User login
SAMHSA releases new guide on the use of medications for alcohol use disorder
A new guide on the use of medications to treat patients with alcohol use disorder has been released by the Substance Abuse and Mental Health Services Administration in conjunction with National Alcohol Awareness Month.
The guide provides an overview of four Food and Drug Administration–approved drugs developed to treat alcohol use disorder: disulfiram, oral naltrexone, extended-release injectable naltrexone, and acamprosate. It also discusses how to screen, treat, and monitor patients based on their individual needs.
“Current evidence shows that medications are underused in the treatment of alcohol use disorder,” the agency said in a statement announcing the new guidance. “As the Patient Protection and Affordable Care Act (ACA) continues to be implemented, there is considerable potential for expanding use of medication-assisted treatment to treat alcohol use disorder,” they concluded.
The guide can be found online at http://store.samhsa.gov.
A new guide on the use of medications to treat patients with alcohol use disorder has been released by the Substance Abuse and Mental Health Services Administration in conjunction with National Alcohol Awareness Month.
The guide provides an overview of four Food and Drug Administration–approved drugs developed to treat alcohol use disorder: disulfiram, oral naltrexone, extended-release injectable naltrexone, and acamprosate. It also discusses how to screen, treat, and monitor patients based on their individual needs.
“Current evidence shows that medications are underused in the treatment of alcohol use disorder,” the agency said in a statement announcing the new guidance. “As the Patient Protection and Affordable Care Act (ACA) continues to be implemented, there is considerable potential for expanding use of medication-assisted treatment to treat alcohol use disorder,” they concluded.
The guide can be found online at http://store.samhsa.gov.
A new guide on the use of medications to treat patients with alcohol use disorder has been released by the Substance Abuse and Mental Health Services Administration in conjunction with National Alcohol Awareness Month.
The guide provides an overview of four Food and Drug Administration–approved drugs developed to treat alcohol use disorder: disulfiram, oral naltrexone, extended-release injectable naltrexone, and acamprosate. It also discusses how to screen, treat, and monitor patients based on their individual needs.
“Current evidence shows that medications are underused in the treatment of alcohol use disorder,” the agency said in a statement announcing the new guidance. “As the Patient Protection and Affordable Care Act (ACA) continues to be implemented, there is considerable potential for expanding use of medication-assisted treatment to treat alcohol use disorder,” they concluded.
The guide can be found online at http://store.samhsa.gov.
Neurology’s archaic tests, past and future
It’s not uncommon to read about neurologists of yore and be stunned, if not horrified, to think of what they had to work with.
Going back perhaps 100 years, it wasn’t uncommon for anyone with a head injury and hemiparesis to have one (or more) burr holes placed in hope of draining a subdural hematoma causing the symptoms.
In more recent memory was the dreaded ventriculogram, or pneumoencephalogram: A painful procedure in which a lumbar puncture was done in order to blow air bubbles into the spinal fluid, then use skull X-rays to watch them outline the ventricles and other structures to look for midline shift.
I remember one of my old teachers (RIP, Al) recalling that imaging in his younger era consisted of a cerebral angiogram to look for displaced vessels and an EEG for focal slowing.
The CT scan obviously changed all that, with its excellent noninvasive imaging of the brain, and the MRI made things even better by several orders of magnitude.
But where are we now? As frightening as the practices of 50-100 years ago may seem now, we have to keep in mind that, to the doctors using them, they were at the cutting edge of medical technology. They weren’t saying “this would be so much easier if the MRI had been invented.”
None of us can clearly see what the next big advances will be. We use what we have, knowing it’s the best we can do. As the leading philosopher of our era (Yogi Berra) said, “It’s tough to make predictions, especially about the future.”
So what will future doctors think of us? What tests will they look at and shudder, asking, “They actually DID that to people?”
I’m sure the CT-myelogram will be one of them. It is perhaps the last descendant of the pneumoencephalogram still in use; it’s done uncommonly, but still has value. For those who can’t have an MRI or where confirmation of an MRI is needed, it’s quite accurate.
What other tests will be considered archaic? The EMG/NCV [electromyogram and nerve conduction studies]? Lumbar puncture? Cerebral angiogram?
Of course, these are just in neurology. Every field is going to have a past test that today is looked upon with horror, and the knowledge that someday another generation will look at us the same way.
Like all scientific disciplines, what we do is based on the foundation laid by those before us, and it’s up to the next generation to push the horizon further back.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
It’s not uncommon to read about neurologists of yore and be stunned, if not horrified, to think of what they had to work with.
Going back perhaps 100 years, it wasn’t uncommon for anyone with a head injury and hemiparesis to have one (or more) burr holes placed in hope of draining a subdural hematoma causing the symptoms.
In more recent memory was the dreaded ventriculogram, or pneumoencephalogram: A painful procedure in which a lumbar puncture was done in order to blow air bubbles into the spinal fluid, then use skull X-rays to watch them outline the ventricles and other structures to look for midline shift.
I remember one of my old teachers (RIP, Al) recalling that imaging in his younger era consisted of a cerebral angiogram to look for displaced vessels and an EEG for focal slowing.
The CT scan obviously changed all that, with its excellent noninvasive imaging of the brain, and the MRI made things even better by several orders of magnitude.
But where are we now? As frightening as the practices of 50-100 years ago may seem now, we have to keep in mind that, to the doctors using them, they were at the cutting edge of medical technology. They weren’t saying “this would be so much easier if the MRI had been invented.”
None of us can clearly see what the next big advances will be. We use what we have, knowing it’s the best we can do. As the leading philosopher of our era (Yogi Berra) said, “It’s tough to make predictions, especially about the future.”
So what will future doctors think of us? What tests will they look at and shudder, asking, “They actually DID that to people?”
I’m sure the CT-myelogram will be one of them. It is perhaps the last descendant of the pneumoencephalogram still in use; it’s done uncommonly, but still has value. For those who can’t have an MRI or where confirmation of an MRI is needed, it’s quite accurate.
What other tests will be considered archaic? The EMG/NCV [electromyogram and nerve conduction studies]? Lumbar puncture? Cerebral angiogram?
Of course, these are just in neurology. Every field is going to have a past test that today is looked upon with horror, and the knowledge that someday another generation will look at us the same way.
Like all scientific disciplines, what we do is based on the foundation laid by those before us, and it’s up to the next generation to push the horizon further back.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
It’s not uncommon to read about neurologists of yore and be stunned, if not horrified, to think of what they had to work with.
Going back perhaps 100 years, it wasn’t uncommon for anyone with a head injury and hemiparesis to have one (or more) burr holes placed in hope of draining a subdural hematoma causing the symptoms.
In more recent memory was the dreaded ventriculogram, or pneumoencephalogram: A painful procedure in which a lumbar puncture was done in order to blow air bubbles into the spinal fluid, then use skull X-rays to watch them outline the ventricles and other structures to look for midline shift.
I remember one of my old teachers (RIP, Al) recalling that imaging in his younger era consisted of a cerebral angiogram to look for displaced vessels and an EEG for focal slowing.
The CT scan obviously changed all that, with its excellent noninvasive imaging of the brain, and the MRI made things even better by several orders of magnitude.
But where are we now? As frightening as the practices of 50-100 years ago may seem now, we have to keep in mind that, to the doctors using them, they were at the cutting edge of medical technology. They weren’t saying “this would be so much easier if the MRI had been invented.”
None of us can clearly see what the next big advances will be. We use what we have, knowing it’s the best we can do. As the leading philosopher of our era (Yogi Berra) said, “It’s tough to make predictions, especially about the future.”
So what will future doctors think of us? What tests will they look at and shudder, asking, “They actually DID that to people?”
I’m sure the CT-myelogram will be one of them. It is perhaps the last descendant of the pneumoencephalogram still in use; it’s done uncommonly, but still has value. For those who can’t have an MRI or where confirmation of an MRI is needed, it’s quite accurate.
What other tests will be considered archaic? The EMG/NCV [electromyogram and nerve conduction studies]? Lumbar puncture? Cerebral angiogram?
Of course, these are just in neurology. Every field is going to have a past test that today is looked upon with horror, and the knowledge that someday another generation will look at us the same way.
Like all scientific disciplines, what we do is based on the foundation laid by those before us, and it’s up to the next generation to push the horizon further back.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Assessing, Managing Delirium in Hospitalized Patients
Summary: Delirium is a common problem in hospitalized patients, and all too often delirium is iatrogenic. Delirium is associated with poor outcomes such as prolonged hospitalization and functional decline, and it increases the risk of nursing home admission. The most common tool to assess the presence of delirium is the Confusion Assessment Method (CAM). Dr. Cumbler educated the audience on a more refined tool, the 3D CAM [PDF], and provided the algorithm for diagnosis and evaluation of hospital-onset delirium.
Where delirium is concerned (as with most conditions), “an ounce of prevention is worth a pound of cure.” Namely, avoid prescribing problem medications such as anticholinergics, sedative/hypnotics (except benzodiazepines for treatment of alcohol withdrawal), and antihistamines; and minimize narcotics, but don’t undertreat pain as uncontrolled pain is a more potent delirium trigger than narcotics.
Avoid sleep deprivation. Do we really require vital signs and phlebotomy between midnight and 6 a.m.? Make sure patients have their glasses and hearing aids, and keep them up and moving during daylight hours. Sleep and sensory deprivation are effective forms of human torture and are known to be rather disorienting.
Finally, antipsychotics are associated with increased mortality in dementia. Patients with agitated delirium may benefit from a low dose of haloperidol. When prescribing haloperidol, remember IV administration requires EKG monitoring (FDA black box warning), and a reasonable starting dose is 0.5 mg, NOT 5 mg.
HM takeaways:
- Use CAM, 3D CAM to diagnose delirium;
- Avoid anticholinergic medications (promethazine, cyclobenzaprine, oxybutynin, amitriptyline, prednisolone, theophylline, dixogin, furosemide);
- Minimize, but do not avoid, narcotics in patients with both pain and delirium;
- Use low-dose antipsychotics, not benzodiazepines, for agitated delirium; and
- STOP antipsychotics ASAP, ideally prior to discharge; if not prior to discharge, then include discontinuation date on discharge medication list. TH
Summary: Delirium is a common problem in hospitalized patients, and all too often delirium is iatrogenic. Delirium is associated with poor outcomes such as prolonged hospitalization and functional decline, and it increases the risk of nursing home admission. The most common tool to assess the presence of delirium is the Confusion Assessment Method (CAM). Dr. Cumbler educated the audience on a more refined tool, the 3D CAM [PDF], and provided the algorithm for diagnosis and evaluation of hospital-onset delirium.
Where delirium is concerned (as with most conditions), “an ounce of prevention is worth a pound of cure.” Namely, avoid prescribing problem medications such as anticholinergics, sedative/hypnotics (except benzodiazepines for treatment of alcohol withdrawal), and antihistamines; and minimize narcotics, but don’t undertreat pain as uncontrolled pain is a more potent delirium trigger than narcotics.
Avoid sleep deprivation. Do we really require vital signs and phlebotomy between midnight and 6 a.m.? Make sure patients have their glasses and hearing aids, and keep them up and moving during daylight hours. Sleep and sensory deprivation are effective forms of human torture and are known to be rather disorienting.
Finally, antipsychotics are associated with increased mortality in dementia. Patients with agitated delirium may benefit from a low dose of haloperidol. When prescribing haloperidol, remember IV administration requires EKG monitoring (FDA black box warning), and a reasonable starting dose is 0.5 mg, NOT 5 mg.
HM takeaways:
- Use CAM, 3D CAM to diagnose delirium;
- Avoid anticholinergic medications (promethazine, cyclobenzaprine, oxybutynin, amitriptyline, prednisolone, theophylline, dixogin, furosemide);
- Minimize, but do not avoid, narcotics in patients with both pain and delirium;
- Use low-dose antipsychotics, not benzodiazepines, for agitated delirium; and
- STOP antipsychotics ASAP, ideally prior to discharge; if not prior to discharge, then include discontinuation date on discharge medication list. TH
Summary: Delirium is a common problem in hospitalized patients, and all too often delirium is iatrogenic. Delirium is associated with poor outcomes such as prolonged hospitalization and functional decline, and it increases the risk of nursing home admission. The most common tool to assess the presence of delirium is the Confusion Assessment Method (CAM). Dr. Cumbler educated the audience on a more refined tool, the 3D CAM [PDF], and provided the algorithm for diagnosis and evaluation of hospital-onset delirium.
