User login
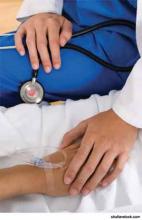
Palliative Care Can Be Incredibly Intense, Richly Rewarding for Hospitalists
After nine years in practice as a hospitalist in community and academic settings, Leonard Noronha, MD, applied for and in July 2012 became the inaugural, full-year, full-time fellow in hospice and palliative medicine (HPM) at the University of New Mexico in Albuquerque, one of approximately 200 such positions nationwide. The fellowship training qualifies him to sit for HPM subspecialty medical board certification.
Dr. Noronha says he was casually acquainted with the concept of palliative care from residency but didn’t know “when to ask for a palliative care consultation or what they offered.”
“I also had a sense that discussions about feeding tubes, for example, could happen better and easier than they typically did,” he says.
His interest piqued as he learned more about palliative care at hospitalist meetings.
“I grew more excited about it and came to realize that it is something I’d find rewarding and enjoyable, if I could get good at it,” Dr. Noronha says. “Over time, I found more satisfaction in palliative care encounters with patients—and became less comfortable with what I perceived as occasionally inappropriate and excessive testing and treatment [for some hospitalized patients who weren’t offered palliative care].”
Palliative care is a medical specialty that focuses on comfort, relief of symptoms, and clarifying patients’ treatment goals. It is commonly provided as an interdisciplinary consultation service in hospitals. Advocates say it can be offered concurrently with other medical therapies for any seriously ill patient, particularly when there are physical, psychosocial, or spiritual complications, and it is not limited to patients approaching death.
Experienced clinicians say palliative care maximizes quality of life and empowers patients and their families to make treatment decisions more in line with their hopes and values. They also say palliative care gives an emotional lift to providers, while reducing hospital expenditures. Some also suggest that palliative care is an additional tool for enhancing care transitions, potentially affecting readmission rates.
For Dr. Noronha, the one-year fellowship required a significant cut in pay, but he was prepared for the financial hardship.
“It was a great decision for me,” he says. “Some of my colleagues had encouraged me to think about using the experiential pathway to HPM board certification, but I knew I’d do better in the structured environment of a fellowship.
“There have been times when I’ve been outside of my comfort zone, sometimes feeling like the least experienced person in the room. But I knew the fellowship would help—and it did.”
He says the training gives him a better appreciation for things like illness trajectories, the nuances of goal clarification, and the benefit of an extra set of eyes and ears to assess the patient.
After completing his fellowship, Dr. Noronha became UNM’s second full-time palliative medicine faculty. He encourages hospitalists to talk to the palliative care service at their institutions and request consultations for complex, seriously ill patients who might benefit.
As for his new career path, he says that often he is asked if palliative care is depressing. “Some of these situations can be tragic, but I find the work very rewarding,” he says.
Service Models
In some settings, palliative care is incorporated into the hospitalist service. Hospitalists are scheduled for palliative care shifts or have palliative care visits incorporated into daily rounds. Such blended positions could be a recruiting incentive for some physicians who want to do both.
In other settings, palliative care is a separate service. Consultations are ordered as needed by hospitalists and other physicians.
Advocates like Marianne Novelli, MD, FHM, FACP, say hospitalists play a pivotal role in providing the basics of palliative care for seriously ill, hospitalized patients.
“Palliative care is part and parcel of what we do as hospitalists with the people we serve—who by definition are very sick, even to get into the hospital,” says Dr. Novelli, formerly the chief of the division of hospital medicine at Kaiser Permanente in Denver, Colo. She rotated off that leadership position in 2011 and has since divided her time between hospital medicine and palliative care shifts in the hospital, although she now does palliative care exclusively.
Initially, she watched palliative care consults and asked for mentorship from the palliative care team. Although it took time to get used to the advisory role of the consultant, and to working with a team, she eventually became board certified in HPM.
“Palliative care is incredibly intense but richly rewarding work,” she says. “The patients you see are never simple. It allows us to practice the type of medicine we originally set out to do, with people at the most vulnerable times in their lives.”
Workforce, Fellowship, Board Certification
In October 2012, 3,356 physicians passed the hospice and palliative medicine subspecialty board exams offered by the American Board of Medical Specialties and 10 of its constituent specialty boards, with the lion’s share of them certified by the American Board of Internal Medicine. That more than doubled the number of physicians earning the HPM credential since its inception in 2008.
Even with the surge in palliative care training, workforce studies suggest the U.S. is woefully short of credentialed palliative care physicians. And many think hospitalists can help fill that void.
The Center to Advance Palliative Care (CAPC, www.capc.org) counts 1,400 hospital-based palliative care programs in the U.S., while the Centers for Medicare & Medicaid Services (CMS) recognizes about 3,500 Medicare-certified hospice programs. A 2010 estimate by Dale Lupu and the American Academy of Hospice and Palliative Medicine (AAHPM), however, suggested a need for between 4,487 and 10,810 palliative care physician FTEs just to staff existing programs at appropriate levels—without considering growth for the field or its spread into outpatient settings.1
In the past, mid-career physicians had an experiential pathway to the HPM board exam, based on hours worked with a hospice or palliative care team, but physicians now must complete an HPM fellowship of at least one year in order to sit for the boards. And, according to AAHPM, only 234 HPM fellowship positions are offered nationwide by 85 approved fellowship programs.
A one-year fellowship is a big commitment for an established hospitalist, according to Stephen Bekanich, MD, co-director of Seton Palliative Care at Seton Healthcare, an 11-hospital system in Austin, Texas. A former hospitalist, Dr. Bekanich says that in his region a fellow stipend is about $70,000, whereas typical hospitalist compensation is in the mid- to upper-$200,000s.
AAHPM is exploring other approaches to expanding the workforce with mid-career physicians. One approach, authored by Timothy Quill, MD, and Amy Abernethy, MD, the past and current AAHPM board presidents, is to develop a two-tiered system in which palliative medicine specialists teach basic palliative care techniques and approaches to primary care physicians, hospitalists, and such specialists as oncologists.2 The article also suggested equipping clinicians with the tools to recognize when more specialized help is needed.
“As in any medical discipline, some core elements of palliative care, such as aligning treatment with a patient’s goals and basic symptom management, should be routine aspects of care delivered by any practitioner,” Drs. Quill and Abernethy wrote. “Other skills are more complex and take years of training to learn and apply, such as negotiating a difficult family meeting, addressing veiled existential distress, and managing refractory symptoms.”
Dr. Bekanich is trying the two-tiered approach at Seton Healthcare. At facilities with no palliative care service, he is transplanting palliative-trained nurse practitioners in hospital medicine groups.
“This model is locked into our budget for fiscal year 2014,” Dr. Bekanich says. “We’ll train folks, starting with hospitalists and primary care physicians.”
The training will start with a pair of three-hour sessions on palliative care techniques for hospitalists and PCPs, followed by homework assignments. “Then we’ll meet again in three months to do some role plays,” he says.
Two final rounds of training will focus on skills, philosophy, values, and practice.

—Marianne Novelli, MD, FHM, FACP, former chief of the division of hospital medicine, Kaiser Permanente, Denver, Colo.
On-the-Job Training
David Weissman, MD, FACP, a palliative care specialist in Milwaukee, Wis., and consultant to the CAPC, recommends hospitalists do what they can to improve their knowledge and skills. “There are a lot of opportunities for palliative care training out there,” he says.
HM conferences often include palliative care content. AAHPM and CAPC offer annual conferences that immerse participants in content, with opportunities to mingle with palliative care colleagues. AAHPM also offers specific content through its “Unipac” series of nine self-study training modules (www.aahpm.org/resources/default/unipac-4th-edition.html.)
On the job, Dr. Weissman says hospitalists should ask for consults for patients with complex needs. Also pay attention to how the service works and what it recommends. Taking a couple of days to round with the palliative care service could be very educational. It may be possible to take a part-time position with the team, providing weekend or vacation coverage. Hospitalists can participate on planning or advisory committees for palliative care in their hospitals or on quality improvement projects.
“If there isn’t a palliative care service, advocate for developing one,” he says.
Local hospice programs, especially those with inpatient hospice facilities that need daily physician coverage, might have part-time staff positions, which could be a great moonlighting opportunity for hospitalists and a way to learn a lot very quickly.
“I can tell the difference between physicians who have spent time working in a hospice, where you can learn about caring for people at the end of life because most of the patients are so sick, and those who have not,” says Porter Storey, MD, FACP, AAHPM’s executive vice president and a practicing palliative care physician in Colorado. “You can learn how to use the medications to get someone comfortable quickly and how to talk to families in crisis. It can be some of the most rewarding work you can possibly do—especially when you have the time and training to do it well for some of the most challenging of patients and families.”
Dr. Storey recommends that hospitalists join AAHPM, use its professional materials, attend its annual meetings, and, if they feel a calling, consider fellowship training as the next big step.
“Palliative care programs are growing in number and size but are chronically understaffed,” says Steven Pantilat, MD, SFHM, hospitalist and director of the Palliative Care Program at the University of California at San Francisco. “This creates a great opportunity for hospitalists. I have heard of places that were having trouble recruiting palliative care physicians but were willing to sponsor a hospitalist to go and do a fellowship, supplementing their salary as an incentive—and a reasonable one—for a hospitalist interested in making a career move.”
He says that palliative care, like hospital medicine, has been a significant value-add in many hospitals and health systems. More importantly, it correlates to positive patient outcomes (see “Research Highlights Palliative Care Contributions,”).
“What’s new is how it connects to current issues like improved care transitions and readmissions reduction,” Dr. Pantilat says.
Advocates say palliative care helps to match medical services to patient preferences, thereby improving patient satisfaction scores, especially for those who aren’t likely to achieve good outcomes. Dr. Pantilat says it puts plans in place for patients to get the right services for the post-discharge period and for responding to anticipated problems like chest pain.
“It’s not just how to get patients out of the hospital as quickly as possible,” he says, “but to do that with a plan that sets them up to succeed at home.”
Larry Beresford is a freelance writer in San Francisco.
References
- Lupu D. American Academy of Hospice and Palliative Medicine Workforce Task Force. Estimate of current hospice and palliative medicine physician workforce shortage. J Pain Symptom Manage. 2010;40(6):899-911.
- Quill TE, Abernethy AP. Generalist plus specialist palliative care—creating a more sustainable model. N Engl J Med. 2013;368(13):1173-1175.
- Morrison RS, Penrod JD, Cassel JB, et al. Palliative Care Leadership Centers' Outcomes Group. Cost savings associated with US hospital palliative care consultation programs. Arch Intern Med. 2008;168(16):1783-1790.
- Penrod JD, Deb P, Dellenbaugh C, et al. Hospital-based palliative care consultation: effects on hospital cost. J Palliat Med. 2010;13(8):973-979.
- Gelfman LP, Meier DE, Morrison RS. Does palliative care improve quality? A survey of bereaved family members. J Pain Symptom Manage. 2008;36(1):22-28.
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742.
- Irwin KE, Greer JA, Khatib J, Temel JS, Pirl WF. Early palliative care and metastatic non-small cell lung cancer: potential mechanisms of prolonged survival. Chron Respir Dis. 2013;10(1):35-47.
- Von Roenn JN, Temel J. The integration of palliative care and oncology: the evidence. Oncology. 2011;25(13):1258-1260,1262,1264-1265.
- Yoong J, Park ER, Greer JA, etc. Early palliative care in advanced lung cancer: a qualitative study. JAMA Intern Med. 2013;173(4):283-290.
