User login
Medical Roundtable: Peripheral T-Cell Lymphomas: A Practical Approach to Newly Diagnosed and Relapsed Patients
Moderator: Steven M. Horwitz, MD1
Discussants: Alison Moskowitz, MD1; Michelle Fanale, MD2; Andrei Shustov, MD3
From Memorial Sloan Kettering Cancer Center, New York, NY1; MD Anderson Cancer Center, Houston, TX2; University of Washington Medical Center, Seattle, WA3
Address for correspondence: Steven M. Horwitz, MD, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065
E-mail: [email protected]
DR. HORWITZ: My name is Dr. Steven Horwitz from Memorial Sloan-Kettering Cancer Center. I’m joined today by Drs. Alison Moskowitz, Memorial Sloan-Kettering, Michelle Fanale of MD Anderson, and Andrei Shustov, University of Washington in Seattle. Thank you all for joining us for this conversation on T-cell lymphoma. My colleagues are all well known as experts in T-cell lymphomas. Those of you who treat these diseases recognize the systemic T-cell lymphomas as one of our greater challenges in hematologic malignancies in terms of the treatment options for patients and the frequent lack of definitive data to guide our decisions.
I thought what we would do today is have a very practical discussion about the way we think about these diseases, the decisions we make, and the way we make those decisions. I'll start off by asking when you get a referral of a patient with a new diagnosis of peripheral T-cell lymphoma (PTCL), what are some of the basic things that you first think about in terms of approaching a new patient?
DR. SHUSTOV: I think one of the biggest challenges in T-cell lymphomas still remains making the proper diagnosis. In general, pathologists in the United States have a pretty good idea when they see T-cell lymphomas, however, subclassification remains a challenge even for expert hematopathologists due to frequent histologic overlap between the subtypes of PTCL, and even with non-malignant autoimmune disorders. I frequently see patients who are diagnosed with or misdiagnosed with a different subtype of T-cell lymphoma. The most challenging is differentiation between angioimmunoblastic T-cell lymphoma (AITL), anaplastic large cell T-cell lymphoma (ALCL), and PTCL not otherwise specified (PTCL-NOS), especially when the latter patients have high expression of CD30 and/or bear features resembling AITL.
Sometimes they are a slam-dunk diagnosis, but frequently our hematopathologists reverse the diagnosis after doing additional studies on the biopsy material. The most recent case I've seen in my clinic for consultation was a patient with a diagnosis of extranodal NK-cell lymphoma that was reclassified as a gamma-delta T-cell lymphoma after additional work-up. I truly believe that it is advisable that majority, if not all PTCL cases are reviewed by expert hematopathology teams at academic centers that see large volumes of these cases.
I think it's very important to educate community physicians and patients about a proper PTCL diagnosis, especially now that more targeted therapies are being developed and the gene expression profiling techniques will probably lead to identification of specific pathways that are amenable to therapy with specific biologic agents.
DR. FANALE: I'd like to expand upon what Andrei just said. For me the next step after confirming the pathology diagnosis is to think about two things. To think about whether or not this patient is a patient who might be eligible for an ongoing front-line trial, typically if the patient meets eligibility criteria for one of our ongoing front-line trials I would really recommend to the patient to consider being enrolled in that trial, and I also think about whether the patient, if he or she enters into remission with front-line therapy, can be considered for a consolidative autologous stem cell transplant.
I think it's very important to educate community physicians and patients about a proper PTCL diagnosis. Right now, our ongoing front-line trial is the ECHELON-2 trial which is evaluating brentuximab vedotin (BV) plus cyclophosphamide, doxorubicin, prednisone (CHP [BV-CHP]) chemotherapy compared to standard of care cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) chemotherapy, and that trial is based on the promising data that we've seen in the initial phase I trial that combined BV plus CHP chemotherapy followed by maintenance BV which demonstrated both high and durable complete remission (CR) rates.1 BV is an antibody drug conjugate that's targeted at CD30 and carries initial US Food and Drug Administration approval for patients who have relapsed or refractory ALCL which has a 100% level of CD30 positivity and then also has a National Comprehensive Cancer Network listing for treating other types of relapsed or refractory CD30 positive PTCL as well.
Also there is another upcoming front-line trial, which is to combine pralatrexate plus CHOP. If a patient isn't eligible for a clinical trial or it's just not feasible for that patient to enroll in a clinical trial, I will then look further at what would be potentially the best standard of care option for that patient.
I'll look at the patient's age and performance status and if they are generally less than age 65 or so and if otherwise well, I'll preferentially treat that patient with CHOEP which is CHOP plus etoposide. And then, for a patient who's either older than this or has multiple other comorbidities, I would treat that patient typically with CHOP alone.
DR. HORWITZ: Thank you, we'll go further into the selection of initial therapies but first circle back, I was curious as to how often your center comes to a different diagnosis than the referring center, and are there pitfalls you see that alert you to be suspicious of diagnosis.
DR. SHUSTOV: I'd say probably 10% of cases that we see at our center will be reclassified by our hematopathologists. In most cases, they do not necessarily reverse the diagnosis, but provide further clarification. It is occurring to me that in the community, pathologists are less likely to call the subtype of T-cell lymphoma and limit the report to the general description of T-cell lymphoprolipherative disorder, or state something like “consistent with T-cell lymphoma with features of AITL, or with features of anaplastic lymphoma.” I would admit though, that sometimes it's very difficult to identify the specific subtype of PTCL even in the expert hands; but I'd say these cases would constitute no more than 5% of PTCL patients.
DR. HORWITZ: And in your experience is it mostly fine tuning a T-cell lymphoma diagnosis, or do you see totally different diagnoses?
DR. MOSKOWITZ: Usually review by expert hematopathologists simply leads to fine-tuning the T-cell lymphoma diagnosis, however, I occasionally see significant changes in diagnosis. Often, alterations or clarification of a diagnosis are made possible only after we provide the pathologist with clinical history. For example, a lymph node biopsy may be interpreted as ALCL, however the knowledge that the particular patient has a history of mycosis fungoides would lead the pathologist to consider the diagnosis of large cell transformation of mycosis fungoides rather than ALCL. In such a case, molecular studies are helpful in confirming that the lymph node findings originate from the same clone as that in the original mycosis fungoides lesions, rather than representing a second primary.
DR. FANALE: Very occasionally, I've seen patients truly “with a misdiagnosis and a complete revision of diagnosis” and, usually, those pitfalls that I've seen them occasionally have been the young patients—the young patients who generally have disease in the thorax and neck, who are treated as though they have classical Hodgkin lymphoma, who have very significant progression of disease on standard of care treatment. So it's important that not all cases that have CD30 positivity are classical Hodgkin lymphoma even if the patient is young.
DR. HORWITZ: I often think the systemic T-cell lymphomas usually behave in an aggressive fashion so when the clinical picture and the diagnosis don't really fit, I think about getting a second biopsy before we finalize the diagnosis. Of course, when people are ill with obviously progressing disease, you may need to move more quickly.
I often think the systemic T-cell lymphomas usually behave in an aggressive fashion so when the clinical picture and the diagnosis don't really fit, I think about getting a second biopsy before we finalize the diagnosis. To move on, when you first see a patient, what is the decision tree in your mind in terms of picking therapy or planning therapy, or what kind of things do you consider?
DR. MOSKOWITZ: The first thing I think about when deciding upon treatment for a patient with T-cell lymphoma is whether or not I plan to use a curative approach to therapy. As was mentioned by Michelle, this decision is partly based upon the patients’ comorbidities and age. For patients who are eligible for curative therapy, our frontline approach for the most common types of T-cell lymphoma is to use CHOP with or without etoposide followed by consideration for autologous stem cell transplant in first remission. There are certainly individuals for whom such an aggressive strategy would not be appropriate due to age or co-morbidities. In these individuals, we may consider CHOP-based therapy alone or sometimes even milder approaches aimed at disease control.
DR. HORWITZ: Andrei, are you similar in the CHOP/CHOEP paradigm? And if so, what do you think of those data? How do you decide between the two and when do you do that regimen versus something different?
DR. SHUSTOV: I think I will double or triple what Michelle and Alison just said; for me, the two most important decisions that I have to make in the first encounter with patients with newly diagnosed PTCL are: 1) whether we're going to pursue curative approach strategy; and 2) whether the patient can tolerate the intensity of treatments that would provide him/her with the best chance of cure or long term remission. Patients who are elderly and have high risk disease would be very hard to cure, especially considering that the consolidative transplant might carry high rates of morbidity or mortality; more conservative strategies might be appropriate in these cases. On the other hand, younger and more fit patients might benefit more from intensified initial regimens—ie, CHOEP—followed by high-dose therapy and either autologous or allogeneic stem cell transplant (ALLO).
I usually have a long initial discussion with patients and families during which we decide on the intent of treatment and what to expect from certain regimens in terms of toxicities. I typically choose CHOEP regimen (or infusional version, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin [EPOCH]) for younger patients based on recent German data, even though this was a retrospective study and benefit of adding etoposide only approached statistical significance for the majority of PTCL subtypes.2 In older patients, I try to avoid anthracyclines, especially in the palliative intent setting, based on the retrospective analysis by the International T-Cell Lymphoma Project, and frequently use CEOP regimen (CHOEP minus anthracycline).3 I find that the majority of older patients tolerate this treatment somewhat better than anthracycline-containing combinations like CHOP.
In the very elderly and frail patients, I try to avoid combination chemotherapy all together. It is a somewhat easier decision for patients with AITL. Some of them are more indolent than other sub-types, and I would treat such patients with immunomodulatory approach, ie with a combination of prednisone and cyclosporine; then, I would consider single agent therapy with one of the recently approved agents for relapsed and refractory PTCL. I would also double what Michelle said in regard to making the best attempt to enroll patients into open clinical trials, because the current standards are not really satisfactory for many T-cell lymphoma patients.
DR. HORWITZ: It sounds like we all approach a new patient similarly. However, several of our up front trials are randomized against CHOP as opposed to CHOEP. When you're consenting a patient to those trials, how do you explain it to a patient or how important do you think the etoposide is?
DR. SHUSTOV: I share your frustration with quite a few of the current study designs for that exact reason. Some of these are confirmatory trials after conditional approvals of the new agents and are important. However, as often happens, in the confirmatory trials, we use controls that are a default from a lot of historical data, or the “common” way that the majority of patients are being treated. It is really a challenge when you consent patients to studies where a control arm is something that you think might not be quite adequate.
Having said that, the CHOEP study that was mentioned several times is a retrospective subgroup analysis and the addition of etoposide had marginal benefit that approached significance, but certainly was not a home run. I don't think we are ready to say that etoposide provides survival advantage in T-cell lymphoma patients and dismiss clinical trials in favor of just giving a patient the CHOEP, even though CHOP is admittedly not the best comparator. I discuss this controversy with patients and tell them frankly that the data that we base addition of etoposide on are not the best evidence one may have. Then, I ask patients to decide which approach sounds more reasonable to them and they make their choice.
DR. FANALE: To expand just slightly upon what Andrei said, I do emphasize to the patient and to the referring doctors that if you look at National Comprehensive Cancer Network guidelines, CHOP, CHOEP, EPOCH, all of these are potential options. So there's really not yet one trial that would say that one is clearly superior to the other at this time. I also emphasize to the patient and to the referring clinician that the only way, let's say, the patient could get the targeted agent plus the chemotherapy and have that 50% chance of, potentially, receiving that combination is really through the randomized clinical trial.
DR. HORWITZ: That was excellent, thank you. So you have alluded to this a bit. How much does subtype specific treatment come into play?
DR. MOSKOWITZ: At this point in time, outside of a clinical trial, for PTCL-NOS, AITL, and ALCL, the front-line approach would typically be CHOEP followed by autologous stem cell transplant. As Michelle mentioned, for a patient with ALCL, I would offer enrollment on the ECHELON-2 study in which patients are randomized to CHOP or BV-CHP, in order to give patients the option of potentially receiving BV.
For AITL, we are now obtaining molecular testing for certain mutations, particularly IDH2, because we currently have a study open with an IDH2 inhibitor that specifically enrolls patients with IDH2-mutant AITL. Because the testing takes some time to get back, we typically test patients’ biopsies at the time of diagnosis, so that we know if they're eligible for future clinical trials in case their disease does not respond to upfront therapy.
There are T-cell lymphoma subtypes that we treat quite differently from the entities discussed so far. For human T-lymphotropic virus-1 associated adult T-cell lymphoma, for example, the data with CHOP are really not good and we typically used EPOCH with the aim to consolidate with an allogeneic stem cell transplant.
Likewise, for hepatosplenic T-cell lymphoma, we have not observed good responses with the CHOP-based therapy and therefore we typically use platinum-based or ifosfamide-based therapy upfront with the aim to consolidate with allogeneic stem cell transplant. Extranodal NK/T-cell lymphoma is another entity for which CHOP is typically not effective and we have adopted asparaginase-based regimens, such as dexamethasone, methotrexate, ifosfamide, L-asparaginase, etoposide (SMILE) for this disease.
DR. SHUSTOV: I completely agree with Alison; for more common nodal lymphomas it is really hard at this point to base a treatment decision on histology, at least, in the front-line setting. However, some of the rare and unique subtypes, we generally treat with a completely different approach (ie, extranodal NK-cell lymphomas, enteropathy-associated T-cell lymphomas, adult T-cell leukemia/lymphomas).
DR. HORWITZ: Excellent. I’m curious, in a patient with ALCL who refused randomization of the ECHELON-2 trial, would you give them BV-CHP off study or would you discuss that as an alternative were they not to participate in the trial?
DR. FANALE: No. I don't urge treatment that's not approved for a particular line of therapy off clinical protocol with the exception that, let's say, if a patient is not a candidate for chemotherapy in second line setting for a particular lymphoma such as Hodgkin lymphoma—then, I would reference published data like Alison's data for a second line use of BV say for classical Hodgkin lymphoma and work with the insurance company to get the drug approved for that patient population, but if a patient just doesn't want to go on trial because they don't like the 50/50 chance of receiving BV-CHP compared to CHOP and they say, “I really want the 100% chance. Why don't you just contact my insurance?” I will explain to them the rationale of why the trial is being conducted including the hope that if the endpoints are met and the regimen is approved for front-line therapy then patients in the future can get BV-CHP at any oncology clinic, but I tell them for now it is a protocol based treatment option.
DR. HORWITZ: That's a very good answer. Can we now touch on the idea of transplant vs no transplant in first remission. Always, sometimes, never; how do you all think about that?
DR. SHUSTOV: I think that the idea of consolidative transplant is very heavily debated and discussed at the majority of specialized PTCL meetings. The reason for that is controversial nature of current clinical evidence. In the absence of randomized trials it is very hard to compare prospective phase II trial data to historical controls. You can interpret this in two ways; you might say, well, these are good data, outcome numbers look better than historical controls, and all patients with PTCL should have a consolidative transplant; or you can say, well, I don't have randomized data, and only a randomized study would really tell us whether post-induction consolidative transplant improves survival.
That is why it is such a controversial subject, and we agree that the phase II perspective studies of transplantation are hampered by significant bias; patients who are able and willing to travel to academic centers, were more robust and able to tolerate high dose therapy. They also have chemosensitive disease and that puts them in a completely different category than those you see in populational or retrospective studies, or other historical control trials.
Having said that, when I talk to patients, and I think that this is where I involve patients into treatment decision very heavily, I present them with the data and say that it is not wrong to do or not do the transplant in first CR. I make patients and family a big part of the decision. When they ask me what would give the patient the best chance being in remission five years from today, my answer is transplant, however, the data are not perfect, and the curative potential of autologous transplant in PTCL is not known.
DR. FANALE: So typically here, we would refer most patients on for front-line consolidative autologous stem cell transplant, I think, some exceptions are the ones that Andrei already touched on. If we're seeing a patient who has PTCL-NOS and this patient is the very rare patient who has early stage disease, with really no significant risk factors, that patient, unless there were pathologic features that showed a higher level of aggressiveness as Alison commented on, this patient might be one where we might defer doing the consolidative stem cell transplant for particularly if the patient’s disease entered into remission very quickly, but this still is controversial.
I'm not sure how you practice within each of your centers, but another patient, or where we would potentially defer, is a patient with fairly limited nasal NK/T-cell lymphoma, a patient that we’ve treated, let's say, with the dexamethasone, etoposide, ifosfamide, carboplatin (DeVIC) chemotherapy regimen plus a concomitant high-dose radiation, we typically historically here have deferred doing consolidative stem cell transplant for that patient population.
In terms of what Andrei commented on for when we might consider an ALLO and for a frontline consolidation, here we typically would not. The exception to that rule is that I've had a couple of patients here who have PTCL with a secondary hemophagocytic lymphohistiocytosis syndrome and then, for those patients because the concern is that the autologous stem cell transplant probably wouldn't be enough and for those patients given that they have such a dismal prognosis from the secondary hemophagocytic lymphohistiocytosis, we send those patients on for an ALLO.
DR. MOSKOWITZ: To expand upon early stage extranodal NK/T-cell lymphoma, I agree with Michelle that we wouldn't use an autologous stem cell transplant in first remission. Typically, for patients with localized disease, we recommend 2 cycles of SMILE followed by involved field radiation. Our patients treated with this approach have typically done quite well without needing a transplant.
DR. HORWITZ: That was great. If there are no further comments on upfront therapy, we can move over to relapse setting. When you see a patient at relapse, what are your strategies and how do you approach that patient?
DR. MOSKOWITZ: The first question in my mind when a patient is either not responding to or relapsing following front-line therapy is: what is my ultimate goal? For a patient who is fit and refractory to CHOP or has relapsed after CHOP or autologous stem cell transplant, my ultimate goal would be to try to get them to an ALLO. My choice of treatment following front-line therapy depends upon whether or not we are aiming for an ALLO as well as whether a donor has been identified for the patient.
Usually, if a patient just relapsed following front-line therapy, we are not necessarily ready to proceed quickly to ALLO. This may be because a donor has not yet been identified or because the patients’ eligibility for an ALLO is not clear. In this situation, my choice of treatment would be something that can be continued, potentially for several months, while we get things in place for the transplant. Treatment options in this situation would include the approved single agents for T-cell lymphoma, such as romidepsin, belinostat, pralatrexate, as well as BV (which would be specific for ALCL). In addition, I would consider enrollment on a promising clinical trial. Among these options, my choice of treatment typically depends upon the side effect profile and/or schedule, which is individualized for the patient. The one caveat would be that I typically will aim to use a histone deacetylase inhibitor (HDAC) inhibitor as my first choice for a patient with AITL.
