User login
Vitamin D levels linked to outcomes in FL

Vitamin D deficiency may negatively impact outcomes in patients with follicular lymphoma (FL), according to research published in the Journal of Clinical Oncology.
The study showed that FL patients with vitamin D deficiency had inferior progression-free survival (PFS) and overall survival (OS) compared to patients with higher vitamin D levels.
According to researchers, this suggests serum vitamin D might be the first potentially modifiable factor to be associated with survival in FL.
However, additional research is needed to determine the effects of vitamin D supplementation in these patients.
Jonathan W. Friedberg, MD, of the Wilmot Cancer Institute at the University of Rochester in New York, and his colleagues conducted this research, analyzing data from 2 cohorts of FL patients.
One cohort consisted of patients derived from 3 SWOG trials (S9800, S9911, and S0016), and the other consisted of patients from a Lymphoma Study Association (LYSA) trial known as PRIMA.
SWOG patients had received CHOP chemotherapy plus an anti-CD20 antibody (rituximab or iodine-131 tositumomab), and LYSA patients had received rituximab plus chemotherapy (and were randomized to rituximab maintenance or observation).
SWOG cohort
After a median follow-up of 5.4 years, patients with vitamin D deficiency (defined as <20 ng/mL) had significantly inferior PFS (hazard ratio [HR]=2.00; P=0.011) and OS (HR=3.57; P=0.003) compared to patients with higher vitamin D levels.
Results were similar when the researchers adjusted for other variables, such as prognostic index (IPI), body mass index, and latitude (≥ vs <35°N). For PFS, the adjusted HR was 1.97 (P=0.023). And for OS, the adjusted HR was 4.16 (P=0.002).
Multivariable analysis of vitamin D by tertile confirmed that the lowest tertile of vitamin D was associated with a greater increase in the risk of either progression or death, but neither result was significant.
LYSA cohort
After a median follow-up of 6.6 years, patients with vitamin D deficiency (defined as <10 ng/mL) had significantly inferior PFS (HR=1.66; P=0.013) but not OS (HR=1.84; P=0.14) compared to patients with higher vitamin D levels.
Results were similar when the researchers adjusted for other variables, such as prognostic index (FLIPI), body mass index, latitude (Europe vs Australia), and hemoglobin. For PFS, the adjusted HR was 1.50 (P=0.095). For OS, the adjusted HR was 1.92 (P=0.192).
Multivariable analysis of vitamin D by tertile confirmed that the lowest tertile of vitamin D was associated with a greater increase in the risk of either progression or death, but only the association with OS reached statistical significance (HR=5.32; P=0.037).
Dr Friedberg said that, taken together, these results suggest vitamin D levels may be a modifiable factor associated with prognosis in patients with FL.
“Our data, replicated internationally, supports other published observations linking vitamin D deficiency with inferior cancer outcomes,” he said. “However, the mechanisms of this link are likely complex and require further study.” ![]()

Vitamin D deficiency may negatively impact outcomes in patients with follicular lymphoma (FL), according to research published in the Journal of Clinical Oncology.
The study showed that FL patients with vitamin D deficiency had inferior progression-free survival (PFS) and overall survival (OS) compared to patients with higher vitamin D levels.
According to researchers, this suggests serum vitamin D might be the first potentially modifiable factor to be associated with survival in FL.
However, additional research is needed to determine the effects of vitamin D supplementation in these patients.
Jonathan W. Friedberg, MD, of the Wilmot Cancer Institute at the University of Rochester in New York, and his colleagues conducted this research, analyzing data from 2 cohorts of FL patients.
One cohort consisted of patients derived from 3 SWOG trials (S9800, S9911, and S0016), and the other consisted of patients from a Lymphoma Study Association (LYSA) trial known as PRIMA.
SWOG patients had received CHOP chemotherapy plus an anti-CD20 antibody (rituximab or iodine-131 tositumomab), and LYSA patients had received rituximab plus chemotherapy (and were randomized to rituximab maintenance or observation).
SWOG cohort
After a median follow-up of 5.4 years, patients with vitamin D deficiency (defined as <20 ng/mL) had significantly inferior PFS (hazard ratio [HR]=2.00; P=0.011) and OS (HR=3.57; P=0.003) compared to patients with higher vitamin D levels.
Results were similar when the researchers adjusted for other variables, such as prognostic index (IPI), body mass index, and latitude (≥ vs <35°N). For PFS, the adjusted HR was 1.97 (P=0.023). And for OS, the adjusted HR was 4.16 (P=0.002).
Multivariable analysis of vitamin D by tertile confirmed that the lowest tertile of vitamin D was associated with a greater increase in the risk of either progression or death, but neither result was significant.
LYSA cohort
After a median follow-up of 6.6 years, patients with vitamin D deficiency (defined as <10 ng/mL) had significantly inferior PFS (HR=1.66; P=0.013) but not OS (HR=1.84; P=0.14) compared to patients with higher vitamin D levels.
Results were similar when the researchers adjusted for other variables, such as prognostic index (FLIPI), body mass index, latitude (Europe vs Australia), and hemoglobin. For PFS, the adjusted HR was 1.50 (P=0.095). For OS, the adjusted HR was 1.92 (P=0.192).
Multivariable analysis of vitamin D by tertile confirmed that the lowest tertile of vitamin D was associated with a greater increase in the risk of either progression or death, but only the association with OS reached statistical significance (HR=5.32; P=0.037).
Dr Friedberg said that, taken together, these results suggest vitamin D levels may be a modifiable factor associated with prognosis in patients with FL.
“Our data, replicated internationally, supports other published observations linking vitamin D deficiency with inferior cancer outcomes,” he said. “However, the mechanisms of this link are likely complex and require further study.” ![]()

Vitamin D deficiency may negatively impact outcomes in patients with follicular lymphoma (FL), according to research published in the Journal of Clinical Oncology.
The study showed that FL patients with vitamin D deficiency had inferior progression-free survival (PFS) and overall survival (OS) compared to patients with higher vitamin D levels.
According to researchers, this suggests serum vitamin D might be the first potentially modifiable factor to be associated with survival in FL.
However, additional research is needed to determine the effects of vitamin D supplementation in these patients.
Jonathan W. Friedberg, MD, of the Wilmot Cancer Institute at the University of Rochester in New York, and his colleagues conducted this research, analyzing data from 2 cohorts of FL patients.
One cohort consisted of patients derived from 3 SWOG trials (S9800, S9911, and S0016), and the other consisted of patients from a Lymphoma Study Association (LYSA) trial known as PRIMA.
SWOG patients had received CHOP chemotherapy plus an anti-CD20 antibody (rituximab or iodine-131 tositumomab), and LYSA patients had received rituximab plus chemotherapy (and were randomized to rituximab maintenance or observation).
SWOG cohort
After a median follow-up of 5.4 years, patients with vitamin D deficiency (defined as <20 ng/mL) had significantly inferior PFS (hazard ratio [HR]=2.00; P=0.011) and OS (HR=3.57; P=0.003) compared to patients with higher vitamin D levels.
Results were similar when the researchers adjusted for other variables, such as prognostic index (IPI), body mass index, and latitude (≥ vs <35°N). For PFS, the adjusted HR was 1.97 (P=0.023). And for OS, the adjusted HR was 4.16 (P=0.002).
Multivariable analysis of vitamin D by tertile confirmed that the lowest tertile of vitamin D was associated with a greater increase in the risk of either progression or death, but neither result was significant.
LYSA cohort
After a median follow-up of 6.6 years, patients with vitamin D deficiency (defined as <10 ng/mL) had significantly inferior PFS (HR=1.66; P=0.013) but not OS (HR=1.84; P=0.14) compared to patients with higher vitamin D levels.
Results were similar when the researchers adjusted for other variables, such as prognostic index (FLIPI), body mass index, latitude (Europe vs Australia), and hemoglobin. For PFS, the adjusted HR was 1.50 (P=0.095). For OS, the adjusted HR was 1.92 (P=0.192).
Multivariable analysis of vitamin D by tertile confirmed that the lowest tertile of vitamin D was associated with a greater increase in the risk of either progression or death, but only the association with OS reached statistical significance (HR=5.32; P=0.037).
Dr Friedberg said that, taken together, these results suggest vitamin D levels may be a modifiable factor associated with prognosis in patients with FL.
“Our data, replicated internationally, supports other published observations linking vitamin D deficiency with inferior cancer outcomes,” he said. “However, the mechanisms of this link are likely complex and require further study.” ![]()
FDA approves new formulation of iron overload drug
The US Food and Drug Administration (FDA) has granted accelerated approval for Jadenu, a new oral formulation of Exjade (deferasirox).
Jadenu is now approved to treat patients 2 years of age and older who have chronic iron overload resulting from blood transfusions. The drug is also approved to treat chronic iron overload in patients 10 years of age and older who have non-transfusion-dependent thalassemia.
Jadenu can be swallowed whole and taken with or without a light meal. Exjade is a dispersible tablet that must be mixed in liquid and taken on an empty stomach.
Jadenu has been approved with a boxed warning, which states that the drug may cause serious and fatal renal toxicity (including failure), hepatic toxicity (including failure), and gastrointestinal hemorrhage. Therefore, treatment with Jadenu requires close patient monitoring, including laboratory tests of renal and hepatic function.
The FDA has granted Jadenu accelerated approval based on the drug showing a reduction of liver iron concentrations and serum ferritin levels. Continued FDA approval for Jadenu may be contingent upon verification and description of clinical benefit in confirmatory trials.
Jadenu has been evaluated in trials of healthy volunteers, but there are no clinical data showing the effects of Jadenu in patients with chronic iron overload.
Exjade, on the other hand, has been evaluated in several trials of patients with chronic iron overload resulting from transfusions and patients with non-transfusion-dependent thalassemia who have chronic iron overload.
Data from these trials can be found in the prescribing information for Jadenu, available at www.jadenu.com. ![]()
The US Food and Drug Administration (FDA) has granted accelerated approval for Jadenu, a new oral formulation of Exjade (deferasirox).
Jadenu is now approved to treat patients 2 years of age and older who have chronic iron overload resulting from blood transfusions. The drug is also approved to treat chronic iron overload in patients 10 years of age and older who have non-transfusion-dependent thalassemia.
Jadenu can be swallowed whole and taken with or without a light meal. Exjade is a dispersible tablet that must be mixed in liquid and taken on an empty stomach.
Jadenu has been approved with a boxed warning, which states that the drug may cause serious and fatal renal toxicity (including failure), hepatic toxicity (including failure), and gastrointestinal hemorrhage. Therefore, treatment with Jadenu requires close patient monitoring, including laboratory tests of renal and hepatic function.
The FDA has granted Jadenu accelerated approval based on the drug showing a reduction of liver iron concentrations and serum ferritin levels. Continued FDA approval for Jadenu may be contingent upon verification and description of clinical benefit in confirmatory trials.
Jadenu has been evaluated in trials of healthy volunteers, but there are no clinical data showing the effects of Jadenu in patients with chronic iron overload.
Exjade, on the other hand, has been evaluated in several trials of patients with chronic iron overload resulting from transfusions and patients with non-transfusion-dependent thalassemia who have chronic iron overload.
Data from these trials can be found in the prescribing information for Jadenu, available at www.jadenu.com. ![]()
The US Food and Drug Administration (FDA) has granted accelerated approval for Jadenu, a new oral formulation of Exjade (deferasirox).
Jadenu is now approved to treat patients 2 years of age and older who have chronic iron overload resulting from blood transfusions. The drug is also approved to treat chronic iron overload in patients 10 years of age and older who have non-transfusion-dependent thalassemia.
Jadenu can be swallowed whole and taken with or without a light meal. Exjade is a dispersible tablet that must be mixed in liquid and taken on an empty stomach.
Jadenu has been approved with a boxed warning, which states that the drug may cause serious and fatal renal toxicity (including failure), hepatic toxicity (including failure), and gastrointestinal hemorrhage. Therefore, treatment with Jadenu requires close patient monitoring, including laboratory tests of renal and hepatic function.
The FDA has granted Jadenu accelerated approval based on the drug showing a reduction of liver iron concentrations and serum ferritin levels. Continued FDA approval for Jadenu may be contingent upon verification and description of clinical benefit in confirmatory trials.
Jadenu has been evaluated in trials of healthy volunteers, but there are no clinical data showing the effects of Jadenu in patients with chronic iron overload.
Exjade, on the other hand, has been evaluated in several trials of patients with chronic iron overload resulting from transfusions and patients with non-transfusion-dependent thalassemia who have chronic iron overload.
Data from these trials can be found in the prescribing information for Jadenu, available at www.jadenu.com. ![]()
Predicting treatment response in CMML

Photo courtesy of
University of Michigan
Newly identified molecular signatures may allow us to predict which patients with chronic myelomonocytic leukemia (CMML) will respond to treatment, according to a study published in The Journal of Clinical Investigation.
Finding effective biomarkers is particularly crucial for CMML, the investigators said, because current treatment is slow-acting. Patients must often undergo as much as 6 months of treatment before there are signs of response.
“The slow kinetics is what gets us,” said study author Maria E. Figueroa, MD, of the University of Michigan Medical School in Ann Arbor.
“It’s not just one week or one dose to see signs of response. A good biomarker test could potentially prevent patients who are unlikely to respond from receiving prolonged, unwarranted treatments.”
With this in mind, Dr Figueroa and her colleagues used next-generation sequencing techniques to analyze 40 CMML samples from patients treated with the DNA methyltransferase inhibitor decitabine.
The researchers found 167 differentially methylated regions of DNA at baseline that distinguished patients who responded to decitabine from those who did not. There was a methylation difference of 25% or more between responders and nonresponders.
The investigators speculated that the baseline differences in DNA methylation could be used to predict treatment response at diagnosis.
So they used the percentage of cytosine methylation at each genomic location among the 40 patients as potential predictors and applied a machine-learning approach to build an epigenetic classifier.
The investigators tested the classifier in 28 additional CMML samples and found it was 87% accurate in predicting a patient’s response to decitabine.
The researchers also investigated why nonresponders were resistant to decitabine and found that 2 proteins, CXCL4 and CXCL7, were overexpressed in nonresponders. In cells exposed to high levels of these chemokines, the effects of decitabine were blocked.
“We are pursuing this to understand why these proteins block the effect of the drug and whether we can develop a new compound that could be used along with decitabine to turn nonresponders into responders,” Dr Figueroa said.
The researchers are also working to refine and translate their findings into a viable biomarker test that could be used in the clinic. ![]()

Photo courtesy of
University of Michigan
Newly identified molecular signatures may allow us to predict which patients with chronic myelomonocytic leukemia (CMML) will respond to treatment, according to a study published in The Journal of Clinical Investigation.
Finding effective biomarkers is particularly crucial for CMML, the investigators said, because current treatment is slow-acting. Patients must often undergo as much as 6 months of treatment before there are signs of response.
“The slow kinetics is what gets us,” said study author Maria E. Figueroa, MD, of the University of Michigan Medical School in Ann Arbor.
“It’s not just one week or one dose to see signs of response. A good biomarker test could potentially prevent patients who are unlikely to respond from receiving prolonged, unwarranted treatments.”
With this in mind, Dr Figueroa and her colleagues used next-generation sequencing techniques to analyze 40 CMML samples from patients treated with the DNA methyltransferase inhibitor decitabine.
The researchers found 167 differentially methylated regions of DNA at baseline that distinguished patients who responded to decitabine from those who did not. There was a methylation difference of 25% or more between responders and nonresponders.
The investigators speculated that the baseline differences in DNA methylation could be used to predict treatment response at diagnosis.
So they used the percentage of cytosine methylation at each genomic location among the 40 patients as potential predictors and applied a machine-learning approach to build an epigenetic classifier.
The investigators tested the classifier in 28 additional CMML samples and found it was 87% accurate in predicting a patient’s response to decitabine.
The researchers also investigated why nonresponders were resistant to decitabine and found that 2 proteins, CXCL4 and CXCL7, were overexpressed in nonresponders. In cells exposed to high levels of these chemokines, the effects of decitabine were blocked.
“We are pursuing this to understand why these proteins block the effect of the drug and whether we can develop a new compound that could be used along with decitabine to turn nonresponders into responders,” Dr Figueroa said.
The researchers are also working to refine and translate their findings into a viable biomarker test that could be used in the clinic. ![]()

Photo courtesy of
University of Michigan
Newly identified molecular signatures may allow us to predict which patients with chronic myelomonocytic leukemia (CMML) will respond to treatment, according to a study published in The Journal of Clinical Investigation.
Finding effective biomarkers is particularly crucial for CMML, the investigators said, because current treatment is slow-acting. Patients must often undergo as much as 6 months of treatment before there are signs of response.
“The slow kinetics is what gets us,” said study author Maria E. Figueroa, MD, of the University of Michigan Medical School in Ann Arbor.
“It’s not just one week or one dose to see signs of response. A good biomarker test could potentially prevent patients who are unlikely to respond from receiving prolonged, unwarranted treatments.”
With this in mind, Dr Figueroa and her colleagues used next-generation sequencing techniques to analyze 40 CMML samples from patients treated with the DNA methyltransferase inhibitor decitabine.
The researchers found 167 differentially methylated regions of DNA at baseline that distinguished patients who responded to decitabine from those who did not. There was a methylation difference of 25% or more between responders and nonresponders.
The investigators speculated that the baseline differences in DNA methylation could be used to predict treatment response at diagnosis.
So they used the percentage of cytosine methylation at each genomic location among the 40 patients as potential predictors and applied a machine-learning approach to build an epigenetic classifier.
The investigators tested the classifier in 28 additional CMML samples and found it was 87% accurate in predicting a patient’s response to decitabine.
The researchers also investigated why nonresponders were resistant to decitabine and found that 2 proteins, CXCL4 and CXCL7, were overexpressed in nonresponders. In cells exposed to high levels of these chemokines, the effects of decitabine were blocked.
“We are pursuing this to understand why these proteins block the effect of the drug and whether we can develop a new compound that could be used along with decitabine to turn nonresponders into responders,” Dr Figueroa said.
The researchers are also working to refine and translate their findings into a viable biomarker test that could be used in the clinic. ![]()
Immunotherapy gets orphan designation

Photo by Graham Colm
The US Food and Drug Administration (FDA) has granted orphan designation to an immunotherapy known as CMD-003, which is under development to treat Epstein-Barr-virus (EBV)-positive non-Hodgkin lymphomas.
CMD-003 consists of T cells derived from blood samples that are activated and expanded through a proprietary process developed for commercial-scale use.
Researchers have treated more than 250 patients with prototypes of CMD-003. And the prototypes have produced promising results in a range of malignancies.
CMD-003 is under development by Cell Medica and the Center for Cell and Gene Therapy (CAGT) at Baylor College of Medicine, Texas Children’s Hospital, and Houston Methodist Hospital.
Orphan designation from the FDA will provide CMD-003’s developers with several benefits, including accessibility to grants to support clinical development, 7 years of market exclusivity if the treatment is approved in the US, and tax credits on US clinical trials.
CMD-003 prototype
Researchers have not published any trials of CMD-003, but they have studied other EBV-specific T-cell products related to CMD-003.
In their most recent study, published in the Journal of Clinical Oncology, the researchers administered cytotoxic T lymphocytes (CTLs) in 50 patients with EBV-associated Hodgkin or non-Hodgkin lymphoma.
Twenty-nine of the patients were in remission when they received CTL infusions, but they were at a high risk of relapse. The remaining 21 patients had relapsed or refractory disease at the time of CTL infusion.
Twenty-seven of the patients who received CTLs as an adjuvant treatment remained in remission from their disease at 3.1 years after treatment.
Their 2-year event-free survival rate was 82%. None of them died of lymphoma, but 9 died from complications associated with the chemotherapy and radiation they had received.
Of the 21 patients with relapsed or refractory disease, 13 responded to CTL infusions, and 11 patients achieved a complete response. In this group, the 2-year event-free survival rate was about 50%.
The researchers said there were no toxicities that were definitively related to CTL infusion.
One patient had central nervous system deterioration 2 weeks after infusion. This was attributed to disease progression but could possibly have been treatment-related.
Another patient developed respiratory complications about 4 weeks after a second CTL infusion that may have been treatment-related. However, the researchers attributed it to an intercurrent infection, and the patient made a complete recovery. ![]()

Photo by Graham Colm
The US Food and Drug Administration (FDA) has granted orphan designation to an immunotherapy known as CMD-003, which is under development to treat Epstein-Barr-virus (EBV)-positive non-Hodgkin lymphomas.
CMD-003 consists of T cells derived from blood samples that are activated and expanded through a proprietary process developed for commercial-scale use.
Researchers have treated more than 250 patients with prototypes of CMD-003. And the prototypes have produced promising results in a range of malignancies.
CMD-003 is under development by Cell Medica and the Center for Cell and Gene Therapy (CAGT) at Baylor College of Medicine, Texas Children’s Hospital, and Houston Methodist Hospital.
Orphan designation from the FDA will provide CMD-003’s developers with several benefits, including accessibility to grants to support clinical development, 7 years of market exclusivity if the treatment is approved in the US, and tax credits on US clinical trials.
CMD-003 prototype
Researchers have not published any trials of CMD-003, but they have studied other EBV-specific T-cell products related to CMD-003.
In their most recent study, published in the Journal of Clinical Oncology, the researchers administered cytotoxic T lymphocytes (CTLs) in 50 patients with EBV-associated Hodgkin or non-Hodgkin lymphoma.
Twenty-nine of the patients were in remission when they received CTL infusions, but they were at a high risk of relapse. The remaining 21 patients had relapsed or refractory disease at the time of CTL infusion.
Twenty-seven of the patients who received CTLs as an adjuvant treatment remained in remission from their disease at 3.1 years after treatment.
Their 2-year event-free survival rate was 82%. None of them died of lymphoma, but 9 died from complications associated with the chemotherapy and radiation they had received.
Of the 21 patients with relapsed or refractory disease, 13 responded to CTL infusions, and 11 patients achieved a complete response. In this group, the 2-year event-free survival rate was about 50%.
The researchers said there were no toxicities that were definitively related to CTL infusion.
One patient had central nervous system deterioration 2 weeks after infusion. This was attributed to disease progression but could possibly have been treatment-related.
Another patient developed respiratory complications about 4 weeks after a second CTL infusion that may have been treatment-related. However, the researchers attributed it to an intercurrent infection, and the patient made a complete recovery. ![]()

Photo by Graham Colm
The US Food and Drug Administration (FDA) has granted orphan designation to an immunotherapy known as CMD-003, which is under development to treat Epstein-Barr-virus (EBV)-positive non-Hodgkin lymphomas.
CMD-003 consists of T cells derived from blood samples that are activated and expanded through a proprietary process developed for commercial-scale use.
Researchers have treated more than 250 patients with prototypes of CMD-003. And the prototypes have produced promising results in a range of malignancies.
CMD-003 is under development by Cell Medica and the Center for Cell and Gene Therapy (CAGT) at Baylor College of Medicine, Texas Children’s Hospital, and Houston Methodist Hospital.
Orphan designation from the FDA will provide CMD-003’s developers with several benefits, including accessibility to grants to support clinical development, 7 years of market exclusivity if the treatment is approved in the US, and tax credits on US clinical trials.
CMD-003 prototype
Researchers have not published any trials of CMD-003, but they have studied other EBV-specific T-cell products related to CMD-003.
In their most recent study, published in the Journal of Clinical Oncology, the researchers administered cytotoxic T lymphocytes (CTLs) in 50 patients with EBV-associated Hodgkin or non-Hodgkin lymphoma.
Twenty-nine of the patients were in remission when they received CTL infusions, but they were at a high risk of relapse. The remaining 21 patients had relapsed or refractory disease at the time of CTL infusion.
Twenty-seven of the patients who received CTLs as an adjuvant treatment remained in remission from their disease at 3.1 years after treatment.
Their 2-year event-free survival rate was 82%. None of them died of lymphoma, but 9 died from complications associated with the chemotherapy and radiation they had received.
Of the 21 patients with relapsed or refractory disease, 13 responded to CTL infusions, and 11 patients achieved a complete response. In this group, the 2-year event-free survival rate was about 50%.
The researchers said there were no toxicities that were definitively related to CTL infusion.
One patient had central nervous system deterioration 2 weeks after infusion. This was attributed to disease progression but could possibly have been treatment-related.
Another patient developed respiratory complications about 4 weeks after a second CTL infusion that may have been treatment-related. However, the researchers attributed it to an intercurrent infection, and the patient made a complete recovery. ![]()
Malpractice Counsel
Carbon Monoxide Poisoning
A 72-year-old man was brought to the ED by paramedics with inability to move his left leg and difficulty speaking. The patient had been heating his home with a generator placed inside the house during an ice storm, and paramedics reported a strong smell of gas inside the house.
The patient was unable to describe the time of onset of his symptoms. He complained of headache, slurred speech, and inability to move his left leg. He also said he felt the urge to urinate, but was unable to do so. He denied chest pain or shortness of breath. His medical history was significant only for hypertension, which was controlled with hydrochlorothiazide and lisinopril. He admitted to smoking a few cigarettes daily, but denied any alcohol use.
On physical examination, the patient’s vital signs were: blood pressure (BP) 162/98 mm Hg; heart rate (HR), 110 beats/minute; respiratory rate (RR), 20 breaths/minute; and temperature (T), 98.6˚F. The patient had 100% oxygen (O2) saturation on 4L O2 via nasal cannula. The head, eyes, ears, nose, and throat examination was normal. There was no facial droop; his speech was slurred, but he was easily understandable. The cardiopulmonary examination revealed tachycardia without murmurs, rubs, or gallop; the lungs were clear to auscultation bilaterally. The neurological examination revealed 5/5 motor strength in the upper extremities and symmetrical; there was no pronator drift. The left leg had 2/5 motor strength compared to 5/5 in the right lower extremity. There was also fullness and tenderness over his suprapubic region.
The emergency physician (EP) ordered a complete blood count, basic metabolic profile, carboxyhemoglobin (COHb) test, electrocardiogram (ECG), portable chest X-ray (CXR), and a noncontrast computed tomography (CT) scan of the head. Since the history and physical examination suggested urinary retention, a Foley catheter was placed; a total of 1,200 cc of clear urine was obtained, after which the patient expressed a feeling of relief.
The patient’s COHb level was 8.5%. The portable CXR and CT scan of the head were both reported as normal by the radiologist. Likewise, the results of the rest of the laboratory evaluation were normal. The ECG revealed sinus tachycardia without evidence of strain or injury.
The EP diagnosed an acute cerebrovascular accident (CVA) and admitted the patient to the hospital. He did not feel that carbon monoxide (CO) contributed to the event given the low level in a cigarette smoker. After an uneventful hospital stay, the patient was transferred to a physical rehabilitation unit. He was ultimately discharged with a neurogenic bladder and weak left leg.
The patient sued the EP for negligence in the failure to diagnose CO poisoning and prompt initiation of 100% O2 therapy. The EP argued that CO poisoning had been properly ruled out and that the diagnosis of CVA was correct. The defense also claimed that even if the patient had suffered CO poisoning, the length of the exposure would have led to the same outcome. A defense verdict was returned.
Discussion
Carbon monoxide poisoning is one of the leading causes of poisoning morbidity and mortality in the United States. This is in part due to the fact that CO is a colorless, odorless, and tasteless gas. The peak incidence for CO poisoning is in the fall and winter, when people are more likely to use space heaters, wood burning stoves, or portable generators inside without adequate ventilation.
The clinical presentation of CO poisoning can range from mild (eg, headache, flu-like symptoms) to devastating (eg, coma, death). The central nervous system is the organ system that is most sensitive to CO poisoning. Symptoms can range from a dull frontal headache, dizziness, and ataxia, to syncope, seizures, focal neurological deficit, and coma. In fact, the most serious complication of CO poisoning may be persistent or delayed neurological or neurocognitive sequelae, which can occur in up to 50% of patients with symptomatic acute poisoning.1 Unfortunately, COHb levels and symptoms do not always correlate well. In fact, particular COHb levels are not predictive of symptoms or outcome.1
The treatment for CO poisoning consists of administering 100% O2 as soon as the diagnosis is considered. If 100% O2 is administered, the half-life of COHb can be reduced from 5 hours (room air) to approximately 1 hour.1 While some argue that treatment with hyperbaric O2 (HBO) therapy should be considered standard of care, it has not yet been determined which patient population benefits from HBO therapy; moreover, there is currently no established optimum timing of therapy. Regardless, the jury came to the correct decision in this case as it is impossible to determine, with any degree of medical certainty, if the patient’s neurological deficits were due to the natural course of an ischemic stroke, or if CO contributed to or was the sole cause of the CVA.
Death in the Emergency Department
A 43-year-old man presented to the ED with the chief complaint of a lower lip laceration. The patient stated he had gotten into an altercation with his girlfriend just prior to arrival. She had punched the patient in the face with her fist, resulting in the lip laceration. The patient denied any loss of consciousness or other pain. He did, however, smell of alcohol and was emotionally labile, crying one moment and yelling the next.
The patient was instructed to remove all of his clothes, change into a hospital gown and give all of his belongings to hospital security. He removed his clothes, but refused to turn them over to security. This prompted a physical altercation between the patient and hospital security. Three hospital security guards wrestled the patient to the ground and placed him face down; one guard placed the patient in a choke hold while the other two guards sat on top of him. Within a few moments, the patient became unresponsive. He was placed immediately on a stretcher and intubated by the EP. After successful intubation and bagging with 100% O2, the patient regained a palpable pulse, but remained unresponsive.
The patient was admitted to the intensive care unit, but never regained consciousness and died 5 days later. The cause of death was thought to be anoxic brain injury due to asphyxiation. The family of the patient sued the hospital and the EP for causing asphyxiation and death in this patient seeking medical care. The hospital denied responsibility for the death because the patient both instigated the altercation and had a preexisting heart condition. According to published reports, a $2.5 million settlement was reached.
Discussion
This unfortunate case did not involve the EP; all of the important events transpired prior to the EP’s initial interaction with the patient. There are not enough details to explain how this situation escalated so rapidly, or why hospital security felt this was the best way to subdue the patient.
Unfortunately, EPs are no strangers to agitated patients. Behavioral emergencies account for approximately 5% of all ED visits, and these usually involve some form of violence or agitation.1 Every physician and nurse working in the ED must be prepared to deal with patients who have the potential to become violent. Clearly, training of all patient-care personnel to handle such patients in the ED is important to ensuring both staff and patient safety. Having the patient undress and change into a hospital gown is the correct first step. This allows for removal of real or potential weapons, and makes it much less likely for the patient to leave before his or her evaluation and management is complete. Doing this properly, however, is key. Providing the patient with a warm blanket or food, or just talking to him or her in a calm and reassuring voice, can often prevent escalation. Simply arguing with the patient rarely works, and often has the opposite desired effect.
If the situation continues to escalate, and it appears either physical or chemical restraint will be necessary, a “show of force” should be made. A restraint team consisting of at least five trained members should be assembled, with the EP acting as the team leader. The team should all enter the room at the same time, explain what will happen, and then move quickly.1 The leader should move to the head of the bed and direct the team, while the remaining four members each take a limb. To preserve the physician-patient relationship, it is best if the EP is not actively involved in placing the physical restraints.
The choke hold should only be considered as a method of last resort. Many police departments in the country prohibit use of the choke hold because of complications such as those observed in this case. The use of choke holds became a topic of intense debate this summer with the death of Eric Garner in Staten Island, New York; it was thought that his pre-existing conditions of obesity, asthma, and heart disease were all aggravated by the choke hold. Although obese patients are often at a higher risk for complications due to pre-existing issues with adequate oxygenation, it is unclear whether the patient in this case was obese.
An alternative strategy in handling an agitated patient would be the use of a taser by trained security personnel. In one study, 99.75% of tasered patients had no significant injury as a result of the device.2 In 2009, the American Medical Association found that tasers, “when used appropriately, can save lives during interventions that would have otherwise involved the use of deadly force.” While the safety of patients and the ED staff (nurses, physicians, and technicians) is paramount, the clinician should always adhere to the principle of “primum non nocere”—“first, do no harm.”
Reference - Carbon Monoxide Poisoning
- Tomaszewski C: Carbon monoxide. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:1658.
Reference - Death in the Emergency Department
- Rossi J, Swan MC, Issacs ED. The violent or agitated patient. Emerg Med Clin North Am. 2010;28(1):235-256.
- Bozeman WP, Hauda WE 2nd, Heck JJ, Graham DD Jr, Martin BP, Winslow JE. Safety and injury profile of conducted electrical weapons used by law enforcement officers against criminal suspects. Ann Emerg Med. 2009;53(4):480-489.
Carbon Monoxide Poisoning
A 72-year-old man was brought to the ED by paramedics with inability to move his left leg and difficulty speaking. The patient had been heating his home with a generator placed inside the house during an ice storm, and paramedics reported a strong smell of gas inside the house.
The patient was unable to describe the time of onset of his symptoms. He complained of headache, slurred speech, and inability to move his left leg. He also said he felt the urge to urinate, but was unable to do so. He denied chest pain or shortness of breath. His medical history was significant only for hypertension, which was controlled with hydrochlorothiazide and lisinopril. He admitted to smoking a few cigarettes daily, but denied any alcohol use.
On physical examination, the patient’s vital signs were: blood pressure (BP) 162/98 mm Hg; heart rate (HR), 110 beats/minute; respiratory rate (RR), 20 breaths/minute; and temperature (T), 98.6˚F. The patient had 100% oxygen (O2) saturation on 4L O2 via nasal cannula. The head, eyes, ears, nose, and throat examination was normal. There was no facial droop; his speech was slurred, but he was easily understandable. The cardiopulmonary examination revealed tachycardia without murmurs, rubs, or gallop; the lungs were clear to auscultation bilaterally. The neurological examination revealed 5/5 motor strength in the upper extremities and symmetrical; there was no pronator drift. The left leg had 2/5 motor strength compared to 5/5 in the right lower extremity. There was also fullness and tenderness over his suprapubic region.
The emergency physician (EP) ordered a complete blood count, basic metabolic profile, carboxyhemoglobin (COHb) test, electrocardiogram (ECG), portable chest X-ray (CXR), and a noncontrast computed tomography (CT) scan of the head. Since the history and physical examination suggested urinary retention, a Foley catheter was placed; a total of 1,200 cc of clear urine was obtained, after which the patient expressed a feeling of relief.
The patient’s COHb level was 8.5%. The portable CXR and CT scan of the head were both reported as normal by the radiologist. Likewise, the results of the rest of the laboratory evaluation were normal. The ECG revealed sinus tachycardia without evidence of strain or injury.
The EP diagnosed an acute cerebrovascular accident (CVA) and admitted the patient to the hospital. He did not feel that carbon monoxide (CO) contributed to the event given the low level in a cigarette smoker. After an uneventful hospital stay, the patient was transferred to a physical rehabilitation unit. He was ultimately discharged with a neurogenic bladder and weak left leg.
The patient sued the EP for negligence in the failure to diagnose CO poisoning and prompt initiation of 100% O2 therapy. The EP argued that CO poisoning had been properly ruled out and that the diagnosis of CVA was correct. The defense also claimed that even if the patient had suffered CO poisoning, the length of the exposure would have led to the same outcome. A defense verdict was returned.
Discussion
Carbon monoxide poisoning is one of the leading causes of poisoning morbidity and mortality in the United States. This is in part due to the fact that CO is a colorless, odorless, and tasteless gas. The peak incidence for CO poisoning is in the fall and winter, when people are more likely to use space heaters, wood burning stoves, or portable generators inside without adequate ventilation.
The clinical presentation of CO poisoning can range from mild (eg, headache, flu-like symptoms) to devastating (eg, coma, death). The central nervous system is the organ system that is most sensitive to CO poisoning. Symptoms can range from a dull frontal headache, dizziness, and ataxia, to syncope, seizures, focal neurological deficit, and coma. In fact, the most serious complication of CO poisoning may be persistent or delayed neurological or neurocognitive sequelae, which can occur in up to 50% of patients with symptomatic acute poisoning.1 Unfortunately, COHb levels and symptoms do not always correlate well. In fact, particular COHb levels are not predictive of symptoms or outcome.1
The treatment for CO poisoning consists of administering 100% O2 as soon as the diagnosis is considered. If 100% O2 is administered, the half-life of COHb can be reduced from 5 hours (room air) to approximately 1 hour.1 While some argue that treatment with hyperbaric O2 (HBO) therapy should be considered standard of care, it has not yet been determined which patient population benefits from HBO therapy; moreover, there is currently no established optimum timing of therapy. Regardless, the jury came to the correct decision in this case as it is impossible to determine, with any degree of medical certainty, if the patient’s neurological deficits were due to the natural course of an ischemic stroke, or if CO contributed to or was the sole cause of the CVA.
Death in the Emergency Department
A 43-year-old man presented to the ED with the chief complaint of a lower lip laceration. The patient stated he had gotten into an altercation with his girlfriend just prior to arrival. She had punched the patient in the face with her fist, resulting in the lip laceration. The patient denied any loss of consciousness or other pain. He did, however, smell of alcohol and was emotionally labile, crying one moment and yelling the next.
The patient was instructed to remove all of his clothes, change into a hospital gown and give all of his belongings to hospital security. He removed his clothes, but refused to turn them over to security. This prompted a physical altercation between the patient and hospital security. Three hospital security guards wrestled the patient to the ground and placed him face down; one guard placed the patient in a choke hold while the other two guards sat on top of him. Within a few moments, the patient became unresponsive. He was placed immediately on a stretcher and intubated by the EP. After successful intubation and bagging with 100% O2, the patient regained a palpable pulse, but remained unresponsive.
The patient was admitted to the intensive care unit, but never regained consciousness and died 5 days later. The cause of death was thought to be anoxic brain injury due to asphyxiation. The family of the patient sued the hospital and the EP for causing asphyxiation and death in this patient seeking medical care. The hospital denied responsibility for the death because the patient both instigated the altercation and had a preexisting heart condition. According to published reports, a $2.5 million settlement was reached.
Discussion
This unfortunate case did not involve the EP; all of the important events transpired prior to the EP’s initial interaction with the patient. There are not enough details to explain how this situation escalated so rapidly, or why hospital security felt this was the best way to subdue the patient.
Unfortunately, EPs are no strangers to agitated patients. Behavioral emergencies account for approximately 5% of all ED visits, and these usually involve some form of violence or agitation.1 Every physician and nurse working in the ED must be prepared to deal with patients who have the potential to become violent. Clearly, training of all patient-care personnel to handle such patients in the ED is important to ensuring both staff and patient safety. Having the patient undress and change into a hospital gown is the correct first step. This allows for removal of real or potential weapons, and makes it much less likely for the patient to leave before his or her evaluation and management is complete. Doing this properly, however, is key. Providing the patient with a warm blanket or food, or just talking to him or her in a calm and reassuring voice, can often prevent escalation. Simply arguing with the patient rarely works, and often has the opposite desired effect.
If the situation continues to escalate, and it appears either physical or chemical restraint will be necessary, a “show of force” should be made. A restraint team consisting of at least five trained members should be assembled, with the EP acting as the team leader. The team should all enter the room at the same time, explain what will happen, and then move quickly.1 The leader should move to the head of the bed and direct the team, while the remaining four members each take a limb. To preserve the physician-patient relationship, it is best if the EP is not actively involved in placing the physical restraints.
The choke hold should only be considered as a method of last resort. Many police departments in the country prohibit use of the choke hold because of complications such as those observed in this case. The use of choke holds became a topic of intense debate this summer with the death of Eric Garner in Staten Island, New York; it was thought that his pre-existing conditions of obesity, asthma, and heart disease were all aggravated by the choke hold. Although obese patients are often at a higher risk for complications due to pre-existing issues with adequate oxygenation, it is unclear whether the patient in this case was obese.
An alternative strategy in handling an agitated patient would be the use of a taser by trained security personnel. In one study, 99.75% of tasered patients had no significant injury as a result of the device.2 In 2009, the American Medical Association found that tasers, “when used appropriately, can save lives during interventions that would have otherwise involved the use of deadly force.” While the safety of patients and the ED staff (nurses, physicians, and technicians) is paramount, the clinician should always adhere to the principle of “primum non nocere”—“first, do no harm.”
Carbon Monoxide Poisoning
A 72-year-old man was brought to the ED by paramedics with inability to move his left leg and difficulty speaking. The patient had been heating his home with a generator placed inside the house during an ice storm, and paramedics reported a strong smell of gas inside the house.
The patient was unable to describe the time of onset of his symptoms. He complained of headache, slurred speech, and inability to move his left leg. He also said he felt the urge to urinate, but was unable to do so. He denied chest pain or shortness of breath. His medical history was significant only for hypertension, which was controlled with hydrochlorothiazide and lisinopril. He admitted to smoking a few cigarettes daily, but denied any alcohol use.
