User login
Defining a New Normal While Awaiting the Pandemic’s Next Wave
Hospitalists have played a central role in the massive response to the coronavirus disease 2019 (COVID-19) pandemic by creating innovative staffing models, rapidly learning about the disease and teaching others, and working closely with hospital executive leadership to create surge capacity.1 Some hospitals and regions have weathered an initial storm and are now experiencing a slower influx of COVID-19 patients, while others are now seeing a surge, which is expected to persist for the foreseeable future—the marathon has begun.2 We have entered a new COVID-19 reality: disrupted care models, harsh financial consequences,3 and uncertainty about which adaptations should be preserved and for how long. Common operational challenges will define the new normal. In this Perspective, we share strategies to address these challenges, focusing on three emerging themes: realigning staffing to patient volumes, safely managing space limitations, and navigating the financial ramifications of COVID-19 for hospital medicine groups.
BALANCING STAFFING AND PATIENT VOLUME
Hospital medicine groups face uncertainty about future patient volumes and their characteristics. It is unclear when, how, or even whether hospital medicine groups should return to “normal” pre-COVID staffing models. The following principles can guide staffing decisions.
First, maintain nonhospitalist backup pools and define triggers to activate these providers. Despite the impulse to return to prior staffing models, this recovery period provides an opportunity for leaders to create transparent activation protocols and provide additional training to enable seamless backup. In preparation for a surge, our hospital medicine group quickly assembled an emergency staffing pool composed of advanced practice providers, primary care providers, medicine subspecialists, and surgeons who were prepared to temporarily assume unfamiliar roles. Thankfully, we were able to manage our COVID-19 patients without much emergency hospitalist staffing, but for other hospitals with larger community outbreaks, the emergency backup workforce proved invaluable.
Second, use appropriate safeguards and delegate certain aspects of COVID-related care to other healthcare team members. As staff are deployed and redeployed, consider how interprofessional team members can be reintegrated into evaluation and triage protocols. For example, registered nurses can determine appropriate isolation precautions for patients with COVID and patients under investigation.
Third, consider hospital-specific specialty care patterns when planning for COVID-19 redeployment to ensure access to equally critical, nonelective services. For example, Level 1 trauma centers may expect seasonal increases in trauma patient volumes, so consider staffing trauma teams (including surgeons, anesthesiologists, and operating room staff) for their usual roles to prevent critical coverage gaps. Concurrently, hospital medicine consulting and comanagement teams must also be available to support the trauma service. These staffing needs affect who will be available for redeployment for future COVID-related care.
MANAGING THE PHYSICAL LIMITATIONS OF SPACE
As the number of COVID cases increased, numerous hospitals created geographic “hot zones” with defined cold (uncontaminated), warm (transitional), and hot (contaminated) areas by either partitioning off a section of an acute care medical ward or repurposing an entire ward as a COVID-19 unit, and similar zones were made in intensive care units. Hot zones required significant early investments to change infrastructure, including equipping rooms for negative pressurization with HEPA filtration towers and training staff on safety protocols for entering these spaces, performing necessary patient care, and exiting. Ultimately, these investments proved worthwhile and allowed for decreased personal protective equipment (PPE) use, as well as improved efficiency and staff safety. However, as hospitals ramp up non-COVID care, deciding how to best reconfigure or downsize these hot zones has become challenging.
With time to regroup, the newly experienced end users of hot zones—hospitalists, other staff who worked in these spaces, and patients—must be included in discussions with engineers, architects, and administrators regarding future construction. Hot zone plans should specifically address how physical separation of COVID and non-COVID patients will be maintained while providing safe and efficient care. With elective surgeries increasing and non-COVID patients returning to hospitals, leaders must consider the psychological effects that seeing hospital staff doffing PPE and crossing an invisible barrier to a ‘‘cold” area of the floor has on patients and their families. It is important to maintain hot zones in areas that can dynamically flex to accommodate waves of the current and future pandemics, especially because hospitals may be asked to care for patients from overwhelmed distant sites even if the pandemic is locally controlled. We are experimenting with modifications to hospital traffic patterns including “no pass through” zones, one-way hallways, and separate entries and exits to clinical floors for COVID and non-COVID patients. With vigilant adherence to infection prevention guidelines and PPE use, we have not seen hospital-acquired infections with this model of care.
Modifying space and flow patterns also enables clustered care for COVID patients, which allows for the temporary use of modular teams.4 This tactic may be especially useful during surge periods, during which PPE conservation is paramount and isolating cohorts of providers provides an extra layer of safety. In the longer run, however, isolating providers from their peers risks worsening morale and increasing burnout.
NAVIGATING THE FINANCIAL CHALLENGES
The path forward must ensure safety but also allow for a financially sustainable balance of COVID and non-COVID care. To prepare for surges, health systems canceled elective surgeries and other services that generate essential revenue. At both private and public hospitals, systemwide measures have been taken to mitigate these financial losses. These measures have included salary, retirement, and continuing medical education benefit reductions for physicians and senior leadership; limits to physician hiring and recruitment; leaner operations with systemwide expense reductions; and mandatory and voluntary staff furloughs. The frontline hospital staff, including physicians, nurses, technologists, and food and environmental service workers, who have made great sacrifices during this pandemic, may also now be facing significant personal financial consequences.
The following recommendations are offered from the perspective that crisis creates opportunity for hospital medicine leaders grappling with budget shortfalls.
First, maximize budget transparency by explicitly defining the principles and priorities that govern budget decisions, which allows hospitalist group members to understand how the organization determines budget cuts. For example, stating that a key priority is to minimize staff layoffs makes consequent salary reductions more understandable.
Second, solicit hospital medicine group members’ input on these shared challenges and invite their help in identifying and prioritizing potential cost-saving or cost-cutting measures.
Third, highlight hospitalists’ nonfiscal contributions, especially in terms of crisis leadership, to continue engagement with executive leaders.5 This may include a dialogue about the disproportionate influence of work relative value unit production on salary and about how to create compensation systems that can also recognize crisis readiness as an important feature of sustainability and quality care. The next pandemic surge may be weeks or months away, and hospitalists will again need to be leaders in the response.
Fourth, use this crisis to foster fiscal innovation and accelerate participation in value improvement work, such as redesigning pay-for-performance metrics. Financially strapped institutions will value hospitalists who are good financial stewards. For example, leverage hospitalist expertise in progression of care to facilitate timely disposition of COVID patients, thereby minimizing costly extended hospitalizations.
Lastly, hospital medicine groups must match staffing to patient volume to the extent possible. Approximately two-thirds of hospitalist groups entered this crisis already understaffed and partially reliant on moonlighters,6 which allowed some variation of labor expenses to match lower patient volume. During the recovery phase, hospital volumes may either be significantly below or above baseline; many patients are understandably avoiding hospitals due to fear of COVID. However, delayed care may create a different kind of peak demand for services. For hospitalists, uncertainty about expected clinical roles, COVID vs non-COVID patient mix, and patient volume can be stressful. We recommend sustained, frequent communication about census trends and how shifts will be covered to ensure adequate, long-term staffing. Maintaining trust and morale will be equally, if not more, important in the next phase.
CONCLUSION
As we settle into the marathon, hospital medicine leadership must balance competing priorities with increasing finesse. Our hospital medicine group has benefited from continually discussing operational challenges and refining our strategies as we plan for what is ahead. We have highlighted three mission-critical themes and recommend that hospital and hospital medicine group leaders remain mindful of these challenges and potential strategies. Each of our four academic hospitals has considered similar trade-offs and will proceed along slightly different trajectories to meet unique needs. Looking to the future, we anticipate additional challenges requiring greater ongoing attention alongside those already identified. These include mitigating provider burnout, optimizing resident and student education, and maintaining scholarly work as COVID unpredictably waxes and wanes. By accumulating confidence and wisdom about post-COVID hospital medicine group functions, we hope to provide hospitalists with the energy to keep the pace in the next phase of the marathon.
- Garg M, Wray CM. Hospital medicine management in the time of COVID-19: preparing for a sprint and a marathon. J Hosp Med . 2020;15(5):305-307. https://doi.org/10.12788/jhm.3427
- COVIDView - A weekly Surveillance Summary of U.S. COVID-19 Activity. US Centers for Disease Control and Prevention. July 9, 2020. Accessed July 13, 2020. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/pdf/covidview-07-10-2020.pdf
- Khullar D, Bond AM, Schpero WL. COVID-19 and the financial health of US hospitals. JAMA. Published online May 4, 2020. https://doi.org/10.1001/jama.2020.6269
- Wang CJ, Bair H, Yeh CC. How to prevent and manage hospital-based infections during coronavirus outbreaks: five lessons from Taiwan. J Hosp Med . 2020;15(6):370-371. https://doi.org/10.12788/jhm.3452
- White AA, McIlraith T, Chivu AM, et al. Collaboration, not calculation: a qualitative study of how hospital executives value hospital medicine groups. J Hosp Med. 2019;14(11):662-667. https://doi.org/10.12788/jhm.3249
- 2018 State of Hospital Medicine: 2018 Report Based on 2017 Data . Society of Hospital Medicine; 2018. Accessed July 27, 2020. https://sohm.hospitalmedicine.org/
Hospitalists have played a central role in the massive response to the coronavirus disease 2019 (COVID-19) pandemic by creating innovative staffing models, rapidly learning about the disease and teaching others, and working closely with hospital executive leadership to create surge capacity.1 Some hospitals and regions have weathered an initial storm and are now experiencing a slower influx of COVID-19 patients, while others are now seeing a surge, which is expected to persist for the foreseeable future—the marathon has begun.2 We have entered a new COVID-19 reality: disrupted care models, harsh financial consequences,3 and uncertainty about which adaptations should be preserved and for how long. Common operational challenges will define the new normal. In this Perspective, we share strategies to address these challenges, focusing on three emerging themes: realigning staffing to patient volumes, safely managing space limitations, and navigating the financial ramifications of COVID-19 for hospital medicine groups.
BALANCING STAFFING AND PATIENT VOLUME
Hospital medicine groups face uncertainty about future patient volumes and their characteristics. It is unclear when, how, or even whether hospital medicine groups should return to “normal” pre-COVID staffing models. The following principles can guide staffing decisions.
First, maintain nonhospitalist backup pools and define triggers to activate these providers. Despite the impulse to return to prior staffing models, this recovery period provides an opportunity for leaders to create transparent activation protocols and provide additional training to enable seamless backup. In preparation for a surge, our hospital medicine group quickly assembled an emergency staffing pool composed of advanced practice providers, primary care providers, medicine subspecialists, and surgeons who were prepared to temporarily assume unfamiliar roles. Thankfully, we were able to manage our COVID-19 patients without much emergency hospitalist staffing, but for other hospitals with larger community outbreaks, the emergency backup workforce proved invaluable.
Second, use appropriate safeguards and delegate certain aspects of COVID-related care to other healthcare team members. As staff are deployed and redeployed, consider how interprofessional team members can be reintegrated into evaluation and triage protocols. For example, registered nurses can determine appropriate isolation precautions for patients with COVID and patients under investigation.
Third, consider hospital-specific specialty care patterns when planning for COVID-19 redeployment to ensure access to equally critical, nonelective services. For example, Level 1 trauma centers may expect seasonal increases in trauma patient volumes, so consider staffing trauma teams (including surgeons, anesthesiologists, and operating room staff) for their usual roles to prevent critical coverage gaps. Concurrently, hospital medicine consulting and comanagement teams must also be available to support the trauma service. These staffing needs affect who will be available for redeployment for future COVID-related care.
MANAGING THE PHYSICAL LIMITATIONS OF SPACE
As the number of COVID cases increased, numerous hospitals created geographic “hot zones” with defined cold (uncontaminated), warm (transitional), and hot (contaminated) areas by either partitioning off a section of an acute care medical ward or repurposing an entire ward as a COVID-19 unit, and similar zones were made in intensive care units. Hot zones required significant early investments to change infrastructure, including equipping rooms for negative pressurization with HEPA filtration towers and training staff on safety protocols for entering these spaces, performing necessary patient care, and exiting. Ultimately, these investments proved worthwhile and allowed for decreased personal protective equipment (PPE) use, as well as improved efficiency and staff safety. However, as hospitals ramp up non-COVID care, deciding how to best reconfigure or downsize these hot zones has become challenging.
With time to regroup, the newly experienced end users of hot zones—hospitalists, other staff who worked in these spaces, and patients—must be included in discussions with engineers, architects, and administrators regarding future construction. Hot zone plans should specifically address how physical separation of COVID and non-COVID patients will be maintained while providing safe and efficient care. With elective surgeries increasing and non-COVID patients returning to hospitals, leaders must consider the psychological effects that seeing hospital staff doffing PPE and crossing an invisible barrier to a ‘‘cold” area of the floor has on patients and their families. It is important to maintain hot zones in areas that can dynamically flex to accommodate waves of the current and future pandemics, especially because hospitals may be asked to care for patients from overwhelmed distant sites even if the pandemic is locally controlled. We are experimenting with modifications to hospital traffic patterns including “no pass through” zones, one-way hallways, and separate entries and exits to clinical floors for COVID and non-COVID patients. With vigilant adherence to infection prevention guidelines and PPE use, we have not seen hospital-acquired infections with this model of care.
Modifying space and flow patterns also enables clustered care for COVID patients, which allows for the temporary use of modular teams.4 This tactic may be especially useful during surge periods, during which PPE conservation is paramount and isolating cohorts of providers provides an extra layer of safety. In the longer run, however, isolating providers from their peers risks worsening morale and increasing burnout.
NAVIGATING THE FINANCIAL CHALLENGES
The path forward must ensure safety but also allow for a financially sustainable balance of COVID and non-COVID care. To prepare for surges, health systems canceled elective surgeries and other services that generate essential revenue. At both private and public hospitals, systemwide measures have been taken to mitigate these financial losses. These measures have included salary, retirement, and continuing medical education benefit reductions for physicians and senior leadership; limits to physician hiring and recruitment; leaner operations with systemwide expense reductions; and mandatory and voluntary staff furloughs. The frontline hospital staff, including physicians, nurses, technologists, and food and environmental service workers, who have made great sacrifices during this pandemic, may also now be facing significant personal financial consequences.
The following recommendations are offered from the perspective that crisis creates opportunity for hospital medicine leaders grappling with budget shortfalls.
First, maximize budget transparency by explicitly defining the principles and priorities that govern budget decisions, which allows hospitalist group members to understand how the organization determines budget cuts. For example, stating that a key priority is to minimize staff layoffs makes consequent salary reductions more understandable.
Second, solicit hospital medicine group members’ input on these shared challenges and invite their help in identifying and prioritizing potential cost-saving or cost-cutting measures.
Third, highlight hospitalists’ nonfiscal contributions, especially in terms of crisis leadership, to continue engagement with executive leaders.5 This may include a dialogue about the disproportionate influence of work relative value unit production on salary and about how to create compensation systems that can also recognize crisis readiness as an important feature of sustainability and quality care. The next pandemic surge may be weeks or months away, and hospitalists will again need to be leaders in the response.
Fourth, use this crisis to foster fiscal innovation and accelerate participation in value improvement work, such as redesigning pay-for-performance metrics. Financially strapped institutions will value hospitalists who are good financial stewards. For example, leverage hospitalist expertise in progression of care to facilitate timely disposition of COVID patients, thereby minimizing costly extended hospitalizations.
Lastly, hospital medicine groups must match staffing to patient volume to the extent possible. Approximately two-thirds of hospitalist groups entered this crisis already understaffed and partially reliant on moonlighters,6 which allowed some variation of labor expenses to match lower patient volume. During the recovery phase, hospital volumes may either be significantly below or above baseline; many patients are understandably avoiding hospitals due to fear of COVID. However, delayed care may create a different kind of peak demand for services. For hospitalists, uncertainty about expected clinical roles, COVID vs non-COVID patient mix, and patient volume can be stressful. We recommend sustained, frequent communication about census trends and how shifts will be covered to ensure adequate, long-term staffing. Maintaining trust and morale will be equally, if not more, important in the next phase.
CONCLUSION
As we settle into the marathon, hospital medicine leadership must balance competing priorities with increasing finesse. Our hospital medicine group has benefited from continually discussing operational challenges and refining our strategies as we plan for what is ahead. We have highlighted three mission-critical themes and recommend that hospital and hospital medicine group leaders remain mindful of these challenges and potential strategies. Each of our four academic hospitals has considered similar trade-offs and will proceed along slightly different trajectories to meet unique needs. Looking to the future, we anticipate additional challenges requiring greater ongoing attention alongside those already identified. These include mitigating provider burnout, optimizing resident and student education, and maintaining scholarly work as COVID unpredictably waxes and wanes. By accumulating confidence and wisdom about post-COVID hospital medicine group functions, we hope to provide hospitalists with the energy to keep the pace in the next phase of the marathon.
Hospitalists have played a central role in the massive response to the coronavirus disease 2019 (COVID-19) pandemic by creating innovative staffing models, rapidly learning about the disease and teaching others, and working closely with hospital executive leadership to create surge capacity.1 Some hospitals and regions have weathered an initial storm and are now experiencing a slower influx of COVID-19 patients, while others are now seeing a surge, which is expected to persist for the foreseeable future—the marathon has begun.2 We have entered a new COVID-19 reality: disrupted care models, harsh financial consequences,3 and uncertainty about which adaptations should be preserved and for how long. Common operational challenges will define the new normal. In this Perspective, we share strategies to address these challenges, focusing on three emerging themes: realigning staffing to patient volumes, safely managing space limitations, and navigating the financial ramifications of COVID-19 for hospital medicine groups.
BALANCING STAFFING AND PATIENT VOLUME
Hospital medicine groups face uncertainty about future patient volumes and their characteristics. It is unclear when, how, or even whether hospital medicine groups should return to “normal” pre-COVID staffing models. The following principles can guide staffing decisions.
First, maintain nonhospitalist backup pools and define triggers to activate these providers. Despite the impulse to return to prior staffing models, this recovery period provides an opportunity for leaders to create transparent activation protocols and provide additional training to enable seamless backup. In preparation for a surge, our hospital medicine group quickly assembled an emergency staffing pool composed of advanced practice providers, primary care providers, medicine subspecialists, and surgeons who were prepared to temporarily assume unfamiliar roles. Thankfully, we were able to manage our COVID-19 patients without much emergency hospitalist staffing, but for other hospitals with larger community outbreaks, the emergency backup workforce proved invaluable.
Second, use appropriate safeguards and delegate certain aspects of COVID-related care to other healthcare team members. As staff are deployed and redeployed, consider how interprofessional team members can be reintegrated into evaluation and triage protocols. For example, registered nurses can determine appropriate isolation precautions for patients with COVID and patients under investigation.
Third, consider hospital-specific specialty care patterns when planning for COVID-19 redeployment to ensure access to equally critical, nonelective services. For example, Level 1 trauma centers may expect seasonal increases in trauma patient volumes, so consider staffing trauma teams (including surgeons, anesthesiologists, and operating room staff) for their usual roles to prevent critical coverage gaps. Concurrently, hospital medicine consulting and comanagement teams must also be available to support the trauma service. These staffing needs affect who will be available for redeployment for future COVID-related care.
MANAGING THE PHYSICAL LIMITATIONS OF SPACE
As the number of COVID cases increased, numerous hospitals created geographic “hot zones” with defined cold (uncontaminated), warm (transitional), and hot (contaminated) areas by either partitioning off a section of an acute care medical ward or repurposing an entire ward as a COVID-19 unit, and similar zones were made in intensive care units. Hot zones required significant early investments to change infrastructure, including equipping rooms for negative pressurization with HEPA filtration towers and training staff on safety protocols for entering these spaces, performing necessary patient care, and exiting. Ultimately, these investments proved worthwhile and allowed for decreased personal protective equipment (PPE) use, as well as improved efficiency and staff safety. However, as hospitals ramp up non-COVID care, deciding how to best reconfigure or downsize these hot zones has become challenging.
With time to regroup, the newly experienced end users of hot zones—hospitalists, other staff who worked in these spaces, and patients—must be included in discussions with engineers, architects, and administrators regarding future construction. Hot zone plans should specifically address how physical separation of COVID and non-COVID patients will be maintained while providing safe and efficient care. With elective surgeries increasing and non-COVID patients returning to hospitals, leaders must consider the psychological effects that seeing hospital staff doffing PPE and crossing an invisible barrier to a ‘‘cold” area of the floor has on patients and their families. It is important to maintain hot zones in areas that can dynamically flex to accommodate waves of the current and future pandemics, especially because hospitals may be asked to care for patients from overwhelmed distant sites even if the pandemic is locally controlled. We are experimenting with modifications to hospital traffic patterns including “no pass through” zones, one-way hallways, and separate entries and exits to clinical floors for COVID and non-COVID patients. With vigilant adherence to infection prevention guidelines and PPE use, we have not seen hospital-acquired infections with this model of care.
Modifying space and flow patterns also enables clustered care for COVID patients, which allows for the temporary use of modular teams.4 This tactic may be especially useful during surge periods, during which PPE conservation is paramount and isolating cohorts of providers provides an extra layer of safety. In the longer run, however, isolating providers from their peers risks worsening morale and increasing burnout.
NAVIGATING THE FINANCIAL CHALLENGES
The path forward must ensure safety but also allow for a financially sustainable balance of COVID and non-COVID care. To prepare for surges, health systems canceled elective surgeries and other services that generate essential revenue. At both private and public hospitals, systemwide measures have been taken to mitigate these financial losses. These measures have included salary, retirement, and continuing medical education benefit reductions for physicians and senior leadership; limits to physician hiring and recruitment; leaner operations with systemwide expense reductions; and mandatory and voluntary staff furloughs. The frontline hospital staff, including physicians, nurses, technologists, and food and environmental service workers, who have made great sacrifices during this pandemic, may also now be facing significant personal financial consequences.
The following recommendations are offered from the perspective that crisis creates opportunity for hospital medicine leaders grappling with budget shortfalls.
First, maximize budget transparency by explicitly defining the principles and priorities that govern budget decisions, which allows hospitalist group members to understand how the organization determines budget cuts. For example, stating that a key priority is to minimize staff layoffs makes consequent salary reductions more understandable.
Second, solicit hospital medicine group members’ input on these shared challenges and invite their help in identifying and prioritizing potential cost-saving or cost-cutting measures.
Third, highlight hospitalists’ nonfiscal contributions, especially in terms of crisis leadership, to continue engagement with executive leaders.5 This may include a dialogue about the disproportionate influence of work relative value unit production on salary and about how to create compensation systems that can also recognize crisis readiness as an important feature of sustainability and quality care. The next pandemic surge may be weeks or months away, and hospitalists will again need to be leaders in the response.
Fourth, use this crisis to foster fiscal innovation and accelerate participation in value improvement work, such as redesigning pay-for-performance metrics. Financially strapped institutions will value hospitalists who are good financial stewards. For example, leverage hospitalist expertise in progression of care to facilitate timely disposition of COVID patients, thereby minimizing costly extended hospitalizations.
Lastly, hospital medicine groups must match staffing to patient volume to the extent possible. Approximately two-thirds of hospitalist groups entered this crisis already understaffed and partially reliant on moonlighters,6 which allowed some variation of labor expenses to match lower patient volume. During the recovery phase, hospital volumes may either be significantly below or above baseline; many patients are understandably avoiding hospitals due to fear of COVID. However, delayed care may create a different kind of peak demand for services. For hospitalists, uncertainty about expected clinical roles, COVID vs non-COVID patient mix, and patient volume can be stressful. We recommend sustained, frequent communication about census trends and how shifts will be covered to ensure adequate, long-term staffing. Maintaining trust and morale will be equally, if not more, important in the next phase.
CONCLUSION
As we settle into the marathon, hospital medicine leadership must balance competing priorities with increasing finesse. Our hospital medicine group has benefited from continually discussing operational challenges and refining our strategies as we plan for what is ahead. We have highlighted three mission-critical themes and recommend that hospital and hospital medicine group leaders remain mindful of these challenges and potential strategies. Each of our four academic hospitals has considered similar trade-offs and will proceed along slightly different trajectories to meet unique needs. Looking to the future, we anticipate additional challenges requiring greater ongoing attention alongside those already identified. These include mitigating provider burnout, optimizing resident and student education, and maintaining scholarly work as COVID unpredictably waxes and wanes. By accumulating confidence and wisdom about post-COVID hospital medicine group functions, we hope to provide hospitalists with the energy to keep the pace in the next phase of the marathon.
- Garg M, Wray CM. Hospital medicine management in the time of COVID-19: preparing for a sprint and a marathon. J Hosp Med . 2020;15(5):305-307. https://doi.org/10.12788/jhm.3427
- COVIDView - A weekly Surveillance Summary of U.S. COVID-19 Activity. US Centers for Disease Control and Prevention. July 9, 2020. Accessed July 13, 2020. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/pdf/covidview-07-10-2020.pdf
- Khullar D, Bond AM, Schpero WL. COVID-19 and the financial health of US hospitals. JAMA. Published online May 4, 2020. https://doi.org/10.1001/jama.2020.6269
- Wang CJ, Bair H, Yeh CC. How to prevent and manage hospital-based infections during coronavirus outbreaks: five lessons from Taiwan. J Hosp Med . 2020;15(6):370-371. https://doi.org/10.12788/jhm.3452
- White AA, McIlraith T, Chivu AM, et al. Collaboration, not calculation: a qualitative study of how hospital executives value hospital medicine groups. J Hosp Med. 2019;14(11):662-667. https://doi.org/10.12788/jhm.3249
- 2018 State of Hospital Medicine: 2018 Report Based on 2017 Data . Society of Hospital Medicine; 2018. Accessed July 27, 2020. https://sohm.hospitalmedicine.org/
- Garg M, Wray CM. Hospital medicine management in the time of COVID-19: preparing for a sprint and a marathon. J Hosp Med . 2020;15(5):305-307. https://doi.org/10.12788/jhm.3427
- COVIDView - A weekly Surveillance Summary of U.S. COVID-19 Activity. US Centers for Disease Control and Prevention. July 9, 2020. Accessed July 13, 2020. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/pdf/covidview-07-10-2020.pdf
- Khullar D, Bond AM, Schpero WL. COVID-19 and the financial health of US hospitals. JAMA. Published online May 4, 2020. https://doi.org/10.1001/jama.2020.6269
- Wang CJ, Bair H, Yeh CC. How to prevent and manage hospital-based infections during coronavirus outbreaks: five lessons from Taiwan. J Hosp Med . 2020;15(6):370-371. https://doi.org/10.12788/jhm.3452
- White AA, McIlraith T, Chivu AM, et al. Collaboration, not calculation: a qualitative study of how hospital executives value hospital medicine groups. J Hosp Med. 2019;14(11):662-667. https://doi.org/10.12788/jhm.3249
- 2018 State of Hospital Medicine: 2018 Report Based on 2017 Data . Society of Hospital Medicine; 2018. Accessed July 27, 2020. https://sohm.hospitalmedicine.org/
© 2021 Society of Hospital Medicine
Email: [email protected].
An Academic Research Coach: An Innovative Approach to Increasing Scholarly Productivity in Medicine
Historically, academic medicine faculty were predominantly physician-scientists.1 During the past decade, the number of clinician-educators and nontenured clinicians has grown.2 Many academically oriented clinical faculty at our institution would like to participate in and learn how to conduct quality scholarship. While institutional requirements vary, scholarly work is often required for promotion,3 and faculty may also desire to support the scholarly work of residents. Moreover, a core program component of the Accreditation Council of Graduate Medical Education standards requires faculty to “maintain an environment of inquiry and scholarship with an active research component.”4 Yet clinical faculty often find academic projects to be challenging. Similar to residents, clinical academic faculty frequently lack formal training in health services research or quality improvement science, have insufficient mentorship, and typically have limited uncommitted time and resources.5
One approach to this problem has been to pair junior clinicians with traditional physician scientists as mentors.6,7 This type of mentorship for clinical faculty is increasingly difficult to access because of growing pressure on physician-scientist faculty to conduct their own research, seek extramural funding, meet clinical expectations, and mentor fellows and faculty in their own disciplines.8 Moreover, senior research faculty may not be prepared or have the time to teach junior faculty how to deal with common stumbling blocks (eg, institutional review board [IRB] applications, statistically testable hypothesis development, and statistical analysis).8,9 Seminars or works-in-progress sessions are another strategy to bolster scholarly work, but the experience at our institution is that such sessions are often not relevant at the time of delivery and can be intimidating to clinical faculty who lack extensive knowledge about research methods and prior research experience.
Another approach to supporting the research efforts of academic clinicians is to fund a consulting statistician. However, without sufficient content expertise, statisticians may be frustrated in their efforts to assist clinicians who struggle to formulate a testable question or to work directly with data collected. Statisticians may be inexperienced in writing IRB applications or implementing protocols in a clinical or educational setting. Furthermore, statistical consultations are often limited in scope10 and, in our setting, rarely produce a durable improvement in the research skills of the faculty member or the enduring partnership required to complete a longer-term project. Because of these shortcomings, we have found that purely statistical support resources are often underutilized and ineffective.
Other models to facilitate scholarship have been employed, but few focus on facilitating scholarship of clinical faculty. One strategy involved supporting hospitalist’s academic productivity by reducing hospitalists’ full-time equivalent (FTE) and providing mentorship.11 For many, this approach is likely cost-prohibitive. Others have focused primarily on resident and fellow scholarships.5,6
In this report, we describe an educational innovation to educate and support the scholarly work of academic hospitalists and internists by using an academic research coach. We recruited a health researcher with extensive experience in research methods and strong interpersonal skills with the ability to explain and teach research concepts in an accessible manner. We sought an individual who would provide high-yield single consultations, join project teams to provide ongoing mentorship from conception to completion, and consequently, bolster scholarly productivity and learning among nonresearch clinicians in our Division. We anticipated that providing support for multiple aspects of a project would be more likely to help faculty overcome barriers to research and disseminate their project results as scholarly output.
METHODS
The coach initiative was implemented in the Division of General Internal Medicine at the University of Washington. The Division has over 200 members (60 hospitalists), including clinical instructors and acting instructors, who have not yet been appointed to the regular faculty (clinician-educators and physician scientists), and full-time clinical faculty. Division members staff clinical services at four area hospitals and 10 affiliated internal medicine and specialty clinics. Eligible clients were all Division members, although the focus of the initial program targeted hospitalists at our three primary teaching hospitals. Fellows, residents, students, and faculty from within and outside the Division were welcome to participate in a project involving coaching as long as a Division faculty member was engaged in the project.
Program Description
The overall goal of the coach initiative was to support the scholarly work of primarily clinical Division members. Given our focus was on clinical faculty with little training on research methodology, we did not expect the coach to secure grant funding for the position. Instead, we aimed to increase the quality and quantity of scholarship through publications, abstracts, and small grants. We defined scholarly work broadly: clinical research, quality improvement, medical education research, and other forms of scientific inquiry or synthesis. The coach was established as a 0.50 FTE position with a 12-month annually renewable appointment. The role was deemed that of a coach instead of a mentor because the coach was available to all Division members and involved task-oriented consultations with check-ins to facilitate projects, rather than a deeper more developmental relationship that typically exists with mentoring. The Division leadership identified support for scholarly activity as a high priority and mentorship as an unmet need based on faculty feedback. Clinical revenue supported the position.
Necessary qualifications, determined prior to hiring, included a PhD in health services or related field (eg, epidemiology) or a master’s degree with five years of experience in project management, clinical research, and study design. The position also called for expertise in articulating research questions, selecting study designs, navigating the IRB approval process, collecting/managing data, analyzing statistics, and mentoring and teaching clinical faculty in their scholarly endeavors. A track record in generating academic output (manuscripts and abstracts at regional/national meetings) was required. We circulated a description of the position to Division faculty and to leadership in our School of Public Health.
Based on these criteria, an inaugural coach was hired (author C.M.M.). The coach had a PhD in epidemiology, 10 years of research experience, 16 publications, and had recently finished a National Institutes of Health (NIH) career development award. At the time of hiring, she was a Clinical Assistant Professor in the School of Dentistry, which provided additional FTE. She had no extramural funding but was applying for NIH-level grants and had received several small grants.
To ensure uptake of the coach’s services, we realized that it was necessary to delineate the scope of services available, clarify availability of the coach, and define expectations regarding authorship. We used an iterative process that took into consideration the coach’s expertise, services most needed by the Division’s clinicians, and discussions with Division leadership and faculty at faculty meetings across hospitals and clinics. A range of services and authorship expectations were defined. Consensus was reached that the coach should be invited to coauthor projects where design, analysis, and/or substantial intellectual content was provided and for which authorship criteria were met.12 Collegial reviews by the coach of already developed manuscripts and time-limited, low-intensity consultations that did not involve substantial intellectual contributions did not warrant authorship.12 On this basis, we created and distributed a flyer to publicize these guidelines and invite Division members to contact the coach (Figure 1).
The coach attended Division, section, and clinical group meetings to publicize the initiative. The coach also individually met with faculty throughout the Division, explained her role, described services available, and answered questions. The marketing effort was continuous and calibrated with more or less exposure depending on existing projects and the coach’s availability. In addition, the coach coordinated with the director of the Division’s faculty development program to cohost works-in-progress seminars, identify coach clients to present at these meetings, and provide brief presentations on a basic research skill at meetings. Faculty built rapport with the coach through these activities and became more comfortable reaching out for assistance. Because of the large size of the Division, it was decided to roll out the initiative in a stepwise fashion, starting with hospitalists before expanding to the rest of the Division.
Most faculty contacted the coach by e-mail to request a consultation, at which time the coach requested that they complete a preconsultation handout (Figure 2). Initial coaching appointments lasted one hour and were in-person. Coaching entailed an in-depth analysis of the project plan and advice on how to move the project forward. The coach provided tailored scholarly project advice and expertise in research methods. After initial consultations, she would review grant proposals, IRB applications, manuscripts, case report forms, abstracts, and other products. Her efforts typically focused on improving the methods and scientific and technical writing. Assistance with statistical analysis was provided on a case-by-case basis to maintain broad availability. To address statistically complex questions, the coach had five hours of monthly access to a PhD biostatistician via an on-campus consulting service. Follow-up appointments were encouraged and provided as needed by e-mail, phone, or in-person. The coach conducted regular reach outs to facilitate projects. However, execution of the research was generally the responsibility of the faculty member.
Program Evaluation
To characterize the reach and scope of the program, the coach tracked the number of faculty supported, types of services provided, status of initiated projects, numbers of grants generated, and the dissemination of scholarly products including papers and abstracts. We used these metrics to create summary reports to identify successes and areas for improvement. Monthly meetings between the coach and Division leadership were used to fine-tune the approach.
We surveyed coach clients anonymously to assess their satisfaction with the coach initiative. Using Likert scale questions where 1 = completely disagree and 5 = completely agree, we asked (1) if they would recommend the coach to colleagues, (2) if their work was higher quality because of the coach, (3) if they were overall satisfied with the coach, (4) whether the Division should continue to support the coach, and (5) if the coach’s lack of clinical training negatively affected their experience. This work was considered a quality improvement initiative for which IRB approval was not required.
RESULTS
Over 18 months, the coach supported a 49 Division members including 30 hospitalists and 63 projects. Projects included a wide range of scholarship: medical education research, qualitative research, clinical quality improvement projects, observational studies, and a randomized clinical trial. Many clients (n = 16) used the coach for more than one project. The scope of work included limited support projects (identifying research resource and brainstorming project feasibility) lasting one to two sessions (n = 25), projects with a limited scope (collegial reviews of manuscripts and assistance with IRB submissions) but requiring more than two consultations (n = 24), and ongoing in-depth support projects (contributions on design, data collection, analysis, and manuscript writing) that required three consultations or more (n = 14). The majority of Division members (75%) supported did not have master’s level training in a health services-related area, six had NIH or other national-level funding, and two had small grants funded by local sources prior to providing support. The number of Division faculty on a given project ranged from one to four.
The coach directly supported 13 manuscripts with coach authorship, seven manuscripts without authorship, 11 abstracts, and four grant submissions (Appendix). The coach was a coauthor on all the abstracts and a coinvestigator on the grant applications. Of the 13 publications the coach coauthored, 11 publications have been accepted to peer-reviewed journals and two are currently in the submission process. The types of articles published included one medical evaluation report, one qualitative study, one randomized clinical trial, three quality assessment/improvement reports, and five epidemiologic studies. The types of abstracts included one qualitative report, one systematic review, one randomized clinical trial, two quality improvement projects, two epidemiologic studies, and four medical education projects. Three of four small grants submitted to local and national funders were funded.
The coach’s influence extended beyond the Division. Forty-eight university faculty, fellows, or students not affiliated with general internal medicine benefited from coach coaching: 26 were authors on papers and/or abstracts coauthored by the coach, 17 on manuscripts the coach reviewed without authorship, and five participated in consultations.
The coach found the experience rewarding. She enjoyed working on the methodologic aspects of projects and benefited from being included as coauthor on papers.
Twenty-nine of the 43 faculty (67%) still at the institution responded to the program assessment survey. Faculty strongly agreed that they would recommend the coach to colleagues (average ± standard deviation [SD]: 4.7 ± 0.5), that it improved the quality of their work (4.5 ± 0.9), that they were overall satisfied with the coaching (4.6 ± 0.7), and that the Division should continue to support the coach (4.9 ± 0.4). Faculty did not agree that the lack of clinical training of the coach was a barrier (2.0 ± 1.3).
DISCUSSION
The coach program was highly utilized, well regarded, and delivered substantial, tangible, and academic output. We anticipate the coach initiative will continue to be a valuable resource for our Division and could prove to be a valuable model for other institutions seeking to bolster the scholarly work of clinical academicians.
Several lessons emerged through the course of this project. First, we realized it is essential to select a coach who is both knowledgeable and approachable. We found that after meeting the coach, many faculty sought her help who otherwise would not have. An explicit, ongoing marketing strategy with regular contact with faculty at meetings was a key to receiving consult requests.
