User login
Incidence, Risk Factors, and Outcome Trends of Acute Kidney Injury in Elective Total Hip and Knee Arthroplasty
Degenerative arthritis is a widespread chronic condition with an incidence of almost 43 million and annual health care costs of $60 billion in the United States alone.1 Although many cases can be managed symptomatically with medical therapy and intra-articular injections,2 many patients experience disease progression resulting in decreased ambulatory ability and work productivity. For these patients, elective hip and knee arthroplasties can drastically improve quality of life and functionality.3,4 Over the past decade, there has been a marked increase in the number of primary and revision total hip and knee arthroplasties performed in the United States. By 2030, the demand for primary total hip arthroplasties will grow an estimated 174%, to 572,000 procedures. Likewise, the demand for primary total knee arthroplasties is projected to grow by 673%, to 3.48 million procedures.5 However, though better surgical techniques and technology have led to improved functional outcomes, there is still substantial risk for complications in the perioperative period, especially in the geriatric population, in which substantial comorbidities are common.6-9
Acute kidney injury (AKI) is a common public health problem in hospitalized patients and in patients undergoing procedures. More than one-third of all AKI cases occur in surgical settings.10,11 Over the past decade, both community-acquired and in-hospital AKIs rapidly increased in incidence in all major clinical settings.12-14 Patients with AKI have high rates of adverse outcomes during hospitalization and discharge.11,15 Sequelae of AKIs include worsening chronic kidney disease (CKD) and progression to end-stage renal disease, necessitating either long-term dialysis or transplantation.12 This in turn leads to exacerbated disability, diminished quality of life, and disproportionate burden on health care resources.
Much of our knowledge about postoperative AKI has been derived from cardiovascular, thoracic, and abdominal surgery settings. However, there is a paucity of data on epidemiology and trends for either AKI or associated outcomes in patients undergoing major orthopedic surgery. The few studies to date either were single-center or had inadequate sample sizes for appropriately powered analysis of the risk factors and outcomes related to AKI.16
In the study reported here, we analyzed a large cohort of patients from a nationwide multicenter database to determine the incidence of and risk factors for AKI. We also examined the mortality and adverse discharges associated with AKI after major joint surgery. Lastly, we assessed temporal trends in both incidence and outcomes of AKI, including the death risk attributable to AKI.
Methods
Database
We extracted our study cohort from the Nationwide Inpatient Sample (NIS) and the National Inpatient Sample of Healthcare Cost and Utilization Project (HCUP) compiled by the Agency for Healthcare Research and Quality.17 NIS, the largest inpatient care database in the United States, stores data from almost 8 million stays in about 1000 hospitals across the country each year. Its participating hospital pool consists of about 20% of US community hospitals, resulting in a sampling frame comprising about 90% of all hospital discharges in the United States. This allows for calculation of precise, weighted nationwide estimates. Data elements within NIS are drawn from hospital discharge abstracts that indicate all procedures performed. NIS also stores information on patient characteristics, length of stay (LOS), discharge disposition, postoperative morbidity, and observed in-hospital mortality. However, it stores no information on long-term follow-up or complications after discharge.
Data Analysis
For the period 2002–2012, we queried the NIS database for hip and knee arthroplasties with primary diagnosis codes for osteoarthritis and secondary codes for AKI. We excluded patients under age 18 years and patients with diagnosis codes for hip and knee fracture/necrosis, inflammatory/infectious arthritis, or bone neoplasms (Table 1). We then extracted baseline characteristics of the study population. Patient-level characteristics included age, sex, race, quartile classification of median household income according to postal (ZIP) code, and primary payer (Medicare/Medicaid, private insurance, self-pay, no charge). Hospital-level characteristics included hospital location (urban, rural), hospital bed size (small, medium, large), region (Northeast, Midwest/North Central, South, West), and teaching status. We defined illness severity and likelihood of death using Deyo’s modification of the Charlson Comorbidity Index (CCI), which draws on principal and secondary ICD-9-CM (International Classification of Diseases, Ninth Revision-Clinical Modification) diagnosis codes, procedure codes, and patient demographics to estimate a patient’s mortality risk. This method reliably predicts mortality and readmission in the orthopedic population.18,19 We assessed the effect of AKI on 4 outcomes, including in-hospital mortality, discharge disposition, LOS, and cost of stay. Discharge disposition was grouped by either (a) home or short-term facility or (b) adverse discharge. Home or short-term facility covered routine, short-term hospital, against medical advice, home intravenous provider, another rehabilitation facility, another institution for outpatient services, institution for outpatient services, discharged alive, and destination unknown; adverse discharge covered skilled nursing facility, intermediate care, hospice home, hospice medical facility, long-term care hospital, and certified nursing facility. This dichotomization of discharge disposition is often used in studies of NIS data.20
Statistical Analyses
We compared the baseline characteristics of hospitalized patients with and without AKI. To test for significance, we used the χ2 test for categorical variables, the Student t test for normally distributed continuous variables, the Wilcoxon rank sum test for non-normally distributed continuous variables, and the Cochran-Armitage test for trends in AKI incidence. We used survey logistic regression models to calculate adjusted odds ratios (ORs) with 95% confidence intervals (95% CIs) in order to estimate the predictors of AKI and the impact of AKI on hospital outcomes. We constructed final models after adjusting for confounders, testing for potential interactions, and ensuring no multicolinearity between covariates. Last, we computed the risk proportion of death attributable to AKI, indicating the proportion of deaths that could potentially be avoided if AKI and its complications were abrogated.21
We performed all statistical analyses with SAS Version 9.3 (SAS Institute) using designated weight values to produce weighted national estimates. The threshold for statistical significance was set at P < .01 (with ORs and 95% CIs that excluded 1).
Results
AKI Incidence, Risk Factors, and Trends
We identified 7,235,251 patients who underwent elective hip or knee arthroplasty for osteoarthritis between 2002 and 2012—an estimate consistent with data from the Centers for Disease Control and Prevention.22 Of that total, 94,367 (1.3%) had AKI. The proportion of discharges diagnosed with AKI increased rapidly over the decade, from 0.5% in 2002 to 1.8% to 1.9% in the period 2010–2012. This upward trend was highly significant (Ptrend < .001) (Figure 1). Patients with AKI (vs patients without AKI) were more likely to be older (mean age, 70 vs 66 years; P < .001), male (50.8% vs 38.4%; P < .001), and black (10.07% vs 5.15%; P<. 001). They were also found to have a significantly higher comorbidity score (mean CCI, 2.8 vs 1.5; P < .001) and higher proportions of comorbidities, including hypertension, CKD, atrial fibrillation, diabetes mellitus (DM), congestive heart failure, chronic liver disease, and hepatitis C virus infection. In addition, AKI was associated with perioperative myocardial infarction (MI), sepsis, cardiac catheterization, and blood transfusion. Regarding socioeconomic characteristics, patients with AKI were more likely to have Medicare/Medicaid insurance (72.26% vs 58.06%; P < .001) and to belong to the extremes of income categories (Table 2).
Using multivariable logistic regression, we found that increased age (1.11 increase in adjusted OR for every year older; 95% CI, 1.09-1.14; P < .001), male sex (adjusted OR, 1.65; 95% CI, 1.60-1.71; P < .001), and black race (adjusted OR, 1.57; 95% CI, 1.45-1.69; P < .001) were significantly associated with postoperative AKI. Regarding comorbidities, baseline CKD (adjusted OR, 8.64; 95% CI, 8.14-9.18; P < .001) and congestive heart failure (adjusted OR, 2.74; 95% CI, 2.57-2.92; P< .0001) were most significantly associated with AKI. Perioperative events, including sepsis (adjusted OR, 35.64; 95% CI, 30.28-41.96; P < .0001), MI (adjusted OR, 6.14; 95% CI, 5.17-7.28; P < .0001), and blood transfusion (adjusted OR, 2.28; 95% CI, 2.15-2.42; P < .0001), were also strongly associated with postoperative AKI. Last, compared with urban hospitals and small hospital bed size, rural hospitals (adjusted OR, 0.70; 95% CI, 0.60-0.81; P< .001) and large bed size (adjusted OR, 0.82; 95% CI, 0.70-0.93; P = .003) were associated with lower probability of developing AKI (Table 3).
Figure 2 elucidates the frequency of AKI based on a combination of key preoperative comorbid conditions and postoperative complications—demonstrating that the proportion of AKI cases associated with other postoperative complications is significantly higher in the CKD and concomitant DM/CKD patient populations. Patients hospitalized with CKD exhibited higher rates of AKI in cases involving blood transfusion (20.9% vs 1.8%; P < .001), acute MI (48.9% vs 13.8%; P < .001), and sepsis (74.7% vs 36.3%;P< .001) relative to patients without CKD. Similarly, patients with concomitant DM/CKD exhibited higher rates of AKI in cases involving blood transfusion (23% vs 1.9%; P< .001), acute MI (51.1% vs 12.1%; P< .001), and sepsis (75% vs 38.2%; P < .001) relative to patients without either condition. However, patients hospitalized with DM alone exhibited only marginally higher rates of AKI in cases involving blood transfusion (4.7% vs 2%; P < .01) and acute MI (19.2% vs 16.7%; P< .01) and a lower rate in cases involving sepsis (38.2% vs 41.7%; P < .01) relative to patients without DM. These data suggest that CKD is the most significant clinically relevant risk factor for AKI and that CKD may synergize with DM to raise the risk for AKI.
Outcomes
We then analyzed the impact of AKI on hospital outcomes, including in-hospital mortality, discharge disposition, LOS, and cost of care. Mortality was significantly higher in patients with AKI than in patients without it (2.08% vs 0.06%; P < .001). Even after adjusting for confounders (eg, demographics, comorbidity burden, perioperative sepsis, hospital-level characteristics), AKI was still associated with strikingly higher odds of in-hospital death (adjusted OR, 11.32; 95% CI, 9.34-13.74; P < .001). However, analysis of temporal trends indicated that the odds for adjusted mortality associated with AKI decreased from 18.09 to 9.45 (Ptrend = .01) over the period 2002–2012 (Figure 3). This decrease in odds of death was countered by an increase in incidence of AKI, resulting in a stable attributable risk proportion (97.9% in 2002 to 97.3% in 2012; Ptrend = .90) (Table 4). Regarding discharge disposition, patients with AKI were much less likely to be discharged home (41.35% vs 62.59%; P < .001) and more likely to be discharged to long-term care (56.37% vs 37.03%; P< .001). After adjustment for confounders, AKI was associated with significantly increased odds of adverse discharge (adjusted OR, 2.24; 95% CI, 2.12-2.36; P< .001). Analysis of temporal trends revealed no appreciable decrease in the adjusted odds of adverse discharge between 2002 (adjusted OR, 1.87; 95% CI, 1.37-2.55; P < .001) and 2012 (adjusted OR, 1.93; 95% CI, 1.76-2.11; P < .001) (Figure 4, Table 5). Last, both mean LOS (5 days vs 3 days; P < .001) and mean cost of hospitalization (US $22,269 vs $15,757; P < .001) were significantly higher in patients with AKI.
Discussion
In this study, we found that the incidence of AKI among hospitalized patients increased 4-fold between 2002 and 2012. Moreover, we identified numerous patient-specific, hospital-specific, perioperative risk factors for AKI. Most important, we found that AKI was associated with a strikingly higher risk of in-hospital death, and surviving patients were more likely to experience adverse discharge. Although the adjusted mortality rate associated with AKI decreased over that decade, the attributable risk proportion remained stable.
Few studies have addressed this significant public health concern. In one recent study in Australia, Kimmel and colleagues16 identified risk factors for AKI but lacked data on AKI outcomes. In a study of complications and mortality occurring after orthopedic surgery, Belmont and colleagues22 categorized complications as either local or systemic but did not examine renal complications. Only 2 other major studies have been conducted on renal outcomes associated with major joint surgery, and both were limited to patients with acute hip fractures. The first included acute fracture surgery patients and omitted elective joint surgery patients, and it evaluated admission renal function but not postoperative AKI.22 The second study had a sample size of only 170 patients.23 Thus, the literature leaves us with a crucial knowledge gap in renal outcomes and their postoperative impact in elective arthroplasties.
The present study filled this information gap by examining the incidence, risk factors, outcomes, and temporal trends of AKI after elective hip and knee arthroplasties. The increasing incidence of AKI in this surgical setting is similar to that of AKI in other surgical settings (cardiac and noncardiac).21 Although our analysis was limited by lack of perioperative management data, patients undergoing elective joint arthroplasty can experience kidney dysfunction for several reasons, including volume depletion, postoperative sepsis, and influence of medications, such as nonsteroidal anti-inflammatory drugs (NSAIDs), especially in older patients with more comorbidities and a higher burden of CKD. Each of these factors can cause renal dysfunction in patients having orthopedic procedures.24 Moreover, NSAID use among elective joint arthroplasty patients is likely higher because of an emphasis on multimodal analgesia, as recent randomized controlled trials have demonstrated the efficacy of NSAID use in controlling pain without increasing bleeding.25-27 Our results also demonstrated that the absolute incidence of AKI after orthopedic surgery is relatively low. One possible explanation for this phenomenon is that the definitions used were based on ICD-9-CM codes that underestimate the true incidence of AKI.
Consistent with other studies, we found that certain key preoperative comorbid conditions and postoperative events were associated with higher AKI risk. We stratified the rate of AKI associated with each postoperative event (sepsis, acute MI, cardiac catheterization, need for transfusion) by DM/CKD comorbidity. CKD was associated with significantly higher AKI risk across all postoperative complications. This information may provide clinicians with bedside information that can be used to determine which patients may be at higher or lower risk for AKI.
Our analysis of patient outcomes revealed that, though AKI was relatively uncommon, it increased the risk for death during hospitalization more than 10-fold between 2002 and 2012. Although the adjusted OR of in-hospital mortality decreased over the decade studied, the concurrent increase in AKI incidence caused the attributable risk of death associated with AKI to essentially remain the same. This observation is consistent with recent reports from cardiac surgery settings.21 These data together suggest that ameliorating occurrences of AKI would decrease mortality and increase quality of care for patients undergoing elective joint surgeries.
We also examined the effect of AKI on resource use by studying LOS, costs, and risk for adverse discharge. Much as in other surgical settings, AKI increased both LOS and overall hospitalization costs. More important, AKI was associated with increased adverse discharge (discharge to long-term care or nursing homes). Although exact reasons are unclear, we can speculate that postoperative renal dysfunction precludes early rehabilitation, impeding desired functional outcome and disposition.28,29 Given the projected increases in primary and revision hip and knee arthroplasties,5 these data predict that the impact of AKI on health outcomes will increase alarmingly in coming years.
There are limitations to our study. First, it was based on administrative data and lacked patient-level and laboratory data. As reported, the sensitivity of AKI codes remains moderate,30 so the true burden may be higher than indicated here. As the definition of AKI was based on administrative coding, we also could not estimate severity, though previous studies have found that administrative codes typically capture a more severe form of disease.31 Another limitation is that, because the data were deidentified, we could not delineate the risk for recurrent AKI in repeated surgical procedures, though this cohort unlikely was large enough to qualitatively affect our results. The third limitation is that, though we used CCI to adjust for the comorbidity burden, we were unable to account for other unmeasured confounders associated with increased AKI incidence, such as specific medication use. In addition, given the lack of patient-level data, we could not analyze the specific factors responsible for AKI in the perioperative period. Nevertheless, the strengths of a nationally representative sample, such as large sample size and generalizability, outweigh these limitations.
Conclusion
AKI is potentially an important quality indicator of elective joint surgery, and reducing its incidence is therefore essential for quality improvement. Given that hip and knee arthroplasties are projected to increase exponentially, as is the burden of comorbid conditions in this population, postoperative AKI will continue to have an incremental impact on health and health care resources. Thus, a carefully planned approach of interdisciplinary perioperative care is warranted to reduce both the risk and the consequences of this devastating condition.
1. Reginster JY. The prevalence and burden of arthritis. Rheumatology. 2002;41(supp 1):3-6.
2. Kullenberg B, Runesson R, Tuvhag R, Olsson C, Resch S. Intraarticular corticosteroid injection: pain relief in osteoarthritis of the hip? J Rheumatol. 2004;31(11):2265-2268.
3. Kawasaki M, Hasegawa Y, Sakano S, Torii Y, Warashina H. Quality of life after several treatments for osteoarthritis of the hip. J Orthop Sci. 2003;8(1):32-35.
4. Ethgen O, Bruyère O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
5. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
6. Matlock D, Earnest M, Epstein A. Utilization of elective hip and knee arthroplasty by age and payer. Clin Orthop Relat Res. 2008;466(4):914-919.
7. Parvizi J, Holiday AD, Ereth MH, Lewallen DG. The Frank Stinchfield Award. Sudden death during primary hip arthroplasty. Clin Orthop Relat Res. 1999;(369):39-48.
8. Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89(1):27-32.
9. Parvizi J, Sullivan TA, Trousdale RT, Lewallen DG. Thirty-day mortality after total knee arthroplasty. J Bone Joint Surg Am. 2001;83(8):1157-1161.
10. Uchino S, Kellum JA, Bellomo R, et al; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813-818.
11. Thakar CV. Perioperative acute kidney injury. Adv Chronic Kidney Dis. 2013;20(1):67-75.
12. Hsu CY, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, Go AS. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4(5):891-898.
13. Rewa O, Bagshaw SM. Acute kidney injury—epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10(4):193-207.
14. Thakar CV, Worley S, Arrigain S, Yared JP, Paganini EP. Influence of renal dysfunction on mortality after cardiac surgery: modifying effect of preoperative renal function. Kidney Int. 2005;67(3):1112-1119.
15. Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9(1):12-20.
16. Kimmel LA, Wilson S, Janardan JD, Liew SM, Walker RG. Incidence of acute kidney injury following total joint arthroplasty: a retrospective review by RIFLE criteria. Clin Kidney J. 2014;7(6):546-551.
17. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) databases, 2002–2012. Rockville, MD: Agency for Healthcare Research and Quality.
18. Bjorgul K, Novicoff WM, Saleh KJ. Evaluating comorbidities in total hip and knee arthroplasty: available instruments. J Orthop Traumatol. 2010;11(4):203-209.
19. Voskuijl T, Hageman M, Ring D. Higher Charlson Comorbidity Index Scores are associated with readmission after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(5):1638-1644.
20. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365-3370.
21. Lenihan CR, Montez-Rath ME, Mora Mangano CT, Chertow GM, Winkelmayer WC. Trends in acute kidney injury, associated use of dialysis, and mortality after cardiac surgery, 1999 to 2008. Ann Thorac Surg. 2013;95(1):20-28.
22. Belmont PJ Jr, Goodman GP, Waterman BR, Bader JO, Schoenfeld AJ. Thirty-day postoperative complications and mortality following total knee arthroplasty: incidence and risk factors among a national sample of 15,321 patients. J Bone Joint Surg Am. 2014;96(1):20-26.
23. Bennet SJ, Berry OM, Goddard J, Keating JF. Acute renal dysfunction following hip fracture. Injury. 2010;41(4):335-338.
24. Kateros K, Doulgerakis C, Galanakos SP, Sakellariou VI, Papadakis SA, Macheras GA. Analysis of kidney dysfunction in orthopaedic patients. BMC Nephrol. 2012;13:101.
25. Huang YM, Wang CM, Wang CT, Lin WP, Horng LC, Jiang CC. Perioperative celecoxib administration for pain management after total knee arthroplasty—a randomized, controlled study. BMC Musculoskelet Disord. 2008;9:77.
26. Kelley TC, Adams MJ, Mulliken BD, Dalury DF. Efficacy of multimodal perioperative analgesia protocol with periarticular medication injection in total knee arthroplasty: a randomized, double-blinded study. J Arthroplasty. 2013;28(8):1274-1277.
27. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329-334.
28. Munin MC, Rudy TE, Glynn NW, Crossett LS, Rubash HE. Early inpatient rehabilitation after elective hip and knee arthroplasty. JAMA. 1998;279(11):847-852.
