User login
Mosquitoes can accumulate malaria infections
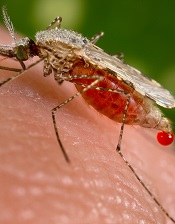
Photo courtesy of the CDC
Repeat infection with malaria parasites might make mosquitoes more dangerous, according to a study published in PLOS Pathogens.
The research showed that individual Anopheles mosquitoes can accumulate infections with different strains of malaria parasites, an existing malaria infection makes mosquitoes more susceptible to a second infection, and infections reach higher densities when another strain is already present.
These phenomena could promote the spread of drug-resistant malaria, according to investigators.
Laura Pollitt, PhD, of the University of Edinburgh in the UK, and her colleagues conducted this research.
They wanted to determine if and how mosquitoes can be infected with different strains of Plasmodium parasites, how such heterogeneous parasites interact in the insects, and whether such interactions affect the transmission of malaria to vertebrate hosts.
The investigators set up cages of female Anopheles stephensi mosquitoes and allowed them, at defined times, to feed on mice infected with 2 different Plasmodium chabaudi strains—AJ and ER.
This study design allowed the team to examine how the presence of a co-infecting strain affects parasites that enter the vector first and second, and to test whether co-infection impacts mosquito survival.
The investigators found that mosquitoes can accumulate mixed-strain malaria infections after feeding on multiple hosts. And parasites have a greater chance of establishing a secondary infection if another Plasmodium strain is already present in a mosquito.
Moreover, the presence of the primary infection facilitated replication of the secondary infection while the first infection developed as normal. This resulted in doubly infected mosquitoes having substantially higher parasite loads.
The investigators noted that the large parasite numbers do not appear to kill the insects. And, because it is expected that mosquitoes carrying more parasites are more likely to transmit malaria to vertebrates, mosquitoes taking multiple infective bites might disproportionally contribute to malaria transmission.
This, in turn, would increase rates of mixed infections in vertebrate hosts, with implications for the evolution of parasite virulence and the spread of drug-resistant strains. ![]()

Photo courtesy of the CDC
Repeat infection with malaria parasites might make mosquitoes more dangerous, according to a study published in PLOS Pathogens.
The research showed that individual Anopheles mosquitoes can accumulate infections with different strains of malaria parasites, an existing malaria infection makes mosquitoes more susceptible to a second infection, and infections reach higher densities when another strain is already present.
These phenomena could promote the spread of drug-resistant malaria, according to investigators.
Laura Pollitt, PhD, of the University of Edinburgh in the UK, and her colleagues conducted this research.
They wanted to determine if and how mosquitoes can be infected with different strains of Plasmodium parasites, how such heterogeneous parasites interact in the insects, and whether such interactions affect the transmission of malaria to vertebrate hosts.
The investigators set up cages of female Anopheles stephensi mosquitoes and allowed them, at defined times, to feed on mice infected with 2 different Plasmodium chabaudi strains—AJ and ER.
This study design allowed the team to examine how the presence of a co-infecting strain affects parasites that enter the vector first and second, and to test whether co-infection impacts mosquito survival.
The investigators found that mosquitoes can accumulate mixed-strain malaria infections after feeding on multiple hosts. And parasites have a greater chance of establishing a secondary infection if another Plasmodium strain is already present in a mosquito.
Moreover, the presence of the primary infection facilitated replication of the secondary infection while the first infection developed as normal. This resulted in doubly infected mosquitoes having substantially higher parasite loads.
The investigators noted that the large parasite numbers do not appear to kill the insects. And, because it is expected that mosquitoes carrying more parasites are more likely to transmit malaria to vertebrates, mosquitoes taking multiple infective bites might disproportionally contribute to malaria transmission.
This, in turn, would increase rates of mixed infections in vertebrate hosts, with implications for the evolution of parasite virulence and the spread of drug-resistant strains. ![]()

Photo courtesy of the CDC
Repeat infection with malaria parasites might make mosquitoes more dangerous, according to a study published in PLOS Pathogens.
The research showed that individual Anopheles mosquitoes can accumulate infections with different strains of malaria parasites, an existing malaria infection makes mosquitoes more susceptible to a second infection, and infections reach higher densities when another strain is already present.
These phenomena could promote the spread of drug-resistant malaria, according to investigators.
Laura Pollitt, PhD, of the University of Edinburgh in the UK, and her colleagues conducted this research.
They wanted to determine if and how mosquitoes can be infected with different strains of Plasmodium parasites, how such heterogeneous parasites interact in the insects, and whether such interactions affect the transmission of malaria to vertebrate hosts.
The investigators set up cages of female Anopheles stephensi mosquitoes and allowed them, at defined times, to feed on mice infected with 2 different Plasmodium chabaudi strains—AJ and ER.
This study design allowed the team to examine how the presence of a co-infecting strain affects parasites that enter the vector first and second, and to test whether co-infection impacts mosquito survival.
The investigators found that mosquitoes can accumulate mixed-strain malaria infections after feeding on multiple hosts. And parasites have a greater chance of establishing a secondary infection if another Plasmodium strain is already present in a mosquito.
Moreover, the presence of the primary infection facilitated replication of the secondary infection while the first infection developed as normal. This resulted in doubly infected mosquitoes having substantially higher parasite loads.
The investigators noted that the large parasite numbers do not appear to kill the insects. And, because it is expected that mosquitoes carrying more parasites are more likely to transmit malaria to vertebrates, mosquitoes taking multiple infective bites might disproportionally contribute to malaria transmission.
This, in turn, would increase rates of mixed infections in vertebrate hosts, with implications for the evolution of parasite virulence and the spread of drug-resistant strains. ![]()
Business associate agreements
Revision of the Health Insurance Portability and Accountability Act (HIPAA) rules has prompted numerous questions about business associates (BAs) and business associate agreements (BAAs). Apparently there is confusion about exactly which businesses qualify as BAs and how your BAAs should be modified to reflect the new provisions.
The criteria for identifying BAs are admittedly vague: The act defines them as nonemployees, performing “functions or activities” on behalf of the “covered entity” (your practice) that involve “creating, receiving, maintaining, or transmitting” personal health information (PHI).
Clearly, answering and billing services, independent transcriptionists, hardware and software companies, and any other vendors involved in creating or maintaining your medical records always qualify as BAs. Other businesses may or may not qualify, depending on whether they need direct access to PHI in order to provide their service. These include practice management consultants, attorneys, companies that store or microfilm medical records, and record-shredding services.
Specialty pharmacies are seldom mentioned in the BA discussion, but they probably should be. Pharmaceutical manufacturers are increasingly using them as intermediaries for their products – particularly the more expensive ones, such as biologics. Many of them ship products directly to patients, for which they require home addresses and other personal information, and in order to file payment paperwork and claim forms, they usually request diagnoses and associated medical information. By any reasonable interpretation of the new rules, this makes them BAs, and you should have BAAs in place before allowing them to fill your prescriptions.
To further complicate the situation, manufacturers and insurers routinely compile information about the real world uses of their products. To that end, they often ask specialty pharmacies to provide them with any patient data that they collect. Under the new rules, patients may restrict any PHI shared with third parties when patients pay for the drugs or services themselves. Your specialty pharmacy BAA should include a provision noting that the pharmacy is forbidden from disclosing any data to pharmaceutical companies or insurers from patients who self-pay and request confidentiality.
Mail carriers, package delivery people, cleaning services, copier repairmen, bank employees, and the like are not considered BAs. While they might conceivably come in contact with PHI on occasion, they don’t need it to do their job. You are required to use “reasonable diligence” in limiting the PHI that these folks may encounter, but you do not need to enter into written BA agreements with them.
Independent contractors who work within your practice – aestheticians and physical therapists, for example – are not considered BAs either, and do not need to sign a BA agreement. Just train them, as you do your employees.
Another source of confusion is the provision in the new rules that makes BAs directly responsible for their own HIPAA violations. While this might seem to eliminate the need for BAAs entirely, unfortunately that is not the case. In fact, even more responsibility has been placed on physicians for confidentiality breaches committed by their BAs. It is not enough to simply have a BAA in place; you are expected to use “reasonable diligence” in monitoring the work of your BAs. While BAs and their subcontractors are responsible for their own actions, the primary responsibility remains with you. Furthermore, you now must assume the worst-case scenario. Previously, when PHI was compromised, you would have to notify affected patients (and the government) only if there was a “significant risk of financial or reputational harm”; but now, any incident involving patient records is assumed to be a breach, and must be reported. Failure to do so could subject your practice, as well as the contractor, to significant fines.
If you haven’t yet revised your Notice of Privacy Practices (NPP) to explain your relationships with BAs, and their status under the new rules, do it now. (You should have done it last September.) You need to explain the breach notification process too, as well as the new patient rights mentioned above. You must post your revised NPP in your office, and make copies available there, but you need not mail a copy to every patient.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News.
Revision of the Health Insurance Portability and Accountability Act (HIPAA) rules has prompted numerous questions about business associates (BAs) and business associate agreements (BAAs). Apparently there is confusion about exactly which businesses qualify as BAs and how your BAAs should be modified to reflect the new provisions.
The criteria for identifying BAs are admittedly vague: The act defines them as nonemployees, performing “functions or activities” on behalf of the “covered entity” (your practice) that involve “creating, receiving, maintaining, or transmitting” personal health information (PHI).
Clearly, answering and billing services, independent transcriptionists, hardware and software companies, and any other vendors involved in creating or maintaining your medical records always qualify as BAs. Other businesses may or may not qualify, depending on whether they need direct access to PHI in order to provide their service. These include practice management consultants, attorneys, companies that store or microfilm medical records, and record-shredding services.
Specialty pharmacies are seldom mentioned in the BA discussion, but they probably should be. Pharmaceutical manufacturers are increasingly using them as intermediaries for their products – particularly the more expensive ones, such as biologics. Many of them ship products directly to patients, for which they require home addresses and other personal information, and in order to file payment paperwork and claim forms, they usually request diagnoses and associated medical information. By any reasonable interpretation of the new rules, this makes them BAs, and you should have BAAs in place before allowing them to fill your prescriptions.
To further complicate the situation, manufacturers and insurers routinely compile information about the real world uses of their products. To that end, they often ask specialty pharmacies to provide them with any patient data that they collect. Under the new rules, patients may restrict any PHI shared with third parties when patients pay for the drugs or services themselves. Your specialty pharmacy BAA should include a provision noting that the pharmacy is forbidden from disclosing any data to pharmaceutical companies or insurers from patients who self-pay and request confidentiality.
Mail carriers, package delivery people, cleaning services, copier repairmen, bank employees, and the like are not considered BAs. While they might conceivably come in contact with PHI on occasion, they don’t need it to do their job. You are required to use “reasonable diligence” in limiting the PHI that these folks may encounter, but you do not need to enter into written BA agreements with them.
Independent contractors who work within your practice – aestheticians and physical therapists, for example – are not considered BAs either, and do not need to sign a BA agreement. Just train them, as you do your employees.
Another source of confusion is the provision in the new rules that makes BAs directly responsible for their own HIPAA violations. While this might seem to eliminate the need for BAAs entirely, unfortunately that is not the case. In fact, even more responsibility has been placed on physicians for confidentiality breaches committed by their BAs. It is not enough to simply have a BAA in place; you are expected to use “reasonable diligence” in monitoring the work of your BAs. While BAs and their subcontractors are responsible for their own actions, the primary responsibility remains with you. Furthermore, you now must assume the worst-case scenario. Previously, when PHI was compromised, you would have to notify affected patients (and the government) only if there was a “significant risk of financial or reputational harm”; but now, any incident involving patient records is assumed to be a breach, and must be reported. Failure to do so could subject your practice, as well as the contractor, to significant fines.
If you haven’t yet revised your Notice of Privacy Practices (NPP) to explain your relationships with BAs, and their status under the new rules, do it now. (You should have done it last September.) You need to explain the breach notification process too, as well as the new patient rights mentioned above. You must post your revised NPP in your office, and make copies available there, but you need not mail a copy to every patient.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News.