Where delirium is concerned (as with most conditions), “an ounce of prevention is worth a pound of cure.” Namely, avoid prescribing problem medications such as anticholinergics, sedative/hypnotics (except benzodiazepines for treatment of alcohol withdrawal), and antihistamines; and minimize narcotics, but don’t undertreat pain as uncontrolled pain is a more potent delirium trigger than narcotics.
Avoid sleep deprivation. Do we really require vital signs and phlebotomy between midnight and 6 a.m.? Make sure patients have their glasses and hearing aids, and keep them up and moving during daylight hours. Sleep and sensory deprivation are effective forms of human torture and are known to be rather disorienting.
Finally, antipsychotics are associated with increased mortality in dementia. Patients with agitated delirium may benefit from a low dose of haloperidol. When prescribing haloperidol, remember IV administration requires EKG monitoring (FDA black box warning), and a reasonable starting dose is 0.5 mg, NOT 5 mg.
HM takeaways:
- Use CAM, 3D CAM to diagnose delirium;
- Avoid anticholinergic medications (promethazine, cyclobenzaprine, oxybutynin, amitriptyline, prednisolone, theophylline, dixogin, furosemide);
- Minimize, but do not avoid, narcotics in patients with both pain and delirium;
- Use low-dose antipsychotics, not benzodiazepines, for agitated delirium; and
- STOP antipsychotics ASAP, ideally prior to discharge; if not prior to discharge, then include discontinuation date on discharge medication list. TH
Mobile Apps to Improve Quality, Value at Point-of-Care for Inpatients
Summary: The panel of high-tech doctors helped a standing-room-only crowd navigate numerous apps to be used at point-of-care [PDF, 458 kb]. Groups worked through case studies utilizing applicable mobile apps. Examples and most useful apps, including occasional user reviews, follow:
Provider-to-Provider Communication, HIPAA secure
- Doximity.
- HIPAA-chat.
- Pros: HIPAA-secure, real-time communication.
- Cons: Both parties must be on app to securely communicate.
Provider-to-Patient Communication, Language Translators
- Google Translate: multiple platforms, free, 90 languages.
- MediBabble: iOS only, free, seven languages, dedicated medical application.
Diagnostic Apps for Providers
- Calculate by QxM.
- PreOpEval14: iOS only.
- PreopRisk Assessment: Android only.
- ASCVD Risk Estimator.
- MDCalc.com in addition to usual formulas, great abg-analyzer (online version only).
- AnticoagEvaluator.
- epocrates: calculators.
Click here for a PDF of useful apps and resource links [PDF, 177 kb]
Resources for Evidence-Based Practice
- ACP Clinical Guidelines.
- ACP Smart Medicine.
- Read by QxMD.
- UpToDate.
- AHRQ ePPS: identifies clinical preventive services.
- epocrates.
Patient Engagement Apps
- Medication reminders: MediSafe, CareZone.
- Pharmaceutical costs: Walmart, Target Healthful, GoodRx.
- Proper inhaler usage: User Inhalers App.
- Smoking cessation: QuitSTART.
HM15 takeaways
- Apps are available to providers and patients to enhance quality, value, and compliance;
- Before “prescribing” any app to patients, vet the application yourself; and
- Use apps to supplement your clinical practice, but be wary of becoming over-reliant upon them, to the detriment of long-term memory. In order to utilize information in critical-thinking processes, it must be stored in long-term memory. TH
Summary: The panel of high-tech doctors helped a standing-room-only crowd navigate numerous apps to be used at point-of-care [PDF, 458 kb]. Groups worked through case studies utilizing applicable mobile apps. Examples and most useful apps, including occasional user reviews, follow:
Provider-to-Provider Communication, HIPAA secure
- Doximity.
- HIPAA-chat.
- Pros: HIPAA-secure, real-time communication.
- Cons: Both parties must be on app to securely communicate.
Provider-to-Patient Communication, Language Translators
- Google Translate: multiple platforms, free, 90 languages.
- MediBabble: iOS only, free, seven languages, dedicated medical application.
Diagnostic Apps for Providers
- Calculate by QxM.
- PreOpEval14: iOS only.
- PreopRisk Assessment: Android only.
- ASCVD Risk Estimator.
- MDCalc.com in addition to usual formulas, great abg-analyzer (online version only).
- AnticoagEvaluator.
- epocrates: calculators.
Click here for a PDF of useful apps and resource links [PDF, 177 kb]
Resources for Evidence-Based Practice
- ACP Clinical Guidelines.
- ACP Smart Medicine.
- Read by QxMD.
- UpToDate.
- AHRQ ePPS: identifies clinical preventive services.
- epocrates.
Patient Engagement Apps
- Medication reminders: MediSafe, CareZone.
- Pharmaceutical costs: Walmart, Target Healthful, GoodRx.
- Proper inhaler usage: User Inhalers App.
- Smoking cessation: QuitSTART.
HM15 takeaways
- Apps are available to providers and patients to enhance quality, value, and compliance;
- Before “prescribing” any app to patients, vet the application yourself; and
- Use apps to supplement your clinical practice, but be wary of becoming over-reliant upon them, to the detriment of long-term memory. In order to utilize information in critical-thinking processes, it must be stored in long-term memory. TH
Summary: The panel of high-tech doctors helped a standing-room-only crowd navigate numerous apps to be used at point-of-care [PDF, 458 kb]. Groups worked through case studies utilizing applicable mobile apps. Examples and most useful apps, including occasional user reviews, follow:
Provider-to-Provider Communication, HIPAA secure
- Doximity.
- HIPAA-chat.
- Pros: HIPAA-secure, real-time communication.
- Cons: Both parties must be on app to securely communicate.
Provider-to-Patient Communication, Language Translators
- Google Translate: multiple platforms, free, 90 languages.
- MediBabble: iOS only, free, seven languages, dedicated medical application.
Diagnostic Apps for Providers
- Calculate by QxM.
- PreOpEval14: iOS only.
- PreopRisk Assessment: Android only.
- ASCVD Risk Estimator.
- MDCalc.com in addition to usual formulas, great abg-analyzer (online version only).
- AnticoagEvaluator.
- epocrates: calculators.
Click here for a PDF of useful apps and resource links [PDF, 177 kb]
Resources for Evidence-Based Practice
- ACP Clinical Guidelines.
- ACP Smart Medicine.
- Read by QxMD.
- UpToDate.
- AHRQ ePPS: identifies clinical preventive services.
- epocrates.
Patient Engagement Apps
- Medication reminders: MediSafe, CareZone.
- Pharmaceutical costs: Walmart, Target Healthful, GoodRx.
- Proper inhaler usage: User Inhalers App.
- Smoking cessation: QuitSTART.
HM15 takeaways
- Apps are available to providers and patients to enhance quality, value, and compliance;
- Before “prescribing” any app to patients, vet the application yourself; and
- Use apps to supplement your clinical practice, but be wary of becoming over-reliant upon them, to the detriment of long-term memory. In order to utilize information in critical-thinking processes, it must be stored in long-term memory. TH
Painful Purpura and Cutaneous Necrosis
The Diagnosis: Levamisole-Contaminated Cocaine
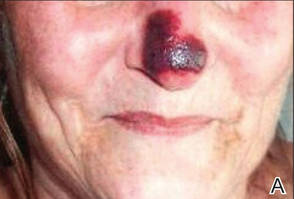
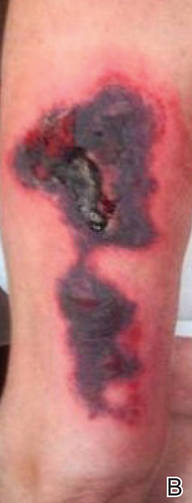
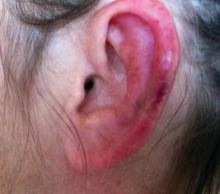
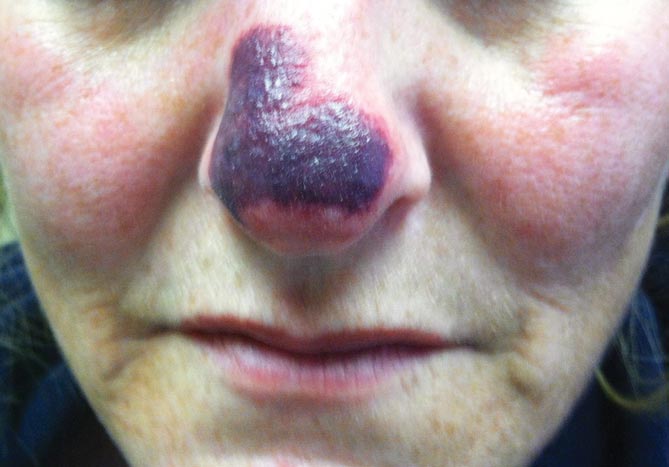
Physical examination revealed scattered palpable purpura including the nasal tip and large necrotizing skin lesions with bullae (Figure 1). Retiform purpura was noted on the patient’s trunk and legs. Purpuric plaques in various stages of necrosis were identified on the arms, trunk, breasts, and thighs, with an early lesion involving the left ear (Figure 2). Vitals revealed a blood pressure of 151/79 mm Hg and a temperature of 37.3°C. Although the patient initially denied illicit drug use, she later admitted to smoking crack cocaine prior to the skin eruption.
|
Levamisole, an agent used in the veterinary setting to deworm cattle and pigs, is a common additive found in nearly 77% of the seized cocaine supplies in the United States.1 Because of its immunomodulator effects, the agent was once used in humans to treat conditions such as colon cancer, rheumatoid arthritis, and nephritic syndrome. Therapeutic levamisole use has been associated with purpura on the external ears, cheeks, and nasal tip. The predilection for purpura on the ears was first described in children using levam-isole as treatment of nephritic syndrome.2 Antinuclear cytoplasmic antibodies (ANCA) and antiphospholipid antibodies also were associated with therapeutic use.3 Reported effects of levamisole in cocaine users include fever, agranulocytosis, and infection. The mechanism is currently unknown but is thought to be an immunological process as evidenced by the presence of positive autoantibodies.4 Characteristic purpura in a cocaine abuser should alert the physician to consider levamisole as the culprit.
The diagnosis is largely a clinical one and other serious etiologies must be ruled out. The differential should include purpura fulminans, a rare hemorrhagic condition caused by severe infection, such as meningococcemia or deficiency of the vitamin K–dependent anticoagulants protein C and protein S. Given our patient’s history of hepatitis, purpura fulminans could have been likely. Purpura fulminans is rapidly progressive and is usually accompanied by disseminated intravascular coagulation. Coagulation studies and evidence of a consumptive coagulopathy such as thrombocytopenia, prolonged partial thromboplastin time, and activated partial thromboplastin time would favor this diagnosis. These studies as well as protein C and protein S were normal in our patient. Mixed cryoglobulinemia also should be suspected in a patient with hepatitis C virus who presents with vasculitis and arthralgia. An undetectable viral load in addition to a negative assay for cryoglobulins and normal complement (C3 and C4) levels make this diagnosis unlikely. Further, the purpura in cryoglobulinemia is typically confined to the lower extremities.5 Warfarin-induced skin necrosis also should be considered in patients who are taking warfarin.
Laboratory tests can be used to confirm the presence of infection, agranulocytosis, and coagulopathy. Although urine drug screen can confirm exposure to cocaine, levamisole is not detected in routine toxicology screening. Its half-life is between 5 and 6 hours; therefore, detection in blood or urine should be done within 48 hours of exposure.3,4 Unfortunately, our patient presented 2 weeks after cocaine use, making detection of levamisole unfeasible. Rheumatologic screening also is appropriate in cases of suspected vasculitis. Our patient had a negative antinuclear antibody but tested positive for lupus anticoagulant and perinuclear ANCA. IgA and IgG anticardiolipin were negative with an indeterminate IgM anticardiolipin level. The literature supports these findings, as ANCA antibodies occurred in more than 90% of reported cases.3 Further, IgM anticardiolipin antibodies and lupus anticoagulant were positive in 65% and 51% of cases, respectively.3 Leukocyte and neutrophil count were normal in our patient.