29. Pua YH, Ong PH. Association of early ambulation with length of stay and costs in total knee arthroplasty: retrospective cohort study. Am J Phys Med Rehabil. 2014;93(11):962-970.
30. Waikar SS, Wald R, Chertow GM, et al. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification codes for acute renal failure. J Am Soc Nephrol. 2006;17(6):1688-1694.
31. Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol. 2014;9(4):682-689.
Degenerative arthritis is a widespread chronic condition with an incidence of almost 43 million and annual health care costs of $60 billion in the United States alone.1 Although many cases can be managed symptomatically with medical therapy and intra-articular injections,2 many patients experience disease progression resulting in decreased ambulatory ability and work productivity. For these patients, elective hip and knee arthroplasties can drastically improve quality of life and functionality.3,4 Over the past decade, there has been a marked increase in the number of primary and revision total hip and knee arthroplasties performed in the United States. By 2030, the demand for primary total hip arthroplasties will grow an estimated 174%, to 572,000 procedures. Likewise, the demand for primary total knee arthroplasties is projected to grow by 673%, to 3.48 million procedures.5 However, though better surgical techniques and technology have led to improved functional outcomes, there is still substantial risk for complications in the perioperative period, especially in the geriatric population, in which substantial comorbidities are common.6-9
Acute kidney injury (AKI) is a common public health problem in hospitalized patients and in patients undergoing procedures. More than one-third of all AKI cases occur in surgical settings.10,11 Over the past decade, both community-acquired and in-hospital AKIs rapidly increased in incidence in all major clinical settings.12-14 Patients with AKI have high rates of adverse outcomes during hospitalization and discharge.11,15 Sequelae of AKIs include worsening chronic kidney disease (CKD) and progression to end-stage renal disease, necessitating either long-term dialysis or transplantation.12 This in turn leads to exacerbated disability, diminished quality of life, and disproportionate burden on health care resources.
Much of our knowledge about postoperative AKI has been derived from cardiovascular, thoracic, and abdominal surgery settings. However, there is a paucity of data on epidemiology and trends for either AKI or associated outcomes in patients undergoing major orthopedic surgery. The few studies to date either were single-center or had inadequate sample sizes for appropriately powered analysis of the risk factors and outcomes related to AKI.16
In the study reported here, we analyzed a large cohort of patients from a nationwide multicenter database to determine the incidence of and risk factors for AKI. We also examined the mortality and adverse discharges associated with AKI after major joint surgery. Lastly, we assessed temporal trends in both incidence and outcomes of AKI, including the death risk attributable to AKI.
Methods
Database
We extracted our study cohort from the Nationwide Inpatient Sample (NIS) and the National Inpatient Sample of Healthcare Cost and Utilization Project (HCUP) compiled by the Agency for Healthcare Research and Quality.17 NIS, the largest inpatient care database in the United States, stores data from almost 8 million stays in about 1000 hospitals across the country each year. Its participating hospital pool consists of about 20% of US community hospitals, resulting in a sampling frame comprising about 90% of all hospital discharges in the United States. This allows for calculation of precise, weighted nationwide estimates. Data elements within NIS are drawn from hospital discharge abstracts that indicate all procedures performed. NIS also stores information on patient characteristics, length of stay (LOS), discharge disposition, postoperative morbidity, and observed in-hospital mortality. However, it stores no information on long-term follow-up or complications after discharge.
Data Analysis
For the period 2002–2012, we queried the NIS database for hip and knee arthroplasties with primary diagnosis codes for osteoarthritis and secondary codes for AKI. We excluded patients under age 18 years and patients with diagnosis codes for hip and knee fracture/necrosis, inflammatory/infectious arthritis, or bone neoplasms (Table 1). We then extracted baseline characteristics of the study population. Patient-level characteristics included age, sex, race, quartile classification of median household income according to postal (ZIP) code, and primary payer (Medicare/Medicaid, private insurance, self-pay, no charge). Hospital-level characteristics included hospital location (urban, rural), hospital bed size (small, medium, large), region (Northeast, Midwest/North Central, South, West), and teaching status. We defined illness severity and likelihood of death using Deyo’s modification of the Charlson Comorbidity Index (CCI), which draws on principal and secondary ICD-9-CM (International Classification of Diseases, Ninth Revision-Clinical Modification) diagnosis codes, procedure codes, and patient demographics to estimate a patient’s mortality risk. This method reliably predicts mortality and readmission in the orthopedic population.18,19 We assessed the effect of AKI on 4 outcomes, including in-hospital mortality, discharge disposition, LOS, and cost of stay. Discharge disposition was grouped by either (a) home or short-term facility or (b) adverse discharge. Home or short-term facility covered routine, short-term hospital, against medical advice, home intravenous provider, another rehabilitation facility, another institution for outpatient services, institution for outpatient services, discharged alive, and destination unknown; adverse discharge covered skilled nursing facility, intermediate care, hospice home, hospice medical facility, long-term care hospital, and certified nursing facility. This dichotomization of discharge disposition is often used in studies of NIS data.20
Statistical Analyses
We compared the baseline characteristics of hospitalized patients with and without AKI. To test for significance, we used the χ2 test for categorical variables, the Student t test for normally distributed continuous variables, the Wilcoxon rank sum test for non-normally distributed continuous variables, and the Cochran-Armitage test for trends in AKI incidence. We used survey logistic regression models to calculate adjusted odds ratios (ORs) with 95% confidence intervals (95% CIs) in order to estimate the predictors of AKI and the impact of AKI on hospital outcomes. We constructed final models after adjusting for confounders, testing for potential interactions, and ensuring no multicolinearity between covariates. Last, we computed the risk proportion of death attributable to AKI, indicating the proportion of deaths that could potentially be avoided if AKI and its complications were abrogated.21
We performed all statistical analyses with SAS Version 9.3 (SAS Institute) using designated weight values to produce weighted national estimates. The threshold for statistical significance was set at P < .01 (with ORs and 95% CIs that excluded 1).
Results
AKI Incidence, Risk Factors, and Trends
We identified 7,235,251 patients who underwent elective hip or knee arthroplasty for osteoarthritis between 2002 and 2012—an estimate consistent with data from the Centers for Disease Control and Prevention.22 Of that total, 94,367 (1.3%) had AKI. The proportion of discharges diagnosed with AKI increased rapidly over the decade, from 0.5% in 2002 to 1.8% to 1.9% in the period 2010–2012. This upward trend was highly significant (Ptrend < .001) (Figure 1). Patients with AKI (vs patients without AKI) were more likely to be older (mean age, 70 vs 66 years; P < .001), male (50.8% vs 38.4%; P < .001), and black (10.07% vs 5.15%; P<. 001). They were also found to have a significantly higher comorbidity score (mean CCI, 2.8 vs 1.5; P < .001) and higher proportions of comorbidities, including hypertension, CKD, atrial fibrillation, diabetes mellitus (DM), congestive heart failure, chronic liver disease, and hepatitis C virus infection. In addition, AKI was associated with perioperative myocardial infarction (MI), sepsis, cardiac catheterization, and blood transfusion. Regarding socioeconomic characteristics, patients with AKI were more likely to have Medicare/Medicaid insurance (72.26% vs 58.06%; P < .001) and to belong to the extremes of income categories (Table 2).
Using multivariable logistic regression, we found that increased age (1.11 increase in adjusted OR for every year older; 95% CI, 1.09-1.14; P < .001), male sex (adjusted OR, 1.65; 95% CI, 1.60-1.71; P < .001), and black race (adjusted OR, 1.57; 95% CI, 1.45-1.69; P < .001) were significantly associated with postoperative AKI. Regarding comorbidities, baseline CKD (adjusted OR, 8.64; 95% CI, 8.14-9.18; P < .001) and congestive heart failure (adjusted OR, 2.74; 95% CI, 2.57-2.92; P< .0001) were most significantly associated with AKI. Perioperative events, including sepsis (adjusted OR, 35.64; 95% CI, 30.28-41.96; P < .0001), MI (adjusted OR, 6.14; 95% CI, 5.17-7.28; P < .0001), and blood transfusion (adjusted OR, 2.28; 95% CI, 2.15-2.42; P < .0001), were also strongly associated with postoperative AKI. Last, compared with urban hospitals and small hospital bed size, rural hospitals (adjusted OR, 0.70; 95% CI, 0.60-0.81; P< .001) and large bed size (adjusted OR, 0.82; 95% CI, 0.70-0.93; P = .003) were associated with lower probability of developing AKI (Table 3).
Figure 2 elucidates the frequency of AKI based on a combination of key preoperative comorbid conditions and postoperative complications—demonstrating that the proportion of AKI cases associated with other postoperative complications is significantly higher in the CKD and concomitant DM/CKD patient populations. Patients hospitalized with CKD exhibited higher rates of AKI in cases involving blood transfusion (20.9% vs 1.8%; P < .001), acute MI (48.9% vs 13.8%; P < .001), and sepsis (74.7% vs 36.3%;P< .001) relative to patients without CKD. Similarly, patients with concomitant DM/CKD exhibited higher rates of AKI in cases involving blood transfusion (23% vs 1.9%; P< .001), acute MI (51.1% vs 12.1%; P< .001), and sepsis (75% vs 38.2%; P < .001) relative to patients without either condition. However, patients hospitalized with DM alone exhibited only marginally higher rates of AKI in cases involving blood transfusion (4.7% vs 2%; P < .01) and acute MI (19.2% vs 16.7%; P< .01) and a lower rate in cases involving sepsis (38.2% vs 41.7%; P < .01) relative to patients without DM. These data suggest that CKD is the most significant clinically relevant risk factor for AKI and that CKD may synergize with DM to raise the risk for AKI.
Outcomes
We then analyzed the impact of AKI on hospital outcomes, including in-hospital mortality, discharge disposition, LOS, and cost of care. Mortality was significantly higher in patients with AKI than in patients without it (2.08% vs 0.06%; P < .001). Even after adjusting for confounders (eg, demographics, comorbidity burden, perioperative sepsis, hospital-level characteristics), AKI was still associated with strikingly higher odds of in-hospital death (adjusted OR, 11.32; 95% CI, 9.34-13.74; P < .001). However, analysis of temporal trends indicated that the odds for adjusted mortality associated with AKI decreased from 18.09 to 9.45 (Ptrend = .01) over the period 2002–2012 (Figure 3). This decrease in odds of death was countered by an increase in incidence of AKI, resulting in a stable attributable risk proportion (97.9% in 2002 to 97.3% in 2012; Ptrend = .90) (Table 4). Regarding discharge disposition, patients with AKI were much less likely to be discharged home (41.35% vs 62.59%; P < .001) and more likely to be discharged to long-term care (56.37% vs 37.03%; P< .001). After adjustment for confounders, AKI was associated with significantly increased odds of adverse discharge (adjusted OR, 2.24; 95% CI, 2.12-2.36; P< .001). Analysis of temporal trends revealed no appreciable decrease in the adjusted odds of adverse discharge between 2002 (adjusted OR, 1.87; 95% CI, 1.37-2.55; P < .001) and 2012 (adjusted OR, 1.93; 95% CI, 1.76-2.11; P < .001) (Figure 4, Table 5). Last, both mean LOS (5 days vs 3 days; P < .001) and mean cost of hospitalization (US $22,269 vs $15,757; P < .001) were significantly higher in patients with AKI.
Discussion
In this study, we found that the incidence of AKI among hospitalized patients increased 4-fold between 2002 and 2012. Moreover, we identified numerous patient-specific, hospital-specific, perioperative risk factors for AKI. Most important, we found that AKI was associated with a strikingly higher risk of in-hospital death, and surviving patients were more likely to experience adverse discharge. Although the adjusted mortality rate associated with AKI decreased over that decade, the attributable risk proportion remained stable.
Few studies have addressed this significant public health concern. In one recent study in Australia, Kimmel and colleagues16 identified risk factors for AKI but lacked data on AKI outcomes. In a study of complications and mortality occurring after orthopedic surgery, Belmont and colleagues22 categorized complications as either local or systemic but did not examine renal complications. Only 2 other major studies have been conducted on renal outcomes associated with major joint surgery, and both were limited to patients with acute hip fractures. The first included acute fracture surgery patients and omitted elective joint surgery patients, and it evaluated admission renal function but not postoperative AKI.22 The second study had a sample size of only 170 patients.23 Thus, the literature leaves us with a crucial knowledge gap in renal outcomes and their postoperative impact in elective arthroplasties.
The present study filled this information gap by examining the incidence, risk factors, outcomes, and temporal trends of AKI after elective hip and knee arthroplasties. The increasing incidence of AKI in this surgical setting is similar to that of AKI in other surgical settings (cardiac and noncardiac).21 Although our analysis was limited by lack of perioperative management data, patients undergoing elective joint arthroplasty can experience kidney dysfunction for several reasons, including volume depletion, postoperative sepsis, and influence of medications, such as nonsteroidal anti-inflammatory drugs (NSAIDs), especially in older patients with more comorbidities and a higher burden of CKD. Each of these factors can cause renal dysfunction in patients having orthopedic procedures.24 Moreover, NSAID use among elective joint arthroplasty patients is likely higher because of an emphasis on multimodal analgesia, as recent randomized controlled trials have demonstrated the efficacy of NSAID use in controlling pain without increasing bleeding.25-27 Our results also demonstrated that the absolute incidence of AKI after orthopedic surgery is relatively low. One possible explanation for this phenomenon is that the definitions used were based on ICD-9-CM codes that underestimate the true incidence of AKI.
Consistent with other studies, we found that certain key preoperative comorbid conditions and postoperative events were associated with higher AKI risk. We stratified the rate of AKI associated with each postoperative event (sepsis, acute MI, cardiac catheterization, need for transfusion) by DM/CKD comorbidity. CKD was associated with significantly higher AKI risk across all postoperative complications. This information may provide clinicians with bedside information that can be used to determine which patients may be at higher or lower risk for AKI.
Our analysis of patient outcomes revealed that, though AKI was relatively uncommon, it increased the risk for death during hospitalization more than 10-fold between 2002 and 2012. Although the adjusted OR of in-hospital mortality decreased over the decade studied, the concurrent increase in AKI incidence caused the attributable risk of death associated with AKI to essentially remain the same. This observation is consistent with recent reports from cardiac surgery settings.21 These data together suggest that ameliorating occurrences of AKI would decrease mortality and increase quality of care for patients undergoing elective joint surgeries.
We also examined the effect of AKI on resource use by studying LOS, costs, and risk for adverse discharge. Much as in other surgical settings, AKI increased both LOS and overall hospitalization costs. More important, AKI was associated with increased adverse discharge (discharge to long-term care or nursing homes). Although exact reasons are unclear, we can speculate that postoperative renal dysfunction precludes early rehabilitation, impeding desired functional outcome and disposition.28,29 Given the projected increases in primary and revision hip and knee arthroplasties,5 these data predict that the impact of AKI on health outcomes will increase alarmingly in coming years.
There are limitations to our study. First, it was based on administrative data and lacked patient-level and laboratory data. As reported, the sensitivity of AKI codes remains moderate,30 so the true burden may be higher than indicated here. As the definition of AKI was based on administrative coding, we also could not estimate severity, though previous studies have found that administrative codes typically capture a more severe form of disease.31 Another limitation is that, because the data were deidentified, we could not delineate the risk for recurrent AKI in repeated surgical procedures, though this cohort unlikely was large enough to qualitatively affect our results. The third limitation is that, though we used CCI to adjust for the comorbidity burden, we were unable to account for other unmeasured confounders associated with increased AKI incidence, such as specific medication use. In addition, given the lack of patient-level data, we could not analyze the specific factors responsible for AKI in the perioperative period. Nevertheless, the strengths of a nationally representative sample, such as large sample size and generalizability, outweigh these limitations.
Conclusion
AKI is potentially an important quality indicator of elective joint surgery, and reducing its incidence is therefore essential for quality improvement. Given that hip and knee arthroplasties are projected to increase exponentially, as is the burden of comorbid conditions in this population, postoperative AKI will continue to have an incremental impact on health and health care resources. Thus, a carefully planned approach of interdisciplinary perioperative care is warranted to reduce both the risk and the consequences of this devastating condition.
Degenerative arthritis is a widespread chronic condition with an incidence of almost 43 million and annual health care costs of $60 billion in the United States alone.1 Although many cases can be managed symptomatically with medical therapy and intra-articular injections,2 many patients experience disease progression resulting in decreased ambulatory ability and work productivity. For these patients, elective hip and knee arthroplasties can drastically improve quality of life and functionality.3,4 Over the past decade, there has been a marked increase in the number of primary and revision total hip and knee arthroplasties performed in the United States. By 2030, the demand for primary total hip arthroplasties will grow an estimated 174%, to 572,000 procedures. Likewise, the demand for primary total knee arthroplasties is projected to grow by 673%, to 3.48 million procedures.5 However, though better surgical techniques and technology have led to improved functional outcomes, there is still substantial risk for complications in the perioperative period, especially in the geriatric population, in which substantial comorbidities are common.6-9
Acute kidney injury (AKI) is a common public health problem in hospitalized patients and in patients undergoing procedures. More than one-third of all AKI cases occur in surgical settings.10,11 Over the past decade, both community-acquired and in-hospital AKIs rapidly increased in incidence in all major clinical settings.12-14 Patients with AKI have high rates of adverse outcomes during hospitalization and discharge.11,15 Sequelae of AKIs include worsening chronic kidney disease (CKD) and progression to end-stage renal disease, necessitating either long-term dialysis or transplantation.12 This in turn leads to exacerbated disability, diminished quality of life, and disproportionate burden on health care resources.
Much of our knowledge about postoperative AKI has been derived from cardiovascular, thoracic, and abdominal surgery settings. However, there is a paucity of data on epidemiology and trends for either AKI or associated outcomes in patients undergoing major orthopedic surgery. The few studies to date either were single-center or had inadequate sample sizes for appropriately powered analysis of the risk factors and outcomes related to AKI.16
In the study reported here, we analyzed a large cohort of patients from a nationwide multicenter database to determine the incidence of and risk factors for AKI. We also examined the mortality and adverse discharges associated with AKI after major joint surgery. Lastly, we assessed temporal trends in both incidence and outcomes of AKI, including the death risk attributable to AKI.
Methods
Database
We extracted our study cohort from the Nationwide Inpatient Sample (NIS) and the National Inpatient Sample of Healthcare Cost and Utilization Project (HCUP) compiled by the Agency for Healthcare Research and Quality.17 NIS, the largest inpatient care database in the United States, stores data from almost 8 million stays in about 1000 hospitals across the country each year. Its participating hospital pool consists of about 20% of US community hospitals, resulting in a sampling frame comprising about 90% of all hospital discharges in the United States. This allows for calculation of precise, weighted nationwide estimates. Data elements within NIS are drawn from hospital discharge abstracts that indicate all procedures performed. NIS also stores information on patient characteristics, length of stay (LOS), discharge disposition, postoperative morbidity, and observed in-hospital mortality. However, it stores no information on long-term follow-up or complications after discharge.