- Enguidanos S, Vesper E, Lorenz K. 30-day readmissions among seriously ill older adults. J Palliat Med. 2012;15(12):1356-1361.
- Eti S, O’Mahony S, McHugh M, Guilbe R, Blank A, Selwyn P. Outcomes of the acute palliative care unit in an academic medical center [published online ahead of print May 10, 2013]. Am J Hosp Palliat Care. PMID: 23666616.
- Smith AK, Thai JN, Bakitas MA, et al. The diverse landscape of palliative care clinics. J Palliat Med. 2013;16(6):661-668.
After nine years in practice as a hospitalist in community and academic settings, Leonard Noronha, MD, applied for and in July 2012 became the inaugural, full-year, full-time fellow in hospice and palliative medicine (HPM) at the University of New Mexico in Albuquerque, one of approximately 200 such positions nationwide. The fellowship training qualifies him to sit for HPM subspecialty medical board certification.
Dr. Noronha says he was casually acquainted with the concept of palliative care from residency but didn’t know “when to ask for a palliative care consultation or what they offered.”
“I also had a sense that discussions about feeding tubes, for example, could happen better and easier than they typically did,” he says.
His interest piqued as he learned more about palliative care at hospitalist meetings.
“I grew more excited about it and came to realize that it is something I’d find rewarding and enjoyable, if I could get good at it,” Dr. Noronha says. “Over time, I found more satisfaction in palliative care encounters with patients—and became less comfortable with what I perceived as occasionally inappropriate and excessive testing and treatment [for some hospitalized patients who weren’t offered palliative care].”
Palliative care is a medical specialty that focuses on comfort, relief of symptoms, and clarifying patients’ treatment goals. It is commonly provided as an interdisciplinary consultation service in hospitals. Advocates say it can be offered concurrently with other medical therapies for any seriously ill patient, particularly when there are physical, psychosocial, or spiritual complications, and it is not limited to patients approaching death.
Experienced clinicians say palliative care maximizes quality of life and empowers patients and their families to make treatment decisions more in line with their hopes and values. They also say palliative care gives an emotional lift to providers, while reducing hospital expenditures. Some also suggest that palliative care is an additional tool for enhancing care transitions, potentially affecting readmission rates.
For Dr. Noronha, the one-year fellowship required a significant cut in pay, but he was prepared for the financial hardship.
“It was a great decision for me,” he says. “Some of my colleagues had encouraged me to think about using the experiential pathway to HPM board certification, but I knew I’d do better in the structured environment of a fellowship.
“There have been times when I’ve been outside of my comfort zone, sometimes feeling like the least experienced person in the room. But I knew the fellowship would help—and it did.”
He says the training gives him a better appreciation for things like illness trajectories, the nuances of goal clarification, and the benefit of an extra set of eyes and ears to assess the patient.
After completing his fellowship, Dr. Noronha became UNM’s second full-time palliative medicine faculty. He encourages hospitalists to talk to the palliative care service at their institutions and request consultations for complex, seriously ill patients who might benefit.
As for his new career path, he says that often he is asked if palliative care is depressing. “Some of these situations can be tragic, but I find the work very rewarding,” he says.
Service Models
In some settings, palliative care is incorporated into the hospitalist service. Hospitalists are scheduled for palliative care shifts or have palliative care visits incorporated into daily rounds. Such blended positions could be a recruiting incentive for some physicians who want to do both.
In other settings, palliative care is a separate service. Consultations are ordered as needed by hospitalists and other physicians.
Advocates like Marianne Novelli, MD, FHM, FACP, say hospitalists play a pivotal role in providing the basics of palliative care for seriously ill, hospitalized patients.
“Palliative care is part and parcel of what we do as hospitalists with the people we serve—who by definition are very sick, even to get into the hospital,” says Dr. Novelli, formerly the chief of the division of hospital medicine at Kaiser Permanente in Denver, Colo. She rotated off that leadership position in 2011 and has since divided her time between hospital medicine and palliative care shifts in the hospital, although she now does palliative care exclusively.
Initially, she watched palliative care consults and asked for mentorship from the palliative care team. Although it took time to get used to the advisory role of the consultant, and to working with a team, she eventually became board certified in HPM.
“Palliative care is incredibly intense but richly rewarding work,” she says. “The patients you see are never simple. It allows us to practice the type of medicine we originally set out to do, with people at the most vulnerable times in their lives.”
Workforce, Fellowship, Board Certification
In October 2012, 3,356 physicians passed the hospice and palliative medicine subspecialty board exams offered by the American Board of Medical Specialties and 10 of its constituent specialty boards, with the lion’s share of them certified by the American Board of Internal Medicine. That more than doubled the number of physicians earning the HPM credential since its inception in 2008.
Even with the surge in palliative care training, workforce studies suggest the U.S. is woefully short of credentialed palliative care physicians. And many think hospitalists can help fill that void.
The Center to Advance Palliative Care (CAPC, www.capc.org) counts 1,400 hospital-based palliative care programs in the U.S., while the Centers for Medicare & Medicaid Services (CMS) recognizes about 3,500 Medicare-certified hospice programs. A 2010 estimate by Dale Lupu and the American Academy of Hospice and Palliative Medicine (AAHPM), however, suggested a need for between 4,487 and 10,810 palliative care physician FTEs just to staff existing programs at appropriate levels—without considering growth for the field or its spread into outpatient settings.1
In the past, mid-career physicians had an experiential pathway to the HPM board exam, based on hours worked with a hospice or palliative care team, but physicians now must complete an HPM fellowship of at least one year in order to sit for the boards. And, according to AAHPM, only 234 HPM fellowship positions are offered nationwide by 85 approved fellowship programs.
A one-year fellowship is a big commitment for an established hospitalist, according to Stephen Bekanich, MD, co-director of Seton Palliative Care at Seton Healthcare, an 11-hospital system in Austin, Texas. A former hospitalist, Dr. Bekanich says that in his region a fellow stipend is about $70,000, whereas typical hospitalist compensation is in the mid- to upper-$200,000s.
AAHPM is exploring other approaches to expanding the workforce with mid-career physicians. One approach, authored by Timothy Quill, MD, and Amy Abernethy, MD, the past and current AAHPM board presidents, is to develop a two-tiered system in which palliative medicine specialists teach basic palliative care techniques and approaches to primary care physicians, hospitalists, and such specialists as oncologists.2 The article also suggested equipping clinicians with the tools to recognize when more specialized help is needed.
“As in any medical discipline, some core elements of palliative care, such as aligning treatment with a patient’s goals and basic symptom management, should be routine aspects of care delivered by any practitioner,” Drs. Quill and Abernethy wrote. “Other skills are more complex and take years of training to learn and apply, such as negotiating a difficult family meeting, addressing veiled existential distress, and managing refractory symptoms.”
Dr. Bekanich is trying the two-tiered approach at Seton Healthcare. At facilities with no palliative care service, he is transplanting palliative-trained nurse practitioners in hospital medicine groups.
“This model is locked into our budget for fiscal year 2014,” Dr. Bekanich says. “We’ll train folks, starting with hospitalists and primary care physicians.”
The training will start with a pair of three-hour sessions on palliative care techniques for hospitalists and PCPs, followed by homework assignments. “Then we’ll meet again in three months to do some role plays,” he says.
Two final rounds of training will focus on skills, philosophy, values, and practice.

—Marianne Novelli, MD, FHM, FACP, former chief of the division of hospital medicine, Kaiser Permanente, Denver, Colo.
On-the-Job Training
David Weissman, MD, FACP, a palliative care specialist in Milwaukee, Wis., and consultant to the CAPC, recommends hospitalists do what they can to improve their knowledge and skills. “There are a lot of opportunities for palliative care training out there,” he says.
HM conferences often include palliative care content. AAHPM and CAPC offer annual conferences that immerse participants in content, with opportunities to mingle with palliative care colleagues. AAHPM also offers specific content through its “Unipac” series of nine self-study training modules (www.aahpm.org/resources/default/unipac-4th-edition.html.)
On the job, Dr. Weissman says hospitalists should ask for consults for patients with complex needs. Also pay attention to how the service works and what it recommends. Taking a couple of days to round with the palliative care service could be very educational. It may be possible to take a part-time position with the team, providing weekend or vacation coverage. Hospitalists can participate on planning or advisory committees for palliative care in their hospitals or on quality improvement projects.
“If there isn’t a palliative care service, advocate for developing one,” he says.
Local hospice programs, especially those with inpatient hospice facilities that need daily physician coverage, might have part-time staff positions, which could be a great moonlighting opportunity for hospitalists and a way to learn a lot very quickly.
“I can tell the difference between physicians who have spent time working in a hospice, where you can learn about caring for people at the end of life because most of the patients are so sick, and those who have not,” says Porter Storey, MD, FACP, AAHPM’s executive vice president and a practicing palliative care physician in Colorado. “You can learn how to use the medications to get someone comfortable quickly and how to talk to families in crisis. It can be some of the most rewarding work you can possibly do—especially when you have the time and training to do it well for some of the most challenging of patients and families.”
Dr. Storey recommends that hospitalists join AAHPM, use its professional materials, attend its annual meetings, and, if they feel a calling, consider fellowship training as the next big step.
“Palliative care programs are growing in number and size but are chronically understaffed,” says Steven Pantilat, MD, SFHM, hospitalist and director of the Palliative Care Program at the University of California at San Francisco. “This creates a great opportunity for hospitalists. I have heard of places that were having trouble recruiting palliative care physicians but were willing to sponsor a hospitalist to go and do a fellowship, supplementing their salary as an incentive—and a reasonable one—for a hospitalist interested in making a career move.”
He says that palliative care, like hospital medicine, has been a significant value-add in many hospitals and health systems. More importantly, it correlates to positive patient outcomes (see “Research Highlights Palliative Care Contributions,”).
“What’s new is how it connects to current issues like improved care transitions and readmissions reduction,” Dr. Pantilat says.
Advocates say palliative care helps to match medical services to patient preferences, thereby improving patient satisfaction scores, especially for those who aren’t likely to achieve good outcomes. Dr. Pantilat says it puts plans in place for patients to get the right services for the post-discharge period and for responding to anticipated problems like chest pain.
“It’s not just how to get patients out of the hospital as quickly as possible,” he says, “but to do that with a plan that sets them up to succeed at home.”
Larry Beresford is a freelance writer in San Francisco.
References
- Lupu D. American Academy of Hospice and Palliative Medicine Workforce Task Force. Estimate of current hospice and palliative medicine physician workforce shortage. J Pain Symptom Manage. 2010;40(6):899-911.
- Quill TE, Abernethy AP. Generalist plus specialist palliative care—creating a more sustainable model. N Engl J Med. 2013;368(13):1173-1175.
- Morrison RS, Penrod JD, Cassel JB, et al. Palliative Care Leadership Centers' Outcomes Group. Cost savings associated with US hospital palliative care consultation programs. Arch Intern Med. 2008;168(16):1783-1790.
- Penrod JD, Deb P, Dellenbaugh C, et al. Hospital-based palliative care consultation: effects on hospital cost. J Palliat Med. 2010;13(8):973-979.
- Gelfman LP, Meier DE, Morrison RS. Does palliative care improve quality? A survey of bereaved family members. J Pain Symptom Manage. 2008;36(1):22-28.
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742.
