User login
AHA: Should BP targets be higher in asymptomatic aortic stenosis?
ORLANDO – Optimal blood pressure in patients with asymptomatic mild to moderate aortic stenosis is 140-159 mm Hg for systolic and 70-89 mm Hg for diastolic, according to an analysis of all-cause mortality in the world’s largest data set of such patients with longitudinal follow-up and endpoint evaluation.
Within those target blood pressure ranges, the nadir in terms of all-cause mortality – and thus the true optimal blood pressure – is about 145/82 mm Hg, Dr. Kristian Wachtell reported at the American Heart Association scientific sessions.
He presented an analysis of 1,873 asymptomatic patients with mild to moderate aortic stenosis (AS) and a peak aortic-jet velocity of 2.5-4.0 M/sec upon enrollment in the Simvastatin Ezetimibe in Aortic Stenosis (SEAS) trial. SEAS was a double-blind, multicenter study in which participants were randomized to 40 mg of simvastatin plus 10 mg of ezetimibe per day or placebo and followed for a mean of 4.3 years. Half of them had a history of hypertension at baseline.
As previously reported (N Engl J Med. 2008 Sep 25;359[13]:1343-56), SEAS was a negative trial. Lipid-lowering therapy didn’t affect AS progression or aortic valve–related outcomes. On the plus side, the SEAS cohort is the world’s largest population of patients with asymptomatic AS followed prospectively for clinical endpoints, noted Dr. Wachtell of Oslo University.
He and his coinvestigators decided to plot all-cause mortality versus average blood pressure in the SEAS cohort because of the paucity of data regarding blood pressure and antihypertensive therapy in patients with asymptomatic AS. Neither the 2014 ACC/AHA guidelines for management of valvular heart disease (Circulation. 2014 Jun 10;129[23]:e521-643) nor the European guidelines provide recommendations for optimal blood pressure targets in patients with asymptomatic AS, even though AS is the third most common cardiac disease, behind hypertension and coronary artery disease.
Moreover, nearly 100,000 aortic valve replacements are done per year in the United States as a consequence of AS. And AS and hypertension go hand in hand, with the prevalence of hypertension among patients with AS cited as 50% or more in multiple studies, the cardiologist continued.
In a multivariate analysis adjusted for aortic valve area index and peak velocity, heart failure, MI, and aortic valve replacement during follow-up, all-cause mortality showed a U-shaped relationship with blood pressure. A systolic blood pressure below 120 mm Hg was associated with a 5-fold increased risk of mortality, a systolic of 120-139 mm Hg carried a 1.5-fold increased risk, and a diastolic blood pressure of 90 mm Hg or more was associated with a 1.9-fold increased risk.
“Patients with low systolic blood pressure had an increased mortality risk and should probably undertake individual clinical assessment for blood pressure control and evaluation of their AS,” Dr. Wachtell said.
The first question audience members asked was, “What about SPRINT?” The SPRINT trial, presented elsewhere at the meeting, was a practice-changing study that was the talk of the conference. It redefined the systolic blood pressure treatment target as less than 120 mm Hg instead of less than 140 mm Hg in hypertensive patients (N Engl J Med. 2015 Nov 9. doi: 10.1056/NEJMoa1511939).
“I think it’s most likely that for blood pressure, one size does not fit all,” Dr. Wachtell replied. “The extent of target organ damage – and you could say that aortic stenosis is actually target organ damage, like atrial fibrillation or left ventricular hypertrophy – warrants a different level of blood pressure.”
He added, however, that the SEAS analysis was based on observational data, and that’s a limitation.
“This is a qualified guess as to what blood pressures should be in patients with aortic stenosis,” he cautioned.
Dr. Wachtell reported having no conflicts of interest regarding his presentation.
ORLANDO – Optimal blood pressure in patients with asymptomatic mild to moderate aortic stenosis is 140-159 mm Hg for systolic and 70-89 mm Hg for diastolic, according to an analysis of all-cause mortality in the world’s largest data set of such patients with longitudinal follow-up and endpoint evaluation.
Within those target blood pressure ranges, the nadir in terms of all-cause mortality – and thus the true optimal blood pressure – is about 145/82 mm Hg, Dr. Kristian Wachtell reported at the American Heart Association scientific sessions.
He presented an analysis of 1,873 asymptomatic patients with mild to moderate aortic stenosis (AS) and a peak aortic-jet velocity of 2.5-4.0 M/sec upon enrollment in the Simvastatin Ezetimibe in Aortic Stenosis (SEAS) trial. SEAS was a double-blind, multicenter study in which participants were randomized to 40 mg of simvastatin plus 10 mg of ezetimibe per day or placebo and followed for a mean of 4.3 years. Half of them had a history of hypertension at baseline.
As previously reported (N Engl J Med. 2008 Sep 25;359[13]:1343-56), SEAS was a negative trial. Lipid-lowering therapy didn’t affect AS progression or aortic valve–related outcomes. On the plus side, the SEAS cohort is the world’s largest population of patients with asymptomatic AS followed prospectively for clinical endpoints, noted Dr. Wachtell of Oslo University.
He and his coinvestigators decided to plot all-cause mortality versus average blood pressure in the SEAS cohort because of the paucity of data regarding blood pressure and antihypertensive therapy in patients with asymptomatic AS. Neither the 2014 ACC/AHA guidelines for management of valvular heart disease (Circulation. 2014 Jun 10;129[23]:e521-643) nor the European guidelines provide recommendations for optimal blood pressure targets in patients with asymptomatic AS, even though AS is the third most common cardiac disease, behind hypertension and coronary artery disease.
Moreover, nearly 100,000 aortic valve replacements are done per year in the United States as a consequence of AS. And AS and hypertension go hand in hand, with the prevalence of hypertension among patients with AS cited as 50% or more in multiple studies, the cardiologist continued.
In a multivariate analysis adjusted for aortic valve area index and peak velocity, heart failure, MI, and aortic valve replacement during follow-up, all-cause mortality showed a U-shaped relationship with blood pressure. A systolic blood pressure below 120 mm Hg was associated with a 5-fold increased risk of mortality, a systolic of 120-139 mm Hg carried a 1.5-fold increased risk, and a diastolic blood pressure of 90 mm Hg or more was associated with a 1.9-fold increased risk.
“Patients with low systolic blood pressure had an increased mortality risk and should probably undertake individual clinical assessment for blood pressure control and evaluation of their AS,” Dr. Wachtell said.
The first question audience members asked was, “What about SPRINT?” The SPRINT trial, presented elsewhere at the meeting, was a practice-changing study that was the talk of the conference. It redefined the systolic blood pressure treatment target as less than 120 mm Hg instead of less than 140 mm Hg in hypertensive patients (N Engl J Med. 2015 Nov 9. doi: 10.1056/NEJMoa1511939).
“I think it’s most likely that for blood pressure, one size does not fit all,” Dr. Wachtell replied. “The extent of target organ damage – and you could say that aortic stenosis is actually target organ damage, like atrial fibrillation or left ventricular hypertrophy – warrants a different level of blood pressure.”
He added, however, that the SEAS analysis was based on observational data, and that’s a limitation.
“This is a qualified guess as to what blood pressures should be in patients with aortic stenosis,” he cautioned.
Dr. Wachtell reported having no conflicts of interest regarding his presentation.
ORLANDO – Optimal blood pressure in patients with asymptomatic mild to moderate aortic stenosis is 140-159 mm Hg for systolic and 70-89 mm Hg for diastolic, according to an analysis of all-cause mortality in the world’s largest data set of such patients with longitudinal follow-up and endpoint evaluation.
Within those target blood pressure ranges, the nadir in terms of all-cause mortality – and thus the true optimal blood pressure – is about 145/82 mm Hg, Dr. Kristian Wachtell reported at the American Heart Association scientific sessions.
He presented an analysis of 1,873 asymptomatic patients with mild to moderate aortic stenosis (AS) and a peak aortic-jet velocity of 2.5-4.0 M/sec upon enrollment in the Simvastatin Ezetimibe in Aortic Stenosis (SEAS) trial. SEAS was a double-blind, multicenter study in which participants were randomized to 40 mg of simvastatin plus 10 mg of ezetimibe per day or placebo and followed for a mean of 4.3 years. Half of them had a history of hypertension at baseline.
As previously reported (N Engl J Med. 2008 Sep 25;359[13]:1343-56), SEAS was a negative trial. Lipid-lowering therapy didn’t affect AS progression or aortic valve–related outcomes. On the plus side, the SEAS cohort is the world’s largest population of patients with asymptomatic AS followed prospectively for clinical endpoints, noted Dr. Wachtell of Oslo University.
He and his coinvestigators decided to plot all-cause mortality versus average blood pressure in the SEAS cohort because of the paucity of data regarding blood pressure and antihypertensive therapy in patients with asymptomatic AS. Neither the 2014 ACC/AHA guidelines for management of valvular heart disease (Circulation. 2014 Jun 10;129[23]:e521-643) nor the European guidelines provide recommendations for optimal blood pressure targets in patients with asymptomatic AS, even though AS is the third most common cardiac disease, behind hypertension and coronary artery disease.