Second, the lack of a clinical background did not seem to hinder the coach’s ability to coach clinicians. The coach acknowledged her lack of clinical experience and relied on clients to explain the clinical context of projects. We also learned that the coach’s substantial experience with the logistics of research was invaluable. For example, the coach had substantial experience with the IRB process and her pre-reviews of IRB applications made for a short and relatively seamless experience navigating the IRB process. The coach also facilitated collaborations and leveraged existing resources at our institution. For example, for a qualitative research project, the coach helped identify a health services faculty member with this specific expertise, which led to a successful collaboration and publication. Although a more junior coach with less established qualifications may be helpful with research methods and with the research process, our endeavor suggests that having a more highly trained and experienced researcher was extremely valuable. Finally, we learned that for a Division of our size, the 0.50 FTE allotted to the coach is a minimum requirement. The coach spent approximately four hours a week on marketing, attending faculty meetings and conducting brief didactics, two hours per week on administration, and 14 hours per week on consultations. Faculty generally received support soon after their requests, but there were occasional wait times, which may have delayed some projects.
Academic leaders at our institution have noted the success of our coach initiative and have created a demand for coach services. We are exploring funding models that would allow for the expansion of coach services to other departments and divisions. We are in the initial stages of creating an Academic Scholarship Support Core under the supervision of the coach. Within this Core, we envision that various research support services will be triaged to staff with appropriate expertise; for example, a regulatory coordinator would review IRB applications while a master’s level statistician would conduct statistical analyses.
We have also transitioned to a new coach and have continued to experience success with the program. Our initial coach (author C.M.M.) obtained an NIH R01, a foundation grant, and took over a summer program that trains dental faculty in clinical research methods leaving insufficient time for coaching. Our new coach also has a PhD in epidemiology with NIH R01 funding but has more available FTE. Both of our coaches are graduates of our School of Public Health and institutions with such schools may have good access to the expertise needed. Nonclinical PhDs are often almost entirely reliant on grants, and some nongrant support is often attractive to these researchers. Additionally, PhDs who are junior or mid-career faculty that have the needed training are relatively affordable, particularly when the resource is made available to large number of faculty.
A limitation to our assessment of the coach initiative was the lack of pre- and postintervention metrics of scholarly productivity. We cannot definitively say that the Division’s scholarly output has increased because of the coach. Nevertheless, we are confident that the coach’s coaching has enhanced the scholarly work of individual clinicians and provided value to the Division as a whole. The coach program has been a success in our Division. Other institutions facing the challenge of supporting the research efforts of academic clinicians may consider this model as a worthy investment.
Disclosures
The authors have nothing to disclose.
1. Marks AR. Physician-scientist, heal thyself. J Clin Invest. 2007;117(1):2. https://doi.org/10.1172/JCI31031.
2. Bunton SA, Corrice AM. Trends in tenure for clinical M.D. faculty in U.S. medical schools: a 25-year review. Association of American Medical Colleges: Analysis in Brief. 2010;9(9):1-2; https://www.aamc.org/download/139778/data/aibvol9_no9.pdf. Accessed March 7, 2019.
3. Bunton SA, Mallon WT. The continued evolution of faculty appointment and tenure policies at U.S. medical schools. Acad Med. 2007;82(3):281-289. https://doi.org/10.1097/ACM.0b013e3180307e87.
4. Accreditation Council for Graduate Medical Education. ACGME Common Program Requirements. 2017; http://www.acgme.org/What-We-Do/Accreditation/Common-Program-Requirements. Accessed March 7, 2019.
5. Penrose LL, Yeomans ER, Praderio C, Prien SD. An incremental approach to improving scholarly activity. J Grad Med Educ. 2012;4(4):496-499. https://doi.org/10.4300/JGME-D-11-00185.1.
6. Manring MM, Panzo JA, Mayerson JL. A framework for improving resident research participation and scholarly output. J Surg Educ. 2014;71(1):8-13. https://doi.org/10.1016/j.jsurg.2013.07.011.
7. Palacio A, Campbell DT, Moore M, Symes S, Tamariz L. Predictors of scholarly success among internal medicine residents. Am J Med. 2013;126(2):181-185. https:doi.org/10.1016/j.amjmed.2012.10.003.
8. Physician-Scientist Workforce Working Group. Physician-scientist workforce (PSW) report 2014. https://report.nih.gov/Workforce/PSW/challenges.aspx. Accessed December 27, 2018.
9. Straus SE, Johnson MO, Marquez C, Feldman MD. Characteristics of successful and failed mentoring relationships: a qualitative study across two academic health centers. Acad Med. 2013;88(1):82-89. https://doi.org/10.1097/ACM.0b013e31827647a0.
10. Altman DG, Goodman SN, Schroter S. How statistical expertise is used in medical research. JAMA. 2002;287(21):2817-2820. https://doi.org/10.1001/jama.287.21.2817.
11. Howell E, Kravet S, Kisuule F, Wright SM. An innovative approach to supporting hospitalist physicians towards academic success. J Hosp Med. 2008;3(4):314-318. https://doi.org/10.1002/jhm.327.
12. Kripalani S, Williams MV. Author responsibilities and disclosures at the Journal of Hospital Medicine. J Hosp Med. 2010;5(6):320-322. https://doi.org/10.1002/jhm.715.
Historically, academic medicine faculty were predominantly physician-scientists.1 During the past decade, the number of clinician-educators and nontenured clinicians has grown.2 Many academically oriented clinical faculty at our institution would like to participate in and learn how to conduct quality scholarship. While institutional requirements vary, scholarly work is often required for promotion,3 and faculty may also desire to support the scholarly work of residents. Moreover, a core program component of the Accreditation Council of Graduate Medical Education standards requires faculty to “maintain an environment of inquiry and scholarship with an active research component.”4 Yet clinical faculty often find academic projects to be challenging. Similar to residents, clinical academic faculty frequently lack formal training in health services research or quality improvement science, have insufficient mentorship, and typically have limited uncommitted time and resources.5
One approach to this problem has been to pair junior clinicians with traditional physician scientists as mentors.6,7 This type of mentorship for clinical faculty is increasingly difficult to access because of growing pressure on physician-scientist faculty to conduct their own research, seek extramural funding, meet clinical expectations, and mentor fellows and faculty in their own disciplines.8 Moreover, senior research faculty may not be prepared or have the time to teach junior faculty how to deal with common stumbling blocks (eg, institutional review board [IRB] applications, statistically testable hypothesis development, and statistical analysis).8,9 Seminars or works-in-progress sessions are another strategy to bolster scholarly work, but the experience at our institution is that such sessions are often not relevant at the time of delivery and can be intimidating to clinical faculty who lack extensive knowledge about research methods and prior research experience.
Another approach to supporting the research efforts of academic clinicians is to fund a consulting statistician. However, without sufficient content expertise, statisticians may be frustrated in their efforts to assist clinicians who struggle to formulate a testable question or to work directly with data collected. Statisticians may be inexperienced in writing IRB applications or implementing protocols in a clinical or educational setting. Furthermore, statistical consultations are often limited in scope10 and, in our setting, rarely produce a durable improvement in the research skills of the faculty member or the enduring partnership required to complete a longer-term project. Because of these shortcomings, we have found that purely statistical support resources are often underutilized and ineffective.
Other models to facilitate scholarship have been employed, but few focus on facilitating scholarship of clinical faculty. One strategy involved supporting hospitalist’s academic productivity by reducing hospitalists’ full-time equivalent (FTE) and providing mentorship.11 For many, this approach is likely cost-prohibitive. Others have focused primarily on resident and fellow scholarships.5,6
In this report, we describe an educational innovation to educate and support the scholarly work of academic hospitalists and internists by using an academic research coach. We recruited a health researcher with extensive experience in research methods and strong interpersonal skills with the ability to explain and teach research concepts in an accessible manner. We sought an individual who would provide high-yield single consultations, join project teams to provide ongoing mentorship from conception to completion, and consequently, bolster scholarly productivity and learning among nonresearch clinicians in our Division. We anticipated that providing support for multiple aspects of a project would be more likely to help faculty overcome barriers to research and disseminate their project results as scholarly output.
METHODS
The coach initiative was implemented in the Division of General Internal Medicine at the University of Washington. The Division has over 200 members (60 hospitalists), including clinical instructors and acting instructors, who have not yet been appointed to the regular faculty (clinician-educators and physician scientists), and full-time clinical faculty. Division members staff clinical services at four area hospitals and 10 affiliated internal medicine and specialty clinics. Eligible clients were all Division members, although the focus of the initial program targeted hospitalists at our three primary teaching hospitals. Fellows, residents, students, and faculty from within and outside the Division were welcome to participate in a project involving coaching as long as a Division faculty member was engaged in the project.
Program Description
The overall goal of the coach initiative was to support the scholarly work of primarily clinical Division members. Given our focus was on clinical faculty with little training on research methodology, we did not expect the coach to secure grant funding for the position. Instead, we aimed to increase the quality and quantity of scholarship through publications, abstracts, and small grants. We defined scholarly work broadly: clinical research, quality improvement, medical education research, and other forms of scientific inquiry or synthesis. The coach was established as a 0.50 FTE position with a 12-month annually renewable appointment. The role was deemed that of a coach instead of a mentor because the coach was available to all Division members and involved task-oriented consultations with check-ins to facilitate projects, rather than a deeper more developmental relationship that typically exists with mentoring. The Division leadership identified support for scholarly activity as a high priority and mentorship as an unmet need based on faculty feedback. Clinical revenue supported the position.
Necessary qualifications, determined prior to hiring, included a PhD in health services or related field (eg, epidemiology) or a master’s degree with five years of experience in project management, clinical research, and study design. The position also called for expertise in articulating research questions, selecting study designs, navigating the IRB approval process, collecting/managing data, analyzing statistics, and mentoring and teaching clinical faculty in their scholarly endeavors. A track record in generating academic output (manuscripts and abstracts at regional/national meetings) was required. We circulated a description of the position to Division faculty and to leadership in our School of Public Health.
Based on these criteria, an inaugural coach was hired (author C.M.M.). The coach had a PhD in epidemiology, 10 years of research experience, 16 publications, and had recently finished a National Institutes of Health (NIH) career development award. At the time of hiring, she was a Clinical Assistant Professor in the School of Dentistry, which provided additional FTE. She had no extramural funding but was applying for NIH-level grants and had received several small grants.
To ensure uptake of the coach’s services, we realized that it was necessary to delineate the scope of services available, clarify availability of the coach, and define expectations regarding authorship. We used an iterative process that took into consideration the coach’s expertise, services most needed by the Division’s clinicians, and discussions with Division leadership and faculty at faculty meetings across hospitals and clinics. A range of services and authorship expectations were defined. Consensus was reached that the coach should be invited to coauthor projects where design, analysis, and/or substantial intellectual content was provided and for which authorship criteria were met.12 Collegial reviews by the coach of already developed manuscripts and time-limited, low-intensity consultations that did not involve substantial intellectual contributions did not warrant authorship.12 On this basis, we created and distributed a flyer to publicize these guidelines and invite Division members to contact the coach (Figure 1).
The coach attended Division, section, and clinical group meetings to publicize the initiative. The coach also individually met with faculty throughout the Division, explained her role, described services available, and answered questions. The marketing effort was continuous and calibrated with more or less exposure depending on existing projects and the coach’s availability. In addition, the coach coordinated with the director of the Division’s faculty development program to cohost works-in-progress seminars, identify coach clients to present at these meetings, and provide brief presentations on a basic research skill at meetings. Faculty built rapport with the coach through these activities and became more comfortable reaching out for assistance. Because of the large size of the Division, it was decided to roll out the initiative in a stepwise fashion, starting with hospitalists before expanding to the rest of the Division.
Most faculty contacted the coach by e-mail to request a consultation, at which time the coach requested that they complete a preconsultation handout (Figure 2). Initial coaching appointments lasted one hour and were in-person. Coaching entailed an in-depth analysis of the project plan and advice on how to move the project forward. The coach provided tailored scholarly project advice and expertise in research methods. After initial consultations, she would review grant proposals, IRB applications, manuscripts, case report forms, abstracts, and other products. Her efforts typically focused on improving the methods and scientific and technical writing. Assistance with statistical analysis was provided on a case-by-case basis to maintain broad availability. To address statistically complex questions, the coach had five hours of monthly access to a PhD biostatistician via an on-campus consulting service. Follow-up appointments were encouraged and provided as needed by e-mail, phone, or in-person. The coach conducted regular reach outs to facilitate projects. However, execution of the research was generally the responsibility of the faculty member.
Program Evaluation
To characterize the reach and scope of the program, the coach tracked the number of faculty supported, types of services provided, status of initiated projects, numbers of grants generated, and the dissemination of scholarly products including papers and abstracts. We used these metrics to create summary reports to identify successes and areas for improvement. Monthly meetings between the coach and Division leadership were used to fine-tune the approach.
We surveyed coach clients anonymously to assess their satisfaction with the coach initiative. Using Likert scale questions where 1 = completely disagree and 5 = completely agree, we asked (1) if they would recommend the coach to colleagues, (2) if their work was higher quality because of the coach, (3) if they were overall satisfied with the coach, (4) whether the Division should continue to support the coach, and (5) if the coach’s lack of clinical training negatively affected their experience. This work was considered a quality improvement initiative for which IRB approval was not required.
RESULTS
Over 18 months, the coach supported a 49 Division members including 30 hospitalists and 63 projects. Projects included a wide range of scholarship: medical education research, qualitative research, clinical quality improvement projects, observational studies, and a randomized clinical trial. Many clients (n = 16) used the coach for more than one project. The scope of work included limited support projects (identifying research resource and brainstorming project feasibility) lasting one to two sessions (n = 25), projects with a limited scope (collegial reviews of manuscripts and assistance with IRB submissions) but requiring more than two consultations (n = 24), and ongoing in-depth support projects (contributions on design, data collection, analysis, and manuscript writing) that required three consultations or more (n = 14). The majority of Division members (75%) supported did not have master’s level training in a health services-related area, six had NIH or other national-level funding, and two had small grants funded by local sources prior to providing support. The number of Division faculty on a given project ranged from one to four.
The coach directly supported 13 manuscripts with coach authorship, seven manuscripts without authorship, 11 abstracts, and four grant submissions (Appendix). The coach was a coauthor on all the abstracts and a coinvestigator on the grant applications. Of the 13 publications the coach coauthored, 11 publications have been accepted to peer-reviewed journals and two are currently in the submission process. The types of articles published included one medical evaluation report, one qualitative study, one randomized clinical trial, three quality assessment/improvement reports, and five epidemiologic studies. The types of abstracts included one qualitative report, one systematic review, one randomized clinical trial, two quality improvement projects, two epidemiologic studies, and four medical education projects. Three of four small grants submitted to local and national funders were funded.
The coach’s influence extended beyond the Division. Forty-eight university faculty, fellows, or students not affiliated with general internal medicine benefited from coach coaching: 26 were authors on papers and/or abstracts coauthored by the coach, 17 on manuscripts the coach reviewed without authorship, and five participated in consultations.
The coach found the experience rewarding. She enjoyed working on the methodologic aspects of projects and benefited from being included as coauthor on papers.
Twenty-nine of the 43 faculty (67%) still at the institution responded to the program assessment survey. Faculty strongly agreed that they would recommend the coach to colleagues (average ± standard deviation [SD]: 4.7 ± 0.5), that it improved the quality of their work (4.5 ± 0.9), that they were overall satisfied with the coaching (4.6 ± 0.7), and that the Division should continue to support the coach (4.9 ± 0.4). Faculty did not agree that the lack of clinical training of the coach was a barrier (2.0 ± 1.3).
DISCUSSION
The coach program was highly utilized, well regarded, and delivered substantial, tangible, and academic output. We anticipate the coach initiative will continue to be a valuable resource for our Division and could prove to be a valuable model for other institutions seeking to bolster the scholarly work of clinical academicians.
Several lessons emerged through the course of this project. First, we realized it is essential to select a coach who is both knowledgeable and approachable. We found that after meeting the coach, many faculty sought her help who otherwise would not have. An explicit, ongoing marketing strategy with regular contact with faculty at meetings was a key to receiving consult requests.
Second, the lack of a clinical background did not seem to hinder the coach’s ability to coach clinicians. The coach acknowledged her lack of clinical experience and relied on clients to explain the clinical context of projects. We also learned that the coach’s substantial experience with the logistics of research was invaluable. For example, the coach had substantial experience with the IRB process and her pre-reviews of IRB applications made for a short and relatively seamless experience navigating the IRB process. The coach also facilitated collaborations and leveraged existing resources at our institution. For example, for a qualitative research project, the coach helped identify a health services faculty member with this specific expertise, which led to a successful collaboration and publication. Although a more junior coach with less established qualifications may be helpful with research methods and with the research process, our endeavor suggests that having a more highly trained and experienced researcher was extremely valuable. Finally, we learned that for a Division of our size, the 0.50 FTE allotted to the coach is a minimum requirement. The coach spent approximately four hours a week on marketing, attending faculty meetings and conducting brief didactics, two hours per week on administration, and 14 hours per week on consultations. Faculty generally received support soon after their requests, but there were occasional wait times, which may have delayed some projects.
Academic leaders at our institution have noted the success of our coach initiative and have created a demand for coach services. We are exploring funding models that would allow for the expansion of coach services to other departments and divisions. We are in the initial stages of creating an Academic Scholarship Support Core under the supervision of the coach. Within this Core, we envision that various research support services will be triaged to staff with appropriate expertise; for example, a regulatory coordinator would review IRB applications while a master’s level statistician would conduct statistical analyses.
We have also transitioned to a new coach and have continued to experience success with the program. Our initial coach (author C.M.M.) obtained an NIH R01, a foundation grant, and took over a summer program that trains dental faculty in clinical research methods leaving insufficient time for coaching. Our new coach also has a PhD in epidemiology with NIH R01 funding but has more available FTE. Both of our coaches are graduates of our School of Public Health and institutions with such schools may have good access to the expertise needed. Nonclinical PhDs are often almost entirely reliant on grants, and some nongrant support is often attractive to these researchers. Additionally, PhDs who are junior or mid-career faculty that have the needed training are relatively affordable, particularly when the resource is made available to large number of faculty.
A limitation to our assessment of the coach initiative was the lack of pre- and postintervention metrics of scholarly productivity. We cannot definitively say that the Division’s scholarly output has increased because of the coach. Nevertheless, we are confident that the coach’s coaching has enhanced the scholarly work of individual clinicians and provided value to the Division as a whole. The coach program has been a success in our Division. Other institutions facing the challenge of supporting the research efforts of academic clinicians may consider this model as a worthy investment.
Disclosures
The authors have nothing to disclose.
Historically, academic medicine faculty were predominantly physician-scientists.1 During the past decade, the number of clinician-educators and nontenured clinicians has grown.2 Many academically oriented clinical faculty at our institution would like to participate in and learn how to conduct quality scholarship. While institutional requirements vary, scholarly work is often required for promotion,3 and faculty may also desire to support the scholarly work of residents. Moreover, a core program component of the Accreditation Council of Graduate Medical Education standards requires faculty to “maintain an environment of inquiry and scholarship with an active research component.”4 Yet clinical faculty often find academic projects to be challenging. Similar to residents, clinical academic faculty frequently lack formal training in health services research or quality improvement science, have insufficient mentorship, and typically have limited uncommitted time and resources.5
One approach to this problem has been to pair junior clinicians with traditional physician scientists as mentors.6,7 This type of mentorship for clinical faculty is increasingly difficult to access because of growing pressure on physician-scientist faculty to conduct their own research, seek extramural funding, meet clinical expectations, and mentor fellows and faculty in their own disciplines.8 Moreover, senior research faculty may not be prepared or have the time to teach junior faculty how to deal with common stumbling blocks (eg, institutional review board [IRB] applications, statistically testable hypothesis development, and statistical analysis).8,9 Seminars or works-in-progress sessions are another strategy to bolster scholarly work, but the experience at our institution is that such sessions are often not relevant at the time of delivery and can be intimidating to clinical faculty who lack extensive knowledge about research methods and prior research experience.
Another approach to supporting the research efforts of academic clinicians is to fund a consulting statistician. However, without sufficient content expertise, statisticians may be frustrated in their efforts to assist clinicians who struggle to formulate a testable question or to work directly with data collected. Statisticians may be inexperienced in writing IRB applications or implementing protocols in a clinical or educational setting. Furthermore, statistical consultations are often limited in scope10 and, in our setting, rarely produce a durable improvement in the research skills of the faculty member or the enduring partnership required to complete a longer-term project. Because of these shortcomings, we have found that purely statistical support resources are often underutilized and ineffective.
Other models to facilitate scholarship have been employed, but few focus on facilitating scholarship of clinical faculty. One strategy involved supporting hospitalist’s academic productivity by reducing hospitalists’ full-time equivalent (FTE) and providing mentorship.11 For many, this approach is likely cost-prohibitive. Others have focused primarily on resident and fellow scholarships.5,6
In this report, we describe an educational innovation to educate and support the scholarly work of academic hospitalists and internists by using an academic research coach. We recruited a health researcher with extensive experience in research methods and strong interpersonal skills with the ability to explain and teach research concepts in an accessible manner. We sought an individual who would provide high-yield single consultations, join project teams to provide ongoing mentorship from conception to completion, and consequently, bolster scholarly productivity and learning among nonresearch clinicians in our Division. We anticipated that providing support for multiple aspects of a project would be more likely to help faculty overcome barriers to research and disseminate their project results as scholarly output.
METHODS
The coach initiative was implemented in the Division of General Internal Medicine at the University of Washington. The Division has over 200 members (60 hospitalists), including clinical instructors and acting instructors, who have not yet been appointed to the regular faculty (clinician-educators and physician scientists), and full-time clinical faculty. Division members staff clinical services at four area hospitals and 10 affiliated internal medicine and specialty clinics. Eligible clients were all Division members, although the focus of the initial program targeted hospitalists at our three primary teaching hospitals. Fellows, residents, students, and faculty from within and outside the Division were welcome to participate in a project involving coaching as long as a Division faculty member was engaged in the project.
Program Description
The overall goal of the coach initiative was to support the scholarly work of primarily clinical Division members. Given our focus was on clinical faculty with little training on research methodology, we did not expect the coach to secure grant funding for the position. Instead, we aimed to increase the quality and quantity of scholarship through publications, abstracts, and small grants. We defined scholarly work broadly: clinical research, quality improvement, medical education research, and other forms of scientific inquiry or synthesis. The coach was established as a 0.50 FTE position with a 12-month annually renewable appointment. The role was deemed that of a coach instead of a mentor because the coach was available to all Division members and involved task-oriented consultations with check-ins to facilitate projects, rather than a deeper more developmental relationship that typically exists with mentoring. The Division leadership identified support for scholarly activity as a high priority and mentorship as an unmet need based on faculty feedback. Clinical revenue supported the position.
Necessary qualifications, determined prior to hiring, included a PhD in health services or related field (eg, epidemiology) or a master’s degree with five years of experience in project management, clinical research, and study design. The position also called for expertise in articulating research questions, selecting study designs, navigating the IRB approval process, collecting/managing data, analyzing statistics, and mentoring and teaching clinical faculty in their scholarly endeavors. A track record in generating academic output (manuscripts and abstracts at regional/national meetings) was required. We circulated a description of the position to Division faculty and to leadership in our School of Public Health.
Based on these criteria, an inaugural coach was hired (author C.M.M.). The coach had a PhD in epidemiology, 10 years of research experience, 16 publications, and had recently finished a National Institutes of Health (NIH) career development award. At the time of hiring, she was a Clinical Assistant Professor in the School of Dentistry, which provided additional FTE. She had no extramural funding but was applying for NIH-level grants and had received several small grants.
To ensure uptake of the coach’s services, we realized that it was necessary to delineate the scope of services available, clarify availability of the coach, and define expectations regarding authorship. We used an iterative process that took into consideration the coach’s expertise, services most needed by the Division’s clinicians, and discussions with Division leadership and faculty at faculty meetings across hospitals and clinics. A range of services and authorship expectations were defined. Consensus was reached that the coach should be invited to coauthor projects where design, analysis, and/or substantial intellectual content was provided and for which authorship criteria were met.12 Collegial reviews by the coach of already developed manuscripts and time-limited, low-intensity consultations that did not involve substantial intellectual contributions did not warrant authorship.12 On this basis, we created and distributed a flyer to publicize these guidelines and invite Division members to contact the coach (Figure 1).
The coach attended Division, section, and clinical group meetings to publicize the initiative. The coach also individually met with faculty throughout the Division, explained her role, described services available, and answered questions. The marketing effort was continuous and calibrated with more or less exposure depending on existing projects and the coach’s availability. In addition, the coach coordinated with the director of the Division’s faculty development program to cohost works-in-progress seminars, identify coach clients to present at these meetings, and provide brief presentations on a basic research skill at meetings. Faculty built rapport with the coach through these activities and became more comfortable reaching out for assistance. Because of the large size of the Division, it was decided to roll out the initiative in a stepwise fashion, starting with hospitalists before expanding to the rest of the Division.
Most faculty contacted the coach by e-mail to request a consultation, at which time the coach requested that they complete a preconsultation handout (Figure 2). Initial coaching appointments lasted one hour and were in-person. Coaching entailed an in-depth analysis of the project plan and advice on how to move the project forward. The coach provided tailored scholarly project advice and expertise in research methods. After initial consultations, she would review grant proposals, IRB applications, manuscripts, case report forms, abstracts, and other products. Her efforts typically focused on improving the methods and scientific and technical writing. Assistance with statistical analysis was provided on a case-by-case basis to maintain broad availability. To address statistically complex questions, the coach had five hours of monthly access to a PhD biostatistician via an on-campus consulting service. Follow-up appointments were encouraged and provided as needed by e-mail, phone, or in-person. The coach conducted regular reach outs to facilitate projects. However, execution of the research was generally the responsibility of the faculty member.
Program Evaluation
To characterize the reach and scope of the program, the coach tracked the number of faculty supported, types of services provided, status of initiated projects, numbers of grants generated, and the dissemination of scholarly products including papers and abstracts. We used these metrics to create summary reports to identify successes and areas for improvement. Monthly meetings between the coach and Division leadership were used to fine-tune the approach.
We surveyed coach clients anonymously to assess their satisfaction with the coach initiative. Using Likert scale questions where 1 = completely disagree and 5 = completely agree, we asked (1) if they would recommend the coach to colleagues, (2) if their work was higher quality because of the coach, (3) if they were overall satisfied with the coach, (4) whether the Division should continue to support the coach, and (5) if the coach’s lack of clinical training negatively affected their experience. This work was considered a quality improvement initiative for which IRB approval was not required.
RESULTS
Over 18 months, the coach supported a 49 Division members including 30 hospitalists and 63 projects. Projects included a wide range of scholarship: medical education research, qualitative research, clinical quality improvement projects, observational studies, and a randomized clinical trial. Many clients (n = 16) used the coach for more than one project. The scope of work included limited support projects (identifying research resource and brainstorming project feasibility) lasting one to two sessions (n = 25), projects with a limited scope (collegial reviews of manuscripts and assistance with IRB submissions) but requiring more than two consultations (n = 24), and ongoing in-depth support projects (contributions on design, data collection, analysis, and manuscript writing) that required three consultations or more (n = 14). The majority of Division members (75%) supported did not have master’s level training in a health services-related area, six had NIH or other national-level funding, and two had small grants funded by local sources prior to providing support. The number of Division faculty on a given project ranged from one to four.
The coach directly supported 13 manuscripts with coach authorship, seven manuscripts without authorship, 11 abstracts, and four grant submissions (Appendix). The coach was a coauthor on all the abstracts and a coinvestigator on the grant applications. Of the 13 publications the coach coauthored, 11 publications have been accepted to peer-reviewed journals and two are currently in the submission process. The types of articles published included one medical evaluation report, one qualitative study, one randomized clinical trial, three quality assessment/improvement reports, and five epidemiologic studies. The types of abstracts included one qualitative report, one systematic review, one randomized clinical trial, two quality improvement projects, two epidemiologic studies, and four medical education projects. Three of four small grants submitted to local and national funders were funded.
The coach’s influence extended beyond the Division. Forty-eight university faculty, fellows, or students not affiliated with general internal medicine benefited from coach coaching: 26 were authors on papers and/or abstracts coauthored by the coach, 17 on manuscripts the coach reviewed without authorship, and five participated in consultations.
The coach found the experience rewarding. She enjoyed working on the methodologic aspects of projects and benefited from being included as coauthor on papers.
Twenty-nine of the 43 faculty (67%) still at the institution responded to the program assessment survey. Faculty strongly agreed that they would recommend the coach to colleagues (average ± standard deviation [SD]: 4.7 ± 0.5), that it improved the quality of their work (4.5 ± 0.9), that they were overall satisfied with the coaching (4.6 ± 0.7), and that the Division should continue to support the coach (4.9 ± 0.4). Faculty did not agree that the lack of clinical training of the coach was a barrier (2.0 ± 1.3).
DISCUSSION
The coach program was highly utilized, well regarded, and delivered substantial, tangible, and academic output. We anticipate the coach initiative will continue to be a valuable resource for our Division and could prove to be a valuable model for other institutions seeking to bolster the scholarly work of clinical academicians.
Several lessons emerged through the course of this project. First, we realized it is essential to select a coach who is both knowledgeable and approachable. We found that after meeting the coach, many faculty sought her help who otherwise would not have. An explicit, ongoing marketing strategy with regular contact with faculty at meetings was a key to receiving consult requests.
Second, the lack of a clinical background did not seem to hinder the coach’s ability to coach clinicians. The coach acknowledged her lack of clinical experience and relied on clients to explain the clinical context of projects. We also learned that the coach’s substantial experience with the logistics of research was invaluable. For example, the coach had substantial experience with the IRB process and her pre-reviews of IRB applications made for a short and relatively seamless experience navigating the IRB process. The coach also facilitated collaborations and leveraged existing resources at our institution. For example, for a qualitative research project, the coach helped identify a health services faculty member with this specific expertise, which led to a successful collaboration and publication. Although a more junior coach with less established qualifications may be helpful with research methods and with the research process, our endeavor suggests that having a more highly trained and experienced researcher was extremely valuable. Finally, we learned that for a Division of our size, the 0.50 FTE allotted to the coach is a minimum requirement. The coach spent approximately four hours a week on marketing, attending faculty meetings and conducting brief didactics, two hours per week on administration, and 14 hours per week on consultations. Faculty generally received support soon after their requests, but there were occasional wait times, which may have delayed some projects.
Academic leaders at our institution have noted the success of our coach initiative and have created a demand for coach services. We are exploring funding models that would allow for the expansion of coach services to other departments and divisions. We are in the initial stages of creating an Academic Scholarship Support Core under the supervision of the coach. Within this Core, we envision that various research support services will be triaged to staff with appropriate expertise; for example, a regulatory coordinator would review IRB applications while a master’s level statistician would conduct statistical analyses.
We have also transitioned to a new coach and have continued to experience success with the program. Our initial coach (author C.M.M.) obtained an NIH R01, a foundation grant, and took over a summer program that trains dental faculty in clinical research methods leaving insufficient time for coaching. Our new coach also has a PhD in epidemiology with NIH R01 funding but has more available FTE. Both of our coaches are graduates of our School of Public Health and institutions with such schools may have good access to the expertise needed. Nonclinical PhDs are often almost entirely reliant on grants, and some nongrant support is often attractive to these researchers. Additionally, PhDs who are junior or mid-career faculty that have the needed training are relatively affordable, particularly when the resource is made available to large number of faculty.
A limitation to our assessment of the coach initiative was the lack of pre- and postintervention metrics of scholarly productivity. We cannot definitively say that the Division’s scholarly output has increased because of the coach. Nevertheless, we are confident that the coach’s coaching has enhanced the scholarly work of individual clinicians and provided value to the Division as a whole. The coach program has been a success in our Division. Other institutions facing the challenge of supporting the research efforts of academic clinicians may consider this model as a worthy investment.
Disclosures
The authors have nothing to disclose.
1. Marks AR. Physician-scientist, heal thyself. J Clin Invest. 2007;117(1):2. https://doi.org/10.1172/JCI31031.
2. Bunton SA, Corrice AM. Trends in tenure for clinical M.D. faculty in U.S. medical schools: a 25-year review. Association of American Medical Colleges: Analysis in Brief. 2010;9(9):1-2; https://www.aamc.org/download/139778/data/aibvol9_no9.pdf. Accessed March 7, 2019.
3. Bunton SA, Mallon WT. The continued evolution of faculty appointment and tenure policies at U.S. medical schools. Acad Med. 2007;82(3):281-289. https://doi.org/10.1097/ACM.0b013e3180307e87.
4. Accreditation Council for Graduate Medical Education. ACGME Common Program Requirements. 2017; http://www.acgme.org/What-We-Do/Accreditation/Common-Program-Requirements. Accessed March 7, 2019.
5. Penrose LL, Yeomans ER, Praderio C, Prien SD. An incremental approach to improving scholarly activity. J Grad Med Educ. 2012;4(4):496-499. https://doi.org/10.4300/JGME-D-11-00185.1.
6. Manring MM, Panzo JA, Mayerson JL. A framework for improving resident research participation and scholarly output. J Surg Educ. 2014;71(1):8-13. https://doi.org/10.1016/j.jsurg.2013.07.011.
7. Palacio A, Campbell DT, Moore M, Symes S, Tamariz L. Predictors of scholarly success among internal medicine residents. Am J Med. 2013;126(2):181-185. https:doi.org/10.1016/j.amjmed.2012.10.003.
8. Physician-Scientist Workforce Working Group. Physician-scientist workforce (PSW) report 2014. https://report.nih.gov/Workforce/PSW/challenges.aspx. Accessed December 27, 2018.
9. Straus SE, Johnson MO, Marquez C, Feldman MD. Characteristics of successful and failed mentoring relationships: a qualitative study across two academic health centers. Acad Med. 2013;88(1):82-89. https://doi.org/10.1097/ACM.0b013e31827647a0.
10. Altman DG, Goodman SN, Schroter S. How statistical expertise is used in medical research. JAMA. 2002;287(21):2817-2820. https://doi.org/10.1001/jama.287.21.2817.
11. Howell E, Kravet S, Kisuule F, Wright SM. An innovative approach to supporting hospitalist physicians towards academic success. J Hosp Med. 2008;3(4):314-318. https://doi.org/10.1002/jhm.327.
12. Kripalani S, Williams MV. Author responsibilities and disclosures at the Journal of Hospital Medicine. J Hosp Med. 2010;5(6):320-322. https://doi.org/10.1002/jhm.715.
1. Marks AR. Physician-scientist, heal thyself. J Clin Invest. 2007;117(1):2. https://doi.org/10.1172/JCI31031.
2. Bunton SA, Corrice AM. Trends in tenure for clinical M.D. faculty in U.S. medical schools: a 25-year review. Association of American Medical Colleges: Analysis in Brief. 2010;9(9):1-2; https://www.aamc.org/download/139778/data/aibvol9_no9.pdf. Accessed March 7, 2019.
3. Bunton SA, Mallon WT. The continued evolution of faculty appointment and tenure policies at U.S. medical schools. Acad Med. 2007;82(3):281-289. https://doi.org/10.1097/ACM.0b013e3180307e87.
4. Accreditation Council for Graduate Medical Education. ACGME Common Program Requirements. 2017; http://www.acgme.org/What-We-Do/Accreditation/Common-Program-Requirements. Accessed March 7, 2019.
5. Penrose LL, Yeomans ER, Praderio C, Prien SD. An incremental approach to improving scholarly activity. J Grad Med Educ. 2012;4(4):496-499. https://doi.org/10.4300/JGME-D-11-00185.1.
6. Manring MM, Panzo JA, Mayerson JL. A framework for improving resident research participation and scholarly output. J Surg Educ. 2014;71(1):8-13. https://doi.org/10.1016/j.jsurg.2013.07.011.
7. Palacio A, Campbell DT, Moore M, Symes S, Tamariz L. Predictors of scholarly success among internal medicine residents. Am J Med. 2013;126(2):181-185. https:doi.org/10.1016/j.amjmed.2012.10.003.
8. Physician-Scientist Workforce Working Group. Physician-scientist workforce (PSW) report 2014. https://report.nih.gov/Workforce/PSW/challenges.aspx. Accessed December 27, 2018.
9. Straus SE, Johnson MO, Marquez C, Feldman MD. Characteristics of successful and failed mentoring relationships: a qualitative study across two academic health centers. Acad Med. 2013;88(1):82-89. https://doi.org/10.1097/ACM.0b013e31827647a0.
10. Altman DG, Goodman SN, Schroter S. How statistical expertise is used in medical research. JAMA. 2002;287(21):2817-2820. https://doi.org/10.1001/jama.287.21.2817.
11. Howell E, Kravet S, Kisuule F, Wright SM. An innovative approach to supporting hospitalist physicians towards academic success. J Hosp Med. 2008;3(4):314-318. https://doi.org/10.1002/jhm.327.
12. Kripalani S, Williams MV. Author responsibilities and disclosures at the Journal of Hospital Medicine. J Hosp Med. 2010;5(6):320-322. https://doi.org/10.1002/jhm.715.
© 2019 Society of Hospital Medicine
Off Target But Hitting the Mark
A 32-year-old woman presented to the emergency department (ED) with 3 months of abdominal pain and 1 week of vomiting.
The differential diagnosis of abdominal pain is broad. This presentation could be caused by disorders of the gastrointestinal (GI), gynecologic, urinary, or, less likely, the neuromuscular systems. The presence of vomiting supports a GI cause. Pregnancy should be excluded in any woman of childbearing age presenting with abdominal pain.
Characteristics of the pain, including location, temporal characteristics, severity, and aggravating and alleviating factors, can narrow the differential diagnosis. The past medical history, including prior surgeries, menstrual, and obstetric history, is also critical.
Approximately 3 months prior to presentation, she reported a tick bite that had evolved into a circumferential targetoid rash. Her primary care provider performed serologic testing for Lyme disease, which was negative, and prescribed doxycycline, which she stopped after a week because of nausea and diffuse, achy, and constant abdominal pain. After initial improvement, symptoms recurred a week prior to presentation. The nausea was now associated with intractable vomiting and anorexia. She denied hematemesis or coffee ground emesis. Her abdominal pain intensified and radiated to her back. She lost 10 pounds over the past week. She denied headache, constipation, diarrhea, blood per rectum, melena, dysuria, vaginal discharge, or rash. She reported chills and temperatures up to 37.8 ° C at home.
