User login
Patients with ASD may have lower cancer risk

Photo by Darren Baker
New research suggests that patients diagnosed with an autism spectrum disorder (ASD) have a higher burden of mutations in oncogenes but lower rates of cancer than the rest of the population.
Investigators analyzed large, publicly available genomic databases of patients with ASD and found that, compared to a set of control subjects, ASD patients had significantly higher rates of DNA variation in oncogenes.
The team followed up this result with an analysis of the University of Iowa Hospitals and Clinics’ electronic medical record and discovered that ASD patients were also significantly less likely to have a co-occurring diagnosis of cancer.
“It’s a very provocative result that makes sense on one level and is extremely perplexing on another,” said Benjamin Darbro, MD, PhD, of the University of Iowa Carver College of Medicine in Iowa City.
Dr Darbro and his colleagues discussed the result, and the research that led to it, in a paper published in PLOS ONE.
The investigators used exome sequencing data from the ARRA Autism Sequencing Collaboration and compared that data to a control cohort from the Exome Variant Server database.
This revealed that rare, coding variants within oncogenes were greatly enriched in the ARRA ASD cohort. By comparison, variants were not significantly enriched in tumor suppressor genes.
To ensure the genetic differences were not technical artifacts but actually bona fide differences in genetic architecture in ASD, the investigators ran numerous controls.
As expected, they found that individuals with ASD had many more DNA variations in genes previously associated with autism, epilepsy, and intellectual disability compared to control individuals.
However, there was no difference between the ASD and control groups when it came to genes involved in other, unrelated conditions such as skeletal dysplasia, retinitis pigmentosa, dilated cardiomyopathy, and non-syndromic hearing loss.
The investigators then turned their attention to the electronic medical record at the University of Iowa Hospitals and Clinics and conducted a retrospective case-control analysis comparing 1837 patients with ASD to 9336 patients with any other diagnosis, and determined what proportion of each group carried a cancer diagnosis.
The team found that, for children and adults with ASD, there appeared to be a protective effect against cancer. The cancer incidence was 1.3% for patients with ASD and 3.9% for controls.
The protective effect was evident in both males and females with ASD, but it was strongest for the youngest group of patients and decreased with age. For ASD patients who were under 14 years of age, the odds of having cancer were reduced by 94% compared to controls.
When the investigators determined the rates of other systemic diseases—such as high blood pressure and diabetes—in the ASD population, they found no relationship.
Furthermore, the team found no relationship with cancer when they examined the rates of other common conditions such as esophageal reflux, allergic rhinitis, atopic dermatitis, and short stature. They said this demonstrated that the inverse relationship observed between ASD and cancer is not due to a technical artifact. ![]()

Photo by Darren Baker
New research suggests that patients diagnosed with an autism spectrum disorder (ASD) have a higher burden of mutations in oncogenes but lower rates of cancer than the rest of the population.
Investigators analyzed large, publicly available genomic databases of patients with ASD and found that, compared to a set of control subjects, ASD patients had significantly higher rates of DNA variation in oncogenes.
The team followed up this result with an analysis of the University of Iowa Hospitals and Clinics’ electronic medical record and discovered that ASD patients were also significantly less likely to have a co-occurring diagnosis of cancer.
“It’s a very provocative result that makes sense on one level and is extremely perplexing on another,” said Benjamin Darbro, MD, PhD, of the University of Iowa Carver College of Medicine in Iowa City.
Dr Darbro and his colleagues discussed the result, and the research that led to it, in a paper published in PLOS ONE.
The investigators used exome sequencing data from the ARRA Autism Sequencing Collaboration and compared that data to a control cohort from the Exome Variant Server database.
This revealed that rare, coding variants within oncogenes were greatly enriched in the ARRA ASD cohort. By comparison, variants were not significantly enriched in tumor suppressor genes.
To ensure the genetic differences were not technical artifacts but actually bona fide differences in genetic architecture in ASD, the investigators ran numerous controls.
As expected, they found that individuals with ASD had many more DNA variations in genes previously associated with autism, epilepsy, and intellectual disability compared to control individuals.
However, there was no difference between the ASD and control groups when it came to genes involved in other, unrelated conditions such as skeletal dysplasia, retinitis pigmentosa, dilated cardiomyopathy, and non-syndromic hearing loss.
The investigators then turned their attention to the electronic medical record at the University of Iowa Hospitals and Clinics and conducted a retrospective case-control analysis comparing 1837 patients with ASD to 9336 patients with any other diagnosis, and determined what proportion of each group carried a cancer diagnosis.
The team found that, for children and adults with ASD, there appeared to be a protective effect against cancer. The cancer incidence was 1.3% for patients with ASD and 3.9% for controls.
The protective effect was evident in both males and females with ASD, but it was strongest for the youngest group of patients and decreased with age. For ASD patients who were under 14 years of age, the odds of having cancer were reduced by 94% compared to controls.
When the investigators determined the rates of other systemic diseases—such as high blood pressure and diabetes—in the ASD population, they found no relationship.
Furthermore, the team found no relationship with cancer when they examined the rates of other common conditions such as esophageal reflux, allergic rhinitis, atopic dermatitis, and short stature. They said this demonstrated that the inverse relationship observed between ASD and cancer is not due to a technical artifact. ![]()

Photo by Darren Baker
New research suggests that patients diagnosed with an autism spectrum disorder (ASD) have a higher burden of mutations in oncogenes but lower rates of cancer than the rest of the population.
Investigators analyzed large, publicly available genomic databases of patients with ASD and found that, compared to a set of control subjects, ASD patients had significantly higher rates of DNA variation in oncogenes.
The team followed up this result with an analysis of the University of Iowa Hospitals and Clinics’ electronic medical record and discovered that ASD patients were also significantly less likely to have a co-occurring diagnosis of cancer.
“It’s a very provocative result that makes sense on one level and is extremely perplexing on another,” said Benjamin Darbro, MD, PhD, of the University of Iowa Carver College of Medicine in Iowa City.
Dr Darbro and his colleagues discussed the result, and the research that led to it, in a paper published in PLOS ONE.
The investigators used exome sequencing data from the ARRA Autism Sequencing Collaboration and compared that data to a control cohort from the Exome Variant Server database.
This revealed that rare, coding variants within oncogenes were greatly enriched in the ARRA ASD cohort. By comparison, variants were not significantly enriched in tumor suppressor genes.
To ensure the genetic differences were not technical artifacts but actually bona fide differences in genetic architecture in ASD, the investigators ran numerous controls.
As expected, they found that individuals with ASD had many more DNA variations in genes previously associated with autism, epilepsy, and intellectual disability compared to control individuals.
However, there was no difference between the ASD and control groups when it came to genes involved in other, unrelated conditions such as skeletal dysplasia, retinitis pigmentosa, dilated cardiomyopathy, and non-syndromic hearing loss.
The investigators then turned their attention to the electronic medical record at the University of Iowa Hospitals and Clinics and conducted a retrospective case-control analysis comparing 1837 patients with ASD to 9336 patients with any other diagnosis, and determined what proportion of each group carried a cancer diagnosis.
The team found that, for children and adults with ASD, there appeared to be a protective effect against cancer. The cancer incidence was 1.3% for patients with ASD and 3.9% for controls.
The protective effect was evident in both males and females with ASD, but it was strongest for the youngest group of patients and decreased with age. For ASD patients who were under 14 years of age, the odds of having cancer were reduced by 94% compared to controls.
When the investigators determined the rates of other systemic diseases—such as high blood pressure and diabetes—in the ASD population, they found no relationship.
Furthermore, the team found no relationship with cancer when they examined the rates of other common conditions such as esophageal reflux, allergic rhinitis, atopic dermatitis, and short stature. They said this demonstrated that the inverse relationship observed between ASD and cancer is not due to a technical artifact. ![]()
Short transfusion delays can increase risk of death

Photo courtesy of UAB Hospital
Even a short delay in the administration of packed red blood cells (pRBCs) can increase the risk of death for some traumatically injured patients, according to research published in the Journal of Trauma and Acute Care Surgery.
The study showed that a delay of 10 minutes was associated with a higher risk of death among patients who required pRBCs early.
However, a 10-minute delay did not increase the risk of death for the entire study cohort.
For this study, researchers tracked trauma patients taken from the scene of their injury to the University of Cincinnati Medical Center by a helicopter service known as Air Care. The service carries 2 units of pRBCs for protocol-driven prehospital transfusion.
“Air Care is the only helicopter in the area to carry blood (and plasma), so we had the research platform to study how early blood transfusions impact outcomes,” said study author Elizabeth Powell, MD, of the University of Cincinnati Medical Center in Ohio.
Dr Powell and her colleagues studied 94 patients who had received at least 1 unit of pRBCs within 24 hours of arriving at the hospital. Ninety-three percent of patients (n=87) were Caucasian, 70% (n=66) were male, and they had a mean age of 43.
Ninety-four percent of patients (n=88) had sustained blunt force injuries, and 33% (n=31) died within 30 days of hospital arrival.
Thirty-three percent of patients (n=31) received a transfusion during transport, 54% (n=51) were transfused within an hour of hospital arrival, and 13% (n=12) were transfused after the first hour but within 24 hours of hospital arrival.
When considering all 94 patients together, the researchers found that a 10-minute increase in time to pRBC administration did not significantly affect the odds of death, even when adjusting for injury severity. The odds ratio was 1.00 (P=0.575).
However, among the 82 patients who received their first pRBC transfusion during transport or within an hour of hospital arrival, each 10 minute increase in time to transfusion increased the odds of death. When the researchers controlled for Trauma Injury Severity Score, the odds ratio was 1.27 (P=0.044).
“Delays in the time to blood transfusion are associated with increased chances of dying,” Dr Powell said. “Shortening the time to transfusion, including having blood available in the prehospital setting, may improve outcomes.” ![]()

Photo courtesy of UAB Hospital
Even a short delay in the administration of packed red blood cells (pRBCs) can increase the risk of death for some traumatically injured patients, according to research published in the Journal of Trauma and Acute Care Surgery.
The study showed that a delay of 10 minutes was associated with a higher risk of death among patients who required pRBCs early.
However, a 10-minute delay did not increase the risk of death for the entire study cohort.
For this study, researchers tracked trauma patients taken from the scene of their injury to the University of Cincinnati Medical Center by a helicopter service known as Air Care. The service carries 2 units of pRBCs for protocol-driven prehospital transfusion.
“Air Care is the only helicopter in the area to carry blood (and plasma), so we had the research platform to study how early blood transfusions impact outcomes,” said study author Elizabeth Powell, MD, of the University of Cincinnati Medical Center in Ohio.
Dr Powell and her colleagues studied 94 patients who had received at least 1 unit of pRBCs within 24 hours of arriving at the hospital. Ninety-three percent of patients (n=87) were Caucasian, 70% (n=66) were male, and they had a mean age of 43.
Ninety-four percent of patients (n=88) had sustained blunt force injuries, and 33% (n=31) died within 30 days of hospital arrival.
Thirty-three percent of patients (n=31) received a transfusion during transport, 54% (n=51) were transfused within an hour of hospital arrival, and 13% (n=12) were transfused after the first hour but within 24 hours of hospital arrival.
When considering all 94 patients together, the researchers found that a 10-minute increase in time to pRBC administration did not significantly affect the odds of death, even when adjusting for injury severity. The odds ratio was 1.00 (P=0.575).
However, among the 82 patients who received their first pRBC transfusion during transport or within an hour of hospital arrival, each 10 minute increase in time to transfusion increased the odds of death. When the researchers controlled for Trauma Injury Severity Score, the odds ratio was 1.27 (P=0.044).
“Delays in the time to blood transfusion are associated with increased chances of dying,” Dr Powell said. “Shortening the time to transfusion, including having blood available in the prehospital setting, may improve outcomes.” ![]()

Photo courtesy of UAB Hospital
Even a short delay in the administration of packed red blood cells (pRBCs) can increase the risk of death for some traumatically injured patients, according to research published in the Journal of Trauma and Acute Care Surgery.
The study showed that a delay of 10 minutes was associated with a higher risk of death among patients who required pRBCs early.
However, a 10-minute delay did not increase the risk of death for the entire study cohort.
For this study, researchers tracked trauma patients taken from the scene of their injury to the University of Cincinnati Medical Center by a helicopter service known as Air Care. The service carries 2 units of pRBCs for protocol-driven prehospital transfusion.
“Air Care is the only helicopter in the area to carry blood (and plasma), so we had the research platform to study how early blood transfusions impact outcomes,” said study author Elizabeth Powell, MD, of the University of Cincinnati Medical Center in Ohio.
Dr Powell and her colleagues studied 94 patients who had received at least 1 unit of pRBCs within 24 hours of arriving at the hospital. Ninety-three percent of patients (n=87) were Caucasian, 70% (n=66) were male, and they had a mean age of 43.
Ninety-four percent of patients (n=88) had sustained blunt force injuries, and 33% (n=31) died within 30 days of hospital arrival.
Thirty-three percent of patients (n=31) received a transfusion during transport, 54% (n=51) were transfused within an hour of hospital arrival, and 13% (n=12) were transfused after the first hour but within 24 hours of hospital arrival.
When considering all 94 patients together, the researchers found that a 10-minute increase in time to pRBC administration did not significantly affect the odds of death, even when adjusting for injury severity. The odds ratio was 1.00 (P=0.575).
However, among the 82 patients who received their first pRBC transfusion during transport or within an hour of hospital arrival, each 10 minute increase in time to transfusion increased the odds of death. When the researchers controlled for Trauma Injury Severity Score, the odds ratio was 1.27 (P=0.044).
“Delays in the time to blood transfusion are associated with increased chances of dying,” Dr Powell said. “Shortening the time to transfusion, including having blood available in the prehospital setting, may improve outcomes.” ![]()
CASE REPORTS: Transient neutrophilia in acute mania
A description of two bipolar I disorder cases presents examples of the phenomenon of transient neutrophilia that occurred during admission into a state psychiatric hospital. A brief review of the mechanisms that may explain this hematologic response is included.
Background
In 1889, the U.S. territory of New Mexico established the New Mexico Insane Asylum, and it was known as such until 1955, when it became the State Hospital. In 1970, it became the Las Vegas Medical Center but changed its name in 2005 to the New Mexico Behavioral Health Institute (NMBHI), which services the entire state for inpatient and long-term care patients. On average, it accepts two admissions per day, of which two patients per month present with neutrophilia (white blood cell [WBC] count greater than 11,000), which resolves after 1-4 days in the hospital.
Case presentations
Case one. A 21-year-old Native American man presented with multiple psychiatric admissions for bipolar I disorder and major depression with suicidal ideation. He was brought into the local emergency department by police, who found him walking down the interstate highway trying to hitch a ride back to his native pueblo after a disagreement with a fellow resident at a local boarding home. He had discontinued his Seroquel and lithium 2 weeks earlier because he felt he no longer needed them and required medical clearance for admission.1 His presenting hemogram in the ED was normal except for an elevated WBC count of 20,000. His vital signs were normal except for tachycardia of 110 beats per minute. On exam the patient demonstrated a flat affect and anxiety but other than mild ingrown toenails and tachycardia, there were no abnormal findings.
He received a chest x-ray and abdominal computed tomography scan that were both normal, and the patient was cleared for admission. He was cooperative with staff and restarted his lithium. A repeat WBC at day 5 was 9,700.

