User login
CAR T-cell start-up launched
Dr. Siddhartha Mukherjee has partnered with Puretech Health to launch a new biotechnology and immuno-oncology company to broaden the use of chimeric antigen receptor (CAR) T-cell therapy. Dr. Mukherjee, a Columbia University researcher, hematologist, oncologist, and Pulitzer Prize–winning author of “The Emperor of All Maladies: A Biography of Cancer,” (New York: Scribner, a division of Simon & Schuster, 2011) is licensing his CAR T-cell technology to the joint venture, called Vor BioPharma.
Vor BioPharma will focus on advancing and expanding CAR T-cell therapy, a relatively new cancer treatment where T cells are first collected from a patient’s blood and then genetically engineered to produce CAR proteins on their surface. The CAR proteins are designed to bind specific antigens found on the patient’s cancer cells. These genetically engineered T cells are grown in a laboratory and then infused into the patient. As of now, CAR T-cell therapy is primarily used to treat B-cell leukemias and other chronic lymphocytic leukemia.
“We continue to make great strides in developing new ways to treat cancer using the body’s immune system,” said Dr. Mukherjee in a written statement announcing the partnership. “The positive clinical response researchers have achieved with CAR T-cell therapies in B-cell leukemias has led to great interest within the oncology community and is something we hope to achieve in other cancers over time,” he said.
“CAR T-cell therapies have shown remarkable progress in the clinic, yet their applicability beyond a small subset of cancers is currently very limited,” said Dr. Sanjiv Sam Gambhir of Stanford University and a member of the Vor Scientific Advisory Board. “This technology seeks to address bottlenecks that prevent CAR T-cell therapy from becoming more broadly useful in treating cancers outside of B-cell cancers.”
Other Vor BioPharma employees and Scientific Advisory Board members include Dr. Joseph Bolen, former President and Chief Scientific Officer of Moderna Therapeutics; Dr. Dan Littman of the Howard Hughes Medical Institute; and Dr. Derrick Rossi of Harvard University.
On Twitter @jess_craig94
Dr. Siddhartha Mukherjee has partnered with Puretech Health to launch a new biotechnology and immuno-oncology company to broaden the use of chimeric antigen receptor (CAR) T-cell therapy. Dr. Mukherjee, a Columbia University researcher, hematologist, oncologist, and Pulitzer Prize–winning author of “The Emperor of All Maladies: A Biography of Cancer,” (New York: Scribner, a division of Simon & Schuster, 2011) is licensing his CAR T-cell technology to the joint venture, called Vor BioPharma.
Vor BioPharma will focus on advancing and expanding CAR T-cell therapy, a relatively new cancer treatment where T cells are first collected from a patient’s blood and then genetically engineered to produce CAR proteins on their surface. The CAR proteins are designed to bind specific antigens found on the patient’s cancer cells. These genetically engineered T cells are grown in a laboratory and then infused into the patient. As of now, CAR T-cell therapy is primarily used to treat B-cell leukemias and other chronic lymphocytic leukemia.
“We continue to make great strides in developing new ways to treat cancer using the body’s immune system,” said Dr. Mukherjee in a written statement announcing the partnership. “The positive clinical response researchers have achieved with CAR T-cell therapies in B-cell leukemias has led to great interest within the oncology community and is something we hope to achieve in other cancers over time,” he said.
“CAR T-cell therapies have shown remarkable progress in the clinic, yet their applicability beyond a small subset of cancers is currently very limited,” said Dr. Sanjiv Sam Gambhir of Stanford University and a member of the Vor Scientific Advisory Board. “This technology seeks to address bottlenecks that prevent CAR T-cell therapy from becoming more broadly useful in treating cancers outside of B-cell cancers.”
Other Vor BioPharma employees and Scientific Advisory Board members include Dr. Joseph Bolen, former President and Chief Scientific Officer of Moderna Therapeutics; Dr. Dan Littman of the Howard Hughes Medical Institute; and Dr. Derrick Rossi of Harvard University.
On Twitter @jess_craig94
Dr. Siddhartha Mukherjee has partnered with Puretech Health to launch a new biotechnology and immuno-oncology company to broaden the use of chimeric antigen receptor (CAR) T-cell therapy. Dr. Mukherjee, a Columbia University researcher, hematologist, oncologist, and Pulitzer Prize–winning author of “The Emperor of All Maladies: A Biography of Cancer,” (New York: Scribner, a division of Simon & Schuster, 2011) is licensing his CAR T-cell technology to the joint venture, called Vor BioPharma.
Vor BioPharma will focus on advancing and expanding CAR T-cell therapy, a relatively new cancer treatment where T cells are first collected from a patient’s blood and then genetically engineered to produce CAR proteins on their surface. The CAR proteins are designed to bind specific antigens found on the patient’s cancer cells. These genetically engineered T cells are grown in a laboratory and then infused into the patient. As of now, CAR T-cell therapy is primarily used to treat B-cell leukemias and other chronic lymphocytic leukemia.
“We continue to make great strides in developing new ways to treat cancer using the body’s immune system,” said Dr. Mukherjee in a written statement announcing the partnership. “The positive clinical response researchers have achieved with CAR T-cell therapies in B-cell leukemias has led to great interest within the oncology community and is something we hope to achieve in other cancers over time,” he said.
“CAR T-cell therapies have shown remarkable progress in the clinic, yet their applicability beyond a small subset of cancers is currently very limited,” said Dr. Sanjiv Sam Gambhir of Stanford University and a member of the Vor Scientific Advisory Board. “This technology seeks to address bottlenecks that prevent CAR T-cell therapy from becoming more broadly useful in treating cancers outside of B-cell cancers.”
Other Vor BioPharma employees and Scientific Advisory Board members include Dr. Joseph Bolen, former President and Chief Scientific Officer of Moderna Therapeutics; Dr. Dan Littman of the Howard Hughes Medical Institute; and Dr. Derrick Rossi of Harvard University.
On Twitter @jess_craig94
Exercise is protective but underutilized in atrial fib patients
CHICAGO – Efforts to encourage even modest amounts of physical activity in sedentary patients with atrial fibrillation are likely to pay off in reduced risks of cardiovascular and all-cause mortality, according to a report from the EurObservational Research Program Pilot Survey on Atrial Fibrillation General Registry.
“Clearly we would recommend regular physical activity for patients with atrial fibrillation on the basis of the mortality benefit we see in the registry. If we give patients with atrial fibrillation oral anticoagulation, they are protected against stroke risk, but clearly they are still dying a lot,” Dr. Marco Proietti said at the annual meeting of the American College of Cardiology.
He presented 1-year follow-up data on 2,442 “real world” patients enrolled in the nine-country, observational, prospective registry, known as EORP-AF, shortly after being diagnosed with AF. One of the goals of EORP-AF is to learn whether physical exercise protects against cardiovascular events and all-cause mortality in AF patients, as has been well established in the general population and in patients at high cardiovascular risk.
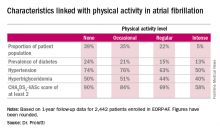
One striking finding was that nearly 40% of patients in EORP-AF reported engaging in no physical activity, defined for study purposes as zero to less than 3 hours of physical activity per week for less than 2 years.
The other three categories employed by investigators were “occasional,” meaning less than 3 hours per week but for 2 years or more; “regular,” defined as at least 3 hours weekly for at least 2 years; and “intense,” which required more than 7 hours of physical activity per week for at least 2 years. Levels of cardiovascular and stroke risk factors decreased progressively with increasing levels of physical activity. Only 5% of the AF patients met the ‘intense’ standard, noted Dr. Proietti of the University of Birmingham (England).
The 1-year cardiovascular mortality rate approached 6% in the no physical activity group and hovered around 1% in the other three groups. The 1-year all-cause mortality rate exceeded 12% in the no-exercise group, was 4%% in the occasional exercisers, and 1%-2% in the groups reporting regular or intense physical activity.
The 1-year composite endpoint of cardiovascular death, any thromboembolism, or a bleeding event occurred in 12% of the sedentary patients, a rate two-to-three times higher than in the others.
Updated outcomes are to be reported from the EORP-AF pilot registry after 2 and 3 years of follow-up. Meanwhile, on the basis of the success of the pilot registry, more than 10,000 patients with AF have been enrolled in the EORP-AF main registry, according to Dr. Proietti.
A study limitation, he conceded, is that the registry includes no objective measure of physical capacity, such as METS.
Session co-chair Dr. Brian Olshansky, emeritus professor of internal medicine at the University of Iowa, Iowa City, observed that the registry data raise a classic chicken-versus-egg issue: Do the sedentary patients do worse because they’re inactive, or are they inactive because they are sicker and hence have worse outcomes?
Dr. Proietti said the registry data provide some support for the latter idea, since the no-physical-activity group had higher prevalences of coronary artery disease and heart failure.
Dr. Olshansky raised another point: “It’s interesting to me that there’s a whole bunch of literature showing that elite endurance athletes – bike racers, cross country skiers – have a very high incidence of atrial fibrillation. It seems to be either an inflammatory or an autonomic issue.”
Dr. Proietti replied that he’s familiar with that extensive literature, but the EORP-AF data through 1 year don’t provide validation. While the intense physical activity group tended to have more symptomatic AF than the other groups, they were no more likely to show progression from paroxysmal to permanent AF. The much larger main registry now underway may be able to better clarify the relationship between physical activity and incidence and progression of AF, including the possibility of a U-shaped dose-response curve.
The EORP-AF registry is supported by the European Society of Cardiology. Dr. Proietti reported having no financial conflicts of interest.
CHICAGO – Efforts to encourage even modest amounts of physical activity in sedentary patients with atrial fibrillation are likely to pay off in reduced risks of cardiovascular and all-cause mortality, according to a report from the EurObservational Research Program Pilot Survey on Atrial Fibrillation General Registry.
“Clearly we would recommend regular physical activity for patients with atrial fibrillation on the basis of the mortality benefit we see in the registry. If we give patients with atrial fibrillation oral anticoagulation, they are protected against stroke risk, but clearly they are still dying a lot,” Dr. Marco Proietti said at the annual meeting of the American College of Cardiology.
He presented 1-year follow-up data on 2,442 “real world” patients enrolled in the nine-country, observational, prospective registry, known as EORP-AF, shortly after being diagnosed with AF. One of the goals of EORP-AF is to learn whether physical exercise protects against cardiovascular events and all-cause mortality in AF patients, as has been well established in the general population and in patients at high cardiovascular risk.
One striking finding was that nearly 40% of patients in EORP-AF reported engaging in no physical activity, defined for study purposes as zero to less than 3 hours of physical activity per week for less than 2 years.
The other three categories employed by investigators were “occasional,” meaning less than 3 hours per week but for 2 years or more; “regular,” defined as at least 3 hours weekly for at least 2 years; and “intense,” which required more than 7 hours of physical activity per week for at least 2 years. Levels of cardiovascular and stroke risk factors decreased progressively with increasing levels of physical activity. Only 5% of the AF patients met the ‘intense’ standard, noted Dr. Proietti of the University of Birmingham (England).
The 1-year cardiovascular mortality rate approached 6% in the no physical activity group and hovered around 1% in the other three groups. The 1-year all-cause mortality rate exceeded 12% in the no-exercise group, was 4%% in the occasional exercisers, and 1%-2% in the groups reporting regular or intense physical activity.
The 1-year composite endpoint of cardiovascular death, any thromboembolism, or a bleeding event occurred in 12% of the sedentary patients, a rate two-to-three times higher than in the others.
Updated outcomes are to be reported from the EORP-AF pilot registry after 2 and 3 years of follow-up. Meanwhile, on the basis of the success of the pilot registry, more than 10,000 patients with AF have been enrolled in the EORP-AF main registry, according to Dr. Proietti.
A study limitation, he conceded, is that the registry includes no objective measure of physical capacity, such as METS.
Session co-chair Dr. Brian Olshansky, emeritus professor of internal medicine at the University of Iowa, Iowa City, observed that the registry data raise a classic chicken-versus-egg issue: Do the sedentary patients do worse because they’re inactive, or are they inactive because they are sicker and hence have worse outcomes?
Dr. Proietti said the registry data provide some support for the latter idea, since the no-physical-activity group had higher prevalences of coronary artery disease and heart failure.
Dr. Olshansky raised another point: “It’s interesting to me that there’s a whole bunch of literature showing that elite endurance athletes – bike racers, cross country skiers – have a very high incidence of atrial fibrillation. It seems to be either an inflammatory or an autonomic issue.”
Dr. Proietti replied that he’s familiar with that extensive literature, but the EORP-AF data through 1 year don’t provide validation. While the intense physical activity group tended to have more symptomatic AF than the other groups, they were no more likely to show progression from paroxysmal to permanent AF. The much larger main registry now underway may be able to better clarify the relationship between physical activity and incidence and progression of AF, including the possibility of a U-shaped dose-response curve.
The EORP-AF registry is supported by the European Society of Cardiology. Dr. Proietti reported having no financial conflicts of interest.
CHICAGO – Efforts to encourage even modest amounts of physical activity in sedentary patients with atrial fibrillation are likely to pay off in reduced risks of cardiovascular and all-cause mortality, according to a report from the EurObservational Research Program Pilot Survey on Atrial Fibrillation General Registry.
“Clearly we would recommend regular physical activity for patients with atrial fibrillation on the basis of the mortality benefit we see in the registry. If we give patients with atrial fibrillation oral anticoagulation, they are protected against stroke risk, but clearly they are still dying a lot,” Dr. Marco Proietti said at the annual meeting of the American College of Cardiology.
He presented 1-year follow-up data on 2,442 “real world” patients enrolled in the nine-country, observational, prospective registry, known as EORP-AF, shortly after being diagnosed with AF. One of the goals of EORP-AF is to learn whether physical exercise protects against cardiovascular events and all-cause mortality in AF patients, as has been well established in the general population and in patients at high cardiovascular risk.
One striking finding was that nearly 40% of patients in EORP-AF reported engaging in no physical activity, defined for study purposes as zero to less than 3 hours of physical activity per week for less than 2 years.
The other three categories employed by investigators were “occasional,” meaning less than 3 hours per week but for 2 years or more; “regular,” defined as at least 3 hours weekly for at least 2 years; and “intense,” which required more than 7 hours of physical activity per week for at least 2 years. Levels of cardiovascular and stroke risk factors decreased progressively with increasing levels of physical activity. Only 5% of the AF patients met the ‘intense’ standard, noted Dr. Proietti of the University of Birmingham (England).
The 1-year cardiovascular mortality rate approached 6% in the no physical activity group and hovered around 1% in the other three groups. The 1-year all-cause mortality rate exceeded 12% in the no-exercise group, was 4%% in the occasional exercisers, and 1%-2% in the groups reporting regular or intense physical activity.
The 1-year composite endpoint of cardiovascular death, any thromboembolism, or a bleeding event occurred in 12% of the sedentary patients, a rate two-to-three times higher than in the others.
Updated outcomes are to be reported from the EORP-AF pilot registry after 2 and 3 years of follow-up. Meanwhile, on the basis of the success of the pilot registry, more than 10,000 patients with AF have been enrolled in the EORP-AF main registry, according to Dr. Proietti.
A study limitation, he conceded, is that the registry includes no objective measure of physical capacity, such as METS.
Session co-chair Dr. Brian Olshansky, emeritus professor of internal medicine at the University of Iowa, Iowa City, observed that the registry data raise a classic chicken-versus-egg issue: Do the sedentary patients do worse because they’re inactive, or are they inactive because they are sicker and hence have worse outcomes?