Management of these patients is mainly supportive. Complete avoidance of the offending agent is absolutely essential for resolution and avoidance of recurrence. Provided that the patient abstains from cocaine, the skin findings typically resolve in 2 to 3 weeks.3,6 The antibodies, however, may be present for up to 14 months.2,3,6 Treatment with steroids and other immunosuppressants has been reported, but evidence is lacking on their role in the resolution of the lesions.3 In patients who develop a temporary antiphospholipid syndrome, aspirin may be recommended as a preventative measure.6 Pain management, treatment of secondary infection especially in situations with agranulocytosis, and surgical debridement of wounds are all potential problems that can arise.
Our patient had complete resolution of the purpura involving the nose and ear over the course of 3 weeks. She did, however, have to undergo surgical debridement of the nonhealing full-thickness necrotic lesions on her legs and arms.
The 2013 National Survey on Drug Use and Health estimates 1.5 million individuals aged 12 years or older were current (within the last month) users of cocaine.7 With levamisole becoming more prevalent in cocaine supplies, clinicians should be aware of this emerging condition. Rapid recognition can spare the patient unnecessary testing and inappropriate treatment regimens. Because testing for levamisole is difficult and not routinely performed, this case highlights the importance of clinical clues and an accurate social history in clinching the diagnosis.
1. National drug threat assessment: 2011. National Drug Intelligence Center Web site. http://www.justice.gov/archive/ndic/pubs44/44849/44849p.pdf. Published August 2011. Accessed March 9, 2015.
2. Rongioletti F, Gio L, Ginevri F, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating long-term treatment with levamisole in children. Br J Dermatol. 1999;40:948-951.
3. Gulati S, Donato AA. Lupus anticoagulant and ANCA associated thrombotic vasculopathy due to cocaine contaminated with levamisole: a case report and review of the literature. J Thromb Thrombolysis. 2012;34:7-10.
4. de la Hera I, Sanz V, Cullen D, et al. Necrosis of ears after cocaine probably adulterated with levamisole. Dermatology. 2011;223:25-28.
5. Seo P. Immune complex-mediated vasculitis. In: Klippel JH. Primer on the Rheumatic Diseases. 13th ed. New York, NY: Springer Science+Business Media, LLC; 2008:427-443.
6. Ching JA, Smith DS. Levamisole-induced necrosis of skin, soft tissue, and bone: case report and review of literature. J Burn Care Res. 2012;33:e1-e5.
7. Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
The Diagnosis: Levamisole-Contaminated Cocaine
Physical examination revealed scattered palpable purpura including the nasal tip and large necrotizing skin lesions with bullae (Figure 1). Retiform purpura was noted on the patient’s trunk and legs. Purpuric plaques in various stages of necrosis were identified on the arms, trunk, breasts, and thighs, with an early lesion involving the left ear (Figure 2). Vitals revealed a blood pressure of 151/79 mm Hg and a temperature of 37.3°C. Although the patient initially denied illicit drug use, she later admitted to smoking crack cocaine prior to the skin eruption.
|
Levamisole, an agent used in the veterinary setting to deworm cattle and pigs, is a common additive found in nearly 77% of the seized cocaine supplies in the United States.1 Because of its immunomodulator effects, the agent was once used in humans to treat conditions such as colon cancer, rheumatoid arthritis, and nephritic syndrome. Therapeutic levamisole use has been associated with purpura on the external ears, cheeks, and nasal tip. The predilection for purpura on the ears was first described in children using levam-isole as treatment of nephritic syndrome.2 Antinuclear cytoplasmic antibodies (ANCA) and antiphospholipid antibodies also were associated with therapeutic use.3 Reported effects of levamisole in cocaine users include fever, agranulocytosis, and infection. The mechanism is currently unknown but is thought to be an immunological process as evidenced by the presence of positive autoantibodies.4 Characteristic purpura in a cocaine abuser should alert the physician to consider levamisole as the culprit.
The diagnosis is largely a clinical one and other serious etiologies must be ruled out. The differential should include purpura fulminans, a rare hemorrhagic condition caused by severe infection, such as meningococcemia or deficiency of the vitamin K–dependent anticoagulants protein C and protein S. Given our patient’s history of hepatitis, purpura fulminans could have been likely. Purpura fulminans is rapidly progressive and is usually accompanied by disseminated intravascular coagulation. Coagulation studies and evidence of a consumptive coagulopathy such as thrombocytopenia, prolonged partial thromboplastin time, and activated partial thromboplastin time would favor this diagnosis. These studies as well as protein C and protein S were normal in our patient. Mixed cryoglobulinemia also should be suspected in a patient with hepatitis C virus who presents with vasculitis and arthralgia. An undetectable viral load in addition to a negative assay for cryoglobulins and normal complement (C3 and C4) levels make this diagnosis unlikely. Further, the purpura in cryoglobulinemia is typically confined to the lower extremities.5 Warfarin-induced skin necrosis also should be considered in patients who are taking warfarin.
Laboratory tests can be used to confirm the presence of infection, agranulocytosis, and coagulopathy. Although urine drug screen can confirm exposure to cocaine, levamisole is not detected in routine toxicology screening. Its half-life is between 5 and 6 hours; therefore, detection in blood or urine should be done within 48 hours of exposure.3,4 Unfortunately, our patient presented 2 weeks after cocaine use, making detection of levamisole unfeasible. Rheumatologic screening also is appropriate in cases of suspected vasculitis. Our patient had a negative antinuclear antibody but tested positive for lupus anticoagulant and perinuclear ANCA. IgA and IgG anticardiolipin were negative with an indeterminate IgM anticardiolipin level. The literature supports these findings, as ANCA antibodies occurred in more than 90% of reported cases.3 Further, IgM anticardiolipin antibodies and lupus anticoagulant were positive in 65% and 51% of cases, respectively.3 Leukocyte and neutrophil count were normal in our patient.
Management of these patients is mainly supportive. Complete avoidance of the offending agent is absolutely essential for resolution and avoidance of recurrence. Provided that the patient abstains from cocaine, the skin findings typically resolve in 2 to 3 weeks.3,6 The antibodies, however, may be present for up to 14 months.2,3,6 Treatment with steroids and other immunosuppressants has been reported, but evidence is lacking on their role in the resolution of the lesions.3 In patients who develop a temporary antiphospholipid syndrome, aspirin may be recommended as a preventative measure.6 Pain management, treatment of secondary infection especially in situations with agranulocytosis, and surgical debridement of wounds are all potential problems that can arise.
Our patient had complete resolution of the purpura involving the nose and ear over the course of 3 weeks. She did, however, have to undergo surgical debridement of the nonhealing full-thickness necrotic lesions on her legs and arms.
The 2013 National Survey on Drug Use and Health estimates 1.5 million individuals aged 12 years or older were current (within the last month) users of cocaine.7 With levamisole becoming more prevalent in cocaine supplies, clinicians should be aware of this emerging condition. Rapid recognition can spare the patient unnecessary testing and inappropriate treatment regimens. Because testing for levamisole is difficult and not routinely performed, this case highlights the importance of clinical clues and an accurate social history in clinching the diagnosis.
The Diagnosis: Levamisole-Contaminated Cocaine
Physical examination revealed scattered palpable purpura including the nasal tip and large necrotizing skin lesions with bullae (Figure 1). Retiform purpura was noted on the patient’s trunk and legs. Purpuric plaques in various stages of necrosis were identified on the arms, trunk, breasts, and thighs, with an early lesion involving the left ear (Figure 2). Vitals revealed a blood pressure of 151/79 mm Hg and a temperature of 37.3°C. Although the patient initially denied illicit drug use, she later admitted to smoking crack cocaine prior to the skin eruption.
|
Levamisole, an agent used in the veterinary setting to deworm cattle and pigs, is a common additive found in nearly 77% of the seized cocaine supplies in the United States.1 Because of its immunomodulator effects, the agent was once used in humans to treat conditions such as colon cancer, rheumatoid arthritis, and nephritic syndrome. Therapeutic levamisole use has been associated with purpura on the external ears, cheeks, and nasal tip. The predilection for purpura on the ears was first described in children using levam-isole as treatment of nephritic syndrome.2 Antinuclear cytoplasmic antibodies (ANCA) and antiphospholipid antibodies also were associated with therapeutic use.3 Reported effects of levamisole in cocaine users include fever, agranulocytosis, and infection. The mechanism is currently unknown but is thought to be an immunological process as evidenced by the presence of positive autoantibodies.4 Characteristic purpura in a cocaine abuser should alert the physician to consider levamisole as the culprit.
The diagnosis is largely a clinical one and other serious etiologies must be ruled out. The differential should include purpura fulminans, a rare hemorrhagic condition caused by severe infection, such as meningococcemia or deficiency of the vitamin K–dependent anticoagulants protein C and protein S. Given our patient’s history of hepatitis, purpura fulminans could have been likely. Purpura fulminans is rapidly progressive and is usually accompanied by disseminated intravascular coagulation. Coagulation studies and evidence of a consumptive coagulopathy such as thrombocytopenia, prolonged partial thromboplastin time, and activated partial thromboplastin time would favor this diagnosis. These studies as well as protein C and protein S were normal in our patient. Mixed cryoglobulinemia also should be suspected in a patient with hepatitis C virus who presents with vasculitis and arthralgia. An undetectable viral load in addition to a negative assay for cryoglobulins and normal complement (C3 and C4) levels make this diagnosis unlikely. Further, the purpura in cryoglobulinemia is typically confined to the lower extremities.5 Warfarin-induced skin necrosis also should be considered in patients who are taking warfarin.
Laboratory tests can be used to confirm the presence of infection, agranulocytosis, and coagulopathy. Although urine drug screen can confirm exposure to cocaine, levamisole is not detected in routine toxicology screening. Its half-life is between 5 and 6 hours; therefore, detection in blood or urine should be done within 48 hours of exposure.3,4 Unfortunately, our patient presented 2 weeks after cocaine use, making detection of levamisole unfeasible. Rheumatologic screening also is appropriate in cases of suspected vasculitis. Our patient had a negative antinuclear antibody but tested positive for lupus anticoagulant and perinuclear ANCA. IgA and IgG anticardiolipin were negative with an indeterminate IgM anticardiolipin level. The literature supports these findings, as ANCA antibodies occurred in more than 90% of reported cases.3 Further, IgM anticardiolipin antibodies and lupus anticoagulant were positive in 65% and 51% of cases, respectively.3 Leukocyte and neutrophil count were normal in our patient.
Management of these patients is mainly supportive. Complete avoidance of the offending agent is absolutely essential for resolution and avoidance of recurrence. Provided that the patient abstains from cocaine, the skin findings typically resolve in 2 to 3 weeks.3,6 The antibodies, however, may be present for up to 14 months.2,3,6 Treatment with steroids and other immunosuppressants has been reported, but evidence is lacking on their role in the resolution of the lesions.3 In patients who develop a temporary antiphospholipid syndrome, aspirin may be recommended as a preventative measure.6 Pain management, treatment of secondary infection especially in situations with agranulocytosis, and surgical debridement of wounds are all potential problems that can arise.
Our patient had complete resolution of the purpura involving the nose and ear over the course of 3 weeks. She did, however, have to undergo surgical debridement of the nonhealing full-thickness necrotic lesions on her legs and arms.
The 2013 National Survey on Drug Use and Health estimates 1.5 million individuals aged 12 years or older were current (within the last month) users of cocaine.7 With levamisole becoming more prevalent in cocaine supplies, clinicians should be aware of this emerging condition. Rapid recognition can spare the patient unnecessary testing and inappropriate treatment regimens. Because testing for levamisole is difficult and not routinely performed, this case highlights the importance of clinical clues and an accurate social history in clinching the diagnosis.
1. National drug threat assessment: 2011. National Drug Intelligence Center Web site. http://www.justice.gov/archive/ndic/pubs44/44849/44849p.pdf. Published August 2011. Accessed March 9, 2015.
