User login
KIR2DL5B genotype predicts outcome in chronic phase–CML
The presence of KIR2DL5B was associated with lower rates of major molecular response (MMR), transformation-free survival, and event-free survival (but not overall survival) in patients with chronic phase–chronic myeloid leukemia (CP-CML) treated with sequential imatinib/nilotinib, according to researchers.
Univariate analysis demonstrated a significant association between KIR2DL5B and achievement of a major molecular response, with hazard ratio 0.423 (95% CI, 0.262-0.682; P less than .001). Other KIR genotypes, KIR2DL2pos and KIR2DS3pos, were also associated with inferior achievement of MMR, probably because of their association with KIR2DL5B due to linkage disequilibrium among KIR genes, according to the investigators.
“Our findings suggest that even with the potent second-generation TKI [tyrosine kinase inhibitor] nilotinib, KIR genotypes, a predetermined genetic host factor, may still be one of the most discriminatory prognostic markers available at baseline,” wrote Dr. David T. Yeung of the department of genetics and molecular pathology, Centre for Cancer Biology and the University of Adelaide, South Australia, and colleagues (Blood 2015 Dec 17. doi:10.1182/blood-2015-07-655589).
Killer immunoglobulin-like receptors (KIRs) contribute to natural killer (NK) cell–mediated killing of tumor cells, in both activating and inhibitory roles. Normal cells are spared through actions of inhibitory KIRs. Although the mechanism underlying the association between KIR2DL5B and CP-CML treatment outcomes is still unclear, the gene encodes an inhibitory KIR receptor, the absence of which may increase efficiency of NK-mediated killing of leukemic stem cells, researchers suggested.
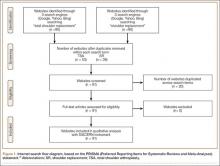
The Therapeutic Intensification in De Novo Leukaemia (TIDEL-II) study included 210 patients with CP-CML who were treated with imatinib initially, and nilotinib subsequently if predetermined molecular targets were not met. The KIR substudy included 148 patients with samples available for genotyping.
KIR genotype frequencies observed in this study were similar to other white populations reported in the Allele Frequency Database.
Early molecular response was also significantly associated with treatment outcomes, independent of KIR prognostic significance, and may add additional prognostic information, available 3 months after treatment commences.
“In contrast, KIR2DL5B can identify, at baseline, the 20% of patients with a transformation risk of [about] 10% over 2 years versus the 80% of patients with a transformation risk of less than 3%,” the authors wrote. They suggest that KIR2DL5B, combined with other predictive markers, may enable targeted early interventions to improve outcomes.
The presence of KIR2DL5B was associated with lower rates of major molecular response (MMR), transformation-free survival, and event-free survival (but not overall survival) in patients with chronic phase–chronic myeloid leukemia (CP-CML) treated with sequential imatinib/nilotinib, according to researchers.
Univariate analysis demonstrated a significant association between KIR2DL5B and achievement of a major molecular response, with hazard ratio 0.423 (95% CI, 0.262-0.682; P less than .001). Other KIR genotypes, KIR2DL2pos and KIR2DS3pos, were also associated with inferior achievement of MMR, probably because of their association with KIR2DL5B due to linkage disequilibrium among KIR genes, according to the investigators.
“Our findings suggest that even with the potent second-generation TKI [tyrosine kinase inhibitor] nilotinib, KIR genotypes, a predetermined genetic host factor, may still be one of the most discriminatory prognostic markers available at baseline,” wrote Dr. David T. Yeung of the department of genetics and molecular pathology, Centre for Cancer Biology and the University of Adelaide, South Australia, and colleagues (Blood 2015 Dec 17. doi:10.1182/blood-2015-07-655589).
Killer immunoglobulin-like receptors (KIRs) contribute to natural killer (NK) cell–mediated killing of tumor cells, in both activating and inhibitory roles. Normal cells are spared through actions of inhibitory KIRs. Although the mechanism underlying the association between KIR2DL5B and CP-CML treatment outcomes is still unclear, the gene encodes an inhibitory KIR receptor, the absence of which may increase efficiency of NK-mediated killing of leukemic stem cells, researchers suggested.
The Therapeutic Intensification in De Novo Leukaemia (TIDEL-II) study included 210 patients with CP-CML who were treated with imatinib initially, and nilotinib subsequently if predetermined molecular targets were not met. The KIR substudy included 148 patients with samples available for genotyping.
KIR genotype frequencies observed in this study were similar to other white populations reported in the Allele Frequency Database.
Early molecular response was also significantly associated with treatment outcomes, independent of KIR prognostic significance, and may add additional prognostic information, available 3 months after treatment commences.
“In contrast, KIR2DL5B can identify, at baseline, the 20% of patients with a transformation risk of [about] 10% over 2 years versus the 80% of patients with a transformation risk of less than 3%,” the authors wrote. They suggest that KIR2DL5B, combined with other predictive markers, may enable targeted early interventions to improve outcomes.
The presence of KIR2DL5B was associated with lower rates of major molecular response (MMR), transformation-free survival, and event-free survival (but not overall survival) in patients with chronic phase–chronic myeloid leukemia (CP-CML) treated with sequential imatinib/nilotinib, according to researchers.
Univariate analysis demonstrated a significant association between KIR2DL5B and achievement of a major molecular response, with hazard ratio 0.423 (95% CI, 0.262-0.682; P less than .001). Other KIR genotypes, KIR2DL2pos and KIR2DS3pos, were also associated with inferior achievement of MMR, probably because of their association with KIR2DL5B due to linkage disequilibrium among KIR genes, according to the investigators.
“Our findings suggest that even with the potent second-generation TKI [tyrosine kinase inhibitor] nilotinib, KIR genotypes, a predetermined genetic host factor, may still be one of the most discriminatory prognostic markers available at baseline,” wrote Dr. David T. Yeung of the department of genetics and molecular pathology, Centre for Cancer Biology and the University of Adelaide, South Australia, and colleagues (Blood 2015 Dec 17. doi:10.1182/blood-2015-07-655589).
Killer immunoglobulin-like receptors (KIRs) contribute to natural killer (NK) cell–mediated killing of tumor cells, in both activating and inhibitory roles. Normal cells are spared through actions of inhibitory KIRs. Although the mechanism underlying the association between KIR2DL5B and CP-CML treatment outcomes is still unclear, the gene encodes an inhibitory KIR receptor, the absence of which may increase efficiency of NK-mediated killing of leukemic stem cells, researchers suggested.
The Therapeutic Intensification in De Novo Leukaemia (TIDEL-II) study included 210 patients with CP-CML who were treated with imatinib initially, and nilotinib subsequently if predetermined molecular targets were not met. The KIR substudy included 148 patients with samples available for genotyping.
KIR genotype frequencies observed in this study were similar to other white populations reported in the Allele Frequency Database.
Early molecular response was also significantly associated with treatment outcomes, independent of KIR prognostic significance, and may add additional prognostic information, available 3 months after treatment commences.
“In contrast, KIR2DL5B can identify, at baseline, the 20% of patients with a transformation risk of [about] 10% over 2 years versus the 80% of patients with a transformation risk of less than 3%,” the authors wrote. They suggest that KIR2DL5B, combined with other predictive markers, may enable targeted early interventions to improve outcomes.
FROM BLOOD
Key clinical point: The presence of KIR2DL5B was associated with worse outcomes in patients with chronic phase–chronic myeloid leukemia treated with sequential imatinib/nilotinib.
Major finding: Achievement of a major molecular response was associated with the KIR2DL5B genotype (HR, 0.423; 95% CI, 0.262-0.682; P less than .001).
Data source: A substudy of the Therapeutic Intensification in De Novo Leukaemia (TIDEL-II) study that included 148 patients with KIR genotype data available.
Disclosures: Support for the study was provided in part by Novartis. Dr. Yeung reported consulting or advisory roles with Novartis, BMS, and Ariad. Several coauthors reported ties to industry.
ACR: The pain of inflammatory disease goes beyond the physical
SAN FRANCISCO – The most common fear of rheumatoid arthritis (RA) and axial spondyloarthritis (axSpA) patients is that their future suffering will be as bad as their past suffering, according to a French survey of 474 patients.
Overall, 182 of the 303 RA patients (60%) and 122 of the 171 axSpA patients (71%) in the study ranked that fear as at least a 7 on a 10-point scale, and it remained a serious and common concern even among the roughly half of patients who were in remission.
Majorities in both groups were highly concerned about deformity, wheel chair dependence, burdening loved ones, losing autonomy, and disease spread to other joints. Less common fears, but still ranked at least a 7 by about one-third to well over half of patients, were more frequent flares, inability to care for others, losing friends, loss of treatment effectiveness, fear of treatment side effects, and not finding help if independence is lost.
In general, axSpA was perceived as the more frightening disease, with patients more likely than those with RA to give fears presented on the survey a score of 7 or higher; axSpA patients also were more likely to fear the impact of disease on pregnancy and work, and more worried about passing disease onto their children. Fears about joints seizing up, bone and spine fusion, and increased flare activity were far more prevalent in the axSpA group.
The findings are from a test run of a new questionnaire being developed in France to capture the psychological burden of chronic inflammatory disease. The idea is to make patients’ fears and convictions explicit so that providers know what they are and can help patients cope.
“We’ve had this idea for a long time. Patients have fears and beliefs that” are difficult to express, and they get in the way of effective office communication. The questionnaire might break down the walls, so “patients know their doctors understand and are concerned” about their overall well-being, said senior investigator Dr. Francis Berenbaum, chief of rheumatology at Saint Antoine Hospital in Paris.
It’s hoped that the efforts will improve trust and dialogue between patients and doctors and lead to better treatment adherence and follow-up, more effective counseling, and perhaps even new patient-related outcomes for clinical trials, he said at the annual meeting of the American College of Rheumatology.
To create the survey, the team conducted semi-structured patient interviews at rheumatology practices across France. They whittled the responses down to identify 23 common fears and 19 disease-related beliefs in RA and axSpA. The resulting 44-item survey – there are two additional items about pregnancy and work-related concerns – asks patients to rate each one on a scale of 1-10. The team hopes to have data soon to show whether or not the efforts improve outcomes.
Common beliefs in both groups were that fatigue, over-exertion, stress, and weather changes trigger flares, but that moderate physical activity reduces them.
Almost half of RA patients, versus about a quarter of axSpA patients, believed that their disease was triggered by an emotional shock or stressful event, and small minorities in both groups attributed their disease to pollution, vaccines, or passive or active smoking. About 70% of patients in both groups were on biologics, and about one-third in each were very worried that their treatments would cause cancer.
Some “disease perceptions may not be accurate” and “provide a basis for discussion … to dispel misconceptions, align treatment expectations, and provide reassurance,” the investigators noted,
The RA patients were 60 years old on average, and about three-quarters were women. The median disease duration was 10 years, and mean Disease Activity Score (DAS28) was 2.7; axSpA patients were a mean age of 47 years, 43% were women, and the median disease duration was 12 years. Their mean Bath Ankylosing Spondylitis Disease Activity Index score was 3.2.
Foundation Arthritis Jacques Courtin and UCB Pharma funded the work. Dr. Berenbaum has no relevant disclosures. Two investigators are UCB employees.
SAN FRANCISCO – The most common fear of rheumatoid arthritis (RA) and axial spondyloarthritis (axSpA) patients is that their future suffering will be as bad as their past suffering, according to a French survey of 474 patients.
Overall, 182 of the 303 RA patients (60%) and 122 of the 171 axSpA patients (71%) in the study ranked that fear as at least a 7 on a 10-point scale, and it remained a serious and common concern even among the roughly half of patients who were in remission.
Majorities in both groups were highly concerned about deformity, wheel chair dependence, burdening loved ones, losing autonomy, and disease spread to other joints. Less common fears, but still ranked at least a 7 by about one-third to well over half of patients, were more frequent flares, inability to care for others, losing friends, loss of treatment effectiveness, fear of treatment side effects, and not finding help if independence is lost.
In general, axSpA was perceived as the more frightening disease, with patients more likely than those with RA to give fears presented on the survey a score of 7 or higher; axSpA patients also were more likely to fear the impact of disease on pregnancy and work, and more worried about passing disease onto their children. Fears about joints seizing up, bone and spine fusion, and increased flare activity were far more prevalent in the axSpA group.
The findings are from a test run of a new questionnaire being developed in France to capture the psychological burden of chronic inflammatory disease. The idea is to make patients’ fears and convictions explicit so that providers know what they are and can help patients cope.
“We’ve had this idea for a long time. Patients have fears and beliefs that” are difficult to express, and they get in the way of effective office communication. The questionnaire might break down the walls, so “patients know their doctors understand and are concerned” about their overall well-being, said senior investigator Dr. Francis Berenbaum, chief of rheumatology at Saint Antoine Hospital in Paris.
It’s hoped that the efforts will improve trust and dialogue between patients and doctors and lead to better treatment adherence and follow-up, more effective counseling, and perhaps even new patient-related outcomes for clinical trials, he said at the annual meeting of the American College of Rheumatology.
To create the survey, the team conducted semi-structured patient interviews at rheumatology practices across France. They whittled the responses down to identify 23 common fears and 19 disease-related beliefs in RA and axSpA. The resulting 44-item survey – there are two additional items about pregnancy and work-related concerns – asks patients to rate each one on a scale of 1-10. The team hopes to have data soon to show whether or not the efforts improve outcomes.
Common beliefs in both groups were that fatigue, over-exertion, stress, and weather changes trigger flares, but that moderate physical activity reduces them.
Almost half of RA patients, versus about a quarter of axSpA patients, believed that their disease was triggered by an emotional shock or stressful event, and small minorities in both groups attributed their disease to pollution, vaccines, or passive or active smoking. About 70% of patients in both groups were on biologics, and about one-third in each were very worried that their treatments would cause cancer.
Some “disease perceptions may not be accurate” and “provide a basis for discussion … to dispel misconceptions, align treatment expectations, and provide reassurance,” the investigators noted,
The RA patients were 60 years old on average, and about three-quarters were women. The median disease duration was 10 years, and mean Disease Activity Score (DAS28) was 2.7; axSpA patients were a mean age of 47 years, 43% were women, and the median disease duration was 12 years. Their mean Bath Ankylosing Spondylitis Disease Activity Index score was 3.2.
Foundation Arthritis Jacques Courtin and UCB Pharma funded the work. Dr. Berenbaum has no relevant disclosures. Two investigators are UCB employees.
SAN FRANCISCO – The most common fear of rheumatoid arthritis (RA) and axial spondyloarthritis (axSpA) patients is that their future suffering will be as bad as their past suffering, according to a French survey of 474 patients.
Overall, 182 of the 303 RA patients (60%) and 122 of the 171 axSpA patients (71%) in the study ranked that fear as at least a 7 on a 10-point scale, and it remained a serious and common concern even among the roughly half of patients who were in remission.
Majorities in both groups were highly concerned about deformity, wheel chair dependence, burdening loved ones, losing autonomy, and disease spread to other joints. Less common fears, but still ranked at least a 7 by about one-third to well over half of patients, were more frequent flares, inability to care for others, losing friends, loss of treatment effectiveness, fear of treatment side effects, and not finding help if independence is lost.
In general, axSpA was perceived as the more frightening disease, with patients more likely than those with RA to give fears presented on the survey a score of 7 or higher; axSpA patients also were more likely to fear the impact of disease on pregnancy and work, and more worried about passing disease onto their children. Fears about joints seizing up, bone and spine fusion, and increased flare activity were far more prevalent in the axSpA group.
The findings are from a test run of a new questionnaire being developed in France to capture the psychological burden of chronic inflammatory disease. The idea is to make patients’ fears and convictions explicit so that providers know what they are and can help patients cope.
“We’ve had this idea for a long time. Patients have fears and beliefs that” are difficult to express, and they get in the way of effective office communication. The questionnaire might break down the walls, so “patients know their doctors understand and are concerned” about their overall well-being, said senior investigator Dr. Francis Berenbaum, chief of rheumatology at Saint Antoine Hospital in Paris.
It’s hoped that the efforts will improve trust and dialogue between patients and doctors and lead to better treatment adherence and follow-up, more effective counseling, and perhaps even new patient-related outcomes for clinical trials, he said at the annual meeting of the American College of Rheumatology.
To create the survey, the team conducted semi-structured patient interviews at rheumatology practices across France. They whittled the responses down to identify 23 common fears and 19 disease-related beliefs in RA and axSpA. The resulting 44-item survey – there are two additional items about pregnancy and work-related concerns – asks patients to rate each one on a scale of 1-10. The team hopes to have data soon to show whether or not the efforts improve outcomes.
Common beliefs in both groups were that fatigue, over-exertion, stress, and weather changes trigger flares, but that moderate physical activity reduces them.
Almost half of RA patients, versus about a quarter of axSpA patients, believed that their disease was triggered by an emotional shock or stressful event, and small minorities in both groups attributed their disease to pollution, vaccines, or passive or active smoking. About 70% of patients in both groups were on biologics, and about one-third in each were very worried that their treatments would cause cancer.
Some “disease perceptions may not be accurate” and “provide a basis for discussion … to dispel misconceptions, align treatment expectations, and provide reassurance,” the investigators noted,
The RA patients were 60 years old on average, and about three-quarters were women. The median disease duration was 10 years, and mean Disease Activity Score (DAS28) was 2.7; axSpA patients were a mean age of 47 years, 43% were women, and the median disease duration was 12 years. Their mean Bath Ankylosing Spondylitis Disease Activity Index score was 3.2.