Revision of the Health Insurance Portability and Accountability Act (HIPAA) rules has prompted numerous questions about business associates (BAs) and business associate agreements (BAAs). Apparently there is confusion about exactly which businesses qualify as BAs and how your BAAs should be modified to reflect the new provisions.
The criteria for identifying BAs are admittedly vague: The act defines them as nonemployees, performing “functions or activities” on behalf of the “covered entity” (your practice) that involve “creating, receiving, maintaining, or transmitting” personal health information (PHI).
Clearly, answering and billing services, independent transcriptionists, hardware and software companies, and any other vendors involved in creating or maintaining your medical records always qualify as BAs. Other businesses may or may not qualify, depending on whether they need direct access to PHI in order to provide their service. These include practice management consultants, attorneys, companies that store or microfilm medical records, and record-shredding services.
Specialty pharmacies are seldom mentioned in the BA discussion, but they probably should be. Pharmaceutical manufacturers are increasingly using them as intermediaries for their products – particularly the more expensive ones, such as biologics. Many of them ship products directly to patients, for which they require home addresses and other personal information, and in order to file payment paperwork and claim forms, they usually request diagnoses and associated medical information. By any reasonable interpretation of the new rules, this makes them BAs, and you should have BAAs in place before allowing them to fill your prescriptions.
To further complicate the situation, manufacturers and insurers routinely compile information about the real world uses of their products. To that end, they often ask specialty pharmacies to provide them with any patient data that they collect. Under the new rules, patients may restrict any PHI shared with third parties when patients pay for the drugs or services themselves. Your specialty pharmacy BAA should include a provision noting that the pharmacy is forbidden from disclosing any data to pharmaceutical companies or insurers from patients who self-pay and request confidentiality.
Mail carriers, package delivery people, cleaning services, copier repairmen, bank employees, and the like are not considered BAs. While they might conceivably come in contact with PHI on occasion, they don’t need it to do their job. You are required to use “reasonable diligence” in limiting the PHI that these folks may encounter, but you do not need to enter into written BA agreements with them.
Independent contractors who work within your practice – aestheticians and physical therapists, for example – are not considered BAs either, and do not need to sign a BA agreement. Just train them, as you do your employees.
Another source of confusion is the provision in the new rules that makes BAs directly responsible for their own HIPAA violations. While this might seem to eliminate the need for BAAs entirely, unfortunately that is not the case. In fact, even more responsibility has been placed on physicians for confidentiality breaches committed by their BAs. It is not enough to simply have a BAA in place; you are expected to use “reasonable diligence” in monitoring the work of your BAs. While BAs and their subcontractors are responsible for their own actions, the primary responsibility remains with you. Furthermore, you now must assume the worst-case scenario. Previously, when PHI was compromised, you would have to notify affected patients (and the government) only if there was a “significant risk of financial or reputational harm”; but now, any incident involving patient records is assumed to be a breach, and must be reported. Failure to do so could subject your practice, as well as the contractor, to significant fines.
If you haven’t yet revised your Notice of Privacy Practices (NPP) to explain your relationships with BAs, and their status under the new rules, do it now. (You should have done it last September.) You need to explain the breach notification process too, as well as the new patient rights mentioned above. You must post your revised NPP in your office, and make copies available there, but you need not mail a copy to every patient.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News.
Adolescents with eating disorders have high rates of psychiatric medication use
Adolescents with restrictive eating disorders had high rates of psychiatric comorbidity and medication use, reported Dr. Maria C. Monge of the division of adolescent/young adult medicine at Boston Children’s Hospital, and her coauthors.
In an analysis of 635 patients (359 of whom had follow-up after 1 year), 20.4% were taking psychopharmacologic medication at intake and 58.7% were taking medication at 1 year (P < .0001). The presence of psychiatric comorbidity was significantly associated with medication use (odds ratio, 10.0).
In 256 patients in which there was additional information about psychopharmacologic medication use, selective serotonin reuptake inhibitors (SSRIs)/serotonin-norepinephrine reuptake inhibitors (SNRIs) was the most common (83%), followed by anxiolytic (18%), antipsychotic (17%), other antidepressant (15%), mood stabilizer (8%), other psychopharmacologic medication not listed elsewhere (cyproheptadine, gabapentin, and guanfacine; 6%), stimulant (6%), and atomoxetine (1%). A total of 30% of patients on these medications were taking two or more at 1-year follow-up.
At 1-year follow-up, 63% of patients had a psychiatric comorbidity. Anxiety was the most common at 44%, followed by depression in 26%. Less-common diagnoses were attention-deficit/hyperactivity disorder in 2%, bipolar disorder in 0.5%, and other diagnoses in 2% including posttraumatic stress disorder, personality disorder, drug abuse, conversion disorder, trichotillomania, oppositional defiant disorder, and body dysmorphic disorder.
“Psychopharmacologic medications may be appropriate for treatment of comorbid conditions in these patients; however, it is unclear from existing studies if the addition of medication changes patients’ trajectories and provides an effective adjunct to weight restoration,” Dr. Monge and her colleagues said. “Continued investigation is needed to ensure [whether] the desired outcomes of the medications are being met, and the risks and rewards of such medications are fully understood in these patients.”
Read the full article here
Adolescents with restrictive eating disorders had high rates of psychiatric comorbidity and medication use, reported Dr. Maria C. Monge of the division of adolescent/young adult medicine at Boston Children’s Hospital, and her coauthors.
In an analysis of 635 patients (359 of whom had follow-up after 1 year), 20.4% were taking psychopharmacologic medication at intake and 58.7% were taking medication at 1 year (P < .0001). The presence of psychiatric comorbidity was significantly associated with medication use (odds ratio, 10.0).
In 256 patients in which there was additional information about psychopharmacologic medication use, selective serotonin reuptake inhibitors (SSRIs)/serotonin-norepinephrine reuptake inhibitors (SNRIs) was the most common (83%), followed by anxiolytic (18%), antipsychotic (17%), other antidepressant (15%), mood stabilizer (8%), other psychopharmacologic medication not listed elsewhere (cyproheptadine, gabapentin, and guanfacine; 6%), stimulant (6%), and atomoxetine (1%). A total of 30% of patients on these medications were taking two or more at 1-year follow-up.
At 1-year follow-up, 63% of patients had a psychiatric comorbidity. Anxiety was the most common at 44%, followed by depression in 26%. Less-common diagnoses were attention-deficit/hyperactivity disorder in 2%, bipolar disorder in 0.5%, and other diagnoses in 2% including posttraumatic stress disorder, personality disorder, drug abuse, conversion disorder, trichotillomania, oppositional defiant disorder, and body dysmorphic disorder.
“Psychopharmacologic medications may be appropriate for treatment of comorbid conditions in these patients; however, it is unclear from existing studies if the addition of medication changes patients’ trajectories and provides an effective adjunct to weight restoration,” Dr. Monge and her colleagues said. “Continued investigation is needed to ensure [whether] the desired outcomes of the medications are being met, and the risks and rewards of such medications are fully understood in these patients.”
Read the full article here
Adolescents with restrictive eating disorders had high rates of psychiatric comorbidity and medication use, reported Dr. Maria C. Monge of the division of adolescent/young adult medicine at Boston Children’s Hospital, and her coauthors.
In an analysis of 635 patients (359 of whom had follow-up after 1 year), 20.4% were taking psychopharmacologic medication at intake and 58.7% were taking medication at 1 year (P < .0001). The presence of psychiatric comorbidity was significantly associated with medication use (odds ratio, 10.0).
In 256 patients in which there was additional information about psychopharmacologic medication use, selective serotonin reuptake inhibitors (SSRIs)/serotonin-norepinephrine reuptake inhibitors (SNRIs) was the most common (83%), followed by anxiolytic (18%), antipsychotic (17%), other antidepressant (15%), mood stabilizer (8%), other psychopharmacologic medication not listed elsewhere (cyproheptadine, gabapentin, and guanfacine; 6%), stimulant (6%), and atomoxetine (1%). A total of 30% of patients on these medications were taking two or more at 1-year follow-up.
At 1-year follow-up, 63% of patients had a psychiatric comorbidity. Anxiety was the most common at 44%, followed by depression in 26%. Less-common diagnoses were attention-deficit/hyperactivity disorder in 2%, bipolar disorder in 0.5%, and other diagnoses in 2% including posttraumatic stress disorder, personality disorder, drug abuse, conversion disorder, trichotillomania, oppositional defiant disorder, and body dysmorphic disorder.
“Psychopharmacologic medications may be appropriate for treatment of comorbid conditions in these patients; however, it is unclear from existing studies if the addition of medication changes patients’ trajectories and provides an effective adjunct to weight restoration,” Dr. Monge and her colleagues said. “Continued investigation is needed to ensure [whether] the desired outcomes of the medications are being met, and the risks and rewards of such medications are fully understood in these patients.”
Read the full article here
The outlander’s tale
“Alien Physician” – thus am I ordained by the document that allows me to train in America. I am the International Medical Graduate. This is my story.
I came from poverty and hardship. In other cases, I came from wealth and privilege. Regardless, I clawed my way over staggering numbers of competitors to earn my shot at a medical education. Back home, I was top of my class, fresh out of medical school. In other cases, I was a respected practitioner with a wealth of experience. Now I am a blank slate.
I am here because I heard of a place where questions were encouraged and boundaries pushed. Or I am here because I learned of the American dream. They told me I would be judged for my merit here and nothing else. I have escaped persecution, war, nepotism, or perhaps just a bogged-down system to get here.
Although I have taken the same tests as you, my resume comes with an asterisk. I have had to prove myself by rising to a standard higher than that expected of my peers. Much of my time and peace of mind are consumed jumping through bureaucratic hoops in order to continue my stay. For every one of me you see, there is at least one more who was forced to give up on his or her dream midway.
Know this, however – I appreciate working in a system where the team is greater than the individual, where no job is menial, where the ability to make choices about your health is yours alone, to be shared should you need to.
Know that I am good company, although my jokes sometimes fall flat in the space between our two cultures. Learning new things about your country and its people makes me feel young again.
Know that I have a keen appreciation of how important cultural nuance is to the doctor-patient relationship, even if I come across as ignorant. I have gained this understanding not by reading about it in a book, but by living it.
Know that nothing is more important to me than the health of my patients, and that my being a foreigner does not mean your child will receive anything less than my very best.
Most importantly, know that I feel blessed to be here. While my journey is still uphill, I have no regrets. If I were not made of hard stuff, I would not be here. Although I may grumble, I shall endeavor to accept my lot with grace and humility in the knowledge that I have earned the right to practice the subject that I love and the cognizance that not everyone would open their arms to outsiders the way you have. It has meant the world to me. Working with you has opened my eyes to things I did not see before and has inspired me to be a better doctor. I hope in some small way my presence here affords you that same inspiration.
Dr. Behere was a pediatric resident at the Children’s Hospital at Dartmouth, Lebanon, N.H. when he wrote this article. He is currently a first-year fellow in pediatric cardiology at the Nemours Cardiac Center at the Nemours/Alfred I. duPont Hospital for Children, Wilmington, Del.
“Alien Physician” – thus am I ordained by the document that allows me to train in America. I am the International Medical Graduate. This is my story.
I came from poverty and hardship. In other cases, I came from wealth and privilege. Regardless, I clawed my way over staggering numbers of competitors to earn my shot at a medical education. Back home, I was top of my class, fresh out of medical school. In other cases, I was a respected practitioner with a wealth of experience. Now I am a blank slate.
I am here because I heard of a place where questions were encouraged and boundaries pushed. Or I am here because I learned of the American dream. They told me I would be judged for my merit here and nothing else. I have escaped persecution, war, nepotism, or perhaps just a bogged-down system to get here.
Although I have taken the same tests as you, my resume comes with an asterisk. I have had to prove myself by rising to a standard higher than that expected of my peers. Much of my time and peace of mind are consumed jumping through bureaucratic hoops in order to continue my stay. For every one of me you see, there is at least one more who was forced to give up on his or her dream midway.
Know this, however – I appreciate working in a system where the team is greater than the individual, where no job is menial, where the ability to make choices about your health is yours alone, to be shared should you need to.