Usually, if a patient just relapsed following front-line therapy, we are not necessarily ready to proceed quickly to ALLO. This may be because a donor has not yet been identified or because the patients’ eligibility for an ALLO is not clear. If my patient has a donor available and we are ready to move quickly to transplant, I would use one of the multi-agent regimens, such as ifosfamide, carboplatin, etoposide (ICE) or cisplatin, cytosine arabinoside, dexamethasone (DHAP), with the aim to try to get them into remission and relatively quickly proceed to transplant. The problem with using one of these types of regimens for everyone in the second-line setting is that the treatment cannot be continued indefinitely due to cumulative toxicity; therefore we need to know that we are ready to proceed to transplant if we are going to use one of the more intense regimens.
DR. HORWITZ: How do the others approach relapse?
DR. FANALE: Generally, a lot of similarities with what Alison discussed. Typically, it's if we're going to be sending the patient on for consideration for ALLO, I think the favor would be to try to use a regimen that has a high CR rate knowing that there is a trend toward improved progression free survival for the patient who enters into CR when compared to those who did not. I think the only slight difference might be just the timing and the selection, so, typically, here, even if the patient doesn't actually have a definite established donor quite yet we'll typically favor a combination type of a treatment approach, so whether or not that would be a platinum-based regimen such as ICE, as Alison mentioned, or gemcitabine-based chemotherapy regimen or similar.
I would generally favor one of those options, usually, over a single-agent therapeutic approach just knowing that the CR rates, generally, with exception of BV for ALCL are, generally, ranging about the 10%–15% range. And then, of course, first priority would be if there is a clinical trial available whether that's with a doublet of targeted agents or with the targeted agent plus chemotherapy in the relapse setting, we would typically favor that approach for a patient being considered for a stem cell transplant, ALLO approach.
DR. SHUSTOV: I'll consolidate what Michelle and Alison just said. I think we're on the same page. To me, the most important decision (even more important in the relapse setting) is to figure out whether we are still going to try to cure the lymphoma or we will pursue a palliative approach for which now we have several treatment options. So again, I’d have a long discussion with the patient, whether we're going to try and cure the disease, or we will pursue a palliative approach.
If this is a curative approach strategy and we're heading toward ALLO, we would start searching for the donor immediately. The choice of salvage therapy depends on duration of first remission. Primary refractory disease patients who failed CHOP, CHOEP, or EPOCH outright or within 3 months, I typically consider using the single agent approach, because even ICE and DHAP type regimens don’t work well for these patients. I put my “money” into HDAC inhibitors or antifolates, or other targeted therapy, if this is anaplastic lymphoma.
For patients who had at least 6 months remission from the initial therapy, younger patients who are still fit, I agree, I would consider standard salvage regimens like DHAP, etoposide, methylprednisolone, high-dosecytarabine, cisplatin (ESHAP) and ICE, and then, follow this with allograft.
DR. HORWITZ: You have all talked about allografting, I have two questions. What is your best guess in terms of percentage of patients you see with relapsed T-cell lymphoma who actually undergo ALLO at some point and do you ever consider autologous transplantation in those who didn't have it as part of their initial therapy?
DR. SHUSTOV: It certainly depends on the type of the academic center that patients are referred to, and we all practice at academic institutions with very strong ALLO programs. In patients who we select for a curative approach (one half to two thirds) we will at least make the best effort to take them to allogeneic grafting. Patients might fall off this track if they don't respond to salvage therapies or develop significant treatment/disease-related complications, ie fungal infections, organ toxicities, poor performance status, etc. Overall, I'd say that we successfully take about half of the patients with relapsed PTCL that are willing to pursue curative approach to allograft.
In addition, I consider autologous stem cell transplant in relapse setting for three situations. One is relapsed ALK-positive ALCL, some patients with ALK-negative ALCL who have had long initial remission and after relapse, and achieved second remission with BV or other salvage therapy. Finally, I’d consider auto-transplant in patients with AITL who achieved second CR with salvage therapy.
DR. HORWITZ: In terms of the relapse setting—some of you talked about angioimmunoblastic and HDAC inhibitors or BV for ALCL—when you have a relapse patient with PTCL-NOS, how do you pick a second line agent if you're not going directly to transplant, and are there new drugs that you're particularly excited about?
DR. FANALE: To answer your questions in terms of how I might choose a targeted agent for a patient who has PTCL-NOS and doesn't have AITL or ALCL, if the patient isn't eligible for trial or trial isn't feasible for that patient, I'll typically look at schedule, and then I'll look at side effect profile. For instance, for comparison, there are two HDAC inhibitor drugs right now approved, romidepsin and belinostat, so I think that potentially in a private practice setting, belinostat might be one when you look at the efficacy, being generally, around equivalent on where a patient might prefer that regimen because of the fact that they come in for five days in a row and then, their treatment is done for that cycle.
At a larger referral center to which patients are often traveling back and forth, it can be quite difficult for a patient to stay in town to do five days of treatment in a row. Often, these patients will prefer the romidepsin schedule because of the fact that they're coming back in once per week for three weeks in a row per cycle. The other way that I'll look at it is side effect profile, so, let's say, pralatrexate compared to the HDAC inhibitors, I will usually favor the HDAC inhibitors because of the potential side effect of mucositis with pralatrexate, although I have been able to manage through the mucositis by giving the patients more of a cutaneous T-cell lymphoma dosing format.
There had been a high level of interest in new emerging agents such as the aurora A-kinase inhibitors, alisertib, and that trial is now complete and the data are to be presented at The American Society of Hematology’s Annual Meeting this year.4 Other new therapies that are emerging are the PI3K inhibitors such as duvelisib and others. One advantage for many of the drugs in current trials is that they are oral agents.
My interest also lies in the combination approach, such as two or three targeted agent combinations, with the goal that if you see favorable CR rates and progression-free survival rates in the relapsed setting that you can then potentially move these treatments to the front-line setting to potentially move beyond the CHOP-based platform of therapies.
DR. SHUSTOV: For patients outside anaplastic lymphoma where, obviously, most of us would likely use BV, given the response rate to this agent, the decision is really based on convenience of the schedule, toxicity profile, and—sometimes—patient’s preference. So, toxicity and quality of life become kind of more decision-driving factors than disease biology. I’d have a discussion with patients about all three drugs, how they are administered, and what toxicities can be expected. Four-hour infusion of romidepsin might not be acceptable for some patients who have to travel long distances to treatment centers. They may prefer more rapid infusion (they would favor a trial of pralatrexate; or, as Alison mentioned, patient prefers a five-day/daily versus weekly administration.
It's kind of a coin toss decision outside clinical trials. Certainly, participation in studies in relapse setting is the high priority. We are all really looking forward to developing doublet combinations of novel agents, ie studies like the one Michelle is running at MD Anderson with a combination of alisertib and romidepsin.
DR. MOSKOWITZ: I'll echo what both Andrei and Michelle said that our choice of treatment is individualized and depends upon side-effect profile and the schedule. With regard to novel agents, I am also very excited about the studies that will be opening with the PI-3 kinase inhibitor, IPI-145. There have been promising results seen with single-agent IPI-1455 and we will be opening a study evaluating it in combination with bortezomib or romidepsin.
In addition, I mentioned earlier the IDH2 inhibitor, which we're studying right now in patients with AITL that is characterized by the presence of the IDH2 mutation. It would be exciting to see if targeting a specific mutation in this disease translates into responses.
DR. HORWITZ: Great, I think that was fantastic. I would like to thank Drs. Moskowitz, Shustov, and Fanale for a very thorough impractical discussion on how they approach and manage patients with T-cell lymphoma. I’m impressed that there is significant consistency among these experts in terms of how they manage these uncommon and often challenging lymphomas in terms of upfront combination chemotherapy approaches, combination considerations for the use of transplantation, as well as their enthusiasm for and dedication to incorporating clinical trials as part of everyday management.
References
1. Fanale MA, Horwitz SM, Forero-Torres A, et al. Brentuximab vedotin in the front-line treatment of patients with CD30+ peripheral T-cell lymphomas: results of a phase I study. J Clin Oncol. 2014;32(28):3137–3143.
2. Schmitz N, Trümper L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116(18):3418–3425. doi: 10.1182/blood-2010-02-270785. Epub 2010 Jul 21.
3. Vose J, Armitage J, Weisenburger D, for the International T-Cell Lymphoma Project. J Clin Oncol. 2008;26(25):4124–4130. doi: 10.1200/JCO.2008.16.4558. Epub 2008 Jul 14.
4. O’Connor OA, Ozcan M, Jacobsen ED, et al. First multicenter, randomized phase 3 study in patients with relapsed/refractory peripheral T-cell lymphoma: alisertib versus investigator’s choice. Paper to be presented at: American Society of Hematology 57th Annual Meeting & Exposition; December 5–8, 2015; Orlando, FL.
5. Horwitz SM, Porcu P, Flinn I, et al. Duvelisib (IPI-145), a phosphoinositide-3-kinsae-γδ inhibitor, shows activity in patients with relapsed/refractory T-cell lymphoma. Paper presented at: American Society of Hematology 56th Annual Meeting & Exposition; December 6–9, 2014; San Francisco, CA.
Moderator: Steven M. Horwitz, MD1
Discussants: Alison Moskowitz, MD1; Michelle Fanale, MD2; Andrei Shustov, MD3
From Memorial Sloan Kettering Cancer Center, New York, NY1; MD Anderson Cancer Center, Houston, TX2; University of Washington Medical Center, Seattle, WA3
Address for correspondence: Steven M. Horwitz, MD, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065
E-mail: [email protected]
DR. HORWITZ: My name is Dr. Steven Horwitz from Memorial Sloan-Kettering Cancer Center. I’m joined today by Drs. Alison Moskowitz, Memorial Sloan-Kettering, Michelle Fanale of MD Anderson, and Andrei Shustov, University of Washington in Seattle. Thank you all for joining us for this conversation on T-cell lymphoma. My colleagues are all well known as experts in T-cell lymphomas. Those of you who treat these diseases recognize the systemic T-cell lymphomas as one of our greater challenges in hematologic malignancies in terms of the treatment options for patients and the frequent lack of definitive data to guide our decisions.
I thought what we would do today is have a very practical discussion about the way we think about these diseases, the decisions we make, and the way we make those decisions. I'll start off by asking when you get a referral of a patient with a new diagnosis of peripheral T-cell lymphoma (PTCL), what are some of the basic things that you first think about in terms of approaching a new patient?
DR. SHUSTOV: I think one of the biggest challenges in T-cell lymphomas still remains making the proper diagnosis. In general, pathologists in the United States have a pretty good idea when they see T-cell lymphomas, however, subclassification remains a challenge even for expert hematopathologists due to frequent histologic overlap between the subtypes of PTCL, and even with non-malignant autoimmune disorders. I frequently see patients who are diagnosed with or misdiagnosed with a different subtype of T-cell lymphoma. The most challenging is differentiation between angioimmunoblastic T-cell lymphoma (AITL), anaplastic large cell T-cell lymphoma (ALCL), and PTCL not otherwise specified (PTCL-NOS), especially when the latter patients have high expression of CD30 and/or bear features resembling AITL.
Sometimes they are a slam-dunk diagnosis, but frequently our hematopathologists reverse the diagnosis after doing additional studies on the biopsy material. The most recent case I've seen in my clinic for consultation was a patient with a diagnosis of extranodal NK-cell lymphoma that was reclassified as a gamma-delta T-cell lymphoma after additional work-up. I truly believe that it is advisable that majority, if not all PTCL cases are reviewed by expert hematopathology teams at academic centers that see large volumes of these cases.
I think it's very important to educate community physicians and patients about a proper PTCL diagnosis, especially now that more targeted therapies are being developed and the gene expression profiling techniques will probably lead to identification of specific pathways that are amenable to therapy with specific biologic agents.
DR. FANALE: I'd like to expand upon what Andrei just said. For me the next step after confirming the pathology diagnosis is to think about two things. To think about whether or not this patient is a patient who might be eligible for an ongoing front-line trial, typically if the patient meets eligibility criteria for one of our ongoing front-line trials I would really recommend to the patient to consider being enrolled in that trial, and I also think about whether the patient, if he or she enters into remission with front-line therapy, can be considered for a consolidative autologous stem cell transplant.
I think it's very important to educate community physicians and patients about a proper PTCL diagnosis. Right now, our ongoing front-line trial is the ECHELON-2 trial which is evaluating brentuximab vedotin (BV) plus cyclophosphamide, doxorubicin, prednisone (CHP [BV-CHP]) chemotherapy compared to standard of care cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) chemotherapy, and that trial is based on the promising data that we've seen in the initial phase I trial that combined BV plus CHP chemotherapy followed by maintenance BV which demonstrated both high and durable complete remission (CR) rates.1 BV is an antibody drug conjugate that's targeted at CD30 and carries initial US Food and Drug Administration approval for patients who have relapsed or refractory ALCL which has a 100% level of CD30 positivity and then also has a National Comprehensive Cancer Network listing for treating other types of relapsed or refractory CD30 positive PTCL as well.
Also there is another upcoming front-line trial, which is to combine pralatrexate plus CHOP. If a patient isn't eligible for a clinical trial or it's just not feasible for that patient to enroll in a clinical trial, I will then look further at what would be potentially the best standard of care option for that patient.
I'll look at the patient's age and performance status and if they are generally less than age 65 or so and if otherwise well, I'll preferentially treat that patient with CHOEP which is CHOP plus etoposide. And then, for a patient who's either older than this or has multiple other comorbidities, I would treat that patient typically with CHOP alone.
DR. HORWITZ: Thank you, we'll go further into the selection of initial therapies but first circle back, I was curious as to how often your center comes to a different diagnosis than the referring center, and are there pitfalls you see that alert you to be suspicious of diagnosis.
DR. SHUSTOV: I'd say probably 10% of cases that we see at our center will be reclassified by our hematopathologists. In most cases, they do not necessarily reverse the diagnosis, but provide further clarification. It is occurring to me that in the community, pathologists are less likely to call the subtype of T-cell lymphoma and limit the report to the general description of T-cell lymphoprolipherative disorder, or state something like “consistent with T-cell lymphoma with features of AITL, or with features of anaplastic lymphoma.” I would admit though, that sometimes it's very difficult to identify the specific subtype of PTCL even in the expert hands; but I'd say these cases would constitute no more than 5% of PTCL patients.
DR. HORWITZ: And in your experience is it mostly fine tuning a T-cell lymphoma diagnosis, or do you see totally different diagnoses?
DR. MOSKOWITZ: Usually review by expert hematopathologists simply leads to fine-tuning the T-cell lymphoma diagnosis, however, I occasionally see significant changes in diagnosis. Often, alterations or clarification of a diagnosis are made possible only after we provide the pathologist with clinical history. For example, a lymph node biopsy may be interpreted as ALCL, however the knowledge that the particular patient has a history of mycosis fungoides would lead the pathologist to consider the diagnosis of large cell transformation of mycosis fungoides rather than ALCL. In such a case, molecular studies are helpful in confirming that the lymph node findings originate from the same clone as that in the original mycosis fungoides lesions, rather than representing a second primary.
DR. FANALE: Very occasionally, I've seen patients truly “with a misdiagnosis and a complete revision of diagnosis” and, usually, those pitfalls that I've seen them occasionally have been the young patients—the young patients who generally have disease in the thorax and neck, who are treated as though they have classical Hodgkin lymphoma, who have very significant progression of disease on standard of care treatment. So it's important that not all cases that have CD30 positivity are classical Hodgkin lymphoma even if the patient is young.
DR. HORWITZ: I often think the systemic T-cell lymphomas usually behave in an aggressive fashion so when the clinical picture and the diagnosis don't really fit, I think about getting a second biopsy before we finalize the diagnosis. Of course, when people are ill with obviously progressing disease, you may need to move more quickly.
I often think the systemic T-cell lymphomas usually behave in an aggressive fashion so when the clinical picture and the diagnosis don't really fit, I think about getting a second biopsy before we finalize the diagnosis. To move on, when you first see a patient, what is the decision tree in your mind in terms of picking therapy or planning therapy, or what kind of things do you consider?
DR. MOSKOWITZ: The first thing I think about when deciding upon treatment for a patient with T-cell lymphoma is whether or not I plan to use a curative approach to therapy. As was mentioned by Michelle, this decision is partly based upon the patients’ comorbidities and age. For patients who are eligible for curative therapy, our frontline approach for the most common types of T-cell lymphoma is to use CHOP with or without etoposide followed by consideration for autologous stem cell transplant in first remission. There are certainly individuals for whom such an aggressive strategy would not be appropriate due to age or co-morbidities. In these individuals, we may consider CHOP-based therapy alone or sometimes even milder approaches aimed at disease control.
DR. HORWITZ: Andrei, are you similar in the CHOP/CHOEP paradigm? And if so, what do you think of those data? How do you decide between the two and when do you do that regimen versus something different?
DR. SHUSTOV: I think I will double or triple what Michelle and Alison just said; for me, the two most important decisions that I have to make in the first encounter with patients with newly diagnosed PTCL are: 1) whether we're going to pursue curative approach strategy; and 2) whether the patient can tolerate the intensity of treatments that would provide him/her with the best chance of cure or long term remission. Patients who are elderly and have high risk disease would be very hard to cure, especially considering that the consolidative transplant might carry high rates of morbidity or mortality; more conservative strategies might be appropriate in these cases. On the other hand, younger and more fit patients might benefit more from intensified initial regimens—ie, CHOEP—followed by high-dose therapy and either autologous or allogeneic stem cell transplant (ALLO).
I usually have a long initial discussion with patients and families during which we decide on the intent of treatment and what to expect from certain regimens in terms of toxicities. I typically choose CHOEP regimen (or infusional version, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin [EPOCH]) for younger patients based on recent German data, even though this was a retrospective study and benefit of adding etoposide only approached statistical significance for the majority of PTCL subtypes.2 In older patients, I try to avoid anthracyclines, especially in the palliative intent setting, based on the retrospective analysis by the International T-Cell Lymphoma Project, and frequently use CEOP regimen (CHOEP minus anthracycline).3 I find that the majority of older patients tolerate this treatment somewhat better than anthracycline-containing combinations like CHOP.
In the very elderly and frail patients, I try to avoid combination chemotherapy all together. It is a somewhat easier decision for patients with AITL. Some of them are more indolent than other sub-types, and I would treat such patients with immunomodulatory approach, ie with a combination of prednisone and cyclosporine; then, I would consider single agent therapy with one of the recently approved agents for relapsed and refractory PTCL. I would also double what Michelle said in regard to making the best attempt to enroll patients into open clinical trials, because the current standards are not really satisfactory for many T-cell lymphoma patients.
