User login
PICC Use in Adults With Pneumonia
Pneumonia is the most common cause of unplanned hospitalization in the United States.[1] Despite its clinical toll, the management of this disease has evolved markedly. Expanding vaccination programs, efforts to improve timeliness of antibiotic therapy, and improved processes of care are but a few developments that have improved outcomes for patients afflicted with this illness.[2, 3]
Use of peripherally inserted central catheters (PICCs) is an example of a modern development in the management of patients with pneumonia.[4, 5, 6, 7] PICCs provide many of the benefits associated with central venous catheters (CVCs) including reliable venous access for delivery of antibiotics, phlebotomy, and invasive hemodynamic monitoring. However, as they are placed in veins of the upper extremity, PICCs bypass insertion risks (eg, injury to the carotid vessels or pneumothorax) associated with placement of traditional CVCs.[8] Because they offer durable venous access, PICCs also facilitate care transitions while continuing intravenous antimicrobial therapy in patients with pneumonia.
However, accumulating evidence also suggests that PICCs are associated with important complications, including central lineassociated bloodstream infectionand venous thromboembolism.[9, 10] Furthermore, knowledge gaps in clinicians regarding indications for appropriate use and management of complications associated with PICCs have been recognized.[10, 11] These elements are problematic because reports of unjustified and inappropriate PICC use are growing in the literature.[12, 13] Such concerns have prompted a number of policy calls to improve PICC use, including Choosing Wisely recommendations by various professional societies.[14, 15]
As little is known about the prevalence or patterns of PICC use in adults hospitalized with pneumonia, we conducted a retrospective cohort study using data from a large network of US hospitals.
METHODS
Setting and Participants
We included patients from hospitals that participated in Premier's inpatient dataset, a large, fee‐supported, multipayer administrative database that has been used extensively in health services research to measure quality of care and comparative effectiveness of interventions.[16] Participating hospitals represent all regions of the United States and include teaching and nonteaching facilities in rural and urban locations. In addition to variables found in the uniform billing form, the Premier inpatient database also includes a date‐stamped list of charges for procedures conducted during hospitalization such as PICC placement. As PICC‐specific data are not available in most nationally representative datasets, Premier offers unique insights into utilization, timing, and factors associated with use of PICCs in hospitalized settings.
We included adult patients aged 18 years who were (1) admitted with a principal diagnosis of pneumonia present on admission, or secondary diagnosis of pneumonia if paired with a principal diagnosis of sepsis, respiratory failure, or influenza; (2) received at least 1 day of antibiotics between July 1, 2007 and November 30, 2011, and (3) underwent chest x‐ray or computed tomography (CT) at the time of admission. International Classification of Disease, 9th Revision, Clinical Modification (ICD‐9‐CM) codes were used for patient selection. Patients who were not admitted (eg, observation cases), had cystic fibrosis, or marked as pneumonia not present on admission were excluded. For patients who had more than 1 hospitalization during the study period, a single admission was randomly selected for inclusion.
Patient, Physician, and Hospital Data
For all patients, age, gender, marital status, insurance, race, and ethnicity were captured. Using software provided by the Healthcare Costs and Utilization Project, we categorized information on 29 comorbid conditions and computed a combined comorbidity score as described by Gagne et al.[17] Patients were considered to have healthcare‐associated pneumonia (HCAP) if they were: (1) admitted from a skilled nursing or a long‐term care facility, (2) hospitalized in the previous 90 days, (3) on dialysis, or (4) receiving immunosuppressing medications (eg, chemotherapy or steroids equivalent to at least 20 mg of prednisone per day) at the time of admission. Information on specialty of the admitting physician and hospital characteristics (eg, size, location, teaching status) were sourced through Premier data.
Receipt of PICCs and Related Therapies
Among eligible adult patients hospitalized with pneumonia, we identified patients who received a PICC at any time during hospitalization via PICC‐specific billing codes. Non‐PICC devices (eg, midlines, Hickman catheters) were not included. For all insertions, we assessed day of PICC placement relative to admission date. Data on type of PICC (eg, power‐injection capable, antibiotic coating) or PICC characteristics (size, number of lumens) were not available. We used billing codes to assess use of invasive or noninvasive ventilation, vasopressors, and administration of pneumonia‐specific antibiotics (eg, ‐lactams, macrolides, fluoroquinolones). Early exposure was defined when a billing code appeared within 2 days of hospital admission.
Outcomes of Interest
The primary outcome of interest was receipt of a PICC. Additionally, we assessed factors associated with PICC placement and variation in risk‐standardized rates of PICC use between hospitals.
Statistical Analyses
Patient and hospital characteristics were summarized using frequencies for categorical variables and medians with interquartile ranges for continuous variables. We examined association of individual patient and hospital characteristics with use of PICCs using generalized estimating equations models with a logit link for categorical variables and identity link for continuous variables, accounting for patient clustering within hospitals.
| Characteristic | Total, No. (%) | No PICC, No. (%) | PICC, No. (%) | P Value* |
|---|---|---|---|---|
| ||||
| 545,250 (100) | 503,401 (92.3) | 41,849 (7.7) | ||
| Demographics | ||||
| Age, median (Q1Q3), y | 71 (5782) | 72 (5782) | 69 (5780) | 0.001 |
| Gender | 0.001 | |||
| Male | 256,448 (47.0) | 237,232 (47.1) | 19,216 (45.9) | |
| Female | 288,802 (53.0) | 266,169 (52.9) | 22,633 (54.1) | |
| Race/ethnicity | 0.001 | |||
| White | 377,255 (69.2) | 346,689 (68.9) | 30,566 (73.0) | |
| Black | 63,345 (11.6) | 58,407 (11.6) | 4,938 (11.8) | |
| Hispanic | 22,855 (4.2) | 21,716 (4.3) | 1,139 (2.7) | |
| Other | 81,795 (15.0) | 76,589 (15.2) | 5,206 (12.4) | |
| Admitting specialty | 0.001 | |||
| Internal medicine | 236,859 (43.4) | 218,689 (43.4) | 18,170 (43.4) | |
| Hospital medicine | 116,499 (21.4) | 107,671 (21.4) | 8,828 (21.1) | |
| Family practice | 80,388 (14.7) | 75,482 (15.0) | 4,906 (11.7) | |
| Critical care and pulmonary | 35,670 (6.5) | 30,529 (6.1) | 41,849 (12.3) | |
| Geriatrics | 4,812 (0.9) | 4,098 (0.8) | 714 (1.7) | |
| Other | 71,022 (13.0) | 66,932 (13.3) | 4,090 (9.8) | |
| Insurance | 0.001 | |||
| Medicare | 370,303 (67.9) | 341,379 (67.8) | 28,924 (69.1) | |
| Medicaid | 45,505 (8.3) | 41,100 (8.2) | 4,405 (10.5) | |
| Managed care | 69,984 (12.8) | 65,280 (13.0) | 4,704 (11.2) | |
| Commercialindemnity | 20,672 (3.8) | 19,251 (3.8) | 1,421 (3.4) | |
| Other | 38,786 (7.1) | 36,391 (7.2) | 2,395 (5.7) | |
| Comorbidities | ||||
| Gagne combined comorbidity score, median (Q1Q3) | 2 (15) | 2 (14) | 4 (26) | 0.001 |
| Hypertension | 332,347 (60.9) | 306,964 (61.0) | 25,383 (60.7) | 0.13 |
| Chronic pulmonary disease | 255,403 (46.8) | 234,619 (46.6) | 20,784 (49.7) | 0.001 |
| Diabetes | 171,247 (31.4) | 155,540 (30.9) | 15,707 (37.5) | 0.001 |
| Congestive heart failure | 146,492 (26.9) | 131,041 (26.0) | 15,451 (36.9) | 0.001 |
| Atrial fibrillation | 108,405 (19.9) | 97,124 (19.3) | 11,281 (27.0) | 0.001 |
| Renal failure | 104,404 (19.1) | 94,277 (18.7) | 10,127 (24.2) | 0.001 |
| Nicotine replacement therapy/tobacco use | 89,938 (16.5) | 83,247 (16.5) | 6,691 (16.0) | 0.001 |
| Obesity | 60,242 (11.0) | 53,268 (10.6) | 6,974 (16.7) | 0.001 |
| Coagulopathy | 41,717 (7.6) | 35,371 (7.0) | 6,346 (15.2) | 0.001 |
| Prior stroke (1 year) | 26,787 (4.9) | 24,046 (4.78) | 2,741 (6.55) | 0.001 |
| Metastatic cancer | 21,868 (4.0) | 20,244 (4.0) | 1,624 (3.9) | 0.16 |
| Solid tumor w/out metastasis | 21,083 (3.9) | 19,380 (3.8) | 1,703 (4.1) | 0.12 |
| Prior VTE (1 year) | 19,090 (3.5) | 16,906 (3.4) | 2,184 (5.2) | 0.001 |
| Chronic liver disease | 16,273 (3.0) | 14,207 (2.8) | 2,066 (4.9) | 0.001 |
| Prior bacteremia (1 year) | 4,106 (0.7) | 3,584 (0.7) | 522 (1.2) | 0.001 |
| Nephrotic syndrome | 671 (0.1) | 607 (0.1) | 64 (0.2) | 0.03 |
| Morbidity markers | ||||
| Type of pneumonia | 0.001 | |||
| CAP | 376,370 (69.1) | 352,900 (70.1) | 23,830 (56.9) | |
| HCAP | 168,520 (30.9) | 150,501 (29.9) | 18,019 (43.1) | |
| Sepsis present on admission | 114,578 (21.0) | 96,467 (19.2) | 18,111 (43.3) | 0.001 |
| Non‐invasive ventilation | 47,913(8.8) | 40,599 (8.1) | 7,314 (17.5) | 0.001 |
| Invasive mechanical ventilation | 56,179 (10.3) | 44,228 (8.8) | 11,951 (28.6) | 0.001 |
| ICU status | 97,703 (17.9) | 80,380 (16.0) | 17,323 (41.4) | 0.001 |
| Vasopressor use | 48,353 (8.9) | 38,030 (7.6) | 10,323 (24.7) | 0.001 |
| Antibiotic/medication use | ||||
| Anti‐MRSA agent (vancomycin) | 146,068 (26.8) | 123,327 (24.5) | 22,741 (54.3) | 0.001 |
| Third‐generation cephalosporin | 250,782 (46.0) | 235,556 (46.8) | 15,226 (36.4) | 0.001 |
| Anti‐Pseudomonal cephalosporin | 41,798 (7.7) | 36,982 (7.3) | 4,816 (11.5) | 0.001 |
| Anti‐Pseudomonal ‐lactam | 122,215 (22.4) | 105,741 (21.0) | 16,474 (39.4) | 0.001 |
| Fluroquinolone | 288,051 (52.8) | 267,131 (53.1) | 20,920 (50.0) | 0.001 |
| Macrolide | 223,737 (41.0) | 210,954 (41.9) | 12,783 (30.5) | 0.001 |
| Aminoglycoside | 15,415 (2.8) | 12,661 (2.5) | 2,754 (6.6) | 0.001 |
| Oral steroids | 44,486 (8.2) | 41,586 (8.3) | 2,900 (6.9) | 0.001 |
| Intravenous steroids | 146,308 (26.8) | 133,920 (26.6) | 12,388 (29.6) | 0.001 |
| VTE prophylaxis with LMWH | 190,735 (35.0) | 174,612 (34.7) | 16,123 (38.5) | 0.01 |
| Discharge disposition | ||||
| Home | 282,146 (51.7) | 272,604(54.1) | 9,542 (22.8) | 0.001 |
| Home with home health | 71,977 (13.2) | 65,289 (13.0) | 6,688 (16.0) | 0.001 |
| Skilled nursing facility | 111,541 (20.5) | 97,113 (19.3) | 14,428 (34.5) | 0.001 |
| Hospice | 20,428 (3.7) | 17,902 (3.6) | 2,526 (6.0) | 0.001 |
| Expired | 47,733 (8.7) | 40,768 (8.1) | 6,965 (16.6) | 0.001 |
| Other | 11,425 (2.1) | 9,725 (1.9) | 1,700 (4.1) | 0.001 |
We then developed a multivariable hierarchical generalized linear model (HGLM) for PICC placement with a random effect for hospital. In this model, we included patient demographics, comorbidities, sepsis on admission, type of pneumonia (eg, HCAP vs community‐associated pneumonia [CAP]), admitting physician specialty, and indicators for early receipt of specific treatments such as guideline‐recommended antibiotics, vasopressors, ventilation (invasive or noninvasive), and pneumatic compression devices for prophylaxis of deep vein thrombosis.
To understand and estimate between‐hospital variation in PICC use, we calculated risk‐standardized rates of PICC use (RSPICC) across hospitals using HGLM methods. These methods are also employed by the Centers for Medicare and Medicaid Services to calculate risk‐standardized measures for public reporting.[18] Because hospital rates of PICC use were highly skewed (21.2% [n = 105] of hospitals had no patients with PICCs), we restricted this model to the 343 hospitals that had at least 5 patients with a PICC to obtain stable estimates. For each hospital, we estimated a predicted rate of PICC use (pPICC) as the sum of predicted probabilities of PICC receipt from patient factors and the random intercept for hospital in which they were admitted. We then calculated an expected rate of PICC use (ePICC) per hospital as the sum of expected probabilities of PICC receipt from patient factors only. RSPICC for each hospital was then computed as the product of the overall unadjusted mean PICC rate (PICC) from all patients and the ratio of the predicted to expected PICC rate (uPICC*[pPICC/ePICC]).[19] Kruskal‐Wallis tests were used to evaluate the association between hospital characteristics with RSPICC rates. To evaluate the impact of the hospital in variation in PICC use, we assessed the change in likelihood ratio of a hierarchical model with hospital random effects compared to a logistic regression model with patient factors only. In addition, we estimated the intraclass correlation (ICC) to assess the proportion of variation in PICC use associated with the hospital, and the median odds ratio (MOR) from the hierarchical model. The MOR is the median of a set of odds ratios comparing 2 patients with the same set of characteristics treated at 2 randomly selected hospitals.[20, 21, 22] All analyses were performed using the Statistical Analysis System version 9.3 (SAS Institute, Inc., Cary, NC) and Stata 13 (StataCorp Inc., College Station, TX).
Ethical and Regulatory Oversight
Permission to conduct this study was obtained from the institutional review board at Baystate Medical Center, Springfield, Massachusetts. The study did not qualify as human subjects research and made use of fully deidentified data.
RESULTS
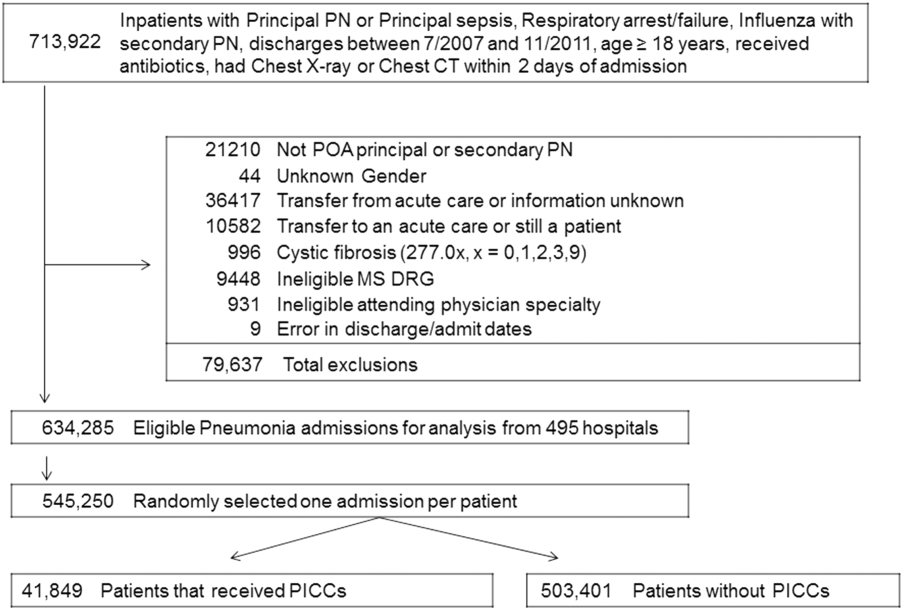
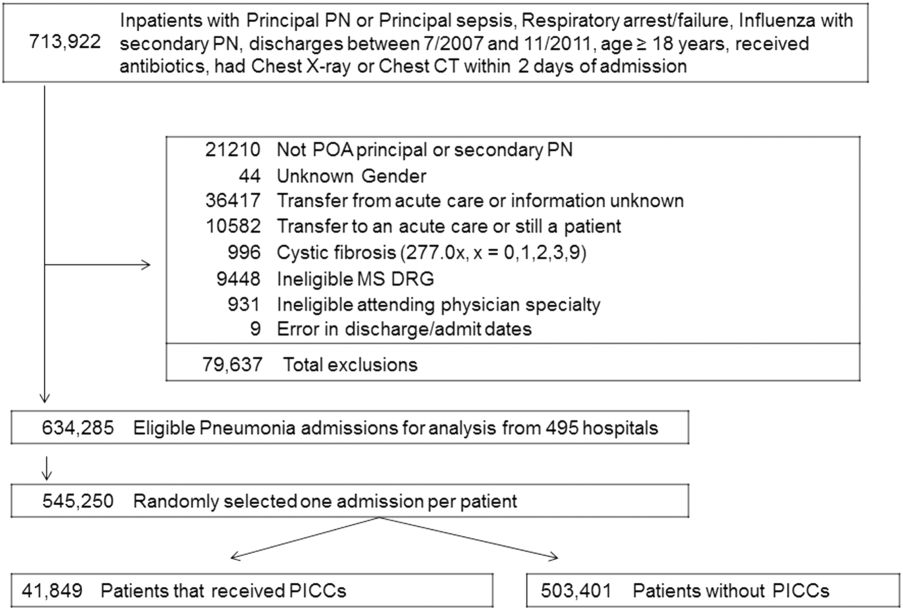
Between July 2007 and November 2011, 634,285 admissions representing 545,250 unique patients from 495 hospitals met eligibility criteria and were included in the study (Figure 1). Included patients had a median age of 71 years (interquartile range [IQR]: 5782), and 53.0% were female. Most patients were Caucasian (69.2%), unmarried (51.6%), and insured by Medicare (67.9%). Patients were admitted to the hospital by internal medicine providers (43.4%), hospitalists (21.4%), and family practice providers (14.7%); notably, critical care and pulmonary medicine providers admitted 6.5% of patients. The median Gagne comorbidity score was 2 (IQR: 15). Hypertension, chronic obstructive pulmonary disease, diabetes, and congestive heart failure were among the most common comorbidities observed (Table 1).
Among eligible patients, 41,849 (7.7%) received a PICC during hospitalization. Approximately a quarter of all patients who received PICCs did so by hospital day 2; 90% underwent insertion by hospital day 11 (mean = 5.4 days, median = 4 days). Patients who received PICCs were younger (median IQR: 69 years, 5780 years) but otherwise demographically similar to those that did not receive PICCs (median IQR: 72 years, 5782 years). Compared to other specialties, patients admitted by critical care/pulmonary providers were twice as likely to receive PICCs (12.3% vs 6.1%, P .001). Patients who received PICCs had higher comorbidity scores than those who did not (median Gagne comorbidity score 4 vs 2, P 0.001) and were more likely to be diagnosed with HCAP (43.1% vs 29.9%, P 0.001) than CAP (56.9% vs 70.1%, P 0.001).
PICC recipients were also more likely to receive intensive care unit (ICU) level of care (41.4% vs 16%, P 0.001) and both noninvasive (17.5% vs 8.1%, P 0.001) and invasive ventilation (28.6% vs 8.8%, P 0.001) upon admission. Vasopressor use was also significantly more frequent in patients who received PICCs (24.7% vs 7.6%, P 0.001) compared to those who did not receive these devices. Patients with PICCs were more often discharged to skilled nursing facilities (34.5% vs 19.3%) than those without PICCs.
Characteristics Associated With PICC Use Following Multivariable Modeling
Using HGLM with a random hospital effect, multiple patient characteristics were associated with PICC use (Table 2). Patients 65 years of age were less likely to receive a PICC compared to younger patients (odds ratio [OR]: 0.81, 95% confidence interval [CI]: 0.79‐0.84). Weight loss (OR: 2.03, 95% CI: 1.97‐2.10), sepsis on admission (OR: 1.80, 95% CI: 1.75‐1.85), and ICU status on hospital day 1 or 2 (OR: 1.70, 95% CI: 1.64‐1.75) represented 3 factors most strongly associated with PICC use.
| Patient Characteristic | Odds Ratio | 95% Confidence Intervals |
|---|---|---|
| ||
| Age group (>66 vs 65 years) | 0.82 | 0.790.84 |
| Race/ethnicity | ||
| Other | 1.02 | 0.971.06 |
| Black | 0.99 | 0.951.03 |
| Hispanic | 0.82 | 0.760.88 |
| White | Referent | |
| Marital status | ||
| Other/missing | 1.07 | 1.011.14 |
| Single | 1.02 | 1.001.05 |
| Married | Referent | |
| Insurance payor | ||
| Other | 0.85 | 0.800.89 |
| Medicaid | 1.13 | 1.081.18 |
| Managed care | 0.95 | 0.910.99 |
| Commercialindemnity | 0.93 | 0.871.00 |
| Medicare | Referent | |
| Admitting physician specialty | ||
| Pulmonary/critical care medicine | 1.18 | 1.131.24 |
| Family practice | 1.01 | 0.971.05 |
| Geriatric medicine (FP and IM) | 1.85 | 1.662.05 |
| Hospitalist | 0.94 | 0.910.98 |
| Other specialties | 1.02 | 0.971.06 |
| Internal medicine | Referent | |
| Comorbidities | ||
| Congestive heart failure | 1.27 | 1.241.31 |
| Valvular disease | 1.11 | 1.071.15 |
| Pulmonary circulation disorders | 1.37 | 1.321.42 |
| Peripheral vascular disease | 1.09 | 1.051.13 |
| Hypertension | 0.94 | 0.920.97 |
| Paralysis | 1.59 | 1.511.67 |
| Other neurological disorders | 1.20 | 1.161.23 |
| Chronic lung disease | 1.10 | 1.071.12 |
| Diabetes | 1.13 | 1.101.16 |
| Hypothyroidism | 1.03 | 1.001.06 |
| Liver disease | 1.16 | 1.101.23 |
| Ulcer | 1.86 | 1.153.02 |
| Lymphoma | 0.88 | 0.810.96 |
| Metastatic cancer | 0.75 | 0.710.80 |
| Solid tumor without metastasis | 0.93 | 0.880.98 |
| Arthritis | 1.22 | 1.161.28 |
| Obesity | 1.47 | 1.421.52 |
| Weight loss | 2.03 | 1.972.10 |
| Blood loss | 1.69 | 1.551.85 |
| Deficiency anemias | 1.40 | 1.371.44 |
| Alcohol abuse | 1.19 | 1.131.26 |
| Drug abuse | 1.31 | 1.231.39 |
| Psychoses | 1.16 | 1.111.21 |
| Depression | 1.10 | 1.061.13 |
| Renal failure | 0.96 | 0.930.98 |
| Type of pneumonia | ||
| HCAP | 1.03 | 1.011.06 |
| CAP | Referent | |
| Sepsis (POA) | 1.80 | 1.751.85 |
| Antibiotic exposure | ||
| Anti‐MRSA agent | 1.72 | 1.671.76 |
| Anti‐Pseudomonal carbapenem | 1.37 | 1.311.44 |
| Non‐Pseudomonal carbapenem | 1.48 | 1.331.66 |
| Third‐generation cephalosporin | 1.04 | 1.011.07 |
| Anti‐Pseudomonal cephalosporin | 1.25 | 1.201.30 |
| Anti‐Pseudomonal ‐lactam | 1.27 | 1.231.31 |
| Aztreonam | 1.31 | 1.231.40 |
| Non‐Pseudomonal ‐lactam | 1.36 | 1.231.50 |
| ‐lactam | 1.55 | 1.261.90 |
| Respiratory quinolone | 0.90 | 0.870.92 |
| Macrolide | 0.85 | 0.820.88 |
| Doxycycline | 0.94 | 0.871.01 |
| Aminoglycoside | 1.21 | 1.141.27 |
| Vasopressors | 1.06 | 1.031.10 |
| Noninvasive ventilation | 1.29 | 1.251.34 |
| Invasive ventilation | 1.66 | 1.611.72 |
| Intensive care unit on admission | 1.70 | 1.641.75 |
| Atrial fibrillation | 1.26 | 1.221.29 |
| Upper extremity chronic DVT | 1.61 | 1.132.28 |
| Nicotine replacement therapy/tobacco abuse | 0.91 | 0.880.94 |
| Aspirin | 0.94 | 0.920.97 |
| Warfarin | 0.90 | 0.860.94 |
| LMWH, prophylactic dose | 1.10 | 1.081.13 |
| LMWH, treatment dose | 1.22 | 1.161.29 |
| Intravenous steroids | 1.05 | 1.021.08 |
| Bacteremia (prior year) | 1.14 | 1.021.27 |
| VTE (prior year) | 1.11 | 1.061.18 |
| Pneumatic compression device | 1.25 | 1.081.45 |
| Invasive ventilation (prior year) | 1.17 | 1.111.24 |
| Irritable bowel disease | 1.19 | 1.051.36 |
Therapy with potent parenteral antimicrobials including antimethicillin‐resistant Staphylococcus aureus agents (OR: 1.72, 95% CI: 1.67‐1.76), antipseudomonal ‐lactamases (OR: 1.27, 95% CI: 1.23‐1.31), and carbapenems (OR: 1.37, 95% CI: 1.31‐1.44) were significantly associated with PICC use. Conversely, use of macrolides (OR: 0.85, 95% CI: 0.82‐0.88) or respiratory fluoroquinolones (OR: 0.90, 95% CI: 0.87‐0.92) were associated with lower likelihood of PICC use. After adjusting for antimicrobial therapy, HCAP was only slightly more likely to result in PICC use than CAP (OR: 1.03, 95% CI: 1.01‐1.06). Compared to internal medicine providers, admission by geriatricians and critical care/pulmonary specialists was associated with greater likelihood of PICC use (OR: 1.85, 95% CI: 1.66‐2.05 and OR: 1.18, 95% CI: =1.13‐1.24, respectively). Admission by hospitalists was associated with a modestly lower likelihood of PICC placement (OR: 0.94, 95% CI: 0.91‐0.98).
Hospital Level Variation in PICC Use
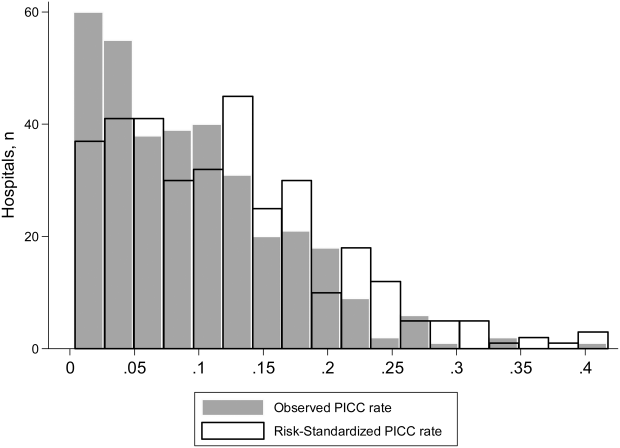
To ensure stable estimates of hospital PICC use, we excluded 152 facilities (31%): 10% had no patients with PICCs and 21% had 5 patients who received a PICC. Therefore, RSPICC was estimated for 343 of 495 facilities (69%) (Figure 2). In these facilities, RSPICC varied from 0.3% to 41.7%. Hospital RSPICC was significantly associated with hospital location (median 11.9% vs 7.8% for urban vs rural hospitals respectively, P = 0.05). RSPICCs were also greater among hospitals in Southern (11.3%), Western (12.7%), and Midwest (12.0%) regions of the nation compared to those in the Northeast (8.4%) (P = 0.02) (Table 3).
| Hospital Characteristic (No.) | Median (IQR), % | P Value |
|---|---|---|
| ||
| Bed size | 0.12 | |
| 200 beds (106) | 9.1 (4.816.3) | |
| 201 beds (237) | 11.6 (5.817.6) | |
| Rural/urban | 0.05 | |
| Urban (275) | 11.9 (5.517.4) | |
| Rural (68) | 7.8 (5.014.0) | |
| Region | 0.02 | |
| Northeast (50) | 8.4 (3.913.0) | |
| Midwest (69) | 12.0 (5.817.4) | |
| West (57) | 12.7 (7.617.0) | |
| South (167) | 11.3 (4.817.8) | |
| Teaching status | 0.77 | |
| Nonteaching (246) | 10.9 (5.017.4) | |
| Teaching (97) | 12.0 (5.816.9) | |
A likelihood ratio test comparing the hierarchical model to a logistic model with patient factors only was highly significant (P 0.001), indicating that the hospital where the patient was treated had a major impact on receipt of PICC after accounting for patient factors. The MOR was 2.71, which is a larger effect than we found for any of the individual patient characteristics. The proportion of variance explained by hospitals was 25% (95% CI: 22%‐28%), as measured by the ICC.
DISCUSSION
In this study of 545,250 adults hospitalized with pneumonia, we found that approximately 8% of patients received a PICC. Patients who received PICCs had more comorbidities, were more frequently diagnosed with HCAP, and were more often admitted to the ICU, where they experienced greater rates of mechanical ventilation, noninvasive ventilation, and vasopressor use compared to those who did not receive a PICC. Additionally, risk‐adjusted rates of PICC use varied as much as 10‐fold across institutions. In fact, almost 70% of the total variation in rates of PICC use remained unexplained by hospital or patient characteristics. Although use of PICCs is often clinically nuanced in ways that are difficult to capture in large datasets (eg, difficult venous access or inability to tolerate oral medications), the substantial variation of PICC use observed suggests that physician and institutional practice styles are the major determinants of PICC placement during a hospitalization for pneumonia. Because PICCs are associated with serious complications, and evidence regarding discretionary use is accumulating, a research agenda examining reasons for such use and related outcomes appears necessary.
