David Hopkins, MS, PhD, Senior Advisor, Pacific Business Group on Health; Fred Martin Jacobs, MD, JD, FACP, FCCP, FCLM, Executive Vice President and Director, Saint Barnabas Quality Institute; Natalie Napolitano, MPH, RRT‐NPS, Respiratory Therapist, Inova Fairfax Hospital; Russell Robbins, MD, MBA, Principal and Senior Clinical Consultant, Mercer. In addition, the authors acknowledge and thank Angela Merrill, Sandi Nelson, Marian Wrobel, and Eric Schone from Mathematica Policy Research, Inc., Sharon‐Lise T. Normand from Harvard Medical School, and Lein Han and Michael Rapp at The Centers for Medicare & Medicaid Services for their contributions to this work.
Disclosures
Peter K. Lindenauer, MD, MSc, is the guarantor of this article, taking responsibility for the integrity of the work as a whole, from inception to published article, and takes responsibility for the content of the manuscript, including the data and data analysis. All authors have made substantial contributions to the conception and design, or acquisition of data, or analysis and interpretation of data; have drafted the submitted article or revised it critically for important intellectual content; and have provided final approval of the version to be published. Preparation of this manuscript was completed under Contract Number: HHSM‐5002008‐0025I/HHSM‐500‐T0001, Modification No. 000007, Option Year 2 Measure Instrument Development and Support (MIDS). Sponsors did not contribute to the development of the research or manuscript. Dr. Au reports being an unpaid research consultant for Bosch Inc. He receives research funding from the NIH, Department of Veterans Affairs, AHRQ, and Gilead Sciences. The views of the this manuscript represent the authors and do not necessarily represent those of the Department of Veterans Affairs. Drs. Drye and Bernheim report receiving contract funding from CMS to develop and maintain quality measures.
- FASTSTATS—chronic lower respiratory disease. Available at: http://www.cdc.gov/nchs/fastats/copd.htm. Accessed September 18, 2010.
- National Heart, Lung and Blood Institute. Morbidity and mortality chartbook. Available at: http://www.nhlbi.nih.gov/resources/docs/cht‐book.htm. Accessed April 27, 2010.
- , , , . In‐hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003;163(10):1180–1186.
- , , , , . Mortality and need for mechanical ventilation in acute exacerbations of chronic obstructive pulmonary disease: development and validation of a simple risk score. Arch Intern Med. 2009;169(17):1595–1602.
- , , , , , . Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2006;144(12):894–903.
- , , , , . Use of beta blockers and the risk of death in hospitalised patients with acute exacerbations of COPD. Thorax. 2008;63(4):301–305.
- , , , , . HCUP facts and figures: statistics on hospital‐based care in the United States, 2007. 2009. Available at: http://www.hcup‐us.ahrq.gov/reports.jsp. Accessed August 6, 2012.
- , . Predictors of long‐term survival in elderly patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Respirology. 2008;13(6):851–855.
- , , , , , . The impact on risk‐factor analysis of different mortality outcomes in COPD patients. Eur Respir J 2008;32(3):629–636.
- , , , , , . Clinical audit indicators of outcome following admission to hospital with acute exacerbation of chronic obstructive pulmonary disease. Thorax. 2002;57(2):137–141.
- , , , et al. The quality of obstructive lung disease care for adults in the United States as measured by adherence to recommended processes. Chest. 2006;130(6):1844–1850.
- , , , , , . Management of acute exacerbations of chronic obstructive pulmonary disease in the elderly: physician practices in the community hospital setting. J Okla State Med Assoc. 2004;97(6):227–232.
- , , . Leadership by Example: Coordinating Government Roles in Improving Health Care Quality. Washington, DC: National Academies Press; 2002.
- Patient Protection and Affordable Care Act [H.R. 3590], Pub. L. No. 111–148, §2702, 124 Stat. 119, 318–319 (March 23, 2010). Available at: http://www.gpo.gov/fdsys/pkg/PLAW‐111publ148/html/PLAW‐111publ148.htm. Accessed July 15, 2012.
- National Quality Forum. NQF Endorses Additional Pulmonary Measure. 2013. Available at: http://www.qualityforum.org/News_And_Resources/Press_Releases/2013/NQF_Endorses_Additional_Pulmonary_Measure.aspx. Accessed January 11, 2013.
- National Quality Forum. National voluntary consensus standards for patient outcomes: a consensus report. Washington, DC: National Quality Forum; 2011.
- The Measures Management System. The Centers for Medicare and Medicaid Services. Available at: http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/MMS/index.html?redirect=/MMS/. Accessed August 6, 2012.
- , , , et al. Standards for statistical models used for public reporting of health outcomes: an American Heart Association Scientific Statement from the Quality of Care and Outcomes Research Interdisciplinary Writing Group: cosponsored by the Council on Epidemiology and Prevention and the Stroke Council. Endorsed by the American College of Cardiology Foundation. Circulation. 2006;113(3):456–462.
- , , , et al. Comparison of hospital risk‐standardized mortality rates calculated by using in‐hospital and 30‐day models: an observational study with implications for hospital profiling. Ann Intern Med. 2012;156(1 pt 1):19–26.
- , , , et al. Diagnostic cost group hierarchical condition category models for Medicare risk adjustment. Report prepared for the Health Care Financing Administration. Health Economics Research, Inc.; 2000. Available at: http://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/Reports/downloads/pope_2000_2.pdf. Accessed November 7, 2009.
- , . Statistical and clinical aspects of hospital outcomes profiling. Stat Sci. 2007;22(2):206–226.
- , . Using full probability models to compute probabilities of actual interest to decision makers. Int J Technol Assess Health Care. 2001;17(1):17–26.
- , , . COPD performance measures: missing opportunities for improving care. Chest. 2010;137(5):1181–1189.
- , , , , . Measuring Performance For Treating Heart Attacks And Heart Failure: The Case For Outcomes Measurement. Health Aff. 2007;26(1):75–85.
- , , , et al. Hospital quality for acute myocardial infarction: correlation among process measures and relationship with short‐term mortality. JAMA. 2006;296(1):72–78.
- , , , et al. Profiling hospital performance to monitor the quality of care: the case of COPD. Eur Respir J. 2010;35(5):1031–1038.
- , , , et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30‐day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2(5):407–413.
- , , , , . Geographic variation in diagnosis frequency and risk of death among Medicare beneficiaries. JAMA. 2011;305(11):1113–1118.
- , , , et al. An administrative claims model for profiling hospital 30‐day mortality rates for pneumonia patients. PLoS ONE. 2011;6(4):e17401.
- , , , et al. An Administrative Claims Model Suitable for Profiling Hospital Performance Based on 30‐Day Mortality Rates Among Patients With Heart Failure. Circulation. 2006;113(13):1693–1701.
Chronic obstructive pulmonary disease (COPD) affects as many as 24 million individuals in the United States, is responsible for more than 700,000 annual hospital admissions, and is currently the nation's third leading cause of death, accounting for nearly $49.9 billion in medical spending in 2010.[1, 2] Reported in‐hospital mortality rates for patients hospitalized for exacerbations of COPD range from 2% to 5%.[3, 4, 5, 6, 7] Information about 30‐day mortality rates following hospitalization for COPD is more limited; however, international studies suggest that rates range from 3% to 9%,[8, 9] and 90‐day mortality rates exceed 15%.[10]
Despite this significant clinical and economic impact, there have been no large‐scale, sustained efforts to measure the quality or outcomes of hospital care for patients with COPD in the United States. What little is known about the treatment of patients with COPD suggests widespread opportunities to increase adherence to guideline‐recommended therapies, to reduce the use of ineffective treatments and tests, and to address variation in care across institutions.[5, 11, 12]
Public reporting of hospital performance is a key strategy for improving the quality and safety of hospital care, both in the United States and internationally.[13] Since 2007, the Centers for Medicare and Medicaid Services (CMS) has reported hospital mortality rates on the Hospital Compare Web site, and COPD is 1 of the conditions highlighted in the Affordable Care Act for future consideration.[14] Such initiatives rely on validated, risk‐adjusted performance measures for comparisons across institutions and to enable outcomes to be tracked over time. We present the development, validation, and results of a model intended for public reporting of risk‐standardized mortality rates for patients hospitalized with exacerbations of COPD that has been endorsed by the National Quality Forum.[15]
METHODS
Approach to Measure Development
We developed this measure in accordance with guidelines described by the National Quality Forum,[16] CMS' Measure Management System,[17] and the American Heart Association scientific statement, Standards for Statistical Models Used for Public Reporting of Health Outcomes.[18] Throughout the process we obtained expert clinical and stakeholder input through meetings with a clinical advisory group and a national technical expert panel (see Acknowledgments). Last, we presented the proposed measure specifications and a summary of the technical expert panel discussions online and made a widely distributed call for public comments. We took the comments into consideration during the final stages of measure development (available at
Data Sources
We used claims data from Medicare inpatient, outpatient, and carrier (physician) Standard Analytic Files from 2008 to develop and validate the model, and examined model reliability using data from 2007 and 2009. The Medicare enrollment database was used to determine Medicare Fee‐for‐Service enrollment and mortality.
Study Cohort
Admissions were considered eligible for inclusion if the patient was 65 years or older, was admitted to a nonfederal acute care hospital in the United States, and had a principal diagnosis of COPD or a principal diagnosis of acute respiratory failure or respiratory arrest when paired with a secondary diagnosis of COPD with exacerbation (Table 1).
| ICD‐9‐CM | Description |
|---|---|
| |
| 491.21 | Obstructive chronic bronchitis; with (acute) exacerbation; acute exacerbation of COPD, decompensated COPD, decompensated COPD with exacerbation |
| 491.22 | Obstructive chronic bronchitis; with acute bronchitis |
| 491.8 | Other chronic bronchitis; chronic: tracheitis, tracheobronchitis |
| 491.9 | Unspecified chronic bronchitis |
| 492.8 | Other emphysema; emphysema (lung or pulmonary): NOS, centriacinar, centrilobular, obstructive, panacinar, panlobular, unilateral, vesicular; MacLeod's syndrome; Swyer‐James syndrome; unilateral hyperlucent lung |
| 493.20 | Chronic obstructive asthma; asthma with COPD, chronic asthmatic bronchitis, unspecified |
| 493.21 | Chronic obstructive asthma; asthma with COPD, chronic asthmatic bronchitis, with status asthmaticus |
| 493.22 | Chronic obstructive asthma; asthma with COPD, chronic asthmatic bronchitis, with (acute) exacerbation |
| 496 | Chronic: nonspecific lung disease, obstructive lung disease, obstructive pulmonary disease (COPD) NOS. (Note: This code is not to be used with any code from categories 491493.) |
| 518.81a | Other diseases of lung; acute respiratory failure; respiratory failure NOS |
| 518.82a | Other diseases of lung; acute respiratory failure; other pulmonary insufficiency, acute respiratory distress |
| 518.84a | Other diseases of lung; acute respiratory failure; acute and chronic respiratory failure |
| 799.1a | Other ill‐defined and unknown causes of morbidity and mortality; respiratory arrest, cardiorespiratory failure |
If a patient was discharged and readmitted to a second hospital on the same or the next day, we combined the 2 acute care admissions into a single episode of care and assigned the mortality outcome to the first admitting hospital. We excluded admissions for patients who were enrolled in Medicare Hospice in the 12 months prior to or on the first day of the index hospitalization. An index admission was any eligible admission assessed in the measure for the outcome. We also excluded admissions for patients who were discharged against medical advice, those for whom vital status at 30 days was unknown or recorded inconsistently, and patients with unreliable data (eg, age >115 years). For patients with multiple hospitalizations during a single year, we randomly selected 1 admission per patient to avoid survival bias. Finally, to assure adequate risk adjustment we limited the analysis to patients who had continuous enrollment in Medicare Fee‐for‐Service Parts A and B for the 12 months prior to their index admission so that we could identify comorbid conditions coded during all prior encounters.
Outcomes
The outcome of 30‐day mortality was defined as death from any cause within 30 days of the admission date for the index hospitalization. Mortality was assessed at 30 days to standardize the period of outcome ascertainment,[19] and because 30 days is a clinically meaningful time frame, during which differences in the quality of hospital care may be revealed.
Risk‐Adjustment Variables
We randomly selected half of all COPD admissions in 2008 that met the inclusion and exclusion criteria to create a model development sample. Candidate variables for inclusion in the risk‐standardized model were selected by a clinician team from diagnostic groups included in the Hierarchical Condition Category clinical classification system[20] and included age and comorbid conditions. Sleep apnea (International Classification of Diseases, 9th Revision, Clinical Modification [ICD‐9‐CM] condition codes 327.20, 327.21, 327.23, 327.27, 327.29, 780.51, 780.53, and 780.57) and mechanical ventilation (ICD‐9‐CM procedure codes 93.90, 96.70, 96.71, and 96.72) were also included as candidate variables.
We defined a condition as present for a given patient if it was coded in the inpatient, outpatient, or physician claims data sources in the preceding 12 months, including the index admission. Because a subset of the condition category variables can represent a complication of care, we did not consider them to be risk factors if they appeared only as secondary diagnosis codes for the index admission and not in claims submitted during the prior year.
We selected final variables for inclusion in the risk‐standardized model based on clinical considerations and a modified approach to stepwise logistic regression. The final patient‐level risk‐adjustment model included 42 variables (Table 2).
