User login
Plasma microRNA assay differentiates colorectal neoplasia
CHICAGO – A novel plasma microRNA assay and prediction model appears to successfully differentiate colorectal neoplasia from other neoplasms and from controls.
The assay includes seven microRNAs that were selected, based on P value, area under the curve (AUC), fold change, and biological plausibility, from among 380 microRNAs screened using microfluidic array technology from a “training” cohort of 60 patients. The training cohort included groups of patients, 10 each, with colorectal cancer, advanced adenoma, breast cancer, pancreatic cancer, and lung cancer – cancers chosen because they frequently develop at similar ages as colon cancer – and 10 controls.
A panel of seven “uniquely dysregulated” microRNAs specific for colorectal neoplasia was evaluated using single assays in a “test” cohort of 120 patients. A mathematical model was developed to predict sample identity in a 150-patient blinded “validation” cohort using repeat-subsampling validation of the testing dataset with 1,000 iterations each to assess model detection accuracy, Dr. Jane V. Carter of the University of Louisville (Ky.) explained at the annual meeting of the American Surgical Association.
The area under the curve for test cohort comparisons with the assay was 0.91, 0.79, and 0.98 for comparison No. 1 (comparing any neoplasia vs. controls), comparison No. 2 (comparing colorectal neoplasia with other cancers) and comparison No. 3 (comparing colorectal cancer with colorectal adenomas) respectively, Dr. Carter reported.
“Our prediction model identified blinded sample identity with 69%-77% accuracy in comparison No. 1, 66%-76% accuracy in comparison No. 2, and 86%-90% accuracy in comparison No. 3,” she said, noting that the sensitivity and specificity of the assay compare very well with current clinical standards.
Colorectal neoplasms frequently develop in individuals at ages when other common cancers also occur. Current screening methods, including endoscopic and imaging studies and fecal testing have poor patient compliance. Fecal and blood tests lack sensitivity and specificity for the detection of adenomas, limiting their use as screening methods, she said.
But this novel assay, which builds on the earlier work identifying miR-21 as a potential marker for colorectal cancer, provides a useful tool for identifying colorectal neoplasms, she said.
Efforts are underway to confirm the findings in a larger study population. If the findings are confirmed, the assay may have other potential uses such as monitoring therapy by comparing microRNA expression before and after treatment, and also for predicting response to treatment such as following preoperative neoadjuvant chemoradiation, Dr. Carter suggested.
The current findings have significant implications for the development of a noninvasive, reliable, and reproducible screening test for detection of colorectal neoplasia.
“If we can improve early-stage detection, we can improve survival,” she said.
Dr. Carter reported having no relevant disclosures.
The complete manuscript of this presentation is anticipated to be published in the Annals of Surgery pending editorial review.
CHICAGO – A novel plasma microRNA assay and prediction model appears to successfully differentiate colorectal neoplasia from other neoplasms and from controls.
The assay includes seven microRNAs that were selected, based on P value, area under the curve (AUC), fold change, and biological plausibility, from among 380 microRNAs screened using microfluidic array technology from a “training” cohort of 60 patients. The training cohort included groups of patients, 10 each, with colorectal cancer, advanced adenoma, breast cancer, pancreatic cancer, and lung cancer – cancers chosen because they frequently develop at similar ages as colon cancer – and 10 controls.
A panel of seven “uniquely dysregulated” microRNAs specific for colorectal neoplasia was evaluated using single assays in a “test” cohort of 120 patients. A mathematical model was developed to predict sample identity in a 150-patient blinded “validation” cohort using repeat-subsampling validation of the testing dataset with 1,000 iterations each to assess model detection accuracy, Dr. Jane V. Carter of the University of Louisville (Ky.) explained at the annual meeting of the American Surgical Association.
The area under the curve for test cohort comparisons with the assay was 0.91, 0.79, and 0.98 for comparison No. 1 (comparing any neoplasia vs. controls), comparison No. 2 (comparing colorectal neoplasia with other cancers) and comparison No. 3 (comparing colorectal cancer with colorectal adenomas) respectively, Dr. Carter reported.
“Our prediction model identified blinded sample identity with 69%-77% accuracy in comparison No. 1, 66%-76% accuracy in comparison No. 2, and 86%-90% accuracy in comparison No. 3,” she said, noting that the sensitivity and specificity of the assay compare very well with current clinical standards.
Colorectal neoplasms frequently develop in individuals at ages when other common cancers also occur. Current screening methods, including endoscopic and imaging studies and fecal testing have poor patient compliance. Fecal and blood tests lack sensitivity and specificity for the detection of adenomas, limiting their use as screening methods, she said.
But this novel assay, which builds on the earlier work identifying miR-21 as a potential marker for colorectal cancer, provides a useful tool for identifying colorectal neoplasms, she said.
Efforts are underway to confirm the findings in a larger study population. If the findings are confirmed, the assay may have other potential uses such as monitoring therapy by comparing microRNA expression before and after treatment, and also for predicting response to treatment such as following preoperative neoadjuvant chemoradiation, Dr. Carter suggested.
The current findings have significant implications for the development of a noninvasive, reliable, and reproducible screening test for detection of colorectal neoplasia.
“If we can improve early-stage detection, we can improve survival,” she said.
Dr. Carter reported having no relevant disclosures.
The complete manuscript of this presentation is anticipated to be published in the Annals of Surgery pending editorial review.
CHICAGO – A novel plasma microRNA assay and prediction model appears to successfully differentiate colorectal neoplasia from other neoplasms and from controls.
The assay includes seven microRNAs that were selected, based on P value, area under the curve (AUC), fold change, and biological plausibility, from among 380 microRNAs screened using microfluidic array technology from a “training” cohort of 60 patients. The training cohort included groups of patients, 10 each, with colorectal cancer, advanced adenoma, breast cancer, pancreatic cancer, and lung cancer – cancers chosen because they frequently develop at similar ages as colon cancer – and 10 controls.
A panel of seven “uniquely dysregulated” microRNAs specific for colorectal neoplasia was evaluated using single assays in a “test” cohort of 120 patients. A mathematical model was developed to predict sample identity in a 150-patient blinded “validation” cohort using repeat-subsampling validation of the testing dataset with 1,000 iterations each to assess model detection accuracy, Dr. Jane V. Carter of the University of Louisville (Ky.) explained at the annual meeting of the American Surgical Association.
The area under the curve for test cohort comparisons with the assay was 0.91, 0.79, and 0.98 for comparison No. 1 (comparing any neoplasia vs. controls), comparison No. 2 (comparing colorectal neoplasia with other cancers) and comparison No. 3 (comparing colorectal cancer with colorectal adenomas) respectively, Dr. Carter reported.
“Our prediction model identified blinded sample identity with 69%-77% accuracy in comparison No. 1, 66%-76% accuracy in comparison No. 2, and 86%-90% accuracy in comparison No. 3,” she said, noting that the sensitivity and specificity of the assay compare very well with current clinical standards.
Colorectal neoplasms frequently develop in individuals at ages when other common cancers also occur. Current screening methods, including endoscopic and imaging studies and fecal testing have poor patient compliance. Fecal and blood tests lack sensitivity and specificity for the detection of adenomas, limiting their use as screening methods, she said.
But this novel assay, which builds on the earlier work identifying miR-21 as a potential marker for colorectal cancer, provides a useful tool for identifying colorectal neoplasms, she said.
Efforts are underway to confirm the findings in a larger study population. If the findings are confirmed, the assay may have other potential uses such as monitoring therapy by comparing microRNA expression before and after treatment, and also for predicting response to treatment such as following preoperative neoadjuvant chemoradiation, Dr. Carter suggested.
The current findings have significant implications for the development of a noninvasive, reliable, and reproducible screening test for detection of colorectal neoplasia.
“If we can improve early-stage detection, we can improve survival,” she said.
Dr. Carter reported having no relevant disclosures.
The complete manuscript of this presentation is anticipated to be published in the Annals of Surgery pending editorial review.
AT THE ASA ANNUAL MEETING
Key clinical point: A novel plasma microRNA assay and prediction model appears to successfully differentiate colorectal neoplasia from other neoplasms and from controls.
Major finding: The prediction model identified sample identity with 69%-77% accuracy when comparing any neoplasia vs. controls, 66%-76% accuracy when comparing colorectal neoplasia with other cancers, and 86%-90% accuracy when comparing colorectal cancer with colorectal adenomas.
Data source: A prediction model used in a 60-person training cohort, a 120-person testing cohort, and a 150-person validation cohort.
Disclosures: Dr. Carter reported having no relevant disclosures.
Smoking gun: DNA methylation in prostate cancer
More reason, if any is needed, to encourage patients to kick the habit comes from a study showing an association between cigarette smoking and tumor DNA methylation changes.
In a study of tumor tissue from men with prostate cancer (PCa) who underwent radical prostatectomy, smoking was associated with differential methylation across 40 genetic regions, and at least 10 of the regions significantly correlated with levels of messenger RNA (mRNA) expression in corresponding genes.
Men whose tumors had the highest levels of smoking-associated methylation were more likely to have higher Gleason grade tumors or regional vs. local stage disease, reported Dr. Irene M. Shui of the Fred Hutchinson Cancer Research Center in Seattle and her colleagues.
“[O]ur results provide support for the hypothesis that smoking-induced changes in DNA methylation may underlie the association of smoking with PCa recurrence and mortality,” they wrote (Cancer 2016 May 3. doi: 10.1002/cncr.30045).
To see whether DNA methylation could at least partly explain the association of smoking with increased PCa progression and mortality, the investigators looked at tumor methylation and long-term follow-up data on 523 patients, 469 of whom (90%) had matched tumor gene expression data available. In all, 43% of the men were never smokers, 47% were former smokers, and 10% were current smokers.
The investigators examined tumor methylation profiles by smoking status, with the goals of determining whether smoking-associated changes in methylation are linked to mRNA expression, and whether they are related to disease prognosis.
They found that 40 DNA methylation regions were associated with smoking, and that 10 of the regions were strongly correlated with mRNA expression. They then used these 10 regions to create a smoking-related methylation score.
As noted before, the score was associated with adverse outcomes, with men in the highest third having an odds ratio (OR) for disease recurrence of 2.29 (P = .0007), and an OR of 4.21 for death from prostate cancer (P = .004)
The associations between smoking-related methylation scores and worse outcomes were slightly less strong but still significant after adjustment for Gleason score and pathologic stage.
“Importantly, there is evidence that smoking-related methylation changes in blood may be reversible; men who quit smoking for longer periods of time have methylation profiles similar to those of never-smokers,” the authors wrote.
More reason, if any is needed, to encourage patients to kick the habit comes from a study showing an association between cigarette smoking and tumor DNA methylation changes.
In a study of tumor tissue from men with prostate cancer (PCa) who underwent radical prostatectomy, smoking was associated with differential methylation across 40 genetic regions, and at least 10 of the regions significantly correlated with levels of messenger RNA (mRNA) expression in corresponding genes.
Men whose tumors had the highest levels of smoking-associated methylation were more likely to have higher Gleason grade tumors or regional vs. local stage disease, reported Dr. Irene M. Shui of the Fred Hutchinson Cancer Research Center in Seattle and her colleagues.
“[O]ur results provide support for the hypothesis that smoking-induced changes in DNA methylation may underlie the association of smoking with PCa recurrence and mortality,” they wrote (Cancer 2016 May 3. doi: 10.1002/cncr.30045).
To see whether DNA methylation could at least partly explain the association of smoking with increased PCa progression and mortality, the investigators looked at tumor methylation and long-term follow-up data on 523 patients, 469 of whom (90%) had matched tumor gene expression data available. In all, 43% of the men were never smokers, 47% were former smokers, and 10% were current smokers.
The investigators examined tumor methylation profiles by smoking status, with the goals of determining whether smoking-associated changes in methylation are linked to mRNA expression, and whether they are related to disease prognosis.
They found that 40 DNA methylation regions were associated with smoking, and that 10 of the regions were strongly correlated with mRNA expression. They then used these 10 regions to create a smoking-related methylation score.
As noted before, the score was associated with adverse outcomes, with men in the highest third having an odds ratio (OR) for disease recurrence of 2.29 (P = .0007), and an OR of 4.21 for death from prostate cancer (P = .004)
The associations between smoking-related methylation scores and worse outcomes were slightly less strong but still significant after adjustment for Gleason score and pathologic stage.
“Importantly, there is evidence that smoking-related methylation changes in blood may be reversible; men who quit smoking for longer periods of time have methylation profiles similar to those of never-smokers,” the authors wrote.
More reason, if any is needed, to encourage patients to kick the habit comes from a study showing an association between cigarette smoking and tumor DNA methylation changes.
In a study of tumor tissue from men with prostate cancer (PCa) who underwent radical prostatectomy, smoking was associated with differential methylation across 40 genetic regions, and at least 10 of the regions significantly correlated with levels of messenger RNA (mRNA) expression in corresponding genes.
Men whose tumors had the highest levels of smoking-associated methylation were more likely to have higher Gleason grade tumors or regional vs. local stage disease, reported Dr. Irene M. Shui of the Fred Hutchinson Cancer Research Center in Seattle and her colleagues.
“[O]ur results provide support for the hypothesis that smoking-induced changes in DNA methylation may underlie the association of smoking with PCa recurrence and mortality,” they wrote (Cancer 2016 May 3. doi: 10.1002/cncr.30045).
To see whether DNA methylation could at least partly explain the association of smoking with increased PCa progression and mortality, the investigators looked at tumor methylation and long-term follow-up data on 523 patients, 469 of whom (90%) had matched tumor gene expression data available. In all, 43% of the men were never smokers, 47% were former smokers, and 10% were current smokers.
The investigators examined tumor methylation profiles by smoking status, with the goals of determining whether smoking-associated changes in methylation are linked to mRNA expression, and whether they are related to disease prognosis.
They found that 40 DNA methylation regions were associated with smoking, and that 10 of the regions were strongly correlated with mRNA expression. They then used these 10 regions to create a smoking-related methylation score.
As noted before, the score was associated with adverse outcomes, with men in the highest third having an odds ratio (OR) for disease recurrence of 2.29 (P = .0007), and an OR of 4.21 for death from prostate cancer (P = .004)
The associations between smoking-related methylation scores and worse outcomes were slightly less strong but still significant after adjustment for Gleason score and pathologic stage.
