User login
Patient safety is a healthcare provider's top priority. Drug allergies are instated into an electronic medical record (EMR) to avoid potential adverse events in the future. Despite the intention to provide safety, healthcare providers frequently document antimicrobial allergies incorrectly.[1] In turn, this may lead to decreased antibiotic choices, increased healthcare costs, potential adverse reactions, and unnecessary avoidance of optimal, first‐line agents.
Several strategies have been developed to help improve the accuracy of allergy documentation, including pharmacy‐based interventions, but the persistence of corrections, once performed, is unknown.[2] Although most antibiotic allergy errors are identified upon review of prior medication history (eg, penicillin allergy listed in a patient who previously received piperacillintazobactam), no prior studies have evaluated penicillin allergy errors directly after a proven tolerance with a penicillin skin testing (PST) and penicillin confirmatory challenge.[3, 4, 5] We hereby assess factors for erroneous allergy documentation in a cohort of patients with a negative PST.
METHODS
We retrospectively reviewed charts under a protocol approved by the university and medical center institutional review board. Following a PST intervention we have previously described, penicillin was removed from the patients' EMR (Epic, Verona, WI) allergy list from March 2012 through July 2012.[6] We then invested a brief procedure note into the allergy section describing the negative PST and subsequent tolerance of a penicillin agent. During the PST intervention, there was no attempt to convey the result of the PST and corrected allergy information to the outpatient clinicians.
As a follow‐up to our previous study, we reviewed the charts of the 150 subjects who represented the entire population of patients who underwent PST in the March 2012 through July 2012 intervention time period. From August 2012 through July 2013, charts were reviewed to gauge reappearances at Vidant Health, a system of 10 hospitals in eastern North Carolina. Collected data also included demographics, drug allergy or intolerance, penicillin allergy redocumentation, residence, antimicrobial use, and presence of dementia or altered mentation.
Outpatient physician and long‐term care facility (LTCF) allergy records were obtained via EMR records, patient or family inquiry, and referring documents that accompanied the patient upon arrival. In addition to reviewing the LTCF and/or outpatient physician referring documents, the outpatient physician(s) and LTCFs were contacted and asked to review other electronic or paper records that may not have been delivered with the referring documents. Inpatient and outpatient records were reviewed for penicillin allergy, as defined by the drug allergy practice parameters.[7] Fischer exact tests were used to identify significant associated factors.
RESULTS
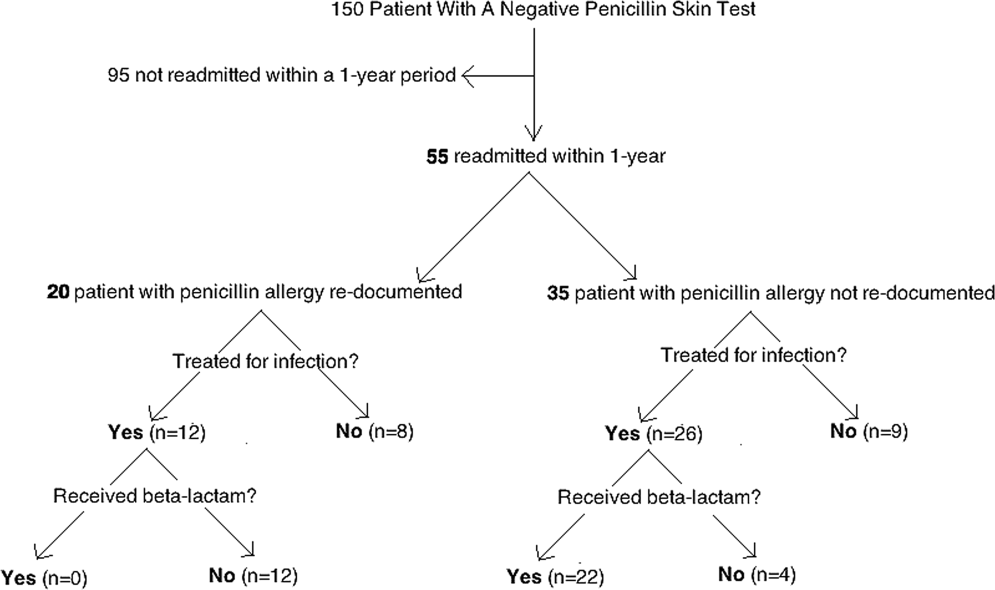
Of the 150 patients with proven penicillin tolerance, 55 (37%) revisited a Vidant Health hospital within a year period, of which 22 (40%) received a ‐lactam agent once again without adverse effects (Table 1). Twenty (36%) of the 55 patients had penicillin allergy redocumented (Figure 1). There was no description of any allergy after the PST in any of the 20 EMR, LTCF records, or outpatient primary care physician records. Factors associated with penicillin allergy redocumentation (vs those not redocumented) included age >65 years (P = 0.011), residence in a LTCF (P = 0.0001), acutely altered mentation (P < 0.0001), and dementia (P < 0.0001). Penicillin allergy was still reported in all 21 (100%) of the LTCF patient records.