2. Rongioletti F, Gio L, Ginevri F, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating long-term treatment with levamisole in children. Br J Dermatol. 1999;40:948-951.
3. Gulati S, Donato AA. Lupus anticoagulant and ANCA associated thrombotic vasculopathy due to cocaine contaminated with levamisole: a case report and review of the literature. J Thromb Thrombolysis. 2012;34:7-10.
4. de la Hera I, Sanz V, Cullen D, et al. Necrosis of ears after cocaine probably adulterated with levamisole. Dermatology. 2011;223:25-28.
5. Seo P. Immune complex-mediated vasculitis. In: Klippel JH. Primer on the Rheumatic Diseases. 13th ed. New York, NY: Springer Science+Business Media, LLC; 2008:427-443.
6. Ching JA, Smith DS. Levamisole-induced necrosis of skin, soft tissue, and bone: case report and review of literature. J Burn Care Res. 2012;33:e1-e5.
7. Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
1. National drug threat assessment: 2011. National Drug Intelligence Center Web site. http://www.justice.gov/archive/ndic/pubs44/44849/44849p.pdf. Published August 2011. Accessed March 9, 2015.
2. Rongioletti F, Gio L, Ginevri F, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating long-term treatment with levamisole in children. Br J Dermatol. 1999;40:948-951.
3. Gulati S, Donato AA. Lupus anticoagulant and ANCA associated thrombotic vasculopathy due to cocaine contaminated with levamisole: a case report and review of the literature. J Thromb Thrombolysis. 2012;34:7-10.
4. de la Hera I, Sanz V, Cullen D, et al. Necrosis of ears after cocaine probably adulterated with levamisole. Dermatology. 2011;223:25-28.
5. Seo P. Immune complex-mediated vasculitis. In: Klippel JH. Primer on the Rheumatic Diseases. 13th ed. New York, NY: Springer Science+Business Media, LLC; 2008:427-443.
6. Ching JA, Smith DS. Levamisole-induced necrosis of skin, soft tissue, and bone: case report and review of literature. J Burn Care Res. 2012;33:e1-e5.
7. Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
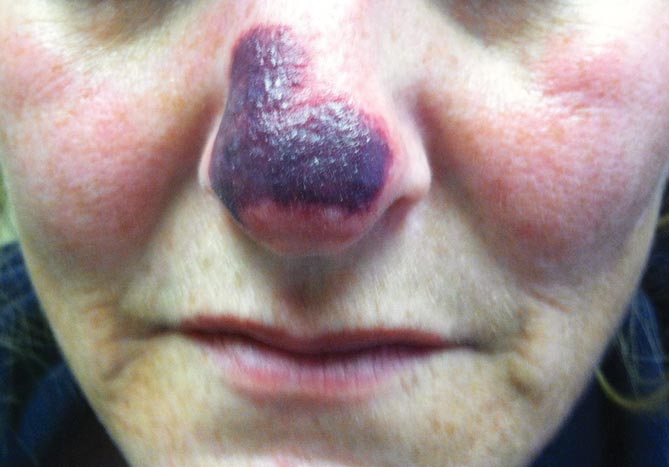
A 44-year-old woman with a history of hepatitis C virus and cocaine abuse presented with painful bruising and skin breakdown. Three weeks prior she was treated at an urgent care clinic with oral clindamycin for a recurrent Staphylococcus infection of her finger. One week later she started developing purpura and painful skin necrosis and ulceration. Most notable was the purpura on the tip of the nose. She also reported arthralgia and low-grade fever.
AML evolution proves unexpectedly complex

Results of single-cell genotyping suggest the evolution of acute myeloid leukemia (AML) is more complex than we thought.
In screening for mutations in 2 genes, researchers identified at least 9 distinct clonal populations, each harboring unique mutational patterns.
Some mutations seemed to arise not sequentially, but independently and at different points in time.
These results suggest single-cell analysis may be more effective than bulk-tumor analysis for assessing cancer evolution.
Carlo Maley, PhD, of Arizona State University in Tempe, and his colleagues recounted the results in Science Translational Medicine.
The researchers set out to provide a more accurate picture of what takes place at the genetic level when an AML patient experiences relapse or metastasis. So they examined individual cells, screening them for mutations in FLT3 and NPM1.
The results suggested the same mutation was occurring multiple times within an AML patient. The team examined individual cells from 6 AML patients, and the results showed all combinations of homozygous and heterozygous mutations of FLT3 and NPM1.
“There’s no way to explain that with each mutation only happening once,” Dr Maley said. “That’s scary because it means that these cancers have access to many mutations and can find the same mutation over and over.”
Dr Maley noted that the process of convergent evolution, in which separate lineages develop similar features, appears to account for some of the observed diversity. And influences from the environment may drive convergent evolution, but identical mutations can also arise through pure coincidence, simply by virtue of the enormous numbers involved.
For example, a 1 cm3 AML tumor may contain a billion cells, each containing some 3 billion base pairs in its genome. Mutations are estimated to occur at a rate of 1 mutation in every billion base pairs.
“That means every time the population of cells in a 1 cm3 tumor undergoes 1 generation, which we think takes just a couple days, every possible mutation of the genome is happening somewhere in that tumor,” Dr Maley said.
This alone would lead to the same mutation likely occurring independently multiple times.
Curbing cancer’s lethality
Given AML’s near-limitless capacity for creating novel variants, what can clinicians do to halt the disease’s advance? According to Dr Maley, one approach would be to use cancer’s ability to evolve to our advantage, rather than attempt to fight it head on.
This paradigm draws on a branch of ecology known as life history theory. The idea is to carefully study the environmental factors that may lead organisms to favor either a fast or slow reproducing strategy to maximize their ability to survive.
According to the theory, fast reproduction tends to occur in environments with high extrinsic mortality. Aggressive cancer treatment creates just such an environment, favoring those cells able to reproduce quickly, producing large numbers of daughter cells, with a few evading extrinsic mortality to repopulate the tumor.
On the other hand, a very stable environment often favors slow reproduction, because organisms reach a carrying capacity of their surrounding environment. In this case, the limiting factor becomes competition between like organisms. Here, a slow reproducing strategy favoring greater investment in maintenance and survivability wins the competition.
“This approach would say, ‘Let’s keep tumors as stable as possible and keep their resources limited,’” Dr Maley said. “If we are able to keep the tumor cells contained and let them fight it out, we would expect to see more competitively fit cells that are growing very slowly.” ![]()

Results of single-cell genotyping suggest the evolution of acute myeloid leukemia (AML) is more complex than we thought.
In screening for mutations in 2 genes, researchers identified at least 9 distinct clonal populations, each harboring unique mutational patterns.
Some mutations seemed to arise not sequentially, but independently and at different points in time.
These results suggest single-cell analysis may be more effective than bulk-tumor analysis for assessing cancer evolution.
Carlo Maley, PhD, of Arizona State University in Tempe, and his colleagues recounted the results in Science Translational Medicine.
The researchers set out to provide a more accurate picture of what takes place at the genetic level when an AML patient experiences relapse or metastasis. So they examined individual cells, screening them for mutations in FLT3 and NPM1.
The results suggested the same mutation was occurring multiple times within an AML patient. The team examined individual cells from 6 AML patients, and the results showed all combinations of homozygous and heterozygous mutations of FLT3 and NPM1.
“There’s no way to explain that with each mutation only happening once,” Dr Maley said. “That’s scary because it means that these cancers have access to many mutations and can find the same mutation over and over.”
Dr Maley noted that the process of convergent evolution, in which separate lineages develop similar features, appears to account for some of the observed diversity. And influences from the environment may drive convergent evolution, but identical mutations can also arise through pure coincidence, simply by virtue of the enormous numbers involved.
For example, a 1 cm3 AML tumor may contain a billion cells, each containing some 3 billion base pairs in its genome. Mutations are estimated to occur at a rate of 1 mutation in every billion base pairs.
“That means every time the population of cells in a 1 cm3 tumor undergoes 1 generation, which we think takes just a couple days, every possible mutation of the genome is happening somewhere in that tumor,” Dr Maley said.
This alone would lead to the same mutation likely occurring independently multiple times.
Curbing cancer’s lethality
Given AML’s near-limitless capacity for creating novel variants, what can clinicians do to halt the disease’s advance? According to Dr Maley, one approach would be to use cancer’s ability to evolve to our advantage, rather than attempt to fight it head on.
This paradigm draws on a branch of ecology known as life history theory. The idea is to carefully study the environmental factors that may lead organisms to favor either a fast or slow reproducing strategy to maximize their ability to survive.
According to the theory, fast reproduction tends to occur in environments with high extrinsic mortality. Aggressive cancer treatment creates just such an environment, favoring those cells able to reproduce quickly, producing large numbers of daughter cells, with a few evading extrinsic mortality to repopulate the tumor.
On the other hand, a very stable environment often favors slow reproduction, because organisms reach a carrying capacity of their surrounding environment. In this case, the limiting factor becomes competition between like organisms. Here, a slow reproducing strategy favoring greater investment in maintenance and survivability wins the competition.
“This approach would say, ‘Let’s keep tumors as stable as possible and keep their resources limited,’” Dr Maley said. “If we are able to keep the tumor cells contained and let them fight it out, we would expect to see more competitively fit cells that are growing very slowly.” ![]()

Results of single-cell genotyping suggest the evolution of acute myeloid leukemia (AML) is more complex than we thought.
In screening for mutations in 2 genes, researchers identified at least 9 distinct clonal populations, each harboring unique mutational patterns.
Some mutations seemed to arise not sequentially, but independently and at different points in time.
These results suggest single-cell analysis may be more effective than bulk-tumor analysis for assessing cancer evolution.
Carlo Maley, PhD, of Arizona State University in Tempe, and his colleagues recounted the results in Science Translational Medicine.
The researchers set out to provide a more accurate picture of what takes place at the genetic level when an AML patient experiences relapse or metastasis. So they examined individual cells, screening them for mutations in FLT3 and NPM1.
The results suggested the same mutation was occurring multiple times within an AML patient. The team examined individual cells from 6 AML patients, and the results showed all combinations of homozygous and heterozygous mutations of FLT3 and NPM1.
“There’s no way to explain that with each mutation only happening once,” Dr Maley said. “That’s scary because it means that these cancers have access to many mutations and can find the same mutation over and over.”
Dr Maley noted that the process of convergent evolution, in which separate lineages develop similar features, appears to account for some of the observed diversity. And influences from the environment may drive convergent evolution, but identical mutations can also arise through pure coincidence, simply by virtue of the enormous numbers involved.
For example, a 1 cm3 AML tumor may contain a billion cells, each containing some 3 billion base pairs in its genome. Mutations are estimated to occur at a rate of 1 mutation in every billion base pairs.
“That means every time the population of cells in a 1 cm3 tumor undergoes 1 generation, which we think takes just a couple days, every possible mutation of the genome is happening somewhere in that tumor,” Dr Maley said.
This alone would lead to the same mutation likely occurring independently multiple times.
Curbing cancer’s lethality
Given AML’s near-limitless capacity for creating novel variants, what can clinicians do to halt the disease’s advance? According to Dr Maley, one approach would be to use cancer’s ability to evolve to our advantage, rather than attempt to fight it head on.
This paradigm draws on a branch of ecology known as life history theory. The idea is to carefully study the environmental factors that may lead organisms to favor either a fast or slow reproducing strategy to maximize their ability to survive.
According to the theory, fast reproduction tends to occur in environments with high extrinsic mortality. Aggressive cancer treatment creates just such an environment, favoring those cells able to reproduce quickly, producing large numbers of daughter cells, with a few evading extrinsic mortality to repopulate the tumor.
On the other hand, a very stable environment often favors slow reproduction, because organisms reach a carrying capacity of their surrounding environment. In this case, the limiting factor becomes competition between like organisms. Here, a slow reproducing strategy favoring greater investment in maintenance and survivability wins the competition.
“This approach would say, ‘Let’s keep tumors as stable as possible and keep their resources limited,’” Dr Maley said. “If we are able to keep the tumor cells contained and let them fight it out, we would expect to see more competitively fit cells that are growing very slowly.” ![]()
‘Junk’ RNA produces lymphoma phenotype
New research suggests the non-coding BRAF pseudogene can produce a lymphoma phenotype in mice.
