User login
Extra education about apixaban may be unnecessary

Photo courtesy of Pfizer
and Bristol-Myers Squibb
LONDON—An educational program designed to improve adherence to the anticoagulant apixaban proved ineffective in a phase 4 trial of patients with atrial fibrillation (AF).
But that’s because adherence was high whether patients completed the program or not.
This suggests current measures used to inform patients about apixaban may be sufficient to ensure treatment adherence, researchers said.
They presented these findings at the ESC Congress 2015 (abstract 2191).
The research, known as the AEGEAN trial, was sponsored by Bristol-Myers Squibb, the company co-developing apixaban (Eliquis) with Pfizer.
“We used the best possible tools for the educational program, including the usual staff and procedures of the anticoagulation clinics, and all of this was useless,” said study investigator Gilles Montalescot, MD, PhD, of Hospitalier Universitaire Pitié-Salpêtrière in Paris, France.
“However, the trial showed very good adherence to apixaban, leaving little room for improvement with an educational program, suggesting one more advantage of prescribing non-vitamin K antagonists (VKAs) over VKAs in that there is apparently no need for additional education and information.”
Dr Montalescot and his colleagues conducted this study in 1162 AF patients receiving apixaban as stroke prophylaxis.
Roughly half of the patients (n=579) completed an educational program promoting treatment adherence, and the other half (n=583) received the usual information about their disease and the treatment.
The educational program included a patient information booklet explaining AF and anticoagulant treatment for stroke prevention, reminder tools (eg, a key ring and mobile phone alerts), and access to a virtual clinic utilizing staff from existing VKA monitoring clinics.
The researchers assessed differences between the 2 patient groups with regard to treatment adherence (defined as continuous, twice-daily dosing, with an occasional missed dose allowed) and treatment persistence (defined as absence of discontinuation for 30 consecutive days) over a 6-month observational period.
Adherence/persistence was measured using an electronic device that holds a blister pack of medication and records each time the pack is removed.
The researchers found no additional value of the educational program for either outcome.
At 24 weeks, the adherence rate was 88.5% in the control group and 88.3% in the education group (P=0.89). Treatment persistence rates were 90.5% and 91.1%, respectively (P=0.76).
For the second part of this study, the researchers are investigating long-term treatment adherence and the value of an educational program beyond 6 months.
“Future studies may want to test more aggressive and more costly educational programs,” Dr Montalescot noted. “But, in the meantime, the adherence and persistence rates we measured are quite reassuring with the common practice and usual mode of prescription of this medication.” ![]()

Photo courtesy of Pfizer
and Bristol-Myers Squibb
LONDON—An educational program designed to improve adherence to the anticoagulant apixaban proved ineffective in a phase 4 trial of patients with atrial fibrillation (AF).
But that’s because adherence was high whether patients completed the program or not.
This suggests current measures used to inform patients about apixaban may be sufficient to ensure treatment adherence, researchers said.
They presented these findings at the ESC Congress 2015 (abstract 2191).
The research, known as the AEGEAN trial, was sponsored by Bristol-Myers Squibb, the company co-developing apixaban (Eliquis) with Pfizer.
“We used the best possible tools for the educational program, including the usual staff and procedures of the anticoagulation clinics, and all of this was useless,” said study investigator Gilles Montalescot, MD, PhD, of Hospitalier Universitaire Pitié-Salpêtrière in Paris, France.
“However, the trial showed very good adherence to apixaban, leaving little room for improvement with an educational program, suggesting one more advantage of prescribing non-vitamin K antagonists (VKAs) over VKAs in that there is apparently no need for additional education and information.”
Dr Montalescot and his colleagues conducted this study in 1162 AF patients receiving apixaban as stroke prophylaxis.
Roughly half of the patients (n=579) completed an educational program promoting treatment adherence, and the other half (n=583) received the usual information about their disease and the treatment.
The educational program included a patient information booklet explaining AF and anticoagulant treatment for stroke prevention, reminder tools (eg, a key ring and mobile phone alerts), and access to a virtual clinic utilizing staff from existing VKA monitoring clinics.
The researchers assessed differences between the 2 patient groups with regard to treatment adherence (defined as continuous, twice-daily dosing, with an occasional missed dose allowed) and treatment persistence (defined as absence of discontinuation for 30 consecutive days) over a 6-month observational period.
Adherence/persistence was measured using an electronic device that holds a blister pack of medication and records each time the pack is removed.
The researchers found no additional value of the educational program for either outcome.
At 24 weeks, the adherence rate was 88.5% in the control group and 88.3% in the education group (P=0.89). Treatment persistence rates were 90.5% and 91.1%, respectively (P=0.76).
For the second part of this study, the researchers are investigating long-term treatment adherence and the value of an educational program beyond 6 months.
“Future studies may want to test more aggressive and more costly educational programs,” Dr Montalescot noted. “But, in the meantime, the adherence and persistence rates we measured are quite reassuring with the common practice and usual mode of prescription of this medication.” ![]()

Photo courtesy of Pfizer
and Bristol-Myers Squibb
LONDON—An educational program designed to improve adherence to the anticoagulant apixaban proved ineffective in a phase 4 trial of patients with atrial fibrillation (AF).
But that’s because adherence was high whether patients completed the program or not.
This suggests current measures used to inform patients about apixaban may be sufficient to ensure treatment adherence, researchers said.
They presented these findings at the ESC Congress 2015 (abstract 2191).
The research, known as the AEGEAN trial, was sponsored by Bristol-Myers Squibb, the company co-developing apixaban (Eliquis) with Pfizer.
“We used the best possible tools for the educational program, including the usual staff and procedures of the anticoagulation clinics, and all of this was useless,” said study investigator Gilles Montalescot, MD, PhD, of Hospitalier Universitaire Pitié-Salpêtrière in Paris, France.
“However, the trial showed very good adherence to apixaban, leaving little room for improvement with an educational program, suggesting one more advantage of prescribing non-vitamin K antagonists (VKAs) over VKAs in that there is apparently no need for additional education and information.”
Dr Montalescot and his colleagues conducted this study in 1162 AF patients receiving apixaban as stroke prophylaxis.
Roughly half of the patients (n=579) completed an educational program promoting treatment adherence, and the other half (n=583) received the usual information about their disease and the treatment.
The educational program included a patient information booklet explaining AF and anticoagulant treatment for stroke prevention, reminder tools (eg, a key ring and mobile phone alerts), and access to a virtual clinic utilizing staff from existing VKA monitoring clinics.
The researchers assessed differences between the 2 patient groups with regard to treatment adherence (defined as continuous, twice-daily dosing, with an occasional missed dose allowed) and treatment persistence (defined as absence of discontinuation for 30 consecutive days) over a 6-month observational period.
Adherence/persistence was measured using an electronic device that holds a blister pack of medication and records each time the pack is removed.
The researchers found no additional value of the educational program for either outcome.
At 24 weeks, the adherence rate was 88.5% in the control group and 88.3% in the education group (P=0.89). Treatment persistence rates were 90.5% and 91.1%, respectively (P=0.76).
For the second part of this study, the researchers are investigating long-term treatment adherence and the value of an educational program beyond 6 months.
“Future studies may want to test more aggressive and more costly educational programs,” Dr Montalescot noted. “But, in the meantime, the adherence and persistence rates we measured are quite reassuring with the common practice and usual mode of prescription of this medication.” ![]()
Early intervention may forestall menopause-related skin aging
NEW YORK – Evidence is mounting that early intervention in the menopausal transition could help forestall some of the skin aging associated with estrogen decline.
Estrogen supplementation and collagen stimulation both seem effective in preserving the integrity of a woman’s skin as levels of the hormone decrease, Dr. Diane Madfes said at the American Academy of Dermatology summer meeting.
Type 3 collagen decreases by up to 50% within a few years of menopause, said Dr. Madfes, a dermatologist in New York. This is directly related to a loss of estrogen receptor beta in the dermal matrix, which promotes collagen formation.
“There is a theory – the timing hypothesis – that we have a window of opportunity to intervene. If we can stimulate the collagen before the receptors go down, maybe we can have a beneficial effect on skin.”
Any method of collagen stimulation should work, she said: laser resurfacing, microneedling, or radiofrequency. “We are very good about being able to stimulate collagen. The method doesn’t matter as much as the timing. The important thing is to intervene early. If you see your patients starting to sag, see a loss of elasticity, that is the time to intervene. Get at the collagen while it’s still receptive.”
Estrogen exerts a plethora of antiaging, skin-preserving effects. “We know that a decrease in estrogen is related to telomere shortening. Estrogen protects against oxidative damage. It signals keratinocytes through IGF-1,” she said.
The hormone also protects skin’s water-binding qualities by promoting mucopolysaccharides, sebum production, barrier function, and hyaluronic acid. It may even play a role in protecting against ultraviolet light. Estrogen downregulation affects healing by inhibiting the proliferation of keratinocytes and the proliferation and migration of fibroblasts.
All these add up to rapid skin aging after estrogen levels drop.
“The visible effects of aging on women’s skin are not so much related to her chronological age as to the years after menopause,” Dr. Madfes said – a finding that is particularly illustrated in young women with surgical menopause and those with breast cancer who take tamoxifen. The observation seems to suggest that early intervention with estrogen might help prevent at least some of the signs of aging.
The ongoing KEEPS trial (Kronos Early Estrogen Prevention Study) may shed some light on the issue. KEEPS has randomized 729 women aged 42-58 years to oral or transdermal estrogen; the primary endpoint is rate of atherosclerosis. But an ancillary study is looking at the effect of estrogen on skin wrinkles and skin rigidity.
The substudy is based on positive findings of a 1996 study, which found evidence for facial application of topical estrogen designed for vulvar use. After 6 months, elasticity and firmness significantly improved. Skin moisture increased, as did type 3 collagen and collagen fibers.
Some women do use topical estrogens on their faces. “It seems to promote skin thickening and tightening,“ Dr. Madfes said, although a recent editorial suggested that using the product anywhere but on the genitals can cause estrogen-mediated side effects in both children and pets.
But recommending estrogen is fraught with controversy. Large studies have come to conflicting conclusions about its benefit and safety. And prescribing estrogen is not really within a dermatologist’s purview.
“It’s not for us to suggest that women go on hormone therapy. But we can explain these things and ask if she is taking it, or if she’s talked to her gynecologist about it.”
Dr. Madfes has no financial disclosures to report.
On Twitter @Alz_Gal
NEW YORK – Evidence is mounting that early intervention in the menopausal transition could help forestall some of the skin aging associated with estrogen decline.
Estrogen supplementation and collagen stimulation both seem effective in preserving the integrity of a woman’s skin as levels of the hormone decrease, Dr. Diane Madfes said at the American Academy of Dermatology summer meeting.
Type 3 collagen decreases by up to 50% within a few years of menopause, said Dr. Madfes, a dermatologist in New York. This is directly related to a loss of estrogen receptor beta in the dermal matrix, which promotes collagen formation.
“There is a theory – the timing hypothesis – that we have a window of opportunity to intervene. If we can stimulate the collagen before the receptors go down, maybe we can have a beneficial effect on skin.”
Any method of collagen stimulation should work, she said: laser resurfacing, microneedling, or radiofrequency. “We are very good about being able to stimulate collagen. The method doesn’t matter as much as the timing. The important thing is to intervene early. If you see your patients starting to sag, see a loss of elasticity, that is the time to intervene. Get at the collagen while it’s still receptive.”
Estrogen exerts a plethora of antiaging, skin-preserving effects. “We know that a decrease in estrogen is related to telomere shortening. Estrogen protects against oxidative damage. It signals keratinocytes through IGF-1,” she said.
The hormone also protects skin’s water-binding qualities by promoting mucopolysaccharides, sebum production, barrier function, and hyaluronic acid. It may even play a role in protecting against ultraviolet light. Estrogen downregulation affects healing by inhibiting the proliferation of keratinocytes and the proliferation and migration of fibroblasts.
All these add up to rapid skin aging after estrogen levels drop.
“The visible effects of aging on women’s skin are not so much related to her chronological age as to the years after menopause,” Dr. Madfes said – a finding that is particularly illustrated in young women with surgical menopause and those with breast cancer who take tamoxifen. The observation seems to suggest that early intervention with estrogen might help prevent at least some of the signs of aging.
The ongoing KEEPS trial (Kronos Early Estrogen Prevention Study) may shed some light on the issue. KEEPS has randomized 729 women aged 42-58 years to oral or transdermal estrogen; the primary endpoint is rate of atherosclerosis. But an ancillary study is looking at the effect of estrogen on skin wrinkles and skin rigidity.
The substudy is based on positive findings of a 1996 study, which found evidence for facial application of topical estrogen designed for vulvar use. After 6 months, elasticity and firmness significantly improved. Skin moisture increased, as did type 3 collagen and collagen fibers.
Some women do use topical estrogens on their faces. “It seems to promote skin thickening and tightening,“ Dr. Madfes said, although a recent editorial suggested that using the product anywhere but on the genitals can cause estrogen-mediated side effects in both children and pets.
But recommending estrogen is fraught with controversy. Large studies have come to conflicting conclusions about its benefit and safety. And prescribing estrogen is not really within a dermatologist’s purview.
“It’s not for us to suggest that women go on hormone therapy. But we can explain these things and ask if she is taking it, or if she’s talked to her gynecologist about it.”
Dr. Madfes has no financial disclosures to report.
On Twitter @Alz_Gal
NEW YORK – Evidence is mounting that early intervention in the menopausal transition could help forestall some of the skin aging associated with estrogen decline.
Estrogen supplementation and collagen stimulation both seem effective in preserving the integrity of a woman’s skin as levels of the hormone decrease, Dr. Diane Madfes said at the American Academy of Dermatology summer meeting.
Type 3 collagen decreases by up to 50% within a few years of menopause, said Dr. Madfes, a dermatologist in New York. This is directly related to a loss of estrogen receptor beta in the dermal matrix, which promotes collagen formation.
“There is a theory – the timing hypothesis – that we have a window of opportunity to intervene. If we can stimulate the collagen before the receptors go down, maybe we can have a beneficial effect on skin.”
Any method of collagen stimulation should work, she said: laser resurfacing, microneedling, or radiofrequency. “We are very good about being able to stimulate collagen. The method doesn’t matter as much as the timing. The important thing is to intervene early. If you see your patients starting to sag, see a loss of elasticity, that is the time to intervene. Get at the collagen while it’s still receptive.”
Estrogen exerts a plethora of antiaging, skin-preserving effects. “We know that a decrease in estrogen is related to telomere shortening. Estrogen protects against oxidative damage. It signals keratinocytes through IGF-1,” she said.
The hormone also protects skin’s water-binding qualities by promoting mucopolysaccharides, sebum production, barrier function, and hyaluronic acid. It may even play a role in protecting against ultraviolet light. Estrogen downregulation affects healing by inhibiting the proliferation of keratinocytes and the proliferation and migration of fibroblasts.
All these add up to rapid skin aging after estrogen levels drop.
“The visible effects of aging on women’s skin are not so much related to her chronological age as to the years after menopause,” Dr. Madfes said – a finding that is particularly illustrated in young women with surgical menopause and those with breast cancer who take tamoxifen. The observation seems to suggest that early intervention with estrogen might help prevent at least some of the signs of aging.
The ongoing KEEPS trial (Kronos Early Estrogen Prevention Study) may shed some light on the issue. KEEPS has randomized 729 women aged 42-58 years to oral or transdermal estrogen; the primary endpoint is rate of atherosclerosis. But an ancillary study is looking at the effect of estrogen on skin wrinkles and skin rigidity.
The substudy is based on positive findings of a 1996 study, which found evidence for facial application of topical estrogen designed for vulvar use. After 6 months, elasticity and firmness significantly improved. Skin moisture increased, as did type 3 collagen and collagen fibers.
Some women do use topical estrogens on their faces. “It seems to promote skin thickening and tightening,“ Dr. Madfes said, although a recent editorial suggested that using the product anywhere but on the genitals can cause estrogen-mediated side effects in both children and pets.
But recommending estrogen is fraught with controversy. Large studies have come to conflicting conclusions about its benefit and safety. And prescribing estrogen is not really within a dermatologist’s purview.
“It’s not for us to suggest that women go on hormone therapy. But we can explain these things and ask if she is taking it, or if she’s talked to her gynecologist about it.”
Dr. Madfes has no financial disclosures to report.
On Twitter @Alz_Gal
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2015
Public uninformed about cancer therapies, survey suggests