Foundation Arthritis Jacques Courtin and UCB Pharma funded the work. Dr. Berenbaum has no relevant disclosures. Two investigators are UCB employees.
AT THE ACR ANNUAL MEETING
Key clinical point: Ask patients what they are worried about; you might put them at ease by addressing their misconceptions.
Major finding: Overall, 60% of RA patients and 71% of axSpA patients were fearful that their future suffering would be as bad as their past suffering.
Data source: A French survey of 474 patients.
Disclosures: Foundation Arthritis Jacques Courtin and UCB Pharma funded the study. The senior investigator has no relevant disclosures. Two investigators are UCB employees.
Hospital-acquired pneumonia threatens cervical spinal cord injury patients
SAN DIEGO – The overall rate of hospital-acquired pneumonia following cervical spinal cord injury is about 20%, results from a study of national data demonstrated.
“Cervical spinal cord injury patients are at an increased risk for the development of hospital-acquired pneumonia,” lead study author Dr. Pablo J. Diaz-Collado said in an interview after the annual meeting of the Cervical Spine Research Society.
“Complete cord injuries, longer length of stay, ICU stay and ventilation time lead to significantly increased risk of HAP, which then leads to poor inpatient outcomes,” he said. “It is of crucial importance to keep these risk factors in mind when treating patients with cervical spinal cord injuries. There is a need to optimize the management protocols for these patients to help prevent the development of HAPs.”
Dr. Diaz-Collado, an orthopedic surgery resident at Yale–New Haven (Conn.) Hospital, and his associates identified 5,198 cervical spinal cord injury patients in the 2011 and 2012 National Trauma Data Bank (NTDB) to analyze risk factors for the development of HAP and inpatient outcomes in this population. They used multivariate logistic regression to identify independent associations of various risk factors with the occurrence of HAP.
The researchers found that the overall incidence of HAP among cervical spinal cord injury patients was 20.5%, which amounted to 1,065 patients. Factors independently associated with HAP were complete spinal cord injuries (compared to central cord injuries; OR 1.44; P = .009); longer inpatient length of stay (OR 3.08 for a stay that lasted 7-13 days, OR 10.21 for 21-27 days, and OR 14.89 for 35 days or more; P = .001 or less for all associations); longer ICU stay (OR 2.86 for a stay that lasted 9-11 days, OR 3.05 for 12-14 days, and OR 2.94 for 15 days or more; P less than .001 for all associations), and longer time on mechanical ventilation (OR 2.68 for ventilation that lasted 3-6 days, OR 3.76 for 7-13 days, OR 3.98 for 14-20 days, and OR 3.99 for 21 days or more; P less than .001 for all associations).
After the researchers controlled for all other risk factors, including patient comorbidities, Injury Severity Score, and other inpatient complications, HAP was associated with increased odds of death (OR 1.60; P = .005), inpatient adverse events (OR 1.65; P less than .001), discharge to an extended-care facility (OR 1.93; P = .001), and longer length of stay (a mean of an additional 10.93 days; P less than .001).
Dr. Diaz-Collado acknowledged that the study is “limited by the quality of the data entry. In addition, the database does not include classifications of fractures, and thus stratification of the analysis in terms of the different kinds of fractures in the cervical spine is not possible. Finally, procedural codes are less accurate and thus including whether or not patients underwent a surgical intervention is less reliable.”
Dr. Diaz-Collado reported having no financial disclosures.
SAN DIEGO – The overall rate of hospital-acquired pneumonia following cervical spinal cord injury is about 20%, results from a study of national data demonstrated.
“Cervical spinal cord injury patients are at an increased risk for the development of hospital-acquired pneumonia,” lead study author Dr. Pablo J. Diaz-Collado said in an interview after the annual meeting of the Cervical Spine Research Society.
“Complete cord injuries, longer length of stay, ICU stay and ventilation time lead to significantly increased risk of HAP, which then leads to poor inpatient outcomes,” he said. “It is of crucial importance to keep these risk factors in mind when treating patients with cervical spinal cord injuries. There is a need to optimize the management protocols for these patients to help prevent the development of HAPs.”
Dr. Diaz-Collado, an orthopedic surgery resident at Yale–New Haven (Conn.) Hospital, and his associates identified 5,198 cervical spinal cord injury patients in the 2011 and 2012 National Trauma Data Bank (NTDB) to analyze risk factors for the development of HAP and inpatient outcomes in this population. They used multivariate logistic regression to identify independent associations of various risk factors with the occurrence of HAP.
The researchers found that the overall incidence of HAP among cervical spinal cord injury patients was 20.5%, which amounted to 1,065 patients. Factors independently associated with HAP were complete spinal cord injuries (compared to central cord injuries; OR 1.44; P = .009); longer inpatient length of stay (OR 3.08 for a stay that lasted 7-13 days, OR 10.21 for 21-27 days, and OR 14.89 for 35 days or more; P = .001 or less for all associations); longer ICU stay (OR 2.86 for a stay that lasted 9-11 days, OR 3.05 for 12-14 days, and OR 2.94 for 15 days or more; P less than .001 for all associations), and longer time on mechanical ventilation (OR 2.68 for ventilation that lasted 3-6 days, OR 3.76 for 7-13 days, OR 3.98 for 14-20 days, and OR 3.99 for 21 days or more; P less than .001 for all associations).
After the researchers controlled for all other risk factors, including patient comorbidities, Injury Severity Score, and other inpatient complications, HAP was associated with increased odds of death (OR 1.60; P = .005), inpatient adverse events (OR 1.65; P less than .001), discharge to an extended-care facility (OR 1.93; P = .001), and longer length of stay (a mean of an additional 10.93 days; P less than .001).
Dr. Diaz-Collado acknowledged that the study is “limited by the quality of the data entry. In addition, the database does not include classifications of fractures, and thus stratification of the analysis in terms of the different kinds of fractures in the cervical spine is not possible. Finally, procedural codes are less accurate and thus including whether or not patients underwent a surgical intervention is less reliable.”
Dr. Diaz-Collado reported having no financial disclosures.
SAN DIEGO – The overall rate of hospital-acquired pneumonia following cervical spinal cord injury is about 20%, results from a study of national data demonstrated.
“Cervical spinal cord injury patients are at an increased risk for the development of hospital-acquired pneumonia,” lead study author Dr. Pablo J. Diaz-Collado said in an interview after the annual meeting of the Cervical Spine Research Society.
“Complete cord injuries, longer length of stay, ICU stay and ventilation time lead to significantly increased risk of HAP, which then leads to poor inpatient outcomes,” he said. “It is of crucial importance to keep these risk factors in mind when treating patients with cervical spinal cord injuries. There is a need to optimize the management protocols for these patients to help prevent the development of HAPs.”
Dr. Diaz-Collado, an orthopedic surgery resident at Yale–New Haven (Conn.) Hospital, and his associates identified 5,198 cervical spinal cord injury patients in the 2011 and 2012 National Trauma Data Bank (NTDB) to analyze risk factors for the development of HAP and inpatient outcomes in this population. They used multivariate logistic regression to identify independent associations of various risk factors with the occurrence of HAP.
The researchers found that the overall incidence of HAP among cervical spinal cord injury patients was 20.5%, which amounted to 1,065 patients. Factors independently associated with HAP were complete spinal cord injuries (compared to central cord injuries; OR 1.44; P = .009); longer inpatient length of stay (OR 3.08 for a stay that lasted 7-13 days, OR 10.21 for 21-27 days, and OR 14.89 for 35 days or more; P = .001 or less for all associations); longer ICU stay (OR 2.86 for a stay that lasted 9-11 days, OR 3.05 for 12-14 days, and OR 2.94 for 15 days or more; P less than .001 for all associations), and longer time on mechanical ventilation (OR 2.68 for ventilation that lasted 3-6 days, OR 3.76 for 7-13 days, OR 3.98 for 14-20 days, and OR 3.99 for 21 days or more; P less than .001 for all associations).
After the researchers controlled for all other risk factors, including patient comorbidities, Injury Severity Score, and other inpatient complications, HAP was associated with increased odds of death (OR 1.60; P = .005), inpatient adverse events (OR 1.65; P less than .001), discharge to an extended-care facility (OR 1.93; P = .001), and longer length of stay (a mean of an additional 10.93 days; P less than .001).
Dr. Diaz-Collado acknowledged that the study is “limited by the quality of the data entry. In addition, the database does not include classifications of fractures, and thus stratification of the analysis in terms of the different kinds of fractures in the cervical spine is not possible. Finally, procedural codes are less accurate and thus including whether or not patients underwent a surgical intervention is less reliable.”
Dr. Diaz-Collado reported having no financial disclosures.
AT CSRS 2015
Key clinical point: About one in five cervical spinal cord injury patients develop hospital-acquired pneumonia.
Major finding: The overall incidence of HAP among cervical spinal cord injury patients was 20.5%.
Data source: A study of 5,198 cervical spinal cord injury patients in the 2011 and 2012 National Trauma Data Bank.
Disclosures: Dr. Diaz-Collado reported having no financial disclosures.
Drug produces ‘encouraging efficacy’ in MM

© ASCO/Todd Buchanan
Single-agent daratumumab has exhibited “encouraging efficacy” and a “favorable safety profile” in patients with heavily pretreated and refractory multiple myeloma (MM), according to investigators from the phase 2 SIRIUS trial.
The drug produced an overall response rate of 30% in MM patients who had received 3 or more prior lines of therapy. The median progression-free survival was close to 4 months, and the median overall survival was nearly 18 months.
Thirty percent of patients had treatment-emergent serious adverse events (AEs), and 23% had grade 3 or 4 treatment-emergent serious AEs.
“This represents the first single-agent activity we have for a monoclonal antibody in treating multiple myeloma,” said study author Sagar Lonial, MD, of Emory University School of Medicine in Atlanta, Georgia.
“The future hope for daratumumab is in our ability to bring this active agent to earlier lines of therapy and combine it with drugs where you may get synergy.”
Dr Lonial and his colleagues reported results from the ongoing SIRIUS trial in The Lancet. Results from the trial were previously presented at the 2015 ASCO Annual Meeting. The research was funded by Janssen Research & Development, the company developing daratumumab.
In part 1 of the trial, 34 MM patients were randomized to receive either 8 mg/kg of daratumumab once every 4 weeks or 16 mg/kg once a week for 8 weeks, then once every 2 weeks for 16 weeks and once every 4 weeks after that, until disease progression or unacceptable toxicity.
In part 2, an additional 90 MM patients were enrolled and received 16 mg/kg of daratumumab on the same dosing schedule as in part 1.
Dr Lonial and his colleagues reported results for all patients who received 16 mg/kg of daratumumab. At the first interim analysis, the 8 mg/kg arm did not meet the criteria for expansion because the overall response rate was 11.1%.
The 106 patients who received the 16 mg/kg dose of daratumumab had received a median of 5 prior lines of therapy, including a proteasome inhibitor and an immunomodulatory drug. Ninety-seven percent of these patients were refractory to their last line of therapy, and 95% were refractory to both a proteasome inhibitor and an immunomodulatory drug.
Response and survival
According to an independent review committee, 29.2% of patients responded to daratumumab. Eighteen patients had a partial response, 10 had a very good partial response, and 3 had a stringent complete response.
The median duration of response was 7.4 months, and the median time to first response was 1 month.
The median overall survival was 17.5 months, and the 12-month overall survival was 64.8%. The median progression-free survival was 3.7 months.
Safety
The most common AEs were fatigue (40%), anemia (33%), nausea (29%), thrombocytopenia (25%), neutropenia (23%), back pain (22%), and cough (21%). Thirty percent of patients experienced serious AEs, and 23% had serious grade 3/4 AEs.
Infusion-related reactions were reported in 42% of patients and were predominantly grade 1 or 2 (5% grade 3; no grade 4). The most common infusion-related reactions were nasal congestion (12%), throat irritation (7%), cough (6%), dyspnea (6%), chills (6%), and vomiting (6%)—all of which were treated with standard of care and slower infusion rates.
None of the patients discontinued daratumumab because of drug-related treatment-emergent AEs, infusion-related reactions, or death. However, 5% of patients discontinued treatment because of treatment-emergent AEs—2 cases of progressive disease and 1 case each of H1N1 influenza, hypercalcemia, and spinal cord compression.
Twenty-nine percent of patients died after treatment—27% due to progressive disease and 2% due to AEs. The 2 AEs were cardiorespiratory failure secondary to H1N1 influenza complications and general health deterioration secondary to complications of aspiration pneumonia. ![]()

© ASCO/Todd Buchanan
Single-agent daratumumab has exhibited “encouraging efficacy” and a “favorable safety profile” in patients with heavily pretreated and refractory multiple myeloma (MM), according to investigators from the phase 2 SIRIUS trial.
The drug produced an overall response rate of 30% in MM patients who had received 3 or more prior lines of therapy. The median progression-free survival was close to 4 months, and the median overall survival was nearly 18 months.
Thirty percent of patients had treatment-emergent serious adverse events (AEs), and 23% had grade 3 or 4 treatment-emergent serious AEs.
“This represents the first single-agent activity we have for a monoclonal antibody in treating multiple myeloma,” said study author Sagar Lonial, MD, of Emory University School of Medicine in Atlanta, Georgia.
“The future hope for daratumumab is in our ability to bring this active agent to earlier lines of therapy and combine it with drugs where you may get synergy.”
Dr Lonial and his colleagues reported results from the ongoing SIRIUS trial in The Lancet. Results from the trial were previously presented at the 2015 ASCO Annual Meeting. The research was funded by Janssen Research & Development, the company developing daratumumab.
In part 1 of the trial, 34 MM patients were randomized to receive either 8 mg/kg of daratumumab once every 4 weeks or 16 mg/kg once a week for 8 weeks, then once every 2 weeks for 16 weeks and once every 4 weeks after that, until disease progression or unacceptable toxicity.
In part 2, an additional 90 MM patients were enrolled and received 16 mg/kg of daratumumab on the same dosing schedule as in part 1.
Dr Lonial and his colleagues reported results for all patients who received 16 mg/kg of daratumumab. At the first interim analysis, the 8 mg/kg arm did not meet the criteria for expansion because the overall response rate was 11.1%.
The 106 patients who received the 16 mg/kg dose of daratumumab had received a median of 5 prior lines of therapy, including a proteasome inhibitor and an immunomodulatory drug. Ninety-seven percent of these patients were refractory to their last line of therapy, and 95% were refractory to both a proteasome inhibitor and an immunomodulatory drug.
Response and survival
According to an independent review committee, 29.2% of patients responded to daratumumab. Eighteen patients had a partial response, 10 had a very good partial response, and 3 had a stringent complete response.
The median duration of response was 7.4 months, and the median time to first response was 1 month.
The median overall survival was 17.5 months, and the 12-month overall survival was 64.8%. The median progression-free survival was 3.7 months.
Safety
The most common AEs were fatigue (40%), anemia (33%), nausea (29%), thrombocytopenia (25%), neutropenia (23%), back pain (22%), and cough (21%). Thirty percent of patients experienced serious AEs, and 23% had serious grade 3/4 AEs.
Infusion-related reactions were reported in 42% of patients and were predominantly grade 1 or 2 (5% grade 3; no grade 4). The most common infusion-related reactions were nasal congestion (12%), throat irritation (7%), cough (6%), dyspnea (6%), chills (6%), and vomiting (6%)—all of which were treated with standard of care and slower infusion rates.
None of the patients discontinued daratumumab because of drug-related treatment-emergent AEs, infusion-related reactions, or death. However, 5% of patients discontinued treatment because of treatment-emergent AEs—2 cases of progressive disease and 1 case each of H1N1 influenza, hypercalcemia, and spinal cord compression.
Twenty-nine percent of patients died after treatment—27% due to progressive disease and 2% due to AEs. The 2 AEs were cardiorespiratory failure secondary to H1N1 influenza complications and general health deterioration secondary to complications of aspiration pneumonia. ![]()

© ASCO/Todd Buchanan
Single-agent daratumumab has exhibited “encouraging efficacy” and a “favorable safety profile” in patients with heavily pretreated and refractory multiple myeloma (MM), according to investigators from the phase 2 SIRIUS trial.
The drug produced an overall response rate of 30% in MM patients who had received 3 or more prior lines of therapy. The median progression-free survival was close to 4 months, and the median overall survival was nearly 18 months.
Thirty percent of patients had treatment-emergent serious adverse events (AEs), and 23% had grade 3 or 4 treatment-emergent serious AEs.
“This represents the first single-agent activity we have for a monoclonal antibody in treating multiple myeloma,” said study author Sagar Lonial, MD, of Emory University School of Medicine in Atlanta, Georgia.
“The future hope for daratumumab is in our ability to bring this active agent to earlier lines of therapy and combine it with drugs where you may get synergy.”
Dr Lonial and his colleagues reported results from the ongoing SIRIUS trial in The Lancet. Results from the trial were previously presented at the 2015 ASCO Annual Meeting. The research was funded by Janssen Research & Development, the company developing daratumumab.
In part 1 of the trial, 34 MM patients were randomized to receive either 8 mg/kg of daratumumab once every 4 weeks or 16 mg/kg once a week for 8 weeks, then once every 2 weeks for 16 weeks and once every 4 weeks after that, until disease progression or unacceptable toxicity.