- Irwin KE, Greer JA, Khatib J, Temel JS, Pirl WF. Early palliative care and metastatic non-small cell lung cancer: potential mechanisms of prolonged survival. Chron Respir Dis. 2013;10(1):35-47.
- Von Roenn JN, Temel J. The integration of palliative care and oncology: the evidence. Oncology. 2011;25(13):1258-1260,1262,1264-1265.
- Yoong J, Park ER, Greer JA, etc. Early palliative care in advanced lung cancer: a qualitative study. JAMA Intern Med. 2013;173(4):283-290.
- Enguidanos S, Vesper E, Lorenz K. 30-day readmissions among seriously ill older adults. J Palliat Med. 2012;15(12):1356-1361.
- Eti S, O’Mahony S, McHugh M, Guilbe R, Blank A, Selwyn P. Outcomes of the acute palliative care unit in an academic medical center [published online ahead of print May 10, 2013]. Am J Hosp Palliat Care. PMID: 23666616.
- Smith AK, Thai JN, Bakitas MA, et al. The diverse landscape of palliative care clinics. J Palliat Med. 2013;16(6):661-668.
After nine years in practice as a hospitalist in community and academic settings, Leonard Noronha, MD, applied for and in July 2012 became the inaugural, full-year, full-time fellow in hospice and palliative medicine (HPM) at the University of New Mexico in Albuquerque, one of approximately 200 such positions nationwide. The fellowship training qualifies him to sit for HPM subspecialty medical board certification.
Dr. Noronha says he was casually acquainted with the concept of palliative care from residency but didn’t know “when to ask for a palliative care consultation or what they offered.”
“I also had a sense that discussions about feeding tubes, for example, could happen better and easier than they typically did,” he says.
His interest piqued as he learned more about palliative care at hospitalist meetings.
“I grew more excited about it and came to realize that it is something I’d find rewarding and enjoyable, if I could get good at it,” Dr. Noronha says. “Over time, I found more satisfaction in palliative care encounters with patients—and became less comfortable with what I perceived as occasionally inappropriate and excessive testing and treatment [for some hospitalized patients who weren’t offered palliative care].”
Palliative care is a medical specialty that focuses on comfort, relief of symptoms, and clarifying patients’ treatment goals. It is commonly provided as an interdisciplinary consultation service in hospitals. Advocates say it can be offered concurrently with other medical therapies for any seriously ill patient, particularly when there are physical, psychosocial, or spiritual complications, and it is not limited to patients approaching death.
Experienced clinicians say palliative care maximizes quality of life and empowers patients and their families to make treatment decisions more in line with their hopes and values. They also say palliative care gives an emotional lift to providers, while reducing hospital expenditures. Some also suggest that palliative care is an additional tool for enhancing care transitions, potentially affecting readmission rates.
For Dr. Noronha, the one-year fellowship required a significant cut in pay, but he was prepared for the financial hardship.
“It was a great decision for me,” he says. “Some of my colleagues had encouraged me to think about using the experiential pathway to HPM board certification, but I knew I’d do better in the structured environment of a fellowship.
“There have been times when I’ve been outside of my comfort zone, sometimes feeling like the least experienced person in the room. But I knew the fellowship would help—and it did.”
He says the training gives him a better appreciation for things like illness trajectories, the nuances of goal clarification, and the benefit of an extra set of eyes and ears to assess the patient.
After completing his fellowship, Dr. Noronha became UNM’s second full-time palliative medicine faculty. He encourages hospitalists to talk to the palliative care service at their institutions and request consultations for complex, seriously ill patients who might benefit.
As for his new career path, he says that often he is asked if palliative care is depressing. “Some of these situations can be tragic, but I find the work very rewarding,” he says.
Service Models
In some settings, palliative care is incorporated into the hospitalist service. Hospitalists are scheduled for palliative care shifts or have palliative care visits incorporated into daily rounds. Such blended positions could be a recruiting incentive for some physicians who want to do both.
In other settings, palliative care is a separate service. Consultations are ordered as needed by hospitalists and other physicians.
Advocates like Marianne Novelli, MD, FHM, FACP, say hospitalists play a pivotal role in providing the basics of palliative care for seriously ill, hospitalized patients.
“Palliative care is part and parcel of what we do as hospitalists with the people we serve—who by definition are very sick, even to get into the hospital,” says Dr. Novelli, formerly the chief of the division of hospital medicine at Kaiser Permanente in Denver, Colo. She rotated off that leadership position in 2011 and has since divided her time between hospital medicine and palliative care shifts in the hospital, although she now does palliative care exclusively.
Initially, she watched palliative care consults and asked for mentorship from the palliative care team. Although it took time to get used to the advisory role of the consultant, and to working with a team, she eventually became board certified in HPM.
“Palliative care is incredibly intense but richly rewarding work,” she says. “The patients you see are never simple. It allows us to practice the type of medicine we originally set out to do, with people at the most vulnerable times in their lives.”
Workforce, Fellowship, Board Certification
In October 2012, 3,356 physicians passed the hospice and palliative medicine subspecialty board exams offered by the American Board of Medical Specialties and 10 of its constituent specialty boards, with the lion’s share of them certified by the American Board of Internal Medicine. That more than doubled the number of physicians earning the HPM credential since its inception in 2008.
Even with the surge in palliative care training, workforce studies suggest the U.S. is woefully short of credentialed palliative care physicians. And many think hospitalists can help fill that void.
The Center to Advance Palliative Care (CAPC, www.capc.org) counts 1,400 hospital-based palliative care programs in the U.S., while the Centers for Medicare & Medicaid Services (CMS) recognizes about 3,500 Medicare-certified hospice programs. A 2010 estimate by Dale Lupu and the American Academy of Hospice and Palliative Medicine (AAHPM), however, suggested a need for between 4,487 and 10,810 palliative care physician FTEs just to staff existing programs at appropriate levels—without considering growth for the field or its spread into outpatient settings.1
In the past, mid-career physicians had an experiential pathway to the HPM board exam, based on hours worked with a hospice or palliative care team, but physicians now must complete an HPM fellowship of at least one year in order to sit for the boards. And, according to AAHPM, only 234 HPM fellowship positions are offered nationwide by 85 approved fellowship programs.
A one-year fellowship is a big commitment for an established hospitalist, according to Stephen Bekanich, MD, co-director of Seton Palliative Care at Seton Healthcare, an 11-hospital system in Austin, Texas. A former hospitalist, Dr. Bekanich says that in his region a fellow stipend is about $70,000, whereas typical hospitalist compensation is in the mid- to upper-$200,000s.
AAHPM is exploring other approaches to expanding the workforce with mid-career physicians. One approach, authored by Timothy Quill, MD, and Amy Abernethy, MD, the past and current AAHPM board presidents, is to develop a two-tiered system in which palliative medicine specialists teach basic palliative care techniques and approaches to primary care physicians, hospitalists, and such specialists as oncologists.2 The article also suggested equipping clinicians with the tools to recognize when more specialized help is needed.
“As in any medical discipline, some core elements of palliative care, such as aligning treatment with a patient’s goals and basic symptom management, should be routine aspects of care delivered by any practitioner,” Drs. Quill and Abernethy wrote. “Other skills are more complex and take years of training to learn and apply, such as negotiating a difficult family meeting, addressing veiled existential distress, and managing refractory symptoms.”
Dr. Bekanich is trying the two-tiered approach at Seton Healthcare. At facilities with no palliative care service, he is transplanting palliative-trained nurse practitioners in hospital medicine groups.
“This model is locked into our budget for fiscal year 2014,” Dr. Bekanich says. “We’ll train folks, starting with hospitalists and primary care physicians.”
The training will start with a pair of three-hour sessions on palliative care techniques for hospitalists and PCPs, followed by homework assignments. “Then we’ll meet again in three months to do some role plays,” he says.
Two final rounds of training will focus on skills, philosophy, values, and practice.

—Marianne Novelli, MD, FHM, FACP, former chief of the division of hospital medicine, Kaiser Permanente, Denver, Colo.
On-the-Job Training
David Weissman, MD, FACP, a palliative care specialist in Milwaukee, Wis., and consultant to the CAPC, recommends hospitalists do what they can to improve their knowledge and skills. “There are a lot of opportunities for palliative care training out there,” he says.
HM conferences often include palliative care content. AAHPM and CAPC offer annual conferences that immerse participants in content, with opportunities to mingle with palliative care colleagues. AAHPM also offers specific content through its “Unipac” series of nine self-study training modules (www.aahpm.org/resources/default/unipac-4th-edition.html.)
On the job, Dr. Weissman says hospitalists should ask for consults for patients with complex needs. Also pay attention to how the service works and what it recommends. Taking a couple of days to round with the palliative care service could be very educational. It may be possible to take a part-time position with the team, providing weekend or vacation coverage. Hospitalists can participate on planning or advisory committees for palliative care in their hospitals or on quality improvement projects.
“If there isn’t a palliative care service, advocate for developing one,” he says.
Local hospice programs, especially those with inpatient hospice facilities that need daily physician coverage, might have part-time staff positions, which could be a great moonlighting opportunity for hospitalists and a way to learn a lot very quickly.
“I can tell the difference between physicians who have spent time working in a hospice, where you can learn about caring for people at the end of life because most of the patients are so sick, and those who have not,” says Porter Storey, MD, FACP, AAHPM’s executive vice president and a practicing palliative care physician in Colorado. “You can learn how to use the medications to get someone comfortable quickly and how to talk to families in crisis. It can be some of the most rewarding work you can possibly do—especially when you have the time and training to do it well for some of the most challenging of patients and families.”
Dr. Storey recommends that hospitalists join AAHPM, use its professional materials, attend its annual meetings, and, if they feel a calling, consider fellowship training as the next big step.
“Palliative care programs are growing in number and size but are chronically understaffed,” says Steven Pantilat, MD, SFHM, hospitalist and director of the Palliative Care Program at the University of California at San Francisco. “This creates a great opportunity for hospitalists. I have heard of places that were having trouble recruiting palliative care physicians but were willing to sponsor a hospitalist to go and do a fellowship, supplementing their salary as an incentive—and a reasonable one—for a hospitalist interested in making a career move.”
He says that palliative care, like hospital medicine, has been a significant value-add in many hospitals and health systems. More importantly, it correlates to positive patient outcomes (see “Research Highlights Palliative Care Contributions,”).
“What’s new is how it connects to current issues like improved care transitions and readmissions reduction,” Dr. Pantilat says.
Advocates say palliative care helps to match medical services to patient preferences, thereby improving patient satisfaction scores, especially for those who aren’t likely to achieve good outcomes. Dr. Pantilat says it puts plans in place for patients to get the right services for the post-discharge period and for responding to anticipated problems like chest pain.
“It’s not just how to get patients out of the hospital as quickly as possible,” he says, “but to do that with a plan that sets them up to succeed at home.”
Larry Beresford is a freelance writer in San Francisco.
References
- Lupu D. American Academy of Hospice and Palliative Medicine Workforce Task Force. Estimate of current hospice and palliative medicine physician workforce shortage. J Pain Symptom Manage. 2010;40(6):899-911.
- Quill TE, Abernethy AP. Generalist plus specialist palliative care—creating a more sustainable model. N Engl J Med. 2013;368(13):1173-1175.
- Morrison RS, Penrod JD, Cassel JB, et al. Palliative Care Leadership Centers' Outcomes Group. Cost savings associated with US hospital palliative care consultation programs. Arch Intern Med. 2008;168(16):1783-1790.