Dr. Proietti said the registry data provide some support for the latter idea, since the no-physical-activity group had higher prevalences of coronary artery disease and heart failure.
Dr. Olshansky raised another point: “It’s interesting to me that there’s a whole bunch of literature showing that elite endurance athletes – bike racers, cross country skiers – have a very high incidence of atrial fibrillation. It seems to be either an inflammatory or an autonomic issue.”
Dr. Proietti replied that he’s familiar with that extensive literature, but the EORP-AF data through 1 year don’t provide validation. While the intense physical activity group tended to have more symptomatic AF than the other groups, they were no more likely to show progression from paroxysmal to permanent AF. The much larger main registry now underway may be able to better clarify the relationship between physical activity and incidence and progression of AF, including the possibility of a U-shaped dose-response curve.
The EORP-AF registry is supported by the European Society of Cardiology. Dr. Proietti reported having no financial conflicts of interest.
AT ACC 16
Key clinical point: Atrial fibrillation patients who report engaging in even occasional physical activity have a markedly lower risk of all-cause mortality than those who are sedentary.
Major finding: The 1-year composite outcome of cardiovascular death, any thromboembolism, or a bleeding event occurred in 12% in patients with atrial fibrillation who were sedentary, a rate two to three times greater than in those who engaged in various amounts of physical activity.
Data source: An analysis of 1-year outcomes in 2,442 patients with AF enrolled in the prospective, observational EORP-AF pilot registry.
Disclosures: The EORP-AF registry is supported by the European Society of Cardiology. The presenter reported having no financial conflicts of interest.
Optimal timing of CRC postop colonoscopy studied
LOS ANGELES – The detection rate of significant polyps was highest for the first postoperative surveillance colonoscopies performed at 1 year following curative resection for colorectal cancer, results from a single-center study demonstrated.
“There’s no consensus on when to perform the first surveillance colonoscopy post curative resection for colorectal cancer,” lead study author Dr. Noura Alhassan said at the annual meeting of the American Society of Colon and Rectal Surgeons. For example, the American Society of Colon and Rectal Surgeons and National Carcinoma Comprehensive Network guidelines recommend a colonoscopy at 1 year, while the Canadian Association of Gastroenterology recommends surveillance at 3 years postoperatively.
In an effort to determine the optimal timing of the first surveillance colonoscopy following curative colorectal carcinoma resection, Dr. Alhassan and her associates retrospectively reviewed the charts of all patients who underwent colorectal resection from 2007 to 2012 at Jewish General Hospital, a tertiary care center affiliated with McGill University, Montreal. The study included patients who had a complete preoperative colonoscopy, those who had a complete postoperative colonoscopy performed by one of the Jewish General Hospital colorectal surgeons, and those who had colorectal cancer resection with curative intent. Excluded from the study were patients with stage IV colorectal cancer, those with a prior history of colorectal cancer, those who underwent total abdominal colectomies or proctocolectomies, those who underwent local excision, and those with familial cancer syndromes and inflammatory bowel disease.
Dr. Alhassan, a fourth-year resident in the division of general surgery at McGill University, said that the researchers classified the colonoscopic findings as normal, nonsignificant polyps, significant polyps, and recurrence. Significant polyps consisted of adenomas 1 cm or greater in size, villous or tubulovillous adenoma, adenoma with high-grade dysplasia, three or more adenomas, or sessile serrated polyps at least 1 cm in size or with dysplasia. Of the 857 colorectal resections performed during the study period, 181 met inclusion criteria. The tumor stage was evenly distributed among study participants and 57% of the resections were colon operations, while the remaining 43% were proctectomies.
The preoperative colonoscopy was done by one of the Jewish General Hospital gastroenterologists 43% of the time, by one of the Jewish General Hospital colorectal surgeons 41% of the time, and by an outside hospital 16% of the time. The median time to postoperative colonoscopy was 421 days (1.1 years). Specifically, 25.90% of patients underwent their first surveillance colonoscopy in the first postoperative year, 48.10% in the second year, 14.40% in the third year, 8.5% in the fourth year, and 2.7% in the fifth year.
Dr. Alhassan reported that the all-polyp detection rate was 30.1%; 21.3% were detected in postoperative year 1, 33.3% in year 2, and 34.6% in year 3.
The overall significant polyp detection rate was 10.5%, but the detection rate was 12.8% in postoperative year 1, 8% in postoperative year 2, and 7.7% in postoperative year 3. There were two anastomotic recurrences: one in year 1 (2.1%) and one in year 3 (3.8%).
On univariate analysis, factors associated with significant polyp detection were male gender, poor bowel preparation on preoperative colonoscopy, and concomitant use of metformin, while having stage III disease was associated with a lower significant polyp detection rate.
On multivariate analysis only male gender was associated with a higher significant polyp detection rate, while stage III disease was associated with a lower significant polyp detection rate.
“Significant polyp detection rate of 12.8% at postoperative year 1 justifies surveillance colonoscopy at 1 year post curative colon cancer resection,” Dr. Alhassan concluded. She reported having no financial disclosures.
LOS ANGELES – The detection rate of significant polyps was highest for the first postoperative surveillance colonoscopies performed at 1 year following curative resection for colorectal cancer, results from a single-center study demonstrated.
“There’s no consensus on when to perform the first surveillance colonoscopy post curative resection for colorectal cancer,” lead study author Dr. Noura Alhassan said at the annual meeting of the American Society of Colon and Rectal Surgeons. For example, the American Society of Colon and Rectal Surgeons and National Carcinoma Comprehensive Network guidelines recommend a colonoscopy at 1 year, while the Canadian Association of Gastroenterology recommends surveillance at 3 years postoperatively.
In an effort to determine the optimal timing of the first surveillance colonoscopy following curative colorectal carcinoma resection, Dr. Alhassan and her associates retrospectively reviewed the charts of all patients who underwent colorectal resection from 2007 to 2012 at Jewish General Hospital, a tertiary care center affiliated with McGill University, Montreal. The study included patients who had a complete preoperative colonoscopy, those who had a complete postoperative colonoscopy performed by one of the Jewish General Hospital colorectal surgeons, and those who had colorectal cancer resection with curative intent. Excluded from the study were patients with stage IV colorectal cancer, those with a prior history of colorectal cancer, those who underwent total abdominal colectomies or proctocolectomies, those who underwent local excision, and those with familial cancer syndromes and inflammatory bowel disease.
Dr. Alhassan, a fourth-year resident in the division of general surgery at McGill University, said that the researchers classified the colonoscopic findings as normal, nonsignificant polyps, significant polyps, and recurrence. Significant polyps consisted of adenomas 1 cm or greater in size, villous or tubulovillous adenoma, adenoma with high-grade dysplasia, three or more adenomas, or sessile serrated polyps at least 1 cm in size or with dysplasia. Of the 857 colorectal resections performed during the study period, 181 met inclusion criteria. The tumor stage was evenly distributed among study participants and 57% of the resections were colon operations, while the remaining 43% were proctectomies.
The preoperative colonoscopy was done by one of the Jewish General Hospital gastroenterologists 43% of the time, by one of the Jewish General Hospital colorectal surgeons 41% of the time, and by an outside hospital 16% of the time. The median time to postoperative colonoscopy was 421 days (1.1 years). Specifically, 25.90% of patients underwent their first surveillance colonoscopy in the first postoperative year, 48.10% in the second year, 14.40% in the third year, 8.5% in the fourth year, and 2.7% in the fifth year.
Dr. Alhassan reported that the all-polyp detection rate was 30.1%; 21.3% were detected in postoperative year 1, 33.3% in year 2, and 34.6% in year 3.
The overall significant polyp detection rate was 10.5%, but the detection rate was 12.8% in postoperative year 1, 8% in postoperative year 2, and 7.7% in postoperative year 3. There were two anastomotic recurrences: one in year 1 (2.1%) and one in year 3 (3.8%).
On univariate analysis, factors associated with significant polyp detection were male gender, poor bowel preparation on preoperative colonoscopy, and concomitant use of metformin, while having stage III disease was associated with a lower significant polyp detection rate.
On multivariate analysis only male gender was associated with a higher significant polyp detection rate, while stage III disease was associated with a lower significant polyp detection rate.
“Significant polyp detection rate of 12.8% at postoperative year 1 justifies surveillance colonoscopy at 1 year post curative colon cancer resection,” Dr. Alhassan concluded. She reported having no financial disclosures.
LOS ANGELES – The detection rate of significant polyps was highest for the first postoperative surveillance colonoscopies performed at 1 year following curative resection for colorectal cancer, results from a single-center study demonstrated.
“There’s no consensus on when to perform the first surveillance colonoscopy post curative resection for colorectal cancer,” lead study author Dr. Noura Alhassan said at the annual meeting of the American Society of Colon and Rectal Surgeons. For example, the American Society of Colon and Rectal Surgeons and National Carcinoma Comprehensive Network guidelines recommend a colonoscopy at 1 year, while the Canadian Association of Gastroenterology recommends surveillance at 3 years postoperatively.
In an effort to determine the optimal timing of the first surveillance colonoscopy following curative colorectal carcinoma resection, Dr. Alhassan and her associates retrospectively reviewed the charts of all patients who underwent colorectal resection from 2007 to 2012 at Jewish General Hospital, a tertiary care center affiliated with McGill University, Montreal. The study included patients who had a complete preoperative colonoscopy, those who had a complete postoperative colonoscopy performed by one of the Jewish General Hospital colorectal surgeons, and those who had colorectal cancer resection with curative intent. Excluded from the study were patients with stage IV colorectal cancer, those with a prior history of colorectal cancer, those who underwent total abdominal colectomies or proctocolectomies, those who underwent local excision, and those with familial cancer syndromes and inflammatory bowel disease.
Dr. Alhassan, a fourth-year resident in the division of general surgery at McGill University, said that the researchers classified the colonoscopic findings as normal, nonsignificant polyps, significant polyps, and recurrence. Significant polyps consisted of adenomas 1 cm or greater in size, villous or tubulovillous adenoma, adenoma with high-grade dysplasia, three or more adenomas, or sessile serrated polyps at least 1 cm in size or with dysplasia. Of the 857 colorectal resections performed during the study period, 181 met inclusion criteria. The tumor stage was evenly distributed among study participants and 57% of the resections were colon operations, while the remaining 43% were proctectomies.
The preoperative colonoscopy was done by one of the Jewish General Hospital gastroenterologists 43% of the time, by one of the Jewish General Hospital colorectal surgeons 41% of the time, and by an outside hospital 16% of the time. The median time to postoperative colonoscopy was 421 days (1.1 years). Specifically, 25.90% of patients underwent their first surveillance colonoscopy in the first postoperative year, 48.10% in the second year, 14.40% in the third year, 8.5% in the fourth year, and 2.7% in the fifth year.
Dr. Alhassan reported that the all-polyp detection rate was 30.1%; 21.3% were detected in postoperative year 1, 33.3% in year 2, and 34.6% in year 3.
The overall significant polyp detection rate was 10.5%, but the detection rate was 12.8% in postoperative year 1, 8% in postoperative year 2, and 7.7% in postoperative year 3. There were two anastomotic recurrences: one in year 1 (2.1%) and one in year 3 (3.8%).
On univariate analysis, factors associated with significant polyp detection were male gender, poor bowel preparation on preoperative colonoscopy, and concomitant use of metformin, while having stage III disease was associated with a lower significant polyp detection rate.
On multivariate analysis only male gender was associated with a higher significant polyp detection rate, while stage III disease was associated with a lower significant polyp detection rate.
“Significant polyp detection rate of 12.8% at postoperative year 1 justifies surveillance colonoscopy at 1 year post curative colon cancer resection,” Dr. Alhassan concluded. She reported having no financial disclosures.
AT THE ASCRS ANNUAL MEETING
Key clinical point: The highest proportion of significant polyps on surveillance colonoscopy after curative resection was detected in postoperative year 1.
Major finding: The overall significant polyp detection rate was 10.5%, but 12.8% were detected in postoperative year 1, 8% in postoperative year 2, and 7.7% in postoperative year 3.
Data source: A retrospective study of 181 patients who underwent colorectal resection from 2007 to 2012 at Jewish General Hospital, Montreal.
Disclosures: Dr. Alhassan reported having no financial disclosures.
Anticoagulation therapy after VT ablation yields fewer thrombotic events
San Francisco – Anticoagulation therapy is probably a good idea after ventricular tachycardia ablation in patients with risk factors or stroke, even if they don’t have atrial fibrillation, according to investigators from the University of Kansas Medical Center in Kansas City.
The advice comes from a review of 2,235 ventricular tachycardia (VT) ablation cases from the university and other members of the International VT Ablation Center Collaborative; about a quarter of the patients (604) were prescribed oral anticoagulation therapy at baseline and at discharge, nearly all for atrial fibrillation (AF) and most with warfarin. Over the next year, just 0.3% (2) had a subsequent thromboembolic complication, one of which was an ischemic stroke.
The remaining patients (1,631) did not have a diagnosis of AF and were not on anticoagulants at baseline or after discharge. They were more likely to have New York Heart Association class I or II heart failure and higher ejection fractions, and to otherwise be in better shape compared with the patients who received anticoagulation therapy. Even so, within a year, 1.3% (21) had a thromboembolic event, almost half of which were ischemic strokes, a substantial increase in relative risk (P = .05).
Maybe those patients had undiagnosed AF at baseline, or perhaps a clot formed over the ablation scar, Dr. Rizwan Afzal said at the annual scientific sessions of the Heart Rhythm Society. Regardless, “this observation has changed our practice. If VT ablation patients have low ejection fractions, if they’re elderly, or have other risk factors for stroke, we put them on blood thinners [afterward] “even if they don’t have atrial fibrillation. We are not sure how long they should be on anticoagulation [therapy] to counteract the increased risk of stroke,” but probably at least for a few weeks, he said.
Dr. Afzal and his colleagues generally opt for warfarin; the use is off label for newer oral anticoagulants, and a tough sell to insurance companies.
There were no predictors of increased thromboembolic risk in the group that was not on anticoagulation therapy. During follow-up, about 2.2% (13) of patients on anticoagulation therapy had bleeding complications, including one intracranial hemorrhage, compared with 2.5% (41) of the patients not treated with an anticoagulant; most of them were on aspirin after the procedure, and the rest were on dual antiplatelet therapy (P = .7), reported Dr. Afzal, a cardiology fellow at the University of Kansas.
The median age of the study patients was 65 years, and 87% were men. In the group on anticoagulation therapy, the mean baseline left ventricular ejection fraction was 31%; 35% had prior cardiac surgery, 29% were on cardiac resynchronization therapy, and 44% had NYHA class III or IV heart failure. The mean baseline ejection fraction among patients who were not on anticoagulation therapy was 35%; 29% had prior heart surgery, 24% were on CRT, and 32.5% had NYHA class III or IV heart failure.
There was no industry funding for the work, and the investigators had no disclosures.
San Francisco – Anticoagulation therapy is probably a good idea after ventricular tachycardia ablation in patients with risk factors or stroke, even if they don’t have atrial fibrillation, according to investigators from the University of Kansas Medical Center in Kansas City.
The advice comes from a review of 2,235 ventricular tachycardia (VT) ablation cases from the university and other members of the International VT Ablation Center Collaborative; about a quarter of the patients (604) were prescribed oral anticoagulation therapy at baseline and at discharge, nearly all for atrial fibrillation (AF) and most with warfarin. Over the next year, just 0.3% (2) had a subsequent thromboembolic complication, one of which was an ischemic stroke.
