User login
EMA grants vaccine orphan status for MM

showing MM
The European Medicines Agency (EMA) has given a novel vaccine orphan designation as a treatment for multiple myeloma (MM).
The vaccine, known as ImMucin, targets the signal peptide domain of the MUC1 tumor antigen.
ImMucin works by “teaching” the immune system to identify and destroy cells that display a short, specific, 21-mer portion from MUC1, which appears on 90% of all cancer cells but not in patients’ blood.
Results of a phase 1/2 trial suggested that ImMucin was safe and active in MM patients. The trial included 15 MUC1-positive patients who had residual or biochemically progressive disease after autologous stem cell transplant.
The patients received 6 or 12 bi-weekly intradermal doses of ImMucin co-administered with human granulocyte-macrophage colony-stimulating factor.
The researchers said the vaccine was well-tolerated, as all adverse events were temporary, grade 1-2 in nature, and resolved spontaneously.
There was a significant decrease in soluble MUC1 levels in 9 patients, and 11 patients had stable disease or clinical improvement that persisted for 17.5 months to more than 41.3 months.
A follow-on study (which is ongoing) in patients who responded to ImMucin has shown that some patients can go more than 4 years without requiring any further treatment for their disease.
ImMucin is also being evaluated in a phase 1/2 study to treat patients with metastatic breast cancer who are receiving first-line hormone therapy.
ImMucin is under development by Vaxil Biotherapeutics Ltd.
About orphan designation
The EMA grants orphan designation to promote the clinical development of drugs that treat rare, life-threatening, or debilitating conditions and are expected to provide significant therapeutic advantage over existing treatments.
Orphan designation provides the company developing a drug with significant benefits, including 10 years of market exclusivity following approval, reductions in the fees and costs of the regulatory process, and scientific assistance from the EMA in clinical development. ![]()

showing MM
The European Medicines Agency (EMA) has given a novel vaccine orphan designation as a treatment for multiple myeloma (MM).
The vaccine, known as ImMucin, targets the signal peptide domain of the MUC1 tumor antigen.
ImMucin works by “teaching” the immune system to identify and destroy cells that display a short, specific, 21-mer portion from MUC1, which appears on 90% of all cancer cells but not in patients’ blood.
Results of a phase 1/2 trial suggested that ImMucin was safe and active in MM patients. The trial included 15 MUC1-positive patients who had residual or biochemically progressive disease after autologous stem cell transplant.
The patients received 6 or 12 bi-weekly intradermal doses of ImMucin co-administered with human granulocyte-macrophage colony-stimulating factor.
The researchers said the vaccine was well-tolerated, as all adverse events were temporary, grade 1-2 in nature, and resolved spontaneously.
There was a significant decrease in soluble MUC1 levels in 9 patients, and 11 patients had stable disease or clinical improvement that persisted for 17.5 months to more than 41.3 months.
A follow-on study (which is ongoing) in patients who responded to ImMucin has shown that some patients can go more than 4 years without requiring any further treatment for their disease.
ImMucin is also being evaluated in a phase 1/2 study to treat patients with metastatic breast cancer who are receiving first-line hormone therapy.
ImMucin is under development by Vaxil Biotherapeutics Ltd.
About orphan designation
The EMA grants orphan designation to promote the clinical development of drugs that treat rare, life-threatening, or debilitating conditions and are expected to provide significant therapeutic advantage over existing treatments.
Orphan designation provides the company developing a drug with significant benefits, including 10 years of market exclusivity following approval, reductions in the fees and costs of the regulatory process, and scientific assistance from the EMA in clinical development. ![]()

showing MM
The European Medicines Agency (EMA) has given a novel vaccine orphan designation as a treatment for multiple myeloma (MM).
The vaccine, known as ImMucin, targets the signal peptide domain of the MUC1 tumor antigen.
ImMucin works by “teaching” the immune system to identify and destroy cells that display a short, specific, 21-mer portion from MUC1, which appears on 90% of all cancer cells but not in patients’ blood.
Results of a phase 1/2 trial suggested that ImMucin was safe and active in MM patients. The trial included 15 MUC1-positive patients who had residual or biochemically progressive disease after autologous stem cell transplant.
The patients received 6 or 12 bi-weekly intradermal doses of ImMucin co-administered with human granulocyte-macrophage colony-stimulating factor.
The researchers said the vaccine was well-tolerated, as all adverse events were temporary, grade 1-2 in nature, and resolved spontaneously.
There was a significant decrease in soluble MUC1 levels in 9 patients, and 11 patients had stable disease or clinical improvement that persisted for 17.5 months to more than 41.3 months.
A follow-on study (which is ongoing) in patients who responded to ImMucin has shown that some patients can go more than 4 years without requiring any further treatment for their disease.
ImMucin is also being evaluated in a phase 1/2 study to treat patients with metastatic breast cancer who are receiving first-line hormone therapy.
ImMucin is under development by Vaxil Biotherapeutics Ltd.
About orphan designation
The EMA grants orphan designation to promote the clinical development of drugs that treat rare, life-threatening, or debilitating conditions and are expected to provide significant therapeutic advantage over existing treatments.
Orphan designation provides the company developing a drug with significant benefits, including 10 years of market exclusivity following approval, reductions in the fees and costs of the regulatory process, and scientific assistance from the EMA in clinical development. ![]()
New approvals, genetic testing, maintenance therapy, and DFS in ovarian cancer
Recent study findings have indicated that women with ovarian cancer may have BRCA1 or BRCA2 mutations despite a negative family history, and current NCCN (National Comprehensive Cancer Network) guidelines endorse genetic testing for all women with epithelial cancer of the ovary. Despite this, recent reports indicate that most women with ovarian cancer are not being tested, particularly those who are elderly or without a family history. In this paper by Daniels and colleagues, the investigators examined targeted versus universal genetic testing to see if the use of a well-regarded risk model (BRCAPRO) based on personal and family history could discriminate among patients with high-grade serous ovarian cancer. Targeted genetic testing in this group might help lower costs and encourage testing for those women who actually have a significant chance of carrying a deleterious gene mutation.
Click on the PDF icon at the top of this introduction to read the full article.
Recent study findings have indicated that women with ovarian cancer may have BRCA1 or BRCA2 mutations despite a negative family history, and current NCCN (National Comprehensive Cancer Network) guidelines endorse genetic testing for all women with epithelial cancer of the ovary. Despite this, recent reports indicate that most women with ovarian cancer are not being tested, particularly those who are elderly or without a family history. In this paper by Daniels and colleagues, the investigators examined targeted versus universal genetic testing to see if the use of a well-regarded risk model (BRCAPRO) based on personal and family history could discriminate among patients with high-grade serous ovarian cancer. Targeted genetic testing in this group might help lower costs and encourage testing for those women who actually have a significant chance of carrying a deleterious gene mutation.
Click on the PDF icon at the top of this introduction to read the full article.
Recent study findings have indicated that women with ovarian cancer may have BRCA1 or BRCA2 mutations despite a negative family history, and current NCCN (National Comprehensive Cancer Network) guidelines endorse genetic testing for all women with epithelial cancer of the ovary. Despite this, recent reports indicate that most women with ovarian cancer are not being tested, particularly those who are elderly or without a family history. In this paper by Daniels and colleagues, the investigators examined targeted versus universal genetic testing to see if the use of a well-regarded risk model (BRCAPRO) based on personal and family history could discriminate among patients with high-grade serous ovarian cancer. Targeted genetic testing in this group might help lower costs and encourage testing for those women who actually have a significant chance of carrying a deleterious gene mutation.
Click on the PDF icon at the top of this introduction to read the full article.
Pelvic pleomorphic rhabdomyosarcoma presenting as oliguria in a 61-year-old woman
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
Guideline recommends combination therapy for smoking cessation in cancer patients
The National Comprehensive Cancer Network has published a new guideline on smoking cessation for cancer patients that recommends combining pharmacologic therapy with counseling as the most effective approach, along with rigorous review and close follow-ups to prevent relapses.
“Although the medical community recognizes the importance of smoking cessation, supporting patients in ceasing to smoke is generally not done well. Our hope is that by addressing smoking cessation in a cancer patient population, we can make it easier for oncologists to effectively support their patients in achieving their smoking cessation goals,” Dr. Peter Shields, deputy director of the Ohio State University Comprehensive Cancer Center, said in a written statement. Of the estimated 590,000 cancer deaths in 2015, about 170,000, or nearly 30%, will be caused by tobacco smoking. Quitting tobacco improves cancer treatment effectiveness and reduces cancer recurrence, according to the NCCN.
Read the full statement on the NCCN website.
The National Comprehensive Cancer Network has published a new guideline on smoking cessation for cancer patients that recommends combining pharmacologic therapy with counseling as the most effective approach, along with rigorous review and close follow-ups to prevent relapses.
“Although the medical community recognizes the importance of smoking cessation, supporting patients in ceasing to smoke is generally not done well. Our hope is that by addressing smoking cessation in a cancer patient population, we can make it easier for oncologists to effectively support their patients in achieving their smoking cessation goals,” Dr. Peter Shields, deputy director of the Ohio State University Comprehensive Cancer Center, said in a written statement. Of the estimated 590,000 cancer deaths in 2015, about 170,000, or nearly 30%, will be caused by tobacco smoking. Quitting tobacco improves cancer treatment effectiveness and reduces cancer recurrence, according to the NCCN.
Read the full statement on the NCCN website.
The National Comprehensive Cancer Network has published a new guideline on smoking cessation for cancer patients that recommends combining pharmacologic therapy with counseling as the most effective approach, along with rigorous review and close follow-ups to prevent relapses.
“Although the medical community recognizes the importance of smoking cessation, supporting patients in ceasing to smoke is generally not done well. Our hope is that by addressing smoking cessation in a cancer patient population, we can make it easier for oncologists to effectively support their patients in achieving their smoking cessation goals,” Dr. Peter Shields, deputy director of the Ohio State University Comprehensive Cancer Center, said in a written statement. Of the estimated 590,000 cancer deaths in 2015, about 170,000, or nearly 30%, will be caused by tobacco smoking. Quitting tobacco improves cancer treatment effectiveness and reduces cancer recurrence, according to the NCCN.
Read the full statement on the NCCN website.
Stem cells from sickle cell disease patients used to generate gene-corrected cells
Cells from patients with sickle cell disease have been used to generate mature red blood cells without the genetic mutation that causes the disease, investigators at Johns Hopkins University reported.
This advance may lead to improved treatment of sickle cell disease patients, who frequently need blood transfusions from healthy donors but often reject foreign blood.
The corrected cells were created through genome editing of human induced pluripotent stem cells (iPSCs), which can make any cell in the body and grow indefinitely in the laboratory.
“Our results represent a significant step towards the clinical applications of genome editing using patient-derived iPSCs to generate disease-free cells for cell and gene therapy,” according to the study’s lead researcher, Xiaosong Huang, and his associates.
The edited iPSCs, which were derived from the patients’ blood cells, produced blood cells as “efficiently” as stem cells that had not been edited, according to a written statement from Johns Hopkins. Still, “to become medically useful, the technique … will have to be made even more efficient and scaled up significantly,” study investigator Linzhao Cheng, Ph.D., noted in the statement.
Find the full study in Stem Cells (2015 Feb. 20 [doi:10.1002/stem.1969]).
Cells from patients with sickle cell disease have been used to generate mature red blood cells without the genetic mutation that causes the disease, investigators at Johns Hopkins University reported.
This advance may lead to improved treatment of sickle cell disease patients, who frequently need blood transfusions from healthy donors but often reject foreign blood.
The corrected cells were created through genome editing of human induced pluripotent stem cells (iPSCs), which can make any cell in the body and grow indefinitely in the laboratory.
“Our results represent a significant step towards the clinical applications of genome editing using patient-derived iPSCs to generate disease-free cells for cell and gene therapy,” according to the study’s lead researcher, Xiaosong Huang, and his associates.
The edited iPSCs, which were derived from the patients’ blood cells, produced blood cells as “efficiently” as stem cells that had not been edited, according to a written statement from Johns Hopkins. Still, “to become medically useful, the technique … will have to be made even more efficient and scaled up significantly,” study investigator Linzhao Cheng, Ph.D., noted in the statement.
Find the full study in Stem Cells (2015 Feb. 20 [doi:10.1002/stem.1969]).
Cells from patients with sickle cell disease have been used to generate mature red blood cells without the genetic mutation that causes the disease, investigators at Johns Hopkins University reported.
This advance may lead to improved treatment of sickle cell disease patients, who frequently need blood transfusions from healthy donors but often reject foreign blood.
The corrected cells were created through genome editing of human induced pluripotent stem cells (iPSCs), which can make any cell in the body and grow indefinitely in the laboratory.
“Our results represent a significant step towards the clinical applications of genome editing using patient-derived iPSCs to generate disease-free cells for cell and gene therapy,” according to the study’s lead researcher, Xiaosong Huang, and his associates.
The edited iPSCs, which were derived from the patients’ blood cells, produced blood cells as “efficiently” as stem cells that had not been edited, according to a written statement from Johns Hopkins. Still, “to become medically useful, the technique … will have to be made even more efficient and scaled up significantly,” study investigator Linzhao Cheng, Ph.D., noted in the statement.
Find the full study in Stem Cells (2015 Feb. 20 [doi:10.1002/stem.1969]).
Immunotoxin could treat B-cell malignancies, team says

Photo courtesy of the
University of Minnesota
A bispecific ligand-directed diphtheria toxin known as DT2219 shows promise for treating patients with relapsed/refractory B-cell malignancies, according to researchers.
DT2219 produced responses in 2 of 25 patients analyzed in a phase 1 study. The maximum tolerated dose of DT2219 was not reached, although 2 patients experienced dose-limiting toxicities.
“In this phase 1 trial, we found a safe dose of the drug that has biological activity,” said Daniel Vallera, PhD, of the University of Minnesota in Minneapolis.
“We are planning a phase 2 trial with this drug. It will focus on giving more cycles of treatment, which we believe will dramatically enhance the response rates.”
Dr Vallera and his colleagues detailed the phase 1 results in Clinical Cancer Research.
To develop DT2219, the researchers chose 2 antibody fragments that each selectively bind to CD19 and CD22. They used genetic engineering to attach these two antibodies to the bacterial diphtheria toxin.
When the antibody fragments bind to the two targets on the cancer cell, the entire drug enters the cell, and the toxin kills the cell.
To test DT2219, the researchers enrolled 25 patients with chemo-refractory pre-B acute lymphoblastic leukemia (n=10), chronic lymphocytic leukemia (n=5), or non-Hodgkin lymphoma (n=10). All tumors had CD19 and/or CD22 proteins.
Patients had received a median of 3 prior therapies (range, 2 to 5), and 8 patients had undergone an unsuccessful stem cell transplant (5 autologous and 3 allogeneic).
Patients received DT2219 intravenously over 2 hours every other day for 4 total doses. The dose was escalated from 0.5 μg/kg/day to 80 μg/kg/day in 9 dose cohorts until a dose-limiting toxicity occurred.
All but 1 patient received a single course of DT2219. That patient received a second, 4-dose course after attaining a partial response.
Outcomes
The 12 patients who received doses ranging from 0.5 mg/kg/day to 20 mg/kg/day had minimal or no adverse events (AEs). But all 13 patients who received 4 doses of DT2219 at 40 mg/kg or greater every other day experienced treatment-related AEs.
Grade 1-2 treatment-related AEs included capillary leak syndrome (n=7), ALT/AST elevation (n=4), fatigue (n=3), fever (n=3), hypokalemia (n=2), hypoalbuminemia (n=1), hearing loss (n=1), hypocalcemia (n=1), anemia (n=1), and vomiting (n=1).
Grade 3-4 treatment-related AEs were thrombocytopenia (n=2), neutropenia (n=1), neutropenic fever (n=1), capillary leak syndrome (n=1), hypokalemia (n=1), and leg weakness (n=1). The grade 3 leg weakness and grade 3 capillary leak syndrome were dose-limiting toxicities.
The maximum tolerated dose was not reached, but clinical responses occurred between doses of 40 to 80 µg/kg.
All 25 patients were evaluable for response, but only 9 patients in the highest dose cohorts had measurable drug levels.
Two patients had durable, objective responses. One patient had chronic lymphocytic leukemia, and the other had diffuse large B-cell lymphoma. The latter patient’s response was a complete remission that occurred after 2 treatment cycles.
“We were surprised that the drug was effective enough to entirely eliminate the cancer in one of our patients,” Dr Vallera said. “Further, we expected the patients to make antibodies against the bacterial toxin and, thus, reject our drug. Surprisingly, this did not occur in the majority of our patients [70%].”
“We need to study more patients to understand why they did not produce neutralizing antibodies. However, we also have been working to create a less immunogenic form of the toxin for the next-generation drug.”
“Another important fact about our drug is that it was home-grown, meaning there was no commercial partner, which is rare. The drug was funded mostly with private donations, including individuals that have lost loved ones to cancer.” ![]()

Photo courtesy of the
University of Minnesota
A bispecific ligand-directed diphtheria toxin known as DT2219 shows promise for treating patients with relapsed/refractory B-cell malignancies, according to researchers.
DT2219 produced responses in 2 of 25 patients analyzed in a phase 1 study. The maximum tolerated dose of DT2219 was not reached, although 2 patients experienced dose-limiting toxicities.
“In this phase 1 trial, we found a safe dose of the drug that has biological activity,” said Daniel Vallera, PhD, of the University of Minnesota in Minneapolis.
“We are planning a phase 2 trial with this drug. It will focus on giving more cycles of treatment, which we believe will dramatically enhance the response rates.”
Dr Vallera and his colleagues detailed the phase 1 results in Clinical Cancer Research.
To develop DT2219, the researchers chose 2 antibody fragments that each selectively bind to CD19 and CD22. They used genetic engineering to attach these two antibodies to the bacterial diphtheria toxin.
When the antibody fragments bind to the two targets on the cancer cell, the entire drug enters the cell, and the toxin kills the cell.
To test DT2219, the researchers enrolled 25 patients with chemo-refractory pre-B acute lymphoblastic leukemia (n=10), chronic lymphocytic leukemia (n=5), or non-Hodgkin lymphoma (n=10). All tumors had CD19 and/or CD22 proteins.
Patients had received a median of 3 prior therapies (range, 2 to 5), and 8 patients had undergone an unsuccessful stem cell transplant (5 autologous and 3 allogeneic).
Patients received DT2219 intravenously over 2 hours every other day for 4 total doses. The dose was escalated from 0.5 μg/kg/day to 80 μg/kg/day in 9 dose cohorts until a dose-limiting toxicity occurred.
All but 1 patient received a single course of DT2219. That patient received a second, 4-dose course after attaining a partial response.
Outcomes
The 12 patients who received doses ranging from 0.5 mg/kg/day to 20 mg/kg/day had minimal or no adverse events (AEs). But all 13 patients who received 4 doses of DT2219 at 40 mg/kg or greater every other day experienced treatment-related AEs.
Grade 1-2 treatment-related AEs included capillary leak syndrome (n=7), ALT/AST elevation (n=4), fatigue (n=3), fever (n=3), hypokalemia (n=2), hypoalbuminemia (n=1), hearing loss (n=1), hypocalcemia (n=1), anemia (n=1), and vomiting (n=1).
Grade 3-4 treatment-related AEs were thrombocytopenia (n=2), neutropenia (n=1), neutropenic fever (n=1), capillary leak syndrome (n=1), hypokalemia (n=1), and leg weakness (n=1). The grade 3 leg weakness and grade 3 capillary leak syndrome were dose-limiting toxicities.
The maximum tolerated dose was not reached, but clinical responses occurred between doses of 40 to 80 µg/kg.
All 25 patients were evaluable for response, but only 9 patients in the highest dose cohorts had measurable drug levels.
Two patients had durable, objective responses. One patient had chronic lymphocytic leukemia, and the other had diffuse large B-cell lymphoma. The latter patient’s response was a complete remission that occurred after 2 treatment cycles.
“We were surprised that the drug was effective enough to entirely eliminate the cancer in one of our patients,” Dr Vallera said. “Further, we expected the patients to make antibodies against the bacterial toxin and, thus, reject our drug. Surprisingly, this did not occur in the majority of our patients [70%].”
“We need to study more patients to understand why they did not produce neutralizing antibodies. However, we also have been working to create a less immunogenic form of the toxin for the next-generation drug.”
“Another important fact about our drug is that it was home-grown, meaning there was no commercial partner, which is rare. The drug was funded mostly with private donations, including individuals that have lost loved ones to cancer.” ![]()

Photo courtesy of the
University of Minnesota
A bispecific ligand-directed diphtheria toxin known as DT2219 shows promise for treating patients with relapsed/refractory B-cell malignancies, according to researchers.
DT2219 produced responses in 2 of 25 patients analyzed in a phase 1 study. The maximum tolerated dose of DT2219 was not reached, although 2 patients experienced dose-limiting toxicities.
“In this phase 1 trial, we found a safe dose of the drug that has biological activity,” said Daniel Vallera, PhD, of the University of Minnesota in Minneapolis.
“We are planning a phase 2 trial with this drug. It will focus on giving more cycles of treatment, which we believe will dramatically enhance the response rates.”
Dr Vallera and his colleagues detailed the phase 1 results in Clinical Cancer Research.
To develop DT2219, the researchers chose 2 antibody fragments that each selectively bind to CD19 and CD22. They used genetic engineering to attach these two antibodies to the bacterial diphtheria toxin.
When the antibody fragments bind to the two targets on the cancer cell, the entire drug enters the cell, and the toxin kills the cell.
To test DT2219, the researchers enrolled 25 patients with chemo-refractory pre-B acute lymphoblastic leukemia (n=10), chronic lymphocytic leukemia (n=5), or non-Hodgkin lymphoma (n=10). All tumors had CD19 and/or CD22 proteins.
Patients had received a median of 3 prior therapies (range, 2 to 5), and 8 patients had undergone an unsuccessful stem cell transplant (5 autologous and 3 allogeneic).
Patients received DT2219 intravenously over 2 hours every other day for 4 total doses. The dose was escalated from 0.5 μg/kg/day to 80 μg/kg/day in 9 dose cohorts until a dose-limiting toxicity occurred.
All but 1 patient received a single course of DT2219. That patient received a second, 4-dose course after attaining a partial response.
Outcomes
The 12 patients who received doses ranging from 0.5 mg/kg/day to 20 mg/kg/day had minimal or no adverse events (AEs). But all 13 patients who received 4 doses of DT2219 at 40 mg/kg or greater every other day experienced treatment-related AEs.
Grade 1-2 treatment-related AEs included capillary leak syndrome (n=7), ALT/AST elevation (n=4), fatigue (n=3), fever (n=3), hypokalemia (n=2), hypoalbuminemia (n=1), hearing loss (n=1), hypocalcemia (n=1), anemia (n=1), and vomiting (n=1).
Grade 3-4 treatment-related AEs were thrombocytopenia (n=2), neutropenia (n=1), neutropenic fever (n=1), capillary leak syndrome (n=1), hypokalemia (n=1), and leg weakness (n=1). The grade 3 leg weakness and grade 3 capillary leak syndrome were dose-limiting toxicities.
The maximum tolerated dose was not reached, but clinical responses occurred between doses of 40 to 80 µg/kg.
All 25 patients were evaluable for response, but only 9 patients in the highest dose cohorts had measurable drug levels.
Two patients had durable, objective responses. One patient had chronic lymphocytic leukemia, and the other had diffuse large B-cell lymphoma. The latter patient’s response was a complete remission that occurred after 2 treatment cycles.
“We were surprised that the drug was effective enough to entirely eliminate the cancer in one of our patients,” Dr Vallera said. “Further, we expected the patients to make antibodies against the bacterial toxin and, thus, reject our drug. Surprisingly, this did not occur in the majority of our patients [70%].”
“We need to study more patients to understand why they did not produce neutralizing antibodies. However, we also have been working to create a less immunogenic form of the toxin for the next-generation drug.”
“Another important fact about our drug is that it was home-grown, meaning there was no commercial partner, which is rare. The drug was funded mostly with private donations, including individuals that have lost loved ones to cancer.” ![]()
Risks and benefits of long-term ticagrelor