receiving chemotherapy
Photo by Rhoda Baer
Results of a new survey suggest many adults in the UK may be uninformed about cancer treatment options, despite broad media coverage of these therapies.
Personalized drug treatment, immunotherapy, and proton beam therapy have all been covered by the lay media and featured in news stories across the globe.
But a survey of more than 2000 UK adults showed that most respondents were not aware of these treatment types.
Only 19% of respondents said they had heard about immunotherapy, 29% had heard of personalized drug treatment, and 30% had heard of proton beam therapy.
The survey, which included 2081 adults, was conducted online by YouGov in June. It was commissioned by Cancer Research UK and other members of the Radiotherapy Awareness Programme.
The primary goal of the survey was to examine public awareness of radiotherapy. And the results showed that many respondents were unaware of newer, more targeted radiotherapy options.
Respondents were largely uninformed about other types of cancer treatment as well. However, of the respondents who elected to give their opinion (n=1877), most said the National Health Service (NHS) should fund chemotherapy and other drug treatments over radiotherapy.
Survey questions and responses were as follows.
Radiotherapy
Before taking this survey, which, if any, of the following types of radiotherapy had you heard of?
| Intensity-modulated radiotherapy | 4% |
| Stereotactic radiotherapy/
stereotactic ablative radiotherapy |
3% |
| Image-guided radiotherapy | 9% |
| Proton beam therapy | 30% |
| Brachytherapy | 5% |
| Radiofrequency ablation | 7% |
| Cyberknife | 4% |
| Gammaknife | 6% |
| Higgs-boson radiotherapy
(red herring option) |
6% |
| Carbon ion radiotherapy
(red herring option) |
3% |
| None of these | 50% |
| Prefer not to say | 11% |
Other cancer treatments
Which, if any, of the following specific types of cancer treatments/tests had you heard of before taking this survey?
| Immunotherapy | 19% |
| Personalized drugs | 29% |
| Monoclonal antibodies | 5% |
| High-dose chemotherapy
with stem cell transplant |
26% |
| Tablet chemotherapy | 28% |
| Molecular diagnostic tests | 6% |
| Robotically assisted surgery/Da Vinci robot | 12% |
| Laparoscopic (keyhole) surgery | 39% |
| None of these | 32% |
| Prefer not to say | 11% |
NHS funding
What level of priority do you think the NHS should give to funding each of the following 4 types of cancer treatments?
| Treatment | 1st priority | 2nd priority | 3rd priority | Lowest priority |
| Chemotherapy &
other drug treatments |
57% | 29% | 10% | 4% |
| Surgery | 29% | 35% | 31% | 5% |
| Radiotherapy | 9% | 32% | 53% | 5% |
| Alternative treatments | 5% | 4% | 6% | 86% |
![]()

receiving chemotherapy
Photo by Rhoda Baer
Results of a new survey suggest many adults in the UK may be uninformed about cancer treatment options, despite broad media coverage of these therapies.
Personalized drug treatment, immunotherapy, and proton beam therapy have all been covered by the lay media and featured in news stories across the globe.
But a survey of more than 2000 UK adults showed that most respondents were not aware of these treatment types.
Only 19% of respondents said they had heard about immunotherapy, 29% had heard of personalized drug treatment, and 30% had heard of proton beam therapy.
The survey, which included 2081 adults, was conducted online by YouGov in June. It was commissioned by Cancer Research UK and other members of the Radiotherapy Awareness Programme.
The primary goal of the survey was to examine public awareness of radiotherapy. And the results showed that many respondents were unaware of newer, more targeted radiotherapy options.
Respondents were largely uninformed about other types of cancer treatment as well. However, of the respondents who elected to give their opinion (n=1877), most said the National Health Service (NHS) should fund chemotherapy and other drug treatments over radiotherapy.
Survey questions and responses were as follows.
Radiotherapy
Before taking this survey, which, if any, of the following types of radiotherapy had you heard of?
| Intensity-modulated radiotherapy | 4% |
| Stereotactic radiotherapy/
stereotactic ablative radiotherapy |
3% |
| Image-guided radiotherapy | 9% |
| Proton beam therapy | 30% |
| Brachytherapy | 5% |
| Radiofrequency ablation | 7% |
| Cyberknife | 4% |
| Gammaknife | 6% |
| Higgs-boson radiotherapy
(red herring option) |
6% |
| Carbon ion radiotherapy
(red herring option) |
3% |
| None of these | 50% |
| Prefer not to say | 11% |
Other cancer treatments
Which, if any, of the following specific types of cancer treatments/tests had you heard of before taking this survey?
| Immunotherapy | 19% |
| Personalized drugs | 29% |
| Monoclonal antibodies | 5% |
| High-dose chemotherapy
with stem cell transplant |
26% |
| Tablet chemotherapy | 28% |
| Molecular diagnostic tests | 6% |
| Robotically assisted surgery/Da Vinci robot | 12% |
| Laparoscopic (keyhole) surgery | 39% |
| None of these | 32% |
| Prefer not to say | 11% |
NHS funding
What level of priority do you think the NHS should give to funding each of the following 4 types of cancer treatments?
| Treatment | 1st priority | 2nd priority | 3rd priority | Lowest priority |
| Chemotherapy &
other drug treatments |
57% | 29% | 10% | 4% |
| Surgery | 29% | 35% | 31% | 5% |
| Radiotherapy | 9% | 32% | 53% | 5% |
| Alternative treatments | 5% | 4% | 6% | 86% |
![]()