She had a history of migraine headaches for which she took ibuprofen occasionally but took no other prescription or over-the-counter medications. She had never smoked, consumed 2 alcoholic beverages a month, and denied illicit drug use. She lived with her boyfriend on a farm in Indiana where she raised chickens, rabbits, and ducks.
The patient dates the onset of nausea and abdominal pain to a course of doxycycline, presumably prescribed for early Lyme disease, which was stopped after only 1 week. GI side effects, including nausea, vomiting, and upper abdominal pain, are common with doxycycline and may account for the early symptoms. However, these symptoms typically resolve promptly with drug discontinuation. Doxycycline may rarely cause esophageal and gastric ulcers, which could explain her symptoms.
Fewer than half of patients with erythema migrans caused by Lyme disease are seropositive at presentation, as there has been insufficient time for antibodies to develop. Lyme disease typically affects the skin, joints, heart, and nervous system and only rarely affects the GI tract. Acute Lyme disease can cause intestinal pseudoobstruction, splenomegaly, and mild hepatitis. Although Lyme disease is unlikely to be the cause of the current symptoms, serologic testing should be repeated and should be positive if the patient now has early disseminated disease.
Patients with Lyme disease are occasionally coinfected with a second organism. Ixodes scapularis, the tick that transmits Lyme disease in the Northeast and Midwest, can be coinfected with Babesia microti, a red cell parasite. Babesiosis can persist for months and presents with fever, malaise, and many other nonspecific symptoms, including some that this patient has: anorexia, weight loss, abdominal pain, and vomiting.
The history of migraine and intractable vomiting suggests the possibility of cyclic vomiting syndrome. This syndrome is characterized by episodic bouts of vomiting lasting from hours to as long as a week. The vomiting is often accompanied by abdominal pain and occasionally headaches. Episodes are separated by asymptomatic periods that may last months. Cyclic vomiting syndrome can occur at any age but is more common in children, those with a personal or family history of migraines, and heavy users of cannabis. At least 3 stereotypical episodes are required to make the diagnosis, so a history of prior similar symptoms should be explored.
The differential diagnosis of abdominal pain and vomiting should stay broad until a comprehensive physical exam and initial laboratory tests are performed. Volume status should be assessed by estimating jugular venous pressure and by obtaining supine and standing blood pressure measurements. The abdomen should be examined carefully, and the presence or absence of hepatomegaly, splenomegaly, masses, and ascites should be specifically noted. The presence of bradycardia, oligoarticular arthritis, or neuropathy could provide supporting evidence for Lyme disease. Pregnancy is less likely given the diffuse and persistent nature of the pain but should still be excluded.
On physical examination, she was distressed, writhing on the bed, and appearing comfortable only on her side with her knees flexed. Her temperature was 36.5 ° C, heart rate 83 beats per minute, respiratory rate 18 breaths per minute, blood pressure 143/77 mmHg, and oxygen saturation 94% while breathing ambient air. Her abdomen was diffusely tender, most markedly in the epigastrium. Abdominal rigidity, rebound tenderness, and costovertebral tenderness were absent. There was no rash; the previously reported targetoid skin lesion was no longer present. The remainder of the exam was normal.
Laboratory evaluation showed a white count of 7900/mm3, hemoglobin 14.3 gm/dL with normocytic indices, and a platelet count of 175,000/mm3. Sodium was 130 mmol/L, potassium was 3.1 mmol/L, bicarbonate 26 mmol/L, blood urea nitrogen 15 mg/dL, creatinine 0.6 mg/dL, and glucose 92 mg/dL. Serum calcium, aspartate aminotransferase, alanine aminotransferase, bilirubin, and lipase were normal. A urine pregnancy test was negative. Urine analysis was negative for nitrites and leukocyte esterase. Abdominal and pelvic computed tomography (CT) scan with intravenous (IV) contrast performed 3 days prior at an outside ED revealed a 3.4 centimeter left ovarian cyst. A subsequent transvaginal ultrasound was negative for cyst torsion and confirmed appropriate placement of an intrauterine device.
The absence of abdominal rigidity and rebound tenderness does not exclude peritonitis. A normal white blood cell count also does not reliably exclude serious intraabdominal pathology. However, the CT scan argues strongly against many common causes of abdominal pain, including appendicitis, diverticulitis, perforated ulcer, intestinal obstruction, and malignancy, assuming the symptoms have not changed since it was performed.
The patient’s laboratory studies argue against biliary obstruction, pancreatitis, pregnancy, hypercalcemia, and ongoing urinary tract infection. Patients with functional gallbladder disorders may have normal laboratory and CT findings but typically have recurrent, biliary-colic-type pain. The low serum potassium, a high blood urea nitrogen to creatinine ratio, and a low serum sodium reflect her significant vomiting. The hyponatremia is consistent with the appropriate release of antidiuretic hormone (ADH) in the setting of volume depletion. She should receive isotonic fluids plus potassium in addition to symptomatic treatment of pain and nausea. Given the severity and duration of symptoms, an esophagogastroduodenoscopy (EGD) should be performed to exclude GI mucosal disease, including peptic ulcer disease and gastritis, which may not be evident on the CT scan.
Additional diagnoses should be considered at this point. This patient has exposure to chickens, ducks, rabbits, and ticks as well as reported chills and mild temperature elevation at home. Tularemia, which can be transmitted by tick bites or exposure to infected rabbits, can cause a prolonged illness. Some patients have abdominal pain, anorexia, nausea, and weight loss, although fever is usually more prominent. Tularemia is uncommon and most frequently seen in the south-central part of the United States but has been reported throughout the country. She should be queried regarding additional exposures, including well water to assess her risk for Campylobacter infection.
Opiate withdrawal can present with pain and vomiting, but she reports no opiate use and lacks other findings such pupillary dilation or piloerection. Given the prevalence of opiate abuse, however, a toxicology screen should be performed. Hypercalcemia and diabetic ketoacidosis as metabolic causes of abdominal pain have been ruled out by her laboratory values. If no other cause is identified, other metabolic etiologies like Addison disease, familial Mediterranean fever, or porphyria should be considered.
Cyclic vomiting syndrome should still be on the differential. It is a diagnosis of exclusion requiring a history of recurrent, stereotypical episodes, which should be explicitly explored.
The patient was admitted to a medical unit by the hospitalist service and received IV normal saline, parenteral potassium, and IV pantoprazole. She underwent an EGD that revealed minor erosions in the antrum of the stomach. Biopsies were obtained.
Seven hours after the endoscopy, the patient had a brief period of confusion followed by a generalized tonic-clonic seizure lasting 1 minute. A head CT without contrast was negative for any focal abnormality. Repeat laboratory evaluation revealed that serum sodium was 125 mmol/L, and serum glucose was 113 mg/dL. She was transferred to the progressive care unit and received IV levetiracetam.
The endoscopy excluded structural abnormalities of the stomach and duodenum. The patient now has an additional problem, seizure, which needs to be incorporated in the diagnostic reasoning.
Seizures can be caused by the rapid development of severe hyponatremia, with serum sodium levels usually less than 120 mmol/L. Seizures caused by hyponatremia are typically preceded by headache and lethargy, as the intracellular movement of excess water causes cerebral edema. Hyponatremia is unlikely to be the cause of her seizure but should nevertheless be evaluated with a urine sodium concentration and serum and urine osmolality. If she is euvolemic, the IV fluids should be stopped and her free water intake should be restricted to avoid worsening the hyponatremia, as it is potentially caused by the syndrome of inappropriate ADH (SIADH).
There are many other possible causes for new onset seizures in adults, including brain tumor, head trauma, alcohol withdrawal, medications, and central nervous system infection, including Lyme disease. Lyme serologies should be repeated.
In this patient, it is likely that the seizure is a manifestation of the same illness that is causing her vomiting and abdominal pain. Seizure is not a feature of cyclic vomiting syndrome in adults. It is also not a feature of tularemia, adrenal insufficiency, or opioid withdrawal.
Acute intermittent porphyria (AIP) can cause both abdominal and neurologic problems. Hyponatremia is common during acute attacks, caused by either the inappropriate release of ADH or the appropriate release of the hormone if there is fluid loss. AIP is a rare diagnosis but could explain the uncommon combination of abdominal pain, vomiting, seizure, and hyponatremia. A spot urine porphobilinogen test should be sent to assess for AIP.
Additional laboratory studies were sent. Serum osmolality was 269 mosm/kg with a corresponding urine osmolality of 699 mosm/kg. A random urine sodium was 145 mEq/L. Thyroid stimulating hormone and cosyntropin stimulating testing were normal. IgM and IgG antibodies to Borrelia burgdorferi were negative. Urine porphobilinogen was sent. An electroencephalogram did not reveal epileptiform discharges. Magnetic resonance imaging (MRI) of the brain was significant for T2/FLAIR hyperintensity in the cortex and subcortical white matter of the occipital lobes bilaterally. Hypertonic saline and fluid restriction were initiated.
The patient’s labs are consistent with SIADH. Excessive ADH release because of volume depletion and consequent hyponatremia should have improved rapidly with the administration of saline. The high urine sodium suggests that she is now volume replete, while the high urine osmolality is consistent with the presence of excessive ADH in the absence of appropriate stimuli. In the context of normal thyroid and adrenal function, the hyponatremia is likely due to the SIADH.
Negative serologic testing for Lyme disease, 3 months after the onset of rash, excludes this diagnosis.
The MRI findings are consistent with posterior reversible encephalopathy syndrome (PRES), a clinicoradiographic syndrome of headache, altered mental status, seizure, and/or vision loss with associated white matter abnormalities of the posterior cerebral hemispheres. PRES has been reported with AIP as well as other disorders, most commonly hypertensive encephalopathy, eclampsia, and immunosuppressive drug use.
The patient’s sodium improved with fluid restriction and the administration of hypertonic saline. There was no recurrence of seizure activity. Amlodipine was initiated for blood pressure readings as high as 156/106 mmHg. A hepatobiliary scan revealed a gallbladder ejection fraction of 13%. Biopsies from her endoscopy revealed nonspecific inflammation without the presence of Helicobacter pylori. The patient was discharged home 7 days after admission after stabilization of serum sodium, improvement in her abdominal pain, and tolerance of oral intake. A plan was made for outpatient cholecystectomy.
Many causes of abdominal pain have been excluded and the remaining diagnostic possibility, porphyria, is rare. The clinicians have revisited their differential and considered other causes of abdominal pain, including functional gallbladder disorders. However, chronic cholecystitis (or functional gallbladder disorder) is not this patient’s primary problem. The diffuse, severe, and constant abdominal pain prior to admission is not typical of biliary pain, and many medical conditions and drugs, including amlodipine, can lead to a positive hepatobiliary scan. Chronic cholecystitis would not explain her seizure.
AIP remains at the top of the differential for this young woman. A urine porphobilinogen has been sent and must be followed up prior to any further workup or surgery.
One week after discharge, the patient’s urine porphobilinogen resulted at 172.8 mCmol/ (upper limits of normal 8.8). Sequencing analysis for genes coding the enzymes involved in the synthetic pathway for heme were sent. Hydroxymethylbilane synthase, coproporphyrinogen oxidase, and protoporphyrinogen oxidase mutation assays were all normal. Despite the normal genetic assays, the diagnosis of AIP was made on the basis of the clinical presentation and elevated urine porphobilinogen. The patient was referred to a hematologist and initiated on oral glucose supplements and hematin infusions.
DISCUSSION
Although abdominal pain has a broad differential, the combination of abdominal pain and neurologic or psychiatric symptoms should suggest the possibility of porphyria, especially if symptoms are recurrent or unexplained. The porphyrias are a group of disorders caused by defects in the synthetic pathway of heme, leading to an overproduction and accumulation of precursors. Heme is a component of multiple proteins, including hemoglobin, myoglobin, and the cytochrome P450 enzymes. Although it is synthesized in all tissues, the bone marrow and liver are the organs most actively involved. The porphyrias can be classified according to the primary site of the overproduction and accumulation of heme precursors (liver vs bone marrow). Although there is overlap between the 2 groups, hepatic porphyrias often present with acute neurovisceral symptoms, while the erythropoietic porphyrias often cause cutaneous photosensitivity.1
AIP is the most common hepatic porphyria with a prevalence of 1 in 20,000 in Caucasians of Western European descent.1 AIP is caused by a defect in the gene that encodes porphobilinogen deaminase, leading to the accumulation of porphobilinogen.1 The cardinal manifestation is an acute porphyric attack. While the precise mechanisms underlying the symptoms are unknown, the accumulating metabolites may be directly neurotoxic.2 Attacks are precipitated by factors that induce heme synthesis, including caloric restriction, alcohol, and certain medications, particularly those that upregulate cyP450. The most commonly implicated drugs are anesthetics, antiepileptics, sulfonamides, rifampin, and estrogen and progesterone. Attacks can also be precipitated by changes in endogenous sex hormone levels, like the increase in progesterone seen in the luteal phase of the menstrual cycle, which may account for the higher incidence of symptomatic attacks in women.3
Acute attacks of AIP may have a wide variety of presentations; the disease was referred to as the “little imitator” in the early 20th century.4 The most common symptom is acute, severe abdominal pain, which may mimic an acute abdomen. Because the pain is neuropathic rather than inflammatory, abdominal tenderness, rebound, fever, and leukocytosis are usually absent, as they were in this patient. Abdominal pain is often accompanied by neuropsychiatric symptoms, including sensory and motor neuropathy, anxiety, hallucinations, delirium, and altered level of consciousness. Seizure occurs in 20% of cases. Involvement of the autonomic nervous system causes tachycardia and new onset hypertension in the majority of patients as well as restlessness and tremor. Hyponatremia, mediated by the syndrome of inappropriate ADH secretion, occurs in nearly a third of patients.5,6 MRI findings consistent with PRES have also been described in AIP.7
The diagnosis of AIP is often delayed; diagnosis later in the disease course is associated with a poorer prognosis.8 Reported intervals between presentation and diagnosis range from several months to as long as 20 years.9 Associating the use of medications, caloric restriction, or the menstrual cycle with the exacerbation of symptoms or darkening of urine can help prompt an earlier diagnosis.6
AIP can be diagnosed by detecting a greater than 5-fold elevation of urinary porphobilinogen excretion in conjunction with the typical symptoms of an acute attack.5 Renal dysfunction causes urinary excretion of PBG to fall and serum levels to rise.10 Serum PBG levels should therefore be sent when AIP is suspected in the setting of renal dysfunction. The primary role of genetic testing in a patient who has AIP confirmed clinically and biochemically is to assist in genetic counseling and to identify asymptomatic family members.11 Genetic testing is not required to confirm the diagnosis and does not help prognosticate. It is unusual that a mutation was not detected in this case, as the current sensitivity of genetic testing is 97% to 100%.11
There are 4 principles of management of an acute porphyric attack. First, any precipitating factors such as medications should be stopped. Second, abdominal pain should be treated appropriately with opioids, if necessary. Third, if autonomic dysfunction is present, beta-blockers or clonidine should be given to treat hypertension.5 Finally, glucose and/or hemin should be administered to downregulate aminolevulinic acid (ALA) synthase by negative feedback. Downregulation of ALA synthase decreases the accumulation of the neurotoxic porphyrin precursors ALA and PBG.5 For patients with mild symptoms, glucose alone (300-500 g/d) may be enough to abort the attack.12 This can be achieved via a high-carbohydrate diet in those able to tolerate oral intake or via continuous infusions of dextrose containing fluids.5 For more severe attacks with associated polyneuropathy, respiratory muscle weakness, or seizures, or for attacks that are not resolving, heme preparations dosed at 3 to 4 mg/kg/d for 3 to 4 days are indicated.5
The recent diagnosis of acute Lyme disease was a distractor in this presentation. In Lyme endemic areas, patients with erythema migrans are treated based on the clinical presentation rather than serologic testing.13 Although this patient took only 1 week of doxycycline, testing during this hospitalization showed that she had either been cured early or had not had Lyme disease in the first place. There is no known association between Lyme disease and the porphyrias, and doxycycline is not a common precipitant of AIP attacks.14 However, the GI side effects of doxycycline may have decreased caloric intake and ultimately provoked the patient’s first attack of AIP. The clinicians in this case appropriately avoided the “target” but hit the mark by correctly diagnosing AIP.
KEY POINTS
- Consider AIP in patients with unexplained abdominal pain, especially when accompanied by neuropsychiatric symptoms and autonomic lability.
- Diagnose AIP by sending a urine PBG during a suspected acute attack.
- Treat AIP acutely by removing precipitants, treating abdominal pain, and initiating dextrose-containing fluids and hemin infusions to downregulate ALA synthase.
Acknowledgments
The authors thank the patient who enthusiastically supported the writing of this report.
Disclosure
Warren Gavin, MD has disclosed participation in expert testimony. The authors have no financial or other conflicts of interest to disclose.
1. Desnick RJ, Balwani M. The Porphyrias. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J, eds. Harrison’s Principles of Internal Medicine, 19th Edition. New York: McGraw-Hill; 2015. http://accessmedicine.mhmedical.com.proxy.medlib.uits.iu.edu/content.aspx?bookid=1130&Sectionid=79754263. Accessed June 14, 2016.
2. Bissell DM, Lai JC, Meister RK, Blanc PD. Role of Delta-aminolevulinic Acid in the Symptoms of Acute Porphyria. Am J Med. 2015;128(3):313-317. PubMed
3. Bonkovsky HL, Guo JT, Hou W, Li T, Narang T, Thapar M. Porphyrin and Heme Metabolism and the Porphyrias. Compr Physiol. 2013;3(1):365-401. PubMed
4. Crimlisk HL. The little imitator--porphyria: a neuropsychiatric disorder. J Neurol Neurosurg Psychiatry. 1997;62(4):319-328. PubMed
5. Pischik E, Kauppinen R. An update of clinical management of acute intermittent porphyria. Appl Clin Genet. 2015;8:201-214. PubMed
6. Ventura P, Cappellini MD, Biolcati G, Guida CC, Rocchi E; Gruppo Italiano Porfiria (GrIP). A challenging diagnosis for potential fatal diseases: recommendations for diagnosing acute porphyrias. Eur J Intern Med. 2014;25(6):497-505. PubMed
7. Dagens A, Gilhooley MJ. Acute intermittent porphyria leading to posterior reversible encephalopathy syndrome (PRES): a rare cause of abdominal pain and seizures. BMJ Case Rep. 2016:bcr2016215350. PubMed
8. Pischik E, Bulyanitsa A, Kazakov V, Kauppinen R. Clinical features predictive of a poor prognosis in acute porphyria. J Neurol. 2004;251(12):1538-1541. PubMed
9. Sack GH. Acute intermittent porphyria. JAMA. 1990;264(10):1290-1293. PubMed
10. Sardh E, Andersson DEH, Henrichson A, Harper P. Porphyrin precursors and porphyrins in three patients with acute intermittent porphyria and end-stage renal disease under different therapy regimes. Cell Mol Biol (Noisy-le-grand). 2009;55(1):66-71. PubMed
11. Whatley SD, Badminton MN. Role of genetic testing in the management of patients with inherited porphyria and their families. Ann Clin Biochem. 2013;50(3):204-216. PubMed
12. Anderson KE, Bloomer JR, Bonkovsky HL, et al. Recommendations for the diagnosis and treatment of the acute porphyrias. Ann Intern Med. 2005;142(6):439-450. PubMed
13. Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43(9):1089-1134. PubMed
14. American Porphyria Foundation. Drug database. http://www.porphyriafoundation.com/drug-database. Accessed July 21, 2017.
A 32-year-old woman presented to the emergency department (ED) with 3 months of abdominal pain and 1 week of vomiting.
The differential diagnosis of abdominal pain is broad. This presentation could be caused by disorders of the gastrointestinal (GI), gynecologic, urinary, or, less likely, the neuromuscular systems. The presence of vomiting supports a GI cause. Pregnancy should be excluded in any woman of childbearing age presenting with abdominal pain.
Characteristics of the pain, including location, temporal characteristics, severity, and aggravating and alleviating factors, can narrow the differential diagnosis. The past medical history, including prior surgeries, menstrual, and obstetric history, is also critical.
Approximately 3 months prior to presentation, she reported a tick bite that had evolved into a circumferential targetoid rash. Her primary care provider performed serologic testing for Lyme disease, which was negative, and prescribed doxycycline, which she stopped after a week because of nausea and diffuse, achy, and constant abdominal pain. After initial improvement, symptoms recurred a week prior to presentation. The nausea was now associated with intractable vomiting and anorexia. She denied hematemesis or coffee ground emesis. Her abdominal pain intensified and radiated to her back. She lost 10 pounds over the past week. She denied headache, constipation, diarrhea, blood per rectum, melena, dysuria, vaginal discharge, or rash. She reported chills and temperatures up to 37.8 ° C at home.
She had a history of migraine headaches for which she took ibuprofen occasionally but took no other prescription or over-the-counter medications. She had never smoked, consumed 2 alcoholic beverages a month, and denied illicit drug use. She lived with her boyfriend on a farm in Indiana where she raised chickens, rabbits, and ducks.
The patient dates the onset of nausea and abdominal pain to a course of doxycycline, presumably prescribed for early Lyme disease, which was stopped after only 1 week. GI side effects, including nausea, vomiting, and upper abdominal pain, are common with doxycycline and may account for the early symptoms. However, these symptoms typically resolve promptly with drug discontinuation. Doxycycline may rarely cause esophageal and gastric ulcers, which could explain her symptoms.
Fewer than half of patients with erythema migrans caused by Lyme disease are seropositive at presentation, as there has been insufficient time for antibodies to develop. Lyme disease typically affects the skin, joints, heart, and nervous system and only rarely affects the GI tract. Acute Lyme disease can cause intestinal pseudoobstruction, splenomegaly, and mild hepatitis. Although Lyme disease is unlikely to be the cause of the current symptoms, serologic testing should be repeated and should be positive if the patient now has early disseminated disease.
Patients with Lyme disease are occasionally coinfected with a second organism. Ixodes scapularis, the tick that transmits Lyme disease in the Northeast and Midwest, can be coinfected with Babesia microti, a red cell parasite. Babesiosis can persist for months and presents with fever, malaise, and many other nonspecific symptoms, including some that this patient has: anorexia, weight loss, abdominal pain, and vomiting.
The history of migraine and intractable vomiting suggests the possibility of cyclic vomiting syndrome. This syndrome is characterized by episodic bouts of vomiting lasting from hours to as long as a week. The vomiting is often accompanied by abdominal pain and occasionally headaches. Episodes are separated by asymptomatic periods that may last months. Cyclic vomiting syndrome can occur at any age but is more common in children, those with a personal or family history of migraines, and heavy users of cannabis. At least 3 stereotypical episodes are required to make the diagnosis, so a history of prior similar symptoms should be explored.
The differential diagnosis of abdominal pain and vomiting should stay broad until a comprehensive physical exam and initial laboratory tests are performed. Volume status should be assessed by estimating jugular venous pressure and by obtaining supine and standing blood pressure measurements. The abdomen should be examined carefully, and the presence or absence of hepatomegaly, splenomegaly, masses, and ascites should be specifically noted. The presence of bradycardia, oligoarticular arthritis, or neuropathy could provide supporting evidence for Lyme disease. Pregnancy is less likely given the diffuse and persistent nature of the pain but should still be excluded.
On physical examination, she was distressed, writhing on the bed, and appearing comfortable only on her side with her knees flexed. Her temperature was 36.5 ° C, heart rate 83 beats per minute, respiratory rate 18 breaths per minute, blood pressure 143/77 mmHg, and oxygen saturation 94% while breathing ambient air. Her abdomen was diffusely tender, most markedly in the epigastrium. Abdominal rigidity, rebound tenderness, and costovertebral tenderness were absent. There was no rash; the previously reported targetoid skin lesion was no longer present. The remainder of the exam was normal.
Laboratory evaluation showed a white count of 7900/mm3, hemoglobin 14.3 gm/dL with normocytic indices, and a platelet count of 175,000/mm3. Sodium was 130 mmol/L, potassium was 3.1 mmol/L, bicarbonate 26 mmol/L, blood urea nitrogen 15 mg/dL, creatinine 0.6 mg/dL, and glucose 92 mg/dL. Serum calcium, aspartate aminotransferase, alanine aminotransferase, bilirubin, and lipase were normal. A urine pregnancy test was negative. Urine analysis was negative for nitrites and leukocyte esterase. Abdominal and pelvic computed tomography (CT) scan with intravenous (IV) contrast performed 3 days prior at an outside ED revealed a 3.4 centimeter left ovarian cyst. A subsequent transvaginal ultrasound was negative for cyst torsion and confirmed appropriate placement of an intrauterine device.
The absence of abdominal rigidity and rebound tenderness does not exclude peritonitis. A normal white blood cell count also does not reliably exclude serious intraabdominal pathology. However, the CT scan argues strongly against many common causes of abdominal pain, including appendicitis, diverticulitis, perforated ulcer, intestinal obstruction, and malignancy, assuming the symptoms have not changed since it was performed.
The patient’s laboratory studies argue against biliary obstruction, pancreatitis, pregnancy, hypercalcemia, and ongoing urinary tract infection. Patients with functional gallbladder disorders may have normal laboratory and CT findings but typically have recurrent, biliary-colic-type pain. The low serum potassium, a high blood urea nitrogen to creatinine ratio, and a low serum sodium reflect her significant vomiting. The hyponatremia is consistent with the appropriate release of antidiuretic hormone (ADH) in the setting of volume depletion. She should receive isotonic fluids plus potassium in addition to symptomatic treatment of pain and nausea. Given the severity and duration of symptoms, an esophagogastroduodenoscopy (EGD) should be performed to exclude GI mucosal disease, including peptic ulcer disease and gastritis, which may not be evident on the CT scan.
Additional diagnoses should be considered at this point. This patient has exposure to chickens, ducks, rabbits, and ticks as well as reported chills and mild temperature elevation at home. Tularemia, which can be transmitted by tick bites or exposure to infected rabbits, can cause a prolonged illness. Some patients have abdominal pain, anorexia, nausea, and weight loss, although fever is usually more prominent. Tularemia is uncommon and most frequently seen in the south-central part of the United States but has been reported throughout the country. She should be queried regarding additional exposures, including well water to assess her risk for Campylobacter infection.
Opiate withdrawal can present with pain and vomiting, but she reports no opiate use and lacks other findings such pupillary dilation or piloerection. Given the prevalence of opiate abuse, however, a toxicology screen should be performed. Hypercalcemia and diabetic ketoacidosis as metabolic causes of abdominal pain have been ruled out by her laboratory values. If no other cause is identified, other metabolic etiologies like Addison disease, familial Mediterranean fever, or porphyria should be considered.
Cyclic vomiting syndrome should still be on the differential. It is a diagnosis of exclusion requiring a history of recurrent, stereotypical episodes, which should be explicitly explored.
The patient was admitted to a medical unit by the hospitalist service and received IV normal saline, parenteral potassium, and IV pantoprazole. She underwent an EGD that revealed minor erosions in the antrum of the stomach. Biopsies were obtained.
Seven hours after the endoscopy, the patient had a brief period of confusion followed by a generalized tonic-clonic seizure lasting 1 minute. A head CT without contrast was negative for any focal abnormality. Repeat laboratory evaluation revealed that serum sodium was 125 mmol/L, and serum glucose was 113 mg/dL. She was transferred to the progressive care unit and received IV levetiracetam.
The endoscopy excluded structural abnormalities of the stomach and duodenum. The patient now has an additional problem, seizure, which needs to be incorporated in the diagnostic reasoning.
Seizures can be caused by the rapid development of severe hyponatremia, with serum sodium levels usually less than 120 mmol/L. Seizures caused by hyponatremia are typically preceded by headache and lethargy, as the intracellular movement of excess water causes cerebral edema. Hyponatremia is unlikely to be the cause of her seizure but should nevertheless be evaluated with a urine sodium concentration and serum and urine osmolality. If she is euvolemic, the IV fluids should be stopped and her free water intake should be restricted to avoid worsening the hyponatremia, as it is potentially caused by the syndrome of inappropriate ADH (SIADH).
There are many other possible causes for new onset seizures in adults, including brain tumor, head trauma, alcohol withdrawal, medications, and central nervous system infection, including Lyme disease. Lyme serologies should be repeated.
In this patient, it is likely that the seizure is a manifestation of the same illness that is causing her vomiting and abdominal pain. Seizure is not a feature of cyclic vomiting syndrome in adults. It is also not a feature of tularemia, adrenal insufficiency, or opioid withdrawal.
Acute intermittent porphyria (AIP) can cause both abdominal and neurologic problems. Hyponatremia is common during acute attacks, caused by either the inappropriate release of ADH or the appropriate release of the hormone if there is fluid loss. AIP is a rare diagnosis but could explain the uncommon combination of abdominal pain, vomiting, seizure, and hyponatremia. A spot urine porphobilinogen test should be sent to assess for AIP.
Additional laboratory studies were sent. Serum osmolality was 269 mosm/kg with a corresponding urine osmolality of 699 mosm/kg. A random urine sodium was 145 mEq/L. Thyroid stimulating hormone and cosyntropin stimulating testing were normal. IgM and IgG antibodies to Borrelia burgdorferi were negative. Urine porphobilinogen was sent. An electroencephalogram did not reveal epileptiform discharges. Magnetic resonance imaging (MRI) of the brain was significant for T2/FLAIR hyperintensity in the cortex and subcortical white matter of the occipital lobes bilaterally. Hypertonic saline and fluid restriction were initiated.
The patient’s labs are consistent with SIADH. Excessive ADH release because of volume depletion and consequent hyponatremia should have improved rapidly with the administration of saline. The high urine sodium suggests that she is now volume replete, while the high urine osmolality is consistent with the presence of excessive ADH in the absence of appropriate stimuli. In the context of normal thyroid and adrenal function, the hyponatremia is likely due to the SIADH.
Negative serologic testing for Lyme disease, 3 months after the onset of rash, excludes this diagnosis.
The MRI findings are consistent with posterior reversible encephalopathy syndrome (PRES), a clinicoradiographic syndrome of headache, altered mental status, seizure, and/or vision loss with associated white matter abnormalities of the posterior cerebral hemispheres. PRES has been reported with AIP as well as other disorders, most commonly hypertensive encephalopathy, eclampsia, and immunosuppressive drug use.
The patient’s sodium improved with fluid restriction and the administration of hypertonic saline. There was no recurrence of seizure activity. Amlodipine was initiated for blood pressure readings as high as 156/106 mmHg. A hepatobiliary scan revealed a gallbladder ejection fraction of 13%. Biopsies from her endoscopy revealed nonspecific inflammation without the presence of Helicobacter pylori. The patient was discharged home 7 days after admission after stabilization of serum sodium, improvement in her abdominal pain, and tolerance of oral intake. A plan was made for outpatient cholecystectomy.
Many causes of abdominal pain have been excluded and the remaining diagnostic possibility, porphyria, is rare. The clinicians have revisited their differential and considered other causes of abdominal pain, including functional gallbladder disorders. However, chronic cholecystitis (or functional gallbladder disorder) is not this patient’s primary problem. The diffuse, severe, and constant abdominal pain prior to admission is not typical of biliary pain, and many medical conditions and drugs, including amlodipine, can lead to a positive hepatobiliary scan. Chronic cholecystitis would not explain her seizure.
AIP remains at the top of the differential for this young woman. A urine porphobilinogen has been sent and must be followed up prior to any further workup or surgery.
One week after discharge, the patient’s urine porphobilinogen resulted at 172.8 mCmol/ (upper limits of normal 8.8). Sequencing analysis for genes coding the enzymes involved in the synthetic pathway for heme were sent. Hydroxymethylbilane synthase, coproporphyrinogen oxidase, and protoporphyrinogen oxidase mutation assays were all normal. Despite the normal genetic assays, the diagnosis of AIP was made on the basis of the clinical presentation and elevated urine porphobilinogen. The patient was referred to a hematologist and initiated on oral glucose supplements and hematin infusions.
DISCUSSION
Although abdominal pain has a broad differential, the combination of abdominal pain and neurologic or psychiatric symptoms should suggest the possibility of porphyria, especially if symptoms are recurrent or unexplained. The porphyrias are a group of disorders caused by defects in the synthetic pathway of heme, leading to an overproduction and accumulation of precursors. Heme is a component of multiple proteins, including hemoglobin, myoglobin, and the cytochrome P450 enzymes. Although it is synthesized in all tissues, the bone marrow and liver are the organs most actively involved. The porphyrias can be classified according to the primary site of the overproduction and accumulation of heme precursors (liver vs bone marrow). Although there is overlap between the 2 groups, hepatic porphyrias often present with acute neurovisceral symptoms, while the erythropoietic porphyrias often cause cutaneous photosensitivity.1
AIP is the most common hepatic porphyria with a prevalence of 1 in 20,000 in Caucasians of Western European descent.1 AIP is caused by a defect in the gene that encodes porphobilinogen deaminase, leading to the accumulation of porphobilinogen.1 The cardinal manifestation is an acute porphyric attack. While the precise mechanisms underlying the symptoms are unknown, the accumulating metabolites may be directly neurotoxic.2 Attacks are precipitated by factors that induce heme synthesis, including caloric restriction, alcohol, and certain medications, particularly those that upregulate cyP450. The most commonly implicated drugs are anesthetics, antiepileptics, sulfonamides, rifampin, and estrogen and progesterone. Attacks can also be precipitated by changes in endogenous sex hormone levels, like the increase in progesterone seen in the luteal phase of the menstrual cycle, which may account for the higher incidence of symptomatic attacks in women.3
Acute attacks of AIP may have a wide variety of presentations; the disease was referred to as the “little imitator” in the early 20th century.4 The most common symptom is acute, severe abdominal pain, which may mimic an acute abdomen. Because the pain is neuropathic rather than inflammatory, abdominal tenderness, rebound, fever, and leukocytosis are usually absent, as they were in this patient. Abdominal pain is often accompanied by neuropsychiatric symptoms, including sensory and motor neuropathy, anxiety, hallucinations, delirium, and altered level of consciousness. Seizure occurs in 20% of cases. Involvement of the autonomic nervous system causes tachycardia and new onset hypertension in the majority of patients as well as restlessness and tremor. Hyponatremia, mediated by the syndrome of inappropriate ADH secretion, occurs in nearly a third of patients.5,6 MRI findings consistent with PRES have also been described in AIP.7
The diagnosis of AIP is often delayed; diagnosis later in the disease course is associated with a poorer prognosis.8 Reported intervals between presentation and diagnosis range from several months to as long as 20 years.9 Associating the use of medications, caloric restriction, or the menstrual cycle with the exacerbation of symptoms or darkening of urine can help prompt an earlier diagnosis.6
AIP can be diagnosed by detecting a greater than 5-fold elevation of urinary porphobilinogen excretion in conjunction with the typical symptoms of an acute attack.5 Renal dysfunction causes urinary excretion of PBG to fall and serum levels to rise.10 Serum PBG levels should therefore be sent when AIP is suspected in the setting of renal dysfunction. The primary role of genetic testing in a patient who has AIP confirmed clinically and biochemically is to assist in genetic counseling and to identify asymptomatic family members.11 Genetic testing is not required to confirm the diagnosis and does not help prognosticate. It is unusual that a mutation was not detected in this case, as the current sensitivity of genetic testing is 97% to 100%.11
There are 4 principles of management of an acute porphyric attack. First, any precipitating factors such as medications should be stopped. Second, abdominal pain should be treated appropriately with opioids, if necessary. Third, if autonomic dysfunction is present, beta-blockers or clonidine should be given to treat hypertension.5 Finally, glucose and/or hemin should be administered to downregulate aminolevulinic acid (ALA) synthase by negative feedback. Downregulation of ALA synthase decreases the accumulation of the neurotoxic porphyrin precursors ALA and PBG.5 For patients with mild symptoms, glucose alone (300-500 g/d) may be enough to abort the attack.12 This can be achieved via a high-carbohydrate diet in those able to tolerate oral intake or via continuous infusions of dextrose containing fluids.5 For more severe attacks with associated polyneuropathy, respiratory muscle weakness, or seizures, or for attacks that are not resolving, heme preparations dosed at 3 to 4 mg/kg/d for 3 to 4 days are indicated.5
The recent diagnosis of acute Lyme disease was a distractor in this presentation. In Lyme endemic areas, patients with erythema migrans are treated based on the clinical presentation rather than serologic testing.13 Although this patient took only 1 week of doxycycline, testing during this hospitalization showed that she had either been cured early or had not had Lyme disease in the first place. There is no known association between Lyme disease and the porphyrias, and doxycycline is not a common precipitant of AIP attacks.14 However, the GI side effects of doxycycline may have decreased caloric intake and ultimately provoked the patient’s first attack of AIP. The clinicians in this case appropriately avoided the “target” but hit the mark by correctly diagnosing AIP.
KEY POINTS
- Consider AIP in patients with unexplained abdominal pain, especially when accompanied by neuropsychiatric symptoms and autonomic lability.
- Diagnose AIP by sending a urine PBG during a suspected acute attack.
- Treat AIP acutely by removing precipitants, treating abdominal pain, and initiating dextrose-containing fluids and hemin infusions to downregulate ALA synthase.
Acknowledgments
The authors thank the patient who enthusiastically supported the writing of this report.
Disclosure
Warren Gavin, MD has disclosed participation in expert testimony. The authors have no financial or other conflicts of interest to disclose.
A 32-year-old woman presented to the emergency department (ED) with 3 months of abdominal pain and 1 week of vomiting.
The differential diagnosis of abdominal pain is broad. This presentation could be caused by disorders of the gastrointestinal (GI), gynecologic, urinary, or, less likely, the neuromuscular systems. The presence of vomiting supports a GI cause. Pregnancy should be excluded in any woman of childbearing age presenting with abdominal pain.
Characteristics of the pain, including location, temporal characteristics, severity, and aggravating and alleviating factors, can narrow the differential diagnosis. The past medical history, including prior surgeries, menstrual, and obstetric history, is also critical.