| Variable | Development Sample (150,035 Admissions at 4537 Hospitals) | Validation Sample (149,646 Admissions at 4535 Hospitals) | ||||
|---|---|---|---|---|---|---|
| Frequency, % | OR | 95% CI | Frequency, % | OR | 95% CI | |
| ||||||
| Demographics | ||||||
| Age 65 years (continuous) | 1.03 | 1.03‐1.04 | 1.03 | 1.03‐1.04 | ||
| Cardiovascular/respiratory | ||||||
| Sleep apnea (ICD‐9‐CM: 327.20, 327.21, 327.23, 327.27, 327.29, 780.51, 780.53, 780.57)a | 9.57 | 0.87 | 0.81‐0.94 | 9.72 | 0.84 | 0.78‐0.90 |
| History of mechanical ventilation (ICD‐9‐CM: 93.90, 96.70, 96.71, 96.72)a | 6.00 | 1.19 | 1.11‐1.27 | 6.00 | 1.15 | 1.08‐1.24 |
| Respirator dependence/respiratory failure (CC 7778)a | 1.15 | 0.89 | 0.77‐1.02 | 1.20 | 0.78 | 0.68‐0.91 |
| Cardiorespiratory failure and shock (CC 79) | 26.35 | 1.60 | 1.53‐1.68 | 26.34 | 1.59 | 1.52‐1.66 |
| Congestive heart failure (CC 80) | 41.50 | 1.34 | 1.28‐1.39 | 41.39 | 1.31 | 1.25‐1.36 |
| Chronic atherosclerosis (CC 8384)a | 50.44 | 0.87 | 0.83‐0.90 | 50.12 | 0.91 | 0.87‐0.94 |
| Arrhythmias (CC 9293) | 37.15 | 1.17 | 1.12‐1.22 | 37.06 | 1.15 | 1.10‐1.20 |
| Vascular or circulatory disease (CC 104106) | 38.20 | 1.09 | 1.05‐1.14 | 38.09 | 1.02 | 0.98‐1.06 |
| Fibrosis of lung and other chronic lung disorder (CC 109) | 16.96 | 1.08 | 1.03‐1.13 | 17.08 | 1.11 | 1.06‐1.17 |
| Asthma (CC 110) | 17.05 | 0.67 | 0.63‐0.70 | 16.90 | 0.67 | 0.63‐0.70 |
| Pneumonia (CC 111113) | 49.46 | 1.29 | 1.24‐1.35 | 49.41 | 1.27 | 1.22‐1.33 |
| Pleural effusion/pneumothorax (CC 114) | 11.78 | 1.17 | 1.11‐1.23 | 11.54 | 1.18 | 1.12‐1.25 |
| Other lung disorders (CC 115) | 53.07 | 0.80 | 0.77‐0.83 | 53.17 | 0.83 | 0.80‐0.87 |
| Other comorbid conditions | ||||||
| Metastatic cancer and acute leukemia (CC 7) | 2.76 | 2.34 | 2.14‐2.56 | 2.79 | 2.15 | 1.97‐2.35 |
| Lung, upper digestive tract, and other severe cancers (CC 8)a | 5.98 | 1.80 | 1.68‐1.92 | 6.02 | 1.98 | 1.85‐2.11 |
| Lymphatic, head and neck, brain, and other major cancers; breast, prostate, colorectal and other cancers and tumors; other respiratory and heart neoplasms (CC 911) | 14.13 | 1.03 | 0.97‐1.08 | 14.19 | 1.01 | 0.95‐1.06 |
| Other digestive and urinary neoplasms (CC 12) | 6.91 | 0.91 | 0.84‐0.98 | 7.05 | 0.85 | 0.79‐0.92 |
| Diabetes and DM complications (CC 1520, 119120) | 38.31 | 0.91 | 0.87‐0.94 | 38.29 | 0.91 | 0.87‐0.94 |
| Protein‐calorie malnutrition (CC 21) | 7.40 | 2.18 | 2.07‐2.30 | 7.44 | 2.09 | 1.98‐2.20 |
| Disorders of fluid/electrolyte/acid‐base (CC 2223) | 32.05 | 1.13 | 1.08‐1.18 | 32.16 | 1.24 | 1.19‐1.30 |
| Other endocrine/metabolic/nutritional disorders (CC 24) | 67.99 | 0.75 | 0.72‐0.78 | 67.88 | 0.76 | 0.73‐0.79 |
| Other gastrointestinal disorders (CC 36) | 56.21 | 0.81 | 0.78‐0.84 | 56.18 | 0.78 | 0.75‐0.81 |
| Osteoarthritis of hip or knee (CC 40) | 9.32 | 0.74 | 0.69‐0.79 | 9.33 | 0.80 | 0.74‐0.85 |
| Other musculoskeletal and connective tissue disorders (CC 43) | 64.14 | 0.83 | 0.80‐0.86 | 64.20 | 0.83 | 0.80‐0.87 |
| Iron deficiency and other/unspecified anemias and blood disease (CC 47) | 40.80 | 1.08 | 1.04‐1.12 | 40.72 | 1.08 | 1.04‐1.13 |
| Dementia and senility (CC 4950) | 17.06 | 1.09 | 1.04‐1.14 | 16.97 | 1.09 | 1.04‐1.15 |
| Drug/alcohol abuse, without dependence (CC 53)a | 23.51 | 0.78 | 0.75‐0.82 | 23.38 | 0.76 | 0.72‐0.80 |
| Other psychiatric disorders (CC 60)a | 16.49 | 1.12 | 1.07‐1.18 | 16.43 | 1.12 | 1.06‐1.17 |
| Quadriplegia, paraplegia, functional disability (CC 6769, 100102, 177178) | 4.92 | 1.03 | 0.95‐1.12 | 4.92 | 1.08 | 0.99‐1.17 |
| Mononeuropathy, other neurological conditions/emnjuries (CC 76) | 11.35 | 0.85 | 0.80‐0.91 | 11.28 | 0.88 | 0.83‐0.93 |
| Hypertension and hypertensive disease (CC 9091) | 80.40 | 0.78 | 0.75‐0.82 | 80.35 | 0.79 | 0.75‐0.83 |
| Stroke (CC 9596)a | 6.77 | 1.00 | 0.93‐1.08 | 6.73 | 0.98 | 0.91‐1.05 |
| Retinal disorders, except detachment and vascular retinopathies (CC 121) | 10.79 | 0.87 | 0.82‐0.93 | 10.69 | 0.90 | 0.85‐0.96 |
| Other eye disorders (CC 124)a | 19.05 | 0.90 | 0.86‐0.95 | 19.13 | 0.98 | 0.85‐0.93 |
| Other ear, nose, throat, and mouth disorders (CC 127) | 35.21 | 0.83 | 0.80‐0.87 | 35.02 | 0.80 | 0.77‐0.83 |
| Renal failure (CC 131)a | 17.92 | 1.12 | 1.07‐1.18 | 18.16 | 1.13 | 1.08‐1.19 |
| Decubitus ulcer or chronic skin ulcer (CC 148149) | 7.42 | 1.27 | 1.19‐1.35 | 7.42 | 1.33 | 1.25‐1.42 |
| Other dermatological disorders (CC 153) | 28.46 | 0.90 | 0.87‐0.94 | 28.32 | 0.89 | 0.86‐0.93 |
| Trauma (CC 154156, 158161) | 9.04 | 1.09 | 1.03‐1.16 | 8.99 | 1.15 | 1.08‐1.22 |
| Vertebral fractures (CC 157) | 5.01 | 1.33 | 1.24‐1.44 | 4.97 | 1.29 | 1.20‐1.39 |
| Major complications of medical care and trauma (CC 164) | 5.47 | 0.81 | 0.75‐0.88 | 5.55 | 0.82 | 0.76‐0.89 |
Model Derivation
We used hierarchical logistic regression models to model the log‐odds of mortality as a function of patient‐level clinical characteristics and a random hospital‐level intercept. At the patient level, each model adjusts the log‐odds of mortality for age and the selected clinical covariates. The second level models the hospital‐specific intercepts as arising from a normal distribution. The hospital intercept represents the underlying risk of mortality, after accounting for patient risk. If there were no differences among hospitals, then after adjusting for patient risk, the hospital intercepts should be identical across all hospitals.
Estimation of Hospital Risk‐Standardized Mortality Rate
We calculated a risk‐standardized mortality rate, defined as the ratio of predicted to expected deaths (similar to observed‐to‐expected), multiplied by the national unadjusted mortality rate.[21] The expected number of deaths for each hospital was estimated by applying the estimated regression coefficients to the characteristics of each hospital's patients, adding the average of the hospital‐specific intercepts, transforming the data by using an inverse logit function, and summing the data from all patients in the hospital to obtain the count. The predicted number of deaths was calculated in the same way, substituting the hospital‐specific intercept for the average hospital‐specific intercept.
Model Performance, Validation, and Reliability Testing
We used the remaining admissions in 2008 as the model validation sample. We computed several summary statistics to assess the patient‐level model performance in both the development and validation samples,[22] including over‐fitting indices, predictive ability, area under the receiver operating characteristic (ROC) curve, distribution of residuals, and model 2. In addition, we assessed face validity through a survey of members of the technical expert panel. To assess reliability of the model across data years, we repeated the modeling process using qualifying COPD admissions in both 2007 and 2009. Finally, to assess generalizability we evaluated the model's performance in an all‐payer sample of data from patients admitted to California hospitals in 2006.
Analyses were conducted using SAS version 9.1.3 (SAS Institute Inc., Cary, NC). We estimated the hierarchical models using the GLIMMIX procedure in SAS.
The Human Investigation Committee at the Yale University School of Medicine/Yale New Haven Hospital approved an exemption (HIC#0903004927) for the authors to use CMS claims and enrollment data for research analyses and publication.
RESULTS
Model Derivation
After exclusions were applied, the development sample included 150,035 admissions in 2008 at 4537 US hospitals (Figure 1). Factors that were most strongly associated with the risk of mortality included metastatic cancer (odds ratio [OR] 2.34), protein calorie malnutrition (OR 2.18), nonmetastatic cancers of the lung and upper digestive tract, (OR 1.80) cardiorespiratory failure and shock (OR 1.60), and congestive heart failure (OR 1.34) (Table 2).

Model Performance, Validation, and Reliability
The model had a C statistic of 0.72, indicating good discrimination, and predicted mortality in the development sample ranged from 1.52% in the lowest decile to 23.74% in the highest. The model validation sample, using the remaining cases from 2008, included 149,646 admissions from 4535 hospitals. Variable frequencies and ORs were similar in both samples (Table 2). Model performance was also similar in the validation samples, with good model discrimination and fit (Table 3). Ten of 12 technical expert panel members responded to the survey, of whom 90% at least somewhat agreed with the statement, the COPD mortality measure provides an accurate reflection of quality. When the model was applied to patients age 18 years and older in the 2006 California Patient Discharge Data, overall discrimination was good (C statistic, 0.74), including in those age 18 to 64 years (C statistic, 0.75; 65 and above C statistic, 0.70).
| Development | Validation | Data Years | ||
|---|---|---|---|---|
| Indices | Sample, 2008 | Sample, 2008 | 2007 | 2009 |
| ||||
| Number of admissions | 150,035 | 149,646 | 259,911 | 279,377 |
| Number of hospitals | 4537 | 4535 | 4636 | 4571 |
| Mean risk‐standardized mortality rate, % (SD) | 8.62 (0.94) | 8.64 (1.07) | 8.97 (1.12) | 8.08 (1.09) |
| Calibration, 0, 1 | 0.034, 0.985 | 0.009, 1.004 | 0.095, 1.022 | 0.120, 0.981 |
| Discriminationpredictive ability, lowest decile %highest decile % | 1.5223.74 | 1.6023.78 | 1.5424.64 | 1.4222.36 |
| Discriminationarea under the ROC curve, C statistic | 0.720 | 0.723 | 0.728 | 0.722 |
| Residuals lack of fit, Pearson residual fall % | ||||
| 2 | 0 | 0 | 0 | 0 |
| 2, 0 | 91.14 | 91.4 | 91.08 | 91.93 |
| 0, 2 | 1.66 | 1.7 | 1.96 | 1.42 |
| 2+ | 6.93 | 6.91 | 6.96 | 6.65 |
| Model Wald 2 (number of covariates) | 6982.11 (42) | 7051.50 (42) | 13042.35 (42) | 12542.15 (42) |
| P value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Between‐hospital variance, (standard error) | 0.067 (0.008) | 0.078 (0.009) | 0.067 (0.006) | 0.072 (0.006) |
Reliability testing demonstrated consistent performance over several years. The frequency and ORs of the variables included in the model showed only minor changes over time. The area under the ROC curve (C statistic) was 0.73 for the model in the 2007 sample and 0.72 for the model using 2009 data (Table 3).
Hospital Risk‐Standardized Mortality Rates
The mean unadjusted hospital 30‐day mortality rate was 8.6% and ranged from 0% to 100% (Figure 2a). Risk‐standardized mortality rates varied across hospitals (Figure 2b). The mean risk‐standardized mortality rate was 8.6% and ranged from 5.9% to 13.5%. The odds of mortality at a hospital 1 standard deviation above average was 1.20 times that of a hospital 1 standard deviation below average.

DISCUSSION
We present a hospital‐level risk‐standardized mortality measure for patients admitted with COPD based on administrative claims data that are intended for public reporting and that have achieved endorsement by the National Quality Forum, a voluntary consensus standards‐setting organization. Across more than 4500 US hospitals, the mean 30‐day risk‐standardized mortality rate in 2008 was 8.6%, and we observed considerable variation across institutions, despite adjustment for case mix, suggesting that improvement by lower‐performing institutions may be an achievable goal.
Although improving the delivery of evidence‐based care processes and outcomes of patients with acute myocardial infarction, heart failure, and pneumonia has been the focus of national quality improvement efforts for more than a decade, COPD has largely been overlooked.[23] Within this context, this analysis represents the first attempt to systematically measure, at the hospital level, 30‐day all‐cause mortality for patients admitted to US hospitals for exacerbation of COPD. The model we have developed and validated is intended to be used to compare the performance of hospitals while controlling for differences in the pretreatment risk of mortality of patients and accounting for the clustering of patients within hospitals, and will facilitate surveillance of hospital‐level risk‐adjusted outcomes over time.
In contrast to process‐based measures of quality, such as the percentage of patients with pneumonia who receive appropriate antibiotic therapy, performance measures based on patient outcomes provide a more comprehensive view of care and are more consistent with patients' goals.[24] Additionally, it is well established that hospital performance on individual and composite process measures explains only a small amount of the observed variation in patient outcomes between institutions.[25] In this regard, outcome measures incorporate important, but difficult to measure aspects of care, such as diagnostic accuracy and timing, communication and teamwork, the recognition and response to complications, care coordination at the time of transfers between levels of care, and care settings. Nevertheless, when used for making inferences about the quality of hospital care, individual measures such as the risk‐standardized hospital mortality rate should be interpreted in the context of other performance measures, including readmission, patient experience, and costs of care.
A number of prior investigators have described the outcomes of care for patients hospitalized with exacerbations of COPD, including identifying risk factors for mortality. Patil et al. carried out an analysis of the 1996 Nationwide Inpatient Sample and described an overall in‐hospital mortality rate of 2.5% among patients with COPD, and reported that a multivariable model containing sociodemographic characteristics about the patient and comorbidities had an area under the ROC curve of 0.70.[3] In contrast, this hospital‐level measure includes patients with a principal diagnosis of respiratory failure and focuses on 30‐day rather than inpatient mortality, accounting for the nearly 3‐fold higher mortality rate we observed. In a more recent study that used clinical from a large multistate database, Tabak et al. developed a prediction model for inpatient mortality for patients with COPD that contained only 4 factors: age, blood urea nitrogen, mental status, and pulse, and achieved an area under the ROC curve of 0.72.[4] The simplicity of such a model and its reliance on clinical measurements makes it particularly well suited for bedside application by clinicians, but less valuable for large‐scale public reporting programs that rely on administrative data. In the only other study identified that focused on the assessment of hospital mortality rates, Agabiti et al. analyzed the outcomes of 12,756 patients hospitalized for exacerbations of COPD, using similar ICD‐9‐CM diagnostic criteria as in this study, at 21 hospitals in Rome, Italy.[26] They reported an average crude 30‐day mortality rate of 3.8% among a group of 5 benchmark hospitals and an average mortality of 7.5% (range, 5.2%17.2%) among the remaining institutions.
To put the variation we observed in mortality rates into a broader context, the relative difference in the risk‐standardized hospital mortality rates across the 10th to 90th percentiles of hospital performance was 25% for acute myocardial infarction and 39% for heart failure, whereas rates varied 30% for COPD, from 7.6% to 9.9%.[27] Model discrimination in COPD (C statistic, 0.72) was also similar to that reported for models used for public reporting of hospital mortality in acute myocardial infarction (C statistic, 0.71) and pneumonia (C statistic, 0.72).
This study has a number of important strengths. First, the model was developed from a large sample of recent Medicare claims, achieved good discrimination, and was validated in samples not limited to Medicare beneficiaries. Second, by including patients with a principal diagnosis of COPD, as well as those with a principal diagnosis of acute respiratory failure when accompanied by a secondary diagnosis of COPD with acute exacerbation, this model can be used to assess hospital performance across the full spectrum of disease severity. This broad set of ICD‐9‐CM codes used to define the cohort also ensures that efforts to measure hospital performance will be less influenced by differences in documentation and coding practices across hospitals relating to the diagnosis or sequencing of acute respiratory failure diagnoses. Moreover, the inclusion of patients with respiratory failure is important because these patients have the greatest risk of mortality, and are those in whom efforts to improve the quality and safety of care may have the greatest impact. Third, rather than relying solely on information documented during the index admission, we used ambulatory and inpatient claims from the full year prior to the index admission to identify comorbidities and to distinguish them from potential complications of care. Finally, we did not include factors such as hospital characteristics (eg, number of beds, teaching status) in the model. Although they might have improved overall predictive ability, the goal of the hospital mortality measure is to enable comparisons of mortality rates among hospitals while controlling for differences in patient characteristics. To the extent that factors such as size or teaching status might be independently associated with hospital outcomes, it would be inappropriate to adjust away their effects, because mortality risk should not be influenced by hospital characteristics other than through their effects on quality.