Know that I am good company, although my jokes sometimes fall flat in the space between our two cultures. Learning new things about your country and its people makes me feel young again.
Know that I have a keen appreciation of how important cultural nuance is to the doctor-patient relationship, even if I come across as ignorant. I have gained this understanding not by reading about it in a book, but by living it.
Know that nothing is more important to me than the health of my patients, and that my being a foreigner does not mean your child will receive anything less than my very best.
Most importantly, know that I feel blessed to be here. While my journey is still uphill, I have no regrets. If I were not made of hard stuff, I would not be here. Although I may grumble, I shall endeavor to accept my lot with grace and humility in the knowledge that I have earned the right to practice the subject that I love and the cognizance that not everyone would open their arms to outsiders the way you have. It has meant the world to me. Working with you has opened my eyes to things I did not see before and has inspired me to be a better doctor. I hope in some small way my presence here affords you that same inspiration.
Dr. Behere was a pediatric resident at the Children’s Hospital at Dartmouth, Lebanon, N.H. when he wrote this article. He is currently a first-year fellow in pediatric cardiology at the Nemours Cardiac Center at the Nemours/Alfred I. duPont Hospital for Children, Wilmington, Del.
“Alien Physician” – thus am I ordained by the document that allows me to train in America. I am the International Medical Graduate. This is my story.
I came from poverty and hardship. In other cases, I came from wealth and privilege. Regardless, I clawed my way over staggering numbers of competitors to earn my shot at a medical education. Back home, I was top of my class, fresh out of medical school. In other cases, I was a respected practitioner with a wealth of experience. Now I am a blank slate.
I am here because I heard of a place where questions were encouraged and boundaries pushed. Or I am here because I learned of the American dream. They told me I would be judged for my merit here and nothing else. I have escaped persecution, war, nepotism, or perhaps just a bogged-down system to get here.
Although I have taken the same tests as you, my resume comes with an asterisk. I have had to prove myself by rising to a standard higher than that expected of my peers. Much of my time and peace of mind are consumed jumping through bureaucratic hoops in order to continue my stay. For every one of me you see, there is at least one more who was forced to give up on his or her dream midway.
Know this, however – I appreciate working in a system where the team is greater than the individual, where no job is menial, where the ability to make choices about your health is yours alone, to be shared should you need to.
Know that I am good company, although my jokes sometimes fall flat in the space between our two cultures. Learning new things about your country and its people makes me feel young again.
Know that I have a keen appreciation of how important cultural nuance is to the doctor-patient relationship, even if I come across as ignorant. I have gained this understanding not by reading about it in a book, but by living it.
Know that nothing is more important to me than the health of my patients, and that my being a foreigner does not mean your child will receive anything less than my very best.
Most importantly, know that I feel blessed to be here. While my journey is still uphill, I have no regrets. If I were not made of hard stuff, I would not be here. Although I may grumble, I shall endeavor to accept my lot with grace and humility in the knowledge that I have earned the right to practice the subject that I love and the cognizance that not everyone would open their arms to outsiders the way you have. It has meant the world to me. Working with you has opened my eyes to things I did not see before and has inspired me to be a better doctor. I hope in some small way my presence here affords you that same inspiration.
Dr. Behere was a pediatric resident at the Children’s Hospital at Dartmouth, Lebanon, N.H. when he wrote this article. He is currently a first-year fellow in pediatric cardiology at the Nemours Cardiac Center at the Nemours/Alfred I. duPont Hospital for Children, Wilmington, Del.
Allergic Contact Dermatitis for Residents
Allergic contact dermatitis (ACD) is a common inflammatory skin condition that affects more than 14 million Americans each year.1 It has been estimated that the economic burden of ACD is nearly $3 billion per year due to school absences, work time lost, and medical expenditures.1,2 In fact, skin diseases rank second to traumatic injuries as the most common type of occupational disease.3 As dermatology residents, we will encounter many patients with ACD, a potentially debilitating skin condition. In this column, I will discuss the different types of ACD as well as their differential diagnoses and management options according to the American Academy of Allergy, Asthma & Immunology’s updated practice parameter for contact dermatitis.4 The 2015 American Contact Dermatitis Society (ACDS) Allergen of the Year and the ACDS’s Contact Allergen Management Program also will be discussed.
Clinical Presentation and Pathophysiology
Allergic contact dermatitis is a widespread skin condition characterized by erythematous and pruritic skin lesions that occur after contact with external stimuli.5 It is caused by a type IV, T cell–mediated, delayed hypersensitivity reaction in which a foreign substance comes into contact with the skin and forms an antigen complex that subsequently leads to sensitization. Upon reexposure to the antigen, the sensitized T cells induce an inflammatory cascade causing the skin changes associated with ACD. Clinical presentations of ACD include vesicles and bullae with distinct angles, lines, and borders.6
Differential Diagnosis
In contrast to ACD, irritant contact dermatitis (the more common form of contact dermatitis) is a non–immune-modulated skin reaction that occurs when an individual is exposed to a substance that causes irritation and damage to the keratinocytes.6,7 It can be an acute reaction to a household cleaning product or a chronic reaction to soap if the patient has had exposure to the product for a prolonged period of time.7 The clinical presentation of irritant contact dermatitis includes dry and fissured skin with less distinct borders and negative patch test results.6
Some other skin diseases that should be considered in the differential diagnosis for suspected ACD include atopic dermatitis, dyshidrotic eczema, inverse psoriasis, latex allergy, palmoplantar psoriasis, scabies, and tinea pedis.5 When ACD is suspected, our diagnostic approach as dermatology residents should be based on a combination of the following factors: the clinical features of the skin reaction (eg, morphology, location, symptoms), the patient’s history of exposure to an alleged allergen and lack of exposure after treatment and/or avoidance, patch test results, laboratory test results, and/or histopathologic examination to exclude other disorders with similar clinical features.8
Management
Localized acute lesions of ACD can be successfully treated with mid- or high-potency topical steroids such as triamcinolone 0.1% or clobetasol 0.05%. If an extensive area of the skin (>20%) is affected, systemic steroid therapy often is required, generally offering relief within 12 to 24 hours. Caution should be taken when prescribing oral prednisone, such as for poison ivy, as it should be tapered over a few weeks to prevent rebound dermatitis. If treatment fails and the diagnosis or specific allergen remains unknown, patch testing should be performed.3,5
Updated Practice Parameter
Practice parameters for contact dermatitis were updated in 2015, as commissioned by the Joint Task Force on Practice Parameters, to address recent advances in the field of contact dermatitis and the most recommended methods for diagnosis and management based on the current scientific literature.4 Prior to this update, the most recent recommendations were from 2006.3
Since the publication of the original practice parameter, new questions have been addressed related to emerging clinical problems such as preoperative screening and postimplantation patch testing for metal allergy in patients undergoing joint replacement surgery. In the updated practice parameter, statements have been added that more comprehensively address evaluation and management of occupational contact dermatitis.4 The potential benefits and limitations of drug patch testing in patients with maculopapular rashes, erythroderma, and nonimmediate cutaneous reactions also have been addressed. New summary statements have been included that make recommendations on the management of ACD, particularly avoidance and prevention.4
ACDS Allergen of the Year
The purpose of this “award” is to recognize the agents that cause the most remarkable clinical effects, those that draw less attention, or those that exhibit exposure patterns that have changed. The ACDS’s 2015 Allergen of the Year is formaldehyde, an inexpensive biocidal preservative used in a wide range of products such as tissue specimen and cadaveric preservation solutions, nail polish, hair-smoothing treatments, and wrinkle-free fabrics.9
Formaldehyde-releasing preservatives (FRPs) are among the leading contact allergens and are found in many personal hygiene products, medications, and household cleansers.8 Specific sources of FRPs include shampoos, bodywashes, hand soaps, lotions, creams, baby wipes, mascara, disinfectants, fabric softeners, topical wart remedies, adhesives, and tissue specimen preservation solutions.10-13 According to de Groot et al,14 the US Food and Drug Administration’s Voluntary Cosmetic Registration Program database has estimated that approximately 20% of personal hygiene products and cosmetics contain an FRP, with imidazolidinyl urea as the most common.
It is important for patients to be aware of sources of formaldehyde exposure and understand that many products containing formaldehyde or FRPs may not list this information on their labels. In fact, one study reported that 33% of 67 moisturizers evaluated did not have proper labeling with regard to their formaldehyde/FRP content.15
Contact Allergen Management Program
During medical school I served as the Dermatology Interest Group Contact Dermatitis Awareness Chair at the University of Texas Medical Branch (Galveston, Texas) and was fortunate to have attended the annual meeting of the ACDS where I learned about the ACDS Contact Allergen Management Program (CAMP), an online resource for dermatologists to access that provides patients a printout list of allergen and cross-reactivity information for more than 1200 products (http://www.contactderm.org/i4a/pages/indexcfm?pageID=3489). This information helps consumers to choose the right products based on their allergies.
Final Thoughts
A thorough review of a patient’s medical history and, if needed, skin patch testing can identify the responsible allergen and initiate an appropriate avoidance plan for the patient. With appropriate avoidance, patients can achieve resolution of their dermatitis and prevent further episodes to substantially improve their quality of life and decrease health care costs.1 If left untreated, ACD can evolve from an acute form to a subacute form and eventually chronic eczematous dermatitis or progression to systemic disease.16,17 Allergic contact dermatitis can negatively impact an individual’s health-related quality of life, particularly in social functioning and psychological well-being.18,19 Therefore, it is pertinent in our role as dermatology residents to recognize ACD before its progression to a chronic state.
1. Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
2. Jacob SE. The lanolin-wool wax alcohol update. The Dermatologist. February 2014;22. http://www.the-dermatologist.com/content/lanolin-wool-wax-alcohol-update. Accessed June 26, 2015.
3. Beltrani VS, Bernstein IL, Cohen DE, et al. Contact dermatitis: a practice parameter [published correction appears in Ann Allergy Asthma Immunol. 2006;97:819]. Ann Allergy Asthma Immunol. 2006;97(3, suppl 2):S1-S38.
4. Fonacier L, Bernstein DI, Pacheco K, et al. Contact dermatitis: a practice parameter—update 2015. J Allergy Clin Immunol Pract. 2015;3(suppl 3):S1-S39.
5. Usatine R, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
6. Usatine RP. Contact dermatitis. In: Usatine RP, Smith M, Mayeaux EJ Jr, et al, eds. Color Atlas of Family Medicine. New York, NY: McGraw-Hill; 2009. http://accessmedicine.mhmedical.com/content.aspx?bookid=378&Sectionid=40419504. Accessed June 26, 2015.
7. Vazirnia A, Jacob SE. Review of ACDS’ allergen of the year 2010-2015. The Dermatologist. November 2014;22. http://www.the-dermatologist.com/content/review-acds%E2%80%99-allergen-od-year-2000-2015. Accessed June 26, 2015.
8. Yiannias J. Clinical features and diagnosis of allergic contact dermatitis. UpToDate Web site. http://www.uptodate.com/contents/clinical-features-and-diagnosis-of-allergic-contact-dermatitis?source=search_result&search=allergic+contact+dermatitis&selectedTitle=2~142#. Updated May 20, 2014. Accessed June 18, 2015.
9. Pontén A, Bruze M. Formaldehyde. Dermatitis. 2015;26:3-6.
10. Maier LE, Lampel HP, Bhutani T, et al. Hand dermatitis: a focus on allergic contact dermatitis to biocides. Dermatol Clin. 2009;27:251-264.
11. Marks JG, Elsner P, DeLeo VA. Contact & Occupational Dermatology. 3rd ed. St. Louis, MO: Mosby; 2002.
12. Rietschel RL, Fowler JF Jr, eds. Fisher’s Contact Dermatitis. 6th ed. Hamilton, ON: BC Decker Inc; 2008.
13. Sasseville D. Hypersensitivity to preservatives. Dermatol Ther. 2004;17:251-263.
14. de Groot AC, White IR, Flyvholm MA, et al. Formaldehyde-releasers in cosmetics: relationship to formaldehyde contact allergy. part 1. characterization, frequency and relevance of sensitization, and frequency of use in cosmetics. Contact Dermatitis. 2010;62:2-17.