The placement of PICCs has grown substantially in hospitalized patients all over the world.[23, 24] Although originally developed for total parenteral nutrition in surgical patients,[25] contemporary reports of PICC use in critical illness,[26] diseases such as cystic fibrosis,[27] and even pregnancy[28] are now common. Although PICCs are clinically invaluable in many of these conditions, growing use of these devices has led to the realization that benefits may be offset by complications.[9, 10, 29, 30] Additionally, recent data suggest that not all PICCs may be used for appropriate reasons. For instance, in a decade‐long study at a tertiary care center, changes in patterns of PICC use including shortened dwell times, multiple insertions in a single patient, and unclear indications for use were reported.[11] In another study at an academic medical center, a substantial proportion of PICCs were found to be idle or unjustified.[12] It comes as little surprise, then, that a recent multicenter study found that 1 out of every 5 clinicians did not even know that their patient had a PICC.[29] Although calls to improve PICC use in the hospital setting have emerged, strategies to do so are limited by data that emanate from single‐center reports or retrospective designs. No other studies reporting use of PICCs across US hospitals for any clinical condition currently exist.[31]
We found that patients with weight loss, those with greater combined comorbidity scores, and those who were critically ill or diagnosed with sepsis were more likely to receive PICCs than others. These observations suggest that PICC use may reflect underlying severity of illness, as advanced care such as ventilator support was often associated with PICC use. Additionally, discharge to a skilled nursing facility was frequently associated with PICC placement, a finding consistent with a recent study evaluating the use of PICCs in these settings.[32] However, a substantial proportion of PICC use remained unexplained by available patient or hospital factors. Although our study was not specifically designed to examine this question, a possible reason may relate to unmeasured institutional factors that influence the propensity to use a PICC, recently termed as PICC culture.[33] For example, it is plausible that hospitals with nursing‐led PICC teams or interventional radiology (such as teaching hospitals) are more likely to use PICCs than those without such operators. This hypothesis may explain why urban, larger, and teaching hospitals exhibited higher rates of PICC use. Conversely, providers may have an affinity toward PICC use that is predicated not just by operator availability, but also local hospital norms. Understanding why some facilities use PICCs at higher rates than others and implications of such variation with respect to patient safety, cost, and outcomes is important. Study designs that use mixed‐methods approaches or seek to qualitatively understand reasons behind PICC use are likely to be valuable in this enquiry.
Our study has limitations. First, we used an administrative dataset and ICD‐9‐CM codes rather than clinical data from medical records to identify cases of pneumonia or comorbidities. Our estimates of PICC use across hospitals thus may not fully account for differences in severity of illness, and it is possible that patients needed a PICC for reasons that we could not observe. However, the substantial variation observed in rates of PICC use across hospitals is unlikely to be explained by differences in patient severity of illness, documentation, or coding practices. Second, as PICC removal codes were not available, we are unable to comment on how often hospitalized pneumonia patients were discharged with PICCs or received antimicrobial therapy beyond their inpatient stay. Third, although we observed that a number of patient and hospital factors were associated with PICC receipt, our study was not designed to determine the reasons underlying these patterns.
These limitations aside, our study has important strengths. To our knowledge, this is the first study to report utilization and outcomes associated with PICC use among those hospitalized with pneumonia across the United States. The inclusion of a large number of patients receiving care in diverse facilities lends a high degree of external validity to our findings. Second, we used advanced modeling to identify factors associated with PICC use in hospitalized patients with pneumonia, producing innovative and novel findings. Third, our study is the first to show the existence of substantial variation in rates of PICC use across US hospitals within the single disease state of pneumonia. Understanding the drivers of this variability is important as it may inform future studies, policies, and practices to improve PICC use in hospitalized patients.
In conclusion, we found that PICC use in patients hospitalized with pneumonia is common and highly variable. Future studies examining the contextual factors behind PICC use and their association with outcomes are needed to facilitate efforts to standardize PICC use across hospitals.
Disclosures
Dr. Chopra is supported by a career development award (1‐K08‐HS022835‐01) from the Agency of Healthcare Research and Quality. The authors report no conflicts of interest.
- , . Reasons for being admitted to the hospital through the emergency department, 2003. Healthcare Cost and Utilization Project Statistical Brief 2. Rockville, MD: Agency for Healthcare Research and Quality. Available at: www.hcup‐us.ahrq.gov/reports/statbriefs/sb2.pdf. Published February 2006. Accessed June 27, 2014.
- , , , et al. National patterns of risk‐standardized mortality and readmission after hospitalization for acute myocardial infarction, heart failure, and pneumonia: update on publicly reported outcomes measures based on the 2013 release. J Gen Intern Med. 2014;29(10):1333–1340.
- , , , et al. Quality of care for elderly patients hospitalized for pneumonia in the United States, 2006 to 2010. JAMA Intern Med. 2014;174(11):1806–1814.
- , . PICC lines: the latest home care challenge. RN. 1990;53(1):44–51.
- , , , . Peripherally inserted central catheters in an acute‐care hospital. Arch Intern Med. 1994;154(16):1833–1837.
- , . The peripherally inserted central catheter: a retrospective look at three years of insertions. J Intraven Nurs. 1993;16(2):92–103.
- , , , . Peripherally inserted central catheters in general medicine. Mayo Clin Proc. 1997;72(3):225–233.
- , , . Two‐year trends of peripherally inserted central catheter‐line complications at a tertiary‐care hospital: role of nursing expertise. Infect Control Hosp Epidemiol. 2001;22(6):377–379.
- , , , , , . PICC‐associated bloodstream infections: prevalence, patterns, and predictors. Am J Med. 2014;127(4):319–328.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013;34(9):908–918.
- , , , , , . Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323–1331.
- , , , et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter”. Infect Control Hosp Epidemiol. 2012;33(1):50–57.
- , , , , . Inappropriate intravascular device use: a prospective study. Journal Hosp Infect. 2011;78(2):128–132.
- , , , et al. Enhancing patient‐centered care: SGIM and choosing wisely. J Gen Intern Med. 2014;29(3):432–433.
- , , , et al. Critical and honest conversations: the evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol. 2012;7(10):1664–1672.
- , , , et al. Using highly detailed administrative data to predict pneumonia mortality. PLoS One. 2014;9(1):e87382.
- , , , , . A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–759.
- , , , , . Hospitals with the highest intensive care utilization provide lower quality pneumonia care to the elderly. Crit Care Med. 2015;43(6):1178–1186.
- , . Statistical and clinical aspects of hospital outcomes profiling. Stat Sci. 2007;22(2):206–226.
- , . Appropriate assessment of neighborhood effects on individual health: integrating random and fixed effects in multilevel logistic regression. Am J Epidemiol. 2005;161(1):81–88.
- , , , . Interpreting parameters in the logistic regression model with random effects. Biometrics. 2000;56(3):909–914.
- , , , . Hospital‐level associations with 30‐day patient mortality after cardiac surgery: a tutorial on the application and interpretation of marginal and multilevel logistic regression. BMC Med Res Methodol. 2012;12:28.
- , , , . Experiences of the first PICC team in the Czech Republic. Br J Nurs. 2015;24(suppl 2):S4–S10.
- , , , et al. Greece reports prototype intervention with first peripherally inserted central catheter: case report and literature review. J Vasc Nurs. 2012;30(3):88–93.
- Total intravenous nutrition with peripherally inserted silicone elastomer central venous catheters. Arch Surg. 1975;110(5):644–646.
- , . Focus on peripherally inserted central catheters in critically ill patients. World J Crit Care Med. 2014;3(4):80–94.
- , , , et al. Quality improvement initiative to reduce deep vein thrombosis associated with peripherally inserted central catheters in adults with cystic fibrosis. Ann Am Thorac Soc. 2014;11(9):1404–1410.
- , , , . Peripherally Inserted central catheter (PICC) complications during pregnancy. JPEN J Parenter Enteral Nutr. 2013;38(5):595–601.
- , , , et al. Do clinicians know which of their patients have central venous catheters?: a multicenter observational study. Ann Intern Med. 2014;161(8):562–567.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
- , , , et al. Peripherally inserted central catheter use in skilled nursing facilities: a pilot study. J Am Geriatr Soc. 2015;63(9):1894–1899.
- , , , , . Inpatient venous access practices: PICC culture and the kidney patient. J Vasc Access. 2015;16(3):206–210.
Pneumonia is the most common cause of unplanned hospitalization in the United States.[1] Despite its clinical toll, the management of this disease has evolved markedly. Expanding vaccination programs, efforts to improve timeliness of antibiotic therapy, and improved processes of care are but a few developments that have improved outcomes for patients afflicted with this illness.[2, 3]
Use of peripherally inserted central catheters (PICCs) is an example of a modern development in the management of patients with pneumonia.[4, 5, 6, 7] PICCs provide many of the benefits associated with central venous catheters (CVCs) including reliable venous access for delivery of antibiotics, phlebotomy, and invasive hemodynamic monitoring. However, as they are placed in veins of the upper extremity, PICCs bypass insertion risks (eg, injury to the carotid vessels or pneumothorax) associated with placement of traditional CVCs.[8] Because they offer durable venous access, PICCs also facilitate care transitions while continuing intravenous antimicrobial therapy in patients with pneumonia.
However, accumulating evidence also suggests that PICCs are associated with important complications, including central lineassociated bloodstream infectionand venous thromboembolism.[9, 10] Furthermore, knowledge gaps in clinicians regarding indications for appropriate use and management of complications associated with PICCs have been recognized.[10, 11] These elements are problematic because reports of unjustified and inappropriate PICC use are growing in the literature.[12, 13] Such concerns have prompted a number of policy calls to improve PICC use, including Choosing Wisely recommendations by various professional societies.[14, 15]
As little is known about the prevalence or patterns of PICC use in adults hospitalized with pneumonia, we conducted a retrospective cohort study using data from a large network of US hospitals.
METHODS
Setting and Participants
We included patients from hospitals that participated in Premier's inpatient dataset, a large, fee‐supported, multipayer administrative database that has been used extensively in health services research to measure quality of care and comparative effectiveness of interventions.[16] Participating hospitals represent all regions of the United States and include teaching and nonteaching facilities in rural and urban locations. In addition to variables found in the uniform billing form, the Premier inpatient database also includes a date‐stamped list of charges for procedures conducted during hospitalization such as PICC placement. As PICC‐specific data are not available in most nationally representative datasets, Premier offers unique insights into utilization, timing, and factors associated with use of PICCs in hospitalized settings.
We included adult patients aged 18 years who were (1) admitted with a principal diagnosis of pneumonia present on admission, or secondary diagnosis of pneumonia if paired with a principal diagnosis of sepsis, respiratory failure, or influenza; (2) received at least 1 day of antibiotics between July 1, 2007 and November 30, 2011, and (3) underwent chest x‐ray or computed tomography (CT) at the time of admission. International Classification of Disease, 9th Revision, Clinical Modification (ICD‐9‐CM) codes were used for patient selection. Patients who were not admitted (eg, observation cases), had cystic fibrosis, or marked as pneumonia not present on admission were excluded. For patients who had more than 1 hospitalization during the study period, a single admission was randomly selected for inclusion.
Patient, Physician, and Hospital Data
For all patients, age, gender, marital status, insurance, race, and ethnicity were captured. Using software provided by the Healthcare Costs and Utilization Project, we categorized information on 29 comorbid conditions and computed a combined comorbidity score as described by Gagne et al.[17] Patients were considered to have healthcare‐associated pneumonia (HCAP) if they were: (1) admitted from a skilled nursing or a long‐term care facility, (2) hospitalized in the previous 90 days, (3) on dialysis, or (4) receiving immunosuppressing medications (eg, chemotherapy or steroids equivalent to at least 20 mg of prednisone per day) at the time of admission. Information on specialty of the admitting physician and hospital characteristics (eg, size, location, teaching status) were sourced through Premier data.
Receipt of PICCs and Related Therapies
Among eligible adult patients hospitalized with pneumonia, we identified patients who received a PICC at any time during hospitalization via PICC‐specific billing codes. Non‐PICC devices (eg, midlines, Hickman catheters) were not included. For all insertions, we assessed day of PICC placement relative to admission date. Data on type of PICC (eg, power‐injection capable, antibiotic coating) or PICC characteristics (size, number of lumens) were not available. We used billing codes to assess use of invasive or noninvasive ventilation, vasopressors, and administration of pneumonia‐specific antibiotics (eg, ‐lactams, macrolides, fluoroquinolones). Early exposure was defined when a billing code appeared within 2 days of hospital admission.
Outcomes of Interest
The primary outcome of interest was receipt of a PICC. Additionally, we assessed factors associated with PICC placement and variation in risk‐standardized rates of PICC use between hospitals.
Statistical Analyses
Patient and hospital characteristics were summarized using frequencies for categorical variables and medians with interquartile ranges for continuous variables. We examined association of individual patient and hospital characteristics with use of PICCs using generalized estimating equations models with a logit link for categorical variables and identity link for continuous variables, accounting for patient clustering within hospitals.
| Characteristic | Total, No. (%) | No PICC, No. (%) | PICC, No. (%) | P Value* |
|---|---|---|---|---|
| ||||
| 545,250 (100) | 503,401 (92.3) | 41,849 (7.7) | ||
| Demographics | ||||
| Age, median (Q1Q3), y | 71 (5782) | 72 (5782) | 69 (5780) | 0.001 |
| Gender | 0.001 | |||
| Male | 256,448 (47.0) | 237,232 (47.1) | 19,216 (45.9) | |
| Female | 288,802 (53.0) | 266,169 (52.9) | 22,633 (54.1) | |
| Race/ethnicity | 0.001 | |||
| White | 377,255 (69.2) | 346,689 (68.9) | 30,566 (73.0) | |
| Black | 63,345 (11.6) | 58,407 (11.6) | 4,938 (11.8) | |
| Hispanic | 22,855 (4.2) | 21,716 (4.3) | 1,139 (2.7) | |
| Other | 81,795 (15.0) | 76,589 (15.2) | 5,206 (12.4) | |
| Admitting specialty | 0.001 | |||
| Internal medicine | 236,859 (43.4) | 218,689 (43.4) | 18,170 (43.4) | |
| Hospital medicine | 116,499 (21.4) | 107,671 (21.4) | 8,828 (21.1) | |
| Family practice | 80,388 (14.7) | 75,482 (15.0) | 4,906 (11.7) | |
| Critical care and pulmonary | 35,670 (6.5) | 30,529 (6.1) | 41,849 (12.3) | |
| Geriatrics | 4,812 (0.9) | 4,098 (0.8) | 714 (1.7) | |
| Other | 71,022 (13.0) | 66,932 (13.3) | 4,090 (9.8) | |
| Insurance | 0.001 | |||
| Medicare | 370,303 (67.9) | 341,379 (67.8) | 28,924 (69.1) | |
| Medicaid | 45,505 (8.3) | 41,100 (8.2) | 4,405 (10.5) | |
| Managed care | 69,984 (12.8) | 65,280 (13.0) | 4,704 (11.2) | |
| Commercialindemnity | 20,672 (3.8) | 19,251 (3.8) | 1,421 (3.4) | |
| Other | 38,786 (7.1) | 36,391 (7.2) | 2,395 (5.7) | |
| Comorbidities | ||||
| Gagne combined comorbidity score, median (Q1Q3) | 2 (15) | 2 (14) | 4 (26) | 0.001 |
| Hypertension | 332,347 (60.9) | 306,964 (61.0) | 25,383 (60.7) | 0.13 |
| Chronic pulmonary disease | 255,403 (46.8) | 234,619 (46.6) | 20,784 (49.7) | 0.001 |
| Diabetes | 171,247 (31.4) | 155,540 (30.9) | 15,707 (37.5) | 0.001 |
| Congestive heart failure | 146,492 (26.9) | 131,041 (26.0) | 15,451 (36.9) | 0.001 |
| Atrial fibrillation | 108,405 (19.9) | 97,124 (19.3) | 11,281 (27.0) | 0.001 |
| Renal failure | 104,404 (19.1) | 94,277 (18.7) | 10,127 (24.2) | 0.001 |
| Nicotine replacement therapy/tobacco use | 89,938 (16.5) | 83,247 (16.5) | 6,691 (16.0) | 0.001 |
| Obesity | 60,242 (11.0) | 53,268 (10.6) | 6,974 (16.7) | 0.001 |
| Coagulopathy | 41,717 (7.6) | 35,371 (7.0) | 6,346 (15.2) | 0.001 |
| Prior stroke (1 year) | 26,787 (4.9) | 24,046 (4.78) | 2,741 (6.55) | 0.001 |
| Metastatic cancer | 21,868 (4.0) | 20,244 (4.0) | 1,624 (3.9) | 0.16 |
| Solid tumor w/out metastasis | 21,083 (3.9) | 19,380 (3.8) | 1,703 (4.1) | 0.12 |
| Prior VTE (1 year) | 19,090 (3.5) | 16,906 (3.4) | 2,184 (5.2) | 0.001 |
| Chronic liver disease | 16,273 (3.0) | 14,207 (2.8) | 2,066 (4.9) | 0.001 |
| Prior bacteremia (1 year) | 4,106 (0.7) | 3,584 (0.7) | 522 (1.2) | 0.001 |
| Nephrotic syndrome | 671 (0.1) | 607 (0.1) | 64 (0.2) | 0.03 |
| Morbidity markers | ||||
| Type of pneumonia | 0.001 | |||
| CAP | 376,370 (69.1) | 352,900 (70.1) | 23,830 (56.9) | |
| HCAP | 168,520 (30.9) | 150,501 (29.9) | 18,019 (43.1) | |
| Sepsis present on admission | 114,578 (21.0) | 96,467 (19.2) | 18,111 (43.3) | 0.001 |
| Non‐invasive ventilation | 47,913(8.8) | 40,599 (8.1) | 7,314 (17.5) | 0.001 |
| Invasive mechanical ventilation | 56,179 (10.3) | 44,228 (8.8) | 11,951 (28.6) | 0.001 |
| ICU status | 97,703 (17.9) | 80,380 (16.0) | 17,323 (41.4) | 0.001 |
| Vasopressor use | 48,353 (8.9) | 38,030 (7.6) | 10,323 (24.7) | 0.001 |
| Antibiotic/medication use | ||||
| Anti‐MRSA agent (vancomycin) | 146,068 (26.8) | 123,327 (24.5) | 22,741 (54.3) | 0.001 |
| Third‐generation cephalosporin | 250,782 (46.0) | 235,556 (46.8) | 15,226 (36.4) | 0.001 |
| Anti‐Pseudomonal cephalosporin | 41,798 (7.7) | 36,982 (7.3) | 4,816 (11.5) | 0.001 |
| Anti‐Pseudomonal ‐lactam | 122,215 (22.4) | 105,741 (21.0) | 16,474 (39.4) | 0.001 |
| Fluroquinolone | 288,051 (52.8) | 267,131 (53.1) | 20,920 (50.0) | 0.001 |
| Macrolide | 223,737 (41.0) | 210,954 (41.9) | 12,783 (30.5) | 0.001 |
| Aminoglycoside | 15,415 (2.8) | 12,661 (2.5) | 2,754 (6.6) | 0.001 |
| Oral steroids | 44,486 (8.2) | 41,586 (8.3) | 2,900 (6.9) | 0.001 |
| Intravenous steroids | 146,308 (26.8) | 133,920 (26.6) | 12,388 (29.6) | 0.001 |
| VTE prophylaxis with LMWH | 190,735 (35.0) | 174,612 (34.7) | 16,123 (38.5) | 0.01 |
| Discharge disposition | ||||
| Home | 282,146 (51.7) | 272,604(54.1) | 9,542 (22.8) | 0.001 |
| Home with home health | 71,977 (13.2) | 65,289 (13.0) | 6,688 (16.0) | 0.001 |
| Skilled nursing facility | 111,541 (20.5) | 97,113 (19.3) | 14,428 (34.5) | 0.001 |
| Hospice | 20,428 (3.7) | 17,902 (3.6) | 2,526 (6.0) | 0.001 |
| Expired | 47,733 (8.7) | 40,768 (8.1) | 6,965 (16.6) | 0.001 |
| Other | 11,425 (2.1) | 9,725 (1.9) | 1,700 (4.1) | 0.001 |
We then developed a multivariable hierarchical generalized linear model (HGLM) for PICC placement with a random effect for hospital. In this model, we included patient demographics, comorbidities, sepsis on admission, type of pneumonia (eg, HCAP vs community‐associated pneumonia [CAP]), admitting physician specialty, and indicators for early receipt of specific treatments such as guideline‐recommended antibiotics, vasopressors, ventilation (invasive or noninvasive), and pneumatic compression devices for prophylaxis of deep vein thrombosis.
To understand and estimate between‐hospital variation in PICC use, we calculated risk‐standardized rates of PICC use (RSPICC) across hospitals using HGLM methods. These methods are also employed by the Centers for Medicare and Medicaid Services to calculate risk‐standardized measures for public reporting.[18] Because hospital rates of PICC use were highly skewed (21.2% [n = 105] of hospitals had no patients with PICCs), we restricted this model to the 343 hospitals that had at least 5 patients with a PICC to obtain stable estimates. For each hospital, we estimated a predicted rate of PICC use (pPICC) as the sum of predicted probabilities of PICC receipt from patient factors and the random intercept for hospital in which they were admitted. We then calculated an expected rate of PICC use (ePICC) per hospital as the sum of expected probabilities of PICC receipt from patient factors only. RSPICC for each hospital was then computed as the product of the overall unadjusted mean PICC rate (PICC) from all patients and the ratio of the predicted to expected PICC rate (uPICC*[pPICC/ePICC]).[19] Kruskal‐Wallis tests were used to evaluate the association between hospital characteristics with RSPICC rates. To evaluate the impact of the hospital in variation in PICC use, we assessed the change in likelihood ratio of a hierarchical model with hospital random effects compared to a logistic regression model with patient factors only. In addition, we estimated the intraclass correlation (ICC) to assess the proportion of variation in PICC use associated with the hospital, and the median odds ratio (MOR) from the hierarchical model. The MOR is the median of a set of odds ratios comparing 2 patients with the same set of characteristics treated at 2 randomly selected hospitals.[20, 21, 22] All analyses were performed using the Statistical Analysis System version 9.3 (SAS Institute, Inc., Cary, NC) and Stata 13 (StataCorp Inc., College Station, TX).
Ethical and Regulatory Oversight
Permission to conduct this study was obtained from the institutional review board at Baystate Medical Center, Springfield, Massachusetts. The study did not qualify as human subjects research and made use of fully deidentified data.
RESULTS
Between July 2007 and November 2011, 634,285 admissions representing 545,250 unique patients from 495 hospitals met eligibility criteria and were included in the study (Figure 1). Included patients had a median age of 71 years (interquartile range [IQR]: 5782), and 53.0% were female. Most patients were Caucasian (69.2%), unmarried (51.6%), and insured by Medicare (67.9%). Patients were admitted to the hospital by internal medicine providers (43.4%), hospitalists (21.4%), and family practice providers (14.7%); notably, critical care and pulmonary medicine providers admitted 6.5% of patients. The median Gagne comorbidity score was 2 (IQR: 15). Hypertension, chronic obstructive pulmonary disease, diabetes, and congestive heart failure were among the most common comorbidities observed (Table 1).

Among eligible patients, 41,849 (7.7%) received a PICC during hospitalization. Approximately a quarter of all patients who received PICCs did so by hospital day 2; 90% underwent insertion by hospital day 11 (mean = 5.4 days, median = 4 days). Patients who received PICCs were younger (median IQR: 69 years, 5780 years) but otherwise demographically similar to those that did not receive PICCs (median IQR: 72 years, 5782 years). Compared to other specialties, patients admitted by critical care/pulmonary providers were twice as likely to receive PICCs (12.3% vs 6.1%, P .001). Patients who received PICCs had higher comorbidity scores than those who did not (median Gagne comorbidity score 4 vs 2, P 0.001) and were more likely to be diagnosed with HCAP (43.1% vs 29.9%, P 0.001) than CAP (56.9% vs 70.1%, P 0.001).
PICC recipients were also more likely to receive intensive care unit (ICU) level of care (41.4% vs 16%, P 0.001) and both noninvasive (17.5% vs 8.1%, P 0.001) and invasive ventilation (28.6% vs 8.8%, P 0.001) upon admission. Vasopressor use was also significantly more frequent in patients who received PICCs (24.7% vs 7.6%, P 0.001) compared to those who did not receive these devices. Patients with PICCs were more often discharged to skilled nursing facilities (34.5% vs 19.3%) than those without PICCs.
Characteristics Associated With PICC Use Following Multivariable Modeling
Using HGLM with a random hospital effect, multiple patient characteristics were associated with PICC use (Table 2). Patients 65 years of age were less likely to receive a PICC compared to younger patients (odds ratio [OR]: 0.81, 95% confidence interval [CI]: 0.79‐0.84). Weight loss (OR: 2.03, 95% CI: 1.97‐2.10), sepsis on admission (OR: 1.80, 95% CI: 1.75‐1.85), and ICU status on hospital day 1 or 2 (OR: 1.70, 95% CI: 1.64‐1.75) represented 3 factors most strongly associated with PICC use.
| Patient Characteristic | Odds Ratio | 95% Confidence Intervals |
|---|---|---|
| ||
| Age group (>66 vs 65 years) | 0.82 | 0.790.84 |
| Race/ethnicity | ||
| Other | 1.02 | 0.971.06 |
| Black | 0.99 | 0.951.03 |
| Hispanic | 0.82 | 0.760.88 |
| White | Referent | |
| Marital status | ||
| Other/missing | 1.07 | 1.011.14 |
| Single | 1.02 | 1.001.05 |
| Married | Referent | |
| Insurance payor | ||
| Other | 0.85 | 0.800.89 |
| Medicaid | 1.13 | 1.081.18 |
| Managed care | 0.95 | 0.910.99 |
| Commercialindemnity | 0.93 | 0.871.00 |
| Medicare | Referent | |
| Admitting physician specialty | ||
| Pulmonary/critical care medicine | 1.18 | 1.131.24 |
| Family practice | 1.01 | 0.971.05 |
| Geriatric medicine (FP and IM) | 1.85 | 1.662.05 |
| Hospitalist | 0.94 | 0.910.98 |
| Other specialties | 1.02 | 0.971.06 |
| Internal medicine | Referent | |
| Comorbidities | ||
| Congestive heart failure | 1.27 | 1.241.31 |
| Valvular disease | 1.11 | 1.071.15 |
| Pulmonary circulation disorders | 1.37 | 1.321.42 |
| Peripheral vascular disease | 1.09 | 1.051.13 |
| Hypertension | 0.94 | 0.920.97 |
| Paralysis | 1.59 | 1.511.67 |
| Other neurological disorders | 1.20 | 1.161.23 |
| Chronic lung disease | 1.10 | 1.071.12 |
| Diabetes | 1.13 | 1.101.16 |
| Hypothyroidism | 1.03 | 1.001.06 |
| Liver disease | 1.16 | 1.101.23 |
| Ulcer | 1.86 | 1.153.02 |
| Lymphoma | 0.88 | 0.810.96 |
| Metastatic cancer | 0.75 | 0.710.80 |
| Solid tumor without metastasis | 0.93 | 0.880.98 |
| Arthritis | 1.22 | 1.161.28 |
| Obesity | 1.47 | 1.421.52 |
| Weight loss | 2.03 | 1.972.10 |
| Blood loss | 1.69 | 1.551.85 |
| Deficiency anemias | 1.40 | 1.371.44 |
| Alcohol abuse | 1.19 | 1.131.26 |
| Drug abuse | 1.31 | 1.231.39 |
| Psychoses | 1.16 | 1.111.21 |
| Depression | 1.10 | 1.061.13 |
| Renal failure | 0.96 | 0.930.98 |
| Type of pneumonia | ||
| HCAP | 1.03 | 1.011.06 |
| CAP | Referent | |
| Sepsis (POA) | 1.80 | 1.751.85 |
| Antibiotic exposure | ||
| Anti‐MRSA agent | 1.72 | 1.671.76 |
| Anti‐Pseudomonal carbapenem | 1.37 | 1.311.44 |
| Non‐Pseudomonal carbapenem | 1.48 | 1.331.66 |
| Third‐generation cephalosporin | 1.04 | 1.011.07 |
| Anti‐Pseudomonal cephalosporin | 1.25 | 1.201.30 |
| Anti‐Pseudomonal ‐lactam | 1.27 | 1.231.31 |
| Aztreonam | 1.31 | 1.231.40 |
| Non‐Pseudomonal ‐lactam | 1.36 | 1.231.50 |
| ‐lactam | 1.55 | 1.261.90 |
| Respiratory quinolone | 0.90 | 0.870.92 |
| Macrolide | 0.85 | 0.820.88 |
| Doxycycline | 0.94 | 0.871.01 |
| Aminoglycoside | 1.21 | 1.141.27 |
| Vasopressors | 1.06 | 1.031.10 |
| Noninvasive ventilation | 1.29 | 1.251.34 |
| Invasive ventilation | 1.66 | 1.611.72 |
| Intensive care unit on admission | 1.70 | 1.641.75 |
| Atrial fibrillation | 1.26 | 1.221.29 |
| Upper extremity chronic DVT | 1.61 | 1.132.28 |
| Nicotine replacement therapy/tobacco abuse | 0.91 | 0.880.94 |
| Aspirin | 0.94 | 0.920.97 |
| Warfarin | 0.90 | 0.860.94 |
| LMWH, prophylactic dose | 1.10 | 1.081.13 |
| LMWH, treatment dose | 1.22 | 1.161.29 |
| Intravenous steroids | 1.05 | 1.021.08 |
| Bacteremia (prior year) | 1.14 | 1.021.27 |
| VTE (prior year) | 1.11 | 1.061.18 |
| Pneumatic compression device | 1.25 | 1.081.45 |
| Invasive ventilation (prior year) | 1.17 | 1.111.24 |
| Irritable bowel disease | 1.19 | 1.051.36 |
Therapy with potent parenteral antimicrobials including antimethicillin‐resistant Staphylococcus aureus agents (OR: 1.72, 95% CI: 1.67‐1.76), antipseudomonal ‐lactamases (OR: 1.27, 95% CI: 1.23‐1.31), and carbapenems (OR: 1.37, 95% CI: 1.31‐1.44) were significantly associated with PICC use. Conversely, use of macrolides (OR: 0.85, 95% CI: 0.82‐0.88) or respiratory fluoroquinolones (OR: 0.90, 95% CI: 0.87‐0.92) were associated with lower likelihood of PICC use. After adjusting for antimicrobial therapy, HCAP was only slightly more likely to result in PICC use than CAP (OR: 1.03, 95% CI: 1.01‐1.06). Compared to internal medicine providers, admission by geriatricians and critical care/pulmonary specialists was associated with greater likelihood of PICC use (OR: 1.85, 95% CI: 1.66‐2.05 and OR: 1.18, 95% CI: =1.13‐1.24, respectively). Admission by hospitalists was associated with a modestly lower likelihood of PICC placement (OR: 0.94, 95% CI: 0.91‐0.98).