Case two. A 24-year-old white man with a history of bipolar I disorder and dependency on benzodiazepines and Ritalin was transferred from a distant county jail after 10 days of incarceration. He started screaming in his cell, praying, and perseverating that he “needed to kill himself,” which triggered his transfer to the NMBHI. His aggressive behavior upon arrival necessitated a transfer to the local ED for sedation and four-point restraints. He received Versed and Ativan IVP before allowing a blood collection, which revealed dehydration and a WBC count of 17,100. After 4 L of normal saline, his labs normalized with a WBC of 10,100, and he was admitted for a 7-day committal.
Discussion
Neutrophilia can result from granulocytes moving from pericapillary tissue margins into the circulating pool.2 It may occur in association with vigorous exercise, seizures, paroxysmal tachycardia, and adrenergic stress.3 The duration is fewer than 30 minutes and usually results in WBC counts of 15,000-20,000.4 Beta receptors on endothelial cells may mediate neutrophil adherence and release from marginal sites. A left shift is absent, because there is no change of the inflow of cells from the marrow.
In these two cases, a transient neutrophilia and tachycardia were observed. Neither case was febrile, and the platelet count remained normal. Both patients voluntarily stopped taking their lithium about 2 weeks before decompensating from bipolar I disorders. Stress was evident in both cases, one from walking on a cold December night after a disagreement, while the other patient in case two was highly agitated and aggressive requiring four-point restraints and intravenous sedation in the ED before admission to NMBHI. Past histories of psychiatric admissions were noted in both cases, and neither subject smoked tobacco – which can increase WBC by 25%-50% with the use of one-two packs per day, respectively.5
These two cases show that clinicians should consider stress in its many permutations to the long list of causes to explain elevated WBC, particularly in the ED. They also illustrate the power of antianxiety medications for some patients with acute mania who present to the ED.
References
1. J Emerg Med. 2012;43(5):866-70.
2. “Wintrobe’s Clinical Hematology,” Philadelphia: Lea & Febiger, 1981, p.1292.
3. “Diagnostic Hematology,” London: Springer, 2009, p. 324.
4. Gen Hosp Psychiatry. 2005;27(6):454-56.
5. Euro Heart J. 2003 Jul;24(14)1365-72.
Dr. Taylor is a staff physician affiliated with the New Mexico Behavioral Health Institute, New Mexico Department of Health, Santa Fe. He reports no financial disclosures or conflicts of interest. The author wishes to thank Dr. Dan Collins from the NMBHI for recommending that he research and write about this topic. In addition, document access was greatly aided by Lisa Apodaca and Mary Bunker, CNP, from the NMBHI, and Karen Ebler and Dr. Irwin Hoffman from Christus St. Vincent Hospital in Santa Fe. Finally, the following colleagues helped by proofreading the manuscript: Dr. Wendy Dimmette, Dr. Richard Nail, and Dr. Matt Streicherz. Eva Romero and Dr. Troy Jones provided useful historical documentation.
A description of two bipolar I disorder cases presents examples of the phenomenon of transient neutrophilia that occurred during admission into a state psychiatric hospital. A brief review of the mechanisms that may explain this hematologic response is included.
Background
In 1889, the U.S. territory of New Mexico established the New Mexico Insane Asylum, and it was known as such until 1955, when it became the State Hospital. In 1970, it became the Las Vegas Medical Center but changed its name in 2005 to the New Mexico Behavioral Health Institute (NMBHI), which services the entire state for inpatient and long-term care patients. On average, it accepts two admissions per day, of which two patients per month present with neutrophilia (white blood cell [WBC] count greater than 11,000), which resolves after 1-4 days in the hospital.
Case presentations
Case one. A 21-year-old Native American man presented with multiple psychiatric admissions for bipolar I disorder and major depression with suicidal ideation. He was brought into the local emergency department by police, who found him walking down the interstate highway trying to hitch a ride back to his native pueblo after a disagreement with a fellow resident at a local boarding home. He had discontinued his Seroquel and lithium 2 weeks earlier because he felt he no longer needed them and required medical clearance for admission.1 His presenting hemogram in the ED was normal except for an elevated WBC count of 20,000. His vital signs were normal except for tachycardia of 110 beats per minute. On exam the patient demonstrated a flat affect and anxiety but other than mild ingrown toenails and tachycardia, there were no abnormal findings.
He received a chest x-ray and abdominal computed tomography scan that were both normal, and the patient was cleared for admission. He was cooperative with staff and restarted his lithium. A repeat WBC at day 5 was 9,700.

Case two. A 24-year-old white man with a history of bipolar I disorder and dependency on benzodiazepines and Ritalin was transferred from a distant county jail after 10 days of incarceration. He started screaming in his cell, praying, and perseverating that he “needed to kill himself,” which triggered his transfer to the NMBHI. His aggressive behavior upon arrival necessitated a transfer to the local ED for sedation and four-point restraints. He received Versed and Ativan IVP before allowing a blood collection, which revealed dehydration and a WBC count of 17,100. After 4 L of normal saline, his labs normalized with a WBC of 10,100, and he was admitted for a 7-day committal.
Discussion
Neutrophilia can result from granulocytes moving from pericapillary tissue margins into the circulating pool.2 It may occur in association with vigorous exercise, seizures, paroxysmal tachycardia, and adrenergic stress.3 The duration is fewer than 30 minutes and usually results in WBC counts of 15,000-20,000.4 Beta receptors on endothelial cells may mediate neutrophil adherence and release from marginal sites. A left shift is absent, because there is no change of the inflow of cells from the marrow.
In these two cases, a transient neutrophilia and tachycardia were observed. Neither case was febrile, and the platelet count remained normal. Both patients voluntarily stopped taking their lithium about 2 weeks before decompensating from bipolar I disorders. Stress was evident in both cases, one from walking on a cold December night after a disagreement, while the other patient in case two was highly agitated and aggressive requiring four-point restraints and intravenous sedation in the ED before admission to NMBHI. Past histories of psychiatric admissions were noted in both cases, and neither subject smoked tobacco – which can increase WBC by 25%-50% with the use of one-two packs per day, respectively.5
These two cases show that clinicians should consider stress in its many permutations to the long list of causes to explain elevated WBC, particularly in the ED. They also illustrate the power of antianxiety medications for some patients with acute mania who present to the ED.
References
1. J Emerg Med. 2012;43(5):866-70.
2. “Wintrobe’s Clinical Hematology,” Philadelphia: Lea & Febiger, 1981, p.1292.
3. “Diagnostic Hematology,” London: Springer, 2009, p. 324.
4. Gen Hosp Psychiatry. 2005;27(6):454-56.
5. Euro Heart J. 2003 Jul;24(14)1365-72.
Dr. Taylor is a staff physician affiliated with the New Mexico Behavioral Health Institute, New Mexico Department of Health, Santa Fe. He reports no financial disclosures or conflicts of interest. The author wishes to thank Dr. Dan Collins from the NMBHI for recommending that he research and write about this topic. In addition, document access was greatly aided by Lisa Apodaca and Mary Bunker, CNP, from the NMBHI, and Karen Ebler and Dr. Irwin Hoffman from Christus St. Vincent Hospital in Santa Fe. Finally, the following colleagues helped by proofreading the manuscript: Dr. Wendy Dimmette, Dr. Richard Nail, and Dr. Matt Streicherz. Eva Romero and Dr. Troy Jones provided useful historical documentation.
A description of two bipolar I disorder cases presents examples of the phenomenon of transient neutrophilia that occurred during admission into a state psychiatric hospital. A brief review of the mechanisms that may explain this hematologic response is included.
Background
In 1889, the U.S. territory of New Mexico established the New Mexico Insane Asylum, and it was known as such until 1955, when it became the State Hospital. In 1970, it became the Las Vegas Medical Center but changed its name in 2005 to the New Mexico Behavioral Health Institute (NMBHI), which services the entire state for inpatient and long-term care patients. On average, it accepts two admissions per day, of which two patients per month present with neutrophilia (white blood cell [WBC] count greater than 11,000), which resolves after 1-4 days in the hospital.
Case presentations
Case one. A 21-year-old Native American man presented with multiple psychiatric admissions for bipolar I disorder and major depression with suicidal ideation. He was brought into the local emergency department by police, who found him walking down the interstate highway trying to hitch a ride back to his native pueblo after a disagreement with a fellow resident at a local boarding home. He had discontinued his Seroquel and lithium 2 weeks earlier because he felt he no longer needed them and required medical clearance for admission.1 His presenting hemogram in the ED was normal except for an elevated WBC count of 20,000. His vital signs were normal except for tachycardia of 110 beats per minute. On exam the patient demonstrated a flat affect and anxiety but other than mild ingrown toenails and tachycardia, there were no abnormal findings.
He received a chest x-ray and abdominal computed tomography scan that were both normal, and the patient was cleared for admission. He was cooperative with staff and restarted his lithium. A repeat WBC at day 5 was 9,700.