Pseudogenes, a sub-class of long non-coding RNA that developed from the genome’s protein-coding genes but lost the ability to produce proteins, have long been considered genomic “junk.”
Yet the retention of pseudogenes during evolution has suggested they may have biological functions and contribute to disease development.
Now, researchers have provided evidence that one of these pseudogenes does have a role in causing cancer, and they described the discovery in Cell.
The investigators found that, independent of any other mutations, abnormal amounts of the BRAF pseudogene led to the development of an aggressive, lymphoma-like disease in mice.
The team said this finding suggests pseudogenes may play a primary role in a variety of diseases, and the functional genome could be much larger than we thought.
“Our mouse model of the BRAF pseudogene developed cancer as rapidly and aggressively as it would if you were to express the protein-coding BRAF oncogene,” said study author Pier Paolo Pandolfi, MD, PhD, of Harvard Medical School in Boston, Massachusetts.
“It’s remarkable that this very aggressive phenotype, resembling human diffuse large B-cell lymphoma, was driven by a piece of so-called ‘junk RNA.’”
The researchers’ discovery hinges on the concept of competing endogenous RNAs (ceRNA), a functional capability for pseudogenes Dr Pandolfi and his colleagues described almost 5 years ago. The team discovered that pseudogenes and other noncoding RNAs could act as “decoys” to divert and sequester microRNAs away from their protein-coding counterparts to regulate gene expression.
“Our discovery of these ‘decoys’ revealed a new role for messenger RNA, demonstrating that, beyond serving as a genetic intermediary in the protein-making process, messenger RNAs could actually regulate expression of one another through this sophisticated new ceRNA ‘language,’” Dr Pandolfi said.
He and his colleagues showed that, when these decoys prevented microRNAs from fulfilling their regulatory function, there could be severe consequences, including making cancer cells more aggressive.
With their new research, the investigators wanted to determine if this same ceRNA cross-talk took place in a living organism and if it would result in similar consequences.
“We conducted a proof-of-principle experiment using the BRAF pseudogene,” explained Florian Karreth, PhD, a fellow in the Pandolfi lab. “We investigated whether this pseudogene exerts critical functions in the context of a whole organism and whether its disruption contributes to the development of disease.”
The researchers focused on the BRAF pseudogene because of its potential ability to regulate levels of the BRAF protein, a well-known proto-oncogene linked to numerous cancer types. In addition, the BRAF pseudogene is known to exist in both humans and mice.
The team began by testing the BRAF pseudogene in tissue culture. Their findings showed that, when overexpressed, the pseudogene operated as a microRNA decoy that increased amounts of the BRAF protein.
This, in turn, stimulated the MAPK signaling cascade, a pathway through which the BRAF protein controls cell proliferation, differentiation, and survival and which is commonly found to be hyperactive in cancer.
The investigators went on to create a mouse model in which the BRAF pseudogene was overexpressed. And they found these mice developed an aggressive, lymphoma-like cancer.
“This cancer of B-lymphocytes manifested primarily in the spleens of the animals but also infiltrated other organs, including the kidneys and liver,” Dr Karreth said. “We were particularly surprised by the development of such a dramatic phenotype in response to BRAF pseudogene overexpression alone since the development of full-blown cancer usually requires two or more mutational events.”
Mice overexpressing the BRAF pseudogene displayed higher levels of the BRAF protein and hyperactivation of the MAPK pathway, which suggests this axis is critical to cancer development.
The researchers confirmed this by inhibiting the MAPK pathway with GSK1120212, a MEK inhibitor that dramatically reduced the cancer cells’ ability to infiltrate the liver in transplantation experiments.
The investigators further validated the microRNA decoy function of the BRAF pseudogene by creating two additional transgenic mice, one overexpressing the front half of the BRAF pseudogene, and the other overexpressing the back half.
Both of these mouse models developed the same lymphoma phenotype as the mice overexpressing the full-length pseudogene, a result the researchers described as “astonishing.”
“We never expected that portions of the BRAF pseudogene could elicit a phenotype,” Dr Karreth said. “[W]hen both front and back halves induced lymphomas, we were certain the BRAF pseudogene was functioning as a microRNA decoy.”
The investigators also found the BRAF pseudogene is overexpressed in human B-cell lymphomas, and the genomic region containing the BRAF pseudogene is commonly amplified in a variety of human cancers. This suggests the group’s murine findings are of relevance to human cancer development.
Moreover, the researchers found that silencing the BRAF pseudogene in human cancer cell lines that expressed higher levels led to reduced cell proliferation. This reinforces the importance of the pseudogene and suggests a therapy that reduces BRAF pseudogene levels may be beneficial to certain cancer patients.
“While we have been busy focusing on the genome’s 20,000 coding genes, we have neglected perhaps as many as 100,000 noncoding genetic units,” Dr Pandolfi noted. “Our new findings not only tell us that we need to characterize the role of all of these non-coding pseudogenes in cancer, but, more urgently, suggest that we need to increase our understanding of the non-coding ‘junk’ of the genome and incorporate this information into our personalized medicine assays.”
“The game has to start now. We have to sequence and analyze the genome and the RNA transcripts from the non-coding space.” ![]()

New research suggests the non-coding BRAF pseudogene can produce a lymphoma phenotype in mice.
Pseudogenes, a sub-class of long non-coding RNA that developed from the genome’s protein-coding genes but lost the ability to produce proteins, have long been considered genomic “junk.”
Yet the retention of pseudogenes during evolution has suggested they may have biological functions and contribute to disease development.
Now, researchers have provided evidence that one of these pseudogenes does have a role in causing cancer, and they described the discovery in Cell.
The investigators found that, independent of any other mutations, abnormal amounts of the BRAF pseudogene led to the development of an aggressive, lymphoma-like disease in mice.
The team said this finding suggests pseudogenes may play a primary role in a variety of diseases, and the functional genome could be much larger than we thought.
“Our mouse model of the BRAF pseudogene developed cancer as rapidly and aggressively as it would if you were to express the protein-coding BRAF oncogene,” said study author Pier Paolo Pandolfi, MD, PhD, of Harvard Medical School in Boston, Massachusetts.
“It’s remarkable that this very aggressive phenotype, resembling human diffuse large B-cell lymphoma, was driven by a piece of so-called ‘junk RNA.’”
The researchers’ discovery hinges on the concept of competing endogenous RNAs (ceRNA), a functional capability for pseudogenes Dr Pandolfi and his colleagues described almost 5 years ago. The team discovered that pseudogenes and other noncoding RNAs could act as “decoys” to divert and sequester microRNAs away from their protein-coding counterparts to regulate gene expression.
“Our discovery of these ‘decoys’ revealed a new role for messenger RNA, demonstrating that, beyond serving as a genetic intermediary in the protein-making process, messenger RNAs could actually regulate expression of one another through this sophisticated new ceRNA ‘language,’” Dr Pandolfi said.
He and his colleagues showed that, when these decoys prevented microRNAs from fulfilling their regulatory function, there could be severe consequences, including making cancer cells more aggressive.
With their new research, the investigators wanted to determine if this same ceRNA cross-talk took place in a living organism and if it would result in similar consequences.
“We conducted a proof-of-principle experiment using the BRAF pseudogene,” explained Florian Karreth, PhD, a fellow in the Pandolfi lab. “We investigated whether this pseudogene exerts critical functions in the context of a whole organism and whether its disruption contributes to the development of disease.”
The researchers focused on the BRAF pseudogene because of its potential ability to regulate levels of the BRAF protein, a well-known proto-oncogene linked to numerous cancer types. In addition, the BRAF pseudogene is known to exist in both humans and mice.
The team began by testing the BRAF pseudogene in tissue culture. Their findings showed that, when overexpressed, the pseudogene operated as a microRNA decoy that increased amounts of the BRAF protein.
This, in turn, stimulated the MAPK signaling cascade, a pathway through which the BRAF protein controls cell proliferation, differentiation, and survival and which is commonly found to be hyperactive in cancer.
The investigators went on to create a mouse model in which the BRAF pseudogene was overexpressed. And they found these mice developed an aggressive, lymphoma-like cancer.
“This cancer of B-lymphocytes manifested primarily in the spleens of the animals but also infiltrated other organs, including the kidneys and liver,” Dr Karreth said. “We were particularly surprised by the development of such a dramatic phenotype in response to BRAF pseudogene overexpression alone since the development of full-blown cancer usually requires two or more mutational events.”
Mice overexpressing the BRAF pseudogene displayed higher levels of the BRAF protein and hyperactivation of the MAPK pathway, which suggests this axis is critical to cancer development.
The researchers confirmed this by inhibiting the MAPK pathway with GSK1120212, a MEK inhibitor that dramatically reduced the cancer cells’ ability to infiltrate the liver in transplantation experiments.
The investigators further validated the microRNA decoy function of the BRAF pseudogene by creating two additional transgenic mice, one overexpressing the front half of the BRAF pseudogene, and the other overexpressing the back half.
Both of these mouse models developed the same lymphoma phenotype as the mice overexpressing the full-length pseudogene, a result the researchers described as “astonishing.”
“We never expected that portions of the BRAF pseudogene could elicit a phenotype,” Dr Karreth said. “[W]hen both front and back halves induced lymphomas, we were certain the BRAF pseudogene was functioning as a microRNA decoy.”
The investigators also found the BRAF pseudogene is overexpressed in human B-cell lymphomas, and the genomic region containing the BRAF pseudogene is commonly amplified in a variety of human cancers. This suggests the group’s murine findings are of relevance to human cancer development.
Moreover, the researchers found that silencing the BRAF pseudogene in human cancer cell lines that expressed higher levels led to reduced cell proliferation. This reinforces the importance of the pseudogene and suggests a therapy that reduces BRAF pseudogene levels may be beneficial to certain cancer patients.
“While we have been busy focusing on the genome’s 20,000 coding genes, we have neglected perhaps as many as 100,000 noncoding genetic units,” Dr Pandolfi noted. “Our new findings not only tell us that we need to characterize the role of all of these non-coding pseudogenes in cancer, but, more urgently, suggest that we need to increase our understanding of the non-coding ‘junk’ of the genome and incorporate this information into our personalized medicine assays.”
“The game has to start now. We have to sequence and analyze the genome and the RNA transcripts from the non-coding space.” ![]()

New research suggests the non-coding BRAF pseudogene can produce a lymphoma phenotype in mice.
Pseudogenes, a sub-class of long non-coding RNA that developed from the genome’s protein-coding genes but lost the ability to produce proteins, have long been considered genomic “junk.”
Yet the retention of pseudogenes during evolution has suggested they may have biological functions and contribute to disease development.
Now, researchers have provided evidence that one of these pseudogenes does have a role in causing cancer, and they described the discovery in Cell.
The investigators found that, independent of any other mutations, abnormal amounts of the BRAF pseudogene led to the development of an aggressive, lymphoma-like disease in mice.
The team said this finding suggests pseudogenes may play a primary role in a variety of diseases, and the functional genome could be much larger than we thought.
“Our mouse model of the BRAF pseudogene developed cancer as rapidly and aggressively as it would if you were to express the protein-coding BRAF oncogene,” said study author Pier Paolo Pandolfi, MD, PhD, of Harvard Medical School in Boston, Massachusetts.
“It’s remarkable that this very aggressive phenotype, resembling human diffuse large B-cell lymphoma, was driven by a piece of so-called ‘junk RNA.’”
The researchers’ discovery hinges on the concept of competing endogenous RNAs (ceRNA), a functional capability for pseudogenes Dr Pandolfi and his colleagues described almost 5 years ago. The team discovered that pseudogenes and other noncoding RNAs could act as “decoys” to divert and sequester microRNAs away from their protein-coding counterparts to regulate gene expression.
“Our discovery of these ‘decoys’ revealed a new role for messenger RNA, demonstrating that, beyond serving as a genetic intermediary in the protein-making process, messenger RNAs could actually regulate expression of one another through this sophisticated new ceRNA ‘language,’” Dr Pandolfi said.
He and his colleagues showed that, when these decoys prevented microRNAs from fulfilling their regulatory function, there could be severe consequences, including making cancer cells more aggressive.