Data Analysis
For the period 2002–2012, we queried the NIS database for hip and knee arthroplasties with primary diagnosis codes for osteoarthritis and secondary codes for AKI. We excluded patients under age 18 years and patients with diagnosis codes for hip and knee fracture/necrosis, inflammatory/infectious arthritis, or bone neoplasms (Table 1). We then extracted baseline characteristics of the study population. Patient-level characteristics included age, sex, race, quartile classification of median household income according to postal (ZIP) code, and primary payer (Medicare/Medicaid, private insurance, self-pay, no charge). Hospital-level characteristics included hospital location (urban, rural), hospital bed size (small, medium, large), region (Northeast, Midwest/North Central, South, West), and teaching status. We defined illness severity and likelihood of death using Deyo’s modification of the Charlson Comorbidity Index (CCI), which draws on principal and secondary ICD-9-CM (International Classification of Diseases, Ninth Revision-Clinical Modification) diagnosis codes, procedure codes, and patient demographics to estimate a patient’s mortality risk. This method reliably predicts mortality and readmission in the orthopedic population.18,19 We assessed the effect of AKI on 4 outcomes, including in-hospital mortality, discharge disposition, LOS, and cost of stay. Discharge disposition was grouped by either (a) home or short-term facility or (b) adverse discharge. Home or short-term facility covered routine, short-term hospital, against medical advice, home intravenous provider, another rehabilitation facility, another institution for outpatient services, institution for outpatient services, discharged alive, and destination unknown; adverse discharge covered skilled nursing facility, intermediate care, hospice home, hospice medical facility, long-term care hospital, and certified nursing facility. This dichotomization of discharge disposition is often used in studies of NIS data.20
Statistical Analyses
We compared the baseline characteristics of hospitalized patients with and without AKI. To test for significance, we used the χ2 test for categorical variables, the Student t test for normally distributed continuous variables, the Wilcoxon rank sum test for non-normally distributed continuous variables, and the Cochran-Armitage test for trends in AKI incidence. We used survey logistic regression models to calculate adjusted odds ratios (ORs) with 95% confidence intervals (95% CIs) in order to estimate the predictors of AKI and the impact of AKI on hospital outcomes. We constructed final models after adjusting for confounders, testing for potential interactions, and ensuring no multicolinearity between covariates. Last, we computed the risk proportion of death attributable to AKI, indicating the proportion of deaths that could potentially be avoided if AKI and its complications were abrogated.21
We performed all statistical analyses with SAS Version 9.3 (SAS Institute) using designated weight values to produce weighted national estimates. The threshold for statistical significance was set at P < .01 (with ORs and 95% CIs that excluded 1).
Results
AKI Incidence, Risk Factors, and Trends
We identified 7,235,251 patients who underwent elective hip or knee arthroplasty for osteoarthritis between 2002 and 2012—an estimate consistent with data from the Centers for Disease Control and Prevention.22 Of that total, 94,367 (1.3%) had AKI. The proportion of discharges diagnosed with AKI increased rapidly over the decade, from 0.5% in 2002 to 1.8% to 1.9% in the period 2010–2012. This upward trend was highly significant (Ptrend < .001) (Figure 1). Patients with AKI (vs patients without AKI) were more likely to be older (mean age, 70 vs 66 years; P < .001), male (50.8% vs 38.4%; P < .001), and black (10.07% vs 5.15%; P<. 001). They were also found to have a significantly higher comorbidity score (mean CCI, 2.8 vs 1.5; P < .001) and higher proportions of comorbidities, including hypertension, CKD, atrial fibrillation, diabetes mellitus (DM), congestive heart failure, chronic liver disease, and hepatitis C virus infection. In addition, AKI was associated with perioperative myocardial infarction (MI), sepsis, cardiac catheterization, and blood transfusion. Regarding socioeconomic characteristics, patients with AKI were more likely to have Medicare/Medicaid insurance (72.26% vs 58.06%; P < .001) and to belong to the extremes of income categories (Table 2).
Using multivariable logistic regression, we found that increased age (1.11 increase in adjusted OR for every year older; 95% CI, 1.09-1.14; P < .001), male sex (adjusted OR, 1.65; 95% CI, 1.60-1.71; P < .001), and black race (adjusted OR, 1.57; 95% CI, 1.45-1.69; P < .001) were significantly associated with postoperative AKI. Regarding comorbidities, baseline CKD (adjusted OR, 8.64; 95% CI, 8.14-9.18; P < .001) and congestive heart failure (adjusted OR, 2.74; 95% CI, 2.57-2.92; P< .0001) were most significantly associated with AKI. Perioperative events, including sepsis (adjusted OR, 35.64; 95% CI, 30.28-41.96; P < .0001), MI (adjusted OR, 6.14; 95% CI, 5.17-7.28; P < .0001), and blood transfusion (adjusted OR, 2.28; 95% CI, 2.15-2.42; P < .0001), were also strongly associated with postoperative AKI. Last, compared with urban hospitals and small hospital bed size, rural hospitals (adjusted OR, 0.70; 95% CI, 0.60-0.81; P< .001) and large bed size (adjusted OR, 0.82; 95% CI, 0.70-0.93; P = .003) were associated with lower probability of developing AKI (Table 3).
Figure 2 elucidates the frequency of AKI based on a combination of key preoperative comorbid conditions and postoperative complications—demonstrating that the proportion of AKI cases associated with other postoperative complications is significantly higher in the CKD and concomitant DM/CKD patient populations. Patients hospitalized with CKD exhibited higher rates of AKI in cases involving blood transfusion (20.9% vs 1.8%; P < .001), acute MI (48.9% vs 13.8%; P < .001), and sepsis (74.7% vs 36.3%;P< .001) relative to patients without CKD. Similarly, patients with concomitant DM/CKD exhibited higher rates of AKI in cases involving blood transfusion (23% vs 1.9%; P< .001), acute MI (51.1% vs 12.1%; P< .001), and sepsis (75% vs 38.2%; P < .001) relative to patients without either condition. However, patients hospitalized with DM alone exhibited only marginally higher rates of AKI in cases involving blood transfusion (4.7% vs 2%; P < .01) and acute MI (19.2% vs 16.7%; P< .01) and a lower rate in cases involving sepsis (38.2% vs 41.7%; P < .01) relative to patients without DM. These data suggest that CKD is the most significant clinically relevant risk factor for AKI and that CKD may synergize with DM to raise the risk for AKI.
Outcomes
We then analyzed the impact of AKI on hospital outcomes, including in-hospital mortality, discharge disposition, LOS, and cost of care. Mortality was significantly higher in patients with AKI than in patients without it (2.08% vs 0.06%; P < .001). Even after adjusting for confounders (eg, demographics, comorbidity burden, perioperative sepsis, hospital-level characteristics), AKI was still associated with strikingly higher odds of in-hospital death (adjusted OR, 11.32; 95% CI, 9.34-13.74; P < .001). However, analysis of temporal trends indicated that the odds for adjusted mortality associated with AKI decreased from 18.09 to 9.45 (Ptrend = .01) over the period 2002–2012 (Figure 3). This decrease in odds of death was countered by an increase in incidence of AKI, resulting in a stable attributable risk proportion (97.9% in 2002 to 97.3% in 2012; Ptrend = .90) (Table 4). Regarding discharge disposition, patients with AKI were much less likely to be discharged home (41.35% vs 62.59%; P < .001) and more likely to be discharged to long-term care (56.37% vs 37.03%; P< .001). After adjustment for confounders, AKI was associated with significantly increased odds of adverse discharge (adjusted OR, 2.24; 95% CI, 2.12-2.36; P< .001). Analysis of temporal trends revealed no appreciable decrease in the adjusted odds of adverse discharge between 2002 (adjusted OR, 1.87; 95% CI, 1.37-2.55; P < .001) and 2012 (adjusted OR, 1.93; 95% CI, 1.76-2.11; P < .001) (Figure 4, Table 5). Last, both mean LOS (5 days vs 3 days; P < .001) and mean cost of hospitalization (US $22,269 vs $15,757; P < .001) were significantly higher in patients with AKI.
Discussion
In this study, we found that the incidence of AKI among hospitalized patients increased 4-fold between 2002 and 2012. Moreover, we identified numerous patient-specific, hospital-specific, perioperative risk factors for AKI. Most important, we found that AKI was associated with a strikingly higher risk of in-hospital death, and surviving patients were more likely to experience adverse discharge. Although the adjusted mortality rate associated with AKI decreased over that decade, the attributable risk proportion remained stable.
Few studies have addressed this significant public health concern. In one recent study in Australia, Kimmel and colleagues16 identified risk factors for AKI but lacked data on AKI outcomes. In a study of complications and mortality occurring after orthopedic surgery, Belmont and colleagues22 categorized complications as either local or systemic but did not examine renal complications. Only 2 other major studies have been conducted on renal outcomes associated with major joint surgery, and both were limited to patients with acute hip fractures. The first included acute fracture surgery patients and omitted elective joint surgery patients, and it evaluated admission renal function but not postoperative AKI.22 The second study had a sample size of only 170 patients.23 Thus, the literature leaves us with a crucial knowledge gap in renal outcomes and their postoperative impact in elective arthroplasties.
The present study filled this information gap by examining the incidence, risk factors, outcomes, and temporal trends of AKI after elective hip and knee arthroplasties. The increasing incidence of AKI in this surgical setting is similar to that of AKI in other surgical settings (cardiac and noncardiac).21 Although our analysis was limited by lack of perioperative management data, patients undergoing elective joint arthroplasty can experience kidney dysfunction for several reasons, including volume depletion, postoperative sepsis, and influence of medications, such as nonsteroidal anti-inflammatory drugs (NSAIDs), especially in older patients with more comorbidities and a higher burden of CKD. Each of these factors can cause renal dysfunction in patients having orthopedic procedures.24 Moreover, NSAID use among elective joint arthroplasty patients is likely higher because of an emphasis on multimodal analgesia, as recent randomized controlled trials have demonstrated the efficacy of NSAID use in controlling pain without increasing bleeding.25-27 Our results also demonstrated that the absolute incidence of AKI after orthopedic surgery is relatively low. One possible explanation for this phenomenon is that the definitions used were based on ICD-9-CM codes that underestimate the true incidence of AKI.
Consistent with other studies, we found that certain key preoperative comorbid conditions and postoperative events were associated with higher AKI risk. We stratified the rate of AKI associated with each postoperative event (sepsis, acute MI, cardiac catheterization, need for transfusion) by DM/CKD comorbidity. CKD was associated with significantly higher AKI risk across all postoperative complications. This information may provide clinicians with bedside information that can be used to determine which patients may be at higher or lower risk for AKI.
Our analysis of patient outcomes revealed that, though AKI was relatively uncommon, it increased the risk for death during hospitalization more than 10-fold between 2002 and 2012. Although the adjusted OR of in-hospital mortality decreased over the decade studied, the concurrent increase in AKI incidence caused the attributable risk of death associated with AKI to essentially remain the same. This observation is consistent with recent reports from cardiac surgery settings.21 These data together suggest that ameliorating occurrences of AKI would decrease mortality and increase quality of care for patients undergoing elective joint surgeries.
We also examined the effect of AKI on resource use by studying LOS, costs, and risk for adverse discharge. Much as in other surgical settings, AKI increased both LOS and overall hospitalization costs. More important, AKI was associated with increased adverse discharge (discharge to long-term care or nursing homes). Although exact reasons are unclear, we can speculate that postoperative renal dysfunction precludes early rehabilitation, impeding desired functional outcome and disposition.28,29 Given the projected increases in primary and revision hip and knee arthroplasties,5 these data predict that the impact of AKI on health outcomes will increase alarmingly in coming years.
There are limitations to our study. First, it was based on administrative data and lacked patient-level and laboratory data. As reported, the sensitivity of AKI codes remains moderate,30 so the true burden may be higher than indicated here. As the definition of AKI was based on administrative coding, we also could not estimate severity, though previous studies have found that administrative codes typically capture a more severe form of disease.31 Another limitation is that, because the data were deidentified, we could not delineate the risk for recurrent AKI in repeated surgical procedures, though this cohort unlikely was large enough to qualitatively affect our results. The third limitation is that, though we used CCI to adjust for the comorbidity burden, we were unable to account for other unmeasured confounders associated with increased AKI incidence, such as specific medication use. In addition, given the lack of patient-level data, we could not analyze the specific factors responsible for AKI in the perioperative period. Nevertheless, the strengths of a nationally representative sample, such as large sample size and generalizability, outweigh these limitations.
Conclusion
AKI is potentially an important quality indicator of elective joint surgery, and reducing its incidence is therefore essential for quality improvement. Given that hip and knee arthroplasties are projected to increase exponentially, as is the burden of comorbid conditions in this population, postoperative AKI will continue to have an incremental impact on health and health care resources. Thus, a carefully planned approach of interdisciplinary perioperative care is warranted to reduce both the risk and the consequences of this devastating condition.
1. Reginster JY. The prevalence and burden of arthritis. Rheumatology. 2002;41(supp 1):3-6.
2. Kullenberg B, Runesson R, Tuvhag R, Olsson C, Resch S. Intraarticular corticosteroid injection: pain relief in osteoarthritis of the hip? J Rheumatol. 2004;31(11):2265-2268.
3. Kawasaki M, Hasegawa Y, Sakano S, Torii Y, Warashina H. Quality of life after several treatments for osteoarthritis of the hip. J Orthop Sci. 2003;8(1):32-35.
4. Ethgen O, Bruyère O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
5. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
6. Matlock D, Earnest M, Epstein A. Utilization of elective hip and knee arthroplasty by age and payer. Clin Orthop Relat Res. 2008;466(4):914-919.
7. Parvizi J, Holiday AD, Ereth MH, Lewallen DG. The Frank Stinchfield Award. Sudden death during primary hip arthroplasty. Clin Orthop Relat Res. 1999;(369):39-48.
8. Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89(1):27-32.
9. Parvizi J, Sullivan TA, Trousdale RT, Lewallen DG. Thirty-day mortality after total knee arthroplasty. J Bone Joint Surg Am. 2001;83(8):1157-1161.
10. Uchino S, Kellum JA, Bellomo R, et al; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813-818.
11. Thakar CV. Perioperative acute kidney injury. Adv Chronic Kidney Dis. 2013;20(1):67-75.
12. Hsu CY, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, Go AS. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4(5):891-898.
13. Rewa O, Bagshaw SM. Acute kidney injury—epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10(4):193-207.
14. Thakar CV, Worley S, Arrigain S, Yared JP, Paganini EP. Influence of renal dysfunction on mortality after cardiac surgery: modifying effect of preoperative renal function. Kidney Int. 2005;67(3):1112-1119.
15. Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9(1):12-20.
16. Kimmel LA, Wilson S, Janardan JD, Liew SM, Walker RG. Incidence of acute kidney injury following total joint arthroplasty: a retrospective review by RIFLE criteria. Clin Kidney J. 2014;7(6):546-551.
17. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) databases, 2002–2012. Rockville, MD: Agency for Healthcare Research and Quality.
18. Bjorgul K, Novicoff WM, Saleh KJ. Evaluating comorbidities in total hip and knee arthroplasty: available instruments. J Orthop Traumatol. 2010;11(4):203-209.
19. Voskuijl T, Hageman M, Ring D. Higher Charlson Comorbidity Index Scores are associated with readmission after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(5):1638-1644.
20. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365-3370.
21. Lenihan CR, Montez-Rath ME, Mora Mangano CT, Chertow GM, Winkelmayer WC. Trends in acute kidney injury, associated use of dialysis, and mortality after cardiac surgery, 1999 to 2008. Ann Thorac Surg. 2013;95(1):20-28.
22. Belmont PJ Jr, Goodman GP, Waterman BR, Bader JO, Schoenfeld AJ. Thirty-day postoperative complications and mortality following total knee arthroplasty: incidence and risk factors among a national sample of 15,321 patients. J Bone Joint Surg Am. 2014;96(1):20-26.
23. Bennet SJ, Berry OM, Goddard J, Keating JF. Acute renal dysfunction following hip fracture. Injury. 2010;41(4):335-338.
24. Kateros K, Doulgerakis C, Galanakos SP, Sakellariou VI, Papadakis SA, Macheras GA. Analysis of kidney dysfunction in orthopaedic patients. BMC Nephrol. 2012;13:101.
25. Huang YM, Wang CM, Wang CT, Lin WP, Horng LC, Jiang CC. Perioperative celecoxib administration for pain management after total knee arthroplasty—a randomized, controlled study. BMC Musculoskelet Disord. 2008;9:77.
26. Kelley TC, Adams MJ, Mulliken BD, Dalury DF. Efficacy of multimodal perioperative analgesia protocol with periarticular medication injection in total knee arthroplasty: a randomized, double-blinded study. J Arthroplasty. 2013;28(8):1274-1277.
27. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329-334.
28. Munin MC, Rudy TE, Glynn NW, Crossett LS, Rubash HE. Early inpatient rehabilitation after elective hip and knee arthroplasty. JAMA. 1998;279(11):847-852.
29. Pua YH, Ong PH. Association of early ambulation with length of stay and costs in total knee arthroplasty: retrospective cohort study. Am J Phys Med Rehabil. 2014;93(11):962-970.
30. Waikar SS, Wald R, Chertow GM, et al. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification codes for acute renal failure. J Am Soc Nephrol. 2006;17(6):1688-1694.
31. Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol. 2014;9(4):682-689.
1. Reginster JY. The prevalence and burden of arthritis. Rheumatology. 2002;41(supp 1):3-6.
2. Kullenberg B, Runesson R, Tuvhag R, Olsson C, Resch S. Intraarticular corticosteroid injection: pain relief in osteoarthritis of the hip? J Rheumatol. 2004;31(11):2265-2268.
3. Kawasaki M, Hasegawa Y, Sakano S, Torii Y, Warashina H. Quality of life after several treatments for osteoarthritis of the hip. J Orthop Sci. 2003;8(1):32-35.
4. Ethgen O, Bruyère O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
5. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
6. Matlock D, Earnest M, Epstein A. Utilization of elective hip and knee arthroplasty by age and payer. Clin Orthop Relat Res. 2008;466(4):914-919.
7. Parvizi J, Holiday AD, Ereth MH, Lewallen DG. The Frank Stinchfield Award. Sudden death during primary hip arthroplasty. Clin Orthop Relat Res. 1999;(369):39-48.
8. Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89(1):27-32.
9. Parvizi J, Sullivan TA, Trousdale RT, Lewallen DG. Thirty-day mortality after total knee arthroplasty. J Bone Joint Surg Am. 2001;83(8):1157-1161.
10. Uchino S, Kellum JA, Bellomo R, et al; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813-818.
11. Thakar CV. Perioperative acute kidney injury. Adv Chronic Kidney Dis. 2013;20(1):67-75.
12. Hsu CY, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, Go AS. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4(5):891-898.
13. Rewa O, Bagshaw SM. Acute kidney injury—epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10(4):193-207.
14. Thakar CV, Worley S, Arrigain S, Yared JP, Paganini EP. Influence of renal dysfunction on mortality after cardiac surgery: modifying effect of preoperative renal function. Kidney Int. 2005;67(3):1112-1119.
15. Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9(1):12-20.
16. Kimmel LA, Wilson S, Janardan JD, Liew SM, Walker RG. Incidence of acute kidney injury following total joint arthroplasty: a retrospective review by RIFLE criteria. Clin Kidney J. 2014;7(6):546-551.
17. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) databases, 2002–2012. Rockville, MD: Agency for Healthcare Research and Quality.
18. Bjorgul K, Novicoff WM, Saleh KJ. Evaluating comorbidities in total hip and knee arthroplasty: available instruments. J Orthop Traumatol. 2010;11(4):203-209.
19. Voskuijl T, Hageman M, Ring D. Higher Charlson Comorbidity Index Scores are associated with readmission after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(5):1638-1644.
20. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365-3370.
21. Lenihan CR, Montez-Rath ME, Mora Mangano CT, Chertow GM, Winkelmayer WC. Trends in acute kidney injury, associated use of dialysis, and mortality after cardiac surgery, 1999 to 2008. Ann Thorac Surg. 2013;95(1):20-28.
22. Belmont PJ Jr, Goodman GP, Waterman BR, Bader JO, Schoenfeld AJ. Thirty-day postoperative complications and mortality following total knee arthroplasty: incidence and risk factors among a national sample of 15,321 patients. J Bone Joint Surg Am. 2014;96(1):20-26.
23. Bennet SJ, Berry OM, Goddard J, Keating JF. Acute renal dysfunction following hip fracture. Injury. 2010;41(4):335-338.
24. Kateros K, Doulgerakis C, Galanakos SP, Sakellariou VI, Papadakis SA, Macheras GA. Analysis of kidney dysfunction in orthopaedic patients. BMC Nephrol. 2012;13:101.