In part 2, an additional 90 MM patients were enrolled and received 16 mg/kg of daratumumab on the same dosing schedule as in part 1.
Dr Lonial and his colleagues reported results for all patients who received 16 mg/kg of daratumumab. At the first interim analysis, the 8 mg/kg arm did not meet the criteria for expansion because the overall response rate was 11.1%.
The 106 patients who received the 16 mg/kg dose of daratumumab had received a median of 5 prior lines of therapy, including a proteasome inhibitor and an immunomodulatory drug. Ninety-seven percent of these patients were refractory to their last line of therapy, and 95% were refractory to both a proteasome inhibitor and an immunomodulatory drug.
Response and survival
According to an independent review committee, 29.2% of patients responded to daratumumab. Eighteen patients had a partial response, 10 had a very good partial response, and 3 had a stringent complete response.
The median duration of response was 7.4 months, and the median time to first response was 1 month.
The median overall survival was 17.5 months, and the 12-month overall survival was 64.8%. The median progression-free survival was 3.7 months.
Safety
The most common AEs were fatigue (40%), anemia (33%), nausea (29%), thrombocytopenia (25%), neutropenia (23%), back pain (22%), and cough (21%). Thirty percent of patients experienced serious AEs, and 23% had serious grade 3/4 AEs.
Infusion-related reactions were reported in 42% of patients and were predominantly grade 1 or 2 (5% grade 3; no grade 4). The most common infusion-related reactions were nasal congestion (12%), throat irritation (7%), cough (6%), dyspnea (6%), chills (6%), and vomiting (6%)—all of which were treated with standard of care and slower infusion rates.
None of the patients discontinued daratumumab because of drug-related treatment-emergent AEs, infusion-related reactions, or death. However, 5% of patients discontinued treatment because of treatment-emergent AEs—2 cases of progressive disease and 1 case each of H1N1 influenza, hypercalcemia, and spinal cord compression.
Twenty-nine percent of patients died after treatment—27% due to progressive disease and 2% due to AEs. The 2 AEs were cardiorespiratory failure secondary to H1N1 influenza complications and general health deterioration secondary to complications of aspiration pneumonia. ![]()
Study links leukemia to low UVB exposure

People residing at higher latitudes, with lower exposure to sunlight/ultraviolet B (UVB) rays, have at least a 2-fold greater risk of developing leukemia than equatorial populations, according to research published in PLOS ONE.
“These results suggest that much of the burden of leukemia worldwide is due to the epidemic of vitamin D deficiency we are experiencing in winter in populations distant from the equator,” said Cedric Garland, DrPH, of the University of California San Diego in La Jolla, California.
“People who live in areas with low solar ultraviolet B exposure tend to have low levels of vitamin D metabolites in their blood. These low levels place them at high risk of certain cancers, including leukemia.”
Dr Garland and his colleagues analyzed age-adjusted incidence rates of leukemia in 172 countries and compared that information with cloud cover data from the International Satellite Cloud Climatology Project.
The team found that leukemia rates were highest in countries relatively closer to the poles, such as Australia, New Zealand, Chile, Ireland, Canada, and the United States.
And leukemia rates were lowest in countries closer to the equator, such as Bolivia, Samoa, Madagascar, and Nigeria.
The researchers also discovered that leukemia incidence was inversely associated with cloud-adjusted UVB irradiance in males (P≤0.01) and females (P≤0.01) in both hemispheres.
The association persisted in males (P≤0.05) and females (P≤0.01) after the team controlled for elevation and life expectancy.
The researchers said it’s plausible that the association is due to vitamin D deficiency.
This study follows similar investigations by Dr Garland and his colleagues in which they looked at other cancers, including breast, colon, pancreas, bladder, and multiple myeloma. In each study, the team found that reduced UVB radiation exposure and lower vitamin D levels were associated with higher risks of cancer.
“These studies do not necessarily provide final evidence,” Dr Garland said, “but they have been helpful in the past in identifying associations that have helped minimize cancer risk.” ![]()

People residing at higher latitudes, with lower exposure to sunlight/ultraviolet B (UVB) rays, have at least a 2-fold greater risk of developing leukemia than equatorial populations, according to research published in PLOS ONE.
“These results suggest that much of the burden of leukemia worldwide is due to the epidemic of vitamin D deficiency we are experiencing in winter in populations distant from the equator,” said Cedric Garland, DrPH, of the University of California San Diego in La Jolla, California.
“People who live in areas with low solar ultraviolet B exposure tend to have low levels of vitamin D metabolites in their blood. These low levels place them at high risk of certain cancers, including leukemia.”
Dr Garland and his colleagues analyzed age-adjusted incidence rates of leukemia in 172 countries and compared that information with cloud cover data from the International Satellite Cloud Climatology Project.
The team found that leukemia rates were highest in countries relatively closer to the poles, such as Australia, New Zealand, Chile, Ireland, Canada, and the United States.
And leukemia rates were lowest in countries closer to the equator, such as Bolivia, Samoa, Madagascar, and Nigeria.
The researchers also discovered that leukemia incidence was inversely associated with cloud-adjusted UVB irradiance in males (P≤0.01) and females (P≤0.01) in both hemispheres.
The association persisted in males (P≤0.05) and females (P≤0.01) after the team controlled for elevation and life expectancy.
The researchers said it’s plausible that the association is due to vitamin D deficiency.
This study follows similar investigations by Dr Garland and his colleagues in which they looked at other cancers, including breast, colon, pancreas, bladder, and multiple myeloma. In each study, the team found that reduced UVB radiation exposure and lower vitamin D levels were associated with higher risks of cancer.
“These studies do not necessarily provide final evidence,” Dr Garland said, “but they have been helpful in the past in identifying associations that have helped minimize cancer risk.” ![]()

People residing at higher latitudes, with lower exposure to sunlight/ultraviolet B (UVB) rays, have at least a 2-fold greater risk of developing leukemia than equatorial populations, according to research published in PLOS ONE.
“These results suggest that much of the burden of leukemia worldwide is due to the epidemic of vitamin D deficiency we are experiencing in winter in populations distant from the equator,” said Cedric Garland, DrPH, of the University of California San Diego in La Jolla, California.
“People who live in areas with low solar ultraviolet B exposure tend to have low levels of vitamin D metabolites in their blood. These low levels place them at high risk of certain cancers, including leukemia.”
Dr Garland and his colleagues analyzed age-adjusted incidence rates of leukemia in 172 countries and compared that information with cloud cover data from the International Satellite Cloud Climatology Project.
The team found that leukemia rates were highest in countries relatively closer to the poles, such as Australia, New Zealand, Chile, Ireland, Canada, and the United States.
And leukemia rates were lowest in countries closer to the equator, such as Bolivia, Samoa, Madagascar, and Nigeria.
The researchers also discovered that leukemia incidence was inversely associated with cloud-adjusted UVB irradiance in males (P≤0.01) and females (P≤0.01) in both hemispheres.
The association persisted in males (P≤0.05) and females (P≤0.01) after the team controlled for elevation and life expectancy.
The researchers said it’s plausible that the association is due to vitamin D deficiency.
This study follows similar investigations by Dr Garland and his colleagues in which they looked at other cancers, including breast, colon, pancreas, bladder, and multiple myeloma. In each study, the team found that reduced UVB radiation exposure and lower vitamin D levels were associated with higher risks of cancer.
“These studies do not necessarily provide final evidence,” Dr Garland said, “but they have been helpful in the past in identifying associations that have helped minimize cancer risk.” ![]()
How microbes drive progression of CTCL

New research indicates that toxins in Staphylococcus bacteria help malignant cells gain control over healthy cells in patients with cutaneous T-cell lymphoma (CTCL).
Investigators found that staphylococcal enterotoxin-A (SEA) induces STAT3 activation and IL-17 expression in malignant T cells via engagement of non-malignant CD4 T cells.
As STAT3 activation has been implicated in CTCL pathogenesis, the discovery suggests bacterial toxins play a key role in activating an oncogenic pathway in CTCL.
“We have gained important insight into the processes that activate cancer cells and make them grow,” said Niels Oedum, MD, of the University of Copenhagen in Denmark.
“[CTCL] patients’ frequent bacterial infections might not be a mere side effect of the disease. On the contrary, toxins in the bacteria actually ‘benefit’ cancer cells. Our next step is examining whether combatting infections can slow down the growth of cancer cells and thus stop the disease.”
Dr Oedum and his colleagues described their research in Blood.
The investigators knew that, in CTCL, CD4 T cells become malignant and turn parasitic on the rest of the immune system. In addition to using healthy cells to do their work for them, the malignant cells slowly destroy the skin’s immune defense mechanism.
The team’s new discoveries indicate that bacterial toxins in some patients enable malignant cells to send off signals that obstruct and change the immune defense mechanism, which would otherwise fight the malignant cells. What was believed to be an overly active immune defense mechanism could, in other words, turn out to be a malignant infection brought on by bacteria, which only worsens the disease.
Dr Oedum and his colleagues found that SEA-positive bacteria isolatated from the skin of CTCL patients stimulated activation of STAT3 and upregulation of IL-17 in malignant and non-malignant T cells.
Malignant T cells expressing an SEA non-responsive T-cell receptor V beta chain did not respond to SEA when cultured alone but exhibited STAT3 activation and IL-17 expression in co-cultures with SEA-responsive, non-malignant T cells.
The investigators found evidence to suggest the response is induced via IL-2Rg cytokines and a JAK3-dependent pathway in malignant T cells. The JAK3 inhibitor tofacitinib inhibited SEA-induced IL-17 production in co-cultures of malignant and non-malignant T cells.
Dr Oedum and his colleagues plan to continue their work investigating how bacteria might affect the balance between the immune defense mechanism and the disease in patients with CTCL.
In the long-term, the investigators’ aim is to understand how bacteria and their toxins can worsen CTCL, knowledge that may be used to develop new targeted treatments.
As only some of the bacteria produce toxins, the team said it will also be important to develop methods to determine which patients may benefit from treatment with antibiotics. ![]()

New research indicates that toxins in Staphylococcus bacteria help malignant cells gain control over healthy cells in patients with cutaneous T-cell lymphoma (CTCL).
Investigators found that staphylococcal enterotoxin-A (SEA) induces STAT3 activation and IL-17 expression in malignant T cells via engagement of non-malignant CD4 T cells.
As STAT3 activation has been implicated in CTCL pathogenesis, the discovery suggests bacterial toxins play a key role in activating an oncogenic pathway in CTCL.
“We have gained important insight into the processes that activate cancer cells and make them grow,” said Niels Oedum, MD, of the University of Copenhagen in Denmark.
“[CTCL] patients’ frequent bacterial infections might not be a mere side effect of the disease. On the contrary, toxins in the bacteria actually ‘benefit’ cancer cells. Our next step is examining whether combatting infections can slow down the growth of cancer cells and thus stop the disease.”
Dr Oedum and his colleagues described their research in Blood.
The investigators knew that, in CTCL, CD4 T cells become malignant and turn parasitic on the rest of the immune system. In addition to using healthy cells to do their work for them, the malignant cells slowly destroy the skin’s immune defense mechanism.
The team’s new discoveries indicate that bacterial toxins in some patients enable malignant cells to send off signals that obstruct and change the immune defense mechanism, which would otherwise fight the malignant cells. What was believed to be an overly active immune defense mechanism could, in other words, turn out to be a malignant infection brought on by bacteria, which only worsens the disease.
Dr Oedum and his colleagues found that SEA-positive bacteria isolatated from the skin of CTCL patients stimulated activation of STAT3 and upregulation of IL-17 in malignant and non-malignant T cells.
Malignant T cells expressing an SEA non-responsive T-cell receptor V beta chain did not respond to SEA when cultured alone but exhibited STAT3 activation and IL-17 expression in co-cultures with SEA-responsive, non-malignant T cells.
The investigators found evidence to suggest the response is induced via IL-2Rg cytokines and a JAK3-dependent pathway in malignant T cells. The JAK3 inhibitor tofacitinib inhibited SEA-induced IL-17 production in co-cultures of malignant and non-malignant T cells.
Dr Oedum and his colleagues plan to continue their work investigating how bacteria might affect the balance between the immune defense mechanism and the disease in patients with CTCL.
In the long-term, the investigators’ aim is to understand how bacteria and their toxins can worsen CTCL, knowledge that may be used to develop new targeted treatments.
As only some of the bacteria produce toxins, the team said it will also be important to develop methods to determine which patients may benefit from treatment with antibiotics. ![]()

New research indicates that toxins in Staphylococcus bacteria help malignant cells gain control over healthy cells in patients with cutaneous T-cell lymphoma (CTCL).
Investigators found that staphylococcal enterotoxin-A (SEA) induces STAT3 activation and IL-17 expression in malignant T cells via engagement of non-malignant CD4 T cells.
As STAT3 activation has been implicated in CTCL pathogenesis, the discovery suggests bacterial toxins play a key role in activating an oncogenic pathway in CTCL.
“We have gained important insight into the processes that activate cancer cells and make them grow,” said Niels Oedum, MD, of the University of Copenhagen in Denmark.
“[CTCL] patients’ frequent bacterial infections might not be a mere side effect of the disease. On the contrary, toxins in the bacteria actually ‘benefit’ cancer cells. Our next step is examining whether combatting infections can slow down the growth of cancer cells and thus stop the disease.”
Dr Oedum and his colleagues described their research in Blood.
The investigators knew that, in CTCL, CD4 T cells become malignant and turn parasitic on the rest of the immune system. In addition to using healthy cells to do their work for them, the malignant cells slowly destroy the skin’s immune defense mechanism.
The team’s new discoveries indicate that bacterial toxins in some patients enable malignant cells to send off signals that obstruct and change the immune defense mechanism, which would otherwise fight the malignant cells. What was believed to be an overly active immune defense mechanism could, in other words, turn out to be a malignant infection brought on by bacteria, which only worsens the disease.
Dr Oedum and his colleagues found that SEA-positive bacteria isolatated from the skin of CTCL patients stimulated activation of STAT3 and upregulation of IL-17 in malignant and non-malignant T cells.
Malignant T cells expressing an SEA non-responsive T-cell receptor V beta chain did not respond to SEA when cultured alone but exhibited STAT3 activation and IL-17 expression in co-cultures with SEA-responsive, non-malignant T cells.
The investigators found evidence to suggest the response is induced via IL-2Rg cytokines and a JAK3-dependent pathway in malignant T cells. The JAK3 inhibitor tofacitinib inhibited SEA-induced IL-17 production in co-cultures of malignant and non-malignant T cells.
Dr Oedum and his colleagues plan to continue their work investigating how bacteria might affect the balance between the immune defense mechanism and the disease in patients with CTCL.
In the long-term, the investigators’ aim is to understand how bacteria and their toxins can worsen CTCL, knowledge that may be used to develop new targeted treatments.
As only some of the bacteria produce toxins, the team said it will also be important to develop methods to determine which patients may benefit from treatment with antibiotics. ![]()
Cutting costs for cancer pts with comorbidities

Photo courtesy of the CDC
Patients with incurable cancer and multiple comorbidities who consulted with a palliative care team within 2 days of hospitalization had significant savings in hospital costs, according to a new study.
The study also showed that the higher number of comorbidities a patient had, the greater the reduction in direct hospital costs with early palliative care as opposed to standard care.
Previous studies have shown a link between palliative care and lower costs, but this is the first to examine whether the effect of palliative care consultation varies by the number of co-existing chronic conditions.
“We already know that coordinated, patient-centered palliative care improves care quality, enhances survival, and reduces costs for persons with cancer,” said R. Sean Morrison, MD, of the Icahn School of Medicine at Mount Sinai in New York, New York.
“Our latest research now shows the strong association between cost and the number of co-occurring conditions. Among patients with advanced cancer and other serious illnesses, aggressive treatments are often inconsistent with patients’ wishes and are associated with worse quality of life compared to other treatments. It is imperative that policymakers act to expand access to palliative care.”
Dr Morrison and his colleagues described their research in Health Affairs.
The study included 906 patients with advanced cancer and multiple comorbidities who were treated at 6 hospitals. One hundred and ninety-three patients were seen by a palliative care team within 2 days of hospitalization, while the remaining 713 patients received usual care.
Patients from the palliative care group had significantly lower total direct hospital costs if they had multimorbidity. For patients with a comorbidity score of 0–1, the estimated mean treatment effect was not significant.
However, patients with a comorbidity score of 2–3 had a 22% reduction in costs, or a reduction of $2321. Patients with a score of 4 or higher had a cost reduction of 32%, or $3515.
“The fact that we found greater cost savings for cancer patients with more comorbidities than for those with fewer comorbidities raises the question of whether similar results would be observed in patients with other serious illnesses and multimorbidity,” said Peter May, of Trinity College Dublin in Ireland.
“Future research is also needed to determine when in the course of illness palliative care is most cost-effective.” ![]()

Photo courtesy of the CDC
Patients with incurable cancer and multiple comorbidities who consulted with a palliative care team within 2 days of hospitalization had significant savings in hospital costs, according to a new study.
The study also showed that the higher number of comorbidities a patient had, the greater the reduction in direct hospital costs with early palliative care as opposed to standard care.
Previous studies have shown a link between palliative care and lower costs, but this is the first to examine whether the effect of palliative care consultation varies by the number of co-existing chronic conditions.
“We already know that coordinated, patient-centered palliative care improves care quality, enhances survival, and reduces costs for persons with cancer,” said R. Sean Morrison, MD, of the Icahn School of Medicine at Mount Sinai in New York, New York.
