User login
EC approves drug for polycythemia vera

Image courtesy of AFIP
The European Commission (EC) has approved ruxolitinib (Jakavi) to treat adults with polycythemia vera (PV) who are resistant to or cannot tolerate hydroxyurea.
This is the first targeted treatment the EC has approved for these patients.
The approval applies to all 28 member states of the European Union (EU), plus Iceland, Norway, and Liechtenstein.
Ruxolitinib is already approved to treat PV in the US, and additional regulatory applications for ruxolitinib in PV are ongoing worldwide.
The drug is also approved to treat adults with primary myelofibrosis (MF), post-PV MF, or post-essential thrombocythemia MF in more than 70 countries, including EU member states and the US.
“The European Commission’s approval of Jakavi [for PV] is encouraging news for patients,” said Claire Harrison, MD, a consultant hematologist at Guy’s and St Thomas’ NHS Foundation Trust in London, England.
“Jakavi will fill an unmet need as the first treatment shown to significantly improve hematocrit, as well as symptom control and reduce spleen size in patients with polycythemia vera resistant to or intolerant of hydroxyurea.”
RESPONSE trial
The EC’s approval is based on data from the phase 3 RESPONSE trial. The study included 222 patients who had PV for at least 24 weeks. All patients had an inadequate response to or could not tolerate hydroxyurea, had undergone a phlebotomy, and had splenomegaly.
Patients were randomized to receive ruxolitinib starting at 10 mg twice daily or best available therapy (BAT) as determined by the investigator. The ruxolitinib dose was adjusted as needed.
The study’s primary endpoint was a composite of hematocrit control and spleen reduction. To meet the endpoint, patients had to experience a 35% or greater reduction in spleen volume from baseline, as assessed by imaging at week 32.
And a patient’s hematocrit was considered under control if he was not eligible for phlebotomy from week 8 through 32 (and had no more than one instance of phlebotomy eligibility between randomization and week 8). Patients who were deemed eligible for phlebotomy had hematocrit that was greater than 45% or had increased 3 or more percentage points from the time they entered the study.
Twenty-one percent of ruxolitinib-treated patients met this endpoint (achieving hematocrit control and spleen reduction), compared to 1% of patients who received BAT (P<0.001).
And the researchers said ruxolitinib was well-tolerated. Common adverse events included headache, diarrhea, and fatigue.
Grade 3/4 anemia, grade 3/4 thrombocytopenia, and herpes zoster infections of all grades were more common in the ruxolitinib arm than the BAT arm. But thromboembolic events were more common with BAT than ruxolitinib.
This trial was funded by the Incyte Corporation, which markets ruxolitinib in the US. Novartis licensed ruxolitinib from Incyte for development and commercialization outside the US.
For more details on ruxolitinib, see the full prescribing information, available at www.jakavi.com. ![]()

Image courtesy of AFIP
The European Commission (EC) has approved ruxolitinib (Jakavi) to treat adults with polycythemia vera (PV) who are resistant to or cannot tolerate hydroxyurea.
This is the first targeted treatment the EC has approved for these patients.
The approval applies to all 28 member states of the European Union (EU), plus Iceland, Norway, and Liechtenstein.
Ruxolitinib is already approved to treat PV in the US, and additional regulatory applications for ruxolitinib in PV are ongoing worldwide.
The drug is also approved to treat adults with primary myelofibrosis (MF), post-PV MF, or post-essential thrombocythemia MF in more than 70 countries, including EU member states and the US.
“The European Commission’s approval of Jakavi [for PV] is encouraging news for patients,” said Claire Harrison, MD, a consultant hematologist at Guy’s and St Thomas’ NHS Foundation Trust in London, England.
“Jakavi will fill an unmet need as the first treatment shown to significantly improve hematocrit, as well as symptom control and reduce spleen size in patients with polycythemia vera resistant to or intolerant of hydroxyurea.”
RESPONSE trial
The EC’s approval is based on data from the phase 3 RESPONSE trial. The study included 222 patients who had PV for at least 24 weeks. All patients had an inadequate response to or could not tolerate hydroxyurea, had undergone a phlebotomy, and had splenomegaly.
Patients were randomized to receive ruxolitinib starting at 10 mg twice daily or best available therapy (BAT) as determined by the investigator. The ruxolitinib dose was adjusted as needed.
The study’s primary endpoint was a composite of hematocrit control and spleen reduction. To meet the endpoint, patients had to experience a 35% or greater reduction in spleen volume from baseline, as assessed by imaging at week 32.
And a patient’s hematocrit was considered under control if he was not eligible for phlebotomy from week 8 through 32 (and had no more than one instance of phlebotomy eligibility between randomization and week 8). Patients who were deemed eligible for phlebotomy had hematocrit that was greater than 45% or had increased 3 or more percentage points from the time they entered the study.
Twenty-one percent of ruxolitinib-treated patients met this endpoint (achieving hematocrit control and spleen reduction), compared to 1% of patients who received BAT (P<0.001).
And the researchers said ruxolitinib was well-tolerated. Common adverse events included headache, diarrhea, and fatigue.
Grade 3/4 anemia, grade 3/4 thrombocytopenia, and herpes zoster infections of all grades were more common in the ruxolitinib arm than the BAT arm. But thromboembolic events were more common with BAT than ruxolitinib.
This trial was funded by the Incyte Corporation, which markets ruxolitinib in the US. Novartis licensed ruxolitinib from Incyte for development and commercialization outside the US.
For more details on ruxolitinib, see the full prescribing information, available at www.jakavi.com. ![]()

Image courtesy of AFIP
The European Commission (EC) has approved ruxolitinib (Jakavi) to treat adults with polycythemia vera (PV) who are resistant to or cannot tolerate hydroxyurea.
This is the first targeted treatment the EC has approved for these patients.
The approval applies to all 28 member states of the European Union (EU), plus Iceland, Norway, and Liechtenstein.
Ruxolitinib is already approved to treat PV in the US, and additional regulatory applications for ruxolitinib in PV are ongoing worldwide.
The drug is also approved to treat adults with primary myelofibrosis (MF), post-PV MF, or post-essential thrombocythemia MF in more than 70 countries, including EU member states and the US.
“The European Commission’s approval of Jakavi [for PV] is encouraging news for patients,” said Claire Harrison, MD, a consultant hematologist at Guy’s and St Thomas’ NHS Foundation Trust in London, England.
“Jakavi will fill an unmet need as the first treatment shown to significantly improve hematocrit, as well as symptom control and reduce spleen size in patients with polycythemia vera resistant to or intolerant of hydroxyurea.”
RESPONSE trial
The EC’s approval is based on data from the phase 3 RESPONSE trial. The study included 222 patients who had PV for at least 24 weeks. All patients had an inadequate response to or could not tolerate hydroxyurea, had undergone a phlebotomy, and had splenomegaly.
Patients were randomized to receive ruxolitinib starting at 10 mg twice daily or best available therapy (BAT) as determined by the investigator. The ruxolitinib dose was adjusted as needed.
The study’s primary endpoint was a composite of hematocrit control and spleen reduction. To meet the endpoint, patients had to experience a 35% or greater reduction in spleen volume from baseline, as assessed by imaging at week 32.
And a patient’s hematocrit was considered under control if he was not eligible for phlebotomy from week 8 through 32 (and had no more than one instance of phlebotomy eligibility between randomization and week 8). Patients who were deemed eligible for phlebotomy had hematocrit that was greater than 45% or had increased 3 or more percentage points from the time they entered the study.
Twenty-one percent of ruxolitinib-treated patients met this endpoint (achieving hematocrit control and spleen reduction), compared to 1% of patients who received BAT (P<0.001).
And the researchers said ruxolitinib was well-tolerated. Common adverse events included headache, diarrhea, and fatigue.
Grade 3/4 anemia, grade 3/4 thrombocytopenia, and herpes zoster infections of all grades were more common in the ruxolitinib arm than the BAT arm. But thromboembolic events were more common with BAT than ruxolitinib.
This trial was funded by the Incyte Corporation, which markets ruxolitinib in the US. Novartis licensed ruxolitinib from Incyte for development and commercialization outside the US.
For more details on ruxolitinib, see the full prescribing information, available at www.jakavi.com. ![]()
4F-PCC proves more effective than plasma

Photo by Cristina Granados
Results of a phase 3 trial indicate that a 4-factor prothrombin complex concentrate (4F-PCC) is more effective than plasma for reversing acquired coagulation factor deficiency induced by vitamin K antagonist therapy in adults who require urgent surgery or an invasive procedure.
4F-PCC induced hemostasis in more patients and reduced international normalized ratios (INRs) more quickly than plasma.
And the rates of adverse events (AEs) were similar in the 2 groups.
“[4F-PCC] is more effective than plasma for INR reduction and periprocedural hemostasis in adults who are taking warfarin and require an urgent procedure,” said Joshua N. Goldstein, MD, PhD, of Massachusetts General Hospital in Boston.
He and his colleagues reported these findings in The Lancet.
The study included 181 patients, but only 168 were evaluable for efficacy. Eighty-seven of these patients received 4F-PCC, and 81 received plasma.
Ninety percent of patients treated with 4F-PCC achieved effective hemostasis, compared to 75% of patients treated with plasma (P=0.0142).
Fifty-five percent of patients who received 4F-PCC achieved rapid INR reduction (to ≤ 1.3 at 30 minutes after the end of infusion), compared to 10% of patients treated with plasma (P<0.0001).
In post-hoc analysis, the median time from the start of infusion to the start of the urgent surgical procedure was shorter in the 4F-PCC group than in the plasma group—3.6 hours and 8.5 hours, respectively (P=0.0098).