Photo courtesy of AstraZeneca
SAN DIEGO—New research suggests that adding the antiplatelet drug ticagrelor to aspirin as long-term therapy after a heart attack can significantly reduce the risk of cardiovascular death, heart attack, or stroke, but it can also increase the risk of major bleeding.
Marc S. Sabatine, MD, of Brigham and Women’s Hospital in Boston, Massachusetts, presented these results at the American College of Cardiology’s 64th Annual Scientific Session (abstract 400-18). The findings were published in NEJM as well.
The research was sponsored by AstraZeneca, the company developing ticagrelor.
The trial, known as PEGASUS-TIMI 54, included 21,162 patients who had experienced a heart attack in the previous 1 to 3 years. Each had another factor, such as age or diabetes, that put them at risk for a second heart attack.
The patients were randomized to receive aspirin plus twice-daily doses of ticagrelor at 90 mg, ticagrelor at 60 mg, or placebo.
The twice-daily 90 mg dose of ticagrelor is already approved for patients with acute coronary syndrome. The researchers included a lower dose in this study to investigate whether platelet inhibition needed 2 years after a heart attack might be different from what is needed 2 hours after a heart attack.
The team found that both ticagrelor doses reduced the rate of the primary endpoint, which was a composite of cardiovascular death, heart attack, and stroke. At 3 years, the rate was 7.85% in the 90 mg group, 7.77% in the 60 mg group, and 9.04% in the placebo group (P=0.008 for 90 mg vs placebo and P=0.004 for 60 mg vs placebo).
“The benefit we saw was remarkably consistent across the individual components of the endpoint and in all the major subgroups of patients,” Dr Sabatine said. “Moreover, we followed patients for an average of just under 3 years, and our event curves continue to spread out over time, suggesting that the benefit continues to accrue over time.”
“Efficacy was virtually identical with both ticagrelor doses,” he added. “Risk of bleeding and dyspnea tended to be, as predicted, a bit more with the 90 mg than the 60 mg dose, but the trial wasn’t designed to compare those two dose levels.”
The rates of TIMI major bleeding were 2.60% in the 90 mg group, 2.30% in the 60 mg group, and 1.06% in the placebo group (P<0.001 for each ticagrelor dose vs placebo).
The rates of dyspnea were 18.93% in the 90 mg group, 15.84% in 60 mg group, and 6.38% in the placebo group (P<0.001 for each ticagrelor dose vs placebo).
The rates of dyspnea leading to treatment discontinuation were 6.5% in the 90 mg group, 4.55% in the 60 mg group, and 0.79% in the placebo group (P<0.001 for each ticagrelor dose vs placebo).
Although the differences in dyspnea and TIMI major bleeding between the 2 ticagrelor dose groups were not statistically significant, Dr Sabatine and his colleagues said the 60 mg dose may offer a more attractive benefit-risk profile.
“Now that we have the evidence, when faced with a patient who has had a heart attack, based on these data, I would continue treatment with ticagrelor as long as the patient tolerated it,” Dr Sabatine concluded. ![]()

Photo courtesy of AstraZeneca
SAN DIEGO—New research suggests that adding the antiplatelet drug ticagrelor to aspirin as long-term therapy after a heart attack can significantly reduce the risk of cardiovascular death, heart attack, or stroke, but it can also increase the risk of major bleeding.
Marc S. Sabatine, MD, of Brigham and Women’s Hospital in Boston, Massachusetts, presented these results at the American College of Cardiology’s 64th Annual Scientific Session (abstract 400-18). The findings were published in NEJM as well.
The research was sponsored by AstraZeneca, the company developing ticagrelor.
The trial, known as PEGASUS-TIMI 54, included 21,162 patients who had experienced a heart attack in the previous 1 to 3 years. Each had another factor, such as age or diabetes, that put them at risk for a second heart attack.
The patients were randomized to receive aspirin plus twice-daily doses of ticagrelor at 90 mg, ticagrelor at 60 mg, or placebo.
The twice-daily 90 mg dose of ticagrelor is already approved for patients with acute coronary syndrome. The researchers included a lower dose in this study to investigate whether platelet inhibition needed 2 years after a heart attack might be different from what is needed 2 hours after a heart attack.
The team found that both ticagrelor doses reduced the rate of the primary endpoint, which was a composite of cardiovascular death, heart attack, and stroke. At 3 years, the rate was 7.85% in the 90 mg group, 7.77% in the 60 mg group, and 9.04% in the placebo group (P=0.008 for 90 mg vs placebo and P=0.004 for 60 mg vs placebo).
“The benefit we saw was remarkably consistent across the individual components of the endpoint and in all the major subgroups of patients,” Dr Sabatine said. “Moreover, we followed patients for an average of just under 3 years, and our event curves continue to spread out over time, suggesting that the benefit continues to accrue over time.”
“Efficacy was virtually identical with both ticagrelor doses,” he added. “Risk of bleeding and dyspnea tended to be, as predicted, a bit more with the 90 mg than the 60 mg dose, but the trial wasn’t designed to compare those two dose levels.”
The rates of TIMI major bleeding were 2.60% in the 90 mg group, 2.30% in the 60 mg group, and 1.06% in the placebo group (P<0.001 for each ticagrelor dose vs placebo).
The rates of dyspnea were 18.93% in the 90 mg group, 15.84% in 60 mg group, and 6.38% in the placebo group (P<0.001 for each ticagrelor dose vs placebo).
The rates of dyspnea leading to treatment discontinuation were 6.5% in the 90 mg group, 4.55% in the 60 mg group, and 0.79% in the placebo group (P<0.001 for each ticagrelor dose vs placebo).
Although the differences in dyspnea and TIMI major bleeding between the 2 ticagrelor dose groups were not statistically significant, Dr Sabatine and his colleagues said the 60 mg dose may offer a more attractive benefit-risk profile.
“Now that we have the evidence, when faced with a patient who has had a heart attack, based on these data, I would continue treatment with ticagrelor as long as the patient tolerated it,” Dr Sabatine concluded. ![]()

