User login
Hospital Safety Grade
The Institute of Medicine (IOM) reported over a decade ago that between 44,000 and 98,000 deaths occurred every year due to preventable medical errors.[1] The report sparked an intense interest in identifying, measuring, and reporting hospital performance in patient safety.[2] The report also sparked the implementation of many initiatives aiming to improve patient safety.[3] Despite these efforts, there is still much room for improvement in the area of patient safety.[4] As the public has become more aware of patient safety issues, there has been an increased demand for information on hospital safety. The Leapfrog Group, a leading organization that examines and reports on hospital performance in patient safety, cites the IOM report as providing the focus that their newly formed organization required.[5]
Using 26 national measures of safety, The Leapfrog Group calculates a numeric Hospital Safety Score for over 2,600 acute care hospitals in the United States.[6] The primary data used to calculate this score are collected through the Leapfrog Hospital Survey, the Agency for Healthcare Research and Quality, the Centers for Disease Control and Prevention, and the Centers for Medicare and Medicaid Services (CMS). The American Hospital Association's (AHA) Annual Survey is used as a secondary data source as necessary. The Leapfrog Group conducts the survey annually, and substantial efforts are put forth to invite hospital administrators to participate in the survey. Participation in the Leapfrog survey is optional and free of charge.
Leapfrog recently moved a step further in their evaluation of hospital safety by releasing the Hidden Surcharge Calculator to enable employers to estimate the hidden surcharge they pay for their employees and dependents because of hospital errors.[7] The calculation depends largely on the letter grade (AF) that the hospital received from Leapfrog's Hospital Safety Score. For example, Leapfrog estimated a commercially insured patient admitted to a hospital with a grade of C or lower would incur $1845 additional cost per admission than if the same patient was admitted to a hospital with a grade of A.[7] The Leapfrog group encourages employers and payers to use this information to adjust benefits structures so that employees are discouraged from using hospitals that receive lower hospital safety scores. Leapfrog also encourages payers to negotiate lower reimbursement rates for hospitals with lower hospital safety scores.
The accuracy of Leapfrog's hospital safety grades warrants attention because of the methodology used to score hospitals that do not participate in the Leapfrog Survey. One common barrier that prevents hospitals from participating is the amount of effort required to complete the annual survey, including extensive inputs from hospital executives and staff. According to Leapfrog, 4 to 6 days are required for a hospital to compile the necessary survey data.[8] Leapfrog estimates a 90‐minute commitment for the hospital chief executive officer or designated administrator to enter the information into the online questionnaire. This is a significant commitment for many hospitals. As a result, among the approximately 2600 acute care hospitals covered by Leapfrog's 2012 to 2013 safety grading, only 1100 (or 42.3%) actually participated in the Leapfrog hospital survey. This limits Leapfrog's ability to provide accurate scores and assign fair safety grades to many hospitals.
METHODS
Leapfrog Hospital Safety Score
Leapfrog's designated Hospital Safety Score is determined by 26 measures. The set of safety measures and their relative weight are determined by a 9‐member Leapfrog expert panel of patient safety experts.[9] The hospital safety score is divided equally into 2 domains of safety measures: process/structural and outcomes.[6] The process measures represent how often a hospital gives patients recommended treatment for a given medical condition or procedure, whereas structural measures represent the environment in which patients receive care.[10] The process/structural measures include computerized physician order entry (CPOE), intensive care unit (ICU) physician staffing (IPS), 8 Leapfrog safety practices, and 5 surgical care improvement project measures. The outcome measures represent what happens to a patient while receiving care. The outcomes domain includes 5 hospital‐acquired conditions and 6 patient safety indicators. A score is assigned and weighted for each measure. All scores are then summed to produce a single number denoting the safety performance score received by each hospital. Every hospital is assigned 1 of 5 letter grades depending on how the hospital's numeric score stands in safety performance relative to all other hospitals. The letter grade A denotes the best hospital safety performance, followed in order by letter grades B through F. The cutoffs for A and B grades represent the first and second quartile of hospital safety scores. The cutoff for the C grade represents the hospitals that were between the mean and 1.5 standard deviations below the mean. The cutoff for the D grade represents the hospitals that were between 1.5 and 3.0 standard deviations below the mean. F grades indicate safety scores more than 3.0 standard deviations below the mean.[11]
Nonparticipating Hospitals
The Leapfrog Survey contributes values for 11 of the 26 measures utilized to calculate the Hospital Safety Score. The score of a nonparticipating hospital will not reflect 8 of these 11 measures. For the 3 remaining measures, CPOE, IPS, and central line‐associated blood stream infection, secondary data from the AHA Survey, AHA Information Technology Supplement Survey, and CMS Hospital Compare were used as proxies, respectively (Table 1). The use of a proxy effectively limits the maximum score attainable by nonparticipating hospitals. For instance, 2 of these 3 measures, CPOE and IPS, are calculated on different scales depending on hospital survey participation status. For CPOE, nonparticipating hospitals are limited to a maximum of 65 out of 100 points; for IPS, they are limited to 85 out of 100 points.[6] Because the actual weight for each of these proxy measures is increased for nonparticipating hospitals in the calculation of the final score, their effective impact is exacerbated. The weight of CPOE and IPS measures in the overall weighted score are increased from 6.1% and 7.0% to 11.0% and 12.6%, respectively.
| Participants | Nonparticipants | |
|---|---|---|
| ||
| Process/structural measures (50% of score) | ||
| Computerized Physician Order Entry | 2012 Leapfrog Hospital Survey | 2010 IT Supplement (AHA) |
| ICU Physician Staffing (IPS) | 2012 Leapfrog Hospital Survey | 2011 AHA Annual Survey |
| Safe Practice 1: Leadership Structures and Systems | 2012 Leapfrog Hospital Survey | Excluded |
| Safe Practice 2: Culture Measurement, Feedback, and Intervention | 2012 Leapfrog Hospital Survey | Excluded |
| Safe Practice 3: Teamwork Training and Skill Building | 2012 Leapfrog Hospital Survey | Excluded |
| Safe Practice 4: Identification and Mitigation of Risks and Hazards | 2012 Leapfrog Hospital Survey | Excluded |
| Safe Practice 9: Nursing Workforce | 2012 Leapfrog Hospital Survey | Excluded |
| Safe Practice 17: Medication Reconciliation | 2012 Leapfrog Hospital Survey | Excluded |
| Safe Practice 19: Hand Hygiene | 2012 Leapfrog Hospital Survey | Excluded |
| Safe Practice 23: Care of the Ventilated Patient | 2012 Leapfrog Hospital Survey | Excluded |
| SCIP‐INF‐1: Antibiotic Within 1 Hour | CMS Hospital Compare | CMS Hospital Compare |
| SCIP‐INF‐2: Antibiotic Selection | CMS Hospital Compare | CMS Hospital Compare |
| SCIP‐INF‐3: Antibiotic Discontinued After 24 Hours | CMS Hospital Compare | CMS Hospital Compare |
| SCIP‐INF‐9: Catheter Removal | CMS Hospital Compare | CMS Hospital Compare |
| SCIP‐VTE‐2: VTE Prophylaxis | CMS Hospital Compare | CMS Hospital Compare |
| Outcome measures (50% of score) | ||
| HAC: Foreign Object Retained | CMS HACs | CMS HACs |
| HAC: Air Embolism | CMS HACs | CMS HACs |
| HAC: Pressure Ulcers | CMS HACs | CMS HACs |
| HAC: Falls and Trauma | CMS HACs | CMS HACs |
| Central Line‐Associated Bloodstream Infection | 2012 Leapfrog Hospital Survey | CMS HAIs |
| PSI 4: Death Among Surgical Inpatients With Serious Treatable Complications | CMS Hospital Compare | CMS Hospital Compare |
| PSI 6: Collapsed Lung Due to Medical Treatment | CMS Hospital Compare | CMS Hospital Compare |
| PSI 12: Postoperative PE/DVT | CMS Hospital Compare | CMS Hospital Compare |
| PSI 14: Wounds Split Open After Surgery | CMS Hospital Compare | CMS Hospital Compare |
| PSI 15: Accidental Cuts or Tears From Medical Treatment | CMS Hospital Compare | CMS Hospital Compare |
Study Sample
We examined the Leapfrog safety grades for top hospitals," as ranked by U.S. News & World Report. Included in this sample were the top 15 ranked hospitals in each of the specialties, excluding those specialties whose ranks are based solely on reputation. Hospitals ranked in more than 1 specialty were only included once in the sample. This resulted in a final study sample of 35 top hospitals. Eighteen of these top hospitals participated in the Leapfrog Survey, whereas 17 did not.
Utilizing Leapfrog's spring 2013 methodology,[6] the Hospital Safety Scores for the 35 top hospitals were calculated. The mean safety score for the 18 participating hospitals was then compared with the mean score for the 17 nonparticipating hospitals. Finally, the safety scores for each of the 17 nonparticipating hospitals, listed in Table 2, were estimated as if they had participated in the Leapfrog Survey. To do this, we assumed that the 17 nonparticipating hospitals could each earn average scores for the CPOE, IPS, and 8 process/structural Leapfrog measures as received by their 18 participating counterparts.
| Participants | Leapfrog Grade | Nonparticipants | Leapfrog Grade |
|---|---|---|---|
| |||
| Brigham and Women's Hospital, Boston, MA | A | Abbott Northwestern Hospital, Minneapolis, MN | A |
| Duke University Medical Center, Durham, NC | A | Barnes‐Jewish Hospital/Washington University, St. Louis, MO | C |
| Massachusetts General Hospital, Boston, MA | B | Baylor University Medical Center, Dallas, TX | C |
| Mayo Clinic, Rochester, MN | A | Cedars‐Sinai Medical Center, Los Angeles, CA | C |
| Methodist Hospital, Houston, TX | A | Cleveland Clinic, Cleveland, OH | C |
| Northwestern Memorial Hospital, Chicago, IL | A | Florida Hospital, Orlando, FL | B |
| Ronald Reagan UCLA Medical Center, Los Angeles, CA | D | Hospital of the University of Pennsylvania, Philadelphia, PA | A |
| Rush University Medical Center, Chicago, IL | A | Indiana University Health, Indianapolis, IN | A |
| St. Francis Hospital, Roslyn, NY | A | Mount Sinai Medical Center, New York, NY | B |
| St. Joseph's Hospital and Medical Center, Phoenix, AZ | B | New York‐Presbyterian Hospital, New York, NY | C |
| Stanford Hospital and Clinics, Stanford, CA | A | NYU Langone Medical Center, New York, NY | A |
| Thomas Jefferson University Hospital, Philadelphia, PA | C | Ochsner Medical Center, New Orleans, LA | A |
| UCSF Medical Center, San Francisco, CA | B | Tampa General Hospital, Tampa, FL | C |
| University Hospitals Case Medical Center, Cleveland, OH | A | University of Iowa Hospitals and Clinics, Iowa City, IA | C |
| University of Michigan Hospitals and Health Centers, Ann Arbor, MI | A | University of Kansas Hospital, Kansas City, KS | A |
| University of Washington Medical Center, Seattle, WA | C | UPMC, Pittsburgh, PA | B |
| Vanderbilt University Medical Center, Nashville, TN | A | Yale‐New Haven Hospital, New Haven, CT | B |
| Wake Forest Baptist Medical Center, Winston‐Salem, NC | A | ||
RESULTS
Out of these 35 top hospitals, those that participated in the Leapfrog Survey generally received higher scores than the nonparticipants (Table 2). The group of participating hospitals received an average grade of A (mean safety score, 3.165; standard error of the mean [SE], 0.081), whereas the nonparticipating hospitals received an average grade of B (mean safety score, 3.012; SE, 0.047). These grades were consistent whether mean or median scores were used.
To further examine the potential bias against nonparticipating hospitals, the safety scores for each of the 17 nonparticipating hospitals were estimated as if they had participated in the Leapfrog Survey. The letter grade of this group increased from an average of B (mean safety score, 3.012; SE, 0.047) to an average of A (mean safety score, 3.216; SE, 0.046). Among the 17 nonparticipating hospitals, 15 showed an increase in safety score, of which 8 hospitals rescored a change in score significant enough to receive 1 or 2 letter grades higher (Table 3). Only 2 hospitals had slight decreases in safety score, without any impact on letter grade.
| Hospital | Original Score (Grade) | Estimated Scorea (Grade) |
|---|---|---|
| ||
| Abbott Northwestern Hospital, Minneapolis, MN | 3.17 (A) | 3.44 (A) |
| Barnes‐Jewish Hospital/Washington University, St. Louis, MO | 2.83 (C) | 3.11 (B) |
| Baylor University Medical Center, Dallas, TX | 2.90 (C) | 3.25 (A) |
| Cedars‐Sinai Medical Center, Los Angeles, CA | 2.92 (C) | 3.30 (A) |
| Cleveland Clinic, Cleveland, OH | 2.76 (C) | 2.78 (C) |
| Florida Hospital, Orlando, FL | 2.98 (B) | 3.38 (A) |
| Hospital of the University of Pennsylvania, Philadelphia, PA | 3.29 (A) | 3.26 (A) |
| Indiana University Health, Indianapolis, IN | 3.14 (A) | 3.37 (A) |
| Mount Sinai Medical Center, New York, NY | 3.01 (B) | 3.02 (B) |
| New York‐Presbyterian Hospital, New York, NY | 2.76 (C) | 3.15 (A) |
| NYU Langone Medical Center, New York, NY | 3.26 (A) | 3.30 (A) |
| Ochsner Medical Center, New Orleans, LA | 3.19 (A) | 3.59 (A) |
| Tampa General Hospital, Tampa, FL | 2.86 (C) | 3.05 (B) |
| University of Iowa Hospitals and Clinics, Iowa City, IA | 2.70 (C) | 3.00 (B) |
| University of Kansas Hospital, Kansas City, KS | 3.29 (A) | 3.35 (A) |
| UPMC, Pittsburgh, PA | 3.04 (B) | 3.24 (A) |
| Yale‐New Haven Hospital, New Haven, CT | 3.10 (B) | 3.08 (B) |
We applied the same methods to test the top 17 Honor Roll Hospitals as designated by US News & World Report; among them, half are participating hospitals and another half nonparticipating hospitals. One hospital, Johns Hopkins Hospital was not scored by Leapfrog because no relevant Medicare data are available for Leapfrog to calculate its safety score. For this reason, Johns Hopkins was excluded from our comparison. The results persist even with this smaller sample of top hospitals. The group of 8 participating hospitals had an average grade of A (mean safety score, 3.145; SE, 0.146), whereas another 8 nonparticipating hospitals received an average grade of B (mean safety score, 3.011; SE, 0.075).
DISCUSSION
The Leapfrog Group's intent to provide patient safety information to patients, physicians, healthcare purchasers, and hospital executives should be commended. However, the current methodology may disadvantage nonparticipating hospitals. The combination of lower maximum scores and increased weight of the CPOE and IPS scores may result in a lower hospital safety score than is justified. Nonparticipating hospitals may also face more intensive pressure from employers and payors to lower their reimbursement rates due to the newly released Leapfrog Hidden Surcharge Calculator.
Leapfrog acknowledges that the more data points a hospital has to be scored on, the better its opportunity to achieve a higher score.[8] This justification may lead to bias against nonparticipating hospitals. On the other hand, it is possible that hospitals with good safety records are more likely to participate in the Leapfrog Survey than those with poorer safety records. Without detailed nonresponse analysis from Leapfrog, it is impossible to know if there is a selection bias. Regardless, the Leapfrog result can subsequently misguide the payment rate negotiation between insurers and hospitals.
With this consideration in mind, Leapfrog should explicitly acknowledge the limitations of its methodology and consider revising it in future studies. For example, Leapfrog could only report on those measures for which there are data available for both participating and nonparticipating hospitals. Pending this revision, every effort must be made to distinguish between participating and nonparticipating hospitals. The outcomes of Leapfrog's hospital safety grades are made available online to consumers without distinguishing between participating and nonparticipating hospitals. The only method to differentiate the categories is to examine the data sources in detail amid a large volume of data. It is unlikely that consumers comparing hospital safety grades will take note of this caveat. Thus, Leapfrog's grading system can drastically misrepresent many nonparticipating hospitals' patient safety performances.
This study of The Leapfrog Group's Hospital Safety Score is not without limitations. The small sample utilized in this study limited the power of statistical testing. The difference in mean scores between participating and nonparticipating hospitals is not statistically significant. However, The Leapfrog Group uses specific numerical cutoff points for each letter grade classification. In this classification system statistical significance is not considered when assigning hospitals with different letter grades. It was clear that nonparticipating hospitals were more likely to receive lower letter grades than participating hospitals.
The small sample also posed challenges when attempting to account for missing data when comparing participating hospitals versus nonparticipating hospitals. Although a multiple imputation approach may have been ideal to address this, the small sample size coupled with the large amount of missing data (58% of hospitals did not participate in the Leapfrog Survey) led us to question the accuracy of this approach in this situation.[12] Instead, a crude, mean imputation approach was utilized, relying on the assumption that nonresponding hospitals had the same mean performance as responding hospitals on those domains where data were missing. In this study, we purposely selected a sample of hospitals from U.S. News & World Report's top hospitals. We believe the mean imputation approach, although not perfect, is appropriate for this sample of hospitals. Future study, however, should examine if hospitals that anticipated lower performance scores would be less likely to participate in the Leapfrog Survey. This would help strengthen Leapfrog's methodology in dealing with nonresponsive hospitals.
ACKNOWLEDGMENTS
Disclosures: Harold Paz is the CEO of Penn State Hershey Medical Center, which did not participate in the Leapfrog Survey. The authors have no financial conflicts of interest to report.
- , , . To Err Is Human: Building a Safer Health System. Washington, DC: National Academy Press; 2000.
- , , , , . The “To Err is Human” report and the patient safety literature. Qual Saf Health Care. 2006;15(3):174–178.
- , . A call to excellence. Health Aff (Millwood). 2003;22(2):113–115.
- US Department of Health and Human Services. Adverse events in hospitals: national incidence among Medicare beneficiaries. Available at: http://oig.hhs.gov/oei/reports/oei‐06‐09‐00090.pdf. Published November 2010. Accessed on August 2, 2013.
- The Leapfrog Group. The Leapfrog Group—fact sheet 2013. Available at: https://leapfroghospitalsurvey.org/web/wp‐content/uploads/Fsleapfrog.pdf. Accessed October 9, 2013.
- The Leapfrog Group. Hospital Safety score scoring methodology. Available at: http://www.hospitalsafetyscore.org/media/file/HospitalSafetyScore_ScoringMethodology_May2013.pdf. Published May 2013. Accessed June 17, 2013.
- The Leapfrog Group. The Hidden Surcharge Americans Pay for Hospital Errors 2013. Available at: http://www.leapfroggroup.org/employers_purchasers/HiddenSurchargeCalculator. Accessed August 2, 2013.
- The Leapfrog Group. 2013 Leapfrog Hospital Survey Reference Book 2013. https://leapfroghospitalsurvey.org/web/wp‐content/uploads/reference.pdf. Published April 1, 2013. Accessed June 17, 2013.
- , , , et al. Safety in numbers: the development of Leapfrog's composite patient safety score for U.S. hospitals [published online ahead of print September 27, 2013]. J Patient Saf. doi: 10.1097/PTS.0b013e3182952644.
- The Leapfrog Group. Measures in detail. Available at: http://www. hospitalsafetyscore.org/about‐the‐score/measures‐in‐detail. Accessed June 17, 2013.
- The Leapfrog Group. Explanation of safety score grades. Available at: http://www.hospitalsafetyscore.org/media/file/ExplanationofSafety ScoreGrades_May2013.pdf. Published May 2013. Accessed June 17, 2013.
- , , , et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393.
The Institute of Medicine (IOM) reported over a decade ago that between 44,000 and 98,000 deaths occurred every year due to preventable medical errors.[1] The report sparked an intense interest in identifying, measuring, and reporting hospital performance in patient safety.[2] The report also sparked the implementation of many initiatives aiming to improve patient safety.[3] Despite these efforts, there is still much room for improvement in the area of patient safety.[4] As the public has become more aware of patient safety issues, there has been an increased demand for information on hospital safety. The Leapfrog Group, a leading organization that examines and reports on hospital performance in patient safety, cites the IOM report as providing the focus that their newly formed organization required.[5]
Using 26 national measures of safety, The Leapfrog Group calculates a numeric Hospital Safety Score for over 2,600 acute care hospitals in the United States.[6] The primary data used to calculate this score are collected through the Leapfrog Hospital Survey, the Agency for Healthcare Research and Quality, the Centers for Disease Control and Prevention, and the Centers for Medicare and Medicaid Services (CMS). The American Hospital Association's (AHA) Annual Survey is used as a secondary data source as necessary. The Leapfrog Group conducts the survey annually, and substantial efforts are put forth to invite hospital administrators to participate in the survey. Participation in the Leapfrog survey is optional and free of charge.
Leapfrog recently moved a step further in their evaluation of hospital safety by releasing the Hidden Surcharge Calculator to enable employers to estimate the hidden surcharge they pay for their employees and dependents because of hospital errors.[7] The calculation depends largely on the letter grade (AF) that the hospital received from Leapfrog's Hospital Safety Score. For example, Leapfrog estimated a commercially insured patient admitted to a hospital with a grade of C or lower would incur $1845 additional cost per admission than if the same patient was admitted to a hospital with a grade of A.[7] The Leapfrog group encourages employers and payers to use this information to adjust benefits structures so that employees are discouraged from using hospitals that receive lower hospital safety scores. Leapfrog also encourages payers to negotiate lower reimbursement rates for hospitals with lower hospital safety scores.
The accuracy of Leapfrog's hospital safety grades warrants attention because of the methodology used to score hospitals that do not participate in the Leapfrog Survey. One common barrier that prevents hospitals from participating is the amount of effort required to complete the annual survey, including extensive inputs from hospital executives and staff. According to Leapfrog, 4 to 6 days are required for a hospital to compile the necessary survey data.[8] Leapfrog estimates a 90‐minute commitment for the hospital chief executive officer or designated administrator to enter the information into the online questionnaire. This is a significant commitment for many hospitals. As a result, among the approximately 2600 acute care hospitals covered by Leapfrog's 2012 to 2013 safety grading, only 1100 (or 42.3%) actually participated in the Leapfrog hospital survey. This limits Leapfrog's ability to provide accurate scores and assign fair safety grades to many hospitals.
METHODS
Leapfrog Hospital Safety Score
Leapfrog's designated Hospital Safety Score is determined by 26 measures. The set of safety measures and their relative weight are determined by a 9‐member Leapfrog expert panel of patient safety experts.[9] The hospital safety score is divided equally into 2 domains of safety measures: process/structural and outcomes.[6] The process measures represent how often a hospital gives patients recommended treatment for a given medical condition or procedure, whereas structural measures represent the environment in which patients receive care.[10] The process/structural measures include computerized physician order entry (CPOE), intensive care unit (ICU) physician staffing (IPS), 8 Leapfrog safety practices, and 5 surgical care improvement project measures. The outcome measures represent what happens to a patient while receiving care. The outcomes domain includes 5 hospital‐acquired conditions and 6 patient safety indicators. A score is assigned and weighted for each measure. All scores are then summed to produce a single number denoting the safety performance score received by each hospital. Every hospital is assigned 1 of 5 letter grades depending on how the hospital's numeric score stands in safety performance relative to all other hospitals. The letter grade A denotes the best hospital safety performance, followed in order by letter grades B through F. The cutoffs for A and B grades represent the first and second quartile of hospital safety scores. The cutoff for the C grade represents the hospitals that were between the mean and 1.5 standard deviations below the mean. The cutoff for the D grade represents the hospitals that were between 1.5 and 3.0 standard deviations below the mean. F grades indicate safety scores more than 3.0 standard deviations below the mean.[11]
Nonparticipating Hospitals
The Leapfrog Survey contributes values for 11 of the 26 measures utilized to calculate the Hospital Safety Score. The score of a nonparticipating hospital will not reflect 8 of these 11 measures. For the 3 remaining measures, CPOE, IPS, and central line‐associated blood stream infection, secondary data from the AHA Survey, AHA Information Technology Supplement Survey, and CMS Hospital Compare were used as proxies, respectively (Table 1). The use of a proxy effectively limits the maximum score attainable by nonparticipating hospitals. For instance, 2 of these 3 measures, CPOE and IPS, are calculated on different scales depending on hospital survey participation status. For CPOE, nonparticipating hospitals are limited to a maximum of 65 out of 100 points; for IPS, they are limited to 85 out of 100 points.[6] Because the actual weight for each of these proxy measures is increased for nonparticipating hospitals in the calculation of the final score, their effective impact is exacerbated. The weight of CPOE and IPS measures in the overall weighted score are increased from 6.1% and 7.0% to 11.0% and 12.6%, respectively.
| Participants | Nonparticipants | |
|---|---|---|
| ||
| Process/structural measures (50% of score) | ||
| Computerized Physician Order Entry | 2012 Leapfrog Hospital Survey | 2010 IT Supplement (AHA) |
| ICU Physician Staffing (IPS) | 2012 Leapfrog Hospital Survey | 2011 AHA Annual Survey |
| Safe Practice 1: Leadership Structures and Systems | 2012 Leapfrog Hospital Survey | Excluded |
| Safe Practice 2: Culture Measurement, Feedback, and Intervention | 2012 Leapfrog Hospital Survey | Excluded |
| Safe Practice 3: Teamwork Training and Skill Building | 2012 Leapfrog Hospital Survey | Excluded |
| Safe Practice 4: Identification and Mitigation of Risks and Hazards | 2012 Leapfrog Hospital Survey | Excluded |
| Safe Practice 9: Nursing Workforce | 2012 Leapfrog Hospital Survey | Excluded |
| Safe Practice 17: Medication Reconciliation | 2012 Leapfrog Hospital Survey | Excluded |
| Safe Practice 19: Hand Hygiene | 2012 Leapfrog Hospital Survey | Excluded |
| Safe Practice 23: Care of the Ventilated Patient | 2012 Leapfrog Hospital Survey | Excluded |
| SCIP‐INF‐1: Antibiotic Within 1 Hour | CMS Hospital Compare | CMS Hospital Compare |
| SCIP‐INF‐2: Antibiotic Selection | CMS Hospital Compare | CMS Hospital Compare |
| SCIP‐INF‐3: Antibiotic Discontinued After 24 Hours | CMS Hospital Compare | CMS Hospital Compare |
| SCIP‐INF‐9: Catheter Removal | CMS Hospital Compare | CMS Hospital Compare |
| SCIP‐VTE‐2: VTE Prophylaxis | CMS Hospital Compare | CMS Hospital Compare |
| Outcome measures (50% of score) | ||
| HAC: Foreign Object Retained | CMS HACs | CMS HACs |
| HAC: Air Embolism | CMS HACs | CMS HACs |
| HAC: Pressure Ulcers | CMS HACs | CMS HACs |
| HAC: Falls and Trauma | CMS HACs | CMS HACs |
| Central Line‐Associated Bloodstream Infection | 2012 Leapfrog Hospital Survey | CMS HAIs |
| PSI 4: Death Among Surgical Inpatients With Serious Treatable Complications | CMS Hospital Compare | CMS Hospital Compare |
| PSI 6: Collapsed Lung Due to Medical Treatment | CMS Hospital Compare | CMS Hospital Compare |
| PSI 12: Postoperative PE/DVT | CMS Hospital Compare | CMS Hospital Compare |
| PSI 14: Wounds Split Open After Surgery | CMS Hospital Compare | CMS Hospital Compare |
| PSI 15: Accidental Cuts or Tears From Medical Treatment | CMS Hospital Compare | CMS Hospital Compare |
Study Sample
We examined the Leapfrog safety grades for top hospitals," as ranked by U.S. News & World Report. Included in this sample were the top 15 ranked hospitals in each of the specialties, excluding those specialties whose ranks are based solely on reputation. Hospitals ranked in more than 1 specialty were only included once in the sample. This resulted in a final study sample of 35 top hospitals. Eighteen of these top hospitals participated in the Leapfrog Survey, whereas 17 did not.
Utilizing Leapfrog's spring 2013 methodology,[6] the Hospital Safety Scores for the 35 top hospitals were calculated. The mean safety score for the 18 participating hospitals was then compared with the mean score for the 17 nonparticipating hospitals. Finally, the safety scores for each of the 17 nonparticipating hospitals, listed in Table 2, were estimated as if they had participated in the Leapfrog Survey. To do this, we assumed that the 17 nonparticipating hospitals could each earn average scores for the CPOE, IPS, and 8 process/structural Leapfrog measures as received by their 18 participating counterparts.
| Participants | Leapfrog Grade | Nonparticipants | Leapfrog Grade |
|---|---|---|---|
| |||
| Brigham and Women's Hospital, Boston, MA | A | Abbott Northwestern Hospital, Minneapolis, MN | A |
| Duke University Medical Center, Durham, NC | A | Barnes‐Jewish Hospital/Washington University, St. Louis, MO | C |
| Massachusetts General Hospital, Boston, MA | B | Baylor University Medical Center, Dallas, TX | C |
| Mayo Clinic, Rochester, MN | A | Cedars‐Sinai Medical Center, Los Angeles, CA | C |
| Methodist Hospital, Houston, TX | A | Cleveland Clinic, Cleveland, OH | C |
| Northwestern Memorial Hospital, Chicago, IL | A | Florida Hospital, Orlando, FL | B |
| Ronald Reagan UCLA Medical Center, Los Angeles, CA | D | Hospital of the University of Pennsylvania, Philadelphia, PA | A |
| Rush University Medical Center, Chicago, IL | A | Indiana University Health, Indianapolis, IN | A |
| St. Francis Hospital, Roslyn, NY | A | Mount Sinai Medical Center, New York, NY | B |
| St. Joseph's Hospital and Medical Center, Phoenix, AZ | B | New York‐Presbyterian Hospital, New York, NY | C |
| Stanford Hospital and Clinics, Stanford, CA | A | NYU Langone Medical Center, New York, NY | A |
| Thomas Jefferson University Hospital, Philadelphia, PA | C | Ochsner Medical Center, New Orleans, LA | A |
| UCSF Medical Center, San Francisco, CA | B | Tampa General Hospital, Tampa, FL | C |
| University Hospitals Case Medical Center, Cleveland, OH | A | University of Iowa Hospitals and Clinics, Iowa City, IA | C |
| University of Michigan Hospitals and Health Centers, Ann Arbor, MI | A | University of Kansas Hospital, Kansas City, KS | A |
| University of Washington Medical Center, Seattle, WA | C | UPMC, Pittsburgh, PA | B |
| Vanderbilt University Medical Center, Nashville, TN | A | Yale‐New Haven Hospital, New Haven, CT | B |
| Wake Forest Baptist Medical Center, Winston‐Salem, NC | A | ||
RESULTS
Out of these 35 top hospitals, those that participated in the Leapfrog Survey generally received higher scores than the nonparticipants (Table 2). The group of participating hospitals received an average grade of A (mean safety score, 3.165; standard error of the mean [SE], 0.081), whereas the nonparticipating hospitals received an average grade of B (mean safety score, 3.012; SE, 0.047). These grades were consistent whether mean or median scores were used.
To further examine the potential bias against nonparticipating hospitals, the safety scores for each of the 17 nonparticipating hospitals were estimated as if they had participated in the Leapfrog Survey. The letter grade of this group increased from an average of B (mean safety score, 3.012; SE, 0.047) to an average of A (mean safety score, 3.216; SE, 0.046). Among the 17 nonparticipating hospitals, 15 showed an increase in safety score, of which 8 hospitals rescored a change in score significant enough to receive 1 or 2 letter grades higher (Table 3). Only 2 hospitals had slight decreases in safety score, without any impact on letter grade.
| Hospital | Original Score (Grade) | Estimated Scorea (Grade) |
|---|---|---|
| ||
| Abbott Northwestern Hospital, Minneapolis, MN | 3.17 (A) | 3.44 (A) |
| Barnes‐Jewish Hospital/Washington University, St. Louis, MO | 2.83 (C) | 3.11 (B) |
| Baylor University Medical Center, Dallas, TX | 2.90 (C) | 3.25 (A) |
| Cedars‐Sinai Medical Center, Los Angeles, CA | 2.92 (C) | 3.30 (A) |
| Cleveland Clinic, Cleveland, OH | 2.76 (C) | 2.78 (C) |
| Florida Hospital, Orlando, FL | 2.98 (B) | 3.38 (A) |
| Hospital of the University of Pennsylvania, Philadelphia, PA | 3.29 (A) | 3.26 (A) |
| Indiana University Health, Indianapolis, IN | 3.14 (A) | 3.37 (A) |
| Mount Sinai Medical Center, New York, NY | 3.01 (B) | 3.02 (B) |
| New York‐Presbyterian Hospital, New York, NY | 2.76 (C) | 3.15 (A) |
| NYU Langone Medical Center, New York, NY | 3.26 (A) | 3.30 (A) |
| Ochsner Medical Center, New Orleans, LA | 3.19 (A) | 3.59 (A) |
| Tampa General Hospital, Tampa, FL | 2.86 (C) | 3.05 (B) |
| University of Iowa Hospitals and Clinics, Iowa City, IA | 2.70 (C) | 3.00 (B) |
| University of Kansas Hospital, Kansas City, KS | 3.29 (A) | 3.35 (A) |
| UPMC, Pittsburgh, PA | 3.04 (B) | 3.24 (A) |
| Yale‐New Haven Hospital, New Haven, CT | 3.10 (B) | 3.08 (B) |
We applied the same methods to test the top 17 Honor Roll Hospitals as designated by US News & World Report; among them, half are participating hospitals and another half nonparticipating hospitals. One hospital, Johns Hopkins Hospital was not scored by Leapfrog because no relevant Medicare data are available for Leapfrog to calculate its safety score. For this reason, Johns Hopkins was excluded from our comparison. The results persist even with this smaller sample of top hospitals. The group of 8 participating hospitals had an average grade of A (mean safety score, 3.145; SE, 0.146), whereas another 8 nonparticipating hospitals received an average grade of B (mean safety score, 3.011; SE, 0.075).
DISCUSSION
The Leapfrog Group's intent to provide patient safety information to patients, physicians, healthcare purchasers, and hospital executives should be commended. However, the current methodology may disadvantage nonparticipating hospitals. The combination of lower maximum scores and increased weight of the CPOE and IPS scores may result in a lower hospital safety score than is justified. Nonparticipating hospitals may also face more intensive pressure from employers and payors to lower their reimbursement rates due to the newly released Leapfrog Hidden Surcharge Calculator.
Leapfrog acknowledges that the more data points a hospital has to be scored on, the better its opportunity to achieve a higher score.[8] This justification may lead to bias against nonparticipating hospitals. On the other hand, it is possible that hospitals with good safety records are more likely to participate in the Leapfrog Survey than those with poorer safety records. Without detailed nonresponse analysis from Leapfrog, it is impossible to know if there is a selection bias. Regardless, the Leapfrog result can subsequently misguide the payment rate negotiation between insurers and hospitals.
With this consideration in mind, Leapfrog should explicitly acknowledge the limitations of its methodology and consider revising it in future studies. For example, Leapfrog could only report on those measures for which there are data available for both participating and nonparticipating hospitals. Pending this revision, every effort must be made to distinguish between participating and nonparticipating hospitals. The outcomes of Leapfrog's hospital safety grades are made available online to consumers without distinguishing between participating and nonparticipating hospitals. The only method to differentiate the categories is to examine the data sources in detail amid a large volume of data. It is unlikely that consumers comparing hospital safety grades will take note of this caveat. Thus, Leapfrog's grading system can drastically misrepresent many nonparticipating hospitals' patient safety performances.
This study of The Leapfrog Group's Hospital Safety Score is not without limitations. The small sample utilized in this study limited the power of statistical testing. The difference in mean scores between participating and nonparticipating hospitals is not statistically significant. However, The Leapfrog Group uses specific numerical cutoff points for each letter grade classification. In this classification system statistical significance is not considered when assigning hospitals with different letter grades. It was clear that nonparticipating hospitals were more likely to receive lower letter grades than participating hospitals.
The small sample also posed challenges when attempting to account for missing data when comparing participating hospitals versus nonparticipating hospitals. Although a multiple imputation approach may have been ideal to address this, the small sample size coupled with the large amount of missing data (58% of hospitals did not participate in the Leapfrog Survey) led us to question the accuracy of this approach in this situation.[12] Instead, a crude, mean imputation approach was utilized, relying on the assumption that nonresponding hospitals had the same mean performance as responding hospitals on those domains where data were missing. In this study, we purposely selected a sample of hospitals from U.S. News & World Report's top hospitals. We believe the mean imputation approach, although not perfect, is appropriate for this sample of hospitals. Future study, however, should examine if hospitals that anticipated lower performance scores would be less likely to participate in the Leapfrog Survey. This would help strengthen Leapfrog's methodology in dealing with nonresponsive hospitals.
ACKNOWLEDGMENTS
Disclosures: Harold Paz is the CEO of Penn State Hershey Medical Center, which did not participate in the Leapfrog Survey. The authors have no financial conflicts of interest to report.
The Institute of Medicine (IOM) reported over a decade ago that between 44,000 and 98,000 deaths occurred every year due to preventable medical errors.[1] The report sparked an intense interest in identifying, measuring, and reporting hospital performance in patient safety.[2] The report also sparked the implementation of many initiatives aiming to improve patient safety.[3] Despite these efforts, there is still much room for improvement in the area of patient safety.[4] As the public has become more aware of patient safety issues, there has been an increased demand for information on hospital safety. The Leapfrog Group, a leading organization that examines and reports on hospital performance in patient safety, cites the IOM report as providing the focus that their newly formed organization required.[5]
Using 26 national measures of safety, The Leapfrog Group calculates a numeric Hospital Safety Score for over 2,600 acute care hospitals in the United States.[6] The primary data used to calculate this score are collected through the Leapfrog Hospital Survey, the Agency for Healthcare Research and Quality, the Centers for Disease Control and Prevention, and the Centers for Medicare and Medicaid Services (CMS). The American Hospital Association's (AHA) Annual Survey is used as a secondary data source as necessary. The Leapfrog Group conducts the survey annually, and substantial efforts are put forth to invite hospital administrators to participate in the survey. Participation in the Leapfrog survey is optional and free of charge.
Leapfrog recently moved a step further in their evaluation of hospital safety by releasing the Hidden Surcharge Calculator to enable employers to estimate the hidden surcharge they pay for their employees and dependents because of hospital errors.[7] The calculation depends largely on the letter grade (AF) that the hospital received from Leapfrog's Hospital Safety Score. For example, Leapfrog estimated a commercially insured patient admitted to a hospital with a grade of C or lower would incur $1845 additional cost per admission than if the same patient was admitted to a hospital with a grade of A.[7] The Leapfrog group encourages employers and payers to use this information to adjust benefits structures so that employees are discouraged from using hospitals that receive lower hospital safety scores. Leapfrog also encourages payers to negotiate lower reimbursement rates for hospitals with lower hospital safety scores.
The accuracy of Leapfrog's hospital safety grades warrants attention because of the methodology used to score hospitals that do not participate in the Leapfrog Survey. One common barrier that prevents hospitals from participating is the amount of effort required to complete the annual survey, including extensive inputs from hospital executives and staff. According to Leapfrog, 4 to 6 days are required for a hospital to compile the necessary survey data.[8] Leapfrog estimates a 90‐minute commitment for the hospital chief executive officer or designated administrator to enter the information into the online questionnaire. This is a significant commitment for many hospitals. As a result, among the approximately 2600 acute care hospitals covered by Leapfrog's 2012 to 2013 safety grading, only 1100 (or 42.3%) actually participated in the Leapfrog hospital survey. This limits Leapfrog's ability to provide accurate scores and assign fair safety grades to many hospitals.
METHODS
Leapfrog Hospital Safety Score
Leapfrog's designated Hospital Safety Score is determined by 26 measures. The set of safety measures and their relative weight are determined by a 9‐member Leapfrog expert panel of patient safety experts.[9] The hospital safety score is divided equally into 2 domains of safety measures: process/structural and outcomes.[6] The process measures represent how often a hospital gives patients recommended treatment for a given medical condition or procedure, whereas structural measures represent the environment in which patients receive care.[10] The process/structural measures include computerized physician order entry (CPOE), intensive care unit (ICU) physician staffing (IPS), 8 Leapfrog safety practices, and 5 surgical care improvement project measures. The outcome measures represent what happens to a patient while receiving care. The outcomes domain includes 5 hospital‐acquired conditions and 6 patient safety indicators. A score is assigned and weighted for each measure. All scores are then summed to produce a single number denoting the safety performance score received by each hospital. Every hospital is assigned 1 of 5 letter grades depending on how the hospital's numeric score stands in safety performance relative to all other hospitals. The letter grade A denotes the best hospital safety performance, followed in order by letter grades B through F. The cutoffs for A and B grades represent the first and second quartile of hospital safety scores. The cutoff for the C grade represents the hospitals that were between the mean and 1.5 standard deviations below the mean. The cutoff for the D grade represents the hospitals that were between 1.5 and 3.0 standard deviations below the mean. F grades indicate safety scores more than 3.0 standard deviations below the mean.[11]
Nonparticipating Hospitals
The Leapfrog Survey contributes values for 11 of the 26 measures utilized to calculate the Hospital Safety Score. The score of a nonparticipating hospital will not reflect 8 of these 11 measures. For the 3 remaining measures, CPOE, IPS, and central line‐associated blood stream infection, secondary data from the AHA Survey, AHA Information Technology Supplement Survey, and CMS Hospital Compare were used as proxies, respectively (Table 1). The use of a proxy effectively limits the maximum score attainable by nonparticipating hospitals. For instance, 2 of these 3 measures, CPOE and IPS, are calculated on different scales depending on hospital survey participation status. For CPOE, nonparticipating hospitals are limited to a maximum of 65 out of 100 points; for IPS, they are limited to 85 out of 100 points.[6] Because the actual weight for each of these proxy measures is increased for nonparticipating hospitals in the calculation of the final score, their effective impact is exacerbated. The weight of CPOE and IPS measures in the overall weighted score are increased from 6.1% and 7.0% to 11.0% and 12.6%, respectively.
| Participants | Nonparticipants | |
|---|---|---|
| ||
| Process/structural measures (50% of score) | ||
| Computerized Physician Order Entry | 2012 Leapfrog Hospital Survey | 2010 IT Supplement (AHA) |
| ICU Physician Staffing (IPS) | 2012 Leapfrog Hospital Survey | 2011 AHA Annual Survey |
| Safe Practice 1: Leadership Structures and Systems | 2012 Leapfrog Hospital Survey | Excluded |
| Safe Practice 2: Culture Measurement, Feedback, and Intervention | 2012 Leapfrog Hospital Survey | Excluded |
| Safe Practice 3: Teamwork Training and Skill Building | 2012 Leapfrog Hospital Survey | Excluded |
| Safe Practice 4: Identification and Mitigation of Risks and Hazards | 2012 Leapfrog Hospital Survey | Excluded |
| Safe Practice 9: Nursing Workforce | 2012 Leapfrog Hospital Survey | Excluded |
| Safe Practice 17: Medication Reconciliation | 2012 Leapfrog Hospital Survey | Excluded |
| Safe Practice 19: Hand Hygiene | 2012 Leapfrog Hospital Survey | Excluded |
| Safe Practice 23: Care of the Ventilated Patient | 2012 Leapfrog Hospital Survey | Excluded |
| SCIP‐INF‐1: Antibiotic Within 1 Hour | CMS Hospital Compare | CMS Hospital Compare |
| SCIP‐INF‐2: Antibiotic Selection | CMS Hospital Compare | CMS Hospital Compare |
| SCIP‐INF‐3: Antibiotic Discontinued After 24 Hours | CMS Hospital Compare | CMS Hospital Compare |
| SCIP‐INF‐9: Catheter Removal | CMS Hospital Compare | CMS Hospital Compare |
| SCIP‐VTE‐2: VTE Prophylaxis | CMS Hospital Compare | CMS Hospital Compare |
| Outcome measures (50% of score) | ||
| HAC: Foreign Object Retained | CMS HACs | CMS HACs |
| HAC: Air Embolism | CMS HACs | CMS HACs |
| HAC: Pressure Ulcers | CMS HACs | CMS HACs |
| HAC: Falls and Trauma | CMS HACs | CMS HACs |
| Central Line‐Associated Bloodstream Infection | 2012 Leapfrog Hospital Survey | CMS HAIs |
| PSI 4: Death Among Surgical Inpatients With Serious Treatable Complications | CMS Hospital Compare | CMS Hospital Compare |
| PSI 6: Collapsed Lung Due to Medical Treatment | CMS Hospital Compare | CMS Hospital Compare |
| PSI 12: Postoperative PE/DVT | CMS Hospital Compare | CMS Hospital Compare |
| PSI 14: Wounds Split Open After Surgery | CMS Hospital Compare | CMS Hospital Compare |
| PSI 15: Accidental Cuts or Tears From Medical Treatment | CMS Hospital Compare | CMS Hospital Compare |
Study Sample
We examined the Leapfrog safety grades for top hospitals," as ranked by U.S. News & World Report. Included in this sample were the top 15 ranked hospitals in each of the specialties, excluding those specialties whose ranks are based solely on reputation. Hospitals ranked in more than 1 specialty were only included once in the sample. This resulted in a final study sample of 35 top hospitals. Eighteen of these top hospitals participated in the Leapfrog Survey, whereas 17 did not.
Utilizing Leapfrog's spring 2013 methodology,[6] the Hospital Safety Scores for the 35 top hospitals were calculated. The mean safety score for the 18 participating hospitals was then compared with the mean score for the 17 nonparticipating hospitals. Finally, the safety scores for each of the 17 nonparticipating hospitals, listed in Table 2, were estimated as if they had participated in the Leapfrog Survey. To do this, we assumed that the 17 nonparticipating hospitals could each earn average scores for the CPOE, IPS, and 8 process/structural Leapfrog measures as received by their 18 participating counterparts.
| Participants | Leapfrog Grade | Nonparticipants | Leapfrog Grade |
|---|---|---|---|
| |||
| Brigham and Women's Hospital, Boston, MA | A | Abbott Northwestern Hospital, Minneapolis, MN | A |
| Duke University Medical Center, Durham, NC | A | Barnes‐Jewish Hospital/Washington University, St. Louis, MO | C |
| Massachusetts General Hospital, Boston, MA | B | Baylor University Medical Center, Dallas, TX | C |
| Mayo Clinic, Rochester, MN | A | Cedars‐Sinai Medical Center, Los Angeles, CA | C |
| Methodist Hospital, Houston, TX | A | Cleveland Clinic, Cleveland, OH | C |
| Northwestern Memorial Hospital, Chicago, IL | A | Florida Hospital, Orlando, FL | B |
| Ronald Reagan UCLA Medical Center, Los Angeles, CA | D | Hospital of the University of Pennsylvania, Philadelphia, PA | A |
| Rush University Medical Center, Chicago, IL | A | Indiana University Health, Indianapolis, IN | A |
| St. Francis Hospital, Roslyn, NY | A | Mount Sinai Medical Center, New York, NY | B |
| St. Joseph's Hospital and Medical Center, Phoenix, AZ | B | New York‐Presbyterian Hospital, New York, NY | C |
| Stanford Hospital and Clinics, Stanford, CA | A | NYU Langone Medical Center, New York, NY | A |
| Thomas Jefferson University Hospital, Philadelphia, PA | C | Ochsner Medical Center, New Orleans, LA | A |
| UCSF Medical Center, San Francisco, CA | B | Tampa General Hospital, Tampa, FL | C |
| University Hospitals Case Medical Center, Cleveland, OH | A | University of Iowa Hospitals and Clinics, Iowa City, IA | C |
| University of Michigan Hospitals and Health Centers, Ann Arbor, MI | A | University of Kansas Hospital, Kansas City, KS | A |
| University of Washington Medical Center, Seattle, WA | C | UPMC, Pittsburgh, PA | B |
| Vanderbilt University Medical Center, Nashville, TN | A | Yale‐New Haven Hospital, New Haven, CT | B |
| Wake Forest Baptist Medical Center, Winston‐Salem, NC | A | ||
RESULTS
Out of these 35 top hospitals, those that participated in the Leapfrog Survey generally received higher scores than the nonparticipants (Table 2). The group of participating hospitals received an average grade of A (mean safety score, 3.165; standard error of the mean [SE], 0.081), whereas the nonparticipating hospitals received an average grade of B (mean safety score, 3.012; SE, 0.047). These grades were consistent whether mean or median scores were used.
To further examine the potential bias against nonparticipating hospitals, the safety scores for each of the 17 nonparticipating hospitals were estimated as if they had participated in the Leapfrog Survey. The letter grade of this group increased from an average of B (mean safety score, 3.012; SE, 0.047) to an average of A (mean safety score, 3.216; SE, 0.046). Among the 17 nonparticipating hospitals, 15 showed an increase in safety score, of which 8 hospitals rescored a change in score significant enough to receive 1 or 2 letter grades higher (Table 3). Only 2 hospitals had slight decreases in safety score, without any impact on letter grade.
| Hospital | Original Score (Grade) | Estimated Scorea (Grade) |
|---|---|---|
| ||
| Abbott Northwestern Hospital, Minneapolis, MN | 3.17 (A) | 3.44 (A) |
| Barnes‐Jewish Hospital/Washington University, St. Louis, MO | 2.83 (C) | 3.11 (B) |
| Baylor University Medical Center, Dallas, TX | 2.90 (C) | 3.25 (A) |
| Cedars‐Sinai Medical Center, Los Angeles, CA | 2.92 (C) | 3.30 (A) |
| Cleveland Clinic, Cleveland, OH | 2.76 (C) | 2.78 (C) |
| Florida Hospital, Orlando, FL | 2.98 (B) | 3.38 (A) |
| Hospital of the University of Pennsylvania, Philadelphia, PA | 3.29 (A) | 3.26 (A) |
| Indiana University Health, Indianapolis, IN | 3.14 (A) | 3.37 (A) |
| Mount Sinai Medical Center, New York, NY | 3.01 (B) | 3.02 (B) |
| New York‐Presbyterian Hospital, New York, NY | 2.76 (C) | 3.15 (A) |
| NYU Langone Medical Center, New York, NY | 3.26 (A) | 3.30 (A) |
| Ochsner Medical Center, New Orleans, LA | 3.19 (A) | 3.59 (A) |
| Tampa General Hospital, Tampa, FL | 2.86 (C) | 3.05 (B) |
| University of Iowa Hospitals and Clinics, Iowa City, IA | 2.70 (C) | 3.00 (B) |
| University of Kansas Hospital, Kansas City, KS | 3.29 (A) | 3.35 (A) |
| UPMC, Pittsburgh, PA | 3.04 (B) | 3.24 (A) |
| Yale‐New Haven Hospital, New Haven, CT | 3.10 (B) | 3.08 (B) |
We applied the same methods to test the top 17 Honor Roll Hospitals as designated by US News & World Report; among them, half are participating hospitals and another half nonparticipating hospitals. One hospital, Johns Hopkins Hospital was not scored by Leapfrog because no relevant Medicare data are available for Leapfrog to calculate its safety score. For this reason, Johns Hopkins was excluded from our comparison. The results persist even with this smaller sample of top hospitals. The group of 8 participating hospitals had an average grade of A (mean safety score, 3.145; SE, 0.146), whereas another 8 nonparticipating hospitals received an average grade of B (mean safety score, 3.011; SE, 0.075).
DISCUSSION
The Leapfrog Group's intent to provide patient safety information to patients, physicians, healthcare purchasers, and hospital executives should be commended. However, the current methodology may disadvantage nonparticipating hospitals. The combination of lower maximum scores and increased weight of the CPOE and IPS scores may result in a lower hospital safety score than is justified. Nonparticipating hospitals may also face more intensive pressure from employers and payors to lower their reimbursement rates due to the newly released Leapfrog Hidden Surcharge Calculator.
Leapfrog acknowledges that the more data points a hospital has to be scored on, the better its opportunity to achieve a higher score.[8] This justification may lead to bias against nonparticipating hospitals. On the other hand, it is possible that hospitals with good safety records are more likely to participate in the Leapfrog Survey than those with poorer safety records. Without detailed nonresponse analysis from Leapfrog, it is impossible to know if there is a selection bias. Regardless, the Leapfrog result can subsequently misguide the payment rate negotiation between insurers and hospitals.
With this consideration in mind, Leapfrog should explicitly acknowledge the limitations of its methodology and consider revising it in future studies. For example, Leapfrog could only report on those measures for which there are data available for both participating and nonparticipating hospitals. Pending this revision, every effort must be made to distinguish between participating and nonparticipating hospitals. The outcomes of Leapfrog's hospital safety grades are made available online to consumers without distinguishing between participating and nonparticipating hospitals. The only method to differentiate the categories is to examine the data sources in detail amid a large volume of data. It is unlikely that consumers comparing hospital safety grades will take note of this caveat. Thus, Leapfrog's grading system can drastically misrepresent many nonparticipating hospitals' patient safety performances.
This study of The Leapfrog Group's Hospital Safety Score is not without limitations. The small sample utilized in this study limited the power of statistical testing. The difference in mean scores between participating and nonparticipating hospitals is not statistically significant. However, The Leapfrog Group uses specific numerical cutoff points for each letter grade classification. In this classification system statistical significance is not considered when assigning hospitals with different letter grades. It was clear that nonparticipating hospitals were more likely to receive lower letter grades than participating hospitals.
The small sample also posed challenges when attempting to account for missing data when comparing participating hospitals versus nonparticipating hospitals. Although a multiple imputation approach may have been ideal to address this, the small sample size coupled with the large amount of missing data (58% of hospitals did not participate in the Leapfrog Survey) led us to question the accuracy of this approach in this situation.[12] Instead, a crude, mean imputation approach was utilized, relying on the assumption that nonresponding hospitals had the same mean performance as responding hospitals on those domains where data were missing. In this study, we purposely selected a sample of hospitals from U.S. News & World Report's top hospitals. We believe the mean imputation approach, although not perfect, is appropriate for this sample of hospitals. Future study, however, should examine if hospitals that anticipated lower performance scores would be less likely to participate in the Leapfrog Survey. This would help strengthen Leapfrog's methodology in dealing with nonresponsive hospitals.
ACKNOWLEDGMENTS
Disclosures: Harold Paz is the CEO of Penn State Hershey Medical Center, which did not participate in the Leapfrog Survey. The authors have no financial conflicts of interest to report.
- , , . To Err Is Human: Building a Safer Health System. Washington, DC: National Academy Press; 2000.
- , , , , . The “To Err is Human” report and the patient safety literature. Qual Saf Health Care. 2006;15(3):174–178.
- , . A call to excellence. Health Aff (Millwood). 2003;22(2):113–115.
- US Department of Health and Human Services. Adverse events in hospitals: national incidence among Medicare beneficiaries. Available at: http://oig.hhs.gov/oei/reports/oei‐06‐09‐00090.pdf. Published November 2010. Accessed on August 2, 2013.
- The Leapfrog Group. The Leapfrog Group—fact sheet 2013. Available at: https://leapfroghospitalsurvey.org/web/wp‐content/uploads/Fsleapfrog.pdf. Accessed October 9, 2013.
- The Leapfrog Group. Hospital Safety score scoring methodology. Available at: http://www.hospitalsafetyscore.org/media/file/HospitalSafetyScore_ScoringMethodology_May2013.pdf. Published May 2013. Accessed June 17, 2013.
- The Leapfrog Group. The Hidden Surcharge Americans Pay for Hospital Errors 2013. Available at: http://www.leapfroggroup.org/employers_purchasers/HiddenSurchargeCalculator. Accessed August 2, 2013.
- The Leapfrog Group. 2013 Leapfrog Hospital Survey Reference Book 2013. https://leapfroghospitalsurvey.org/web/wp‐content/uploads/reference.pdf. Published April 1, 2013. Accessed June 17, 2013.
- , , , et al. Safety in numbers: the development of Leapfrog's composite patient safety score for U.S. hospitals [published online ahead of print September 27, 2013]. J Patient Saf. doi: 10.1097/PTS.0b013e3182952644.
- The Leapfrog Group. Measures in detail. Available at: http://www. hospitalsafetyscore.org/about‐the‐score/measures‐in‐detail. Accessed June 17, 2013.
- The Leapfrog Group. Explanation of safety score grades. Available at: http://www.hospitalsafetyscore.org/media/file/ExplanationofSafety ScoreGrades_May2013.pdf. Published May 2013. Accessed June 17, 2013.
- , , , et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393.
- , , . To Err Is Human: Building a Safer Health System. Washington, DC: National Academy Press; 2000.
- , , , , . The “To Err is Human” report and the patient safety literature. Qual Saf Health Care. 2006;15(3):174–178.
- , . A call to excellence. Health Aff (Millwood). 2003;22(2):113–115.
- US Department of Health and Human Services. Adverse events in hospitals: national incidence among Medicare beneficiaries. Available at: http://oig.hhs.gov/oei/reports/oei‐06‐09‐00090.pdf. Published November 2010. Accessed on August 2, 2013.
- The Leapfrog Group. The Leapfrog Group—fact sheet 2013. Available at: https://leapfroghospitalsurvey.org/web/wp‐content/uploads/Fsleapfrog.pdf. Accessed October 9, 2013.
- The Leapfrog Group. Hospital Safety score scoring methodology. Available at: http://www.hospitalsafetyscore.org/media/file/HospitalSafetyScore_ScoringMethodology_May2013.pdf. Published May 2013. Accessed June 17, 2013.
- The Leapfrog Group. The Hidden Surcharge Americans Pay for Hospital Errors 2013. Available at: http://www.leapfroggroup.org/employers_purchasers/HiddenSurchargeCalculator. Accessed August 2, 2013.
- The Leapfrog Group. 2013 Leapfrog Hospital Survey Reference Book 2013. https://leapfroghospitalsurvey.org/web/wp‐content/uploads/reference.pdf. Published April 1, 2013. Accessed June 17, 2013.
- , , , et al. Safety in numbers: the development of Leapfrog's composite patient safety score for U.S. hospitals [published online ahead of print September 27, 2013]. J Patient Saf. doi: 10.1097/PTS.0b013e3182952644.
- The Leapfrog Group. Measures in detail. Available at: http://www. hospitalsafetyscore.org/about‐the‐score/measures‐in‐detail. Accessed June 17, 2013.
- The Leapfrog Group. Explanation of safety score grades. Available at: http://www.hospitalsafetyscore.org/media/file/ExplanationofSafety ScoreGrades_May2013.pdf. Published May 2013. Accessed June 17, 2013.
- , , , et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393.
Discovery may aid vaccine design for P vivax malaria