The remaining patients (1,631) did not have a diagnosis of AF and were not on anticoagulants at baseline or after discharge. They were more likely to have New York Heart Association class I or II heart failure and higher ejection fractions, and to otherwise be in better shape compared with the patients who received anticoagulation therapy. Even so, within a year, 1.3% (21) had a thromboembolic event, almost half of which were ischemic strokes, a substantial increase in relative risk (P = .05).
Maybe those patients had undiagnosed AF at baseline, or perhaps a clot formed over the ablation scar, Dr. Rizwan Afzal said at the annual scientific sessions of the Heart Rhythm Society. Regardless, “this observation has changed our practice. If VT ablation patients have low ejection fractions, if they’re elderly, or have other risk factors for stroke, we put them on blood thinners [afterward] “even if they don’t have atrial fibrillation. We are not sure how long they should be on anticoagulation [therapy] to counteract the increased risk of stroke,” but probably at least for a few weeks, he said.
Dr. Afzal and his colleagues generally opt for warfarin; the use is off label for newer oral anticoagulants, and a tough sell to insurance companies.
There were no predictors of increased thromboembolic risk in the group that was not on anticoagulation therapy. During follow-up, about 2.2% (13) of patients on anticoagulation therapy had bleeding complications, including one intracranial hemorrhage, compared with 2.5% (41) of the patients not treated with an anticoagulant; most of them were on aspirin after the procedure, and the rest were on dual antiplatelet therapy (P = .7), reported Dr. Afzal, a cardiology fellow at the University of Kansas.
The median age of the study patients was 65 years, and 87% were men. In the group on anticoagulation therapy, the mean baseline left ventricular ejection fraction was 31%; 35% had prior cardiac surgery, 29% were on cardiac resynchronization therapy, and 44% had NYHA class III or IV heart failure. The mean baseline ejection fraction among patients who were not on anticoagulation therapy was 35%; 29% had prior heart surgery, 24% were on CRT, and 32.5% had NYHA class III or IV heart failure.
There was no industry funding for the work, and the investigators had no disclosures.
San Francisco – Anticoagulation therapy is probably a good idea after ventricular tachycardia ablation in patients with risk factors or stroke, even if they don’t have atrial fibrillation, according to investigators from the University of Kansas Medical Center in Kansas City.
The advice comes from a review of 2,235 ventricular tachycardia (VT) ablation cases from the university and other members of the International VT Ablation Center Collaborative; about a quarter of the patients (604) were prescribed oral anticoagulation therapy at baseline and at discharge, nearly all for atrial fibrillation (AF) and most with warfarin. Over the next year, just 0.3% (2) had a subsequent thromboembolic complication, one of which was an ischemic stroke.
The remaining patients (1,631) did not have a diagnosis of AF and were not on anticoagulants at baseline or after discharge. They were more likely to have New York Heart Association class I or II heart failure and higher ejection fractions, and to otherwise be in better shape compared with the patients who received anticoagulation therapy. Even so, within a year, 1.3% (21) had a thromboembolic event, almost half of which were ischemic strokes, a substantial increase in relative risk (P = .05).
Maybe those patients had undiagnosed AF at baseline, or perhaps a clot formed over the ablation scar, Dr. Rizwan Afzal said at the annual scientific sessions of the Heart Rhythm Society. Regardless, “this observation has changed our practice. If VT ablation patients have low ejection fractions, if they’re elderly, or have other risk factors for stroke, we put them on blood thinners [afterward] “even if they don’t have atrial fibrillation. We are not sure how long they should be on anticoagulation [therapy] to counteract the increased risk of stroke,” but probably at least for a few weeks, he said.
Dr. Afzal and his colleagues generally opt for warfarin; the use is off label for newer oral anticoagulants, and a tough sell to insurance companies.
There were no predictors of increased thromboembolic risk in the group that was not on anticoagulation therapy. During follow-up, about 2.2% (13) of patients on anticoagulation therapy had bleeding complications, including one intracranial hemorrhage, compared with 2.5% (41) of the patients not treated with an anticoagulant; most of them were on aspirin after the procedure, and the rest were on dual antiplatelet therapy (P = .7), reported Dr. Afzal, a cardiology fellow at the University of Kansas.
The median age of the study patients was 65 years, and 87% were men. In the group on anticoagulation therapy, the mean baseline left ventricular ejection fraction was 31%; 35% had prior cardiac surgery, 29% were on cardiac resynchronization therapy, and 44% had NYHA class III or IV heart failure. The mean baseline ejection fraction among patients who were not on anticoagulation therapy was 35%; 29% had prior heart surgery, 24% were on CRT, and 32.5% had NYHA class III or IV heart failure.
There was no industry funding for the work, and the investigators had no disclosures.
AT HEART RHYTHM 2016
Key clinical point: Anticoagulant therapy may be a good idea after ventricular tachycardia ablation in patients with risk factors for stroke, even if they don’t have atrial fibrillation.
Major finding: About 0.3% of patients on oral anticoagulant therapy after VT ablation had a thromboembolic event within a year, compared with 1.3% of those who were not on such therapy.
Data source: Review of 2,245 VT ablation cases.
Disclosures: There was no industry funding for the work, and the investigators had no disclosures.
Low vasculitis risk with TNF inhibitors
GLASGOW – Treatment with tumor necrosis factor (TNF) inhibitors for rheumatoid arthritis is associated with a low risk of vasculitis-like events, according to a large analysis of data from the United Kingdom.
Investigators using data from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis (BSRBR-RA) found that the crude incidence rate was 16 cases per 10,000 person-years among TNF-inhibitor users versus seven cases per 10,000 person-years among users of nonbiologic disease-modifying antirheumatic drugs (nbDMARDs) such as methotrexate and sulfasalazine.
Although the risk was slightly higher among anti-TNF than nbDMARD users, the propensity score fully adjusted hazard ratio for a first vasculitis-like event was 1.27, comparing the anti-TNF drugs with nbDMARDs, with a 95% confidence interval of 0.40-4.04.
“This is the first prospective observational study to systematically look at the risk of vasculitis-like events” in patients with RA treated with anti-TNF agents, Dr. Meghna Jani said at the British Society for Rheumatology annual conference.
Dr. Jani of the Arthritis Research UK Centre for Epidemiology at the University of Manchester (England) explained that the reason for looking at this topic was that vasculitis-like events had been reported in case series and single-center studies, but these prior reports were too small to be able to estimate exactly how big a problem this was.
“Anti-TNF agents are associated with the development of a number of autoantibodies, including antinuclear antibodies and antidrug antibodies, and ANCA [antineutrophil cytoplasmic antibody],” she observed.
“We know that a small proportion of these patients may then go on to develop autoimmune diseases, some independent of autoantibodies,” she added. The most common of these is vasculitis, including cutaneous vasculitis.
Vasculitis is a somewhat paradoxical adverse event, she noted, in that it has been associated with anti-TNF therapy, but these drugs can also be used to treat it.
Now in its 15th year, the BSRBR-RA is the largest ongoing cohort of patients treated with biologic agents for rheumatic disease and provides one of the best sources of data to examine the risk for vasculitis-like events Dr. Jani observed. The aims were to look at the respective risks as well as to see if there were any particular predictive factors.
The current analysis included more than 16,000 patients enrolled in the BSRBR-RA between 2001 and 2015, of whom 12,745 were newly started on an anti-TNF drug and 3,640 were receiving nbDMARDs and were also biologic naive. The mean age of patients in the two groups was 56 and 60 years, 76% and 72% were female, with a mean Disease Activity Score (DAS28) of 6.5 and 5.1 and median disease duration of 11 and 6 years, respectively.
After more than 52,428 person-years of exposure and a median of 5.1 years of follow-up, 81 vasculitis-like events occurred in the anti-TNF therapy group. Vasculitis-like events were attributed to treatment only if they had occurred within 90 days of starting the drug. Follow-up stopped after a first event; if there was a switch to another biologic drug; and at death, the last clinical follow-up, or the end of the analysis period (May 31, 2015).
In comparison, there were 20,635 person-years of exposure and 6.5 years’ follow-up in the nbDMARD group, with 14 vasculitis-like events reported during this time.
A sensitivity analysis was performed excluding patients who had nail-fold vasculitis at baseline, had vasculitis due to a possible secondary cause such as infection, and were taking any other medications associated with vasculitis-like events. Results showed a similar risk for a first vasculitis event between anti-TNF and nbDMARD users (aHR = 1.05; 95% CI, 0.32-3.45).
Looking at the risk of vasculitis events for individual anti-TNF drugs, there initially appeared to be a higher risk for patients taking infliximab (n = 3,292) and etanercept (n = 4,450) but not for those taking adalimumab (n = 4,312) versus nbDMARDs, with crude incidence rates of 10, 17, and 11 per 10,000 person-years; after adjustment, these differences were not significant (aHRs of 1.55, 1.72, and 0.77, respectively, with 95% CIs crossing 1.0). A crude rate for certolizumab could not be calculated as there were no vasculitis events reported but there were only 691 patients enrolled in the BSRBR-RA at the time of the analysis who had been exposed to the drug.
“The risk of the event was highest in the first year of treatment, followed by reduction over time,” Dr. Jani reported. “Reassuringly, up to two-thirds of patients in both cohorts had manifestations that were just limited to cutaneous involvement,” she said.
The most common systemic presentation was digital ischemia, affecting 14% of patients treated with anti-TNFs and 14% of those given nbDMARDs. Neurologic involvement was also seen in both groups of patients (7% vs. 7%), but new nail-fold vasculitis (17% vs. 0%), respiratory involvement (4% vs. 0%), associated thrombotic events (5% vs. 0%), and renal involvement (2.5% vs. 0%) were seen in TNF inhibitor-treated patients only.
Ten anti-TNF–treated patients and one nbDMARD-treated patient needed treatment for the vasculitis-like event, and three patients in the anti-TNF cohort died as a result of the event, all of whom had multisystem organ involvement and one of whom had cytoplasmic ANCA-positive vasculitis.
Treatment with methotrexate or sulfasalazine at baseline was associated with a lower risk for vasculitis-like events, while seropositive status, disease duration, DAS28, and HAQ scores were associated with an increased risk for such events.
The BSRBR-RA receives restricted income financial support from Abbvie, Amgen, Swedish Orphan Biovitram (SOBI), Merck, Pfizer, Roche, and UCB Pharma. Dr. Jani disclosed she has received honoraria from Pfizer, Abbvie, and UCB Pharma.
GLASGOW – Treatment with tumor necrosis factor (TNF) inhibitors for rheumatoid arthritis is associated with a low risk of vasculitis-like events, according to a large analysis of data from the United Kingdom.
Investigators using data from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis (BSRBR-RA) found that the crude incidence rate was 16 cases per 10,000 person-years among TNF-inhibitor users versus seven cases per 10,000 person-years among users of nonbiologic disease-modifying antirheumatic drugs (nbDMARDs) such as methotrexate and sulfasalazine.
Although the risk was slightly higher among anti-TNF than nbDMARD users, the propensity score fully adjusted hazard ratio for a first vasculitis-like event was 1.27, comparing the anti-TNF drugs with nbDMARDs, with a 95% confidence interval of 0.40-4.04.
“This is the first prospective observational study to systematically look at the risk of vasculitis-like events” in patients with RA treated with anti-TNF agents, Dr. Meghna Jani said at the British Society for Rheumatology annual conference.
Dr. Jani of the Arthritis Research UK Centre for Epidemiology at the University of Manchester (England) explained that the reason for looking at this topic was that vasculitis-like events had been reported in case series and single-center studies, but these prior reports were too small to be able to estimate exactly how big a problem this was.
“Anti-TNF agents are associated with the development of a number of autoantibodies, including antinuclear antibodies and antidrug antibodies, and ANCA [antineutrophil cytoplasmic antibody],” she observed.
“We know that a small proportion of these patients may then go on to develop autoimmune diseases, some independent of autoantibodies,” she added. The most common of these is vasculitis, including cutaneous vasculitis.
Vasculitis is a somewhat paradoxical adverse event, she noted, in that it has been associated with anti-TNF therapy, but these drugs can also be used to treat it.
Now in its 15th year, the BSRBR-RA is the largest ongoing cohort of patients treated with biologic agents for rheumatic disease and provides one of the best sources of data to examine the risk for vasculitis-like events Dr. Jani observed. The aims were to look at the respective risks as well as to see if there were any particular predictive factors.
The current analysis included more than 16,000 patients enrolled in the BSRBR-RA between 2001 and 2015, of whom 12,745 were newly started on an anti-TNF drug and 3,640 were receiving nbDMARDs and were also biologic naive. The mean age of patients in the two groups was 56 and 60 years, 76% and 72% were female, with a mean Disease Activity Score (DAS28) of 6.5 and 5.1 and median disease duration of 11 and 6 years, respectively.
After more than 52,428 person-years of exposure and a median of 5.1 years of follow-up, 81 vasculitis-like events occurred in the anti-TNF therapy group. Vasculitis-like events were attributed to treatment only if they had occurred within 90 days of starting the drug. Follow-up stopped after a first event; if there was a switch to another biologic drug; and at death, the last clinical follow-up, or the end of the analysis period (May 31, 2015).
In comparison, there were 20,635 person-years of exposure and 6.5 years’ follow-up in the nbDMARD group, with 14 vasculitis-like events reported during this time.
A sensitivity analysis was performed excluding patients who had nail-fold vasculitis at baseline, had vasculitis due to a possible secondary cause such as infection, and were taking any other medications associated with vasculitis-like events. Results showed a similar risk for a first vasculitis event between anti-TNF and nbDMARD users (aHR = 1.05; 95% CI, 0.32-3.45).
Looking at the risk of vasculitis events for individual anti-TNF drugs, there initially appeared to be a higher risk for patients taking infliximab (n = 3,292) and etanercept (n = 4,450) but not for those taking adalimumab (n = 4,312) versus nbDMARDs, with crude incidence rates of 10, 17, and 11 per 10,000 person-years; after adjustment, these differences were not significant (aHRs of 1.55, 1.72, and 0.77, respectively, with 95% CIs crossing 1.0). A crude rate for certolizumab could not be calculated as there were no vasculitis events reported but there were only 691 patients enrolled in the BSRBR-RA at the time of the analysis who had been exposed to the drug.
“The risk of the event was highest in the first year of treatment, followed by reduction over time,” Dr. Jani reported. “Reassuringly, up to two-thirds of patients in both cohorts had manifestations that were just limited to cutaneous involvement,” she said.
The most common systemic presentation was digital ischemia, affecting 14% of patients treated with anti-TNFs and 14% of those given nbDMARDs. Neurologic involvement was also seen in both groups of patients (7% vs. 7%), but new nail-fold vasculitis (17% vs. 0%), respiratory involvement (4% vs. 0%), associated thrombotic events (5% vs. 0%), and renal involvement (2.5% vs. 0%) were seen in TNF inhibitor-treated patients only.
Ten anti-TNF–treated patients and one nbDMARD-treated patient needed treatment for the vasculitis-like event, and three patients in the anti-TNF cohort died as a result of the event, all of whom had multisystem organ involvement and one of whom had cytoplasmic ANCA-positive vasculitis.
Treatment with methotrexate or sulfasalazine at baseline was associated with a lower risk for vasculitis-like events, while seropositive status, disease duration, DAS28, and HAQ scores were associated with an increased risk for such events.
