User login
Hospitalist Compensation Models Evolve Toward Production, Performance-Based Variables
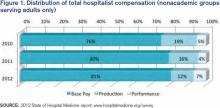
Hospitalists have long recognized that compensation varies significantly by geographic location and by the type of hospitalist medicine group (HMG) you work in: private vs. hospital-owned vs. national-management-owned. A review of SHM’s 2012 State of Hospital Medicine report suggests that hospitalist compensation is also evolving toward a model that more routinely includes both some production variable and performance-based pay (see Figure 1). Although the proportion of compensation paid as a base salary has been trending up over the last few years, so has the proportion paid as a performance incentive.
Source: 2012 State of Hospital Medicine report; www.hospitalmedicine.org/survey
The pay distribution of adult-medicine hospitalists employed by management companies is composed of a high base percentage (mean 88.3% by survey data) and relatively low production and performance variables (mean 6.8% and 4.9%, respectively) compared with other employment models. Contrast that with private hospitalist-only groups, where the mean base is 76.3% with an emphasis on a production component (19.4%) and slightly less on performance pay at 4.2%.
Of the three employment models, however, hospital-/health-system-employed groups have the highest proportion of compensation based on performance metrics with a mean of 7.8%. This makes sense given the financial penalties hospitals and health systems are facing from the Centers for Medicare & Medicaid Services (CMS) around pay-for-performance measures. Hospitals are looking for help from hospitalists in improving quality of care and patient satisfaction and avoiding incurring future penalties. Compensation models in these groups reflect the goals of aligning performance on these measures with financial incentives/risk for hospitalists working in these environments.
What are the top performance metrics hospitalists are being compensated for? CMS’ hospital value-based purchasing (HVBP) core measures and patient satisfaction scores are at the top of the list. More than 70% of all HMGs identify these two measures as part of their performance pay incentive, which is seen consistently by geographic location and by type of hospitalist group.
Beyond these top two metrics, management-company-employed groups also focus on ED throughput measures and early morning discharge times, with more than 70% of these groups having pay incentives aligned with these goals. They also have a higher proportion of their groups participating in several other measures, such as clinical protocols, medication reconciliation, EHR utilization, transitions of care, and readmission rates. In comparison, both hospital-employed and private groups have a wider variety of performance measures in which they participate. Differences are seen geographically, too, with hospitalists located in the Western region having a wider variety of performance measures than other regions.
How hospitalists are compensated for their work will likely continue to evolve. Overall, for nonacademic HMGs serving adults only, we are seeing an upward trend in percentage paid as base pay (from 76% in 2010 to 81% in 2012) and in performance (from 5% in 2010 to 7% in 2012). Hospitalists should anticipate that performance-based pay will continue to account for an increasingly larger percentage of their overall compensation, especially as CMS’ pay-for-performance measures for hospital systems really start to take effect.
Hospital CEOs and CFOs are looking to hospitalists to help deliver on quality, satisfaction, and other performance measures. Incentives will be put in place to reward those groups who do it well.
Dr. Sites is senior medical director of hospitalist programs at Providence Health and Services in Oregon. She is a member of SHM’s Practice Analysis Committee.
Hospitalists have long recognized that compensation varies significantly by geographic location and by the type of hospitalist medicine group (HMG) you work in: private vs. hospital-owned vs. national-management-owned. A review of SHM’s 2012 State of Hospital Medicine report suggests that hospitalist compensation is also evolving toward a model that more routinely includes both some production variable and performance-based pay (see Figure 1). Although the proportion of compensation paid as a base salary has been trending up over the last few years, so has the proportion paid as a performance incentive.
Source: 2012 State of Hospital Medicine report; www.hospitalmedicine.org/survey
The pay distribution of adult-medicine hospitalists employed by management companies is composed of a high base percentage (mean 88.3% by survey data) and relatively low production and performance variables (mean 6.8% and 4.9%, respectively) compared with other employment models. Contrast that with private hospitalist-only groups, where the mean base is 76.3% with an emphasis on a production component (19.4%) and slightly less on performance pay at 4.2%.
Of the three employment models, however, hospital-/health-system-employed groups have the highest proportion of compensation based on performance metrics with a mean of 7.8%. This makes sense given the financial penalties hospitals and health systems are facing from the Centers for Medicare & Medicaid Services (CMS) around pay-for-performance measures. Hospitals are looking for help from hospitalists in improving quality of care and patient satisfaction and avoiding incurring future penalties. Compensation models in these groups reflect the goals of aligning performance on these measures with financial incentives/risk for hospitalists working in these environments.
What are the top performance metrics hospitalists are being compensated for? CMS’ hospital value-based purchasing (HVBP) core measures and patient satisfaction scores are at the top of the list. More than 70% of all HMGs identify these two measures as part of their performance pay incentive, which is seen consistently by geographic location and by type of hospitalist group.
Beyond these top two metrics, management-company-employed groups also focus on ED throughput measures and early morning discharge times, with more than 70% of these groups having pay incentives aligned with these goals. They also have a higher proportion of their groups participating in several other measures, such as clinical protocols, medication reconciliation, EHR utilization, transitions of care, and readmission rates. In comparison, both hospital-employed and private groups have a wider variety of performance measures in which they participate. Differences are seen geographically, too, with hospitalists located in the Western region having a wider variety of performance measures than other regions.
How hospitalists are compensated for their work will likely continue to evolve. Overall, for nonacademic HMGs serving adults only, we are seeing an upward trend in percentage paid as base pay (from 76% in 2010 to 81% in 2012) and in performance (from 5% in 2010 to 7% in 2012). Hospitalists should anticipate that performance-based pay will continue to account for an increasingly larger percentage of their overall compensation, especially as CMS’ pay-for-performance measures for hospital systems really start to take effect.
Hospital CEOs and CFOs are looking to hospitalists to help deliver on quality, satisfaction, and other performance measures. Incentives will be put in place to reward those groups who do it well.
Dr. Sites is senior medical director of hospitalist programs at Providence Health and Services in Oregon. She is a member of SHM’s Practice Analysis Committee.
Hospitalists have long recognized that compensation varies significantly by geographic location and by the type of hospitalist medicine group (HMG) you work in: private vs. hospital-owned vs. national-management-owned. A review of SHM’s 2012 State of Hospital Medicine report suggests that hospitalist compensation is also evolving toward a model that more routinely includes both some production variable and performance-based pay (see Figure 1). Although the proportion of compensation paid as a base salary has been trending up over the last few years, so has the proportion paid as a performance incentive.
Source: 2012 State of Hospital Medicine report; www.hospitalmedicine.org/survey
The pay distribution of adult-medicine hospitalists employed by management companies is composed of a high base percentage (mean 88.3% by survey data) and relatively low production and performance variables (mean 6.8% and 4.9%, respectively) compared with other employment models. Contrast that with private hospitalist-only groups, where the mean base is 76.3% with an emphasis on a production component (19.4%) and slightly less on performance pay at 4.2%.
Of the three employment models, however, hospital-/health-system-employed groups have the highest proportion of compensation based on performance metrics with a mean of 7.8%. This makes sense given the financial penalties hospitals and health systems are facing from the Centers for Medicare & Medicaid Services (CMS) around pay-for-performance measures. Hospitals are looking for help from hospitalists in improving quality of care and patient satisfaction and avoiding incurring future penalties. Compensation models in these groups reflect the goals of aligning performance on these measures with financial incentives/risk for hospitalists working in these environments.
What are the top performance metrics hospitalists are being compensated for? CMS’ hospital value-based purchasing (HVBP) core measures and patient satisfaction scores are at the top of the list. More than 70% of all HMGs identify these two measures as part of their performance pay incentive, which is seen consistently by geographic location and by type of hospitalist group.
Beyond these top two metrics, management-company-employed groups also focus on ED throughput measures and early morning discharge times, with more than 70% of these groups having pay incentives aligned with these goals. They also have a higher proportion of their groups participating in several other measures, such as clinical protocols, medication reconciliation, EHR utilization, transitions of care, and readmission rates. In comparison, both hospital-employed and private groups have a wider variety of performance measures in which they participate. Differences are seen geographically, too, with hospitalists located in the Western region having a wider variety of performance measures than other regions.
How hospitalists are compensated for their work will likely continue to evolve. Overall, for nonacademic HMGs serving adults only, we are seeing an upward trend in percentage paid as base pay (from 76% in 2010 to 81% in 2012) and in performance (from 5% in 2010 to 7% in 2012). Hospitalists should anticipate that performance-based pay will continue to account for an increasingly larger percentage of their overall compensation, especially as CMS’ pay-for-performance measures for hospital systems really start to take effect.
Hospital CEOs and CFOs are looking to hospitalists to help deliver on quality, satisfaction, and other performance measures. Incentives will be put in place to reward those groups who do it well.
Dr. Sites is senior medical director of hospitalist programs at Providence Health and Services in Oregon. She is a member of SHM’s Practice Analysis Committee.
SHM Advocates for Medicare to Cover Skilled-Nursing Facilities
The Centers for Medicare & Medicaid Services (CMS) recently issued a Final Rule for the Inpatient Prospective Payment System, which guides payment and programs associated with inpatient hospitalizations. In this year’s rule, CMS adjusted the criteria for inpatient admissions in an attempt to simplify and clarify the decision-making process.
The policy would allow physicians to admit a patient if they reasonably expect and document in the medical record that a beneficiary will need to stay in the hospital for more than two midnights. Admissions based on this time-limited expectation will be presumed to be appropriate for Medicare Part A payment. CMS cited concerns about the growing trend of longer observation stays to support this change.
With observation stays, there are two major financial concerns for patients: whether the hospital stay is paid under Medicare Part A or Part B, and whether Medicare will pay for post-acute care in a skilled-nursing facility (SNF). Medicare Part A reimburses for inpatient admissions, with a one-time deductible for the benefit period. Outpatient services, such as observation care and physician services, are covered under Medicare Part B, which has copays and co-insurance that greatly increase the costs for beneficiaries. In addition, SNF coverage through Medicare Part A is determined by the three-day rule; a patient must be an inpatient for three days to qualify for coverage.
While the long-term impacts of this regulatory change to the admission criteria remain to be seen, SHM is concerned that the rule does not adequately address the broader problems associated with inpatient and observation status. As we note in our comments to CMS on the new rule:1
Even with these changes, the central tension created by the bifurcation in admission status still remains.…Other policies and programs, such as the attempts to reduce admissions, may inadvertently add pressure to the admission decision.
Indeed, for beneficiaries, the barrier to SNF coverage remains. CMS takes care to note that, while time under emergency care and observation care count toward the two-midnight presumption for inpatient admission, it does not count toward the three-day rule for SNF coverage. This is particularly problematic; as advances in medicine allow for the treatment of higher-acuity and -severity conditions with observation stays or shorter inpatient stays, patients might not be getting the follow-up care they need. This puts them at risk for additional complications and, ultimately, readmissions to the hospital.