| Category | Variables | Penicillin Allergy Not Reinstated, n = 35 | Penicillin Allergy Reinstated, n = 20 | P Value |
|---|---|---|---|---|
| ||||
| Age, y | 1830 | 5 (14%) | 0 (0%) | 0.011 |
| 3164 | 17 (49%) | 5 (37%) | ||
| >65 | 13 (37%) | 15 (75%) | ||
| Gender | Male | 12 (34%) | 10 (50%) | 0.19 |
| Female | 23 (66%) | 10 (50%) | ||
| Race | White | 20 (57%) | 11 (55%) | 0.36 |
| Black | 14 (40%) | 8 (40%) | ||
| Hispanic | 1 (3%) | 1 (5%) | ||
| Residence | Home | 28 (80%) | 5 (25%) | 0.0001 |
| LTCF | 7 (20%) | 15 (75%) | ||
| Acutely altered mentation | Yes | 8 (23%) | 16 (80%) | <0.0001 |
| No | 27 (77%) | 4 (20%) | ||
| Dementia | Yes | 1 (3%) | 10 (50%) | <0.0001 |
| No | 34 (97%) | 10 (50%) | ||
| Primary service | Residenta | 18 (51%) | 5 (25%) | 0.18 |
| Hospitalist | 8 (23%) | 10 (50%) | ||
| Surgery | 3 (9%) | 3 (15%) | ||
| Emergency medicine | 6 (17%) | 2 (10%) | ||
| Primary language | English | 34 (97%) | 19 (95%) | 0.59 |
| Spanish | 1 (3%) | 1 (5%) | ||
| Hospital diagnosis | Infectious | 19 (54%) | 14 (70%) | 0.20 |
| Noninfectious | 16 (46%) | 6 (30%) | ||
| Antibiotic received | ‐lactamb | 22 (63%) | 0 (0%) | 0.07 |
| Non‐lactamc | 4 (11%) | 12 (60%) | ||
| None | 9 (26%) | 8 (40%) | ||
CONCLUSION
Errors in medication documentation are a major cause of potential harm and death.[8] In the United States, up to 14% of patient harm is due to a preventable medication error, a rate that exceeds death related to breast cancer, vehicular accidents, and AIDS.[9, 10] Inaccurate drug allergy reporting can result in a cascade of consequential medical errors, including medication prescribing (eg, use of less effective, potentially more toxic and/or more expensive agents), and diagnostic errors (eg, repeat PST, unnecessary medication desensitization).
Although EMR systems are designed to improve allergy documentation, they may also increase the risk of inaccurate or out‐of‐date data. Providers may be reluctant to permanently alter the electronic record by removing an allergy from the EMR. Chart lore, the persistence of inaccurate or outdated information, may contribute to error, particularly when the patient is unable to provide information directly. We found, for example, that dementia and acutely altered mentation were associated with allergy reporting errors, likely related to the inability of the patient to give a reliable history. Finally, the EMR does not typically include a function for noting that an allergy does not exist, making it easier to reinstate incorrect allergies. To address this problem, we subsequently began listing a negative PST as an other allergy in the EMR allergy section to improve visibility.
We also found that residence in an LTCF was associated with allergy reporting error, in part perhaps because all LTCF records still included penicillin as an allergy. This finding highlights the need for direct communication of a proven PST tolerance with the primary care physician or LTCF provider, which was not part of our initial intervention. Previous studies have described the benefit of removing incorrectly reported allergies from community pharmacy records as well.[2, 11] Simply recording it into a transfer summary may not suffice, as LTCF providers may not read, or misread, the PST result. Healthcare providers performing PST should attempt to maintain consistent inpatient and outpatient drug allergy reports to avoid drug allergies.
Another possible modality to reduce inaccurate drug allergy documentation is repetitive review of the allergy list. In the Epic EMR system, the allergy list will illustrate when the healthcare provider(s) reviewed the patients' allergies last. At Vidant Health, the allergy list is generally only reviewed during nursing triage in the emergency department. Healthcare providers should avoid chart lore or relying on nursing notes and routinely review allergies directly with the patient. Obtaining allergy information only during routine nursing triage assessment is substandard.[12] This should not substitute acquisition of allergy information from the patient using a structured, direct interview. Supervision and repeated EMR review may help to avoid overlooking an inaccurate history acquisition.[13] This may help not only help to remove drug allergies that were erroneously added to the patient's list, but also to possibly add agents that may have been missed by the triaging team.