Hospital Level Variation in PICC Use
To ensure stable estimates of hospital PICC use, we excluded 152 facilities (31%): 10% had no patients with PICCs and 21% had 5 patients who received a PICC. Therefore, RSPICC was estimated for 343 of 495 facilities (69%) (Figure 2). In these facilities, RSPICC varied from 0.3% to 41.7%. Hospital RSPICC was significantly associated with hospital location (median 11.9% vs 7.8% for urban vs rural hospitals respectively, P = 0.05). RSPICCs were also greater among hospitals in Southern (11.3%), Western (12.7%), and Midwest (12.0%) regions of the nation compared to those in the Northeast (8.4%) (P = 0.02) (Table 3).
| Hospital Characteristic (No.) | Median (IQR), % | P Value |
|---|---|---|
| ||
| Bed size | 0.12 | |
| 200 beds (106) | 9.1 (4.816.3) | |
| 201 beds (237) | 11.6 (5.817.6) | |
| Rural/urban | 0.05 | |
| Urban (275) | 11.9 (5.517.4) | |
| Rural (68) | 7.8 (5.014.0) | |
| Region | 0.02 | |
| Northeast (50) | 8.4 (3.913.0) | |
| Midwest (69) | 12.0 (5.817.4) | |
| West (57) | 12.7 (7.617.0) | |
| South (167) | 11.3 (4.817.8) | |
| Teaching status | 0.77 | |
| Nonteaching (246) | 10.9 (5.017.4) | |
| Teaching (97) | 12.0 (5.816.9) | |

A likelihood ratio test comparing the hierarchical model to a logistic model with patient factors only was highly significant (P 0.001), indicating that the hospital where the patient was treated had a major impact on receipt of PICC after accounting for patient factors. The MOR was 2.71, which is a larger effect than we found for any of the individual patient characteristics. The proportion of variance explained by hospitals was 25% (95% CI: 22%‐28%), as measured by the ICC.
DISCUSSION
In this study of 545,250 adults hospitalized with pneumonia, we found that approximately 8% of patients received a PICC. Patients who received PICCs had more comorbidities, were more frequently diagnosed with HCAP, and were more often admitted to the ICU, where they experienced greater rates of mechanical ventilation, noninvasive ventilation, and vasopressor use compared to those who did not receive a PICC. Additionally, risk‐adjusted rates of PICC use varied as much as 10‐fold across institutions. In fact, almost 70% of the total variation in rates of PICC use remained unexplained by hospital or patient characteristics. Although use of PICCs is often clinically nuanced in ways that are difficult to capture in large datasets (eg, difficult venous access or inability to tolerate oral medications), the substantial variation of PICC use observed suggests that physician and institutional practice styles are the major determinants of PICC placement during a hospitalization for pneumonia. Because PICCs are associated with serious complications, and evidence regarding discretionary use is accumulating, a research agenda examining reasons for such use and related outcomes appears necessary.
The placement of PICCs has grown substantially in hospitalized patients all over the world.[23, 24] Although originally developed for total parenteral nutrition in surgical patients,[25] contemporary reports of PICC use in critical illness,[26] diseases such as cystic fibrosis,[27] and even pregnancy[28] are now common. Although PICCs are clinically invaluable in many of these conditions, growing use of these devices has led to the realization that benefits may be offset by complications.[9, 10, 29, 30] Additionally, recent data suggest that not all PICCs may be used for appropriate reasons. For instance, in a decade‐long study at a tertiary care center, changes in patterns of PICC use including shortened dwell times, multiple insertions in a single patient, and unclear indications for use were reported.[11] In another study at an academic medical center, a substantial proportion of PICCs were found to be idle or unjustified.[12] It comes as little surprise, then, that a recent multicenter study found that 1 out of every 5 clinicians did not even know that their patient had a PICC.[29] Although calls to improve PICC use in the hospital setting have emerged, strategies to do so are limited by data that emanate from single‐center reports or retrospective designs. No other studies reporting use of PICCs across US hospitals for any clinical condition currently exist.[31]
We found that patients with weight loss, those with greater combined comorbidity scores, and those who were critically ill or diagnosed with sepsis were more likely to receive PICCs than others. These observations suggest that PICC use may reflect underlying severity of illness, as advanced care such as ventilator support was often associated with PICC use. Additionally, discharge to a skilled nursing facility was frequently associated with PICC placement, a finding consistent with a recent study evaluating the use of PICCs in these settings.[32] However, a substantial proportion of PICC use remained unexplained by available patient or hospital factors. Although our study was not specifically designed to examine this question, a possible reason may relate to unmeasured institutional factors that influence the propensity to use a PICC, recently termed as PICC culture.[33] For example, it is plausible that hospitals with nursing‐led PICC teams or interventional radiology (such as teaching hospitals) are more likely to use PICCs than those without such operators. This hypothesis may explain why urban, larger, and teaching hospitals exhibited higher rates of PICC use. Conversely, providers may have an affinity toward PICC use that is predicated not just by operator availability, but also local hospital norms. Understanding why some facilities use PICCs at higher rates than others and implications of such variation with respect to patient safety, cost, and outcomes is important. Study designs that use mixed‐methods approaches or seek to qualitatively understand reasons behind PICC use are likely to be valuable in this enquiry.
Our study has limitations. First, we used an administrative dataset and ICD‐9‐CM codes rather than clinical data from medical records to identify cases of pneumonia or comorbidities. Our estimates of PICC use across hospitals thus may not fully account for differences in severity of illness, and it is possible that patients needed a PICC for reasons that we could not observe. However, the substantial variation observed in rates of PICC use across hospitals is unlikely to be explained by differences in patient severity of illness, documentation, or coding practices. Second, as PICC removal codes were not available, we are unable to comment on how often hospitalized pneumonia patients were discharged with PICCs or received antimicrobial therapy beyond their inpatient stay. Third, although we observed that a number of patient and hospital factors were associated with PICC receipt, our study was not designed to determine the reasons underlying these patterns.
These limitations aside, our study has important strengths. To our knowledge, this is the first study to report utilization and outcomes associated with PICC use among those hospitalized with pneumonia across the United States. The inclusion of a large number of patients receiving care in diverse facilities lends a high degree of external validity to our findings. Second, we used advanced modeling to identify factors associated with PICC use in hospitalized patients with pneumonia, producing innovative and novel findings. Third, our study is the first to show the existence of substantial variation in rates of PICC use across US hospitals within the single disease state of pneumonia. Understanding the drivers of this variability is important as it may inform future studies, policies, and practices to improve PICC use in hospitalized patients.
In conclusion, we found that PICC use in patients hospitalized with pneumonia is common and highly variable. Future studies examining the contextual factors behind PICC use and their association with outcomes are needed to facilitate efforts to standardize PICC use across hospitals.
Disclosures
Dr. Chopra is supported by a career development award (1‐K08‐HS022835‐01) from the Agency of Healthcare Research and Quality. The authors report no conflicts of interest.
Pneumonia is the most common cause of unplanned hospitalization in the United States.[1] Despite its clinical toll, the management of this disease has evolved markedly. Expanding vaccination programs, efforts to improve timeliness of antibiotic therapy, and improved processes of care are but a few developments that have improved outcomes for patients afflicted with this illness.[2, 3]
Use of peripherally inserted central catheters (PICCs) is an example of a modern development in the management of patients with pneumonia.[4, 5, 6, 7] PICCs provide many of the benefits associated with central venous catheters (CVCs) including reliable venous access for delivery of antibiotics, phlebotomy, and invasive hemodynamic monitoring. However, as they are placed in veins of the upper extremity, PICCs bypass insertion risks (eg, injury to the carotid vessels or pneumothorax) associated with placement of traditional CVCs.[8] Because they offer durable venous access, PICCs also facilitate care transitions while continuing intravenous antimicrobial therapy in patients with pneumonia.
However, accumulating evidence also suggests that PICCs are associated with important complications, including central lineassociated bloodstream infectionand venous thromboembolism.[9, 10] Furthermore, knowledge gaps in clinicians regarding indications for appropriate use and management of complications associated with PICCs have been recognized.[10, 11] These elements are problematic because reports of unjustified and inappropriate PICC use are growing in the literature.[12, 13] Such concerns have prompted a number of policy calls to improve PICC use, including Choosing Wisely recommendations by various professional societies.[14, 15]
As little is known about the prevalence or patterns of PICC use in adults hospitalized with pneumonia, we conducted a retrospective cohort study using data from a large network of US hospitals.
METHODS
Setting and Participants
We included patients from hospitals that participated in Premier's inpatient dataset, a large, fee‐supported, multipayer administrative database that has been used extensively in health services research to measure quality of care and comparative effectiveness of interventions.[16] Participating hospitals represent all regions of the United States and include teaching and nonteaching facilities in rural and urban locations. In addition to variables found in the uniform billing form, the Premier inpatient database also includes a date‐stamped list of charges for procedures conducted during hospitalization such as PICC placement. As PICC‐specific data are not available in most nationally representative datasets, Premier offers unique insights into utilization, timing, and factors associated with use of PICCs in hospitalized settings.
We included adult patients aged 18 years who were (1) admitted with a principal diagnosis of pneumonia present on admission, or secondary diagnosis of pneumonia if paired with a principal diagnosis of sepsis, respiratory failure, or influenza; (2) received at least 1 day of antibiotics between July 1, 2007 and November 30, 2011, and (3) underwent chest x‐ray or computed tomography (CT) at the time of admission. International Classification of Disease, 9th Revision, Clinical Modification (ICD‐9‐CM) codes were used for patient selection. Patients who were not admitted (eg, observation cases), had cystic fibrosis, or marked as pneumonia not present on admission were excluded. For patients who had more than 1 hospitalization during the study period, a single admission was randomly selected for inclusion.
Patient, Physician, and Hospital Data
For all patients, age, gender, marital status, insurance, race, and ethnicity were captured. Using software provided by the Healthcare Costs and Utilization Project, we categorized information on 29 comorbid conditions and computed a combined comorbidity score as described by Gagne et al.[17] Patients were considered to have healthcare‐associated pneumonia (HCAP) if they were: (1) admitted from a skilled nursing or a long‐term care facility, (2) hospitalized in the previous 90 days, (3) on dialysis, or (4) receiving immunosuppressing medications (eg, chemotherapy or steroids equivalent to at least 20 mg of prednisone per day) at the time of admission. Information on specialty of the admitting physician and hospital characteristics (eg, size, location, teaching status) were sourced through Premier data.
Receipt of PICCs and Related Therapies
Among eligible adult patients hospitalized with pneumonia, we identified patients who received a PICC at any time during hospitalization via PICC‐specific billing codes. Non‐PICC devices (eg, midlines, Hickman catheters) were not included. For all insertions, we assessed day of PICC placement relative to admission date. Data on type of PICC (eg, power‐injection capable, antibiotic coating) or PICC characteristics (size, number of lumens) were not available. We used billing codes to assess use of invasive or noninvasive ventilation, vasopressors, and administration of pneumonia‐specific antibiotics (eg, ‐lactams, macrolides, fluoroquinolones). Early exposure was defined when a billing code appeared within 2 days of hospital admission.
Outcomes of Interest
The primary outcome of interest was receipt of a PICC. Additionally, we assessed factors associated with PICC placement and variation in risk‐standardized rates of PICC use between hospitals.
Statistical Analyses
Patient and hospital characteristics were summarized using frequencies for categorical variables and medians with interquartile ranges for continuous variables. We examined association of individual patient and hospital characteristics with use of PICCs using generalized estimating equations models with a logit link for categorical variables and identity link for continuous variables, accounting for patient clustering within hospitals.
| Characteristic | Total, No. (%) | No PICC, No. (%) | PICC, No. (%) | P Value* |
|---|---|---|---|---|
| ||||
| 545,250 (100) | 503,401 (92.3) | 41,849 (7.7) | ||
| Demographics | ||||
| Age, median (Q1Q3), y | 71 (5782) | 72 (5782) | 69 (5780) | 0.001 |
| Gender | 0.001 | |||
| Male | 256,448 (47.0) | 237,232 (47.1) | 19,216 (45.9) | |
| Female | 288,802 (53.0) | 266,169 (52.9) | 22,633 (54.1) | |
| Race/ethnicity | 0.001 | |||
| White | 377,255 (69.2) | 346,689 (68.9) | 30,566 (73.0) | |
| Black | 63,345 (11.6) | 58,407 (11.6) | 4,938 (11.8) | |
| Hispanic | 22,855 (4.2) | 21,716 (4.3) | 1,139 (2.7) | |
| Other | 81,795 (15.0) | 76,589 (15.2) | 5,206 (12.4) | |
| Admitting specialty | 0.001 | |||
| Internal medicine | 236,859 (43.4) | 218,689 (43.4) | 18,170 (43.4) | |
| Hospital medicine | 116,499 (21.4) | 107,671 (21.4) | 8,828 (21.1) | |
| Family practice | 80,388 (14.7) | 75,482 (15.0) | 4,906 (11.7) | |
| Critical care and pulmonary | 35,670 (6.5) | 30,529 (6.1) | 41,849 (12.3) | |
| Geriatrics | 4,812 (0.9) | 4,098 (0.8) | 714 (1.7) | |
| Other | 71,022 (13.0) | 66,932 (13.3) | 4,090 (9.8) | |
| Insurance | 0.001 | |||
| Medicare | 370,303 (67.9) | 341,379 (67.8) | 28,924 (69.1) | |
| Medicaid | 45,505 (8.3) | 41,100 (8.2) | 4,405 (10.5) | |
| Managed care | 69,984 (12.8) | 65,280 (13.0) | 4,704 (11.2) | |
| Commercialindemnity | 20,672 (3.8) | 19,251 (3.8) | 1,421 (3.4) | |
| Other | 38,786 (7.1) | 36,391 (7.2) | 2,395 (5.7) | |
| Comorbidities | ||||
| Gagne combined comorbidity score, median (Q1Q3) | 2 (15) | 2 (14) | 4 (26) | 0.001 |
| Hypertension | 332,347 (60.9) | 306,964 (61.0) | 25,383 (60.7) | 0.13 |
| Chronic pulmonary disease | 255,403 (46.8) | 234,619 (46.6) | 20,784 (49.7) | 0.001 |
| Diabetes | 171,247 (31.4) | 155,540 (30.9) | 15,707 (37.5) | 0.001 |
| Congestive heart failure | 146,492 (26.9) | 131,041 (26.0) | 15,451 (36.9) | 0.001 |
| Atrial fibrillation | 108,405 (19.9) | 97,124 (19.3) | 11,281 (27.0) | 0.001 |
| Renal failure | 104,404 (19.1) | 94,277 (18.7) | 10,127 (24.2) | 0.001 |
| Nicotine replacement therapy/tobacco use | 89,938 (16.5) | 83,247 (16.5) | 6,691 (16.0) | 0.001 |
| Obesity | 60,242 (11.0) | 53,268 (10.6) | 6,974 (16.7) | 0.001 |
| Coagulopathy | 41,717 (7.6) | 35,371 (7.0) | 6,346 (15.2) | 0.001 |
| Prior stroke (1 year) | 26,787 (4.9) | 24,046 (4.78) | 2,741 (6.55) | 0.001 |
| Metastatic cancer | 21,868 (4.0) | 20,244 (4.0) | 1,624 (3.9) | 0.16 |
| Solid tumor w/out metastasis | 21,083 (3.9) | 19,380 (3.8) | 1,703 (4.1) | 0.12 |
| Prior VTE (1 year) | 19,090 (3.5) | 16,906 (3.4) | 2,184 (5.2) | 0.001 |
| Chronic liver disease | 16,273 (3.0) | 14,207 (2.8) | 2,066 (4.9) | 0.001 |
| Prior bacteremia (1 year) | 4,106 (0.7) | 3,584 (0.7) | 522 (1.2) | 0.001 |
| Nephrotic syndrome | 671 (0.1) | 607 (0.1) | 64 (0.2) | 0.03 |
| Morbidity markers | ||||
| Type of pneumonia | 0.001 | |||
| CAP | 376,370 (69.1) | 352,900 (70.1) | 23,830 (56.9) | |
| HCAP | 168,520 (30.9) | 150,501 (29.9) | 18,019 (43.1) | |
| Sepsis present on admission | 114,578 (21.0) | 96,467 (19.2) | 18,111 (43.3) | 0.001 |
| Non‐invasive ventilation | 47,913(8.8) | 40,599 (8.1) | 7,314 (17.5) | 0.001 |
| Invasive mechanical ventilation | 56,179 (10.3) | 44,228 (8.8) | 11,951 (28.6) | 0.001 |
| ICU status | 97,703 (17.9) | 80,380 (16.0) | 17,323 (41.4) | 0.001 |
| Vasopressor use | 48,353 (8.9) | 38,030 (7.6) | 10,323 (24.7) | 0.001 |
| Antibiotic/medication use | ||||
| Anti‐MRSA agent (vancomycin) | 146,068 (26.8) | 123,327 (24.5) | 22,741 (54.3) | 0.001 |
| Third‐generation cephalosporin | 250,782 (46.0) | 235,556 (46.8) | 15,226 (36.4) | 0.001 |
| Anti‐Pseudomonal cephalosporin | 41,798 (7.7) | 36,982 (7.3) | 4,816 (11.5) | 0.001 |
| Anti‐Pseudomonal ‐lactam | 122,215 (22.4) | 105,741 (21.0) | 16,474 (39.4) | 0.001 |
| Fluroquinolone | 288,051 (52.8) | 267,131 (53.1) | 20,920 (50.0) | 0.001 |
| Macrolide | 223,737 (41.0) | 210,954 (41.9) | 12,783 (30.5) | 0.001 |
| Aminoglycoside | 15,415 (2.8) | 12,661 (2.5) | 2,754 (6.6) | 0.001 |
| Oral steroids | 44,486 (8.2) | 41,586 (8.3) | 2,900 (6.9) | 0.001 |
| Intravenous steroids | 146,308 (26.8) | 133,920 (26.6) | 12,388 (29.6) | 0.001 |
| VTE prophylaxis with LMWH | 190,735 (35.0) | 174,612 (34.7) | 16,123 (38.5) | 0.01 |
| Discharge disposition | ||||
| Home | 282,146 (51.7) | 272,604(54.1) | 9,542 (22.8) | 0.001 |
| Home with home health | 71,977 (13.2) | 65,289 (13.0) | 6,688 (16.0) | 0.001 |
| Skilled nursing facility | 111,541 (20.5) | 97,113 (19.3) | 14,428 (34.5) | 0.001 |
| Hospice | 20,428 (3.7) | 17,902 (3.6) | 2,526 (6.0) | 0.001 |
| Expired | 47,733 (8.7) | 40,768 (8.1) | 6,965 (16.6) | 0.001 |
| Other | 11,425 (2.1) | 9,725 (1.9) | 1,700 (4.1) | 0.001 |
We then developed a multivariable hierarchical generalized linear model (HGLM) for PICC placement with a random effect for hospital. In this model, we included patient demographics, comorbidities, sepsis on admission, type of pneumonia (eg, HCAP vs community‐associated pneumonia [CAP]), admitting physician specialty, and indicators for early receipt of specific treatments such as guideline‐recommended antibiotics, vasopressors, ventilation (invasive or noninvasive), and pneumatic compression devices for prophylaxis of deep vein thrombosis.
To understand and estimate between‐hospital variation in PICC use, we calculated risk‐standardized rates of PICC use (RSPICC) across hospitals using HGLM methods. These methods are also employed by the Centers for Medicare and Medicaid Services to calculate risk‐standardized measures for public reporting.[18] Because hospital rates of PICC use were highly skewed (21.2% [n = 105] of hospitals had no patients with PICCs), we restricted this model to the 343 hospitals that had at least 5 patients with a PICC to obtain stable estimates. For each hospital, we estimated a predicted rate of PICC use (pPICC) as the sum of predicted probabilities of PICC receipt from patient factors and the random intercept for hospital in which they were admitted. We then calculated an expected rate of PICC use (ePICC) per hospital as the sum of expected probabilities of PICC receipt from patient factors only. RSPICC for each hospital was then computed as the product of the overall unadjusted mean PICC rate (PICC) from all patients and the ratio of the predicted to expected PICC rate (uPICC*[pPICC/ePICC]).[19] Kruskal‐Wallis tests were used to evaluate the association between hospital characteristics with RSPICC rates. To evaluate the impact of the hospital in variation in PICC use, we assessed the change in likelihood ratio of a hierarchical model with hospital random effects compared to a logistic regression model with patient factors only. In addition, we estimated the intraclass correlation (ICC) to assess the proportion of variation in PICC use associated with the hospital, and the median odds ratio (MOR) from the hierarchical model. The MOR is the median of a set of odds ratios comparing 2 patients with the same set of characteristics treated at 2 randomly selected hospitals.[20, 21, 22] All analyses were performed using the Statistical Analysis System version 9.3 (SAS Institute, Inc., Cary, NC) and Stata 13 (StataCorp Inc., College Station, TX).
Ethical and Regulatory Oversight
Permission to conduct this study was obtained from the institutional review board at Baystate Medical Center, Springfield, Massachusetts. The study did not qualify as human subjects research and made use of fully deidentified data.
RESULTS
Between July 2007 and November 2011, 634,285 admissions representing 545,250 unique patients from 495 hospitals met eligibility criteria and were included in the study (Figure 1). Included patients had a median age of 71 years (interquartile range [IQR]: 5782), and 53.0% were female. Most patients were Caucasian (69.2%), unmarried (51.6%), and insured by Medicare (67.9%). Patients were admitted to the hospital by internal medicine providers (43.4%), hospitalists (21.4%), and family practice providers (14.7%); notably, critical care and pulmonary medicine providers admitted 6.5% of patients. The median Gagne comorbidity score was 2 (IQR: 15). Hypertension, chronic obstructive pulmonary disease, diabetes, and congestive heart failure were among the most common comorbidities observed (Table 1).

Among eligible patients, 41,849 (7.7%) received a PICC during hospitalization. Approximately a quarter of all patients who received PICCs did so by hospital day 2; 90% underwent insertion by hospital day 11 (mean = 5.4 days, median = 4 days). Patients who received PICCs were younger (median IQR: 69 years, 5780 years) but otherwise demographically similar to those that did not receive PICCs (median IQR: 72 years, 5782 years). Compared to other specialties, patients admitted by critical care/pulmonary providers were twice as likely to receive PICCs (12.3% vs 6.1%, P .001). Patients who received PICCs had higher comorbidity scores than those who did not (median Gagne comorbidity score 4 vs 2, P 0.001) and were more likely to be diagnosed with HCAP (43.1% vs 29.9%, P 0.001) than CAP (56.9% vs 70.1%, P 0.001).
PICC recipients were also more likely to receive intensive care unit (ICU) level of care (41.4% vs 16%, P 0.001) and both noninvasive (17.5% vs 8.1%, P 0.001) and invasive ventilation (28.6% vs 8.8%, P 0.001) upon admission. Vasopressor use was also significantly more frequent in patients who received PICCs (24.7% vs 7.6%, P 0.001) compared to those who did not receive these devices. Patients with PICCs were more often discharged to skilled nursing facilities (34.5% vs 19.3%) than those without PICCs.
Characteristics Associated With PICC Use Following Multivariable Modeling
Using HGLM with a random hospital effect, multiple patient characteristics were associated with PICC use (Table 2). Patients 65 years of age were less likely to receive a PICC compared to younger patients (odds ratio [OR]: 0.81, 95% confidence interval [CI]: 0.79‐0.84). Weight loss (OR: 2.03, 95% CI: 1.97‐2.10), sepsis on admission (OR: 1.80, 95% CI: 1.75‐1.85), and ICU status on hospital day 1 or 2 (OR: 1.70, 95% CI: 1.64‐1.75) represented 3 factors most strongly associated with PICC use.
| Patient Characteristic | Odds Ratio | 95% Confidence Intervals |
|---|---|---|
| ||
| Age group (>66 vs 65 years) | 0.82 | 0.790.84 |
| Race/ethnicity | ||
| Other | 1.02 | 0.971.06 |
| Black | 0.99 | 0.951.03 |
| Hispanic | 0.82 | 0.760.88 |
| White | Referent | |
| Marital status | ||
| Other/missing | 1.07 | 1.011.14 |
| Single | 1.02 | 1.001.05 |
| Married | Referent | |
| Insurance payor | ||
| Other | 0.85 | 0.800.89 |
| Medicaid | 1.13 | 1.081.18 |
| Managed care | 0.95 | 0.910.99 |
| Commercialindemnity | 0.93 | 0.871.00 |
| Medicare | Referent | |
| Admitting physician specialty | ||
| Pulmonary/critical care medicine | 1.18 | 1.131.24 |
| Family practice | 1.01 | 0.971.05 |
| Geriatric medicine (FP and IM) | 1.85 | 1.662.05 |
| Hospitalist | 0.94 | 0.910.98 |
| Other specialties | 1.02 | 0.971.06 |
| Internal medicine | Referent | |
| Comorbidities | ||
| Congestive heart failure | 1.27 | 1.241.31 |
| Valvular disease | 1.11 | 1.071.15 |
| Pulmonary circulation disorders | 1.37 | 1.321.42 |
| Peripheral vascular disease | 1.09 | 1.051.13 |
| Hypertension | 0.94 | 0.920.97 |
| Paralysis | 1.59 | 1.511.67 |
| Other neurological disorders | 1.20 | 1.161.23 |
| Chronic lung disease | 1.10 | 1.071.12 |
| Diabetes | 1.13 | 1.101.16 |
| Hypothyroidism | 1.03 | 1.001.06 |
| Liver disease | 1.16 | 1.101.23 |
| Ulcer | 1.86 | 1.153.02 |
| Lymphoma | 0.88 | 0.810.96 |
| Metastatic cancer | 0.75 | 0.710.80 |
| Solid tumor without metastasis | 0.93 | 0.880.98 |
| Arthritis | 1.22 | 1.161.28 |
| Obesity | 1.47 | 1.421.52 |
| Weight loss | 2.03 | 1.972.10 |
| Blood loss | 1.69 | 1.551.85 |
| Deficiency anemias | 1.40 | 1.371.44 |
| Alcohol abuse | 1.19 | 1.131.26 |
| Drug abuse | 1.31 | 1.231.39 |
| Psychoses | 1.16 | 1.111.21 |
| Depression | 1.10 | 1.061.13 |
| Renal failure | 0.96 | 0.930.98 |
| Type of pneumonia | ||
| HCAP | 1.03 | 1.011.06 |
| CAP | Referent | |
| Sepsis (POA) | 1.80 | 1.751.85 |
| Antibiotic exposure | ||
| Anti‐MRSA agent | 1.72 | 1.671.76 |
| Anti‐Pseudomonal carbapenem | 1.37 | 1.311.44 |
| Non‐Pseudomonal carbapenem | 1.48 | 1.331.66 |
| Third‐generation cephalosporin | 1.04 | 1.011.07 |
| Anti‐Pseudomonal cephalosporin | 1.25 | 1.201.30 |
| Anti‐Pseudomonal ‐lactam | 1.27 | 1.231.31 |
| Aztreonam | 1.31 | 1.231.40 |
| Non‐Pseudomonal ‐lactam | 1.36 | 1.231.50 |
| ‐lactam | 1.55 | 1.261.90 |
| Respiratory quinolone | 0.90 | 0.870.92 |
| Macrolide | 0.85 | 0.820.88 |
| Doxycycline | 0.94 | 0.871.01 |
| Aminoglycoside | 1.21 | 1.141.27 |
| Vasopressors | 1.06 | 1.031.10 |
| Noninvasive ventilation | 1.29 | 1.251.34 |
| Invasive ventilation | 1.66 | 1.611.72 |
| Intensive care unit on admission | 1.70 | 1.641.75 |
| Atrial fibrillation | 1.26 | 1.221.29 |
| Upper extremity chronic DVT | 1.61 | 1.132.28 |
| Nicotine replacement therapy/tobacco abuse | 0.91 | 0.880.94 |
| Aspirin | 0.94 | 0.920.97 |
| Warfarin | 0.90 | 0.860.94 |
| LMWH, prophylactic dose | 1.10 | 1.081.13 |
| LMWH, treatment dose | 1.22 | 1.161.29 |
| Intravenous steroids | 1.05 | 1.021.08 |
| Bacteremia (prior year) | 1.14 | 1.021.27 |
| VTE (prior year) | 1.11 | 1.061.18 |
| Pneumatic compression device | 1.25 | 1.081.45 |
| Invasive ventilation (prior year) | 1.17 | 1.111.24 |
| Irritable bowel disease | 1.19 | 1.051.36 |
Therapy with potent parenteral antimicrobials including antimethicillin‐resistant Staphylococcus aureus agents (OR: 1.72, 95% CI: 1.67‐1.76), antipseudomonal ‐lactamases (OR: 1.27, 95% CI: 1.23‐1.31), and carbapenems (OR: 1.37, 95% CI: 1.31‐1.44) were significantly associated with PICC use. Conversely, use of macrolides (OR: 0.85, 95% CI: 0.82‐0.88) or respiratory fluoroquinolones (OR: 0.90, 95% CI: 0.87‐0.92) were associated with lower likelihood of PICC use. After adjusting for antimicrobial therapy, HCAP was only slightly more likely to result in PICC use than CAP (OR: 1.03, 95% CI: 1.01‐1.06). Compared to internal medicine providers, admission by geriatricians and critical care/pulmonary specialists was associated with greater likelihood of PICC use (OR: 1.85, 95% CI: 1.66‐2.05 and OR: 1.18, 95% CI: =1.13‐1.24, respectively). Admission by hospitalists was associated with a modestly lower likelihood of PICC placement (OR: 0.94, 95% CI: 0.91‐0.98).
