User login
A new standard of care for rel/ref MM?

Photo courtesy of ASH
ORLANDO, FL—Adding the oral proteasome inhibitor ixazomib to treatment with lenalidomide and dexamethasone can prolong progression-free survival (PFS) in patients with relapsed and/or refractory multiple myeloma (MM), according to interim results of the phase 3 TOURMALINE-MM1 trial.
It is not yet clear if the 3-drug combination can prolong overall survival when compared to treatment with lenalidomide and dexamethasone.
However, researchers believe the triplet shows promise and could become a new standard of care for relapsed/refractory MM.
Philippe Moreau, MD, of the University of Nantes in France, discussed this possibility while presenting results from TOURMALINE-MM1 at the 2015 ASH Annual Meeting (abstract 727*). The study was sponsored by Millennium Pharmaceuticals, Inc.
The trial included 722 MM patients enrolled at 147 centers in 26 countries. Patients were randomized to receive ixazomib, lenalidomide, and dexamethasone (IRd, n=360) or placebo, lenalidomide, and dexamethasone (Rd, n=362).
Baseline patient characteristics were similar between the arms. The median age was 66 in both arms (overall range, 30-91), and nearly 60% of patients in both arms were male.
Fifty percent of patients in the IRd arm and 47% in the Rd arm had an ECOG performance status of 0. Forty-three percent and 45%, respectively, had a status of 1, and 5% and 7%, respectively, had a status of 2.
Eighty-seven percent and 88%, respectively, had an ISS stage of I or II. Fifty-five percent of patients in the IRd arm had standard-risk cytogenetics, as did 60% in the Rd arm.
Fifty-nine percent of patients in both arms had received 1 prior line of therapy, and 41% in both arms had 2 or 3 prior lines.
Response and survival
“Ixazomib, when combined with len-dex . . . , was associated with a significant and meaningful improvement in progression-free survival, improved time to progression, and [higher] response rate as well,” Dr Moreau said.
At a median follow-up of about 15 months, the median PFS was 20.6 months in the IRd arm and 14.7 months in the Rd arm. The hazard ratio was 0.742 (P=0.012).
Dr Moreau said the PFS benefit was consistent across pre-specified subgroups. So the benefit was present regardless of age, ISS stage, cytogenetic risk, number of prior therapies, prior exposure to a proteasome inhibitor, prior immunomodulatory therapy, whether the patient was refractory to his last therapy, and whether the patient had relapsed or refractory disease.
Dr Moreau also pointed out that, in the IRd arm, the median PFS in high-risk patients was similar to that in the overall patient population and in patients with standard-risk cytogenetics. This suggests ixazomib may overcome the negative impact of cytogenetic alterations.
Whether IRd confers an overall survival benefit is not clear, as those data are not yet mature. At a median follow-up of about 23 months, the median overall survival was not reached in either treatment arm.
The researchers conducted a non-inferential PFS analysis at the same time point (23 months) and found the median PFS was 20 months in the IRd arm and 15.9 months in the Rd arm. The hazard ratio was 0.82.
As for other efficacy endpoints, the overall response rate was 78.3% in the IRd arm and 71.5% in the Rd arm (P=0.035). The rates of complete response were 11.7% and 6.6%, respectively (P=0.019). And the rates of very good partial response or greater were 48.1% and 39%, respectively (P=0.014).
The median time to response was 1.1 months in the IRd arm and 1.9 months in the Rd arm. The median duration of response was 20.5 months and 15 months, respectively. And the median time to progression was 21.4 months and 15.7 months, respectively.
Adverse events
At a median follow-up of about 23 months, patients had received a median of 17 cycles of IRd and a median of 15 cycles of Rd.
The incidence of any adverse event (AE) was 98% in the IRd arm and 99% in the Rd arm. The incidence of grade 3 or higher AEs was 74% and 69%, respectively. The incidence of serious AEs was 47% and 49%, respectively.
The incidence of AEs resulting in discontinuation was 17% and 14%, respectively. And the incidence of on-study deaths (occurring within 30 days of the last dose) was 4% and 6%, respectively.
Common AEs in the IRd and Rd arms, respectively, were diarrhea (45% vs 39%), constipation (35% vs 26%), nausea (29% vs 22%), vomiting (23% vs 12%), rash (36% vs 23%), back pain (24% vs 17%), upper respiratory tract infection (23% vs 19%), thrombocytopenia (31% vs 16%), peripheral neuropathy (27% vs 22%), peripheral edema (28% vs 20%), thromboembolism (8% vs 11%), and neutropenia (33% vs 31%).
“Ixazomib is adding limited toxicity to lenalidomide and dex, with a very low rate of peripheral neuropathy and no cardiovascular or renal adverse signals,” Dr Moreau said.
“This all-oral triplet regimen may become one of the new standards of care in the relapsed setting. [It has] a very safe profile, [is] a very effective combination, [and is] simple and convenient.” ![]()
*Data in the abstract differ from the presentation.

Photo courtesy of ASH
ORLANDO, FL—Adding the oral proteasome inhibitor ixazomib to treatment with lenalidomide and dexamethasone can prolong progression-free survival (PFS) in patients with relapsed and/or refractory multiple myeloma (MM), according to interim results of the phase 3 TOURMALINE-MM1 trial.
It is not yet clear if the 3-drug combination can prolong overall survival when compared to treatment with lenalidomide and dexamethasone.
However, researchers believe the triplet shows promise and could become a new standard of care for relapsed/refractory MM.
Philippe Moreau, MD, of the University of Nantes in France, discussed this possibility while presenting results from TOURMALINE-MM1 at the 2015 ASH Annual Meeting (abstract 727*). The study was sponsored by Millennium Pharmaceuticals, Inc.
The trial included 722 MM patients enrolled at 147 centers in 26 countries. Patients were randomized to receive ixazomib, lenalidomide, and dexamethasone (IRd, n=360) or placebo, lenalidomide, and dexamethasone (Rd, n=362).
Baseline patient characteristics were similar between the arms. The median age was 66 in both arms (overall range, 30-91), and nearly 60% of patients in both arms were male.
Fifty percent of patients in the IRd arm and 47% in the Rd arm had an ECOG performance status of 0. Forty-three percent and 45%, respectively, had a status of 1, and 5% and 7%, respectively, had a status of 2.
Eighty-seven percent and 88%, respectively, had an ISS stage of I or II. Fifty-five percent of patients in the IRd arm had standard-risk cytogenetics, as did 60% in the Rd arm.
Fifty-nine percent of patients in both arms had received 1 prior line of therapy, and 41% in both arms had 2 or 3 prior lines.
Response and survival
“Ixazomib, when combined with len-dex . . . , was associated with a significant and meaningful improvement in progression-free survival, improved time to progression, and [higher] response rate as well,” Dr Moreau said.
At a median follow-up of about 15 months, the median PFS was 20.6 months in the IRd arm and 14.7 months in the Rd arm. The hazard ratio was 0.742 (P=0.012).
Dr Moreau said the PFS benefit was consistent across pre-specified subgroups. So the benefit was present regardless of age, ISS stage, cytogenetic risk, number of prior therapies, prior exposure to a proteasome inhibitor, prior immunomodulatory therapy, whether the patient was refractory to his last therapy, and whether the patient had relapsed or refractory disease.
Dr Moreau also pointed out that, in the IRd arm, the median PFS in high-risk patients was similar to that in the overall patient population and in patients with standard-risk cytogenetics. This suggests ixazomib may overcome the negative impact of cytogenetic alterations.
Whether IRd confers an overall survival benefit is not clear, as those data are not yet mature. At a median follow-up of about 23 months, the median overall survival was not reached in either treatment arm.
The researchers conducted a non-inferential PFS analysis at the same time point (23 months) and found the median PFS was 20 months in the IRd arm and 15.9 months in the Rd arm. The hazard ratio was 0.82.
As for other efficacy endpoints, the overall response rate was 78.3% in the IRd arm and 71.5% in the Rd arm (P=0.035). The rates of complete response were 11.7% and 6.6%, respectively (P=0.019). And the rates of very good partial response or greater were 48.1% and 39%, respectively (P=0.014).
The median time to response was 1.1 months in the IRd arm and 1.9 months in the Rd arm. The median duration of response was 20.5 months and 15 months, respectively. And the median time to progression was 21.4 months and 15.7 months, respectively.
Adverse events
At a median follow-up of about 23 months, patients had received a median of 17 cycles of IRd and a median of 15 cycles of Rd.
The incidence of any adverse event (AE) was 98% in the IRd arm and 99% in the Rd arm. The incidence of grade 3 or higher AEs was 74% and 69%, respectively. The incidence of serious AEs was 47% and 49%, respectively.
The incidence of AEs resulting in discontinuation was 17% and 14%, respectively. And the incidence of on-study deaths (occurring within 30 days of the last dose) was 4% and 6%, respectively.
Common AEs in the IRd and Rd arms, respectively, were diarrhea (45% vs 39%), constipation (35% vs 26%), nausea (29% vs 22%), vomiting (23% vs 12%), rash (36% vs 23%), back pain (24% vs 17%), upper respiratory tract infection (23% vs 19%), thrombocytopenia (31% vs 16%), peripheral neuropathy (27% vs 22%), peripheral edema (28% vs 20%), thromboembolism (8% vs 11%), and neutropenia (33% vs 31%).
“Ixazomib is adding limited toxicity to lenalidomide and dex, with a very low rate of peripheral neuropathy and no cardiovascular or renal adverse signals,” Dr Moreau said.
“This all-oral triplet regimen may become one of the new standards of care in the relapsed setting. [It has] a very safe profile, [is] a very effective combination, [and is] simple and convenient.” ![]()
*Data in the abstract differ from the presentation.

Photo courtesy of ASH
ORLANDO, FL—Adding the oral proteasome inhibitor ixazomib to treatment with lenalidomide and dexamethasone can prolong progression-free survival (PFS) in patients with relapsed and/or refractory multiple myeloma (MM), according to interim results of the phase 3 TOURMALINE-MM1 trial.
It is not yet clear if the 3-drug combination can prolong overall survival when compared to treatment with lenalidomide and dexamethasone.
However, researchers believe the triplet shows promise and could become a new standard of care for relapsed/refractory MM.
Philippe Moreau, MD, of the University of Nantes in France, discussed this possibility while presenting results from TOURMALINE-MM1 at the 2015 ASH Annual Meeting (abstract 727*). The study was sponsored by Millennium Pharmaceuticals, Inc.
The trial included 722 MM patients enrolled at 147 centers in 26 countries. Patients were randomized to receive ixazomib, lenalidomide, and dexamethasone (IRd, n=360) or placebo, lenalidomide, and dexamethasone (Rd, n=362).
Baseline patient characteristics were similar between the arms. The median age was 66 in both arms (overall range, 30-91), and nearly 60% of patients in both arms were male.
Fifty percent of patients in the IRd arm and 47% in the Rd arm had an ECOG performance status of 0. Forty-three percent and 45%, respectively, had a status of 1, and 5% and 7%, respectively, had a status of 2.
Eighty-seven percent and 88%, respectively, had an ISS stage of I or II. Fifty-five percent of patients in the IRd arm had standard-risk cytogenetics, as did 60% in the Rd arm.
Fifty-nine percent of patients in both arms had received 1 prior line of therapy, and 41% in both arms had 2 or 3 prior lines.
Response and survival
“Ixazomib, when combined with len-dex . . . , was associated with a significant and meaningful improvement in progression-free survival, improved time to progression, and [higher] response rate as well,” Dr Moreau said.
At a median follow-up of about 15 months, the median PFS was 20.6 months in the IRd arm and 14.7 months in the Rd arm. The hazard ratio was 0.742 (P=0.012).
Dr Moreau said the PFS benefit was consistent across pre-specified subgroups. So the benefit was present regardless of age, ISS stage, cytogenetic risk, number of prior therapies, prior exposure to a proteasome inhibitor, prior immunomodulatory therapy, whether the patient was refractory to his last therapy, and whether the patient had relapsed or refractory disease.
Dr Moreau also pointed out that, in the IRd arm, the median PFS in high-risk patients was similar to that in the overall patient population and in patients with standard-risk cytogenetics. This suggests ixazomib may overcome the negative impact of cytogenetic alterations.
Whether IRd confers an overall survival benefit is not clear, as those data are not yet mature. At a median follow-up of about 23 months, the median overall survival was not reached in either treatment arm.
The researchers conducted a non-inferential PFS analysis at the same time point (23 months) and found the median PFS was 20 months in the IRd arm and 15.9 months in the Rd arm. The hazard ratio was 0.82.
As for other efficacy endpoints, the overall response rate was 78.3% in the IRd arm and 71.5% in the Rd arm (P=0.035). The rates of complete response were 11.7% and 6.6%, respectively (P=0.019). And the rates of very good partial response or greater were 48.1% and 39%, respectively (P=0.014).
The median time to response was 1.1 months in the IRd arm and 1.9 months in the Rd arm. The median duration of response was 20.5 months and 15 months, respectively. And the median time to progression was 21.4 months and 15.7 months, respectively.
Adverse events
At a median follow-up of about 23 months, patients had received a median of 17 cycles of IRd and a median of 15 cycles of Rd.
The incidence of any adverse event (AE) was 98% in the IRd arm and 99% in the Rd arm. The incidence of grade 3 or higher AEs was 74% and 69%, respectively. The incidence of serious AEs was 47% and 49%, respectively.
The incidence of AEs resulting in discontinuation was 17% and 14%, respectively. And the incidence of on-study deaths (occurring within 30 days of the last dose) was 4% and 6%, respectively.
Common AEs in the IRd and Rd arms, respectively, were diarrhea (45% vs 39%), constipation (35% vs 26%), nausea (29% vs 22%), vomiting (23% vs 12%), rash (36% vs 23%), back pain (24% vs 17%), upper respiratory tract infection (23% vs 19%), thrombocytopenia (31% vs 16%), peripheral neuropathy (27% vs 22%), peripheral edema (28% vs 20%), thromboembolism (8% vs 11%), and neutropenia (33% vs 31%).
“Ixazomib is adding limited toxicity to lenalidomide and dex, with a very low rate of peripheral neuropathy and no cardiovascular or renal adverse signals,” Dr Moreau said.
“This all-oral triplet regimen may become one of the new standards of care in the relapsed setting. [It has] a very safe profile, [is] a very effective combination, [and is] simple and convenient.” ![]()
*Data in the abstract differ from the presentation.
Disease Education
Q) The billing consultant who came to our office said we can increase our reimbursements if we also provide education to our patients with chronic kidney disease (CKD). Is she right?
In 2010, under an omnibus bill, kidney disease education (KDE) classes were added as a Medicare benefit. These are for patients with stage 4 CKD (glomerular filtration rate, 15-30 mL/min) and are to be taught by a qualified instructor (MD, PA, NP, or CNS).
The classes can be taught on the same day as an evaluation/management visit (ie, a regular office visit) and are compensated by the hour. (Side note: Medicare defines an hour as 31 minutes—yes, 31 minutes; Medicare takes for granted that you will also need time to chart!) You can teach two classes in the same day. Thus, if you wanted to, you could have a patient arrive for an office visit, then teach two 31-minute classes, and bill all three for the same day. The entire visit could be 75 minutes (although this may be exhausting for this population).
You can conduct the classes in a number of settings, including nursing homes, hospitals, skilled nursing facilities, the office, or even the patient’s home. Many PAs and NPs have taught these classes to hospitalized patients who have lost kidney function due to an acute insult (ie, medications, dehydration, contrast).
Each Medicare recipient has a lifetime benefit of six KDE classes. The CPT billing code is G0420 for an individual class and G0421 for a group class. You must make sure you also code for the stage 4 CKD diagnosis (code: 585.4).
Congress stipulated KDE classes must include information on causes, symptoms, and treatments and comprise a posttest at a specific health literacy level. To make it simple, the National Kidney Foundation Council of Advanced Practitioners (NKF-CAP) has developed two free Power-Point slide decks for clinicians to use in KDE classes (available at www.kidney.org/professionals/CAP/sub_resources#kde). References and updated peer-reviewed guidelines are included. You can print the slides for your patients and/or share the program with your colleagues.
Many nephrology practitioners teach the two slide sets over and over, because patients only retain one-third of the info we provide them on a given day. So if you teach each slide set three times, you have six lifetime classes—and hopefully the patient will have retained everything.
One caveat: Before you initiate KDE classes for a specific patient, check with the patient’s nephrology group (we hope at stage 4 the patient has a nephrologist) to see if they are providing the education. —KZ and JD
Kim Zuber, PA-C, MSPS, DFAAPA
American Academy of Nephrology PAs
Jane S. Davis, CRNP, DNP
Division of Nephrology at the University of Alabama
National Kidney Foundation's Council of Advanced Practitioners
Q) The billing consultant who came to our office said we can increase our reimbursements if we also provide education to our patients with chronic kidney disease (CKD). Is she right?
In 2010, under an omnibus bill, kidney disease education (KDE) classes were added as a Medicare benefit. These are for patients with stage 4 CKD (glomerular filtration rate, 15-30 mL/min) and are to be taught by a qualified instructor (MD, PA, NP, or CNS).
The classes can be taught on the same day as an evaluation/management visit (ie, a regular office visit) and are compensated by the hour. (Side note: Medicare defines an hour as 31 minutes—yes, 31 minutes; Medicare takes for granted that you will also need time to chart!) You can teach two classes in the same day. Thus, if you wanted to, you could have a patient arrive for an office visit, then teach two 31-minute classes, and bill all three for the same day. The entire visit could be 75 minutes (although this may be exhausting for this population).
You can conduct the classes in a number of settings, including nursing homes, hospitals, skilled nursing facilities, the office, or even the patient’s home. Many PAs and NPs have taught these classes to hospitalized patients who have lost kidney function due to an acute insult (ie, medications, dehydration, contrast).
Each Medicare recipient has a lifetime benefit of six KDE classes. The CPT billing code is G0420 for an individual class and G0421 for a group class. You must make sure you also code for the stage 4 CKD diagnosis (code: 585.4).
Congress stipulated KDE classes must include information on causes, symptoms, and treatments and comprise a posttest at a specific health literacy level. To make it simple, the National Kidney Foundation Council of Advanced Practitioners (NKF-CAP) has developed two free Power-Point slide decks for clinicians to use in KDE classes (available at www.kidney.org/professionals/CAP/sub_resources#kde). References and updated peer-reviewed guidelines are included. You can print the slides for your patients and/or share the program with your colleagues.
Many nephrology practitioners teach the two slide sets over and over, because patients only retain one-third of the info we provide them on a given day. So if you teach each slide set three times, you have six lifetime classes—and hopefully the patient will have retained everything.