Another means by which inaccurate redocumentation of drug allergies can be avoided is avoidance of placing nonallergic drug reactions in the allergy section of the EMR. Antimicrobial agents are often added to the allergy list because of a drug intolerance (eg, gastrointestinal symptoms), and/or pharmacologic effect (eg, electrolyte abnormality). Although these are not true reactions, healthcare providers often avoid rechallenging these agents. These adverse reactions should be placed within the problem list or past medical history section of the EMR, and not within the allergy section. Therefore, healthcare providers should accurately describe the behavior of the allergic reaction(s).[14]
A limitation of our study is our small sample size and single‐site design. This may have limited the ability to analyze the data in a multivariable way and the ability to learn about risk factors across a variety of EMR and workflow settings. Furthermore, we reviewed only the 55 patients who were readmitted, and therefore do not know how accurate records were for the other 95 patients.
In summary, this work highlights the challenges of successful implementation of quality improvement projects in an electronic health record‐based world. Although PST can expand antimicrobial choices and reduce healthcare costs, the benefits may be limited by inadequately removing the allergy from the hospital and outpatient record(s). From the novel data gathered from our study, primary care physicians and LTCFs are now promptly notified of a negative PST to reduce these medical errors, and we believe this process should become a standard of care.
Acknowledgments
The authors thank Dr. Muhammad S. Ashraf for his assistance in preparing this manuscript.
Disclosures: Ramzy H. Rimawi, MD, has a potential conflict of interest with Alk‐Abello (speakers' bureau), the manufacturer of the Pre‐PEN penicillin skin test. Alk‐Abello was not involved in the production of this article. Paul P. Cook, MD has potential conflicts of interest with Gilead (investigator), Pfizer (investigator), Merck (investigator and speakers' bureau) and Forest (speakers' bureau), none of which relate to the use of penicillin or penicillin skin tests. None of the authors have received any source(s) of funding for this article. The corresponding author, Ramzy Rimawi, MD, had full access to all of the data in the study and had final responsibility for the decision to submit for publication. The manuscript is not under review by any other publication.
- , . Accuracy of drug allergy documentation. Am J Health Syst Pharm. 1997;54(14):1627–1629.
- , , , et al. Program to remove incorrect allergy documentation in pediatrics medical records. Am J Health Syst Pharm. 2001;58(18):1722–1727.
- , , . Electronic medication ordering with integrated drug database and clinical decision support system. Stud Health Technol Inform. 2012;180:693–697.
- , , , . Pharmacy‐controlled documentation of drug allergies. Am J Health‐Syst Pharm. 1991;48:260–264.
- , , , et al. Systems analysis of adverse drug events. JAMA. 1995;274:35–43.
- , , , et al. The impact of penicillin skin testing on clinical practice and antimicrobial stewardship. J Hosp Med. 2013;8(6):341–345.
- , , , et al.Joint Task Force on Practice Parameters; American College of Allergy, Asthma and Immunology;Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259–273.
- , , . Prescription quality in an acute medical ward. Pharmacoepidemiol Drug Saf. 2009;18(12):1158–1165.
- . Make no mistake! Medical errors can be deadly serious. FDA Consum. 2000;34(5):13–18.
- . Medication errors. J R Coll Physicians Edinb. 2007;37:343–346.
- , , , et al. A pharmacist‐led information technology intervention for medication errors (PINCER): a multicenter, cluster randomized, controlled trial and cost‐effectiveness analysis. Lancet. 2012;379(9823):1301–1309.
- , , , . Getting the data right: information accuracy in pediatric emergency medicine. Qual Saf Health Care. 2006;15(4):296–301.
- , . Antibiotic allergy: inaccurate history taking in a teaching hospital. South Med J. 1994;87(8):805–807.
- , , . Drug allergy documentation—time for a change? Int J Clin Pharm. 2011;33(4):610–613.
Patient safety is a healthcare provider's top priority. Drug allergies are instated into an electronic medical record (EMR) to avoid potential adverse events in the future. Despite the intention to provide safety, healthcare providers frequently document antimicrobial allergies incorrectly.[1] In turn, this may lead to decreased antibiotic choices, increased healthcare costs, potential adverse reactions, and unnecessary avoidance of optimal, first‐line agents.
Several strategies have been developed to help improve the accuracy of allergy documentation, including pharmacy‐based interventions, but the persistence of corrections, once performed, is unknown.[2] Although most antibiotic allergy errors are identified upon review of prior medication history (eg, penicillin allergy listed in a patient who previously received piperacillintazobactam), no prior studies have evaluated penicillin allergy errors directly after a proven tolerance with a penicillin skin testing (PST) and penicillin confirmatory challenge.[3, 4, 5] We hereby assess factors for erroneous allergy documentation in a cohort of patients with a negative PST.