In an era of seeking value in the healthcare system, it seems like an opportunity lost to streamline and coordinate care across settings and to ensure that patients are getting the follow-up care they require. It is for this reason that hospitalists continue to push for passage of the Improving Access to Medicare Coverage Act, a bill sponsored by Rep. Joe Courtney (D-Conn.), Rep. Tom Latham (R-Iowa), and Sen. Sherrod Brown (D-Ohio) that would count observation status as time toward the three-day requirement for SNF coverage.
A recent Office of Inspector General (OIG) report for the U.S. Department of Health and Human Services on observation status sums up the problem succinctly.2 The OIG states that “CMS should consider how to ensure that beneficiaries with similar post-hospital care needs have the same access and cost-sharing for SNF services.”2
SHM concurs.
Joshua Lapps is SHM’s government relations specialist.
References
- Society of Hospital Medicine. SHM submits comments in response to FY2014 inpatient prospective payment system proposed rule. Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Letters_to_Congress_and_Regulatory_Agencies&Template=/CM/ContentDisplay.cfm&ContentID=34044. Accessed Sept. 9, 2013.
- Office of Inspector General. Memorandum report: Hospitals’ use of observations stays and short inpatient stays for Medicare beneficiaries, OEI-02-12-00040. U.S. Department of Health and Human Services website. Available at: http://oig.hhs.gov/oei/reports/oei-02-12-00040.pdf. Accessed Sept. 9, 2013.
The Centers for Medicare & Medicaid Services (CMS) recently issued a Final Rule for the Inpatient Prospective Payment System, which guides payment and programs associated with inpatient hospitalizations. In this year’s rule, CMS adjusted the criteria for inpatient admissions in an attempt to simplify and clarify the decision-making process.
The policy would allow physicians to admit a patient if they reasonably expect and document in the medical record that a beneficiary will need to stay in the hospital for more than two midnights. Admissions based on this time-limited expectation will be presumed to be appropriate for Medicare Part A payment. CMS cited concerns about the growing trend of longer observation stays to support this change.
With observation stays, there are two major financial concerns for patients: whether the hospital stay is paid under Medicare Part A or Part B, and whether Medicare will pay for post-acute care in a skilled-nursing facility (SNF). Medicare Part A reimburses for inpatient admissions, with a one-time deductible for the benefit period. Outpatient services, such as observation care and physician services, are covered under Medicare Part B, which has copays and co-insurance that greatly increase the costs for beneficiaries. In addition, SNF coverage through Medicare Part A is determined by the three-day rule; a patient must be an inpatient for three days to qualify for coverage.
While the long-term impacts of this regulatory change to the admission criteria remain to be seen, SHM is concerned that the rule does not adequately address the broader problems associated with inpatient and observation status. As we note in our comments to CMS on the new rule:1
Even with these changes, the central tension created by the bifurcation in admission status still remains.…Other policies and programs, such as the attempts to reduce admissions, may inadvertently add pressure to the admission decision.
Indeed, for beneficiaries, the barrier to SNF coverage remains. CMS takes care to note that, while time under emergency care and observation care count toward the two-midnight presumption for inpatient admission, it does not count toward the three-day rule for SNF coverage. This is particularly problematic; as advances in medicine allow for the treatment of higher-acuity and -severity conditions with observation stays or shorter inpatient stays, patients might not be getting the follow-up care they need. This puts them at risk for additional complications and, ultimately, readmissions to the hospital.
In an era of seeking value in the healthcare system, it seems like an opportunity lost to streamline and coordinate care across settings and to ensure that patients are getting the follow-up care they require. It is for this reason that hospitalists continue to push for passage of the Improving Access to Medicare Coverage Act, a bill sponsored by Rep. Joe Courtney (D-Conn.), Rep. Tom Latham (R-Iowa), and Sen. Sherrod Brown (D-Ohio) that would count observation status as time toward the three-day requirement for SNF coverage.
A recent Office of Inspector General (OIG) report for the U.S. Department of Health and Human Services on observation status sums up the problem succinctly.2 The OIG states that “CMS should consider how to ensure that beneficiaries with similar post-hospital care needs have the same access and cost-sharing for SNF services.”2
SHM concurs.
Joshua Lapps is SHM’s government relations specialist.
References
- Society of Hospital Medicine. SHM submits comments in response to FY2014 inpatient prospective payment system proposed rule. Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Letters_to_Congress_and_Regulatory_Agencies&Template=/CM/ContentDisplay.cfm&ContentID=34044. Accessed Sept. 9, 2013.
- Office of Inspector General. Memorandum report: Hospitals’ use of observations stays and short inpatient stays for Medicare beneficiaries, OEI-02-12-00040. U.S. Department of Health and Human Services website. Available at: http://oig.hhs.gov/oei/reports/oei-02-12-00040.pdf. Accessed Sept. 9, 2013.
The Centers for Medicare & Medicaid Services (CMS) recently issued a Final Rule for the Inpatient Prospective Payment System, which guides payment and programs associated with inpatient hospitalizations. In this year’s rule, CMS adjusted the criteria for inpatient admissions in an attempt to simplify and clarify the decision-making process.
The policy would allow physicians to admit a patient if they reasonably expect and document in the medical record that a beneficiary will need to stay in the hospital for more than two midnights. Admissions based on this time-limited expectation will be presumed to be appropriate for Medicare Part A payment. CMS cited concerns about the growing trend of longer observation stays to support this change.
With observation stays, there are two major financial concerns for patients: whether the hospital stay is paid under Medicare Part A or Part B, and whether Medicare will pay for post-acute care in a skilled-nursing facility (SNF). Medicare Part A reimburses for inpatient admissions, with a one-time deductible for the benefit period. Outpatient services, such as observation care and physician services, are covered under Medicare Part B, which has copays and co-insurance that greatly increase the costs for beneficiaries. In addition, SNF coverage through Medicare Part A is determined by the three-day rule; a patient must be an inpatient for three days to qualify for coverage.
While the long-term impacts of this regulatory change to the admission criteria remain to be seen, SHM is concerned that the rule does not adequately address the broader problems associated with inpatient and observation status. As we note in our comments to CMS on the new rule:1
Even with these changes, the central tension created by the bifurcation in admission status still remains.…Other policies and programs, such as the attempts to reduce admissions, may inadvertently add pressure to the admission decision.
Indeed, for beneficiaries, the barrier to SNF coverage remains. CMS takes care to note that, while time under emergency care and observation care count toward the two-midnight presumption for inpatient admission, it does not count toward the three-day rule for SNF coverage. This is particularly problematic; as advances in medicine allow for the treatment of higher-acuity and -severity conditions with observation stays or shorter inpatient stays, patients might not be getting the follow-up care they need. This puts them at risk for additional complications and, ultimately, readmissions to the hospital.
In an era of seeking value in the healthcare system, it seems like an opportunity lost to streamline and coordinate care across settings and to ensure that patients are getting the follow-up care they require. It is for this reason that hospitalists continue to push for passage of the Improving Access to Medicare Coverage Act, a bill sponsored by Rep. Joe Courtney (D-Conn.), Rep. Tom Latham (R-Iowa), and Sen. Sherrod Brown (D-Ohio) that would count observation status as time toward the three-day requirement for SNF coverage.
A recent Office of Inspector General (OIG) report for the U.S. Department of Health and Human Services on observation status sums up the problem succinctly.2 The OIG states that “CMS should consider how to ensure that beneficiaries with similar post-hospital care needs have the same access and cost-sharing for SNF services.”2
SHM concurs.
Joshua Lapps is SHM’s government relations specialist.
References
- Society of Hospital Medicine. SHM submits comments in response to FY2014 inpatient prospective payment system proposed rule. Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Letters_to_Congress_and_Regulatory_Agencies&Template=/CM/ContentDisplay.cfm&ContentID=34044. Accessed Sept. 9, 2013.
- Office of Inspector General. Memorandum report: Hospitals’ use of observations stays and short inpatient stays for Medicare beneficiaries, OEI-02-12-00040. U.S. Department of Health and Human Services website. Available at: http://oig.hhs.gov/oei/reports/oei-02-12-00040.pdf. Accessed Sept. 9, 2013.
Bleeding Risks, Anticoagulants, Hospital-Acquired Infections Among Can't Miss Topics at HM14
What breakout-session and pre-course topics are HM14 course director Daniel Brotman, MD, SFHM, and assistant course director Efren Manjarrez, MD, SFHM, looking forward to showcasing? Here is a sampling:
- Bleeding risks: a crucial yet misunderstood area.
- New anticoagulants: a quickly evolving area that will affect lots of hospitalists and patients.
- What keeps your CFO up at night: a financial perspective from a hospital president and hospitalist.
- Choosing Wisely: Learn how SHM turned the ABIM Foundation’s Choosing Wisely initiative into practical recommendations for hospitalists.
- Pediatric clinical conundrums.
- Updates in key specialty and content areas.
- Hospital-acquired infection control by Sanjay Saint.
- CMS’ meaningful use.
What breakout-session and pre-course topics are HM14 course director Daniel Brotman, MD, SFHM, and assistant course director Efren Manjarrez, MD, SFHM, looking forward to showcasing? Here is a sampling:
- Bleeding risks: a crucial yet misunderstood area.
- New anticoagulants: a quickly evolving area that will affect lots of hospitalists and patients.
- What keeps your CFO up at night: a financial perspective from a hospital president and hospitalist.
- Choosing Wisely: Learn how SHM turned the ABIM Foundation’s Choosing Wisely initiative into practical recommendations for hospitalists.
- Pediatric clinical conundrums.
- Updates in key specialty and content areas.
- Hospital-acquired infection control by Sanjay Saint.
- CMS’ meaningful use.
What breakout-session and pre-course topics are HM14 course director Daniel Brotman, MD, SFHM, and assistant course director Efren Manjarrez, MD, SFHM, looking forward to showcasing? Here is a sampling:
- Bleeding risks: a crucial yet misunderstood area.
- New anticoagulants: a quickly evolving area that will affect lots of hospitalists and patients.
- What keeps your CFO up at night: a financial perspective from a hospital president and hospitalist.
- Choosing Wisely: Learn how SHM turned the ABIM Foundation’s Choosing Wisely initiative into practical recommendations for hospitalists.
- Pediatric clinical conundrums.
- Updates in key specialty and content areas.
- Hospital-acquired infection control by Sanjay Saint.
- CMS’ meaningful use.
Make Plans Now for HM14
SHM’s next annual meeting, HM14, is only six months away. So today is the day to make scheduling requests and book a room. And, for the first time, the biggest annual event in hospital medicine will be in Las Vegas.
HM14 will be held March 24-27 at Mandalay Bay Resort and Casino in Las Vegas. Meeting registration is now open at www.hospitalmedicine2014.org. The early registration discount ends Jan. 26.
Who should attend HM14? Bring the whole team: hospitalists, pediatricians, academic hospitalists, general internists, family physicians, nurse practitioners, physician assistants, administrators, and providers practicing in acute-care settings.
SHM’s next annual meeting, HM14, is only six months away. So today is the day to make scheduling requests and book a room. And, for the first time, the biggest annual event in hospital medicine will be in Las Vegas.
HM14 will be held March 24-27 at Mandalay Bay Resort and Casino in Las Vegas. Meeting registration is now open at www.hospitalmedicine2014.org. The early registration discount ends Jan. 26.
