User login
Bleomycin can be safely omitted after negative PET2 in HL
Photo by Jens Maus
LUGANO—Results of the RATHL trial indicate that bleomycin can be omitted from ABVD therapy following a negative interim FDG-PET scan in patients with Hodgkin lymphoma.
Progression-free survival (PFS) and overall survival (OS) were the same at 3 years for patients who were PET-negative after 2 cycles of ABVD and then continued therapy with or without bleomycin.
These results were presented at the 13th International Conference on Malignant Lymphoma (13-ICML).
Investigators based the RATHL study on the principles that it’s desirable to de-escalate treatment in the best responders to avoid late toxicity and that PET scans after 2 cycles of ABVD are highly predictive.
The team enrolled 1214 patients from 6 countries, 861 of whom were in the UK. Patients received a PET scan at staging, 2 cycles of ABVD, and then a second PET scan (PET2).
If patients were negative after PET2, they were randomized to receive 4 more cycles of ABVD or AVD and no radiotherapy.
If they were positive after PET2, patients received 4 cycles of BEACOPP-14 or 3 cycles of escalated BEACOPP. These patients then received a third PET scan, and the positive patients went on to receive radiotherapy or a salvage regimen.
The PET3-negative patients received 2 more cycles of BEACOPP-14 or one of escalated BEACOPP without radiotherapy.
Peter W. Johnson, MD, of the University of Southampton in the UK, presented the results of these treatment regimens during the plenary session of 13-ICML as abstract 008.
Patient characteristics
Patients were a median age of 33 (range, 18-79), and 55% were male. They had disease stages of II (41%), III (31%), or IV (28%).
Seventy-four percent of patients had a performance status of 0. Almost half (49%) had an IPS score of 2 to 3, and 18% had an IPS score of 4 or more. Thirty-two percent had bulky disease.
Investigators followed the patients for a median of 34.7 months (range, 1 day to 68.2 months).
Results after PET2
Seventy-seven patients were missing a second PET scan, mostly due to PET protocol violations of having to use the same scanner for the baseline and second scan and the same acquisition time.
“We were very strict on our quality control,” Dr Johnson said, “because we wished to make sure this was reproducible data.”
So the results after 2 cycles of ABVD treatment were based on 1137 patients.
PET-negative patients
More than 80% of patients were PET-negative after 2 cycles. Four hundred and sixty-nine patients were randomized to receive ABVD and 466 to AVD.
The groups were well-balanced in terms of median age, performance status, stage, B symptoms, bulky disease, and IPS score.
There was a significant excess of neutropenic fever (P=0.032) and infection (P=0.040) in those patients continuing on ABVD compared to AVD. And any hematologic toxicity was highly significantly different between the 2 arms (P<0.001).
“So we have demonstrated that continuing with bleomycin beyond cycle 2 is accompanied by significantly more toxicity,” Dr Johnson said.
Ninety-eight percent of patients in both cohorts received at least 6 cycles of therapy post-randomization.
At a median follow-up of 36.3 months, 65% of patients in the ABVD arm and 69% in the AVD arm achieved a complete remission (CR) or unconfirmed CR (CRu).
Fourteen patients died in each of the arms. Seven patients died of their disease in the AVD arm, compared with 1 in the ABVD arm. Slightly more patients died from toxicity in the ABVD arm.
The primary endpoint of PFS showed very little difference between the 2 arms. The 3-year PFS in the intent-to-treat analysis was 85.4% for patients in the ABVD arm and 84.4% for those in the AVD arm.
The investigators observed that the PFS of 85% was somewhat lower than the 95% PFS observed in the literature. So they looked at the association between baseline factors and PFS after negative PET2.
“And what stands out from this is that if you have high-stage disease at presentation, there is a slightly higher chance of treatment failure following a negative PET scan,” Dr Johnson said. “And you can see the trend here, from early stage disease up to advanced-stage disease, the PET scan becomes a less reliable indicator of result.”
The investigators also conducted a subgroup analysis of the PET2-negative patients and found there was no difference in outcome between treatment arms in patients with more advanced disease, with bulky disease, with a high IPS score, or according to the PET score.
“So we have not succeeded in finding any subgroup where it appears to be beneficial to continue bleomycin,” Dr Johnson said.
The OS rate was also the same between the 2 arms, at 97%.
PET2-positive patients
One hundred and seventy-four patients who were positive after the second PET scan received either BEACOPP for 14 days or escalated BEACOPP.
The percentage of patients who experienced grade 3-4 toxicities was largely similar between the 2 regimens, although the patients receiving escalated BEACOPP had more neutropenia (P=0.057), thrombocytopenia (P=0.001), and neutropenic fever (P=0.08).
In terms of efficacy, two-thirds of patients became PET-negative by the third PET scan, and 48% of patients achieved a CR or CRu.
Twenty-one patients died, 8 due to Hodgkin lymphoma.
The PFS was 66.0% in the BEACOPP-14 group and 71.1% in the escalated-BEACOPP group. The 3-year OS was 89.6% in the BEACOPP-14 group and 82.8% in the escalated-BEACOPP group.
For the entire group of 1214 patients, the 3-year PFS was 82.5%, and the OS was 95.4%.
Based on these results, the investigators concluded that it is safe to omit bleomycin and consolidation radiotherapy from subsequent ABVD therapy after a negative interim PET scan. And doing so reduces toxicity, especially dyspnea, thromboembolism, and neutropenic fever.
“[B]y using more selective chemotherapy and much less radiotherapy than we have previously used in our studies, where we’re giving less than 3% of patients consolidation radiotherapy, the results appear to be favorable and an improvement over what we have seen previously,” Dr Johnson said.
Details on lung toxicity in this study were presented separately at 13-ICML as abstract 041. ![]()

Photo by Jens Maus
LUGANO—Results of the RATHL trial indicate that bleomycin can be omitted from ABVD therapy following a negative interim FDG-PET scan in patients with Hodgkin lymphoma.
Progression-free survival (PFS) and overall survival (OS) were the same at 3 years for patients who were PET-negative after 2 cycles of ABVD and then continued therapy with or without bleomycin.
These results were presented at the 13th International Conference on Malignant Lymphoma (13-ICML).
Investigators based the RATHL study on the principles that it’s desirable to de-escalate treatment in the best responders to avoid late toxicity and that PET scans after 2 cycles of ABVD are highly predictive.
The team enrolled 1214 patients from 6 countries, 861 of whom were in the UK. Patients received a PET scan at staging, 2 cycles of ABVD, and then a second PET scan (PET2).
If patients were negative after PET2, they were randomized to receive 4 more cycles of ABVD or AVD and no radiotherapy.
If they were positive after PET2, patients received 4 cycles of BEACOPP-14 or 3 cycles of escalated BEACOPP. These patients then received a third PET scan, and the positive patients went on to receive radiotherapy or a salvage regimen.
The PET3-negative patients received 2 more cycles of BEACOPP-14 or one of escalated BEACOPP without radiotherapy.
Peter W. Johnson, MD, of the University of Southampton in the UK, presented the results of these treatment regimens during the plenary session of 13-ICML as abstract 008.
Patient characteristics
Patients were a median age of 33 (range, 18-79), and 55% were male. They had disease stages of II (41%), III (31%), or IV (28%).
Seventy-four percent of patients had a performance status of 0. Almost half (49%) had an IPS score of 2 to 3, and 18% had an IPS score of 4 or more. Thirty-two percent had bulky disease.
Investigators followed the patients for a median of 34.7 months (range, 1 day to 68.2 months).
Results after PET2
Seventy-seven patients were missing a second PET scan, mostly due to PET protocol violations of having to use the same scanner for the baseline and second scan and the same acquisition time.
“We were very strict on our quality control,” Dr Johnson said, “because we wished to make sure this was reproducible data.”
So the results after 2 cycles of ABVD treatment were based on 1137 patients.
PET-negative patients
More than 80% of patients were PET-negative after 2 cycles. Four hundred and sixty-nine patients were randomized to receive ABVD and 466 to AVD.
The groups were well-balanced in terms of median age, performance status, stage, B symptoms, bulky disease, and IPS score.
There was a significant excess of neutropenic fever (P=0.032) and infection (P=0.040) in those patients continuing on ABVD compared to AVD. And any hematologic toxicity was highly significantly different between the 2 arms (P<0.001).
“So we have demonstrated that continuing with bleomycin beyond cycle 2 is accompanied by significantly more toxicity,” Dr Johnson said.
Ninety-eight percent of patients in both cohorts received at least 6 cycles of therapy post-randomization.
At a median follow-up of 36.3 months, 65% of patients in the ABVD arm and 69% in the AVD arm achieved a complete remission (CR) or unconfirmed CR (CRu).
Fourteen patients died in each of the arms. Seven patients died of their disease in the AVD arm, compared with 1 in the ABVD arm. Slightly more patients died from toxicity in the ABVD arm.
The primary endpoint of PFS showed very little difference between the 2 arms. The 3-year PFS in the intent-to-treat analysis was 85.4% for patients in the ABVD arm and 84.4% for those in the AVD arm.
The investigators observed that the PFS of 85% was somewhat lower than the 95% PFS observed in the literature. So they looked at the association between baseline factors and PFS after negative PET2.
“And what stands out from this is that if you have high-stage disease at presentation, there is a slightly higher chance of treatment failure following a negative PET scan,” Dr Johnson said. “And you can see the trend here, from early stage disease up to advanced-stage disease, the PET scan becomes a less reliable indicator of result.”
The investigators also conducted a subgroup analysis of the PET2-negative patients and found there was no difference in outcome between treatment arms in patients with more advanced disease, with bulky disease, with a high IPS score, or according to the PET score.
“So we have not succeeded in finding any subgroup where it appears to be beneficial to continue bleomycin,” Dr Johnson said.
The OS rate was also the same between the 2 arms, at 97%.
PET2-positive patients
One hundred and seventy-four patients who were positive after the second PET scan received either BEACOPP for 14 days or escalated BEACOPP.
The percentage of patients who experienced grade 3-4 toxicities was largely similar between the 2 regimens, although the patients receiving escalated BEACOPP had more neutropenia (P=0.057), thrombocytopenia (P=0.001), and neutropenic fever (P=0.08).
In terms of efficacy, two-thirds of patients became PET-negative by the third PET scan, and 48% of patients achieved a CR or CRu.
Twenty-one patients died, 8 due to Hodgkin lymphoma.
The PFS was 66.0% in the BEACOPP-14 group and 71.1% in the escalated-BEACOPP group. The 3-year OS was 89.6% in the BEACOPP-14 group and 82.8% in the escalated-BEACOPP group.
For the entire group of 1214 patients, the 3-year PFS was 82.5%, and the OS was 95.4%.
Based on these results, the investigators concluded that it is safe to omit bleomycin and consolidation radiotherapy from subsequent ABVD therapy after a negative interim PET scan. And doing so reduces toxicity, especially dyspnea, thromboembolism, and neutropenic fever.
“[B]y using more selective chemotherapy and much less radiotherapy than we have previously used in our studies, where we’re giving less than 3% of patients consolidation radiotherapy, the results appear to be favorable and an improvement over what we have seen previously,” Dr Johnson said.
Details on lung toxicity in this study were presented separately at 13-ICML as abstract 041. ![]()