METHODS
We retrospectively reviewed charts under a protocol approved by the university and medical center institutional review board. Following a PST intervention we have previously described, penicillin was removed from the patients' EMR (Epic, Verona, WI) allergy list from March 2012 through July 2012.[6] We then invested a brief procedure note into the allergy section describing the negative PST and subsequent tolerance of a penicillin agent. During the PST intervention, there was no attempt to convey the result of the PST and corrected allergy information to the outpatient clinicians.
As a follow‐up to our previous study, we reviewed the charts of the 150 subjects who represented the entire population of patients who underwent PST in the March 2012 through July 2012 intervention time period. From August 2012 through July 2013, charts were reviewed to gauge reappearances at Vidant Health, a system of 10 hospitals in eastern North Carolina. Collected data also included demographics, drug allergy or intolerance, penicillin allergy redocumentation, residence, antimicrobial use, and presence of dementia or altered mentation.
Outpatient physician and long‐term care facility (LTCF) allergy records were obtained via EMR records, patient or family inquiry, and referring documents that accompanied the patient upon arrival. In addition to reviewing the LTCF and/or outpatient physician referring documents, the outpatient physician(s) and LTCFs were contacted and asked to review other electronic or paper records that may not have been delivered with the referring documents. Inpatient and outpatient records were reviewed for penicillin allergy, as defined by the drug allergy practice parameters.[7] Fischer exact tests were used to identify significant associated factors.
RESULTS
Of the 150 patients with proven penicillin tolerance, 55 (37%) revisited a Vidant Health hospital within a year period, of which 22 (40%) received a ‐lactam agent once again without adverse effects (Table 1). Twenty (36%) of the 55 patients had penicillin allergy redocumented (Figure 1). There was no description of any allergy after the PST in any of the 20 EMR, LTCF records, or outpatient primary care physician records. Factors associated with penicillin allergy redocumentation (vs those not redocumented) included age >65 years (P = 0.011), residence in a LTCF (P = 0.0001), acutely altered mentation (P < 0.0001), and dementia (P < 0.0001). Penicillin allergy was still reported in all 21 (100%) of the LTCF patient records.

| Category | Variables | Penicillin Allergy Not Reinstated, n = 35 | Penicillin Allergy Reinstated, n = 20 | P Value |
|---|---|---|---|---|
| ||||
| Age, y | 1830 | 5 (14%) | 0 (0%) | 0.011 |
| 3164 | 17 (49%) | 5 (37%) | ||
| >65 | 13 (37%) | 15 (75%) | ||
| Gender | Male | 12 (34%) | 10 (50%) | 0.19 |
| Female | 23 (66%) | 10 (50%) | ||
| Race | White | 20 (57%) | 11 (55%) | 0.36 |
| Black | 14 (40%) | 8 (40%) | ||
| Hispanic | 1 (3%) | 1 (5%) | ||
| Residence | Home | 28 (80%) | 5 (25%) | 0.0001 |
| LTCF | 7 (20%) | 15 (75%) | ||
| Acutely altered mentation | Yes | 8 (23%) | 16 (80%) | <0.0001 |
| No | 27 (77%) | 4 (20%) | ||
| Dementia | Yes | 1 (3%) | 10 (50%) | <0.0001 |
| No | 34 (97%) | 10 (50%) | ||
| Primary service | Residenta | 18 (51%) | 5 (25%) | 0.18 |
| Hospitalist | 8 (23%) | 10 (50%) | ||
| Surgery | 3 (9%) | 3 (15%) | ||
| Emergency medicine | 6 (17%) | 2 (10%) | ||
| Primary language | English | 34 (97%) | 19 (95%) | 0.59 |
| Spanish | 1 (3%) | 1 (5%) | ||
| Hospital diagnosis | Infectious | 19 (54%) | 14 (70%) | 0.20 |
| Noninfectious | 16 (46%) | 6 (30%) | ||
| Antibiotic received | ‐lactamb | 22 (63%) | 0 (0%) | 0.07 |
| Non‐lactamc | 4 (11%) | 12 (60%) | ||
| None | 9 (26%) | 8 (40%) | ||
CONCLUSION
Errors in medication documentation are a major cause of potential harm and death.[8] In the United States, up to 14% of patient harm is due to a preventable medication error, a rate that exceeds death related to breast cancer, vehicular accidents, and AIDS.[9, 10] Inaccurate drug allergy reporting can result in a cascade of consequential medical errors, including medication prescribing (eg, use of less effective, potentially more toxic and/or more expensive agents), and diagnostic errors (eg, repeat PST, unnecessary medication desensitization).