attached to syncytiotrophoblast
Credit: Fabio T.M. Costa
Plasmodium vivax malaria attacks red blood cells by clamping down on the cells with a pair of proteins, researchers have found.
Earlier studies suggested that a single P vivax protein binds to a protein on the surface of red blood cells.
But the new study showed that binding is a 2-step process that involves 2 copies of a parasite protein coming together like tongs around 2 copies of a host protein.
The researchers believe this discovery, detailed in PLOS Pathogens, could help scientists design better vaccines and treatments for P vivax, which is common in India, Southeast Asia, and South America.
“More people live at risk of infection by this strain of malaria than any other,” said senior study author Niraj Tolia, PhD, of the Washington University School of Medicine in St Louis, Missouri.
“We now are using what we have learned to create vaccines tailored to stop the infectious process by preventing the parasite from attaching to red blood cells.”
Dr Tolia and his colleagues knew that P vivax Duffy binding protein (DBP) recognizes the receptor Duffy antigen/receptor for chemokines (DARC) during the parasite’s invasion of red blood cells. But the team wanted to identify binding contacts during invasion and determine the molecular basis of DBP receptor recognition.
So they conducted structural studies on the minimal binding domain of DBP in complex with the minimal region from DARC. And they found that 2 DBP molecules bind 2 DARC molecules.
The researchers also performed erythrocyte binding assays with binding site mutants and identified essential receptor contacts.
“It’s a very intricate and chemically strong interaction that was not easily understood before,” Dr Tolia said. “We have had hints that other forms of malaria, including the African strain, may be binding in a similar fashion to host cells, but this is one of the first definitive proofs of this kind of attack.”
Dr Tolia suspects that blocking any of the proteins with drugs or vaccines will stop the infectious process.
“For example, some people have a mutation that eliminates the protein on red blood cell surfaces that P vivax binds to, and they tend to be resistant to the parasite,” he said. “This is why this strain isn’t prevalent in Africa. Evolutionary pressure has caused most of the populations there to stop making this protein.”
Dr Tolia and his colleagues also found evidence that other people with immunity to P vivax have developed naturally occurring antibodies that attach to a key part of the parasite’s binding protein, preventing infection.
“The parasite protein is very large, and human antibodies bind to it at many different points along its length,” Dr Tolia explained. “We have observed that the ones that are most effective, so far, are the antibodies that bind to the protein at the region highlighted by our new research.” ![]()