Photo by Jens Maus
LUGANO—Results of the RATHL trial indicate that bleomycin can be omitted from ABVD therapy following a negative interim FDG-PET scan in patients with Hodgkin lymphoma.
Progression-free survival (PFS) and overall survival (OS) were the same at 3 years for patients who were PET-negative after 2 cycles of ABVD and then continued therapy with or without bleomycin.
These results were presented at the 13th International Conference on Malignant Lymphoma (13-ICML).
Investigators based the RATHL study on the principles that it’s desirable to de-escalate treatment in the best responders to avoid late toxicity and that PET scans after 2 cycles of ABVD are highly predictive.
The team enrolled 1214 patients from 6 countries, 861 of whom were in the UK. Patients received a PET scan at staging, 2 cycles of ABVD, and then a second PET scan (PET2).
If patients were negative after PET2, they were randomized to receive 4 more cycles of ABVD or AVD and no radiotherapy.
If they were positive after PET2, patients received 4 cycles of BEACOPP-14 or 3 cycles of escalated BEACOPP. These patients then received a third PET scan, and the positive patients went on to receive radiotherapy or a salvage regimen.
The PET3-negative patients received 2 more cycles of BEACOPP-14 or one of escalated BEACOPP without radiotherapy.
Peter W. Johnson, MD, of the University of Southampton in the UK, presented the results of these treatment regimens during the plenary session of 13-ICML as abstract 008.
Patient characteristics
Patients were a median age of 33 (range, 18-79), and 55% were male. They had disease stages of II (41%), III (31%), or IV (28%).
Seventy-four percent of patients had a performance status of 0. Almost half (49%) had an IPS score of 2 to 3, and 18% had an IPS score of 4 or more. Thirty-two percent had bulky disease.
Investigators followed the patients for a median of 34.7 months (range, 1 day to 68.2 months).
Results after PET2
Seventy-seven patients were missing a second PET scan, mostly due to PET protocol violations of having to use the same scanner for the baseline and second scan and the same acquisition time.
“We were very strict on our quality control,” Dr Johnson said, “because we wished to make sure this was reproducible data.”
So the results after 2 cycles of ABVD treatment were based on 1137 patients.
PET-negative patients
More than 80% of patients were PET-negative after 2 cycles. Four hundred and sixty-nine patients were randomized to receive ABVD and 466 to AVD.
The groups were well-balanced in terms of median age, performance status, stage, B symptoms, bulky disease, and IPS score.
There was a significant excess of neutropenic fever (P=0.032) and infection (P=0.040) in those patients continuing on ABVD compared to AVD. And any hematologic toxicity was highly significantly different between the 2 arms (P<0.001).
“So we have demonstrated that continuing with bleomycin beyond cycle 2 is accompanied by significantly more toxicity,” Dr Johnson said.
Ninety-eight percent of patients in both cohorts received at least 6 cycles of therapy post-randomization.
At a median follow-up of 36.3 months, 65% of patients in the ABVD arm and 69% in the AVD arm achieved a complete remission (CR) or unconfirmed CR (CRu).
Fourteen patients died in each of the arms. Seven patients died of their disease in the AVD arm, compared with 1 in the ABVD arm. Slightly more patients died from toxicity in the ABVD arm.
The primary endpoint of PFS showed very little difference between the 2 arms. The 3-year PFS in the intent-to-treat analysis was 85.4% for patients in the ABVD arm and 84.4% for those in the AVD arm.
The investigators observed that the PFS of 85% was somewhat lower than the 95% PFS observed in the literature. So they looked at the association between baseline factors and PFS after negative PET2.
“And what stands out from this is that if you have high-stage disease at presentation, there is a slightly higher chance of treatment failure following a negative PET scan,” Dr Johnson said. “And you can see the trend here, from early stage disease up to advanced-stage disease, the PET scan becomes a less reliable indicator of result.”
The investigators also conducted a subgroup analysis of the PET2-negative patients and found there was no difference in outcome between treatment arms in patients with more advanced disease, with bulky disease, with a high IPS score, or according to the PET score.
“So we have not succeeded in finding any subgroup where it appears to be beneficial to continue bleomycin,” Dr Johnson said.
The OS rate was also the same between the 2 arms, at 97%.
PET2-positive patients
One hundred and seventy-four patients who were positive after the second PET scan received either BEACOPP for 14 days or escalated BEACOPP.
The percentage of patients who experienced grade 3-4 toxicities was largely similar between the 2 regimens, although the patients receiving escalated BEACOPP had more neutropenia (P=0.057), thrombocytopenia (P=0.001), and neutropenic fever (P=0.08).
In terms of efficacy, two-thirds of patients became PET-negative by the third PET scan, and 48% of patients achieved a CR or CRu.
Twenty-one patients died, 8 due to Hodgkin lymphoma.
The PFS was 66.0% in the BEACOPP-14 group and 71.1% in the escalated-BEACOPP group. The 3-year OS was 89.6% in the BEACOPP-14 group and 82.8% in the escalated-BEACOPP group.
For the entire group of 1214 patients, the 3-year PFS was 82.5%, and the OS was 95.4%.
Based on these results, the investigators concluded that it is safe to omit bleomycin and consolidation radiotherapy from subsequent ABVD therapy after a negative interim PET scan. And doing so reduces toxicity, especially dyspnea, thromboembolism, and neutropenic fever.
“[B]y using more selective chemotherapy and much less radiotherapy than we have previously used in our studies, where we’re giving less than 3% of patients consolidation radiotherapy, the results appear to be favorable and an improvement over what we have seen previously,” Dr Johnson said.
Details on lung toxicity in this study were presented separately at 13-ICML as abstract 041. ![]()
Combo shows promise for heavily pretreated MM

Image by Louis Heiser
and Robert Ackland
VIENNA—Combining a novel agent with dexamethasone can produce successful results where other treatments have failed, according to a presentation at the 20th Congress of the European Hematology Association.
Researchers tested low-dose dexamethasone in combination with melflufen, a peptidase-targeted therapy and antiangiogenic compound, in a phase 1/2 study of patients with relapsed or relapsed-refractory multiple myeloma (MM).
The treatment produced an overall response rate of 52%, and, although grade 3/4 hematologic adverse events were common, there were few serious adverse events related to melflufen.
These results were presented at the meeting as abstract P285.* The research was sponsored by Oncopeptides AB, the company developing melflufen.
Drug dosing and patient characteristics
In the phase 1 portion of this study, researchers evaluated 4 dose levels of melflufen—15 mg, 25 mg, 40 mg, and 55 mg—on day 1 with 40 mg of dexamethasone on days 1, 8, and 15 of 21-day cycles in a standard 3+3 design. Eight cycles of therapy were planned, but patients could continue on treatment if they experienced a clinical benefit.
There were no-dose limiting toxicities (DLTs) when melflufen was given at the 3 lower doses. However, 4 of the 6 patients who received the 55 mg dose experienced DLTs of prolonged and severe neutropenia and thrombocytopenia.
So the researchers said the MTD of melflufen, when combined with 40 mg of weekly dexamethasone, was 40 mg every 21 days.
In the ongoing phase 2 portion of the study, 29 patients have received melflufen at the MTD. The median time from the patients’ MM diagnosis to the first dose of melflufen was 5.5 years (range, 1-15), and their median number of prior therapies was 4 (range, 2-11).
Nineteen of the patients were refractory to an immunomodulatory drug (IMiD) or a proteasome inhibitor (PI); 11 were refractory to an alkylator; 10 were refractory to both an IMiD and a PI; and 5 were refractory to an IMiD, a PI, and an alkylator.
Safety data
Among the 29 patients in the phase 2 portion of the study, 22 had treatment-related grade 3-4 adverse events. These included thrombocytopenia (59%), neutropenia (48%), anemia (31%), leukopenia (21%), asthenia (7%), fatigue (7%), hyperglycemia (7%), and pyrexia/fever (7%).
To date, 12 serious adverse events have been reported in 8 of the phase 2 patients. Three events in 3 patients were considered related to melflufen—2 cases of febrile neutropenia and 1 case of pyrexia.
Fifteen patients discontinued therapy, 8 due to adverse events, 6 due to disease progression, and 1 after completing all planned cycles of treatment.
Efficacy data
Twenty-one patients were evaluable for efficacy according to the protocol, meaning they had received 2 or more cycles of therapy and completed response assessments after cycle 2.
Four patients withdrew from treatment after 1 cycle due to rapid disease progression and were included in a second response assessment (n=25). It was too early to evaluate the remaining 4 patients.
Among the 21 protocol-evaluable patients, the overall response rate was 52%, and the clinical benefit rate was 67%. Eleven patients achieved a partial response, 3 had a minimal response, 6 had stable disease, and 1 had progressive disease.
Among all 25 evaluable patients, the overall response rate was 44%, and the clinical benefit rate was 56%.
The median progression-free survival for these patients was 7.6 months (range, 3.4 months to not reached).
The researchers said enrollment in this trial is ongoing, with the goal of reaching 55 patients to further characterize the safety and efficacy of melflufen in heavily pretreated MM patients. ![]()
*Information in the abstract differs from that presented at the meeting.

Image by Louis Heiser
and Robert Ackland
VIENNA—Combining a novel agent with dexamethasone can produce successful results where other treatments have failed, according to a presentation at the 20th Congress of the European Hematology Association.
Researchers tested low-dose dexamethasone in combination with melflufen, a peptidase-targeted therapy and antiangiogenic compound, in a phase 1/2 study of patients with relapsed or relapsed-refractory multiple myeloma (MM).
The treatment produced an overall response rate of 52%, and, although grade 3/4 hematologic adverse events were common, there were few serious adverse events related to melflufen.
These results were presented at the meeting as abstract P285.* The research was sponsored by Oncopeptides AB, the company developing melflufen.
Drug dosing and patient characteristics
In the phase 1 portion of this study, researchers evaluated 4 dose levels of melflufen—15 mg, 25 mg, 40 mg, and 55 mg—on day 1 with 40 mg of dexamethasone on days 1, 8, and 15 of 21-day cycles in a standard 3+3 design. Eight cycles of therapy were planned, but patients could continue on treatment if they experienced a clinical benefit.
There were no-dose limiting toxicities (DLTs) when melflufen was given at the 3 lower doses. However, 4 of the 6 patients who received the 55 mg dose experienced DLTs of prolonged and severe neutropenia and thrombocytopenia.
So the researchers said the MTD of melflufen, when combined with 40 mg of weekly dexamethasone, was 40 mg every 21 days.
In the ongoing phase 2 portion of the study, 29 patients have received melflufen at the MTD. The median time from the patients’ MM diagnosis to the first dose of melflufen was 5.5 years (range, 1-15), and their median number of prior therapies was 4 (range, 2-11).
Nineteen of the patients were refractory to an immunomodulatory drug (IMiD) or a proteasome inhibitor (PI); 11 were refractory to an alkylator; 10 were refractory to both an IMiD and a PI; and 5 were refractory to an IMiD, a PI, and an alkylator.
Safety data
Among the 29 patients in the phase 2 portion of the study, 22 had treatment-related grade 3-4 adverse events. These included thrombocytopenia (59%), neutropenia (48%), anemia (31%), leukopenia (21%), asthenia (7%), fatigue (7%), hyperglycemia (7%), and pyrexia/fever (7%).
To date, 12 serious adverse events have been reported in 8 of the phase 2 patients. Three events in 3 patients were considered related to melflufen—2 cases of febrile neutropenia and 1 case of pyrexia.
Fifteen patients discontinued therapy, 8 due to adverse events, 6 due to disease progression, and 1 after completing all planned cycles of treatment.
Efficacy data
Twenty-one patients were evaluable for efficacy according to the protocol, meaning they had received 2 or more cycles of therapy and completed response assessments after cycle 2.
Four patients withdrew from treatment after 1 cycle due to rapid disease progression and were included in a second response assessment (n=25). It was too early to evaluate the remaining 4 patients.
Among the 21 protocol-evaluable patients, the overall response rate was 52%, and the clinical benefit rate was 67%. Eleven patients achieved a partial response, 3 had a minimal response, 6 had stable disease, and 1 had progressive disease.
Among all 25 evaluable patients, the overall response rate was 44%, and the clinical benefit rate was 56%.
The median progression-free survival for these patients was 7.6 months (range, 3.4 months to not reached).
The researchers said enrollment in this trial is ongoing, with the goal of reaching 55 patients to further characterize the safety and efficacy of melflufen in heavily pretreated MM patients. ![]()
*Information in the abstract differs from that presented at the meeting.