“Importantly, there is evidence that smoking-related methylation changes in blood may be reversible; men who quit smoking for longer periods of time have methylation profiles similar to those of never-smokers,” the authors wrote.
FROM CANCER
Key clinical point: This study demonstrates an association between smoking, DNA methylation, and potentially pathogenic genetic changes.
Major finding: Men with the highest smoking-related methylation scores were at increased risk for worse prostate cancer outcomes.
Data source: A retrospective study of tumor methylation and the association with outcomes in 523 men who underwent radical prostatectomy for adenocarcinoma of the prostate.
Disclosures: The study was supported by grants from the National Institutes of Health, Fred Hutchinson Cancer Research Center, and Prostate Cancer Foundation. The authors made no conflict of interest disclosures.
Total Hip Arthroplasty After Proximal Femoral Osteotomy: A Technique That Can Be Used to Address Presence of a Retained Intracortical Plate
Total hip arthroplasty (THA) is an effective treatment for advanced hip arthritis from a variety of causes, including osteoarthritis, inflammatory arthritis, posttraumatic arthritis, and sequelae of developmental disorders. It is not uncommon to perform THA in the presence of a previous proximal femoral osteotomy that may have been performed for slipped capital femoral epiphysis (SCFE), Legg-Calvé-Perthes disease, or developmental dysplasia of the hip, among other conditions. These osteotomies are commonly combined with internal fixation, a plate-and-screw device. These patients are at risk for developing degenerative arthritis at an earlier age than patients with other types of arthritis and subsequently may undergo THA at a younger age.1-3 Presence of a plate can pose a technical challenge during THA surgery. THA performed after intertrochanteric osteotomy has higher rates of perioperative and postoperative complications.4 Ferguson and colleagues4 noted difficulty during hardware removal in 24% of cases. Among the complications encountered were broken hardware, stripped screws, greater trochanteric fracture, stress risers from previous screw holes, canal narrowing from endosteal hypertrophy around hardware, and lateral cortical deficiency after removal of the side plate. As intertrochanteric osteotomies are often performed in patients who have yet to reach skeletal maturity, cortical hypertrophy can lead to complete coverage of the side plate and an “intracortical” position.
This article reports on 2 THA cases in which a technique was used to avoid intracortical plate removal and the resulting problems of lateral cortical deficiency. During each THA, the plate was left in place to avoid compromise of the lateral femoral cortex. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
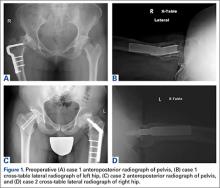
An adolescent with bilateral SCFE was treated first with internal fixation of the right hip and subsequently with left proximal femoral osteotomy with internal fixation. He did well until age 31 years, when he developed progressively worsening pain about the left hip. Clinical findings and imaging studies were consistent with advanced degenerative arthritis of the left hip. Radiographs showed a sliding hip screw in place, with proximal femoral deformity consisting of femoral neck shortening and posterior angulation (Figures 1A, 1B). Preoperative Harris Hip Score was 54.5.
Case 2
A 51-year-old woman presented with a history of right hip problems dating back to age 13 years, when she sustained a fracture of the right hip and was treated with internal fixation. At age 15 years, she underwent proximal femoral osteotomy to correct residual deformity. She did well until age 45 years, when she developed worsening hip symptoms. Clinical findings and imaging studies were consistent with advanced degenerative arthritis of the right hip. Radiographs showed a fixed-angle blade plate in the proximal femur, with significant proximal femoral deformity (Figures 1C, 1D). Preoperative Harris Hip Score was 53.6.
Surgical Technique
In both cases, a standard series of radiographs was obtained—an anteroposterior (AP) radiograph of the pelvis and AP and cross-table lateral radiographs of the operative hip (Figure 1). Computed tomography (CT) with a metal-artifact-reducing technique may be useful in determining amount of cortical bone remaining under the plate. CT showed limited lateral cortex beneath the side plate and bony overgrowth covering the side plate. Preoperative templating was performed using previously described techniques.5
During THA, before removing any portion of any retained hardware, the surgeon should perform 3 important actions: Dislocate the hip, perform all appropriate capsular releases, and reduce the hip. Dislocating the hip before hardware removal significantly decreases the risk for fracture caused by stress risers, as the force required for dislocation is much more controlled because of the capsular releases. After hardware removal, the hip can be easily redislocated, and the femoral neck osteotomy can be performed.
When plate and screws are in an intracortical position, the screws can be removed only after removing the small shell of cortical bone covering them. The amount of bone to be removed is minimal. After the screws are removed, the plate remains in place. A motorized device with a metal-cutting attachment is used to transect the construct at the junction of the plate and barrel (case 1) or at the bend of a fixed-angle device (case 2). Laparotomy sponges are placed around the proximal femur to minimize the amount of soft tissue that could be exposed to metal shavings. Copious irrigation is used throughout this part of the procedure. Osteotomes are used to elevate the proximal portion of the plate and the barrel, preserving the distal portion of the plate on the lateral cortex of the femoral shaft.
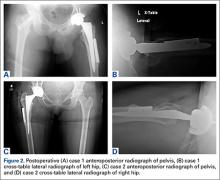
After the head is removed, the rest of the THA can be performed using standard press-fit insertion technique (Figures 2A-2D). Care must be taken to ensure that the distal aspect of the femoral stem bypasses the most distal screw hole by at least 2 cortical diameters in order to reduce the risk for periprosthetic fracture.
By 2-year follow-up, both patients had regained excellent range of motion, ambulation, and overall function. Postoperative Harris Hip Scores were 86.6 and 83.8, respectively. There were no radiographic signs of complications.
Discussion
THA can be challenging in the setting of previously placed internal fixation devices, particularly devices inserted during a patient’s adolescence, as significant bony overgrowth can occur. The standard approach has been to remove the internal fixation device and then perform the THA. In most cases, and particularly when the internal fixation device is in an intracortical position, the result is significant compromise of bone. This article describes a technique in which a portion of the hardware is retained to avoid compromise of the lateral femoral cortex, thereby allowing insertion of a noncemented femoral component.
THA is the most effective procedure for reducing hip pain and disability in the setting of degenerative changes.6 Patients with SCFE, Legg-Calvé-Perthes disease, or developmental dysplasia of the hip generally are younger at the time they may be sufficiently symptomatic to consider THA.7,8 Many have had previous surgery using internal fixation devices. THAs after previous osteotomies with internal fixation devices are more technically demanding, require more operative time, are subject to more blood loss, and have a higher rate of complications, including femoral fracture. Ferguson and colleagues4 and Boos and colleagues9 found these surgeries were more difficult 33.8% and 36.8% of the time, respectively. For these reasons, some authors have recommended removing the internal fixation device as soon as the osteotomy is healed.4 However, this has not become the standard of care, and surgeons continue to perform THAs in the presence of a previous osteotomy with an internal fixation device in place.
The technique described in this article was used successfully in 2 cases. In each case, leaving the intracortical plate in place avoided compromise of the lateral femoral cortex and allowed insertion of a noncemented femoral component without complication. Of course, with the screw holes representing stress risers, careful insertion of the femoral component was required. Retaining the intracortical plate allowed it to function as part of the lateral femoral cortex, thereby maintaining the structural integrity of the femoral canal. As has been described for the 2 cases, a blade plate and plate and barrel were converted to a limited intracortical plate by removing the proximal portion of the plates—a modification that could be applied to other types of internal fixation devices that extend into the femoral neck as long as appropriate cutting tools are available.
Conclusion
THA in the setting of a retained internal fixation device is relatively common. This article describes a technique that can be used when a plate applied to the lateral femoral cortex has become intracortical as a result of extensive bony overgrowth. In using this technique to avoid plate removal, the surgeon eliminates the need for more extensive procedures aimed at compensating for deficiency of the femoral cortex in the area of plate removal. Although only 2 cases are presented here, this technique potentially can be used more broadly in these specific clinical situations.
1. Engesæter LB, Engesæter IØ, Fenstad AM, et al. Low revision rate after total hip arthroplasty in patients with pediatric hip diseases. Acta Orthop. 2012;83(5):436-441.
2. Froberg L, Christensen F, Pedersen NW, Overgaard S. The need for total hip arthroplasty in Perthes disease: a long-term study. Clin Orthop Relat Res. 2011;469(4):1134-1140.
3. Furnes O, Lie SA, Espehaug B, Vollset SE, Engesæter LB, Havelin LI. Hip disease and the prognosis of total hip replacements. A review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987-99. J Bone Joint Surg Br. 2001;83(4):579-586.
4. Ferguson GM, Cabanela ME, Ilstrup DM. Total hip arthroplasty after failed intertrochanteric osteotomy. J Bone Joint Surg Br. 1994;76(2):252-257.
5. Scheerlinck T. Primary hip arthroplasty templating on standard radiographs. A stepwise approach. Acta Orthop Belg. 2010;76(4):432-442.
6. Wroblewski BM, Siney PD. Charnley low-friction arthroplasty of the hip. Long-term results. Clin Orthop Relat Res. 1993;(292):191-201.
7. Chandler HP, Reineck FT, Wixson RL, McCarthy JC. Total hip replacement in patients younger than thirty years old. A five-year follow-up study. J Bone Joint Surg Am. 1981;63(9):1426-1434.
8. Dorr LD, Luckett M, Conaty JP. Total hip arthroplasties in patients younger than 45 years. A nine- to ten-year follow-up study. Clin Orthop Relat Res. 1990;(260):215-219.
9. Boos N, Krushell R, Ganz R, Müller ME. Total hip arthroplasty after previous proximal femoral osteotomy. J Bone Joint Surg Br. 1997;79(2):247-253.
Total hip arthroplasty (THA) is an effective treatment for advanced hip arthritis from a variety of causes, including osteoarthritis, inflammatory arthritis, posttraumatic arthritis, and sequelae of developmental disorders. It is not uncommon to perform THA in the presence of a previous proximal femoral osteotomy that may have been performed for slipped capital femoral epiphysis (SCFE), Legg-Calvé-Perthes disease, or developmental dysplasia of the hip, among other conditions. These osteotomies are commonly combined with internal fixation, a plate-and-screw device. These patients are at risk for developing degenerative arthritis at an earlier age than patients with other types of arthritis and subsequently may undergo THA at a younger age.1-3 Presence of a plate can pose a technical challenge during THA surgery. THA performed after intertrochanteric osteotomy has higher rates of perioperative and postoperative complications.4 Ferguson and colleagues4 noted difficulty during hardware removal in 24% of cases. Among the complications encountered were broken hardware, stripped screws, greater trochanteric fracture, stress risers from previous screw holes, canal narrowing from endosteal hypertrophy around hardware, and lateral cortical deficiency after removal of the side plate. As intertrochanteric osteotomies are often performed in patients who have yet to reach skeletal maturity, cortical hypertrophy can lead to complete coverage of the side plate and an “intracortical” position.
This article reports on 2 THA cases in which a technique was used to avoid intracortical plate removal and the resulting problems of lateral cortical deficiency. During each THA, the plate was left in place to avoid compromise of the lateral femoral cortex. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
An adolescent with bilateral SCFE was treated first with internal fixation of the right hip and subsequently with left proximal femoral osteotomy with internal fixation. He did well until age 31 years, when he developed progressively worsening pain about the left hip. Clinical findings and imaging studies were consistent with advanced degenerative arthritis of the left hip. Radiographs showed a sliding hip screw in place, with proximal femoral deformity consisting of femoral neck shortening and posterior angulation (Figures 1A, 1B). Preoperative Harris Hip Score was 54.5.
Case 2
A 51-year-old woman presented with a history of right hip problems dating back to age 13 years, when she sustained a fracture of the right hip and was treated with internal fixation. At age 15 years, she underwent proximal femoral osteotomy to correct residual deformity. She did well until age 45 years, when she developed worsening hip symptoms. Clinical findings and imaging studies were consistent with advanced degenerative arthritis of the right hip. Radiographs showed a fixed-angle blade plate in the proximal femur, with significant proximal femoral deformity (Figures 1C, 1D). Preoperative Harris Hip Score was 53.6.
Surgical Technique
In both cases, a standard series of radiographs was obtained—an anteroposterior (AP) radiograph of the pelvis and AP and cross-table lateral radiographs of the operative hip (Figure 1). Computed tomography (CT) with a metal-artifact-reducing technique may be useful in determining amount of cortical bone remaining under the plate. CT showed limited lateral cortex beneath the side plate and bony overgrowth covering the side plate. Preoperative templating was performed using previously described techniques.5
During THA, before removing any portion of any retained hardware, the surgeon should perform 3 important actions: Dislocate the hip, perform all appropriate capsular releases, and reduce the hip. Dislocating the hip before hardware removal significantly decreases the risk for fracture caused by stress risers, as the force required for dislocation is much more controlled because of the capsular releases. After hardware removal, the hip can be easily redislocated, and the femoral neck osteotomy can be performed.
When plate and screws are in an intracortical position, the screws can be removed only after removing the small shell of cortical bone covering them. The amount of bone to be removed is minimal. After the screws are removed, the plate remains in place. A motorized device with a metal-cutting attachment is used to transect the construct at the junction of the plate and barrel (case 1) or at the bend of a fixed-angle device (case 2). Laparotomy sponges are placed around the proximal femur to minimize the amount of soft tissue that could be exposed to metal shavings. Copious irrigation is used throughout this part of the procedure. Osteotomes are used to elevate the proximal portion of the plate and the barrel, preserving the distal portion of the plate on the lateral cortex of the femoral shaft.
After the head is removed, the rest of the THA can be performed using standard press-fit insertion technique (Figures 2A-2D). Care must be taken to ensure that the distal aspect of the femoral stem bypasses the most distal screw hole by at least 2 cortical diameters in order to reduce the risk for periprosthetic fracture.
By 2-year follow-up, both patients had regained excellent range of motion, ambulation, and overall function. Postoperative Harris Hip Scores were 86.6 and 83.8, respectively. There were no radiographic signs of complications.
Discussion
THA can be challenging in the setting of previously placed internal fixation devices, particularly devices inserted during a patient’s adolescence, as significant bony overgrowth can occur. The standard approach has been to remove the internal fixation device and then perform the THA. In most cases, and particularly when the internal fixation device is in an intracortical position, the result is significant compromise of bone. This article describes a technique in which a portion of the hardware is retained to avoid compromise of the lateral femoral cortex, thereby allowing insertion of a noncemented femoral component.