“Our latest research now shows the strong association between cost and the number of co-occurring conditions. Among patients with advanced cancer and other serious illnesses, aggressive treatments are often inconsistent with patients’ wishes and are associated with worse quality of life compared to other treatments. It is imperative that policymakers act to expand access to palliative care.”
Dr Morrison and his colleagues described their research in Health Affairs.
The study included 906 patients with advanced cancer and multiple comorbidities who were treated at 6 hospitals. One hundred and ninety-three patients were seen by a palliative care team within 2 days of hospitalization, while the remaining 713 patients received usual care.
Patients from the palliative care group had significantly lower total direct hospital costs if they had multimorbidity. For patients with a comorbidity score of 0–1, the estimated mean treatment effect was not significant.
However, patients with a comorbidity score of 2–3 had a 22% reduction in costs, or a reduction of $2321. Patients with a score of 4 or higher had a cost reduction of 32%, or $3515.
“The fact that we found greater cost savings for cancer patients with more comorbidities than for those with fewer comorbidities raises the question of whether similar results would be observed in patients with other serious illnesses and multimorbidity,” said Peter May, of Trinity College Dublin in Ireland.
“Future research is also needed to determine when in the course of illness palliative care is most cost-effective.” ![]()

Photo courtesy of the CDC
Patients with incurable cancer and multiple comorbidities who consulted with a palliative care team within 2 days of hospitalization had significant savings in hospital costs, according to a new study.
The study also showed that the higher number of comorbidities a patient had, the greater the reduction in direct hospital costs with early palliative care as opposed to standard care.
Previous studies have shown a link between palliative care and lower costs, but this is the first to examine whether the effect of palliative care consultation varies by the number of co-existing chronic conditions.
“We already know that coordinated, patient-centered palliative care improves care quality, enhances survival, and reduces costs for persons with cancer,” said R. Sean Morrison, MD, of the Icahn School of Medicine at Mount Sinai in New York, New York.
“Our latest research now shows the strong association between cost and the number of co-occurring conditions. Among patients with advanced cancer and other serious illnesses, aggressive treatments are often inconsistent with patients’ wishes and are associated with worse quality of life compared to other treatments. It is imperative that policymakers act to expand access to palliative care.”
Dr Morrison and his colleagues described their research in Health Affairs.
The study included 906 patients with advanced cancer and multiple comorbidities who were treated at 6 hospitals. One hundred and ninety-three patients were seen by a palliative care team within 2 days of hospitalization, while the remaining 713 patients received usual care.
Patients from the palliative care group had significantly lower total direct hospital costs if they had multimorbidity. For patients with a comorbidity score of 0–1, the estimated mean treatment effect was not significant.
However, patients with a comorbidity score of 2–3 had a 22% reduction in costs, or a reduction of $2321. Patients with a score of 4 or higher had a cost reduction of 32%, or $3515.
“The fact that we found greater cost savings for cancer patients with more comorbidities than for those with fewer comorbidities raises the question of whether similar results would be observed in patients with other serious illnesses and multimorbidity,” said Peter May, of Trinity College Dublin in Ireland.
“Future research is also needed to determine when in the course of illness palliative care is most cost-effective.” ![]()
A Perfect Storm: The current climate in breast cancer
This is the first installment of a five-part monthly series that will discuss the pathologic, genomic, and clinical factors that contribute to the racial survival disparity in breast cancer. The series, which is adapted from an article that originally appeared in CA: A Cancer Journal for Clinicians,1 a journal of the American Cancer Society, will also review exciting and innovative interventions to close this survival gap. This month’s column reviews the scope of this important health care issue.
The National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results Program (SEER) has estimated that 231,840 new cases of female breast cancer will be diagnosed in 2015, representing 14% of all new cancer cases among women. The NCI also has estimated 40,290 deaths from breast cancer, representing 6.8% of all cancer deaths among women.2 Breast cancer is the second leading cause of cancer death among women after lung cancer. It is well known that there has historically been a significant racial divide in breast cancer incidence (rate of new occurrences of breast cancer) and mortality (death) rates. Caucasian women were more likely to be diagnosed with breast cancer, but African American women were more likely to die from it.
However, in a recently released study by DeSantis et al. this incidence trend no longer holds, and in 2012 there was a convergence of breast cancer incidence rates at 135 cases per 100,000 women for both Caucasian and African American women.3 In addition, this recent analysis revealed that the mortality disparity between African American and Caucasian women has continued to increase, with a death rate 42% higher in African American than in Caucasian women in 2012. While overall improvements in therapy have led to a decrease in breast cancer death rates in the United States since 1990, the decreases in death rates began earlier and have been larger in proportionate terms for Caucasians than for African Americans.4,5 According to SEER data from 1975 to 2011, Caucasian women had a 23% increase in breast cancer incidence and a 34% decrease in mortality, whereas African American women experienced a 35% increase in incidence and a 2% increase in mortality.6
Beyond national statistics and on a more-local level, several studies have explored regional variations in breast cancer mortality by race. One such study analyzed mortality data from the National Center for Health Statistics from 1975 to 2004.5 The researchers discovered that trends in breast cancer death rates varied widely by region. While breast cancer death rates in Caucasian women decreased in all 50 states, among African American women in 37 states analyzed, breast cancer death rates increased in 2 states, were level in 24 states, and decreased in only 11 states. Many of the states in which African American breast cancer death rates were level or rising were in the South and Midwest.
There are also differences in age and stage at diagnosis between African American and Caucasian women. Although the overall incidence of breast cancer has been historically higher in Caucasians, the incidence profile changes when the data are looked at by age. Among African American women with breast cancer, 33% are diagnosed at an age younger than 50 years, compared with 21.9% among Caucasian women.7
In women younger than 35 years, the incidence of breast cancer in African Americans is 1.4-2.0 times that of Caucasians.8 In addition, African American women present with more advanced-stage disease. Again, using the SEER program and examining data from 2005-2011, 62% of Caucasians had localized disease (cancer confined to the breast and potentially curable) versus 53% of African Americans. In all, 5% of Caucasians had distant disease (cancer outside the breast and treatable but not curable), compared with 9% of African Americans.9 A recent study in JAMA of 373,563 women with breast cancer during 2004-2011 found that African American women were less likely to be diagnosed with stage I breast cancer than were non-Hispanic white women across all age groups (non-Hispanic white women, 50.8%; African American women, 37.0%).10
The researchers examined further those women with small breast cancers (breast tumors ≤ 2 cm) and the percentages of nodal metastases (cancer in the lymph nodes) and distant metastases (cancer outside the breast) by race/ethnicity. The authors found that an African American woman with a small-sized breast tumor was more likely to present with lymph node metastases and distant metastases. Significantly, African American women were also more likely to die of breast cancer with small-sized tumors than were non-Hispanic white women.
These differences in age and stage highlight important differences in tumor biology, genomics, and patterns of care that contribute to the disparity in breast cancer survival between Caucasian and African American women. The February installment of this column will explore tumor biology – the first element in the perfect storm.
Other installments of this column can be found in the Related Content box.
1. Daly B, Olopade OI: A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 65:221-38, 2015.
2. National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program Stat fact sheets: Breast cancer. Surveillance, Epidemiology, and End Results Program. http://seer.cancer.gov/statfacts/html/breast.html. Accessed Nov. 20, 2015.
3. DeSantis C, Fedewa S, Goding Sauer A, et al., Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA: A Cancer Journal for Clinicians. doi: 10.3322/caac.21320
4. DeLancey JO, Thun MJ, Jemal A, et al.: Recent trends in Black-White disparities in cancer mortality. Cancer Epidemiol Biomarkers Prev. 17:2908-12, 2008.
5. DeSantis C, Jemal A, Ward E, et al.: Temporal trends in breast cancer mortality by state and race. Cancer Causes Control. 19:537-45, 2008.
6. Howlander N NA, Krapcho M, et al. eds.: SEER Cancer Statistics Review, 1975-2011, 2014.
7. Clarke CA, West DW, Edwards BK, et al.: Existing data on breast cancer in African-American women: what we know and what we need to know. Cancer. 97:211-21, 2003.
8. Marie Swanson G, Haslam SZ, Azzouz F: Breast cancer among young African-American women: a summary of data and literature and of issues discussed during the Summit Meeting on Breast Cancer Among African American Women, Washington, DC, September 8-10, 2000. Cancer. 97:273-9, 2003.
9. National Cancer Institute. SEER Cancer Statistics Review, 1975-2012. http://seer.cancer.gov/csr/1975_2012/results_single/sect_04_table.13.pdf. Accessed, Nov. 20, 2015.
10. Iqbal J, Ginsburg O, Rochon PA, et al: Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA 313:165-73, 2015.
Bobby Daly, MD, MBA, is the chief fellow in the section of hematology/oncology at the University of Chicago Medicine. His clinical focus is breast and thoracic oncology, and his research focus is health services. Specifically, Dr. Daly researches disparities in oncology care delivery, oncology health care utilization, aggressive end-of-life oncology care, and oncology payment models. He received his MD and MBA from Harvard Medical School and Harvard Business School, both in Boston, and a BA in Economics and History from Stanford (Calif.) University. He was the recipient of the Dean’s Award at Harvard Medical and Business Schools.
Olufunmilayo Olopade, MD, FACP, OON, is the Walter L. Palmer Distinguished Service Professor of Medicine and Human Genetics, and director, Center for Global Health at the University of Chicago. She is adopting emerging high throughput genomic and informatics strategies to identify genetic and nongenetic risk factors for breast cancer in order to implement precision health care in diverse populations. This innovative approach has the potential to improve the quality of care and reduce costs while saving more lives.
Disclosures: Dr. Olopade serves on the Medical Advisory Board for CancerIQ. Dr. Daly serves as a director of Quadrant Holdings Corporation and receives compensation from this entity. Frontline Medical Communications is a subsidiary of Quadrant Holdings Corporation.
Published in conjunction with Susan G. Komen®.
This is the first installment of a five-part monthly series that will discuss the pathologic, genomic, and clinical factors that contribute to the racial survival disparity in breast cancer. The series, which is adapted from an article that originally appeared in CA: A Cancer Journal for Clinicians,1 a journal of the American Cancer Society, will also review exciting and innovative interventions to close this survival gap. This month’s column reviews the scope of this important health care issue.
The National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results Program (SEER) has estimated that 231,840 new cases of female breast cancer will be diagnosed in 2015, representing 14% of all new cancer cases among women. The NCI also has estimated 40,290 deaths from breast cancer, representing 6.8% of all cancer deaths among women.2 Breast cancer is the second leading cause of cancer death among women after lung cancer. It is well known that there has historically been a significant racial divide in breast cancer incidence (rate of new occurrences of breast cancer) and mortality (death) rates. Caucasian women were more likely to be diagnosed with breast cancer, but African American women were more likely to die from it.
However, in a recently released study by DeSantis et al. this incidence trend no longer holds, and in 2012 there was a convergence of breast cancer incidence rates at 135 cases per 100,000 women for both Caucasian and African American women.3 In addition, this recent analysis revealed that the mortality disparity between African American and Caucasian women has continued to increase, with a death rate 42% higher in African American than in Caucasian women in 2012. While overall improvements in therapy have led to a decrease in breast cancer death rates in the United States since 1990, the decreases in death rates began earlier and have been larger in proportionate terms for Caucasians than for African Americans.4,5 According to SEER data from 1975 to 2011, Caucasian women had a 23% increase in breast cancer incidence and a 34% decrease in mortality, whereas African American women experienced a 35% increase in incidence and a 2% increase in mortality.6
Beyond national statistics and on a more-local level, several studies have explored regional variations in breast cancer mortality by race. One such study analyzed mortality data from the National Center for Health Statistics from 1975 to 2004.5 The researchers discovered that trends in breast cancer death rates varied widely by region. While breast cancer death rates in Caucasian women decreased in all 50 states, among African American women in 37 states analyzed, breast cancer death rates increased in 2 states, were level in 24 states, and decreased in only 11 states. Many of the states in which African American breast cancer death rates were level or rising were in the South and Midwest.
There are also differences in age and stage at diagnosis between African American and Caucasian women. Although the overall incidence of breast cancer has been historically higher in Caucasians, the incidence profile changes when the data are looked at by age. Among African American women with breast cancer, 33% are diagnosed at an age younger than 50 years, compared with 21.9% among Caucasian women.7
In women younger than 35 years, the incidence of breast cancer in African Americans is 1.4-2.0 times that of Caucasians.8 In addition, African American women present with more advanced-stage disease. Again, using the SEER program and examining data from 2005-2011, 62% of Caucasians had localized disease (cancer confined to the breast and potentially curable) versus 53% of African Americans. In all, 5% of Caucasians had distant disease (cancer outside the breast and treatable but not curable), compared with 9% of African Americans.9 A recent study in JAMA of 373,563 women with breast cancer during 2004-2011 found that African American women were less likely to be diagnosed with stage I breast cancer than were non-Hispanic white women across all age groups (non-Hispanic white women, 50.8%; African American women, 37.0%).10
The researchers examined further those women with small breast cancers (breast tumors ≤ 2 cm) and the percentages of nodal metastases (cancer in the lymph nodes) and distant metastases (cancer outside the breast) by race/ethnicity. The authors found that an African American woman with a small-sized breast tumor was more likely to present with lymph node metastases and distant metastases. Significantly, African American women were also more likely to die of breast cancer with small-sized tumors than were non-Hispanic white women.
These differences in age and stage highlight important differences in tumor biology, genomics, and patterns of care that contribute to the disparity in breast cancer survival between Caucasian and African American women. The February installment of this column will explore tumor biology – the first element in the perfect storm.
Other installments of this column can be found in the Related Content box.
1. Daly B, Olopade OI: A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 65:221-38, 2015.
2. National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program Stat fact sheets: Breast cancer. Surveillance, Epidemiology, and End Results Program. http://seer.cancer.gov/statfacts/html/breast.html. Accessed Nov. 20, 2015.
3. DeSantis C, Fedewa S, Goding Sauer A, et al., Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA: A Cancer Journal for Clinicians. doi: 10.3322/caac.21320
4. DeLancey JO, Thun MJ, Jemal A, et al.: Recent trends in Black-White disparities in cancer mortality. Cancer Epidemiol Biomarkers Prev. 17:2908-12, 2008.
5. DeSantis C, Jemal A, Ward E, et al.: Temporal trends in breast cancer mortality by state and race. Cancer Causes Control. 19:537-45, 2008.
6. Howlander N NA, Krapcho M, et al. eds.: SEER Cancer Statistics Review, 1975-2011, 2014.
7. Clarke CA, West DW, Edwards BK, et al.: Existing data on breast cancer in African-American women: what we know and what we need to know. Cancer. 97:211-21, 2003.
8. Marie Swanson G, Haslam SZ, Azzouz F: Breast cancer among young African-American women: a summary of data and literature and of issues discussed during the Summit Meeting on Breast Cancer Among African American Women, Washington, DC, September 8-10, 2000. Cancer. 97:273-9, 2003.
9. National Cancer Institute. SEER Cancer Statistics Review, 1975-2012. http://seer.cancer.gov/csr/1975_2012/results_single/sect_04_table.13.pdf. Accessed, Nov. 20, 2015.
10. Iqbal J, Ginsburg O, Rochon PA, et al: Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA 313:165-73, 2015.
Bobby Daly, MD, MBA, is the chief fellow in the section of hematology/oncology at the University of Chicago Medicine. His clinical focus is breast and thoracic oncology, and his research focus is health services. Specifically, Dr. Daly researches disparities in oncology care delivery, oncology health care utilization, aggressive end-of-life oncology care, and oncology payment models. He received his MD and MBA from Harvard Medical School and Harvard Business School, both in Boston, and a BA in Economics and History from Stanford (Calif.) University. He was the recipient of the Dean’s Award at Harvard Medical and Business Schools.
Olufunmilayo Olopade, MD, FACP, OON, is the Walter L. Palmer Distinguished Service Professor of Medicine and Human Genetics, and director, Center for Global Health at the University of Chicago. She is adopting emerging high throughput genomic and informatics strategies to identify genetic and nongenetic risk factors for breast cancer in order to implement precision health care in diverse populations. This innovative approach has the potential to improve the quality of care and reduce costs while saving more lives.
Disclosures: Dr. Olopade serves on the Medical Advisory Board for CancerIQ. Dr. Daly serves as a director of Quadrant Holdings Corporation and receives compensation from this entity. Frontline Medical Communications is a subsidiary of Quadrant Holdings Corporation.
Published in conjunction with Susan G. Komen®.
This is the first installment of a five-part monthly series that will discuss the pathologic, genomic, and clinical factors that contribute to the racial survival disparity in breast cancer. The series, which is adapted from an article that originally appeared in CA: A Cancer Journal for Clinicians,1 a journal of the American Cancer Society, will also review exciting and innovative interventions to close this survival gap. This month’s column reviews the scope of this important health care issue.
The National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results Program (SEER) has estimated that 231,840 new cases of female breast cancer will be diagnosed in 2015, representing 14% of all new cancer cases among women. The NCI also has estimated 40,290 deaths from breast cancer, representing 6.8% of all cancer deaths among women.2 Breast cancer is the second leading cause of cancer death among women after lung cancer. It is well known that there has historically been a significant racial divide in breast cancer incidence (rate of new occurrences of breast cancer) and mortality (death) rates. Caucasian women were more likely to be diagnosed with breast cancer, but African American women were more likely to die from it.