Case two. A 24-year-old white man with a history of bipolar I disorder and dependency on benzodiazepines and Ritalin was transferred from a distant county jail after 10 days of incarceration. He started screaming in his cell, praying, and perseverating that he “needed to kill himself,” which triggered his transfer to the NMBHI. His aggressive behavior upon arrival necessitated a transfer to the local ED for sedation and four-point restraints. He received Versed and Ativan IVP before allowing a blood collection, which revealed dehydration and a WBC count of 17,100. After 4 L of normal saline, his labs normalized with a WBC of 10,100, and he was admitted for a 7-day committal.
Discussion
Neutrophilia can result from granulocytes moving from pericapillary tissue margins into the circulating pool.2 It may occur in association with vigorous exercise, seizures, paroxysmal tachycardia, and adrenergic stress.3 The duration is fewer than 30 minutes and usually results in WBC counts of 15,000-20,000.4 Beta receptors on endothelial cells may mediate neutrophil adherence and release from marginal sites. A left shift is absent, because there is no change of the inflow of cells from the marrow.
In these two cases, a transient neutrophilia and tachycardia were observed. Neither case was febrile, and the platelet count remained normal. Both patients voluntarily stopped taking their lithium about 2 weeks before decompensating from bipolar I disorders. Stress was evident in both cases, one from walking on a cold December night after a disagreement, while the other patient in case two was highly agitated and aggressive requiring four-point restraints and intravenous sedation in the ED before admission to NMBHI. Past histories of psychiatric admissions were noted in both cases, and neither subject smoked tobacco – which can increase WBC by 25%-50% with the use of one-two packs per day, respectively.5
These two cases show that clinicians should consider stress in its many permutations to the long list of causes to explain elevated WBC, particularly in the ED. They also illustrate the power of antianxiety medications for some patients with acute mania who present to the ED.
References
1. J Emerg Med. 2012;43(5):866-70.
2. “Wintrobe’s Clinical Hematology,” Philadelphia: Lea & Febiger, 1981, p.1292.
3. “Diagnostic Hematology,” London: Springer, 2009, p. 324.
4. Gen Hosp Psychiatry. 2005;27(6):454-56.
5. Euro Heart J. 2003 Jul;24(14)1365-72.
Dr. Taylor is a staff physician affiliated with the New Mexico Behavioral Health Institute, New Mexico Department of Health, Santa Fe. He reports no financial disclosures or conflicts of interest. The author wishes to thank Dr. Dan Collins from the NMBHI for recommending that he research and write about this topic. In addition, document access was greatly aided by Lisa Apodaca and Mary Bunker, CNP, from the NMBHI, and Karen Ebler and Dr. Irwin Hoffman from Christus St. Vincent Hospital in Santa Fe. Finally, the following colleagues helped by proofreading the manuscript: Dr. Wendy Dimmette, Dr. Richard Nail, and Dr. Matt Streicherz. Eva Romero and Dr. Troy Jones provided useful historical documentation.
In severe hemophilia B, rIX-FP prophylaxis gets good results with less frequent dosing
Recombinant fusion protein linking coagulation factor IX with albumin (rIX-FP) could result in a “paradigm shift” in prophylaxis regimens for patients with hemophilia B, according to researchers reporting results from a phase III trial.
At dosing intervals of up to 14 days, rIX-FP was a safe and effective factor IX (FIX) replacement product for preventing and treating bleeding episodes in an open-label study of 63 previously treated adolescents and adults with severe hemophilia B.
Compared to standard FIX products, rIX-FP is more active, has a longer half-life, and has better clearance. A study is now underway to see whether a 21-day prophylaxis regimen provides even better results than the 14-day regimen, reported Dr. Elena Santagostino of Istituto di Ricovero e Cura a Carattere Scientifico Ca’ Granda Foundation, Maggiore Hospital Policlinico, Milan, and her associates in the PROLONG-9FP Investigators Study Group.
For prophylaxis, standard FIX products are administered two times per week. Patients are at increased risk of bleeding when their FIX activity is low, particularly just before their next scheduled dose. With the prolonged FIX activity of rIX-FP, the dosing is less frequent and that could potentially improve adherence, the researchers wrote (Blood. 2016;127[14]:1761-9).
In addition, rIX-FP was associated with a reduced risk of spontaneous and trauma-related bleeding episodes.
The findings came from a study of patients who had FIX activity levels of 2% or less and were assigned to one of two groups. The first group received routine prophylaxis once every 7 days for 26 weeks; they were then placed on either a 7-, 10-, or 14-day prophylaxis regimen for a mean of 50, 38, or 51 weeks, respectively. The second group received on-demand treatment of bleeding episodes for 26 weeks and were then switched to a 7-day prophylaxis regimen for a mean of 45 weeks. Only patients who were previously receiving on-demand treatment were eligible for group 2 assignment.
Patients self-administered rIX-FP for routine prophylaxis and on-demand treatment of bleeding episodes; all home administrations were recorded in an electronic diary. A second dose of rIX-FP was administered at least 24 hours after the first injection, if needed, to achieve hemostasis. Efficacy and safety assessments were performed at study sites on a monthly basis.
The study design controlled for the variability of bleeding frequency within the hemophilia B patient population, as group 2 patients started rIX-FP treatment on-demand and continued on 7-day prophylaxis. During the on-demand phase, rIX-FP controlled 98.6% of bleeding episodes, and 93.6% of bleeds were controlled with one infusion.
Once the group was switched to 7-day prophylaxis, the median annualized spontaneous bleeding rate dropped to 0.0 and all target joints resolved.(P less than .0001). No patient developed an inhibitor, and no safety concerns were identified.
The annualized bleeding rate decreased slightly in group 2 patients during on-demand treatment with rIX-FP, compared with the rate in the 12-month period before they entered the study. The researchers speculated that on-demand treatment with rIX-FP “might provide, to a certain extent, some protection against a subsequent bleeding episode. Therefore, patients with a severe bleeding phenotype treated on-demand might experience fewer bleeding episodes if switched to rIX-FP. Furthermore, such on-demand patients who require more than one infusion per month may have the benefit of a reduction of ABR by administering a similar number of infusions according to a 14-day prophylaxis regimen with rIX-FP.”
The sample size was small, however, and differences of care in the clinical study could have affected the results.
Compared to other FIX products, rIX-FP had an extended terminal half-life of 102 hours, 4.3-fold longer than seen with the patients’ previous FIX treatment. Other pharmacokinetic measures such as area under the curve (7176 h × IU/dL), clearance (0.77 mL/h per kg), and incremental recovery (1.27 IU/dL per IU/kg) also were improved. Patients maintained a mean trough of 20 and 12 IU/dL FIX activity on prophylaxis with rIX-FP 40 IU/kg every 7 days and 75 IU/kg every 14 days, respectively. Rather than half-life alone, a slower clearance may be a factor in the success of prophylaxis regimens, the researchers said.
The principal investigators received research support from CSL Behring, which sponsored the study and was responsible for trial operations and data analysis.
On Twitter @maryjodales
Recombinant fusion protein linking coagulation factor IX with albumin (rIX-FP) could result in a “paradigm shift” in prophylaxis regimens for patients with hemophilia B, according to researchers reporting results from a phase III trial.
At dosing intervals of up to 14 days, rIX-FP was a safe and effective factor IX (FIX) replacement product for preventing and treating bleeding episodes in an open-label study of 63 previously treated adolescents and adults with severe hemophilia B.
Compared to standard FIX products, rIX-FP is more active, has a longer half-life, and has better clearance. A study is now underway to see whether a 21-day prophylaxis regimen provides even better results than the 14-day regimen, reported Dr. Elena Santagostino of Istituto di Ricovero e Cura a Carattere Scientifico Ca’ Granda Foundation, Maggiore Hospital Policlinico, Milan, and her associates in the PROLONG-9FP Investigators Study Group.
For prophylaxis, standard FIX products are administered two times per week. Patients are at increased risk of bleeding when their FIX activity is low, particularly just before their next scheduled dose. With the prolonged FIX activity of rIX-FP, the dosing is less frequent and that could potentially improve adherence, the researchers wrote (Blood. 2016;127[14]:1761-9).
In addition, rIX-FP was associated with a reduced risk of spontaneous and trauma-related bleeding episodes.
The findings came from a study of patients who had FIX activity levels of 2% or less and were assigned to one of two groups. The first group received routine prophylaxis once every 7 days for 26 weeks; they were then placed on either a 7-, 10-, or 14-day prophylaxis regimen for a mean of 50, 38, or 51 weeks, respectively. The second group received on-demand treatment of bleeding episodes for 26 weeks and were then switched to a 7-day prophylaxis regimen for a mean of 45 weeks. Only patients who were previously receiving on-demand treatment were eligible for group 2 assignment.
Patients self-administered rIX-FP for routine prophylaxis and on-demand treatment of bleeding episodes; all home administrations were recorded in an electronic diary. A second dose of rIX-FP was administered at least 24 hours after the first injection, if needed, to achieve hemostasis. Efficacy and safety assessments were performed at study sites on a monthly basis.
The study design controlled for the variability of bleeding frequency within the hemophilia B patient population, as group 2 patients started rIX-FP treatment on-demand and continued on 7-day prophylaxis. During the on-demand phase, rIX-FP controlled 98.6% of bleeding episodes, and 93.6% of bleeds were controlled with one infusion.
Once the group was switched to 7-day prophylaxis, the median annualized spontaneous bleeding rate dropped to 0.0 and all target joints resolved.(P less than .0001). No patient developed an inhibitor, and no safety concerns were identified.
The annualized bleeding rate decreased slightly in group 2 patients during on-demand treatment with rIX-FP, compared with the rate in the 12-month period before they entered the study. The researchers speculated that on-demand treatment with rIX-FP “might provide, to a certain extent, some protection against a subsequent bleeding episode. Therefore, patients with a severe bleeding phenotype treated on-demand might experience fewer bleeding episodes if switched to rIX-FP. Furthermore, such on-demand patients who require more than one infusion per month may have the benefit of a reduction of ABR by administering a similar number of infusions according to a 14-day prophylaxis regimen with rIX-FP.”
The sample size was small, however, and differences of care in the clinical study could have affected the results.
Compared to other FIX products, rIX-FP had an extended terminal half-life of 102 hours, 4.3-fold longer than seen with the patients’ previous FIX treatment. Other pharmacokinetic measures such as area under the curve (7176 h × IU/dL), clearance (0.77 mL/h per kg), and incremental recovery (1.27 IU/dL per IU/kg) also were improved. Patients maintained a mean trough of 20 and 12 IU/dL FIX activity on prophylaxis with rIX-FP 40 IU/kg every 7 days and 75 IU/kg every 14 days, respectively. Rather than half-life alone, a slower clearance may be a factor in the success of prophylaxis regimens, the researchers said.
The principal investigators received research support from CSL Behring, which sponsored the study and was responsible for trial operations and data analysis.
On Twitter @maryjodales
Recombinant fusion protein linking coagulation factor IX with albumin (rIX-FP) could result in a “paradigm shift” in prophylaxis regimens for patients with hemophilia B, according to researchers reporting results from a phase III trial.
At dosing intervals of up to 14 days, rIX-FP was a safe and effective factor IX (FIX) replacement product for preventing and treating bleeding episodes in an open-label study of 63 previously treated adolescents and adults with severe hemophilia B.
Compared to standard FIX products, rIX-FP is more active, has a longer half-life, and has better clearance. A study is now underway to see whether a 21-day prophylaxis regimen provides even better results than the 14-day regimen, reported Dr. Elena Santagostino of Istituto di Ricovero e Cura a Carattere Scientifico Ca’ Granda Foundation, Maggiore Hospital Policlinico, Milan, and her associates in the PROLONG-9FP Investigators Study Group.
For prophylaxis, standard FIX products are administered two times per week. Patients are at increased risk of bleeding when their FIX activity is low, particularly just before their next scheduled dose. With the prolonged FIX activity of rIX-FP, the dosing is less frequent and that could potentially improve adherence, the researchers wrote (Blood. 2016;127[14]:1761-9).
In addition, rIX-FP was associated with a reduced risk of spontaneous and trauma-related bleeding episodes.
The findings came from a study of patients who had FIX activity levels of 2% or less and were assigned to one of two groups. The first group received routine prophylaxis once every 7 days for 26 weeks; they were then placed on either a 7-, 10-, or 14-day prophylaxis regimen for a mean of 50, 38, or 51 weeks, respectively. The second group received on-demand treatment of bleeding episodes for 26 weeks and were then switched to a 7-day prophylaxis regimen for a mean of 45 weeks. Only patients who were previously receiving on-demand treatment were eligible for group 2 assignment.
Patients self-administered rIX-FP for routine prophylaxis and on-demand treatment of bleeding episodes; all home administrations were recorded in an electronic diary. A second dose of rIX-FP was administered at least 24 hours after the first injection, if needed, to achieve hemostasis. Efficacy and safety assessments were performed at study sites on a monthly basis.
The study design controlled for the variability of bleeding frequency within the hemophilia B patient population, as group 2 patients started rIX-FP treatment on-demand and continued on 7-day prophylaxis. During the on-demand phase, rIX-FP controlled 98.6% of bleeding episodes, and 93.6% of bleeds were controlled with one infusion.
Once the group was switched to 7-day prophylaxis, the median annualized spontaneous bleeding rate dropped to 0.0 and all target joints resolved.(P less than .0001). No patient developed an inhibitor, and no safety concerns were identified.
The annualized bleeding rate decreased slightly in group 2 patients during on-demand treatment with rIX-FP, compared with the rate in the 12-month period before they entered the study. The researchers speculated that on-demand treatment with rIX-FP “might provide, to a certain extent, some protection against a subsequent bleeding episode. Therefore, patients with a severe bleeding phenotype treated on-demand might experience fewer bleeding episodes if switched to rIX-FP. Furthermore, such on-demand patients who require more than one infusion per month may have the benefit of a reduction of ABR by administering a similar number of infusions according to a 14-day prophylaxis regimen with rIX-FP.”
The sample size was small, however, and differences of care in the clinical study could have affected the results.
Compared to other FIX products, rIX-FP had an extended terminal half-life of 102 hours, 4.3-fold longer than seen with the patients’ previous FIX treatment. Other pharmacokinetic measures such as area under the curve (7176 h × IU/dL), clearance (0.77 mL/h per kg), and incremental recovery (1.27 IU/dL per IU/kg) also were improved. Patients maintained a mean trough of 20 and 12 IU/dL FIX activity on prophylaxis with rIX-FP 40 IU/kg every 7 days and 75 IU/kg every 14 days, respectively. Rather than half-life alone, a slower clearance may be a factor in the success of prophylaxis regimens, the researchers said.
The principal investigators received research support from CSL Behring, which sponsored the study and was responsible for trial operations and data analysis.
On Twitter @maryjodales
FROM BLOOD
Key clinical point: Weekly and 14-day prophylaxis regimens with rIX-FP were well tolerated and provided low bleeding rates and target joint improvement.
Major finding: On 7-day prophylaxis, the median annualized spontaneous bleeding rate dropped to 0.0 and all target joints resolved (P less than .0001).
Data source: An open-label study of 63 previously treated adolescents and adults with severe hemophilia B.
Disclosures: The principal investigators received research support from CSL Behring, which sponsored the study and was responsible for trial operations and data analysis.
Merkel Cell Carcinoma: A Review
Merkel cells originally were described by German histopathologist Friedrich Sigmund Merkel in 1875. These unique tactile cells were described as epidermal, nondendritic, and nonkeratinizing. Merkel cells are thought to arise from the neural crest and are believed to be primary neural cells found within the basal layer of the epidermis.1,2 They likely function primarily as slowly adapting type I mechanoreceptors. Origin from the neural crest is controversial, as other investigators have suggested derivation from epidermal keratinocytes.1,2 Tumor cells have been linked to the amine precursor uptake and decarboxylation system.3 In 1972, Toker4 described several cases of trabecular or sweat gland carcinomas of the skin. Upon further investigation, the cells that comprised these tumors were found to have dense core granules on electron microscopy, typical of Merkel cells.1,2 Other terms such as neuroendocrine carcinoma of the skin, small cell carcinoma of the skin, and anaplastic carcinoma of the skin have been used to describe Merkel cell carcinoma (MCC),1 which was suggested by De Wolf-Peeters et al5 in 1980.
Despite being a rare malignancy, MCC follows an aggressive clinical course. Upon presentation, approximately 66% of patients have local disease, 27% have nodal involvement, and 7% have distant metastasis.1 Future treatments will likely center around the novel Merkel cell polyomavirus (MCPyV) and modification of immune responses toward tumor cells. Standardization continues to be lacking in both staging and treatment of this aggressive tumor.
Epidemiology of MCC