15. Rastogi SC. Analytical control of preservative labeling on skin creams. Contact Dermatitis. 2000;43:339-343.
16. Hsu JW, Matiz C, Jacob SE. Nickel allergy: localized, id, and systemic manifestations in children. Pediatr Dermatol. 2011;28:276-280.
17. Salam TN, Fowler JF Jr. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
18. Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158.
19. Hutchings CV, Shum KW, Gawkrodger DJ. Occupational contact dermatitis has an appreciable impact on quality of life. Contact Dermatitis. 2001;45:17-20.
Allergic contact dermatitis (ACD) is a common inflammatory skin condition that affects more than 14 million Americans each year.1 It has been estimated that the economic burden of ACD is nearly $3 billion per year due to school absences, work time lost, and medical expenditures.1,2 In fact, skin diseases rank second to traumatic injuries as the most common type of occupational disease.3 As dermatology residents, we will encounter many patients with ACD, a potentially debilitating skin condition. In this column, I will discuss the different types of ACD as well as their differential diagnoses and management options according to the American Academy of Allergy, Asthma & Immunology’s updated practice parameter for contact dermatitis.4 The 2015 American Contact Dermatitis Society (ACDS) Allergen of the Year and the ACDS’s Contact Allergen Management Program also will be discussed.
Clinical Presentation and Pathophysiology
Allergic contact dermatitis is a widespread skin condition characterized by erythematous and pruritic skin lesions that occur after contact with external stimuli.5 It is caused by a type IV, T cell–mediated, delayed hypersensitivity reaction in which a foreign substance comes into contact with the skin and forms an antigen complex that subsequently leads to sensitization. Upon reexposure to the antigen, the sensitized T cells induce an inflammatory cascade causing the skin changes associated with ACD. Clinical presentations of ACD include vesicles and bullae with distinct angles, lines, and borders.6
Differential Diagnosis
In contrast to ACD, irritant contact dermatitis (the more common form of contact dermatitis) is a non–immune-modulated skin reaction that occurs when an individual is exposed to a substance that causes irritation and damage to the keratinocytes.6,7 It can be an acute reaction to a household cleaning product or a chronic reaction to soap if the patient has had exposure to the product for a prolonged period of time.7 The clinical presentation of irritant contact dermatitis includes dry and fissured skin with less distinct borders and negative patch test results.6
Some other skin diseases that should be considered in the differential diagnosis for suspected ACD include atopic dermatitis, dyshidrotic eczema, inverse psoriasis, latex allergy, palmoplantar psoriasis, scabies, and tinea pedis.5 When ACD is suspected, our diagnostic approach as dermatology residents should be based on a combination of the following factors: the clinical features of the skin reaction (eg, morphology, location, symptoms), the patient’s history of exposure to an alleged allergen and lack of exposure after treatment and/or avoidance, patch test results, laboratory test results, and/or histopathologic examination to exclude other disorders with similar clinical features.8
Management
Localized acute lesions of ACD can be successfully treated with mid- or high-potency topical steroids such as triamcinolone 0.1% or clobetasol 0.05%. If an extensive area of the skin (>20%) is affected, systemic steroid therapy often is required, generally offering relief within 12 to 24 hours. Caution should be taken when prescribing oral prednisone, such as for poison ivy, as it should be tapered over a few weeks to prevent rebound dermatitis. If treatment fails and the diagnosis or specific allergen remains unknown, patch testing should be performed.3,5
Updated Practice Parameter
Practice parameters for contact dermatitis were updated in 2015, as commissioned by the Joint Task Force on Practice Parameters, to address recent advances in the field of contact dermatitis and the most recommended methods for diagnosis and management based on the current scientific literature.4 Prior to this update, the most recent recommendations were from 2006.3
Since the publication of the original practice parameter, new questions have been addressed related to emerging clinical problems such as preoperative screening and postimplantation patch testing for metal allergy in patients undergoing joint replacement surgery. In the updated practice parameter, statements have been added that more comprehensively address evaluation and management of occupational contact dermatitis.4 The potential benefits and limitations of drug patch testing in patients with maculopapular rashes, erythroderma, and nonimmediate cutaneous reactions also have been addressed. New summary statements have been included that make recommendations on the management of ACD, particularly avoidance and prevention.4
ACDS Allergen of the Year
The purpose of this “award” is to recognize the agents that cause the most remarkable clinical effects, those that draw less attention, or those that exhibit exposure patterns that have changed. The ACDS’s 2015 Allergen of the Year is formaldehyde, an inexpensive biocidal preservative used in a wide range of products such as tissue specimen and cadaveric preservation solutions, nail polish, hair-smoothing treatments, and wrinkle-free fabrics.9
Formaldehyde-releasing preservatives (FRPs) are among the leading contact allergens and are found in many personal hygiene products, medications, and household cleansers.8 Specific sources of FRPs include shampoos, bodywashes, hand soaps, lotions, creams, baby wipes, mascara, disinfectants, fabric softeners, topical wart remedies, adhesives, and tissue specimen preservation solutions.10-13 According to de Groot et al,14 the US Food and Drug Administration’s Voluntary Cosmetic Registration Program database has estimated that approximately 20% of personal hygiene products and cosmetics contain an FRP, with imidazolidinyl urea as the most common.
It is important for patients to be aware of sources of formaldehyde exposure and understand that many products containing formaldehyde or FRPs may not list this information on their labels. In fact, one study reported that 33% of 67 moisturizers evaluated did not have proper labeling with regard to their formaldehyde/FRP content.15
Contact Allergen Management Program
During medical school I served as the Dermatology Interest Group Contact Dermatitis Awareness Chair at the University of Texas Medical Branch (Galveston, Texas) and was fortunate to have attended the annual meeting of the ACDS where I learned about the ACDS Contact Allergen Management Program (CAMP), an online resource for dermatologists to access that provides patients a printout list of allergen and cross-reactivity information for more than 1200 products (http://www.contactderm.org/i4a/pages/indexcfm?pageID=3489). This information helps consumers to choose the right products based on their allergies.
Final Thoughts
A thorough review of a patient’s medical history and, if needed, skin patch testing can identify the responsible allergen and initiate an appropriate avoidance plan for the patient. With appropriate avoidance, patients can achieve resolution of their dermatitis and prevent further episodes to substantially improve their quality of life and decrease health care costs.1 If left untreated, ACD can evolve from an acute form to a subacute form and eventually chronic eczematous dermatitis or progression to systemic disease.16,17 Allergic contact dermatitis can negatively impact an individual’s health-related quality of life, particularly in social functioning and psychological well-being.18,19 Therefore, it is pertinent in our role as dermatology residents to recognize ACD before its progression to a chronic state.
Allergic contact dermatitis (ACD) is a common inflammatory skin condition that affects more than 14 million Americans each year.1 It has been estimated that the economic burden of ACD is nearly $3 billion per year due to school absences, work time lost, and medical expenditures.1,2 In fact, skin diseases rank second to traumatic injuries as the most common type of occupational disease.3 As dermatology residents, we will encounter many patients with ACD, a potentially debilitating skin condition. In this column, I will discuss the different types of ACD as well as their differential diagnoses and management options according to the American Academy of Allergy, Asthma & Immunology’s updated practice parameter for contact dermatitis.4 The 2015 American Contact Dermatitis Society (ACDS) Allergen of the Year and the ACDS’s Contact Allergen Management Program also will be discussed.
Clinical Presentation and Pathophysiology
Allergic contact dermatitis is a widespread skin condition characterized by erythematous and pruritic skin lesions that occur after contact with external stimuli.5 It is caused by a type IV, T cell–mediated, delayed hypersensitivity reaction in which a foreign substance comes into contact with the skin and forms an antigen complex that subsequently leads to sensitization. Upon reexposure to the antigen, the sensitized T cells induce an inflammatory cascade causing the skin changes associated with ACD. Clinical presentations of ACD include vesicles and bullae with distinct angles, lines, and borders.6
Differential Diagnosis
In contrast to ACD, irritant contact dermatitis (the more common form of contact dermatitis) is a non–immune-modulated skin reaction that occurs when an individual is exposed to a substance that causes irritation and damage to the keratinocytes.6,7 It can be an acute reaction to a household cleaning product or a chronic reaction to soap if the patient has had exposure to the product for a prolonged period of time.7 The clinical presentation of irritant contact dermatitis includes dry and fissured skin with less distinct borders and negative patch test results.6
Some other skin diseases that should be considered in the differential diagnosis for suspected ACD include atopic dermatitis, dyshidrotic eczema, inverse psoriasis, latex allergy, palmoplantar psoriasis, scabies, and tinea pedis.5 When ACD is suspected, our diagnostic approach as dermatology residents should be based on a combination of the following factors: the clinical features of the skin reaction (eg, morphology, location, symptoms), the patient’s history of exposure to an alleged allergen and lack of exposure after treatment and/or avoidance, patch test results, laboratory test results, and/or histopathologic examination to exclude other disorders with similar clinical features.8
Management
Localized acute lesions of ACD can be successfully treated with mid- or high-potency topical steroids such as triamcinolone 0.1% or clobetasol 0.05%. If an extensive area of the skin (>20%) is affected, systemic steroid therapy often is required, generally offering relief within 12 to 24 hours. Caution should be taken when prescribing oral prednisone, such as for poison ivy, as it should be tapered over a few weeks to prevent rebound dermatitis. If treatment fails and the diagnosis or specific allergen remains unknown, patch testing should be performed.3,5
Updated Practice Parameter
Practice parameters for contact dermatitis were updated in 2015, as commissioned by the Joint Task Force on Practice Parameters, to address recent advances in the field of contact dermatitis and the most recommended methods for diagnosis and management based on the current scientific literature.4 Prior to this update, the most recent recommendations were from 2006.3
Since the publication of the original practice parameter, new questions have been addressed related to emerging clinical problems such as preoperative screening and postimplantation patch testing for metal allergy in patients undergoing joint replacement surgery. In the updated practice parameter, statements have been added that more comprehensively address evaluation and management of occupational contact dermatitis.4 The potential benefits and limitations of drug patch testing in patients with maculopapular rashes, erythroderma, and nonimmediate cutaneous reactions also have been addressed. New summary statements have been included that make recommendations on the management of ACD, particularly avoidance and prevention.4
ACDS Allergen of the Year
The purpose of this “award” is to recognize the agents that cause the most remarkable clinical effects, those that draw less attention, or those that exhibit exposure patterns that have changed. The ACDS’s 2015 Allergen of the Year is formaldehyde, an inexpensive biocidal preservative used in a wide range of products such as tissue specimen and cadaveric preservation solutions, nail polish, hair-smoothing treatments, and wrinkle-free fabrics.9
Formaldehyde-releasing preservatives (FRPs) are among the leading contact allergens and are found in many personal hygiene products, medications, and household cleansers.8 Specific sources of FRPs include shampoos, bodywashes, hand soaps, lotions, creams, baby wipes, mascara, disinfectants, fabric softeners, topical wart remedies, adhesives, and tissue specimen preservation solutions.10-13 According to de Groot et al,14 the US Food and Drug Administration’s Voluntary Cosmetic Registration Program database has estimated that approximately 20% of personal hygiene products and cosmetics contain an FRP, with imidazolidinyl urea as the most common.
It is important for patients to be aware of sources of formaldehyde exposure and understand that many products containing formaldehyde or FRPs may not list this information on their labels. In fact, one study reported that 33% of 67 moisturizers evaluated did not have proper labeling with regard to their formaldehyde/FRP content.15
Contact Allergen Management Program
During medical school I served as the Dermatology Interest Group Contact Dermatitis Awareness Chair at the University of Texas Medical Branch (Galveston, Texas) and was fortunate to have attended the annual meeting of the ACDS where I learned about the ACDS Contact Allergen Management Program (CAMP), an online resource for dermatologists to access that provides patients a printout list of allergen and cross-reactivity information for more than 1200 products (http://www.contactderm.org/i4a/pages/indexcfm?pageID=3489). This information helps consumers to choose the right products based on their allergies.