- Penrod JD, Deb P, Dellenbaugh C, et al. Hospital-based palliative care consultation: effects on hospital cost. J Palliat Med. 2010;13(8):973-979.
- Gelfman LP, Meier DE, Morrison RS. Does palliative care improve quality? A survey of bereaved family members. J Pain Symptom Manage. 2008;36(1):22-28.
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742.
- Irwin KE, Greer JA, Khatib J, Temel JS, Pirl WF. Early palliative care and metastatic non-small cell lung cancer: potential mechanisms of prolonged survival. Chron Respir Dis. 2013;10(1):35-47.
- Von Roenn JN, Temel J. The integration of palliative care and oncology: the evidence. Oncology. 2011;25(13):1258-1260,1262,1264-1265.
- Yoong J, Park ER, Greer JA, etc. Early palliative care in advanced lung cancer: a qualitative study. JAMA Intern Med. 2013;173(4):283-290.
- Enguidanos S, Vesper E, Lorenz K. 30-day readmissions among seriously ill older adults. J Palliat Med. 2012;15(12):1356-1361.
- Eti S, O’Mahony S, McHugh M, Guilbe R, Blank A, Selwyn P. Outcomes of the acute palliative care unit in an academic medical center [published online ahead of print May 10, 2013]. Am J Hosp Palliat Care. PMID: 23666616.
- Smith AK, Thai JN, Bakitas MA, et al. The diverse landscape of palliative care clinics. J Palliat Med. 2013;16(6):661-668.
Listening to the voice of patients
The stories of patients and families, like this one presented by Kimberly Lastinger, offer powerful lessons for health professionals about the human experience of illness and its impact on the person and family. Furthermore, patient narratives present ‘experiential truth and passion’ that compel us to re-examine medical practices and ethical perceptions of care.1 However much we think we know from our years of medical practice and our observation of many patients and families, we are not ‘in the patient’s shoes’. The content of patient narratives supports ethical decisions by helping us listen and hear what patients say, how they say it, and by clarifying why it matters. The patient story can help us focus healthcare on the patient and to recognize that the patient is the ultimate authority when it comes to the interpretation of his or her illness experience. Until one has been there, it is impossible to imagine the impact of a life-threatening illness. I am reminded of my own surprise at seeing, feeling, and experiencing the loss of a loved one to cancer. I have been a medical oncologist and palliative care physician for more than 25 years, and I thought I understood the experience I could expect when my husband died. Instead, I was stunned to find that I didn’t have a clue! It has taught me to listen more carefully and ask more questions. When listening occurs, understanding increases, and narratives can be jointly constructed by the patient and healthcare provider. This leads to power that is shared and the sharing of power constitutes an important ethical safeguard within the relationship.2
The narrative presented here suggests a remarkably positive experience of a devastating illness and its potential impact on the patient and family. The patient was someone with tremendous resiliency and optimism. She was commited to living on her terms and for caring for her daughter. Her story is an inspiring one. Ms Lastinger recalls her mother’s amazing support system, but also her mother’s fear of dying. The latter learned only years after her mother’s death from reading her journal. I wonder if, as too often happens, we failed to offer adequate psychosocial support. This service is too often not offered.3,4 This occurs for many reasons including, I suspect, when there is the perception of a supportive social network embracing the patient. It behooves us as healthcare professionals to remember that patients may not want or be able to share some of their deepest fears, the threat of dying or of being dependent, with the people they love. The patients who seem to be doing well emotionally, those with ‘great support’ may also benefit from professional counseling.
The stories of patients and families, like this one presented by Kimberly Lastinger, offer powerful lessons for health professionals about the human experience of illness and its impact on the person and family. Furthermore, patient narratives present ‘experiential truth and passion’ that compel us to re-examine medical practices and ethical perceptions of care.1 However much we think we know from our years of medical practice and our observation of many patients and families, we are not ‘in the patient’s shoes’. The content of patient narratives supports ethical decisions by helping us listen and hear what patients say, how they say it, and by clarifying why it matters. The patient story can help us focus healthcare on the patient and to recognize that the patient is the ultimate authority when it comes to the interpretation of his or her illness experience. Until one has been there, it is impossible to imagine the impact of a life-threatening illness. I am reminded of my own surprise at seeing, feeling, and experiencing the loss of a loved one to cancer. I have been a medical oncologist and palliative care physician for more than 25 years, and I thought I understood the experience I could expect when my husband died. Instead, I was stunned to find that I didn’t have a clue! It has taught me to listen more carefully and ask more questions. When listening occurs, understanding increases, and narratives can be jointly constructed by the patient and healthcare provider. This leads to power that is shared and the sharing of power constitutes an important ethical safeguard within the relationship.2
The narrative presented here suggests a remarkably positive experience of a devastating illness and its potential impact on the patient and family. The patient was someone with tremendous resiliency and optimism. She was commited to living on her terms and for caring for her daughter. Her story is an inspiring one. Ms Lastinger recalls her mother’s amazing support system, but also her mother’s fear of dying. The latter learned only years after her mother’s death from reading her journal. I wonder if, as too often happens, we failed to offer adequate psychosocial support. This service is too often not offered.3,4 This occurs for many reasons including, I suspect, when there is the perception of a supportive social network embracing the patient. It behooves us as healthcare professionals to remember that patients may not want or be able to share some of their deepest fears, the threat of dying or of being dependent, with the people they love. The patients who seem to be doing well emotionally, those with ‘great support’ may also benefit from professional counseling.
The stories of patients and families, like this one presented by Kimberly Lastinger, offer powerful lessons for health professionals about the human experience of illness and its impact on the person and family. Furthermore, patient narratives present ‘experiential truth and passion’ that compel us to re-examine medical practices and ethical perceptions of care.1 However much we think we know from our years of medical practice and our observation of many patients and families, we are not ‘in the patient’s shoes’. The content of patient narratives supports ethical decisions by helping us listen and hear what patients say, how they say it, and by clarifying why it matters. The patient story can help us focus healthcare on the patient and to recognize that the patient is the ultimate authority when it comes to the interpretation of his or her illness experience. Until one has been there, it is impossible to imagine the impact of a life-threatening illness. I am reminded of my own surprise at seeing, feeling, and experiencing the loss of a loved one to cancer. I have been a medical oncologist and palliative care physician for more than 25 years, and I thought I understood the experience I could expect when my husband died. Instead, I was stunned to find that I didn’t have a clue! It has taught me to listen more carefully and ask more questions. When listening occurs, understanding increases, and narratives can be jointly constructed by the patient and healthcare provider. This leads to power that is shared and the sharing of power constitutes an important ethical safeguard within the relationship.2
The narrative presented here suggests a remarkably positive experience of a devastating illness and its potential impact on the patient and family. The patient was someone with tremendous resiliency and optimism. She was commited to living on her terms and for caring for her daughter. Her story is an inspiring one. Ms Lastinger recalls her mother’s amazing support system, but also her mother’s fear of dying. The latter learned only years after her mother’s death from reading her journal. I wonder if, as too often happens, we failed to offer adequate psychosocial support. This service is too often not offered.3,4 This occurs for many reasons including, I suspect, when there is the perception of a supportive social network embracing the patient. It behooves us as healthcare professionals to remember that patients may not want or be able to share some of their deepest fears, the threat of dying or of being dependent, with the people they love. The patients who seem to be doing well emotionally, those with ‘great support’ may also benefit from professional counseling.
To treat or not to treat: balancing therapeutic outcomes, toxicity and quality of life in patients with recurrent and/or metastatic head and neck cancer
Squamous cell carcinomas of the head and neck account for 3% of all new cancers diagnosed annually within the United States.1 According to the Surveillance Epidemiology
and Ends Reports (SEER) database, 79% of patients in the US present with local or regional advanced disease and are treated with combinedmodality therapy.2 Factors that influence treatment decision making include the following: resectability, function preservation, local patterns of care, and patient characteristics or preferences. In this cohort of patients, disease eradication is the goal of therapy. Conversely, for approximately 16% of patients who are diagnosed with metastatic disease at presentation, or the substantial portion of patients who develop non-curable disease recurrence, the main therapeutic objectives are palliation and prolongation of survival (accessible at http://seer.cancer.gov/statfacts/html/oralcav.html).2,3 We define patients as having non-curable recurrence if development of metastatic disease or development of local recurrence is not amenable to either surgical resection or re-irradiation therapy. Several changes in the epidemiology and treatment of metastatic and recurrent head and neck cancer (M/RHNC) have resulted in paradigm shifts that effect treatment decision making in this population. First, a combination of standard chemotherapy with cetuximab has demonstrated a survival advantage. This is the first time that any agent or combination of agents has demonstrated superiority in the treatment of M/RHNC.4 Second, human papilloma virus (HPV)-associated oropharyngeal cancers are epidemic in many areas of the world. The cohort of HPV-positive patients has an excellent prognosis with currently available primary treatment regimens. The recurrence rate in this population is low; however, data regarding the treatment responsiveness of HPV-associated tumors that recur after primary therapy is lacking. Finally, with the increased use of aggressive combined modality regimens as primary therapy, patients with recurrent disease are often heavily pretreated and suffer from symptoms secondary to their initial therapy. It is important to understand how the evolving epidemiology and treatment paradigms affect decision making for our patients. This requires an understanding of how these changes affect both the benefits and risks to the patient.
Squamous cell carcinomas of the head and neck account for 3% of all new cancers diagnosed annually within the United States.1 According to the Surveillance Epidemiology
and Ends Reports (SEER) database, 79% of patients in the US present with local or regional advanced disease and are treated with combinedmodality therapy.2 Factors that influence treatment decision making include the following: resectability, function preservation, local patterns of care, and patient characteristics or preferences. In this cohort of patients, disease eradication is the goal of therapy. Conversely, for approximately 16% of patients who are diagnosed with metastatic disease at presentation, or the substantial portion of patients who develop non-curable disease recurrence, the main therapeutic objectives are palliation and prolongation of survival (accessible at http://seer.cancer.gov/statfacts/html/oralcav.html).2,3 We define patients as having non-curable recurrence if development of metastatic disease or development of local recurrence is not amenable to either surgical resection or re-irradiation therapy. Several changes in the epidemiology and treatment of metastatic and recurrent head and neck cancer (M/RHNC) have resulted in paradigm shifts that effect treatment decision making in this population. First, a combination of standard chemotherapy with cetuximab has demonstrated a survival advantage. This is the first time that any agent or combination of agents has demonstrated superiority in the treatment of M/RHNC.4 Second, human papilloma virus (HPV)-associated oropharyngeal cancers are epidemic in many areas of the world. The cohort of HPV-positive patients has an excellent prognosis with currently available primary treatment regimens. The recurrence rate in this population is low; however, data regarding the treatment responsiveness of HPV-associated tumors that recur after primary therapy is lacking. Finally, with the increased use of aggressive combined modality regimens as primary therapy, patients with recurrent disease are often heavily pretreated and suffer from symptoms secondary to their initial therapy. It is important to understand how the evolving epidemiology and treatment paradigms affect decision making for our patients. This requires an understanding of how these changes affect both the benefits and risks to the patient.