Who should attend HM14? Bring the whole team: hospitalists, pediatricians, academic hospitalists, general internists, family physicians, nurse practitioners, physician assistants, administrators, and providers practicing in acute-care settings.
SHM’s next annual meeting, HM14, is only six months away. So today is the day to make scheduling requests and book a room. And, for the first time, the biggest annual event in hospital medicine will be in Las Vegas.
HM14 will be held March 24-27 at Mandalay Bay Resort and Casino in Las Vegas. Meeting registration is now open at www.hospitalmedicine2014.org. The early registration discount ends Jan. 26.
Who should attend HM14? Bring the whole team: hospitalists, pediatricians, academic hospitalists, general internists, family physicians, nurse practitioners, physician assistants, administrators, and providers practicing in acute-care settings.
SHM Fellow in Hospital Medicine Spotlight: Janet Nagamine, MD, BSN, SFHM
Dr. Nagamine is a hospitalist physician at Kaiser Permanente Medical Center in Santa Clara, Calif., where she previously was a quality chief and safety officer. She is a member of the National Quality Forum Patient Safety Complications Steering Committee, an SHM board member, and a member of SHM’s Hospital Quality and Patient Safety Committee, which she chaired for four years.
Undergraduate education: University of Hawaii, Honolulu.
Medical school: University of Hawaii.
Notable: In the early stages of the patient-safety movement, Dr. Nagamine worked with aviation-safety experts and Kaiser colleagues to develop innovative patient-safety programs. She has led numerous quality-improvement (QI) projects, including the development of a patient-safety curriculum for staff and anonymous reporting mechanisms that focus on identifying problems in the system rather than focusing simply on individuals. For her efforts, she was awarded SHM’s Clinical Award of Excellence in 2002.
After leading many other QI initiatives on a national level, she was named a Senior Fellow in Hospital Medicine in 2010. Dr. Nagamine has been with SHM’s Project BOOST since its inception, serving on the advisory board and as a mentor. She says the SFHM designation acknowledges that she has contributed to hospital medicine in a meaningful way.
FYI: A doctor who enjoys creative projects outside of the workplace, Dr. Nagamine is working on a documentary about her father’s struggles as a World War II soldier and a poor immigrant starting a new life in California.
Quotable: “Creating a culture of safety and transparency allows us to get real about the fact that we’re human and fallible. Ultimately, we get much more information about where the problems are and how to fix them.”
Dr. Nagamine is a hospitalist physician at Kaiser Permanente Medical Center in Santa Clara, Calif., where she previously was a quality chief and safety officer. She is a member of the National Quality Forum Patient Safety Complications Steering Committee, an SHM board member, and a member of SHM’s Hospital Quality and Patient Safety Committee, which she chaired for four years.
Undergraduate education: University of Hawaii, Honolulu.
Medical school: University of Hawaii.
Notable: In the early stages of the patient-safety movement, Dr. Nagamine worked with aviation-safety experts and Kaiser colleagues to develop innovative patient-safety programs. She has led numerous quality-improvement (QI) projects, including the development of a patient-safety curriculum for staff and anonymous reporting mechanisms that focus on identifying problems in the system rather than focusing simply on individuals. For her efforts, she was awarded SHM’s Clinical Award of Excellence in 2002.
After leading many other QI initiatives on a national level, she was named a Senior Fellow in Hospital Medicine in 2010. Dr. Nagamine has been with SHM’s Project BOOST since its inception, serving on the advisory board and as a mentor. She says the SFHM designation acknowledges that she has contributed to hospital medicine in a meaningful way.
FYI: A doctor who enjoys creative projects outside of the workplace, Dr. Nagamine is working on a documentary about her father’s struggles as a World War II soldier and a poor immigrant starting a new life in California.
Quotable: “Creating a culture of safety and transparency allows us to get real about the fact that we’re human and fallible. Ultimately, we get much more information about where the problems are and how to fix them.”
Dr. Nagamine is a hospitalist physician at Kaiser Permanente Medical Center in Santa Clara, Calif., where she previously was a quality chief and safety officer. She is a member of the National Quality Forum Patient Safety Complications Steering Committee, an SHM board member, and a member of SHM’s Hospital Quality and Patient Safety Committee, which she chaired for four years.
Undergraduate education: University of Hawaii, Honolulu.
Medical school: University of Hawaii.
Notable: In the early stages of the patient-safety movement, Dr. Nagamine worked with aviation-safety experts and Kaiser colleagues to develop innovative patient-safety programs. She has led numerous quality-improvement (QI) projects, including the development of a patient-safety curriculum for staff and anonymous reporting mechanisms that focus on identifying problems in the system rather than focusing simply on individuals. For her efforts, she was awarded SHM’s Clinical Award of Excellence in 2002.
After leading many other QI initiatives on a national level, she was named a Senior Fellow in Hospital Medicine in 2010. Dr. Nagamine has been with SHM’s Project BOOST since its inception, serving on the advisory board and as a mentor. She says the SFHM designation acknowledges that she has contributed to hospital medicine in a meaningful way.
FYI: A doctor who enjoys creative projects outside of the workplace, Dr. Nagamine is working on a documentary about her father’s struggles as a World War II soldier and a poor immigrant starting a new life in California.
Quotable: “Creating a culture of safety and transparency allows us to get real about the fact that we’re human and fallible. Ultimately, we get much more information about where the problems are and how to fix them.”
SHM President to Outline Benefits of Becoming a Career Hospitalist
The care of hospitalized patients in the future rests with the young physicians of today. That’s the message of an upcoming presentation, “Target 1000: Creating the Pipeline to the Future,” by SHM President Eric Howell, MD, SFHM, set for Oct. 23 at Jefferson University Hospital in Philadelphia.
Intended for local and regional residents in the Philadelphia area, Dr. Howell will present the many benefits of becoming a career hospitalist and the resources available to medical students, residents, and early-career hospitalists. Exhibits and networking begin at 6 p.m., with Dr. Howell presenting at 7 p.m., followed by a question-and-answer session.
Attendees will be eligible to win an iPad. Medical students in attendance will receive a complimentary, one-year membership to SHM. Resident membership fees will be discounted to $100.
For more information, visit www.hospitalmedicine.org/events.
The care of hospitalized patients in the future rests with the young physicians of today. That’s the message of an upcoming presentation, “Target 1000: Creating the Pipeline to the Future,” by SHM President Eric Howell, MD, SFHM, set for Oct. 23 at Jefferson University Hospital in Philadelphia.
Intended for local and regional residents in the Philadelphia area, Dr. Howell will present the many benefits of becoming a career hospitalist and the resources available to medical students, residents, and early-career hospitalists. Exhibits and networking begin at 6 p.m., with Dr. Howell presenting at 7 p.m., followed by a question-and-answer session.
Attendees will be eligible to win an iPad. Medical students in attendance will receive a complimentary, one-year membership to SHM. Resident membership fees will be discounted to $100.
For more information, visit www.hospitalmedicine.org/events.
The care of hospitalized patients in the future rests with the young physicians of today. That’s the message of an upcoming presentation, “Target 1000: Creating the Pipeline to the Future,” by SHM President Eric Howell, MD, SFHM, set for Oct. 23 at Jefferson University Hospital in Philadelphia.
Intended for local and regional residents in the Philadelphia area, Dr. Howell will present the many benefits of becoming a career hospitalist and the resources available to medical students, residents, and early-career hospitalists. Exhibits and networking begin at 6 p.m., with Dr. Howell presenting at 7 p.m., followed by a question-and-answer session.
Attendees will be eligible to win an iPad. Medical students in attendance will receive a complimentary, one-year membership to SHM. Resident membership fees will be discounted to $100.
For more information, visit www.hospitalmedicine.org/events.
SHM Introduces Discounted PQRS Through New Learning Portal
First, SHM’s new Learning Portal was the one-stop shop for free and discounted continuing medical education (CME) credits online. Now, the Learning Portal can help hospitalists report into the physician quality reporting system (PQRS) at a discounted individual rate.
And the time to start reporting measures in PQRS is now.
The PQRS was developed by the Centers for Medicare & Medicaid Services (CMS) in 2007 as a voluntary reporting program that provides a financial incentive to physicians and other eligible professionals who report data on quality measures for covered services furnished to Medicare beneficiaries. Starting in 2013, reporting in PQRS becomes mandatory for all eligible professionals.
SHM has encouraged its members to participate in the PQRS since the system’s inception in 2007. With the exciting launch of the SHM Learning Portal, it is easier than ever to get started. If you or your group are not currently reporting, there are still incentive payments available in 2013 and 2014. Beginning in 2015, there will be a penalty for not reporting quality measures based on 2013 performance.
Access the PQRIwizard through the SHM Learning Portal
SHM has secured a significant discount for members to report PQRS through the PQRIwizard. Located within the SHM Learning Portal, this online tool is a fast, convenient, and cost-effective solution to help collect and report quality measures data for the PQRS program. Similar to online tax-preparation software, the PQRIwizard guides you through a few easy steps to help rapidly collect, validate, report, and submit your results to CMS. The tool is powered by the CECity Registry, a CMS-qualified registry for PQRS reporting.
What Measures Are Available?
The SHM PQRIwizard features six individual quality measures in the areas of stroke and stroke rehabilitation, including measures on screening for dysphagia and thrombolytic therapy. To report on any of these measures, simply select three measures and report on 80 percent of your Medicare Part B fee-for-services patients who apply to the measures you selected.
PQRIwizard has a built-in progress monitor that validates your report by checking for missing data. The monitor also tracks your data to provide you with continuous feedback regarding valid patients. The system even calculates your measures and provides a printable report of your measure results in real time.
First, SHM’s new Learning Portal was the one-stop shop for free and discounted continuing medical education (CME) credits online. Now, the Learning Portal can help hospitalists report into the physician quality reporting system (PQRS) at a discounted individual rate.
And the time to start reporting measures in PQRS is now.
The PQRS was developed by the Centers for Medicare & Medicaid Services (CMS) in 2007 as a voluntary reporting program that provides a financial incentive to physicians and other eligible professionals who report data on quality measures for covered services furnished to Medicare beneficiaries. Starting in 2013, reporting in PQRS becomes mandatory for all eligible professionals.
SHM has encouraged its members to participate in the PQRS since the system’s inception in 2007. With the exciting launch of the SHM Learning Portal, it is easier than ever to get started. If you or your group are not currently reporting, there are still incentive payments available in 2013 and 2014. Beginning in 2015, there will be a penalty for not reporting quality measures based on 2013 performance.
Access the PQRIwizard through the SHM Learning Portal
SHM has secured a significant discount for members to report PQRS through the PQRIwizard. Located within the SHM Learning Portal, this online tool is a fast, convenient, and cost-effective solution to help collect and report quality measures data for the PQRS program. Similar to online tax-preparation software, the PQRIwizard guides you through a few easy steps to help rapidly collect, validate, report, and submit your results to CMS. The tool is powered by the CECity Registry, a CMS-qualified registry for PQRS reporting.
What Measures Are Available?