Hospital Level Variation in PICC Use
To ensure stable estimates of hospital PICC use, we excluded 152 facilities (31%): 10% had no patients with PICCs and 21% had 5 patients who received a PICC. Therefore, RSPICC was estimated for 343 of 495 facilities (69%) (Figure 2). In these facilities, RSPICC varied from 0.3% to 41.7%. Hospital RSPICC was significantly associated with hospital location (median 11.9% vs 7.8% for urban vs rural hospitals respectively, P = 0.05). RSPICCs were also greater among hospitals in Southern (11.3%), Western (12.7%), and Midwest (12.0%) regions of the nation compared to those in the Northeast (8.4%) (P = 0.02) (Table 3).
| Hospital Characteristic (No.) | Median (IQR), % | P Value |
|---|---|---|
| ||
| Bed size | 0.12 | |
| 200 beds (106) | 9.1 (4.816.3) | |
| 201 beds (237) | 11.6 (5.817.6) | |
| Rural/urban | 0.05 | |
| Urban (275) | 11.9 (5.517.4) | |
| Rural (68) | 7.8 (5.014.0) | |
| Region | 0.02 | |
| Northeast (50) | 8.4 (3.913.0) | |
| Midwest (69) | 12.0 (5.817.4) | |
| West (57) | 12.7 (7.617.0) | |
| South (167) | 11.3 (4.817.8) | |
| Teaching status | 0.77 | |
| Nonteaching (246) | 10.9 (5.017.4) | |
| Teaching (97) | 12.0 (5.816.9) | |

A likelihood ratio test comparing the hierarchical model to a logistic model with patient factors only was highly significant (P 0.001), indicating that the hospital where the patient was treated had a major impact on receipt of PICC after accounting for patient factors. The MOR was 2.71, which is a larger effect than we found for any of the individual patient characteristics. The proportion of variance explained by hospitals was 25% (95% CI: 22%‐28%), as measured by the ICC.
DISCUSSION
In this study of 545,250 adults hospitalized with pneumonia, we found that approximately 8% of patients received a PICC. Patients who received PICCs had more comorbidities, were more frequently diagnosed with HCAP, and were more often admitted to the ICU, where they experienced greater rates of mechanical ventilation, noninvasive ventilation, and vasopressor use compared to those who did not receive a PICC. Additionally, risk‐adjusted rates of PICC use varied as much as 10‐fold across institutions. In fact, almost 70% of the total variation in rates of PICC use remained unexplained by hospital or patient characteristics. Although use of PICCs is often clinically nuanced in ways that are difficult to capture in large datasets (eg, difficult venous access or inability to tolerate oral medications), the substantial variation of PICC use observed suggests that physician and institutional practice styles are the major determinants of PICC placement during a hospitalization for pneumonia. Because PICCs are associated with serious complications, and evidence regarding discretionary use is accumulating, a research agenda examining reasons for such use and related outcomes appears necessary.
The placement of PICCs has grown substantially in hospitalized patients all over the world.[23, 24] Although originally developed for total parenteral nutrition in surgical patients,[25] contemporary reports of PICC use in critical illness,[26] diseases such as cystic fibrosis,[27] and even pregnancy[28] are now common. Although PICCs are clinically invaluable in many of these conditions, growing use of these devices has led to the realization that benefits may be offset by complications.[9, 10, 29, 30] Additionally, recent data suggest that not all PICCs may be used for appropriate reasons. For instance, in a decade‐long study at a tertiary care center, changes in patterns of PICC use including shortened dwell times, multiple insertions in a single patient, and unclear indications for use were reported.[11] In another study at an academic medical center, a substantial proportion of PICCs were found to be idle or unjustified.[12] It comes as little surprise, then, that a recent multicenter study found that 1 out of every 5 clinicians did not even know that their patient had a PICC.[29] Although calls to improve PICC use in the hospital setting have emerged, strategies to do so are limited by data that emanate from single‐center reports or retrospective designs. No other studies reporting use of PICCs across US hospitals for any clinical condition currently exist.[31]
We found that patients with weight loss, those with greater combined comorbidity scores, and those who were critically ill or diagnosed with sepsis were more likely to receive PICCs than others. These observations suggest that PICC use may reflect underlying severity of illness, as advanced care such as ventilator support was often associated with PICC use. Additionally, discharge to a skilled nursing facility was frequently associated with PICC placement, a finding consistent with a recent study evaluating the use of PICCs in these settings.[32] However, a substantial proportion of PICC use remained unexplained by available patient or hospital factors. Although our study was not specifically designed to examine this question, a possible reason may relate to unmeasured institutional factors that influence the propensity to use a PICC, recently termed as PICC culture.[33] For example, it is plausible that hospitals with nursing‐led PICC teams or interventional radiology (such as teaching hospitals) are more likely to use PICCs than those without such operators. This hypothesis may explain why urban, larger, and teaching hospitals exhibited higher rates of PICC use. Conversely, providers may have an affinity toward PICC use that is predicated not just by operator availability, but also local hospital norms. Understanding why some facilities use PICCs at higher rates than others and implications of such variation with respect to patient safety, cost, and outcomes is important. Study designs that use mixed‐methods approaches or seek to qualitatively understand reasons behind PICC use are likely to be valuable in this enquiry.
Our study has limitations. First, we used an administrative dataset and ICD‐9‐CM codes rather than clinical data from medical records to identify cases of pneumonia or comorbidities. Our estimates of PICC use across hospitals thus may not fully account for differences in severity of illness, and it is possible that patients needed a PICC for reasons that we could not observe. However, the substantial variation observed in rates of PICC use across hospitals is unlikely to be explained by differences in patient severity of illness, documentation, or coding practices. Second, as PICC removal codes were not available, we are unable to comment on how often hospitalized pneumonia patients were discharged with PICCs or received antimicrobial therapy beyond their inpatient stay. Third, although we observed that a number of patient and hospital factors were associated with PICC receipt, our study was not designed to determine the reasons underlying these patterns.
These limitations aside, our study has important strengths. To our knowledge, this is the first study to report utilization and outcomes associated with PICC use among those hospitalized with pneumonia across the United States. The inclusion of a large number of patients receiving care in diverse facilities lends a high degree of external validity to our findings. Second, we used advanced modeling to identify factors associated with PICC use in hospitalized patients with pneumonia, producing innovative and novel findings. Third, our study is the first to show the existence of substantial variation in rates of PICC use across US hospitals within the single disease state of pneumonia. Understanding the drivers of this variability is important as it may inform future studies, policies, and practices to improve PICC use in hospitalized patients.
In conclusion, we found that PICC use in patients hospitalized with pneumonia is common and highly variable. Future studies examining the contextual factors behind PICC use and their association with outcomes are needed to facilitate efforts to standardize PICC use across hospitals.
Disclosures
Dr. Chopra is supported by a career development award (1‐K08‐HS022835‐01) from the Agency of Healthcare Research and Quality. The authors report no conflicts of interest.
- , . Reasons for being admitted to the hospital through the emergency department, 2003. Healthcare Cost and Utilization Project Statistical Brief 2. Rockville, MD: Agency for Healthcare Research and Quality. Available at: www.hcup‐us.ahrq.gov/reports/statbriefs/sb2.pdf. Published February 2006. Accessed June 27, 2014.
- , , , et al. National patterns of risk‐standardized mortality and readmission after hospitalization for acute myocardial infarction, heart failure, and pneumonia: update on publicly reported outcomes measures based on the 2013 release. J Gen Intern Med. 2014;29(10):1333–1340.
- , , , et al. Quality of care for elderly patients hospitalized for pneumonia in the United States, 2006 to 2010. JAMA Intern Med. 2014;174(11):1806–1814.
- , . PICC lines: the latest home care challenge. RN. 1990;53(1):44–51.
- , , , . Peripherally inserted central catheters in an acute‐care hospital. Arch Intern Med. 1994;154(16):1833–1837.
- , . The peripherally inserted central catheter: a retrospective look at three years of insertions. J Intraven Nurs. 1993;16(2):92–103.
- , , , . Peripherally inserted central catheters in general medicine. Mayo Clin Proc. 1997;72(3):225–233.
- , , . Two‐year trends of peripherally inserted central catheter‐line complications at a tertiary‐care hospital: role of nursing expertise. Infect Control Hosp Epidemiol. 2001;22(6):377–379.
- , , , , , . PICC‐associated bloodstream infections: prevalence, patterns, and predictors. Am J Med. 2014;127(4):319–328.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013;34(9):908–918.
- , , , , , . Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323–1331.
- , , , et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter”. Infect Control Hosp Epidemiol. 2012;33(1):50–57.
- , , , , . Inappropriate intravascular device use: a prospective study. Journal Hosp Infect. 2011;78(2):128–132.
- , , , et al. Enhancing patient‐centered care: SGIM and choosing wisely. J Gen Intern Med. 2014;29(3):432–433.
- , , , et al. Critical and honest conversations: the evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol. 2012;7(10):1664–1672.
- , , , et al. Using highly detailed administrative data to predict pneumonia mortality. PLoS One. 2014;9(1):e87382.
- , , , , . A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–759.
- , , , , . Hospitals with the highest intensive care utilization provide lower quality pneumonia care to the elderly. Crit Care Med. 2015;43(6):1178–1186.
- , . Statistical and clinical aspects of hospital outcomes profiling. Stat Sci. 2007;22(2):206–226.
- , . Appropriate assessment of neighborhood effects on individual health: integrating random and fixed effects in multilevel logistic regression. Am J Epidemiol. 2005;161(1):81–88.
- , , , . Interpreting parameters in the logistic regression model with random effects. Biometrics. 2000;56(3):909–914.
- , , , . Hospital‐level associations with 30‐day patient mortality after cardiac surgery: a tutorial on the application and interpretation of marginal and multilevel logistic regression. BMC Med Res Methodol. 2012;12:28.
- , , , . Experiences of the first PICC team in the Czech Republic. Br J Nurs. 2015;24(suppl 2):S4–S10.
- , , , et al. Greece reports prototype intervention with first peripherally inserted central catheter: case report and literature review. J Vasc Nurs. 2012;30(3):88–93.
- Total intravenous nutrition with peripherally inserted silicone elastomer central venous catheters. Arch Surg. 1975;110(5):644–646.
- , . Focus on peripherally inserted central catheters in critically ill patients. World J Crit Care Med. 2014;3(4):80–94.
- , , , et al. Quality improvement initiative to reduce deep vein thrombosis associated with peripherally inserted central catheters in adults with cystic fibrosis. Ann Am Thorac Soc. 2014;11(9):1404–1410.
- , , , . Peripherally Inserted central catheter (PICC) complications during pregnancy. JPEN J Parenter Enteral Nutr. 2013;38(5):595–601.
- , , , et al. Do clinicians know which of their patients have central venous catheters?: a multicenter observational study. Ann Intern Med. 2014;161(8):562–567.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
- , , , et al. Peripherally inserted central catheter use in skilled nursing facilities: a pilot study. J Am Geriatr Soc. 2015;63(9):1894–1899.
- , , , , . Inpatient venous access practices: PICC culture and the kidney patient. J Vasc Access. 2015;16(3):206–210.
- , . Reasons for being admitted to the hospital through the emergency department, 2003. Healthcare Cost and Utilization Project Statistical Brief 2. Rockville, MD: Agency for Healthcare Research and Quality. Available at: www.hcup‐us.ahrq.gov/reports/statbriefs/sb2.pdf. Published February 2006. Accessed June 27, 2014.
- , , , et al. National patterns of risk‐standardized mortality and readmission after hospitalization for acute myocardial infarction, heart failure, and pneumonia: update on publicly reported outcomes measures based on the 2013 release. J Gen Intern Med. 2014;29(10):1333–1340.
- , , , et al. Quality of care for elderly patients hospitalized for pneumonia in the United States, 2006 to 2010. JAMA Intern Med. 2014;174(11):1806–1814.
- , . PICC lines: the latest home care challenge. RN. 1990;53(1):44–51.
- , , , . Peripherally inserted central catheters in an acute‐care hospital. Arch Intern Med. 1994;154(16):1833–1837.
- , . The peripherally inserted central catheter: a retrospective look at three years of insertions. J Intraven Nurs. 1993;16(2):92–103.
- , , , . Peripherally inserted central catheters in general medicine. Mayo Clin Proc. 1997;72(3):225–233.
- , , . Two‐year trends of peripherally inserted central catheter‐line complications at a tertiary‐care hospital: role of nursing expertise. Infect Control Hosp Epidemiol. 2001;22(6):377–379.
- , , , , , . PICC‐associated bloodstream infections: prevalence, patterns, and predictors. Am J Med. 2014;127(4):319–328.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013;34(9):908–918.
- , , , , , . Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323–1331.
- , , , et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter”. Infect Control Hosp Epidemiol. 2012;33(1):50–57.
- , , , , . Inappropriate intravascular device use: a prospective study. Journal Hosp Infect. 2011;78(2):128–132.
- , , , et al. Enhancing patient‐centered care: SGIM and choosing wisely. J Gen Intern Med. 2014;29(3):432–433.
- , , , et al. Critical and honest conversations: the evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol. 2012;7(10):1664–1672.
- , , , et al. Using highly detailed administrative data to predict pneumonia mortality. PLoS One. 2014;9(1):e87382.
- , , , , . A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–759.
- , , , , . Hospitals with the highest intensive care utilization provide lower quality pneumonia care to the elderly. Crit Care Med. 2015;43(6):1178–1186.
- , . Statistical and clinical aspects of hospital outcomes profiling. Stat Sci. 2007;22(2):206–226.
- , . Appropriate assessment of neighborhood effects on individual health: integrating random and fixed effects in multilevel logistic regression. Am J Epidemiol. 2005;161(1):81–88.
- , , , . Interpreting parameters in the logistic regression model with random effects. Biometrics. 2000;56(3):909–914.
- , , , . Hospital‐level associations with 30‐day patient mortality after cardiac surgery: a tutorial on the application and interpretation of marginal and multilevel logistic regression. BMC Med Res Methodol. 2012;12:28.
- , , , . Experiences of the first PICC team in the Czech Republic. Br J Nurs. 2015;24(suppl 2):S4–S10.
- , , , et al. Greece reports prototype intervention with first peripherally inserted central catheter: case report and literature review. J Vasc Nurs. 2012;30(3):88–93.
- Total intravenous nutrition with peripherally inserted silicone elastomer central venous catheters. Arch Surg. 1975;110(5):644–646.
- , . Focus on peripherally inserted central catheters in critically ill patients. World J Crit Care Med. 2014;3(4):80–94.
- , , , et al. Quality improvement initiative to reduce deep vein thrombosis associated with peripherally inserted central catheters in adults with cystic fibrosis. Ann Am Thorac Soc. 2014;11(9):1404–1410.
- , , , . Peripherally Inserted central catheter (PICC) complications during pregnancy. JPEN J Parenter Enteral Nutr. 2013;38(5):595–601.
- , , , et al. Do clinicians know which of their patients have central venous catheters?: a multicenter observational study. Ann Intern Med. 2014;161(8):562–567.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
- , , , et al. Peripherally inserted central catheter use in skilled nursing facilities: a pilot study. J Am Geriatr Soc. 2015;63(9):1894–1899.
- , , , , . Inpatient venous access practices: PICC culture and the kidney patient. J Vasc Access. 2015;16(3):206–210.
Hospital Admission Service Structure
Hospital admission represents a time period during which patients are at risk for poor clinical outcomes. Although some risk is directly generated by illness pathophysiology, some additive risk is generated by the emergency department (ED)inpatient service handover inherent in the admission process.[1] Increased risk of suboptimal outcomes can result from ED overcrowding, which has been associated with increased mortality, difficulty in patient disposition, and delays in provision of care.[2] Inpatient bed occupancy, as well as availability and organization of accepting inpatient service healthcare staff, can affect ED overcrowding as well.[3, 4]
The overwhelming majority of hospitalist groups accept a significant portion of their admissions via the ED.[5] Hospitalist services must balance their daily group workload between ongoing care and discharge of inpatients and the activity of admitting new patients to their service. Two major models of admission processing exist for hospitalist groups to accomplish these competing tasks. One model, called the general model, employs the use of individual hospitalists to simultaneously perform admission activity as well as ongoing ward‐based care for inpatients during their workday. In the general model, a hospitalist who admits patients on their first hospital day will generally continue to see them on their second hospital day. The other model, called the admitter‐rounder model, divides the hospitalist daily group workflow between hospitalists who are assigned to perform only admission activity (admitters), and hospitalists who are assigned to perform only ongoing care for patients who are already admitted (rounders). In the admitter‐rounder model, the admitter on a patient's first hospital day will generally not serve as the patient's rounder on subsequent hospital days.
Limited evidence exists to guide hospitalist groups on which model their service design should adopt. Conflicting evidence exists as to whether the fragmentation of care generated by an admitter‐rounder admission model is beneficial or harmful.[6, 7, 8, 9] Increased availability of attending inpatient physicians during the EDinpatient admission process has been associated with improved hospital mortality and decreased readmissions in hospital settings outside the United States, where attending availability may otherwise be limited.[10, 11, 12] Separation of admission and rounding activity within a hospitalist workforce may allow each group of hospitalists to provide more timely and effective care related to their respective tasks. Our division implemented a change from a general model to an admitter‐rounder model of care on January 2, 2012. We hypothesized that changing from a general admission model to an admitter‐rounder model of care would be associated with a decreased rate of transfer to the intensive care unit (ICU) 24 hours after floor arrival and shortened ED length of stay (LOS), due to improved availability of hospitalists during the admission process. Due to the introduction of discontinuity, we hypothesized that adoption of the admitter‐rounder model would be associated with a prolongation of hospital LOS and no overall effect on 30 day postdischarge readmission rate. We sought to examine the relationship between our division's service design change and our hypothesized variables of interest.
METHODS
Setting and Study Design
We retrospectively evaluated electronic medical records of patients admitted between July 1, 2010 and June 30, 2013 from the ED to medical floor beds at Northwestern Memorial Hospital, an academic tertiary care teaching hospital located in Chicago, Illinois, under care of either a hospital medicine independent service or a medical teaching service. Admissions for care in observation units, service intake via interhospital or intrahospital transfers of care, or direct admissions from outpatient clinics that bypassed the ED were excluded, as was any patient with incomplete data, leaving 19,270 hospitalizations available for analysis. Each hospital medicine service was comprised of a single hospitalist with only clinical care responsibilities for the workday and no ICU or outpatient clinic responsibilities, with routine handover of the service to a hospitalist colleague every 7 days. Each medical teaching service was comprised of a supervising attending (often a hospitalist), a resident, 1 to 2 interns, and 1 to 3 medical students; the residents and interns maintained outpatient clinic responsibilities of 1 to 2 half days per service week. Inpatients on all teams were localized to hospital beds assigned to their care team. Regardless of hospitalist service design, 3 or more hospitalists were available each day to perform daytime admissions. Throughout the study period, both the hospital medicine and medicine teaching services utilized a group of physicians separate from the day teams to perform admissions and cross‐coverage at night, and the teaching services maintained a generalist model of daytime admission practice. All teams accepted new admissions every day. All ED admissions involved a phone‐based signout of transfer of care to the person admitting for the accepting ward team, followed by transfer of the patient to the floor, independent of whether the accepting team met the patient in the ED prior to transfer. None of the accepting inpatient services in the study had a formal right to refuse acceptance of patients referred for admission by the ED. The time period evaluated was constrained to avoid the effect of other service changes that took place before or after the study period. The Northwestern University Institutional Review Board approved the study (STU00087387).
Data Acquisition and Measures
Data were obtained from the Northwestern Memorial Hospital Enterprise Data Warehouse, an integrated repository of all clinical and research data for patients receiving care in the system. For analysis, the patients were separated into 4 groups: a prechange general admission hospitalist group (group 1), a postchange admitter‐rounder hospitalist group (group 2), and 2 teaching service control groups separated according to the prechange or postchange time period (groups 3 and 4, respectively). The primary outcome variable for the study was transfer of the patient to the ICU within 24 hours of inpatient floor arrival, which has been previously reported as an adverse outcome related to the admission process due to its association with increased inpatient mortality.[13] Secondary outcome variables included ED LOS, total hospital LOS, and readmission to Northwestern Memorial Hospital within 30 days of hospital discharge. Data on unexpected transfer to the operating room, discharge against medical advice (all within 24 hours of arrival to the ward), as well as mortality during the hospital stay were collected but not further analyzed due to the extremely low incidence of each. Covariables measured included each admitted patient's age, sex, race, Elixhauser composite score (a patient comorbidity score associated with inpatient mortality, described by van Walraven et al.[14]), case mix, insurance payer status, patient census on the accepting service for day 2 of the admitted patient's hospitalization, and hospital occupancy on the day of admission.[7, 14, 15, 16] Hospital occupancy was calculated as the sum of the number of beds occupied at midnight plus the number of patients discharged during the previous 24 hours, divided by the number of hospital beds, as defined by Forster et al.[16]
Statistical Analysis
Prestudy sample size calculation using an value of 0.05 and value of 0.2 to detect a 1.5% absolute difference in ICU transfer rate between postchange study groups, with a patient distribution ratio of 3.3:1 or higher between the admitter‐rounder and teaching postchange groups, and an assumed higher transfer rate in the teaching postchange group, revealed a requirement of at least 1068 hospitalizations in the teaching postchange group for our evaluation. Descriptive statistics were calculated for each patient group. Firth's logistic regressions were used to model the odds of patient being transfer to ICU within 24 hours after arrival and the odds of hospital readmission within 30 days after discharge, adjusting for confounders.[17] Quantile regressions were used to model the change in the median of ED LOS and the median of hospital LOS due to the right‐skewed distributions of LOS. Based on the clinical relevance to the outcomes, models were adjusted for patients' measured covariates. All covariates that were significant at = 0.05 level were considered significant. All statistical analyses were performed in SAS version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Patient Characteristics
The characteristics of the 4 patient populations are listed in Table 1. Compared to the general admission hospitalist group, the admitter‐rounder hospitalist group was more likely to be older (admitter‐rounder 61.9 19.0 vs 61.2 18.4, P = 0.03), a Medicare beneficiary (56.0% vs 52.9%, P 0.001), have a higher Elixhauser composite score (6.6 7.3 vs 5.3 6.7, P 0.001), and less likely to be white (46.5% vs 48.4%, P = 0.03). The teaching service patient characteristics changed over time only with regard to Elixhauser composite score (teaching postchange 6.4 7.3 vs 5.6 7.0, P 0.001); except for case mix, all other covariates did not change significantly between prechange and postchange teaching services. There was no significant difference in Elixhauser composite score between hospitalist and teaching services during the study period. Hospitalist groups were more likely than teaching service groups to have older patients, both before (hospitalist 61.2 18.4 vs teaching 60.1 19.1, P = 0.009) and after (hospitalist 61.9 18.0 vs teaching 60.0 18.6, P 0.001) the hospitalist admission system change. Compared to teaching groups, hospitalist groups were less likely to have female patients before the system change (hospitalist 52.3% vs 54.6%, P = 0.03), and more likely to have Medicare beneficiaries after the system change (hospitalist 56.0% vs 51.1%, P 0.001). Significant differences in case mix existed in all comparisons among all 4 study groups.
| Group 1 Hospitalist General, N = 8,465 | Group 2 Hospitalist Admitter‐Rounder, N = 6,291 | Group 3 Teaching Prechange, N = 2,636 | Group 4 Teaching Postchange, N = 1,878 | Group 2 vs Group 1, P Value | Group 4 vs Group 3, P Value | Group 1 vs Group 3, P Value | Group 2 vs Group 4, P Value | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Age, y, mean (SD) | 61.2 (18.4) | 61.9 (19.0) | 60.1 (19.1) | 60.0 (18.6) | 0.03 | 0.88 | 0.009 | 0.001 |
| Female sex, n (%) | 4,423 (52.3) | 3,298 (52.4) | 1,440 (54.6) | 1,031 (54.9) | 0.83 | 0.86 | 0.03 | 0.06 |
| White race, n (%) | 4,096 (48.4) | 2,927 (46.5) | 1,261 (47.8) | 880 (46.9) | 0.03 | 0.52 | 0.62 | 0.80 |
| Payer status | 0.001 | 0.001 | 0.07 | 0.001 | ||||
| Medicaid, n (%) | 1,121 (13.2) | 811 (12.9) | 393 (14.9) | 222 (11.8) | ||||
| Medicare, n (%) | 4,475 (52.9) | 3,521 (56.0) | 1,394 (52.9) | 961 (51.2) | ||||
| Private, n (%) | 2,218 (26.2) | 1,442 (22.9) | 674 (25.6) | 525 (28.0) | ||||
| Self‐pay, n (%) | 299 (3.5) | 273 (4.3) | 72 (2.7) | 88 (4.7) | ||||
| Other, n (%) | 352 (4.2) | 244 (3.9) | 103 (3.9) | 82 (4.4) | ||||
| Elixhauser composite score, mean (SD) | 5.3 (6.7) | 6.6 (7.3) | 5.6 (7.0) | 6.4 (7.3) | 0.001 | 0.007 | 0.05 | 0.30 |
| Inpatient mortality, n (%) | 74 (0.9) | 70 (1.1) | 31 (1.2) | 18 (1.0) | 0.14 | 0.51 | 0.15 | 0.62 |
| No. of patients seen by accepting service, mean (SD) | 10.2 (3.8) | 12.0 (3.1) | 6.3 (3.2) | 7.0 (3.3) | 0.001 | 0.001 | 0.001 | 0.001 |
| Hospital % occupancy at admission, mean (SD) | 1.23 (0.18) | 1.20 (0.17) | 1.23 (0.18) | 1.20 (0.17) | 0.001 | 0.001 | 0.61 | 0.43 |
| Case mix, n (%) | 0.001 | 0.001 | 0.001 | 0.001 | ||||
| Diseases of the circulatory system | 2,695 (31.8) | 1,173 (18.9) | 396 (15.0) | 292 (15.6) | ||||
| Other | 1,139 (13.5) | 1,151 (18.3) | 423 (16.1) | 292 (15.6) | ||||
| Diseases of the respiratory system | 883 (10.4) | 612 (9.7) | 314 (11.9) | 541 (28.9) | ||||
| Diseases of the digestive system | 923 (10.9) | 889 (14.1) | 420 (15.9) | 196 (10.4) | ||||
| Diseases of the genitourinary system | 492 (5.8) | 525 (8.4) | 230 (8.7) | 122 (6.5) | ||||
| Injury and poisoning | 517 (6.1) | 451 (7.2) | 182 (6.9) | 80 (4.3) | ||||
| Endocrine, nutritional, and metabolic diseases and immunity disorders | 473 (5.6) | 357 (5.7) | 194 (7.4) | 76 (4.1) | ||||
| Symptoms, signs, and ill‐defined conditions and factors influencing health status | 470 (5.6) | 267 (4.2) | 141 (5.4) | 63 (3.4) | ||||
| Diseases of the musculoskeletal system and connective tissue | 371 (4.4) | 281 (4.5) | 136 (5.1) | 58 (3.1) | ||||
| Infectious and parasitic diseases | 234 (2.8) | 288 (4.6) | 108 (4.1) | 98 (5.2) | ||||
| Diseases of the blood and blood‐forming organs | 268 (3.2) | 297 (4.7) | 92 (3.5) | 60 (3.2) | ||||
Impact of the Admission System on Outcomes
Measured unadjusted primary and secondary outcomes for the 4 study groups, as well as inpatient mortality, are listed in Table 2. Comparative odds ratios (ORs) for the outcomes of transfer to ICU 24 hours of floor arrival and readmission to hospital 30 days after discharge, median (50% quantile) regression results for the outcomes of ED and hospital LOS, each adjusted by all study covariates, as well as associated difference‐in‐difference parameter estimates with associated standard error (SE) ranges and P values, are listed in Table 3. Difference‐in‐difference analysis of outcomes associated with adoption of the hospitalist admitter‐rounder system compared to the time‐matched teaching service revealed no statistically significant difference in associated ICU transfer outcome between hospitalist or teaching services (admitter‐rounder OR difference of +0.22, SE 0.22, P = 0.32). A significant decrease in associated odds for hospital readmission 30 days postdischarge was noted when adoption of the hospitalist admitter‐rounder system was compared to the time‐matched teaching service (admitter‐rounder OR difference of 0.21, SE 0.08, P = 0.01). Adoption of the hospitalist admitter‐rounder system, compared to the time‐matched teaching service, was associated with a significant increase in ED LOS (admitter‐rounder difference of +0.49 hours, SE 0.09, P 0.001). Difference‐in‐difference analysis revealed no significant difference in associated hospital LOS between the hospitalist and time‐matched teaching services over the study period (admitter‐rounder difference of 0.39 hours, SE 2.44, P = 0.87).
|
Group 1, Hospitalist General, N = 8,465 |
Group 2, Hospitalist Admitter‐Rounder, N = 6,291 |
Group 3, Teaching Prechange, N = 2,636 |
Group 4. Teaching Postchange, N = 1,878 |
|
|---|---|---|---|---|
| ||||
| Transfer to ICU 24 hours after ward arrival, n (%) | 235 (2.8) | 139 (2.2) | 75 (2.9) | 59 (3.1) |
| Hospital readmission 30 days after discharge, n (%) | 1,924 (22.7) | 1,546 (24.6) | 608 (23.1) | 504 (26.8) |
| Emergency department length of stay, h | ||||
| Mean (SD) | 6.9 (3.36) | 7.39 (3.9) | 7.05 (2.98) | 6.89 (3.03) |
| Median [range] | 6.22 [0.2262.47] | 6.68 [0.62149.52] | 6.53 [1.9833.63] | 6.3 [2.0224.17] |
| Hospital length of stay, h | ||||
| Mean (SD) | 102.46 (120.14) | 125.94 (153.41) | 114.07 (165.62) | 122.89 (125.55) |
| Median [range] | 67.37 [0.521,964.07] | 88.18 [0.285,801.28] | 71.5 [4.575,131.37] | 88.08 [4.731,262.58] |
| Hospitalist Admitter‐Rounder vs Hospitalist General | Teaching Postchange vs Teaching Prechange | Difference‐in‐Difference Value Parameter Estimate [Standard Error], P Value | |
|---|---|---|---|
| |||
| Transfer to ICU 24 hours after floor arrival, OR (95% confidence interval) | 1.292 (1.0261.629) | 1.029 (0.7211.468) | OR: +0.22 [ 0.22], 0.32 |
| Hospital readmission 30 days after discharge, OR (95% confidence interval) | 1.048 (0.9661.136) | 1.298 (1.1271.495) | OR: 0.21 [ 0.08], 0.01 |
| Emergency department length of stay, median hours | +0.40 | 0.09 | +0.49 [ 0.09], 0.001 |
| Hospital length of stay, median hours | +12.96 | +13.36 | 0.39 [ 2.44], 0.87 |
DISCUSSION
Our observations were revealing for a statistically nonsignificant trend toward increased ICU transfers 24 hours after floor arrival after adoption of the admitter‐rounder model by the hospital medicine service. Despite prior publication of early transfer to the ICU being associated with adverse outcomes, including increased inpatient mortality, we observed no difference in mortality in our study groups.[13] We suspect that earlier transfer to the ICU in our study cohort may instead represent a protective action taken more frequently by admitting hospitalists in the admitter‐rounder model in response to provider discontinuity risks embedded in the admission process. Requests for transfer to the ICU at our institution require approval by the ICU team, and requests from attending hospitalists may be responded to differently from requests enacted by teaching team members, which as a factor also may account for some of the adjusted differences in transfer incidence. Taken together, increased availability of hospitalists during the admission process may result in earlier implementation of an overall lower threshold for implementation of ICU transfer. Our conclusion is limited by our study cohort's overall inpatient mortality rate, which is sufficiently low to preclude further assessment of the relationship of adverse outcomes with ICU transfer rate in our study groups. Therefore, clinical significance of our primary outcome findings, as well as the workload factors that impact ICU transfers initiated by hospitalist and teaching services, require further examination.