Squamous cell carcinomas of the head and neck account for 3% of all new cancers diagnosed annually within the United States.1 According to the Surveillance Epidemiology
and Ends Reports (SEER) database, 79% of patients in the US present with local or regional advanced disease and are treated with combinedmodality therapy.2 Factors that influence treatment decision making include the following: resectability, function preservation, local patterns of care, and patient characteristics or preferences. In this cohort of patients, disease eradication is the goal of therapy. Conversely, for approximately 16% of patients who are diagnosed with metastatic disease at presentation, or the substantial portion of patients who develop non-curable disease recurrence, the main therapeutic objectives are palliation and prolongation of survival (accessible at http://seer.cancer.gov/statfacts/html/oralcav.html).2,3 We define patients as having non-curable recurrence if development of metastatic disease or development of local recurrence is not amenable to either surgical resection or re-irradiation therapy. Several changes in the epidemiology and treatment of metastatic and recurrent head and neck cancer (M/RHNC) have resulted in paradigm shifts that effect treatment decision making in this population. First, a combination of standard chemotherapy with cetuximab has demonstrated a survival advantage. This is the first time that any agent or combination of agents has demonstrated superiority in the treatment of M/RHNC.4 Second, human papilloma virus (HPV)-associated oropharyngeal cancers are epidemic in many areas of the world. The cohort of HPV-positive patients has an excellent prognosis with currently available primary treatment regimens. The recurrence rate in this population is low; however, data regarding the treatment responsiveness of HPV-associated tumors that recur after primary therapy is lacking. Finally, with the increased use of aggressive combined modality regimens as primary therapy, patients with recurrent disease are often heavily pretreated and suffer from symptoms secondary to their initial therapy. It is important to understand how the evolving epidemiology and treatment paradigms affect decision making for our patients. This requires an understanding of how these changes affect both the benefits and risks to the patient.
My mom, the cancer warrior
I often daydream that my mom is still here and living cancer free. I like to imagine her teaching art at a prestigious private school in New York City, or maybe retired quilting in a little cottage in Vermont, or at home sketching on her back porch watching her dog playing in the yard. My mother battled cancer for over 20 years; and I find myself wondering what would it have been like to grow up without living under the shadow of the “c” word. How would things have been different? Would my mom still have been the fierce, strong, and passionate woman I remember? She never allowed herself to be a cancer patient. She was a mother, an artist, a friend, a teacher, and a cancer warrior. My mother was diagnosed with Stage II breast cancer in July of 1991. I was 7 years old and my younger sister was 2. She found a pea-sized lump in her left breast by self examination. Her treatment was to be a lumpectomy and radiation. While in surgery, they found numerous lumps in her left breast as well as her right breast and lymph nodes. My father had to make the decision for a radical mastectomy of her left breast and a partial of her right. It was very hard to see her going through all of it. I can remember having to spend a lot of time overnight with friends and family. There was a lot of crying and adults whispering. My mom was in bed most of the time and I remember waking up at night to her vomiting. At 7 years old, I knew words like mastectomy and chemotherapy. One night I couldn’t sleep and I went up stairs to ask my mom if she was dying. How do you answer that? My pediatrician told my mother to give me a journal so I could draw and write my thoughts and emotions. In the afternoons, the two of us would write in our journals. She kept my composition notebook filled with funny round people and lots of “x”s over my mommy’s “bueb”.
I often daydream that my mom is still here and living cancer free. I like to imagine her teaching art at a prestigious private school in New York City, or maybe retired quilting in a little cottage in Vermont, or at home sketching on her back porch watching her dog playing in the yard. My mother battled cancer for over 20 years; and I find myself wondering what would it have been like to grow up without living under the shadow of the “c” word. How would things have been different? Would my mom still have been the fierce, strong, and passionate woman I remember? She never allowed herself to be a cancer patient. She was a mother, an artist, a friend, a teacher, and a cancer warrior. My mother was diagnosed with Stage II breast cancer in July of 1991. I was 7 years old and my younger sister was 2. She found a pea-sized lump in her left breast by self examination. Her treatment was to be a lumpectomy and radiation. While in surgery, they found numerous lumps in her left breast as well as her right breast and lymph nodes. My father had to make the decision for a radical mastectomy of her left breast and a partial of her right. It was very hard to see her going through all of it. I can remember having to spend a lot of time overnight with friends and family. There was a lot of crying and adults whispering. My mom was in bed most of the time and I remember waking up at night to her vomiting. At 7 years old, I knew words like mastectomy and chemotherapy. One night I couldn’t sleep and I went up stairs to ask my mom if she was dying. How do you answer that? My pediatrician told my mother to give me a journal so I could draw and write my thoughts and emotions. In the afternoons, the two of us would write in our journals. She kept my composition notebook filled with funny round people and lots of “x”s over my mommy’s “bueb”.
I often daydream that my mom is still here and living cancer free. I like to imagine her teaching art at a prestigious private school in New York City, or maybe retired quilting in a little cottage in Vermont, or at home sketching on her back porch watching her dog playing in the yard. My mother battled cancer for over 20 years; and I find myself wondering what would it have been like to grow up without living under the shadow of the “c” word. How would things have been different? Would my mom still have been the fierce, strong, and passionate woman I remember? She never allowed herself to be a cancer patient. She was a mother, an artist, a friend, a teacher, and a cancer warrior. My mother was diagnosed with Stage II breast cancer in July of 1991. I was 7 years old and my younger sister was 2. She found a pea-sized lump in her left breast by self examination. Her treatment was to be a lumpectomy and radiation. While in surgery, they found numerous lumps in her left breast as well as her right breast and lymph nodes. My father had to make the decision for a radical mastectomy of her left breast and a partial of her right. It was very hard to see her going through all of it. I can remember having to spend a lot of time overnight with friends and family. There was a lot of crying and adults whispering. My mom was in bed most of the time and I remember waking up at night to her vomiting. At 7 years old, I knew words like mastectomy and chemotherapy. One night I couldn’t sleep and I went up stairs to ask my mom if she was dying. How do you answer that? My pediatrician told my mother to give me a journal so I could draw and write my thoughts and emotions. In the afternoons, the two of us would write in our journals. She kept my composition notebook filled with funny round people and lots of “x”s over my mommy’s “bueb”.
Characterization of skin reactions and pain reported by patients receiving radiation therapy for cancer at different sites
Background Skin reactions and pain are commonly reported side effects of radiation therapy (RT).
Objective To characterize RT-induced symptoms according to treatment site subgroups and identify skin symptoms that correlate with pain.
Methods A self-report survey—adapted from the MD Anderson Symptom Inventory and the McGill Pain Questionnaire—assessed RT-induced skin problems, pain, and specific skin symptoms. Wilcoxon Sign Ranked tests compared mean severity of pre- and post-RT pain and skin problems within each RT-site subgroup. Multiple linear regression (MLR) investigated associations between skin symptoms and pain.
Results Survey respondents (N = 106) were 58% female and on average 64 years old. RT sites included lung, breast, lower abdomen, head/neck/brain, and upper abdomen. Only patients receiving breast RT reported significant increases in treatment site pain and skin problems (P ≤ .007). Patients receiving head/neck/brain RT reported increased skin problems (P < .0009). MLR showed that post-RT skin tenderness and tightness were most strongly associated with post-RT pain (P = .066 and P = .122, respectively).
Limitations Small sample size, exploratory analyses, and nonvalidated measure.
Conclusions Only patients receiving breast RT reported significant increases in pain and skin problems at the RT site while patients receiving head/neck/brain RT had increased skin problems but not pain. These findings suggest that the severity of skin problems is not the only factor that contributes to pain and that interventions should be tailored to specifically target pain at the RT site, possibly by targeting tenderness and tightness. These findings should be confirmed in a larger sampling of RT patients.
Click on the PDF icon at the top of this introduction to read the full article.
Background Skin reactions and pain are commonly reported side effects of radiation therapy (RT).
Objective To characterize RT-induced symptoms according to treatment site subgroups and identify skin symptoms that correlate with pain.
Methods A self-report survey—adapted from the MD Anderson Symptom Inventory and the McGill Pain Questionnaire—assessed RT-induced skin problems, pain, and specific skin symptoms. Wilcoxon Sign Ranked tests compared mean severity of pre- and post-RT pain and skin problems within each RT-site subgroup. Multiple linear regression (MLR) investigated associations between skin symptoms and pain.
Results Survey respondents (N = 106) were 58% female and on average 64 years old. RT sites included lung, breast, lower abdomen, head/neck/brain, and upper abdomen. Only patients receiving breast RT reported significant increases in treatment site pain and skin problems (P ≤ .007). Patients receiving head/neck/brain RT reported increased skin problems (P < .0009). MLR showed that post-RT skin tenderness and tightness were most strongly associated with post-RT pain (P = .066 and P = .122, respectively).
Limitations Small sample size, exploratory analyses, and nonvalidated measure.
Conclusions Only patients receiving breast RT reported significant increases in pain and skin problems at the RT site while patients receiving head/neck/brain RT had increased skin problems but not pain. These findings suggest that the severity of skin problems is not the only factor that contributes to pain and that interventions should be tailored to specifically target pain at the RT site, possibly by targeting tenderness and tightness. These findings should be confirmed in a larger sampling of RT patients.
Click on the PDF icon at the top of this introduction to read the full article.
Background Skin reactions and pain are commonly reported side effects of radiation therapy (RT).
Objective To characterize RT-induced symptoms according to treatment site subgroups and identify skin symptoms that correlate with pain.
Methods A self-report survey—adapted from the MD Anderson Symptom Inventory and the McGill Pain Questionnaire—assessed RT-induced skin problems, pain, and specific skin symptoms. Wilcoxon Sign Ranked tests compared mean severity of pre- and post-RT pain and skin problems within each RT-site subgroup. Multiple linear regression (MLR) investigated associations between skin symptoms and pain.
Results Survey respondents (N = 106) were 58% female and on average 64 years old. RT sites included lung, breast, lower abdomen, head/neck/brain, and upper abdomen. Only patients receiving breast RT reported significant increases in treatment site pain and skin problems (P ≤ .007). Patients receiving head/neck/brain RT reported increased skin problems (P < .0009). MLR showed that post-RT skin tenderness and tightness were most strongly associated with post-RT pain (P = .066 and P = .122, respectively).
Limitations Small sample size, exploratory analyses, and nonvalidated measure.
Conclusions Only patients receiving breast RT reported significant increases in pain and skin problems at the RT site while patients receiving head/neck/brain RT had increased skin problems but not pain. These findings suggest that the severity of skin problems is not the only factor that contributes to pain and that interventions should be tailored to specifically target pain at the RT site, possibly by targeting tenderness and tightness. These findings should be confirmed in a larger sampling of RT patients.
Click on the PDF icon at the top of this introduction to read the full article.
Whole brain radiotherapy for poor prognosis patients with brain metastases: predictably poor results Neil C.