receiving chemotherapy
Photo by Rhoda Baer
Results of a new survey suggest many adults in the UK may be uninformed about cancer treatment options, despite broad media coverage of these therapies.
Personalized drug treatment, immunotherapy, and proton beam therapy have all been covered by the lay media and featured in news stories across the globe.
But a survey of more than 2000 UK adults showed that most respondents were not aware of these treatment types.
Only 19% of respondents said they had heard about immunotherapy, 29% had heard of personalized drug treatment, and 30% had heard of proton beam therapy.
The survey, which included 2081 adults, was conducted online by YouGov in June. It was commissioned by Cancer Research UK and other members of the Radiotherapy Awareness Programme.
The primary goal of the survey was to examine public awareness of radiotherapy. And the results showed that many respondents were unaware of newer, more targeted radiotherapy options.
Respondents were largely uninformed about other types of cancer treatment as well. However, of the respondents who elected to give their opinion (n=1877), most said the National Health Service (NHS) should fund chemotherapy and other drug treatments over radiotherapy.
Survey questions and responses were as follows.
Radiotherapy
Before taking this survey, which, if any, of the following types of radiotherapy had you heard of?
| Intensity-modulated radiotherapy | 4% |
| Stereotactic radiotherapy/
stereotactic ablative radiotherapy |
3% |
| Image-guided radiotherapy | 9% |
| Proton beam therapy | 30% |
| Brachytherapy | 5% |
| Radiofrequency ablation | 7% |
| Cyberknife | 4% |
| Gammaknife | 6% |
| Higgs-boson radiotherapy
(red herring option) |
6% |
| Carbon ion radiotherapy
(red herring option) |
3% |
| None of these | 50% |
| Prefer not to say | 11% |
Other cancer treatments
Which, if any, of the following specific types of cancer treatments/tests had you heard of before taking this survey?
| Immunotherapy | 19% |
| Personalized drugs | 29% |
| Monoclonal antibodies | 5% |
| High-dose chemotherapy
with stem cell transplant |
26% |
| Tablet chemotherapy | 28% |
| Molecular diagnostic tests | 6% |
| Robotically assisted surgery/Da Vinci robot | 12% |
| Laparoscopic (keyhole) surgery | 39% |
| None of these | 32% |
| Prefer not to say | 11% |
NHS funding
What level of priority do you think the NHS should give to funding each of the following 4 types of cancer treatments?
| Treatment | 1st priority | 2nd priority | 3rd priority | Lowest priority |
| Chemotherapy &
other drug treatments |
57% | 29% | 10% | 4% |
| Surgery | 29% | 35% | 31% | 5% |
| Radiotherapy | 9% | 32% | 53% | 5% |
| Alternative treatments | 5% | 4% | 6% | 86% |
![]()
Less posttransplant primary biliary cirrhosis with preventive UDCA
Only 21% of liver transplantation patients who received ursodeoxycholic acid (UDCA) after surgery developed recurrent primary biliary cirrhosis, compared with 62% of patients who did not receive the bile acid, researchers reported in the Journal of Hepatology.
The results provide strong evidence that routinely giving liver transplant patients UDCA can prevent or delay recurrent primary biliary cirrhosis, said Alexie Bosch at Hôpital Edouard Herriot in Lyon, France, and his associates.
Primary biliary cirrhosis can recur after liver transplantation and increases the chances of graft dysfunction, the researchers noted. UDCA is the only approved medical treatment for primary biliary cirrhosis in the United States or Europe, but no research team has studied its potential to prevent recurrent primary biliary cirrhosis after liver transplantation, they added. Therefore, they retrospectively studied 90 patients with primary biliary cirrhosis who underwent liver transplantation at five centers in France and Switzerland between 1988 and 2010. In all, 21% of patients received oral UDCA (10-15 mg/kg per day in two divided doses) within 2 weeks after their operation, while the rest received it only if they developed biopsy-confirmed recurrent primary biliary cirrhosis. Biopsies were taken at posttransplant year 1 and every 5 years after that, or when clinically indicated, the investigators noted (J Hepatol. 2015 Aug. 14. doi: 10.1016/j.jhep.2015.07.038).
Patients who received preventive UDCA had a lower cumulative rate of recurrence throughout 15 years of postsurgical follow-up (P = .014), the researchers reported. The chances of recurrent primary biliary cirrhosis at 5, 10, and 15 years after transplantation were 11%, 21%, and 40% in the UDCA group, compared with 32%, 53%, and 70% for patients who did not receive prophylactic UDCA, they added. A multivariable analysis showed that recurrent primary biliary cirrhosis was associated with not receiving prophylactic UDCA (hazard ratio, 0.32; 95% confidence interval, 0.11, 0.91), but was not linked to donor age, Model For End-Stage Liver Disease (MELD) score, or sex mismatch between donor and recipient, the investigators said. Preventive UDCA also was tied to a 1.6-year longer median time to recurrence, although the trend did not reach statistical significance.
Although the study was retrospective and most patients who received UDCA were treated at one transplant center, all centers had similar histologic findings for recurrent primary biliary cirrhosis, said the researchers. Biopsies also were histologically similar regardless of whether they were event driven or obtained based on the study protocol, and time to recurrence did not vary based on biopsy type, they added. “In our multivariate analysis, we took care to account for all risk factors and confounders, as well as to test multilevel models in order to exclude potential misleading results and center effects,” they emphasized. “Given the extremely limited feasibility of prospective studies and the good tolerance and acceptability of long-term UDCA therapy, these results support the extended use of UDCA as prophylaxis for primary biliary cirrhosis recurrence after liver transplantation.”
The researchers declared no funding sources and reported having no conflicts of interest.
Only 21% of liver transplantation patients who received ursodeoxycholic acid (UDCA) after surgery developed recurrent primary biliary cirrhosis, compared with 62% of patients who did not receive the bile acid, researchers reported in the Journal of Hepatology.
The results provide strong evidence that routinely giving liver transplant patients UDCA can prevent or delay recurrent primary biliary cirrhosis, said Alexie Bosch at Hôpital Edouard Herriot in Lyon, France, and his associates.
Primary biliary cirrhosis can recur after liver transplantation and increases the chances of graft dysfunction, the researchers noted. UDCA is the only approved medical treatment for primary biliary cirrhosis in the United States or Europe, but no research team has studied its potential to prevent recurrent primary biliary cirrhosis after liver transplantation, they added. Therefore, they retrospectively studied 90 patients with primary biliary cirrhosis who underwent liver transplantation at five centers in France and Switzerland between 1988 and 2010. In all, 21% of patients received oral UDCA (10-15 mg/kg per day in two divided doses) within 2 weeks after their operation, while the rest received it only if they developed biopsy-confirmed recurrent primary biliary cirrhosis. Biopsies were taken at posttransplant year 1 and every 5 years after that, or when clinically indicated, the investigators noted (J Hepatol. 2015 Aug. 14. doi: 10.1016/j.jhep.2015.07.038).
Patients who received preventive UDCA had a lower cumulative rate of recurrence throughout 15 years of postsurgical follow-up (P = .014), the researchers reported. The chances of recurrent primary biliary cirrhosis at 5, 10, and 15 years after transplantation were 11%, 21%, and 40% in the UDCA group, compared with 32%, 53%, and 70% for patients who did not receive prophylactic UDCA, they added. A multivariable analysis showed that recurrent primary biliary cirrhosis was associated with not receiving prophylactic UDCA (hazard ratio, 0.32; 95% confidence interval, 0.11, 0.91), but was not linked to donor age, Model For End-Stage Liver Disease (MELD) score, or sex mismatch between donor and recipient, the investigators said. Preventive UDCA also was tied to a 1.6-year longer median time to recurrence, although the trend did not reach statistical significance.
Although the study was retrospective and most patients who received UDCA were treated at one transplant center, all centers had similar histologic findings for recurrent primary biliary cirrhosis, said the researchers. Biopsies also were histologically similar regardless of whether they were event driven or obtained based on the study protocol, and time to recurrence did not vary based on biopsy type, they added. “In our multivariate analysis, we took care to account for all risk factors and confounders, as well as to test multilevel models in order to exclude potential misleading results and center effects,” they emphasized. “Given the extremely limited feasibility of prospective studies and the good tolerance and acceptability of long-term UDCA therapy, these results support the extended use of UDCA as prophylaxis for primary biliary cirrhosis recurrence after liver transplantation.”
The researchers declared no funding sources and reported having no conflicts of interest.
Only 21% of liver transplantation patients who received ursodeoxycholic acid (UDCA) after surgery developed recurrent primary biliary cirrhosis, compared with 62% of patients who did not receive the bile acid, researchers reported in the Journal of Hepatology.
The results provide strong evidence that routinely giving liver transplant patients UDCA can prevent or delay recurrent primary biliary cirrhosis, said Alexie Bosch at Hôpital Edouard Herriot in Lyon, France, and his associates.
Primary biliary cirrhosis can recur after liver transplantation and increases the chances of graft dysfunction, the researchers noted. UDCA is the only approved medical treatment for primary biliary cirrhosis in the United States or Europe, but no research team has studied its potential to prevent recurrent primary biliary cirrhosis after liver transplantation, they added. Therefore, they retrospectively studied 90 patients with primary biliary cirrhosis who underwent liver transplantation at five centers in France and Switzerland between 1988 and 2010. In all, 21% of patients received oral UDCA (10-15 mg/kg per day in two divided doses) within 2 weeks after their operation, while the rest received it only if they developed biopsy-confirmed recurrent primary biliary cirrhosis. Biopsies were taken at posttransplant year 1 and every 5 years after that, or when clinically indicated, the investigators noted (J Hepatol. 2015 Aug. 14. doi: 10.1016/j.jhep.2015.07.038).
Patients who received preventive UDCA had a lower cumulative rate of recurrence throughout 15 years of postsurgical follow-up (P = .014), the researchers reported. The chances of recurrent primary biliary cirrhosis at 5, 10, and 15 years after transplantation were 11%, 21%, and 40% in the UDCA group, compared with 32%, 53%, and 70% for patients who did not receive prophylactic UDCA, they added. A multivariable analysis showed that recurrent primary biliary cirrhosis was associated with not receiving prophylactic UDCA (hazard ratio, 0.32; 95% confidence interval, 0.11, 0.91), but was not linked to donor age, Model For End-Stage Liver Disease (MELD) score, or sex mismatch between donor and recipient, the investigators said. Preventive UDCA also was tied to a 1.6-year longer median time to recurrence, although the trend did not reach statistical significance.
Although the study was retrospective and most patients who received UDCA were treated at one transplant center, all centers had similar histologic findings for recurrent primary biliary cirrhosis, said the researchers. Biopsies also were histologically similar regardless of whether they were event driven or obtained based on the study protocol, and time to recurrence did not vary based on biopsy type, they added. “In our multivariate analysis, we took care to account for all risk factors and confounders, as well as to test multilevel models in order to exclude potential misleading results and center effects,” they emphasized. “Given the extremely limited feasibility of prospective studies and the good tolerance and acceptability of long-term UDCA therapy, these results support the extended use of UDCA as prophylaxis for primary biliary cirrhosis recurrence after liver transplantation.”
The researchers declared no funding sources and reported having no conflicts of interest.
FROM THE JOURNAL OF HEPATOLOGY
Key clinical point: Treatment with prophylactic ursodeoxycholic acid might help prevent recurrent primary biliary cirrhosis after liver transplantation.
Major finding: Patients who received preventive UDCA had a lower cumulative rate of recurrence throughout 15 years of follow-up (P = .014).
Data source: Multicenter retrospective study of 90 patients who underwent liver transplantation for primary biliary cirrhosis.
Disclosures: The researchers declared no funding sources and reported having no conflicts of interest.
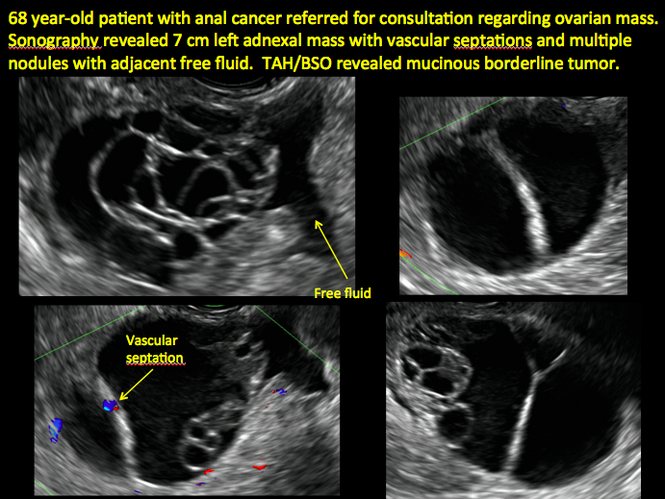
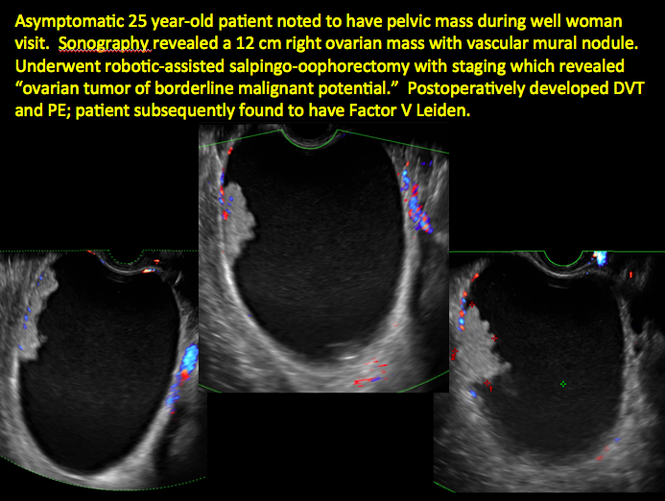
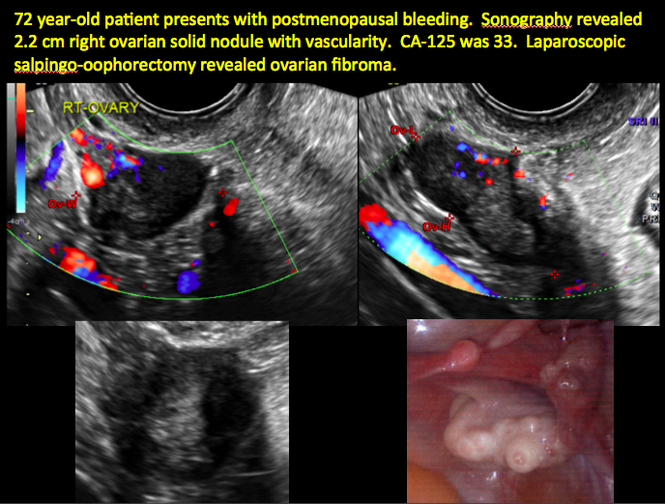
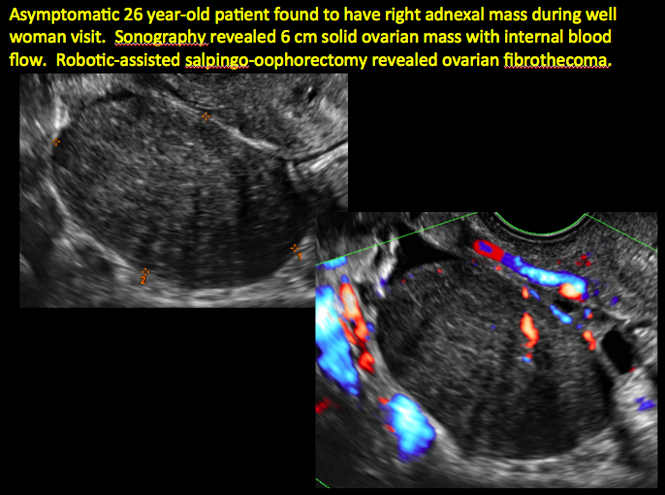
Imaging the suspected ovarian malignancy: 14 cases
Pelvic ultrasonography remains the preferred imaging method to evaluate most adnexal cysts given its ability to characterize such cysts with high resolution and accuracy. Most cystic adnexal masses have characteristic findings that can guide counseling and management decisions. For instance, mature cystic teratomas have hyperechoic lines/dots and acoustic shadowing; hydrosalpinx are tubular or s shaped and show a “waist sign.”
In parts 1 through 3 of this 4-part series on adnexal pathology, we presented images detailing common benign adnexal cysts, including:
- simple and hemorrhagic cysts (Part 1:Telltale sonographic features of simple and hemorrhagic cysts)
- mature cystic teratomas (dermoid cysts) and endometriomas (Part 2: Imaging the endometrioma and mature cystic teratoma)
- hydrosalpinx and pelvic inclusion cysts (Part 3: “Cogwheel” and other signs of hydrosalpinx and pelvic inclusion cysts)
In this conclusion to the series, we detail imaging for ovarian neoplasias (including cystadenoma and cystadenocarcinoma).
OVARIAN NEOPLASIA
A woman’s lifetime risk of undergoing surgery for suspected ovarian malignancy is 5% to 10% in the United States, and only about 13% to 21% of those undergoing surgery will actually be diagnosed with ovarian cancer.1 Therefore, the goal of diagnostic evaluation is to exclude malignancy.
Diagnostic evaluation includes:
- imaging
- lab work
- history
- physical findings.
The preferred imaging modality for a pelvic mass in asymptomatic premenopausal and postmenopausal women is transvaginal ultrasonography according to the American College of Obstetricians and Gynecologists (ACOG) practice bulletin, which was reaffirmed in 2013.1 “No alternative imaging modality has demonstrated sufficient superiority to transvaginal ultrasonography to justify its routine use.”1
Transvaginal ultrasonography with color Doppler interrogation has demonstrated a sensitivity of 0.86% and a specificity of 0.91% for discriminating between malignant and benign ovarian masses.
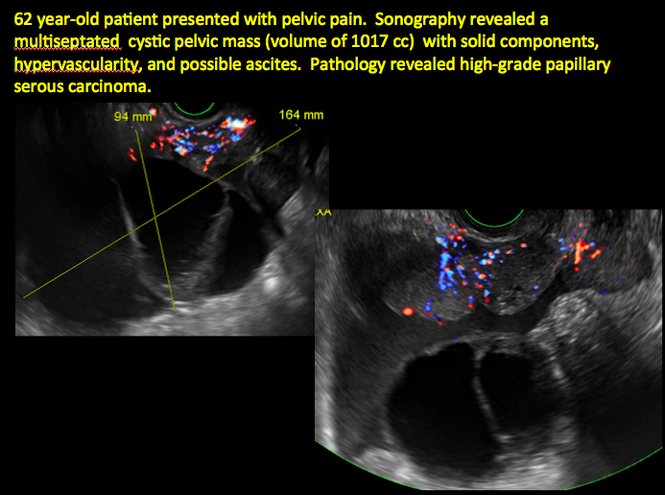
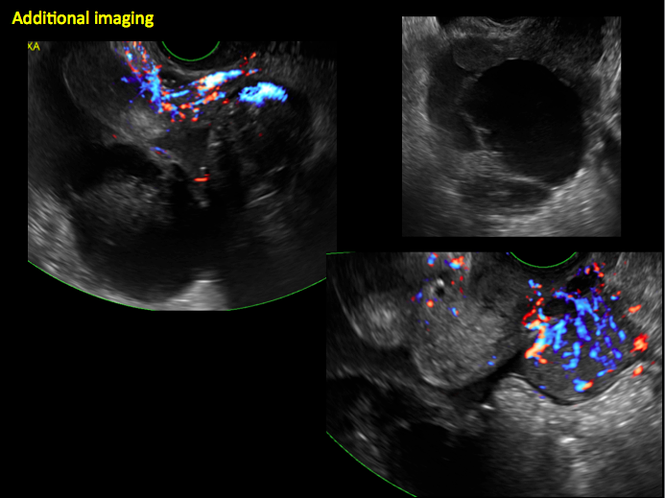
Sonographic features that are worrisome for malignancy include:
- Multiple thin septations (if indeterminate, the mass may possibly be benign)
- Thick (> 3 mm), irregular septations
- Focal areas of wall thickening (> 3 mm)
- Mural nodules or papillary projections
- Levine and colleagues note that a cyst with a mural nodule with internal blood flow on color Doppler has the highest likelihood of being malignant2
- Moderate or large amount of ascitic fluid in pelvis (in conjunction with ovarian mass showing the above characteristics)
Various morphology indices have been developed that combine these criteria with ovarian mass volume to determine the preoperative predictive value for malignancy.
In the images that follow, we present 14 cases that demonstrate cystadenoma, low malignant potential tumors, and ovarian neoplasia.
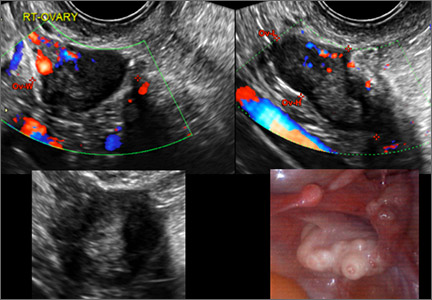
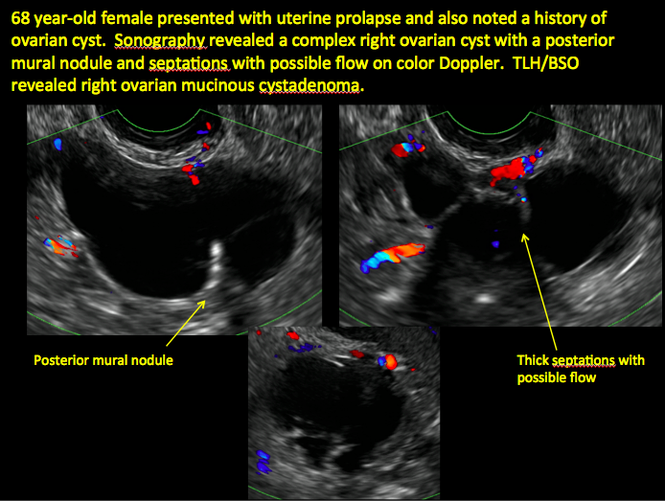
CASE 1. Right ovarian mucinous cystadenoma in 68-year-old woman with uterine prolapse and history of ovarian cyst
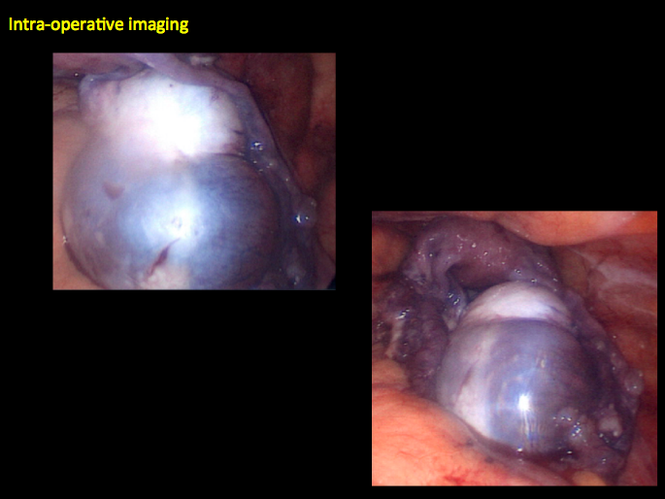
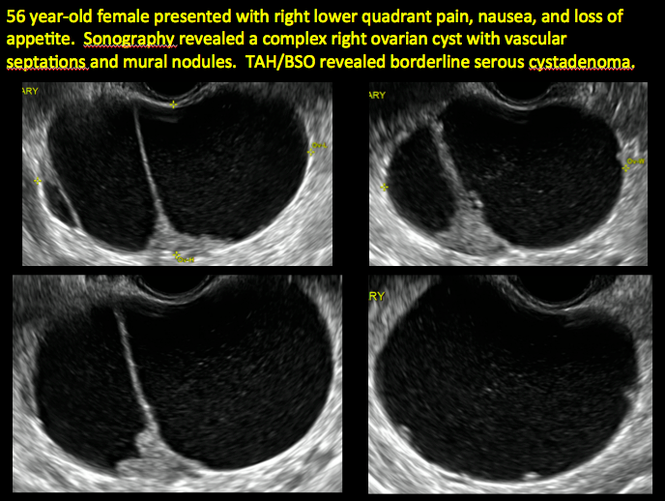
CASE 2. Borderline serous cystadenoma in 56-year-old woman with right lower quadrant pain, nausea, and loss of appetite