Despite a hypothesized increase in hospital LOS caused by additional discontinuity of hospitalist care in the admitter‐rounder model, adoption of the admitter‐rounder model was not associated with an increased hospital LOS. We suspect this finding may represent the presence of action(s) proximal to the admission process, on the part of either admission and/or rounding hospitalists, which decrease hospital LOS to a degree offsetting the expected LOS increase generated by provider discontinuity. Examples of such actions include more efficient testing or consultation, or improved detection of diagnostic errors.
Adoption of the admitter‐rounder model by the hospital medicine service was also associated with decreased hospital readmission rates compared to the time‐matched teaching service. We suspect that assignment of daily discharge and admission service activity to separate hospitalists in the admitter‐rounder model may allow more opportunity for rounder hospitalists to engage in activity protective against readmissions, such as greater direct engagement with postdischarge resources, or improved hospitalist availability for multidisciplinary inpatient efforts focused on discharge planning.
Adoption of the admitter‐rounder model was found to be associated with a median 29‐minute increase in ED LOS compared to the time‐matched teaching service. As a floor team member's physical presence in the ED was not required for ED‐floor transfer during the study period, increased physical availability of admitting hospitalists in the admitter‐rounder model may allow for increased opportunity for a hospitalist to disrupt ED‐specific workflows related to patient transfer (eg, disruption of transportation service activity by an earlier bedside visit from the admitting hospitalist). Hospitalists in the general model were allowed to leave after performing their daily duties, whereas admitting hospitalists in the admitter‐rounder model were assigned to stay for a timed shift, regardless of the completion of admissions; the difference in duty assignment may be associated with different hospitalist behaviors during the admission process. Improved ease for ED staff to contact hospitalist staff in the admitter‐rounder model may have led ED staff to prioritize other tasks more demanding of their continuous engagement at the expense of initiating admissions, thereby paradoxically delaying admissions to hospital medicine.
Other studies exist that attempt to describe changes in admission service structure, particularly with regard to housestaff admission activity in relation to changes in resident work hours. Many of these studies vary with regard to implementation of separate physician teams for day and night coverage, or are focused on a specific medical condition, thereby limiting their applicability to a hospital medicine service free of work‐hour restrictions and engaged in care of a wide variety of medical conditions.[18, 19, 20] In contrast, our study is an attempt to examine, in isolation, outcomes associated with adoption of an admitter‐rounder model of care as a specific discontinuity risk during the admission process, within the context of a stable system of night coverage in place for all medical teams engaged in admission activity of undifferentiated medical patients.
Limitations of our study include the inability to ascertain causality of observed outcomes, due to our observational study design. Our study was of a single hospital, which may limit applicability of our results to other hospital environments. However, the admission models examined in our study are common among hospital medicine groups. Clinically relevant outcome metrics, such as mortality and unexpected transfer to the operating room, were measured but of too low incidence to allow for further meaningful analysis. The clinical consequences and workflow practices that correlate with our study's findings likely require case review and time‐motion analyses, respectively, to further delineate the relevance of our findings; these analyses were outside of the scope of our study, and further investigation is required. In summary, our observations suggest that adoption by hospitalist services of an admitter‐rounder model of care for admissions is associated with a decreased rate of hospital readmission 30 days after discharge, with no effect on median hospital LOS, a statistically nonsignificant trend toward more ICU transfers in the first 24 hours of a patient's hospital stay, and a slight increase in median ED LOS.
Acknowledgements
This study was conducted with logistical support, software, and computer hardware provided by the Division of Hospital Medicine, Department of Medicine, Northwestern University Feinberg School of Medicine, and by the Biostatistics Collaboration Center, Northwestern University Feinberg School of Medicine.
Disclosure: Nothing to report.
- , , , et al. Residents' and attending physicians' handoffs: a systematic review of the literature. Acad Med. 2009;84(12):1775–1787.
- , , , et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16:1–10.
- , , , et al. Time series analysis of variables associated with daily mean emergency department length of stay. Ann Emerg Med. 2007;49:265–271.
- , , , et al. Active bed management by hospitalists and emergency department throughput. Ann Intern Med. 2008;149:804–810.
- Society of Hospital Medicine. 2014 state of hospital medicine report. 2014:22.
- , , , , . The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5:335–338.
- , , , et al. The effect of hospitalist discontinuity on adverse events. J Hosp Med. 2015;10:147–151.
- , , , . Liability impact of the hospitalist model of care. J Hosp Med. 2014;9:750–755.
- . Does continuity of care matter? No: discontinuity can improve patient care. West J Med. 2001;175(1):5.
- , , , , . Consultant input in acute medical admissions and patient outcomes in hospitals in England: a multivariate analysis. PLoS One. 2013;8(4):e61476.
- , , . Effectiveness of acute medical units in hospitals: a systematic review. Int J Qual Health Care. 2009;21(6):397–407.
- , , . Acute medicine in the United Kingdom: first‐hand perspectives on a parallel evolution of inpatient medical care. J Hosp Med. 2012:7(3);254–257.
- , , , et al. Adverse outcomes associated with delayed intensive care unit transfers in an integrated healthcare system. J Hosp Med. 2012;7(3):224–230.
- , , , , . A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626–633.
- , , , , . Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174(5):786–793.
- , , , , . The effect of hospital occupancy on emergency department length of stay and patient disposition. Acad Emerg Med. 2003;10(2):127–133.
- . Bias reduction of maximum likelihood estimates. Biometrika. 1993;80(1):27–38.
- , , , et al. Effect of the 2011 vs 2003 duty hour regulation‐compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff. JAMA Intern Med. 2013;173(8):649–655.
- , , , . Post‐call transfer of resident responsibility: Its effect on patient care. J Gen Intern Med. 1990;5:501–505.
- , , , et al. Effect of short call admission on length of stay and quality of care for acute decompensated heart failure. Circulation. 2008;117:2637–2644.
Hospital admission represents a time period during which patients are at risk for poor clinical outcomes. Although some risk is directly generated by illness pathophysiology, some additive risk is generated by the emergency department (ED)inpatient service handover inherent in the admission process.[1] Increased risk of suboptimal outcomes can result from ED overcrowding, which has been associated with increased mortality, difficulty in patient disposition, and delays in provision of care.[2] Inpatient bed occupancy, as well as availability and organization of accepting inpatient service healthcare staff, can affect ED overcrowding as well.[3, 4]
The overwhelming majority of hospitalist groups accept a significant portion of their admissions via the ED.[5] Hospitalist services must balance their daily group workload between ongoing care and discharge of inpatients and the activity of admitting new patients to their service. Two major models of admission processing exist for hospitalist groups to accomplish these competing tasks. One model, called the general model, employs the use of individual hospitalists to simultaneously perform admission activity as well as ongoing ward‐based care for inpatients during their workday. In the general model, a hospitalist who admits patients on their first hospital day will generally continue to see them on their second hospital day. The other model, called the admitter‐rounder model, divides the hospitalist daily group workflow between hospitalists who are assigned to perform only admission activity (admitters), and hospitalists who are assigned to perform only ongoing care for patients who are already admitted (rounders). In the admitter‐rounder model, the admitter on a patient's first hospital day will generally not serve as the patient's rounder on subsequent hospital days.
Limited evidence exists to guide hospitalist groups on which model their service design should adopt. Conflicting evidence exists as to whether the fragmentation of care generated by an admitter‐rounder admission model is beneficial or harmful.[6, 7, 8, 9] Increased availability of attending inpatient physicians during the EDinpatient admission process has been associated with improved hospital mortality and decreased readmissions in hospital settings outside the United States, where attending availability may otherwise be limited.[10, 11, 12] Separation of admission and rounding activity within a hospitalist workforce may allow each group of hospitalists to provide more timely and effective care related to their respective tasks. Our division implemented a change from a general model to an admitter‐rounder model of care on January 2, 2012. We hypothesized that changing from a general admission model to an admitter‐rounder model of care would be associated with a decreased rate of transfer to the intensive care unit (ICU) 24 hours after floor arrival and shortened ED length of stay (LOS), due to improved availability of hospitalists during the admission process. Due to the introduction of discontinuity, we hypothesized that adoption of the admitter‐rounder model would be associated with a prolongation of hospital LOS and no overall effect on 30 day postdischarge readmission rate. We sought to examine the relationship between our division's service design change and our hypothesized variables of interest.
METHODS
Setting and Study Design
We retrospectively evaluated electronic medical records of patients admitted between July 1, 2010 and June 30, 2013 from the ED to medical floor beds at Northwestern Memorial Hospital, an academic tertiary care teaching hospital located in Chicago, Illinois, under care of either a hospital medicine independent service or a medical teaching service. Admissions for care in observation units, service intake via interhospital or intrahospital transfers of care, or direct admissions from outpatient clinics that bypassed the ED were excluded, as was any patient with incomplete data, leaving 19,270 hospitalizations available for analysis. Each hospital medicine service was comprised of a single hospitalist with only clinical care responsibilities for the workday and no ICU or outpatient clinic responsibilities, with routine handover of the service to a hospitalist colleague every 7 days. Each medical teaching service was comprised of a supervising attending (often a hospitalist), a resident, 1 to 2 interns, and 1 to 3 medical students; the residents and interns maintained outpatient clinic responsibilities of 1 to 2 half days per service week. Inpatients on all teams were localized to hospital beds assigned to their care team. Regardless of hospitalist service design, 3 or more hospitalists were available each day to perform daytime admissions. Throughout the study period, both the hospital medicine and medicine teaching services utilized a group of physicians separate from the day teams to perform admissions and cross‐coverage at night, and the teaching services maintained a generalist model of daytime admission practice. All teams accepted new admissions every day. All ED admissions involved a phone‐based signout of transfer of care to the person admitting for the accepting ward team, followed by transfer of the patient to the floor, independent of whether the accepting team met the patient in the ED prior to transfer. None of the accepting inpatient services in the study had a formal right to refuse acceptance of patients referred for admission by the ED. The time period evaluated was constrained to avoid the effect of other service changes that took place before or after the study period. The Northwestern University Institutional Review Board approved the study (STU00087387).
Data Acquisition and Measures
Data were obtained from the Northwestern Memorial Hospital Enterprise Data Warehouse, an integrated repository of all clinical and research data for patients receiving care in the system. For analysis, the patients were separated into 4 groups: a prechange general admission hospitalist group (group 1), a postchange admitter‐rounder hospitalist group (group 2), and 2 teaching service control groups separated according to the prechange or postchange time period (groups 3 and 4, respectively). The primary outcome variable for the study was transfer of the patient to the ICU within 24 hours of inpatient floor arrival, which has been previously reported as an adverse outcome related to the admission process due to its association with increased inpatient mortality.[13] Secondary outcome variables included ED LOS, total hospital LOS, and readmission to Northwestern Memorial Hospital within 30 days of hospital discharge. Data on unexpected transfer to the operating room, discharge against medical advice (all within 24 hours of arrival to the ward), as well as mortality during the hospital stay were collected but not further analyzed due to the extremely low incidence of each. Covariables measured included each admitted patient's age, sex, race, Elixhauser composite score (a patient comorbidity score associated with inpatient mortality, described by van Walraven et al.[14]), case mix, insurance payer status, patient census on the accepting service for day 2 of the admitted patient's hospitalization, and hospital occupancy on the day of admission.[7, 14, 15, 16] Hospital occupancy was calculated as the sum of the number of beds occupied at midnight plus the number of patients discharged during the previous 24 hours, divided by the number of hospital beds, as defined by Forster et al.[16]
Statistical Analysis
Prestudy sample size calculation using an value of 0.05 and value of 0.2 to detect a 1.5% absolute difference in ICU transfer rate between postchange study groups, with a patient distribution ratio of 3.3:1 or higher between the admitter‐rounder and teaching postchange groups, and an assumed higher transfer rate in the teaching postchange group, revealed a requirement of at least 1068 hospitalizations in the teaching postchange group for our evaluation. Descriptive statistics were calculated for each patient group. Firth's logistic regressions were used to model the odds of patient being transfer to ICU within 24 hours after arrival and the odds of hospital readmission within 30 days after discharge, adjusting for confounders.[17] Quantile regressions were used to model the change in the median of ED LOS and the median of hospital LOS due to the right‐skewed distributions of LOS. Based on the clinical relevance to the outcomes, models were adjusted for patients' measured covariates. All covariates that were significant at = 0.05 level were considered significant. All statistical analyses were performed in SAS version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Patient Characteristics
The characteristics of the 4 patient populations are listed in Table 1. Compared to the general admission hospitalist group, the admitter‐rounder hospitalist group was more likely to be older (admitter‐rounder 61.9 19.0 vs 61.2 18.4, P = 0.03), a Medicare beneficiary (56.0% vs 52.9%, P 0.001), have a higher Elixhauser composite score (6.6 7.3 vs 5.3 6.7, P 0.001), and less likely to be white (46.5% vs 48.4%, P = 0.03). The teaching service patient characteristics changed over time only with regard to Elixhauser composite score (teaching postchange 6.4 7.3 vs 5.6 7.0, P 0.001); except for case mix, all other covariates did not change significantly between prechange and postchange teaching services. There was no significant difference in Elixhauser composite score between hospitalist and teaching services during the study period. Hospitalist groups were more likely than teaching service groups to have older patients, both before (hospitalist 61.2 18.4 vs teaching 60.1 19.1, P = 0.009) and after (hospitalist 61.9 18.0 vs teaching 60.0 18.6, P 0.001) the hospitalist admission system change. Compared to teaching groups, hospitalist groups were less likely to have female patients before the system change (hospitalist 52.3% vs 54.6%, P = 0.03), and more likely to have Medicare beneficiaries after the system change (hospitalist 56.0% vs 51.1%, P 0.001). Significant differences in case mix existed in all comparisons among all 4 study groups.
| Group 1 Hospitalist General, N = 8,465 | Group 2 Hospitalist Admitter‐Rounder, N = 6,291 | Group 3 Teaching Prechange, N = 2,636 | Group 4 Teaching Postchange, N = 1,878 | Group 2 vs Group 1, P Value | Group 4 vs Group 3, P Value | Group 1 vs Group 3, P Value | Group 2 vs Group 4, P Value | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Age, y, mean (SD) | 61.2 (18.4) | 61.9 (19.0) | 60.1 (19.1) | 60.0 (18.6) | 0.03 | 0.88 | 0.009 | 0.001 |
| Female sex, n (%) | 4,423 (52.3) | 3,298 (52.4) | 1,440 (54.6) | 1,031 (54.9) | 0.83 | 0.86 | 0.03 | 0.06 |
| White race, n (%) | 4,096 (48.4) | 2,927 (46.5) | 1,261 (47.8) | 880 (46.9) | 0.03 | 0.52 | 0.62 | 0.80 |
| Payer status | 0.001 | 0.001 | 0.07 | 0.001 | ||||
| Medicaid, n (%) | 1,121 (13.2) | 811 (12.9) | 393 (14.9) | 222 (11.8) | ||||
| Medicare, n (%) | 4,475 (52.9) | 3,521 (56.0) | 1,394 (52.9) | 961 (51.2) | ||||
| Private, n (%) | 2,218 (26.2) | 1,442 (22.9) | 674 (25.6) | 525 (28.0) | ||||
| Self‐pay, n (%) | 299 (3.5) | 273 (4.3) | 72 (2.7) | 88 (4.7) | ||||
| Other, n (%) | 352 (4.2) | 244 (3.9) | 103 (3.9) | 82 (4.4) | ||||
| Elixhauser composite score, mean (SD) | 5.3 (6.7) | 6.6 (7.3) | 5.6 (7.0) | 6.4 (7.3) | 0.001 | 0.007 | 0.05 | 0.30 |
| Inpatient mortality, n (%) | 74 (0.9) | 70 (1.1) | 31 (1.2) | 18 (1.0) | 0.14 | 0.51 | 0.15 | 0.62 |
| No. of patients seen by accepting service, mean (SD) | 10.2 (3.8) | 12.0 (3.1) | 6.3 (3.2) | 7.0 (3.3) | 0.001 | 0.001 | 0.001 | 0.001 |
| Hospital % occupancy at admission, mean (SD) | 1.23 (0.18) | 1.20 (0.17) | 1.23 (0.18) | 1.20 (0.17) | 0.001 | 0.001 | 0.61 | 0.43 |
| Case mix, n (%) | 0.001 | 0.001 | 0.001 | 0.001 | ||||
| Diseases of the circulatory system | 2,695 (31.8) | 1,173 (18.9) | 396 (15.0) | 292 (15.6) | ||||
| Other | 1,139 (13.5) | 1,151 (18.3) | 423 (16.1) | 292 (15.6) | ||||
| Diseases of the respiratory system | 883 (10.4) | 612 (9.7) | 314 (11.9) | 541 (28.9) | ||||
| Diseases of the digestive system | 923 (10.9) | 889 (14.1) | 420 (15.9) | 196 (10.4) | ||||
| Diseases of the genitourinary system | 492 (5.8) | 525 (8.4) | 230 (8.7) | 122 (6.5) | ||||
| Injury and poisoning | 517 (6.1) | 451 (7.2) | 182 (6.9) | 80 (4.3) | ||||
| Endocrine, nutritional, and metabolic diseases and immunity disorders | 473 (5.6) | 357 (5.7) | 194 (7.4) | 76 (4.1) | ||||
| Symptoms, signs, and ill‐defined conditions and factors influencing health status | 470 (5.6) | 267 (4.2) | 141 (5.4) | 63 (3.4) | ||||
| Diseases of the musculoskeletal system and connective tissue | 371 (4.4) | 281 (4.5) | 136 (5.1) | 58 (3.1) | ||||
| Infectious and parasitic diseases | 234 (2.8) | 288 (4.6) | 108 (4.1) | 98 (5.2) | ||||
| Diseases of the blood and blood‐forming organs | 268 (3.2) | 297 (4.7) | 92 (3.5) | 60 (3.2) | ||||
Impact of the Admission System on Outcomes
Measured unadjusted primary and secondary outcomes for the 4 study groups, as well as inpatient mortality, are listed in Table 2. Comparative odds ratios (ORs) for the outcomes of transfer to ICU 24 hours of floor arrival and readmission to hospital 30 days after discharge, median (50% quantile) regression results for the outcomes of ED and hospital LOS, each adjusted by all study covariates, as well as associated difference‐in‐difference parameter estimates with associated standard error (SE) ranges and P values, are listed in Table 3. Difference‐in‐difference analysis of outcomes associated with adoption of the hospitalist admitter‐rounder system compared to the time‐matched teaching service revealed no statistically significant difference in associated ICU transfer outcome between hospitalist or teaching services (admitter‐rounder OR difference of +0.22, SE 0.22, P = 0.32). A significant decrease in associated odds for hospital readmission 30 days postdischarge was noted when adoption of the hospitalist admitter‐rounder system was compared to the time‐matched teaching service (admitter‐rounder OR difference of 0.21, SE 0.08, P = 0.01). Adoption of the hospitalist admitter‐rounder system, compared to the time‐matched teaching service, was associated with a significant increase in ED LOS (admitter‐rounder difference of +0.49 hours, SE 0.09, P 0.001). Difference‐in‐difference analysis revealed no significant difference in associated hospital LOS between the hospitalist and time‐matched teaching services over the study period (admitter‐rounder difference of 0.39 hours, SE 2.44, P = 0.87).
|
Group 1, Hospitalist General, N = 8,465 |
Group 2, Hospitalist Admitter‐Rounder, N = 6,291 |
Group 3, Teaching Prechange, N = 2,636 |
Group 4. Teaching Postchange, N = 1,878 |
|
|---|---|---|---|---|
| ||||
| Transfer to ICU 24 hours after ward arrival, n (%) | 235 (2.8) | 139 (2.2) | 75 (2.9) | 59 (3.1) |
| Hospital readmission 30 days after discharge, n (%) | 1,924 (22.7) | 1,546 (24.6) | 608 (23.1) | 504 (26.8) |
| Emergency department length of stay, h | ||||
| Mean (SD) | 6.9 (3.36) | 7.39 (3.9) | 7.05 (2.98) | 6.89 (3.03) |
| Median [range] | 6.22 [0.2262.47] | 6.68 [0.62149.52] | 6.53 [1.9833.63] | 6.3 [2.0224.17] |
| Hospital length of stay, h | ||||
| Mean (SD) | 102.46 (120.14) | 125.94 (153.41) | 114.07 (165.62) | 122.89 (125.55) |
| Median [range] | 67.37 [0.521,964.07] | 88.18 [0.285,801.28] | 71.5 [4.575,131.37] | 88.08 [4.731,262.58] |
| Hospitalist Admitter‐Rounder vs Hospitalist General | Teaching Postchange vs Teaching Prechange | Difference‐in‐Difference Value Parameter Estimate [Standard Error], P Value | |
|---|---|---|---|
| |||
| Transfer to ICU 24 hours after floor arrival, OR (95% confidence interval) | 1.292 (1.0261.629) | 1.029 (0.7211.468) | OR: +0.22 [ 0.22], 0.32 |
| Hospital readmission 30 days after discharge, OR (95% confidence interval) | 1.048 (0.9661.136) | 1.298 (1.1271.495) | OR: 0.21 [ 0.08], 0.01 |
| Emergency department length of stay, median hours | +0.40 | 0.09 | +0.49 [ 0.09], 0.001 |
| Hospital length of stay, median hours | +12.96 | +13.36 | 0.39 [ 2.44], 0.87 |
DISCUSSION
Our observations were revealing for a statistically nonsignificant trend toward increased ICU transfers 24 hours after floor arrival after adoption of the admitter‐rounder model by the hospital medicine service. Despite prior publication of early transfer to the ICU being associated with adverse outcomes, including increased inpatient mortality, we observed no difference in mortality in our study groups.[13] We suspect that earlier transfer to the ICU in our study cohort may instead represent a protective action taken more frequently by admitting hospitalists in the admitter‐rounder model in response to provider discontinuity risks embedded in the admission process. Requests for transfer to the ICU at our institution require approval by the ICU team, and requests from attending hospitalists may be responded to differently from requests enacted by teaching team members, which as a factor also may account for some of the adjusted differences in transfer incidence. Taken together, increased availability of hospitalists during the admission process may result in earlier implementation of an overall lower threshold for implementation of ICU transfer. Our conclusion is limited by our study cohort's overall inpatient mortality rate, which is sufficiently low to preclude further assessment of the relationship of adverse outcomes with ICU transfer rate in our study groups. Therefore, clinical significance of our primary outcome findings, as well as the workload factors that impact ICU transfers initiated by hospitalist and teaching services, require further examination.
Despite a hypothesized increase in hospital LOS caused by additional discontinuity of hospitalist care in the admitter‐rounder model, adoption of the admitter‐rounder model was not associated with an increased hospital LOS. We suspect this finding may represent the presence of action(s) proximal to the admission process, on the part of either admission and/or rounding hospitalists, which decrease hospital LOS to a degree offsetting the expected LOS increase generated by provider discontinuity. Examples of such actions include more efficient testing or consultation, or improved detection of diagnostic errors.
Adoption of the admitter‐rounder model by the hospital medicine service was also associated with decreased hospital readmission rates compared to the time‐matched teaching service. We suspect that assignment of daily discharge and admission service activity to separate hospitalists in the admitter‐rounder model may allow more opportunity for rounder hospitalists to engage in activity protective against readmissions, such as greater direct engagement with postdischarge resources, or improved hospitalist availability for multidisciplinary inpatient efforts focused on discharge planning.
Adoption of the admitter‐rounder model was found to be associated with a median 29‐minute increase in ED LOS compared to the time‐matched teaching service. As a floor team member's physical presence in the ED was not required for ED‐floor transfer during the study period, increased physical availability of admitting hospitalists in the admitter‐rounder model may allow for increased opportunity for a hospitalist to disrupt ED‐specific workflows related to patient transfer (eg, disruption of transportation service activity by an earlier bedside visit from the admitting hospitalist). Hospitalists in the general model were allowed to leave after performing their daily duties, whereas admitting hospitalists in the admitter‐rounder model were assigned to stay for a timed shift, regardless of the completion of admissions; the difference in duty assignment may be associated with different hospitalist behaviors during the admission process. Improved ease for ED staff to contact hospitalist staff in the admitter‐rounder model may have led ED staff to prioritize other tasks more demanding of their continuous engagement at the expense of initiating admissions, thereby paradoxically delaying admissions to hospital medicine.
Other studies exist that attempt to describe changes in admission service structure, particularly with regard to housestaff admission activity in relation to changes in resident work hours. Many of these studies vary with regard to implementation of separate physician teams for day and night coverage, or are focused on a specific medical condition, thereby limiting their applicability to a hospital medicine service free of work‐hour restrictions and engaged in care of a wide variety of medical conditions.[18, 19, 20] In contrast, our study is an attempt to examine, in isolation, outcomes associated with adoption of an admitter‐rounder model of care as a specific discontinuity risk during the admission process, within the context of a stable system of night coverage in place for all medical teams engaged in admission activity of undifferentiated medical patients.
Limitations of our study include the inability to ascertain causality of observed outcomes, due to our observational study design. Our study was of a single hospital, which may limit applicability of our results to other hospital environments. However, the admission models examined in our study are common among hospital medicine groups. Clinically relevant outcome metrics, such as mortality and unexpected transfer to the operating room, were measured but of too low incidence to allow for further meaningful analysis. The clinical consequences and workflow practices that correlate with our study's findings likely require case review and time‐motion analyses, respectively, to further delineate the relevance of our findings; these analyses were outside of the scope of our study, and further investigation is required. In summary, our observations suggest that adoption by hospitalist services of an admitter‐rounder model of care for admissions is associated with a decreased rate of hospital readmission 30 days after discharge, with no effect on median hospital LOS, a statistically nonsignificant trend toward more ICU transfers in the first 24 hours of a patient's hospital stay, and a slight increase in median ED LOS.
Acknowledgements
This study was conducted with logistical support, software, and computer hardware provided by the Division of Hospital Medicine, Department of Medicine, Northwestern University Feinberg School of Medicine, and by the Biostatistics Collaboration Center, Northwestern University Feinberg School of Medicine.
Disclosure: Nothing to report.
Hospital admission represents a time period during which patients are at risk for poor clinical outcomes. Although some risk is directly generated by illness pathophysiology, some additive risk is generated by the emergency department (ED)inpatient service handover inherent in the admission process.[1] Increased risk of suboptimal outcomes can result from ED overcrowding, which has been associated with increased mortality, difficulty in patient disposition, and delays in provision of care.[2] Inpatient bed occupancy, as well as availability and organization of accepting inpatient service healthcare staff, can affect ED overcrowding as well.[3, 4]
The overwhelming majority of hospitalist groups accept a significant portion of their admissions via the ED.[5] Hospitalist services must balance their daily group workload between ongoing care and discharge of inpatients and the activity of admitting new patients to their service. Two major models of admission processing exist for hospitalist groups to accomplish these competing tasks. One model, called the general model, employs the use of individual hospitalists to simultaneously perform admission activity as well as ongoing ward‐based care for inpatients during their workday. In the general model, a hospitalist who admits patients on their first hospital day will generally continue to see them on their second hospital day. The other model, called the admitter‐rounder model, divides the hospitalist daily group workflow between hospitalists who are assigned to perform only admission activity (admitters), and hospitalists who are assigned to perform only ongoing care for patients who are already admitted (rounders). In the admitter‐rounder model, the admitter on a patient's first hospital day will generally not serve as the patient's rounder on subsequent hospital days.