On physical examination, the patient’s vital signs were: blood pressure (BP) 162/98 mm Hg; heart rate (HR), 110 beats/minute; respiratory rate (RR), 20 breaths/minute; and temperature (T), 98.6˚F. The patient had 100% oxygen (O2) saturation on 4L O2 via nasal cannula. The head, eyes, ears, nose, and throat examination was normal. There was no facial droop; his speech was slurred, but he was easily understandable. The cardiopulmonary examination revealed tachycardia without murmurs, rubs, or gallop; the lungs were clear to auscultation bilaterally. The neurological examination revealed 5/5 motor strength in the upper extremities and symmetrical; there was no pronator drift. The left leg had 2/5 motor strength compared to 5/5 in the right lower extremity. There was also fullness and tenderness over his suprapubic region.
The emergency physician (EP) ordered a complete blood count, basic metabolic profile, carboxyhemoglobin (COHb) test, electrocardiogram (ECG), portable chest X-ray (CXR), and a noncontrast computed tomography (CT) scan of the head. Since the history and physical examination suggested urinary retention, a Foley catheter was placed; a total of 1,200 cc of clear urine was obtained, after which the patient expressed a feeling of relief.
The patient’s COHb level was 8.5%. The portable CXR and CT scan of the head were both reported as normal by the radiologist. Likewise, the results of the rest of the laboratory evaluation were normal. The ECG revealed sinus tachycardia without evidence of strain or injury.
The EP diagnosed an acute cerebrovascular accident (CVA) and admitted the patient to the hospital. He did not feel that carbon monoxide (CO) contributed to the event given the low level in a cigarette smoker. After an uneventful hospital stay, the patient was transferred to a physical rehabilitation unit. He was ultimately discharged with a neurogenic bladder and weak left leg.
The patient sued the EP for negligence in the failure to diagnose CO poisoning and prompt initiation of 100% O2 therapy. The EP argued that CO poisoning had been properly ruled out and that the diagnosis of CVA was correct. The defense also claimed that even if the patient had suffered CO poisoning, the length of the exposure would have led to the same outcome. A defense verdict was returned.
Discussion
Carbon monoxide poisoning is one of the leading causes of poisoning morbidity and mortality in the United States. This is in part due to the fact that CO is a colorless, odorless, and tasteless gas. The peak incidence for CO poisoning is in the fall and winter, when people are more likely to use space heaters, wood burning stoves, or portable generators inside without adequate ventilation.
The clinical presentation of CO poisoning can range from mild (eg, headache, flu-like symptoms) to devastating (eg, coma, death). The central nervous system is the organ system that is most sensitive to CO poisoning. Symptoms can range from a dull frontal headache, dizziness, and ataxia, to syncope, seizures, focal neurological deficit, and coma. In fact, the most serious complication of CO poisoning may be persistent or delayed neurological or neurocognitive sequelae, which can occur in up to 50% of patients with symptomatic acute poisoning.1 Unfortunately, COHb levels and symptoms do not always correlate well. In fact, particular COHb levels are not predictive of symptoms or outcome.1
The treatment for CO poisoning consists of administering 100% O2 as soon as the diagnosis is considered. If 100% O2 is administered, the half-life of COHb can be reduced from 5 hours (room air) to approximately 1 hour.1 While some argue that treatment with hyperbaric O2 (HBO) therapy should be considered standard of care, it has not yet been determined which patient population benefits from HBO therapy; moreover, there is currently no established optimum timing of therapy. Regardless, the jury came to the correct decision in this case as it is impossible to determine, with any degree of medical certainty, if the patient’s neurological deficits were due to the natural course of an ischemic stroke, or if CO contributed to or was the sole cause of the CVA.
Death in the Emergency Department
A 43-year-old man presented to the ED with the chief complaint of a lower lip laceration. The patient stated he had gotten into an altercation with his girlfriend just prior to arrival. She had punched the patient in the face with her fist, resulting in the lip laceration. The patient denied any loss of consciousness or other pain. He did, however, smell of alcohol and was emotionally labile, crying one moment and yelling the next.
The patient was instructed to remove all of his clothes, change into a hospital gown and give all of his belongings to hospital security. He removed his clothes, but refused to turn them over to security. This prompted a physical altercation between the patient and hospital security. Three hospital security guards wrestled the patient to the ground and placed him face down; one guard placed the patient in a choke hold while the other two guards sat on top of him. Within a few moments, the patient became unresponsive. He was placed immediately on a stretcher and intubated by the EP. After successful intubation and bagging with 100% O2, the patient regained a palpable pulse, but remained unresponsive.
The patient was admitted to the intensive care unit, but never regained consciousness and died 5 days later. The cause of death was thought to be anoxic brain injury due to asphyxiation. The family of the patient sued the hospital and the EP for causing asphyxiation and death in this patient seeking medical care. The hospital denied responsibility for the death because the patient both instigated the altercation and had a preexisting heart condition. According to published reports, a $2.5 million settlement was reached.
Discussion
This unfortunate case did not involve the EP; all of the important events transpired prior to the EP’s initial interaction with the patient. There are not enough details to explain how this situation escalated so rapidly, or why hospital security felt this was the best way to subdue the patient.
Unfortunately, EPs are no strangers to agitated patients. Behavioral emergencies account for approximately 5% of all ED visits, and these usually involve some form of violence or agitation.1 Every physician and nurse working in the ED must be prepared to deal with patients who have the potential to become violent. Clearly, training of all patient-care personnel to handle such patients in the ED is important to ensuring both staff and patient safety. Having the patient undress and change into a hospital gown is the correct first step. This allows for removal of real or potential weapons, and makes it much less likely for the patient to leave before his or her evaluation and management is complete. Doing this properly, however, is key. Providing the patient with a warm blanket or food, or just talking to him or her in a calm and reassuring voice, can often prevent escalation. Simply arguing with the patient rarely works, and often has the opposite desired effect.
If the situation continues to escalate, and it appears either physical or chemical restraint will be necessary, a “show of force” should be made. A restraint team consisting of at least five trained members should be assembled, with the EP acting as the team leader. The team should all enter the room at the same time, explain what will happen, and then move quickly.1 The leader should move to the head of the bed and direct the team, while the remaining four members each take a limb. To preserve the physician-patient relationship, it is best if the EP is not actively involved in placing the physical restraints.
The choke hold should only be considered as a method of last resort. Many police departments in the country prohibit use of the choke hold because of complications such as those observed in this case. The use of choke holds became a topic of intense debate this summer with the death of Eric Garner in Staten Island, New York; it was thought that his pre-existing conditions of obesity, asthma, and heart disease were all aggravated by the choke hold. Although obese patients are often at a higher risk for complications due to pre-existing issues with adequate oxygenation, it is unclear whether the patient in this case was obese.
An alternative strategy in handling an agitated patient would be the use of a taser by trained security personnel. In one study, 99.75% of tasered patients had no significant injury as a result of the device.2 In 2009, the American Medical Association found that tasers, “when used appropriately, can save lives during interventions that would have otherwise involved the use of deadly force.” While the safety of patients and the ED staff (nurses, physicians, and technicians) is paramount, the clinician should always adhere to the principle of “primum non nocere”—“first, do no harm.”
Reference - Carbon Monoxide Poisoning
- Tomaszewski C: Carbon monoxide. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:1658.
Reference - Death in the Emergency Department
- Rossi J, Swan MC, Issacs ED. The violent or agitated patient. Emerg Med Clin North Am. 2010;28(1):235-256.
- Bozeman WP, Hauda WE 2nd, Heck JJ, Graham DD Jr, Martin BP, Winslow JE. Safety and injury profile of conducted electrical weapons used by law enforcement officers against criminal suspects. Ann Emerg Med. 2009;53(4):480-489.
Reference - Carbon Monoxide Poisoning
- Tomaszewski C: Carbon monoxide. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:1658.
Reference - Death in the Emergency Department
- Rossi J, Swan MC, Issacs ED. The violent or agitated patient. Emerg Med Clin North Am. 2010;28(1):235-256.
- Bozeman WP, Hauda WE 2nd, Heck JJ, Graham DD Jr, Martin BP, Winslow JE. Safety and injury profile of conducted electrical weapons used by law enforcement officers against criminal suspects. Ann Emerg Med. 2009;53(4):480-489.
The Surviving Sepsis Campaign: Where have we been and where are we going?
Sepsis is familiar to most physicians in clinical practice, but guidance from the medical literature on how best to manage it has traditionally been confusing.
Starting in 2002, the Surviving Sepsis Campaign has worked to reduce worldwide mortality from severe sepsis and septic shock by developing and publicizing guidelines of best practices based on evidence from the literature. The campaign published its first management guidelines in 2004.
In this article, I review the most recent guidelines1,2 (published in 2013) and discuss the campaign’s ongoing performance-improvement program.
DEFINING SEPSIS
Sepsis is a known or suspected infection plus systemic manifestations of infection. This includes the sepsis inflammatory response syndrome. Criteria include:
- Tachycardia (heart rate > 90 beats per minute)
- Tachypnea (> 20 breaths/minute or Paco2 < 32 mm Hg)
- Fever (temperature > 38.3°C [100.9°F]) or hypothermia (core temperature < 36°C [96.8°F])
- High or low white blood cell count (> 12.0 × 109/L or < 4.0 × 109/L), or a normal count with more than 10% immature cells.
The definition of sepsis was broadened in 2002 to include other systemic manifestations of infection, such as changes in blood glucose level and organ dysfunction.
Severe sepsis is defined as sepsis plus either acute organ dysfunction or tissue hypoperfusion due to infection, with tissue hypoperfusion defined as:
- Hypotension (systolic blood pressure < 90 mm Hg, or a drop in systolic blood pressure of > 40 mm Hg)
- Elevated lactate
- Low urine output
- Altered mental status.
In severe sepsis, organ dysfunction is caused by blood-borne toxins and involves acute lung and kidney injury, coagulopathy (thrombocytopenia and increased international normalized ratio), and liver dysfunction.
Septic shock is present when a patient requires vasopressors after adequate intravascular volume repletion.
SEPSIS IS DEADLY AND COSTLY
Severe sepsis is the leading cause of hospital death. Patients admitted with severe sepsis are eight times more likely to die than those admitted with other conditions.3 The economic burden is enormous: it is the most expensive condition treated in US hospitals, costing an estimated $20.3 billion in 2011, of which $12.7 billion came from Medicare.
THE SURVIVING SEPSIS CAMPAIGN
The Surviving Sepsis Campaign is a global effort to reduce the rate of death from severe sepsis. The campaign’s methods include:
- Educating physicians, the public, the media, and government about the high rates of morbidity and death in severe sepsis
- Creating evidence-based guidelines for managing sepsis and establishing global best-practice standards
- Facilitating the transfer of knowledge by developing performance-improvement programs to change bedside practice.
The campaign is funded with a grant from the Gordon and Betty Moore Foundation. The campaign’s guidelines are not associated with any direct or indirect industry support. The 2013 guidelines were backed by 30 international organizations.1,2
All recommendations are ranked with numerical and letter scores, according to the GRADE system: 1 indicates a strong recommendation and 2 a weak one. The letters A through D reflect the quality of evidence, ranging from high (A) to very low (D).
GIVING ANTIBIOTICS EARLY IMPROVES OUTCOMES
A number of studies have suggested that starting appropriate antibiotics early improves outcomes in severe sepsis and septic shock. The death rate increases with each hour of delay.4
Recommendation. Intravenous antibiotic therapy should be started as soon as possible, and within the first hour after recognition of septic shock (grade 1B) and severe sepsis without septic shock (grade 1C).
The feasibility of achieving this goal has not been scientifically validated, and the recommendation should not be misinterpreted as the current standard of care. Even hospitals that participate in performance-improvement programs often struggle to start antibiotics, even within 6 hours of recognition. Nevertheless, the goal is a good one.
Some have questioned the early antibiotic recommendation because of concerns about antibiotic overuse and resistance. For a patient with some manifestation of systemic inflammation, such as organ dysfunction or hypotension with no clear cause, the campaign’s position is to provide empiric antibiotics early and then, if a noninfectious cause is found, to stop the antibiotics. Moreover, as soon as a causative pathogen has been identified, the regimen should be switched to the most appropriate antimicrobial that covers the pathogen and is safe and cost-effective. Collaboration with an antimicrobial stewardship program, if available, is encouraged.
FIND THE INFECTION SOURCE PROMPTLY: SOURCE CONTROL MAY BE REQUIRED
Recommendation. A specific anatomic diagnosis of infection (eg, necrotizing soft-tissue infection, peritonitis complicated by intra-abdominal infection, cholangitis, intestinal infarction) requiring consideration of emergency source control should be confirmed or excluded as soon as possible. If needed, surgical drainage should be undertaken for source control within the first 12 hours after a diagnosis is made (grade 1C).
FLUID THERAPY: CRYSTALLOIDS FIRST
Recommendation. In fluid resuscitation of severe sepsis, use crystalloids first (grade 1B).
No head-to-head trial has shown albumin to be superior to crystalloids, and crystalloids are less expensive. However, normal saline has a higher chloride content than plasma, which leads to non-anion-gap metabolic acidosis. It is called an unbalanced crystalloid, having a high chloride content and no buffer. There is concern that this reduces renal blood flow and the glomerular filtration rate, creating the potential for acute kidney injury. Although no high-level evidence supports this concern, some animal studies and historical control studies suggest that a balanced crystalloid such as Ringer’s lactate, Ringer’s acetate, or PlasmaLyte (having a chloride content close to that of plasma and the buffers acetate or lactate) may be associated with better outcome in resuscitation of severe sepsis.
Use albumin solution if necessary
Recommendation. Albumin should be used in the fluid resuscitation of severe sepsis and septic shock for patients who require substantial amounts of crystalloids (grade 2C).
Finfer et al5 compared the effect of fluid resuscitation with either an albumin or saline solution in nearly 7,000 patients in intensive care and found that death rates over 28 days were nearly identical between the two groups. Although this study was not designed to measure an effect in subsets of patients, the subgroup with severe sepsis had a lower mortality rate with albumin (relative risk 0.87, 95% confidence interval 0.74–1.02). In a meta-analysis of 17 studies of albumin vs crystalloids or albumin vs saline, Delaney et al6 found a significant survival advantage with an albumin solution in patients with sepsis and severe septic shock.
Sometimes, in patients admitted to intensive care with septic shock and receiving two or three vasopressors and large amounts of a crystalloid solution, vasopressors can be reduced when fluid is being given, but as soon as the fluid infusion rate is decreased, the need for increasing vasopressors returns. This scenario is an indication for changing to an albumin solution.
Recommendation. Initial fluid challenge in sepsis-induced tissue hypoperfusion (as evidenced by hypotension or elevated lactate) with suspicion of hypovolemia should be a minimum of 30 mL/kg of crystalloids, a portion of which can be an albumin equivalent. Some patients require more rapid administration and greater amounts of fluid (grade 1B).
Other fluid resuscitation considerations
Recommendation. Hydroxyethyl starch (hetastarch) should not be used for fluid resuscitation of severe sepsis and septic shock (grade 1B).
Five large clinical trials7–11 compared hetastarch with crystalloids in the resuscitation of severe sepsis or septic shock. None found an advantage to using hetastarch, and three found it to be associated with higher rates of acute kidney injury and renal-replacement therapy.
Blood is not considered a resuscitation fluid.
Full fluid replacement is still needed in heart or kidney disease
Often, doctors hesitate to administer full fluid resuscitation to patients with septic shock or sepsis-induced hypotension who have baseline cardiomyopathy with a low ejection fraction or who have end-stage renal disease and are anuric. However, these patients’ baseline intravascular volume status has changed because of venodilation and capillary leak leading to reduced blood return to the heart. They require the same amount of fluids as other patients to return to their baseline state.
To avoid fluid overload in these patients, however, we recommend providing fluid in smaller boluses. For a young, previously healthy patient, 2 L of crystalloid should be provided as quickly as possible. Patients with heart or kidney disease should receive smaller (250- or 500-mL) boluses, with oxygen saturation checked after each dose, as hypoxemia is one of only two potential downsides of aggressive fluid resuscitation (the other being the further raising of intra-abdominal pressure in the intra-abdominal compartment syndrome).
WHAT DRIVES HYPOTENSION IN SEPTIC SHOCK?
In septic shock, mechanisms that can lower the blood pressure include capillary leakage (loss of intravascular volume), decreased arteriolar resistance, decreased cardiac contractility, increased ventricular compliance, and increased venous capacitance (loss of intra-arterial volume).
Capillary leakage ranges from moderate to severe, and it is difficult to know the severity early on during resuscitation. The extent of capillary leakage is often apparent only after 24 hours of fluid resuscitation, when the large amount of fluid needed to maintain intravascular volume produces significant tissue edema. Within the first 24 hours of resuscitation of a patient with septic shock or in the presence of ongoing inflammation, one cannot use intake and output to judge the adequacy of fluid resuscitation.
Reduced arteriolar resistance may be an advantage in the nonhypotensive severely septic patient, compensating for the decreased ejection fraction, but it becomes problematic in the presence of hypotension. In addition, venodilation increases venous capacitance, producing a “sink” for blood and inadequate return of blood volume to the heart.
Decreased contractility of the left and right ventricles leads to compensatory sinus tachycardia.12 Reduced heart contractility can be seen by radionuclide angiography: little difference in chamber size is apparent in systole (immediately before contraction) vs diastole (immediately after contraction) (Figure 1).
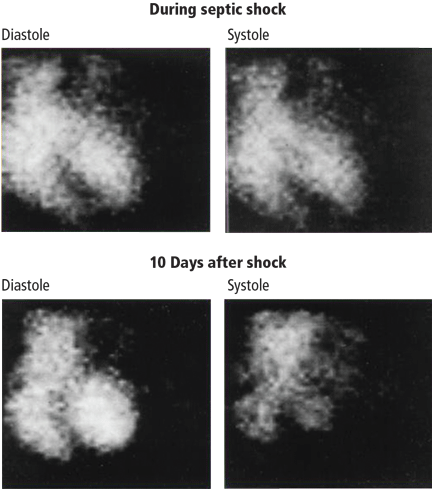
NOREPINEPHRINE IS THE FIRST-CHOICE VASOPRESSOR
If a patient remains hypotensive after replacement of intravascular volume, the hypotension is due to a combination of vasodilation and reduced contractility, and a combined inotrope-vasopressor is an appropriate drug to raise blood pressure. Therefore, the drug of first choice for raising blood pressure should be a combined inotrope-vasopressor.
There are three combined inotrope-vasopressors: dopamine, norepinephrine, and epinephrine. Head-to-head comparisons of norepinephrine and dopamine have supported a survival advantage with norepinephrine in patients with shock, including septic shock.13 A meta-analysis of six randomized trials totaling 2,768 patients also supports norepinephrine over dopamine in septic shock. Dopamine has been associated with a higher incidence of tachyarrhythmic events.14
Recommendations. Norepinephrine is the first choice for vasopressor therapy (grade 1B). If an additional agent is needed to maintain blood pressure, epinephrine should be added to norepinephrine (grade 2B). Alternatively, vasopressin (0.03 U/minute) can be added to norepinephrine to raise mean arterial pressure to target or to decrease the norepinephrine dose (ungraded recommendation).
Dopamine is not recommended as empiric or additive therapy for septic shock. It may be considered, however, in the presence of septic shock with sinus bradycardia.
Phenylephrine for special cases
Phenylephrine is a pure vasopressor: it decreases stroke volume and is particularly disadvantageous in patients with low cardiac output.
Recommendation. Phenylephrine is not recommended as empiric or additive therapy in the treatment of septic shock, with these exceptions (grade 1C):
- In unusual cases in which norepinephrine is associated with serious tachyarrhythmia, phenylephrine would be the least likely vasopressor to exacerbate arrhythmia
- If cardiac output is known to be high and blood pressure is persistently low
- If it is used as salvage therapy when combined inotrope-vasopressor drugs and low-dose vasopressin have failed to achieve the mean arterial pressure target.
RESUSCITATION OF SEPSIS-INDUCED TISSUE HYPOPERFUSION
A more severe form of sepsis-induced tissue hypoperfusion occurs in patients with severe sepsis, who require vasopressors after fluid challenge or have a lactate level of at least 4 mmol/L (36 mg/dL). Initial resuscitation is of utmost importance in these patients and often is done in the emergency department or regular hospital unit. These patients are targeted for “quantitative resuscitation,” ie, a protocol of fluid therapy and vasoactive agent support to achieve predefined end points.
Rivers et al15 published a landmark study of “early goal-directed therapy” targeting the early management of sepsis-induced tissue hypoperfusion (vasopressor requirement after fluid resuscitation or lactate > 4 mmol/L) and reported significant improvement in the survival rate when resuscitation was targeted to a superior vena cava oxygen saturation of 70%. Both control-group and active-treatment-group patients had central venous pressure targets of 8 mm Hg or greater. The Surviving Sepsis Campaign adopted these targets as recommendations in the original 2004 guidelines and continued through the 2013 guidelines, although the campaign’s sepsis management “bundles” that had originally included specific targets for central venous pressure and central venous oxygen saturation as above were changed in the 2013 guidelines to only measuring these variables (see discussion below).
Jones et al16 analyzed studies that involved early (within 24 hours of presentation) vs late (after 24 hours or unknown) quantitative resuscitation for sepsis-induced tissue hypoperfusion and found a significant reduction in the rate of death with early resuscitation but no difference with late resuscitation compared with standard therapy.
ALTERNATIVES TO MEASURING PRESSURE TO PREDICT RESPONSE TO FLUID
The campaign recognizes the limitation of pressure measurements to predict the response to fluid resuscitation. Some clinicians have objected to the guidelines, arguing that new bedside technology provides better information than central venous pressure or superior vena cava oxygen saturation.
It is useful to recall the Starling principle, which is based on the behavior of isolated myocardial fibrils that are put under the strain of graduated weights and then are stimulated to contract, modeling the contractility of the heart. The more the fibril is stretched, the more intense the contraction. Increased contractility explains why fluid resuscitation increases cardiac output; it is not simply a matter of increasing fluid volume in the veins. Increased volume in the left ventricle increases stretch, causing more intense contractility and higher stroke-volume cardiac output.
The guidelines are based on pressure measurements, but volume is the important measure that drives contractility. For this reason, the 2013 guidelines encourage the use of alternative measures if a hospital has the capability to assess and use them. These alternative measures include changes in pulse pressure, systolic pressure, and stroke volume during the respiratory cycle or with fluid bolus. The greater the variation in these measures, the more likely the patient will respond to additional fluid therapy.17 Normal values:
- Pulse pressure variation: < 13%
- Systolic pressure variation: < 10 mm Hg
- Stroke volume variation: < 10%.
The problem with the more sophisticated technologies is that they tend to be available only in academic centers and not at hospitals doing the critical early resuscitation of septic shock.
The serum lactate level
Measuring serum lactate levels is an alternative method for monitoring resuscitation of early septic shock. This method is widely available even with point-of-care testing. If the lactate level is elevated, quantitative resuscitation, fluids, inotropes, and oxygen delivery can be targeted to lactate clearance.
Recommendation. In patients in whom elevated lactate levels are used as a marker of tissue hypoperfusion, resuscitation should be targeted to normalize lactate as rapidly as possible (grade 2C).
STEROID THERAPY IS CONTROVERSIAL
Corticosteroid therapy for septic shock remains controversial. Although it has been deemphasized, it likely has a role in select patients.
Recommendation. Intravenous corticosteroids should not be used in adults with septic shock if adequate fluid resuscitation and vasopressor therapy restore hemodynamic stability (grade 2C). However, a patient on high doses of multiple vasopressors after adequate fluid resuscitation would likely benefit.
Recommendation. If corticosteroid therapy is used, hydrocortisone 200 mg should be given over 24 hours, preferentially by continuous intravenous infusion but alternatively 50 mg every 6 hours (grade 2D). This regimen can be continued for up to 7 days or tapered when shock resolves.
SURVIVING SEPSIS CAMPAIGN PERFORMANCE-IMPROVEMENT PROGRAM
By themselves, guidelines change bedside care very slowly. To effect change, protocols must be put in place and quality indicators must be measured. Beginning in 2005, the Surviving Sepsis Campaign converted its guidelines to selected sets of quality indicators, ie, severe sepsis bundles. The campaign published tools that hospitals could use to initiate performance improvement programs for diagnosis and management of severe sepsis and septic shock. The information was disseminated worldwide with a free software program. The program allowed data collection at the bedside to record performance with quality indicators.
In addition, the campaign requested user data so that performance could be tracked over time. In 2010, data on more than 10,000 patients in participating hospitals showed improved ability to achieve quality indicators. The longer a hospital continued the program, the better its compliance with management bundles; in addition, there was a concomitant reduction in hospital mortality rates.18
At this time, the database holds records for more than 30,000 patients. Mortality rates among campaign participants decreased from 37% in the first quarter to 26% in the 16th quarter worldwide, with a reduced relative risk of mortality of 28%.19 To assess whether background factors unrelated to campaign participation were contributing to the reduced rates, mortality rates of long-term participants were compared with those of new program participants; the finding supported the association with program participation.
Bundles revised
The campaign published updated performance bundles in the 2013 guidelines.
The 3-hour bundle remains the same. Within the first 3 hours of presentation with sepsis:
- Measure the serum lactate level.
- Obtain blood cultures before starting antibiotics.
- Start broad-spectrum antibiotics.
- Give a crystalloid (30 mL/kg) for hypotension or for lactate ≥ 4 mmol/L.
The 6-hour bundle has changed somewhat. Within 6 hours of presentation:
- If hypotension does not respond to initial fluid resuscitation, apply vasopressors to maintain mean arterial pressure ≥ 65 mm Hg.
- In the event of persistent arterial hypotension despite volume resuscitation (septic shock) or initial lactate ≥ 4 mmol/L, measure central venous pressure and central venous oxygen saturation.
- Remeasure lactate if the initial lactate level was elevated.
In light of the campaign’s recognition of alternatives to central venous pressure and central venous oxygen saturation for quantitative resuscitation targets, specific targets for these measures were not defined, allowing institutions the flexibility to base decisions on other technologies, such as inferior vena cava ultrasonography, systolic pressure variation, and changes in flow measures or estimates with fluid boluses if they have the capability.
Moreover, the second point in the 6-hour bundle is being further revised. The Protocolized Care for Early Septic Shock (ProCESS) trial20 and the Australasian Resuscitation in Sepsis Evaluation (ARISE) trial,21 both published in 2013, demonstrated that measuring central venous pressure and central venous oxygen saturation, although safe, is not necessary for successful resuscitation of patients with septic shock. Therefore, newer versions of the 6-hour bundle propose that physicians reassess intravascular volume status and tissue perfusion, after initial 30 mL/kg crystalloid administration, in the event of persistent hypotension (mean arterial pressure < 65 mm Hg, ie, vasopressor requirement) or an initial lactate level of 4 mmol/L or higher, and then document the findings. To meet the requirements, one must document either a repeat focused examination by a licensed independent practitioner (to include vital signs, cardiopulmonary, capillary refill, pulse, and skin findings) or two alternative items from the following options: central venous pressure, central venous oxygen saturation, bedside cardiovascular ultrasonography, and dynamic assessment of fluid responsiveness with passive leg-raising or fluid challenge.
Of interest, the ProCESS20 and ARISE21 trials supported early identification of septic shock, early use of antibiotics, and early aggressive fluid resuscitation as the likely reasons for the reduced mortality rates across all treatment groups in these studies.
REDUCING HOSPITAL MORTALITY RATES
Phase 3 of the campaign involves data from 30,000 patients with severe sepsis or septic shock in emergency departments (52%), medical and surgical units (35%), and critical care units (13%).
Hospital mortality rates were 28% for those who presented to the emergency department with sepsis vs 47% for those who developed it in the hospital.22 The reason for the substantial difference is unclear; possibly, diagnosis takes longer in medical and surgical units because of a lower nurse-to-patient ratio, leading to delay in diagnosis and treatment.
The finding of the greater risk of dying from sepsis in those who develop severe sepsis on medical and surgical floors has led to initiation of phase 4 of the campaign, conducted in four US-based collaborative groups in California, Illinois, New Jersey, and Florida, with 12 to 20 sites per collaborative. The collaborative is funded by the Moore Foundation and sponsored by the Society of Critical Care Medicine and the Society of Hospital Medicine. The purpose is to improve early recognition of severe sepsis through nurse screening of every patient during every shift of every day of hospitalization. The program empowers nurses to recognize and report sepsis, severe sepsis, and septic shock. The response differs depending on the hospital: some employ a rapid response or “sepsis alert,” others have a designated hospitalist on each shift who is informed, and hospitals that use private doctors may have a call-in system.
MUCH REMAINS TO BE DONE
The Surviving Sepsis Campaign has come far since the initial guidelines published in 2004. Thirty international organizations now sponsor and support the evidence-based guidelines. The sepsis performance improvement program deployed internationally has been associated with significant improvement in outcome in patients with severe sepsis.
How much of this is related to the campaign as opposed to other changes in health care cannot be clearly ascertained. In addition, how much of the Surviving Sepsis Campaign’s performance-improvement program effect is from attention to this patient group or from precise indicators is difficult to deduce. However, most experts in the field believe the Surviving Sepsis Campaign has significantly improved outcomes since its inception in 2002. Much still needs to be done as new evidence evolves.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41:580–637.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39:165–228.
- Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. HCHS Data Brief No. 62, June 2011. https://www.cdc.gov/nchs/products/databriefs/db62.htm.
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–1596.
- Finfer S, Bellomo R, Boyce N, Frency J, Myburgh J, Norton R; SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 2004; 350:2247–2256.
- Delaney AP, Dan A, McCaffrey J, et al. The role of albumin as a resuscitation fluid for patients with sepsis: a systematic review and meta-analysis. Crit Care Med 2011; 39:389–391.
- Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008; 358:125–139.
- Guidet B, Martinet O, Boulain T, et al. Assessment of haemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 vs. 0.9% NaCl fluid replacement in patients with severe sepsis: the CRYSTMAS study. Crit Care 2012; 16:R94.
- Perner A, Haase N, Guttormsen AB, et al; the 6S Trial Group and the Scandinavian Critical Care Trials Group. Hydroxyethyl starch 130.0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med 2012; 367:124–134.
- Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012; 367:1901–1911.
- Annane D, Siami S, Jaber S, et al; CRISTAL Investigators. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA 2013; 310:1809–1817.
- Dellinger RP. Cardiovascular management of septic shock. Crit Care Med 2003; 31:946–955.
- De Backer D, Biston P, Devriendt J, et al; SOAP II Investigators. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010; 362:779–789.
- De Backer D, Aldecoa C, Njimi H, Vincent JL. Dopamine versus norepinephrine in the treatment of septic shock: a meta-analysis. Crit Care Med 2012; 40:725–730.
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345:1368–1377.
- Jones AE, Brown MD, Trzeciak S, et al; Emergency Medicine Shock Research Network Investigators. The effect of a quantitative resuscitation strategy on mortality in patients with sepsis: a meta-analysis. Crit Care Med 2008; 36:2734–2739.
- Parry-Jones AJD, Pittman JAL. Arterial pressure and stroke volume variability as measurements for cardiovascular optimisation. Int J Intensive Care 2003; 2:67–72.
- Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med 2010; 38:367–374.
- Levy M, Artigas A, Phillips GS, et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis 2012; 12:919–924.
- ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014; 370:1683–1693.
- ARISE Investigators; ANZICS Clinical Trials Group, Peake SL, Delaney A, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014; 371:1496–1506.
- Levy MM, Dellinger RP, Townsend SA, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 2010; 36:222-231.
Sepsis is familiar to most physicians in clinical practice, but guidance from the medical literature on how best to manage it has traditionally been confusing.
Starting in 2002, the Surviving Sepsis Campaign has worked to reduce worldwide mortality from severe sepsis and septic shock by developing and publicizing guidelines of best practices based on evidence from the literature. The campaign published its first management guidelines in 2004.
In this article, I review the most recent guidelines1,2 (published in 2013) and discuss the campaign’s ongoing performance-improvement program.
DEFINING SEPSIS
Sepsis is a known or suspected infection plus systemic manifestations of infection. This includes the sepsis inflammatory response syndrome. Criteria include:
- Tachycardia (heart rate > 90 beats per minute)
- Tachypnea (> 20 breaths/minute or Paco2 < 32 mm Hg)
- Fever (temperature > 38.3°C [100.9°F]) or hypothermia (core temperature < 36°C [96.8°F])
- High or low white blood cell count (> 12.0 × 109/L or < 4.0 × 109/L), or a normal count with more than 10% immature cells.
The definition of sepsis was broadened in 2002 to include other systemic manifestations of infection, such as changes in blood glucose level and organ dysfunction.
Severe sepsis is defined as sepsis plus either acute organ dysfunction or tissue hypoperfusion due to infection, with tissue hypoperfusion defined as:
- Hypotension (systolic blood pressure < 90 mm Hg, or a drop in systolic blood pressure of > 40 mm Hg)
- Elevated lactate
- Low urine output
- Altered mental status.
In severe sepsis, organ dysfunction is caused by blood-borne toxins and involves acute lung and kidney injury, coagulopathy (thrombocytopenia and increased international normalized ratio), and liver dysfunction.
Septic shock is present when a patient requires vasopressors after adequate intravascular volume repletion.
SEPSIS IS DEADLY AND COSTLY
Severe sepsis is the leading cause of hospital death. Patients admitted with severe sepsis are eight times more likely to die than those admitted with other conditions.3 The economic burden is enormous: it is the most expensive condition treated in US hospitals, costing an estimated $20.3 billion in 2011, of which $12.7 billion came from Medicare.
THE SURVIVING SEPSIS CAMPAIGN
The Surviving Sepsis Campaign is a global effort to reduce the rate of death from severe sepsis. The campaign’s methods include:
- Educating physicians, the public, the media, and government about the high rates of morbidity and death in severe sepsis
- Creating evidence-based guidelines for managing sepsis and establishing global best-practice standards
- Facilitating the transfer of knowledge by developing performance-improvement programs to change bedside practice.
The campaign is funded with a grant from the Gordon and Betty Moore Foundation. The campaign’s guidelines are not associated with any direct or indirect industry support. The 2013 guidelines were backed by 30 international organizations.1,2
All recommendations are ranked with numerical and letter scores, according to the GRADE system: 1 indicates a strong recommendation and 2 a weak one. The letters A through D reflect the quality of evidence, ranging from high (A) to very low (D).
GIVING ANTIBIOTICS EARLY IMPROVES OUTCOMES
A number of studies have suggested that starting appropriate antibiotics early improves outcomes in severe sepsis and septic shock. The death rate increases with each hour of delay.4
Recommendation. Intravenous antibiotic therapy should be started as soon as possible, and within the first hour after recognition of septic shock (grade 1B) and severe sepsis without septic shock (grade 1C).
The feasibility of achieving this goal has not been scientifically validated, and the recommendation should not be misinterpreted as the current standard of care. Even hospitals that participate in performance-improvement programs often struggle to start antibiotics, even within 6 hours of recognition. Nevertheless, the goal is a good one.
Some have questioned the early antibiotic recommendation because of concerns about antibiotic overuse and resistance. For a patient with some manifestation of systemic inflammation, such as organ dysfunction or hypotension with no clear cause, the campaign’s position is to provide empiric antibiotics early and then, if a noninfectious cause is found, to stop the antibiotics. Moreover, as soon as a causative pathogen has been identified, the regimen should be switched to the most appropriate antimicrobial that covers the pathogen and is safe and cost-effective. Collaboration with an antimicrobial stewardship program, if available, is encouraged.
FIND THE INFECTION SOURCE PROMPTLY: SOURCE CONTROL MAY BE REQUIRED
Recommendation. A specific anatomic diagnosis of infection (eg, necrotizing soft-tissue infection, peritonitis complicated by intra-abdominal infection, cholangitis, intestinal infarction) requiring consideration of emergency source control should be confirmed or excluded as soon as possible. If needed, surgical drainage should be undertaken for source control within the first 12 hours after a diagnosis is made (grade 1C).
FLUID THERAPY: CRYSTALLOIDS FIRST
Recommendation. In fluid resuscitation of severe sepsis, use crystalloids first (grade 1B).
No head-to-head trial has shown albumin to be superior to crystalloids, and crystalloids are less expensive. However, normal saline has a higher chloride content than plasma, which leads to non-anion-gap metabolic acidosis. It is called an unbalanced crystalloid, having a high chloride content and no buffer. There is concern that this reduces renal blood flow and the glomerular filtration rate, creating the potential for acute kidney injury. Although no high-level evidence supports this concern, some animal studies and historical control studies suggest that a balanced crystalloid such as Ringer’s lactate, Ringer’s acetate, or PlasmaLyte (having a chloride content close to that of plasma and the buffers acetate or lactate) may be associated with better outcome in resuscitation of severe sepsis.
Use albumin solution if necessary
Recommendation. Albumin should be used in the fluid resuscitation of severe sepsis and septic shock for patients who require substantial amounts of crystalloids (grade 2C).
Finfer et al5 compared the effect of fluid resuscitation with either an albumin or saline solution in nearly 7,000 patients in intensive care and found that death rates over 28 days were nearly identical between the two groups. Although this study was not designed to measure an effect in subsets of patients, the subgroup with severe sepsis had a lower mortality rate with albumin (relative risk 0.87, 95% confidence interval 0.74–1.02). In a meta-analysis of 17 studies of albumin vs crystalloids or albumin vs saline, Delaney et al6 found a significant survival advantage with an albumin solution in patients with sepsis and severe septic shock.
Sometimes, in patients admitted to intensive care with septic shock and receiving two or three vasopressors and large amounts of a crystalloid solution, vasopressors can be reduced when fluid is being given, but as soon as the fluid infusion rate is decreased, the need for increasing vasopressors returns. This scenario is an indication for changing to an albumin solution.
Recommendation. Initial fluid challenge in sepsis-induced tissue hypoperfusion (as evidenced by hypotension or elevated lactate) with suspicion of hypovolemia should be a minimum of 30 mL/kg of crystalloids, a portion of which can be an albumin equivalent. Some patients require more rapid administration and greater amounts of fluid (grade 1B).
Other fluid resuscitation considerations
Recommendation. Hydroxyethyl starch (hetastarch) should not be used for fluid resuscitation of severe sepsis and septic shock (grade 1B).
Five large clinical trials7–11 compared hetastarch with crystalloids in the resuscitation of severe sepsis or septic shock. None found an advantage to using hetastarch, and three found it to be associated with higher rates of acute kidney injury and renal-replacement therapy.
Blood is not considered a resuscitation fluid.
Full fluid replacement is still needed in heart or kidney disease
Often, doctors hesitate to administer full fluid resuscitation to patients with septic shock or sepsis-induced hypotension who have baseline cardiomyopathy with a low ejection fraction or who have end-stage renal disease and are anuric. However, these patients’ baseline intravascular volume status has changed because of venodilation and capillary leak leading to reduced blood return to the heart. They require the same amount of fluids as other patients to return to their baseline state.
To avoid fluid overload in these patients, however, we recommend providing fluid in smaller boluses. For a young, previously healthy patient, 2 L of crystalloid should be provided as quickly as possible. Patients with heart or kidney disease should receive smaller (250- or 500-mL) boluses, with oxygen saturation checked after each dose, as hypoxemia is one of only two potential downsides of aggressive fluid resuscitation (the other being the further raising of intra-abdominal pressure in the intra-abdominal compartment syndrome).
WHAT DRIVES HYPOTENSION IN SEPTIC SHOCK?
In septic shock, mechanisms that can lower the blood pressure include capillary leakage (loss of intravascular volume), decreased arteriolar resistance, decreased cardiac contractility, increased ventricular compliance, and increased venous capacitance (loss of intra-arterial volume).
Capillary leakage ranges from moderate to severe, and it is difficult to know the severity early on during resuscitation. The extent of capillary leakage is often apparent only after 24 hours of fluid resuscitation, when the large amount of fluid needed to maintain intravascular volume produces significant tissue edema. Within the first 24 hours of resuscitation of a patient with septic shock or in the presence of ongoing inflammation, one cannot use intake and output to judge the adequacy of fluid resuscitation.
Reduced arteriolar resistance may be an advantage in the nonhypotensive severely septic patient, compensating for the decreased ejection fraction, but it becomes problematic in the presence of hypotension. In addition, venodilation increases venous capacitance, producing a “sink” for blood and inadequate return of blood volume to the heart.
Decreased contractility of the left and right ventricles leads to compensatory sinus tachycardia.12 Reduced heart contractility can be seen by radionuclide angiography: little difference in chamber size is apparent in systole (immediately before contraction) vs diastole (immediately after contraction) (Figure 1).