25. Huang YM, Wang CM, Wang CT, Lin WP, Horng LC, Jiang CC. Perioperative celecoxib administration for pain management after total knee arthroplasty—a randomized, controlled study. BMC Musculoskelet Disord. 2008;9:77.
26. Kelley TC, Adams MJ, Mulliken BD, Dalury DF. Efficacy of multimodal perioperative analgesia protocol with periarticular medication injection in total knee arthroplasty: a randomized, double-blinded study. J Arthroplasty. 2013;28(8):1274-1277.
27. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329-334.
28. Munin MC, Rudy TE, Glynn NW, Crossett LS, Rubash HE. Early inpatient rehabilitation after elective hip and knee arthroplasty. JAMA. 1998;279(11):847-852.
29. Pua YH, Ong PH. Association of early ambulation with length of stay and costs in total knee arthroplasty: retrospective cohort study. Am J Phys Med Rehabil. 2014;93(11):962-970.
30. Waikar SS, Wald R, Chertow GM, et al. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification codes for acute renal failure. J Am Soc Nephrol. 2006;17(6):1688-1694.
31. Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol. 2014;9(4):682-689.
Analysis of Direct Costs of Outpatient Arthroscopic Rotator Cuff Repair
Musculoskeletal disorders, the leading cause of disability in the United States,1 account for more than half of all persons reporting missing a workday because of a medical condition.2 Shoulder disorders in particular play a significant role in the burden of musculoskeletal disorders and cost of care. In 2008, 18.9 million adults (8.2% of the US adult population) reported chronic shoulder pain.1 Among shoulder disorders, rotator cuff pathology is the most common cause of shoulder-related disability found by orthopedic surgeons.3 Rotator cuff surgery (RCS) is one of the most commonly performed orthopedic surgical procedures, and surgery volume is on the rise. One study found a 141% increase in rotator cuff repairs between the years 1996 (~41 per 100,000 population) and 2006 (~98 per 100,000 population).4
US health care costs are also increasing. In 2011, $2.7 trillion was spent on health care, representing 17.9% of the national gross domestic product (GDP). According to projections, costs will rise to $4.6 trillion by 2020.5 In particular, as patients continue to live longer and remain more active into their later years, the costs of treating and managing musculoskeletal disorders become more important from a public policy standpoint. In 2006, the cost of treating musculoskeletal disorders alone was $576 billion, representing 4.5% of that year’s GDP.2
Paramount in this era of rising costs is the idea of maximizing the value of health care dollars. Health care economists Porter and Teisberg6 defined value as patient health outcomes achieved per dollar of cost expended in a care cycle (diagnosis, treatment, ongoing management) for a particular disease or disorder. For proper management of value, outcomes and costs for an entire cycle of care must be determined. From a practical standpoint, this first requires determining the true cost of a care cycle—dollars spent on personnel, equipment, materials, and other resources required to deliver a particular service—rather than the amount charged or reimbursed for providing the service in question.7
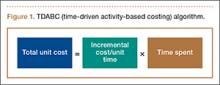
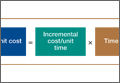
Kaplan and Anderson8,9 described the TDABC (time-driven activity-based costing) algorithm for calculating the cost of delivering a service based on 2 parameters: unit cost of a particular resource, and time required to supply it. These parameters apply to material costs and labor costs. In the medical setting, the TDABC algorithm can be applied by defining a care delivery value chain for each aspect of patient care and then multiplying incremental cost per unit time by time required to deliver that resource (Figure 1). Tabulating the overall unit cost for each resource then yields the overall cost of the care cycle. Clinical outcomes data can then be determined and used to calculate overall value for the patient care cycle.
In the study reported here, we used the TDABC algorithm to calculate the direct financial costs of surgical treatment of rotator cuff tears confirmed by magnetic resonance imaging (MRI) in an academic medical center.
Methods
Per our institution’s Office for the Protection of Research Subjects, institutional review board (IRB) approval is required only for projects using “human subjects” as defined by federal policy. In the present study, no private information could be identified, and all data were obtained from hospital billing records without intervention or interaction with individual patients. Accordingly, IRB approval was deemed unnecessary for our economic cost analysis.
Billing records of a single academic fellowship-trained sports surgeon were reviewed to identify patients who underwent primary repair of an MRI-confirmed rotator cuff tear between April 1, 2009, and July 31, 2012. Patients who had undergone prior shoulder surgery of any type were excluded from the study. Operative reports were reviewed, and exact surgical procedures performed were noted. The operating surgeon selected the specific repair techniques, including single- or double-row repair, with emphasis on restoring footprint coverage and avoiding overtensioning.
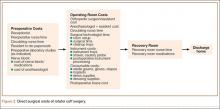
All surgeries were performed in an outpatient surgical center owned and operated by the surgeon’s home university. Surgeries were performed by the attending physician assisted by a senior orthopedic resident. The RCS care cycle was divided into 3 phases (Figure 2):
1. Preoperative. Patient’s interaction with receptionist in surgery center, time with preoperative nurse and circulating nurse in preoperative area, resident check-in time, and time placing preoperative nerve block and consumable materials used during block placement.
2. Operative. Time in operating room with surgical team for RCS, consumable materials used during surgery (eg, anchors, shavers, drapes), anesthetic medications, shoulder abduction pillow placed on completion of surgery, and cost of instrument processing.
3. Postoperative. Time in postoperative recovery area with recovery room nursing staff.
Time in each portion of the care cycle was directly observed and tabulated by hospital volunteers in the surgery center. Institutional billing data were used to identify material resources consumed, and the actual cost paid by the hospital for these resources was obtained from internal records. Mean hourly salary data and standard benefit rates were obtained for surgery center staff. Attending physician salary was extrapolated from published mean market salary data for academic physicians and mean hours worked,10,11 and resident physician costs were tabulated from publically available institutional payroll data and average resident work hours at our institution. These cost data and times were then used to tabulate total cost for the RCS care cycle using the TDABC algorithm.
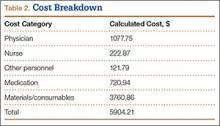
Results
We identified 28 shoulders in 26 patients (mean age, 54.5 years) who met the inclusion criteria. Of these 28 shoulders, 18 (64.3%) had an isolated supraspinatus tear, 8 (28.6%) had combined supraspinatus and infraspinatus tears, 1 (3.6%) had combined supraspinatus and subscapularis tears, and 1 (3.6%) had an isolated infraspinatus tear. Demographic data are listed in Table 1.
All patients received an interscalene nerve block in the preoperative area before being brought into the operating room. In our analysis, we included nerve block supply costs and the anesthesiologist’s mean time placing the nerve block.
In all cases, primary rotator cuff repair was performed with suture anchors (Parcus Medical) with the patient in the lateral decubitus position. In 13 (46%) of the 28 shoulders, this repair was described as “complex,” requiring double-row technique. Subacromial decompression and bursectomy were performed in addition to the rotator cuff repair. Labral débridement was performed in 23 patients, synovectomy in 10, biceps tenodesis with anchor (Smith & Nephew) in 1, and biceps tenotomy in 1. Mean time in operating room was 148 minutes; mean time in postoperative recovery unit was 105 minutes.
Directly observing the care cycle, hospital volunteers found that patients spent a mean of 15 minutes with the receptionist when they arrived in the outpatient surgical center, 25 minutes with nurses for check-in in the preoperative holding area, and 10 minutes with the anesthesiology resident and 15 minutes with the orthopedic surgery resident for preoperative evaluation and paperwork. Mean nerve block time was 20 minutes. Mean electrocardiogram (ECG) time (12 patients) was 15 minutes. The surgical technician spent a mean time of 20 minutes setting up the operating room before the patient was brought in and 15 minutes cleaning up after the patient was transferred to the recovery room. Costs of postoperative care in the recovery room were based on a 2:1 patient-to-nurse ratio, as is the standard practice in our outpatient surgery center.
Using the times mentioned and our hospital’s salary data—including standard hospital benefits rates of 33.5% for nonphysicians and 17.65% for physicians—we determined, using the TDABC algorithm, a direct cost of $5904.21 for this process cycle, excluding hospital overhead and indirect costs. Table 2 provides the overall cost breakdown. Compared with the direct economic cost, the mean hospital charge to insurers for the procedure was $31,459.35. Mean reimbursement from insurers was $9679.08.
Overall attending and resident physician costs were $1077.75, which consisted of $623.66 for the surgeon and $454.09 for the anesthesiologist (included placement of nerve block and administration of anesthesia during surgery). Preoperative bloodwork was obtained in 23 cases, adding a mean cost of $111.04 after adjusting for standard hospital markup. Preoperative ECG was performed in 12 cases, for an added mean cost of $7.30 based on the TDABC algorithm.
We also broke down costs by care cycle phase. The preoperative phase, excluding the preoperative laboratory studies and ECGs (not performed in all cases), cost $134.34 (2.3% of total costs); the operative phase cost $5718.01 (96.8% of total costs); and the postoperative phase cost $51.86 (0.9% of total costs). Within the operative phase, the cost of consumables (specifically, suture anchors) was the main cost driver. Mean anchor cost per case was $3432.67. “Complex” tears involving a double-row repair averaged $4570.25 in anchor cost per patient, as compared with $2522.60 in anchor costs for simple repairs.
Discussion
US health care costs continue to increase unsustainably, with rising pressure on hospitals and providers to deliver the highest value for each health care dollar. The present study is the first to calculate (using the TDABC algorithm) the direct economic cost ($5904.21) of the entire RCS care cycle at a university-based outpatient surgery center. Rent, utility costs, administrative costs, overhead, and other indirect costs at the surgery center were not included in this cost analysis, as they would be incurred irrespective of type of surgery performed. As such, our data isolate the procedure-specific costs of rotator cuff repair in order to provide a more meaningful comparison for other institutions, where indirect costs may be different.
In the literature, rigorous economic analysis of shoulder pathology is sparse. Kuye and colleagues12 systematically reviewed economic evaluations in shoulder surgery for the period 1980–2010 and noted more than 50% of the papers were published between 2005 and 2010.12 They also noted the poor quality of these studies and concluded more rigorous economic evaluations are needed to help justify the rising costs of shoulder-related treatments.
Several studies have directly evaluated costs associated with RCS. Cordasco and colleagues13 detailed the success of open rotator cuff repair as an outpatient procedure—noting its 43% cost savings ($4300 for outpatient vs $7500 for inpatient) and high patient satisfaction—using hospital charge data for operating room time, supplies, instruments, and postoperative slings. Churchill and Ghorai14 evaluated costs of mini-open and arthroscopic rotator cuff repairs in a statewide database and estimated the arthroscopic repair cost at $8985, compared with $7841 for the mini-open repair. They used reported hospital charge data, which were not itemized and did not include physician professional fees. Adla and colleagues,15 in a similar analysis of open versus arthroscopic cuff repair, estimated direct material costs of $1609.50 (arthroscopic) and $360.75 (open); these figures were converted from 2005 UK currency using the exchange rate cited in their paper. Salaries of surgeon, anesthesiologist, and other operating room personnel were said to be included in the operating room cost, but the authors’ paper did not include these data.
Two studies directly estimated the costs of arthroscopic rotator cuff repair. Hearnden and Tennent16 calculated the cost of RCS at their UK institution to be £2672, which included cost of operating room consumable materials, medication, and salaries of operating room personnel, including surgeon and anesthesiologist. Using online currency conversion from 2008 exchange rates and adjusting for inflation gave a corresponding US cost of $5449.63.17 Vitale and colleagues18 prospectively calculated costs of arthroscopic rotator cuff repair over a 1-year period using a cost-to-charge ratio from tabulated inpatient charges, procedure charges, and physician fees and payments abstracted from medical records, hospital billing, and administrative databases. Mean total cost for this cycle was $10,605.20, which included several costs (physical therapy, radiologist fees) not included in the present study. These studies, though more comprehensive than prior work, did not capture the entire cycle of surgical care.
Our study was designed to provide initial data on the direct costs of arthroscopic repair of the rotator cuff for the entire process cycle. Our overall cost estimate of $5904.21 differs significantly from prior work—not unexpected given the completely different cost methodology used.
Our study had several limitations. First, it was a single-surgeon evaluation, and a number of operating room variables (eg, use of adjunct instrumentation such as radiofrequency probes, differences in draping preferences) as well as surgeon volume in performing rotator cuff repairs might have substantially affected the reproducibility and generalizability of our data. Similarly, the large number of adjunctive procedures (eg, subacromial decompression, labral débridement) performed in conjunction with the rotator cuff repairs added operative time and therefore increased overall cost. Double-row repairs added operative time and increased the cost of consumable materials as well. Differences in surgeon preference for suture anchors may also be important, as anchors are a major cost driver and can vary significantly between vendors and institutions. Tear-related variables (eg, tear size, tear chronicity, degree of fatty cuff degeneration) were not controlled for and might have significantly affected operative time and associated cost. Resident involvement in the surgical procedure and anesthesia process in an academic setting prolongs surgical time and thus directly impacts costs.
In addition, we used the patient’s time in the operating room as a proxy for actual surgical time, as this was the only reliable and reproducible data point available in our electronic medical record. As such, an unquantifiable amount of surgeon time may have been overallocated to our cost estimate for time spent inducing anesthesia, positioning, helping take the patient off the operating table, and so on. However, as typical surgeon practice is to be involved in these tasks in the operating room, the possible overestimate of surgeon cost is likely minimal.
Our salary data for the TDABC algorithm were based on national averages for work hours and gross income for physicians and on hospital-based wage structure and may not be generalizable to other institutions. There may also be regional differences in work hours and salaries, which in turn would factor into a different per-minute cost for surgeon and anesthesiologist, depending on the exact geographic area where the surgery is performed. Costs may be higher at institutions that use certified nurse anesthetists rather than resident physicians because of the salary differences between these practitioners.
Moreover, the time that patients spend in the holding area—waiting to go into surgery and, after surgery, waiting for their ride home, for their prescriptions to be ready, and so forth—is an important variable to consider from a cost standpoint. However, as this time varied significantly and involved minimal contact with hospital personnel, we excluded its associated costs from our analysis. Similarly, and as already noted, hospital overhead and other indirect costs were excluded from analysis as well.
Conclusion
Using the TDABC algorithm, we found a direct economic cost of $5904.21 for RCS at our academic outpatient surgical center, with anchor cost the main cost driver. Judicious use of consumable resources is a key focus for cost containment in arthroscopic shoulder surgery, particularly with respect to implantable suture anchors. However, in the setting of more complex tears that require multiple anchors in a double-row repair construct, our pilot data may be useful to hospitals and surgery centers negotiating procedural reimbursement for the increased cost of complex repairs. Use of the TDABC algorithm for RCS and other procedures may also help in identifying opportunities to deliver more cost-effective health care.
1. American Academy of Orthopaedic Surgeons. The Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2011.
2. National health expenditure data. Centers for Medicare & Medicare Services website. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/index.html. Updated May 5, 2014. Accessed December 1, 2015.
3. Tashjian RZ. Epidemiology, natural history, and indications for treatment of rotator cuff tears. Clin Sports Med. 2012;31(4):589-604.
4. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
5. Black EM, Higgins LD, Warner JJ. Value-based shoulder surgery: practicing outcomes-driven, cost-conscious care. J Shoulder Elbow Surg. 2013;22(7):1000-1009.
6. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006.
7. Kaplan RS, Porter ME. How to solve the cost crisis in health care. Harv Bus Rev. 2011;89(9):46-52, 54, 56-61 passim.
8. Kaplan RS, Anderson SR. Time-driven activity-based costing. Harv Bus Rev. 2004;82(11):131-138, 150.
9. Kaplan RS, Anderson SR. Time-Driven Activity-Based Costing: A Simpler and More Powerful Path to Higher Profits. Boston, MA: Harvard Business Review Press; 2007.
10. American Academy of Orthopaedic Surgeons. Orthopaedic Practice in the U.S. 2012. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2012.
11. Medical Group Management Association. Physician Compensation and Production Survey: 2012 Report Based on 2011 Data. Englewood, CO: Medical Group Management Association; 2012.
12. Kuye IO, Jain NB, Warner L, Herndon JH, Warner JJ. Economic evaluations in shoulder pathologies: a systematic review of the literature. J Shoulder Elbow Surg. 2012;21(3):367-375.
13. Cordasco FA, McGinley BJ, Charlton T. Rotator cuff repair as an outpatient procedure. J Shoulder Elbow Surg. 2000;9(1):27-30.
14. Churchill RS, Ghorai JK. Total cost and operating room time comparison of rotator cuff repair techniques at low, intermediate, and high volume centers: mini-open versus all-arthroscopic. J Shoulder Elbow Surg. 2010;19(5):716-721.
15. Adla DN, Rowsell M, Pandey R. Cost-effectiveness of open versus arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2010;19(2):258-261.
16. Hearnden A, Tennent D. The cost of shoulder arthroscopy: a comparison with national tariff. Ann R Coll Surg Engl. 2008;90(7):587-591.
17. Xrates currency conversion. http://www.x-rates.com/historical/?from=GBP&amount=1&date=2015-12-03. Accessed December 13, 2015.
18. Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181-187.
Musculoskeletal disorders, the leading cause of disability in the United States,1 account for more than half of all persons reporting missing a workday because of a medical condition.2 Shoulder disorders in particular play a significant role in the burden of musculoskeletal disorders and cost of care. In 2008, 18.9 million adults (8.2% of the US adult population) reported chronic shoulder pain.1 Among shoulder disorders, rotator cuff pathology is the most common cause of shoulder-related disability found by orthopedic surgeons.3 Rotator cuff surgery (RCS) is one of the most commonly performed orthopedic surgical procedures, and surgery volume is on the rise. One study found a 141% increase in rotator cuff repairs between the years 1996 (~41 per 100,000 population) and 2006 (~98 per 100,000 population).4
US health care costs are also increasing. In 2011, $2.7 trillion was spent on health care, representing 17.9% of the national gross domestic product (GDP). According to projections, costs will rise to $4.6 trillion by 2020.5 In particular, as patients continue to live longer and remain more active into their later years, the costs of treating and managing musculoskeletal disorders become more important from a public policy standpoint. In 2006, the cost of treating musculoskeletal disorders alone was $576 billion, representing 4.5% of that year’s GDP.2
Paramount in this era of rising costs is the idea of maximizing the value of health care dollars. Health care economists Porter and Teisberg6 defined value as patient health outcomes achieved per dollar of cost expended in a care cycle (diagnosis, treatment, ongoing management) for a particular disease or disorder. For proper management of value, outcomes and costs for an entire cycle of care must be determined. From a practical standpoint, this first requires determining the true cost of a care cycle—dollars spent on personnel, equipment, materials, and other resources required to deliver a particular service—rather than the amount charged or reimbursed for providing the service in question.7
Kaplan and Anderson8,9 described the TDABC (time-driven activity-based costing) algorithm for calculating the cost of delivering a service based on 2 parameters: unit cost of a particular resource, and time required to supply it. These parameters apply to material costs and labor costs. In the medical setting, the TDABC algorithm can be applied by defining a care delivery value chain for each aspect of patient care and then multiplying incremental cost per unit time by time required to deliver that resource (Figure 1). Tabulating the overall unit cost for each resource then yields the overall cost of the care cycle. Clinical outcomes data can then be determined and used to calculate overall value for the patient care cycle.
In the study reported here, we used the TDABC algorithm to calculate the direct financial costs of surgical treatment of rotator cuff tears confirmed by magnetic resonance imaging (MRI) in an academic medical center.
Methods
Per our institution’s Office for the Protection of Research Subjects, institutional review board (IRB) approval is required only for projects using “human subjects” as defined by federal policy. In the present study, no private information could be identified, and all data were obtained from hospital billing records without intervention or interaction with individual patients. Accordingly, IRB approval was deemed unnecessary for our economic cost analysis.