Eighty-eight patients in each group were evaluable for safety. And the incidence of AEs was similar in the 4F-PCC and plasma groups, at 56% and 60%, respectively.
Treatment-related AEs occurred in 9% of 4F-PCC-treated patients and 17% of plasma-treated patients. In both groups, 3% of these AEs were serious.
The rate of death at day 45 was 3% in the 4F-PCC group and 9% in the plasma group. The rates of thromboembolic AEs were 7% and 8%, respectively.
The rates of fluid overload or similar cardiac events were 3% and 13%, respectively. And the rates of bleeding after the primary outcome assessment were 3% and 5%, respectively.
This study was funded by CSL Behring, makers of 4F-PCC, which is marketed as Kcentra, Beriplex, or Confidex. ![]()

Photo by Cristina Granados
Results of a phase 3 trial indicate that a 4-factor prothrombin complex concentrate (4F-PCC) is more effective than plasma for reversing acquired coagulation factor deficiency induced by vitamin K antagonist therapy in adults who require urgent surgery or an invasive procedure.
4F-PCC induced hemostasis in more patients and reduced international normalized ratios (INRs) more quickly than plasma.
And the rates of adverse events (AEs) were similar in the 2 groups.
“[4F-PCC] is more effective than plasma for INR reduction and periprocedural hemostasis in adults who are taking warfarin and require an urgent procedure,” said Joshua N. Goldstein, MD, PhD, of Massachusetts General Hospital in Boston.
He and his colleagues reported these findings in The Lancet.
The study included 181 patients, but only 168 were evaluable for efficacy. Eighty-seven of these patients received 4F-PCC, and 81 received plasma.
Ninety percent of patients treated with 4F-PCC achieved effective hemostasis, compared to 75% of patients treated with plasma (P=0.0142).
Fifty-five percent of patients who received 4F-PCC achieved rapid INR reduction (to ≤ 1.3 at 30 minutes after the end of infusion), compared to 10% of patients treated with plasma (P<0.0001).
In post-hoc analysis, the median time from the start of infusion to the start of the urgent surgical procedure was shorter in the 4F-PCC group than in the plasma group—3.6 hours and 8.5 hours, respectively (P=0.0098).
Eighty-eight patients in each group were evaluable for safety. And the incidence of AEs was similar in the 4F-PCC and plasma groups, at 56% and 60%, respectively.
Treatment-related AEs occurred in 9% of 4F-PCC-treated patients and 17% of plasma-treated patients. In both groups, 3% of these AEs were serious.
The rate of death at day 45 was 3% in the 4F-PCC group and 9% in the plasma group. The rates of thromboembolic AEs were 7% and 8%, respectively.
The rates of fluid overload or similar cardiac events were 3% and 13%, respectively. And the rates of bleeding after the primary outcome assessment were 3% and 5%, respectively.
This study was funded by CSL Behring, makers of 4F-PCC, which is marketed as Kcentra, Beriplex, or Confidex. ![]()

Photo by Cristina Granados
Results of a phase 3 trial indicate that a 4-factor prothrombin complex concentrate (4F-PCC) is more effective than plasma for reversing acquired coagulation factor deficiency induced by vitamin K antagonist therapy in adults who require urgent surgery or an invasive procedure.
4F-PCC induced hemostasis in more patients and reduced international normalized ratios (INRs) more quickly than plasma.
And the rates of adverse events (AEs) were similar in the 2 groups.
“[4F-PCC] is more effective than plasma for INR reduction and periprocedural hemostasis in adults who are taking warfarin and require an urgent procedure,” said Joshua N. Goldstein, MD, PhD, of Massachusetts General Hospital in Boston.
He and his colleagues reported these findings in The Lancet.
The study included 181 patients, but only 168 were evaluable for efficacy. Eighty-seven of these patients received 4F-PCC, and 81 received plasma.
Ninety percent of patients treated with 4F-PCC achieved effective hemostasis, compared to 75% of patients treated with plasma (P=0.0142).
Fifty-five percent of patients who received 4F-PCC achieved rapid INR reduction (to ≤ 1.3 at 30 minutes after the end of infusion), compared to 10% of patients treated with plasma (P<0.0001).
In post-hoc analysis, the median time from the start of infusion to the start of the urgent surgical procedure was shorter in the 4F-PCC group than in the plasma group—3.6 hours and 8.5 hours, respectively (P=0.0098).
Eighty-eight patients in each group were evaluable for safety. And the incidence of AEs was similar in the 4F-PCC and plasma groups, at 56% and 60%, respectively.
Treatment-related AEs occurred in 9% of 4F-PCC-treated patients and 17% of plasma-treated patients. In both groups, 3% of these AEs were serious.
The rate of death at day 45 was 3% in the 4F-PCC group and 9% in the plasma group. The rates of thromboembolic AEs were 7% and 8%, respectively.
The rates of fluid overload or similar cardiac events were 3% and 13%, respectively. And the rates of bleeding after the primary outcome assessment were 3% and 5%, respectively.
This study was funded by CSL Behring, makers of 4F-PCC, which is marketed as Kcentra, Beriplex, or Confidex. ![]()
LISTEN NOW: My iPad Went to Medical School
Mobile devices put information in the palm of your hand. For hospitalists, this presents real opportunities to engage patients, improve care, and streamline hospital workflows. Two hospitalists who were early adopters of mobile tech in their practices, Dr. Henry Feldman of Beth Israel Deaconess and Dr. Richard Pittman of Emory University/Grady share their lessons learned, and their advice for other hospital clinicians and informaticists on using mobile tech in their practices.
Mobile devices put information in the palm of your hand. For hospitalists, this presents real opportunities to engage patients, improve care, and streamline hospital workflows. Two hospitalists who were early adopters of mobile tech in their practices, Dr. Henry Feldman of Beth Israel Deaconess and Dr. Richard Pittman of Emory University/Grady share their lessons learned, and their advice for other hospital clinicians and informaticists on using mobile tech in their practices.
Mobile devices put information in the palm of your hand. For hospitalists, this presents real opportunities to engage patients, improve care, and streamline hospital workflows. Two hospitalists who were early adopters of mobile tech in their practices, Dr. Henry Feldman of Beth Israel Deaconess and Dr. Richard Pittman of Emory University/Grady share their lessons learned, and their advice for other hospital clinicians and informaticists on using mobile tech in their practices.
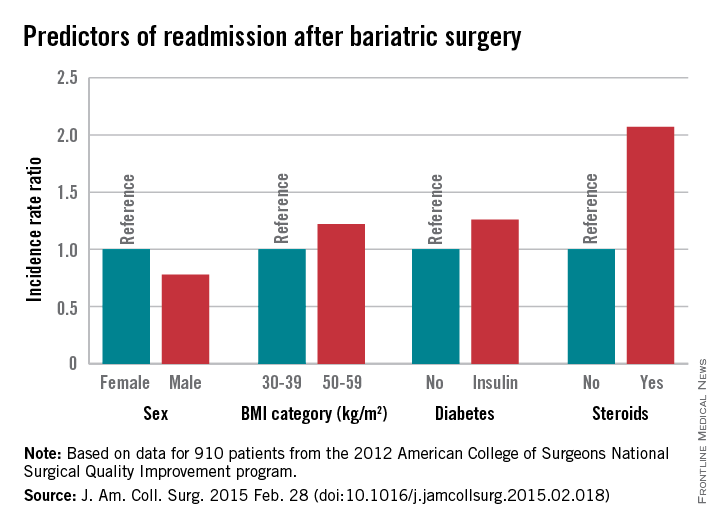
Several factors predict postbariatric surgery readmission
Bariatric surgery is generally safe and readmissions are rare, but prolonged operative time, operation complexity, and major postoperative complications are among several risk factors for readmission identified in a large retrospective cohort.
Of 18,186 patients from the 2012 American College of Surgeons National Surgical Quality Improvement program (ACS NSQIP) database who had bariatric surgery as a primary procedure, 5% were readmitted. Of 815 patients with any major complication, 31% were readmitted. Factors found on multivariate analysis to significantly predict readmission within 30 days were age, sex, body mass index, American Society of Anesthesiology (ASA) risk class, diabetes status, hypertension, and steroid use, Dr. Christa R. Abraham of Albany (N.Y.) Medical College and her colleagues reported online in the Journal of the American College of Surgeons.
Further, all major postoperative complications were significant predictors of readmission, including bleeding requiring transfusion, urinary tract infections, and superficial surgical site infection (SSI). Other significant predictors were deep SSI, organ space SSI, wound disruption, pneumonia, unplanned intubation, mechanical ventilation for more than 48 hours, pulmonary embolism, deep vein thrombosis, and sepsis, the investigators said (J. Am. Coll. Surg. 2015 [doi:10.1016/j.jamcollsurg.2015.02.018]).
Of the patients included in the study, 1,819 had a laparoscopic gastric band, 9,613 had laparoscopic Roux-en-Y gastric bypass, 6,439 had gastroplasties, and 315 had open Roux-en-Y gastric bypass. All had a BMI of at least 30 kg/m2, and had a postsurgery length of stay of 14 days or fewer. Most were ASA risk class 3 or lower, and most were functionally independent.
Complications were more common with laparoscopic and open Roux-en-y gastric bypass (5.5% and 11.8%, respectively) rather than with gastroplasty and sleeve (3.4%) and laparoscopic banding (1.4%).
The findings are of value, because while bariatric surgery is a low-risk procedure, and it is extremely common; in 2013 there were 179,000 such surgeries performed in the United States.
“Bariatric surgery is one of the fastest-growing surgical interest areas, making analysis of patient outcomes and reasons for readmission important,” the investigators explained.