Moreover, nearly 100,000 aortic valve replacements are done per year in the United States as a consequence of AS. And AS and hypertension go hand in hand, with the prevalence of hypertension among patients with AS cited as 50% or more in multiple studies, the cardiologist continued.
In a multivariate analysis adjusted for aortic valve area index and peak velocity, heart failure, MI, and aortic valve replacement during follow-up, all-cause mortality showed a U-shaped relationship with blood pressure. A systolic blood pressure below 120 mm Hg was associated with a 5-fold increased risk of mortality, a systolic of 120-139 mm Hg carried a 1.5-fold increased risk, and a diastolic blood pressure of 90 mm Hg or more was associated with a 1.9-fold increased risk.
“Patients with low systolic blood pressure had an increased mortality risk and should probably undertake individual clinical assessment for blood pressure control and evaluation of their AS,” Dr. Wachtell said.
The first question audience members asked was, “What about SPRINT?” The SPRINT trial, presented elsewhere at the meeting, was a practice-changing study that was the talk of the conference. It redefined the systolic blood pressure treatment target as less than 120 mm Hg instead of less than 140 mm Hg in hypertensive patients (N Engl J Med. 2015 Nov 9. doi: 10.1056/NEJMoa1511939).
“I think it’s most likely that for blood pressure, one size does not fit all,” Dr. Wachtell replied. “The extent of target organ damage – and you could say that aortic stenosis is actually target organ damage, like atrial fibrillation or left ventricular hypertrophy – warrants a different level of blood pressure.”
He added, however, that the SEAS analysis was based on observational data, and that’s a limitation.
“This is a qualified guess as to what blood pressures should be in patients with aortic stenosis,” he cautioned.
Dr. Wachtell reported having no conflicts of interest regarding his presentation.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Optimal blood pressures in patients with asymptomatic aortic stenosis appear to be significantly higher than those identified for the general hypertensive population in the recent SPRINT trial.
Major finding: All-cause mortality in patients with asymptomatic mild to moderate aortic stenosis is lowest at a blood pressure of 145/82 mm Hg.
Data source: This was an analysis of all-cause mortality over a mean follow-up of 4.3 years in 1,873 patients with asymptomatic mild to moderate aortic stenosis.
Disclosures: This secondary analysis of a completed clinical trial was conducted without commercial support. The presenter reported having no financial conflicts.
Lipoma of the Tendon Sheath in the Fourth Extensor Compartment of the Hand
Lipomas are relatively common benign tumors composed primarily of adipose tissue. They can occur anywhere on the body and are seen often in the hands and forearm. Typically localized to the subcutaneous fat layer, a lipoma is rarely associated with a tendon sheath or tendon compartment.1,2 When this uncommon event occurs, the lipoma is appropriately labeled lipoma of the tendon sheath.
While there are numerous case reports of lipomas of the tendon sheath occurring in association with tendons in the lower extremity, there are no reports, to our knowledge, of their occurrence in the extensor compartments of the hand.1 We report a rare case of lipoma of the tendon sheath localized to the fourth dorsal compartment of the hand, which was successfully treated with surgical excision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 33-year-old right hand–dominant waitress presented with a chief complaint of a painful, slowly enlarging right dorsal hand mass of 5 years’ duration. The mass was particularly bothersome with activities involving grip and finger extension. Physical examination revealed a mobile, rubbery mass on the dorsum of the hand that moved slightly with fist formation. There were no signs of neurovascular compromise. She had normal hand and wrist range of motion.
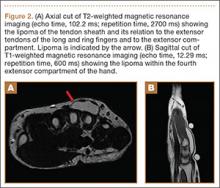
Plain radiographs were unremarkable (Figures 1A, 1B). Magnetic resonance imaging (MRI) with and without contrast revealed a 4×2-cm mass consistent with a diagnosis of lipoma. However, it was unique in that it appeared to extend from the long- and ring-finger extensor tendon sheaths in the fourth dorsal compartment of the hand (Figures 2A, 2B) and was deemed a lipoma of the tendon sheath. Representative MRI also showed the lipoma to be present within the fourth extensor compartment of the hand (Figure 2B). Because of the mass’s increasing size and interference with hand function, the patient elected to have the mass excised.
Surgical Technique
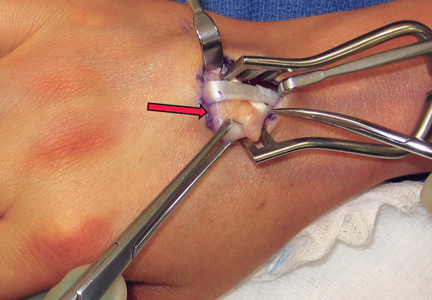
A 3-cm longitudinal incision was made over the dorsum of the hand centered directly over the mass. Dissection was carried through the subcutaneous tissue to the distal margin of the extensor retinaculum. The fourth dorsal compartment was entered and the tendons of the fourth extensor compartment were identified. Immediately beneath the extensor tendons to the long and ring fingers was a yellow, rubbery mass consistent with lipoma (Figure 3). This mass was strongly adherent to the underlying tendons and had to be dissected carefully with tenotomy scissors. Fortunately, the mass could be excised as a single unit (Figure 4). It was sent to the pathology department for histologic examination, which revealed mature adipose tissue and confirmed the diagnosis of lipoma. The wound was closed with absorbable suture, and a soft, sterile dressing was applied.
Postoperative Care
The patient was seen in follow-up 2 weeks later for routine evaluation. She had an intact wound with minimal hand pain, and full wrist and hand range of motion. She returned to work as a waitress approximately 3 weeks after surgery without difficulty. At her 6-week postoperative mark, she had a pain-free wrist with a well-healed incision and no signs of recurrence.
Discussion
Tendon sheath lipomas, whether in the upper or lower extremities, are exceedingly rare entities. Further, lipomas of an individual extensor compartment of the hand (as in our case) have yet to be described, in contrast to lipomas of flexor tendon sheaths.3 There are only a handful of case reports in the literature of lipomas of the tendon sheath, and none to our knowledge of their existence in the extensor compartments of the hand. Nevertheless, it is important for the treating surgeon to be aware of their existence and know some basics about them and their treatment.
There are 2 types of tendon sheath lipomas: discrete solid masses of adipose tissue (which we encountered) and adipose tissue coupled with hypertrophic synovial villi (or, lipoma arborescens).4,5 Of note, the latter is significantly more common than the former, which makes our case even more uncommon. Although both types of lipoma of the tendon sheath are benign, they can cause symptoms such as pain, finger stiffness, and nerve compression.6 Thus, they frequently merit surgical removal, as in our case.
The appropriate workup for lipoma of the tendon sheath generally includes thorough history, physical examination, and advanced imaging, such as MRI. MRI is usually diagnostic of such a lesion and can aid in surgical planning.1 Regarding their overall prognosis, all lipomas (even large ones) are benign by definition but can transform into liposarcomas in rare cases.4 Lipomas are typically treated surgically by simple excision, and lipoma of the tendon sheath is no different. As long as complete excision of a tendon sheath lipoma is performed, recurrence rates are less than 5%.2,3
Surgeons should also be aware that, with long-standing lipomas of the tendon sheath, weakening of a tendon secondary to irritation from the mass is a possibility, especially in the lower extremities. All tendons should be inspected carefully at the time of surgery to ensure that other procedures, such as tendon grafting or side-to-side tenodesis, are not required. Although lipomas of the tendon sheath and extensor compartments are quite rare, all surgeons evaluating masses for possible surgical excision should be aware of their existence and know how to manage them appropriately.
1. Khan AZ, Shafafy M, Latimer MD, Crosby J. A lipoma within the Achilles tendon sheath. Foot Ankle Surg. 2012;18(1):e16-e17.
2. Bryan RS, Dahlin DC, Sullivan CR. Lipoma of the tendon sheath. J Bone Joint Surg Am. 1956;38(6):1275-1280.
3. Kremchek TE, Kremchek EJ. Carpal tunnel syndrome caused by flexor tendon sheath lipoma. Orthop Rev. 1998;17(11):1083-1085.
4. Murphey MD, Caroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24(5):1433-1466.
5. Chronopoulous E, Nicholas P, Karanikas C, et al. Patient presenting with lipoma of the index finger: a case report. Cases J. 2010;3:20.
6. Elbardouni A, Kharmaz M, Salah Berrada M, Mahfoud M, Eylaacoubi M. Well-circumscribed deep-seated lesions of the upper extremity. A report of 13 cases. Orthop Traumatol: Surg Res. 2011;97(2):152-158.
Lipomas are relatively common benign tumors composed primarily of adipose tissue. They can occur anywhere on the body and are seen often in the hands and forearm. Typically localized to the subcutaneous fat layer, a lipoma is rarely associated with a tendon sheath or tendon compartment.1,2 When this uncommon event occurs, the lipoma is appropriately labeled lipoma of the tendon sheath.