With their new research, the investigators wanted to determine if this same ceRNA cross-talk took place in a living organism and if it would result in similar consequences.
“We conducted a proof-of-principle experiment using the BRAF pseudogene,” explained Florian Karreth, PhD, a fellow in the Pandolfi lab. “We investigated whether this pseudogene exerts critical functions in the context of a whole organism and whether its disruption contributes to the development of disease.”
The researchers focused on the BRAF pseudogene because of its potential ability to regulate levels of the BRAF protein, a well-known proto-oncogene linked to numerous cancer types. In addition, the BRAF pseudogene is known to exist in both humans and mice.
The team began by testing the BRAF pseudogene in tissue culture. Their findings showed that, when overexpressed, the pseudogene operated as a microRNA decoy that increased amounts of the BRAF protein.
This, in turn, stimulated the MAPK signaling cascade, a pathway through which the BRAF protein controls cell proliferation, differentiation, and survival and which is commonly found to be hyperactive in cancer.
The investigators went on to create a mouse model in which the BRAF pseudogene was overexpressed. And they found these mice developed an aggressive, lymphoma-like cancer.
“This cancer of B-lymphocytes manifested primarily in the spleens of the animals but also infiltrated other organs, including the kidneys and liver,” Dr Karreth said. “We were particularly surprised by the development of such a dramatic phenotype in response to BRAF pseudogene overexpression alone since the development of full-blown cancer usually requires two or more mutational events.”
Mice overexpressing the BRAF pseudogene displayed higher levels of the BRAF protein and hyperactivation of the MAPK pathway, which suggests this axis is critical to cancer development.
The researchers confirmed this by inhibiting the MAPK pathway with GSK1120212, a MEK inhibitor that dramatically reduced the cancer cells’ ability to infiltrate the liver in transplantation experiments.
The investigators further validated the microRNA decoy function of the BRAF pseudogene by creating two additional transgenic mice, one overexpressing the front half of the BRAF pseudogene, and the other overexpressing the back half.
Both of these mouse models developed the same lymphoma phenotype as the mice overexpressing the full-length pseudogene, a result the researchers described as “astonishing.”
“We never expected that portions of the BRAF pseudogene could elicit a phenotype,” Dr Karreth said. “[W]hen both front and back halves induced lymphomas, we were certain the BRAF pseudogene was functioning as a microRNA decoy.”
The investigators also found the BRAF pseudogene is overexpressed in human B-cell lymphomas, and the genomic region containing the BRAF pseudogene is commonly amplified in a variety of human cancers. This suggests the group’s murine findings are of relevance to human cancer development.
Moreover, the researchers found that silencing the BRAF pseudogene in human cancer cell lines that expressed higher levels led to reduced cell proliferation. This reinforces the importance of the pseudogene and suggests a therapy that reduces BRAF pseudogene levels may be beneficial to certain cancer patients.
“While we have been busy focusing on the genome’s 20,000 coding genes, we have neglected perhaps as many as 100,000 noncoding genetic units,” Dr Pandolfi noted. “Our new findings not only tell us that we need to characterize the role of all of these non-coding pseudogenes in cancer, but, more urgently, suggest that we need to increase our understanding of the non-coding ‘junk’ of the genome and incorporate this information into our personalized medicine assays.”
“The game has to start now. We have to sequence and analyze the genome and the RNA transcripts from the non-coding space.” ![]()
Fish oil may cause chemoresistance

Consuming certain types of fish and taking fish oil supplements may induce chemoresistance, according to research published in JAMA Oncology.
Researchers found that herring and mackerel, as well as 6 different types of fish oil supplements, raised blood levels of the fatty acid 16:4(n-3).
And experiments in mice showed that small amounts of either purified 16:4(n-3) or fish oil induced resistance to the chemotherapy drug cisplatin.
Emile E. Voest, MD, PhD, of the Netherlands Cancer Institute in Amsterdam, and his colleagues conducted this multi-part study.
In one part, the team conducted a survey to determine the rate of fish oil use among patients undergoing cancer treatment (n=118). Thirty-five patients (30%) reported regular use of nutritional supplements, and 13 (11%) said they used supplements containing omega-3 fatty acids.
For another part of the study, the researchers evaluated 6 types of fish oil supplements. All of them contained relevant amounts of 16:4(n-3), ranging from 0.2 µM to 5.7 µM.
The team also recruited healthy volunteers to examine blood levels of 16:4(n-3) after the ingestion of fish oil supplements (n=30) and fish (n=20).
Volunteers had increased blood levels of 16:4(n-3) after the recommended daily amount of 10 mL of fish oil and after a 50 mL dose. Subjects had an almost-complete normalization of blood levels 8 hours after a 10 mL fish oil dose, but they had a more prolonged elevation of fatty acid levels after a 50 mL dose.
Eating 100 grams of herring and mackerel also increased blood levels of 16:4(n-3) compared with tuna, which did not affect blood levels, and salmon,
which resulted in a small, short-lived peak.
Finally, experiments in mice showed that as little as 2.5 pmol of purified 16:4(n-3) or 1 µL of fish oil was sufficient to induce resistance to the chemotherapy drug cisplatin.
The fish oil/cisplatin combination had no significant impact on mouse tumors when compared to vehicle control treatment. The estimated tumor volume difference was 44.1 mm3 (P >0.99).
When the researchers compared cisplatin alone to cisplatin plus 16:4(n-3), the estimated tumor volume difference was 95.5 mm3 (P=0 .04). When they compared vehicle control to cisplatin alone, there was an estimated tumor volume difference of 142.4 mm3 (P=0.001).
The team said these results suggest that simultaneous exposure to chemotherapy and fish oil may be detrimental to cancer patients. So until further data become available, patients should avoid consuming fish oil from the day before chemotherapy until the day after. ![]()

Consuming certain types of fish and taking fish oil supplements may induce chemoresistance, according to research published in JAMA Oncology.
Researchers found that herring and mackerel, as well as 6 different types of fish oil supplements, raised blood levels of the fatty acid 16:4(n-3).
And experiments in mice showed that small amounts of either purified 16:4(n-3) or fish oil induced resistance to the chemotherapy drug cisplatin.
Emile E. Voest, MD, PhD, of the Netherlands Cancer Institute in Amsterdam, and his colleagues conducted this multi-part study.
In one part, the team conducted a survey to determine the rate of fish oil use among patients undergoing cancer treatment (n=118). Thirty-five patients (30%) reported regular use of nutritional supplements, and 13 (11%) said they used supplements containing omega-3 fatty acids.
For another part of the study, the researchers evaluated 6 types of fish oil supplements. All of them contained relevant amounts of 16:4(n-3), ranging from 0.2 µM to 5.7 µM.
The team also recruited healthy volunteers to examine blood levels of 16:4(n-3) after the ingestion of fish oil supplements (n=30) and fish (n=20).
Volunteers had increased blood levels of 16:4(n-3) after the recommended daily amount of 10 mL of fish oil and after a 50 mL dose. Subjects had an almost-complete normalization of blood levels 8 hours after a 10 mL fish oil dose, but they had a more prolonged elevation of fatty acid levels after a 50 mL dose.
Eating 100 grams of herring and mackerel also increased blood levels of 16:4(n-3) compared with tuna, which did not affect blood levels, and salmon,
which resulted in a small, short-lived peak.
Finally, experiments in mice showed that as little as 2.5 pmol of purified 16:4(n-3) or 1 µL of fish oil was sufficient to induce resistance to the chemotherapy drug cisplatin.
The fish oil/cisplatin combination had no significant impact on mouse tumors when compared to vehicle control treatment. The estimated tumor volume difference was 44.1 mm3 (P >0.99).
When the researchers compared cisplatin alone to cisplatin plus 16:4(n-3), the estimated tumor volume difference was 95.5 mm3 (P=0 .04). When they compared vehicle control to cisplatin alone, there was an estimated tumor volume difference of 142.4 mm3 (P=0.001).
The team said these results suggest that simultaneous exposure to chemotherapy and fish oil may be detrimental to cancer patients. So until further data become available, patients should avoid consuming fish oil from the day before chemotherapy until the day after. ![]()

Consuming certain types of fish and taking fish oil supplements may induce chemoresistance, according to research published in JAMA Oncology.
Researchers found that herring and mackerel, as well as 6 different types of fish oil supplements, raised blood levels of the fatty acid 16:4(n-3).
And experiments in mice showed that small amounts of either purified 16:4(n-3) or fish oil induced resistance to the chemotherapy drug cisplatin.
Emile E. Voest, MD, PhD, of the Netherlands Cancer Institute in Amsterdam, and his colleagues conducted this multi-part study.
In one part, the team conducted a survey to determine the rate of fish oil use among patients undergoing cancer treatment (n=118). Thirty-five patients (30%) reported regular use of nutritional supplements, and 13 (11%) said they used supplements containing omega-3 fatty acids.
For another part of the study, the researchers evaluated 6 types of fish oil supplements. All of them contained relevant amounts of 16:4(n-3), ranging from 0.2 µM to 5.7 µM.
The team also recruited healthy volunteers to examine blood levels of 16:4(n-3) after the ingestion of fish oil supplements (n=30) and fish (n=20).
Volunteers had increased blood levels of 16:4(n-3) after the recommended daily amount of 10 mL of fish oil and after a 50 mL dose. Subjects had an almost-complete normalization of blood levels 8 hours after a 10 mL fish oil dose, but they had a more prolonged elevation of fatty acid levels after a 50 mL dose.
Eating 100 grams of herring and mackerel also increased blood levels of 16:4(n-3) compared with tuna, which did not affect blood levels, and salmon,
which resulted in a small, short-lived peak.
Finally, experiments in mice showed that as little as 2.5 pmol of purified 16:4(n-3) or 1 µL of fish oil was sufficient to induce resistance to the chemotherapy drug cisplatin.
The fish oil/cisplatin combination had no significant impact on mouse tumors when compared to vehicle control treatment. The estimated tumor volume difference was 44.1 mm3 (P >0.99).
When the researchers compared cisplatin alone to cisplatin plus 16:4(n-3), the estimated tumor volume difference was 95.5 mm3 (P=0 .04). When they compared vehicle control to cisplatin alone, there was an estimated tumor volume difference of 142.4 mm3 (P=0.001).
The team said these results suggest that simultaneous exposure to chemotherapy and fish oil may be detrimental to cancer patients. So until further data become available, patients should avoid consuming fish oil from the day before chemotherapy until the day after. ![]()
Japan approves pomalidomide for MM

Photo by Steven Harbour
Japan’s Ministry of Health, Labour and Welfare (MHLW) has granted full marketing authorization for pomalidomide (Pomalyst) to treat patients with relapsed or refractory multiple myeloma (MM) who have previously received lenalidomide and bortezomib.
Pomalidomide will be available in Japan through RevMate, a restricted distribution program that should ensure the proper use of pomalidomide and prevent fetal exposure to the drug.
The MHLW approved pomalidomide based on results of trials in patients with previously treated MM, including the phase 3 MM-003 trial and the phase 2 MM-011 trial.
Phase 2 trial
In the MM-011 trial, which has not yet been published, researchers evaluated the safety and efficacy of pomalidomide in combination with dexamethasone in 36 patients with relapsed/refractory MM.
The patients had received at least 2 prior therapies, including lenalidomide and bortezomib, and had disease progression within 60 days of completing their last therapy.
Twenty-five percent of patients responded to treatment with pomalidomide and dexamethasone, 1 with a complete response and 8 with a partial response.
About 89% of patients (32/36) experienced adverse events, including lab test abnormalities. The most common events were neutropenia (69.4%), thrombocytopenia (33.3%), rash (22.2%), leukocytopenia (13.9%), pyrexia (13.9%), anemia (11.1%), lymphopenia (11.1%), and constipation (11.1%).
Phase 3 trial
The MM-003 study included 455 patients with relapsed or refractory MM. They were randomized to receive either pomalidomide plus low-dose dexamethasone (POM-LoDEX, n=302) or high-dose dexamethasone (HiDEX, n=153). The median follow-up was 10 months.
The overall response rate was 31% (n=95) in the POM-LoDEX arm and 10% (n=15) in the HiDEX arm. The median duration of response was 7.0 months (range, 6 to 9 months) and 6.1 months (range, 1.4 to 8.5 months), respectively.