These results should be viewed in light of several limitations. First, we used ICD‐9‐CM codes derived from claims files to define the patient populations included in the measure rather than collecting clinical or physiologic information prospectively or through manual review of medical records, such as the forced expiratory volume in 1 second or whether the patient required long‐term oxygen therapy. Nevertheless, we included a broad set of potential diagnosis codes to capture the full spectrum of COPD exacerbations and to minimize differences in coding across hospitals. Second, because the risk‐adjustment included diagnoses coded in the year prior to the index admission, it is potentially subject to bias due to regional differences in medical care utilization that are not driven by underlying differences in patient illness.[28] Third, using administrative claims data, we observed some paradoxical associations in the model that are difficult to explain on clinical grounds, such as a protective effect of substance and alcohol abuse or prior episodes of respiratory failure. Fourth, although we excluded patients from the analysis who were enrolled in hospice prior to, or on the day of, the index admission, we did not exclude those who choose to withdraw support, transition to comfort measures only, or enrolled in hospice care during a hospitalization. We do not seek to penalize hospitals for being sensitive to the preferences of patients at the end of life. At the same time, it is equally important that the measure is capable of detecting the outcomes of suboptimal care that may in some instances lead a patient or their family to withdraw support or choose hospice. Finally, we did not have the opportunity to validate the model against a clinical registry of patients with COPD, because such data do not currently exist. Nevertheless, the use of claims as a surrogate for chart data for risk adjustment has been validated for several conditions, including acute myocardial infarction, heart failure, and pneumonia.[29, 30]
CONCLUSIONS
Risk‐standardized 30‐day mortality rates for Medicare beneficiaries with COPD vary across hospitals in the US. Calculating and reporting hospital outcomes using validated performance measures may catalyze quality improvement activities and lead to better outcomes. Additional research would be helpful to confirm that hospitals with lower mortality rates achieve care that meets the goals of patients and their families better than at hospitals with higher mortality rates.
Acknowledgment
The authors thank the following members of the technical expert panel: Darlene Bainbridge, RN, MS, NHA, CPHQ, CPHRM, President/CEO, Darlene D. Bainbridge & Associates, Inc.; Robert A. Balk, MD, Director of Pulmonary and Critical Care Medicine, Rush University Medical Center; Dale Bratzler, DO, MPH, President and CEO, Oklahoma Foundation for Medical Quality; Scott Cerreta, RRT, Director of Education, COPD Foundation; Gerard J. Criner, MD, Director of Temple Lung Center and Divisions of Pulmonary and Critical Care Medicine, Temple University; Guy D'Andrea, MBA, President, Discern Consulting; Jonathan Fine, MD, Director of Pulmonary Fellowship, Research and Medical Education, Norwalk Hospital; David Hopkins, MS, PhD, Senior Advisor, Pacific Business Group on Health; Fred Martin Jacobs, MD, JD, FACP, FCCP, FCLM, Executive Vice President and Director, Saint Barnabas Quality Institute; Natalie Napolitano, MPH, RRT‐NPS, Respiratory Therapist, Inova Fairfax Hospital; Russell Robbins, MD, MBA, Principal and Senior Clinical Consultant, Mercer. In addition, the authors acknowledge and thank Angela Merrill, Sandi Nelson, Marian Wrobel, and Eric Schone from Mathematica Policy Research, Inc., Sharon‐Lise T. Normand from Harvard Medical School, and Lein Han and Michael Rapp at The Centers for Medicare & Medicaid Services for their contributions to this work.
Disclosures
Peter K. Lindenauer, MD, MSc, is the guarantor of this article, taking responsibility for the integrity of the work as a whole, from inception to published article, and takes responsibility for the content of the manuscript, including the data and data analysis. All authors have made substantial contributions to the conception and design, or acquisition of data, or analysis and interpretation of data; have drafted the submitted article or revised it critically for important intellectual content; and have provided final approval of the version to be published. Preparation of this manuscript was completed under Contract Number: HHSM‐5002008‐0025I/HHSM‐500‐T0001, Modification No. 000007, Option Year 2 Measure Instrument Development and Support (MIDS). Sponsors did not contribute to the development of the research or manuscript. Dr. Au reports being an unpaid research consultant for Bosch Inc. He receives research funding from the NIH, Department of Veterans Affairs, AHRQ, and Gilead Sciences. The views of the this manuscript represent the authors and do not necessarily represent those of the Department of Veterans Affairs. Drs. Drye and Bernheim report receiving contract funding from CMS to develop and maintain quality measures.
Chronic obstructive pulmonary disease (COPD) affects as many as 24 million individuals in the United States, is responsible for more than 700,000 annual hospital admissions, and is currently the nation's third leading cause of death, accounting for nearly $49.9 billion in medical spending in 2010.[1, 2] Reported in‐hospital mortality rates for patients hospitalized for exacerbations of COPD range from 2% to 5%.[3, 4, 5, 6, 7] Information about 30‐day mortality rates following hospitalization for COPD is more limited; however, international studies suggest that rates range from 3% to 9%,[8, 9] and 90‐day mortality rates exceed 15%.[10]
Despite this significant clinical and economic impact, there have been no large‐scale, sustained efforts to measure the quality or outcomes of hospital care for patients with COPD in the United States. What little is known about the treatment of patients with COPD suggests widespread opportunities to increase adherence to guideline‐recommended therapies, to reduce the use of ineffective treatments and tests, and to address variation in care across institutions.[5, 11, 12]
Public reporting of hospital performance is a key strategy for improving the quality and safety of hospital care, both in the United States and internationally.[13] Since 2007, the Centers for Medicare and Medicaid Services (CMS) has reported hospital mortality rates on the Hospital Compare Web site, and COPD is 1 of the conditions highlighted in the Affordable Care Act for future consideration.[14] Such initiatives rely on validated, risk‐adjusted performance measures for comparisons across institutions and to enable outcomes to be tracked over time. We present the development, validation, and results of a model intended for public reporting of risk‐standardized mortality rates for patients hospitalized with exacerbations of COPD that has been endorsed by the National Quality Forum.[15]
METHODS
Approach to Measure Development
We developed this measure in accordance with guidelines described by the National Quality Forum,[16] CMS' Measure Management System,[17] and the American Heart Association scientific statement, Standards for Statistical Models Used for Public Reporting of Health Outcomes.[18] Throughout the process we obtained expert clinical and stakeholder input through meetings with a clinical advisory group and a national technical expert panel (see Acknowledgments). Last, we presented the proposed measure specifications and a summary of the technical expert panel discussions online and made a widely distributed call for public comments. We took the comments into consideration during the final stages of measure development (available at
Data Sources
We used claims data from Medicare inpatient, outpatient, and carrier (physician) Standard Analytic Files from 2008 to develop and validate the model, and examined model reliability using data from 2007 and 2009. The Medicare enrollment database was used to determine Medicare Fee‐for‐Service enrollment and mortality.
Study Cohort
Admissions were considered eligible for inclusion if the patient was 65 years or older, was admitted to a nonfederal acute care hospital in the United States, and had a principal diagnosis of COPD or a principal diagnosis of acute respiratory failure or respiratory arrest when paired with a secondary diagnosis of COPD with exacerbation (Table 1).
| ICD‐9‐CM | Description |
|---|---|
| |
| 491.21 | Obstructive chronic bronchitis; with (acute) exacerbation; acute exacerbation of COPD, decompensated COPD, decompensated COPD with exacerbation |
| 491.22 | Obstructive chronic bronchitis; with acute bronchitis |
| 491.8 | Other chronic bronchitis; chronic: tracheitis, tracheobronchitis |
| 491.9 | Unspecified chronic bronchitis |
| 492.8 | Other emphysema; emphysema (lung or pulmonary): NOS, centriacinar, centrilobular, obstructive, panacinar, panlobular, unilateral, vesicular; MacLeod's syndrome; Swyer‐James syndrome; unilateral hyperlucent lung |
| 493.20 | Chronic obstructive asthma; asthma with COPD, chronic asthmatic bronchitis, unspecified |
| 493.21 | Chronic obstructive asthma; asthma with COPD, chronic asthmatic bronchitis, with status asthmaticus |
| 493.22 | Chronic obstructive asthma; asthma with COPD, chronic asthmatic bronchitis, with (acute) exacerbation |
| 496 | Chronic: nonspecific lung disease, obstructive lung disease, obstructive pulmonary disease (COPD) NOS. (Note: This code is not to be used with any code from categories 491493.) |
| 518.81a | Other diseases of lung; acute respiratory failure; respiratory failure NOS |
| 518.82a | Other diseases of lung; acute respiratory failure; other pulmonary insufficiency, acute respiratory distress |
| 518.84a | Other diseases of lung; acute respiratory failure; acute and chronic respiratory failure |
| 799.1a | Other ill‐defined and unknown causes of morbidity and mortality; respiratory arrest, cardiorespiratory failure |
If a patient was discharged and readmitted to a second hospital on the same or the next day, we combined the 2 acute care admissions into a single episode of care and assigned the mortality outcome to the first admitting hospital. We excluded admissions for patients who were enrolled in Medicare Hospice in the 12 months prior to or on the first day of the index hospitalization. An index admission was any eligible admission assessed in the measure for the outcome. We also excluded admissions for patients who were discharged against medical advice, those for whom vital status at 30 days was unknown or recorded inconsistently, and patients with unreliable data (eg, age >115 years). For patients with multiple hospitalizations during a single year, we randomly selected 1 admission per patient to avoid survival bias. Finally, to assure adequate risk adjustment we limited the analysis to patients who had continuous enrollment in Medicare Fee‐for‐Service Parts A and B for the 12 months prior to their index admission so that we could identify comorbid conditions coded during all prior encounters.
Outcomes
The outcome of 30‐day mortality was defined as death from any cause within 30 days of the admission date for the index hospitalization. Mortality was assessed at 30 days to standardize the period of outcome ascertainment,[19] and because 30 days is a clinically meaningful time frame, during which differences in the quality of hospital care may be revealed.
Risk‐Adjustment Variables
We randomly selected half of all COPD admissions in 2008 that met the inclusion and exclusion criteria to create a model development sample. Candidate variables for inclusion in the risk‐standardized model were selected by a clinician team from diagnostic groups included in the Hierarchical Condition Category clinical classification system[20] and included age and comorbid conditions. Sleep apnea (International Classification of Diseases, 9th Revision, Clinical Modification [ICD‐9‐CM] condition codes 327.20, 327.21, 327.23, 327.27, 327.29, 780.51, 780.53, and 780.57) and mechanical ventilation (ICD‐9‐CM procedure codes 93.90, 96.70, 96.71, and 96.72) were also included as candidate variables.
We defined a condition as present for a given patient if it was coded in the inpatient, outpatient, or physician claims data sources in the preceding 12 months, including the index admission. Because a subset of the condition category variables can represent a complication of care, we did not consider them to be risk factors if they appeared only as secondary diagnosis codes for the index admission and not in claims submitted during the prior year.
We selected final variables for inclusion in the risk‐standardized model based on clinical considerations and a modified approach to stepwise logistic regression. The final patient‐level risk‐adjustment model included 42 variables (Table 2).
| Variable | Development Sample (150,035 Admissions at 4537 Hospitals) | Validation Sample (149,646 Admissions at 4535 Hospitals) | ||||
|---|---|---|---|---|---|---|
| Frequency, % | OR | 95% CI | Frequency, % | OR | 95% CI | |
| ||||||
| Demographics | ||||||
| Age 65 years (continuous) | 1.03 | 1.03‐1.04 | 1.03 | 1.03‐1.04 | ||
| Cardiovascular/respiratory | ||||||
| Sleep apnea (ICD‐9‐CM: 327.20, 327.21, 327.23, 327.27, 327.29, 780.51, 780.53, 780.57)a | 9.57 | 0.87 | 0.81‐0.94 | 9.72 | 0.84 | 0.78‐0.90 |
| History of mechanical ventilation (ICD‐9‐CM: 93.90, 96.70, 96.71, 96.72)a | 6.00 | 1.19 | 1.11‐1.27 | 6.00 | 1.15 | 1.08‐1.24 |
| Respirator dependence/respiratory failure (CC 7778)a | 1.15 | 0.89 | 0.77‐1.02 | 1.20 | 0.78 | 0.68‐0.91 |
| Cardiorespiratory failure and shock (CC 79) | 26.35 | 1.60 | 1.53‐1.68 | 26.34 | 1.59 | 1.52‐1.66 |
| Congestive heart failure (CC 80) | 41.50 | 1.34 | 1.28‐1.39 | 41.39 | 1.31 | 1.25‐1.36 |
| Chronic atherosclerosis (CC 8384)a | 50.44 | 0.87 | 0.83‐0.90 | 50.12 | 0.91 | 0.87‐0.94 |
| Arrhythmias (CC 9293) | 37.15 | 1.17 | 1.12‐1.22 | 37.06 | 1.15 | 1.10‐1.20 |
| Vascular or circulatory disease (CC 104106) | 38.20 | 1.09 | 1.05‐1.14 | 38.09 | 1.02 | 0.98‐1.06 |
| Fibrosis of lung and other chronic lung disorder (CC 109) | 16.96 | 1.08 | 1.03‐1.13 | 17.08 | 1.11 | 1.06‐1.17 |
| Asthma (CC 110) | 17.05 | 0.67 | 0.63‐0.70 | 16.90 | 0.67 | 0.63‐0.70 |
| Pneumonia (CC 111113) | 49.46 | 1.29 | 1.24‐1.35 | 49.41 | 1.27 | 1.22‐1.33 |
| Pleural effusion/pneumothorax (CC 114) | 11.78 | 1.17 | 1.11‐1.23 | 11.54 | 1.18 | 1.12‐1.25 |
| Other lung disorders (CC 115) | 53.07 | 0.80 | 0.77‐0.83 | 53.17 | 0.83 | 0.80‐0.87 |
| Other comorbid conditions | ||||||
| Metastatic cancer and acute leukemia (CC 7) | 2.76 | 2.34 | 2.14‐2.56 | 2.79 | 2.15 | 1.97‐2.35 |
| Lung, upper digestive tract, and other severe cancers (CC 8)a | 5.98 | 1.80 | 1.68‐1.92 | 6.02 | 1.98 | 1.85‐2.11 |
| Lymphatic, head and neck, brain, and other major cancers; breast, prostate, colorectal and other cancers and tumors; other respiratory and heart neoplasms (CC 911) | 14.13 | 1.03 | 0.97‐1.08 | 14.19 | 1.01 | 0.95‐1.06 |
| Other digestive and urinary neoplasms (CC 12) | 6.91 | 0.91 | 0.84‐0.98 | 7.05 | 0.85 | 0.79‐0.92 |
| Diabetes and DM complications (CC 1520, 119120) | 38.31 | 0.91 | 0.87‐0.94 | 38.29 | 0.91 | 0.87‐0.94 |
| Protein‐calorie malnutrition (CC 21) | 7.40 | 2.18 | 2.07‐2.30 | 7.44 | 2.09 | 1.98‐2.20 |
| Disorders of fluid/electrolyte/acid‐base (CC 2223) | 32.05 | 1.13 | 1.08‐1.18 | 32.16 | 1.24 | 1.19‐1.30 |
| Other endocrine/metabolic/nutritional disorders (CC 24) | 67.99 | 0.75 | 0.72‐0.78 | 67.88 | 0.76 | 0.73‐0.79 |
| Other gastrointestinal disorders (CC 36) | 56.21 | 0.81 | 0.78‐0.84 | 56.18 | 0.78 | 0.75‐0.81 |
| Osteoarthritis of hip or knee (CC 40) | 9.32 | 0.74 | 0.69‐0.79 | 9.33 | 0.80 | 0.74‐0.85 |
| Other musculoskeletal and connective tissue disorders (CC 43) | 64.14 | 0.83 | 0.80‐0.86 | 64.20 | 0.83 | 0.80‐0.87 |
| Iron deficiency and other/unspecified anemias and blood disease (CC 47) | 40.80 | 1.08 | 1.04‐1.12 | 40.72 | 1.08 | 1.04‐1.13 |
| Dementia and senility (CC 4950) | 17.06 | 1.09 | 1.04‐1.14 | 16.97 | 1.09 | 1.04‐1.15 |
| Drug/alcohol abuse, without dependence (CC 53)a | 23.51 | 0.78 | 0.75‐0.82 | 23.38 | 0.76 | 0.72‐0.80 |
| Other psychiatric disorders (CC 60)a | 16.49 | 1.12 | 1.07‐1.18 | 16.43 | 1.12 | 1.06‐1.17 |
| Quadriplegia, paraplegia, functional disability (CC 6769, 100102, 177178) | 4.92 | 1.03 | 0.95‐1.12 | 4.92 | 1.08 | 0.99‐1.17 |
| Mononeuropathy, other neurological conditions/emnjuries (CC 76) | 11.35 | 0.85 | 0.80‐0.91 | 11.28 | 0.88 | 0.83‐0.93 |
| Hypertension and hypertensive disease (CC 9091) | 80.40 | 0.78 | 0.75‐0.82 | 80.35 | 0.79 | 0.75‐0.83 |
| Stroke (CC 9596)a | 6.77 | 1.00 | 0.93‐1.08 | 6.73 | 0.98 | 0.91‐1.05 |
| Retinal disorders, except detachment and vascular retinopathies (CC 121) | 10.79 | 0.87 | 0.82‐0.93 | 10.69 | 0.90 | 0.85‐0.96 |
| Other eye disorders (CC 124)a | 19.05 | 0.90 | 0.86‐0.95 | 19.13 | 0.98 | 0.85‐0.93 |
| Other ear, nose, throat, and mouth disorders (CC 127) | 35.21 | 0.83 | 0.80‐0.87 | 35.02 | 0.80 | 0.77‐0.83 |
| Renal failure (CC 131)a | 17.92 | 1.12 | 1.07‐1.18 | 18.16 | 1.13 | 1.08‐1.19 |
| Decubitus ulcer or chronic skin ulcer (CC 148149) | 7.42 | 1.27 | 1.19‐1.35 | 7.42 | 1.33 | 1.25‐1.42 |
| Other dermatological disorders (CC 153) | 28.46 | 0.90 | 0.87‐0.94 | 28.32 | 0.89 | 0.86‐0.93 |
| Trauma (CC 154156, 158161) | 9.04 | 1.09 | 1.03‐1.16 | 8.99 | 1.15 | 1.08‐1.22 |
| Vertebral fractures (CC 157) | 5.01 | 1.33 | 1.24‐1.44 | 4.97 | 1.29 | 1.20‐1.39 |
| Major complications of medical care and trauma (CC 164) | 5.47 | 0.81 | 0.75‐0.88 | 5.55 | 0.82 | 0.76‐0.89 |
Model Derivation
We used hierarchical logistic regression models to model the log‐odds of mortality as a function of patient‐level clinical characteristics and a random hospital‐level intercept. At the patient level, each model adjusts the log‐odds of mortality for age and the selected clinical covariates. The second level models the hospital‐specific intercepts as arising from a normal distribution. The hospital intercept represents the underlying risk of mortality, after accounting for patient risk. If there were no differences among hospitals, then after adjusting for patient risk, the hospital intercepts should be identical across all hospitals.