One caveat: Before you initiate KDE classes for a specific patient, check with the patient’s nephrology group (we hope at stage 4 the patient has a nephrologist) to see if they are providing the education. —KZ and JD
Kim Zuber, PA-C, MSPS, DFAAPA
American Academy of Nephrology PAs
Jane S. Davis, CRNP, DNP
Division of Nephrology at the University of Alabama
National Kidney Foundation's Council of Advanced Practitioners
Q) The billing consultant who came to our office said we can increase our reimbursements if we also provide education to our patients with chronic kidney disease (CKD). Is she right?
In 2010, under an omnibus bill, kidney disease education (KDE) classes were added as a Medicare benefit. These are for patients with stage 4 CKD (glomerular filtration rate, 15-30 mL/min) and are to be taught by a qualified instructor (MD, PA, NP, or CNS).
The classes can be taught on the same day as an evaluation/management visit (ie, a regular office visit) and are compensated by the hour. (Side note: Medicare defines an hour as 31 minutes—yes, 31 minutes; Medicare takes for granted that you will also need time to chart!) You can teach two classes in the same day. Thus, if you wanted to, you could have a patient arrive for an office visit, then teach two 31-minute classes, and bill all three for the same day. The entire visit could be 75 minutes (although this may be exhausting for this population).
You can conduct the classes in a number of settings, including nursing homes, hospitals, skilled nursing facilities, the office, or even the patient’s home. Many PAs and NPs have taught these classes to hospitalized patients who have lost kidney function due to an acute insult (ie, medications, dehydration, contrast).
Each Medicare recipient has a lifetime benefit of six KDE classes. The CPT billing code is G0420 for an individual class and G0421 for a group class. You must make sure you also code for the stage 4 CKD diagnosis (code: 585.4).
Congress stipulated KDE classes must include information on causes, symptoms, and treatments and comprise a posttest at a specific health literacy level. To make it simple, the National Kidney Foundation Council of Advanced Practitioners (NKF-CAP) has developed two free Power-Point slide decks for clinicians to use in KDE classes (available at www.kidney.org/professionals/CAP/sub_resources#kde). References and updated peer-reviewed guidelines are included. You can print the slides for your patients and/or share the program with your colleagues.
Many nephrology practitioners teach the two slide sets over and over, because patients only retain one-third of the info we provide them on a given day. So if you teach each slide set three times, you have six lifetime classes—and hopefully the patient will have retained everything.
One caveat: Before you initiate KDE classes for a specific patient, check with the patient’s nephrology group (we hope at stage 4 the patient has a nephrologist) to see if they are providing the education. —KZ and JD
Kim Zuber, PA-C, MSPS, DFAAPA
American Academy of Nephrology PAs
Jane S. Davis, CRNP, DNP
Division of Nephrology at the University of Alabama
National Kidney Foundation's Council of Advanced Practitioners
Advances in Hematology and Oncology (May 2014)
High-risk B-ALL subgroup has ‘outstanding outcomes’

Photo courtesy of ASH
ORLANDO, FL—A subgroup of young patients with high-risk B-cell acute lymphoblastic leukemia (B-ALL) can have “outstanding outcomes” with contemporary therapy, according to researchers.
Results of a large study suggested that patients ages 1 to 30 who have high-risk B-ALL according to National Cancer Institute (NCI) classification can have high rates of event-free survival (EFS) and overall survival (OS) if they have favorable cytogenetic features, have no evidence of CNS disease, and have rapid minimal residual disease (MRD) responses.
The research suggested these patients will not benefit from further chemotherapy intensification.
Elizabeth Raetz, MD, of the University of Utah in Salt Lake City, presented these results at the 2015 ASH Annual Meeting (abstract 807).
She and her colleagues analyzed patients enrolled on the Children’s Oncology Group (COG) AALL03B1 classification study at the time of B-ALL diagnosis. From December 2003 to September 2011, there were 11,144 eligible patients enrolled on this trial.
Eighty-nine percent of these patients were also enrolled on a frontline ALL therapeutic trial, and 96% of these patients were evaluable for post-induction treatment assignment. Sixty-five percent of these patients were treated on a trial for NCI standard-risk B-ALL (COG-AALL0331), and 35% were treated on a trial for high-risk B-ALL (COG-AALL0232).
At the end of induction therapy, patients were classified into low-risk (29%), standard-risk (33%), high-risk (34%), and very-high-risk (4%) groups for further treatment allocation. The variables used for risk classification were age, initial white blood cell count, extramedullary disease status, blast cytogenetics, and early treatment response based on bone marrow morphology and day 29 MRD.
Patients with very-high-risk features (BCR-ABL1, hypodiploidy, induction failure, or poor response at day 43) did not continue on AALL0232/AALL0331 post-induction but did have outcome data captured for analysis.
Response and survival
Rapid early response was defined as M1 (<5% blasts) bone marrow by day 15 plus flow cytometry-based MRD <0.1% on day 29 of induction. Patients with either M2/M3 (≥5% blasts) day 15 marrow or MRD ≥0.1% at day 29 were deemed slow early responders.
Eighty-four percent of patients had a rapid early response to induction, and 16% had a slow early response.
For rapid early responders, the 5-year EFS was 89.3%, and the 5-year OS was 95.2%. For slow early responders, the EFS and OS rates were 67.9% and 84.3%, respectively (P<0.0001 for both EFS and OS comparisons).
Survival according to cytogenetics
Having favorable cytogenetic abnormalities (triple trisomies of chromosomes 4, 10, and 17 or ETV6-RUNX1 fusion) was associated with significantly better EFS and OS than having unfavorable cytogenetics (hypodiploidy [DNA index <0.81 or chromosomes < 44], MLL rearrangements, BCR-ABL1, or iAMP21).
And Dr Raetz pointed out that the 5-year OS exceeded 98% for patients with either standard- or high-risk disease who had favorable cytogenetics.
For patients who were ETV6-RUNX1-positive, the EFS was 93.2% and the OS was 98.3%. For patients who were ETV6-RUNX1 negative, the rates were 83.5% and 92%, respectively (P<0.0001).
For patients with triple trisomy, EFS was 94.7% and OS was 98.7%. For those without triple trisomy, the rates were 83.6% and 92.2%, respectively (P<0.0001).
For patients with MLL rearrangement, the EFS was 73.9% and the OS was 83.1%. For patients without MLL rearrangement, the rates were 85.9% and 93.6%, respectively (P<0.0001).
For patients who were positive for iAMP21, the EFS was 69.5% and the OS was 90.1%. For iAMP21-negative patients, the rates were 86.1% and 93.4%, respectively (P<0.0001 for PFS comparison and P=0.0026 for OS comparison).
Survival according to risk group and MRD
The researchers also assessed EFS and OS among patients with favorable cytogenetics according to NCI risk group and MRD at days 8 and 29.
“One thing to point out is that, regardless of having favorable cytogenetics, those individuals who had end-induction MRD values of greater than 0.01% had inferior outcomes, so that was still a prognostic marker,” Dr Raetz said.
“And one thing that we were pleasantly surprised to see was that, among the NCI high-risk patients, those who had very rapid MRD responses—so less than 1% at day 8 in the blood and less than 0.01% in the marrow on day 29—had a 94.9% 5-year event-free survival and 98.1% overall survival.”
The researchers also divided this group according to age—patients younger than 10 and those 10 years or older. There was no significant difference in EFS or OS between the age groups (P=0.126 and P=0.411).
Standard-risk group
Among patients with <1% MRD on day 8 and <0.01% MRD on day 29, the EFS was 95.7% and the OS was 99.1%.
Among patients with ≥1% MRD on day 8 and <0.01% MRD on day 29, the EFS was 91.7% and the OS was 99.4%.
Among patients with any MRD on day 8 and ≥0.01% MRD on day 29, the EFS was 88.1% and the OS was 96.8%.
High-risk group
Among patients with <1% MRD on day 8 and <0.01% MRD on day 29, the EFS was 94.9% and the OS was 98.1%.
Among patients with ≥1% MRD on day 8 and <0.01% MRD on day 29, the EFS was 93.6% and the OS was 95.5%.
Among patients with any MRD on day 8 and ≥0.01% MRD on day 29, the EFS was 75.4% and the OS was 90.4%.
In closing, Dr Raetz said this study showed that real‐time classification incorporating clinical features, blast cytogenetics, and early response was feasible in a large group of patients enrolled on COG ALL trials and identified patients with varying outcomes for risk‐based treatment allocation.
She noted that early response by marrow morphology was not prognostic when MRD response was used and is therefore no longer used in COG studies.
And although favorable cytogenetic features were not prognostic in NCI high-risk B‐ALL patients in prior COG studies, the current study indicates that these patients can have “excellent outcomes” if they have no evidence of CNS leukemia and are rapid MRD responders. So these patients will not benefit from further chemotherapy intensification. ![]()

Photo courtesy of ASH
ORLANDO, FL—A subgroup of young patients with high-risk B-cell acute lymphoblastic leukemia (B-ALL) can have “outstanding outcomes” with contemporary therapy, according to researchers.
Results of a large study suggested that patients ages 1 to 30 who have high-risk B-ALL according to National Cancer Institute (NCI) classification can have high rates of event-free survival (EFS) and overall survival (OS) if they have favorable cytogenetic features, have no evidence of CNS disease, and have rapid minimal residual disease (MRD) responses.
The research suggested these patients will not benefit from further chemotherapy intensification.
Elizabeth Raetz, MD, of the University of Utah in Salt Lake City, presented these results at the 2015 ASH Annual Meeting (abstract 807).
She and her colleagues analyzed patients enrolled on the Children’s Oncology Group (COG) AALL03B1 classification study at the time of B-ALL diagnosis. From December 2003 to September 2011, there were 11,144 eligible patients enrolled on this trial.
Eighty-nine percent of these patients were also enrolled on a frontline ALL therapeutic trial, and 96% of these patients were evaluable for post-induction treatment assignment. Sixty-five percent of these patients were treated on a trial for NCI standard-risk B-ALL (COG-AALL0331), and 35% were treated on a trial for high-risk B-ALL (COG-AALL0232).
At the end of induction therapy, patients were classified into low-risk (29%), standard-risk (33%), high-risk (34%), and very-high-risk (4%) groups for further treatment allocation. The variables used for risk classification were age, initial white blood cell count, extramedullary disease status, blast cytogenetics, and early treatment response based on bone marrow morphology and day 29 MRD.
Patients with very-high-risk features (BCR-ABL1, hypodiploidy, induction failure, or poor response at day 43) did not continue on AALL0232/AALL0331 post-induction but did have outcome data captured for analysis.
Response and survival
Rapid early response was defined as M1 (<5% blasts) bone marrow by day 15 plus flow cytometry-based MRD <0.1% on day 29 of induction. Patients with either M2/M3 (≥5% blasts) day 15 marrow or MRD ≥0.1% at day 29 were deemed slow early responders.
Eighty-four percent of patients had a rapid early response to induction, and 16% had a slow early response.
For rapid early responders, the 5-year EFS was 89.3%, and the 5-year OS was 95.2%. For slow early responders, the EFS and OS rates were 67.9% and 84.3%, respectively (P<0.0001 for both EFS and OS comparisons).
Survival according to cytogenetics
Having favorable cytogenetic abnormalities (triple trisomies of chromosomes 4, 10, and 17 or ETV6-RUNX1 fusion) was associated with significantly better EFS and OS than having unfavorable cytogenetics (hypodiploidy [DNA index <0.81 or chromosomes < 44], MLL rearrangements, BCR-ABL1, or iAMP21).
And Dr Raetz pointed out that the 5-year OS exceeded 98% for patients with either standard- or high-risk disease who had favorable cytogenetics.
For patients who were ETV6-RUNX1-positive, the EFS was 93.2% and the OS was 98.3%. For patients who were ETV6-RUNX1 negative, the rates were 83.5% and 92%, respectively (P<0.0001).
For patients with triple trisomy, EFS was 94.7% and OS was 98.7%. For those without triple trisomy, the rates were 83.6% and 92.2%, respectively (P<0.0001).
For patients with MLL rearrangement, the EFS was 73.9% and the OS was 83.1%. For patients without MLL rearrangement, the rates were 85.9% and 93.6%, respectively (P<0.0001).
For patients who were positive for iAMP21, the EFS was 69.5% and the OS was 90.1%. For iAMP21-negative patients, the rates were 86.1% and 93.4%, respectively (P<0.0001 for PFS comparison and P=0.0026 for OS comparison).
Survival according to risk group and MRD
The researchers also assessed EFS and OS among patients with favorable cytogenetics according to NCI risk group and MRD at days 8 and 29.
“One thing to point out is that, regardless of having favorable cytogenetics, those individuals who had end-induction MRD values of greater than 0.01% had inferior outcomes, so that was still a prognostic marker,” Dr Raetz said.
“And one thing that we were pleasantly surprised to see was that, among the NCI high-risk patients, those who had very rapid MRD responses—so less than 1% at day 8 in the blood and less than 0.01% in the marrow on day 29—had a 94.9% 5-year event-free survival and 98.1% overall survival.”
The researchers also divided this group according to age—patients younger than 10 and those 10 years or older. There was no significant difference in EFS or OS between the age groups (P=0.126 and P=0.411).
Standard-risk group
Among patients with <1% MRD on day 8 and <0.01% MRD on day 29, the EFS was 95.7% and the OS was 99.1%.
Among patients with ≥1% MRD on day 8 and <0.01% MRD on day 29, the EFS was 91.7% and the OS was 99.4%.
Among patients with any MRD on day 8 and ≥0.01% MRD on day 29, the EFS was 88.1% and the OS was 96.8%.
High-risk group
Among patients with <1% MRD on day 8 and <0.01% MRD on day 29, the EFS was 94.9% and the OS was 98.1%.
Among patients with ≥1% MRD on day 8 and <0.01% MRD on day 29, the EFS was 93.6% and the OS was 95.5%.
Among patients with any MRD on day 8 and ≥0.01% MRD on day 29, the EFS was 75.4% and the OS was 90.4%.
In closing, Dr Raetz said this study showed that real‐time classification incorporating clinical features, blast cytogenetics, and early response was feasible in a large group of patients enrolled on COG ALL trials and identified patients with varying outcomes for risk‐based treatment allocation.
She noted that early response by marrow morphology was not prognostic when MRD response was used and is therefore no longer used in COG studies.
And although favorable cytogenetic features were not prognostic in NCI high-risk B‐ALL patients in prior COG studies, the current study indicates that these patients can have “excellent outcomes” if they have no evidence of CNS leukemia and are rapid MRD responders. So these patients will not benefit from further chemotherapy intensification. ![]()

Photo courtesy of ASH
ORLANDO, FL—A subgroup of young patients with high-risk B-cell acute lymphoblastic leukemia (B-ALL) can have “outstanding outcomes” with contemporary therapy, according to researchers.
Results of a large study suggested that patients ages 1 to 30 who have high-risk B-ALL according to National Cancer Institute (NCI) classification can have high rates of event-free survival (EFS) and overall survival (OS) if they have favorable cytogenetic features, have no evidence of CNS disease, and have rapid minimal residual disease (MRD) responses.
The research suggested these patients will not benefit from further chemotherapy intensification.
Elizabeth Raetz, MD, of the University of Utah in Salt Lake City, presented these results at the 2015 ASH Annual Meeting (abstract 807).
She and her colleagues analyzed patients enrolled on the Children’s Oncology Group (COG) AALL03B1 classification study at the time of B-ALL diagnosis. From December 2003 to September 2011, there were 11,144 eligible patients enrolled on this trial.
Eighty-nine percent of these patients were also enrolled on a frontline ALL therapeutic trial, and 96% of these patients were evaluable for post-induction treatment assignment. Sixty-five percent of these patients were treated on a trial for NCI standard-risk B-ALL (COG-AALL0331), and 35% were treated on a trial for high-risk B-ALL (COG-AALL0232).
At the end of induction therapy, patients were classified into low-risk (29%), standard-risk (33%), high-risk (34%), and very-high-risk (4%) groups for further treatment allocation. The variables used for risk classification were age, initial white blood cell count, extramedullary disease status, blast cytogenetics, and early treatment response based on bone marrow morphology and day 29 MRD.
Patients with very-high-risk features (BCR-ABL1, hypodiploidy, induction failure, or poor response at day 43) did not continue on AALL0232/AALL0331 post-induction but did have outcome data captured for analysis.
Response and survival
Rapid early response was defined as M1 (<5% blasts) bone marrow by day 15 plus flow cytometry-based MRD <0.1% on day 29 of induction. Patients with either M2/M3 (≥5% blasts) day 15 marrow or MRD ≥0.1% at day 29 were deemed slow early responders.
Eighty-four percent of patients had a rapid early response to induction, and 16% had a slow early response.
For rapid early responders, the 5-year EFS was 89.3%, and the 5-year OS was 95.2%. For slow early responders, the EFS and OS rates were 67.9% and 84.3%, respectively (P<0.0001 for both EFS and OS comparisons).
Survival according to cytogenetics
Having favorable cytogenetic abnormalities (triple trisomies of chromosomes 4, 10, and 17 or ETV6-RUNX1 fusion) was associated with significantly better EFS and OS than having unfavorable cytogenetics (hypodiploidy [DNA index <0.81 or chromosomes < 44], MLL rearrangements, BCR-ABL1, or iAMP21).
And Dr Raetz pointed out that the 5-year OS exceeded 98% for patients with either standard- or high-risk disease who had favorable cytogenetics.
For patients who were ETV6-RUNX1-positive, the EFS was 93.2% and the OS was 98.3%. For patients who were ETV6-RUNX1 negative, the rates were 83.5% and 92%, respectively (P<0.0001).
For patients with triple trisomy, EFS was 94.7% and OS was 98.7%. For those without triple trisomy, the rates were 83.6% and 92.2%, respectively (P<0.0001).
For patients with MLL rearrangement, the EFS was 73.9% and the OS was 83.1%. For patients without MLL rearrangement, the rates were 85.9% and 93.6%, respectively (P<0.0001).
For patients who were positive for iAMP21, the EFS was 69.5% and the OS was 90.1%. For iAMP21-negative patients, the rates were 86.1% and 93.4%, respectively (P<0.0001 for PFS comparison and P=0.0026 for OS comparison).
Survival according to risk group and MRD
The researchers also assessed EFS and OS among patients with favorable cytogenetics according to NCI risk group and MRD at days 8 and 29.
“One thing to point out is that, regardless of having favorable cytogenetics, those individuals who had end-induction MRD values of greater than 0.01% had inferior outcomes, so that was still a prognostic marker,” Dr Raetz said.
“And one thing that we were pleasantly surprised to see was that, among the NCI high-risk patients, those who had very rapid MRD responses—so less than 1% at day 8 in the blood and less than 0.01% in the marrow on day 29—had a 94.9% 5-year event-free survival and 98.1% overall survival.”