NOREPINEPHRINE IS THE FIRST-CHOICE VASOPRESSOR
If a patient remains hypotensive after replacement of intravascular volume, the hypotension is due to a combination of vasodilation and reduced contractility, and a combined inotrope-vasopressor is an appropriate drug to raise blood pressure. Therefore, the drug of first choice for raising blood pressure should be a combined inotrope-vasopressor.
There are three combined inotrope-vasopressors: dopamine, norepinephrine, and epinephrine. Head-to-head comparisons of norepinephrine and dopamine have supported a survival advantage with norepinephrine in patients with shock, including septic shock.13 A meta-analysis of six randomized trials totaling 2,768 patients also supports norepinephrine over dopamine in septic shock. Dopamine has been associated with a higher incidence of tachyarrhythmic events.14
Recommendations. Norepinephrine is the first choice for vasopressor therapy (grade 1B). If an additional agent is needed to maintain blood pressure, epinephrine should be added to norepinephrine (grade 2B). Alternatively, vasopressin (0.03 U/minute) can be added to norepinephrine to raise mean arterial pressure to target or to decrease the norepinephrine dose (ungraded recommendation).
Dopamine is not recommended as empiric or additive therapy for septic shock. It may be considered, however, in the presence of septic shock with sinus bradycardia.
Phenylephrine for special cases
Phenylephrine is a pure vasopressor: it decreases stroke volume and is particularly disadvantageous in patients with low cardiac output.
Recommendation. Phenylephrine is not recommended as empiric or additive therapy in the treatment of septic shock, with these exceptions (grade 1C):
- In unusual cases in which norepinephrine is associated with serious tachyarrhythmia, phenylephrine would be the least likely vasopressor to exacerbate arrhythmia
- If cardiac output is known to be high and blood pressure is persistently low
- If it is used as salvage therapy when combined inotrope-vasopressor drugs and low-dose vasopressin have failed to achieve the mean arterial pressure target.
RESUSCITATION OF SEPSIS-INDUCED TISSUE HYPOPERFUSION
A more severe form of sepsis-induced tissue hypoperfusion occurs in patients with severe sepsis, who require vasopressors after fluid challenge or have a lactate level of at least 4 mmol/L (36 mg/dL). Initial resuscitation is of utmost importance in these patients and often is done in the emergency department or regular hospital unit. These patients are targeted for “quantitative resuscitation,” ie, a protocol of fluid therapy and vasoactive agent support to achieve predefined end points.
Rivers et al15 published a landmark study of “early goal-directed therapy” targeting the early management of sepsis-induced tissue hypoperfusion (vasopressor requirement after fluid resuscitation or lactate > 4 mmol/L) and reported significant improvement in the survival rate when resuscitation was targeted to a superior vena cava oxygen saturation of 70%. Both control-group and active-treatment-group patients had central venous pressure targets of 8 mm Hg or greater. The Surviving Sepsis Campaign adopted these targets as recommendations in the original 2004 guidelines and continued through the 2013 guidelines, although the campaign’s sepsis management “bundles” that had originally included specific targets for central venous pressure and central venous oxygen saturation as above were changed in the 2013 guidelines to only measuring these variables (see discussion below).
Jones et al16 analyzed studies that involved early (within 24 hours of presentation) vs late (after 24 hours or unknown) quantitative resuscitation for sepsis-induced tissue hypoperfusion and found a significant reduction in the rate of death with early resuscitation but no difference with late resuscitation compared with standard therapy.
ALTERNATIVES TO MEASURING PRESSURE TO PREDICT RESPONSE TO FLUID
The campaign recognizes the limitation of pressure measurements to predict the response to fluid resuscitation. Some clinicians have objected to the guidelines, arguing that new bedside technology provides better information than central venous pressure or superior vena cava oxygen saturation.
It is useful to recall the Starling principle, which is based on the behavior of isolated myocardial fibrils that are put under the strain of graduated weights and then are stimulated to contract, modeling the contractility of the heart. The more the fibril is stretched, the more intense the contraction. Increased contractility explains why fluid resuscitation increases cardiac output; it is not simply a matter of increasing fluid volume in the veins. Increased volume in the left ventricle increases stretch, causing more intense contractility and higher stroke-volume cardiac output.
The guidelines are based on pressure measurements, but volume is the important measure that drives contractility. For this reason, the 2013 guidelines encourage the use of alternative measures if a hospital has the capability to assess and use them. These alternative measures include changes in pulse pressure, systolic pressure, and stroke volume during the respiratory cycle or with fluid bolus. The greater the variation in these measures, the more likely the patient will respond to additional fluid therapy.17 Normal values:
- Pulse pressure variation: < 13%
- Systolic pressure variation: < 10 mm Hg
- Stroke volume variation: < 10%.
The problem with the more sophisticated technologies is that they tend to be available only in academic centers and not at hospitals doing the critical early resuscitation of septic shock.
The serum lactate level
Measuring serum lactate levels is an alternative method for monitoring resuscitation of early septic shock. This method is widely available even with point-of-care testing. If the lactate level is elevated, quantitative resuscitation, fluids, inotropes, and oxygen delivery can be targeted to lactate clearance.
Recommendation. In patients in whom elevated lactate levels are used as a marker of tissue hypoperfusion, resuscitation should be targeted to normalize lactate as rapidly as possible (grade 2C).
STEROID THERAPY IS CONTROVERSIAL
Corticosteroid therapy for septic shock remains controversial. Although it has been deemphasized, it likely has a role in select patients.
Recommendation. Intravenous corticosteroids should not be used in adults with septic shock if adequate fluid resuscitation and vasopressor therapy restore hemodynamic stability (grade 2C). However, a patient on high doses of multiple vasopressors after adequate fluid resuscitation would likely benefit.
Recommendation. If corticosteroid therapy is used, hydrocortisone 200 mg should be given over 24 hours, preferentially by continuous intravenous infusion but alternatively 50 mg every 6 hours (grade 2D). This regimen can be continued for up to 7 days or tapered when shock resolves.
SURVIVING SEPSIS CAMPAIGN PERFORMANCE-IMPROVEMENT PROGRAM
By themselves, guidelines change bedside care very slowly. To effect change, protocols must be put in place and quality indicators must be measured. Beginning in 2005, the Surviving Sepsis Campaign converted its guidelines to selected sets of quality indicators, ie, severe sepsis bundles. The campaign published tools that hospitals could use to initiate performance improvement programs for diagnosis and management of severe sepsis and septic shock. The information was disseminated worldwide with a free software program. The program allowed data collection at the bedside to record performance with quality indicators.
In addition, the campaign requested user data so that performance could be tracked over time. In 2010, data on more than 10,000 patients in participating hospitals showed improved ability to achieve quality indicators. The longer a hospital continued the program, the better its compliance with management bundles; in addition, there was a concomitant reduction in hospital mortality rates.18
At this time, the database holds records for more than 30,000 patients. Mortality rates among campaign participants decreased from 37% in the first quarter to 26% in the 16th quarter worldwide, with a reduced relative risk of mortality of 28%.19 To assess whether background factors unrelated to campaign participation were contributing to the reduced rates, mortality rates of long-term participants were compared with those of new program participants; the finding supported the association with program participation.
Bundles revised
The campaign published updated performance bundles in the 2013 guidelines.
The 3-hour bundle remains the same. Within the first 3 hours of presentation with sepsis:
- Measure the serum lactate level.
- Obtain blood cultures before starting antibiotics.
- Start broad-spectrum antibiotics.
- Give a crystalloid (30 mL/kg) for hypotension or for lactate ≥ 4 mmol/L.
The 6-hour bundle has changed somewhat. Within 6 hours of presentation:
- If hypotension does not respond to initial fluid resuscitation, apply vasopressors to maintain mean arterial pressure ≥ 65 mm Hg.
- In the event of persistent arterial hypotension despite volume resuscitation (septic shock) or initial lactate ≥ 4 mmol/L, measure central venous pressure and central venous oxygen saturation.
- Remeasure lactate if the initial lactate level was elevated.
In light of the campaign’s recognition of alternatives to central venous pressure and central venous oxygen saturation for quantitative resuscitation targets, specific targets for these measures were not defined, allowing institutions the flexibility to base decisions on other technologies, such as inferior vena cava ultrasonography, systolic pressure variation, and changes in flow measures or estimates with fluid boluses if they have the capability.
Moreover, the second point in the 6-hour bundle is being further revised. The Protocolized Care for Early Septic Shock (ProCESS) trial20 and the Australasian Resuscitation in Sepsis Evaluation (ARISE) trial,21 both published in 2013, demonstrated that measuring central venous pressure and central venous oxygen saturation, although safe, is not necessary for successful resuscitation of patients with septic shock. Therefore, newer versions of the 6-hour bundle propose that physicians reassess intravascular volume status and tissue perfusion, after initial 30 mL/kg crystalloid administration, in the event of persistent hypotension (mean arterial pressure < 65 mm Hg, ie, vasopressor requirement) or an initial lactate level of 4 mmol/L or higher, and then document the findings. To meet the requirements, one must document either a repeat focused examination by a licensed independent practitioner (to include vital signs, cardiopulmonary, capillary refill, pulse, and skin findings) or two alternative items from the following options: central venous pressure, central venous oxygen saturation, bedside cardiovascular ultrasonography, and dynamic assessment of fluid responsiveness with passive leg-raising or fluid challenge.
Of interest, the ProCESS20 and ARISE21 trials supported early identification of septic shock, early use of antibiotics, and early aggressive fluid resuscitation as the likely reasons for the reduced mortality rates across all treatment groups in these studies.
REDUCING HOSPITAL MORTALITY RATES
Phase 3 of the campaign involves data from 30,000 patients with severe sepsis or septic shock in emergency departments (52%), medical and surgical units (35%), and critical care units (13%).
Hospital mortality rates were 28% for those who presented to the emergency department with sepsis vs 47% for those who developed it in the hospital.22 The reason for the substantial difference is unclear; possibly, diagnosis takes longer in medical and surgical units because of a lower nurse-to-patient ratio, leading to delay in diagnosis and treatment.
The finding of the greater risk of dying from sepsis in those who develop severe sepsis on medical and surgical floors has led to initiation of phase 4 of the campaign, conducted in four US-based collaborative groups in California, Illinois, New Jersey, and Florida, with 12 to 20 sites per collaborative. The collaborative is funded by the Moore Foundation and sponsored by the Society of Critical Care Medicine and the Society of Hospital Medicine. The purpose is to improve early recognition of severe sepsis through nurse screening of every patient during every shift of every day of hospitalization. The program empowers nurses to recognize and report sepsis, severe sepsis, and septic shock. The response differs depending on the hospital: some employ a rapid response or “sepsis alert,” others have a designated hospitalist on each shift who is informed, and hospitals that use private doctors may have a call-in system.
MUCH REMAINS TO BE DONE
The Surviving Sepsis Campaign has come far since the initial guidelines published in 2004. Thirty international organizations now sponsor and support the evidence-based guidelines. The sepsis performance improvement program deployed internationally has been associated with significant improvement in outcome in patients with severe sepsis.
How much of this is related to the campaign as opposed to other changes in health care cannot be clearly ascertained. In addition, how much of the Surviving Sepsis Campaign’s performance-improvement program effect is from attention to this patient group or from precise indicators is difficult to deduce. However, most experts in the field believe the Surviving Sepsis Campaign has significantly improved outcomes since its inception in 2002. Much still needs to be done as new evidence evolves.
Sepsis is familiar to most physicians in clinical practice, but guidance from the medical literature on how best to manage it has traditionally been confusing.
Starting in 2002, the Surviving Sepsis Campaign has worked to reduce worldwide mortality from severe sepsis and septic shock by developing and publicizing guidelines of best practices based on evidence from the literature. The campaign published its first management guidelines in 2004.
In this article, I review the most recent guidelines1,2 (published in 2013) and discuss the campaign’s ongoing performance-improvement program.
DEFINING SEPSIS
Sepsis is a known or suspected infection plus systemic manifestations of infection. This includes the sepsis inflammatory response syndrome. Criteria include:
- Tachycardia (heart rate > 90 beats per minute)
- Tachypnea (> 20 breaths/minute or Paco2 < 32 mm Hg)
- Fever (temperature > 38.3°C [100.9°F]) or hypothermia (core temperature < 36°C [96.8°F])
- High or low white blood cell count (> 12.0 × 109/L or < 4.0 × 109/L), or a normal count with more than 10% immature cells.
The definition of sepsis was broadened in 2002 to include other systemic manifestations of infection, such as changes in blood glucose level and organ dysfunction.
Severe sepsis is defined as sepsis plus either acute organ dysfunction or tissue hypoperfusion due to infection, with tissue hypoperfusion defined as:
- Hypotension (systolic blood pressure < 90 mm Hg, or a drop in systolic blood pressure of > 40 mm Hg)
- Elevated lactate
- Low urine output
- Altered mental status.
In severe sepsis, organ dysfunction is caused by blood-borne toxins and involves acute lung and kidney injury, coagulopathy (thrombocytopenia and increased international normalized ratio), and liver dysfunction.
Septic shock is present when a patient requires vasopressors after adequate intravascular volume repletion.
SEPSIS IS DEADLY AND COSTLY
Severe sepsis is the leading cause of hospital death. Patients admitted with severe sepsis are eight times more likely to die than those admitted with other conditions.3 The economic burden is enormous: it is the most expensive condition treated in US hospitals, costing an estimated $20.3 billion in 2011, of which $12.7 billion came from Medicare.
THE SURVIVING SEPSIS CAMPAIGN
The Surviving Sepsis Campaign is a global effort to reduce the rate of death from severe sepsis. The campaign’s methods include:
- Educating physicians, the public, the media, and government about the high rates of morbidity and death in severe sepsis
- Creating evidence-based guidelines for managing sepsis and establishing global best-practice standards
- Facilitating the transfer of knowledge by developing performance-improvement programs to change bedside practice.
The campaign is funded with a grant from the Gordon and Betty Moore Foundation. The campaign’s guidelines are not associated with any direct or indirect industry support. The 2013 guidelines were backed by 30 international organizations.1,2
All recommendations are ranked with numerical and letter scores, according to the GRADE system: 1 indicates a strong recommendation and 2 a weak one. The letters A through D reflect the quality of evidence, ranging from high (A) to very low (D).
GIVING ANTIBIOTICS EARLY IMPROVES OUTCOMES
A number of studies have suggested that starting appropriate antibiotics early improves outcomes in severe sepsis and septic shock. The death rate increases with each hour of delay.4
Recommendation. Intravenous antibiotic therapy should be started as soon as possible, and within the first hour after recognition of septic shock (grade 1B) and severe sepsis without septic shock (grade 1C).
The feasibility of achieving this goal has not been scientifically validated, and the recommendation should not be misinterpreted as the current standard of care. Even hospitals that participate in performance-improvement programs often struggle to start antibiotics, even within 6 hours of recognition. Nevertheless, the goal is a good one.
Some have questioned the early antibiotic recommendation because of concerns about antibiotic overuse and resistance. For a patient with some manifestation of systemic inflammation, such as organ dysfunction or hypotension with no clear cause, the campaign’s position is to provide empiric antibiotics early and then, if a noninfectious cause is found, to stop the antibiotics. Moreover, as soon as a causative pathogen has been identified, the regimen should be switched to the most appropriate antimicrobial that covers the pathogen and is safe and cost-effective. Collaboration with an antimicrobial stewardship program, if available, is encouraged.
FIND THE INFECTION SOURCE PROMPTLY: SOURCE CONTROL MAY BE REQUIRED
Recommendation. A specific anatomic diagnosis of infection (eg, necrotizing soft-tissue infection, peritonitis complicated by intra-abdominal infection, cholangitis, intestinal infarction) requiring consideration of emergency source control should be confirmed or excluded as soon as possible. If needed, surgical drainage should be undertaken for source control within the first 12 hours after a diagnosis is made (grade 1C).
FLUID THERAPY: CRYSTALLOIDS FIRST
Recommendation. In fluid resuscitation of severe sepsis, use crystalloids first (grade 1B).
No head-to-head trial has shown albumin to be superior to crystalloids, and crystalloids are less expensive. However, normal saline has a higher chloride content than plasma, which leads to non-anion-gap metabolic acidosis. It is called an unbalanced crystalloid, having a high chloride content and no buffer. There is concern that this reduces renal blood flow and the glomerular filtration rate, creating the potential for acute kidney injury. Although no high-level evidence supports this concern, some animal studies and historical control studies suggest that a balanced crystalloid such as Ringer’s lactate, Ringer’s acetate, or PlasmaLyte (having a chloride content close to that of plasma and the buffers acetate or lactate) may be associated with better outcome in resuscitation of severe sepsis.
Use albumin solution if necessary
Recommendation. Albumin should be used in the fluid resuscitation of severe sepsis and septic shock for patients who require substantial amounts of crystalloids (grade 2C).
Finfer et al5 compared the effect of fluid resuscitation with either an albumin or saline solution in nearly 7,000 patients in intensive care and found that death rates over 28 days were nearly identical between the two groups. Although this study was not designed to measure an effect in subsets of patients, the subgroup with severe sepsis had a lower mortality rate with albumin (relative risk 0.87, 95% confidence interval 0.74–1.02). In a meta-analysis of 17 studies of albumin vs crystalloids or albumin vs saline, Delaney et al6 found a significant survival advantage with an albumin solution in patients with sepsis and severe septic shock.
Sometimes, in patients admitted to intensive care with septic shock and receiving two or three vasopressors and large amounts of a crystalloid solution, vasopressors can be reduced when fluid is being given, but as soon as the fluid infusion rate is decreased, the need for increasing vasopressors returns. This scenario is an indication for changing to an albumin solution.
Recommendation. Initial fluid challenge in sepsis-induced tissue hypoperfusion (as evidenced by hypotension or elevated lactate) with suspicion of hypovolemia should be a minimum of 30 mL/kg of crystalloids, a portion of which can be an albumin equivalent. Some patients require more rapid administration and greater amounts of fluid (grade 1B).
Other fluid resuscitation considerations
Recommendation. Hydroxyethyl starch (hetastarch) should not be used for fluid resuscitation of severe sepsis and septic shock (grade 1B).
Five large clinical trials7–11 compared hetastarch with crystalloids in the resuscitation of severe sepsis or septic shock. None found an advantage to using hetastarch, and three found it to be associated with higher rates of acute kidney injury and renal-replacement therapy.
Blood is not considered a resuscitation fluid.
Full fluid replacement is still needed in heart or kidney disease
Often, doctors hesitate to administer full fluid resuscitation to patients with septic shock or sepsis-induced hypotension who have baseline cardiomyopathy with a low ejection fraction or who have end-stage renal disease and are anuric. However, these patients’ baseline intravascular volume status has changed because of venodilation and capillary leak leading to reduced blood return to the heart. They require the same amount of fluids as other patients to return to their baseline state.
To avoid fluid overload in these patients, however, we recommend providing fluid in smaller boluses. For a young, previously healthy patient, 2 L of crystalloid should be provided as quickly as possible. Patients with heart or kidney disease should receive smaller (250- or 500-mL) boluses, with oxygen saturation checked after each dose, as hypoxemia is one of only two potential downsides of aggressive fluid resuscitation (the other being the further raising of intra-abdominal pressure in the intra-abdominal compartment syndrome).
WHAT DRIVES HYPOTENSION IN SEPTIC SHOCK?
In septic shock, mechanisms that can lower the blood pressure include capillary leakage (loss of intravascular volume), decreased arteriolar resistance, decreased cardiac contractility, increased ventricular compliance, and increased venous capacitance (loss of intra-arterial volume).
Capillary leakage ranges from moderate to severe, and it is difficult to know the severity early on during resuscitation. The extent of capillary leakage is often apparent only after 24 hours of fluid resuscitation, when the large amount of fluid needed to maintain intravascular volume produces significant tissue edema. Within the first 24 hours of resuscitation of a patient with septic shock or in the presence of ongoing inflammation, one cannot use intake and output to judge the adequacy of fluid resuscitation.
Reduced arteriolar resistance may be an advantage in the nonhypotensive severely septic patient, compensating for the decreased ejection fraction, but it becomes problematic in the presence of hypotension. In addition, venodilation increases venous capacitance, producing a “sink” for blood and inadequate return of blood volume to the heart.
Decreased contractility of the left and right ventricles leads to compensatory sinus tachycardia.12 Reduced heart contractility can be seen by radionuclide angiography: little difference in chamber size is apparent in systole (immediately before contraction) vs diastole (immediately after contraction) (Figure 1).

NOREPINEPHRINE IS THE FIRST-CHOICE VASOPRESSOR
If a patient remains hypotensive after replacement of intravascular volume, the hypotension is due to a combination of vasodilation and reduced contractility, and a combined inotrope-vasopressor is an appropriate drug to raise blood pressure. Therefore, the drug of first choice for raising blood pressure should be a combined inotrope-vasopressor.
There are three combined inotrope-vasopressors: dopamine, norepinephrine, and epinephrine. Head-to-head comparisons of norepinephrine and dopamine have supported a survival advantage with norepinephrine in patients with shock, including septic shock.13 A meta-analysis of six randomized trials totaling 2,768 patients also supports norepinephrine over dopamine in septic shock. Dopamine has been associated with a higher incidence of tachyarrhythmic events.14
Recommendations. Norepinephrine is the first choice for vasopressor therapy (grade 1B). If an additional agent is needed to maintain blood pressure, epinephrine should be added to norepinephrine (grade 2B). Alternatively, vasopressin (0.03 U/minute) can be added to norepinephrine to raise mean arterial pressure to target or to decrease the norepinephrine dose (ungraded recommendation).
Dopamine is not recommended as empiric or additive therapy for septic shock. It may be considered, however, in the presence of septic shock with sinus bradycardia.
Phenylephrine for special cases
Phenylephrine is a pure vasopressor: it decreases stroke volume and is particularly disadvantageous in patients with low cardiac output.
Recommendation. Phenylephrine is not recommended as empiric or additive therapy in the treatment of septic shock, with these exceptions (grade 1C):
- In unusual cases in which norepinephrine is associated with serious tachyarrhythmia, phenylephrine would be the least likely vasopressor to exacerbate arrhythmia
- If cardiac output is known to be high and blood pressure is persistently low
- If it is used as salvage therapy when combined inotrope-vasopressor drugs and low-dose vasopressin have failed to achieve the mean arterial pressure target.
RESUSCITATION OF SEPSIS-INDUCED TISSUE HYPOPERFUSION
A more severe form of sepsis-induced tissue hypoperfusion occurs in patients with severe sepsis, who require vasopressors after fluid challenge or have a lactate level of at least 4 mmol/L (36 mg/dL). Initial resuscitation is of utmost importance in these patients and often is done in the emergency department or regular hospital unit. These patients are targeted for “quantitative resuscitation,” ie, a protocol of fluid therapy and vasoactive agent support to achieve predefined end points.
Rivers et al15 published a landmark study of “early goal-directed therapy” targeting the early management of sepsis-induced tissue hypoperfusion (vasopressor requirement after fluid resuscitation or lactate > 4 mmol/L) and reported significant improvement in the survival rate when resuscitation was targeted to a superior vena cava oxygen saturation of 70%. Both control-group and active-treatment-group patients had central venous pressure targets of 8 mm Hg or greater. The Surviving Sepsis Campaign adopted these targets as recommendations in the original 2004 guidelines and continued through the 2013 guidelines, although the campaign’s sepsis management “bundles” that had originally included specific targets for central venous pressure and central venous oxygen saturation as above were changed in the 2013 guidelines to only measuring these variables (see discussion below).
Jones et al16 analyzed studies that involved early (within 24 hours of presentation) vs late (after 24 hours or unknown) quantitative resuscitation for sepsis-induced tissue hypoperfusion and found a significant reduction in the rate of death with early resuscitation but no difference with late resuscitation compared with standard therapy.
ALTERNATIVES TO MEASURING PRESSURE TO PREDICT RESPONSE TO FLUID
The campaign recognizes the limitation of pressure measurements to predict the response to fluid resuscitation. Some clinicians have objected to the guidelines, arguing that new bedside technology provides better information than central venous pressure or superior vena cava oxygen saturation.
It is useful to recall the Starling principle, which is based on the behavior of isolated myocardial fibrils that are put under the strain of graduated weights and then are stimulated to contract, modeling the contractility of the heart. The more the fibril is stretched, the more intense the contraction. Increased contractility explains why fluid resuscitation increases cardiac output; it is not simply a matter of increasing fluid volume in the veins. Increased volume in the left ventricle increases stretch, causing more intense contractility and higher stroke-volume cardiac output.
The guidelines are based on pressure measurements, but volume is the important measure that drives contractility. For this reason, the 2013 guidelines encourage the use of alternative measures if a hospital has the capability to assess and use them. These alternative measures include changes in pulse pressure, systolic pressure, and stroke volume during the respiratory cycle or with fluid bolus. The greater the variation in these measures, the more likely the patient will respond to additional fluid therapy.17 Normal values:
- Pulse pressure variation: < 13%
- Systolic pressure variation: < 10 mm Hg
- Stroke volume variation: < 10%.
The problem with the more sophisticated technologies is that they tend to be available only in academic centers and not at hospitals doing the critical early resuscitation of septic shock.
The serum lactate level
Measuring serum lactate levels is an alternative method for monitoring resuscitation of early septic shock. This method is widely available even with point-of-care testing. If the lactate level is elevated, quantitative resuscitation, fluids, inotropes, and oxygen delivery can be targeted to lactate clearance.
Recommendation. In patients in whom elevated lactate levels are used as a marker of tissue hypoperfusion, resuscitation should be targeted to normalize lactate as rapidly as possible (grade 2C).
STEROID THERAPY IS CONTROVERSIAL
Corticosteroid therapy for septic shock remains controversial. Although it has been deemphasized, it likely has a role in select patients.
Recommendation. Intravenous corticosteroids should not be used in adults with septic shock if adequate fluid resuscitation and vasopressor therapy restore hemodynamic stability (grade 2C). However, a patient on high doses of multiple vasopressors after adequate fluid resuscitation would likely benefit.
Recommendation. If corticosteroid therapy is used, hydrocortisone 200 mg should be given over 24 hours, preferentially by continuous intravenous infusion but alternatively 50 mg every 6 hours (grade 2D). This regimen can be continued for up to 7 days or tapered when shock resolves.
SURVIVING SEPSIS CAMPAIGN PERFORMANCE-IMPROVEMENT PROGRAM
By themselves, guidelines change bedside care very slowly. To effect change, protocols must be put in place and quality indicators must be measured. Beginning in 2005, the Surviving Sepsis Campaign converted its guidelines to selected sets of quality indicators, ie, severe sepsis bundles. The campaign published tools that hospitals could use to initiate performance improvement programs for diagnosis and management of severe sepsis and septic shock. The information was disseminated worldwide with a free software program. The program allowed data collection at the bedside to record performance with quality indicators.
In addition, the campaign requested user data so that performance could be tracked over time. In 2010, data on more than 10,000 patients in participating hospitals showed improved ability to achieve quality indicators. The longer a hospital continued the program, the better its compliance with management bundles; in addition, there was a concomitant reduction in hospital mortality rates.18
At this time, the database holds records for more than 30,000 patients. Mortality rates among campaign participants decreased from 37% in the first quarter to 26% in the 16th quarter worldwide, with a reduced relative risk of mortality of 28%.19 To assess whether background factors unrelated to campaign participation were contributing to the reduced rates, mortality rates of long-term participants were compared with those of new program participants; the finding supported the association with program participation.
Bundles revised
The campaign published updated performance bundles in the 2013 guidelines.
The 3-hour bundle remains the same. Within the first 3 hours of presentation with sepsis:
- Measure the serum lactate level.
- Obtain blood cultures before starting antibiotics.
- Start broad-spectrum antibiotics.
- Give a crystalloid (30 mL/kg) for hypotension or for lactate ≥ 4 mmol/L.
The 6-hour bundle has changed somewhat. Within 6 hours of presentation:
- If hypotension does not respond to initial fluid resuscitation, apply vasopressors to maintain mean arterial pressure ≥ 65 mm Hg.
- In the event of persistent arterial hypotension despite volume resuscitation (septic shock) or initial lactate ≥ 4 mmol/L, measure central venous pressure and central venous oxygen saturation.
- Remeasure lactate if the initial lactate level was elevated.
In light of the campaign’s recognition of alternatives to central venous pressure and central venous oxygen saturation for quantitative resuscitation targets, specific targets for these measures were not defined, allowing institutions the flexibility to base decisions on other technologies, such as inferior vena cava ultrasonography, systolic pressure variation, and changes in flow measures or estimates with fluid boluses if they have the capability.
Moreover, the second point in the 6-hour bundle is being further revised. The Protocolized Care for Early Septic Shock (ProCESS) trial20 and the Australasian Resuscitation in Sepsis Evaluation (ARISE) trial,21 both published in 2013, demonstrated that measuring central venous pressure and central venous oxygen saturation, although safe, is not necessary for successful resuscitation of patients with septic shock. Therefore, newer versions of the 6-hour bundle propose that physicians reassess intravascular volume status and tissue perfusion, after initial 30 mL/kg crystalloid administration, in the event of persistent hypotension (mean arterial pressure < 65 mm Hg, ie, vasopressor requirement) or an initial lactate level of 4 mmol/L or higher, and then document the findings. To meet the requirements, one must document either a repeat focused examination by a licensed independent practitioner (to include vital signs, cardiopulmonary, capillary refill, pulse, and skin findings) or two alternative items from the following options: central venous pressure, central venous oxygen saturation, bedside cardiovascular ultrasonography, and dynamic assessment of fluid responsiveness with passive leg-raising or fluid challenge.
Of interest, the ProCESS20 and ARISE21 trials supported early identification of septic shock, early use of antibiotics, and early aggressive fluid resuscitation as the likely reasons for the reduced mortality rates across all treatment groups in these studies.
REDUCING HOSPITAL MORTALITY RATES
Phase 3 of the campaign involves data from 30,000 patients with severe sepsis or septic shock in emergency departments (52%), medical and surgical units (35%), and critical care units (13%).
Hospital mortality rates were 28% for those who presented to the emergency department with sepsis vs 47% for those who developed it in the hospital.22 The reason for the substantial difference is unclear; possibly, diagnosis takes longer in medical and surgical units because of a lower nurse-to-patient ratio, leading to delay in diagnosis and treatment.
The finding of the greater risk of dying from sepsis in those who develop severe sepsis on medical and surgical floors has led to initiation of phase 4 of the campaign, conducted in four US-based collaborative groups in California, Illinois, New Jersey, and Florida, with 12 to 20 sites per collaborative. The collaborative is funded by the Moore Foundation and sponsored by the Society of Critical Care Medicine and the Society of Hospital Medicine. The purpose is to improve early recognition of severe sepsis through nurse screening of every patient during every shift of every day of hospitalization. The program empowers nurses to recognize and report sepsis, severe sepsis, and septic shock. The response differs depending on the hospital: some employ a rapid response or “sepsis alert,” others have a designated hospitalist on each shift who is informed, and hospitals that use private doctors may have a call-in system.
MUCH REMAINS TO BE DONE
The Surviving Sepsis Campaign has come far since the initial guidelines published in 2004. Thirty international organizations now sponsor and support the evidence-based guidelines. The sepsis performance improvement program deployed internationally has been associated with significant improvement in outcome in patients with severe sepsis.
How much of this is related to the campaign as opposed to other changes in health care cannot be clearly ascertained. In addition, how much of the Surviving Sepsis Campaign’s performance-improvement program effect is from attention to this patient group or from precise indicators is difficult to deduce. However, most experts in the field believe the Surviving Sepsis Campaign has significantly improved outcomes since its inception in 2002. Much still needs to be done as new evidence evolves.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41:580–637.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39:165–228.
- Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. HCHS Data Brief No. 62, June 2011. https://www.cdc.gov/nchs/products/databriefs/db62.htm.
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–1596.
- Finfer S, Bellomo R, Boyce N, Frency J, Myburgh J, Norton R; SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 2004; 350:2247–2256.
- Delaney AP, Dan A, McCaffrey J, et al. The role of albumin as a resuscitation fluid for patients with sepsis: a systematic review and meta-analysis. Crit Care Med 2011; 39:389–391.
- Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008; 358:125–139.
- Guidet B, Martinet O, Boulain T, et al. Assessment of haemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 vs. 0.9% NaCl fluid replacement in patients with severe sepsis: the CRYSTMAS study. Crit Care 2012; 16:R94.
- Perner A, Haase N, Guttormsen AB, et al; the 6S Trial Group and the Scandinavian Critical Care Trials Group. Hydroxyethyl starch 130.0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med 2012; 367:124–134.
- Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012; 367:1901–1911.
- Annane D, Siami S, Jaber S, et al; CRISTAL Investigators. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA 2013; 310:1809–1817.
- Dellinger RP. Cardiovascular management of septic shock. Crit Care Med 2003; 31:946–955.
- De Backer D, Biston P, Devriendt J, et al; SOAP II Investigators. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010; 362:779–789.
- De Backer D, Aldecoa C, Njimi H, Vincent JL. Dopamine versus norepinephrine in the treatment of septic shock: a meta-analysis. Crit Care Med 2012; 40:725–730.
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345:1368–1377.
- Jones AE, Brown MD, Trzeciak S, et al; Emergency Medicine Shock Research Network Investigators. The effect of a quantitative resuscitation strategy on mortality in patients with sepsis: a meta-analysis. Crit Care Med 2008; 36:2734–2739.
- Parry-Jones AJD, Pittman JAL. Arterial pressure and stroke volume variability as measurements for cardiovascular optimisation. Int J Intensive Care 2003; 2:67–72.
- Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med 2010; 38:367–374.
- Levy M, Artigas A, Phillips GS, et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis 2012; 12:919–924.
- ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014; 370:1683–1693.
- ARISE Investigators; ANZICS Clinical Trials Group, Peake SL, Delaney A, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014; 371:1496–1506.
- Levy MM, Dellinger RP, Townsend SA, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 2010; 36:222-231.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41:580–637.
- Dellinger RP, Levy MM, Rhodes A, et al; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39:165–228.
- Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. HCHS Data Brief No. 62, June 2011. https://www.cdc.gov/nchs/products/databriefs/db62.htm.
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–1596.
- Finfer S, Bellomo R, Boyce N, Frency J, Myburgh J, Norton R; SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 2004; 350:2247–2256.
- Delaney AP, Dan A, McCaffrey J, et al. The role of albumin as a resuscitation fluid for patients with sepsis: a systematic review and meta-analysis. Crit Care Med 2011; 39:389–391.
- Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008; 358:125–139.
- Guidet B, Martinet O, Boulain T, et al. Assessment of haemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 vs. 0.9% NaCl fluid replacement in patients with severe sepsis: the CRYSTMAS study. Crit Care 2012; 16:R94.
- Perner A, Haase N, Guttormsen AB, et al; the 6S Trial Group and the Scandinavian Critical Care Trials Group. Hydroxyethyl starch 130.0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med 2012; 367:124–134.
- Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012; 367:1901–1911.
- Annane D, Siami S, Jaber S, et al; CRISTAL Investigators. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA 2013; 310:1809–1817.
- Dellinger RP. Cardiovascular management of septic shock. Crit Care Med 2003; 31:946–955.
- De Backer D, Biston P, Devriendt J, et al; SOAP II Investigators. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010; 362:779–789.
- De Backer D, Aldecoa C, Njimi H, Vincent JL. Dopamine versus norepinephrine in the treatment of septic shock: a meta-analysis. Crit Care Med 2012; 40:725–730.
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345:1368–1377.
- Jones AE, Brown MD, Trzeciak S, et al; Emergency Medicine Shock Research Network Investigators. The effect of a quantitative resuscitation strategy on mortality in patients with sepsis: a meta-analysis. Crit Care Med 2008; 36:2734–2739.
- Parry-Jones AJD, Pittman JAL. Arterial pressure and stroke volume variability as measurements for cardiovascular optimisation. Int J Intensive Care 2003; 2:67–72.
- Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med 2010; 38:367–374.
- Levy M, Artigas A, Phillips GS, et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis 2012; 12:919–924.
- ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014; 370:1683–1693.
- ARISE Investigators; ANZICS Clinical Trials Group, Peake SL, Delaney A, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014; 371:1496–1506.
- Levy MM, Dellinger RP, Townsend SA, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 2010; 36:222-231.
KEY POINTS
- Ideally, intravenous antibiotic therapy should start within the first hour after sepsis is recognized; performance improvement protocols set a target of within 3 hours.
- A specific source of infection that requires source control measures should be sought, diagnosed or excluded, and if located, treated as rapidly as possible.
- Crystalloids should be used for initial fluid resuscitation. Adding an albumin-based solution is suggested for patients who require substantial amounts of crystalloids.
- Vasopressors are indicated for those who remain hypotensive despite fluid resuscitation. Norepinephrine should be used initially, and if the target mean arterial pressure cannot be achieved, then epinephrine or low-dose vasopressin is added.
- Corticosteroids should be considered only for patients who remain unstable despite adequate fluid resuscitation and vasopressor therapy.
A 57-year-old woman with abdominal pain
A 57-year-old woman presented to the emergency department with left lower quadrant pain, which had started 1 week earlier and was constant, dull, aching, and nonradiating. There were no aggravating or alleviating factors. The pain was associated with low-grade fever and nausea. She reported no vomiting, no change in bowel habits, and no hematemesis, hematochezia, or melena. She did not have urinary urgency, frequency, or dysuria. She had no cardiac, respiratory, or neurologic symptoms.
Her medical history included hypothyroidism, type 2 diabetes mellitus, diverticulosis, and chronic obstructive pulmonary disease. Her medications included metformin, insulin, levothyroxine, and inhaled tiotropium. She had no allergies. She had never undergone surgery, including cesarean delivery. She was postmenopausal. She had two children, both of whom had been born vaginally at full term. She denied using alcohol, tobacco, and illicit drugs. Her family history was noncontributory.
On examination, she was not in acute distress. Her temperature was 36.7°C (98.1°F), blood pressure 130/90 mm Hg, heart rate 86 beats per minute and regular, respiratory rate 16 breaths per minute, and oxygen saturation 98% on ambient air. Examination of her head and neck was unremarkable. Cardiopulmonary examination was normal. Abdominal examination revealed normal bowel sounds, mild tenderness in the left lower quadrant with localized guarding, and rebound tenderness. Neurologic examination was unremarkable.
Initial laboratory data showed mild leukocytosis. Computed tomography with contrast of the abdomen and pelvis suggested acute diverticulitis.
ATRIAL FIBRILLATION, AND THEN A TURN FOR THE WORSE
The patient was admitted with an initial diagnosis of acute diverticulitis. She was started on antibiotics, hydration, and pain medications, and her abdominal pain gradually improved.
On the third hospital day, she suddenly experienced shortness of breath and palpitations. At the time of admission her electrocardiogram had been normal, but it now showed atrial fibrillation with a rapid ventricular response. She also developed elevated troponin levels, which were thought to represent type 2 non-ST-elevation myocardial infarction.
She was started on aspirin, clopidogrel, and anticoagulation with heparin bridged with warfarin for the new-onset atrial fibrillation. Her heart rate was controlled with metoprolol, and her shortness of breath improved. An echocardiogram was normal.
On the seventh hospital day, she developed severe right-sided lower abdominal pain and bruising. Her blood pressure was 90/60 mm Hg, heart rate 110 beats per minute and irregularly irregular, respiratory rate 22 breaths per minute, and oxygen saturation 97% on room air. Her abdomen was diffusely tender with a palpable mass in the right lower quadrant and hypoactive bowel sounds. Ecchymosis was noted (Figure 1).
DIFFERENTIAL DIAGNOSIS
1. What is the likely cause of her decompensation?
- Acute mesenteric ischemia
- Perforation of the gastrointestinal tract
- Rectus sheath hematoma
- Abdominal compartment syndrome due to acute pancreatitis
Acute mesenteric ischemia
Signs and symptoms of acute mesenteric ischemia can be vague. Moreover, when it leads to bowel necrosis the mortality rate is high, ranging from 30% to 65%.1 Hence, one should suspect it and try to diagnose it early.