Estimation of Hospital Risk‐Standardized Mortality Rate
We calculated a risk‐standardized mortality rate, defined as the ratio of predicted to expected deaths (similar to observed‐to‐expected), multiplied by the national unadjusted mortality rate.[21] The expected number of deaths for each hospital was estimated by applying the estimated regression coefficients to the characteristics of each hospital's patients, adding the average of the hospital‐specific intercepts, transforming the data by using an inverse logit function, and summing the data from all patients in the hospital to obtain the count. The predicted number of deaths was calculated in the same way, substituting the hospital‐specific intercept for the average hospital‐specific intercept.
Model Performance, Validation, and Reliability Testing
We used the remaining admissions in 2008 as the model validation sample. We computed several summary statistics to assess the patient‐level model performance in both the development and validation samples,[22] including over‐fitting indices, predictive ability, area under the receiver operating characteristic (ROC) curve, distribution of residuals, and model 2. In addition, we assessed face validity through a survey of members of the technical expert panel. To assess reliability of the model across data years, we repeated the modeling process using qualifying COPD admissions in both 2007 and 2009. Finally, to assess generalizability we evaluated the model's performance in an all‐payer sample of data from patients admitted to California hospitals in 2006.
Analyses were conducted using SAS version 9.1.3 (SAS Institute Inc., Cary, NC). We estimated the hierarchical models using the GLIMMIX procedure in SAS.
The Human Investigation Committee at the Yale University School of Medicine/Yale New Haven Hospital approved an exemption (HIC#0903004927) for the authors to use CMS claims and enrollment data for research analyses and publication.
RESULTS
Model Derivation
After exclusions were applied, the development sample included 150,035 admissions in 2008 at 4537 US hospitals (Figure 1). Factors that were most strongly associated with the risk of mortality included metastatic cancer (odds ratio [OR] 2.34), protein calorie malnutrition (OR 2.18), nonmetastatic cancers of the lung and upper digestive tract, (OR 1.80) cardiorespiratory failure and shock (OR 1.60), and congestive heart failure (OR 1.34) (Table 2).

Model Performance, Validation, and Reliability
The model had a C statistic of 0.72, indicating good discrimination, and predicted mortality in the development sample ranged from 1.52% in the lowest decile to 23.74% in the highest. The model validation sample, using the remaining cases from 2008, included 149,646 admissions from 4535 hospitals. Variable frequencies and ORs were similar in both samples (Table 2). Model performance was also similar in the validation samples, with good model discrimination and fit (Table 3). Ten of 12 technical expert panel members responded to the survey, of whom 90% at least somewhat agreed with the statement, the COPD mortality measure provides an accurate reflection of quality. When the model was applied to patients age 18 years and older in the 2006 California Patient Discharge Data, overall discrimination was good (C statistic, 0.74), including in those age 18 to 64 years (C statistic, 0.75; 65 and above C statistic, 0.70).
| Development | Validation | Data Years | ||
|---|---|---|---|---|
| Indices | Sample, 2008 | Sample, 2008 | 2007 | 2009 |
| ||||
| Number of admissions | 150,035 | 149,646 | 259,911 | 279,377 |
| Number of hospitals | 4537 | 4535 | 4636 | 4571 |
| Mean risk‐standardized mortality rate, % (SD) | 8.62 (0.94) | 8.64 (1.07) | 8.97 (1.12) | 8.08 (1.09) |
| Calibration, 0, 1 | 0.034, 0.985 | 0.009, 1.004 | 0.095, 1.022 | 0.120, 0.981 |
| Discriminationpredictive ability, lowest decile %highest decile % | 1.5223.74 | 1.6023.78 | 1.5424.64 | 1.4222.36 |
| Discriminationarea under the ROC curve, C statistic | 0.720 | 0.723 | 0.728 | 0.722 |
| Residuals lack of fit, Pearson residual fall % | ||||
| 2 | 0 | 0 | 0 | 0 |
| 2, 0 | 91.14 | 91.4 | 91.08 | 91.93 |
| 0, 2 | 1.66 | 1.7 | 1.96 | 1.42 |
| 2+ | 6.93 | 6.91 | 6.96 | 6.65 |
| Model Wald 2 (number of covariates) | 6982.11 (42) | 7051.50 (42) | 13042.35 (42) | 12542.15 (42) |
| P value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Between‐hospital variance, (standard error) | 0.067 (0.008) | 0.078 (0.009) | 0.067 (0.006) | 0.072 (0.006) |
Reliability testing demonstrated consistent performance over several years. The frequency and ORs of the variables included in the model showed only minor changes over time. The area under the ROC curve (C statistic) was 0.73 for the model in the 2007 sample and 0.72 for the model using 2009 data (Table 3).
Hospital Risk‐Standardized Mortality Rates
The mean unadjusted hospital 30‐day mortality rate was 8.6% and ranged from 0% to 100% (Figure 2a). Risk‐standardized mortality rates varied across hospitals (Figure 2b). The mean risk‐standardized mortality rate was 8.6% and ranged from 5.9% to 13.5%. The odds of mortality at a hospital 1 standard deviation above average was 1.20 times that of a hospital 1 standard deviation below average.

DISCUSSION
We present a hospital‐level risk‐standardized mortality measure for patients admitted with COPD based on administrative claims data that are intended for public reporting and that have achieved endorsement by the National Quality Forum, a voluntary consensus standards‐setting organization. Across more than 4500 US hospitals, the mean 30‐day risk‐standardized mortality rate in 2008 was 8.6%, and we observed considerable variation across institutions, despite adjustment for case mix, suggesting that improvement by lower‐performing institutions may be an achievable goal.
Although improving the delivery of evidence‐based care processes and outcomes of patients with acute myocardial infarction, heart failure, and pneumonia has been the focus of national quality improvement efforts for more than a decade, COPD has largely been overlooked.[23] Within this context, this analysis represents the first attempt to systematically measure, at the hospital level, 30‐day all‐cause mortality for patients admitted to US hospitals for exacerbation of COPD. The model we have developed and validated is intended to be used to compare the performance of hospitals while controlling for differences in the pretreatment risk of mortality of patients and accounting for the clustering of patients within hospitals, and will facilitate surveillance of hospital‐level risk‐adjusted outcomes over time.
In contrast to process‐based measures of quality, such as the percentage of patients with pneumonia who receive appropriate antibiotic therapy, performance measures based on patient outcomes provide a more comprehensive view of care and are more consistent with patients' goals.[24] Additionally, it is well established that hospital performance on individual and composite process measures explains only a small amount of the observed variation in patient outcomes between institutions.[25] In this regard, outcome measures incorporate important, but difficult to measure aspects of care, such as diagnostic accuracy and timing, communication and teamwork, the recognition and response to complications, care coordination at the time of transfers between levels of care, and care settings. Nevertheless, when used for making inferences about the quality of hospital care, individual measures such as the risk‐standardized hospital mortality rate should be interpreted in the context of other performance measures, including readmission, patient experience, and costs of care.
A number of prior investigators have described the outcomes of care for patients hospitalized with exacerbations of COPD, including identifying risk factors for mortality. Patil et al. carried out an analysis of the 1996 Nationwide Inpatient Sample and described an overall in‐hospital mortality rate of 2.5% among patients with COPD, and reported that a multivariable model containing sociodemographic characteristics about the patient and comorbidities had an area under the ROC curve of 0.70.[3] In contrast, this hospital‐level measure includes patients with a principal diagnosis of respiratory failure and focuses on 30‐day rather than inpatient mortality, accounting for the nearly 3‐fold higher mortality rate we observed. In a more recent study that used clinical from a large multistate database, Tabak et al. developed a prediction model for inpatient mortality for patients with COPD that contained only 4 factors: age, blood urea nitrogen, mental status, and pulse, and achieved an area under the ROC curve of 0.72.[4] The simplicity of such a model and its reliance on clinical measurements makes it particularly well suited for bedside application by clinicians, but less valuable for large‐scale public reporting programs that rely on administrative data. In the only other study identified that focused on the assessment of hospital mortality rates, Agabiti et al. analyzed the outcomes of 12,756 patients hospitalized for exacerbations of COPD, using similar ICD‐9‐CM diagnostic criteria as in this study, at 21 hospitals in Rome, Italy.[26] They reported an average crude 30‐day mortality rate of 3.8% among a group of 5 benchmark hospitals and an average mortality of 7.5% (range, 5.2%17.2%) among the remaining institutions.
To put the variation we observed in mortality rates into a broader context, the relative difference in the risk‐standardized hospital mortality rates across the 10th to 90th percentiles of hospital performance was 25% for acute myocardial infarction and 39% for heart failure, whereas rates varied 30% for COPD, from 7.6% to 9.9%.[27] Model discrimination in COPD (C statistic, 0.72) was also similar to that reported for models used for public reporting of hospital mortality in acute myocardial infarction (C statistic, 0.71) and pneumonia (C statistic, 0.72).
This study has a number of important strengths. First, the model was developed from a large sample of recent Medicare claims, achieved good discrimination, and was validated in samples not limited to Medicare beneficiaries. Second, by including patients with a principal diagnosis of COPD, as well as those with a principal diagnosis of acute respiratory failure when accompanied by a secondary diagnosis of COPD with acute exacerbation, this model can be used to assess hospital performance across the full spectrum of disease severity. This broad set of ICD‐9‐CM codes used to define the cohort also ensures that efforts to measure hospital performance will be less influenced by differences in documentation and coding practices across hospitals relating to the diagnosis or sequencing of acute respiratory failure diagnoses. Moreover, the inclusion of patients with respiratory failure is important because these patients have the greatest risk of mortality, and are those in whom efforts to improve the quality and safety of care may have the greatest impact. Third, rather than relying solely on information documented during the index admission, we used ambulatory and inpatient claims from the full year prior to the index admission to identify comorbidities and to distinguish them from potential complications of care. Finally, we did not include factors such as hospital characteristics (eg, number of beds, teaching status) in the model. Although they might have improved overall predictive ability, the goal of the hospital mortality measure is to enable comparisons of mortality rates among hospitals while controlling for differences in patient characteristics. To the extent that factors such as size or teaching status might be independently associated with hospital outcomes, it would be inappropriate to adjust away their effects, because mortality risk should not be influenced by hospital characteristics other than through their effects on quality.
These results should be viewed in light of several limitations. First, we used ICD‐9‐CM codes derived from claims files to define the patient populations included in the measure rather than collecting clinical or physiologic information prospectively or through manual review of medical records, such as the forced expiratory volume in 1 second or whether the patient required long‐term oxygen therapy. Nevertheless, we included a broad set of potential diagnosis codes to capture the full spectrum of COPD exacerbations and to minimize differences in coding across hospitals. Second, because the risk‐adjustment included diagnoses coded in the year prior to the index admission, it is potentially subject to bias due to regional differences in medical care utilization that are not driven by underlying differences in patient illness.[28] Third, using administrative claims data, we observed some paradoxical associations in the model that are difficult to explain on clinical grounds, such as a protective effect of substance and alcohol abuse or prior episodes of respiratory failure. Fourth, although we excluded patients from the analysis who were enrolled in hospice prior to, or on the day of, the index admission, we did not exclude those who choose to withdraw support, transition to comfort measures only, or enrolled in hospice care during a hospitalization. We do not seek to penalize hospitals for being sensitive to the preferences of patients at the end of life. At the same time, it is equally important that the measure is capable of detecting the outcomes of suboptimal care that may in some instances lead a patient or their family to withdraw support or choose hospice. Finally, we did not have the opportunity to validate the model against a clinical registry of patients with COPD, because such data do not currently exist. Nevertheless, the use of claims as a surrogate for chart data for risk adjustment has been validated for several conditions, including acute myocardial infarction, heart failure, and pneumonia.[29, 30]
CONCLUSIONS
Risk‐standardized 30‐day mortality rates for Medicare beneficiaries with COPD vary across hospitals in the US. Calculating and reporting hospital outcomes using validated performance measures may catalyze quality improvement activities and lead to better outcomes. Additional research would be helpful to confirm that hospitals with lower mortality rates achieve care that meets the goals of patients and their families better than at hospitals with higher mortality rates.