CASE 3. Mucinous cystadenoma in 38-year-old woman undergoing sonography for spontaneous abortion

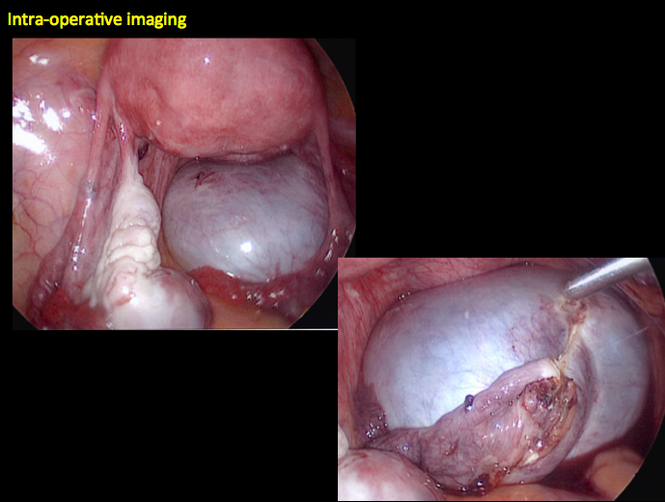
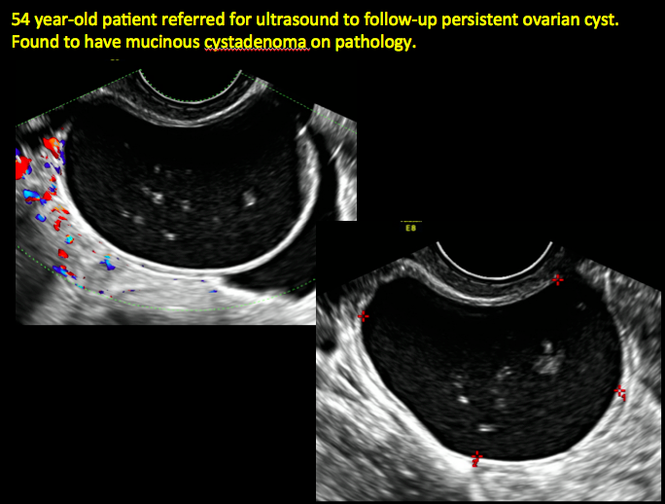
CASE 4. Mucinous cystadenoma in 54-year-old woman undergoing follow-up ultrasound for persistent ovarian cyst
CASE 7. Mature cystic teratoma in 31-year-old woman with progressively heavier bleeding and pelvic pain