Approximately 3 months prior to presentation, she reported a tick bite that had evolved into a circumferential targetoid rash. Her primary care provider performed serologic testing for Lyme disease, which was negative, and prescribed doxycycline, which she stopped after a week because of nausea and diffuse, achy, and constant abdominal pain. After initial improvement, symptoms recurred a week prior to presentation. The nausea was now associated with intractable vomiting and anorexia. She denied hematemesis or coffee ground emesis. Her abdominal pain intensified and radiated to her back. She lost 10 pounds over the past week. She denied headache, constipation, diarrhea, blood per rectum, melena, dysuria, vaginal discharge, or rash. She reported chills and temperatures up to 37.8 ° C at home.
She had a history of migraine headaches for which she took ibuprofen occasionally but took no other prescription or over-the-counter medications. She had never smoked, consumed 2 alcoholic beverages a month, and denied illicit drug use. She lived with her boyfriend on a farm in Indiana where she raised chickens, rabbits, and ducks.
The patient dates the onset of nausea and abdominal pain to a course of doxycycline, presumably prescribed for early Lyme disease, which was stopped after only 1 week. GI side effects, including nausea, vomiting, and upper abdominal pain, are common with doxycycline and may account for the early symptoms. However, these symptoms typically resolve promptly with drug discontinuation. Doxycycline may rarely cause esophageal and gastric ulcers, which could explain her symptoms.
Fewer than half of patients with erythema migrans caused by Lyme disease are seropositive at presentation, as there has been insufficient time for antibodies to develop. Lyme disease typically affects the skin, joints, heart, and nervous system and only rarely affects the GI tract. Acute Lyme disease can cause intestinal pseudoobstruction, splenomegaly, and mild hepatitis. Although Lyme disease is unlikely to be the cause of the current symptoms, serologic testing should be repeated and should be positive if the patient now has early disseminated disease.
Patients with Lyme disease are occasionally coinfected with a second organism. Ixodes scapularis, the tick that transmits Lyme disease in the Northeast and Midwest, can be coinfected with Babesia microti, a red cell parasite. Babesiosis can persist for months and presents with fever, malaise, and many other nonspecific symptoms, including some that this patient has: anorexia, weight loss, abdominal pain, and vomiting.
The history of migraine and intractable vomiting suggests the possibility of cyclic vomiting syndrome. This syndrome is characterized by episodic bouts of vomiting lasting from hours to as long as a week. The vomiting is often accompanied by abdominal pain and occasionally headaches. Episodes are separated by asymptomatic periods that may last months. Cyclic vomiting syndrome can occur at any age but is more common in children, those with a personal or family history of migraines, and heavy users of cannabis. At least 3 stereotypical episodes are required to make the diagnosis, so a history of prior similar symptoms should be explored.
The differential diagnosis of abdominal pain and vomiting should stay broad until a comprehensive physical exam and initial laboratory tests are performed. Volume status should be assessed by estimating jugular venous pressure and by obtaining supine and standing blood pressure measurements. The abdomen should be examined carefully, and the presence or absence of hepatomegaly, splenomegaly, masses, and ascites should be specifically noted. The presence of bradycardia, oligoarticular arthritis, or neuropathy could provide supporting evidence for Lyme disease. Pregnancy is less likely given the diffuse and persistent nature of the pain but should still be excluded.
On physical examination, she was distressed, writhing on the bed, and appearing comfortable only on her side with her knees flexed. Her temperature was 36.5 ° C, heart rate 83 beats per minute, respiratory rate 18 breaths per minute, blood pressure 143/77 mmHg, and oxygen saturation 94% while breathing ambient air. Her abdomen was diffusely tender, most markedly in the epigastrium. Abdominal rigidity, rebound tenderness, and costovertebral tenderness were absent. There was no rash; the previously reported targetoid skin lesion was no longer present. The remainder of the exam was normal.
Laboratory evaluation showed a white count of 7900/mm3, hemoglobin 14.3 gm/dL with normocytic indices, and a platelet count of 175,000/mm3. Sodium was 130 mmol/L, potassium was 3.1 mmol/L, bicarbonate 26 mmol/L, blood urea nitrogen 15 mg/dL, creatinine 0.6 mg/dL, and glucose 92 mg/dL. Serum calcium, aspartate aminotransferase, alanine aminotransferase, bilirubin, and lipase were normal. A urine pregnancy test was negative. Urine analysis was negative for nitrites and leukocyte esterase. Abdominal and pelvic computed tomography (CT) scan with intravenous (IV) contrast performed 3 days prior at an outside ED revealed a 3.4 centimeter left ovarian cyst. A subsequent transvaginal ultrasound was negative for cyst torsion and confirmed appropriate placement of an intrauterine device.
The absence of abdominal rigidity and rebound tenderness does not exclude peritonitis. A normal white blood cell count also does not reliably exclude serious intraabdominal pathology. However, the CT scan argues strongly against many common causes of abdominal pain, including appendicitis, diverticulitis, perforated ulcer, intestinal obstruction, and malignancy, assuming the symptoms have not changed since it was performed.
The patient’s laboratory studies argue against biliary obstruction, pancreatitis, pregnancy, hypercalcemia, and ongoing urinary tract infection. Patients with functional gallbladder disorders may have normal laboratory and CT findings but typically have recurrent, biliary-colic-type pain. The low serum potassium, a high blood urea nitrogen to creatinine ratio, and a low serum sodium reflect her significant vomiting. The hyponatremia is consistent with the appropriate release of antidiuretic hormone (ADH) in the setting of volume depletion. She should receive isotonic fluids plus potassium in addition to symptomatic treatment of pain and nausea. Given the severity and duration of symptoms, an esophagogastroduodenoscopy (EGD) should be performed to exclude GI mucosal disease, including peptic ulcer disease and gastritis, which may not be evident on the CT scan.
Additional diagnoses should be considered at this point. This patient has exposure to chickens, ducks, rabbits, and ticks as well as reported chills and mild temperature elevation at home. Tularemia, which can be transmitted by tick bites or exposure to infected rabbits, can cause a prolonged illness. Some patients have abdominal pain, anorexia, nausea, and weight loss, although fever is usually more prominent. Tularemia is uncommon and most frequently seen in the south-central part of the United States but has been reported throughout the country. She should be queried regarding additional exposures, including well water to assess her risk for Campylobacter infection.
Opiate withdrawal can present with pain and vomiting, but she reports no opiate use and lacks other findings such pupillary dilation or piloerection. Given the prevalence of opiate abuse, however, a toxicology screen should be performed. Hypercalcemia and diabetic ketoacidosis as metabolic causes of abdominal pain have been ruled out by her laboratory values. If no other cause is identified, other metabolic etiologies like Addison disease, familial Mediterranean fever, or porphyria should be considered.
Cyclic vomiting syndrome should still be on the differential. It is a diagnosis of exclusion requiring a history of recurrent, stereotypical episodes, which should be explicitly explored.
The patient was admitted to a medical unit by the hospitalist service and received IV normal saline, parenteral potassium, and IV pantoprazole. She underwent an EGD that revealed minor erosions in the antrum of the stomach. Biopsies were obtained.
Seven hours after the endoscopy, the patient had a brief period of confusion followed by a generalized tonic-clonic seizure lasting 1 minute. A head CT without contrast was negative for any focal abnormality. Repeat laboratory evaluation revealed that serum sodium was 125 mmol/L, and serum glucose was 113 mg/dL. She was transferred to the progressive care unit and received IV levetiracetam.
The endoscopy excluded structural abnormalities of the stomach and duodenum. The patient now has an additional problem, seizure, which needs to be incorporated in the diagnostic reasoning.
Seizures can be caused by the rapid development of severe hyponatremia, with serum sodium levels usually less than 120 mmol/L. Seizures caused by hyponatremia are typically preceded by headache and lethargy, as the intracellular movement of excess water causes cerebral edema. Hyponatremia is unlikely to be the cause of her seizure but should nevertheless be evaluated with a urine sodium concentration and serum and urine osmolality. If she is euvolemic, the IV fluids should be stopped and her free water intake should be restricted to avoid worsening the hyponatremia, as it is potentially caused by the syndrome of inappropriate ADH (SIADH).
There are many other possible causes for new onset seizures in adults, including brain tumor, head trauma, alcohol withdrawal, medications, and central nervous system infection, including Lyme disease. Lyme serologies should be repeated.
In this patient, it is likely that the seizure is a manifestation of the same illness that is causing her vomiting and abdominal pain. Seizure is not a feature of cyclic vomiting syndrome in adults. It is also not a feature of tularemia, adrenal insufficiency, or opioid withdrawal.
Acute intermittent porphyria (AIP) can cause both abdominal and neurologic problems. Hyponatremia is common during acute attacks, caused by either the inappropriate release of ADH or the appropriate release of the hormone if there is fluid loss. AIP is a rare diagnosis but could explain the uncommon combination of abdominal pain, vomiting, seizure, and hyponatremia. A spot urine porphobilinogen test should be sent to assess for AIP.
Additional laboratory studies were sent. Serum osmolality was 269 mosm/kg with a corresponding urine osmolality of 699 mosm/kg. A random urine sodium was 145 mEq/L. Thyroid stimulating hormone and cosyntropin stimulating testing were normal. IgM and IgG antibodies to Borrelia burgdorferi were negative. Urine porphobilinogen was sent. An electroencephalogram did not reveal epileptiform discharges. Magnetic resonance imaging (MRI) of the brain was significant for T2/FLAIR hyperintensity in the cortex and subcortical white matter of the occipital lobes bilaterally. Hypertonic saline and fluid restriction were initiated.
The patient’s labs are consistent with SIADH. Excessive ADH release because of volume depletion and consequent hyponatremia should have improved rapidly with the administration of saline. The high urine sodium suggests that she is now volume replete, while the high urine osmolality is consistent with the presence of excessive ADH in the absence of appropriate stimuli. In the context of normal thyroid and adrenal function, the hyponatremia is likely due to the SIADH.
Negative serologic testing for Lyme disease, 3 months after the onset of rash, excludes this diagnosis.
The MRI findings are consistent with posterior reversible encephalopathy syndrome (PRES), a clinicoradiographic syndrome of headache, altered mental status, seizure, and/or vision loss with associated white matter abnormalities of the posterior cerebral hemispheres. PRES has been reported with AIP as well as other disorders, most commonly hypertensive encephalopathy, eclampsia, and immunosuppressive drug use.
The patient’s sodium improved with fluid restriction and the administration of hypertonic saline. There was no recurrence of seizure activity. Amlodipine was initiated for blood pressure readings as high as 156/106 mmHg. A hepatobiliary scan revealed a gallbladder ejection fraction of 13%. Biopsies from her endoscopy revealed nonspecific inflammation without the presence of Helicobacter pylori. The patient was discharged home 7 days after admission after stabilization of serum sodium, improvement in her abdominal pain, and tolerance of oral intake. A plan was made for outpatient cholecystectomy.
Many causes of abdominal pain have been excluded and the remaining diagnostic possibility, porphyria, is rare. The clinicians have revisited their differential and considered other causes of abdominal pain, including functional gallbladder disorders. However, chronic cholecystitis (or functional gallbladder disorder) is not this patient’s primary problem. The diffuse, severe, and constant abdominal pain prior to admission is not typical of biliary pain, and many medical conditions and drugs, including amlodipine, can lead to a positive hepatobiliary scan. Chronic cholecystitis would not explain her seizure.
AIP remains at the top of the differential for this young woman. A urine porphobilinogen has been sent and must be followed up prior to any further workup or surgery.
One week after discharge, the patient’s urine porphobilinogen resulted at 172.8 mCmol/ (upper limits of normal 8.8). Sequencing analysis for genes coding the enzymes involved in the synthetic pathway for heme were sent. Hydroxymethylbilane synthase, coproporphyrinogen oxidase, and protoporphyrinogen oxidase mutation assays were all normal. Despite the normal genetic assays, the diagnosis of AIP was made on the basis of the clinical presentation and elevated urine porphobilinogen. The patient was referred to a hematologist and initiated on oral glucose supplements and hematin infusions.
DISCUSSION
Although abdominal pain has a broad differential, the combination of abdominal pain and neurologic or psychiatric symptoms should suggest the possibility of porphyria, especially if symptoms are recurrent or unexplained. The porphyrias are a group of disorders caused by defects in the synthetic pathway of heme, leading to an overproduction and accumulation of precursors. Heme is a component of multiple proteins, including hemoglobin, myoglobin, and the cytochrome P450 enzymes. Although it is synthesized in all tissues, the bone marrow and liver are the organs most actively involved. The porphyrias can be classified according to the primary site of the overproduction and accumulation of heme precursors (liver vs bone marrow). Although there is overlap between the 2 groups, hepatic porphyrias often present with acute neurovisceral symptoms, while the erythropoietic porphyrias often cause cutaneous photosensitivity.1
AIP is the most common hepatic porphyria with a prevalence of 1 in 20,000 in Caucasians of Western European descent.1 AIP is caused by a defect in the gene that encodes porphobilinogen deaminase, leading to the accumulation of porphobilinogen.1 The cardinal manifestation is an acute porphyric attack. While the precise mechanisms underlying the symptoms are unknown, the accumulating metabolites may be directly neurotoxic.2 Attacks are precipitated by factors that induce heme synthesis, including caloric restriction, alcohol, and certain medications, particularly those that upregulate cyP450. The most commonly implicated drugs are anesthetics, antiepileptics, sulfonamides, rifampin, and estrogen and progesterone. Attacks can also be precipitated by changes in endogenous sex hormone levels, like the increase in progesterone seen in the luteal phase of the menstrual cycle, which may account for the higher incidence of symptomatic attacks in women.3
Acute attacks of AIP may have a wide variety of presentations; the disease was referred to as the “little imitator” in the early 20th century.4 The most common symptom is acute, severe abdominal pain, which may mimic an acute abdomen. Because the pain is neuropathic rather than inflammatory, abdominal tenderness, rebound, fever, and leukocytosis are usually absent, as they were in this patient. Abdominal pain is often accompanied by neuropsychiatric symptoms, including sensory and motor neuropathy, anxiety, hallucinations, delirium, and altered level of consciousness. Seizure occurs in 20% of cases. Involvement of the autonomic nervous system causes tachycardia and new onset hypertension in the majority of patients as well as restlessness and tremor. Hyponatremia, mediated by the syndrome of inappropriate ADH secretion, occurs in nearly a third of patients.5,6 MRI findings consistent with PRES have also been described in AIP.7
The diagnosis of AIP is often delayed; diagnosis later in the disease course is associated with a poorer prognosis.8 Reported intervals between presentation and diagnosis range from several months to as long as 20 years.9 Associating the use of medications, caloric restriction, or the menstrual cycle with the exacerbation of symptoms or darkening of urine can help prompt an earlier diagnosis.6
AIP can be diagnosed by detecting a greater than 5-fold elevation of urinary porphobilinogen excretion in conjunction with the typical symptoms of an acute attack.5 Renal dysfunction causes urinary excretion of PBG to fall and serum levels to rise.10 Serum PBG levels should therefore be sent when AIP is suspected in the setting of renal dysfunction. The primary role of genetic testing in a patient who has AIP confirmed clinically and biochemically is to assist in genetic counseling and to identify asymptomatic family members.11 Genetic testing is not required to confirm the diagnosis and does not help prognosticate. It is unusual that a mutation was not detected in this case, as the current sensitivity of genetic testing is 97% to 100%.11
There are 4 principles of management of an acute porphyric attack. First, any precipitating factors such as medications should be stopped. Second, abdominal pain should be treated appropriately with opioids, if necessary. Third, if autonomic dysfunction is present, beta-blockers or clonidine should be given to treat hypertension.5 Finally, glucose and/or hemin should be administered to downregulate aminolevulinic acid (ALA) synthase by negative feedback. Downregulation of ALA synthase decreases the accumulation of the neurotoxic porphyrin precursors ALA and PBG.5 For patients with mild symptoms, glucose alone (300-500 g/d) may be enough to abort the attack.12 This can be achieved via a high-carbohydrate diet in those able to tolerate oral intake or via continuous infusions of dextrose containing fluids.5 For more severe attacks with associated polyneuropathy, respiratory muscle weakness, or seizures, or for attacks that are not resolving, heme preparations dosed at 3 to 4 mg/kg/d for 3 to 4 days are indicated.5
The recent diagnosis of acute Lyme disease was a distractor in this presentation. In Lyme endemic areas, patients with erythema migrans are treated based on the clinical presentation rather than serologic testing.13 Although this patient took only 1 week of doxycycline, testing during this hospitalization showed that she had either been cured early or had not had Lyme disease in the first place. There is no known association between Lyme disease and the porphyrias, and doxycycline is not a common precipitant of AIP attacks.14 However, the GI side effects of doxycycline may have decreased caloric intake and ultimately provoked the patient’s first attack of AIP. The clinicians in this case appropriately avoided the “target” but hit the mark by correctly diagnosing AIP.
KEY POINTS
- Consider AIP in patients with unexplained abdominal pain, especially when accompanied by neuropsychiatric symptoms and autonomic lability.
- Diagnose AIP by sending a urine PBG during a suspected acute attack.
- Treat AIP acutely by removing precipitants, treating abdominal pain, and initiating dextrose-containing fluids and hemin infusions to downregulate ALA synthase.
Acknowledgments
The authors thank the patient who enthusiastically supported the writing of this report.
Disclosure
Warren Gavin, MD has disclosed participation in expert testimony. The authors have no financial or other conflicts of interest to disclose.
1. Desnick RJ, Balwani M. The Porphyrias. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J, eds. Harrison’s Principles of Internal Medicine, 19th Edition. New York: McGraw-Hill; 2015. http://accessmedicine.mhmedical.com.proxy.medlib.uits.iu.edu/content.aspx?bookid=1130&Sectionid=79754263. Accessed June 14, 2016.
2. Bissell DM, Lai JC, Meister RK, Blanc PD. Role of Delta-aminolevulinic Acid in the Symptoms of Acute Porphyria. Am J Med. 2015;128(3):313-317. PubMed
3. Bonkovsky HL, Guo JT, Hou W, Li T, Narang T, Thapar M. Porphyrin and Heme Metabolism and the Porphyrias. Compr Physiol. 2013;3(1):365-401. PubMed
4. Crimlisk HL. The little imitator--porphyria: a neuropsychiatric disorder. J Neurol Neurosurg Psychiatry. 1997;62(4):319-328. PubMed
5. Pischik E, Kauppinen R. An update of clinical management of acute intermittent porphyria. Appl Clin Genet. 2015;8:201-214. PubMed
6. Ventura P, Cappellini MD, Biolcati G, Guida CC, Rocchi E; Gruppo Italiano Porfiria (GrIP). A challenging diagnosis for potential fatal diseases: recommendations for diagnosing acute porphyrias. Eur J Intern Med. 2014;25(6):497-505. PubMed
7. Dagens A, Gilhooley MJ. Acute intermittent porphyria leading to posterior reversible encephalopathy syndrome (PRES): a rare cause of abdominal pain and seizures. BMJ Case Rep. 2016:bcr2016215350. PubMed
8. Pischik E, Bulyanitsa A, Kazakov V, Kauppinen R. Clinical features predictive of a poor prognosis in acute porphyria. J Neurol. 2004;251(12):1538-1541. PubMed
9. Sack GH. Acute intermittent porphyria. JAMA. 1990;264(10):1290-1293. PubMed
10. Sardh E, Andersson DEH, Henrichson A, Harper P. Porphyrin precursors and porphyrins in three patients with acute intermittent porphyria and end-stage renal disease under different therapy regimes. Cell Mol Biol (Noisy-le-grand). 2009;55(1):66-71. PubMed
11. Whatley SD, Badminton MN. Role of genetic testing in the management of patients with inherited porphyria and their families. Ann Clin Biochem. 2013;50(3):204-216. PubMed
12. Anderson KE, Bloomer JR, Bonkovsky HL, et al. Recommendations for the diagnosis and treatment of the acute porphyrias. Ann Intern Med. 2005;142(6):439-450. PubMed
13. Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43(9):1089-1134. PubMed
14. American Porphyria Foundation. Drug database. http://www.porphyriafoundation.com/drug-database. Accessed July 21, 2017.
1. Desnick RJ, Balwani M. The Porphyrias. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J, eds. Harrison’s Principles of Internal Medicine, 19th Edition. New York: McGraw-Hill; 2015. http://accessmedicine.mhmedical.com.proxy.medlib.uits.iu.edu/content.aspx?bookid=1130&Sectionid=79754263. Accessed June 14, 2016.
2. Bissell DM, Lai JC, Meister RK, Blanc PD. Role of Delta-aminolevulinic Acid in the Symptoms of Acute Porphyria. Am J Med. 2015;128(3):313-317. PubMed
3. Bonkovsky HL, Guo JT, Hou W, Li T, Narang T, Thapar M. Porphyrin and Heme Metabolism and the Porphyrias. Compr Physiol. 2013;3(1):365-401. PubMed
4. Crimlisk HL. The little imitator--porphyria: a neuropsychiatric disorder. J Neurol Neurosurg Psychiatry. 1997;62(4):319-328. PubMed
5. Pischik E, Kauppinen R. An update of clinical management of acute intermittent porphyria. Appl Clin Genet. 2015;8:201-214. PubMed
6. Ventura P, Cappellini MD, Biolcati G, Guida CC, Rocchi E; Gruppo Italiano Porfiria (GrIP). A challenging diagnosis for potential fatal diseases: recommendations for diagnosing acute porphyrias. Eur J Intern Med. 2014;25(6):497-505. PubMed
7. Dagens A, Gilhooley MJ. Acute intermittent porphyria leading to posterior reversible encephalopathy syndrome (PRES): a rare cause of abdominal pain and seizures. BMJ Case Rep. 2016:bcr2016215350. PubMed
8. Pischik E, Bulyanitsa A, Kazakov V, Kauppinen R. Clinical features predictive of a poor prognosis in acute porphyria. J Neurol. 2004;251(12):1538-1541. PubMed
9. Sack GH. Acute intermittent porphyria. JAMA. 1990;264(10):1290-1293. PubMed
10. Sardh E, Andersson DEH, Henrichson A, Harper P. Porphyrin precursors and porphyrins in three patients with acute intermittent porphyria and end-stage renal disease under different therapy regimes. Cell Mol Biol (Noisy-le-grand). 2009;55(1):66-71. PubMed
11. Whatley SD, Badminton MN. Role of genetic testing in the management of patients with inherited porphyria and their families. Ann Clin Biochem. 2013;50(3):204-216. PubMed
12. Anderson KE, Bloomer JR, Bonkovsky HL, et al. Recommendations for the diagnosis and treatment of the acute porphyrias. Ann Intern Med. 2005;142(6):439-450. PubMed
13. Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43(9):1089-1134. PubMed
14. American Porphyria Foundation. Drug database. http://www.porphyriafoundation.com/drug-database. Accessed July 21, 2017.
© 2018 Society of Hospital Medicine
What are the chances?
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
Two weeks after undergoing a below-knee amputation (BKA) and 10 days after being discharged to a skilled nursing facility (SNF), an 87-year-old man returned to the emergency department (ED) for evaluation of somnolence and altered mental state. In the ED, he was disoriented and unable to provide a detailed history.
The differential diagnosis for acute confusion and altered consciousness is broad. Initial possibilities include toxic-metabolic abnormalities, medication side effects, and infections. Urinary tract infection, pneumonia, and surgical-site infection should be assessed for first, as they are common causes of postoperative altered mentation. Next to be considered are subclinical seizure, ischemic stroke, and infectious encephalitis or meningitis, along with hemorrhagic stroke and subdural hematoma.
During initial assessment, the clinician should ascertain baseline mental state, the timeline of the change in mental status, recent medication changes, history of substance abuse, and concern about any recent trauma, such as a fall. Performing the physical examination, the clinician should assess vital signs and then focus on identifying localizing neurologic deficits.
First steps in the work-up include a complete metabolic panel, complete blood cell count, urinalysis with culture, and a urine toxicology screen. If the patient has a “toxic” appearance, blood cultures should be obtained. An electrocardiogram should be used to screen for drug toxicity or evidence of cardiac ischemia. If laboratory test results do not reveal an obvious infectious or metabolic cause, a noncontrast computed tomography (CT) of the head should be obtained. In terms of early interventions, a low glucose level should be treated with thiamine and then glucose, and naloxone should be given if there is any suspicion of narcotic overdose.
More history was obtained from the patient’s records. The BKA was performed to address a nonhealing transmetatarsal amputation. Two months earlier, the transmetatarsal amputation had been performed as treatment for a diabetic forefoot ulcer with chronic osteomyelitis. The patient’s post-BKA course was uncomplicated. He was started on intravenous (IV) ertapenem on postoperative day 1, and on postoperative day 4 was discharged to the SNF to complete a 6-week course of antibiotics for osteomyelitis. Past medical history included paroxysmal atrial fibrillation, coronary artery disease, congestive heart failure (ejection fraction 40%), and type 2 diabetes mellitus. Medications given at the SNF were oxycodone, acetaminophen, cholecalciferol, melatonin, digoxin, ondansetron, furosemide, gabapentin, correctional insulin, tamsulosin, senna, docusate, warfarin, and metoprolol. While there, the patient’s family expressed concern about his diminishing “mental ability.” They reported he had been fully alert and oriented on arrival at the SNF, and living independently with his wife before the BKA. Then, a week before the ED presentation, he started becoming more somnolent and forgetful. The gabapentin and oxycodone dosages were reduced to minimize their sedative effects, but he showed no improvement. At the SNF, a somnolence work-up was not performed.
Several of the patient’s medications can contribute to altered mental state. Ertapenem can cause seizures as well as profound mental status changes, though these are more likely in the setting of poor renal function. The mental status changes were noticed about a week into the patient’s course of antibiotics, which suggests a possible temporal correlation with the initiation of ertapenem. An electroencephalogram is required to diagnose nonconvulsive seizure activity. Narcotic overdose should still be considered, despite the recent reduction in oxycodone dosage. Digoxin toxicity, though less likely when the dose is stable and there are no changes in renal function, can cause a confused state. Concurrent use of furosemide could potentiate the toxic effects of digoxin.
Non-medication-related concerns include hypoglycemia, hyperglycemia, and, given his history of atrial fibrillation, cardioembolic stroke. Although generalized confusion is not a common manifestation of stroke, a thalamic stroke can alter mental state but be easily missed if not specifically considered. Additional lab work-up should include a digoxin level and, since he is taking warfarin, a prothrombin time/international normalized ratio (PT/INR). If the initial laboratory studies and head CT do not explain the altered mental state, magnetic resonance imaging (MRI) of the brain should be performed to further assess for stroke.
On physical examination in the ED, the patient was resting comfortably with eyes closed, and arousing to voice. He obeyed commands and participated in the examination. His Glasgow Coma Scale score was 13; temperature, 36.8°C, heart rate, 80 beats per minute; respiratory rate, 16 breaths per minute; blood pressure, 90/57 mm Hg; and 100% peripheral capillary oxygen saturation while breathing ambient air. He appeared well developed. His heart rhythm was irregularly irregular, without murmurs, rubs, or gallops. Respiratory and abdominal examination findings were normal. The left BKA incision was well approximated, with no drainage, dehiscence, fluctuance, or erythema. On neurologic examination, the patient was intermittently oriented only to self. Pupils were equal, round, and reactive to light; extraocular movements were intact; face was symmetric; tongue was midline; sensation on face was equal bilaterally; and shoulder shrug was intact. Strength was 5/5 and symmetric in the elbow and hip and 5/5 in the right knee and ankle (not tested on left because of BKA). Deep tendon reflexes were 3+ and symmetrical at the biceps, brachioradialis, and triceps tendons and 3+ in the right patellar and Achilles tendons. Sensation was intact and symmetrical in the upper and lower extremities. The patient’s speech was slow and slurred, and his answers were unrelated to the questions being asked.
The patient’s mental state is best described as lethargic. As he is only intermittently oriented, he meets the criteria for delirium. He is not obtunded or comatose, and his pupils are at least reactive, not pinpoint, so narcotic overdose is less likely. Thalamic stroke remains in the differential diagnosis; despite the seemingly symmetrical sensation examination, hemisensory deficits cannot be definitively ruled out given the patient’s mental state. A rare entity such as carcinomatosis meningitis or another diffuse, infiltrative neoplastic process could be causing his condition. However, because focal deficits other than abnormal speech and diffuse hyperreflexia are absent, toxic, infectious, or metabolic causes are more likely than structural abnormalities. Still possible is a medication toxicity, such as ertapenem toxicity or, less likely, digoxin toxicity. In terms of infectious possibilities, urinary tract infection could certainly present in this fashion, especially if the patient had a somewhat low neurologic reserve at baseline, and hypotension could be secondary to sepsis. Encephalitis or meningitis remains in the differential diagnosis, though the patient appears nontoxic, and therefore a bacterial etiology is very unlikely.
The patient’s hyperreflexia may be an important clue. Although the strength of his reflexes at baseline is unknown, seizures can cause transiently increased reflexes as well as a confused, lethargic mental state. Reflexes can also be increased by a drug overdose that has caused serotonin syndrome. Of the patient’s medications, only ondansetron can cause this reaction. Hyperthyroidism can cause brisk reflexes and confusion, though more typically it causes agitated confusion. A thyroid-stimulating hormone level should be added to the initial laboratory panel.
A complete blood count revealed white blood cell count 11.86 K/uL with neutrophilic predominance and immature granulocytes, hemoglobin 11.5 g/dL, and platelet count 323 K/uL. Serum sodium was 141 mEq/L, potassium 4.2 mEq/L, chloride 103 mEq/L, bicarbonate 30 mEq/L, creatinine 1.14 mg/dL (prior baseline of 0.8-1.0 mg/dL), blood urea nitrogen 26 mg/dL, blood glucose 159 mg/dL, and calcium 9.1 mg/dL. His digoxin level was 1.3 ng/mL (reference range 0.5-1.9 mg/mL) and troponin was undetectable. INR was 2.7 and partial thromboplastin time (PTT) 60 seconds. Vitamin B12 level was 674 pg/mL (reference range >180). A urinalysis had 1+ hyaline casts and was negative for nitrites, leukocyte esterase, blood, and bacteria. An ECG revealed atrial fibrillation with a ventricular rate of 80 beats per minute. A chest radiograph showed clear lung fields. A CT of the head without IV contrast had no evidence of an acute intracranial abnormality. In the ED, 1 liter of IV normal saline was given and blood pressure improved to 127/72 mm Hg.
The head CT does not show intracranial bleeding, and, though it is reassuring that INR is in the therapeutic range, ischemic stroke must remain in the differential diagnosis. Sepsis is less likely given that the criteria for systemic inflammatory response syndrome are not met, and hypotension was rapidly corrected with administration of IV fluids. Urinary tract infection was ruled out with the negative urinalysis. Subclinical seizures remain possible, as does medication-related or other toxicity. A medication overdose, intentional or otherwise, should also be considered.
The patient was admitted to the hospital. On reassessment by the inpatient team, he was oriented only to self, frequently falling asleep, and not recalling earlier conversations when aroused. His speech remained slurred and difficult to understand. Neurologic examination findings were unchanged since the ED examination. On additional cerebellar examination, he had dysmetria with finger-to-nose testing bilaterally and dysdiadochokinesia (impaired rapid alternating movements) of the left hand.
His handedness is not mentioned; the dysdiadochokinesia of the left hand may reflect the patient’s being right-handed, or may signify a focal cerebellar lesion. The cerebellum is also implicated by the bilateral dysmetria. Persistent somnolence in the absence of CT findings suggests a metabolic or infectious process. Metabolic processes that can cause bilateral cerebellar ataxia and somnolence include overdose of a drug or medication. Use of alcohol or a medication such as phenytoin, valproic acid, or a benzodiazepine can cause the symptoms in this case, but was not reported by the family, and there was no documentation of it in the SNF records. Wernicke encephalopathy is rare and is not well supported by the patient’s presentation but should be considered, as it can be easily treated with thiamine. Meningoencephalitis affecting the cerebellum remains possible, but infection is less likely. Both electroencephalogram and brain MRI should be performed, with a specific interest in possible cerebellar lesions. If the MRI is unremarkable, a lumbar puncture should be performed to assess opening pressure and investigate for infectious etiologies.
MRI of the brain showed age-related volume loss and nonspecific white matter disease without acute changes. Lack of a clear explanation for the neurologic findings led to suspicion of a medication side effect. Ertapenem was stopped on admission because it has been reported to rarely cause altered mental status. IV moxifloxacin was started for the osteomyelitis. Over the next 2 days, symptoms began resolving; within 24 hours of ertapenem discontinuation, the patient was awake, alert, and talkative. On examination, he remained dysarthric but was no longer dysmetric. Within 48 hours, the dysarthria was completely resolved, and he was returned to the SNF to complete a course of IV moxifloxacin.
DISCUSSION
Among elderly patients presenting to the ED, altered mental status is a common complaint, accounting for 10% to 30% of visits.1 Medications are a common cause of altered mental status among the elderly and are responsible for 40% of delirium cases.1 The risk of adverse drug events (ADEs) rises with the number of medications prescribed.1-3 Among patients older than 60 years, the incidence of polypharmacy (defined as taking >5 prescription medications) increased from roughly 20% in 1999 to 40% in 2012.4,5 The most common ADEs in the ambulatory setting (25%) are central nervous system (CNS) symptoms, including dizziness, sleep disturbances, and mood changes.6 A medication effect should be suspected in any elderly patient presenting with altered mental state.
The present patient developed a constellation of neurologic symptoms after starting ertapenem, one of the carbapenem antibiotics, which is a class of medications that can cause CNS ADEs. Carbapenems are renally cleared, and adjustments must be made for acute or chronic changes in kidney function. Carbapenems are associated with increased risk of seizure; the incidence of seizure with ertapenem is 0.2%.7,8 Food and Drug Administration postmarketing reports have noted ertapenem can cause somnolence and dyskinesia,9 and several case reports have described ertapenem-associated CNS side effects, including psychosis and encephalopathy.10-13 Symptoms and examination findings can include confusion, disorientation, garbled speech, dysphagia, hallucinations, miosis, myoclonus, tremor, and agitation.10-13 Although reports of dysmetria and dysdiadochokinesia are lacking, suspicion of an ADE in this case was heightened by the timing of the exposure and the absence of alternative infectious, metabolic, and vascular explanations for bilateral cerebellar dysfunction.
The Naranjo Adverse Drug Reaction (ADR) scale may help clinicians differentiate ADEs from other etiologies of symptoms. It uses 10 weighted questions (Table) to estimate the probability that an adverse clinical event is caused by a drug reaction.14 The present case was assigned 1 point for prior reports of neurologic ADEs associated with ertapenem, 2 for the temporal association, 1 for resolution after medication withdrawal, 2 for lack of alternative causes, and 1 for objective evidence of neurologic dysfunction—for a total of 7 points, indicating ertapenem was probably the cause of the patient’s neurologic symptoms. Of 4 prior cases in which carbapenem toxicity was suspected and the Naranjo scale was used, 3 found a probable relationship, and the fourth a highly probable one.10,12 Confusion, disorientation, hallucinations, tangential thoughts, and garbled speech were reported in the 3 probable cases of ADEs. In the highly probable case, tangential thoughts, garbled speech, and miosis were noted on examination, and these findings returned after re-exposure to ertapenem. Of note, these ADEs occurred in patients with normal and abnormal renal function, and in middle-aged and elderly patients.10,11,13
Most medications have a long list of low-frequency and rarely reported adverse effects. The present case reminds clinicians to consider rare adverse effects, or variants of previously reported adverse effects, in a patient with unexplained symptoms. To estimate the probability that a drug is causing harm to a patient, using a validated tool such as the Naranjo scale helps answer the question, What are the chances?
KEY TEACHING POINTS
Clinicians should include rare adverse effects of common medications in the differential diagnosis.
The Naranjo score is a validated tool that can be used to systematically assess the probability of an adverse drug effect at the bedside.
- The presentation of ertapenem-associated neurotoxicity may include features of bilateral cerebellar dysfunction.
Disclosure
Nothing to report.
1. Inouye SK, Fearing MA, Marcantonio ER. Delirium. In: Halter JB, Ouslander JG, Tinetti ME, Studenski S, High KP, Asthana S, eds. Hazzard’s Geriatric Medicine and Gerontology. 6th ed. New York, NY: McGraw-Hill; 2009.
2. Sarkar U, López A, Maselli JH, Gonzales R. Adverse drug events in U.S. adult ambulatory medical care. Health Serv Res. 2011;46(5):1517-1533. PubMed
3. Chrischilles E, Rubenstein L, Van Gilder R, Voelker M, Wright K, Wallace R. Risk factors for adverse drug events in older adults with mobility limitations in the community setting. J Am Geriatr Soc. 2007;55(1):29-34. PubMed
4. Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA. 2002;287(3):337-344. PubMed
5. Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831. PubMed
6. Thomsen LA, Winterstein AG, Søndergaard B, Haugbølle LS, Melander A. Systematic review of the incidence and characteristics of preventable adverse drug events in ambulatory care. Ann Pharmacother. 2007;41(9):1411-1426. PubMed
7. Zhanel GG, Wiebe R, Dilay L, et al. Comparative review of the carbapenems. Drugs. 2007;67(7):1027-1052. PubMed
8. Cannon JP, Lee TA, Clark NM, Setlak P, Grim SA. The risk of seizures among the carbapenems: a meta-analysis. J Antimicrob Chemother. 2014;69(8):2043-2055. PubMed
9. US Food and Drug Administration. Invanz (ertapenem) injection [safety information]. http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm196605.htm. Published July 2013. Accessed July 6, 2015.
10. Oo Y, Packham D, Yau W, Munckhof WJ. Ertapenem-associated psychosis and encephalopathy. Intern Med J. 2014;44(8):817-819. PubMed
11. Wen MJ, Sung CC, Chau T, Lin SH. Acute prolonged neurotoxicity associated with recommended doses of ertapenem in 2 patients with advanced renal failure. Clin Nephrol. 2013;80(6):474-478. PubMed
12. Duquaine S, Kitchell E, Tate T, Tannen RC, Wickremasinghe IM. Central nervous system toxicity associated with ertapenem use. Ann Pharmacother. 2011;45(1):e6. PubMed
13. Kong V, Beckert L, Awunor-Renner C. A case of beta lactam-induced visual hallucination. N Z Med J. 2009;122(1298):76-77. PubMed
14. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245. PubMed
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
Two weeks after undergoing a below-knee amputation (BKA) and 10 days after being discharged to a skilled nursing facility (SNF), an 87-year-old man returned to the emergency department (ED) for evaluation of somnolence and altered mental state. In the ED, he was disoriented and unable to provide a detailed history.