Acknowledgment
The authors thank the following members of the technical expert panel: Darlene Bainbridge, RN, MS, NHA, CPHQ, CPHRM, President/CEO, Darlene D. Bainbridge & Associates, Inc.; Robert A. Balk, MD, Director of Pulmonary and Critical Care Medicine, Rush University Medical Center; Dale Bratzler, DO, MPH, President and CEO, Oklahoma Foundation for Medical Quality; Scott Cerreta, RRT, Director of Education, COPD Foundation; Gerard J. Criner, MD, Director of Temple Lung Center and Divisions of Pulmonary and Critical Care Medicine, Temple University; Guy D'Andrea, MBA, President, Discern Consulting; Jonathan Fine, MD, Director of Pulmonary Fellowship, Research and Medical Education, Norwalk Hospital; David Hopkins, MS, PhD, Senior Advisor, Pacific Business Group on Health; Fred Martin Jacobs, MD, JD, FACP, FCCP, FCLM, Executive Vice President and Director, Saint Barnabas Quality Institute; Natalie Napolitano, MPH, RRT‐NPS, Respiratory Therapist, Inova Fairfax Hospital; Russell Robbins, MD, MBA, Principal and Senior Clinical Consultant, Mercer. In addition, the authors acknowledge and thank Angela Merrill, Sandi Nelson, Marian Wrobel, and Eric Schone from Mathematica Policy Research, Inc., Sharon‐Lise T. Normand from Harvard Medical School, and Lein Han and Michael Rapp at The Centers for Medicare & Medicaid Services for their contributions to this work.
Disclosures
Peter K. Lindenauer, MD, MSc, is the guarantor of this article, taking responsibility for the integrity of the work as a whole, from inception to published article, and takes responsibility for the content of the manuscript, including the data and data analysis. All authors have made substantial contributions to the conception and design, or acquisition of data, or analysis and interpretation of data; have drafted the submitted article or revised it critically for important intellectual content; and have provided final approval of the version to be published. Preparation of this manuscript was completed under Contract Number: HHSM‐5002008‐0025I/HHSM‐500‐T0001, Modification No. 000007, Option Year 2 Measure Instrument Development and Support (MIDS). Sponsors did not contribute to the development of the research or manuscript. Dr. Au reports being an unpaid research consultant for Bosch Inc. He receives research funding from the NIH, Department of Veterans Affairs, AHRQ, and Gilead Sciences. The views of the this manuscript represent the authors and do not necessarily represent those of the Department of Veterans Affairs. Drs. Drye and Bernheim report receiving contract funding from CMS to develop and maintain quality measures.
- FASTSTATS—chronic lower respiratory disease. Available at: http://www.cdc.gov/nchs/fastats/copd.htm. Accessed September 18, 2010.
- National Heart, Lung and Blood Institute. Morbidity and mortality chartbook. Available at: http://www.nhlbi.nih.gov/resources/docs/cht‐book.htm. Accessed April 27, 2010.
- , , , . In‐hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003;163(10):1180–1186.
- , , , , . Mortality and need for mechanical ventilation in acute exacerbations of chronic obstructive pulmonary disease: development and validation of a simple risk score. Arch Intern Med. 2009;169(17):1595–1602.
- , , , , , . Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2006;144(12):894–903.
- , , , , . Use of beta blockers and the risk of death in hospitalised patients with acute exacerbations of COPD. Thorax. 2008;63(4):301–305.
- , , , , . HCUP facts and figures: statistics on hospital‐based care in the United States, 2007. 2009. Available at: http://www.hcup‐us.ahrq.gov/reports.jsp. Accessed August 6, 2012.
- , . Predictors of long‐term survival in elderly patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Respirology. 2008;13(6):851–855.
- , , , , , . The impact on risk‐factor analysis of different mortality outcomes in COPD patients. Eur Respir J 2008;32(3):629–636.
- , , , , , . Clinical audit indicators of outcome following admission to hospital with acute exacerbation of chronic obstructive pulmonary disease. Thorax. 2002;57(2):137–141.
- , , , et al. The quality of obstructive lung disease care for adults in the United States as measured by adherence to recommended processes. Chest. 2006;130(6):1844–1850.
- , , , , , . Management of acute exacerbations of chronic obstructive pulmonary disease in the elderly: physician practices in the community hospital setting. J Okla State Med Assoc. 2004;97(6):227–232.
- , , . Leadership by Example: Coordinating Government Roles in Improving Health Care Quality. Washington, DC: National Academies Press; 2002.
- Patient Protection and Affordable Care Act [H.R. 3590], Pub. L. No. 111–148, §2702, 124 Stat. 119, 318–319 (March 23, 2010). Available at: http://www.gpo.gov/fdsys/pkg/PLAW‐111publ148/html/PLAW‐111publ148.htm. Accessed July 15, 2012.
- National Quality Forum. NQF Endorses Additional Pulmonary Measure. 2013. Available at: http://www.qualityforum.org/News_And_Resources/Press_Releases/2013/NQF_Endorses_Additional_Pulmonary_Measure.aspx. Accessed January 11, 2013.
- National Quality Forum. National voluntary consensus standards for patient outcomes: a consensus report. Washington, DC: National Quality Forum; 2011.
- The Measures Management System. The Centers for Medicare and Medicaid Services. Available at: http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/MMS/index.html?redirect=/MMS/. Accessed August 6, 2012.
- , , , et al. Standards for statistical models used for public reporting of health outcomes: an American Heart Association Scientific Statement from the Quality of Care and Outcomes Research Interdisciplinary Writing Group: cosponsored by the Council on Epidemiology and Prevention and the Stroke Council. Endorsed by the American College of Cardiology Foundation. Circulation. 2006;113(3):456–462.
- , , , et al. Comparison of hospital risk‐standardized mortality rates calculated by using in‐hospital and 30‐day models: an observational study with implications for hospital profiling. Ann Intern Med. 2012;156(1 pt 1):19–26.
- , , , et al. Diagnostic cost group hierarchical condition category models for Medicare risk adjustment. Report prepared for the Health Care Financing Administration. Health Economics Research, Inc.; 2000. Available at: http://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/Reports/downloads/pope_2000_2.pdf. Accessed November 7, 2009.
- , . Statistical and clinical aspects of hospital outcomes profiling. Stat Sci. 2007;22(2):206–226.
- , . Using full probability models to compute probabilities of actual interest to decision makers. Int J Technol Assess Health Care. 2001;17(1):17–26.
- , , . COPD performance measures: missing opportunities for improving care. Chest. 2010;137(5):1181–1189.
- , , , , . Measuring Performance For Treating Heart Attacks And Heart Failure: The Case For Outcomes Measurement. Health Aff. 2007;26(1):75–85.
- , , , et al. Hospital quality for acute myocardial infarction: correlation among process measures and relationship with short‐term mortality. JAMA. 2006;296(1):72–78.
- , , , et al. Profiling hospital performance to monitor the quality of care: the case of COPD. Eur Respir J. 2010;35(5):1031–1038.
- , , , et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30‐day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2(5):407–413.
- , , , , . Geographic variation in diagnosis frequency and risk of death among Medicare beneficiaries. JAMA. 2011;305(11):1113–1118.
- , , , et al. An administrative claims model for profiling hospital 30‐day mortality rates for pneumonia patients. PLoS ONE. 2011;6(4):e17401.
- , , , et al. An Administrative Claims Model Suitable for Profiling Hospital Performance Based on 30‐Day Mortality Rates Among Patients With Heart Failure. Circulation. 2006;113(13):1693–1701.
- FASTSTATS—chronic lower respiratory disease. Available at: http://www.cdc.gov/nchs/fastats/copd.htm. Accessed September 18, 2010.
- National Heart, Lung and Blood Institute. Morbidity and mortality chartbook. Available at: http://www.nhlbi.nih.gov/resources/docs/cht‐book.htm. Accessed April 27, 2010.
- , , , . In‐hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003;163(10):1180–1186.
- , , , , . Mortality and need for mechanical ventilation in acute exacerbations of chronic obstructive pulmonary disease: development and validation of a simple risk score. Arch Intern Med. 2009;169(17):1595–1602.
- , , , , , . Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2006;144(12):894–903.
- , , , , . Use of beta blockers and the risk of death in hospitalised patients with acute exacerbations of COPD. Thorax. 2008;63(4):301–305.
- , , , , . HCUP facts and figures: statistics on hospital‐based care in the United States, 2007. 2009. Available at: http://www.hcup‐us.ahrq.gov/reports.jsp. Accessed August 6, 2012.
- , . Predictors of long‐term survival in elderly patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Respirology. 2008;13(6):851–855.
- , , , , , . The impact on risk‐factor analysis of different mortality outcomes in COPD patients. Eur Respir J 2008;32(3):629–636.
- , , , , , . Clinical audit indicators of outcome following admission to hospital with acute exacerbation of chronic obstructive pulmonary disease. Thorax. 2002;57(2):137–141.
- , , , et al. The quality of obstructive lung disease care for adults in the United States as measured by adherence to recommended processes. Chest. 2006;130(6):1844–1850.
- , , , , , . Management of acute exacerbations of chronic obstructive pulmonary disease in the elderly: physician practices in the community hospital setting. J Okla State Med Assoc. 2004;97(6):227–232.
- , , . Leadership by Example: Coordinating Government Roles in Improving Health Care Quality. Washington, DC: National Academies Press; 2002.
- Patient Protection and Affordable Care Act [H.R. 3590], Pub. L. No. 111–148, §2702, 124 Stat. 119, 318–319 (March 23, 2010). Available at: http://www.gpo.gov/fdsys/pkg/PLAW‐111publ148/html/PLAW‐111publ148.htm. Accessed July 15, 2012.
- National Quality Forum. NQF Endorses Additional Pulmonary Measure. 2013. Available at: http://www.qualityforum.org/News_And_Resources/Press_Releases/2013/NQF_Endorses_Additional_Pulmonary_Measure.aspx. Accessed January 11, 2013.
- National Quality Forum. National voluntary consensus standards for patient outcomes: a consensus report. Washington, DC: National Quality Forum; 2011.
- The Measures Management System. The Centers for Medicare and Medicaid Services. Available at: http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/MMS/index.html?redirect=/MMS/. Accessed August 6, 2012.
- , , , et al. Standards for statistical models used for public reporting of health outcomes: an American Heart Association Scientific Statement from the Quality of Care and Outcomes Research Interdisciplinary Writing Group: cosponsored by the Council on Epidemiology and Prevention and the Stroke Council. Endorsed by the American College of Cardiology Foundation. Circulation. 2006;113(3):456–462.
- , , , et al. Comparison of hospital risk‐standardized mortality rates calculated by using in‐hospital and 30‐day models: an observational study with implications for hospital profiling. Ann Intern Med. 2012;156(1 pt 1):19–26.
- , , , et al. Diagnostic cost group hierarchical condition category models for Medicare risk adjustment. Report prepared for the Health Care Financing Administration. Health Economics Research, Inc.; 2000. Available at: http://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/Reports/downloads/pope_2000_2.pdf. Accessed November 7, 2009.
- , . Statistical and clinical aspects of hospital outcomes profiling. Stat Sci. 2007;22(2):206–226.
- , . Using full probability models to compute probabilities of actual interest to decision makers. Int J Technol Assess Health Care. 2001;17(1):17–26.
- , , . COPD performance measures: missing opportunities for improving care. Chest. 2010;137(5):1181–1189.
- , , , , . Measuring Performance For Treating Heart Attacks And Heart Failure: The Case For Outcomes Measurement. Health Aff. 2007;26(1):75–85.
- , , , et al. Hospital quality for acute myocardial infarction: correlation among process measures and relationship with short‐term mortality. JAMA. 2006;296(1):72–78.
- , , , et al. Profiling hospital performance to monitor the quality of care: the case of COPD. Eur Respir J. 2010;35(5):1031–1038.
- , , , et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30‐day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2(5):407–413.
- , , , , . Geographic variation in diagnosis frequency and risk of death among Medicare beneficiaries. JAMA. 2011;305(11):1113–1118.
- , , , et al. An administrative claims model for profiling hospital 30‐day mortality rates for pneumonia patients. PLoS ONE. 2011;6(4):e17401.
- , , , et al. An Administrative Claims Model Suitable for Profiling Hospital Performance Based on 30‐Day Mortality Rates Among Patients With Heart Failure. Circulation. 2006;113(13):1693–1701.
Copyright © 2013 Society of Hospital Medicine
Guidelines issued on radiation-induced heart disease
Cancer patients undergoing radiation therapy need to have baseline studies of cardiac function and routine screening for heart disease, according to recommendations from the European Society of Cardiology and the American Society of Echocardiography published July 16 in the European Heart Journal–Cardiovascular Imaging.
The groups recommend baseline preradiation echocardiography along with a cardiac exam as well as screening for risk factors. An annual cardiac history and physical should be performed to check for new-onset heart problems.
Within 10 years of treatment, 10%-30% of patients who undergo radiation therapy develop radiation-induced heart diseases (RIHD), including chronic pericarditis, myocardial fibrosis, coronary artery disease, aortic calcification, and valve regurgitation or stenosis. The hope of screening is to catch early RIHD, but screening is not currently routine.
"We wrote the expert consensus to raise the alarm that the risks of radiation-induced heart disease should not be ignored. The prevalence ... is increasing because the rate of cancer survival has improved," said Dr. Patrizio Lancellotti, who is a professor of cardiology at the University Hospital of Liège, Belgium, and led the recommendations task force.
Radiotherapy is given in more targeted form and at lower doses than it once was, but "patients are still at increased risk of RIHD, particularly when the heart is in the radiation field. This applies to patients treated for lymphoma, breast cancer, and esophageal cancer. Patients who receive radiotherapy for neck cancer are also at risk because lesions can develop on the carotid artery and increase the risk of stroke," Dr. Lancellotti said in a statement.
Using targeted radiation and alternate radiation fields, with avoidance and shielding of the heart, remain "the most important interventions to prevent" cardiac complications, the authors noted.
The task force advises that high-risk patients without evidence of heart disease on history and physical should have screening echocardiography every 5 years and noninvasive stress testing every 5-10 years; low-risk patients should have screening echocardiography every 10 years. If heart disorders are detected, routine monitoring should include echocardiography, cardiac magnetic resonance imaging, or carotid ultrasound as appropriate.
High-risk patients include those who received radiotherapy at younger ages; those who have cardiovascular risk factors or preexisting heart disease; and those who receive high-dose radiation (greater than 30 Gy), concomitant chemotherapy, radiation without shielding, or anterior or left chest radiation (Eur. Heart J. Cardiovasc. Imaging 2013;14:721-40).
The recommendations are based on an extensive literature review and analysis by Dr. Lancellotti and other specialists.
The authors reported no financial conflicts or outside funding for their work.
Cancer patients undergoing radiation therapy need to have baseline studies of cardiac function and routine screening for heart disease, according to recommendations from the European Society of Cardiology and the American Society of Echocardiography published July 16 in the European Heart Journal–Cardiovascular Imaging.
The groups recommend baseline preradiation echocardiography along with a cardiac exam as well as screening for risk factors. An annual cardiac history and physical should be performed to check for new-onset heart problems.
Within 10 years of treatment, 10%-30% of patients who undergo radiation therapy develop radiation-induced heart diseases (RIHD), including chronic pericarditis, myocardial fibrosis, coronary artery disease, aortic calcification, and valve regurgitation or stenosis. The hope of screening is to catch early RIHD, but screening is not currently routine.
"We wrote the expert consensus to raise the alarm that the risks of radiation-induced heart disease should not be ignored. The prevalence ... is increasing because the rate of cancer survival has improved," said Dr. Patrizio Lancellotti, who is a professor of cardiology at the University Hospital of Liège, Belgium, and led the recommendations task force.
Radiotherapy is given in more targeted form and at lower doses than it once was, but "patients are still at increased risk of RIHD, particularly when the heart is in the radiation field. This applies to patients treated for lymphoma, breast cancer, and esophageal cancer. Patients who receive radiotherapy for neck cancer are also at risk because lesions can develop on the carotid artery and increase the risk of stroke," Dr. Lancellotti said in a statement.