However, in a recently released study by DeSantis et al. this incidence trend no longer holds, and in 2012 there was a convergence of breast cancer incidence rates at 135 cases per 100,000 women for both Caucasian and African American women.3 In addition, this recent analysis revealed that the mortality disparity between African American and Caucasian women has continued to increase, with a death rate 42% higher in African American than in Caucasian women in 2012. While overall improvements in therapy have led to a decrease in breast cancer death rates in the United States since 1990, the decreases in death rates began earlier and have been larger in proportionate terms for Caucasians than for African Americans.4,5 According to SEER data from 1975 to 2011, Caucasian women had a 23% increase in breast cancer incidence and a 34% decrease in mortality, whereas African American women experienced a 35% increase in incidence and a 2% increase in mortality.6
Beyond national statistics and on a more-local level, several studies have explored regional variations in breast cancer mortality by race. One such study analyzed mortality data from the National Center for Health Statistics from 1975 to 2004.5 The researchers discovered that trends in breast cancer death rates varied widely by region. While breast cancer death rates in Caucasian women decreased in all 50 states, among African American women in 37 states analyzed, breast cancer death rates increased in 2 states, were level in 24 states, and decreased in only 11 states. Many of the states in which African American breast cancer death rates were level or rising were in the South and Midwest.
There are also differences in age and stage at diagnosis between African American and Caucasian women. Although the overall incidence of breast cancer has been historically higher in Caucasians, the incidence profile changes when the data are looked at by age. Among African American women with breast cancer, 33% are diagnosed at an age younger than 50 years, compared with 21.9% among Caucasian women.7
In women younger than 35 years, the incidence of breast cancer in African Americans is 1.4-2.0 times that of Caucasians.8 In addition, African American women present with more advanced-stage disease. Again, using the SEER program and examining data from 2005-2011, 62% of Caucasians had localized disease (cancer confined to the breast and potentially curable) versus 53% of African Americans. In all, 5% of Caucasians had distant disease (cancer outside the breast and treatable but not curable), compared with 9% of African Americans.9 A recent study in JAMA of 373,563 women with breast cancer during 2004-2011 found that African American women were less likely to be diagnosed with stage I breast cancer than were non-Hispanic white women across all age groups (non-Hispanic white women, 50.8%; African American women, 37.0%).10
The researchers examined further those women with small breast cancers (breast tumors ≤ 2 cm) and the percentages of nodal metastases (cancer in the lymph nodes) and distant metastases (cancer outside the breast) by race/ethnicity. The authors found that an African American woman with a small-sized breast tumor was more likely to present with lymph node metastases and distant metastases. Significantly, African American women were also more likely to die of breast cancer with small-sized tumors than were non-Hispanic white women.
These differences in age and stage highlight important differences in tumor biology, genomics, and patterns of care that contribute to the disparity in breast cancer survival between Caucasian and African American women. The February installment of this column will explore tumor biology – the first element in the perfect storm.
Other installments of this column can be found in the Related Content box.
1. Daly B, Olopade OI: A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 65:221-38, 2015.
2. National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program Stat fact sheets: Breast cancer. Surveillance, Epidemiology, and End Results Program. http://seer.cancer.gov/statfacts/html/breast.html. Accessed Nov. 20, 2015.
3. DeSantis C, Fedewa S, Goding Sauer A, et al., Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA: A Cancer Journal for Clinicians. doi: 10.3322/caac.21320
4. DeLancey JO, Thun MJ, Jemal A, et al.: Recent trends in Black-White disparities in cancer mortality. Cancer Epidemiol Biomarkers Prev. 17:2908-12, 2008.
5. DeSantis C, Jemal A, Ward E, et al.: Temporal trends in breast cancer mortality by state and race. Cancer Causes Control. 19:537-45, 2008.
6. Howlander N NA, Krapcho M, et al. eds.: SEER Cancer Statistics Review, 1975-2011, 2014.
7. Clarke CA, West DW, Edwards BK, et al.: Existing data on breast cancer in African-American women: what we know and what we need to know. Cancer. 97:211-21, 2003.
8. Marie Swanson G, Haslam SZ, Azzouz F: Breast cancer among young African-American women: a summary of data and literature and of issues discussed during the Summit Meeting on Breast Cancer Among African American Women, Washington, DC, September 8-10, 2000. Cancer. 97:273-9, 2003.
9. National Cancer Institute. SEER Cancer Statistics Review, 1975-2012. http://seer.cancer.gov/csr/1975_2012/results_single/sect_04_table.13.pdf. Accessed, Nov. 20, 2015.
10. Iqbal J, Ginsburg O, Rochon PA, et al: Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA 313:165-73, 2015.
Bobby Daly, MD, MBA, is the chief fellow in the section of hematology/oncology at the University of Chicago Medicine. His clinical focus is breast and thoracic oncology, and his research focus is health services. Specifically, Dr. Daly researches disparities in oncology care delivery, oncology health care utilization, aggressive end-of-life oncology care, and oncology payment models. He received his MD and MBA from Harvard Medical School and Harvard Business School, both in Boston, and a BA in Economics and History from Stanford (Calif.) University. He was the recipient of the Dean’s Award at Harvard Medical and Business Schools.
Olufunmilayo Olopade, MD, FACP, OON, is the Walter L. Palmer Distinguished Service Professor of Medicine and Human Genetics, and director, Center for Global Health at the University of Chicago. She is adopting emerging high throughput genomic and informatics strategies to identify genetic and nongenetic risk factors for breast cancer in order to implement precision health care in diverse populations. This innovative approach has the potential to improve the quality of care and reduce costs while saving more lives.
Disclosures: Dr. Olopade serves on the Medical Advisory Board for CancerIQ. Dr. Daly serves as a director of Quadrant Holdings Corporation and receives compensation from this entity. Frontline Medical Communications is a subsidiary of Quadrant Holdings Corporation.
Published in conjunction with Susan G. Komen®.
Pure Intrathoracic Scapular Dislocation
Scapular dislocation, which is also termed locked scapula or scapulothoracic dislocation, is an unusual condition that can be described as extrathoracic or intrathoracic dislocation, depending on the penetration of scapula into the thoracic cavity.
There have been 3 reported cases of intrathoracic scapular dislocations in the literature,1-3all associated with a preexisting condition (eg, sternoclavicular separation, prior rib fracture, thoracotomy for a lung transplant procedure, or surgical resection of superior ribs during breast or pulmonary tumor excisions). Three published cases of intrathoracic scapular impaction involve comminuted scapular fractures with intrathoracic impaction of the inferior fragment through intercostal space.4-6
Here we report an intrathoracic scapular dislocation that was not associated with fracture of the scapula or predisposing factors. To our knowledge, this is the first case of pure intrathoracic dislocation. The possibility of intrathoracic scapular dislocation should be considered as part of the differential diagnosis even in patients with a negative anamnesis for predisposing factors, such as lung or chest surgery. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 29-year-old woman presented to the emergency department after a motor vehicle accident. She had tenderness over the left shoulder and left elbow with decreased range of motion; however, motor and sensory examination of the wrist and fingers were normal. No distal neurovascular deficit was noted.
Physical examination revealed pain on pelvic compression. We observed an asymmetrical appearance between shoulders; the left shoulder was superior when compared with the right side (Figure 1). Palpation of the scapula aggravated the pain. The inferior angle of the left scapula was not palpable, and the medial border was palpated through the intercostal space between the third and fourth ribs.
Initial radiographs showed additional left olecranon and bilateral ramus pubis fractures. A chest radiograph showed nondisplaced fractures of the second and third ribs without any obvious hemothorax or pneumothorax. No other pathology involving the chest, such as resection of the ribs or congenital anomaly, was observed. The patient reported no history of thoracic trauma or lung surgery. There were no fractures of the scapula, humerus, or clavicles. Thoracic computed tomography was performed, and 3-dimensional (3D) reconstruction showed that the inferior angle of scapula penetrated into the thoracic cavity through the third intercostal space (Figure 2).
Given the intrathoracic scapular dislocation diagnosis, closed reduction under sedation was planned. The patient was placed in the supine position, and reduction was performed by applying pressure on the shoulder anteriorly. This maneuver increased deformity. At the same time, another physician pulled the spine of the scapula superiorly, releasing the scapula out of the thoracic cavity. When the arm was slightly lowered to neutral position, scapular deformity was no longer present (Figure 3). A shoulder sling was applied, and the patient was hospitalized for surgical fixation of pelvic and olecranon fractures. The arm was immobilized in a sling for 1 week, and shoulder exercises were started immediately afterward.
At 1-month follow-up, full shoulder range of motion was achieved, although rehabilitation for the elbow continued. Final follow-up examination at 4 months revealed no difference between shoulders, and no recurrence occurred.
Discussion
Intrathoracic scapular dislocation is a rare injury. There are only a few cases reported in the literature, and most of them are well associated with a predisposing factor. Nettrour and colleagues1 described the first intrathoracic scapular dislocation, which occurred 6 weeks after sternoclavicular separation and fracture of a rib. In the case reports of Ward and colleagues2 and Fowler and colleagues,3 the predisposing factor was resection of the ribs due to pancoast tumor and breast carcinoma, respectively. The mechanism of these dislocations depends on a weak area over the thoracic cage, creating a fulcrum point for levering the scapula into the thoracic cavity.
There are other cases of scapular dislocations that are accompanied by a comminuted fracture of scapula; a review of the literature revealed 3 cases.4-6 In our opinion, fracture of the inferior pole of the scapula leads to injury of the soft tissues and also results in intrathoracic impaction by creating a weak area over the thoracic cavity. This mechanism can be referred to as penetration.
Our case is singular because it is the first case that is not associated with fracture of the scapula or predisposing factors. Consequently, we report the first pure intrathoracic scapular dislocation in the literature. It is important to suspect intrathoracic scapular dislocation in the case of deformity (Figure 1), even in the absence of any predisposing factors or scapular fracture.
Although plain radiographs may not be elucidative, 3D reconstruction of computed tomography (Figure 2) reveals the pathology and plays an important role in guiding treatment.
In the treatment of our patient, relying on the unique dislocation mechanism without any fracture of the scapula or ribs, we started early active shoulder movement after 1 week of immobilization in a shoulder sling, which prevented recurrence of dislocation. In addition to presenting the first pure intrathoracic scapular dislocation, this case demonstrated satisfactory clinical results with short-term immobilization and early rehabilitation.
Conclusion
Contrary to the literature, the possibility of intrathoracic scapular dislocation should be considered in the differential diagnosis even in patients with a negative anamnesis for predisposing factors, such as lung or chest surgery, and when no fractures are detected. Shoulder or thorax computed tomography, especially 3D reconstructions, are helpful in diagnosing the condition and in guiding treatment. Closed reduction under sedation followed by early rehabilitation is an appropriate treatment method, which resulted in a full return of function in 1 month in our patient.
1. Nettrour LF, Krufky EL, Mueller RE, Raycroft JF. Locked scapula: intrathoracic dislocation of the inferior angle. A case report. J Bone Joint Surg Am. 1972;54(2):413-416.
2. Ward WG, Weaver JP, Garrett WE Jr. Locked scapula: A case report. J Bone Joint Surg Am. 1989;71(10):1558-1159.
3. Fowler TT, Taylor BC, Fankhauser RA. Recurrent low-energy intrathoracic dislocation of the scapula. Am J Orthop. 2013;42(1):E1-E4.
4. Blue JM, Anglen JO, Helikson MA. Fracture of the scapula with intrathoracic penetration. A case report. J Bone Joint Surg Am. 1997;79(7):1076-1078.
5. Schwartzbach CC, Seoudi H, Ross AE, Hendershot K, Robinson L, Malekzadeh A. Fracture of the scapula with intrathoracic penetration in a skeletally mature patient. A case report. J Bone Joint Surg Am. 2006;88(12):2735-2738.
6. Porte AN, Wirtzfeld DA, Mann C. Intrathoracic scapular impaction: an unusual complication of scapular fractures. Can J Surg. 2009;52(3):E62-E63.
Scapular dislocation, which is also termed locked scapula or scapulothoracic dislocation, is an unusual condition that can be described as extrathoracic or intrathoracic dislocation, depending on the penetration of scapula into the thoracic cavity.
There have been 3 reported cases of intrathoracic scapular dislocations in the literature,1-3all associated with a preexisting condition (eg, sternoclavicular separation, prior rib fracture, thoracotomy for a lung transplant procedure, or surgical resection of superior ribs during breast or pulmonary tumor excisions). Three published cases of intrathoracic scapular impaction involve comminuted scapular fractures with intrathoracic impaction of the inferior fragment through intercostal space.4-6
Here we report an intrathoracic scapular dislocation that was not associated with fracture of the scapula or predisposing factors. To our knowledge, this is the first case of pure intrathoracic dislocation. The possibility of intrathoracic scapular dislocation should be considered as part of the differential diagnosis even in patients with a negative anamnesis for predisposing factors, such as lung or chest surgery. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 29-year-old woman presented to the emergency department after a motor vehicle accident. She had tenderness over the left shoulder and left elbow with decreased range of motion; however, motor and sensory examination of the wrist and fingers were normal. No distal neurovascular deficit was noted.
Physical examination revealed pain on pelvic compression. We observed an asymmetrical appearance between shoulders; the left shoulder was superior when compared with the right side (Figure 1). Palpation of the scapula aggravated the pain. The inferior angle of the left scapula was not palpable, and the medial border was palpated through the intercostal space between the third and fourth ribs.
Initial radiographs showed additional left olecranon and bilateral ramus pubis fractures. A chest radiograph showed nondisplaced fractures of the second and third ribs without any obvious hemothorax or pneumothorax. No other pathology involving the chest, such as resection of the ribs or congenital anomaly, was observed. The patient reported no history of thoracic trauma or lung surgery. There were no fractures of the scapula, humerus, or clavicles. Thoracic computed tomography was performed, and 3-dimensional (3D) reconstruction showed that the inferior angle of scapula penetrated into the thoracic cavity through the third intercostal space (Figure 2).
Given the intrathoracic scapular dislocation diagnosis, closed reduction under sedation was planned. The patient was placed in the supine position, and reduction was performed by applying pressure on the shoulder anteriorly. This maneuver increased deformity. At the same time, another physician pulled the spine of the scapula superiorly, releasing the scapula out of the thoracic cavity. When the arm was slightly lowered to neutral position, scapular deformity was no longer present (Figure 3). A shoulder sling was applied, and the patient was hospitalized for surgical fixation of pelvic and olecranon fractures. The arm was immobilized in a sling for 1 week, and shoulder exercises were started immediately afterward.
At 1-month follow-up, full shoulder range of motion was achieved, although rehabilitation for the elbow continued. Final follow-up examination at 4 months revealed no difference between shoulders, and no recurrence occurred.
Discussion
Intrathoracic scapular dislocation is a rare injury. There are only a few cases reported in the literature, and most of them are well associated with a predisposing factor. Nettrour and colleagues1 described the first intrathoracic scapular dislocation, which occurred 6 weeks after sternoclavicular separation and fracture of a rib. In the case reports of Ward and colleagues2 and Fowler and colleagues,3 the predisposing factor was resection of the ribs due to pancoast tumor and breast carcinoma, respectively. The mechanism of these dislocations depends on a weak area over the thoracic cage, creating a fulcrum point for levering the scapula into the thoracic cavity.
There are other cases of scapular dislocations that are accompanied by a comminuted fracture of scapula; a review of the literature revealed 3 cases.4-6 In our opinion, fracture of the inferior pole of the scapula leads to injury of the soft tissues and also results in intrathoracic impaction by creating a weak area over the thoracic cavity. This mechanism can be referred to as penetration.
Our case is singular because it is the first case that is not associated with fracture of the scapula or predisposing factors. Consequently, we report the first pure intrathoracic scapular dislocation in the literature. It is important to suspect intrathoracic scapular dislocation in the case of deformity (Figure 1), even in the absence of any predisposing factors or scapular fracture.
Although plain radiographs may not be elucidative, 3D reconstruction of computed tomography (Figure 2) reveals the pathology and plays an important role in guiding treatment.
In the treatment of our patient, relying on the unique dislocation mechanism without any fracture of the scapula or ribs, we started early active shoulder movement after 1 week of immobilization in a shoulder sling, which prevented recurrence of dislocation. In addition to presenting the first pure intrathoracic scapular dislocation, this case demonstrated satisfactory clinical results with short-term immobilization and early rehabilitation.
Conclusion
Contrary to the literature, the possibility of intrathoracic scapular dislocation should be considered in the differential diagnosis even in patients with a negative anamnesis for predisposing factors, such as lung or chest surgery, and when no fractures are detected. Shoulder or thorax computed tomography, especially 3D reconstructions, are helpful in diagnosing the condition and in guiding treatment. Closed reduction under sedation followed by early rehabilitation is an appropriate treatment method, which resulted in a full return of function in 1 month in our patient.
Scapular dislocation, which is also termed locked scapula or scapulothoracic dislocation, is an unusual condition that can be described as extrathoracic or intrathoracic dislocation, depending on the penetration of scapula into the thoracic cavity.