Limited evidence exists to guide hospitalist groups on which model their service design should adopt. Conflicting evidence exists as to whether the fragmentation of care generated by an admitter‐rounder admission model is beneficial or harmful.[6, 7, 8, 9] Increased availability of attending inpatient physicians during the EDinpatient admission process has been associated with improved hospital mortality and decreased readmissions in hospital settings outside the United States, where attending availability may otherwise be limited.[10, 11, 12] Separation of admission and rounding activity within a hospitalist workforce may allow each group of hospitalists to provide more timely and effective care related to their respective tasks. Our division implemented a change from a general model to an admitter‐rounder model of care on January 2, 2012. We hypothesized that changing from a general admission model to an admitter‐rounder model of care would be associated with a decreased rate of transfer to the intensive care unit (ICU) 24 hours after floor arrival and shortened ED length of stay (LOS), due to improved availability of hospitalists during the admission process. Due to the introduction of discontinuity, we hypothesized that adoption of the admitter‐rounder model would be associated with a prolongation of hospital LOS and no overall effect on 30 day postdischarge readmission rate. We sought to examine the relationship between our division's service design change and our hypothesized variables of interest.
METHODS
Setting and Study Design
We retrospectively evaluated electronic medical records of patients admitted between July 1, 2010 and June 30, 2013 from the ED to medical floor beds at Northwestern Memorial Hospital, an academic tertiary care teaching hospital located in Chicago, Illinois, under care of either a hospital medicine independent service or a medical teaching service. Admissions for care in observation units, service intake via interhospital or intrahospital transfers of care, or direct admissions from outpatient clinics that bypassed the ED were excluded, as was any patient with incomplete data, leaving 19,270 hospitalizations available for analysis. Each hospital medicine service was comprised of a single hospitalist with only clinical care responsibilities for the workday and no ICU or outpatient clinic responsibilities, with routine handover of the service to a hospitalist colleague every 7 days. Each medical teaching service was comprised of a supervising attending (often a hospitalist), a resident, 1 to 2 interns, and 1 to 3 medical students; the residents and interns maintained outpatient clinic responsibilities of 1 to 2 half days per service week. Inpatients on all teams were localized to hospital beds assigned to their care team. Regardless of hospitalist service design, 3 or more hospitalists were available each day to perform daytime admissions. Throughout the study period, both the hospital medicine and medicine teaching services utilized a group of physicians separate from the day teams to perform admissions and cross‐coverage at night, and the teaching services maintained a generalist model of daytime admission practice. All teams accepted new admissions every day. All ED admissions involved a phone‐based signout of transfer of care to the person admitting for the accepting ward team, followed by transfer of the patient to the floor, independent of whether the accepting team met the patient in the ED prior to transfer. None of the accepting inpatient services in the study had a formal right to refuse acceptance of patients referred for admission by the ED. The time period evaluated was constrained to avoid the effect of other service changes that took place before or after the study period. The Northwestern University Institutional Review Board approved the study (STU00087387).
Data Acquisition and Measures
Data were obtained from the Northwestern Memorial Hospital Enterprise Data Warehouse, an integrated repository of all clinical and research data for patients receiving care in the system. For analysis, the patients were separated into 4 groups: a prechange general admission hospitalist group (group 1), a postchange admitter‐rounder hospitalist group (group 2), and 2 teaching service control groups separated according to the prechange or postchange time period (groups 3 and 4, respectively). The primary outcome variable for the study was transfer of the patient to the ICU within 24 hours of inpatient floor arrival, which has been previously reported as an adverse outcome related to the admission process due to its association with increased inpatient mortality.[13] Secondary outcome variables included ED LOS, total hospital LOS, and readmission to Northwestern Memorial Hospital within 30 days of hospital discharge. Data on unexpected transfer to the operating room, discharge against medical advice (all within 24 hours of arrival to the ward), as well as mortality during the hospital stay were collected but not further analyzed due to the extremely low incidence of each. Covariables measured included each admitted patient's age, sex, race, Elixhauser composite score (a patient comorbidity score associated with inpatient mortality, described by van Walraven et al.[14]), case mix, insurance payer status, patient census on the accepting service for day 2 of the admitted patient's hospitalization, and hospital occupancy on the day of admission.[7, 14, 15, 16] Hospital occupancy was calculated as the sum of the number of beds occupied at midnight plus the number of patients discharged during the previous 24 hours, divided by the number of hospital beds, as defined by Forster et al.[16]
Statistical Analysis
Prestudy sample size calculation using an value of 0.05 and value of 0.2 to detect a 1.5% absolute difference in ICU transfer rate between postchange study groups, with a patient distribution ratio of 3.3:1 or higher between the admitter‐rounder and teaching postchange groups, and an assumed higher transfer rate in the teaching postchange group, revealed a requirement of at least 1068 hospitalizations in the teaching postchange group for our evaluation. Descriptive statistics were calculated for each patient group. Firth's logistic regressions were used to model the odds of patient being transfer to ICU within 24 hours after arrival and the odds of hospital readmission within 30 days after discharge, adjusting for confounders.[17] Quantile regressions were used to model the change in the median of ED LOS and the median of hospital LOS due to the right‐skewed distributions of LOS. Based on the clinical relevance to the outcomes, models were adjusted for patients' measured covariates. All covariates that were significant at = 0.05 level were considered significant. All statistical analyses were performed in SAS version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Patient Characteristics
The characteristics of the 4 patient populations are listed in Table 1. Compared to the general admission hospitalist group, the admitter‐rounder hospitalist group was more likely to be older (admitter‐rounder 61.9 19.0 vs 61.2 18.4, P = 0.03), a Medicare beneficiary (56.0% vs 52.9%, P 0.001), have a higher Elixhauser composite score (6.6 7.3 vs 5.3 6.7, P 0.001), and less likely to be white (46.5% vs 48.4%, P = 0.03). The teaching service patient characteristics changed over time only with regard to Elixhauser composite score (teaching postchange 6.4 7.3 vs 5.6 7.0, P 0.001); except for case mix, all other covariates did not change significantly between prechange and postchange teaching services. There was no significant difference in Elixhauser composite score between hospitalist and teaching services during the study period. Hospitalist groups were more likely than teaching service groups to have older patients, both before (hospitalist 61.2 18.4 vs teaching 60.1 19.1, P = 0.009) and after (hospitalist 61.9 18.0 vs teaching 60.0 18.6, P 0.001) the hospitalist admission system change. Compared to teaching groups, hospitalist groups were less likely to have female patients before the system change (hospitalist 52.3% vs 54.6%, P = 0.03), and more likely to have Medicare beneficiaries after the system change (hospitalist 56.0% vs 51.1%, P 0.001). Significant differences in case mix existed in all comparisons among all 4 study groups.
| Group 1 Hospitalist General, N = 8,465 | Group 2 Hospitalist Admitter‐Rounder, N = 6,291 | Group 3 Teaching Prechange, N = 2,636 | Group 4 Teaching Postchange, N = 1,878 | Group 2 vs Group 1, P Value | Group 4 vs Group 3, P Value | Group 1 vs Group 3, P Value | Group 2 vs Group 4, P Value | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Age, y, mean (SD) | 61.2 (18.4) | 61.9 (19.0) | 60.1 (19.1) | 60.0 (18.6) | 0.03 | 0.88 | 0.009 | 0.001 |
| Female sex, n (%) | 4,423 (52.3) | 3,298 (52.4) | 1,440 (54.6) | 1,031 (54.9) | 0.83 | 0.86 | 0.03 | 0.06 |
| White race, n (%) | 4,096 (48.4) | 2,927 (46.5) | 1,261 (47.8) | 880 (46.9) | 0.03 | 0.52 | 0.62 | 0.80 |
| Payer status | 0.001 | 0.001 | 0.07 | 0.001 | ||||
| Medicaid, n (%) | 1,121 (13.2) | 811 (12.9) | 393 (14.9) | 222 (11.8) | ||||
| Medicare, n (%) | 4,475 (52.9) | 3,521 (56.0) | 1,394 (52.9) | 961 (51.2) | ||||
| Private, n (%) | 2,218 (26.2) | 1,442 (22.9) | 674 (25.6) | 525 (28.0) | ||||
| Self‐pay, n (%) | 299 (3.5) | 273 (4.3) | 72 (2.7) | 88 (4.7) | ||||
| Other, n (%) | 352 (4.2) | 244 (3.9) | 103 (3.9) | 82 (4.4) | ||||
| Elixhauser composite score, mean (SD) | 5.3 (6.7) | 6.6 (7.3) | 5.6 (7.0) | 6.4 (7.3) | 0.001 | 0.007 | 0.05 | 0.30 |
| Inpatient mortality, n (%) | 74 (0.9) | 70 (1.1) | 31 (1.2) | 18 (1.0) | 0.14 | 0.51 | 0.15 | 0.62 |
| No. of patients seen by accepting service, mean (SD) | 10.2 (3.8) | 12.0 (3.1) | 6.3 (3.2) | 7.0 (3.3) | 0.001 | 0.001 | 0.001 | 0.001 |
| Hospital % occupancy at admission, mean (SD) | 1.23 (0.18) | 1.20 (0.17) | 1.23 (0.18) | 1.20 (0.17) | 0.001 | 0.001 | 0.61 | 0.43 |
| Case mix, n (%) | 0.001 | 0.001 | 0.001 | 0.001 | ||||
| Diseases of the circulatory system | 2,695 (31.8) | 1,173 (18.9) | 396 (15.0) | 292 (15.6) | ||||
| Other | 1,139 (13.5) | 1,151 (18.3) | 423 (16.1) | 292 (15.6) | ||||
| Diseases of the respiratory system | 883 (10.4) | 612 (9.7) | 314 (11.9) | 541 (28.9) | ||||
| Diseases of the digestive system | 923 (10.9) | 889 (14.1) | 420 (15.9) | 196 (10.4) | ||||
| Diseases of the genitourinary system | 492 (5.8) | 525 (8.4) | 230 (8.7) | 122 (6.5) | ||||
| Injury and poisoning | 517 (6.1) | 451 (7.2) | 182 (6.9) | 80 (4.3) | ||||
| Endocrine, nutritional, and metabolic diseases and immunity disorders | 473 (5.6) | 357 (5.7) | 194 (7.4) | 76 (4.1) | ||||
| Symptoms, signs, and ill‐defined conditions and factors influencing health status | 470 (5.6) | 267 (4.2) | 141 (5.4) | 63 (3.4) | ||||
| Diseases of the musculoskeletal system and connective tissue | 371 (4.4) | 281 (4.5) | 136 (5.1) | 58 (3.1) | ||||
| Infectious and parasitic diseases | 234 (2.8) | 288 (4.6) | 108 (4.1) | 98 (5.2) | ||||
| Diseases of the blood and blood‐forming organs | 268 (3.2) | 297 (4.7) | 92 (3.5) | 60 (3.2) | ||||
Impact of the Admission System on Outcomes
Measured unadjusted primary and secondary outcomes for the 4 study groups, as well as inpatient mortality, are listed in Table 2. Comparative odds ratios (ORs) for the outcomes of transfer to ICU 24 hours of floor arrival and readmission to hospital 30 days after discharge, median (50% quantile) regression results for the outcomes of ED and hospital LOS, each adjusted by all study covariates, as well as associated difference‐in‐difference parameter estimates with associated standard error (SE) ranges and P values, are listed in Table 3. Difference‐in‐difference analysis of outcomes associated with adoption of the hospitalist admitter‐rounder system compared to the time‐matched teaching service revealed no statistically significant difference in associated ICU transfer outcome between hospitalist or teaching services (admitter‐rounder OR difference of +0.22, SE 0.22, P = 0.32). A significant decrease in associated odds for hospital readmission 30 days postdischarge was noted when adoption of the hospitalist admitter‐rounder system was compared to the time‐matched teaching service (admitter‐rounder OR difference of 0.21, SE 0.08, P = 0.01). Adoption of the hospitalist admitter‐rounder system, compared to the time‐matched teaching service, was associated with a significant increase in ED LOS (admitter‐rounder difference of +0.49 hours, SE 0.09, P 0.001). Difference‐in‐difference analysis revealed no significant difference in associated hospital LOS between the hospitalist and time‐matched teaching services over the study period (admitter‐rounder difference of 0.39 hours, SE 2.44, P = 0.87).
|
Group 1, Hospitalist General, N = 8,465 |
Group 2, Hospitalist Admitter‐Rounder, N = 6,291 |
Group 3, Teaching Prechange, N = 2,636 |
Group 4. Teaching Postchange, N = 1,878 |
|
|---|---|---|---|---|
| ||||
| Transfer to ICU 24 hours after ward arrival, n (%) | 235 (2.8) | 139 (2.2) | 75 (2.9) | 59 (3.1) |
| Hospital readmission 30 days after discharge, n (%) | 1,924 (22.7) | 1,546 (24.6) | 608 (23.1) | 504 (26.8) |
| Emergency department length of stay, h | ||||
| Mean (SD) | 6.9 (3.36) | 7.39 (3.9) | 7.05 (2.98) | 6.89 (3.03) |
| Median [range] | 6.22 [0.2262.47] | 6.68 [0.62149.52] | 6.53 [1.9833.63] | 6.3 [2.0224.17] |
| Hospital length of stay, h | ||||
| Mean (SD) | 102.46 (120.14) | 125.94 (153.41) | 114.07 (165.62) | 122.89 (125.55) |
| Median [range] | 67.37 [0.521,964.07] | 88.18 [0.285,801.28] | 71.5 [4.575,131.37] | 88.08 [4.731,262.58] |
| Hospitalist Admitter‐Rounder vs Hospitalist General | Teaching Postchange vs Teaching Prechange | Difference‐in‐Difference Value Parameter Estimate [Standard Error], P Value | |
|---|---|---|---|
| |||
| Transfer to ICU 24 hours after floor arrival, OR (95% confidence interval) | 1.292 (1.0261.629) | 1.029 (0.7211.468) | OR: +0.22 [ 0.22], 0.32 |
| Hospital readmission 30 days after discharge, OR (95% confidence interval) | 1.048 (0.9661.136) | 1.298 (1.1271.495) | OR: 0.21 [ 0.08], 0.01 |
| Emergency department length of stay, median hours | +0.40 | 0.09 | +0.49 [ 0.09], 0.001 |
| Hospital length of stay, median hours | +12.96 | +13.36 | 0.39 [ 2.44], 0.87 |
DISCUSSION
Our observations were revealing for a statistically nonsignificant trend toward increased ICU transfers 24 hours after floor arrival after adoption of the admitter‐rounder model by the hospital medicine service. Despite prior publication of early transfer to the ICU being associated with adverse outcomes, including increased inpatient mortality, we observed no difference in mortality in our study groups.[13] We suspect that earlier transfer to the ICU in our study cohort may instead represent a protective action taken more frequently by admitting hospitalists in the admitter‐rounder model in response to provider discontinuity risks embedded in the admission process. Requests for transfer to the ICU at our institution require approval by the ICU team, and requests from attending hospitalists may be responded to differently from requests enacted by teaching team members, which as a factor also may account for some of the adjusted differences in transfer incidence. Taken together, increased availability of hospitalists during the admission process may result in earlier implementation of an overall lower threshold for implementation of ICU transfer. Our conclusion is limited by our study cohort's overall inpatient mortality rate, which is sufficiently low to preclude further assessment of the relationship of adverse outcomes with ICU transfer rate in our study groups. Therefore, clinical significance of our primary outcome findings, as well as the workload factors that impact ICU transfers initiated by hospitalist and teaching services, require further examination.
Despite a hypothesized increase in hospital LOS caused by additional discontinuity of hospitalist care in the admitter‐rounder model, adoption of the admitter‐rounder model was not associated with an increased hospital LOS. We suspect this finding may represent the presence of action(s) proximal to the admission process, on the part of either admission and/or rounding hospitalists, which decrease hospital LOS to a degree offsetting the expected LOS increase generated by provider discontinuity. Examples of such actions include more efficient testing or consultation, or improved detection of diagnostic errors.
Adoption of the admitter‐rounder model by the hospital medicine service was also associated with decreased hospital readmission rates compared to the time‐matched teaching service. We suspect that assignment of daily discharge and admission service activity to separate hospitalists in the admitter‐rounder model may allow more opportunity for rounder hospitalists to engage in activity protective against readmissions, such as greater direct engagement with postdischarge resources, or improved hospitalist availability for multidisciplinary inpatient efforts focused on discharge planning.
Adoption of the admitter‐rounder model was found to be associated with a median 29‐minute increase in ED LOS compared to the time‐matched teaching service. As a floor team member's physical presence in the ED was not required for ED‐floor transfer during the study period, increased physical availability of admitting hospitalists in the admitter‐rounder model may allow for increased opportunity for a hospitalist to disrupt ED‐specific workflows related to patient transfer (eg, disruption of transportation service activity by an earlier bedside visit from the admitting hospitalist). Hospitalists in the general model were allowed to leave after performing their daily duties, whereas admitting hospitalists in the admitter‐rounder model were assigned to stay for a timed shift, regardless of the completion of admissions; the difference in duty assignment may be associated with different hospitalist behaviors during the admission process. Improved ease for ED staff to contact hospitalist staff in the admitter‐rounder model may have led ED staff to prioritize other tasks more demanding of their continuous engagement at the expense of initiating admissions, thereby paradoxically delaying admissions to hospital medicine.
Other studies exist that attempt to describe changes in admission service structure, particularly with regard to housestaff admission activity in relation to changes in resident work hours. Many of these studies vary with regard to implementation of separate physician teams for day and night coverage, or are focused on a specific medical condition, thereby limiting their applicability to a hospital medicine service free of work‐hour restrictions and engaged in care of a wide variety of medical conditions.[18, 19, 20] In contrast, our study is an attempt to examine, in isolation, outcomes associated with adoption of an admitter‐rounder model of care as a specific discontinuity risk during the admission process, within the context of a stable system of night coverage in place for all medical teams engaged in admission activity of undifferentiated medical patients.
Limitations of our study include the inability to ascertain causality of observed outcomes, due to our observational study design. Our study was of a single hospital, which may limit applicability of our results to other hospital environments. However, the admission models examined in our study are common among hospital medicine groups. Clinically relevant outcome metrics, such as mortality and unexpected transfer to the operating room, were measured but of too low incidence to allow for further meaningful analysis. The clinical consequences and workflow practices that correlate with our study's findings likely require case review and time‐motion analyses, respectively, to further delineate the relevance of our findings; these analyses were outside of the scope of our study, and further investigation is required. In summary, our observations suggest that adoption by hospitalist services of an admitter‐rounder model of care for admissions is associated with a decreased rate of hospital readmission 30 days after discharge, with no effect on median hospital LOS, a statistically nonsignificant trend toward more ICU transfers in the first 24 hours of a patient's hospital stay, and a slight increase in median ED LOS.
Acknowledgements
This study was conducted with logistical support, software, and computer hardware provided by the Division of Hospital Medicine, Department of Medicine, Northwestern University Feinberg School of Medicine, and by the Biostatistics Collaboration Center, Northwestern University Feinberg School of Medicine.
Disclosure: Nothing to report.
- , , , et al. Residents' and attending physicians' handoffs: a systematic review of the literature. Acad Med. 2009;84(12):1775–1787.
- , , , et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16:1–10.
- , , , et al. Time series analysis of variables associated with daily mean emergency department length of stay. Ann Emerg Med. 2007;49:265–271.
- , , , et al. Active bed management by hospitalists and emergency department throughput. Ann Intern Med. 2008;149:804–810.
- Society of Hospital Medicine. 2014 state of hospital medicine report. 2014:22.
- , , , , . The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5:335–338.
- , , , et al. The effect of hospitalist discontinuity on adverse events. J Hosp Med. 2015;10:147–151.
- , , , . Liability impact of the hospitalist model of care. J Hosp Med. 2014;9:750–755.
- . Does continuity of care matter? No: discontinuity can improve patient care. West J Med. 2001;175(1):5.
- , , , , . Consultant input in acute medical admissions and patient outcomes in hospitals in England: a multivariate analysis. PLoS One. 2013;8(4):e61476.
- , , . Effectiveness of acute medical units in hospitals: a systematic review. Int J Qual Health Care. 2009;21(6):397–407.
- , , . Acute medicine in the United Kingdom: first‐hand perspectives on a parallel evolution of inpatient medical care. J Hosp Med. 2012:7(3);254–257.
- , , , et al. Adverse outcomes associated with delayed intensive care unit transfers in an integrated healthcare system. J Hosp Med. 2012;7(3):224–230.
- , , , , . A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626–633.
- , , , , . Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174(5):786–793.
- , , , , . The effect of hospital occupancy on emergency department length of stay and patient disposition. Acad Emerg Med. 2003;10(2):127–133.
- . Bias reduction of maximum likelihood estimates. Biometrika. 1993;80(1):27–38.
- , , , et al. Effect of the 2011 vs 2003 duty hour regulation‐compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff. JAMA Intern Med. 2013;173(8):649–655.
- , , , . Post‐call transfer of resident responsibility: Its effect on patient care. J Gen Intern Med. 1990;5:501–505.
- , , , et al. Effect of short call admission on length of stay and quality of care for acute decompensated heart failure. Circulation. 2008;117:2637–2644.
- , , , et al. Residents' and attending physicians' handoffs: a systematic review of the literature. Acad Med. 2009;84(12):1775–1787.
- , , , et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16:1–10.
- , , , et al. Time series analysis of variables associated with daily mean emergency department length of stay. Ann Emerg Med. 2007;49:265–271.
- , , , et al. Active bed management by hospitalists and emergency department throughput. Ann Intern Med. 2008;149:804–810.
- Society of Hospital Medicine. 2014 state of hospital medicine report. 2014:22.
- , , , , . The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5:335–338.
- , , , et al. The effect of hospitalist discontinuity on adverse events. J Hosp Med. 2015;10:147–151.
- , , , . Liability impact of the hospitalist model of care. J Hosp Med. 2014;9:750–755.
- . Does continuity of care matter? No: discontinuity can improve patient care. West J Med. 2001;175(1):5.
- , , , , . Consultant input in acute medical admissions and patient outcomes in hospitals in England: a multivariate analysis. PLoS One. 2013;8(4):e61476.
- , , . Effectiveness of acute medical units in hospitals: a systematic review. Int J Qual Health Care. 2009;21(6):397–407.
- , , . Acute medicine in the United Kingdom: first‐hand perspectives on a parallel evolution of inpatient medical care. J Hosp Med. 2012:7(3);254–257.
- , , , et al. Adverse outcomes associated with delayed intensive care unit transfers in an integrated healthcare system. J Hosp Med. 2012;7(3):224–230.
- , , , , . A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626–633.
- , , , , . Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174(5):786–793.
- , , , , . The effect of hospital occupancy on emergency department length of stay and patient disposition. Acad Emerg Med. 2003;10(2):127–133.
- . Bias reduction of maximum likelihood estimates. Biometrika. 1993;80(1):27–38.
- , , , et al. Effect of the 2011 vs 2003 duty hour regulation‐compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff. JAMA Intern Med. 2013;173(8):649–655.
- , , , . Post‐call transfer of resident responsibility: Its effect on patient care. J Gen Intern Med. 1990;5:501–505.
- , , , et al. Effect of short call admission on length of stay and quality of care for acute decompensated heart failure. Circulation. 2008;117:2637–2644.
The Power of Quiet
In his insightful book “The Wisdom of Crowds” (New York: Anchor Books, 2004), James Surowiecke makes the convincing argument that many heads are wiser than one, even if that one is the sole expert regarding the subject under discussion. As long as the decision-making group is diverse, with each individual being allowed to come to an independent conclusion, this tenet appears to hold, whether the group is estimating the number of jelly beans in a jar or resolving a difficult issue. The message is clear: As a leader your leadership will be more effective if you solicit input from all members of your group, including those who may be reluctant to offer it.
In another excellent book, “Quiet” (New York: Crown Publishers, 2012), Susan Cain posits that, from early in the 20th century on, despite the considerable value it has to offer, introversion has become a “second-class personality trait.” Although highly valued earlier in our history, the thoughtful, introspective temperament was replaced by the aggressive, decisive character as the ideal.
Cain delves deeply into the substantial differences between extroverts and introverts, acknowledging that there are many gradations between the extremes. Extroverts tend to be loquacious and are seldom hesitant to offer their opinions on complex, difficult issues, even when their understanding of them is limited. They don’t always think before speaking and are less skilled listeners than introverts. They prefer to come to decisions rapidly, sometimes with incomplete data, and are much more decisive than introverts.
Introverts, on the other hand, prefer to listen rather than talk and to thoroughly vet an issue before reaching a decision. When they do, they are uncomfortable expressing it in a group setting. They prefer to work alone rather than in groups and, because of their thoughtful approach, their solutions to problems may be more innovative and sound than the shoot-from-the-hip, rapid answers that extroverts frequently propose. They abhor conflict and are likely to remain silent during controversy. In sum, although more difficult to elicit, obtaining input from the quiet members of the group is very worthwhile.
Often the most timely and ideal resolution is reached by balanced contributions from both personality types, the decision-making extroverts and the more thoughtful but reticent introverts. In fact, some of the best team members are those who are not on either extreme of the extrovert-introvert scale. But considering the fact that one-third to one-half of Americans are introverts (I suspect the fraction is a bit less among surgeons) and hesitant to offer their opinions in a group setting, how is this to be accomplished?
First, as a leader, you need to be sensitive to the fact that the introverts in your group are likely out of their comfort zone during communal meetings. It may even be embarrassing for them if they are called upon to offer their advice or opinion. To some degree this reluctance can be overcome by a leader who always attempts to reach consensus by valuing everyone’s opinion. Even the arrangement of the meeting room is important. The ideal is for all participants to be situated around a table rather than facing an imposing leader at the front of the room. This “leveling of the play field” emphasizes equality, de-emphasizes hierarchy, and encourages all to participate. The least likely to contribute can often be nudged from their quiet solitude by gentle urging from the leader with a statement such as: “Joe, I know you have a thoughtful perspective on this. Can you share it with the group?”
However, even the best-run meeting may not result in satisfactory resolution of difficult issues. In my experience, even those toward the extrovert end of the spectrum may be hesitant to offer their honest opinion in a meeting if it is in conflict with that of the leader. It is not uncommon to come to a consensus resolution of a controversial issue in a group meeting only to find out from hallway chatter that many disagree with the agreement reached. It is essential that the leader have access to this hallway chatter. This can be accomplished by way of confidantes who have the trust of both the troops and the leader.
During my years of leadership, a useful and productive technique I fostered to prompt input from introverts and honest assessments from all was to visit individual offices after the busy work day had quieted down, usually after 5 p.m. Meeting with individual faculty in their offices rather than in mine lent an informality to the conversation that could not be duplicated in the office of the chairman. In these one-on-one encounters, I found that even my relatively quiet faculty members felt comfortable in expressing their views regarding controversial issues facing our department. These informal chats also allowed me to become aware of problems they were facing in their professional and personal lives. They were great opportunities for mentoring and bonding as well. When these individual discussions precede what is anticipated to be a contentious group meeting, the likelihood of a successful conclusion is significantly enhanced.
Although my leadership experience was confined within the walls of academe, I believe these principles apply to anyone invited to lead a group in virtually any setting. Individual meetings are not an efficient way to lead, but they may provide a more effective and, in some cases, more rapid means of reaching consensus than innumerable group meetings with follow-up emails. When the group is too large to conference with everyone individually, one-on-one meetings with several key players may achieve the same result. During the process, don’t forget the quiet ones. They sometimes contribute the best and most innovative solutions to complex problems. There is power in quiet.
Dr. Rikkers is Editor in Chief of ACS Surgery News.
In his insightful book “The Wisdom of Crowds” (New York: Anchor Books, 2004), James Surowiecke makes the convincing argument that many heads are wiser than one, even if that one is the sole expert regarding the subject under discussion. As long as the decision-making group is diverse, with each individual being allowed to come to an independent conclusion, this tenet appears to hold, whether the group is estimating the number of jelly beans in a jar or resolving a difficult issue. The message is clear: As a leader your leadership will be more effective if you solicit input from all members of your group, including those who may be reluctant to offer it.
In another excellent book, “Quiet” (New York: Crown Publishers, 2012), Susan Cain posits that, from early in the 20th century on, despite the considerable value it has to offer, introversion has become a “second-class personality trait.” Although highly valued earlier in our history, the thoughtful, introspective temperament was replaced by the aggressive, decisive character as the ideal.
Cain delves deeply into the substantial differences between extroverts and introverts, acknowledging that there are many gradations between the extremes. Extroverts tend to be loquacious and are seldom hesitant to offer their opinions on complex, difficult issues, even when their understanding of them is limited. They don’t always think before speaking and are less skilled listeners than introverts. They prefer to come to decisions rapidly, sometimes with incomplete data, and are much more decisive than introverts.
Introverts, on the other hand, prefer to listen rather than talk and to thoroughly vet an issue before reaching a decision. When they do, they are uncomfortable expressing it in a group setting. They prefer to work alone rather than in groups and, because of their thoughtful approach, their solutions to problems may be more innovative and sound than the shoot-from-the-hip, rapid answers that extroverts frequently propose. They abhor conflict and are likely to remain silent during controversy. In sum, although more difficult to elicit, obtaining input from the quiet members of the group is very worthwhile.
Often the most timely and ideal resolution is reached by balanced contributions from both personality types, the decision-making extroverts and the more thoughtful but reticent introverts. In fact, some of the best team members are those who are not on either extreme of the extrovert-introvert scale. But considering the fact that one-third to one-half of Americans are introverts (I suspect the fraction is a bit less among surgeons) and hesitant to offer their opinions in a group setting, how is this to be accomplished?
First, as a leader, you need to be sensitive to the fact that the introverts in your group are likely out of their comfort zone during communal meetings. It may even be embarrassing for them if they are called upon to offer their advice or opinion. To some degree this reluctance can be overcome by a leader who always attempts to reach consensus by valuing everyone’s opinion. Even the arrangement of the meeting room is important. The ideal is for all participants to be situated around a table rather than facing an imposing leader at the front of the room. This “leveling of the play field” emphasizes equality, de-emphasizes hierarchy, and encourages all to participate. The least likely to contribute can often be nudged from their quiet solitude by gentle urging from the leader with a statement such as: “Joe, I know you have a thoughtful perspective on this. Can you share it with the group?”