The differential diagnosis for acute confusion and altered consciousness is broad. Initial possibilities include toxic-metabolic abnormalities, medication side effects, and infections. Urinary tract infection, pneumonia, and surgical-site infection should be assessed for first, as they are common causes of postoperative altered mentation. Next to be considered are subclinical seizure, ischemic stroke, and infectious encephalitis or meningitis, along with hemorrhagic stroke and subdural hematoma.
During initial assessment, the clinician should ascertain baseline mental state, the timeline of the change in mental status, recent medication changes, history of substance abuse, and concern about any recent trauma, such as a fall. Performing the physical examination, the clinician should assess vital signs and then focus on identifying localizing neurologic deficits.
First steps in the work-up include a complete metabolic panel, complete blood cell count, urinalysis with culture, and a urine toxicology screen. If the patient has a “toxic” appearance, blood cultures should be obtained. An electrocardiogram should be used to screen for drug toxicity or evidence of cardiac ischemia. If laboratory test results do not reveal an obvious infectious or metabolic cause, a noncontrast computed tomography (CT) of the head should be obtained. In terms of early interventions, a low glucose level should be treated with thiamine and then glucose, and naloxone should be given if there is any suspicion of narcotic overdose.
More history was obtained from the patient’s records. The BKA was performed to address a nonhealing transmetatarsal amputation. Two months earlier, the transmetatarsal amputation had been performed as treatment for a diabetic forefoot ulcer with chronic osteomyelitis. The patient’s post-BKA course was uncomplicated. He was started on intravenous (IV) ertapenem on postoperative day 1, and on postoperative day 4 was discharged to the SNF to complete a 6-week course of antibiotics for osteomyelitis. Past medical history included paroxysmal atrial fibrillation, coronary artery disease, congestive heart failure (ejection fraction 40%), and type 2 diabetes mellitus. Medications given at the SNF were oxycodone, acetaminophen, cholecalciferol, melatonin, digoxin, ondansetron, furosemide, gabapentin, correctional insulin, tamsulosin, senna, docusate, warfarin, and metoprolol. While there, the patient’s family expressed concern about his diminishing “mental ability.” They reported he had been fully alert and oriented on arrival at the SNF, and living independently with his wife before the BKA. Then, a week before the ED presentation, he started becoming more somnolent and forgetful. The gabapentin and oxycodone dosages were reduced to minimize their sedative effects, but he showed no improvement. At the SNF, a somnolence work-up was not performed.
Several of the patient’s medications can contribute to altered mental state. Ertapenem can cause seizures as well as profound mental status changes, though these are more likely in the setting of poor renal function. The mental status changes were noticed about a week into the patient’s course of antibiotics, which suggests a possible temporal correlation with the initiation of ertapenem. An electroencephalogram is required to diagnose nonconvulsive seizure activity. Narcotic overdose should still be considered, despite the recent reduction in oxycodone dosage. Digoxin toxicity, though less likely when the dose is stable and there are no changes in renal function, can cause a confused state. Concurrent use of furosemide could potentiate the toxic effects of digoxin.
Non-medication-related concerns include hypoglycemia, hyperglycemia, and, given his history of atrial fibrillation, cardioembolic stroke. Although generalized confusion is not a common manifestation of stroke, a thalamic stroke can alter mental state but be easily missed if not specifically considered. Additional lab work-up should include a digoxin level and, since he is taking warfarin, a prothrombin time/international normalized ratio (PT/INR). If the initial laboratory studies and head CT do not explain the altered mental state, magnetic resonance imaging (MRI) of the brain should be performed to further assess for stroke.
On physical examination in the ED, the patient was resting comfortably with eyes closed, and arousing to voice. He obeyed commands and participated in the examination. His Glasgow Coma Scale score was 13; temperature, 36.8°C, heart rate, 80 beats per minute; respiratory rate, 16 breaths per minute; blood pressure, 90/57 mm Hg; and 100% peripheral capillary oxygen saturation while breathing ambient air. He appeared well developed. His heart rhythm was irregularly irregular, without murmurs, rubs, or gallops. Respiratory and abdominal examination findings were normal. The left BKA incision was well approximated, with no drainage, dehiscence, fluctuance, or erythema. On neurologic examination, the patient was intermittently oriented only to self. Pupils were equal, round, and reactive to light; extraocular movements were intact; face was symmetric; tongue was midline; sensation on face was equal bilaterally; and shoulder shrug was intact. Strength was 5/5 and symmetric in the elbow and hip and 5/5 in the right knee and ankle (not tested on left because of BKA). Deep tendon reflexes were 3+ and symmetrical at the biceps, brachioradialis, and triceps tendons and 3+ in the right patellar and Achilles tendons. Sensation was intact and symmetrical in the upper and lower extremities. The patient’s speech was slow and slurred, and his answers were unrelated to the questions being asked.
The patient’s mental state is best described as lethargic. As he is only intermittently oriented, he meets the criteria for delirium. He is not obtunded or comatose, and his pupils are at least reactive, not pinpoint, so narcotic overdose is less likely. Thalamic stroke remains in the differential diagnosis; despite the seemingly symmetrical sensation examination, hemisensory deficits cannot be definitively ruled out given the patient’s mental state. A rare entity such as carcinomatosis meningitis or another diffuse, infiltrative neoplastic process could be causing his condition. However, because focal deficits other than abnormal speech and diffuse hyperreflexia are absent, toxic, infectious, or metabolic causes are more likely than structural abnormalities. Still possible is a medication toxicity, such as ertapenem toxicity or, less likely, digoxin toxicity. In terms of infectious possibilities, urinary tract infection could certainly present in this fashion, especially if the patient had a somewhat low neurologic reserve at baseline, and hypotension could be secondary to sepsis. Encephalitis or meningitis remains in the differential diagnosis, though the patient appears nontoxic, and therefore a bacterial etiology is very unlikely.
The patient’s hyperreflexia may be an important clue. Although the strength of his reflexes at baseline is unknown, seizures can cause transiently increased reflexes as well as a confused, lethargic mental state. Reflexes can also be increased by a drug overdose that has caused serotonin syndrome. Of the patient’s medications, only ondansetron can cause this reaction. Hyperthyroidism can cause brisk reflexes and confusion, though more typically it causes agitated confusion. A thyroid-stimulating hormone level should be added to the initial laboratory panel.
A complete blood count revealed white blood cell count 11.86 K/uL with neutrophilic predominance and immature granulocytes, hemoglobin 11.5 g/dL, and platelet count 323 K/uL. Serum sodium was 141 mEq/L, potassium 4.2 mEq/L, chloride 103 mEq/L, bicarbonate 30 mEq/L, creatinine 1.14 mg/dL (prior baseline of 0.8-1.0 mg/dL), blood urea nitrogen 26 mg/dL, blood glucose 159 mg/dL, and calcium 9.1 mg/dL. His digoxin level was 1.3 ng/mL (reference range 0.5-1.9 mg/mL) and troponin was undetectable. INR was 2.7 and partial thromboplastin time (PTT) 60 seconds. Vitamin B12 level was 674 pg/mL (reference range >180). A urinalysis had 1+ hyaline casts and was negative for nitrites, leukocyte esterase, blood, and bacteria. An ECG revealed atrial fibrillation with a ventricular rate of 80 beats per minute. A chest radiograph showed clear lung fields. A CT of the head without IV contrast had no evidence of an acute intracranial abnormality. In the ED, 1 liter of IV normal saline was given and blood pressure improved to 127/72 mm Hg.
The head CT does not show intracranial bleeding, and, though it is reassuring that INR is in the therapeutic range, ischemic stroke must remain in the differential diagnosis. Sepsis is less likely given that the criteria for systemic inflammatory response syndrome are not met, and hypotension was rapidly corrected with administration of IV fluids. Urinary tract infection was ruled out with the negative urinalysis. Subclinical seizures remain possible, as does medication-related or other toxicity. A medication overdose, intentional or otherwise, should also be considered.
The patient was admitted to the hospital. On reassessment by the inpatient team, he was oriented only to self, frequently falling asleep, and not recalling earlier conversations when aroused. His speech remained slurred and difficult to understand. Neurologic examination findings were unchanged since the ED examination. On additional cerebellar examination, he had dysmetria with finger-to-nose testing bilaterally and dysdiadochokinesia (impaired rapid alternating movements) of the left hand.
His handedness is not mentioned; the dysdiadochokinesia of the left hand may reflect the patient’s being right-handed, or may signify a focal cerebellar lesion. The cerebellum is also implicated by the bilateral dysmetria. Persistent somnolence in the absence of CT findings suggests a metabolic or infectious process. Metabolic processes that can cause bilateral cerebellar ataxia and somnolence include overdose of a drug or medication. Use of alcohol or a medication such as phenytoin, valproic acid, or a benzodiazepine can cause the symptoms in this case, but was not reported by the family, and there was no documentation of it in the SNF records. Wernicke encephalopathy is rare and is not well supported by the patient’s presentation but should be considered, as it can be easily treated with thiamine. Meningoencephalitis affecting the cerebellum remains possible, but infection is less likely. Both electroencephalogram and brain MRI should be performed, with a specific interest in possible cerebellar lesions. If the MRI is unremarkable, a lumbar puncture should be performed to assess opening pressure and investigate for infectious etiologies.
MRI of the brain showed age-related volume loss and nonspecific white matter disease without acute changes. Lack of a clear explanation for the neurologic findings led to suspicion of a medication side effect. Ertapenem was stopped on admission because it has been reported to rarely cause altered mental status. IV moxifloxacin was started for the osteomyelitis. Over the next 2 days, symptoms began resolving; within 24 hours of ertapenem discontinuation, the patient was awake, alert, and talkative. On examination, he remained dysarthric but was no longer dysmetric. Within 48 hours, the dysarthria was completely resolved, and he was returned to the SNF to complete a course of IV moxifloxacin.
DISCUSSION
Among elderly patients presenting to the ED, altered mental status is a common complaint, accounting for 10% to 30% of visits.1 Medications are a common cause of altered mental status among the elderly and are responsible for 40% of delirium cases.1 The risk of adverse drug events (ADEs) rises with the number of medications prescribed.1-3 Among patients older than 60 years, the incidence of polypharmacy (defined as taking >5 prescription medications) increased from roughly 20% in 1999 to 40% in 2012.4,5 The most common ADEs in the ambulatory setting (25%) are central nervous system (CNS) symptoms, including dizziness, sleep disturbances, and mood changes.6 A medication effect should be suspected in any elderly patient presenting with altered mental state.
The present patient developed a constellation of neurologic symptoms after starting ertapenem, one of the carbapenem antibiotics, which is a class of medications that can cause CNS ADEs. Carbapenems are renally cleared, and adjustments must be made for acute or chronic changes in kidney function. Carbapenems are associated with increased risk of seizure; the incidence of seizure with ertapenem is 0.2%.7,8 Food and Drug Administration postmarketing reports have noted ertapenem can cause somnolence and dyskinesia,9 and several case reports have described ertapenem-associated CNS side effects, including psychosis and encephalopathy.10-13 Symptoms and examination findings can include confusion, disorientation, garbled speech, dysphagia, hallucinations, miosis, myoclonus, tremor, and agitation.10-13 Although reports of dysmetria and dysdiadochokinesia are lacking, suspicion of an ADE in this case was heightened by the timing of the exposure and the absence of alternative infectious, metabolic, and vascular explanations for bilateral cerebellar dysfunction.
The Naranjo Adverse Drug Reaction (ADR) scale may help clinicians differentiate ADEs from other etiologies of symptoms. It uses 10 weighted questions (Table) to estimate the probability that an adverse clinical event is caused by a drug reaction.14 The present case was assigned 1 point for prior reports of neurologic ADEs associated with ertapenem, 2 for the temporal association, 1 for resolution after medication withdrawal, 2 for lack of alternative causes, and 1 for objective evidence of neurologic dysfunction—for a total of 7 points, indicating ertapenem was probably the cause of the patient’s neurologic symptoms. Of 4 prior cases in which carbapenem toxicity was suspected and the Naranjo scale was used, 3 found a probable relationship, and the fourth a highly probable one.10,12 Confusion, disorientation, hallucinations, tangential thoughts, and garbled speech were reported in the 3 probable cases of ADEs. In the highly probable case, tangential thoughts, garbled speech, and miosis were noted on examination, and these findings returned after re-exposure to ertapenem. Of note, these ADEs occurred in patients with normal and abnormal renal function, and in middle-aged and elderly patients.10,11,13
Most medications have a long list of low-frequency and rarely reported adverse effects. The present case reminds clinicians to consider rare adverse effects, or variants of previously reported adverse effects, in a patient with unexplained symptoms. To estimate the probability that a drug is causing harm to a patient, using a validated tool such as the Naranjo scale helps answer the question, What are the chances?
KEY TEACHING POINTS
Clinicians should include rare adverse effects of common medications in the differential diagnosis.
The Naranjo score is a validated tool that can be used to systematically assess the probability of an adverse drug effect at the bedside.
- The presentation of ertapenem-associated neurotoxicity may include features of bilateral cerebellar dysfunction.
Disclosure
Nothing to report.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
Two weeks after undergoing a below-knee amputation (BKA) and 10 days after being discharged to a skilled nursing facility (SNF), an 87-year-old man returned to the emergency department (ED) for evaluation of somnolence and altered mental state. In the ED, he was disoriented and unable to provide a detailed history.
The differential diagnosis for acute confusion and altered consciousness is broad. Initial possibilities include toxic-metabolic abnormalities, medication side effects, and infections. Urinary tract infection, pneumonia, and surgical-site infection should be assessed for first, as they are common causes of postoperative altered mentation. Next to be considered are subclinical seizure, ischemic stroke, and infectious encephalitis or meningitis, along with hemorrhagic stroke and subdural hematoma.
During initial assessment, the clinician should ascertain baseline mental state, the timeline of the change in mental status, recent medication changes, history of substance abuse, and concern about any recent trauma, such as a fall. Performing the physical examination, the clinician should assess vital signs and then focus on identifying localizing neurologic deficits.
First steps in the work-up include a complete metabolic panel, complete blood cell count, urinalysis with culture, and a urine toxicology screen. If the patient has a “toxic” appearance, blood cultures should be obtained. An electrocardiogram should be used to screen for drug toxicity or evidence of cardiac ischemia. If laboratory test results do not reveal an obvious infectious or metabolic cause, a noncontrast computed tomography (CT) of the head should be obtained. In terms of early interventions, a low glucose level should be treated with thiamine and then glucose, and naloxone should be given if there is any suspicion of narcotic overdose.
More history was obtained from the patient’s records. The BKA was performed to address a nonhealing transmetatarsal amputation. Two months earlier, the transmetatarsal amputation had been performed as treatment for a diabetic forefoot ulcer with chronic osteomyelitis. The patient’s post-BKA course was uncomplicated. He was started on intravenous (IV) ertapenem on postoperative day 1, and on postoperative day 4 was discharged to the SNF to complete a 6-week course of antibiotics for osteomyelitis. Past medical history included paroxysmal atrial fibrillation, coronary artery disease, congestive heart failure (ejection fraction 40%), and type 2 diabetes mellitus. Medications given at the SNF were oxycodone, acetaminophen, cholecalciferol, melatonin, digoxin, ondansetron, furosemide, gabapentin, correctional insulin, tamsulosin, senna, docusate, warfarin, and metoprolol. While there, the patient’s family expressed concern about his diminishing “mental ability.” They reported he had been fully alert and oriented on arrival at the SNF, and living independently with his wife before the BKA. Then, a week before the ED presentation, he started becoming more somnolent and forgetful. The gabapentin and oxycodone dosages were reduced to minimize their sedative effects, but he showed no improvement. At the SNF, a somnolence work-up was not performed.
Several of the patient’s medications can contribute to altered mental state. Ertapenem can cause seizures as well as profound mental status changes, though these are more likely in the setting of poor renal function. The mental status changes were noticed about a week into the patient’s course of antibiotics, which suggests a possible temporal correlation with the initiation of ertapenem. An electroencephalogram is required to diagnose nonconvulsive seizure activity. Narcotic overdose should still be considered, despite the recent reduction in oxycodone dosage. Digoxin toxicity, though less likely when the dose is stable and there are no changes in renal function, can cause a confused state. Concurrent use of furosemide could potentiate the toxic effects of digoxin.
Non-medication-related concerns include hypoglycemia, hyperglycemia, and, given his history of atrial fibrillation, cardioembolic stroke. Although generalized confusion is not a common manifestation of stroke, a thalamic stroke can alter mental state but be easily missed if not specifically considered. Additional lab work-up should include a digoxin level and, since he is taking warfarin, a prothrombin time/international normalized ratio (PT/INR). If the initial laboratory studies and head CT do not explain the altered mental state, magnetic resonance imaging (MRI) of the brain should be performed to further assess for stroke.
On physical examination in the ED, the patient was resting comfortably with eyes closed, and arousing to voice. He obeyed commands and participated in the examination. His Glasgow Coma Scale score was 13; temperature, 36.8°C, heart rate, 80 beats per minute; respiratory rate, 16 breaths per minute; blood pressure, 90/57 mm Hg; and 100% peripheral capillary oxygen saturation while breathing ambient air. He appeared well developed. His heart rhythm was irregularly irregular, without murmurs, rubs, or gallops. Respiratory and abdominal examination findings were normal. The left BKA incision was well approximated, with no drainage, dehiscence, fluctuance, or erythema. On neurologic examination, the patient was intermittently oriented only to self. Pupils were equal, round, and reactive to light; extraocular movements were intact; face was symmetric; tongue was midline; sensation on face was equal bilaterally; and shoulder shrug was intact. Strength was 5/5 and symmetric in the elbow and hip and 5/5 in the right knee and ankle (not tested on left because of BKA). Deep tendon reflexes were 3+ and symmetrical at the biceps, brachioradialis, and triceps tendons and 3+ in the right patellar and Achilles tendons. Sensation was intact and symmetrical in the upper and lower extremities. The patient’s speech was slow and slurred, and his answers were unrelated to the questions being asked.
The patient’s mental state is best described as lethargic. As he is only intermittently oriented, he meets the criteria for delirium. He is not obtunded or comatose, and his pupils are at least reactive, not pinpoint, so narcotic overdose is less likely. Thalamic stroke remains in the differential diagnosis; despite the seemingly symmetrical sensation examination, hemisensory deficits cannot be definitively ruled out given the patient’s mental state. A rare entity such as carcinomatosis meningitis or another diffuse, infiltrative neoplastic process could be causing his condition. However, because focal deficits other than abnormal speech and diffuse hyperreflexia are absent, toxic, infectious, or metabolic causes are more likely than structural abnormalities. Still possible is a medication toxicity, such as ertapenem toxicity or, less likely, digoxin toxicity. In terms of infectious possibilities, urinary tract infection could certainly present in this fashion, especially if the patient had a somewhat low neurologic reserve at baseline, and hypotension could be secondary to sepsis. Encephalitis or meningitis remains in the differential diagnosis, though the patient appears nontoxic, and therefore a bacterial etiology is very unlikely.
The patient’s hyperreflexia may be an important clue. Although the strength of his reflexes at baseline is unknown, seizures can cause transiently increased reflexes as well as a confused, lethargic mental state. Reflexes can also be increased by a drug overdose that has caused serotonin syndrome. Of the patient’s medications, only ondansetron can cause this reaction. Hyperthyroidism can cause brisk reflexes and confusion, though more typically it causes agitated confusion. A thyroid-stimulating hormone level should be added to the initial laboratory panel.
A complete blood count revealed white blood cell count 11.86 K/uL with neutrophilic predominance and immature granulocytes, hemoglobin 11.5 g/dL, and platelet count 323 K/uL. Serum sodium was 141 mEq/L, potassium 4.2 mEq/L, chloride 103 mEq/L, bicarbonate 30 mEq/L, creatinine 1.14 mg/dL (prior baseline of 0.8-1.0 mg/dL), blood urea nitrogen 26 mg/dL, blood glucose 159 mg/dL, and calcium 9.1 mg/dL. His digoxin level was 1.3 ng/mL (reference range 0.5-1.9 mg/mL) and troponin was undetectable. INR was 2.7 and partial thromboplastin time (PTT) 60 seconds. Vitamin B12 level was 674 pg/mL (reference range >180). A urinalysis had 1+ hyaline casts and was negative for nitrites, leukocyte esterase, blood, and bacteria. An ECG revealed atrial fibrillation with a ventricular rate of 80 beats per minute. A chest radiograph showed clear lung fields. A CT of the head without IV contrast had no evidence of an acute intracranial abnormality. In the ED, 1 liter of IV normal saline was given and blood pressure improved to 127/72 mm Hg.
The head CT does not show intracranial bleeding, and, though it is reassuring that INR is in the therapeutic range, ischemic stroke must remain in the differential diagnosis. Sepsis is less likely given that the criteria for systemic inflammatory response syndrome are not met, and hypotension was rapidly corrected with administration of IV fluids. Urinary tract infection was ruled out with the negative urinalysis. Subclinical seizures remain possible, as does medication-related or other toxicity. A medication overdose, intentional or otherwise, should also be considered.
The patient was admitted to the hospital. On reassessment by the inpatient team, he was oriented only to self, frequently falling asleep, and not recalling earlier conversations when aroused. His speech remained slurred and difficult to understand. Neurologic examination findings were unchanged since the ED examination. On additional cerebellar examination, he had dysmetria with finger-to-nose testing bilaterally and dysdiadochokinesia (impaired rapid alternating movements) of the left hand.
His handedness is not mentioned; the dysdiadochokinesia of the left hand may reflect the patient’s being right-handed, or may signify a focal cerebellar lesion. The cerebellum is also implicated by the bilateral dysmetria. Persistent somnolence in the absence of CT findings suggests a metabolic or infectious process. Metabolic processes that can cause bilateral cerebellar ataxia and somnolence include overdose of a drug or medication. Use of alcohol or a medication such as phenytoin, valproic acid, or a benzodiazepine can cause the symptoms in this case, but was not reported by the family, and there was no documentation of it in the SNF records. Wernicke encephalopathy is rare and is not well supported by the patient’s presentation but should be considered, as it can be easily treated with thiamine. Meningoencephalitis affecting the cerebellum remains possible, but infection is less likely. Both electroencephalogram and brain MRI should be performed, with a specific interest in possible cerebellar lesions. If the MRI is unremarkable, a lumbar puncture should be performed to assess opening pressure and investigate for infectious etiologies.
MRI of the brain showed age-related volume loss and nonspecific white matter disease without acute changes. Lack of a clear explanation for the neurologic findings led to suspicion of a medication side effect. Ertapenem was stopped on admission because it has been reported to rarely cause altered mental status. IV moxifloxacin was started for the osteomyelitis. Over the next 2 days, symptoms began resolving; within 24 hours of ertapenem discontinuation, the patient was awake, alert, and talkative. On examination, he remained dysarthric but was no longer dysmetric. Within 48 hours, the dysarthria was completely resolved, and he was returned to the SNF to complete a course of IV moxifloxacin.
DISCUSSION
Among elderly patients presenting to the ED, altered mental status is a common complaint, accounting for 10% to 30% of visits.1 Medications are a common cause of altered mental status among the elderly and are responsible for 40% of delirium cases.1 The risk of adverse drug events (ADEs) rises with the number of medications prescribed.1-3 Among patients older than 60 years, the incidence of polypharmacy (defined as taking >5 prescription medications) increased from roughly 20% in 1999 to 40% in 2012.4,5 The most common ADEs in the ambulatory setting (25%) are central nervous system (CNS) symptoms, including dizziness, sleep disturbances, and mood changes.6 A medication effect should be suspected in any elderly patient presenting with altered mental state.
The present patient developed a constellation of neurologic symptoms after starting ertapenem, one of the carbapenem antibiotics, which is a class of medications that can cause CNS ADEs. Carbapenems are renally cleared, and adjustments must be made for acute or chronic changes in kidney function. Carbapenems are associated with increased risk of seizure; the incidence of seizure with ertapenem is 0.2%.7,8 Food and Drug Administration postmarketing reports have noted ertapenem can cause somnolence and dyskinesia,9 and several case reports have described ertapenem-associated CNS side effects, including psychosis and encephalopathy.10-13 Symptoms and examination findings can include confusion, disorientation, garbled speech, dysphagia, hallucinations, miosis, myoclonus, tremor, and agitation.10-13 Although reports of dysmetria and dysdiadochokinesia are lacking, suspicion of an ADE in this case was heightened by the timing of the exposure and the absence of alternative infectious, metabolic, and vascular explanations for bilateral cerebellar dysfunction.
The Naranjo Adverse Drug Reaction (ADR) scale may help clinicians differentiate ADEs from other etiologies of symptoms. It uses 10 weighted questions (Table) to estimate the probability that an adverse clinical event is caused by a drug reaction.14 The present case was assigned 1 point for prior reports of neurologic ADEs associated with ertapenem, 2 for the temporal association, 1 for resolution after medication withdrawal, 2 for lack of alternative causes, and 1 for objective evidence of neurologic dysfunction—for a total of 7 points, indicating ertapenem was probably the cause of the patient’s neurologic symptoms. Of 4 prior cases in which carbapenem toxicity was suspected and the Naranjo scale was used, 3 found a probable relationship, and the fourth a highly probable one.10,12 Confusion, disorientation, hallucinations, tangential thoughts, and garbled speech were reported in the 3 probable cases of ADEs. In the highly probable case, tangential thoughts, garbled speech, and miosis were noted on examination, and these findings returned after re-exposure to ertapenem. Of note, these ADEs occurred in patients with normal and abnormal renal function, and in middle-aged and elderly patients.10,11,13
Most medications have a long list of low-frequency and rarely reported adverse effects. The present case reminds clinicians to consider rare adverse effects, or variants of previously reported adverse effects, in a patient with unexplained symptoms. To estimate the probability that a drug is causing harm to a patient, using a validated tool such as the Naranjo scale helps answer the question, What are the chances?
KEY TEACHING POINTS
Clinicians should include rare adverse effects of common medications in the differential diagnosis.
The Naranjo score is a validated tool that can be used to systematically assess the probability of an adverse drug effect at the bedside.
- The presentation of ertapenem-associated neurotoxicity may include features of bilateral cerebellar dysfunction.
Disclosure
Nothing to report.
1. Inouye SK, Fearing MA, Marcantonio ER. Delirium. In: Halter JB, Ouslander JG, Tinetti ME, Studenski S, High KP, Asthana S, eds. Hazzard’s Geriatric Medicine and Gerontology. 6th ed. New York, NY: McGraw-Hill; 2009.
2. Sarkar U, López A, Maselli JH, Gonzales R. Adverse drug events in U.S. adult ambulatory medical care. Health Serv Res. 2011;46(5):1517-1533. PubMed
3. Chrischilles E, Rubenstein L, Van Gilder R, Voelker M, Wright K, Wallace R. Risk factors for adverse drug events in older adults with mobility limitations in the community setting. J Am Geriatr Soc. 2007;55(1):29-34. PubMed
4. Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA. 2002;287(3):337-344. PubMed
5. Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831. PubMed
6. Thomsen LA, Winterstein AG, Søndergaard B, Haugbølle LS, Melander A. Systematic review of the incidence and characteristics of preventable adverse drug events in ambulatory care. Ann Pharmacother. 2007;41(9):1411-1426. PubMed
7. Zhanel GG, Wiebe R, Dilay L, et al. Comparative review of the carbapenems. Drugs. 2007;67(7):1027-1052. PubMed
8. Cannon JP, Lee TA, Clark NM, Setlak P, Grim SA. The risk of seizures among the carbapenems: a meta-analysis. J Antimicrob Chemother. 2014;69(8):2043-2055. PubMed
9. US Food and Drug Administration. Invanz (ertapenem) injection [safety information]. http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm196605.htm. Published July 2013. Accessed July 6, 2015.
10. Oo Y, Packham D, Yau W, Munckhof WJ. Ertapenem-associated psychosis and encephalopathy. Intern Med J. 2014;44(8):817-819. PubMed
11. Wen MJ, Sung CC, Chau T, Lin SH. Acute prolonged neurotoxicity associated with recommended doses of ertapenem in 2 patients with advanced renal failure. Clin Nephrol. 2013;80(6):474-478. PubMed
12. Duquaine S, Kitchell E, Tate T, Tannen RC, Wickremasinghe IM. Central nervous system toxicity associated with ertapenem use. Ann Pharmacother. 2011;45(1):e6. PubMed
13. Kong V, Beckert L, Awunor-Renner C. A case of beta lactam-induced visual hallucination. N Z Med J. 2009;122(1298):76-77. PubMed
14. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245. PubMed
1. Inouye SK, Fearing MA, Marcantonio ER. Delirium. In: Halter JB, Ouslander JG, Tinetti ME, Studenski S, High KP, Asthana S, eds. Hazzard’s Geriatric Medicine and Gerontology. 6th ed. New York, NY: McGraw-Hill; 2009.
2. Sarkar U, López A, Maselli JH, Gonzales R. Adverse drug events in U.S. adult ambulatory medical care. Health Serv Res. 2011;46(5):1517-1533. PubMed
3. Chrischilles E, Rubenstein L, Van Gilder R, Voelker M, Wright K, Wallace R. Risk factors for adverse drug events in older adults with mobility limitations in the community setting. J Am Geriatr Soc. 2007;55(1):29-34. PubMed
4. Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA. 2002;287(3):337-344. PubMed
5. Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831. PubMed
6. Thomsen LA, Winterstein AG, Søndergaard B, Haugbølle LS, Melander A. Systematic review of the incidence and characteristics of preventable adverse drug events in ambulatory care. Ann Pharmacother. 2007;41(9):1411-1426. PubMed
7. Zhanel GG, Wiebe R, Dilay L, et al. Comparative review of the carbapenems. Drugs. 2007;67(7):1027-1052. PubMed
8. Cannon JP, Lee TA, Clark NM, Setlak P, Grim SA. The risk of seizures among the carbapenems: a meta-analysis. J Antimicrob Chemother. 2014;69(8):2043-2055. PubMed
9. US Food and Drug Administration. Invanz (ertapenem) injection [safety information]. http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm196605.htm. Published July 2013. Accessed July 6, 2015.
10. Oo Y, Packham D, Yau W, Munckhof WJ. Ertapenem-associated psychosis and encephalopathy. Intern Med J. 2014;44(8):817-819. PubMed
11. Wen MJ, Sung CC, Chau T, Lin SH. Acute prolonged neurotoxicity associated with recommended doses of ertapenem in 2 patients with advanced renal failure. Clin Nephrol. 2013;80(6):474-478. PubMed
12. Duquaine S, Kitchell E, Tate T, Tannen RC, Wickremasinghe IM. Central nervous system toxicity associated with ertapenem use. Ann Pharmacother. 2011;45(1):e6. PubMed
13. Kong V, Beckert L, Awunor-Renner C. A case of beta lactam-induced visual hallucination. N Z Med J. 2009;122(1298):76-77. PubMed
14. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245. PubMed
© 2017 Society of Hospital Medicine
Taking the Detour
A 60‐year‐old woman presented to a community hospital's emergency department with 4 days of right‐sided abdominal pain and multiple episodes of black stools. She reported nausea without vomiting. She denied light‐headedness, chest pain, or shortness of breath. She also denied difficulty in swallowing, weight loss, jaundice, or other bleeding.
The first priority when assessing a patient with gastrointestinal (GI) bleeding is to ensure hemodynamic stability. Next, it is important to carefully characterize the stools to help narrow the differential diagnosis. As blood is a cathartic, frequent, loose, and black stools suggest vigorous bleeding. It is essential to establish that the stools are actually black, as some patients will mistake dark brown stools for melena. Using a visual aid like a black pen or shoes as a point of reference can help the patient differentiate between dark stool and melena. It is also important to obtain a thorough medication history because iron supplements or bismuth‐containing remedies can turn stool black. The use of any antiplatelet agents or anticoagulants should also be noted. The right‐sided abdominal pain should be characterized by establishing the frequency, severity, and association with eating, movement, and position. For this patient's presentation, increased pain with eating would rapidly heighten concern for mesenteric ischemia.
The patient reported having 1 to 2 semiformed, tarry, black bowel movements per day. The night prior to admission she had passed some bright red blood along with the melena. The abdominal pain had increased gradually over 4 days, was dull, constant, did not radiate, and there were no evident aggravating or relieving factors. She rated the pain as 4 out of 10 in intensity, worst in her right upper quadrant.
Her past medical history was notable for recurrent deep venous thromboses and pulmonary emboli that had occurred even while on oral anticoagulation. Inferior vena cava (IVC) filters had twice been placed many years prior; anticoagulation had been subsequently discontinued. Additionally, she was known to have chronic superior vena cava (SVC) occlusion, presumably related to hypercoagulability. Previous evaluation had identified only hyperhomocysteinemia as a risk factor for recurrent thromboses. Other medical problems included hemorrhoids, gastroesophageal reflux disease, and asthma. Her only surgical history was an abdominal hysterectomy and bilateral oophorectomy many years ago for nonmalignant disease. Home medications were omeprazole, ranitidine, albuterol, and fluticasone‐salmeterol. She denied using nonsteroidal anti‐inflammatory drugs, aspirin, or any dietary supplements. She denied smoking, alcohol, or recreational drug use.
Because melena is confirmed, an upper GI tract bleeding source is most likely. The more recent appearance of bright red blood is concerning for acceleration of bleeding, or may point to a distal small bowel or right colonic source. Given the history of thromboembolic disease and likely underlying hypercoagulability, vascular occlusion is a leading possibility. Thus, mesenteric arterial insufficiency or mesenteric venous thrombosis should be considered, even though the patient does not report the characteristic postprandial exacerbation of pain. Ischemic colitis due to arterial insufficiency typically presents with severe, acute pain, with or without hematochezia. This syndrome is typically manifested in vascular watershed areas such as the splenic flexure, but can also affect the right colon. Mesenteric venous thrombosis is a rare condition that most often occurs in patients with hypercoagulability. Patients present with variable degrees of abdominal pain and often with GI bleeding. Finally, portal venous thrombosis may be seen alongside thromboses of other mesenteric veins or may occur independently. Portal hypertension due to portal vein thrombosis can result in esophageal and/or gastric varices. Although variceal bleeding classically presents with dramatic hematemesis, the absence of hematemesis does not rule out a variceal bleed in this patient.
On physical examination, the patient had a temperature of 37.1C with a pulse of 90 beats per minute and blood pressure of 161/97 mm Hg. Orthostatics were not performed. No blood was seen on nasal and oropharyngeal exam. Respiratory and cardiovascular exams were normal. On abdominal exam, there was tenderness to palpation of the right upper quadrant without rebound or guarding. The spleen and the liver were not palpable. There was a lower midline incisional scar. Rectal exam revealed nonbleeding hemorrhoids and heme‐positive stool without gross blood. Bilateral lower extremities had trace pitting edema, hyperpigmentation, and superficial venous varicosities. On skin exam, there were distended subcutaneous veins radiating outward from around the umbilicus as well as prominent subcutaneous venous collaterals over the chest and lateral abdomen.
The collateral veins over the chest and lateral abdomen are consistent with central venous obstruction from the patient's known SVC thrombus. However, the presence of paraumbilical venous collaterals (caput medusa) is highly suggestive of portal hypertension. This evidence, in addition to the known central venous occlusion and history of thromboembolic disease, raises the suspicion for mesenteric thrombosis as a cause of her bleeding and pain. The first diagnostic procedure should be an esophagogastroduodenoscopy (EGD) to identify and potentially treat the source of bleeding, whether it is portal hypertension related (portal gastropathy, variceal bleed) or from a more common cause (peptic ulcer disease, stress gastritis). If the EGD is not diagnostic, the next step should be to obtain computed tomography (CT) of the abdomen and pelvis with intravenous (IV) and oral contrast. In many patients with GI bleed, a colonoscopy would typically be performed as the next diagnostic study after EGD. However, in this patient, a CT scan is likely to be of higher yield because it could help assess the mesenteric and portal vessels for patency and characterize the appearance of the small intestine and colon. Depending on the findings of the CT, additional dedicated vascular diagnostics might be needed.
Hemoglobin was 8.5 g/dL (12.4 g/dL 6 weeks prior) with a normal mean corpuscular volume and red cell distribution. The white cell count was normal, and the platelet count was 142,000/mm3. The blood urea nitrogen was 27 mg/dL, with a creatinine of 1.1 mg/dL. Routine chemistries, liver enzymes, bilirubin, and coagulation parameters were normal. Ferritin was 15 ng/mL (normal: 15200 ng/mL).
The patient was admitted to the intensive care unit. An EGD revealed a hiatal hernia and grade II nonbleeding esophageal varices with normal=appearing stomach and duodenum. The varices did not have stigmata of a recent bleed and were not ligated. The patient continued to bleed and received 2 U of packed red blood cells (RBCs), as her hemoglobin had decreased to 7.3 g/dL. On hospital day 3, a colonoscopy was done that showed blood clots in the ascending colon but was otherwise normal. The patient had ongoing abdominal pain, melena, and hematochezia, and continued to require blood transfusions every other day.
Esophageal varices were confirmed on EGD. However, no high‐risk stigmata were seen. Findings that suggest either recent bleeding or are risk factors for subsequent bleeding include large size of the varices, nipple sign referring to a protruding vessel from an underlying varix, or red wale sign, referring to a longitudinal red streak on a varix. The lack of evidence for an esophageal, gastric, or duodenal bleeding source correlates with lack of clinical signs of upper GI tract hemorrhage such as hematemesis or coffee ground emesis. Because the colonoscopy also did not identify a bleeding source, the bleeding remains unexplained. The absence of significant abnormalities in liver function or liver inflammation labs suggests that the patient does not have advanced cirrhosis and supports the suspicion of a vascular cause of the portal hypertension. At this point, it would be most useful to obtain a CT scan of the abdomen and pelvis.