Image by Louis Heiser
and Robert Ackland
VIENNA—Combining a novel agent with dexamethasone can produce successful results where other treatments have failed, according to a presentation at the 20th Congress of the European Hematology Association.
Researchers tested low-dose dexamethasone in combination with melflufen, a peptidase-targeted therapy and antiangiogenic compound, in a phase 1/2 study of patients with relapsed or relapsed-refractory multiple myeloma (MM).
The treatment produced an overall response rate of 52%, and, although grade 3/4 hematologic adverse events were common, there were few serious adverse events related to melflufen.
These results were presented at the meeting as abstract P285.* The research was sponsored by Oncopeptides AB, the company developing melflufen.
Drug dosing and patient characteristics
In the phase 1 portion of this study, researchers evaluated 4 dose levels of melflufen—15 mg, 25 mg, 40 mg, and 55 mg—on day 1 with 40 mg of dexamethasone on days 1, 8, and 15 of 21-day cycles in a standard 3+3 design. Eight cycles of therapy were planned, but patients could continue on treatment if they experienced a clinical benefit.
There were no-dose limiting toxicities (DLTs) when melflufen was given at the 3 lower doses. However, 4 of the 6 patients who received the 55 mg dose experienced DLTs of prolonged and severe neutropenia and thrombocytopenia.
So the researchers said the MTD of melflufen, when combined with 40 mg of weekly dexamethasone, was 40 mg every 21 days.
In the ongoing phase 2 portion of the study, 29 patients have received melflufen at the MTD. The median time from the patients’ MM diagnosis to the first dose of melflufen was 5.5 years (range, 1-15), and their median number of prior therapies was 4 (range, 2-11).
Nineteen of the patients were refractory to an immunomodulatory drug (IMiD) or a proteasome inhibitor (PI); 11 were refractory to an alkylator; 10 were refractory to both an IMiD and a PI; and 5 were refractory to an IMiD, a PI, and an alkylator.
Safety data
Among the 29 patients in the phase 2 portion of the study, 22 had treatment-related grade 3-4 adverse events. These included thrombocytopenia (59%), neutropenia (48%), anemia (31%), leukopenia (21%), asthenia (7%), fatigue (7%), hyperglycemia (7%), and pyrexia/fever (7%).
To date, 12 serious adverse events have been reported in 8 of the phase 2 patients. Three events in 3 patients were considered related to melflufen—2 cases of febrile neutropenia and 1 case of pyrexia.
Fifteen patients discontinued therapy, 8 due to adverse events, 6 due to disease progression, and 1 after completing all planned cycles of treatment.
Efficacy data
Twenty-one patients were evaluable for efficacy according to the protocol, meaning they had received 2 or more cycles of therapy and completed response assessments after cycle 2.
Four patients withdrew from treatment after 1 cycle due to rapid disease progression and were included in a second response assessment (n=25). It was too early to evaluate the remaining 4 patients.
Among the 21 protocol-evaluable patients, the overall response rate was 52%, and the clinical benefit rate was 67%. Eleven patients achieved a partial response, 3 had a minimal response, 6 had stable disease, and 1 had progressive disease.
Among all 25 evaluable patients, the overall response rate was 44%, and the clinical benefit rate was 56%.
The median progression-free survival for these patients was 7.6 months (range, 3.4 months to not reached).
The researchers said enrollment in this trial is ongoing, with the goal of reaching 55 patients to further characterize the safety and efficacy of melflufen in heavily pretreated MM patients. ![]()
*Information in the abstract differs from that presented at the meeting.
FDA approves antiplatelet drug for PCI patients
Photo courtesy of
The Medicines Company
The US Food and Drug Administration (FDA) has approved the intravenous antiplatelet agent cangrelor (Kengreal) for use in adults undergoing percutaneous coronary intervention (PCI).
The drug can now be used to reduce periprocedural thrombotic events in patients who have not been treated with a P2Y12 inhibitor and are not receiving a glycoprotein IIb/IIIa inhibitor.
The FDA’s approval of cangrelor was based on results of the CHAMPION PHOENIX trial.
In this phase 3 trial, researchers compared cangrelor to clopidogrel in 11,145 patients undergoing PCI.
The study’s primary efficacy endpoint was the incidence of death, myocardial infarction, ischemia-driven revascularization, or stent thrombosis.
At 48 hours, 4.7% of patients in the cangrelor arm had met this endpoint, compared to 5.9% of patients in the clopidogrel arm (P=0.005). At 30 days, the incidence was 6.0% in the cangrelor arm and 7.0% in the clopidogrel arm (P=0.03).
The study’s primary safety endpoint was severe bleeding according to GUSTO criteria. At 48 hours, major bleeding had occurred in 0.16% of patients in the cangrelor arm and 0.11% of patients in the clopidogrel arm (P=0.44).
Major bleeding occurred in 4.3% of patients on cangrelor and 2.5% of patients on clopidogrel (P<0.001). And minor bleeding occurred in 11.8% of patients on cangrelor and 8.6% of patients on clopidogrel (P<0.001).
Cangrelor is manufactured by The Medicines Company, which is based in Parsippany, New Jersey. ![]()

Photo courtesy of
The Medicines Company
The US Food and Drug Administration (FDA) has approved the intravenous antiplatelet agent cangrelor (Kengreal) for use in adults undergoing percutaneous coronary intervention (PCI).
The drug can now be used to reduce periprocedural thrombotic events in patients who have not been treated with a P2Y12 inhibitor and are not receiving a glycoprotein IIb/IIIa inhibitor.
The FDA’s approval of cangrelor was based on results of the CHAMPION PHOENIX trial.
In this phase 3 trial, researchers compared cangrelor to clopidogrel in 11,145 patients undergoing PCI.
The study’s primary efficacy endpoint was the incidence of death, myocardial infarction, ischemia-driven revascularization, or stent thrombosis.
At 48 hours, 4.7% of patients in the cangrelor arm had met this endpoint, compared to 5.9% of patients in the clopidogrel arm (P=0.005). At 30 days, the incidence was 6.0% in the cangrelor arm and 7.0% in the clopidogrel arm (P=0.03).
The study’s primary safety endpoint was severe bleeding according to GUSTO criteria. At 48 hours, major bleeding had occurred in 0.16% of patients in the cangrelor arm and 0.11% of patients in the clopidogrel arm (P=0.44).
Major bleeding occurred in 4.3% of patients on cangrelor and 2.5% of patients on clopidogrel (P<0.001). And minor bleeding occurred in 11.8% of patients on cangrelor and 8.6% of patients on clopidogrel (P<0.001).
Cangrelor is manufactured by The Medicines Company, which is based in Parsippany, New Jersey. ![]()