Although EMR systems are designed to improve allergy documentation, they may also increase the risk of inaccurate or out‐of‐date data. Providers may be reluctant to permanently alter the electronic record by removing an allergy from the EMR. Chart lore, the persistence of inaccurate or outdated information, may contribute to error, particularly when the patient is unable to provide information directly. We found, for example, that dementia and acutely altered mentation were associated with allergy reporting errors, likely related to the inability of the patient to give a reliable history. Finally, the EMR does not typically include a function for noting that an allergy does not exist, making it easier to reinstate incorrect allergies. To address this problem, we subsequently began listing a negative PST as an other allergy in the EMR allergy section to improve visibility.
We also found that residence in an LTCF was associated with allergy reporting error, in part perhaps because all LTCF records still included penicillin as an allergy. This finding highlights the need for direct communication of a proven PST tolerance with the primary care physician or LTCF provider, which was not part of our initial intervention. Previous studies have described the benefit of removing incorrectly reported allergies from community pharmacy records as well.[2, 11] Simply recording it into a transfer summary may not suffice, as LTCF providers may not read, or misread, the PST result. Healthcare providers performing PST should attempt to maintain consistent inpatient and outpatient drug allergy reports to avoid drug allergies.
Another possible modality to reduce inaccurate drug allergy documentation is repetitive review of the allergy list. In the Epic EMR system, the allergy list will illustrate when the healthcare provider(s) reviewed the patients' allergies last. At Vidant Health, the allergy list is generally only reviewed during nursing triage in the emergency department. Healthcare providers should avoid chart lore or relying on nursing notes and routinely review allergies directly with the patient. Obtaining allergy information only during routine nursing triage assessment is substandard.[12] This should not substitute acquisition of allergy information from the patient using a structured, direct interview. Supervision and repeated EMR review may help to avoid overlooking an inaccurate history acquisition.[13] This may help not only help to remove drug allergies that were erroneously added to the patient's list, but also to possibly add agents that may have been missed by the triaging team.
Another means by which inaccurate redocumentation of drug allergies can be avoided is avoidance of placing nonallergic drug reactions in the allergy section of the EMR. Antimicrobial agents are often added to the allergy list because of a drug intolerance (eg, gastrointestinal symptoms), and/or pharmacologic effect (eg, electrolyte abnormality). Although these are not true reactions, healthcare providers often avoid rechallenging these agents. These adverse reactions should be placed within the problem list or past medical history section of the EMR, and not within the allergy section. Therefore, healthcare providers should accurately describe the behavior of the allergic reaction(s).[14]
A limitation of our study is our small sample size and single‐site design. This may have limited the ability to analyze the data in a multivariable way and the ability to learn about risk factors across a variety of EMR and workflow settings. Furthermore, we reviewed only the 55 patients who were readmitted, and therefore do not know how accurate records were for the other 95 patients.
In summary, this work highlights the challenges of successful implementation of quality improvement projects in an electronic health record‐based world. Although PST can expand antimicrobial choices and reduce healthcare costs, the benefits may be limited by inadequately removing the allergy from the hospital and outpatient record(s). From the novel data gathered from our study, primary care physicians and LTCFs are now promptly notified of a negative PST to reduce these medical errors, and we believe this process should become a standard of care.
Acknowledgments
The authors thank Dr. Muhammad S. Ashraf for his assistance in preparing this manuscript.
Disclosures: Ramzy H. Rimawi, MD, has a potential conflict of interest with Alk‐Abello (speakers' bureau), the manufacturer of the Pre‐PEN penicillin skin test. Alk‐Abello was not involved in the production of this article. Paul P. Cook, MD has potential conflicts of interest with Gilead (investigator), Pfizer (investigator), Merck (investigator and speakers' bureau) and Forest (speakers' bureau), none of which relate to the use of penicillin or penicillin skin tests. None of the authors have received any source(s) of funding for this article. The corresponding author, Ramzy Rimawi, MD, had full access to all of the data in the study and had final responsibility for the decision to submit for publication. The manuscript is not under review by any other publication.
Patient safety is a healthcare provider's top priority. Drug allergies are instated into an electronic medical record (EMR) to avoid potential adverse events in the future. Despite the intention to provide safety, healthcare providers frequently document antimicrobial allergies incorrectly.[1] In turn, this may lead to decreased antibiotic choices, increased healthcare costs, potential adverse reactions, and unnecessary avoidance of optimal, first‐line agents.
Several strategies have been developed to help improve the accuracy of allergy documentation, including pharmacy‐based interventions, but the persistence of corrections, once performed, is unknown.[2] Although most antibiotic allergy errors are identified upon review of prior medication history (eg, penicillin allergy listed in a patient who previously received piperacillintazobactam), no prior studies have evaluated penicillin allergy errors directly after a proven tolerance with a penicillin skin testing (PST) and penicillin confirmatory challenge.[3, 4, 5] We hereby assess factors for erroneous allergy documentation in a cohort of patients with a negative PST.