Photo courtesy of AstraZeneca
SAN DIEGO—New research suggests that adding the antiplatelet drug ticagrelor to aspirin as long-term therapy after a heart attack can significantly reduce the risk of cardiovascular death, heart attack, or stroke, but it can also increase the risk of major bleeding.
Marc S. Sabatine, MD, of Brigham and Women’s Hospital in Boston, Massachusetts, presented these results at the American College of Cardiology’s 64th Annual Scientific Session (abstract 400-18). The findings were published in NEJM as well.
The research was sponsored by AstraZeneca, the company developing ticagrelor.
The trial, known as PEGASUS-TIMI 54, included 21,162 patients who had experienced a heart attack in the previous 1 to 3 years. Each had another factor, such as age or diabetes, that put them at risk for a second heart attack.
The patients were randomized to receive aspirin plus twice-daily doses of ticagrelor at 90 mg, ticagrelor at 60 mg, or placebo.
The twice-daily 90 mg dose of ticagrelor is already approved for patients with acute coronary syndrome. The researchers included a lower dose in this study to investigate whether platelet inhibition needed 2 years after a heart attack might be different from what is needed 2 hours after a heart attack.
The team found that both ticagrelor doses reduced the rate of the primary endpoint, which was a composite of cardiovascular death, heart attack, and stroke. At 3 years, the rate was 7.85% in the 90 mg group, 7.77% in the 60 mg group, and 9.04% in the placebo group (P=0.008 for 90 mg vs placebo and P=0.004 for 60 mg vs placebo).
“The benefit we saw was remarkably consistent across the individual components of the endpoint and in all the major subgroups of patients,” Dr Sabatine said. “Moreover, we followed patients for an average of just under 3 years, and our event curves continue to spread out over time, suggesting that the benefit continues to accrue over time.”
“Efficacy was virtually identical with both ticagrelor doses,” he added. “Risk of bleeding and dyspnea tended to be, as predicted, a bit more with the 90 mg than the 60 mg dose, but the trial wasn’t designed to compare those two dose levels.”
The rates of TIMI major bleeding were 2.60% in the 90 mg group, 2.30% in the 60 mg group, and 1.06% in the placebo group (P<0.001 for each ticagrelor dose vs placebo).
The rates of dyspnea were 18.93% in the 90 mg group, 15.84% in 60 mg group, and 6.38% in the placebo group (P<0.001 for each ticagrelor dose vs placebo).
The rates of dyspnea leading to treatment discontinuation were 6.5% in the 90 mg group, 4.55% in the 60 mg group, and 0.79% in the placebo group (P<0.001 for each ticagrelor dose vs placebo).
Although the differences in dyspnea and TIMI major bleeding between the 2 ticagrelor dose groups were not statistically significant, Dr Sabatine and his colleagues said the 60 mg dose may offer a more attractive benefit-risk profile.
“Now that we have the evidence, when faced with a patient who has had a heart attack, based on these data, I would continue treatment with ticagrelor as long as the patient tolerated it,” Dr Sabatine concluded. ![]()
Opioid Risk Measure for Hospitalization
Longer term and higher doses of opioid analgesics (OAs) have been associated with multiple adverse outcomes such as loss of work, cognitive decline, and poor function.[1, 2, 3, 4] One of the most widely reported complications of opioid therapy is drug overdose.[5, 6, 7, 8, 9] In population‐based studies, daily morphine equivalent doses >100 mg have been associated with significantly increased risk of drug overdose.[5, 6, 7, 8, 9, 10] Among health maintenance organization (HMO) enrollees filling at least 2 prescriptions for opioids, our group reported that daily opioid doses 100 mg were associated with approximately threefold greater adjusted odds of drug overdose.[10] We also observed over a twofold increase in odds of drug overdose for lower daily doses of 50 to 99 mg if the patient also received a high total opioid dose (>1830 mg) over a 6‐month period. This analysis suggests that clinicians may need to monitor not only daily dose but also total dose of opioids to reduce the risk of drug overdose.
Yet drug overdose represents only a small subset of all hospitalizations for persons receiving long‐term or higher doses of opioids for noncancer pain. These patients have significant demand for urgent care services, including hospitalization, for diverse reasons such as adverse effects of opioids, underlying cause of chronic pain, and comorbidities such as mental health disorders.[11] In a cohort of elderly primary care patients who were high hospital utilizers, Freund and colleagues reported that chronic pain and depression were the most common conditions co‐occurring with their other comorbidities.[12] However, little is known about the association of opioid dose with the risk of all‐cause hospitalization for patients with noncancer pain.
In this article we examined hospitalizations for a national cohort of HMO enrollees with noncancer pain who filled at least 2 prescriptions for schedule II or III opioids over a 3.5‐year timeframe. This retrospective cohort analysis aims to identify clinically useful opioid dose measures for clinicians, administrators, and policymakers to use in identifying patients at increased risk of future hospitalization who may warrant interventions to reduce this risk.
METHODS
Study Sample
From Aetna administrative databases including enrollment files and paid claims for services, we identified 261,528 subjects aged 18 to 64 years who had at least 2 paid claims for schedule II or III noninjectable OA prescriptions from January 2009 through July 2012.[10] For individuals meeting these criteria, study cohort eligibility required at least 12 months of enrollment and complete data on demographics and OA prescriptions as well as clinical conditions from at least 1 encounter (see Supporting Information, Appendix 1, in the online version of this article).[10] We excluded subjects with a cancer diagnosis who have high hospital utilization and those younger than 45 years because of a higher likelihood of pregnancy‐related hospitalization. To afford sufficient observation time for outcomes, subjects with <12 months follow‐up after the first opioid prescription were excluded. The resultant study cohort totaled 87,688 subjects.
To capture the changing nature of medication utilization and clinical conditions in this longitudinal study, we divided the study timeframe into 6‐month intervals starting with the first opioid prescription and ending with the subject's last enrollment or end of the study (see Supporting Information, Appendix 2, in the online version of this article). Six‐month intervals were studied because this is the maximum duration of benefit from randomized trials of opioid therapy for noncancer pain.[13] This study was approved by the University of Texas Health Science Center at San Antonio's institutional review board.
Outcome Variables
Study outcomes were all‐cause hospitalization (binary) and hospital days (discrete) per 6‐month interval and were measured repeatedly for up to 6, 6‐month intervals.
Primary Independent Variables
We examined 2 opioid dose measures within a 6‐month interval and hospitalization outcomes in the next 6 months (see Supporting Information, Appendix 2, in the online version of this article). We did not examine OA use in the last 6 months of the study timeframe because subsequent hospitalization outcomes were not available. We defined the total morphine equivalent dose of OA prescriptions filled within a 6‐month interval based on the method used by Edlund et al.[14] and adapted by our group.[10] We also defined the daily dose of OAs that is a widely used metric used in chronic pain management guidelines.[10, 15]
To calculate the total opioid dose, all filled schedule II or III OA prescriptions (noninjectable formulations) were identified from claims for filled prescriptions for each 6‐month interval. The morphine equivalent dose for each opioid prescription was calculated from the number of pills dispensed multiplied by strength (in milligrams) and by a morphine equivalent conversion factor derived from several sources including published data,[16, 17] conversion tables from Internet sources, and drug information resources.[18, 19] A clinical pharmacist reviewed and finalized conversions. When an opioid prescription spanned two, 6‐month intervals, the dose was divided proportionate to time in each interval. The total dose for all opioid prescriptions within an interval was summed and categorized by quartile of nonzero total dose as: 1 to 190, 191 to 450, 451 to 1830, and >1830 mg.[10]
To calculate the daily opioid dose in each interval, the total dose was divided by total nonoverlapping days' supply covered by all prescriptions. The average daily dose was categorized as in other studies: 1 to 19, 20 to 49, 50 to 99, and 100 mg.[5, 6, 10] In each 6‐month interval, the percentage of days covered by filled prescriptions was calculated as total days' supply/180.
Other Independent Variables
Demographic data included age as of July 2012, sex, and US region. From available diagnosis codes for encounters, pain‐related conditions were identified including: back pain, other osteoarthritis, neuropathic pain, chronic pain unspecified, or chronic headache (International Classification of Diseases, Ninth Revision, Clinical Modification codes available from authors). Mental health/substance use disorders were similarly identified: anxiety or post‐traumatic stress disorder (PTSD), depression, psychosis, drug abuse, and alcohol abuse. Once a psychiatric condition or substance use disorder was diagnosed, it was considered to persist because these are usually not transient. We examined filled prescriptions for psychoactive drugs in 6‐month intervals including: benzodiazepines (i.e., clonazepam, alprazolam, lorazepam, diazepam, chlordiazepine, temazepam, flurazepam), antidepressants (i.e., selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors, tricyclics [complete list available from authors]), and sedatives (i.e., zolpidem, eszopiclone). For these drugs, time‐varying variables were created as follows: benzodiazepines (0, 130, 3190, 91180 days), sedatives (0, 130, 3190, 91180 days), and antidepressants (0, 160, 61180 days). Categories for duration of antidepressants differed because a clinical response can take up to 6 to 8 weeks.
Statistical Analyses
Descriptive statistics were examined for study cohort characteristics. For the binary all‐cause hospitalization outcome, repeated measures logistic regression models were estimated using generalized estimating equations (GEE) to examine associations of daily opioid dose, total opioid dose, and their interaction with all‐cause hospitalization. The fully adjusted model includes demographics, chronic pain conditions, mental health conditions, substance use disorders, other psychoactive drugs, and current hospitalization (yes/no). For the hospital days per 6‐month outcome, a series of repeated measures Poisson regressions were estimated using the GEE approach.
In a post hoc sensitivity analysis, we examined the association of the percentage of days covered by prescribed opioids, categorized based on approximate quartiles and clinical judgment, with hospitalization among subjects with a high total dose (>1830 mg). For this analysis, we created a composite measure of opioid treatment for each 6‐month interval that has 6 categories: (1) none, (2) low total dose 1 to 1830 mg, (3) high total dose >1830 mg with 50% of days on opioids, (4) total dose >1830 mg with >50% to 75% of days on opioids, (5) total dose >1830 mg with >75% to 90% of days on opioids, and (6) total dose >1830 mg and >90% of days on opioids. Adjusted regression analyses described above were repeated for both outcomes and included this composite measure. All statistical tests were performed with a 2‐sided significance level of 0.05, and analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Of 87,688 study subjects, 54.8% were women, and the mean age was 53.8 years (standard deviation [SD]=5.5). Nearly half of the cohort resided in Southern states (Table 1). In the baseline 6‐month interval, the most common chronic noncancer pain conditions were musculoskeletal involving large joint arthritis/arthralgia (38.4%) and back pain (28.2%). In regard to mental health and substance use conditions, both anxiety/PTSD and depression were diagnosed in approximately 7% of the cohort, whereas psychosis, and alcohol and other substance use disorders were each diagnosed in <2%. In the baseline interval, 12.7% of subjects were hospitalized. The majority of patients received a daily opioid dose of 20 to 49 mg, and the median total dose was 450 mg. The median percent of time exposed to opioids was 6.7% among all study subjects and 70% for those with a high total dose (>1830 mg).
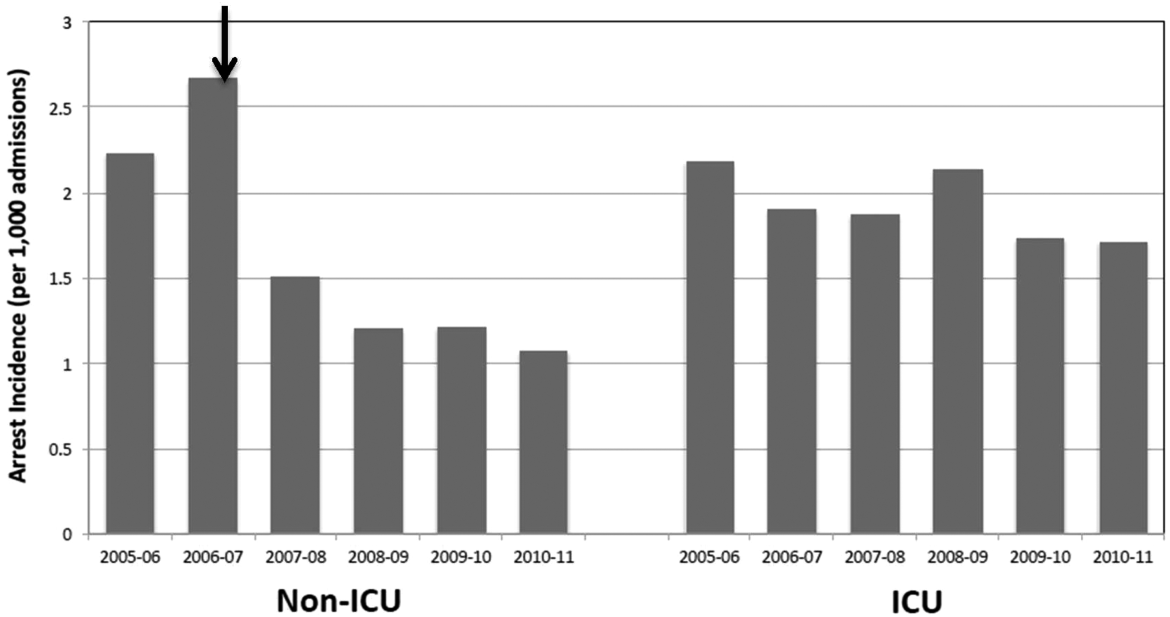
In the 3 study years, an average of 12% of the cohort was hospitalized yearly (Table 2), or 1120 hospitalizations per 10,000 person‐years. Among those who were hospitalized, inpatient days averaged 6.5 (SD=8.5). The highest proportion of hospitalized subjects was 6.5%, occurring in the 6‐month interval immediately following the first opioid treatment interval. In subsequent 6‐month intervals, hospitalization rates were relatively stable, ranging from 5.2% to 6.1% (Table 2). As shown, future hospitalization rates increased monotonically, with increasing total or daily dose within each 6‐month interval.
| Characteristics | Total, N=87,688 |
|---|---|
| |
| Demographics | |
| Women, n (%) | 48,077 (54.8) |
| Age, mean (SD) | 53.8 (5.5) |
| US region, n (%) | |
| Midwest | 4,609 (5.3) |
| Northeast | 27,568 (31.4) |
| South | 40,767 (46.5) |
| West | 14,744 (16.8) |
| Clinical conditions, n (%)b | |
| Noncancer pain conditions | |
| Back pain | 24,767 (28.2) |
| Large joint arthritis, other musculoskeletalc | 33,689 (38.4) |
| Neuropathy | 1,519 (1.7) |
| Chronic pain (unspecified) | 3,229 (3.7) |
| Headache | 2,837 (3.2) |
| Mental health and substance use disorders | |
| Anxiety or post‐traumatic stress disorder | 6,006 (6.9) |
| Depression | 6,111 (7.0) |
| Psychosis | 1,259 (1.4) |
| Alcohol abuse | 877 (1.0) |
| Other substance abuse | 615 (0.7) |
| Current hospitalization, n (%) | 11,165 (12.7) |
| Opioid measures, n (%) | |
| Daily MED dose, mg | |
| 0 | |
| 119 | 9,870 (11.3) |
| 2049 | 50,050 (57.1) |
| 5099 | 21,188 (24.2) |
| 100 | 6,580 (7.5) |
| Total MED dose, mg | |
| 0 | |
| 1190 | 20,276 (23.1) |
| 191450 | 26,000 (29.7) |
| 4511,830 | 23,551 (26.9) |
| >1,830 | 17,861 (20.4) |
| Percent time exposed to opioid therapy, median (Q1, Q3) | |
| Among any total MED | 6.7 (2.8, 22.2) |
| Among total MED >1,830 mg | 70 (42.8, 93.9) |
| Subjects | 6‐Month Interval | |||||
|---|---|---|---|---|---|---|
| 1 (Baseline), N=87,688 | 2, N=65,835 | 3, N=46,041 | 4, N=31,550 | 5, N=18,915 | 6, N=3,502 | |
| ||||||
| Overall (%) | 6.5 | 5.9 | 5.9 | 5.4 | 5.2 | 6.1 |
| Opioid dose measure | ||||||
| Daily dose (%) | ||||||
| 0 mg | 4.8 | 4.4 | 4.0 | 3.6 | 3.2 | |
| 119 mg | 5.9 | 5.6 | 6.0 | 5.6 | 5.6 | 4.4 |
| 2049 mg | 6.2 | 6.5 | 7.1 | 6.6 | 6.1 | 6.1 |
| 5099 mg | 6.8 | 7.9 | 7.5 | 7.6 | 7.6 | 9.8 |
| 100 mg | 9.0 | 9.3 | 10.3 | 9.2 | 9.5 | 9.5 |
| Total dose (%) a | ||||||
| 0 mg | 4.8 | 4.4 | 4.0 | 3.6 | 3.2 | |
| 1190 mg | 5.5 | 4.7 | 5.0 | 4.1 | 4.0 | 2.7 |
| 191450 mg | 5.1 | 5.1 | 6.3 | 6.7 | 5.0 | 3.2 |
| 4511,830 mg | 6.5 | 7.4 | 7.9 | 7.2 | 7.1 | 7.0 |
| >1,830 mg | 9.8 | 9.6 | 9.6 | 8.9 | 8.8 | 9.0 |
In unadjusted analyses, a significant interaction between daily dose and total dose (P<0.001) revealed that, within each daily dose category, the odds of hospitalization differed by total dose (all P<0.05, Table 3). When the total dose was >1830 mg, the odds of future hospitalization rose monotonically with increasing daily dose (i.e., <20, 2049, 5099, 100 mg): 1.33, 1.84, 1.96, and 2.08 (P<0.05 for all comparisons vs no opioids). On the other hand, when the total dose was 450 mg or less, all daily dose categories including a very high daily dose (100 mg) were not associated with future hospitalization (all P>0.05 vs no opioids). When the total dose was 451 to 1830 mg, a nonlinear association with hospitalization appeared with higher odds for lower daily doses. For the outcome of hospital days per 6‐month interval, increasing daily dose was also associated with more hospital days per 6‐month interval when the total dose was high (>1830 mg), whereas for lower total doses, daily dose was weakly positive or even protective versus no opioids.
| All‐Cause Hospitalization (Yes/No), Odds Ratio (95% CI) | |||||
|---|---|---|---|---|---|
| Total Morphine Equivalent Dose, mg | Daily Morphine Equivalent Dose, mg | ||||
| 0 | 19 | 2049 | 5099 | 100 | |
| Hospital Days per 6‐Month, Incident Rate Ratio (95% CI) | |||||
| Total Morphine Equivalent Dose, mg | Daily Morphine Equivalent Dose, mg | ||||
| 0 | 119 | 2049 | 5099 | 100 | |
| |||||
| 0 | 1 | ||||
| 1190 | 1.06 (0.95‐1.19) | 1.01 (0.95‐1.08) | 1.07 (0.95‐1.19) | 0.73 (0.44‐1.21) | |
| 191450 | 1.08 (0.96‐1.22) | 1.03 (0.96‐1.10) | 0.99 (0.9‐1.10) | 0.88 (0.67‐1.15) | |
| 4511,830 | 1.34 (1.21‐1.48)a | 1.37 (1.28‐1.46)a | 1.16 (1.05‐1.27)a | 1.25 (0.98‐1.59) | |
| >1,830 | 1.33 (1.09‐1.62)a | 1.84 (1.73‐1.97)a | 1.96 (1.82‐2.11)a | 2.08 (1.93‐2.24)a | |
| 0 | 1 | ||||
| 1190 | 0.95 (0.79‐1.14) | 0.90 (0.82‐0.99)a | 1.03 (0.87‐1.23) | 0.63 (0.36‐1.12) | |
| 191450 | 0.92 (0.77‐1.10) | 0.93 (0.84‐1.02) | 0.79 (0.69‐0.91)a | 0.69 (0.49‐0.98)a | |
| 4511,830 | 1.31 (1.10‐1.57)a | 1.26 (1.13‐1.40)a | 1.01 (0.86‐1.19) | 0.99 (0.71‐1.37) | |
| >1,830 | 1.32 (0.93‐1.89) | 1.79 (1.60‐2.01)a | 1.76 (1.54‐2.01)a | 2.09 (1.85‐2.36)a | |
In the model adjusting for all covariates (Table 4), the interaction between total dose and daily dose was also significant (P=0.002). When the total dose was high (>1830 mg), the adjusted odds of future hospitalization were significantly increased by 35% to 44% for daily doses of 20 to 49 mg or greater versus no opioids (P<0.05 for all comparisons). When the total dose was <1830 mg, the majority of daily dose categories were not significantly associated with hospitalization. Similarly, in the fully adjusted analysis of hospital days, the number of inpatient days were increased by 28% to 48% when the total dose was >1830 mg and daily dose was >20 mg, but these associations were nonsignificant or protective when the total dose was lower.
| All‐Cause Hospitalization (Yes/No), Odds Ratio (95% CI) | |||||
|---|---|---|---|---|---|
| Total Morphine Equivalent Dose, mg | Daily Morphine Equivalent Dose, mg | ||||
| 0 | 119 | 2049 | 5099 | 100 | |
| Hospital Days per 6‐Month, Incident Rate Ratio (95% CI) | |||||
| Total Morphine Equivalent Dose, mg | Daily Morphine Equivalent Dose, mg | ||||
| 0 | 119 | 2049 | 5099 | 100 | |
| |||||
| 0 | 1 | ||||
| 1190 | 1.09 (0.97‐1.23) | 1.07 (1.001.14) | 1.12 (1.001.26)b | 0.75 (0.45‐1.23) | |
| 191450 | 1.00 (0.88‐1.13) | 0.99 (0.92‐1.06) | 0.97 (0.88‐1.08) | 0.87 (0.68‐1.12) | |
| 4511,830 | 1.16 (1.04‐1.29) | 1.14 (1.07‐1.22) | 0.94 (0.85‐1.03) | 1.08 (0.85‐1.35) | |
| >1,830 | 1.10 (0.90‐1.34) | 1.41 (1.32‐1.51) | 1.35 (1.25‐1.46) | 1.44 (1.34‐1.55) | |
| 0 | 1 | ||||
| 1190 | 0.97 (0.8‐1.18) | 0.94 (0.85‐1.04) | 1.06 (0.88‐1.27) | 0.60 (0.33‐1.1) | |
| 191450 | 0.85 (0.71‐1.02) | 0.88 (0.79‐0.98) | 0.75 (0.65‐0.86) | 0.65 (0.46‐0.92) | |
| 4511,830 | 1.16 (0.97‐1.4) | 1.09 (0.97‐1.22) | 0.83 (0.71‐0.98) | 0.81 (0.59‐1.13) | |
| >1,830 | 1.12 (0.77‐1.63) | 1.41 (1.25‐1.58) | 1.28 (1.12‐1.46) | 1.48 (1.29‐1.69) | |
In a sensitivity analysis, we examined the percentage of days covered by filled opioid prescriptions within a 6‐month interval for subjects receiving high‐dose therapy (Table 5). Compared with no opioid therapy, the adjusted odds of future hospitalization were 5% greater for low total opioid dose (11830 mg) and 21% greater for high total dose (>1830 mg) when the duration of treatment was shorter (50% of the 6‐month interval). However, the odds were increased by 41% to 51% for a high total dose (>1830 mg), with longer periods of treatment (>50% of the interval). For hospital days as the outcome, subjects with high total doses (>1830 mg) and longer periods of treatment (>50% of the interval) had 41% to 71% more hospital days per 6‐month interval than those with no opioid therapy.
| Opioid Analgesic Category | All‐Cause Hospitalization | Hospital Days per 6 Months |
|---|---|---|
| Odds Ratio (95% CI) | Incident Rate Ratio (95% CI) | |
| ||
| 0 mg | 1 | 1 |
| 11,830 mg | 1.05 (1.001.10)b | 0.94 (0.87‐1.01) |
| >1,830 mg and 50% days on opioids | 1.21 (1.11‐1.31)b | 1.10 (0.96‐1.26) |
| >1,830 mg and >50 to 75% days on opioids | 1.51 (1.40‐1.64)b | 1.45 (1.26‐1.67) |
| >1,830 mg and >75 to 90% days on opioids | 1.50 (1.38‐1.64)b | 1.71 (1.46‐1.99) |
| >1,830 mg and >90% days on opioids | 1.41 (1.31‐1.52)b | 1.41 (1.26‐1.58) |
DISCUSSION
In a national cohort of HMO enrollees who filled at least 2 prescriptions for OAs, 12% were hospitalized annually. Other studies of opioid users have focused on only a fraction of these hospitalizations. For example, a recent Agency for Healthcare Research and Quality study reported that the rate of hospitalization for complications from accidental or deliberate overuse of opioids more than doubled from 11.7/10,000 in 1993 to 29.5/10,000 in 2010.[20] However, in our cohort, the all‐cause hospitalization rate was 1120 per 10,000 person‐years, or over 40 times greater than the rate for complications from overuse of opioids. By comparison, hospitalization for heart failure was only 32.8/10,000 nationally in 2010.[21] Thus, our study confirms the significant demand for hospital care by patients treated with opioids. A novel finding of our study is that the total dose of prescriptions filled over 6 months is significantly associated with an increased risk of future hospitalization. When the total dose within 6 months was in the top quartile (>1830 mg in our cohort), the adjusted odds of future hospitalization ranged from 35% to 44% greater than no opioids for daily opioid doses above 20 mg/day. On the other hand, when the total dose was 1830 mg, the daily opioid dose was only weakly associated with future hospitalization. These associations were similar for hospital days per 6‐month interval as the outcome.
Edlund and colleagues examined the total dose of opioids in a national cohort of veterans with chronic noncancer pain who filled at least 1 opioid prescription.[22] In 2011, the 60th percentile for the total opioid dose for these veterans was 3610 mg within a year, which is roughly equivalent to our top quartile (1830 mg) over a 6‐month interval. These data support replicating our study in veterans to evaluate whether a similarly increased risk of hospitalization appears for those with high total opioid doses. In support of a concern among veterans, a population‐based, cross‐sectional study of hospitalized veterans reported a high rate of chronic opioid therapy (90 days) in the 6 months prior to hospitalization.[23]
Other studies have reported increased risk of hospitalization with chronic opioid therapy. Among 1045 patients followed up to 1‐year post‐transplantation, long‐term opioids were associated with up to a sixfold greater risk of at least 4 admissions within that year.[24] Among 13,127 Danish adults on opioid therapy, the odds of future hospitalization from injuries were increased by 74% for long‐term therapy and 46% for short‐term therapy versus no opioids and by threefold and 1.6‐fold, respectively, for hospitalization due to toxicity/poisoning.[9] However, none of these studies examined the dose of opioids.
In a sensitivity analysis, we found that when a subject received a high total opioid dose within 6 months, treatment for more than 50% of the interval (i.e., >3 months) was associated with a significantly increased risk of future hospitalization and significantly more hospital days. Because the strongest evidence for the benefit of opioids for chronic noncancer pain comes from trials of <3 months,[25] these data lend additional support to recommendations to minimize both dose and duration of opioid therapy.
Our study has several limitations. First, we did not assess the immediate risk of hospitalization after starting opioid therapy. Second, our outcome of hospitalization represents only 1 measure of risk. Thus, our data should not be regarded as supporting short‐term use of high‐dose opioids over 100 to 120 mg per day.[26] In an earlier study, we reported that either a high daily dose (100 mg) or a moderately high daily dose (5099 mg) plus a high total dose (>1830 mg) increased the risk of drug overdose.[10] Third, we could not examine the reason for hospitalizations in this analysis. Therefore, we cannot presume that opioid therapy caused these hospitalizations, but it likely serves as a proxy for other factors such as disability and mental health disorders that increase risk of hospitalization. However, we did adjust for pain conditions as well as mental health and substance abuse disorders that are known to increase the risk of hospitalization in other cohorts.[27, 28, 29, 30] In a national veterans study, the most common clinical conditions associated with long‐term opioid therapy were major depression and PTSD.[22] Last, we did also not consider the number of prescribers of opioids. In a Medicare study, 1 versus 4 prescribers of OAs increased patients' annual hospitalization rate from 1.6% to 4.8%, respectively.[31]
Although the total opioid dose categories observed for our study population may differ from those in other cohorts, these data offer additional evidence for clinicians to consider this measure when assessing risk for hospitalization, and among subjects on high total doses, the percentage of time on opioids offers an additional measure of risk. Because opioid users with noncancer pain are heavy consumers of healthcare services,32,33 public health benefits and reductions in costs of care may be substantial if opportunities can be identified to reduce hospital utilization by persons treated with higher doses of OAs.
Disclosures
The work on this project was supported by an intramural grant from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 1UL TR001120. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors report no conflicts of interest.
- , , . Higher opioid doses predict poorer functional outcome in patients with chronic disabling occupational musculoskeletal disorders. J Bone Joint Surg Am. 2009;91:919–927.
- , , . Opioid therapy for nonspecific low back pain and the outcome of chronic work loss. Pain. 2009;142:194–201.
- , , , , , . The comparative safety of analgesics in older adults with arthritis. Arch Intern Med. 2010;170:1968–1976.
- , , , et al. Evidence of specific cognitive deficits in patients with chronic low back pain under long‐term substitution treatment of opioids. Pain Physician. 2014;17:9–20.
- , , , et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152:85–92.
- , , , et al. Association between opioid prescribing patterns and opioid overdose‐related deaths. JAMA. 2011;305:1315–1321.
- , , , , , . Trends in opioid use and dosing among socio‐economically disadvantaged patients. Open Med. 2011;5:e13–e22.
- , , , et al. A history of being prescribed controlled substances and risk of drug overdose death. Pain Med. 2012;13:87–95.
- , , , , . Chronic pain, opioid prescriptions and mortality in Denmark: a population‐based cohort study. Pain. 2014;155:2486–2490.
- , . Assessing risk for drug overdose in a national cohort: Role for both daily and total opioid dose [published online ahead of print December 5, 2015]? J Pain. doi: 10.1016/j.jpain.2014.11.007.
- Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Available at: http://www.ncbi.nlm.nih.gov/books/NBK91497. Accessed December 12, 2014.
- , , , , . Patterns of multimorbidity in primary care patients at high risk of future hospitalization. Popul Health Manag. 2012;15:119–124.
- , , , et al. Long‐term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;(1):CD006605.
- , , , , , . An analysis of heavy utilizers of opioids for chronic noncancer pain in the TROUP study. J Pain Symptom Manage. 2010;40:279–289.
- , , , et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38–47.
- . The treatment of cancer pain. N Engl J Med. 1985;313:84–95.
- , , , , . Opioid rotation in the management of chronic pain: where is the evidence? Pain Pract. 2010;10:85–93.
- . Palliative Care Perspectives. New York, NY: Oxford University Press; 2003:36–74.
- Agency Medical Director's Group. Web‐based opioid dose calculator. Available at http://agencymeddirectors.wa.govmobile.html. Accessed April 7, 2014.
- Agency for Healthcare Research and Quality. Hospital inpatient utilization related to opioid overuse among adults, 1993–2012. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb177-Hospitalizations-for-Opioid-Overuse.pdf. Accessed December 12, 2014.
- National Center for Health Statistics. Hospitalization for congestive heart failure: United States, 2000–2010. Available at: http://www.cdc.gov/nchs/data/databriefs/db108.htm#trends. Accessed December 12, 2014.
- , , , et al. Patterns of opioid use for chronic non‐cancer pain in the veterans health administration from 2009 to 2011. Pain. 2014;155:2337–2343.
- , , , , , . Prevalence and characteristics of hospitalized adults on chronic opioid therapy. J Hosp Med. 2014;9:82–87.
- , , , . Chronic opioid analgesic usage post‐kidney transplantation and clinical outcomes. Clin Transplant. 2014;28:1041–1046.
- , , , . A comparison between enriched and nonenriched enrollment randomized withdrawal trials of opioids for chronic noncancer pain. Pain Res Manag. 2011;16:337–351.
- , , , , . Redesigning delivery of opioids to optimize pain management, improve outcomes, and contain costs. Pain Med. 2013;14:36–42.
- , , , , . Interaction of age and opioid dependence on length of hospital stay for spine surgery patients. Psychol Rep. 2009;105:361–364.
- , , , et al. Sex, depression, and risk of hospitalization and mortality in chronic obstructive pulmonary disease. Arch Intern Med. 2007;167:2345–2353.
- , , , et al. Risk of fractures requiring hospitalization after an initial prescription for zolpidem, alprazolam, lorazepam, or diazepam in older adults. J Am Geriatr Soc. 2011;59:1883–1890.
- , , , et al. Relationship between potential opioid‐related adverse effects and hospital length of stay in patients receiving opioids after orthopedic surgery. Pharmacotherapy. 2012;32:502–514.
- , , , . Opioid prescribing by multiple providers in Medicare: Retrospective observational study of insurance claims. BMJ. 2014;348:g1393.
- , , , , , . Analgesic usage for low back pain: Impact on health care costs and service use. Spine (Phila Pa 1976). 2005;30:1075–1081.
- . Economic burden of prescription opioid misuse and abuse. J Manag Care Pharm. 2009;15:556–562.
Longer term and higher doses of opioid analgesics (OAs) have been associated with multiple adverse outcomes such as loss of work, cognitive decline, and poor function.[1, 2, 3, 4] One of the most widely reported complications of opioid therapy is drug overdose.[5, 6, 7, 8, 9] In population‐based studies, daily morphine equivalent doses >100 mg have been associated with significantly increased risk of drug overdose.[5, 6, 7, 8, 9, 10] Among health maintenance organization (HMO) enrollees filling at least 2 prescriptions for opioids, our group reported that daily opioid doses 100 mg were associated with approximately threefold greater adjusted odds of drug overdose.[10] We also observed over a twofold increase in odds of drug overdose for lower daily doses of 50 to 99 mg if the patient also received a high total opioid dose (>1830 mg) over a 6‐month period. This analysis suggests that clinicians may need to monitor not only daily dose but also total dose of opioids to reduce the risk of drug overdose.
Yet drug overdose represents only a small subset of all hospitalizations for persons receiving long‐term or higher doses of opioids for noncancer pain. These patients have significant demand for urgent care services, including hospitalization, for diverse reasons such as adverse effects of opioids, underlying cause of chronic pain, and comorbidities such as mental health disorders.[11] In a cohort of elderly primary care patients who were high hospital utilizers, Freund and colleagues reported that chronic pain and depression were the most common conditions co‐occurring with their other comorbidities.[12] However, little is known about the association of opioid dose with the risk of all‐cause hospitalization for patients with noncancer pain.
In this article we examined hospitalizations for a national cohort of HMO enrollees with noncancer pain who filled at least 2 prescriptions for schedule II or III opioids over a 3.5‐year timeframe. This retrospective cohort analysis aims to identify clinically useful opioid dose measures for clinicians, administrators, and policymakers to use in identifying patients at increased risk of future hospitalization who may warrant interventions to reduce this risk.
METHODS
Study Sample
From Aetna administrative databases including enrollment files and paid claims for services, we identified 261,528 subjects aged 18 to 64 years who had at least 2 paid claims for schedule II or III noninjectable OA prescriptions from January 2009 through July 2012.[10] For individuals meeting these criteria, study cohort eligibility required at least 12 months of enrollment and complete data on demographics and OA prescriptions as well as clinical conditions from at least 1 encounter (see Supporting Information, Appendix 1, in the online version of this article).[10] We excluded subjects with a cancer diagnosis who have high hospital utilization and those younger than 45 years because of a higher likelihood of pregnancy‐related hospitalization. To afford sufficient observation time for outcomes, subjects with <12 months follow‐up after the first opioid prescription were excluded. The resultant study cohort totaled 87,688 subjects.
To capture the changing nature of medication utilization and clinical conditions in this longitudinal study, we divided the study timeframe into 6‐month intervals starting with the first opioid prescription and ending with the subject's last enrollment or end of the study (see Supporting Information, Appendix 2, in the online version of this article). Six‐month intervals were studied because this is the maximum duration of benefit from randomized trials of opioid therapy for noncancer pain.[13] This study was approved by the University of Texas Health Science Center at San Antonio's institutional review board.
Outcome Variables
Study outcomes were all‐cause hospitalization (binary) and hospital days (discrete) per 6‐month interval and were measured repeatedly for up to 6, 6‐month intervals.
Primary Independent Variables
We examined 2 opioid dose measures within a 6‐month interval and hospitalization outcomes in the next 6 months (see Supporting Information, Appendix 2, in the online version of this article). We did not examine OA use in the last 6 months of the study timeframe because subsequent hospitalization outcomes were not available. We defined the total morphine equivalent dose of OA prescriptions filled within a 6‐month interval based on the method used by Edlund et al.[14] and adapted by our group.[10] We also defined the daily dose of OAs that is a widely used metric used in chronic pain management guidelines.[10, 15]
To calculate the total opioid dose, all filled schedule II or III OA prescriptions (noninjectable formulations) were identified from claims for filled prescriptions for each 6‐month interval. The morphine equivalent dose for each opioid prescription was calculated from the number of pills dispensed multiplied by strength (in milligrams) and by a morphine equivalent conversion factor derived from several sources including published data,[16, 17] conversion tables from Internet sources, and drug information resources.[18, 19] A clinical pharmacist reviewed and finalized conversions. When an opioid prescription spanned two, 6‐month intervals, the dose was divided proportionate to time in each interval. The total dose for all opioid prescriptions within an interval was summed and categorized by quartile of nonzero total dose as: 1 to 190, 191 to 450, 451 to 1830, and >1830 mg.[10]
To calculate the daily opioid dose in each interval, the total dose was divided by total nonoverlapping days' supply covered by all prescriptions. The average daily dose was categorized as in other studies: 1 to 19, 20 to 49, 50 to 99, and 100 mg.[5, 6, 10] In each 6‐month interval, the percentage of days covered by filled prescriptions was calculated as total days' supply/180.
Other Independent Variables
Demographic data included age as of July 2012, sex, and US region. From available diagnosis codes for encounters, pain‐related conditions were identified including: back pain, other osteoarthritis, neuropathic pain, chronic pain unspecified, or chronic headache (International Classification of Diseases, Ninth Revision, Clinical Modification codes available from authors). Mental health/substance use disorders were similarly identified: anxiety or post‐traumatic stress disorder (PTSD), depression, psychosis, drug abuse, and alcohol abuse. Once a psychiatric condition or substance use disorder was diagnosed, it was considered to persist because these are usually not transient. We examined filled prescriptions for psychoactive drugs in 6‐month intervals including: benzodiazepines (i.e., clonazepam, alprazolam, lorazepam, diazepam, chlordiazepine, temazepam, flurazepam), antidepressants (i.e., selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors, tricyclics [complete list available from authors]), and sedatives (i.e., zolpidem, eszopiclone). For these drugs, time‐varying variables were created as follows: benzodiazepines (0, 130, 3190, 91180 days), sedatives (0, 130, 3190, 91180 days), and antidepressants (0, 160, 61180 days). Categories for duration of antidepressants differed because a clinical response can take up to 6 to 8 weeks.
Statistical Analyses
Descriptive statistics were examined for study cohort characteristics. For the binary all‐cause hospitalization outcome, repeated measures logistic regression models were estimated using generalized estimating equations (GEE) to examine associations of daily opioid dose, total opioid dose, and their interaction with all‐cause hospitalization. The fully adjusted model includes demographics, chronic pain conditions, mental health conditions, substance use disorders, other psychoactive drugs, and current hospitalization (yes/no). For the hospital days per 6‐month outcome, a series of repeated measures Poisson regressions were estimated using the GEE approach.
In a post hoc sensitivity analysis, we examined the association of the percentage of days covered by prescribed opioids, categorized based on approximate quartiles and clinical judgment, with hospitalization among subjects with a high total dose (>1830 mg). For this analysis, we created a composite measure of opioid treatment for each 6‐month interval that has 6 categories: (1) none, (2) low total dose 1 to 1830 mg, (3) high total dose >1830 mg with 50% of days on opioids, (4) total dose >1830 mg with >50% to 75% of days on opioids, (5) total dose >1830 mg with >75% to 90% of days on opioids, and (6) total dose >1830 mg and >90% of days on opioids. Adjusted regression analyses described above were repeated for both outcomes and included this composite measure. All statistical tests were performed with a 2‐sided significance level of 0.05, and analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Of 87,688 study subjects, 54.8% were women, and the mean age was 53.8 years (standard deviation [SD]=5.5). Nearly half of the cohort resided in Southern states (Table 1). In the baseline 6‐month interval, the most common chronic noncancer pain conditions were musculoskeletal involving large joint arthritis/arthralgia (38.4%) and back pain (28.2%). In regard to mental health and substance use conditions, both anxiety/PTSD and depression were diagnosed in approximately 7% of the cohort, whereas psychosis, and alcohol and other substance use disorders were each diagnosed in <2%. In the baseline interval, 12.7% of subjects were hospitalized. The majority of patients received a daily opioid dose of 20 to 49 mg, and the median total dose was 450 mg. The median percent of time exposed to opioids was 6.7% among all study subjects and 70% for those with a high total dose (>1830 mg).
In the 3 study years, an average of 12% of the cohort was hospitalized yearly (Table 2), or 1120 hospitalizations per 10,000 person‐years. Among those who were hospitalized, inpatient days averaged 6.5 (SD=8.5). The highest proportion of hospitalized subjects was 6.5%, occurring in the 6‐month interval immediately following the first opioid treatment interval. In subsequent 6‐month intervals, hospitalization rates were relatively stable, ranging from 5.2% to 6.1% (Table 2). As shown, future hospitalization rates increased monotonically, with increasing total or daily dose within each 6‐month interval.
| Characteristics | Total, N=87,688 |
|---|---|
| |
| Demographics | |
| Women, n (%) | 48,077 (54.8) |
| Age, mean (SD) | 53.8 (5.5) |
| US region, n (%) | |
| Midwest | 4,609 (5.3) |
| Northeast | 27,568 (31.4) |
| South | 40,767 (46.5) |
| West | 14,744 (16.8) |
| Clinical conditions, n (%)b | |
| Noncancer pain conditions | |
| Back pain | 24,767 (28.2) |
| Large joint arthritis, other musculoskeletalc | 33,689 (38.4) |
| Neuropathy | 1,519 (1.7) |
| Chronic pain (unspecified) | 3,229 (3.7) |
| Headache | 2,837 (3.2) |
| Mental health and substance use disorders | |
| Anxiety or post‐traumatic stress disorder | 6,006 (6.9) |
| Depression | 6,111 (7.0) |
| Psychosis | 1,259 (1.4) |
| Alcohol abuse | 877 (1.0) |
| Other substance abuse | 615 (0.7) |
| Current hospitalization, n (%) | 11,165 (12.7) |
| Opioid measures, n (%) | |
| Daily MED dose, mg | |
| 0 | |
| 119 | 9,870 (11.3) |
| 2049 | 50,050 (57.1) |
| 5099 | 21,188 (24.2) |
| 100 | 6,580 (7.5) |
| Total MED dose, mg | |
| 0 | |
| 1190 | 20,276 (23.1) |
| 191450 | 26,000 (29.7) |
| 4511,830 | 23,551 (26.9) |
| >1,830 | 17,861 (20.4) |
| Percent time exposed to opioid therapy, median (Q1, Q3) | |
| Among any total MED | 6.7 (2.8, 22.2) |
| Among total MED >1,830 mg | 70 (42.8, 93.9) |
| Subjects | 6‐Month Interval | |||||
|---|---|---|---|---|---|---|
| 1 (Baseline), N=87,688 | 2, N=65,835 | 3, N=46,041 | 4, N=31,550 | 5, N=18,915 | 6, N=3,502 | |
| ||||||
| Overall (%) | 6.5 | 5.9 | 5.9 | 5.4 | 5.2 | 6.1 |
| Opioid dose measure | ||||||
| Daily dose (%) | ||||||
| 0 mg | 4.8 | 4.4 | 4.0 | 3.6 | 3.2 | |
| 119 mg | 5.9 | 5.6 | 6.0 | 5.6 | 5.6 | 4.4 |
| 2049 mg | 6.2 | 6.5 | 7.1 | 6.6 | 6.1 | 6.1 |
| 5099 mg | 6.8 | 7.9 | 7.5 | 7.6 | 7.6 | 9.8 |
| 100 mg | 9.0 | 9.3 | 10.3 | 9.2 | 9.5 | 9.5 |
| Total dose (%) a | ||||||
| 0 mg | 4.8 | 4.4 | 4.0 | 3.6 | 3.2 | |
| 1190 mg | 5.5 | 4.7 | 5.0 | 4.1 | 4.0 | 2.7 |
| 191450 mg | 5.1 | 5.1 | 6.3 | 6.7 | 5.0 | 3.2 |
| 4511,830 mg | 6.5 | 7.4 | 7.9 | 7.2 | 7.1 | 7.0 |
| >1,830 mg | 9.8 | 9.6 | 9.6 | 8.9 | 8.8 | 9.0 |
In unadjusted analyses, a significant interaction between daily dose and total dose (P<0.001) revealed that, within each daily dose category, the odds of hospitalization differed by total dose (all P<0.05, Table 3). When the total dose was >1830 mg, the odds of future hospitalization rose monotonically with increasing daily dose (i.e., <20, 2049, 5099, 100 mg): 1.33, 1.84, 1.96, and 2.08 (P<0.05 for all comparisons vs no opioids). On the other hand, when the total dose was 450 mg or less, all daily dose categories including a very high daily dose (100 mg) were not associated with future hospitalization (all P>0.05 vs no opioids). When the total dose was 451 to 1830 mg, a nonlinear association with hospitalization appeared with higher odds for lower daily doses. For the outcome of hospital days per 6‐month interval, increasing daily dose was also associated with more hospital days per 6‐month interval when the total dose was high (>1830 mg), whereas for lower total doses, daily dose was weakly positive or even protective versus no opioids.
| All‐Cause Hospitalization (Yes/No), Odds Ratio (95% CI) | |||||
|---|---|---|---|---|---|
| Total Morphine Equivalent Dose, mg | Daily Morphine Equivalent Dose, mg | ||||
| 0 | 19 | 2049 | 5099 | 100 | |
| Hospital Days per 6‐Month, Incident Rate Ratio (95% CI) | |||||
| Total Morphine Equivalent Dose, mg | Daily Morphine Equivalent Dose, mg | ||||
| 0 | 119 | 2049 | 5099 | 100 | |
| |||||
| 0 | 1 | ||||
| 1190 | 1.06 (0.95‐1.19) | 1.01 (0.95‐1.08) | 1.07 (0.95‐1.19) | 0.73 (0.44‐1.21) | |
| 191450 | 1.08 (0.96‐1.22) | 1.03 (0.96‐1.10) | 0.99 (0.9‐1.10) | 0.88 (0.67‐1.15) | |
| 4511,830 | 1.34 (1.21‐1.48)a | 1.37 (1.28‐1.46)a | 1.16 (1.05‐1.27)a | 1.25 (0.98‐1.59) | |
| >1,830 | 1.33 (1.09‐1.62)a | 1.84 (1.73‐1.97)a | 1.96 (1.82‐2.11)a | 2.08 (1.93‐2.24)a | |
| 0 | 1 | ||||
| 1190 | 0.95 (0.79‐1.14) | 0.90 (0.82‐0.99)a | 1.03 (0.87‐1.23) | 0.63 (0.36‐1.12) | |
| 191450 | 0.92 (0.77‐1.10) | 0.93 (0.84‐1.02) | 0.79 (0.69‐0.91)a | 0.69 (0.49‐0.98)a | |
| 4511,830 | 1.31 (1.10‐1.57)a | 1.26 (1.13‐1.40)a | 1.01 (0.86‐1.19) | 0.99 (0.71‐1.37) | |
| >1,830 | 1.32 (0.93‐1.89) | 1.79 (1.60‐2.01)a | 1.76 (1.54‐2.01)a | 2.09 (1.85‐2.36)a | |
In the model adjusting for all covariates (Table 4), the interaction between total dose and daily dose was also significant (P=0.002). When the total dose was high (>1830 mg), the adjusted odds of future hospitalization were significantly increased by 35% to 44% for daily doses of 20 to 49 mg or greater versus no opioids (P<0.05 for all comparisons). When the total dose was <1830 mg, the majority of daily dose categories were not significantly associated with hospitalization. Similarly, in the fully adjusted analysis of hospital days, the number of inpatient days were increased by 28% to 48% when the total dose was >1830 mg and daily dose was >20 mg, but these associations were nonsignificant or protective when the total dose was lower.
| All‐Cause Hospitalization (Yes/No), Odds Ratio (95% CI) | |||||
|---|---|---|---|---|---|
| Total Morphine Equivalent Dose, mg | Daily Morphine Equivalent Dose, mg | ||||
| 0 | 119 | 2049 | 5099 | 100 | |
| Hospital Days per 6‐Month, Incident Rate Ratio (95% CI) | |||||
| Total Morphine Equivalent Dose, mg | Daily Morphine Equivalent Dose, mg | ||||
| 0 | 119 | 2049 | 5099 | 100 | |
| |||||
| 0 | 1 | ||||