While there are numerous case reports of lipomas of the tendon sheath occurring in association with tendons in the lower extremity, there are no reports, to our knowledge, of their occurrence in the extensor compartments of the hand.1 We report a rare case of lipoma of the tendon sheath localized to the fourth dorsal compartment of the hand, which was successfully treated with surgical excision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 33-year-old right hand–dominant waitress presented with a chief complaint of a painful, slowly enlarging right dorsal hand mass of 5 years’ duration. The mass was particularly bothersome with activities involving grip and finger extension. Physical examination revealed a mobile, rubbery mass on the dorsum of the hand that moved slightly with fist formation. There were no signs of neurovascular compromise. She had normal hand and wrist range of motion.
Plain radiographs were unremarkable (Figures 1A, 1B). Magnetic resonance imaging (MRI) with and without contrast revealed a 4×2-cm mass consistent with a diagnosis of lipoma. However, it was unique in that it appeared to extend from the long- and ring-finger extensor tendon sheaths in the fourth dorsal compartment of the hand (Figures 2A, 2B) and was deemed a lipoma of the tendon sheath. Representative MRI also showed the lipoma to be present within the fourth extensor compartment of the hand (Figure 2B). Because of the mass’s increasing size and interference with hand function, the patient elected to have the mass excised.
Surgical Technique
A 3-cm longitudinal incision was made over the dorsum of the hand centered directly over the mass. Dissection was carried through the subcutaneous tissue to the distal margin of the extensor retinaculum. The fourth dorsal compartment was entered and the tendons of the fourth extensor compartment were identified. Immediately beneath the extensor tendons to the long and ring fingers was a yellow, rubbery mass consistent with lipoma (Figure 3). This mass was strongly adherent to the underlying tendons and had to be dissected carefully with tenotomy scissors. Fortunately, the mass could be excised as a single unit (Figure 4). It was sent to the pathology department for histologic examination, which revealed mature adipose tissue and confirmed the diagnosis of lipoma. The wound was closed with absorbable suture, and a soft, sterile dressing was applied.
Postoperative Care
The patient was seen in follow-up 2 weeks later for routine evaluation. She had an intact wound with minimal hand pain, and full wrist and hand range of motion. She returned to work as a waitress approximately 3 weeks after surgery without difficulty. At her 6-week postoperative mark, she had a pain-free wrist with a well-healed incision and no signs of recurrence.
Discussion
Tendon sheath lipomas, whether in the upper or lower extremities, are exceedingly rare entities. Further, lipomas of an individual extensor compartment of the hand (as in our case) have yet to be described, in contrast to lipomas of flexor tendon sheaths.3 There are only a handful of case reports in the literature of lipomas of the tendon sheath, and none to our knowledge of their existence in the extensor compartments of the hand. Nevertheless, it is important for the treating surgeon to be aware of their existence and know some basics about them and their treatment.
There are 2 types of tendon sheath lipomas: discrete solid masses of adipose tissue (which we encountered) and adipose tissue coupled with hypertrophic synovial villi (or, lipoma arborescens).4,5 Of note, the latter is significantly more common than the former, which makes our case even more uncommon. Although both types of lipoma of the tendon sheath are benign, they can cause symptoms such as pain, finger stiffness, and nerve compression.6 Thus, they frequently merit surgical removal, as in our case.
The appropriate workup for lipoma of the tendon sheath generally includes thorough history, physical examination, and advanced imaging, such as MRI. MRI is usually diagnostic of such a lesion and can aid in surgical planning.1 Regarding their overall prognosis, all lipomas (even large ones) are benign by definition but can transform into liposarcomas in rare cases.4 Lipomas are typically treated surgically by simple excision, and lipoma of the tendon sheath is no different. As long as complete excision of a tendon sheath lipoma is performed, recurrence rates are less than 5%.2,3
Surgeons should also be aware that, with long-standing lipomas of the tendon sheath, weakening of a tendon secondary to irritation from the mass is a possibility, especially in the lower extremities. All tendons should be inspected carefully at the time of surgery to ensure that other procedures, such as tendon grafting or side-to-side tenodesis, are not required. Although lipomas of the tendon sheath and extensor compartments are quite rare, all surgeons evaluating masses for possible surgical excision should be aware of their existence and know how to manage them appropriately.
Lipomas are relatively common benign tumors composed primarily of adipose tissue. They can occur anywhere on the body and are seen often in the hands and forearm. Typically localized to the subcutaneous fat layer, a lipoma is rarely associated with a tendon sheath or tendon compartment.1,2 When this uncommon event occurs, the lipoma is appropriately labeled lipoma of the tendon sheath.
While there are numerous case reports of lipomas of the tendon sheath occurring in association with tendons in the lower extremity, there are no reports, to our knowledge, of their occurrence in the extensor compartments of the hand.1 We report a rare case of lipoma of the tendon sheath localized to the fourth dorsal compartment of the hand, which was successfully treated with surgical excision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 33-year-old right hand–dominant waitress presented with a chief complaint of a painful, slowly enlarging right dorsal hand mass of 5 years’ duration. The mass was particularly bothersome with activities involving grip and finger extension. Physical examination revealed a mobile, rubbery mass on the dorsum of the hand that moved slightly with fist formation. There were no signs of neurovascular compromise. She had normal hand and wrist range of motion.
Plain radiographs were unremarkable (Figures 1A, 1B). Magnetic resonance imaging (MRI) with and without contrast revealed a 4×2-cm mass consistent with a diagnosis of lipoma. However, it was unique in that it appeared to extend from the long- and ring-finger extensor tendon sheaths in the fourth dorsal compartment of the hand (Figures 2A, 2B) and was deemed a lipoma of the tendon sheath. Representative MRI also showed the lipoma to be present within the fourth extensor compartment of the hand (Figure 2B). Because of the mass’s increasing size and interference with hand function, the patient elected to have the mass excised.
Surgical Technique
A 3-cm longitudinal incision was made over the dorsum of the hand centered directly over the mass. Dissection was carried through the subcutaneous tissue to the distal margin of the extensor retinaculum. The fourth dorsal compartment was entered and the tendons of the fourth extensor compartment were identified. Immediately beneath the extensor tendons to the long and ring fingers was a yellow, rubbery mass consistent with lipoma (Figure 3). This mass was strongly adherent to the underlying tendons and had to be dissected carefully with tenotomy scissors. Fortunately, the mass could be excised as a single unit (Figure 4). It was sent to the pathology department for histologic examination, which revealed mature adipose tissue and confirmed the diagnosis of lipoma. The wound was closed with absorbable suture, and a soft, sterile dressing was applied.
Postoperative Care
The patient was seen in follow-up 2 weeks later for routine evaluation. She had an intact wound with minimal hand pain, and full wrist and hand range of motion. She returned to work as a waitress approximately 3 weeks after surgery without difficulty. At her 6-week postoperative mark, she had a pain-free wrist with a well-healed incision and no signs of recurrence.
Discussion
Tendon sheath lipomas, whether in the upper or lower extremities, are exceedingly rare entities. Further, lipomas of an individual extensor compartment of the hand (as in our case) have yet to be described, in contrast to lipomas of flexor tendon sheaths.3 There are only a handful of case reports in the literature of lipomas of the tendon sheath, and none to our knowledge of their existence in the extensor compartments of the hand. Nevertheless, it is important for the treating surgeon to be aware of their existence and know some basics about them and their treatment.
There are 2 types of tendon sheath lipomas: discrete solid masses of adipose tissue (which we encountered) and adipose tissue coupled with hypertrophic synovial villi (or, lipoma arborescens).4,5 Of note, the latter is significantly more common than the former, which makes our case even more uncommon. Although both types of lipoma of the tendon sheath are benign, they can cause symptoms such as pain, finger stiffness, and nerve compression.6 Thus, they frequently merit surgical removal, as in our case.
The appropriate workup for lipoma of the tendon sheath generally includes thorough history, physical examination, and advanced imaging, such as MRI. MRI is usually diagnostic of such a lesion and can aid in surgical planning.1 Regarding their overall prognosis, all lipomas (even large ones) are benign by definition but can transform into liposarcomas in rare cases.4 Lipomas are typically treated surgically by simple excision, and lipoma of the tendon sheath is no different. As long as complete excision of a tendon sheath lipoma is performed, recurrence rates are less than 5%.2,3
Surgeons should also be aware that, with long-standing lipomas of the tendon sheath, weakening of a tendon secondary to irritation from the mass is a possibility, especially in the lower extremities. All tendons should be inspected carefully at the time of surgery to ensure that other procedures, such as tendon grafting or side-to-side tenodesis, are not required. Although lipomas of the tendon sheath and extensor compartments are quite rare, all surgeons evaluating masses for possible surgical excision should be aware of their existence and know how to manage them appropriately.
1. Khan AZ, Shafafy M, Latimer MD, Crosby J. A lipoma within the Achilles tendon sheath. Foot Ankle Surg. 2012;18(1):e16-e17.