The BSRBR-RA receives restricted income financial support from Abbvie, Amgen, Swedish Orphan Biovitram (SOBI), Merck, Pfizer, Roche, and UCB Pharma. Dr. Jani disclosed she has received honoraria from Pfizer, Abbvie, and UCB Pharma.
GLASGOW – Treatment with tumor necrosis factor (TNF) inhibitors for rheumatoid arthritis is associated with a low risk of vasculitis-like events, according to a large analysis of data from the United Kingdom.
Investigators using data from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis (BSRBR-RA) found that the crude incidence rate was 16 cases per 10,000 person-years among TNF-inhibitor users versus seven cases per 10,000 person-years among users of nonbiologic disease-modifying antirheumatic drugs (nbDMARDs) such as methotrexate and sulfasalazine.
Although the risk was slightly higher among anti-TNF than nbDMARD users, the propensity score fully adjusted hazard ratio for a first vasculitis-like event was 1.27, comparing the anti-TNF drugs with nbDMARDs, with a 95% confidence interval of 0.40-4.04.
“This is the first prospective observational study to systematically look at the risk of vasculitis-like events” in patients with RA treated with anti-TNF agents, Dr. Meghna Jani said at the British Society for Rheumatology annual conference.
Dr. Jani of the Arthritis Research UK Centre for Epidemiology at the University of Manchester (England) explained that the reason for looking at this topic was that vasculitis-like events had been reported in case series and single-center studies, but these prior reports were too small to be able to estimate exactly how big a problem this was.
“Anti-TNF agents are associated with the development of a number of autoantibodies, including antinuclear antibodies and antidrug antibodies, and ANCA [antineutrophil cytoplasmic antibody],” she observed.
“We know that a small proportion of these patients may then go on to develop autoimmune diseases, some independent of autoantibodies,” she added. The most common of these is vasculitis, including cutaneous vasculitis.
Vasculitis is a somewhat paradoxical adverse event, she noted, in that it has been associated with anti-TNF therapy, but these drugs can also be used to treat it.
Now in its 15th year, the BSRBR-RA is the largest ongoing cohort of patients treated with biologic agents for rheumatic disease and provides one of the best sources of data to examine the risk for vasculitis-like events Dr. Jani observed. The aims were to look at the respective risks as well as to see if there were any particular predictive factors.
The current analysis included more than 16,000 patients enrolled in the BSRBR-RA between 2001 and 2015, of whom 12,745 were newly started on an anti-TNF drug and 3,640 were receiving nbDMARDs and were also biologic naive. The mean age of patients in the two groups was 56 and 60 years, 76% and 72% were female, with a mean Disease Activity Score (DAS28) of 6.5 and 5.1 and median disease duration of 11 and 6 years, respectively.
After more than 52,428 person-years of exposure and a median of 5.1 years of follow-up, 81 vasculitis-like events occurred in the anti-TNF therapy group. Vasculitis-like events were attributed to treatment only if they had occurred within 90 days of starting the drug. Follow-up stopped after a first event; if there was a switch to another biologic drug; and at death, the last clinical follow-up, or the end of the analysis period (May 31, 2015).
In comparison, there were 20,635 person-years of exposure and 6.5 years’ follow-up in the nbDMARD group, with 14 vasculitis-like events reported during this time.
A sensitivity analysis was performed excluding patients who had nail-fold vasculitis at baseline, had vasculitis due to a possible secondary cause such as infection, and were taking any other medications associated with vasculitis-like events. Results showed a similar risk for a first vasculitis event between anti-TNF and nbDMARD users (aHR = 1.05; 95% CI, 0.32-3.45).
Looking at the risk of vasculitis events for individual anti-TNF drugs, there initially appeared to be a higher risk for patients taking infliximab (n = 3,292) and etanercept (n = 4,450) but not for those taking adalimumab (n = 4,312) versus nbDMARDs, with crude incidence rates of 10, 17, and 11 per 10,000 person-years; after adjustment, these differences were not significant (aHRs of 1.55, 1.72, and 0.77, respectively, with 95% CIs crossing 1.0). A crude rate for certolizumab could not be calculated as there were no vasculitis events reported but there were only 691 patients enrolled in the BSRBR-RA at the time of the analysis who had been exposed to the drug.
“The risk of the event was highest in the first year of treatment, followed by reduction over time,” Dr. Jani reported. “Reassuringly, up to two-thirds of patients in both cohorts had manifestations that were just limited to cutaneous involvement,” she said.
The most common systemic presentation was digital ischemia, affecting 14% of patients treated with anti-TNFs and 14% of those given nbDMARDs. Neurologic involvement was also seen in both groups of patients (7% vs. 7%), but new nail-fold vasculitis (17% vs. 0%), respiratory involvement (4% vs. 0%), associated thrombotic events (5% vs. 0%), and renal involvement (2.5% vs. 0%) were seen in TNF inhibitor-treated patients only.
Ten anti-TNF–treated patients and one nbDMARD-treated patient needed treatment for the vasculitis-like event, and three patients in the anti-TNF cohort died as a result of the event, all of whom had multisystem organ involvement and one of whom had cytoplasmic ANCA-positive vasculitis.
Treatment with methotrexate or sulfasalazine at baseline was associated with a lower risk for vasculitis-like events, while seropositive status, disease duration, DAS28, and HAQ scores were associated with an increased risk for such events.
The BSRBR-RA receives restricted income financial support from Abbvie, Amgen, Swedish Orphan Biovitram (SOBI), Merck, Pfizer, Roche, and UCB Pharma. Dr. Jani disclosed she has received honoraria from Pfizer, Abbvie, and UCB Pharma.
AT RHEUMATOLOGY 2016
Key clinical point: There is a low risk of vasculitis-like events with tumor necrosis factor inhibitors.
Major finding: Crude incidence rates for vasculitis-like events were 16/10,000 person-years with TNF-inhibitor therapy and 7/10,000 person-years with nonbiologic disease-modifying antirheumatic drugs.
Data source: British Society for Rheumatology Biologics Register for Rheumatoid Arthritis of 12,745 TNF-inhibitor and 3,640 nbDMARD users.
Disclosures: The BSRBR-RA receives restricted income financial support from Abbvie, Amgen, Swedish Orphan Biovitram (SOBI), Merck, Pfizer, Roche, and UCB Pharma. Dr. Jani disclosed she has received honoraria from Pfizer, Abbvie, and UCB Pharma.
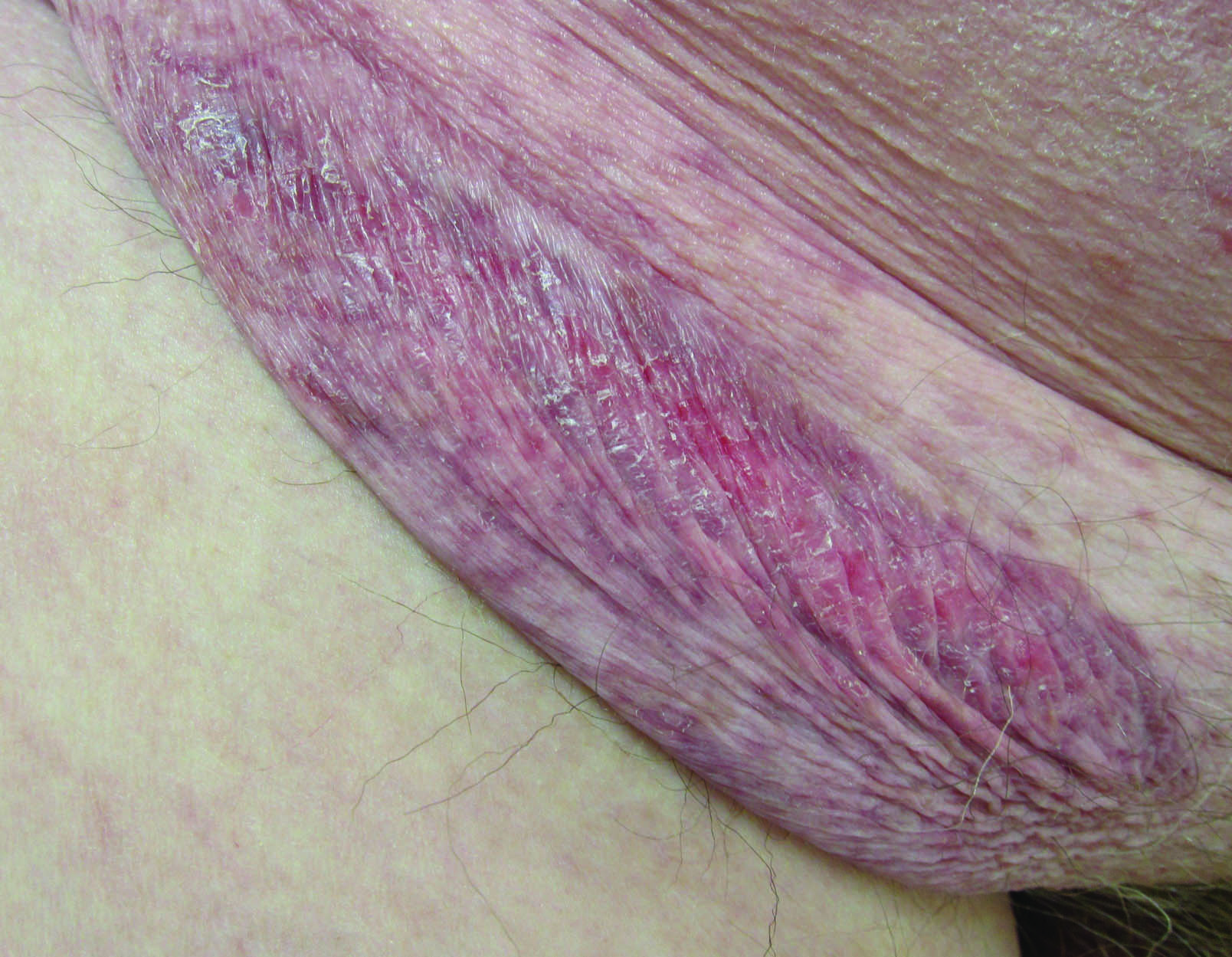
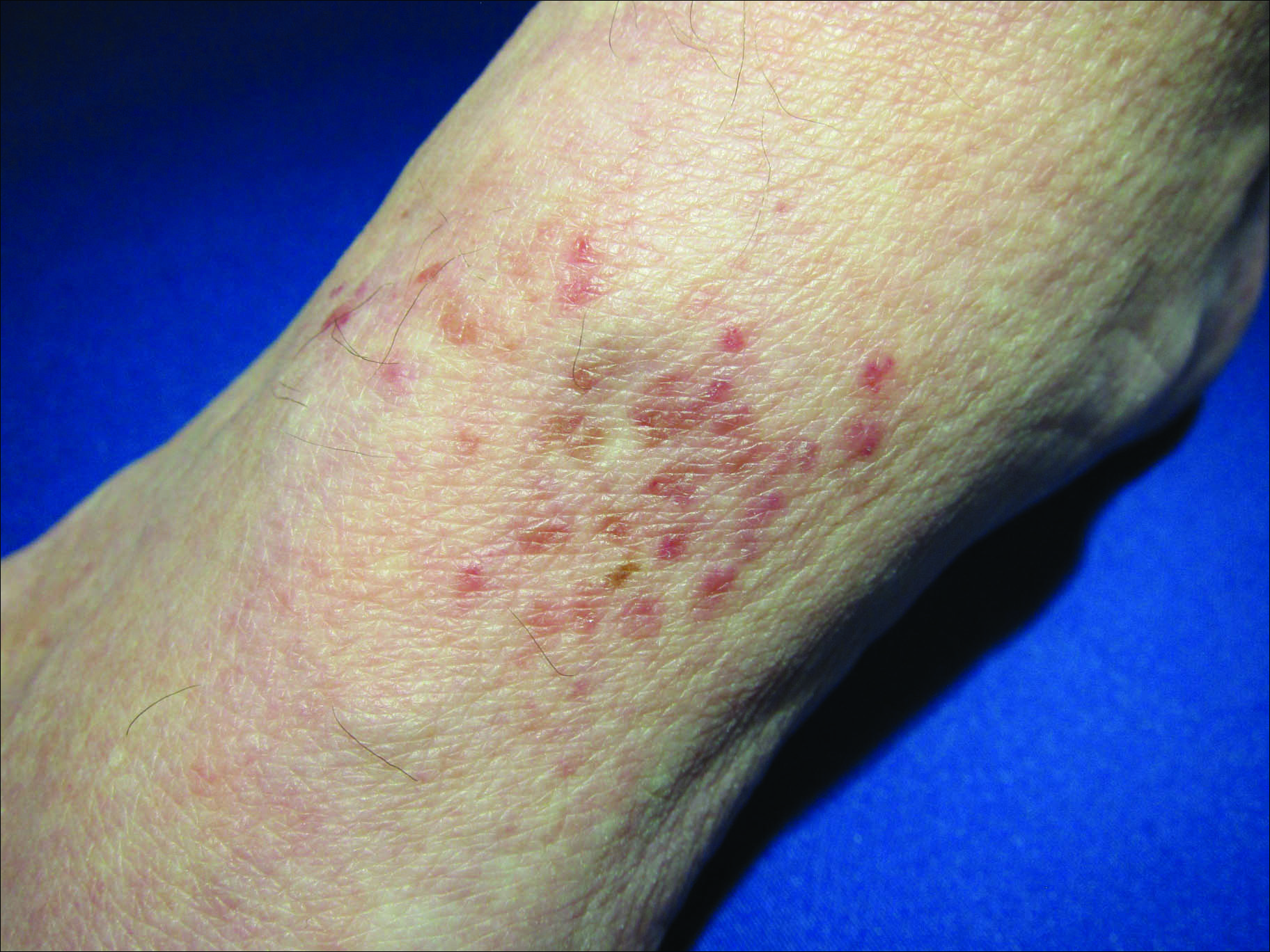
Erythematous Atrophic Plaque in the Inguinal Fold
The Diagnosis: Granulomatous Slack Skin Disease
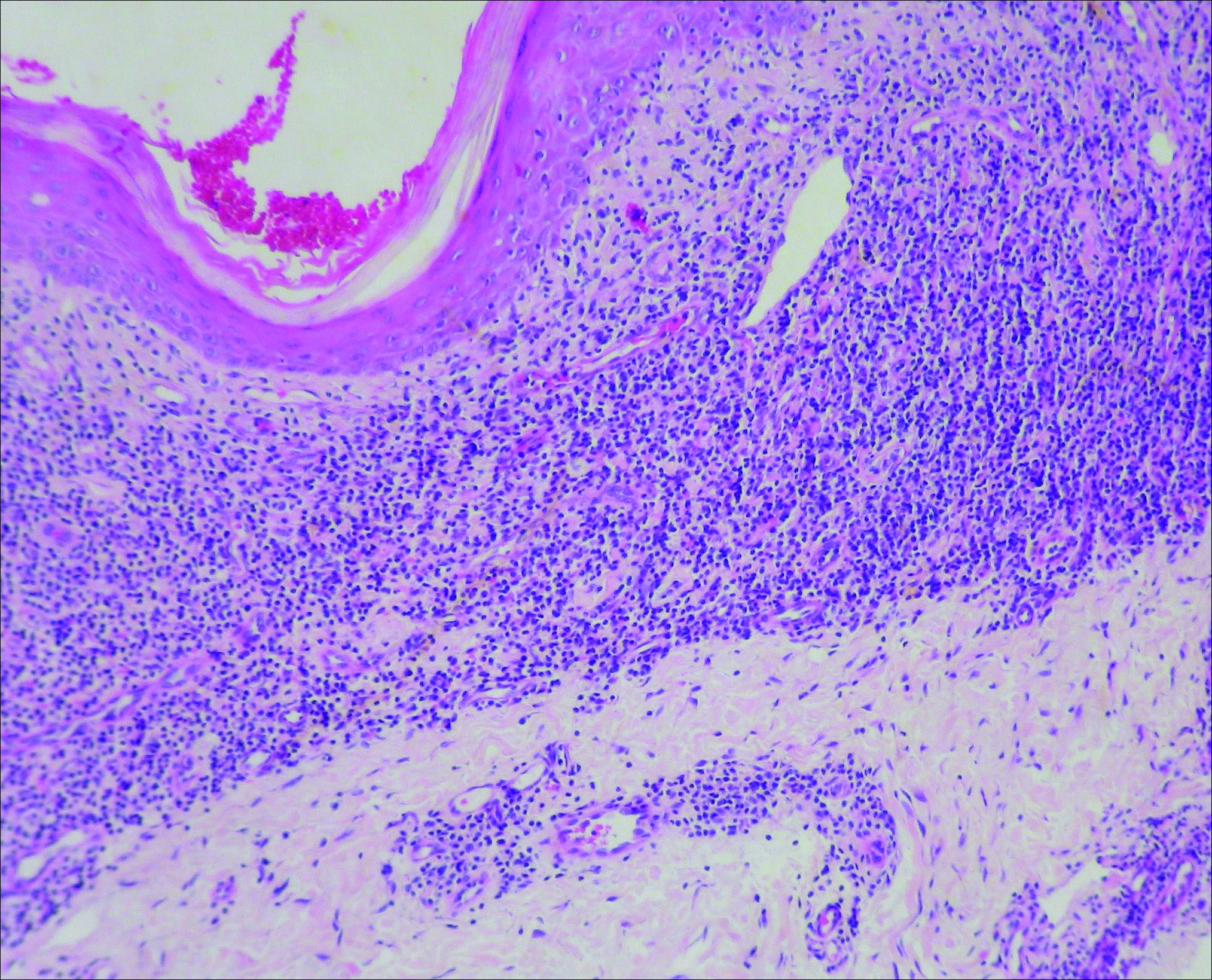
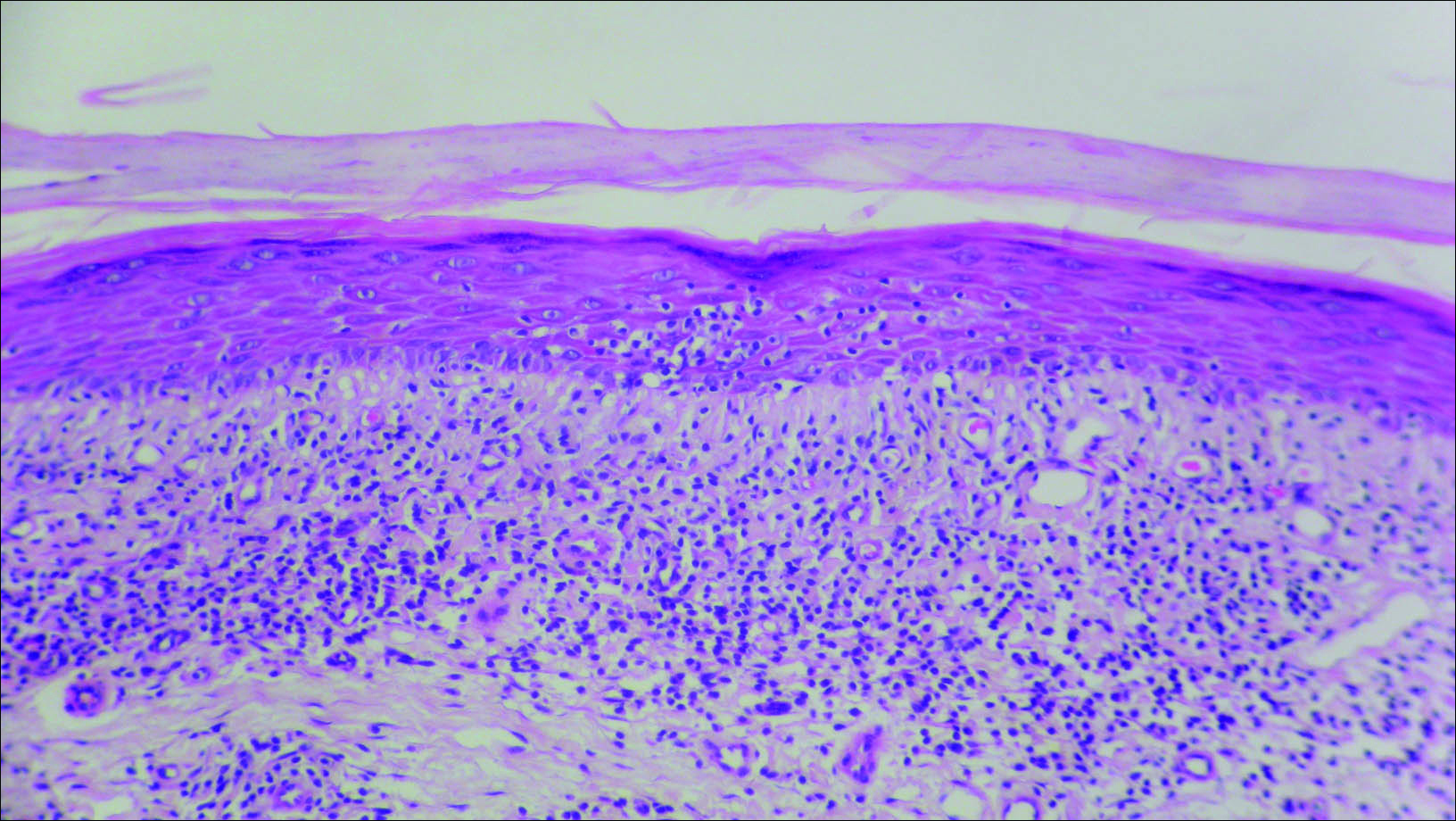
Initial biopsy revealed a lichenoid lymphohistiocytic infiltrate with scattered epidermotropism, papillary dermal sclerosis, and lymphocyte atypia (Figure 1). A repeat biopsy showed a lichenoid granulomatous infiltrate with histiocytes and rare giant cells, superficially located in the dermis, without a deeper dense infiltration. Focal lymphocytic epidermotropism also was present (Figure 2). The infiltrate was CD3+CD4+ with a minority of cells also staining for CD8. An elastin stain demonstrated diminished elastin fibers in the superficial dermis. A clonal T-cell receptor gene rearrangement was identified by polymerase chain reaction. One group of pink and brown papules was present on the dorsal aspect of the right foot (Figure 3). A biopsy of this area showed similar findings. The patient was treated with a trial of carmustine 20-mg% ointment over the following year with some improvement of the mild pruritus but without notable change in the clinical findings.