attached to syncytiotrophoblast
Credit: Fabio T.M. Costa
Plasmodium vivax malaria attacks red blood cells by clamping down on the cells with a pair of proteins, researchers have found.
Earlier studies suggested that a single P vivax protein binds to a protein on the surface of red blood cells.
But the new study showed that binding is a 2-step process that involves 2 copies of a parasite protein coming together like tongs around 2 copies of a host protein.
The researchers believe this discovery, detailed in PLOS Pathogens, could help scientists design better vaccines and treatments for P vivax, which is common in India, Southeast Asia, and South America.
“More people live at risk of infection by this strain of malaria than any other,” said senior study author Niraj Tolia, PhD, of the Washington University School of Medicine in St Louis, Missouri.
“We now are using what we have learned to create vaccines tailored to stop the infectious process by preventing the parasite from attaching to red blood cells.”
Dr Tolia and his colleagues knew that P vivax Duffy binding protein (DBP) recognizes the receptor Duffy antigen/receptor for chemokines (DARC) during the parasite’s invasion of red blood cells. But the team wanted to identify binding contacts during invasion and determine the molecular basis of DBP receptor recognition.
So they conducted structural studies on the minimal binding domain of DBP in complex with the minimal region from DARC. And they found that 2 DBP molecules bind 2 DARC molecules.
The researchers also performed erythrocyte binding assays with binding site mutants and identified essential receptor contacts.
“It’s a very intricate and chemically strong interaction that was not easily understood before,” Dr Tolia said. “We have had hints that other forms of malaria, including the African strain, may be binding in a similar fashion to host cells, but this is one of the first definitive proofs of this kind of attack.”
Dr Tolia suspects that blocking any of the proteins with drugs or vaccines will stop the infectious process.
“For example, some people have a mutation that eliminates the protein on red blood cell surfaces that P vivax binds to, and they tend to be resistant to the parasite,” he said. “This is why this strain isn’t prevalent in Africa. Evolutionary pressure has caused most of the populations there to stop making this protein.”
Dr Tolia and his colleagues also found evidence that other people with immunity to P vivax have developed naturally occurring antibodies that attach to a key part of the parasite’s binding protein, preventing infection.
“The parasite protein is very large, and human antibodies bind to it at many different points along its length,” Dr Tolia explained. “We have observed that the ones that are most effective, so far, are the antibodies that bind to the protein at the region highlighted by our new research.” ![]()