Billing records of a single academic fellowship-trained sports surgeon were reviewed to identify patients who underwent primary repair of an MRI-confirmed rotator cuff tear between April 1, 2009, and July 31, 2012. Patients who had undergone prior shoulder surgery of any type were excluded from the study. Operative reports were reviewed, and exact surgical procedures performed were noted. The operating surgeon selected the specific repair techniques, including single- or double-row repair, with emphasis on restoring footprint coverage and avoiding overtensioning.
All surgeries were performed in an outpatient surgical center owned and operated by the surgeon’s home university. Surgeries were performed by the attending physician assisted by a senior orthopedic resident. The RCS care cycle was divided into 3 phases (Figure 2):
1. Preoperative. Patient’s interaction with receptionist in surgery center, time with preoperative nurse and circulating nurse in preoperative area, resident check-in time, and time placing preoperative nerve block and consumable materials used during block placement.
2. Operative. Time in operating room with surgical team for RCS, consumable materials used during surgery (eg, anchors, shavers, drapes), anesthetic medications, shoulder abduction pillow placed on completion of surgery, and cost of instrument processing.
3. Postoperative. Time in postoperative recovery area with recovery room nursing staff.
Time in each portion of the care cycle was directly observed and tabulated by hospital volunteers in the surgery center. Institutional billing data were used to identify material resources consumed, and the actual cost paid by the hospital for these resources was obtained from internal records. Mean hourly salary data and standard benefit rates were obtained for surgery center staff. Attending physician salary was extrapolated from published mean market salary data for academic physicians and mean hours worked,10,11 and resident physician costs were tabulated from publically available institutional payroll data and average resident work hours at our institution. These cost data and times were then used to tabulate total cost for the RCS care cycle using the TDABC algorithm.
Results
We identified 28 shoulders in 26 patients (mean age, 54.5 years) who met the inclusion criteria. Of these 28 shoulders, 18 (64.3%) had an isolated supraspinatus tear, 8 (28.6%) had combined supraspinatus and infraspinatus tears, 1 (3.6%) had combined supraspinatus and subscapularis tears, and 1 (3.6%) had an isolated infraspinatus tear. Demographic data are listed in Table 1.
All patients received an interscalene nerve block in the preoperative area before being brought into the operating room. In our analysis, we included nerve block supply costs and the anesthesiologist’s mean time placing the nerve block.
In all cases, primary rotator cuff repair was performed with suture anchors (Parcus Medical) with the patient in the lateral decubitus position. In 13 (46%) of the 28 shoulders, this repair was described as “complex,” requiring double-row technique. Subacromial decompression and bursectomy were performed in addition to the rotator cuff repair. Labral débridement was performed in 23 patients, synovectomy in 10, biceps tenodesis with anchor (Smith & Nephew) in 1, and biceps tenotomy in 1. Mean time in operating room was 148 minutes; mean time in postoperative recovery unit was 105 minutes.
Directly observing the care cycle, hospital volunteers found that patients spent a mean of 15 minutes with the receptionist when they arrived in the outpatient surgical center, 25 minutes with nurses for check-in in the preoperative holding area, and 10 minutes with the anesthesiology resident and 15 minutes with the orthopedic surgery resident for preoperative evaluation and paperwork. Mean nerve block time was 20 minutes. Mean electrocardiogram (ECG) time (12 patients) was 15 minutes. The surgical technician spent a mean time of 20 minutes setting up the operating room before the patient was brought in and 15 minutes cleaning up after the patient was transferred to the recovery room. Costs of postoperative care in the recovery room were based on a 2:1 patient-to-nurse ratio, as is the standard practice in our outpatient surgery center.
Using the times mentioned and our hospital’s salary data—including standard hospital benefits rates of 33.5% for nonphysicians and 17.65% for physicians—we determined, using the TDABC algorithm, a direct cost of $5904.21 for this process cycle, excluding hospital overhead and indirect costs. Table 2 provides the overall cost breakdown. Compared with the direct economic cost, the mean hospital charge to insurers for the procedure was $31,459.35. Mean reimbursement from insurers was $9679.08.
Overall attending and resident physician costs were $1077.75, which consisted of $623.66 for the surgeon and $454.09 for the anesthesiologist (included placement of nerve block and administration of anesthesia during surgery). Preoperative bloodwork was obtained in 23 cases, adding a mean cost of $111.04 after adjusting for standard hospital markup. Preoperative ECG was performed in 12 cases, for an added mean cost of $7.30 based on the TDABC algorithm.
We also broke down costs by care cycle phase. The preoperative phase, excluding the preoperative laboratory studies and ECGs (not performed in all cases), cost $134.34 (2.3% of total costs); the operative phase cost $5718.01 (96.8% of total costs); and the postoperative phase cost $51.86 (0.9% of total costs). Within the operative phase, the cost of consumables (specifically, suture anchors) was the main cost driver. Mean anchor cost per case was $3432.67. “Complex” tears involving a double-row repair averaged $4570.25 in anchor cost per patient, as compared with $2522.60 in anchor costs for simple repairs.
Discussion
US health care costs continue to increase unsustainably, with rising pressure on hospitals and providers to deliver the highest value for each health care dollar. The present study is the first to calculate (using the TDABC algorithm) the direct economic cost ($5904.21) of the entire RCS care cycle at a university-based outpatient surgery center. Rent, utility costs, administrative costs, overhead, and other indirect costs at the surgery center were not included in this cost analysis, as they would be incurred irrespective of type of surgery performed. As such, our data isolate the procedure-specific costs of rotator cuff repair in order to provide a more meaningful comparison for other institutions, where indirect costs may be different.
In the literature, rigorous economic analysis of shoulder pathology is sparse. Kuye and colleagues12 systematically reviewed economic evaluations in shoulder surgery for the period 1980–2010 and noted more than 50% of the papers were published between 2005 and 2010.12 They also noted the poor quality of these studies and concluded more rigorous economic evaluations are needed to help justify the rising costs of shoulder-related treatments.
Several studies have directly evaluated costs associated with RCS. Cordasco and colleagues13 detailed the success of open rotator cuff repair as an outpatient procedure—noting its 43% cost savings ($4300 for outpatient vs $7500 for inpatient) and high patient satisfaction—using hospital charge data for operating room time, supplies, instruments, and postoperative slings. Churchill and Ghorai14 evaluated costs of mini-open and arthroscopic rotator cuff repairs in a statewide database and estimated the arthroscopic repair cost at $8985, compared with $7841 for the mini-open repair. They used reported hospital charge data, which were not itemized and did not include physician professional fees. Adla and colleagues,15 in a similar analysis of open versus arthroscopic cuff repair, estimated direct material costs of $1609.50 (arthroscopic) and $360.75 (open); these figures were converted from 2005 UK currency using the exchange rate cited in their paper. Salaries of surgeon, anesthesiologist, and other operating room personnel were said to be included in the operating room cost, but the authors’ paper did not include these data.
Two studies directly estimated the costs of arthroscopic rotator cuff repair. Hearnden and Tennent16 calculated the cost of RCS at their UK institution to be £2672, which included cost of operating room consumable materials, medication, and salaries of operating room personnel, including surgeon and anesthesiologist. Using online currency conversion from 2008 exchange rates and adjusting for inflation gave a corresponding US cost of $5449.63.17 Vitale and colleagues18 prospectively calculated costs of arthroscopic rotator cuff repair over a 1-year period using a cost-to-charge ratio from tabulated inpatient charges, procedure charges, and physician fees and payments abstracted from medical records, hospital billing, and administrative databases. Mean total cost for this cycle was $10,605.20, which included several costs (physical therapy, radiologist fees) not included in the present study. These studies, though more comprehensive than prior work, did not capture the entire cycle of surgical care.
Our study was designed to provide initial data on the direct costs of arthroscopic repair of the rotator cuff for the entire process cycle. Our overall cost estimate of $5904.21 differs significantly from prior work—not unexpected given the completely different cost methodology used.
Our study had several limitations. First, it was a single-surgeon evaluation, and a number of operating room variables (eg, use of adjunct instrumentation such as radiofrequency probes, differences in draping preferences) as well as surgeon volume in performing rotator cuff repairs might have substantially affected the reproducibility and generalizability of our data. Similarly, the large number of adjunctive procedures (eg, subacromial decompression, labral débridement) performed in conjunction with the rotator cuff repairs added operative time and therefore increased overall cost. Double-row repairs added operative time and increased the cost of consumable materials as well. Differences in surgeon preference for suture anchors may also be important, as anchors are a major cost driver and can vary significantly between vendors and institutions. Tear-related variables (eg, tear size, tear chronicity, degree of fatty cuff degeneration) were not controlled for and might have significantly affected operative time and associated cost. Resident involvement in the surgical procedure and anesthesia process in an academic setting prolongs surgical time and thus directly impacts costs.
In addition, we used the patient’s time in the operating room as a proxy for actual surgical time, as this was the only reliable and reproducible data point available in our electronic medical record. As such, an unquantifiable amount of surgeon time may have been overallocated to our cost estimate for time spent inducing anesthesia, positioning, helping take the patient off the operating table, and so on. However, as typical surgeon practice is to be involved in these tasks in the operating room, the possible overestimate of surgeon cost is likely minimal.
Our salary data for the TDABC algorithm were based on national averages for work hours and gross income for physicians and on hospital-based wage structure and may not be generalizable to other institutions. There may also be regional differences in work hours and salaries, which in turn would factor into a different per-minute cost for surgeon and anesthesiologist, depending on the exact geographic area where the surgery is performed. Costs may be higher at institutions that use certified nurse anesthetists rather than resident physicians because of the salary differences between these practitioners.
Moreover, the time that patients spend in the holding area—waiting to go into surgery and, after surgery, waiting for their ride home, for their prescriptions to be ready, and so forth—is an important variable to consider from a cost standpoint. However, as this time varied significantly and involved minimal contact with hospital personnel, we excluded its associated costs from our analysis. Similarly, and as already noted, hospital overhead and other indirect costs were excluded from analysis as well.
Conclusion
Using the TDABC algorithm, we found a direct economic cost of $5904.21 for RCS at our academic outpatient surgical center, with anchor cost the main cost driver. Judicious use of consumable resources is a key focus for cost containment in arthroscopic shoulder surgery, particularly with respect to implantable suture anchors. However, in the setting of more complex tears that require multiple anchors in a double-row repair construct, our pilot data may be useful to hospitals and surgery centers negotiating procedural reimbursement for the increased cost of complex repairs. Use of the TDABC algorithm for RCS and other procedures may also help in identifying opportunities to deliver more cost-effective health care.
Musculoskeletal disorders, the leading cause of disability in the United States,1 account for more than half of all persons reporting missing a workday because of a medical condition.2 Shoulder disorders in particular play a significant role in the burden of musculoskeletal disorders and cost of care. In 2008, 18.9 million adults (8.2% of the US adult population) reported chronic shoulder pain.1 Among shoulder disorders, rotator cuff pathology is the most common cause of shoulder-related disability found by orthopedic surgeons.3 Rotator cuff surgery (RCS) is one of the most commonly performed orthopedic surgical procedures, and surgery volume is on the rise. One study found a 141% increase in rotator cuff repairs between the years 1996 (~41 per 100,000 population) and 2006 (~98 per 100,000 population).4
US health care costs are also increasing. In 2011, $2.7 trillion was spent on health care, representing 17.9% of the national gross domestic product (GDP). According to projections, costs will rise to $4.6 trillion by 2020.5 In particular, as patients continue to live longer and remain more active into their later years, the costs of treating and managing musculoskeletal disorders become more important from a public policy standpoint. In 2006, the cost of treating musculoskeletal disorders alone was $576 billion, representing 4.5% of that year’s GDP.2
Paramount in this era of rising costs is the idea of maximizing the value of health care dollars. Health care economists Porter and Teisberg6 defined value as patient health outcomes achieved per dollar of cost expended in a care cycle (diagnosis, treatment, ongoing management) for a particular disease or disorder. For proper management of value, outcomes and costs for an entire cycle of care must be determined. From a practical standpoint, this first requires determining the true cost of a care cycle—dollars spent on personnel, equipment, materials, and other resources required to deliver a particular service—rather than the amount charged or reimbursed for providing the service in question.7
Kaplan and Anderson8,9 described the TDABC (time-driven activity-based costing) algorithm for calculating the cost of delivering a service based on 2 parameters: unit cost of a particular resource, and time required to supply it. These parameters apply to material costs and labor costs. In the medical setting, the TDABC algorithm can be applied by defining a care delivery value chain for each aspect of patient care and then multiplying incremental cost per unit time by time required to deliver that resource (Figure 1). Tabulating the overall unit cost for each resource then yields the overall cost of the care cycle. Clinical outcomes data can then be determined and used to calculate overall value for the patient care cycle.
In the study reported here, we used the TDABC algorithm to calculate the direct financial costs of surgical treatment of rotator cuff tears confirmed by magnetic resonance imaging (MRI) in an academic medical center.
Methods
Per our institution’s Office for the Protection of Research Subjects, institutional review board (IRB) approval is required only for projects using “human subjects” as defined by federal policy. In the present study, no private information could be identified, and all data were obtained from hospital billing records without intervention or interaction with individual patients. Accordingly, IRB approval was deemed unnecessary for our economic cost analysis.
Billing records of a single academic fellowship-trained sports surgeon were reviewed to identify patients who underwent primary repair of an MRI-confirmed rotator cuff tear between April 1, 2009, and July 31, 2012. Patients who had undergone prior shoulder surgery of any type were excluded from the study. Operative reports were reviewed, and exact surgical procedures performed were noted. The operating surgeon selected the specific repair techniques, including single- or double-row repair, with emphasis on restoring footprint coverage and avoiding overtensioning.
All surgeries were performed in an outpatient surgical center owned and operated by the surgeon’s home university. Surgeries were performed by the attending physician assisted by a senior orthopedic resident. The RCS care cycle was divided into 3 phases (Figure 2):
1. Preoperative. Patient’s interaction with receptionist in surgery center, time with preoperative nurse and circulating nurse in preoperative area, resident check-in time, and time placing preoperative nerve block and consumable materials used during block placement.
2. Operative. Time in operating room with surgical team for RCS, consumable materials used during surgery (eg, anchors, shavers, drapes), anesthetic medications, shoulder abduction pillow placed on completion of surgery, and cost of instrument processing.
3. Postoperative. Time in postoperative recovery area with recovery room nursing staff.
Time in each portion of the care cycle was directly observed and tabulated by hospital volunteers in the surgery center. Institutional billing data were used to identify material resources consumed, and the actual cost paid by the hospital for these resources was obtained from internal records. Mean hourly salary data and standard benefit rates were obtained for surgery center staff. Attending physician salary was extrapolated from published mean market salary data for academic physicians and mean hours worked,10,11 and resident physician costs were tabulated from publically available institutional payroll data and average resident work hours at our institution. These cost data and times were then used to tabulate total cost for the RCS care cycle using the TDABC algorithm.
Results
We identified 28 shoulders in 26 patients (mean age, 54.5 years) who met the inclusion criteria. Of these 28 shoulders, 18 (64.3%) had an isolated supraspinatus tear, 8 (28.6%) had combined supraspinatus and infraspinatus tears, 1 (3.6%) had combined supraspinatus and subscapularis tears, and 1 (3.6%) had an isolated infraspinatus tear. Demographic data are listed in Table 1.
All patients received an interscalene nerve block in the preoperative area before being brought into the operating room. In our analysis, we included nerve block supply costs and the anesthesiologist’s mean time placing the nerve block.
In all cases, primary rotator cuff repair was performed with suture anchors (Parcus Medical) with the patient in the lateral decubitus position. In 13 (46%) of the 28 shoulders, this repair was described as “complex,” requiring double-row technique. Subacromial decompression and bursectomy were performed in addition to the rotator cuff repair. Labral débridement was performed in 23 patients, synovectomy in 10, biceps tenodesis with anchor (Smith & Nephew) in 1, and biceps tenotomy in 1. Mean time in operating room was 148 minutes; mean time in postoperative recovery unit was 105 minutes.
Directly observing the care cycle, hospital volunteers found that patients spent a mean of 15 minutes with the receptionist when they arrived in the outpatient surgical center, 25 minutes with nurses for check-in in the preoperative holding area, and 10 minutes with the anesthesiology resident and 15 minutes with the orthopedic surgery resident for preoperative evaluation and paperwork. Mean nerve block time was 20 minutes. Mean electrocardiogram (ECG) time (12 patients) was 15 minutes. The surgical technician spent a mean time of 20 minutes setting up the operating room before the patient was brought in and 15 minutes cleaning up after the patient was transferred to the recovery room. Costs of postoperative care in the recovery room were based on a 2:1 patient-to-nurse ratio, as is the standard practice in our outpatient surgery center.
Using the times mentioned and our hospital’s salary data—including standard hospital benefits rates of 33.5% for nonphysicians and 17.65% for physicians—we determined, using the TDABC algorithm, a direct cost of $5904.21 for this process cycle, excluding hospital overhead and indirect costs. Table 2 provides the overall cost breakdown. Compared with the direct economic cost, the mean hospital charge to insurers for the procedure was $31,459.35. Mean reimbursement from insurers was $9679.08.
Overall attending and resident physician costs were $1077.75, which consisted of $623.66 for the surgeon and $454.09 for the anesthesiologist (included placement of nerve block and administration of anesthesia during surgery). Preoperative bloodwork was obtained in 23 cases, adding a mean cost of $111.04 after adjusting for standard hospital markup. Preoperative ECG was performed in 12 cases, for an added mean cost of $7.30 based on the TDABC algorithm.
We also broke down costs by care cycle phase. The preoperative phase, excluding the preoperative laboratory studies and ECGs (not performed in all cases), cost $134.34 (2.3% of total costs); the operative phase cost $5718.01 (96.8% of total costs); and the postoperative phase cost $51.86 (0.9% of total costs). Within the operative phase, the cost of consumables (specifically, suture anchors) was the main cost driver. Mean anchor cost per case was $3432.67. “Complex” tears involving a double-row repair averaged $4570.25 in anchor cost per patient, as compared with $2522.60 in anchor costs for simple repairs.
Discussion
US health care costs continue to increase unsustainably, with rising pressure on hospitals and providers to deliver the highest value for each health care dollar. The present study is the first to calculate (using the TDABC algorithm) the direct economic cost ($5904.21) of the entire RCS care cycle at a university-based outpatient surgery center. Rent, utility costs, administrative costs, overhead, and other indirect costs at the surgery center were not included in this cost analysis, as they would be incurred irrespective of type of surgery performed. As such, our data isolate the procedure-specific costs of rotator cuff repair in order to provide a more meaningful comparison for other institutions, where indirect costs may be different.
In the literature, rigorous economic analysis of shoulder pathology is sparse. Kuye and colleagues12 systematically reviewed economic evaluations in shoulder surgery for the period 1980–2010 and noted more than 50% of the papers were published between 2005 and 2010.12 They also noted the poor quality of these studies and concluded more rigorous economic evaluations are needed to help justify the rising costs of shoulder-related treatments.
Several studies have directly evaluated costs associated with RCS. Cordasco and colleagues13 detailed the success of open rotator cuff repair as an outpatient procedure—noting its 43% cost savings ($4300 for outpatient vs $7500 for inpatient) and high patient satisfaction—using hospital charge data for operating room time, supplies, instruments, and postoperative slings. Churchill and Ghorai14 evaluated costs of mini-open and arthroscopic rotator cuff repairs in a statewide database and estimated the arthroscopic repair cost at $8985, compared with $7841 for the mini-open repair. They used reported hospital charge data, which were not itemized and did not include physician professional fees. Adla and colleagues,15 in a similar analysis of open versus arthroscopic cuff repair, estimated direct material costs of $1609.50 (arthroscopic) and $360.75 (open); these figures were converted from 2005 UK currency using the exchange rate cited in their paper. Salaries of surgeon, anesthesiologist, and other operating room personnel were said to be included in the operating room cost, but the authors’ paper did not include these data.