Granulomatous slack skin disease (GSSD) is a rare form of mycosis fungoides–type cutaneous T-cell lymphoma. It usually presents as well-demarcated, atrophic, poikilodermatous patches and plaques with a predilection for the inguinal and axillary regions.1 The affected areas tend to be asymptomatic and enlarge gradually over years to become pendulous with lax skin and wrinkles. In contrast to other forms of cutaneous T-cell lymphoma, extracutaneous spread is rare. The disease shows a slow progression over many years and by itself is not life threatening. However, affected patients have a risk for developing secondary lymphoproliferative neoplasms, which have been documented in approximately 50% of reported cases.2 These lymphoproliferative neoplasms may arise concurrently, precede, or follow the development of GSSD lesions. Hodgkin lymphoma, seen in 33% of cases, is the most common association, with others being non-Hodgkin lymphoma, mycosis fungoides, acute myeloid leukemia, and Langerhans cell histiocytosis.1-3
Histologically, GSSD is characterized by a dense, dermal, granulomatous proliferation of atypical T lymphocytes with scattered multinucleated giant cells.1,4 There is a loss of elastin fibers in the infiltrated areas, and occasional elastophagocytosis can be seen.1,2,4 Immunoprofiling of the infiltrate has shown CD3+CD4+CD45RO+ T-helper cells with occasional loss of CD5 and CD7.3 A clonal T-cell receptor rearrangement of the g and b genes frequently is described.1,4,5
At this time no treatment has been found to be reliably curative. Varying success in treating GSSD has been achieved with topical nitrogen mustard, carmustine, topical and systemic corticosteroids, psoralen plus UVA, radiotherapy, azathioprine, IFN-g, and combinations of these agents.1-3,6-9 Excision of the diseased skin has been performed for cosmetically or functionally disturbing lesions, but in all but one case the lesions recurred within months.1,10 A consistently reliable treatment of GSSD has not been established; treatment should be tailored to the individual patient.
- Shah A, Safaya A. Granulomatous slack skin disease: a review, in comparison with mycosis fungoides. J Eur Acad Dermatol Venereol. 2012;26:1472-1478.
- Teixeira M, Alves R, Lima M, et al. Granulomatous slack skin. Eur J Dermatol. 2007;17:435-438.
- van Haselen CW, Toonstra J, van der Putte SJ, et al. Granulomatous slack skin: report of three patients with an updated review of the literature. Dermatology. 1998;196:382-391.
- Kempf W, Ostheeren-Michaelis S, Paulli M, et al. Granulomatous mycosis fungoides and granulomatous slack skin: a multicenter study of the Cutaneous Lymphoma Histopathology Task Force Group of the European Organization for Research and Treatment of Cancer (EORTC). Arch Dermatol. 2008;144:1609-1617.
- LeBoit PE, Zackheim HS, White CR Jr. Granulomatous variants of cutaneous t-cell lymphoma: the histopathology of granulomatous mycosis fungoides and granulomatous slack skin. Am J Surg Pathol. 1988;12:83-95.
- Hultgren TL, Jones D, Duvic M. Topical nitrogen mustard for the treatment of granulomatous slack skin. Am J Clin Dermatol. 2007;8:51-54.
- Camacho FM, Burg G, Moreno JC, et al. Granulomatous slack skin in childhood. Pediatr Dermatol. 1997;14:204-208.
- Liu Z, Huang C, Li J. Prednisone combined with interferon for the treatment of one case of generalized granulomatous slack skin. J Huazhong Univ Sci Technolo Med Sci. 2005;25:617-618.
- Oberholzer PA, Cozzio A, Dummer R, et al. Granulomatous slack skin responds to UVA1 phototherapy. Dermatology. 2009;219:268-271.
- Clarijis M, Poot F, Laka A, et al. Granulomatous slack skin: treatment with extensive surgery and review of the literature. Dermatology. 2003;206:393-397.
The Diagnosis: Granulomatous Slack Skin Disease
Initial biopsy revealed a lichenoid lymphohistiocytic infiltrate with scattered epidermotropism, papillary dermal sclerosis, and lymphocyte atypia (Figure 1). A repeat biopsy showed a lichenoid granulomatous infiltrate with histiocytes and rare giant cells, superficially located in the dermis, without a deeper dense infiltration. Focal lymphocytic epidermotropism also was present (Figure 2). The infiltrate was CD3+CD4+ with a minority of cells also staining for CD8. An elastin stain demonstrated diminished elastin fibers in the superficial dermis. A clonal T-cell receptor gene rearrangement was identified by polymerase chain reaction. One group of pink and brown papules was present on the dorsal aspect of the right foot (Figure 3). A biopsy of this area showed similar findings. The patient was treated with a trial of carmustine 20-mg% ointment over the following year with some improvement of the mild pruritus but without notable change in the clinical findings.



Granulomatous slack skin disease (GSSD) is a rare form of mycosis fungoides–type cutaneous T-cell lymphoma. It usually presents as well-demarcated, atrophic, poikilodermatous patches and plaques with a predilection for the inguinal and axillary regions.1 The affected areas tend to be asymptomatic and enlarge gradually over years to become pendulous with lax skin and wrinkles. In contrast to other forms of cutaneous T-cell lymphoma, extracutaneous spread is rare. The disease shows a slow progression over many years and by itself is not life threatening. However, affected patients have a risk for developing secondary lymphoproliferative neoplasms, which have been documented in approximately 50% of reported cases.2 These lymphoproliferative neoplasms may arise concurrently, precede, or follow the development of GSSD lesions. Hodgkin lymphoma, seen in 33% of cases, is the most common association, with others being non-Hodgkin lymphoma, mycosis fungoides, acute myeloid leukemia, and Langerhans cell histiocytosis.1-3
Histologically, GSSD is characterized by a dense, dermal, granulomatous proliferation of atypical T lymphocytes with scattered multinucleated giant cells.1,4 There is a loss of elastin fibers in the infiltrated areas, and occasional elastophagocytosis can be seen.1,2,4 Immunoprofiling of the infiltrate has shown CD3+CD4+CD45RO+ T-helper cells with occasional loss of CD5 and CD7.3 A clonal T-cell receptor rearrangement of the g and b genes frequently is described.1,4,5
At this time no treatment has been found to be reliably curative. Varying success in treating GSSD has been achieved with topical nitrogen mustard, carmustine, topical and systemic corticosteroids, psoralen plus UVA, radiotherapy, azathioprine, IFN-g, and combinations of these agents.1-3,6-9 Excision of the diseased skin has been performed for cosmetically or functionally disturbing lesions, but in all but one case the lesions recurred within months.1,10 A consistently reliable treatment of GSSD has not been established; treatment should be tailored to the individual patient.
The Diagnosis: Granulomatous Slack Skin Disease
Initial biopsy revealed a lichenoid lymphohistiocytic infiltrate with scattered epidermotropism, papillary dermal sclerosis, and lymphocyte atypia (Figure 1). A repeat biopsy showed a lichenoid granulomatous infiltrate with histiocytes and rare giant cells, superficially located in the dermis, without a deeper dense infiltration. Focal lymphocytic epidermotropism also was present (Figure 2). The infiltrate was CD3+CD4+ with a minority of cells also staining for CD8. An elastin stain demonstrated diminished elastin fibers in the superficial dermis. A clonal T-cell receptor gene rearrangement was identified by polymerase chain reaction. One group of pink and brown papules was present on the dorsal aspect of the right foot (Figure 3). A biopsy of this area showed similar findings. The patient was treated with a trial of carmustine 20-mg% ointment over the following year with some improvement of the mild pruritus but without notable change in the clinical findings.