Most patients with this condition have comorbidities; risk factors include atherosclerotic disease, cardiac conditions (congestive heart failure, recent myocardial infarction, and atrial fibrillation), systemic illness, and inherited or acquired hypercoagulable states.2
The four major causes are:
- Acute thromboembolic occlusion of the superior mesenteric artery (the most common site of occlusion because of the acute angle of origin from the aorta)
- Acute thrombosis of the mesenteric vein
- Acute thrombosis of the mesenteric artery
- Nonocclusive disease affecting the mesenteric vessels2
Nonocclusive disease is seen in conditions in which the mesenteric vessels are already compromised due to background stenosis owing to atherosclerosis. Also, conditions such as septic and cardiogenic shock can compromise these arteries, leading to ischemia, which, if it persists, can lead to bowel infarction. Ischemic colitis falls under this category. It commonly involves the descending and sigmoid colon.3
The initial symptom of ischemia may be abdominal pain that is brought on by eating large meals (“postprandial intestinal angina.”2 When the ischemia worsens to infarction, patients may have a diffusely tender abdomen and constant pain that does not vary with palpation. Surprisingly, patients do not exhibit peritoneal signs early on. This gives rise to the description of “pain out of proportion to the physical findings” traditionally associated with acute mesenteric ischemia.2
Diagnosis. Supportive laboratory data include marked leukocytosis, elevated hematocrit due to hemoconcentration, metabolic acidosis, and elevated lactate.4 Newer markers such as serum alpha-glutathione S-transferase (alpha-GST) and intestinal fatty acid-binding protein (I-FABP) may be used to support the diagnosis.
Elevated alpha-GST has 72% sensitivity and 77% specificity in the diagnosis of acute mesenteric ischemia.5 The caveat is that it cannot reliably differentiate ischemia from infarction. Its sensitivity can be improved to 97% to 100% by using the white blood cell count and lactate levels in combination.5
An I-FABP level higher than 100 ng/mL has 100% sensitivity for diagnosing mesenteric infarction but only 25% sensitivity for bowel strangulation.6
Early use of abdominal computed tomography with contrast can aid in recognizing this diagnosis.7 Thus, it should be ordered in suspected cases, even in patients who have elevated creatinine levels (which would normally preclude the use of contrast), since early diagnosis followed by endovascular therapy is associated with survival benefit, and the risk of contrast-induced nephropathy appears to be small.8 Computed tomography helps to determine the state of mesenteric vessels and bowel perfusion before ischemia progresses to infarction. It also helps to rule out other common diagnoses. Findings that suggest acute mesenteric ischemia include segmental bowel wall thickening, intestinal pneumatosis with gas in the portal vein, bowel dilation, mesenteric stranding, portomesenteric thrombosis, and solid-organ infarction.9
Treatment. If superior mesenteric artery occlusion is diagnosed on computed tomography, the next step is to determine if there is peritonitis.10 In patients who have evidence of peritonitis, exploratory laparotomy is performed. For emboli in such patients, open embolectomy followed by on-table angiography is carried out in combination with damage-control surgery. For patients with peritonitis and acute thrombosis, stenting along with damage-control surgery is preferred.10
On the other hand, if there is no peritonitis, the thrombosis may be amenable to endovascular intervention. For patients with acute embolic occlusion with no contraindications to thrombolysis, aspiration embolectomy in combination with local catheter-directed thrombolysis with recombinant tissue plasminogen activator can be performed. This can be combined with endovascular mechanical embolectomy for more complete management.10 Patients with contraindications to thrombolysis can be treated either with aspiration and mechanical embolectomy or with open embolectomy with angiography.10
During laparotomy, the surgeon carefully inspects the bowel for signs of necrosis. Signs that bowel is still viable include pink color, bleeding from cut surfaces, good peristalsis, and visible pulsations in the arterial arcade of the mesentery.
Acute mesenteric artery thrombosis arising from chronic atherosclerotic disease can be treated with stenting of the stenotic lesion.10 Patients with this condition would also benefit from aggressive management of atherosclerotic disease with statins along with antiplatelet agents.10
Mesenteric vein thrombosis requires prompt institution of anticoagulation. However, in advanced cases leading to bowel infarction, exploratory laparotomy with resection of the necrotic bowel may be required. Anticoagulation should be continued for at least 6 months, and further therapy should be determined by the underlying precipitating condition.10
Critically ill patients who develop mesenteric ischemia secondary to persistent hypotension usually respond to adequate volume resuscitation, cessation of vasopressors, and overall improvement in their hemodynamic status. These patients must be closely monitored for development of gangrene of the bowel because they may be intubated and not able to complain. Any sudden deterioration in their condition should prompt physicians to consider bowel necrosis developing in these patients. Elevation of lactate levels out of proportion to the degree of hypotension may be corroborative evidence.4
Our patient had risk factors for acute mesenteric ischemia that included atrial fibrillation and recent non-ST-elevation myocardial infarction. She could have had arterial emboli due to atrial fibrillation, in situ superior mesenteric arterial thrombosis, or splanchnic arterial vasoconstriction due to the myocardial infarction associated with transient hypotension.
Arguing against this diagnosis, although she had a grossly distended abdomen, abdominal bruising usually is not seen. Also, a palpable mass in the right lower quadrant is uncommon except when acute mesenteric ischemia occurs due to segmental intestinal strangulation, as with strangulated hernia or volvulus. She also had therapeutic international normalized ratio (INR) levels constantly while on anticoagulation. Nevertheless, acute mesenteric ischemia should be strongly considered in the initial differential diagnosis of this patient’s acute decompensation.
Perforation of the gastrointestinal tract
Diverticulitis is the acute inflammation of one or more diverticula, which are small pouches created by herniation of the mucosa into the wall of the colon. The condition is caused by microscopic or macroscopic perforation of the diverticula. Microscopic perforation is usually self-limited (uncomplicated diverticulitis) and responds to conservative treatment, whereas macroscopic perforation can be associated with fecal or purulent peritonitis, abscess, enteric fistula, bowel obstruction, and stricture (complicated diverticulitis), in which case surgery may be necessary.
Patients with peritonitis due to free perforation present with generalized tenderness with rebound tenderness and guarding on abdominal examination. The abdomen may be distended and tympanic to percussion, with diminished or absent bowel sounds. Patients may have hemodynamic compromise.
Plain upright abdominal radiographs may show free air under the diaphragm. Computed tomography may show oral contrast outside the lumen and detect even small amounts of free intraperitoneal air (more clearly seen on a lung window setting).
Our patient initially presented with acute diverticulitis. She later developed diffuse abdominal tenderness with hypoactive bowel sounds. Bowel perforation is certainly a possibility at this stage, though it is usually not associated with abdominal bruising. She would need additional imaging to rule out this complication.
Other differential diagnoses to be considered in this patient with right lower-quadrant pain include acute appendicitis, incarcerated inguinal hernia, volvulus (particularly cecal volvulus), small-bowel obstruction, pyelonephritis, and gynecologic causes such as adnexal torsion, ruptured ovarian cyst, and tubo-ovarian abscess. Computed tomography helps to differentiate most of these causes.
Rectus sheath hematoma
Rectus sheath hematoma is relatively uncommon and often not considered in the initial differential diagnosis of an acute abdomen. This gives it the rightful term “a great masquerader.” It usually results from bleeding into the rectus sheath from damage to the superior (more common) or inferior epigastric arteries and occasionally from a direct tear of the rectus abdominis muscle. Predisposing factors include anticoagulant therapy (most common), advanced age, hypertension, previous abdominal surgery, trauma, paroxysmal coughing, medication injections, pregnancy, blood dyscrasias, severe vomiting, violent physical activity, and leukemia.11
Clinical manifestations include acute abdominal pain, often associated with fever, nausea, and vomiting. Physical examination may reveal signs of hypovolemic shock, a palpable nonpulsatile abdominal mass, and signs of local peritoneal irritation. The Carnett sign11 (tenderness within the abdominal wall that persists and does not improve with raising the head) and the Fothergill sign11 (a tender abdominal mass that does not cross the midline and remains palpable with tensing of the rectus sheath) may be elicited.
Computed tomography is more sensitive than abdominal ultrasonography in differentiating rectus sheath hematoma from an intra-abdominal pathology.11 In addition, computed tomography also helps to determine if the bleeding is active or not, which has therapeutic implications.
In our patient, rectus sheath hematoma is a possibility because of her ongoing anticoagulation, findings of localized abdominal bruising, and palpable right lower-quadrant mass, and it is high on the list of differential diagnoses. Rectus sheath hematoma should be considered in the differential diagnosis of lower abdominal pain particularly in elderly women who are on anticoagulation and in whom the onset of pain coincides with a paroxysm of cough.12 Women are twice as likely as men to develop rectus sheath hematoma, owing to their different muscle mass.13 In addition, anterior abdominal wall muscles are stretched during pregnancy.13
Abdominal compartment syndrome
Abdominal compartment syndrome has been classically associated with surgical patients. However, it is being increasingly recognized in critically ill medical patients, in whom detecting and treating it early may result in significant reduction in rates of morbidity and death.14
Abdominal compartment syndrome is of three types: primary, secondary, and recurrent. Primary abdominal compartment syndrome refers to the classic surgical patients with evidence of direct injury to the abdominal or pelvic organs through major trauma or extensive abdominal surgeries. Secondary abdominal compartment syndrome refers to its development in critically ill intensive care patients in whom the pathology does not directly involve the abdominal or pelvic organs.
Various medical conditions can culminate in abdominal compartment syndrome and result in multiorgan failure. Recurrent abdominal compartment syndrome refers to its development after management of either primary or secondary intra-abdominal hypertension or abdominal compartment syndrome.15 Clinicians thus must be aware of secondary and recurrent abdominal compartment syndrome occurring in critically ill patients.
The normal intra-abdominal pressure is around 5 to 7 mm Hg, even in most critically ill patients. Persistent elevation, ie, higher than 12 mm Hg, is referred to as intra-abdominal hypertension.16–18 Intra-abdominal hypertension is subdivided into four grades:
- Grade I: 12–15 mm Hg
- Grade II: 16–20 mm Hg
- Grade III: 21–25 mm Hg
- Grade IV: > 25 mm Hg.
The World Society of the Abdominal Compartment Syndrome (WSACS) defines abdominal compartment syndrome as pressure higher than 20 mm Hg along with organ damage.18 It may or may not be associated with an abdominal perfusion pressure less than 60 mm Hg.18
Risk factors associated with abdominal compartment syndrome include conditions causing decreased gut motility (gastroparesis, ileus, and colonic pseudo-obstruction), intra-abdominal or retroperitoneal masses or abscesses, ascites, hemoperitoneum, acute pancreatitis, third-spacing due to massive fluid resuscitation with transfusions, peritoneal dialysis, and shock.18,19
Physical examination has a sensitivity of only 40% to 60% in detecting intra-abdominal hypertension.20 The gold-standard method of measuring the intra-abdominal pressure is the modified Kron technique,18 using a Foley catheter in the bladder connected to a pressure transducer. With the patient in the supine position, the transducer is zeroed at the mid-axillary line at the level of the iliac crest, and 25 mL of normal saline is instilled into the bladder and maintained for 30 to 60 seconds to let the detrusor muscle relax.15 Pressure tracings are then recorded at the end of expiration. Factors that are known to affect the transbladder pressure include patient position, respiratory movement, and body mass index, and should be taken into account when reading the pressure recordings.15,21 Other techniques that can be used include intragastric, intra-inferior vena cava, and intrarectal approaches.15
The WSACS recommends that any patient admitted to a critical care unit or in whom new organ failure develops should be screened for risk factors for intra-abdominal hypertension and abdominal compartment syndrome. If a patient has at least two of the risk factors suggested by WSACS, a baseline intra-abdominal pressure measurement should be obtained. Patients at risk for intra-abdominal hypertension should have the intra-abdominal pressure measured every 4 to 6 hours. However, in the face of hemodynamic instability and worsening multiorgan failure, the pressure may need to be measured hourly.18
Clinicians managing patients in the intensive care unit should think of intra-abdominal pressure alongside blood pressure, urine output, and mental status when evaluating hemodynamic status. Clinical manifestations of abdominal compartment syndrome reflect the underlying organ dysfunction and include hypotension, refractory shock, decreased urine output, intracranial hypertension, progressive hypoxemia and hypercarbia, elevated pulmonary peak pressures, and worsening of metabolic acidosis.22
Treatment. The standard treatment for primary abdominal compartment syndrome is surgical decompression. According to WSACS guidelines, insertion of a percutaneous drainage catheter should be advocated in patients with gross ascites and in whom decompressive surgery is not feasible. A damage-control resuscitation strategy used for patients undergoing damage-control laparotomy has been found to increase the 30-day survival rate.23 A damage-control resuscitation strategy consists of increasing the use of plasma and platelet transfusions over packed red cell transfusions, limiting the use of crystalloid solutions in early fluid resuscitation, and allowing for permissive hypotension.
Secondary abdominal compartment syndrome is treated conservatively in most cases, since patients with this condition are very poor surgical candidates owing to their comorbidities.18 However, in patients with progressive organ dysfunction in whom medical management has failed, surgical decompression should be considered.18 Medical management of secondary abdominal compartment syndrome depends on the underlying etiology. Strategies include nasogastric or colonic decompression, use of prokinetic agents, paracentesis in cases with gross ascites, and maintaining a cumulative negative fluid balance. The WSACS does not recommend routine use of diuretics, albumin infusion, or renal replacement strategies. Pain should be adequately controlled to improve abdominal wall compliance.18,24 Neuromuscular blockade agents may be used to aid this process. Neostigmine may be used to treat colonic pseudo-obstruction when other conservative methods fail. Use of enteral nutrition should be minimized.18
Our patient might have abdominal compartment syndrome, but a definitive diagnosis cannot be made at this point without measuring the intra-abdominal pressure.
WHICH IMAGING TEST WOULD BE BEST?
2. Which imaging test would be best for establishing the diagnosis in this patient?
- Plain abdominal radiography
- Abdominal ultrasonography
- Computed tomography of the abdomen and pelvis with contrast
- Magnetic resonance imaging of the abdomen and pelvis
Plain abdominal radiography
Plain abdominal radiography can help to determine if there is free gas under the diaphragm (due to bowel perforation), obstructed bowel, sentinel loop, volvulus, or fecoliths causing the abdominal pain. It cannot diagnose rectus sheath hematoma or acute mesenteric ischemia.
Abdominal ultrasonography
Abdominal ultrasonography can be used as the first diagnostic test, as it is widely available, safe, effective, and tolerable. It does not expose the patient to radiation or intravenous contrast agents. It helps to diagnose rectus sheath hematoma and helps to follow its maturation and resolution once a diagnosis is made. It can provide a rapid assessment of the size, location, extent, and physical characteristics of the mass.
Rectus sheath hematoma appears spindle-shaped on sagittal sections and ovoid on coronal sections. It often appears sonolucent in the early stages and sonodense in the late stage, but the appearance may be heterogeneous depending on the combined presence of clot and fresh blood. These findings are sufficient to make the diagnosis.
Abdominal ultrasonography has 85% to 96% sensitivity in diagnosing rectus sheath hematoma.25 It can help diagnose other causes of the abdominal pain, such as renal stones and cholecystitis. It is the preferred imaging test in pediatric patients, pregnant patients, and those with renal insufficiency.
Abdominal computed tomography
Abdominal computed tomography has a sensitivity and specificity of 100% for diagnosing acute rectus sheath hematoma with a duration of less than 5 days.25 It not only helps to determine the precise location and extent, but also helps to determine if there is active extravasation. Even in patients with renal insufficiency, noncontrast computed tomography will help to confirm the diagnosis, although it may not show extravasation or it may miss certain abdominal pathologies because of the lack of contrast.
Acute rectus sheath hematoma appears as a hyperdense mass posterior to the rectus abdominis muscle with ipsilateral anterolateral muscular enlargement. Chronic rectus sheath hematoma appears isodense or hypodense relative to the surrounding muscle. Above the arcuate line, rectus sheath hematoma has a spindle shape; below the arcuate line, it is typically spherical.
In 1996, Berná et al26 classified rectus sheath hematoma into three grades based on findings of computed tomography:
- Grade I is intramuscular and unilateral
- Grade II may involve bilateral rectus muscles without extension into the prevesicular space
- Grade III extends into the peritoneum and prevesicular space
Magnetic resonance imaging
Magnetic resonance imaging is useful to differentiate chronic rectus sheath hematoma (greater than 5-day duration) from an anterior abdominal wall mass. Chronic rectus sheath hematoma will have high signal intensity on both T1- and T2-weighted images up to 10 months after the onset of the hematoma.27
Back to our patient
Since our patient’s symptoms are acute and of less than 5 days’ duration, computed tomography of the abdomen and pelvis would be the best diagnostic test, with therapeutic implications if there is ongoing extravasation.
Computed tomography of the abdomen with contrast showed a new hematoma, measuring 25 by 14 by 13.5 cm, in the right rectus sheath (Figure 2), with no other findings. The hematoma was grade I, since it was intramuscular and unilateral without extension elsewhere.
Laboratory workup showed that the patient’s hematocrit was falling, from 34% to 24%, and her INR was elevated at 2.5. She was resuscitated with fluids, blood transfusion, and fresh-frozen plasma. Anticoagulation was withheld. In spite of resuscitation, her hematocrit kept falling, though she remained hemodynamically stable.
THE WAY FORWARD
3. At this point, what would be the best approach to management in this patient?
- Serial clinical examinations and frequent monitoring of the complete blood cell count
- Urgent surgical consult for exploratory laparotomy with evaluation of the hematoma and ligation of the bleeding vessel
- Repeat computed tomographic angiography to identify a possible bleeding vessel; consideration of radiographically guided arterial embolization
- Measuring the intra-abdominal pressure using the intrabladder pressure for abdominal compartment syndrome and consideration of surgical drainage
The key clinical concern in a patient with a rectus sheath hematoma who is hemodynamically stable is whether the hematoma is expanding. This patient responded to initial resuscitation, but her falling hematocrit was evidence of ongoing bleeding leading to an expanding rectus sheath hematoma. Thus, serial clinical examinations and frequent monitoring of the complete blood cell count would not be enough, as it could miss fatal ongoing bleeding.
Radiographically guided embolization with Gelfoam, thrombin, or coils should be attempted first, as this is less invasive than exploratory laparotomy.28 It can achieve hemostasis, decrease the size of the hematoma, limit the need for blood products, and prevent rupture into the abdomen. If this is unsuccessful, the next step is ligation of the bleeding vessel.29
Surgical treatment includes evacuation of the hematoma, repair of the rectus sheath, ligation of bleeding vessels, and abdominal wall closure. Surgical evacuation or guided drainage of a rectus sheath hematoma on its own is not normally indicated and may indeed cause persistent bleeding by diminishing a potential tamponade effect. However, it may become necessary if the hematoma is very large or infected, if it causes marked respiratory impairment, or if abdominal compartment syndrome is suspected.
Abdominal compartment syndrome is very rare but is associated with a 50% mortality rate.30 It should be suspected in patients with oliguria, low cardiac output, changes in minute ventilation, and altered splanchnic blood flow. The diagnosis is confirmed with indwelling catheter manometry of the bladder to measure intra-abdominal pressure. Intra-abominal pressure above 25 mm Hg should be treated with decompressive laparotomy.30 However, the clinical suspicion of abdominal compartment syndrome was low in this patient.
The best choice at this point would be urgent computed tomographic angiography to identify a bleeding vessel, along with consideration of radiographically guided arterial embolization.
TREATMENT IS USUALLY CONSERVATIVE
Treatment of rectus sheath hematoma is conservative in most hemodynamically stable patients, with embolization or surgical intervention reserved for unstable patients or those in whom the hematoma is expanding.
Knowledge of the grading system of Berná et al26 helps to assess the patient’s risk and to anticipate potential complications. Grade I hematomas are mild and do not necessitate admission. Patients with grade II hematoma can be admitted to the floor for 24 to 48 hours for observation. Grade III usually occurs in patients receiving anticoagulant therapy and frequently requires blood products. These patients have a prolonged hospital stay and more complications, including hypovolemic shock, myonecrosis, acute coronary syndrome, arrhythmias, acute renal failure, small-bowel infarction, and abdominal compartment syndrome—all of which increases the risk of morbidity and death. Thus, patients who are on anticoagulation who develop grade III rectus sheath hematoma should be admitted to the hospital, preferably to the intensive care unit, to ensure that the hematoma is not expanding and to plan reinstitution of anticoagulation as appropriate.
In most cases, rectus sheath hematomas resolve within 1 to 3 months. Resolution of large hematomas may be hastened with the use of pulsed ultrasound.31 However, this treatment should be used only after the acute phase is over, when there is evidence of an organized thrombus and coagulation measures have returned to the target range. This helps to reduce the risk of bleeding and to prevent symptoms from worsening.31
OUR PATIENT’S COURSE
Our patient underwent urgent computed tomographic angiography, which showed a modest increase in the size of the rectus sheath hematoma. However, no definitive blush of contrast was seen to suggest active arterial bleeding. Her hematocrit stabilized, and she remained hemodynamically stable without requiring additional intervention. Most likely her bleeding was self-contained. She had normal intra-abdominal pressure on serial monitoring. She was later transferred to acute inpatient rehabilitation in view of deconditioning and is currently doing well. The hematoma persisted, decreasing only slightly in size over the next 3 weeks.
- Kougias P, Lau D, El Sayed HF, Zhou W, Huynh TT, Lin PH. Determinants of mortality and treatment outcome following surgical interventions for acute mesenteric ischemia. J Vasc Surg 2007; 46:467–474.
- Sise MJ. Acute mesenteric ischemia. Surg Clin North Am 2014; 94:165–181.
- Scharff JR, Longo WE, Vartanian SM, Jacobs DL, Bahadursingh AN, Kaminski DL. Ischemic colitis: spectrum of disease and outcome. Surgery 2003; 134:624–629.
- Lange H, Jäckel R. Usefulness of plasma lactate concentration in the diagnosis of acute abdominal disease. Eur J Surg 1994; 160:381–384.
- Gearhart SL, Delaney CP, Senagore AJ, et al. Prospective assessment of the predictive value of alpha-glutathione S-transferase for intestinal ischemia. Am Surg 2003; 69:324–329.
- Kanda T, Fujii H, Tani T, et al. Intestinal fatty acid-binding protein is a useful diagnostic marker for mesenteric infarction in humans. Gastroenterology 1996; 110:339–343.
- Menke J. Diagnostic accuracy of multidetector CT in acute mesenteric ischemia: systematic review and meta-analysis. Radiology 2010; 256:93–101.
- Acosta S, Björnsson S, Ekberg O, Resch T. CT angiography followed by endovascular intervention for acute superior mesenteric artery occlusion does not increase risk of contrast-induced renal failure. Eur J Vasc Endovasc Surg 2010; 39:726–730.
- Clark RA. Computed tomography of bowel infarction. J Comput Assist Tomogr 1987; 11:757–762.
- Acosta S, Björck M. Modern treatment of acute mesenteric ischaemia. Br J Surg 2014; 101:e100–e108.
- Smithson A, Ruiz J, Perello R, Valverde M, Ramos J, Garzo L. Diagnostic and management of spontaneous rectus sheath hematoma. Eur J Intern Med 2013; 24:579–582.
- Moreno Gallego A, Aguayo JL, Flores B, et al. Ultrasonography and computed tomography reduce unnecessary surgery in abdominal rectus sheath haematoma. Br J Surg 1997; 84:1295–1297.
- Dubinsky IL. Hematoma of the rectus abdominis muscle: case report and review of the literature. J Emerg Med 1997; 15:165–167.
- Yi M, Yao G, Bai Y. The monitoring of intra-abdominal pressure in critically ill patients. (In Chinese.) Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2014; 26:175–178.
- Hunt L, Frost SA, Hillman K, Newton PJ, Davidson PM. Management of intra-abdominal hypertension and abdominal compartment syndrome: a review. J Trauma Manag Outcomes 2014; 8:2.
- Malbrain ML, Cheatham ML, Kirkpatrick A, et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med 2006; 32:1722–1732.
- Malbrain ML, Chiumello D, Cesana BM, et al; WAKE-Up! Investigators. A systematic review and individual patient data meta-analysis on intra-abdominal hypertension in critically ill patients: the wake-up project. World initiative on Abdominal Hypertension Epidemiology, a Unifying Project (WAKE-Up!). Minerva Anestesiol 2014; 80:293–306.
- Kirkpatrick AW, Roberts DJ, De Waele J, et al; Pediatric Guidelines Sub-Committee for the World Society of the Abdominal Compartment Syndrome. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med 2013; 39:1190–1206.
- Holodinsky JK, Roberts DJ, Ball CG, et al. Risk factors for intra-abdominal hypertension and abdominal compartment syndrome among adult intensive care unit patients: a systematic review and meta-analysis. Crit Care 2013; 17:R249.
- Sugrue M, Bauman A, Jones F, et al. Clinical examination is an inaccurate predictor of intraabdominal pressure. World J Surg 2002; 26:1428–1431.
- Cheatham ML, De Waele JJ, De Laet I, et al; World Society of the Abdominal Compartment Syndrome (WSACS) Clinical Trials Working Group. The impact of body position on intra-abdominal pressure measurement: a multicenter analysis. Crit Care Med 2009; 37:2187–2190.
- Ortiz-Diaz E, Lan CK. Intra-abdominal hypertension in medical critically ill patients: a narrative review. Shock 2014; 41:175–180.
- Cotton BA, Reddy N, Hatch QM, et al. Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg 2011; 254:598–605.
- An G, West MA. Abdominal compartment syndrome: a concise clinical review. Crit Care Med 2008; 36:1304–1310.
- Tolcher MC, Nitsche JF, Arendt KW, Rose CH. Spontaneous rectus sheath hematoma pregnancy: case report and review of the literature. Obstet Gynecol Surv 2010; 65:517–522.
- Berná JD, Garcia-Medina V, Guirao J, Garcia-Medina J. Rectus sheath hematoma: diagnostic classification by CT. Abdom Imaging 1996; 21:62–64.
- Unger EC, Glazer HS, Lee JK, Ling D. MRI of extracranial hematomas: preliminary observations. AJR Am J Roentgenol 1986; 146:403–407.
- Rimola J, Perendreu J, Falcó J, Fortuño JR, Massuet A, Branera J. Percutaneous arterial embolization in the management of rectus sheath hematoma. AJR Am J Roentgenol 2007; 188:W497–W502.
- Titone C, Lipsius M, Krakauer JS. “Spontaneous” hematoma of the rectus abdominis muscle: critical review of 50 cases with emphasis on early diagnosis and treatment. Surgery 1972; 72:568–572.
- Osinbowale O, Bartholomew JR. Rectus sheath hematoma. Vasc Med 2008; 13:275–279.
- Berná-Serna JD, Sánchez-Garre J, Madrigal M, Zuazu I, Berná-Mestre JD. Ultrasound therapy in rectus sheath hematoma. Phys Ther 2005; 85:352–357.
A 57-year-old woman presented to the emergency department with left lower quadrant pain, which had started 1 week earlier and was constant, dull, aching, and nonradiating. There were no aggravating or alleviating factors. The pain was associated with low-grade fever and nausea. She reported no vomiting, no change in bowel habits, and no hematemesis, hematochezia, or melena. She did not have urinary urgency, frequency, or dysuria. She had no cardiac, respiratory, or neurologic symptoms.
Her medical history included hypothyroidism, type 2 diabetes mellitus, diverticulosis, and chronic obstructive pulmonary disease. Her medications included metformin, insulin, levothyroxine, and inhaled tiotropium. She had no allergies. She had never undergone surgery, including cesarean delivery. She was postmenopausal. She had two children, both of whom had been born vaginally at full term. She denied using alcohol, tobacco, and illicit drugs. Her family history was noncontributory.
On examination, she was not in acute distress. Her temperature was 36.7°C (98.1°F), blood pressure 130/90 mm Hg, heart rate 86 beats per minute and regular, respiratory rate 16 breaths per minute, and oxygen saturation 98% on ambient air. Examination of her head and neck was unremarkable. Cardiopulmonary examination was normal. Abdominal examination revealed normal bowel sounds, mild tenderness in the left lower quadrant with localized guarding, and rebound tenderness. Neurologic examination was unremarkable.
Initial laboratory data showed mild leukocytosis. Computed tomography with contrast of the abdomen and pelvis suggested acute diverticulitis.
ATRIAL FIBRILLATION, AND THEN A TURN FOR THE WORSE
The patient was admitted with an initial diagnosis of acute diverticulitis. She was started on antibiotics, hydration, and pain medications, and her abdominal pain gradually improved.
On the third hospital day, she suddenly experienced shortness of breath and palpitations. At the time of admission her electrocardiogram had been normal, but it now showed atrial fibrillation with a rapid ventricular response. She also developed elevated troponin levels, which were thought to represent type 2 non-ST-elevation myocardial infarction.
She was started on aspirin, clopidogrel, and anticoagulation with heparin bridged with warfarin for the new-onset atrial fibrillation. Her heart rate was controlled with metoprolol, and her shortness of breath improved. An echocardiogram was normal.
On the seventh hospital day, she developed severe right-sided lower abdominal pain and bruising. Her blood pressure was 90/60 mm Hg, heart rate 110 beats per minute and irregularly irregular, respiratory rate 22 breaths per minute, and oxygen saturation 97% on room air. Her abdomen was diffusely tender with a palpable mass in the right lower quadrant and hypoactive bowel sounds. Ecchymosis was noted (Figure 1).
DIFFERENTIAL DIAGNOSIS
1. What is the likely cause of her decompensation?
- Acute mesenteric ischemia
- Perforation of the gastrointestinal tract
- Rectus sheath hematoma
- Abdominal compartment syndrome due to acute pancreatitis
Acute mesenteric ischemia
Signs and symptoms of acute mesenteric ischemia can be vague. Moreover, when it leads to bowel necrosis the mortality rate is high, ranging from 30% to 65%.1 Hence, one should suspect it and try to diagnose it early.
Most patients with this condition have comorbidities; risk factors include atherosclerotic disease, cardiac conditions (congestive heart failure, recent myocardial infarction, and atrial fibrillation), systemic illness, and inherited or acquired hypercoagulable states.2
The four major causes are:
- Acute thromboembolic occlusion of the superior mesenteric artery (the most common site of occlusion because of the acute angle of origin from the aorta)
- Acute thrombosis of the mesenteric vein
- Acute thrombosis of the mesenteric artery
- Nonocclusive disease affecting the mesenteric vessels2
Nonocclusive disease is seen in conditions in which the mesenteric vessels are already compromised due to background stenosis owing to atherosclerosis. Also, conditions such as septic and cardiogenic shock can compromise these arteries, leading to ischemia, which, if it persists, can lead to bowel infarction. Ischemic colitis falls under this category. It commonly involves the descending and sigmoid colon.3
The initial symptom of ischemia may be abdominal pain that is brought on by eating large meals (“postprandial intestinal angina.”2 When the ischemia worsens to infarction, patients may have a diffusely tender abdomen and constant pain that does not vary with palpation. Surprisingly, patients do not exhibit peritoneal signs early on. This gives rise to the description of “pain out of proportion to the physical findings” traditionally associated with acute mesenteric ischemia.2
Diagnosis. Supportive laboratory data include marked leukocytosis, elevated hematocrit due to hemoconcentration, metabolic acidosis, and elevated lactate.4 Newer markers such as serum alpha-glutathione S-transferase (alpha-GST) and intestinal fatty acid-binding protein (I-FABP) may be used to support the diagnosis.
Elevated alpha-GST has 72% sensitivity and 77% specificity in the diagnosis of acute mesenteric ischemia.5 The caveat is that it cannot reliably differentiate ischemia from infarction. Its sensitivity can be improved to 97% to 100% by using the white blood cell count and lactate levels in combination.5
An I-FABP level higher than 100 ng/mL has 100% sensitivity for diagnosing mesenteric infarction but only 25% sensitivity for bowel strangulation.6
Early use of abdominal computed tomography with contrast can aid in recognizing this diagnosis.7 Thus, it should be ordered in suspected cases, even in patients who have elevated creatinine levels (which would normally preclude the use of contrast), since early diagnosis followed by endovascular therapy is associated with survival benefit, and the risk of contrast-induced nephropathy appears to be small.8 Computed tomography helps to determine the state of mesenteric vessels and bowel perfusion before ischemia progresses to infarction. It also helps to rule out other common diagnoses. Findings that suggest acute mesenteric ischemia include segmental bowel wall thickening, intestinal pneumatosis with gas in the portal vein, bowel dilation, mesenteric stranding, portomesenteric thrombosis, and solid-organ infarction.9
Treatment. If superior mesenteric artery occlusion is diagnosed on computed tomography, the next step is to determine if there is peritonitis.10 In patients who have evidence of peritonitis, exploratory laparotomy is performed. For emboli in such patients, open embolectomy followed by on-table angiography is carried out in combination with damage-control surgery. For patients with peritonitis and acute thrombosis, stenting along with damage-control surgery is preferred.10
On the other hand, if there is no peritonitis, the thrombosis may be amenable to endovascular intervention. For patients with acute embolic occlusion with no contraindications to thrombolysis, aspiration embolectomy in combination with local catheter-directed thrombolysis with recombinant tissue plasminogen activator can be performed. This can be combined with endovascular mechanical embolectomy for more complete management.10 Patients with contraindications to thrombolysis can be treated either with aspiration and mechanical embolectomy or with open embolectomy with angiography.10
During laparotomy, the surgeon carefully inspects the bowel for signs of necrosis. Signs that bowel is still viable include pink color, bleeding from cut surfaces, good peristalsis, and visible pulsations in the arterial arcade of the mesentery.
Acute mesenteric artery thrombosis arising from chronic atherosclerotic disease can be treated with stenting of the stenotic lesion.10 Patients with this condition would also benefit from aggressive management of atherosclerotic disease with statins along with antiplatelet agents.10
Mesenteric vein thrombosis requires prompt institution of anticoagulation. However, in advanced cases leading to bowel infarction, exploratory laparotomy with resection of the necrotic bowel may be required. Anticoagulation should be continued for at least 6 months, and further therapy should be determined by the underlying precipitating condition.10
Critically ill patients who develop mesenteric ischemia secondary to persistent hypotension usually respond to adequate volume resuscitation, cessation of vasopressors, and overall improvement in their hemodynamic status. These patients must be closely monitored for development of gangrene of the bowel because they may be intubated and not able to complain. Any sudden deterioration in their condition should prompt physicians to consider bowel necrosis developing in these patients. Elevation of lactate levels out of proportion to the degree of hypotension may be corroborative evidence.4
Our patient had risk factors for acute mesenteric ischemia that included atrial fibrillation and recent non-ST-elevation myocardial infarction. She could have had arterial emboli due to atrial fibrillation, in situ superior mesenteric arterial thrombosis, or splanchnic arterial vasoconstriction due to the myocardial infarction associated with transient hypotension.
Arguing against this diagnosis, although she had a grossly distended abdomen, abdominal bruising usually is not seen. Also, a palpable mass in the right lower quadrant is uncommon except when acute mesenteric ischemia occurs due to segmental intestinal strangulation, as with strangulated hernia or volvulus. She also had therapeutic international normalized ratio (INR) levels constantly while on anticoagulation. Nevertheless, acute mesenteric ischemia should be strongly considered in the initial differential diagnosis of this patient’s acute decompensation.
Perforation of the gastrointestinal tract
Diverticulitis is the acute inflammation of one or more diverticula, which are small pouches created by herniation of the mucosa into the wall of the colon. The condition is caused by microscopic or macroscopic perforation of the diverticula. Microscopic perforation is usually self-limited (uncomplicated diverticulitis) and responds to conservative treatment, whereas macroscopic perforation can be associated with fecal or purulent peritonitis, abscess, enteric fistula, bowel obstruction, and stricture (complicated diverticulitis), in which case surgery may be necessary.
Patients with peritonitis due to free perforation present with generalized tenderness with rebound tenderness and guarding on abdominal examination. The abdomen may be distended and tympanic to percussion, with diminished or absent bowel sounds. Patients may have hemodynamic compromise.
Plain upright abdominal radiographs may show free air under the diaphragm. Computed tomography may show oral contrast outside the lumen and detect even small amounts of free intraperitoneal air (more clearly seen on a lung window setting).
Our patient initially presented with acute diverticulitis. She later developed diffuse abdominal tenderness with hypoactive bowel sounds. Bowel perforation is certainly a possibility at this stage, though it is usually not associated with abdominal bruising. She would need additional imaging to rule out this complication.
Other differential diagnoses to be considered in this patient with right lower-quadrant pain include acute appendicitis, incarcerated inguinal hernia, volvulus (particularly cecal volvulus), small-bowel obstruction, pyelonephritis, and gynecologic causes such as adnexal torsion, ruptured ovarian cyst, and tubo-ovarian abscess. Computed tomography helps to differentiate most of these causes.
Rectus sheath hematoma
Rectus sheath hematoma is relatively uncommon and often not considered in the initial differential diagnosis of an acute abdomen. This gives it the rightful term “a great masquerader.” It usually results from bleeding into the rectus sheath from damage to the superior (more common) or inferior epigastric arteries and occasionally from a direct tear of the rectus abdominis muscle. Predisposing factors include anticoagulant therapy (most common), advanced age, hypertension, previous abdominal surgery, trauma, paroxysmal coughing, medication injections, pregnancy, blood dyscrasias, severe vomiting, violent physical activity, and leukemia.11
Clinical manifestations include acute abdominal pain, often associated with fever, nausea, and vomiting. Physical examination may reveal signs of hypovolemic shock, a palpable nonpulsatile abdominal mass, and signs of local peritoneal irritation. The Carnett sign11 (tenderness within the abdominal wall that persists and does not improve with raising the head) and the Fothergill sign11 (a tender abdominal mass that does not cross the midline and remains palpable with tensing of the rectus sheath) may be elicited.
Computed tomography is more sensitive than abdominal ultrasonography in differentiating rectus sheath hematoma from an intra-abdominal pathology.11 In addition, computed tomography also helps to determine if the bleeding is active or not, which has therapeutic implications.
In our patient, rectus sheath hematoma is a possibility because of her ongoing anticoagulation, findings of localized abdominal bruising, and palpable right lower-quadrant mass, and it is high on the list of differential diagnoses. Rectus sheath hematoma should be considered in the differential diagnosis of lower abdominal pain particularly in elderly women who are on anticoagulation and in whom the onset of pain coincides with a paroxysm of cough.12 Women are twice as likely as men to develop rectus sheath hematoma, owing to their different muscle mass.13 In addition, anterior abdominal wall muscles are stretched during pregnancy.13
Abdominal compartment syndrome
Abdominal compartment syndrome has been classically associated with surgical patients. However, it is being increasingly recognized in critically ill medical patients, in whom detecting and treating it early may result in significant reduction in rates of morbidity and death.14
Abdominal compartment syndrome is of three types: primary, secondary, and recurrent. Primary abdominal compartment syndrome refers to the classic surgical patients with evidence of direct injury to the abdominal or pelvic organs through major trauma or extensive abdominal surgeries. Secondary abdominal compartment syndrome refers to its development in critically ill intensive care patients in whom the pathology does not directly involve the abdominal or pelvic organs.
Various medical conditions can culminate in abdominal compartment syndrome and result in multiorgan failure. Recurrent abdominal compartment syndrome refers to its development after management of either primary or secondary intra-abdominal hypertension or abdominal compartment syndrome.15 Clinicians thus must be aware of secondary and recurrent abdominal compartment syndrome occurring in critically ill patients.
The normal intra-abdominal pressure is around 5 to 7 mm Hg, even in most critically ill patients. Persistent elevation, ie, higher than 12 mm Hg, is referred to as intra-abdominal hypertension.16–18 Intra-abdominal hypertension is subdivided into four grades:
- Grade I: 12–15 mm Hg
- Grade II: 16–20 mm Hg
- Grade III: 21–25 mm Hg
- Grade IV: > 25 mm Hg.
The World Society of the Abdominal Compartment Syndrome (WSACS) defines abdominal compartment syndrome as pressure higher than 20 mm Hg along with organ damage.18 It may or may not be associated with an abdominal perfusion pressure less than 60 mm Hg.18
Risk factors associated with abdominal compartment syndrome include conditions causing decreased gut motility (gastroparesis, ileus, and colonic pseudo-obstruction), intra-abdominal or retroperitoneal masses or abscesses, ascites, hemoperitoneum, acute pancreatitis, third-spacing due to massive fluid resuscitation with transfusions, peritoneal dialysis, and shock.18,19
Physical examination has a sensitivity of only 40% to 60% in detecting intra-abdominal hypertension.20 The gold-standard method of measuring the intra-abdominal pressure is the modified Kron technique,18 using a Foley catheter in the bladder connected to a pressure transducer. With the patient in the supine position, the transducer is zeroed at the mid-axillary line at the level of the iliac crest, and 25 mL of normal saline is instilled into the bladder and maintained for 30 to 60 seconds to let the detrusor muscle relax.15 Pressure tracings are then recorded at the end of expiration. Factors that are known to affect the transbladder pressure include patient position, respiratory movement, and body mass index, and should be taken into account when reading the pressure recordings.15,21 Other techniques that can be used include intragastric, intra-inferior vena cava, and intrarectal approaches.15
The WSACS recommends that any patient admitted to a critical care unit or in whom new organ failure develops should be screened for risk factors for intra-abdominal hypertension and abdominal compartment syndrome. If a patient has at least two of the risk factors suggested by WSACS, a baseline intra-abdominal pressure measurement should be obtained. Patients at risk for intra-abdominal hypertension should have the intra-abdominal pressure measured every 4 to 6 hours. However, in the face of hemodynamic instability and worsening multiorgan failure, the pressure may need to be measured hourly.18
Clinicians managing patients in the intensive care unit should think of intra-abdominal pressure alongside blood pressure, urine output, and mental status when evaluating hemodynamic status. Clinical manifestations of abdominal compartment syndrome reflect the underlying organ dysfunction and include hypotension, refractory shock, decreased urine output, intracranial hypertension, progressive hypoxemia and hypercarbia, elevated pulmonary peak pressures, and worsening of metabolic acidosis.22
Treatment. The standard treatment for primary abdominal compartment syndrome is surgical decompression. According to WSACS guidelines, insertion of a percutaneous drainage catheter should be advocated in patients with gross ascites and in whom decompressive surgery is not feasible. A damage-control resuscitation strategy used for patients undergoing damage-control laparotomy has been found to increase the 30-day survival rate.23 A damage-control resuscitation strategy consists of increasing the use of plasma and platelet transfusions over packed red cell transfusions, limiting the use of crystalloid solutions in early fluid resuscitation, and allowing for permissive hypotension.