The patient continued to bleed, requiring a total of 7 U of packed RBCs over 7 days. On hospital day 4, a repeat EGD showed nonbleeding varices with a red wale sign that were banded. Despite this, the hemoglobin continued to drop. A technetium‐tagged RBC study showed a small area of subumbilical activity, which appeared to indicate transverse colonic or small bowel bleeding (Figure 1). A subsequent mesenteric angiogram failed to show active bleeding.

A red wale sign confers a higher risk of bleeding from esophageal varices. However, this finding can be subjective, and the endoscopist must individualize the decision for banding based on the size and appearance of the varices. It was reasonable to proceed with banding this time because the varices were large, had a red wale sign, and there was otherwise unexplained ongoing bleeding. Because her hemoglobin continued to drop after the banding and a tagged RBC study best localized the bleeding to the small intestine or transverse colon, it is unlikely that the varices are the primary source of bleeding. It is not surprising that the mesenteric angiogram did not show a source of bleeding, because this study requires active bleeding at a sufficient rate to radiographically identify the source.
The leading diagnosis remains an as yet uncharacterized small bowel bleeding source related to mesenteric thrombotic disease. Cross‐sectional imaging with IV contrast to identify significant vascular occlusion should be the next diagnostic step. Capsule endoscopy would be a more expensive and time‐consuming option, and although this could reveal the source of bleeding, it might not characterize the underlying vascular nature of the problem.
Due to persistent abdominal pain, a CT without intravenous contrast was done on hospital day 10. This showed extensive collateral vessels along the chest and abdominal wall with a distended azygos vein. The study was otherwise unrevealing. Her bloody stools cleared, so she was discharged with a plan for capsule endoscopy and outpatient follow‐up with her gastroenterologist. On the day of discharge (hospital day 11), hemoglobin was 7.5 g/dL and she received an eighth unit of packed RBCs. Overt bleeding was absent.
As an outpatient, intermittent hematochezia and melena recurred. The capsule endoscopy showed active bleeding approximately 45 minutes after the capsule exited the stomach. The lesion was not precisely located or characterized, but was believed to be in the distal small bowel.
The capsule finding supports the growing body of evidence implicating a small bowel source of bleeding. Furthermore, the ongoing but slow rate of blood loss makes a venous bleed more likely than an arterial bleed. A CT scan was performed prior to capsule study, but this was done without intravenous contrast. The brief description of the CT findings emphasizes the subcutaneous venous changes; a contraindication to IV contrast is not mentioned. Certainly IV contrast would have been very helpful to characterize the mesenteric arterial and venous vasculature. If there is no contraindication, a repeat CT scan with IV contrast should be performed. If there is a contraindication to IV contrast, it would be beneficial to revisit the noncontrast study with the specific purpose of searching for clues suggesting mesenteric or portal thrombosis. If the source still remains unclear, the next steps should be to perform push enteroscopy to assess the small intestine from the luminal side and magnetic resonance angiogram with venous phase imaging (or CT venogram if there is no contraindication to contrast) to evaluate the venous circulation.
The patient was readmitted 9 days after discharge with persistent melena and hematochezia. Her hemoglobin was 7.2 g/dL. Given the lack of a diagnosis, the patient was transferred to a tertiary care hospital, where a second colonoscopy and mesenteric angiogram were negative for bleeding. Small bowel enteroscopy showed no source of bleeding up to 60 cm past the pylorus. A third colonoscopy was performed due to recurrent bleeding; this showed a large amount of dark blood and clots throughout the entire colon including the cecum (Figure 2). After copious irrigation, the underlying mucosa was seen to be normal. At this point, a CT angiogram with both venous and arterial phases was done due to the high suspicion for a distal jejunal bleeding source. The CT angiogram showed numerous venous collaterals encasing a loop of midsmall bowel demonstrating progressive submucosal venous enhancement. In addition, a venous collateral ran down the right side of the sternum to the infraumbilical area and drained through the encasing collaterals into the portal venous system (Figure 3). The CT scan also revealed IVC obstruction below the distal IVC filter and an enlarged portal vein measuring 18 mm (normal 12 mm).
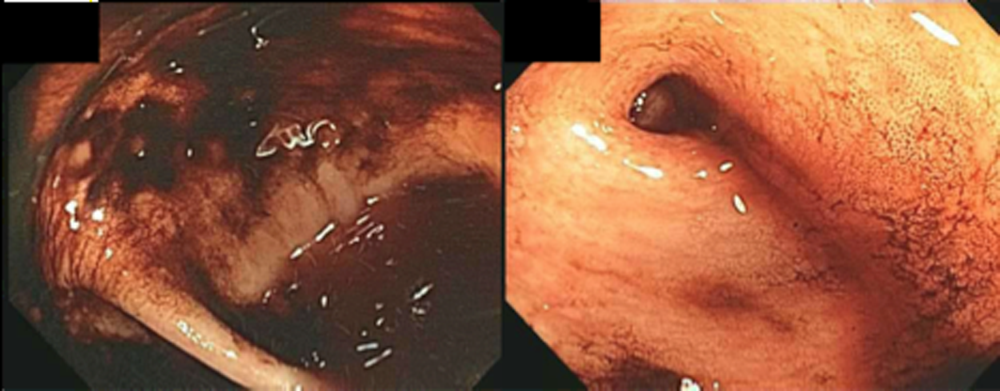
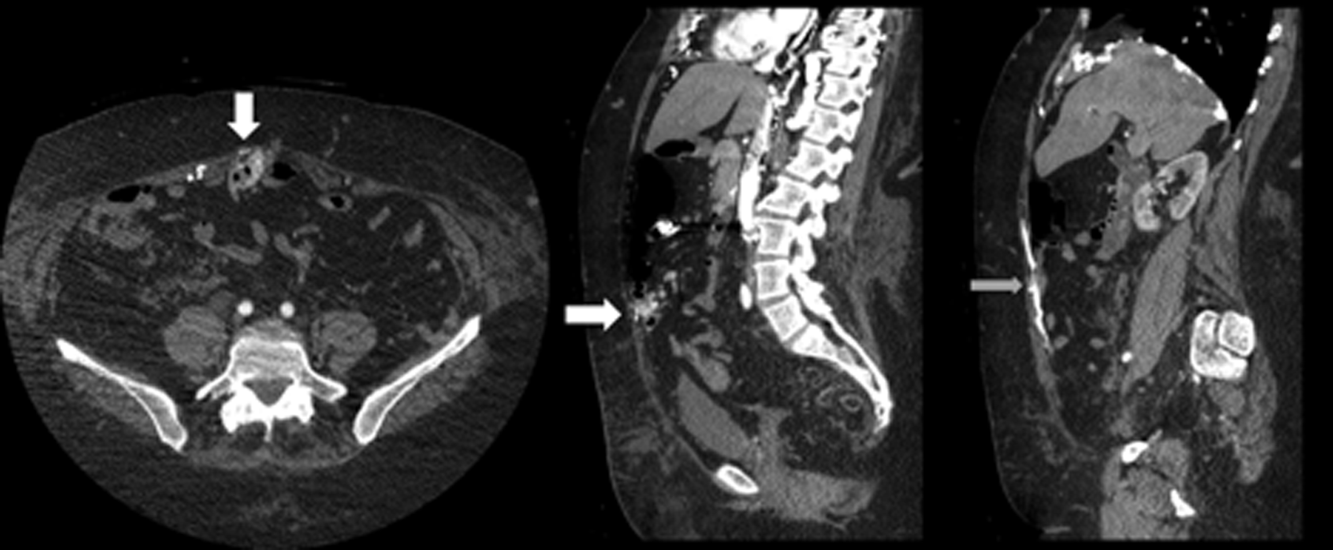
The CT angiogram provides much‐needed clarity. The continued bleeding is likely due to ectopic varices in the small bowel. The venous phase of the CT angiogram shows thrombosis of key venous structures and evidence of a dilated portal vein (indicating portal hypertension) leading to ectopic varices in the abdominal wall and jejunum. Given the prior studies that suggest a small bowel source of bleeding, jejunal varices are the most likely cause of recurrent GI bleeding in this patient.
The patient underwent exploratory laparotomy. Loops of small bowel were found to be adherent to the hysterectomy scar. There were many venous collaterals from the abdominal wall to these loops of bowel, dilating the veins both in intestinal walls and those in the adjacent mesentery. After clamping these veins, the small bowel was detached from the abdominal wall. On unclamping, the collaterals bled with a high venous pressure. Because these systemic‐portal shunts were responsible for the bleeding, the collaterals were sutured, stopping the bleeding. Thus, partial small bowel resection was not necessary. Postoperatively, her bleeding resolved completely and she maintained normal hemoglobin at 1‐year follow‐up.
COMMENTARY
The axiom common ailments are encountered most frequently underpins the classical stepwise approach to GI bleeding. First, a focused history helps localize the source of bleeding to the upper or lower GI tract. Next, endoscopy is performed to identify and treat the cause of bleeding. Finally, advanced tests such as angiography and capsule endoscopy are performed if needed. For this patient, following the usual algorithm failed to make the diagnosis or stop the bleeding. Despite historical and examination features suggesting that her case fell outside of the common patterns of GI bleeding, this patient underwent 3 upper endoscopies, 3 colonoscopies, a capsule endoscopy, a technetium‐tagged RBC study, 2 mesenteric angiograms, and a noncontrast CT scan before the study that was ultimately diagnostic was performed. The clinicians caring for this patient struggled to incorporate the atypical features of her history and presentation and failed to take an earlier detour from the usual algorithm. Instead, the same studies that had not previously led to the diagnosis were repeated multiple times.
Ectopic varices are enlarged portosystemic venous collaterals located anywhere outside the gastroesophageal region.[1] They occur in the setting of portal hypertension, surgical procedures involving abdominal viscera and vasculature, and venous occlusion. Ectopic varices account for 4% to 5% of all variceal bleeding episodes.[1] The most common sites include the anorectal junction (44%), duodenum (17%33%), jejunum/emleum (5%17%), colon (3.5%14%), and sites of previous abdominal surgery.[2, 3] Ectopic varices can cause either luminal or extraluminal (i.e., peritoneal) bleeding.[3] Luminal bleeding, seen in this case, is caused by venous protrusion into the submucosa. Ectopic varices present as a slow venous ooze, which explains this patient's ongoing requirement for recurrent blood transfusions.[4]
In this patient, submucosal ectopic varices developed as a result of a combination of known risk factors: portal hypertension in the setting of chronic venous occlusion from her hypercoagulability and a history of abdominal surgery (hysterectomy). [5] The apposition of her abdominal wall structures (drained by the systemic veins) to the bowel (drained by the portal veins) resulted in adhesion formation, detour of venous flow, collateralization, and submucosal varix formation.[1, 2, 6]
The key diagnostic study for this patient was a CT angiogram, with both arterial and venous phases. The prior 2 mesenteric angiograms had been limited to the arterial phase, which had missed identifying the venous abnormalities altogether. This highlights an important lesson from this case: contrast‐enhanced CT may have a higher yield in diagnosing ectopic varices compared to repeated endoscopiesespecially when captured in the late venous phaseand should strongly be considered for unexplained bleeding in patients with stigmata of liver disease or portal hypertension.[7, 8] Another clue for ectopic varices in a bleeding patient are nonbleeding esophageal or gastric varices, as was the case in this patient.[9]
The initial management of ectopic varices is similar to bleeding secondary to esophageal varices.[1] Definitive treatment includes endoscopic embolization or ligation, interventional radiological procedures such as portosystemic shunting or percutaneous embolization, and exploratory laparotomy to either resect the segment of bowel that is the source of bleeding or to decompress the collaterals surgically.[9] Although endoscopic ligation has been shown to have a lower rebleeding rate and mortality compared to endoscopic injection sclerotherapy in patients with esophageal varices, the data are too sparse in jejunal varices to recommend 1 treatment over another. Both have been used successfully either alone or in combination with each other, and can be useful alternatives for patients who are unable to undergo laparotomy.[9]
Diagnostic errors due to cognitive biases can be avoided by following diagnostic algorithms. However, over‐reliance on algorithms can result in vertical line failure, a form of cognitive bias in which the clinician subconsciously adheres to an inflexible diagnostic approach.[10] To overcome this bias, clinicians need to think laterally and consider alternative diagnoses when algorithms do not lead to expected outcomes. This case highlights the challenges of knowing when to break free of conventional approaches and the rewards of taking a well‐chosen detour that leads to the diagnosis.
KEY POINTS
- Recurrent, occult gastrointestinal bleeding should raise concern for a small bowel source, and clinicians may need to take a detour away from the usual workup to arrive at a diagnosis.
- CT angiography of the abdomen and pelvis may miss venous sources of bleeding, unless a venous phase is specifically requested.
- Ectopic varices can occur in patients with portal hypertension who have had a history of abdominal surgery; these patients can develop venous collaterals for decompression into the systemic circulation through the abdominal wall.
Disclosure
Nothing to report.
- , , . Updates in the pathogenesis, diagnosis and management of ectopic varices. Hepatol Int. 2008;2:322–334.
- , , . Management of ectopic varices. Hepatology. 1998;28:1154–1158.
- , , , et al. Current status of ectopic varices in Japan: results of a survey by the Japan Society for Portal Hypertension. Hepatol Res. 2010;40:763–766.
- , , . Stomal Varices: Management with decompression TIPS and transvenous obliteration or sclerosis. Tech Vasc Interv Radiol. 2013;16:126–134.
- , , , et al. Jejunal varices as a cause of massive gastrointestinal bleeding. Am J Gastroenterol. 1992;87:514–517.
- , . Ectopic varices in portal hypertension. Clin Gastroenterol. 1985;14:105–121.
- , , , et al. Ectopic varices in portal hypertension: computed tomographic angiography instead of repeated endoscopies for diagnosis. Eur J Gastroenterol Hepatol. 2011;23:620–622.
- , , , et al. ACR appropriateness criteria. Radiologic management of lower gastrointestinal tract bleeding. Reston, VA: American College of Radiology; 2011. Available at: http://www.acr.org/Quality‐Safety/Appropriateness‐Criteria/∼/media/5F9CB95C164E4DA19DCBCFBBA790BB3C.pdf. Accessed January 28, 2015.
- , . Diagnosis and management of ectopic varices. Gastrointest Interv. 2012;1:3–10.
- . Achieving quality in clinical decision making: cognitive strategies and detection of bias. Acad Emerg Med. 2002;9:1184–1204.
A 60‐year‐old woman presented to a community hospital's emergency department with 4 days of right‐sided abdominal pain and multiple episodes of black stools. She reported nausea without vomiting. She denied light‐headedness, chest pain, or shortness of breath. She also denied difficulty in swallowing, weight loss, jaundice, or other bleeding.
The first priority when assessing a patient with gastrointestinal (GI) bleeding is to ensure hemodynamic stability. Next, it is important to carefully characterize the stools to help narrow the differential diagnosis. As blood is a cathartic, frequent, loose, and black stools suggest vigorous bleeding. It is essential to establish that the stools are actually black, as some patients will mistake dark brown stools for melena. Using a visual aid like a black pen or shoes as a point of reference can help the patient differentiate between dark stool and melena. It is also important to obtain a thorough medication history because iron supplements or bismuth‐containing remedies can turn stool black. The use of any antiplatelet agents or anticoagulants should also be noted. The right‐sided abdominal pain should be characterized by establishing the frequency, severity, and association with eating, movement, and position. For this patient's presentation, increased pain with eating would rapidly heighten concern for mesenteric ischemia.
The patient reported having 1 to 2 semiformed, tarry, black bowel movements per day. The night prior to admission she had passed some bright red blood along with the melena. The abdominal pain had increased gradually over 4 days, was dull, constant, did not radiate, and there were no evident aggravating or relieving factors. She rated the pain as 4 out of 10 in intensity, worst in her right upper quadrant.
Her past medical history was notable for recurrent deep venous thromboses and pulmonary emboli that had occurred even while on oral anticoagulation. Inferior vena cava (IVC) filters had twice been placed many years prior; anticoagulation had been subsequently discontinued. Additionally, she was known to have chronic superior vena cava (SVC) occlusion, presumably related to hypercoagulability. Previous evaluation had identified only hyperhomocysteinemia as a risk factor for recurrent thromboses. Other medical problems included hemorrhoids, gastroesophageal reflux disease, and asthma. Her only surgical history was an abdominal hysterectomy and bilateral oophorectomy many years ago for nonmalignant disease. Home medications were omeprazole, ranitidine, albuterol, and fluticasone‐salmeterol. She denied using nonsteroidal anti‐inflammatory drugs, aspirin, or any dietary supplements. She denied smoking, alcohol, or recreational drug use.
Because melena is confirmed, an upper GI tract bleeding source is most likely. The more recent appearance of bright red blood is concerning for acceleration of bleeding, or may point to a distal small bowel or right colonic source. Given the history of thromboembolic disease and likely underlying hypercoagulability, vascular occlusion is a leading possibility. Thus, mesenteric arterial insufficiency or mesenteric venous thrombosis should be considered, even though the patient does not report the characteristic postprandial exacerbation of pain. Ischemic colitis due to arterial insufficiency typically presents with severe, acute pain, with or without hematochezia. This syndrome is typically manifested in vascular watershed areas such as the splenic flexure, but can also affect the right colon. Mesenteric venous thrombosis is a rare condition that most often occurs in patients with hypercoagulability. Patients present with variable degrees of abdominal pain and often with GI bleeding. Finally, portal venous thrombosis may be seen alongside thromboses of other mesenteric veins or may occur independently. Portal hypertension due to portal vein thrombosis can result in esophageal and/or gastric varices. Although variceal bleeding classically presents with dramatic hematemesis, the absence of hematemesis does not rule out a variceal bleed in this patient.
On physical examination, the patient had a temperature of 37.1C with a pulse of 90 beats per minute and blood pressure of 161/97 mm Hg. Orthostatics were not performed. No blood was seen on nasal and oropharyngeal exam. Respiratory and cardiovascular exams were normal. On abdominal exam, there was tenderness to palpation of the right upper quadrant without rebound or guarding. The spleen and the liver were not palpable. There was a lower midline incisional scar. Rectal exam revealed nonbleeding hemorrhoids and heme‐positive stool without gross blood. Bilateral lower extremities had trace pitting edema, hyperpigmentation, and superficial venous varicosities. On skin exam, there were distended subcutaneous veins radiating outward from around the umbilicus as well as prominent subcutaneous venous collaterals over the chest and lateral abdomen.
The collateral veins over the chest and lateral abdomen are consistent with central venous obstruction from the patient's known SVC thrombus. However, the presence of paraumbilical venous collaterals (caput medusa) is highly suggestive of portal hypertension. This evidence, in addition to the known central venous occlusion and history of thromboembolic disease, raises the suspicion for mesenteric thrombosis as a cause of her bleeding and pain. The first diagnostic procedure should be an esophagogastroduodenoscopy (EGD) to identify and potentially treat the source of bleeding, whether it is portal hypertension related (portal gastropathy, variceal bleed) or from a more common cause (peptic ulcer disease, stress gastritis). If the EGD is not diagnostic, the next step should be to obtain computed tomography (CT) of the abdomen and pelvis with intravenous (IV) and oral contrast. In many patients with GI bleed, a colonoscopy would typically be performed as the next diagnostic study after EGD. However, in this patient, a CT scan is likely to be of higher yield because it could help assess the mesenteric and portal vessels for patency and characterize the appearance of the small intestine and colon. Depending on the findings of the CT, additional dedicated vascular diagnostics might be needed.
Hemoglobin was 8.5 g/dL (12.4 g/dL 6 weeks prior) with a normal mean corpuscular volume and red cell distribution. The white cell count was normal, and the platelet count was 142,000/mm3. The blood urea nitrogen was 27 mg/dL, with a creatinine of 1.1 mg/dL. Routine chemistries, liver enzymes, bilirubin, and coagulation parameters were normal. Ferritin was 15 ng/mL (normal: 15200 ng/mL).
The patient was admitted to the intensive care unit. An EGD revealed a hiatal hernia and grade II nonbleeding esophageal varices with normal=appearing stomach and duodenum. The varices did not have stigmata of a recent bleed and were not ligated. The patient continued to bleed and received 2 U of packed red blood cells (RBCs), as her hemoglobin had decreased to 7.3 g/dL. On hospital day 3, a colonoscopy was done that showed blood clots in the ascending colon but was otherwise normal. The patient had ongoing abdominal pain, melena, and hematochezia, and continued to require blood transfusions every other day.
Esophageal varices were confirmed on EGD. However, no high‐risk stigmata were seen. Findings that suggest either recent bleeding or are risk factors for subsequent bleeding include large size of the varices, nipple sign referring to a protruding vessel from an underlying varix, or red wale sign, referring to a longitudinal red streak on a varix. The lack of evidence for an esophageal, gastric, or duodenal bleeding source correlates with lack of clinical signs of upper GI tract hemorrhage such as hematemesis or coffee ground emesis. Because the colonoscopy also did not identify a bleeding source, the bleeding remains unexplained. The absence of significant abnormalities in liver function or liver inflammation labs suggests that the patient does not have advanced cirrhosis and supports the suspicion of a vascular cause of the portal hypertension. At this point, it would be most useful to obtain a CT scan of the abdomen and pelvis.
The patient continued to bleed, requiring a total of 7 U of packed RBCs over 7 days. On hospital day 4, a repeat EGD showed nonbleeding varices with a red wale sign that were banded. Despite this, the hemoglobin continued to drop. A technetium‐tagged RBC study showed a small area of subumbilical activity, which appeared to indicate transverse colonic or small bowel bleeding (Figure 1). A subsequent mesenteric angiogram failed to show active bleeding.

A red wale sign confers a higher risk of bleeding from esophageal varices. However, this finding can be subjective, and the endoscopist must individualize the decision for banding based on the size and appearance of the varices. It was reasonable to proceed with banding this time because the varices were large, had a red wale sign, and there was otherwise unexplained ongoing bleeding. Because her hemoglobin continued to drop after the banding and a tagged RBC study best localized the bleeding to the small intestine or transverse colon, it is unlikely that the varices are the primary source of bleeding. It is not surprising that the mesenteric angiogram did not show a source of bleeding, because this study requires active bleeding at a sufficient rate to radiographically identify the source.
The leading diagnosis remains an as yet uncharacterized small bowel bleeding source related to mesenteric thrombotic disease. Cross‐sectional imaging with IV contrast to identify significant vascular occlusion should be the next diagnostic step. Capsule endoscopy would be a more expensive and time‐consuming option, and although this could reveal the source of bleeding, it might not characterize the underlying vascular nature of the problem.
Due to persistent abdominal pain, a CT without intravenous contrast was done on hospital day 10. This showed extensive collateral vessels along the chest and abdominal wall with a distended azygos vein. The study was otherwise unrevealing. Her bloody stools cleared, so she was discharged with a plan for capsule endoscopy and outpatient follow‐up with her gastroenterologist. On the day of discharge (hospital day 11), hemoglobin was 7.5 g/dL and she received an eighth unit of packed RBCs. Overt bleeding was absent.
As an outpatient, intermittent hematochezia and melena recurred. The capsule endoscopy showed active bleeding approximately 45 minutes after the capsule exited the stomach. The lesion was not precisely located or characterized, but was believed to be in the distal small bowel.
The capsule finding supports the growing body of evidence implicating a small bowel source of bleeding. Furthermore, the ongoing but slow rate of blood loss makes a venous bleed more likely than an arterial bleed. A CT scan was performed prior to capsule study, but this was done without intravenous contrast. The brief description of the CT findings emphasizes the subcutaneous venous changes; a contraindication to IV contrast is not mentioned. Certainly IV contrast would have been very helpful to characterize the mesenteric arterial and venous vasculature. If there is no contraindication, a repeat CT scan with IV contrast should be performed. If there is a contraindication to IV contrast, it would be beneficial to revisit the noncontrast study with the specific purpose of searching for clues suggesting mesenteric or portal thrombosis. If the source still remains unclear, the next steps should be to perform push enteroscopy to assess the small intestine from the luminal side and magnetic resonance angiogram with venous phase imaging (or CT venogram if there is no contraindication to contrast) to evaluate the venous circulation.
The patient was readmitted 9 days after discharge with persistent melena and hematochezia. Her hemoglobin was 7.2 g/dL. Given the lack of a diagnosis, the patient was transferred to a tertiary care hospital, where a second colonoscopy and mesenteric angiogram were negative for bleeding. Small bowel enteroscopy showed no source of bleeding up to 60 cm past the pylorus. A third colonoscopy was performed due to recurrent bleeding; this showed a large amount of dark blood and clots throughout the entire colon including the cecum (Figure 2). After copious irrigation, the underlying mucosa was seen to be normal. At this point, a CT angiogram with both venous and arterial phases was done due to the high suspicion for a distal jejunal bleeding source. The CT angiogram showed numerous venous collaterals encasing a loop of midsmall bowel demonstrating progressive submucosal venous enhancement. In addition, a venous collateral ran down the right side of the sternum to the infraumbilical area and drained through the encasing collaterals into the portal venous system (Figure 3). The CT scan also revealed IVC obstruction below the distal IVC filter and an enlarged portal vein measuring 18 mm (normal 12 mm).


The CT angiogram provides much‐needed clarity. The continued bleeding is likely due to ectopic varices in the small bowel. The venous phase of the CT angiogram shows thrombosis of key venous structures and evidence of a dilated portal vein (indicating portal hypertension) leading to ectopic varices in the abdominal wall and jejunum. Given the prior studies that suggest a small bowel source of bleeding, jejunal varices are the most likely cause of recurrent GI bleeding in this patient.
The patient underwent exploratory laparotomy. Loops of small bowel were found to be adherent to the hysterectomy scar. There were many venous collaterals from the abdominal wall to these loops of bowel, dilating the veins both in intestinal walls and those in the adjacent mesentery. After clamping these veins, the small bowel was detached from the abdominal wall. On unclamping, the collaterals bled with a high venous pressure. Because these systemic‐portal shunts were responsible for the bleeding, the collaterals were sutured, stopping the bleeding. Thus, partial small bowel resection was not necessary. Postoperatively, her bleeding resolved completely and she maintained normal hemoglobin at 1‐year follow‐up.
COMMENTARY
The axiom common ailments are encountered most frequently underpins the classical stepwise approach to GI bleeding. First, a focused history helps localize the source of bleeding to the upper or lower GI tract. Next, endoscopy is performed to identify and treat the cause of bleeding. Finally, advanced tests such as angiography and capsule endoscopy are performed if needed. For this patient, following the usual algorithm failed to make the diagnosis or stop the bleeding. Despite historical and examination features suggesting that her case fell outside of the common patterns of GI bleeding, this patient underwent 3 upper endoscopies, 3 colonoscopies, a capsule endoscopy, a technetium‐tagged RBC study, 2 mesenteric angiograms, and a noncontrast CT scan before the study that was ultimately diagnostic was performed. The clinicians caring for this patient struggled to incorporate the atypical features of her history and presentation and failed to take an earlier detour from the usual algorithm. Instead, the same studies that had not previously led to the diagnosis were repeated multiple times.
Ectopic varices are enlarged portosystemic venous collaterals located anywhere outside the gastroesophageal region.[1] They occur in the setting of portal hypertension, surgical procedures involving abdominal viscera and vasculature, and venous occlusion. Ectopic varices account for 4% to 5% of all variceal bleeding episodes.[1] The most common sites include the anorectal junction (44%), duodenum (17%33%), jejunum/emleum (5%17%), colon (3.5%14%), and sites of previous abdominal surgery.[2, 3] Ectopic varices can cause either luminal or extraluminal (i.e., peritoneal) bleeding.[3] Luminal bleeding, seen in this case, is caused by venous protrusion into the submucosa. Ectopic varices present as a slow venous ooze, which explains this patient's ongoing requirement for recurrent blood transfusions.[4]
In this patient, submucosal ectopic varices developed as a result of a combination of known risk factors: portal hypertension in the setting of chronic venous occlusion from her hypercoagulability and a history of abdominal surgery (hysterectomy). [5] The apposition of her abdominal wall structures (drained by the systemic veins) to the bowel (drained by the portal veins) resulted in adhesion formation, detour of venous flow, collateralization, and submucosal varix formation.[1, 2, 6]
The key diagnostic study for this patient was a CT angiogram, with both arterial and venous phases. The prior 2 mesenteric angiograms had been limited to the arterial phase, which had missed identifying the venous abnormalities altogether. This highlights an important lesson from this case: contrast‐enhanced CT may have a higher yield in diagnosing ectopic varices compared to repeated endoscopiesespecially when captured in the late venous phaseand should strongly be considered for unexplained bleeding in patients with stigmata of liver disease or portal hypertension.[7, 8] Another clue for ectopic varices in a bleeding patient are nonbleeding esophageal or gastric varices, as was the case in this patient.[9]
The initial management of ectopic varices is similar to bleeding secondary to esophageal varices.[1] Definitive treatment includes endoscopic embolization or ligation, interventional radiological procedures such as portosystemic shunting or percutaneous embolization, and exploratory laparotomy to either resect the segment of bowel that is the source of bleeding or to decompress the collaterals surgically.[9] Although endoscopic ligation has been shown to have a lower rebleeding rate and mortality compared to endoscopic injection sclerotherapy in patients with esophageal varices, the data are too sparse in jejunal varices to recommend 1 treatment over another. Both have been used successfully either alone or in combination with each other, and can be useful alternatives for patients who are unable to undergo laparotomy.[9]
Diagnostic errors due to cognitive biases can be avoided by following diagnostic algorithms. However, over‐reliance on algorithms can result in vertical line failure, a form of cognitive bias in which the clinician subconsciously adheres to an inflexible diagnostic approach.[10] To overcome this bias, clinicians need to think laterally and consider alternative diagnoses when algorithms do not lead to expected outcomes. This case highlights the challenges of knowing when to break free of conventional approaches and the rewards of taking a well‐chosen detour that leads to the diagnosis.
KEY POINTS
- Recurrent, occult gastrointestinal bleeding should raise concern for a small bowel source, and clinicians may need to take a detour away from the usual workup to arrive at a diagnosis.
- CT angiography of the abdomen and pelvis may miss venous sources of bleeding, unless a venous phase is specifically requested.
- Ectopic varices can occur in patients with portal hypertension who have had a history of abdominal surgery; these patients can develop venous collaterals for decompression into the systemic circulation through the abdominal wall.
Disclosure
Nothing to report.
A 60‐year‐old woman presented to a community hospital's emergency department with 4 days of right‐sided abdominal pain and multiple episodes of black stools. She reported nausea without vomiting. She denied light‐headedness, chest pain, or shortness of breath. She also denied difficulty in swallowing, weight loss, jaundice, or other bleeding.
The first priority when assessing a patient with gastrointestinal (GI) bleeding is to ensure hemodynamic stability. Next, it is important to carefully characterize the stools to help narrow the differential diagnosis. As blood is a cathartic, frequent, loose, and black stools suggest vigorous bleeding. It is essential to establish that the stools are actually black, as some patients will mistake dark brown stools for melena. Using a visual aid like a black pen or shoes as a point of reference can help the patient differentiate between dark stool and melena. It is also important to obtain a thorough medication history because iron supplements or bismuth‐containing remedies can turn stool black. The use of any antiplatelet agents or anticoagulants should also be noted. The right‐sided abdominal pain should be characterized by establishing the frequency, severity, and association with eating, movement, and position. For this patient's presentation, increased pain with eating would rapidly heighten concern for mesenteric ischemia.
The patient reported having 1 to 2 semiformed, tarry, black bowel movements per day. The night prior to admission she had passed some bright red blood along with the melena. The abdominal pain had increased gradually over 4 days, was dull, constant, did not radiate, and there were no evident aggravating or relieving factors. She rated the pain as 4 out of 10 in intensity, worst in her right upper quadrant.
Her past medical history was notable for recurrent deep venous thromboses and pulmonary emboli that had occurred even while on oral anticoagulation. Inferior vena cava (IVC) filters had twice been placed many years prior; anticoagulation had been subsequently discontinued. Additionally, she was known to have chronic superior vena cava (SVC) occlusion, presumably related to hypercoagulability. Previous evaluation had identified only hyperhomocysteinemia as a risk factor for recurrent thromboses. Other medical problems included hemorrhoids, gastroesophageal reflux disease, and asthma. Her only surgical history was an abdominal hysterectomy and bilateral oophorectomy many years ago for nonmalignant disease. Home medications were omeprazole, ranitidine, albuterol, and fluticasone‐salmeterol. She denied using nonsteroidal anti‐inflammatory drugs, aspirin, or any dietary supplements. She denied smoking, alcohol, or recreational drug use.
Because melena is confirmed, an upper GI tract bleeding source is most likely. The more recent appearance of bright red blood is concerning for acceleration of bleeding, or may point to a distal small bowel or right colonic source. Given the history of thromboembolic disease and likely underlying hypercoagulability, vascular occlusion is a leading possibility. Thus, mesenteric arterial insufficiency or mesenteric venous thrombosis should be considered, even though the patient does not report the characteristic postprandial exacerbation of pain. Ischemic colitis due to arterial insufficiency typically presents with severe, acute pain, with or without hematochezia. This syndrome is typically manifested in vascular watershed areas such as the splenic flexure, but can also affect the right colon. Mesenteric venous thrombosis is a rare condition that most often occurs in patients with hypercoagulability. Patients present with variable degrees of abdominal pain and often with GI bleeding. Finally, portal venous thrombosis may be seen alongside thromboses of other mesenteric veins or may occur independently. Portal hypertension due to portal vein thrombosis can result in esophageal and/or gastric varices. Although variceal bleeding classically presents with dramatic hematemesis, the absence of hematemesis does not rule out a variceal bleed in this patient.
On physical examination, the patient had a temperature of 37.1C with a pulse of 90 beats per minute and blood pressure of 161/97 mm Hg. Orthostatics were not performed. No blood was seen on nasal and oropharyngeal exam. Respiratory and cardiovascular exams were normal. On abdominal exam, there was tenderness to palpation of the right upper quadrant without rebound or guarding. The spleen and the liver were not palpable. There was a lower midline incisional scar. Rectal exam revealed nonbleeding hemorrhoids and heme‐positive stool without gross blood. Bilateral lower extremities had trace pitting edema, hyperpigmentation, and superficial venous varicosities. On skin exam, there were distended subcutaneous veins radiating outward from around the umbilicus as well as prominent subcutaneous venous collaterals over the chest and lateral abdomen.
The collateral veins over the chest and lateral abdomen are consistent with central venous obstruction from the patient's known SVC thrombus. However, the presence of paraumbilical venous collaterals (caput medusa) is highly suggestive of portal hypertension. This evidence, in addition to the known central venous occlusion and history of thromboembolic disease, raises the suspicion for mesenteric thrombosis as a cause of her bleeding and pain. The first diagnostic procedure should be an esophagogastroduodenoscopy (EGD) to identify and potentially treat the source of bleeding, whether it is portal hypertension related (portal gastropathy, variceal bleed) or from a more common cause (peptic ulcer disease, stress gastritis). If the EGD is not diagnostic, the next step should be to obtain computed tomography (CT) of the abdomen and pelvis with intravenous (IV) and oral contrast. In many patients with GI bleed, a colonoscopy would typically be performed as the next diagnostic study after EGD. However, in this patient, a CT scan is likely to be of higher yield because it could help assess the mesenteric and portal vessels for patency and characterize the appearance of the small intestine and colon. Depending on the findings of the CT, additional dedicated vascular diagnostics might be needed.
Hemoglobin was 8.5 g/dL (12.4 g/dL 6 weeks prior) with a normal mean corpuscular volume and red cell distribution. The white cell count was normal, and the platelet count was 142,000/mm3. The blood urea nitrogen was 27 mg/dL, with a creatinine of 1.1 mg/dL. Routine chemistries, liver enzymes, bilirubin, and coagulation parameters were normal. Ferritin was 15 ng/mL (normal: 15200 ng/mL).
The patient was admitted to the intensive care unit. An EGD revealed a hiatal hernia and grade II nonbleeding esophageal varices with normal=appearing stomach and duodenum. The varices did not have stigmata of a recent bleed and were not ligated. The patient continued to bleed and received 2 U of packed red blood cells (RBCs), as her hemoglobin had decreased to 7.3 g/dL. On hospital day 3, a colonoscopy was done that showed blood clots in the ascending colon but was otherwise normal. The patient had ongoing abdominal pain, melena, and hematochezia, and continued to require blood transfusions every other day.
Esophageal varices were confirmed on EGD. However, no high‐risk stigmata were seen. Findings that suggest either recent bleeding or are risk factors for subsequent bleeding include large size of the varices, nipple sign referring to a protruding vessel from an underlying varix, or red wale sign, referring to a longitudinal red streak on a varix. The lack of evidence for an esophageal, gastric, or duodenal bleeding source correlates with lack of clinical signs of upper GI tract hemorrhage such as hematemesis or coffee ground emesis. Because the colonoscopy also did not identify a bleeding source, the bleeding remains unexplained. The absence of significant abnormalities in liver function or liver inflammation labs suggests that the patient does not have advanced cirrhosis and supports the suspicion of a vascular cause of the portal hypertension. At this point, it would be most useful to obtain a CT scan of the abdomen and pelvis.
The patient continued to bleed, requiring a total of 7 U of packed RBCs over 7 days. On hospital day 4, a repeat EGD showed nonbleeding varices with a red wale sign that were banded. Despite this, the hemoglobin continued to drop. A technetium‐tagged RBC study showed a small area of subumbilical activity, which appeared to indicate transverse colonic or small bowel bleeding (Figure 1). A subsequent mesenteric angiogram failed to show active bleeding.

A red wale sign confers a higher risk of bleeding from esophageal varices. However, this finding can be subjective, and the endoscopist must individualize the decision for banding based on the size and appearance of the varices. It was reasonable to proceed with banding this time because the varices were large, had a red wale sign, and there was otherwise unexplained ongoing bleeding. Because her hemoglobin continued to drop after the banding and a tagged RBC study best localized the bleeding to the small intestine or transverse colon, it is unlikely that the varices are the primary source of bleeding. It is not surprising that the mesenteric angiogram did not show a source of bleeding, because this study requires active bleeding at a sufficient rate to radiographically identify the source.
The leading diagnosis remains an as yet uncharacterized small bowel bleeding source related to mesenteric thrombotic disease. Cross‐sectional imaging with IV contrast to identify significant vascular occlusion should be the next diagnostic step. Capsule endoscopy would be a more expensive and time‐consuming option, and although this could reveal the source of bleeding, it might not characterize the underlying vascular nature of the problem.