Using targeted radiation and alternate radiation fields, with avoidance and shielding of the heart, remain "the most important interventions to prevent" cardiac complications, the authors noted.
The task force advises that high-risk patients without evidence of heart disease on history and physical should have screening echocardiography every 5 years and noninvasive stress testing every 5-10 years; low-risk patients should have screening echocardiography every 10 years. If heart disorders are detected, routine monitoring should include echocardiography, cardiac magnetic resonance imaging, or carotid ultrasound as appropriate.
High-risk patients include those who received radiotherapy at younger ages; those who have cardiovascular risk factors or preexisting heart disease; and those who receive high-dose radiation (greater than 30 Gy), concomitant chemotherapy, radiation without shielding, or anterior or left chest radiation (Eur. Heart J. Cardiovasc. Imaging 2013;14:721-40).
The recommendations are based on an extensive literature review and analysis by Dr. Lancellotti and other specialists.
The authors reported no financial conflicts or outside funding for their work.
Cancer patients undergoing radiation therapy need to have baseline studies of cardiac function and routine screening for heart disease, according to recommendations from the European Society of Cardiology and the American Society of Echocardiography published July 16 in the European Heart Journal–Cardiovascular Imaging.
The groups recommend baseline preradiation echocardiography along with a cardiac exam as well as screening for risk factors. An annual cardiac history and physical should be performed to check for new-onset heart problems.
Within 10 years of treatment, 10%-30% of patients who undergo radiation therapy develop radiation-induced heart diseases (RIHD), including chronic pericarditis, myocardial fibrosis, coronary artery disease, aortic calcification, and valve regurgitation or stenosis. The hope of screening is to catch early RIHD, but screening is not currently routine.
"We wrote the expert consensus to raise the alarm that the risks of radiation-induced heart disease should not be ignored. The prevalence ... is increasing because the rate of cancer survival has improved," said Dr. Patrizio Lancellotti, who is a professor of cardiology at the University Hospital of Liège, Belgium, and led the recommendations task force.
Radiotherapy is given in more targeted form and at lower doses than it once was, but "patients are still at increased risk of RIHD, particularly when the heart is in the radiation field. This applies to patients treated for lymphoma, breast cancer, and esophageal cancer. Patients who receive radiotherapy for neck cancer are also at risk because lesions can develop on the carotid artery and increase the risk of stroke," Dr. Lancellotti said in a statement.
Using targeted radiation and alternate radiation fields, with avoidance and shielding of the heart, remain "the most important interventions to prevent" cardiac complications, the authors noted.
The task force advises that high-risk patients without evidence of heart disease on history and physical should have screening echocardiography every 5 years and noninvasive stress testing every 5-10 years; low-risk patients should have screening echocardiography every 10 years. If heart disorders are detected, routine monitoring should include echocardiography, cardiac magnetic resonance imaging, or carotid ultrasound as appropriate.
High-risk patients include those who received radiotherapy at younger ages; those who have cardiovascular risk factors or preexisting heart disease; and those who receive high-dose radiation (greater than 30 Gy), concomitant chemotherapy, radiation without shielding, or anterior or left chest radiation (Eur. Heart J. Cardiovasc. Imaging 2013;14:721-40).
The recommendations are based on an extensive literature review and analysis by Dr. Lancellotti and other specialists.
The authors reported no financial conflicts or outside funding for their work.
FROM THE EUROPEAN HEART JOURNAL – CARDIOVASCULAR IMAGING
Family therapy in Romania and lessons for the West
In the United States, family psychiatrists continue to deal with the fallout from the 1950s and 1960s, when the early family therapists located mental illness within the family and then touted family therapy as the cure. Families felt blamed and shied away from "family therapy."
Yet, research shows that family treatment for many psychiatric and medical illnesses, whether it is family inclusion or psychoeducation, is very effective in reducing morbidity. Stigma and fear about family involvement have resulted in family treatment lagging behind other psychotherapies in its acceptance as a valid therapeutic intervention.
As a contrast, it is therefore interesting to look at Romania, a postcommunist country, where all psychotherapies were deemed "unnecessary" under communism. According to Dr. Ileana-Mihaela Botezat-Antonescu, "Psychotherapy and psychoanalysis were known as the studies of the soul during the communist regime and went underground. Secret psychotherapy meetings were held in Sibiu and Timisoara, but after the 1989 revolution, we had access to information from abroad," she said during a presentation this year at the World Psychiatric Association meeting in Bucharest, Romania.
"In 1990, freedom occurs, but nobody tells you what to do. You cannot count on anything. It alienated people seeking help. It was a process that took time," said Dr. Botezat-Antonescu, a psychiatrist and psychoanalyst who was trained in the mid-1990s by trainers from the Dutch Psychoanalytic Association and serves as chair of the National Center for Mental Health.
Psychotherapists in Romania must somehow address the traumatic environment that lasted a generation. Young people strive to gain their sense of identity and belonging and, at the same time, are challenged with reestablishing a connection between the generations. The sense of intergenerational trauma and loss extends back to grandparents who lost their farms, houses, and social position.
An understanding of the intergenerational transmission of trauma can inform psychotherapists across the globe in their care of young people. Family therapy is a type of psychotherapy that is well suited to address this intergenerational trauma.
Psychological trauma is passed down through the generations in subtle and unspoken ways. It is important for therapists to recognize when this is occurring and work with the whole family. Family therapy that specifically addresses the intergenerational transmission of trauma can help move a family from feelings of helplessness toward resilience.
Development of family therapy
Family therapy developed in Romania through training courses in Cluj, Târgu Mures, and Timisoara. As there were no Romanian trainers, these courses were taught by family therapists from countries such as Ireland, France, and Yugoslavia. Families and trainees spoke Romanian or Hungarian, and during live supervision, simultaneous translation occurred. All courses, readings, and assignments were in English. Family therapy developed in Romania through training courses in Cluj, Târgu Mures and Timisoara.
Trainees saw families in their own work contexts, for example, psychotherapy centers; psychiatry hospitals; and community centers, such as family planning clinics and domestic violence shelters. Currently, there are 16 family therapy professional organizations (Contemp. Fam. Ther. 2013;35:275-87), including:
• Systemic Family Therapy Association in Cluj
• Association of Family Therapy in Bucharest
• Romanian Association for Family and Systemic Therapy in Timisoara
• Association Crisdu Areopagus in Timisoara
• Pro Familia – Family Therapy Association in Miercurea-Ciuc
• Association for Couple and Family Psychotherapy in Iasi
• Association for Family Counselling and Therapy in Iasi
Dr. Zoltán Kónya and Dr. Ágnes Kónya run the family therapy center in Cluj and have written about the challenges of practicing family therapy in Romania (Context 2007;92:2-4 and Contemporary Family Therapy 2013;35:1). Since family therapy training courses developed at different times, in different places, with trainers invited from different countries, the sense of what constitutes family therapy varies across Romania.
The meaning of the word "systemic" has proved particularly contentious. For some, systemic is synonymous with the Milan approach – which is based on the notion that "families are self-regulating systems that function based on self-developed rules tested over time through a process of trial and error" (Case Conceptualization in Family Therapy, Boston: Pearson, 2013). However, others consider family therapy as more than a systemic approach. Some promote systemic thinking as an all-encompassing epistemological frame for consultation with individuals, families, and organizations, but others do not attach much importance to the term "systemic."
The challenge of organizing into one Romanian family therapy institution with one outlook is great. This challenge also replicates one of the major problems in our field – the idea that family therapy means different things to different people. In Romania, well-meaning outside attempts ended up in a fractured national family therapy identity.
The Kónyas, trained by Irish family therapists, identify additional challenges of introducing family therapy into a culture unfamiliar with the concept. "The fit between systemic therapy and Romanian culture has been a concern of ours since the beginning of our training," they wrote in 2003. "There had been no tradition of people seeing psychotherapists in times of distress. Also, the vast majority of health care professionals had not even heard about family therapy. Would families come to therapy?"
They found that not only did clients come to therapy, but they also were ready to work hard when they did.
"We admire our clients’ courage in facing a series of challenges involved in the therapy process: consulting an outsider for a family problem, participating in sessions as a family, being asked unusual questions, being videotaped and, sometimes, being observed by a team and/or supervisors from abroad," they said.
In their work with patients, the Kónyas write, they have encountered difficulties tied to the use of words and phrases used in systemic therapy.
"For example, the Batesonian phrase 'a difference that makes a difference' is very difficult, if not impossible to properly translate into Romanian – of course, this may only be a problem in a training context, not in therapy. In response to the Romanian or Hungarian translation of the question: ‘And how has this been a problem for you?’ clients almost invariably demonstrate a lack of comprehension: ‘Would you please say that again? I didn’t understand.’ The difficulty here is not that the question makes no sense, but that it is culturally unusual – and therefore potentially therapeutic," they write (Context 2007)
"Also, certain words that might sound neutral in the West sometimes trigger strong emotions in our country. For example, monitoring progress on a 1-10 scale might recall painful experiences connected with school, because in Romania, marks are from 1 to 10. Also, talking about ‘systems’ may trigger discomfort, as this is the word people used during communism to describe the oppressive dictatorial regime. People who challenged the dominant ideas used to be called ‘the enemies of the system.’ "
The communist ideal of "systemization" broke families. Women were encouraged to give birth in a pronatal policy that resulted in orphans and unwanted children. After the 1989 revolution, more than 300,000 Romanians were living in psychiatric institutions. Communist factories were closed, and displaced workers had no place. Raising a voice against the regime risked imprisonment as an enemy of the system. The word "system" is associated with oppression in Romania.
Are there lessons for us in the West? I think the answer is yes and that the Romanian experience highlights several imperatives that are useful for Western mental health professionals. Among those imperatives:
• Family psychiatry needs to agree on a definition of family therapy. Is it any approach that includes families? Do we need to be systemic to be considered family therapists? Does family support qualify as family therapy? Does family psychoeducation qualify as family therapy? Can we embrace these two levels, as well as a third, more-skilled level, a systemic family therapy? Can we accept a three-level definition of family treatment?
• Can we incorporate all the family therapy models into one approach that people will recognize as a generic approach to families? Can we use the common factors approach described by Douglas H. Sprenkle, Ph.D., Sean D. Davis, Ph.D., and Jay L. Lebow, Ph.D., in "Common Factors in Couple and Family Therapy" (New York: The Guilford Press, 2009)? For couples and family therapists, common factors over and above the well-recognized individual psychotherapy factors are conceptualizing the problems in relational terms, using therapy that aims to disrupt dysfunctional relational patterns, expanding treatment to include family members of the index patient, and fostering an expanded therapeutic alliance, according to Dr. Sprenkle, Dr. Davis, and Dr. Lebow.
• Can we develop a protocol that beginners can follow? Aaron Beck’s cognitive-behavioral therapy (CBT) provides a basic template that is easy for the novice therapist and the patient to use, yet brings a unique perspective to psychotherapy. CBT has gone on to develop in several diverse directions, but all CBT models have the same basic set of beliefs. Can a family approach or protocol be both simple AND allow for more sophisticated elaboration? Can we develop a set of basic steps that define family treatment?
• Should there be an approach to families that all disciplines can follow? Family therapy is practiced by physicians, nurses, social workers, and marriage and family therapists. Each discipline tends to work with different populations. Physicians tend to see families in which one person is the identified patient. Social workers tend to see families that have been referred for social services, families who are frequently struggling with such problems as housing, financial, and legal difficulties. Marriage and family therapists are often employed by community and hospital agencies as health care extenders and might work alongside other health care professionals. Would a single approach to families be useful?
In summary, if family therapy is to endure, it must be teachable, translatable, and relevant across disciplines and national boundaries. The systemic paradigm is an important perspective from which all practitioners can benefit. We must continue to disseminate evidence-based family treatments and teach family principles that can be incorporated on a daily basis by all mental health professionals.
Dr. Heru is with the department of psychiatry at the University of Colorado at Denver, Aurora. She is editor of the recently published book, "Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals" (New York: Routledge, 2013).
In the United States, family psychiatrists continue to deal with the fallout from the 1950s and 1960s, when the early family therapists located mental illness within the family and then touted family therapy as the cure. Families felt blamed and shied away from "family therapy."
Yet, research shows that family treatment for many psychiatric and medical illnesses, whether it is family inclusion or psychoeducation, is very effective in reducing morbidity. Stigma and fear about family involvement have resulted in family treatment lagging behind other psychotherapies in its acceptance as a valid therapeutic intervention.
As a contrast, it is therefore interesting to look at Romania, a postcommunist country, where all psychotherapies were deemed "unnecessary" under communism. According to Dr. Ileana-Mihaela Botezat-Antonescu, "Psychotherapy and psychoanalysis were known as the studies of the soul during the communist regime and went underground. Secret psychotherapy meetings were held in Sibiu and Timisoara, but after the 1989 revolution, we had access to information from abroad," she said during a presentation this year at the World Psychiatric Association meeting in Bucharest, Romania.
"In 1990, freedom occurs, but nobody tells you what to do. You cannot count on anything. It alienated people seeking help. It was a process that took time," said Dr. Botezat-Antonescu, a psychiatrist and psychoanalyst who was trained in the mid-1990s by trainers from the Dutch Psychoanalytic Association and serves as chair of the National Center for Mental Health.
Psychotherapists in Romania must somehow address the traumatic environment that lasted a generation. Young people strive to gain their sense of identity and belonging and, at the same time, are challenged with reestablishing a connection between the generations. The sense of intergenerational trauma and loss extends back to grandparents who lost their farms, houses, and social position.
An understanding of the intergenerational transmission of trauma can inform psychotherapists across the globe in their care of young people. Family therapy is a type of psychotherapy that is well suited to address this intergenerational trauma.
Psychological trauma is passed down through the generations in subtle and unspoken ways. It is important for therapists to recognize when this is occurring and work with the whole family. Family therapy that specifically addresses the intergenerational transmission of trauma can help move a family from feelings of helplessness toward resilience.
Development of family therapy
Family therapy developed in Romania through training courses in Cluj, Târgu Mures, and Timisoara. As there were no Romanian trainers, these courses were taught by family therapists from countries such as Ireland, France, and Yugoslavia. Families and trainees spoke Romanian or Hungarian, and during live supervision, simultaneous translation occurred. All courses, readings, and assignments were in English. Family therapy developed in Romania through training courses in Cluj, Târgu Mures and Timisoara.
Trainees saw families in their own work contexts, for example, psychotherapy centers; psychiatry hospitals; and community centers, such as family planning clinics and domestic violence shelters. Currently, there are 16 family therapy professional organizations (Contemp. Fam. Ther. 2013;35:275-87), including:
• Systemic Family Therapy Association in Cluj
• Association of Family Therapy in Bucharest
• Romanian Association for Family and Systemic Therapy in Timisoara
• Association Crisdu Areopagus in Timisoara
• Pro Familia – Family Therapy Association in Miercurea-Ciuc
• Association for Couple and Family Psychotherapy in Iasi
• Association for Family Counselling and Therapy in Iasi
Dr. Zoltán Kónya and Dr. Ágnes Kónya run the family therapy center in Cluj and have written about the challenges of practicing family therapy in Romania (Context 2007;92:2-4 and Contemporary Family Therapy 2013;35:1). Since family therapy training courses developed at different times, in different places, with trainers invited from different countries, the sense of what constitutes family therapy varies across Romania.
The meaning of the word "systemic" has proved particularly contentious. For some, systemic is synonymous with the Milan approach – which is based on the notion that "families are self-regulating systems that function based on self-developed rules tested over time through a process of trial and error" (Case Conceptualization in Family Therapy, Boston: Pearson, 2013). However, others consider family therapy as more than a systemic approach. Some promote systemic thinking as an all-encompassing epistemological frame for consultation with individuals, families, and organizations, but others do not attach much importance to the term "systemic."