The median progression-free survival was 4.0 months in the POM-LoDEX arm and 1.9 months in the HiDEX arm (P<0.001). And the median overall survival was 12.7 months in the POM-LoDEX arm and 8.1 months in the HiDEX arm (P=0.028).
Patients in the POM-LoDEX arm experienced more grade 3/4 neutropenia than patients in the HiDEX arm. But rates of grade 3/4 anemia and thrombocytopenia were similar between the 2 arms.
Rates of grade 3/4 non-hematologic toxicities were also similar between the arms and included infection, pneumonia, hemorrhage, glucose intolerance, and fatigue. Other adverse events of note included venous thromboembolism and peripheral neuropathy, which occurred at similar rates in both arms. ![]()

Photo by Steven Harbour
Japan’s Ministry of Health, Labour and Welfare (MHLW) has granted full marketing authorization for pomalidomide (Pomalyst) to treat patients with relapsed or refractory multiple myeloma (MM) who have previously received lenalidomide and bortezomib.
Pomalidomide will be available in Japan through RevMate, a restricted distribution program that should ensure the proper use of pomalidomide and prevent fetal exposure to the drug.
The MHLW approved pomalidomide based on results of trials in patients with previously treated MM, including the phase 3 MM-003 trial and the phase 2 MM-011 trial.
Phase 2 trial
In the MM-011 trial, which has not yet been published, researchers evaluated the safety and efficacy of pomalidomide in combination with dexamethasone in 36 patients with relapsed/refractory MM.
The patients had received at least 2 prior therapies, including lenalidomide and bortezomib, and had disease progression within 60 days of completing their last therapy.
Twenty-five percent of patients responded to treatment with pomalidomide and dexamethasone, 1 with a complete response and 8 with a partial response.
About 89% of patients (32/36) experienced adverse events, including lab test abnormalities. The most common events were neutropenia (69.4%), thrombocytopenia (33.3%), rash (22.2%), leukocytopenia (13.9%), pyrexia (13.9%), anemia (11.1%), lymphopenia (11.1%), and constipation (11.1%).
Phase 3 trial
The MM-003 study included 455 patients with relapsed or refractory MM. They were randomized to receive either pomalidomide plus low-dose dexamethasone (POM-LoDEX, n=302) or high-dose dexamethasone (HiDEX, n=153). The median follow-up was 10 months.
The overall response rate was 31% (n=95) in the POM-LoDEX arm and 10% (n=15) in the HiDEX arm. The median duration of response was 7.0 months (range, 6 to 9 months) and 6.1 months (range, 1.4 to 8.5 months), respectively.
The median progression-free survival was 4.0 months in the POM-LoDEX arm and 1.9 months in the HiDEX arm (P<0.001). And the median overall survival was 12.7 months in the POM-LoDEX arm and 8.1 months in the HiDEX arm (P=0.028).
Patients in the POM-LoDEX arm experienced more grade 3/4 neutropenia than patients in the HiDEX arm. But rates of grade 3/4 anemia and thrombocytopenia were similar between the 2 arms.
Rates of grade 3/4 non-hematologic toxicities were also similar between the arms and included infection, pneumonia, hemorrhage, glucose intolerance, and fatigue. Other adverse events of note included venous thromboembolism and peripheral neuropathy, which occurred at similar rates in both arms. ![]()

Photo by Steven Harbour
Japan’s Ministry of Health, Labour and Welfare (MHLW) has granted full marketing authorization for pomalidomide (Pomalyst) to treat patients with relapsed or refractory multiple myeloma (MM) who have previously received lenalidomide and bortezomib.
Pomalidomide will be available in Japan through RevMate, a restricted distribution program that should ensure the proper use of pomalidomide and prevent fetal exposure to the drug.
The MHLW approved pomalidomide based on results of trials in patients with previously treated MM, including the phase 3 MM-003 trial and the phase 2 MM-011 trial.
Phase 2 trial
In the MM-011 trial, which has not yet been published, researchers evaluated the safety and efficacy of pomalidomide in combination with dexamethasone in 36 patients with relapsed/refractory MM.
The patients had received at least 2 prior therapies, including lenalidomide and bortezomib, and had disease progression within 60 days of completing their last therapy.
Twenty-five percent of patients responded to treatment with pomalidomide and dexamethasone, 1 with a complete response and 8 with a partial response.
About 89% of patients (32/36) experienced adverse events, including lab test abnormalities. The most common events were neutropenia (69.4%), thrombocytopenia (33.3%), rash (22.2%), leukocytopenia (13.9%), pyrexia (13.9%), anemia (11.1%), lymphopenia (11.1%), and constipation (11.1%).
Phase 3 trial
The MM-003 study included 455 patients with relapsed or refractory MM. They were randomized to receive either pomalidomide plus low-dose dexamethasone (POM-LoDEX, n=302) or high-dose dexamethasone (HiDEX, n=153). The median follow-up was 10 months.
The overall response rate was 31% (n=95) in the POM-LoDEX arm and 10% (n=15) in the HiDEX arm. The median duration of response was 7.0 months (range, 6 to 9 months) and 6.1 months (range, 1.4 to 8.5 months), respectively.
The median progression-free survival was 4.0 months in the POM-LoDEX arm and 1.9 months in the HiDEX arm (P<0.001). And the median overall survival was 12.7 months in the POM-LoDEX arm and 8.1 months in the HiDEX arm (P=0.028).
Patients in the POM-LoDEX arm experienced more grade 3/4 neutropenia than patients in the HiDEX arm. But rates of grade 3/4 anemia and thrombocytopenia were similar between the 2 arms.
Rates of grade 3/4 non-hematologic toxicities were also similar between the arms and included infection, pneumonia, hemorrhage, glucose intolerance, and fatigue. Other adverse events of note included venous thromboembolism and peripheral neuropathy, which occurred at similar rates in both arms. ![]()
Low mortality, good outcomes in octogenarian AAA repair sparks QOL vs. utility debate
SCOTTSDALE, ARIZ. – Abdominal aortic aneurysm repair in patients 80 years and older can be performed safely and with good medium-term survival rates, a prospective single-site study has shown.
Perioperative mortality in elective and emergent AAA repair for octogenarians was 2% and 35%, respectively, with a median survival rate of 19 months in both groups.
According to these data, “Patients shouldn’t be turned down for aneurysm repair on the basis of their age alone,” Dr. Christopher M. Lamb, a vascular surgery fellow at the University of California Davis Medical Center in Sacramento, said during a presentation at this year’s Southern Association for Vascular Surgery annual meeting. “However, whether should we be doing these procedures is a different question, and I don’t think these data allow us to answer that question properly.”
Dr. Lamb and his colleagues reviewed the records of 847 consecutive patients aged 80 years or older, seen between April 2005 and February 2014 for any type of AAA repair. Cases were sorted according to whether they were elective, ruptured, or urgent but unruptured. A total of 226 patients met the study’s age criteria, there were nearly seven men for every woman, all with a median age of 83 years.
Of the elective AAA repair arm of the study, 131 patients (116 men) with a median age of 82 years had an endovascular repair, while the rest underwent open surgical repair. The combined 30-day mortality rate for these patients was 2.3%, with no significant difference between either the endovascular aneurysm repair (EVAR) or the open surgical repair (OSR) patients (1.9% vs. 4.2%; P = .458). The median survival of all elective repair patients was 19 months (interquartile range, 10-35), with no difference seen between the two groups (P = .113)
Of the 65 patients (53 men) with ruptured AAA, the median age was 83 years. A third had open repair (32.3%), while the rest had EVAR. The combined 30-day mortality rate was 35.4% but was significantly higher after OSR (52.4% vs. 27.3%; P = .048). The median survival rate was 6 months (IQR, 6-42) when 30-day mortality rates were excluded. The median survival rates in patients who lived longer than 30 days was significantly higher in OSR patients (42.5 months vs. 11 months; P = .019).
Of the 23 men and 7 women with symptomatic but unruptured AAA, all but 1 had EVAR. At 30 days, there was one diverticular perforation-related postoperative death in the EVAR group, which had a median survival rate of 29 months. There being only a single patient in the OSR group obviated a comparative median survival rate analysis.
A subanalysis of the final 20 months of the study showed that 41% of octogenarians seeking any type of AAA repair at the site were rejected (48 rejections vs. 69 repairs). Those who were rejected for repair tended to be older, with a median age of 86 years vs. 83 years for patients who underwent repair (P = .0004).
Dr. Lamb noted that although the findings demonstrate acceptable overall safety rates for the entire cohort, without a control group of patients that did not have AAA repair, it would be hard to draw a definite conclusion about the utility of the findings, and that more data was warranted; however, the potential for limited long-term survival with what previous reports have suggested may include “a reduced quality of life for a good part of it, possibly raises the question that these patients should be treated conservatively, more often.”
The rejection rate data prompted the presentation’s discussant, Dr. William D. Jordan Jr., section chief of vascular surgery at the University of Alabama at Birmingham and the presentation’s discussant, to challenge the findings and asked whether a single surgeon selected the patients.
“You said there is not a selection bias in your study, but I beg to differ. Perhaps all these kinds of studies have a selection bias, and I believe they should. We should select the appropriate patients for the appropriate procedure at the appropriate time, with the appropriate expectation of outcome. Bias in this setting may be seen as good,” Dr. Jordan said.
Dr. Lamb responded that the treatment algorithm at the site for all patients with a confirmed AAA of 5.5 cm or greater included CT imaging that is reviewed by a multidisciplinary team comprising vascular surgeons and interventional radiologists, who then evaluated the patients according to their physiology and anatomy, as well as their comorbidities, with the intention that whenever possible, EVAR rather than open repair would be performed.
As to whether there was a bias toward not repairing AAA in older patients, Dr. Lamb said it was incumbent on any health system to evaluate a procedure’s cost effectiveness, but that, “the life expectancy of a vascular patient is often more limited than I think we’d like to believe ... we don’t know what the natural history of these patients’ life expectancy is. We don’t know from these data what the cause of death was, but anecdotally, we didn’t see hundreds of patients return with ruptured aneurysms after an EVAR.”
“I would truly like to see how many [of these patients] who make it out of the hospital return to normal living within six months,” Dr. Samuel R. Money, chair of surgery at the Mayo Clinic in Scottsdale, Ariz., said in an interview following the presentation. “At some point, the question becomes ‘Can we afford to spend $100,000 dollars to keep a 90-year-old patient alive for 6 more months?’ Can this society sustain the cost of that?”
[email protected]
On Twitter @whitneymcknight
This discussion is provocative and raises some interesting points. Obviously cost effectiveness considerations are important, and our country does not have unlimited funds to spend on medical care. And perhaps there are some elderly and frail individuals who should not have their AAAs repaired electively because the risk of rupture during the patients’ remaining months or years of life is small.
This is particularly true if the patient’s AAA is less than 7 cm and his or her anatomy and condition are unsuitable for an easy repair. However, if the AAA is large and threatening, and the patient has the possibility of living several years, elective repair is justified and reasonable – especially if it can be accomplished endovascularly. As someone who is near 80 [years old], I could not feel more strongly about this, and I would maintain this view if I were near 90 and healthy.

|
Dr. Frank J. Veith |
I hold the same view even more strongly regarding a ruptured AAA. In this setting, the alternative management is nontreatment, which is uniformly fatal. The common term “palliative treatment” for such nontreatment is a misleading misnomer. No sane, reasonably healthy elderly patient would knowingly choose such nontreatment when a good alternative with well over an even chance of living a lot longer is offered. That good alternative – again especially if it can be performed endovascularly – should be offered, and our health system should pay for it and compensate by saving money on unnecessary SFA [superficial femoral artery] stents and carotid procedures.
Dr. Frank J. Veith is professor of surgery at New York University Medical Center and the Cleveland Clinic and is an associate medical editor for Vascular Specialist.
This discussion is provocative and raises some interesting points. Obviously cost effectiveness considerations are important, and our country does not have unlimited funds to spend on medical care. And perhaps there are some elderly and frail individuals who should not have their AAAs repaired electively because the risk of rupture during the patients’ remaining months or years of life is small.