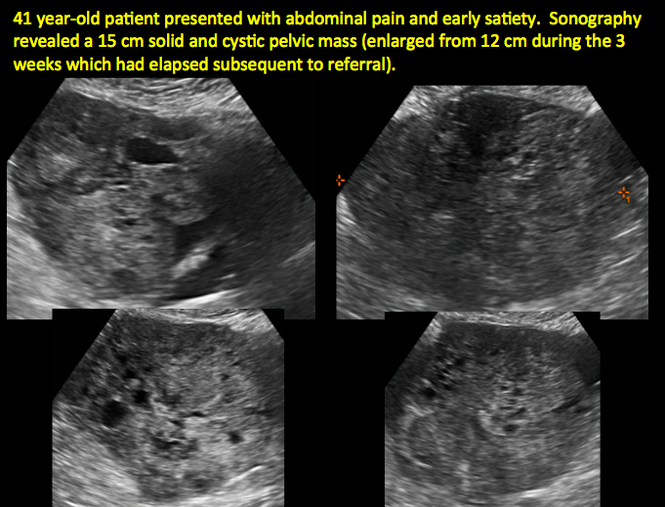
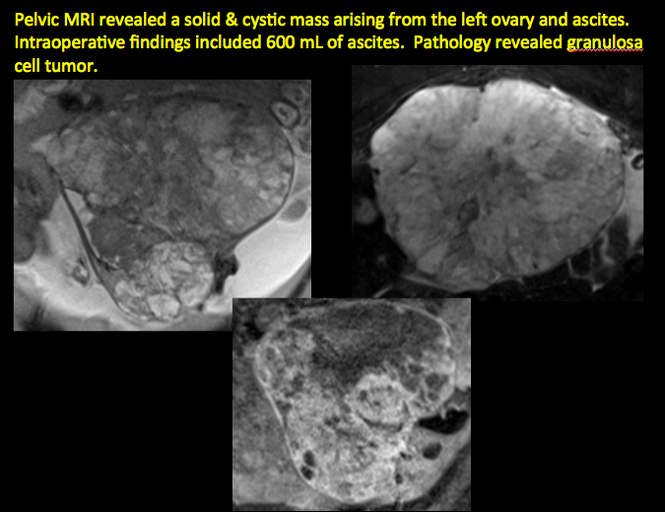
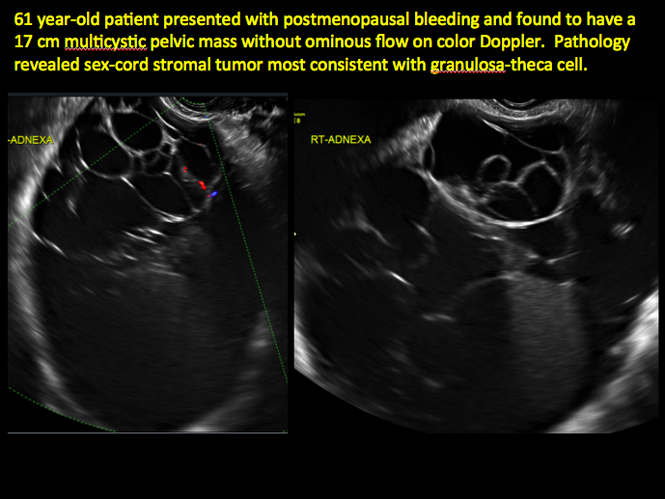
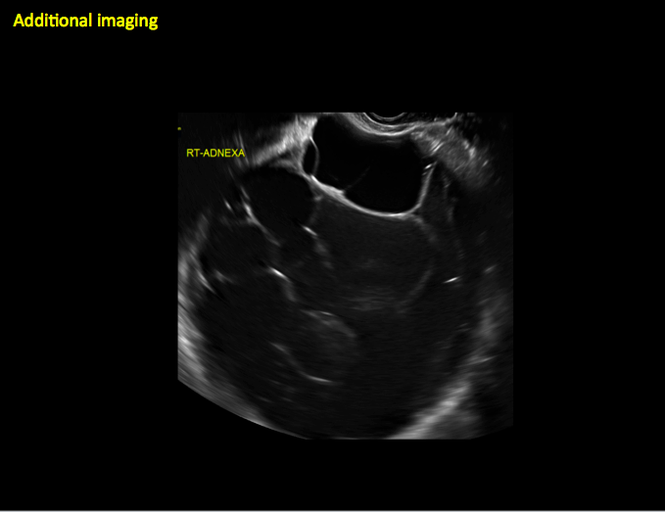
CASE 11. Sex-cord stromal tumor in 61-year-old woman with postmenopausal bleeding
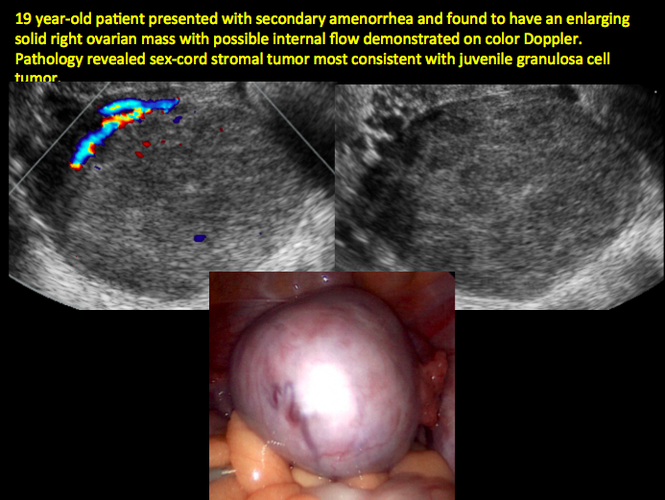
CASE 12. Juvenile granulosa cell tumor in 19-year-old patient with secondary amenorrhea
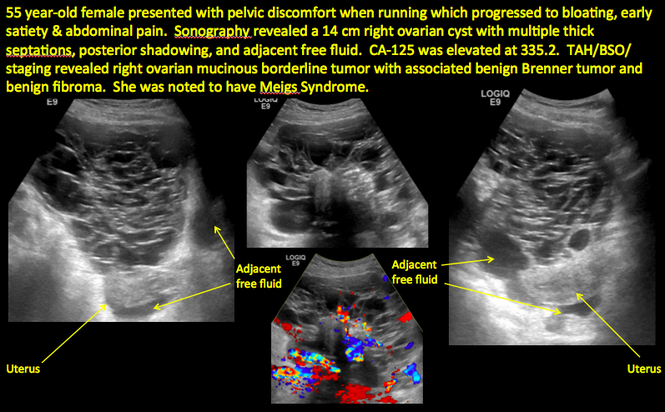
CASE 14. Mucinous borderline tumor in 55-year-old woman with pelvic discomfort
Pelvic ultrasonography remains the preferred imaging method to evaluate most adnexal cysts given its ability to characterize such cysts with high resolution and accuracy. Most cystic adnexal masses have characteristic findings that can guide counseling and management decisions. For instance, mature cystic teratomas have hyperechoic lines/dots and acoustic shadowing; hydrosalpinx are tubular or s shaped and show a “waist sign.”
In parts 1 through 3 of this 4-part series on adnexal pathology, we presented images detailing common benign adnexal cysts, including:
- simple and hemorrhagic cysts (Part 1:Telltale sonographic features of simple and hemorrhagic cysts)
- mature cystic teratomas (dermoid cysts) and endometriomas (Part 2: Imaging the endometrioma and mature cystic teratoma)
- hydrosalpinx and pelvic inclusion cysts (Part 3: “Cogwheel” and other signs of hydrosalpinx and pelvic inclusion cysts)
In this conclusion to the series, we detail imaging for ovarian neoplasias (including cystadenoma and cystadenocarcinoma).
OVARIAN NEOPLASIA
A woman’s lifetime risk of undergoing surgery for suspected ovarian malignancy is 5% to 10% in the United States, and only about 13% to 21% of those undergoing surgery will actually be diagnosed with ovarian cancer.1 Therefore, the goal of diagnostic evaluation is to exclude malignancy.
Diagnostic evaluation includes:
- imaging
- lab work
- history
- physical findings.
The preferred imaging modality for a pelvic mass in asymptomatic premenopausal and postmenopausal women is transvaginal ultrasonography according to the American College of Obstetricians and Gynecologists (ACOG) practice bulletin, which was reaffirmed in 2013.1 “No alternative imaging modality has demonstrated sufficient superiority to transvaginal ultrasonography to justify its routine use.”1
Transvaginal ultrasonography with color Doppler interrogation has demonstrated a sensitivity of 0.86% and a specificity of 0.91% for discriminating between malignant and benign ovarian masses.
Sonographic features that are worrisome for malignancy include:
- Multiple thin septations (if indeterminate, the mass may possibly be benign)
- Thick (> 3 mm), irregular septations
- Focal areas of wall thickening (> 3 mm)
- Mural nodules or papillary projections
- Levine and colleagues note that a cyst with a mural nodule with internal blood flow on color Doppler has the highest likelihood of being malignant2
- Moderate or large amount of ascitic fluid in pelvis (in conjunction with ovarian mass showing the above characteristics)
Various morphology indices have been developed that combine these criteria with ovarian mass volume to determine the preoperative predictive value for malignancy.
In the images that follow, we present 14 cases that demonstrate cystadenoma, low malignant potential tumors, and ovarian neoplasia.
CASE 1. Right ovarian mucinous cystadenoma in 68-year-old woman with uterine prolapse and history of ovarian cyst


CASE 2. Borderline serous cystadenoma in 56-year-old woman with right lower quadrant pain, nausea, and loss of appetite


CASE 3. Mucinous cystadenoma in 38-year-old woman undergoing sonography for spontaneous abortion


CASE 4. Mucinous cystadenoma in 54-year-old woman undergoing follow-up ultrasound for persistent ovarian cyst



CASE 7. Mature cystic teratoma in 31-year-old woman with progressively heavier bleeding and pelvic pain





CASE 11. Sex-cord stromal tumor in 61-year-old woman with postmenopausal bleeding


CASE 12. Juvenile granulosa cell tumor in 19-year-old patient with secondary amenorrhea



CASE 14. Mucinous borderline tumor in 55-year-old woman with pelvic discomfort

Pelvic ultrasonography remains the preferred imaging method to evaluate most adnexal cysts given its ability to characterize such cysts with high resolution and accuracy. Most cystic adnexal masses have characteristic findings that can guide counseling and management decisions. For instance, mature cystic teratomas have hyperechoic lines/dots and acoustic shadowing; hydrosalpinx are tubular or s shaped and show a “waist sign.”
In parts 1 through 3 of this 4-part series on adnexal pathology, we presented images detailing common benign adnexal cysts, including:
- simple and hemorrhagic cysts (Part 1:Telltale sonographic features of simple and hemorrhagic cysts)
- mature cystic teratomas (dermoid cysts) and endometriomas (Part 2: Imaging the endometrioma and mature cystic teratoma)
- hydrosalpinx and pelvic inclusion cysts (Part 3: “Cogwheel” and other signs of hydrosalpinx and pelvic inclusion cysts)
In this conclusion to the series, we detail imaging for ovarian neoplasias (including cystadenoma and cystadenocarcinoma).
OVARIAN NEOPLASIA
A woman’s lifetime risk of undergoing surgery for suspected ovarian malignancy is 5% to 10% in the United States, and only about 13% to 21% of those undergoing surgery will actually be diagnosed with ovarian cancer.1 Therefore, the goal of diagnostic evaluation is to exclude malignancy.
Diagnostic evaluation includes:
- imaging
- lab work
- history
- physical findings.
The preferred imaging modality for a pelvic mass in asymptomatic premenopausal and postmenopausal women is transvaginal ultrasonography according to the American College of Obstetricians and Gynecologists (ACOG) practice bulletin, which was reaffirmed in 2013.1 “No alternative imaging modality has demonstrated sufficient superiority to transvaginal ultrasonography to justify its routine use.”1
Transvaginal ultrasonography with color Doppler interrogation has demonstrated a sensitivity of 0.86% and a specificity of 0.91% for discriminating between malignant and benign ovarian masses.
Sonographic features that are worrisome for malignancy include:
- Multiple thin septations (if indeterminate, the mass may possibly be benign)
- Thick (> 3 mm), irregular septations
- Focal areas of wall thickening (> 3 mm)
- Mural nodules or papillary projections
- Levine and colleagues note that a cyst with a mural nodule with internal blood flow on color Doppler has the highest likelihood of being malignant2
- Moderate or large amount of ascitic fluid in pelvis (in conjunction with ovarian mass showing the above characteristics)
Various morphology indices have been developed that combine these criteria with ovarian mass volume to determine the preoperative predictive value for malignancy.
In the images that follow, we present 14 cases that demonstrate cystadenoma, low malignant potential tumors, and ovarian neoplasia.
CASE 1. Right ovarian mucinous cystadenoma in 68-year-old woman with uterine prolapse and history of ovarian cyst