Final Thoughts
A thorough review of a patient’s medical history and, if needed, skin patch testing can identify the responsible allergen and initiate an appropriate avoidance plan for the patient. With appropriate avoidance, patients can achieve resolution of their dermatitis and prevent further episodes to substantially improve their quality of life and decrease health care costs.1 If left untreated, ACD can evolve from an acute form to a subacute form and eventually chronic eczematous dermatitis or progression to systemic disease.16,17 Allergic contact dermatitis can negatively impact an individual’s health-related quality of life, particularly in social functioning and psychological well-being.18,19 Therefore, it is pertinent in our role as dermatology residents to recognize ACD before its progression to a chronic state.
1. Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
2. Jacob SE. The lanolin-wool wax alcohol update. The Dermatologist. February 2014;22. http://www.the-dermatologist.com/content/lanolin-wool-wax-alcohol-update. Accessed June 26, 2015.
3. Beltrani VS, Bernstein IL, Cohen DE, et al. Contact dermatitis: a practice parameter [published correction appears in Ann Allergy Asthma Immunol. 2006;97:819]. Ann Allergy Asthma Immunol. 2006;97(3, suppl 2):S1-S38.
4. Fonacier L, Bernstein DI, Pacheco K, et al. Contact dermatitis: a practice parameter—update 2015. J Allergy Clin Immunol Pract. 2015;3(suppl 3):S1-S39.
5. Usatine R, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
6. Usatine RP. Contact dermatitis. In: Usatine RP, Smith M, Mayeaux EJ Jr, et al, eds. Color Atlas of Family Medicine. New York, NY: McGraw-Hill; 2009. http://accessmedicine.mhmedical.com/content.aspx?bookid=378&Sectionid=40419504. Accessed June 26, 2015.
7. Vazirnia A, Jacob SE. Review of ACDS’ allergen of the year 2010-2015. The Dermatologist. November 2014;22. http://www.the-dermatologist.com/content/review-acds%E2%80%99-allergen-od-year-2000-2015. Accessed June 26, 2015.
8. Yiannias J. Clinical features and diagnosis of allergic contact dermatitis. UpToDate Web site. http://www.uptodate.com/contents/clinical-features-and-diagnosis-of-allergic-contact-dermatitis?source=search_result&search=allergic+contact+dermatitis&selectedTitle=2~142#. Updated May 20, 2014. Accessed June 18, 2015.
9. Pontén A, Bruze M. Formaldehyde. Dermatitis. 2015;26:3-6.
10. Maier LE, Lampel HP, Bhutani T, et al. Hand dermatitis: a focus on allergic contact dermatitis to biocides. Dermatol Clin. 2009;27:251-264.
11. Marks JG, Elsner P, DeLeo VA. Contact & Occupational Dermatology. 3rd ed. St. Louis, MO: Mosby; 2002.
12. Rietschel RL, Fowler JF Jr, eds. Fisher’s Contact Dermatitis. 6th ed. Hamilton, ON: BC Decker Inc; 2008.
13. Sasseville D. Hypersensitivity to preservatives. Dermatol Ther. 2004;17:251-263.
14. de Groot AC, White IR, Flyvholm MA, et al. Formaldehyde-releasers in cosmetics: relationship to formaldehyde contact allergy. part 1. characterization, frequency and relevance of sensitization, and frequency of use in cosmetics. Contact Dermatitis. 2010;62:2-17.
15. Rastogi SC. Analytical control of preservative labeling on skin creams. Contact Dermatitis. 2000;43:339-343.
16. Hsu JW, Matiz C, Jacob SE. Nickel allergy: localized, id, and systemic manifestations in children. Pediatr Dermatol. 2011;28:276-280.
17. Salam TN, Fowler JF Jr. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
18. Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158.
19. Hutchings CV, Shum KW, Gawkrodger DJ. Occupational contact dermatitis has an appreciable impact on quality of life. Contact Dermatitis. 2001;45:17-20.
1. Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
2. Jacob SE. The lanolin-wool wax alcohol update. The Dermatologist. February 2014;22. http://www.the-dermatologist.com/content/lanolin-wool-wax-alcohol-update. Accessed June 26, 2015.
3. Beltrani VS, Bernstein IL, Cohen DE, et al. Contact dermatitis: a practice parameter [published correction appears in Ann Allergy Asthma Immunol. 2006;97:819]. Ann Allergy Asthma Immunol. 2006;97(3, suppl 2):S1-S38.
4. Fonacier L, Bernstein DI, Pacheco K, et al. Contact dermatitis: a practice parameter—update 2015. J Allergy Clin Immunol Pract. 2015;3(suppl 3):S1-S39.
5. Usatine R, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
6. Usatine RP. Contact dermatitis. In: Usatine RP, Smith M, Mayeaux EJ Jr, et al, eds. Color Atlas of Family Medicine. New York, NY: McGraw-Hill; 2009. http://accessmedicine.mhmedical.com/content.aspx?bookid=378&Sectionid=40419504. Accessed June 26, 2015.
7. Vazirnia A, Jacob SE. Review of ACDS’ allergen of the year 2010-2015. The Dermatologist. November 2014;22. http://www.the-dermatologist.com/content/review-acds%E2%80%99-allergen-od-year-2000-2015. Accessed June 26, 2015.
8. Yiannias J. Clinical features and diagnosis of allergic contact dermatitis. UpToDate Web site. http://www.uptodate.com/contents/clinical-features-and-diagnosis-of-allergic-contact-dermatitis?source=search_result&search=allergic+contact+dermatitis&selectedTitle=2~142#. Updated May 20, 2014. Accessed June 18, 2015.
9. Pontén A, Bruze M. Formaldehyde. Dermatitis. 2015;26:3-6.
10. Maier LE, Lampel HP, Bhutani T, et al. Hand dermatitis: a focus on allergic contact dermatitis to biocides. Dermatol Clin. 2009;27:251-264.
11. Marks JG, Elsner P, DeLeo VA. Contact & Occupational Dermatology. 3rd ed. St. Louis, MO: Mosby; 2002.
12. Rietschel RL, Fowler JF Jr, eds. Fisher’s Contact Dermatitis. 6th ed. Hamilton, ON: BC Decker Inc; 2008.
13. Sasseville D. Hypersensitivity to preservatives. Dermatol Ther. 2004;17:251-263.
14. de Groot AC, White IR, Flyvholm MA, et al. Formaldehyde-releasers in cosmetics: relationship to formaldehyde contact allergy. part 1. characterization, frequency and relevance of sensitization, and frequency of use in cosmetics. Contact Dermatitis. 2010;62:2-17.
15. Rastogi SC. Analytical control of preservative labeling on skin creams. Contact Dermatitis. 2000;43:339-343.
16. Hsu JW, Matiz C, Jacob SE. Nickel allergy: localized, id, and systemic manifestations in children. Pediatr Dermatol. 2011;28:276-280.
17. Salam TN, Fowler JF Jr. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
18. Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158.
19. Hutchings CV, Shum KW, Gawkrodger DJ. Occupational contact dermatitis has an appreciable impact on quality of life. Contact Dermatitis. 2001;45:17-20.
New antimalarial progresses to clinical trials

falciparum
in a mouse liverImage courtesy of Seattle
Biomedical Research Institute
A new compound can fight multidrug-resistant malaria, according to preclinical research published in Science Translational Medicine.
The compound, DSM265, targets DHODH, an enzyme involved in the malaria parasite’s production of nucleotides.
Experiments showed that DSM265 can target the parasite at both the blood and liver stages of infection. And the compound appeared to be safe.
Based on these results, DSM265 has advanced to clinical trials.
Investigators believe the drug could potentially be partnered with other antimalarials for a single-dose treatment or once-weekly prophylaxis.
“This is the first of a new class of molecules that’s going into humans,” said study author Pradipsinh Rathod, PhD, of the University of Washington in Seattle.
“Until now, everything else in humans has been variations of drugs that have been developed in the distant past.”
The investigators tested DSM265 in multiple models, including human cells, rodents, dogs, and monkeys.
DSM265 arrested growth of the Plasmodium falciparum parasite at both the blood and liver stages. And the drug was active against P falciparum strains that were resistant to chloroquine and pyrimethamine.
DSM265 also proved to be long-acting. Experiments suggested a dose of 200 mg to 400 mg would maintain therapeutic concentrations in humans for at least 8 days.
Repeated doses of DSM265, given to mice and dogs, did not cause major side effects. The researchers said DSM265 was well tolerated at all dose levels given to mice (25 to 200 mg/kg per day once daily for 7 days) and dogs (30 to 480 mg/kg every other day for 10 days).
None of the animals died, and there were no clinical effects observed in the mice. Dogs receiving the highest dose of DSM265 experienced vomiting, apparent loss of appetite, and a slight increase in bilirubin that was not accompanied by changes in any other liver function markers.
Dr Rathod said he hopes the development and discovery pipeline for DSM265 will pave the way for a faster and more collaborative drug development process in “the long war against malaria.”
In an attempt to accelerate the drug’s development, the investigators transferred their patent rights for DSM265 to the Medicines for Malaria Venture, a Bill & Melinda Gates Foundation-supported nonprofit that is leading some of the clinical and field trials. ![]()

falciparum
in a mouse liverImage courtesy of Seattle
Biomedical Research Institute
A new compound can fight multidrug-resistant malaria, according to preclinical research published in Science Translational Medicine.
The compound, DSM265, targets DHODH, an enzyme involved in the malaria parasite’s production of nucleotides.
Experiments showed that DSM265 can target the parasite at both the blood and liver stages of infection. And the compound appeared to be safe.
Based on these results, DSM265 has advanced to clinical trials.
Investigators believe the drug could potentially be partnered with other antimalarials for a single-dose treatment or once-weekly prophylaxis.
“This is the first of a new class of molecules that’s going into humans,” said study author Pradipsinh Rathod, PhD, of the University of Washington in Seattle.
“Until now, everything else in humans has been variations of drugs that have been developed in the distant past.”
The investigators tested DSM265 in multiple models, including human cells, rodents, dogs, and monkeys.
DSM265 arrested growth of the Plasmodium falciparum parasite at both the blood and liver stages. And the drug was active against P falciparum strains that were resistant to chloroquine and pyrimethamine.
DSM265 also proved to be long-acting. Experiments suggested a dose of 200 mg to 400 mg would maintain therapeutic concentrations in humans for at least 8 days.
Repeated doses of DSM265, given to mice and dogs, did not cause major side effects. The researchers said DSM265 was well tolerated at all dose levels given to mice (25 to 200 mg/kg per day once daily for 7 days) and dogs (30 to 480 mg/kg every other day for 10 days).
None of the animals died, and there were no clinical effects observed in the mice. Dogs receiving the highest dose of DSM265 experienced vomiting, apparent loss of appetite, and a slight increase in bilirubin that was not accompanied by changes in any other liver function markers.
Dr Rathod said he hopes the development and discovery pipeline for DSM265 will pave the way for a faster and more collaborative drug development process in “the long war against malaria.”
In an attempt to accelerate the drug’s development, the investigators transferred their patent rights for DSM265 to the Medicines for Malaria Venture, a Bill & Melinda Gates Foundation-supported nonprofit that is leading some of the clinical and field trials. ![]()

falciparum
in a mouse liverImage courtesy of Seattle
Biomedical Research Institute
A new compound can fight multidrug-resistant malaria, according to preclinical research published in Science Translational Medicine.
The compound, DSM265, targets DHODH, an enzyme involved in the malaria parasite’s production of nucleotides.
Experiments showed that DSM265 can target the parasite at both the blood and liver stages of infection. And the compound appeared to be safe.
Based on these results, DSM265 has advanced to clinical trials.
Investigators believe the drug could potentially be partnered with other antimalarials for a single-dose treatment or once-weekly prophylaxis.
“This is the first of a new class of molecules that’s going into humans,” said study author Pradipsinh Rathod, PhD, of the University of Washington in Seattle.
“Until now, everything else in humans has been variations of drugs that have been developed in the distant past.”