Over 170,000 cases of metastatic brain tumors are diagnosed in the United States each year; and the length of survival for patients with brain metastases is often quite limited, ranging from a few weeks to several months.1 The Radiation Therapy Oncology Group (RTOG) Recursive Partitioning Analysis (RPA) and the Graded Prognostic Assessment (GPA) are 2 prognostic indices that have been validated to predict survival and guide the treatment of these patients.2-5 The RPA and GPA indices were formulated by comparing survival to patient and tumor characteristics compiled from RTOG brain metastasis treatment protocols spanning greater than 3 decades. The RPA has 3 classes of patients enumerated as “I”, “II”, and “III,” with class I patients having the longest predicted survival and class III patients having the worst prognosis. The RPA classes are based upon factors that include patient age and Karnofsky Performance Status (KPS) as well as control of the primary tumor and evidence of extra-cranial metastases (Table 1).2 The GPA has 4 classes of patients with a score that may be considered analogous to a grade point average achieved by students in school. The classes are arranged into 4 groupings, which are divided from best to worst prognosis as follows: 3.5 to 4.0, 3.0, 1.5 to 2.5, and 0.0 to 1.0. The GPA employs criteria similar to but slightly different from those used in the RPA, estimating survival by patient age and performance status as well as the number of brain metastases and evidence of extracranial metastases (Table 2).4
Treatment options for patients with brain metastases include surgery, stereotactic radiosurgery (SRS), whole brain radiation therapy (WBRT), supportive measures such as corticosteroids, or a combination of these modalities. The survival of the worst prognosis brain metastases patients treated with WBRT and steroids is estimated by the RPA and GPA tools to be 2.3 months and 2.6 months, respectively.2,4 As noted above, the patient data from which the RPA and GPA indices were created included patients treated on clinical trials. This could have resulted in the selection of patients more fit than average patients and lead to an overestimation of survival when applied to all patients. The clinical trial data used were drawn from over 3 decades, during which supportive care and chemotherapy treatments improved. This could result in an underestimation of survival when applied to patients treated with current systemic therapies and supportive care. It is important for physicians to have an accurate method to predict survival in patients to ensure that appropriate treatments can be recommended.
Over 170,000 cases of metastatic brain tumors are diagnosed in the United States each year; and the length of survival for patients with brain metastases is often quite limited, ranging from a few weeks to several months.1 The Radiation Therapy Oncology Group (RTOG) Recursive Partitioning Analysis (RPA) and the Graded Prognostic Assessment (GPA) are 2 prognostic indices that have been validated to predict survival and guide the treatment of these patients.2-5 The RPA and GPA indices were formulated by comparing survival to patient and tumor characteristics compiled from RTOG brain metastasis treatment protocols spanning greater than 3 decades. The RPA has 3 classes of patients enumerated as “I”, “II”, and “III,” with class I patients having the longest predicted survival and class III patients having the worst prognosis. The RPA classes are based upon factors that include patient age and Karnofsky Performance Status (KPS) as well as control of the primary tumor and evidence of extra-cranial metastases (Table 1).2 The GPA has 4 classes of patients with a score that may be considered analogous to a grade point average achieved by students in school. The classes are arranged into 4 groupings, which are divided from best to worst prognosis as follows: 3.5 to 4.0, 3.0, 1.5 to 2.5, and 0.0 to 1.0. The GPA employs criteria similar to but slightly different from those used in the RPA, estimating survival by patient age and performance status as well as the number of brain metastases and evidence of extracranial metastases (Table 2).4
Treatment options for patients with brain metastases include surgery, stereotactic radiosurgery (SRS), whole brain radiation therapy (WBRT), supportive measures such as corticosteroids, or a combination of these modalities. The survival of the worst prognosis brain metastases patients treated with WBRT and steroids is estimated by the RPA and GPA tools to be 2.3 months and 2.6 months, respectively.2,4 As noted above, the patient data from which the RPA and GPA indices were created included patients treated on clinical trials. This could have resulted in the selection of patients more fit than average patients and lead to an overestimation of survival when applied to all patients. The clinical trial data used were drawn from over 3 decades, during which supportive care and chemotherapy treatments improved. This could result in an underestimation of survival when applied to patients treated with current systemic therapies and supportive care. It is important for physicians to have an accurate method to predict survival in patients to ensure that appropriate treatments can be recommended.
Over 170,000 cases of metastatic brain tumors are diagnosed in the United States each year; and the length of survival for patients with brain metastases is often quite limited, ranging from a few weeks to several months.1 The Radiation Therapy Oncology Group (RTOG) Recursive Partitioning Analysis (RPA) and the Graded Prognostic Assessment (GPA) are 2 prognostic indices that have been validated to predict survival and guide the treatment of these patients.2-5 The RPA and GPA indices were formulated by comparing survival to patient and tumor characteristics compiled from RTOG brain metastasis treatment protocols spanning greater than 3 decades. The RPA has 3 classes of patients enumerated as “I”, “II”, and “III,” with class I patients having the longest predicted survival and class III patients having the worst prognosis. The RPA classes are based upon factors that include patient age and Karnofsky Performance Status (KPS) as well as control of the primary tumor and evidence of extra-cranial metastases (Table 1).2 The GPA has 4 classes of patients with a score that may be considered analogous to a grade point average achieved by students in school. The classes are arranged into 4 groupings, which are divided from best to worst prognosis as follows: 3.5 to 4.0, 3.0, 1.5 to 2.5, and 0.0 to 1.0. The GPA employs criteria similar to but slightly different from those used in the RPA, estimating survival by patient age and performance status as well as the number of brain metastases and evidence of extracranial metastases (Table 2).4
Treatment options for patients with brain metastases include surgery, stereotactic radiosurgery (SRS), whole brain radiation therapy (WBRT), supportive measures such as corticosteroids, or a combination of these modalities. The survival of the worst prognosis brain metastases patients treated with WBRT and steroids is estimated by the RPA and GPA tools to be 2.3 months and 2.6 months, respectively.2,4 As noted above, the patient data from which the RPA and GPA indices were created included patients treated on clinical trials. This could have resulted in the selection of patients more fit than average patients and lead to an overestimation of survival when applied to all patients. The clinical trial data used were drawn from over 3 decades, during which supportive care and chemotherapy treatments improved. This could result in an underestimation of survival when applied to patients treated with current systemic therapies and supportive care. It is important for physicians to have an accurate method to predict survival in patients to ensure that appropriate treatments can be recommended.
Measuring the quality of palliative care and supportive oncology: principles and practice
Palliative care quality indicators should be part of oncology performance assessment initiatives. Palliative care programs should also include initiatives to address the overall quality of palliative care issues, such as pain management, in the settings where the programs are located.1 Measuring quality facilitates justifying palliative care initiatives and documenting their impact, targeting quality improvement efforts, monitoring care for deficiencies, and evaluating providers (Table 1). However, measurement in this field is often not straightforward. Potential challenges include defining the population to measure and data sources, collection and analysis, as well as choosing among many potentially relevant issues and quality measures. This article describes an approach to quality measurement in palliative care, beginning with a description of key frameworks to guide the measurement approach. The article also reviews key steps in designing a quality measurement program, which include defining the quality problem and population to measure and choosing domains and specific measures. Finally, the article addresses other key considerations, such as considering unintended consequences and using data for quality improvement.
Frameworks for evaluating quality
The Donabedian framework of structure (stable elements of the health care system), process (what health care services are provided), and outcome (end results for the patient and family) can be
applied to relevant domains to guide evaluation design (Table 2).2-8 Key structural elements may include characteristics of programs (eg, palliative clinic availability), providers (eg, multidisciplinary members of the palliative care team), and tools (eg, do-not-resuscitate policies). Processes may include technical aspects of care, such as appropriate prescribing and interpersonal aspects of care (eg, coordination among providers). Outcomes may include patient quality of life or symptoms, perceptions of care, or caregiver outcomes such as burden. Outcomes may also be categorized as overuse (eg, use of chemotherapy at the end of life compared to national benchmarks), underuse (eg, lower rates of hospice care or use of antinausea drugs), or appropriateness of care (eg, accurately documenting patients’ preferences for care).
Palliative care quality indicators should be part of oncology performance assessment initiatives. Palliative care programs should also include initiatives to address the overall quality of palliative care issues, such as pain management, in the settings where the programs are located.1 Measuring quality facilitates justifying palliative care initiatives and documenting their impact, targeting quality improvement efforts, monitoring care for deficiencies, and evaluating providers (Table 1). However, measurement in this field is often not straightforward. Potential challenges include defining the population to measure and data sources, collection and analysis, as well as choosing among many potentially relevant issues and quality measures. This article describes an approach to quality measurement in palliative care, beginning with a description of key frameworks to guide the measurement approach. The article also reviews key steps in designing a quality measurement program, which include defining the quality problem and population to measure and choosing domains and specific measures. Finally, the article addresses other key considerations, such as considering unintended consequences and using data for quality improvement.
Frameworks for evaluating quality
The Donabedian framework of structure (stable elements of the health care system), process (what health care services are provided), and outcome (end results for the patient and family) can be
applied to relevant domains to guide evaluation design (Table 2).2-8 Key structural elements may include characteristics of programs (eg, palliative clinic availability), providers (eg, multidisciplinary members of the palliative care team), and tools (eg, do-not-resuscitate policies). Processes may include technical aspects of care, such as appropriate prescribing and interpersonal aspects of care (eg, coordination among providers). Outcomes may include patient quality of life or symptoms, perceptions of care, or caregiver outcomes such as burden. Outcomes may also be categorized as overuse (eg, use of chemotherapy at the end of life compared to national benchmarks), underuse (eg, lower rates of hospice care or use of antinausea drugs), or appropriateness of care (eg, accurately documenting patients’ preferences for care).
Palliative care quality indicators should be part of oncology performance assessment initiatives. Palliative care programs should also include initiatives to address the overall quality of palliative care issues, such as pain management, in the settings where the programs are located.1 Measuring quality facilitates justifying palliative care initiatives and documenting their impact, targeting quality improvement efforts, monitoring care for deficiencies, and evaluating providers (Table 1). However, measurement in this field is often not straightforward. Potential challenges include defining the population to measure and data sources, collection and analysis, as well as choosing among many potentially relevant issues and quality measures. This article describes an approach to quality measurement in palliative care, beginning with a description of key frameworks to guide the measurement approach. The article also reviews key steps in designing a quality measurement program, which include defining the quality problem and population to measure and choosing domains and specific measures. Finally, the article addresses other key considerations, such as considering unintended consequences and using data for quality improvement.
Frameworks for evaluating quality
The Donabedian framework of structure (stable elements of the health care system), process (what health care services are provided), and outcome (end results for the patient and family) can be
applied to relevant domains to guide evaluation design (Table 2).2-8 Key structural elements may include characteristics of programs (eg, palliative clinic availability), providers (eg, multidisciplinary members of the palliative care team), and tools (eg, do-not-resuscitate policies). Processes may include technical aspects of care, such as appropriate prescribing and interpersonal aspects of care (eg, coordination among providers). Outcomes may include patient quality of life or symptoms, perceptions of care, or caregiver outcomes such as burden. Outcomes may also be categorized as overuse (eg, use of chemotherapy at the end of life compared to national benchmarks), underuse (eg, lower rates of hospice care or use of antinausea drugs), or appropriateness of care (eg, accurately documenting patients’ preferences for care).