The SHM PQRIwizard features six individual quality measures in the areas of stroke and stroke rehabilitation, including measures on screening for dysphagia and thrombolytic therapy. To report on any of these measures, simply select three measures and report on 80 percent of your Medicare Part B fee-for-services patients who apply to the measures you selected.
PQRIwizard has a built-in progress monitor that validates your report by checking for missing data. The monitor also tracks your data to provide you with continuous feedback regarding valid patients. The system even calculates your measures and provides a printable report of your measure results in real time.
First, SHM’s new Learning Portal was the one-stop shop for free and discounted continuing medical education (CME) credits online. Now, the Learning Portal can help hospitalists report into the physician quality reporting system (PQRS) at a discounted individual rate.
And the time to start reporting measures in PQRS is now.
The PQRS was developed by the Centers for Medicare & Medicaid Services (CMS) in 2007 as a voluntary reporting program that provides a financial incentive to physicians and other eligible professionals who report data on quality measures for covered services furnished to Medicare beneficiaries. Starting in 2013, reporting in PQRS becomes mandatory for all eligible professionals.
SHM has encouraged its members to participate in the PQRS since the system’s inception in 2007. With the exciting launch of the SHM Learning Portal, it is easier than ever to get started. If you or your group are not currently reporting, there are still incentive payments available in 2013 and 2014. Beginning in 2015, there will be a penalty for not reporting quality measures based on 2013 performance.
Access the PQRIwizard through the SHM Learning Portal
SHM has secured a significant discount for members to report PQRS through the PQRIwizard. Located within the SHM Learning Portal, this online tool is a fast, convenient, and cost-effective solution to help collect and report quality measures data for the PQRS program. Similar to online tax-preparation software, the PQRIwizard guides you through a few easy steps to help rapidly collect, validate, report, and submit your results to CMS. The tool is powered by the CECity Registry, a CMS-qualified registry for PQRS reporting.
What Measures Are Available?
The SHM PQRIwizard features six individual quality measures in the areas of stroke and stroke rehabilitation, including measures on screening for dysphagia and thrombolytic therapy. To report on any of these measures, simply select three measures and report on 80 percent of your Medicare Part B fee-for-services patients who apply to the measures you selected.
PQRIwizard has a built-in progress monitor that validates your report by checking for missing data. The monitor also tracks your data to provide you with continuous feedback regarding valid patients. The system even calculates your measures and provides a printable report of your measure results in real time.
Nominations for SHM Board of Directors, Committees due Oct. 21
Are you ready to shape the future of hospital medicine, collaborate with leaders in the field, and advance your career? Now is the time—by nominating yourself (or a colleague) for any one of dozens of SHM committees or the board of directors. But don’t delay: The deadline for nominations is Oct. 21.
To learn more about SHM’s 20-plus committees and submit a nomination, visit www.hospitalmedicine.org/committees.
To learn about board eligibility, visit the “About SHM” section at www.hospitalmedicine.org and select “Election Information.”
Why get involved in committees or SHM’s board of directors? Here are some of the reasons current leaders in the field got involved:
Eric Howell, MD, SFHM, SHM president; chief of the division of hospital medicine, John Hopkins Bayview Hospital, Baltimore
The most valuable thing to me is interacting with the nation’s HM leaders, not just other board members. Serving on the board provides connections with many of the best and brightest in our field, from “masters” to brilliant staff, and many, many insightful and thoughtful members.
Serving on the board has been a huge help in my career. The networking is fabulous and absolutely cannot be understated. Plus, you learn a ton from serving on the board, from cutting-edge topics to being involved in areas of HM that might not be present at your home institution. There are multiple opportunities to grow and advance your own leadership skills, from running for a board of directors officer position (treasurer, secretary, president) to opportunities to participate in the Leadership Academy to the AHA to QSEA and more.
Nasim Afsar, MD, SFHM, SHM board member; associate chief medical officer, assistant clinical professor, medicine and neurosurgery, executive director of quality and safety, medicine and neurosurgery, UCLA Hospitals, Los Angeles.
If you want to work on challenges facing our specialty, with an incredibly insightful, dedicated, and thoughtful group, come on board. Participating as an SHM board member is invaluable. We have such a dedicated and accomplished group of colleagues focused on the challenges in health care, and we are working toward solutions for the future.
It has enabled me to have a broader perspective on the field of hospital medicine as well as the various roles hospitalists play locally and nationally.
Alexander Carbo, MD, SFHM, SHM Membership Committee chair; assistant professor of medicine, Beth Israel Deaconess Medical Center, Boston
There are several benefits to serving on an SHM committee. It allows you to meet and collaborate with a fantastic group of individuals, and easily establishes connections that would otherwise take much longer to foster. It also allows you to participate in the field at a national level: If there is something that you are passionate about, committee service can provide a platform for that passion.
It is great fun to participate in SHM committees and to be a part of the process in which this society shapes policy and provides educational opportunities for hospitalists.
Serving on an SHM committee has certainly expanded my network of contacts within hospital medicine!
We are trying to listen to what front-line providers want and need to know about patient safety and quality improvement, and to provide that information for them.
Kim Dickinson, MA, RRT, SFHM, SHM Administrators Committee chair; executive vice president, Acute Services Hospitalists Now Inc., Tucson, Ariz.
I have appreciated the opportunity for continued personal leadership development and the ability to interact closely with others in our industry. I have found hospitalists to be very transparent regarding improving patient care best practices.
It is fun. You meet a subset of people you may have never known. Deep friendships are formed. HM is a large specialty in a very small world. People I have worked with in past committees resurface in my life with regularity.
Our committee has been active in providing broad education about the best practices in HM administration, as well as providing a fellowship track for nonphysicians. This is a landmark achievement for us. Recognition for being part of the HM transformation of health care is immensely satisfying.
Tierza Stephan, MD, FACP, SFHM, SHM Practice Analysis Committee member, hospitalist regional medical director, Allina Health, Minneapolis
Serving on the Practice Analysis Committee has helped me to be a more informed, credible source of hospitalist information for senior leaders in my organization. It has definitely provided me a set of knowledgeable hospitalist colleagues outside of my health system to whom I can turn to for advice and help with problem-solving.
Hospitalists across the country share an amazing number of similar issues despite every hospital having its own unique culture. It’s helpful to hear others talk about the solutions they’ve considered and tried, what went well, and what didn’t. I’ve learned more about the complexity of analyzing a hospitalist practice.
Kendall M. Rogers, MD, CPE, FACP, SFHM, SHM IT Executive Committee chair, associate professor of medicine, chief of the division of hospital medicine, University of New Mexico Health Sciences Center, Albuquerque
My time on SHM committees has been one of the most professionally satisfying activities I have engaged in. In addition to meeting and working closely with national leaders and role models, it has expanded my local idea of what our HM group was capable of achieving by seeing the accomplishments of others and allowing me to incorporate many aspects of these practices without having to develop it from scratch. It also has given me a great sense of pride in our specialty, which has also added to my job satisfaction.
Much of this would not have been possible without the support structure I have built through SHM, which all began with serving on one committee. That has grown to chairing committees, serving as SHM faculty, being a mentee, then mentor, then lead mentor in SHM’s mentored implementation programs.
Committee membership gives you a source of professional satisfaction that is different from your local work. It ties you into a network of people with similar interests while also making you more effective in your local work.
As chair of the Information Technology (IT) Executive Committee, I received a message from my administrative assistant that stated: “The Society of Hospital Medicine called and they need you to go to the White House next week.” I was invited to represent SHM at a town hall meeting on IT with the ONC director at the White House with SHM’s senior advisor for advocacy and government affairs. I have traveled with the CEO, Larry Wellikson, to visit major [electronic medical records] vendors and advocate for the IT tools we need for our members to provide the highest quality of care.
Are you ready to shape the future of hospital medicine, collaborate with leaders in the field, and advance your career? Now is the time—by nominating yourself (or a colleague) for any one of dozens of SHM committees or the board of directors. But don’t delay: The deadline for nominations is Oct. 21.
To learn more about SHM’s 20-plus committees and submit a nomination, visit www.hospitalmedicine.org/committees.
To learn about board eligibility, visit the “About SHM” section at www.hospitalmedicine.org and select “Election Information.”
Why get involved in committees or SHM’s board of directors? Here are some of the reasons current leaders in the field got involved:
Eric Howell, MD, SFHM, SHM president; chief of the division of hospital medicine, John Hopkins Bayview Hospital, Baltimore
The most valuable thing to me is interacting with the nation’s HM leaders, not just other board members. Serving on the board provides connections with many of the best and brightest in our field, from “masters” to brilliant staff, and many, many insightful and thoughtful members.
Serving on the board has been a huge help in my career. The networking is fabulous and absolutely cannot be understated. Plus, you learn a ton from serving on the board, from cutting-edge topics to being involved in areas of HM that might not be present at your home institution. There are multiple opportunities to grow and advance your own leadership skills, from running for a board of directors officer position (treasurer, secretary, president) to opportunities to participate in the Leadership Academy to the AHA to QSEA and more.
Nasim Afsar, MD, SFHM, SHM board member; associate chief medical officer, assistant clinical professor, medicine and neurosurgery, executive director of quality and safety, medicine and neurosurgery, UCLA Hospitals, Los Angeles.
If you want to work on challenges facing our specialty, with an incredibly insightful, dedicated, and thoughtful group, come on board. Participating as an SHM board member is invaluable. We have such a dedicated and accomplished group of colleagues focused on the challenges in health care, and we are working toward solutions for the future.
It has enabled me to have a broader perspective on the field of hospital medicine as well as the various roles hospitalists play locally and nationally.
Alexander Carbo, MD, SFHM, SHM Membership Committee chair; assistant professor of medicine, Beth Israel Deaconess Medical Center, Boston
There are several benefits to serving on an SHM committee. It allows you to meet and collaborate with a fantastic group of individuals, and easily establishes connections that would otherwise take much longer to foster. It also allows you to participate in the field at a national level: If there is something that you are passionate about, committee service can provide a platform for that passion.
It is great fun to participate in SHM committees and to be a part of the process in which this society shapes policy and provides educational opportunities for hospitalists.
Serving on an SHM committee has certainly expanded my network of contacts within hospital medicine!
We are trying to listen to what front-line providers want and need to know about patient safety and quality improvement, and to provide that information for them.
Kim Dickinson, MA, RRT, SFHM, SHM Administrators Committee chair; executive vice president, Acute Services Hospitalists Now Inc., Tucson, Ariz.
I have appreciated the opportunity for continued personal leadership development and the ability to interact closely with others in our industry. I have found hospitalists to be very transparent regarding improving patient care best practices.
It is fun. You meet a subset of people you may have never known. Deep friendships are formed. HM is a large specialty in a very small world. People I have worked with in past committees resurface in my life with regularity.
Our committee has been active in providing broad education about the best practices in HM administration, as well as providing a fellowship track for nonphysicians. This is a landmark achievement for us. Recognition for being part of the HM transformation of health care is immensely satisfying.