Secondary abdominal compartment syndrome is treated conservatively in most cases, since patients with this condition are very poor surgical candidates owing to their comorbidities.18 However, in patients with progressive organ dysfunction in whom medical management has failed, surgical decompression should be considered.18 Medical management of secondary abdominal compartment syndrome depends on the underlying etiology. Strategies include nasogastric or colonic decompression, use of prokinetic agents, paracentesis in cases with gross ascites, and maintaining a cumulative negative fluid balance. The WSACS does not recommend routine use of diuretics, albumin infusion, or renal replacement strategies. Pain should be adequately controlled to improve abdominal wall compliance.18,24 Neuromuscular blockade agents may be used to aid this process. Neostigmine may be used to treat colonic pseudo-obstruction when other conservative methods fail. Use of enteral nutrition should be minimized.18
Our patient might have abdominal compartment syndrome, but a definitive diagnosis cannot be made at this point without measuring the intra-abdominal pressure.
WHICH IMAGING TEST WOULD BE BEST?
2. Which imaging test would be best for establishing the diagnosis in this patient?
- Plain abdominal radiography
- Abdominal ultrasonography
- Computed tomography of the abdomen and pelvis with contrast
- Magnetic resonance imaging of the abdomen and pelvis
Plain abdominal radiography
Plain abdominal radiography can help to determine if there is free gas under the diaphragm (due to bowel perforation), obstructed bowel, sentinel loop, volvulus, or fecoliths causing the abdominal pain. It cannot diagnose rectus sheath hematoma or acute mesenteric ischemia.
Abdominal ultrasonography
Abdominal ultrasonography can be used as the first diagnostic test, as it is widely available, safe, effective, and tolerable. It does not expose the patient to radiation or intravenous contrast agents. It helps to diagnose rectus sheath hematoma and helps to follow its maturation and resolution once a diagnosis is made. It can provide a rapid assessment of the size, location, extent, and physical characteristics of the mass.
Rectus sheath hematoma appears spindle-shaped on sagittal sections and ovoid on coronal sections. It often appears sonolucent in the early stages and sonodense in the late stage, but the appearance may be heterogeneous depending on the combined presence of clot and fresh blood. These findings are sufficient to make the diagnosis.
Abdominal ultrasonography has 85% to 96% sensitivity in diagnosing rectus sheath hematoma.25 It can help diagnose other causes of the abdominal pain, such as renal stones and cholecystitis. It is the preferred imaging test in pediatric patients, pregnant patients, and those with renal insufficiency.
Abdominal computed tomography
Abdominal computed tomography has a sensitivity and specificity of 100% for diagnosing acute rectus sheath hematoma with a duration of less than 5 days.25 It not only helps to determine the precise location and extent, but also helps to determine if there is active extravasation. Even in patients with renal insufficiency, noncontrast computed tomography will help to confirm the diagnosis, although it may not show extravasation or it may miss certain abdominal pathologies because of the lack of contrast.
Acute rectus sheath hematoma appears as a hyperdense mass posterior to the rectus abdominis muscle with ipsilateral anterolateral muscular enlargement. Chronic rectus sheath hematoma appears isodense or hypodense relative to the surrounding muscle. Above the arcuate line, rectus sheath hematoma has a spindle shape; below the arcuate line, it is typically spherical.
In 1996, Berná et al26 classified rectus sheath hematoma into three grades based on findings of computed tomography:
- Grade I is intramuscular and unilateral
- Grade II may involve bilateral rectus muscles without extension into the prevesicular space
- Grade III extends into the peritoneum and prevesicular space
Magnetic resonance imaging
Magnetic resonance imaging is useful to differentiate chronic rectus sheath hematoma (greater than 5-day duration) from an anterior abdominal wall mass. Chronic rectus sheath hematoma will have high signal intensity on both T1- and T2-weighted images up to 10 months after the onset of the hematoma.27
Back to our patient
Since our patient’s symptoms are acute and of less than 5 days’ duration, computed tomography of the abdomen and pelvis would be the best diagnostic test, with therapeutic implications if there is ongoing extravasation.
Computed tomography of the abdomen with contrast showed a new hematoma, measuring 25 by 14 by 13.5 cm, in the right rectus sheath (Figure 2), with no other findings. The hematoma was grade I, since it was intramuscular and unilateral without extension elsewhere.
Laboratory workup showed that the patient’s hematocrit was falling, from 34% to 24%, and her INR was elevated at 2.5. She was resuscitated with fluids, blood transfusion, and fresh-frozen plasma. Anticoagulation was withheld. In spite of resuscitation, her hematocrit kept falling, though she remained hemodynamically stable.
THE WAY FORWARD
3. At this point, what would be the best approach to management in this patient?
- Serial clinical examinations and frequent monitoring of the complete blood cell count
- Urgent surgical consult for exploratory laparotomy with evaluation of the hematoma and ligation of the bleeding vessel
- Repeat computed tomographic angiography to identify a possible bleeding vessel; consideration of radiographically guided arterial embolization
- Measuring the intra-abdominal pressure using the intrabladder pressure for abdominal compartment syndrome and consideration of surgical drainage
The key clinical concern in a patient with a rectus sheath hematoma who is hemodynamically stable is whether the hematoma is expanding. This patient responded to initial resuscitation, but her falling hematocrit was evidence of ongoing bleeding leading to an expanding rectus sheath hematoma. Thus, serial clinical examinations and frequent monitoring of the complete blood cell count would not be enough, as it could miss fatal ongoing bleeding.
Radiographically guided embolization with Gelfoam, thrombin, or coils should be attempted first, as this is less invasive than exploratory laparotomy.28 It can achieve hemostasis, decrease the size of the hematoma, limit the need for blood products, and prevent rupture into the abdomen. If this is unsuccessful, the next step is ligation of the bleeding vessel.29
Surgical treatment includes evacuation of the hematoma, repair of the rectus sheath, ligation of bleeding vessels, and abdominal wall closure. Surgical evacuation or guided drainage of a rectus sheath hematoma on its own is not normally indicated and may indeed cause persistent bleeding by diminishing a potential tamponade effect. However, it may become necessary if the hematoma is very large or infected, if it causes marked respiratory impairment, or if abdominal compartment syndrome is suspected.
Abdominal compartment syndrome is very rare but is associated with a 50% mortality rate.30 It should be suspected in patients with oliguria, low cardiac output, changes in minute ventilation, and altered splanchnic blood flow. The diagnosis is confirmed with indwelling catheter manometry of the bladder to measure intra-abdominal pressure. Intra-abominal pressure above 25 mm Hg should be treated with decompressive laparotomy.30 However, the clinical suspicion of abdominal compartment syndrome was low in this patient.
The best choice at this point would be urgent computed tomographic angiography to identify a bleeding vessel, along with consideration of radiographically guided arterial embolization.
TREATMENT IS USUALLY CONSERVATIVE
Treatment of rectus sheath hematoma is conservative in most hemodynamically stable patients, with embolization or surgical intervention reserved for unstable patients or those in whom the hematoma is expanding.
Knowledge of the grading system of Berná et al26 helps to assess the patient’s risk and to anticipate potential complications. Grade I hematomas are mild and do not necessitate admission. Patients with grade II hematoma can be admitted to the floor for 24 to 48 hours for observation. Grade III usually occurs in patients receiving anticoagulant therapy and frequently requires blood products. These patients have a prolonged hospital stay and more complications, including hypovolemic shock, myonecrosis, acute coronary syndrome, arrhythmias, acute renal failure, small-bowel infarction, and abdominal compartment syndrome—all of which increases the risk of morbidity and death. Thus, patients who are on anticoagulation who develop grade III rectus sheath hematoma should be admitted to the hospital, preferably to the intensive care unit, to ensure that the hematoma is not expanding and to plan reinstitution of anticoagulation as appropriate.
In most cases, rectus sheath hematomas resolve within 1 to 3 months. Resolution of large hematomas may be hastened with the use of pulsed ultrasound.31 However, this treatment should be used only after the acute phase is over, when there is evidence of an organized thrombus and coagulation measures have returned to the target range. This helps to reduce the risk of bleeding and to prevent symptoms from worsening.31
OUR PATIENT’S COURSE
Our patient underwent urgent computed tomographic angiography, which showed a modest increase in the size of the rectus sheath hematoma. However, no definitive blush of contrast was seen to suggest active arterial bleeding. Her hematocrit stabilized, and she remained hemodynamically stable without requiring additional intervention. Most likely her bleeding was self-contained. She had normal intra-abdominal pressure on serial monitoring. She was later transferred to acute inpatient rehabilitation in view of deconditioning and is currently doing well. The hematoma persisted, decreasing only slightly in size over the next 3 weeks.
A 57-year-old woman presented to the emergency department with left lower quadrant pain, which had started 1 week earlier and was constant, dull, aching, and nonradiating. There were no aggravating or alleviating factors. The pain was associated with low-grade fever and nausea. She reported no vomiting, no change in bowel habits, and no hematemesis, hematochezia, or melena. She did not have urinary urgency, frequency, or dysuria. She had no cardiac, respiratory, or neurologic symptoms.
Her medical history included hypothyroidism, type 2 diabetes mellitus, diverticulosis, and chronic obstructive pulmonary disease. Her medications included metformin, insulin, levothyroxine, and inhaled tiotropium. She had no allergies. She had never undergone surgery, including cesarean delivery. She was postmenopausal. She had two children, both of whom had been born vaginally at full term. She denied using alcohol, tobacco, and illicit drugs. Her family history was noncontributory.
On examination, she was not in acute distress. Her temperature was 36.7°C (98.1°F), blood pressure 130/90 mm Hg, heart rate 86 beats per minute and regular, respiratory rate 16 breaths per minute, and oxygen saturation 98% on ambient air. Examination of her head and neck was unremarkable. Cardiopulmonary examination was normal. Abdominal examination revealed normal bowel sounds, mild tenderness in the left lower quadrant with localized guarding, and rebound tenderness. Neurologic examination was unremarkable.
Initial laboratory data showed mild leukocytosis. Computed tomography with contrast of the abdomen and pelvis suggested acute diverticulitis.
ATRIAL FIBRILLATION, AND THEN A TURN FOR THE WORSE
The patient was admitted with an initial diagnosis of acute diverticulitis. She was started on antibiotics, hydration, and pain medications, and her abdominal pain gradually improved.
On the third hospital day, she suddenly experienced shortness of breath and palpitations. At the time of admission her electrocardiogram had been normal, but it now showed atrial fibrillation with a rapid ventricular response. She also developed elevated troponin levels, which were thought to represent type 2 non-ST-elevation myocardial infarction.
She was started on aspirin, clopidogrel, and anticoagulation with heparin bridged with warfarin for the new-onset atrial fibrillation. Her heart rate was controlled with metoprolol, and her shortness of breath improved. An echocardiogram was normal.
On the seventh hospital day, she developed severe right-sided lower abdominal pain and bruising. Her blood pressure was 90/60 mm Hg, heart rate 110 beats per minute and irregularly irregular, respiratory rate 22 breaths per minute, and oxygen saturation 97% on room air. Her abdomen was diffusely tender with a palpable mass in the right lower quadrant and hypoactive bowel sounds. Ecchymosis was noted (Figure 1).
DIFFERENTIAL DIAGNOSIS
1. What is the likely cause of her decompensation?
- Acute mesenteric ischemia
- Perforation of the gastrointestinal tract
- Rectus sheath hematoma
- Abdominal compartment syndrome due to acute pancreatitis
Acute mesenteric ischemia
Signs and symptoms of acute mesenteric ischemia can be vague. Moreover, when it leads to bowel necrosis the mortality rate is high, ranging from 30% to 65%.1 Hence, one should suspect it and try to diagnose it early.
Most patients with this condition have comorbidities; risk factors include atherosclerotic disease, cardiac conditions (congestive heart failure, recent myocardial infarction, and atrial fibrillation), systemic illness, and inherited or acquired hypercoagulable states.2
The four major causes are:
- Acute thromboembolic occlusion of the superior mesenteric artery (the most common site of occlusion because of the acute angle of origin from the aorta)
- Acute thrombosis of the mesenteric vein
- Acute thrombosis of the mesenteric artery
- Nonocclusive disease affecting the mesenteric vessels2
Nonocclusive disease is seen in conditions in which the mesenteric vessels are already compromised due to background stenosis owing to atherosclerosis. Also, conditions such as septic and cardiogenic shock can compromise these arteries, leading to ischemia, which, if it persists, can lead to bowel infarction. Ischemic colitis falls under this category. It commonly involves the descending and sigmoid colon.3
The initial symptom of ischemia may be abdominal pain that is brought on by eating large meals (“postprandial intestinal angina.”2 When the ischemia worsens to infarction, patients may have a diffusely tender abdomen and constant pain that does not vary with palpation. Surprisingly, patients do not exhibit peritoneal signs early on. This gives rise to the description of “pain out of proportion to the physical findings” traditionally associated with acute mesenteric ischemia.2
Diagnosis. Supportive laboratory data include marked leukocytosis, elevated hematocrit due to hemoconcentration, metabolic acidosis, and elevated lactate.4 Newer markers such as serum alpha-glutathione S-transferase (alpha-GST) and intestinal fatty acid-binding protein (I-FABP) may be used to support the diagnosis.
Elevated alpha-GST has 72% sensitivity and 77% specificity in the diagnosis of acute mesenteric ischemia.5 The caveat is that it cannot reliably differentiate ischemia from infarction. Its sensitivity can be improved to 97% to 100% by using the white blood cell count and lactate levels in combination.5
An I-FABP level higher than 100 ng/mL has 100% sensitivity for diagnosing mesenteric infarction but only 25% sensitivity for bowel strangulation.6
Early use of abdominal computed tomography with contrast can aid in recognizing this diagnosis.7 Thus, it should be ordered in suspected cases, even in patients who have elevated creatinine levels (which would normally preclude the use of contrast), since early diagnosis followed by endovascular therapy is associated with survival benefit, and the risk of contrast-induced nephropathy appears to be small.8 Computed tomography helps to determine the state of mesenteric vessels and bowel perfusion before ischemia progresses to infarction. It also helps to rule out other common diagnoses. Findings that suggest acute mesenteric ischemia include segmental bowel wall thickening, intestinal pneumatosis with gas in the portal vein, bowel dilation, mesenteric stranding, portomesenteric thrombosis, and solid-organ infarction.9
Treatment. If superior mesenteric artery occlusion is diagnosed on computed tomography, the next step is to determine if there is peritonitis.10 In patients who have evidence of peritonitis, exploratory laparotomy is performed. For emboli in such patients, open embolectomy followed by on-table angiography is carried out in combination with damage-control surgery. For patients with peritonitis and acute thrombosis, stenting along with damage-control surgery is preferred.10
On the other hand, if there is no peritonitis, the thrombosis may be amenable to endovascular intervention. For patients with acute embolic occlusion with no contraindications to thrombolysis, aspiration embolectomy in combination with local catheter-directed thrombolysis with recombinant tissue plasminogen activator can be performed. This can be combined with endovascular mechanical embolectomy for more complete management.10 Patients with contraindications to thrombolysis can be treated either with aspiration and mechanical embolectomy or with open embolectomy with angiography.10
During laparotomy, the surgeon carefully inspects the bowel for signs of necrosis. Signs that bowel is still viable include pink color, bleeding from cut surfaces, good peristalsis, and visible pulsations in the arterial arcade of the mesentery.
Acute mesenteric artery thrombosis arising from chronic atherosclerotic disease can be treated with stenting of the stenotic lesion.10 Patients with this condition would also benefit from aggressive management of atherosclerotic disease with statins along with antiplatelet agents.10
Mesenteric vein thrombosis requires prompt institution of anticoagulation. However, in advanced cases leading to bowel infarction, exploratory laparotomy with resection of the necrotic bowel may be required. Anticoagulation should be continued for at least 6 months, and further therapy should be determined by the underlying precipitating condition.10
Critically ill patients who develop mesenteric ischemia secondary to persistent hypotension usually respond to adequate volume resuscitation, cessation of vasopressors, and overall improvement in their hemodynamic status. These patients must be closely monitored for development of gangrene of the bowel because they may be intubated and not able to complain. Any sudden deterioration in their condition should prompt physicians to consider bowel necrosis developing in these patients. Elevation of lactate levels out of proportion to the degree of hypotension may be corroborative evidence.4
Our patient had risk factors for acute mesenteric ischemia that included atrial fibrillation and recent non-ST-elevation myocardial infarction. She could have had arterial emboli due to atrial fibrillation, in situ superior mesenteric arterial thrombosis, or splanchnic arterial vasoconstriction due to the myocardial infarction associated with transient hypotension.
Arguing against this diagnosis, although she had a grossly distended abdomen, abdominal bruising usually is not seen. Also, a palpable mass in the right lower quadrant is uncommon except when acute mesenteric ischemia occurs due to segmental intestinal strangulation, as with strangulated hernia or volvulus. She also had therapeutic international normalized ratio (INR) levels constantly while on anticoagulation. Nevertheless, acute mesenteric ischemia should be strongly considered in the initial differential diagnosis of this patient’s acute decompensation.
Perforation of the gastrointestinal tract
Diverticulitis is the acute inflammation of one or more diverticula, which are small pouches created by herniation of the mucosa into the wall of the colon. The condition is caused by microscopic or macroscopic perforation of the diverticula. Microscopic perforation is usually self-limited (uncomplicated diverticulitis) and responds to conservative treatment, whereas macroscopic perforation can be associated with fecal or purulent peritonitis, abscess, enteric fistula, bowel obstruction, and stricture (complicated diverticulitis), in which case surgery may be necessary.
Patients with peritonitis due to free perforation present with generalized tenderness with rebound tenderness and guarding on abdominal examination. The abdomen may be distended and tympanic to percussion, with diminished or absent bowel sounds. Patients may have hemodynamic compromise.
Plain upright abdominal radiographs may show free air under the diaphragm. Computed tomography may show oral contrast outside the lumen and detect even small amounts of free intraperitoneal air (more clearly seen on a lung window setting).
Our patient initially presented with acute diverticulitis. She later developed diffuse abdominal tenderness with hypoactive bowel sounds. Bowel perforation is certainly a possibility at this stage, though it is usually not associated with abdominal bruising. She would need additional imaging to rule out this complication.
Other differential diagnoses to be considered in this patient with right lower-quadrant pain include acute appendicitis, incarcerated inguinal hernia, volvulus (particularly cecal volvulus), small-bowel obstruction, pyelonephritis, and gynecologic causes such as adnexal torsion, ruptured ovarian cyst, and tubo-ovarian abscess. Computed tomography helps to differentiate most of these causes.
Rectus sheath hematoma
Rectus sheath hematoma is relatively uncommon and often not considered in the initial differential diagnosis of an acute abdomen. This gives it the rightful term “a great masquerader.” It usually results from bleeding into the rectus sheath from damage to the superior (more common) or inferior epigastric arteries and occasionally from a direct tear of the rectus abdominis muscle. Predisposing factors include anticoagulant therapy (most common), advanced age, hypertension, previous abdominal surgery, trauma, paroxysmal coughing, medication injections, pregnancy, blood dyscrasias, severe vomiting, violent physical activity, and leukemia.11
Clinical manifestations include acute abdominal pain, often associated with fever, nausea, and vomiting. Physical examination may reveal signs of hypovolemic shock, a palpable nonpulsatile abdominal mass, and signs of local peritoneal irritation. The Carnett sign11 (tenderness within the abdominal wall that persists and does not improve with raising the head) and the Fothergill sign11 (a tender abdominal mass that does not cross the midline and remains palpable with tensing of the rectus sheath) may be elicited.
Computed tomography is more sensitive than abdominal ultrasonography in differentiating rectus sheath hematoma from an intra-abdominal pathology.11 In addition, computed tomography also helps to determine if the bleeding is active or not, which has therapeutic implications.
In our patient, rectus sheath hematoma is a possibility because of her ongoing anticoagulation, findings of localized abdominal bruising, and palpable right lower-quadrant mass, and it is high on the list of differential diagnoses. Rectus sheath hematoma should be considered in the differential diagnosis of lower abdominal pain particularly in elderly women who are on anticoagulation and in whom the onset of pain coincides with a paroxysm of cough.12 Women are twice as likely as men to develop rectus sheath hematoma, owing to their different muscle mass.13 In addition, anterior abdominal wall muscles are stretched during pregnancy.13
Abdominal compartment syndrome
Abdominal compartment syndrome has been classically associated with surgical patients. However, it is being increasingly recognized in critically ill medical patients, in whom detecting and treating it early may result in significant reduction in rates of morbidity and death.14
Abdominal compartment syndrome is of three types: primary, secondary, and recurrent. Primary abdominal compartment syndrome refers to the classic surgical patients with evidence of direct injury to the abdominal or pelvic organs through major trauma or extensive abdominal surgeries. Secondary abdominal compartment syndrome refers to its development in critically ill intensive care patients in whom the pathology does not directly involve the abdominal or pelvic organs.
Various medical conditions can culminate in abdominal compartment syndrome and result in multiorgan failure. Recurrent abdominal compartment syndrome refers to its development after management of either primary or secondary intra-abdominal hypertension or abdominal compartment syndrome.15 Clinicians thus must be aware of secondary and recurrent abdominal compartment syndrome occurring in critically ill patients.
The normal intra-abdominal pressure is around 5 to 7 mm Hg, even in most critically ill patients. Persistent elevation, ie, higher than 12 mm Hg, is referred to as intra-abdominal hypertension.16–18 Intra-abdominal hypertension is subdivided into four grades:
- Grade I: 12–15 mm Hg
- Grade II: 16–20 mm Hg
- Grade III: 21–25 mm Hg
- Grade IV: > 25 mm Hg.
The World Society of the Abdominal Compartment Syndrome (WSACS) defines abdominal compartment syndrome as pressure higher than 20 mm Hg along with organ damage.18 It may or may not be associated with an abdominal perfusion pressure less than 60 mm Hg.18
Risk factors associated with abdominal compartment syndrome include conditions causing decreased gut motility (gastroparesis, ileus, and colonic pseudo-obstruction), intra-abdominal or retroperitoneal masses or abscesses, ascites, hemoperitoneum, acute pancreatitis, third-spacing due to massive fluid resuscitation with transfusions, peritoneal dialysis, and shock.18,19
Physical examination has a sensitivity of only 40% to 60% in detecting intra-abdominal hypertension.20 The gold-standard method of measuring the intra-abdominal pressure is the modified Kron technique,18 using a Foley catheter in the bladder connected to a pressure transducer. With the patient in the supine position, the transducer is zeroed at the mid-axillary line at the level of the iliac crest, and 25 mL of normal saline is instilled into the bladder and maintained for 30 to 60 seconds to let the detrusor muscle relax.15 Pressure tracings are then recorded at the end of expiration. Factors that are known to affect the transbladder pressure include patient position, respiratory movement, and body mass index, and should be taken into account when reading the pressure recordings.15,21 Other techniques that can be used include intragastric, intra-inferior vena cava, and intrarectal approaches.15
The WSACS recommends that any patient admitted to a critical care unit or in whom new organ failure develops should be screened for risk factors for intra-abdominal hypertension and abdominal compartment syndrome. If a patient has at least two of the risk factors suggested by WSACS, a baseline intra-abdominal pressure measurement should be obtained. Patients at risk for intra-abdominal hypertension should have the intra-abdominal pressure measured every 4 to 6 hours. However, in the face of hemodynamic instability and worsening multiorgan failure, the pressure may need to be measured hourly.18
Clinicians managing patients in the intensive care unit should think of intra-abdominal pressure alongside blood pressure, urine output, and mental status when evaluating hemodynamic status. Clinical manifestations of abdominal compartment syndrome reflect the underlying organ dysfunction and include hypotension, refractory shock, decreased urine output, intracranial hypertension, progressive hypoxemia and hypercarbia, elevated pulmonary peak pressures, and worsening of metabolic acidosis.22
Treatment. The standard treatment for primary abdominal compartment syndrome is surgical decompression. According to WSACS guidelines, insertion of a percutaneous drainage catheter should be advocated in patients with gross ascites and in whom decompressive surgery is not feasible. A damage-control resuscitation strategy used for patients undergoing damage-control laparotomy has been found to increase the 30-day survival rate.23 A damage-control resuscitation strategy consists of increasing the use of plasma and platelet transfusions over packed red cell transfusions, limiting the use of crystalloid solutions in early fluid resuscitation, and allowing for permissive hypotension.
Secondary abdominal compartment syndrome is treated conservatively in most cases, since patients with this condition are very poor surgical candidates owing to their comorbidities.18 However, in patients with progressive organ dysfunction in whom medical management has failed, surgical decompression should be considered.18 Medical management of secondary abdominal compartment syndrome depends on the underlying etiology. Strategies include nasogastric or colonic decompression, use of prokinetic agents, paracentesis in cases with gross ascites, and maintaining a cumulative negative fluid balance. The WSACS does not recommend routine use of diuretics, albumin infusion, or renal replacement strategies. Pain should be adequately controlled to improve abdominal wall compliance.18,24 Neuromuscular blockade agents may be used to aid this process. Neostigmine may be used to treat colonic pseudo-obstruction when other conservative methods fail. Use of enteral nutrition should be minimized.18
Our patient might have abdominal compartment syndrome, but a definitive diagnosis cannot be made at this point without measuring the intra-abdominal pressure.
WHICH IMAGING TEST WOULD BE BEST?
2. Which imaging test would be best for establishing the diagnosis in this patient?
- Plain abdominal radiography
- Abdominal ultrasonography
- Computed tomography of the abdomen and pelvis with contrast
- Magnetic resonance imaging of the abdomen and pelvis
Plain abdominal radiography
Plain abdominal radiography can help to determine if there is free gas under the diaphragm (due to bowel perforation), obstructed bowel, sentinel loop, volvulus, or fecoliths causing the abdominal pain. It cannot diagnose rectus sheath hematoma or acute mesenteric ischemia.
Abdominal ultrasonography
Abdominal ultrasonography can be used as the first diagnostic test, as it is widely available, safe, effective, and tolerable. It does not expose the patient to radiation or intravenous contrast agents. It helps to diagnose rectus sheath hematoma and helps to follow its maturation and resolution once a diagnosis is made. It can provide a rapid assessment of the size, location, extent, and physical characteristics of the mass.
Rectus sheath hematoma appears spindle-shaped on sagittal sections and ovoid on coronal sections. It often appears sonolucent in the early stages and sonodense in the late stage, but the appearance may be heterogeneous depending on the combined presence of clot and fresh blood. These findings are sufficient to make the diagnosis.
Abdominal ultrasonography has 85% to 96% sensitivity in diagnosing rectus sheath hematoma.25 It can help diagnose other causes of the abdominal pain, such as renal stones and cholecystitis. It is the preferred imaging test in pediatric patients, pregnant patients, and those with renal insufficiency.
Abdominal computed tomography
Abdominal computed tomography has a sensitivity and specificity of 100% for diagnosing acute rectus sheath hematoma with a duration of less than 5 days.25 It not only helps to determine the precise location and extent, but also helps to determine if there is active extravasation. Even in patients with renal insufficiency, noncontrast computed tomography will help to confirm the diagnosis, although it may not show extravasation or it may miss certain abdominal pathologies because of the lack of contrast.
Acute rectus sheath hematoma appears as a hyperdense mass posterior to the rectus abdominis muscle with ipsilateral anterolateral muscular enlargement. Chronic rectus sheath hematoma appears isodense or hypodense relative to the surrounding muscle. Above the arcuate line, rectus sheath hematoma has a spindle shape; below the arcuate line, it is typically spherical.
In 1996, Berná et al26 classified rectus sheath hematoma into three grades based on findings of computed tomography:
- Grade I is intramuscular and unilateral
- Grade II may involve bilateral rectus muscles without extension into the prevesicular space
- Grade III extends into the peritoneum and prevesicular space
Magnetic resonance imaging
Magnetic resonance imaging is useful to differentiate chronic rectus sheath hematoma (greater than 5-day duration) from an anterior abdominal wall mass. Chronic rectus sheath hematoma will have high signal intensity on both T1- and T2-weighted images up to 10 months after the onset of the hematoma.27
Back to our patient
Since our patient’s symptoms are acute and of less than 5 days’ duration, computed tomography of the abdomen and pelvis would be the best diagnostic test, with therapeutic implications if there is ongoing extravasation.
Computed tomography of the abdomen with contrast showed a new hematoma, measuring 25 by 14 by 13.5 cm, in the right rectus sheath (Figure 2), with no other findings. The hematoma was grade I, since it was intramuscular and unilateral without extension elsewhere.
Laboratory workup showed that the patient’s hematocrit was falling, from 34% to 24%, and her INR was elevated at 2.5. She was resuscitated with fluids, blood transfusion, and fresh-frozen plasma. Anticoagulation was withheld. In spite of resuscitation, her hematocrit kept falling, though she remained hemodynamically stable.
THE WAY FORWARD
3. At this point, what would be the best approach to management in this patient?
- Serial clinical examinations and frequent monitoring of the complete blood cell count
- Urgent surgical consult for exploratory laparotomy with evaluation of the hematoma and ligation of the bleeding vessel
- Repeat computed tomographic angiography to identify a possible bleeding vessel; consideration of radiographically guided arterial embolization
- Measuring the intra-abdominal pressure using the intrabladder pressure for abdominal compartment syndrome and consideration of surgical drainage
The key clinical concern in a patient with a rectus sheath hematoma who is hemodynamically stable is whether the hematoma is expanding. This patient responded to initial resuscitation, but her falling hematocrit was evidence of ongoing bleeding leading to an expanding rectus sheath hematoma. Thus, serial clinical examinations and frequent monitoring of the complete blood cell count would not be enough, as it could miss fatal ongoing bleeding.
Radiographically guided embolization with Gelfoam, thrombin, or coils should be attempted first, as this is less invasive than exploratory laparotomy.28 It can achieve hemostasis, decrease the size of the hematoma, limit the need for blood products, and prevent rupture into the abdomen. If this is unsuccessful, the next step is ligation of the bleeding vessel.29
Surgical treatment includes evacuation of the hematoma, repair of the rectus sheath, ligation of bleeding vessels, and abdominal wall closure. Surgical evacuation or guided drainage of a rectus sheath hematoma on its own is not normally indicated and may indeed cause persistent bleeding by diminishing a potential tamponade effect. However, it may become necessary if the hematoma is very large or infected, if it causes marked respiratory impairment, or if abdominal compartment syndrome is suspected.
Abdominal compartment syndrome is very rare but is associated with a 50% mortality rate.30 It should be suspected in patients with oliguria, low cardiac output, changes in minute ventilation, and altered splanchnic blood flow. The diagnosis is confirmed with indwelling catheter manometry of the bladder to measure intra-abdominal pressure. Intra-abominal pressure above 25 mm Hg should be treated with decompressive laparotomy.30 However, the clinical suspicion of abdominal compartment syndrome was low in this patient.
The best choice at this point would be urgent computed tomographic angiography to identify a bleeding vessel, along with consideration of radiographically guided arterial embolization.
TREATMENT IS USUALLY CONSERVATIVE
Treatment of rectus sheath hematoma is conservative in most hemodynamically stable patients, with embolization or surgical intervention reserved for unstable patients or those in whom the hematoma is expanding.
Knowledge of the grading system of Berná et al26 helps to assess the patient’s risk and to anticipate potential complications. Grade I hematomas are mild and do not necessitate admission. Patients with grade II hematoma can be admitted to the floor for 24 to 48 hours for observation. Grade III usually occurs in patients receiving anticoagulant therapy and frequently requires blood products. These patients have a prolonged hospital stay and more complications, including hypovolemic shock, myonecrosis, acute coronary syndrome, arrhythmias, acute renal failure, small-bowel infarction, and abdominal compartment syndrome—all of which increases the risk of morbidity and death. Thus, patients who are on anticoagulation who develop grade III rectus sheath hematoma should be admitted to the hospital, preferably to the intensive care unit, to ensure that the hematoma is not expanding and to plan reinstitution of anticoagulation as appropriate.
In most cases, rectus sheath hematomas resolve within 1 to 3 months. Resolution of large hematomas may be hastened with the use of pulsed ultrasound.31 However, this treatment should be used only after the acute phase is over, when there is evidence of an organized thrombus and coagulation measures have returned to the target range. This helps to reduce the risk of bleeding and to prevent symptoms from worsening.31
OUR PATIENT’S COURSE
Our patient underwent urgent computed tomographic angiography, which showed a modest increase in the size of the rectus sheath hematoma. However, no definitive blush of contrast was seen to suggest active arterial bleeding. Her hematocrit stabilized, and she remained hemodynamically stable without requiring additional intervention. Most likely her bleeding was self-contained. She had normal intra-abdominal pressure on serial monitoring. She was later transferred to acute inpatient rehabilitation in view of deconditioning and is currently doing well. The hematoma persisted, decreasing only slightly in size over the next 3 weeks.
- Kougias P, Lau D, El Sayed HF, Zhou W, Huynh TT, Lin PH. Determinants of mortality and treatment outcome following surgical interventions for acute mesenteric ischemia. J Vasc Surg 2007; 46:467–474.
- Sise MJ. Acute mesenteric ischemia. Surg Clin North Am 2014; 94:165–181.
- Scharff JR, Longo WE, Vartanian SM, Jacobs DL, Bahadursingh AN, Kaminski DL. Ischemic colitis: spectrum of disease and outcome. Surgery 2003; 134:624–629.
- Lange H, Jäckel R. Usefulness of plasma lactate concentration in the diagnosis of acute abdominal disease. Eur J Surg 1994; 160:381–384.
- Gearhart SL, Delaney CP, Senagore AJ, et al. Prospective assessment of the predictive value of alpha-glutathione S-transferase for intestinal ischemia. Am Surg 2003; 69:324–329.
- Kanda T, Fujii H, Tani T, et al. Intestinal fatty acid-binding protein is a useful diagnostic marker for mesenteric infarction in humans. Gastroenterology 1996; 110:339–343.
- Menke J. Diagnostic accuracy of multidetector CT in acute mesenteric ischemia: systematic review and meta-analysis. Radiology 2010; 256:93–101.
- Acosta S, Björnsson S, Ekberg O, Resch T. CT angiography followed by endovascular intervention for acute superior mesenteric artery occlusion does not increase risk of contrast-induced renal failure. Eur J Vasc Endovasc Surg 2010; 39:726–730.
- Clark RA. Computed tomography of bowel infarction. J Comput Assist Tomogr 1987; 11:757–762.
- Acosta S, Björck M. Modern treatment of acute mesenteric ischaemia. Br J Surg 2014; 101:e100–e108.
- Smithson A, Ruiz J, Perello R, Valverde M, Ramos J, Garzo L. Diagnostic and management of spontaneous rectus sheath hematoma. Eur J Intern Med 2013; 24:579–582.
- Moreno Gallego A, Aguayo JL, Flores B, et al. Ultrasonography and computed tomography reduce unnecessary surgery in abdominal rectus sheath haematoma. Br J Surg 1997; 84:1295–1297.
- Dubinsky IL. Hematoma of the rectus abdominis muscle: case report and review of the literature. J Emerg Med 1997; 15:165–167.
- Yi M, Yao G, Bai Y. The monitoring of intra-abdominal pressure in critically ill patients. (In Chinese.) Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2014; 26:175–178.
- Hunt L, Frost SA, Hillman K, Newton PJ, Davidson PM. Management of intra-abdominal hypertension and abdominal compartment syndrome: a review. J Trauma Manag Outcomes 2014; 8:2.
- Malbrain ML, Cheatham ML, Kirkpatrick A, et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med 2006; 32:1722–1732.
- Malbrain ML, Chiumello D, Cesana BM, et al; WAKE-Up! Investigators. A systematic review and individual patient data meta-analysis on intra-abdominal hypertension in critically ill patients: the wake-up project. World initiative on Abdominal Hypertension Epidemiology, a Unifying Project (WAKE-Up!). Minerva Anestesiol 2014; 80:293–306.
- Kirkpatrick AW, Roberts DJ, De Waele J, et al; Pediatric Guidelines Sub-Committee for the World Society of the Abdominal Compartment Syndrome. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med 2013; 39:1190–1206.
- Holodinsky JK, Roberts DJ, Ball CG, et al. Risk factors for intra-abdominal hypertension and abdominal compartment syndrome among adult intensive care unit patients: a systematic review and meta-analysis. Crit Care 2013; 17:R249.
- Sugrue M, Bauman A, Jones F, et al. Clinical examination is an inaccurate predictor of intraabdominal pressure. World J Surg 2002; 26:1428–1431.
- Cheatham ML, De Waele JJ, De Laet I, et al; World Society of the Abdominal Compartment Syndrome (WSACS) Clinical Trials Working Group. The impact of body position on intra-abdominal pressure measurement: a multicenter analysis. Crit Care Med 2009; 37:2187–2190.
- Ortiz-Diaz E, Lan CK. Intra-abdominal hypertension in medical critically ill patients: a narrative review. Shock 2014; 41:175–180.
- Cotton BA, Reddy N, Hatch QM, et al. Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg 2011; 254:598–605.
- An G, West MA. Abdominal compartment syndrome: a concise clinical review. Crit Care Med 2008; 36:1304–1310.
- Tolcher MC, Nitsche JF, Arendt KW, Rose CH. Spontaneous rectus sheath hematoma pregnancy: case report and review of the literature. Obstet Gynecol Surv 2010; 65:517–522.
- Berná JD, Garcia-Medina V, Guirao J, Garcia-Medina J. Rectus sheath hematoma: diagnostic classification by CT. Abdom Imaging 1996; 21:62–64.
- Unger EC, Glazer HS, Lee JK, Ling D. MRI of extracranial hematomas: preliminary observations. AJR Am J Roentgenol 1986; 146:403–407.
- Rimola J, Perendreu J, Falcó J, Fortuño JR, Massuet A, Branera J. Percutaneous arterial embolization in the management of rectus sheath hematoma. AJR Am J Roentgenol 2007; 188:W497–W502.
- Titone C, Lipsius M, Krakauer JS. “Spontaneous” hematoma of the rectus abdominis muscle: critical review of 50 cases with emphasis on early diagnosis and treatment. Surgery 1972; 72:568–572.
- Osinbowale O, Bartholomew JR. Rectus sheath hematoma. Vasc Med 2008; 13:275–279.
- Berná-Serna JD, Sánchez-Garre J, Madrigal M, Zuazu I, Berná-Mestre JD. Ultrasound therapy in rectus sheath hematoma. Phys Ther 2005; 85:352–357.
- Kougias P, Lau D, El Sayed HF, Zhou W, Huynh TT, Lin PH. Determinants of mortality and treatment outcome following surgical interventions for acute mesenteric ischemia. J Vasc Surg 2007; 46:467–474.
- Sise MJ. Acute mesenteric ischemia. Surg Clin North Am 2014; 94:165–181.
- Scharff JR, Longo WE, Vartanian SM, Jacobs DL, Bahadursingh AN, Kaminski DL. Ischemic colitis: spectrum of disease and outcome. Surgery 2003; 134:624–629.
- Lange H, Jäckel R. Usefulness of plasma lactate concentration in the diagnosis of acute abdominal disease. Eur J Surg 1994; 160:381–384.
- Gearhart SL, Delaney CP, Senagore AJ, et al. Prospective assessment of the predictive value of alpha-glutathione S-transferase for intestinal ischemia. Am Surg 2003; 69:324–329.
- Kanda T, Fujii H, Tani T, et al. Intestinal fatty acid-binding protein is a useful diagnostic marker for mesenteric infarction in humans. Gastroenterology 1996; 110:339–343.