The challenge of organizing into one Romanian family therapy institution with one outlook is great. This challenge also replicates one of the major problems in our field – the idea that family therapy means different things to different people. In Romania, well-meaning outside attempts ended up in a fractured national family therapy identity.
The Kónyas, trained by Irish family therapists, identify additional challenges of introducing family therapy into a culture unfamiliar with the concept. "The fit between systemic therapy and Romanian culture has been a concern of ours since the beginning of our training," they wrote in 2003. "There had been no tradition of people seeing psychotherapists in times of distress. Also, the vast majority of health care professionals had not even heard about family therapy. Would families come to therapy?"
They found that not only did clients come to therapy, but they also were ready to work hard when they did.
"We admire our clients’ courage in facing a series of challenges involved in the therapy process: consulting an outsider for a family problem, participating in sessions as a family, being asked unusual questions, being videotaped and, sometimes, being observed by a team and/or supervisors from abroad," they said.
In their work with patients, the Kónyas write, they have encountered difficulties tied to the use of words and phrases used in systemic therapy.
"For example, the Batesonian phrase 'a difference that makes a difference' is very difficult, if not impossible to properly translate into Romanian – of course, this may only be a problem in a training context, not in therapy. In response to the Romanian or Hungarian translation of the question: ‘And how has this been a problem for you?’ clients almost invariably demonstrate a lack of comprehension: ‘Would you please say that again? I didn’t understand.’ The difficulty here is not that the question makes no sense, but that it is culturally unusual – and therefore potentially therapeutic," they write (Context 2007)
"Also, certain words that might sound neutral in the West sometimes trigger strong emotions in our country. For example, monitoring progress on a 1-10 scale might recall painful experiences connected with school, because in Romania, marks are from 1 to 10. Also, talking about ‘systems’ may trigger discomfort, as this is the word people used during communism to describe the oppressive dictatorial regime. People who challenged the dominant ideas used to be called ‘the enemies of the system.’ "
The communist ideal of "systemization" broke families. Women were encouraged to give birth in a pronatal policy that resulted in orphans and unwanted children. After the 1989 revolution, more than 300,000 Romanians were living in psychiatric institutions. Communist factories were closed, and displaced workers had no place. Raising a voice against the regime risked imprisonment as an enemy of the system. The word "system" is associated with oppression in Romania.
Are there lessons for us in the West? I think the answer is yes and that the Romanian experience highlights several imperatives that are useful for Western mental health professionals. Among those imperatives:
• Family psychiatry needs to agree on a definition of family therapy. Is it any approach that includes families? Do we need to be systemic to be considered family therapists? Does family support qualify as family therapy? Does family psychoeducation qualify as family therapy? Can we embrace these two levels, as well as a third, more-skilled level, a systemic family therapy? Can we accept a three-level definition of family treatment?
• Can we incorporate all the family therapy models into one approach that people will recognize as a generic approach to families? Can we use the common factors approach described by Douglas H. Sprenkle, Ph.D., Sean D. Davis, Ph.D., and Jay L. Lebow, Ph.D., in "Common Factors in Couple and Family Therapy" (New York: The Guilford Press, 2009)? For couples and family therapists, common factors over and above the well-recognized individual psychotherapy factors are conceptualizing the problems in relational terms, using therapy that aims to disrupt dysfunctional relational patterns, expanding treatment to include family members of the index patient, and fostering an expanded therapeutic alliance, according to Dr. Sprenkle, Dr. Davis, and Dr. Lebow.
• Can we develop a protocol that beginners can follow? Aaron Beck’s cognitive-behavioral therapy (CBT) provides a basic template that is easy for the novice therapist and the patient to use, yet brings a unique perspective to psychotherapy. CBT has gone on to develop in several diverse directions, but all CBT models have the same basic set of beliefs. Can a family approach or protocol be both simple AND allow for more sophisticated elaboration? Can we develop a set of basic steps that define family treatment?
• Should there be an approach to families that all disciplines can follow? Family therapy is practiced by physicians, nurses, social workers, and marriage and family therapists. Each discipline tends to work with different populations. Physicians tend to see families in which one person is the identified patient. Social workers tend to see families that have been referred for social services, families who are frequently struggling with such problems as housing, financial, and legal difficulties. Marriage and family therapists are often employed by community and hospital agencies as health care extenders and might work alongside other health care professionals. Would a single approach to families be useful?
In summary, if family therapy is to endure, it must be teachable, translatable, and relevant across disciplines and national boundaries. The systemic paradigm is an important perspective from which all practitioners can benefit. We must continue to disseminate evidence-based family treatments and teach family principles that can be incorporated on a daily basis by all mental health professionals.
Dr. Heru is with the department of psychiatry at the University of Colorado at Denver, Aurora. She is editor of the recently published book, "Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals" (New York: Routledge, 2013).
In the United States, family psychiatrists continue to deal with the fallout from the 1950s and 1960s, when the early family therapists located mental illness within the family and then touted family therapy as the cure. Families felt blamed and shied away from "family therapy."
Yet, research shows that family treatment for many psychiatric and medical illnesses, whether it is family inclusion or psychoeducation, is very effective in reducing morbidity. Stigma and fear about family involvement have resulted in family treatment lagging behind other psychotherapies in its acceptance as a valid therapeutic intervention.
As a contrast, it is therefore interesting to look at Romania, a postcommunist country, where all psychotherapies were deemed "unnecessary" under communism. According to Dr. Ileana-Mihaela Botezat-Antonescu, "Psychotherapy and psychoanalysis were known as the studies of the soul during the communist regime and went underground. Secret psychotherapy meetings were held in Sibiu and Timisoara, but after the 1989 revolution, we had access to information from abroad," she said during a presentation this year at the World Psychiatric Association meeting in Bucharest, Romania.
"In 1990, freedom occurs, but nobody tells you what to do. You cannot count on anything. It alienated people seeking help. It was a process that took time," said Dr. Botezat-Antonescu, a psychiatrist and psychoanalyst who was trained in the mid-1990s by trainers from the Dutch Psychoanalytic Association and serves as chair of the National Center for Mental Health.
Psychotherapists in Romania must somehow address the traumatic environment that lasted a generation. Young people strive to gain their sense of identity and belonging and, at the same time, are challenged with reestablishing a connection between the generations. The sense of intergenerational trauma and loss extends back to grandparents who lost their farms, houses, and social position.
An understanding of the intergenerational transmission of trauma can inform psychotherapists across the globe in their care of young people. Family therapy is a type of psychotherapy that is well suited to address this intergenerational trauma.
Psychological trauma is passed down through the generations in subtle and unspoken ways. It is important for therapists to recognize when this is occurring and work with the whole family. Family therapy that specifically addresses the intergenerational transmission of trauma can help move a family from feelings of helplessness toward resilience.
Development of family therapy
Family therapy developed in Romania through training courses in Cluj, Târgu Mures, and Timisoara. As there were no Romanian trainers, these courses were taught by family therapists from countries such as Ireland, France, and Yugoslavia. Families and trainees spoke Romanian or Hungarian, and during live supervision, simultaneous translation occurred. All courses, readings, and assignments were in English. Family therapy developed in Romania through training courses in Cluj, Târgu Mures and Timisoara.
Trainees saw families in their own work contexts, for example, psychotherapy centers; psychiatry hospitals; and community centers, such as family planning clinics and domestic violence shelters. Currently, there are 16 family therapy professional organizations (Contemp. Fam. Ther. 2013;35:275-87), including:
• Systemic Family Therapy Association in Cluj
• Association of Family Therapy in Bucharest
• Romanian Association for Family and Systemic Therapy in Timisoara
• Association Crisdu Areopagus in Timisoara
• Pro Familia – Family Therapy Association in Miercurea-Ciuc
• Association for Couple and Family Psychotherapy in Iasi
• Association for Family Counselling and Therapy in Iasi
Dr. Zoltán Kónya and Dr. Ágnes Kónya run the family therapy center in Cluj and have written about the challenges of practicing family therapy in Romania (Context 2007;92:2-4 and Contemporary Family Therapy 2013;35:1). Since family therapy training courses developed at different times, in different places, with trainers invited from different countries, the sense of what constitutes family therapy varies across Romania.
The meaning of the word "systemic" has proved particularly contentious. For some, systemic is synonymous with the Milan approach – which is based on the notion that "families are self-regulating systems that function based on self-developed rules tested over time through a process of trial and error" (Case Conceptualization in Family Therapy, Boston: Pearson, 2013). However, others consider family therapy as more than a systemic approach. Some promote systemic thinking as an all-encompassing epistemological frame for consultation with individuals, families, and organizations, but others do not attach much importance to the term "systemic."
The challenge of organizing into one Romanian family therapy institution with one outlook is great. This challenge also replicates one of the major problems in our field – the idea that family therapy means different things to different people. In Romania, well-meaning outside attempts ended up in a fractured national family therapy identity.
The Kónyas, trained by Irish family therapists, identify additional challenges of introducing family therapy into a culture unfamiliar with the concept. "The fit between systemic therapy and Romanian culture has been a concern of ours since the beginning of our training," they wrote in 2003. "There had been no tradition of people seeing psychotherapists in times of distress. Also, the vast majority of health care professionals had not even heard about family therapy. Would families come to therapy?"
They found that not only did clients come to therapy, but they also were ready to work hard when they did.
"We admire our clients’ courage in facing a series of challenges involved in the therapy process: consulting an outsider for a family problem, participating in sessions as a family, being asked unusual questions, being videotaped and, sometimes, being observed by a team and/or supervisors from abroad," they said.
In their work with patients, the Kónyas write, they have encountered difficulties tied to the use of words and phrases used in systemic therapy.
"For example, the Batesonian phrase 'a difference that makes a difference' is very difficult, if not impossible to properly translate into Romanian – of course, this may only be a problem in a training context, not in therapy. In response to the Romanian or Hungarian translation of the question: ‘And how has this been a problem for you?’ clients almost invariably demonstrate a lack of comprehension: ‘Would you please say that again? I didn’t understand.’ The difficulty here is not that the question makes no sense, but that it is culturally unusual – and therefore potentially therapeutic," they write (Context 2007)
"Also, certain words that might sound neutral in the West sometimes trigger strong emotions in our country. For example, monitoring progress on a 1-10 scale might recall painful experiences connected with school, because in Romania, marks are from 1 to 10. Also, talking about ‘systems’ may trigger discomfort, as this is the word people used during communism to describe the oppressive dictatorial regime. People who challenged the dominant ideas used to be called ‘the enemies of the system.’ "
The communist ideal of "systemization" broke families. Women were encouraged to give birth in a pronatal policy that resulted in orphans and unwanted children. After the 1989 revolution, more than 300,000 Romanians were living in psychiatric institutions. Communist factories were closed, and displaced workers had no place. Raising a voice against the regime risked imprisonment as an enemy of the system. The word "system" is associated with oppression in Romania.
Are there lessons for us in the West? I think the answer is yes and that the Romanian experience highlights several imperatives that are useful for Western mental health professionals. Among those imperatives:
• Family psychiatry needs to agree on a definition of family therapy. Is it any approach that includes families? Do we need to be systemic to be considered family therapists? Does family support qualify as family therapy? Does family psychoeducation qualify as family therapy? Can we embrace these two levels, as well as a third, more-skilled level, a systemic family therapy? Can we accept a three-level definition of family treatment?
• Can we incorporate all the family therapy models into one approach that people will recognize as a generic approach to families? Can we use the common factors approach described by Douglas H. Sprenkle, Ph.D., Sean D. Davis, Ph.D., and Jay L. Lebow, Ph.D., in "Common Factors in Couple and Family Therapy" (New York: The Guilford Press, 2009)? For couples and family therapists, common factors over and above the well-recognized individual psychotherapy factors are conceptualizing the problems in relational terms, using therapy that aims to disrupt dysfunctional relational patterns, expanding treatment to include family members of the index patient, and fostering an expanded therapeutic alliance, according to Dr. Sprenkle, Dr. Davis, and Dr. Lebow.
• Can we develop a protocol that beginners can follow? Aaron Beck’s cognitive-behavioral therapy (CBT) provides a basic template that is easy for the novice therapist and the patient to use, yet brings a unique perspective to psychotherapy. CBT has gone on to develop in several diverse directions, but all CBT models have the same basic set of beliefs. Can a family approach or protocol be both simple AND allow for more sophisticated elaboration? Can we develop a set of basic steps that define family treatment?
• Should there be an approach to families that all disciplines can follow? Family therapy is practiced by physicians, nurses, social workers, and marriage and family therapists. Each discipline tends to work with different populations. Physicians tend to see families in which one person is the identified patient. Social workers tend to see families that have been referred for social services, families who are frequently struggling with such problems as housing, financial, and legal difficulties. Marriage and family therapists are often employed by community and hospital agencies as health care extenders and might work alongside other health care professionals. Would a single approach to families be useful?
In summary, if family therapy is to endure, it must be teachable, translatable, and relevant across disciplines and national boundaries. The systemic paradigm is an important perspective from which all practitioners can benefit. We must continue to disseminate evidence-based family treatments and teach family principles that can be incorporated on a daily basis by all mental health professionals.
Dr. Heru is with the department of psychiatry at the University of Colorado at Denver, Aurora. She is editor of the recently published book, "Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals" (New York: Routledge, 2013).
BEST PRACTICES IN: Thermal Integrity of Shipping Containers Used by Private Cord Blood Banks
A supplement to Ob.Gyn. News. This supplement was sponsored by CORD:USE Cord Blood Bank, Inc.
• Introduction
• Materials and methods
• Results
• Discussion
Faculty/Faculty Disclosure
Robert N. Wolfson, MD, PhD, FACOG, FAIUM
Specialists in Women’s Health, LLC
Colorado Springs, Colorado
Dr Wolfson has nothing to disclose.
Copyright ©2013 by Frontline Medical Communications Inc.
A supplement to Ob.Gyn. News. This supplement was sponsored by CORD:USE Cord Blood Bank, Inc.
• Introduction
• Materials and methods
• Results
• Discussion
Faculty/Faculty Disclosure
Robert N. Wolfson, MD, PhD, FACOG, FAIUM
Specialists in Women’s Health, LLC
Colorado Springs, Colorado
Dr Wolfson has nothing to disclose.
Copyright ©2013 by Frontline Medical Communications Inc.
A supplement to Ob.Gyn. News. This supplement was sponsored by CORD:USE Cord Blood Bank, Inc.
• Introduction
• Materials and methods
• Results
• Discussion
Faculty/Faculty Disclosure
Robert N. Wolfson, MD, PhD, FACOG, FAIUM
Specialists in Women’s Health, LLC
Colorado Springs, Colorado
Dr Wolfson has nothing to disclose.
Copyright ©2013 by Frontline Medical Communications Inc.
From the Vascular Community
Please submit your short meeting reports, comings and goings, upcoming meetings, obituary announcements, etc., to From the Vascular Community in care of vascularspecialist@frontlinemedcom.com.
Meeting News
Reports
The South-Asian Association for Vascular Surgery (SAAVS) held their second annual meeting on May 30, 2013. Founded in 2011, the SAAVS is a member organization of the SVS with a mission to promote vascular health and disseminate the latest in vascular surgical techniques throughout South Asia. In just 2 years, the SAAVS has 100 registered members including 23 from overseas. During the meeting, Dr. Anil Hingorani began his tenure as President and Dr. Dipankar Mukherjee was voted President-Elect. Dr. Anahit Dua was presented an $800 prize for the outstanding resident research award. Dr. Krishna Jain and Dr. Bhagwan Satiani spoke on current issues facing vascular surgeons in the United States while Dr. Kumud Rai and Dr. Ramesh Tripathi spoke on the status of the field in India. The SAAVS is focusing its energy on establishing a "vascular update" with a 2-week didactic and practical course in South Asia. It is actively partnering with vascular societies in India to fulfill its mission. Medical students, trainees, and vascular surgeons from all backgrounds and geographic areas who are interested in advancing vascular care in South Asia are welcome to join. Visit http://saavsociety.org for more information.