attached to syncytiotrophoblast
Credit: Fabio T.M. Costa
Plasmodium vivax malaria attacks red blood cells by clamping down on the cells with a pair of proteins, researchers have found.
Earlier studies suggested that a single P vivax protein binds to a protein on the surface of red blood cells.
But the new study showed that binding is a 2-step process that involves 2 copies of a parasite protein coming together like tongs around 2 copies of a host protein.
The researchers believe this discovery, detailed in PLOS Pathogens, could help scientists design better vaccines and treatments for P vivax, which is common in India, Southeast Asia, and South America.
“More people live at risk of infection by this strain of malaria than any other,” said senior study author Niraj Tolia, PhD, of the Washington University School of Medicine in St Louis, Missouri.
“We now are using what we have learned to create vaccines tailored to stop the infectious process by preventing the parasite from attaching to red blood cells.”
Dr Tolia and his colleagues knew that P vivax Duffy binding protein (DBP) recognizes the receptor Duffy antigen/receptor for chemokines (DARC) during the parasite’s invasion of red blood cells. But the team wanted to identify binding contacts during invasion and determine the molecular basis of DBP receptor recognition.
So they conducted structural studies on the minimal binding domain of DBP in complex with the minimal region from DARC. And they found that 2 DBP molecules bind 2 DARC molecules.
The researchers also performed erythrocyte binding assays with binding site mutants and identified essential receptor contacts.
“It’s a very intricate and chemically strong interaction that was not easily understood before,” Dr Tolia said. “We have had hints that other forms of malaria, including the African strain, may be binding in a similar fashion to host cells, but this is one of the first definitive proofs of this kind of attack.”
Dr Tolia suspects that blocking any of the proteins with drugs or vaccines will stop the infectious process.
“For example, some people have a mutation that eliminates the protein on red blood cell surfaces that P vivax binds to, and they tend to be resistant to the parasite,” he said. “This is why this strain isn’t prevalent in Africa. Evolutionary pressure has caused most of the populations there to stop making this protein.”
Dr Tolia and his colleagues also found evidence that other people with immunity to P vivax have developed naturally occurring antibodies that attach to a key part of the parasite’s binding protein, preventing infection.
“The parasite protein is very large, and human antibodies bind to it at many different points along its length,” Dr Tolia explained. “We have observed that the ones that are most effective, so far, are the antibodies that bind to the protein at the region highlighted by our new research.” ![]()
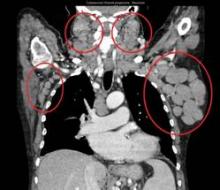
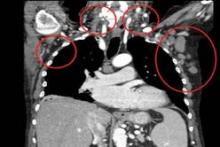
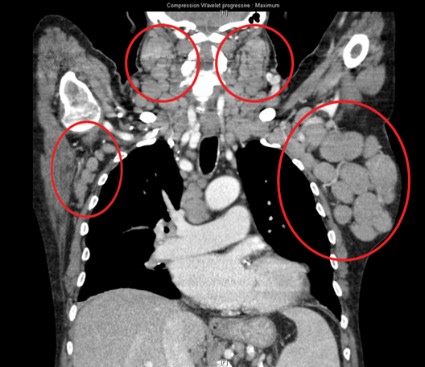
Team identifies mutations that may drive FL

Genetic profiling has provided a clearer picture of follicular lymphoma (FL) development and progression, according to research published in Nature Genetics.
Investigators performed whole-genome and whole-exome sequencing of samples from FL patients and found a number of mutations that appeared to be responsible for disease onset.
The team also identified mutations that seemed to drive FL toward a more aggressive form.
They said these findings provide a number of new therapeutic targets that may stop FL from becoming aggressive or developing resistance to treatment.
“Resistance to treatment is a major problem for follicular lymphoma patients, as they often respond well to treatment and later relapse,” said study author Jude Fitzgibbon, PhD, of Barts Cancer Institute in London, England.
“[This] gives the cancer multiple opportunities to evolve into a more aggressive and more difficult-to-treat form of the disease. We’ve been able to chronicle the chain of genetic events that leads to aggressive forms of the disease. If we can develop treatments to prevent some of these changes from taking place, we should be able to stop the cancer in its tracks.”
Dr Fitzgibbon and his colleagues performed whole-genome or whole-exome sequencing of sequential FL and transformed FL pairs and matched germline samples from 10 FL cases with deep-targeted sequencing of 28 genes in an extension cohort.
Among the 10 cases, the researchers identified 1560 protein-altering variants affecting 908 genes, including missense changes (84.8%), short indels (8.9%), and nonsense mutations (6.3%).
Patterns of evolution
The investigators constructed phylogenetic trees for the 10 FL cases and discovered a common progenitor clone (CPC), as well as 2 patterns of evolution.
Eight of the cases exhibited evolution through a “rich” ancestral CPC, showing high clonal semblance between the FL and transformed-FL tumors. The other 2 cases showed evolution through a “sparse” CPC, with only 4 nonsynonymous mutations shared by the FL and transformed-FL samples.
These 2 patterns of evolution shared mutations in 3 genes—KMT2D, TNFRSF14, and CREBBP. According to the researchers, this suggests tumor dependency on these alterations during lymphomagenesis and progression.
Mutation prevalence, timing
The investigators then set out to determine the prevalence of the mutations they identified in the 10 cases. They performed deep-targeted resequencing of 28 candidate genes in an extension cohort of 100 independent FL biopsies and 32 paired FL-transformed FL cases (including the 10 index cases).
More than 70% of cases had concurrent mutations in at least 2 of the histone-modifying enzymes screened (CREBBP, EZH2, MEF2B, and KMT2D).
Twenty-eight percent of cases had mutations affecting at least one histone H1 gene. HIST1H1C and HIST1H1E were the most frequently mutated.
The researchers also saw frequent mutations in components of the JAK-STAT signaling pathway, including STAT6 (12%) and SOCS1 (8%).
They found mutually exclusive mutations in the NF-κB signaling pathway in a third of FLs, including CARD11 (11%) and TNFAIP3 (11%).
And 17% of cases had mutations in genes important for B-cell development, including Ebf1.
Finally, the investigators set out to differentiate early genetic events from late ones. They found that mutations in histone-modifying genes—KMT2D, CREBBP, and EZH2—as well as mutations in STAT6 and TNFRSF14 were predominantly clonal events.
On the other hand, mutations in EBF1 and regulators of NF-κB signaling—MYD88 and TNFAIP3—were gained at transformation.
“This study has uncovered some of the key molecular changes taking place [in FL] and offers new targets for treating the disease,” said Nell Barrie, of Cancer Research UK, the organization that funded this study.
“Research into the genetics that underpin cancer is helping us to better know the enemy and find new ways in which we might beat it.” ![]()

Genetic profiling has provided a clearer picture of follicular lymphoma (FL) development and progression, according to research published in Nature Genetics.
Investigators performed whole-genome and whole-exome sequencing of samples from FL patients and found a number of mutations that appeared to be responsible for disease onset.
The team also identified mutations that seemed to drive FL toward a more aggressive form.
They said these findings provide a number of new therapeutic targets that may stop FL from becoming aggressive or developing resistance to treatment.
“Resistance to treatment is a major problem for follicular lymphoma patients, as they often respond well to treatment and later relapse,” said study author Jude Fitzgibbon, PhD, of Barts Cancer Institute in London, England.
“[This] gives the cancer multiple opportunities to evolve into a more aggressive and more difficult-to-treat form of the disease. We’ve been able to chronicle the chain of genetic events that leads to aggressive forms of the disease. If we can develop treatments to prevent some of these changes from taking place, we should be able to stop the cancer in its tracks.”
Dr Fitzgibbon and his colleagues performed whole-genome or whole-exome sequencing of sequential FL and transformed FL pairs and matched germline samples from 10 FL cases with deep-targeted sequencing of 28 genes in an extension cohort.
Among the 10 cases, the researchers identified 1560 protein-altering variants affecting 908 genes, including missense changes (84.8%), short indels (8.9%), and nonsense mutations (6.3%).
Patterns of evolution
The investigators constructed phylogenetic trees for the 10 FL cases and discovered a common progenitor clone (CPC), as well as 2 patterns of evolution.
Eight of the cases exhibited evolution through a “rich” ancestral CPC, showing high clonal semblance between the FL and transformed-FL tumors. The other 2 cases showed evolution through a “sparse” CPC, with only 4 nonsynonymous mutations shared by the FL and transformed-FL samples.
These 2 patterns of evolution shared mutations in 3 genes—KMT2D, TNFRSF14, and CREBBP. According to the researchers, this suggests tumor dependency on these alterations during lymphomagenesis and progression.
Mutation prevalence, timing
The investigators then set out to determine the prevalence of the mutations they identified in the 10 cases. They performed deep-targeted resequencing of 28 candidate genes in an extension cohort of 100 independent FL biopsies and 32 paired FL-transformed FL cases (including the 10 index cases).
More than 70% of cases had concurrent mutations in at least 2 of the histone-modifying enzymes screened (CREBBP, EZH2, MEF2B, and KMT2D).
Twenty-eight percent of cases had mutations affecting at least one histone H1 gene. HIST1H1C and HIST1H1E were the most frequently mutated.
The researchers also saw frequent mutations in components of the JAK-STAT signaling pathway, including STAT6 (12%) and SOCS1 (8%).
They found mutually exclusive mutations in the NF-κB signaling pathway in a third of FLs, including CARD11 (11%) and TNFAIP3 (11%).
And 17% of cases had mutations in genes important for B-cell development, including Ebf1.
Finally, the investigators set out to differentiate early genetic events from late ones. They found that mutations in histone-modifying genes—KMT2D, CREBBP, and EZH2—as well as mutations in STAT6 and TNFRSF14 were predominantly clonal events.
On the other hand, mutations in EBF1 and regulators of NF-κB signaling—MYD88 and TNFAIP3—were gained at transformation.
“This study has uncovered some of the key molecular changes taking place [in FL] and offers new targets for treating the disease,” said Nell Barrie, of Cancer Research UK, the organization that funded this study.
“Research into the genetics that underpin cancer is helping us to better know the enemy and find new ways in which we might beat it.” ![]()