THA is the most effective procedure for reducing hip pain and disability in the setting of degenerative changes.6 Patients with SCFE, Legg-Calvé-Perthes disease, or developmental dysplasia of the hip generally are younger at the time they may be sufficiently symptomatic to consider THA.7,8 Many have had previous surgery using internal fixation devices. THAs after previous osteotomies with internal fixation devices are more technically demanding, require more operative time, are subject to more blood loss, and have a higher rate of complications, including femoral fracture. Ferguson and colleagues4 and Boos and colleagues9 found these surgeries were more difficult 33.8% and 36.8% of the time, respectively. For these reasons, some authors have recommended removing the internal fixation device as soon as the osteotomy is healed.4 However, this has not become the standard of care, and surgeons continue to perform THAs in the presence of a previous osteotomy with an internal fixation device in place.
The technique described in this article was used successfully in 2 cases. In each case, leaving the intracortical plate in place avoided compromise of the lateral femoral cortex and allowed insertion of a noncemented femoral component without complication. Of course, with the screw holes representing stress risers, careful insertion of the femoral component was required. Retaining the intracortical plate allowed it to function as part of the lateral femoral cortex, thereby maintaining the structural integrity of the femoral canal. As has been described for the 2 cases, a blade plate and plate and barrel were converted to a limited intracortical plate by removing the proximal portion of the plates—a modification that could be applied to other types of internal fixation devices that extend into the femoral neck as long as appropriate cutting tools are available.
Conclusion
THA in the setting of a retained internal fixation device is relatively common. This article describes a technique that can be used when a plate applied to the lateral femoral cortex has become intracortical as a result of extensive bony overgrowth. In using this technique to avoid plate removal, the surgeon eliminates the need for more extensive procedures aimed at compensating for deficiency of the femoral cortex in the area of plate removal. Although only 2 cases are presented here, this technique potentially can be used more broadly in these specific clinical situations.
Total hip arthroplasty (THA) is an effective treatment for advanced hip arthritis from a variety of causes, including osteoarthritis, inflammatory arthritis, posttraumatic arthritis, and sequelae of developmental disorders. It is not uncommon to perform THA in the presence of a previous proximal femoral osteotomy that may have been performed for slipped capital femoral epiphysis (SCFE), Legg-Calvé-Perthes disease, or developmental dysplasia of the hip, among other conditions. These osteotomies are commonly combined with internal fixation, a plate-and-screw device. These patients are at risk for developing degenerative arthritis at an earlier age than patients with other types of arthritis and subsequently may undergo THA at a younger age.1-3 Presence of a plate can pose a technical challenge during THA surgery. THA performed after intertrochanteric osteotomy has higher rates of perioperative and postoperative complications.4 Ferguson and colleagues4 noted difficulty during hardware removal in 24% of cases. Among the complications encountered were broken hardware, stripped screws, greater trochanteric fracture, stress risers from previous screw holes, canal narrowing from endosteal hypertrophy around hardware, and lateral cortical deficiency after removal of the side plate. As intertrochanteric osteotomies are often performed in patients who have yet to reach skeletal maturity, cortical hypertrophy can lead to complete coverage of the side plate and an “intracortical” position.
This article reports on 2 THA cases in which a technique was used to avoid intracortical plate removal and the resulting problems of lateral cortical deficiency. During each THA, the plate was left in place to avoid compromise of the lateral femoral cortex. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
An adolescent with bilateral SCFE was treated first with internal fixation of the right hip and subsequently with left proximal femoral osteotomy with internal fixation. He did well until age 31 years, when he developed progressively worsening pain about the left hip. Clinical findings and imaging studies were consistent with advanced degenerative arthritis of the left hip. Radiographs showed a sliding hip screw in place, with proximal femoral deformity consisting of femoral neck shortening and posterior angulation (Figures 1A, 1B). Preoperative Harris Hip Score was 54.5.
Case 2
A 51-year-old woman presented with a history of right hip problems dating back to age 13 years, when she sustained a fracture of the right hip and was treated with internal fixation. At age 15 years, she underwent proximal femoral osteotomy to correct residual deformity. She did well until age 45 years, when she developed worsening hip symptoms. Clinical findings and imaging studies were consistent with advanced degenerative arthritis of the right hip. Radiographs showed a fixed-angle blade plate in the proximal femur, with significant proximal femoral deformity (Figures 1C, 1D). Preoperative Harris Hip Score was 53.6.
Surgical Technique
In both cases, a standard series of radiographs was obtained—an anteroposterior (AP) radiograph of the pelvis and AP and cross-table lateral radiographs of the operative hip (Figure 1). Computed tomography (CT) with a metal-artifact-reducing technique may be useful in determining amount of cortical bone remaining under the plate. CT showed limited lateral cortex beneath the side plate and bony overgrowth covering the side plate. Preoperative templating was performed using previously described techniques.5
During THA, before removing any portion of any retained hardware, the surgeon should perform 3 important actions: Dislocate the hip, perform all appropriate capsular releases, and reduce the hip. Dislocating the hip before hardware removal significantly decreases the risk for fracture caused by stress risers, as the force required for dislocation is much more controlled because of the capsular releases. After hardware removal, the hip can be easily redislocated, and the femoral neck osteotomy can be performed.
When plate and screws are in an intracortical position, the screws can be removed only after removing the small shell of cortical bone covering them. The amount of bone to be removed is minimal. After the screws are removed, the plate remains in place. A motorized device with a metal-cutting attachment is used to transect the construct at the junction of the plate and barrel (case 1) or at the bend of a fixed-angle device (case 2). Laparotomy sponges are placed around the proximal femur to minimize the amount of soft tissue that could be exposed to metal shavings. Copious irrigation is used throughout this part of the procedure. Osteotomes are used to elevate the proximal portion of the plate and the barrel, preserving the distal portion of the plate on the lateral cortex of the femoral shaft.
After the head is removed, the rest of the THA can be performed using standard press-fit insertion technique (Figures 2A-2D). Care must be taken to ensure that the distal aspect of the femoral stem bypasses the most distal screw hole by at least 2 cortical diameters in order to reduce the risk for periprosthetic fracture.
By 2-year follow-up, both patients had regained excellent range of motion, ambulation, and overall function. Postoperative Harris Hip Scores were 86.6 and 83.8, respectively. There were no radiographic signs of complications.
Discussion
THA can be challenging in the setting of previously placed internal fixation devices, particularly devices inserted during a patient’s adolescence, as significant bony overgrowth can occur. The standard approach has been to remove the internal fixation device and then perform the THA. In most cases, and particularly when the internal fixation device is in an intracortical position, the result is significant compromise of bone. This article describes a technique in which a portion of the hardware is retained to avoid compromise of the lateral femoral cortex, thereby allowing insertion of a noncemented femoral component.
THA is the most effective procedure for reducing hip pain and disability in the setting of degenerative changes.6 Patients with SCFE, Legg-Calvé-Perthes disease, or developmental dysplasia of the hip generally are younger at the time they may be sufficiently symptomatic to consider THA.7,8 Many have had previous surgery using internal fixation devices. THAs after previous osteotomies with internal fixation devices are more technically demanding, require more operative time, are subject to more blood loss, and have a higher rate of complications, including femoral fracture. Ferguson and colleagues4 and Boos and colleagues9 found these surgeries were more difficult 33.8% and 36.8% of the time, respectively. For these reasons, some authors have recommended removing the internal fixation device as soon as the osteotomy is healed.4 However, this has not become the standard of care, and surgeons continue to perform THAs in the presence of a previous osteotomy with an internal fixation device in place.
The technique described in this article was used successfully in 2 cases. In each case, leaving the intracortical plate in place avoided compromise of the lateral femoral cortex and allowed insertion of a noncemented femoral component without complication. Of course, with the screw holes representing stress risers, careful insertion of the femoral component was required. Retaining the intracortical plate allowed it to function as part of the lateral femoral cortex, thereby maintaining the structural integrity of the femoral canal. As has been described for the 2 cases, a blade plate and plate and barrel were converted to a limited intracortical plate by removing the proximal portion of the plates—a modification that could be applied to other types of internal fixation devices that extend into the femoral neck as long as appropriate cutting tools are available.
Conclusion
THA in the setting of a retained internal fixation device is relatively common. This article describes a technique that can be used when a plate applied to the lateral femoral cortex has become intracortical as a result of extensive bony overgrowth. In using this technique to avoid plate removal, the surgeon eliminates the need for more extensive procedures aimed at compensating for deficiency of the femoral cortex in the area of plate removal. Although only 2 cases are presented here, this technique potentially can be used more broadly in these specific clinical situations.
1. Engesæter LB, Engesæter IØ, Fenstad AM, et al. Low revision rate after total hip arthroplasty in patients with pediatric hip diseases. Acta Orthop. 2012;83(5):436-441.
2. Froberg L, Christensen F, Pedersen NW, Overgaard S. The need for total hip arthroplasty in Perthes disease: a long-term study. Clin Orthop Relat Res. 2011;469(4):1134-1140.
3. Furnes O, Lie SA, Espehaug B, Vollset SE, Engesæter LB, Havelin LI. Hip disease and the prognosis of total hip replacements. A review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987-99. J Bone Joint Surg Br. 2001;83(4):579-586.
4. Ferguson GM, Cabanela ME, Ilstrup DM. Total hip arthroplasty after failed intertrochanteric osteotomy. J Bone Joint Surg Br. 1994;76(2):252-257.
5. Scheerlinck T. Primary hip arthroplasty templating on standard radiographs. A stepwise approach. Acta Orthop Belg. 2010;76(4):432-442.
6. Wroblewski BM, Siney PD. Charnley low-friction arthroplasty of the hip. Long-term results. Clin Orthop Relat Res. 1993;(292):191-201.
7. Chandler HP, Reineck FT, Wixson RL, McCarthy JC. Total hip replacement in patients younger than thirty years old. A five-year follow-up study. J Bone Joint Surg Am. 1981;63(9):1426-1434.
8. Dorr LD, Luckett M, Conaty JP. Total hip arthroplasties in patients younger than 45 years. A nine- to ten-year follow-up study. Clin Orthop Relat Res. 1990;(260):215-219.
9. Boos N, Krushell R, Ganz R, Müller ME. Total hip arthroplasty after previous proximal femoral osteotomy. J Bone Joint Surg Br. 1997;79(2):247-253.
1. Engesæter LB, Engesæter IØ, Fenstad AM, et al. Low revision rate after total hip arthroplasty in patients with pediatric hip diseases. Acta Orthop. 2012;83(5):436-441.
2. Froberg L, Christensen F, Pedersen NW, Overgaard S. The need for total hip arthroplasty in Perthes disease: a long-term study. Clin Orthop Relat Res. 2011;469(4):1134-1140.
3. Furnes O, Lie SA, Espehaug B, Vollset SE, Engesæter LB, Havelin LI. Hip disease and the prognosis of total hip replacements. A review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987-99. J Bone Joint Surg Br. 2001;83(4):579-586.
4. Ferguson GM, Cabanela ME, Ilstrup DM. Total hip arthroplasty after failed intertrochanteric osteotomy. J Bone Joint Surg Br. 1994;76(2):252-257.
5. Scheerlinck T. Primary hip arthroplasty templating on standard radiographs. A stepwise approach. Acta Orthop Belg. 2010;76(4):432-442.
6. Wroblewski BM, Siney PD. Charnley low-friction arthroplasty of the hip. Long-term results. Clin Orthop Relat Res. 1993;(292):191-201.
7. Chandler HP, Reineck FT, Wixson RL, McCarthy JC. Total hip replacement in patients younger than thirty years old. A five-year follow-up study. J Bone Joint Surg Am. 1981;63(9):1426-1434.
8. Dorr LD, Luckett M, Conaty JP. Total hip arthroplasties in patients younger than 45 years. A nine- to ten-year follow-up study. Clin Orthop Relat Res. 1990;(260):215-219.
9. Boos N, Krushell R, Ganz R, Müller ME. Total hip arthroplasty after previous proximal femoral osteotomy. J Bone Joint Surg Br. 1997;79(2):247-253.
A Retrospective Analysis of Hemostatic Techniques in Primary Total Knee Arthroplasty: Traditional Electrocautery, Bipolar Sealer, and Argon Beam Coagulation
Total knee arthroplasty (TKA) is a reliable and successful treatment for end-stage degenerative joint disease of the knee. Given the reproducibility of its generally excellent outcomes, TKA is increasingly being performed.1 However, the potential complications of this procedure can be devastating.2-4 The arthroplasty literature has shed light on the detrimental effects of postoperative blood loss and anemia.5,6 In addition, the increase in transfusion burden among patients is not without risk.7 Given these concerns, surgeons have been tasked with determining the ideal methods for minimizing blood transfusions and postoperative hematomas and anemia. Several strategies have been described.8-11 Hemostasis can be achieved with use of intravenous medications, intra-articular agents, or electrocautery devices. Electrocautery technologies include traditional electrocautery (TE), saline-coupled bipolar sealer (BS), and argon beam coagulation (ABC). There is controversy as to whether outcomes are better with one hemostasis method over another and whether these methods are worth the additional cost.
In traditional (Bovie) electrocautery, a unipolar device delivers an electrical current to tissues through a pencil-like instrument. Intraoperative tissue temperatures can exceed 400°C.12 In BS, radiofrequency energy is delivered through a saline medium, which increases the contact area, acts as an electrode, and maintains a cooler environment during electrocautery. Proposed advantages are reduced tissue destruction and absence of smoke.12 There is evidence both for10,12-16 and against17-20 use of BS in total joint arthroplasty. ABC, a novel hemostasis method, has been studied in the context of orthopedics21,22 but not TKA specifically. ABC establishes a monopolar electric circuit between a handheld device and the target tissues by channeling electrons through ionized argon gas. Hemostasis is achieved through thermal coagulation. Tissue penetration can be adjusted by changing power, probe-to-target distance, and duration of use.23 We conducted a study to assess the efficacy of all 3 electrocautery methods during TKA. We hypothesized the 3 methods would be clinically equivalent with respect to estimated blood loss (EBL), 48-hour wound drainage, operative time, and change from preoperative hemoglobin (Hb) level.
Methods
We conducted a retrospective cohort study of consecutive primary TKAs performed by Dr. Levine between October 2010 and November 2011. Patients were identified by querying an internal database. Exclusion criteria were prior ipsilateral open knee procedure, prior fracture, nonuse of our standard hemostatic protocol, and either tourniquet time under 40 minutes or intraoperative documentation of tourniquet failure. As only 9 patients were initially identified for the TE cohort, the same database was used to add 32 patients treated between April 2009 and October 2009 (before our institution began using BS and ABC).