Tierza Stephan, MD, FACP, SFHM, SHM Practice Analysis Committee member, hospitalist regional medical director, Allina Health, Minneapolis
Serving on the Practice Analysis Committee has helped me to be a more informed, credible source of hospitalist information for senior leaders in my organization. It has definitely provided me a set of knowledgeable hospitalist colleagues outside of my health system to whom I can turn to for advice and help with problem-solving.
Hospitalists across the country share an amazing number of similar issues despite every hospital having its own unique culture. It’s helpful to hear others talk about the solutions they’ve considered and tried, what went well, and what didn’t. I’ve learned more about the complexity of analyzing a hospitalist practice.
Kendall M. Rogers, MD, CPE, FACP, SFHM, SHM IT Executive Committee chair, associate professor of medicine, chief of the division of hospital medicine, University of New Mexico Health Sciences Center, Albuquerque
My time on SHM committees has been one of the most professionally satisfying activities I have engaged in. In addition to meeting and working closely with national leaders and role models, it has expanded my local idea of what our HM group was capable of achieving by seeing the accomplishments of others and allowing me to incorporate many aspects of these practices without having to develop it from scratch. It also has given me a great sense of pride in our specialty, which has also added to my job satisfaction.
Much of this would not have been possible without the support structure I have built through SHM, which all began with serving on one committee. That has grown to chairing committees, serving as SHM faculty, being a mentee, then mentor, then lead mentor in SHM’s mentored implementation programs.
Committee membership gives you a source of professional satisfaction that is different from your local work. It ties you into a network of people with similar interests while also making you more effective in your local work.
As chair of the Information Technology (IT) Executive Committee, I received a message from my administrative assistant that stated: “The Society of Hospital Medicine called and they need you to go to the White House next week.” I was invited to represent SHM at a town hall meeting on IT with the ONC director at the White House with SHM’s senior advisor for advocacy and government affairs. I have traveled with the CEO, Larry Wellikson, to visit major [electronic medical records] vendors and advocate for the IT tools we need for our members to provide the highest quality of care.
Are you ready to shape the future of hospital medicine, collaborate with leaders in the field, and advance your career? Now is the time—by nominating yourself (or a colleague) for any one of dozens of SHM committees or the board of directors. But don’t delay: The deadline for nominations is Oct. 21.
To learn more about SHM’s 20-plus committees and submit a nomination, visit www.hospitalmedicine.org/committees.
To learn about board eligibility, visit the “About SHM” section at www.hospitalmedicine.org and select “Election Information.”
Why get involved in committees or SHM’s board of directors? Here are some of the reasons current leaders in the field got involved:
Eric Howell, MD, SFHM, SHM president; chief of the division of hospital medicine, John Hopkins Bayview Hospital, Baltimore
The most valuable thing to me is interacting with the nation’s HM leaders, not just other board members. Serving on the board provides connections with many of the best and brightest in our field, from “masters” to brilliant staff, and many, many insightful and thoughtful members.
Serving on the board has been a huge help in my career. The networking is fabulous and absolutely cannot be understated. Plus, you learn a ton from serving on the board, from cutting-edge topics to being involved in areas of HM that might not be present at your home institution. There are multiple opportunities to grow and advance your own leadership skills, from running for a board of directors officer position (treasurer, secretary, president) to opportunities to participate in the Leadership Academy to the AHA to QSEA and more.
Nasim Afsar, MD, SFHM, SHM board member; associate chief medical officer, assistant clinical professor, medicine and neurosurgery, executive director of quality and safety, medicine and neurosurgery, UCLA Hospitals, Los Angeles.
If you want to work on challenges facing our specialty, with an incredibly insightful, dedicated, and thoughtful group, come on board. Participating as an SHM board member is invaluable. We have such a dedicated and accomplished group of colleagues focused on the challenges in health care, and we are working toward solutions for the future.
It has enabled me to have a broader perspective on the field of hospital medicine as well as the various roles hospitalists play locally and nationally.
Alexander Carbo, MD, SFHM, SHM Membership Committee chair; assistant professor of medicine, Beth Israel Deaconess Medical Center, Boston
There are several benefits to serving on an SHM committee. It allows you to meet and collaborate with a fantastic group of individuals, and easily establishes connections that would otherwise take much longer to foster. It also allows you to participate in the field at a national level: If there is something that you are passionate about, committee service can provide a platform for that passion.
It is great fun to participate in SHM committees and to be a part of the process in which this society shapes policy and provides educational opportunities for hospitalists.
Serving on an SHM committee has certainly expanded my network of contacts within hospital medicine!
We are trying to listen to what front-line providers want and need to know about patient safety and quality improvement, and to provide that information for them.
Kim Dickinson, MA, RRT, SFHM, SHM Administrators Committee chair; executive vice president, Acute Services Hospitalists Now Inc., Tucson, Ariz.
I have appreciated the opportunity for continued personal leadership development and the ability to interact closely with others in our industry. I have found hospitalists to be very transparent regarding improving patient care best practices.
It is fun. You meet a subset of people you may have never known. Deep friendships are formed. HM is a large specialty in a very small world. People I have worked with in past committees resurface in my life with regularity.
Our committee has been active in providing broad education about the best practices in HM administration, as well as providing a fellowship track for nonphysicians. This is a landmark achievement for us. Recognition for being part of the HM transformation of health care is immensely satisfying.
Tierza Stephan, MD, FACP, SFHM, SHM Practice Analysis Committee member, hospitalist regional medical director, Allina Health, Minneapolis
Serving on the Practice Analysis Committee has helped me to be a more informed, credible source of hospitalist information for senior leaders in my organization. It has definitely provided me a set of knowledgeable hospitalist colleagues outside of my health system to whom I can turn to for advice and help with problem-solving.
Hospitalists across the country share an amazing number of similar issues despite every hospital having its own unique culture. It’s helpful to hear others talk about the solutions they’ve considered and tried, what went well, and what didn’t. I’ve learned more about the complexity of analyzing a hospitalist practice.
Kendall M. Rogers, MD, CPE, FACP, SFHM, SHM IT Executive Committee chair, associate professor of medicine, chief of the division of hospital medicine, University of New Mexico Health Sciences Center, Albuquerque
My time on SHM committees has been one of the most professionally satisfying activities I have engaged in. In addition to meeting and working closely with national leaders and role models, it has expanded my local idea of what our HM group was capable of achieving by seeing the accomplishments of others and allowing me to incorporate many aspects of these practices without having to develop it from scratch. It also has given me a great sense of pride in our specialty, which has also added to my job satisfaction.
Much of this would not have been possible without the support structure I have built through SHM, which all began with serving on one committee. That has grown to chairing committees, serving as SHM faculty, being a mentee, then mentor, then lead mentor in SHM’s mentored implementation programs.
Committee membership gives you a source of professional satisfaction that is different from your local work. It ties you into a network of people with similar interests while also making you more effective in your local work.
As chair of the Information Technology (IT) Executive Committee, I received a message from my administrative assistant that stated: “The Society of Hospital Medicine called and they need you to go to the White House next week.” I was invited to represent SHM at a town hall meeting on IT with the ONC director at the White House with SHM’s senior advisor for advocacy and government affairs. I have traveled with the CEO, Larry Wellikson, to visit major [electronic medical records] vendors and advocate for the IT tools we need for our members to provide the highest quality of care.
Movers and Shakers in Hospital Medicine
Robert Wachter, MD, MHM, has been named to the board of directors and chair of the quality committee for IPC: The Hospitalist Company, based in North Hollywood, Calif. Dr. Wachter currently serves as director of the division of hospital medicine and associate chair of the department of medicine at the University of California at San Francisco. A well-known and respected authority on quality and safety, he was recognized with the John M. Eisenberg Award for excellence in patient safety in 2004. He also pens the Wachter’s World blog at www.wachtersworld.com.
Raffi Hodikian, MD, a hospitalist and longtime member of SHM, was named the 2013 Physician of the Year at Foothill Presbyterian Hospital in Glendora, Calif. “Not only was this the greatest honor of my career, but I thought it further reaffirmed the vital role hospitalists play in our community hospitals,” Dr. Hodikian said of the award.
Kimberly A. Bell, MD, is the new associate vice president of hospital medicine for Franciscan Health System (FHS) in Tacoma, Wash. In her new role, Dr. Bell will oversee hospitalist services at five of FHS’ seven area hospitals. FHS employs nearly 100 hospital medicine providers, including physicians and physician extenders.
Felix T. Cabrera, MD, has been named associate medical director at Guam Memorial Hospital (GMH) in Tamuning, Guam, after working as a hospitalist at GMH for more than two years. In his new role, Dr. Cabrera hopes to improve the technological infrastructure within the hospital. He will continue with his regular hospitalist rounds and private practice at International Health Providers Medical Group in Dededo, Guam. GMH is a 158-bed acute-care facility and the only hospital dedicated to civilian care on the island.
Troy Martin, MD, has been appointed chief medical officer for Questcare Hospitalists, based in Dallas. Dr. Martin comes to the Questcare executive team from the Medical Center of McKinney in McKinney, Texas, where he served as medical director of Questcare’s hospitalist program.
Robert Wachter, MD, MHM, has been named to the board of directors and chair of the quality committee for IPC: The Hospitalist Company, based in North Hollywood, Calif. Dr. Wachter currently serves as director of the division of hospital medicine and associate chair of the department of medicine at the University of California at San Francisco. A well-known and respected authority on quality and safety, he was recognized with the John M. Eisenberg Award for excellence in patient safety in 2004. He also pens the Wachter’s World blog at www.wachtersworld.com.
Raffi Hodikian, MD, a hospitalist and longtime member of SHM, was named the 2013 Physician of the Year at Foothill Presbyterian Hospital in Glendora, Calif. “Not only was this the greatest honor of my career, but I thought it further reaffirmed the vital role hospitalists play in our community hospitals,” Dr. Hodikian said of the award.
Kimberly A. Bell, MD, is the new associate vice president of hospital medicine for Franciscan Health System (FHS) in Tacoma, Wash. In her new role, Dr. Bell will oversee hospitalist services at five of FHS’ seven area hospitals. FHS employs nearly 100 hospital medicine providers, including physicians and physician extenders.
Felix T. Cabrera, MD, has been named associate medical director at Guam Memorial Hospital (GMH) in Tamuning, Guam, after working as a hospitalist at GMH for more than two years. In his new role, Dr. Cabrera hopes to improve the technological infrastructure within the hospital. He will continue with his regular hospitalist rounds and private practice at International Health Providers Medical Group in Dededo, Guam. GMH is a 158-bed acute-care facility and the only hospital dedicated to civilian care on the island.
Troy Martin, MD, has been appointed chief medical officer for Questcare Hospitalists, based in Dallas. Dr. Martin comes to the Questcare executive team from the Medical Center of McKinney in McKinney, Texas, where he served as medical director of Questcare’s hospitalist program.
Robert Wachter, MD, MHM, has been named to the board of directors and chair of the quality committee for IPC: The Hospitalist Company, based in North Hollywood, Calif. Dr. Wachter currently serves as director of the division of hospital medicine and associate chair of the department of medicine at the University of California at San Francisco. A well-known and respected authority on quality and safety, he was recognized with the John M. Eisenberg Award for excellence in patient safety in 2004. He also pens the Wachter’s World blog at www.wachtersworld.com.