The ability to identify high-risk patients could allow for targeted interventions to prevent readmission, they said.
For example, steroid use, which was identified as a risk factor in the current study, is modifiable.
“In our practice, steroids are discontinued for 6 weeks prior to bariatric surgery and patients who are steroid dependent are unlikely to undergo bariatric surgery,” they said.
Additionally, they “try to minimize readmission for patients with infections by treating with antibiotics following operation and continuing antibiotics at discharge.”
The investigators noted that the ACS NSQIP MORBPROB (estimated probability of morbidity) tool is a good tool for predicting readmission among prospective bariatric patients, although it may not fully capture the effect of preexisting conditions.
“These data led us to change our own practice by risk-stratifying patients with higher ASA and BMI to consider surgical options, and to begin early surveillance soon after discharge,” they said.
The authors reported having no disclosures.
Bariatric surgery is generally safe and readmissions are rare, but prolonged operative time, operation complexity, and major postoperative complications are among several risk factors for readmission identified in a large retrospective cohort.
Of 18,186 patients from the 2012 American College of Surgeons National Surgical Quality Improvement program (ACS NSQIP) database who had bariatric surgery as a primary procedure, 5% were readmitted. Of 815 patients with any major complication, 31% were readmitted. Factors found on multivariate analysis to significantly predict readmission within 30 days were age, sex, body mass index, American Society of Anesthesiology (ASA) risk class, diabetes status, hypertension, and steroid use, Dr. Christa R. Abraham of Albany (N.Y.) Medical College and her colleagues reported online in the Journal of the American College of Surgeons.
Further, all major postoperative complications were significant predictors of readmission, including bleeding requiring transfusion, urinary tract infections, and superficial surgical site infection (SSI). Other significant predictors were deep SSI, organ space SSI, wound disruption, pneumonia, unplanned intubation, mechanical ventilation for more than 48 hours, pulmonary embolism, deep vein thrombosis, and sepsis, the investigators said (J. Am. Coll. Surg. 2015 [doi:10.1016/j.jamcollsurg.2015.02.018]).
Of the patients included in the study, 1,819 had a laparoscopic gastric band, 9,613 had laparoscopic Roux-en-Y gastric bypass, 6,439 had gastroplasties, and 315 had open Roux-en-Y gastric bypass. All had a BMI of at least 30 kg/m2, and had a postsurgery length of stay of 14 days or fewer. Most were ASA risk class 3 or lower, and most were functionally independent.
Complications were more common with laparoscopic and open Roux-en-y gastric bypass (5.5% and 11.8%, respectively) rather than with gastroplasty and sleeve (3.4%) and laparoscopic banding (1.4%).
The findings are of value, because while bariatric surgery is a low-risk procedure, and it is extremely common; in 2013 there were 179,000 such surgeries performed in the United States.

“Bariatric surgery is one of the fastest-growing surgical interest areas, making analysis of patient outcomes and reasons for readmission important,” the investigators explained.
The ability to identify high-risk patients could allow for targeted interventions to prevent readmission, they said.
For example, steroid use, which was identified as a risk factor in the current study, is modifiable.
“In our practice, steroids are discontinued for 6 weeks prior to bariatric surgery and patients who are steroid dependent are unlikely to undergo bariatric surgery,” they said.
Additionally, they “try to minimize readmission for patients with infections by treating with antibiotics following operation and continuing antibiotics at discharge.”
The investigators noted that the ACS NSQIP MORBPROB (estimated probability of morbidity) tool is a good tool for predicting readmission among prospective bariatric patients, although it may not fully capture the effect of preexisting conditions.
“These data led us to change our own practice by risk-stratifying patients with higher ASA and BMI to consider surgical options, and to begin early surveillance soon after discharge,” they said.
The authors reported having no disclosures.
Bariatric surgery is generally safe and readmissions are rare, but prolonged operative time, operation complexity, and major postoperative complications are among several risk factors for readmission identified in a large retrospective cohort.
Of 18,186 patients from the 2012 American College of Surgeons National Surgical Quality Improvement program (ACS NSQIP) database who had bariatric surgery as a primary procedure, 5% were readmitted. Of 815 patients with any major complication, 31% were readmitted. Factors found on multivariate analysis to significantly predict readmission within 30 days were age, sex, body mass index, American Society of Anesthesiology (ASA) risk class, diabetes status, hypertension, and steroid use, Dr. Christa R. Abraham of Albany (N.Y.) Medical College and her colleagues reported online in the Journal of the American College of Surgeons.
Further, all major postoperative complications were significant predictors of readmission, including bleeding requiring transfusion, urinary tract infections, and superficial surgical site infection (SSI). Other significant predictors were deep SSI, organ space SSI, wound disruption, pneumonia, unplanned intubation, mechanical ventilation for more than 48 hours, pulmonary embolism, deep vein thrombosis, and sepsis, the investigators said (J. Am. Coll. Surg. 2015 [doi:10.1016/j.jamcollsurg.2015.02.018]).
Of the patients included in the study, 1,819 had a laparoscopic gastric band, 9,613 had laparoscopic Roux-en-Y gastric bypass, 6,439 had gastroplasties, and 315 had open Roux-en-Y gastric bypass. All had a BMI of at least 30 kg/m2, and had a postsurgery length of stay of 14 days or fewer. Most were ASA risk class 3 or lower, and most were functionally independent.
Complications were more common with laparoscopic and open Roux-en-y gastric bypass (5.5% and 11.8%, respectively) rather than with gastroplasty and sleeve (3.4%) and laparoscopic banding (1.4%).
The findings are of value, because while bariatric surgery is a low-risk procedure, and it is extremely common; in 2013 there were 179,000 such surgeries performed in the United States.

“Bariatric surgery is one of the fastest-growing surgical interest areas, making analysis of patient outcomes and reasons for readmission important,” the investigators explained.
The ability to identify high-risk patients could allow for targeted interventions to prevent readmission, they said.
For example, steroid use, which was identified as a risk factor in the current study, is modifiable.
“In our practice, steroids are discontinued for 6 weeks prior to bariatric surgery and patients who are steroid dependent are unlikely to undergo bariatric surgery,” they said.
Additionally, they “try to minimize readmission for patients with infections by treating with antibiotics following operation and continuing antibiotics at discharge.”
The investigators noted that the ACS NSQIP MORBPROB (estimated probability of morbidity) tool is a good tool for predicting readmission among prospective bariatric patients, although it may not fully capture the effect of preexisting conditions.
“These data led us to change our own practice by risk-stratifying patients with higher ASA and BMI to consider surgical options, and to begin early surveillance soon after discharge,” they said.
The authors reported having no disclosures.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS
Key clinical point: Knowing risk factors for readmission after bariatric surgery can allow for targeted interventions.
Major finding: Steroid use is among several risk factors for readmission following bariatric surgery (incidence rate ratio, 2.07)
Data source: A retrospective cohort study involving 18,186 patients.
Disclosures: The authors reported having no disclosures.
Huntington’s Disease: Emerging Concepts in Diagnosis and Treatment
Trading in work-life balance for a well-balanced life
My residency supervisor candidly asked me today – Isn’t stressing out about writing an article on work-life balance kind of missing the point? Well, yeah, that’s why she’s my supervisor. This brings me to one of the lesser advertised tips to avoiding burnout, which is: Get yourself a great mensch. But I’m getting ahead of myself here. The plan was to have 10 perfectly delineated rules, because if it worked for Letterman and Moses, it should work for residency. More to come on that.
Another part of the plan was to have this article finished by last weekend, but long call was Saturday. This was followed by long call recovery consisting of sleeping in so late my dad texted and left a voicemail asking, what happened? I haven’t heard from you all weekend. Then there was the obligatory run on the treadmill so the gooey cinnamon rolls the nurses baked and generously invited me to on Thursday would not stick around long enough for my husband to wonder if this was the beginning of me letting myself go. Isn’t that a lovely phrase?
Monday was Monday. How does anyone get anything done on Mondays? I had a new team, two new patients to learn and discharge. Plus, it was the first day that cracked 50 degrees in 5 months. I had to meet up with a friend, grab some coffee, and gossip walk around the lake. This was before we found out another friend was being slammed with consults in the emergency room. So there I was right back at the hospital Monday night with a cream cheese cherry pastry to cheer up my compatriot in the struggle.
This brings me to Tuesday. I had planned to be at the editing stage of this article on Tuesday. But didactics ran long due to everyone being so engaged in our formulations lecture, I didn’t have a shot at looking at this thing until lunchtime. Lunchtime came, and as I opened Microsoft Word among my dollar turkey sandwich and mini Purell bottles stationed around me like glorious little sergeants, I heard the gingerly utterings of a medical student: Um, if you have a moment, could you tell me the difference between the side-effect profile of first-generation and second-generation antipsychotics?
An hour later, I was informed that an admit was on the way and was traveling from out of state, set to arrive a half-hour before shift’s end. Did I mention he arrived with two family members in tow who wanted to talk about how things went wrong starting 20 years ago? Then there was the patient to see who I knew would pout if I didn’t spend at least a half-hour checking in. You know, the one the nurses always try to save me from even though I secretly never wanted to be saved.
I finally drove home 2 hours later than anticipated with a smile on my face. I should repeat that, WITH A SMILE ON MY FACE. I felt good because I’d done good. After all, there’s even a little sunlight left. When I walk in the front door, I kiss my husband and then immediately delve into a new story from the day. We laugh. We warm up leftovers, sit on the couch with our bare feet on the table, and catch an hour of American Idol (talent never gets old). Then it’s time to meet this maker.