The researchers also divided this group according to age—patients younger than 10 and those 10 years or older. There was no significant difference in EFS or OS between the age groups (P=0.126 and P=0.411).
Standard-risk group
Among patients with <1% MRD on day 8 and <0.01% MRD on day 29, the EFS was 95.7% and the OS was 99.1%.
Among patients with ≥1% MRD on day 8 and <0.01% MRD on day 29, the EFS was 91.7% and the OS was 99.4%.
Among patients with any MRD on day 8 and ≥0.01% MRD on day 29, the EFS was 88.1% and the OS was 96.8%.
High-risk group
Among patients with <1% MRD on day 8 and <0.01% MRD on day 29, the EFS was 94.9% and the OS was 98.1%.
Among patients with ≥1% MRD on day 8 and <0.01% MRD on day 29, the EFS was 93.6% and the OS was 95.5%.
Among patients with any MRD on day 8 and ≥0.01% MRD on day 29, the EFS was 75.4% and the OS was 90.4%.
In closing, Dr Raetz said this study showed that real‐time classification incorporating clinical features, blast cytogenetics, and early response was feasible in a large group of patients enrolled on COG ALL trials and identified patients with varying outcomes for risk‐based treatment allocation.
She noted that early response by marrow morphology was not prognostic when MRD response was used and is therefore no longer used in COG studies.
And although favorable cytogenetic features were not prognostic in NCI high-risk B‐ALL patients in prior COG studies, the current study indicates that these patients can have “excellent outcomes” if they have no evidence of CNS leukemia and are rapid MRD responders. So these patients will not benefit from further chemotherapy intensification. ![]()
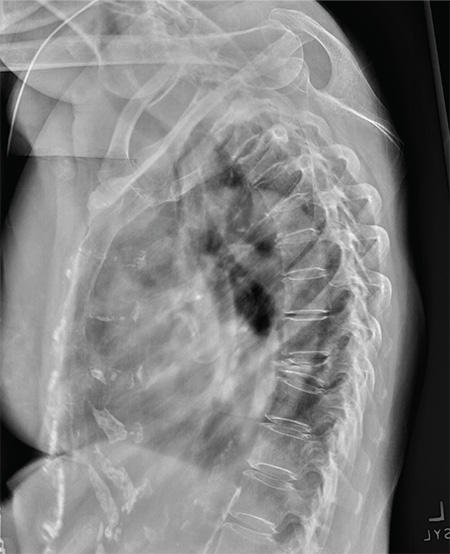
Pulmonary nodule on x-ray: An algorithmic approach
› Order a computed tomography chest scan, preferably with thin sections through the nodule, to help characterize an indeterminate pulmonary nodule identified on x-ray. B
› Estimate the pretest probability of malignancy for a patient with a pulmonary nodule using your clinical judgment and/or by using a validated model. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
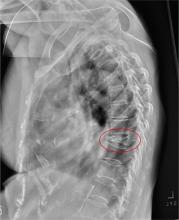
CASE 1 › George D is a 67-year-old patient who has never smoked and who has no history of malignancy. An x-ray of his ribs performed after a fall shows a 13-mm solitary nodule in his right upper lung.
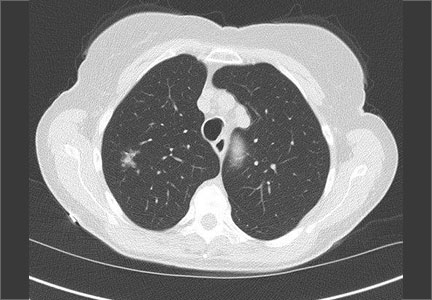
CASE 2 › Cathy B is a healthy 80-year-old with no history of smoking. During a trip to the emergency department for chest pain, she had a computed tomography (CT) scan of her chest. While the chest pain was subsequently attributed to gastroesophageal reflux, the CT revealed a 9-mm part solid nodule that was 75% solid.
How should the physicians caring for each of these patients proceed with their care?
The widespread use of sensitive imaging techniques often leads to the incidental discovery of unrelated—but possibly significant—pulmonary findings. Pulmonary nodules are incidentally discovered on an estimated 0.09% to 0.2% of all chest x-rays, 13% of all chest CT angiograms,1 31% of all cardiac CTs performed for coronary calcium scoring,2 and up to 50% of thin-section chest CT scans.1
The widespread implementation of the US Preventive Services Task Force recommendations on lung cancer screening has further expanded the number of patients in whom asymptomatic pulmonary nodules will be detected. As a result, family physicians (FPs) will frequently encounter this challenging clinical dilemma and will need to:
- assess the patient’s risk profile
- address the patient’s concerns about malignancy while eliciting his or her preference for management
- minimize the risks of surveillance testing
- minimize patient distress while ensuring compliance with a follow-up that may extend up to 4 years
- determine when it’s appropriate to refer the patient to a pulmonologist and/or pulmonary nodule clinic or registry.
Taking these steps, however, can be challenging. In interviews, 15 primary care clinicians who care for patients with pulmonary nodules expressed concerns about limitations in time, knowledge, and resources, as well as a fear about such patients “falling through the cracks.”3 Familiarity with current evidence-based guidelines such as those from the American College of Chest Physicians (ACCP) and knowledge of emerging data on the management of various types of nodules are imperative.
To that end, this review will fill in the information gaps and provide guidance on how best to communicate what is known about a particular type of nodule with the patient who has one. (See “What to say to improve joint decision-making.”4-7) But first, a word about terminology.
What to say to improve joint decision-making4-7
Diagnosis and follow-up of a pulmonary nodule takes an emotional toll on patients, who often have a poor sense of what the presence of a nodule signifies. When caring for a patient with a pulmonary nodule, it’s essential to have an effective communication strategy to ensure that he or she is a well-informed partner in decision-making.
Specifically, you'll need to describe the type of nodule that the patient has, how fast it might grow and its malignancy potential, steps that will need to be taken, and the importance of smoking cessation (if the patient smokes).
Ask the patient about any concerns/fears he or she may have, and provide resources to reduce them. Emphasize shared decision-making and discuss the rationale for various management plans and the limitations of diagnostic tests. Do not minimize the issue; emphasize the need for, and importance of, prolonged follow-up—even for a patient who has a small, low-risk nodule.
Solid vs subsolid pulmonary nodules
Solid pulmonary nodules. Traditionally, the term “solitary pulmonary nodule” has been used to describe a single, well-circumscribed, radiographic opacity that measures up to 3 cm in diameter and is completely surrounded by aerated lung.1,8 The term “solitary” is now less useful because increasingly sensitive imaging techniques often reveal more than one nodule. In the absence of evidence of features that strongly suggest a benign etiology, these are now commonly referred to as indeterminate solid nodules.
Subsolid nodules are pulmonary nodules that have unique characteristics and require separate guidelines for management. Subsolid nodules include pure ground glass nodules (GGNs) and part solid nodules. GGNs are focal nodular areas of increased lung attenuation through which normal parenchymal structures such as airways, vessels, and interlobular septa can be visualized.1,8 Part solid nodules have a solid component. They are usually, but not necessarily, >50% ground glass in appearance.
Lung masses. Focal pulmonary lesions >3 cm in diameter are called lung masses and are presumed to be malignant (bronchogenic carcinoma) unless proven otherwise.1,8
The specific approach to evaluating and monitoring a pulmonary nodule varies depending on whether the nodule is solid or subsolid and other factors, including the nodule’s size.
Monitoring solid nodules
Monitoring of an indeterminate solid nodule is largely determined by the patient’s risk profile and the characteristics of the identified nodule.1 Independent patient predictors of malignancy include older age, smoking status, and history of prior malignancy (>5 years ago). Less established predictors are the presence of moderate or severe obstructive lung disease and exposure to particulate or sulfur oxide-related pollution.9
Patients who have an indeterminate nodule identified on chest x-ray should undergo a chest CT scan, preferably with thin sections through the nodule to help characterize it.1 Nodule characteristics that can help predict a patient’s risk of malignancy include the size (>8 mm confers higher risk), malignant rate of growth, edge characteristics (spiculation or irregular edges), thickness of the wall of a cavitary pulmonary nodule (≥16 mm has a likelihood ratio [LR] 37.97 of malignancy), and the location of the nodule (upper or middle lobe [LR=1.2 to 1.6]). 10 A lack of growth over 2 years and a benign pattern of calcification eliminate the need for further evaluation.
Validated tools to help guide decision-making. Although many physicians estimate pretest probability of malignancy intuitively, validated tools are readily available and can help in clinical decision-making.11 One such tool is the Mayo model, which is available at http://reference.medscape.com/calculator/solitary-pulmonary-nodule-risk. This model takes into account the patient’s age, smoking status, history of cancer, and characteristics of the nodule.
Solid nodules >8 mm to 3 cm
For a patient with a solid nodule >8 mm to 3 cm, ACCP guidelines suggest that physicians estimate the pretest probability of malignancy qualitatively using their clinical judgment and/or quantitatively by using a validated model, such as the Mayo model described above.
Based on the patient’s probability of malignancy, management options include continued CT surveillance, positron emission tomography (PET) imaging, CT-guided needle lung biopsy, bronchoscopy with biopsy, or surgical wedge resection (ALGORITHM 1).1
CT surveillance is recommended for individuals:
- with very low (<5%) probability of malignancy
- with low to moderate (5% to 30%) or moderate to high (31% to 65%) probability of malignancy with negative functional imaging (PET)
- with high probability of malignancy (>65%) when needle biopsy is nondiagnostic and the lesion is not hypermetabolic on PET scan.
Surveillance is also recommended when a fully informed patient prefers nonaggressive management. The intervals for serial CT in this population are at 3 to 6 months, 9 to 12 months, and 18 to 24 months.
In an individual with a solid indeterminate nodule with a high probability of malignancy (>65%), functional imaging should not be performed to characterize the nodule. It may, however, be performed for staging.
Time for biopsy or resection? If a nodule shows evidence of malignant growth on serial imaging, nonsurgical biopsy (CT scan-guided transthoracic needle biopsy, bronchoscopy guided by fluoroscopy, endobronchial ultrasound, electromagnetic navigation bronchoscopy, or virtual bronchoscopy navigation) or surgical resection is recommended.
Nonsurgical biopsy is also recommended when the patient’s pretest probability and imaging test results are discordant, when a benign diagnosis requires specific medical treatment, or if a fully informed patient desires proof of diagnosis prior to surgery.
Thoracoscopy with wedge resection is the gold standard for diagnosis of a malignant nodule. It is recommended:
- when the clinical probability of malignancy is high (>65%)
- when the nodule is intensely hypermetabolic by PET scan or positive by other functional imaging tests
- when nonsurgical biopsy is suggestive of malignancy
- when a fully informed patient prefers a definitive diagnostic procedure.
CASE 1 › The FP contacts Mr. D and advises that he get a chest CT to better characterize his pulmonary nodule. A thin-slice CT of the lung reveals that the 13-mm solid nodule in the right upper lobe has spiculated margins. According to the Mayo risk calculator, Mr. D is at moderate risk of malignancy (32.5%). Mr. D and his physician discuss the findings and possible management options, and Mr. D opts to have a PET scan. The FP gives Mr. D literature on pulmonary nodules and contact information for the provider team. A PET scan shows negative uptake. Mr. D and his physician discuss CT surveillance and nonsurgical biopsy. He opts for CT surveillance. The next CT is scheduled for 3 months.
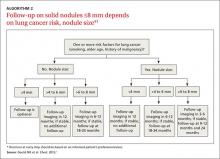
Solid nodules ≤8 mm
Management of these lesions generally follows the consensus-based guidelines that were first published by the Fleischner Society and subsequently endorsed by the ACCP.1 The 2 main determinants that guide management of nodules ≤8 mm are the patient’s risk factors for cancer and nodule size (ALGORITHM 2).1 The Fleischner guidelines pertain only to patients older than age 35 with no current extra pulmonary malignancy or unexplained fevers. The ACCP guidelines, although similar, do not include these limitations. Patient risk factors include history of smoking, older age, and a history of malignancy.1
Patients with no risk factors for malignancy. The frequency of surveillance CT is determined by the size of the nodule. Nodules ≤4 mm do not need to be followed. For nodules >4 mm to 6 mm, a repeat CT in 12 months is recommended with no follow-up if stable. For nodules >6 to <8 mm, repeat CT is recommended at 6 to 12 months, and again between 18 and 24 months if unchanged.1Patients with one or more risk factors for malignancy. Nodules ≤4 mm should be reevaluated at 12 months in patients with one or more risk factors; no additional follow-up is needed if unchanged. For nodules >4 mm to 6 mm, CT should be repeated between 6 and 12 months and again between 18 and 24 months. Nodules >6 mm to <8 mm should be followed initially between 3 to 6 months, then between 9 and 12 months and again at 24 months if unchanged.1
Subsolid nodules require a different approach
Subsolid nodules have a high prevalence of premalignant and malignant disease (adenocarcinoma in situ, minimally invasive adenocarcinoma, and adenocarcinoma). Studies have reported subsolid nodule malignancy rates ranging from 20% to 75%.11-15 This wide range may be a function of different nodule sizes or rates of biopsy. The prevalence increases even further in nodules with a part solid component.
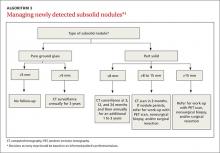
These factors, plus challenges in measuring serial growth on CT and the uncertain prognosis of untreated premalignant disease, make it necessary to have separate guidelines for managing subsolid nodules. The Fleischner Society, National Comprehensive Cancer Network, and the American College of Radiology (LungRads) each have differing recommendations on the frequency of follow-up for different-sized subsolid nodules. Newer studies favor a more conservative approach.16 Here we describe the current ACCP guidelines for managing subsolid nodules (ALGORITHM 3).1
GGNs. In an individual with a pure GGN ≤5 mm in diameter, no further evaluation is recommended. In an individual with a pure GGN >5 mm in diameter, annual surveillance with chest CT for at least 3 years is recommended.1
Part solid nodules. In an individual with a part solid nodule ≤8 mm, conduct CT surveillance at 3, 12, and 24 months and then annually for an additional one to 3 years. In a patient with a part solid nodule >8 mm to 15 mm, repeat chest CT at 3 months followed by a PET scan, nonsurgical biopsy, and/or surgical resection if the nodule persists. A patient with a part solid nodule >15 mm should undergo a PET scan, nonsurgical biopsy, and/or surgical resection.
CASE 2 › Ms. G is seen in the office by her FP, and they discuss management options. A repeat CT is done in 3 months and shows a persistent, unchanged nodule. Ms. G opts for a transthoracic biopsy, which reveals adenocarcinoma. Following a PET scan, which shows no evidence of metastasis, curative surgical wedge resection is done.
Multiple subsolid nodules. In a patient who has a dominant nodule and one or more additional nodules, each nodule should be evaluated individually, according to recommendations from the Fleischner Society (the ACCP currently does not have guidelines for managing multiple subsolid nodules). An individual with multiple GGNs that all measure ≤5 mm should receive CT exams at 2 and 4 years.13 A patient with multiple GGNs that include at least one nodule >5 mm but no dominant nodule should undergo follow-up CT at 3 months and annual CT surveillance for at least 3 years.13
CORRESPONDENCE
Samina Yunus, MD, MPH, Cleveland Clinic, Family Medicine, 551 East Washington Street, Chagrin Falls, OH 44022; [email protected].
1. Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e93S-e120S.
2. Burt JR, Iribarren C, Fair JM, et al; Atherosclerotic Disease, Vascular Function, and Genetic Epidemiology (ADVANCE) Study. Incidental findings on cardiac multidetector row computed tomography among healthy older adults: prevalence and clinical correlates. Arch Intern Med. 2008;168:756-761.
3. Golden SE, Wiener RS, Sullivan D, et al. Primary care providers and a system problem: A qualitative study of clinicians caring for patients with incidental pulmonary nodules. Chest. 2015;148:1422-1429.
4. Sullivan DR, Golden SE, Ganzini L, et al. ‘I still don’t know diddly’: a longitudinal qualitative study of patients’ knowledge and distress while undergoing evaluation of incidental pulmonary nodules. NPJ Prim Care Respir Med. 2015;25:15028.
5. van den Bergh KA, Essink-Bot ML, Borsboom GJ, et al. Long-term effects of lung cancer computed tomography screening on health-related quality of life: the NELSON trial. Eur Respir J. 2011;38:154-161.
6. Wiener RS, Gould MK, Woloshin S, et al. What do you mean, a spot?: A qualitative analysis of patients’ reactions to discussions with their physicians about pulmonary nodules. Chest. 2013;143:672-677.
7. Wiener RS, Gould MK, Woloshin S, et al. ‘The thing is not knowing’: patients’ perspectives on surveillance of an indeterminate pulmonary nodule. Health Expect. 2015;18:355-365.
8. Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697-722.
9. Pope CA 3rd, Burnett RT, Thun MJ, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132-1141.
10. Winer-Muram HT. The solitary pulmonary nodule. Radiology. 2006;239:34-49.
11. Gould MK, Ananth L, Barnett PG; Veterans Affairs SNAP Cooperative Study Group. A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules. Chest. 2007;131:383-388.
12. Seidelman JL, Myers JL, Quint LE. Incidental, subsolid pulmonary nodules at CT: etiology and management. Cancer Imaging. 2013;13:365-373.
13. Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013;266:304-317.
14. Oh JY, Kwon SY, Yoon HI, et al. Clinical significance of a solitary ground-glass opacity (GGO) lesion of the lung detected by chest CT. Lung Cancer. 2007;55:67-73.
15. Park CM, Goo JM, Lee HJ, et al. Nodular ground-glass opacity at thin-section CT: histologic correlation and evaluation of change at follow-up. Radiographics. 2007;27:391-408.
16. Heuvelmans MA, Oudkerk M. Management of subsolid pulmonary nodules in CT lung cancer screening. J Thorac Dis. 2015;7:1103-1106.
› Order a computed tomography chest scan, preferably with thin sections through the nodule, to help characterize an indeterminate pulmonary nodule identified on x-ray. B
› Estimate the pretest probability of malignancy for a patient with a pulmonary nodule using your clinical judgment and/or by using a validated model. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › George D is a 67-year-old patient who has never smoked and who has no history of malignancy. An x-ray of his ribs performed after a fall shows a 13-mm solitary nodule in his right upper lung.
CASE 2 › Cathy B is a healthy 80-year-old with no history of smoking. During a trip to the emergency department for chest pain, she had a computed tomography (CT) scan of her chest. While the chest pain was subsequently attributed to gastroesophageal reflux, the CT revealed a 9-mm part solid nodule that was 75% solid.
How should the physicians caring for each of these patients proceed with their care?