2. Bryan RS, Dahlin DC, Sullivan CR. Lipoma of the tendon sheath. J Bone Joint Surg Am. 1956;38(6):1275-1280.
3. Kremchek TE, Kremchek EJ. Carpal tunnel syndrome caused by flexor tendon sheath lipoma. Orthop Rev. 1998;17(11):1083-1085.
4. Murphey MD, Caroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24(5):1433-1466.
5. Chronopoulous E, Nicholas P, Karanikas C, et al. Patient presenting with lipoma of the index finger: a case report. Cases J. 2010;3:20.
6. Elbardouni A, Kharmaz M, Salah Berrada M, Mahfoud M, Eylaacoubi M. Well-circumscribed deep-seated lesions of the upper extremity. A report of 13 cases. Orthop Traumatol: Surg Res. 2011;97(2):152-158.
1. Khan AZ, Shafafy M, Latimer MD, Crosby J. A lipoma within the Achilles tendon sheath. Foot Ankle Surg. 2012;18(1):e16-e17.
2. Bryan RS, Dahlin DC, Sullivan CR. Lipoma of the tendon sheath. J Bone Joint Surg Am. 1956;38(6):1275-1280.
3. Kremchek TE, Kremchek EJ. Carpal tunnel syndrome caused by flexor tendon sheath lipoma. Orthop Rev. 1998;17(11):1083-1085.
4. Murphey MD, Caroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24(5):1433-1466.
5. Chronopoulous E, Nicholas P, Karanikas C, et al. Patient presenting with lipoma of the index finger: a case report. Cases J. 2010;3:20.
6. Elbardouni A, Kharmaz M, Salah Berrada M, Mahfoud M, Eylaacoubi M. Well-circumscribed deep-seated lesions of the upper extremity. A report of 13 cases. Orthop Traumatol: Surg Res. 2011;97(2):152-158.
Study: Children with pet dogs less likely to have anxiety
A higher percentage of children without pet dogs (21%) than children with pet dogs (12%) tested positive for anxiety, in a cross-sectional study.
Researchers conducted the study at a general pediatric clinic in an academic medical center in Upstate New York. All parents of children enrolled in the study completed SCARED-5, a 5-item scale adapted from the Screen for Child Anxiety and Related Disorders, a tool validated in both psychiatric and primary care settings.
Dr. Anne M. Gadomski, attending pediatrician and research scientist at Bassett Medical Center in Cooperstown, N.Y., and her colleagues analyzed the mean SCARED-5 score and the proportion of children meeting the SCARED-5 clinical score threshold of 3 or more, a point at which further assessment is indicated to diagnose anxiety. Their final analysis involved 370 children with a pet dog and 273 children with no pet dog. The children were aged 4-10 years. Ill or developmentally disabled children were excluded from the study.
In a univariate analysis, the mean SCARED-5 score was significantly lower among children with a pet dog, compared with children without a pet dog. The average score for children with dogs was 1.13 vs. 1.40 for children without dogs (P = .01). The predicted probability of a SCARE-5 score of 3 or higher was 0.20 for children without pet dogs, compared with 0.11 for children with pet dogs. Further demonstrating the link between children with pet dogs and a decreased likelihood of childhood anxiety was the study’s finding of a pet dog having been associated with a 9% decreased probability of child scoring greater than or equal to 3 in the SCARED-5.
“Our study results suggest that children who have a pet dog in the home have a lower anxiety screening score than children who do not,” wrote Dr. Gadomski and her colleagues.
Further research on the anxiety levels of children with pet dogs should determine whether having a pet dog prevents a child from being anxious, and if so, how pets contribute to this absence of anxiety in children, they noted.
Read the full study in Preventing Chronic Disease (doi: 10.5888/pcd12.150204).
A higher percentage of children without pet dogs (21%) than children with pet dogs (12%) tested positive for anxiety, in a cross-sectional study.
Researchers conducted the study at a general pediatric clinic in an academic medical center in Upstate New York. All parents of children enrolled in the study completed SCARED-5, a 5-item scale adapted from the Screen for Child Anxiety and Related Disorders, a tool validated in both psychiatric and primary care settings.
Dr. Anne M. Gadomski, attending pediatrician and research scientist at Bassett Medical Center in Cooperstown, N.Y., and her colleagues analyzed the mean SCARED-5 score and the proportion of children meeting the SCARED-5 clinical score threshold of 3 or more, a point at which further assessment is indicated to diagnose anxiety. Their final analysis involved 370 children with a pet dog and 273 children with no pet dog. The children were aged 4-10 years. Ill or developmentally disabled children were excluded from the study.
In a univariate analysis, the mean SCARED-5 score was significantly lower among children with a pet dog, compared with children without a pet dog. The average score for children with dogs was 1.13 vs. 1.40 for children without dogs (P = .01). The predicted probability of a SCARE-5 score of 3 or higher was 0.20 for children without pet dogs, compared with 0.11 for children with pet dogs. Further demonstrating the link between children with pet dogs and a decreased likelihood of childhood anxiety was the study’s finding of a pet dog having been associated with a 9% decreased probability of child scoring greater than or equal to 3 in the SCARED-5.
“Our study results suggest that children who have a pet dog in the home have a lower anxiety screening score than children who do not,” wrote Dr. Gadomski and her colleagues.
Further research on the anxiety levels of children with pet dogs should determine whether having a pet dog prevents a child from being anxious, and if so, how pets contribute to this absence of anxiety in children, they noted.
Read the full study in Preventing Chronic Disease (doi: 10.5888/pcd12.150204).
A higher percentage of children without pet dogs (21%) than children with pet dogs (12%) tested positive for anxiety, in a cross-sectional study.
Researchers conducted the study at a general pediatric clinic in an academic medical center in Upstate New York. All parents of children enrolled in the study completed SCARED-5, a 5-item scale adapted from the Screen for Child Anxiety and Related Disorders, a tool validated in both psychiatric and primary care settings.
Dr. Anne M. Gadomski, attending pediatrician and research scientist at Bassett Medical Center in Cooperstown, N.Y., and her colleagues analyzed the mean SCARED-5 score and the proportion of children meeting the SCARED-5 clinical score threshold of 3 or more, a point at which further assessment is indicated to diagnose anxiety. Their final analysis involved 370 children with a pet dog and 273 children with no pet dog. The children were aged 4-10 years. Ill or developmentally disabled children were excluded from the study.
In a univariate analysis, the mean SCARED-5 score was significantly lower among children with a pet dog, compared with children without a pet dog. The average score for children with dogs was 1.13 vs. 1.40 for children without dogs (P = .01). The predicted probability of a SCARE-5 score of 3 or higher was 0.20 for children without pet dogs, compared with 0.11 for children with pet dogs. Further demonstrating the link between children with pet dogs and a decreased likelihood of childhood anxiety was the study’s finding of a pet dog having been associated with a 9% decreased probability of child scoring greater than or equal to 3 in the SCARED-5.
“Our study results suggest that children who have a pet dog in the home have a lower anxiety screening score than children who do not,” wrote Dr. Gadomski and her colleagues.
Further research on the anxiety levels of children with pet dogs should determine whether having a pet dog prevents a child from being anxious, and if so, how pets contribute to this absence of anxiety in children, they noted.
Read the full study in Preventing Chronic Disease (doi: 10.5888/pcd12.150204).
FROM PREVENTING CHRONIC DISEASE
Heparin Bridging for Atrial Fibrillation
Clinical question: In patients with atrial fibrillation (AF) or flutter, is heparin bridging needed during interruption of warfarin therapy for surgery or invasive procedures?
Background: Bridging is intended to decrease the risk of stroke or other arterial thromboembolism by minimizing time off anticoagulation. Bridging may increase the risk of serious bleeding, offsetting any benefit. Guidelines have provided weak and inconsistent recommendations due to a lack of randomized trials.
Study design: Randomized, double blind, placebo-controlled trial.
Setting: More than 100 centers in the U.S. and Canada, from 2009-2014.
Synopsis: Investigators randomized 1,884 patients on warfarin with a CHADS2 risk factor of one or higher undergoing elective surgery or procedure to dalteparin or placebo, from three days to 24 hours before the procedure and for five to 10 days after. Mean CHADS2 score was 2.3; 3% of patients had scores of five to six. Approximately one-third of patients were on aspirin, and most procedures (89%) were classified as minor. Patients with mechanical heart valves, stroke/transient ischemic attack (TIA)/systemic embolization within 12 weeks, major bleeding within six weeks, renal insufficiency, thrombocytopenia or planned cardiac, intracranial, or intraspinal surgery were excluded.
Thirty-day incidence of arterial thromboembolism (stroke, TIA, systemic embolization) was 0.4% in the non-bridging group and 0.3% in the bridging group (P=0.01 for noninferiority). Patients suffering arterial thromboembolism had mean CHADS2 scores of 2.6; most events occurred after minor procedures. Major bleeding was less common with no bridge (1.3% vs. 3.2%, relative risk 0.41, P=0.005 for superiority).
In this trial, most patients underwent minor procedures and few CHADS2 5-6 patients were enrolled; however, this well-designed, randomized trial adds important evidence to existing observational data.
Bottom line: Bridging is not warranted for most AF patients with CHADS2 scores of four or lower, at least for low-risk procedures.