CASE 2. Borderline serous cystadenoma in 56-year-old woman with right lower quadrant pain, nausea, and loss of appetite


CASE 3. Mucinous cystadenoma in 38-year-old woman undergoing sonography for spontaneous abortion


CASE 4. Mucinous cystadenoma in 54-year-old woman undergoing follow-up ultrasound for persistent ovarian cyst



CASE 7. Mature cystic teratoma in 31-year-old woman with progressively heavier bleeding and pelvic pain





CASE 11. Sex-cord stromal tumor in 61-year-old woman with postmenopausal bleeding


CASE 12. Juvenile granulosa cell tumor in 19-year-old patient with secondary amenorrhea



CASE 14. Mucinous borderline tumor in 55-year-old woman with pelvic discomfort

Protein may be key in virus, cancer research

Image courtesy of the
University of North Carolina
Researchers say they have uncovered a viral protein that inhibits cGAS, the principal cytosolic DNA sensor that detects invading viral DNA and triggers antiviral responses.
The protein, Kaposi’s sarcoma-associated herpesvirus (KSHV) ORF52, subverts cytosolic DNA sensing by directly inhibiting cGAS enzymatic activity.
The team believes this finding could have a range of therapeutic implications.
“We can manipulate the protein and/or the sensor to boost or tune down the immune response in order to fight infectious and autoimmune diseases, as well as cancers,” said Fanxiu Zhu, PhD, of Florida State University in Tallahassee.
Dr Zhu and his colleagues described this research in Cell Host and Microbe.
The authors noted that, although cGAS senses several DNA viruses, viral strategies targeting cGAS are “virtually unknown.”
To uncover a cGAS inhibitor, the researchers screened every protein in a KSHV cell—90 in total. This revealed KSHV ORF52, which the team renamed “KicGas,” an abbreviation for “KSHV inhibitor of cGAS.”
Further investigation revealed how KicGas inhibits cGAS activity: it must bind to both DNA and cGAS.
The researchers then found that ORF52 homologs in other gammaherpesviruses also inhibit cGAS activity and similarly bind cGAS and DNA.
Finally, the team infected human cell lines with KSHV to mimic natural infection. They found that KSHV triggers a cGAS-dependent immune response that can be partially mitigated by KicGas.
When the researchers eliminated KicGas from infected cells, the cells produced a much stronger immune response.
For the next phase of research, the team is building a 3-dimensional model to help them better understand how KicGas functions. They hope this will help them utilize KicGas to fight disease. ![]()

Image courtesy of the
University of North Carolina
Researchers say they have uncovered a viral protein that inhibits cGAS, the principal cytosolic DNA sensor that detects invading viral DNA and triggers antiviral responses.
The protein, Kaposi’s sarcoma-associated herpesvirus (KSHV) ORF52, subverts cytosolic DNA sensing by directly inhibiting cGAS enzymatic activity.
The team believes this finding could have a range of therapeutic implications.
“We can manipulate the protein and/or the sensor to boost or tune down the immune response in order to fight infectious and autoimmune diseases, as well as cancers,” said Fanxiu Zhu, PhD, of Florida State University in Tallahassee.
Dr Zhu and his colleagues described this research in Cell Host and Microbe.
The authors noted that, although cGAS senses several DNA viruses, viral strategies targeting cGAS are “virtually unknown.”
To uncover a cGAS inhibitor, the researchers screened every protein in a KSHV cell—90 in total. This revealed KSHV ORF52, which the team renamed “KicGas,” an abbreviation for “KSHV inhibitor of cGAS.”
Further investigation revealed how KicGas inhibits cGAS activity: it must bind to both DNA and cGAS.
The researchers then found that ORF52 homologs in other gammaherpesviruses also inhibit cGAS activity and similarly bind cGAS and DNA.
Finally, the team infected human cell lines with KSHV to mimic natural infection. They found that KSHV triggers a cGAS-dependent immune response that can be partially mitigated by KicGas.
When the researchers eliminated KicGas from infected cells, the cells produced a much stronger immune response.
For the next phase of research, the team is building a 3-dimensional model to help them better understand how KicGas functions. They hope this will help them utilize KicGas to fight disease. ![]()

Image courtesy of the
University of North Carolina
Researchers say they have uncovered a viral protein that inhibits cGAS, the principal cytosolic DNA sensor that detects invading viral DNA and triggers antiviral responses.
The protein, Kaposi’s sarcoma-associated herpesvirus (KSHV) ORF52, subverts cytosolic DNA sensing by directly inhibiting cGAS enzymatic activity.
The team believes this finding could have a range of therapeutic implications.
“We can manipulate the protein and/or the sensor to boost or tune down the immune response in order to fight infectious and autoimmune diseases, as well as cancers,” said Fanxiu Zhu, PhD, of Florida State University in Tallahassee.
Dr Zhu and his colleagues described this research in Cell Host and Microbe.
The authors noted that, although cGAS senses several DNA viruses, viral strategies targeting cGAS are “virtually unknown.”
To uncover a cGAS inhibitor, the researchers screened every protein in a KSHV cell—90 in total. This revealed KSHV ORF52, which the team renamed “KicGas,” an abbreviation for “KSHV inhibitor of cGAS.”
Further investigation revealed how KicGas inhibits cGAS activity: it must bind to both DNA and cGAS.
The researchers then found that ORF52 homologs in other gammaherpesviruses also inhibit cGAS activity and similarly bind cGAS and DNA.
Finally, the team infected human cell lines with KSHV to mimic natural infection. They found that KSHV triggers a cGAS-dependent immune response that can be partially mitigated by KicGas.
When the researchers eliminated KicGas from infected cells, the cells produced a much stronger immune response.
For the next phase of research, the team is building a 3-dimensional model to help them better understand how KicGas functions. They hope this will help them utilize KicGas to fight disease. ![]()
FDA warns of disabling joint pain from DPP-4 inhibitors
Multiple reports of severe and disabling joint pain in some patients taking dipeptidyl peptidase-4 (DPP-4) inhibitors for type 2 diabetes have prompted the Food and Drug Administration to add a new warning and precaution for this class of drugs. Some cases were severe enough to require hospitalization, though symptoms eventually resolved after patients stopped taking the medication.
In a MedWatch Bulletin, the FDA advises that physicians should be alert for DPP-4 inhibitors as a causative factor for patients who present with severe, persistent joint pain, even for those who have been on the medication for some time.
Most patients developed symptoms within a month of beginning treatment; however, some patients had been on a DPP-4 inhibitor for as long as a year before the onset of joint pain. When the medication was stopped, arthralgia resolved within a month in all reported cases.
Of the 33 cases of severe arthralgia found in the FDA adverse events reporting database, 28 were associated with the use of sitagliptin (Januvia), with some cases also reported with saxagliptin (Onglyza), linagliptin (Tradjenta), alogliptin (Nesina), and vildagliptin (Galvus). Ten patients’ symptoms were severe enough to require hospitalization; eight experienced recurrent arthralgia when rechallenged.
A literature search conducted by FDA officials revealed seven reports of DPP-4 inhibitor–associated arthralgia, two of which also were in their reporting database.
Patients taking DPP-4 inhibitors should continue taking their medication but consult their health care providers if they experience severe, persistent joint pain, according to the FDA advisory.
On Twitter @karioakes
Multiple reports of severe and disabling joint pain in some patients taking dipeptidyl peptidase-4 (DPP-4) inhibitors for type 2 diabetes have prompted the Food and Drug Administration to add a new warning and precaution for this class of drugs. Some cases were severe enough to require hospitalization, though symptoms eventually resolved after patients stopped taking the medication.
In a MedWatch Bulletin, the FDA advises that physicians should be alert for DPP-4 inhibitors as a causative factor for patients who present with severe, persistent joint pain, even for those who have been on the medication for some time.
Most patients developed symptoms within a month of beginning treatment; however, some patients had been on a DPP-4 inhibitor for as long as a year before the onset of joint pain. When the medication was stopped, arthralgia resolved within a month in all reported cases.
Of the 33 cases of severe arthralgia found in the FDA adverse events reporting database, 28 were associated with the use of sitagliptin (Januvia), with some cases also reported with saxagliptin (Onglyza), linagliptin (Tradjenta), alogliptin (Nesina), and vildagliptin (Galvus). Ten patients’ symptoms were severe enough to require hospitalization; eight experienced recurrent arthralgia when rechallenged.
A literature search conducted by FDA officials revealed seven reports of DPP-4 inhibitor–associated arthralgia, two of which also were in their reporting database.
Patients taking DPP-4 inhibitors should continue taking their medication but consult their health care providers if they experience severe, persistent joint pain, according to the FDA advisory.
On Twitter @karioakes
Multiple reports of severe and disabling joint pain in some patients taking dipeptidyl peptidase-4 (DPP-4) inhibitors for type 2 diabetes have prompted the Food and Drug Administration to add a new warning and precaution for this class of drugs. Some cases were severe enough to require hospitalization, though symptoms eventually resolved after patients stopped taking the medication.
In a MedWatch Bulletin, the FDA advises that physicians should be alert for DPP-4 inhibitors as a causative factor for patients who present with severe, persistent joint pain, even for those who have been on the medication for some time.
Most patients developed symptoms within a month of beginning treatment; however, some patients had been on a DPP-4 inhibitor for as long as a year before the onset of joint pain. When the medication was stopped, arthralgia resolved within a month in all reported cases.
Of the 33 cases of severe arthralgia found in the FDA adverse events reporting database, 28 were associated with the use of sitagliptin (Januvia), with some cases also reported with saxagliptin (Onglyza), linagliptin (Tradjenta), alogliptin (Nesina), and vildagliptin (Galvus). Ten patients’ symptoms were severe enough to require hospitalization; eight experienced recurrent arthralgia when rechallenged.
A literature search conducted by FDA officials revealed seven reports of DPP-4 inhibitor–associated arthralgia, two of which also were in their reporting database.
Patients taking DPP-4 inhibitors should continue taking their medication but consult their health care providers if they experience severe, persistent joint pain, according to the FDA advisory.
On Twitter @karioakes
Retinoids, FAK inhibitors may aid TKIs in treating ALL subtype