| 1190 | 1.09 (0.97‐1.23) | 1.07 (1.001.14) | 1.12 (1.001.26)b | 0.75 (0.45‐1.23) | |
| 191450 | 1.00 (0.88‐1.13) | 0.99 (0.92‐1.06) | 0.97 (0.88‐1.08) | 0.87 (0.68‐1.12) | |
| 4511,830 | 1.16 (1.04‐1.29) | 1.14 (1.07‐1.22) | 0.94 (0.85‐1.03) | 1.08 (0.85‐1.35) | |
| >1,830 | 1.10 (0.90‐1.34) | 1.41 (1.32‐1.51) | 1.35 (1.25‐1.46) | 1.44 (1.34‐1.55) | |
| 0 | 1 | ||||
| 1190 | 0.97 (0.8‐1.18) | 0.94 (0.85‐1.04) | 1.06 (0.88‐1.27) | 0.60 (0.33‐1.1) | |
| 191450 | 0.85 (0.71‐1.02) | 0.88 (0.79‐0.98) | 0.75 (0.65‐0.86) | 0.65 (0.46‐0.92) | |
| 4511,830 | 1.16 (0.97‐1.4) | 1.09 (0.97‐1.22) | 0.83 (0.71‐0.98) | 0.81 (0.59‐1.13) | |
| >1,830 | 1.12 (0.77‐1.63) | 1.41 (1.25‐1.58) | 1.28 (1.12‐1.46) | 1.48 (1.29‐1.69) | |
In a sensitivity analysis, we examined the percentage of days covered by filled opioid prescriptions within a 6‐month interval for subjects receiving high‐dose therapy (Table 5). Compared with no opioid therapy, the adjusted odds of future hospitalization were 5% greater for low total opioid dose (11830 mg) and 21% greater for high total dose (>1830 mg) when the duration of treatment was shorter (50% of the 6‐month interval). However, the odds were increased by 41% to 51% for a high total dose (>1830 mg), with longer periods of treatment (>50% of the interval). For hospital days as the outcome, subjects with high total doses (>1830 mg) and longer periods of treatment (>50% of the interval) had 41% to 71% more hospital days per 6‐month interval than those with no opioid therapy.
| Opioid Analgesic Category | All‐Cause Hospitalization | Hospital Days per 6 Months |
|---|---|---|
| Odds Ratio (95% CI) | Incident Rate Ratio (95% CI) | |
| ||
| 0 mg | 1 | 1 |
| 11,830 mg | 1.05 (1.001.10)b | 0.94 (0.87‐1.01) |
| >1,830 mg and 50% days on opioids | 1.21 (1.11‐1.31)b | 1.10 (0.96‐1.26) |
| >1,830 mg and >50 to 75% days on opioids | 1.51 (1.40‐1.64)b | 1.45 (1.26‐1.67) |
| >1,830 mg and >75 to 90% days on opioids | 1.50 (1.38‐1.64)b | 1.71 (1.46‐1.99) |
| >1,830 mg and >90% days on opioids | 1.41 (1.31‐1.52)b | 1.41 (1.26‐1.58) |
DISCUSSION
In a national cohort of HMO enrollees who filled at least 2 prescriptions for OAs, 12% were hospitalized annually. Other studies of opioid users have focused on only a fraction of these hospitalizations. For example, a recent Agency for Healthcare Research and Quality study reported that the rate of hospitalization for complications from accidental or deliberate overuse of opioids more than doubled from 11.7/10,000 in 1993 to 29.5/10,000 in 2010.[20] However, in our cohort, the all‐cause hospitalization rate was 1120 per 10,000 person‐years, or over 40 times greater than the rate for complications from overuse of opioids. By comparison, hospitalization for heart failure was only 32.8/10,000 nationally in 2010.[21] Thus, our study confirms the significant demand for hospital care by patients treated with opioids. A novel finding of our study is that the total dose of prescriptions filled over 6 months is significantly associated with an increased risk of future hospitalization. When the total dose within 6 months was in the top quartile (>1830 mg in our cohort), the adjusted odds of future hospitalization ranged from 35% to 44% greater than no opioids for daily opioid doses above 20 mg/day. On the other hand, when the total dose was 1830 mg, the daily opioid dose was only weakly associated with future hospitalization. These associations were similar for hospital days per 6‐month interval as the outcome.
Edlund and colleagues examined the total dose of opioids in a national cohort of veterans with chronic noncancer pain who filled at least 1 opioid prescription.[22] In 2011, the 60th percentile for the total opioid dose for these veterans was 3610 mg within a year, which is roughly equivalent to our top quartile (1830 mg) over a 6‐month interval. These data support replicating our study in veterans to evaluate whether a similarly increased risk of hospitalization appears for those with high total opioid doses. In support of a concern among veterans, a population‐based, cross‐sectional study of hospitalized veterans reported a high rate of chronic opioid therapy (90 days) in the 6 months prior to hospitalization.[23]
Other studies have reported increased risk of hospitalization with chronic opioid therapy. Among 1045 patients followed up to 1‐year post‐transplantation, long‐term opioids were associated with up to a sixfold greater risk of at least 4 admissions within that year.[24] Among 13,127 Danish adults on opioid therapy, the odds of future hospitalization from injuries were increased by 74% for long‐term therapy and 46% for short‐term therapy versus no opioids and by threefold and 1.6‐fold, respectively, for hospitalization due to toxicity/poisoning.[9] However, none of these studies examined the dose of opioids.
In a sensitivity analysis, we found that when a subject received a high total opioid dose within 6 months, treatment for more than 50% of the interval (i.e., >3 months) was associated with a significantly increased risk of future hospitalization and significantly more hospital days. Because the strongest evidence for the benefit of opioids for chronic noncancer pain comes from trials of <3 months,[25] these data lend additional support to recommendations to minimize both dose and duration of opioid therapy.
Our study has several limitations. First, we did not assess the immediate risk of hospitalization after starting opioid therapy. Second, our outcome of hospitalization represents only 1 measure of risk. Thus, our data should not be regarded as supporting short‐term use of high‐dose opioids over 100 to 120 mg per day.[26] In an earlier study, we reported that either a high daily dose (100 mg) or a moderately high daily dose (5099 mg) plus a high total dose (>1830 mg) increased the risk of drug overdose.[10] Third, we could not examine the reason for hospitalizations in this analysis. Therefore, we cannot presume that opioid therapy caused these hospitalizations, but it likely serves as a proxy for other factors such as disability and mental health disorders that increase risk of hospitalization. However, we did adjust for pain conditions as well as mental health and substance abuse disorders that are known to increase the risk of hospitalization in other cohorts.[27, 28, 29, 30] In a national veterans study, the most common clinical conditions associated with long‐term opioid therapy were major depression and PTSD.[22] Last, we did also not consider the number of prescribers of opioids. In a Medicare study, 1 versus 4 prescribers of OAs increased patients' annual hospitalization rate from 1.6% to 4.8%, respectively.[31]
Although the total opioid dose categories observed for our study population may differ from those in other cohorts, these data offer additional evidence for clinicians to consider this measure when assessing risk for hospitalization, and among subjects on high total doses, the percentage of time on opioids offers an additional measure of risk. Because opioid users with noncancer pain are heavy consumers of healthcare services,32,33 public health benefits and reductions in costs of care may be substantial if opportunities can be identified to reduce hospital utilization by persons treated with higher doses of OAs.
Disclosures
The work on this project was supported by an intramural grant from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 1UL TR001120. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors report no conflicts of interest.
Longer term and higher doses of opioid analgesics (OAs) have been associated with multiple adverse outcomes such as loss of work, cognitive decline, and poor function.[1, 2, 3, 4] One of the most widely reported complications of opioid therapy is drug overdose.[5, 6, 7, 8, 9] In population‐based studies, daily morphine equivalent doses >100 mg have been associated with significantly increased risk of drug overdose.[5, 6, 7, 8, 9, 10] Among health maintenance organization (HMO) enrollees filling at least 2 prescriptions for opioids, our group reported that daily opioid doses 100 mg were associated with approximately threefold greater adjusted odds of drug overdose.[10] We also observed over a twofold increase in odds of drug overdose for lower daily doses of 50 to 99 mg if the patient also received a high total opioid dose (>1830 mg) over a 6‐month period. This analysis suggests that clinicians may need to monitor not only daily dose but also total dose of opioids to reduce the risk of drug overdose.
Yet drug overdose represents only a small subset of all hospitalizations for persons receiving long‐term or higher doses of opioids for noncancer pain. These patients have significant demand for urgent care services, including hospitalization, for diverse reasons such as adverse effects of opioids, underlying cause of chronic pain, and comorbidities such as mental health disorders.[11] In a cohort of elderly primary care patients who were high hospital utilizers, Freund and colleagues reported that chronic pain and depression were the most common conditions co‐occurring with their other comorbidities.[12] However, little is known about the association of opioid dose with the risk of all‐cause hospitalization for patients with noncancer pain.
In this article we examined hospitalizations for a national cohort of HMO enrollees with noncancer pain who filled at least 2 prescriptions for schedule II or III opioids over a 3.5‐year timeframe. This retrospective cohort analysis aims to identify clinically useful opioid dose measures for clinicians, administrators, and policymakers to use in identifying patients at increased risk of future hospitalization who may warrant interventions to reduce this risk.
METHODS
Study Sample
From Aetna administrative databases including enrollment files and paid claims for services, we identified 261,528 subjects aged 18 to 64 years who had at least 2 paid claims for schedule II or III noninjectable OA prescriptions from January 2009 through July 2012.[10] For individuals meeting these criteria, study cohort eligibility required at least 12 months of enrollment and complete data on demographics and OA prescriptions as well as clinical conditions from at least 1 encounter (see Supporting Information, Appendix 1, in the online version of this article).[10] We excluded subjects with a cancer diagnosis who have high hospital utilization and those younger than 45 years because of a higher likelihood of pregnancy‐related hospitalization. To afford sufficient observation time for outcomes, subjects with <12 months follow‐up after the first opioid prescription were excluded. The resultant study cohort totaled 87,688 subjects.
To capture the changing nature of medication utilization and clinical conditions in this longitudinal study, we divided the study timeframe into 6‐month intervals starting with the first opioid prescription and ending with the subject's last enrollment or end of the study (see Supporting Information, Appendix 2, in the online version of this article). Six‐month intervals were studied because this is the maximum duration of benefit from randomized trials of opioid therapy for noncancer pain.[13] This study was approved by the University of Texas Health Science Center at San Antonio's institutional review board.
Outcome Variables
Study outcomes were all‐cause hospitalization (binary) and hospital days (discrete) per 6‐month interval and were measured repeatedly for up to 6, 6‐month intervals.
Primary Independent Variables
We examined 2 opioid dose measures within a 6‐month interval and hospitalization outcomes in the next 6 months (see Supporting Information, Appendix 2, in the online version of this article). We did not examine OA use in the last 6 months of the study timeframe because subsequent hospitalization outcomes were not available. We defined the total morphine equivalent dose of OA prescriptions filled within a 6‐month interval based on the method used by Edlund et al.[14] and adapted by our group.[10] We also defined the daily dose of OAs that is a widely used metric used in chronic pain management guidelines.[10, 15]
To calculate the total opioid dose, all filled schedule II or III OA prescriptions (noninjectable formulations) were identified from claims for filled prescriptions for each 6‐month interval. The morphine equivalent dose for each opioid prescription was calculated from the number of pills dispensed multiplied by strength (in milligrams) and by a morphine equivalent conversion factor derived from several sources including published data,[16, 17] conversion tables from Internet sources, and drug information resources.[18, 19] A clinical pharmacist reviewed and finalized conversions. When an opioid prescription spanned two, 6‐month intervals, the dose was divided proportionate to time in each interval. The total dose for all opioid prescriptions within an interval was summed and categorized by quartile of nonzero total dose as: 1 to 190, 191 to 450, 451 to 1830, and >1830 mg.[10]
To calculate the daily opioid dose in each interval, the total dose was divided by total nonoverlapping days' supply covered by all prescriptions. The average daily dose was categorized as in other studies: 1 to 19, 20 to 49, 50 to 99, and 100 mg.[5, 6, 10] In each 6‐month interval, the percentage of days covered by filled prescriptions was calculated as total days' supply/180.
Other Independent Variables
Demographic data included age as of July 2012, sex, and US region. From available diagnosis codes for encounters, pain‐related conditions were identified including: back pain, other osteoarthritis, neuropathic pain, chronic pain unspecified, or chronic headache (International Classification of Diseases, Ninth Revision, Clinical Modification codes available from authors). Mental health/substance use disorders were similarly identified: anxiety or post‐traumatic stress disorder (PTSD), depression, psychosis, drug abuse, and alcohol abuse. Once a psychiatric condition or substance use disorder was diagnosed, it was considered to persist because these are usually not transient. We examined filled prescriptions for psychoactive drugs in 6‐month intervals including: benzodiazepines (i.e., clonazepam, alprazolam, lorazepam, diazepam, chlordiazepine, temazepam, flurazepam), antidepressants (i.e., selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors, tricyclics [complete list available from authors]), and sedatives (i.e., zolpidem, eszopiclone). For these drugs, time‐varying variables were created as follows: benzodiazepines (0, 130, 3190, 91180 days), sedatives (0, 130, 3190, 91180 days), and antidepressants (0, 160, 61180 days). Categories for duration of antidepressants differed because a clinical response can take up to 6 to 8 weeks.
Statistical Analyses
Descriptive statistics were examined for study cohort characteristics. For the binary all‐cause hospitalization outcome, repeated measures logistic regression models were estimated using generalized estimating equations (GEE) to examine associations of daily opioid dose, total opioid dose, and their interaction with all‐cause hospitalization. The fully adjusted model includes demographics, chronic pain conditions, mental health conditions, substance use disorders, other psychoactive drugs, and current hospitalization (yes/no). For the hospital days per 6‐month outcome, a series of repeated measures Poisson regressions were estimated using the GEE approach.
In a post hoc sensitivity analysis, we examined the association of the percentage of days covered by prescribed opioids, categorized based on approximate quartiles and clinical judgment, with hospitalization among subjects with a high total dose (>1830 mg). For this analysis, we created a composite measure of opioid treatment for each 6‐month interval that has 6 categories: (1) none, (2) low total dose 1 to 1830 mg, (3) high total dose >1830 mg with 50% of days on opioids, (4) total dose >1830 mg with >50% to 75% of days on opioids, (5) total dose >1830 mg with >75% to 90% of days on opioids, and (6) total dose >1830 mg and >90% of days on opioids. Adjusted regression analyses described above were repeated for both outcomes and included this composite measure. All statistical tests were performed with a 2‐sided significance level of 0.05, and analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Of 87,688 study subjects, 54.8% were women, and the mean age was 53.8 years (standard deviation [SD]=5.5). Nearly half of the cohort resided in Southern states (Table 1). In the baseline 6‐month interval, the most common chronic noncancer pain conditions were musculoskeletal involving large joint arthritis/arthralgia (38.4%) and back pain (28.2%). In regard to mental health and substance use conditions, both anxiety/PTSD and depression were diagnosed in approximately 7% of the cohort, whereas psychosis, and alcohol and other substance use disorders were each diagnosed in <2%. In the baseline interval, 12.7% of subjects were hospitalized. The majority of patients received a daily opioid dose of 20 to 49 mg, and the median total dose was 450 mg. The median percent of time exposed to opioids was 6.7% among all study subjects and 70% for those with a high total dose (>1830 mg).
In the 3 study years, an average of 12% of the cohort was hospitalized yearly (Table 2), or 1120 hospitalizations per 10,000 person‐years. Among those who were hospitalized, inpatient days averaged 6.5 (SD=8.5). The highest proportion of hospitalized subjects was 6.5%, occurring in the 6‐month interval immediately following the first opioid treatment interval. In subsequent 6‐month intervals, hospitalization rates were relatively stable, ranging from 5.2% to 6.1% (Table 2). As shown, future hospitalization rates increased monotonically, with increasing total or daily dose within each 6‐month interval.
| Characteristics | Total, N=87,688 |
|---|---|
| |
| Demographics | |
| Women, n (%) | 48,077 (54.8) |
| Age, mean (SD) | 53.8 (5.5) |
| US region, n (%) | |
| Midwest | 4,609 (5.3) |
| Northeast | 27,568 (31.4) |
| South | 40,767 (46.5) |
| West | 14,744 (16.8) |
| Clinical conditions, n (%)b | |
| Noncancer pain conditions | |
| Back pain | 24,767 (28.2) |
| Large joint arthritis, other musculoskeletalc | 33,689 (38.4) |
| Neuropathy | 1,519 (1.7) |
| Chronic pain (unspecified) | 3,229 (3.7) |
| Headache | 2,837 (3.2) |
| Mental health and substance use disorders | |
| Anxiety or post‐traumatic stress disorder | 6,006 (6.9) |
| Depression | 6,111 (7.0) |
| Psychosis | 1,259 (1.4) |
| Alcohol abuse | 877 (1.0) |
| Other substance abuse | 615 (0.7) |
| Current hospitalization, n (%) | 11,165 (12.7) |
| Opioid measures, n (%) | |
| Daily MED dose, mg | |
| 0 | |
| 119 | 9,870 (11.3) |
| 2049 | 50,050 (57.1) |
| 5099 | 21,188 (24.2) |
| 100 | 6,580 (7.5) |
| Total MED dose, mg | |
| 0 | |
| 1190 | 20,276 (23.1) |
| 191450 | 26,000 (29.7) |
| 4511,830 | 23,551 (26.9) |
| >1,830 | 17,861 (20.4) |
| Percent time exposed to opioid therapy, median (Q1, Q3) | |
| Among any total MED | 6.7 (2.8, 22.2) |
| Among total MED >1,830 mg | 70 (42.8, 93.9) |
| Subjects | 6‐Month Interval | |||||
|---|---|---|---|---|---|---|
| 1 (Baseline), N=87,688 | 2, N=65,835 | 3, N=46,041 | 4, N=31,550 | 5, N=18,915 | 6, N=3,502 | |
| ||||||
| Overall (%) | 6.5 | 5.9 | 5.9 | 5.4 | 5.2 | 6.1 |
| Opioid dose measure | ||||||
| Daily dose (%) | ||||||
| 0 mg | 4.8 | 4.4 | 4.0 | 3.6 | 3.2 | |
| 119 mg | 5.9 | 5.6 | 6.0 | 5.6 | 5.6 | 4.4 |
| 2049 mg | 6.2 | 6.5 | 7.1 | 6.6 | 6.1 | 6.1 |
| 5099 mg | 6.8 | 7.9 | 7.5 | 7.6 | 7.6 | 9.8 |
| 100 mg | 9.0 | 9.3 | 10.3 | 9.2 | 9.5 | 9.5 |
| Total dose (%) a | ||||||
| 0 mg | 4.8 | 4.4 | 4.0 | 3.6 | 3.2 | |
| 1190 mg | 5.5 | 4.7 | 5.0 | 4.1 | 4.0 | 2.7 |
| 191450 mg | 5.1 | 5.1 | 6.3 | 6.7 | 5.0 | 3.2 |
| 4511,830 mg | 6.5 | 7.4 | 7.9 | 7.2 | 7.1 | 7.0 |
| >1,830 mg | 9.8 | 9.6 | 9.6 | 8.9 | 8.8 | 9.0 |
In unadjusted analyses, a significant interaction between daily dose and total dose (P<0.001) revealed that, within each daily dose category, the odds of hospitalization differed by total dose (all P<0.05, Table 3). When the total dose was >1830 mg, the odds of future hospitalization rose monotonically with increasing daily dose (i.e., <20, 2049, 5099, 100 mg): 1.33, 1.84, 1.96, and 2.08 (P<0.05 for all comparisons vs no opioids). On the other hand, when the total dose was 450 mg or less, all daily dose categories including a very high daily dose (100 mg) were not associated with future hospitalization (all P>0.05 vs no opioids). When the total dose was 451 to 1830 mg, a nonlinear association with hospitalization appeared with higher odds for lower daily doses. For the outcome of hospital days per 6‐month interval, increasing daily dose was also associated with more hospital days per 6‐month interval when the total dose was high (>1830 mg), whereas for lower total doses, daily dose was weakly positive or even protective versus no opioids.
| All‐Cause Hospitalization (Yes/No), Odds Ratio (95% CI) | |||||
|---|---|---|---|---|---|
| Total Morphine Equivalent Dose, mg | Daily Morphine Equivalent Dose, mg | ||||
| 0 | 19 | 2049 | 5099 | 100 | |
| Hospital Days per 6‐Month, Incident Rate Ratio (95% CI) | |||||
| Total Morphine Equivalent Dose, mg | Daily Morphine Equivalent Dose, mg | ||||
| 0 | 119 | 2049 | 5099 | 100 | |
| |||||
| 0 | 1 | ||||
| 1190 | 1.06 (0.95‐1.19) | 1.01 (0.95‐1.08) | 1.07 (0.95‐1.19) | 0.73 (0.44‐1.21) | |
| 191450 | 1.08 (0.96‐1.22) | 1.03 (0.96‐1.10) | 0.99 (0.9‐1.10) | 0.88 (0.67‐1.15) | |
| 4511,830 | 1.34 (1.21‐1.48)a | 1.37 (1.28‐1.46)a | 1.16 (1.05‐1.27)a | 1.25 (0.98‐1.59) | |
| >1,830 | 1.33 (1.09‐1.62)a | 1.84 (1.73‐1.97)a | 1.96 (1.82‐2.11)a | 2.08 (1.93‐2.24)a | |
| 0 | 1 | ||||
| 1190 | 0.95 (0.79‐1.14) | 0.90 (0.82‐0.99)a | 1.03 (0.87‐1.23) | 0.63 (0.36‐1.12) | |
| 191450 | 0.92 (0.77‐1.10) | 0.93 (0.84‐1.02) | 0.79 (0.69‐0.91)a | 0.69 (0.49‐0.98)a | |
| 4511,830 | 1.31 (1.10‐1.57)a | 1.26 (1.13‐1.40)a | 1.01 (0.86‐1.19) | 0.99 (0.71‐1.37) | |
| >1,830 | 1.32 (0.93‐1.89) | 1.79 (1.60‐2.01)a | 1.76 (1.54‐2.01)a | 2.09 (1.85‐2.36)a | |
In the model adjusting for all covariates (Table 4), the interaction between total dose and daily dose was also significant (P=0.002). When the total dose was high (>1830 mg), the adjusted odds of future hospitalization were significantly increased by 35% to 44% for daily doses of 20 to 49 mg or greater versus no opioids (P<0.05 for all comparisons). When the total dose was <1830 mg, the majority of daily dose categories were not significantly associated with hospitalization. Similarly, in the fully adjusted analysis of hospital days, the number of inpatient days were increased by 28% to 48% when the total dose was >1830 mg and daily dose was >20 mg, but these associations were nonsignificant or protective when the total dose was lower.
| All‐Cause Hospitalization (Yes/No), Odds Ratio (95% CI) | |||||
|---|---|---|---|---|---|
| Total Morphine Equivalent Dose, mg | Daily Morphine Equivalent Dose, mg | ||||
| 0 | 119 | 2049 | 5099 | 100 | |
| Hospital Days per 6‐Month, Incident Rate Ratio (95% CI) | |||||
| Total Morphine Equivalent Dose, mg | Daily Morphine Equivalent Dose, mg | ||||
| 0 | 119 | 2049 | 5099 | 100 | |
| |||||
| 0 | 1 | ||||
| 1190 | 1.09 (0.97‐1.23) | 1.07 (1.001.14) | 1.12 (1.001.26)b | 0.75 (0.45‐1.23) | |
| 191450 | 1.00 (0.88‐1.13) | 0.99 (0.92‐1.06) | 0.97 (0.88‐1.08) | 0.87 (0.68‐1.12) | |
| 4511,830 | 1.16 (1.04‐1.29) | 1.14 (1.07‐1.22) | 0.94 (0.85‐1.03) | 1.08 (0.85‐1.35) | |
| >1,830 | 1.10 (0.90‐1.34) | 1.41 (1.32‐1.51) | 1.35 (1.25‐1.46) | 1.44 (1.34‐1.55) | |
| 0 | 1 | ||||
| 1190 | 0.97 (0.8‐1.18) | 0.94 (0.85‐1.04) | 1.06 (0.88‐1.27) | 0.60 (0.33‐1.1) | |
| 191450 | 0.85 (0.71‐1.02) | 0.88 (0.79‐0.98) | 0.75 (0.65‐0.86) | 0.65 (0.46‐0.92) | |
| 4511,830 | 1.16 (0.97‐1.4) | 1.09 (0.97‐1.22) | 0.83 (0.71‐0.98) | 0.81 (0.59‐1.13) | |
| >1,830 | 1.12 (0.77‐1.63) | 1.41 (1.25‐1.58) | 1.28 (1.12‐1.46) | 1.48 (1.29‐1.69) | |
In a sensitivity analysis, we examined the percentage of days covered by filled opioid prescriptions within a 6‐month interval for subjects receiving high‐dose therapy (Table 5). Compared with no opioid therapy, the adjusted odds of future hospitalization were 5% greater for low total opioid dose (11830 mg) and 21% greater for high total dose (>1830 mg) when the duration of treatment was shorter (50% of the 6‐month interval). However, the odds were increased by 41% to 51% for a high total dose (>1830 mg), with longer periods of treatment (>50% of the interval). For hospital days as the outcome, subjects with high total doses (>1830 mg) and longer periods of treatment (>50% of the interval) had 41% to 71% more hospital days per 6‐month interval than those with no opioid therapy.
| Opioid Analgesic Category | All‐Cause Hospitalization | Hospital Days per 6 Months |
|---|---|---|
| Odds Ratio (95% CI) | Incident Rate Ratio (95% CI) | |
| ||
| 0 mg | 1 | 1 |
| 11,830 mg | 1.05 (1.001.10)b | 0.94 (0.87‐1.01) |
| >1,830 mg and 50% days on opioids | 1.21 (1.11‐1.31)b | 1.10 (0.96‐1.26) |
| >1,830 mg and >50 to 75% days on opioids | 1.51 (1.40‐1.64)b | 1.45 (1.26‐1.67) |
| >1,830 mg and >75 to 90% days on opioids | 1.50 (1.38‐1.64)b | 1.71 (1.46‐1.99) |
| >1,830 mg and >90% days on opioids | 1.41 (1.31‐1.52)b | 1.41 (1.26‐1.58) |
DISCUSSION
In a national cohort of HMO enrollees who filled at least 2 prescriptions for OAs, 12% were hospitalized annually. Other studies of opioid users have focused on only a fraction of these hospitalizations. For example, a recent Agency for Healthcare Research and Quality study reported that the rate of hospitalization for complications from accidental or deliberate overuse of opioids more than doubled from 11.7/10,000 in 1993 to 29.5/10,000 in 2010.[20] However, in our cohort, the all‐cause hospitalization rate was 1120 per 10,000 person‐years, or over 40 times greater than the rate for complications from overuse of opioids. By comparison, hospitalization for heart failure was only 32.8/10,000 nationally in 2010.[21] Thus, our study confirms the significant demand for hospital care by patients treated with opioids. A novel finding of our study is that the total dose of prescriptions filled over 6 months is significantly associated with an increased risk of future hospitalization. When the total dose within 6 months was in the top quartile (>1830 mg in our cohort), the adjusted odds of future hospitalization ranged from 35% to 44% greater than no opioids for daily opioid doses above 20 mg/day. On the other hand, when the total dose was 1830 mg, the daily opioid dose was only weakly associated with future hospitalization. These associations were similar for hospital days per 6‐month interval as the outcome.
Edlund and colleagues examined the total dose of opioids in a national cohort of veterans with chronic noncancer pain who filled at least 1 opioid prescription.[22] In 2011, the 60th percentile for the total opioid dose for these veterans was 3610 mg within a year, which is roughly equivalent to our top quartile (1830 mg) over a 6‐month interval. These data support replicating our study in veterans to evaluate whether a similarly increased risk of hospitalization appears for those with high total opioid doses. In support of a concern among veterans, a population‐based, cross‐sectional study of hospitalized veterans reported a high rate of chronic opioid therapy (90 days) in the 6 months prior to hospitalization.[23]
Other studies have reported increased risk of hospitalization with chronic opioid therapy. Among 1045 patients followed up to 1‐year post‐transplantation, long‐term opioids were associated with up to a sixfold greater risk of at least 4 admissions within that year.[24] Among 13,127 Danish adults on opioid therapy, the odds of future hospitalization from injuries were increased by 74% for long‐term therapy and 46% for short‐term therapy versus no opioids and by threefold and 1.6‐fold, respectively, for hospitalization due to toxicity/poisoning.[9] However, none of these studies examined the dose of opioids.
In a sensitivity analysis, we found that when a subject received a high total opioid dose within 6 months, treatment for more than 50% of the interval (i.e., >3 months) was associated with a significantly increased risk of future hospitalization and significantly more hospital days. Because the strongest evidence for the benefit of opioids for chronic noncancer pain comes from trials of <3 months,[25] these data lend additional support to recommendations to minimize both dose and duration of opioid therapy.
Our study has several limitations. First, we did not assess the immediate risk of hospitalization after starting opioid therapy. Second, our outcome of hospitalization represents only 1 measure of risk. Thus, our data should not be regarded as supporting short‐term use of high‐dose opioids over 100 to 120 mg per day.[26] In an earlier study, we reported that either a high daily dose (100 mg) or a moderately high daily dose (5099 mg) plus a high total dose (>1830 mg) increased the risk of drug overdose.[10] Third, we could not examine the reason for hospitalizations in this analysis. Therefore, we cannot presume that opioid therapy caused these hospitalizations, but it likely serves as a proxy for other factors such as disability and mental health disorders that increase risk of hospitalization. However, we did adjust for pain conditions as well as mental health and substance abuse disorders that are known to increase the risk of hospitalization in other cohorts.[27, 28, 29, 30] In a national veterans study, the most common clinical conditions associated with long‐term opioid therapy were major depression and PTSD.[22] Last, we did also not consider the number of prescribers of opioids. In a Medicare study, 1 versus 4 prescribers of OAs increased patients' annual hospitalization rate from 1.6% to 4.8%, respectively.[31]
Although the total opioid dose categories observed for our study population may differ from those in other cohorts, these data offer additional evidence for clinicians to consider this measure when assessing risk for hospitalization, and among subjects on high total doses, the percentage of time on opioids offers an additional measure of risk. Because opioid users with noncancer pain are heavy consumers of healthcare services,32,33 public health benefits and reductions in costs of care may be substantial if opportunities can be identified to reduce hospital utilization by persons treated with higher doses of OAs.
Disclosures
The work on this project was supported by an intramural grant from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 1UL TR001120. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors report no conflicts of interest.
- , , . Higher opioid doses predict poorer functional outcome in patients with chronic disabling occupational musculoskeletal disorders. J Bone Joint Surg Am. 2009;91:919–927.
- , , . Opioid therapy for nonspecific low back pain and the outcome of chronic work loss. Pain. 2009;142:194–201.
- , , , , , . The comparative safety of analgesics in older adults with arthritis. Arch Intern Med. 2010;170:1968–1976.
- , , , et al. Evidence of specific cognitive deficits in patients with chronic low back pain under long‐term substitution treatment of opioids. Pain Physician. 2014;17:9–20.
- , , , et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152:85–92.
- , , , et al. Association between opioid prescribing patterns and opioid overdose‐related deaths. JAMA. 2011;305:1315–1321.
- , , , , , . Trends in opioid use and dosing among socio‐economically disadvantaged patients. Open Med. 2011;5:e13–e22.
- , , , et al. A history of being prescribed controlled substances and risk of drug overdose death. Pain Med. 2012;13:87–95.
- , , , , . Chronic pain, opioid prescriptions and mortality in Denmark: a population‐based cohort study. Pain. 2014;155:2486–2490.
- , . Assessing risk for drug overdose in a national cohort: Role for both daily and total opioid dose [published online ahead of print December 5, 2015]? J Pain. doi: 10.1016/j.jpain.2014.11.007.
- Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Available at: http://www.ncbi.nlm.nih.gov/books/NBK91497. Accessed December 12, 2014.
- , , , , . Patterns of multimorbidity in primary care patients at high risk of future hospitalization. Popul Health Manag. 2012;15:119–124.
- , , , et al. Long‐term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;(1):CD006605.
- , , , , , . An analysis of heavy utilizers of opioids for chronic noncancer pain in the TROUP study. J Pain Symptom Manage. 2010;40:279–289.
- , , , et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38–47.
- . The treatment of cancer pain. N Engl J Med. 1985;313:84–95.
- , , , , . Opioid rotation in the management of chronic pain: where is the evidence? Pain Pract. 2010;10:85–93.
- . Palliative Care Perspectives. New York, NY: Oxford University Press; 2003:36–74.
- Agency Medical Director's Group. Web‐based opioid dose calculator. Available at http://agencymeddirectors.wa.govmobile.html. Accessed April 7, 2014.
- Agency for Healthcare Research and Quality. Hospital inpatient utilization related to opioid overuse among adults, 1993–2012. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb177-Hospitalizations-for-Opioid-Overuse.pdf. Accessed December 12, 2014.
- National Center for Health Statistics. Hospitalization for congestive heart failure: United States, 2000–2010. Available at: http://www.cdc.gov/nchs/data/databriefs/db108.htm#trends. Accessed December 12, 2014.
- , , , et al. Patterns of opioid use for chronic non‐cancer pain in the veterans health administration from 2009 to 2011. Pain. 2014;155:2337–2343.
- , , , , , . Prevalence and characteristics of hospitalized adults on chronic opioid therapy. J Hosp Med. 2014;9:82–87.
- , , , . Chronic opioid analgesic usage post‐kidney transplantation and clinical outcomes. Clin Transplant. 2014;28:1041–1046.
- , , , . A comparison between enriched and nonenriched enrollment randomized withdrawal trials of opioids for chronic noncancer pain. Pain Res Manag. 2011;16:337–351.
- , , , , . Redesigning delivery of opioids to optimize pain management, improve outcomes, and contain costs. Pain Med. 2013;14:36–42.
- , , , , . Interaction of age and opioid dependence on length of hospital stay for spine surgery patients. Psychol Rep. 2009;105:361–364.
- , , , et al. Sex, depression, and risk of hospitalization and mortality in chronic obstructive pulmonary disease. Arch Intern Med. 2007;167:2345–2353.
- , , , et al. Risk of fractures requiring hospitalization after an initial prescription for zolpidem, alprazolam, lorazepam, or diazepam in older adults. J Am Geriatr Soc. 2011;59:1883–1890.
- , , , et al. Relationship between potential opioid‐related adverse effects and hospital length of stay in patients receiving opioids after orthopedic surgery. Pharmacotherapy. 2012;32:502–514.
- , , , . Opioid prescribing by multiple providers in Medicare: Retrospective observational study of insurance claims. BMJ. 2014;348:g1393.
- , , , , , . Analgesic usage for low back pain: Impact on health care costs and service use. Spine (Phila Pa 1976). 2005;30:1075–1081.
- . Economic burden of prescription opioid misuse and abuse. J Manag Care Pharm. 2009;15:556–562.
- , , . Higher opioid doses predict poorer functional outcome in patients with chronic disabling occupational musculoskeletal disorders. J Bone Joint Surg Am. 2009;91:919–927.
- , , . Opioid therapy for nonspecific low back pain and the outcome of chronic work loss. Pain. 2009;142:194–201.
- , , , , , . The comparative safety of analgesics in older adults with arthritis. Arch Intern Med. 2010;170:1968–1976.
- , , , et al. Evidence of specific cognitive deficits in patients with chronic low back pain under long‐term substitution treatment of opioids. Pain Physician. 2014;17:9–20.
- , , , et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152:85–92.
- , , , et al. Association between opioid prescribing patterns and opioid overdose‐related deaths. JAMA. 2011;305:1315–1321.
- , , , , , . Trends in opioid use and dosing among socio‐economically disadvantaged patients. Open Med. 2011;5:e13–e22.
- , , , et al. A history of being prescribed controlled substances and risk of drug overdose death. Pain Med. 2012;13:87–95.
- , , , , . Chronic pain, opioid prescriptions and mortality in Denmark: a population‐based cohort study. Pain. 2014;155:2486–2490.
- , . Assessing risk for drug overdose in a national cohort: Role for both daily and total opioid dose [published online ahead of print December 5, 2015]? J Pain. doi: 10.1016/j.jpain.2014.11.007.
- Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Available at: http://www.ncbi.nlm.nih.gov/books/NBK91497. Accessed December 12, 2014.
- , , , , . Patterns of multimorbidity in primary care patients at high risk of future hospitalization. Popul Health Manag. 2012;15:119–124.
- , , , et al. Long‐term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;(1):CD006605.
- , , , , , . An analysis of heavy utilizers of opioids for chronic noncancer pain in the TROUP study. J Pain Symptom Manage. 2010;40:279–289.
- , , , et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38–47.
- . The treatment of cancer pain. N Engl J Med. 1985;313:84–95.
- , , , , . Opioid rotation in the management of chronic pain: where is the evidence? Pain Pract. 2010;10:85–93.
- . Palliative Care Perspectives. New York, NY: Oxford University Press; 2003:36–74.
- Agency Medical Director's Group. Web‐based opioid dose calculator. Available at http://agencymeddirectors.wa.govmobile.html. Accessed April 7, 2014.
- Agency for Healthcare Research and Quality. Hospital inpatient utilization related to opioid overuse among adults, 1993–2012. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb177-Hospitalizations-for-Opioid-Overuse.pdf. Accessed December 12, 2014.
- National Center for Health Statistics. Hospitalization for congestive heart failure: United States, 2000–2010. Available at: http://www.cdc.gov/nchs/data/databriefs/db108.htm#trends. Accessed December 12, 2014.
- , , , et al. Patterns of opioid use for chronic non‐cancer pain in the veterans health administration from 2009 to 2011. Pain. 2014;155:2337–2343.
- , , , , , . Prevalence and characteristics of hospitalized adults on chronic opioid therapy. J Hosp Med. 2014;9:82–87.
- , , , . Chronic opioid analgesic usage post‐kidney transplantation and clinical outcomes. Clin Transplant. 2014;28:1041–1046.
- , , , . A comparison between enriched and nonenriched enrollment randomized withdrawal trials of opioids for chronic noncancer pain. Pain Res Manag. 2011;16:337–351.
- , , , , . Redesigning delivery of opioids to optimize pain management, improve outcomes, and contain costs. Pain Med. 2013;14:36–42.
- , , , , . Interaction of age and opioid dependence on length of hospital stay for spine surgery patients. Psychol Rep. 2009;105:361–364.
- , , , et al. Sex, depression, and risk of hospitalization and mortality in chronic obstructive pulmonary disease. Arch Intern Med. 2007;167:2345–2353.
- , , , et al. Risk of fractures requiring hospitalization after an initial prescription for zolpidem, alprazolam, lorazepam, or diazepam in older adults. J Am Geriatr Soc. 2011;59:1883–1890.
- , , , et al. Relationship between potential opioid‐related adverse effects and hospital length of stay in patients receiving opioids after orthopedic surgery. Pharmacotherapy. 2012;32:502–514.
- , , , . Opioid prescribing by multiple providers in Medicare: Retrospective observational study of insurance claims. BMJ. 2014;348:g1393.
- , , , , , . Analgesic usage for low back pain: Impact on health care costs and service use. Spine (Phila Pa 1976). 2005;30:1075–1081.
- . Economic burden of prescription opioid misuse and abuse. J Manag Care Pharm. 2009;15:556–562.
© 2015 Society of Hospital Medicine
Novel Configuration of a Traditional RRT
Cardiopulmonary arrest (CPA) is a major cause of morbidity and mortality, both in the out‐of‐hospital environment as well as the inpatient setting.[1, 2] Unlike the out‐of‐hospital environment, inpatient CPA is unique in that healthcare providers are present during the prearrest period. In theory, this allows the opportunity to intervene and potentially prevent arrest. However, multiple investigators have demonstrated that the vast majority of inpatient CPA victims demonstrate abnormal vital signs prior to arrest without antecedent therapeutic intervention.[3, 4, 5]
Rapid response teams (RRTs) were created to institutionalize the response to at‐risk patients based on chief complaint or vital sign abnormalities. Early evaluation by a critical care team and initiation of appropriate therapy based on defined activation criteria should prevent deterioration in a substantial portion of patients at risk for CPA. Unfortunately, RRTs have not consistently demonstrated improved outcomes on the incidence of CPA and hospital mortality.[6, 7, 8, 9, 10, 11, 12, 13, 14] Although some investigators have reported a decrease in nonintensive care unit (ICU) arrests, it has been posited that this finding appears to be highly associated with either an increase in ICU arrests or more aggressive do not attempt resuscitation (DNAR) orders.[8]
Another potential explanation is an absence of training models that focus on the primary inpatient healthcare providers who are directly responsible for the afferent portion of an RRT program. Here we describe our experience with a novel RRT curriculum, in which unit managers (ie, charge nurse) play an essential role, and substantial education is directed toward primary responders such as bedside nurses and respiratory therapists. This approach is implemented through our novel resuscitation curriculum, which represents a comprehensive approach to inpatient resuscitation management built around critical links between continuous quality improvement (CQI) data, training, and special initiatives.
METHODS
Setting
This study was conducted in 2 urban university hospitals totaling approximately 500 medical/surgical beds starting fiscal year June 2005 through June 2011. Beds in the emergency department were not included. The primary medical center is comprised of 392 inpatient beds, whereas the sister campus consists of 119 inpatient beds. Waiver of informed consent was granted from our investigational review board. In 2007, our hospitals implemented the advanced resuscitation training (ART) program as an alternative to Advanced Cardiac Life Support and Basic Life Support. The ART program at the University of California San Diego consists of 5 key components: an institutional algorithm for arrest and nonarrest resuscitation, annual advanced resuscitation training for inpatient providers, the RRT as described below, an aggressive CQI program linked with training and inpatient special projects, and advanced defibrillators (Zoll E Series; Zoll Corp, Chelmsford, MA). By end of postimplementation year 1, all inpatient providers were required to have undergone training.
In November 2007, the RRT was initiated and is comprised of a dedicated critical care nurse and respiratory therapist. The third member of the team is the unit charge nurse who is not a dedicated primary responder but rather acts only if the response is activated in their specific unit. As part of our curriculum, it is the responsibility of the charge nurse on each inpatient unit to conduct rounds on at‐risk patients throughout each shift. Additionally, each inpatient provider undergoes several hours of RRT education on patient surveillance and the recognition of deterioration as part of annual training in our novel hospital‐wide resuscitation curriculum. The content of this training is frequently modified based on institutional CQI data. Instructors include critical care physicians and designated Code Blue/RRT nurses with extensive training and exposure to our novel curriculum. A conceptual model is used to present RRT activation criteria, with specific parameters provided as a guide (see Supporting Table 1 in the online version of this article). The Code Blue physician leader is available to the primary RRT responders based on their initial assessment. Emergency standing orders allow the RRT nurse to implement particular therapies under institutional protocols.
Data Collection
Data from all inpatient Code Blue and RRT activations are entered into an electronic CQI database by the responding nurse. Rapid response data include the etiology or chief complaint for the activation, relevant clinical findings, therapeutic interventions, disposition, and duration of the response. Additional clinical data, including a comprehensive process of classification and targeted CQI data collection, are provided by a dedicated resuscitation CQI team. Outcomes are obtained from the electronic patient care record and from hospital admissions so that all events can be normalized to patient discharge volume.
Data Analysis
To evaluate the effectiveness of the RRT, we compared the yearly incidence of non‐ICU CPA (per 1000 patient discharges) on all units starting fiscal year July 2005 through June 2011. Hospital discharge and mortality data were available after July 2006, whereas complete Code Blue activation data were available starting fiscal year July 2005. The incidence of ICU arrests was also determined to assess the RRT impact on the ICU and overall hospital mortality. The number and year‐over‐year change in RRT versus Code Blue activations for each individual inpatient unit starting November (quarter [Q] 3) 2007 through 20011 were compared using linear regression and described by Pearson correlation coefficient.[15] Patient acuity over the course of observation was monitored hospital wide through case mix index (CMI).[16, 17, 18] StatsDirect (StatsDirect Software Inc., Ashwell, UK) statistical software was used for all comparisons. P values <0.05 were considered statistically significant.
RESULTS
Starting preimplementation year 2006 through postimplementation years 2007 to 2011, the incidence of non‐ICU CPA decreased from 2.7 to 1.1 arrests per 1000 discharges (P<0.0001). The incidence of ICU CPA remained unchanged following program implementation (P=0.532) (Figure 1). Overall hospital mortality also decreased over the study period 2006 to 2011 (2.12%1.74%, P<0.001) (Figure 2). Overall hospital CMI for fiscal years 2005/2006 through 2011/2012 were significantly different (1.47 vs 1.67, P<0.0001). No difference was observed in the pre‐ and postimplementation period likelihood of DNAR status among nonsurvivors with initial return of spontaneous circulation at the time of CPA resuscitation (76% vs 75%, P=0.841).
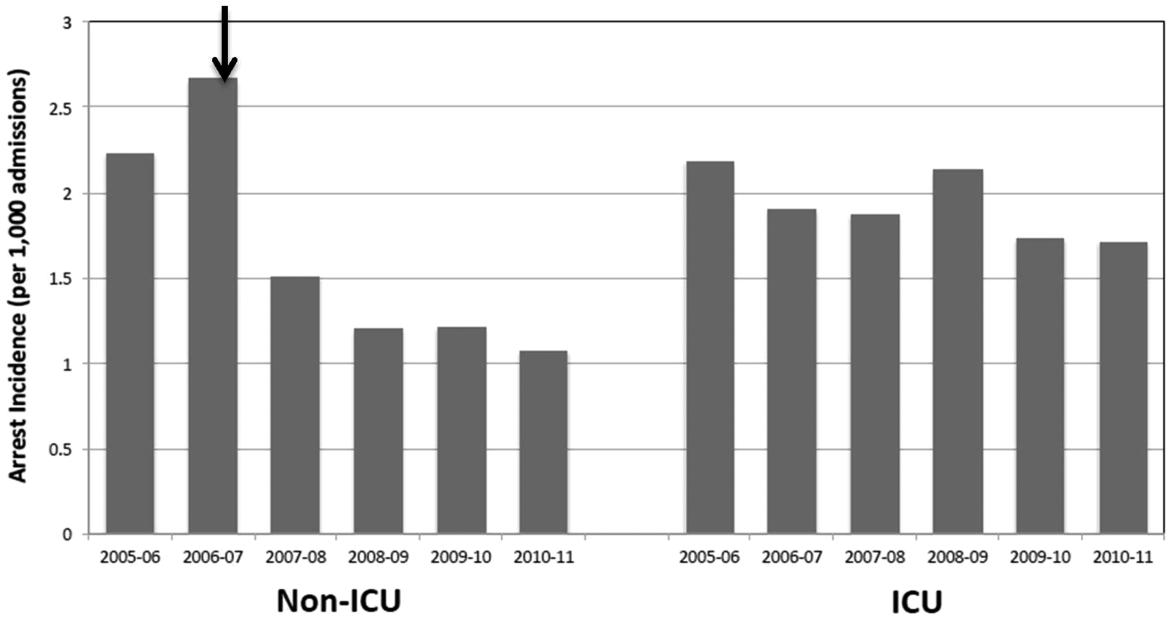