There have been 3 reported cases of intrathoracic scapular dislocations in the literature,1-3all associated with a preexisting condition (eg, sternoclavicular separation, prior rib fracture, thoracotomy for a lung transplant procedure, or surgical resection of superior ribs during breast or pulmonary tumor excisions). Three published cases of intrathoracic scapular impaction involve comminuted scapular fractures with intrathoracic impaction of the inferior fragment through intercostal space.4-6
Here we report an intrathoracic scapular dislocation that was not associated with fracture of the scapula or predisposing factors. To our knowledge, this is the first case of pure intrathoracic dislocation. The possibility of intrathoracic scapular dislocation should be considered as part of the differential diagnosis even in patients with a negative anamnesis for predisposing factors, such as lung or chest surgery. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 29-year-old woman presented to the emergency department after a motor vehicle accident. She had tenderness over the left shoulder and left elbow with decreased range of motion; however, motor and sensory examination of the wrist and fingers were normal. No distal neurovascular deficit was noted.
Physical examination revealed pain on pelvic compression. We observed an asymmetrical appearance between shoulders; the left shoulder was superior when compared with the right side (Figure 1). Palpation of the scapula aggravated the pain. The inferior angle of the left scapula was not palpable, and the medial border was palpated through the intercostal space between the third and fourth ribs.
Initial radiographs showed additional left olecranon and bilateral ramus pubis fractures. A chest radiograph showed nondisplaced fractures of the second and third ribs without any obvious hemothorax or pneumothorax. No other pathology involving the chest, such as resection of the ribs or congenital anomaly, was observed. The patient reported no history of thoracic trauma or lung surgery. There were no fractures of the scapula, humerus, or clavicles. Thoracic computed tomography was performed, and 3-dimensional (3D) reconstruction showed that the inferior angle of scapula penetrated into the thoracic cavity through the third intercostal space (Figure 2).
Given the intrathoracic scapular dislocation diagnosis, closed reduction under sedation was planned. The patient was placed in the supine position, and reduction was performed by applying pressure on the shoulder anteriorly. This maneuver increased deformity. At the same time, another physician pulled the spine of the scapula superiorly, releasing the scapula out of the thoracic cavity. When the arm was slightly lowered to neutral position, scapular deformity was no longer present (Figure 3). A shoulder sling was applied, and the patient was hospitalized for surgical fixation of pelvic and olecranon fractures. The arm was immobilized in a sling for 1 week, and shoulder exercises were started immediately afterward.
At 1-month follow-up, full shoulder range of motion was achieved, although rehabilitation for the elbow continued. Final follow-up examination at 4 months revealed no difference between shoulders, and no recurrence occurred.
Discussion
Intrathoracic scapular dislocation is a rare injury. There are only a few cases reported in the literature, and most of them are well associated with a predisposing factor. Nettrour and colleagues1 described the first intrathoracic scapular dislocation, which occurred 6 weeks after sternoclavicular separation and fracture of a rib. In the case reports of Ward and colleagues2 and Fowler and colleagues,3 the predisposing factor was resection of the ribs due to pancoast tumor and breast carcinoma, respectively. The mechanism of these dislocations depends on a weak area over the thoracic cage, creating a fulcrum point for levering the scapula into the thoracic cavity.
There are other cases of scapular dislocations that are accompanied by a comminuted fracture of scapula; a review of the literature revealed 3 cases.4-6 In our opinion, fracture of the inferior pole of the scapula leads to injury of the soft tissues and also results in intrathoracic impaction by creating a weak area over the thoracic cavity. This mechanism can be referred to as penetration.
Our case is singular because it is the first case that is not associated with fracture of the scapula or predisposing factors. Consequently, we report the first pure intrathoracic scapular dislocation in the literature. It is important to suspect intrathoracic scapular dislocation in the case of deformity (Figure 1), even in the absence of any predisposing factors or scapular fracture.
Although plain radiographs may not be elucidative, 3D reconstruction of computed tomography (Figure 2) reveals the pathology and plays an important role in guiding treatment.
In the treatment of our patient, relying on the unique dislocation mechanism without any fracture of the scapula or ribs, we started early active shoulder movement after 1 week of immobilization in a shoulder sling, which prevented recurrence of dislocation. In addition to presenting the first pure intrathoracic scapular dislocation, this case demonstrated satisfactory clinical results with short-term immobilization and early rehabilitation.
Conclusion
Contrary to the literature, the possibility of intrathoracic scapular dislocation should be considered in the differential diagnosis even in patients with a negative anamnesis for predisposing factors, such as lung or chest surgery, and when no fractures are detected. Shoulder or thorax computed tomography, especially 3D reconstructions, are helpful in diagnosing the condition and in guiding treatment. Closed reduction under sedation followed by early rehabilitation is an appropriate treatment method, which resulted in a full return of function in 1 month in our patient.
1. Nettrour LF, Krufky EL, Mueller RE, Raycroft JF. Locked scapula: intrathoracic dislocation of the inferior angle. A case report. J Bone Joint Surg Am. 1972;54(2):413-416.
2. Ward WG, Weaver JP, Garrett WE Jr. Locked scapula: A case report. J Bone Joint Surg Am. 1989;71(10):1558-1159.
3. Fowler TT, Taylor BC, Fankhauser RA. Recurrent low-energy intrathoracic dislocation of the scapula. Am J Orthop. 2013;42(1):E1-E4.
4. Blue JM, Anglen JO, Helikson MA. Fracture of the scapula with intrathoracic penetration. A case report. J Bone Joint Surg Am. 1997;79(7):1076-1078.
5. Schwartzbach CC, Seoudi H, Ross AE, Hendershot K, Robinson L, Malekzadeh A. Fracture of the scapula with intrathoracic penetration in a skeletally mature patient. A case report. J Bone Joint Surg Am. 2006;88(12):2735-2738.
6. Porte AN, Wirtzfeld DA, Mann C. Intrathoracic scapular impaction: an unusual complication of scapular fractures. Can J Surg. 2009;52(3):E62-E63.
1. Nettrour LF, Krufky EL, Mueller RE, Raycroft JF. Locked scapula: intrathoracic dislocation of the inferior angle. A case report. J Bone Joint Surg Am. 1972;54(2):413-416.
2. Ward WG, Weaver JP, Garrett WE Jr. Locked scapula: A case report. J Bone Joint Surg Am. 1989;71(10):1558-1159.
3. Fowler TT, Taylor BC, Fankhauser RA. Recurrent low-energy intrathoracic dislocation of the scapula. Am J Orthop. 2013;42(1):E1-E4.
4. Blue JM, Anglen JO, Helikson MA. Fracture of the scapula with intrathoracic penetration. A case report. J Bone Joint Surg Am. 1997;79(7):1076-1078.
5. Schwartzbach CC, Seoudi H, Ross AE, Hendershot K, Robinson L, Malekzadeh A. Fracture of the scapula with intrathoracic penetration in a skeletally mature patient. A case report. J Bone Joint Surg Am. 2006;88(12):2735-2738.
6. Porte AN, Wirtzfeld DA, Mann C. Intrathoracic scapular impaction: an unusual complication of scapular fractures. Can J Surg. 2009;52(3):E62-E63.
Web Page Content and Quality Assessed for Shoulder Replacement
The Internet is becoming a primary source for obtaining medical information. This growing trend may have serious implications for the medical field. As patients increasingly regard the Internet as an essential tool for obtaining health-related information, questions have been raised regarding the quality of medical information available on the Internet.1 Studies have shown that health-related sites often present inaccurate, inconsistent, and outdated information that may have a negative impact on health care decisions made by patients.2
According to the US Census Bureau, 71.7% of American households report having access to the Internet.3 Of those who have access to Internet, approximately 72% have sought health information online over the last year.4 Among people older than age 65 years living in the United States, there has been a growing trend toward using the Internet, from 14% in 2000 to almost 60% in 2013, according to the Pew Research Internet Project.5 Most medical websites are viewed for information on diseases and treatment options.6 Since most patients want to be informed about treatment options, as well as risks and benefits for each treatment, access to credible information is essential for proper decision-making.7
To assess the quality of information on the Internet, we used DISCERN, a standardized questionnaire to aid consumers in judging Internet content.8 The DISCERN instrument, available at www.discern.org.uk, was designed by an expert group in the United Kingdom. First, an expert panel developed and tested the instrument, and then health care providers and self-help group members tested it further.8,9 The questionnaire had been found to have good interrater reliability, regardless of use by health professionals or consumers.8-10
More than 53,000 shoulder arthroplasties are performed in the United States annually, and the number is growing, with the main goal of pain relief from glenohumeral degenerative joint disease.11,12 The Internet has become a quasi–second opinion for patients trying to participate in their care. Given the prevalence of shoulder-related surgeries, it is critical to analyze and become familiar with the quality of information that patients read online in order to direct them to nonbiased, all-inclusive websites. In this study, we provide a summary assessment and comparison of the quality of online information pertaining to shoulder replacement, using medical (total shoulder replacement) and nontechnical (shoulder replacement) search terms.
Methods
Websites were identified using 3 search engines (Google, Yahoo, and Bing) and 2 search terms, shoulder replacement (SR) and total shoulder arthroplasty (TSA), on January 17, 2014. These 3 search engines were used because 77% of health care–related information online searches begin through a search engine (Google, Bing, Yahoo); only 13% begin at a health care–specialized website.4 These search terms were used after consulting with orthopedic residents and attending physicians in a focus group regarding the terminology used with patients. The first 30 websites in each search engine were identified consecutively and evaluated for category and quality of information using the DISCERN instrument.
A total of 180 websites (90 per search term) were reviewed. Each website was evaluated independently by 3 medical students. In the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram, we recorded how websites were identified, screened, and included (Figure 1).13 Websites that were duplicated within each search term and those that were inaccessible were used to determine the total number of noncommercial versus commercial websites, but were excluded from the final analysis. The first part of the analysis involved determining the type of website (eg, commercial vs noncommercial) based upon the html endings. All .com endings were classified as commercial websites; noncommercial included .gov, .org, .edu, and .net endings. Next, each website was categorized based on the target audience. Websites were grouped into health professional–oriented information, patient-oriented, advertisement, or “other.” These classifications were based on those described in previous works.14,15 The “other” category included images, YouTube videos, another search engine, and open forums, which were also excluded from the final analysis because they were not easily evaluable with the DISCERN instrument. Websites were considered health professional–oriented if they included journal articles, scholarly articles, and/or rehabilitation protocols. Patient-directed websites clearly stated the information was directed to patients or provided a general overview. Advertisement included sites that displayed ads or products for sale. Websites were evaluated for quality using the DISCERN instrument (Figure 2).
DISCERN has 3 subdivision scores: the reliable score (composed of the first 8 questions), the treatment options (the next 7 questions), and 1 final question that addresses the overall quality of the website and is rated independently of the first 15 questions. DISCERN uses 2 scales, a binary scale anchored on both extremes with the number 1 equaling complete absence of the criteria being measured, and the number 5 at the upper extreme, representing completeness of the quality being assessed. In between 1 and 5 is a partial ordinal scale measuring from 2 to 4, which indicates the information is present to some extent but not complete. The ordinal scale allows ranking of the criteria being assessed. Summarizing values from each of the 2 scales poses some concern: the scale is not a true binary scale because of the ordinal scale of the middle numbers (2-4), and as such, is not amenable to being an interval scale to calculate arithmetic means. To summarize the values from the 2 scales, we calculated the harmonic mean, the arithmetic mean, the geometric mean, and the median. The means were empirically compared with the median, and we used the harmonic mean to summarize scale values because it was the best approximation of the medians.
Results
A total of 90 websites were assessed with the search term total shoulder arthroplasty and another 90 with shoulder replacement. When 37 duplicate websites for TSA and 52 for SR were eliminated, 53 (59%) and 38 (42%) unique websites were evaluated for each search term, respectively (Figure 1). (These unique websites are included in the Appendix.) Between the 2 search terms, 20 websites were duplicated. Figure 3 shows the distribution of websites by category. Total shoulder arthroplasty provided the highest percentage of health professional–oriented information; SR had the greatest percentage of patient-oriented information. Both TSA and SR had nearly the same number of advertisements and websites labeled “other.” The percentage of noncommercial websites from each search engine is represented in Figure 4. For SR, Google had 40% (12/30) noncommercial websites compared with Yahoo at 53% (16/30) and Bing at 46% (14/30). Total shoulder arthroplasty had 43% (13/30) noncommercial websites on Google, 27% (8/30) on Yahoo, and 40% (12/30) on Bing. In total, SR had more noncommercial websites, 47% (42/90), compared with 37% (33/90) for TSA.
The mean of all 3 raters for reliablity (DISCERN questions 1-8) and treatment options (DISCERN questions 9-15) is represented in the Table. For both search terms, we found that websites identified as health professional–oriented had the highest reliable mean scores, followed by patient-oriented, and advertisement at the lowest (SR: P = .054; TSA: P = .134). For SR, treatment mean scores demonstrated similar results with health professional–oriented websites receiving the highest, followed by patient-oriented and advertisement (P = .005). However, the treatment mean scores for TSA differed with patient-oriented websites receiving higher scores than health professional–oriented websites, but this was not statistically significant (P= .407). Regarding search terms, there were no significant differences between mean reliable and treatment scores across all categories.
The average overall DISCERN score for TSA websites was 2.5 (range, 1-5), compared with 2.3 (range, 1-5) for SR websites. The overall reliable score (DISCERN questions 1-8) for TSA websites was 2.6 and 2.5 for SR websites (P < .001). For TSA websites, 38% (20/53) were classified as good, having an overall DISCERN score ≥3, versus 26% (10/38) of SR websites. The overall DISCERN score for health professional–oriented websites was 2.7, patient-oriented websites received a score of 2.6, and advertisements had the lowest score at 2.4.
Discussion
Both patients and health professionals obtain information on health care subjects through the Internet, which has become the primary resource for patients.15,16 However, there are no strict regulations of the content being written. This creates a challenge for the typical user to find credible and evidence-based information, which is important because misleading information could cause undue anxiety, among other effects.17,18 The aims of this study were to determine the quality of Internet information for shoulder replacement surgeries using the medical terminology total shoulder arthroplasty (TSA) and the nontechnical term shoulder replacement (SR), and to compare the results.
After analyzing the types of websites returned for both total shoulder arthroplasty and shoulder replacement (Figure 4), it was interesting to find that using nonmedical terminology as the search term provided more noncommercial websites compared with total shoulder arthroplasty. Furthermore, Yahoo provided the highest yield of noncommercial websites at 16, with Bing at 14, when using SR as the search term. We believe the increase in noncommercial websites returned for SR was greater than for TSA because SR yielded more patient-oriented websites, which usually had html endings of .edu and .org, as shown in Figure 3 (48% of SR websites offered patient-oriented information).
Although there were more noncommercial websites for SR, the majority of the DISCERN values between the 2 search terms did not differ significantly. This is a direct result of the number of sites (20) that were duplicated across both search terms. However as seen in the Table, TSA had similar reliable mean scores for advertisements and patient-oriented websites but a slightly higher reliable score for health professional–oriented websites. We correlated this with the increased number of health professional–oriented websites returned when using TSA as the search term (Figure 3). The health professional–oriented websites explained their aims and cited their sources more consistently than did patient-oriented sites and advertisements, resulting in higher reliable scores. Although patient-oriented websites frequently lacked citations, they provided information about multiple treatment options, which were more relevant to consumers. This resulted in nearly equivalent reliable scores. Treatment means for advertisements in both SR and TSA were similar. However, treatment means for professional-oriented websites in TSA were lower than those for SR because health professional–oriented websites often were only moderately relevant to consumers, with their focus usually on 1 treatment option or on rehabilitation protocols. Although the DISCERN scores were similar between the search terms, total shoulder arthroplasty provided more websites (20) classified as good—overall DISCERN score, ≥3—than SR did (10). Advertisement websites had similar overall DISCERN scores, which we anticipated because most of the advertisements were duplicated across the search terms.
Using the 2 search terms, academic websites and commercial websites, such as WebMD, consistently received higher reliable and overall DISCERN scores. Advertisement websites, which need to deliver a clear message, frequently scored high on explicitly stating their aims and relevance to consumers, but focused on their products without discussing the benefits of other treatment options. This is significant because Internet search engines, such as Google, offer sponsor links for which organizations pay to appear at the top of the search results. This creates the potential for consumers to receive biased information because most individuals only visit the top 10 websites generated by a search engine.19
We concluded that the quality of online information relating to SR and TSA was highly variable and frequently of moderate-to-poor quality, with most overall DISCERN scores <3. The quality of information found online for this study using the DISCERN instrument is consistent with those studies using DISCERN to evaluate other medical conditions (eg, bunions, chronic pain, general anesthesia, and anterior cruciate ligament reconstruction).2,9,15,19 These studies also concluded that online information varies tremendously in quality and completeness.
This study has several limitations. Websites were searched at a single time point and, because Internet resources are frequently updated, the results of this study could vary. Furthermore, although Google, Yahoo, and Bing are 3 of the most popular search engines, these are not the only resources patients use when searching the Internet for health-related information. Other search engines, such as Pubmed.gov and MSN.com, could provide additional websites for Internet users. Lastly, although DISCERN is validated to address the quality of information available online, it does not evaluate the accuracy of the information.8 Our use of DISCERN involves 2 scales, a binary yes/no (ratings, 1 and 5) and an ordinal scale (ratings, 2-4). As such, a single mean summary statistic cannot be calculated.