and Charles Mullighan
Photo courtesy of St. Jude
Children’s Research Hospital
and Peter Barta
Retinoids and FAK inhibitors may override resistance to tyrosine kinase inhibitors (TKIs) in IKZF1-mutated, Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL), according to preclinical research published in Cancer Cell.
Experiments showed that, in Ph+ ALL, IKZF1 mutations prompt changes that reduce responsiveness to TKIs. But combining a TKI with a retinoid or FAK inhibitor can overcome this problem.
“The research shows why, in this era of targeted therapies, Ph+ ALL patients who also have IKZF1 mutations fare so poorly,” said study author Charles Mullighan, MD, MBBS, of St. Jude Children’s Research Hospital in Memphis, Tennessee. “The insight also led us to a promising new treatment strategy.”
To conduct this research, Dr Mullighan and his colleagues began with mouse models of IKZF1-mutated Ph+ ALL, with and without mutations in the ARF gene. ARF encodes a tumor suppressor protein and is altered in about half of Ph+ ALL cases.
With these models, the researchers showed that the addition of IKZF1 mutations, particularly in combination with ARF mutations, was a central event in driving ALL.
In pre-B cells with BCR-ABL1, IKZF1 mutations induced a stem cell-like phenotype, increased stromal bone marrow adhesion, and reduced responsiveness to the TKI dasatinib.
When the researchers investigated the increased adhesion of mutated cells, they found overexpression of FAK and other molecules implicated in leukemic and stem cell adherence. This led the researchers to speculate that FAK inhibitors might prove useful against IKZF1-mutated Ph+ ALL.
But the team also found, through a screen of 483 compounds, that retinoids can reverse the effects of IKZF1 mutations. The antineoplastic agent bexarotene and 4 nuclear hormone receptor effectors—carbacyclin, all-trans retinoic acid (ATRA), 9-cis RA, and 13-cis RA—proved particularly effective.
The drugs worked, in part, by inducing expression of wild-type IKZF1. But they also worked in other ways to reverse the stem-cell phenotype, halt cell proliferation, and promote differentiation of altered cells.
The researchers then tested bexarotene and dasatinib, alone and in combination, in mice transplanted with ARF-/- BCR-ABL1 pre-B cells, with or without IK6 expression. Bexarotene alone produced “significant benefit without detectable toxicity.”
Dasatinib alone increased survival, but dasatinib and bexarotene in combination resulted in a greater survival advantage. The combination nearly doubled the survival time of mice with IK6 tumors, when compared to dasatinib alone.
The researchers also established xenografts of Ph+ ALL that recapitulate a range of IKZF1 genotypes. They administered dasatinib plus bexarotene, ATRA, or the FAK inhibitors PF-562271, NVPTAE226, or PF-573228 ex vivo and observed “significant potentiation of cell killing.”
The team is currently investigating how to incorporate retinoids or FAK inhibitors into the existing treatment of IKZF1-mutated Ph+ ALL. ![]()

and Charles Mullighan
Photo courtesy of St. Jude
Children’s Research Hospital
and Peter Barta
Retinoids and FAK inhibitors may override resistance to tyrosine kinase inhibitors (TKIs) in IKZF1-mutated, Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL), according to preclinical research published in Cancer Cell.
Experiments showed that, in Ph+ ALL, IKZF1 mutations prompt changes that reduce responsiveness to TKIs. But combining a TKI with a retinoid or FAK inhibitor can overcome this problem.
“The research shows why, in this era of targeted therapies, Ph+ ALL patients who also have IKZF1 mutations fare so poorly,” said study author Charles Mullighan, MD, MBBS, of St. Jude Children’s Research Hospital in Memphis, Tennessee. “The insight also led us to a promising new treatment strategy.”
To conduct this research, Dr Mullighan and his colleagues began with mouse models of IKZF1-mutated Ph+ ALL, with and without mutations in the ARF gene. ARF encodes a tumor suppressor protein and is altered in about half of Ph+ ALL cases.
With these models, the researchers showed that the addition of IKZF1 mutations, particularly in combination with ARF mutations, was a central event in driving ALL.
In pre-B cells with BCR-ABL1, IKZF1 mutations induced a stem cell-like phenotype, increased stromal bone marrow adhesion, and reduced responsiveness to the TKI dasatinib.
When the researchers investigated the increased adhesion of mutated cells, they found overexpression of FAK and other molecules implicated in leukemic and stem cell adherence. This led the researchers to speculate that FAK inhibitors might prove useful against IKZF1-mutated Ph+ ALL.
But the team also found, through a screen of 483 compounds, that retinoids can reverse the effects of IKZF1 mutations. The antineoplastic agent bexarotene and 4 nuclear hormone receptor effectors—carbacyclin, all-trans retinoic acid (ATRA), 9-cis RA, and 13-cis RA—proved particularly effective.
The drugs worked, in part, by inducing expression of wild-type IKZF1. But they also worked in other ways to reverse the stem-cell phenotype, halt cell proliferation, and promote differentiation of altered cells.
The researchers then tested bexarotene and dasatinib, alone and in combination, in mice transplanted with ARF-/- BCR-ABL1 pre-B cells, with or without IK6 expression. Bexarotene alone produced “significant benefit without detectable toxicity.”
Dasatinib alone increased survival, but dasatinib and bexarotene in combination resulted in a greater survival advantage. The combination nearly doubled the survival time of mice with IK6 tumors, when compared to dasatinib alone.
The researchers also established xenografts of Ph+ ALL that recapitulate a range of IKZF1 genotypes. They administered dasatinib plus bexarotene, ATRA, or the FAK inhibitors PF-562271, NVPTAE226, or PF-573228 ex vivo and observed “significant potentiation of cell killing.”
The team is currently investigating how to incorporate retinoids or FAK inhibitors into the existing treatment of IKZF1-mutated Ph+ ALL. ![]()

and Charles Mullighan
Photo courtesy of St. Jude
Children’s Research Hospital
and Peter Barta
Retinoids and FAK inhibitors may override resistance to tyrosine kinase inhibitors (TKIs) in IKZF1-mutated, Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL), according to preclinical research published in Cancer Cell.
Experiments showed that, in Ph+ ALL, IKZF1 mutations prompt changes that reduce responsiveness to TKIs. But combining a TKI with a retinoid or FAK inhibitor can overcome this problem.
“The research shows why, in this era of targeted therapies, Ph+ ALL patients who also have IKZF1 mutations fare so poorly,” said study author Charles Mullighan, MD, MBBS, of St. Jude Children’s Research Hospital in Memphis, Tennessee. “The insight also led us to a promising new treatment strategy.”
To conduct this research, Dr Mullighan and his colleagues began with mouse models of IKZF1-mutated Ph+ ALL, with and without mutations in the ARF gene. ARF encodes a tumor suppressor protein and is altered in about half of Ph+ ALL cases.
With these models, the researchers showed that the addition of IKZF1 mutations, particularly in combination with ARF mutations, was a central event in driving ALL.
In pre-B cells with BCR-ABL1, IKZF1 mutations induced a stem cell-like phenotype, increased stromal bone marrow adhesion, and reduced responsiveness to the TKI dasatinib.
When the researchers investigated the increased adhesion of mutated cells, they found overexpression of FAK and other molecules implicated in leukemic and stem cell adherence. This led the researchers to speculate that FAK inhibitors might prove useful against IKZF1-mutated Ph+ ALL.
But the team also found, through a screen of 483 compounds, that retinoids can reverse the effects of IKZF1 mutations. The antineoplastic agent bexarotene and 4 nuclear hormone receptor effectors—carbacyclin, all-trans retinoic acid (ATRA), 9-cis RA, and 13-cis RA—proved particularly effective.
The drugs worked, in part, by inducing expression of wild-type IKZF1. But they also worked in other ways to reverse the stem-cell phenotype, halt cell proliferation, and promote differentiation of altered cells.
The researchers then tested bexarotene and dasatinib, alone and in combination, in mice transplanted with ARF-/- BCR-ABL1 pre-B cells, with or without IK6 expression. Bexarotene alone produced “significant benefit without detectable toxicity.”
Dasatinib alone increased survival, but dasatinib and bexarotene in combination resulted in a greater survival advantage. The combination nearly doubled the survival time of mice with IK6 tumors, when compared to dasatinib alone.
The researchers also established xenografts of Ph+ ALL that recapitulate a range of IKZF1 genotypes. They administered dasatinib plus bexarotene, ATRA, or the FAK inhibitors PF-562271, NVPTAE226, or PF-573228 ex vivo and observed “significant potentiation of cell killing.”
The team is currently investigating how to incorporate retinoids or FAK inhibitors into the existing treatment of IKZF1-mutated Ph+ ALL. ![]()
Malaria tests underused despite training

Photo courtesy of USAID
A study conducted in Nigeria has shown that health providers continue to prescribe malaria medicines inappropriately, even after they learn to test for malaria and receive testing kits free of charge.
Health providers were given rapid diagnostic tests (RDT) for malaria and learned to use the tests via 3 different methods.
However, the use of RDTs was “critically low” in all 3 groups, as was the proportion of patients treated appropriately.
“This study confirms that treating malaria based on signs and symptoms alone remains an ingrained behavior that is difficult to change,” said Obinna Onwujekwe, MD, PhD, of the University of Nigeria in Enugu.
Dr Onwujekwe and his colleagues reported these findings in PLOS ONE.
Interventions
Their study included health workers and patients from 40 communities in the Nigerian state of Enugu. Health workers received free RDT kits and were taught to use the tests in 3 different ways.
The first group received comprehensive RDT training, which included instructions on how to use an RDT, guidelines on malaria diagnosis and treatment, information about other causes of fever, and help with communications skills, especially for patients whose test results were negative.
The second group received the same training plus a school-based intervention that involved training 2 teachers per school. The aim was to influence the attitudes of school children and their families as well as the wider community.
And health workers in the third group—the control arm—were invited to a demonstration and practical on how to safely use RDTs and supplied with written instructions on their use.
Results
The primary outcome was the proportion of patients who were treated according to guidelines. In other words, they presented with symptoms consistent with malaria, were tested for malaria, and received treatment consistent with the test result.
The researchers assessed the primary outcome in 4946 patients from 40 communities—12 in the control arm and 14 in each intervention arm.
There was no significant difference between the arms with regard to this outcome. The proportion of patients treated according to guidelines was 36% in the comprehensive training arm, 24% in the training-school arm, and 23% in the control arm (P=0.36).
Likewise, the use of testing was low in all arms—34% in the control arm, 48% in the training arm, and 37% in the training-school arm (P=0.47).
The use of testing was lower at private facilities than public ones. Cost may have been a factor here, as public facilities were asked to offer testing free of charge, but private facilities could charge 100 Naira (0.6 USD).
“We have shown that training alone is not enough to realize the full potential of an RDT,” said Virginia Wiseman, PhD, of the London School of Hygiene & Tropical Medicine in the UK.
“We must continue to explore alternative ways of encouraging providers to deliver appropriate treatment and avoid the misuse of valuable medicines, especially in the private sector, where we found levels of testing to be lowest.” ![]()

Photo courtesy of USAID
A study conducted in Nigeria has shown that health providers continue to prescribe malaria medicines inappropriately, even after they learn to test for malaria and receive testing kits free of charge.
Health providers were given rapid diagnostic tests (RDT) for malaria and learned to use the tests via 3 different methods.
However, the use of RDTs was “critically low” in all 3 groups, as was the proportion of patients treated appropriately.
“This study confirms that treating malaria based on signs and symptoms alone remains an ingrained behavior that is difficult to change,” said Obinna Onwujekwe, MD, PhD, of the University of Nigeria in Enugu.
Dr Onwujekwe and his colleagues reported these findings in PLOS ONE.
Interventions
Their study included health workers and patients from 40 communities in the Nigerian state of Enugu. Health workers received free RDT kits and were taught to use the tests in 3 different ways.
The first group received comprehensive RDT training, which included instructions on how to use an RDT, guidelines on malaria diagnosis and treatment, information about other causes of fever, and help with communications skills, especially for patients whose test results were negative.
The second group received the same training plus a school-based intervention that involved training 2 teachers per school. The aim was to influence the attitudes of school children and their families as well as the wider community.
And health workers in the third group—the control arm—were invited to a demonstration and practical on how to safely use RDTs and supplied with written instructions on their use.
Results
The primary outcome was the proportion of patients who were treated according to guidelines. In other words, they presented with symptoms consistent with malaria, were tested for malaria, and received treatment consistent with the test result.
The researchers assessed the primary outcome in 4946 patients from 40 communities—12 in the control arm and 14 in each intervention arm.
There was no significant difference between the arms with regard to this outcome. The proportion of patients treated according to guidelines was 36% in the comprehensive training arm, 24% in the training-school arm, and 23% in the control arm (P=0.36).
Likewise, the use of testing was low in all arms—34% in the control arm, 48% in the training arm, and 37% in the training-school arm (P=0.47).
The use of testing was lower at private facilities than public ones. Cost may have been a factor here, as public facilities were asked to offer testing free of charge, but private facilities could charge 100 Naira (0.6 USD).
“We have shown that training alone is not enough to realize the full potential of an RDT,” said Virginia Wiseman, PhD, of the London School of Hygiene & Tropical Medicine in the UK.
“We must continue to explore alternative ways of encouraging providers to deliver appropriate treatment and avoid the misuse of valuable medicines, especially in the private sector, where we found levels of testing to be lowest.” ![]()