Starting fiscal year 2005to 2011, there were a total of 546 total CPAs with 247 non‐ICU CPAs observed between both hospital systems. Since its implementation starting at Q3 of 2007, a total of 1729 RRT activations throughout all inpatient areas were observed through 2011. The overall relationship between Code Blue activations starting July 2005 and the RRT since its implementation is displayed in Figure 3. No relationship was detected between the number of Code Blue and RRT activations on each unit (r=0.17, P=0.242). However, the year‐over‐year (fiscal years 2007/20082008/2009) change in RRT activations for each unit was inversely related to the change in Code Blue activations; the individual units with an increase in RRT activations experienced a decrease in Code Blue activations, and units with a decrease in RRT activations experienced an increase in Code Blue activations (r=0.68, P<0.001) (Figure 4).
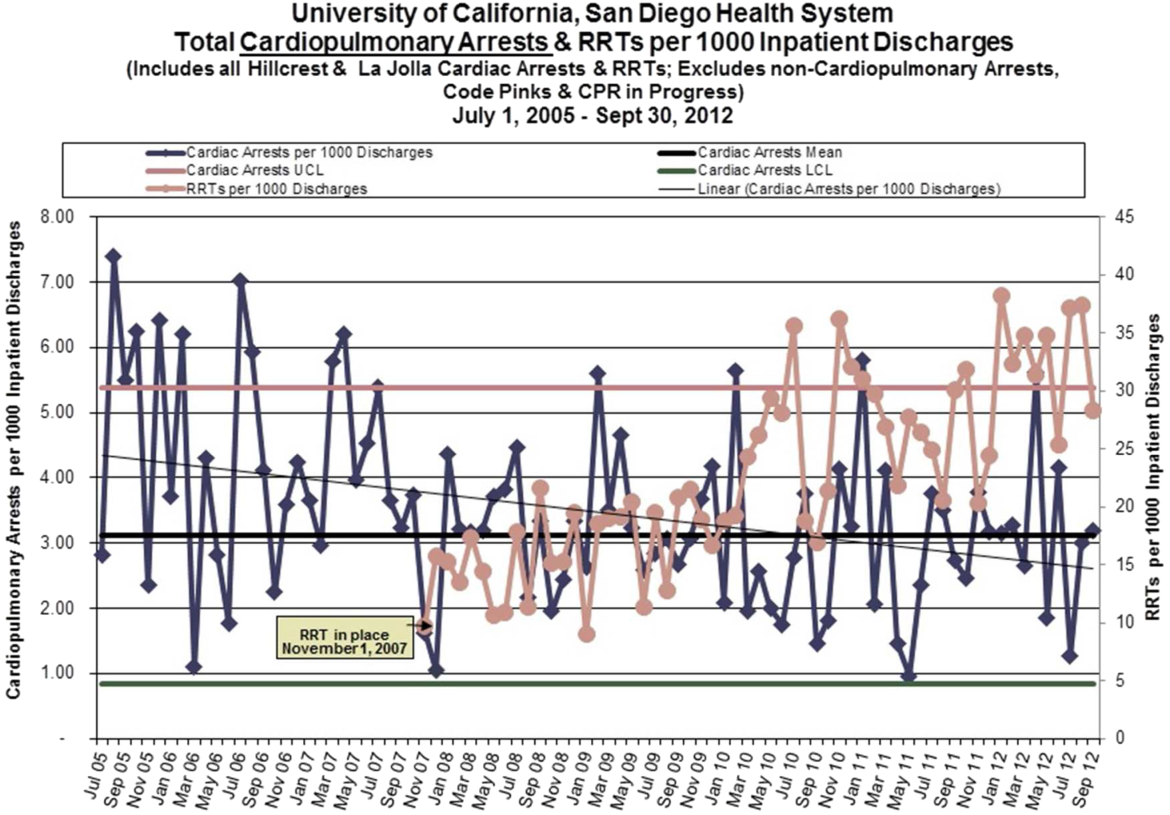
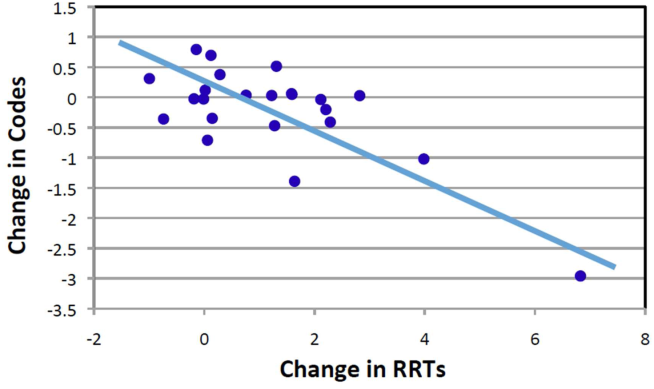
The time per RRT activation appeared to stabilize after the first program year, whereas the number of RRT activations per month has increased over time. The RRT activation etiologies based on chief complaint reported to the RRT nurse are displayed in Figure 5. Only 3% of activations resulted in no intervention; most of these represented seizures that resolved prior to RRT arrival or syncope episodes for noninpatients. The most common interventions were airway management (27%), fluid therapy (18%), and respiratory treatments (15%). The vast majority of patients (99%) survived the RRT response. A total of 56% of RRT activations resulted in disposition to a higher level of care (43% upgraded to ICU status, 13% upgraded to intermediate care unit status), whereas the remaining 44% of patients stayed on their original unit.
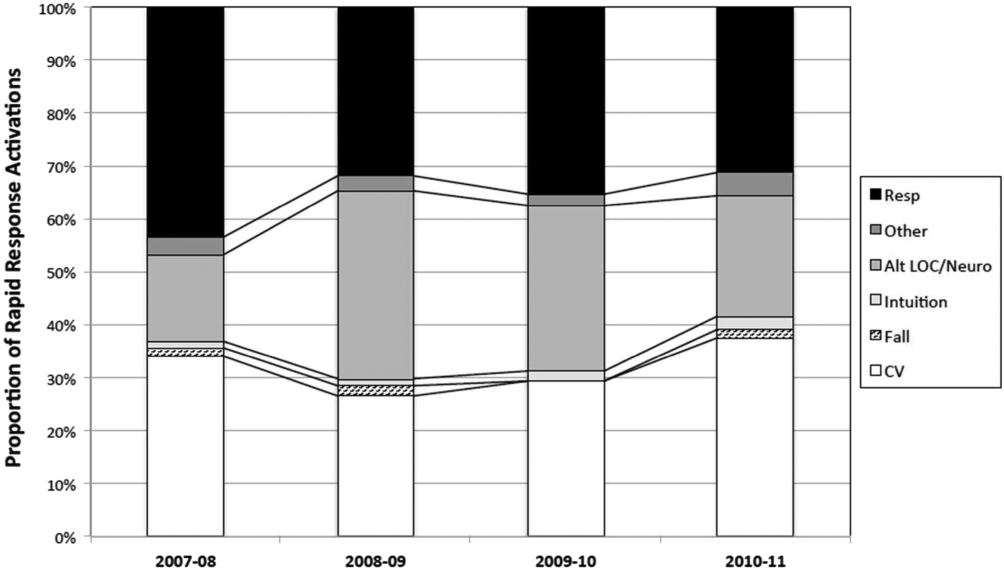
DISCUSSION
One of the primary rationales for hospital admission is the ability to observe and monitor patients to identify deterioration and prevent CPA. Thus, the failure of RRT programs to consistently demonstrate improvements in overall hospital mortality is somewhat perplexing. Here we present 4 years of data starting at the initiation of our RRT program. During our period of observation, we noted a significant inverse relationship between the activations of the RRT and incidence of CPA. Additionally, we noticed a significant decrease in non‐ICU arrests and in overall hospital mortality that appears to be associated with initiation of our novel RRT (Figures 1 and 2, respectively). In postintervention year 1, the unadjusted implementation of our RRT was associated with approximately 57% of the improvement of in‐hospital mortality. We noted a decrease of non‐ICU mortality from 45 deaths in fiscal year 2006 to 2007 to 21 deaths in fiscal year 2007 to 2008. Additionally, the number of non‐ICU Code Blue activations also declined during this period, from 56 to 34, with a survival to hospital discharge rate of 38% (fiscal year 2007 to 2008). In the second full year postimplementation, the activation of the RRT appears to be associated with a similar amount of reduction in in‐hospital mortality (52%) (see Supporting Table 2 in the online version of this article). We do not believe that initiation of our novel RRT accounts for all the variance in reduction of overall mortality. However, we posit that much of our success likely reflects our unique combined approach to a multifaceted life‐support training curriculum. Finally, though not statistically investigated, RRT activation etiology appears to be relatively uniform with respiratory, suspected cardiovascular, and clinical intuition accounting for a large majority of activations through the study period (Figure 5).
Multiple potential explanations exist to account for the lack of outcomes data to fully support RRT programs, with a failure to follow published RRT activation guidelines listed as a key factor.[7, 8, 19] It is unclear whether this reflects inconsistent activation protocols, as many hospitals lack specific published guidelines, whereas others may have more have rigid protocols. Another consideration is possible reluctance to activate a specialized team by primary in‐hospital caregivers due in part to intimidation or a lack of specific training. Interestingly, the routine presence of a physician with RRT activations has been postulated to be inhibitory in this regard and result in critical delays to resuscitative care.[20, 21, 22]
The vast majority of our RRT activations survived the initial response. This is an important metric not only for determining the competency of RRT providers in initiating therapies but also reflects the willingness of inpatient staff to activate RRT early in the course of a patient's deterioration, as delayed activation has been previously associated with increased mortality.[23, 24, 25] The relatively high RRT survival rate could be interpreted as reflecting some degree of overactivation. Of note, based upon hospital CMI, overall patient acuity appeared to continuously increase during and after the observation period. However, the incidence of RRT activations in which no therapies were initiated was extremely low, and more than half of patients were transferred to a higher level of care. We posit that benchmarking such metrics in the future may help institutions guide their resuscitation programs.
Our current RRT configuration of a dedicated critical care trained nurse and respiratory therapist plus unit manager (ie, charge nurse) is a departure from the traditional RRT historically consisting of a dedicated critical care trained nurse and/or respiratory therapist, and physician(s).[26] Perhaps more important than team configuration is our novel concept of individual unit managers regularly rounding on their own most at‐risk patients, which may add a layer of familiarity and increased likelihood of identifying even subtle changes associated with eventual decompensation. Unfortunately, we did not assess the charge nurse decision‐making process regarding RRT activation. This approach differs from Gerdik et al., who have demonstrated a positive association with the ability of the patient and/or family to activate the RRT. [27] Though similar, our approach also differs from the strategy of a dedicated RRT nurse rounding on high risk patients identified through physician and nurse surveys that have also shown a significant reduction in admission deaths.[26, 28, 29] Although the strategies may differ on the specific team members initiating the deployment of the RRT, what they appear to have in common is the proactive component of the identification of at‐risk patients. Additionally, we employ an annual RRT educational seminar for potential primary responders including bedside nurses, respiratory therapists, and physical therapists. Our novel resuscitation program and RRT education allows modulation of the life‐support curriculum to emphasize the importance of early recognition and response to at‐risk patients based upon our activation criteria and evaluation of activation trends (see Supporting Table 1 in the online version of this article). Finally, the presence of the charge nurse as part of the RRT is important, not only as part of the afferent arm of the program but also to enhance unit responsibility for detecting deterioration. It is our belief that an aggressive resuscitation CQI program with efferent links to unit managers amplifies the perception of ownership by primary providers and enhances the culture of resuscitation.
A lack of understanding as to the etiology of CPA in the inpatient environment may also limit the effectiveness of protocol and monitoring strategies. Our current resuscitation program places great emphasis on the taxonomy of our in‐hospital cardiac arrests and classifying inpatient events to help guide CQI efforts. These classifications provide a scaffolding for life‐support education and can result in changes to treatment algorithms or initiate new programs to target particular patient populations. An example includes the implementation of respiratory monitoring strategies in perioperative patients at high risk for obstructive sleep apnea.
Several limitations to this analysis must be considered. The study was not a randomized prospective trial and lacks internal validation. The before‐and‐after study was limited by the inclusion of only 1 complete preimplementation year (2006), which may have introduced a bias related to the inherent inability to properly evaluate secular trends. As such, this study cannot compare the relative effectiveness of our novel RRT participants and curriculum versus the traditional RRTs previously described.[26] Additionally, we also excluded data from both the emergency department and operating arena. Both are reported to have overall higher survival rates due to differences in arrest etiology, monitoring, anticipation, and available personal.[30, 31] We used CMI coefficients to explore the possibility of a decrease in patient acuity during the study. However, we noticed an increasing case‐mix coefficient value suggesting higher patient acuity, which would predict increased mortality rather than the decrease we observed.
One must also consider how the introduction of an RRT may increase DNAR orders and subsequently affect overall mortality by artificially lowering it.[32] Trends in DNAR during our period of observation were not significantly different. However, if an increase in DNAR orders did artificially improve non‐ICU CPA outcomes, one would expect unchanged or increased overall hospital mortality. In contrast, we found improvement in all outcomes including overall hospital mortality (Figure 2).
CONCLUSIONS
Our novel RRT program, with an emphasis on inclusion of non‐ICU charge nurses as part of the team and universal RRT education integrated within life support training, appears to be effective at decreasing the incidence of non‐ICU CPA and overall hospital mortality.
Disclosure: Nothing to report.
- , , , et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486.
- , , , et al. Out‐of‐hospital cardiac arrest surveillance—Cardiac Arrest Registry to Enhance Survival (CARES), United States, October 1, 2005—December 31, 2010. MMWR Surveill Summ. 2011;60:1–19.
- , , , et al. Antecedent bradycardia and in‐hospital cardiopulmonary arrest mortality in telemetry‐monitored patients outside the ICU. Resuscitation. 2012;83:1106–1110.
- , , , , . In‐hospital cardiac arrest: impact of monitoring and witnessed event on patient survival and neurologic status at hospital discharge. Resuscitation. 2011;82:845–852.
- , , , , . ST changes on continuous telemetry monitoring before in‐hospital cardiac arrests. Paper presented at: Resuscitation Science Symposium, Los Angeles, Ca. November 3–4, 2012.
- , , , , , . Rapid‐response systems as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158:417–425.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial. Lancet. 2005;365:2091–2097.
- , , , . Evaluation of a medical emergency team one year after implementation. Resuscitation. 2004;61:257–263.
- , , , , . Rapid response teams: a systematic review and meta‐analysis. Arch Intern Med. 2010;170:18–26.
- , , , , , . Hospital‐wide code rates and mortality before and after implementation of a rapid response team. JAMA. 2008;300:2506–2513.
- , , , , . Rapid response team implementation and in‐hospital mortality. Crit Care Med. 2014;42(9):2001–2006.
- , , , , , . Effect of a rapid response team on patient outcomes in a community‐based teaching hospital. J Grad Med Educ. 2014;6:61–64.
- , , , , . Reduction in hospital‐wide mortality after implementation of a rapid response team: a long‐term cohort study. Crit Care. 2011;15:R269.
- , , , , , . Rapid response systems: a systematic review. Crit Care Med. 2007;35:1238–1243.
- , , , , . Baseline hospital performance and the impact of medical emergency teams: modelling vs. conventional subgroup analysis. Trials. 2009;10:117.
- . The evolution of case‐mix measurement using DRGs: past, present and future. Stud Health Technol Inform. 1994;14:75–83.
- , , , et al. Variability in case‐mix adjusted in‐hospital cardiac arrest rates. Med Care. 2012;50:124–130.
- , , , , . Impact of socioeconomic adjustment on physicians' relative cost of care. Med Care. 2013;51:454–460.
- , , , , , . Reducing in‐hospital cardiac arrests and hospital mortality by introducing a medical emergency team. Intensive Care Med. 2010;36:100–106.
- , , , et al. Barriers to calling for urgent assistance despite a comprehensive pediatric rapid response system. Am J Crit Care. 2014;23:223–229.
- , , , et al. What stops hospital clinical staff from following protocols? An analysis of the incidence and factors behind the failure of bedside clinical staff to activate the rapid response system in a multi‐campus Australian metropolitan healthcare service. BMJ Qual Saf. 2012;21:569–575.
- , , , , , . Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ. 2002;324:387–390.
- , , , , . The relationship between early emergency team calls and serious adverse events. Crit Care Med. 2009;37:148–153.
- , , , , , . Characteristics and outcomes of patients receiving a medical emergency team review for acute change in conscious state or arrhythmias. Crit Care Med. 2008;36:477–481.
- , , , , , . Characteristics and outcomes of patients receiving a medical emergency team review for respiratory distress or hypotension. J Crit Care. 2008;23:325–331.
- , , , et al. Proactive rounding by the rapid response team reduces inpatient cardiac arrests. Resuscitation. 2013;84:1668–1673.
- , , , , , . Successful implementation of a family and patient activated rapid response team in an adult level 1 trauma center. Resuscitation. 2014;81:1676–1681.
- , , . Effect of the critical care outreach team on patient survival to discharge from hospital and readmission to critical care: non‐randomised population based study. BMJ. 2003;327:1014.
- , , , , , . Beyond rapid response teams: instituting a “rover team” improves the management of at‐risk patients, facilitates proactive interventions, and improves outcomes. In: Henriksen K, Battles JB, Keyes MA, Grady ML, eds. Advances in Patient Safety: New Directions and Alternative Approaches. Vol. 3. Performance and Tools. Rockville, MD: Agency for Healthcare Research and Quality; 2008:1–12.
- , , . Cardiac arrest in the emergency department: a report from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2008;78:151–160.
- , , , , . Delayed time to defibrillation after intraoperative and periprocedural cardiac arrest. Anesthesiology. 2010;113:782–793.
- , , , , . The Medical Emergency Team System and not‐for‐resuscitation orders: results from the MERIT study. Resuscitation. 2008;79:391–397.
Cardiopulmonary arrest (CPA) is a major cause of morbidity and mortality, both in the out‐of‐hospital environment as well as the inpatient setting.[1, 2] Unlike the out‐of‐hospital environment, inpatient CPA is unique in that healthcare providers are present during the prearrest period. In theory, this allows the opportunity to intervene and potentially prevent arrest. However, multiple investigators have demonstrated that the vast majority of inpatient CPA victims demonstrate abnormal vital signs prior to arrest without antecedent therapeutic intervention.[3, 4, 5]
Rapid response teams (RRTs) were created to institutionalize the response to at‐risk patients based on chief complaint or vital sign abnormalities. Early evaluation by a critical care team and initiation of appropriate therapy based on defined activation criteria should prevent deterioration in a substantial portion of patients at risk for CPA. Unfortunately, RRTs have not consistently demonstrated improved outcomes on the incidence of CPA and hospital mortality.[6, 7, 8, 9, 10, 11, 12, 13, 14] Although some investigators have reported a decrease in nonintensive care unit (ICU) arrests, it has been posited that this finding appears to be highly associated with either an increase in ICU arrests or more aggressive do not attempt resuscitation (DNAR) orders.[8]
Another potential explanation is an absence of training models that focus on the primary inpatient healthcare providers who are directly responsible for the afferent portion of an RRT program. Here we describe our experience with a novel RRT curriculum, in which unit managers (ie, charge nurse) play an essential role, and substantial education is directed toward primary responders such as bedside nurses and respiratory therapists. This approach is implemented through our novel resuscitation curriculum, which represents a comprehensive approach to inpatient resuscitation management built around critical links between continuous quality improvement (CQI) data, training, and special initiatives.
METHODS
Setting
This study was conducted in 2 urban university hospitals totaling approximately 500 medical/surgical beds starting fiscal year June 2005 through June 2011. Beds in the emergency department were not included. The primary medical center is comprised of 392 inpatient beds, whereas the sister campus consists of 119 inpatient beds. Waiver of informed consent was granted from our investigational review board. In 2007, our hospitals implemented the advanced resuscitation training (ART) program as an alternative to Advanced Cardiac Life Support and Basic Life Support. The ART program at the University of California San Diego consists of 5 key components: an institutional algorithm for arrest and nonarrest resuscitation, annual advanced resuscitation training for inpatient providers, the RRT as described below, an aggressive CQI program linked with training and inpatient special projects, and advanced defibrillators (Zoll E Series; Zoll Corp, Chelmsford, MA). By end of postimplementation year 1, all inpatient providers were required to have undergone training.
In November 2007, the RRT was initiated and is comprised of a dedicated critical care nurse and respiratory therapist. The third member of the team is the unit charge nurse who is not a dedicated primary responder but rather acts only if the response is activated in their specific unit. As part of our curriculum, it is the responsibility of the charge nurse on each inpatient unit to conduct rounds on at‐risk patients throughout each shift. Additionally, each inpatient provider undergoes several hours of RRT education on patient surveillance and the recognition of deterioration as part of annual training in our novel hospital‐wide resuscitation curriculum. The content of this training is frequently modified based on institutional CQI data. Instructors include critical care physicians and designated Code Blue/RRT nurses with extensive training and exposure to our novel curriculum. A conceptual model is used to present RRT activation criteria, with specific parameters provided as a guide (see Supporting Table 1 in the online version of this article). The Code Blue physician leader is available to the primary RRT responders based on their initial assessment. Emergency standing orders allow the RRT nurse to implement particular therapies under institutional protocols.
Data Collection
Data from all inpatient Code Blue and RRT activations are entered into an electronic CQI database by the responding nurse. Rapid response data include the etiology or chief complaint for the activation, relevant clinical findings, therapeutic interventions, disposition, and duration of the response. Additional clinical data, including a comprehensive process of classification and targeted CQI data collection, are provided by a dedicated resuscitation CQI team. Outcomes are obtained from the electronic patient care record and from hospital admissions so that all events can be normalized to patient discharge volume.
Data Analysis
To evaluate the effectiveness of the RRT, we compared the yearly incidence of non‐ICU CPA (per 1000 patient discharges) on all units starting fiscal year July 2005 through June 2011. Hospital discharge and mortality data were available after July 2006, whereas complete Code Blue activation data were available starting fiscal year July 2005. The incidence of ICU arrests was also determined to assess the RRT impact on the ICU and overall hospital mortality. The number and year‐over‐year change in RRT versus Code Blue activations for each individual inpatient unit starting November (quarter [Q] 3) 2007 through 20011 were compared using linear regression and described by Pearson correlation coefficient.[15] Patient acuity over the course of observation was monitored hospital wide through case mix index (CMI).[16, 17, 18] StatsDirect (StatsDirect Software Inc., Ashwell, UK) statistical software was used for all comparisons. P values <0.05 were considered statistically significant.
RESULTS
Starting preimplementation year 2006 through postimplementation years 2007 to 2011, the incidence of non‐ICU CPA decreased from 2.7 to 1.1 arrests per 1000 discharges (P<0.0001). The incidence of ICU CPA remained unchanged following program implementation (P=0.532) (Figure 1). Overall hospital mortality also decreased over the study period 2006 to 2011 (2.12%1.74%, P<0.001) (Figure 2). Overall hospital CMI for fiscal years 2005/2006 through 2011/2012 were significantly different (1.47 vs 1.67, P<0.0001). No difference was observed in the pre‐ and postimplementation period likelihood of DNAR status among nonsurvivors with initial return of spontaneous circulation at the time of CPA resuscitation (76% vs 75%, P=0.841).