Due to persistent abdominal pain, a CT without intravenous contrast was done on hospital day 10. This showed extensive collateral vessels along the chest and abdominal wall with a distended azygos vein. The study was otherwise unrevealing. Her bloody stools cleared, so she was discharged with a plan for capsule endoscopy and outpatient follow‐up with her gastroenterologist. On the day of discharge (hospital day 11), hemoglobin was 7.5 g/dL and she received an eighth unit of packed RBCs. Overt bleeding was absent.
As an outpatient, intermittent hematochezia and melena recurred. The capsule endoscopy showed active bleeding approximately 45 minutes after the capsule exited the stomach. The lesion was not precisely located or characterized, but was believed to be in the distal small bowel.
The capsule finding supports the growing body of evidence implicating a small bowel source of bleeding. Furthermore, the ongoing but slow rate of blood loss makes a venous bleed more likely than an arterial bleed. A CT scan was performed prior to capsule study, but this was done without intravenous contrast. The brief description of the CT findings emphasizes the subcutaneous venous changes; a contraindication to IV contrast is not mentioned. Certainly IV contrast would have been very helpful to characterize the mesenteric arterial and venous vasculature. If there is no contraindication, a repeat CT scan with IV contrast should be performed. If there is a contraindication to IV contrast, it would be beneficial to revisit the noncontrast study with the specific purpose of searching for clues suggesting mesenteric or portal thrombosis. If the source still remains unclear, the next steps should be to perform push enteroscopy to assess the small intestine from the luminal side and magnetic resonance angiogram with venous phase imaging (or CT venogram if there is no contraindication to contrast) to evaluate the venous circulation.
The patient was readmitted 9 days after discharge with persistent melena and hematochezia. Her hemoglobin was 7.2 g/dL. Given the lack of a diagnosis, the patient was transferred to a tertiary care hospital, where a second colonoscopy and mesenteric angiogram were negative for bleeding. Small bowel enteroscopy showed no source of bleeding up to 60 cm past the pylorus. A third colonoscopy was performed due to recurrent bleeding; this showed a large amount of dark blood and clots throughout the entire colon including the cecum (Figure 2). After copious irrigation, the underlying mucosa was seen to be normal. At this point, a CT angiogram with both venous and arterial phases was done due to the high suspicion for a distal jejunal bleeding source. The CT angiogram showed numerous venous collaterals encasing a loop of midsmall bowel demonstrating progressive submucosal venous enhancement. In addition, a venous collateral ran down the right side of the sternum to the infraumbilical area and drained through the encasing collaterals into the portal venous system (Figure 3). The CT scan also revealed IVC obstruction below the distal IVC filter and an enlarged portal vein measuring 18 mm (normal 12 mm).


The CT angiogram provides much‐needed clarity. The continued bleeding is likely due to ectopic varices in the small bowel. The venous phase of the CT angiogram shows thrombosis of key venous structures and evidence of a dilated portal vein (indicating portal hypertension) leading to ectopic varices in the abdominal wall and jejunum. Given the prior studies that suggest a small bowel source of bleeding, jejunal varices are the most likely cause of recurrent GI bleeding in this patient.
The patient underwent exploratory laparotomy. Loops of small bowel were found to be adherent to the hysterectomy scar. There were many venous collaterals from the abdominal wall to these loops of bowel, dilating the veins both in intestinal walls and those in the adjacent mesentery. After clamping these veins, the small bowel was detached from the abdominal wall. On unclamping, the collaterals bled with a high venous pressure. Because these systemic‐portal shunts were responsible for the bleeding, the collaterals were sutured, stopping the bleeding. Thus, partial small bowel resection was not necessary. Postoperatively, her bleeding resolved completely and she maintained normal hemoglobin at 1‐year follow‐up.
COMMENTARY
The axiom common ailments are encountered most frequently underpins the classical stepwise approach to GI bleeding. First, a focused history helps localize the source of bleeding to the upper or lower GI tract. Next, endoscopy is performed to identify and treat the cause of bleeding. Finally, advanced tests such as angiography and capsule endoscopy are performed if needed. For this patient, following the usual algorithm failed to make the diagnosis or stop the bleeding. Despite historical and examination features suggesting that her case fell outside of the common patterns of GI bleeding, this patient underwent 3 upper endoscopies, 3 colonoscopies, a capsule endoscopy, a technetium‐tagged RBC study, 2 mesenteric angiograms, and a noncontrast CT scan before the study that was ultimately diagnostic was performed. The clinicians caring for this patient struggled to incorporate the atypical features of her history and presentation and failed to take an earlier detour from the usual algorithm. Instead, the same studies that had not previously led to the diagnosis were repeated multiple times.
Ectopic varices are enlarged portosystemic venous collaterals located anywhere outside the gastroesophageal region.[1] They occur in the setting of portal hypertension, surgical procedures involving abdominal viscera and vasculature, and venous occlusion. Ectopic varices account for 4% to 5% of all variceal bleeding episodes.[1] The most common sites include the anorectal junction (44%), duodenum (17%33%), jejunum/emleum (5%17%), colon (3.5%14%), and sites of previous abdominal surgery.[2, 3] Ectopic varices can cause either luminal or extraluminal (i.e., peritoneal) bleeding.[3] Luminal bleeding, seen in this case, is caused by venous protrusion into the submucosa. Ectopic varices present as a slow venous ooze, which explains this patient's ongoing requirement for recurrent blood transfusions.[4]
In this patient, submucosal ectopic varices developed as a result of a combination of known risk factors: portal hypertension in the setting of chronic venous occlusion from her hypercoagulability and a history of abdominal surgery (hysterectomy). [5] The apposition of her abdominal wall structures (drained by the systemic veins) to the bowel (drained by the portal veins) resulted in adhesion formation, detour of venous flow, collateralization, and submucosal varix formation.[1, 2, 6]
The key diagnostic study for this patient was a CT angiogram, with both arterial and venous phases. The prior 2 mesenteric angiograms had been limited to the arterial phase, which had missed identifying the venous abnormalities altogether. This highlights an important lesson from this case: contrast‐enhanced CT may have a higher yield in diagnosing ectopic varices compared to repeated endoscopiesespecially when captured in the late venous phaseand should strongly be considered for unexplained bleeding in patients with stigmata of liver disease or portal hypertension.[7, 8] Another clue for ectopic varices in a bleeding patient are nonbleeding esophageal or gastric varices, as was the case in this patient.[9]
The initial management of ectopic varices is similar to bleeding secondary to esophageal varices.[1] Definitive treatment includes endoscopic embolization or ligation, interventional radiological procedures such as portosystemic shunting or percutaneous embolization, and exploratory laparotomy to either resect the segment of bowel that is the source of bleeding or to decompress the collaterals surgically.[9] Although endoscopic ligation has been shown to have a lower rebleeding rate and mortality compared to endoscopic injection sclerotherapy in patients with esophageal varices, the data are too sparse in jejunal varices to recommend 1 treatment over another. Both have been used successfully either alone or in combination with each other, and can be useful alternatives for patients who are unable to undergo laparotomy.[9]
Diagnostic errors due to cognitive biases can be avoided by following diagnostic algorithms. However, over‐reliance on algorithms can result in vertical line failure, a form of cognitive bias in which the clinician subconsciously adheres to an inflexible diagnostic approach.[10] To overcome this bias, clinicians need to think laterally and consider alternative diagnoses when algorithms do not lead to expected outcomes. This case highlights the challenges of knowing when to break free of conventional approaches and the rewards of taking a well‐chosen detour that leads to the diagnosis.
KEY POINTS
- Recurrent, occult gastrointestinal bleeding should raise concern for a small bowel source, and clinicians may need to take a detour away from the usual workup to arrive at a diagnosis.
- CT angiography of the abdomen and pelvis may miss venous sources of bleeding, unless a venous phase is specifically requested.
- Ectopic varices can occur in patients with portal hypertension who have had a history of abdominal surgery; these patients can develop venous collaterals for decompression into the systemic circulation through the abdominal wall.
Disclosure
Nothing to report.
- , , . Updates in the pathogenesis, diagnosis and management of ectopic varices. Hepatol Int. 2008;2:322–334.
- , , . Management of ectopic varices. Hepatology. 1998;28:1154–1158.
- , , , et al. Current status of ectopic varices in Japan: results of a survey by the Japan Society for Portal Hypertension. Hepatol Res. 2010;40:763–766.
- , , . Stomal Varices: Management with decompression TIPS and transvenous obliteration or sclerosis. Tech Vasc Interv Radiol. 2013;16:126–134.
- , , , et al. Jejunal varices as a cause of massive gastrointestinal bleeding. Am J Gastroenterol. 1992;87:514–517.
- , . Ectopic varices in portal hypertension. Clin Gastroenterol. 1985;14:105–121.
- , , , et al. Ectopic varices in portal hypertension: computed tomographic angiography instead of repeated endoscopies for diagnosis. Eur J Gastroenterol Hepatol. 2011;23:620–622.
- , , , et al. ACR appropriateness criteria. Radiologic management of lower gastrointestinal tract bleeding. Reston, VA: American College of Radiology; 2011. Available at: http://www.acr.org/Quality‐Safety/Appropriateness‐Criteria/∼/media/5F9CB95C164E4DA19DCBCFBBA790BB3C.pdf. Accessed January 28, 2015.
- , . Diagnosis and management of ectopic varices. Gastrointest Interv. 2012;1:3–10.
- . Achieving quality in clinical decision making: cognitive strategies and detection of bias. Acad Emerg Med. 2002;9:1184–1204.
- , , . Updates in the pathogenesis, diagnosis and management of ectopic varices. Hepatol Int. 2008;2:322–334.
- , , . Management of ectopic varices. Hepatology. 1998;28:1154–1158.
- , , , et al. Current status of ectopic varices in Japan: results of a survey by the Japan Society for Portal Hypertension. Hepatol Res. 2010;40:763–766.
- , , . Stomal Varices: Management with decompression TIPS and transvenous obliteration or sclerosis. Tech Vasc Interv Radiol. 2013;16:126–134.
- , , , et al. Jejunal varices as a cause of massive gastrointestinal bleeding. Am J Gastroenterol. 1992;87:514–517.
- , . Ectopic varices in portal hypertension. Clin Gastroenterol. 1985;14:105–121.
- , , , et al. Ectopic varices in portal hypertension: computed tomographic angiography instead of repeated endoscopies for diagnosis. Eur J Gastroenterol Hepatol. 2011;23:620–622.
- , , , et al. ACR appropriateness criteria. Radiologic management of lower gastrointestinal tract bleeding. Reston, VA: American College of Radiology; 2011. Available at: http://www.acr.org/Quality‐Safety/Appropriateness‐Criteria/∼/media/5F9CB95C164E4DA19DCBCFBBA790BB3C.pdf. Accessed January 28, 2015.
- , . Diagnosis and management of ectopic varices. Gastrointest Interv. 2012;1:3–10.
- . Achieving quality in clinical decision making: cognitive strategies and detection of bias. Acad Emerg Med. 2002;9:1184–1204.
Structured Peer Observation of Teaching
Hospitalists are increasingly responsible for educating students and housestaff in internal medicine.[1] Because the quality of teaching is an important factor in learning,[2, 3, 4] leaders in medical education have expressed concern over the rapid shift of teaching responsibilities to this new group of educators.[5, 6, 7, 8] Moreover, recent changes in duty hour restrictions have strained both student and resident education,[9, 10] necessitating the optimization of inpatient teaching.[11, 12] Many hospitalists have recently finished residency and have not had formal training in clinical teaching. Collectively, most hospital medicine groups are early in their careers, have significant clinical obligations,[13] and may not have the bandwidth or expertise to provide faculty development for improving clinical teaching.
Rationally designed and theoretically sound faculty development to improve inpatient clinical teaching is required to meet this challenge. There are a limited number of reports describing faculty development focused on strengthening the teaching of hospitalists, and only 3 utilized direct observation and feedback, 1 of which involved peer observation in the clinical setting.[14, 15, 16] This 2011 report described a narrative method of peer observation and feedback but did not assess for efficacy of the program.[16] To our knowledge, there have been no studies of structured peer observation and feedback to optimize hospitalist attendings' teaching which have evaluated the efficacy of the intervention.
We developed a faculty development program based on peer observation and feedback based on actual teaching practices, using structured feedback anchored in validated and observable measures of effective teaching. We hypothesized that participation in the program would increase confidence in key teaching skills, increase confidence in the ability to give and receive peer feedback, and strengthen attitudes toward peer observation and feedback.
METHODS
Subjects and Setting
The study was conducted at a 570‐bed academic, tertiary care medical center affiliated with an internal medicine residency program of 180 housestaff. Internal medicine ward attendings rotate during 2‐week blocks, and are asked to give formal teaching rounds 3 or 4 times a week (these sessions are distinct from teaching which may happen while rounding on patients). Ward teams are composed of 1 senior resident, 2 interns, and 1 to 2 medical students. The majority of internal medicine ward attendings are hospitalist faculty, hospital medicine fellows, or medicine chief residents. Because outpatient general internists and subspecialists only occasionally attend on the wards, we refer to ward attendings as attending hospitalists in this article. All attending hospitalists were eligible to participate if they attended on the wards at least twice during the academic year. The institutional review board at the University of California, San Francisco approved this study.
Theoretical Framework
We reviewed the literature to optimize our program in 3 conceptual domains: (1) overall structure of the program, (2) definition of effective teaching and (3) effective delivery of feedback.
Over‐reliance on didactics that are disconnected from the work environment is a weakness of traditional faculty development. Individuals may attempt to apply what they have learned, but receiving feedback on their actual workplace practices may be difficult. A recent perspective responds to this fragmentation by conceptualizing faculty development as embedded in both a faculty development community and a workplace community. This model emphasizes translating what faculty have learned in the classroom into practice, and highlights the importance of coaching in the workplace.[17] In accordance with this framework, we designed our program to reach beyond isolated workshops to effectively penetrate the workplace community.
We selected the Stanford Faculty Development Program (SFDP) framework for optimal clinical teaching as our model for recognizing and improving teaching skills. The SFDP was developed as a theory‐based intensive feedback method to improve teaching skills,[18, 19] and has been shown to improve teaching in the ambulatory[20] and inpatient settings.[21, 22] In this widely disseminated framework,[23, 24] excellent clinical teaching is grounded in optimizing observable behaviors organized around 7 domains.[18] A 26‐item instrument to evaluate clinical teaching (SFDP‐26) has been developed based on this framework[25] and has been validated in multiple settings.[26, 27] High‐quality teaching, as defined by the SFDP framework, has been correlated with improved educational outcomes in internal medicine clerkship students.[4]
Feedback is crucial to optimizing teaching,[28, 29, 30] particularly when it incorporates consultation[31] and narrative comments.[32] Peer feedback has several advantages over feedback from learners or from other non‐peer observers (such as supervisors or other evaluators). First, the observers benefit by gaining insight into their own weaknesses and potential areas for growth as teachers.[33, 34] Additionally, collegial observation and feedback may promote supportive teaching relationships between faculty.[35] Furthermore, peer review overcomes the biases that may be present in learner evaluations.[36] We established a 3‐stage feedback technique based on a previously described method.[37] In the first step, the observer elicits self‐appraisal from the speaker. Next, the observer provides specific, behaviorally anchored feedback in the form of 3 reinforcing comments and 2 constructive comments. Finally, the observer elicits a reflection on the feedback and helps develop a plan to improve teaching in future opportunities. We used a dyad model (paired participants repeatedly observe and give feedback to each other) to support mutual benefit and reciprocity between attendings.
Intervention
Using a modified Delphi approach, 5 medical education experts selected the 10 items that are most easily observable and salient to formal attending teaching rounds from the SFDP‐26 teaching assessment tool. A structured observation form was created, which included a checklist of the 10 selected items, space for note taking, and a template for narrative feedback (Figure 1).
We introduced the SFDP framework during a 2‐hour initial training session. Participants watched videos of teaching, learned to identify the 10 selected teaching behaviors, developed appropriate constructive and reinforcing comments, and practiced giving and receiving peer feedback.
Dyads were created on the basis of predetermined attending schedules. Participants were asked to observe and be observed twice during attending teaching rounds over the course of the academic year. Attending teaching rounds were defined as any preplanned didactic activity for ward teams. The structured observation forms were returned to the study coordinators after the observer had given feedback to the presenter. A copy of the feedback without the observer's notes was also given to each speaker. At the midpoint of the academic year, a refresher session was offered to reinforce those teaching behaviors that were the least frequently performed to date. All participants received a $50.00
Measurements and Data Collection
Participants were given a pre‐ and post‐program survey. The surveys included questions assessing confidence in ability to give feedback, receive feedback without feeling defensive, and teach effectively, as well as attitudes toward peer observation. The postprogram survey was administered at the end of the year and additionally assessed the self‐rated performance of the 10 selected teaching behaviors. A retrospective pre‐ and post‐program assessment was used for this outcome, because this method can be more reliable when participants initially may not have sufficient insight to accurately assess their own competence in specific measures.[21] The post‐program survey also included 4 questions assessing satisfaction with aspects of the program. All questions were structured as statements to which the respondent indicated degree of agreement using a 5‐point Likert scale, where 1=strongly disagree and 5=strongly agree. Structured observation forms used by participants were collected throughout the year to assess frequency of performance of the 10 selected teaching behaviors.
Statistical Analysis
We only analyzed the pre‐ and post‐program surveys that could be matched using anonymous identifiers provided by participants. For both prospective and retrospective measures, mean values and standard deviations were calculated. Wilcoxon signed rank tests for nonparametric data were performed to obtain P values. For all comparisons, a P value of <0.05 was considered significant. All comparisons were performed using Stata version 10 (StataCorp, College Station, TX).
RESULTS
Participant Characteristics and Participation in Program
Of the 37 eligible attending hospitalists, 22 (59%) enrolled. Fourteen were hospital medicine faculty, 6 were hospital medicine fellows, and 2 were internal medicine chief residents. The averagestandard deviation (SD) number of years as a ward attending was 2.2 years2.1. Seventeen (77%) reported previously having been observed and given feedback by a colleague, and 9 (41%) reported previously observing a colleague for the purpose of giving feedback.
All 22 participants attended 1 of 2, 2‐hour training sessions. Ten participants attended an hour‐long midyear refresher session. A total of 19 observation and feedback sessions took place; 15 of them occurred in the first half of the academic year. Fifteen attending hospitalists participated in at least 1 observed teaching session. Of the 11 dyads, 6 completed at least 1 observation of each other. Two dyads performed 2 observations of each other.
Fifteen participants (68% of those enrolled) completed both the pre‐ and post‐program surveys. Among these respondents, the average number of years attending was 2.92.2 years. Eight (53%) reported previously having been observed and given feedback by a colleague, and 7 (47%) reported previously observing a colleague for the purpose of giving feedback. For this subset of participants, the averageSD frequency of being observed during the program was 1.30.7, and observing was 1.10.8.
Confidence in Ability to Give Feedback, Receive Feedback, and Teach Effectively
In comparison of pre‐ and post‐intervention measures, participants indicated increased confidence in their ability to evaluate their colleagues and provide feedback in all domains queried. Participants also indicated increased confidence in the efficacy of their feedback to improve their colleagues' teaching skills. Participating in the program did not significantly change pre‐intervention levels of confidence in ability to receive feedback without being defensive or confidence in ability to use feedback to improve teaching skills (Table 1).
| Statement | Mean Pre | SD | Mean Post | SD | P |
|---|---|---|---|---|---|
| |||||
| I can accurately assess my colleagues' teaching skills. | 3.20 | 0.86 | 4.07 | 0.59 | 0.004 |
| I can give accurate feedback to my colleagues regarding their teaching skills. | 3.40 | 0.63 | 4.20 | 0.56 | 0.002 |
| I can give feedback in a way that that my colleague will not feel defensive about their teaching skills. | 3.60 | 0.63 | 4.20 | 0.56 | 0.046 |
| My feedback will improve my colleagues' teaching skills. | 3.40 | 0.51 | 3.93 | 0.59 | 0.011 |
| I can receive feedback from a colleague without being defensive about my teaching skills. | 3.87 | 0.92 | 4.27 | 0.59 | 0.156 |
| I can use feedback from a colleague to improve my teaching skills. | 4.33 | 0.82 | 4.47 | 0.64 | 0.607 |
| I am confident in my ability to teach students and residents during attending rounds.a | 3.21 | 0.89 | 3.71 | 0.83 | 0.026 |
| I am confident in my knowledge of components of effective teaching.a | 3.21 | 0.89 | 3.71 | 0.99 | 0.035 |
| Learners regard me as an effective teacher.a | 3.14 | 0.66 | 3.64 | 0.74 | 0.033 |
Self‐Rated Performance of 10 Selected Teaching Behaviors
In retrospective assessment, participants felt that their performance had improved in all 10 teaching behaviors after the intervention. This perceived improvement reached statistical significance in 8 of the 10 selected behaviors (Table 2).
| SFDP Framework Category From Skeff et al.[18] | When I Give Attending Rounds, I Generally . | Mean Pre | SD | Mean Post | SD | P |
|---|---|---|---|---|---|---|
| ||||||
| 1. Establishing a positive learning climate | Listen to learners | 4.27 | 0.59 | 4.53 | 0.52 | 0.046 |
| Encourage learners to participate actively in the discussion | 4.07 | 0.70 | 4.60 | 0.51 | 0.009 | |
| 2. Controlling the teaching session | Call attention to time | 3.33 | 0.98 | 4.27 | 0.59 | 0.004 |
| 3. Communicating goals | State goals clearly and concisely | 3.40 | 0.63 | 4.27 | 0.59 | 0.001 |
| State relevance of goals to learners | 3.40 | 0.74 | 4.20 | 0.68 | 0.002 | |
| 4. Promoting understanding and retention | Present well‐organized material | 3.87 | 0.64 | 4.07 | 0.70 | 0.083 |
| Use blackboard or other visual aids | 4.27 | 0.88 | 4.47 | 0.74 | 0.158 | |
| 5. Evaluating the learners | Evaluate learners' ability to apply medical knowledge to specific patients | 3.33 | 0.98 | 4.00 | 0.76 | 0.005 |
| 6. Providing feedback to the learners | Explain to learners why he/she was correct or incorrect | 3.47 | 1.13 | 4.13 | 0.64 | 0.009 |
| 7. Promoting self‐directed learning | Motivate learners to learn on their own | 3.20 | 0.86 | 3.73 | 0.70 | 0.005 |
Attitudes Toward Peer Observation and Feedback
There were no significant changes in attitudes toward observation and feedback on teaching. A strong preprogram belief that observation and feedback can improve teaching skills increased slightly, but not significantly, after the program. Participants remained largely neutral in expectation of discomfort with giving or receiving peer feedback. Prior to the program, there was a slight tendency to believe that observation and feedback is more effective when done by more skilled and experienced colleagues; this belief diminished, but not significantly (Table 3).
| Statement | Mean Pre | SD | Mean Post | SD | P |
|---|---|---|---|---|---|
| |||||
| Being observed and receiving feedback can improve my teaching skills. | 4.47 | 1.06 | 4.60 | 0.51 | 0.941 |
| My teaching skills cannot improve without observation with feedback. | 2.93 | 1.39 | 3.47 | 1.30 | 0.188 |
| Observation with feedback is most effective when done by colleagues who are expert educators. | 3.53 | 0.83 | 3.33 | 0.98 | 0.180 |
| Observation with feedback is most effective when done by colleagues who have been teaching many years. | 3.40 | 0.91 | 3.07 | 1.03 | 0.143 |
| The thought of observing and giving feedback to my colleagues makes me uncomfortable. | 3.13 | 0.92 | 3.00 | 1.13 | 0.565 |
| The thought of being observed by a colleague and receiving feedback makes me uncomfortable. | 3.20 | 0.94 | 3.27 | 1.22 | 0.747 |
Program Evaluation
There were a variable number of responses to the program evaluation questions. The majority of participants found the program to be very beneficial (1=strongly disagree, 5=strongly agree [n, meanSD]): My teaching has improved as a result of this program (n=14, 4.90.3). Both giving (n=11, 4.21.6) and receiving (n=13, 4.61.1) feedback were felt to have improved teaching skills. There was strong agreement from respondents that they would participate in the program in the future: I am likely to participate in this program in the future (n=12, 4.60.9).
DISCUSSION
Previous studies have shown that teaching skills are unlikely to improve without feedback,[28, 29, 30] yet feedback for hospitalists is usually limited to summative, end‐rotation evaluations from learners, disconnected from the teaching encounter. Our theory‐based, rationally designed peer observation and feedback program resulted in increased confidence in the ability to give feedback, receive feedback, and teach effectively. Participation did not result in negative attitudes toward giving and receiving feedback from colleagues. Participants self‐reported increased performance of important teaching behaviors. Most participants rated the program very highly, and endorsed improved teaching skills as a result of the program.
Our experience provides several lessons for other groups considering the implementation of peer feedback to strengthen teaching. First, we suggest that hospitalist groups may expect variable degrees of participation in a voluntary peer feedback program. In our program, 41% of eligible attendings did not participate. We did not specifically investigate why; we speculate that they may not have had the time, believed that their teaching skills were already strong, or they may have been daunted at the idea of peer review. It is also possible that participants were a self‐selected group who were the most motivated to strengthen their teaching. Second, we note the steep decline in the number of observations in the second half of the year. Informal assessment for reasons for the drop‐off suggested that after initial enthusiasm for the program, navigating the logistics of observing the same peer in the second half of the year proved to be prohibitive to many participants. Therefore, future versions of peer feedback programs may benefit from removing the dyad requirement and encouraging all participants to observe one another whenever possible.
With these lessons in mind, we believe that a peer observation program could be implemented by other hospital medicine groups. The program does not require extensive content expertise or senior faculty but does require engaged leadership and interested and motivated faculty. Groups could identify an individual in their group with an interest in clinical teaching who could then be responsible for creating the training session (materials available upon request). We believe that with only a small upfront investment, most hospital medicine groups could use this as a model to build a peer observation program aimed at improving clinical teaching.
Our study has several limitations. As noted above, our participation rate was 59%, and the number of participating attendings declined through the year. We did not examine whether our program resulted in advances in the knowledge, skills, or attitudes of the learners; because each attending teaching session was unique, it was not possible to measure changes in learner knowledge. Our primary outcome measures relied on self‐assessment rather than higher order and more objective measures of teaching efficacy. Furthermore, our results may not be generalizable to other programs, given the heterogeneity in service structures and teaching practices across the country. This was an uncontrolled study; some of the outcomes may have naturally occurred independent of the intervention due to the natural evolution of clinical teaching. As with any educational intervention that integrates multiple strategies, we are not able to discern if the improved outcomes were the result of the initial didactic sessions, the refresher sessions, or the peer feedback itself. Serial assessments of frequency of teaching behaviors were not done due to the low number of observations in the second half of the program. Finally, our 10‐item tool derived from the validated SFDP‐26 tool is not itself a validated assessment of teaching.
We acknowledge that the increased confidence seen in our participants does not necessarily predict improved performance. Although increased confidence in core skills is a necessary step that can lead to changes in behavior, further studies are needed to determine whether the increase in faculty confidence that results from peer observation and feedback translates into improved educational outcomes.
The pressure on hospitalists to be excellent teachers is here to stay. Resources to train these faculty are scarce, yet we must prioritize faculty development in teaching to optimize the training of future physicians. Our data illustrate the benefits of peer observation and feedback. Hospitalist programs should consider this option in addressing the professional development needs of their faculty.
Acknowledgements
The authors thank Zachary Martin for administrative support for the program; Gurpreet Dhaliwal, MD, and Patricia O'Sullivan, PhD, for aid in program development; and John Amory, MD, MPH, for critical review of the manuscript. The authors thank the University of California, San Francisco Office of Medical Education for funding this work with an Educational Research Grant.
Disclosures: Funding: UCSF Office of Medical Education Educational Research Grant. Ethics approval: approved by UCSF Committee on Human Research. Previous presentations: Previous versions of this work were presented as an oral presentation at the University of California at San Francisco Medical Education Day, San Francisco, California, April 27, 2012, and as a poster presentation at the Society for General Internal Medicine 35th Annual Meeting, Orlando, Florida, May 912, 2012. The authors report no conflicts of interest.
- , , . Hospitalist involvement in internal medicine residencies. J Hosp Med. 2009;4(8):471–475.
- , , , , , . Is there a relationship between attending physicians' and residents' teaching skills and students' examination scores? Acad Med. 2000;75(11):1144–1146.
- , , . Six‐year documentation of the association between excellent clinical teaching and improved students' examination performances. Acad Med. 2000;75(10 suppl):S62–S64.
- , . Effect of clinical teaching on student performance during a medicine clerkship. Am J Med. 2001;110(3):205–209.
- , . Implications of the hospitalist model for medical students' education. Acad Med. 2001;76(4):324–330.
- . On educating and being a physician in the hospitalist era. Am J Med. 2001;111(9B):45S–47S.
- , . The role of hospitalists in medical education. Am J Med. 1999;107(4):305–309.
- , , , , , . Challenges and opportunities in academic hospital medicine: report from the academic hospital medicine summit. J Gen Intern Med. 2009;24(5):636–641.
- , , , et al. Impact of duty hour regulations on medical students' education: views of key clinical faculty. J Gen Intern Med. 2008;23(7):1084–1089.
- , , , , , . The impact of resident duty hours reform on the internal medicine core clerkship: results from the clerkship directors in internal medicine survey. Acad Med. 2006;81(12):1038–1044.
- , , , . Effects of resident work hour limitations on faculty professional lives. J Gen Intern Med. 2008;23(7):1077–1083.
- , . Teaching internal medicine residents in the new era. Inpatient attending with duty‐hour regulations. J Gen Intern Med. 2006;21(5):447–452.
- , , , . Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5–9.
- , , , . Using observed structured teaching exercises (OSTE) to enhance hospitalist teaching during family centered rounds. J Hosp Med. 2011;6(7):423–427.
- , , , . Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6(3):161–166.
- , , , . How to become a better clinical teacher: a collaborative peer observation process. Med Teach. 2011;33(2):151–155.
- , . Reframing research on faculty development. Acad Med. 2011;86(4):421–428.
- , , , et al. The Stanford faculty development program: a dissemination approach to faculty development for medical teachers. Teach Learn Med. 1992;4(3):180–187.
- . Evaluation of a method for improving the teaching performance of attending physicians. Am J Med. 1983;75(3):465–470.
- , , , . The impact of the Stanford Faculty Development Program on ambulatory teaching behavior. J Gen Intern Med. 2006;21(5):430–434.
- , , . Evaluation of a medical faculty development program: a comparison of traditional pre/post and retrospective pre/post self‐assessment ratings. Eval Health Prof. 1992;15(3):350–366.
- , , , , . Evaluation of the seminar method to improve clinical teaching. J Gen Intern Med. 1986;1(5):315–322.
- , , , , . Regional teaching improvement programs for community‐based teachers. Am J Med. 1999;106(1):76–80.
- , , , . Improving clinical teaching. Evaluation of a national dissemination program. Arch Intern Med. 1992;152(6):1156–1161.
- , , , . Factorial validation of a widely disseminated educational framework for evaluating clinical teachers. Acad Med. 1998;73(6):688–695.
- , , , . Student and resident evaluations of faculty—how reliable are they? Factorial validation of an educational framework using residents' evaluations of clinician‐educators. Acad Med. 1999;74(10):S25–S27.
- , . Students' global assessments of clinical teachers: a reliable and valid measure of teaching effectiveness. Acad Med. 1998;73(10 suppl):S72–S74.
- . The practice of giving feedback to improve teaching: what is effective? J Higher Educ. 1993;64(5):574–593.
- , , , et al. Faculty development. A resource for clinical teachers. J Gen Intern Med. 1997;12(suppl 2):S56–S63.
- , , , et al. A systematic review of faculty development initiatives designed to improve teaching effectiveness in medical education: BEME guide no. 8. Med Teach. 2006;28(6):497–526.
- , . Strategies for improving teaching practices: a comprehensive approach to faculty development. Acad Med. 1998;73(4):387–396.
- , . Relationship between systematic feedback to faculty and ratings of clinical teaching. Acad Med. 1996;71(10):1100–1102.
- . Lessons learned from a peer review of bedside teaching. Acad Med. 2004;79(4):343–346.
- , , , . Evaluating an instrument for the peer review of inpatient teaching. Med Teach. 2003;25(2):131–135.
- , , . Twelve tips for peer observation of teaching. Med Teach. 2007;29(4):297–300.
- , . Assessing the quality of teaching. Am J Med. 1999;106(4):381–384.
- , , , , , . To the point: medical education reviews—providing feedback. Am J Obstet Gynecol. 2007;196(6):508–513.
Hospitalists are increasingly responsible for educating students and housestaff in internal medicine.[1] Because the quality of teaching is an important factor in learning,[2, 3, 4] leaders in medical education have expressed concern over the rapid shift of teaching responsibilities to this new group of educators.[5, 6, 7, 8] Moreover, recent changes in duty hour restrictions have strained both student and resident education,[9, 10] necessitating the optimization of inpatient teaching.[11, 12] Many hospitalists have recently finished residency and have not had formal training in clinical teaching. Collectively, most hospital medicine groups are early in their careers, have significant clinical obligations,[13] and may not have the bandwidth or expertise to provide faculty development for improving clinical teaching.
Rationally designed and theoretically sound faculty development to improve inpatient clinical teaching is required to meet this challenge. There are a limited number of reports describing faculty development focused on strengthening the teaching of hospitalists, and only 3 utilized direct observation and feedback, 1 of which involved peer observation in the clinical setting.[14, 15, 16] This 2011 report described a narrative method of peer observation and feedback but did not assess for efficacy of the program.[16] To our knowledge, there have been no studies of structured peer observation and feedback to optimize hospitalist attendings' teaching which have evaluated the efficacy of the intervention.
We developed a faculty development program based on peer observation and feedback based on actual teaching practices, using structured feedback anchored in validated and observable measures of effective teaching. We hypothesized that participation in the program would increase confidence in key teaching skills, increase confidence in the ability to give and receive peer feedback, and strengthen attitudes toward peer observation and feedback.
METHODS
Subjects and Setting
The study was conducted at a 570‐bed academic, tertiary care medical center affiliated with an internal medicine residency program of 180 housestaff. Internal medicine ward attendings rotate during 2‐week blocks, and are asked to give formal teaching rounds 3 or 4 times a week (these sessions are distinct from teaching which may happen while rounding on patients). Ward teams are composed of 1 senior resident, 2 interns, and 1 to 2 medical students. The majority of internal medicine ward attendings are hospitalist faculty, hospital medicine fellows, or medicine chief residents. Because outpatient general internists and subspecialists only occasionally attend on the wards, we refer to ward attendings as attending hospitalists in this article. All attending hospitalists were eligible to participate if they attended on the wards at least twice during the academic year. The institutional review board at the University of California, San Francisco approved this study.
Theoretical Framework
We reviewed the literature to optimize our program in 3 conceptual domains: (1) overall structure of the program, (2) definition of effective teaching and (3) effective delivery of feedback.
Over‐reliance on didactics that are disconnected from the work environment is a weakness of traditional faculty development. Individuals may attempt to apply what they have learned, but receiving feedback on their actual workplace practices may be difficult. A recent perspective responds to this fragmentation by conceptualizing faculty development as embedded in both a faculty development community and a workplace community. This model emphasizes translating what faculty have learned in the classroom into practice, and highlights the importance of coaching in the workplace.[17] In accordance with this framework, we designed our program to reach beyond isolated workshops to effectively penetrate the workplace community.
We selected the Stanford Faculty Development Program (SFDP) framework for optimal clinical teaching as our model for recognizing and improving teaching skills. The SFDP was developed as a theory‐based intensive feedback method to improve teaching skills,[18, 19] and has been shown to improve teaching in the ambulatory[20] and inpatient settings.[21, 22] In this widely disseminated framework,[23, 24] excellent clinical teaching is grounded in optimizing observable behaviors organized around 7 domains.[18] A 26‐item instrument to evaluate clinical teaching (SFDP‐26) has been developed based on this framework[25] and has been validated in multiple settings.[26, 27] High‐quality teaching, as defined by the SFDP framework, has been correlated with improved educational outcomes in internal medicine clerkship students.[4]
Feedback is crucial to optimizing teaching,[28, 29, 30] particularly when it incorporates consultation[31] and narrative comments.[32] Peer feedback has several advantages over feedback from learners or from other non‐peer observers (such as supervisors or other evaluators). First, the observers benefit by gaining insight into their own weaknesses and potential areas for growth as teachers.[33, 34] Additionally, collegial observation and feedback may promote supportive teaching relationships between faculty.[35] Furthermore, peer review overcomes the biases that may be present in learner evaluations.[36] We established a 3‐stage feedback technique based on a previously described method.[37] In the first step, the observer elicits self‐appraisal from the speaker. Next, the observer provides specific, behaviorally anchored feedback in the form of 3 reinforcing comments and 2 constructive comments. Finally, the observer elicits a reflection on the feedback and helps develop a plan to improve teaching in future opportunities. We used a dyad model (paired participants repeatedly observe and give feedback to each other) to support mutual benefit and reciprocity between attendings.
Intervention
Using a modified Delphi approach, 5 medical education experts selected the 10 items that are most easily observable and salient to formal attending teaching rounds from the SFDP‐26 teaching assessment tool. A structured observation form was created, which included a checklist of the 10 selected items, space for note taking, and a template for narrative feedback (Figure 1).

We introduced the SFDP framework during a 2‐hour initial training session. Participants watched videos of teaching, learned to identify the 10 selected teaching behaviors, developed appropriate constructive and reinforcing comments, and practiced giving and receiving peer feedback.