| ||
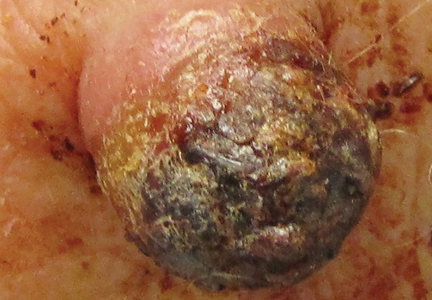
Figure 1. A 2.3×1.5×1.2-cm, hemorrhagic, crusted, | ||
| ||
| ||
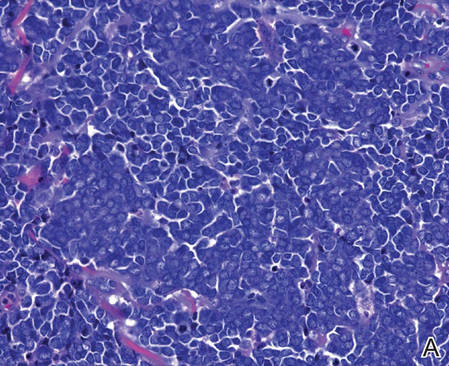
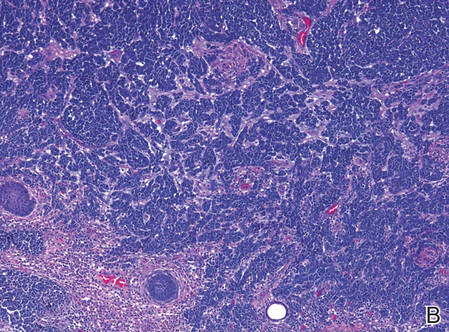
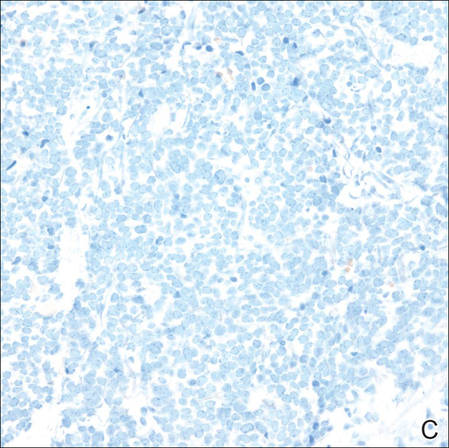
| Figure 2. Merkel cells are small- to medium-sized cells with round nuclei and scant cytoplasm. Granular or stippled chromatin can be seen (A)(H&E, original magnification ×40). Merkel cell carcinoma with trabecular pattern (B) (H&E, original magnification ×10). | ||
Between 1986 and 2006, the incidence of MCC grew substantially.1,2 Figures have been reported at 0.15 cases per 100,000 individuals to 0.6 cases per 100,000 individuals worldwide. In the United States, the age-adjusted incidence of MCC is estimated at 0.24 per 100,000 person-years, which is higher than the estimated 0.13 per 100,000 person-years found in Europe.3 The highest incidence worldwide has been noted in Western Australia, likely due to high levels of UV exposure.1 The incidence of MCC in psoriasis patients who are treated with oral methoxsalen (psoralen) and UVA photochemotherapy is 100 times greater than in the general population, further supporting the role of UV light in the development of MCC.1 White individuals have the highest incidence of MCC worldwide, with men being affected more frequently than women.1,3 The majority of patients with MCC are diagnosed at 70 years or older.1 Approximately 5% of reported MCC patients are diagnosed before 50 years of age.2 Immunosuppression and immunodeficiency likely play a role in the pathogenesis of MCC, and the incidence is increased in solid organ transplant recipients, most commonly renal transplant recipients,1 as well as individuals with chronic lymphocytic leukemia, human immunodeficiency virus infection, and AIDS.1,3 Patients with autoimmune diseases such as rheumatoid arthritis also are at increased risk for MCC.3 Individuals who are diagnosed with MCC are at an increased risk for development of other malignancies including nonmelanoma skin cancers, chronic lymphocytic leukemia, Hodgkin lymphoma, and non-Hodgkin lymphoma.3
Clinical Presentation of MCC
The clinical presentation of MCC can be variable. Most tumors present as firm, red to purple, nontender papules or nodules (Figure 1).1 Tumor size may range from 2 to 200 mm but is most commonly less than 20 mm.2 Growth can be rapid, and tumors are most commonly located on sun-exposed skin. The head and neck areas account for 48% of all MCC cases,1 with the eyelids being frequently involved.2 Merkel cell carcinoma also has been reported on the arms, legs, trunk, back, and buttocks.1 Non–sun-exposed areas are less commonly affected. Mucosal sites (eg, larynx, nasal cavity, pharynx, mouth) account for 5% of primary MCCs.1 Merkel cell carcinoma also has been reported to affect the vulva and penis. Subcutaneous primary MCC has presented without overlying epidermal changes.1 In a case series by Heath et al,6 14% (27/195) of MCC patients presented with nodal disease without any identifiable primary tumor, with the inguinal nodal chain being the most common for this presentation. It currently is not known whether these nodal tumors are primary tumors or metastatic disease with a regression of the primary tumor.1
|
Histopathology of MCC
| ||
| ||
| ||
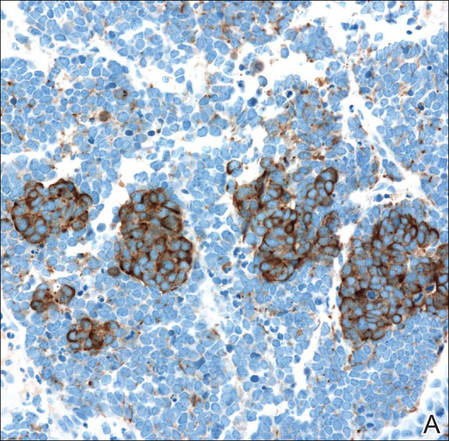
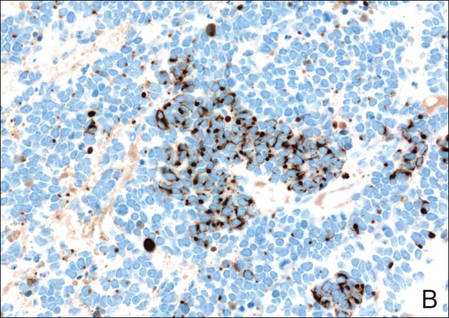
Figure 3. Positive chromogranin staining (A)(original | ||
Merkel cells are small- to medium-sized basophilic cells with round nuclei and scant cytoplasm. Granular or stippled chromatin can be seen on histopathology (Figure 2A).1 Some tumor cells have more vesicular chromatin, multiple small nucleoli, irregular contours, and more abundant cytoplasm. In some reports, irregular contours and abundant cytoplasm were associated with no detectible MCPyV infection.1,3 Merkel cell carcinomas have a primarily nodular architecture, and classification is based on growth pattern and cell size. Three histopathologic growth patterns have been described: nodular, infiltrative, and trabecular. The trabecular pattern is composed of interconnecting strands ofcells (Figure 2B). Tumors with solely intraepidermal involvement (MCC in situ) have been described but are exceedingly rare.1 Cell types are classified according to size, with the intermediate cell type being the most common. The small cell variant may be mistaken for a lymphocytic infiltrate due to the similar size and appearance of both types of cells.1,3
Merkel cell carcinomas can have histopathological overlap with lymphomas, small cell lung cancers, carcinoid tumors, primitive neuroectodermal tumors, neuroblastomas, small cell osteosarcomas, rhabdomyosarcomas, or Ewing sarcomas.1,3 Specifically, differentiation from small cell carcinoma of the lung is of utmost importance. Merkel cell carcinoma stains positively for cytokeratins 8, 18, 19, and 20. The neuroendocrine markers chromogranin (Figure 3A), synaptophysin, and neuron-specific enolase also may show positive staining. Cytokeratin 20, low-molecular-weight cytokeratins (CAM 5.2), and neurofilament immunostains have a high sensitivity for MCC and are the most frequently used.1 Cytokeratin 20 stains in the characteristic paranuclear dot–like pattern, which is a hallmark of MCC (Figure 3B). Cytokeratin 20 positivity in conjunction with negative staining for thyroid transcription factor 1 (Figure 3C) and cytokeratin 7 can aid in differentiation from small cell carcinoma of the lung.1,3
Pathogenesis of MCC
In 2008, Feng et al7 discovered a novel polyomavirus associated with the development of MCC. This novel polyomavirus, MCPyV, is found in approximately 80% of all cases of MCC. Seventeen members of the polyomavirus family have been identified, 9 of which have been found to infect humans, including BK virus, JC virus, WU, MCPyV, human polyomavirus 6, human polyomavirus 7, trichodysplasia spinulosa–associated polyomavirus, human polyomavirus 9, and Simian virus 40.1 Merkel cell polyomavirus infection is found in approximately 60% of the general population and exposure likely occurs early in life. The virus likely is transmitted through skin shedding and nasal secretions, though it also has been found in urine specimens.3 Currently, there is no evidence to suggest vertical viral transmission from mother to fetus.
Merkel cell polyomavirus is composed of early and late gene regions. The early gene region contains both large T antigen (LT) and small T antigen reading frames, which are necessary for viral replication.8 The late region is responsible for encoding viral proteins necessary for viral capsid assembly. Mutations found in viral protein 1 prevent formation of viral particles.9 Large T antigen is substantially overexpressed in MCC and is responsible for tumor suppression through retinoblastoma tumor suppressor protein. It also serves as a binding domain for both heat shock proteins and helicases.8,10 These domains allow the polyomaviruses to use host-cell machinery for viral genome replication while targeting tumor suppressor proteins.8 Upon viral integration into host DNA, viral replication ceases while oncogenic function persists.
The exact mechanism by which the MCPyV contributes to the development of MCC still has yet to be identified. Hypotheses suggest a combination of viral infection with external mutagens (eg, UV radiation). Experimental observations suggest viral contribution is likely due to the large percentage of MCCs that are positive for MCPyV, the identification of LT antigen expression and the role it plays in preserving cell cycle progression, and the role persistent LT antigen expression plays in continued growth of MCC cell lines in vitro.8 Two important cell line preservation mechanisms ensure continued tumor growth, including prevention of apoptosis triggered by DNA damage response mechanisms following UV damage and interaction with the retinoblastoma tumor suppressor protein allowing continued growth.8,11 Other important factors in tumor growth and survival may be the inhibition of apoptosis through the BCL2 (B-cell chronic lymphocytic leukemia/lymphoma 2) proto-oncogene and survivin (baculoviral inhibitor of apoptosis repeat-containing 5 [BIRC5]).12 Survivin has been found to play an important role in MCPyV-positive MCCs.12,13 It has been suggested that lymphangiogenesis in MCC likely is driven by vascular endothelial growth factor-C+CD68+CD163+ M2 macrophages.14 Another survival mechanism specific to polyomaviruses is their ability to interfere with the p53 tumor suppressor pathway.8 Loss of p53 expression by tumor cell nuclei has been associated with poor prognosis.15
Immune Response
Immune response as a role in tumor progression can be primarily centered on the concept of persistent antigen expression as a means of immune downregulation. Dunn et al16 suggested that cancer cells must interact through 3 consecutive phases with the host immune system (immunoediting hypothesis). In the elimination phase, the host immune system is able to recognize and destroy newly transformed cells through both the innate and adaptive immune systems. The second equilibrium phase allows the tumor to remain dormant and growth remains stagnant. Lastly, the tumor is allowed to evade the immune system through the escape phase.8
Host immune responses play an important role in both the progression and prognosis of MCC. High anti-MCPyV capsid antibody titers have been associated with better progression-free survival in some patients.8 Patients with high antibody titers (>10,000) likely have better progression-free survival than those with low antibody titers (<10,000).17 Antibody titers to the LT antigen may serve as a biomarker of MCC disease burden in the future. Rising LT antigen titers have been shown to correlate with disease progression and falling titers correlate with successful treatment.8 Tumoral infiltration of CD8+ T lymphocytes has been shown to be a predictor of survival compared to no intratumoral infiltration.6 Sihto et al18 suggested that this better prognosis from high intratumoral infiltration is not specific to MCPyV-positive MCC; however, it does highlight an important aspect of tumor evasion through the downregulation of cell surface expression of class I major histocompatibility complex antigens, which allows presentation of tumor intracellular peptides to CD8+ T lymphocytes.8 Upregulation of this specific immune response may play a role in the future treatment of MCC.
Staging and Prognosis
Due to the extremely aggressive nature of MCC, patients with local disease and tumors 2 cm or smaller in diameter have a 66% survival at 5 years.1,3 The 5-year survival rate for patients with local metastasis to regional lymph nodes ranges from 26% to 42%. Patients with distant metastasis have an 18% survival rate at 5 years.1,3 Data suggest that sentinel lymph node biopsy should be performed on all patients with MCC regardless of tumor size.1 There are no consensus guidelines to date regarding imaging for the staging of MCC patients. It is suggested that (18F)fluorodeoxyglucose positron emission tomography alone or in combination with computed tomography (CT) may be of value as a single whole-body diagnostic tool for accurate staging.10 It also has been suggested that (18F)fluorodeoxyglucose positron emission tomography and CT may offer more accurate staging than other screening modalities such as CT alone or magnetic resonance imaging.14,19
Treatment of MCC
Surgery remains the mainstay of treatment of MCC. Current National Comprehensive Cancer Network guidelines20 recommend 1- to 2-cm margins for wide local excision or treatment with Mohs micrographic surgery. Sentinel lymph node biopsy should be performed intraoperatively in patients undergoing wide local excision and preoperatively for patients undergoing Mohs micrographic surgery due to potential alterations in lymphatic drainage that may affect lymphoscintigraphy.1
Radiation may be used as primary or adjuvant therapy in patients with MCC. Radiation as primary therapy generally is reserved for patients who are not surgical candidates. It has been suggested that there was no difference in outcome in a small group of patients treated with radiation alone compared to patients who underwent surgery and radiation to the tumor bed.1 Current guidelines suggest a small group of patients may not require adjuvant therapy following adequate resection of some small tumors, and clinical observation may be appropriate.1,3 Chemotherapy may play a palliative role in patients with metastatic MCC. Merkel cell carcinoma has been shown to be chemosensitive but with a high recurrence rate.1 Because the immune system plays an important role in disease prognosis, having an intact immune system likely is paramount in the prevention of further disease progression.
Future Treatments of MCC
Future treatment of MCC may be focused on the viral etiology of most tumors and upregulation of the immune response, which may lead to the possibility of specifically interfering with virus-specific oncoproteins and stimulation of immune responses to virally infected tumor cells.8 The MCPyV large T antigen has been found to be overexpressed in some tumors and may serve as a specific target of therapy.10,21 Survivin, a key cell cycle protein encoded by LT antigen, may be an interesting target given its implication in other cancers.13 Other potential nonviral molecular target antigens include the oncoprotein H1P1 that interacts with c-KIT.8 Specific immunostimulatory cytokines that may be used to upregulate the immune response to tumoral cells may include IL-2, IL-12, IL-15, or IL-21. Therapeutic agents that may be studied in the future to target the immune exhaustion phenomenon associated with tumorigenesis include ipilimumab (cytotoxic T lymphocyte antigen 4 receptor-blocking agent) as well as programmed cell death 1 and programmed cell death 1 ligand 1 (PD-1/PD-L1).8 Neuroendocrine tumors including MCC tend to be highly vascular and express vascular endothelial growth factors and platelet-derived growth factors, which may be other potential therapeutic targets. It has been reported that approximately 95% of MCC patients have CD56+ tumors, and current clinical trials suggest a promising therapeutic response with the immunogen anti-CD56 monoclonal antibody.3
Conclusion
Merkel cell carcinoma is a rare aggressive neuroendocrine tumor that has been associated with a novel polyomavirus. Merkel cell carcinoma tends to affect elderly and immunocompromised patients as well as white individuals. Tumors are most often found in areas of high UV exposure and clinically on sun-exposed skin. Merkel cell polyomavirus is associated with approximately 80% of tumors, and tumorigenesis likely is caused by a number of sequential steps from viral integration into host DNA, mutagenic events, and specific immune responses. Currently there are no consensus guidelines for using imaging for staging of MCCs, but sentinel lymph node biopsy is recommended for all cases due to the aggressive nature of even smaller tumors. Surgery remains the mainstay of treatment, and radiation therapy may be used as a primary or adjuvant treatment. Chemotherapy usually is reserved for patients with metastatic disease purely for palliation. Future treatments of MCC likely will center on the viral etiology of MCC and upregulation of immune responses to virally infected tumor cells.
1. Han S, North J, Canavan T, et al. Merkel cell carcinoma. Hematol Oncol Clin N Am. 2012;26:1351-1374.
2. Goessling W, McKee P, Mayer R. Merkel cell carcinoma. J Clin Oncol. 2002;20:588-598.
3. Donepudi S, DeConti R, Samlowski W. Recent advances in the understanding of the genetics, etiology, and treatment of merkel cell carcinoma. Semin Oncol. 2012;39:163-172.
4. Toker C. Trabecular carcinoma of the skin. Arch Dermatol. 1972;105:107-110.
5. De Wolff-Peeters C, Marien K, Mebis J, et al. A cutaneous APUDoma or Merkel cell tumor? a morphologically recognizable tumor with a biological and histological malignant aspect in contrast with its clinical behavior. Cancer. 1980;46:810-816.
6. Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
7. Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096-1100.
8. Bhatia S, Afanasiev O, Nghiem P. Immunobiology of Merkel cell carcinoma: implications for immunotherapy of a polyomavirus-associated cancer. Curr Oncol Rep. 2011;13:488-497.
9. Amber K, McLeod M, Nouri K. The Merkel cell polyomavirus and its involvement in Merkel cell carcinoma. Dermatol Surg. 2013;39:232-238.
10. Erovic B, Al Habeeb A, Harris L, et al. Significant overexpression of the Merkel cell polyomavirus (MCPyV) large T antigen in Merkel cell carcinoma. Head Neck. 2013;35:184-189.
11. Demetriou S, Ona-Vu K, Sullivan E, et al. Defective DNA repair and cell cycle arrest in cells expressing Merkel cell polyomavirus T antigen. Int J Cancer. 2012;131:1818-1827.
12. Sahi H, Koljonen V, Kavola H, et al. Bcl-2 expression indicates better prognosis of Merkel cell carcinoma regardless of the presence of Merkel cell polyomavirus. Virchows Arch. 2012;461:553-559.
13. Arora R, Shuda M, Guastafierro A, et al. Survivin is a therapeutic target in Merkel cell carcinoma. Sci Transl Med. 2012;4:1-11.
14. Hawryluk E, O’Regan K, Sheehy N, et al. Positron emission tomography/computed tomography imaging in Merkel cell carcinoma: a study of 270 scans in 97 patients at the Dana-Farber/Brigham and Women’s Cancer Center. J Am Acad Dermatol. 2013;68:592-599.
15. Hall B, Pincus L, Yu S, et al. Immunohistochemical prognostication of Merkel cell carcinoma: p63 expression but not polyomavirus status correlates with outcome. J Cutan Pathol. 2012;39:911-917.
16. Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991-998.
17. Touze A, Le Bidre E, Laude H, et al. High levels of antibodies against Merkel cell polyomavirus identify a subset of patients with Merkel cell carcinoma with better clinical outcome. J Clin Oncol. 2011;29:1612-1619.
18. Sihto H, Bohling T, Kavola H, et al. Tumor infiltrating immune cells and outcome of Merkel cell carcinoma: a population-based study. Clin Cancer Res. 2012;18:2872-2881.
19. Colgan M, Tarantola T, Weaver A, et al. The predictive value of imaging studies in evaluating regional lymph node involvement in Merkel cell carcinoma. J Am Acad Dermatol. 2012;67:1250-1256.
20. NCCN Clinical Practice Guidelines in Oncology. National Comprehensive Cancer Network website. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site. Accessed March 22, 2016.
21. Angermeyer S, Hesbacher S, Becker J, et al. Merkel cell polyomavirus-positive Merkel cell carcinoma cells do not require expression of the viral small T antigen. J Invest Dermatol. 2013;133:1-6.
Merkel cells originally were described by German histopathologist Friedrich Sigmund Merkel in 1875. These unique tactile cells were described as epidermal, nondendritic, and nonkeratinizing. Merkel cells are thought to arise from the neural crest and are believed to be primary neural cells found within the basal layer of the epidermis.1,2 They likely function primarily as slowly adapting type I mechanoreceptors. Origin from the neural crest is controversial, as other investigators have suggested derivation from epidermal keratinocytes.1,2 Tumor cells have been linked to the amine precursor uptake and decarboxylation system.3 In 1972, Toker4 described several cases of trabecular or sweat gland carcinomas of the skin. Upon further investigation, the cells that comprised these tumors were found to have dense core granules on electron microscopy, typical of Merkel cells.1,2 Other terms such as neuroendocrine carcinoma of the skin, small cell carcinoma of the skin, and anaplastic carcinoma of the skin have been used to describe Merkel cell carcinoma (MCC),1 which was suggested by De Wolf-Peeters et al5 in 1980.
Despite being a rare malignancy, MCC follows an aggressive clinical course. Upon presentation, approximately 66% of patients have local disease, 27% have nodal involvement, and 7% have distant metastasis.1 Future treatments will likely center around the novel Merkel cell polyomavirus (MCPyV) and modification of immune responses toward tumor cells. Standardization continues to be lacking in both staging and treatment of this aggressive tumor.
Epidemiology of MCC

| ||
Figure 1. A 2.3×1.5×1.2-cm, hemorrhagic, crusted, | ||

| ||

| ||
| Figure 2. Merkel cells are small- to medium-sized cells with round nuclei and scant cytoplasm. Granular or stippled chromatin can be seen (A)(H&E, original magnification ×40). Merkel cell carcinoma with trabecular pattern (B) (H&E, original magnification ×10). | ||
Between 1986 and 2006, the incidence of MCC grew substantially.1,2 Figures have been reported at 0.15 cases per 100,000 individuals to 0.6 cases per 100,000 individuals worldwide. In the United States, the age-adjusted incidence of MCC is estimated at 0.24 per 100,000 person-years, which is higher than the estimated 0.13 per 100,000 person-years found in Europe.3 The highest incidence worldwide has been noted in Western Australia, likely due to high levels of UV exposure.1 The incidence of MCC in psoriasis patients who are treated with oral methoxsalen (psoralen) and UVA photochemotherapy is 100 times greater than in the general population, further supporting the role of UV light in the development of MCC.1 White individuals have the highest incidence of MCC worldwide, with men being affected more frequently than women.1,3 The majority of patients with MCC are diagnosed at 70 years or older.1 Approximately 5% of reported MCC patients are diagnosed before 50 years of age.2 Immunosuppression and immunodeficiency likely play a role in the pathogenesis of MCC, and the incidence is increased in solid organ transplant recipients, most commonly renal transplant recipients,1 as well as individuals with chronic lymphocytic leukemia, human immunodeficiency virus infection, and AIDS.1,3 Patients with autoimmune diseases such as rheumatoid arthritis also are at increased risk for MCC.3 Individuals who are diagnosed with MCC are at an increased risk for development of other malignancies including nonmelanoma skin cancers, chronic lymphocytic leukemia, Hodgkin lymphoma, and non-Hodgkin lymphoma.3
Clinical Presentation of MCC
The clinical presentation of MCC can be variable. Most tumors present as firm, red to purple, nontender papules or nodules (Figure 1).1 Tumor size may range from 2 to 200 mm but is most commonly less than 20 mm.2 Growth can be rapid, and tumors are most commonly located on sun-exposed skin. The head and neck areas account for 48% of all MCC cases,1 with the eyelids being frequently involved.2 Merkel cell carcinoma also has been reported on the arms, legs, trunk, back, and buttocks.1 Non–sun-exposed areas are less commonly affected. Mucosal sites (eg, larynx, nasal cavity, pharynx, mouth) account for 5% of primary MCCs.1 Merkel cell carcinoma also has been reported to affect the vulva and penis. Subcutaneous primary MCC has presented without overlying epidermal changes.1 In a case series by Heath et al,6 14% (27/195) of MCC patients presented with nodal disease without any identifiable primary tumor, with the inguinal nodal chain being the most common for this presentation. It currently is not known whether these nodal tumors are primary tumors or metastatic disease with a regression of the primary tumor.1
|
Histopathology of MCC