METHODS
We retrospectively reviewed charts under a protocol approved by the university and medical center institutional review board. Following a PST intervention we have previously described, penicillin was removed from the patients' EMR (Epic, Verona, WI) allergy list from March 2012 through July 2012.[6] We then invested a brief procedure note into the allergy section describing the negative PST and subsequent tolerance of a penicillin agent. During the PST intervention, there was no attempt to convey the result of the PST and corrected allergy information to the outpatient clinicians.
As a follow‐up to our previous study, we reviewed the charts of the 150 subjects who represented the entire population of patients who underwent PST in the March 2012 through July 2012 intervention time period. From August 2012 through July 2013, charts were reviewed to gauge reappearances at Vidant Health, a system of 10 hospitals in eastern North Carolina. Collected data also included demographics, drug allergy or intolerance, penicillin allergy redocumentation, residence, antimicrobial use, and presence of dementia or altered mentation.
Outpatient physician and long‐term care facility (LTCF) allergy records were obtained via EMR records, patient or family inquiry, and referring documents that accompanied the patient upon arrival. In addition to reviewing the LTCF and/or outpatient physician referring documents, the outpatient physician(s) and LTCFs were contacted and asked to review other electronic or paper records that may not have been delivered with the referring documents. Inpatient and outpatient records were reviewed for penicillin allergy, as defined by the drug allergy practice parameters.[7] Fischer exact tests were used to identify significant associated factors.
RESULTS
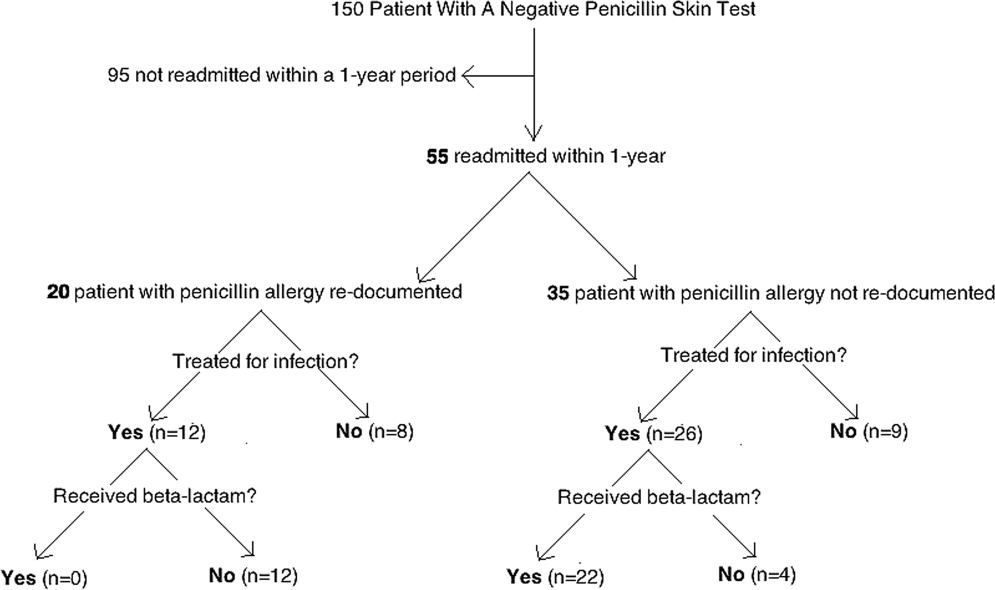
Of the 150 patients with proven penicillin tolerance, 55 (37%) revisited a Vidant Health hospital within a year period, of which 22 (40%) received a ‐lactam agent once again without adverse effects (Table 1). Twenty (36%) of the 55 patients had penicillin allergy redocumented (Figure 1). There was no description of any allergy after the PST in any of the 20 EMR, LTCF records, or outpatient primary care physician records. Factors associated with penicillin allergy redocumentation (vs those not redocumented) included age >65 years (P = 0.011), residence in a LTCF (P = 0.0001), acutely altered mentation (P < 0.0001), and dementia (P < 0.0001). Penicillin allergy was still reported in all 21 (100%) of the LTCF patient records.