Granulomatous slack skin disease (GSSD) is a rare form of mycosis fungoides–type cutaneous T-cell lymphoma. It usually presents as well-demarcated, atrophic, poikilodermatous patches and plaques with a predilection for the inguinal and axillary regions.1 The affected areas tend to be asymptomatic and enlarge gradually over years to become pendulous with lax skin and wrinkles. In contrast to other forms of cutaneous T-cell lymphoma, extracutaneous spread is rare. The disease shows a slow progression over many years and by itself is not life threatening. However, affected patients have a risk for developing secondary lymphoproliferative neoplasms, which have been documented in approximately 50% of reported cases.2 These lymphoproliferative neoplasms may arise concurrently, precede, or follow the development of GSSD lesions. Hodgkin lymphoma, seen in 33% of cases, is the most common association, with others being non-Hodgkin lymphoma, mycosis fungoides, acute myeloid leukemia, and Langerhans cell histiocytosis.1-3
Histologically, GSSD is characterized by a dense, dermal, granulomatous proliferation of atypical T lymphocytes with scattered multinucleated giant cells.1,4 There is a loss of elastin fibers in the infiltrated areas, and occasional elastophagocytosis can be seen.1,2,4 Immunoprofiling of the infiltrate has shown CD3+CD4+CD45RO+ T-helper cells with occasional loss of CD5 and CD7.3 A clonal T-cell receptor rearrangement of the g and b genes frequently is described.1,4,5
At this time no treatment has been found to be reliably curative. Varying success in treating GSSD has been achieved with topical nitrogen mustard, carmustine, topical and systemic corticosteroids, psoralen plus UVA, radiotherapy, azathioprine, IFN-g, and combinations of these agents.1-3,6-9 Excision of the diseased skin has been performed for cosmetically or functionally disturbing lesions, but in all but one case the lesions recurred within months.1,10 A consistently reliable treatment of GSSD has not been established; treatment should be tailored to the individual patient.
- Shah A, Safaya A. Granulomatous slack skin disease: a review, in comparison with mycosis fungoides. J Eur Acad Dermatol Venereol. 2012;26:1472-1478.
- Teixeira M, Alves R, Lima M, et al. Granulomatous slack skin. Eur J Dermatol. 2007;17:435-438.
- van Haselen CW, Toonstra J, van der Putte SJ, et al. Granulomatous slack skin: report of three patients with an updated review of the literature. Dermatology. 1998;196:382-391.
- Kempf W, Ostheeren-Michaelis S, Paulli M, et al. Granulomatous mycosis fungoides and granulomatous slack skin: a multicenter study of the Cutaneous Lymphoma Histopathology Task Force Group of the European Organization for Research and Treatment of Cancer (EORTC). Arch Dermatol. 2008;144:1609-1617.
- LeBoit PE, Zackheim HS, White CR Jr. Granulomatous variants of cutaneous t-cell lymphoma: the histopathology of granulomatous mycosis fungoides and granulomatous slack skin. Am J Surg Pathol. 1988;12:83-95.
- Hultgren TL, Jones D, Duvic M. Topical nitrogen mustard for the treatment of granulomatous slack skin. Am J Clin Dermatol. 2007;8:51-54.
- Camacho FM, Burg G, Moreno JC, et al. Granulomatous slack skin in childhood. Pediatr Dermatol. 1997;14:204-208.
- Liu Z, Huang C, Li J. Prednisone combined with interferon for the treatment of one case of generalized granulomatous slack skin. J Huazhong Univ Sci Technolo Med Sci. 2005;25:617-618.
- Oberholzer PA, Cozzio A, Dummer R, et al. Granulomatous slack skin responds to UVA1 phototherapy. Dermatology. 2009;219:268-271.
- Clarijis M, Poot F, Laka A, et al. Granulomatous slack skin: treatment with extensive surgery and review of the literature. Dermatology. 2003;206:393-397.
- Shah A, Safaya A. Granulomatous slack skin disease: a review, in comparison with mycosis fungoides. J Eur Acad Dermatol Venereol. 2012;26:1472-1478.
- Teixeira M, Alves R, Lima M, et al. Granulomatous slack skin. Eur J Dermatol. 2007;17:435-438.
- van Haselen CW, Toonstra J, van der Putte SJ, et al. Granulomatous slack skin: report of three patients with an updated review of the literature. Dermatology. 1998;196:382-391.
- Kempf W, Ostheeren-Michaelis S, Paulli M, et al. Granulomatous mycosis fungoides and granulomatous slack skin: a multicenter study of the Cutaneous Lymphoma Histopathology Task Force Group of the European Organization for Research and Treatment of Cancer (EORTC). Arch Dermatol. 2008;144:1609-1617.
- LeBoit PE, Zackheim HS, White CR Jr. Granulomatous variants of cutaneous t-cell lymphoma: the histopathology of granulomatous mycosis fungoides and granulomatous slack skin. Am J Surg Pathol. 1988;12:83-95.
- Hultgren TL, Jones D, Duvic M. Topical nitrogen mustard for the treatment of granulomatous slack skin. Am J Clin Dermatol. 2007;8:51-54.
- Camacho FM, Burg G, Moreno JC, et al. Granulomatous slack skin in childhood. Pediatr Dermatol. 1997;14:204-208.
- Liu Z, Huang C, Li J. Prednisone combined with interferon for the treatment of one case of generalized granulomatous slack skin. J Huazhong Univ Sci Technolo Med Sci. 2005;25:617-618.
- Oberholzer PA, Cozzio A, Dummer R, et al. Granulomatous slack skin responds to UVA1 phototherapy. Dermatology. 2009;219:268-271.
- Clarijis M, Poot F, Laka A, et al. Granulomatous slack skin: treatment with extensive surgery and review of the literature. Dermatology. 2003;206:393-397.
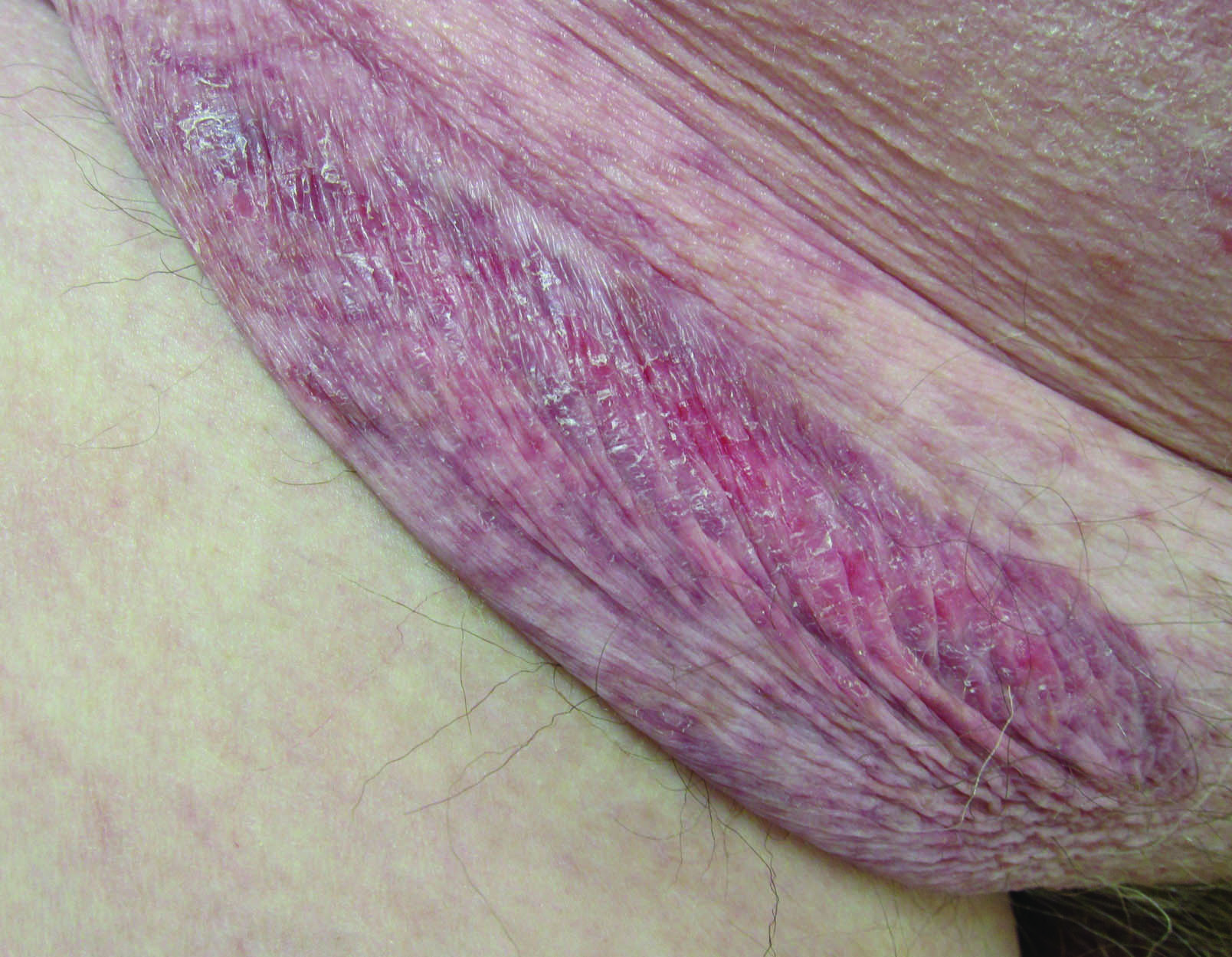
A 66-year-old man presented with a rash on the groin of more than 6 years’ duration. The eruption was asymptomatic, except for occasional pruritus during the summer months. Numerous over-the-counter ointments, creams, and powders, as well as prescription topical corticosteroids, had failed to provide improvement. An outside biopsy performed 1 year earlier was considered nondiagnostic. Physical examination revealed a pink to violaceous, pendulous, atrophic plaque with slight scale on the right side of the lower abdomen running just superior to the right inguinal fold; the left inguinal fold was unaffected. Inguinal lymph nodes were not palpable. A 4-mm punch biopsy of the plaque in the inguinal fold was performed.
Summer colds
Most viral infections in summer months are caused by enteroviruses. We studied illnesses in about 400 kids aged 4-18 years seen in private pediatric practice and were surprised by what we found.
Our impression was that summer colds lasted for a shorter time span than winter colds. What we found was that the median duration of illness was about 8 days. Among the various syndromes, the most common was stomatitis (viral blisters in the throat), accounting for 58% of all cases seen. A flulike illness with fever, myalgias, and malaise was second most common (28% of cases), followed by hand/foot/mouth syndrome (8%), pleurodynia (3%), fever with viral rash (3%), and aseptic meningitis (1%). Most of the cases occurred among children 4-12 years old.
The most prevalent symptoms were fever, headache, sore throat, tiredness, muscle aches, and crankiness. Fever was present in about 85% of cases of children with stomatitis, in 95% of cases with myalgias and malaise, but in only 50% of cases of hand/foot/mouth. Headache was very common as well, occurring in about 40% of children with stomatitis, 70% of children with myalgias and malaise, and in 30% of children with hand/foot/mouth.
Illness within a household was quite common. About 50% of the children who came for care had a sibling or parent ill with a summer cold. However, while the symptoms of the family members often were the same as the child who presented for care, that was not always the case. As anticipated, most illness within a household occurred within a 2-week time span. Hand/foot/mouth was most easily recognized by parents to have spread among their children. When a parent became ill, it was almost always the mother because she was almost always the primary parent caretaker.
Summer colds took a toll on families in terms of loss of work by parents. Most of the children were ill enough to stay out of day care or school for about 2-4 days. Virtually all the children with hand/foot/mouth and stomatitis with classic viral blister lesions had a single visit to the pediatric practice, and very limited or no tests done or medications prescribed other than acetaminophen or ibuprofen. But for the children with higher fevers without hand/foot/mouth or stomatitis, the costs of care escalated as tests were much more often performed (CBC, chest x-ray), and medications prescribed (antibiotics for uncertain diagnosis in the context of high fever), and occasional referrals made to the emergency department for further work-up (100% of cases of aseptic meningitis and 50% of cases of pleurodynia).
Overall, summer colds are not so insignificant as presumed at first glance. What interests me now is why summer colds so infrequently are followed by an acute otitis media or sinusitis, whereas winter colds caused by respiratory syncytial virus, influenza, and rhinoviruses are followed by an acute otitis media in about one-third of cases. A new study is underway!
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has no disclosures.
Most viral infections in summer months are caused by enteroviruses. We studied illnesses in about 400 kids aged 4-18 years seen in private pediatric practice and were surprised by what we found.
Our impression was that summer colds lasted for a shorter time span than winter colds. What we found was that the median duration of illness was about 8 days. Among the various syndromes, the most common was stomatitis (viral blisters in the throat), accounting for 58% of all cases seen. A flulike illness with fever, myalgias, and malaise was second most common (28% of cases), followed by hand/foot/mouth syndrome (8%), pleurodynia (3%), fever with viral rash (3%), and aseptic meningitis (1%). Most of the cases occurred among children 4-12 years old.
The most prevalent symptoms were fever, headache, sore throat, tiredness, muscle aches, and crankiness. Fever was present in about 85% of cases of children with stomatitis, in 95% of cases with myalgias and malaise, but in only 50% of cases of hand/foot/mouth. Headache was very common as well, occurring in about 40% of children with stomatitis, 70% of children with myalgias and malaise, and in 30% of children with hand/foot/mouth.
Illness within a household was quite common. About 50% of the children who came for care had a sibling or parent ill with a summer cold. However, while the symptoms of the family members often were the same as the child who presented for care, that was not always the case. As anticipated, most illness within a household occurred within a 2-week time span. Hand/foot/mouth was most easily recognized by parents to have spread among their children. When a parent became ill, it was almost always the mother because she was almost always the primary parent caretaker.
Summer colds took a toll on families in terms of loss of work by parents. Most of the children were ill enough to stay out of day care or school for about 2-4 days. Virtually all the children with hand/foot/mouth and stomatitis with classic viral blister lesions had a single visit to the pediatric practice, and very limited or no tests done or medications prescribed other than acetaminophen or ibuprofen. But for the children with higher fevers without hand/foot/mouth or stomatitis, the costs of care escalated as tests were much more often performed (CBC, chest x-ray), and medications prescribed (antibiotics for uncertain diagnosis in the context of high fever), and occasional referrals made to the emergency department for further work-up (100% of cases of aseptic meningitis and 50% of cases of pleurodynia).
Overall, summer colds are not so insignificant as presumed at first glance. What interests me now is why summer colds so infrequently are followed by an acute otitis media or sinusitis, whereas winter colds caused by respiratory syncytial virus, influenza, and rhinoviruses are followed by an acute otitis media in about one-third of cases. A new study is underway!
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has no disclosures.
Most viral infections in summer months are caused by enteroviruses. We studied illnesses in about 400 kids aged 4-18 years seen in private pediatric practice and were surprised by what we found.
Our impression was that summer colds lasted for a shorter time span than winter colds. What we found was that the median duration of illness was about 8 days. Among the various syndromes, the most common was stomatitis (viral blisters in the throat), accounting for 58% of all cases seen. A flulike illness with fever, myalgias, and malaise was second most common (28% of cases), followed by hand/foot/mouth syndrome (8%), pleurodynia (3%), fever with viral rash (3%), and aseptic meningitis (1%). Most of the cases occurred among children 4-12 years old.
The most prevalent symptoms were fever, headache, sore throat, tiredness, muscle aches, and crankiness. Fever was present in about 85% of cases of children with stomatitis, in 95% of cases with myalgias and malaise, but in only 50% of cases of hand/foot/mouth. Headache was very common as well, occurring in about 40% of children with stomatitis, 70% of children with myalgias and malaise, and in 30% of children with hand/foot/mouth.
Illness within a household was quite common. About 50% of the children who came for care had a sibling or parent ill with a summer cold. However, while the symptoms of the family members often were the same as the child who presented for care, that was not always the case. As anticipated, most illness within a household occurred within a 2-week time span. Hand/foot/mouth was most easily recognized by parents to have spread among their children. When a parent became ill, it was almost always the mother because she was almost always the primary parent caretaker.