| ||

| ||

| ||
Figure 3. Positive chromogranin staining (A)(original | ||
Merkel cells are small- to medium-sized basophilic cells with round nuclei and scant cytoplasm. Granular or stippled chromatin can be seen on histopathology (Figure 2A).1 Some tumor cells have more vesicular chromatin, multiple small nucleoli, irregular contours, and more abundant cytoplasm. In some reports, irregular contours and abundant cytoplasm were associated with no detectible MCPyV infection.1,3 Merkel cell carcinomas have a primarily nodular architecture, and classification is based on growth pattern and cell size. Three histopathologic growth patterns have been described: nodular, infiltrative, and trabecular. The trabecular pattern is composed of interconnecting strands ofcells (Figure 2B). Tumors with solely intraepidermal involvement (MCC in situ) have been described but are exceedingly rare.1 Cell types are classified according to size, with the intermediate cell type being the most common. The small cell variant may be mistaken for a lymphocytic infiltrate due to the similar size and appearance of both types of cells.1,3
Merkel cell carcinomas can have histopathological overlap with lymphomas, small cell lung cancers, carcinoid tumors, primitive neuroectodermal tumors, neuroblastomas, small cell osteosarcomas, rhabdomyosarcomas, or Ewing sarcomas.1,3 Specifically, differentiation from small cell carcinoma of the lung is of utmost importance. Merkel cell carcinoma stains positively for cytokeratins 8, 18, 19, and 20. The neuroendocrine markers chromogranin (Figure 3A), synaptophysin, and neuron-specific enolase also may show positive staining. Cytokeratin 20, low-molecular-weight cytokeratins (CAM 5.2), and neurofilament immunostains have a high sensitivity for MCC and are the most frequently used.1 Cytokeratin 20 stains in the characteristic paranuclear dot–like pattern, which is a hallmark of MCC (Figure 3B). Cytokeratin 20 positivity in conjunction with negative staining for thyroid transcription factor 1 (Figure 3C) and cytokeratin 7 can aid in differentiation from small cell carcinoma of the lung.1,3
Pathogenesis of MCC
In 2008, Feng et al7 discovered a novel polyomavirus associated with the development of MCC. This novel polyomavirus, MCPyV, is found in approximately 80% of all cases of MCC. Seventeen members of the polyomavirus family have been identified, 9 of which have been found to infect humans, including BK virus, JC virus, WU, MCPyV, human polyomavirus 6, human polyomavirus 7, trichodysplasia spinulosa–associated polyomavirus, human polyomavirus 9, and Simian virus 40.1 Merkel cell polyomavirus infection is found in approximately 60% of the general population and exposure likely occurs early in life. The virus likely is transmitted through skin shedding and nasal secretions, though it also has been found in urine specimens.3 Currently, there is no evidence to suggest vertical viral transmission from mother to fetus.
Merkel cell polyomavirus is composed of early and late gene regions. The early gene region contains both large T antigen (LT) and small T antigen reading frames, which are necessary for viral replication.8 The late region is responsible for encoding viral proteins necessary for viral capsid assembly. Mutations found in viral protein 1 prevent formation of viral particles.9 Large T antigen is substantially overexpressed in MCC and is responsible for tumor suppression through retinoblastoma tumor suppressor protein. It also serves as a binding domain for both heat shock proteins and helicases.8,10 These domains allow the polyomaviruses to use host-cell machinery for viral genome replication while targeting tumor suppressor proteins.8 Upon viral integration into host DNA, viral replication ceases while oncogenic function persists.
The exact mechanism by which the MCPyV contributes to the development of MCC still has yet to be identified. Hypotheses suggest a combination of viral infection with external mutagens (eg, UV radiation). Experimental observations suggest viral contribution is likely due to the large percentage of MCCs that are positive for MCPyV, the identification of LT antigen expression and the role it plays in preserving cell cycle progression, and the role persistent LT antigen expression plays in continued growth of MCC cell lines in vitro.8 Two important cell line preservation mechanisms ensure continued tumor growth, including prevention of apoptosis triggered by DNA damage response mechanisms following UV damage and interaction with the retinoblastoma tumor suppressor protein allowing continued growth.8,11 Other important factors in tumor growth and survival may be the inhibition of apoptosis through the BCL2 (B-cell chronic lymphocytic leukemia/lymphoma 2) proto-oncogene and survivin (baculoviral inhibitor of apoptosis repeat-containing 5 [BIRC5]).12 Survivin has been found to play an important role in MCPyV-positive MCCs.12,13 It has been suggested that lymphangiogenesis in MCC likely is driven by vascular endothelial growth factor-C+CD68+CD163+ M2 macrophages.14 Another survival mechanism specific to polyomaviruses is their ability to interfere with the p53 tumor suppressor pathway.8 Loss of p53 expression by tumor cell nuclei has been associated with poor prognosis.15
Immune Response
Immune response as a role in tumor progression can be primarily centered on the concept of persistent antigen expression as a means of immune downregulation. Dunn et al16 suggested that cancer cells must interact through 3 consecutive phases with the host immune system (immunoediting hypothesis). In the elimination phase, the host immune system is able to recognize and destroy newly transformed cells through both the innate and adaptive immune systems. The second equilibrium phase allows the tumor to remain dormant and growth remains stagnant. Lastly, the tumor is allowed to evade the immune system through the escape phase.8
Host immune responses play an important role in both the progression and prognosis of MCC. High anti-MCPyV capsid antibody titers have been associated with better progression-free survival in some patients.8 Patients with high antibody titers (>10,000) likely have better progression-free survival than those with low antibody titers (<10,000).17 Antibody titers to the LT antigen may serve as a biomarker of MCC disease burden in the future. Rising LT antigen titers have been shown to correlate with disease progression and falling titers correlate with successful treatment.8 Tumoral infiltration of CD8+ T lymphocytes has been shown to be a predictor of survival compared to no intratumoral infiltration.6 Sihto et al18 suggested that this better prognosis from high intratumoral infiltration is not specific to MCPyV-positive MCC; however, it does highlight an important aspect of tumor evasion through the downregulation of cell surface expression of class I major histocompatibility complex antigens, which allows presentation of tumor intracellular peptides to CD8+ T lymphocytes.8 Upregulation of this specific immune response may play a role in the future treatment of MCC.
Staging and Prognosis
Due to the extremely aggressive nature of MCC, patients with local disease and tumors 2 cm or smaller in diameter have a 66% survival at 5 years.1,3 The 5-year survival rate for patients with local metastasis to regional lymph nodes ranges from 26% to 42%. Patients with distant metastasis have an 18% survival rate at 5 years.1,3 Data suggest that sentinel lymph node biopsy should be performed on all patients with MCC regardless of tumor size.1 There are no consensus guidelines to date regarding imaging for the staging of MCC patients. It is suggested that (18F)fluorodeoxyglucose positron emission tomography alone or in combination with computed tomography (CT) may be of value as a single whole-body diagnostic tool for accurate staging.10 It also has been suggested that (18F)fluorodeoxyglucose positron emission tomography and CT may offer more accurate staging than other screening modalities such as CT alone or magnetic resonance imaging.14,19
Treatment of MCC
Surgery remains the mainstay of treatment of MCC. Current National Comprehensive Cancer Network guidelines20 recommend 1- to 2-cm margins for wide local excision or treatment with Mohs micrographic surgery. Sentinel lymph node biopsy should be performed intraoperatively in patients undergoing wide local excision and preoperatively for patients undergoing Mohs micrographic surgery due to potential alterations in lymphatic drainage that may affect lymphoscintigraphy.1
Radiation may be used as primary or adjuvant therapy in patients with MCC. Radiation as primary therapy generally is reserved for patients who are not surgical candidates. It has been suggested that there was no difference in outcome in a small group of patients treated with radiation alone compared to patients who underwent surgery and radiation to the tumor bed.1 Current guidelines suggest a small group of patients may not require adjuvant therapy following adequate resection of some small tumors, and clinical observation may be appropriate.1,3 Chemotherapy may play a palliative role in patients with metastatic MCC. Merkel cell carcinoma has been shown to be chemosensitive but with a high recurrence rate.1 Because the immune system plays an important role in disease prognosis, having an intact immune system likely is paramount in the prevention of further disease progression.
Future Treatments of MCC
Future treatment of MCC may be focused on the viral etiology of most tumors and upregulation of the immune response, which may lead to the possibility of specifically interfering with virus-specific oncoproteins and stimulation of immune responses to virally infected tumor cells.8 The MCPyV large T antigen has been found to be overexpressed in some tumors and may serve as a specific target of therapy.10,21 Survivin, a key cell cycle protein encoded by LT antigen, may be an interesting target given its implication in other cancers.13 Other potential nonviral molecular target antigens include the oncoprotein H1P1 that interacts with c-KIT.8 Specific immunostimulatory cytokines that may be used to upregulate the immune response to tumoral cells may include IL-2, IL-12, IL-15, or IL-21. Therapeutic agents that may be studied in the future to target the immune exhaustion phenomenon associated with tumorigenesis include ipilimumab (cytotoxic T lymphocyte antigen 4 receptor-blocking agent) as well as programmed cell death 1 and programmed cell death 1 ligand 1 (PD-1/PD-L1).8 Neuroendocrine tumors including MCC tend to be highly vascular and express vascular endothelial growth factors and platelet-derived growth factors, which may be other potential therapeutic targets. It has been reported that approximately 95% of MCC patients have CD56+ tumors, and current clinical trials suggest a promising therapeutic response with the immunogen anti-CD56 monoclonal antibody.3
Conclusion
Merkel cell carcinoma is a rare aggressive neuroendocrine tumor that has been associated with a novel polyomavirus. Merkel cell carcinoma tends to affect elderly and immunocompromised patients as well as white individuals. Tumors are most often found in areas of high UV exposure and clinically on sun-exposed skin. Merkel cell polyomavirus is associated with approximately 80% of tumors, and tumorigenesis likely is caused by a number of sequential steps from viral integration into host DNA, mutagenic events, and specific immune responses. Currently there are no consensus guidelines for using imaging for staging of MCCs, but sentinel lymph node biopsy is recommended for all cases due to the aggressive nature of even smaller tumors. Surgery remains the mainstay of treatment, and radiation therapy may be used as a primary or adjuvant treatment. Chemotherapy usually is reserved for patients with metastatic disease purely for palliation. Future treatments of MCC likely will center on the viral etiology of MCC and upregulation of immune responses to virally infected tumor cells.
Merkel cells originally were described by German histopathologist Friedrich Sigmund Merkel in 1875. These unique tactile cells were described as epidermal, nondendritic, and nonkeratinizing. Merkel cells are thought to arise from the neural crest and are believed to be primary neural cells found within the basal layer of the epidermis.1,2 They likely function primarily as slowly adapting type I mechanoreceptors. Origin from the neural crest is controversial, as other investigators have suggested derivation from epidermal keratinocytes.1,2 Tumor cells have been linked to the amine precursor uptake and decarboxylation system.3 In 1972, Toker4 described several cases of trabecular or sweat gland carcinomas of the skin. Upon further investigation, the cells that comprised these tumors were found to have dense core granules on electron microscopy, typical of Merkel cells.1,2 Other terms such as neuroendocrine carcinoma of the skin, small cell carcinoma of the skin, and anaplastic carcinoma of the skin have been used to describe Merkel cell carcinoma (MCC),1 which was suggested by De Wolf-Peeters et al5 in 1980.
Despite being a rare malignancy, MCC follows an aggressive clinical course. Upon presentation, approximately 66% of patients have local disease, 27% have nodal involvement, and 7% have distant metastasis.1 Future treatments will likely center around the novel Merkel cell polyomavirus (MCPyV) and modification of immune responses toward tumor cells. Standardization continues to be lacking in both staging and treatment of this aggressive tumor.
Epidemiology of MCC

| ||
Figure 1. A 2.3×1.5×1.2-cm, hemorrhagic, crusted, | ||

| ||

| ||
| Figure 2. Merkel cells are small- to medium-sized cells with round nuclei and scant cytoplasm. Granular or stippled chromatin can be seen (A)(H&E, original magnification ×40). Merkel cell carcinoma with trabecular pattern (B) (H&E, original magnification ×10). | ||
Between 1986 and 2006, the incidence of MCC grew substantially.1,2 Figures have been reported at 0.15 cases per 100,000 individuals to 0.6 cases per 100,000 individuals worldwide. In the United States, the age-adjusted incidence of MCC is estimated at 0.24 per 100,000 person-years, which is higher than the estimated 0.13 per 100,000 person-years found in Europe.3 The highest incidence worldwide has been noted in Western Australia, likely due to high levels of UV exposure.1 The incidence of MCC in psoriasis patients who are treated with oral methoxsalen (psoralen) and UVA photochemotherapy is 100 times greater than in the general population, further supporting the role of UV light in the development of MCC.1 White individuals have the highest incidence of MCC worldwide, with men being affected more frequently than women.1,3 The majority of patients with MCC are diagnosed at 70 years or older.1 Approximately 5% of reported MCC patients are diagnosed before 50 years of age.2 Immunosuppression and immunodeficiency likely play a role in the pathogenesis of MCC, and the incidence is increased in solid organ transplant recipients, most commonly renal transplant recipients,1 as well as individuals with chronic lymphocytic leukemia, human immunodeficiency virus infection, and AIDS.1,3 Patients with autoimmune diseases such as rheumatoid arthritis also are at increased risk for MCC.3 Individuals who are diagnosed with MCC are at an increased risk for development of other malignancies including nonmelanoma skin cancers, chronic lymphocytic leukemia, Hodgkin lymphoma, and non-Hodgkin lymphoma.3
Clinical Presentation of MCC
The clinical presentation of MCC can be variable. Most tumors present as firm, red to purple, nontender papules or nodules (Figure 1).1 Tumor size may range from 2 to 200 mm but is most commonly less than 20 mm.2 Growth can be rapid, and tumors are most commonly located on sun-exposed skin. The head and neck areas account for 48% of all MCC cases,1 with the eyelids being frequently involved.2 Merkel cell carcinoma also has been reported on the arms, legs, trunk, back, and buttocks.1 Non–sun-exposed areas are less commonly affected. Mucosal sites (eg, larynx, nasal cavity, pharynx, mouth) account for 5% of primary MCCs.1 Merkel cell carcinoma also has been reported to affect the vulva and penis. Subcutaneous primary MCC has presented without overlying epidermal changes.1 In a case series by Heath et al,6 14% (27/195) of MCC patients presented with nodal disease without any identifiable primary tumor, with the inguinal nodal chain being the most common for this presentation. It currently is not known whether these nodal tumors are primary tumors or metastatic disease with a regression of the primary tumor.1
|
Histopathology of MCC