The FREEDOM trial
To the Editor: We would like to raise the following points about the paper by Dr. Aggarwal et al1 interpreting the Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial.2
The patients enrolled in the FREEDOM trial do not in our opinion completely reflect the real patients that we meet in our daily “real-world” practice.2 The patients in the FREEDOM trial did not have a high-risk profile. Rather, the mean European System for Cardiac Operative Risk Evaluation score (EuroSCORE) was 2.7 ± 2.4 in the percutaneous coronary intervention (PCI) group and 2.8 ± 2.5 in the coronary artery bypass grafting group—whereas a score of 5 or more on the EuroSCORE is associated with decreased rates of survival.2
Furthermore, patients with left main coronary artery stenosis were completely excluded from the FREEDOM trial,2 but this type of stenosis, with different grades, is found in about 30% of diabetic patients with multivessel coronary artery disease, a fact that may significantly influence the decision regarding the revascularization strategy (bypass grafting or PCI), especially in a clinical setting such as acute coronary syndrome.3–5
In addition, the authors did not clearly highlight that diabetes mellitus is an independent risk factor for coronary lesion progression, coronary bypass graft occlusion, and cardiac mortality after bypass grafting surgery.6–8 Clinical outcomes after bypass grafting in diabetic patients are worse than in nondiabetic patients; diabetic patients have higher rates of morbidity (deep sternal instability, wound infection, stroke, renal dysfunction, and respiratory problems), longer intensive care unit and hospital stays, and poorer postoperative physical functioning and quality of life.6–8
The authors correctly explain the reasons for the superiority of coronary artery bypass grafting vs PCI in diabetic patients, either by the ability to achieve complete revascularization or by using more arterial grafts, and especially the left internal thoracic artery.1 However, clarifying details on the strategy of revascularization in the FREEDOM trial are scarcely provided.2 All we know from the provided details in this regard is that “for CABG surgery, arterial revascularization was encouraged” and 94.4% of the patients undergoing bypass grafting received left internal thoracic artery grafts.2
In addition, whereas off-pump coronary artery bypass grafting surgery is superior to conventional bypass grafting in terms of lower rates of death and major adverse cardiac and cerebrovascular events in diabetic patients with multivessel coronary artery disease,3 only 165 (18.5%) of the 893 patients who underwent bypass grafting in the FREEDOM trial underwent an off-pump procedure.2,3
Therefore, all these considerations should be taken into account as the physician team discusses the therapeutic options (PCI and bypass grafting surgery) with diabetic patients who have multivessel coronary artery disease.
- Aggarwal B, Goel S, Sabik JF, Shishehbor MH. The FREEDOM trial: in appropriate patients with diabetes and multivessel coronary artery disease, CABG beats PCI. Cleve Clin J Med 2013; 80:515–523.
- Farkouh ME, Domanski M, Sleeper LA, et al; FREEDOM Trial Investigators. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Emmert MY, Salzberg SP, Seifert B, et al. Is off-pump superior to conventional coronary artery bypass grafting in diabetic patients with multivessel disease? Eur J Cardiothorac Surg 2011; 40:233–239.
- Perrier S, Kindo M, Gerelli S, Mazzucotelli JP. Coronary artery bypass grafting or percutaneous revascularization in acute myocardial infarction? Interact Cardiovasc Thorac Surg 2013 Aug 20 [Epub ahead of print]
- Sabik JF, Blackstone EH, Firstenberg M, Lytle BW. A benchmark for evaluating innovative treatment of left main coronary disease. Circulation 2007; 116(11 Suppl):I232–I239.
- Lu JC, Grayson AD, Jha P, Srinivasan AK, Fabri BM. Risk factors for sternal wound infection and mid-term survival following coronary artery bypass surgery. Euro J Cardiothorac Surg 2003; 23:943–949.
- Ji Q, Mei Y, Wang X, Feng J, Cai J, Sun Y. Impact of diabetes mellitus on old patients undergoing coronary artery bypass grafting. Int Heart J 2009; 50:693–700.
- Stevens LM, Carrier M, Perrault LP, et al. Influence of diabetes and bilateral internal thoracic artery grafts on long-term outcome for multivessel coronary artery bypass grafting. Eur J Cardiothorac Surg 2005; 27:281–288.
To the Editor: We would like to raise the following points about the paper by Dr. Aggarwal et al1 interpreting the Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial.2
The patients enrolled in the FREEDOM trial do not in our opinion completely reflect the real patients that we meet in our daily “real-world” practice.2 The patients in the FREEDOM trial did not have a high-risk profile. Rather, the mean European System for Cardiac Operative Risk Evaluation score (EuroSCORE) was 2.7 ± 2.4 in the percutaneous coronary intervention (PCI) group and 2.8 ± 2.5 in the coronary artery bypass grafting group—whereas a score of 5 or more on the EuroSCORE is associated with decreased rates of survival.2
Furthermore, patients with left main coronary artery stenosis were completely excluded from the FREEDOM trial,2 but this type of stenosis, with different grades, is found in about 30% of diabetic patients with multivessel coronary artery disease, a fact that may significantly influence the decision regarding the revascularization strategy (bypass grafting or PCI), especially in a clinical setting such as acute coronary syndrome.3–5
In addition, the authors did not clearly highlight that diabetes mellitus is an independent risk factor for coronary lesion progression, coronary bypass graft occlusion, and cardiac mortality after bypass grafting surgery.6–8 Clinical outcomes after bypass grafting in diabetic patients are worse than in nondiabetic patients; diabetic patients have higher rates of morbidity (deep sternal instability, wound infection, stroke, renal dysfunction, and respiratory problems), longer intensive care unit and hospital stays, and poorer postoperative physical functioning and quality of life.6–8
The authors correctly explain the reasons for the superiority of coronary artery bypass grafting vs PCI in diabetic patients, either by the ability to achieve complete revascularization or by using more arterial grafts, and especially the left internal thoracic artery.1 However, clarifying details on the strategy of revascularization in the FREEDOM trial are scarcely provided.2 All we know from the provided details in this regard is that “for CABG surgery, arterial revascularization was encouraged” and 94.4% of the patients undergoing bypass grafting received left internal thoracic artery grafts.2
In addition, whereas off-pump coronary artery bypass grafting surgery is superior to conventional bypass grafting in terms of lower rates of death and major adverse cardiac and cerebrovascular events in diabetic patients with multivessel coronary artery disease,3 only 165 (18.5%) of the 893 patients who underwent bypass grafting in the FREEDOM trial underwent an off-pump procedure.2,3
Therefore, all these considerations should be taken into account as the physician team discusses the therapeutic options (PCI and bypass grafting surgery) with diabetic patients who have multivessel coronary artery disease.
To the Editor: We would like to raise the following points about the paper by Dr. Aggarwal et al1 interpreting the Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial.2
The patients enrolled in the FREEDOM trial do not in our opinion completely reflect the real patients that we meet in our daily “real-world” practice.2 The patients in the FREEDOM trial did not have a high-risk profile. Rather, the mean European System for Cardiac Operative Risk Evaluation score (EuroSCORE) was 2.7 ± 2.4 in the percutaneous coronary intervention (PCI) group and 2.8 ± 2.5 in the coronary artery bypass grafting group—whereas a score of 5 or more on the EuroSCORE is associated with decreased rates of survival.2
Furthermore, patients with left main coronary artery stenosis were completely excluded from the FREEDOM trial,2 but this type of stenosis, with different grades, is found in about 30% of diabetic patients with multivessel coronary artery disease, a fact that may significantly influence the decision regarding the revascularization strategy (bypass grafting or PCI), especially in a clinical setting such as acute coronary syndrome.3–5
In addition, the authors did not clearly highlight that diabetes mellitus is an independent risk factor for coronary lesion progression, coronary bypass graft occlusion, and cardiac mortality after bypass grafting surgery.6–8 Clinical outcomes after bypass grafting in diabetic patients are worse than in nondiabetic patients; diabetic patients have higher rates of morbidity (deep sternal instability, wound infection, stroke, renal dysfunction, and respiratory problems), longer intensive care unit and hospital stays, and poorer postoperative physical functioning and quality of life.6–8
The authors correctly explain the reasons for the superiority of coronary artery bypass grafting vs PCI in diabetic patients, either by the ability to achieve complete revascularization or by using more arterial grafts, and especially the left internal thoracic artery.1 However, clarifying details on the strategy of revascularization in the FREEDOM trial are scarcely provided.2 All we know from the provided details in this regard is that “for CABG surgery, arterial revascularization was encouraged” and 94.4% of the patients undergoing bypass grafting received left internal thoracic artery grafts.2
In addition, whereas off-pump coronary artery bypass grafting surgery is superior to conventional bypass grafting in terms of lower rates of death and major adverse cardiac and cerebrovascular events in diabetic patients with multivessel coronary artery disease,3 only 165 (18.5%) of the 893 patients who underwent bypass grafting in the FREEDOM trial underwent an off-pump procedure.2,3
Therefore, all these considerations should be taken into account as the physician team discusses the therapeutic options (PCI and bypass grafting surgery) with diabetic patients who have multivessel coronary artery disease.
- Aggarwal B, Goel S, Sabik JF, Shishehbor MH. The FREEDOM trial: in appropriate patients with diabetes and multivessel coronary artery disease, CABG beats PCI. Cleve Clin J Med 2013; 80:515–523.
- Farkouh ME, Domanski M, Sleeper LA, et al; FREEDOM Trial Investigators. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Emmert MY, Salzberg SP, Seifert B, et al. Is off-pump superior to conventional coronary artery bypass grafting in diabetic patients with multivessel disease? Eur J Cardiothorac Surg 2011; 40:233–239.
- Perrier S, Kindo M, Gerelli S, Mazzucotelli JP. Coronary artery bypass grafting or percutaneous revascularization in acute myocardial infarction? Interact Cardiovasc Thorac Surg 2013 Aug 20 [Epub ahead of print]
- Sabik JF, Blackstone EH, Firstenberg M, Lytle BW. A benchmark for evaluating innovative treatment of left main coronary disease. Circulation 2007; 116(11 Suppl):I232–I239.
- Lu JC, Grayson AD, Jha P, Srinivasan AK, Fabri BM. Risk factors for sternal wound infection and mid-term survival following coronary artery bypass surgery. Euro J Cardiothorac Surg 2003; 23:943–949.
- Ji Q, Mei Y, Wang X, Feng J, Cai J, Sun Y. Impact of diabetes mellitus on old patients undergoing coronary artery bypass grafting. Int Heart J 2009; 50:693–700.
- Stevens LM, Carrier M, Perrault LP, et al. Influence of diabetes and bilateral internal thoracic artery grafts on long-term outcome for multivessel coronary artery bypass grafting. Eur J Cardiothorac Surg 2005; 27:281–288.
- Aggarwal B, Goel S, Sabik JF, Shishehbor MH. The FREEDOM trial: in appropriate patients with diabetes and multivessel coronary artery disease, CABG beats PCI. Cleve Clin J Med 2013; 80:515–523.
- Farkouh ME, Domanski M, Sleeper LA, et al; FREEDOM Trial Investigators. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Emmert MY, Salzberg SP, Seifert B, et al. Is off-pump superior to conventional coronary artery bypass grafting in diabetic patients with multivessel disease? Eur J Cardiothorac Surg 2011; 40:233–239.
- Perrier S, Kindo M, Gerelli S, Mazzucotelli JP. Coronary artery bypass grafting or percutaneous revascularization in acute myocardial infarction? Interact Cardiovasc Thorac Surg 2013 Aug 20 [Epub ahead of print]
- Sabik JF, Blackstone EH, Firstenberg M, Lytle BW. A benchmark for evaluating innovative treatment of left main coronary disease. Circulation 2007; 116(11 Suppl):I232–I239.
- Lu JC, Grayson AD, Jha P, Srinivasan AK, Fabri BM. Risk factors for sternal wound infection and mid-term survival following coronary artery bypass surgery. Euro J Cardiothorac Surg 2003; 23:943–949.
- Ji Q, Mei Y, Wang X, Feng J, Cai J, Sun Y. Impact of diabetes mellitus on old patients undergoing coronary artery bypass grafting. Int Heart J 2009; 50:693–700.
- Stevens LM, Carrier M, Perrault LP, et al. Influence of diabetes and bilateral internal thoracic artery grafts on long-term outcome for multivessel coronary artery bypass grafting. Eur J Cardiothorac Surg 2005; 27:281–288.