Summer colds took a toll on families in terms of loss of work by parents. Most of the children were ill enough to stay out of day care or school for about 2-4 days. Virtually all the children with hand/foot/mouth and stomatitis with classic viral blister lesions had a single visit to the pediatric practice, and very limited or no tests done or medications prescribed other than acetaminophen or ibuprofen. But for the children with higher fevers without hand/foot/mouth or stomatitis, the costs of care escalated as tests were much more often performed (CBC, chest x-ray), and medications prescribed (antibiotics for uncertain diagnosis in the context of high fever), and occasional referrals made to the emergency department for further work-up (100% of cases of aseptic meningitis and 50% of cases of pleurodynia).
Overall, summer colds are not so insignificant as presumed at first glance. What interests me now is why summer colds so infrequently are followed by an acute otitis media or sinusitis, whereas winter colds caused by respiratory syncytial virus, influenza, and rhinoviruses are followed by an acute otitis media in about one-third of cases. A new study is underway!
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has no disclosures.
Sports safety
The National Athletic Trainers Association and the American Medical Society for Sports Medicine have developed a new program they call Collaborative Solutions for Safety in Sports, with the goal of establishing a suite of safety rules, policies, and possibly laws to protect high school athletes from injury (“School Athletes Often Lack Adequate Protection” by Jane Brody, New York Times, April 18, 2016).
After its second meeting, Dr. Jonathan Drezner, director of the Center for Sports Cardiology at the University of Washington, Seattle, said that the collaborative hopes that eventually every high school in the country will have an athletic trainer at every practice and game; an emergency action plan to respond appropriately to an athlete in distress; a publicly accessible automated external defibrillator (AED) and a school-based program in its use; and climatization policies to prevent heat injury and heat stroke.
I suspect that in communities in which the athletic facilities are located on a single campus that these guideline might be achievable. But here in Brunswick, Maine, and all of the other communities that I am familiar with, having a trainer at every practice and game is logistically impractical and financially unsustainable.
For example, on a given weekday afternoon in the spring here in Brunswick, there may be boys and girls varsity and junior varsity lacrosse, baseball, and softball practices or games on fields scattered around town – many of which are miles apart. The track team may be running on the roads and in the woods, who knows where. Staffing all of these events might be a financial windfall for the athletic trainers, if they could be found. But the money just isn’t there. Although the 50 high school athletes who died last year according to the National Athletic Trainers Association is 50 too many, I doubt that having a trainer at every practice and game would be a cost-effective solution.
When I opened my practice, Brunswick’s only pediatrician eagerly relinquished the job of school physician. Along with doing annual physical assessments on the bus drivers, I was expected to attend all of the home varsity football games. Trotting out on the field to evaluate the bumped and bruised players provided good visibility and helped build my practice. But I wondered who was going to attend to the junior varsity players and to the soccer players, who in my experience were more likely to be injured than the varsity football players. I certainly didn’t have the time. Nor could I be in five places at once.
After a couple of years, the athletic director and I hatched a plan to ask the school board to require that the coaches in every sport be certified in CPR. Our plan was quickly adopted, in part because a 15-year-old in a neighboring town had recently suffered a cardiac event during a track practice. Later, it was discovered that she had short QT syndrome, but there was an unfortunate delay in finding someone skilled in CPR.
In the 35 years since the CPR requirement was initially adopted, there has not been a single player who required resuscitation. However, last year one of the track coaches was running with a friend at dawn on a rural road when his friend dropped, pulseless. The coach’s school-required CPR training saved the man’s life.
While having a trainer at every high school practice and game is an unrealistic goal, educating coaches and players on how to identify and manage an athlete in distress makes a lot of sense. But the collaborative’s recommendation that is my personal favorite is having AEDs publicly accessible at games and practices. As a grandparent who spends a lot of time watching his grandchildren compete, I want the equipment available should I get a little too excited.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
The National Athletic Trainers Association and the American Medical Society for Sports Medicine have developed a new program they call Collaborative Solutions for Safety in Sports, with the goal of establishing a suite of safety rules, policies, and possibly laws to protect high school athletes from injury (“School Athletes Often Lack Adequate Protection” by Jane Brody, New York Times, April 18, 2016).
After its second meeting, Dr. Jonathan Drezner, director of the Center for Sports Cardiology at the University of Washington, Seattle, said that the collaborative hopes that eventually every high school in the country will have an athletic trainer at every practice and game; an emergency action plan to respond appropriately to an athlete in distress; a publicly accessible automated external defibrillator (AED) and a school-based program in its use; and climatization policies to prevent heat injury and heat stroke.
I suspect that in communities in which the athletic facilities are located on a single campus that these guideline might be achievable. But here in Brunswick, Maine, and all of the other communities that I am familiar with, having a trainer at every practice and game is logistically impractical and financially unsustainable.
For example, on a given weekday afternoon in the spring here in Brunswick, there may be boys and girls varsity and junior varsity lacrosse, baseball, and softball practices or games on fields scattered around town – many of which are miles apart. The track team may be running on the roads and in the woods, who knows where. Staffing all of these events might be a financial windfall for the athletic trainers, if they could be found. But the money just isn’t there. Although the 50 high school athletes who died last year according to the National Athletic Trainers Association is 50 too many, I doubt that having a trainer at every practice and game would be a cost-effective solution.
When I opened my practice, Brunswick’s only pediatrician eagerly relinquished the job of school physician. Along with doing annual physical assessments on the bus drivers, I was expected to attend all of the home varsity football games. Trotting out on the field to evaluate the bumped and bruised players provided good visibility and helped build my practice. But I wondered who was going to attend to the junior varsity players and to the soccer players, who in my experience were more likely to be injured than the varsity football players. I certainly didn’t have the time. Nor could I be in five places at once.
After a couple of years, the athletic director and I hatched a plan to ask the school board to require that the coaches in every sport be certified in CPR. Our plan was quickly adopted, in part because a 15-year-old in a neighboring town had recently suffered a cardiac event during a track practice. Later, it was discovered that she had short QT syndrome, but there was an unfortunate delay in finding someone skilled in CPR.
In the 35 years since the CPR requirement was initially adopted, there has not been a single player who required resuscitation. However, last year one of the track coaches was running with a friend at dawn on a rural road when his friend dropped, pulseless. The coach’s school-required CPR training saved the man’s life.
While having a trainer at every high school practice and game is an unrealistic goal, educating coaches and players on how to identify and manage an athlete in distress makes a lot of sense. But the collaborative’s recommendation that is my personal favorite is having AEDs publicly accessible at games and practices. As a grandparent who spends a lot of time watching his grandchildren compete, I want the equipment available should I get a little too excited.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
The National Athletic Trainers Association and the American Medical Society for Sports Medicine have developed a new program they call Collaborative Solutions for Safety in Sports, with the goal of establishing a suite of safety rules, policies, and possibly laws to protect high school athletes from injury (“School Athletes Often Lack Adequate Protection” by Jane Brody, New York Times, April 18, 2016).
After its second meeting, Dr. Jonathan Drezner, director of the Center for Sports Cardiology at the University of Washington, Seattle, said that the collaborative hopes that eventually every high school in the country will have an athletic trainer at every practice and game; an emergency action plan to respond appropriately to an athlete in distress; a publicly accessible automated external defibrillator (AED) and a school-based program in its use; and climatization policies to prevent heat injury and heat stroke.
I suspect that in communities in which the athletic facilities are located on a single campus that these guideline might be achievable. But here in Brunswick, Maine, and all of the other communities that I am familiar with, having a trainer at every practice and game is logistically impractical and financially unsustainable.
For example, on a given weekday afternoon in the spring here in Brunswick, there may be boys and girls varsity and junior varsity lacrosse, baseball, and softball practices or games on fields scattered around town – many of which are miles apart. The track team may be running on the roads and in the woods, who knows where. Staffing all of these events might be a financial windfall for the athletic trainers, if they could be found. But the money just isn’t there. Although the 50 high school athletes who died last year according to the National Athletic Trainers Association is 50 too many, I doubt that having a trainer at every practice and game would be a cost-effective solution.
When I opened my practice, Brunswick’s only pediatrician eagerly relinquished the job of school physician. Along with doing annual physical assessments on the bus drivers, I was expected to attend all of the home varsity football games. Trotting out on the field to evaluate the bumped and bruised players provided good visibility and helped build my practice. But I wondered who was going to attend to the junior varsity players and to the soccer players, who in my experience were more likely to be injured than the varsity football players. I certainly didn’t have the time. Nor could I be in five places at once.
After a couple of years, the athletic director and I hatched a plan to ask the school board to require that the coaches in every sport be certified in CPR. Our plan was quickly adopted, in part because a 15-year-old in a neighboring town had recently suffered a cardiac event during a track practice. Later, it was discovered that she had short QT syndrome, but there was an unfortunate delay in finding someone skilled in CPR.
In the 35 years since the CPR requirement was initially adopted, there has not been a single player who required resuscitation. However, last year one of the track coaches was running with a friend at dawn on a rural road when his friend dropped, pulseless. The coach’s school-required CPR training saved the man’s life.
While having a trainer at every high school practice and game is an unrealistic goal, educating coaches and players on how to identify and manage an athlete in distress makes a lot of sense. But the collaborative’s recommendation that is my personal favorite is having AEDs publicly accessible at games and practices. As a grandparent who spends a lot of time watching his grandchildren compete, I want the equipment available should I get a little too excited.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
Focus on patient-level factors, postop complications to reduce readmissions
CHICAGO – Preadmission and postdischarge factors were important predictors of postoperative readmission in a large cohort of surgical patients, but the hospital course had little incremental impact on either readmissions or postdischarge complications in the cohort, according to a retrospective study of Veterans Affairs data.
The findings suggest that efforts to reduce postoperative readmissions should focus on enhanced postdischarge surveillance and early intervention, Dr. Melanie S. Morris of the University of Alabama at Birmingham reported at the annual meeting of the American Surgical Association.
To assess the relative contributions of patient factors, operative characteristics, and postoperative hospital course on readmissions, she and her colleagues evaluated 243,956 general, vascular, and orthopedic surgery patients in 121 VA hospitals. The overall readmission rate among the cohort was 11.1%, and for general, vascular, and orthopedic surgeries, the rates were 12.9%, 15.4%, and 7.6%, respectively; the average postoperative length of stay was 6.9 days, and 6.1% of patients experienced a predischarge complication.
Almost all readmissions occurred within 2 weeks of discharge, and for general surgery patients, most occurred within 1 week. The readmission rate for vascular surgery patients remained high beyond the 2-week mark.
An examination of the reasons for readmission showed that wound complications were the most common reason for readmission, and this was particularly true for vascular surgery patients, in whom 44% of readmissions were for wound complications, Dr. Morris said.
Gastrointestinal complications including ileus and obstruction were also common, accounting for nearly 28% of readmissions among general surgery patients, she said.
Importantly, when including preoperative data (such as demographics, comorbidities, social and behavioral factors, labs and vital signs, and planned procedure type), the variability in readmissions could only be explained 8.6% of the time, she said.
“Adding in operative data, such as procedure complexity and intraoperative blood transfusions, as well as postoperative course, added very little to our predictive ability. Including both of those groups, we could only explain 10% of the variation in readmission,” she said.
Including postdischarge data such as complications and emergency department utilization in the model increased predictive ability to 18%.
R2 and C-statistics comparing the sequentially built model showed that demographics and comorbidities contributed the most to predicting readmission risk, Dr. Morris said.
Modeling based on readmission reason and specialty improved predictive ability. For example, almost 12% of readmissions for wound complications among vascular surgery patients were predictable.
“Our best predictive ability was for orthopedic patients who were readmitted with pneumonia. We were able to predict that 14% of the time,” she said.
The findings were derived by merging VA Surgical Quality Improvement Program data from inpatient operations performed between 2007 and 2014 and involving at least a 2-day postoperative hospital stay, with clinical data including laboratory findings, vitals, prior health care utilization, and postoperative complications.
“We then grouped our variables of interest into the following categories: preoperative, operative, postoperative but predischarge, and postdischarge,” she explained, noting that logistic models predicting 30-day readmission were constructed by sequentially adding groups into the model. Models were compared by way of adjusted R2 and C-statistics.
Assuming postoperative readmissions are preventable suggests that they are linked to the quality of care during the index hospitalization. The current findings demonstrate the challenges in predicting readmissions, and are important given that hospitals with higher-than-expected readmission rates for certain diagnoses and procedures are fined by the Centers for Medicare & Medicaid Services; 54% of hospitals were fined in 2015, she said.
“Readmission is difficult to predict at the time of discharge despite exhaustive statistical modeling with very granular clinical patient-level detail. Preoperative patient factors and postdischarge complications contribute the most to predictive models. Efforts to decrease readmissions should focus on modifiable patient-level factors, transitions of care, and minimizing postoperative complications,” she concluded.
Dr. Morris reported having no disclosures.
The complete manuscript of this presentation is anticipated to be published in Annals of Surgery pending editorial review.
CHICAGO – Preadmission and postdischarge factors were important predictors of postoperative readmission in a large cohort of surgical patients, but the hospital course had little incremental impact on either readmissions or postdischarge complications in the cohort, according to a retrospective study of Veterans Affairs data.
The findings suggest that efforts to reduce postoperative readmissions should focus on enhanced postdischarge surveillance and early intervention, Dr. Melanie S. Morris of the University of Alabama at Birmingham reported at the annual meeting of the American Surgical Association.
To assess the relative contributions of patient factors, operative characteristics, and postoperative hospital course on readmissions, she and her colleagues evaluated 243,956 general, vascular, and orthopedic surgery patients in 121 VA hospitals. The overall readmission rate among the cohort was 11.1%, and for general, vascular, and orthopedic surgeries, the rates were 12.9%, 15.4%, and 7.6%, respectively; the average postoperative length of stay was 6.9 days, and 6.1% of patients experienced a predischarge complication.
Almost all readmissions occurred within 2 weeks of discharge, and for general surgery patients, most occurred within 1 week. The readmission rate for vascular surgery patients remained high beyond the 2-week mark.
An examination of the reasons for readmission showed that wound complications were the most common reason for readmission, and this was particularly true for vascular surgery patients, in whom 44% of readmissions were for wound complications, Dr. Morris said.
Gastrointestinal complications including ileus and obstruction were also common, accounting for nearly 28% of readmissions among general surgery patients, she said.
Importantly, when including preoperative data (such as demographics, comorbidities, social and behavioral factors, labs and vital signs, and planned procedure type), the variability in readmissions could only be explained 8.6% of the time, she said.
“Adding in operative data, such as procedure complexity and intraoperative blood transfusions, as well as postoperative course, added very little to our predictive ability. Including both of those groups, we could only explain 10% of the variation in readmission,” she said.
Including postdischarge data such as complications and emergency department utilization in the model increased predictive ability to 18%.
R2 and C-statistics comparing the sequentially built model showed that demographics and comorbidities contributed the most to predicting readmission risk, Dr. Morris said.
Modeling based on readmission reason and specialty improved predictive ability. For example, almost 12% of readmissions for wound complications among vascular surgery patients were predictable.
“Our best predictive ability was for orthopedic patients who were readmitted with pneumonia. We were able to predict that 14% of the time,” she said.
The findings were derived by merging VA Surgical Quality Improvement Program data from inpatient operations performed between 2007 and 2014 and involving at least a 2-day postoperative hospital stay, with clinical data including laboratory findings, vitals, prior health care utilization, and postoperative complications.