Conclusion
The information available on the Internet pertaining to TSA and SR is highly variable and provides mostly moderate-to-poor quality information based on the DISCERN instrument. Many websites failed to describe the benefits and the risks of different treatment options, including nonoperative management. Health care professionals should be aware that patients often refer to the Internet as a primary resource for obtaining medical information. It is important to direct patients to websites that provide accurate information, because patients who educate themselves about their conditions and actively participate in decision-making may have improved health outcomes.20-22 Overall, academic websites and commercial websites, such as WebMD and OrthoInfo, generally had higher DISCERN scores when using either search term. Of major concern is the potential for misleading advertisements or incorrect information that can negatively affect health outcomes. This study found that using nonmedical terminology (SR) provided more noncommercial and patient-oriented websites, especially through Yahoo. This study highlights the need for more comprehensive online information pertaining to shoulder replacement that can better serve as a resource for Internet users.
1. Eysenbach G, Powell J, Kuss O, Sa ER. Empirical studies assessing the quality of health information for consumers on the world wide web: a systematic review. JAMA. 2002;287(20):2691-2700.
2. Bruce-Brand RA, Baker JF, Byrne DP, Hogan NA, McCarthy T. Assessment of the quality and content of information on anterior cruciate ligament reconstruction on the internet. Arthroscopy. 2013;29(6):1095-1100.
3. Computer and internet use in the United States: population characteristics. US Census Bureau website. http://www.census.gov/hhes/computer/. Accessed December 11, 2015.
4. Fox S, Duggan M. Health online 2013. Pew Research Center website. http://pewinternet.org/Reports/2013/Health-online.aspx. Published January 15, 2013. Accessed November 24, 2015.
5. Smith A. Older adults and technology use. Pew Research Center website. http://www.pewinternet.org/2014/04/03/older-adults-and-technology-use. Published April 3, 2014. Accessed November 24, 2015.
6. Shuyler KS, Knight KM. What are patients seeking when they turn to the internet? Qualitative content analysis of questions asked by visitors to an orthopaedics web site. J Med Internet Res. 2003;5(4):e24.
7. Meredith P, Emberton M, Wood C, Smith J. Comparison of patients’ needs for information on prostate surgery with printed materials provided by surgeons. Qual Health Care. 1995;4(1):18-23.
8. Charnock D, Shepperd S, Needham G, Gann R. DISCERN: An instrument for judging the quality of written consumer health information on treatment choices. J Epidemiol Community Health. 1999;53(2):105-111.
9. Kaicker J, Debono VB, Dang W, Buckley N, Thabane L. Assessment of the quality and variability of health information on chronic pain websites using the DISCERN instrument. BMC Med. 2010;8(1):59.
10. Griffiths KM, Christensen H. Website quality indicators for consumers. J Med Internet Res. 2005;7(5):e55.
11. Wiater JM. Shoulder joint replacement. American Academy of Orthopedic Surgeons website. http://orthoinfo.aaos.org/topic.cfm?topic=A00094. Updated December 2011. Accessed November 24, 2015.
12. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the united states. J Bone Joint Surg Am. 2011;93(24):2249-2254.
13. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65-W94.
14. Nason GJ, Baker JF, Byrne DP, Noel J, Moore D, Kiely PJ. Scoliosis-specific information on the internet: has the “information highway” led to better information provision? Spine. 2012;37(21):E1364-E1369.
15. Starman JS, Gettys FK, Capo JA, Fleischli JE, Norton HJ, Karunakar MA. Quality and content of internet-based information for ten common orthopaedic sports medicine diagnoses. J Bone Joint Surg Am. 2010;92(7):1612-1618.
16. Bernstein J, Ahn J, Veillette C. The future of orthopaedic information management. J Bone Joint Surg Am. 2012;94(13):e95.
17. Berland GK, Elliott MN, Morales LS, et al. Health information on the Internet: accessibility, quality, and readability in English and Spanish. JAMA. 2001;285(20):2612-2621.
18. Fallowfield LJ, Hall A, Maguire GP, Baum M. Psychological outcomes of different treatment policies in women with early breast cancer outside a clinical trial. BMJ. 1990;301(6752):575-580.
19. Chong YM, Fraval A, Chandrananth J, Plunkett V, Tran P. Assessment of the quality of web-based information on bunions. Foot Ankle Int. 2013;34(8):1134-1139.
20. Brody DS, Miller SM, Lerman CE, Smith DG, Caputo GC. Patient perception of involvement in medical care. J Gen Intern Med. 1989;4(6):506-511.
21. Greenfield S, Kaplan S, Ware JE Jr. Expanding patient involvement in care. Effects on patient outcomes. Ann Intern Med. 1985;102(4):520-528.
22. Kaplan SH, Greenfield S, Ware JE Jr. Assessing the effects of physician-patient interactions on the outcomes of chronic disease. Med Care. 1989;27(3 suppl):S110-S127.
The Internet is becoming a primary source for obtaining medical information. This growing trend may have serious implications for the medical field. As patients increasingly regard the Internet as an essential tool for obtaining health-related information, questions have been raised regarding the quality of medical information available on the Internet.1 Studies have shown that health-related sites often present inaccurate, inconsistent, and outdated information that may have a negative impact on health care decisions made by patients.2
According to the US Census Bureau, 71.7% of American households report having access to the Internet.3 Of those who have access to Internet, approximately 72% have sought health information online over the last year.4 Among people older than age 65 years living in the United States, there has been a growing trend toward using the Internet, from 14% in 2000 to almost 60% in 2013, according to the Pew Research Internet Project.5 Most medical websites are viewed for information on diseases and treatment options.6 Since most patients want to be informed about treatment options, as well as risks and benefits for each treatment, access to credible information is essential for proper decision-making.7
To assess the quality of information on the Internet, we used DISCERN, a standardized questionnaire to aid consumers in judging Internet content.8 The DISCERN instrument, available at www.discern.org.uk, was designed by an expert group in the United Kingdom. First, an expert panel developed and tested the instrument, and then health care providers and self-help group members tested it further.8,9 The questionnaire had been found to have good interrater reliability, regardless of use by health professionals or consumers.8-10
More than 53,000 shoulder arthroplasties are performed in the United States annually, and the number is growing, with the main goal of pain relief from glenohumeral degenerative joint disease.11,12 The Internet has become a quasi–second opinion for patients trying to participate in their care. Given the prevalence of shoulder-related surgeries, it is critical to analyze and become familiar with the quality of information that patients read online in order to direct them to nonbiased, all-inclusive websites. In this study, we provide a summary assessment and comparison of the quality of online information pertaining to shoulder replacement, using medical (total shoulder replacement) and nontechnical (shoulder replacement) search terms.
Methods
Websites were identified using 3 search engines (Google, Yahoo, and Bing) and 2 search terms, shoulder replacement (SR) and total shoulder arthroplasty (TSA), on January 17, 2014. These 3 search engines were used because 77% of health care–related information online searches begin through a search engine (Google, Bing, Yahoo); only 13% begin at a health care–specialized website.4 These search terms were used after consulting with orthopedic residents and attending physicians in a focus group regarding the terminology used with patients. The first 30 websites in each search engine were identified consecutively and evaluated for category and quality of information using the DISCERN instrument.
A total of 180 websites (90 per search term) were reviewed. Each website was evaluated independently by 3 medical students. In the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram, we recorded how websites were identified, screened, and included (Figure 1).13 Websites that were duplicated within each search term and those that were inaccessible were used to determine the total number of noncommercial versus commercial websites, but were excluded from the final analysis. The first part of the analysis involved determining the type of website (eg, commercial vs noncommercial) based upon the html endings. All .com endings were classified as commercial websites; noncommercial included .gov, .org, .edu, and .net endings. Next, each website was categorized based on the target audience. Websites were grouped into health professional–oriented information, patient-oriented, advertisement, or “other.” These classifications were based on those described in previous works.14,15 The “other” category included images, YouTube videos, another search engine, and open forums, which were also excluded from the final analysis because they were not easily evaluable with the DISCERN instrument. Websites were considered health professional–oriented if they included journal articles, scholarly articles, and/or rehabilitation protocols. Patient-directed websites clearly stated the information was directed to patients or provided a general overview. Advertisement included sites that displayed ads or products for sale. Websites were evaluated for quality using the DISCERN instrument (Figure 2).
DISCERN has 3 subdivision scores: the reliable score (composed of the first 8 questions), the treatment options (the next 7 questions), and 1 final question that addresses the overall quality of the website and is rated independently of the first 15 questions. DISCERN uses 2 scales, a binary scale anchored on both extremes with the number 1 equaling complete absence of the criteria being measured, and the number 5 at the upper extreme, representing completeness of the quality being assessed. In between 1 and 5 is a partial ordinal scale measuring from 2 to 4, which indicates the information is present to some extent but not complete. The ordinal scale allows ranking of the criteria being assessed. Summarizing values from each of the 2 scales poses some concern: the scale is not a true binary scale because of the ordinal scale of the middle numbers (2-4), and as such, is not amenable to being an interval scale to calculate arithmetic means. To summarize the values from the 2 scales, we calculated the harmonic mean, the arithmetic mean, the geometric mean, and the median. The means were empirically compared with the median, and we used the harmonic mean to summarize scale values because it was the best approximation of the medians.
Results
A total of 90 websites were assessed with the search term total shoulder arthroplasty and another 90 with shoulder replacement. When 37 duplicate websites for TSA and 52 for SR were eliminated, 53 (59%) and 38 (42%) unique websites were evaluated for each search term, respectively (Figure 1). (These unique websites are included in the Appendix.) Between the 2 search terms, 20 websites were duplicated. Figure 3 shows the distribution of websites by category. Total shoulder arthroplasty provided the highest percentage of health professional–oriented information; SR had the greatest percentage of patient-oriented information. Both TSA and SR had nearly the same number of advertisements and websites labeled “other.” The percentage of noncommercial websites from each search engine is represented in Figure 4. For SR, Google had 40% (12/30) noncommercial websites compared with Yahoo at 53% (16/30) and Bing at 46% (14/30). Total shoulder arthroplasty had 43% (13/30) noncommercial websites on Google, 27% (8/30) on Yahoo, and 40% (12/30) on Bing. In total, SR had more noncommercial websites, 47% (42/90), compared with 37% (33/90) for TSA.
The mean of all 3 raters for reliablity (DISCERN questions 1-8) and treatment options (DISCERN questions 9-15) is represented in the Table. For both search terms, we found that websites identified as health professional–oriented had the highest reliable mean scores, followed by patient-oriented, and advertisement at the lowest (SR: P = .054; TSA: P = .134). For SR, treatment mean scores demonstrated similar results with health professional–oriented websites receiving the highest, followed by patient-oriented and advertisement (P = .005). However, the treatment mean scores for TSA differed with patient-oriented websites receiving higher scores than health professional–oriented websites, but this was not statistically significant (P= .407). Regarding search terms, there were no significant differences between mean reliable and treatment scores across all categories.
The average overall DISCERN score for TSA websites was 2.5 (range, 1-5), compared with 2.3 (range, 1-5) for SR websites. The overall reliable score (DISCERN questions 1-8) for TSA websites was 2.6 and 2.5 for SR websites (P < .001). For TSA websites, 38% (20/53) were classified as good, having an overall DISCERN score ≥3, versus 26% (10/38) of SR websites. The overall DISCERN score for health professional–oriented websites was 2.7, patient-oriented websites received a score of 2.6, and advertisements had the lowest score at 2.4.
Discussion
Both patients and health professionals obtain information on health care subjects through the Internet, which has become the primary resource for patients.15,16 However, there are no strict regulations of the content being written. This creates a challenge for the typical user to find credible and evidence-based information, which is important because misleading information could cause undue anxiety, among other effects.17,18 The aims of this study were to determine the quality of Internet information for shoulder replacement surgeries using the medical terminology total shoulder arthroplasty (TSA) and the nontechnical term shoulder replacement (SR), and to compare the results.
After analyzing the types of websites returned for both total shoulder arthroplasty and shoulder replacement (Figure 4), it was interesting to find that using nonmedical terminology as the search term provided more noncommercial websites compared with total shoulder arthroplasty. Furthermore, Yahoo provided the highest yield of noncommercial websites at 16, with Bing at 14, when using SR as the search term. We believe the increase in noncommercial websites returned for SR was greater than for TSA because SR yielded more patient-oriented websites, which usually had html endings of .edu and .org, as shown in Figure 3 (48% of SR websites offered patient-oriented information).
Although there were more noncommercial websites for SR, the majority of the DISCERN values between the 2 search terms did not differ significantly. This is a direct result of the number of sites (20) that were duplicated across both search terms. However as seen in the Table, TSA had similar reliable mean scores for advertisements and patient-oriented websites but a slightly higher reliable score for health professional–oriented websites. We correlated this with the increased number of health professional–oriented websites returned when using TSA as the search term (Figure 3). The health professional–oriented websites explained their aims and cited their sources more consistently than did patient-oriented sites and advertisements, resulting in higher reliable scores. Although patient-oriented websites frequently lacked citations, they provided information about multiple treatment options, which were more relevant to consumers. This resulted in nearly equivalent reliable scores. Treatment means for advertisements in both SR and TSA were similar. However, treatment means for professional-oriented websites in TSA were lower than those for SR because health professional–oriented websites often were only moderately relevant to consumers, with their focus usually on 1 treatment option or on rehabilitation protocols. Although the DISCERN scores were similar between the search terms, total shoulder arthroplasty provided more websites (20) classified as good—overall DISCERN score, ≥3—than SR did (10). Advertisement websites had similar overall DISCERN scores, which we anticipated because most of the advertisements were duplicated across the search terms.
Using the 2 search terms, academic websites and commercial websites, such as WebMD, consistently received higher reliable and overall DISCERN scores. Advertisement websites, which need to deliver a clear message, frequently scored high on explicitly stating their aims and relevance to consumers, but focused on their products without discussing the benefits of other treatment options. This is significant because Internet search engines, such as Google, offer sponsor links for which organizations pay to appear at the top of the search results. This creates the potential for consumers to receive biased information because most individuals only visit the top 10 websites generated by a search engine.19
We concluded that the quality of online information relating to SR and TSA was highly variable and frequently of moderate-to-poor quality, with most overall DISCERN scores <3. The quality of information found online for this study using the DISCERN instrument is consistent with those studies using DISCERN to evaluate other medical conditions (eg, bunions, chronic pain, general anesthesia, and anterior cruciate ligament reconstruction).2,9,15,19 These studies also concluded that online information varies tremendously in quality and completeness.
This study has several limitations. Websites were searched at a single time point and, because Internet resources are frequently updated, the results of this study could vary. Furthermore, although Google, Yahoo, and Bing are 3 of the most popular search engines, these are not the only resources patients use when searching the Internet for health-related information. Other search engines, such as Pubmed.gov and MSN.com, could provide additional websites for Internet users. Lastly, although DISCERN is validated to address the quality of information available online, it does not evaluate the accuracy of the information.8 Our use of DISCERN involves 2 scales, a binary yes/no (ratings, 1 and 5) and an ordinal scale (ratings, 2-4). As such, a single mean summary statistic cannot be calculated.
Conclusion
The information available on the Internet pertaining to TSA and SR is highly variable and provides mostly moderate-to-poor quality information based on the DISCERN instrument. Many websites failed to describe the benefits and the risks of different treatment options, including nonoperative management. Health care professionals should be aware that patients often refer to the Internet as a primary resource for obtaining medical information. It is important to direct patients to websites that provide accurate information, because patients who educate themselves about their conditions and actively participate in decision-making may have improved health outcomes.20-22 Overall, academic websites and commercial websites, such as WebMD and OrthoInfo, generally had higher DISCERN scores when using either search term. Of major concern is the potential for misleading advertisements or incorrect information that can negatively affect health outcomes. This study found that using nonmedical terminology (SR) provided more noncommercial and patient-oriented websites, especially through Yahoo. This study highlights the need for more comprehensive online information pertaining to shoulder replacement that can better serve as a resource for Internet users.
The Internet is becoming a primary source for obtaining medical information. This growing trend may have serious implications for the medical field. As patients increasingly regard the Internet as an essential tool for obtaining health-related information, questions have been raised regarding the quality of medical information available on the Internet.1 Studies have shown that health-related sites often present inaccurate, inconsistent, and outdated information that may have a negative impact on health care decisions made by patients.2
According to the US Census Bureau, 71.7% of American households report having access to the Internet.3 Of those who have access to Internet, approximately 72% have sought health information online over the last year.4 Among people older than age 65 years living in the United States, there has been a growing trend toward using the Internet, from 14% in 2000 to almost 60% in 2013, according to the Pew Research Internet Project.5 Most medical websites are viewed for information on diseases and treatment options.6 Since most patients want to be informed about treatment options, as well as risks and benefits for each treatment, access to credible information is essential for proper decision-making.7
To assess the quality of information on the Internet, we used DISCERN, a standardized questionnaire to aid consumers in judging Internet content.8 The DISCERN instrument, available at www.discern.org.uk, was designed by an expert group in the United Kingdom. First, an expert panel developed and tested the instrument, and then health care providers and self-help group members tested it further.8,9 The questionnaire had been found to have good interrater reliability, regardless of use by health professionals or consumers.8-10
More than 53,000 shoulder arthroplasties are performed in the United States annually, and the number is growing, with the main goal of pain relief from glenohumeral degenerative joint disease.11,12 The Internet has become a quasi–second opinion for patients trying to participate in their care. Given the prevalence of shoulder-related surgeries, it is critical to analyze and become familiar with the quality of information that patients read online in order to direct them to nonbiased, all-inclusive websites. In this study, we provide a summary assessment and comparison of the quality of online information pertaining to shoulder replacement, using medical (total shoulder replacement) and nontechnical (shoulder replacement) search terms.