DR. HORWITZ: It sounds like we all approach a new patient similarly. However, several of our up front trials are randomized against CHOP as opposed to CHOEP. When you're consenting a patient to those trials, how do you explain it to a patient or how important do you think the etoposide is?
DR. SHUSTOV: I share your frustration with quite a few of the current study designs for that exact reason. Some of these are confirmatory trials after conditional approvals of the new agents and are important. However, as often happens, in the confirmatory trials, we use controls that are a default from a lot of historical data, or the “common” way that the majority of patients are being treated. It is really a challenge when you consent patients to studies where a control arm is something that you think might not be quite adequate.
Having said that, the CHOEP study that was mentioned several times is a retrospective subgroup analysis and the addition of etoposide had marginal benefit that approached significance, but certainly was not a home run. I don't think we are ready to say that etoposide provides survival advantage in T-cell lymphoma patients and dismiss clinical trials in favor of just giving a patient the CHOEP, even though CHOP is admittedly not the best comparator. I discuss this controversy with patients and tell them frankly that the data that we base addition of etoposide on are not the best evidence one may have. Then, I ask patients to decide which approach sounds more reasonable to them and they make their choice.
DR. FANALE: To expand just slightly upon what Andrei said, I do emphasize to the patient and to the referring doctors that if you look at National Comprehensive Cancer Network guidelines, CHOP, CHOEP, EPOCH, all of these are potential options. So there's really not yet one trial that would say that one is clearly superior to the other at this time. I also emphasize to the patient and to the referring clinician that the only way, let's say, the patient could get the targeted agent plus the chemotherapy and have that 50% chance of, potentially, receiving that combination is really through the randomized clinical trial.
DR. HORWITZ: That was excellent, thank you. So you have alluded to this a bit. How much does subtype specific treatment come into play?
DR. MOSKOWITZ: At this point in time, outside of a clinical trial, for PTCL-NOS, AITL, and ALCL, the front-line approach would typically be CHOEP followed by autologous stem cell transplant. As Michelle mentioned, for a patient with ALCL, I would offer enrollment on the ECHELON-2 study in which patients are randomized to CHOP or BV-CHP, in order to give patients the option of potentially receiving BV.
For AITL, we are now obtaining molecular testing for certain mutations, particularly IDH2, because we currently have a study open with an IDH2 inhibitor that specifically enrolls patients with IDH2-mutant AITL. Because the testing takes some time to get back, we typically test patients’ biopsies at the time of diagnosis, so that we know if they're eligible for future clinical trials in case their disease does not respond to upfront therapy.
There are T-cell lymphoma subtypes that we treat quite differently from the entities discussed so far. For human T-lymphotropic virus-1 associated adult T-cell lymphoma, for example, the data with CHOP are really not good and we typically used EPOCH with the aim to consolidate with an allogeneic stem cell transplant.
Likewise, for hepatosplenic T-cell lymphoma, we have not observed good responses with the CHOP-based therapy and therefore we typically use platinum-based or ifosfamide-based therapy upfront with the aim to consolidate with allogeneic stem cell transplant. Extranodal NK/T-cell lymphoma is another entity for which CHOP is typically not effective and we have adopted asparaginase-based regimens, such as dexamethasone, methotrexate, ifosfamide, L-asparaginase, etoposide (SMILE) for this disease.
DR. SHUSTOV: I completely agree with Alison; for more common nodal lymphomas it is really hard at this point to base a treatment decision on histology, at least, in the front-line setting. However, some of the rare and unique subtypes, we generally treat with a completely different approach (ie, extranodal NK-cell lymphomas, enteropathy-associated T-cell lymphomas, adult T-cell leukemia/lymphomas).
DR. HORWITZ: Excellent. I’m curious, in a patient with ALCL who refused randomization of the ECHELON-2 trial, would you give them BV-CHP off study or would you discuss that as an alternative were they not to participate in the trial?
DR. FANALE: No. I don't urge treatment that's not approved for a particular line of therapy off clinical protocol with the exception that, let's say, if a patient is not a candidate for chemotherapy in second line setting for a particular lymphoma such as Hodgkin lymphoma—then, I would reference published data like Alison's data for a second line use of BV say for classical Hodgkin lymphoma and work with the insurance company to get the drug approved for that patient population, but if a patient just doesn't want to go on trial because they don't like the 50/50 chance of receiving BV-CHP compared to CHOP and they say, “I really want the 100% chance. Why don't you just contact my insurance?” I will explain to them the rationale of why the trial is being conducted including the hope that if the endpoints are met and the regimen is approved for front-line therapy then patients in the future can get BV-CHP at any oncology clinic, but I tell them for now it is a protocol based treatment option.
DR. HORWITZ: That's a very good answer. Can we now touch on the idea of transplant vs no transplant in first remission. Always, sometimes, never; how do you all think about that?
DR. SHUSTOV: I think that the idea of consolidative transplant is very heavily debated and discussed at the majority of specialized PTCL meetings. The reason for that is controversial nature of current clinical evidence. In the absence of randomized trials it is very hard to compare prospective phase II trial data to historical controls. You can interpret this in two ways; you might say, well, these are good data, outcome numbers look better than historical controls, and all patients with PTCL should have a consolidative transplant; or you can say, well, I don't have randomized data, and only a randomized study would really tell us whether post-induction consolidative transplant improves survival.
That is why it is such a controversial subject, and we agree that the phase II perspective studies of transplantation are hampered by significant bias; patients who are able and willing to travel to academic centers, were more robust and able to tolerate high dose therapy. They also have chemosensitive disease and that puts them in a completely different category than those you see in populational or retrospective studies, or other historical control trials.
Having said that, when I talk to patients, and I think that this is where I involve patients into treatment decision very heavily, I present them with the data and say that it is not wrong to do or not do the transplant in first CR. I make patients and family a big part of the decision. When they ask me what would give the patient the best chance being in remission five years from today, my answer is transplant, however, the data are not perfect, and the curative potential of autologous transplant in PTCL is not known.
DR. FANALE: So typically here, we would refer most patients on for front-line consolidative autologous stem cell transplant, I think, some exceptions are the ones that Andrei already touched on. If we're seeing a patient who has PTCL-NOS and this patient is the very rare patient who has early stage disease, with really no significant risk factors, that patient, unless there were pathologic features that showed a higher level of aggressiveness as Alison commented on, this patient might be one where we might defer doing the consolidative stem cell transplant for particularly if the patient’s disease entered into remission very quickly, but this still is controversial.
I'm not sure how you practice within each of your centers, but another patient, or where we would potentially defer, is a patient with fairly limited nasal NK/T-cell lymphoma, a patient that we’ve treated, let's say, with the dexamethasone, etoposide, ifosfamide, carboplatin (DeVIC) chemotherapy regimen plus a concomitant high-dose radiation, we typically historically here have deferred doing consolidative stem cell transplant for that patient population.
In terms of what Andrei commented on for when we might consider an ALLO and for a frontline consolidation, here we typically would not. The exception to that rule is that I've had a couple of patients here who have PTCL with a secondary hemophagocytic lymphohistiocytosis syndrome and then, for those patients because the concern is that the autologous stem cell transplant probably wouldn't be enough and for those patients given that they have such a dismal prognosis from the secondary hemophagocytic lymphohistiocytosis, we send those patients on for an ALLO.
DR. MOSKOWITZ: To expand upon early stage extranodal NK/T-cell lymphoma, I agree with Michelle that we wouldn't use an autologous stem cell transplant in first remission. Typically, for patients with localized disease, we recommend 2 cycles of SMILE followed by involved field radiation. Our patients treated with this approach have typically done quite well without needing a transplant.
DR. HORWITZ: That was great. If there are no further comments on upfront therapy, we can move over to relapse setting. When you see a patient at relapse, what are your strategies and how do you approach that patient?
DR. MOSKOWITZ: The first question in my mind when a patient is either not responding to or relapsing following front-line therapy is: what is my ultimate goal? For a patient who is fit and refractory to CHOP or has relapsed after CHOP or autologous stem cell transplant, my ultimate goal would be to try to get them to an ALLO. My choice of treatment following front-line therapy depends upon whether or not we are aiming for an ALLO as well as whether a donor has been identified for the patient.
Usually, if a patient just relapsed following front-line therapy, we are not necessarily ready to proceed quickly to ALLO. This may be because a donor has not yet been identified or because the patients’ eligibility for an ALLO is not clear. In this situation, my choice of treatment would be something that can be continued, potentially for several months, while we get things in place for the transplant. Treatment options in this situation would include the approved single agents for T-cell lymphoma, such as romidepsin, belinostat, pralatrexate, as well as BV (which would be specific for ALCL). In addition, I would consider enrollment on a promising clinical trial. Among these options, my choice of treatment typically depends upon the side effect profile and/or schedule, which is individualized for the patient. The one caveat would be that I typically will aim to use a histone deacetylase inhibitor (HDAC) inhibitor as my first choice for a patient with AITL.
Usually, if a patient just relapsed following front-line therapy, we are not necessarily ready to proceed quickly to ALLO. This may be because a donor has not yet been identified or because the patients’ eligibility for an ALLO is not clear. If my patient has a donor available and we are ready to move quickly to transplant, I would use one of the multi-agent regimens, such as ifosfamide, carboplatin, etoposide (ICE) or cisplatin, cytosine arabinoside, dexamethasone (DHAP), with the aim to try to get them into remission and relatively quickly proceed to transplant. The problem with using one of these types of regimens for everyone in the second-line setting is that the treatment cannot be continued indefinitely due to cumulative toxicity; therefore we need to know that we are ready to proceed to transplant if we are going to use one of the more intense regimens.
DR. HORWITZ: How do the others approach relapse?
DR. FANALE: Generally, a lot of similarities with what Alison discussed. Typically, it's if we're going to be sending the patient on for consideration for ALLO, I think the favor would be to try to use a regimen that has a high CR rate knowing that there is a trend toward improved progression free survival for the patient who enters into CR when compared to those who did not. I think the only slight difference might be just the timing and the selection, so, typically, here, even if the patient doesn't actually have a definite established donor quite yet we'll typically favor a combination type of a treatment approach, so whether or not that would be a platinum-based regimen such as ICE, as Alison mentioned, or gemcitabine-based chemotherapy regimen or similar.
I would generally favor one of those options, usually, over a single-agent therapeutic approach just knowing that the CR rates, generally, with exception of BV for ALCL are, generally, ranging about the 10%–15% range. And then, of course, first priority would be if there is a clinical trial available whether that's with a doublet of targeted agents or with the targeted agent plus chemotherapy in the relapse setting, we would typically favor that approach for a patient being considered for a stem cell transplant, ALLO approach.
DR. SHUSTOV: I'll consolidate what Michelle and Alison just said. I think we're on the same page. To me, the most important decision (even more important in the relapse setting) is to figure out whether we are still going to try to cure the lymphoma or we will pursue a palliative approach for which now we have several treatment options. So again, I’d have a long discussion with the patient, whether we're going to try and cure the disease, or we will pursue a palliative approach.
If this is a curative approach strategy and we're heading toward ALLO, we would start searching for the donor immediately. The choice of salvage therapy depends on duration of first remission. Primary refractory disease patients who failed CHOP, CHOEP, or EPOCH outright or within 3 months, I typically consider using the single agent approach, because even ICE and DHAP type regimens don’t work well for these patients. I put my “money” into HDAC inhibitors or antifolates, or other targeted therapy, if this is anaplastic lymphoma.
For patients who had at least 6 months remission from the initial therapy, younger patients who are still fit, I agree, I would consider standard salvage regimens like DHAP, etoposide, methylprednisolone, high-dosecytarabine, cisplatin (ESHAP) and ICE, and then, follow this with allograft.
DR. HORWITZ: You have all talked about allografting, I have two questions. What is your best guess in terms of percentage of patients you see with relapsed T-cell lymphoma who actually undergo ALLO at some point and do you ever consider autologous transplantation in those who didn't have it as part of their initial therapy?
DR. SHUSTOV: It certainly depends on the type of the academic center that patients are referred to, and we all practice at academic institutions with very strong ALLO programs. In patients who we select for a curative approach (one half to two thirds) we will at least make the best effort to take them to allogeneic grafting. Patients might fall off this track if they don't respond to salvage therapies or develop significant treatment/disease-related complications, ie fungal infections, organ toxicities, poor performance status, etc. Overall, I'd say that we successfully take about half of the patients with relapsed PTCL that are willing to pursue curative approach to allograft.
In addition, I consider autologous stem cell transplant in relapse setting for three situations. One is relapsed ALK-positive ALCL, some patients with ALK-negative ALCL who have had long initial remission and after relapse, and achieved second remission with BV or other salvage therapy. Finally, I’d consider auto-transplant in patients with AITL who achieved second CR with salvage therapy.
DR. HORWITZ: In terms of the relapse setting—some of you talked about angioimmunoblastic and HDAC inhibitors or BV for ALCL—when you have a relapse patient with PTCL-NOS, how do you pick a second line agent if you're not going directly to transplant, and are there new drugs that you're particularly excited about?
DR. FANALE: To answer your questions in terms of how I might choose a targeted agent for a patient who has PTCL-NOS and doesn't have AITL or ALCL, if the patient isn't eligible for trial or trial isn't feasible for that patient, I'll typically look at schedule, and then I'll look at side effect profile. For instance, for comparison, there are two HDAC inhibitor drugs right now approved, romidepsin and belinostat, so I think that potentially in a private practice setting, belinostat might be one when you look at the efficacy, being generally, around equivalent on where a patient might prefer that regimen because of the fact that they come in for five days in a row and then, their treatment is done for that cycle.
At a larger referral center to which patients are often traveling back and forth, it can be quite difficult for a patient to stay in town to do five days of treatment in a row. Often, these patients will prefer the romidepsin schedule because of the fact that they're coming back in once per week for three weeks in a row per cycle. The other way that I'll look at it is side effect profile, so, let's say, pralatrexate compared to the HDAC inhibitors, I will usually favor the HDAC inhibitors because of the potential side effect of mucositis with pralatrexate, although I have been able to manage through the mucositis by giving the patients more of a cutaneous T-cell lymphoma dosing format.
There had been a high level of interest in new emerging agents such as the aurora A-kinase inhibitors, alisertib, and that trial is now complete and the data are to be presented at The American Society of Hematology’s Annual Meeting this year.4 Other new therapies that are emerging are the PI3K inhibitors such as duvelisib and others. One advantage for many of the drugs in current trials is that they are oral agents.
My interest also lies in the combination approach, such as two or three targeted agent combinations, with the goal that if you see favorable CR rates and progression-free survival rates in the relapsed setting that you can then potentially move these treatments to the front-line setting to potentially move beyond the CHOP-based platform of therapies.
DR. SHUSTOV: For patients outside anaplastic lymphoma where, obviously, most of us would likely use BV, given the response rate to this agent, the decision is really based on convenience of the schedule, toxicity profile, and—sometimes—patient’s preference. So, toxicity and quality of life become kind of more decision-driving factors than disease biology. I’d have a discussion with patients about all three drugs, how they are administered, and what toxicities can be expected. Four-hour infusion of romidepsin might not be acceptable for some patients who have to travel long distances to treatment centers. They may prefer more rapid infusion (they would favor a trial of pralatrexate; or, as Alison mentioned, patient prefers a five-day/daily versus weekly administration.
It's kind of a coin toss decision outside clinical trials. Certainly, participation in studies in relapse setting is the high priority. We are all really looking forward to developing doublet combinations of novel agents, ie studies like the one Michelle is running at MD Anderson with a combination of alisertib and romidepsin.
DR. MOSKOWITZ: I'll echo what both Andrei and Michelle said that our choice of treatment is individualized and depends upon side-effect profile and the schedule. With regard to novel agents, I am also very excited about the studies that will be opening with the PI-3 kinase inhibitor, IPI-145. There have been promising results seen with single-agent IPI-1455 and we will be opening a study evaluating it in combination with bortezomib or romidepsin.
In addition, I mentioned earlier the IDH2 inhibitor, which we're studying right now in patients with AITL that is characterized by the presence of the IDH2 mutation. It would be exciting to see if targeting a specific mutation in this disease translates into responses.
DR. HORWITZ: Great, I think that was fantastic. I would like to thank Drs. Moskowitz, Shustov, and Fanale for a very thorough impractical discussion on how they approach and manage patients with T-cell lymphoma. I’m impressed that there is significant consistency among these experts in terms of how they manage these uncommon and often challenging lymphomas in terms of upfront combination chemotherapy approaches, combination considerations for the use of transplantation, as well as their enthusiasm for and dedication to incorporating clinical trials as part of everyday management.
References
1. Fanale MA, Horwitz SM, Forero-Torres A, et al. Brentuximab vedotin in the front-line treatment of patients with CD30+ peripheral T-cell lymphomas: results of a phase I study. J Clin Oncol. 2014;32(28):3137–3143.
2. Schmitz N, Trümper L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116(18):3418–3425. doi: 10.1182/blood-2010-02-270785. Epub 2010 Jul 21.
3. Vose J, Armitage J, Weisenburger D, for the International T-Cell Lymphoma Project. J Clin Oncol. 2008;26(25):4124–4130. doi: 10.1200/JCO.2008.16.4558. Epub 2008 Jul 14.
4. O’Connor OA, Ozcan M, Jacobsen ED, et al. First multicenter, randomized phase 3 study in patients with relapsed/refractory peripheral T-cell lymphoma: alisertib versus investigator’s choice. Paper to be presented at: American Society of Hematology 57th Annual Meeting & Exposition; December 5–8, 2015; Orlando, FL.
5. Horwitz SM, Porcu P, Flinn I, et al. Duvelisib (IPI-145), a phosphoinositide-3-kinsae-γδ inhibitor, shows activity in patients with relapsed/refractory T-cell lymphoma. Paper presented at: American Society of Hematology 56th Annual Meeting & Exposition; December 6–9, 2014; San Francisco, CA.
Moderator: Steven M. Horwitz, MD1
Discussants: Alison Moskowitz, MD1; Michelle Fanale, MD2; Andrei Shustov, MD3
From Memorial Sloan Kettering Cancer Center, New York, NY1; MD Anderson Cancer Center, Houston, TX2; University of Washington Medical Center, Seattle, WA3
Address for correspondence: Steven M. Horwitz, MD, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065
E-mail: [email protected]
DR. HORWITZ: My name is Dr. Steven Horwitz from Memorial Sloan-Kettering Cancer Center. I’m joined today by Drs. Alison Moskowitz, Memorial Sloan-Kettering, Michelle Fanale of MD Anderson, and Andrei Shustov, University of Washington in Seattle. Thank you all for joining us for this conversation on T-cell lymphoma. My colleagues are all well known as experts in T-cell lymphomas. Those of you who treat these diseases recognize the systemic T-cell lymphomas as one of our greater challenges in hematologic malignancies in terms of the treatment options for patients and the frequent lack of definitive data to guide our decisions.