Citation: Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373(9):823-833.
Clinical question: In patients with atrial fibrillation (AF) or flutter, is heparin bridging needed during interruption of warfarin therapy for surgery or invasive procedures?
Background: Bridging is intended to decrease the risk of stroke or other arterial thromboembolism by minimizing time off anticoagulation. Bridging may increase the risk of serious bleeding, offsetting any benefit. Guidelines have provided weak and inconsistent recommendations due to a lack of randomized trials.
Study design: Randomized, double blind, placebo-controlled trial.
Setting: More than 100 centers in the U.S. and Canada, from 2009-2014.
Synopsis: Investigators randomized 1,884 patients on warfarin with a CHADS2 risk factor of one or higher undergoing elective surgery or procedure to dalteparin or placebo, from three days to 24 hours before the procedure and for five to 10 days after. Mean CHADS2 score was 2.3; 3% of patients had scores of five to six. Approximately one-third of patients were on aspirin, and most procedures (89%) were classified as minor. Patients with mechanical heart valves, stroke/transient ischemic attack (TIA)/systemic embolization within 12 weeks, major bleeding within six weeks, renal insufficiency, thrombocytopenia or planned cardiac, intracranial, or intraspinal surgery were excluded.
Thirty-day incidence of arterial thromboembolism (stroke, TIA, systemic embolization) was 0.4% in the non-bridging group and 0.3% in the bridging group (P=0.01 for noninferiority). Patients suffering arterial thromboembolism had mean CHADS2 scores of 2.6; most events occurred after minor procedures. Major bleeding was less common with no bridge (1.3% vs. 3.2%, relative risk 0.41, P=0.005 for superiority).
In this trial, most patients underwent minor procedures and few CHADS2 5-6 patients were enrolled; however, this well-designed, randomized trial adds important evidence to existing observational data.
Bottom line: Bridging is not warranted for most AF patients with CHADS2 scores of four or lower, at least for low-risk procedures.
Citation: Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373(9):823-833.
Clinical question: In patients with atrial fibrillation (AF) or flutter, is heparin bridging needed during interruption of warfarin therapy for surgery or invasive procedures?
Background: Bridging is intended to decrease the risk of stroke or other arterial thromboembolism by minimizing time off anticoagulation. Bridging may increase the risk of serious bleeding, offsetting any benefit. Guidelines have provided weak and inconsistent recommendations due to a lack of randomized trials.
Study design: Randomized, double blind, placebo-controlled trial.
Setting: More than 100 centers in the U.S. and Canada, from 2009-2014.
Synopsis: Investigators randomized 1,884 patients on warfarin with a CHADS2 risk factor of one or higher undergoing elective surgery or procedure to dalteparin or placebo, from three days to 24 hours before the procedure and for five to 10 days after. Mean CHADS2 score was 2.3; 3% of patients had scores of five to six. Approximately one-third of patients were on aspirin, and most procedures (89%) were classified as minor. Patients with mechanical heart valves, stroke/transient ischemic attack (TIA)/systemic embolization within 12 weeks, major bleeding within six weeks, renal insufficiency, thrombocytopenia or planned cardiac, intracranial, or intraspinal surgery were excluded.
Thirty-day incidence of arterial thromboembolism (stroke, TIA, systemic embolization) was 0.4% in the non-bridging group and 0.3% in the bridging group (P=0.01 for noninferiority). Patients suffering arterial thromboembolism had mean CHADS2 scores of 2.6; most events occurred after minor procedures. Major bleeding was less common with no bridge (1.3% vs. 3.2%, relative risk 0.41, P=0.005 for superiority).
In this trial, most patients underwent minor procedures and few CHADS2 5-6 patients were enrolled; however, this well-designed, randomized trial adds important evidence to existing observational data.
Bottom line: Bridging is not warranted for most AF patients with CHADS2 scores of four or lower, at least for low-risk procedures.
Citation: Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373(9):823-833.
Hospital Medicine Exchange Adds Mobile App to Mark Its 3rd Birthday
Three years ago, SHM launched Hospital Medicine Exchange (HMX), an online collaborative forum designed to foster thoughtful discussions related to the hospital medicine movement, facilitate networking among SHM members, and house shared resources and best practices in the field.
In celebration of this milestone, SHM has unveiled a new native mobile app for HMX, ensuring that SHM members have access to insight and answers from thousands of other members, wherever they are.
The redesigned app features a new user interface, including easy access to your communities as well as SHM’s website, social media, and The Hospital Leader blog. Not only is the new app easy to use, but, once you are online, you can be a part of the vibrant conversations taking place among thousands of hospitalists across the country.
After you download the new app from the iTunes or Google Play stores, log in using your SHM username and password on your tablet or smartphone to:
- Quickly scan through and engage in discussions in your favorite communities, including HMX Open Forum;
- Connect and network with fellow SHM members across the country by clicking on “People”;
- Check your private messages with Inbox; and
- Access resources relevant to your everyday practice in the Libraries.
“It is a great app, and I love the shortcuts on the home screen.” —Masoumeh Ghaffari, MD, hospitalist, Piedmont Healthcare, Acworth, Ga., in a posting to the HMX Open Forum
Members already are responding to the new and improved HMX mobile app. Hospitalist Masoumeh Ghaffari, MD of Piedmont Healthcare in Acworth, Ga., posted in HMX Open Forum, “It is a great app, and I love the shortcuts on the home screen.”
The new HMX mobile app is one of many recent HMX enhancements. HMX is constantly growing and launching new communities for members. For example, the Patient Experience community and the Women in Hospital Medicine community were most recently launched. Join these and other communities to share your thoughts on topics ranging from admissions to pediatrics and everything in between.
Get involved now by downloading the new app.
Brett Radler is SHM’s communications coordinator.
Three years ago, SHM launched Hospital Medicine Exchange (HMX), an online collaborative forum designed to foster thoughtful discussions related to the hospital medicine movement, facilitate networking among SHM members, and house shared resources and best practices in the field.
In celebration of this milestone, SHM has unveiled a new native mobile app for HMX, ensuring that SHM members have access to insight and answers from thousands of other members, wherever they are.
The redesigned app features a new user interface, including easy access to your communities as well as SHM’s website, social media, and The Hospital Leader blog. Not only is the new app easy to use, but, once you are online, you can be a part of the vibrant conversations taking place among thousands of hospitalists across the country.
After you download the new app from the iTunes or Google Play stores, log in using your SHM username and password on your tablet or smartphone to:
- Quickly scan through and engage in discussions in your favorite communities, including HMX Open Forum;
- Connect and network with fellow SHM members across the country by clicking on “People”;
- Check your private messages with Inbox; and
- Access resources relevant to your everyday practice in the Libraries.
“It is a great app, and I love the shortcuts on the home screen.” —Masoumeh Ghaffari, MD, hospitalist, Piedmont Healthcare, Acworth, Ga., in a posting to the HMX Open Forum
Members already are responding to the new and improved HMX mobile app. Hospitalist Masoumeh Ghaffari, MD of Piedmont Healthcare in Acworth, Ga., posted in HMX Open Forum, “It is a great app, and I love the shortcuts on the home screen.”
The new HMX mobile app is one of many recent HMX enhancements. HMX is constantly growing and launching new communities for members. For example, the Patient Experience community and the Women in Hospital Medicine community were most recently launched. Join these and other communities to share your thoughts on topics ranging from admissions to pediatrics and everything in between.
Get involved now by downloading the new app.
Brett Radler is SHM’s communications coordinator.
Three years ago, SHM launched Hospital Medicine Exchange (HMX), an online collaborative forum designed to foster thoughtful discussions related to the hospital medicine movement, facilitate networking among SHM members, and house shared resources and best practices in the field.
In celebration of this milestone, SHM has unveiled a new native mobile app for HMX, ensuring that SHM members have access to insight and answers from thousands of other members, wherever they are.
The redesigned app features a new user interface, including easy access to your communities as well as SHM’s website, social media, and The Hospital Leader blog. Not only is the new app easy to use, but, once you are online, you can be a part of the vibrant conversations taking place among thousands of hospitalists across the country.
After you download the new app from the iTunes or Google Play stores, log in using your SHM username and password on your tablet or smartphone to:
- Quickly scan through and engage in discussions in your favorite communities, including HMX Open Forum;
- Connect and network with fellow SHM members across the country by clicking on “People”;
- Check your private messages with Inbox; and
- Access resources relevant to your everyday practice in the Libraries.
“It is a great app, and I love the shortcuts on the home screen.” —Masoumeh Ghaffari, MD, hospitalist, Piedmont Healthcare, Acworth, Ga., in a posting to the HMX Open Forum
Members already are responding to the new and improved HMX mobile app. Hospitalist Masoumeh Ghaffari, MD of Piedmont Healthcare in Acworth, Ga., posted in HMX Open Forum, “It is a great app, and I love the shortcuts on the home screen.”