| ||

| ||

| ||
Figure 3. Positive chromogranin staining (A)(original | ||
Merkel cells are small- to medium-sized basophilic cells with round nuclei and scant cytoplasm. Granular or stippled chromatin can be seen on histopathology (Figure 2A).1 Some tumor cells have more vesicular chromatin, multiple small nucleoli, irregular contours, and more abundant cytoplasm. In some reports, irregular contours and abundant cytoplasm were associated with no detectible MCPyV infection.1,3 Merkel cell carcinomas have a primarily nodular architecture, and classification is based on growth pattern and cell size. Three histopathologic growth patterns have been described: nodular, infiltrative, and trabecular. The trabecular pattern is composed of interconnecting strands ofcells (Figure 2B). Tumors with solely intraepidermal involvement (MCC in situ) have been described but are exceedingly rare.1 Cell types are classified according to size, with the intermediate cell type being the most common. The small cell variant may be mistaken for a lymphocytic infiltrate due to the similar size and appearance of both types of cells.1,3
Merkel cell carcinomas can have histopathological overlap with lymphomas, small cell lung cancers, carcinoid tumors, primitive neuroectodermal tumors, neuroblastomas, small cell osteosarcomas, rhabdomyosarcomas, or Ewing sarcomas.1,3 Specifically, differentiation from small cell carcinoma of the lung is of utmost importance. Merkel cell carcinoma stains positively for cytokeratins 8, 18, 19, and 20. The neuroendocrine markers chromogranin (Figure 3A), synaptophysin, and neuron-specific enolase also may show positive staining. Cytokeratin 20, low-molecular-weight cytokeratins (CAM 5.2), and neurofilament immunostains have a high sensitivity for MCC and are the most frequently used.1 Cytokeratin 20 stains in the characteristic paranuclear dot–like pattern, which is a hallmark of MCC (Figure 3B). Cytokeratin 20 positivity in conjunction with negative staining for thyroid transcription factor 1 (Figure 3C) and cytokeratin 7 can aid in differentiation from small cell carcinoma of the lung.1,3
Pathogenesis of MCC
In 2008, Feng et al7 discovered a novel polyomavirus associated with the development of MCC. This novel polyomavirus, MCPyV, is found in approximately 80% of all cases of MCC. Seventeen members of the polyomavirus family have been identified, 9 of which have been found to infect humans, including BK virus, JC virus, WU, MCPyV, human polyomavirus 6, human polyomavirus 7, trichodysplasia spinulosa–associated polyomavirus, human polyomavirus 9, and Simian virus 40.1 Merkel cell polyomavirus infection is found in approximately 60% of the general population and exposure likely occurs early in life. The virus likely is transmitted through skin shedding and nasal secretions, though it also has been found in urine specimens.3 Currently, there is no evidence to suggest vertical viral transmission from mother to fetus.
Merkel cell polyomavirus is composed of early and late gene regions. The early gene region contains both large T antigen (LT) and small T antigen reading frames, which are necessary for viral replication.8 The late region is responsible for encoding viral proteins necessary for viral capsid assembly. Mutations found in viral protein 1 prevent formation of viral particles.9 Large T antigen is substantially overexpressed in MCC and is responsible for tumor suppression through retinoblastoma tumor suppressor protein. It also serves as a binding domain for both heat shock proteins and helicases.8,10 These domains allow the polyomaviruses to use host-cell machinery for viral genome replication while targeting tumor suppressor proteins.8 Upon viral integration into host DNA, viral replication ceases while oncogenic function persists.
The exact mechanism by which the MCPyV contributes to the development of MCC still has yet to be identified. Hypotheses suggest a combination of viral infection with external mutagens (eg, UV radiation). Experimental observations suggest viral contribution is likely due to the large percentage of MCCs that are positive for MCPyV, the identification of LT antigen expression and the role it plays in preserving cell cycle progression, and the role persistent LT antigen expression plays in continued growth of MCC cell lines in vitro.8 Two important cell line preservation mechanisms ensure continued tumor growth, including prevention of apoptosis triggered by DNA damage response mechanisms following UV damage and interaction with the retinoblastoma tumor suppressor protein allowing continued growth.8,11 Other important factors in tumor growth and survival may be the inhibition of apoptosis through the BCL2 (B-cell chronic lymphocytic leukemia/lymphoma 2) proto-oncogene and survivin (baculoviral inhibitor of apoptosis repeat-containing 5 [BIRC5]).12 Survivin has been found to play an important role in MCPyV-positive MCCs.12,13 It has been suggested that lymphangiogenesis in MCC likely is driven by vascular endothelial growth factor-C+CD68+CD163+ M2 macrophages.14 Another survival mechanism specific to polyomaviruses is their ability to interfere with the p53 tumor suppressor pathway.8 Loss of p53 expression by tumor cell nuclei has been associated with poor prognosis.15
Immune Response
Immune response as a role in tumor progression can be primarily centered on the concept of persistent antigen expression as a means of immune downregulation. Dunn et al16 suggested that cancer cells must interact through 3 consecutive phases with the host immune system (immunoediting hypothesis). In the elimination phase, the host immune system is able to recognize and destroy newly transformed cells through both the innate and adaptive immune systems. The second equilibrium phase allows the tumor to remain dormant and growth remains stagnant. Lastly, the tumor is allowed to evade the immune system through the escape phase.8
Host immune responses play an important role in both the progression and prognosis of MCC. High anti-MCPyV capsid antibody titers have been associated with better progression-free survival in some patients.8 Patients with high antibody titers (>10,000) likely have better progression-free survival than those with low antibody titers (<10,000).17 Antibody titers to the LT antigen may serve as a biomarker of MCC disease burden in the future. Rising LT antigen titers have been shown to correlate with disease progression and falling titers correlate with successful treatment.8 Tumoral infiltration of CD8+ T lymphocytes has been shown to be a predictor of survival compared to no intratumoral infiltration.6 Sihto et al18 suggested that this better prognosis from high intratumoral infiltration is not specific to MCPyV-positive MCC; however, it does highlight an important aspect of tumor evasion through the downregulation of cell surface expression of class I major histocompatibility complex antigens, which allows presentation of tumor intracellular peptides to CD8+ T lymphocytes.8 Upregulation of this specific immune response may play a role in the future treatment of MCC.
Staging and Prognosis
Due to the extremely aggressive nature of MCC, patients with local disease and tumors 2 cm or smaller in diameter have a 66% survival at 5 years.1,3 The 5-year survival rate for patients with local metastasis to regional lymph nodes ranges from 26% to 42%. Patients with distant metastasis have an 18% survival rate at 5 years.1,3 Data suggest that sentinel lymph node biopsy should be performed on all patients with MCC regardless of tumor size.1 There are no consensus guidelines to date regarding imaging for the staging of MCC patients. It is suggested that (18F)fluorodeoxyglucose positron emission tomography alone or in combination with computed tomography (CT) may be of value as a single whole-body diagnostic tool for accurate staging.10 It also has been suggested that (18F)fluorodeoxyglucose positron emission tomography and CT may offer more accurate staging than other screening modalities such as CT alone or magnetic resonance imaging.14,19
Treatment of MCC
Surgery remains the mainstay of treatment of MCC. Current National Comprehensive Cancer Network guidelines20 recommend 1- to 2-cm margins for wide local excision or treatment with Mohs micrographic surgery. Sentinel lymph node biopsy should be performed intraoperatively in patients undergoing wide local excision and preoperatively for patients undergoing Mohs micrographic surgery due to potential alterations in lymphatic drainage that may affect lymphoscintigraphy.1
Radiation may be used as primary or adjuvant therapy in patients with MCC. Radiation as primary therapy generally is reserved for patients who are not surgical candidates. It has been suggested that there was no difference in outcome in a small group of patients treated with radiation alone compared to patients who underwent surgery and radiation to the tumor bed.1 Current guidelines suggest a small group of patients may not require adjuvant therapy following adequate resection of some small tumors, and clinical observation may be appropriate.1,3 Chemotherapy may play a palliative role in patients with metastatic MCC. Merkel cell carcinoma has been shown to be chemosensitive but with a high recurrence rate.1 Because the immune system plays an important role in disease prognosis, having an intact immune system likely is paramount in the prevention of further disease progression.
Future Treatments of MCC
Future treatment of MCC may be focused on the viral etiology of most tumors and upregulation of the immune response, which may lead to the possibility of specifically interfering with virus-specific oncoproteins and stimulation of immune responses to virally infected tumor cells.8 The MCPyV large T antigen has been found to be overexpressed in some tumors and may serve as a specific target of therapy.10,21 Survivin, a key cell cycle protein encoded by LT antigen, may be an interesting target given its implication in other cancers.13 Other potential nonviral molecular target antigens include the oncoprotein H1P1 that interacts with c-KIT.8 Specific immunostimulatory cytokines that may be used to upregulate the immune response to tumoral cells may include IL-2, IL-12, IL-15, or IL-21. Therapeutic agents that may be studied in the future to target the immune exhaustion phenomenon associated with tumorigenesis include ipilimumab (cytotoxic T lymphocyte antigen 4 receptor-blocking agent) as well as programmed cell death 1 and programmed cell death 1 ligand 1 (PD-1/PD-L1).8 Neuroendocrine tumors including MCC tend to be highly vascular and express vascular endothelial growth factors and platelet-derived growth factors, which may be other potential therapeutic targets. It has been reported that approximately 95% of MCC patients have CD56+ tumors, and current clinical trials suggest a promising therapeutic response with the immunogen anti-CD56 monoclonal antibody.3
Conclusion
Merkel cell carcinoma is a rare aggressive neuroendocrine tumor that has been associated with a novel polyomavirus. Merkel cell carcinoma tends to affect elderly and immunocompromised patients as well as white individuals. Tumors are most often found in areas of high UV exposure and clinically on sun-exposed skin. Merkel cell polyomavirus is associated with approximately 80% of tumors, and tumorigenesis likely is caused by a number of sequential steps from viral integration into host DNA, mutagenic events, and specific immune responses. Currently there are no consensus guidelines for using imaging for staging of MCCs, but sentinel lymph node biopsy is recommended for all cases due to the aggressive nature of even smaller tumors. Surgery remains the mainstay of treatment, and radiation therapy may be used as a primary or adjuvant treatment. Chemotherapy usually is reserved for patients with metastatic disease purely for palliation. Future treatments of MCC likely will center on the viral etiology of MCC and upregulation of immune responses to virally infected tumor cells.
1. Han S, North J, Canavan T, et al. Merkel cell carcinoma. Hematol Oncol Clin N Am. 2012;26:1351-1374.
2. Goessling W, McKee P, Mayer R. Merkel cell carcinoma. J Clin Oncol. 2002;20:588-598.
3. Donepudi S, DeConti R, Samlowski W. Recent advances in the understanding of the genetics, etiology, and treatment of merkel cell carcinoma. Semin Oncol. 2012;39:163-172.
4. Toker C. Trabecular carcinoma of the skin. Arch Dermatol. 1972;105:107-110.
5. De Wolff-Peeters C, Marien K, Mebis J, et al. A cutaneous APUDoma or Merkel cell tumor? a morphologically recognizable tumor with a biological and histological malignant aspect in contrast with its clinical behavior. Cancer. 1980;46:810-816.
6. Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
7. Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096-1100.
8. Bhatia S, Afanasiev O, Nghiem P. Immunobiology of Merkel cell carcinoma: implications for immunotherapy of a polyomavirus-associated cancer. Curr Oncol Rep. 2011;13:488-497.
9. Amber K, McLeod M, Nouri K. The Merkel cell polyomavirus and its involvement in Merkel cell carcinoma. Dermatol Surg. 2013;39:232-238.
10. Erovic B, Al Habeeb A, Harris L, et al. Significant overexpression of the Merkel cell polyomavirus (MCPyV) large T antigen in Merkel cell carcinoma. Head Neck. 2013;35:184-189.
11. Demetriou S, Ona-Vu K, Sullivan E, et al. Defective DNA repair and cell cycle arrest in cells expressing Merkel cell polyomavirus T antigen. Int J Cancer. 2012;131:1818-1827.
12. Sahi H, Koljonen V, Kavola H, et al. Bcl-2 expression indicates better prognosis of Merkel cell carcinoma regardless of the presence of Merkel cell polyomavirus. Virchows Arch. 2012;461:553-559.
13. Arora R, Shuda M, Guastafierro A, et al. Survivin is a therapeutic target in Merkel cell carcinoma. Sci Transl Med. 2012;4:1-11.
14. Hawryluk E, O’Regan K, Sheehy N, et al. Positron emission tomography/computed tomography imaging in Merkel cell carcinoma: a study of 270 scans in 97 patients at the Dana-Farber/Brigham and Women’s Cancer Center. J Am Acad Dermatol. 2013;68:592-599.
15. Hall B, Pincus L, Yu S, et al. Immunohistochemical prognostication of Merkel cell carcinoma: p63 expression but not polyomavirus status correlates with outcome. J Cutan Pathol. 2012;39:911-917.
16. Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991-998.
17. Touze A, Le Bidre E, Laude H, et al. High levels of antibodies against Merkel cell polyomavirus identify a subset of patients with Merkel cell carcinoma with better clinical outcome. J Clin Oncol. 2011;29:1612-1619.
18. Sihto H, Bohling T, Kavola H, et al. Tumor infiltrating immune cells and outcome of Merkel cell carcinoma: a population-based study. Clin Cancer Res. 2012;18:2872-2881.
19. Colgan M, Tarantola T, Weaver A, et al. The predictive value of imaging studies in evaluating regional lymph node involvement in Merkel cell carcinoma. J Am Acad Dermatol. 2012;67:1250-1256.
20. NCCN Clinical Practice Guidelines in Oncology. National Comprehensive Cancer Network website. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site. Accessed March 22, 2016.
21. Angermeyer S, Hesbacher S, Becker J, et al. Merkel cell polyomavirus-positive Merkel cell carcinoma cells do not require expression of the viral small T antigen. J Invest Dermatol. 2013;133:1-6.
1. Han S, North J, Canavan T, et al. Merkel cell carcinoma. Hematol Oncol Clin N Am. 2012;26:1351-1374.
2. Goessling W, McKee P, Mayer R. Merkel cell carcinoma. J Clin Oncol. 2002;20:588-598.
3. Donepudi S, DeConti R, Samlowski W. Recent advances in the understanding of the genetics, etiology, and treatment of merkel cell carcinoma. Semin Oncol. 2012;39:163-172.
4. Toker C. Trabecular carcinoma of the skin. Arch Dermatol. 1972;105:107-110.
5. De Wolff-Peeters C, Marien K, Mebis J, et al. A cutaneous APUDoma or Merkel cell tumor? a morphologically recognizable tumor with a biological and histological malignant aspect in contrast with its clinical behavior. Cancer. 1980;46:810-816.
6. Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
7. Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096-1100.
8. Bhatia S, Afanasiev O, Nghiem P. Immunobiology of Merkel cell carcinoma: implications for immunotherapy of a polyomavirus-associated cancer. Curr Oncol Rep. 2011;13:488-497.
9. Amber K, McLeod M, Nouri K. The Merkel cell polyomavirus and its involvement in Merkel cell carcinoma. Dermatol Surg. 2013;39:232-238.
10. Erovic B, Al Habeeb A, Harris L, et al. Significant overexpression of the Merkel cell polyomavirus (MCPyV) large T antigen in Merkel cell carcinoma. Head Neck. 2013;35:184-189.
11. Demetriou S, Ona-Vu K, Sullivan E, et al. Defective DNA repair and cell cycle arrest in cells expressing Merkel cell polyomavirus T antigen. Int J Cancer. 2012;131:1818-1827.
12. Sahi H, Koljonen V, Kavola H, et al. Bcl-2 expression indicates better prognosis of Merkel cell carcinoma regardless of the presence of Merkel cell polyomavirus. Virchows Arch. 2012;461:553-559.
13. Arora R, Shuda M, Guastafierro A, et al. Survivin is a therapeutic target in Merkel cell carcinoma. Sci Transl Med. 2012;4:1-11.
14. Hawryluk E, O’Regan K, Sheehy N, et al. Positron emission tomography/computed tomography imaging in Merkel cell carcinoma: a study of 270 scans in 97 patients at the Dana-Farber/Brigham and Women’s Cancer Center. J Am Acad Dermatol. 2013;68:592-599.
15. Hall B, Pincus L, Yu S, et al. Immunohistochemical prognostication of Merkel cell carcinoma: p63 expression but not polyomavirus status correlates with outcome. J Cutan Pathol. 2012;39:911-917.
16. Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991-998.
17. Touze A, Le Bidre E, Laude H, et al. High levels of antibodies against Merkel cell polyomavirus identify a subset of patients with Merkel cell carcinoma with better clinical outcome. J Clin Oncol. 2011;29:1612-1619.
18. Sihto H, Bohling T, Kavola H, et al. Tumor infiltrating immune cells and outcome of Merkel cell carcinoma: a population-based study. Clin Cancer Res. 2012;18:2872-2881.
19. Colgan M, Tarantola T, Weaver A, et al. The predictive value of imaging studies in evaluating regional lymph node involvement in Merkel cell carcinoma. J Am Acad Dermatol. 2012;67:1250-1256.
20. NCCN Clinical Practice Guidelines in Oncology. National Comprehensive Cancer Network website. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site. Accessed March 22, 2016.
21. Angermeyer S, Hesbacher S, Becker J, et al. Merkel cell polyomavirus-positive Merkel cell carcinoma cells do not require expression of the viral small T antigen. J Invest Dermatol. 2013;133:1-6.
Practice Points
- Merkel cell carcinoma has been associated with a novel polyomavirus.
- Merkel cell carcinoma follows a very aggressive course and is most likely metastatic at diagnosis.
3 Hospitalists Honored on Modern Healthcare’s List of 50 Most Influential Physician Executives and Leaders
The Society of Hospital Medicine (SHM) congratulates three hospitalists who appear on Modern Healthcare’s list of the 50 Most Influential Physician Executives and Leaders of 2016. Pediatric hospitalist Patrick Conway, MD, MSc, MHM, Deputy Administrator for Innovation and Quality and Chief Medical Officer of the Centers for Medicare and Medicaid Services (CMS), takes the No. 3 spot; national leader in healthcare quality and an architect of the hospital medicine movement, Robert M. Wachter, MD, MHM, of the University of California, San Francisco (UCSF), comes in at No. 4; and US Surgeon General and hospitalist Vivek Murthy, MD, MBA, ranks at No. 22.
In this Year of the Hospitalist, SHM’s celebration of the 20th anniversary of the New England Journal of Medicine paper by Drs. Robert Wachter and Lee Goldman that first used the term “hospitalist” to describe physicians who care for hospitalized patients, this distinct honor demonstrates hospitalists’ integral role in the hospital and health system, particularly in this era of monumental change. By nature of their work, hospitalists possess a deep understanding of how hospitals operate and how to add value to their institutions, improve patient outcomes and lead teams to solve the most complex problems both inside hospitals’ walls and in the U.S. healthcare system.
“I’m always proud to be a hospitalist, but it is in moments like these when hospitalists are recognized on the national stage that my pride is confirmed and amplified, validating that we are the change agents for improved quality of care and teamwork across the care continuum,” SHM President Brian Harte, MD, SFHM, said. “Congratulations to our colleagues and respected leaders, Drs. Conway, Wachter, and Murthy!”
The Society of Hospital Medicine (SHM) congratulates three hospitalists who appear on Modern Healthcare’s list of the 50 Most Influential Physician Executives and Leaders of 2016. Pediatric hospitalist Patrick Conway, MD, MSc, MHM, Deputy Administrator for Innovation and Quality and Chief Medical Officer of the Centers for Medicare and Medicaid Services (CMS), takes the No. 3 spot; national leader in healthcare quality and an architect of the hospital medicine movement, Robert M. Wachter, MD, MHM, of the University of California, San Francisco (UCSF), comes in at No. 4; and US Surgeon General and hospitalist Vivek Murthy, MD, MBA, ranks at No. 22.
In this Year of the Hospitalist, SHM’s celebration of the 20th anniversary of the New England Journal of Medicine paper by Drs. Robert Wachter and Lee Goldman that first used the term “hospitalist” to describe physicians who care for hospitalized patients, this distinct honor demonstrates hospitalists’ integral role in the hospital and health system, particularly in this era of monumental change. By nature of their work, hospitalists possess a deep understanding of how hospitals operate and how to add value to their institutions, improve patient outcomes and lead teams to solve the most complex problems both inside hospitals’ walls and in the U.S. healthcare system.
“I’m always proud to be a hospitalist, but it is in moments like these when hospitalists are recognized on the national stage that my pride is confirmed and amplified, validating that we are the change agents for improved quality of care and teamwork across the care continuum,” SHM President Brian Harte, MD, SFHM, said. “Congratulations to our colleagues and respected leaders, Drs. Conway, Wachter, and Murthy!”
The Society of Hospital Medicine (SHM) congratulates three hospitalists who appear on Modern Healthcare’s list of the 50 Most Influential Physician Executives and Leaders of 2016. Pediatric hospitalist Patrick Conway, MD, MSc, MHM, Deputy Administrator for Innovation and Quality and Chief Medical Officer of the Centers for Medicare and Medicaid Services (CMS), takes the No. 3 spot; national leader in healthcare quality and an architect of the hospital medicine movement, Robert M. Wachter, MD, MHM, of the University of California, San Francisco (UCSF), comes in at No. 4; and US Surgeon General and hospitalist Vivek Murthy, MD, MBA, ranks at No. 22.
In this Year of the Hospitalist, SHM’s celebration of the 20th anniversary of the New England Journal of Medicine paper by Drs. Robert Wachter and Lee Goldman that first used the term “hospitalist” to describe physicians who care for hospitalized patients, this distinct honor demonstrates hospitalists’ integral role in the hospital and health system, particularly in this era of monumental change. By nature of their work, hospitalists possess a deep understanding of how hospitals operate and how to add value to their institutions, improve patient outcomes and lead teams to solve the most complex problems both inside hospitals’ walls and in the U.S. healthcare system.
“I’m always proud to be a hospitalist, but it is in moments like these when hospitalists are recognized on the national stage that my pride is confirmed and amplified, validating that we are the change agents for improved quality of care and teamwork across the care continuum,” SHM President Brian Harte, MD, SFHM, said. “Congratulations to our colleagues and respected leaders, Drs. Conway, Wachter, and Murthy!”