| Category | Variables | Penicillin Allergy Not Reinstated, n = 35 | Penicillin Allergy Reinstated, n = 20 | P Value |
|---|---|---|---|---|
| ||||
| Age, y | 1830 | 5 (14%) | 0 (0%) | 0.011 |
| 3164 | 17 (49%) | 5 (37%) | ||
| >65 | 13 (37%) | 15 (75%) | ||
| Gender | Male | 12 (34%) | 10 (50%) | 0.19 |
| Female | 23 (66%) | 10 (50%) | ||
| Race | White | 20 (57%) | 11 (55%) | 0.36 |
| Black | 14 (40%) | 8 (40%) | ||
| Hispanic | 1 (3%) | 1 (5%) | ||
| Residence | Home | 28 (80%) | 5 (25%) | 0.0001 |
| LTCF | 7 (20%) | 15 (75%) | ||
| Acutely altered mentation | Yes | 8 (23%) | 16 (80%) | <0.0001 |
| No | 27 (77%) | 4 (20%) | ||
| Dementia | Yes | 1 (3%) | 10 (50%) | <0.0001 |
| No | 34 (97%) | 10 (50%) | ||
| Primary service | Residenta | 18 (51%) | 5 (25%) | 0.18 |
| Hospitalist | 8 (23%) | 10 (50%) | ||
| Surgery | 3 (9%) | 3 (15%) | ||
| Emergency medicine | 6 (17%) | 2 (10%) | ||
| Primary language | English | 34 (97%) | 19 (95%) | 0.59 |
| Spanish | 1 (3%) | 1 (5%) | ||
| Hospital diagnosis | Infectious | 19 (54%) | 14 (70%) | 0.20 |
| Noninfectious | 16 (46%) | 6 (30%) | ||
| Antibiotic received | ‐lactamb | 22 (63%) | 0 (0%) | 0.07 |
| Non‐lactamc | 4 (11%) | 12 (60%) | ||
| None | 9 (26%) | 8 (40%) | ||
CONCLUSION
Errors in medication documentation are a major cause of potential harm and death.[8] In the United States, up to 14% of patient harm is due to a preventable medication error, a rate that exceeds death related to breast cancer, vehicular accidents, and AIDS.[9, 10] Inaccurate drug allergy reporting can result in a cascade of consequential medical errors, including medication prescribing (eg, use of less effective, potentially more toxic and/or more expensive agents), and diagnostic errors (eg, repeat PST, unnecessary medication desensitization).
Although EMR systems are designed to improve allergy documentation, they may also increase the risk of inaccurate or out‐of‐date data. Providers may be reluctant to permanently alter the electronic record by removing an allergy from the EMR. Chart lore, the persistence of inaccurate or outdated information, may contribute to error, particularly when the patient is unable to provide information directly. We found, for example, that dementia and acutely altered mentation were associated with allergy reporting errors, likely related to the inability of the patient to give a reliable history. Finally, the EMR does not typically include a function for noting that an allergy does not exist, making it easier to reinstate incorrect allergies. To address this problem, we subsequently began listing a negative PST as an other allergy in the EMR allergy section to improve visibility.
We also found that residence in an LTCF was associated with allergy reporting error, in part perhaps because all LTCF records still included penicillin as an allergy. This finding highlights the need for direct communication of a proven PST tolerance with the primary care physician or LTCF provider, which was not part of our initial intervention. Previous studies have described the benefit of removing incorrectly reported allergies from community pharmacy records as well.[2, 11] Simply recording it into a transfer summary may not suffice, as LTCF providers may not read, or misread, the PST result. Healthcare providers performing PST should attempt to maintain consistent inpatient and outpatient drug allergy reports to avoid drug allergies.
Another possible modality to reduce inaccurate drug allergy documentation is repetitive review of the allergy list. In the Epic EMR system, the allergy list will illustrate when the healthcare provider(s) reviewed the patients' allergies last. At Vidant Health, the allergy list is generally only reviewed during nursing triage in the emergency department. Healthcare providers should avoid chart lore or relying on nursing notes and routinely review allergies directly with the patient. Obtaining allergy information only during routine nursing triage assessment is substandard.[12] This should not substitute acquisition of allergy information from the patient using a structured, direct interview. Supervision and repeated EMR review may help to avoid overlooking an inaccurate history acquisition.[13] This may help not only help to remove drug allergies that were erroneously added to the patient's list, but also to possibly add agents that may have been missed by the triaging team.
Another means by which inaccurate redocumentation of drug allergies can be avoided is avoidance of placing nonallergic drug reactions in the allergy section of the EMR. Antimicrobial agents are often added to the allergy list because of a drug intolerance (eg, gastrointestinal symptoms), and/or pharmacologic effect (eg, electrolyte abnormality). Although these are not true reactions, healthcare providers often avoid rechallenging these agents. These adverse reactions should be placed within the problem list or past medical history section of the EMR, and not within the allergy section. Therefore, healthcare providers should accurately describe the behavior of the allergic reaction(s).[14]
A limitation of our study is our small sample size and single‐site design. This may have limited the ability to analyze the data in a multivariable way and the ability to learn about risk factors across a variety of EMR and workflow settings. Furthermore, we reviewed only the 55 patients who were readmitted, and therefore do not know how accurate records were for the other 95 patients.