The widespread use of sensitive imaging techniques often leads to the incidental discovery of unrelated—but possibly significant—pulmonary findings. Pulmonary nodules are incidentally discovered on an estimated 0.09% to 0.2% of all chest x-rays, 13% of all chest CT angiograms,1 31% of all cardiac CTs performed for coronary calcium scoring,2 and up to 50% of thin-section chest CT scans.1
The widespread implementation of the US Preventive Services Task Force recommendations on lung cancer screening has further expanded the number of patients in whom asymptomatic pulmonary nodules will be detected. As a result, family physicians (FPs) will frequently encounter this challenging clinical dilemma and will need to:
- assess the patient’s risk profile
- address the patient’s concerns about malignancy while eliciting his or her preference for management
- minimize the risks of surveillance testing
- minimize patient distress while ensuring compliance with a follow-up that may extend up to 4 years
- determine when it’s appropriate to refer the patient to a pulmonologist and/or pulmonary nodule clinic or registry.
Taking these steps, however, can be challenging. In interviews, 15 primary care clinicians who care for patients with pulmonary nodules expressed concerns about limitations in time, knowledge, and resources, as well as a fear about such patients “falling through the cracks.”3 Familiarity with current evidence-based guidelines such as those from the American College of Chest Physicians (ACCP) and knowledge of emerging data on the management of various types of nodules are imperative.
To that end, this review will fill in the information gaps and provide guidance on how best to communicate what is known about a particular type of nodule with the patient who has one. (See “What to say to improve joint decision-making.”4-7) But first, a word about terminology.
What to say to improve joint decision-making4-7
Diagnosis and follow-up of a pulmonary nodule takes an emotional toll on patients, who often have a poor sense of what the presence of a nodule signifies. When caring for a patient with a pulmonary nodule, it’s essential to have an effective communication strategy to ensure that he or she is a well-informed partner in decision-making.
Specifically, you'll need to describe the type of nodule that the patient has, how fast it might grow and its malignancy potential, steps that will need to be taken, and the importance of smoking cessation (if the patient smokes).
Ask the patient about any concerns/fears he or she may have, and provide resources to reduce them. Emphasize shared decision-making and discuss the rationale for various management plans and the limitations of diagnostic tests. Do not minimize the issue; emphasize the need for, and importance of, prolonged follow-up—even for a patient who has a small, low-risk nodule.
Solid vs subsolid pulmonary nodules
Solid pulmonary nodules. Traditionally, the term “solitary pulmonary nodule” has been used to describe a single, well-circumscribed, radiographic opacity that measures up to 3 cm in diameter and is completely surrounded by aerated lung.1,8 The term “solitary” is now less useful because increasingly sensitive imaging techniques often reveal more than one nodule. In the absence of evidence of features that strongly suggest a benign etiology, these are now commonly referred to as indeterminate solid nodules.
Subsolid nodules are pulmonary nodules that have unique characteristics and require separate guidelines for management. Subsolid nodules include pure ground glass nodules (GGNs) and part solid nodules. GGNs are focal nodular areas of increased lung attenuation through which normal parenchymal structures such as airways, vessels, and interlobular septa can be visualized.1,8 Part solid nodules have a solid component. They are usually, but not necessarily, >50% ground glass in appearance.
Lung masses. Focal pulmonary lesions >3 cm in diameter are called lung masses and are presumed to be malignant (bronchogenic carcinoma) unless proven otherwise.1,8
The specific approach to evaluating and monitoring a pulmonary nodule varies depending on whether the nodule is solid or subsolid and other factors, including the nodule’s size.
Monitoring solid nodules
Monitoring of an indeterminate solid nodule is largely determined by the patient’s risk profile and the characteristics of the identified nodule.1 Independent patient predictors of malignancy include older age, smoking status, and history of prior malignancy (>5 years ago). Less established predictors are the presence of moderate or severe obstructive lung disease and exposure to particulate or sulfur oxide-related pollution.9
Patients who have an indeterminate nodule identified on chest x-ray should undergo a chest CT scan, preferably with thin sections through the nodule to help characterize it.1 Nodule characteristics that can help predict a patient’s risk of malignancy include the size (>8 mm confers higher risk), malignant rate of growth, edge characteristics (spiculation or irregular edges), thickness of the wall of a cavitary pulmonary nodule (≥16 mm has a likelihood ratio [LR] 37.97 of malignancy), and the location of the nodule (upper or middle lobe [LR=1.2 to 1.6]). 10 A lack of growth over 2 years and a benign pattern of calcification eliminate the need for further evaluation.
Validated tools to help guide decision-making. Although many physicians estimate pretest probability of malignancy intuitively, validated tools are readily available and can help in clinical decision-making.11 One such tool is the Mayo model, which is available at http://reference.medscape.com/calculator/solitary-pulmonary-nodule-risk. This model takes into account the patient’s age, smoking status, history of cancer, and characteristics of the nodule.
Solid nodules >8 mm to 3 cm
For a patient with a solid nodule >8 mm to 3 cm, ACCP guidelines suggest that physicians estimate the pretest probability of malignancy qualitatively using their clinical judgment and/or quantitatively by using a validated model, such as the Mayo model described above.
Based on the patient’s probability of malignancy, management options include continued CT surveillance, positron emission tomography (PET) imaging, CT-guided needle lung biopsy, bronchoscopy with biopsy, or surgical wedge resection (ALGORITHM 1).1
CT surveillance is recommended for individuals:
- with very low (<5%) probability of malignancy
- with low to moderate (5% to 30%) or moderate to high (31% to 65%) probability of malignancy with negative functional imaging (PET)
- with high probability of malignancy (>65%) when needle biopsy is nondiagnostic and the lesion is not hypermetabolic on PET scan.
Surveillance is also recommended when a fully informed patient prefers nonaggressive management. The intervals for serial CT in this population are at 3 to 6 months, 9 to 12 months, and 18 to 24 months.
In an individual with a solid indeterminate nodule with a high probability of malignancy (>65%), functional imaging should not be performed to characterize the nodule. It may, however, be performed for staging.
Time for biopsy or resection? If a nodule shows evidence of malignant growth on serial imaging, nonsurgical biopsy (CT scan-guided transthoracic needle biopsy, bronchoscopy guided by fluoroscopy, endobronchial ultrasound, electromagnetic navigation bronchoscopy, or virtual bronchoscopy navigation) or surgical resection is recommended.
Nonsurgical biopsy is also recommended when the patient’s pretest probability and imaging test results are discordant, when a benign diagnosis requires specific medical treatment, or if a fully informed patient desires proof of diagnosis prior to surgery.
Thoracoscopy with wedge resection is the gold standard for diagnosis of a malignant nodule. It is recommended:
- when the clinical probability of malignancy is high (>65%)
- when the nodule is intensely hypermetabolic by PET scan or positive by other functional imaging tests
- when nonsurgical biopsy is suggestive of malignancy
- when a fully informed patient prefers a definitive diagnostic procedure.
CASE 1 › The FP contacts Mr. D and advises that he get a chest CT to better characterize his pulmonary nodule. A thin-slice CT of the lung reveals that the 13-mm solid nodule in the right upper lobe has spiculated margins. According to the Mayo risk calculator, Mr. D is at moderate risk of malignancy (32.5%). Mr. D and his physician discuss the findings and possible management options, and Mr. D opts to have a PET scan. The FP gives Mr. D literature on pulmonary nodules and contact information for the provider team. A PET scan shows negative uptake. Mr. D and his physician discuss CT surveillance and nonsurgical biopsy. He opts for CT surveillance. The next CT is scheduled for 3 months.
Solid nodules ≤8 mm
Management of these lesions generally follows the consensus-based guidelines that were first published by the Fleischner Society and subsequently endorsed by the ACCP.1 The 2 main determinants that guide management of nodules ≤8 mm are the patient’s risk factors for cancer and nodule size (ALGORITHM 2).1 The Fleischner guidelines pertain only to patients older than age 35 with no current extra pulmonary malignancy or unexplained fevers. The ACCP guidelines, although similar, do not include these limitations. Patient risk factors include history of smoking, older age, and a history of malignancy.1
Patients with no risk factors for malignancy. The frequency of surveillance CT is determined by the size of the nodule. Nodules ≤4 mm do not need to be followed. For nodules >4 mm to 6 mm, a repeat CT in 12 months is recommended with no follow-up if stable. For nodules >6 to <8 mm, repeat CT is recommended at 6 to 12 months, and again between 18 and 24 months if unchanged.1Patients with one or more risk factors for malignancy. Nodules ≤4 mm should be reevaluated at 12 months in patients with one or more risk factors; no additional follow-up is needed if unchanged. For nodules >4 mm to 6 mm, CT should be repeated between 6 and 12 months and again between 18 and 24 months. Nodules >6 mm to <8 mm should be followed initially between 3 to 6 months, then between 9 and 12 months and again at 24 months if unchanged.1
Subsolid nodules require a different approach
Subsolid nodules have a high prevalence of premalignant and malignant disease (adenocarcinoma in situ, minimally invasive adenocarcinoma, and adenocarcinoma). Studies have reported subsolid nodule malignancy rates ranging from 20% to 75%.11-15 This wide range may be a function of different nodule sizes or rates of biopsy. The prevalence increases even further in nodules with a part solid component.
These factors, plus challenges in measuring serial growth on CT and the uncertain prognosis of untreated premalignant disease, make it necessary to have separate guidelines for managing subsolid nodules. The Fleischner Society, National Comprehensive Cancer Network, and the American College of Radiology (LungRads) each have differing recommendations on the frequency of follow-up for different-sized subsolid nodules. Newer studies favor a more conservative approach.16 Here we describe the current ACCP guidelines for managing subsolid nodules (ALGORITHM 3).1
GGNs. In an individual with a pure GGN ≤5 mm in diameter, no further evaluation is recommended. In an individual with a pure GGN >5 mm in diameter, annual surveillance with chest CT for at least 3 years is recommended.1
Part solid nodules. In an individual with a part solid nodule ≤8 mm, conduct CT surveillance at 3, 12, and 24 months and then annually for an additional one to 3 years. In a patient with a part solid nodule >8 mm to 15 mm, repeat chest CT at 3 months followed by a PET scan, nonsurgical biopsy, and/or surgical resection if the nodule persists. A patient with a part solid nodule >15 mm should undergo a PET scan, nonsurgical biopsy, and/or surgical resection.
CASE 2 › Ms. G is seen in the office by her FP, and they discuss management options. A repeat CT is done in 3 months and shows a persistent, unchanged nodule. Ms. G opts for a transthoracic biopsy, which reveals adenocarcinoma. Following a PET scan, which shows no evidence of metastasis, curative surgical wedge resection is done.
Multiple subsolid nodules. In a patient who has a dominant nodule and one or more additional nodules, each nodule should be evaluated individually, according to recommendations from the Fleischner Society (the ACCP currently does not have guidelines for managing multiple subsolid nodules). An individual with multiple GGNs that all measure ≤5 mm should receive CT exams at 2 and 4 years.13 A patient with multiple GGNs that include at least one nodule >5 mm but no dominant nodule should undergo follow-up CT at 3 months and annual CT surveillance for at least 3 years.13
CORRESPONDENCE
Samina Yunus, MD, MPH, Cleveland Clinic, Family Medicine, 551 East Washington Street, Chagrin Falls, OH 44022; [email protected].
› Order a computed tomography chest scan, preferably with thin sections through the nodule, to help characterize an indeterminate pulmonary nodule identified on x-ray. B
› Estimate the pretest probability of malignancy for a patient with a pulmonary nodule using your clinical judgment and/or by using a validated model. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › George D is a 67-year-old patient who has never smoked and who has no history of malignancy. An x-ray of his ribs performed after a fall shows a 13-mm solitary nodule in his right upper lung.
CASE 2 › Cathy B is a healthy 80-year-old with no history of smoking. During a trip to the emergency department for chest pain, she had a computed tomography (CT) scan of her chest. While the chest pain was subsequently attributed to gastroesophageal reflux, the CT revealed a 9-mm part solid nodule that was 75% solid.
How should the physicians caring for each of these patients proceed with their care?
The widespread use of sensitive imaging techniques often leads to the incidental discovery of unrelated—but possibly significant—pulmonary findings. Pulmonary nodules are incidentally discovered on an estimated 0.09% to 0.2% of all chest x-rays, 13% of all chest CT angiograms,1 31% of all cardiac CTs performed for coronary calcium scoring,2 and up to 50% of thin-section chest CT scans.1
The widespread implementation of the US Preventive Services Task Force recommendations on lung cancer screening has further expanded the number of patients in whom asymptomatic pulmonary nodules will be detected. As a result, family physicians (FPs) will frequently encounter this challenging clinical dilemma and will need to:
- assess the patient’s risk profile
- address the patient’s concerns about malignancy while eliciting his or her preference for management
- minimize the risks of surveillance testing
- minimize patient distress while ensuring compliance with a follow-up that may extend up to 4 years
- determine when it’s appropriate to refer the patient to a pulmonologist and/or pulmonary nodule clinic or registry.
Taking these steps, however, can be challenging. In interviews, 15 primary care clinicians who care for patients with pulmonary nodules expressed concerns about limitations in time, knowledge, and resources, as well as a fear about such patients “falling through the cracks.”3 Familiarity with current evidence-based guidelines such as those from the American College of Chest Physicians (ACCP) and knowledge of emerging data on the management of various types of nodules are imperative.
To that end, this review will fill in the information gaps and provide guidance on how best to communicate what is known about a particular type of nodule with the patient who has one. (See “What to say to improve joint decision-making.”4-7) But first, a word about terminology.
What to say to improve joint decision-making4-7
Diagnosis and follow-up of a pulmonary nodule takes an emotional toll on patients, who often have a poor sense of what the presence of a nodule signifies. When caring for a patient with a pulmonary nodule, it’s essential to have an effective communication strategy to ensure that he or she is a well-informed partner in decision-making.
Specifically, you'll need to describe the type of nodule that the patient has, how fast it might grow and its malignancy potential, steps that will need to be taken, and the importance of smoking cessation (if the patient smokes).
Ask the patient about any concerns/fears he or she may have, and provide resources to reduce them. Emphasize shared decision-making and discuss the rationale for various management plans and the limitations of diagnostic tests. Do not minimize the issue; emphasize the need for, and importance of, prolonged follow-up—even for a patient who has a small, low-risk nodule.
Solid vs subsolid pulmonary nodules
Solid pulmonary nodules. Traditionally, the term “solitary pulmonary nodule” has been used to describe a single, well-circumscribed, radiographic opacity that measures up to 3 cm in diameter and is completely surrounded by aerated lung.1,8 The term “solitary” is now less useful because increasingly sensitive imaging techniques often reveal more than one nodule. In the absence of evidence of features that strongly suggest a benign etiology, these are now commonly referred to as indeterminate solid nodules.
Subsolid nodules are pulmonary nodules that have unique characteristics and require separate guidelines for management. Subsolid nodules include pure ground glass nodules (GGNs) and part solid nodules. GGNs are focal nodular areas of increased lung attenuation through which normal parenchymal structures such as airways, vessels, and interlobular septa can be visualized.1,8 Part solid nodules have a solid component. They are usually, but not necessarily, >50% ground glass in appearance.
Lung masses. Focal pulmonary lesions >3 cm in diameter are called lung masses and are presumed to be malignant (bronchogenic carcinoma) unless proven otherwise.1,8
The specific approach to evaluating and monitoring a pulmonary nodule varies depending on whether the nodule is solid or subsolid and other factors, including the nodule’s size.
Monitoring solid nodules
Monitoring of an indeterminate solid nodule is largely determined by the patient’s risk profile and the characteristics of the identified nodule.1 Independent patient predictors of malignancy include older age, smoking status, and history of prior malignancy (>5 years ago). Less established predictors are the presence of moderate or severe obstructive lung disease and exposure to particulate or sulfur oxide-related pollution.9
Patients who have an indeterminate nodule identified on chest x-ray should undergo a chest CT scan, preferably with thin sections through the nodule to help characterize it.1 Nodule characteristics that can help predict a patient’s risk of malignancy include the size (>8 mm confers higher risk), malignant rate of growth, edge characteristics (spiculation or irregular edges), thickness of the wall of a cavitary pulmonary nodule (≥16 mm has a likelihood ratio [LR] 37.97 of malignancy), and the location of the nodule (upper or middle lobe [LR=1.2 to 1.6]). 10 A lack of growth over 2 years and a benign pattern of calcification eliminate the need for further evaluation.
Validated tools to help guide decision-making. Although many physicians estimate pretest probability of malignancy intuitively, validated tools are readily available and can help in clinical decision-making.11 One such tool is the Mayo model, which is available at http://reference.medscape.com/calculator/solitary-pulmonary-nodule-risk. This model takes into account the patient’s age, smoking status, history of cancer, and characteristics of the nodule.
Solid nodules >8 mm to 3 cm
For a patient with a solid nodule >8 mm to 3 cm, ACCP guidelines suggest that physicians estimate the pretest probability of malignancy qualitatively using their clinical judgment and/or quantitatively by using a validated model, such as the Mayo model described above.
Based on the patient’s probability of malignancy, management options include continued CT surveillance, positron emission tomography (PET) imaging, CT-guided needle lung biopsy, bronchoscopy with biopsy, or surgical wedge resection (ALGORITHM 1).1
CT surveillance is recommended for individuals:
- with very low (<5%) probability of malignancy
- with low to moderate (5% to 30%) or moderate to high (31% to 65%) probability of malignancy with negative functional imaging (PET)
- with high probability of malignancy (>65%) when needle biopsy is nondiagnostic and the lesion is not hypermetabolic on PET scan.
Surveillance is also recommended when a fully informed patient prefers nonaggressive management. The intervals for serial CT in this population are at 3 to 6 months, 9 to 12 months, and 18 to 24 months.
In an individual with a solid indeterminate nodule with a high probability of malignancy (>65%), functional imaging should not be performed to characterize the nodule. It may, however, be performed for staging.
Time for biopsy or resection? If a nodule shows evidence of malignant growth on serial imaging, nonsurgical biopsy (CT scan-guided transthoracic needle biopsy, bronchoscopy guided by fluoroscopy, endobronchial ultrasound, electromagnetic navigation bronchoscopy, or virtual bronchoscopy navigation) or surgical resection is recommended.
Nonsurgical biopsy is also recommended when the patient’s pretest probability and imaging test results are discordant, when a benign diagnosis requires specific medical treatment, or if a fully informed patient desires proof of diagnosis prior to surgery.
Thoracoscopy with wedge resection is the gold standard for diagnosis of a malignant nodule. It is recommended:
- when the clinical probability of malignancy is high (>65%)
- when the nodule is intensely hypermetabolic by PET scan or positive by other functional imaging tests
- when nonsurgical biopsy is suggestive of malignancy
- when a fully informed patient prefers a definitive diagnostic procedure.