Photo courtesy of
The Medicines Company
The US Food and Drug Administration (FDA) has approved the intravenous antiplatelet agent cangrelor (Kengreal) for use in adults undergoing percutaneous coronary intervention (PCI).
The drug can now be used to reduce periprocedural thrombotic events in patients who have not been treated with a P2Y12 inhibitor and are not receiving a glycoprotein IIb/IIIa inhibitor.
The FDA’s approval of cangrelor was based on results of the CHAMPION PHOENIX trial.
In this phase 3 trial, researchers compared cangrelor to clopidogrel in 11,145 patients undergoing PCI.
The study’s primary efficacy endpoint was the incidence of death, myocardial infarction, ischemia-driven revascularization, or stent thrombosis.
At 48 hours, 4.7% of patients in the cangrelor arm had met this endpoint, compared to 5.9% of patients in the clopidogrel arm (P=0.005). At 30 days, the incidence was 6.0% in the cangrelor arm and 7.0% in the clopidogrel arm (P=0.03).
The study’s primary safety endpoint was severe bleeding according to GUSTO criteria. At 48 hours, major bleeding had occurred in 0.16% of patients in the cangrelor arm and 0.11% of patients in the clopidogrel arm (P=0.44).
Major bleeding occurred in 4.3% of patients on cangrelor and 2.5% of patients on clopidogrel (P<0.001). And minor bleeding occurred in 11.8% of patients on cangrelor and 8.6% of patients on clopidogrel (P<0.001).
Cangrelor is manufactured by The Medicines Company, which is based in Parsippany, New Jersey. ![]()
Perioperative factors influenced open TAAA repair
Open thoracoabdominal aortic aneurysm (TAAA) repair produced respectable early outcomes, although preoperative and intraoperative factors were found to influence risk, according to Dr. Joseph S. Coselli, who presented the results of the study he and his colleagues at the Baylor College of Medicine in Houston performed at the annual meeting of the American Association for Thoracic Surgery.
They analyzed data from 3,309 open TAAA repairs performed between October 1986 and December 2014.
“I have been very fortunate to have spent my entire career at Baylor College of Medicine, the epicenter of aortic surgery in the 1950s, ’60s, and ’70s, as well as to have been mentored by Dr. E. Stanley Crawford, who was arguably the finest aortic surgeon of his era. Since transitioning from Dr. Crawford’s surgical practice to my own surgical practice, we have kept his pioneering spirit alive by developing a multimodal strategy for thoracoabdominal aortic aneurysm repair that is based on the Crawford extent of repair and our evolving investigation. We sought to describe our series of over 3,000 TAAA repairs and to identify predictors of early death and other adverse postoperative outcomes,” said Dr. Coselli.
The median patient age was around 67 years, and the repairs involved acute or subacute aortic dissection in about 5% of the cases. Nearly 31% of the case involved chronic dissection, with nearly 22% emergent or urgent repairs and around 5% ruptured aneurysms. Connective tissue disorders were present in roughly 10% of patients. “Operatively, we tend to reserve surgical adjuncts for use in the most-extensive repairs, namely extents I and II TAAA repair; intercostal or lumbar artery reattachment was used in just over half of the repairs, left heart bypass (LHB) was used in around 45% of patients, cold renal perfusion was performed in 58%. and cerebrospinal fluid drainage (CSFD) was used in 45%,” said Dr. Coselli.
There was substantial atherosclerotic disease in older patients, and in nearly 41% of repairs, a visceral vessel procedure was performed.
Unlike many aortic centers that routinely use deep hypothermic circulatory arrest (HCA) for extensive TAAA repair, Dr. Coselli reserved this approach for a small number of highly complex repairs (1.4%) in which the aorta could not be safely clamped.
Of the more than a thousand most extensive (i.e., Crawford extent II) repairs, intercostal/lumbar artery reattachment was used in the vast majority (88%), LHB in 82%, and CSFD in 61%. They used multivariable analysis to identify predictors of operative (30-day or in-hospital) mortality and adverse event, a composite outcome comprising operative death and permanent (present at discharge) spinal cord deficit, renal failure, or stroke, according to Dr. Coselli.
Their results showed an operative mortality rate of 7.5%, a 30-day death rate of 4.8%, with the adverse event outcome occurring in about 14% of repairs. A video of his presentation is available at the AATS website.
The statistically significant predictors of operative death were rupture; renal insufficiency, symptoms, procedures targeting visceral vessels, increasing age, and increasing clamp time, while extent IV repair (the least extensive form of TAAA repair) was inversely associated with death. Their analysis showed that the significant predictors of adverse event were use of HCA, renal insufficiency, rupture, extent II repair, visceral vessel procedures, urgent or emergent repair, increasing age, and increasing clamp time. In addition, they used multivariable analysis to identify predictors of renal failure and paraplegia.
In the 3,060 early survivors, roughly 7% had a life-altering complication at discharge: Nearly 3% of patients had renal failure necessitating dialysis, slightly more than 1% had a unresolved stroke, and about 4% had unresolved paraplegia or paraparesis. Repair failure, primarily pseudoaneurysm, or patch aneurysm, occurred after nearly 3% of repairs, said Dr. Coselli.
Outcomes differed by extent of repair, with the risk being greatest in extent II repair. Actuarial survival was 63.6% at 5 years, 36.8% at 10 years, and 18.3% at 15 years. Freedom from repair failure was nearly 98% at 5 years, around 95% at 10 years, and 94% at 15 years.
“Along with respectable early outcomes, after repair, patients have acceptable long-term survival, and late repair failure was uncommon. Notably, there are several subgroups of patients that do exceedingly well. Paraplegia in young patients with connective tissue disorders, even in the most-extensive repair (extent II), is remarkably rare – these patients do extremely well across the board,” he concluded.
Dr. Cosselli reported that he is a principal investigator and consultant for Medtronic and W.L. Gore & Assoc., as well as being a principal investigator, consultant, and having various financial relationships with Vascutek.
Open thoracoabdominal aortic aneurysm (TAAA) repair produced respectable early outcomes, although preoperative and intraoperative factors were found to influence risk, according to Dr. Joseph S. Coselli, who presented the results of the study he and his colleagues at the Baylor College of Medicine in Houston performed at the annual meeting of the American Association for Thoracic Surgery.
They analyzed data from 3,309 open TAAA repairs performed between October 1986 and December 2014.
“I have been very fortunate to have spent my entire career at Baylor College of Medicine, the epicenter of aortic surgery in the 1950s, ’60s, and ’70s, as well as to have been mentored by Dr. E. Stanley Crawford, who was arguably the finest aortic surgeon of his era. Since transitioning from Dr. Crawford’s surgical practice to my own surgical practice, we have kept his pioneering spirit alive by developing a multimodal strategy for thoracoabdominal aortic aneurysm repair that is based on the Crawford extent of repair and our evolving investigation. We sought to describe our series of over 3,000 TAAA repairs and to identify predictors of early death and other adverse postoperative outcomes,” said Dr. Coselli.
The median patient age was around 67 years, and the repairs involved acute or subacute aortic dissection in about 5% of the cases. Nearly 31% of the case involved chronic dissection, with nearly 22% emergent or urgent repairs and around 5% ruptured aneurysms. Connective tissue disorders were present in roughly 10% of patients. “Operatively, we tend to reserve surgical adjuncts for use in the most-extensive repairs, namely extents I and II TAAA repair; intercostal or lumbar artery reattachment was used in just over half of the repairs, left heart bypass (LHB) was used in around 45% of patients, cold renal perfusion was performed in 58%. and cerebrospinal fluid drainage (CSFD) was used in 45%,” said Dr. Coselli.
There was substantial atherosclerotic disease in older patients, and in nearly 41% of repairs, a visceral vessel procedure was performed.
Unlike many aortic centers that routinely use deep hypothermic circulatory arrest (HCA) for extensive TAAA repair, Dr. Coselli reserved this approach for a small number of highly complex repairs (1.4%) in which the aorta could not be safely clamped.
Of the more than a thousand most extensive (i.e., Crawford extent II) repairs, intercostal/lumbar artery reattachment was used in the vast majority (88%), LHB in 82%, and CSFD in 61%. They used multivariable analysis to identify predictors of operative (30-day or in-hospital) mortality and adverse event, a composite outcome comprising operative death and permanent (present at discharge) spinal cord deficit, renal failure, or stroke, according to Dr. Coselli.
Their results showed an operative mortality rate of 7.5%, a 30-day death rate of 4.8%, with the adverse event outcome occurring in about 14% of repairs. A video of his presentation is available at the AATS website.
The statistically significant predictors of operative death were rupture; renal insufficiency, symptoms, procedures targeting visceral vessels, increasing age, and increasing clamp time, while extent IV repair (the least extensive form of TAAA repair) was inversely associated with death. Their analysis showed that the significant predictors of adverse event were use of HCA, renal insufficiency, rupture, extent II repair, visceral vessel procedures, urgent or emergent repair, increasing age, and increasing clamp time. In addition, they used multivariable analysis to identify predictors of renal failure and paraplegia.
In the 3,060 early survivors, roughly 7% had a life-altering complication at discharge: Nearly 3% of patients had renal failure necessitating dialysis, slightly more than 1% had a unresolved stroke, and about 4% had unresolved paraplegia or paraparesis. Repair failure, primarily pseudoaneurysm, or patch aneurysm, occurred after nearly 3% of repairs, said Dr. Coselli.
Outcomes differed by extent of repair, with the risk being greatest in extent II repair. Actuarial survival was 63.6% at 5 years, 36.8% at 10 years, and 18.3% at 15 years. Freedom from repair failure was nearly 98% at 5 years, around 95% at 10 years, and 94% at 15 years.
“Along with respectable early outcomes, after repair, patients have acceptable long-term survival, and late repair failure was uncommon. Notably, there are several subgroups of patients that do exceedingly well. Paraplegia in young patients with connective tissue disorders, even in the most-extensive repair (extent II), is remarkably rare – these patients do extremely well across the board,” he concluded.
Dr. Cosselli reported that he is a principal investigator and consultant for Medtronic and W.L. Gore & Assoc., as well as being a principal investigator, consultant, and having various financial relationships with Vascutek.
Open thoracoabdominal aortic aneurysm (TAAA) repair produced respectable early outcomes, although preoperative and intraoperative factors were found to influence risk, according to Dr. Joseph S. Coselli, who presented the results of the study he and his colleagues at the Baylor College of Medicine in Houston performed at the annual meeting of the American Association for Thoracic Surgery.
They analyzed data from 3,309 open TAAA repairs performed between October 1986 and December 2014.
“I have been very fortunate to have spent my entire career at Baylor College of Medicine, the epicenter of aortic surgery in the 1950s, ’60s, and ’70s, as well as to have been mentored by Dr. E. Stanley Crawford, who was arguably the finest aortic surgeon of his era. Since transitioning from Dr. Crawford’s surgical practice to my own surgical practice, we have kept his pioneering spirit alive by developing a multimodal strategy for thoracoabdominal aortic aneurysm repair that is based on the Crawford extent of repair and our evolving investigation. We sought to describe our series of over 3,000 TAAA repairs and to identify predictors of early death and other adverse postoperative outcomes,” said Dr. Coselli.
The median patient age was around 67 years, and the repairs involved acute or subacute aortic dissection in about 5% of the cases. Nearly 31% of the case involved chronic dissection, with nearly 22% emergent or urgent repairs and around 5% ruptured aneurysms. Connective tissue disorders were present in roughly 10% of patients. “Operatively, we tend to reserve surgical adjuncts for use in the most-extensive repairs, namely extents I and II TAAA repair; intercostal or lumbar artery reattachment was used in just over half of the repairs, left heart bypass (LHB) was used in around 45% of patients, cold renal perfusion was performed in 58%. and cerebrospinal fluid drainage (CSFD) was used in 45%,” said Dr. Coselli.
There was substantial atherosclerotic disease in older patients, and in nearly 41% of repairs, a visceral vessel procedure was performed.
Unlike many aortic centers that routinely use deep hypothermic circulatory arrest (HCA) for extensive TAAA repair, Dr. Coselli reserved this approach for a small number of highly complex repairs (1.4%) in which the aorta could not be safely clamped.
Of the more than a thousand most extensive (i.e., Crawford extent II) repairs, intercostal/lumbar artery reattachment was used in the vast majority (88%), LHB in 82%, and CSFD in 61%. They used multivariable analysis to identify predictors of operative (30-day or in-hospital) mortality and adverse event, a composite outcome comprising operative death and permanent (present at discharge) spinal cord deficit, renal failure, or stroke, according to Dr. Coselli.
Their results showed an operative mortality rate of 7.5%, a 30-day death rate of 4.8%, with the adverse event outcome occurring in about 14% of repairs. A video of his presentation is available at the AATS website.
The statistically significant predictors of operative death were rupture; renal insufficiency, symptoms, procedures targeting visceral vessels, increasing age, and increasing clamp time, while extent IV repair (the least extensive form of TAAA repair) was inversely associated with death. Their analysis showed that the significant predictors of adverse event were use of HCA, renal insufficiency, rupture, extent II repair, visceral vessel procedures, urgent or emergent repair, increasing age, and increasing clamp time. In addition, they used multivariable analysis to identify predictors of renal failure and paraplegia.
In the 3,060 early survivors, roughly 7% had a life-altering complication at discharge: Nearly 3% of patients had renal failure necessitating dialysis, slightly more than 1% had a unresolved stroke, and about 4% had unresolved paraplegia or paraparesis. Repair failure, primarily pseudoaneurysm, or patch aneurysm, occurred after nearly 3% of repairs, said Dr. Coselli.
Outcomes differed by extent of repair, with the risk being greatest in extent II repair. Actuarial survival was 63.6% at 5 years, 36.8% at 10 years, and 18.3% at 15 years. Freedom from repair failure was nearly 98% at 5 years, around 95% at 10 years, and 94% at 15 years.
“Along with respectable early outcomes, after repair, patients have acceptable long-term survival, and late repair failure was uncommon. Notably, there are several subgroups of patients that do exceedingly well. Paraplegia in young patients with connective tissue disorders, even in the most-extensive repair (extent II), is remarkably rare – these patients do extremely well across the board,” he concluded.
Dr. Cosselli reported that he is a principal investigator and consultant for Medtronic and W.L. Gore & Assoc., as well as being a principal investigator, consultant, and having various financial relationships with Vascutek.
AT THE AATS ANNUAL MEETING
Weight in America: Abnormal is the new normal
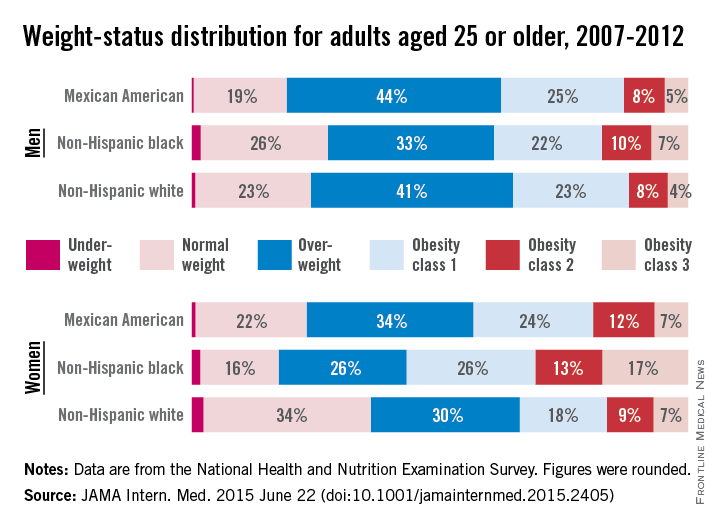
Three-quarters of men and two-thirds of women aged 25 years or older in the United States are either overweight or obese, according to a study published online June 22 in JAMA Internal Medicine.
During 2007-2012, about 40% of men were overweight and 35% were obese. For women, almost 30% were overweight and 37% were obese, Lin Yang, Ph.D., and Dr. Graham A. Colditz of Washington University, St. Louis, reported in a research letter (JAMA Intern. Med. 2015 June 22 [doi:10.1001/jamainternmed.2015.2405]).
Weight-status distributions across racial groups show that non-Hispanic whites were generally less obese than non-Hispanic blacks and Mexican Americans – an effect that was more pronounced among women. About 34% of white women were obese, compared with 57% of black women, with over 17% occupying the heaviest of three obesity levels, according to the investigators’ analysis of the most recent data from the National Health and Nutrition Examination Survey.
Weight groups in the study were defined by body mass index: underweight (< 18.5 kg/m2), normal weight (18.5-24.9), overweight (25.0-29.9), obesity class 1 (30.0-34.9), obesity class 2 (35.0-39.9), and obesity class 3 (≥ 40).
Compared with an earlier study reporting data for 1988-1994, “the greatest increase in the proportion of patients in the obesity class 3 category was among non-Hispanic black women,” Dr. Yang and Dr. Colditz wrote. Health and policy decision-makers should consider “population-based strategies helping to reduce modifiable risk factors such as physical environment interventions, enhancing primary care efforts to prevent and treat obesity, and altering societal norms of behavior,” they added.
The study was funded by the Washington University Transdiscliplinary Research on Energetics and Cancer Center, the Foundation for Barnes-Jewish Hospital, and the Breast Cancer Research Foundation. The investigators did not report any conflicts.
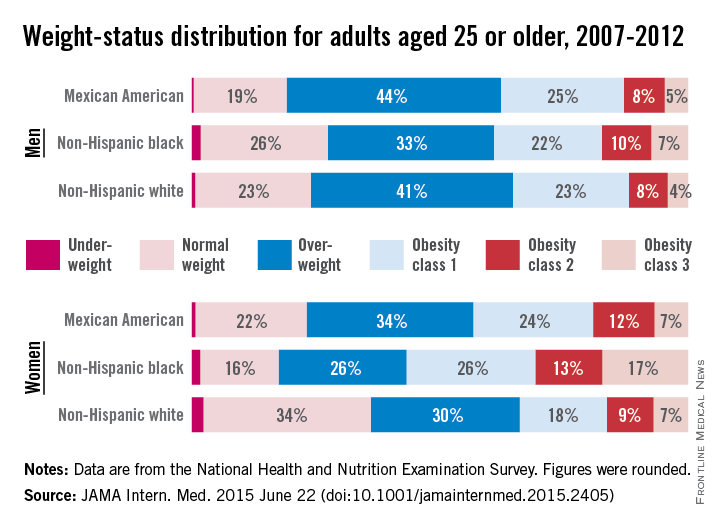
Three-quarters of men and two-thirds of women aged 25 years or older in the United States are either overweight or obese, according to a study published online June 22 in JAMA Internal Medicine.
During 2007-2012, about 40% of men were overweight and 35% were obese. For women, almost 30% were overweight and 37% were obese, Lin Yang, Ph.D., and Dr. Graham A. Colditz of Washington University, St. Louis, reported in a research letter (JAMA Intern. Med. 2015 June 22 [doi:10.1001/jamainternmed.2015.2405]).
Weight-status distributions across racial groups show that non-Hispanic whites were generally less obese than non-Hispanic blacks and Mexican Americans – an effect that was more pronounced among women. About 34% of white women were obese, compared with 57% of black women, with over 17% occupying the heaviest of three obesity levels, according to the investigators’ analysis of the most recent data from the National Health and Nutrition Examination Survey.
Weight groups in the study were defined by body mass index: underweight (< 18.5 kg/m2), normal weight (18.5-24.9), overweight (25.0-29.9), obesity class 1 (30.0-34.9), obesity class 2 (35.0-39.9), and obesity class 3 (≥ 40).
Compared with an earlier study reporting data for 1988-1994, “the greatest increase in the proportion of patients in the obesity class 3 category was among non-Hispanic black women,” Dr. Yang and Dr. Colditz wrote. Health and policy decision-makers should consider “population-based strategies helping to reduce modifiable risk factors such as physical environment interventions, enhancing primary care efforts to prevent and treat obesity, and altering societal norms of behavior,” they added.
The study was funded by the Washington University Transdiscliplinary Research on Energetics and Cancer Center, the Foundation for Barnes-Jewish Hospital, and the Breast Cancer Research Foundation. The investigators did not report any conflicts.