Two studies directly estimated the costs of arthroscopic rotator cuff repair. Hearnden and Tennent16 calculated the cost of RCS at their UK institution to be £2672, which included cost of operating room consumable materials, medication, and salaries of operating room personnel, including surgeon and anesthesiologist. Using online currency conversion from 2008 exchange rates and adjusting for inflation gave a corresponding US cost of $5449.63.17 Vitale and colleagues18 prospectively calculated costs of arthroscopic rotator cuff repair over a 1-year period using a cost-to-charge ratio from tabulated inpatient charges, procedure charges, and physician fees and payments abstracted from medical records, hospital billing, and administrative databases. Mean total cost for this cycle was $10,605.20, which included several costs (physical therapy, radiologist fees) not included in the present study. These studies, though more comprehensive than prior work, did not capture the entire cycle of surgical care.
Our study was designed to provide initial data on the direct costs of arthroscopic repair of the rotator cuff for the entire process cycle. Our overall cost estimate of $5904.21 differs significantly from prior work—not unexpected given the completely different cost methodology used.
Our study had several limitations. First, it was a single-surgeon evaluation, and a number of operating room variables (eg, use of adjunct instrumentation such as radiofrequency probes, differences in draping preferences) as well as surgeon volume in performing rotator cuff repairs might have substantially affected the reproducibility and generalizability of our data. Similarly, the large number of adjunctive procedures (eg, subacromial decompression, labral débridement) performed in conjunction with the rotator cuff repairs added operative time and therefore increased overall cost. Double-row repairs added operative time and increased the cost of consumable materials as well. Differences in surgeon preference for suture anchors may also be important, as anchors are a major cost driver and can vary significantly between vendors and institutions. Tear-related variables (eg, tear size, tear chronicity, degree of fatty cuff degeneration) were not controlled for and might have significantly affected operative time and associated cost. Resident involvement in the surgical procedure and anesthesia process in an academic setting prolongs surgical time and thus directly impacts costs.
In addition, we used the patient’s time in the operating room as a proxy for actual surgical time, as this was the only reliable and reproducible data point available in our electronic medical record. As such, an unquantifiable amount of surgeon time may have been overallocated to our cost estimate for time spent inducing anesthesia, positioning, helping take the patient off the operating table, and so on. However, as typical surgeon practice is to be involved in these tasks in the operating room, the possible overestimate of surgeon cost is likely minimal.
Our salary data for the TDABC algorithm were based on national averages for work hours and gross income for physicians and on hospital-based wage structure and may not be generalizable to other institutions. There may also be regional differences in work hours and salaries, which in turn would factor into a different per-minute cost for surgeon and anesthesiologist, depending on the exact geographic area where the surgery is performed. Costs may be higher at institutions that use certified nurse anesthetists rather than resident physicians because of the salary differences between these practitioners.
Moreover, the time that patients spend in the holding area—waiting to go into surgery and, after surgery, waiting for their ride home, for their prescriptions to be ready, and so forth—is an important variable to consider from a cost standpoint. However, as this time varied significantly and involved minimal contact with hospital personnel, we excluded its associated costs from our analysis. Similarly, and as already noted, hospital overhead and other indirect costs were excluded from analysis as well.
Conclusion
Using the TDABC algorithm, we found a direct economic cost of $5904.21 for RCS at our academic outpatient surgical center, with anchor cost the main cost driver. Judicious use of consumable resources is a key focus for cost containment in arthroscopic shoulder surgery, particularly with respect to implantable suture anchors. However, in the setting of more complex tears that require multiple anchors in a double-row repair construct, our pilot data may be useful to hospitals and surgery centers negotiating procedural reimbursement for the increased cost of complex repairs. Use of the TDABC algorithm for RCS and other procedures may also help in identifying opportunities to deliver more cost-effective health care.
1. American Academy of Orthopaedic Surgeons. The Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2011.
2. National health expenditure data. Centers for Medicare & Medicare Services website. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/index.html. Updated May 5, 2014. Accessed December 1, 2015.
3. Tashjian RZ. Epidemiology, natural history, and indications for treatment of rotator cuff tears. Clin Sports Med. 2012;31(4):589-604.
4. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
5. Black EM, Higgins LD, Warner JJ. Value-based shoulder surgery: practicing outcomes-driven, cost-conscious care. J Shoulder Elbow Surg. 2013;22(7):1000-1009.
6. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006.
7. Kaplan RS, Porter ME. How to solve the cost crisis in health care. Harv Bus Rev. 2011;89(9):46-52, 54, 56-61 passim.
8. Kaplan RS, Anderson SR. Time-driven activity-based costing. Harv Bus Rev. 2004;82(11):131-138, 150.
9. Kaplan RS, Anderson SR. Time-Driven Activity-Based Costing: A Simpler and More Powerful Path to Higher Profits. Boston, MA: Harvard Business Review Press; 2007.
10. American Academy of Orthopaedic Surgeons. Orthopaedic Practice in the U.S. 2012. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2012.
11. Medical Group Management Association. Physician Compensation and Production Survey: 2012 Report Based on 2011 Data. Englewood, CO: Medical Group Management Association; 2012.
12. Kuye IO, Jain NB, Warner L, Herndon JH, Warner JJ. Economic evaluations in shoulder pathologies: a systematic review of the literature. J Shoulder Elbow Surg. 2012;21(3):367-375.
13. Cordasco FA, McGinley BJ, Charlton T. Rotator cuff repair as an outpatient procedure. J Shoulder Elbow Surg. 2000;9(1):27-30.
14. Churchill RS, Ghorai JK. Total cost and operating room time comparison of rotator cuff repair techniques at low, intermediate, and high volume centers: mini-open versus all-arthroscopic. J Shoulder Elbow Surg. 2010;19(5):716-721.
15. Adla DN, Rowsell M, Pandey R. Cost-effectiveness of open versus arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2010;19(2):258-261.
16. Hearnden A, Tennent D. The cost of shoulder arthroscopy: a comparison with national tariff. Ann R Coll Surg Engl. 2008;90(7):587-591.
17. Xrates currency conversion. http://www.x-rates.com/historical/?from=GBP&amount=1&date=2015-12-03. Accessed December 13, 2015.
18. Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181-187.
1. American Academy of Orthopaedic Surgeons. The Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2011.
2. National health expenditure data. Centers for Medicare & Medicare Services website. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/index.html. Updated May 5, 2014. Accessed December 1, 2015.
3. Tashjian RZ. Epidemiology, natural history, and indications for treatment of rotator cuff tears. Clin Sports Med. 2012;31(4):589-604.
4. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
5. Black EM, Higgins LD, Warner JJ. Value-based shoulder surgery: practicing outcomes-driven, cost-conscious care. J Shoulder Elbow Surg. 2013;22(7):1000-1009.
6. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006.
7. Kaplan RS, Porter ME. How to solve the cost crisis in health care. Harv Bus Rev. 2011;89(9):46-52, 54, 56-61 passim.
8. Kaplan RS, Anderson SR. Time-driven activity-based costing. Harv Bus Rev. 2004;82(11):131-138, 150.
9. Kaplan RS, Anderson SR. Time-Driven Activity-Based Costing: A Simpler and More Powerful Path to Higher Profits. Boston, MA: Harvard Business Review Press; 2007.
10. American Academy of Orthopaedic Surgeons. Orthopaedic Practice in the U.S. 2012. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2012.
11. Medical Group Management Association. Physician Compensation and Production Survey: 2012 Report Based on 2011 Data. Englewood, CO: Medical Group Management Association; 2012.
12. Kuye IO, Jain NB, Warner L, Herndon JH, Warner JJ. Economic evaluations in shoulder pathologies: a systematic review of the literature. J Shoulder Elbow Surg. 2012;21(3):367-375.
13. Cordasco FA, McGinley BJ, Charlton T. Rotator cuff repair as an outpatient procedure. J Shoulder Elbow Surg. 2000;9(1):27-30.
14. Churchill RS, Ghorai JK. Total cost and operating room time comparison of rotator cuff repair techniques at low, intermediate, and high volume centers: mini-open versus all-arthroscopic. J Shoulder Elbow Surg. 2010;19(5):716-721.
15. Adla DN, Rowsell M, Pandey R. Cost-effectiveness of open versus arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2010;19(2):258-261.
16. Hearnden A, Tennent D. The cost of shoulder arthroscopy: a comparison with national tariff. Ann R Coll Surg Engl. 2008;90(7):587-591.
17. Xrates currency conversion. http://www.x-rates.com/historical/?from=GBP&amount=1&date=2015-12-03. Accessed December 13, 2015.
18. Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181-187.
The Right Choice? Kindness and Surgical Ethics: Reflections on a Friend and Mentor
As I sit down to write this column, I reflect on the news that my mentor and friend, Norman W. Thompson, M.D, FACS, passed away yesterday. I had the good fortune to spend 1 year as an endocrine surgery fellow with Dr. Thompson at the University of Michigan in 1995-96. That year was certainly the most significant of my training in terms of defining my professional life as an endocrine surgeon. However, as I think back on my time with Dr. Thompson, I am struck by how much more I learned from him than how to take out a thyroid or a parathyroid or manage multiple endocrine neoplasia.
Dr. Thompson was an excellent technical surgeon, and he would have had a tremendous career helping thousands of patients if that was all that he had done. However, he was much more than an excellent technician. He was also a great doctor. In order for a surgeon to be a great doctor, it is necessary to be technically excellent, but that alone is not sufficient. I believe that what makes a surgeon a great doctor is the combination of technical mastery with outstanding interpersonal skills and ethically sound clinical judgment. Dr. Thompson had all of that, and he was exceptionally kind.
Kindness is not a word that we commonly use in describing surgeons today. In an era of surgeons being pressured to see more patients and generate more RVUs [relative value units], it is unusual to hear kindness mentioned as an essential attribute of a great surgeon. However, Dr. Thompson’s kindness was immediately apparent to all who spent time with him. He treated each patient as a unique individual. In addition, he treated his trainees and his colleagues in Ann Arbor and around the world with respect and incredible humility. He was generous with his time and was always approachable no matter how inexperienced the surgeon asking him a question. Dr. Thompson was kind to all of us and made us feel that he valued spending time with us.
What does kindness have to do with a column that traditionally focuses on ethical issues in the practice of surgery? Although acting with kindness is not the same as acting in an ethical manner, I believe that there is more overlap of the terms than we often imagine. The kind surgeon is the one who treats people – whether they are patients or colleagues – as though they matter. The ethical surgeon respects the patient’s wishes and acts to benefit the patient as much as possible in all circumstances. I am certain that I have met ethical surgeons who were not kind, but I have met very few kind surgeons who are not ethical.
As someone who has spent significant time and energy in the last 19 years as a surgery faculty member trying to teach ethics, I am also struck by a clear truth. Actions always speak louder than words. It may be valuable to talk about the ethical principles that may come to play in a particularly difficult surgical case. Defining the competing interests and assessing the patient’s wishes are important components of the ethical practice of surgery. However, no amount of discussion of these issues can substitute for the value of behavior. Treating patients and colleagues with kindness and respect is modeling the behaviors of an ethical surgeon – perhaps learned from a wise and thoughtful mentor.
Dr. Thompson was an excellent role model for me and so many others in how he treated patients and everyone around him. As I see patients and perform surgery, I still hear myself saying many of the same things that he said many years ago. His genuine expressions of optimism before difficult operations, honesty in communicating, and sadness when things did not go well were tremendous examples to me of how a great doctor treats those around him. These lessons that I learned from Dr. Thompson have influenced my practice significantly, and I am grateful for the opportunity to try to model them on a daily basis.
Although I remain convinced that formal curricula in ethics and professionalism remain important in the education of today’s surgeons, it is valuable to remember the impact that the behaviors of those we respect have on us. Perhaps we surgeons more than other physicians are molded by the people who train us, but there is no question that the ethical behaviors of our teachers and mentors will have a greater impact than any lecture or manuscript. I want to acknowledge and commemorate the kindness and ethical behaviors that Dr. Thompson modeled daily for all who were fortunate enough to work with him.
Dr. Angelos is an ACS Fellow; the Linda Kohler Anderson Professor of Surgery and Surgical Ethics; chief, endocrine surgery; and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
As I sit down to write this column, I reflect on the news that my mentor and friend, Norman W. Thompson, M.D, FACS, passed away yesterday. I had the good fortune to spend 1 year as an endocrine surgery fellow with Dr. Thompson at the University of Michigan in 1995-96. That year was certainly the most significant of my training in terms of defining my professional life as an endocrine surgeon. However, as I think back on my time with Dr. Thompson, I am struck by how much more I learned from him than how to take out a thyroid or a parathyroid or manage multiple endocrine neoplasia.
Dr. Thompson was an excellent technical surgeon, and he would have had a tremendous career helping thousands of patients if that was all that he had done. However, he was much more than an excellent technician. He was also a great doctor. In order for a surgeon to be a great doctor, it is necessary to be technically excellent, but that alone is not sufficient. I believe that what makes a surgeon a great doctor is the combination of technical mastery with outstanding interpersonal skills and ethically sound clinical judgment. Dr. Thompson had all of that, and he was exceptionally kind.
Kindness is not a word that we commonly use in describing surgeons today. In an era of surgeons being pressured to see more patients and generate more RVUs [relative value units], it is unusual to hear kindness mentioned as an essential attribute of a great surgeon. However, Dr. Thompson’s kindness was immediately apparent to all who spent time with him. He treated each patient as a unique individual. In addition, he treated his trainees and his colleagues in Ann Arbor and around the world with respect and incredible humility. He was generous with his time and was always approachable no matter how inexperienced the surgeon asking him a question. Dr. Thompson was kind to all of us and made us feel that he valued spending time with us.
What does kindness have to do with a column that traditionally focuses on ethical issues in the practice of surgery? Although acting with kindness is not the same as acting in an ethical manner, I believe that there is more overlap of the terms than we often imagine. The kind surgeon is the one who treats people – whether they are patients or colleagues – as though they matter. The ethical surgeon respects the patient’s wishes and acts to benefit the patient as much as possible in all circumstances. I am certain that I have met ethical surgeons who were not kind, but I have met very few kind surgeons who are not ethical.
As someone who has spent significant time and energy in the last 19 years as a surgery faculty member trying to teach ethics, I am also struck by a clear truth. Actions always speak louder than words. It may be valuable to talk about the ethical principles that may come to play in a particularly difficult surgical case. Defining the competing interests and assessing the patient’s wishes are important components of the ethical practice of surgery. However, no amount of discussion of these issues can substitute for the value of behavior. Treating patients and colleagues with kindness and respect is modeling the behaviors of an ethical surgeon – perhaps learned from a wise and thoughtful mentor.
Dr. Thompson was an excellent role model for me and so many others in how he treated patients and everyone around him. As I see patients and perform surgery, I still hear myself saying many of the same things that he said many years ago. His genuine expressions of optimism before difficult operations, honesty in communicating, and sadness when things did not go well were tremendous examples to me of how a great doctor treats those around him. These lessons that I learned from Dr. Thompson have influenced my practice significantly, and I am grateful for the opportunity to try to model them on a daily basis.
Although I remain convinced that formal curricula in ethics and professionalism remain important in the education of today’s surgeons, it is valuable to remember the impact that the behaviors of those we respect have on us. Perhaps we surgeons more than other physicians are molded by the people who train us, but there is no question that the ethical behaviors of our teachers and mentors will have a greater impact than any lecture or manuscript. I want to acknowledge and commemorate the kindness and ethical behaviors that Dr. Thompson modeled daily for all who were fortunate enough to work with him.
Dr. Angelos is an ACS Fellow; the Linda Kohler Anderson Professor of Surgery and Surgical Ethics; chief, endocrine surgery; and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
As I sit down to write this column, I reflect on the news that my mentor and friend, Norman W. Thompson, M.D, FACS, passed away yesterday. I had the good fortune to spend 1 year as an endocrine surgery fellow with Dr. Thompson at the University of Michigan in 1995-96. That year was certainly the most significant of my training in terms of defining my professional life as an endocrine surgeon. However, as I think back on my time with Dr. Thompson, I am struck by how much more I learned from him than how to take out a thyroid or a parathyroid or manage multiple endocrine neoplasia.
Dr. Thompson was an excellent technical surgeon, and he would have had a tremendous career helping thousands of patients if that was all that he had done. However, he was much more than an excellent technician. He was also a great doctor. In order for a surgeon to be a great doctor, it is necessary to be technically excellent, but that alone is not sufficient. I believe that what makes a surgeon a great doctor is the combination of technical mastery with outstanding interpersonal skills and ethically sound clinical judgment. Dr. Thompson had all of that, and he was exceptionally kind.
Kindness is not a word that we commonly use in describing surgeons today. In an era of surgeons being pressured to see more patients and generate more RVUs [relative value units], it is unusual to hear kindness mentioned as an essential attribute of a great surgeon. However, Dr. Thompson’s kindness was immediately apparent to all who spent time with him. He treated each patient as a unique individual. In addition, he treated his trainees and his colleagues in Ann Arbor and around the world with respect and incredible humility. He was generous with his time and was always approachable no matter how inexperienced the surgeon asking him a question. Dr. Thompson was kind to all of us and made us feel that he valued spending time with us.
What does kindness have to do with a column that traditionally focuses on ethical issues in the practice of surgery? Although acting with kindness is not the same as acting in an ethical manner, I believe that there is more overlap of the terms than we often imagine. The kind surgeon is the one who treats people – whether they are patients or colleagues – as though they matter. The ethical surgeon respects the patient’s wishes and acts to benefit the patient as much as possible in all circumstances. I am certain that I have met ethical surgeons who were not kind, but I have met very few kind surgeons who are not ethical.
As someone who has spent significant time and energy in the last 19 years as a surgery faculty member trying to teach ethics, I am also struck by a clear truth. Actions always speak louder than words. It may be valuable to talk about the ethical principles that may come to play in a particularly difficult surgical case. Defining the competing interests and assessing the patient’s wishes are important components of the ethical practice of surgery. However, no amount of discussion of these issues can substitute for the value of behavior. Treating patients and colleagues with kindness and respect is modeling the behaviors of an ethical surgeon – perhaps learned from a wise and thoughtful mentor.
Dr. Thompson was an excellent role model for me and so many others in how he treated patients and everyone around him. As I see patients and perform surgery, I still hear myself saying many of the same things that he said many years ago. His genuine expressions of optimism before difficult operations, honesty in communicating, and sadness when things did not go well were tremendous examples to me of how a great doctor treats those around him. These lessons that I learned from Dr. Thompson have influenced my practice significantly, and I am grateful for the opportunity to try to model them on a daily basis.
Although I remain convinced that formal curricula in ethics and professionalism remain important in the education of today’s surgeons, it is valuable to remember the impact that the behaviors of those we respect have on us. Perhaps we surgeons more than other physicians are molded by the people who train us, but there is no question that the ethical behaviors of our teachers and mentors will have a greater impact than any lecture or manuscript. I want to acknowledge and commemorate the kindness and ethical behaviors that Dr. Thompson modeled daily for all who were fortunate enough to work with him.
Dr. Angelos is an ACS Fellow; the Linda Kohler Anderson Professor of Surgery and Surgical Ethics; chief, endocrine surgery; and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
T-VEC: Advancing the Fight Against Melanoma

Following a phase III, open-label trial conducted by Andtbacka et al (J Clin Oncol. 2015;33:2780-2788), the US Food and Drug Administration recently approved the first oncolytic immunotherapy talimogene laherparepvec (T-VEC) for the treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with advanced melanoma (stage IIIB/C–stage IV) following initial surgery.
A group of 436 patients with injectable melanomas (melanomas that are accessible via a percutaneous injection) that were not surgically resectable were randomly assigned (2:1) to treatment with intralesional T-VEC or subcutaneous granulocyte macrophage colony-stimulating factor (GM-CSF). The primary endpoint of the study was durable response rate (DRR), defined as objective response lasting continuously for 6 months or longer. Secondary endpoints included overall survival (OS) and overall response rate.
Talimogene laherparepvec was shown to extend DRRs compared to GM-CSF. Durable response rates were significantly higher with T-VEC (16.3%; 95% confidence interval [CI], 12.1%–20.5%) versus GM-CSF (2.1%; 95% CI, 0%–4.5%)(odds ratio, 8.9; P<.001).