The new HMX mobile app is one of many recent HMX enhancements. HMX is constantly growing and launching new communities for members. For example, the Patient Experience community and the Women in Hospital Medicine community were most recently launched. Join these and other communities to share your thoughts on topics ranging from admissions to pediatrics and everything in between.
Get involved now by downloading the new app.
Brett Radler is SHM’s communications coordinator.
Study: Exposure history critical to design of universal flu vaccine
In a study with implications for the development of new influenza vaccine strategies, researchers discovered that – among patients who received the 2009 H1N1 influenza vaccine – individuals with low levels of H1N1-specific antibodies prior to vaccination produced a more broadly protective immune response against the influenza virus than patients with high levels of H1N1-specific antibodies prior to vaccination.
A research team led by Patrick C. Wilson, Ph.D., of the Knapp Center for Lupus and Immunology Research at the University of Chicago, studied the B cell response in patients who received the pandemic 2009 H1N1 vaccine 2 years in a row and had varied histories of influenza exposure. All patients were 18 years or older, healthy, and had not received the yearly influenza vaccine prior to participating in the study. The researchers compared the patients’ “vaccine-induced plasmablast response upon first vaccination with the pandemic H1N1 strain in 2009-2010” with the patients’ plasmablast response upon revaccination with this same strain in 2010-2011 or 2011-2012. Each of the 21 study participants provided the researchers with at least four H1N1-specific plasmablasts.
The researchers “analyzed the immunoglobulin regions, strain specificity, and functional properties of the antibodies produced by this plasmablast population at the single-cell level across multiple years,” which allowed them to directly evaluate the effect of immune memory on the specificity of the current response to the virus.
Among the study’s findings was that “only individuals with low preexisting serological levels of pandemic H1N1 specific antibodies generated a broadly neutralizing plasmablast response directed toward the [hemagglutinin] stalk,” which is part of the hemagglutinin protein located on the surface of the influenza virus.
“[W]e demonstrate that the immune subdominance of the [hemagglutinin] stalk is a function of both the poor accessibility to the broadly protective epitopes and the inherent polyreactivity of the antibodies that can bind. We conclude that immunological memory profoundly shapes the viral epitopes targeted upon exposure with divergent influenza strains and determines the likelihood of generating a broadly protective response,” said Dr. Wilson and his coauthors. The authors reported no conflicts of interest.
Read the full study in Science Translational Medicine (doi: 10.1126/scitranslmed.aad0522).
In a study with implications for the development of new influenza vaccine strategies, researchers discovered that – among patients who received the 2009 H1N1 influenza vaccine – individuals with low levels of H1N1-specific antibodies prior to vaccination produced a more broadly protective immune response against the influenza virus than patients with high levels of H1N1-specific antibodies prior to vaccination.
A research team led by Patrick C. Wilson, Ph.D., of the Knapp Center for Lupus and Immunology Research at the University of Chicago, studied the B cell response in patients who received the pandemic 2009 H1N1 vaccine 2 years in a row and had varied histories of influenza exposure. All patients were 18 years or older, healthy, and had not received the yearly influenza vaccine prior to participating in the study. The researchers compared the patients’ “vaccine-induced plasmablast response upon first vaccination with the pandemic H1N1 strain in 2009-2010” with the patients’ plasmablast response upon revaccination with this same strain in 2010-2011 or 2011-2012. Each of the 21 study participants provided the researchers with at least four H1N1-specific plasmablasts.
The researchers “analyzed the immunoglobulin regions, strain specificity, and functional properties of the antibodies produced by this plasmablast population at the single-cell level across multiple years,” which allowed them to directly evaluate the effect of immune memory on the specificity of the current response to the virus.
Among the study’s findings was that “only individuals with low preexisting serological levels of pandemic H1N1 specific antibodies generated a broadly neutralizing plasmablast response directed toward the [hemagglutinin] stalk,” which is part of the hemagglutinin protein located on the surface of the influenza virus.
“[W]e demonstrate that the immune subdominance of the [hemagglutinin] stalk is a function of both the poor accessibility to the broadly protective epitopes and the inherent polyreactivity of the antibodies that can bind. We conclude that immunological memory profoundly shapes the viral epitopes targeted upon exposure with divergent influenza strains and determines the likelihood of generating a broadly protective response,” said Dr. Wilson and his coauthors. The authors reported no conflicts of interest.
Read the full study in Science Translational Medicine (doi: 10.1126/scitranslmed.aad0522).
In a study with implications for the development of new influenza vaccine strategies, researchers discovered that – among patients who received the 2009 H1N1 influenza vaccine – individuals with low levels of H1N1-specific antibodies prior to vaccination produced a more broadly protective immune response against the influenza virus than patients with high levels of H1N1-specific antibodies prior to vaccination.
A research team led by Patrick C. Wilson, Ph.D., of the Knapp Center for Lupus and Immunology Research at the University of Chicago, studied the B cell response in patients who received the pandemic 2009 H1N1 vaccine 2 years in a row and had varied histories of influenza exposure. All patients were 18 years or older, healthy, and had not received the yearly influenza vaccine prior to participating in the study. The researchers compared the patients’ “vaccine-induced plasmablast response upon first vaccination with the pandemic H1N1 strain in 2009-2010” with the patients’ plasmablast response upon revaccination with this same strain in 2010-2011 or 2011-2012. Each of the 21 study participants provided the researchers with at least four H1N1-specific plasmablasts.
The researchers “analyzed the immunoglobulin regions, strain specificity, and functional properties of the antibodies produced by this plasmablast population at the single-cell level across multiple years,” which allowed them to directly evaluate the effect of immune memory on the specificity of the current response to the virus.
Among the study’s findings was that “only individuals with low preexisting serological levels of pandemic H1N1 specific antibodies generated a broadly neutralizing plasmablast response directed toward the [hemagglutinin] stalk,” which is part of the hemagglutinin protein located on the surface of the influenza virus.
“[W]e demonstrate that the immune subdominance of the [hemagglutinin] stalk is a function of both the poor accessibility to the broadly protective epitopes and the inherent polyreactivity of the antibodies that can bind. We conclude that immunological memory profoundly shapes the viral epitopes targeted upon exposure with divergent influenza strains and determines the likelihood of generating a broadly protective response,” said Dr. Wilson and his coauthors. The authors reported no conflicts of interest.
Read the full study in Science Translational Medicine (doi: 10.1126/scitranslmed.aad0522).
FROM SCIENCE TRANSLATIONAL MEDICINE
Psoriasis Cohort Reveals High Arthritis Risk
Psoriatic arthritis may occur more frequently among people with psoriasis than previously reported, and risk factors include having severe psoriasis, nail pitting, low education levels, and uveitis, according to findings from a Canadian cohort study.
Beginning in 2006, Dr. Lihi Eder of the University of Toronto and coinvestigators recruited 464 patients (mean age 47, 56% male, 77% white) mainly from phototherapy and dermatology outpatient clinics in Toronto, and followed them 8 years. All had psoriasis of varying type and severity at baseline, but not inflammatory arthritis or spondylitis (Arthritis Rheumatol. 2015 Nov 10 doi: 10.1002/art.39494).
During the 8-year follow-up, 51 patients developed rheumatologist-confirmed psoriatic arthritis (PsA). Dr. Eder and colleagues reported an annual incidence rate of 2.7 confirmed cases of psoriatic arthritis per 100 psoriasis patients per year, which is considerably higher than previous published estimates, the investigators noted. The independent predictors of confirmed psoriatic arthritis were severe psoriasis (relative risk, 5.4; P = .006), not finishing high school (vs. finishing college RR, 4.5, P = .005; and vs. finishing high school RR, 3.3; P = .049), and use of systemic retinoids (RR, 3.4; P = .02). Time-dependent predictive variables included psoriatic nail pitting (RR, 2.5; P = .002) and uveitis (RR, 31.5; P = .001). Disease severity and nail pitting have been found in previous studies to be associated with a higher risk of psoriatic arthritis.
This study confirmed this association and also identified low education levels and uveitis as predictors. Low education is a marker of socioeconomic status that has been associated with lifestyle habits and possibly occupations that may increase PsA risk, the study authors noted, but the link requires further investigation. The authors cautioned that only three uveitis cases occurred in the cohort and that confidence intervals were wide. They also noted as a limitation that most participants were recruited from dermatology clinics, leading to overrepresentation of moderate-severe psoriasis and possibly patients with longer disease duration. Nevertheless, it “is likely that the true incidence of PsA in patients with psoriasis, particularly those attending dermatology clinics, is higher than previously reported,” the investigators wrote. “This highlights the role of dermatologists as key players in identifying psoriasis patients who are at higher risk of developing PsA.”
Krembil Foundation, the Canadian Institutes of Health Research, and The Arthritis Society supported the study.
Psoriatic arthritis may occur more frequently among people with psoriasis than previously reported, and risk factors include having severe psoriasis, nail pitting, low education levels, and uveitis, according to findings from a Canadian cohort study.