The strange thing is, the person who began this column with all of her well-intentioned plans feels very different from the person who has made it to the deadline. There is a whole life lived in between. All of the readings I had done, notations I had made, seem kind of beside the point. I could pepper you with statistics and evidence-based outcomes warning of divorce, substance abuse, physician suicide, patient errors, and the like, which are all very real outcomes of poorly balanced lives. But I think we know all of that. It’s the in between space, the living part where so many of us lose our way. So instead of referenced journals, I offer you my journey. Because I can truly say that for the last 3 months of the most difficult year of residency, I have been happy. May this piece be also with you.
Dr. Schmidt, a second-year psychiatry resident at the Mayo Clinic in Rochester, Minn., is interested in psychodynamic therapy and in pursuing a fellowship in addictions. After obtaining a bachelor of arts at the University of California, Berkeley, she earned a master of arts degree in philosophy and humanities at the University of Chicago. She attended medical school at the University of Illinois College of Medicine at Peoria.
My residency supervisor candidly asked me today – Isn’t stressing out about writing an article on work-life balance kind of missing the point? Well, yeah, that’s why she’s my supervisor. This brings me to one of the lesser advertised tips to avoiding burnout, which is: Get yourself a great mensch. But I’m getting ahead of myself here. The plan was to have 10 perfectly delineated rules, because if it worked for Letterman and Moses, it should work for residency. More to come on that.
Another part of the plan was to have this article finished by last weekend, but long call was Saturday. This was followed by long call recovery consisting of sleeping in so late my dad texted and left a voicemail asking, what happened? I haven’t heard from you all weekend. Then there was the obligatory run on the treadmill so the gooey cinnamon rolls the nurses baked and generously invited me to on Thursday would not stick around long enough for my husband to wonder if this was the beginning of me letting myself go. Isn’t that a lovely phrase?
Monday was Monday. How does anyone get anything done on Mondays? I had a new team, two new patients to learn and discharge. Plus, it was the first day that cracked 50 degrees in 5 months. I had to meet up with a friend, grab some coffee, and gossip walk around the lake. This was before we found out another friend was being slammed with consults in the emergency room. So there I was right back at the hospital Monday night with a cream cheese cherry pastry to cheer up my compatriot in the struggle.
This brings me to Tuesday. I had planned to be at the editing stage of this article on Tuesday. But didactics ran long due to everyone being so engaged in our formulations lecture, I didn’t have a shot at looking at this thing until lunchtime. Lunchtime came, and as I opened Microsoft Word among my dollar turkey sandwich and mini Purell bottles stationed around me like glorious little sergeants, I heard the gingerly utterings of a medical student: Um, if you have a moment, could you tell me the difference between the side-effect profile of first-generation and second-generation antipsychotics?
An hour later, I was informed that an admit was on the way and was traveling from out of state, set to arrive a half-hour before shift’s end. Did I mention he arrived with two family members in tow who wanted to talk about how things went wrong starting 20 years ago? Then there was the patient to see who I knew would pout if I didn’t spend at least a half-hour checking in. You know, the one the nurses always try to save me from even though I secretly never wanted to be saved.
I finally drove home 2 hours later than anticipated with a smile on my face. I should repeat that, WITH A SMILE ON MY FACE. I felt good because I’d done good. After all, there’s even a little sunlight left. When I walk in the front door, I kiss my husband and then immediately delve into a new story from the day. We laugh. We warm up leftovers, sit on the couch with our bare feet on the table, and catch an hour of American Idol (talent never gets old). Then it’s time to meet this maker.
The strange thing is, the person who began this column with all of her well-intentioned plans feels very different from the person who has made it to the deadline. There is a whole life lived in between. All of the readings I had done, notations I had made, seem kind of beside the point. I could pepper you with statistics and evidence-based outcomes warning of divorce, substance abuse, physician suicide, patient errors, and the like, which are all very real outcomes of poorly balanced lives. But I think we know all of that. It’s the in between space, the living part where so many of us lose our way. So instead of referenced journals, I offer you my journey. Because I can truly say that for the last 3 months of the most difficult year of residency, I have been happy. May this piece be also with you.
Dr. Schmidt, a second-year psychiatry resident at the Mayo Clinic in Rochester, Minn., is interested in psychodynamic therapy and in pursuing a fellowship in addictions. After obtaining a bachelor of arts at the University of California, Berkeley, she earned a master of arts degree in philosophy and humanities at the University of Chicago. She attended medical school at the University of Illinois College of Medicine at Peoria.
My residency supervisor candidly asked me today – Isn’t stressing out about writing an article on work-life balance kind of missing the point? Well, yeah, that’s why she’s my supervisor. This brings me to one of the lesser advertised tips to avoiding burnout, which is: Get yourself a great mensch. But I’m getting ahead of myself here. The plan was to have 10 perfectly delineated rules, because if it worked for Letterman and Moses, it should work for residency. More to come on that.
Another part of the plan was to have this article finished by last weekend, but long call was Saturday. This was followed by long call recovery consisting of sleeping in so late my dad texted and left a voicemail asking, what happened? I haven’t heard from you all weekend. Then there was the obligatory run on the treadmill so the gooey cinnamon rolls the nurses baked and generously invited me to on Thursday would not stick around long enough for my husband to wonder if this was the beginning of me letting myself go. Isn’t that a lovely phrase?
Monday was Monday. How does anyone get anything done on Mondays? I had a new team, two new patients to learn and discharge. Plus, it was the first day that cracked 50 degrees in 5 months. I had to meet up with a friend, grab some coffee, and gossip walk around the lake. This was before we found out another friend was being slammed with consults in the emergency room. So there I was right back at the hospital Monday night with a cream cheese cherry pastry to cheer up my compatriot in the struggle.
This brings me to Tuesday. I had planned to be at the editing stage of this article on Tuesday. But didactics ran long due to everyone being so engaged in our formulations lecture, I didn’t have a shot at looking at this thing until lunchtime. Lunchtime came, and as I opened Microsoft Word among my dollar turkey sandwich and mini Purell bottles stationed around me like glorious little sergeants, I heard the gingerly utterings of a medical student: Um, if you have a moment, could you tell me the difference between the side-effect profile of first-generation and second-generation antipsychotics?
An hour later, I was informed that an admit was on the way and was traveling from out of state, set to arrive a half-hour before shift’s end. Did I mention he arrived with two family members in tow who wanted to talk about how things went wrong starting 20 years ago? Then there was the patient to see who I knew would pout if I didn’t spend at least a half-hour checking in. You know, the one the nurses always try to save me from even though I secretly never wanted to be saved.
I finally drove home 2 hours later than anticipated with a smile on my face. I should repeat that, WITH A SMILE ON MY FACE. I felt good because I’d done good. After all, there’s even a little sunlight left. When I walk in the front door, I kiss my husband and then immediately delve into a new story from the day. We laugh. We warm up leftovers, sit on the couch with our bare feet on the table, and catch an hour of American Idol (talent never gets old). Then it’s time to meet this maker.
The strange thing is, the person who began this column with all of her well-intentioned plans feels very different from the person who has made it to the deadline. There is a whole life lived in between. All of the readings I had done, notations I had made, seem kind of beside the point. I could pepper you with statistics and evidence-based outcomes warning of divorce, substance abuse, physician suicide, patient errors, and the like, which are all very real outcomes of poorly balanced lives. But I think we know all of that. It’s the in between space, the living part where so many of us lose our way. So instead of referenced journals, I offer you my journey. Because I can truly say that for the last 3 months of the most difficult year of residency, I have been happy. May this piece be also with you.
Dr. Schmidt, a second-year psychiatry resident at the Mayo Clinic in Rochester, Minn., is interested in psychodynamic therapy and in pursuing a fellowship in addictions. After obtaining a bachelor of arts at the University of California, Berkeley, she earned a master of arts degree in philosophy and humanities at the University of Chicago. She attended medical school at the University of Illinois College of Medicine at Peoria.
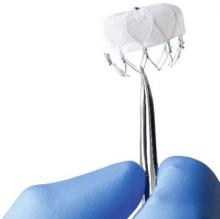
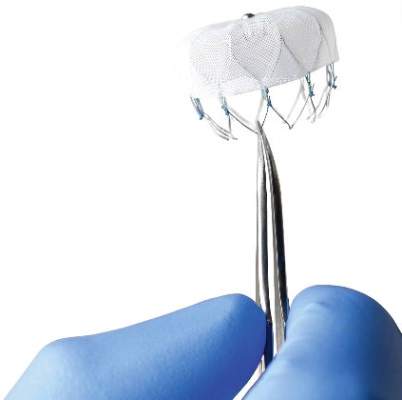
Novel Watchman device approved as warfarin alternative in atrial fib
The Watchman left atrial appendage (LAA) closure device has been approved in the United States as an alternative to warfarin for patients with nonvalvular atrial fibrillation, for a narrower indication than the one submitted for approval to the Food and Drug Administration.
The device is a percutaneously delivered permanent cardiac implant placed in the LAA to prevent the embolization of thrombi formed in the LAA, and is manufactured by Boston Scientific. The FDA approved the Watchman for reducing the risk of thromboembolism from the LAA in patients with nonvalvular atrial fibrillation “who are at increased risk for stroke and systemic embolism based on CHADS2 or CHA2DS2-VASc scores, are deemed by their physicians to be suitable for warfarin; and have an appropriate rationale to seek a nonpharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device, compared to warfarin,” according to a statement issued by the company on March 13.
The approved indication is worded differently than the proposed indication that was submitted to the FDA for approval and discussed at an FDA panel meeting in October, to “prevent thromboembolism from the left atrial appendage.” The changes in the indication include the replacement of “prevent” with “reduce the risk” of thromboembolism, and the addition of the following qualifiers: In patients who “are deemed by their physicians to be suitable for warfarin,” and who have “an appropriate rationale to seek a nonpharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device compared to warfarin.”