CASE 1 › The FP contacts Mr. D and advises that he get a chest CT to better characterize his pulmonary nodule. A thin-slice CT of the lung reveals that the 13-mm solid nodule in the right upper lobe has spiculated margins. According to the Mayo risk calculator, Mr. D is at moderate risk of malignancy (32.5%). Mr. D and his physician discuss the findings and possible management options, and Mr. D opts to have a PET scan. The FP gives Mr. D literature on pulmonary nodules and contact information for the provider team. A PET scan shows negative uptake. Mr. D and his physician discuss CT surveillance and nonsurgical biopsy. He opts for CT surveillance. The next CT is scheduled for 3 months.
Solid nodules ≤8 mm
Management of these lesions generally follows the consensus-based guidelines that were first published by the Fleischner Society and subsequently endorsed by the ACCP.1 The 2 main determinants that guide management of nodules ≤8 mm are the patient’s risk factors for cancer and nodule size (ALGORITHM 2).1 The Fleischner guidelines pertain only to patients older than age 35 with no current extra pulmonary malignancy or unexplained fevers. The ACCP guidelines, although similar, do not include these limitations. Patient risk factors include history of smoking, older age, and a history of malignancy.1
Patients with no risk factors for malignancy. The frequency of surveillance CT is determined by the size of the nodule. Nodules ≤4 mm do not need to be followed. For nodules >4 mm to 6 mm, a repeat CT in 12 months is recommended with no follow-up if stable. For nodules >6 to <8 mm, repeat CT is recommended at 6 to 12 months, and again between 18 and 24 months if unchanged.1Patients with one or more risk factors for malignancy. Nodules ≤4 mm should be reevaluated at 12 months in patients with one or more risk factors; no additional follow-up is needed if unchanged. For nodules >4 mm to 6 mm, CT should be repeated between 6 and 12 months and again between 18 and 24 months. Nodules >6 mm to <8 mm should be followed initially between 3 to 6 months, then between 9 and 12 months and again at 24 months if unchanged.1
Subsolid nodules require a different approach
Subsolid nodules have a high prevalence of premalignant and malignant disease (adenocarcinoma in situ, minimally invasive adenocarcinoma, and adenocarcinoma). Studies have reported subsolid nodule malignancy rates ranging from 20% to 75%.11-15 This wide range may be a function of different nodule sizes or rates of biopsy. The prevalence increases even further in nodules with a part solid component.
These factors, plus challenges in measuring serial growth on CT and the uncertain prognosis of untreated premalignant disease, make it necessary to have separate guidelines for managing subsolid nodules. The Fleischner Society, National Comprehensive Cancer Network, and the American College of Radiology (LungRads) each have differing recommendations on the frequency of follow-up for different-sized subsolid nodules. Newer studies favor a more conservative approach.16 Here we describe the current ACCP guidelines for managing subsolid nodules (ALGORITHM 3).1
GGNs. In an individual with a pure GGN ≤5 mm in diameter, no further evaluation is recommended. In an individual with a pure GGN >5 mm in diameter, annual surveillance with chest CT for at least 3 years is recommended.1
Part solid nodules. In an individual with a part solid nodule ≤8 mm, conduct CT surveillance at 3, 12, and 24 months and then annually for an additional one to 3 years. In a patient with a part solid nodule >8 mm to 15 mm, repeat chest CT at 3 months followed by a PET scan, nonsurgical biopsy, and/or surgical resection if the nodule persists. A patient with a part solid nodule >15 mm should undergo a PET scan, nonsurgical biopsy, and/or surgical resection.
CASE 2 › Ms. G is seen in the office by her FP, and they discuss management options. A repeat CT is done in 3 months and shows a persistent, unchanged nodule. Ms. G opts for a transthoracic biopsy, which reveals adenocarcinoma. Following a PET scan, which shows no evidence of metastasis, curative surgical wedge resection is done.
Multiple subsolid nodules. In a patient who has a dominant nodule and one or more additional nodules, each nodule should be evaluated individually, according to recommendations from the Fleischner Society (the ACCP currently does not have guidelines for managing multiple subsolid nodules). An individual with multiple GGNs that all measure ≤5 mm should receive CT exams at 2 and 4 years.13 A patient with multiple GGNs that include at least one nodule >5 mm but no dominant nodule should undergo follow-up CT at 3 months and annual CT surveillance for at least 3 years.13
CORRESPONDENCE
Samina Yunus, MD, MPH, Cleveland Clinic, Family Medicine, 551 East Washington Street, Chagrin Falls, OH 44022; [email protected].
1. Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e93S-e120S.
2. Burt JR, Iribarren C, Fair JM, et al; Atherosclerotic Disease, Vascular Function, and Genetic Epidemiology (ADVANCE) Study. Incidental findings on cardiac multidetector row computed tomography among healthy older adults: prevalence and clinical correlates. Arch Intern Med. 2008;168:756-761.
3. Golden SE, Wiener RS, Sullivan D, et al. Primary care providers and a system problem: A qualitative study of clinicians caring for patients with incidental pulmonary nodules. Chest. 2015;148:1422-1429.
4. Sullivan DR, Golden SE, Ganzini L, et al. ‘I still don’t know diddly’: a longitudinal qualitative study of patients’ knowledge and distress while undergoing evaluation of incidental pulmonary nodules. NPJ Prim Care Respir Med. 2015;25:15028.
5. van den Bergh KA, Essink-Bot ML, Borsboom GJ, et al. Long-term effects of lung cancer computed tomography screening on health-related quality of life: the NELSON trial. Eur Respir J. 2011;38:154-161.
6. Wiener RS, Gould MK, Woloshin S, et al. What do you mean, a spot?: A qualitative analysis of patients’ reactions to discussions with their physicians about pulmonary nodules. Chest. 2013;143:672-677.
7. Wiener RS, Gould MK, Woloshin S, et al. ‘The thing is not knowing’: patients’ perspectives on surveillance of an indeterminate pulmonary nodule. Health Expect. 2015;18:355-365.
8. Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697-722.
9. Pope CA 3rd, Burnett RT, Thun MJ, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132-1141.
10. Winer-Muram HT. The solitary pulmonary nodule. Radiology. 2006;239:34-49.
11. Gould MK, Ananth L, Barnett PG; Veterans Affairs SNAP Cooperative Study Group. A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules. Chest. 2007;131:383-388.
12. Seidelman JL, Myers JL, Quint LE. Incidental, subsolid pulmonary nodules at CT: etiology and management. Cancer Imaging. 2013;13:365-373.
13. Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013;266:304-317.
14. Oh JY, Kwon SY, Yoon HI, et al. Clinical significance of a solitary ground-glass opacity (GGO) lesion of the lung detected by chest CT. Lung Cancer. 2007;55:67-73.
15. Park CM, Goo JM, Lee HJ, et al. Nodular ground-glass opacity at thin-section CT: histologic correlation and evaluation of change at follow-up. Radiographics. 2007;27:391-408.
16. Heuvelmans MA, Oudkerk M. Management of subsolid pulmonary nodules in CT lung cancer screening. J Thorac Dis. 2015;7:1103-1106.
1. Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e93S-e120S.
2. Burt JR, Iribarren C, Fair JM, et al; Atherosclerotic Disease, Vascular Function, and Genetic Epidemiology (ADVANCE) Study. Incidental findings on cardiac multidetector row computed tomography among healthy older adults: prevalence and clinical correlates. Arch Intern Med. 2008;168:756-761.
3. Golden SE, Wiener RS, Sullivan D, et al. Primary care providers and a system problem: A qualitative study of clinicians caring for patients with incidental pulmonary nodules. Chest. 2015;148:1422-1429.
4. Sullivan DR, Golden SE, Ganzini L, et al. ‘I still don’t know diddly’: a longitudinal qualitative study of patients’ knowledge and distress while undergoing evaluation of incidental pulmonary nodules. NPJ Prim Care Respir Med. 2015;25:15028.
5. van den Bergh KA, Essink-Bot ML, Borsboom GJ, et al. Long-term effects of lung cancer computed tomography screening on health-related quality of life: the NELSON trial. Eur Respir J. 2011;38:154-161.
6. Wiener RS, Gould MK, Woloshin S, et al. What do you mean, a spot?: A qualitative analysis of patients’ reactions to discussions with their physicians about pulmonary nodules. Chest. 2013;143:672-677.
7. Wiener RS, Gould MK, Woloshin S, et al. ‘The thing is not knowing’: patients’ perspectives on surveillance of an indeterminate pulmonary nodule. Health Expect. 2015;18:355-365.
8. Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697-722.
9. Pope CA 3rd, Burnett RT, Thun MJ, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132-1141.
10. Winer-Muram HT. The solitary pulmonary nodule. Radiology. 2006;239:34-49.
11. Gould MK, Ananth L, Barnett PG; Veterans Affairs SNAP Cooperative Study Group. A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules. Chest. 2007;131:383-388.
12. Seidelman JL, Myers JL, Quint LE. Incidental, subsolid pulmonary nodules at CT: etiology and management. Cancer Imaging. 2013;13:365-373.
13. Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013;266:304-317.
14. Oh JY, Kwon SY, Yoon HI, et al. Clinical significance of a solitary ground-glass opacity (GGO) lesion of the lung detected by chest CT. Lung Cancer. 2007;55:67-73.
15. Park CM, Goo JM, Lee HJ, et al. Nodular ground-glass opacity at thin-section CT: histologic correlation and evaluation of change at follow-up. Radiographics. 2007;27:391-408.
16. Heuvelmans MA, Oudkerk M. Management of subsolid pulmonary nodules in CT lung cancer screening. J Thorac Dis. 2015;7:1103-1106.
Patient Has No Complaints, But Family Is Concerned
ANSWER
The radiograph shows diffuse osteopenia and spondylosis. Of note is a moderate to severe compression deformity of the T8 vertebral body. However, by plain radiograph, it is age indeterminate as to its acuity. For definitive diagnosis, MRI without contrast is required to assess for marrow edema, which then suggests an acute fracture.
This patient was admitted for further workup. MRI was ultimately obtained and revealed marrow edema within that vertebral body. She was treated with a rigid custom-made brace.
ANSWER
The radiograph shows diffuse osteopenia and spondylosis. Of note is a moderate to severe compression deformity of the T8 vertebral body. However, by plain radiograph, it is age indeterminate as to its acuity. For definitive diagnosis, MRI without contrast is required to assess for marrow edema, which then suggests an acute fracture.
This patient was admitted for further workup. MRI was ultimately obtained and revealed marrow edema within that vertebral body. She was treated with a rigid custom-made brace.
ANSWER
The radiograph shows diffuse osteopenia and spondylosis. Of note is a moderate to severe compression deformity of the T8 vertebral body. However, by plain radiograph, it is age indeterminate as to its acuity. For definitive diagnosis, MRI without contrast is required to assess for marrow edema, which then suggests an acute fracture.
This patient was admitted for further workup. MRI was ultimately obtained and revealed marrow edema within that vertebral body. She was treated with a rigid custom-made brace.
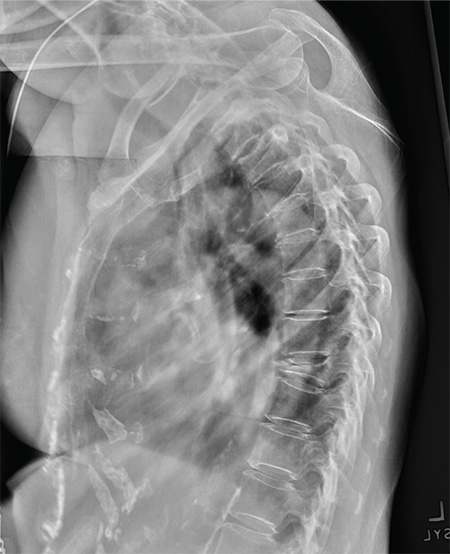
An 80-year-old woman presents to the emergency department for evaluation. Her family reports that she has baseline dementia and resides in an assisted living facility. Staff there report that recently the patient has fallen multiple times. The patient herself does not voice any specific complaints, but her family has noticed she is not as active or walking as much as usual. Medical history is significant for mild hypertension. On physical exam, you note that the patient is awake and alert but oriented only to person. Her vital signs are stable. Primary and secondary survey do not demonstrate any obvious injury or trauma. You order some basic blood work, as well as some imaging studies—including thoracic and lumbar radiographs. The lateral thoracic radiograph is shown. What is your impression?
The Ears Have It (and She Doesn’t Want It)
ANSWER
The correct answer is to obtain a biopsy (choice “d”), the results of which would likely dictate rational and effective treatment. The other choices are largely empirical and not based on available evidence.
DISCUSSION
In this case, the biopsy (with samples from the arm rash as well as from the ears) showed unequivocal evidence of connective tissue disease—almost certainly lupus.
Systemic lupus erythematosus (SLE) has protean manifestations because it can affect so many different organs in so many different ways. Reduced to its simplest elements, lupus is an autoimmune process that results in a form of vasculitis that can affect any perfused tissue.
In terms of the skin, the visible manifestations of lupus are numerous and not always obvious. Sun is a known exacerbating factor, as this case demonstrated quite well: The patient’s rash was pronounced on sun-exposed skin but spared covered skin. When this was brought to her attention, the patient recalled a baby-sitting job earlier in the year (summer) that had required her to spend time outdoors. She also acknowledged that her smoking habit often took her into the backyard, where she would stand in the sun.
That being said, neither the arm rash nor the ear changes are “typical” of lupus (although the latter did include patulous follicular orifices, enlarged pores often seen focally with lupus). The effect is simply the result of the patient’s normal dark skin color; on a white person, the discoloration would have been pink or red.
Although these changes were suspicious for lupus, it was necessary to establish the diagnosis by biopsy—especially since the patient was already being treated for the disease. With that accomplished, the patient was sent back to her rheumatologist, who indicated he would probably treat her with a biologic, plus or minus methotrexate.
ANSWER
The correct answer is to obtain a biopsy (choice “d”), the results of which would likely dictate rational and effective treatment. The other choices are largely empirical and not based on available evidence.
DISCUSSION
In this case, the biopsy (with samples from the arm rash as well as from the ears) showed unequivocal evidence of connective tissue disease—almost certainly lupus.
Systemic lupus erythematosus (SLE) has protean manifestations because it can affect so many different organs in so many different ways. Reduced to its simplest elements, lupus is an autoimmune process that results in a form of vasculitis that can affect any perfused tissue.
In terms of the skin, the visible manifestations of lupus are numerous and not always obvious. Sun is a known exacerbating factor, as this case demonstrated quite well: The patient’s rash was pronounced on sun-exposed skin but spared covered skin. When this was brought to her attention, the patient recalled a baby-sitting job earlier in the year (summer) that had required her to spend time outdoors. She also acknowledged that her smoking habit often took her into the backyard, where she would stand in the sun.
That being said, neither the arm rash nor the ear changes are “typical” of lupus (although the latter did include patulous follicular orifices, enlarged pores often seen focally with lupus). The effect is simply the result of the patient’s normal dark skin color; on a white person, the discoloration would have been pink or red.
Although these changes were suspicious for lupus, it was necessary to establish the diagnosis by biopsy—especially since the patient was already being treated for the disease. With that accomplished, the patient was sent back to her rheumatologist, who indicated he would probably treat her with a biologic, plus or minus methotrexate.
ANSWER
The correct answer is to obtain a biopsy (choice “d”), the results of which would likely dictate rational and effective treatment. The other choices are largely empirical and not based on available evidence.
DISCUSSION
In this case, the biopsy (with samples from the arm rash as well as from the ears) showed unequivocal evidence of connective tissue disease—almost certainly lupus.
Systemic lupus erythematosus (SLE) has protean manifestations because it can affect so many different organs in so many different ways. Reduced to its simplest elements, lupus is an autoimmune process that results in a form of vasculitis that can affect any perfused tissue.
In terms of the skin, the visible manifestations of lupus are numerous and not always obvious. Sun is a known exacerbating factor, as this case demonstrated quite well: The patient’s rash was pronounced on sun-exposed skin but spared covered skin. When this was brought to her attention, the patient recalled a baby-sitting job earlier in the year (summer) that had required her to spend time outdoors. She also acknowledged that her smoking habit often took her into the backyard, where she would stand in the sun.
That being said, neither the arm rash nor the ear changes are “typical” of lupus (although the latter did include patulous follicular orifices, enlarged pores often seen focally with lupus). The effect is simply the result of the patient’s normal dark skin color; on a white person, the discoloration would have been pink or red.
Although these changes were suspicious for lupus, it was necessary to establish the diagnosis by biopsy—especially since the patient was already being treated for the disease. With that accomplished, the patient was sent back to her rheumatologist, who indicated he would probably treat her with a biologic, plus or minus methotrexate.
A 34-year-old black woman is sent to dermatology by her rheumatologist for evaluation of changes to her ears that began several months ago. The patient reports no symptoms, but she is quite distressed by the appearance of her ears. She has been under the care of the rheumatologist for several years for her systemic lupus erythematosus. She takes hydroxychloroquine (400 mg/d), which she says controls most of her systemic symptoms (ie, joint pain and malaise). Further history taking reveals that, within the time frame of the ear changes, the patient also developed an itchy rash on both arms. Application of triamcinolone 0.1% cream has not helped. On examination, the changes to the patient’s ears are immediately obvious: the dark brown to black discoloration contrasts sharply with her light brown skin. In addition to the color change, the surface of the ears is scaly and rough, with enlarged pores evident. There is no redness or swelling noted; palpation elicits neither increased warmth nor adenopathy around the ears or on the adjacent neck. The scaly rash on the patient’s arms is remarkably symmetrical. It affects the sun-exposed lateral portions of both arms, sparing the skin on the medial aspects and on the proximal portions normally covered by clothing.
The simple lab test is sometimes more complex than we think, if we think about it at all
We are all exposed to initiatives from multiple stakeholders telling us to order fewer tests. Many of these efforts to control costs and improve efficiency and quality of care are directed at populations of patients and are broad concepts: provide screening only to those most likely to benefit (eg, don’t screen for prostate cancer in men with a lifespan < 10 years); avoid procedures that provided limited benefit in controlled trials (eg, limit routine arthroscopic treatment of knee osteoarthritis); and avoid reflexive practices unlikely to improve patient outcomes (eg, eliminate routine preoperative testing before elective procedures in otherwise healthy patients).
Whether system-based changes will be implemented and have an impact remains to be determined. But I sense that with all the attention being focused on population management and healthcare practices, including an emphasis on documenting and coding our encounters with patients, whether substantive or simply digital housekeeping, we are increasingly distracted from the patient in front of us and are spending less time reviewing the principles underlying the diagnoses we make and the tests we order—just as we are taking less time to perform relevant physical examinations.1 The latter may mostly relate to time pressures. The former, I believe, is a product of both time pressures and a false sense of confidence in our knowledge of seemingly commonplace laboratory tests.