Photo courtesy of USAID
A study conducted in Nigeria has shown that health providers continue to prescribe malaria medicines inappropriately, even after they learn to test for malaria and receive testing kits free of charge.
Health providers were given rapid diagnostic tests (RDT) for malaria and learned to use the tests via 3 different methods.
However, the use of RDTs was “critically low” in all 3 groups, as was the proportion of patients treated appropriately.
“This study confirms that treating malaria based on signs and symptoms alone remains an ingrained behavior that is difficult to change,” said Obinna Onwujekwe, MD, PhD, of the University of Nigeria in Enugu.
Dr Onwujekwe and his colleagues reported these findings in PLOS ONE.
Interventions
Their study included health workers and patients from 40 communities in the Nigerian state of Enugu. Health workers received free RDT kits and were taught to use the tests in 3 different ways.
The first group received comprehensive RDT training, which included instructions on how to use an RDT, guidelines on malaria diagnosis and treatment, information about other causes of fever, and help with communications skills, especially for patients whose test results were negative.
The second group received the same training plus a school-based intervention that involved training 2 teachers per school. The aim was to influence the attitudes of school children and their families as well as the wider community.
And health workers in the third group—the control arm—were invited to a demonstration and practical on how to safely use RDTs and supplied with written instructions on their use.
Results
The primary outcome was the proportion of patients who were treated according to guidelines. In other words, they presented with symptoms consistent with malaria, were tested for malaria, and received treatment consistent with the test result.
The researchers assessed the primary outcome in 4946 patients from 40 communities—12 in the control arm and 14 in each intervention arm.
There was no significant difference between the arms with regard to this outcome. The proportion of patients treated according to guidelines was 36% in the comprehensive training arm, 24% in the training-school arm, and 23% in the control arm (P=0.36).
Likewise, the use of testing was low in all arms—34% in the control arm, 48% in the training arm, and 37% in the training-school arm (P=0.47).
The use of testing was lower at private facilities than public ones. Cost may have been a factor here, as public facilities were asked to offer testing free of charge, but private facilities could charge 100 Naira (0.6 USD).
“We have shown that training alone is not enough to realize the full potential of an RDT,” said Virginia Wiseman, PhD, of the London School of Hygiene & Tropical Medicine in the UK.
“We must continue to explore alternative ways of encouraging providers to deliver appropriate treatment and avoid the misuse of valuable medicines, especially in the private sector, where we found levels of testing to be lowest.” ![]()
Inhibitors can target CML stem cells

Preclinical research has revealed nutrients that support the activity of chronic myelogenous leukemia (CML) stem cells and suggests these nutrients may be promising targets for CML therapy.
Investigators discovered that CML stem cells accumulate high levels of certain dipeptide species, and these dipeptides act as nutrients for the cells.
When the team inhibited dipeptide uptake, they observed decreased CML stem cell activity.
Combining agents that inhibit dipeptide uptake with tyrosine kinase inhibitors (TKIs) proved more effective against CML than TKI treatment alone, both in vitro and in vivo.
Kazuhito Naka, PhD, of Hiroshima University in Japan, and his colleagues conducted this research and disclosed the results in Nature Communications.
The team began by analyzing CML stem cells isolated from a mouse model of the disease. They found that CML stem cells accumulate significantly higher levels of certain dipeptide species—such as Ala-Leu, Asp-Leu, Ser-Tyr, and Thr-Val—than normal hematopoietic stem cells.
Additional investigation revealed that CML stem cells take up dipeptides via the Slc15A2 transporter. And once internalized, the dipeptides act as nutrients and play a role in CML stem cell maintenance.
The dipeptides activate amino-acid signaling via a pathway involving p38MAPK and Smad3, and this promotes CML stem cell maintenance.
To build upon these findings, the investigators assessed the effects of cefadroxil, which inhibits Slc15A2-mediated nutrient signaling, in combination with imatinib as CML treatment.
In murine CML stem cell cultures, the 2 drugs in combination reduced colony formation more effectively than imatinib alone.
In vivo, mice with CML responded to imatinib alone but eventually experienced disease recurrence. And cefadroxil alone promoted disease development. But when cefadroxil was given in combination with imatinib, disease recurrence was significantly lower than in mice that received imatinib alone.
The investigators then showed that cefadroxil decreases the number of CML stem cells in CML-affected mice. And cefadroxil combined with imatinib reduces CML stem cell numbers more effectively than imatinib alone.
In serial transplantation experiments, CML stem cells isolated from cefadroxil-treated mice completely lost their ability to drive BCR-ABL1+ disease in new recipients. These animals survived for more than 90 days, whereas mice that received CML stem cells from vehicle-treated mice developed BCR-ABL1+ disease and died before 80 days.
The investigators also tested cefadroxil in stem cells derived from humans with chronic CML. Cefadroxil suppressed the colony-forming capacity of all 3 samples tested. And combining cefadroxil with imatinib or dasatinib reduced colony formation more effectively than either TKI alone.
Lastly, the team decided to test 3 clinical-grade p38MAPK inhibitors that are already approved for use in the US—Ly2228820 (ralimetinib), VX-702, and BIRB796 (doramapimod). When cultured with CML stem cells, each of these drugs significantly decreased colony formation.
In addition, Ly2228820 combined with dasatinib delayed CML onset in mice and improved their survival when compared with dasatinib alone.
These results suggest p38MAPK inhibitors and cefadroxil may be useful additions to TKI therapy in CML, the investigators said.
“Our proposed approach of using inhibitors to shut down a key nutrient uptake process specific to CML stem cells, in combination with TKI therapy, may thus provide concrete therapeutic benefits to patients with CML,” Dr Naka said. “It will open up a novel avenue for curative CML therapy.” ![]()

Preclinical research has revealed nutrients that support the activity of chronic myelogenous leukemia (CML) stem cells and suggests these nutrients may be promising targets for CML therapy.
Investigators discovered that CML stem cells accumulate high levels of certain dipeptide species, and these dipeptides act as nutrients for the cells.
When the team inhibited dipeptide uptake, they observed decreased CML stem cell activity.
Combining agents that inhibit dipeptide uptake with tyrosine kinase inhibitors (TKIs) proved more effective against CML than TKI treatment alone, both in vitro and in vivo.
Kazuhito Naka, PhD, of Hiroshima University in Japan, and his colleagues conducted this research and disclosed the results in Nature Communications.
The team began by analyzing CML stem cells isolated from a mouse model of the disease. They found that CML stem cells accumulate significantly higher levels of certain dipeptide species—such as Ala-Leu, Asp-Leu, Ser-Tyr, and Thr-Val—than normal hematopoietic stem cells.
Additional investigation revealed that CML stem cells take up dipeptides via the Slc15A2 transporter. And once internalized, the dipeptides act as nutrients and play a role in CML stem cell maintenance.
The dipeptides activate amino-acid signaling via a pathway involving p38MAPK and Smad3, and this promotes CML stem cell maintenance.
To build upon these findings, the investigators assessed the effects of cefadroxil, which inhibits Slc15A2-mediated nutrient signaling, in combination with imatinib as CML treatment.
In murine CML stem cell cultures, the 2 drugs in combination reduced colony formation more effectively than imatinib alone.
In vivo, mice with CML responded to imatinib alone but eventually experienced disease recurrence. And cefadroxil alone promoted disease development. But when cefadroxil was given in combination with imatinib, disease recurrence was significantly lower than in mice that received imatinib alone.
The investigators then showed that cefadroxil decreases the number of CML stem cells in CML-affected mice. And cefadroxil combined with imatinib reduces CML stem cell numbers more effectively than imatinib alone.
In serial transplantation experiments, CML stem cells isolated from cefadroxil-treated mice completely lost their ability to drive BCR-ABL1+ disease in new recipients. These animals survived for more than 90 days, whereas mice that received CML stem cells from vehicle-treated mice developed BCR-ABL1+ disease and died before 80 days.
The investigators also tested cefadroxil in stem cells derived from humans with chronic CML. Cefadroxil suppressed the colony-forming capacity of all 3 samples tested. And combining cefadroxil with imatinib or dasatinib reduced colony formation more effectively than either TKI alone.
Lastly, the team decided to test 3 clinical-grade p38MAPK inhibitors that are already approved for use in the US—Ly2228820 (ralimetinib), VX-702, and BIRB796 (doramapimod). When cultured with CML stem cells, each of these drugs significantly decreased colony formation.
In addition, Ly2228820 combined with dasatinib delayed CML onset in mice and improved their survival when compared with dasatinib alone.
These results suggest p38MAPK inhibitors and cefadroxil may be useful additions to TKI therapy in CML, the investigators said.
“Our proposed approach of using inhibitors to shut down a key nutrient uptake process specific to CML stem cells, in combination with TKI therapy, may thus provide concrete therapeutic benefits to patients with CML,” Dr Naka said. “It will open up a novel avenue for curative CML therapy.” ![]()

Preclinical research has revealed nutrients that support the activity of chronic myelogenous leukemia (CML) stem cells and suggests these nutrients may be promising targets for CML therapy.
Investigators discovered that CML stem cells accumulate high levels of certain dipeptide species, and these dipeptides act as nutrients for the cells.
When the team inhibited dipeptide uptake, they observed decreased CML stem cell activity.
Combining agents that inhibit dipeptide uptake with tyrosine kinase inhibitors (TKIs) proved more effective against CML than TKI treatment alone, both in vitro and in vivo.
Kazuhito Naka, PhD, of Hiroshima University in Japan, and his colleagues conducted this research and disclosed the results in Nature Communications.
The team began by analyzing CML stem cells isolated from a mouse model of the disease. They found that CML stem cells accumulate significantly higher levels of certain dipeptide species—such as Ala-Leu, Asp-Leu, Ser-Tyr, and Thr-Val—than normal hematopoietic stem cells.
Additional investigation revealed that CML stem cells take up dipeptides via the Slc15A2 transporter. And once internalized, the dipeptides act as nutrients and play a role in CML stem cell maintenance.
The dipeptides activate amino-acid signaling via a pathway involving p38MAPK and Smad3, and this promotes CML stem cell maintenance.
To build upon these findings, the investigators assessed the effects of cefadroxil, which inhibits Slc15A2-mediated nutrient signaling, in combination with imatinib as CML treatment.
In murine CML stem cell cultures, the 2 drugs in combination reduced colony formation more effectively than imatinib alone.
In vivo, mice with CML responded to imatinib alone but eventually experienced disease recurrence. And cefadroxil alone promoted disease development. But when cefadroxil was given in combination with imatinib, disease recurrence was significantly lower than in mice that received imatinib alone.
The investigators then showed that cefadroxil decreases the number of CML stem cells in CML-affected mice. And cefadroxil combined with imatinib reduces CML stem cell numbers more effectively than imatinib alone.
In serial transplantation experiments, CML stem cells isolated from cefadroxil-treated mice completely lost their ability to drive BCR-ABL1+ disease in new recipients. These animals survived for more than 90 days, whereas mice that received CML stem cells from vehicle-treated mice developed BCR-ABL1+ disease and died before 80 days.
The investigators also tested cefadroxil in stem cells derived from humans with chronic CML. Cefadroxil suppressed the colony-forming capacity of all 3 samples tested. And combining cefadroxil with imatinib or dasatinib reduced colony formation more effectively than either TKI alone.
Lastly, the team decided to test 3 clinical-grade p38MAPK inhibitors that are already approved for use in the US—Ly2228820 (ralimetinib), VX-702, and BIRB796 (doramapimod). When cultured with CML stem cells, each of these drugs significantly decreased colony formation.
In addition, Ly2228820 combined with dasatinib delayed CML onset in mice and improved their survival when compared with dasatinib alone.
These results suggest p38MAPK inhibitors and cefadroxil may be useful additions to TKI therapy in CML, the investigators said.
“Our proposed approach of using inhibitors to shut down a key nutrient uptake process specific to CML stem cells, in combination with TKI therapy, may thus provide concrete therapeutic benefits to patients with CML,” Dr Naka said. “It will open up a novel avenue for curative CML therapy.” ![]()