“These changes were made to more accurately reflect the appropriate patient population for this device,” according to an FDA spokesperson.
At a meeting in October 2014, the FDA’s Circulatory System Devices Panel voted 6-5 with one abstention that the benefits of the device outweighed its risks for the proposed indication, but several panelists who voted no said they would support approval of a second-line indication. In addition, panelists voting on both sides of this question said that the indication was too broad and should be revised to describe the device as a second-line alternative to warfarin, making clear it is not appropriate for all warfarin-eligible patients. (At the meeting, the panel unanimously agreed that there was “reasonable assurance” that the device was safe for use in this population.)
At the first advisory panel meeting on the device, in December 2013, the panel voted 13-1 to recommend approval, based on data from the PREVAIL and PROTECT-AF studies, which compared the device to chronic warfarin, and information from the Continued Access to PREVAIL (CAP2) registry.PREVAIL compared implantation of the device – with 45 days of warfarin plus 81 mg of aspirin for 45 days, followed by 325 mg of aspirin and 75 mg of clopidogrel through 6 months, followed by 325 mg of aspirin a day indefinitely – to chronic warfarin.
The October meeting was convened by the FDA to review longer follow-up data from PREVAIL, which found additional cases of ischemic strokes in the Watchman group and none in the warfarin-treated group.
The Watchman device has been available outside of the United States since 2009, is registered in 75 countries, and has been used to treat more than 10,000 patients, according to Boston Scientific.
The Watchman left atrial appendage (LAA) closure device has been approved in the United States as an alternative to warfarin for patients with nonvalvular atrial fibrillation, for a narrower indication than the one submitted for approval to the Food and Drug Administration.
The device is a percutaneously delivered permanent cardiac implant placed in the LAA to prevent the embolization of thrombi formed in the LAA, and is manufactured by Boston Scientific. The FDA approved the Watchman for reducing the risk of thromboembolism from the LAA in patients with nonvalvular atrial fibrillation “who are at increased risk for stroke and systemic embolism based on CHADS2 or CHA2DS2-VASc scores, are deemed by their physicians to be suitable for warfarin; and have an appropriate rationale to seek a nonpharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device, compared to warfarin,” according to a statement issued by the company on March 13.
The approved indication is worded differently than the proposed indication that was submitted to the FDA for approval and discussed at an FDA panel meeting in October, to “prevent thromboembolism from the left atrial appendage.” The changes in the indication include the replacement of “prevent” with “reduce the risk” of thromboembolism, and the addition of the following qualifiers: In patients who “are deemed by their physicians to be suitable for warfarin,” and who have “an appropriate rationale to seek a nonpharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device compared to warfarin.”
“These changes were made to more accurately reflect the appropriate patient population for this device,” according to an FDA spokesperson.
At a meeting in October 2014, the FDA’s Circulatory System Devices Panel voted 6-5 with one abstention that the benefits of the device outweighed its risks for the proposed indication, but several panelists who voted no said they would support approval of a second-line indication. In addition, panelists voting on both sides of this question said that the indication was too broad and should be revised to describe the device as a second-line alternative to warfarin, making clear it is not appropriate for all warfarin-eligible patients. (At the meeting, the panel unanimously agreed that there was “reasonable assurance” that the device was safe for use in this population.)
At the first advisory panel meeting on the device, in December 2013, the panel voted 13-1 to recommend approval, based on data from the PREVAIL and PROTECT-AF studies, which compared the device to chronic warfarin, and information from the Continued Access to PREVAIL (CAP2) registry.PREVAIL compared implantation of the device – with 45 days of warfarin plus 81 mg of aspirin for 45 days, followed by 325 mg of aspirin and 75 mg of clopidogrel through 6 months, followed by 325 mg of aspirin a day indefinitely – to chronic warfarin.
The October meeting was convened by the FDA to review longer follow-up data from PREVAIL, which found additional cases of ischemic strokes in the Watchman group and none in the warfarin-treated group.
The Watchman device has been available outside of the United States since 2009, is registered in 75 countries, and has been used to treat more than 10,000 patients, according to Boston Scientific.
The Watchman left atrial appendage (LAA) closure device has been approved in the United States as an alternative to warfarin for patients with nonvalvular atrial fibrillation, for a narrower indication than the one submitted for approval to the Food and Drug Administration.
The device is a percutaneously delivered permanent cardiac implant placed in the LAA to prevent the embolization of thrombi formed in the LAA, and is manufactured by Boston Scientific. The FDA approved the Watchman for reducing the risk of thromboembolism from the LAA in patients with nonvalvular atrial fibrillation “who are at increased risk for stroke and systemic embolism based on CHADS2 or CHA2DS2-VASc scores, are deemed by their physicians to be suitable for warfarin; and have an appropriate rationale to seek a nonpharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device, compared to warfarin,” according to a statement issued by the company on March 13.
The approved indication is worded differently than the proposed indication that was submitted to the FDA for approval and discussed at an FDA panel meeting in October, to “prevent thromboembolism from the left atrial appendage.” The changes in the indication include the replacement of “prevent” with “reduce the risk” of thromboembolism, and the addition of the following qualifiers: In patients who “are deemed by their physicians to be suitable for warfarin,” and who have “an appropriate rationale to seek a nonpharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device compared to warfarin.”
“These changes were made to more accurately reflect the appropriate patient population for this device,” according to an FDA spokesperson.
At a meeting in October 2014, the FDA’s Circulatory System Devices Panel voted 6-5 with one abstention that the benefits of the device outweighed its risks for the proposed indication, but several panelists who voted no said they would support approval of a second-line indication. In addition, panelists voting on both sides of this question said that the indication was too broad and should be revised to describe the device as a second-line alternative to warfarin, making clear it is not appropriate for all warfarin-eligible patients. (At the meeting, the panel unanimously agreed that there was “reasonable assurance” that the device was safe for use in this population.)
At the first advisory panel meeting on the device, in December 2013, the panel voted 13-1 to recommend approval, based on data from the PREVAIL and PROTECT-AF studies, which compared the device to chronic warfarin, and information from the Continued Access to PREVAIL (CAP2) registry.PREVAIL compared implantation of the device – with 45 days of warfarin plus 81 mg of aspirin for 45 days, followed by 325 mg of aspirin and 75 mg of clopidogrel through 6 months, followed by 325 mg of aspirin a day indefinitely – to chronic warfarin.
The October meeting was convened by the FDA to review longer follow-up data from PREVAIL, which found additional cases of ischemic strokes in the Watchman group and none in the warfarin-treated group.
The Watchman device has been available outside of the United States since 2009, is registered in 75 countries, and has been used to treat more than 10,000 patients, according to Boston Scientific.
Apple’s ResearchKit
Doctors have been conjecturing about how the new Apple Watch, with its spectacular fitness and wellness tracking features, will transform health care. The real rock star at Apple’s March 9 “Spring Forward” event, however, was the opening band, ResearchKit.
What is it?
ResearchKit is Apple’s (beautiful) solution to one of the great problems of medical research: recruiting subjects. ResearchKit allows researchers to collect data in a way that before today was impossible: with just a click from their smartphones. The open-source software platform allows developers to design studies and to recruit subjects right from the app store. Researchers can leverage high-tech smartphone sensors and can push out surveys, collecting both objective and subjective data from thousands (heck, potentially millions) of participants.
Five apps were developed for the launch: mPower for Parkinson’s disease, from the University of Rochester, N.Y.; GlucoSuccess for diabetes, from Massachusetts General Hospital, Boston; MyHeart Counts for cardiovascular disease, from Stanford (Calif.) University and the University of Oxford, England; Asthma Health from Mount Sinai and Weill Medical College of Cornell University, New York, N.Y.; and Share the Journey for breast cancer, from the Dana-Farber Cancer Institute, Boston; the University of California, Los Angeles Fielding School of Public Health; and Penn Medicine, Philadelphia.
My take
I took a closer look at MyHeart Counts, which evaluates how patients’ activity levels influence their cardiovascular health. According to Stanford University, a mere 4 days after its release, the MyHeart Counts app had been downloaded 52,900 times in the United States and Canada and had more than 22,000 users who had consented to the study. Try getting that kind of response to your research study with a flyer with tear-off phone number posted in your hospital cafeteria.
I was impressed with its beautiful interface and ease of use. Designed to gather sensor and health data from your iPhone and personal devices, this app is designed to help researchers (and you) detect patterns or details about your heart health. To start, you download the app, give your consent, answer questions about your health and lifestyle, and begin recording your activity with your phone or wearable device. You do a walk test to determine your heart health and potential health risk.
What happens to the data you input? It is sent (with your permission) to a secure database, and your name is replaced with a random code. Your coded and encrypted data are then shared with scientists and physicians to use in medical research.
For this particular study, they ask you to participate 10-15 minutes per day for 1 week, then hope that you can contribute further for 1 week every 3 months answering surveys about your health, lifestyle, and physical activity. Apple reassures users that they can withdraw at any time.
Why? Who cares?
The value proposition for researchers is obvious: The platform provides access to many more subjects than even imaginable. The accelerometer, barometer, gyroscope, and GPS send interesting data to researchers friction free. The Parkinson’s app, for example, uses a cool algorithm and the phone’s microphone to detect symptoms by having patients say “ahhhh.” By pushing out questionnaires regularly, you can collect much more data with shorter intervals for longer periods of time.