In the OS analysis, a 4.4-month extension with T-VEC was observed; however, this was not deemed to be statistically significant (P=.051). The median OS was 23.3 months (95% CI, 19.5–29.6 months) with T-VEC and 18.9 months (95% CI, 16.0–23.7 months) with GM-CSF (hazard ratio, 0.79; 95% CI, 0.62–1.00; P=.051). Overall response rate also was higher in the T-VEC arm (26.4%; 95% CI, 21.4%–31.5%) versus GM-CSF (5.7%; 95% CI, 1.9%–9.5%).
Talimogene laherparepvec is a herpes simplex virus type 1–derived oncolytic immunotherapy designed to replicate within tumors and produce GM-CSF, which enhances systemic antitumor immune responses and induces tumor lysis.
In this study, T-VEC efficacy was greatest in patients with stage IIIB, IIIC, or IVM1a melanomas and in patients with treatment-naive disease. Differences in DRRs in patients with stage IIIB/C melanomas were 33% in the T-VEC group versus 0% for patients treated with GM-CSF alone. In the stage IVM1a group, DRR was 16% with T-VEC versus 2% with GM-CSF. The difference between both treatments was smaller in more advanced melanomas (IVM1b group, 3% vs 4%; IVM1c, 7% vs 3%). In the first-line treatment, the DRR with T-VEC was 24% versus 0% with GM-CSF. In the second-line and beyond, the DRR with T-VEC was 10% compared to 4% for GM-CSF.
The main adverse events seen in this study were fatigue, chills, and pyrexia. Serious adverse events occurred in 25.7% and 13.4% of participants in the T-VEC and GM-CSF arms, respectively, with disease progression (3.1% vs 1.6%) and cellulitis (2.4% vs 0.8%) being the most common. Six immune-mediated events occurred in the T-VEC group compared to 3 in the GM-CSF group.
Twelve patient deaths occurred within 30 days of the last dose of T-VEC; 9 were associated with progressive disease and the other 3 were associated with myocardial infarction, cardiac arrest, and sepsis, respectively. Four patient deaths were reported in the GM-CSF arm within the same 30 days.
What’s the Issue?
Immunotherapy represents a promising treatment option for metastatic melanoma. These promising results along with the US Food and Drug Administration’s approval of T-VEC will lead to further studies of the uses of T-VEC in combination with other therapies, including a phase I/II study to assess T-VEC in combination with ipilimumab for unresected melanomas (NCT01740297) and a phase III study of T-VEC in combination with pembrolizumab for unresected melanomas (NCT02263508). It is important for dermatologists to be familiar with the new frontier of melanoma treatments. How will these new immunotherapies affect your treatment of melanoma?

Following a phase III, open-label trial conducted by Andtbacka et al (J Clin Oncol. 2015;33:2780-2788), the US Food and Drug Administration recently approved the first oncolytic immunotherapy talimogene laherparepvec (T-VEC) for the treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with advanced melanoma (stage IIIB/C–stage IV) following initial surgery.
A group of 436 patients with injectable melanomas (melanomas that are accessible via a percutaneous injection) that were not surgically resectable were randomly assigned (2:1) to treatment with intralesional T-VEC or subcutaneous granulocyte macrophage colony-stimulating factor (GM-CSF). The primary endpoint of the study was durable response rate (DRR), defined as objective response lasting continuously for 6 months or longer. Secondary endpoints included overall survival (OS) and overall response rate.
Talimogene laherparepvec was shown to extend DRRs compared to GM-CSF. Durable response rates were significantly higher with T-VEC (16.3%; 95% confidence interval [CI], 12.1%–20.5%) versus GM-CSF (2.1%; 95% CI, 0%–4.5%)(odds ratio, 8.9; P<.001).
In the OS analysis, a 4.4-month extension with T-VEC was observed; however, this was not deemed to be statistically significant (P=.051). The median OS was 23.3 months (95% CI, 19.5–29.6 months) with T-VEC and 18.9 months (95% CI, 16.0–23.7 months) with GM-CSF (hazard ratio, 0.79; 95% CI, 0.62–1.00; P=.051). Overall response rate also was higher in the T-VEC arm (26.4%; 95% CI, 21.4%–31.5%) versus GM-CSF (5.7%; 95% CI, 1.9%–9.5%).
Talimogene laherparepvec is a herpes simplex virus type 1–derived oncolytic immunotherapy designed to replicate within tumors and produce GM-CSF, which enhances systemic antitumor immune responses and induces tumor lysis.
In this study, T-VEC efficacy was greatest in patients with stage IIIB, IIIC, or IVM1a melanomas and in patients with treatment-naive disease. Differences in DRRs in patients with stage IIIB/C melanomas were 33% in the T-VEC group versus 0% for patients treated with GM-CSF alone. In the stage IVM1a group, DRR was 16% with T-VEC versus 2% with GM-CSF. The difference between both treatments was smaller in more advanced melanomas (IVM1b group, 3% vs 4%; IVM1c, 7% vs 3%). In the first-line treatment, the DRR with T-VEC was 24% versus 0% with GM-CSF. In the second-line and beyond, the DRR with T-VEC was 10% compared to 4% for GM-CSF.
The main adverse events seen in this study were fatigue, chills, and pyrexia. Serious adverse events occurred in 25.7% and 13.4% of participants in the T-VEC and GM-CSF arms, respectively, with disease progression (3.1% vs 1.6%) and cellulitis (2.4% vs 0.8%) being the most common. Six immune-mediated events occurred in the T-VEC group compared to 3 in the GM-CSF group.
Twelve patient deaths occurred within 30 days of the last dose of T-VEC; 9 were associated with progressive disease and the other 3 were associated with myocardial infarction, cardiac arrest, and sepsis, respectively. Four patient deaths were reported in the GM-CSF arm within the same 30 days.
What’s the Issue?
Immunotherapy represents a promising treatment option for metastatic melanoma. These promising results along with the US Food and Drug Administration’s approval of T-VEC will lead to further studies of the uses of T-VEC in combination with other therapies, including a phase I/II study to assess T-VEC in combination with ipilimumab for unresected melanomas (NCT01740297) and a phase III study of T-VEC in combination with pembrolizumab for unresected melanomas (NCT02263508). It is important for dermatologists to be familiar with the new frontier of melanoma treatments. How will these new immunotherapies affect your treatment of melanoma?

Following a phase III, open-label trial conducted by Andtbacka et al (J Clin Oncol. 2015;33:2780-2788), the US Food and Drug Administration recently approved the first oncolytic immunotherapy talimogene laherparepvec (T-VEC) for the treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with advanced melanoma (stage IIIB/C–stage IV) following initial surgery.
A group of 436 patients with injectable melanomas (melanomas that are accessible via a percutaneous injection) that were not surgically resectable were randomly assigned (2:1) to treatment with intralesional T-VEC or subcutaneous granulocyte macrophage colony-stimulating factor (GM-CSF). The primary endpoint of the study was durable response rate (DRR), defined as objective response lasting continuously for 6 months or longer. Secondary endpoints included overall survival (OS) and overall response rate.
Talimogene laherparepvec was shown to extend DRRs compared to GM-CSF. Durable response rates were significantly higher with T-VEC (16.3%; 95% confidence interval [CI], 12.1%–20.5%) versus GM-CSF (2.1%; 95% CI, 0%–4.5%)(odds ratio, 8.9; P<.001).
In the OS analysis, a 4.4-month extension with T-VEC was observed; however, this was not deemed to be statistically significant (P=.051). The median OS was 23.3 months (95% CI, 19.5–29.6 months) with T-VEC and 18.9 months (95% CI, 16.0–23.7 months) with GM-CSF (hazard ratio, 0.79; 95% CI, 0.62–1.00; P=.051). Overall response rate also was higher in the T-VEC arm (26.4%; 95% CI, 21.4%–31.5%) versus GM-CSF (5.7%; 95% CI, 1.9%–9.5%).
Talimogene laherparepvec is a herpes simplex virus type 1–derived oncolytic immunotherapy designed to replicate within tumors and produce GM-CSF, which enhances systemic antitumor immune responses and induces tumor lysis.
In this study, T-VEC efficacy was greatest in patients with stage IIIB, IIIC, or IVM1a melanomas and in patients with treatment-naive disease. Differences in DRRs in patients with stage IIIB/C melanomas were 33% in the T-VEC group versus 0% for patients treated with GM-CSF alone. In the stage IVM1a group, DRR was 16% with T-VEC versus 2% with GM-CSF. The difference between both treatments was smaller in more advanced melanomas (IVM1b group, 3% vs 4%; IVM1c, 7% vs 3%). In the first-line treatment, the DRR with T-VEC was 24% versus 0% with GM-CSF. In the second-line and beyond, the DRR with T-VEC was 10% compared to 4% for GM-CSF.
The main adverse events seen in this study were fatigue, chills, and pyrexia. Serious adverse events occurred in 25.7% and 13.4% of participants in the T-VEC and GM-CSF arms, respectively, with disease progression (3.1% vs 1.6%) and cellulitis (2.4% vs 0.8%) being the most common. Six immune-mediated events occurred in the T-VEC group compared to 3 in the GM-CSF group.
Twelve patient deaths occurred within 30 days of the last dose of T-VEC; 9 were associated with progressive disease and the other 3 were associated with myocardial infarction, cardiac arrest, and sepsis, respectively. Four patient deaths were reported in the GM-CSF arm within the same 30 days.
What’s the Issue?
Immunotherapy represents a promising treatment option for metastatic melanoma. These promising results along with the US Food and Drug Administration’s approval of T-VEC will lead to further studies of the uses of T-VEC in combination with other therapies, including a phase I/II study to assess T-VEC in combination with ipilimumab for unresected melanomas (NCT01740297) and a phase III study of T-VEC in combination with pembrolizumab for unresected melanomas (NCT02263508). It is important for dermatologists to be familiar with the new frontier of melanoma treatments. How will these new immunotherapies affect your treatment of melanoma?
Video etiquette
FaceTime with my mother would be better described as ForeheadTime. She loves to use video for our Sunday calls, yet when she does, she always talks into her iPhone as if it’s a speakerphone. As a result, all I see is the top of her head. “Mom. Lower the phone. Mom, I can’t see you,” I must repeat weekly.
Video provides a richer experience compared with telephone. It allows for a deeper, emotional connection. That’s why moms like mine prefer it to telephone conversations. In medicine, video visits are uncommon, but that’s changing as payers are now reimbursing and patients are demanding the service. For many, they offer a far more convenient and still effective method to receive medical care. Psychiatry is an obvious example. Less obvious, but still effective examples, include endocrinology, pediatrics, primary care, surgery (post operatively), and dermatology.
Like the example with my mom, quality of the experience matters, and issues often arise not from the technology, but from the technique. Making eye contact is more difficult on video, and not looking patients in the eye can harm doctor-patient bonding. Here are a few basic tips when using video with your patients:
• Be sure the light source is in front of you. Having windows behind you often puts you in shadow.
• The best place for the camera is at the top of your screen. It’s nearly impossible to look into the camera and see the patient if the camera is next to the screen instead of above.
• Remember, to look directly at the patient, you have to look into the camera. This is tricky and easy to forget.
• Be sure your entire head and upper torso are in the frame. Talking heads can be intimidating.
• When possible, use a headset with a microphone. Headsets help both you and your patient hear better and give the patient an increased sense of privacy.
• Generally speaking, video visits take as long or longer than in-person visits. Remember to be patient as some of your patients may experience technical difficulties. Our IT colleagues have a word for it: “picnic,” which stands for “Problem In Chair Not In Computer.” You should also train your staff to aid you and the patients. For instance, if a patient is struggling with the computer, you might have your assistant help him or her while you move on to the next patient.
• Although the patient can be home, it is best for you to be in your office. It’s possible to do video consults from home, but it is more difficult because you have to ensure that both your technology and your environment are secure and private. Otherwise, you risk violating HIPAA or other compliance requirements.
• Be sure to get the appropriate consent before conducting a virtual visit. In California, it requires only verbal consent, but your state’s requirements might be different.
• As for your appearance, there’s a reason why Kennedy won the Kennedy-Nixon debates. Video does reveal details that you might not want emphasized. A two-day beard might appear hip in person but unkempt and uncaring online. Bold stripes or checks on your shirt sometimes appear distorted, so opt for solids in soft shades. Scrubs are okay, but be sure to check your neckline, particularly as you move about. Whether it’s clothing or accessories, avoid anything overly distracting.
Video visits have had a long, slow ramp-up, but they seem to be gaining momentum. You may not use them in your practice now, but it’s likely we all will someday. Soon.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego, and a volunteer clinical assistant professor at the University of California, San Diego. He is @dermdoc on Twitter.
FaceTime with my mother would be better described as ForeheadTime. She loves to use video for our Sunday calls, yet when she does, she always talks into her iPhone as if it’s a speakerphone. As a result, all I see is the top of her head. “Mom. Lower the phone. Mom, I can’t see you,” I must repeat weekly.
Video provides a richer experience compared with telephone. It allows for a deeper, emotional connection. That’s why moms like mine prefer it to telephone conversations. In medicine, video visits are uncommon, but that’s changing as payers are now reimbursing and patients are demanding the service. For many, they offer a far more convenient and still effective method to receive medical care. Psychiatry is an obvious example. Less obvious, but still effective examples, include endocrinology, pediatrics, primary care, surgery (post operatively), and dermatology.
Like the example with my mom, quality of the experience matters, and issues often arise not from the technology, but from the technique. Making eye contact is more difficult on video, and not looking patients in the eye can harm doctor-patient bonding. Here are a few basic tips when using video with your patients:
• Be sure the light source is in front of you. Having windows behind you often puts you in shadow.
• The best place for the camera is at the top of your screen. It’s nearly impossible to look into the camera and see the patient if the camera is next to the screen instead of above.
• Remember, to look directly at the patient, you have to look into the camera. This is tricky and easy to forget.
• Be sure your entire head and upper torso are in the frame. Talking heads can be intimidating.
• When possible, use a headset with a microphone. Headsets help both you and your patient hear better and give the patient an increased sense of privacy.
• Generally speaking, video visits take as long or longer than in-person visits. Remember to be patient as some of your patients may experience technical difficulties. Our IT colleagues have a word for it: “picnic,” which stands for “Problem In Chair Not In Computer.” You should also train your staff to aid you and the patients. For instance, if a patient is struggling with the computer, you might have your assistant help him or her while you move on to the next patient.
• Although the patient can be home, it is best for you to be in your office. It’s possible to do video consults from home, but it is more difficult because you have to ensure that both your technology and your environment are secure and private. Otherwise, you risk violating HIPAA or other compliance requirements.
• Be sure to get the appropriate consent before conducting a virtual visit. In California, it requires only verbal consent, but your state’s requirements might be different.
• As for your appearance, there’s a reason why Kennedy won the Kennedy-Nixon debates. Video does reveal details that you might not want emphasized. A two-day beard might appear hip in person but unkempt and uncaring online. Bold stripes or checks on your shirt sometimes appear distorted, so opt for solids in soft shades. Scrubs are okay, but be sure to check your neckline, particularly as you move about. Whether it’s clothing or accessories, avoid anything overly distracting.
Video visits have had a long, slow ramp-up, but they seem to be gaining momentum. You may not use them in your practice now, but it’s likely we all will someday. Soon.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego, and a volunteer clinical assistant professor at the University of California, San Diego. He is @dermdoc on Twitter.
FaceTime with my mother would be better described as ForeheadTime. She loves to use video for our Sunday calls, yet when she does, she always talks into her iPhone as if it’s a speakerphone. As a result, all I see is the top of her head. “Mom. Lower the phone. Mom, I can’t see you,” I must repeat weekly.
Video provides a richer experience compared with telephone. It allows for a deeper, emotional connection. That’s why moms like mine prefer it to telephone conversations. In medicine, video visits are uncommon, but that’s changing as payers are now reimbursing and patients are demanding the service. For many, they offer a far more convenient and still effective method to receive medical care. Psychiatry is an obvious example. Less obvious, but still effective examples, include endocrinology, pediatrics, primary care, surgery (post operatively), and dermatology.
Like the example with my mom, quality of the experience matters, and issues often arise not from the technology, but from the technique. Making eye contact is more difficult on video, and not looking patients in the eye can harm doctor-patient bonding. Here are a few basic tips when using video with your patients:
• Be sure the light source is in front of you. Having windows behind you often puts you in shadow.
• The best place for the camera is at the top of your screen. It’s nearly impossible to look into the camera and see the patient if the camera is next to the screen instead of above.
• Remember, to look directly at the patient, you have to look into the camera. This is tricky and easy to forget.
• Be sure your entire head and upper torso are in the frame. Talking heads can be intimidating.
• When possible, use a headset with a microphone. Headsets help both you and your patient hear better and give the patient an increased sense of privacy.
• Generally speaking, video visits take as long or longer than in-person visits. Remember to be patient as some of your patients may experience technical difficulties. Our IT colleagues have a word for it: “picnic,” which stands for “Problem In Chair Not In Computer.” You should also train your staff to aid you and the patients. For instance, if a patient is struggling with the computer, you might have your assistant help him or her while you move on to the next patient.
• Although the patient can be home, it is best for you to be in your office. It’s possible to do video consults from home, but it is more difficult because you have to ensure that both your technology and your environment are secure and private. Otherwise, you risk violating HIPAA or other compliance requirements.
• Be sure to get the appropriate consent before conducting a virtual visit. In California, it requires only verbal consent, but your state’s requirements might be different.
• As for your appearance, there’s a reason why Kennedy won the Kennedy-Nixon debates. Video does reveal details that you might not want emphasized. A two-day beard might appear hip in person but unkempt and uncaring online. Bold stripes or checks on your shirt sometimes appear distorted, so opt for solids in soft shades. Scrubs are okay, but be sure to check your neckline, particularly as you move about. Whether it’s clothing or accessories, avoid anything overly distracting.
Video visits have had a long, slow ramp-up, but they seem to be gaining momentum. You may not use them in your practice now, but it’s likely we all will someday. Soon.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego, and a volunteer clinical assistant professor at the University of California, San Diego. He is @dermdoc on Twitter.
Neurosurgery at the End of Life
The juxtaposition between my first 2 days of neurosurgery could not have been more profound. On my first day as a third-year medical student, the attending and chief resident let me take the lead on the first case: a straightforward brain biopsy. I got to make the incision, drill the burr hole, and perform the needle biopsy. I still remember the thrill of the technical challenge, the controlled violence of drilling into the skull, and the finesse of accessing the tumor core.
The buzz was so strong that I barely registered the diagnosis that was called back from the pathologist: glioblastoma. It was not until I saw the face of the disease the next morning that I understood the reality of a GBM diagnosis. That face belonged to a 47-year-old man who hadn’t slept all night, wide eyed with apprehension at what news I might bring. He beseeched me with questions, and though his aphasia left him stammering to get the words out, I knew exactly what he was asking: Would he live or die? It was a question I was in no position to answer. Instead, I reassured him that we were waiting on the final pathology, all the while trying to forget the fact that the frozen section suggested an aggressive subtype, surely heralding a poor prognosis.
In his poignant memoir, “Do No Harm: Stories of Life, Death and Brain Surgery” (New York: Thomas Dunne Book, 2015), Dr. Henry Marsh writes beautifully about how difficult it can be to find the balance between optimism and realism. In one memorable passage, Dr. Marsh shows a house officer a scan of a highly malignant brain tumor and asks him what he would say to the patient. The trainee reflexively hides behind jargon, skirting around what he knew to be the truth: This tumor would kill her. Marsh presses him to admit that he’s lying, before lamenting at how hard it is to improve these critical communication skills: “When I have had to break bad news I never know whether I have done it well or not. The patients aren’t going to ring me up afterward and say, ‘Mr. Marsh, I really liked the way you told me that I was going to die,’ or ‘Mr. Marsh, you were crap.’ You can only hope that you haven’t made too much of a mess of it.”