Clinical charts were reviewed, and baseline demographics (age, body mass index [BMI], preoperative Hb level) were abstracted, as were outcome metrics (EBL, 48-hour wound drainage, operative time, postoperative transfusions, adverse events (AEs) before discharge, and change in Hb level from before surgery to after surgery, in recovery room and on discharge). Statistical analyses were performed with JMP Version 10.0.0 (SAS Institute). Given the hypothesis that the 3 hemostasis methods would be clinically equivalent, 2 one-sided tests (TOSTs) of equivalence were performed with an α of 0.05. With TOST, the traditional null and alternative hypotheses are reversed; thus, P < .05 identifies statistical equivalence. The advantage of this study design is that equivalence can be identified, whereas traditional study designs can identify only a lack of statistical difference.24 We used our consensus opinions to set clinical insignificance thresholds for EBL (150 mL), wound drainage (150 mL), decrease from postoperative Hb level (1 g/dL), and operative time (10 minutes). Patients who received a blood transfusion were subsequently excluded from analysis in order to avoid skewing Hb-level depreciation calculations. Analysis of variance (ANOVA) and χ2 tests were used to compare preoperative variables, transfusion requirements, hospital length of stay, and AE rates by hemostasis type.
Cautery Technique
In all cases, TE was used for surgical dissection, which followed a standard midvastus approach. Then, for meniscal excision, the capsule and meniscal attachment sites were treated with TE, BS, or ABC. During cement hardening, an available supplemental cautery option was used to achieve hemostasis of the suprapatellar fat pad and visible meniscal attachment sites. All other aspects of the procedure and the postoperative protocols—including the anticoagulation and rapid rehabilitation (early ambulation and therapy) protocols—were similar for all patients. The standard anticoagulation protocol was to use low-molecular-weight heparin, unless contraindicated. Tranexamic acid was not used at our institution during the study period.
Results
For the study period, 280 cases (41 TE, 203 BS, 36 ABC) met the inclusion criteria. Of the 280 TKAs, 261 (93.21%) were performed for degenerative arthritis. There was no statistically significant difference among cohorts in indication (χ2 = 1.841, P = .398) or sex (χ2 = 1.176, P= .555).
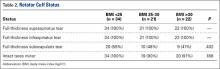
Table 1 lists the cohorts’ baseline demographics (mean age, BMI, preoperative Hb level) and comparative ANOVA results. TOSTs of equivalence were performed to compare operative time, EBL, 48-hour wound drainage, and postoperative Hb-level depreciation among hemostasis types. Changes in Hb level were calculated for the immediate postoperative period and time of discharge (Table 2). ANOVA of hospital length of stay demonstrated no significant difference in means among groups (P = .09).
The cohorts were compared with respect to use of postoperative transfusions and incidence of postoperative AEs (Table 3). The TE cohort did not have any AEs. Of the 203 BS patients, 14 (7%) had 1 or more AEs, which included acute kidney injury (3 cases), electrolyte disturbance (3), urinary tract infection (2), oxygen desaturation (2), altered mental status (1), pneumonia (1), arrhythmia (1), congestive heart failure exacerbation (1), dehiscence (1), pulmonary embolism (2), and hypotension (1). Of the 36 ABC patients, 1 (3%) had arrhythmia, pneumonia, sepsis, and altered mental status.
Discussion
With the population aging, the demand for TKA is greater than ever.1 As surgical volume increases, the ability to minimize the rates of intraoperative bleeding, postoperative anemia, and transfusion is becoming increasingly important to patients and the healthcare system. There is no consensus as to which cautery method is ideal. Other investigators have identified differences in clinical outcomes between cautery systems, but reported results are largely conflicting.10,12-20 In addition, no one has studied the utility of ABC in TKA. In the present retrospective cohort analysis, we hypothesized that TE, BS, and ABC would be clinically equivalent in primary TKA with respect to EBL, 48-hour wound drainage, operative time, and change from preoperative Hb level.
The data on hemostatic technology in primary TKA are inconclusive. In an age- and sex-matched study comparing TE and BS in primary TKA, BS used with shed blood autotransfusion reduced homologous blood transfusions by a factor of 5.16 In addition, BS patients lost significantly less total visible blood (intraoperative EBL, postoperative drain output), and their magnitude of postoperative Hb-level depreciations at time of discharge was significantly lower. In a multicenter, prospective randomized trial comparing TE with BS, adjusted blood loss and need for autologous blood transfusions were lower in BS patients,10 though there was no significant difference in Knee Society Scale scores between the 2 treatment arms. However, analysis was potentially biased in that multiple authors had financial ties to Salient Surgical Technologies, the manufacturer of the BS device used in the study. Other prospective randomized trials of patients who had primary TKA with either TE or BS did not find any significant difference in postoperative Hb level, postoperative drainage, or transfusion requirements.19 ABC has been studied in the context of orthopedics but not joint arthroplasty specifically. This technology was anecdotally identified as a means of attaining hemostasis in foot and ankle surgery after failure of TE and other conventional means.22 ABC has also been identified as a successful adjuvant to curettage in the treatment of aneurysmal bone cysts.21 However, ABC has not been compared with TE or BS in the orthopedic literature.
In the present study, analysis of preoperative variables revealed a statistically but not clinically significant difference in BMI among cohorts. Mean (SD) BMI was 35.6 (6.5) for TE patients, 35.8 (9.7) for BS patients, and 40.9 (11.3) for ABC patients. (Previously, BMI did not correlate with intraoperative blood loss in TKA.25) Analysis also revealed a statistically significant but clinically insignificant and inconsequential difference in Hb level among cohorts. Mean (SD) preoperative Hb level was 13.5 (1.6) g/dL for TE patients, 12.8 (1.4) g/dL for BS patients, and 13.0 (1.6) g/dL for ABC patients. As decreases from preoperative baseline Hb levels were the intended focus of analysis—not absolute Hb levels—this finding does not refute postoperative analyses.
Our results suggest that, though TE may have relatively longer operative times in primary TKA, it is clinically equivalent to BS and ABC with respect to EBL and postoperative change in Hb levels. In addition, postoperative drainage was lower in TE than in BS and ABC, which were equivalent. No significant differences were found among hemostasis types with respect to postoperative transfusion requirements.
The prevalence distribution of predischarge AEs trended toward significance (χ2 = 5.957, P = .051), despite not meeting the predetermined α level. Rates of predischarge AEs were 0% (0/41) for TE patients, 7% (14/203) for BS patients, and 3% (1/36) for ABC patients. AEs included acute kidney injuries, electrolyte disturbances, urinary tract infections, oxygen desaturation, altered mental status, sepsis/infections, arrhythmias, congestive heart failure exacerbation, dehiscence, pulmonary embolism, and hypotension. Clearly, many of these AEs are not attributable to the hemostasis system used.
Limitations of this study include its retrospective design, documentation inadequate to account for drainage amount reinfused, and limited data on which clinical insignificance thresholds were based. In addition, reliance on historical data may have introduced bias into the analysis. The historical data used to increase the size of the TE cohort may reflect a period of relative inexperience and may have contributed to the longer operative times relative to those of the ABC cohort (Dr. Levine used ABC later in his career).
Traditional electrocautery remains a viable option in primary TKA. With its low cost and hemostasis equivalent to that of BS and ABC, TE deserves consideration equal to that given to these more modern hemostasis technologies. Cost per case is about $10 for TE versus $500 for BS and $110 for ABC.17 Soaring healthcare expenditures may warrant returning to TE or combining cautery techniques and other agents in primary TKA in order to reduce the number of transfusions and associated surgical costs.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991-2010. JAMA. 2012;308(12):1227-1236.
2. Leijtens B, Kremers van de Hei K, Jansen J, Koëter S. High complication rate after total knee and hip replacement due to perioperative bridging of anticoagulant therapy based on the 2012 ACCP guideline. Arch Orthop Trauma Surg. 2014;134(9):1335-1341.
3. Park CH, Lee SH, Kang DG, Cho KY, Lee SH, Kim KI. Compartment syndrome following total knee arthroplasty: clinical results of late fasciotomy. Knee Surg Relat Res. 2014;26(3):177-181.
4. Pedersen AB, Mehnert F, Sorensen HT, Emmeluth C, Overgaard S, Johnsen SP. The risk of venous thromboembolism, myocardial infarction, stroke, major bleeding and death in patients undergoing total hip and knee replacement: a 15-year retrospective cohort study of routine clinical practice. Bone Joint J. 2014;96-B(4):479-485.
5. Carson JL, Poses RM, Spence RK, Bonavita G. Severity of anaemia and operative mortality and morbidity. Lancet. 1988;1(8588):727-729.
6. Carson JL, Duff A, Poses RM, et al. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348(9034):1055-1060.
7. Dodd RY. Current risk for transfusion transmitted infections. Curr Opin Hematol. 2007;14(6):671-676.
8. Kang DG, Khurana S, Baek JH, Park YS, Lee SH, Kim KI. Efficacy and safety using autotransfusion system with postoperative shed blood following total knee arthroplasty in haemophilia. Haemophilia. 2014;20(1):129-132.
9. Aguilera X, Martinez-Zapata MJ, Bosch A, et al. Efficacy and safety of fibrin glue and tranexamic acid to prevent postoperative blood loss in total knee arthroplasty: a randomized controlled clinical trial. J Bone Joint Surg Am. 2013;95(22):2001-2007.
10. Marulanda GA, Krebs VE, Bierbaum BE, et al. Hemostasis using a bipolar sealer in primary unilateral total knee arthroplasty. Am J Orthop. 2009;38(12):E179-E183.
11. Katkhouda N, Friedlander M, Darehzereshki A, et al. Argon beam coagulation versus fibrin sealant for hemostasis following liver resection: a randomized study in a porcine model. Hepatogastroenterology. 2013;60(125):1110-1116.
12. Marulanda GA, Ulrich SD, Seyler TM, Delanois RE, Mont MA. Reductions in blood loss with a bipolar sealer in total hip arthroplasty. Expert Rev Med Devices. 2008;5(2):125-131.
13. Morris MJ, Berend KR, Lombardi AV Jr. Hemostasis in anterior supine intermuscular total hip arthroplasty: pilot study comparing standard electrocautery and a bipolar sealer. Surg Technol Int. 2010;20:352-356.
14. Clement RC, Kamath AF, Derman PB, Garino JP, Lee GC. Bipolar sealing in revision total hip arthroplasty for infection: efficacy and cost analysis. J Arthroplasty. 2012;27(7):1376-1381.
15. Rosenberg AG. Reducing blood loss in total joint surgery with a saline-coupled bipolar sealing technology. J Arthroplasty. 2007;22(4 suppl 1):82-85.
16. Pfeiffer M, Bräutigam H, Draws D, Sigg A. A new bipolar blood sealing system embedded in perioperative strategies vs. a conventional regimen for total knee arthroplasty: results of a matched-pair study. Ger Med Sci. 2005;3:Doc10.
17. Morris MJ, Barrett M, Lombardi AV Jr, Tucker TL, Berend KR. Randomized blinded study comparing a bipolar sealer and standard electrocautery in reducing transfusion requirements in anterior supine intermuscular total hip arthroplasty. J Arthroplasty. 2013;28(9):1614-1617.
18. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518.
19. Plymale MF, Capogna BM, Lovy AJ, Adler ML, Hirsh DM, Kim SJ. Unipolar vs bipolar hemostasis in total knee arthroplasty: a prospective randomized trial. J Arthroplasty. 2012;27(6):1133-1137.e1.
20. Zeh A, Messer J, Davis J, Vasarhelyi A, Wohlrab D. The Aquamantys system—an alternative to reduce blood loss in primary total hip arthroplasty? J Arthroplasty. 2010;25(7):1072-1077.
21. Cummings JE, Smith RA, Heck RK Jr. Argon beam coagulation as adjuvant treatment after curettage of aneurysmal bone cysts: a preliminary study. Clin Orthop Relat Res. 2010;468(1):231-237.
22. Adams ML, Steinberg JS. Argon beam coagulation in foot and ankle surgery. J Foot Ankle Surg. 2011;50(6):780-782.
23. Neumayer L, Vargo D. Principles of preoperative and operative surgery. In: Townsend CM Jr, Beauchamp RD, Evers BM, Mattox KL, eds. Sabiston Textbook of Surgery. 19th ed. Philadelphia, PA: Elsevier Saunders; 2012:211-239.
24. Walker E, Nowacki AS. Understanding equivalence and noninferiority testing. J Gen Intern Med. 2011;26(2):192-196.
25. Hrnack SA, Skeen N, Xu T, Rosenstein AD. Correlation of body mass index and blood loss during total knee and total hip arthroplasty. Am J Orthop. 2012;41(10):467-471.
Total knee arthroplasty (TKA) is a reliable and successful treatment for end-stage degenerative joint disease of the knee. Given the reproducibility of its generally excellent outcomes, TKA is increasingly being performed.1 However, the potential complications of this procedure can be devastating.2-4 The arthroplasty literature has shed light on the detrimental effects of postoperative blood loss and anemia.5,6 In addition, the increase in transfusion burden among patients is not without risk.7 Given these concerns, surgeons have been tasked with determining the ideal methods for minimizing blood transfusions and postoperative hematomas and anemia. Several strategies have been described.8-11 Hemostasis can be achieved with use of intravenous medications, intra-articular agents, or electrocautery devices. Electrocautery technologies include traditional electrocautery (TE), saline-coupled bipolar sealer (BS), and argon beam coagulation (ABC). There is controversy as to whether outcomes are better with one hemostasis method over another and whether these methods are worth the additional cost.
In traditional (Bovie) electrocautery, a unipolar device delivers an electrical current to tissues through a pencil-like instrument. Intraoperative tissue temperatures can exceed 400°C.12 In BS, radiofrequency energy is delivered through a saline medium, which increases the contact area, acts as an electrode, and maintains a cooler environment during electrocautery. Proposed advantages are reduced tissue destruction and absence of smoke.12 There is evidence both for10,12-16 and against17-20 use of BS in total joint arthroplasty. ABC, a novel hemostasis method, has been studied in the context of orthopedics21,22 but not TKA specifically. ABC establishes a monopolar electric circuit between a handheld device and the target tissues by channeling electrons through ionized argon gas. Hemostasis is achieved through thermal coagulation. Tissue penetration can be adjusted by changing power, probe-to-target distance, and duration of use.23 We conducted a study to assess the efficacy of all 3 electrocautery methods during TKA. We hypothesized the 3 methods would be clinically equivalent with respect to estimated blood loss (EBL), 48-hour wound drainage, operative time, and change from preoperative hemoglobin (Hb) level.