Three-quarters of men and two-thirds of women aged 25 years or older in the United States are either overweight or obese, according to a study published online June 22 in JAMA Internal Medicine.
During 2007-2012, about 40% of men were overweight and 35% were obese. For women, almost 30% were overweight and 37% were obese, Lin Yang, Ph.D., and Dr. Graham A. Colditz of Washington University, St. Louis, reported in a research letter (JAMA Intern. Med. 2015 June 22 [doi:10.1001/jamainternmed.2015.2405]).
Weight-status distributions across racial groups show that non-Hispanic whites were generally less obese than non-Hispanic blacks and Mexican Americans – an effect that was more pronounced among women. About 34% of white women were obese, compared with 57% of black women, with over 17% occupying the heaviest of three obesity levels, according to the investigators’ analysis of the most recent data from the National Health and Nutrition Examination Survey.
Weight groups in the study were defined by body mass index: underweight (< 18.5 kg/m2), normal weight (18.5-24.9), overweight (25.0-29.9), obesity class 1 (30.0-34.9), obesity class 2 (35.0-39.9), and obesity class 3 (≥ 40).
Compared with an earlier study reporting data for 1988-1994, “the greatest increase in the proportion of patients in the obesity class 3 category was among non-Hispanic black women,” Dr. Yang and Dr. Colditz wrote. Health and policy decision-makers should consider “population-based strategies helping to reduce modifiable risk factors such as physical environment interventions, enhancing primary care efforts to prevent and treat obesity, and altering societal norms of behavior,” they added.
The study was funded by the Washington University Transdiscliplinary Research on Energetics and Cancer Center, the Foundation for Barnes-Jewish Hospital, and the Breast Cancer Research Foundation. The investigators did not report any conflicts.

FROM JAMA INTERNAL MEDICINE
Activin receptors continue to show efficacy in ß-thalassemia

Congress of the European
Hematology Association
VIENNA—Two very similar activin receptors, luspatercept and sotatercept, continue to show efficacy in patients with ß-thalassemia, according to research presented at the 20th Congress of the European Hematology Association (EHA).
The “twin compounds” basically differ from each other in the receptor type, but both can increase hemoglobin levels in non-transfusion-dependent (NTD) patients and reduce transfusion burden in transfusion-dependent (TD) patients.
Luspatercept is a recombinant fusion protein containing a modified extracellular domain of the activin receptor type IIB, while sotatercept is an activin receptor type IIA.
Antonio Piga, MD, of Turin University in Italy, presented the most recent results with luspatercept at EHA as abstract S136*. An update on sotatercept was also presented at the meeting.
Acceleron Pharma Inc. and Celgene Corporation are jointly developing both compounds.
The phase 2, multicenter, open-label trial of luspatercept now has results on 39 patients, 35 of whom were in the dose-escalation cohorts and 4 who are in the expansion cohort that is currently underway.
Adult patients age 18 and older received luspatercept subcutaneously every 3 weeks for 3 months. Doses in the dose-escalation cohorts increased from 0.2 mg/kg to 1.25 mg/kg.
Patients in the expansion cohort received a starting dose of 0.8 mg/kg, with an individual dose titration up to 1.25 mg/kg, for an additional 12 months of treatment. Twenty of 30 patients were enrolled in the expansion cohort as of June 8.
For all patients, the median age was 40, 82% had had a splenectomy, and 49% were male. Twenty-five patients were NTD, and 14 were TD.
NTD patients
NTD patients had a median baseline hemoglobin level of 8.4 g/dL and liver iron concentration (LIC) of 5.8 ± 3.8 mg/g dry weight (dw).
Four of 8 patients who received luspatercept at doses ranging from 0.8 mg/kg to 1.25 mg/kg saw an increase in their hemoglobin levels of 1.5 g/dL or more for 2 weeks or longer, and 3 patients (38%) had a mean hemoglobin increase of 1.5 g/dL for 9 weeks or longer.
Patients on the higher doses of luspatercept had larger hemoglobin increases, and, with continued treatment, patients sustained their hemoglobin increases.
Increases in hemoglobin correlated with reductions in LIC.
“There was a trend to lower liver iron concentration,” Dr Piga said, “a trend that seems important.”
Patients achieved reductions in LIC with and without iron chelation therapy.
Eight of 12 patients with a baseline LIC of 5 mg/g dw or greater had decreases of 1 mg or more at month 4. And 10 of 10 patients with a baseline LIC of less than 5 mg/g dw were able to maintain that concentration.
TD patients
At baseline, TD patients required a median of 7.5 red blood cell (RBC) units every 12 weeks and had an LIC of 5.2 ± 5.7 mg/g dw.
Ten of 14 patients were treated for 12 weeks or longer and were evaluable for changes in transfusion burden. And all 10 patients had a 40% or greater reduction in transfusion burden.
Two of 3 patients with an LIC of 7 mg/g dw had decreases of 1 mg/g dw or more at month 4. And all 7 patients with a baseline LIC less than 7 mg/g dw were able to maintain that level.
Leg ulcers
Three patients with long-term, persistent leg ulcers experienced rapid healing with luspatercept treatment.
One NTD patient on the 0.4 mg/kg dose experienced complete healing after 6 weeks, and 1 TD patient on the 1.0 mg/kg dose experienced complete healing after 18 weeks.
Safety
Bone pain (23%), myalgia (18%), headache (15%), and asthenia (10%) were the most common drug-related adverse events. None of the related adverse events were serious.
Two patients had treatment-related, grade 3 adverse events of bone pain (2 events) and asthenia (1 event). Six of 39 patients discontinued treatment early due to an adverse event of headache, ankle pain, back pain, spider nevi, superficial thrombosis, or bone pain.
The US Food and Drug Administration recently granted luspatercept fast track designation for the treatment of patients with TD or NTD β-thalassemia.
Dr Piga said a pivotal phase 3 trial of luspatercept in patients with β -thalassemia and myelodysplastic syndromes is planned. ![]()
*Data in the abstract differ from the presentation.

Congress of the European
Hematology Association
VIENNA—Two very similar activin receptors, luspatercept and sotatercept, continue to show efficacy in patients with ß-thalassemia, according to research presented at the 20th Congress of the European Hematology Association (EHA).
The “twin compounds” basically differ from each other in the receptor type, but both can increase hemoglobin levels in non-transfusion-dependent (NTD) patients and reduce transfusion burden in transfusion-dependent (TD) patients.
Luspatercept is a recombinant fusion protein containing a modified extracellular domain of the activin receptor type IIB, while sotatercept is an activin receptor type IIA.
Antonio Piga, MD, of Turin University in Italy, presented the most recent results with luspatercept at EHA as abstract S136*. An update on sotatercept was also presented at the meeting.
Acceleron Pharma Inc. and Celgene Corporation are jointly developing both compounds.
The phase 2, multicenter, open-label trial of luspatercept now has results on 39 patients, 35 of whom were in the dose-escalation cohorts and 4 who are in the expansion cohort that is currently underway.
Adult patients age 18 and older received luspatercept subcutaneously every 3 weeks for 3 months. Doses in the dose-escalation cohorts increased from 0.2 mg/kg to 1.25 mg/kg.
Patients in the expansion cohort received a starting dose of 0.8 mg/kg, with an individual dose titration up to 1.25 mg/kg, for an additional 12 months of treatment. Twenty of 30 patients were enrolled in the expansion cohort as of June 8.
For all patients, the median age was 40, 82% had had a splenectomy, and 49% were male. Twenty-five patients were NTD, and 14 were TD.
NTD patients
NTD patients had a median baseline hemoglobin level of 8.4 g/dL and liver iron concentration (LIC) of 5.8 ± 3.8 mg/g dry weight (dw).
Four of 8 patients who received luspatercept at doses ranging from 0.8 mg/kg to 1.25 mg/kg saw an increase in their hemoglobin levels of 1.5 g/dL or more for 2 weeks or longer, and 3 patients (38%) had a mean hemoglobin increase of 1.5 g/dL for 9 weeks or longer.
Patients on the higher doses of luspatercept had larger hemoglobin increases, and, with continued treatment, patients sustained their hemoglobin increases.
Increases in hemoglobin correlated with reductions in LIC.
“There was a trend to lower liver iron concentration,” Dr Piga said, “a trend that seems important.”
Patients achieved reductions in LIC with and without iron chelation therapy.
Eight of 12 patients with a baseline LIC of 5 mg/g dw or greater had decreases of 1 mg or more at month 4. And 10 of 10 patients with a baseline LIC of less than 5 mg/g dw were able to maintain that concentration.
TD patients
At baseline, TD patients required a median of 7.5 red blood cell (RBC) units every 12 weeks and had an LIC of 5.2 ± 5.7 mg/g dw.
Ten of 14 patients were treated for 12 weeks or longer and were evaluable for changes in transfusion burden. And all 10 patients had a 40% or greater reduction in transfusion burden.
Two of 3 patients with an LIC of 7 mg/g dw had decreases of 1 mg/g dw or more at month 4. And all 7 patients with a baseline LIC less than 7 mg/g dw were able to maintain that level.
Leg ulcers
Three patients with long-term, persistent leg ulcers experienced rapid healing with luspatercept treatment.
One NTD patient on the 0.4 mg/kg dose experienced complete healing after 6 weeks, and 1 TD patient on the 1.0 mg/kg dose experienced complete healing after 18 weeks.
Safety
Bone pain (23%), myalgia (18%), headache (15%), and asthenia (10%) were the most common drug-related adverse events. None of the related adverse events were serious.
Two patients had treatment-related, grade 3 adverse events of bone pain (2 events) and asthenia (1 event). Six of 39 patients discontinued treatment early due to an adverse event of headache, ankle pain, back pain, spider nevi, superficial thrombosis, or bone pain.
The US Food and Drug Administration recently granted luspatercept fast track designation for the treatment of patients with TD or NTD β-thalassemia.
Dr Piga said a pivotal phase 3 trial of luspatercept in patients with β -thalassemia and myelodysplastic syndromes is planned. ![]()
*Data in the abstract differ from the presentation.