Pediatric Dermatology Consult - April 2016
By Catalina Matiz, M.D., and David Ginsberg
Eczema cocksackium
Eczema coxsackium (EC) is an atypical variant of hand, foot, and mouth disease (HFMD) that occurs in patients with atopic dermatitis lesions. HFMD is a common viral exanthema seen in pediatric populations, predominantly in children under 5 years of age, and is most commonly caused by coxsackievirus A16 in the United States.1-3 Enterovirus 71 is also a common cause of HFMD in Asian countries, and in rare cases has been linked to more severe neurologic manifestations.2,3
Over the past several years, infection by coxsackievirus A6 (CVA6) has been responsible for HFMD outbreaks in multiple countries around the globe.1-5 This particular strain of coxsackievirus is known for causing atypical HFMD, which has several overlapping variations. The variants of atypical HFMD caused by CVA6 include eczema coxsackium (EC), widespread vesiculobullous and erosive lesions, purpuric lesions, and Gianotti-Crosti-like eruptions.2 EC is the result of a CVA6 infection in a child with a history of atopic dermatitis (AD). The infection in these patients is a more severe form of HFMD, presenting with eczema herpeticum (EH)-like lesions, with some combination of vesicles, papules, and erosions, in areas affected by atopic dermatitis.2,3 This is in contrast to classic HFMD, which commonly presents with oral erosions, and small vesicles limited to the hands, feet, and buttocks.1,2
In addition to the dermatologic manifestations, patients with EC also can present with other symptoms including fever and oropharyngeal pain.2,3 Any of the variants of atypical HFMD can present with lesions, including erosions, vesicles, and bullae, in areas of skin irritation such as sunburns, irritant dermatitis, lacerations, and tinea pedis.2
Differential diagnosis
The differential diagnosis for EC includes EH, impetiginized atopic dermatitis, varicella, and erythema multiforme major.2-4 EH is typically the highest on the differential because the presentation of EC is described as an EH-like rash and on occasion it is difficult to differentiate the two.2 When diagnosing EC, the history plays an important role, and it is imperative to question whether the patient has come into contact with anyone with cold sores to help rule out EH. It is recommended to perform testing for herpes simplex virus (HSV), either by culture, polymerase chain reaction (PCR), or direct immunofluorescence if the diagnosis is not straightforward.2,5 To confirm the diagnosis of EC, CVA6 PCR can be performed.4 The ideal sample would be from vesicular fluid because the same sample also could be used to test for HSV to help rule out EH, but throat swabs and stool samples also can be used.2 As lesions in EH can sometimes be superinfected with bacteria such as Staphylococcus aureus or streptococcus, a bacterial swab for culture and sensitivities is also recommended prior to starting empiric antimicrobial therapy.
Treatment
Because EC was only characterized several years ago, and the majority of the cases reported have been self-limiting, there has been little research done looking into the best treatment. The majority of the literature recommends treating symptomatically in a similar fashion to an eczema flare including daily moisturizing, bleach baths, and topical corticosteroids as needed.6 There has been a report that using wet wraps with lower-strength corticosteroids is an effective treatment method for severe cases.6 Treatment with acyclovir and oral antimicrobials is recommended if the diagnosis of EH is in question and HSV and bacterial testing are pending.
References
- JAMA. 2012;308(4):335.
- Pediatrics. 2013 Jul;132(1):e149-57.
- PIDJ. 2014 Apr;33(4):e92-98.
- Virol J. 2013 Jun 24;10:209.
- Lancet Infect Dis. 2014 Jan;14(1):83-6.
- J Allergy Clin Immunol Pract. 2014 Nov-Dec;2(6):803-4.
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California, San Diego and Mr. Ginsberg is a research associate at the hospital. Dr. Matiz and Mr. Ginsberg said they have no relevant financial disclosures.
By Catalina Matiz, M.D., and David Ginsberg
Eczema cocksackium
Eczema coxsackium (EC) is an atypical variant of hand, foot, and mouth disease (HFMD) that occurs in patients with atopic dermatitis lesions. HFMD is a common viral exanthema seen in pediatric populations, predominantly in children under 5 years of age, and is most commonly caused by coxsackievirus A16 in the United States.1-3 Enterovirus 71 is also a common cause of HFMD in Asian countries, and in rare cases has been linked to more severe neurologic manifestations.2,3
Over the past several years, infection by coxsackievirus A6 (CVA6) has been responsible for HFMD outbreaks in multiple countries around the globe.1-5 This particular strain of coxsackievirus is known for causing atypical HFMD, which has several overlapping variations. The variants of atypical HFMD caused by CVA6 include eczema coxsackium (EC), widespread vesiculobullous and erosive lesions, purpuric lesions, and Gianotti-Crosti-like eruptions.2 EC is the result of a CVA6 infection in a child with a history of atopic dermatitis (AD). The infection in these patients is a more severe form of HFMD, presenting with eczema herpeticum (EH)-like lesions, with some combination of vesicles, papules, and erosions, in areas affected by atopic dermatitis.2,3 This is in contrast to classic HFMD, which commonly presents with oral erosions, and small vesicles limited to the hands, feet, and buttocks.1,2
In addition to the dermatologic manifestations, patients with EC also can present with other symptoms including fever and oropharyngeal pain.2,3 Any of the variants of atypical HFMD can present with lesions, including erosions, vesicles, and bullae, in areas of skin irritation such as sunburns, irritant dermatitis, lacerations, and tinea pedis.2
Differential diagnosis
The differential diagnosis for EC includes EH, impetiginized atopic dermatitis, varicella, and erythema multiforme major.2-4 EH is typically the highest on the differential because the presentation of EC is described as an EH-like rash and on occasion it is difficult to differentiate the two.2 When diagnosing EC, the history plays an important role, and it is imperative to question whether the patient has come into contact with anyone with cold sores to help rule out EH. It is recommended to perform testing for herpes simplex virus (HSV), either by culture, polymerase chain reaction (PCR), or direct immunofluorescence if the diagnosis is not straightforward.2,5 To confirm the diagnosis of EC, CVA6 PCR can be performed.4 The ideal sample would be from vesicular fluid because the same sample also could be used to test for HSV to help rule out EH, but throat swabs and stool samples also can be used.2 As lesions in EH can sometimes be superinfected with bacteria such as Staphylococcus aureus or streptococcus, a bacterial swab for culture and sensitivities is also recommended prior to starting empiric antimicrobial therapy.
Treatment
Because EC was only characterized several years ago, and the majority of the cases reported have been self-limiting, there has been little research done looking into the best treatment. The majority of the literature recommends treating symptomatically in a similar fashion to an eczema flare including daily moisturizing, bleach baths, and topical corticosteroids as needed.6 There has been a report that using wet wraps with lower-strength corticosteroids is an effective treatment method for severe cases.6 Treatment with acyclovir and oral antimicrobials is recommended if the diagnosis of EH is in question and HSV and bacterial testing are pending.
References
- JAMA. 2012;308(4):335.
- Pediatrics. 2013 Jul;132(1):e149-57.
- PIDJ. 2014 Apr;33(4):e92-98.
- Virol J. 2013 Jun 24;10:209.
- Lancet Infect Dis. 2014 Jan;14(1):83-6.
- J Allergy Clin Immunol Pract. 2014 Nov-Dec;2(6):803-4.
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California, San Diego and Mr. Ginsberg is a research associate at the hospital. Dr. Matiz and Mr. Ginsberg said they have no relevant financial disclosures.
By Catalina Matiz, M.D., and David Ginsberg
Eczema cocksackium
Eczema coxsackium (EC) is an atypical variant of hand, foot, and mouth disease (HFMD) that occurs in patients with atopic dermatitis lesions. HFMD is a common viral exanthema seen in pediatric populations, predominantly in children under 5 years of age, and is most commonly caused by coxsackievirus A16 in the United States.1-3 Enterovirus 71 is also a common cause of HFMD in Asian countries, and in rare cases has been linked to more severe neurologic manifestations.2,3
Over the past several years, infection by coxsackievirus A6 (CVA6) has been responsible for HFMD outbreaks in multiple countries around the globe.1-5 This particular strain of coxsackievirus is known for causing atypical HFMD, which has several overlapping variations. The variants of atypical HFMD caused by CVA6 include eczema coxsackium (EC), widespread vesiculobullous and erosive lesions, purpuric lesions, and Gianotti-Crosti-like eruptions.2 EC is the result of a CVA6 infection in a child with a history of atopic dermatitis (AD). The infection in these patients is a more severe form of HFMD, presenting with eczema herpeticum (EH)-like lesions, with some combination of vesicles, papules, and erosions, in areas affected by atopic dermatitis.2,3 This is in contrast to classic HFMD, which commonly presents with oral erosions, and small vesicles limited to the hands, feet, and buttocks.1,2
In addition to the dermatologic manifestations, patients with EC also can present with other symptoms including fever and oropharyngeal pain.2,3 Any of the variants of atypical HFMD can present with lesions, including erosions, vesicles, and bullae, in areas of skin irritation such as sunburns, irritant dermatitis, lacerations, and tinea pedis.2
Differential diagnosis
The differential diagnosis for EC includes EH, impetiginized atopic dermatitis, varicella, and erythema multiforme major.2-4 EH is typically the highest on the differential because the presentation of EC is described as an EH-like rash and on occasion it is difficult to differentiate the two.2 When diagnosing EC, the history plays an important role, and it is imperative to question whether the patient has come into contact with anyone with cold sores to help rule out EH. It is recommended to perform testing for herpes simplex virus (HSV), either by culture, polymerase chain reaction (PCR), or direct immunofluorescence if the diagnosis is not straightforward.2,5 To confirm the diagnosis of EC, CVA6 PCR can be performed.4 The ideal sample would be from vesicular fluid because the same sample also could be used to test for HSV to help rule out EH, but throat swabs and stool samples also can be used.2 As lesions in EH can sometimes be superinfected with bacteria such as Staphylococcus aureus or streptococcus, a bacterial swab for culture and sensitivities is also recommended prior to starting empiric antimicrobial therapy.
Treatment
Because EC was only characterized several years ago, and the majority of the cases reported have been self-limiting, there has been little research done looking into the best treatment. The majority of the literature recommends treating symptomatically in a similar fashion to an eczema flare including daily moisturizing, bleach baths, and topical corticosteroids as needed.6 There has been a report that using wet wraps with lower-strength corticosteroids is an effective treatment method for severe cases.6 Treatment with acyclovir and oral antimicrobials is recommended if the diagnosis of EH is in question and HSV and bacterial testing are pending.
References
- JAMA. 2012;308(4):335.
- Pediatrics. 2013 Jul;132(1):e149-57.
- PIDJ. 2014 Apr;33(4):e92-98.
- Virol J. 2013 Jun 24;10:209.
- Lancet Infect Dis. 2014 Jan;14(1):83-6.
- J Allergy Clin Immunol Pract. 2014 Nov-Dec;2(6):803-4.
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California, San Diego and Mr. Ginsberg is a research associate at the hospital. Dr. Matiz and Mr. Ginsberg said they have no relevant financial disclosures.
A 16-month-old male with a history of mild atopic dermatitis presents with a 2-day history of a rash on his lower extremities, arms, abdomen, and face. His father reports that it began as a single spot on his leg and has spread over the past 2 days. His other symptoms included a fever of 101.5 F, hypoactivity, and an unwillingness to eat solid foods. The patient has not had contact with anyone with cold sores. The sister is developing similar lesions on her face and knees. Physical exam On exam there are hemorrhagic crusted papules with an erythematous base, as well as grouped vesicles and eroded punched-out papules scattered on the lower extremities bilaterally. The area of involvement extends from his ankles to his hips, involving the posterior thighs, inguinal folds, and calves. There also are some hemorrhagic crusted erythematous grouped papules scattered on the upper extremities. On the face there are few crusted erythematous papules around the mouth. On the soles there are few erythematous oval papules.
AATS Graham Foundation Programs Open with July Deadlines
Two AATS Graham Foundation programs are now open for application.
Third John Alexander Research Scholarship provides research, training and experience for North American surgeons committed to pursuing an academic career in cardiothoracic surgery. This two-year scholarship will run from July 1, 2017 - June 30, 2019 and provides an annual $80,000 stipend to the host institution.
* Applications must be submitted during the candidate’s first two years in an academic position.
* Research must occur within the first three years after completion of an approved North American cardiothoracic residency.
Deadline: July 1, 2016
Evarts A. Graham Memorial Traveling Fellowship supports training of international surgeons with the potential for future thoracic surgical leadership. The fellowship will run from 2017-2018 and provide a stipend of $75,000 to help cover living and travel expenses for fellows while visiting North American medical centers.
* Candidates must be from other than North America, who have not limited (six months or less) clinical training in North American prior to submitting their application.
* Candidates must have completed their formal training in general and thoracic/cardiovascular surgery but not yet reached a senior position.
Deadline: July 1, 2016
Two AATS Graham Foundation programs are now open for application.
Third John Alexander Research Scholarship provides research, training and experience for North American surgeons committed to pursuing an academic career in cardiothoracic surgery. This two-year scholarship will run from July 1, 2017 - June 30, 2019 and provides an annual $80,000 stipend to the host institution.
* Applications must be submitted during the candidate’s first two years in an academic position.
* Research must occur within the first three years after completion of an approved North American cardiothoracic residency.
Deadline: July 1, 2016
Evarts A. Graham Memorial Traveling Fellowship supports training of international surgeons with the potential for future thoracic surgical leadership. The fellowship will run from 2017-2018 and provide a stipend of $75,000 to help cover living and travel expenses for fellows while visiting North American medical centers.
* Candidates must be from other than North America, who have not limited (six months or less) clinical training in North American prior to submitting their application.
* Candidates must have completed their formal training in general and thoracic/cardiovascular surgery but not yet reached a senior position.
Deadline: July 1, 2016
Two AATS Graham Foundation programs are now open for application.
Third John Alexander Research Scholarship provides research, training and experience for North American surgeons committed to pursuing an academic career in cardiothoracic surgery. This two-year scholarship will run from July 1, 2017 - June 30, 2019 and provides an annual $80,000 stipend to the host institution.
* Applications must be submitted during the candidate’s first two years in an academic position.
* Research must occur within the first three years after completion of an approved North American cardiothoracic residency.
Deadline: July 1, 2016
Evarts A. Graham Memorial Traveling Fellowship supports training of international surgeons with the potential for future thoracic surgical leadership. The fellowship will run from 2017-2018 and provide a stipend of $75,000 to help cover living and travel expenses for fellows while visiting North American medical centers.
* Candidates must be from other than North America, who have not limited (six months or less) clinical training in North American prior to submitting their application.
* Candidates must have completed their formal training in general and thoracic/cardiovascular surgery but not yet reached a senior position.
Deadline: July 1, 2016
Housing & Registration Open: Inaugural Patient Safety Course
June 24-25, 2016
Renaissance Boston Waterfront Hotel
Boston, MA
Co-Directors
Thoralf M. Sundt, III, MD
Steven Yule, PhD
Program Committee
David J. Bunnell, PA-C, APACVS
David C. Fitzgerald, CCP, AMSECT
Jake Jaquiss, MD
M. Blair Marshall, MD
Shannon Pengel, RN
Kenneth Shann, CCP, LP, AMSECT
Marco Zenati, MD
Patient Safety is essential for successful surgical care — in cardiothoracic surgery — and all disciplines. In the more than 10 years since the Institute of Medicine made patient safety a priority, little progress has been made in changing culture and practice on the front lines.
This two-day course — including team training and simulation experiences — will make a difference, offering surgical team members practical safety behaviors for improving patient outcomes.
Special Benefits for Team Registration
The Patient Safety program includes team training and simulation experiences. Register three or more members from the same institution and receive one Healthcare Professional registration COMPLIMENTARY. We encourage you to send the entire team to join us in Boston this June.
June 24-25, 2016
Renaissance Boston Waterfront Hotel
Boston, MA
Co-Directors
Thoralf M. Sundt, III, MD
Steven Yule, PhD
Program Committee
David J. Bunnell, PA-C, APACVS
David C. Fitzgerald, CCP, AMSECT
Jake Jaquiss, MD
M. Blair Marshall, MD
Shannon Pengel, RN
Kenneth Shann, CCP, LP, AMSECT
Marco Zenati, MD
Patient Safety is essential for successful surgical care — in cardiothoracic surgery — and all disciplines. In the more than 10 years since the Institute of Medicine made patient safety a priority, little progress has been made in changing culture and practice on the front lines.
This two-day course — including team training and simulation experiences — will make a difference, offering surgical team members practical safety behaviors for improving patient outcomes.
Special Benefits for Team Registration
The Patient Safety program includes team training and simulation experiences. Register three or more members from the same institution and receive one Healthcare Professional registration COMPLIMENTARY. We encourage you to send the entire team to join us in Boston this June.
June 24-25, 2016
Renaissance Boston Waterfront Hotel
Boston, MA
Co-Directors
Thoralf M. Sundt, III, MD
Steven Yule, PhD
Program Committee
David J. Bunnell, PA-C, APACVS
David C. Fitzgerald, CCP, AMSECT
Jake Jaquiss, MD
M. Blair Marshall, MD
Shannon Pengel, RN
Kenneth Shann, CCP, LP, AMSECT
Marco Zenati, MD
Patient Safety is essential for successful surgical care — in cardiothoracic surgery — and all disciplines. In the more than 10 years since the Institute of Medicine made patient safety a priority, little progress has been made in changing culture and practice on the front lines.
This two-day course — including team training and simulation experiences — will make a difference, offering surgical team members practical safety behaviors for improving patient outcomes.
Special Benefits for Team Registration
The Patient Safety program includes team training and simulation experiences. Register three or more members from the same institution and receive one Healthcare Professional registration COMPLIMENTARY. We encourage you to send the entire team to join us in Boston this June.
Clinical Pearl: Increasing Utility of Isopropyl Alcohol for Cutaneous Dyschromia
Practice Gap
Conditions with dyschromia including terra firma-forme dermatosis (TFFD), confluent and reticulate papillomatosis (CARP), and acanthosis nigricans are difficult to distinguish from one another.
Diagnostic Tools
Since its development in 1920, dermatologists have utilized isopropyl alcohol in ways that exceed conventional antimicrobial purposes. If TFFP, CARP, and acanthosis nigricans are suspected, the first step in any algorithmic approach should be to rub the skin with an alcohol pad using firm continuous pressure in an attempt to remove pigmentation. Complete resolution of dyspigmentation strongly supports a diagnosis of TFFD1 and can be curative (Figure). Alcohol can similarly lighten CARP but to a lesser degree than TFFD.2 In contrast, acanthosis nigricans will display minimal to no improvement with isopropyl alcohol.
Practice Implications
Isopropyl alcohol has few side effects and each swab costs less than a dime. It is extremely cost effective compared to biopsy and subsequent pathology and laboratory costs. Patients appreciate a noninvasive initial approach, and it is rewarding to treat a cosmetically disturbing condition with ease.
Swabbing the skin with alcohol pads reflects light and improves visualization of veins that should be avoided during surgery. Alcohol-based gel inhibits bacterial colonization, reduces dermatoscope-related nosocomial infection, and enhances dermoscopic resolution.3 Alcohol swabs quickly remove gentian violet, which aids in porokeratosis diagnosis; the pathognomonic cornoid lamella of porokeratosis retains gentian violet.4 A solution of 70% isopropyl alcohol preserves myiasis larvae better than formalin, which causes larval tissue hardening. Alcohol also can be squeezed into the central punctum in myiasis as a form of treatment.5 In conclusion, alcohol represents a convenient, inexpensive, and helpful tool in the dermatologist’s armamentarium that should not be forgotten.
- Browning J, Rosen T. Terra firma-forme dermatosis revisited. Dermatol Online J. 2005;11:15.
- Berk DR. Confluent and reticulated papillomatosis response to 70% alcohol swabbing. Arch Dermatol. 2011;147:247-248.
- Kelly SC, Purcell SM. Prevention of nosocomial infection during dermoscopy? Dermatol Surg. 2006;32:552-555.
- Thomas CJ, Elston DM. Medical pearl: Gentian violet to highlight the cornoid lamella in disseminated superficial actinic porokeratosis. J Am Acad Dermatol. 2005;52(3, pt 1):513-514.
- Meinking TL, Burkhart CN, Burkhart CG. Changing paradigms in parasitic infections: common dermatological helminthic infections and cutaneous myiasis. Clin Dermatol. 2003;21:407-416.
Practice Gap
Conditions with dyschromia including terra firma-forme dermatosis (TFFD), confluent and reticulate papillomatosis (CARP), and acanthosis nigricans are difficult to distinguish from one another.
Diagnostic Tools
Since its development in 1920, dermatologists have utilized isopropyl alcohol in ways that exceed conventional antimicrobial purposes. If TFFP, CARP, and acanthosis nigricans are suspected, the first step in any algorithmic approach should be to rub the skin with an alcohol pad using firm continuous pressure in an attempt to remove pigmentation. Complete resolution of dyspigmentation strongly supports a diagnosis of TFFD1 and can be curative (Figure). Alcohol can similarly lighten CARP but to a lesser degree than TFFD.2 In contrast, acanthosis nigricans will display minimal to no improvement with isopropyl alcohol.