Raffi Hodikian, MD, a hospitalist and longtime member of SHM, was named the 2013 Physician of the Year at Foothill Presbyterian Hospital in Glendora, Calif. “Not only was this the greatest honor of my career, but I thought it further reaffirmed the vital role hospitalists play in our community hospitals,” Dr. Hodikian said of the award.
Kimberly A. Bell, MD, is the new associate vice president of hospital medicine for Franciscan Health System (FHS) in Tacoma, Wash. In her new role, Dr. Bell will oversee hospitalist services at five of FHS’ seven area hospitals. FHS employs nearly 100 hospital medicine providers, including physicians and physician extenders.
Felix T. Cabrera, MD, has been named associate medical director at Guam Memorial Hospital (GMH) in Tamuning, Guam, after working as a hospitalist at GMH for more than two years. In his new role, Dr. Cabrera hopes to improve the technological infrastructure within the hospital. He will continue with his regular hospitalist rounds and private practice at International Health Providers Medical Group in Dededo, Guam. GMH is a 158-bed acute-care facility and the only hospital dedicated to civilian care on the island.
Troy Martin, MD, has been appointed chief medical officer for Questcare Hospitalists, based in Dallas. Dr. Martin comes to the Questcare executive team from the Medical Center of McKinney in McKinney, Texas, where he served as medical director of Questcare’s hospitalist program.
Nine Things Chronic-Pain Specialists Think Hospitalists Need To Know
In This Edition
9 Things: At a Glance
An occasional series providing specialty-specific advice for hospitalists from experts in the field.
- Recognize the differential diagnosis for pain exacerbation in a chronic opioid therapy (COT) patient/chronic pain patient.
- Know where the opioids are going.
- Sometimes stopping pills, rather than adding them, can cure pain.
- Take time to educate patients about methadone and its risk of mortality if not used as prescribed.
- A little local anesthetic (and some steroid) goes a long way.
- Addiction to opioids is not rare.
- Safely changing opioid regimens requires good math and good judgment.
- For a low-risk chronic pain patient on low-dose opioids, don’t change the regimen, even if the indication for opioids isn’t clear.
- If a patient has pain all the time, they need to be on a medication that works all the time.
The differential diagnosis for pain exacerbation in a chronic opioid therapy (COT) patient/chronic pain patient is:
- Worsening medical problem;
- New medical problem;
- Nonopioid problem (side effect);
- Opioid problem (resistance/tolerance/side effect); and
- Opioid-induced hyperalgesia.
The search for an etiology and treatment for chronic pain should not end, even if a patient is labeled with “chronic pain syndrome.” The patient could simply be chronically undiagnosed or on an incorrect therapy.
Know where the opioids are going.
Whether it’s auditing a prescription-monitoring program (PMP), checking a urine drug screen, or calling a pharmacist, try to ensure that chronic pain patients are taking the opioids as prescribed. A phone call to the primary opioid prescriber or chronic pain provider could save a busy hospitalist a lot of time.
Using PMP data can consume a lot of time. Typically, only prescribing providers can access PMPs, so delegating this responsibility to someone else is not possible. If your state PMP does not help, simply call the patient’s pharmacy and ask for the last three fill dates on an opioid prescription. This also works well in case the patient’s pharmacy doesn’t participate in a PMP or is delayed in uploading recent prescriber data. Many COT patients have an opioid treatment agreement with their prescriber and must use only one pharmacy to fill opioids.
In January 2013, the University of North Carolina Injury Prevention Center published an analysis of three years of North Carolina PMP data.1 Patients followed by providers who consistently used the state PMP were five times more likely to receive treatment for opioid dependence compared with patients of providers who never used the state PMP.1
Why go through all this trouble if a chronic pain specialist is also doing it? It’s good documentation and good care, like monitoring levels of transplant meds or making sure hemoglobin A1Cs are up to date and trending toward goal. It may only take one misused or diverted opioid pill to result in a serious adverse event.
Sometimes stopping pills, rather than adding them, can cure pain.
Many chronic pain patients accumulate a patchwork of pills (e.g. benzodiazepines, opioids, muscle relaxants, and antidepressants). Many interpret noxious symptoms associated with the drug burden as “uncontrolled pain.” Two conditions that might afflict the pain sufferer who takes multiple medications are opioid-induced hyperalgesia (OIH) and medication-overuse headaches (MOH). They are uncommon but should be on a hospitalist’s differential for difficult-to-control chronic pain. Opioids commonly are implicated in causing MOH, a chronic headache occurring at least 15 days a month, four hours a day if untreated, and for at least three consecutive months. OIH is a nociceptive sensitization caused by opioids that can occur suddenly or insidiously.
If a drug isn’t absolutely necessary, stop it. If you and the patient start by agreeing to the shared goal of improving health, the conversation should go better. An axiom we learned from mentors at the University of Washington is: “There is no pain that cannot be made worse with inappropriate therapy.”
Take time to educate patients about methadone and its risk of mortality if not used as prescribed.
Methadone is less frequently prescribed than other opioids, yet it is more frequently associated with death from overdose. Though there is a risk of overdose and death with any opioid, managing methadone is more difficult. A desperate chronic pain patient may self-escalate their methadone without proper insight into the consequences.
Remember the logarithmic relationship methadone doses have with their morphine equivalency. The following highlights how deceiving the numbers are: 50 mg of methadone is about 100 mg of morphine-equivalent, but 100 mg of methadone is about 1,000 mg of morphine-equivalent, or 10 times as strong.
From 1999 to 2005, methadone-related deaths increased by 468%.2 If the patient doesn’t seem to understand these risks, they are not a good candidate for methadone treatment.
A little local anesthetic (and some steroid) goes a long way.
Hospitalists should offer an assortment of diagnostic and therapeutic injections to chronic pain patients. First, be sure you’ve done your due diligence:
- What procedures do you have privileges to do?
- Do you need to be proctored first?
- How do your local specialists feel about you doing injections?
In light of these considerations, hospitalists should be able to train and be credentialed to offer such procedures as trigger-point injections, joint injections (knees, shoulders), or even a peripheral nerve injection (e.g. lateral femoral cutaneous nerve or ilioinguinal nerve injection). Some hospitalists might even want to learn ultrasound-guided sacroiliac joint injections for chronic unexplained back pain.
Offering an indicated and effective injection is a good nonopioid option. And local anesthetic injections can help hospitalists establish an elusive diagnosis. For example, many patients spend years getting worked up for head and neck pain when dry-needling with a small volume (1 cc) of local anesthetic into a neck muscle trigger point can break their pain generator, eliminating their pain.
Addiction to opioids is not rare.
The use, misuse, and diversion of opioids and all the associated complications have appropriately received considerable media attention. A seminal paper by Porter and Jick titled “Addiction Is Rare in Patients Treated with Narcotics” is one of many tipping points associated with the boom in opioid prescribing.3 Whether it’s a three-day supply of hydrocodone, 24 hours on a PCA, or an opioid rotation, any exposure to opioids can put a patient on the runway to addiction.
There are only 3,071 board-certified addiction specialists certified by the American Board of Addiction Medicine, so access to an addiction specialist might be difficult.4
Nonetheless, do not become complacent and just continue the opioid therapy in a difficult opioid-addicted patient. Express your concerns to the primary opioid prescriber, or help patients who don’t have an opioid prescriber get access and treatment. Otherwise, you have no choice but to taper the opioids.
Ideally, chronic pain management should be delivered in the outpatient arena where long-term monitoring can take place.
Safely changing opioid regimens requires good math and good judgment.
During training and practice, hospitalists become accustomed to rapidly analyze objective data, such as ABGs, ECGs, anion gaps, and vent settings. A hospitalist should become similarly efficient at calculating morphine equivalencies (cautiously with methadone and fentanyl), making dose reductions, and rotating opioids. The more comfortable you are with morphine equivalencies, the faster and safer you will be at rotating opioids. Whatever morphine-equivalence table you feel comfortable with is the one you should use consistently.
We see many providers who unwittingly take, for example, a patient who has become resistant to hydrocodone/acetaminophen 10/325 mg PO TID (30 mg of morphine) and convert them to oxycodone/acetaminophen 10/325 mg PO QID (60 mg of morphine—a doubling), when doubling could cause respiratory depression or a faster path to addiction and dependency.
But there are cases in which judgment should trump math, such as when converting from an IV to an oral regimen. We frequently see patients in the clinic requesting refills for more than 100 mg of hydromorphone because “that’s what I was on when I was hospitalized and on the pump.” If the IV-to-oral conversion leaves you prescribing high doses of oral opioids, plan for a rapid taper and a smooth handoff to the outpatient setting.
One strategy to decrease an error in math and judgment is to use IV PCAs as infrequently as possible if a patient isn’t post-operative and they are able to take oral meds. And never hesitate to consult with your inpatient pharmacist or a chronic pain specialist.
For a low-risk chronic pain patient on low-dose opioids, don’t change the regimen, even if the indication for opioids isn’t clear.
Although it is tempting to become an opioid prohibitionist, if a patient has been taking an opioid for years and is functioning, working, compliant, and has no risk factors for complications from COT, it is likely fine to continue their current regimen. Touch base with the primary opioid prescriber, and if you’re concerned, use some of the monitoring instruments described earlier (PMP, urine drug screen, opioid treatment agreement, pill counts).
If a patient has pain all the time, they need to be on a medication that works all the time.
A good pain history followed by a good neurological and mental health assessment is of incalculable value, especially because physicians often underestimate a patient’s pain intensity and its impact on a patient’s quality of life.5,6 A patient’s pain intensity, quality of life, and function can be dramatically improved by starting a long-acting medication for “constant pain.”
If a patient hurts “24 hours a day” and cannot function on hydrocodone/acetaminophen 10/325 QID, it’s probably because they are constantly reacting to spikes in pain and using a “some of the time” medicine to treat “all the time” pain. Switching to a long-acting medication—and it doesn’t have to be an opioid—could improve control and decrease how much narcotic the patient needs.
If you choose a long-acting opioid (in this case, you could try morphine sulphate extended-release 15 mg BID and satisfy 50% of the hydrocodone need), then you could titrate slowly upwards to where the patient would not need hydrocodone. If the patient still had uncontrolled pain, then either morphine is the wrong compound for them or they are benefiting from the “nonanalgesic properties” of the opioids.
Give the patient the benefit of the doubt; because of genetic polymorphisms, a patient may need several opioid rotations before the right opioid compound is found.
Dr. Schultz is a hospitalist and assistant professor in the department of internal medicine at the University of Miami Miller School of Medicine. She is board-certified in hospice and palliative care and specializes in chronic pain management. Dr. Ajam is a hospitalist and a clinical assistant professor in the department of anesthesiology at Wake Forest University Baptist Medical Center and the Carolinas Pain Institute. He is board-certified in chronic pain management.