As I lecture, work with trainees, and reflect on my own patients, I realize that we are slowly but progressively minimizing the importance of a working knowledge of the basic foundations of clinical practice—perhaps because facts can always be looked up. I am not referring to knowledge of arcane biochemical pathways, eponymous references, or the latest recommended treatment of inclusion body myositis. I am thinking instead of the value of regularly refreshing our knowledge of laboratory tests and diagnoses we frequently encounter.
Having access to multiple clinical databases literally in our pockets is likely bolstering a false sense of confidence in our knowledge. The National Library of Medicine may be only a tap on a smart phone away, but accessing it regularly is a different thing. Attending conferences and reading educational journals help to keep our broad-based knowledge of internal medicine refreshed, but time pressures may significantly limit our ability to regularly pursue these activities.
In this issue of the Journal, Drs. Moghadam-Kia et al discuss their approach to asymptomatic elevations in creatine kinase (CK). Although no longer included in the most commonly used lab panels, CK measurement is often ordered in patients taking statins, even if they have no relevant muscle-related symptoms. Thus, evaluating a patient with an asymptomatic elevated CK level is not rare. The authors delve into the clinically relevant test characteristics, and their important caveats about interpreting elevated CK levels are germane not only to the asymptomatic patient, but also to the patient being evaluated for myalgia or weakness. This latter situation is one I frequently face in both the hospital and the outpatient clinic. I am often asked to consult on patients who have incompletely defined symptoms and elevated CK.
As discussed in the article, the laboratory definition of “normal” must first be considered. Laboratory test results must always be interpreted in the clinical context. An isolated elevation in parathyroid hormone cannot be interpreted without knowing the patient’s vitamin D level. Nor can “normal” low-density lipoprotein or serum urate levels be interpreted properly without knowing if the patient is accumulating excess cholesterol or urate deposits. As we order and interpret test results, we must consider the biology of the substance being measured as well as the test characteristics; too often, we react to abnormal laboratory results with an incomplete understanding of these aspects.
Moghadam-Kia et al do not dwell on the organ involvement causing CK elevations, but specificity is another very important aspect when clinically interpreting the results of a CK test. Many patients with muscle damage or inflammation have elevations in serum aspartate aminotransferase and alanine aminotransferase levels (the ratio of elevation depends on the time course of the muscle damage and on the relative clearance rate of the two enzymes). Without knowing that the CK is elevated, one might assume that an aminotransferase elevation reflects hepatitis. I have seen several patients with elevated aminotransferases and complaints of weakness and fatigue who were subjected to liver biopsy before it was recognized that the source of the enzyme elevation (“liver function test changes”) was muscle (or hemolysis). Frequently unrecognized is that aldolase, which has a cell distribution similar to that of lactate dehydrogenase, does not have the relative specificity of localization to muscle that CK has. CK is quite useful in distinguishing myocyte from hepatocyte damage.
This paper presents a wonderful reminder of the value of updating and reviewing what we know about tests that we order, even if we feel comfortable when ordering them. Before initiating a cascade of additional tests and consultations to explore the cause of an abnormal test result, a little time spent reviewing its basic characteristics and biology may pay dividends.
As 2015 comes to a close, we at the Journal share with you our sincere wishes for personal satisfaction and a globally more peaceful 2016.
- Verghese A, Charlton B, Kassirer JP, Ramsey M, Ioannidis JP. Inadequacies of physical examination as a cause of medical errors and adverse events: a collection of vignettes. Am J Med 2015; 128:1322–1324.
We are all exposed to initiatives from multiple stakeholders telling us to order fewer tests. Many of these efforts to control costs and improve efficiency and quality of care are directed at populations of patients and are broad concepts: provide screening only to those most likely to benefit (eg, don’t screen for prostate cancer in men with a lifespan < 10 years); avoid procedures that provided limited benefit in controlled trials (eg, limit routine arthroscopic treatment of knee osteoarthritis); and avoid reflexive practices unlikely to improve patient outcomes (eg, eliminate routine preoperative testing before elective procedures in otherwise healthy patients).
Whether system-based changes will be implemented and have an impact remains to be determined. But I sense that with all the attention being focused on population management and healthcare practices, including an emphasis on documenting and coding our encounters with patients, whether substantive or simply digital housekeeping, we are increasingly distracted from the patient in front of us and are spending less time reviewing the principles underlying the diagnoses we make and the tests we order—just as we are taking less time to perform relevant physical examinations.1 The latter may mostly relate to time pressures. The former, I believe, is a product of both time pressures and a false sense of confidence in our knowledge of seemingly commonplace laboratory tests.
As I lecture, work with trainees, and reflect on my own patients, I realize that we are slowly but progressively minimizing the importance of a working knowledge of the basic foundations of clinical practice—perhaps because facts can always be looked up. I am not referring to knowledge of arcane biochemical pathways, eponymous references, or the latest recommended treatment of inclusion body myositis. I am thinking instead of the value of regularly refreshing our knowledge of laboratory tests and diagnoses we frequently encounter.
Having access to multiple clinical databases literally in our pockets is likely bolstering a false sense of confidence in our knowledge. The National Library of Medicine may be only a tap on a smart phone away, but accessing it regularly is a different thing. Attending conferences and reading educational journals help to keep our broad-based knowledge of internal medicine refreshed, but time pressures may significantly limit our ability to regularly pursue these activities.
In this issue of the Journal, Drs. Moghadam-Kia et al discuss their approach to asymptomatic elevations in creatine kinase (CK). Although no longer included in the most commonly used lab panels, CK measurement is often ordered in patients taking statins, even if they have no relevant muscle-related symptoms. Thus, evaluating a patient with an asymptomatic elevated CK level is not rare. The authors delve into the clinically relevant test characteristics, and their important caveats about interpreting elevated CK levels are germane not only to the asymptomatic patient, but also to the patient being evaluated for myalgia or weakness. This latter situation is one I frequently face in both the hospital and the outpatient clinic. I am often asked to consult on patients who have incompletely defined symptoms and elevated CK.
As discussed in the article, the laboratory definition of “normal” must first be considered. Laboratory test results must always be interpreted in the clinical context. An isolated elevation in parathyroid hormone cannot be interpreted without knowing the patient’s vitamin D level. Nor can “normal” low-density lipoprotein or serum urate levels be interpreted properly without knowing if the patient is accumulating excess cholesterol or urate deposits. As we order and interpret test results, we must consider the biology of the substance being measured as well as the test characteristics; too often, we react to abnormal laboratory results with an incomplete understanding of these aspects.
Moghadam-Kia et al do not dwell on the organ involvement causing CK elevations, but specificity is another very important aspect when clinically interpreting the results of a CK test. Many patients with muscle damage or inflammation have elevations in serum aspartate aminotransferase and alanine aminotransferase levels (the ratio of elevation depends on the time course of the muscle damage and on the relative clearance rate of the two enzymes). Without knowing that the CK is elevated, one might assume that an aminotransferase elevation reflects hepatitis. I have seen several patients with elevated aminotransferases and complaints of weakness and fatigue who were subjected to liver biopsy before it was recognized that the source of the enzyme elevation (“liver function test changes”) was muscle (or hemolysis). Frequently unrecognized is that aldolase, which has a cell distribution similar to that of lactate dehydrogenase, does not have the relative specificity of localization to muscle that CK has. CK is quite useful in distinguishing myocyte from hepatocyte damage.
This paper presents a wonderful reminder of the value of updating and reviewing what we know about tests that we order, even if we feel comfortable when ordering them. Before initiating a cascade of additional tests and consultations to explore the cause of an abnormal test result, a little time spent reviewing its basic characteristics and biology may pay dividends.
As 2015 comes to a close, we at the Journal share with you our sincere wishes for personal satisfaction and a globally more peaceful 2016.
We are all exposed to initiatives from multiple stakeholders telling us to order fewer tests. Many of these efforts to control costs and improve efficiency and quality of care are directed at populations of patients and are broad concepts: provide screening only to those most likely to benefit (eg, don’t screen for prostate cancer in men with a lifespan < 10 years); avoid procedures that provided limited benefit in controlled trials (eg, limit routine arthroscopic treatment of knee osteoarthritis); and avoid reflexive practices unlikely to improve patient outcomes (eg, eliminate routine preoperative testing before elective procedures in otherwise healthy patients).
Whether system-based changes will be implemented and have an impact remains to be determined. But I sense that with all the attention being focused on population management and healthcare practices, including an emphasis on documenting and coding our encounters with patients, whether substantive or simply digital housekeeping, we are increasingly distracted from the patient in front of us and are spending less time reviewing the principles underlying the diagnoses we make and the tests we order—just as we are taking less time to perform relevant physical examinations.1 The latter may mostly relate to time pressures. The former, I believe, is a product of both time pressures and a false sense of confidence in our knowledge of seemingly commonplace laboratory tests.
As I lecture, work with trainees, and reflect on my own patients, I realize that we are slowly but progressively minimizing the importance of a working knowledge of the basic foundations of clinical practice—perhaps because facts can always be looked up. I am not referring to knowledge of arcane biochemical pathways, eponymous references, or the latest recommended treatment of inclusion body myositis. I am thinking instead of the value of regularly refreshing our knowledge of laboratory tests and diagnoses we frequently encounter.
Having access to multiple clinical databases literally in our pockets is likely bolstering a false sense of confidence in our knowledge. The National Library of Medicine may be only a tap on a smart phone away, but accessing it regularly is a different thing. Attending conferences and reading educational journals help to keep our broad-based knowledge of internal medicine refreshed, but time pressures may significantly limit our ability to regularly pursue these activities.
In this issue of the Journal, Drs. Moghadam-Kia et al discuss their approach to asymptomatic elevations in creatine kinase (CK). Although no longer included in the most commonly used lab panels, CK measurement is often ordered in patients taking statins, even if they have no relevant muscle-related symptoms. Thus, evaluating a patient with an asymptomatic elevated CK level is not rare. The authors delve into the clinically relevant test characteristics, and their important caveats about interpreting elevated CK levels are germane not only to the asymptomatic patient, but also to the patient being evaluated for myalgia or weakness. This latter situation is one I frequently face in both the hospital and the outpatient clinic. I am often asked to consult on patients who have incompletely defined symptoms and elevated CK.
As discussed in the article, the laboratory definition of “normal” must first be considered. Laboratory test results must always be interpreted in the clinical context. An isolated elevation in parathyroid hormone cannot be interpreted without knowing the patient’s vitamin D level. Nor can “normal” low-density lipoprotein or serum urate levels be interpreted properly without knowing if the patient is accumulating excess cholesterol or urate deposits. As we order and interpret test results, we must consider the biology of the substance being measured as well as the test characteristics; too often, we react to abnormal laboratory results with an incomplete understanding of these aspects.
Moghadam-Kia et al do not dwell on the organ involvement causing CK elevations, but specificity is another very important aspect when clinically interpreting the results of a CK test. Many patients with muscle damage or inflammation have elevations in serum aspartate aminotransferase and alanine aminotransferase levels (the ratio of elevation depends on the time course of the muscle damage and on the relative clearance rate of the two enzymes). Without knowing that the CK is elevated, one might assume that an aminotransferase elevation reflects hepatitis. I have seen several patients with elevated aminotransferases and complaints of weakness and fatigue who were subjected to liver biopsy before it was recognized that the source of the enzyme elevation (“liver function test changes”) was muscle (or hemolysis). Frequently unrecognized is that aldolase, which has a cell distribution similar to that of lactate dehydrogenase, does not have the relative specificity of localization to muscle that CK has. CK is quite useful in distinguishing myocyte from hepatocyte damage.
This paper presents a wonderful reminder of the value of updating and reviewing what we know about tests that we order, even if we feel comfortable when ordering them. Before initiating a cascade of additional tests and consultations to explore the cause of an abnormal test result, a little time spent reviewing its basic characteristics and biology may pay dividends.
As 2015 comes to a close, we at the Journal share with you our sincere wishes for personal satisfaction and a globally more peaceful 2016.
- Verghese A, Charlton B, Kassirer JP, Ramsey M, Ioannidis JP. Inadequacies of physical examination as a cause of medical errors and adverse events: a collection of vignettes. Am J Med 2015; 128:1322–1324.
- Verghese A, Charlton B, Kassirer JP, Ramsey M, Ioannidis JP. Inadequacies of physical examination as a cause of medical errors and adverse events: a collection of vignettes. Am J Med 2015; 128:1322–1324.
Obstructive sleep apnea: Who should be tested, and how?
Patients who have risk factors for obstructive sleep apnea (OSA) or who report symptoms of OSA should be screened for it, first with a complete sleep history and standardized questionnaire, and then by objective testing if indicated. The gold standard test for OSA is polysomnography performed overnight in a sleep laboratory. Home testing is an option in certain instances.
Common risk factors include obesity, resistant hypertension, retrognathia, large neck circumference (> 17 inches in men, > 16 inches in women), and history of stroke, atrial fibrillation, nocturnal arrhythmias, heart failure, and pulmonary hypertension. Screening is also recommended for any patient who is found on physical examination to have upper-airway narrowing or who reports symptoms such as loud snoring, observed episodes of apnea, gasping or choking at night, unrefreshing sleep, morning headaches, unexplained fatigue, and excessive tiredness during the day.
The American Academy of Sleep Medicine suggests three opportunities to screen for OSA1:
- At routine health maintenance visits
- If the patient reports clinical symptoms of OSA
- If the patient has risk factors.
A DISMAL STATISTIC
The prevalence of OSA in the United States is high, estimated to be 2% in women and 4% in men in the middle-aged work force,2 and even more in blacks, Asians, and older adults.3 Yet only 10% of people with OSA are diagnosed4—a dismal statistic considering the association of OSA with resistant hypertension5 and with a greater risk of stroke,6 cardiovascular disease, and death.7
CONSEQUENCES OF UNTREATED OSA
Untreated OSA is associated with a number of conditions7:
- Hypertension. OSA is one of the most common conditions associated with resistant hypertension. Patients with severe OSA and resistant hypertension who comply with continuous positive airway pressure (CPAP) treatment have significant reductions in blood pressure.
- Coronary artery disease. OSA is twice as common in people with coronary artery disease as in those with no coronary artery disease. In patients with coronary artery disease and OSA, CPAP may reduce the rate of nonfatal and fatal cardiovascular events.
- Heart failure. OSA is common in patients with systolic dysfunction (11% to 37%). OSA also has been detected in more than 50% of patients with heart failure with preserved systolic function. CPAP treatment can improve ejection fraction in patients with systolic dysfunction.
- Arrythmias. Atrial fibrillation, nonsustained ventricular tachycardia, and complex ventricular ectopy have been reported to be significantly more common in people with OSA.8 If the underlying cardiac conduction system is normal and there is no significant thyroid dysfunction, bradyarrhythmias and heart block may be treated effectively with CPAP.7 Treatment of OSA may decrease the incidence and severity of ventricular arrhythmias.7
- Sudden cardiac death. OSA was independently associated with sudden cardiac death in a longitudinal study.9
- Stroke. The Sleep Heart Health Study6 showed that OSA is 30% more common in patients who developed ischemic stroke. Long-term CPAP treatment in moderate to severe OSA and ischemic stroke is associated with a reduction in the mortality rate.10
- Diabetes. The Sleep Heart Health Study showed that OSA is independently associated with glucose intolerance and insulin resistance and may lead to type 2 diabetes mellitus.11
A QUESTIONNAIRE HELPS IDENTIFY WHO NEEDS TESTING
If you suspect OSA, consider administering a sleep disorder questionnaire such as the Berlin,12 the Epworth Sleepiness Scale, or the STOP-Bang questionnaire (Table 1). The STOP-Bang questionnaire is an easy-to-use tool that expands on the STOP questionnaire (snoring, tiredness, observed apnea, high blood pressure) with the addition of body mass index, age, neck size, and gender. The Berlin questionnaire has been validated in the primary care setting.12 The STOP-Bang questionnaire has been validated in preoperative settings13 but not in the primary care setting (although it has been commonly used in primary care).
WHICH TEST TO ORDER?
If the score on the questionnaire indicates a moderate or high risk of OSA, the patient should undergo objective testing with polysomnography or, in certain instances, home testing.1 Polysomnography is the gold standard. Home testing costs less and is easier to arrange, but the American Academy of Sleep Medicine recommends it as an alternative to polysomnography, in conjunction with a comprehensive sleep evaluation, only in the following situations14:
- If the patient has a high pretest probability of moderate to severe OSA
- If immobility or critical illness makes polysomnography unfeasible
- If direct monitoring of the response to non-CPAP treatments for sleep apnea is needed.
Home testing for OSA should not be used in the following situations:
- If the patient has significant morbidity such as moderate to severe pulmonary disease, neuromuscular disease, or congestive heart failure
- In evaluating a patient suspected of having comorbid sleep disorders such as central sleep apnea, periodic limb movement disorder, insomnia, parasomnias, circadian rhythm disorder, or narcolepsy
- In screening of asymptomatic patients.
Home testing has important drawbacks. It may underestimate the severity of sleep apnea. The rate of false-negative results may be as high as 17%. If the home test was thought to be technically inadequate or the results were inconsistent with those that were expected—ie, if the patient has a high pretest probability of OSA based on risk factors or symptoms but negative results on home testing—then the patient should undergo polysomnography.14
DIAGNOSIS
The diagnosis of OSA is confirmed if the number of apnea events per hour (ie, the apnea-hypopnea index) on polysomnography or home testing is more than 15, regardless of symptoms, or more than 5 in a patient who reports OSA symptoms. An apnea-hypopnea index of 5 to 14 indicates mild OSA, 15 to 30 indicates moderate OSA, and greater than 30 indicates severe OSA.
BENEFITS OF TREATMENT
Treatment of OSA with CPAP reduces the 10-year risk of fatal and nonfatal motor vehicle accidents by 52%, the 10-year expected number of myocardial infarctions by 49%, and the 10-year risk of stroke by 31%.7 It has also been found to be cost-effective, for men and women of all ages with moderate to severe OSA.15
- Epstein LJ, Kristo D, Strollo PJ Jr, et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009; 5:263–276.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328:1230–1235.
- Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 2008; 5:136–143.
- Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997; 20:705–706.
- Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension 2011; 58:811–817.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med 2010; 182:269–277.
- Somers VK, White DP, Amin R, et al; American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology; American Heart Association Stroke Council; American Heart Association Council on Cardiovascular Nursing; American College of Cardiology Foundation. Sleep apnea and cardiovascular disease: an American Heart Association/American College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. in collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 2008; 118:1080–1111.
- Mehra R, Benjamin EJ, Shahar E, et al; Sleep Heart Health Study. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med 2006; 173:910–916.
- Gami AS, Olson EJ, Shen WK, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol 2013; 62:610–616.
- Martinez-Garcia MA, Soler-Cataluna JJ, Ejarque-Martinez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med 2009; 180:36–41.
- Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE; Sleep Heart Health Study Investigators. Sleep-disordered breathing, glucose intolerance, and insulin resistance: The Sleep Heart Health Study. Am J Epidemiol 2004; 160:521–530.
- Netzer NC, Hoegel JJ, Loube D, et al; Sleep in Primary Care International Study Group. Prevalence of symptoms and risk of sleep apnea in primary care. Chest 2003; 124:1406–1414.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Collop NA, Anderson WM, Boehlecke B, et al; Portable Monitoring Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2007; 3:737–747.
- Pietzsch JB, Garner A, Cipriano LE, Linehan JH. An integrated health-economic analysis of diagnostic and therapeutic strategies in the treatment of moderate-to-severe obstructive sleep apnea. Sleep 2011; 34:695–709.
Patients who have risk factors for obstructive sleep apnea (OSA) or who report symptoms of OSA should be screened for it, first with a complete sleep history and standardized questionnaire, and then by objective testing if indicated. The gold standard test for OSA is polysomnography performed overnight in a sleep laboratory. Home testing is an option in certain instances.
Common risk factors include obesity, resistant hypertension, retrognathia, large neck circumference (> 17 inches in men, > 16 inches in women), and history of stroke, atrial fibrillation, nocturnal arrhythmias, heart failure, and pulmonary hypertension. Screening is also recommended for any patient who is found on physical examination to have upper-airway narrowing or who reports symptoms such as loud snoring, observed episodes of apnea, gasping or choking at night, unrefreshing sleep, morning headaches, unexplained fatigue, and excessive tiredness during the day.
The American Academy of Sleep Medicine suggests three opportunities to screen for OSA1:
- At routine health maintenance visits
- If the patient reports clinical symptoms of OSA
- If the patient has risk factors.
A DISMAL STATISTIC
The prevalence of OSA in the United States is high, estimated to be 2% in women and 4% in men in the middle-aged work force,2 and even more in blacks, Asians, and older adults.3 Yet only 10% of people with OSA are diagnosed4—a dismal statistic considering the association of OSA with resistant hypertension5 and with a greater risk of stroke,6 cardiovascular disease, and death.7
CONSEQUENCES OF UNTREATED OSA
Untreated OSA is associated with a number of conditions7:
- Hypertension. OSA is one of the most common conditions associated with resistant hypertension. Patients with severe OSA and resistant hypertension who comply with continuous positive airway pressure (CPAP) treatment have significant reductions in blood pressure.
- Coronary artery disease. OSA is twice as common in people with coronary artery disease as in those with no coronary artery disease. In patients with coronary artery disease and OSA, CPAP may reduce the rate of nonfatal and fatal cardiovascular events.
- Heart failure. OSA is common in patients with systolic dysfunction (11% to 37%). OSA also has been detected in more than 50% of patients with heart failure with preserved systolic function. CPAP treatment can improve ejection fraction in patients with systolic dysfunction.
- Arrythmias. Atrial fibrillation, nonsustained ventricular tachycardia, and complex ventricular ectopy have been reported to be significantly more common in people with OSA.8 If the underlying cardiac conduction system is normal and there is no significant thyroid dysfunction, bradyarrhythmias and heart block may be treated effectively with CPAP.7 Treatment of OSA may decrease the incidence and severity of ventricular arrhythmias.7
- Sudden cardiac death. OSA was independently associated with sudden cardiac death in a longitudinal study.9
- Stroke. The Sleep Heart Health Study6 showed that OSA is 30% more common in patients who developed ischemic stroke. Long-term CPAP treatment in moderate to severe OSA and ischemic stroke is associated with a reduction in the mortality rate.10
- Diabetes. The Sleep Heart Health Study showed that OSA is independently associated with glucose intolerance and insulin resistance and may lead to type 2 diabetes mellitus.11
A QUESTIONNAIRE HELPS IDENTIFY WHO NEEDS TESTING
If you suspect OSA, consider administering a sleep disorder questionnaire such as the Berlin,12 the Epworth Sleepiness Scale, or the STOP-Bang questionnaire (Table 1). The STOP-Bang questionnaire is an easy-to-use tool that expands on the STOP questionnaire (snoring, tiredness, observed apnea, high blood pressure) with the addition of body mass index, age, neck size, and gender. The Berlin questionnaire has been validated in the primary care setting.12 The STOP-Bang questionnaire has been validated in preoperative settings13 but not in the primary care setting (although it has been commonly used in primary care).
WHICH TEST TO ORDER?
If the score on the questionnaire indicates a moderate or high risk of OSA, the patient should undergo objective testing with polysomnography or, in certain instances, home testing.1 Polysomnography is the gold standard. Home testing costs less and is easier to arrange, but the American Academy of Sleep Medicine recommends it as an alternative to polysomnography, in conjunction with a comprehensive sleep evaluation, only in the following situations14:
- If the patient has a high pretest probability of moderate to severe OSA
- If immobility or critical illness makes polysomnography unfeasible
- If direct monitoring of the response to non-CPAP treatments for sleep apnea is needed.
Home testing for OSA should not be used in the following situations:
- If the patient has significant morbidity such as moderate to severe pulmonary disease, neuromuscular disease, or congestive heart failure
- In evaluating a patient suspected of having comorbid sleep disorders such as central sleep apnea, periodic limb movement disorder, insomnia, parasomnias, circadian rhythm disorder, or narcolepsy
- In screening of asymptomatic patients.
Home testing has important drawbacks. It may underestimate the severity of sleep apnea. The rate of false-negative results may be as high as 17%. If the home test was thought to be technically inadequate or the results were inconsistent with those that were expected—ie, if the patient has a high pretest probability of OSA based on risk factors or symptoms but negative results on home testing—then the patient should undergo polysomnography.14
DIAGNOSIS
The diagnosis of OSA is confirmed if the number of apnea events per hour (ie, the apnea-hypopnea index) on polysomnography or home testing is more than 15, regardless of symptoms, or more than 5 in a patient who reports OSA symptoms. An apnea-hypopnea index of 5 to 14 indicates mild OSA, 15 to 30 indicates moderate OSA, and greater than 30 indicates severe OSA.
BENEFITS OF TREATMENT
Treatment of OSA with CPAP reduces the 10-year risk of fatal and nonfatal motor vehicle accidents by 52%, the 10-year expected number of myocardial infarctions by 49%, and the 10-year risk of stroke by 31%.7 It has also been found to be cost-effective, for men and women of all ages with moderate to severe OSA.15
Patients who have risk factors for obstructive sleep apnea (OSA) or who report symptoms of OSA should be screened for it, first with a complete sleep history and standardized questionnaire, and then by objective testing if indicated. The gold standard test for OSA is polysomnography performed overnight in a sleep laboratory. Home testing is an option in certain instances.
Common risk factors include obesity, resistant hypertension, retrognathia, large neck circumference (> 17 inches in men, > 16 inches in women), and history of stroke, atrial fibrillation, nocturnal arrhythmias, heart failure, and pulmonary hypertension. Screening is also recommended for any patient who is found on physical examination to have upper-airway narrowing or who reports symptoms such as loud snoring, observed episodes of apnea, gasping or choking at night, unrefreshing sleep, morning headaches, unexplained fatigue, and excessive tiredness during the day.
The American Academy of Sleep Medicine suggests three opportunities to screen for OSA1:
- At routine health maintenance visits
- If the patient reports clinical symptoms of OSA
- If the patient has risk factors.
A DISMAL STATISTIC
The prevalence of OSA in the United States is high, estimated to be 2% in women and 4% in men in the middle-aged work force,2 and even more in blacks, Asians, and older adults.3 Yet only 10% of people with OSA are diagnosed4—a dismal statistic considering the association of OSA with resistant hypertension5 and with a greater risk of stroke,6 cardiovascular disease, and death.7
CONSEQUENCES OF UNTREATED OSA
Untreated OSA is associated with a number of conditions7:
- Hypertension. OSA is one of the most common conditions associated with resistant hypertension. Patients with severe OSA and resistant hypertension who comply with continuous positive airway pressure (CPAP) treatment have significant reductions in blood pressure.
- Coronary artery disease. OSA is twice as common in people with coronary artery disease as in those with no coronary artery disease. In patients with coronary artery disease and OSA, CPAP may reduce the rate of nonfatal and fatal cardiovascular events.
- Heart failure. OSA is common in patients with systolic dysfunction (11% to 37%). OSA also has been detected in more than 50% of patients with heart failure with preserved systolic function. CPAP treatment can improve ejection fraction in patients with systolic dysfunction.
- Arrythmias. Atrial fibrillation, nonsustained ventricular tachycardia, and complex ventricular ectopy have been reported to be significantly more common in people with OSA.8 If the underlying cardiac conduction system is normal and there is no significant thyroid dysfunction, bradyarrhythmias and heart block may be treated effectively with CPAP.7 Treatment of OSA may decrease the incidence and severity of ventricular arrhythmias.7
- Sudden cardiac death. OSA was independently associated with sudden cardiac death in a longitudinal study.9
- Stroke. The Sleep Heart Health Study6 showed that OSA is 30% more common in patients who developed ischemic stroke. Long-term CPAP treatment in moderate to severe OSA and ischemic stroke is associated with a reduction in the mortality rate.10
- Diabetes. The Sleep Heart Health Study showed that OSA is independently associated with glucose intolerance and insulin resistance and may lead to type 2 diabetes mellitus.11
A QUESTIONNAIRE HELPS IDENTIFY WHO NEEDS TESTING
If you suspect OSA, consider administering a sleep disorder questionnaire such as the Berlin,12 the Epworth Sleepiness Scale, or the STOP-Bang questionnaire (Table 1). The STOP-Bang questionnaire is an easy-to-use tool that expands on the STOP questionnaire (snoring, tiredness, observed apnea, high blood pressure) with the addition of body mass index, age, neck size, and gender. The Berlin questionnaire has been validated in the primary care setting.12 The STOP-Bang questionnaire has been validated in preoperative settings13 but not in the primary care setting (although it has been commonly used in primary care).
WHICH TEST TO ORDER?
If the score on the questionnaire indicates a moderate or high risk of OSA, the patient should undergo objective testing with polysomnography or, in certain instances, home testing.1 Polysomnography is the gold standard. Home testing costs less and is easier to arrange, but the American Academy of Sleep Medicine recommends it as an alternative to polysomnography, in conjunction with a comprehensive sleep evaluation, only in the following situations14:
- If the patient has a high pretest probability of moderate to severe OSA
- If immobility or critical illness makes polysomnography unfeasible
- If direct monitoring of the response to non-CPAP treatments for sleep apnea is needed.
Home testing for OSA should not be used in the following situations:
- If the patient has significant morbidity such as moderate to severe pulmonary disease, neuromuscular disease, or congestive heart failure
- In evaluating a patient suspected of having comorbid sleep disorders such as central sleep apnea, periodic limb movement disorder, insomnia, parasomnias, circadian rhythm disorder, or narcolepsy
- In screening of asymptomatic patients.
Home testing has important drawbacks. It may underestimate the severity of sleep apnea. The rate of false-negative results may be as high as 17%. If the home test was thought to be technically inadequate or the results were inconsistent with those that were expected—ie, if the patient has a high pretest probability of OSA based on risk factors or symptoms but negative results on home testing—then the patient should undergo polysomnography.14
DIAGNOSIS
The diagnosis of OSA is confirmed if the number of apnea events per hour (ie, the apnea-hypopnea index) on polysomnography or home testing is more than 15, regardless of symptoms, or more than 5 in a patient who reports OSA symptoms. An apnea-hypopnea index of 5 to 14 indicates mild OSA, 15 to 30 indicates moderate OSA, and greater than 30 indicates severe OSA.
BENEFITS OF TREATMENT
Treatment of OSA with CPAP reduces the 10-year risk of fatal and nonfatal motor vehicle accidents by 52%, the 10-year expected number of myocardial infarctions by 49%, and the 10-year risk of stroke by 31%.7 It has also been found to be cost-effective, for men and women of all ages with moderate to severe OSA.15
- Epstein LJ, Kristo D, Strollo PJ Jr, et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009; 5:263–276.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328:1230–1235.
- Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 2008; 5:136–143.
- Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997; 20:705–706.
- Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension 2011; 58:811–817.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med 2010; 182:269–277.
- Somers VK, White DP, Amin R, et al; American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology; American Heart Association Stroke Council; American Heart Association Council on Cardiovascular Nursing; American College of Cardiology Foundation. Sleep apnea and cardiovascular disease: an American Heart Association/American College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. in collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 2008; 118:1080–1111.
- Mehra R, Benjamin EJ, Shahar E, et al; Sleep Heart Health Study. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med 2006; 173:910–916.
- Gami AS, Olson EJ, Shen WK, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol 2013; 62:610–616.
- Martinez-Garcia MA, Soler-Cataluna JJ, Ejarque-Martinez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med 2009; 180:36–41.
- Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE; Sleep Heart Health Study Investigators. Sleep-disordered breathing, glucose intolerance, and insulin resistance: The Sleep Heart Health Study. Am J Epidemiol 2004; 160:521–530.
- Netzer NC, Hoegel JJ, Loube D, et al; Sleep in Primary Care International Study Group. Prevalence of symptoms and risk of sleep apnea in primary care. Chest 2003; 124:1406–1414.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Collop NA, Anderson WM, Boehlecke B, et al; Portable Monitoring Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2007; 3:737–747.
- Pietzsch JB, Garner A, Cipriano LE, Linehan JH. An integrated health-economic analysis of diagnostic and therapeutic strategies in the treatment of moderate-to-severe obstructive sleep apnea. Sleep 2011; 34:695–709.
- Epstein LJ, Kristo D, Strollo PJ Jr, et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009; 5:263–276.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328:1230–1235.
- Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 2008; 5:136–143.
- Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997; 20:705–706.
- Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension 2011; 58:811–817.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med 2010; 182:269–277.
- Somers VK, White DP, Amin R, et al; American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology; American Heart Association Stroke Council; American Heart Association Council on Cardiovascular Nursing; American College of Cardiology Foundation. Sleep apnea and cardiovascular disease: an American Heart Association/American College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. in collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 2008; 118:1080–1111.
- Mehra R, Benjamin EJ, Shahar E, et al; Sleep Heart Health Study. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med 2006; 173:910–916.
- Gami AS, Olson EJ, Shen WK, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol 2013; 62:610–616.
- Martinez-Garcia MA, Soler-Cataluna JJ, Ejarque-Martinez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med 2009; 180:36–41.
- Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE; Sleep Heart Health Study Investigators. Sleep-disordered breathing, glucose intolerance, and insulin resistance: The Sleep Heart Health Study. Am J Epidemiol 2004; 160:521–530.
- Netzer NC, Hoegel JJ, Loube D, et al; Sleep in Primary Care International Study Group. Prevalence of symptoms and risk of sleep apnea in primary care. Chest 2003; 124:1406–1414.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Collop NA, Anderson WM, Boehlecke B, et al; Portable Monitoring Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2007; 3:737–747.
- Pietzsch JB, Garner A, Cipriano LE, Linehan JH. An integrated health-economic analysis of diagnostic and therapeutic strategies in the treatment of moderate-to-severe obstructive sleep apnea. Sleep 2011; 34:695–709.
Serum allergen-specific IgE testing: How much is too much?
A 25-year-old man is evaluated for angioedema (swelling of lips and tongue) after eating paella at a Spanish restaurant. He has no history of allergies, but he says he had never eaten such a large variety of seafood before, especially shellfish.
He suspects that he is allergic to shellfish and asks the attending physician to order blood tests for seafood allergies, as he heard from a friend that blood tests are superior to other types of tests for allergy. The physician requests a serum immunoglobulin E (IgE) food panel test for this patient.
SERUM ALLERGEN-SPECIFIC IgE TESTING
Many methods of testing for allergy are available, including the skin-prick test, double-blind and single-blind placebo-controlled food challenges, open food challenges, inhalant challenges, drug challenges, and serum IgE tests. In clinical practice, these tests are often used in combination because when used individually, few of them are both highly sensitive and specific (Table 1).1–6
Skin-prick testing is generally the method of choice for the preliminary evaluation of IgE-mediated allergies because it is more sensitive and requires less time to get a result.1 But it is not the preferred test if the patient is at risk of a systemic reaction or has widespread dermatitis, nor is it useful if the patient is taking drugs that suppress the histamine response, such as antihistamines or tricyclic antidepressants.6 Moreover, skin-prick testing is more invasive and time-consuming than serum IgE testing.
Serum IgE testing is an attractive alternative, and it is more convenient because it requires only a single blood draw and poses a lower risk of adverse effects.
NOT A RELIABLE DIAGNOSTIC TOOL
As serum IgE testing has gained popularity, researchers have tried to improve its diagnostic power (ie, maximize its sensitivity and specificity) by determining the best cutoff values for IgE against specific antigens. Unfortunately, these values are difficult to determine because of confounding factors such as the lack of a reference standard, population diversity, patient atopy, and the overwhelming number of allergens that must be examined.
In addition, some researchers have used positive and negative predictive values to evaluate diagnostic cutoffs for serum antigen-specific IgE values. But these are not the most suitable performance measure to evaluate because they depend on disease prevalence and population characteristics.
Despite these efforts, results are still conflicting, and serum antigen-specific IgE testing is not a reliable diagnostic tool.
In an effort to gain insight from the available research data, we evaluated the clinical usefulness of 89 antigen-specific IgE tests, using an approach of summing their sensitivity and specificity. Previously, Wians7 proposed that a test is likely to be clinically useful if the sum of its sensitivity and specificity is equal to or greater than 170. Figure 1 shows the 89 tests, grouped into categories, and their summed sensitivities and specificities. The dashed line indicates a cutoff of 170; any bar that touches or crosses that line indicates that the test may be clinically useful, according to Wians.7
Only 7 of the 89 tests (cow, buckwheat, hazelnut, latex, Alternaria alternata, honey bee venom, and Johnson grass) satisfied this criterion. This suggests that a significant number of serum antigen-specific IgE tests perform suboptimally, and we are left with the question of why they are so commonly ordered.
Inappropriate use can lead to false-positive results, a situation in which patients may be subjected to unnecessary food avoidance that can result in nutritional deficiencies and decreased quality of life. It can also lead to false-negative results, when life-threatening diagnoses are missed and further excessive downstream testing is required—all leading; to negative outcomes for both patients and healthcare providers.
CHOOSING WISELY
The Choosing Wisely campaign in the United States has partnered with the American Academy of Allergy, Asthma, and Immunology to advocate against indiscriminate IgE testing in evaluating allergy.8 Allergy diagnosis and evaluation should be based on a combination of clinical history and judicious ordering of specific IgE tests, whether through skin or blood testing. Ordering of serum allergen-specific IgE tests for food allergies should be consistent with a clinical history of potential IgE-mediated food allergy8 and not food intolerance (Table 2).4,5
Some jurisdictions in Canada have followed suit by restricting the number of serum IgE tests each physician is allowed to order per patient, to encourage more responsible ordering and to lower the number of potential false-positive results, which can lead to increased downstream costs as well as unnecessary patient worry and lifestyle modification.