I thought what we would do today is have a very practical discussion about the way we think about these diseases, the decisions we make, and the way we make those decisions. I'll start off by asking when you get a referral of a patient with a new diagnosis of peripheral T-cell lymphoma (PTCL), what are some of the basic things that you first think about in terms of approaching a new patient?
DR. SHUSTOV: I think one of the biggest challenges in T-cell lymphomas still remains making the proper diagnosis. In general, pathologists in the United States have a pretty good idea when they see T-cell lymphomas, however, subclassification remains a challenge even for expert hematopathologists due to frequent histologic overlap between the subtypes of PTCL, and even with non-malignant autoimmune disorders. I frequently see patients who are diagnosed with or misdiagnosed with a different subtype of T-cell lymphoma. The most challenging is differentiation between angioimmunoblastic T-cell lymphoma (AITL), anaplastic large cell T-cell lymphoma (ALCL), and PTCL not otherwise specified (PTCL-NOS), especially when the latter patients have high expression of CD30 and/or bear features resembling AITL.
Sometimes they are a slam-dunk diagnosis, but frequently our hematopathologists reverse the diagnosis after doing additional studies on the biopsy material. The most recent case I've seen in my clinic for consultation was a patient with a diagnosis of extranodal NK-cell lymphoma that was reclassified as a gamma-delta T-cell lymphoma after additional work-up. I truly believe that it is advisable that majority, if not all PTCL cases are reviewed by expert hematopathology teams at academic centers that see large volumes of these cases.
I think it's very important to educate community physicians and patients about a proper PTCL diagnosis, especially now that more targeted therapies are being developed and the gene expression profiling techniques will probably lead to identification of specific pathways that are amenable to therapy with specific biologic agents.
DR. FANALE: I'd like to expand upon what Andrei just said. For me the next step after confirming the pathology diagnosis is to think about two things. To think about whether or not this patient is a patient who might be eligible for an ongoing front-line trial, typically if the patient meets eligibility criteria for one of our ongoing front-line trials I would really recommend to the patient to consider being enrolled in that trial, and I also think about whether the patient, if he or she enters into remission with front-line therapy, can be considered for a consolidative autologous stem cell transplant.
I think it's very important to educate community physicians and patients about a proper PTCL diagnosis. Right now, our ongoing front-line trial is the ECHELON-2 trial which is evaluating brentuximab vedotin (BV) plus cyclophosphamide, doxorubicin, prednisone (CHP [BV-CHP]) chemotherapy compared to standard of care cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) chemotherapy, and that trial is based on the promising data that we've seen in the initial phase I trial that combined BV plus CHP chemotherapy followed by maintenance BV which demonstrated both high and durable complete remission (CR) rates.1 BV is an antibody drug conjugate that's targeted at CD30 and carries initial US Food and Drug Administration approval for patients who have relapsed or refractory ALCL which has a 100% level of CD30 positivity and then also has a National Comprehensive Cancer Network listing for treating other types of relapsed or refractory CD30 positive PTCL as well.
Also there is another upcoming front-line trial, which is to combine pralatrexate plus CHOP. If a patient isn't eligible for a clinical trial or it's just not feasible for that patient to enroll in a clinical trial, I will then look further at what would be potentially the best standard of care option for that patient.
I'll look at the patient's age and performance status and if they are generally less than age 65 or so and if otherwise well, I'll preferentially treat that patient with CHOEP which is CHOP plus etoposide. And then, for a patient who's either older than this or has multiple other comorbidities, I would treat that patient typically with CHOP alone.
DR. HORWITZ: Thank you, we'll go further into the selection of initial therapies but first circle back, I was curious as to how often your center comes to a different diagnosis than the referring center, and are there pitfalls you see that alert you to be suspicious of diagnosis.
DR. SHUSTOV: I'd say probably 10% of cases that we see at our center will be reclassified by our hematopathologists. In most cases, they do not necessarily reverse the diagnosis, but provide further clarification. It is occurring to me that in the community, pathologists are less likely to call the subtype of T-cell lymphoma and limit the report to the general description of T-cell lymphoprolipherative disorder, or state something like “consistent with T-cell lymphoma with features of AITL, or with features of anaplastic lymphoma.” I would admit though, that sometimes it's very difficult to identify the specific subtype of PTCL even in the expert hands; but I'd say these cases would constitute no more than 5% of PTCL patients.
DR. HORWITZ: And in your experience is it mostly fine tuning a T-cell lymphoma diagnosis, or do you see totally different diagnoses?
DR. MOSKOWITZ: Usually review by expert hematopathologists simply leads to fine-tuning the T-cell lymphoma diagnosis, however, I occasionally see significant changes in diagnosis. Often, alterations or clarification of a diagnosis are made possible only after we provide the pathologist with clinical history. For example, a lymph node biopsy may be interpreted as ALCL, however the knowledge that the particular patient has a history of mycosis fungoides would lead the pathologist to consider the diagnosis of large cell transformation of mycosis fungoides rather than ALCL. In such a case, molecular studies are helpful in confirming that the lymph node findings originate from the same clone as that in the original mycosis fungoides lesions, rather than representing a second primary.
DR. FANALE: Very occasionally, I've seen patients truly “with a misdiagnosis and a complete revision of diagnosis” and, usually, those pitfalls that I've seen them occasionally have been the young patients—the young patients who generally have disease in the thorax and neck, who are treated as though they have classical Hodgkin lymphoma, who have very significant progression of disease on standard of care treatment. So it's important that not all cases that have CD30 positivity are classical Hodgkin lymphoma even if the patient is young.
DR. HORWITZ: I often think the systemic T-cell lymphomas usually behave in an aggressive fashion so when the clinical picture and the diagnosis don't really fit, I think about getting a second biopsy before we finalize the diagnosis. Of course, when people are ill with obviously progressing disease, you may need to move more quickly.
I often think the systemic T-cell lymphomas usually behave in an aggressive fashion so when the clinical picture and the diagnosis don't really fit, I think about getting a second biopsy before we finalize the diagnosis. To move on, when you first see a patient, what is the decision tree in your mind in terms of picking therapy or planning therapy, or what kind of things do you consider?
DR. MOSKOWITZ: The first thing I think about when deciding upon treatment for a patient with T-cell lymphoma is whether or not I plan to use a curative approach to therapy. As was mentioned by Michelle, this decision is partly based upon the patients’ comorbidities and age. For patients who are eligible for curative therapy, our frontline approach for the most common types of T-cell lymphoma is to use CHOP with or without etoposide followed by consideration for autologous stem cell transplant in first remission. There are certainly individuals for whom such an aggressive strategy would not be appropriate due to age or co-morbidities. In these individuals, we may consider CHOP-based therapy alone or sometimes even milder approaches aimed at disease control.
DR. HORWITZ: Andrei, are you similar in the CHOP/CHOEP paradigm? And if so, what do you think of those data? How do you decide between the two and when do you do that regimen versus something different?
DR. SHUSTOV: I think I will double or triple what Michelle and Alison just said; for me, the two most important decisions that I have to make in the first encounter with patients with newly diagnosed PTCL are: 1) whether we're going to pursue curative approach strategy; and 2) whether the patient can tolerate the intensity of treatments that would provide him/her with the best chance of cure or long term remission. Patients who are elderly and have high risk disease would be very hard to cure, especially considering that the consolidative transplant might carry high rates of morbidity or mortality; more conservative strategies might be appropriate in these cases. On the other hand, younger and more fit patients might benefit more from intensified initial regimens—ie, CHOEP—followed by high-dose therapy and either autologous or allogeneic stem cell transplant (ALLO).
I usually have a long initial discussion with patients and families during which we decide on the intent of treatment and what to expect from certain regimens in terms of toxicities. I typically choose CHOEP regimen (or infusional version, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin [EPOCH]) for younger patients based on recent German data, even though this was a retrospective study and benefit of adding etoposide only approached statistical significance for the majority of PTCL subtypes.2 In older patients, I try to avoid anthracyclines, especially in the palliative intent setting, based on the retrospective analysis by the International T-Cell Lymphoma Project, and frequently use CEOP regimen (CHOEP minus anthracycline).3 I find that the majority of older patients tolerate this treatment somewhat better than anthracycline-containing combinations like CHOP.
In the very elderly and frail patients, I try to avoid combination chemotherapy all together. It is a somewhat easier decision for patients with AITL. Some of them are more indolent than other sub-types, and I would treat such patients with immunomodulatory approach, ie with a combination of prednisone and cyclosporine; then, I would consider single agent therapy with one of the recently approved agents for relapsed and refractory PTCL. I would also double what Michelle said in regard to making the best attempt to enroll patients into open clinical trials, because the current standards are not really satisfactory for many T-cell lymphoma patients.
DR. HORWITZ: It sounds like we all approach a new patient similarly. However, several of our up front trials are randomized against CHOP as opposed to CHOEP. When you're consenting a patient to those trials, how do you explain it to a patient or how important do you think the etoposide is?
DR. SHUSTOV: I share your frustration with quite a few of the current study designs for that exact reason. Some of these are confirmatory trials after conditional approvals of the new agents and are important. However, as often happens, in the confirmatory trials, we use controls that are a default from a lot of historical data, or the “common” way that the majority of patients are being treated. It is really a challenge when you consent patients to studies where a control arm is something that you think might not be quite adequate.
Having said that, the CHOEP study that was mentioned several times is a retrospective subgroup analysis and the addition of etoposide had marginal benefit that approached significance, but certainly was not a home run. I don't think we are ready to say that etoposide provides survival advantage in T-cell lymphoma patients and dismiss clinical trials in favor of just giving a patient the CHOEP, even though CHOP is admittedly not the best comparator. I discuss this controversy with patients and tell them frankly that the data that we base addition of etoposide on are not the best evidence one may have. Then, I ask patients to decide which approach sounds more reasonable to them and they make their choice.
DR. FANALE: To expand just slightly upon what Andrei said, I do emphasize to the patient and to the referring doctors that if you look at National Comprehensive Cancer Network guidelines, CHOP, CHOEP, EPOCH, all of these are potential options. So there's really not yet one trial that would say that one is clearly superior to the other at this time. I also emphasize to the patient and to the referring clinician that the only way, let's say, the patient could get the targeted agent plus the chemotherapy and have that 50% chance of, potentially, receiving that combination is really through the randomized clinical trial.
DR. HORWITZ: That was excellent, thank you. So you have alluded to this a bit. How much does subtype specific treatment come into play?
DR. MOSKOWITZ: At this point in time, outside of a clinical trial, for PTCL-NOS, AITL, and ALCL, the front-line approach would typically be CHOEP followed by autologous stem cell transplant. As Michelle mentioned, for a patient with ALCL, I would offer enrollment on the ECHELON-2 study in which patients are randomized to CHOP or BV-CHP, in order to give patients the option of potentially receiving BV.
For AITL, we are now obtaining molecular testing for certain mutations, particularly IDH2, because we currently have a study open with an IDH2 inhibitor that specifically enrolls patients with IDH2-mutant AITL. Because the testing takes some time to get back, we typically test patients’ biopsies at the time of diagnosis, so that we know if they're eligible for future clinical trials in case their disease does not respond to upfront therapy.
There are T-cell lymphoma subtypes that we treat quite differently from the entities discussed so far. For human T-lymphotropic virus-1 associated adult T-cell lymphoma, for example, the data with CHOP are really not good and we typically used EPOCH with the aim to consolidate with an allogeneic stem cell transplant.
Likewise, for hepatosplenic T-cell lymphoma, we have not observed good responses with the CHOP-based therapy and therefore we typically use platinum-based or ifosfamide-based therapy upfront with the aim to consolidate with allogeneic stem cell transplant. Extranodal NK/T-cell lymphoma is another entity for which CHOP is typically not effective and we have adopted asparaginase-based regimens, such as dexamethasone, methotrexate, ifosfamide, L-asparaginase, etoposide (SMILE) for this disease.
DR. SHUSTOV: I completely agree with Alison; for more common nodal lymphomas it is really hard at this point to base a treatment decision on histology, at least, in the front-line setting. However, some of the rare and unique subtypes, we generally treat with a completely different approach (ie, extranodal NK-cell lymphomas, enteropathy-associated T-cell lymphomas, adult T-cell leukemia/lymphomas).
DR. HORWITZ: Excellent. I’m curious, in a patient with ALCL who refused randomization of the ECHELON-2 trial, would you give them BV-CHP off study or would you discuss that as an alternative were they not to participate in the trial?
DR. FANALE: No. I don't urge treatment that's not approved for a particular line of therapy off clinical protocol with the exception that, let's say, if a patient is not a candidate for chemotherapy in second line setting for a particular lymphoma such as Hodgkin lymphoma—then, I would reference published data like Alison's data for a second line use of BV say for classical Hodgkin lymphoma and work with the insurance company to get the drug approved for that patient population, but if a patient just doesn't want to go on trial because they don't like the 50/50 chance of receiving BV-CHP compared to CHOP and they say, “I really want the 100% chance. Why don't you just contact my insurance?” I will explain to them the rationale of why the trial is being conducted including the hope that if the endpoints are met and the regimen is approved for front-line therapy then patients in the future can get BV-CHP at any oncology clinic, but I tell them for now it is a protocol based treatment option.
DR. HORWITZ: That's a very good answer. Can we now touch on the idea of transplant vs no transplant in first remission. Always, sometimes, never; how do you all think about that?
DR. SHUSTOV: I think that the idea of consolidative transplant is very heavily debated and discussed at the majority of specialized PTCL meetings. The reason for that is controversial nature of current clinical evidence. In the absence of randomized trials it is very hard to compare prospective phase II trial data to historical controls. You can interpret this in two ways; you might say, well, these are good data, outcome numbers look better than historical controls, and all patients with PTCL should have a consolidative transplant; or you can say, well, I don't have randomized data, and only a randomized study would really tell us whether post-induction consolidative transplant improves survival.
That is why it is such a controversial subject, and we agree that the phase II perspective studies of transplantation are hampered by significant bias; patients who are able and willing to travel to academic centers, were more robust and able to tolerate high dose therapy. They also have chemosensitive disease and that puts them in a completely different category than those you see in populational or retrospective studies, or other historical control trials.
Having said that, when I talk to patients, and I think that this is where I involve patients into treatment decision very heavily, I present them with the data and say that it is not wrong to do or not do the transplant in first CR. I make patients and family a big part of the decision. When they ask me what would give the patient the best chance being in remission five years from today, my answer is transplant, however, the data are not perfect, and the curative potential of autologous transplant in PTCL is not known.
DR. FANALE: So typically here, we would refer most patients on for front-line consolidative autologous stem cell transplant, I think, some exceptions are the ones that Andrei already touched on. If we're seeing a patient who has PTCL-NOS and this patient is the very rare patient who has early stage disease, with really no significant risk factors, that patient, unless there were pathologic features that showed a higher level of aggressiveness as Alison commented on, this patient might be one where we might defer doing the consolidative stem cell transplant for particularly if the patient’s disease entered into remission very quickly, but this still is controversial.
I'm not sure how you practice within each of your centers, but another patient, or where we would potentially defer, is a patient with fairly limited nasal NK/T-cell lymphoma, a patient that we’ve treated, let's say, with the dexamethasone, etoposide, ifosfamide, carboplatin (DeVIC) chemotherapy regimen plus a concomitant high-dose radiation, we typically historically here have deferred doing consolidative stem cell transplant for that patient population.
In terms of what Andrei commented on for when we might consider an ALLO and for a frontline consolidation, here we typically would not. The exception to that rule is that I've had a couple of patients here who have PTCL with a secondary hemophagocytic lymphohistiocytosis syndrome and then, for those patients because the concern is that the autologous stem cell transplant probably wouldn't be enough and for those patients given that they have such a dismal prognosis from the secondary hemophagocytic lymphohistiocytosis, we send those patients on for an ALLO.
DR. MOSKOWITZ: To expand upon early stage extranodal NK/T-cell lymphoma, I agree with Michelle that we wouldn't use an autologous stem cell transplant in first remission. Typically, for patients with localized disease, we recommend 2 cycles of SMILE followed by involved field radiation. Our patients treated with this approach have typically done quite well without needing a transplant.
DR. HORWITZ: That was great. If there are no further comments on upfront therapy, we can move over to relapse setting. When you see a patient at relapse, what are your strategies and how do you approach that patient?
DR. MOSKOWITZ: The first question in my mind when a patient is either not responding to or relapsing following front-line therapy is: what is my ultimate goal? For a patient who is fit and refractory to CHOP or has relapsed after CHOP or autologous stem cell transplant, my ultimate goal would be to try to get them to an ALLO. My choice of treatment following front-line therapy depends upon whether or not we are aiming for an ALLO as well as whether a donor has been identified for the patient.
Usually, if a patient just relapsed following front-line therapy, we are not necessarily ready to proceed quickly to ALLO. This may be because a donor has not yet been identified or because the patients’ eligibility for an ALLO is not clear. In this situation, my choice of treatment would be something that can be continued, potentially for several months, while we get things in place for the transplant. Treatment options in this situation would include the approved single agents for T-cell lymphoma, such as romidepsin, belinostat, pralatrexate, as well as BV (which would be specific for ALCL). In addition, I would consider enrollment on a promising clinical trial. Among these options, my choice of treatment typically depends upon the side effect profile and/or schedule, which is individualized for the patient. The one caveat would be that I typically will aim to use a histone deacetylase inhibitor (HDAC) inhibitor as my first choice for a patient with AITL.
Usually, if a patient just relapsed following front-line therapy, we are not necessarily ready to proceed quickly to ALLO. This may be because a donor has not yet been identified or because the patients’ eligibility for an ALLO is not clear. If my patient has a donor available and we are ready to move quickly to transplant, I would use one of the multi-agent regimens, such as ifosfamide, carboplatin, etoposide (ICE) or cisplatin, cytosine arabinoside, dexamethasone (DHAP), with the aim to try to get them into remission and relatively quickly proceed to transplant. The problem with using one of these types of regimens for everyone in the second-line setting is that the treatment cannot be continued indefinitely due to cumulative toxicity; therefore we need to know that we are ready to proceed to transplant if we are going to use one of the more intense regimens.