This is particularly true if the patient’s AAA is less than 7 cm and his or her anatomy and condition are unsuitable for an easy repair. However, if the AAA is large and threatening, and the patient has the possibility of living several years, elective repair is justified and reasonable – especially if it can be accomplished endovascularly. As someone who is near 80 [years old], I could not feel more strongly about this, and I would maintain this view if I were near 90 and healthy.

|
Dr. Frank J. Veith |
I hold the same view even more strongly regarding a ruptured AAA. In this setting, the alternative management is nontreatment, which is uniformly fatal. The common term “palliative treatment” for such nontreatment is a misleading misnomer. No sane, reasonably healthy elderly patient would knowingly choose such nontreatment when a good alternative with well over an even chance of living a lot longer is offered. That good alternative – again especially if it can be performed endovascularly – should be offered, and our health system should pay for it and compensate by saving money on unnecessary SFA [superficial femoral artery] stents and carotid procedures.
Dr. Frank J. Veith is professor of surgery at New York University Medical Center and the Cleveland Clinic and is an associate medical editor for Vascular Specialist.
This discussion is provocative and raises some interesting points. Obviously cost effectiveness considerations are important, and our country does not have unlimited funds to spend on medical care. And perhaps there are some elderly and frail individuals who should not have their AAAs repaired electively because the risk of rupture during the patients’ remaining months or years of life is small.
This is particularly true if the patient’s AAA is less than 7 cm and his or her anatomy and condition are unsuitable for an easy repair. However, if the AAA is large and threatening, and the patient has the possibility of living several years, elective repair is justified and reasonable – especially if it can be accomplished endovascularly. As someone who is near 80 [years old], I could not feel more strongly about this, and I would maintain this view if I were near 90 and healthy.

|
Dr. Frank J. Veith |
I hold the same view even more strongly regarding a ruptured AAA. In this setting, the alternative management is nontreatment, which is uniformly fatal. The common term “palliative treatment” for such nontreatment is a misleading misnomer. No sane, reasonably healthy elderly patient would knowingly choose such nontreatment when a good alternative with well over an even chance of living a lot longer is offered. That good alternative – again especially if it can be performed endovascularly – should be offered, and our health system should pay for it and compensate by saving money on unnecessary SFA [superficial femoral artery] stents and carotid procedures.
Dr. Frank J. Veith is professor of surgery at New York University Medical Center and the Cleveland Clinic and is an associate medical editor for Vascular Specialist.
SCOTTSDALE, ARIZ. – Abdominal aortic aneurysm repair in patients 80 years and older can be performed safely and with good medium-term survival rates, a prospective single-site study has shown.
Perioperative mortality in elective and emergent AAA repair for octogenarians was 2% and 35%, respectively, with a median survival rate of 19 months in both groups.
According to these data, “Patients shouldn’t be turned down for aneurysm repair on the basis of their age alone,” Dr. Christopher M. Lamb, a vascular surgery fellow at the University of California Davis Medical Center in Sacramento, said during a presentation at this year’s Southern Association for Vascular Surgery annual meeting. “However, whether should we be doing these procedures is a different question, and I don’t think these data allow us to answer that question properly.”
Dr. Lamb and his colleagues reviewed the records of 847 consecutive patients aged 80 years or older, seen between April 2005 and February 2014 for any type of AAA repair. Cases were sorted according to whether they were elective, ruptured, or urgent but unruptured. A total of 226 patients met the study’s age criteria, there were nearly seven men for every woman, all with a median age of 83 years.
Of the elective AAA repair arm of the study, 131 patients (116 men) with a median age of 82 years had an endovascular repair, while the rest underwent open surgical repair. The combined 30-day mortality rate for these patients was 2.3%, with no significant difference between either the endovascular aneurysm repair (EVAR) or the open surgical repair (OSR) patients (1.9% vs. 4.2%; P = .458). The median survival of all elective repair patients was 19 months (interquartile range, 10-35), with no difference seen between the two groups (P = .113)
Of the 65 patients (53 men) with ruptured AAA, the median age was 83 years. A third had open repair (32.3%), while the rest had EVAR. The combined 30-day mortality rate was 35.4% but was significantly higher after OSR (52.4% vs. 27.3%; P = .048). The median survival rate was 6 months (IQR, 6-42) when 30-day mortality rates were excluded. The median survival rates in patients who lived longer than 30 days was significantly higher in OSR patients (42.5 months vs. 11 months; P = .019).
Of the 23 men and 7 women with symptomatic but unruptured AAA, all but 1 had EVAR. At 30 days, there was one diverticular perforation-related postoperative death in the EVAR group, which had a median survival rate of 29 months. There being only a single patient in the OSR group obviated a comparative median survival rate analysis.
A subanalysis of the final 20 months of the study showed that 41% of octogenarians seeking any type of AAA repair at the site were rejected (48 rejections vs. 69 repairs). Those who were rejected for repair tended to be older, with a median age of 86 years vs. 83 years for patients who underwent repair (P = .0004).
Dr. Lamb noted that although the findings demonstrate acceptable overall safety rates for the entire cohort, without a control group of patients that did not have AAA repair, it would be hard to draw a definite conclusion about the utility of the findings, and that more data was warranted; however, the potential for limited long-term survival with what previous reports have suggested may include “a reduced quality of life for a good part of it, possibly raises the question that these patients should be treated conservatively, more often.”
The rejection rate data prompted the presentation’s discussant, Dr. William D. Jordan Jr., section chief of vascular surgery at the University of Alabama at Birmingham and the presentation’s discussant, to challenge the findings and asked whether a single surgeon selected the patients.
“You said there is not a selection bias in your study, but I beg to differ. Perhaps all these kinds of studies have a selection bias, and I believe they should. We should select the appropriate patients for the appropriate procedure at the appropriate time, with the appropriate expectation of outcome. Bias in this setting may be seen as good,” Dr. Jordan said.
Dr. Lamb responded that the treatment algorithm at the site for all patients with a confirmed AAA of 5.5 cm or greater included CT imaging that is reviewed by a multidisciplinary team comprising vascular surgeons and interventional radiologists, who then evaluated the patients according to their physiology and anatomy, as well as their comorbidities, with the intention that whenever possible, EVAR rather than open repair would be performed.
As to whether there was a bias toward not repairing AAA in older patients, Dr. Lamb said it was incumbent on any health system to evaluate a procedure’s cost effectiveness, but that, “the life expectancy of a vascular patient is often more limited than I think we’d like to believe ... we don’t know what the natural history of these patients’ life expectancy is. We don’t know from these data what the cause of death was, but anecdotally, we didn’t see hundreds of patients return with ruptured aneurysms after an EVAR.”
“I would truly like to see how many [of these patients] who make it out of the hospital return to normal living within six months,” Dr. Samuel R. Money, chair of surgery at the Mayo Clinic in Scottsdale, Ariz., said in an interview following the presentation. “At some point, the question becomes ‘Can we afford to spend $100,000 dollars to keep a 90-year-old patient alive for 6 more months?’ Can this society sustain the cost of that?”
[email protected]
On Twitter @whitneymcknight
SCOTTSDALE, ARIZ. – Abdominal aortic aneurysm repair in patients 80 years and older can be performed safely and with good medium-term survival rates, a prospective single-site study has shown.
Perioperative mortality in elective and emergent AAA repair for octogenarians was 2% and 35%, respectively, with a median survival rate of 19 months in both groups.
According to these data, “Patients shouldn’t be turned down for aneurysm repair on the basis of their age alone,” Dr. Christopher M. Lamb, a vascular surgery fellow at the University of California Davis Medical Center in Sacramento, said during a presentation at this year’s Southern Association for Vascular Surgery annual meeting. “However, whether should we be doing these procedures is a different question, and I don’t think these data allow us to answer that question properly.”
Dr. Lamb and his colleagues reviewed the records of 847 consecutive patients aged 80 years or older, seen between April 2005 and February 2014 for any type of AAA repair. Cases were sorted according to whether they were elective, ruptured, or urgent but unruptured. A total of 226 patients met the study’s age criteria, there were nearly seven men for every woman, all with a median age of 83 years.
Of the elective AAA repair arm of the study, 131 patients (116 men) with a median age of 82 years had an endovascular repair, while the rest underwent open surgical repair. The combined 30-day mortality rate for these patients was 2.3%, with no significant difference between either the endovascular aneurysm repair (EVAR) or the open surgical repair (OSR) patients (1.9% vs. 4.2%; P = .458). The median survival of all elective repair patients was 19 months (interquartile range, 10-35), with no difference seen between the two groups (P = .113)
Of the 65 patients (53 men) with ruptured AAA, the median age was 83 years. A third had open repair (32.3%), while the rest had EVAR. The combined 30-day mortality rate was 35.4% but was significantly higher after OSR (52.4% vs. 27.3%; P = .048). The median survival rate was 6 months (IQR, 6-42) when 30-day mortality rates were excluded. The median survival rates in patients who lived longer than 30 days was significantly higher in OSR patients (42.5 months vs. 11 months; P = .019).
Of the 23 men and 7 women with symptomatic but unruptured AAA, all but 1 had EVAR. At 30 days, there was one diverticular perforation-related postoperative death in the EVAR group, which had a median survival rate of 29 months. There being only a single patient in the OSR group obviated a comparative median survival rate analysis.
A subanalysis of the final 20 months of the study showed that 41% of octogenarians seeking any type of AAA repair at the site were rejected (48 rejections vs. 69 repairs). Those who were rejected for repair tended to be older, with a median age of 86 years vs. 83 years for patients who underwent repair (P = .0004).
Dr. Lamb noted that although the findings demonstrate acceptable overall safety rates for the entire cohort, without a control group of patients that did not have AAA repair, it would be hard to draw a definite conclusion about the utility of the findings, and that more data was warranted; however, the potential for limited long-term survival with what previous reports have suggested may include “a reduced quality of life for a good part of it, possibly raises the question that these patients should be treated conservatively, more often.”
The rejection rate data prompted the presentation’s discussant, Dr. William D. Jordan Jr., section chief of vascular surgery at the University of Alabama at Birmingham and the presentation’s discussant, to challenge the findings and asked whether a single surgeon selected the patients.
“You said there is not a selection bias in your study, but I beg to differ. Perhaps all these kinds of studies have a selection bias, and I believe they should. We should select the appropriate patients for the appropriate procedure at the appropriate time, with the appropriate expectation of outcome. Bias in this setting may be seen as good,” Dr. Jordan said.
Dr. Lamb responded that the treatment algorithm at the site for all patients with a confirmed AAA of 5.5 cm or greater included CT imaging that is reviewed by a multidisciplinary team comprising vascular surgeons and interventional radiologists, who then evaluated the patients according to their physiology and anatomy, as well as their comorbidities, with the intention that whenever possible, EVAR rather than open repair would be performed.
As to whether there was a bias toward not repairing AAA in older patients, Dr. Lamb said it was incumbent on any health system to evaluate a procedure’s cost effectiveness, but that, “the life expectancy of a vascular patient is often more limited than I think we’d like to believe ... we don’t know what the natural history of these patients’ life expectancy is. We don’t know from these data what the cause of death was, but anecdotally, we didn’t see hundreds of patients return with ruptured aneurysms after an EVAR.”
“I would truly like to see how many [of these patients] who make it out of the hospital return to normal living within six months,” Dr. Samuel R. Money, chair of surgery at the Mayo Clinic in Scottsdale, Ariz., said in an interview following the presentation. “At some point, the question becomes ‘Can we afford to spend $100,000 dollars to keep a 90-year-old patient alive for 6 more months?’ Can this society sustain the cost of that?”
[email protected]
On Twitter @whitneymcknight
AT THE SAVS ANNUAL MEETING 2015
Key clinical point: EVAR and OSR outcomes for AAA were both shown safe and effective at 30 days and 6 months in patients 80 years and older.
Major finding: Perioperative mortality in elective and emergent AAA repair was 2% and 35%, respectively, with a median survival rate of 19 months in both groups.
Data source: Prospective study of 847 consecutive AAA-repair patients at a single site between May 2005 and February 2014.
Disclosures: Dr. Lamb did not have any relevant disclosures.