CLINICAL BOTTOM LINE
Ordering diagnostic tests that have little clinical utility has long-term detrimental effects on both patient safety and healthcare sustainability.
In the case of the 25-year-old evaluated for shellfish allergy, the clinician should first explain that the swelling of the lips and tongue (angioedema) does suggest an IgE-mediated allergic reaction and not a non–IgE-mediated allergic reaction or a food intolerance. Non–IgE-mediated food allergies and food intolerances are marked by symptoms relating mainly to nonimmune aspects of the digestive system, whereas IgE-mediated food allergies affect the immune system and can involve a multitude of organs, including the skin and the respiratory and digestive systems (Table 2).
However, clinicians should avoid indiscriminately ordering food allergen IgE panels and instead should focus on foods likely to be the culprits based on the clinical history.9 Indiscriminate testing can lead to false-positive results and unnecessary food avoidance.
Since the patient developed symptoms of angioedema when he was exposed to his allergen, he may be apprehensive about a skin- prick test and the possibility of being subjected to the same discomfort. Therefore, in this situation, it may be best to perform serum IgE tests, but on a few targeted seafoods rather than the food panel the physician had ordered. A patient can be sensitized to an allergen (possess IgE antibodies) but not experience symptoms when exposed to it (ie, have tolerance).5 Also, false-negative results may occur, so a negative serum allergen-specific IgE test should likewise be interpreted in light of the pretest probability of allergy to a specific antigen.
If the history and the results of testing are not clear and congruent, the patient should be referred to an allergist for diagnosis or for management. The allergist can provide management techniques and periodic assessment as to the progression and resolution of the allergy. Table 2 highlights symptoms that differentiate an IgE-mediated from a non–IgE-mediated food allergy.10,11 Table 1 presents clinical indications and suggested diagnostic methods to the five most common allergen groups and the diagnostically invalid tests.1–6
The bottom line is that we must consider the poor performance of serum allergen-specific IgE tests when diagnosing and treating suspected type I allergies and avoid ordering food allergen IgE panels whenever possible.
- Bernstein IL, Li JT, Bernstein DI, et al; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol 2008; 100(suppl 3):S1–S148.
- Bird JA, Crain M, Varshney P. Food allergen panel testing often results in misdiagnosis of food allergy. J Pediatr 2015; 166:97–100.
- Kattan JD, Sicherer SH. Optimizing the diagnosis of food allergy. Immunol Allergy Clin North Am 2015; 35:61–76.
- Sampson HA, Aceves S, Bock SA, et al. Food allergy: a practice parameter update-2014. J Allergy Clin Immunol 2014; 134:1016–1025.e43.
- Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol 2014; 133:291–308.
- Siles RI, Hsieh FH. Allergy blood testing: a practical guide for clinicians. Cleve Clin J Med 2011; 78:585–592.
- Wians FH Jr. Clinical laboratory tests: which, why, and what do the results mean? Lab Medicine 2009; 40:105–113.
- Choosing Wisely. American Academy of Allergy, Asthma & Immunology. Ten Things Physicians and Patients Should Question. www.choosingwisely.org/doctor-patient-lists/american-academy-of-allergy-asthma-immunology/. Accessed December 3, 2015.
- Fleischer DM, Burks AW. Pitfalls in food allergy diagnosis: serum IgE testing. J Pediatr 2015; 166: 8-10.
- Boyce JA, Assa'ad A, Burks AW, et al; NIAID-Sponsored Expert Panel. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel report. J Allergy Clin Immunol 2010; 126:1105–1118.
- Stiefel G, Roberts G. How to use serum-specific IgE measurements in diagnosing and monitoring food allergy. Arch Dis Child Educ Pract Ed 2012; 97:29–36.
A 25-year-old man is evaluated for angioedema (swelling of lips and tongue) after eating paella at a Spanish restaurant. He has no history of allergies, but he says he had never eaten such a large variety of seafood before, especially shellfish.
He suspects that he is allergic to shellfish and asks the attending physician to order blood tests for seafood allergies, as he heard from a friend that blood tests are superior to other types of tests for allergy. The physician requests a serum immunoglobulin E (IgE) food panel test for this patient.
SERUM ALLERGEN-SPECIFIC IgE TESTING
Many methods of testing for allergy are available, including the skin-prick test, double-blind and single-blind placebo-controlled food challenges, open food challenges, inhalant challenges, drug challenges, and serum IgE tests. In clinical practice, these tests are often used in combination because when used individually, few of them are both highly sensitive and specific (Table 1).1–6
Skin-prick testing is generally the method of choice for the preliminary evaluation of IgE-mediated allergies because it is more sensitive and requires less time to get a result.1 But it is not the preferred test if the patient is at risk of a systemic reaction or has widespread dermatitis, nor is it useful if the patient is taking drugs that suppress the histamine response, such as antihistamines or tricyclic antidepressants.6 Moreover, skin-prick testing is more invasive and time-consuming than serum IgE testing.
Serum IgE testing is an attractive alternative, and it is more convenient because it requires only a single blood draw and poses a lower risk of adverse effects.
NOT A RELIABLE DIAGNOSTIC TOOL
As serum IgE testing has gained popularity, researchers have tried to improve its diagnostic power (ie, maximize its sensitivity and specificity) by determining the best cutoff values for IgE against specific antigens. Unfortunately, these values are difficult to determine because of confounding factors such as the lack of a reference standard, population diversity, patient atopy, and the overwhelming number of allergens that must be examined.
In addition, some researchers have used positive and negative predictive values to evaluate diagnostic cutoffs for serum antigen-specific IgE values. But these are not the most suitable performance measure to evaluate because they depend on disease prevalence and population characteristics.
Despite these efforts, results are still conflicting, and serum antigen-specific IgE testing is not a reliable diagnostic tool.
In an effort to gain insight from the available research data, we evaluated the clinical usefulness of 89 antigen-specific IgE tests, using an approach of summing their sensitivity and specificity. Previously, Wians7 proposed that a test is likely to be clinically useful if the sum of its sensitivity and specificity is equal to or greater than 170. Figure 1 shows the 89 tests, grouped into categories, and their summed sensitivities and specificities. The dashed line indicates a cutoff of 170; any bar that touches or crosses that line indicates that the test may be clinically useful, according to Wians.7
Only 7 of the 89 tests (cow, buckwheat, hazelnut, latex, Alternaria alternata, honey bee venom, and Johnson grass) satisfied this criterion. This suggests that a significant number of serum antigen-specific IgE tests perform suboptimally, and we are left with the question of why they are so commonly ordered.
Inappropriate use can lead to false-positive results, a situation in which patients may be subjected to unnecessary food avoidance that can result in nutritional deficiencies and decreased quality of life. It can also lead to false-negative results, when life-threatening diagnoses are missed and further excessive downstream testing is required—all leading; to negative outcomes for both patients and healthcare providers.
CHOOSING WISELY
The Choosing Wisely campaign in the United States has partnered with the American Academy of Allergy, Asthma, and Immunology to advocate against indiscriminate IgE testing in evaluating allergy.8 Allergy diagnosis and evaluation should be based on a combination of clinical history and judicious ordering of specific IgE tests, whether through skin or blood testing. Ordering of serum allergen-specific IgE tests for food allergies should be consistent with a clinical history of potential IgE-mediated food allergy8 and not food intolerance (Table 2).4,5
Some jurisdictions in Canada have followed suit by restricting the number of serum IgE tests each physician is allowed to order per patient, to encourage more responsible ordering and to lower the number of potential false-positive results, which can lead to increased downstream costs as well as unnecessary patient worry and lifestyle modification.
CLINICAL BOTTOM LINE
Ordering diagnostic tests that have little clinical utility has long-term detrimental effects on both patient safety and healthcare sustainability.
In the case of the 25-year-old evaluated for shellfish allergy, the clinician should first explain that the swelling of the lips and tongue (angioedema) does suggest an IgE-mediated allergic reaction and not a non–IgE-mediated allergic reaction or a food intolerance. Non–IgE-mediated food allergies and food intolerances are marked by symptoms relating mainly to nonimmune aspects of the digestive system, whereas IgE-mediated food allergies affect the immune system and can involve a multitude of organs, including the skin and the respiratory and digestive systems (Table 2).
However, clinicians should avoid indiscriminately ordering food allergen IgE panels and instead should focus on foods likely to be the culprits based on the clinical history.9 Indiscriminate testing can lead to false-positive results and unnecessary food avoidance.
Since the patient developed symptoms of angioedema when he was exposed to his allergen, he may be apprehensive about a skin- prick test and the possibility of being subjected to the same discomfort. Therefore, in this situation, it may be best to perform serum IgE tests, but on a few targeted seafoods rather than the food panel the physician had ordered. A patient can be sensitized to an allergen (possess IgE antibodies) but not experience symptoms when exposed to it (ie, have tolerance).5 Also, false-negative results may occur, so a negative serum allergen-specific IgE test should likewise be interpreted in light of the pretest probability of allergy to a specific antigen.
If the history and the results of testing are not clear and congruent, the patient should be referred to an allergist for diagnosis or for management. The allergist can provide management techniques and periodic assessment as to the progression and resolution of the allergy. Table 2 highlights symptoms that differentiate an IgE-mediated from a non–IgE-mediated food allergy.10,11 Table 1 presents clinical indications and suggested diagnostic methods to the five most common allergen groups and the diagnostically invalid tests.1–6
The bottom line is that we must consider the poor performance of serum allergen-specific IgE tests when diagnosing and treating suspected type I allergies and avoid ordering food allergen IgE panels whenever possible.
A 25-year-old man is evaluated for angioedema (swelling of lips and tongue) after eating paella at a Spanish restaurant. He has no history of allergies, but he says he had never eaten such a large variety of seafood before, especially shellfish.
He suspects that he is allergic to shellfish and asks the attending physician to order blood tests for seafood allergies, as he heard from a friend that blood tests are superior to other types of tests for allergy. The physician requests a serum immunoglobulin E (IgE) food panel test for this patient.
SERUM ALLERGEN-SPECIFIC IgE TESTING
Many methods of testing for allergy are available, including the skin-prick test, double-blind and single-blind placebo-controlled food challenges, open food challenges, inhalant challenges, drug challenges, and serum IgE tests. In clinical practice, these tests are often used in combination because when used individually, few of them are both highly sensitive and specific (Table 1).1–6
Skin-prick testing is generally the method of choice for the preliminary evaluation of IgE-mediated allergies because it is more sensitive and requires less time to get a result.1 But it is not the preferred test if the patient is at risk of a systemic reaction or has widespread dermatitis, nor is it useful if the patient is taking drugs that suppress the histamine response, such as antihistamines or tricyclic antidepressants.6 Moreover, skin-prick testing is more invasive and time-consuming than serum IgE testing.
Serum IgE testing is an attractive alternative, and it is more convenient because it requires only a single blood draw and poses a lower risk of adverse effects.
NOT A RELIABLE DIAGNOSTIC TOOL
As serum IgE testing has gained popularity, researchers have tried to improve its diagnostic power (ie, maximize its sensitivity and specificity) by determining the best cutoff values for IgE against specific antigens. Unfortunately, these values are difficult to determine because of confounding factors such as the lack of a reference standard, population diversity, patient atopy, and the overwhelming number of allergens that must be examined.
In addition, some researchers have used positive and negative predictive values to evaluate diagnostic cutoffs for serum antigen-specific IgE values. But these are not the most suitable performance measure to evaluate because they depend on disease prevalence and population characteristics.
Despite these efforts, results are still conflicting, and serum antigen-specific IgE testing is not a reliable diagnostic tool.
In an effort to gain insight from the available research data, we evaluated the clinical usefulness of 89 antigen-specific IgE tests, using an approach of summing their sensitivity and specificity. Previously, Wians7 proposed that a test is likely to be clinically useful if the sum of its sensitivity and specificity is equal to or greater than 170. Figure 1 shows the 89 tests, grouped into categories, and their summed sensitivities and specificities. The dashed line indicates a cutoff of 170; any bar that touches or crosses that line indicates that the test may be clinically useful, according to Wians.7
Only 7 of the 89 tests (cow, buckwheat, hazelnut, latex, Alternaria alternata, honey bee venom, and Johnson grass) satisfied this criterion. This suggests that a significant number of serum antigen-specific IgE tests perform suboptimally, and we are left with the question of why they are so commonly ordered.
Inappropriate use can lead to false-positive results, a situation in which patients may be subjected to unnecessary food avoidance that can result in nutritional deficiencies and decreased quality of life. It can also lead to false-negative results, when life-threatening diagnoses are missed and further excessive downstream testing is required—all leading; to negative outcomes for both patients and healthcare providers.
CHOOSING WISELY
The Choosing Wisely campaign in the United States has partnered with the American Academy of Allergy, Asthma, and Immunology to advocate against indiscriminate IgE testing in evaluating allergy.8 Allergy diagnosis and evaluation should be based on a combination of clinical history and judicious ordering of specific IgE tests, whether through skin or blood testing. Ordering of serum allergen-specific IgE tests for food allergies should be consistent with a clinical history of potential IgE-mediated food allergy8 and not food intolerance (Table 2).4,5
Some jurisdictions in Canada have followed suit by restricting the number of serum IgE tests each physician is allowed to order per patient, to encourage more responsible ordering and to lower the number of potential false-positive results, which can lead to increased downstream costs as well as unnecessary patient worry and lifestyle modification.
CLINICAL BOTTOM LINE
Ordering diagnostic tests that have little clinical utility has long-term detrimental effects on both patient safety and healthcare sustainability.
In the case of the 25-year-old evaluated for shellfish allergy, the clinician should first explain that the swelling of the lips and tongue (angioedema) does suggest an IgE-mediated allergic reaction and not a non–IgE-mediated allergic reaction or a food intolerance. Non–IgE-mediated food allergies and food intolerances are marked by symptoms relating mainly to nonimmune aspects of the digestive system, whereas IgE-mediated food allergies affect the immune system and can involve a multitude of organs, including the skin and the respiratory and digestive systems (Table 2).
However, clinicians should avoid indiscriminately ordering food allergen IgE panels and instead should focus on foods likely to be the culprits based on the clinical history.9 Indiscriminate testing can lead to false-positive results and unnecessary food avoidance.
Since the patient developed symptoms of angioedema when he was exposed to his allergen, he may be apprehensive about a skin- prick test and the possibility of being subjected to the same discomfort. Therefore, in this situation, it may be best to perform serum IgE tests, but on a few targeted seafoods rather than the food panel the physician had ordered. A patient can be sensitized to an allergen (possess IgE antibodies) but not experience symptoms when exposed to it (ie, have tolerance).5 Also, false-negative results may occur, so a negative serum allergen-specific IgE test should likewise be interpreted in light of the pretest probability of allergy to a specific antigen.
If the history and the results of testing are not clear and congruent, the patient should be referred to an allergist for diagnosis or for management. The allergist can provide management techniques and periodic assessment as to the progression and resolution of the allergy. Table 2 highlights symptoms that differentiate an IgE-mediated from a non–IgE-mediated food allergy.10,11 Table 1 presents clinical indications and suggested diagnostic methods to the five most common allergen groups and the diagnostically invalid tests.1–6
The bottom line is that we must consider the poor performance of serum allergen-specific IgE tests when diagnosing and treating suspected type I allergies and avoid ordering food allergen IgE panels whenever possible.
- Bernstein IL, Li JT, Bernstein DI, et al; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol 2008; 100(suppl 3):S1–S148.
- Bird JA, Crain M, Varshney P. Food allergen panel testing often results in misdiagnosis of food allergy. J Pediatr 2015; 166:97–100.
- Kattan JD, Sicherer SH. Optimizing the diagnosis of food allergy. Immunol Allergy Clin North Am 2015; 35:61–76.
- Sampson HA, Aceves S, Bock SA, et al. Food allergy: a practice parameter update-2014. J Allergy Clin Immunol 2014; 134:1016–1025.e43.
- Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol 2014; 133:291–308.
- Siles RI, Hsieh FH. Allergy blood testing: a practical guide for clinicians. Cleve Clin J Med 2011; 78:585–592.
- Wians FH Jr. Clinical laboratory tests: which, why, and what do the results mean? Lab Medicine 2009; 40:105–113.
- Choosing Wisely. American Academy of Allergy, Asthma & Immunology. Ten Things Physicians and Patients Should Question. www.choosingwisely.org/doctor-patient-lists/american-academy-of-allergy-asthma-immunology/. Accessed December 3, 2015.
- Fleischer DM, Burks AW. Pitfalls in food allergy diagnosis: serum IgE testing. J Pediatr 2015; 166: 8-10.
- Boyce JA, Assa'ad A, Burks AW, et al; NIAID-Sponsored Expert Panel. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel report. J Allergy Clin Immunol 2010; 126:1105–1118.
- Stiefel G, Roberts G. How to use serum-specific IgE measurements in diagnosing and monitoring food allergy. Arch Dis Child Educ Pract Ed 2012; 97:29–36.
- Bernstein IL, Li JT, Bernstein DI, et al; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol 2008; 100(suppl 3):S1–S148.
- Bird JA, Crain M, Varshney P. Food allergen panel testing often results in misdiagnosis of food allergy. J Pediatr 2015; 166:97–100.
- Kattan JD, Sicherer SH. Optimizing the diagnosis of food allergy. Immunol Allergy Clin North Am 2015; 35:61–76.
- Sampson HA, Aceves S, Bock SA, et al. Food allergy: a practice parameter update-2014. J Allergy Clin Immunol 2014; 134:1016–1025.e43.
- Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol 2014; 133:291–308.
- Siles RI, Hsieh FH. Allergy blood testing: a practical guide for clinicians. Cleve Clin J Med 2011; 78:585–592.
- Wians FH Jr. Clinical laboratory tests: which, why, and what do the results mean? Lab Medicine 2009; 40:105–113.
- Choosing Wisely. American Academy of Allergy, Asthma & Immunology. Ten Things Physicians and Patients Should Question. www.choosingwisely.org/doctor-patient-lists/american-academy-of-allergy-asthma-immunology/. Accessed December 3, 2015.
- Fleischer DM, Burks AW. Pitfalls in food allergy diagnosis: serum IgE testing. J Pediatr 2015; 166: 8-10.
- Boyce JA, Assa'ad A, Burks AW, et al; NIAID-Sponsored Expert Panel. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel report. J Allergy Clin Immunol 2010; 126:1105–1118.
- Stiefel G, Roberts G. How to use serum-specific IgE measurements in diagnosing and monitoring food allergy. Arch Dis Child Educ Pract Ed 2012; 97:29–36.