“We then grouped our variables of interest into the following categories: preoperative, operative, postoperative but predischarge, and postdischarge,” she explained, noting that logistic models predicting 30-day readmission were constructed by sequentially adding groups into the model. Models were compared by way of adjusted R2 and C-statistics.
Assuming postoperative readmissions are preventable suggests that they are linked to the quality of care during the index hospitalization. The current findings demonstrate the challenges in predicting readmissions, and are important given that hospitals with higher-than-expected readmission rates for certain diagnoses and procedures are fined by the Centers for Medicare & Medicaid Services; 54% of hospitals were fined in 2015, she said.
“Readmission is difficult to predict at the time of discharge despite exhaustive statistical modeling with very granular clinical patient-level detail. Preoperative patient factors and postdischarge complications contribute the most to predictive models. Efforts to decrease readmissions should focus on modifiable patient-level factors, transitions of care, and minimizing postoperative complications,” she concluded.
Dr. Morris reported having no disclosures.
The complete manuscript of this presentation is anticipated to be published in Annals of Surgery pending editorial review.
CHICAGO – Preadmission and postdischarge factors were important predictors of postoperative readmission in a large cohort of surgical patients, but the hospital course had little incremental impact on either readmissions or postdischarge complications in the cohort, according to a retrospective study of Veterans Affairs data.
The findings suggest that efforts to reduce postoperative readmissions should focus on enhanced postdischarge surveillance and early intervention, Dr. Melanie S. Morris of the University of Alabama at Birmingham reported at the annual meeting of the American Surgical Association.
To assess the relative contributions of patient factors, operative characteristics, and postoperative hospital course on readmissions, she and her colleagues evaluated 243,956 general, vascular, and orthopedic surgery patients in 121 VA hospitals. The overall readmission rate among the cohort was 11.1%, and for general, vascular, and orthopedic surgeries, the rates were 12.9%, 15.4%, and 7.6%, respectively; the average postoperative length of stay was 6.9 days, and 6.1% of patients experienced a predischarge complication.
Almost all readmissions occurred within 2 weeks of discharge, and for general surgery patients, most occurred within 1 week. The readmission rate for vascular surgery patients remained high beyond the 2-week mark.
An examination of the reasons for readmission showed that wound complications were the most common reason for readmission, and this was particularly true for vascular surgery patients, in whom 44% of readmissions were for wound complications, Dr. Morris said.
Gastrointestinal complications including ileus and obstruction were also common, accounting for nearly 28% of readmissions among general surgery patients, she said.
Importantly, when including preoperative data (such as demographics, comorbidities, social and behavioral factors, labs and vital signs, and planned procedure type), the variability in readmissions could only be explained 8.6% of the time, she said.
“Adding in operative data, such as procedure complexity and intraoperative blood transfusions, as well as postoperative course, added very little to our predictive ability. Including both of those groups, we could only explain 10% of the variation in readmission,” she said.
Including postdischarge data such as complications and emergency department utilization in the model increased predictive ability to 18%.
R2 and C-statistics comparing the sequentially built model showed that demographics and comorbidities contributed the most to predicting readmission risk, Dr. Morris said.
Modeling based on readmission reason and specialty improved predictive ability. For example, almost 12% of readmissions for wound complications among vascular surgery patients were predictable.
“Our best predictive ability was for orthopedic patients who were readmitted with pneumonia. We were able to predict that 14% of the time,” she said.
The findings were derived by merging VA Surgical Quality Improvement Program data from inpatient operations performed between 2007 and 2014 and involving at least a 2-day postoperative hospital stay, with clinical data including laboratory findings, vitals, prior health care utilization, and postoperative complications.
“We then grouped our variables of interest into the following categories: preoperative, operative, postoperative but predischarge, and postdischarge,” she explained, noting that logistic models predicting 30-day readmission were constructed by sequentially adding groups into the model. Models were compared by way of adjusted R2 and C-statistics.
Assuming postoperative readmissions are preventable suggests that they are linked to the quality of care during the index hospitalization. The current findings demonstrate the challenges in predicting readmissions, and are important given that hospitals with higher-than-expected readmission rates for certain diagnoses and procedures are fined by the Centers for Medicare & Medicaid Services; 54% of hospitals were fined in 2015, she said.
“Readmission is difficult to predict at the time of discharge despite exhaustive statistical modeling with very granular clinical patient-level detail. Preoperative patient factors and postdischarge complications contribute the most to predictive models. Efforts to decrease readmissions should focus on modifiable patient-level factors, transitions of care, and minimizing postoperative complications,” she concluded.
Dr. Morris reported having no disclosures.
The complete manuscript of this presentation is anticipated to be published in Annals of Surgery pending editorial review.
AT THE ASA ANNUAL MEETING
Key clinical point: Preadmission and postdischarge factors were important predictors of postoperative readmission in a large cohort of surgical patients, but the hospital course had little incremental impact on either readmissions or postdischarge complications.
Major finding: Including both preoperative and operative data in the model predicted only 10% of the variability in readmission rates.
Data source: A retrospective study of data for nearly 244,000 VA patients.
Disclosures: Dr. Morris reported having no disclosures.
Daptomycin beats infective endocarditis caused by several pathogens
AT ECCMID 2016
AMSTERDAM – Daptomycin successfully treated infective endocarditis in 90% of patients who developed it after undergoing heart valve replacement, according to a report presented at the annual congress of the European Society of Clinical Microbiology and Infectious Diseases.
Dr. Achyut Guleri, clinical director of laboratory medicine at Blackpool Victoria Hospital, Lancashire, England, said the lipopeptide antibiotic was equally effective against methicillin- and penicillin-resistant Staphylococcus aureus, coagulase-negative staphylococcus, and enterococci.
“This is particularly good to know because sometimes in real life, on the shop floor, you don’t always have a very clear insight into what you’re trying to treat,” said Dr. Guleri. “It’s reassuring to see that the success rate is similar in all these infections.”
He presented a subgroup analysis of patients enrolled in European Cubicin Outcomes Registry and Experience (EUCORE), a retrospective, noninterventional, postmarketing registry. The 4-year study reported real-world clinical experience of daptomycin use for the treatment of Gram-positive infections in patients with infective endocarditis who had undergone heart valve replacement.
Typically, Dr. Guleri said, vancomycin, either alone or with rifampicin, is recommended for the infection. “However, with increasing antibiotic resistance, vancomycin doesn’t inspire much confidence, especially for MRSA infections,” he noted.
Daptomycin is increasingly employed as an alternative treatment. It exhibits rapid bactericidal activity against a wide range of Gram-positive pathogens, including MRSA. It’s approved for the treatment of right-sided infective endocarditis due to S. aureus, at a dose of 6 mg/kg per day. However, higher doses are now recommended by several international guidelines and are often used for hard-to-treat infections, Dr. Guleri said.
EUCORE comprised 6,075 patients from 18 countries who were enrolled from 2006 to 2012. Patients were followed until 2014. Of this group, 610 had infective endocarditis and 198 underwent valve replacement. Most were male (70%); mean age was 58 years. Medical comorbidities were common and included renal disease, sepsis, diabetes, pulmonary disease, gastrointestinal disease, cerebrovascular disease and inflammatory diseases.
Culture results were available for 87%. Of these, 68% were positive. The most common pathogen was S. aureus (37%). Half of these isolates were penicillin resistant and 35% were methicillin resistant. Enterococci were responsible for 14% of the infections, and coagulase-negative staph for 32%. The rest were caused by other pathogens.
Before trying daptomycin, most patients (83%) had already been treated with an antibiotic, which was employed in conjunction with another antibiotic in 77% of cases. The concomitant medications included rifampicin (31%), aminoglycosides (29%) and carbapenems (18%).
The overall clinical cure rate at 2 years was 90%. Daptomycin was equally effective in left- and right-sided disease, and was more effective in penicillin-resistant staph (95%) than methicillin-resistant staph (80%). The cure rate was also good in coagulase-negative staph (81%) and enterococci (75%).
High doses were more effective than low doses. At 4 mg/kg per day, the cure rate was 61%. At 6 mg/kg per day, it was 86%, and at more than 6 mg/kg per day, it was 90%.
Adverse events were rare (3%). Three patients developed increased creatine phosphokinase levels; one patient developed rhabdomyolysis and one developed cholestasis. Agranulocytosis developed in three patients and eosinophilic pneumonia in three. One patient developed a rash. No one discontinued the drug due to a side effect.
Dr. Guleri had no financial disclosures.
On Twitter @Alz_Gal
AT ECCMID 2016
AMSTERDAM – Daptomycin successfully treated infective endocarditis in 90% of patients who developed it after undergoing heart valve replacement, according to a report presented at the annual congress of the European Society of Clinical Microbiology and Infectious Diseases.
Dr. Achyut Guleri, clinical director of laboratory medicine at Blackpool Victoria Hospital, Lancashire, England, said the lipopeptide antibiotic was equally effective against methicillin- and penicillin-resistant Staphylococcus aureus, coagulase-negative staphylococcus, and enterococci.
“This is particularly good to know because sometimes in real life, on the shop floor, you don’t always have a very clear insight into what you’re trying to treat,” said Dr. Guleri. “It’s reassuring to see that the success rate is similar in all these infections.”
He presented a subgroup analysis of patients enrolled in European Cubicin Outcomes Registry and Experience (EUCORE), a retrospective, noninterventional, postmarketing registry. The 4-year study reported real-world clinical experience of daptomycin use for the treatment of Gram-positive infections in patients with infective endocarditis who had undergone heart valve replacement.
Typically, Dr. Guleri said, vancomycin, either alone or with rifampicin, is recommended for the infection. “However, with increasing antibiotic resistance, vancomycin doesn’t inspire much confidence, especially for MRSA infections,” he noted.
Daptomycin is increasingly employed as an alternative treatment. It exhibits rapid bactericidal activity against a wide range of Gram-positive pathogens, including MRSA. It’s approved for the treatment of right-sided infective endocarditis due to S. aureus, at a dose of 6 mg/kg per day. However, higher doses are now recommended by several international guidelines and are often used for hard-to-treat infections, Dr. Guleri said.
EUCORE comprised 6,075 patients from 18 countries who were enrolled from 2006 to 2012. Patients were followed until 2014. Of this group, 610 had infective endocarditis and 198 underwent valve replacement. Most were male (70%); mean age was 58 years. Medical comorbidities were common and included renal disease, sepsis, diabetes, pulmonary disease, gastrointestinal disease, cerebrovascular disease and inflammatory diseases.
Culture results were available for 87%. Of these, 68% were positive. The most common pathogen was S. aureus (37%). Half of these isolates were penicillin resistant and 35% were methicillin resistant. Enterococci were responsible for 14% of the infections, and coagulase-negative staph for 32%. The rest were caused by other pathogens.
Before trying daptomycin, most patients (83%) had already been treated with an antibiotic, which was employed in conjunction with another antibiotic in 77% of cases. The concomitant medications included rifampicin (31%), aminoglycosides (29%) and carbapenems (18%).
The overall clinical cure rate at 2 years was 90%. Daptomycin was equally effective in left- and right-sided disease, and was more effective in penicillin-resistant staph (95%) than methicillin-resistant staph (80%). The cure rate was also good in coagulase-negative staph (81%) and enterococci (75%).
High doses were more effective than low doses. At 4 mg/kg per day, the cure rate was 61%. At 6 mg/kg per day, it was 86%, and at more than 6 mg/kg per day, it was 90%.
Adverse events were rare (3%). Three patients developed increased creatine phosphokinase levels; one patient developed rhabdomyolysis and one developed cholestasis. Agranulocytosis developed in three patients and eosinophilic pneumonia in three. One patient developed a rash. No one discontinued the drug due to a side effect.
Dr. Guleri had no financial disclosures.
On Twitter @Alz_Gal
AT ECCMID 2016
AMSTERDAM – Daptomycin successfully treated infective endocarditis in 90% of patients who developed it after undergoing heart valve replacement, according to a report presented at the annual congress of the European Society of Clinical Microbiology and Infectious Diseases.
Dr. Achyut Guleri, clinical director of laboratory medicine at Blackpool Victoria Hospital, Lancashire, England, said the lipopeptide antibiotic was equally effective against methicillin- and penicillin-resistant Staphylococcus aureus, coagulase-negative staphylococcus, and enterococci.
“This is particularly good to know because sometimes in real life, on the shop floor, you don’t always have a very clear insight into what you’re trying to treat,” said Dr. Guleri. “It’s reassuring to see that the success rate is similar in all these infections.”
He presented a subgroup analysis of patients enrolled in European Cubicin Outcomes Registry and Experience (EUCORE), a retrospective, noninterventional, postmarketing registry. The 4-year study reported real-world clinical experience of daptomycin use for the treatment of Gram-positive infections in patients with infective endocarditis who had undergone heart valve replacement.
Typically, Dr. Guleri said, vancomycin, either alone or with rifampicin, is recommended for the infection. “However, with increasing antibiotic resistance, vancomycin doesn’t inspire much confidence, especially for MRSA infections,” he noted.
Daptomycin is increasingly employed as an alternative treatment. It exhibits rapid bactericidal activity against a wide range of Gram-positive pathogens, including MRSA. It’s approved for the treatment of right-sided infective endocarditis due to S. aureus, at a dose of 6 mg/kg per day. However, higher doses are now recommended by several international guidelines and are often used for hard-to-treat infections, Dr. Guleri said.
EUCORE comprised 6,075 patients from 18 countries who were enrolled from 2006 to 2012. Patients were followed until 2014. Of this group, 610 had infective endocarditis and 198 underwent valve replacement. Most were male (70%); mean age was 58 years. Medical comorbidities were common and included renal disease, sepsis, diabetes, pulmonary disease, gastrointestinal disease, cerebrovascular disease and inflammatory diseases.
Culture results were available for 87%. Of these, 68% were positive. The most common pathogen was S. aureus (37%). Half of these isolates were penicillin resistant and 35% were methicillin resistant. Enterococci were responsible for 14% of the infections, and coagulase-negative staph for 32%. The rest were caused by other pathogens.
Before trying daptomycin, most patients (83%) had already been treated with an antibiotic, which was employed in conjunction with another antibiotic in 77% of cases. The concomitant medications included rifampicin (31%), aminoglycosides (29%) and carbapenems (18%).
The overall clinical cure rate at 2 years was 90%. Daptomycin was equally effective in left- and right-sided disease, and was more effective in penicillin-resistant staph (95%) than methicillin-resistant staph (80%). The cure rate was also good in coagulase-negative staph (81%) and enterococci (75%).
High doses were more effective than low doses. At 4 mg/kg per day, the cure rate was 61%. At 6 mg/kg per day, it was 86%, and at more than 6 mg/kg per day, it was 90%.
Adverse events were rare (3%). Three patients developed increased creatine phosphokinase levels; one patient developed rhabdomyolysis and one developed cholestasis. Agranulocytosis developed in three patients and eosinophilic pneumonia in three. One patient developed a rash. No one discontinued the drug due to a side effect.
Dr. Guleri had no financial disclosures.
On Twitter @Alz_Gal
Key clinical point: Daptomycin had a high cure rate for infective endocarditis caused by MRSA, MSSA, coagulase-negative staph, and enterococci.
Major finding: The 2-year clinical cure rate was 90% for S. aureus infections.
Data source: Retrospective analysis of EUCORE, which comprised 198 patients.
Disclosures: Dr. Guleri had no financial disclosures.