- Menke J. Diagnostic accuracy of multidetector CT in acute mesenteric ischemia: systematic review and meta-analysis. Radiology 2010; 256:93–101.
- Acosta S, Björnsson S, Ekberg O, Resch T. CT angiography followed by endovascular intervention for acute superior mesenteric artery occlusion does not increase risk of contrast-induced renal failure. Eur J Vasc Endovasc Surg 2010; 39:726–730.
- Clark RA. Computed tomography of bowel infarction. J Comput Assist Tomogr 1987; 11:757–762.
- Acosta S, Björck M. Modern treatment of acute mesenteric ischaemia. Br J Surg 2014; 101:e100–e108.
- Smithson A, Ruiz J, Perello R, Valverde M, Ramos J, Garzo L. Diagnostic and management of spontaneous rectus sheath hematoma. Eur J Intern Med 2013; 24:579–582.
- Moreno Gallego A, Aguayo JL, Flores B, et al. Ultrasonography and computed tomography reduce unnecessary surgery in abdominal rectus sheath haematoma. Br J Surg 1997; 84:1295–1297.
- Dubinsky IL. Hematoma of the rectus abdominis muscle: case report and review of the literature. J Emerg Med 1997; 15:165–167.
- Yi M, Yao G, Bai Y. The monitoring of intra-abdominal pressure in critically ill patients. (In Chinese.) Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2014; 26:175–178.
- Hunt L, Frost SA, Hillman K, Newton PJ, Davidson PM. Management of intra-abdominal hypertension and abdominal compartment syndrome: a review. J Trauma Manag Outcomes 2014; 8:2.
- Malbrain ML, Cheatham ML, Kirkpatrick A, et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med 2006; 32:1722–1732.
- Malbrain ML, Chiumello D, Cesana BM, et al; WAKE-Up! Investigators. A systematic review and individual patient data meta-analysis on intra-abdominal hypertension in critically ill patients: the wake-up project. World initiative on Abdominal Hypertension Epidemiology, a Unifying Project (WAKE-Up!). Minerva Anestesiol 2014; 80:293–306.
- Kirkpatrick AW, Roberts DJ, De Waele J, et al; Pediatric Guidelines Sub-Committee for the World Society of the Abdominal Compartment Syndrome. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med 2013; 39:1190–1206.
- Holodinsky JK, Roberts DJ, Ball CG, et al. Risk factors for intra-abdominal hypertension and abdominal compartment syndrome among adult intensive care unit patients: a systematic review and meta-analysis. Crit Care 2013; 17:R249.
- Sugrue M, Bauman A, Jones F, et al. Clinical examination is an inaccurate predictor of intraabdominal pressure. World J Surg 2002; 26:1428–1431.
- Cheatham ML, De Waele JJ, De Laet I, et al; World Society of the Abdominal Compartment Syndrome (WSACS) Clinical Trials Working Group. The impact of body position on intra-abdominal pressure measurement: a multicenter analysis. Crit Care Med 2009; 37:2187–2190.
- Ortiz-Diaz E, Lan CK. Intra-abdominal hypertension in medical critically ill patients: a narrative review. Shock 2014; 41:175–180.
- Cotton BA, Reddy N, Hatch QM, et al. Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg 2011; 254:598–605.
- An G, West MA. Abdominal compartment syndrome: a concise clinical review. Crit Care Med 2008; 36:1304–1310.
- Tolcher MC, Nitsche JF, Arendt KW, Rose CH. Spontaneous rectus sheath hematoma pregnancy: case report and review of the literature. Obstet Gynecol Surv 2010; 65:517–522.
- Berná JD, Garcia-Medina V, Guirao J, Garcia-Medina J. Rectus sheath hematoma: diagnostic classification by CT. Abdom Imaging 1996; 21:62–64.
- Unger EC, Glazer HS, Lee JK, Ling D. MRI of extracranial hematomas: preliminary observations. AJR Am J Roentgenol 1986; 146:403–407.
- Rimola J, Perendreu J, Falcó J, Fortuño JR, Massuet A, Branera J. Percutaneous arterial embolization in the management of rectus sheath hematoma. AJR Am J Roentgenol 2007; 188:W497–W502.
- Titone C, Lipsius M, Krakauer JS. “Spontaneous” hematoma of the rectus abdominis muscle: critical review of 50 cases with emphasis on early diagnosis and treatment. Surgery 1972; 72:568–572.
- Osinbowale O, Bartholomew JR. Rectus sheath hematoma. Vasc Med 2008; 13:275–279.
- Berná-Serna JD, Sánchez-Garre J, Madrigal M, Zuazu I, Berná-Mestre JD. Ultrasound therapy in rectus sheath hematoma. Phys Ther 2005; 85:352–357.
Alcoholic hepatitis: Challenges in diagnosis and management
Alcoholic hepatitis, a severe manifestation of alcoholic liver disease, is rising in incidence. Complete abstinence from alcohol remains the cornerstone of treatment, while other specific interventions aim to decrease short-term mortality rates.
Despite current treatments, about 25% of patients with severe alcoholic hepatitis eventually die of it. For those who survive hospitalization, measures need to be taken to prevent recidivism. Although liver transplantation seems to hold promise, early transplantation is still largely experimental in alcoholic hepatitis and will likely be available to only a small subset of patients, especially in view of ethical issues and the possible wider implications for transplant centers.
New treatments will largely depend on a better understanding of the disease’s pathophysiology, and future clinical trials should evaluate therapies that improve short-term as well as long-term outcomes.
ACUTE HEPATIC DECOMPENSATION IN A HEAVY DRINKER
Excessive alcohol consumption is very common worldwide, is a major risk factor for liver disease, and is a leading cause of preventable death. Alcoholic cirrhosis is the eighth most common cause of death in the United States and in 2010 was responsible for nearly half of cirrhosis-related deaths worldwide.1
Alcoholic liver disease is a spectrum. Nearly all heavy drinkers (ie, those consuming 40 g or more of alcohol per day, Table 1) have fatty liver changes, 20% to 40% develop fibrosis, 10% to 20% progress to cirrhosis, and of those with cirrhosis, 1% to 2% are diagnosed with hepatocellular carcinoma every year.2
Within this spectrum, alcoholic hepatitis is a well-defined clinical syndrome characterized by acute hepatic decompensation that typically results from long-standing alcohol abuse. Binge drinkers may also be at risk for alcoholic hepatitis, but good data on the association between drinking patterns and the risk of alcoholic hepatitis are limited.
Alcoholic hepatitis varies in severity from mild to life-threatening.3 Although its exact incidence is unknown, its prevalence in alcoholics has been estimated at 20%.4 Nearly half of patients with alcoholic hepatitis have cirrhosis at the time of their acute presentation, and these patients generally have a poor prognosis, with a 28-day death rate as high as 50% in severe cases.5,6 Moreover, although alcoholic hepatitis develops in only a subset of patients with alcoholic liver disease, hospitalizations for it are increasing in the United States.7
Women are at higher risk of developing alcoholic hepatitis, an observation attributed to the effect of estrogens on oxidative stress and inflammation, lower gastric alcohol dehydrogenase levels resulting in slower first-pass metabolism of alcohol, and higher body fat content causing a lower volume of distribution for alcohol than in men.8 The incidence of alcoholic hepatitis is also influenced by a number of demographic and genetic factors as well as nutritional status and coexistence of other liver diseases.9 Most patients diagnosed with alcoholic hepatitis are active drinkers, but it can develop even after significantly reducing or stopping alcohol consumption.
FATTY ACIDS, ENZYMES, CYTOKINES, INFLAMMATION
Alcohol consumption induces fatty acid synthesis and inhibits fatty acid oxidation, thereby promoting fat deposition in the liver.
The major enzymes involved in alcohol metabolism are cytochrome P450 2E1 (CYP2E1) and alcohol dehydrogenase. CYP2E1 is inducible and is up-regulated when excess alcohol is ingested, while alcohol dehydrogen-
ase function is relatively stable. Oxidative degradation of alcohol by these enzymes generates reactive oxygen species and acetaldehyde, inducing liver injury.10 Interestingly, it has been proposed that variations in the genes for these enzymes influence alcohol consumption and dependency as well as alcohol-driven tissue damage.
In addition, alcohol disrupts the intestinal mucosal barrier, allowing lipopolysaccharides from gram-negative bacteria to travel to the liver via the portal vein. These lipopolysaccharides then bind to and activate sinusoidal Kupffer cells, leading to production of several cytokines such as tumor necrosis factor alpha, interleukin 1, and transforming growth factor beta. These cytokines promote hepatocyte inflammation, apoptosis, and necrosis (Figure 1).11
Besides activating the innate immune system, the reactive oxygen species resulting from alcohol metabolism interact with cellular components, leading to production of protein adducts. These act as antigens that activate the adaptive immune response, followed by B- and T-lymphocyte infiltration, which in turn contribute to liver injury and inflammation.12
THE DIAGNOSIS IS MAINLY CLINICAL
The diagnosis of alcoholic hepatitis is mainly clinical. In its usual presentation, jaundice develops rapidly in a person with a known history of heavy alcohol use. Other symptoms and signs may include ascites, encephalopathy, and fever. On examination, the liver may be enlarged and tender, and a hepatic bruit has been reported.13
Other classic signs of liver disease such as parotid enlargement, Dupuytren contracture, dilated abdominal wall veins, and spider nevi can be present, but none is highly specific or sensitive for alcoholic hepatitis.
Elevated liver enzymes and other clues
Laboratory tests are important in evaluating potential alcoholic hepatitis, although no single laboratory marker can definitively establish alcohol as the cause of liver disease. To detect alcohol consumption, biochemical markers such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), mean corpuscular volume, carbohydrate-deficient transferrin, and, more commonly, gamma-glutamyl transpeptidase are used.
In the acute setting, typical biochemical derangements in alcoholic hepatitis include elevated AST (up to 2 to 6 times the upper limit of normal; usually less than 300 IU/L) and elevated ALT to a lesser extent,14 with an AST-to-ALT ratio greater than 2. Neutrophilia, anemia, hyperbilirubinemia, and coagulopathy with an elevated international normalized ratio are common.
Patients with alcoholic hepatitis are also prone to develop bacterial infections, and about 7% develop hepatorenal syndrome, itself an ominous sign.15
Imaging studies are valuable in excluding other causes of abnormal liver test results in patients who abuse alcohol, such as biliary obstruction, infiltrative liver diseases, and hepatocellular carcinoma.
Screen for alcohol intake
During the initial evaluation of suspected alcoholic hepatitis, one should screen for excessive drinking. In a US Centers for Disease Control and Prevention study, only one of six US adults, including binge drinkers, said they had ever discussed alcohol consumption with a health professional.16 Many patients with alcoholic liver disease in general and alcoholic hepatitis in particular deny alcohol abuse or underreport their intake.17
Screening tests such as the CAGE questionnaire and the Alcohol Use Disorders Identification Test can be used to assess alcohol dependence or abuse.18,19 The CAGE questionnaire consists of four questions:
- Have you ever felt you should cut down on your drinking?
- Have people annoyed you by criticizing your drinking?
- Have you ever felt guilty about your drinking?
- Have you ever had a drink first thing in the morning (an eye-opener) to steady your nerves or to get rid of a hangover?
A yes answer to two or more questions is considered clinically significant.
Is liver biopsy always needed?
Although alcoholic hepatitis can be suspected on the basis of clinical and biochemical clues, liver biopsy remains the gold standard diagnostic tool. It confirms the clinical diagnosis of alcoholic hepatitis in about 85% of all patients and in up to 95% when significant hyperbilirubinemia is present.20
However, whether a particular patient needs a biopsy is not always clear. The American Association for the Study of Liver Diseases (AASLD) recommends biopsy in patients who have a clinical diagnosis of severe alcoholic hepatitis for whom medical treatment is being considered and in those with an uncertain underlying diagnosis.
Findings on liver biopsy in alcoholic hepatitis include steatosis, hepatocyte ballooning, neutrophilic infiltration, Mallory bodies (which represent aggregated cytokeratin intermediate filaments and other proteins), and scarring with a typical perivenular distribution as opposed to the periportal fibrosis seen in chronic viral hepatitis. Some histologic findings, such as centrilobular necrosis, may overlap alcoholic hepatitis and nonalcoholic steatohepatitis.
In addition to confirming the diagnosis and staging the disease, liver biopsy has prognostic value. The severity of inflammation and cholestatic changes correlates with poor prognosis and may also predict response to corticosteroid treatment in severe cases of alcoholic hepatitis.21
However, the utility of liver biopsy in confirming the diagnosis and assessing the prognosis of alcoholic hepatitis is controversial for several reasons. Coagulopathy, thrombocytopenia, and ascites are all common in patients with alcoholic hepatitis, often making percutaneous liver biopsy contraindicated. Trans-
jugular liver biopsy is not universally available outside tertiary care centers.
Needed is a minimally invasive test for assessing this disease. Breath analysis might be such a test, offering a noninvasive means to study the composition of volatile organic compounds and elemental gases and an attractive method to evaluate health and disease in a patient-friendly manner. Our group devised a model based on breath levels of trimethylamine and pentane. When we tested it, we found that it distinguishes patients with alcoholic hepatitis from those with acute liver decompensation from causes other than alcohol and controls without liver disease with up to 90% sensitivity and 80% specificity.22
ASSESSING THE SEVERITY OF ALCOHOLIC HEPATITIS
Several models have been developed to assess the severity of alcoholic hepatitis and guide treatment decisions (Table 2).
The MDF (Maddrey Discriminant Function)6 system was the first scoring system developed and is still the most widely used. A score of 32 or higher indicates severe alcoholic hepatitis and has been used as the threshold for starting treatment with corticosteroids.6
The MDF has limitations. Patients with a score lower than 32 are considered not to have severe alcoholic hepatitis, but up to 17% of them still die. Also, since it uses the prothrombin time, its results can vary considerably among laboratories, depending on the sensitivity of the thromboplastin reagent used.
The MELD (Model for End-stage Liver Disease) score. Sheth et al23 compared the MELD and the MDF scores in assessing the severity of alcoholic hepatitis. They found that the MELD performed as well as the MDF in predicting 30-day mortality. A MELD score of greater than 11 had a sensitivity in predicting 30-day mortality of 86% and a specificity of 81%, compared with 86% and 48%, respectively, for MDF scores greater than 32.
Another study found a MELD score of 21 to have the highest sensitivity and specificity in predicting mortality (an estimated 90-day death rate of 20%). Thus, a MELD score of 21 is an appropriate threshold for prompt consideration of specific therapies such as corticosteroids.24
The MELD score has become increasingly important in patients with alcoholic hepatitis, as some of them may become candidates for liver transplantation (see below). Also, serial MELD scores in hospitalized patients have prognostic implications, since an increase of 2 or more points in the first week has been shown to predict in-hospital mortality.25
The GAHS (Glasgow Alcoholic Hepatitis Score)26 was shown to identify patients with alcoholic hepatitis who have an especially poor prognosis and need corticosteroid therapy. In those with a GAHS of 9 or higher, the 28-day survival rate was 78% with corticosteroid treatment and 52% without corticosteroid treatment; survival rates at 84 days were 59% and 38%, respectively.26
The ABIC scoring system (Age, Serum Bilirubin, INR, and Serum Creatinine) stratifies patients by risk of death at 90 days27:
- Score less than 6.71: low risk (100% survival)
- A score 6.71–8.99: intermediate risk (70% survival)
- A score 9.0 or higher: high risk (25% survival).
Both the GAHS and ABIC score are limited by lack of external validation.
The Lille score.28 While the above scores are used to identify patients at risk of death from alcoholic hepatitis and to decide on starting corticosteroids, the Lille score is designed to assess response to corticosteroids after 1 week of treatment. It is calculated based on five pretreatment variables and the change in serum bilirubin level at day 7 of corticosteroid therapy. Lille scores range from 0 to 1; a score higher than 0.45 is associated with a 75% mortality rate at 6 months and indicates a lack of response to corticosteroids and that these drugs should be discontinued.28
MANAGEMENT
Supportive treatment
Abstinence from alcohol is the cornerstone of treatment of alcoholic hepatitis. Early management of alcohol abuse or dependence is, therefore, warranted in all patients with alcoholic hepatitis. Referral to addiction specialists, motivational therapies, and anticraving drugs such as baclofen can be utilized.
Treat alcohol withdrawal. Alcoholics who suddenly decrease or discontinue their alcohol use are at high risk of alcohol withdrawal syndrome. Within 24 hours after the last drink, patients can experience increases in their heart rate and blood pressure, along with irritability and hyperreflexia. Within the next few days, more dangerous complications including seizures and delirium tremens can arise.
Alcohol withdrawal symptoms should be treated with short-acting benzodiazepines or clomethiazole, keeping the risk of worsening encephalopathy in mind.29 If present, complications of cirrhosis such as encephalopathy, ascites, and variceal bleeding should be managed.
Nutritional support is important. Protein-calorie malnutrition is common in alcoholics, as are deficiencies of vitamin A, vitamin D, thiamine, folate, pyridoxine, and zinc.30 Although a randomized controlled trial comparing enteral nutrition (2,000 kcal/day) vs corticosteroids (prednisolone 40 mg/day) in patients with alcoholic hepatitis did not show any difference in the 28-day mortality rate, those who received nutritional support and survived the first month had a lower mortality rate than those treated with corticosteroids (8% vs 37%).31 A daily protein intake of 1.5 g per kilogram of body weight is therefore recommended, even in patients with hepatic encephalopathy.15
Combining enteral nutrition and corticosteroid treatment may have a synergistic effect but is yet to be investigated.
Screen for infection. Patients with alcoholic hepatitis should be screened for infection, as about 25% of those with severe alcoholic hepatitis have an infection at admission.32 Since many of these patients meet the criteria for systemic inflammatory response syndrome, infections can be particularly difficult to diagnose. Patients require close clinical monitoring as well as regular pancultures for early detection. Antibiotics are frequently started empirically even though we lack specific evidence-based guidelines on this practice.33
Corticosteroids
Various studies have evaluated the role of corticosteroids in treating alcoholic hepatitis, differing considerably in sample populations, methods, and end points. Although the results of individual trials differ, meta-analyses indicate that corticosteroids have a moderate beneficial effect in patients with severe alcoholic hepatitis.
For example, Rambaldi et al34 performed a meta-analysis that concluded the mortality rate was lower in alcoholic hepatitis patients with MDF scores of at least 32 or hepatic encephalopathy who were treated with corticosteroids than in controls (relative risk 0.37, 95% confidence interval 0.16–0.86).
Therefore, in the absence of contraindications, the AASLD recommends starting corticosteroids in patients with severe alcoholic hepatitis, defined as an MDF score of 32 or higher.21 The preferred agent is oral prednisolone 40 mg daily or parenteral methylprednisolone 32 mg daily for 4 weeks and then tapered over the next 2 to 4 weeks or abruptly discontinued. Because activation of prednisone is decreased in patients with liver disease, prednisolone (the active form) is preferred over prednisone (the inactive precursor).35 In alcoholic hepatitis, the number needed to treat with corticosteroids to prevent one death has been calculated36 at 5.
As mentioned, response to corticosteroids is commonly assessed at 1 week of treatment using the Lille score. A score higher than 0.45 predicts a poor response and should trigger discontinuation of corticosteroids, particularly in those classified as null responders (Lille score > 0.56).
Adverse effects of steroids include sepsis, gastrointestinal bleeding, and steroid psychosis. Of note, patients who have evidence of hepatorenal syndrome or gastrointestinal bleeding tend to have a less favorable response to corticosteroids. Also, while infections were once considered a contraindication to steroid therapy, recent evidence suggests that steroid use might not be precluded in infected patients after appropriate antibiotic therapy. Infections occur in about a quarter of all alcoholic hepatitis patients treated with steroids, more frequently in null responders (42.5%) than in responders (11.1%), which supports corticosteroid discontinuance at 1 week in null responders.32
Pentoxifylline
An oral phosphodiesterase inhibitor, pentoxifylline, also inhibits production of several cytokines, including tumor necrosis factor alpha. At a dose of 400 mg orally three times daily for 4 weeks, pentoxifylline has been used in treating severe alcoholic hepatitis (MDF score ≥ 32) and is recommended especially if corticosteroids are contraindicated, as with sepsis.21
An early double-blind clinical trial randomized patients with severe alcoholic hepatitis to receive either pentoxifylline 400 mg orally three times daily or placebo. Of the patients who received pentoxifylline, 24.5% died during the index hospitalization, compared with 46.1% of patients who received placebo. This survival benefit was mainly related to a markedly lower incidence of hepatorenal syndrome as the cause of death in the pentoxifylline group than in the placebo group (50% vs 91.7% of deaths).37
In a small clinical trial in patients with severe alcoholic hepatitis, pentoxifylline recipients had a higher 3-month survival rate than prednisolone recipients (35.29% vs 14.71%, P = .04).38 However, a larger trial showed no improvement in 6-month survival with the combination of prednisolone and pentoxifylline compared with prednisolone alone (69.9% vs 69.2%, P = .91).39 Also, a meta-analysis of five randomized clinical trials found no survival benefit with pentoxifylline therapy.40
Of note, in the unfortunate subgroup of patients who have a poor response to corticosteroids, no alternative treatment, including pentoxifylline, has been shown to be effective.41
Prednisone or pentoxifylline? Very recently, results of the Steroids or Pentoxifylline for Alcoholic Hepatitis (STOPAH) trial have been released.42 This is a large, multicenter, double-blinded clinical trial that aimed to provide a definitive answer to whether corticosteroids or pentoxifylline (or both) are beneficial in patients with alcoholic hepatitis. The study included 1,103 adult patients with severe alcoholic hepatitis (MDF score ≥ 32) who were randomized to monotherapy with prednisolone or pentoxifylline, combination therapy, or placebo. The primary end point was mortality at 28 days, and secondary end points included mortality at 90 days and at 1 year. Prednisolone reduced 28-day mortality by about 39%. In contrast, the 28-day mortality rate was similar in patients who received pentoxifylline and those who did not. Also, neither drug was significantly associated with a survival benefit beyond 28 days. The investigators concluded that pentoxifylline has no impact on disease progression and should not be used for the treatment of severe alcoholic hepatitis.42
Other tumor necrosis factor alpha inhibitors not recommended
Two other tumor necrosis factor alpha inhibitors, infliximab and etanercept, have been tested in clinical trials in alcoholic hepatitis. Unfortunately, the results were not encouraging, with no major reduction in mortality.43–45 In fact, these trials demonstrated a significantly increased risk of infections in the treatment groups. Therefore, these drugs are not recommended for treating alcoholic hepatitis.
A possible explanation is that tumor necrosis factor alpha plays an important role in liver regeneration, aiding in recovery from alcohol-induced liver injury, and inhibiting it can have deleterious consequences.
Other agents
A number of other agents have undergone clinical trials in alcoholic hepatitis.
N-acetylcysteine, an antioxidant that replenishes glutathione stores in hepatocytes, was evaluated in a randomized clinical trial in combination with prednisolone.46 Although the 1-month mortality rate was significantly lower in the combination group than in the prednisolone-only group (8% vs 24%, P = .006), 3-month and 6-month mortality rates were not. Nonetheless, the rates of infection and hepatorenal syndrome were lower in the combination group. Therefore, corticosteroids and N-acetylcysteine may have synergistic effects, but the optimum duration of N-acetylcysteine therapy needs to be determined in further studies.
Vitamin E, silymarin, propylthiouracil, colchicine, and oxandrolone (an anabolic steroid) have also been studied, but with no convincing benefit.21
Role of liver transplantation
Liver transplantation for alcoholic liver disease has been a topic of great medical and social controversy. The view that alcoholic patients are responsible for their own illness led to caution when contemplating liver transplantation. Many countries require 6 months of abstinence from alcohol before placing a patient on the liver transplant list, posing a major obstacle to patients with alcoholic hepatitis, as almost all are active drinkers at the time of presentation and many will die within 6 months. Reasons for this 6-month rule include donor shortage and risk of recidivism.47
With regard to survival following alcoholic hepatitis, a study utilizing the United Network for Organ Sharing database matched patients with alcoholic hepatitis and alcoholic cirrhosis who underwent liver transplantation. Rates of 5-year graft survival were 75% in those with alcoholic hepatitis and 73% in those with alcoholic cirrhosis (P = .97), and rates of patient survival were 80% and 78% (P = .90), respectively. Proportional regression analysis adjusting for other variables showed no impact of the etiology of liver disease on graft or patient survival. The investigators concluded that liver transplantation could be considered in a select group of patients with alcoholic hepatitis who do not improve with medical therapy.48
In a pivotal case-control prospective study,49 26 patients with Lille scores greater than 0.45 were listed for liver transplantation within a median of 13 days after nonresponse to medical therapy. The cumulative 6-month survival rate was higher in patients who received a liver transplant early than in those who did not (77% vs 23%, P < .001). This benefit was maintained through 2 years of follow-up (hazard ratio 6.08, P = .004). Of note, all these patients had supportive family members, no severe coexisting conditions, and a commitment to alcohol abstinence (although 3 patients resumed drinking after liver transplantation).49
Although these studies support early liver transplantation in carefully selected patients with severe alcoholic hepatitis, the criteria for transplantation in this group need to be refined. Views on alcoholism also need to be reconciled, as strong evidence is emerging that implicates genetic and environmental influences on alcohol dependence.
Management algorithm
Figure 2 shows a suggested management algorithm for alcoholic hepatitis, adapted from the guidelines of the AASLD and European Association for the Study of the Liver.
NEW THERAPIES NEEDED
Novel therapies for severe alcoholic hepatitis are urgently needed to help combat this devastating condition. Advances in understanding its pathophysiology have uncovered several new therapeutic targets, and new agents are already being evaluated in clinical trials.
IMM 124-E, a hyperimmune bovine colostrum enriched with immunoglobulin G anti-lipopolysaccharide, is going to be evaluated in combination with prednisolone in patients with severe alcoholic hepatitis.
Anakinra, an interleukin 1 receptor antagonist, has significant anti-inflammatory activity and is used to treat rheumatoid arthritis. A clinical trial to evaluate its role in alcoholic hepatitis has been designed in which patients with severe alcoholic hepatitis (defined as a MELD score ≥ 21) will be randomized to receive either methylprednisolone or a combination of anakinra, pentoxifylline, and zinc (a mineral that improves gut integrity).
Emricasan, an orally active caspase protease inhibitor, is another agent currently being tested in a phase 2 clinical trial in patients with severe alcoholic hepatitis. Since caspases induce apoptosis, inhibiting them should theoretically dampen alcohol-induced hepatocyte injury.
Interleukin 22, a hepatoprotective cytokine, shows promise as a treatment and will soon be evaluated in alcoholic hepatitis.
- Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol 2013; 59:160–168.
- Teli MR, Day CP, Burt AD, Bennett MK, James OF. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet 1995; 346:987–990.
- Alcoholic liver disease: morphological manifestations. Review by an international group. Lancet 1981; 1:707–711.
- Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput JC. Excess weight risk factor for alcoholic liver disease. Hepatology 1997; 25:108–111.
- Basra S, Anand BS. Definition, epidemiology and magnitude of alcoholic hepatitis. World J Hepatol 2011; 3:108–113.
- Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978; 75:193–199.
- Jinjuvadia R, Liangpunsakul S, for the Translational Research and Evolving Alcoholic Hepatitis Treatment Consortium. Trends in alcoholic hepatitis-related hospitalizations, financial burden, and mortality in the United States. J Clin Gastroenterol 2014 Jun 25 (Epub ahead of print).
- Sato N, Lindros KO, Baraona E, et al. Sex difference in alcohol-related organ injury. Alcohol Clin Exp Res 2001; 25(suppl s1):40S–45S.
- Singal AK, Kamath PS, Gores GJ, Shah VH. Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol 2014; 12:555–564.
- Seitz HK, Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol Chem 2006; 387:349–360.
- Thurman RG. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol 1998; 275:G605–G611.
- Duddempudi AT. Immunology in alcoholic liver disease. Clin Liver Dis 2012; 16:687–698.
- Lischner MW, Alexander JF, Galambos JT. Natural history of alcoholic hepatitis. I. The acute disease. Am J Dig Dis 1971; 16:481–494.
- Cohen JA, Kaplan MM. The SGOT/SGPT ratio—an indicator of alcoholic liver disease. Dig Dis Sci 1979; 24:835–838.
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 2009; 360:2758–2769.
- McKnight-Eily LR, Liu Y, Brewer RD, et al; Centers for Disease Control and Prevention (CDC). Vital signs: communication between health professionals and their patients about alcohol use—44 states and the District of Columbia, 2011. MMWR Morb Mortal Wkly Rep 2014; 63:16–22.
- Grant BF. Barriers to alcoholism treatment: reasons for not seeking treatment in a general population sample. J Stud Alcohol 1997; 58:365–371.
- Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol 2004; 57:30–39.
- The Alcohol Use Disorders Identification Test Guidelines for Use in Primary Care. Second Edition. World Health Organization. Department of Mental Health and Substance Dependence. http://whqlibdoc.who.int/hq/2001/who_msd_msb_01.6a.pdf. Accessed February 3, 2015.
- Hamid R, Forrest EH. Is histology required for the diagnosis of alcoholic hepatitis? A review of published randomised controlled trials. Gut 2011; 60(suppl 1):A233.
- O’Shea RS, Dasarathy S, McCullough AJ; Practice Guideline Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Alcoholic liver disease. Hepatology 2010; 51:307–328.
- Hanouneh IA, Zein NN, Cikach F, et al. The breathprints in patients with liver disease identify novel breath biomarkers in alcoholic hepatitis. Clin Gastroenterol Hepatol 2014; 12:516–523.
- Sheth M, Riggs M, Patel T. Utility of the Mayo End-Stage Liver Disease (MELD) score in assessing prognosis of patients with alcoholic hepatitis. BMC Gastroenterol 2002; 2:2.
- Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology 2005; 41:353–358.
- Srikureja W, Kyulo NL, Runyon BA, Hu KQ. MELD score is a better prognostic model than Child-Turcotte-Pugh score or Discriminant Function score in patients with alcoholic hepatitis. J Hepatol 2005; 42:700–706.
- Forrest EH, Morris AJ, Stewart S, et al. The Glasgow alcoholic hepatitis score identifies patients who may benefit from corticosteroids. Gut 2007; 56:1743–1746.
- Dominguez M, Rincón D, Abraldes JG, et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol 2008; 103:2747–2756.
- Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007; 45:1348–1354.
- Mayo-Smith MF, Beecher LH, Fischer TL, et al; Working Group on the Management of Alcohol Withdrawal Delirium, Practice Guidelines Committee, American Society of Addiction Medicine. Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med 2004; 164:1405–1412.
- Mezey E. Interaction between alcohol and nutrition in the pathogenesis of alcoholic liver disease. Semin Liver Dis 1991; 11:340–348.
- Cabré E, Rodríguez-Iglesias P, Caballería J, et al. Short- and long-term outcome of severe alcohol-induced hepatitis treated with steroids or enteral nutrition: a multicenter randomized trial. Hepatology 2000; 32:36–42.
- Louvet A, Wartel F, Castel H, et al. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology 2009; 137:541–548.
- European Association for the Study of Liver. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol 2012; 57:399–420.
- Rambaldi A, Saconato HH, Christensen E, Thorlund K, Wetterslev J, Gluud C. Systematic review: glucocorticosteroids for alcoholic hepatitis—a Cochrane Hepato-Biliary Group systematic review with meta-analyses and trial sequential analyses of randomized clinical trials. Aliment Pharmacol Ther 2008; 27:1167–1178.
- Powell LW, Axelsen E. Corticosteroids in liver disease: studies on the biological conversion of prednisone to prednisolone and plasma protein binding. Gut 1972; 13:690–696.
- Mathurin P, O’Grady J, Carithers RL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut 2011; 60:255–260.
- Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology 2000; 119:1637–1648.
- De BK, Gangopadhyay S, Dutta D, Baksi SD, Pani A, Ghosh P. Pentoxifylline versus prednisolone for severe alcoholic hepatitis: a randomized controlled trial. World J Gastroenterol 2009; 15:1613–1619.
- Mathurin P, Louvet A, Dao T, et al. Addition of pentoxifylline to prednisolone for severe alcoholic hepatitis does not improve 6-month survival: results of the CORPENTOX trial (abstract). Hepatology 2011; 54(suppl 1):81A.
- Whitfield K, Rambaldi A, Wetterslev J, Gluud C. Pentoxifylline for alcoholic hepatitis. Cochrane Database Syst Rev 2009; CD007339.
- Louvet A, Diaz E, Dharancy S, et al. Early switch to pentoxifylline in patients with severe alcoholic hepatitis is inefficient in non-responders to corticosteroids. J Hepatol 2008; 48:465–470.
- Thursz MR, Richardson P, Allison ME, et al. Steroids or pentoxifylline for alcoholic hepatitis: results of the STOPAH trial [abstract LB-1]. 65th Annual Meeting of the American Association for the Study of Liver Diseases; November 7–11, 2014; Boston, MA.
- Naveau S, Chollet-Martin S, Dharancy S, et al; Foie-Alcool group of the Association Française pour l’Etude du Foie. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology 2004; 39:1390–1397.
- Menon KV, Stadheim L, Kamath PS, et al. A pilot study of the safety and tolerability of etanercept in patients with alcoholic hepatitis. Am J Gastroenterol 2004; 99:255–260.
- Boetticher NC, Peine CJ, Kwo P, et al. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology 2008; 135:1953–1960.
- Nguyen-Khac E, Thevenot T, Piquet MA, et al; AAH-NAC Study Group. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med 2011; 365:1781–1789.
- Singal AK, Duchini A. Liver transplantation in acute alcoholic hepatitis: current status and future development. World J Hepatol 2011; 3:215–218.
- Singal AK, Bashar H, Anand BS, Jampana SC, Singal V, Kuo YF. Outcomes after liver transplantation for alcoholic hepatitis are similar to alcoholic cirrhosis: exploratory analysis from the UNOS database. Hepatology 2012; 55:1398–1405.
- Mathurin P, Moreno C, Samuel D, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med 2011; 365:1790–1800.
Alcoholic hepatitis, a severe manifestation of alcoholic liver disease, is rising in incidence. Complete abstinence from alcohol remains the cornerstone of treatment, while other specific interventions aim to decrease short-term mortality rates.
Despite current treatments, about 25% of patients with severe alcoholic hepatitis eventually die of it. For those who survive hospitalization, measures need to be taken to prevent recidivism. Although liver transplantation seems to hold promise, early transplantation is still largely experimental in alcoholic hepatitis and will likely be available to only a small subset of patients, especially in view of ethical issues and the possible wider implications for transplant centers.
New treatments will largely depend on a better understanding of the disease’s pathophysiology, and future clinical trials should evaluate therapies that improve short-term as well as long-term outcomes.
ACUTE HEPATIC DECOMPENSATION IN A HEAVY DRINKER
Excessive alcohol consumption is very common worldwide, is a major risk factor for liver disease, and is a leading cause of preventable death. Alcoholic cirrhosis is the eighth most common cause of death in the United States and in 2010 was responsible for nearly half of cirrhosis-related deaths worldwide.1
Alcoholic liver disease is a spectrum. Nearly all heavy drinkers (ie, those consuming 40 g or more of alcohol per day, Table 1) have fatty liver changes, 20% to 40% develop fibrosis, 10% to 20% progress to cirrhosis, and of those with cirrhosis, 1% to 2% are diagnosed with hepatocellular carcinoma every year.2
Within this spectrum, alcoholic hepatitis is a well-defined clinical syndrome characterized by acute hepatic decompensation that typically results from long-standing alcohol abuse. Binge drinkers may also be at risk for alcoholic hepatitis, but good data on the association between drinking patterns and the risk of alcoholic hepatitis are limited.
Alcoholic hepatitis varies in severity from mild to life-threatening.3 Although its exact incidence is unknown, its prevalence in alcoholics has been estimated at 20%.4 Nearly half of patients with alcoholic hepatitis have cirrhosis at the time of their acute presentation, and these patients generally have a poor prognosis, with a 28-day death rate as high as 50% in severe cases.5,6 Moreover, although alcoholic hepatitis develops in only a subset of patients with alcoholic liver disease, hospitalizations for it are increasing in the United States.7
Women are at higher risk of developing alcoholic hepatitis, an observation attributed to the effect of estrogens on oxidative stress and inflammation, lower gastric alcohol dehydrogenase levels resulting in slower first-pass metabolism of alcohol, and higher body fat content causing a lower volume of distribution for alcohol than in men.8 The incidence of alcoholic hepatitis is also influenced by a number of demographic and genetic factors as well as nutritional status and coexistence of other liver diseases.9 Most patients diagnosed with alcoholic hepatitis are active drinkers, but it can develop even after significantly reducing or stopping alcohol consumption.
FATTY ACIDS, ENZYMES, CYTOKINES, INFLAMMATION
Alcohol consumption induces fatty acid synthesis and inhibits fatty acid oxidation, thereby promoting fat deposition in the liver.
The major enzymes involved in alcohol metabolism are cytochrome P450 2E1 (CYP2E1) and alcohol dehydrogenase. CYP2E1 is inducible and is up-regulated when excess alcohol is ingested, while alcohol dehydrogen-
ase function is relatively stable. Oxidative degradation of alcohol by these enzymes generates reactive oxygen species and acetaldehyde, inducing liver injury.10 Interestingly, it has been proposed that variations in the genes for these enzymes influence alcohol consumption and dependency as well as alcohol-driven tissue damage.
In addition, alcohol disrupts the intestinal mucosal barrier, allowing lipopolysaccharides from gram-negative bacteria to travel to the liver via the portal vein. These lipopolysaccharides then bind to and activate sinusoidal Kupffer cells, leading to production of several cytokines such as tumor necrosis factor alpha, interleukin 1, and transforming growth factor beta. These cytokines promote hepatocyte inflammation, apoptosis, and necrosis (Figure 1).11
Besides activating the innate immune system, the reactive oxygen species resulting from alcohol metabolism interact with cellular components, leading to production of protein adducts. These act as antigens that activate the adaptive immune response, followed by B- and T-lymphocyte infiltration, which in turn contribute to liver injury and inflammation.12
THE DIAGNOSIS IS MAINLY CLINICAL
The diagnosis of alcoholic hepatitis is mainly clinical. In its usual presentation, jaundice develops rapidly in a person with a known history of heavy alcohol use. Other symptoms and signs may include ascites, encephalopathy, and fever. On examination, the liver may be enlarged and tender, and a hepatic bruit has been reported.13
Other classic signs of liver disease such as parotid enlargement, Dupuytren contracture, dilated abdominal wall veins, and spider nevi can be present, but none is highly specific or sensitive for alcoholic hepatitis.
Elevated liver enzymes and other clues
Laboratory tests are important in evaluating potential alcoholic hepatitis, although no single laboratory marker can definitively establish alcohol as the cause of liver disease. To detect alcohol consumption, biochemical markers such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), mean corpuscular volume, carbohydrate-deficient transferrin, and, more commonly, gamma-glutamyl transpeptidase are used.
In the acute setting, typical biochemical derangements in alcoholic hepatitis include elevated AST (up to 2 to 6 times the upper limit of normal; usually less than 300 IU/L) and elevated ALT to a lesser extent,14 with an AST-to-ALT ratio greater than 2. Neutrophilia, anemia, hyperbilirubinemia, and coagulopathy with an elevated international normalized ratio are common.
Patients with alcoholic hepatitis are also prone to develop bacterial infections, and about 7% develop hepatorenal syndrome, itself an ominous sign.15
Imaging studies are valuable in excluding other causes of abnormal liver test results in patients who abuse alcohol, such as biliary obstruction, infiltrative liver diseases, and hepatocellular carcinoma.
Screen for alcohol intake
During the initial evaluation of suspected alcoholic hepatitis, one should screen for excessive drinking. In a US Centers for Disease Control and Prevention study, only one of six US adults, including binge drinkers, said they had ever discussed alcohol consumption with a health professional.16 Many patients with alcoholic liver disease in general and alcoholic hepatitis in particular deny alcohol abuse or underreport their intake.17
Screening tests such as the CAGE questionnaire and the Alcohol Use Disorders Identification Test can be used to assess alcohol dependence or abuse.18,19 The CAGE questionnaire consists of four questions:
- Have you ever felt you should cut down on your drinking?
- Have people annoyed you by criticizing your drinking?
- Have you ever felt guilty about your drinking?
- Have you ever had a drink first thing in the morning (an eye-opener) to steady your nerves or to get rid of a hangover?
A yes answer to two or more questions is considered clinically significant.
Is liver biopsy always needed?