The investigators tested DSM265 in multiple models, including human cells, rodents, dogs, and monkeys.
DSM265 arrested growth of the Plasmodium falciparum parasite at both the blood and liver stages. And the drug was active against P falciparum strains that were resistant to chloroquine and pyrimethamine.
DSM265 also proved to be long-acting. Experiments suggested a dose of 200 mg to 400 mg would maintain therapeutic concentrations in humans for at least 8 days.
Repeated doses of DSM265, given to mice and dogs, did not cause major side effects. The researchers said DSM265 was well tolerated at all dose levels given to mice (25 to 200 mg/kg per day once daily for 7 days) and dogs (30 to 480 mg/kg every other day for 10 days).
None of the animals died, and there were no clinical effects observed in the mice. Dogs receiving the highest dose of DSM265 experienced vomiting, apparent loss of appetite, and a slight increase in bilirubin that was not accompanied by changes in any other liver function markers.
Dr Rathod said he hopes the development and discovery pipeline for DSM265 will pave the way for a faster and more collaborative drug development process in “the long war against malaria.”
In an attempt to accelerate the drug’s development, the investigators transferred their patent rights for DSM265 to the Medicines for Malaria Venture, a Bill & Melinda Gates Foundation-supported nonprofit that is leading some of the clinical and field trials. ![]()
Neutrophils cause hemorrhage in thrombocytopenia

Image by Volker Brinkmann
Researchers have found that hemorrhage occurs in the context of thrombocytopenia when neutrophils cross the endothelial barrier.
The team also identified ways to inhibit this diapedesis and prevent hemorrhage in mouse models.
If these results can be replicated in humans, such interventions could prevent bleeding in patients with immune thrombocytopenia and certain patients receiving chemotherapy or hematopoietic stem cell transplant.
Carina Hillgruber, PhD, of the University of Munster in Germany, and her colleagues conducted this research and detailed the results in the Journal of Experimental Medicine.
Previous studies showed that an absence of platelets alone was not sufficient to cause hemorrhage. Inflammation was required. But the exact cause of bleeding complications in thrombocytopenia was unknown.
With their research, Dr Hillgruber and her colleagues found that neutrophils are recruited to inflammatory sites to induce thrombocytopenic tissue hemorrhage. The process consists of Gαi2-mediated neutrophil transmigratory activity and opening of the endothelial barrier via VE-cadherin.
So the researchers speculated that preventing neutrophil diapedesis by either tightening the endothelial barrier or targeting neutrophil transmigratory activity would prevent hemorrhage.
The team showed they could inhibit the interaction between neutrophils and the endothelium by interfering with P-selectin, β2integrin-mediated adhesion, and chemokine signaling. This led to reduced inflammatory bleeding in thrombocytopenic mice.
The researchers also found they could interfere with Gαi signaling in neutrophils (which is critical for the cells’ transmigration) via treatment with pertussis toxin or gene ablation. And this protected thrombocytopenic mice from cutaneous hemorrhage.
Finally, the team showed that mice harboring the VE-cadherin mutation Y731F were protected from hemorrhage despite having low platelet counts and immune complex-mediated vasculitis.
The researchers said these findings suggest therapeutically targeting neutrophil diapedesis through the endothelial barrier could potentially prevent hemorrhage in patients with thrombocytopenia.
The team noted that platelets must seal the damage induced by neutrophil diapedesis, but they could only speculate as to how that occurs. ![]()

Image by Volker Brinkmann
Researchers have found that hemorrhage occurs in the context of thrombocytopenia when neutrophils cross the endothelial barrier.
The team also identified ways to inhibit this diapedesis and prevent hemorrhage in mouse models.
If these results can be replicated in humans, such interventions could prevent bleeding in patients with immune thrombocytopenia and certain patients receiving chemotherapy or hematopoietic stem cell transplant.
Carina Hillgruber, PhD, of the University of Munster in Germany, and her colleagues conducted this research and detailed the results in the Journal of Experimental Medicine.
Previous studies showed that an absence of platelets alone was not sufficient to cause hemorrhage. Inflammation was required. But the exact cause of bleeding complications in thrombocytopenia was unknown.
With their research, Dr Hillgruber and her colleagues found that neutrophils are recruited to inflammatory sites to induce thrombocytopenic tissue hemorrhage. The process consists of Gαi2-mediated neutrophil transmigratory activity and opening of the endothelial barrier via VE-cadherin.
So the researchers speculated that preventing neutrophil diapedesis by either tightening the endothelial barrier or targeting neutrophil transmigratory activity would prevent hemorrhage.
The team showed they could inhibit the interaction between neutrophils and the endothelium by interfering with P-selectin, β2integrin-mediated adhesion, and chemokine signaling. This led to reduced inflammatory bleeding in thrombocytopenic mice.
The researchers also found they could interfere with Gαi signaling in neutrophils (which is critical for the cells’ transmigration) via treatment with pertussis toxin or gene ablation. And this protected thrombocytopenic mice from cutaneous hemorrhage.
Finally, the team showed that mice harboring the VE-cadherin mutation Y731F were protected from hemorrhage despite having low platelet counts and immune complex-mediated vasculitis.
The researchers said these findings suggest therapeutically targeting neutrophil diapedesis through the endothelial barrier could potentially prevent hemorrhage in patients with thrombocytopenia.
The team noted that platelets must seal the damage induced by neutrophil diapedesis, but they could only speculate as to how that occurs. ![]()

Image by Volker Brinkmann
Researchers have found that hemorrhage occurs in the context of thrombocytopenia when neutrophils cross the endothelial barrier.
The team also identified ways to inhibit this diapedesis and prevent hemorrhage in mouse models.
If these results can be replicated in humans, such interventions could prevent bleeding in patients with immune thrombocytopenia and certain patients receiving chemotherapy or hematopoietic stem cell transplant.
Carina Hillgruber, PhD, of the University of Munster in Germany, and her colleagues conducted this research and detailed the results in the Journal of Experimental Medicine.
Previous studies showed that an absence of platelets alone was not sufficient to cause hemorrhage. Inflammation was required. But the exact cause of bleeding complications in thrombocytopenia was unknown.
With their research, Dr Hillgruber and her colleagues found that neutrophils are recruited to inflammatory sites to induce thrombocytopenic tissue hemorrhage. The process consists of Gαi2-mediated neutrophil transmigratory activity and opening of the endothelial barrier via VE-cadherin.
So the researchers speculated that preventing neutrophil diapedesis by either tightening the endothelial barrier or targeting neutrophil transmigratory activity would prevent hemorrhage.
The team showed they could inhibit the interaction between neutrophils and the endothelium by interfering with P-selectin, β2integrin-mediated adhesion, and chemokine signaling. This led to reduced inflammatory bleeding in thrombocytopenic mice.
The researchers also found they could interfere with Gαi signaling in neutrophils (which is critical for the cells’ transmigration) via treatment with pertussis toxin or gene ablation. And this protected thrombocytopenic mice from cutaneous hemorrhage.
Finally, the team showed that mice harboring the VE-cadherin mutation Y731F were protected from hemorrhage despite having low platelet counts and immune complex-mediated vasculitis.
The researchers said these findings suggest therapeutically targeting neutrophil diapedesis through the endothelial barrier could potentially prevent hemorrhage in patients with thrombocytopenia.
The team noted that platelets must seal the damage induced by neutrophil diapedesis, but they could only speculate as to how that occurs. ![]()
Drug granted orphan designation for MM

The US Food and Drug Administration (FDA) has granted orphan designation for the drug CB-5083 to treat multiple myeloma (MM).
CB-5083 works by inhibiting p97, an enzyme that controls various aspects of protein homeostasis.
Inhibiting p97 function in cancers has been shown to generate irresolvable endoplasmic reticulum stress that induces a lethal unfolded protein response, which leads to apoptosis and antitumor activity.
CB-5083 has exhibited anticancer activity in preclinical research, and the drug is currently under investigation in two phase 1 studies—one in MM and one in solid tumor malignancies.
Research in MM
Preclinical research presented at ASH 2014 showed that CB-5083 inhibits MM cell viability in vitro. The drug also inhibited tumor growth in xenograft models of MM and in an orthometastatic model of MM.
In addition, CB-5083 demonstrated synergy with carfilzomib, bortezomib, and combination lenalidomide-dexamethasone.
CB-5083 is now under investigation in a phase 1 dose-escalation/dose-expansion trial of MM patients.
Researchers are evaluating the safety, pharmacokinetics, pharmacodynamics, and antitumor activity of the drug in patients who have relapsed/refractory or refractory MM after receiving 2 or more lines of therapy, including an immunomodulatory agent and a proteasome inhibitor.
The goal is to enroll up to 60 patients in this trial at multiple US cancer centers that are part of the Multiple Myeloma Research Consortium. More information about the trial is available at www.clinicaltrials.gov (NCT02223598).
About orphan designation
The FDA grants orphan designation to drugs that are intended to treat diseases or conditions affecting fewer than 200,000 patients in the US. Orphan designation provides the sponsor of a drug with various development incentives.
The orphan designation for CB-5083 provides Cleave Biosciences with opportunities to apply for research-related tax credits and grant funding, assistance in designing clinical trials, 7 years of US marketing exclusivity if the drug is approved, and other benefits. ![]()

The US Food and Drug Administration (FDA) has granted orphan designation for the drug CB-5083 to treat multiple myeloma (MM).
CB-5083 works by inhibiting p97, an enzyme that controls various aspects of protein homeostasis.
Inhibiting p97 function in cancers has been shown to generate irresolvable endoplasmic reticulum stress that induces a lethal unfolded protein response, which leads to apoptosis and antitumor activity.
CB-5083 has exhibited anticancer activity in preclinical research, and the drug is currently under investigation in two phase 1 studies—one in MM and one in solid tumor malignancies.
Research in MM
Preclinical research presented at ASH 2014 showed that CB-5083 inhibits MM cell viability in vitro. The drug also inhibited tumor growth in xenograft models of MM and in an orthometastatic model of MM.
In addition, CB-5083 demonstrated synergy with carfilzomib, bortezomib, and combination lenalidomide-dexamethasone.
CB-5083 is now under investigation in a phase 1 dose-escalation/dose-expansion trial of MM patients.
Researchers are evaluating the safety, pharmacokinetics, pharmacodynamics, and antitumor activity of the drug in patients who have relapsed/refractory or refractory MM after receiving 2 or more lines of therapy, including an immunomodulatory agent and a proteasome inhibitor.
The goal is to enroll up to 60 patients in this trial at multiple US cancer centers that are part of the Multiple Myeloma Research Consortium. More information about the trial is available at www.clinicaltrials.gov (NCT02223598).
About orphan designation
The FDA grants orphan designation to drugs that are intended to treat diseases or conditions affecting fewer than 200,000 patients in the US. Orphan designation provides the sponsor of a drug with various development incentives.
The orphan designation for CB-5083 provides Cleave Biosciences with opportunities to apply for research-related tax credits and grant funding, assistance in designing clinical trials, 7 years of US marketing exclusivity if the drug is approved, and other benefits. ![]()

The US Food and Drug Administration (FDA) has granted orphan designation for the drug CB-5083 to treat multiple myeloma (MM).
CB-5083 works by inhibiting p97, an enzyme that controls various aspects of protein homeostasis.
Inhibiting p97 function in cancers has been shown to generate irresolvable endoplasmic reticulum stress that induces a lethal unfolded protein response, which leads to apoptosis and antitumor activity.
CB-5083 has exhibited anticancer activity in preclinical research, and the drug is currently under investigation in two phase 1 studies—one in MM and one in solid tumor malignancies.
Research in MM
Preclinical research presented at ASH 2014 showed that CB-5083 inhibits MM cell viability in vitro. The drug also inhibited tumor growth in xenograft models of MM and in an orthometastatic model of MM.
In addition, CB-5083 demonstrated synergy with carfilzomib, bortezomib, and combination lenalidomide-dexamethasone.
CB-5083 is now under investigation in a phase 1 dose-escalation/dose-expansion trial of MM patients.