In summary, this work highlights the challenges of successful implementation of quality improvement projects in an electronic health record‐based world. Although PST can expand antimicrobial choices and reduce healthcare costs, the benefits may be limited by inadequately removing the allergy from the hospital and outpatient record(s). From the novel data gathered from our study, primary care physicians and LTCFs are now promptly notified of a negative PST to reduce these medical errors, and we believe this process should become a standard of care.
Acknowledgments
The authors thank Dr. Muhammad S. Ashraf for his assistance in preparing this manuscript.
Disclosures: Ramzy H. Rimawi, MD, has a potential conflict of interest with Alk‐Abello (speakers' bureau), the manufacturer of the Pre‐PEN penicillin skin test. Alk‐Abello was not involved in the production of this article. Paul P. Cook, MD has potential conflicts of interest with Gilead (investigator), Pfizer (investigator), Merck (investigator and speakers' bureau) and Forest (speakers' bureau), none of which relate to the use of penicillin or penicillin skin tests. None of the authors have received any source(s) of funding for this article. The corresponding author, Ramzy Rimawi, MD, had full access to all of the data in the study and had final responsibility for the decision to submit for publication. The manuscript is not under review by any other publication.
- , . Accuracy of drug allergy documentation. Am J Health Syst Pharm. 1997;54(14):1627–1629.
- , , , et al. Program to remove incorrect allergy documentation in pediatrics medical records. Am J Health Syst Pharm. 2001;58(18):1722–1727.
- , , . Electronic medication ordering with integrated drug database and clinical decision support system. Stud Health Technol Inform. 2012;180:693–697.
- , , , . Pharmacy‐controlled documentation of drug allergies. Am J Health‐Syst Pharm. 1991;48:260–264.
- , , , et al. Systems analysis of adverse drug events. JAMA. 1995;274:35–43.
- , , , et al. The impact of penicillin skin testing on clinical practice and antimicrobial stewardship. J Hosp Med. 2013;8(6):341–345.
- , , , et al.Joint Task Force on Practice Parameters; American College of Allergy, Asthma and Immunology;Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259–273.
- , , . Prescription quality in an acute medical ward. Pharmacoepidemiol Drug Saf. 2009;18(12):1158–1165.
- . Make no mistake! Medical errors can be deadly serious. FDA Consum. 2000;34(5):13–18.
- . Medication errors. J R Coll Physicians Edinb. 2007;37:343–346.
- , , , et al. A pharmacist‐led information technology intervention for medication errors (PINCER): a multicenter, cluster randomized, controlled trial and cost‐effectiveness analysis. Lancet. 2012;379(9823):1301–1309.
- , , , . Getting the data right: information accuracy in pediatric emergency medicine. Qual Saf Health Care. 2006;15(4):296–301.
- , . Antibiotic allergy: inaccurate history taking in a teaching hospital. South Med J. 1994;87(8):805–807.
- , , . Drug allergy documentation—time for a change? Int J Clin Pharm. 2011;33(4):610–613.
- , . Accuracy of drug allergy documentation. Am J Health Syst Pharm. 1997;54(14):1627–1629.
- , , , et al. Program to remove incorrect allergy documentation in pediatrics medical records. Am J Health Syst Pharm. 2001;58(18):1722–1727.
- , , . Electronic medication ordering with integrated drug database and clinical decision support system. Stud Health Technol Inform. 2012;180:693–697.
- , , , . Pharmacy‐controlled documentation of drug allergies. Am J Health‐Syst Pharm. 1991;48:260–264.
- , , , et al. Systems analysis of adverse drug events. JAMA. 1995;274:35–43.
- , , , et al. The impact of penicillin skin testing on clinical practice and antimicrobial stewardship. J Hosp Med. 2013;8(6):341–345.
- , , , et al.Joint Task Force on Practice Parameters; American College of Allergy, Asthma and Immunology;Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259–273.
- , , . Prescription quality in an acute medical ward. Pharmacoepidemiol Drug Saf. 2009;18(12):1158–1165.
- . Make no mistake! Medical errors can be deadly serious. FDA Consum. 2000;34(5):13–18.
- . Medication errors. J R Coll Physicians Edinb. 2007;37:343–346.
- , , , et al. A pharmacist‐led information technology intervention for medication errors (PINCER): a multicenter, cluster randomized, controlled trial and cost‐effectiveness analysis. Lancet. 2012;379(9823):1301–1309.
- , , , . Getting the data right: information accuracy in pediatric emergency medicine. Qual Saf Health Care. 2006;15(4):296–301.
- , . Antibiotic allergy: inaccurate history taking in a teaching hospital. South Med J. 1994;87(8):805–807.
- , , . Drug allergy documentation—time for a change? Int J Clin Pharm. 2011;33(4):610–613.
© 2013 Society of Hospital Medicine