Methods
Websites were identified using 3 search engines (Google, Yahoo, and Bing) and 2 search terms, shoulder replacement (SR) and total shoulder arthroplasty (TSA), on January 17, 2014. These 3 search engines were used because 77% of health care–related information online searches begin through a search engine (Google, Bing, Yahoo); only 13% begin at a health care–specialized website.4 These search terms were used after consulting with orthopedic residents and attending physicians in a focus group regarding the terminology used with patients. The first 30 websites in each search engine were identified consecutively and evaluated for category and quality of information using the DISCERN instrument.
A total of 180 websites (90 per search term) were reviewed. Each website was evaluated independently by 3 medical students. In the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram, we recorded how websites were identified, screened, and included (Figure 1).13 Websites that were duplicated within each search term and those that were inaccessible were used to determine the total number of noncommercial versus commercial websites, but were excluded from the final analysis. The first part of the analysis involved determining the type of website (eg, commercial vs noncommercial) based upon the html endings. All .com endings were classified as commercial websites; noncommercial included .gov, .org, .edu, and .net endings. Next, each website was categorized based on the target audience. Websites were grouped into health professional–oriented information, patient-oriented, advertisement, or “other.” These classifications were based on those described in previous works.14,15 The “other” category included images, YouTube videos, another search engine, and open forums, which were also excluded from the final analysis because they were not easily evaluable with the DISCERN instrument. Websites were considered health professional–oriented if they included journal articles, scholarly articles, and/or rehabilitation protocols. Patient-directed websites clearly stated the information was directed to patients or provided a general overview. Advertisement included sites that displayed ads or products for sale. Websites were evaluated for quality using the DISCERN instrument (Figure 2).
DISCERN has 3 subdivision scores: the reliable score (composed of the first 8 questions), the treatment options (the next 7 questions), and 1 final question that addresses the overall quality of the website and is rated independently of the first 15 questions. DISCERN uses 2 scales, a binary scale anchored on both extremes with the number 1 equaling complete absence of the criteria being measured, and the number 5 at the upper extreme, representing completeness of the quality being assessed. In between 1 and 5 is a partial ordinal scale measuring from 2 to 4, which indicates the information is present to some extent but not complete. The ordinal scale allows ranking of the criteria being assessed. Summarizing values from each of the 2 scales poses some concern: the scale is not a true binary scale because of the ordinal scale of the middle numbers (2-4), and as such, is not amenable to being an interval scale to calculate arithmetic means. To summarize the values from the 2 scales, we calculated the harmonic mean, the arithmetic mean, the geometric mean, and the median. The means were empirically compared with the median, and we used the harmonic mean to summarize scale values because it was the best approximation of the medians.
Results
A total of 90 websites were assessed with the search term total shoulder arthroplasty and another 90 with shoulder replacement. When 37 duplicate websites for TSA and 52 for SR were eliminated, 53 (59%) and 38 (42%) unique websites were evaluated for each search term, respectively (Figure 1). (These unique websites are included in the Appendix.) Between the 2 search terms, 20 websites were duplicated. Figure 3 shows the distribution of websites by category. Total shoulder arthroplasty provided the highest percentage of health professional–oriented information; SR had the greatest percentage of patient-oriented information. Both TSA and SR had nearly the same number of advertisements and websites labeled “other.” The percentage of noncommercial websites from each search engine is represented in Figure 4. For SR, Google had 40% (12/30) noncommercial websites compared with Yahoo at 53% (16/30) and Bing at 46% (14/30). Total shoulder arthroplasty had 43% (13/30) noncommercial websites on Google, 27% (8/30) on Yahoo, and 40% (12/30) on Bing. In total, SR had more noncommercial websites, 47% (42/90), compared with 37% (33/90) for TSA.
The mean of all 3 raters for reliablity (DISCERN questions 1-8) and treatment options (DISCERN questions 9-15) is represented in the Table. For both search terms, we found that websites identified as health professional–oriented had the highest reliable mean scores, followed by patient-oriented, and advertisement at the lowest (SR: P = .054; TSA: P = .134). For SR, treatment mean scores demonstrated similar results with health professional–oriented websites receiving the highest, followed by patient-oriented and advertisement (P = .005). However, the treatment mean scores for TSA differed with patient-oriented websites receiving higher scores than health professional–oriented websites, but this was not statistically significant (P= .407). Regarding search terms, there were no significant differences between mean reliable and treatment scores across all categories.
The average overall DISCERN score for TSA websites was 2.5 (range, 1-5), compared with 2.3 (range, 1-5) for SR websites. The overall reliable score (DISCERN questions 1-8) for TSA websites was 2.6 and 2.5 for SR websites (P < .001). For TSA websites, 38% (20/53) were classified as good, having an overall DISCERN score ≥3, versus 26% (10/38) of SR websites. The overall DISCERN score for health professional–oriented websites was 2.7, patient-oriented websites received a score of 2.6, and advertisements had the lowest score at 2.4.
Discussion
Both patients and health professionals obtain information on health care subjects through the Internet, which has become the primary resource for patients.15,16 However, there are no strict regulations of the content being written. This creates a challenge for the typical user to find credible and evidence-based information, which is important because misleading information could cause undue anxiety, among other effects.17,18 The aims of this study were to determine the quality of Internet information for shoulder replacement surgeries using the medical terminology total shoulder arthroplasty (TSA) and the nontechnical term shoulder replacement (SR), and to compare the results.
After analyzing the types of websites returned for both total shoulder arthroplasty and shoulder replacement (Figure 4), it was interesting to find that using nonmedical terminology as the search term provided more noncommercial websites compared with total shoulder arthroplasty. Furthermore, Yahoo provided the highest yield of noncommercial websites at 16, with Bing at 14, when using SR as the search term. We believe the increase in noncommercial websites returned for SR was greater than for TSA because SR yielded more patient-oriented websites, which usually had html endings of .edu and .org, as shown in Figure 3 (48% of SR websites offered patient-oriented information).
Although there were more noncommercial websites for SR, the majority of the DISCERN values between the 2 search terms did not differ significantly. This is a direct result of the number of sites (20) that were duplicated across both search terms. However as seen in the Table, TSA had similar reliable mean scores for advertisements and patient-oriented websites but a slightly higher reliable score for health professional–oriented websites. We correlated this with the increased number of health professional–oriented websites returned when using TSA as the search term (Figure 3). The health professional–oriented websites explained their aims and cited their sources more consistently than did patient-oriented sites and advertisements, resulting in higher reliable scores. Although patient-oriented websites frequently lacked citations, they provided information about multiple treatment options, which were more relevant to consumers. This resulted in nearly equivalent reliable scores. Treatment means for advertisements in both SR and TSA were similar. However, treatment means for professional-oriented websites in TSA were lower than those for SR because health professional–oriented websites often were only moderately relevant to consumers, with their focus usually on 1 treatment option or on rehabilitation protocols. Although the DISCERN scores were similar between the search terms, total shoulder arthroplasty provided more websites (20) classified as good—overall DISCERN score, ≥3—than SR did (10). Advertisement websites had similar overall DISCERN scores, which we anticipated because most of the advertisements were duplicated across the search terms.
Using the 2 search terms, academic websites and commercial websites, such as WebMD, consistently received higher reliable and overall DISCERN scores. Advertisement websites, which need to deliver a clear message, frequently scored high on explicitly stating their aims and relevance to consumers, but focused on their products without discussing the benefits of other treatment options. This is significant because Internet search engines, such as Google, offer sponsor links for which organizations pay to appear at the top of the search results. This creates the potential for consumers to receive biased information because most individuals only visit the top 10 websites generated by a search engine.19
We concluded that the quality of online information relating to SR and TSA was highly variable and frequently of moderate-to-poor quality, with most overall DISCERN scores <3. The quality of information found online for this study using the DISCERN instrument is consistent with those studies using DISCERN to evaluate other medical conditions (eg, bunions, chronic pain, general anesthesia, and anterior cruciate ligament reconstruction).2,9,15,19 These studies also concluded that online information varies tremendously in quality and completeness.
This study has several limitations. Websites were searched at a single time point and, because Internet resources are frequently updated, the results of this study could vary. Furthermore, although Google, Yahoo, and Bing are 3 of the most popular search engines, these are not the only resources patients use when searching the Internet for health-related information. Other search engines, such as Pubmed.gov and MSN.com, could provide additional websites for Internet users. Lastly, although DISCERN is validated to address the quality of information available online, it does not evaluate the accuracy of the information.8 Our use of DISCERN involves 2 scales, a binary yes/no (ratings, 1 and 5) and an ordinal scale (ratings, 2-4). As such, a single mean summary statistic cannot be calculated.
Conclusion
The information available on the Internet pertaining to TSA and SR is highly variable and provides mostly moderate-to-poor quality information based on the DISCERN instrument. Many websites failed to describe the benefits and the risks of different treatment options, including nonoperative management. Health care professionals should be aware that patients often refer to the Internet as a primary resource for obtaining medical information. It is important to direct patients to websites that provide accurate information, because patients who educate themselves about their conditions and actively participate in decision-making may have improved health outcomes.20-22 Overall, academic websites and commercial websites, such as WebMD and OrthoInfo, generally had higher DISCERN scores when using either search term. Of major concern is the potential for misleading advertisements or incorrect information that can negatively affect health outcomes. This study found that using nonmedical terminology (SR) provided more noncommercial and patient-oriented websites, especially through Yahoo. This study highlights the need for more comprehensive online information pertaining to shoulder replacement that can better serve as a resource for Internet users.
1. Eysenbach G, Powell J, Kuss O, Sa ER. Empirical studies assessing the quality of health information for consumers on the world wide web: a systematic review. JAMA. 2002;287(20):2691-2700.
2. Bruce-Brand RA, Baker JF, Byrne DP, Hogan NA, McCarthy T. Assessment of the quality and content of information on anterior cruciate ligament reconstruction on the internet. Arthroscopy. 2013;29(6):1095-1100.
3. Computer and internet use in the United States: population characteristics. US Census Bureau website. http://www.census.gov/hhes/computer/. Accessed December 11, 2015.
4. Fox S, Duggan M. Health online 2013. Pew Research Center website. http://pewinternet.org/Reports/2013/Health-online.aspx. Published January 15, 2013. Accessed November 24, 2015.
5. Smith A. Older adults and technology use. Pew Research Center website. http://www.pewinternet.org/2014/04/03/older-adults-and-technology-use. Published April 3, 2014. Accessed November 24, 2015.
6. Shuyler KS, Knight KM. What are patients seeking when they turn to the internet? Qualitative content analysis of questions asked by visitors to an orthopaedics web site. J Med Internet Res. 2003;5(4):e24.
7. Meredith P, Emberton M, Wood C, Smith J. Comparison of patients’ needs for information on prostate surgery with printed materials provided by surgeons. Qual Health Care. 1995;4(1):18-23.
8. Charnock D, Shepperd S, Needham G, Gann R. DISCERN: An instrument for judging the quality of written consumer health information on treatment choices. J Epidemiol Community Health. 1999;53(2):105-111.
9. Kaicker J, Debono VB, Dang W, Buckley N, Thabane L. Assessment of the quality and variability of health information on chronic pain websites using the DISCERN instrument. BMC Med. 2010;8(1):59.
10. Griffiths KM, Christensen H. Website quality indicators for consumers. J Med Internet Res. 2005;7(5):e55.
11. Wiater JM. Shoulder joint replacement. American Academy of Orthopedic Surgeons website. http://orthoinfo.aaos.org/topic.cfm?topic=A00094. Updated December 2011. Accessed November 24, 2015.
12. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the united states. J Bone Joint Surg Am. 2011;93(24):2249-2254.
13. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65-W94.
14. Nason GJ, Baker JF, Byrne DP, Noel J, Moore D, Kiely PJ. Scoliosis-specific information on the internet: has the “information highway” led to better information provision? Spine. 2012;37(21):E1364-E1369.
15. Starman JS, Gettys FK, Capo JA, Fleischli JE, Norton HJ, Karunakar MA. Quality and content of internet-based information for ten common orthopaedic sports medicine diagnoses. J Bone Joint Surg Am. 2010;92(7):1612-1618.
16. Bernstein J, Ahn J, Veillette C. The future of orthopaedic information management. J Bone Joint Surg Am. 2012;94(13):e95.
17. Berland GK, Elliott MN, Morales LS, et al. Health information on the Internet: accessibility, quality, and readability in English and Spanish. JAMA. 2001;285(20):2612-2621.
18. Fallowfield LJ, Hall A, Maguire GP, Baum M. Psychological outcomes of different treatment policies in women with early breast cancer outside a clinical trial. BMJ. 1990;301(6752):575-580.
19. Chong YM, Fraval A, Chandrananth J, Plunkett V, Tran P. Assessment of the quality of web-based information on bunions. Foot Ankle Int. 2013;34(8):1134-1139.
20. Brody DS, Miller SM, Lerman CE, Smith DG, Caputo GC. Patient perception of involvement in medical care. J Gen Intern Med. 1989;4(6):506-511.
21. Greenfield S, Kaplan S, Ware JE Jr. Expanding patient involvement in care. Effects on patient outcomes. Ann Intern Med. 1985;102(4):520-528.
22. Kaplan SH, Greenfield S, Ware JE Jr. Assessing the effects of physician-patient interactions on the outcomes of chronic disease. Med Care. 1989;27(3 suppl):S110-S127.
1. Eysenbach G, Powell J, Kuss O, Sa ER. Empirical studies assessing the quality of health information for consumers on the world wide web: a systematic review. JAMA. 2002;287(20):2691-2700.
2. Bruce-Brand RA, Baker JF, Byrne DP, Hogan NA, McCarthy T. Assessment of the quality and content of information on anterior cruciate ligament reconstruction on the internet. Arthroscopy. 2013;29(6):1095-1100.
3. Computer and internet use in the United States: population characteristics. US Census Bureau website. http://www.census.gov/hhes/computer/. Accessed December 11, 2015.
4. Fox S, Duggan M. Health online 2013. Pew Research Center website. http://pewinternet.org/Reports/2013/Health-online.aspx. Published January 15, 2013. Accessed November 24, 2015.
5. Smith A. Older adults and technology use. Pew Research Center website. http://www.pewinternet.org/2014/04/03/older-adults-and-technology-use. Published April 3, 2014. Accessed November 24, 2015.
6. Shuyler KS, Knight KM. What are patients seeking when they turn to the internet? Qualitative content analysis of questions asked by visitors to an orthopaedics web site. J Med Internet Res. 2003;5(4):e24.
7. Meredith P, Emberton M, Wood C, Smith J. Comparison of patients’ needs for information on prostate surgery with printed materials provided by surgeons. Qual Health Care. 1995;4(1):18-23.
8. Charnock D, Shepperd S, Needham G, Gann R. DISCERN: An instrument for judging the quality of written consumer health information on treatment choices. J Epidemiol Community Health. 1999;53(2):105-111.
9. Kaicker J, Debono VB, Dang W, Buckley N, Thabane L. Assessment of the quality and variability of health information on chronic pain websites using the DISCERN instrument. BMC Med. 2010;8(1):59.
10. Griffiths KM, Christensen H. Website quality indicators for consumers. J Med Internet Res. 2005;7(5):e55.
11. Wiater JM. Shoulder joint replacement. American Academy of Orthopedic Surgeons website. http://orthoinfo.aaos.org/topic.cfm?topic=A00094. Updated December 2011. Accessed November 24, 2015.
12. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the united states. J Bone Joint Surg Am. 2011;93(24):2249-2254.
13. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65-W94.
14. Nason GJ, Baker JF, Byrne DP, Noel J, Moore D, Kiely PJ. Scoliosis-specific information on the internet: has the “information highway” led to better information provision? Spine. 2012;37(21):E1364-E1369.
15. Starman JS, Gettys FK, Capo JA, Fleischli JE, Norton HJ, Karunakar MA. Quality and content of internet-based information for ten common orthopaedic sports medicine diagnoses. J Bone Joint Surg Am. 2010;92(7):1612-1618.
16. Bernstein J, Ahn J, Veillette C. The future of orthopaedic information management. J Bone Joint Surg Am. 2012;94(13):e95.
17. Berland GK, Elliott MN, Morales LS, et al. Health information on the Internet: accessibility, quality, and readability in English and Spanish. JAMA. 2001;285(20):2612-2621.
18. Fallowfield LJ, Hall A, Maguire GP, Baum M. Psychological outcomes of different treatment policies in women with early breast cancer outside a clinical trial. BMJ. 1990;301(6752):575-580.
19. Chong YM, Fraval A, Chandrananth J, Plunkett V, Tran P. Assessment of the quality of web-based information on bunions. Foot Ankle Int. 2013;34(8):1134-1139.
20. Brody DS, Miller SM, Lerman CE, Smith DG, Caputo GC. Patient perception of involvement in medical care. J Gen Intern Med. 1989;4(6):506-511.
21. Greenfield S, Kaplan S, Ware JE Jr. Expanding patient involvement in care. Effects on patient outcomes. Ann Intern Med. 1985;102(4):520-528.
22. Kaplan SH, Greenfield S, Ware JE Jr. Assessing the effects of physician-patient interactions on the outcomes of chronic disease. Med Care. 1989;27(3 suppl):S110-S127.