Methods
We conducted a retrospective cohort study of consecutive primary TKAs performed by Dr. Levine between October 2010 and November 2011. Patients were identified by querying an internal database. Exclusion criteria were prior ipsilateral open knee procedure, prior fracture, nonuse of our standard hemostatic protocol, and either tourniquet time under 40 minutes or intraoperative documentation of tourniquet failure. As only 9 patients were initially identified for the TE cohort, the same database was used to add 32 patients treated between April 2009 and October 2009 (before our institution began using BS and ABC).
Clinical charts were reviewed, and baseline demographics (age, body mass index [BMI], preoperative Hb level) were abstracted, as were outcome metrics (EBL, 48-hour wound drainage, operative time, postoperative transfusions, adverse events (AEs) before discharge, and change in Hb level from before surgery to after surgery, in recovery room and on discharge). Statistical analyses were performed with JMP Version 10.0.0 (SAS Institute). Given the hypothesis that the 3 hemostasis methods would be clinically equivalent, 2 one-sided tests (TOSTs) of equivalence were performed with an α of 0.05. With TOST, the traditional null and alternative hypotheses are reversed; thus, P < .05 identifies statistical equivalence. The advantage of this study design is that equivalence can be identified, whereas traditional study designs can identify only a lack of statistical difference.24 We used our consensus opinions to set clinical insignificance thresholds for EBL (150 mL), wound drainage (150 mL), decrease from postoperative Hb level (1 g/dL), and operative time (10 minutes). Patients who received a blood transfusion were subsequently excluded from analysis in order to avoid skewing Hb-level depreciation calculations. Analysis of variance (ANOVA) and χ2 tests were used to compare preoperative variables, transfusion requirements, hospital length of stay, and AE rates by hemostasis type.
Cautery Technique
In all cases, TE was used for surgical dissection, which followed a standard midvastus approach. Then, for meniscal excision, the capsule and meniscal attachment sites were treated with TE, BS, or ABC. During cement hardening, an available supplemental cautery option was used to achieve hemostasis of the suprapatellar fat pad and visible meniscal attachment sites. All other aspects of the procedure and the postoperative protocols—including the anticoagulation and rapid rehabilitation (early ambulation and therapy) protocols—were similar for all patients. The standard anticoagulation protocol was to use low-molecular-weight heparin, unless contraindicated. Tranexamic acid was not used at our institution during the study period.
Results
For the study period, 280 cases (41 TE, 203 BS, 36 ABC) met the inclusion criteria. Of the 280 TKAs, 261 (93.21%) were performed for degenerative arthritis. There was no statistically significant difference among cohorts in indication (χ2 = 1.841, P = .398) or sex (χ2 = 1.176, P= .555).
Table 1 lists the cohorts’ baseline demographics (mean age, BMI, preoperative Hb level) and comparative ANOVA results. TOSTs of equivalence were performed to compare operative time, EBL, 48-hour wound drainage, and postoperative Hb-level depreciation among hemostasis types. Changes in Hb level were calculated for the immediate postoperative period and time of discharge (Table 2). ANOVA of hospital length of stay demonstrated no significant difference in means among groups (P = .09).
The cohorts were compared with respect to use of postoperative transfusions and incidence of postoperative AEs (Table 3). The TE cohort did not have any AEs. Of the 203 BS patients, 14 (7%) had 1 or more AEs, which included acute kidney injury (3 cases), electrolyte disturbance (3), urinary tract infection (2), oxygen desaturation (2), altered mental status (1), pneumonia (1), arrhythmia (1), congestive heart failure exacerbation (1), dehiscence (1), pulmonary embolism (2), and hypotension (1). Of the 36 ABC patients, 1 (3%) had arrhythmia, pneumonia, sepsis, and altered mental status.
Discussion
With the population aging, the demand for TKA is greater than ever.1 As surgical volume increases, the ability to minimize the rates of intraoperative bleeding, postoperative anemia, and transfusion is becoming increasingly important to patients and the healthcare system. There is no consensus as to which cautery method is ideal. Other investigators have identified differences in clinical outcomes between cautery systems, but reported results are largely conflicting.10,12-20 In addition, no one has studied the utility of ABC in TKA. In the present retrospective cohort analysis, we hypothesized that TE, BS, and ABC would be clinically equivalent in primary TKA with respect to EBL, 48-hour wound drainage, operative time, and change from preoperative Hb level.
The data on hemostatic technology in primary TKA are inconclusive. In an age- and sex-matched study comparing TE and BS in primary TKA, BS used with shed blood autotransfusion reduced homologous blood transfusions by a factor of 5.16 In addition, BS patients lost significantly less total visible blood (intraoperative EBL, postoperative drain output), and their magnitude of postoperative Hb-level depreciations at time of discharge was significantly lower. In a multicenter, prospective randomized trial comparing TE with BS, adjusted blood loss and need for autologous blood transfusions were lower in BS patients,10 though there was no significant difference in Knee Society Scale scores between the 2 treatment arms. However, analysis was potentially biased in that multiple authors had financial ties to Salient Surgical Technologies, the manufacturer of the BS device used in the study. Other prospective randomized trials of patients who had primary TKA with either TE or BS did not find any significant difference in postoperative Hb level, postoperative drainage, or transfusion requirements.19 ABC has been studied in the context of orthopedics but not joint arthroplasty specifically. This technology was anecdotally identified as a means of attaining hemostasis in foot and ankle surgery after failure of TE and other conventional means.22 ABC has also been identified as a successful adjuvant to curettage in the treatment of aneurysmal bone cysts.21 However, ABC has not been compared with TE or BS in the orthopedic literature.
In the present study, analysis of preoperative variables revealed a statistically but not clinically significant difference in BMI among cohorts. Mean (SD) BMI was 35.6 (6.5) for TE patients, 35.8 (9.7) for BS patients, and 40.9 (11.3) for ABC patients. (Previously, BMI did not correlate with intraoperative blood loss in TKA.25) Analysis also revealed a statistically significant but clinically insignificant and inconsequential difference in Hb level among cohorts. Mean (SD) preoperative Hb level was 13.5 (1.6) g/dL for TE patients, 12.8 (1.4) g/dL for BS patients, and 13.0 (1.6) g/dL for ABC patients. As decreases from preoperative baseline Hb levels were the intended focus of analysis—not absolute Hb levels—this finding does not refute postoperative analyses.
Our results suggest that, though TE may have relatively longer operative times in primary TKA, it is clinically equivalent to BS and ABC with respect to EBL and postoperative change in Hb levels. In addition, postoperative drainage was lower in TE than in BS and ABC, which were equivalent. No significant differences were found among hemostasis types with respect to postoperative transfusion requirements.
The prevalence distribution of predischarge AEs trended toward significance (χ2 = 5.957, P = .051), despite not meeting the predetermined α level. Rates of predischarge AEs were 0% (0/41) for TE patients, 7% (14/203) for BS patients, and 3% (1/36) for ABC patients. AEs included acute kidney injuries, electrolyte disturbances, urinary tract infections, oxygen desaturation, altered mental status, sepsis/infections, arrhythmias, congestive heart failure exacerbation, dehiscence, pulmonary embolism, and hypotension. Clearly, many of these AEs are not attributable to the hemostasis system used.
Limitations of this study include its retrospective design, documentation inadequate to account for drainage amount reinfused, and limited data on which clinical insignificance thresholds were based. In addition, reliance on historical data may have introduced bias into the analysis. The historical data used to increase the size of the TE cohort may reflect a period of relative inexperience and may have contributed to the longer operative times relative to those of the ABC cohort (Dr. Levine used ABC later in his career).
Traditional electrocautery remains a viable option in primary TKA. With its low cost and hemostasis equivalent to that of BS and ABC, TE deserves consideration equal to that given to these more modern hemostasis technologies. Cost per case is about $10 for TE versus $500 for BS and $110 for ABC.17 Soaring healthcare expenditures may warrant returning to TE or combining cautery techniques and other agents in primary TKA in order to reduce the number of transfusions and associated surgical costs.
Total knee arthroplasty (TKA) is a reliable and successful treatment for end-stage degenerative joint disease of the knee. Given the reproducibility of its generally excellent outcomes, TKA is increasingly being performed.1 However, the potential complications of this procedure can be devastating.2-4 The arthroplasty literature has shed light on the detrimental effects of postoperative blood loss and anemia.5,6 In addition, the increase in transfusion burden among patients is not without risk.7 Given these concerns, surgeons have been tasked with determining the ideal methods for minimizing blood transfusions and postoperative hematomas and anemia. Several strategies have been described.8-11 Hemostasis can be achieved with use of intravenous medications, intra-articular agents, or electrocautery devices. Electrocautery technologies include traditional electrocautery (TE), saline-coupled bipolar sealer (BS), and argon beam coagulation (ABC). There is controversy as to whether outcomes are better with one hemostasis method over another and whether these methods are worth the additional cost.
In traditional (Bovie) electrocautery, a unipolar device delivers an electrical current to tissues through a pencil-like instrument. Intraoperative tissue temperatures can exceed 400°C.12 In BS, radiofrequency energy is delivered through a saline medium, which increases the contact area, acts as an electrode, and maintains a cooler environment during electrocautery. Proposed advantages are reduced tissue destruction and absence of smoke.12 There is evidence both for10,12-16 and against17-20 use of BS in total joint arthroplasty. ABC, a novel hemostasis method, has been studied in the context of orthopedics21,22 but not TKA specifically. ABC establishes a monopolar electric circuit between a handheld device and the target tissues by channeling electrons through ionized argon gas. Hemostasis is achieved through thermal coagulation. Tissue penetration can be adjusted by changing power, probe-to-target distance, and duration of use.23 We conducted a study to assess the efficacy of all 3 electrocautery methods during TKA. We hypothesized the 3 methods would be clinically equivalent with respect to estimated blood loss (EBL), 48-hour wound drainage, operative time, and change from preoperative hemoglobin (Hb) level.
Methods
We conducted a retrospective cohort study of consecutive primary TKAs performed by Dr. Levine between October 2010 and November 2011. Patients were identified by querying an internal database. Exclusion criteria were prior ipsilateral open knee procedure, prior fracture, nonuse of our standard hemostatic protocol, and either tourniquet time under 40 minutes or intraoperative documentation of tourniquet failure. As only 9 patients were initially identified for the TE cohort, the same database was used to add 32 patients treated between April 2009 and October 2009 (before our institution began using BS and ABC).
Clinical charts were reviewed, and baseline demographics (age, body mass index [BMI], preoperative Hb level) were abstracted, as were outcome metrics (EBL, 48-hour wound drainage, operative time, postoperative transfusions, adverse events (AEs) before discharge, and change in Hb level from before surgery to after surgery, in recovery room and on discharge). Statistical analyses were performed with JMP Version 10.0.0 (SAS Institute). Given the hypothesis that the 3 hemostasis methods would be clinically equivalent, 2 one-sided tests (TOSTs) of equivalence were performed with an α of 0.05. With TOST, the traditional null and alternative hypotheses are reversed; thus, P < .05 identifies statistical equivalence. The advantage of this study design is that equivalence can be identified, whereas traditional study designs can identify only a lack of statistical difference.24 We used our consensus opinions to set clinical insignificance thresholds for EBL (150 mL), wound drainage (150 mL), decrease from postoperative Hb level (1 g/dL), and operative time (10 minutes). Patients who received a blood transfusion were subsequently excluded from analysis in order to avoid skewing Hb-level depreciation calculations. Analysis of variance (ANOVA) and χ2 tests were used to compare preoperative variables, transfusion requirements, hospital length of stay, and AE rates by hemostasis type.
Cautery Technique
In all cases, TE was used for surgical dissection, which followed a standard midvastus approach. Then, for meniscal excision, the capsule and meniscal attachment sites were treated with TE, BS, or ABC. During cement hardening, an available supplemental cautery option was used to achieve hemostasis of the suprapatellar fat pad and visible meniscal attachment sites. All other aspects of the procedure and the postoperative protocols—including the anticoagulation and rapid rehabilitation (early ambulation and therapy) protocols—were similar for all patients. The standard anticoagulation protocol was to use low-molecular-weight heparin, unless contraindicated. Tranexamic acid was not used at our institution during the study period.
Results
For the study period, 280 cases (41 TE, 203 BS, 36 ABC) met the inclusion criteria. Of the 280 TKAs, 261 (93.21%) were performed for degenerative arthritis. There was no statistically significant difference among cohorts in indication (χ2 = 1.841, P = .398) or sex (χ2 = 1.176, P= .555).
Table 1 lists the cohorts’ baseline demographics (mean age, BMI, preoperative Hb level) and comparative ANOVA results. TOSTs of equivalence were performed to compare operative time, EBL, 48-hour wound drainage, and postoperative Hb-level depreciation among hemostasis types. Changes in Hb level were calculated for the immediate postoperative period and time of discharge (Table 2). ANOVA of hospital length of stay demonstrated no significant difference in means among groups (P = .09).
The cohorts were compared with respect to use of postoperative transfusions and incidence of postoperative AEs (Table 3). The TE cohort did not have any AEs. Of the 203 BS patients, 14 (7%) had 1 or more AEs, which included acute kidney injury (3 cases), electrolyte disturbance (3), urinary tract infection (2), oxygen desaturation (2), altered mental status (1), pneumonia (1), arrhythmia (1), congestive heart failure exacerbation (1), dehiscence (1), pulmonary embolism (2), and hypotension (1). Of the 36 ABC patients, 1 (3%) had arrhythmia, pneumonia, sepsis, and altered mental status.
Discussion
With the population aging, the demand for TKA is greater than ever.1 As surgical volume increases, the ability to minimize the rates of intraoperative bleeding, postoperative anemia, and transfusion is becoming increasingly important to patients and the healthcare system. There is no consensus as to which cautery method is ideal. Other investigators have identified differences in clinical outcomes between cautery systems, but reported results are largely conflicting.10,12-20 In addition, no one has studied the utility of ABC in TKA. In the present retrospective cohort analysis, we hypothesized that TE, BS, and ABC would be clinically equivalent in primary TKA with respect to EBL, 48-hour wound drainage, operative time, and change from preoperative Hb level.