Congress of the European
Hematology Association
VIENNA—Two very similar activin receptors, luspatercept and sotatercept, continue to show efficacy in patients with ß-thalassemia, according to research presented at the 20th Congress of the European Hematology Association (EHA).
The “twin compounds” basically differ from each other in the receptor type, but both can increase hemoglobin levels in non-transfusion-dependent (NTD) patients and reduce transfusion burden in transfusion-dependent (TD) patients.
Luspatercept is a recombinant fusion protein containing a modified extracellular domain of the activin receptor type IIB, while sotatercept is an activin receptor type IIA.
Antonio Piga, MD, of Turin University in Italy, presented the most recent results with luspatercept at EHA as abstract S136*. An update on sotatercept was also presented at the meeting.
Acceleron Pharma Inc. and Celgene Corporation are jointly developing both compounds.
The phase 2, multicenter, open-label trial of luspatercept now has results on 39 patients, 35 of whom were in the dose-escalation cohorts and 4 who are in the expansion cohort that is currently underway.
Adult patients age 18 and older received luspatercept subcutaneously every 3 weeks for 3 months. Doses in the dose-escalation cohorts increased from 0.2 mg/kg to 1.25 mg/kg.
Patients in the expansion cohort received a starting dose of 0.8 mg/kg, with an individual dose titration up to 1.25 mg/kg, for an additional 12 months of treatment. Twenty of 30 patients were enrolled in the expansion cohort as of June 8.
For all patients, the median age was 40, 82% had had a splenectomy, and 49% were male. Twenty-five patients were NTD, and 14 were TD.
NTD patients
NTD patients had a median baseline hemoglobin level of 8.4 g/dL and liver iron concentration (LIC) of 5.8 ± 3.8 mg/g dry weight (dw).
Four of 8 patients who received luspatercept at doses ranging from 0.8 mg/kg to 1.25 mg/kg saw an increase in their hemoglobin levels of 1.5 g/dL or more for 2 weeks or longer, and 3 patients (38%) had a mean hemoglobin increase of 1.5 g/dL for 9 weeks or longer.
Patients on the higher doses of luspatercept had larger hemoglobin increases, and, with continued treatment, patients sustained their hemoglobin increases.
Increases in hemoglobin correlated with reductions in LIC.
“There was a trend to lower liver iron concentration,” Dr Piga said, “a trend that seems important.”
Patients achieved reductions in LIC with and without iron chelation therapy.
Eight of 12 patients with a baseline LIC of 5 mg/g dw or greater had decreases of 1 mg or more at month 4. And 10 of 10 patients with a baseline LIC of less than 5 mg/g dw were able to maintain that concentration.
TD patients
At baseline, TD patients required a median of 7.5 red blood cell (RBC) units every 12 weeks and had an LIC of 5.2 ± 5.7 mg/g dw.
Ten of 14 patients were treated for 12 weeks or longer and were evaluable for changes in transfusion burden. And all 10 patients had a 40% or greater reduction in transfusion burden.
Two of 3 patients with an LIC of 7 mg/g dw had decreases of 1 mg/g dw or more at month 4. And all 7 patients with a baseline LIC less than 7 mg/g dw were able to maintain that level.
Leg ulcers
Three patients with long-term, persistent leg ulcers experienced rapid healing with luspatercept treatment.
One NTD patient on the 0.4 mg/kg dose experienced complete healing after 6 weeks, and 1 TD patient on the 1.0 mg/kg dose experienced complete healing after 18 weeks.
Safety
Bone pain (23%), myalgia (18%), headache (15%), and asthenia (10%) were the most common drug-related adverse events. None of the related adverse events were serious.
Two patients had treatment-related, grade 3 adverse events of bone pain (2 events) and asthenia (1 event). Six of 39 patients discontinued treatment early due to an adverse event of headache, ankle pain, back pain, spider nevi, superficial thrombosis, or bone pain.
The US Food and Drug Administration recently granted luspatercept fast track designation for the treatment of patients with TD or NTD β-thalassemia.
Dr Piga said a pivotal phase 3 trial of luspatercept in patients with β -thalassemia and myelodysplastic syndromes is planned. ![]()
*Data in the abstract differ from the presentation.
Drug won’t advance to phase 3 in ß-thalassemia

thalassemia
VIENNA—Data from a phase 2a trial suggest the activin receptor sotatercept can effectively treat patients with ß-thalassemia.
However, the companies developing the drug have decided not to advance sotatercept to phase 3 trials in this patient population.
Instead, the companies are initiating a phase 3 program with sotatercept’s “twin” activin receptor, luspatercept, in patients with ß-thalassemia or myelodysplastic syndromes.
Acceleron Pharma Inc. and Celgene Corporation are jointly developing both compounds. The companies plan to continue developing sotatercept for patients with chronic kidney disease.
Maria Domenica Cappellini, MD, of the University of Milan in Italy, presented results from the phase 2a study of sotatercept in ß-thalassemia at the 20th Congress of the European Hematology Association (abstract S137*).
Phase 2 results with luspatercept in ß-thalassemia were also presented at the meeting.
The dose-finding study of sotatercept had enrolled 46 patients at the time of Dr Cappellini’s presentation. The drug was given subcutaneously at doses ranging from 0.1 mg/kg to 1.0 mg/kg every 3 weeks.
The 30 non-transfusion-dependent (NTD) patients received 4 or fewer red blood cell (RBC) units in the 6 months prior to study enrollment. The 16 transfusion-dependent (TD) patients had received 2 or more RBC units every 30 days for 6 months or more prior to study enrollment.
Twenty-five of the 46 patients remain on treatment, with a median exposure time of 12.4 months.
NTD patients
The patients’ median age was 42, and 53% were female. Fifty-three percent had had a splenectomy, and the median baseline hemoglobin level was 8.4 g/dL.
Treatment with sotatercept produced a dose-dependent hemoglobin increase.
“[W]hat I found quite interesting is that the increase is consistent and persistent, [even] after 1 year,” Dr Cappellini said. “And they are still maintained on treatment, of course, but it is a sustained response.”
At the 0.75 mg/kg dose, 86% of patients had a hemoglobin increase of 1 g/dL for 12 weeks or more, and 71% had an increase of 1.5 g/dL.
TD patients
The patients’ median age was 36 years, and 38% were female. Three-quarters of patients had ß-thalassemia major, and a quarter had ß-thalassemia intermedia.
Nearly a third of patients had had a splenectomy, and their transfusion burden at baseline ranged from 8 RBC units to 35 RBC units every 24 weeks.
The mean reduction in transfusion burden among patients treated with sotatercept at doses of 0.5 mg/kg or higher was 32.25%. And the 1.0 mg/kg dose of sotatercept reduced one patient’s transfusion burden by 61.6%.
Dr Cappellini noted that the pharmacokinetic analysis showed a correlation with exposure. The investigators observed no apparent effects of weight, sex, age, or transfusion burden on drug clearance.
“So the relationship was probably more related to long-term exposure than to the real dosage,” she said.
Safety
Hypertension and bone pain were the most common grade 2-4, treatment-related adverse events.
Seven patients discontinued the study due to adverse events, one patient each with bone pain, superficial thrombophlebitis, ventricular extrasystole, spinal extramedullary erythropoietic tissue, and erythema at the injection site/allergic reaction. Two patients discontinued due to hypertension.
Dr Cappellini concluded that sotatercept and the related molecule, luspatercept, may provide a favorable risk-benefit profile for patients with ß-thalassemia.
“These 2 molecules are merging now for a phase 3 trial in either TD or NTD thalassemia cohorts,” she said. “The two drugs are actually very, very similar, and, in fact, for the trial, we decided to use only one. There is no way to go on with 2 molecules.”
In April, Acceleron and Celgene announced plans to initiate a phase 3 program with luspatercept in myelodysplastic syndromes and β-thalassemia by the end of this year. The companies said they will continue to develop sotatercept for patients with chronic kidney disease. ![]()
*Data in the abstract differ from the presentation.

thalassemia
VIENNA—Data from a phase 2a trial suggest the activin receptor sotatercept can effectively treat patients with ß-thalassemia.
However, the companies developing the drug have decided not to advance sotatercept to phase 3 trials in this patient population.
Instead, the companies are initiating a phase 3 program with sotatercept’s “twin” activin receptor, luspatercept, in patients with ß-thalassemia or myelodysplastic syndromes.
Acceleron Pharma Inc. and Celgene Corporation are jointly developing both compounds. The companies plan to continue developing sotatercept for patients with chronic kidney disease.
Maria Domenica Cappellini, MD, of the University of Milan in Italy, presented results from the phase 2a study of sotatercept in ß-thalassemia at the 20th Congress of the European Hematology Association (abstract S137*).
Phase 2 results with luspatercept in ß-thalassemia were also presented at the meeting.
The dose-finding study of sotatercept had enrolled 46 patients at the time of Dr Cappellini’s presentation. The drug was given subcutaneously at doses ranging from 0.1 mg/kg to 1.0 mg/kg every 3 weeks.
The 30 non-transfusion-dependent (NTD) patients received 4 or fewer red blood cell (RBC) units in the 6 months prior to study enrollment. The 16 transfusion-dependent (TD) patients had received 2 or more RBC units every 30 days for 6 months or more prior to study enrollment.
Twenty-five of the 46 patients remain on treatment, with a median exposure time of 12.4 months.
NTD patients
The patients’ median age was 42, and 53% were female. Fifty-three percent had had a splenectomy, and the median baseline hemoglobin level was 8.4 g/dL.
Treatment with sotatercept produced a dose-dependent hemoglobin increase.
“[W]hat I found quite interesting is that the increase is consistent and persistent, [even] after 1 year,” Dr Cappellini said. “And they are still maintained on treatment, of course, but it is a sustained response.”
At the 0.75 mg/kg dose, 86% of patients had a hemoglobin increase of 1 g/dL for 12 weeks or more, and 71% had an increase of 1.5 g/dL.
TD patients
The patients’ median age was 36 years, and 38% were female. Three-quarters of patients had ß-thalassemia major, and a quarter had ß-thalassemia intermedia.
Nearly a third of patients had had a splenectomy, and their transfusion burden at baseline ranged from 8 RBC units to 35 RBC units every 24 weeks.
The mean reduction in transfusion burden among patients treated with sotatercept at doses of 0.5 mg/kg or higher was 32.25%. And the 1.0 mg/kg dose of sotatercept reduced one patient’s transfusion burden by 61.6%.
Dr Cappellini noted that the pharmacokinetic analysis showed a correlation with exposure. The investigators observed no apparent effects of weight, sex, age, or transfusion burden on drug clearance.
“So the relationship was probably more related to long-term exposure than to the real dosage,” she said.
Safety
Hypertension and bone pain were the most common grade 2-4, treatment-related adverse events.
Seven patients discontinued the study due to adverse events, one patient each with bone pain, superficial thrombophlebitis, ventricular extrasystole, spinal extramedullary erythropoietic tissue, and erythema at the injection site/allergic reaction. Two patients discontinued due to hypertension.
Dr Cappellini concluded that sotatercept and the related molecule, luspatercept, may provide a favorable risk-benefit profile for patients with ß-thalassemia.
“These 2 molecules are merging now for a phase 3 trial in either TD or NTD thalassemia cohorts,” she said. “The two drugs are actually very, very similar, and, in fact, for the trial, we decided to use only one. There is no way to go on with 2 molecules.”
In April, Acceleron and Celgene announced plans to initiate a phase 3 program with luspatercept in myelodysplastic syndromes and β-thalassemia by the end of this year. The companies said they will continue to develop sotatercept for patients with chronic kidney disease. ![]()
*Data in the abstract differ from the presentation.