DR. HORWITZ: How do the others approach relapse?
DR. FANALE: Generally, a lot of similarities with what Alison discussed. Typically, it's if we're going to be sending the patient on for consideration for ALLO, I think the favor would be to try to use a regimen that has a high CR rate knowing that there is a trend toward improved progression free survival for the patient who enters into CR when compared to those who did not. I think the only slight difference might be just the timing and the selection, so, typically, here, even if the patient doesn't actually have a definite established donor quite yet we'll typically favor a combination type of a treatment approach, so whether or not that would be a platinum-based regimen such as ICE, as Alison mentioned, or gemcitabine-based chemotherapy regimen or similar.
I would generally favor one of those options, usually, over a single-agent therapeutic approach just knowing that the CR rates, generally, with exception of BV for ALCL are, generally, ranging about the 10%–15% range. And then, of course, first priority would be if there is a clinical trial available whether that's with a doublet of targeted agents or with the targeted agent plus chemotherapy in the relapse setting, we would typically favor that approach for a patient being considered for a stem cell transplant, ALLO approach.
DR. SHUSTOV: I'll consolidate what Michelle and Alison just said. I think we're on the same page. To me, the most important decision (even more important in the relapse setting) is to figure out whether we are still going to try to cure the lymphoma or we will pursue a palliative approach for which now we have several treatment options. So again, I’d have a long discussion with the patient, whether we're going to try and cure the disease, or we will pursue a palliative approach.
If this is a curative approach strategy and we're heading toward ALLO, we would start searching for the donor immediately. The choice of salvage therapy depends on duration of first remission. Primary refractory disease patients who failed CHOP, CHOEP, or EPOCH outright or within 3 months, I typically consider using the single agent approach, because even ICE and DHAP type regimens don’t work well for these patients. I put my “money” into HDAC inhibitors or antifolates, or other targeted therapy, if this is anaplastic lymphoma.
For patients who had at least 6 months remission from the initial therapy, younger patients who are still fit, I agree, I would consider standard salvage regimens like DHAP, etoposide, methylprednisolone, high-dosecytarabine, cisplatin (ESHAP) and ICE, and then, follow this with allograft.
DR. HORWITZ: You have all talked about allografting, I have two questions. What is your best guess in terms of percentage of patients you see with relapsed T-cell lymphoma who actually undergo ALLO at some point and do you ever consider autologous transplantation in those who didn't have it as part of their initial therapy?
DR. SHUSTOV: It certainly depends on the type of the academic center that patients are referred to, and we all practice at academic institutions with very strong ALLO programs. In patients who we select for a curative approach (one half to two thirds) we will at least make the best effort to take them to allogeneic grafting. Patients might fall off this track if they don't respond to salvage therapies or develop significant treatment/disease-related complications, ie fungal infections, organ toxicities, poor performance status, etc. Overall, I'd say that we successfully take about half of the patients with relapsed PTCL that are willing to pursue curative approach to allograft.
In addition, I consider autologous stem cell transplant in relapse setting for three situations. One is relapsed ALK-positive ALCL, some patients with ALK-negative ALCL who have had long initial remission and after relapse, and achieved second remission with BV or other salvage therapy. Finally, I’d consider auto-transplant in patients with AITL who achieved second CR with salvage therapy.
DR. HORWITZ: In terms of the relapse setting—some of you talked about angioimmunoblastic and HDAC inhibitors or BV for ALCL—when you have a relapse patient with PTCL-NOS, how do you pick a second line agent if you're not going directly to transplant, and are there new drugs that you're particularly excited about?
DR. FANALE: To answer your questions in terms of how I might choose a targeted agent for a patient who has PTCL-NOS and doesn't have AITL or ALCL, if the patient isn't eligible for trial or trial isn't feasible for that patient, I'll typically look at schedule, and then I'll look at side effect profile. For instance, for comparison, there are two HDAC inhibitor drugs right now approved, romidepsin and belinostat, so I think that potentially in a private practice setting, belinostat might be one when you look at the efficacy, being generally, around equivalent on where a patient might prefer that regimen because of the fact that they come in for five days in a row and then, their treatment is done for that cycle.
At a larger referral center to which patients are often traveling back and forth, it can be quite difficult for a patient to stay in town to do five days of treatment in a row. Often, these patients will prefer the romidepsin schedule because of the fact that they're coming back in once per week for three weeks in a row per cycle. The other way that I'll look at it is side effect profile, so, let's say, pralatrexate compared to the HDAC inhibitors, I will usually favor the HDAC inhibitors because of the potential side effect of mucositis with pralatrexate, although I have been able to manage through the mucositis by giving the patients more of a cutaneous T-cell lymphoma dosing format.
There had been a high level of interest in new emerging agents such as the aurora A-kinase inhibitors, alisertib, and that trial is now complete and the data are to be presented at The American Society of Hematology’s Annual Meeting this year.4 Other new therapies that are emerging are the PI3K inhibitors such as duvelisib and others. One advantage for many of the drugs in current trials is that they are oral agents.
My interest also lies in the combination approach, such as two or three targeted agent combinations, with the goal that if you see favorable CR rates and progression-free survival rates in the relapsed setting that you can then potentially move these treatments to the front-line setting to potentially move beyond the CHOP-based platform of therapies.
DR. SHUSTOV: For patients outside anaplastic lymphoma where, obviously, most of us would likely use BV, given the response rate to this agent, the decision is really based on convenience of the schedule, toxicity profile, and—sometimes—patient’s preference. So, toxicity and quality of life become kind of more decision-driving factors than disease biology. I’d have a discussion with patients about all three drugs, how they are administered, and what toxicities can be expected. Four-hour infusion of romidepsin might not be acceptable for some patients who have to travel long distances to treatment centers. They may prefer more rapid infusion (they would favor a trial of pralatrexate; or, as Alison mentioned, patient prefers a five-day/daily versus weekly administration.
It's kind of a coin toss decision outside clinical trials. Certainly, participation in studies in relapse setting is the high priority. We are all really looking forward to developing doublet combinations of novel agents, ie studies like the one Michelle is running at MD Anderson with a combination of alisertib and romidepsin.
DR. MOSKOWITZ: I'll echo what both Andrei and Michelle said that our choice of treatment is individualized and depends upon side-effect profile and the schedule. With regard to novel agents, I am also very excited about the studies that will be opening with the PI-3 kinase inhibitor, IPI-145. There have been promising results seen with single-agent IPI-1455 and we will be opening a study evaluating it in combination with bortezomib or romidepsin.
In addition, I mentioned earlier the IDH2 inhibitor, which we're studying right now in patients with AITL that is characterized by the presence of the IDH2 mutation. It would be exciting to see if targeting a specific mutation in this disease translates into responses.
DR. HORWITZ: Great, I think that was fantastic. I would like to thank Drs. Moskowitz, Shustov, and Fanale for a very thorough impractical discussion on how they approach and manage patients with T-cell lymphoma. I’m impressed that there is significant consistency among these experts in terms of how they manage these uncommon and often challenging lymphomas in terms of upfront combination chemotherapy approaches, combination considerations for the use of transplantation, as well as their enthusiasm for and dedication to incorporating clinical trials as part of everyday management.
References
1. Fanale MA, Horwitz SM, Forero-Torres A, et al. Brentuximab vedotin in the front-line treatment of patients with CD30+ peripheral T-cell lymphomas: results of a phase I study. J Clin Oncol. 2014;32(28):3137–3143.
2. Schmitz N, Trümper L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116(18):3418–3425. doi: 10.1182/blood-2010-02-270785. Epub 2010 Jul 21.
3. Vose J, Armitage J, Weisenburger D, for the International T-Cell Lymphoma Project. J Clin Oncol. 2008;26(25):4124–4130. doi: 10.1200/JCO.2008.16.4558. Epub 2008 Jul 14.
4. O’Connor OA, Ozcan M, Jacobsen ED, et al. First multicenter, randomized phase 3 study in patients with relapsed/refractory peripheral T-cell lymphoma: alisertib versus investigator’s choice. Paper to be presented at: American Society of Hematology 57th Annual Meeting & Exposition; December 5–8, 2015; Orlando, FL.
5. Horwitz SM, Porcu P, Flinn I, et al. Duvelisib (IPI-145), a phosphoinositide-3-kinsae-γδ inhibitor, shows activity in patients with relapsed/refractory T-cell lymphoma. Paper presented at: American Society of Hematology 56th Annual Meeting & Exposition; December 6–9, 2014; San Francisco, CA.
67% of teens have substantial cardiometabolic risk burden, blood donor survey shows
ORLANDO – Fully two-thirds of nearly 25,000 Dallas-area volunteer blood donors ages 16-19 had elevated or borderline total cholesterol, blood pressure, and/or hemoglobin A1c, Dr. Merlyn H. Sayers reported at the American Heart Association scientific sessions.
“It is startling that such a significant percentage of these young, ostensibly healthy volunteers have abnormal cardiometabolic health metrics,” observed Dr. Sayers, president and chief executive officer of Carter BloodCare of Bedford, Tex., a nonprofit organization that is the largest blood bank in the state.
After all, he noted, longitudinal studies have clearly shown that cardiometabolic risk factors present in adolescence will persist into adulthood and are associated with increased risks of cardiovascular disease and diabetes. Moreover, it’s troubling, albeit not really surprising, that for the most part these adolescents don’t seem to care about their cardiometabolic risk, the hematologist-oncologist added.
“We give all these youngsters an opportunity to go to the Carter BloodCare website and confidentially retrieve their values. But despite all manner of urging on our part that these results are important, at best only about 20% of the individuals actually do so, and that rate varies substantially by race and ethnicity,” according to Dr. Sayers. “Where appropriate, we need to find ways to impose behavior modification on a group that is relatively resistant to guidance and intervention. Even the best kids, as teenagers, really don’t take this sort of advice about their health risk very seriously. They regard themselves as immortal during their teenage years.”
Noting that behavioral change is not a core strength among transfusion medicine specialists, Dr. Sayers appealed to his audience of cardiologists for suggestions as to how to encourage lifestyle modification in this youthful group without browbeating them to the point that they’re driven off from becoming serial blood donors.
It’s not widely appreciated that across the U.S. during the school year, 20% of all unpaid blood donors are high school students. These high school blood drives provide an as-yet untapped opportunity to screen adolescents for cardiometabolic risk at low cost and minimal inconvenience to participants, said Dr. Sayers of the University of Texas, Dallas.
“We need allies to help us to ensure we get the kids’ attention better,” he explained. “I want to leave you with the sense that perhaps you will see these blood drives as an opportunity to find interventions that might address primordial prevention of cardiometabolic risk.”
He presented a study of 24,925 youths aged 16-19 who donated blood to Carter BloodCare during 2011-2012. Since blood is drawn for obligatory infectious diseases screening at each donation, Dr. Sayers and coinvestigators were able to measure nonfasting total cholesterol and HbA1c in every teen donor. Blood pressure is also measured at every donation.
The investigators used widely accepted definitions of elevated blood pressure, cholesterol, and HbA1c: namely, at least 140/80 mm Hg, 200 mg/dL, and 6.5%, respectively.
While the percentage of teen blood donors with borderline or elevated levels of all three cardiometabolic risk factors was in the low single figures, 21% of boys and 15% of girls were positive for two out of the three.
The prevalence of cardiometabolic risk factors varied by ethnicity. Sixteen percent of white adolescents had elevated or borderline levels of two risk factors. So did 24% of African Americans, 22% of Asian Americans, and 18% of Hispanics.
“These are really staggering results,” commented session chair Dr. Seth S. Martin of Johns Hopkins University, Baltimore. “This is a call to action now that you’ve identified all these kids who are on a trajectory that doesn’t look good.”
As to how physicians can help to favorably alter that trajectory, however, audience members admitted to being stumped, especially since many young people stop going to a primary care physician for preventive care during their teenage years.
“The big problem here is how to use this information to initiate lifestyle change,” observed Dr. Lewis H. Kuller, professor and past chair of epidemiology at the University of Pittsburgh.
Dr. Sayers reported having no financial conflicts regarding his study.
ORLANDO – Fully two-thirds of nearly 25,000 Dallas-area volunteer blood donors ages 16-19 had elevated or borderline total cholesterol, blood pressure, and/or hemoglobin A1c, Dr. Merlyn H. Sayers reported at the American Heart Association scientific sessions.
“It is startling that such a significant percentage of these young, ostensibly healthy volunteers have abnormal cardiometabolic health metrics,” observed Dr. Sayers, president and chief executive officer of Carter BloodCare of Bedford, Tex., a nonprofit organization that is the largest blood bank in the state.
After all, he noted, longitudinal studies have clearly shown that cardiometabolic risk factors present in adolescence will persist into adulthood and are associated with increased risks of cardiovascular disease and diabetes. Moreover, it’s troubling, albeit not really surprising, that for the most part these adolescents don’t seem to care about their cardiometabolic risk, the hematologist-oncologist added.
“We give all these youngsters an opportunity to go to the Carter BloodCare website and confidentially retrieve their values. But despite all manner of urging on our part that these results are important, at best only about 20% of the individuals actually do so, and that rate varies substantially by race and ethnicity,” according to Dr. Sayers. “Where appropriate, we need to find ways to impose behavior modification on a group that is relatively resistant to guidance and intervention. Even the best kids, as teenagers, really don’t take this sort of advice about their health risk very seriously. They regard themselves as immortal during their teenage years.”
Noting that behavioral change is not a core strength among transfusion medicine specialists, Dr. Sayers appealed to his audience of cardiologists for suggestions as to how to encourage lifestyle modification in this youthful group without browbeating them to the point that they’re driven off from becoming serial blood donors.
It’s not widely appreciated that across the U.S. during the school year, 20% of all unpaid blood donors are high school students. These high school blood drives provide an as-yet untapped opportunity to screen adolescents for cardiometabolic risk at low cost and minimal inconvenience to participants, said Dr. Sayers of the University of Texas, Dallas.
“We need allies to help us to ensure we get the kids’ attention better,” he explained. “I want to leave you with the sense that perhaps you will see these blood drives as an opportunity to find interventions that might address primordial prevention of cardiometabolic risk.”
He presented a study of 24,925 youths aged 16-19 who donated blood to Carter BloodCare during 2011-2012. Since blood is drawn for obligatory infectious diseases screening at each donation, Dr. Sayers and coinvestigators were able to measure nonfasting total cholesterol and HbA1c in every teen donor. Blood pressure is also measured at every donation.
The investigators used widely accepted definitions of elevated blood pressure, cholesterol, and HbA1c: namely, at least 140/80 mm Hg, 200 mg/dL, and 6.5%, respectively.
While the percentage of teen blood donors with borderline or elevated levels of all three cardiometabolic risk factors was in the low single figures, 21% of boys and 15% of girls were positive for two out of the three.
The prevalence of cardiometabolic risk factors varied by ethnicity. Sixteen percent of white adolescents had elevated or borderline levels of two risk factors. So did 24% of African Americans, 22% of Asian Americans, and 18% of Hispanics.
“These are really staggering results,” commented session chair Dr. Seth S. Martin of Johns Hopkins University, Baltimore. “This is a call to action now that you’ve identified all these kids who are on a trajectory that doesn’t look good.”
As to how physicians can help to favorably alter that trajectory, however, audience members admitted to being stumped, especially since many young people stop going to a primary care physician for preventive care during their teenage years.
“The big problem here is how to use this information to initiate lifestyle change,” observed Dr. Lewis H. Kuller, professor and past chair of epidemiology at the University of Pittsburgh.
Dr. Sayers reported having no financial conflicts regarding his study.
ORLANDO – Fully two-thirds of nearly 25,000 Dallas-area volunteer blood donors ages 16-19 had elevated or borderline total cholesterol, blood pressure, and/or hemoglobin A1c, Dr. Merlyn H. Sayers reported at the American Heart Association scientific sessions.
“It is startling that such a significant percentage of these young, ostensibly healthy volunteers have abnormal cardiometabolic health metrics,” observed Dr. Sayers, president and chief executive officer of Carter BloodCare of Bedford, Tex., a nonprofit organization that is the largest blood bank in the state.
After all, he noted, longitudinal studies have clearly shown that cardiometabolic risk factors present in adolescence will persist into adulthood and are associated with increased risks of cardiovascular disease and diabetes. Moreover, it’s troubling, albeit not really surprising, that for the most part these adolescents don’t seem to care about their cardiometabolic risk, the hematologist-oncologist added.
“We give all these youngsters an opportunity to go to the Carter BloodCare website and confidentially retrieve their values. But despite all manner of urging on our part that these results are important, at best only about 20% of the individuals actually do so, and that rate varies substantially by race and ethnicity,” according to Dr. Sayers. “Where appropriate, we need to find ways to impose behavior modification on a group that is relatively resistant to guidance and intervention. Even the best kids, as teenagers, really don’t take this sort of advice about their health risk very seriously. They regard themselves as immortal during their teenage years.”
Noting that behavioral change is not a core strength among transfusion medicine specialists, Dr. Sayers appealed to his audience of cardiologists for suggestions as to how to encourage lifestyle modification in this youthful group without browbeating them to the point that they’re driven off from becoming serial blood donors.
It’s not widely appreciated that across the U.S. during the school year, 20% of all unpaid blood donors are high school students. These high school blood drives provide an as-yet untapped opportunity to screen adolescents for cardiometabolic risk at low cost and minimal inconvenience to participants, said Dr. Sayers of the University of Texas, Dallas.
“We need allies to help us to ensure we get the kids’ attention better,” he explained. “I want to leave you with the sense that perhaps you will see these blood drives as an opportunity to find interventions that might address primordial prevention of cardiometabolic risk.”
He presented a study of 24,925 youths aged 16-19 who donated blood to Carter BloodCare during 2011-2012. Since blood is drawn for obligatory infectious diseases screening at each donation, Dr. Sayers and coinvestigators were able to measure nonfasting total cholesterol and HbA1c in every teen donor. Blood pressure is also measured at every donation.
The investigators used widely accepted definitions of elevated blood pressure, cholesterol, and HbA1c: namely, at least 140/80 mm Hg, 200 mg/dL, and 6.5%, respectively.
While the percentage of teen blood donors with borderline or elevated levels of all three cardiometabolic risk factors was in the low single figures, 21% of boys and 15% of girls were positive for two out of the three.
The prevalence of cardiometabolic risk factors varied by ethnicity. Sixteen percent of white adolescents had elevated or borderline levels of two risk factors. So did 24% of African Americans, 22% of Asian Americans, and 18% of Hispanics.