The advantages for patients are equally compelling. In addition to sending their data to researchers, they also receive information back from the researchers, helping them monitor their cardiovascular health. In fact, just knowing they are participating in the study might be of benefit. As dermatologist Dr. Steve Feldman of Wake Forest Baptist Medical Center, Winston Salem, N.C., has shown, patients are more likely to adhere to therapies when they know they are being watched, a manifestation of the Hawthorne effect.
Shortcomings
Surely there is a catch? And there is. With potentially millions of participants sending self-reported data, there is the potential that ResearchKit studies glean big, beautiful, bad data. How, for example, could you verify that self-reported asthma patients actually have asthma? Maybe they just read about ResearchKit and wanted to be part of the fun.
For patients, privacy concerns are paramount. Apple promised that no one, not even Apple, will see your data without your permission. But with privacy breaches reported in the news weekly, what can Apple’s assurance mean? Didn’t Target and Aetna promise to keep your data safe as well?
The potential for interesting research is enormous. By the time you read this, I wouldn’t be surprised if a psoriasis study had already launched. In fact, a year from now, the problem might be a dozen or more interesting psoriasis studies all competing for the same patients. Ah, maybe we should be glad if we should be so lucky.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego, and volunteer clinical assistant professor at the University of California, San Diego. Dr. Benabio is @dermdoc on Twitter.
Doctors have been conjecturing about how the new Apple Watch, with its spectacular fitness and wellness tracking features, will transform health care. The real rock star at Apple’s March 9 “Spring Forward” event, however, was the opening band, ResearchKit.
What is it?
ResearchKit is Apple’s (beautiful) solution to one of the great problems of medical research: recruiting subjects. ResearchKit allows researchers to collect data in a way that before today was impossible: with just a click from their smartphones. The open-source software platform allows developers to design studies and to recruit subjects right from the app store. Researchers can leverage high-tech smartphone sensors and can push out surveys, collecting both objective and subjective data from thousands (heck, potentially millions) of participants.
Five apps were developed for the launch: mPower for Parkinson’s disease, from the University of Rochester, N.Y.; GlucoSuccess for diabetes, from Massachusetts General Hospital, Boston; MyHeart Counts for cardiovascular disease, from Stanford (Calif.) University and the University of Oxford, England; Asthma Health from Mount Sinai and Weill Medical College of Cornell University, New York, N.Y.; and Share the Journey for breast cancer, from the Dana-Farber Cancer Institute, Boston; the University of California, Los Angeles Fielding School of Public Health; and Penn Medicine, Philadelphia.
My take
I took a closer look at MyHeart Counts, which evaluates how patients’ activity levels influence their cardiovascular health. According to Stanford University, a mere 4 days after its release, the MyHeart Counts app had been downloaded 52,900 times in the United States and Canada and had more than 22,000 users who had consented to the study. Try getting that kind of response to your research study with a flyer with tear-off phone number posted in your hospital cafeteria.
I was impressed with its beautiful interface and ease of use. Designed to gather sensor and health data from your iPhone and personal devices, this app is designed to help researchers (and you) detect patterns or details about your heart health. To start, you download the app, give your consent, answer questions about your health and lifestyle, and begin recording your activity with your phone or wearable device. You do a walk test to determine your heart health and potential health risk.
What happens to the data you input? It is sent (with your permission) to a secure database, and your name is replaced with a random code. Your coded and encrypted data are then shared with scientists and physicians to use in medical research.
For this particular study, they ask you to participate 10-15 minutes per day for 1 week, then hope that you can contribute further for 1 week every 3 months answering surveys about your health, lifestyle, and physical activity. Apple reassures users that they can withdraw at any time.
Why? Who cares?
The value proposition for researchers is obvious: The platform provides access to many more subjects than even imaginable. The accelerometer, barometer, gyroscope, and GPS send interesting data to researchers friction free. The Parkinson’s app, for example, uses a cool algorithm and the phone’s microphone to detect symptoms by having patients say “ahhhh.” By pushing out questionnaires regularly, you can collect much more data with shorter intervals for longer periods of time.
The advantages for patients are equally compelling. In addition to sending their data to researchers, they also receive information back from the researchers, helping them monitor their cardiovascular health. In fact, just knowing they are participating in the study might be of benefit. As dermatologist Dr. Steve Feldman of Wake Forest Baptist Medical Center, Winston Salem, N.C., has shown, patients are more likely to adhere to therapies when they know they are being watched, a manifestation of the Hawthorne effect.
Shortcomings
Surely there is a catch? And there is. With potentially millions of participants sending self-reported data, there is the potential that ResearchKit studies glean big, beautiful, bad data. How, for example, could you verify that self-reported asthma patients actually have asthma? Maybe they just read about ResearchKit and wanted to be part of the fun.
For patients, privacy concerns are paramount. Apple promised that no one, not even Apple, will see your data without your permission. But with privacy breaches reported in the news weekly, what can Apple’s assurance mean? Didn’t Target and Aetna promise to keep your data safe as well?
The potential for interesting research is enormous. By the time you read this, I wouldn’t be surprised if a psoriasis study had already launched. In fact, a year from now, the problem might be a dozen or more interesting psoriasis studies all competing for the same patients. Ah, maybe we should be glad if we should be so lucky.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego, and volunteer clinical assistant professor at the University of California, San Diego. Dr. Benabio is @dermdoc on Twitter.
Doctors have been conjecturing about how the new Apple Watch, with its spectacular fitness and wellness tracking features, will transform health care. The real rock star at Apple’s March 9 “Spring Forward” event, however, was the opening band, ResearchKit.
What is it?
ResearchKit is Apple’s (beautiful) solution to one of the great problems of medical research: recruiting subjects. ResearchKit allows researchers to collect data in a way that before today was impossible: with just a click from their smartphones. The open-source software platform allows developers to design studies and to recruit subjects right from the app store. Researchers can leverage high-tech smartphone sensors and can push out surveys, collecting both objective and subjective data from thousands (heck, potentially millions) of participants.
Five apps were developed for the launch: mPower for Parkinson’s disease, from the University of Rochester, N.Y.; GlucoSuccess for diabetes, from Massachusetts General Hospital, Boston; MyHeart Counts for cardiovascular disease, from Stanford (Calif.) University and the University of Oxford, England; Asthma Health from Mount Sinai and Weill Medical College of Cornell University, New York, N.Y.; and Share the Journey for breast cancer, from the Dana-Farber Cancer Institute, Boston; the University of California, Los Angeles Fielding School of Public Health; and Penn Medicine, Philadelphia.
My take
I took a closer look at MyHeart Counts, which evaluates how patients’ activity levels influence their cardiovascular health. According to Stanford University, a mere 4 days after its release, the MyHeart Counts app had been downloaded 52,900 times in the United States and Canada and had more than 22,000 users who had consented to the study. Try getting that kind of response to your research study with a flyer with tear-off phone number posted in your hospital cafeteria.
I was impressed with its beautiful interface and ease of use. Designed to gather sensor and health data from your iPhone and personal devices, this app is designed to help researchers (and you) detect patterns or details about your heart health. To start, you download the app, give your consent, answer questions about your health and lifestyle, and begin recording your activity with your phone or wearable device. You do a walk test to determine your heart health and potential health risk.
What happens to the data you input? It is sent (with your permission) to a secure database, and your name is replaced with a random code. Your coded and encrypted data are then shared with scientists and physicians to use in medical research.
For this particular study, they ask you to participate 10-15 minutes per day for 1 week, then hope that you can contribute further for 1 week every 3 months answering surveys about your health, lifestyle, and physical activity. Apple reassures users that they can withdraw at any time.
Why? Who cares?
The value proposition for researchers is obvious: The platform provides access to many more subjects than even imaginable. The accelerometer, barometer, gyroscope, and GPS send interesting data to researchers friction free. The Parkinson’s app, for example, uses a cool algorithm and the phone’s microphone to detect symptoms by having patients say “ahhhh.” By pushing out questionnaires regularly, you can collect much more data with shorter intervals for longer periods of time.
The advantages for patients are equally compelling. In addition to sending their data to researchers, they also receive information back from the researchers, helping them monitor their cardiovascular health. In fact, just knowing they are participating in the study might be of benefit. As dermatologist Dr. Steve Feldman of Wake Forest Baptist Medical Center, Winston Salem, N.C., has shown, patients are more likely to adhere to therapies when they know they are being watched, a manifestation of the Hawthorne effect.
Shortcomings
Surely there is a catch? And there is. With potentially millions of participants sending self-reported data, there is the potential that ResearchKit studies glean big, beautiful, bad data. How, for example, could you verify that self-reported asthma patients actually have asthma? Maybe they just read about ResearchKit and wanted to be part of the fun.
For patients, privacy concerns are paramount. Apple promised that no one, not even Apple, will see your data without your permission. But with privacy breaches reported in the news weekly, what can Apple’s assurance mean? Didn’t Target and Aetna promise to keep your data safe as well?
The potential for interesting research is enormous. By the time you read this, I wouldn’t be surprised if a psoriasis study had already launched. In fact, a year from now, the problem might be a dozen or more interesting psoriasis studies all competing for the same patients. Ah, maybe we should be glad if we should be so lucky.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego, and volunteer clinical assistant professor at the University of California, San Diego. Dr. Benabio is @dermdoc on Twitter.
Addressing pain at the end of life
A few months ago, a colleague asked me about treating a patient’s pain that he was managing for months both in and out of the hospital for what was now an incurable condition. This very skilled surgeon believed that the patient should “not require” such high doses of opioids based on the clinical picture of a healed surgical wound but felt at a loss of what else to do. He did not want to abandon his relationship with the patient. He considered referral to the anesthesia pain clinic as escalating pain requirements were exceeding his comfort level.