Starting fiscal year 2005to 2011, there were a total of 546 total CPAs with 247 non‐ICU CPAs observed between both hospital systems. Since its implementation starting at Q3 of 2007, a total of 1729 RRT activations throughout all inpatient areas were observed through 2011. The overall relationship between Code Blue activations starting July 2005 and the RRT since its implementation is displayed in Figure 3. No relationship was detected between the number of Code Blue and RRT activations on each unit (r=0.17, P=0.242). However, the year‐over‐year (fiscal years 2007/20082008/2009) change in RRT activations for each unit was inversely related to the change in Code Blue activations; the individual units with an increase in RRT activations experienced a decrease in Code Blue activations, and units with a decrease in RRT activations experienced an increase in Code Blue activations (r=0.68, P<0.001) (Figure 4).


The time per RRT activation appeared to stabilize after the first program year, whereas the number of RRT activations per month has increased over time. The RRT activation etiologies based on chief complaint reported to the RRT nurse are displayed in Figure 5. Only 3% of activations resulted in no intervention; most of these represented seizures that resolved prior to RRT arrival or syncope episodes for noninpatients. The most common interventions were airway management (27%), fluid therapy (18%), and respiratory treatments (15%). The vast majority of patients (99%) survived the RRT response. A total of 56% of RRT activations resulted in disposition to a higher level of care (43% upgraded to ICU status, 13% upgraded to intermediate care unit status), whereas the remaining 44% of patients stayed on their original unit.