“These are really staggering results,” commented session chair Dr. Seth S. Martin of Johns Hopkins University, Baltimore. “This is a call to action now that you’ve identified all these kids who are on a trajectory that doesn’t look good.”
As to how physicians can help to favorably alter that trajectory, however, audience members admitted to being stumped, especially since many young people stop going to a primary care physician for preventive care during their teenage years.
“The big problem here is how to use this information to initiate lifestyle change,” observed Dr. Lewis H. Kuller, professor and past chair of epidemiology at the University of Pittsburgh.
Dr. Sayers reported having no financial conflicts regarding his study.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Two-thirds of 16- to 19-year-olds have borderline or frank hypertension, hypercholesterolemia, and/or high blood glucose.
Major finding: Of a very large group of 16- to 19-year-old blood donors, 67% had borderline or elevated total cholesterol, blood pressure, and/or hemoglobin A1c levels.
Data source: A retrospective analysis of total cholesterol, blood pressure, and HbA1c levels in 24,925 Dallas-area blood donors aged 16-19.
Disclosures: The presenter reported having no financial conflicts of interest regarding this study.
Cognitive impairment varies according to mood disorder
The nature and severity of cognitive alterations can vary significantly between different mood disorders, according to Dr. Charles Cotrena and his associates.
The study of 205 Brazilians comprised patients with major depressive disorder (MDD), patients with bipolar disorder I (BDI) and II (BDII), and a healthy control group, all of whom took a battery of neurocognitive tests. MDD patients performed poorly in tests involving attention and timed tasks, compared with the control group, but had less motor inhibition than did patients with BDI. Patients with BDI tended to perform worse across all executive functions, compared with patients with MDD, BDII, and the control group; however, BDII patients were the only ones who performed worse on the Iowa Gambling Task than did the control group, and performed worse on the Stroop Color–Word Test than did BDI patients.
While MDD patients had worse psychological quality of life (QOL) than that of controls, there was no difference in other QOL measures. MDD patients reported better physical health and lower disability rates than did BD patients. BDII patients had worse QOL than did control patients, but had lower disability rates than did BDI patients.
The investigators found that differences in cognitive function and quality of life still existed in patients with mood disorder, even after adjustment for mania and depressive symptoms.
“The importance of a detailed assessment of [executive function] and disability levels within each of these diagnostic categories, while controlling for demographic variables, was especially evident from the current results. Additionally, the comparison of impairment rates between groups – which is not a usual measure in the literature – provided important contributions to current knowledge regarding cognition and mood disorders,” the investigators noted.
Find the study in the Journal of Affective Disorders (doi: 10.1016/j.jad.2015.11.007).
The nature and severity of cognitive alterations can vary significantly between different mood disorders, according to Dr. Charles Cotrena and his associates.
The study of 205 Brazilians comprised patients with major depressive disorder (MDD), patients with bipolar disorder I (BDI) and II (BDII), and a healthy control group, all of whom took a battery of neurocognitive tests. MDD patients performed poorly in tests involving attention and timed tasks, compared with the control group, but had less motor inhibition than did patients with BDI. Patients with BDI tended to perform worse across all executive functions, compared with patients with MDD, BDII, and the control group; however, BDII patients were the only ones who performed worse on the Iowa Gambling Task than did the control group, and performed worse on the Stroop Color–Word Test than did BDI patients.
While MDD patients had worse psychological quality of life (QOL) than that of controls, there was no difference in other QOL measures. MDD patients reported better physical health and lower disability rates than did BD patients. BDII patients had worse QOL than did control patients, but had lower disability rates than did BDI patients.
The investigators found that differences in cognitive function and quality of life still existed in patients with mood disorder, even after adjustment for mania and depressive symptoms.
“The importance of a detailed assessment of [executive function] and disability levels within each of these diagnostic categories, while controlling for demographic variables, was especially evident from the current results. Additionally, the comparison of impairment rates between groups – which is not a usual measure in the literature – provided important contributions to current knowledge regarding cognition and mood disorders,” the investigators noted.
Find the study in the Journal of Affective Disorders (doi: 10.1016/j.jad.2015.11.007).
The nature and severity of cognitive alterations can vary significantly between different mood disorders, according to Dr. Charles Cotrena and his associates.
The study of 205 Brazilians comprised patients with major depressive disorder (MDD), patients with bipolar disorder I (BDI) and II (BDII), and a healthy control group, all of whom took a battery of neurocognitive tests. MDD patients performed poorly in tests involving attention and timed tasks, compared with the control group, but had less motor inhibition than did patients with BDI. Patients with BDI tended to perform worse across all executive functions, compared with patients with MDD, BDII, and the control group; however, BDII patients were the only ones who performed worse on the Iowa Gambling Task than did the control group, and performed worse on the Stroop Color–Word Test than did BDI patients.
While MDD patients had worse psychological quality of life (QOL) than that of controls, there was no difference in other QOL measures. MDD patients reported better physical health and lower disability rates than did BD patients. BDII patients had worse QOL than did control patients, but had lower disability rates than did BDI patients.
The investigators found that differences in cognitive function and quality of life still existed in patients with mood disorder, even after adjustment for mania and depressive symptoms.
“The importance of a detailed assessment of [executive function] and disability levels within each of these diagnostic categories, while controlling for demographic variables, was especially evident from the current results. Additionally, the comparison of impairment rates between groups – which is not a usual measure in the literature – provided important contributions to current knowledge regarding cognition and mood disorders,” the investigators noted.
Find the study in the Journal of Affective Disorders (doi: 10.1016/j.jad.2015.11.007).
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Webcast: Obesity and contraceptive efficacy and risks
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channelAccess Dr. Burkman's Webcasts on contraception: Helpful resources for your practice: |
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channelAccess Dr. Burkman's Webcasts on contraception: Helpful resources for your practice: |
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channelAccess Dr. Burkman's Webcasts on contraception: Helpful resources for your practice: |
8 tips to help patients cope with insomnia
Patients who struggle with insomnia may be better able to cope with their condition if they follow some of the tips listed in this patient handout from Neurology Reviews. Resisting naps, exercising earlier in the day, and avoiding the use of certain electronic devices before bedtime are among the tips offered at: http://cdn4.imng.com/fileadmin/qhi/nr/pdfs/53_TIPS_Insomnia_1115.pdf.
Patients who struggle with insomnia may be better able to cope with their condition if they follow some of the tips listed in this patient handout from Neurology Reviews. Resisting naps, exercising earlier in the day, and avoiding the use of certain electronic devices before bedtime are among the tips offered at: http://cdn4.imng.com/fileadmin/qhi/nr/pdfs/53_TIPS_Insomnia_1115.pdf.
Patients who struggle with insomnia may be better able to cope with their condition if they follow some of the tips listed in this patient handout from Neurology Reviews. Resisting naps, exercising earlier in the day, and avoiding the use of certain electronic devices before bedtime are among the tips offered at: http://cdn4.imng.com/fileadmin/qhi/nr/pdfs/53_TIPS_Insomnia_1115.pdf.
Proportion of HCV patients with cirrhosis is climbing
Despite a decline in the overall prevalence of hepatitis C virus (HCV) in the United States, the number of HCV patients with cirrhosis has risen significantly. Researchers report that the estimated prevalence of cirrhosis in HCV-infected patients has climbed from 6.6% in the early 1990s to more than 17% in the present era. Read more about this study, including the factors contributing to this increase, at Family Practice News. http://www.familypracticenews.com/specialty-focus/infectious-diseases/single-article-page/proportion-of-hcv-patients-in-us-with-cirrhosis-climbing/0a460b7fef6d035236f12b94f8aafc32.html
Despite a decline in the overall prevalence of hepatitis C virus (HCV) in the United States, the number of HCV patients with cirrhosis has risen significantly. Researchers report that the estimated prevalence of cirrhosis in HCV-infected patients has climbed from 6.6% in the early 1990s to more than 17% in the present era. Read more about this study, including the factors contributing to this increase, at Family Practice News. http://www.familypracticenews.com/specialty-focus/infectious-diseases/single-article-page/proportion-of-hcv-patients-in-us-with-cirrhosis-climbing/0a460b7fef6d035236f12b94f8aafc32.html
Despite a decline in the overall prevalence of hepatitis C virus (HCV) in the United States, the number of HCV patients with cirrhosis has risen significantly. Researchers report that the estimated prevalence of cirrhosis in HCV-infected patients has climbed from 6.6% in the early 1990s to more than 17% in the present era. Read more about this study, including the factors contributing to this increase, at Family Practice News. http://www.familypracticenews.com/specialty-focus/infectious-diseases/single-article-page/proportion-of-hcv-patients-in-us-with-cirrhosis-climbing/0a460b7fef6d035236f12b94f8aafc32.html
High-dose vitamin D increases fall risk in study of 200 patients
Older adults treated with high-dose vitamin D as part of a double-blind, randomized clinical trial achieved target 25-hydroxyvitamin D levels at 6 and 12 months, but did not achieve the primary endpoint of improved lower extremity function.
In fact, the highest dose used in the study – 60,000 IU of vitamin D3 monthly – was associated with an increased risk of falls, according to Dr. Heike A. Bischoff-Ferrari of University Hospital Zurich, and her colleagues.
In the 200 home-dwelling adults aged older than 70 years who participated in the 1-year study, monthly doses of 60,000 IU of vitamin D3 and 24,000 IU of vitamin D3 plus 300 mcg of calcifediol were significantly more likely than a 24,000 IU dose of vitamin D3 alone to result in 25-hydroxyvitamin D levels of at least 30 ng/mL at 6 and 12 months, but lower extremity function did not differ among the groups, the investigators reported in a study published online Jan. 4 in JAMA Internal Medicine.
For example, the adjusted changes in Short Physical Performance Battery scores – a measure of walking speed, successive chair stands, and balance – were 0.17, 0.16, and 0.16 at 6 months in the 24,000 IU, 60,000 IU, and 24,000 IU plus calcifediol groups, respectively, and 0.38, 0.10, and 0.11 at 12 months (JAMA Intern Med. 2016 Jan 4. doi: 10.1001/jamainternmed.2015.7148).
However, the incidence of falls was 66.9%, 66.1%, and 47.9% in the groups, respectively, and the mean number of falls was higher in the 60,000 IU group (1.47) and 24,000 IU plus calcifediol group (1.24) than in the 24,000 IU group (0.94), they said, noting that a similar pattern was observed during study months 0-6 and months 7-12.
Study subjects had a mean age of 78 years, 58% were vitamin D deficient with levels of less than 20 ng/mL at baseline, and 13% were severely deficient (levels less than 10 ng/mL). All had experienced a low-trauma fall in the previous 12 months and were thus considered a high-risk group for vitamin D deficiency and functional decline. Vitamin D supplementation was given via one 5-mL drink solution each month that provided 24,000 IU of vitamin D3 – equivalent to the current recommendation of 800 IU/day – plus three placebo capsules; a 5-mL drink solution that provided 60,000 IU of vitamin D3 – the equivalent of 2,000 IU/day – plus three placebo capsules; or a 5-mL placebo drink, two capsules containing 12,000 IU of vitamin D3 each, and one capsule containing 300 mcg of calcifediol, which is a potent liver metabolite of vitamin D.
Although vitamin D supplementation has been proposed as a possible strategy for delaying functional decline because of its effects on muscle strength, the current findings, which are consistent with some prior studies, suggest that high-doses of vitamin D confer no benefit with respect to function decline, compared with a standard-of-care dose of 24,000 IU of D3, and that high doses may increase falls in those with a prior fall event. Further research is needed to confirm the findings for daily dosing regimens, as well as to explore the physiology behind a possible detrimental effect of a high monthly bolus dose of vitamin D on muscle function and falls, they concluded.
This study was funded by the Swiss National Science Foundation and the VELUX Foundations, as well as by investigator-initiated funds from Merck Sharp & Dohme AG, WILD, and DSM Nutritional Products. Dr. Bischoff-Ferrari reported receiving speaker fees from and serving on advisory boards for Merck Sharp & Dohme AG, Amgen, WILD, DSM Nutritional Products, Roche Diagnostics, Nestle, Pfizer, and Sanofi.
The vitamin D storyline may be similar to that of antioxidant vitamins, Dr. Steven R. Cummings and his colleagues wrote in an editorial.
“Enthusiasm for the health benefits of vitamin supplements is coupled with the belief that ‘vitamins’ are inherently safe and reinforced by observational studies showing, essentially, that healthy people have higher vitamin levels. Then [randomized, controlled trials] and meta-analyses proved that the supplements in fact increase mortality (beta carotene, vitamin E), or have no health benefits (vitamin A, vitamin C),” they wrote (JAMA Intern Med. 2016 Jan 4. doi: 10.1001/jamainternmed.2015.7568).
The strategy of supplementation with vitamin D to achieve serum levels of at least 30 ng/mL to reduce the risk of falls and fractures has not been established, and according to the findings by Bischoff-Ferrari et al., it may increase the risk of falling.
“Until that approach is supported by randomized trials with updated meta-analyses, it would be prudent to follow recommendation from the Institute of Medicine (IOM) that people 70 years or older have a total daily intake of 800 IU of vitamin D without routine measurement of serum 25(OH)D levels,” they wrote, adding that recommended intakes are best achieved through a balanced diet.
Dr. Cummings is with the California Pacific Medical Center Research Institute, San Francisco, and the University of California, San Francisco. He and his coauthors reported having no disclosures.
The vitamin D storyline may be similar to that of antioxidant vitamins, Dr. Steven R. Cummings and his colleagues wrote in an editorial.
“Enthusiasm for the health benefits of vitamin supplements is coupled with the belief that ‘vitamins’ are inherently safe and reinforced by observational studies showing, essentially, that healthy people have higher vitamin levels. Then [randomized, controlled trials] and meta-analyses proved that the supplements in fact increase mortality (beta carotene, vitamin E), or have no health benefits (vitamin A, vitamin C),” they wrote (JAMA Intern Med. 2016 Jan 4. doi: 10.1001/jamainternmed.2015.7568).
The strategy of supplementation with vitamin D to achieve serum levels of at least 30 ng/mL to reduce the risk of falls and fractures has not been established, and according to the findings by Bischoff-Ferrari et al., it may increase the risk of falling.
“Until that approach is supported by randomized trials with updated meta-analyses, it would be prudent to follow recommendation from the Institute of Medicine (IOM) that people 70 years or older have a total daily intake of 800 IU of vitamin D without routine measurement of serum 25(OH)D levels,” they wrote, adding that recommended intakes are best achieved through a balanced diet.
Dr. Cummings is with the California Pacific Medical Center Research Institute, San Francisco, and the University of California, San Francisco. He and his coauthors reported having no disclosures.
The vitamin D storyline may be similar to that of antioxidant vitamins, Dr. Steven R. Cummings and his colleagues wrote in an editorial.
“Enthusiasm for the health benefits of vitamin supplements is coupled with the belief that ‘vitamins’ are inherently safe and reinforced by observational studies showing, essentially, that healthy people have higher vitamin levels. Then [randomized, controlled trials] and meta-analyses proved that the supplements in fact increase mortality (beta carotene, vitamin E), or have no health benefits (vitamin A, vitamin C),” they wrote (JAMA Intern Med. 2016 Jan 4. doi: 10.1001/jamainternmed.2015.7568).
The strategy of supplementation with vitamin D to achieve serum levels of at least 30 ng/mL to reduce the risk of falls and fractures has not been established, and according to the findings by Bischoff-Ferrari et al., it may increase the risk of falling.
“Until that approach is supported by randomized trials with updated meta-analyses, it would be prudent to follow recommendation from the Institute of Medicine (IOM) that people 70 years or older have a total daily intake of 800 IU of vitamin D without routine measurement of serum 25(OH)D levels,” they wrote, adding that recommended intakes are best achieved through a balanced diet.
Dr. Cummings is with the California Pacific Medical Center Research Institute, San Francisco, and the University of California, San Francisco. He and his coauthors reported having no disclosures.
Older adults treated with high-dose vitamin D as part of a double-blind, randomized clinical trial achieved target 25-hydroxyvitamin D levels at 6 and 12 months, but did not achieve the primary endpoint of improved lower extremity function.
In fact, the highest dose used in the study – 60,000 IU of vitamin D3 monthly – was associated with an increased risk of falls, according to Dr. Heike A. Bischoff-Ferrari of University Hospital Zurich, and her colleagues.
In the 200 home-dwelling adults aged older than 70 years who participated in the 1-year study, monthly doses of 60,000 IU of vitamin D3 and 24,000 IU of vitamin D3 plus 300 mcg of calcifediol were significantly more likely than a 24,000 IU dose of vitamin D3 alone to result in 25-hydroxyvitamin D levels of at least 30 ng/mL at 6 and 12 months, but lower extremity function did not differ among the groups, the investigators reported in a study published online Jan. 4 in JAMA Internal Medicine.
For example, the adjusted changes in Short Physical Performance Battery scores – a measure of walking speed, successive chair stands, and balance – were 0.17, 0.16, and 0.16 at 6 months in the 24,000 IU, 60,000 IU, and 24,000 IU plus calcifediol groups, respectively, and 0.38, 0.10, and 0.11 at 12 months (JAMA Intern Med. 2016 Jan 4. doi: 10.1001/jamainternmed.2015.7148).
However, the incidence of falls was 66.9%, 66.1%, and 47.9% in the groups, respectively, and the mean number of falls was higher in the 60,000 IU group (1.47) and 24,000 IU plus calcifediol group (1.24) than in the 24,000 IU group (0.94), they said, noting that a similar pattern was observed during study months 0-6 and months 7-12.
Study subjects had a mean age of 78 years, 58% were vitamin D deficient with levels of less than 20 ng/mL at baseline, and 13% were severely deficient (levels less than 10 ng/mL). All had experienced a low-trauma fall in the previous 12 months and were thus considered a high-risk group for vitamin D deficiency and functional decline. Vitamin D supplementation was given via one 5-mL drink solution each month that provided 24,000 IU of vitamin D3 – equivalent to the current recommendation of 800 IU/day – plus three placebo capsules; a 5-mL drink solution that provided 60,000 IU of vitamin D3 – the equivalent of 2,000 IU/day – plus three placebo capsules; or a 5-mL placebo drink, two capsules containing 12,000 IU of vitamin D3 each, and one capsule containing 300 mcg of calcifediol, which is a potent liver metabolite of vitamin D.
Although vitamin D supplementation has been proposed as a possible strategy for delaying functional decline because of its effects on muscle strength, the current findings, which are consistent with some prior studies, suggest that high-doses of vitamin D confer no benefit with respect to function decline, compared with a standard-of-care dose of 24,000 IU of D3, and that high doses may increase falls in those with a prior fall event. Further research is needed to confirm the findings for daily dosing regimens, as well as to explore the physiology behind a possible detrimental effect of a high monthly bolus dose of vitamin D on muscle function and falls, they concluded.