thalassemia
VIENNA—Data from a phase 2a trial suggest the activin receptor sotatercept can effectively treat patients with ß-thalassemia.
However, the companies developing the drug have decided not to advance sotatercept to phase 3 trials in this patient population.
Instead, the companies are initiating a phase 3 program with sotatercept’s “twin” activin receptor, luspatercept, in patients with ß-thalassemia or myelodysplastic syndromes.
Acceleron Pharma Inc. and Celgene Corporation are jointly developing both compounds. The companies plan to continue developing sotatercept for patients with chronic kidney disease.
Maria Domenica Cappellini, MD, of the University of Milan in Italy, presented results from the phase 2a study of sotatercept in ß-thalassemia at the 20th Congress of the European Hematology Association (abstract S137*).
Phase 2 results with luspatercept in ß-thalassemia were also presented at the meeting.
The dose-finding study of sotatercept had enrolled 46 patients at the time of Dr Cappellini’s presentation. The drug was given subcutaneously at doses ranging from 0.1 mg/kg to 1.0 mg/kg every 3 weeks.
The 30 non-transfusion-dependent (NTD) patients received 4 or fewer red blood cell (RBC) units in the 6 months prior to study enrollment. The 16 transfusion-dependent (TD) patients had received 2 or more RBC units every 30 days for 6 months or more prior to study enrollment.
Twenty-five of the 46 patients remain on treatment, with a median exposure time of 12.4 months.
NTD patients
The patients’ median age was 42, and 53% were female. Fifty-three percent had had a splenectomy, and the median baseline hemoglobin level was 8.4 g/dL.
Treatment with sotatercept produced a dose-dependent hemoglobin increase.
“[W]hat I found quite interesting is that the increase is consistent and persistent, [even] after 1 year,” Dr Cappellini said. “And they are still maintained on treatment, of course, but it is a sustained response.”
At the 0.75 mg/kg dose, 86% of patients had a hemoglobin increase of 1 g/dL for 12 weeks or more, and 71% had an increase of 1.5 g/dL.
TD patients
The patients’ median age was 36 years, and 38% were female. Three-quarters of patients had ß-thalassemia major, and a quarter had ß-thalassemia intermedia.
Nearly a third of patients had had a splenectomy, and their transfusion burden at baseline ranged from 8 RBC units to 35 RBC units every 24 weeks.
The mean reduction in transfusion burden among patients treated with sotatercept at doses of 0.5 mg/kg or higher was 32.25%. And the 1.0 mg/kg dose of sotatercept reduced one patient’s transfusion burden by 61.6%.
Dr Cappellini noted that the pharmacokinetic analysis showed a correlation with exposure. The investigators observed no apparent effects of weight, sex, age, or transfusion burden on drug clearance.
“So the relationship was probably more related to long-term exposure than to the real dosage,” she said.
Safety
Hypertension and bone pain were the most common grade 2-4, treatment-related adverse events.
Seven patients discontinued the study due to adverse events, one patient each with bone pain, superficial thrombophlebitis, ventricular extrasystole, spinal extramedullary erythropoietic tissue, and erythema at the injection site/allergic reaction. Two patients discontinued due to hypertension.
Dr Cappellini concluded that sotatercept and the related molecule, luspatercept, may provide a favorable risk-benefit profile for patients with ß-thalassemia.
“These 2 molecules are merging now for a phase 3 trial in either TD or NTD thalassemia cohorts,” she said. “The two drugs are actually very, very similar, and, in fact, for the trial, we decided to use only one. There is no way to go on with 2 molecules.”
In April, Acceleron and Celgene announced plans to initiate a phase 3 program with luspatercept in myelodysplastic syndromes and β-thalassemia by the end of this year. The companies said they will continue to develop sotatercept for patients with chronic kidney disease. ![]()
*Data in the abstract differ from the presentation.
Veterans with TBI have higher rates of unemployment
WASHINGTON – Soldiers who experience traumatic brain injury (TBI) while deployed have a much higher rate of unemployment than those who experience no TBI during their tour of duty, according to a study presented at the annual meeting of the American Headache Society.
“We know that TBI and PTSD [post-traumatic stress disorder] can lead to headaches, depression, and loss of libido, but there’s very little data on the long-term effects of TBI on [veterans], and therefore very little control data,” explained Dr. James R. Couch of the University of Oklahoma in Oklahoma City.
Dr. Couch and his coinvestigators looked at 5,743 veterans of Operation Iraqi Freedom and Operation Enduring Freedom who had been deployed between June 2008 and April 2011, 1,325 (23%) of whom had experienced TBI while serving. The first 500 subjects seen were then frequency matched with a control subject who did not experience TBI, based on age, sex, race, and deployment length.
From this pool, 67 pairs were finally selected for inclusion, all of whom were 2-11 years post TBI: 39 pairs were 2-7 years post TBI and the remaining 28 pairs were 8-11 years post TBI. All subjects were 25-60 years old. Marital and employment data were collected from each subject, and each subject completed a TBI questionnaire, headache questionnaire, Beck Depression Inventory 2, and post-traumatic stress disorder questionnaire, from which bivariate analyses were performed.
Although marital status was not found to be significantly tied to TBI experience while deployed, unemployment rates were consistently and significantly higher in the TBI cohort, compared with their non-TBI counterparts. In the 2-7 years post-TBI group, 35.9% of subjects were unemployed, while only 10.3% of non-TBI subjects were without a job (P = .014). In the 8-11 years post-TBI cohort, 50.0% of subjects were unemployed, compared with 7.1% of controls who were unemployed. No significant association, however, was found between frequency of headache or severity of TBI with either unemployment or marital status.
The most important thing to take away, said Dr. Couch, is “the marked difference in unemployment from 4-11 years after TBI, and that things seem to be getting worse as time goes along. Headache, depression, PTSD – none of these stand out as singular causes but are all known to be related to [TBI], and severity of TBI was not strongly associated.”
Dr. Couch disclosed receiving consulting fees/honoraria from St. Jude Medical.
WASHINGTON – Soldiers who experience traumatic brain injury (TBI) while deployed have a much higher rate of unemployment than those who experience no TBI during their tour of duty, according to a study presented at the annual meeting of the American Headache Society.
“We know that TBI and PTSD [post-traumatic stress disorder] can lead to headaches, depression, and loss of libido, but there’s very little data on the long-term effects of TBI on [veterans], and therefore very little control data,” explained Dr. James R. Couch of the University of Oklahoma in Oklahoma City.
Dr. Couch and his coinvestigators looked at 5,743 veterans of Operation Iraqi Freedom and Operation Enduring Freedom who had been deployed between June 2008 and April 2011, 1,325 (23%) of whom had experienced TBI while serving. The first 500 subjects seen were then frequency matched with a control subject who did not experience TBI, based on age, sex, race, and deployment length.
From this pool, 67 pairs were finally selected for inclusion, all of whom were 2-11 years post TBI: 39 pairs were 2-7 years post TBI and the remaining 28 pairs were 8-11 years post TBI. All subjects were 25-60 years old. Marital and employment data were collected from each subject, and each subject completed a TBI questionnaire, headache questionnaire, Beck Depression Inventory 2, and post-traumatic stress disorder questionnaire, from which bivariate analyses were performed.
Although marital status was not found to be significantly tied to TBI experience while deployed, unemployment rates were consistently and significantly higher in the TBI cohort, compared with their non-TBI counterparts. In the 2-7 years post-TBI group, 35.9% of subjects were unemployed, while only 10.3% of non-TBI subjects were without a job (P = .014). In the 8-11 years post-TBI cohort, 50.0% of subjects were unemployed, compared with 7.1% of controls who were unemployed. No significant association, however, was found between frequency of headache or severity of TBI with either unemployment or marital status.
The most important thing to take away, said Dr. Couch, is “the marked difference in unemployment from 4-11 years after TBI, and that things seem to be getting worse as time goes along. Headache, depression, PTSD – none of these stand out as singular causes but are all known to be related to [TBI], and severity of TBI was not strongly associated.”
Dr. Couch disclosed receiving consulting fees/honoraria from St. Jude Medical.
WASHINGTON – Soldiers who experience traumatic brain injury (TBI) while deployed have a much higher rate of unemployment than those who experience no TBI during their tour of duty, according to a study presented at the annual meeting of the American Headache Society.
“We know that TBI and PTSD [post-traumatic stress disorder] can lead to headaches, depression, and loss of libido, but there’s very little data on the long-term effects of TBI on [veterans], and therefore very little control data,” explained Dr. James R. Couch of the University of Oklahoma in Oklahoma City.
Dr. Couch and his coinvestigators looked at 5,743 veterans of Operation Iraqi Freedom and Operation Enduring Freedom who had been deployed between June 2008 and April 2011, 1,325 (23%) of whom had experienced TBI while serving. The first 500 subjects seen were then frequency matched with a control subject who did not experience TBI, based on age, sex, race, and deployment length.
From this pool, 67 pairs were finally selected for inclusion, all of whom were 2-11 years post TBI: 39 pairs were 2-7 years post TBI and the remaining 28 pairs were 8-11 years post TBI. All subjects were 25-60 years old. Marital and employment data were collected from each subject, and each subject completed a TBI questionnaire, headache questionnaire, Beck Depression Inventory 2, and post-traumatic stress disorder questionnaire, from which bivariate analyses were performed.
Although marital status was not found to be significantly tied to TBI experience while deployed, unemployment rates were consistently and significantly higher in the TBI cohort, compared with their non-TBI counterparts. In the 2-7 years post-TBI group, 35.9% of subjects were unemployed, while only 10.3% of non-TBI subjects were without a job (P = .014). In the 8-11 years post-TBI cohort, 50.0% of subjects were unemployed, compared with 7.1% of controls who were unemployed. No significant association, however, was found between frequency of headache or severity of TBI with either unemployment or marital status.
The most important thing to take away, said Dr. Couch, is “the marked difference in unemployment from 4-11 years after TBI, and that things seem to be getting worse as time goes along. Headache, depression, PTSD – none of these stand out as singular causes but are all known to be related to [TBI], and severity of TBI was not strongly associated.”
Dr. Couch disclosed receiving consulting fees/honoraria from St. Jude Medical.
AT THE AHS ANNUAL MEETING
Key clinical point: Soldiers who experience traumatic brain injury while deployed have significantly lower rates of employment than soldiers who experience no traumatic brain injury.
Major finding: At 2-7 years post TBI, 39.1% of soldiers who experienced TBI were unemployed vs. 10.3% unemployment for soldiers with no TBI (P = .014); at 8-11 years post TBI, unemployment was 50.0% for TBI soldiers vs. 7.1% for non-TBI soldiers.
Data source: Interview-based study of 67 soldiers, aged 25-60 years, who experienced TBI during deployment between June 2008 and April 2011, all of whom were paired with a control of similar age, sex, race, and deployment time who did not experience TBI.
Disclosures: Dr. Couch disclosed receiving consulting fees/honoraria from St. Jude Medical.
Lessons from polio
For those of us who appreciate the value of science and accept its limitations, it is sometimes difficult to understand how parents can choose to not immunize their children against serious and life-threatening diseases. To some extent, the explanation may be that immunizations simply have become victims of their own success.
How many adults have a relative, friend, or neighbor whose child has died as the result of bacterial meningitis or epiglottitis? They might have had a friend whose month-long cough was eventually diagnosed as whooping cough, but how many parents know of an infant who succumbed to pertussis? If you were trained in the last decade, you may not have had first-hand experience with most of the diseases for which we now have immunizations.
Reading a recent review of a new biography of Jonas Salk triggered a stream of memories of what it was like when polio descended on the landscape of North America – unchecked by an effective immunization. Moving through communities, choosing victims seemingly at random, it was every parent’s nightmare.
I grew up in a small town in New York State, so small that its inhabitants refer to it as a “village.” Everyone in Pleasantville knew at least one family that had been touched by polio. I don’t recall being aware of anyone in my family’s extended network of acquaintances who had died of the disease, but I suspect there may have been some fatalities that my parents avoided discussing in my presence. But I knew it was a disease with a significant mortality rate, and I knew of children and adults who had luckily survived several weeks or months in an iron lung. One of my parents’ closest friends walked with a limp as a result of polio.
There was rumor in town that all five members in one family had contracted polio and incredibly survived. Their cat had allegedly died of the disease. Our community was said to be particularly vulnerable because we had a public pool. This gift from the federal government’s Works Progress Administration provided a multi-lane superhighway for the virus to spread from child to child.
Even as a young child, I could sense that a blanket of fear hung over our little village during the summer when the disease was at its most prevalent. Now, as a parent, I am surprised how well my own parents disguised the fears that they and their peers must have harbored. My sister and I were still allowed to go swimming at the pool on the hottest days, but we knew that there were other families who stayed away.
When a vaccine trial began at our school, there was no question that we would participate. In fact, I don’t recall bringing home any permission slips to be signed. Nor do I remember hearing of any families who had opted out. We always wondered whether we had received the real vaccine or the placebo. But when the trials were over and the real vaccine was available, what parent in his or her right mind would even consider depriving his or her child from protection against this scourge that had taken up residence among us? I’m sure that Dr. Blum, my pediatrician, never needed to spend more than 30 seconds trying to convince my parents or any other parents, for that matter, of the need to vaccinate against polio.
My childhood ended before the development of the vaccines against the other common viral illnesses, and as a result I contracted and survived measles, mumps, rubella, and varicella. Of course, there must have been a few children who died of the diseases that had left me unscathed, but the number of fatalities was so small that I’m sure my parents would have wondered why we would need vaccines for these “usual diseases of childhood.”
But polio was different, and while it pales in comparison to Ebola, polio and its successful eradication created a generation of parents with a respect for science and the value of immunization. However, that generation has passed, and with it the stories they could have told the parents of today. Unfortunately, vaccine refusers seem to be immune to education and deaf to the lessons history can teach. I suspect that they would have foolishly ignored my parents’ stories about polio as just so much when-I-was-your-age mumbling.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Coping with a Picky Eater.” E-mail him at [email protected].
For those of us who appreciate the value of science and accept its limitations, it is sometimes difficult to understand how parents can choose to not immunize their children against serious and life-threatening diseases. To some extent, the explanation may be that immunizations simply have become victims of their own success.
How many adults have a relative, friend, or neighbor whose child has died as the result of bacterial meningitis or epiglottitis? They might have had a friend whose month-long cough was eventually diagnosed as whooping cough, but how many parents know of an infant who succumbed to pertussis? If you were trained in the last decade, you may not have had first-hand experience with most of the diseases for which we now have immunizations.
Reading a recent review of a new biography of Jonas Salk triggered a stream of memories of what it was like when polio descended on the landscape of North America – unchecked by an effective immunization. Moving through communities, choosing victims seemingly at random, it was every parent’s nightmare.
I grew up in a small town in New York State, so small that its inhabitants refer to it as a “village.” Everyone in Pleasantville knew at least one family that had been touched by polio. I don’t recall being aware of anyone in my family’s extended network of acquaintances who had died of the disease, but I suspect there may have been some fatalities that my parents avoided discussing in my presence. But I knew it was a disease with a significant mortality rate, and I knew of children and adults who had luckily survived several weeks or months in an iron lung. One of my parents’ closest friends walked with a limp as a result of polio.
There was rumor in town that all five members in one family had contracted polio and incredibly survived. Their cat had allegedly died of the disease. Our community was said to be particularly vulnerable because we had a public pool. This gift from the federal government’s Works Progress Administration provided a multi-lane superhighway for the virus to spread from child to child.
Even as a young child, I could sense that a blanket of fear hung over our little village during the summer when the disease was at its most prevalent. Now, as a parent, I am surprised how well my own parents disguised the fears that they and their peers must have harbored. My sister and I were still allowed to go swimming at the pool on the hottest days, but we knew that there were other families who stayed away.
When a vaccine trial began at our school, there was no question that we would participate. In fact, I don’t recall bringing home any permission slips to be signed. Nor do I remember hearing of any families who had opted out. We always wondered whether we had received the real vaccine or the placebo. But when the trials were over and the real vaccine was available, what parent in his or her right mind would even consider depriving his or her child from protection against this scourge that had taken up residence among us? I’m sure that Dr. Blum, my pediatrician, never needed to spend more than 30 seconds trying to convince my parents or any other parents, for that matter, of the need to vaccinate against polio.
My childhood ended before the development of the vaccines against the other common viral illnesses, and as a result I contracted and survived measles, mumps, rubella, and varicella. Of course, there must have been a few children who died of the diseases that had left me unscathed, but the number of fatalities was so small that I’m sure my parents would have wondered why we would need vaccines for these “usual diseases of childhood.”
But polio was different, and while it pales in comparison to Ebola, polio and its successful eradication created a generation of parents with a respect for science and the value of immunization. However, that generation has passed, and with it the stories they could have told the parents of today. Unfortunately, vaccine refusers seem to be immune to education and deaf to the lessons history can teach. I suspect that they would have foolishly ignored my parents’ stories about polio as just so much when-I-was-your-age mumbling.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Coping with a Picky Eater.” E-mail him at [email protected].
For those of us who appreciate the value of science and accept its limitations, it is sometimes difficult to understand how parents can choose to not immunize their children against serious and life-threatening diseases. To some extent, the explanation may be that immunizations simply have become victims of their own success.
How many adults have a relative, friend, or neighbor whose child has died as the result of bacterial meningitis or epiglottitis? They might have had a friend whose month-long cough was eventually diagnosed as whooping cough, but how many parents know of an infant who succumbed to pertussis? If you were trained in the last decade, you may not have had first-hand experience with most of the diseases for which we now have immunizations.
Reading a recent review of a new biography of Jonas Salk triggered a stream of memories of what it was like when polio descended on the landscape of North America – unchecked by an effective immunization. Moving through communities, choosing victims seemingly at random, it was every parent’s nightmare.
I grew up in a small town in New York State, so small that its inhabitants refer to it as a “village.” Everyone in Pleasantville knew at least one family that had been touched by polio. I don’t recall being aware of anyone in my family’s extended network of acquaintances who had died of the disease, but I suspect there may have been some fatalities that my parents avoided discussing in my presence. But I knew it was a disease with a significant mortality rate, and I knew of children and adults who had luckily survived several weeks or months in an iron lung. One of my parents’ closest friends walked with a limp as a result of polio.
There was rumor in town that all five members in one family had contracted polio and incredibly survived. Their cat had allegedly died of the disease. Our community was said to be particularly vulnerable because we had a public pool. This gift from the federal government’s Works Progress Administration provided a multi-lane superhighway for the virus to spread from child to child.
Even as a young child, I could sense that a blanket of fear hung over our little village during the summer when the disease was at its most prevalent. Now, as a parent, I am surprised how well my own parents disguised the fears that they and their peers must have harbored. My sister and I were still allowed to go swimming at the pool on the hottest days, but we knew that there were other families who stayed away.
When a vaccine trial began at our school, there was no question that we would participate. In fact, I don’t recall bringing home any permission slips to be signed. Nor do I remember hearing of any families who had opted out. We always wondered whether we had received the real vaccine or the placebo. But when the trials were over and the real vaccine was available, what parent in his or her right mind would even consider depriving his or her child from protection against this scourge that had taken up residence among us? I’m sure that Dr. Blum, my pediatrician, never needed to spend more than 30 seconds trying to convince my parents or any other parents, for that matter, of the need to vaccinate against polio.
My childhood ended before the development of the vaccines against the other common viral illnesses, and as a result I contracted and survived measles, mumps, rubella, and varicella. Of course, there must have been a few children who died of the diseases that had left me unscathed, but the number of fatalities was so small that I’m sure my parents would have wondered why we would need vaccines for these “usual diseases of childhood.”
But polio was different, and while it pales in comparison to Ebola, polio and its successful eradication created a generation of parents with a respect for science and the value of immunization. However, that generation has passed, and with it the stories they could have told the parents of today. Unfortunately, vaccine refusers seem to be immune to education and deaf to the lessons history can teach. I suspect that they would have foolishly ignored my parents’ stories about polio as just so much when-I-was-your-age mumbling.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Coping with a Picky Eater.” E-mail him at [email protected].
New insight into blood production