The data on hemostatic technology in primary TKA are inconclusive. In an age- and sex-matched study comparing TE and BS in primary TKA, BS used with shed blood autotransfusion reduced homologous blood transfusions by a factor of 5.16 In addition, BS patients lost significantly less total visible blood (intraoperative EBL, postoperative drain output), and their magnitude of postoperative Hb-level depreciations at time of discharge was significantly lower. In a multicenter, prospective randomized trial comparing TE with BS, adjusted blood loss and need for autologous blood transfusions were lower in BS patients,10 though there was no significant difference in Knee Society Scale scores between the 2 treatment arms. However, analysis was potentially biased in that multiple authors had financial ties to Salient Surgical Technologies, the manufacturer of the BS device used in the study. Other prospective randomized trials of patients who had primary TKA with either TE or BS did not find any significant difference in postoperative Hb level, postoperative drainage, or transfusion requirements.19 ABC has been studied in the context of orthopedics but not joint arthroplasty specifically. This technology was anecdotally identified as a means of attaining hemostasis in foot and ankle surgery after failure of TE and other conventional means.22 ABC has also been identified as a successful adjuvant to curettage in the treatment of aneurysmal bone cysts.21 However, ABC has not been compared with TE or BS in the orthopedic literature.
In the present study, analysis of preoperative variables revealed a statistically but not clinically significant difference in BMI among cohorts. Mean (SD) BMI was 35.6 (6.5) for TE patients, 35.8 (9.7) for BS patients, and 40.9 (11.3) for ABC patients. (Previously, BMI did not correlate with intraoperative blood loss in TKA.25) Analysis also revealed a statistically significant but clinically insignificant and inconsequential difference in Hb level among cohorts. Mean (SD) preoperative Hb level was 13.5 (1.6) g/dL for TE patients, 12.8 (1.4) g/dL for BS patients, and 13.0 (1.6) g/dL for ABC patients. As decreases from preoperative baseline Hb levels were the intended focus of analysis—not absolute Hb levels—this finding does not refute postoperative analyses.
Our results suggest that, though TE may have relatively longer operative times in primary TKA, it is clinically equivalent to BS and ABC with respect to EBL and postoperative change in Hb levels. In addition, postoperative drainage was lower in TE than in BS and ABC, which were equivalent. No significant differences were found among hemostasis types with respect to postoperative transfusion requirements.
The prevalence distribution of predischarge AEs trended toward significance (χ2 = 5.957, P = .051), despite not meeting the predetermined α level. Rates of predischarge AEs were 0% (0/41) for TE patients, 7% (14/203) for BS patients, and 3% (1/36) for ABC patients. AEs included acute kidney injuries, electrolyte disturbances, urinary tract infections, oxygen desaturation, altered mental status, sepsis/infections, arrhythmias, congestive heart failure exacerbation, dehiscence, pulmonary embolism, and hypotension. Clearly, many of these AEs are not attributable to the hemostasis system used.
Limitations of this study include its retrospective design, documentation inadequate to account for drainage amount reinfused, and limited data on which clinical insignificance thresholds were based. In addition, reliance on historical data may have introduced bias into the analysis. The historical data used to increase the size of the TE cohort may reflect a period of relative inexperience and may have contributed to the longer operative times relative to those of the ABC cohort (Dr. Levine used ABC later in his career).
Traditional electrocautery remains a viable option in primary TKA. With its low cost and hemostasis equivalent to that of BS and ABC, TE deserves consideration equal to that given to these more modern hemostasis technologies. Cost per case is about $10 for TE versus $500 for BS and $110 for ABC.17 Soaring healthcare expenditures may warrant returning to TE or combining cautery techniques and other agents in primary TKA in order to reduce the number of transfusions and associated surgical costs.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991-2010. JAMA. 2012;308(12):1227-1236.
2. Leijtens B, Kremers van de Hei K, Jansen J, Koëter S. High complication rate after total knee and hip replacement due to perioperative bridging of anticoagulant therapy based on the 2012 ACCP guideline. Arch Orthop Trauma Surg. 2014;134(9):1335-1341.
3. Park CH, Lee SH, Kang DG, Cho KY, Lee SH, Kim KI. Compartment syndrome following total knee arthroplasty: clinical results of late fasciotomy. Knee Surg Relat Res. 2014;26(3):177-181.
4. Pedersen AB, Mehnert F, Sorensen HT, Emmeluth C, Overgaard S, Johnsen SP. The risk of venous thromboembolism, myocardial infarction, stroke, major bleeding and death in patients undergoing total hip and knee replacement: a 15-year retrospective cohort study of routine clinical practice. Bone Joint J. 2014;96-B(4):479-485.
5. Carson JL, Poses RM, Spence RK, Bonavita G. Severity of anaemia and operative mortality and morbidity. Lancet. 1988;1(8588):727-729.
6. Carson JL, Duff A, Poses RM, et al. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348(9034):1055-1060.
7. Dodd RY. Current risk for transfusion transmitted infections. Curr Opin Hematol. 2007;14(6):671-676.
8. Kang DG, Khurana S, Baek JH, Park YS, Lee SH, Kim KI. Efficacy and safety using autotransfusion system with postoperative shed blood following total knee arthroplasty in haemophilia. Haemophilia. 2014;20(1):129-132.
9. Aguilera X, Martinez-Zapata MJ, Bosch A, et al. Efficacy and safety of fibrin glue and tranexamic acid to prevent postoperative blood loss in total knee arthroplasty: a randomized controlled clinical trial. J Bone Joint Surg Am. 2013;95(22):2001-2007.
10. Marulanda GA, Krebs VE, Bierbaum BE, et al. Hemostasis using a bipolar sealer in primary unilateral total knee arthroplasty. Am J Orthop. 2009;38(12):E179-E183.
11. Katkhouda N, Friedlander M, Darehzereshki A, et al. Argon beam coagulation versus fibrin sealant for hemostasis following liver resection: a randomized study in a porcine model. Hepatogastroenterology. 2013;60(125):1110-1116.
12. Marulanda GA, Ulrich SD, Seyler TM, Delanois RE, Mont MA. Reductions in blood loss with a bipolar sealer in total hip arthroplasty. Expert Rev Med Devices. 2008;5(2):125-131.
13. Morris MJ, Berend KR, Lombardi AV Jr. Hemostasis in anterior supine intermuscular total hip arthroplasty: pilot study comparing standard electrocautery and a bipolar sealer. Surg Technol Int. 2010;20:352-356.
14. Clement RC, Kamath AF, Derman PB, Garino JP, Lee GC. Bipolar sealing in revision total hip arthroplasty for infection: efficacy and cost analysis. J Arthroplasty. 2012;27(7):1376-1381.
15. Rosenberg AG. Reducing blood loss in total joint surgery with a saline-coupled bipolar sealing technology. J Arthroplasty. 2007;22(4 suppl 1):82-85.
16. Pfeiffer M, Bräutigam H, Draws D, Sigg A. A new bipolar blood sealing system embedded in perioperative strategies vs. a conventional regimen for total knee arthroplasty: results of a matched-pair study. Ger Med Sci. 2005;3:Doc10.
17. Morris MJ, Barrett M, Lombardi AV Jr, Tucker TL, Berend KR. Randomized blinded study comparing a bipolar sealer and standard electrocautery in reducing transfusion requirements in anterior supine intermuscular total hip arthroplasty. J Arthroplasty. 2013;28(9):1614-1617.
18. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518.
19. Plymale MF, Capogna BM, Lovy AJ, Adler ML, Hirsh DM, Kim SJ. Unipolar vs bipolar hemostasis in total knee arthroplasty: a prospective randomized trial. J Arthroplasty. 2012;27(6):1133-1137.e1.
20. Zeh A, Messer J, Davis J, Vasarhelyi A, Wohlrab D. The Aquamantys system—an alternative to reduce blood loss in primary total hip arthroplasty? J Arthroplasty. 2010;25(7):1072-1077.
21. Cummings JE, Smith RA, Heck RK Jr. Argon beam coagulation as adjuvant treatment after curettage of aneurysmal bone cysts: a preliminary study. Clin Orthop Relat Res. 2010;468(1):231-237.
22. Adams ML, Steinberg JS. Argon beam coagulation in foot and ankle surgery. J Foot Ankle Surg. 2011;50(6):780-782.
23. Neumayer L, Vargo D. Principles of preoperative and operative surgery. In: Townsend CM Jr, Beauchamp RD, Evers BM, Mattox KL, eds. Sabiston Textbook of Surgery. 19th ed. Philadelphia, PA: Elsevier Saunders; 2012:211-239.
24. Walker E, Nowacki AS. Understanding equivalence and noninferiority testing. J Gen Intern Med. 2011;26(2):192-196.
25. Hrnack SA, Skeen N, Xu T, Rosenstein AD. Correlation of body mass index and blood loss during total knee and total hip arthroplasty. Am J Orthop. 2012;41(10):467-471.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991-2010. JAMA. 2012;308(12):1227-1236.
2. Leijtens B, Kremers van de Hei K, Jansen J, Koëter S. High complication rate after total knee and hip replacement due to perioperative bridging of anticoagulant therapy based on the 2012 ACCP guideline. Arch Orthop Trauma Surg. 2014;134(9):1335-1341.
3. Park CH, Lee SH, Kang DG, Cho KY, Lee SH, Kim KI. Compartment syndrome following total knee arthroplasty: clinical results of late fasciotomy. Knee Surg Relat Res. 2014;26(3):177-181.
4. Pedersen AB, Mehnert F, Sorensen HT, Emmeluth C, Overgaard S, Johnsen SP. The risk of venous thromboembolism, myocardial infarction, stroke, major bleeding and death in patients undergoing total hip and knee replacement: a 15-year retrospective cohort study of routine clinical practice. Bone Joint J. 2014;96-B(4):479-485.
5. Carson JL, Poses RM, Spence RK, Bonavita G. Severity of anaemia and operative mortality and morbidity. Lancet. 1988;1(8588):727-729.
6. Carson JL, Duff A, Poses RM, et al. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348(9034):1055-1060.
7. Dodd RY. Current risk for transfusion transmitted infections. Curr Opin Hematol. 2007;14(6):671-676.
8. Kang DG, Khurana S, Baek JH, Park YS, Lee SH, Kim KI. Efficacy and safety using autotransfusion system with postoperative shed blood following total knee arthroplasty in haemophilia. Haemophilia. 2014;20(1):129-132.
9. Aguilera X, Martinez-Zapata MJ, Bosch A, et al. Efficacy and safety of fibrin glue and tranexamic acid to prevent postoperative blood loss in total knee arthroplasty: a randomized controlled clinical trial. J Bone Joint Surg Am. 2013;95(22):2001-2007.
10. Marulanda GA, Krebs VE, Bierbaum BE, et al. Hemostasis using a bipolar sealer in primary unilateral total knee arthroplasty. Am J Orthop. 2009;38(12):E179-E183.
11. Katkhouda N, Friedlander M, Darehzereshki A, et al. Argon beam coagulation versus fibrin sealant for hemostasis following liver resection: a randomized study in a porcine model. Hepatogastroenterology. 2013;60(125):1110-1116.
12. Marulanda GA, Ulrich SD, Seyler TM, Delanois RE, Mont MA. Reductions in blood loss with a bipolar sealer in total hip arthroplasty. Expert Rev Med Devices. 2008;5(2):125-131.
13. Morris MJ, Berend KR, Lombardi AV Jr. Hemostasis in anterior supine intermuscular total hip arthroplasty: pilot study comparing standard electrocautery and a bipolar sealer. Surg Technol Int. 2010;20:352-356.
14. Clement RC, Kamath AF, Derman PB, Garino JP, Lee GC. Bipolar sealing in revision total hip arthroplasty for infection: efficacy and cost analysis. J Arthroplasty. 2012;27(7):1376-1381.
15. Rosenberg AG. Reducing blood loss in total joint surgery with a saline-coupled bipolar sealing technology. J Arthroplasty. 2007;22(4 suppl 1):82-85.
16. Pfeiffer M, Bräutigam H, Draws D, Sigg A. A new bipolar blood sealing system embedded in perioperative strategies vs. a conventional regimen for total knee arthroplasty: results of a matched-pair study. Ger Med Sci. 2005;3:Doc10.
17. Morris MJ, Barrett M, Lombardi AV Jr, Tucker TL, Berend KR. Randomized blinded study comparing a bipolar sealer and standard electrocautery in reducing transfusion requirements in anterior supine intermuscular total hip arthroplasty. J Arthroplasty. 2013;28(9):1614-1617.
18. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518.
19. Plymale MF, Capogna BM, Lovy AJ, Adler ML, Hirsh DM, Kim SJ. Unipolar vs bipolar hemostasis in total knee arthroplasty: a prospective randomized trial. J Arthroplasty. 2012;27(6):1133-1137.e1.
20. Zeh A, Messer J, Davis J, Vasarhelyi A, Wohlrab D. The Aquamantys system—an alternative to reduce blood loss in primary total hip arthroplasty? J Arthroplasty. 2010;25(7):1072-1077.
21. Cummings JE, Smith RA, Heck RK Jr. Argon beam coagulation as adjuvant treatment after curettage of aneurysmal bone cysts: a preliminary study. Clin Orthop Relat Res. 2010;468(1):231-237.
22. Adams ML, Steinberg JS. Argon beam coagulation in foot and ankle surgery. J Foot Ankle Surg. 2011;50(6):780-782.
23. Neumayer L, Vargo D. Principles of preoperative and operative surgery. In: Townsend CM Jr, Beauchamp RD, Evers BM, Mattox KL, eds. Sabiston Textbook of Surgery. 19th ed. Philadelphia, PA: Elsevier Saunders; 2012:211-239.
24. Walker E, Nowacki AS. Understanding equivalence and noninferiority testing. J Gen Intern Med. 2011;26(2):192-196.
25. Hrnack SA, Skeen N, Xu T, Rosenstein AD. Correlation of body mass index and blood loss during total knee and total hip arthroplasty. Am J Orthop. 2012;41(10):467-471.