This study was funded by the Swiss National Science Foundation and the VELUX Foundations, as well as by investigator-initiated funds from Merck Sharp & Dohme AG, WILD, and DSM Nutritional Products. Dr. Bischoff-Ferrari reported receiving speaker fees from and serving on advisory boards for Merck Sharp & Dohme AG, Amgen, WILD, DSM Nutritional Products, Roche Diagnostics, Nestle, Pfizer, and Sanofi.
Older adults treated with high-dose vitamin D as part of a double-blind, randomized clinical trial achieved target 25-hydroxyvitamin D levels at 6 and 12 months, but did not achieve the primary endpoint of improved lower extremity function.
In fact, the highest dose used in the study – 60,000 IU of vitamin D3 monthly – was associated with an increased risk of falls, according to Dr. Heike A. Bischoff-Ferrari of University Hospital Zurich, and her colleagues.
In the 200 home-dwelling adults aged older than 70 years who participated in the 1-year study, monthly doses of 60,000 IU of vitamin D3 and 24,000 IU of vitamin D3 plus 300 mcg of calcifediol were significantly more likely than a 24,000 IU dose of vitamin D3 alone to result in 25-hydroxyvitamin D levels of at least 30 ng/mL at 6 and 12 months, but lower extremity function did not differ among the groups, the investigators reported in a study published online Jan. 4 in JAMA Internal Medicine.
For example, the adjusted changes in Short Physical Performance Battery scores – a measure of walking speed, successive chair stands, and balance – were 0.17, 0.16, and 0.16 at 6 months in the 24,000 IU, 60,000 IU, and 24,000 IU plus calcifediol groups, respectively, and 0.38, 0.10, and 0.11 at 12 months (JAMA Intern Med. 2016 Jan 4. doi: 10.1001/jamainternmed.2015.7148).
However, the incidence of falls was 66.9%, 66.1%, and 47.9% in the groups, respectively, and the mean number of falls was higher in the 60,000 IU group (1.47) and 24,000 IU plus calcifediol group (1.24) than in the 24,000 IU group (0.94), they said, noting that a similar pattern was observed during study months 0-6 and months 7-12.
Study subjects had a mean age of 78 years, 58% were vitamin D deficient with levels of less than 20 ng/mL at baseline, and 13% were severely deficient (levels less than 10 ng/mL). All had experienced a low-trauma fall in the previous 12 months and were thus considered a high-risk group for vitamin D deficiency and functional decline. Vitamin D supplementation was given via one 5-mL drink solution each month that provided 24,000 IU of vitamin D3 – equivalent to the current recommendation of 800 IU/day – plus three placebo capsules; a 5-mL drink solution that provided 60,000 IU of vitamin D3 – the equivalent of 2,000 IU/day – plus three placebo capsules; or a 5-mL placebo drink, two capsules containing 12,000 IU of vitamin D3 each, and one capsule containing 300 mcg of calcifediol, which is a potent liver metabolite of vitamin D.
Although vitamin D supplementation has been proposed as a possible strategy for delaying functional decline because of its effects on muscle strength, the current findings, which are consistent with some prior studies, suggest that high-doses of vitamin D confer no benefit with respect to function decline, compared with a standard-of-care dose of 24,000 IU of D3, and that high doses may increase falls in those with a prior fall event. Further research is needed to confirm the findings for daily dosing regimens, as well as to explore the physiology behind a possible detrimental effect of a high monthly bolus dose of vitamin D on muscle function and falls, they concluded.
This study was funded by the Swiss National Science Foundation and the VELUX Foundations, as well as by investigator-initiated funds from Merck Sharp & Dohme AG, WILD, and DSM Nutritional Products. Dr. Bischoff-Ferrari reported receiving speaker fees from and serving on advisory boards for Merck Sharp & Dohme AG, Amgen, WILD, DSM Nutritional Products, Roche Diagnostics, Nestle, Pfizer, and Sanofi.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Older adults treated with high-dose vitamin D as part of a double-blind, randomized clinical trial achieved target 25-hydroxyvitamin D levels at 6 and 12 months, but did not achieve the primary endpoint of improved lower extremity function.
Major finding: Fall incidence was 66.9%, 66.1%, and 47.9% with vitamin D3 at doses of 24,000 IU, 60,000 IU, and 24,000 IU, respectively, plus 300 mcg calcifediol.
Data source: A double-blind, randomized trial involving 200 patients.
Disclosures: This study was funded by the Swiss National Science Foundation and the VELUX Foundations, as well as by investigator-initiated funds from Merck Sharp & Dohme AG, WILD, and DSM Nutritional Products. Dr. Bischoff-Ferrari disclosed ties with Merck Sharp & Dohme AG, Amgen, WILD, DSM Nutritional Products, Roche Diagnostics, Nestle, Pfizer, and Sanofi.
Cancer drug discovery database goes 3D

Photo by Rhoda Baer
Researchers have updated the canSAR database, a tool designed to aid cancer drug discovery, by adding 3D structures of faulty proteins and maps of cancer’s communication networks.
The canSAR database brings together biological, chemical, and pharmacological data.
The goal of the database is to make these data accessible to researchers worldwide to help with hypothesis generation and support drug discovery decisions.
Users can search canSAR using text queries, protein/gene name searches, any keyword searches, chemical structure searches, and sequence similarity searches. Users can also explore and filter chemical compound sets, view experimental data, and produce summary plots.
The canSAR database was launched in 2011 with the goal of using Big Data approaches to build a detailed picture of how the majority of known human molecules behave.
The database has already collated billions of experimental measurements, mapping the actions of 1 million drugs and chemicals on human proteins, and it has combined these data with genetic information and results from clinical trials.
The updated version of canSAR uses artificial intelligence to identify nooks and crannies on the surface of faulty cancer-causing molecules as a key step in designing new drugs to block them. It also allows researchers to identify communication lines that can be intercepted within tumor cells, opening up potential new approaches for cancer treatment.
The growing database now holds the 3D structures of almost 3 million cavities on the surface of nearly 110,000 molecules.
“Our database is constantly growing with information and is the largest of its kind, with more than 140,000 users from over 175 countries,” said Bissan Al-Lazikani, PhD, of The Institute of Cancer Research in London, UK.
“And we regularly develop new artificial intelligence technologies that help scientists make predictions and design experiments. Our aim is that cancer scientists will be armed with the data they need to carry out life-saving research into the most exciting drugs of the future.”
“Scientists need to find all the information there is about a faulty gene or protein to understand whether a new drug might work. These data are vast and scattered, but the canSAR database brings them together and adds value by identifying hidden links and presenting the key information easily.”
Details on the updates to canSAR have been published in Nucleic Acid Research. The database is available online at https://cansar.icr.ac.uk/. ![]()

Photo by Rhoda Baer
Researchers have updated the canSAR database, a tool designed to aid cancer drug discovery, by adding 3D structures of faulty proteins and maps of cancer’s communication networks.
The canSAR database brings together biological, chemical, and pharmacological data.
The goal of the database is to make these data accessible to researchers worldwide to help with hypothesis generation and support drug discovery decisions.
Users can search canSAR using text queries, protein/gene name searches, any keyword searches, chemical structure searches, and sequence similarity searches. Users can also explore and filter chemical compound sets, view experimental data, and produce summary plots.
The canSAR database was launched in 2011 with the goal of using Big Data approaches to build a detailed picture of how the majority of known human molecules behave.
The database has already collated billions of experimental measurements, mapping the actions of 1 million drugs and chemicals on human proteins, and it has combined these data with genetic information and results from clinical trials.
The updated version of canSAR uses artificial intelligence to identify nooks and crannies on the surface of faulty cancer-causing molecules as a key step in designing new drugs to block them. It also allows researchers to identify communication lines that can be intercepted within tumor cells, opening up potential new approaches for cancer treatment.
The growing database now holds the 3D structures of almost 3 million cavities on the surface of nearly 110,000 molecules.
“Our database is constantly growing with information and is the largest of its kind, with more than 140,000 users from over 175 countries,” said Bissan Al-Lazikani, PhD, of The Institute of Cancer Research in London, UK.
“And we regularly develop new artificial intelligence technologies that help scientists make predictions and design experiments. Our aim is that cancer scientists will be armed with the data they need to carry out life-saving research into the most exciting drugs of the future.”
“Scientists need to find all the information there is about a faulty gene or protein to understand whether a new drug might work. These data are vast and scattered, but the canSAR database brings them together and adds value by identifying hidden links and presenting the key information easily.”
Details on the updates to canSAR have been published in Nucleic Acid Research. The database is available online at https://cansar.icr.ac.uk/. ![]()

Photo by Rhoda Baer
Researchers have updated the canSAR database, a tool designed to aid cancer drug discovery, by adding 3D structures of faulty proteins and maps of cancer’s communication networks.
The canSAR database brings together biological, chemical, and pharmacological data.
The goal of the database is to make these data accessible to researchers worldwide to help with hypothesis generation and support drug discovery decisions.
Users can search canSAR using text queries, protein/gene name searches, any keyword searches, chemical structure searches, and sequence similarity searches. Users can also explore and filter chemical compound sets, view experimental data, and produce summary plots.
The canSAR database was launched in 2011 with the goal of using Big Data approaches to build a detailed picture of how the majority of known human molecules behave.
The database has already collated billions of experimental measurements, mapping the actions of 1 million drugs and chemicals on human proteins, and it has combined these data with genetic information and results from clinical trials.
The updated version of canSAR uses artificial intelligence to identify nooks and crannies on the surface of faulty cancer-causing molecules as a key step in designing new drugs to block them. It also allows researchers to identify communication lines that can be intercepted within tumor cells, opening up potential new approaches for cancer treatment.
The growing database now holds the 3D structures of almost 3 million cavities on the surface of nearly 110,000 molecules.
“Our database is constantly growing with information and is the largest of its kind, with more than 140,000 users from over 175 countries,” said Bissan Al-Lazikani, PhD, of The Institute of Cancer Research in London, UK.
“And we regularly develop new artificial intelligence technologies that help scientists make predictions and design experiments. Our aim is that cancer scientists will be armed with the data they need to carry out life-saving research into the most exciting drugs of the future.”
“Scientists need to find all the information there is about a faulty gene or protein to understand whether a new drug might work. These data are vast and scattered, but the canSAR database brings them together and adds value by identifying hidden links and presenting the key information easily.”
Details on the updates to canSAR have been published in Nucleic Acid Research. The database is available online at https://cansar.icr.ac.uk/. ![]()
Nonalcoholic fatty liver disease will keep rising ‘in near term’
Nonalcoholic fatty liver disease (NAFLD) almost tripled among United States veterans in a recent 9-year period, investigators reported in the February issue of Clinical Gastroenterology and Hepatology.
The trend “was evident in all racial groups, across all age groups, and in both genders,” said Dr. Fasiha Kanwal of the Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine, both in Houston, and her associates. The increasing prevalence of NAFLD “is likely generalizable to nonveterans,” and will probably persist because of a “fairly steady” 2%-3% overall annual incidence and a steeper rise among younger individuals, they added. “Nonalcoholic fatty liver disease will continue to remain a major public health problem in the United States, at least in the near and intermediate future.”
Although NAFLD is the leading cause of chronic liver failure in the United States, few studies have examined its incidence or prevalence over time, which are key to predicting future disease burden. Therefore, the investigators analyzed data for more than 9.78 million patients who visited the VA at least once between 2003 and 2011. They defined NAFLD as at least two elevated alanine aminotransferase (ALT) values (greater than 40 IU/mL) separated by at least 6 months, with no history of positive serology for hepatitis B surface antigen or hepatitis C virus RNA, and no alcohol-related ICD-9 codes or positive AUDIT-C scores within a year of elevated ALT levels (Clin Gastroenterol Hepatol. 2015 Aug 7. doi: 10.1016/j.cgh.2015.08.010).
During the study period, more than 1.3 million patients, or 13.6%, met the definition of NAFLD, said the researchers. Age-adjusted incidence rates dropped slightly from 3.16% in 2003 to 2.5% in 2011, ranging between 2.3% and 2.7% in most years. Prevalence, however, rose from 6.3% in 2003 (95% confidence interval, 6.26%-6.3%) to 17.6% in 2011 (95% CI, 17.58%-17.65%), a 2.8-fold increase. Moreover, about one in five patients with NAFLD who visited the VA in 2011 was at risk for advanced fibrosis.
Among individuals who were younger than 45 years, the incidence of NAFLD rose from 2.3 to 4.3 cases per 100 persons (annual percentage change, 7.4%; 95% CI, 5.7% to 9.2%), the researchers also found. “Although recent studies show that the rate of increase in both obesity and diabetes, which are both major risk factors for NAFLD, may be slowing down in the U.S., this may not be the case in the VA, where the prevalence of obesity and diabetes is in fact higher than in the U.S. population,” they said.
In general, the findings mirror a recent analysis of the National Health and Nutrition Examination Survey (Aliment Pharmacol Ther. 2015 Jan;41[1]:65-76), according to the investigators. “The VA is the largest integrated health care system in the United States,” they added. “We believe that the sheer size of the veteran cohort, combined with a complete dearth of information regarding the burden of NAFLD in the VA, renders our findings highly significant. Furthermore, the VA is in a unique position to test and implement systemic changes in medical care delivery to improve the health care of NAFLD patients.”
The study was partially supported by the Michael E. DeBakey Veterans Affairs Medical Center. The researchers had no disclosures.
Kanwal and colleagues present an interesting study assessing the trends in the incidence and prevalence of NAFLD in the United States. Findings suggest that the annual incidence of NAFLD has generally been stable (2.2%-3.2%), while the prevalence of NAFLD has increased by 2.8-fold (6.3%-17.6%). These findings are consistent with the literature and provide additional evidence supporting the increasing burden of NAFLD. Although an important study, there are some limitations to the study design. First, the diagnosis of NAFLD was solely based on elevated liver enzymes, which can underestimate the true incidence and prevalence of NAFLD. In fact, in a recent meta-analysis, NAFLD prevalence based on liver enzymes was 13%, while NAFLD prevalence based on radiologic diagnosis was 25% (Hepatology. 2015 Dec 28. doi: 10.1002/hep.28431. [Epub ahead of print]). Second, the study subjects came from the VA system, which may not be representative of the U.S. population (Patrick AFB, FL: Defense Equal Opportunity Management Institute, 2010). This is important because sex-specific differences in the prevalence of NAFLD have been reported (Hepatology. 2015 Dec 28. doi: 10.1002/hep.28431. [Epub ahead of print]). Nevertheless, these limitations do not minimize the important contribution of this study. There appears to be an alarming increase in the burden of NAFLD within all the racial and age groups in the U.S. Further, this increase in the incidence and prevalence of NAFLD is especially significant among the younger age groups (less than 45 years). This finding is in contrast to others who have reported a higher prevalence in older subjects (Presented at AASLD 2015. San Francisco. Abstract #534). If confirmed, this younger cohort of patients with NAFLD can fuel the future burden of liver disease for the next few decades (JAMA. 2012;307:491-7). Given the current lack of an effective treatment for NAFLD, a national strategy to deal with this important and rising cause of chronic liver disease is urgently needed.
Dr. Zobair M. Younossi, MPH, FACG, AGAF, FAASLD, is chairman, department of medicine, Inova Fairfax Hospital; vice president for research, Inova Health System; professor of medicine, VCU-Inova Campus and Beatty Center for Integrated Research, Falls Church, Va. He has consulted for Gilead, AbbVie, Intercept, BMS, and GSK.
Kanwal and colleagues present an interesting study assessing the trends in the incidence and prevalence of NAFLD in the United States. Findings suggest that the annual incidence of NAFLD has generally been stable (2.2%-3.2%), while the prevalence of NAFLD has increased by 2.8-fold (6.3%-17.6%). These findings are consistent with the literature and provide additional evidence supporting the increasing burden of NAFLD. Although an important study, there are some limitations to the study design. First, the diagnosis of NAFLD was solely based on elevated liver enzymes, which can underestimate the true incidence and prevalence of NAFLD. In fact, in a recent meta-analysis, NAFLD prevalence based on liver enzymes was 13%, while NAFLD prevalence based on radiologic diagnosis was 25% (Hepatology. 2015 Dec 28. doi: 10.1002/hep.28431. [Epub ahead of print]). Second, the study subjects came from the VA system, which may not be representative of the U.S. population (Patrick AFB, FL: Defense Equal Opportunity Management Institute, 2010). This is important because sex-specific differences in the prevalence of NAFLD have been reported (Hepatology. 2015 Dec 28. doi: 10.1002/hep.28431. [Epub ahead of print]). Nevertheless, these limitations do not minimize the important contribution of this study. There appears to be an alarming increase in the burden of NAFLD within all the racial and age groups in the U.S. Further, this increase in the incidence and prevalence of NAFLD is especially significant among the younger age groups (less than 45 years). This finding is in contrast to others who have reported a higher prevalence in older subjects (Presented at AASLD 2015. San Francisco. Abstract #534). If confirmed, this younger cohort of patients with NAFLD can fuel the future burden of liver disease for the next few decades (JAMA. 2012;307:491-7). Given the current lack of an effective treatment for NAFLD, a national strategy to deal with this important and rising cause of chronic liver disease is urgently needed.
Dr. Zobair M. Younossi, MPH, FACG, AGAF, FAASLD, is chairman, department of medicine, Inova Fairfax Hospital; vice president for research, Inova Health System; professor of medicine, VCU-Inova Campus and Beatty Center for Integrated Research, Falls Church, Va. He has consulted for Gilead, AbbVie, Intercept, BMS, and GSK.