Dyads were created on the basis of predetermined attending schedules. Participants were asked to observe and be observed twice during attending teaching rounds over the course of the academic year. Attending teaching rounds were defined as any preplanned didactic activity for ward teams. The structured observation forms were returned to the study coordinators after the observer had given feedback to the presenter. A copy of the feedback without the observer's notes was also given to each speaker. At the midpoint of the academic year, a refresher session was offered to reinforce those teaching behaviors that were the least frequently performed to date. All participants received a $50.00
Measurements and Data Collection
Participants were given a pre‐ and post‐program survey. The surveys included questions assessing confidence in ability to give feedback, receive feedback without feeling defensive, and teach effectively, as well as attitudes toward peer observation. The postprogram survey was administered at the end of the year and additionally assessed the self‐rated performance of the 10 selected teaching behaviors. A retrospective pre‐ and post‐program assessment was used for this outcome, because this method can be more reliable when participants initially may not have sufficient insight to accurately assess their own competence in specific measures.[21] The post‐program survey also included 4 questions assessing satisfaction with aspects of the program. All questions were structured as statements to which the respondent indicated degree of agreement using a 5‐point Likert scale, where 1=strongly disagree and 5=strongly agree. Structured observation forms used by participants were collected throughout the year to assess frequency of performance of the 10 selected teaching behaviors.
Statistical Analysis
We only analyzed the pre‐ and post‐program surveys that could be matched using anonymous identifiers provided by participants. For both prospective and retrospective measures, mean values and standard deviations were calculated. Wilcoxon signed rank tests for nonparametric data were performed to obtain P values. For all comparisons, a P value of <0.05 was considered significant. All comparisons were performed using Stata version 10 (StataCorp, College Station, TX).
RESULTS
Participant Characteristics and Participation in Program
Of the 37 eligible attending hospitalists, 22 (59%) enrolled. Fourteen were hospital medicine faculty, 6 were hospital medicine fellows, and 2 were internal medicine chief residents. The averagestandard deviation (SD) number of years as a ward attending was 2.2 years2.1. Seventeen (77%) reported previously having been observed and given feedback by a colleague, and 9 (41%) reported previously observing a colleague for the purpose of giving feedback.
All 22 participants attended 1 of 2, 2‐hour training sessions. Ten participants attended an hour‐long midyear refresher session. A total of 19 observation and feedback sessions took place; 15 of them occurred in the first half of the academic year. Fifteen attending hospitalists participated in at least 1 observed teaching session. Of the 11 dyads, 6 completed at least 1 observation of each other. Two dyads performed 2 observations of each other.
Fifteen participants (68% of those enrolled) completed both the pre‐ and post‐program surveys. Among these respondents, the average number of years attending was 2.92.2 years. Eight (53%) reported previously having been observed and given feedback by a colleague, and 7 (47%) reported previously observing a colleague for the purpose of giving feedback. For this subset of participants, the averageSD frequency of being observed during the program was 1.30.7, and observing was 1.10.8.
Confidence in Ability to Give Feedback, Receive Feedback, and Teach Effectively
In comparison of pre‐ and post‐intervention measures, participants indicated increased confidence in their ability to evaluate their colleagues and provide feedback in all domains queried. Participants also indicated increased confidence in the efficacy of their feedback to improve their colleagues' teaching skills. Participating in the program did not significantly change pre‐intervention levels of confidence in ability to receive feedback without being defensive or confidence in ability to use feedback to improve teaching skills (Table 1).
| Statement | Mean Pre | SD | Mean Post | SD | P |
|---|---|---|---|---|---|
| |||||
| I can accurately assess my colleagues' teaching skills. | 3.20 | 0.86 | 4.07 | 0.59 | 0.004 |
| I can give accurate feedback to my colleagues regarding their teaching skills. | 3.40 | 0.63 | 4.20 | 0.56 | 0.002 |
| I can give feedback in a way that that my colleague will not feel defensive about their teaching skills. | 3.60 | 0.63 | 4.20 | 0.56 | 0.046 |
| My feedback will improve my colleagues' teaching skills. | 3.40 | 0.51 | 3.93 | 0.59 | 0.011 |
| I can receive feedback from a colleague without being defensive about my teaching skills. | 3.87 | 0.92 | 4.27 | 0.59 | 0.156 |
| I can use feedback from a colleague to improve my teaching skills. | 4.33 | 0.82 | 4.47 | 0.64 | 0.607 |
| I am confident in my ability to teach students and residents during attending rounds.a | 3.21 | 0.89 | 3.71 | 0.83 | 0.026 |
| I am confident in my knowledge of components of effective teaching.a | 3.21 | 0.89 | 3.71 | 0.99 | 0.035 |
| Learners regard me as an effective teacher.a | 3.14 | 0.66 | 3.64 | 0.74 | 0.033 |
Self‐Rated Performance of 10 Selected Teaching Behaviors
In retrospective assessment, participants felt that their performance had improved in all 10 teaching behaviors after the intervention. This perceived improvement reached statistical significance in 8 of the 10 selected behaviors (Table 2).
| SFDP Framework Category From Skeff et al.[18] | When I Give Attending Rounds, I Generally . | Mean Pre | SD | Mean Post | SD | P |
|---|---|---|---|---|---|---|
| ||||||
| 1. Establishing a positive learning climate | Listen to learners | 4.27 | 0.59 | 4.53 | 0.52 | 0.046 |
| Encourage learners to participate actively in the discussion | 4.07 | 0.70 | 4.60 | 0.51 | 0.009 | |
| 2. Controlling the teaching session | Call attention to time | 3.33 | 0.98 | 4.27 | 0.59 | 0.004 |
| 3. Communicating goals | State goals clearly and concisely | 3.40 | 0.63 | 4.27 | 0.59 | 0.001 |
| State relevance of goals to learners | 3.40 | 0.74 | 4.20 | 0.68 | 0.002 | |
| 4. Promoting understanding and retention | Present well‐organized material | 3.87 | 0.64 | 4.07 | 0.70 | 0.083 |
| Use blackboard or other visual aids | 4.27 | 0.88 | 4.47 | 0.74 | 0.158 | |
| 5. Evaluating the learners | Evaluate learners' ability to apply medical knowledge to specific patients | 3.33 | 0.98 | 4.00 | 0.76 | 0.005 |
| 6. Providing feedback to the learners | Explain to learners why he/she was correct or incorrect | 3.47 | 1.13 | 4.13 | 0.64 | 0.009 |
| 7. Promoting self‐directed learning | Motivate learners to learn on their own | 3.20 | 0.86 | 3.73 | 0.70 | 0.005 |
Attitudes Toward Peer Observation and Feedback
There were no significant changes in attitudes toward observation and feedback on teaching. A strong preprogram belief that observation and feedback can improve teaching skills increased slightly, but not significantly, after the program. Participants remained largely neutral in expectation of discomfort with giving or receiving peer feedback. Prior to the program, there was a slight tendency to believe that observation and feedback is more effective when done by more skilled and experienced colleagues; this belief diminished, but not significantly (Table 3).
| Statement | Mean Pre | SD | Mean Post | SD | P |
|---|---|---|---|---|---|
| |||||
| Being observed and receiving feedback can improve my teaching skills. | 4.47 | 1.06 | 4.60 | 0.51 | 0.941 |
| My teaching skills cannot improve without observation with feedback. | 2.93 | 1.39 | 3.47 | 1.30 | 0.188 |
| Observation with feedback is most effective when done by colleagues who are expert educators. | 3.53 | 0.83 | 3.33 | 0.98 | 0.180 |
| Observation with feedback is most effective when done by colleagues who have been teaching many years. | 3.40 | 0.91 | 3.07 | 1.03 | 0.143 |
| The thought of observing and giving feedback to my colleagues makes me uncomfortable. | 3.13 | 0.92 | 3.00 | 1.13 | 0.565 |
| The thought of being observed by a colleague and receiving feedback makes me uncomfortable. | 3.20 | 0.94 | 3.27 | 1.22 | 0.747 |
Program Evaluation
There were a variable number of responses to the program evaluation questions. The majority of participants found the program to be very beneficial (1=strongly disagree, 5=strongly agree [n, meanSD]): My teaching has improved as a result of this program (n=14, 4.90.3). Both giving (n=11, 4.21.6) and receiving (n=13, 4.61.1) feedback were felt to have improved teaching skills. There was strong agreement from respondents that they would participate in the program in the future: I am likely to participate in this program in the future (n=12, 4.60.9).
DISCUSSION
Previous studies have shown that teaching skills are unlikely to improve without feedback,[28, 29, 30] yet feedback for hospitalists is usually limited to summative, end‐rotation evaluations from learners, disconnected from the teaching encounter. Our theory‐based, rationally designed peer observation and feedback program resulted in increased confidence in the ability to give feedback, receive feedback, and teach effectively. Participation did not result in negative attitudes toward giving and receiving feedback from colleagues. Participants self‐reported increased performance of important teaching behaviors. Most participants rated the program very highly, and endorsed improved teaching skills as a result of the program.
Our experience provides several lessons for other groups considering the implementation of peer feedback to strengthen teaching. First, we suggest that hospitalist groups may expect variable degrees of participation in a voluntary peer feedback program. In our program, 41% of eligible attendings did not participate. We did not specifically investigate why; we speculate that they may not have had the time, believed that their teaching skills were already strong, or they may have been daunted at the idea of peer review. It is also possible that participants were a self‐selected group who were the most motivated to strengthen their teaching. Second, we note the steep decline in the number of observations in the second half of the year. Informal assessment for reasons for the drop‐off suggested that after initial enthusiasm for the program, navigating the logistics of observing the same peer in the second half of the year proved to be prohibitive to many participants. Therefore, future versions of peer feedback programs may benefit from removing the dyad requirement and encouraging all participants to observe one another whenever possible.
With these lessons in mind, we believe that a peer observation program could be implemented by other hospital medicine groups. The program does not require extensive content expertise or senior faculty but does require engaged leadership and interested and motivated faculty. Groups could identify an individual in their group with an interest in clinical teaching who could then be responsible for creating the training session (materials available upon request). We believe that with only a small upfront investment, most hospital medicine groups could use this as a model to build a peer observation program aimed at improving clinical teaching.
Our study has several limitations. As noted above, our participation rate was 59%, and the number of participating attendings declined through the year. We did not examine whether our program resulted in advances in the knowledge, skills, or attitudes of the learners; because each attending teaching session was unique, it was not possible to measure changes in learner knowledge. Our primary outcome measures relied on self‐assessment rather than higher order and more objective measures of teaching efficacy. Furthermore, our results may not be generalizable to other programs, given the heterogeneity in service structures and teaching practices across the country. This was an uncontrolled study; some of the outcomes may have naturally occurred independent of the intervention due to the natural evolution of clinical teaching. As with any educational intervention that integrates multiple strategies, we are not able to discern if the improved outcomes were the result of the initial didactic sessions, the refresher sessions, or the peer feedback itself. Serial assessments of frequency of teaching behaviors were not done due to the low number of observations in the second half of the program. Finally, our 10‐item tool derived from the validated SFDP‐26 tool is not itself a validated assessment of teaching.
We acknowledge that the increased confidence seen in our participants does not necessarily predict improved performance. Although increased confidence in core skills is a necessary step that can lead to changes in behavior, further studies are needed to determine whether the increase in faculty confidence that results from peer observation and feedback translates into improved educational outcomes.
The pressure on hospitalists to be excellent teachers is here to stay. Resources to train these faculty are scarce, yet we must prioritize faculty development in teaching to optimize the training of future physicians. Our data illustrate the benefits of peer observation and feedback. Hospitalist programs should consider this option in addressing the professional development needs of their faculty.
Acknowledgements
The authors thank Zachary Martin for administrative support for the program; Gurpreet Dhaliwal, MD, and Patricia O'Sullivan, PhD, for aid in program development; and John Amory, MD, MPH, for critical review of the manuscript. The authors thank the University of California, San Francisco Office of Medical Education for funding this work with an Educational Research Grant.
Disclosures: Funding: UCSF Office of Medical Education Educational Research Grant. Ethics approval: approved by UCSF Committee on Human Research. Previous presentations: Previous versions of this work were presented as an oral presentation at the University of California at San Francisco Medical Education Day, San Francisco, California, April 27, 2012, and as a poster presentation at the Society for General Internal Medicine 35th Annual Meeting, Orlando, Florida, May 912, 2012. The authors report no conflicts of interest.
Hospitalists are increasingly responsible for educating students and housestaff in internal medicine.[1] Because the quality of teaching is an important factor in learning,[2, 3, 4] leaders in medical education have expressed concern over the rapid shift of teaching responsibilities to this new group of educators.[5, 6, 7, 8] Moreover, recent changes in duty hour restrictions have strained both student and resident education,[9, 10] necessitating the optimization of inpatient teaching.[11, 12] Many hospitalists have recently finished residency and have not had formal training in clinical teaching. Collectively, most hospital medicine groups are early in their careers, have significant clinical obligations,[13] and may not have the bandwidth or expertise to provide faculty development for improving clinical teaching.
Rationally designed and theoretically sound faculty development to improve inpatient clinical teaching is required to meet this challenge. There are a limited number of reports describing faculty development focused on strengthening the teaching of hospitalists, and only 3 utilized direct observation and feedback, 1 of which involved peer observation in the clinical setting.[14, 15, 16] This 2011 report described a narrative method of peer observation and feedback but did not assess for efficacy of the program.[16] To our knowledge, there have been no studies of structured peer observation and feedback to optimize hospitalist attendings' teaching which have evaluated the efficacy of the intervention.
We developed a faculty development program based on peer observation and feedback based on actual teaching practices, using structured feedback anchored in validated and observable measures of effective teaching. We hypothesized that participation in the program would increase confidence in key teaching skills, increase confidence in the ability to give and receive peer feedback, and strengthen attitudes toward peer observation and feedback.
METHODS
Subjects and Setting
The study was conducted at a 570‐bed academic, tertiary care medical center affiliated with an internal medicine residency program of 180 housestaff. Internal medicine ward attendings rotate during 2‐week blocks, and are asked to give formal teaching rounds 3 or 4 times a week (these sessions are distinct from teaching which may happen while rounding on patients). Ward teams are composed of 1 senior resident, 2 interns, and 1 to 2 medical students. The majority of internal medicine ward attendings are hospitalist faculty, hospital medicine fellows, or medicine chief residents. Because outpatient general internists and subspecialists only occasionally attend on the wards, we refer to ward attendings as attending hospitalists in this article. All attending hospitalists were eligible to participate if they attended on the wards at least twice during the academic year. The institutional review board at the University of California, San Francisco approved this study.
Theoretical Framework
We reviewed the literature to optimize our program in 3 conceptual domains: (1) overall structure of the program, (2) definition of effective teaching and (3) effective delivery of feedback.
Over‐reliance on didactics that are disconnected from the work environment is a weakness of traditional faculty development. Individuals may attempt to apply what they have learned, but receiving feedback on their actual workplace practices may be difficult. A recent perspective responds to this fragmentation by conceptualizing faculty development as embedded in both a faculty development community and a workplace community. This model emphasizes translating what faculty have learned in the classroom into practice, and highlights the importance of coaching in the workplace.[17] In accordance with this framework, we designed our program to reach beyond isolated workshops to effectively penetrate the workplace community.
We selected the Stanford Faculty Development Program (SFDP) framework for optimal clinical teaching as our model for recognizing and improving teaching skills. The SFDP was developed as a theory‐based intensive feedback method to improve teaching skills,[18, 19] and has been shown to improve teaching in the ambulatory[20] and inpatient settings.[21, 22] In this widely disseminated framework,[23, 24] excellent clinical teaching is grounded in optimizing observable behaviors organized around 7 domains.[18] A 26‐item instrument to evaluate clinical teaching (SFDP‐26) has been developed based on this framework[25] and has been validated in multiple settings.[26, 27] High‐quality teaching, as defined by the SFDP framework, has been correlated with improved educational outcomes in internal medicine clerkship students.[4]
Feedback is crucial to optimizing teaching,[28, 29, 30] particularly when it incorporates consultation[31] and narrative comments.[32] Peer feedback has several advantages over feedback from learners or from other non‐peer observers (such as supervisors or other evaluators). First, the observers benefit by gaining insight into their own weaknesses and potential areas for growth as teachers.[33, 34] Additionally, collegial observation and feedback may promote supportive teaching relationships between faculty.[35] Furthermore, peer review overcomes the biases that may be present in learner evaluations.[36] We established a 3‐stage feedback technique based on a previously described method.[37] In the first step, the observer elicits self‐appraisal from the speaker. Next, the observer provides specific, behaviorally anchored feedback in the form of 3 reinforcing comments and 2 constructive comments. Finally, the observer elicits a reflection on the feedback and helps develop a plan to improve teaching in future opportunities. We used a dyad model (paired participants repeatedly observe and give feedback to each other) to support mutual benefit and reciprocity between attendings.
Intervention
Using a modified Delphi approach, 5 medical education experts selected the 10 items that are most easily observable and salient to formal attending teaching rounds from the SFDP‐26 teaching assessment tool. A structured observation form was created, which included a checklist of the 10 selected items, space for note taking, and a template for narrative feedback (Figure 1).

We introduced the SFDP framework during a 2‐hour initial training session. Participants watched videos of teaching, learned to identify the 10 selected teaching behaviors, developed appropriate constructive and reinforcing comments, and practiced giving and receiving peer feedback.
Dyads were created on the basis of predetermined attending schedules. Participants were asked to observe and be observed twice during attending teaching rounds over the course of the academic year. Attending teaching rounds were defined as any preplanned didactic activity for ward teams. The structured observation forms were returned to the study coordinators after the observer had given feedback to the presenter. A copy of the feedback without the observer's notes was also given to each speaker. At the midpoint of the academic year, a refresher session was offered to reinforce those teaching behaviors that were the least frequently performed to date. All participants received a $50.00
Measurements and Data Collection
Participants were given a pre‐ and post‐program survey. The surveys included questions assessing confidence in ability to give feedback, receive feedback without feeling defensive, and teach effectively, as well as attitudes toward peer observation. The postprogram survey was administered at the end of the year and additionally assessed the self‐rated performance of the 10 selected teaching behaviors. A retrospective pre‐ and post‐program assessment was used for this outcome, because this method can be more reliable when participants initially may not have sufficient insight to accurately assess their own competence in specific measures.[21] The post‐program survey also included 4 questions assessing satisfaction with aspects of the program. All questions were structured as statements to which the respondent indicated degree of agreement using a 5‐point Likert scale, where 1=strongly disagree and 5=strongly agree. Structured observation forms used by participants were collected throughout the year to assess frequency of performance of the 10 selected teaching behaviors.
Statistical Analysis
We only analyzed the pre‐ and post‐program surveys that could be matched using anonymous identifiers provided by participants. For both prospective and retrospective measures, mean values and standard deviations were calculated. Wilcoxon signed rank tests for nonparametric data were performed to obtain P values. For all comparisons, a P value of <0.05 was considered significant. All comparisons were performed using Stata version 10 (StataCorp, College Station, TX).
RESULTS
Participant Characteristics and Participation in Program
Of the 37 eligible attending hospitalists, 22 (59%) enrolled. Fourteen were hospital medicine faculty, 6 were hospital medicine fellows, and 2 were internal medicine chief residents. The averagestandard deviation (SD) number of years as a ward attending was 2.2 years2.1. Seventeen (77%) reported previously having been observed and given feedback by a colleague, and 9 (41%) reported previously observing a colleague for the purpose of giving feedback.
All 22 participants attended 1 of 2, 2‐hour training sessions. Ten participants attended an hour‐long midyear refresher session. A total of 19 observation and feedback sessions took place; 15 of them occurred in the first half of the academic year. Fifteen attending hospitalists participated in at least 1 observed teaching session. Of the 11 dyads, 6 completed at least 1 observation of each other. Two dyads performed 2 observations of each other.
Fifteen participants (68% of those enrolled) completed both the pre‐ and post‐program surveys. Among these respondents, the average number of years attending was 2.92.2 years. Eight (53%) reported previously having been observed and given feedback by a colleague, and 7 (47%) reported previously observing a colleague for the purpose of giving feedback. For this subset of participants, the averageSD frequency of being observed during the program was 1.30.7, and observing was 1.10.8.
Confidence in Ability to Give Feedback, Receive Feedback, and Teach Effectively
In comparison of pre‐ and post‐intervention measures, participants indicated increased confidence in their ability to evaluate their colleagues and provide feedback in all domains queried. Participants also indicated increased confidence in the efficacy of their feedback to improve their colleagues' teaching skills. Participating in the program did not significantly change pre‐intervention levels of confidence in ability to receive feedback without being defensive or confidence in ability to use feedback to improve teaching skills (Table 1).
| Statement | Mean Pre | SD | Mean Post | SD | P |
|---|---|---|---|---|---|
| |||||
| I can accurately assess my colleagues' teaching skills. | 3.20 | 0.86 | 4.07 | 0.59 | 0.004 |
| I can give accurate feedback to my colleagues regarding their teaching skills. | 3.40 | 0.63 | 4.20 | 0.56 | 0.002 |
| I can give feedback in a way that that my colleague will not feel defensive about their teaching skills. | 3.60 | 0.63 | 4.20 | 0.56 | 0.046 |
| My feedback will improve my colleagues' teaching skills. | 3.40 | 0.51 | 3.93 | 0.59 | 0.011 |
| I can receive feedback from a colleague without being defensive about my teaching skills. | 3.87 | 0.92 | 4.27 | 0.59 | 0.156 |
| I can use feedback from a colleague to improve my teaching skills. | 4.33 | 0.82 | 4.47 | 0.64 | 0.607 |
| I am confident in my ability to teach students and residents during attending rounds.a | 3.21 | 0.89 | 3.71 | 0.83 | 0.026 |
| I am confident in my knowledge of components of effective teaching.a | 3.21 | 0.89 | 3.71 | 0.99 | 0.035 |
| Learners regard me as an effective teacher.a | 3.14 | 0.66 | 3.64 | 0.74 | 0.033 |
Self‐Rated Performance of 10 Selected Teaching Behaviors
In retrospective assessment, participants felt that their performance had improved in all 10 teaching behaviors after the intervention. This perceived improvement reached statistical significance in 8 of the 10 selected behaviors (Table 2).
| SFDP Framework Category From Skeff et al.[18] | When I Give Attending Rounds, I Generally . | Mean Pre | SD | Mean Post | SD | P |
|---|---|---|---|---|---|---|
| ||||||
| 1. Establishing a positive learning climate | Listen to learners | 4.27 | 0.59 | 4.53 | 0.52 | 0.046 |
| Encourage learners to participate actively in the discussion | 4.07 | 0.70 | 4.60 | 0.51 | 0.009 | |
| 2. Controlling the teaching session | Call attention to time | 3.33 | 0.98 | 4.27 | 0.59 | 0.004 |
| 3. Communicating goals | State goals clearly and concisely | 3.40 | 0.63 | 4.27 | 0.59 | 0.001 |
| State relevance of goals to learners | 3.40 | 0.74 | 4.20 | 0.68 | 0.002 | |
| 4. Promoting understanding and retention | Present well‐organized material | 3.87 | 0.64 | 4.07 | 0.70 | 0.083 |
| Use blackboard or other visual aids | 4.27 | 0.88 | 4.47 | 0.74 | 0.158 | |
| 5. Evaluating the learners | Evaluate learners' ability to apply medical knowledge to specific patients | 3.33 | 0.98 | 4.00 | 0.76 | 0.005 |
| 6. Providing feedback to the learners | Explain to learners why he/she was correct or incorrect | 3.47 | 1.13 | 4.13 | 0.64 | 0.009 |
| 7. Promoting self‐directed learning | Motivate learners to learn on their own | 3.20 | 0.86 | 3.73 | 0.70 | 0.005 |
Attitudes Toward Peer Observation and Feedback
There were no significant changes in attitudes toward observation and feedback on teaching. A strong preprogram belief that observation and feedback can improve teaching skills increased slightly, but not significantly, after the program. Participants remained largely neutral in expectation of discomfort with giving or receiving peer feedback. Prior to the program, there was a slight tendency to believe that observation and feedback is more effective when done by more skilled and experienced colleagues; this belief diminished, but not significantly (Table 3).
| Statement | Mean Pre | SD | Mean Post | SD | P |
|---|---|---|---|---|---|
| |||||
| Being observed and receiving feedback can improve my teaching skills. | 4.47 | 1.06 | 4.60 | 0.51 | 0.941 |
| My teaching skills cannot improve without observation with feedback. | 2.93 | 1.39 | 3.47 | 1.30 | 0.188 |
| Observation with feedback is most effective when done by colleagues who are expert educators. | 3.53 | 0.83 | 3.33 | 0.98 | 0.180 |
| Observation with feedback is most effective when done by colleagues who have been teaching many years. | 3.40 | 0.91 | 3.07 | 1.03 | 0.143 |
| The thought of observing and giving feedback to my colleagues makes me uncomfortable. | 3.13 | 0.92 | 3.00 | 1.13 | 0.565 |
| The thought of being observed by a colleague and receiving feedback makes me uncomfortable. | 3.20 | 0.94 | 3.27 | 1.22 | 0.747 |
Program Evaluation
There were a variable number of responses to the program evaluation questions. The majority of participants found the program to be very beneficial (1=strongly disagree, 5=strongly agree [n, meanSD]): My teaching has improved as a result of this program (n=14, 4.90.3). Both giving (n=11, 4.21.6) and receiving (n=13, 4.61.1) feedback were felt to have improved teaching skills. There was strong agreement from respondents that they would participate in the program in the future: I am likely to participate in this program in the future (n=12, 4.60.9).
DISCUSSION
Previous studies have shown that teaching skills are unlikely to improve without feedback,[28, 29, 30] yet feedback for hospitalists is usually limited to summative, end‐rotation evaluations from learners, disconnected from the teaching encounter. Our theory‐based, rationally designed peer observation and feedback program resulted in increased confidence in the ability to give feedback, receive feedback, and teach effectively. Participation did not result in negative attitudes toward giving and receiving feedback from colleagues. Participants self‐reported increased performance of important teaching behaviors. Most participants rated the program very highly, and endorsed improved teaching skills as a result of the program.
Our experience provides several lessons for other groups considering the implementation of peer feedback to strengthen teaching. First, we suggest that hospitalist groups may expect variable degrees of participation in a voluntary peer feedback program. In our program, 41% of eligible attendings did not participate. We did not specifically investigate why; we speculate that they may not have had the time, believed that their teaching skills were already strong, or they may have been daunted at the idea of peer review. It is also possible that participants were a self‐selected group who were the most motivated to strengthen their teaching. Second, we note the steep decline in the number of observations in the second half of the year. Informal assessment for reasons for the drop‐off suggested that after initial enthusiasm for the program, navigating the logistics of observing the same peer in the second half of the year proved to be prohibitive to many participants. Therefore, future versions of peer feedback programs may benefit from removing the dyad requirement and encouraging all participants to observe one another whenever possible.
With these lessons in mind, we believe that a peer observation program could be implemented by other hospital medicine groups. The program does not require extensive content expertise or senior faculty but does require engaged leadership and interested and motivated faculty. Groups could identify an individual in their group with an interest in clinical teaching who could then be responsible for creating the training session (materials available upon request). We believe that with only a small upfront investment, most hospital medicine groups could use this as a model to build a peer observation program aimed at improving clinical teaching.
Our study has several limitations. As noted above, our participation rate was 59%, and the number of participating attendings declined through the year. We did not examine whether our program resulted in advances in the knowledge, skills, or attitudes of the learners; because each attending teaching session was unique, it was not possible to measure changes in learner knowledge. Our primary outcome measures relied on self‐assessment rather than higher order and more objective measures of teaching efficacy. Furthermore, our results may not be generalizable to other programs, given the heterogeneity in service structures and teaching practices across the country. This was an uncontrolled study; some of the outcomes may have naturally occurred independent of the intervention due to the natural evolution of clinical teaching. As with any educational intervention that integrates multiple strategies, we are not able to discern if the improved outcomes were the result of the initial didactic sessions, the refresher sessions, or the peer feedback itself. Serial assessments of frequency of teaching behaviors were not done due to the low number of observations in the second half of the program. Finally, our 10‐item tool derived from the validated SFDP‐26 tool is not itself a validated assessment of teaching.
We acknowledge that the increased confidence seen in our participants does not necessarily predict improved performance. Although increased confidence in core skills is a necessary step that can lead to changes in behavior, further studies are needed to determine whether the increase in faculty confidence that results from peer observation and feedback translates into improved educational outcomes.
The pressure on hospitalists to be excellent teachers is here to stay. Resources to train these faculty are scarce, yet we must prioritize faculty development in teaching to optimize the training of future physicians. Our data illustrate the benefits of peer observation and feedback. Hospitalist programs should consider this option in addressing the professional development needs of their faculty.
Acknowledgements
The authors thank Zachary Martin for administrative support for the program; Gurpreet Dhaliwal, MD, and Patricia O'Sullivan, PhD, for aid in program development; and John Amory, MD, MPH, for critical review of the manuscript. The authors thank the University of California, San Francisco Office of Medical Education for funding this work with an Educational Research Grant.
Disclosures: Funding: UCSF Office of Medical Education Educational Research Grant. Ethics approval: approved by UCSF Committee on Human Research. Previous presentations: Previous versions of this work were presented as an oral presentation at the University of California at San Francisco Medical Education Day, San Francisco, California, April 27, 2012, and as a poster presentation at the Society for General Internal Medicine 35th Annual Meeting, Orlando, Florida, May 912, 2012. The authors report no conflicts of interest.
- , , . Hospitalist involvement in internal medicine residencies. J Hosp Med. 2009;4(8):471–475.
- , , , , , . Is there a relationship between attending physicians' and residents' teaching skills and students' examination scores? Acad Med. 2000;75(11):1144–1146.
- , , . Six‐year documentation of the association between excellent clinical teaching and improved students' examination performances. Acad Med. 2000;75(10 suppl):S62–S64.
- , . Effect of clinical teaching on student performance during a medicine clerkship. Am J Med. 2001;110(3):205–209.
- , . Implications of the hospitalist model for medical students' education. Acad Med. 2001;76(4):324–330.
- . On educating and being a physician in the hospitalist era. Am J Med. 2001;111(9B):45S–47S.
- , . The role of hospitalists in medical education. Am J Med. 1999;107(4):305–309.
- , , , , , . Challenges and opportunities in academic hospital medicine: report from the academic hospital medicine summit. J Gen Intern Med. 2009;24(5):636–641.
- , , , et al. Impact of duty hour regulations on medical students' education: views of key clinical faculty. J Gen Intern Med. 2008;23(7):1084–1089.
- , , , , , . The impact of resident duty hours reform on the internal medicine core clerkship: results from the clerkship directors in internal medicine survey. Acad Med. 2006;81(12):1038–1044.
- , , , . Effects of resident work hour limitations on faculty professional lives. J Gen Intern Med. 2008;23(7):1077–1083.
- , . Teaching internal medicine residents in the new era. Inpatient attending with duty‐hour regulations. J Gen Intern Med. 2006;21(5):447–452.
- , , , . Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5–9.
- , , , . Using observed structured teaching exercises (OSTE) to enhance hospitalist teaching during family centered rounds. J Hosp Med. 2011;6(7):423–427.
- , , , . Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6(3):161–166.
- , , , . How to become a better clinical teacher: a collaborative peer observation process. Med Teach. 2011;33(2):151–155.
- , . Reframing research on faculty development. Acad Med. 2011;86(4):421–428.
- , , , et al. The Stanford faculty development program: a dissemination approach to faculty development for medical teachers. Teach Learn Med. 1992;4(3):180–187.
- . Evaluation of a method for improving the teaching performance of attending physicians. Am J Med. 1983;75(3):465–470.
- , , , . The impact of the Stanford Faculty Development Program on ambulatory teaching behavior. J Gen Intern Med. 2006;21(5):430–434.
- , , . Evaluation of a medical faculty development program: a comparison of traditional pre/post and retrospective pre/post self‐assessment ratings. Eval Health Prof. 1992;15(3):350–366.
- , , , , . Evaluation of the seminar method to improve clinical teaching. J Gen Intern Med. 1986;1(5):315–322.
- , , , , . Regional teaching improvement programs for community‐based teachers. Am J Med. 1999;106(1):76–80.
- , , , . Improving clinical teaching. Evaluation of a national dissemination program. Arch Intern Med. 1992;152(6):1156–1161.
- , , , . Factorial validation of a widely disseminated educational framework for evaluating clinical teachers. Acad Med. 1998;73(6):688–695.
- , , , . Student and resident evaluations of faculty—how reliable are they? Factorial validation of an educational framework using residents' evaluations of clinician‐educators. Acad Med. 1999;74(10):S25–S27.
- , . Students' global assessments of clinical teachers: a reliable and valid measure of teaching effectiveness. Acad Med. 1998;73(10 suppl):S72–S74.
- . The practice of giving feedback to improve teaching: what is effective? J Higher Educ. 1993;64(5):574–593.
- , , , et al. Faculty development. A resource for clinical teachers. J Gen Intern Med. 1997;12(suppl 2):S56–S63.
- , , , et al. A systematic review of faculty development initiatives designed to improve teaching effectiveness in medical education: BEME guide no. 8. Med Teach. 2006;28(6):497–526.
- , . Strategies for improving teaching practices: a comprehensive approach to faculty development. Acad Med. 1998;73(4):387–396.
- , . Relationship between systematic feedback to faculty and ratings of clinical teaching. Acad Med. 1996;71(10):1100–1102.
- . Lessons learned from a peer review of bedside teaching. Acad Med. 2004;79(4):343–346.
- , , , . Evaluating an instrument for the peer review of inpatient teaching. Med Teach. 2003;25(2):131–135.
- , , . Twelve tips for peer observation of teaching. Med Teach. 2007;29(4):297–300.
- , . Assessing the quality of teaching. Am J Med. 1999;106(4):381–384.
- , , , , , . To the point: medical education reviews—providing feedback. Am J Obstet Gynecol. 2007;196(6):508–513.
- , , . Hospitalist involvement in internal medicine residencies. J Hosp Med. 2009;4(8):471–475.
- , , , , , . Is there a relationship between attending physicians' and residents' teaching skills and students' examination scores? Acad Med. 2000;75(11):1144–1146.
- , , . Six‐year documentation of the association between excellent clinical teaching and improved students' examination performances. Acad Med. 2000;75(10 suppl):S62–S64.
- , . Effect of clinical teaching on student performance during a medicine clerkship. Am J Med. 2001;110(3):205–209.
- , . Implications of the hospitalist model for medical students' education. Acad Med. 2001;76(4):324–330.
- . On educating and being a physician in the hospitalist era. Am J Med. 2001;111(9B):45S–47S.
- , . The role of hospitalists in medical education. Am J Med. 1999;107(4):305–309.
- , , , , , . Challenges and opportunities in academic hospital medicine: report from the academic hospital medicine summit. J Gen Intern Med. 2009;24(5):636–641.
- , , , et al. Impact of duty hour regulations on medical students' education: views of key clinical faculty. J Gen Intern Med. 2008;23(7):1084–1089.
- , , , , , . The impact of resident duty hours reform on the internal medicine core clerkship: results from the clerkship directors in internal medicine survey. Acad Med. 2006;81(12):1038–1044.
- , , , . Effects of resident work hour limitations on faculty professional lives. J Gen Intern Med. 2008;23(7):1077–1083.
- , . Teaching internal medicine residents in the new era. Inpatient attending with duty‐hour regulations. J Gen Intern Med. 2006;21(5):447–452.
- , , , . Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5–9.
- , , , . Using observed structured teaching exercises (OSTE) to enhance hospitalist teaching during family centered rounds. J Hosp Med. 2011;6(7):423–427.
- , , , . Investing in the future: building an academic hospitalist faculty development program. J Hosp Med. 2011;6(3):161–166.
- , , , . How to become a better clinical teacher: a collaborative peer observation process. Med Teach. 2011;33(2):151–155.
- , . Reframing research on faculty development. Acad Med. 2011;86(4):421–428.
- , , , et al. The Stanford faculty development program: a dissemination approach to faculty development for medical teachers. Teach Learn Med. 1992;4(3):180–187.
- . Evaluation of a method for improving the teaching performance of attending physicians. Am J Med. 1983;75(3):465–470.
- , , , . The impact of the Stanford Faculty Development Program on ambulatory teaching behavior. J Gen Intern Med. 2006;21(5):430–434.
- , , . Evaluation of a medical faculty development program: a comparison of traditional pre/post and retrospective pre/post self‐assessment ratings. Eval Health Prof. 1992;15(3):350–366.
- , , , , . Evaluation of the seminar method to improve clinical teaching. J Gen Intern Med. 1986;1(5):315–322.
- , , , , . Regional teaching improvement programs for community‐based teachers. Am J Med. 1999;106(1):76–80.
- , , , . Improving clinical teaching. Evaluation of a national dissemination program. Arch Intern Med. 1992;152(6):1156–1161.
- , , , . Factorial validation of a widely disseminated educational framework for evaluating clinical teachers. Acad Med. 1998;73(6):688–695.
- , , , . Student and resident evaluations of faculty—how reliable are they? Factorial validation of an educational framework using residents' evaluations of clinician‐educators. Acad Med. 1999;74(10):S25–S27.
- , . Students' global assessments of clinical teachers: a reliable and valid measure of teaching effectiveness. Acad Med. 1998;73(10 suppl):S72–S74.
- . The practice of giving feedback to improve teaching: what is effective? J Higher Educ. 1993;64(5):574–593.
- , , , et al. Faculty development. A resource for clinical teachers. J Gen Intern Med. 1997;12(suppl 2):S56–S63.
- , , , et al. A systematic review of faculty development initiatives designed to improve teaching effectiveness in medical education: BEME guide no. 8. Med Teach. 2006;28(6):497–526.
- , . Strategies for improving teaching practices: a comprehensive approach to faculty development. Acad Med. 1998;73(4):387–396.
- , . Relationship between systematic feedback to faculty and ratings of clinical teaching. Acad Med. 1996;71(10):1100–1102.
- . Lessons learned from a peer review of bedside teaching. Acad Med. 2004;79(4):343–346.
- , , , . Evaluating an instrument for the peer review of inpatient teaching. Med Teach. 2003;25(2):131–135.
- , , . Twelve tips for peer observation of teaching. Med Teach. 2007;29(4):297–300.
- , . Assessing the quality of teaching. Am J Med. 1999;106(4):381–384.
- , , , , , . To the point: medical education reviews—providing feedback. Am J Obstet Gynecol. 2007;196(6):508–513.
© 2014 Society of Hospital Medicine