Genetic profiling has provided a clearer picture of follicular lymphoma (FL) development and progression, according to research published in Nature Genetics.
Investigators performed whole-genome and whole-exome sequencing of samples from FL patients and found a number of mutations that appeared to be responsible for disease onset.
The team also identified mutations that seemed to drive FL toward a more aggressive form.
They said these findings provide a number of new therapeutic targets that may stop FL from becoming aggressive or developing resistance to treatment.
“Resistance to treatment is a major problem for follicular lymphoma patients, as they often respond well to treatment and later relapse,” said study author Jude Fitzgibbon, PhD, of Barts Cancer Institute in London, England.
“[This] gives the cancer multiple opportunities to evolve into a more aggressive and more difficult-to-treat form of the disease. We’ve been able to chronicle the chain of genetic events that leads to aggressive forms of the disease. If we can develop treatments to prevent some of these changes from taking place, we should be able to stop the cancer in its tracks.”
Dr Fitzgibbon and his colleagues performed whole-genome or whole-exome sequencing of sequential FL and transformed FL pairs and matched germline samples from 10 FL cases with deep-targeted sequencing of 28 genes in an extension cohort.
Among the 10 cases, the researchers identified 1560 protein-altering variants affecting 908 genes, including missense changes (84.8%), short indels (8.9%), and nonsense mutations (6.3%).
Patterns of evolution
The investigators constructed phylogenetic trees for the 10 FL cases and discovered a common progenitor clone (CPC), as well as 2 patterns of evolution.
Eight of the cases exhibited evolution through a “rich” ancestral CPC, showing high clonal semblance between the FL and transformed-FL tumors. The other 2 cases showed evolution through a “sparse” CPC, with only 4 nonsynonymous mutations shared by the FL and transformed-FL samples.
These 2 patterns of evolution shared mutations in 3 genes—KMT2D, TNFRSF14, and CREBBP. According to the researchers, this suggests tumor dependency on these alterations during lymphomagenesis and progression.
Mutation prevalence, timing
The investigators then set out to determine the prevalence of the mutations they identified in the 10 cases. They performed deep-targeted resequencing of 28 candidate genes in an extension cohort of 100 independent FL biopsies and 32 paired FL-transformed FL cases (including the 10 index cases).
More than 70% of cases had concurrent mutations in at least 2 of the histone-modifying enzymes screened (CREBBP, EZH2, MEF2B, and KMT2D).
Twenty-eight percent of cases had mutations affecting at least one histone H1 gene. HIST1H1C and HIST1H1E were the most frequently mutated.
The researchers also saw frequent mutations in components of the JAK-STAT signaling pathway, including STAT6 (12%) and SOCS1 (8%).
They found mutually exclusive mutations in the NF-κB signaling pathway in a third of FLs, including CARD11 (11%) and TNFAIP3 (11%).
And 17% of cases had mutations in genes important for B-cell development, including Ebf1.
Finally, the investigators set out to differentiate early genetic events from late ones. They found that mutations in histone-modifying genes—KMT2D, CREBBP, and EZH2—as well as mutations in STAT6 and TNFRSF14 were predominantly clonal events.
On the other hand, mutations in EBF1 and regulators of NF-κB signaling—MYD88 and TNFAIP3—were gained at transformation.
“This study has uncovered some of the key molecular changes taking place [in FL] and offers new targets for treating the disease,” said Nell Barrie, of Cancer Research UK, the organization that funded this study.
“Research into the genetics that underpin cancer is helping us to better know the enemy and find new ways in which we might beat it.” ![]()
Vive la difference
The scenario was familiar. Henry looked peeved. Mary looked anxious. Henry spoke first.
"This spot on my nose has been there for months," he said. "I’m concerned because we’ll be in the sun in Aruba next week."
I examined Henry. "It’s not skin cancer," I said. "Just leave it alone, and it’ll be fine.
"Of course," I went on, "you’ll want to take sensible sun precautions while you’re on vacation, a hat, sunscreen, and so forth." That’s when Mary spoke up.
"You know, Doctor," she said, "Henry does not take sensible sun precautions."
"Yes I do!" Henry objected. "At 10 every morning I leave the beach ..." Mary interrupted him. "He abuses the sun, even though I remind him every day." You could tell by Henry’s hangdog expression that "every day" was no exaggeration.
In its many forms, the eternal battle of the sexes has been examined in countless books, plays, movies, and sitcoms. Gender stereotypes don’t tell the whole story, but without some truth they wouldn’t become stereotypes. There is no getting around the fact that men and women often have their own ways of looking at the world. One part of the world they see differently is health in general and skin health in particular.
I don’t know what life is like on other planets, but if it’s true that men are from Mars and women are from Venus, then it follows that:
• People on Venus follow instructions, eat right, and take care of things so they don’t get out of control. People on Mars can’t be bothered with stuff like that.
• People on Venus wash regularly and use good products. On Mars they don’t much care.
• Venusians moisturize and use sunscreen. Not Martians.
Mini-dramas like that of Henry and Mary play themselves out in our offices all the time. Women take health maintenance more seriously than men do (or than men like to pretend they do.) Proper face washing (in adolescents), regular mole checks (in adults), and careful sun care (especially among the older set) are common flashpoints of gender disagreement. By and large, women feel responsible to make sure men do the right thing, while men just want to be left alone. "I’m only here because..." says the man, but I cut him off. I know why he’s here. It’s just a question of which woman got him there. Real men, you see, don’t ask directions or visit doctors.
One of the right things that women feel obliged to encourage is moisturizing. Men are functional: We shop when we need something and we moisturize when we feel dry. Women think you should moisturize every day, regardless, to make skin healthier and ward off aging.
Maybe so, maybe not, but we men as a group really dislike the feel of lotions on our skin and resist applying them. We find the sensation unpleasant, and anyhow don’t get why we should bother in the first place. Women in turn can’t figure why men should be so cussedly defiant about doing what seems to them not just worthwhile but delightful.
Men, accompanied by women or sent in by them against their better judgment, often make a great show of being put upon. They shrug, roll their eyes, and look irritated, much as they did when they were 8 years old and their mother said, "Tell him, Doctor. Tell him to eat his vegetables. Tell him to wash his face." Now that he’s grown up, her plea is more likely to be, "Tell him, Doctor. Tell him he has to get his spots checked and put sunscreen on every day. Maybe he’ll listen to you. I tell him all the time but he never listens to me." When that happens, I try to split the difference when I can and let both parties save face. After all, they have to live with each other, not with me.
Besides, men’s little secret is that we expect the women in our lives to take care of us and make sure we do the right things that we can’t be bothered to do for ourselves. For many couples, that’s the unspoken deal. We men know it, but we keep it quiet, even from ourselves. Shh, don’t tell anybody ...
Besides, we don’t even have to ask directions anymore. We’ve got GPS!
Dr. Rockoff practices dermatology in Brookline, Mass. He is on the clinical faculty at Tufts University School of Medicine, Boston, and has taught senior medical students and other trainees for 30 years. Dr. Rockoff has contributed to the Under My Skin column in Skin & Allergy News since January 2002. Skin & Allergy News is a publication of Frontline Medical Communications.
The scenario was familiar. Henry looked peeved. Mary looked anxious. Henry spoke first.
"This spot on my nose has been there for months," he said. "I’m concerned because we’ll be in the sun in Aruba next week."
I examined Henry. "It’s not skin cancer," I said. "Just leave it alone, and it’ll be fine.
"Of course," I went on, "you’ll want to take sensible sun precautions while you’re on vacation, a hat, sunscreen, and so forth." That’s when Mary spoke up.
"You know, Doctor," she said, "Henry does not take sensible sun precautions."
"Yes I do!" Henry objected. "At 10 every morning I leave the beach ..." Mary interrupted him. "He abuses the sun, even though I remind him every day." You could tell by Henry’s hangdog expression that "every day" was no exaggeration.
In its many forms, the eternal battle of the sexes has been examined in countless books, plays, movies, and sitcoms. Gender stereotypes don’t tell the whole story, but without some truth they wouldn’t become stereotypes. There is no getting around the fact that men and women often have their own ways of looking at the world. One part of the world they see differently is health in general and skin health in particular.
I don’t know what life is like on other planets, but if it’s true that men are from Mars and women are from Venus, then it follows that:
• People on Venus follow instructions, eat right, and take care of things so they don’t get out of control. People on Mars can’t be bothered with stuff like that.
• People on Venus wash regularly and use good products. On Mars they don’t much care.
• Venusians moisturize and use sunscreen. Not Martians.
Mini-dramas like that of Henry and Mary play themselves out in our offices all the time. Women take health maintenance more seriously than men do (or than men like to pretend they do.) Proper face washing (in adolescents), regular mole checks (in adults), and careful sun care (especially among the older set) are common flashpoints of gender disagreement. By and large, women feel responsible to make sure men do the right thing, while men just want to be left alone. "I’m only here because..." says the man, but I cut him off. I know why he’s here. It’s just a question of which woman got him there. Real men, you see, don’t ask directions or visit doctors.
One of the right things that women feel obliged to encourage is moisturizing. Men are functional: We shop when we need something and we moisturize when we feel dry. Women think you should moisturize every day, regardless, to make skin healthier and ward off aging.
Maybe so, maybe not, but we men as a group really dislike the feel of lotions on our skin and resist applying them. We find the sensation unpleasant, and anyhow don’t get why we should bother in the first place. Women in turn can’t figure why men should be so cussedly defiant about doing what seems to them not just worthwhile but delightful.
Men, accompanied by women or sent in by them against their better judgment, often make a great show of being put upon. They shrug, roll their eyes, and look irritated, much as they did when they were 8 years old and their mother said, "Tell him, Doctor. Tell him to eat his vegetables. Tell him to wash his face." Now that he’s grown up, her plea is more likely to be, "Tell him, Doctor. Tell him he has to get his spots checked and put sunscreen on every day. Maybe he’ll listen to you. I tell him all the time but he never listens to me." When that happens, I try to split the difference when I can and let both parties save face. After all, they have to live with each other, not with me.
Besides, men’s little secret is that we expect the women in our lives to take care of us and make sure we do the right things that we can’t be bothered to do for ourselves. For many couples, that’s the unspoken deal. We men know it, but we keep it quiet, even from ourselves. Shh, don’t tell anybody ...
Besides, we don’t even have to ask directions anymore. We’ve got GPS!
Dr. Rockoff practices dermatology in Brookline, Mass. He is on the clinical faculty at Tufts University School of Medicine, Boston, and has taught senior medical students and other trainees for 30 years. Dr. Rockoff has contributed to the Under My Skin column in Skin & Allergy News since January 2002. Skin & Allergy News is a publication of Frontline Medical Communications.
The scenario was familiar. Henry looked peeved. Mary looked anxious. Henry spoke first.
"This spot on my nose has been there for months," he said. "I’m concerned because we’ll be in the sun in Aruba next week."
I examined Henry. "It’s not skin cancer," I said. "Just leave it alone, and it’ll be fine.
"Of course," I went on, "you’ll want to take sensible sun precautions while you’re on vacation, a hat, sunscreen, and so forth." That’s when Mary spoke up.
"You know, Doctor," she said, "Henry does not take sensible sun precautions."
"Yes I do!" Henry objected. "At 10 every morning I leave the beach ..." Mary interrupted him. "He abuses the sun, even though I remind him every day." You could tell by Henry’s hangdog expression that "every day" was no exaggeration.
In its many forms, the eternal battle of the sexes has been examined in countless books, plays, movies, and sitcoms. Gender stereotypes don’t tell the whole story, but without some truth they wouldn’t become stereotypes. There is no getting around the fact that men and women often have their own ways of looking at the world. One part of the world they see differently is health in general and skin health in particular.
I don’t know what life is like on other planets, but if it’s true that men are from Mars and women are from Venus, then it follows that:
• People on Venus follow instructions, eat right, and take care of things so they don’t get out of control. People on Mars can’t be bothered with stuff like that.
• People on Venus wash regularly and use good products. On Mars they don’t much care.
• Venusians moisturize and use sunscreen. Not Martians.
Mini-dramas like that of Henry and Mary play themselves out in our offices all the time. Women take health maintenance more seriously than men do (or than men like to pretend they do.) Proper face washing (in adolescents), regular mole checks (in adults), and careful sun care (especially among the older set) are common flashpoints of gender disagreement. By and large, women feel responsible to make sure men do the right thing, while men just want to be left alone. "I’m only here because..." says the man, but I cut him off. I know why he’s here. It’s just a question of which woman got him there. Real men, you see, don’t ask directions or visit doctors.
One of the right things that women feel obliged to encourage is moisturizing. Men are functional: We shop when we need something and we moisturize when we feel dry. Women think you should moisturize every day, regardless, to make skin healthier and ward off aging.
Maybe so, maybe not, but we men as a group really dislike the feel of lotions on our skin and resist applying them. We find the sensation unpleasant, and anyhow don’t get why we should bother in the first place. Women in turn can’t figure why men should be so cussedly defiant about doing what seems to them not just worthwhile but delightful.
Men, accompanied by women or sent in by them against their better judgment, often make a great show of being put upon. They shrug, roll their eyes, and look irritated, much as they did when they were 8 years old and their mother said, "Tell him, Doctor. Tell him to eat his vegetables. Tell him to wash his face." Now that he’s grown up, her plea is more likely to be, "Tell him, Doctor. Tell him he has to get his spots checked and put sunscreen on every day. Maybe he’ll listen to you. I tell him all the time but he never listens to me." When that happens, I try to split the difference when I can and let both parties save face. After all, they have to live with each other, not with me.
Besides, men’s little secret is that we expect the women in our lives to take care of us and make sure we do the right things that we can’t be bothered to do for ourselves. For many couples, that’s the unspoken deal. We men know it, but we keep it quiet, even from ourselves. Shh, don’t tell anybody ...
Besides, we don’t even have to ask directions anymore. We’ve got GPS!
Dr. Rockoff practices dermatology in Brookline, Mass. He is on the clinical faculty at Tufts University School of Medicine, Boston, and has taught senior medical students and other trainees for 30 years. Dr. Rockoff has contributed to the Under My Skin column in Skin & Allergy News since January 2002. Skin & Allergy News is a publication of Frontline Medical Communications.
Zinc oxide, part 2
Nanotechnology, which applies gathered knowledge on the characteristics of matter to design new products on the nanoscale (<1,000 nm), emerged in the 1980s and has made great strides since then. Dermatology is a prime area of interest for nanotech applications, as numerous products using nanotechnology have been marketed. In fact, the sixth-largest U.S. patent holder in nanotechnology is a cosmetics company (Skin Therapy Lett. 2010;15:1-4). The newest generation of skin products is characterized by improved skin penetration (Arch. Dermatol. Res. 2011;303:533-50), and these products may have a role in enhancing the treatment of several skin disorders; however, toxicological studies must establish the safety of formulations increasingly likely to penetrate multiple skin layers.
Zinc oxide (ZnO) and titanium dioxide (TiO2) are two of the most prominent ingredients in the dermatologic armamentarium that are used in micro- and nanoparticle forms. Efficacy has been well established for these ingredients as inorganic sunscreens, but their relative safety has been debated and remains somewhat controversial. This column discusses findings regarding the safety of ZnO nanoparticles.
Elevated risk
Absorption and effects of zinc ions. In a small study (n = 20) in humans conducted in 2010, Gulson et al. found that small amounts of zinc from ZnO in sunscreens applied for five consecutive days outdoors were absorbed in the skin, with levels of the stable isotope tracer (68)Zn in blood and urine from females receiving the nano sunscreen higher than in males receiving the same sunscreen and higher than in all participants who received the bulk sunscreen (Toxicol. Sci. 2010;118:140-9).
In 2010, Martorano et al. examined the separation of zinc ions from ZnO in commercial sunscreens under UVB exposure and studied the effects of zinc ion accumulation in human epidermal keratinocytes. They noted that UVB light exposure led to a significant concentration-dependent and radiation intensity–dependent rise in zinc ion levels. In turn, a late- or delayed-type cytotoxicity in human epidermal keratinocytes was observed, as was the induction of reactive oxygen species (ROS) in the keratinocytes. The investigators concluded that UVB exposure leads to an elevation in zinc ion dissociation in ZnO sunscreen, yielding cytotoxic effects and oxidative stress (J. Cosmet. Dermatol. 2010;9:276-86).
Genotoxic potential. As Wang and Tooley aptly noted, the concerns regarding the safety of nanoparticles in sunscreens pertain to potential toxicity and capacity to penetrate the skin (Sem. Cutan. Med. Surg. 2011;30:210-13).
In a 2010 in vitro study of the toxicity of ZnO and TiO2 on keratinocytes over short- and long-term application periods, Kocbek et al. found that ZnO nanoparticles conferred more adverse effects than TiO2, with ZnO inhibiting cell viability above 15 mcg/mL after brief exposure while TiO2 exerted no effect up to 100 mcg/mL. Prolonged exposure to ZnO nanoparticles at 10 mcg/mL yielded diminished mitochondrial activity as well as changes in cell morphology and cell-cycle distribution; no such changes were associated with TiO2 nanoparticles. The researchers also reported more nanotubular intercellular structures in keratinocytes exposed to either nanoparticle type as compared to unexposed cells and nanoparticles present in vesicles within the cell cytoplasm. Finally, they observed that partially soluble ZnO spurred the synthesis of ROS, as opposed to insoluble TiO2. They concluded that their findings of an adverse effect on human keratinocytes suggest that long-term exposure to ZnO and TiO2 nanoparticles poses a possible health risk (Small 2010;6:1908-17).
In early 2011, Sharma et al. studied the cytotoxic and genotoxic potential of ZnO nanoparticles in the human liver carcinoma cell line HepG2, given what they argued was the pervasiveness of ZnO in consumer products and industrial applications and the concomitant likelihood of transmission to the liver. Their various assays revealed a significant concentration- and time-dependent toxicity after 12 and 24 hours at 14 and 20 mcg/mL, as well as a significant surge in DNA damage in cells exposed to ZnO nanoparticles for 6 hours (J. Biomed. Nanotechnol. 2011;7:98-9).
Previously, in 2009, Sharma et al. had investigated the potential genotoxicity of ZnO nanoparticles in the human epidermal cell line A431. They found concentration- and time-dependent decreases in cell viability as well as DNA damage potential, as revealed by Comet assay results. In addition, oxidative stress was provoked by ZnO nanoparticles, as evidenced by significant reductions in glutathione, catalase, and superoxide dismutase. The investigators urged caution related to dermatologic formulations containing ZnO nanoparticles, suggesting that their findings indicate a genotoxic potential in human epidermal cells, possibly mediated via lipid peroxidation and oxidative stress (Toxicol. Lett. 2009;185:211-8).
In May 2011, Sharma et al. investigated the biological interactions of ZnO nanoparticles in human epidermal keratinocytes, where electron microscopy showed the internalization of the nanoparticles after 6 hours of exposure at a concentration of 14 mcg/mL. Various assays revealed a time- and concentration-dependent suppression of mitochondrial activity as well as DNA damage in cells, compared with controls. The investigators concluded that ZnO nanoparticles are internalized by human epidermal keratinocytes and provoke a cytotoxic and genotoxic response, providing reason for caution when using consumer products containing nanoparticles. Specifically, they warned that any disruptions in the stratum corneum (SC) could allow the exposure of internal cells to nanoparticles (J. Nanosci. Nanotechnol. 2011;11:3782-8).
Also, in a recent study of the interactions of ZnO nanoparticles with the tumor suppressor p53, Ng et al. found that the p53 pathway was activated in BJ cells (skin fibroblasts) upon treatment with ZnO nanoparticles, leading to a reduction in cell numbers. One implication of this response, the researchers concluded, was that in cells lacking robust p53, the protective response can be turned toward carcinogenesis due to exposure to DNA damage–inducing agents like ZnO nanoparticles (Biomaterials 2011;32:8218-25).
Weight of evidence
However, several researchers contend that current data strongly suggest that nanosized ZnO and TiO2 do not, in fact, pose such risks (Photodermatol. Photoimmunol. Photomed. 2011;27:58-67; Int. J. Dermatol. 2011;50:247-54; Sem. Cutan. Med. Surg. 2011;30:210-13).
In 2009, in response to increasing concerns about the potential adverse effects of ZnO- and TiO2-coated nanoparticles used in physical sunblocks, Filipe et al. evaluated the localization and possible skin penetration of these nanoparticles in three sunscreen formulations under realistic in vivo conditions in normal and altered skin. They tested a hydrophobic formulation containing coated 20-nm TiO2 nanoparticles and two commercially available formulations containing TiO2 alone or in combination with ZnO. The goal was to assess how consumers actually use sunscreens in comparison to the recommended standard condition for the sun protection factor test. Traces of the physical blockers could only be detected at the skin surface and uppermost area of the SC in normal human skin after a 2-hour exposure. No ZnO or TiO2 nanoparticles could be detected in layers deeper than the SC after 48 hours of exposure. The investigators concluded that significant penetration by ZnO or TiO2 nanoparticles into keratinocytes is unlikely (Skin Pharmacol. Physiol. 2009;22:266-75).
According to a safety review by Schilling et al., the current evidence implies that there are minimal risks to human health posed from the use of ZnO or TiO2 nanoparticles at concentrations of up to 25% in cosmetic preparations or sunscreens, regardless of coatings or crystalline structure. The researchers observed that these nanoparticles incorporated in topical products occur as aggregates of primary particles 30-150 nm in size that bond in a way that leaves them impervious to the force of product application. Consequently, their structure is unaffected, and no primary particles are released (Photochem. Photobiol. Sci. 2010;9:495-509).
Newman et al. reviewed studies and position statements from 1980 to 2008 in order to characterize the safety, use, and regulatory conditions related to nanosized ZnO and TiO2 in sunscreens. They reported that, while no data suggested significant penetration of the particles beyond the SC, there is a need for additional studies simulating real-world conditions, especially related to UV exposure and sunburned skin (J. Am. Acad. Dermatol. 2009;61:685-92).
In 2011, Monteiro-Riviere et al. performed in vitro and in vivo studies in which pigs received moderate sunburn from UVB exposure. The researchers found that UVB-damaged skin slightly mediated ZnO or TiO2 nanoparticle penetration in multiple tested sunscreen formulations, but they observed no transdermal absorption (Toxicol. Sci. 2011;123:264-80).
Conclusion
Zinc oxide has long been used as one of the two primary inorganic physical sunscreens. Its use in nanoparticle form has appeared effective, but the different physicochemical qualities of the metal oxide in nanosized form have prompted questions regarding safety. Current data suggest minimal risk to intact skin, but additional studies are needed.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, April 2002), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001 and joined the editorial advisory board in 2004. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
Nanotechnology, which applies gathered knowledge on the characteristics of matter to design new products on the nanoscale (<1,000 nm), emerged in the 1980s and has made great strides since then. Dermatology is a prime area of interest for nanotech applications, as numerous products using nanotechnology have been marketed. In fact, the sixth-largest U.S. patent holder in nanotechnology is a cosmetics company (Skin Therapy Lett. 2010;15:1-4). The newest generation of skin products is characterized by improved skin penetration (Arch. Dermatol. Res. 2011;303:533-50), and these products may have a role in enhancing the treatment of several skin disorders; however, toxicological studies must establish the safety of formulations increasingly likely to penetrate multiple skin layers.
Zinc oxide (ZnO) and titanium dioxide (TiO2) are two of the most prominent ingredients in the dermatologic armamentarium that are used in micro- and nanoparticle forms. Efficacy has been well established for these ingredients as inorganic sunscreens, but their relative safety has been debated and remains somewhat controversial. This column discusses findings regarding the safety of ZnO nanoparticles.
Elevated risk
Absorption and effects of zinc ions. In a small study (n = 20) in humans conducted in 2010, Gulson et al. found that small amounts of zinc from ZnO in sunscreens applied for five consecutive days outdoors were absorbed in the skin, with levels of the stable isotope tracer (68)Zn in blood and urine from females receiving the nano sunscreen higher than in males receiving the same sunscreen and higher than in all participants who received the bulk sunscreen (Toxicol. Sci. 2010;118:140-9).
In 2010, Martorano et al. examined the separation of zinc ions from ZnO in commercial sunscreens under UVB exposure and studied the effects of zinc ion accumulation in human epidermal keratinocytes. They noted that UVB light exposure led to a significant concentration-dependent and radiation intensity–dependent rise in zinc ion levels. In turn, a late- or delayed-type cytotoxicity in human epidermal keratinocytes was observed, as was the induction of reactive oxygen species (ROS) in the keratinocytes. The investigators concluded that UVB exposure leads to an elevation in zinc ion dissociation in ZnO sunscreen, yielding cytotoxic effects and oxidative stress (J. Cosmet. Dermatol. 2010;9:276-86).
Genotoxic potential. As Wang and Tooley aptly noted, the concerns regarding the safety of nanoparticles in sunscreens pertain to potential toxicity and capacity to penetrate the skin (Sem. Cutan. Med. Surg. 2011;30:210-13).
In a 2010 in vitro study of the toxicity of ZnO and TiO2 on keratinocytes over short- and long-term application periods, Kocbek et al. found that ZnO nanoparticles conferred more adverse effects than TiO2, with ZnO inhibiting cell viability above 15 mcg/mL after brief exposure while TiO2 exerted no effect up to 100 mcg/mL. Prolonged exposure to ZnO nanoparticles at 10 mcg/mL yielded diminished mitochondrial activity as well as changes in cell morphology and cell-cycle distribution; no such changes were associated with TiO2 nanoparticles. The researchers also reported more nanotubular intercellular structures in keratinocytes exposed to either nanoparticle type as compared to unexposed cells and nanoparticles present in vesicles within the cell cytoplasm. Finally, they observed that partially soluble ZnO spurred the synthesis of ROS, as opposed to insoluble TiO2. They concluded that their findings of an adverse effect on human keratinocytes suggest that long-term exposure to ZnO and TiO2 nanoparticles poses a possible health risk (Small 2010;6:1908-17).
In early 2011, Sharma et al. studied the cytotoxic and genotoxic potential of ZnO nanoparticles in the human liver carcinoma cell line HepG2, given what they argued was the pervasiveness of ZnO in consumer products and industrial applications and the concomitant likelihood of transmission to the liver. Their various assays revealed a significant concentration- and time-dependent toxicity after 12 and 24 hours at 14 and 20 mcg/mL, as well as a significant surge in DNA damage in cells exposed to ZnO nanoparticles for 6 hours (J. Biomed. Nanotechnol. 2011;7:98-9).
Previously, in 2009, Sharma et al. had investigated the potential genotoxicity of ZnO nanoparticles in the human epidermal cell line A431. They found concentration- and time-dependent decreases in cell viability as well as DNA damage potential, as revealed by Comet assay results. In addition, oxidative stress was provoked by ZnO nanoparticles, as evidenced by significant reductions in glutathione, catalase, and superoxide dismutase. The investigators urged caution related to dermatologic formulations containing ZnO nanoparticles, suggesting that their findings indicate a genotoxic potential in human epidermal cells, possibly mediated via lipid peroxidation and oxidative stress (Toxicol. Lett. 2009;185:211-8).
In May 2011, Sharma et al. investigated the biological interactions of ZnO nanoparticles in human epidermal keratinocytes, where electron microscopy showed the internalization of the nanoparticles after 6 hours of exposure at a concentration of 14 mcg/mL. Various assays revealed a time- and concentration-dependent suppression of mitochondrial activity as well as DNA damage in cells, compared with controls. The investigators concluded that ZnO nanoparticles are internalized by human epidermal keratinocytes and provoke a cytotoxic and genotoxic response, providing reason for caution when using consumer products containing nanoparticles. Specifically, they warned that any disruptions in the stratum corneum (SC) could allow the exposure of internal cells to nanoparticles (J. Nanosci. Nanotechnol. 2011;11:3782-8).
Also, in a recent study of the interactions of ZnO nanoparticles with the tumor suppressor p53, Ng et al. found that the p53 pathway was activated in BJ cells (skin fibroblasts) upon treatment with ZnO nanoparticles, leading to a reduction in cell numbers. One implication of this response, the researchers concluded, was that in cells lacking robust p53, the protective response can be turned toward carcinogenesis due to exposure to DNA damage–inducing agents like ZnO nanoparticles (Biomaterials 2011;32:8218-25).
Weight of evidence
However, several researchers contend that current data strongly suggest that nanosized ZnO and TiO2 do not, in fact, pose such risks (Photodermatol. Photoimmunol. Photomed. 2011;27:58-67; Int. J. Dermatol. 2011;50:247-54; Sem. Cutan. Med. Surg. 2011;30:210-13).
In 2009, in response to increasing concerns about the potential adverse effects of ZnO- and TiO2-coated nanoparticles used in physical sunblocks, Filipe et al. evaluated the localization and possible skin penetration of these nanoparticles in three sunscreen formulations under realistic in vivo conditions in normal and altered skin. They tested a hydrophobic formulation containing coated 20-nm TiO2 nanoparticles and two commercially available formulations containing TiO2 alone or in combination with ZnO. The goal was to assess how consumers actually use sunscreens in comparison to the recommended standard condition for the sun protection factor test. Traces of the physical blockers could only be detected at the skin surface and uppermost area of the SC in normal human skin after a 2-hour exposure. No ZnO or TiO2 nanoparticles could be detected in layers deeper than the SC after 48 hours of exposure. The investigators concluded that significant penetration by ZnO or TiO2 nanoparticles into keratinocytes is unlikely (Skin Pharmacol. Physiol. 2009;22:266-75).
According to a safety review by Schilling et al., the current evidence implies that there are minimal risks to human health posed from the use of ZnO or TiO2 nanoparticles at concentrations of up to 25% in cosmetic preparations or sunscreens, regardless of coatings or crystalline structure. The researchers observed that these nanoparticles incorporated in topical products occur as aggregates of primary particles 30-150 nm in size that bond in a way that leaves them impervious to the force of product application. Consequently, their structure is unaffected, and no primary particles are released (Photochem. Photobiol. Sci. 2010;9:495-509).
Newman et al. reviewed studies and position statements from 1980 to 2008 in order to characterize the safety, use, and regulatory conditions related to nanosized ZnO and TiO2 in sunscreens. They reported that, while no data suggested significant penetration of the particles beyond the SC, there is a need for additional studies simulating real-world conditions, especially related to UV exposure and sunburned skin (J. Am. Acad. Dermatol. 2009;61:685-92).
In 2011, Monteiro-Riviere et al. performed in vitro and in vivo studies in which pigs received moderate sunburn from UVB exposure. The researchers found that UVB-damaged skin slightly mediated ZnO or TiO2 nanoparticle penetration in multiple tested sunscreen formulations, but they observed no transdermal absorption (Toxicol. Sci. 2011;123:264-80).
Conclusion
Zinc oxide has long been used as one of the two primary inorganic physical sunscreens. Its use in nanoparticle form has appeared effective, but the different physicochemical qualities of the metal oxide in nanosized form have prompted questions regarding safety. Current data suggest minimal risk to intact skin, but additional studies are needed.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, April 2002), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001 and joined the editorial advisory board in 2004. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
Nanotechnology, which applies gathered knowledge on the characteristics of matter to design new products on the nanoscale (<1,000 nm), emerged in the 1980s and has made great strides since then. Dermatology is a prime area of interest for nanotech applications, as numerous products using nanotechnology have been marketed. In fact, the sixth-largest U.S. patent holder in nanotechnology is a cosmetics company (Skin Therapy Lett. 2010;15:1-4). The newest generation of skin products is characterized by improved skin penetration (Arch. Dermatol. Res. 2011;303:533-50), and these products may have a role in enhancing the treatment of several skin disorders; however, toxicological studies must establish the safety of formulations increasingly likely to penetrate multiple skin layers.
Zinc oxide (ZnO) and titanium dioxide (TiO2) are two of the most prominent ingredients in the dermatologic armamentarium that are used in micro- and nanoparticle forms. Efficacy has been well established for these ingredients as inorganic sunscreens, but their relative safety has been debated and remains somewhat controversial. This column discusses findings regarding the safety of ZnO nanoparticles.
Elevated risk
Absorption and effects of zinc ions. In a small study (n = 20) in humans conducted in 2010, Gulson et al. found that small amounts of zinc from ZnO in sunscreens applied for five consecutive days outdoors were absorbed in the skin, with levels of the stable isotope tracer (68)Zn in blood and urine from females receiving the nano sunscreen higher than in males receiving the same sunscreen and higher than in all participants who received the bulk sunscreen (Toxicol. Sci. 2010;118:140-9).
In 2010, Martorano et al. examined the separation of zinc ions from ZnO in commercial sunscreens under UVB exposure and studied the effects of zinc ion accumulation in human epidermal keratinocytes. They noted that UVB light exposure led to a significant concentration-dependent and radiation intensity–dependent rise in zinc ion levels. In turn, a late- or delayed-type cytotoxicity in human epidermal keratinocytes was observed, as was the induction of reactive oxygen species (ROS) in the keratinocytes. The investigators concluded that UVB exposure leads to an elevation in zinc ion dissociation in ZnO sunscreen, yielding cytotoxic effects and oxidative stress (J. Cosmet. Dermatol. 2010;9:276-86).
Genotoxic potential. As Wang and Tooley aptly noted, the concerns regarding the safety of nanoparticles in sunscreens pertain to potential toxicity and capacity to penetrate the skin (Sem. Cutan. Med. Surg. 2011;30:210-13).
In a 2010 in vitro study of the toxicity of ZnO and TiO2 on keratinocytes over short- and long-term application periods, Kocbek et al. found that ZnO nanoparticles conferred more adverse effects than TiO2, with ZnO inhibiting cell viability above 15 mcg/mL after brief exposure while TiO2 exerted no effect up to 100 mcg/mL. Prolonged exposure to ZnO nanoparticles at 10 mcg/mL yielded diminished mitochondrial activity as well as changes in cell morphology and cell-cycle distribution; no such changes were associated with TiO2 nanoparticles. The researchers also reported more nanotubular intercellular structures in keratinocytes exposed to either nanoparticle type as compared to unexposed cells and nanoparticles present in vesicles within the cell cytoplasm. Finally, they observed that partially soluble ZnO spurred the synthesis of ROS, as opposed to insoluble TiO2. They concluded that their findings of an adverse effect on human keratinocytes suggest that long-term exposure to ZnO and TiO2 nanoparticles poses a possible health risk (Small 2010;6:1908-17).
In early 2011, Sharma et al. studied the cytotoxic and genotoxic potential of ZnO nanoparticles in the human liver carcinoma cell line HepG2, given what they argued was the pervasiveness of ZnO in consumer products and industrial applications and the concomitant likelihood of transmission to the liver. Their various assays revealed a significant concentration- and time-dependent toxicity after 12 and 24 hours at 14 and 20 mcg/mL, as well as a significant surge in DNA damage in cells exposed to ZnO nanoparticles for 6 hours (J. Biomed. Nanotechnol. 2011;7:98-9).
Previously, in 2009, Sharma et al. had investigated the potential genotoxicity of ZnO nanoparticles in the human epidermal cell line A431. They found concentration- and time-dependent decreases in cell viability as well as DNA damage potential, as revealed by Comet assay results. In addition, oxidative stress was provoked by ZnO nanoparticles, as evidenced by significant reductions in glutathione, catalase, and superoxide dismutase. The investigators urged caution related to dermatologic formulations containing ZnO nanoparticles, suggesting that their findings indicate a genotoxic potential in human epidermal cells, possibly mediated via lipid peroxidation and oxidative stress (Toxicol. Lett. 2009;185:211-8).
In May 2011, Sharma et al. investigated the biological interactions of ZnO nanoparticles in human epidermal keratinocytes, where electron microscopy showed the internalization of the nanoparticles after 6 hours of exposure at a concentration of 14 mcg/mL. Various assays revealed a time- and concentration-dependent suppression of mitochondrial activity as well as DNA damage in cells, compared with controls. The investigators concluded that ZnO nanoparticles are internalized by human epidermal keratinocytes and provoke a cytotoxic and genotoxic response, providing reason for caution when using consumer products containing nanoparticles. Specifically, they warned that any disruptions in the stratum corneum (SC) could allow the exposure of internal cells to nanoparticles (J. Nanosci. Nanotechnol. 2011;11:3782-8).
Also, in a recent study of the interactions of ZnO nanoparticles with the tumor suppressor p53, Ng et al. found that the p53 pathway was activated in BJ cells (skin fibroblasts) upon treatment with ZnO nanoparticles, leading to a reduction in cell numbers. One implication of this response, the researchers concluded, was that in cells lacking robust p53, the protective response can be turned toward carcinogenesis due to exposure to DNA damage–inducing agents like ZnO nanoparticles (Biomaterials 2011;32:8218-25).
Weight of evidence
However, several researchers contend that current data strongly suggest that nanosized ZnO and TiO2 do not, in fact, pose such risks (Photodermatol. Photoimmunol. Photomed. 2011;27:58-67; Int. J. Dermatol. 2011;50:247-54; Sem. Cutan. Med. Surg. 2011;30:210-13).
In 2009, in response to increasing concerns about the potential adverse effects of ZnO- and TiO2-coated nanoparticles used in physical sunblocks, Filipe et al. evaluated the localization and possible skin penetration of these nanoparticles in three sunscreen formulations under realistic in vivo conditions in normal and altered skin. They tested a hydrophobic formulation containing coated 20-nm TiO2 nanoparticles and two commercially available formulations containing TiO2 alone or in combination with ZnO. The goal was to assess how consumers actually use sunscreens in comparison to the recommended standard condition for the sun protection factor test. Traces of the physical blockers could only be detected at the skin surface and uppermost area of the SC in normal human skin after a 2-hour exposure. No ZnO or TiO2 nanoparticles could be detected in layers deeper than the SC after 48 hours of exposure. The investigators concluded that significant penetration by ZnO or TiO2 nanoparticles into keratinocytes is unlikely (Skin Pharmacol. Physiol. 2009;22:266-75).
According to a safety review by Schilling et al., the current evidence implies that there are minimal risks to human health posed from the use of ZnO or TiO2 nanoparticles at concentrations of up to 25% in cosmetic preparations or sunscreens, regardless of coatings or crystalline structure. The researchers observed that these nanoparticles incorporated in topical products occur as aggregates of primary particles 30-150 nm in size that bond in a way that leaves them impervious to the force of product application. Consequently, their structure is unaffected, and no primary particles are released (Photochem. Photobiol. Sci. 2010;9:495-509).
Newman et al. reviewed studies and position statements from 1980 to 2008 in order to characterize the safety, use, and regulatory conditions related to nanosized ZnO and TiO2 in sunscreens. They reported that, while no data suggested significant penetration of the particles beyond the SC, there is a need for additional studies simulating real-world conditions, especially related to UV exposure and sunburned skin (J. Am. Acad. Dermatol. 2009;61:685-92).
In 2011, Monteiro-Riviere et al. performed in vitro and in vivo studies in which pigs received moderate sunburn from UVB exposure. The researchers found that UVB-damaged skin slightly mediated ZnO or TiO2 nanoparticle penetration in multiple tested sunscreen formulations, but they observed no transdermal absorption (Toxicol. Sci. 2011;123:264-80).
Conclusion
Zinc oxide has long been used as one of the two primary inorganic physical sunscreens. Its use in nanoparticle form has appeared effective, but the different physicochemical qualities of the metal oxide in nanosized form have prompted questions regarding safety. Current data suggest minimal risk to intact skin, but additional studies are needed.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, April 2002), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001 and joined the editorial advisory board in 2004. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
ONO-4059 makes waves in heavily pretreated CLL
NEW ORLEANS – Early data suggest that the second-generation oral BTK inhibitor ONO-4059 may give ibrutinib a run for its money in chronic lymphocytic leukemia.
The response rate to ONO-4059 monotherapy was 89% overall and 71% in those with the deleterious 17p deletion among 18 heavily pretreated patients with relapsed/refractory or high-risk CLL in a phase I, dose-escalation study.
Patients had already received a median of three prior therapies, including rituximab (84%) and fludarabine (95%), and had no higher priority therapy available to them, said Dr. Gilles Salles of Hospices Civils de Lyon (France), Universite Claude Bernard Lyon.
All patients had improved hemoglobin and platelet counts after 3 months on treatment and rapid reductions in lymph node size within the first 28-day cycle. Tumor burden was reduced by 50% for most patients, and all but one patient experienced a response that was detectable on a CT scan.
"This was true whatever their FISH status or 17p or 11q deletion status," Dr. Salles said at the annual meeting of the American Society of Hematology.
ONO-4059 is a highly selective Bruton’s tyrosine kinase (BTK) inhibitor with antitumor activity in several preclinical models.
No patients had received prior treatment with a P13 kinase or a BTK inhibitor, including ibrutinib (Imbruvica), which recently gained accelerated approval for previously treated mantle cell lymphoma.
ONO-4059 was given at daily doses ranging from 20 mg to 320 mg for up to 6 months, with the option of additional dosing up to 2 years. Sustained BTK inhibition was established at doses of 40 mg and higher.
Overall, the best response was a partial response in 14 patients, as well as two partial responses with lymphocytosis and one stable disease, he said. No complete responses occurred.
One patient progressed roughly 1 month after showing an initial response and complete disappearance of all palpable disease on physical exam. Richter’s syndrome was suspected.
"It’s very promising efficacy in this highly pretreated population," Dr. Salles said.
Patients with relapsed/refractory mantle cell lymphoma and diffuse large B-cell lymphoma, especially the ABC subtype, also appear sensitive to ONO-4059. Overall response rates were 43% and 75%, respectively, including three complete responses reported from the phase I study in a separate poster presentation at the meeting.
ONO-4059 had a favorable safety profile with a single dose-limiting toxicity observed in a patient who had Waldenstrom’s macroglobulinemia, was on the 320-mg dose, and was intolerant to all prior therapies. The maximum tolerated dose has not yet been reached.
The majority of adverse events in the CLL patients were grades 1 and 2. There were no clinically significant bleeding events or bruising, and there was a low incidence of diarrhea and rash, Dr. Salles said.
ONO-4059–related grade 3-4 events were independent of dose and included one grade 3 neutropenia at 20 mg and two grade 4 events at 20 mg and 320 mg. Four serious adverse events (febrile neutropenia, pyrexia, rash, and neutropenia) occurred in three patients, all of whom are still in the study and showing good clinical response, Dr. Salles said. Of the 30 patients dosed to date, 22 remain in the study.
No other trials are firmly planned, and pharmacokinetics/pharmacodynamics data continue to be explored in order to assess a phase II dosage, he said in an interview.
Dr. Salles reported consulting for and receiving honoraria from Roche. Several coauthors have financial ties, including employment with the study sponsor, Ono Pharmaceutical, which is developing ONO-4059.
NEW ORLEANS – Early data suggest that the second-generation oral BTK inhibitor ONO-4059 may give ibrutinib a run for its money in chronic lymphocytic leukemia.
The response rate to ONO-4059 monotherapy was 89% overall and 71% in those with the deleterious 17p deletion among 18 heavily pretreated patients with relapsed/refractory or high-risk CLL in a phase I, dose-escalation study.
Patients had already received a median of three prior therapies, including rituximab (84%) and fludarabine (95%), and had no higher priority therapy available to them, said Dr. Gilles Salles of Hospices Civils de Lyon (France), Universite Claude Bernard Lyon.
All patients had improved hemoglobin and platelet counts after 3 months on treatment and rapid reductions in lymph node size within the first 28-day cycle. Tumor burden was reduced by 50% for most patients, and all but one patient experienced a response that was detectable on a CT scan.
"This was true whatever their FISH status or 17p or 11q deletion status," Dr. Salles said at the annual meeting of the American Society of Hematology.
ONO-4059 is a highly selective Bruton’s tyrosine kinase (BTK) inhibitor with antitumor activity in several preclinical models.
No patients had received prior treatment with a P13 kinase or a BTK inhibitor, including ibrutinib (Imbruvica), which recently gained accelerated approval for previously treated mantle cell lymphoma.
ONO-4059 was given at daily doses ranging from 20 mg to 320 mg for up to 6 months, with the option of additional dosing up to 2 years. Sustained BTK inhibition was established at doses of 40 mg and higher.
Overall, the best response was a partial response in 14 patients, as well as two partial responses with lymphocytosis and one stable disease, he said. No complete responses occurred.
One patient progressed roughly 1 month after showing an initial response and complete disappearance of all palpable disease on physical exam. Richter’s syndrome was suspected.
"It’s very promising efficacy in this highly pretreated population," Dr. Salles said.
Patients with relapsed/refractory mantle cell lymphoma and diffuse large B-cell lymphoma, especially the ABC subtype, also appear sensitive to ONO-4059. Overall response rates were 43% and 75%, respectively, including three complete responses reported from the phase I study in a separate poster presentation at the meeting.
ONO-4059 had a favorable safety profile with a single dose-limiting toxicity observed in a patient who had Waldenstrom’s macroglobulinemia, was on the 320-mg dose, and was intolerant to all prior therapies. The maximum tolerated dose has not yet been reached.
The majority of adverse events in the CLL patients were grades 1 and 2. There were no clinically significant bleeding events or bruising, and there was a low incidence of diarrhea and rash, Dr. Salles said.
ONO-4059–related grade 3-4 events were independent of dose and included one grade 3 neutropenia at 20 mg and two grade 4 events at 20 mg and 320 mg. Four serious adverse events (febrile neutropenia, pyrexia, rash, and neutropenia) occurred in three patients, all of whom are still in the study and showing good clinical response, Dr. Salles said. Of the 30 patients dosed to date, 22 remain in the study.
No other trials are firmly planned, and pharmacokinetics/pharmacodynamics data continue to be explored in order to assess a phase II dosage, he said in an interview.
Dr. Salles reported consulting for and receiving honoraria from Roche. Several coauthors have financial ties, including employment with the study sponsor, Ono Pharmaceutical, which is developing ONO-4059.
NEW ORLEANS – Early data suggest that the second-generation oral BTK inhibitor ONO-4059 may give ibrutinib a run for its money in chronic lymphocytic leukemia.
The response rate to ONO-4059 monotherapy was 89% overall and 71% in those with the deleterious 17p deletion among 18 heavily pretreated patients with relapsed/refractory or high-risk CLL in a phase I, dose-escalation study.
Patients had already received a median of three prior therapies, including rituximab (84%) and fludarabine (95%), and had no higher priority therapy available to them, said Dr. Gilles Salles of Hospices Civils de Lyon (France), Universite Claude Bernard Lyon.
All patients had improved hemoglobin and platelet counts after 3 months on treatment and rapid reductions in lymph node size within the first 28-day cycle. Tumor burden was reduced by 50% for most patients, and all but one patient experienced a response that was detectable on a CT scan.
"This was true whatever their FISH status or 17p or 11q deletion status," Dr. Salles said at the annual meeting of the American Society of Hematology.
ONO-4059 is a highly selective Bruton’s tyrosine kinase (BTK) inhibitor with antitumor activity in several preclinical models.
No patients had received prior treatment with a P13 kinase or a BTK inhibitor, including ibrutinib (Imbruvica), which recently gained accelerated approval for previously treated mantle cell lymphoma.
ONO-4059 was given at daily doses ranging from 20 mg to 320 mg for up to 6 months, with the option of additional dosing up to 2 years. Sustained BTK inhibition was established at doses of 40 mg and higher.
Overall, the best response was a partial response in 14 patients, as well as two partial responses with lymphocytosis and one stable disease, he said. No complete responses occurred.
One patient progressed roughly 1 month after showing an initial response and complete disappearance of all palpable disease on physical exam. Richter’s syndrome was suspected.
"It’s very promising efficacy in this highly pretreated population," Dr. Salles said.
Patients with relapsed/refractory mantle cell lymphoma and diffuse large B-cell lymphoma, especially the ABC subtype, also appear sensitive to ONO-4059. Overall response rates were 43% and 75%, respectively, including three complete responses reported from the phase I study in a separate poster presentation at the meeting.
ONO-4059 had a favorable safety profile with a single dose-limiting toxicity observed in a patient who had Waldenstrom’s macroglobulinemia, was on the 320-mg dose, and was intolerant to all prior therapies. The maximum tolerated dose has not yet been reached.
The majority of adverse events in the CLL patients were grades 1 and 2. There were no clinically significant bleeding events or bruising, and there was a low incidence of diarrhea and rash, Dr. Salles said.
ONO-4059–related grade 3-4 events were independent of dose and included one grade 3 neutropenia at 20 mg and two grade 4 events at 20 mg and 320 mg. Four serious adverse events (febrile neutropenia, pyrexia, rash, and neutropenia) occurred in three patients, all of whom are still in the study and showing good clinical response, Dr. Salles said. Of the 30 patients dosed to date, 22 remain in the study.
No other trials are firmly planned, and pharmacokinetics/pharmacodynamics data continue to be explored in order to assess a phase II dosage, he said in an interview.
Dr. Salles reported consulting for and receiving honoraria from Roche. Several coauthors have financial ties, including employment with the study sponsor, Ono Pharmaceutical, which is developing ONO-4059.
AT ASH 2013
Major finding: The response rate was 89% overall and 71% for patients with 17p deletion.
Data source: A prospective, phase I dose-escalation study in 18 patients with relapsed/refractory or high-risk CLL.
Disclosures: Dr. Salles reported honoraria from Janssen, Gilead, and Celgene. Several coauthors have financial ties, including employment with the study sponsor, Ono Pharmaceutical, which is developing ONO-4059.
Study reveals RBC function in clot contraction