Obesity Has Minimal Impact on Short-Term Functional Scores After Reverse Shoulder Arthroplasty for Rotator Cuff Tear Arthropathy
Body mass index (BMI) is thought to be a predictor of body composition, with higher values indicating more adipose tissue. BMI is a measure of mass with respect to height. The World Health Organization1 has established health categories based on BMI measurements. Values from 18.5 to 24.9 kg/m2 are deemed to represent normal weight; those from 25 to 30 kg/m2, overweight; and those higher than 30 kg/m2, obesity. BMI is not a perfect tool, but it is the most widely used tool in clinical and research practice because of its relative reliability and ease of use.2 Being overweight or obese (according to BMI) is increasingly common among adults worldwide, and particularly in the United States. An estimated 39% of adults worldwide are overweight, and 13% are obese.1 An estimated 69% of US adults are overweight, including 35.1% who are obese.2
Various pathologies have been treated with reverse shoulder arthroplasty (RSA), and results have been promising,3-9 but little is known about patient demographic and clinical factors that may adversely affect outcomes. Recent work suggests younger age7 and failed prior arthroplasty may adversely affect RSA outcomes.10 Higher BMI has also been implicated as a cause of increased perioperative and immediate postoperative complications of RSA with minimum 90-day follow-up, but no one has examined shoulder function scores at minimum 2-year follow-up.11,12
We conducted a study to examine shoulder function scores, mobility, patient satisfaction, and complications at minimum 2-year follow-up in normal-weight, overweight, and obese patients who underwent RSA. We hypothesized that, compared with normal-weight patients, obese patients would have worse shoulder function scores, worse mobility, and more complications.
Materials and Methods
Inclusion Criteria and Demographics
After obtaining Institutional Review Board approval for this study, we used a prospective shoulder arthroplasty registry to identify patients (N = 77) who had rotator cuff tear arthropathy (RCTA) treated with primary RSA and then had minimum 2-year follow-up. The study period was 2004-2011. All patients had RCTA diagnosed with physical examination findings and anteroposterior, scapular Y, and axillary radiographs. RCTA was graded 1 to 5 using the classification system of Hamada and colleagues.13 Rotator cuff status was determined with preoperative computed tomography arthrogram (CTA) or magnetic resonance imaging (MRI) and confirmed at time of surgery. BMI calculations were based on height and weight measured at initial office visit. Thirty-four patients had normal weight (BMI <25 kg/m2), 21 were overweight (BMI 25-30 kg/m2), and 22 were obese (BMI >30 kg/m2). Patient demographic and clinical characteristics reviewed also included age, sex, follow-up duration, arm dominance, complications, prevalence of depression, and prevalence of diabetes. All RSAs were performed by the same surgeon (Dr. Edwards) at a single high-volume shoulder arthroplasty center.
Shoulder function scores evaluated before surgery and at final follow-up included Constant score,14 American Shoulder and Elbow Surgeons (ASES) score,15 Western Ontario Osteoarthritis Shoulder (WOOS) index,16 Single Assessment Numeric Evaluation (SANE),17 and mobility. Satisfaction was assessed by having patients describe themselves as very dissatisfied, dissatisfied, satisfied, or very satisfied. All intraoperative and postoperative complications were recorded.
Surgical Technique and Postoperative Rehabilitation
The Aequalis RSA system (Tornier) was used for all patients during the study period. The RSA technique used has been well described.18,19 A standard postoperative rehabilitation protocol was followed.19,20
Clinical and Radiographic Assessment
Patients were prospectively enrolled in a shoulder arthroplasty outcomes registry and followed clinically. Mean follow-up was 3.16 years (range, 2-8 years). Before surgery, patients were examined by the surgeon. Examinations were repeated 1 week, 6 weeks, 3 months, 6 months, and 12 months after surgery and annually thereafter. Mobility (active range of motion) was determined with a handheld goniometer. Strength of abduction was measured with a handheld digital dynamometer (Chatillon digital force gauge, 200 lbf; Ametek). Anteroposterior in plane of scapula, scapular Y, and axillary radiographs were obtained at each clinic appointment.
Before surgery, the surgeon reviewed all radiographs. Each RCTA was given a Hamada grade (1-5).13 Glenoid erosion in the coronal plane was classified (E0, E1, E2, E3) according to Sirveaux and colleagues.21 Hamada grades and glenoid erosion types are listed in Table 1. The overall trend in classification by BMI group was statistically significant for Hamada grade (P = .004) but not glenoid erosion type (P = .153).
Before surgery, the surgeon also evaluated rotator cuff status using CTA or MRI. All patients had full-thickness tears of the supraspinatus and infraspinatus. The subscapularis was variably present, and subscapularis repair was performed when the subscapularis was intact. Rotator cuff status is listed in Table 2. There were no significant differences in the distribution of intact subscapularis (P = .402) or teres minor (P = .188) among the normal-weight, overweight, and obese groups. No patient had a latissimus dorsi transfer at time of RSA.
Statistical Analysis
Independent-samples t tests assuming unequal variances were used to compare the 3 BMI groups on age, follow-up duration, preoperative shoulder function scores, and mobility. Chi-square tests were used to identify any significant group differences in comorbidities (eg, complications, arm dominance, prevalence of depression, prevalence of diabetes) and patient satisfaction. Repeated-measures analysis of variance was used to evaluate main effects, changes from before surgery to final follow-up, and BMI group differences, as well as differences in changes from before surgery to final follow-up among the 3 BMI groups.
Results
Among BMI groups (<25 kg/m2, 25-30 kg/m2, >30 kg/m2), there were no statistically significant preoperative differences in age, sex, follow-up duration, complications, arm dominance, prevalence of depression, or prevalence of diabetes (P >. 05) (Table 3). Table 4 lists the groups’ preoperative and final follow-up data (Constant score, ASES score, WOOS index, SANE, mobility). There were no statistically significant preoperative group differences in Constant score, ASES score, WOOS index, SANE, mobility, or patient satisfaction (P > .05) (Tables 5, 6).
All groups’ shoulder function scores and mobility improved significantly from before surgery to final follow-up (P < .001) (Table 5). The groups’ magnitudes of change (improvement) from before surgery to final follow-up were almost identical, with no significant differences in shoulder function scores or mobility (Table 5). The only significant difference was in Constant–Strength, which was higher in the obese group (P = .017) (Table 5). Patient satisfaction ratings improved after surgery, with 79% of the normal-weight group reporting being satisfied or very satisfied with their shoulders (Table 6). The overweight and obese groups gave similar satisfied (81%) and very satisfied (82%) ratings. The small differences between group satisfaction scores were nonsignificant (P = .967).
Complications
The normal-weight group had 4 complications: periprosthetic infection (2 cases), intraoperative humeral fracture (1), and scapular spine stress fracture (1). The overweight group had 1 complication, an acromial stress fracture that was successfully treated with conservative measures. The obese group had 1 patient with 2 postoperative dislocations. The first dislocation was treated with closed reduction and bracing, and the second required revision surgery. There was no statistical difference in complications among the groups (P = .680).
Discussion
To our knowledge, this is the first study of the effects of varying BMI on functional outcomes of RSA with minimum 2-year follow-up. There appears to be minimal impact on shoulder function scores, complications, and patient satisfaction among normal-weight, overweight, and obese patients with RCTA treated by the same surgeon using similar techniques.
The relationship between obesity and increased perioperative risks or poorer surgical outcomes has been well established in orthopedic surgery. In a systematic review, Falagas and Kompoti22 found increased risk for perioperative and nosocomial infections in obese patients. Schoenfeld and colleagues23 and Jiang and colleagues24 reported increased risk for complications in spinal surgery. The total joint arthroplasty literature is rife with evidence suggesting higher BMI leads to increased risk for complications, including infection and deep venous thrombosis, as well as decreased functional outcome scores.25-29 Recent studies on shoulder surgery have shown worse outcomes in rotator cuff repair30 and a higher revision rate in hemiarthroplasy.31
Other RSA studies have examined short-term complications or perioperative risk factors associated with BMI. In a study using slightly different BMI groupings, Gupta and colleagues12 reported significantly higher complication rates for RSA patients with BMI higher than 35 kg/m2 compared to patients with BMI of 25 to 35 kg/m2 and compared to patients with BMI lower than 25 kg/m2. Their study highlighted medical and surgical complications and used a short follow-up period (minimum, 90 days). It did not assess shoulder function scores, and included multiple indications for RSA (eg, RCTA, proximal humerus fracture, inflammatory arthropathy). In another study, higher BMI was reported as a risk factor for early dislocation after RSA, but only 11 patients with a history of dislocation were assessed, and minimum follow-up was 6 months.32 We know of only one study that addressed RSA outcomes in obese patients and used minimum 2-year follow-up, but its primary endpoint was rate of complications, and it did not report shoulder function scores.11 Li and colleagues33 conducted a study similar to ours, but with primary total shoulder arthroplasty (TSA) patients, and reported similar results. Relative to normal BMI, higher BMI did not have a detrimental effect on short-term improvement in shoulder function after TSA.
Given the US obesity epidemic, our study results are encouraging. Depending on many factors, obesity remains a risk factor for poor outcomes in patients who undergo orthopedic surgery. As our results show, however, patients with higher BMI can obtain functional outcomes similar to those experienced by patients with normal-weight BMI after RSA for RCTA.
The primary limitation of this study was its retrospective design. Strengths of the study included its having a single surgeon and a single diagnosis: RCTA. In addition, each group was robust in size, a standard operative/postoperative protocol was used, and clinical results were measured with multiple validated shoulder function scores.
Conclusion
Improved shoulder function scores, mobility, and patient satisfaction can be expected after RSA for RCTA in patients with BMI higher than 30 kg/m2. These patients did not exhibit an increase in complications at short-term follow-up.
1. World Health Organization. Obesity and overweight [factsheet 311]. Updated January 2015. http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed March 27, 2016.
2. National Center for Health Statistics, Centers for Disease Control and Prevention. Obesity and overweight. Updated February 25, 2016. http://www.cdc.gov/nchs/fastats/obesity-overweight.htm. Accessed March 27, 2016.
3. Boileau P, Gonzalez JF, Chuinard C, Bicknell R, Walch G. Reverse total shoulder arthroplasty after failed rotator cuff surgery. J Shoulder Elbow Surg. 2009;18(4):600-606.
4. Drake GN, O’Connor DP, Edwards TB. Indications for reverse total shoulder arthroplasty in rotator cuff disease. Clin Orthop Relat Res. 2010;468(6):1526-1533.
5. Gerber C, Pennington SD, Nyffeler RW. Reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2009;17(5):284-289.
6. Lenarz C, Shishani Y, McCrum C, Nowinski RJ, Edwards TB, Gobezie R. Is reverse shoulder arthroplasty appropriate for the treatment of fractures in the older patient? Early observations. Clin Orthop Relat Res. 2011;469(12):3324-3331.
7. Muh SJ, Streit JJ, Wanner JP, et al. Early follow-up of reverse total shoulder arthroplasty in patients sixty years of age or younger. J Bone Joint Surg Am. 2013;95(20):1877-1883.
8. Wall B, Nové-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485.
9. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):1915-1923.
10. Boileau P, Melis B, Duperron D, Moineau G, Rumian AP, Han Y. Revision surgery of reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(10):1359-1370.
11. Beck JD, Irgit KS, Andreychik CM, Maloney PJ, Tang X, Harter GD. Reverse total shoulder arthroplasty in obese patients. J Hand Surg Am. 2013;38(5):965-970.
12. Gupta AK, Chalmers PN, Rahman Z, et al. Reverse total shoulder arthroplasty in patients of varying body mass index. J Shoulder Elbow Surg. 2014;23(1):35-42.
13. Hamada K, Fukuda H, Mikasa M, Kobayashi Y. Roentgenographic findings in massive rotator cuff tears. A long-term observation. Clin Orthop Relat Res. 1990;(254):92-96.
14. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
15. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons standardized shoulder assessment form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
16. Lo IK, Griffin S, Kirkley A. The development of a disease-specific quality of life measurement tool for osteoarthritis of the shoulder: the Western Ontario Osteoarthritis of the Shoulder (WOOS) index. Osteoarthritis Cartilage. 2001;9(8):771-778.
17. Williams GN, Gangel TJ, Arciero RA, Uhorchak JM, Taylor DC. Comparison of the Single Assessment Numeric Evaluation method and two shoulder rating scales. Outcomes measures after shoulder surgery. Am J Sports Med. 1999;27(2):214-221.
18. Gartsman GM, Edwards TB. Shoulder Arthroplasty. Philadelpia, PA: Saunders Elsevier; 2008.
19. Liotard JP, Edwards TB, Padey A, Walch G, Boulahia A. Hydrotherapy rehabilitation after shoulder surgery. Tech Shoulder Elbow Surg. 2003;4:44-49.
20. Trappey GJ 4th, O’Connor DP, Edwards TB. What are the instability and infection rates after reverse shoulder arthroplasty? Clin Orthop Relat Res. 2011;469(9):2505-2511.
21. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
22. Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis. 2006;6(7):438-446.
23. Schoenfeld AJ, Carey PA, Cleveland AW 3rd, Bader JO, Bono CM. Patient factors, comorbidities, and surgical characteristics that increase mortality and complication risk after spinal arthrodesis: a prognostic study based on 5,887 patients. Spine J. 2013;13(10):1171-1179.
24. Jiang J, Teng Y, Fan Z, Khan S, Xia Y. Does obesity affect the surgical outcome and complication rates of spinal surgery? A meta-analysis. Clin Orthop Relat Res. 2014;472(3):968-975.
25. Bozic KJ, Lau E, Kurtz S, et al. Patient-related risk factors for periprosthetic joint infection and postoperative mortality following total hip arthroplasty in Medicare patients. J Bone Joint Surg Am. 2012;94(9):794-800.
26. Franklin PD, Li W, Ayers DC. The Chitranjan Ranawat Award: functional outcome after total knee replacement varies with patient attributes. Clin Orthop Relat Res. 2008;466(11):2597-2604.
27. Huddleston JI, Wang Y, Uquillas C, Herndon JH, Maloney WJ. Age and obesity are risk factors for adverse events after total hip arthroplasty. Clin Orthop Relat Res. 2012;470(2):490-496.
28. Jämsen E, Nevalainen P, Eskelinen A, Huotari K, Kalliovalkama J, Moilanen T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am. 2012;94(14):e101.
29. Naziri Q, Issa K, Malkani AL, Bonutti PM, Harwin SF, Mont MA. Bariatric orthopaedics: total knee arthroplasty in super-obese patients (BMI > 50 kg/m2). Survivorship and complications. Clin Orthop Relat Res. 2013;471(11):3523-3530.