DISCUSSION
One of the primary rationales for hospital admission is the ability to observe and monitor patients to identify deterioration and prevent CPA. Thus, the failure of RRT programs to consistently demonstrate improvements in overall hospital mortality is somewhat perplexing. Here we present 4 years of data starting at the initiation of our RRT program. During our period of observation, we noted a significant inverse relationship between the activations of the RRT and incidence of CPA. Additionally, we noticed a significant decrease in non‐ICU arrests and in overall hospital mortality that appears to be associated with initiation of our novel RRT (Figures 1 and 2, respectively). In postintervention year 1, the unadjusted implementation of our RRT was associated with approximately 57% of the improvement of in‐hospital mortality. We noted a decrease of non‐ICU mortality from 45 deaths in fiscal year 2006 to 2007 to 21 deaths in fiscal year 2007 to 2008. Additionally, the number of non‐ICU Code Blue activations also declined during this period, from 56 to 34, with a survival to hospital discharge rate of 38% (fiscal year 2007 to 2008). In the second full year postimplementation, the activation of the RRT appears to be associated with a similar amount of reduction in in‐hospital mortality (52%) (see Supporting Table 2 in the online version of this article). We do not believe that initiation of our novel RRT accounts for all the variance in reduction of overall mortality. However, we posit that much of our success likely reflects our unique combined approach to a multifaceted life‐support training curriculum. Finally, though not statistically investigated, RRT activation etiology appears to be relatively uniform with respiratory, suspected cardiovascular, and clinical intuition accounting for a large majority of activations through the study period (Figure 5).
Multiple potential explanations exist to account for the lack of outcomes data to fully support RRT programs, with a failure to follow published RRT activation guidelines listed as a key factor.[7, 8, 19] It is unclear whether this reflects inconsistent activation protocols, as many hospitals lack specific published guidelines, whereas others may have more have rigid protocols. Another consideration is possible reluctance to activate a specialized team by primary in‐hospital caregivers due in part to intimidation or a lack of specific training. Interestingly, the routine presence of a physician with RRT activations has been postulated to be inhibitory in this regard and result in critical delays to resuscitative care.[20, 21, 22]
The vast majority of our RRT activations survived the initial response. This is an important metric not only for determining the competency of RRT providers in initiating therapies but also reflects the willingness of inpatient staff to activate RRT early in the course of a patient's deterioration, as delayed activation has been previously associated with increased mortality.[23, 24, 25] The relatively high RRT survival rate could be interpreted as reflecting some degree of overactivation. Of note, based upon hospital CMI, overall patient acuity appeared to continuously increase during and after the observation period. However, the incidence of RRT activations in which no therapies were initiated was extremely low, and more than half of patients were transferred to a higher level of care. We posit that benchmarking such metrics in the future may help institutions guide their resuscitation programs.
Our current RRT configuration of a dedicated critical care trained nurse and respiratory therapist plus unit manager (ie, charge nurse) is a departure from the traditional RRT historically consisting of a dedicated critical care trained nurse and/or respiratory therapist, and physician(s).[26] Perhaps more important than team configuration is our novel concept of individual unit managers regularly rounding on their own most at‐risk patients, which may add a layer of familiarity and increased likelihood of identifying even subtle changes associated with eventual decompensation. Unfortunately, we did not assess the charge nurse decision‐making process regarding RRT activation. This approach differs from Gerdik et al., who have demonstrated a positive association with the ability of the patient and/or family to activate the RRT. [27] Though similar, our approach also differs from the strategy of a dedicated RRT nurse rounding on high risk patients identified through physician and nurse surveys that have also shown a significant reduction in admission deaths.[26, 28, 29] Although the strategies may differ on the specific team members initiating the deployment of the RRT, what they appear to have in common is the proactive component of the identification of at‐risk patients. Additionally, we employ an annual RRT educational seminar for potential primary responders including bedside nurses, respiratory therapists, and physical therapists. Our novel resuscitation program and RRT education allows modulation of the life‐support curriculum to emphasize the importance of early recognition and response to at‐risk patients based upon our activation criteria and evaluation of activation trends (see Supporting Table 1 in the online version of this article). Finally, the presence of the charge nurse as part of the RRT is important, not only as part of the afferent arm of the program but also to enhance unit responsibility for detecting deterioration. It is our belief that an aggressive resuscitation CQI program with efferent links to unit managers amplifies the perception of ownership by primary providers and enhances the culture of resuscitation.
A lack of understanding as to the etiology of CPA in the inpatient environment may also limit the effectiveness of protocol and monitoring strategies. Our current resuscitation program places great emphasis on the taxonomy of our in‐hospital cardiac arrests and classifying inpatient events to help guide CQI efforts. These classifications provide a scaffolding for life‐support education and can result in changes to treatment algorithms or initiate new programs to target particular patient populations. An example includes the implementation of respiratory monitoring strategies in perioperative patients at high risk for obstructive sleep apnea.
Several limitations to this analysis must be considered. The study was not a randomized prospective trial and lacks internal validation. The before‐and‐after study was limited by the inclusion of only 1 complete preimplementation year (2006), which may have introduced a bias related to the inherent inability to properly evaluate secular trends. As such, this study cannot compare the relative effectiveness of our novel RRT participants and curriculum versus the traditional RRTs previously described.[26] Additionally, we also excluded data from both the emergency department and operating arena. Both are reported to have overall higher survival rates due to differences in arrest etiology, monitoring, anticipation, and available personal.[30, 31] We used CMI coefficients to explore the possibility of a decrease in patient acuity during the study. However, we noticed an increasing case‐mix coefficient value suggesting higher patient acuity, which would predict increased mortality rather than the decrease we observed.
One must also consider how the introduction of an RRT may increase DNAR orders and subsequently affect overall mortality by artificially lowering it.[32] Trends in DNAR during our period of observation were not significantly different. However, if an increase in DNAR orders did artificially improve non‐ICU CPA outcomes, one would expect unchanged or increased overall hospital mortality. In contrast, we found improvement in all outcomes including overall hospital mortality (Figure 2).
CONCLUSIONS
Our novel RRT program, with an emphasis on inclusion of non‐ICU charge nurses as part of the team and universal RRT education integrated within life support training, appears to be effective at decreasing the incidence of non‐ICU CPA and overall hospital mortality.
Disclosure: Nothing to report.
Cardiopulmonary arrest (CPA) is a major cause of morbidity and mortality, both in the out‐of‐hospital environment as well as the inpatient setting.[1, 2] Unlike the out‐of‐hospital environment, inpatient CPA is unique in that healthcare providers are present during the prearrest period. In theory, this allows the opportunity to intervene and potentially prevent arrest. However, multiple investigators have demonstrated that the vast majority of inpatient CPA victims demonstrate abnormal vital signs prior to arrest without antecedent therapeutic intervention.[3, 4, 5]
Rapid response teams (RRTs) were created to institutionalize the response to at‐risk patients based on chief complaint or vital sign abnormalities. Early evaluation by a critical care team and initiation of appropriate therapy based on defined activation criteria should prevent deterioration in a substantial portion of patients at risk for CPA. Unfortunately, RRTs have not consistently demonstrated improved outcomes on the incidence of CPA and hospital mortality.[6, 7, 8, 9, 10, 11, 12, 13, 14] Although some investigators have reported a decrease in nonintensive care unit (ICU) arrests, it has been posited that this finding appears to be highly associated with either an increase in ICU arrests or more aggressive do not attempt resuscitation (DNAR) orders.[8]
Another potential explanation is an absence of training models that focus on the primary inpatient healthcare providers who are directly responsible for the afferent portion of an RRT program. Here we describe our experience with a novel RRT curriculum, in which unit managers (ie, charge nurse) play an essential role, and substantial education is directed toward primary responders such as bedside nurses and respiratory therapists. This approach is implemented through our novel resuscitation curriculum, which represents a comprehensive approach to inpatient resuscitation management built around critical links between continuous quality improvement (CQI) data, training, and special initiatives.
METHODS
Setting
This study was conducted in 2 urban university hospitals totaling approximately 500 medical/surgical beds starting fiscal year June 2005 through June 2011. Beds in the emergency department were not included. The primary medical center is comprised of 392 inpatient beds, whereas the sister campus consists of 119 inpatient beds. Waiver of informed consent was granted from our investigational review board. In 2007, our hospitals implemented the advanced resuscitation training (ART) program as an alternative to Advanced Cardiac Life Support and Basic Life Support. The ART program at the University of California San Diego consists of 5 key components: an institutional algorithm for arrest and nonarrest resuscitation, annual advanced resuscitation training for inpatient providers, the RRT as described below, an aggressive CQI program linked with training and inpatient special projects, and advanced defibrillators (Zoll E Series; Zoll Corp, Chelmsford, MA). By end of postimplementation year 1, all inpatient providers were required to have undergone training.
In November 2007, the RRT was initiated and is comprised of a dedicated critical care nurse and respiratory therapist. The third member of the team is the unit charge nurse who is not a dedicated primary responder but rather acts only if the response is activated in their specific unit. As part of our curriculum, it is the responsibility of the charge nurse on each inpatient unit to conduct rounds on at‐risk patients throughout each shift. Additionally, each inpatient provider undergoes several hours of RRT education on patient surveillance and the recognition of deterioration as part of annual training in our novel hospital‐wide resuscitation curriculum. The content of this training is frequently modified based on institutional CQI data. Instructors include critical care physicians and designated Code Blue/RRT nurses with extensive training and exposure to our novel curriculum. A conceptual model is used to present RRT activation criteria, with specific parameters provided as a guide (see Supporting Table 1 in the online version of this article). The Code Blue physician leader is available to the primary RRT responders based on their initial assessment. Emergency standing orders allow the RRT nurse to implement particular therapies under institutional protocols.
Data Collection
Data from all inpatient Code Blue and RRT activations are entered into an electronic CQI database by the responding nurse. Rapid response data include the etiology or chief complaint for the activation, relevant clinical findings, therapeutic interventions, disposition, and duration of the response. Additional clinical data, including a comprehensive process of classification and targeted CQI data collection, are provided by a dedicated resuscitation CQI team. Outcomes are obtained from the electronic patient care record and from hospital admissions so that all events can be normalized to patient discharge volume.
Data Analysis
To evaluate the effectiveness of the RRT, we compared the yearly incidence of non‐ICU CPA (per 1000 patient discharges) on all units starting fiscal year July 2005 through June 2011. Hospital discharge and mortality data were available after July 2006, whereas complete Code Blue activation data were available starting fiscal year July 2005. The incidence of ICU arrests was also determined to assess the RRT impact on the ICU and overall hospital mortality. The number and year‐over‐year change in RRT versus Code Blue activations for each individual inpatient unit starting November (quarter [Q] 3) 2007 through 20011 were compared using linear regression and described by Pearson correlation coefficient.[15] Patient acuity over the course of observation was monitored hospital wide through case mix index (CMI).[16, 17, 18] StatsDirect (StatsDirect Software Inc., Ashwell, UK) statistical software was used for all comparisons. P values <0.05 were considered statistically significant.
RESULTS
Starting preimplementation year 2006 through postimplementation years 2007 to 2011, the incidence of non‐ICU CPA decreased from 2.7 to 1.1 arrests per 1000 discharges (P<0.0001). The incidence of ICU CPA remained unchanged following program implementation (P=0.532) (Figure 1). Overall hospital mortality also decreased over the study period 2006 to 2011 (2.12%1.74%, P<0.001) (Figure 2). Overall hospital CMI for fiscal years 2005/2006 through 2011/2012 were significantly different (1.47 vs 1.67, P<0.0001). No difference was observed in the pre‐ and postimplementation period likelihood of DNAR status among nonsurvivors with initial return of spontaneous circulation at the time of CPA resuscitation (76% vs 75%, P=0.841).


Starting fiscal year 2005to 2011, there were a total of 546 total CPAs with 247 non‐ICU CPAs observed between both hospital systems. Since its implementation starting at Q3 of 2007, a total of 1729 RRT activations throughout all inpatient areas were observed through 2011. The overall relationship between Code Blue activations starting July 2005 and the RRT since its implementation is displayed in Figure 3. No relationship was detected between the number of Code Blue and RRT activations on each unit (r=0.17, P=0.242). However, the year‐over‐year (fiscal years 2007/20082008/2009) change in RRT activations for each unit was inversely related to the change in Code Blue activations; the individual units with an increase in RRT activations experienced a decrease in Code Blue activations, and units with a decrease in RRT activations experienced an increase in Code Blue activations (r=0.68, P<0.001) (Figure 4).


The time per RRT activation appeared to stabilize after the first program year, whereas the number of RRT activations per month has increased over time. The RRT activation etiologies based on chief complaint reported to the RRT nurse are displayed in Figure 5. Only 3% of activations resulted in no intervention; most of these represented seizures that resolved prior to RRT arrival or syncope episodes for noninpatients. The most common interventions were airway management (27%), fluid therapy (18%), and respiratory treatments (15%). The vast majority of patients (99%) survived the RRT response. A total of 56% of RRT activations resulted in disposition to a higher level of care (43% upgraded to ICU status, 13% upgraded to intermediate care unit status), whereas the remaining 44% of patients stayed on their original unit.