However, even the best-run meeting may not result in satisfactory resolution of difficult issues. In my experience, even those toward the extrovert end of the spectrum may be hesitant to offer their honest opinion in a meeting if it is in conflict with that of the leader. It is not uncommon to come to a consensus resolution of a controversial issue in a group meeting only to find out from hallway chatter that many disagree with the agreement reached. It is essential that the leader have access to this hallway chatter. This can be accomplished by way of confidantes who have the trust of both the troops and the leader.
During my years of leadership, a useful and productive technique I fostered to prompt input from introverts and honest assessments from all was to visit individual offices after the busy work day had quieted down, usually after 5 p.m. Meeting with individual faculty in their offices rather than in mine lent an informality to the conversation that could not be duplicated in the office of the chairman. In these one-on-one encounters, I found that even my relatively quiet faculty members felt comfortable in expressing their views regarding controversial issues facing our department. These informal chats also allowed me to become aware of problems they were facing in their professional and personal lives. They were great opportunities for mentoring and bonding as well. When these individual discussions precede what is anticipated to be a contentious group meeting, the likelihood of a successful conclusion is significantly enhanced.
Although my leadership experience was confined within the walls of academe, I believe these principles apply to anyone invited to lead a group in virtually any setting. Individual meetings are not an efficient way to lead, but they may provide a more effective and, in some cases, more rapid means of reaching consensus than innumerable group meetings with follow-up emails. When the group is too large to conference with everyone individually, one-on-one meetings with several key players may achieve the same result. During the process, don’t forget the quiet ones. They sometimes contribute the best and most innovative solutions to complex problems. There is power in quiet.
Dr. Rikkers is Editor in Chief of ACS Surgery News.
In his insightful book “The Wisdom of Crowds” (New York: Anchor Books, 2004), James Surowiecke makes the convincing argument that many heads are wiser than one, even if that one is the sole expert regarding the subject under discussion. As long as the decision-making group is diverse, with each individual being allowed to come to an independent conclusion, this tenet appears to hold, whether the group is estimating the number of jelly beans in a jar or resolving a difficult issue. The message is clear: As a leader your leadership will be more effective if you solicit input from all members of your group, including those who may be reluctant to offer it.
In another excellent book, “Quiet” (New York: Crown Publishers, 2012), Susan Cain posits that, from early in the 20th century on, despite the considerable value it has to offer, introversion has become a “second-class personality trait.” Although highly valued earlier in our history, the thoughtful, introspective temperament was replaced by the aggressive, decisive character as the ideal.
Cain delves deeply into the substantial differences between extroverts and introverts, acknowledging that there are many gradations between the extremes. Extroverts tend to be loquacious and are seldom hesitant to offer their opinions on complex, difficult issues, even when their understanding of them is limited. They don’t always think before speaking and are less skilled listeners than introverts. They prefer to come to decisions rapidly, sometimes with incomplete data, and are much more decisive than introverts.
Introverts, on the other hand, prefer to listen rather than talk and to thoroughly vet an issue before reaching a decision. When they do, they are uncomfortable expressing it in a group setting. They prefer to work alone rather than in groups and, because of their thoughtful approach, their solutions to problems may be more innovative and sound than the shoot-from-the-hip, rapid answers that extroverts frequently propose. They abhor conflict and are likely to remain silent during controversy. In sum, although more difficult to elicit, obtaining input from the quiet members of the group is very worthwhile.
Often the most timely and ideal resolution is reached by balanced contributions from both personality types, the decision-making extroverts and the more thoughtful but reticent introverts. In fact, some of the best team members are those who are not on either extreme of the extrovert-introvert scale. But considering the fact that one-third to one-half of Americans are introverts (I suspect the fraction is a bit less among surgeons) and hesitant to offer their opinions in a group setting, how is this to be accomplished?
First, as a leader, you need to be sensitive to the fact that the introverts in your group are likely out of their comfort zone during communal meetings. It may even be embarrassing for them if they are called upon to offer their advice or opinion. To some degree this reluctance can be overcome by a leader who always attempts to reach consensus by valuing everyone’s opinion. Even the arrangement of the meeting room is important. The ideal is for all participants to be situated around a table rather than facing an imposing leader at the front of the room. This “leveling of the play field” emphasizes equality, de-emphasizes hierarchy, and encourages all to participate. The least likely to contribute can often be nudged from their quiet solitude by gentle urging from the leader with a statement such as: “Joe, I know you have a thoughtful perspective on this. Can you share it with the group?”
However, even the best-run meeting may not result in satisfactory resolution of difficult issues. In my experience, even those toward the extrovert end of the spectrum may be hesitant to offer their honest opinion in a meeting if it is in conflict with that of the leader. It is not uncommon to come to a consensus resolution of a controversial issue in a group meeting only to find out from hallway chatter that many disagree with the agreement reached. It is essential that the leader have access to this hallway chatter. This can be accomplished by way of confidantes who have the trust of both the troops and the leader.
During my years of leadership, a useful and productive technique I fostered to prompt input from introverts and honest assessments from all was to visit individual offices after the busy work day had quieted down, usually after 5 p.m. Meeting with individual faculty in their offices rather than in mine lent an informality to the conversation that could not be duplicated in the office of the chairman. In these one-on-one encounters, I found that even my relatively quiet faculty members felt comfortable in expressing their views regarding controversial issues facing our department. These informal chats also allowed me to become aware of problems they were facing in their professional and personal lives. They were great opportunities for mentoring and bonding as well. When these individual discussions precede what is anticipated to be a contentious group meeting, the likelihood of a successful conclusion is significantly enhanced.
Although my leadership experience was confined within the walls of academe, I believe these principles apply to anyone invited to lead a group in virtually any setting. Individual meetings are not an efficient way to lead, but they may provide a more effective and, in some cases, more rapid means of reaching consensus than innumerable group meetings with follow-up emails. When the group is too large to conference with everyone individually, one-on-one meetings with several key players may achieve the same result. During the process, don’t forget the quiet ones. They sometimes contribute the best and most innovative solutions to complex problems. There is power in quiet.
Dr. Rikkers is Editor in Chief of ACS Surgery News.
From the Washington Office: A guide to in-district meetings with your representatives and senators
WHY should surgeons take time out of their busy schedules to meet with legislators?
To become an effective surgeon advocate, nothing is more important than establishing a personal relationship with your legislators. Conversely, to a legislator, there is nothing more valuable than the input and support of constituents. After all, constituents are VOTERS. Meeting with policy makers and/or their staff is extremely valuable in advancing the overall advocacy agenda of The American College of Surgeons and provides surgeons with the opportunity to develop key contacts in the offices of their legislators.
WHERE do such meetings take place?
All U.S. Representatives and Senators have one or more offices for constituent service in their home districts or states. These offices serve as a readily accessible meeting point. As an alternative, legislators frequently will schedule meetings with constituents in mutually convenient locations such as a coffee shop, or during a local legislative event such as a town hall.
WHEN is it most feasible to schedule in-district meetings?
You might be surprised to discover how much time is allotted by both the House and Senate for in-district work periods. Typical times include periods around President’s Day in February, Easter/Passover in March/April, Memorial Day, Independence Day, and summer recess (late July and the month of August). If Congress does not officially adjourn in early October, additional work periods include time around Columbus Day in October, Veteran’s Day in November, and Thanksgiving. Congress will usually adjourn for the year in December. A specific schedule for each legislative body for the year 2016 can be found at:
House of Representatives: http://www.majorityleader.gov/wp-content/uploads/2011/07/2016_ANNUAL_CALENDAR.pdf
Senate: http://www.senate.gov/legislative/resources/pdf/2016_calendar.pdf
HOW does one schedule an in-district meeting?
To set up a meeting you should first search the websites of your representatives (www.house.gov) and senators (www.senate.gov) for information as to the preferred scheduling procedures. Expect each office’s procedure to be a bit different. You will be asked to provide your name, address, and basic contact information as well as to briefly describe what issue(s) you wish to discuss. Be sure to mention that you are a surgeon and also whether you have previously met with the representative or senator.
If several days pass and staff from the office have not followed up, you should not hesitate to call or contact the office again. Remember, persistence is key! Keep in mind that legislators typically maintain busy schedules during the in-district work period and accordingly, the scheduled appointment time will be brief and subject to change, perhaps on short notice.
If you experience difficulty or simply would like to have assistance in scheduling an in-district meeting, staff in the ACS Division of Advocacy and Health Policy are available to assist and may be contacted by e-mail at surgeonsvoice.org.
WHAT should one discuss?
As a surgeon advocate, your most powerful tool is frequent contact and meetings with your elected officials. Meetings provide an opportunity to offer knowledge and perspective to educate legislators on key topics important to ensuring access to quality surgical care. Your personal experience brings a personal, human touch to issues about which legislators only have knowledge based upon raw numbers and impersonal policy jargon. Most legislators, as well as their staff, will be grateful to have the reliable resource of a constituent’s experience and perspective on complicated medical issues.
To maximize the opportunity for a successful meeting and thereby lay the foundation for the development of a mutually beneficial future relationship, I would offer the following three tips:
1) KNOW YOUR LEGISLATOR: Visit your legislators’ websites, read their biographies, ascertain to what congressional committees they are assigned, and what leadership roles they may have. All of this serves to help determine what issues are important to them and what positions they have previously taken on such issues.
2) KNOW YOUR ISSUE and be able to FRAME IT: Nothing substitutes for a solid knowledge base of the issue and the position you are trying to convey. Be focused and resist the temptation to try to cover too many topics in any one visit. When presenting your argument, “frame it” in layman’s terms much as you would explain it to a patient. Including examples of real-life, anecdotal experiences demonstrating how the status quo or the proposed legislation (depending upon the circumstance) is impacting providers and patients is particularly important.
3) HAVE AN ASK: It is imperative that you always be clear with your legislators about what you want them to do. This serves to reinforce the importance of your having taken time out of your schedule to communicate with them and also serves to hold the legislator accountable. “Asks” can be as specific as a request to cosponsor and support legislation or simply making the offer to serve as a resource to them as a constituent with expertise in health care.
Lastly, I would respectfully request that when surgeons meet with their legislators they inform the ACS Division of Advocacy and Health Policy. Having basic information about the outcome of the meeting, whether knowing that the legislator committed to taking a specific action or knowing that the legislator has requested additional information, is incredibly valuable to us in our ongoing advocacy efforts on behalf of surgeons and their patients here in Washington, DC.
Until next month ….
Dr. Patrick V. Bailey is an ACS Fellow, a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy, in the ACS offices in Washington, D.C.
WHY should surgeons take time out of their busy schedules to meet with legislators?
To become an effective surgeon advocate, nothing is more important than establishing a personal relationship with your legislators. Conversely, to a legislator, there is nothing more valuable than the input and support of constituents. After all, constituents are VOTERS. Meeting with policy makers and/or their staff is extremely valuable in advancing the overall advocacy agenda of The American College of Surgeons and provides surgeons with the opportunity to develop key contacts in the offices of their legislators.
WHERE do such meetings take place?
All U.S. Representatives and Senators have one or more offices for constituent service in their home districts or states. These offices serve as a readily accessible meeting point. As an alternative, legislators frequently will schedule meetings with constituents in mutually convenient locations such as a coffee shop, or during a local legislative event such as a town hall.
WHEN is it most feasible to schedule in-district meetings?
You might be surprised to discover how much time is allotted by both the House and Senate for in-district work periods. Typical times include periods around President’s Day in February, Easter/Passover in March/April, Memorial Day, Independence Day, and summer recess (late July and the month of August). If Congress does not officially adjourn in early October, additional work periods include time around Columbus Day in October, Veteran’s Day in November, and Thanksgiving. Congress will usually adjourn for the year in December. A specific schedule for each legislative body for the year 2016 can be found at:
House of Representatives: http://www.majorityleader.gov/wp-content/uploads/2011/07/2016_ANNUAL_CALENDAR.pdf
Senate: http://www.senate.gov/legislative/resources/pdf/2016_calendar.pdf
HOW does one schedule an in-district meeting?
To set up a meeting you should first search the websites of your representatives (www.house.gov) and senators (www.senate.gov) for information as to the preferred scheduling procedures. Expect each office’s procedure to be a bit different. You will be asked to provide your name, address, and basic contact information as well as to briefly describe what issue(s) you wish to discuss. Be sure to mention that you are a surgeon and also whether you have previously met with the representative or senator.
If several days pass and staff from the office have not followed up, you should not hesitate to call or contact the office again. Remember, persistence is key! Keep in mind that legislators typically maintain busy schedules during the in-district work period and accordingly, the scheduled appointment time will be brief and subject to change, perhaps on short notice.
If you experience difficulty or simply would like to have assistance in scheduling an in-district meeting, staff in the ACS Division of Advocacy and Health Policy are available to assist and may be contacted by e-mail at surgeonsvoice.org.
WHAT should one discuss?
As a surgeon advocate, your most powerful tool is frequent contact and meetings with your elected officials. Meetings provide an opportunity to offer knowledge and perspective to educate legislators on key topics important to ensuring access to quality surgical care. Your personal experience brings a personal, human touch to issues about which legislators only have knowledge based upon raw numbers and impersonal policy jargon. Most legislators, as well as their staff, will be grateful to have the reliable resource of a constituent’s experience and perspective on complicated medical issues.
To maximize the opportunity for a successful meeting and thereby lay the foundation for the development of a mutually beneficial future relationship, I would offer the following three tips:
1) KNOW YOUR LEGISLATOR: Visit your legislators’ websites, read their biographies, ascertain to what congressional committees they are assigned, and what leadership roles they may have. All of this serves to help determine what issues are important to them and what positions they have previously taken on such issues.
2) KNOW YOUR ISSUE and be able to FRAME IT: Nothing substitutes for a solid knowledge base of the issue and the position you are trying to convey. Be focused and resist the temptation to try to cover too many topics in any one visit. When presenting your argument, “frame it” in layman’s terms much as you would explain it to a patient. Including examples of real-life, anecdotal experiences demonstrating how the status quo or the proposed legislation (depending upon the circumstance) is impacting providers and patients is particularly important.
3) HAVE AN ASK: It is imperative that you always be clear with your legislators about what you want them to do. This serves to reinforce the importance of your having taken time out of your schedule to communicate with them and also serves to hold the legislator accountable. “Asks” can be as specific as a request to cosponsor and support legislation or simply making the offer to serve as a resource to them as a constituent with expertise in health care.
Lastly, I would respectfully request that when surgeons meet with their legislators they inform the ACS Division of Advocacy and Health Policy. Having basic information about the outcome of the meeting, whether knowing that the legislator committed to taking a specific action or knowing that the legislator has requested additional information, is incredibly valuable to us in our ongoing advocacy efforts on behalf of surgeons and their patients here in Washington, DC.
Until next month ….
Dr. Patrick V. Bailey is an ACS Fellow, a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy, in the ACS offices in Washington, D.C.
WHY should surgeons take time out of their busy schedules to meet with legislators?
To become an effective surgeon advocate, nothing is more important than establishing a personal relationship with your legislators. Conversely, to a legislator, there is nothing more valuable than the input and support of constituents. After all, constituents are VOTERS. Meeting with policy makers and/or their staff is extremely valuable in advancing the overall advocacy agenda of The American College of Surgeons and provides surgeons with the opportunity to develop key contacts in the offices of their legislators.
WHERE do such meetings take place?
All U.S. Representatives and Senators have one or more offices for constituent service in their home districts or states. These offices serve as a readily accessible meeting point. As an alternative, legislators frequently will schedule meetings with constituents in mutually convenient locations such as a coffee shop, or during a local legislative event such as a town hall.
WHEN is it most feasible to schedule in-district meetings?
You might be surprised to discover how much time is allotted by both the House and Senate for in-district work periods. Typical times include periods around President’s Day in February, Easter/Passover in March/April, Memorial Day, Independence Day, and summer recess (late July and the month of August). If Congress does not officially adjourn in early October, additional work periods include time around Columbus Day in October, Veteran’s Day in November, and Thanksgiving. Congress will usually adjourn for the year in December. A specific schedule for each legislative body for the year 2016 can be found at:
House of Representatives: http://www.majorityleader.gov/wp-content/uploads/2011/07/2016_ANNUAL_CALENDAR.pdf
Senate: http://www.senate.gov/legislative/resources/pdf/2016_calendar.pdf
HOW does one schedule an in-district meeting?
To set up a meeting you should first search the websites of your representatives (www.house.gov) and senators (www.senate.gov) for information as to the preferred scheduling procedures. Expect each office’s procedure to be a bit different. You will be asked to provide your name, address, and basic contact information as well as to briefly describe what issue(s) you wish to discuss. Be sure to mention that you are a surgeon and also whether you have previously met with the representative or senator.
If several days pass and staff from the office have not followed up, you should not hesitate to call or contact the office again. Remember, persistence is key! Keep in mind that legislators typically maintain busy schedules during the in-district work period and accordingly, the scheduled appointment time will be brief and subject to change, perhaps on short notice.
If you experience difficulty or simply would like to have assistance in scheduling an in-district meeting, staff in the ACS Division of Advocacy and Health Policy are available to assist and may be contacted by e-mail at surgeonsvoice.org.
WHAT should one discuss?
As a surgeon advocate, your most powerful tool is frequent contact and meetings with your elected officials. Meetings provide an opportunity to offer knowledge and perspective to educate legislators on key topics important to ensuring access to quality surgical care. Your personal experience brings a personal, human touch to issues about which legislators only have knowledge based upon raw numbers and impersonal policy jargon. Most legislators, as well as their staff, will be grateful to have the reliable resource of a constituent’s experience and perspective on complicated medical issues.
To maximize the opportunity for a successful meeting and thereby lay the foundation for the development of a mutually beneficial future relationship, I would offer the following three tips:
1) KNOW YOUR LEGISLATOR: Visit your legislators’ websites, read their biographies, ascertain to what congressional committees they are assigned, and what leadership roles they may have. All of this serves to help determine what issues are important to them and what positions they have previously taken on such issues.
2) KNOW YOUR ISSUE and be able to FRAME IT: Nothing substitutes for a solid knowledge base of the issue and the position you are trying to convey. Be focused and resist the temptation to try to cover too many topics in any one visit. When presenting your argument, “frame it” in layman’s terms much as you would explain it to a patient. Including examples of real-life, anecdotal experiences demonstrating how the status quo or the proposed legislation (depending upon the circumstance) is impacting providers and patients is particularly important.
3) HAVE AN ASK: It is imperative that you always be clear with your legislators about what you want them to do. This serves to reinforce the importance of your having taken time out of your schedule to communicate with them and also serves to hold the legislator accountable. “Asks” can be as specific as a request to cosponsor and support legislation or simply making the offer to serve as a resource to them as a constituent with expertise in health care.
Lastly, I would respectfully request that when surgeons meet with their legislators they inform the ACS Division of Advocacy and Health Policy. Having basic information about the outcome of the meeting, whether knowing that the legislator committed to taking a specific action or knowing that the legislator has requested additional information, is incredibly valuable to us in our ongoing advocacy efforts on behalf of surgeons and their patients here in Washington, DC.
Until next month ….
Dr. Patrick V. Bailey is an ACS Fellow, a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy, in the ACS offices in Washington, D.C.
C. difficile transmission linked to antibiotic use in long-term care facilities
Antibiotic use may drive Clostridium difficile transmission within long-term care facilities, according to the results of a recent study published in Annals of Internal Medicine.
Dr. Kevin A. Brown of Public Health Ontario in Toronto, and his coauthors, assessed long-term care–onset C. difficile infection in the largest and most comprehensive study of its kind to date. The retrospective study included 86 Veterans Health Administration health care regions and examined long-term care residents from January 2006 through December 2012. Study results indicated large variations in regional rates of C. difficile infection, regional antibiotic use, and importation of cases of acute care C. difficile infection (Ann Intern Med. 2016 Apr 19. doi: 10.7326/M15-1754).
The total study population included 6,012 cases with a C. difficile infection incidence of 3.7 cases per 10,000 resident days. The regional variability in the incidence of long-term care–onset C. difficile infection was found to be attributable in large part (75%) to antibiotic use and importation from acute care facilities. The data also showed that regional differences in both the prescription of antibiotics and the individual receipt of antibiotics contributed to resident risk, suggesting increased risk for both acquiring and spreading C. difficile.
A potential mechanism offered by the authors for the transmission of C. difficile in facilities with high antibiotic use may be the increased prevalence of residents with asymptomatic C. difficile colonization who become more effective at shedding C. difficile spores when exposed to antibiotics.
Dr. Brown and his colleagues said that efforts designed to reduce C. difficile infection in long-term care should focus on the reduction of total antibiotic use, and that infection control teams may need to take special measures in long-term care facilities that receive residents from hospitals with elevated rates of C. difficile infection.
This study was funded by the U.S. Department of Veterans Affairs and the Centers for Disease Control and Prevention. Dr. Brown reported grants from AstraZeneca outside the submitted work, and another coauthor disclosed grant support from the funding source during the conduct of the study. The remaining coauthors disclosed no conflicts of interest.
Antibiotic use may drive Clostridium difficile transmission within long-term care facilities, according to the results of a recent study published in Annals of Internal Medicine.
Dr. Kevin A. Brown of Public Health Ontario in Toronto, and his coauthors, assessed long-term care–onset C. difficile infection in the largest and most comprehensive study of its kind to date. The retrospective study included 86 Veterans Health Administration health care regions and examined long-term care residents from January 2006 through December 2012. Study results indicated large variations in regional rates of C. difficile infection, regional antibiotic use, and importation of cases of acute care C. difficile infection (Ann Intern Med. 2016 Apr 19. doi: 10.7326/M15-1754).
The total study population included 6,012 cases with a C. difficile infection incidence of 3.7 cases per 10,000 resident days. The regional variability in the incidence of long-term care–onset C. difficile infection was found to be attributable in large part (75%) to antibiotic use and importation from acute care facilities. The data also showed that regional differences in both the prescription of antibiotics and the individual receipt of antibiotics contributed to resident risk, suggesting increased risk for both acquiring and spreading C. difficile.
A potential mechanism offered by the authors for the transmission of C. difficile in facilities with high antibiotic use may be the increased prevalence of residents with asymptomatic C. difficile colonization who become more effective at shedding C. difficile spores when exposed to antibiotics.
Dr. Brown and his colleagues said that efforts designed to reduce C. difficile infection in long-term care should focus on the reduction of total antibiotic use, and that infection control teams may need to take special measures in long-term care facilities that receive residents from hospitals with elevated rates of C. difficile infection.
This study was funded by the U.S. Department of Veterans Affairs and the Centers for Disease Control and Prevention. Dr. Brown reported grants from AstraZeneca outside the submitted work, and another coauthor disclosed grant support from the funding source during the conduct of the study. The remaining coauthors disclosed no conflicts of interest.
Antibiotic use may drive Clostridium difficile transmission within long-term care facilities, according to the results of a recent study published in Annals of Internal Medicine.
Dr. Kevin A. Brown of Public Health Ontario in Toronto, and his coauthors, assessed long-term care–onset C. difficile infection in the largest and most comprehensive study of its kind to date. The retrospective study included 86 Veterans Health Administration health care regions and examined long-term care residents from January 2006 through December 2012. Study results indicated large variations in regional rates of C. difficile infection, regional antibiotic use, and importation of cases of acute care C. difficile infection (Ann Intern Med. 2016 Apr 19. doi: 10.7326/M15-1754).
The total study population included 6,012 cases with a C. difficile infection incidence of 3.7 cases per 10,000 resident days. The regional variability in the incidence of long-term care–onset C. difficile infection was found to be attributable in large part (75%) to antibiotic use and importation from acute care facilities. The data also showed that regional differences in both the prescription of antibiotics and the individual receipt of antibiotics contributed to resident risk, suggesting increased risk for both acquiring and spreading C. difficile.
A potential mechanism offered by the authors for the transmission of C. difficile in facilities with high antibiotic use may be the increased prevalence of residents with asymptomatic C. difficile colonization who become more effective at shedding C. difficile spores when exposed to antibiotics.
Dr. Brown and his colleagues said that efforts designed to reduce C. difficile infection in long-term care should focus on the reduction of total antibiotic use, and that infection control teams may need to take special measures in long-term care facilities that receive residents from hospitals with elevated rates of C. difficile infection.
This study was funded by the U.S. Department of Veterans Affairs and the Centers for Disease Control and Prevention. Dr. Brown reported grants from AstraZeneca outside the submitted work, and another coauthor disclosed grant support from the funding source during the conduct of the study. The remaining coauthors disclosed no conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point:C. difficile transmission may be driven by antibiotic use in long-term care facilities.
Major finding: The majority (75%) of the regional variability in the incidence of long-term care–onset C. difficile infection was attributable to antibiotic use and importation.
Data sources: Retrospective study of long-term care residents from 86 Veterans Health Administration health care regions examined from January 2006 through December 2012.
Disclosures: This study was funded by the U.S. Department of Veterans Affairs and the Centers for Disease Control and Prevention. Dr. Brown reported grants from AstraZeneca outside the submitted work, and another coauthor disclosed grant support from the funding source during the conduct of the study. The remaining coauthors disclosed no conflicts of interest.
New Model May Predict Risk of Acute Kidney Injury in Orthopedic Patients
Clinical question: What is the risk of acute kidney injury after orthopedic surgery, and does it impact mortality?
Background: Current studies show that acute kidney injury is associated with increased long-term mortality, future development of chronic kidney disease, and increased healthcare costs. However, no externally validated models are available to predict patients undergoing non-cardiac surgery at risk of postoperative acute kidney injury.
Study design: Observational, cohort study.
Setting: Teaching and private hospitals in the National Health Service (NHS) in the Tayside region of Scotland.
Synopsis: Investigators enrolled 10,615 adults >18 years of age undergoing orthopedic surgery into two groups: development cohort (6,220 patients) and validation cohort (4,395 patients). Using the development cohort, seven predictors were identified in the risk model: age at operation, male sex, diabetes, lower estimated glomerular filtration rate (GFR), use of ACE inhibitor/ARB, number of prescribing drugs, and American Society of Anesthesiologists (ASA) grade.
The model’s predictive performance for discrimination was good in the development cohort (C statistic 0.74; 95% CI, 0.72–0.76) and validation cohort (C statistic 0.7). Calibration was good in the development cohort but overestimated the risk in the validation cohort. Postoperative acute kidney injury developed in 672 (10.8%) patients in the development cohort and 295 (6.7%) in the validation cohort. Thirty percent (3,166) of the 10,615 patients enrolled in this study died over the median follow-up of 4.58 years. Survival was worse in the patients with acute kidney injury (adjusted hazard ratio 1.53; 95% CI, 1.38–1.70), worse in the short term (90-day adjusted hazard ratio 2.36; 95% CI, 1.94–2.87), and diminished over time.
Bottom line: A predictive model using age, male sex, diabetes, lower GFR, use of ACE inhibitor/ARB, multiple medications, and ASA grades might predict risk of postoperative acute kidney injury in orthopedic patients.
Citation: Bell S, Dekker FW, Vadiveloo T, et al. Risk of postoperative acute kidney injury in patients undergoing orthopaedic surgery—development and validation of a risk score and effect of acute kidney injury on survival: observational cohort study. BMJ 2015; 351:h5639.
Clinical question: What is the risk of acute kidney injury after orthopedic surgery, and does it impact mortality?
Background: Current studies show that acute kidney injury is associated with increased long-term mortality, future development of chronic kidney disease, and increased healthcare costs. However, no externally validated models are available to predict patients undergoing non-cardiac surgery at risk of postoperative acute kidney injury.
Study design: Observational, cohort study.
Setting: Teaching and private hospitals in the National Health Service (NHS) in the Tayside region of Scotland.
Synopsis: Investigators enrolled 10,615 adults >18 years of age undergoing orthopedic surgery into two groups: development cohort (6,220 patients) and validation cohort (4,395 patients). Using the development cohort, seven predictors were identified in the risk model: age at operation, male sex, diabetes, lower estimated glomerular filtration rate (GFR), use of ACE inhibitor/ARB, number of prescribing drugs, and American Society of Anesthesiologists (ASA) grade.
The model’s predictive performance for discrimination was good in the development cohort (C statistic 0.74; 95% CI, 0.72–0.76) and validation cohort (C statistic 0.7). Calibration was good in the development cohort but overestimated the risk in the validation cohort. Postoperative acute kidney injury developed in 672 (10.8%) patients in the development cohort and 295 (6.7%) in the validation cohort. Thirty percent (3,166) of the 10,615 patients enrolled in this study died over the median follow-up of 4.58 years. Survival was worse in the patients with acute kidney injury (adjusted hazard ratio 1.53; 95% CI, 1.38–1.70), worse in the short term (90-day adjusted hazard ratio 2.36; 95% CI, 1.94–2.87), and diminished over time.
Bottom line: A predictive model using age, male sex, diabetes, lower GFR, use of ACE inhibitor/ARB, multiple medications, and ASA grades might predict risk of postoperative acute kidney injury in orthopedic patients.
Citation: Bell S, Dekker FW, Vadiveloo T, et al. Risk of postoperative acute kidney injury in patients undergoing orthopaedic surgery—development and validation of a risk score and effect of acute kidney injury on survival: observational cohort study. BMJ 2015; 351:h5639.
Clinical question: What is the risk of acute kidney injury after orthopedic surgery, and does it impact mortality?
Background: Current studies show that acute kidney injury is associated with increased long-term mortality, future development of chronic kidney disease, and increased healthcare costs. However, no externally validated models are available to predict patients undergoing non-cardiac surgery at risk of postoperative acute kidney injury.
Study design: Observational, cohort study.
Setting: Teaching and private hospitals in the National Health Service (NHS) in the Tayside region of Scotland.