Upcoming
The Canadian Society for Vascular Surgery will be holding its annual meeting September 13-14, 2013, at The Westin Edmonton, Edmonton, Alberta, Canada. The invited guest speaker is Dr. Ronald Lee Dalman II, who is the Dr. Walter C. Chidester Professor of Surgery, at Stanford University School of Medicine. Visit http://canadianvascular.ca for more details.
Obituaries
As we are beginning this new section, we are including obituaries from 2012.
Harold Clifton Urschel, Jr.
Dr. Urschel passed away on Nov. 12, 2012, at the age of 82. At the time of his death he was at the American Heart Association meeting in Los Angeles, where he was presenting material on his latest research interest: the use of stem cells for the treatment of heart failure. He was the past president of the Society of Thoracic Surgeons, the Southern Thoracic Surgical Association, the American College of Chest Physicians, and the Texas Surgical Association and a Distinguished fellow of the Society for Vascular Surgery. He has been a Governor of the American College of Surgeons, Chairman of the American Board of Thoracic Surgery, Chairman of the Residency Review Committee for Thoracic Surgery and also a member of every important national and international medical and surgical society.
Max R. Gaspar
Dr. Gaspar, an internationally reputed vascular surgeon, died Oct. 7, 2012. He was 97. Gaspar, formerly of Long Beach, had been chief of vascular surgery for 25 years at Los Angeles County-USC Medical Center, where he also served as attending surgeon for 50 years. He had a practice in Long Beach and had also performed surgeries at St. Mary Medical Center, Memorial Medical Center, and Community Medical. He attended the University of South Dakota Medical School but finished his training at USC in 1938, and earned his M.D. in 1940. During World War II, he served in the Navy as a doctor in the Pacific. Dr. Gaspar remained active in medicine and teaching. About 17 years ago, USC established the Max R. Gaspar Symposium, which addressed a specific topic of interest to physicians and surgeons who care for patients with vascular disease. He also authored numerous articles and contributed about 14 chapters to various texts. He was one of the early pioneers in our field.
Edwin Salzman
Dr. Salzman, a professor of surgery emeritus at Harvard Medical School, died Oct. 3, 2012, at Beth Israel Deaconess Medical Center, in a room not far from his old office. His surgical career was cut short by Parkinson’s disease in the mid-1970s. Turning full attention to the scientific research that had always been his parallel career, he helped pioneer using aspirin to prevent DVT and spent a dozen years working part-time as deputy editor of the New England Journal of Medicine. Along with the findings in the 1970s about aspirin, he made significant contributions to research involving heparin and other methods that prevent postoperative pulmonary embolism.
Geoffrey Hamilton White
Dr. White died peacefully in Australia on Jan. 26, 2012, at the age of 60. He was at UCLA from 1984 to 1989 as Assistant Professor of Surgery at the UCLA School of Medicine and Chief of Vascular Surgery at the VA Wadsworth Medical Center. He was later appointed head of the department at Royal Prince Alfred Hospital and Professor of Vascular Surgery at Macquarie University Hospital, both in Australia. He had a richly deserved international reputation for his many contributions to the development of the endovascular treatment abdominal aortic aneurysms. He also coined the term "endoleak," which is nowpart of the nomenclature.
Deceased Members
(Reported to the SVS as of April 19, 2013; presented in order of receiving):
• Johann Ehrenhaft, MD Iowa City, IA
• J. Harold Harrison, MD Bartow, GA
• George Kish, MD Henderson, NV
• Malcolm Thomas, MD Phoenix, AZ
• Norman Rosenberg, MD Lantana, FL
• Michael Seremetis, MD Washington, DC
• Andrew Michalski, MD St. Catherines, Ontario, Canada
• Dean Wasserman, MD Paramus, NJ
• Duncan W. Campbell, MD Tucson, AZ
• Edwin Salzman, MD Cambridge, MA
• John Vander Woude, MD Sioux Falls, SD
• William A. Holbrook, MD Chevy Chase, MD
• Lewis H. Bosher, MD Richmond, VA
• Joseph Graham, MD Joplin, MO
• William D. Byrne McLean, VA
• John Waldhausen, MD Lemoyne, PA
• David Wulkan, MD Boca Raton, FL
• Max Gaspar, MD Seal Beach, CA
• Hugh E. Stephenson, MD Columbia, MO
• Harold C. Urschel, Jr., MD Dallas, TX
• Geoffrey H. White, MD Sydney, Australia
• Henning Loeprecht, MD Augsburg, Germany
Please submit your short meeting reports, comings and goings, upcoming meetings, obituary announcements, etc., to From the Vascular Community in care of vascularspecialist@frontlinemedcom.com.
Meeting News
Reports
The South-Asian Association for Vascular Surgery (SAAVS) held their second annual meeting on May 30, 2013. Founded in 2011, the SAAVS is a member organization of the SVS with a mission to promote vascular health and disseminate the latest in vascular surgical techniques throughout South Asia. In just 2 years, the SAAVS has 100 registered members including 23 from overseas. During the meeting, Dr. Anil Hingorani began his tenure as President and Dr. Dipankar Mukherjee was voted President-Elect. Dr. Anahit Dua was presented an $800 prize for the outstanding resident research award. Dr. Krishna Jain and Dr. Bhagwan Satiani spoke on current issues facing vascular surgeons in the United States while Dr. Kumud Rai and Dr. Ramesh Tripathi spoke on the status of the field in India. The SAAVS is focusing its energy on establishing a "vascular update" with a 2-week didactic and practical course in South Asia. It is actively partnering with vascular societies in India to fulfill its mission. Medical students, trainees, and vascular surgeons from all backgrounds and geographic areas who are interested in advancing vascular care in South Asia are welcome to join. Visit http://saavsociety.org for more information.
Upcoming
The Canadian Society for Vascular Surgery will be holding its annual meeting September 13-14, 2013, at The Westin Edmonton, Edmonton, Alberta, Canada. The invited guest speaker is Dr. Ronald Lee Dalman II, who is the Dr. Walter C. Chidester Professor of Surgery, at Stanford University School of Medicine. Visit http://canadianvascular.ca for more details.
Obituaries
As we are beginning this new section, we are including obituaries from 2012.
Harold Clifton Urschel, Jr.
Dr. Urschel passed away on Nov. 12, 2012, at the age of 82. At the time of his death he was at the American Heart Association meeting in Los Angeles, where he was presenting material on his latest research interest: the use of stem cells for the treatment of heart failure. He was the past president of the Society of Thoracic Surgeons, the Southern Thoracic Surgical Association, the American College of Chest Physicians, and the Texas Surgical Association and a Distinguished fellow of the Society for Vascular Surgery. He has been a Governor of the American College of Surgeons, Chairman of the American Board of Thoracic Surgery, Chairman of the Residency Review Committee for Thoracic Surgery and also a member of every important national and international medical and surgical society.
Max R. Gaspar
Dr. Gaspar, an internationally reputed vascular surgeon, died Oct. 7, 2012. He was 97. Gaspar, formerly of Long Beach, had been chief of vascular surgery for 25 years at Los Angeles County-USC Medical Center, where he also served as attending surgeon for 50 years. He had a practice in Long Beach and had also performed surgeries at St. Mary Medical Center, Memorial Medical Center, and Community Medical. He attended the University of South Dakota Medical School but finished his training at USC in 1938, and earned his M.D. in 1940. During World War II, he served in the Navy as a doctor in the Pacific. Dr. Gaspar remained active in medicine and teaching. About 17 years ago, USC established the Max R. Gaspar Symposium, which addressed a specific topic of interest to physicians and surgeons who care for patients with vascular disease. He also authored numerous articles and contributed about 14 chapters to various texts. He was one of the early pioneers in our field.
Edwin Salzman
Dr. Salzman, a professor of surgery emeritus at Harvard Medical School, died Oct. 3, 2012, at Beth Israel Deaconess Medical Center, in a room not far from his old office. His surgical career was cut short by Parkinson’s disease in the mid-1970s. Turning full attention to the scientific research that had always been his parallel career, he helped pioneer using aspirin to prevent DVT and spent a dozen years working part-time as deputy editor of the New England Journal of Medicine. Along with the findings in the 1970s about aspirin, he made significant contributions to research involving heparin and other methods that prevent postoperative pulmonary embolism.
Geoffrey Hamilton White
Dr. White died peacefully in Australia on Jan. 26, 2012, at the age of 60. He was at UCLA from 1984 to 1989 as Assistant Professor of Surgery at the UCLA School of Medicine and Chief of Vascular Surgery at the VA Wadsworth Medical Center. He was later appointed head of the department at Royal Prince Alfred Hospital and Professor of Vascular Surgery at Macquarie University Hospital, both in Australia. He had a richly deserved international reputation for his many contributions to the development of the endovascular treatment abdominal aortic aneurysms. He also coined the term "endoleak," which is nowpart of the nomenclature.
Deceased Members
(Reported to the SVS as of April 19, 2013; presented in order of receiving):
• Johann Ehrenhaft, MD Iowa City, IA
• J. Harold Harrison, MD Bartow, GA
• George Kish, MD Henderson, NV
• Malcolm Thomas, MD Phoenix, AZ
• Norman Rosenberg, MD Lantana, FL
• Michael Seremetis, MD Washington, DC
• Andrew Michalski, MD St. Catherines, Ontario, Canada
• Dean Wasserman, MD Paramus, NJ
• Duncan W. Campbell, MD Tucson, AZ
• Edwin Salzman, MD Cambridge, MA
• John Vander Woude, MD Sioux Falls, SD
• William A. Holbrook, MD Chevy Chase, MD
• Lewis H. Bosher, MD Richmond, VA
• Joseph Graham, MD Joplin, MO
• William D. Byrne McLean, VA
• John Waldhausen, MD Lemoyne, PA
• David Wulkan, MD Boca Raton, FL
• Max Gaspar, MD Seal Beach, CA
• Hugh E. Stephenson, MD Columbia, MO
• Harold C. Urschel, Jr., MD Dallas, TX
• Geoffrey H. White, MD Sydney, Australia
• Henning Loeprecht, MD Augsburg, Germany
Please submit your short meeting reports, comings and goings, upcoming meetings, obituary announcements, etc., to From the Vascular Community in care of vascularspecialist@frontlinemedcom.com.
Meeting News
Reports
The South-Asian Association for Vascular Surgery (SAAVS) held their second annual meeting on May 30, 2013. Founded in 2011, the SAAVS is a member organization of the SVS with a mission to promote vascular health and disseminate the latest in vascular surgical techniques throughout South Asia. In just 2 years, the SAAVS has 100 registered members including 23 from overseas. During the meeting, Dr. Anil Hingorani began his tenure as President and Dr. Dipankar Mukherjee was voted President-Elect. Dr. Anahit Dua was presented an $800 prize for the outstanding resident research award. Dr. Krishna Jain and Dr. Bhagwan Satiani spoke on current issues facing vascular surgeons in the United States while Dr. Kumud Rai and Dr. Ramesh Tripathi spoke on the status of the field in India. The SAAVS is focusing its energy on establishing a "vascular update" with a 2-week didactic and practical course in South Asia. It is actively partnering with vascular societies in India to fulfill its mission. Medical students, trainees, and vascular surgeons from all backgrounds and geographic areas who are interested in advancing vascular care in South Asia are welcome to join. Visit http://saavsociety.org for more information.
Upcoming
The Canadian Society for Vascular Surgery will be holding its annual meeting September 13-14, 2013, at The Westin Edmonton, Edmonton, Alberta, Canada. The invited guest speaker is Dr. Ronald Lee Dalman II, who is the Dr. Walter C. Chidester Professor of Surgery, at Stanford University School of Medicine. Visit http://canadianvascular.ca for more details.
Obituaries
As we are beginning this new section, we are including obituaries from 2012.
Harold Clifton Urschel, Jr.
Dr. Urschel passed away on Nov. 12, 2012, at the age of 82. At the time of his death he was at the American Heart Association meeting in Los Angeles, where he was presenting material on his latest research interest: the use of stem cells for the treatment of heart failure. He was the past president of the Society of Thoracic Surgeons, the Southern Thoracic Surgical Association, the American College of Chest Physicians, and the Texas Surgical Association and a Distinguished fellow of the Society for Vascular Surgery. He has been a Governor of the American College of Surgeons, Chairman of the American Board of Thoracic Surgery, Chairman of the Residency Review Committee for Thoracic Surgery and also a member of every important national and international medical and surgical society.
Max R. Gaspar
Dr. Gaspar, an internationally reputed vascular surgeon, died Oct. 7, 2012. He was 97. Gaspar, formerly of Long Beach, had been chief of vascular surgery for 25 years at Los Angeles County-USC Medical Center, where he also served as attending surgeon for 50 years. He had a practice in Long Beach and had also performed surgeries at St. Mary Medical Center, Memorial Medical Center, and Community Medical. He attended the University of South Dakota Medical School but finished his training at USC in 1938, and earned his M.D. in 1940. During World War II, he served in the Navy as a doctor in the Pacific. Dr. Gaspar remained active in medicine and teaching. About 17 years ago, USC established the Max R. Gaspar Symposium, which addressed a specific topic of interest to physicians and surgeons who care for patients with vascular disease. He also authored numerous articles and contributed about 14 chapters to various texts. He was one of the early pioneers in our field.
Edwin Salzman
Dr. Salzman, a professor of surgery emeritus at Harvard Medical School, died Oct. 3, 2012, at Beth Israel Deaconess Medical Center, in a room not far from his old office. His surgical career was cut short by Parkinson’s disease in the mid-1970s. Turning full attention to the scientific research that had always been his parallel career, he helped pioneer using aspirin to prevent DVT and spent a dozen years working part-time as deputy editor of the New England Journal of Medicine. Along with the findings in the 1970s about aspirin, he made significant contributions to research involving heparin and other methods that prevent postoperative pulmonary embolism.
Geoffrey Hamilton White
Dr. White died peacefully in Australia on Jan. 26, 2012, at the age of 60. He was at UCLA from 1984 to 1989 as Assistant Professor of Surgery at the UCLA School of Medicine and Chief of Vascular Surgery at the VA Wadsworth Medical Center. He was later appointed head of the department at Royal Prince Alfred Hospital and Professor of Vascular Surgery at Macquarie University Hospital, both in Australia. He had a richly deserved international reputation for his many contributions to the development of the endovascular treatment abdominal aortic aneurysms. He also coined the term "endoleak," which is nowpart of the nomenclature.
Deceased Members
(Reported to the SVS as of April 19, 2013; presented in order of receiving):
• Johann Ehrenhaft, MD Iowa City, IA
• J. Harold Harrison, MD Bartow, GA
• George Kish, MD Henderson, NV
• Malcolm Thomas, MD Phoenix, AZ
• Norman Rosenberg, MD Lantana, FL
• Michael Seremetis, MD Washington, DC
• Andrew Michalski, MD St. Catherines, Ontario, Canada
• Dean Wasserman, MD Paramus, NJ
• Duncan W. Campbell, MD Tucson, AZ
• Edwin Salzman, MD Cambridge, MA
• John Vander Woude, MD Sioux Falls, SD
• William A. Holbrook, MD Chevy Chase, MD
• Lewis H. Bosher, MD Richmond, VA
• Joseph Graham, MD Joplin, MO
• William D. Byrne McLean, VA
• John Waldhausen, MD Lemoyne, PA
• David Wulkan, MD Boca Raton, FL
• Max Gaspar, MD Seal Beach, CA
• Hugh E. Stephenson, MD Columbia, MO
• Harold C. Urschel, Jr., MD Dallas, TX
• Geoffrey H. White, MD Sydney, Australia
• Henning Loeprecht, MD Augsburg, Germany