Researchers are evaluating the safety, pharmacokinetics, pharmacodynamics, and antitumor activity of the drug in patients who have relapsed/refractory or refractory MM after receiving 2 or more lines of therapy, including an immunomodulatory agent and a proteasome inhibitor.
The goal is to enroll up to 60 patients in this trial at multiple US cancer centers that are part of the Multiple Myeloma Research Consortium. More information about the trial is available at www.clinicaltrials.gov (NCT02223598).
About orphan designation
The FDA grants orphan designation to drugs that are intended to treat diseases or conditions affecting fewer than 200,000 patients in the US. Orphan designation provides the sponsor of a drug with various development incentives.
The orphan designation for CB-5083 provides Cleave Biosciences with opportunities to apply for research-related tax credits and grant funding, assistance in designing clinical trials, 7 years of US marketing exclusivity if the drug is approved, and other benefits. ![]()
IRB members and industry relationships

Institutional Review Boards (IRBs) at academic health centers have gotten better at managing members’ industry relationships in the last several years, but issues persist, according to a new study.
The research is a follow-up to a study published in 2006.
The new study showed improvements in the management of industry relationships, with increased levels of disclosure and fewer “problematic relationships” than were reported in the previous study.
However, nearly a third of IRB members in the new study said they were unaware of their board’s conflict of interest policies. And a quarter of IRB members reported voting on protocols with which they have conflicts of interest, which is a violation of federal regulations.
“IRBs are the primary mechanisms by which institutions oversee research that involves human participants, and industry relationships of IRB members have the potential to impact their IRB-related activities in both positive and negative ways,” said Eric G. Campbell, PhD, of the Mongan Institute for Health Policy at Massachusetts General Hospital in Boston.
“While our principal findings are that, compared to a decade ago, IRBs appear to be doing a much better job at managing their members’ industry relationships, there still are findings that are concerning.”
Dr Campbell and his colleagues described these findings in JAMA Internal Medicine.
Every US institution that conducts research involving human participants must have an IRB, which reviews proposed studies to ensure their design is scientifically valid and does not violate ethical and regulatory guidelines. IRBs also monitor ongoing studies to make sure they maintain appropriate practices.
The first study to examine industry relationships of IRB members, conducted by Dr Campbell and his colleagues, was based on a survey of IRB members at 100 institutions that was published in NEJM in November 2006.
That study showed that more than a third of IRB members had some sort of financial relationship with commercial firms. And while most respondents did not believe that such relationships had an inappropriate impact on IRB decision making, significant percentages were not aware of institutional guidelines for disclosing industry relationships or defining conflicts of interest.
The current study involved an identical survey administered in 2014 to nearly 500 IRB members at 115 medical schools and teaching hospitals around the country.
As in the 2006 study, respondents were asked about specific types of relationships with commercial companies, whether they received any industry funding and for what purposes, and how many protocols had come before their IRB that involved companies with which they had a relationship.
For protocols with which IRB members had a conflict—relating either to companies they had relationships with or to competitors—they were asked whether they had disclosed their relationships to the IRB, whether they participated in discussion of those protocols, and whether they voted on the protocols.
Results
There was no significant difference between the current study and the previous study in the percentage of IRB members who reported having industry relationships overall—37% and 32%, respectively.
However, the percentage of respondents who reported receiving payments for attending meetings and conferences or for serving on speakers’ bureaus—relationships that are considered problematic—dropped significantly. The percentages fell from 16% to 9% for meeting/conference participation and from 14% to 4% for speakers’ bureaus.
“We were encouraged to see that the prevalence of potentially beneficial relationships—such as industry funding to support research studies—was essentially unchanged, indicating IRBs have not tried to eliminate members’ industry relationships across the board,” said Christine Vogeli, PhD, also of the Mongan Institute.
Survey respondents were more likely in the current study than in the previous one to say that their IRB had a written definition of conflicts of interest—63% compared with 46%. In addition, the percentage of respondents who reported their IRB had no policy related to conflicts dropped from 14% to 5%.
However, the researchers were concerned by the fact that 32% of respondents still did not know whether or not their IRB had a policy on conflicts, even though that number had dropped from 41% in the previous study.
The percentage of respondents who handled their conflicts of interest in an appropriate manner increased. Eighty percent of respondents with conflicts reported them to the IRB, up from 55% in the previous study.
And 68% of respondents said they always left the room when a protocol with which they had a conflict was being discussed, up from 38% in the previous study.
However, a quarter of respondents with conflicts indicated they had voted on protocols with which they had a conflict. While that was a drop from what was reported in the previous study, it was not statistically significant.
The percentage of respondents who felt that at least one protocol had been presented to their IRB in a biased fashion because of another member’s industry relationships dropped from 14% in the previous study to 8% in this study.
And when asked about the types of bias they perceived in the presentation—questions not included in the previous survey—8% of respondents reported a pro-industry bias, and 14% reported an anti-industry bias.
“The fact that we found any bias—either pro- or anti-industry—is an issue, since bias is antithetical to research and should be eliminated,” Dr Campbell said. “IRBs should address that issue, along with increasing efforts to educate their members about what constitutes a conflict of interest and the inappropriateness of voting on protocols with which they have a conflict.” ![]()

Institutional Review Boards (IRBs) at academic health centers have gotten better at managing members’ industry relationships in the last several years, but issues persist, according to a new study.
The research is a follow-up to a study published in 2006.
The new study showed improvements in the management of industry relationships, with increased levels of disclosure and fewer “problematic relationships” than were reported in the previous study.
However, nearly a third of IRB members in the new study said they were unaware of their board’s conflict of interest policies. And a quarter of IRB members reported voting on protocols with which they have conflicts of interest, which is a violation of federal regulations.
“IRBs are the primary mechanisms by which institutions oversee research that involves human participants, and industry relationships of IRB members have the potential to impact their IRB-related activities in both positive and negative ways,” said Eric G. Campbell, PhD, of the Mongan Institute for Health Policy at Massachusetts General Hospital in Boston.
“While our principal findings are that, compared to a decade ago, IRBs appear to be doing a much better job at managing their members’ industry relationships, there still are findings that are concerning.”
Dr Campbell and his colleagues described these findings in JAMA Internal Medicine.
Every US institution that conducts research involving human participants must have an IRB, which reviews proposed studies to ensure their design is scientifically valid and does not violate ethical and regulatory guidelines. IRBs also monitor ongoing studies to make sure they maintain appropriate practices.
The first study to examine industry relationships of IRB members, conducted by Dr Campbell and his colleagues, was based on a survey of IRB members at 100 institutions that was published in NEJM in November 2006.
That study showed that more than a third of IRB members had some sort of financial relationship with commercial firms. And while most respondents did not believe that such relationships had an inappropriate impact on IRB decision making, significant percentages were not aware of institutional guidelines for disclosing industry relationships or defining conflicts of interest.
The current study involved an identical survey administered in 2014 to nearly 500 IRB members at 115 medical schools and teaching hospitals around the country.
As in the 2006 study, respondents were asked about specific types of relationships with commercial companies, whether they received any industry funding and for what purposes, and how many protocols had come before their IRB that involved companies with which they had a relationship.
For protocols with which IRB members had a conflict—relating either to companies they had relationships with or to competitors—they were asked whether they had disclosed their relationships to the IRB, whether they participated in discussion of those protocols, and whether they voted on the protocols.
Results
There was no significant difference between the current study and the previous study in the percentage of IRB members who reported having industry relationships overall—37% and 32%, respectively.
However, the percentage of respondents who reported receiving payments for attending meetings and conferences or for serving on speakers’ bureaus—relationships that are considered problematic—dropped significantly. The percentages fell from 16% to 9% for meeting/conference participation and from 14% to 4% for speakers’ bureaus.
“We were encouraged to see that the prevalence of potentially beneficial relationships—such as industry funding to support research studies—was essentially unchanged, indicating IRBs have not tried to eliminate members’ industry relationships across the board,” said Christine Vogeli, PhD, also of the Mongan Institute.
Survey respondents were more likely in the current study than in the previous one to say that their IRB had a written definition of conflicts of interest—63% compared with 46%. In addition, the percentage of respondents who reported their IRB had no policy related to conflicts dropped from 14% to 5%.
However, the researchers were concerned by the fact that 32% of respondents still did not know whether or not their IRB had a policy on conflicts, even though that number had dropped from 41% in the previous study.
The percentage of respondents who handled their conflicts of interest in an appropriate manner increased. Eighty percent of respondents with conflicts reported them to the IRB, up from 55% in the previous study.
And 68% of respondents said they always left the room when a protocol with which they had a conflict was being discussed, up from 38% in the previous study.
However, a quarter of respondents with conflicts indicated they had voted on protocols with which they had a conflict. While that was a drop from what was reported in the previous study, it was not statistically significant.
The percentage of respondents who felt that at least one protocol had been presented to their IRB in a biased fashion because of another member’s industry relationships dropped from 14% in the previous study to 8% in this study.
And when asked about the types of bias they perceived in the presentation—questions not included in the previous survey—8% of respondents reported a pro-industry bias, and 14% reported an anti-industry bias.
“The fact that we found any bias—either pro- or anti-industry—is an issue, since bias is antithetical to research and should be eliminated,” Dr Campbell said. “IRBs should address that issue, along with increasing efforts to educate their members about what constitutes a conflict of interest and the inappropriateness of voting on protocols with which they have a conflict.” ![]()

Institutional Review Boards (IRBs) at academic health centers have gotten better at managing members’ industry relationships in the last several years, but issues persist, according to a new study.
The research is a follow-up to a study published in 2006.
The new study showed improvements in the management of industry relationships, with increased levels of disclosure and fewer “problematic relationships” than were reported in the previous study.
However, nearly a third of IRB members in the new study said they were unaware of their board’s conflict of interest policies. And a quarter of IRB members reported voting on protocols with which they have conflicts of interest, which is a violation of federal regulations.
“IRBs are the primary mechanisms by which institutions oversee research that involves human participants, and industry relationships of IRB members have the potential to impact their IRB-related activities in both positive and negative ways,” said Eric G. Campbell, PhD, of the Mongan Institute for Health Policy at Massachusetts General Hospital in Boston.
“While our principal findings are that, compared to a decade ago, IRBs appear to be doing a much better job at managing their members’ industry relationships, there still are findings that are concerning.”
Dr Campbell and his colleagues described these findings in JAMA Internal Medicine.
Every US institution that conducts research involving human participants must have an IRB, which reviews proposed studies to ensure their design is scientifically valid and does not violate ethical and regulatory guidelines. IRBs also monitor ongoing studies to make sure they maintain appropriate practices.
The first study to examine industry relationships of IRB members, conducted by Dr Campbell and his colleagues, was based on a survey of IRB members at 100 institutions that was published in NEJM in November 2006.
That study showed that more than a third of IRB members had some sort of financial relationship with commercial firms. And while most respondents did not believe that such relationships had an inappropriate impact on IRB decision making, significant percentages were not aware of institutional guidelines for disclosing industry relationships or defining conflicts of interest.
The current study involved an identical survey administered in 2014 to nearly 500 IRB members at 115 medical schools and teaching hospitals around the country.
As in the 2006 study, respondents were asked about specific types of relationships with commercial companies, whether they received any industry funding and for what purposes, and how many protocols had come before their IRB that involved companies with which they had a relationship.
For protocols with which IRB members had a conflict—relating either to companies they had relationships with or to competitors—they were asked whether they had disclosed their relationships to the IRB, whether they participated in discussion of those protocols, and whether they voted on the protocols.
Results
There was no significant difference between the current study and the previous study in the percentage of IRB members who reported having industry relationships overall—37% and 32%, respectively.
However, the percentage of respondents who reported receiving payments for attending meetings and conferences or for serving on speakers’ bureaus—relationships that are considered problematic—dropped significantly. The percentages fell from 16% to 9% for meeting/conference participation and from 14% to 4% for speakers’ bureaus.
“We were encouraged to see that the prevalence of potentially beneficial relationships—such as industry funding to support research studies—was essentially unchanged, indicating IRBs have not tried to eliminate members’ industry relationships across the board,” said Christine Vogeli, PhD, also of the Mongan Institute.