Beginning in 2006, Dr. Lihi Eder of the University of Toronto and coinvestigators recruited 464 patients (mean age 47, 56% male, 77% white) mainly from phototherapy and dermatology outpatient clinics in Toronto, and followed them 8 years. All had psoriasis of varying type and severity at baseline, but not inflammatory arthritis or spondylitis (Arthritis Rheumatol. 2015 Nov 10 doi: 10.1002/art.39494).
During the 8-year follow-up, 51 patients developed rheumatologist-confirmed psoriatic arthritis (PsA). Dr. Eder and colleagues reported an annual incidence rate of 2.7 confirmed cases of psoriatic arthritis per 100 psoriasis patients per year, which is considerably higher than previous published estimates, the investigators noted. The independent predictors of confirmed psoriatic arthritis were severe psoriasis (relative risk, 5.4; P = .006), not finishing high school (vs. finishing college RR, 4.5, P = .005; and vs. finishing high school RR, 3.3; P = .049), and use of systemic retinoids (RR, 3.4; P = .02). Time-dependent predictive variables included psoriatic nail pitting (RR, 2.5; P = .002) and uveitis (RR, 31.5; P = .001). Disease severity and nail pitting have been found in previous studies to be associated with a higher risk of psoriatic arthritis.
This study confirmed this association and also identified low education levels and uveitis as predictors. Low education is a marker of socioeconomic status that has been associated with lifestyle habits and possibly occupations that may increase PsA risk, the study authors noted, but the link requires further investigation. The authors cautioned that only three uveitis cases occurred in the cohort and that confidence intervals were wide. They also noted as a limitation that most participants were recruited from dermatology clinics, leading to overrepresentation of moderate-severe psoriasis and possibly patients with longer disease duration. Nevertheless, it “is likely that the true incidence of PsA in patients with psoriasis, particularly those attending dermatology clinics, is higher than previously reported,” the investigators wrote. “This highlights the role of dermatologists as key players in identifying psoriasis patients who are at higher risk of developing PsA.”
Krembil Foundation, the Canadian Institutes of Health Research, and The Arthritis Society supported the study.
Psoriatic arthritis may occur more frequently among people with psoriasis than previously reported, and risk factors include having severe psoriasis, nail pitting, low education levels, and uveitis, according to findings from a Canadian cohort study.
Beginning in 2006, Dr. Lihi Eder of the University of Toronto and coinvestigators recruited 464 patients (mean age 47, 56% male, 77% white) mainly from phototherapy and dermatology outpatient clinics in Toronto, and followed them 8 years. All had psoriasis of varying type and severity at baseline, but not inflammatory arthritis or spondylitis (Arthritis Rheumatol. 2015 Nov 10 doi: 10.1002/art.39494).
During the 8-year follow-up, 51 patients developed rheumatologist-confirmed psoriatic arthritis (PsA). Dr. Eder and colleagues reported an annual incidence rate of 2.7 confirmed cases of psoriatic arthritis per 100 psoriasis patients per year, which is considerably higher than previous published estimates, the investigators noted. The independent predictors of confirmed psoriatic arthritis were severe psoriasis (relative risk, 5.4; P = .006), not finishing high school (vs. finishing college RR, 4.5, P = .005; and vs. finishing high school RR, 3.3; P = .049), and use of systemic retinoids (RR, 3.4; P = .02). Time-dependent predictive variables included psoriatic nail pitting (RR, 2.5; P = .002) and uveitis (RR, 31.5; P = .001). Disease severity and nail pitting have been found in previous studies to be associated with a higher risk of psoriatic arthritis.
This study confirmed this association and also identified low education levels and uveitis as predictors. Low education is a marker of socioeconomic status that has been associated with lifestyle habits and possibly occupations that may increase PsA risk, the study authors noted, but the link requires further investigation. The authors cautioned that only three uveitis cases occurred in the cohort and that confidence intervals were wide. They also noted as a limitation that most participants were recruited from dermatology clinics, leading to overrepresentation of moderate-severe psoriasis and possibly patients with longer disease duration. Nevertheless, it “is likely that the true incidence of PsA in patients with psoriasis, particularly those attending dermatology clinics, is higher than previously reported,” the investigators wrote. “This highlights the role of dermatologists as key players in identifying psoriasis patients who are at higher risk of developing PsA.”
Krembil Foundation, the Canadian Institutes of Health Research, and The Arthritis Society supported the study.
FROM ARTHRITIS & RHEUMATOLOGY
Vitamin D improved vascular function in kidney disease
SAN DIEGO – Raising levels of vitamin D, deficient in many patients with chronic kidney disease, improved vascular function and reduced inflammation and vessel wall stiffness after 16 weeks, according to results of a study in a small group of early kidney disease patients in India.
“This tells us that vitamin D has the potential, at least over the short- to intermediate term, to improve vascular function,” with a promise of reducing or preventing cardiovascular outcomes in a larger study, said Dr. Vivekanand Jha, of the George Institute for Global Health in New Delhi.
“Patients with very early kidney disease are at very high risk of developing cardiovascular complications such as heart attacks, strokes, and peripheral vascular disease – and that’s what kills them before they get to the point where they need dialysis,” Dr. Jha said. “So, many die before they need dialysis. But we don’t know the factors that cause these cardiovascular complications.”
His group hypothesized that because vitamin D has biological action on blood vessels, “if we supplemented patients with vitamin D, their vascular function would improve.”
Participants were randomized to receive directly observed oral doses of 300,000 IU of cholecalciferol at baseline and at 8 weeks, or directly observed doses of a placebo in a matched control arm.
“We measured several parameters to look at vascular function after 16 weeks and found that patients who received vitamin D showed normalized vitamin D and decreases in levels of parathyroid hormone,” Dr. Jha said at the meeting sponsored by the American Society of Nephrology.
“But most importantly, we found that vitamin D recipients showed improvement in their vascular function on two or three tests; in flow-mediated dilation, which suggests improved endothelial function; and improved augmentation index, which looks at the stiffness of blood vessels,” he explained.
The next step is a much larger study designed to show that vitamin D supplements in this population can prevent cardiovascular events. “But that’s going to be a long-term study,” Dr. Jha noted.
“I don’t want to get ahead of myself and say that this is going to cure cardiovascular problems,” Dr. Jha cautioned. “But now we know we need to do a definitive study to look at these events, and prove whether supplementation with vitamin D does or does not significantly improve those endpoints.”
SAN DIEGO – Raising levels of vitamin D, deficient in many patients with chronic kidney disease, improved vascular function and reduced inflammation and vessel wall stiffness after 16 weeks, according to results of a study in a small group of early kidney disease patients in India.
“This tells us that vitamin D has the potential, at least over the short- to intermediate term, to improve vascular function,” with a promise of reducing or preventing cardiovascular outcomes in a larger study, said Dr. Vivekanand Jha, of the George Institute for Global Health in New Delhi.
“Patients with very early kidney disease are at very high risk of developing cardiovascular complications such as heart attacks, strokes, and peripheral vascular disease – and that’s what kills them before they get to the point where they need dialysis,” Dr. Jha said. “So, many die before they need dialysis. But we don’t know the factors that cause these cardiovascular complications.”
His group hypothesized that because vitamin D has biological action on blood vessels, “if we supplemented patients with vitamin D, their vascular function would improve.”
Participants were randomized to receive directly observed oral doses of 300,000 IU of cholecalciferol at baseline and at 8 weeks, or directly observed doses of a placebo in a matched control arm.
“We measured several parameters to look at vascular function after 16 weeks and found that patients who received vitamin D showed normalized vitamin D and decreases in levels of parathyroid hormone,” Dr. Jha said at the meeting sponsored by the American Society of Nephrology.
“But most importantly, we found that vitamin D recipients showed improvement in their vascular function on two or three tests; in flow-mediated dilation, which suggests improved endothelial function; and improved augmentation index, which looks at the stiffness of blood vessels,” he explained.
The next step is a much larger study designed to show that vitamin D supplements in this population can prevent cardiovascular events. “But that’s going to be a long-term study,” Dr. Jha noted.
“I don’t want to get ahead of myself and say that this is going to cure cardiovascular problems,” Dr. Jha cautioned. “But now we know we need to do a definitive study to look at these events, and prove whether supplementation with vitamin D does or does not significantly improve those endpoints.”
SAN DIEGO – Raising levels of vitamin D, deficient in many patients with chronic kidney disease, improved vascular function and reduced inflammation and vessel wall stiffness after 16 weeks, according to results of a study in a small group of early kidney disease patients in India.
“This tells us that vitamin D has the potential, at least over the short- to intermediate term, to improve vascular function,” with a promise of reducing or preventing cardiovascular outcomes in a larger study, said Dr. Vivekanand Jha, of the George Institute for Global Health in New Delhi.
“Patients with very early kidney disease are at very high risk of developing cardiovascular complications such as heart attacks, strokes, and peripheral vascular disease – and that’s what kills them before they get to the point where they need dialysis,” Dr. Jha said. “So, many die before they need dialysis. But we don’t know the factors that cause these cardiovascular complications.”
His group hypothesized that because vitamin D has biological action on blood vessels, “if we supplemented patients with vitamin D, their vascular function would improve.”