Alternatively, he considered deferring pain management to the patient’s primary care provider. Instead, we worked together through a rational pain approach and explored external factors that may have been contributing to the patient’s total pain experience. This brief vignette is not atypical and sheds light onto the ongoing need to fill an education gap for surgeons who deal with patients at the end of life.
It has been almost 25 years since the term “pain as the fifth vital sign” was first introduced into the lexicon of clinical practice. The idea was to provide as much zeal to the topic of pain as we do to a patient’s other vital physiological measures. Yet, seriously ill patients with potential life-limiting conditions continue to experience significant pain, especially at the end of life. Among patients with nonmalignant diagnoses, more than 40% experience severe pain within days of their death. For those with malignant conditions, 15%-75% report moderate to severe pain during the final weeks of life. Whether in the ICU, hospital ward, or outpatient setting, our surgical community struggles to provide effective symptomatic pain control in many patients who have transitioned from a curative pathway to one of comfort.
Although we never intend to allow patients to suffer at the end of life, barriers to appropriate pain control persist. In some case cases, patients may feel embarrassed or ashamed to accept escalating opioid doses. In other cases, patients and families may possess misconceptions about addiction to pain medication. It is important to dispel such myths and distinguish tolerance from dependence. Among opioid-naive patients, the risk of dependence (in other words, addiction) is estimated to be 0.1%. Among patients with a history of opioid abuse, the risk of addiction is still only 1%.
Large proportions of physicians continue to report inadequate training in pain control and are reluctant to prescribe high-enough doses of opioids to relieve pain, even at the end of life. One well-described reason has been physician fear of regulatory action and possible litigation for higher than typical opioid dosing.
This was the case for my colleague who was reluctant to escalate pain control.
This in turn leads to undertreating pain which, in fact, has been a source of successful litigation. Because undertreatment of pain may be akin to patient negligence, we should strive to become more comfortable with optimal pain treatment strategies. But pain control is not merely about intravenous opioids or pain tablets. Surgeons should at least have an appreciation for, if not a better understanding, of the modern palliative care approach to “total pain.” This construct consists of four interrelated pain domains: physical, psychological (emotional), spiritual, and social.
Although we tend to focus on physical pain, other domains are influenced by anxiety, depression, and fear. If such an approach seems a bridge too far, optimal care should involve a multidisciplinary team that touches on such areas. This may be most efficiently achieved through consultation and coordination with palliative care services when available. This patient’s surgeon soon discovered that family financial concerns were contributing to the patient’s sleepless nights and worsening somatic pain.
Somewhat outside the scope of typical postoperative care, pain relief at the end of life requires dosing and medication choices for extended periods of time. When establishing a treatment strategy, the surgeon should consider the feasibility and efficacy (half-life, duration, bioavailability, active metabolites) of each modality. In our patient, standard dosing was inadequate; for some, basal doses may increase by 25%-100% for progressive disease. To support the surgeon in learning more about this important area of care, multiple online tools and websites are available to assist with pain management choices. A short while ago, I learned from my colleague that this patient died comfortably and essentially pain free for the last months of his life.
Dr. Zonies is an associate professor of surgery in the trauma/critical care division at Oregon Health & Science University, Portland. He is board certified in hospice and palliative medicine.
A few months ago, a colleague asked me about treating a patient’s pain that he was managing for months both in and out of the hospital for what was now an incurable condition. This very skilled surgeon believed that the patient should “not require” such high doses of opioids based on the clinical picture of a healed surgical wound but felt at a loss of what else to do. He did not want to abandon his relationship with the patient. He considered referral to the anesthesia pain clinic as escalating pain requirements were exceeding his comfort level.
Alternatively, he considered deferring pain management to the patient’s primary care provider. Instead, we worked together through a rational pain approach and explored external factors that may have been contributing to the patient’s total pain experience. This brief vignette is not atypical and sheds light onto the ongoing need to fill an education gap for surgeons who deal with patients at the end of life.
It has been almost 25 years since the term “pain as the fifth vital sign” was first introduced into the lexicon of clinical practice. The idea was to provide as much zeal to the topic of pain as we do to a patient’s other vital physiological measures. Yet, seriously ill patients with potential life-limiting conditions continue to experience significant pain, especially at the end of life. Among patients with nonmalignant diagnoses, more than 40% experience severe pain within days of their death. For those with malignant conditions, 15%-75% report moderate to severe pain during the final weeks of life. Whether in the ICU, hospital ward, or outpatient setting, our surgical community struggles to provide effective symptomatic pain control in many patients who have transitioned from a curative pathway to one of comfort.
Although we never intend to allow patients to suffer at the end of life, barriers to appropriate pain control persist. In some case cases, patients may feel embarrassed or ashamed to accept escalating opioid doses. In other cases, patients and families may possess misconceptions about addiction to pain medication. It is important to dispel such myths and distinguish tolerance from dependence. Among opioid-naive patients, the risk of dependence (in other words, addiction) is estimated to be 0.1%. Among patients with a history of opioid abuse, the risk of addiction is still only 1%.
Large proportions of physicians continue to report inadequate training in pain control and are reluctant to prescribe high-enough doses of opioids to relieve pain, even at the end of life. One well-described reason has been physician fear of regulatory action and possible litigation for higher than typical opioid dosing.
This was the case for my colleague who was reluctant to escalate pain control.
This in turn leads to undertreating pain which, in fact, has been a source of successful litigation. Because undertreatment of pain may be akin to patient negligence, we should strive to become more comfortable with optimal pain treatment strategies. But pain control is not merely about intravenous opioids or pain tablets. Surgeons should at least have an appreciation for, if not a better understanding, of the modern palliative care approach to “total pain.” This construct consists of four interrelated pain domains: physical, psychological (emotional), spiritual, and social.
Although we tend to focus on physical pain, other domains are influenced by anxiety, depression, and fear. If such an approach seems a bridge too far, optimal care should involve a multidisciplinary team that touches on such areas. This may be most efficiently achieved through consultation and coordination with palliative care services when available. This patient’s surgeon soon discovered that family financial concerns were contributing to the patient’s sleepless nights and worsening somatic pain.
Somewhat outside the scope of typical postoperative care, pain relief at the end of life requires dosing and medication choices for extended periods of time. When establishing a treatment strategy, the surgeon should consider the feasibility and efficacy (half-life, duration, bioavailability, active metabolites) of each modality. In our patient, standard dosing was inadequate; for some, basal doses may increase by 25%-100% for progressive disease. To support the surgeon in learning more about this important area of care, multiple online tools and websites are available to assist with pain management choices. A short while ago, I learned from my colleague that this patient died comfortably and essentially pain free for the last months of his life.
Dr. Zonies is an associate professor of surgery in the trauma/critical care division at Oregon Health & Science University, Portland. He is board certified in hospice and palliative medicine.
A few months ago, a colleague asked me about treating a patient’s pain that he was managing for months both in and out of the hospital for what was now an incurable condition. This very skilled surgeon believed that the patient should “not require” such high doses of opioids based on the clinical picture of a healed surgical wound but felt at a loss of what else to do. He did not want to abandon his relationship with the patient. He considered referral to the anesthesia pain clinic as escalating pain requirements were exceeding his comfort level.
Alternatively, he considered deferring pain management to the patient’s primary care provider. Instead, we worked together through a rational pain approach and explored external factors that may have been contributing to the patient’s total pain experience. This brief vignette is not atypical and sheds light onto the ongoing need to fill an education gap for surgeons who deal with patients at the end of life.
It has been almost 25 years since the term “pain as the fifth vital sign” was first introduced into the lexicon of clinical practice. The idea was to provide as much zeal to the topic of pain as we do to a patient’s other vital physiological measures. Yet, seriously ill patients with potential life-limiting conditions continue to experience significant pain, especially at the end of life. Among patients with nonmalignant diagnoses, more than 40% experience severe pain within days of their death. For those with malignant conditions, 15%-75% report moderate to severe pain during the final weeks of life. Whether in the ICU, hospital ward, or outpatient setting, our surgical community struggles to provide effective symptomatic pain control in many patients who have transitioned from a curative pathway to one of comfort.
Although we never intend to allow patients to suffer at the end of life, barriers to appropriate pain control persist. In some case cases, patients may feel embarrassed or ashamed to accept escalating opioid doses. In other cases, patients and families may possess misconceptions about addiction to pain medication. It is important to dispel such myths and distinguish tolerance from dependence. Among opioid-naive patients, the risk of dependence (in other words, addiction) is estimated to be 0.1%. Among patients with a history of opioid abuse, the risk of addiction is still only 1%.
Large proportions of physicians continue to report inadequate training in pain control and are reluctant to prescribe high-enough doses of opioids to relieve pain, even at the end of life. One well-described reason has been physician fear of regulatory action and possible litigation for higher than typical opioid dosing.
This was the case for my colleague who was reluctant to escalate pain control.
This in turn leads to undertreating pain which, in fact, has been a source of successful litigation. Because undertreatment of pain may be akin to patient negligence, we should strive to become more comfortable with optimal pain treatment strategies. But pain control is not merely about intravenous opioids or pain tablets. Surgeons should at least have an appreciation for, if not a better understanding, of the modern palliative care approach to “total pain.” This construct consists of four interrelated pain domains: physical, psychological (emotional), spiritual, and social.
Although we tend to focus on physical pain, other domains are influenced by anxiety, depression, and fear. If such an approach seems a bridge too far, optimal care should involve a multidisciplinary team that touches on such areas. This may be most efficiently achieved through consultation and coordination with palliative care services when available. This patient’s surgeon soon discovered that family financial concerns were contributing to the patient’s sleepless nights and worsening somatic pain.