Survey respondents were more likely in the current study than in the previous one to say that their IRB had a written definition of conflicts of interest—63% compared with 46%. In addition, the percentage of respondents who reported their IRB had no policy related to conflicts dropped from 14% to 5%.
However, the researchers were concerned by the fact that 32% of respondents still did not know whether or not their IRB had a policy on conflicts, even though that number had dropped from 41% in the previous study.
The percentage of respondents who handled their conflicts of interest in an appropriate manner increased. Eighty percent of respondents with conflicts reported them to the IRB, up from 55% in the previous study.
And 68% of respondents said they always left the room when a protocol with which they had a conflict was being discussed, up from 38% in the previous study.
However, a quarter of respondents with conflicts indicated they had voted on protocols with which they had a conflict. While that was a drop from what was reported in the previous study, it was not statistically significant.
The percentage of respondents who felt that at least one protocol had been presented to their IRB in a biased fashion because of another member’s industry relationships dropped from 14% in the previous study to 8% in this study.
And when asked about the types of bias they perceived in the presentation—questions not included in the previous survey—8% of respondents reported a pro-industry bias, and 14% reported an anti-industry bias.
“The fact that we found any bias—either pro- or anti-industry—is an issue, since bias is antithetical to research and should be eliminated,” Dr Campbell said. “IRBs should address that issue, along with increasing efforts to educate their members about what constitutes a conflict of interest and the inappropriateness of voting on protocols with which they have a conflict.” ![]()
‘Choosing Wisely’: Canada vs. U.S.
In 2011, the American Board of Internal Medicine started the Choosing Wisely campaign, a subtly subversive call to curb health care spending. As part of that campaign, the American College of Rheumatology published its own list of five “tests, treatments, or services … whose necessity or value should be questioned” in March 2013.
As it turns out, Canada has also started a Choosing Wisely Canada campaign, and in February of this year the Canadian Rheumatology Association published their list of five. Though the methodology for coming up with the list was the same in these two very similar populations, there is surprisingly little overlap between the two lists. It is likely that the differences are partly explained by how medicine is practiced and paid for in the two countries. How invested physicians are in their respective professional societies may play a role, too. As part of the methodology, surveys were sent out to membership: At the time of the study the ACR had 6,188 members and a 17% response rate, while the CRA had a membership of 484 and a 35% response rate.
The ACR publication reminds us that the initiative is in part a response to a physician charter for medical professionalism, drafted in 2002 by a collaboration of physician organizations, including the ABIM Foundation and the American College of Physicians, outlining principles of professionalism, “including patient welfare, patient autonomy, and social justice” (Ann. Intern. Med. 2002;136:143-6).
Social justice, according to the ACR, “calls on the profession to promote a fair distribution of health care resources and to engage in collective efforts to improve the health care system for the welfare of society.” I wonder if the gap between the survey response rates of the American and the Canadian groups reflects greater indifference to societal welfare, but I certainly hope not.
In any event, I would like to devote some space to reviewing these two lists. They are worth revisiting often. I’ve included some clarifying statements from each publication that I thought were helpful.
The ACR list (Arthritis Care Res. 2013;65:329-39)
• Do not test ANA subserologies without a positive ANA and clinical suspicion of immune-mediated disease. Exceptions include anti-Jo1, which can be positive in some forms of myositis, or occasionally, anti-SSA in the setting of lupus or Sjögren syndrome.
• Do not test for Lyme disease as a cause of musculoskeletal symptoms without an exposure history and appropriate examination findings. Diffuse arthralgias, myalgias, or fibromyalgia alone are not criteria for musculoskeletal Lyme disease.
• Do not perform MRI of the peripheral joints to routinely monitor inflammatory arthritis.
• Do not prescribe biologic agents for RA before a trial of methotrexate (or another conventional nonbiologic DMARD)
• Do not routinely repeat DXA scans more often than once every 2 years. DXA scans should only be repeated if the result will influence clinical management or if rapid changes in bone density are expected.
The CRA list (J. Rheumatol. 2015;42:682-9)
• Do not order ANA as a screening test in patients without specific signs or symptoms of systemic lupus erythematosus or other connective tissue disease. At one center in Canada, ANA testing was positive only 15% of the time and cost more than $800,000 over 3 years when combined with ENA and anti-dsDNA. … An ANA test should be ordered only if the clinician feels there is reasonable clinical suspicion of SLE or CTD based on historical information, physical findings and results of other laboratory tests.
• Do not order an HLA-B27 unless spondyloarthritis is suspected based on specific signs or symptoms. There is no clinical utility to ordering an HLA-B27 in the absence of positive imaging or the minimally required SpA signs or symptoms.
• Do not repeat DXA scans more often than every 2 years. If BMD are stable and/or individuals are at low risk of fracture, then less frequent monitoring up to an interval of 5-10 years can be considered.
• Do not prescribe bisphosphonates for patients at low risk of fracture.
• Do not perform whole body bone scans (e.g., scintigraphy) for diagnostic screening for peripheral and axial arthritis in the adult population.
Dr. Chan practices rheumatology in Pawtucket, R.I.
In 2011, the American Board of Internal Medicine started the Choosing Wisely campaign, a subtly subversive call to curb health care spending. As part of that campaign, the American College of Rheumatology published its own list of five “tests, treatments, or services … whose necessity or value should be questioned” in March 2013.
As it turns out, Canada has also started a Choosing Wisely Canada campaign, and in February of this year the Canadian Rheumatology Association published their list of five. Though the methodology for coming up with the list was the same in these two very similar populations, there is surprisingly little overlap between the two lists. It is likely that the differences are partly explained by how medicine is practiced and paid for in the two countries. How invested physicians are in their respective professional societies may play a role, too. As part of the methodology, surveys were sent out to membership: At the time of the study the ACR had 6,188 members and a 17% response rate, while the CRA had a membership of 484 and a 35% response rate.
The ACR publication reminds us that the initiative is in part a response to a physician charter for medical professionalism, drafted in 2002 by a collaboration of physician organizations, including the ABIM Foundation and the American College of Physicians, outlining principles of professionalism, “including patient welfare, patient autonomy, and social justice” (Ann. Intern. Med. 2002;136:143-6).
Social justice, according to the ACR, “calls on the profession to promote a fair distribution of health care resources and to engage in collective efforts to improve the health care system for the welfare of society.” I wonder if the gap between the survey response rates of the American and the Canadian groups reflects greater indifference to societal welfare, but I certainly hope not.
In any event, I would like to devote some space to reviewing these two lists. They are worth revisiting often. I’ve included some clarifying statements from each publication that I thought were helpful.
The ACR list (Arthritis Care Res. 2013;65:329-39)
• Do not test ANA subserologies without a positive ANA and clinical suspicion of immune-mediated disease. Exceptions include anti-Jo1, which can be positive in some forms of myositis, or occasionally, anti-SSA in the setting of lupus or Sjögren syndrome.
• Do not test for Lyme disease as a cause of musculoskeletal symptoms without an exposure history and appropriate examination findings. Diffuse arthralgias, myalgias, or fibromyalgia alone are not criteria for musculoskeletal Lyme disease.
• Do not perform MRI of the peripheral joints to routinely monitor inflammatory arthritis.
• Do not prescribe biologic agents for RA before a trial of methotrexate (or another conventional nonbiologic DMARD)
• Do not routinely repeat DXA scans more often than once every 2 years. DXA scans should only be repeated if the result will influence clinical management or if rapid changes in bone density are expected.
The CRA list (J. Rheumatol. 2015;42:682-9)
• Do not order ANA as a screening test in patients without specific signs or symptoms of systemic lupus erythematosus or other connective tissue disease. At one center in Canada, ANA testing was positive only 15% of the time and cost more than $800,000 over 3 years when combined with ENA and anti-dsDNA. … An ANA test should be ordered only if the clinician feels there is reasonable clinical suspicion of SLE or CTD based on historical information, physical findings and results of other laboratory tests.
• Do not order an HLA-B27 unless spondyloarthritis is suspected based on specific signs or symptoms. There is no clinical utility to ordering an HLA-B27 in the absence of positive imaging or the minimally required SpA signs or symptoms.
• Do not repeat DXA scans more often than every 2 years. If BMD are stable and/or individuals are at low risk of fracture, then less frequent monitoring up to an interval of 5-10 years can be considered.
• Do not prescribe bisphosphonates for patients at low risk of fracture.
• Do not perform whole body bone scans (e.g., scintigraphy) for diagnostic screening for peripheral and axial arthritis in the adult population.
Dr. Chan practices rheumatology in Pawtucket, R.I.
In 2011, the American Board of Internal Medicine started the Choosing Wisely campaign, a subtly subversive call to curb health care spending. As part of that campaign, the American College of Rheumatology published its own list of five “tests, treatments, or services … whose necessity or value should be questioned” in March 2013.
As it turns out, Canada has also started a Choosing Wisely Canada campaign, and in February of this year the Canadian Rheumatology Association published their list of five. Though the methodology for coming up with the list was the same in these two very similar populations, there is surprisingly little overlap between the two lists. It is likely that the differences are partly explained by how medicine is practiced and paid for in the two countries. How invested physicians are in their respective professional societies may play a role, too. As part of the methodology, surveys were sent out to membership: At the time of the study the ACR had 6,188 members and a 17% response rate, while the CRA had a membership of 484 and a 35% response rate.
The ACR publication reminds us that the initiative is in part a response to a physician charter for medical professionalism, drafted in 2002 by a collaboration of physician organizations, including the ABIM Foundation and the American College of Physicians, outlining principles of professionalism, “including patient welfare, patient autonomy, and social justice” (Ann. Intern. Med. 2002;136:143-6).
Social justice, according to the ACR, “calls on the profession to promote a fair distribution of health care resources and to engage in collective efforts to improve the health care system for the welfare of society.” I wonder if the gap between the survey response rates of the American and the Canadian groups reflects greater indifference to societal welfare, but I certainly hope not.
In any event, I would like to devote some space to reviewing these two lists. They are worth revisiting often. I’ve included some clarifying statements from each publication that I thought were helpful.
The ACR list (Arthritis Care Res. 2013;65:329-39)
• Do not test ANA subserologies without a positive ANA and clinical suspicion of immune-mediated disease. Exceptions include anti-Jo1, which can be positive in some forms of myositis, or occasionally, anti-SSA in the setting of lupus or Sjögren syndrome.
• Do not test for Lyme disease as a cause of musculoskeletal symptoms without an exposure history and appropriate examination findings. Diffuse arthralgias, myalgias, or fibromyalgia alone are not criteria for musculoskeletal Lyme disease.
• Do not perform MRI of the peripheral joints to routinely monitor inflammatory arthritis.
• Do not prescribe biologic agents for RA before a trial of methotrexate (or another conventional nonbiologic DMARD)
• Do not routinely repeat DXA scans more often than once every 2 years. DXA scans should only be repeated if the result will influence clinical management or if rapid changes in bone density are expected.
The CRA list (J. Rheumatol. 2015;42:682-9)
• Do not order ANA as a screening test in patients without specific signs or symptoms of systemic lupus erythematosus or other connective tissue disease. At one center in Canada, ANA testing was positive only 15% of the time and cost more than $800,000 over 3 years when combined with ENA and anti-dsDNA. … An ANA test should be ordered only if the clinician feels there is reasonable clinical suspicion of SLE or CTD based on historical information, physical findings and results of other laboratory tests.
• Do not order an HLA-B27 unless spondyloarthritis is suspected based on specific signs or symptoms. There is no clinical utility to ordering an HLA-B27 in the absence of positive imaging or the minimally required SpA signs or symptoms.
• Do not repeat DXA scans more often than every 2 years. If BMD are stable and/or individuals are at low risk of fracture, then less frequent monitoring up to an interval of 5-10 years can be considered.
• Do not prescribe bisphosphonates for patients at low risk of fracture.
• Do not perform whole body bone scans (e.g., scintigraphy) for diagnostic screening for peripheral and axial arthritis in the adult population.
Dr. Chan practices rheumatology in Pawtucket, R.I.