In reply: The FREEDOM trial
In Reply: We appreciate the comments of Dr. Saeed and colleagues. As stated in our article, given that the patients included in the FREEDOM trial represent a select group with diabetes and multivessel coronary artery disease, they may not represent all patients encountered in a real-world setting. We highlighted that only 10% of the patients screened were included for randomization, which limits the generalizability of the results. Also, the overall patient population may not be at high risk, as evidenced by low mean EuroSCORE and SYNTAX scores and by the low proportion of patients with ejection fractions less than 40%. However, patients with left main coronary artery disease (even without diabetes) have been shown to have better outcomes with coronary artery bypass grafting than with PCI, although a head-to-head trial in a diabetic subgroup is currently not available.1,2 In addition, it is important to realize that the FREEDOM trial deals with stable angina; therefore, the results may not extend to patients with acute coronary syndrome wherein primary PCI remains the most feasible option in most cases.
Diabetes mellitus is independently associated with complex, accelerated, and multifocal coronary artery disease. Therefore, outcomes after revascularization (with bypass grafting or PCI) are worse in diabetic patients than in those without diabetes. However, this association does not prove the superiority of PCI over bypass grafting.
As we stated in our paper, the FREEDOM trial did not clearly define the strategy for arterial grafts in patients undergoing bypass grafting. The mean number of coronary lesions in the bypass grafting group was high (mean = 5.74), but the average number of grafts used was only 2.9, and data were not provided on the use of sequential grafting and multiple arterial conduits. Lastly, it is true that the FREEDOM trial had relatively fewer patients (18.5%) that underwent off-pump bypass grafting surgery; however, this approach has never been shown to be superior in large randomized trials.3,4
In conclusion, no randomized trial should replace clinical judgment to define the targeted revascularization strategy for an individual patient. Rather, results from the FREEDOM trial should help support clinical decision-making in the context of the patient and the institution.
- Hlatky MA, Boothroyd DB, Bravata DM, et al. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet 2009; 373:1190–1197.
- Banning AP, Westaby S, Morice MC, et al. Diabetic and nondiabetic patients with left main and/or 3-vessel coronary artery disease: comparison of outcomes with cardiac surgery and paclitaxel-eluting stents. J Am Coll Cardiol 2010; 55:1067–1075.
- Diegeler A, Börgermann J, Kappert U, et al. Off-pump versus on-pump coronary-artery bypass grafting in elderly patients. N Engl J Med 2013; 368:1189–1198.
- Lamy A, Devereaux PJ, Prabhakaran D, et al; CORONARY Investigators. Effects of off-pump and on-pump coronary-artery bypass grafting at 1 year. N Engl J Med 2013; 368:1179–1188.
In Reply: We appreciate the comments of Dr. Saeed and colleagues. As stated in our article, given that the patients included in the FREEDOM trial represent a select group with diabetes and multivessel coronary artery disease, they may not represent all patients encountered in a real-world setting. We highlighted that only 10% of the patients screened were included for randomization, which limits the generalizability of the results. Also, the overall patient population may not be at high risk, as evidenced by low mean EuroSCORE and SYNTAX scores and by the low proportion of patients with ejection fractions less than 40%. However, patients with left main coronary artery disease (even without diabetes) have been shown to have better outcomes with coronary artery bypass grafting than with PCI, although a head-to-head trial in a diabetic subgroup is currently not available.1,2 In addition, it is important to realize that the FREEDOM trial deals with stable angina; therefore, the results may not extend to patients with acute coronary syndrome wherein primary PCI remains the most feasible option in most cases.
Diabetes mellitus is independently associated with complex, accelerated, and multifocal coronary artery disease. Therefore, outcomes after revascularization (with bypass grafting or PCI) are worse in diabetic patients than in those without diabetes. However, this association does not prove the superiority of PCI over bypass grafting.
As we stated in our paper, the FREEDOM trial did not clearly define the strategy for arterial grafts in patients undergoing bypass grafting. The mean number of coronary lesions in the bypass grafting group was high (mean = 5.74), but the average number of grafts used was only 2.9, and data were not provided on the use of sequential grafting and multiple arterial conduits. Lastly, it is true that the FREEDOM trial had relatively fewer patients (18.5%) that underwent off-pump bypass grafting surgery; however, this approach has never been shown to be superior in large randomized trials.3,4
In conclusion, no randomized trial should replace clinical judgment to define the targeted revascularization strategy for an individual patient. Rather, results from the FREEDOM trial should help support clinical decision-making in the context of the patient and the institution.
In Reply: We appreciate the comments of Dr. Saeed and colleagues. As stated in our article, given that the patients included in the FREEDOM trial represent a select group with diabetes and multivessel coronary artery disease, they may not represent all patients encountered in a real-world setting. We highlighted that only 10% of the patients screened were included for randomization, which limits the generalizability of the results. Also, the overall patient population may not be at high risk, as evidenced by low mean EuroSCORE and SYNTAX scores and by the low proportion of patients with ejection fractions less than 40%. However, patients with left main coronary artery disease (even without diabetes) have been shown to have better outcomes with coronary artery bypass grafting than with PCI, although a head-to-head trial in a diabetic subgroup is currently not available.1,2 In addition, it is important to realize that the FREEDOM trial deals with stable angina; therefore, the results may not extend to patients with acute coronary syndrome wherein primary PCI remains the most feasible option in most cases.
Diabetes mellitus is independently associated with complex, accelerated, and multifocal coronary artery disease. Therefore, outcomes after revascularization (with bypass grafting or PCI) are worse in diabetic patients than in those without diabetes. However, this association does not prove the superiority of PCI over bypass grafting.
As we stated in our paper, the FREEDOM trial did not clearly define the strategy for arterial grafts in patients undergoing bypass grafting. The mean number of coronary lesions in the bypass grafting group was high (mean = 5.74), but the average number of grafts used was only 2.9, and data were not provided on the use of sequential grafting and multiple arterial conduits. Lastly, it is true that the FREEDOM trial had relatively fewer patients (18.5%) that underwent off-pump bypass grafting surgery; however, this approach has never been shown to be superior in large randomized trials.3,4
In conclusion, no randomized trial should replace clinical judgment to define the targeted revascularization strategy for an individual patient. Rather, results from the FREEDOM trial should help support clinical decision-making in the context of the patient and the institution.
- Hlatky MA, Boothroyd DB, Bravata DM, et al. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet 2009; 373:1190–1197.
- Banning AP, Westaby S, Morice MC, et al. Diabetic and nondiabetic patients with left main and/or 3-vessel coronary artery disease: comparison of outcomes with cardiac surgery and paclitaxel-eluting stents. J Am Coll Cardiol 2010; 55:1067–1075.
- Diegeler A, Börgermann J, Kappert U, et al. Off-pump versus on-pump coronary-artery bypass grafting in elderly patients. N Engl J Med 2013; 368:1189–1198.
- Lamy A, Devereaux PJ, Prabhakaran D, et al; CORONARY Investigators. Effects of off-pump and on-pump coronary-artery bypass grafting at 1 year. N Engl J Med 2013; 368:1179–1188.
- Hlatky MA, Boothroyd DB, Bravata DM, et al. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet 2009; 373:1190–1197.
- Banning AP, Westaby S, Morice MC, et al. Diabetic and nondiabetic patients with left main and/or 3-vessel coronary artery disease: comparison of outcomes with cardiac surgery and paclitaxel-eluting stents. J Am Coll Cardiol 2010; 55:1067–1075.
- Diegeler A, Börgermann J, Kappert U, et al. Off-pump versus on-pump coronary-artery bypass grafting in elderly patients. N Engl J Med 2013; 368:1189–1198.
- Lamy A, Devereaux PJ, Prabhakaran D, et al; CORONARY Investigators. Effects of off-pump and on-pump coronary-artery bypass grafting at 1 year. N Engl J Med 2013; 368:1179–1188.
Electronic health records
To the Editor: The July 2013 Cleveland Clinic Journal of Medicine includes timely articles addressing the problems of electronic health records (EHRs). At least to this reader, there is little that is surprising in the observations.
A common inside joke among programmers, sometimes displayed at one’s cubicle, is: “Fast, good, or cheap (pick two).” In other words, there is always a compromise to be had between a good product and one that is punched out on a given timetable and inexpensive. Economists call this the “second best.”
Any truly great software product accomplishes three goals. First, it allows the user to do everything previously doable at least as well or as easily as before. Second, it eliminates drudgery. And third, ideally, it provides new functionality, which previously was difficult or impossible to accomplish or to afford.
The reality is that much software is sold on the basis of the third goal, whereas goal number 1 and sometimes goal number 2 get short shrift. And for EHRs in particular, it is a fallacy for physicians to think that EHRs were brought out primarily for their benefit rather than for the benefit of the front office. This was all the more true a decade ago, when very few physicians were employed by hospitals. Thus, if the physician’s workload was increased because of the hospital’s choice of EHR, the hospital felt no financial pain. With greater reliance on an employment model, we can hope that hospitals will recognize that physicians should not be turned into very expensive secretaries.
To the Editor: The July 2013 Cleveland Clinic Journal of Medicine includes timely articles addressing the problems of electronic health records (EHRs). At least to this reader, there is little that is surprising in the observations.
A common inside joke among programmers, sometimes displayed at one’s cubicle, is: “Fast, good, or cheap (pick two).” In other words, there is always a compromise to be had between a good product and one that is punched out on a given timetable and inexpensive. Economists call this the “second best.”
Any truly great software product accomplishes three goals. First, it allows the user to do everything previously doable at least as well or as easily as before. Second, it eliminates drudgery. And third, ideally, it provides new functionality, which previously was difficult or impossible to accomplish or to afford.
The reality is that much software is sold on the basis of the third goal, whereas goal number 1 and sometimes goal number 2 get short shrift. And for EHRs in particular, it is a fallacy for physicians to think that EHRs were brought out primarily for their benefit rather than for the benefit of the front office. This was all the more true a decade ago, when very few physicians were employed by hospitals. Thus, if the physician’s workload was increased because of the hospital’s choice of EHR, the hospital felt no financial pain. With greater reliance on an employment model, we can hope that hospitals will recognize that physicians should not be turned into very expensive secretaries.
To the Editor: The July 2013 Cleveland Clinic Journal of Medicine includes timely articles addressing the problems of electronic health records (EHRs). At least to this reader, there is little that is surprising in the observations.
A common inside joke among programmers, sometimes displayed at one’s cubicle, is: “Fast, good, or cheap (pick two).” In other words, there is always a compromise to be had between a good product and one that is punched out on a given timetable and inexpensive. Economists call this the “second best.”
Any truly great software product accomplishes three goals. First, it allows the user to do everything previously doable at least as well or as easily as before. Second, it eliminates drudgery. And third, ideally, it provides new functionality, which previously was difficult or impossible to accomplish or to afford.
The reality is that much software is sold on the basis of the third goal, whereas goal number 1 and sometimes goal number 2 get short shrift. And for EHRs in particular, it is a fallacy for physicians to think that EHRs were brought out primarily for their benefit rather than for the benefit of the front office. This was all the more true a decade ago, when very few physicians were employed by hospitals. Thus, if the physician’s workload was increased because of the hospital’s choice of EHR, the hospital felt no financial pain. With greater reliance on an employment model, we can hope that hospitals will recognize that physicians should not be turned into very expensive secretaries.