References
- Garrettson M, Ringwalt C. An evaluation of the North Carolina controlled substances reporting system: part II impact evaluation, January 2013. PDMP Center of Excellence website. Available at: http://pdmpexcellence.org/sites/all/pdfs/NC_control_sub_eval_pt_2.pdf. Accessed Sept. 3, 2013.
- Kung HC, Hoyert DL, Xu JQ, Murphy SL. Deaths: Final data for 2005, national vital statistics reports; Vol. 56 No. 10. Hyattsville, Md.: National Center for Health Statistics; 2008.
- Porter J, Jick H. Addiction rare in patients treated with narcotics. N Engl J Med. 1980;302(2):123.
- American Society of Addiction Medicine; personal communication, 2013.
- Mäntyselkä P, Kumpusalo E, Ahonen R, Takala J. Patients’ versus general practitioners’ assessments of pain intensity in primary care patients with non-cancer pain. Br J Gen Pract. 2001;51(473):995-997.
- Petersen MA, Larsen H, Pedersen L, Sonne N, Groenvold M. Assessing health-related quality of life in palliative care: comparing patient and physician assessments. Eur J Cancer. 2006;42(8):1159-1166.
In This Edition
9 Things: At a Glance
An occasional series providing specialty-specific advice for hospitalists from experts in the field.
- Recognize the differential diagnosis for pain exacerbation in a chronic opioid therapy (COT) patient/chronic pain patient.
- Know where the opioids are going.
- Sometimes stopping pills, rather than adding them, can cure pain.
- Take time to educate patients about methadone and its risk of mortality if not used as prescribed.
- A little local anesthetic (and some steroid) goes a long way.
- Addiction to opioids is not rare.
- Safely changing opioid regimens requires good math and good judgment.
- For a low-risk chronic pain patient on low-dose opioids, don’t change the regimen, even if the indication for opioids isn’t clear.
- If a patient has pain all the time, they need to be on a medication that works all the time.
The differential diagnosis for pain exacerbation in a chronic opioid therapy (COT) patient/chronic pain patient is:
- Worsening medical problem;
- New medical problem;
- Nonopioid problem (side effect);
- Opioid problem (resistance/tolerance/side effect); and
- Opioid-induced hyperalgesia.
The search for an etiology and treatment for chronic pain should not end, even if a patient is labeled with “chronic pain syndrome.” The patient could simply be chronically undiagnosed or on an incorrect therapy.
Know where the opioids are going.
Whether it’s auditing a prescription-monitoring program (PMP), checking a urine drug screen, or calling a pharmacist, try to ensure that chronic pain patients are taking the opioids as prescribed. A phone call to the primary opioid prescriber or chronic pain provider could save a busy hospitalist a lot of time.
Using PMP data can consume a lot of time. Typically, only prescribing providers can access PMPs, so delegating this responsibility to someone else is not possible. If your state PMP does not help, simply call the patient’s pharmacy and ask for the last three fill dates on an opioid prescription. This also works well in case the patient’s pharmacy doesn’t participate in a PMP or is delayed in uploading recent prescriber data. Many COT patients have an opioid treatment agreement with their prescriber and must use only one pharmacy to fill opioids.
In January 2013, the University of North Carolina Injury Prevention Center published an analysis of three years of North Carolina PMP data.1 Patients followed by providers who consistently used the state PMP were five times more likely to receive treatment for opioid dependence compared with patients of providers who never used the state PMP.1
Why go through all this trouble if a chronic pain specialist is also doing it? It’s good documentation and good care, like monitoring levels of transplant meds or making sure hemoglobin A1Cs are up to date and trending toward goal. It may only take one misused or diverted opioid pill to result in a serious adverse event.
Sometimes stopping pills, rather than adding them, can cure pain.
Many chronic pain patients accumulate a patchwork of pills (e.g. benzodiazepines, opioids, muscle relaxants, and antidepressants). Many interpret noxious symptoms associated with the drug burden as “uncontrolled pain.” Two conditions that might afflict the pain sufferer who takes multiple medications are opioid-induced hyperalgesia (OIH) and medication-overuse headaches (MOH). They are uncommon but should be on a hospitalist’s differential for difficult-to-control chronic pain. Opioids commonly are implicated in causing MOH, a chronic headache occurring at least 15 days a month, four hours a day if untreated, and for at least three consecutive months. OIH is a nociceptive sensitization caused by opioids that can occur suddenly or insidiously.
If a drug isn’t absolutely necessary, stop it. If you and the patient start by agreeing to the shared goal of improving health, the conversation should go better. An axiom we learned from mentors at the University of Washington is: “There is no pain that cannot be made worse with inappropriate therapy.”
Take time to educate patients about methadone and its risk of mortality if not used as prescribed.
Methadone is less frequently prescribed than other opioids, yet it is more frequently associated with death from overdose. Though there is a risk of overdose and death with any opioid, managing methadone is more difficult. A desperate chronic pain patient may self-escalate their methadone without proper insight into the consequences.
Remember the logarithmic relationship methadone doses have with their morphine equivalency. The following highlights how deceiving the numbers are: 50 mg of methadone is about 100 mg of morphine-equivalent, but 100 mg of methadone is about 1,000 mg of morphine-equivalent, or 10 times as strong.
From 1999 to 2005, methadone-related deaths increased by 468%.2 If the patient doesn’t seem to understand these risks, they are not a good candidate for methadone treatment.
A little local anesthetic (and some steroid) goes a long way.
Hospitalists should offer an assortment of diagnostic and therapeutic injections to chronic pain patients. First, be sure you’ve done your due diligence:
- What procedures do you have privileges to do?
- Do you need to be proctored first?
- How do your local specialists feel about you doing injections?
In light of these considerations, hospitalists should be able to train and be credentialed to offer such procedures as trigger-point injections, joint injections (knees, shoulders), or even a peripheral nerve injection (e.g. lateral femoral cutaneous nerve or ilioinguinal nerve injection). Some hospitalists might even want to learn ultrasound-guided sacroiliac joint injections for chronic unexplained back pain.
Offering an indicated and effective injection is a good nonopioid option. And local anesthetic injections can help hospitalists establish an elusive diagnosis. For example, many patients spend years getting worked up for head and neck pain when dry-needling with a small volume (1 cc) of local anesthetic into a neck muscle trigger point can break their pain generator, eliminating their pain.
Addiction to opioids is not rare.
The use, misuse, and diversion of opioids and all the associated complications have appropriately received considerable media attention. A seminal paper by Porter and Jick titled “Addiction Is Rare in Patients Treated with Narcotics” is one of many tipping points associated with the boom in opioid prescribing.3 Whether it’s a three-day supply of hydrocodone, 24 hours on a PCA, or an opioid rotation, any exposure to opioids can put a patient on the runway to addiction.
There are only 3,071 board-certified addiction specialists certified by the American Board of Addiction Medicine, so access to an addiction specialist might be difficult.4
Nonetheless, do not become complacent and just continue the opioid therapy in a difficult opioid-addicted patient. Express your concerns to the primary opioid prescriber, or help patients who don’t have an opioid prescriber get access and treatment. Otherwise, you have no choice but to taper the opioids.
Ideally, chronic pain management should be delivered in the outpatient arena where long-term monitoring can take place.
Safely changing opioid regimens requires good math and good judgment.
During training and practice, hospitalists become accustomed to rapidly analyze objective data, such as ABGs, ECGs, anion gaps, and vent settings. A hospitalist should become similarly efficient at calculating morphine equivalencies (cautiously with methadone and fentanyl), making dose reductions, and rotating opioids. The more comfortable you are with morphine equivalencies, the faster and safer you will be at rotating opioids. Whatever morphine-equivalence table you feel comfortable with is the one you should use consistently.
We see many providers who unwittingly take, for example, a patient who has become resistant to hydrocodone/acetaminophen 10/325 mg PO TID (30 mg of morphine) and convert them to oxycodone/acetaminophen 10/325 mg PO QID (60 mg of morphine—a doubling), when doubling could cause respiratory depression or a faster path to addiction and dependency.
But there are cases in which judgment should trump math, such as when converting from an IV to an oral regimen. We frequently see patients in the clinic requesting refills for more than 100 mg of hydromorphone because “that’s what I was on when I was hospitalized and on the pump.” If the IV-to-oral conversion leaves you prescribing high doses of oral opioids, plan for a rapid taper and a smooth handoff to the outpatient setting.
One strategy to decrease an error in math and judgment is to use IV PCAs as infrequently as possible if a patient isn’t post-operative and they are able to take oral meds. And never hesitate to consult with your inpatient pharmacist or a chronic pain specialist.
For a low-risk chronic pain patient on low-dose opioids, don’t change the regimen, even if the indication for opioids isn’t clear.
Although it is tempting to become an opioid prohibitionist, if a patient has been taking an opioid for years and is functioning, working, compliant, and has no risk factors for complications from COT, it is likely fine to continue their current regimen. Touch base with the primary opioid prescriber, and if you’re concerned, use some of the monitoring instruments described earlier (PMP, urine drug screen, opioid treatment agreement, pill counts).
If a patient has pain all the time, they need to be on a medication that works all the time.
A good pain history followed by a good neurological and mental health assessment is of incalculable value, especially because physicians often underestimate a patient’s pain intensity and its impact on a patient’s quality of life.5,6 A patient’s pain intensity, quality of life, and function can be dramatically improved by starting a long-acting medication for “constant pain.”
If a patient hurts “24 hours a day” and cannot function on hydrocodone/acetaminophen 10/325 QID, it’s probably because they are constantly reacting to spikes in pain and using a “some of the time” medicine to treat “all the time” pain. Switching to a long-acting medication—and it doesn’t have to be an opioid—could improve control and decrease how much narcotic the patient needs.
If you choose a long-acting opioid (in this case, you could try morphine sulphate extended-release 15 mg BID and satisfy 50% of the hydrocodone need), then you could titrate slowly upwards to where the patient would not need hydrocodone. If the patient still had uncontrolled pain, then either morphine is the wrong compound for them or they are benefiting from the “nonanalgesic properties” of the opioids.
Give the patient the benefit of the doubt; because of genetic polymorphisms, a patient may need several opioid rotations before the right opioid compound is found.
Dr. Schultz is a hospitalist and assistant professor in the department of internal medicine at the University of Miami Miller School of Medicine. She is board-certified in hospice and palliative care and specializes in chronic pain management. Dr. Ajam is a hospitalist and a clinical assistant professor in the department of anesthesiology at Wake Forest University Baptist Medical Center and the Carolinas Pain Institute. He is board-certified in chronic pain management.
References
- Garrettson M, Ringwalt C. An evaluation of the North Carolina controlled substances reporting system: part II impact evaluation, January 2013. PDMP Center of Excellence website. Available at: http://pdmpexcellence.org/sites/all/pdfs/NC_control_sub_eval_pt_2.pdf. Accessed Sept. 3, 2013.
- Kung HC, Hoyert DL, Xu JQ, Murphy SL. Deaths: Final data for 2005, national vital statistics reports; Vol. 56 No. 10. Hyattsville, Md.: National Center for Health Statistics; 2008.
- Porter J, Jick H. Addiction rare in patients treated with narcotics. N Engl J Med. 1980;302(2):123.