Participants were randomized to receive directly observed oral doses of 300,000 IU of cholecalciferol at baseline and at 8 weeks, or directly observed doses of a placebo in a matched control arm.
“We measured several parameters to look at vascular function after 16 weeks and found that patients who received vitamin D showed normalized vitamin D and decreases in levels of parathyroid hormone,” Dr. Jha said at the meeting sponsored by the American Society of Nephrology.
“But most importantly, we found that vitamin D recipients showed improvement in their vascular function on two or three tests; in flow-mediated dilation, which suggests improved endothelial function; and improved augmentation index, which looks at the stiffness of blood vessels,” he explained.
The next step is a much larger study designed to show that vitamin D supplements in this population can prevent cardiovascular events. “But that’s going to be a long-term study,” Dr. Jha noted.
“I don’t want to get ahead of myself and say that this is going to cure cardiovascular problems,” Dr. Jha cautioned. “But now we know we need to do a definitive study to look at these events, and prove whether supplementation with vitamin D does or does not significantly improve those endpoints.”
AT KIDNEY WEEK 2015
Key clinical point: Vitamin D supplements may improve vascular function in patients with stage 3 and 4 chronic kidney disease who did not have diabetes.
Major finding: 70% of patients in the treatment group achieved a 40% change in endothelial-dependent flow-mediated dilation and vascular function after 16 weeks. All patients achieved sufficient vitamin D levels.
Data source: A randomized, double-blind, placebo-controlled trial of 120 patients in New Delhi.
Disclosures: The researchers reported grant support from the Indian government and the department of biotechnology at the George Institute for Global Health in New Delhi.
Putting the Focus on Quality of Life in Cancer Care
Patient-centered care increasingly means focusing on quality of life. For the past 26 years, Betty Ferrell, PhD, MA, FAAN, FPCN, director and professor, nursing research and education at City of Hope has focused on quality of life research.
Dr. Ferrell recently sat down Federal Practitioner to discuss the components of quality cancer care, the role of family caregivers, and the importance of patient communication.
According to Dr. Ferrell, quality cancer care starts a comprehensive assessment so that care providers understand not only comorbidities, but also family help and psychosocial concerns. Interdisciplinary collaboration is also an essential element of quality care, bringing together an entire team to focus on the patient. Finally, Dr. Ferrell noted, care must include patient and family education.
0:15 Quality of life research
1:35 Quality of life interventions
2:52 Family care givers
3:30 Three components of quality cancer care
4:38 Communication
5:20 VA cancer care
Patient-centered care increasingly means focusing on quality of life. For the past 26 years, Betty Ferrell, PhD, MA, FAAN, FPCN, director and professor, nursing research and education at City of Hope has focused on quality of life research.
Dr. Ferrell recently sat down Federal Practitioner to discuss the components of quality cancer care, the role of family caregivers, and the importance of patient communication.
According to Dr. Ferrell, quality cancer care starts a comprehensive assessment so that care providers understand not only comorbidities, but also family help and psychosocial concerns. Interdisciplinary collaboration is also an essential element of quality care, bringing together an entire team to focus on the patient. Finally, Dr. Ferrell noted, care must include patient and family education.
0:15 Quality of life research
1:35 Quality of life interventions
2:52 Family care givers
3:30 Three components of quality cancer care
4:38 Communication
5:20 VA cancer care
Patient-centered care increasingly means focusing on quality of life. For the past 26 years, Betty Ferrell, PhD, MA, FAAN, FPCN, director and professor, nursing research and education at City of Hope has focused on quality of life research.
Dr. Ferrell recently sat down Federal Practitioner to discuss the components of quality cancer care, the role of family caregivers, and the importance of patient communication.
According to Dr. Ferrell, quality cancer care starts a comprehensive assessment so that care providers understand not only comorbidities, but also family help and psychosocial concerns. Interdisciplinary collaboration is also an essential element of quality care, bringing together an entire team to focus on the patient. Finally, Dr. Ferrell noted, care must include patient and family education.
0:15 Quality of life research
1:35 Quality of life interventions
2:52 Family care givers
3:30 Three components of quality cancer care
4:38 Communication
5:20 VA cancer care
Climate change may alter malaria risk in Africa

Photo by James Gathany
A larger portion of Africa is at high risk for malaria transmission than previously predicted, according to a mapping study published in Vector-Borne and Zoonotic Diseases.
The research also suggests that, under future climate regimes, the area where the disease can be transmitted most easily will shrink, but the total
malaria transmission zone in Africa will expand and move into new territory.
Researchers estimate that, by 2080, the year-round, highest-risk transmission zone will move from coastal West Africa, east to the Albertine Rift, between the Democratic Republic of Congo and Uganda.
The area suitable for seasonal, lower-risk transmission will shift north into coastal sub-Saharan Africa.
In addition, some parts of Africa will become too hot for malaria.
The overall expansion of malaria-vulnerable areas will challenge management of the deadly disease, said study author Sadie Ryan, PhD, of the University of Florida in Gainesville.
She noted that malaria will arrive in new areas, posing a risk to new populations, and the shift of endemic and epidemic areas will require public health management changes.
“Mapping a mathematical predictive model of a climate-driven infectious disease like malaria allows us to develop tools to understand both spatial and seasonal dynamics, and to anticipate the future changes to those dynamics,” Dr Ryan said.
She and her colleagues used a model that takes into account how mosquitoes and the malaria parasite respond to temperature. This model shows an optimal transmission temperature for malaria that, at 25 degrees Celsius, is 6 degrees lower than previous predictive models.
Dr Ryan said this work will play an important role in helping public health officials and non-governmental organizations plan for the efficient deployment of resources and interventions to control future outbreaks of malaria and their associated societal costs.
This study expands upon the team’s prior work at the National Center for Ecological Analysis and Synthesis at the University of California, Santa Barbara. ![]()

Photo by James Gathany
A larger portion of Africa is at high risk for malaria transmission than previously predicted, according to a mapping study published in Vector-Borne and Zoonotic Diseases.
The research also suggests that, under future climate regimes, the area where the disease can be transmitted most easily will shrink, but the total
malaria transmission zone in Africa will expand and move into new territory.
Researchers estimate that, by 2080, the year-round, highest-risk transmission zone will move from coastal West Africa, east to the Albertine Rift, between the Democratic Republic of Congo and Uganda.
The area suitable for seasonal, lower-risk transmission will shift north into coastal sub-Saharan Africa.
In addition, some parts of Africa will become too hot for malaria.
The overall expansion of malaria-vulnerable areas will challenge management of the deadly disease, said study author Sadie Ryan, PhD, of the University of Florida in Gainesville.
She noted that malaria will arrive in new areas, posing a risk to new populations, and the shift of endemic and epidemic areas will require public health management changes.
“Mapping a mathematical predictive model of a climate-driven infectious disease like malaria allows us to develop tools to understand both spatial and seasonal dynamics, and to anticipate the future changes to those dynamics,” Dr Ryan said.
She and her colleagues used a model that takes into account how mosquitoes and the malaria parasite respond to temperature. This model shows an optimal transmission temperature for malaria that, at 25 degrees Celsius, is 6 degrees lower than previous predictive models.
Dr Ryan said this work will play an important role in helping public health officials and non-governmental organizations plan for the efficient deployment of resources and interventions to control future outbreaks of malaria and their associated societal costs.
This study expands upon the team’s prior work at the National Center for Ecological Analysis and Synthesis at the University of California, Santa Barbara. ![]()

Photo by James Gathany
A larger portion of Africa is at high risk for malaria transmission than previously predicted, according to a mapping study published in Vector-Borne and Zoonotic Diseases.
The research also suggests that, under future climate regimes, the area where the disease can be transmitted most easily will shrink, but the total
malaria transmission zone in Africa will expand and move into new territory.
Researchers estimate that, by 2080, the year-round, highest-risk transmission zone will move from coastal West Africa, east to the Albertine Rift, between the Democratic Republic of Congo and Uganda.
The area suitable for seasonal, lower-risk transmission will shift north into coastal sub-Saharan Africa.
In addition, some parts of Africa will become too hot for malaria.
The overall expansion of malaria-vulnerable areas will challenge management of the deadly disease, said study author Sadie Ryan, PhD, of the University of Florida in Gainesville.
She noted that malaria will arrive in new areas, posing a risk to new populations, and the shift of endemic and epidemic areas will require public health management changes.
“Mapping a mathematical predictive model of a climate-driven infectious disease like malaria allows us to develop tools to understand both spatial and seasonal dynamics, and to anticipate the future changes to those dynamics,” Dr Ryan said.
She and her colleagues used a model that takes into account how mosquitoes and the malaria parasite respond to temperature. This model shows an optimal transmission temperature for malaria that, at 25 degrees Celsius, is 6 degrees lower than previous predictive models.
Dr Ryan said this work will play an important role in helping public health officials and non-governmental organizations plan for the efficient deployment of resources and interventions to control future outbreaks of malaria and their associated societal costs.
This study expands upon the team’s prior work at the National Center for Ecological Analysis and Synthesis at the University of California, Santa Barbara. ![]()