Red blood cells (RBCs) take on a new shape and perform important functions in contracted blood clots, a new study suggests.
Researchers found that, during clot contraction, RBCs can be compressed into many-sided, closely packed, polyhedral structures.
These polyhedral RBCs form an impermeable seal within the clot to stem bleeding and help prevent vascular obstruction. And the cells may be the reason fibrinolysis is hampered after clot contraction.
John Weisel, PhD, of the University of Pennsylvania in Philadelphia, and his colleagues described these findings in Blood.
The researchers knew that, after a blood clot forms, the actin and myosin in platelets start the contraction process and cause the clot to shrink to about one-third of its original size. RBCs are caught up in the contraction process and get pulled by platelets toward the interior of the clot.
But little was known about the structure of contracted clots or the role RBCs play in the contraction process. So Dr Weisel and his colleagues decided to study clot contraction using a novel magnetic resonance technology.
“We found that contracted blood clots develop a remarkable structure, with a meshwork of fibrin and platelet aggregates on the exterior of the clot and a close-packed, tessellated array of compressed polyhedral erythrocytes within,” Dr Weisel said.
The team saw the same morphology in clots created from human blood reconstituted with its cellular and plasma components, as well as clots made with mouse blood.
The polyhedral erythrocytes, or polyhedrocytes as the researchers named them, were also present in human arterial thrombi taken from patients with myocardial infarctions.
The researchers believe the RBCs take on the polyhedral shape so as to decrease volume, surface energy, or bending energy.
The team said their findings might have clinical implications. It is well known that, with time, thrombi develop resistance to being broken up by thrombolytic agents.
And the nearly impermeable barrier formed by RBCs within the contracted clots may help to explain why. Clot contraction could be a target of intervention to prevent the formation of the closely packed polyhedrocytes. ![]()