- American Society of Addiction Medicine; personal communication, 2013.
- Mäntyselkä P, Kumpusalo E, Ahonen R, Takala J. Patients’ versus general practitioners’ assessments of pain intensity in primary care patients with non-cancer pain. Br J Gen Pract. 2001;51(473):995-997.
- Petersen MA, Larsen H, Pedersen L, Sonne N, Groenvold M. Assessing health-related quality of life in palliative care: comparing patient and physician assessments. Eur J Cancer. 2006;42(8):1159-1166.
In This Edition
9 Things: At a Glance
An occasional series providing specialty-specific advice for hospitalists from experts in the field.
- Recognize the differential diagnosis for pain exacerbation in a chronic opioid therapy (COT) patient/chronic pain patient.
- Know where the opioids are going.
- Sometimes stopping pills, rather than adding them, can cure pain.
- Take time to educate patients about methadone and its risk of mortality if not used as prescribed.
- A little local anesthetic (and some steroid) goes a long way.
- Addiction to opioids is not rare.
- Safely changing opioid regimens requires good math and good judgment.
- For a low-risk chronic pain patient on low-dose opioids, don’t change the regimen, even if the indication for opioids isn’t clear.
- If a patient has pain all the time, they need to be on a medication that works all the time.
The differential diagnosis for pain exacerbation in a chronic opioid therapy (COT) patient/chronic pain patient is:
- Worsening medical problem;
- New medical problem;
- Nonopioid problem (side effect);
- Opioid problem (resistance/tolerance/side effect); and
- Opioid-induced hyperalgesia.
The search for an etiology and treatment for chronic pain should not end, even if a patient is labeled with “chronic pain syndrome.” The patient could simply be chronically undiagnosed or on an incorrect therapy.
Know where the opioids are going.
Whether it’s auditing a prescription-monitoring program (PMP), checking a urine drug screen, or calling a pharmacist, try to ensure that chronic pain patients are taking the opioids as prescribed. A phone call to the primary opioid prescriber or chronic pain provider could save a busy hospitalist a lot of time.
Using PMP data can consume a lot of time. Typically, only prescribing providers can access PMPs, so delegating this responsibility to someone else is not possible. If your state PMP does not help, simply call the patient’s pharmacy and ask for the last three fill dates on an opioid prescription. This also works well in case the patient’s pharmacy doesn’t participate in a PMP or is delayed in uploading recent prescriber data. Many COT patients have an opioid treatment agreement with their prescriber and must use only one pharmacy to fill opioids.
In January 2013, the University of North Carolina Injury Prevention Center published an analysis of three years of North Carolina PMP data.1 Patients followed by providers who consistently used the state PMP were five times more likely to receive treatment for opioid dependence compared with patients of providers who never used the state PMP.1
Why go through all this trouble if a chronic pain specialist is also doing it? It’s good documentation and good care, like monitoring levels of transplant meds or making sure hemoglobin A1Cs are up to date and trending toward goal. It may only take one misused or diverted opioid pill to result in a serious adverse event.
Sometimes stopping pills, rather than adding them, can cure pain.
Many chronic pain patients accumulate a patchwork of pills (e.g. benzodiazepines, opioids, muscle relaxants, and antidepressants). Many interpret noxious symptoms associated with the drug burden as “uncontrolled pain.” Two conditions that might afflict the pain sufferer who takes multiple medications are opioid-induced hyperalgesia (OIH) and medication-overuse headaches (MOH). They are uncommon but should be on a hospitalist’s differential for difficult-to-control chronic pain. Opioids commonly are implicated in causing MOH, a chronic headache occurring at least 15 days a month, four hours a day if untreated, and for at least three consecutive months. OIH is a nociceptive sensitization caused by opioids that can occur suddenly or insidiously.
If a drug isn’t absolutely necessary, stop it. If you and the patient start by agreeing to the shared goal of improving health, the conversation should go better. An axiom we learned from mentors at the University of Washington is: “There is no pain that cannot be made worse with inappropriate therapy.”
Take time to educate patients about methadone and its risk of mortality if not used as prescribed.
Methadone is less frequently prescribed than other opioids, yet it is more frequently associated with death from overdose. Though there is a risk of overdose and death with any opioid, managing methadone is more difficult. A desperate chronic pain patient may self-escalate their methadone without proper insight into the consequences.
Remember the logarithmic relationship methadone doses have with their morphine equivalency. The following highlights how deceiving the numbers are: 50 mg of methadone is about 100 mg of morphine-equivalent, but 100 mg of methadone is about 1,000 mg of morphine-equivalent, or 10 times as strong.
From 1999 to 2005, methadone-related deaths increased by 468%.2 If the patient doesn’t seem to understand these risks, they are not a good candidate for methadone treatment.
A little local anesthetic (and some steroid) goes a long way.
Hospitalists should offer an assortment of diagnostic and therapeutic injections to chronic pain patients. First, be sure you’ve done your due diligence:
- What procedures do you have privileges to do?
- Do you need to be proctored first?
- How do your local specialists feel about you doing injections?
In light of these considerations, hospitalists should be able to train and be credentialed to offer such procedures as trigger-point injections, joint injections (knees, shoulders), or even a peripheral nerve injection (e.g. lateral femoral cutaneous nerve or ilioinguinal nerve injection). Some hospitalists might even want to learn ultrasound-guided sacroiliac joint injections for chronic unexplained back pain.
Offering an indicated and effective injection is a good nonopioid option. And local anesthetic injections can help hospitalists establish an elusive diagnosis. For example, many patients spend years getting worked up for head and neck pain when dry-needling with a small volume (1 cc) of local anesthetic into a neck muscle trigger point can break their pain generator, eliminating their pain.
Addiction to opioids is not rare.
The use, misuse, and diversion of opioids and all the associated complications have appropriately received considerable media attention. A seminal paper by Porter and Jick titled “Addiction Is Rare in Patients Treated with Narcotics” is one of many tipping points associated with the boom in opioid prescribing.3 Whether it’s a three-day supply of hydrocodone, 24 hours on a PCA, or an opioid rotation, any exposure to opioids can put a patient on the runway to addiction.
There are only 3,071 board-certified addiction specialists certified by the American Board of Addiction Medicine, so access to an addiction specialist might be difficult.4
Nonetheless, do not become complacent and just continue the opioid therapy in a difficult opioid-addicted patient. Express your concerns to the primary opioid prescriber, or help patients who don’t have an opioid prescriber get access and treatment. Otherwise, you have no choice but to taper the opioids.
Ideally, chronic pain management should be delivered in the outpatient arena where long-term monitoring can take place.
Safely changing opioid regimens requires good math and good judgment.
During training and practice, hospitalists become accustomed to rapidly analyze objective data, such as ABGs, ECGs, anion gaps, and vent settings. A hospitalist should become similarly efficient at calculating morphine equivalencies (cautiously with methadone and fentanyl), making dose reductions, and rotating opioids. The more comfortable you are with morphine equivalencies, the faster and safer you will be at rotating opioids. Whatever morphine-equivalence table you feel comfortable with is the one you should use consistently.
We see many providers who unwittingly take, for example, a patient who has become resistant to hydrocodone/acetaminophen 10/325 mg PO TID (30 mg of morphine) and convert them to oxycodone/acetaminophen 10/325 mg PO QID (60 mg of morphine—a doubling), when doubling could cause respiratory depression or a faster path to addiction and dependency.
But there are cases in which judgment should trump math, such as when converting from an IV to an oral regimen. We frequently see patients in the clinic requesting refills for more than 100 mg of hydromorphone because “that’s what I was on when I was hospitalized and on the pump.” If the IV-to-oral conversion leaves you prescribing high doses of oral opioids, plan for a rapid taper and a smooth handoff to the outpatient setting.
One strategy to decrease an error in math and judgment is to use IV PCAs as infrequently as possible if a patient isn’t post-operative and they are able to take oral meds. And never hesitate to consult with your inpatient pharmacist or a chronic pain specialist.
For a low-risk chronic pain patient on low-dose opioids, don’t change the regimen, even if the indication for opioids isn’t clear.
Although it is tempting to become an opioid prohibitionist, if a patient has been taking an opioid for years and is functioning, working, compliant, and has no risk factors for complications from COT, it is likely fine to continue their current regimen. Touch base with the primary opioid prescriber, and if you’re concerned, use some of the monitoring instruments described earlier (PMP, urine drug screen, opioid treatment agreement, pill counts).
If a patient has pain all the time, they need to be on a medication that works all the time.
A good pain history followed by a good neurological and mental health assessment is of incalculable value, especially because physicians often underestimate a patient’s pain intensity and its impact on a patient’s quality of life.5,6 A patient’s pain intensity, quality of life, and function can be dramatically improved by starting a long-acting medication for “constant pain.”
If a patient hurts “24 hours a day” and cannot function on hydrocodone/acetaminophen 10/325 QID, it’s probably because they are constantly reacting to spikes in pain and using a “some of the time” medicine to treat “all the time” pain. Switching to a long-acting medication—and it doesn’t have to be an opioid—could improve control and decrease how much narcotic the patient needs.
If you choose a long-acting opioid (in this case, you could try morphine sulphate extended-release 15 mg BID and satisfy 50% of the hydrocodone need), then you could titrate slowly upwards to where the patient would not need hydrocodone. If the patient still had uncontrolled pain, then either morphine is the wrong compound for them or they are benefiting from the “nonanalgesic properties” of the opioids.
Give the patient the benefit of the doubt; because of genetic polymorphisms, a patient may need several opioid rotations before the right opioid compound is found.
Dr. Schultz is a hospitalist and assistant professor in the department of internal medicine at the University of Miami Miller School of Medicine. She is board-certified in hospice and palliative care and specializes in chronic pain management. Dr. Ajam is a hospitalist and a clinical assistant professor in the department of anesthesiology at Wake Forest University Baptist Medical Center and the Carolinas Pain Institute. He is board-certified in chronic pain management.
References
- Garrettson M, Ringwalt C. An evaluation of the North Carolina controlled substances reporting system: part II impact evaluation, January 2013. PDMP Center of Excellence website. Available at: http://pdmpexcellence.org/sites/all/pdfs/NC_control_sub_eval_pt_2.pdf. Accessed Sept. 3, 2013.
- Kung HC, Hoyert DL, Xu JQ, Murphy SL. Deaths: Final data for 2005, national vital statistics reports; Vol. 56 No. 10. Hyattsville, Md.: National Center for Health Statistics; 2008.
- Porter J, Jick H. Addiction rare in patients treated with narcotics. N Engl J Med. 1980;302(2):123.
- American Society of Addiction Medicine; personal communication, 2013.
- Mäntyselkä P, Kumpusalo E, Ahonen R, Takala J. Patients’ versus general practitioners’ assessments of pain intensity in primary care patients with non-cancer pain. Br J Gen Pract. 2001;51(473):995-997.
- Petersen MA, Larsen H, Pedersen L, Sonne N, Groenvold M. Assessing health-related quality of life in palliative care: comparing patient and physician assessments. Eur J Cancer. 2006;42(8):1159-1166.