Although alcoholic hepatitis can be suspected on the basis of clinical and biochemical clues, liver biopsy remains the gold standard diagnostic tool. It confirms the clinical diagnosis of alcoholic hepatitis in about 85% of all patients and in up to 95% when significant hyperbilirubinemia is present.20
However, whether a particular patient needs a biopsy is not always clear. The American Association for the Study of Liver Diseases (AASLD) recommends biopsy in patients who have a clinical diagnosis of severe alcoholic hepatitis for whom medical treatment is being considered and in those with an uncertain underlying diagnosis.
Findings on liver biopsy in alcoholic hepatitis include steatosis, hepatocyte ballooning, neutrophilic infiltration, Mallory bodies (which represent aggregated cytokeratin intermediate filaments and other proteins), and scarring with a typical perivenular distribution as opposed to the periportal fibrosis seen in chronic viral hepatitis. Some histologic findings, such as centrilobular necrosis, may overlap alcoholic hepatitis and nonalcoholic steatohepatitis.
In addition to confirming the diagnosis and staging the disease, liver biopsy has prognostic value. The severity of inflammation and cholestatic changes correlates with poor prognosis and may also predict response to corticosteroid treatment in severe cases of alcoholic hepatitis.21
However, the utility of liver biopsy in confirming the diagnosis and assessing the prognosis of alcoholic hepatitis is controversial for several reasons. Coagulopathy, thrombocytopenia, and ascites are all common in patients with alcoholic hepatitis, often making percutaneous liver biopsy contraindicated. Trans-
jugular liver biopsy is not universally available outside tertiary care centers.
Needed is a minimally invasive test for assessing this disease. Breath analysis might be such a test, offering a noninvasive means to study the composition of volatile organic compounds and elemental gases and an attractive method to evaluate health and disease in a patient-friendly manner. Our group devised a model based on breath levels of trimethylamine and pentane. When we tested it, we found that it distinguishes patients with alcoholic hepatitis from those with acute liver decompensation from causes other than alcohol and controls without liver disease with up to 90% sensitivity and 80% specificity.22
ASSESSING THE SEVERITY OF ALCOHOLIC HEPATITIS
Several models have been developed to assess the severity of alcoholic hepatitis and guide treatment decisions (Table 2).
The MDF (Maddrey Discriminant Function)6 system was the first scoring system developed and is still the most widely used. A score of 32 or higher indicates severe alcoholic hepatitis and has been used as the threshold for starting treatment with corticosteroids.6
The MDF has limitations. Patients with a score lower than 32 are considered not to have severe alcoholic hepatitis, but up to 17% of them still die. Also, since it uses the prothrombin time, its results can vary considerably among laboratories, depending on the sensitivity of the thromboplastin reagent used.
The MELD (Model for End-stage Liver Disease) score. Sheth et al23 compared the MELD and the MDF scores in assessing the severity of alcoholic hepatitis. They found that the MELD performed as well as the MDF in predicting 30-day mortality. A MELD score of greater than 11 had a sensitivity in predicting 30-day mortality of 86% and a specificity of 81%, compared with 86% and 48%, respectively, for MDF scores greater than 32.
Another study found a MELD score of 21 to have the highest sensitivity and specificity in predicting mortality (an estimated 90-day death rate of 20%). Thus, a MELD score of 21 is an appropriate threshold for prompt consideration of specific therapies such as corticosteroids.24
The MELD score has become increasingly important in patients with alcoholic hepatitis, as some of them may become candidates for liver transplantation (see below). Also, serial MELD scores in hospitalized patients have prognostic implications, since an increase of 2 or more points in the first week has been shown to predict in-hospital mortality.25
The GAHS (Glasgow Alcoholic Hepatitis Score)26 was shown to identify patients with alcoholic hepatitis who have an especially poor prognosis and need corticosteroid therapy. In those with a GAHS of 9 or higher, the 28-day survival rate was 78% with corticosteroid treatment and 52% without corticosteroid treatment; survival rates at 84 days were 59% and 38%, respectively.26
The ABIC scoring system (Age, Serum Bilirubin, INR, and Serum Creatinine) stratifies patients by risk of death at 90 days27:
- Score less than 6.71: low risk (100% survival)
- A score 6.71–8.99: intermediate risk (70% survival)
- A score 9.0 or higher: high risk (25% survival).
Both the GAHS and ABIC score are limited by lack of external validation.
The Lille score.28 While the above scores are used to identify patients at risk of death from alcoholic hepatitis and to decide on starting corticosteroids, the Lille score is designed to assess response to corticosteroids after 1 week of treatment. It is calculated based on five pretreatment variables and the change in serum bilirubin level at day 7 of corticosteroid therapy. Lille scores range from 0 to 1; a score higher than 0.45 is associated with a 75% mortality rate at 6 months and indicates a lack of response to corticosteroids and that these drugs should be discontinued.28
MANAGEMENT
Supportive treatment
Abstinence from alcohol is the cornerstone of treatment of alcoholic hepatitis. Early management of alcohol abuse or dependence is, therefore, warranted in all patients with alcoholic hepatitis. Referral to addiction specialists, motivational therapies, and anticraving drugs such as baclofen can be utilized.
Treat alcohol withdrawal. Alcoholics who suddenly decrease or discontinue their alcohol use are at high risk of alcohol withdrawal syndrome. Within 24 hours after the last drink, patients can experience increases in their heart rate and blood pressure, along with irritability and hyperreflexia. Within the next few days, more dangerous complications including seizures and delirium tremens can arise.
Alcohol withdrawal symptoms should be treated with short-acting benzodiazepines or clomethiazole, keeping the risk of worsening encephalopathy in mind.29 If present, complications of cirrhosis such as encephalopathy, ascites, and variceal bleeding should be managed.
Nutritional support is important. Protein-calorie malnutrition is common in alcoholics, as are deficiencies of vitamin A, vitamin D, thiamine, folate, pyridoxine, and zinc.30 Although a randomized controlled trial comparing enteral nutrition (2,000 kcal/day) vs corticosteroids (prednisolone 40 mg/day) in patients with alcoholic hepatitis did not show any difference in the 28-day mortality rate, those who received nutritional support and survived the first month had a lower mortality rate than those treated with corticosteroids (8% vs 37%).31 A daily protein intake of 1.5 g per kilogram of body weight is therefore recommended, even in patients with hepatic encephalopathy.15
Combining enteral nutrition and corticosteroid treatment may have a synergistic effect but is yet to be investigated.
Screen for infection. Patients with alcoholic hepatitis should be screened for infection, as about 25% of those with severe alcoholic hepatitis have an infection at admission.32 Since many of these patients meet the criteria for systemic inflammatory response syndrome, infections can be particularly difficult to diagnose. Patients require close clinical monitoring as well as regular pancultures for early detection. Antibiotics are frequently started empirically even though we lack specific evidence-based guidelines on this practice.33
Corticosteroids
Various studies have evaluated the role of corticosteroids in treating alcoholic hepatitis, differing considerably in sample populations, methods, and end points. Although the results of individual trials differ, meta-analyses indicate that corticosteroids have a moderate beneficial effect in patients with severe alcoholic hepatitis.
For example, Rambaldi et al34 performed a meta-analysis that concluded the mortality rate was lower in alcoholic hepatitis patients with MDF scores of at least 32 or hepatic encephalopathy who were treated with corticosteroids than in controls (relative risk 0.37, 95% confidence interval 0.16–0.86).
Therefore, in the absence of contraindications, the AASLD recommends starting corticosteroids in patients with severe alcoholic hepatitis, defined as an MDF score of 32 or higher.21 The preferred agent is oral prednisolone 40 mg daily or parenteral methylprednisolone 32 mg daily for 4 weeks and then tapered over the next 2 to 4 weeks or abruptly discontinued. Because activation of prednisone is decreased in patients with liver disease, prednisolone (the active form) is preferred over prednisone (the inactive precursor).35 In alcoholic hepatitis, the number needed to treat with corticosteroids to prevent one death has been calculated36 at 5.
As mentioned, response to corticosteroids is commonly assessed at 1 week of treatment using the Lille score. A score higher than 0.45 predicts a poor response and should trigger discontinuation of corticosteroids, particularly in those classified as null responders (Lille score > 0.56).
Adverse effects of steroids include sepsis, gastrointestinal bleeding, and steroid psychosis. Of note, patients who have evidence of hepatorenal syndrome or gastrointestinal bleeding tend to have a less favorable response to corticosteroids. Also, while infections were once considered a contraindication to steroid therapy, recent evidence suggests that steroid use might not be precluded in infected patients after appropriate antibiotic therapy. Infections occur in about a quarter of all alcoholic hepatitis patients treated with steroids, more frequently in null responders (42.5%) than in responders (11.1%), which supports corticosteroid discontinuance at 1 week in null responders.32
Pentoxifylline
An oral phosphodiesterase inhibitor, pentoxifylline, also inhibits production of several cytokines, including tumor necrosis factor alpha. At a dose of 400 mg orally three times daily for 4 weeks, pentoxifylline has been used in treating severe alcoholic hepatitis (MDF score ≥ 32) and is recommended especially if corticosteroids are contraindicated, as with sepsis.21
An early double-blind clinical trial randomized patients with severe alcoholic hepatitis to receive either pentoxifylline 400 mg orally three times daily or placebo. Of the patients who received pentoxifylline, 24.5% died during the index hospitalization, compared with 46.1% of patients who received placebo. This survival benefit was mainly related to a markedly lower incidence of hepatorenal syndrome as the cause of death in the pentoxifylline group than in the placebo group (50% vs 91.7% of deaths).37
In a small clinical trial in patients with severe alcoholic hepatitis, pentoxifylline recipients had a higher 3-month survival rate than prednisolone recipients (35.29% vs 14.71%, P = .04).38 However, a larger trial showed no improvement in 6-month survival with the combination of prednisolone and pentoxifylline compared with prednisolone alone (69.9% vs 69.2%, P = .91).39 Also, a meta-analysis of five randomized clinical trials found no survival benefit with pentoxifylline therapy.40
Of note, in the unfortunate subgroup of patients who have a poor response to corticosteroids, no alternative treatment, including pentoxifylline, has been shown to be effective.41
Prednisone or pentoxifylline? Very recently, results of the Steroids or Pentoxifylline for Alcoholic Hepatitis (STOPAH) trial have been released.42 This is a large, multicenter, double-blinded clinical trial that aimed to provide a definitive answer to whether corticosteroids or pentoxifylline (or both) are beneficial in patients with alcoholic hepatitis. The study included 1,103 adult patients with severe alcoholic hepatitis (MDF score ≥ 32) who were randomized to monotherapy with prednisolone or pentoxifylline, combination therapy, or placebo. The primary end point was mortality at 28 days, and secondary end points included mortality at 90 days and at 1 year. Prednisolone reduced 28-day mortality by about 39%. In contrast, the 28-day mortality rate was similar in patients who received pentoxifylline and those who did not. Also, neither drug was significantly associated with a survival benefit beyond 28 days. The investigators concluded that pentoxifylline has no impact on disease progression and should not be used for the treatment of severe alcoholic hepatitis.42
Other tumor necrosis factor alpha inhibitors not recommended
Two other tumor necrosis factor alpha inhibitors, infliximab and etanercept, have been tested in clinical trials in alcoholic hepatitis. Unfortunately, the results were not encouraging, with no major reduction in mortality.43–45 In fact, these trials demonstrated a significantly increased risk of infections in the treatment groups. Therefore, these drugs are not recommended for treating alcoholic hepatitis.
A possible explanation is that tumor necrosis factor alpha plays an important role in liver regeneration, aiding in recovery from alcohol-induced liver injury, and inhibiting it can have deleterious consequences.
Other agents
A number of other agents have undergone clinical trials in alcoholic hepatitis.
N-acetylcysteine, an antioxidant that replenishes glutathione stores in hepatocytes, was evaluated in a randomized clinical trial in combination with prednisolone.46 Although the 1-month mortality rate was significantly lower in the combination group than in the prednisolone-only group (8% vs 24%, P = .006), 3-month and 6-month mortality rates were not. Nonetheless, the rates of infection and hepatorenal syndrome were lower in the combination group. Therefore, corticosteroids and N-acetylcysteine may have synergistic effects, but the optimum duration of N-acetylcysteine therapy needs to be determined in further studies.
Vitamin E, silymarin, propylthiouracil, colchicine, and oxandrolone (an anabolic steroid) have also been studied, but with no convincing benefit.21
Role of liver transplantation
Liver transplantation for alcoholic liver disease has been a topic of great medical and social controversy. The view that alcoholic patients are responsible for their own illness led to caution when contemplating liver transplantation. Many countries require 6 months of abstinence from alcohol before placing a patient on the liver transplant list, posing a major obstacle to patients with alcoholic hepatitis, as almost all are active drinkers at the time of presentation and many will die within 6 months. Reasons for this 6-month rule include donor shortage and risk of recidivism.47
With regard to survival following alcoholic hepatitis, a study utilizing the United Network for Organ Sharing database matched patients with alcoholic hepatitis and alcoholic cirrhosis who underwent liver transplantation. Rates of 5-year graft survival were 75% in those with alcoholic hepatitis and 73% in those with alcoholic cirrhosis (P = .97), and rates of patient survival were 80% and 78% (P = .90), respectively. Proportional regression analysis adjusting for other variables showed no impact of the etiology of liver disease on graft or patient survival. The investigators concluded that liver transplantation could be considered in a select group of patients with alcoholic hepatitis who do not improve with medical therapy.48
In a pivotal case-control prospective study,49 26 patients with Lille scores greater than 0.45 were listed for liver transplantation within a median of 13 days after nonresponse to medical therapy. The cumulative 6-month survival rate was higher in patients who received a liver transplant early than in those who did not (77% vs 23%, P < .001). This benefit was maintained through 2 years of follow-up (hazard ratio 6.08, P = .004). Of note, all these patients had supportive family members, no severe coexisting conditions, and a commitment to alcohol abstinence (although 3 patients resumed drinking after liver transplantation).49
Although these studies support early liver transplantation in carefully selected patients with severe alcoholic hepatitis, the criteria for transplantation in this group need to be refined. Views on alcoholism also need to be reconciled, as strong evidence is emerging that implicates genetic and environmental influences on alcohol dependence.
Management algorithm
Figure 2 shows a suggested management algorithm for alcoholic hepatitis, adapted from the guidelines of the AASLD and European Association for the Study of the Liver.
NEW THERAPIES NEEDED
Novel therapies for severe alcoholic hepatitis are urgently needed to help combat this devastating condition. Advances in understanding its pathophysiology have uncovered several new therapeutic targets, and new agents are already being evaluated in clinical trials.
IMM 124-E, a hyperimmune bovine colostrum enriched with immunoglobulin G anti-lipopolysaccharide, is going to be evaluated in combination with prednisolone in patients with severe alcoholic hepatitis.
Anakinra, an interleukin 1 receptor antagonist, has significant anti-inflammatory activity and is used to treat rheumatoid arthritis. A clinical trial to evaluate its role in alcoholic hepatitis has been designed in which patients with severe alcoholic hepatitis (defined as a MELD score ≥ 21) will be randomized to receive either methylprednisolone or a combination of anakinra, pentoxifylline, and zinc (a mineral that improves gut integrity).
Emricasan, an orally active caspase protease inhibitor, is another agent currently being tested in a phase 2 clinical trial in patients with severe alcoholic hepatitis. Since caspases induce apoptosis, inhibiting them should theoretically dampen alcohol-induced hepatocyte injury.
Interleukin 22, a hepatoprotective cytokine, shows promise as a treatment and will soon be evaluated in alcoholic hepatitis.
Alcoholic hepatitis, a severe manifestation of alcoholic liver disease, is rising in incidence. Complete abstinence from alcohol remains the cornerstone of treatment, while other specific interventions aim to decrease short-term mortality rates.
Despite current treatments, about 25% of patients with severe alcoholic hepatitis eventually die of it. For those who survive hospitalization, measures need to be taken to prevent recidivism. Although liver transplantation seems to hold promise, early transplantation is still largely experimental in alcoholic hepatitis and will likely be available to only a small subset of patients, especially in view of ethical issues and the possible wider implications for transplant centers.
New treatments will largely depend on a better understanding of the disease’s pathophysiology, and future clinical trials should evaluate therapies that improve short-term as well as long-term outcomes.
ACUTE HEPATIC DECOMPENSATION IN A HEAVY DRINKER
Excessive alcohol consumption is very common worldwide, is a major risk factor for liver disease, and is a leading cause of preventable death. Alcoholic cirrhosis is the eighth most common cause of death in the United States and in 2010 was responsible for nearly half of cirrhosis-related deaths worldwide.1
Alcoholic liver disease is a spectrum. Nearly all heavy drinkers (ie, those consuming 40 g or more of alcohol per day, Table 1) have fatty liver changes, 20% to 40% develop fibrosis, 10% to 20% progress to cirrhosis, and of those with cirrhosis, 1% to 2% are diagnosed with hepatocellular carcinoma every year.2
Within this spectrum, alcoholic hepatitis is a well-defined clinical syndrome characterized by acute hepatic decompensation that typically results from long-standing alcohol abuse. Binge drinkers may also be at risk for alcoholic hepatitis, but good data on the association between drinking patterns and the risk of alcoholic hepatitis are limited.
Alcoholic hepatitis varies in severity from mild to life-threatening.3 Although its exact incidence is unknown, its prevalence in alcoholics has been estimated at 20%.4 Nearly half of patients with alcoholic hepatitis have cirrhosis at the time of their acute presentation, and these patients generally have a poor prognosis, with a 28-day death rate as high as 50% in severe cases.5,6 Moreover, although alcoholic hepatitis develops in only a subset of patients with alcoholic liver disease, hospitalizations for it are increasing in the United States.7
Women are at higher risk of developing alcoholic hepatitis, an observation attributed to the effect of estrogens on oxidative stress and inflammation, lower gastric alcohol dehydrogenase levels resulting in slower first-pass metabolism of alcohol, and higher body fat content causing a lower volume of distribution for alcohol than in men.8 The incidence of alcoholic hepatitis is also influenced by a number of demographic and genetic factors as well as nutritional status and coexistence of other liver diseases.9 Most patients diagnosed with alcoholic hepatitis are active drinkers, but it can develop even after significantly reducing or stopping alcohol consumption.
FATTY ACIDS, ENZYMES, CYTOKINES, INFLAMMATION
Alcohol consumption induces fatty acid synthesis and inhibits fatty acid oxidation, thereby promoting fat deposition in the liver.
The major enzymes involved in alcohol metabolism are cytochrome P450 2E1 (CYP2E1) and alcohol dehydrogenase. CYP2E1 is inducible and is up-regulated when excess alcohol is ingested, while alcohol dehydrogen-
ase function is relatively stable. Oxidative degradation of alcohol by these enzymes generates reactive oxygen species and acetaldehyde, inducing liver injury.10 Interestingly, it has been proposed that variations in the genes for these enzymes influence alcohol consumption and dependency as well as alcohol-driven tissue damage.
In addition, alcohol disrupts the intestinal mucosal barrier, allowing lipopolysaccharides from gram-negative bacteria to travel to the liver via the portal vein. These lipopolysaccharides then bind to and activate sinusoidal Kupffer cells, leading to production of several cytokines such as tumor necrosis factor alpha, interleukin 1, and transforming growth factor beta. These cytokines promote hepatocyte inflammation, apoptosis, and necrosis (Figure 1).11
Besides activating the innate immune system, the reactive oxygen species resulting from alcohol metabolism interact with cellular components, leading to production of protein adducts. These act as antigens that activate the adaptive immune response, followed by B- and T-lymphocyte infiltration, which in turn contribute to liver injury and inflammation.12
THE DIAGNOSIS IS MAINLY CLINICAL
The diagnosis of alcoholic hepatitis is mainly clinical. In its usual presentation, jaundice develops rapidly in a person with a known history of heavy alcohol use. Other symptoms and signs may include ascites, encephalopathy, and fever. On examination, the liver may be enlarged and tender, and a hepatic bruit has been reported.13
Other classic signs of liver disease such as parotid enlargement, Dupuytren contracture, dilated abdominal wall veins, and spider nevi can be present, but none is highly specific or sensitive for alcoholic hepatitis.
Elevated liver enzymes and other clues
Laboratory tests are important in evaluating potential alcoholic hepatitis, although no single laboratory marker can definitively establish alcohol as the cause of liver disease. To detect alcohol consumption, biochemical markers such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), mean corpuscular volume, carbohydrate-deficient transferrin, and, more commonly, gamma-glutamyl transpeptidase are used.
In the acute setting, typical biochemical derangements in alcoholic hepatitis include elevated AST (up to 2 to 6 times the upper limit of normal; usually less than 300 IU/L) and elevated ALT to a lesser extent,14 with an AST-to-ALT ratio greater than 2. Neutrophilia, anemia, hyperbilirubinemia, and coagulopathy with an elevated international normalized ratio are common.
Patients with alcoholic hepatitis are also prone to develop bacterial infections, and about 7% develop hepatorenal syndrome, itself an ominous sign.15
Imaging studies are valuable in excluding other causes of abnormal liver test results in patients who abuse alcohol, such as biliary obstruction, infiltrative liver diseases, and hepatocellular carcinoma.
Screen for alcohol intake
During the initial evaluation of suspected alcoholic hepatitis, one should screen for excessive drinking. In a US Centers for Disease Control and Prevention study, only one of six US adults, including binge drinkers, said they had ever discussed alcohol consumption with a health professional.16 Many patients with alcoholic liver disease in general and alcoholic hepatitis in particular deny alcohol abuse or underreport their intake.17
Screening tests such as the CAGE questionnaire and the Alcohol Use Disorders Identification Test can be used to assess alcohol dependence or abuse.18,19 The CAGE questionnaire consists of four questions:
- Have you ever felt you should cut down on your drinking?
- Have people annoyed you by criticizing your drinking?
- Have you ever felt guilty about your drinking?
- Have you ever had a drink first thing in the morning (an eye-opener) to steady your nerves or to get rid of a hangover?
A yes answer to two or more questions is considered clinically significant.
Is liver biopsy always needed?
Although alcoholic hepatitis can be suspected on the basis of clinical and biochemical clues, liver biopsy remains the gold standard diagnostic tool. It confirms the clinical diagnosis of alcoholic hepatitis in about 85% of all patients and in up to 95% when significant hyperbilirubinemia is present.20
However, whether a particular patient needs a biopsy is not always clear. The American Association for the Study of Liver Diseases (AASLD) recommends biopsy in patients who have a clinical diagnosis of severe alcoholic hepatitis for whom medical treatment is being considered and in those with an uncertain underlying diagnosis.
Findings on liver biopsy in alcoholic hepatitis include steatosis, hepatocyte ballooning, neutrophilic infiltration, Mallory bodies (which represent aggregated cytokeratin intermediate filaments and other proteins), and scarring with a typical perivenular distribution as opposed to the periportal fibrosis seen in chronic viral hepatitis. Some histologic findings, such as centrilobular necrosis, may overlap alcoholic hepatitis and nonalcoholic steatohepatitis.
In addition to confirming the diagnosis and staging the disease, liver biopsy has prognostic value. The severity of inflammation and cholestatic changes correlates with poor prognosis and may also predict response to corticosteroid treatment in severe cases of alcoholic hepatitis.21
However, the utility of liver biopsy in confirming the diagnosis and assessing the prognosis of alcoholic hepatitis is controversial for several reasons. Coagulopathy, thrombocytopenia, and ascites are all common in patients with alcoholic hepatitis, often making percutaneous liver biopsy contraindicated. Trans-
jugular liver biopsy is not universally available outside tertiary care centers.
Needed is a minimally invasive test for assessing this disease. Breath analysis might be such a test, offering a noninvasive means to study the composition of volatile organic compounds and elemental gases and an attractive method to evaluate health and disease in a patient-friendly manner. Our group devised a model based on breath levels of trimethylamine and pentane. When we tested it, we found that it distinguishes patients with alcoholic hepatitis from those with acute liver decompensation from causes other than alcohol and controls without liver disease with up to 90% sensitivity and 80% specificity.22
ASSESSING THE SEVERITY OF ALCOHOLIC HEPATITIS
Several models have been developed to assess the severity of alcoholic hepatitis and guide treatment decisions (Table 2).
The MDF (Maddrey Discriminant Function)6 system was the first scoring system developed and is still the most widely used. A score of 32 or higher indicates severe alcoholic hepatitis and has been used as the threshold for starting treatment with corticosteroids.6
The MDF has limitations. Patients with a score lower than 32 are considered not to have severe alcoholic hepatitis, but up to 17% of them still die. Also, since it uses the prothrombin time, its results can vary considerably among laboratories, depending on the sensitivity of the thromboplastin reagent used.
The MELD (Model for End-stage Liver Disease) score. Sheth et al23 compared the MELD and the MDF scores in assessing the severity of alcoholic hepatitis. They found that the MELD performed as well as the MDF in predicting 30-day mortality. A MELD score of greater than 11 had a sensitivity in predicting 30-day mortality of 86% and a specificity of 81%, compared with 86% and 48%, respectively, for MDF scores greater than 32.
Another study found a MELD score of 21 to have the highest sensitivity and specificity in predicting mortality (an estimated 90-day death rate of 20%). Thus, a MELD score of 21 is an appropriate threshold for prompt consideration of specific therapies such as corticosteroids.24
The MELD score has become increasingly important in patients with alcoholic hepatitis, as some of them may become candidates for liver transplantation (see below). Also, serial MELD scores in hospitalized patients have prognostic implications, since an increase of 2 or more points in the first week has been shown to predict in-hospital mortality.25
The GAHS (Glasgow Alcoholic Hepatitis Score)26 was shown to identify patients with alcoholic hepatitis who have an especially poor prognosis and need corticosteroid therapy. In those with a GAHS of 9 or higher, the 28-day survival rate was 78% with corticosteroid treatment and 52% without corticosteroid treatment; survival rates at 84 days were 59% and 38%, respectively.26
The ABIC scoring system (Age, Serum Bilirubin, INR, and Serum Creatinine) stratifies patients by risk of death at 90 days27:
- Score less than 6.71: low risk (100% survival)
- A score 6.71–8.99: intermediate risk (70% survival)
- A score 9.0 or higher: high risk (25% survival).
Both the GAHS and ABIC score are limited by lack of external validation.
The Lille score.28 While the above scores are used to identify patients at risk of death from alcoholic hepatitis and to decide on starting corticosteroids, the Lille score is designed to assess response to corticosteroids after 1 week of treatment. It is calculated based on five pretreatment variables and the change in serum bilirubin level at day 7 of corticosteroid therapy. Lille scores range from 0 to 1; a score higher than 0.45 is associated with a 75% mortality rate at 6 months and indicates a lack of response to corticosteroids and that these drugs should be discontinued.28
MANAGEMENT
Supportive treatment
Abstinence from alcohol is the cornerstone of treatment of alcoholic hepatitis. Early management of alcohol abuse or dependence is, therefore, warranted in all patients with alcoholic hepatitis. Referral to addiction specialists, motivational therapies, and anticraving drugs such as baclofen can be utilized.
Treat alcohol withdrawal. Alcoholics who suddenly decrease or discontinue their alcohol use are at high risk of alcohol withdrawal syndrome. Within 24 hours after the last drink, patients can experience increases in their heart rate and blood pressure, along with irritability and hyperreflexia. Within the next few days, more dangerous complications including seizures and delirium tremens can arise.
Alcohol withdrawal symptoms should be treated with short-acting benzodiazepines or clomethiazole, keeping the risk of worsening encephalopathy in mind.29 If present, complications of cirrhosis such as encephalopathy, ascites, and variceal bleeding should be managed.
Nutritional support is important. Protein-calorie malnutrition is common in alcoholics, as are deficiencies of vitamin A, vitamin D, thiamine, folate, pyridoxine, and zinc.30 Although a randomized controlled trial comparing enteral nutrition (2,000 kcal/day) vs corticosteroids (prednisolone 40 mg/day) in patients with alcoholic hepatitis did not show any difference in the 28-day mortality rate, those who received nutritional support and survived the first month had a lower mortality rate than those treated with corticosteroids (8% vs 37%).31 A daily protein intake of 1.5 g per kilogram of body weight is therefore recommended, even in patients with hepatic encephalopathy.15
Combining enteral nutrition and corticosteroid treatment may have a synergistic effect but is yet to be investigated.
Screen for infection. Patients with alcoholic hepatitis should be screened for infection, as about 25% of those with severe alcoholic hepatitis have an infection at admission.32 Since many of these patients meet the criteria for systemic inflammatory response syndrome, infections can be particularly difficult to diagnose. Patients require close clinical monitoring as well as regular pancultures for early detection. Antibiotics are frequently started empirically even though we lack specific evidence-based guidelines on this practice.33
Corticosteroids
Various studies have evaluated the role of corticosteroids in treating alcoholic hepatitis, differing considerably in sample populations, methods, and end points. Although the results of individual trials differ, meta-analyses indicate that corticosteroids have a moderate beneficial effect in patients with severe alcoholic hepatitis.
For example, Rambaldi et al34 performed a meta-analysis that concluded the mortality rate was lower in alcoholic hepatitis patients with MDF scores of at least 32 or hepatic encephalopathy who were treated with corticosteroids than in controls (relative risk 0.37, 95% confidence interval 0.16–0.86).
Therefore, in the absence of contraindications, the AASLD recommends starting corticosteroids in patients with severe alcoholic hepatitis, defined as an MDF score of 32 or higher.21 The preferred agent is oral prednisolone 40 mg daily or parenteral methylprednisolone 32 mg daily for 4 weeks and then tapered over the next 2 to 4 weeks or abruptly discontinued. Because activation of prednisone is decreased in patients with liver disease, prednisolone (the active form) is preferred over prednisone (the inactive precursor).35 In alcoholic hepatitis, the number needed to treat with corticosteroids to prevent one death has been calculated36 at 5.
As mentioned, response to corticosteroids is commonly assessed at 1 week of treatment using the Lille score. A score higher than 0.45 predicts a poor response and should trigger discontinuation of corticosteroids, particularly in those classified as null responders (Lille score > 0.56).
Adverse effects of steroids include sepsis, gastrointestinal bleeding, and steroid psychosis. Of note, patients who have evidence of hepatorenal syndrome or gastrointestinal bleeding tend to have a less favorable response to corticosteroids. Also, while infections were once considered a contraindication to steroid therapy, recent evidence suggests that steroid use might not be precluded in infected patients after appropriate antibiotic therapy. Infections occur in about a quarter of all alcoholic hepatitis patients treated with steroids, more frequently in null responders (42.5%) than in responders (11.1%), which supports corticosteroid discontinuance at 1 week in null responders.32
Pentoxifylline
An oral phosphodiesterase inhibitor, pentoxifylline, also inhibits production of several cytokines, including tumor necrosis factor alpha. At a dose of 400 mg orally three times daily for 4 weeks, pentoxifylline has been used in treating severe alcoholic hepatitis (MDF score ≥ 32) and is recommended especially if corticosteroids are contraindicated, as with sepsis.21
An early double-blind clinical trial randomized patients with severe alcoholic hepatitis to receive either pentoxifylline 400 mg orally three times daily or placebo. Of the patients who received pentoxifylline, 24.5% died during the index hospitalization, compared with 46.1% of patients who received placebo. This survival benefit was mainly related to a markedly lower incidence of hepatorenal syndrome as the cause of death in the pentoxifylline group than in the placebo group (50% vs 91.7% of deaths).37
In a small clinical trial in patients with severe alcoholic hepatitis, pentoxifylline recipients had a higher 3-month survival rate than prednisolone recipients (35.29% vs 14.71%, P = .04).38 However, a larger trial showed no improvement in 6-month survival with the combination of prednisolone and pentoxifylline compared with prednisolone alone (69.9% vs 69.2%, P = .91).39 Also, a meta-analysis of five randomized clinical trials found no survival benefit with pentoxifylline therapy.40
Of note, in the unfortunate subgroup of patients who have a poor response to corticosteroids, no alternative treatment, including pentoxifylline, has been shown to be effective.41
Prednisone or pentoxifylline? Very recently, results of the Steroids or Pentoxifylline for Alcoholic Hepatitis (STOPAH) trial have been released.42 This is a large, multicenter, double-blinded clinical trial that aimed to provide a definitive answer to whether corticosteroids or pentoxifylline (or both) are beneficial in patients with alcoholic hepatitis. The study included 1,103 adult patients with severe alcoholic hepatitis (MDF score ≥ 32) who were randomized to monotherapy with prednisolone or pentoxifylline, combination therapy, or placebo. The primary end point was mortality at 28 days, and secondary end points included mortality at 90 days and at 1 year. Prednisolone reduced 28-day mortality by about 39%. In contrast, the 28-day mortality rate was similar in patients who received pentoxifylline and those who did not. Also, neither drug was significantly associated with a survival benefit beyond 28 days. The investigators concluded that pentoxifylline has no impact on disease progression and should not be used for the treatment of severe alcoholic hepatitis.42
Other tumor necrosis factor alpha inhibitors not recommended
Two other tumor necrosis factor alpha inhibitors, infliximab and etanercept, have been tested in clinical trials in alcoholic hepatitis. Unfortunately, the results were not encouraging, with no major reduction in mortality.43–45 In fact, these trials demonstrated a significantly increased risk of infections in the treatment groups. Therefore, these drugs are not recommended for treating alcoholic hepatitis.
A possible explanation is that tumor necrosis factor alpha plays an important role in liver regeneration, aiding in recovery from alcohol-induced liver injury, and inhibiting it can have deleterious consequences.
Other agents
A number of other agents have undergone clinical trials in alcoholic hepatitis.
N-acetylcysteine, an antioxidant that replenishes glutathione stores in hepatocytes, was evaluated in a randomized clinical trial in combination with prednisolone.46 Although the 1-month mortality rate was significantly lower in the combination group than in the prednisolone-only group (8% vs 24%, P = .006), 3-month and 6-month mortality rates were not. Nonetheless, the rates of infection and hepatorenal syndrome were lower in the combination group. Therefore, corticosteroids and N-acetylcysteine may have synergistic effects, but the optimum duration of N-acetylcysteine therapy needs to be determined in further studies.
Vitamin E, silymarin, propylthiouracil, colchicine, and oxandrolone (an anabolic steroid) have also been studied, but with no convincing benefit.21
Role of liver transplantation
Liver transplantation for alcoholic liver disease has been a topic of great medical and social controversy. The view that alcoholic patients are responsible for their own illness led to caution when contemplating liver transplantation. Many countries require 6 months of abstinence from alcohol before placing a patient on the liver transplant list, posing a major obstacle to patients with alcoholic hepatitis, as almost all are active drinkers at the time of presentation and many will die within 6 months. Reasons for this 6-month rule include donor shortage and risk of recidivism.47
With regard to survival following alcoholic hepatitis, a study utilizing the United Network for Organ Sharing database matched patients with alcoholic hepatitis and alcoholic cirrhosis who underwent liver transplantation. Rates of 5-year graft survival were 75% in those with alcoholic hepatitis and 73% in those with alcoholic cirrhosis (P = .97), and rates of patient survival were 80% and 78% (P = .90), respectively. Proportional regression analysis adjusting for other variables showed no impact of the etiology of liver disease on graft or patient survival. The investigators concluded that liver transplantation could be considered in a select group of patients with alcoholic hepatitis who do not improve with medical therapy.48
In a pivotal case-control prospective study,49 26 patients with Lille scores greater than 0.45 were listed for liver transplantation within a median of 13 days after nonresponse to medical therapy. The cumulative 6-month survival rate was higher in patients who received a liver transplant early than in those who did not (77% vs 23%, P < .001). This benefit was maintained through 2 years of follow-up (hazard ratio 6.08, P = .004). Of note, all these patients had supportive family members, no severe coexisting conditions, and a commitment to alcohol abstinence (although 3 patients resumed drinking after liver transplantation).49
Although these studies support early liver transplantation in carefully selected patients with severe alcoholic hepatitis, the criteria for transplantation in this group need to be refined. Views on alcoholism also need to be reconciled, as strong evidence is emerging that implicates genetic and environmental influences on alcohol dependence.
Management algorithm
Figure 2 shows a suggested management algorithm for alcoholic hepatitis, adapted from the guidelines of the AASLD and European Association for the Study of the Liver.
NEW THERAPIES NEEDED
Novel therapies for severe alcoholic hepatitis are urgently needed to help combat this devastating condition. Advances in understanding its pathophysiology have uncovered several new therapeutic targets, and new agents are already being evaluated in clinical trials.
IMM 124-E, a hyperimmune bovine colostrum enriched with immunoglobulin G anti-lipopolysaccharide, is going to be evaluated in combination with prednisolone in patients with severe alcoholic hepatitis.
Anakinra, an interleukin 1 receptor antagonist, has significant anti-inflammatory activity and is used to treat rheumatoid arthritis. A clinical trial to evaluate its role in alcoholic hepatitis has been designed in which patients with severe alcoholic hepatitis (defined as a MELD score ≥ 21) will be randomized to receive either methylprednisolone or a combination of anakinra, pentoxifylline, and zinc (a mineral that improves gut integrity).
Emricasan, an orally active caspase protease inhibitor, is another agent currently being tested in a phase 2 clinical trial in patients with severe alcoholic hepatitis. Since caspases induce apoptosis, inhibiting them should theoretically dampen alcohol-induced hepatocyte injury.
Interleukin 22, a hepatoprotective cytokine, shows promise as a treatment and will soon be evaluated in alcoholic hepatitis.
- Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol 2013; 59:160–168.
- Teli MR, Day CP, Burt AD, Bennett MK, James OF. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet 1995; 346:987–990.
- Alcoholic liver disease: morphological manifestations. Review by an international group. Lancet 1981; 1:707–711.
- Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput JC. Excess weight risk factor for alcoholic liver disease. Hepatology 1997; 25:108–111.
- Basra S, Anand BS. Definition, epidemiology and magnitude of alcoholic hepatitis. World J Hepatol 2011; 3:108–113.
- Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978; 75:193–199.
- Jinjuvadia R, Liangpunsakul S, for the Translational Research and Evolving Alcoholic Hepatitis Treatment Consortium. Trends in alcoholic hepatitis-related hospitalizations, financial burden, and mortality in the United States. J Clin Gastroenterol 2014 Jun 25 (Epub ahead of print).
- Sato N, Lindros KO, Baraona E, et al. Sex difference in alcohol-related organ injury. Alcohol Clin Exp Res 2001; 25(suppl s1):40S–45S.
- Singal AK, Kamath PS, Gores GJ, Shah VH. Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol 2014; 12:555–564.
- Seitz HK, Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol Chem 2006; 387:349–360.
- Thurman RG. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol 1998; 275:G605–G611.
- Duddempudi AT. Immunology in alcoholic liver disease. Clin Liver Dis 2012; 16:687–698.
- Lischner MW, Alexander JF, Galambos JT. Natural history of alcoholic hepatitis. I. The acute disease. Am J Dig Dis 1971; 16:481–494.
- Cohen JA, Kaplan MM. The SGOT/SGPT ratio—an indicator of alcoholic liver disease. Dig Dis Sci 1979; 24:835–838.
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 2009; 360:2758–2769.
- McKnight-Eily LR, Liu Y, Brewer RD, et al; Centers for Disease Control and Prevention (CDC). Vital signs: communication between health professionals and their patients about alcohol use—44 states and the District of Columbia, 2011. MMWR Morb Mortal Wkly Rep 2014; 63:16–22.
- Grant BF. Barriers to alcoholism treatment: reasons for not seeking treatment in a general population sample. J Stud Alcohol 1997; 58:365–371.
- Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol 2004; 57:30–39.
- The Alcohol Use Disorders Identification Test Guidelines for Use in Primary Care. Second Edition. World Health Organization. Department of Mental Health and Substance Dependence. http://whqlibdoc.who.int/hq/2001/who_msd_msb_01.6a.pdf. Accessed February 3, 2015.
- Hamid R, Forrest EH. Is histology required for the diagnosis of alcoholic hepatitis? A review of published randomised controlled trials. Gut 2011; 60(suppl 1):A233.
- O’Shea RS, Dasarathy S, McCullough AJ; Practice Guideline Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Alcoholic liver disease. Hepatology 2010; 51:307–328.
- Hanouneh IA, Zein NN, Cikach F, et al. The breathprints in patients with liver disease identify novel breath biomarkers in alcoholic hepatitis. Clin Gastroenterol Hepatol 2014; 12:516–523.
- Sheth M, Riggs M, Patel T. Utility of the Mayo End-Stage Liver Disease (MELD) score in assessing prognosis of patients with alcoholic hepatitis. BMC Gastroenterol 2002; 2:2.
- Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology 2005; 41:353–358.
- Srikureja W, Kyulo NL, Runyon BA, Hu KQ. MELD score is a better prognostic model than Child-Turcotte-Pugh score or Discriminant Function score in patients with alcoholic hepatitis. J Hepatol 2005; 42:700–706.