Synopsis: Investigators enrolled 10,615 adults >18 years of age undergoing orthopedic surgery into two groups: development cohort (6,220 patients) and validation cohort (4,395 patients). Using the development cohort, seven predictors were identified in the risk model: age at operation, male sex, diabetes, lower estimated glomerular filtration rate (GFR), use of ACE inhibitor/ARB, number of prescribing drugs, and American Society of Anesthesiologists (ASA) grade.
The model’s predictive performance for discrimination was good in the development cohort (C statistic 0.74; 95% CI, 0.72–0.76) and validation cohort (C statistic 0.7). Calibration was good in the development cohort but overestimated the risk in the validation cohort. Postoperative acute kidney injury developed in 672 (10.8%) patients in the development cohort and 295 (6.7%) in the validation cohort. Thirty percent (3,166) of the 10,615 patients enrolled in this study died over the median follow-up of 4.58 years. Survival was worse in the patients with acute kidney injury (adjusted hazard ratio 1.53; 95% CI, 1.38–1.70), worse in the short term (90-day adjusted hazard ratio 2.36; 95% CI, 1.94–2.87), and diminished over time.
Bottom line: A predictive model using age, male sex, diabetes, lower GFR, use of ACE inhibitor/ARB, multiple medications, and ASA grades might predict risk of postoperative acute kidney injury in orthopedic patients.
Citation: Bell S, Dekker FW, Vadiveloo T, et al. Risk of postoperative acute kidney injury in patients undergoing orthopaedic surgery—development and validation of a risk score and effect of acute kidney injury on survival: observational cohort study. BMJ 2015; 351:h5639.
Treating Asymptomatic Bacteriuria Can Be Dangerous
Clinical question: Does treating asymptomatic bacteriuria (AB) cause harm in women?
Background: In women with recurrent UTIs, AB is often treated, increasing the risk of multi-drug-resistant bacteria. At the same time, little data exist on the relationship between AB treatment and risk of higher antibiotic resistance in women with recurrent UTIs.
Study design: Follow-up observational, analytical, longitudinal study on a previously randomized clinical trial (RCT).
Setting: Sexually transmitted disease (STD) center in Florence, Italy.
Synopsis: Using the patients from the authors’ previous RCT, the study followed 550 women with recurrent UTIs and AB for a mean of 38.8 months in parallel groups: One group had AB treated, and the other group did not. In the group of women treated with antibiotics, the recurrence rate was 69.6% versus 37.7% in the group not treated (P<0.001). In addition, E. coli isolates showed more resistance to amoxicillin/clavulanic acid (P=0.03), trimethoprim/sulfamethazole (P=0.01), and ciprofloxacin (P=0.03) in the group previously treated with antibiotics.
Given the observational design of the study, data must be interpreted with caution in determining a causal relationship. However, prior studies have shown this relationship, and current Infectious Diseases Society of America guidelines support neither screening nor treating AB.
Bottom line: In women with recurrent UTIs, previous treatment of AB is associated with higher rates of antibiotic-resistant bacteria, causing symptomatic UTIs.
Citation: Cai T, Nsei G, Mazzoli S, et al. Asymptomatic bacteriuria treatment is associated with a higher prevalence of antibiotic resistant strains in women with urinary tract infection. Clin Infect Dis. 2015;61(11):1655-1661.
Short Take
National Healthcare Spending Increased in 2014
Led by expansions under the Affordable Care Act, healthcare spending increased 5.3% from the previous year and now totals $3 trillion, which represents 17.5% of the gross domestic product.
Citation: Martin AB, Hartman M, Benson J, Catlin A. National health spending in 2014: faster growth drive by coverage expansion and prescription drug spending. Health Aff. 2016;35(1):150-160.
Clinical question: Does treating asymptomatic bacteriuria (AB) cause harm in women?
Background: In women with recurrent UTIs, AB is often treated, increasing the risk of multi-drug-resistant bacteria. At the same time, little data exist on the relationship between AB treatment and risk of higher antibiotic resistance in women with recurrent UTIs.
Study design: Follow-up observational, analytical, longitudinal study on a previously randomized clinical trial (RCT).
Setting: Sexually transmitted disease (STD) center in Florence, Italy.
Synopsis: Using the patients from the authors’ previous RCT, the study followed 550 women with recurrent UTIs and AB for a mean of 38.8 months in parallel groups: One group had AB treated, and the other group did not. In the group of women treated with antibiotics, the recurrence rate was 69.6% versus 37.7% in the group not treated (P<0.001). In addition, E. coli isolates showed more resistance to amoxicillin/clavulanic acid (P=0.03), trimethoprim/sulfamethazole (P=0.01), and ciprofloxacin (P=0.03) in the group previously treated with antibiotics.
Given the observational design of the study, data must be interpreted with caution in determining a causal relationship. However, prior studies have shown this relationship, and current Infectious Diseases Society of America guidelines support neither screening nor treating AB.
Bottom line: In women with recurrent UTIs, previous treatment of AB is associated with higher rates of antibiotic-resistant bacteria, causing symptomatic UTIs.
Citation: Cai T, Nsei G, Mazzoli S, et al. Asymptomatic bacteriuria treatment is associated with a higher prevalence of antibiotic resistant strains in women with urinary tract infection. Clin Infect Dis. 2015;61(11):1655-1661.
Short Take
National Healthcare Spending Increased in 2014
Led by expansions under the Affordable Care Act, healthcare spending increased 5.3% from the previous year and now totals $3 trillion, which represents 17.5% of the gross domestic product.
Citation: Martin AB, Hartman M, Benson J, Catlin A. National health spending in 2014: faster growth drive by coverage expansion and prescription drug spending. Health Aff. 2016;35(1):150-160.
Clinical question: Does treating asymptomatic bacteriuria (AB) cause harm in women?
Background: In women with recurrent UTIs, AB is often treated, increasing the risk of multi-drug-resistant bacteria. At the same time, little data exist on the relationship between AB treatment and risk of higher antibiotic resistance in women with recurrent UTIs.
Study design: Follow-up observational, analytical, longitudinal study on a previously randomized clinical trial (RCT).
Setting: Sexually transmitted disease (STD) center in Florence, Italy.
Synopsis: Using the patients from the authors’ previous RCT, the study followed 550 women with recurrent UTIs and AB for a mean of 38.8 months in parallel groups: One group had AB treated, and the other group did not. In the group of women treated with antibiotics, the recurrence rate was 69.6% versus 37.7% in the group not treated (P<0.001). In addition, E. coli isolates showed more resistance to amoxicillin/clavulanic acid (P=0.03), trimethoprim/sulfamethazole (P=0.01), and ciprofloxacin (P=0.03) in the group previously treated with antibiotics.
Given the observational design of the study, data must be interpreted with caution in determining a causal relationship. However, prior studies have shown this relationship, and current Infectious Diseases Society of America guidelines support neither screening nor treating AB.
Bottom line: In women with recurrent UTIs, previous treatment of AB is associated with higher rates of antibiotic-resistant bacteria, causing symptomatic UTIs.
Citation: Cai T, Nsei G, Mazzoli S, et al. Asymptomatic bacteriuria treatment is associated with a higher prevalence of antibiotic resistant strains in women with urinary tract infection. Clin Infect Dis. 2015;61(11):1655-1661.
Short Take
National Healthcare Spending Increased in 2014
Led by expansions under the Affordable Care Act, healthcare spending increased 5.3% from the previous year and now totals $3 trillion, which represents 17.5% of the gross domestic product.
Citation: Martin AB, Hartman M, Benson J, Catlin A. National health spending in 2014: faster growth drive by coverage expansion and prescription drug spending. Health Aff. 2016;35(1):150-160.
Targeted corticosteroids cut GVHD incidence
Short-term low-dose corticosteroid prophylaxis reduces the incidence of graft-vs.-host disease in patients who undergo allogeneic haploidentical stem-cell transplantation to treat hematologic neoplasms, according to a report published online April 18 in the Journal of Clinical Oncology.
The key to selecting patients most likely to benefit from the corticosteroid therapy is to identify those at high risk for graft-vs.-host disease (GVHD) using two biomarkers: high levels of CD56bright natural killer cells in allogeneic grafts or high CD4:CD8 ratios in bone marrow grafts, according to Dr. Ying-Jun Chang of Peking University People’s Hospital, Beijing, and associates.
The investigators performed an open-label trial involving 228 patients aged 15-60 years treated at a single medical center during an 18-month period for acute myeloid leukemia, acute lymphoblastic leukemia, chronic myeloid leukemia, myelodysplastic syndrome, or other hematologic neoplasms. Using the two biomarkers, the patients were categorized as either high or low risk for developing GVHD. They were randomly assigned to three study groups: 72 high-risk patients who received short-term low-dose corticosteroids, 73 high-risk patients who received usual care, and 83 low-risk patients who received usual care.
The cumulative 100-day incidence of acute grade-II to grade-IV GVHD was significantly lower in the high-risk patients who received prophylaxis (21%) than in the high-risk patients who did not receive prophylaxis (48%). In fact, corticosteroids decreased the rate of GVHD so that it was comparable with that in the low-risk patients (26%), Dr. Chang and associates said (J Clin Oncol. 2016 Apr 18. doi: 10.1200/JCO.2015.63l.8817).
Moreover, in the high-risk patients the median interval until GVHD developed was 25 days for those who took corticosteroids, compared with only 15 days for those who did not. Median times to myeloid recovery and platelet recovery were significantly shorter for high-risk patients who received corticosteroids than for either of the other study groups. However, 3-year overall survival and leukemia-free survival were comparable among the three study groups.
The short-term low-dose regimen of corticosteroids did not raise the rate of adverse events, including infection, which suggests that it is preferable to standard corticosteroid regimens in this patient population. The incidences of cytomegalovirus or Epstein-Barr virus reactivation, post-transplantation lymphoproliferative disorder, hemorrhagic cystitis, bacteremia, and invasive fungal infections were comparable among the three study groups. Of note, the incidences of osteonecrosis of the femoral head and secondary hypertension were significantly lower among high-risk patients who received corticosteroid prophylaxis than among those who did not.
“These results provide the first test, to our knowledge, of a novel risk-stratification-directed prophylaxis strategy that effectively prevented acute GVHD among patients who were at high risk for GVHD, without unnecessarily exposing patients who were at low risk to excessive toxicity from additional immunosuppressive agents,” Dr. Chang and associates said.
Despite the encouraging results of Chang et al, it would be premature to routinely use corticosteroid prophylaxis to prevent GVHD until further studies are completed.
This study wasn’t sufficiently powered to determine whether corticosteroids reduced treatment-specific mortality or improved overall survival. Future studies must examine these end points, as well as relapse rates, before this method of prophylaxis is widely adopted.
Dr. Edwin P. Alyea is at Dana-Farber Cancer Institute, Boston. He reported having no relevant financial disclosures. Dr. Alyea made these remarks in an editorial accompanying Dr. Chang’s report (J Clin Oncol. 2016 Apr 18. doi: 10.1200/JCO.2015.66.0902).
Despite the encouraging results of Chang et al, it would be premature to routinely use corticosteroid prophylaxis to prevent GVHD until further studies are completed.
This study wasn’t sufficiently powered to determine whether corticosteroids reduced treatment-specific mortality or improved overall survival. Future studies must examine these end points, as well as relapse rates, before this method of prophylaxis is widely adopted.
Dr. Edwin P. Alyea is at Dana-Farber Cancer Institute, Boston. He reported having no relevant financial disclosures. Dr. Alyea made these remarks in an editorial accompanying Dr. Chang’s report (J Clin Oncol. 2016 Apr 18. doi: 10.1200/JCO.2015.66.0902).
Despite the encouraging results of Chang et al, it would be premature to routinely use corticosteroid prophylaxis to prevent GVHD until further studies are completed.
This study wasn’t sufficiently powered to determine whether corticosteroids reduced treatment-specific mortality or improved overall survival. Future studies must examine these end points, as well as relapse rates, before this method of prophylaxis is widely adopted.
Dr. Edwin P. Alyea is at Dana-Farber Cancer Institute, Boston. He reported having no relevant financial disclosures. Dr. Alyea made these remarks in an editorial accompanying Dr. Chang’s report (J Clin Oncol. 2016 Apr 18. doi: 10.1200/JCO.2015.66.0902).
Short-term low-dose corticosteroid prophylaxis reduces the incidence of graft-vs.-host disease in patients who undergo allogeneic haploidentical stem-cell transplantation to treat hematologic neoplasms, according to a report published online April 18 in the Journal of Clinical Oncology.
The key to selecting patients most likely to benefit from the corticosteroid therapy is to identify those at high risk for graft-vs.-host disease (GVHD) using two biomarkers: high levels of CD56bright natural killer cells in allogeneic grafts or high CD4:CD8 ratios in bone marrow grafts, according to Dr. Ying-Jun Chang of Peking University People’s Hospital, Beijing, and associates.
The investigators performed an open-label trial involving 228 patients aged 15-60 years treated at a single medical center during an 18-month period for acute myeloid leukemia, acute lymphoblastic leukemia, chronic myeloid leukemia, myelodysplastic syndrome, or other hematologic neoplasms. Using the two biomarkers, the patients were categorized as either high or low risk for developing GVHD. They were randomly assigned to three study groups: 72 high-risk patients who received short-term low-dose corticosteroids, 73 high-risk patients who received usual care, and 83 low-risk patients who received usual care.
The cumulative 100-day incidence of acute grade-II to grade-IV GVHD was significantly lower in the high-risk patients who received prophylaxis (21%) than in the high-risk patients who did not receive prophylaxis (48%). In fact, corticosteroids decreased the rate of GVHD so that it was comparable with that in the low-risk patients (26%), Dr. Chang and associates said (J Clin Oncol. 2016 Apr 18. doi: 10.1200/JCO.2015.63l.8817).
Moreover, in the high-risk patients the median interval until GVHD developed was 25 days for those who took corticosteroids, compared with only 15 days for those who did not. Median times to myeloid recovery and platelet recovery were significantly shorter for high-risk patients who received corticosteroids than for either of the other study groups. However, 3-year overall survival and leukemia-free survival were comparable among the three study groups.
The short-term low-dose regimen of corticosteroids did not raise the rate of adverse events, including infection, which suggests that it is preferable to standard corticosteroid regimens in this patient population. The incidences of cytomegalovirus or Epstein-Barr virus reactivation, post-transplantation lymphoproliferative disorder, hemorrhagic cystitis, bacteremia, and invasive fungal infections were comparable among the three study groups. Of note, the incidences of osteonecrosis of the femoral head and secondary hypertension were significantly lower among high-risk patients who received corticosteroid prophylaxis than among those who did not.
“These results provide the first test, to our knowledge, of a novel risk-stratification-directed prophylaxis strategy that effectively prevented acute GVHD among patients who were at high risk for GVHD, without unnecessarily exposing patients who were at low risk to excessive toxicity from additional immunosuppressive agents,” Dr. Chang and associates said.
Short-term low-dose corticosteroid prophylaxis reduces the incidence of graft-vs.-host disease in patients who undergo allogeneic haploidentical stem-cell transplantation to treat hematologic neoplasms, according to a report published online April 18 in the Journal of Clinical Oncology.
The key to selecting patients most likely to benefit from the corticosteroid therapy is to identify those at high risk for graft-vs.-host disease (GVHD) using two biomarkers: high levels of CD56bright natural killer cells in allogeneic grafts or high CD4:CD8 ratios in bone marrow grafts, according to Dr. Ying-Jun Chang of Peking University People’s Hospital, Beijing, and associates.
The investigators performed an open-label trial involving 228 patients aged 15-60 years treated at a single medical center during an 18-month period for acute myeloid leukemia, acute lymphoblastic leukemia, chronic myeloid leukemia, myelodysplastic syndrome, or other hematologic neoplasms. Using the two biomarkers, the patients were categorized as either high or low risk for developing GVHD. They were randomly assigned to three study groups: 72 high-risk patients who received short-term low-dose corticosteroids, 73 high-risk patients who received usual care, and 83 low-risk patients who received usual care.
The cumulative 100-day incidence of acute grade-II to grade-IV GVHD was significantly lower in the high-risk patients who received prophylaxis (21%) than in the high-risk patients who did not receive prophylaxis (48%). In fact, corticosteroids decreased the rate of GVHD so that it was comparable with that in the low-risk patients (26%), Dr. Chang and associates said (J Clin Oncol. 2016 Apr 18. doi: 10.1200/JCO.2015.63l.8817).
Moreover, in the high-risk patients the median interval until GVHD developed was 25 days for those who took corticosteroids, compared with only 15 days for those who did not. Median times to myeloid recovery and platelet recovery were significantly shorter for high-risk patients who received corticosteroids than for either of the other study groups. However, 3-year overall survival and leukemia-free survival were comparable among the three study groups.
The short-term low-dose regimen of corticosteroids did not raise the rate of adverse events, including infection, which suggests that it is preferable to standard corticosteroid regimens in this patient population. The incidences of cytomegalovirus or Epstein-Barr virus reactivation, post-transplantation lymphoproliferative disorder, hemorrhagic cystitis, bacteremia, and invasive fungal infections were comparable among the three study groups. Of note, the incidences of osteonecrosis of the femoral head and secondary hypertension were significantly lower among high-risk patients who received corticosteroid prophylaxis than among those who did not.
“These results provide the first test, to our knowledge, of a novel risk-stratification-directed prophylaxis strategy that effectively prevented acute GVHD among patients who were at high risk for GVHD, without unnecessarily exposing patients who were at low risk to excessive toxicity from additional immunosuppressive agents,” Dr. Chang and associates said.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Short-term low-dose corticosteroid prophylaxis reduces the incidence of the GVHD in patients who undergo haploidentical stem-cell transplantation to treat hematologic neoplasms.
Major finding: The 100-day incidence of acute GVHD was significantly lower in the high-risk patients who received corticosteroid prophylaxis (21%) than in the high-risk patients who did not (48%).
Data source: An open-label randomized controlled trial involving 228 Chinese patients who underwent stem-cell transplantation.
Disclosures: This study was supported by the Beijing Committee of Science and Technology, the National High Technology Research and Development Program of China, and the National Natural Science Foundation of China. Dr. Chang and associates reported having no relevant financial disclosures.
Donor EBV status affects recipient graft-vs-host disease risk
In allogeneic hematopoietic stem-cell transplantation, the donor’s status regarding Epstein-Barr virus affects the recipient’s risk of developing graft-vs-host disease – a “completely new and striking” finding, according to a report published online April 18 in the Journal of Clinical Oncology.
Approximately 80% of the general population has been infected with EBV and carries persistent virus in memory B cells. When viral material is transmitted to stem-cell recipients, it is known to cause posttransplantation lymphoproliferative disorder. Until now, however, no data were available to examine EBV serology’s effect on other posttransplantation outcomes, said Dr. Jan Styczynski of the department of pediatric hematology and oncology at Nicolaus Copernicus University, Bydgoszcz, Poland, and his associates.
They analyzed information in the European Society of Blood and Marrow Transplantation database for 11,364 patients with acute lymphoblastic leukemia or acute myeloblastic leukemia who underwent stem-cell transplantation between 1997 and 2012 and who were followed for approximately 5 years. Most of the donors (82%) were seropositive for EBV. Acute graft-vs-host disease (GVHD) developed in 32% and chronic GVHD developed in 40% of these stem cell–transplant recipients.
The incidence of chronic GVHD was significantly higher when the donor was EBV-seropositive (41%) than when the donor was EBV-seronegative (31%). Similarly, the incidence of acute GVHD was significantly higher when the donor was EBV-seropositive (32% vs 30%), but the magnitude of the difference between the two groups was smaller. The risk for GVHD increased even though patients receiving transplants from EBV-seropositive donors underwent more intensive GVHD prophylaxis than did those who had seronegative donors, the investigators said (J Clin Oncol. 2016 Apr 18. doi: 10.1200/JCO.2015.64.2405).
In contrast, the transplant recipients’ EBV status did not affect their risk of developing GVHD.
“Despite the effect of donor EBV serostatus on GVHD, we did not observe a corresponding GVHD-related death rate, and as a result, there was no effect on overall survival, relapse-free survival, relapse incidence, and nonrelapse mortality. However, it should be kept in mind that many other pre- and posttransplantation factors play a role in contributing to final transplantation outcomes,” Dr. Styczynski and his associates noted.
The current recommendation to monitor transplantation recipients for EBV and to give them “preemptive” rituximab to stave off the development of posttransplantation lymphoproliferative disorder might prove useful in also preventing GVHD, they added.
The findings of Dr. Styczynski and his associates raise the possibility that we may be able to prevent or treat GVHD in transplant recipients by controlling EBV infection.
Selecting only EBV-negative donors would be one way to accomplish this, but that would be impractical given the high seroprevalence of EBV in the general population. Depleting memory B cells, the reservoir of EBV infection, using monoclonal antibodies may prove helpful, and these agents might provide additional therapeutic effects. And novel antivirals such as retroviral integrase inhibitors may be more specific at targeting EBV than acyclovir and related agents, which have limited activity against latently infected B cells. These novel drugs, however, are not without risks and adverse effects.
A promising alternative might be to boost immunity to EBV using vaccination or adoptive transfer of ex vivo expanded EBV-specific cytotoxic T cells.
Dr. Katayoun Rezvani and Dr. Richard E. Champlin are with the University of Texas MD Andersen Cancer Center, Houston. Their financial disclosures are available at www.jco.org. They made these remarks in an editorial accompanying Dr. Styczynski’s report (J Clin Oncol. 2016 Apr 18. doi: 10.1200/JCO.2016.66.6099).
The findings of Dr. Styczynski and his associates raise the possibility that we may be able to prevent or treat GVHD in transplant recipients by controlling EBV infection.
Selecting only EBV-negative donors would be one way to accomplish this, but that would be impractical given the high seroprevalence of EBV in the general population. Depleting memory B cells, the reservoir of EBV infection, using monoclonal antibodies may prove helpful, and these agents might provide additional therapeutic effects. And novel antivirals such as retroviral integrase inhibitors may be more specific at targeting EBV than acyclovir and related agents, which have limited activity against latently infected B cells. These novel drugs, however, are not without risks and adverse effects.
A promising alternative might be to boost immunity to EBV using vaccination or adoptive transfer of ex vivo expanded EBV-specific cytotoxic T cells.
Dr. Katayoun Rezvani and Dr. Richard E. Champlin are with the University of Texas MD Andersen Cancer Center, Houston. Their financial disclosures are available at www.jco.org. They made these remarks in an editorial accompanying Dr. Styczynski’s report (J Clin Oncol. 2016 Apr 18. doi: 10.1200/JCO.2016.66.6099).
The findings of Dr. Styczynski and his associates raise the possibility that we may be able to prevent or treat GVHD in transplant recipients by controlling EBV infection.
Selecting only EBV-negative donors would be one way to accomplish this, but that would be impractical given the high seroprevalence of EBV in the general population. Depleting memory B cells, the reservoir of EBV infection, using monoclonal antibodies may prove helpful, and these agents might provide additional therapeutic effects. And novel antivirals such as retroviral integrase inhibitors may be more specific at targeting EBV than acyclovir and related agents, which have limited activity against latently infected B cells. These novel drugs, however, are not without risks and adverse effects.
A promising alternative might be to boost immunity to EBV using vaccination or adoptive transfer of ex vivo expanded EBV-specific cytotoxic T cells.
Dr. Katayoun Rezvani and Dr. Richard E. Champlin are with the University of Texas MD Andersen Cancer Center, Houston. Their financial disclosures are available at www.jco.org. They made these remarks in an editorial accompanying Dr. Styczynski’s report (J Clin Oncol. 2016 Apr 18. doi: 10.1200/JCO.2016.66.6099).
In allogeneic hematopoietic stem-cell transplantation, the donor’s status regarding Epstein-Barr virus affects the recipient’s risk of developing graft-vs-host disease – a “completely new and striking” finding, according to a report published online April 18 in the Journal of Clinical Oncology.
Approximately 80% of the general population has been infected with EBV and carries persistent virus in memory B cells. When viral material is transmitted to stem-cell recipients, it is known to cause posttransplantation lymphoproliferative disorder. Until now, however, no data were available to examine EBV serology’s effect on other posttransplantation outcomes, said Dr. Jan Styczynski of the department of pediatric hematology and oncology at Nicolaus Copernicus University, Bydgoszcz, Poland, and his associates.
They analyzed information in the European Society of Blood and Marrow Transplantation database for 11,364 patients with acute lymphoblastic leukemia or acute myeloblastic leukemia who underwent stem-cell transplantation between 1997 and 2012 and who were followed for approximately 5 years. Most of the donors (82%) were seropositive for EBV. Acute graft-vs-host disease (GVHD) developed in 32% and chronic GVHD developed in 40% of these stem cell–transplant recipients.
The incidence of chronic GVHD was significantly higher when the donor was EBV-seropositive (41%) than when the donor was EBV-seronegative (31%). Similarly, the incidence of acute GVHD was significantly higher when the donor was EBV-seropositive (32% vs 30%), but the magnitude of the difference between the two groups was smaller. The risk for GVHD increased even though patients receiving transplants from EBV-seropositive donors underwent more intensive GVHD prophylaxis than did those who had seronegative donors, the investigators said (J Clin Oncol. 2016 Apr 18. doi: 10.1200/JCO.2015.64.2405).
In contrast, the transplant recipients’ EBV status did not affect their risk of developing GVHD.
“Despite the effect of donor EBV serostatus on GVHD, we did not observe a corresponding GVHD-related death rate, and as a result, there was no effect on overall survival, relapse-free survival, relapse incidence, and nonrelapse mortality. However, it should be kept in mind that many other pre- and posttransplantation factors play a role in contributing to final transplantation outcomes,” Dr. Styczynski and his associates noted.
The current recommendation to monitor transplantation recipients for EBV and to give them “preemptive” rituximab to stave off the development of posttransplantation lymphoproliferative disorder might prove useful in also preventing GVHD, they added.
In allogeneic hematopoietic stem-cell transplantation, the donor’s status regarding Epstein-Barr virus affects the recipient’s risk of developing graft-vs-host disease – a “completely new and striking” finding, according to a report published online April 18 in the Journal of Clinical Oncology.
Approximately 80% of the general population has been infected with EBV and carries persistent virus in memory B cells. When viral material is transmitted to stem-cell recipients, it is known to cause posttransplantation lymphoproliferative disorder. Until now, however, no data were available to examine EBV serology’s effect on other posttransplantation outcomes, said Dr. Jan Styczynski of the department of pediatric hematology and oncology at Nicolaus Copernicus University, Bydgoszcz, Poland, and his associates.
They analyzed information in the European Society of Blood and Marrow Transplantation database for 11,364 patients with acute lymphoblastic leukemia or acute myeloblastic leukemia who underwent stem-cell transplantation between 1997 and 2012 and who were followed for approximately 5 years. Most of the donors (82%) were seropositive for EBV. Acute graft-vs-host disease (GVHD) developed in 32% and chronic GVHD developed in 40% of these stem cell–transplant recipients.
The incidence of chronic GVHD was significantly higher when the donor was EBV-seropositive (41%) than when the donor was EBV-seronegative (31%). Similarly, the incidence of acute GVHD was significantly higher when the donor was EBV-seropositive (32% vs 30%), but the magnitude of the difference between the two groups was smaller. The risk for GVHD increased even though patients receiving transplants from EBV-seropositive donors underwent more intensive GVHD prophylaxis than did those who had seronegative donors, the investigators said (J Clin Oncol. 2016 Apr 18. doi: 10.1200/JCO.2015.64.2405).
In contrast, the transplant recipients’ EBV status did not affect their risk of developing GVHD.
“Despite the effect of donor EBV serostatus on GVHD, we did not observe a corresponding GVHD-related death rate, and as a result, there was no effect on overall survival, relapse-free survival, relapse incidence, and nonrelapse mortality. However, it should be kept in mind that many other pre- and posttransplantation factors play a role in contributing to final transplantation outcomes,” Dr. Styczynski and his associates noted.
The current recommendation to monitor transplantation recipients for EBV and to give them “preemptive” rituximab to stave off the development of posttransplantation lymphoproliferative disorder might prove useful in also preventing GVHD, they added.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: In allogeneic hematopoietic stem-cell transplantation, the donor’s EBV status affects the recipient’s risk of developing GVHD.
Major finding: Chronic GVHD was significantly more likely to develop when the donor was EBV-seropositive (41%) than EBV-seronegative (31%).
Data source: A retrospective analysis of data regarding 11,364 European patients with acute leukemia who underwent stem-cell transplantation and were followed for 5 years.
Disclosures: No study sponsor was identified. Dr. Styczynski reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
Targeting T and B Cells as a Therapeutic Approach for Multiple Sclerosis
Immunotherapies that target abnormally activated T and B cells may represent a unique combination and promising DMT strategy for patients with RRMS and have the greatest potential for long-term success. Targeting T cells in MS may help attenuate initiation and maintenance of inflammatory attacks by reducing the production of pro-inflammatory cytokines, recruitment of innate immune cells, stimulation of antibody production, and direct attack of myelin. Targeting B cells in MS may attenuate secretion of autoantibodies and pro-inflammatory cytokines, as well as presentation of self-antigen to T cells.
Click here to read the digital edition.
Immunotherapies that target abnormally activated T and B cells may represent a unique combination and promising DMT strategy for patients with RRMS and have the greatest potential for long-term success. Targeting T cells in MS may help attenuate initiation and maintenance of inflammatory attacks by reducing the production of pro-inflammatory cytokines, recruitment of innate immune cells, stimulation of antibody production, and direct attack of myelin. Targeting B cells in MS may attenuate secretion of autoantibodies and pro-inflammatory cytokines, as well as presentation of self-antigen to T cells.
Click here to read the digital edition.
Immunotherapies that target abnormally activated T and B cells may represent a unique combination and promising DMT strategy for patients with RRMS and have the greatest potential for long-term success. Targeting T cells in MS may help attenuate initiation and maintenance of inflammatory attacks by reducing the production of pro-inflammatory cytokines, recruitment of innate immune cells, stimulation of antibody production, and direct attack of myelin. Targeting B cells in MS may attenuate secretion of autoantibodies and pro-inflammatory cytokines, as well as presentation of self-antigen to T cells.
Click here to read the digital edition.