in the bone marrow
Scientists say they have identified a family of multipotent progenitor (MPP) cells that are the first to arise from hematopoietic stem cells (HSCs).
The team said their discovery, published in Cell Stem Cell, raises the possibility that, by manipulating the fates of MPPs or HSCs, researchers could one day help overcome imbalances and deficiencies that can arise in the blood system due to aging or leukemia.
Similar imbalances can render patients vulnerable immediately following HSC transplants, especially following umbilical cord transplants, said study author Emmanuelle Passegué, PhD, of the University of California San Francisco.
Via experiments in mice, Dr Passegué and her colleagues found that HSCs “make educated decisions” when it comes time to differentiate.
“Previously, researchers thought that the developmental paths of daughter cells were randomly specified by the HSC, but we conclude that the HSC normally responds appropriately to signals in the environment, making the different MPPs in parallel, but at different speeds and in different amounts to meet the body’s needs,” Dr Passegué said.
To uncover these findings, she and her colleagues investigated patterns of gene expression and cell signaling that determine which developmental paths are favored when relatively rare HSCs spin off daughter cells. The team also explored HSCs’ responses during transplant.
The researchers found that the first daughter cells that arise from HSCs are already distinct, favoring the development of different specialized cell lineages.
The team identified two types of daughter cells, MPP2 and MPP3, which, under normal conditions, are rare. These cells work together with more common daughter cells, MPP4 cells, to control blood production.
MPP2 cells favor the production of platelets and red blood cells, while MPP3 cells favor production of inflammatory cells.
MPP4 cells are the main producers of lymphocytes that fight specific disease pathogens, but the researchers showed that MPP4 cells can easily be re-educated to make many inflammatory cells when regenerative needs are high, as they are following a transplant.
The team found that, during transplant, regenerating HSCs limit their own self-renewal and instead go to work overproducing MPP2 and MPP3 cells that quickly produce needed red blood cells, platelets, and inflammatory cells. Only later does MPP4 production return to normal, enabling the immune system to replenish lymphocytes.
“It will be compelling to test whether the developmental pathways leading to cell specialization can be manipulated to favor production of specific lineage-biased MPPs in order to optimize blood recovery following hematopoietic injury or to rebalance the output of various cell lineages in an aging or deregulated blood system,” Dr Passegué said. ![]()

in the bone marrow
Scientists say they have identified a family of multipotent progenitor (MPP) cells that are the first to arise from hematopoietic stem cells (HSCs).
The team said their discovery, published in Cell Stem Cell, raises the possibility that, by manipulating the fates of MPPs or HSCs, researchers could one day help overcome imbalances and deficiencies that can arise in the blood system due to aging or leukemia.
Similar imbalances can render patients vulnerable immediately following HSC transplants, especially following umbilical cord transplants, said study author Emmanuelle Passegué, PhD, of the University of California San Francisco.
Via experiments in mice, Dr Passegué and her colleagues found that HSCs “make educated decisions” when it comes time to differentiate.
“Previously, researchers thought that the developmental paths of daughter cells were randomly specified by the HSC, but we conclude that the HSC normally responds appropriately to signals in the environment, making the different MPPs in parallel, but at different speeds and in different amounts to meet the body’s needs,” Dr Passegué said.
To uncover these findings, she and her colleagues investigated patterns of gene expression and cell signaling that determine which developmental paths are favored when relatively rare HSCs spin off daughter cells. The team also explored HSCs’ responses during transplant.
The researchers found that the first daughter cells that arise from HSCs are already distinct, favoring the development of different specialized cell lineages.
The team identified two types of daughter cells, MPP2 and MPP3, which, under normal conditions, are rare. These cells work together with more common daughter cells, MPP4 cells, to control blood production.
MPP2 cells favor the production of platelets and red blood cells, while MPP3 cells favor production of inflammatory cells.
MPP4 cells are the main producers of lymphocytes that fight specific disease pathogens, but the researchers showed that MPP4 cells can easily be re-educated to make many inflammatory cells when regenerative needs are high, as they are following a transplant.
The team found that, during transplant, regenerating HSCs limit their own self-renewal and instead go to work overproducing MPP2 and MPP3 cells that quickly produce needed red blood cells, platelets, and inflammatory cells. Only later does MPP4 production return to normal, enabling the immune system to replenish lymphocytes.
“It will be compelling to test whether the developmental pathways leading to cell specialization can be manipulated to favor production of specific lineage-biased MPPs in order to optimize blood recovery following hematopoietic injury or to rebalance the output of various cell lineages in an aging or deregulated blood system,” Dr Passegué said. ![]()

in the bone marrow
Scientists say they have identified a family of multipotent progenitor (MPP) cells that are the first to arise from hematopoietic stem cells (HSCs).
The team said their discovery, published in Cell Stem Cell, raises the possibility that, by manipulating the fates of MPPs or HSCs, researchers could one day help overcome imbalances and deficiencies that can arise in the blood system due to aging or leukemia.
Similar imbalances can render patients vulnerable immediately following HSC transplants, especially following umbilical cord transplants, said study author Emmanuelle Passegué, PhD, of the University of California San Francisco.
Via experiments in mice, Dr Passegué and her colleagues found that HSCs “make educated decisions” when it comes time to differentiate.
“Previously, researchers thought that the developmental paths of daughter cells were randomly specified by the HSC, but we conclude that the HSC normally responds appropriately to signals in the environment, making the different MPPs in parallel, but at different speeds and in different amounts to meet the body’s needs,” Dr Passegué said.
To uncover these findings, she and her colleagues investigated patterns of gene expression and cell signaling that determine which developmental paths are favored when relatively rare HSCs spin off daughter cells. The team also explored HSCs’ responses during transplant.
The researchers found that the first daughter cells that arise from HSCs are already distinct, favoring the development of different specialized cell lineages.
The team identified two types of daughter cells, MPP2 and MPP3, which, under normal conditions, are rare. These cells work together with more common daughter cells, MPP4 cells, to control blood production.
MPP2 cells favor the production of platelets and red blood cells, while MPP3 cells favor production of inflammatory cells.
MPP4 cells are the main producers of lymphocytes that fight specific disease pathogens, but the researchers showed that MPP4 cells can easily be re-educated to make many inflammatory cells when regenerative needs are high, as they are following a transplant.
The team found that, during transplant, regenerating HSCs limit their own self-renewal and instead go to work overproducing MPP2 and MPP3 cells that quickly produce needed red blood cells, platelets, and inflammatory cells. Only later does MPP4 production return to normal, enabling the immune system to replenish lymphocytes.
“It will be compelling to test whether the developmental pathways leading to cell specialization can be manipulated to favor production of specific lineage-biased MPPs in order to optimize blood recovery following hematopoietic injury or to rebalance the output of various cell lineages in an aging or deregulated blood system,” Dr Passegué said. ![]()