Kanwal and colleagues present an interesting study assessing the trends in the incidence and prevalence of NAFLD in the United States. Findings suggest that the annual incidence of NAFLD has generally been stable (2.2%-3.2%), while the prevalence of NAFLD has increased by 2.8-fold (6.3%-17.6%). These findings are consistent with the literature and provide additional evidence supporting the increasing burden of NAFLD. Although an important study, there are some limitations to the study design. First, the diagnosis of NAFLD was solely based on elevated liver enzymes, which can underestimate the true incidence and prevalence of NAFLD. In fact, in a recent meta-analysis, NAFLD prevalence based on liver enzymes was 13%, while NAFLD prevalence based on radiologic diagnosis was 25% (Hepatology. 2015 Dec 28. doi: 10.1002/hep.28431. [Epub ahead of print]). Second, the study subjects came from the VA system, which may not be representative of the U.S. population (Patrick AFB, FL: Defense Equal Opportunity Management Institute, 2010). This is important because sex-specific differences in the prevalence of NAFLD have been reported (Hepatology. 2015 Dec 28. doi: 10.1002/hep.28431. [Epub ahead of print]). Nevertheless, these limitations do not minimize the important contribution of this study. There appears to be an alarming increase in the burden of NAFLD within all the racial and age groups in the U.S. Further, this increase in the incidence and prevalence of NAFLD is especially significant among the younger age groups (less than 45 years). This finding is in contrast to others who have reported a higher prevalence in older subjects (Presented at AASLD 2015. San Francisco. Abstract #534). If confirmed, this younger cohort of patients with NAFLD can fuel the future burden of liver disease for the next few decades (JAMA. 2012;307:491-7). Given the current lack of an effective treatment for NAFLD, a national strategy to deal with this important and rising cause of chronic liver disease is urgently needed.
Dr. Zobair M. Younossi, MPH, FACG, AGAF, FAASLD, is chairman, department of medicine, Inova Fairfax Hospital; vice president for research, Inova Health System; professor of medicine, VCU-Inova Campus and Beatty Center for Integrated Research, Falls Church, Va. He has consulted for Gilead, AbbVie, Intercept, BMS, and GSK.
Nonalcoholic fatty liver disease (NAFLD) almost tripled among United States veterans in a recent 9-year period, investigators reported in the February issue of Clinical Gastroenterology and Hepatology.
The trend “was evident in all racial groups, across all age groups, and in both genders,” said Dr. Fasiha Kanwal of the Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine, both in Houston, and her associates. The increasing prevalence of NAFLD “is likely generalizable to nonveterans,” and will probably persist because of a “fairly steady” 2%-3% overall annual incidence and a steeper rise among younger individuals, they added. “Nonalcoholic fatty liver disease will continue to remain a major public health problem in the United States, at least in the near and intermediate future.”
Although NAFLD is the leading cause of chronic liver failure in the United States, few studies have examined its incidence or prevalence over time, which are key to predicting future disease burden. Therefore, the investigators analyzed data for more than 9.78 million patients who visited the VA at least once between 2003 and 2011. They defined NAFLD as at least two elevated alanine aminotransferase (ALT) values (greater than 40 IU/mL) separated by at least 6 months, with no history of positive serology for hepatitis B surface antigen or hepatitis C virus RNA, and no alcohol-related ICD-9 codes or positive AUDIT-C scores within a year of elevated ALT levels (Clin Gastroenterol Hepatol. 2015 Aug 7. doi: 10.1016/j.cgh.2015.08.010).
During the study period, more than 1.3 million patients, or 13.6%, met the definition of NAFLD, said the researchers. Age-adjusted incidence rates dropped slightly from 3.16% in 2003 to 2.5% in 2011, ranging between 2.3% and 2.7% in most years. Prevalence, however, rose from 6.3% in 2003 (95% confidence interval, 6.26%-6.3%) to 17.6% in 2011 (95% CI, 17.58%-17.65%), a 2.8-fold increase. Moreover, about one in five patients with NAFLD who visited the VA in 2011 was at risk for advanced fibrosis.
Among individuals who were younger than 45 years, the incidence of NAFLD rose from 2.3 to 4.3 cases per 100 persons (annual percentage change, 7.4%; 95% CI, 5.7% to 9.2%), the researchers also found. “Although recent studies show that the rate of increase in both obesity and diabetes, which are both major risk factors for NAFLD, may be slowing down in the U.S., this may not be the case in the VA, where the prevalence of obesity and diabetes is in fact higher than in the U.S. population,” they said.
In general, the findings mirror a recent analysis of the National Health and Nutrition Examination Survey (Aliment Pharmacol Ther. 2015 Jan;41[1]:65-76), according to the investigators. “The VA is the largest integrated health care system in the United States,” they added. “We believe that the sheer size of the veteran cohort, combined with a complete dearth of information regarding the burden of NAFLD in the VA, renders our findings highly significant. Furthermore, the VA is in a unique position to test and implement systemic changes in medical care delivery to improve the health care of NAFLD patients.”
The study was partially supported by the Michael E. DeBakey Veterans Affairs Medical Center. The researchers had no disclosures.
Nonalcoholic fatty liver disease (NAFLD) almost tripled among United States veterans in a recent 9-year period, investigators reported in the February issue of Clinical Gastroenterology and Hepatology.
The trend “was evident in all racial groups, across all age groups, and in both genders,” said Dr. Fasiha Kanwal of the Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine, both in Houston, and her associates. The increasing prevalence of NAFLD “is likely generalizable to nonveterans,” and will probably persist because of a “fairly steady” 2%-3% overall annual incidence and a steeper rise among younger individuals, they added. “Nonalcoholic fatty liver disease will continue to remain a major public health problem in the United States, at least in the near and intermediate future.”
Although NAFLD is the leading cause of chronic liver failure in the United States, few studies have examined its incidence or prevalence over time, which are key to predicting future disease burden. Therefore, the investigators analyzed data for more than 9.78 million patients who visited the VA at least once between 2003 and 2011. They defined NAFLD as at least two elevated alanine aminotransferase (ALT) values (greater than 40 IU/mL) separated by at least 6 months, with no history of positive serology for hepatitis B surface antigen or hepatitis C virus RNA, and no alcohol-related ICD-9 codes or positive AUDIT-C scores within a year of elevated ALT levels (Clin Gastroenterol Hepatol. 2015 Aug 7. doi: 10.1016/j.cgh.2015.08.010).
During the study period, more than 1.3 million patients, or 13.6%, met the definition of NAFLD, said the researchers. Age-adjusted incidence rates dropped slightly from 3.16% in 2003 to 2.5% in 2011, ranging between 2.3% and 2.7% in most years. Prevalence, however, rose from 6.3% in 2003 (95% confidence interval, 6.26%-6.3%) to 17.6% in 2011 (95% CI, 17.58%-17.65%), a 2.8-fold increase. Moreover, about one in five patients with NAFLD who visited the VA in 2011 was at risk for advanced fibrosis.
Among individuals who were younger than 45 years, the incidence of NAFLD rose from 2.3 to 4.3 cases per 100 persons (annual percentage change, 7.4%; 95% CI, 5.7% to 9.2%), the researchers also found. “Although recent studies show that the rate of increase in both obesity and diabetes, which are both major risk factors for NAFLD, may be slowing down in the U.S., this may not be the case in the VA, where the prevalence of obesity and diabetes is in fact higher than in the U.S. population,” they said.
In general, the findings mirror a recent analysis of the National Health and Nutrition Examination Survey (Aliment Pharmacol Ther. 2015 Jan;41[1]:65-76), according to the investigators. “The VA is the largest integrated health care system in the United States,” they added. “We believe that the sheer size of the veteran cohort, combined with a complete dearth of information regarding the burden of NAFLD in the VA, renders our findings highly significant. Furthermore, the VA is in a unique position to test and implement systemic changes in medical care delivery to improve the health care of NAFLD patients.”
The study was partially supported by the Michael E. DeBakey Veterans Affairs Medical Center. The researchers had no disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: The prevalence of nonalcoholic fatty liver disease has risen substantially since 2003, and will probably keep increasing in the near term.
Major finding: Prevalence among veterans rose about 2.8 times between 2003 and 2011, mirroring trends reported in the general population.
Data source: An analysis of data from 9.78 million Veterans Affairs patients.
Disclosures: The study was partially supported by the Michael E. DeBakey Veterans Affairs Medical Center. The researchers had no disclosures.
Is there an increased risk of GI bleeds with SSRIs?
Yes. Selective serotonin reuptake inhibitors (SSRIs) are likely associated with a moderate increased risk of upper gastrointestinal (UGI) bleeding. Use of a nonsteroidal anti-inflammatory drug (NSAID) in combination with the SSRI appears to amplify the risk (strength of recommendation [SOR]: B, meta-analysis of cohort and case control studies).
The increased risk from SSRIs occurs within the first 7 to 28 days after exposure (SOR: B, retrospective study).
SSRIs raise bleeding risk; concurrent NSAIDs raise it more
A 2014 systematic review and meta-analysis of 19 case-control and cohort studies with a total of 446,949 patients investigated the risk of UGI bleeding in patients using SSRIs and NSAIDs.1 The studies, which included both inpatients and outpatients, were done in Europe and North America. Patients were at least 16 years old, but pooled demographics were not reported. Investigators compared SSRI use with or without concurrent NSAID use to placebo or no treatment.
SSRI use was associated with an increased risk of UGI bleeding in 15 case-control studies (393,268 patients; odds ratio [OR]=1.7; 95% confidence interval [CI], 1.4-1.9) and 4 cohort studies (53,681 patients; OR=1.7; 95% CI, 1.1-2.5). The simultaneous use of SSRIs and NSAIDs compared to nonuse of both medications was associated with a larger increase in bleeding risk (10 case-control studies, 223,336 patients; OR=4.3; 95% CI, 2.8-6.4).
The meta-analysis is limited by statistically significant heterogeneity in all of the pooled results and high risk of bias in 9 of the case-control studies and all of the cohort studies. There was no evidence of publication bias, however.
Bleeding risk rises 7 to 28 days after SSRI exposure
A 2014 case-crossover study of 5377 inpatients in Taiwan with a psychiatric diagnosis evaluated the risk of UGI bleeding within the first 28 days after SSRI exposure (SSRI-mediated inhibition of platelets occurs within the first 7 to 14 days).2 The average age of the patients was 58 years and 75% of the study population was male. Each patient served as his or her own control.
ORs were calculated to compare patients who were exposed to SSRIs only during 7-, 14-, and 21-day windows immediately before a UGI bleed to controls exposed to SSRIs only during the control periods before the 7-, 14-, and 21-day windows. The ORs were adjusted through multivariate analysis to account for 7 potential confounding factors.
SSRI use was associated with an increased risk of UGI bleeding in 7-, 14-, and 21-day windows before the index event (TABLE2). An increased bleeding risk in the 14 days after SSRI initiation was observed in men (OR=2.4; 95% CI, 1.8-3.4) but not women (OR=1.0; 95% CI, 0.6-1.6). Increased bleeding risk in the 14 days after SSRI initiation was also observed in patients younger than 55 years (OR=2.1; 95% CI, 1.5-3.1), patients with a history of upper GI disease (OR=3.1; 95% CI, 1.7-6.0), and patients with no previous exposure to SSRIs (OR=2.6; 95% CI, 1.6-4.2).
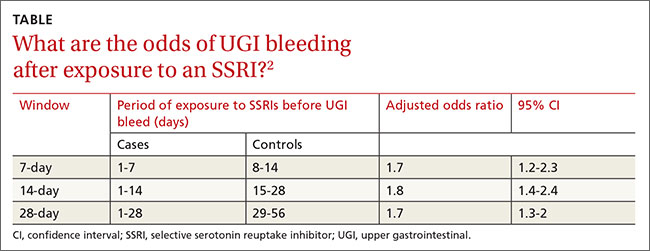
This study didn’t account for SSRI indication as a potential confounder, and the study’s inclusion of inpatients, whose illnesses are typically more severe, may limit generalizability.
1. Anglin R, Yuan Y, Moayyedi P, et al. Risk of upper gastrointestinal bleeding with selective serotonin reuptake inhibitors with or without concurrent nonsteroidal anti-inflammatory use: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:811-819.
2. Wang YP, Chen YT, Tsai C, et al. Short-term use of serotonin reuptake inhibitors and risk of upper gastrointestinal bleeding. Am J Psychiatry. 2014;171:54-61.
Yes. Selective serotonin reuptake inhibitors (SSRIs) are likely associated with a moderate increased risk of upper gastrointestinal (UGI) bleeding. Use of a nonsteroidal anti-inflammatory drug (NSAID) in combination with the SSRI appears to amplify the risk (strength of recommendation [SOR]: B, meta-analysis of cohort and case control studies).
The increased risk from SSRIs occurs within the first 7 to 28 days after exposure (SOR: B, retrospective study).
SSRIs raise bleeding risk; concurrent NSAIDs raise it more
A 2014 systematic review and meta-analysis of 19 case-control and cohort studies with a total of 446,949 patients investigated the risk of UGI bleeding in patients using SSRIs and NSAIDs.1 The studies, which included both inpatients and outpatients, were done in Europe and North America. Patients were at least 16 years old, but pooled demographics were not reported. Investigators compared SSRI use with or without concurrent NSAID use to placebo or no treatment.
SSRI use was associated with an increased risk of UGI bleeding in 15 case-control studies (393,268 patients; odds ratio [OR]=1.7; 95% confidence interval [CI], 1.4-1.9) and 4 cohort studies (53,681 patients; OR=1.7; 95% CI, 1.1-2.5). The simultaneous use of SSRIs and NSAIDs compared to nonuse of both medications was associated with a larger increase in bleeding risk (10 case-control studies, 223,336 patients; OR=4.3; 95% CI, 2.8-6.4).
The meta-analysis is limited by statistically significant heterogeneity in all of the pooled results and high risk of bias in 9 of the case-control studies and all of the cohort studies. There was no evidence of publication bias, however.
Bleeding risk rises 7 to 28 days after SSRI exposure
A 2014 case-crossover study of 5377 inpatients in Taiwan with a psychiatric diagnosis evaluated the risk of UGI bleeding within the first 28 days after SSRI exposure (SSRI-mediated inhibition of platelets occurs within the first 7 to 14 days).2 The average age of the patients was 58 years and 75% of the study population was male. Each patient served as his or her own control.
ORs were calculated to compare patients who were exposed to SSRIs only during 7-, 14-, and 21-day windows immediately before a UGI bleed to controls exposed to SSRIs only during the control periods before the 7-, 14-, and 21-day windows. The ORs were adjusted through multivariate analysis to account for 7 potential confounding factors.
SSRI use was associated with an increased risk of UGI bleeding in 7-, 14-, and 21-day windows before the index event (TABLE2). An increased bleeding risk in the 14 days after SSRI initiation was observed in men (OR=2.4; 95% CI, 1.8-3.4) but not women (OR=1.0; 95% CI, 0.6-1.6). Increased bleeding risk in the 14 days after SSRI initiation was also observed in patients younger than 55 years (OR=2.1; 95% CI, 1.5-3.1), patients with a history of upper GI disease (OR=3.1; 95% CI, 1.7-6.0), and patients with no previous exposure to SSRIs (OR=2.6; 95% CI, 1.6-4.2).

This study didn’t account for SSRI indication as a potential confounder, and the study’s inclusion of inpatients, whose illnesses are typically more severe, may limit generalizability.
Yes. Selective serotonin reuptake inhibitors (SSRIs) are likely associated with a moderate increased risk of upper gastrointestinal (UGI) bleeding. Use of a nonsteroidal anti-inflammatory drug (NSAID) in combination with the SSRI appears to amplify the risk (strength of recommendation [SOR]: B, meta-analysis of cohort and case control studies).
The increased risk from SSRIs occurs within the first 7 to 28 days after exposure (SOR: B, retrospective study).
SSRIs raise bleeding risk; concurrent NSAIDs raise it more
A 2014 systematic review and meta-analysis of 19 case-control and cohort studies with a total of 446,949 patients investigated the risk of UGI bleeding in patients using SSRIs and NSAIDs.1 The studies, which included both inpatients and outpatients, were done in Europe and North America. Patients were at least 16 years old, but pooled demographics were not reported. Investigators compared SSRI use with or without concurrent NSAID use to placebo or no treatment.
SSRI use was associated with an increased risk of UGI bleeding in 15 case-control studies (393,268 patients; odds ratio [OR]=1.7; 95% confidence interval [CI], 1.4-1.9) and 4 cohort studies (53,681 patients; OR=1.7; 95% CI, 1.1-2.5). The simultaneous use of SSRIs and NSAIDs compared to nonuse of both medications was associated with a larger increase in bleeding risk (10 case-control studies, 223,336 patients; OR=4.3; 95% CI, 2.8-6.4).
The meta-analysis is limited by statistically significant heterogeneity in all of the pooled results and high risk of bias in 9 of the case-control studies and all of the cohort studies. There was no evidence of publication bias, however.
Bleeding risk rises 7 to 28 days after SSRI exposure
A 2014 case-crossover study of 5377 inpatients in Taiwan with a psychiatric diagnosis evaluated the risk of UGI bleeding within the first 28 days after SSRI exposure (SSRI-mediated inhibition of platelets occurs within the first 7 to 14 days).2 The average age of the patients was 58 years and 75% of the study population was male. Each patient served as his or her own control.
ORs were calculated to compare patients who were exposed to SSRIs only during 7-, 14-, and 21-day windows immediately before a UGI bleed to controls exposed to SSRIs only during the control periods before the 7-, 14-, and 21-day windows. The ORs were adjusted through multivariate analysis to account for 7 potential confounding factors.
SSRI use was associated with an increased risk of UGI bleeding in 7-, 14-, and 21-day windows before the index event (TABLE2). An increased bleeding risk in the 14 days after SSRI initiation was observed in men (OR=2.4; 95% CI, 1.8-3.4) but not women (OR=1.0; 95% CI, 0.6-1.6). Increased bleeding risk in the 14 days after SSRI initiation was also observed in patients younger than 55 years (OR=2.1; 95% CI, 1.5-3.1), patients with a history of upper GI disease (OR=3.1; 95% CI, 1.7-6.0), and patients with no previous exposure to SSRIs (OR=2.6; 95% CI, 1.6-4.2).

This study didn’t account for SSRI indication as a potential confounder, and the study’s inclusion of inpatients, whose illnesses are typically more severe, may limit generalizability.
1. Anglin R, Yuan Y, Moayyedi P, et al. Risk of upper gastrointestinal bleeding with selective serotonin reuptake inhibitors with or without concurrent nonsteroidal anti-inflammatory use: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:811-819.
2. Wang YP, Chen YT, Tsai C, et al. Short-term use of serotonin reuptake inhibitors and risk of upper gastrointestinal bleeding. Am J Psychiatry. 2014;171:54-61.
1. Anglin R, Yuan Y, Moayyedi P, et al. Risk of upper gastrointestinal bleeding with selective serotonin reuptake inhibitors with or without concurrent nonsteroidal anti-inflammatory use: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:811-819.
2. Wang YP, Chen YT, Tsai C, et al. Short-term use of serotonin reuptake inhibitors and risk of upper gastrointestinal bleeding. Am J Psychiatry. 2014;171:54-61.
Evidence-based answers from the Family Physicians Inquiries Network