- Forrest EH, Morris AJ, Stewart S, et al. The Glasgow alcoholic hepatitis score identifies patients who may benefit from corticosteroids. Gut 2007; 56:1743–1746.
- Dominguez M, Rincón D, Abraldes JG, et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol 2008; 103:2747–2756.
- Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007; 45:1348–1354.
- Mayo-Smith MF, Beecher LH, Fischer TL, et al; Working Group on the Management of Alcohol Withdrawal Delirium, Practice Guidelines Committee, American Society of Addiction Medicine. Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med 2004; 164:1405–1412.
- Mezey E. Interaction between alcohol and nutrition in the pathogenesis of alcoholic liver disease. Semin Liver Dis 1991; 11:340–348.
- Cabré E, Rodríguez-Iglesias P, Caballería J, et al. Short- and long-term outcome of severe alcohol-induced hepatitis treated with steroids or enteral nutrition: a multicenter randomized trial. Hepatology 2000; 32:36–42.
- Louvet A, Wartel F, Castel H, et al. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology 2009; 137:541–548.
- European Association for the Study of Liver. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol 2012; 57:399–420.
- Rambaldi A, Saconato HH, Christensen E, Thorlund K, Wetterslev J, Gluud C. Systematic review: glucocorticosteroids for alcoholic hepatitis—a Cochrane Hepato-Biliary Group systematic review with meta-analyses and trial sequential analyses of randomized clinical trials. Aliment Pharmacol Ther 2008; 27:1167–1178.
- Powell LW, Axelsen E. Corticosteroids in liver disease: studies on the biological conversion of prednisone to prednisolone and plasma protein binding. Gut 1972; 13:690–696.
- Mathurin P, O’Grady J, Carithers RL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut 2011; 60:255–260.
- Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology 2000; 119:1637–1648.
- De BK, Gangopadhyay S, Dutta D, Baksi SD, Pani A, Ghosh P. Pentoxifylline versus prednisolone for severe alcoholic hepatitis: a randomized controlled trial. World J Gastroenterol 2009; 15:1613–1619.
- Mathurin P, Louvet A, Dao T, et al. Addition of pentoxifylline to prednisolone for severe alcoholic hepatitis does not improve 6-month survival: results of the CORPENTOX trial (abstract). Hepatology 2011; 54(suppl 1):81A.
- Whitfield K, Rambaldi A, Wetterslev J, Gluud C. Pentoxifylline for alcoholic hepatitis. Cochrane Database Syst Rev 2009; CD007339.
- Louvet A, Diaz E, Dharancy S, et al. Early switch to pentoxifylline in patients with severe alcoholic hepatitis is inefficient in non-responders to corticosteroids. J Hepatol 2008; 48:465–470.
- Thursz MR, Richardson P, Allison ME, et al. Steroids or pentoxifylline for alcoholic hepatitis: results of the STOPAH trial [abstract LB-1]. 65th Annual Meeting of the American Association for the Study of Liver Diseases; November 7–11, 2014; Boston, MA.
- Naveau S, Chollet-Martin S, Dharancy S, et al; Foie-Alcool group of the Association Française pour l’Etude du Foie. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology 2004; 39:1390–1397.
- Menon KV, Stadheim L, Kamath PS, et al. A pilot study of the safety and tolerability of etanercept in patients with alcoholic hepatitis. Am J Gastroenterol 2004; 99:255–260.
- Boetticher NC, Peine CJ, Kwo P, et al. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology 2008; 135:1953–1960.
- Nguyen-Khac E, Thevenot T, Piquet MA, et al; AAH-NAC Study Group. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med 2011; 365:1781–1789.
- Singal AK, Duchini A. Liver transplantation in acute alcoholic hepatitis: current status and future development. World J Hepatol 2011; 3:215–218.
- Singal AK, Bashar H, Anand BS, Jampana SC, Singal V, Kuo YF. Outcomes after liver transplantation for alcoholic hepatitis are similar to alcoholic cirrhosis: exploratory analysis from the UNOS database. Hepatology 2012; 55:1398–1405.
- Mathurin P, Moreno C, Samuel D, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med 2011; 365:1790–1800.
- Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol 2013; 59:160–168.
- Teli MR, Day CP, Burt AD, Bennett MK, James OF. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet 1995; 346:987–990.
- Alcoholic liver disease: morphological manifestations. Review by an international group. Lancet 1981; 1:707–711.
- Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput JC. Excess weight risk factor for alcoholic liver disease. Hepatology 1997; 25:108–111.
- Basra S, Anand BS. Definition, epidemiology and magnitude of alcoholic hepatitis. World J Hepatol 2011; 3:108–113.
- Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978; 75:193–199.
- Jinjuvadia R, Liangpunsakul S, for the Translational Research and Evolving Alcoholic Hepatitis Treatment Consortium. Trends in alcoholic hepatitis-related hospitalizations, financial burden, and mortality in the United States. J Clin Gastroenterol 2014 Jun 25 (Epub ahead of print).
- Sato N, Lindros KO, Baraona E, et al. Sex difference in alcohol-related organ injury. Alcohol Clin Exp Res 2001; 25(suppl s1):40S–45S.
- Singal AK, Kamath PS, Gores GJ, Shah VH. Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol 2014; 12:555–564.
- Seitz HK, Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol Chem 2006; 387:349–360.
- Thurman RG. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol 1998; 275:G605–G611.
- Duddempudi AT. Immunology in alcoholic liver disease. Clin Liver Dis 2012; 16:687–698.
- Lischner MW, Alexander JF, Galambos JT. Natural history of alcoholic hepatitis. I. The acute disease. Am J Dig Dis 1971; 16:481–494.
- Cohen JA, Kaplan MM. The SGOT/SGPT ratio—an indicator of alcoholic liver disease. Dig Dis Sci 1979; 24:835–838.
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 2009; 360:2758–2769.
- McKnight-Eily LR, Liu Y, Brewer RD, et al; Centers for Disease Control and Prevention (CDC). Vital signs: communication between health professionals and their patients about alcohol use—44 states and the District of Columbia, 2011. MMWR Morb Mortal Wkly Rep 2014; 63:16–22.
- Grant BF. Barriers to alcoholism treatment: reasons for not seeking treatment in a general population sample. J Stud Alcohol 1997; 58:365–371.
- Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol 2004; 57:30–39.
- The Alcohol Use Disorders Identification Test Guidelines for Use in Primary Care. Second Edition. World Health Organization. Department of Mental Health and Substance Dependence. http://whqlibdoc.who.int/hq/2001/who_msd_msb_01.6a.pdf. Accessed February 3, 2015.
- Hamid R, Forrest EH. Is histology required for the diagnosis of alcoholic hepatitis? A review of published randomised controlled trials. Gut 2011; 60(suppl 1):A233.
- O’Shea RS, Dasarathy S, McCullough AJ; Practice Guideline Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Alcoholic liver disease. Hepatology 2010; 51:307–328.
- Hanouneh IA, Zein NN, Cikach F, et al. The breathprints in patients with liver disease identify novel breath biomarkers in alcoholic hepatitis. Clin Gastroenterol Hepatol 2014; 12:516–523.
- Sheth M, Riggs M, Patel T. Utility of the Mayo End-Stage Liver Disease (MELD) score in assessing prognosis of patients with alcoholic hepatitis. BMC Gastroenterol 2002; 2:2.
- Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology 2005; 41:353–358.
- Srikureja W, Kyulo NL, Runyon BA, Hu KQ. MELD score is a better prognostic model than Child-Turcotte-Pugh score or Discriminant Function score in patients with alcoholic hepatitis. J Hepatol 2005; 42:700–706.
- Forrest EH, Morris AJ, Stewart S, et al. The Glasgow alcoholic hepatitis score identifies patients who may benefit from corticosteroids. Gut 2007; 56:1743–1746.
- Dominguez M, Rincón D, Abraldes JG, et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol 2008; 103:2747–2756.
- Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007; 45:1348–1354.
- Mayo-Smith MF, Beecher LH, Fischer TL, et al; Working Group on the Management of Alcohol Withdrawal Delirium, Practice Guidelines Committee, American Society of Addiction Medicine. Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med 2004; 164:1405–1412.
- Mezey E. Interaction between alcohol and nutrition in the pathogenesis of alcoholic liver disease. Semin Liver Dis 1991; 11:340–348.
- Cabré E, Rodríguez-Iglesias P, Caballería J, et al. Short- and long-term outcome of severe alcohol-induced hepatitis treated with steroids or enteral nutrition: a multicenter randomized trial. Hepatology 2000; 32:36–42.
- Louvet A, Wartel F, Castel H, et al. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology 2009; 137:541–548.
- European Association for the Study of Liver. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol 2012; 57:399–420.
- Rambaldi A, Saconato HH, Christensen E, Thorlund K, Wetterslev J, Gluud C. Systematic review: glucocorticosteroids for alcoholic hepatitis—a Cochrane Hepato-Biliary Group systematic review with meta-analyses and trial sequential analyses of randomized clinical trials. Aliment Pharmacol Ther 2008; 27:1167–1178.
- Powell LW, Axelsen E. Corticosteroids in liver disease: studies on the biological conversion of prednisone to prednisolone and plasma protein binding. Gut 1972; 13:690–696.
- Mathurin P, O’Grady J, Carithers RL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut 2011; 60:255–260.
- Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology 2000; 119:1637–1648.
- De BK, Gangopadhyay S, Dutta D, Baksi SD, Pani A, Ghosh P. Pentoxifylline versus prednisolone for severe alcoholic hepatitis: a randomized controlled trial. World J Gastroenterol 2009; 15:1613–1619.
- Mathurin P, Louvet A, Dao T, et al. Addition of pentoxifylline to prednisolone for severe alcoholic hepatitis does not improve 6-month survival: results of the CORPENTOX trial (abstract). Hepatology 2011; 54(suppl 1):81A.
- Whitfield K, Rambaldi A, Wetterslev J, Gluud C. Pentoxifylline for alcoholic hepatitis. Cochrane Database Syst Rev 2009; CD007339.
- Louvet A, Diaz E, Dharancy S, et al. Early switch to pentoxifylline in patients with severe alcoholic hepatitis is inefficient in non-responders to corticosteroids. J Hepatol 2008; 48:465–470.
- Thursz MR, Richardson P, Allison ME, et al. Steroids or pentoxifylline for alcoholic hepatitis: results of the STOPAH trial [abstract LB-1]. 65th Annual Meeting of the American Association for the Study of Liver Diseases; November 7–11, 2014; Boston, MA.
- Naveau S, Chollet-Martin S, Dharancy S, et al; Foie-Alcool group of the Association Française pour l’Etude du Foie. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology 2004; 39:1390–1397.
- Menon KV, Stadheim L, Kamath PS, et al. A pilot study of the safety and tolerability of etanercept in patients with alcoholic hepatitis. Am J Gastroenterol 2004; 99:255–260.
- Boetticher NC, Peine CJ, Kwo P, et al. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology 2008; 135:1953–1960.
- Nguyen-Khac E, Thevenot T, Piquet MA, et al; AAH-NAC Study Group. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med 2011; 365:1781–1789.
- Singal AK, Duchini A. Liver transplantation in acute alcoholic hepatitis: current status and future development. World J Hepatol 2011; 3:215–218.
- Singal AK, Bashar H, Anand BS, Jampana SC, Singal V, Kuo YF. Outcomes after liver transplantation for alcoholic hepatitis are similar to alcoholic cirrhosis: exploratory analysis from the UNOS database. Hepatology 2012; 55:1398–1405.
- Mathurin P, Moreno C, Samuel D, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med 2011; 365:1790–1800.
KEY POINTS
- One should assess the severity of alcoholic hepatitis, using defined scoring systems, to allocate resources and initiate appropriate therapy.
- Supportive care should focus on alcohol withdrawal and enteral nutrition while managing the complications of liver failure.
- Corticosteroids or pentoxifylline are commonly used, but increase the survival rate only by about 50%.
- Opinion is shifting toward allowing some patients with alcoholic hepatitis to receive liver transplants early in the course of their disease.
- Many new therapies are undergoing clinical trials.
Corkscrew hairs
A 22-year-old woman with a 1-year history of mild systemic lupus erythematosus presented with disproportionately severe constitutional symptoms of fatigue and malaise. Physical examination showed multiple follicular-based hyperkeratotic papules with coiled “corkscrew” hairs on the outer surface of the arms and on the front of the legs (Figure 1). The patient reported a diet consisting mainly of white meat and processed foods. Although levels of serum folate, ferritin, zinc, and vitamins A, B1, B2, B6, B12, D, and E were within normal limits, the serum ascorbic acid level was low at 0.2 mg/dL (reference range 0.6–2.0 mg/dL). Ascorbic acid supplementation and dietary modification were recommended.
Ascorbic acid deficiency, or scurvy, is often considered a disease primarily of historical significance, with occurrences today limited to malnutrition or poverty.1 However, 18% of adults in the United States consume less than the recommend daily allowance of ascorbic acid.2 Ascorbic acid is minimally stored in the body,3 and scurvy can develop after 60 to 90 days of a diet free of ascorbic acid.4
Initial symptoms of fatigue, mood changes, and other constitutional symptoms are nonspecific, leading to a delay in diagnosis. Cutaneous manifestations include follicular hyperkeratosis associated with coiled or “corkscrew” hairs. Fragility of cutaneous blood vessels leads to perifollicular hemorrhages, petechiae, purpura, and ecchymoses. Extracutaneous manifestations are diverse and include oral involvement and intramuscular or intra-articular hemorrhage.1 Clinicians should have a high index of suspicion in socially isolated adults, elderly patients, those with alcoholism, mental illness, or chronic illness, and those with restrictive dietary preferences, particularly with predominant intake of processed foods.5
- Nguyen RT, Cowley DM, Muir JB. Scurvy: a cutaneous clinical diagnosis. Australas J Dermatol 2003; 44:48–51.
- Hampl JS, Taylor CA, Johnston CS. Vitamin C deficiency and depletion in the United States: the Third National Health and Nutrition Examination Survey, 1988 to 1994. Am J Public Health 2004; 94:870–875.
- Kluesner NH, Miller DG. Scurvy: malnourishment in the land of plenty. J Emerg Med 2014; 46:530–532.
- Popovich D, McAlhany A, Adewumi AO, Barnes MM. Scurvy: forgotten but definitely not gone. J Pediatr Health Care 2009; 23:405–415.
Velandia B, Centor RM, McConnell V, Shah M. Scurvy is still present in developed countries. J Gen Intern Med 2008; 23:1281–1284.
A 22-year-old woman with a 1-year history of mild systemic lupus erythematosus presented with disproportionately severe constitutional symptoms of fatigue and malaise. Physical examination showed multiple follicular-based hyperkeratotic papules with coiled “corkscrew” hairs on the outer surface of the arms and on the front of the legs (Figure 1). The patient reported a diet consisting mainly of white meat and processed foods. Although levels of serum folate, ferritin, zinc, and vitamins A, B1, B2, B6, B12, D, and E were within normal limits, the serum ascorbic acid level was low at 0.2 mg/dL (reference range 0.6–2.0 mg/dL). Ascorbic acid supplementation and dietary modification were recommended.
Ascorbic acid deficiency, or scurvy, is often considered a disease primarily of historical significance, with occurrences today limited to malnutrition or poverty.1 However, 18% of adults in the United States consume less than the recommend daily allowance of ascorbic acid.2 Ascorbic acid is minimally stored in the body,3 and scurvy can develop after 60 to 90 days of a diet free of ascorbic acid.4
Initial symptoms of fatigue, mood changes, and other constitutional symptoms are nonspecific, leading to a delay in diagnosis. Cutaneous manifestations include follicular hyperkeratosis associated with coiled or “corkscrew” hairs. Fragility of cutaneous blood vessels leads to perifollicular hemorrhages, petechiae, purpura, and ecchymoses. Extracutaneous manifestations are diverse and include oral involvement and intramuscular or intra-articular hemorrhage.1 Clinicians should have a high index of suspicion in socially isolated adults, elderly patients, those with alcoholism, mental illness, or chronic illness, and those with restrictive dietary preferences, particularly with predominant intake of processed foods.5
A 22-year-old woman with a 1-year history of mild systemic lupus erythematosus presented with disproportionately severe constitutional symptoms of fatigue and malaise. Physical examination showed multiple follicular-based hyperkeratotic papules with coiled “corkscrew” hairs on the outer surface of the arms and on the front of the legs (Figure 1). The patient reported a diet consisting mainly of white meat and processed foods. Although levels of serum folate, ferritin, zinc, and vitamins A, B1, B2, B6, B12, D, and E were within normal limits, the serum ascorbic acid level was low at 0.2 mg/dL (reference range 0.6–2.0 mg/dL). Ascorbic acid supplementation and dietary modification were recommended.
Ascorbic acid deficiency, or scurvy, is often considered a disease primarily of historical significance, with occurrences today limited to malnutrition or poverty.1 However, 18% of adults in the United States consume less than the recommend daily allowance of ascorbic acid.2 Ascorbic acid is minimally stored in the body,3 and scurvy can develop after 60 to 90 days of a diet free of ascorbic acid.4
Initial symptoms of fatigue, mood changes, and other constitutional symptoms are nonspecific, leading to a delay in diagnosis. Cutaneous manifestations include follicular hyperkeratosis associated with coiled or “corkscrew” hairs. Fragility of cutaneous blood vessels leads to perifollicular hemorrhages, petechiae, purpura, and ecchymoses. Extracutaneous manifestations are diverse and include oral involvement and intramuscular or intra-articular hemorrhage.1 Clinicians should have a high index of suspicion in socially isolated adults, elderly patients, those with alcoholism, mental illness, or chronic illness, and those with restrictive dietary preferences, particularly with predominant intake of processed foods.5
- Nguyen RT, Cowley DM, Muir JB. Scurvy: a cutaneous clinical diagnosis. Australas J Dermatol 2003; 44:48–51.
- Hampl JS, Taylor CA, Johnston CS. Vitamin C deficiency and depletion in the United States: the Third National Health and Nutrition Examination Survey, 1988 to 1994. Am J Public Health 2004; 94:870–875.
- Kluesner NH, Miller DG. Scurvy: malnourishment in the land of plenty. J Emerg Med 2014; 46:530–532.
- Popovich D, McAlhany A, Adewumi AO, Barnes MM. Scurvy: forgotten but definitely not gone. J Pediatr Health Care 2009; 23:405–415.
Velandia B, Centor RM, McConnell V, Shah M. Scurvy is still present in developed countries. J Gen Intern Med 2008; 23:1281–1284.
- Nguyen RT, Cowley DM, Muir JB. Scurvy: a cutaneous clinical diagnosis. Australas J Dermatol 2003; 44:48–51.
- Hampl JS, Taylor CA, Johnston CS. Vitamin C deficiency and depletion in the United States: the Third National Health and Nutrition Examination Survey, 1988 to 1994. Am J Public Health 2004; 94:870–875.
- Kluesner NH, Miller DG. Scurvy: malnourishment in the land of plenty. J Emerg Med 2014; 46:530–532.
- Popovich D, McAlhany A, Adewumi AO, Barnes MM. Scurvy: forgotten but definitely not gone. J Pediatr Health Care 2009; 23:405–415.
Velandia B, Centor RM, McConnell V, Shah M. Scurvy is still present in developed countries. J Gen Intern Med 2008; 23:1281–1284.
Can the test for human papillomavirus DNA be used as the stand-alone, first-line screening test for cervical cancer?
Yes. Growing evidence demonstrates that the human papillomavirus (HPV) DNA test is more sensitive than the Papanicolaou (Pap) test, with a better negative predictive value—ie, women who have negative test results can be more certain that they are truly free of cervical cancer.1–3
On April 24, 2014, the US Food and Drug Administration (FDA) approved the Cobas HPV test developed by Roche for use as the first-line screening test for cervical cancer in women age 25 and older.4 The approval follows the unanimous recommendation from an independent panel of experts, the Microbiology Devices Panel of the FDA’s Medical Devices Advisory Committee, on March 12, 2014.
PAP-HPV COTESTING IS EFFECTIVE BUT NOT PERFECT
Based on conclusive evidence of a direct link between HPV infection (specifically, infection with certain high-risk HPV genotypes) and almost all cases of invasive cervical cancer,5,6 the American Cancer Society (ACS), American Society for Colposcopy and Cervical Pathology (ASCCP), American Society for Clinical Pathology (ASCP), US Preventive Services Task Force (USPSTF), and American Congress of Obstetricians and Gynecologists (ACOG) issued a consensus recommendation for Pap-HPV cotesting as the preferred screening strategy starting at age 30 and continuing through age 65.7–9
Compared with Pap testing alone, cotesting offers improved detection of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) and the ability to safely extend the screening interval to every 5 years in women who have negative results on both tests. It is an effective screening strategy and remains the standard of care today.
However, this strategy is not perfect and presents several problems for clinicians. The results of the two tests often conflict—the results of the Pap test might be positive while those of the HPV test are negative, or vice versa. Integrating the results of cotesting into triaging can be confusing and complicated. In addition, performing two tests on all women increases the cost of care. And furthermore, the cotesting strategy increases the number of women who require immediate or short-term follow-up,1,2,10–12 such as colposcopy, which is unnecessary for many.
THE HPV TEST DETECTS 14 HIGH-RISK GENOTYPES
The FDA-approved HPV test detects 14 high-risk genotypes. The results for 12 of these are pooled and reported collectively as either positive or negative, while the other two—HPV 16 and HPV 18—are reported separately. (HPV 16 and HPV 18 are the highest-risk genotypes, and together they account for more than two-thirds of cases of invasive cervical cancer.)
ADVANTAGES OF HPV-ONLY TESTING: FINDINGS FROM THE ATHENA TRIAL
The FDA’s decision to approve the Cobas HPV test for use by itself for screening was based on the landmark ATHENA (Addressing the Need for Advanced HPV Diagnostics) trial.13 ATHENA, the largest prospective study of cervical cancer screening performed in the United States to date, enrolled 47,208 women at 61 sites in 23 states. The study revealed the following findings:
- The HPV DNA test had higher sensitivity for detecting CIN3+ (37% higher than the Pap test) and equivalent specificity.
- The HPV test’s positive predictive value was nearly twice as high (12.25% vs 6.47%), and it had a higher negative predictive value (99.58% vs 99.41%) in detecting CIN3+ than with the Pap test.
- HPV testing by itself performed better than Pap-HPV cotesting, with positive predictive values of 12.25% vs 11.04% and negative predictive values of 99.58% vs 99.52% (data presented to the FDA Medical Devices Advisory Committee, Microbiology Panel. March 12, 2014. FDA Executive Summary).
For women whose results were negative for HPV 16 and 18 but positive for the 12-genotype pooled panel, the sample was automatically submitted for cytologic (Pap) testing. Reserving Pap testing for samples in this category improved the specificity of the test and resulted in fewer colposcopy referrals. The ATHENA researchers found that 11.4% of the participants who tested positive for either HPV 16 or 18 had CIN2+.13 Other large cohort studies14,15 also showed that the short-term risk of developing CIN3+ reached 10% over 1 to 5 years in women who tested positive for HPV 16 or 18.
The proposed algorithm for screening (Figure 1) takes advantage of the superior sensitivity of the HPV test, the built-in risk stratification of HPV 16 and 18 genotyping, and the excellent specificity of the Pap test in triaging women whose results are positive for high-risk HPV genotypes other than HPV 16 and 18. Thus, women who have a negative HPV test result can be assured of remaining disease-free for 3 years. The algorithm also identifies women who are at highest risk, ie, those who test positive for HPV 16 or 18. In contrast, the current cotesting approach uses the Qiagen Hybrid Capture HPV testing system, which is a panel of 13 high-risk genotypes, but, if the result is positive, it does not tell you which one the patient has. Furthermore, the new algorithm provides efficient triage, using the Pap test, for women who test positive for the 12 other high-risk HPV genotypes.
Data from large clinical trials other than ATHENA are limited.
FDA APPROVAL DOES NOT CHANGE THE GUIDELINES—YET
The cervical cancer screening guidelines are developed by several organizations other than the FDA. The current guidelines issued by the ACS, ASCCP, ASCP, USPSTF, and ACOG in 2012 call for Pap testing every 3 years in women younger than 30 and Pap-HPV cotesting every 5 years in women ages 30 to 65.7–9 However, FDA approval of the new indication of the HPV DNA test as a stand-alone first-line screening test is an important milestone. It heralds the shifting of the practice paradigm from morphologically based Pap testing to molecular testing in cervical cancer screening.
The ACS and ASCCP have announced that they are reviewing the evidence and may issue updated guidelines for clinicians in the near future.16,17 We anticipate that other organizations may take similar steps. As primary care physicians, we need to stay tuned and follow the most up-to-date evidence-based practice guidelines to provide the best care for our patients.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol 2011; 12:663–672.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomized controlled trial. Lancet Oncol 2010; 11:249–257.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- US Food and Drug Administration. FDA approves first human papillomavirus test for primary cervical cancer screening. www.fda.gov/newsevents/newsroom/pressannouncements/ucm394773.htm. Accessed March 3, 2015.
- Muñoz N, Castellsagué X, de González AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine 2006; 24(suppl 3):S3/1–S3/10.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137:516–542.
- Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 156:880–891.
- Committee on Practice Bulletins—Gynecology. ACOG practice bulletin number 131: screening for cervical cancer. Obstet Gynecol 2012; 120:1222–1238.
- Castle PE, Stoler MH, Wright TC Jr, Sharma A, Wright TL, Behrens CM. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol 2011; 12:880–890.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomized controlled trial. Lancet Oncol 2009; 10:672–682.
- Naucler P, Ryd W, Tornberg S, et al. Efficacy of HPV DNA testing with cytology triage and/or repeat HPV DNA testing in primary cervical cancer screening. J Natl Cancer Inst 2009; 101:88–99.
- Wright TC Jr, Stoler MH, Sharma A, Zhang G, Behrens C, Wright TL; ATHENA (Addressing The Need for Advanced HPV Diagnostics) Study Group. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol 2011; 136:578–586.
- Kjaer SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst 2010; 102:1478–1488.
- Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst 2005; 97:1072–1079.
- American Cancer Society. FDA approves HPV test as first line screening for cervical cancer. www.cancer.org/cancer/news/fda-approves-hpv-test-as-first-line-screening-for-cervical-cancer. Accessed March 3, 2015.
- American Society for Colposcopy and Cervical Pathology. Medical societies recommend consideration of primary HPV testing for cervical cancer screening. www.asccp.org/About-ASCCP/News-Announcements. Accessed March 3, 2015.
Yes. Growing evidence demonstrates that the human papillomavirus (HPV) DNA test is more sensitive than the Papanicolaou (Pap) test, with a better negative predictive value—ie, women who have negative test results can be more certain that they are truly free of cervical cancer.1–3
On April 24, 2014, the US Food and Drug Administration (FDA) approved the Cobas HPV test developed by Roche for use as the first-line screening test for cervical cancer in women age 25 and older.4 The approval follows the unanimous recommendation from an independent panel of experts, the Microbiology Devices Panel of the FDA’s Medical Devices Advisory Committee, on March 12, 2014.
PAP-HPV COTESTING IS EFFECTIVE BUT NOT PERFECT
Based on conclusive evidence of a direct link between HPV infection (specifically, infection with certain high-risk HPV genotypes) and almost all cases of invasive cervical cancer,5,6 the American Cancer Society (ACS), American Society for Colposcopy and Cervical Pathology (ASCCP), American Society for Clinical Pathology (ASCP), US Preventive Services Task Force (USPSTF), and American Congress of Obstetricians and Gynecologists (ACOG) issued a consensus recommendation for Pap-HPV cotesting as the preferred screening strategy starting at age 30 and continuing through age 65.7–9
Compared with Pap testing alone, cotesting offers improved detection of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) and the ability to safely extend the screening interval to every 5 years in women who have negative results on both tests. It is an effective screening strategy and remains the standard of care today.
However, this strategy is not perfect and presents several problems for clinicians. The results of the two tests often conflict—the results of the Pap test might be positive while those of the HPV test are negative, or vice versa. Integrating the results of cotesting into triaging can be confusing and complicated. In addition, performing two tests on all women increases the cost of care. And furthermore, the cotesting strategy increases the number of women who require immediate or short-term follow-up,1,2,10–12 such as colposcopy, which is unnecessary for many.
THE HPV TEST DETECTS 14 HIGH-RISK GENOTYPES
The FDA-approved HPV test detects 14 high-risk genotypes. The results for 12 of these are pooled and reported collectively as either positive or negative, while the other two—HPV 16 and HPV 18—are reported separately. (HPV 16 and HPV 18 are the highest-risk genotypes, and together they account for more than two-thirds of cases of invasive cervical cancer.)
ADVANTAGES OF HPV-ONLY TESTING: FINDINGS FROM THE ATHENA TRIAL
The FDA’s decision to approve the Cobas HPV test for use by itself for screening was based on the landmark ATHENA (Addressing the Need for Advanced HPV Diagnostics) trial.13 ATHENA, the largest prospective study of cervical cancer screening performed in the United States to date, enrolled 47,208 women at 61 sites in 23 states. The study revealed the following findings:
- The HPV DNA test had higher sensitivity for detecting CIN3+ (37% higher than the Pap test) and equivalent specificity.
- The HPV test’s positive predictive value was nearly twice as high (12.25% vs 6.47%), and it had a higher negative predictive value (99.58% vs 99.41%) in detecting CIN3+ than with the Pap test.
- HPV testing by itself performed better than Pap-HPV cotesting, with positive predictive values of 12.25% vs 11.04% and negative predictive values of 99.58% vs 99.52% (data presented to the FDA Medical Devices Advisory Committee, Microbiology Panel. March 12, 2014. FDA Executive Summary).
For women whose results were negative for HPV 16 and 18 but positive for the 12-genotype pooled panel, the sample was automatically submitted for cytologic (Pap) testing. Reserving Pap testing for samples in this category improved the specificity of the test and resulted in fewer colposcopy referrals. The ATHENA researchers found that 11.4% of the participants who tested positive for either HPV 16 or 18 had CIN2+.13 Other large cohort studies14,15 also showed that the short-term risk of developing CIN3+ reached 10% over 1 to 5 years in women who tested positive for HPV 16 or 18.
The proposed algorithm for screening (Figure 1) takes advantage of the superior sensitivity of the HPV test, the built-in risk stratification of HPV 16 and 18 genotyping, and the excellent specificity of the Pap test in triaging women whose results are positive for high-risk HPV genotypes other than HPV 16 and 18. Thus, women who have a negative HPV test result can be assured of remaining disease-free for 3 years. The algorithm also identifies women who are at highest risk, ie, those who test positive for HPV 16 or 18. In contrast, the current cotesting approach uses the Qiagen Hybrid Capture HPV testing system, which is a panel of 13 high-risk genotypes, but, if the result is positive, it does not tell you which one the patient has. Furthermore, the new algorithm provides efficient triage, using the Pap test, for women who test positive for the 12 other high-risk HPV genotypes.
Data from large clinical trials other than ATHENA are limited.
FDA APPROVAL DOES NOT CHANGE THE GUIDELINES—YET
The cervical cancer screening guidelines are developed by several organizations other than the FDA. The current guidelines issued by the ACS, ASCCP, ASCP, USPSTF, and ACOG in 2012 call for Pap testing every 3 years in women younger than 30 and Pap-HPV cotesting every 5 years in women ages 30 to 65.7–9 However, FDA approval of the new indication of the HPV DNA test as a stand-alone first-line screening test is an important milestone. It heralds the shifting of the practice paradigm from morphologically based Pap testing to molecular testing in cervical cancer screening.
The ACS and ASCCP have announced that they are reviewing the evidence and may issue updated guidelines for clinicians in the near future.16,17 We anticipate that other organizations may take similar steps. As primary care physicians, we need to stay tuned and follow the most up-to-date evidence-based practice guidelines to provide the best care for our patients.
Yes. Growing evidence demonstrates that the human papillomavirus (HPV) DNA test is more sensitive than the Papanicolaou (Pap) test, with a better negative predictive value—ie, women who have negative test results can be more certain that they are truly free of cervical cancer.1–3
On April 24, 2014, the US Food and Drug Administration (FDA) approved the Cobas HPV test developed by Roche for use as the first-line screening test for cervical cancer in women age 25 and older.4 The approval follows the unanimous recommendation from an independent panel of experts, the Microbiology Devices Panel of the FDA’s Medical Devices Advisory Committee, on March 12, 2014.
PAP-HPV COTESTING IS EFFECTIVE BUT NOT PERFECT
Based on conclusive evidence of a direct link between HPV infection (specifically, infection with certain high-risk HPV genotypes) and almost all cases of invasive cervical cancer,5,6 the American Cancer Society (ACS), American Society for Colposcopy and Cervical Pathology (ASCCP), American Society for Clinical Pathology (ASCP), US Preventive Services Task Force (USPSTF), and American Congress of Obstetricians and Gynecologists (ACOG) issued a consensus recommendation for Pap-HPV cotesting as the preferred screening strategy starting at age 30 and continuing through age 65.7–9
Compared with Pap testing alone, cotesting offers improved detection of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) and the ability to safely extend the screening interval to every 5 years in women who have negative results on both tests. It is an effective screening strategy and remains the standard of care today.
However, this strategy is not perfect and presents several problems for clinicians. The results of the two tests often conflict—the results of the Pap test might be positive while those of the HPV test are negative, or vice versa. Integrating the results of cotesting into triaging can be confusing and complicated. In addition, performing two tests on all women increases the cost of care. And furthermore, the cotesting strategy increases the number of women who require immediate or short-term follow-up,1,2,10–12 such as colposcopy, which is unnecessary for many.
THE HPV TEST DETECTS 14 HIGH-RISK GENOTYPES
The FDA-approved HPV test detects 14 high-risk genotypes. The results for 12 of these are pooled and reported collectively as either positive or negative, while the other two—HPV 16 and HPV 18—are reported separately. (HPV 16 and HPV 18 are the highest-risk genotypes, and together they account for more than two-thirds of cases of invasive cervical cancer.)
ADVANTAGES OF HPV-ONLY TESTING: FINDINGS FROM THE ATHENA TRIAL
The FDA’s decision to approve the Cobas HPV test for use by itself for screening was based on the landmark ATHENA (Addressing the Need for Advanced HPV Diagnostics) trial.13 ATHENA, the largest prospective study of cervical cancer screening performed in the United States to date, enrolled 47,208 women at 61 sites in 23 states. The study revealed the following findings:
- The HPV DNA test had higher sensitivity for detecting CIN3+ (37% higher than the Pap test) and equivalent specificity.
- The HPV test’s positive predictive value was nearly twice as high (12.25% vs 6.47%), and it had a higher negative predictive value (99.58% vs 99.41%) in detecting CIN3+ than with the Pap test.
- HPV testing by itself performed better than Pap-HPV cotesting, with positive predictive values of 12.25% vs 11.04% and negative predictive values of 99.58% vs 99.52% (data presented to the FDA Medical Devices Advisory Committee, Microbiology Panel. March 12, 2014. FDA Executive Summary).
For women whose results were negative for HPV 16 and 18 but positive for the 12-genotype pooled panel, the sample was automatically submitted for cytologic (Pap) testing. Reserving Pap testing for samples in this category improved the specificity of the test and resulted in fewer colposcopy referrals. The ATHENA researchers found that 11.4% of the participants who tested positive for either HPV 16 or 18 had CIN2+.13 Other large cohort studies14,15 also showed that the short-term risk of developing CIN3+ reached 10% over 1 to 5 years in women who tested positive for HPV 16 or 18.
The proposed algorithm for screening (Figure 1) takes advantage of the superior sensitivity of the HPV test, the built-in risk stratification of HPV 16 and 18 genotyping, and the excellent specificity of the Pap test in triaging women whose results are positive for high-risk HPV genotypes other than HPV 16 and 18. Thus, women who have a negative HPV test result can be assured of remaining disease-free for 3 years. The algorithm also identifies women who are at highest risk, ie, those who test positive for HPV 16 or 18. In contrast, the current cotesting approach uses the Qiagen Hybrid Capture HPV testing system, which is a panel of 13 high-risk genotypes, but, if the result is positive, it does not tell you which one the patient has. Furthermore, the new algorithm provides efficient triage, using the Pap test, for women who test positive for the 12 other high-risk HPV genotypes.
Data from large clinical trials other than ATHENA are limited.
FDA APPROVAL DOES NOT CHANGE THE GUIDELINES—YET
The cervical cancer screening guidelines are developed by several organizations other than the FDA. The current guidelines issued by the ACS, ASCCP, ASCP, USPSTF, and ACOG in 2012 call for Pap testing every 3 years in women younger than 30 and Pap-HPV cotesting every 5 years in women ages 30 to 65.7–9 However, FDA approval of the new indication of the HPV DNA test as a stand-alone first-line screening test is an important milestone. It heralds the shifting of the practice paradigm from morphologically based Pap testing to molecular testing in cervical cancer screening.
The ACS and ASCCP have announced that they are reviewing the evidence and may issue updated guidelines for clinicians in the near future.16,17 We anticipate that other organizations may take similar steps. As primary care physicians, we need to stay tuned and follow the most up-to-date evidence-based practice guidelines to provide the best care for our patients.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol 2011; 12:663–672.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomized controlled trial. Lancet Oncol 2010; 11:249–257.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- US Food and Drug Administration. FDA approves first human papillomavirus test for primary cervical cancer screening. www.fda.gov/newsevents/newsroom/pressannouncements/ucm394773.htm. Accessed March 3, 2015.
- Muñoz N, Castellsagué X, de González AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine 2006; 24(suppl 3):S3/1–S3/10.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137:516–542.
- Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 156:880–891.
- Committee on Practice Bulletins—Gynecology. ACOG practice bulletin number 131: screening for cervical cancer. Obstet Gynecol 2012; 120:1222–1238.
- Castle PE, Stoler MH, Wright TC Jr, Sharma A, Wright TL, Behrens CM. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol 2011; 12:880–890.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomized controlled trial. Lancet Oncol 2009; 10:672–682.
- Naucler P, Ryd W, Tornberg S, et al. Efficacy of HPV DNA testing with cytology triage and/or repeat HPV DNA testing in primary cervical cancer screening. J Natl Cancer Inst 2009; 101:88–99.
- Wright TC Jr, Stoler MH, Sharma A, Zhang G, Behrens C, Wright TL; ATHENA (Addressing The Need for Advanced HPV Diagnostics) Study Group. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol 2011; 136:578–586.
- Kjaer SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst 2010; 102:1478–1488.
- Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst 2005; 97:1072–1079.
- American Cancer Society. FDA approves HPV test as first line screening for cervical cancer. www.cancer.org/cancer/news/fda-approves-hpv-test-as-first-line-screening-for-cervical-cancer. Accessed March 3, 2015.
- American Society for Colposcopy and Cervical Pathology. Medical societies recommend consideration of primary HPV testing for cervical cancer screening. www.asccp.org/About-ASCCP/News-Announcements. Accessed March 3, 2015.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol 2011; 12:663–672.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomized controlled trial. Lancet Oncol 2010; 11:249–257.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- US Food and Drug Administration. FDA approves first human papillomavirus test for primary cervical cancer screening. www.fda.gov/newsevents/newsroom/pressannouncements/ucm394773.htm. Accessed March 3, 2015.
- Muñoz N, Castellsagué X, de González AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine 2006; 24(suppl 3):S3/1–S3/10.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137:516–542.
- Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 156:880–891.
- Committee on Practice Bulletins—Gynecology. ACOG practice bulletin number 131: screening for cervical cancer. Obstet Gynecol 2012; 120:1222–1238.
- Castle PE, Stoler MH, Wright TC Jr, Sharma A, Wright TL, Behrens CM. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol 2011; 12:880–890.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomized controlled trial. Lancet Oncol 2009; 10:672–682.
- Naucler P, Ryd W, Tornberg S, et al. Efficacy of HPV DNA testing with cytology triage and/or repeat HPV DNA testing in primary cervical cancer screening. J Natl Cancer Inst 2009; 101:88–99.
- Wright TC Jr, Stoler MH, Sharma A, Zhang G, Behrens C, Wright TL; ATHENA (Addressing The Need for Advanced HPV Diagnostics) Study Group. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol 2011; 136:578–586.
- Kjaer SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst 2010; 102:1478–1488.
- Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst 2005; 97:1072–1079.
- American Cancer Society. FDA approves HPV test as first line screening for cervical cancer. www.cancer.org/cancer/news/fda-approves-hpv-test-as-first-line-screening-for-cervical-cancer. Accessed March 3, 2015.
- American Society for Colposcopy and Cervical Pathology. Medical societies recommend consideration of primary HPV testing for cervical cancer screening. www.asccp.org/About-ASCCP/News-Announcements. Accessed March 3, 2015.