Red blood cells (RBCs) take on a new shape and perform important functions in contracted blood clots, a new study suggests.
Researchers found that, during clot contraction, RBCs can be compressed into many-sided, closely packed, polyhedral structures.
These polyhedral RBCs form an impermeable seal within the clot to stem bleeding and help prevent vascular obstruction. And the cells may be the reason fibrinolysis is hampered after clot contraction.
John Weisel, PhD, of the University of Pennsylvania in Philadelphia, and his colleagues described these findings in Blood.
The researchers knew that, after a blood clot forms, the actin and myosin in platelets start the contraction process and cause the clot to shrink to about one-third of its original size. RBCs are caught up in the contraction process and get pulled by platelets toward the interior of the clot.
But little was known about the structure of contracted clots or the role RBCs play in the contraction process. So Dr Weisel and his colleagues decided to study clot contraction using a novel magnetic resonance technology.
“We found that contracted blood clots develop a remarkable structure, with a meshwork of fibrin and platelet aggregates on the exterior of the clot and a close-packed, tessellated array of compressed polyhedral erythrocytes within,” Dr Weisel said.
The team saw the same morphology in clots created from human blood reconstituted with its cellular and plasma components, as well as clots made with mouse blood.
The polyhedral erythrocytes, or polyhedrocytes as the researchers named them, were also present in human arterial thrombi taken from patients with myocardial infarctions.
The researchers believe the RBCs take on the polyhedral shape so as to decrease volume, surface energy, or bending energy.
The team said their findings might have clinical implications. It is well known that, with time, thrombi develop resistance to being broken up by thrombolytic agents.
And the nearly impermeable barrier formed by RBCs within the contracted clots may help to explain why. Clot contraction could be a target of intervention to prevent the formation of the closely packed polyhedrocytes. ![]()

Red blood cells (RBCs) take on a new shape and perform important functions in contracted blood clots, a new study suggests.
Researchers found that, during clot contraction, RBCs can be compressed into many-sided, closely packed, polyhedral structures.
These polyhedral RBCs form an impermeable seal within the clot to stem bleeding and help prevent vascular obstruction. And the cells may be the reason fibrinolysis is hampered after clot contraction.
John Weisel, PhD, of the University of Pennsylvania in Philadelphia, and his colleagues described these findings in Blood.
The researchers knew that, after a blood clot forms, the actin and myosin in platelets start the contraction process and cause the clot to shrink to about one-third of its original size. RBCs are caught up in the contraction process and get pulled by platelets toward the interior of the clot.
But little was known about the structure of contracted clots or the role RBCs play in the contraction process. So Dr Weisel and his colleagues decided to study clot contraction using a novel magnetic resonance technology.
“We found that contracted blood clots develop a remarkable structure, with a meshwork of fibrin and platelet aggregates on the exterior of the clot and a close-packed, tessellated array of compressed polyhedral erythrocytes within,” Dr Weisel said.
The team saw the same morphology in clots created from human blood reconstituted with its cellular and plasma components, as well as clots made with mouse blood.
The polyhedral erythrocytes, or polyhedrocytes as the researchers named them, were also present in human arterial thrombi taken from patients with myocardial infarctions.
The researchers believe the RBCs take on the polyhedral shape so as to decrease volume, surface energy, or bending energy.
The team said their findings might have clinical implications. It is well known that, with time, thrombi develop resistance to being broken up by thrombolytic agents.
And the nearly impermeable barrier formed by RBCs within the contracted clots may help to explain why. Clot contraction could be a target of intervention to prevent the formation of the closely packed polyhedrocytes. ![]()
AML scoring system could optimize treatment

Credit: NIGMS
A scoring system that combines genetic and epigenetic changes could help guide therapy for acute myeloid leukemia (AML), according to a study published in the Journal of Clinical Oncology.
The score is based on the presence of 7 mutated genes and DNA methylation.
For each of these genes, lower expression and higher DNA methylation were associated with better patient outcomes.
The investigators therefore believe this scoring system could guide treatment by identifying novel subsets of patients.
“To date, disease classification and prognostication for AML patients have been based largely on chromosomal and genetic markers,” said principal investigator Clara D. Bloomfield, MD, of The Ohio State University in Columbus.
“Epigenetic changes that affect gene expression have not been considered. Here, we show that epigenetic changes in previously recognized and prognostically important mutated genes can identify novel patient subgroups, which might better help guide therapy.”
Creating the score
Dr Bloomfield and her colleagues identified the 7-gene panel in 134 patients who were 60 and older, had cytogenetically normal AML (CN-AML), and had been treated on Cancer and Leukemia Group B/Alliance clinical trials.
The investigators used next-generation sequencing to analyze regions of methylated DNA associated with prognostically important genetic mutations. The 7 genes they identified are CD34, RHOC, SCRN1, F2RL1, FAM92A1, MIR155HG, and VWA8.
The team then developed a summary score based on the number of genes in the panel showing high expression.
And they applied the unweighted score to 126 of the aforementioned patients. Individuals with 1 or no highly expressed genes had a 96% complete remission (CR) rate, a 3-year disease-free survival (DFS) rate of 32%, and a 3-year overall survival (OS) rate of 39%.
Patients with 6 to 7 highly expressed genes, on the other hand, had a 25% CR rate, a 3-year DFS of 0%, and a 3-year OS of 4%.
Validating the system
The investigators also tested the score in 4 validation cohorts: older patients (age 60 and up) with primary AML (n=72), younger patients (59 and under) with primary AML (n=134), older patients with CN-AML (n=65), and younger patients with CN-AML (n=84).
“In both younger and older patients, those who had no highly expressed genes, or had one highly expressed gene, had the best outcomes,” said study author Guido Marcucci, MD, of The Ohio State University Comprehensive Cancer Center.
For the younger patients (with primary or CN-AML), individuals with 1 or no highly expressed genes had a 91% to 100% CR rate, a 3-year DFS of 60% to 65%, and a 3-year OS of 76% to 82%.
But younger patients with 6 to 7 highly expressed genes had a 53% to 71% CR rate, a 3-year DFS of 13% to 17%, and a 3-year OS of 7% to 24%.
For the older patients, individuals with 1 or no highly expressed genes had a 69% to 89% CR rate, a 3-year DFS of 42% (CN-AML only), and a 3-year OS of 44% to 46%.
Older patients with 6 to 7 highly expressed genes had a 50% CR rate (both types of AML), a 3-year DFS of 0% (CN-AML only), and a 3-year OS of 10% to 12%. DFS data were not evaluable for the older patients with primary AML due to the small sample size.
“Overall, our findings suggest that the unweighted summary score is a better model compared with all other prognostic markers and previously reported gene-expression profiles,” Dr Bloomfield concluded. ![]()

Credit: NIGMS
A scoring system that combines genetic and epigenetic changes could help guide therapy for acute myeloid leukemia (AML), according to a study published in the Journal of Clinical Oncology.
The score is based on the presence of 7 mutated genes and DNA methylation.
For each of these genes, lower expression and higher DNA methylation were associated with better patient outcomes.
The investigators therefore believe this scoring system could guide treatment by identifying novel subsets of patients.
“To date, disease classification and prognostication for AML patients have been based largely on chromosomal and genetic markers,” said principal investigator Clara D. Bloomfield, MD, of The Ohio State University in Columbus.
“Epigenetic changes that affect gene expression have not been considered. Here, we show that epigenetic changes in previously recognized and prognostically important mutated genes can identify novel patient subgroups, which might better help guide therapy.”
Creating the score
Dr Bloomfield and her colleagues identified the 7-gene panel in 134 patients who were 60 and older, had cytogenetically normal AML (CN-AML), and had been treated on Cancer and Leukemia Group B/Alliance clinical trials.
The investigators used next-generation sequencing to analyze regions of methylated DNA associated with prognostically important genetic mutations. The 7 genes they identified are CD34, RHOC, SCRN1, F2RL1, FAM92A1, MIR155HG, and VWA8.
The team then developed a summary score based on the number of genes in the panel showing high expression.
And they applied the unweighted score to 126 of the aforementioned patients. Individuals with 1 or no highly expressed genes had a 96% complete remission (CR) rate, a 3-year disease-free survival (DFS) rate of 32%, and a 3-year overall survival (OS) rate of 39%.
Patients with 6 to 7 highly expressed genes, on the other hand, had a 25% CR rate, a 3-year DFS of 0%, and a 3-year OS of 4%.
Validating the system
The investigators also tested the score in 4 validation cohorts: older patients (age 60 and up) with primary AML (n=72), younger patients (59 and under) with primary AML (n=134), older patients with CN-AML (n=65), and younger patients with CN-AML (n=84).
“In both younger and older patients, those who had no highly expressed genes, or had one highly expressed gene, had the best outcomes,” said study author Guido Marcucci, MD, of The Ohio State University Comprehensive Cancer Center.
For the younger patients (with primary or CN-AML), individuals with 1 or no highly expressed genes had a 91% to 100% CR rate, a 3-year DFS of 60% to 65%, and a 3-year OS of 76% to 82%.
But younger patients with 6 to 7 highly expressed genes had a 53% to 71% CR rate, a 3-year DFS of 13% to 17%, and a 3-year OS of 7% to 24%.
For the older patients, individuals with 1 or no highly expressed genes had a 69% to 89% CR rate, a 3-year DFS of 42% (CN-AML only), and a 3-year OS of 44% to 46%.
Older patients with 6 to 7 highly expressed genes had a 50% CR rate (both types of AML), a 3-year DFS of 0% (CN-AML only), and a 3-year OS of 10% to 12%. DFS data were not evaluable for the older patients with primary AML due to the small sample size.
“Overall, our findings suggest that the unweighted summary score is a better model compared with all other prognostic markers and previously reported gene-expression profiles,” Dr Bloomfield concluded. ![]()