I could certainly relate to Dr. Marsh’s house officer as I walked away from my own patient. I felt almost deceitful withholding diagnostic information from him, even if I did the “right” thing. It made me wonder, why did I want to become a neurosurgeon? Surely to help people through some of the most difficult moments of their lives. But is it possible to be a source of comfort when you are required so often to be a harbinger of death? The answer depends on whether one can envision a role for the neurosurgeon beyond the mandate of “life at all costs.”
While the field has become known for its life-saving procedures, neurosurgeons are called just as often to preside over the end of their patient’s lives – work that requires just as much skill as any technical procedure. Dr. Marsh recognized the tremendous human cost of neglecting that work. For cases that appear “hopeless,” he writes, “We often end up operating because it’s easier than being honest, and it means that we can avoid a painful conversation.”
We are only beginning to understand the many issues that neurosurgical patients face at the end of life, but so far it is clear that neurosurgical trainees require substantive training in prognostication, communication, and palliation (Crit Care Med. 2015 Sep;43[9]:1964-77 1,2; J Neurooncol. 2009 Jan;91[1]:39-43). Is there room in the current training paradigm for more formal education in these domains? As we move further into the 21st century, we must embrace the need for masterful clinicians outside of the operating room if we are to ever challenge the axiom set forth by the renowned French surgeon, René Leriche, some 65 years ago: “Every surgeon carries within himself a small cemetery, where from time to time he goes to pray – a place of bitterness and regret, where he must look for an explanation for his failures.” Let us look forward to the day when this is no longer the case.
Stephen Miranda is a medical student from the University of Rochester, who is now working as a research fellow at Ariadne Labs, a joint center for health systems innovation at Brigham & Women’s Hospital and Harvard T.H. Chan School of Public Health, both in Boston.
The juxtaposition between my first 2 days of neurosurgery could not have been more profound. On my first day as a third-year medical student, the attending and chief resident let me take the lead on the first case: a straightforward brain biopsy. I got to make the incision, drill the burr hole, and perform the needle biopsy. I still remember the thrill of the technical challenge, the controlled violence of drilling into the skull, and the finesse of accessing the tumor core.
The buzz was so strong that I barely registered the diagnosis that was called back from the pathologist: glioblastoma. It was not until I saw the face of the disease the next morning that I understood the reality of a GBM diagnosis. That face belonged to a 47-year-old man who hadn’t slept all night, wide eyed with apprehension at what news I might bring. He beseeched me with questions, and though his aphasia left him stammering to get the words out, I knew exactly what he was asking: Would he live or die? It was a question I was in no position to answer. Instead, I reassured him that we were waiting on the final pathology, all the while trying to forget the fact that the frozen section suggested an aggressive subtype, surely heralding a poor prognosis.
In his poignant memoir, “Do No Harm: Stories of Life, Death and Brain Surgery” (New York: Thomas Dunne Book, 2015), Dr. Henry Marsh writes beautifully about how difficult it can be to find the balance between optimism and realism. In one memorable passage, Dr. Marsh shows a house officer a scan of a highly malignant brain tumor and asks him what he would say to the patient. The trainee reflexively hides behind jargon, skirting around what he knew to be the truth: This tumor would kill her. Marsh presses him to admit that he’s lying, before lamenting at how hard it is to improve these critical communication skills: “When I have had to break bad news I never know whether I have done it well or not. The patients aren’t going to ring me up afterward and say, ‘Mr. Marsh, I really liked the way you told me that I was going to die,’ or ‘Mr. Marsh, you were crap.’ You can only hope that you haven’t made too much of a mess of it.”
I could certainly relate to Dr. Marsh’s house officer as I walked away from my own patient. I felt almost deceitful withholding diagnostic information from him, even if I did the “right” thing. It made me wonder, why did I want to become a neurosurgeon? Surely to help people through some of the most difficult moments of their lives. But is it possible to be a source of comfort when you are required so often to be a harbinger of death? The answer depends on whether one can envision a role for the neurosurgeon beyond the mandate of “life at all costs.”
While the field has become known for its life-saving procedures, neurosurgeons are called just as often to preside over the end of their patient’s lives – work that requires just as much skill as any technical procedure. Dr. Marsh recognized the tremendous human cost of neglecting that work. For cases that appear “hopeless,” he writes, “We often end up operating because it’s easier than being honest, and it means that we can avoid a painful conversation.”
We are only beginning to understand the many issues that neurosurgical patients face at the end of life, but so far it is clear that neurosurgical trainees require substantive training in prognostication, communication, and palliation (Crit Care Med. 2015 Sep;43[9]:1964-77 1,2; J Neurooncol. 2009 Jan;91[1]:39-43). Is there room in the current training paradigm for more formal education in these domains? As we move further into the 21st century, we must embrace the need for masterful clinicians outside of the operating room if we are to ever challenge the axiom set forth by the renowned French surgeon, René Leriche, some 65 years ago: “Every surgeon carries within himself a small cemetery, where from time to time he goes to pray – a place of bitterness and regret, where he must look for an explanation for his failures.” Let us look forward to the day when this is no longer the case.
Stephen Miranda is a medical student from the University of Rochester, who is now working as a research fellow at Ariadne Labs, a joint center for health systems innovation at Brigham & Women’s Hospital and Harvard T.H. Chan School of Public Health, both in Boston.
The juxtaposition between my first 2 days of neurosurgery could not have been more profound. On my first day as a third-year medical student, the attending and chief resident let me take the lead on the first case: a straightforward brain biopsy. I got to make the incision, drill the burr hole, and perform the needle biopsy. I still remember the thrill of the technical challenge, the controlled violence of drilling into the skull, and the finesse of accessing the tumor core.
The buzz was so strong that I barely registered the diagnosis that was called back from the pathologist: glioblastoma. It was not until I saw the face of the disease the next morning that I understood the reality of a GBM diagnosis. That face belonged to a 47-year-old man who hadn’t slept all night, wide eyed with apprehension at what news I might bring. He beseeched me with questions, and though his aphasia left him stammering to get the words out, I knew exactly what he was asking: Would he live or die? It was a question I was in no position to answer. Instead, I reassured him that we were waiting on the final pathology, all the while trying to forget the fact that the frozen section suggested an aggressive subtype, surely heralding a poor prognosis.
In his poignant memoir, “Do No Harm: Stories of Life, Death and Brain Surgery” (New York: Thomas Dunne Book, 2015), Dr. Henry Marsh writes beautifully about how difficult it can be to find the balance between optimism and realism. In one memorable passage, Dr. Marsh shows a house officer a scan of a highly malignant brain tumor and asks him what he would say to the patient. The trainee reflexively hides behind jargon, skirting around what he knew to be the truth: This tumor would kill her. Marsh presses him to admit that he’s lying, before lamenting at how hard it is to improve these critical communication skills: “When I have had to break bad news I never know whether I have done it well or not. The patients aren’t going to ring me up afterward and say, ‘Mr. Marsh, I really liked the way you told me that I was going to die,’ or ‘Mr. Marsh, you were crap.’ You can only hope that you haven’t made too much of a mess of it.”
I could certainly relate to Dr. Marsh’s house officer as I walked away from my own patient. I felt almost deceitful withholding diagnostic information from him, even if I did the “right” thing. It made me wonder, why did I want to become a neurosurgeon? Surely to help people through some of the most difficult moments of their lives. But is it possible to be a source of comfort when you are required so often to be a harbinger of death? The answer depends on whether one can envision a role for the neurosurgeon beyond the mandate of “life at all costs.”
While the field has become known for its life-saving procedures, neurosurgeons are called just as often to preside over the end of their patient’s lives – work that requires just as much skill as any technical procedure. Dr. Marsh recognized the tremendous human cost of neglecting that work. For cases that appear “hopeless,” he writes, “We often end up operating because it’s easier than being honest, and it means that we can avoid a painful conversation.”
We are only beginning to understand the many issues that neurosurgical patients face at the end of life, but so far it is clear that neurosurgical trainees require substantive training in prognostication, communication, and palliation (Crit Care Med. 2015 Sep;43[9]:1964-77 1,2; J Neurooncol. 2009 Jan;91[1]:39-43). Is there room in the current training paradigm for more formal education in these domains? As we move further into the 21st century, we must embrace the need for masterful clinicians outside of the operating room if we are to ever challenge the axiom set forth by the renowned French surgeon, René Leriche, some 65 years ago: “Every surgeon carries within himself a small cemetery, where from time to time he goes to pray – a place of bitterness and regret, where he must look for an explanation for his failures.” Let us look forward to the day when this is no longer the case.
Stephen Miranda is a medical student from the University of Rochester, who is now working as a research fellow at Ariadne Labs, a joint center for health systems innovation at Brigham & Women’s Hospital and Harvard T.H. Chan School of Public Health, both in Boston.
Immediate treatment yields best outcomes in NSTEMI MI
Immediate, rather than delayed, angiography reduced by 62% the chance of both recurrent heart attack and death in patients with non–ST-segment myocardial infarction.
The significant advantage of early treatment was apparent at both 30 days and 1 year after myocardial infarction, Dr. Aleksandra Milosevic and colleagues wrote (JACC Cardiovasc Interv. 2016;10.1016/j.jcin.2015.11.018).
RIDDLE-NSTEMI (Randomized Study of Immediate vs. Delayed Invasive Intervention in Patients with Non–ST-Segment Elevation MI) is not the first to examine immediate vs. delayed outcomes in coronary angiography. But it is the first to look at a pure cohort of NSTEMI patients, and the first to use purely clinical, rather than biochemical, outcomes, said Dr. Milosevic of the Clinical Center of Serbia, Belgrade.
The study group comprised 323 patients with a confirmed NSTEMI MI to either immediate or delayed angiography. Those in the immediate treatment group went to catheterization as soon as possible (median, 1.4 hours); those in the delayed-treatment group underwent the procedure within 72 hours of randomization (median, 61 hours).
All patients received a loading dose of dual-antiplatelet therapy. For immediate-treatment patients, this consisted of 300 mg aspirin and 600 mg clopidogrel. Those in the delayed-treatment group received 300 mg aspirin and 300 mg clopidogrel. Everyone then received daily treatment of aspirin 100 mg and clopidogrel 75 mg.
Patients who had already been taking dual-antiplatelet therapy continued their maintenance dose. Low-molecular-weight heparin, nitrates, beta-blockers, and ACE inhibitors were given according to clinical guidelines; glycoprotein IIb/IIIa inhibitors were allowed at the treating physician’s discretion.
The time from symptom onset to randomization was 5 hours in the immediate- and 6.5 hours in the delayed-treatment groups. Manual thrombectomy was more common in the immediate-treatment group (3.9% vs. 1%), as was percutaneous coronary intervention (78% vs. 65%). Coronary artery bypass grafting was more common in the delayed-treatment group (23.8% vs. 12%).
At 30 days, the composite primary endpoint of mortality and new MI was significantly less common in the immediate-treatment group (4.3% vs. 13.0%; hazard ratio, 0.32). Mortality was the same in both groups (5 patients; 3%). The difference was driven by the significantly larger number of nonfatal MIs in the delayed-treatment group (16 vs. 2).
The advantage of early treatment held at 1 year, with significantly lower rates of death and new MI in the immediate-treatment group (16.8% vs. 18.8%). This was entirely driven by early events; there was no significant difference after the first 30 days.
A composite secondary endpoint of death, new MI or recurrent ischemia, was also significantly lower in the immediate-intervention group at 30 days (6.8% vs. 26.7%) and 1 year (15.4% vs. 33%).
A multivariate regression analysis controlled for demographics, past medical history, prior coronary procedures, and electrocardiogram changes. After considering these variables, immediate treatment was associated with a 62% reduction in the chance of a new MI within 30 days (HR, 0.42; P = .052).
There was no between-group difference in major bleeding, which was low both at 30 days and 1 year. One patient in the immediate-treatment group and two in the delayed-treatment group needed a transfusion. One intracranial bleed occurred in an immediately treated patient. Four patients in the delayed treatment group were treated for gastrointestinal bleeding.
The authors had no financial declarations.
Immediate, rather than delayed, angiography reduced by 62% the chance of both recurrent heart attack and death in patients with non–ST-segment myocardial infarction.
The significant advantage of early treatment was apparent at both 30 days and 1 year after myocardial infarction, Dr. Aleksandra Milosevic and colleagues wrote (JACC Cardiovasc Interv. 2016;10.1016/j.jcin.2015.11.018).
RIDDLE-NSTEMI (Randomized Study of Immediate vs. Delayed Invasive Intervention in Patients with Non–ST-Segment Elevation MI) is not the first to examine immediate vs. delayed outcomes in coronary angiography. But it is the first to look at a pure cohort of NSTEMI patients, and the first to use purely clinical, rather than biochemical, outcomes, said Dr. Milosevic of the Clinical Center of Serbia, Belgrade.
The study group comprised 323 patients with a confirmed NSTEMI MI to either immediate or delayed angiography. Those in the immediate treatment group went to catheterization as soon as possible (median, 1.4 hours); those in the delayed-treatment group underwent the procedure within 72 hours of randomization (median, 61 hours).
All patients received a loading dose of dual-antiplatelet therapy. For immediate-treatment patients, this consisted of 300 mg aspirin and 600 mg clopidogrel. Those in the delayed-treatment group received 300 mg aspirin and 300 mg clopidogrel. Everyone then received daily treatment of aspirin 100 mg and clopidogrel 75 mg.
Patients who had already been taking dual-antiplatelet therapy continued their maintenance dose. Low-molecular-weight heparin, nitrates, beta-blockers, and ACE inhibitors were given according to clinical guidelines; glycoprotein IIb/IIIa inhibitors were allowed at the treating physician’s discretion.
The time from symptom onset to randomization was 5 hours in the immediate- and 6.5 hours in the delayed-treatment groups. Manual thrombectomy was more common in the immediate-treatment group (3.9% vs. 1%), as was percutaneous coronary intervention (78% vs. 65%). Coronary artery bypass grafting was more common in the delayed-treatment group (23.8% vs. 12%).
At 30 days, the composite primary endpoint of mortality and new MI was significantly less common in the immediate-treatment group (4.3% vs. 13.0%; hazard ratio, 0.32). Mortality was the same in both groups (5 patients; 3%). The difference was driven by the significantly larger number of nonfatal MIs in the delayed-treatment group (16 vs. 2).
The advantage of early treatment held at 1 year, with significantly lower rates of death and new MI in the immediate-treatment group (16.8% vs. 18.8%). This was entirely driven by early events; there was no significant difference after the first 30 days.
A composite secondary endpoint of death, new MI or recurrent ischemia, was also significantly lower in the immediate-intervention group at 30 days (6.8% vs. 26.7%) and 1 year (15.4% vs. 33%).
A multivariate regression analysis controlled for demographics, past medical history, prior coronary procedures, and electrocardiogram changes. After considering these variables, immediate treatment was associated with a 62% reduction in the chance of a new MI within 30 days (HR, 0.42; P = .052).
There was no between-group difference in major bleeding, which was low both at 30 days and 1 year. One patient in the immediate-treatment group and two in the delayed-treatment group needed a transfusion. One intracranial bleed occurred in an immediately treated patient. Four patients in the delayed treatment group were treated for gastrointestinal bleeding.
The authors had no financial declarations.
Immediate, rather than delayed, angiography reduced by 62% the chance of both recurrent heart attack and death in patients with non–ST-segment myocardial infarction.
The significant advantage of early treatment was apparent at both 30 days and 1 year after myocardial infarction, Dr. Aleksandra Milosevic and colleagues wrote (JACC Cardiovasc Interv. 2016;10.1016/j.jcin.2015.11.018).
RIDDLE-NSTEMI (Randomized Study of Immediate vs. Delayed Invasive Intervention in Patients with Non–ST-Segment Elevation MI) is not the first to examine immediate vs. delayed outcomes in coronary angiography. But it is the first to look at a pure cohort of NSTEMI patients, and the first to use purely clinical, rather than biochemical, outcomes, said Dr. Milosevic of the Clinical Center of Serbia, Belgrade.
The study group comprised 323 patients with a confirmed NSTEMI MI to either immediate or delayed angiography. Those in the immediate treatment group went to catheterization as soon as possible (median, 1.4 hours); those in the delayed-treatment group underwent the procedure within 72 hours of randomization (median, 61 hours).
All patients received a loading dose of dual-antiplatelet therapy. For immediate-treatment patients, this consisted of 300 mg aspirin and 600 mg clopidogrel. Those in the delayed-treatment group received 300 mg aspirin and 300 mg clopidogrel. Everyone then received daily treatment of aspirin 100 mg and clopidogrel 75 mg.
Patients who had already been taking dual-antiplatelet therapy continued their maintenance dose. Low-molecular-weight heparin, nitrates, beta-blockers, and ACE inhibitors were given according to clinical guidelines; glycoprotein IIb/IIIa inhibitors were allowed at the treating physician’s discretion.
The time from symptom onset to randomization was 5 hours in the immediate- and 6.5 hours in the delayed-treatment groups. Manual thrombectomy was more common in the immediate-treatment group (3.9% vs. 1%), as was percutaneous coronary intervention (78% vs. 65%). Coronary artery bypass grafting was more common in the delayed-treatment group (23.8% vs. 12%).
At 30 days, the composite primary endpoint of mortality and new MI was significantly less common in the immediate-treatment group (4.3% vs. 13.0%; hazard ratio, 0.32). Mortality was the same in both groups (5 patients; 3%). The difference was driven by the significantly larger number of nonfatal MIs in the delayed-treatment group (16 vs. 2).
The advantage of early treatment held at 1 year, with significantly lower rates of death and new MI in the immediate-treatment group (16.8% vs. 18.8%). This was entirely driven by early events; there was no significant difference after the first 30 days.
A composite secondary endpoint of death, new MI or recurrent ischemia, was also significantly lower in the immediate-intervention group at 30 days (6.8% vs. 26.7%) and 1 year (15.4% vs. 33%).
A multivariate regression analysis controlled for demographics, past medical history, prior coronary procedures, and electrocardiogram changes. After considering these variables, immediate treatment was associated with a 62% reduction in the chance of a new MI within 30 days (HR, 0.42; P = .052).
There was no between-group difference in major bleeding, which was low both at 30 days and 1 year. One patient in the immediate-treatment group and two in the delayed-treatment group needed a transfusion. One intracranial bleed occurred in an immediately treated patient. Four patients in the delayed treatment group were treated for gastrointestinal bleeding.
The authors had no financial declarations.
FROM JACC CARDIOVASCULAR INTERVENTIONS
Key clinical point: Immediate angiography improved both short- and long-term outcomes in non–ST-segment myocardial infarction.
Major finding: Compared with delayed treatment, an immediate cardiac angiography reduced the risk of recurrent MI and death by more than 60% at both 30 days and 1 year.
Data source: The randomized study comprised 323 patients.
Disclosures: The authors made no financial disclosures.
Nick Fitterman, MD, SFHM, Discusses Population Health and Hospital Medicine's Role
Nick Fitterman, MD, SFHM, vice chair of hospital medicine for the Hofstra North Shore-LIJ School of Medicine in Hempstead, N.Y., and North Shore-Long Island Jewish Health System in New Hyde Park, N.Y., discusses how hospital medicine factors into population health—where is the intersection and what is the hospitalist’s role?
Nick Fitterman, MD, SFHM, vice chair of hospital medicine for the Hofstra North Shore-LIJ School of Medicine in Hempstead, N.Y., and North Shore-Long Island Jewish Health System in New Hyde Park, N.Y., discusses how hospital medicine factors into population health—where is the intersection and what is the hospitalist’s role?
Nick Fitterman, MD, SFHM, vice chair of hospital medicine for the Hofstra North Shore-LIJ School of Medicine in Hempstead, N.Y., and North Shore-Long Island Jewish Health System in New Hyde Park, N.Y., discusses how hospital medicine factors into population health—where is the intersection and what is the hospitalist’s role?