Somewhat outside the scope of typical postoperative care, pain relief at the end of life requires dosing and medication choices for extended periods of time. When establishing a treatment strategy, the surgeon should consider the feasibility and efficacy (half-life, duration, bioavailability, active metabolites) of each modality. In our patient, standard dosing was inadequate; for some, basal doses may increase by 25%-100% for progressive disease. To support the surgeon in learning more about this important area of care, multiple online tools and websites are available to assist with pain management choices. A short while ago, I learned from my colleague that this patient died comfortably and essentially pain free for the last months of his life.
Dr. Zonies is an associate professor of surgery in the trauma/critical care division at Oregon Health & Science University, Portland. He is board certified in hospice and palliative medicine.
Sharpening the saw
Recently, I wrote that springtime is an excellent time to spruce up your office, to check your equipment for malfunctions, to resharpen your curettes and scissors, and to back up your computer files and upgrade software. More important than any of that, though, is reevaluating your most important asset: yourself.
I write this reminder every couple of years because it’s so easy to lose sight of the big picture among the pressures of our daily routines. Sooner or later, no matter how dedicated we are, the grind gets to all of us, leading to fatigue, irritability, and a progressive decline in motivation. And we are too busy to sit down and think about what we might do to break that vicious cycle. This is detrimental to our own well-being, as well as that of our patients.
There are many ways to maintain your intellectual and emotional health, but here’s how I do it: I take individual days off (average of 1 a month) to catch up on journals or taking a CME course; or to try something new – something I’ve been thinking about doing “someday, when there is time” – such as a piano or sailing lesson; or a long weekend away with my wife. And I take no less than 4 weeks vacation per year.
I know how some of you feel about “wasting” a workday. Vacations are even worse, because patients might go elsewhere while we’re gone, and every day the office is idle we “lose money.”
That whole paradigm is wrong. Stop thinking day to day; think year to year instead. You bring in a given amount of revenue per year – more on some days, less on other days, none on weekends and vacation days; it all averages out in the end.
Besides, this is much more important than money. This is breaking the routine, clearing the cobwebs, living your life. Trust me – your practice will still be there when you return.
Last month, my wife and I hiked up a mountain in the Himalayas to the fabled Tiger’s Nest Monastery in Bhutan. As I huffed and puffed up the trail, I didn’t have the time – or the slightest inclination – to worry about the office. When the trek was over, I returned ready to take on the world, and my practice, anew.
And I jotted down some great ideas – practical, medical, and literary. Original thoughts are hard to come by during the daily grind, but they often appear, unannounced, in a new and refreshing environment.
Creative people have long recognized the value of “sharpening the saw.” A classic example is the oft-told story of Swiss physicist K. Alex Müller and German physicist J. Georg Bednorz. In 1986, they reached a major impasse in their superconductivity research; it appeared 2 decades of work might be for naught. The harder they pressed, the more elusive the answer became. So Müller decided to take some time off, put aside his troubles, and research a subject that had always interested him: ceramics.
Nothing could have been further from his research field, of course, since ceramics are among the poorest conductors known. Yet, as he relaxed, it occurred to Müller that a unique property of ceramics might apply to their project. Back in the lab, the team created a ceramic compound that became the first successful “high-temperature” superconductor.
The rest, as they say, is history; Müller and Bednorz won a Nobel Prize and triggered an explosion of research leading to breakthroughs in computing, electricity transmission, magnetically levitated trains, and many other applications that are still being realized.
Sharpening your saw may not change the world, but it will change you; any nudge out of your comfort zone will give you fresh ideas and help you look at the same old problems in completely new ways.
And to those who still can’t bear the thought of taking time off, remember Eastern’s First Law: Your last words will NOT be, “I wish I had spent more time in the office!”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News.
Recently, I wrote that springtime is an excellent time to spruce up your office, to check your equipment for malfunctions, to resharpen your curettes and scissors, and to back up your computer files and upgrade software. More important than any of that, though, is reevaluating your most important asset: yourself.
I write this reminder every couple of years because it’s so easy to lose sight of the big picture among the pressures of our daily routines. Sooner or later, no matter how dedicated we are, the grind gets to all of us, leading to fatigue, irritability, and a progressive decline in motivation. And we are too busy to sit down and think about what we might do to break that vicious cycle. This is detrimental to our own well-being, as well as that of our patients.
There are many ways to maintain your intellectual and emotional health, but here’s how I do it: I take individual days off (average of 1 a month) to catch up on journals or taking a CME course; or to try something new – something I’ve been thinking about doing “someday, when there is time” – such as a piano or sailing lesson; or a long weekend away with my wife. And I take no less than 4 weeks vacation per year.
I know how some of you feel about “wasting” a workday. Vacations are even worse, because patients might go elsewhere while we’re gone, and every day the office is idle we “lose money.”
That whole paradigm is wrong. Stop thinking day to day; think year to year instead. You bring in a given amount of revenue per year – more on some days, less on other days, none on weekends and vacation days; it all averages out in the end.
Besides, this is much more important than money. This is breaking the routine, clearing the cobwebs, living your life. Trust me – your practice will still be there when you return.
Last month, my wife and I hiked up a mountain in the Himalayas to the fabled Tiger’s Nest Monastery in Bhutan. As I huffed and puffed up the trail, I didn’t have the time – or the slightest inclination – to worry about the office. When the trek was over, I returned ready to take on the world, and my practice, anew.
And I jotted down some great ideas – practical, medical, and literary. Original thoughts are hard to come by during the daily grind, but they often appear, unannounced, in a new and refreshing environment.
Creative people have long recognized the value of “sharpening the saw.” A classic example is the oft-told story of Swiss physicist K. Alex Müller and German physicist J. Georg Bednorz. In 1986, they reached a major impasse in their superconductivity research; it appeared 2 decades of work might be for naught. The harder they pressed, the more elusive the answer became. So Müller decided to take some time off, put aside his troubles, and research a subject that had always interested him: ceramics.
Nothing could have been further from his research field, of course, since ceramics are among the poorest conductors known. Yet, as he relaxed, it occurred to Müller that a unique property of ceramics might apply to their project. Back in the lab, the team created a ceramic compound that became the first successful “high-temperature” superconductor.
The rest, as they say, is history; Müller and Bednorz won a Nobel Prize and triggered an explosion of research leading to breakthroughs in computing, electricity transmission, magnetically levitated trains, and many other applications that are still being realized.
Sharpening your saw may not change the world, but it will change you; any nudge out of your comfort zone will give you fresh ideas and help you look at the same old problems in completely new ways.
And to those who still can’t bear the thought of taking time off, remember Eastern’s First Law: Your last words will NOT be, “I wish I had spent more time in the office!”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News.
Recently, I wrote that springtime is an excellent time to spruce up your office, to check your equipment for malfunctions, to resharpen your curettes and scissors, and to back up your computer files and upgrade software. More important than any of that, though, is reevaluating your most important asset: yourself.
I write this reminder every couple of years because it’s so easy to lose sight of the big picture among the pressures of our daily routines. Sooner or later, no matter how dedicated we are, the grind gets to all of us, leading to fatigue, irritability, and a progressive decline in motivation. And we are too busy to sit down and think about what we might do to break that vicious cycle. This is detrimental to our own well-being, as well as that of our patients.
There are many ways to maintain your intellectual and emotional health, but here’s how I do it: I take individual days off (average of 1 a month) to catch up on journals or taking a CME course; or to try something new – something I’ve been thinking about doing “someday, when there is time” – such as a piano or sailing lesson; or a long weekend away with my wife. And I take no less than 4 weeks vacation per year.
I know how some of you feel about “wasting” a workday. Vacations are even worse, because patients might go elsewhere while we’re gone, and every day the office is idle we “lose money.”
That whole paradigm is wrong. Stop thinking day to day; think year to year instead. You bring in a given amount of revenue per year – more on some days, less on other days, none on weekends and vacation days; it all averages out in the end.
Besides, this is much more important than money. This is breaking the routine, clearing the cobwebs, living your life. Trust me – your practice will still be there when you return.
Last month, my wife and I hiked up a mountain in the Himalayas to the fabled Tiger’s Nest Monastery in Bhutan. As I huffed and puffed up the trail, I didn’t have the time – or the slightest inclination – to worry about the office. When the trek was over, I returned ready to take on the world, and my practice, anew.
And I jotted down some great ideas – practical, medical, and literary. Original thoughts are hard to come by during the daily grind, but they often appear, unannounced, in a new and refreshing environment.
Creative people have long recognized the value of “sharpening the saw.” A classic example is the oft-told story of Swiss physicist K. Alex Müller and German physicist J. Georg Bednorz. In 1986, they reached a major impasse in their superconductivity research; it appeared 2 decades of work might be for naught. The harder they pressed, the more elusive the answer became. So Müller decided to take some time off, put aside his troubles, and research a subject that had always interested him: ceramics.
Nothing could have been further from his research field, of course, since ceramics are among the poorest conductors known. Yet, as he relaxed, it occurred to Müller that a unique property of ceramics might apply to their project. Back in the lab, the team created a ceramic compound that became the first successful “high-temperature” superconductor.
The rest, as they say, is history; Müller and Bednorz won a Nobel Prize and triggered an explosion of research leading to breakthroughs in computing, electricity transmission, magnetically levitated trains, and many other applications that are still being realized.
Sharpening your saw may not change the world, but it will change you; any nudge out of your comfort zone will give you fresh ideas and help you look at the same old problems in completely new ways.
And to those who still can’t bear the thought of taking time off, remember Eastern’s First Law: Your last words will NOT be, “I wish I had spent more time in the office!”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News.