DISCUSSION
One of the primary rationales for hospital admission is the ability to observe and monitor patients to identify deterioration and prevent CPA. Thus, the failure of RRT programs to consistently demonstrate improvements in overall hospital mortality is somewhat perplexing. Here we present 4 years of data starting at the initiation of our RRT program. During our period of observation, we noted a significant inverse relationship between the activations of the RRT and incidence of CPA. Additionally, we noticed a significant decrease in non‐ICU arrests and in overall hospital mortality that appears to be associated with initiation of our novel RRT (Figures 1 and 2, respectively). In postintervention year 1, the unadjusted implementation of our RRT was associated with approximately 57% of the improvement of in‐hospital mortality. We noted a decrease of non‐ICU mortality from 45 deaths in fiscal year 2006 to 2007 to 21 deaths in fiscal year 2007 to 2008. Additionally, the number of non‐ICU Code Blue activations also declined during this period, from 56 to 34, with a survival to hospital discharge rate of 38% (fiscal year 2007 to 2008). In the second full year postimplementation, the activation of the RRT appears to be associated with a similar amount of reduction in in‐hospital mortality (52%) (see Supporting Table 2 in the online version of this article). We do not believe that initiation of our novel RRT accounts for all the variance in reduction of overall mortality. However, we posit that much of our success likely reflects our unique combined approach to a multifaceted life‐support training curriculum. Finally, though not statistically investigated, RRT activation etiology appears to be relatively uniform with respiratory, suspected cardiovascular, and clinical intuition accounting for a large majority of activations through the study period (Figure 5).
Multiple potential explanations exist to account for the lack of outcomes data to fully support RRT programs, with a failure to follow published RRT activation guidelines listed as a key factor.[7, 8, 19] It is unclear whether this reflects inconsistent activation protocols, as many hospitals lack specific published guidelines, whereas others may have more have rigid protocols. Another consideration is possible reluctance to activate a specialized team by primary in‐hospital caregivers due in part to intimidation or a lack of specific training. Interestingly, the routine presence of a physician with RRT activations has been postulated to be inhibitory in this regard and result in critical delays to resuscitative care.[20, 21, 22]
The vast majority of our RRT activations survived the initial response. This is an important metric not only for determining the competency of RRT providers in initiating therapies but also reflects the willingness of inpatient staff to activate RRT early in the course of a patient's deterioration, as delayed activation has been previously associated with increased mortality.[23, 24, 25] The relatively high RRT survival rate could be interpreted as reflecting some degree of overactivation. Of note, based upon hospital CMI, overall patient acuity appeared to continuously increase during and after the observation period. However, the incidence of RRT activations in which no therapies were initiated was extremely low, and more than half of patients were transferred to a higher level of care. We posit that benchmarking such metrics in the future may help institutions guide their resuscitation programs.
Our current RRT configuration of a dedicated critical care trained nurse and respiratory therapist plus unit manager (ie, charge nurse) is a departure from the traditional RRT historically consisting of a dedicated critical care trained nurse and/or respiratory therapist, and physician(s).[26] Perhaps more important than team configuration is our novel concept of individual unit managers regularly rounding on their own most at‐risk patients, which may add a layer of familiarity and increased likelihood of identifying even subtle changes associated with eventual decompensation. Unfortunately, we did not assess the charge nurse decision‐making process regarding RRT activation. This approach differs from Gerdik et al., who have demonstrated a positive association with the ability of the patient and/or family to activate the RRT. [27] Though similar, our approach also differs from the strategy of a dedicated RRT nurse rounding on high risk patients identified through physician and nurse surveys that have also shown a significant reduction in admission deaths.[26, 28, 29] Although the strategies may differ on the specific team members initiating the deployment of the RRT, what they appear to have in common is the proactive component of the identification of at‐risk patients. Additionally, we employ an annual RRT educational seminar for potential primary responders including bedside nurses, respiratory therapists, and physical therapists. Our novel resuscitation program and RRT education allows modulation of the life‐support curriculum to emphasize the importance of early recognition and response to at‐risk patients based upon our activation criteria and evaluation of activation trends (see Supporting Table 1 in the online version of this article). Finally, the presence of the charge nurse as part of the RRT is important, not only as part of the afferent arm of the program but also to enhance unit responsibility for detecting deterioration. It is our belief that an aggressive resuscitation CQI program with efferent links to unit managers amplifies the perception of ownership by primary providers and enhances the culture of resuscitation.
A lack of understanding as to the etiology of CPA in the inpatient environment may also limit the effectiveness of protocol and monitoring strategies. Our current resuscitation program places great emphasis on the taxonomy of our in‐hospital cardiac arrests and classifying inpatient events to help guide CQI efforts. These classifications provide a scaffolding for life‐support education and can result in changes to treatment algorithms or initiate new programs to target particular patient populations. An example includes the implementation of respiratory monitoring strategies in perioperative patients at high risk for obstructive sleep apnea.
Several limitations to this analysis must be considered. The study was not a randomized prospective trial and lacks internal validation. The before‐and‐after study was limited by the inclusion of only 1 complete preimplementation year (2006), which may have introduced a bias related to the inherent inability to properly evaluate secular trends. As such, this study cannot compare the relative effectiveness of our novel RRT participants and curriculum versus the traditional RRTs previously described.[26] Additionally, we also excluded data from both the emergency department and operating arena. Both are reported to have overall higher survival rates due to differences in arrest etiology, monitoring, anticipation, and available personal.[30, 31] We used CMI coefficients to explore the possibility of a decrease in patient acuity during the study. However, we noticed an increasing case‐mix coefficient value suggesting higher patient acuity, which would predict increased mortality rather than the decrease we observed.
One must also consider how the introduction of an RRT may increase DNAR orders and subsequently affect overall mortality by artificially lowering it.[32] Trends in DNAR during our period of observation were not significantly different. However, if an increase in DNAR orders did artificially improve non‐ICU CPA outcomes, one would expect unchanged or increased overall hospital mortality. In contrast, we found improvement in all outcomes including overall hospital mortality (Figure 2).
CONCLUSIONS
Our novel RRT program, with an emphasis on inclusion of non‐ICU charge nurses as part of the team and universal RRT education integrated within life support training, appears to be effective at decreasing the incidence of non‐ICU CPA and overall hospital mortality.
Disclosure: Nothing to report.
- , , , et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486.
- , , , et al. Out‐of‐hospital cardiac arrest surveillance—Cardiac Arrest Registry to Enhance Survival (CARES), United States, October 1, 2005—December 31, 2010. MMWR Surveill Summ. 2011;60:1–19.
- , , , et al. Antecedent bradycardia and in‐hospital cardiopulmonary arrest mortality in telemetry‐monitored patients outside the ICU. Resuscitation. 2012;83:1106–1110.
- , , , , . In‐hospital cardiac arrest: impact of monitoring and witnessed event on patient survival and neurologic status at hospital discharge. Resuscitation. 2011;82:845–852.
- , , , , . ST changes on continuous telemetry monitoring before in‐hospital cardiac arrests. Paper presented at: Resuscitation Science Symposium, Los Angeles, Ca. November 3–4, 2012.
- , , , , , . Rapid‐response systems as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158:417–425.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial. Lancet. 2005;365:2091–2097.
- , , , . Evaluation of a medical emergency team one year after implementation. Resuscitation. 2004;61:257–263.
- , , , , . Rapid response teams: a systematic review and meta‐analysis. Arch Intern Med. 2010;170:18–26.
- , , , , , . Hospital‐wide code rates and mortality before and after implementation of a rapid response team. JAMA. 2008;300:2506–2513.
- , , , , . Rapid response team implementation and in‐hospital mortality. Crit Care Med. 2014;42(9):2001–2006.
- , , , , , . Effect of a rapid response team on patient outcomes in a community‐based teaching hospital. J Grad Med Educ. 2014;6:61–64.
- , , , , . Reduction in hospital‐wide mortality after implementation of a rapid response team: a long‐term cohort study. Crit Care. 2011;15:R269.
- , , , , , . Rapid response systems: a systematic review. Crit Care Med. 2007;35:1238–1243.
- , , , , . Baseline hospital performance and the impact of medical emergency teams: modelling vs. conventional subgroup analysis. Trials. 2009;10:117.
- . The evolution of case‐mix measurement using DRGs: past, present and future. Stud Health Technol Inform. 1994;14:75–83.
- , , , et al. Variability in case‐mix adjusted in‐hospital cardiac arrest rates. Med Care. 2012;50:124–130.
- , , , , . Impact of socioeconomic adjustment on physicians' relative cost of care. Med Care. 2013;51:454–460.
- , , , , , . Reducing in‐hospital cardiac arrests and hospital mortality by introducing a medical emergency team. Intensive Care Med. 2010;36:100–106.
- , , , et al. Barriers to calling for urgent assistance despite a comprehensive pediatric rapid response system. Am J Crit Care. 2014;23:223–229.
- , , , et al. What stops hospital clinical staff from following protocols? An analysis of the incidence and factors behind the failure of bedside clinical staff to activate the rapid response system in a multi‐campus Australian metropolitan healthcare service. BMJ Qual Saf. 2012;21:569–575.
- , , , , , . Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ. 2002;324:387–390.
- , , , , . The relationship between early emergency team calls and serious adverse events. Crit Care Med. 2009;37:148–153.
- , , , , , . Characteristics and outcomes of patients receiving a medical emergency team review for acute change in conscious state or arrhythmias. Crit Care Med. 2008;36:477–481.
- , , , , , . Characteristics and outcomes of patients receiving a medical emergency team review for respiratory distress or hypotension. J Crit Care. 2008;23:325–331.
- , , , et al. Proactive rounding by the rapid response team reduces inpatient cardiac arrests. Resuscitation. 2013;84:1668–1673.
- , , , , , . Successful implementation of a family and patient activated rapid response team in an adult level 1 trauma center. Resuscitation. 2014;81:1676–1681.
- , , . Effect of the critical care outreach team on patient survival to discharge from hospital and readmission to critical care: non‐randomised population based study. BMJ. 2003;327:1014.
- , , , , , . Beyond rapid response teams: instituting a “rover team” improves the management of at‐risk patients, facilitates proactive interventions, and improves outcomes. In: Henriksen K, Battles JB, Keyes MA, Grady ML, eds. Advances in Patient Safety: New Directions and Alternative Approaches. Vol. 3. Performance and Tools. Rockville, MD: Agency for Healthcare Research and Quality; 2008:1–12.
- , , . Cardiac arrest in the emergency department: a report from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2008;78:151–160.
- , , , , . Delayed time to defibrillation after intraoperative and periprocedural cardiac arrest. Anesthesiology. 2010;113:782–793.
- , , , , . The Medical Emergency Team System and not‐for‐resuscitation orders: results from the MERIT study. Resuscitation. 2008;79:391–397.
- , , , et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486.
- , , , et al. Out‐of‐hospital cardiac arrest surveillance—Cardiac Arrest Registry to Enhance Survival (CARES), United States, October 1, 2005—December 31, 2010. MMWR Surveill Summ. 2011;60:1–19.
- , , , et al. Antecedent bradycardia and in‐hospital cardiopulmonary arrest mortality in telemetry‐monitored patients outside the ICU. Resuscitation. 2012;83:1106–1110.
- , , , , . In‐hospital cardiac arrest: impact of monitoring and witnessed event on patient survival and neurologic status at hospital discharge. Resuscitation. 2011;82:845–852.
- , , , , . ST changes on continuous telemetry monitoring before in‐hospital cardiac arrests. Paper presented at: Resuscitation Science Symposium, Los Angeles, Ca. November 3–4, 2012.
- , , , , , . Rapid‐response systems as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158:417–425.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial. Lancet. 2005;365:2091–2097.
- , , , . Evaluation of a medical emergency team one year after implementation. Resuscitation. 2004;61:257–263.
- , , , , . Rapid response teams: a systematic review and meta‐analysis. Arch Intern Med. 2010;170:18–26.
- , , , , , . Hospital‐wide code rates and mortality before and after implementation of a rapid response team. JAMA. 2008;300:2506–2513.
- , , , , . Rapid response team implementation and in‐hospital mortality. Crit Care Med. 2014;42(9):2001–2006.
- , , , , , . Effect of a rapid response team on patient outcomes in a community‐based teaching hospital. J Grad Med Educ. 2014;6:61–64.
- , , , , . Reduction in hospital‐wide mortality after implementation of a rapid response team: a long‐term cohort study. Crit Care. 2011;15:R269.
- , , , , , . Rapid response systems: a systematic review. Crit Care Med. 2007;35:1238–1243.
- , , , , . Baseline hospital performance and the impact of medical emergency teams: modelling vs. conventional subgroup analysis. Trials. 2009;10:117.
- . The evolution of case‐mix measurement using DRGs: past, present and future. Stud Health Technol Inform. 1994;14:75–83.
- , , , et al. Variability in case‐mix adjusted in‐hospital cardiac arrest rates. Med Care. 2012;50:124–130.
- , , , , . Impact of socioeconomic adjustment on physicians' relative cost of care. Med Care. 2013;51:454–460.
- , , , , , . Reducing in‐hospital cardiac arrests and hospital mortality by introducing a medical emergency team. Intensive Care Med. 2010;36:100–106.
- , , , et al. Barriers to calling for urgent assistance despite a comprehensive pediatric rapid response system. Am J Crit Care. 2014;23:223–229.
- , , , et al. What stops hospital clinical staff from following protocols? An analysis of the incidence and factors behind the failure of bedside clinical staff to activate the rapid response system in a multi‐campus Australian metropolitan healthcare service. BMJ Qual Saf. 2012;21:569–575.
- , , , , , . Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ. 2002;324:387–390.
- , , , , . The relationship between early emergency team calls and serious adverse events. Crit Care Med. 2009;37:148–153.
- , , , , , . Characteristics and outcomes of patients receiving a medical emergency team review for acute change in conscious state or arrhythmias. Crit Care Med. 2008;36:477–481.
- , , , , , . Characteristics and outcomes of patients receiving a medical emergency team review for respiratory distress or hypotension. J Crit Care. 2008;23:325–331.
- , , , et al. Proactive rounding by the rapid response team reduces inpatient cardiac arrests. Resuscitation. 2013;84:1668–1673.
- , , , , , . Successful implementation of a family and patient activated rapid response team in an adult level 1 trauma center. Resuscitation. 2014;81:1676–1681.
- , , . Effect of the critical care outreach team on patient survival to discharge from hospital and readmission to critical care: non‐randomised population based study. BMJ. 2003;327:1014.
- , , , , , . Beyond rapid response teams: instituting a “rover team” improves the management of at‐risk patients, facilitates proactive interventions, and improves outcomes. In: Henriksen K, Battles JB, Keyes MA, Grady ML, eds. Advances in Patient Safety: New Directions and Alternative Approaches. Vol. 3. Performance and Tools. Rockville, MD: Agency for Healthcare Research and Quality; 2008:1–12.
- , , . Cardiac arrest in the emergency department: a report from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2008;78:151–160.
- , , , , . Delayed time to defibrillation after intraoperative and periprocedural cardiac arrest. Anesthesiology. 2010;113:782–793.
- , , , , . The Medical Emergency Team System and not‐for‐resuscitation orders: results from the MERIT study. Resuscitation. 2008;79:391–397.
© 2015 Society of Hospital Medicine
Optimal Rapid Response System Bundle
The theory behind rapid response teams (RRTs), namely to provide critical care resources to patients with clinical deterioration on the wards, is such common sense that failure to do so seems unethical. This idea, combined with evidence that many cardiac arrests on the wards are predictable and potentially preventable events, led to the proliferation of RRTs across the country and a Joint Commission mandate.[1] However, data from clinical trials have failed to consistently confirm the value of these teams, likely a product of the wide variability in implementation practices across institutions.[2]
In this issue of the Journal of Hospital Medicine, Davis and colleagues demonstrate improvements in both mortality and cardiac arrest rates outside the intensive care unit (ICU) following implementation of their rapid response system in 2 hospitals.[3] Although several other studies have shown similar results, what makes this implementation unique is the bundle approach that included proactive rounding by the charge nurse from each unit, annual focused training of team members and staff, and an integrated, continuous, quality‐improvement feedback loop. Bundles are common in successful quality‐improvement work, but can be challenging for deciphering which of the individual components are driving the results, leaving readers to venture an educated guess. In the current bundle, the novel use of the charge nurse has some significant appeal as a candidate primary driver of the impact, because it likely had 2 distinct actions: (1) proactive rounding and (2) promoting a culture change, both of which are well supported in the literature.4,5
Several studies, including this one, have demonstrated a dose‐response association between the number of RRT activations and patient outcomes, with a low number of RRT activations deemed a major contributor to the neutral results of the large multicenter, randomized, controlled MERIT trial.[6, 7] Additionally, delays in treatment and transfer to the ICU for unstable patients are known to increase mortality.[8] One way to increase the number of patients seen by the RRT and decrease activation delays is by instituting proactive rounding by the team on high‐risk patients. This was the strategy employed in a landmark ward‐randomized trial by Priestley and colleagues, which demonstrated a significant improvement in mortality from proactive rounding on patients deemed to be at high risk of clinical deterioration as calculated by an early warning score or due to caregiver concern.4
Identification of at‐risk patients for proactive rounding can be accomplished with gestalt, as was done by the charge nurse in the current study, or using specific individual criteria such as recent discharge from an ICU. Alternatively, this can be accomplished using composite vital signbased risk scores, such as the Modified Early Warning Score (MEWS).[9] Recently, several newer algorithms that integrate vital signs, laboratory data, and demographics have been shown to outperform the MEWS.[10, 11] Such systems promise an exciting age of real‐time computer‐generated risk stratification, with the ability to automate and standardize the selection of patients for proactive rounding across institutions.
Interestingly, the selection of the charge nurse, rather than someone who did not reside on the unit, to conduct the surveillance rounds likely had another benefit: expediting and facilitating the culture change necessary for a successful implementation. The integration of the charge nurse into the RRT likely led to a local reinforcement of important cultural changes that were already happening at the institutional level. It is clear that culture change is essential in any quality improvement endeavor, and previous literature on RRTs supports this notion.[5]
Rapid response systems are complex and include the activation criteria, team composition and training, and an administrative component. A multifaceted, bundled approach is likely to be required for success. Furthermore, regardless of what risk stratification criteria are used, proactive rounding on high‐risk patients is likely to increase the yield. Utilizing the charge nurse in that effort is a creative use of a preexisting local resource and is worthy of future study.
Disclosures: Dr. Churpek is supported by a career development award from the National Heart, Lung, and Blood Institute (K08 HL121080) and has received honoraria from CHEST for invited speaking engagements. Drs. Churpek and Edelson have a patent pending (ARCD.P0535US.P2) for risk stratification algorithms for hospitalized patients, and Dr. Edelson has an ownership interest in Quant HC (Chicago, IL), which seeks to commercialize those algorithms.
- , , . Rapid‐response teams. N Engl J Med. 2011;365(2):139–146.
- , , , , . Rapid response teams: a systematic review and meta‐analysis. Arch Intern Med. 2010;170(1):18–26.
- , , , et al. A novel configuration of a traditional rapid response team decreases non‐ICU arrests and overall hospital mortality. J Hosp Med. 2015;10(6):352–357
- , , , et al. Introducing Critical Care Outreach: a ward‐randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004;30(7):1398–1404.
- , , , , , . Long‐term culture change related to rapid response system implementation. Med Educ. 2014;48(12):1211–1219.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial. Lancet. 2005;365(9477):2091–2097.
- , , . Effectiveness of the Medical Emergency Team: the importance of dose. Crit Care. 2009;13(5):313.
- , , , , . Inpatient transfers to the intensive care unit: delays are associated with increased mortality and morbidity. J Gen Intern Med. 2003;18(2):77–83.
- , , , . Validation of a modified Early Warning Score in medical admissions. QJM. 2001;94(10):521–526.
- , , , et al. Multicenter development and validation of a risk stratification tool for ward patients. Am J Respir Crit Care Med. 2014;190(6):649–655.
- , , , , , . Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7(5):388–395.
The theory behind rapid response teams (RRTs), namely to provide critical care resources to patients with clinical deterioration on the wards, is such common sense that failure to do so seems unethical. This idea, combined with evidence that many cardiac arrests on the wards are predictable and potentially preventable events, led to the proliferation of RRTs across the country and a Joint Commission mandate.[1] However, data from clinical trials have failed to consistently confirm the value of these teams, likely a product of the wide variability in implementation practices across institutions.[2]
In this issue of the Journal of Hospital Medicine, Davis and colleagues demonstrate improvements in both mortality and cardiac arrest rates outside the intensive care unit (ICU) following implementation of their rapid response system in 2 hospitals.[3] Although several other studies have shown similar results, what makes this implementation unique is the bundle approach that included proactive rounding by the charge nurse from each unit, annual focused training of team members and staff, and an integrated, continuous, quality‐improvement feedback loop. Bundles are common in successful quality‐improvement work, but can be challenging for deciphering which of the individual components are driving the results, leaving readers to venture an educated guess. In the current bundle, the novel use of the charge nurse has some significant appeal as a candidate primary driver of the impact, because it likely had 2 distinct actions: (1) proactive rounding and (2) promoting a culture change, both of which are well supported in the literature.4,5
Several studies, including this one, have demonstrated a dose‐response association between the number of RRT activations and patient outcomes, with a low number of RRT activations deemed a major contributor to the neutral results of the large multicenter, randomized, controlled MERIT trial.[6, 7] Additionally, delays in treatment and transfer to the ICU for unstable patients are known to increase mortality.[8] One way to increase the number of patients seen by the RRT and decrease activation delays is by instituting proactive rounding by the team on high‐risk patients. This was the strategy employed in a landmark ward‐randomized trial by Priestley and colleagues, which demonstrated a significant improvement in mortality from proactive rounding on patients deemed to be at high risk of clinical deterioration as calculated by an early warning score or due to caregiver concern.4
Identification of at‐risk patients for proactive rounding can be accomplished with gestalt, as was done by the charge nurse in the current study, or using specific individual criteria such as recent discharge from an ICU. Alternatively, this can be accomplished using composite vital signbased risk scores, such as the Modified Early Warning Score (MEWS).[9] Recently, several newer algorithms that integrate vital signs, laboratory data, and demographics have been shown to outperform the MEWS.[10, 11] Such systems promise an exciting age of real‐time computer‐generated risk stratification, with the ability to automate and standardize the selection of patients for proactive rounding across institutions.
Interestingly, the selection of the charge nurse, rather than someone who did not reside on the unit, to conduct the surveillance rounds likely had another benefit: expediting and facilitating the culture change necessary for a successful implementation. The integration of the charge nurse into the RRT likely led to a local reinforcement of important cultural changes that were already happening at the institutional level. It is clear that culture change is essential in any quality improvement endeavor, and previous literature on RRTs supports this notion.[5]
Rapid response systems are complex and include the activation criteria, team composition and training, and an administrative component. A multifaceted, bundled approach is likely to be required for success. Furthermore, regardless of what risk stratification criteria are used, proactive rounding on high‐risk patients is likely to increase the yield. Utilizing the charge nurse in that effort is a creative use of a preexisting local resource and is worthy of future study.
Disclosures: Dr. Churpek is supported by a career development award from the National Heart, Lung, and Blood Institute (K08 HL121080) and has received honoraria from CHEST for invited speaking engagements. Drs. Churpek and Edelson have a patent pending (ARCD.P0535US.P2) for risk stratification algorithms for hospitalized patients, and Dr. Edelson has an ownership interest in Quant HC (Chicago, IL), which seeks to commercialize those algorithms.
The theory behind rapid response teams (RRTs), namely to provide critical care resources to patients with clinical deterioration on the wards, is such common sense that failure to do so seems unethical. This idea, combined with evidence that many cardiac arrests on the wards are predictable and potentially preventable events, led to the proliferation of RRTs across the country and a Joint Commission mandate.[1] However, data from clinical trials have failed to consistently confirm the value of these teams, likely a product of the wide variability in implementation practices across institutions.[2]
In this issue of the Journal of Hospital Medicine, Davis and colleagues demonstrate improvements in both mortality and cardiac arrest rates outside the intensive care unit (ICU) following implementation of their rapid response system in 2 hospitals.[3] Although several other studies have shown similar results, what makes this implementation unique is the bundle approach that included proactive rounding by the charge nurse from each unit, annual focused training of team members and staff, and an integrated, continuous, quality‐improvement feedback loop. Bundles are common in successful quality‐improvement work, but can be challenging for deciphering which of the individual components are driving the results, leaving readers to venture an educated guess. In the current bundle, the novel use of the charge nurse has some significant appeal as a candidate primary driver of the impact, because it likely had 2 distinct actions: (1) proactive rounding and (2) promoting a culture change, both of which are well supported in the literature.4,5
Several studies, including this one, have demonstrated a dose‐response association between the number of RRT activations and patient outcomes, with a low number of RRT activations deemed a major contributor to the neutral results of the large multicenter, randomized, controlled MERIT trial.[6, 7] Additionally, delays in treatment and transfer to the ICU for unstable patients are known to increase mortality.[8] One way to increase the number of patients seen by the RRT and decrease activation delays is by instituting proactive rounding by the team on high‐risk patients. This was the strategy employed in a landmark ward‐randomized trial by Priestley and colleagues, which demonstrated a significant improvement in mortality from proactive rounding on patients deemed to be at high risk of clinical deterioration as calculated by an early warning score or due to caregiver concern.4
Identification of at‐risk patients for proactive rounding can be accomplished with gestalt, as was done by the charge nurse in the current study, or using specific individual criteria such as recent discharge from an ICU. Alternatively, this can be accomplished using composite vital signbased risk scores, such as the Modified Early Warning Score (MEWS).[9] Recently, several newer algorithms that integrate vital signs, laboratory data, and demographics have been shown to outperform the MEWS.[10, 11] Such systems promise an exciting age of real‐time computer‐generated risk stratification, with the ability to automate and standardize the selection of patients for proactive rounding across institutions.
Interestingly, the selection of the charge nurse, rather than someone who did not reside on the unit, to conduct the surveillance rounds likely had another benefit: expediting and facilitating the culture change necessary for a successful implementation. The integration of the charge nurse into the RRT likely led to a local reinforcement of important cultural changes that were already happening at the institutional level. It is clear that culture change is essential in any quality improvement endeavor, and previous literature on RRTs supports this notion.[5]
Rapid response systems are complex and include the activation criteria, team composition and training, and an administrative component. A multifaceted, bundled approach is likely to be required for success. Furthermore, regardless of what risk stratification criteria are used, proactive rounding on high‐risk patients is likely to increase the yield. Utilizing the charge nurse in that effort is a creative use of a preexisting local resource and is worthy of future study.
Disclosures: Dr. Churpek is supported by a career development award from the National Heart, Lung, and Blood Institute (K08 HL121080) and has received honoraria from CHEST for invited speaking engagements. Drs. Churpek and Edelson have a patent pending (ARCD.P0535US.P2) for risk stratification algorithms for hospitalized patients, and Dr. Edelson has an ownership interest in Quant HC (Chicago, IL), which seeks to commercialize those algorithms.
- , , . Rapid‐response teams. N Engl J Med. 2011;365(2):139–146.
- , , , , . Rapid response teams: a systematic review and meta‐analysis. Arch Intern Med. 2010;170(1):18–26.
- , , , et al. A novel configuration of a traditional rapid response team decreases non‐ICU arrests and overall hospital mortality. J Hosp Med. 2015;10(6):352–357
- , , , et al. Introducing Critical Care Outreach: a ward‐randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004;30(7):1398–1404.
- , , , , , . Long‐term culture change related to rapid response system implementation. Med Educ. 2014;48(12):1211–1219.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial. Lancet. 2005;365(9477):2091–2097.
- , , . Effectiveness of the Medical Emergency Team: the importance of dose. Crit Care. 2009;13(5):313.
- , , , , . Inpatient transfers to the intensive care unit: delays are associated with increased mortality and morbidity. J Gen Intern Med. 2003;18(2):77–83.
- , , , . Validation of a modified Early Warning Score in medical admissions. QJM. 2001;94(10):521–526.
- , , , et al. Multicenter development and validation of a risk stratification tool for ward patients. Am J Respir Crit Care Med. 2014;190(6):649–655.
- , , , , , . Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7(5):388–395.
- , , . Rapid‐response teams. N Engl J Med. 2011;365(2):139–146.
- , , , , . Rapid response teams: a systematic review and meta‐analysis. Arch Intern Med. 2010;170(1):18–26.
- , , , et al. A novel configuration of a traditional rapid response team decreases non‐ICU arrests and overall hospital mortality. J Hosp Med. 2015;10(6):352–357
- , , , et al. Introducing Critical Care Outreach: a ward‐randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004;30(7):1398–1404.
- , , , , , . Long‐term culture change related to rapid response system implementation. Med Educ. 2014;48(12):1211–1219.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial. Lancet. 2005;365(9477):2091–2097.
- , , . Effectiveness of the Medical Emergency Team: the importance of dose. Crit Care. 2009;13(5):313.
- , , , , . Inpatient transfers to the intensive care unit: delays are associated with increased mortality and morbidity. J Gen Intern Med. 2003;18(2):77–83.
- , , , . Validation of a modified Early Warning Score in medical admissions. QJM. 2001;94(10):521–526.
- , , , et al. Multicenter development and validation of a risk stratification tool for ward patients. Am J Respir Crit Care Med. 2014;190(6):649–655.
- , , , , , . Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7(5):388–395.