Practice Implications
Isopropyl alcohol has few side effects and each swab costs less than a dime. It is extremely cost effective compared to biopsy and subsequent pathology and laboratory costs. Patients appreciate a noninvasive initial approach, and it is rewarding to treat a cosmetically disturbing condition with ease.
Swabbing the skin with alcohol pads reflects light and improves visualization of veins that should be avoided during surgery. Alcohol-based gel inhibits bacterial colonization, reduces dermatoscope-related nosocomial infection, and enhances dermoscopic resolution.3 Alcohol swabs quickly remove gentian violet, which aids in porokeratosis diagnosis; the pathognomonic cornoid lamella of porokeratosis retains gentian violet.4 A solution of 70% isopropyl alcohol preserves myiasis larvae better than formalin, which causes larval tissue hardening. Alcohol also can be squeezed into the central punctum in myiasis as a form of treatment.5 In conclusion, alcohol represents a convenient, inexpensive, and helpful tool in the dermatologist’s armamentarium that should not be forgotten.
Practice Gap
Conditions with dyschromia including terra firma-forme dermatosis (TFFD), confluent and reticulate papillomatosis (CARP), and acanthosis nigricans are difficult to distinguish from one another.
Diagnostic Tools
Since its development in 1920, dermatologists have utilized isopropyl alcohol in ways that exceed conventional antimicrobial purposes. If TFFP, CARP, and acanthosis nigricans are suspected, the first step in any algorithmic approach should be to rub the skin with an alcohol pad using firm continuous pressure in an attempt to remove pigmentation. Complete resolution of dyspigmentation strongly supports a diagnosis of TFFD1 and can be curative (Figure). Alcohol can similarly lighten CARP but to a lesser degree than TFFD.2 In contrast, acanthosis nigricans will display minimal to no improvement with isopropyl alcohol.

Practice Implications
Isopropyl alcohol has few side effects and each swab costs less than a dime. It is extremely cost effective compared to biopsy and subsequent pathology and laboratory costs. Patients appreciate a noninvasive initial approach, and it is rewarding to treat a cosmetically disturbing condition with ease.
Swabbing the skin with alcohol pads reflects light and improves visualization of veins that should be avoided during surgery. Alcohol-based gel inhibits bacterial colonization, reduces dermatoscope-related nosocomial infection, and enhances dermoscopic resolution.3 Alcohol swabs quickly remove gentian violet, which aids in porokeratosis diagnosis; the pathognomonic cornoid lamella of porokeratosis retains gentian violet.4 A solution of 70% isopropyl alcohol preserves myiasis larvae better than formalin, which causes larval tissue hardening. Alcohol also can be squeezed into the central punctum in myiasis as a form of treatment.5 In conclusion, alcohol represents a convenient, inexpensive, and helpful tool in the dermatologist’s armamentarium that should not be forgotten.
- Browning J, Rosen T. Terra firma-forme dermatosis revisited. Dermatol Online J. 2005;11:15.
- Berk DR. Confluent and reticulated papillomatosis response to 70% alcohol swabbing. Arch Dermatol. 2011;147:247-248.
- Kelly SC, Purcell SM. Prevention of nosocomial infection during dermoscopy? Dermatol Surg. 2006;32:552-555.
- Thomas CJ, Elston DM. Medical pearl: Gentian violet to highlight the cornoid lamella in disseminated superficial actinic porokeratosis. J Am Acad Dermatol. 2005;52(3, pt 1):513-514.
- Meinking TL, Burkhart CN, Burkhart CG. Changing paradigms in parasitic infections: common dermatological helminthic infections and cutaneous myiasis. Clin Dermatol. 2003;21:407-416.
- Browning J, Rosen T. Terra firma-forme dermatosis revisited. Dermatol Online J. 2005;11:15.
- Berk DR. Confluent and reticulated papillomatosis response to 70% alcohol swabbing. Arch Dermatol. 2011;147:247-248.
- Kelly SC, Purcell SM. Prevention of nosocomial infection during dermoscopy? Dermatol Surg. 2006;32:552-555.
- Thomas CJ, Elston DM. Medical pearl: Gentian violet to highlight the cornoid lamella in disseminated superficial actinic porokeratosis. J Am Acad Dermatol. 2005;52(3, pt 1):513-514.
- Meinking TL, Burkhart CN, Burkhart CG. Changing paradigms in parasitic infections: common dermatological helminthic infections and cutaneous myiasis. Clin Dermatol. 2003;21:407-416.