Credit: NIGMS
A scoring system that combines genetic and epigenetic changes could help guide therapy for acute myeloid leukemia (AML), according to a study published in the Journal of Clinical Oncology.
The score is based on the presence of 7 mutated genes and DNA methylation.
For each of these genes, lower expression and higher DNA methylation were associated with better patient outcomes.
The investigators therefore believe this scoring system could guide treatment by identifying novel subsets of patients.
“To date, disease classification and prognostication for AML patients have been based largely on chromosomal and genetic markers,” said principal investigator Clara D. Bloomfield, MD, of The Ohio State University in Columbus.
“Epigenetic changes that affect gene expression have not been considered. Here, we show that epigenetic changes in previously recognized and prognostically important mutated genes can identify novel patient subgroups, which might better help guide therapy.”
Creating the score
Dr Bloomfield and her colleagues identified the 7-gene panel in 134 patients who were 60 and older, had cytogenetically normal AML (CN-AML), and had been treated on Cancer and Leukemia Group B/Alliance clinical trials.
The investigators used next-generation sequencing to analyze regions of methylated DNA associated with prognostically important genetic mutations. The 7 genes they identified are CD34, RHOC, SCRN1, F2RL1, FAM92A1, MIR155HG, and VWA8.
The team then developed a summary score based on the number of genes in the panel showing high expression.
And they applied the unweighted score to 126 of the aforementioned patients. Individuals with 1 or no highly expressed genes had a 96% complete remission (CR) rate, a 3-year disease-free survival (DFS) rate of 32%, and a 3-year overall survival (OS) rate of 39%.
Patients with 6 to 7 highly expressed genes, on the other hand, had a 25% CR rate, a 3-year DFS of 0%, and a 3-year OS of 4%.
Validating the system
The investigators also tested the score in 4 validation cohorts: older patients (age 60 and up) with primary AML (n=72), younger patients (59 and under) with primary AML (n=134), older patients with CN-AML (n=65), and younger patients with CN-AML (n=84).
“In both younger and older patients, those who had no highly expressed genes, or had one highly expressed gene, had the best outcomes,” said study author Guido Marcucci, MD, of The Ohio State University Comprehensive Cancer Center.
For the younger patients (with primary or CN-AML), individuals with 1 or no highly expressed genes had a 91% to 100% CR rate, a 3-year DFS of 60% to 65%, and a 3-year OS of 76% to 82%.
But younger patients with 6 to 7 highly expressed genes had a 53% to 71% CR rate, a 3-year DFS of 13% to 17%, and a 3-year OS of 7% to 24%.
For the older patients, individuals with 1 or no highly expressed genes had a 69% to 89% CR rate, a 3-year DFS of 42% (CN-AML only), and a 3-year OS of 44% to 46%.
Older patients with 6 to 7 highly expressed genes had a 50% CR rate (both types of AML), a 3-year DFS of 0% (CN-AML only), and a 3-year OS of 10% to 12%. DFS data were not evaluable for the older patients with primary AML due to the small sample size.
“Overall, our findings suggest that the unweighted summary score is a better model compared with all other prognostic markers and previously reported gene-expression profiles,” Dr Bloomfield concluded. ![]()
Method can detect malaria through the skin

a red blood cell; Credit: St Jude
Children’s Research Hospital
Researchers say they have developed a diagnostic technique that can rapidly detect low levels of malaria infection through the skin.
The approach involves a low-powered laser that creates tiny vapor nanobubbles inside malaria-infected cells.
The bursting bubbles have a unique acoustic signature that allows for a sensitive diagnosis.
This method requires no dyes or diagnostic chemicals, and there is no need to draw blood.
A preclinical study published in PNAS showed that the method could detect a single malaria-infected cell among a million normal cells with 0 false-positive readings.
“Ours is the first through-the-skin method that’s been shown to rapidly and accurately detect malaria in seconds, without the use of blood sampling or reagents,” said lead investigator Dmitri Lapotko, PhD, of Rice University in Houston, Texas.
The transdermal diagnostic method takes advantage of the optical properties and nanosize of hemozoin, a nanoparticle produced by the malaria parasite inside a red blood cell. Hemozoin crystals are not found in normal red blood cells.
Dr Lapotko and his colleagues found that hemozoin absorbs the energy from a short laser pulse and creates a transient vapor nanobubble. This short-lived vapor nanobubble emerges around the hemozoin nanoparticle and is detected both acoustically and optically.
Acoustic detection of nanobubbles made it possible to detect malaria in whole blood and individual red blood cells infected with Plasmodium falciparum. The method also detected malaria infection as low as 0.00034% in mice infected with Plasmodium yoelii.
“The nanobubbles are generated on demand and only by hemozoin,” said study author Ekaterina Lukianova-Hleb, PhD, also of Rice University. “For this reason, we found that our tests never returned a false-positive result . . . .”
To determine the feasibility of this technique in humans, the researchers tested it on human ears.
The laser probe reliably detected capillaries through the skin, located the blood vessel in the ear in less than 10 seconds, and was reproducible in all 4 subjects studied. In addition, the method did not cause any discomfort or morphological damage to the ear skin.
Dr Lapotko said the first clinical trials of this technology are expected to begin in Houston soon.
He and his colleagues have also used nanobubble technology to deliver chemotherapy drugs directly to cancer cells. ![]()

a red blood cell; Credit: St Jude
Children’s Research Hospital
Researchers say they have developed a diagnostic technique that can rapidly detect low levels of malaria infection through the skin.
The approach involves a low-powered laser that creates tiny vapor nanobubbles inside malaria-infected cells.
The bursting bubbles have a unique acoustic signature that allows for a sensitive diagnosis.
This method requires no dyes or diagnostic chemicals, and there is no need to draw blood.
A preclinical study published in PNAS showed that the method could detect a single malaria-infected cell among a million normal cells with 0 false-positive readings.
“Ours is the first through-the-skin method that’s been shown to rapidly and accurately detect malaria in seconds, without the use of blood sampling or reagents,” said lead investigator Dmitri Lapotko, PhD, of Rice University in Houston, Texas.
The transdermal diagnostic method takes advantage of the optical properties and nanosize of hemozoin, a nanoparticle produced by the malaria parasite inside a red blood cell. Hemozoin crystals are not found in normal red blood cells.
Dr Lapotko and his colleagues found that hemozoin absorbs the energy from a short laser pulse and creates a transient vapor nanobubble. This short-lived vapor nanobubble emerges around the hemozoin nanoparticle and is detected both acoustically and optically.
Acoustic detection of nanobubbles made it possible to detect malaria in whole blood and individual red blood cells infected with Plasmodium falciparum. The method also detected malaria infection as low as 0.00034% in mice infected with Plasmodium yoelii.
“The nanobubbles are generated on demand and only by hemozoin,” said study author Ekaterina Lukianova-Hleb, PhD, also of Rice University. “For this reason, we found that our tests never returned a false-positive result . . . .”
To determine the feasibility of this technique in humans, the researchers tested it on human ears.
The laser probe reliably detected capillaries through the skin, located the blood vessel in the ear in less than 10 seconds, and was reproducible in all 4 subjects studied. In addition, the method did not cause any discomfort or morphological damage to the ear skin.
Dr Lapotko said the first clinical trials of this technology are expected to begin in Houston soon.
He and his colleagues have also used nanobubble technology to deliver chemotherapy drugs directly to cancer cells. ![]()

a red blood cell; Credit: St Jude
Children’s Research Hospital
Researchers say they have developed a diagnostic technique that can rapidly detect low levels of malaria infection through the skin.
The approach involves a low-powered laser that creates tiny vapor nanobubbles inside malaria-infected cells.
The bursting bubbles have a unique acoustic signature that allows for a sensitive diagnosis.
This method requires no dyes or diagnostic chemicals, and there is no need to draw blood.
A preclinical study published in PNAS showed that the method could detect a single malaria-infected cell among a million normal cells with 0 false-positive readings.
“Ours is the first through-the-skin method that’s been shown to rapidly and accurately detect malaria in seconds, without the use of blood sampling or reagents,” said lead investigator Dmitri Lapotko, PhD, of Rice University in Houston, Texas.
The transdermal diagnostic method takes advantage of the optical properties and nanosize of hemozoin, a nanoparticle produced by the malaria parasite inside a red blood cell. Hemozoin crystals are not found in normal red blood cells.
Dr Lapotko and his colleagues found that hemozoin absorbs the energy from a short laser pulse and creates a transient vapor nanobubble. This short-lived vapor nanobubble emerges around the hemozoin nanoparticle and is detected both acoustically and optically.
Acoustic detection of nanobubbles made it possible to detect malaria in whole blood and individual red blood cells infected with Plasmodium falciparum. The method also detected malaria infection as low as 0.00034% in mice infected with Plasmodium yoelii.
“The nanobubbles are generated on demand and only by hemozoin,” said study author Ekaterina Lukianova-Hleb, PhD, also of Rice University. “For this reason, we found that our tests never returned a false-positive result . . . .”
To determine the feasibility of this technique in humans, the researchers tested it on human ears.
The laser probe reliably detected capillaries through the skin, located the blood vessel in the ear in less than 10 seconds, and was reproducible in all 4 subjects studied. In addition, the method did not cause any discomfort or morphological damage to the ear skin.
Dr Lapotko said the first clinical trials of this technology are expected to begin in Houston soon.
He and his colleagues have also used nanobubble technology to deliver chemotherapy drugs directly to cancer cells. ![]()
Survey quantifies impact of drug shortages

Credit: Rhoda Baer
Drug shortages remain a serious problem for patient safety, according to a small survey of US pharmacy directors.
Of the nearly 200 directors, 49% said patients received suboptimal treatment as a result of drug shortages.
Fifty-five percent of respondents reported medication errors resulting from shortages. And 45% reported adverse events due to drug shortages, including a small number of disabling events and deaths.
These results appear in the Journal of Managed Care Pharmacy.
“Drug shortages are the first thing I think about when I get up in the morning, and it is the last thing on my mind when I go to bed at night,” said study author Gary Fennessy, of Northwestern Memorial HealthCare in Chicago, Illinois.
“This is not a problem that is going to go away on its own. Healthcare leaders must not lose sight of it as a major contributor to patient harm or consider its adverse effects inevitable.”
With this in mind, Fennessy and his colleagues sent an electronic survey on drug shortages to 1516 directors of pharmacy.
The survey asked respondents to include information on patient demographics, patient complaints, adverse events, medication errors, patient outcomes, and institutional costs related to drug shortages.
Only 193 pharmacy directors responded. The majority were from acute care institutions serving less than 100 patients. The locations were divided evenly among suburban, urban, and rural institutions.
The medications most commonly reported to be in short supply were analgesics/anesthetics (n=176, 92%), anti-emetics (n=171, 89%), and electrolytes/total parenteral nutrition (n=162, 84%).
Respondents said drug shortages contributed to a variety of issues, including medication errors (such as giving the wrong dose, the wrong drug, or the wrong frequency).
Fifty-three percent of respondents reported 1 to 10 medication errors resulting from drug shortages. And 2% reported more than 30 medication errors.
Eighty-five percent of respondents said patients had to use alternative medications due to drug shortages, 71% said patients’ experienced delays in treatment, and 49% said patients received suboptimal treatment.
Thirty-three percent of respondents said drug shortages resulted in an increased stay in the hospital, 16% said drug shortages caused treatment failure, and 12% said shortages caused hospital readmission.
Forty-one percent of respondents reported 1 to 5 possible or probable adverse events related to drug shortages, and 3% reported more than 15 adverse events.
One percent of respondents reported 1 to 5 patient deaths resulting from drug shortages, 2% reported a disabling adverse event in 1 to 5 patients, and 19% reported adverse events requiring intervention in 1 to 5 patients.
Fifty respondents provided numbers on their estimated costs resulting from drug shortages. And 73% of these individuals calculated costs greater than $100,000.
Thirty-eight percent of respondents said their organization had received at least 1 patient complaint related to drug shortages. And of those respondents reporting the actual number of patient complaints, 18% reported more than 10 complaints.
“This survey is the first that we are aware of to describe the effects that drug shortages have on patient complaints,” said study author Despina Kotis, PharmD, also of Northwestern Memorial HealthCare.
“It clearly shows that patients are aware these shortages are happening, and they are upset that their care is being adversely affected by them.” ![]()

Credit: Rhoda Baer
Drug shortages remain a serious problem for patient safety, according to a small survey of US pharmacy directors.
Of the nearly 200 directors, 49% said patients received suboptimal treatment as a result of drug shortages.
Fifty-five percent of respondents reported medication errors resulting from shortages. And 45% reported adverse events due to drug shortages, including a small number of disabling events and deaths.
These results appear in the Journal of Managed Care Pharmacy.
“Drug shortages are the first thing I think about when I get up in the morning, and it is the last thing on my mind when I go to bed at night,” said study author Gary Fennessy, of Northwestern Memorial HealthCare in Chicago, Illinois.
“This is not a problem that is going to go away on its own. Healthcare leaders must not lose sight of it as a major contributor to patient harm or consider its adverse effects inevitable.”
With this in mind, Fennessy and his colleagues sent an electronic survey on drug shortages to 1516 directors of pharmacy.
The survey asked respondents to include information on patient demographics, patient complaints, adverse events, medication errors, patient outcomes, and institutional costs related to drug shortages.
Only 193 pharmacy directors responded. The majority were from acute care institutions serving less than 100 patients. The locations were divided evenly among suburban, urban, and rural institutions.
The medications most commonly reported to be in short supply were analgesics/anesthetics (n=176, 92%), anti-emetics (n=171, 89%), and electrolytes/total parenteral nutrition (n=162, 84%).
Respondents said drug shortages contributed to a variety of issues, including medication errors (such as giving the wrong dose, the wrong drug, or the wrong frequency).
Fifty-three percent of respondents reported 1 to 10 medication errors resulting from drug shortages. And 2% reported more than 30 medication errors.
Eighty-five percent of respondents said patients had to use alternative medications due to drug shortages, 71% said patients’ experienced delays in treatment, and 49% said patients received suboptimal treatment.
Thirty-three percent of respondents said drug shortages resulted in an increased stay in the hospital, 16% said drug shortages caused treatment failure, and 12% said shortages caused hospital readmission.
Forty-one percent of respondents reported 1 to 5 possible or probable adverse events related to drug shortages, and 3% reported more than 15 adverse events.
One percent of respondents reported 1 to 5 patient deaths resulting from drug shortages, 2% reported a disabling adverse event in 1 to 5 patients, and 19% reported adverse events requiring intervention in 1 to 5 patients.
Fifty respondents provided numbers on their estimated costs resulting from drug shortages. And 73% of these individuals calculated costs greater than $100,000.
Thirty-eight percent of respondents said their organization had received at least 1 patient complaint related to drug shortages. And of those respondents reporting the actual number of patient complaints, 18% reported more than 10 complaints.
“This survey is the first that we are aware of to describe the effects that drug shortages have on patient complaints,” said study author Despina Kotis, PharmD, also of Northwestern Memorial HealthCare.
“It clearly shows that patients are aware these shortages are happening, and they are upset that their care is being adversely affected by them.” ![]()

Credit: Rhoda Baer
Drug shortages remain a serious problem for patient safety, according to a small survey of US pharmacy directors.
Of the nearly 200 directors, 49% said patients received suboptimal treatment as a result of drug shortages.
Fifty-five percent of respondents reported medication errors resulting from shortages. And 45% reported adverse events due to drug shortages, including a small number of disabling events and deaths.
These results appear in the Journal of Managed Care Pharmacy.
“Drug shortages are the first thing I think about when I get up in the morning, and it is the last thing on my mind when I go to bed at night,” said study author Gary Fennessy, of Northwestern Memorial HealthCare in Chicago, Illinois.
“This is not a problem that is going to go away on its own. Healthcare leaders must not lose sight of it as a major contributor to patient harm or consider its adverse effects inevitable.”
With this in mind, Fennessy and his colleagues sent an electronic survey on drug shortages to 1516 directors of pharmacy.
The survey asked respondents to include information on patient demographics, patient complaints, adverse events, medication errors, patient outcomes, and institutional costs related to drug shortages.
Only 193 pharmacy directors responded. The majority were from acute care institutions serving less than 100 patients. The locations were divided evenly among suburban, urban, and rural institutions.
The medications most commonly reported to be in short supply were analgesics/anesthetics (n=176, 92%), anti-emetics (n=171, 89%), and electrolytes/total parenteral nutrition (n=162, 84%).
Respondents said drug shortages contributed to a variety of issues, including medication errors (such as giving the wrong dose, the wrong drug, or the wrong frequency).
Fifty-three percent of respondents reported 1 to 10 medication errors resulting from drug shortages. And 2% reported more than 30 medication errors.
Eighty-five percent of respondents said patients had to use alternative medications due to drug shortages, 71% said patients’ experienced delays in treatment, and 49% said patients received suboptimal treatment.
Thirty-three percent of respondents said drug shortages resulted in an increased stay in the hospital, 16% said drug shortages caused treatment failure, and 12% said shortages caused hospital readmission.
Forty-one percent of respondents reported 1 to 5 possible or probable adverse events related to drug shortages, and 3% reported more than 15 adverse events.
One percent of respondents reported 1 to 5 patient deaths resulting from drug shortages, 2% reported a disabling adverse event in 1 to 5 patients, and 19% reported adverse events requiring intervention in 1 to 5 patients.
Fifty respondents provided numbers on their estimated costs resulting from drug shortages. And 73% of these individuals calculated costs greater than $100,000.
Thirty-eight percent of respondents said their organization had received at least 1 patient complaint related to drug shortages. And of those respondents reporting the actual number of patient complaints, 18% reported more than 10 complaints.
“This survey is the first that we are aware of to describe the effects that drug shortages have on patient complaints,” said study author Despina Kotis, PharmD, also of Northwestern Memorial HealthCare.
“It clearly shows that patients are aware these shortages are happening, and they are upset that their care is being adversely affected by them.” ![]()