30. Warrender WJ, Brown OL, Abboud JA. Outcomes of arthroscopic rotator cuff repairs in obese patients. J Shoulder Elbow Surg. 2011;20(6):961-967.
31. Singh JA, Sperling JW, Cofield RH. Risk factors for revision surgery after humeral head replacement: 1,431 shoulders over 3 decades. J Shoulder Elbow Surg. 2012;21(8):1039-1044.
32. Chalmers PN, Rahman Z, Romeo AA, Nicholson GP. Early dislocation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):737-744.
33. Li X, Williams PN, Nguyen JT, Craig EV, Warren RF, Gulotta LV. Functional outcomes after total shoulder arthroplasty in obese patients. J Bone Joint Surg Am. 2013;95(21):e160.
Body mass index (BMI) is thought to be a predictor of body composition, with higher values indicating more adipose tissue. BMI is a measure of mass with respect to height. The World Health Organization1 has established health categories based on BMI measurements. Values from 18.5 to 24.9 kg/m2 are deemed to represent normal weight; those from 25 to 30 kg/m2, overweight; and those higher than 30 kg/m2, obesity. BMI is not a perfect tool, but it is the most widely used tool in clinical and research practice because of its relative reliability and ease of use.2 Being overweight or obese (according to BMI) is increasingly common among adults worldwide, and particularly in the United States. An estimated 39% of adults worldwide are overweight, and 13% are obese.1 An estimated 69% of US adults are overweight, including 35.1% who are obese.2
Various pathologies have been treated with reverse shoulder arthroplasty (RSA), and results have been promising,3-9 but little is known about patient demographic and clinical factors that may adversely affect outcomes. Recent work suggests younger age7 and failed prior arthroplasty may adversely affect RSA outcomes.10 Higher BMI has also been implicated as a cause of increased perioperative and immediate postoperative complications of RSA with minimum 90-day follow-up, but no one has examined shoulder function scores at minimum 2-year follow-up.11,12
We conducted a study to examine shoulder function scores, mobility, patient satisfaction, and complications at minimum 2-year follow-up in normal-weight, overweight, and obese patients who underwent RSA. We hypothesized that, compared with normal-weight patients, obese patients would have worse shoulder function scores, worse mobility, and more complications.
Materials and Methods
Inclusion Criteria and Demographics
After obtaining Institutional Review Board approval for this study, we used a prospective shoulder arthroplasty registry to identify patients (N = 77) who had rotator cuff tear arthropathy (RCTA) treated with primary RSA and then had minimum 2-year follow-up. The study period was 2004-2011. All patients had RCTA diagnosed with physical examination findings and anteroposterior, scapular Y, and axillary radiographs. RCTA was graded 1 to 5 using the classification system of Hamada and colleagues.13 Rotator cuff status was determined with preoperative computed tomography arthrogram (CTA) or magnetic resonance imaging (MRI) and confirmed at time of surgery. BMI calculations were based on height and weight measured at initial office visit. Thirty-four patients had normal weight (BMI <25 kg/m2), 21 were overweight (BMI 25-30 kg/m2), and 22 were obese (BMI >30 kg/m2). Patient demographic and clinical characteristics reviewed also included age, sex, follow-up duration, arm dominance, complications, prevalence of depression, and prevalence of diabetes. All RSAs were performed by the same surgeon (Dr. Edwards) at a single high-volume shoulder arthroplasty center.
Shoulder function scores evaluated before surgery and at final follow-up included Constant score,14 American Shoulder and Elbow Surgeons (ASES) score,15 Western Ontario Osteoarthritis Shoulder (WOOS) index,16 Single Assessment Numeric Evaluation (SANE),17 and mobility. Satisfaction was assessed by having patients describe themselves as very dissatisfied, dissatisfied, satisfied, or very satisfied. All intraoperative and postoperative complications were recorded.
Surgical Technique and Postoperative Rehabilitation
The Aequalis RSA system (Tornier) was used for all patients during the study period. The RSA technique used has been well described.18,19 A standard postoperative rehabilitation protocol was followed.19,20
Clinical and Radiographic Assessment
Patients were prospectively enrolled in a shoulder arthroplasty outcomes registry and followed clinically. Mean follow-up was 3.16 years (range, 2-8 years). Before surgery, patients were examined by the surgeon. Examinations were repeated 1 week, 6 weeks, 3 months, 6 months, and 12 months after surgery and annually thereafter. Mobility (active range of motion) was determined with a handheld goniometer. Strength of abduction was measured with a handheld digital dynamometer (Chatillon digital force gauge, 200 lbf; Ametek). Anteroposterior in plane of scapula, scapular Y, and axillary radiographs were obtained at each clinic appointment.
Before surgery, the surgeon reviewed all radiographs. Each RCTA was given a Hamada grade (1-5).13 Glenoid erosion in the coronal plane was classified (E0, E1, E2, E3) according to Sirveaux and colleagues.21 Hamada grades and glenoid erosion types are listed in Table 1. The overall trend in classification by BMI group was statistically significant for Hamada grade (P = .004) but not glenoid erosion type (P = .153).
Before surgery, the surgeon also evaluated rotator cuff status using CTA or MRI. All patients had full-thickness tears of the supraspinatus and infraspinatus. The subscapularis was variably present, and subscapularis repair was performed when the subscapularis was intact. Rotator cuff status is listed in Table 2. There were no significant differences in the distribution of intact subscapularis (P = .402) or teres minor (P = .188) among the normal-weight, overweight, and obese groups. No patient had a latissimus dorsi transfer at time of RSA.
Statistical Analysis
Independent-samples t tests assuming unequal variances were used to compare the 3 BMI groups on age, follow-up duration, preoperative shoulder function scores, and mobility. Chi-square tests were used to identify any significant group differences in comorbidities (eg, complications, arm dominance, prevalence of depression, prevalence of diabetes) and patient satisfaction. Repeated-measures analysis of variance was used to evaluate main effects, changes from before surgery to final follow-up, and BMI group differences, as well as differences in changes from before surgery to final follow-up among the 3 BMI groups.
Results
Among BMI groups (<25 kg/m2, 25-30 kg/m2, >30 kg/m2), there were no statistically significant preoperative differences in age, sex, follow-up duration, complications, arm dominance, prevalence of depression, or prevalence of diabetes (P >. 05) (Table 3). Table 4 lists the groups’ preoperative and final follow-up data (Constant score, ASES score, WOOS index, SANE, mobility). There were no statistically significant preoperative group differences in Constant score, ASES score, WOOS index, SANE, mobility, or patient satisfaction (P > .05) (Tables 5, 6).
All groups’ shoulder function scores and mobility improved significantly from before surgery to final follow-up (P < .001) (Table 5). The groups’ magnitudes of change (improvement) from before surgery to final follow-up were almost identical, with no significant differences in shoulder function scores or mobility (Table 5). The only significant difference was in Constant–Strength, which was higher in the obese group (P = .017) (Table 5). Patient satisfaction ratings improved after surgery, with 79% of the normal-weight group reporting being satisfied or very satisfied with their shoulders (Table 6). The overweight and obese groups gave similar satisfied (81%) and very satisfied (82%) ratings. The small differences between group satisfaction scores were nonsignificant (P = .967).
Complications
The normal-weight group had 4 complications: periprosthetic infection (2 cases), intraoperative humeral fracture (1), and scapular spine stress fracture (1). The overweight group had 1 complication, an acromial stress fracture that was successfully treated with conservative measures. The obese group had 1 patient with 2 postoperative dislocations. The first dislocation was treated with closed reduction and bracing, and the second required revision surgery. There was no statistical difference in complications among the groups (P = .680).
Discussion
To our knowledge, this is the first study of the effects of varying BMI on functional outcomes of RSA with minimum 2-year follow-up. There appears to be minimal impact on shoulder function scores, complications, and patient satisfaction among normal-weight, overweight, and obese patients with RCTA treated by the same surgeon using similar techniques.
The relationship between obesity and increased perioperative risks or poorer surgical outcomes has been well established in orthopedic surgery. In a systematic review, Falagas and Kompoti22 found increased risk for perioperative and nosocomial infections in obese patients. Schoenfeld and colleagues23 and Jiang and colleagues24 reported increased risk for complications in spinal surgery. The total joint arthroplasty literature is rife with evidence suggesting higher BMI leads to increased risk for complications, including infection and deep venous thrombosis, as well as decreased functional outcome scores.25-29 Recent studies on shoulder surgery have shown worse outcomes in rotator cuff repair30 and a higher revision rate in hemiarthroplasy.31
Other RSA studies have examined short-term complications or perioperative risk factors associated with BMI. In a study using slightly different BMI groupings, Gupta and colleagues12 reported significantly higher complication rates for RSA patients with BMI higher than 35 kg/m2 compared to patients with BMI of 25 to 35 kg/m2 and compared to patients with BMI lower than 25 kg/m2. Their study highlighted medical and surgical complications and used a short follow-up period (minimum, 90 days). It did not assess shoulder function scores, and included multiple indications for RSA (eg, RCTA, proximal humerus fracture, inflammatory arthropathy). In another study, higher BMI was reported as a risk factor for early dislocation after RSA, but only 11 patients with a history of dislocation were assessed, and minimum follow-up was 6 months.32 We know of only one study that addressed RSA outcomes in obese patients and used minimum 2-year follow-up, but its primary endpoint was rate of complications, and it did not report shoulder function scores.11 Li and colleagues33 conducted a study similar to ours, but with primary total shoulder arthroplasty (TSA) patients, and reported similar results. Relative to normal BMI, higher BMI did not have a detrimental effect on short-term improvement in shoulder function after TSA.
Given the US obesity epidemic, our study results are encouraging. Depending on many factors, obesity remains a risk factor for poor outcomes in patients who undergo orthopedic surgery. As our results show, however, patients with higher BMI can obtain functional outcomes similar to those experienced by patients with normal-weight BMI after RSA for RCTA.
The primary limitation of this study was its retrospective design. Strengths of the study included its having a single surgeon and a single diagnosis: RCTA. In addition, each group was robust in size, a standard operative/postoperative protocol was used, and clinical results were measured with multiple validated shoulder function scores.
Conclusion
Improved shoulder function scores, mobility, and patient satisfaction can be expected after RSA for RCTA in patients with BMI higher than 30 kg/m2. These patients did not exhibit an increase in complications at short-term follow-up.
Body mass index (BMI) is thought to be a predictor of body composition, with higher values indicating more adipose tissue. BMI is a measure of mass with respect to height. The World Health Organization1 has established health categories based on BMI measurements. Values from 18.5 to 24.9 kg/m2 are deemed to represent normal weight; those from 25 to 30 kg/m2, overweight; and those higher than 30 kg/m2, obesity. BMI is not a perfect tool, but it is the most widely used tool in clinical and research practice because of its relative reliability and ease of use.2 Being overweight or obese (according to BMI) is increasingly common among adults worldwide, and particularly in the United States. An estimated 39% of adults worldwide are overweight, and 13% are obese.1 An estimated 69% of US adults are overweight, including 35.1% who are obese.2
Various pathologies have been treated with reverse shoulder arthroplasty (RSA), and results have been promising,3-9 but little is known about patient demographic and clinical factors that may adversely affect outcomes. Recent work suggests younger age7 and failed prior arthroplasty may adversely affect RSA outcomes.10 Higher BMI has also been implicated as a cause of increased perioperative and immediate postoperative complications of RSA with minimum 90-day follow-up, but no one has examined shoulder function scores at minimum 2-year follow-up.11,12
We conducted a study to examine shoulder function scores, mobility, patient satisfaction, and complications at minimum 2-year follow-up in normal-weight, overweight, and obese patients who underwent RSA. We hypothesized that, compared with normal-weight patients, obese patients would have worse shoulder function scores, worse mobility, and more complications.
Materials and Methods
Inclusion Criteria and Demographics
After obtaining Institutional Review Board approval for this study, we used a prospective shoulder arthroplasty registry to identify patients (N = 77) who had rotator cuff tear arthropathy (RCTA) treated with primary RSA and then had minimum 2-year follow-up. The study period was 2004-2011. All patients had RCTA diagnosed with physical examination findings and anteroposterior, scapular Y, and axillary radiographs. RCTA was graded 1 to 5 using the classification system of Hamada and colleagues.13 Rotator cuff status was determined with preoperative computed tomography arthrogram (CTA) or magnetic resonance imaging (MRI) and confirmed at time of surgery. BMI calculations were based on height and weight measured at initial office visit. Thirty-four patients had normal weight (BMI <25 kg/m2), 21 were overweight (BMI 25-30 kg/m2), and 22 were obese (BMI >30 kg/m2). Patient demographic and clinical characteristics reviewed also included age, sex, follow-up duration, arm dominance, complications, prevalence of depression, and prevalence of diabetes. All RSAs were performed by the same surgeon (Dr. Edwards) at a single high-volume shoulder arthroplasty center.
Shoulder function scores evaluated before surgery and at final follow-up included Constant score,14 American Shoulder and Elbow Surgeons (ASES) score,15 Western Ontario Osteoarthritis Shoulder (WOOS) index,16 Single Assessment Numeric Evaluation (SANE),17 and mobility. Satisfaction was assessed by having patients describe themselves as very dissatisfied, dissatisfied, satisfied, or very satisfied. All intraoperative and postoperative complications were recorded.
Surgical Technique and Postoperative Rehabilitation
The Aequalis RSA system (Tornier) was used for all patients during the study period. The RSA technique used has been well described.18,19 A standard postoperative rehabilitation protocol was followed.19,20
Clinical and Radiographic Assessment
Patients were prospectively enrolled in a shoulder arthroplasty outcomes registry and followed clinically. Mean follow-up was 3.16 years (range, 2-8 years). Before surgery, patients were examined by the surgeon. Examinations were repeated 1 week, 6 weeks, 3 months, 6 months, and 12 months after surgery and annually thereafter. Mobility (active range of motion) was determined with a handheld goniometer. Strength of abduction was measured with a handheld digital dynamometer (Chatillon digital force gauge, 200 lbf; Ametek). Anteroposterior in plane of scapula, scapular Y, and axillary radiographs were obtained at each clinic appointment.
Before surgery, the surgeon reviewed all radiographs. Each RCTA was given a Hamada grade (1-5).13 Glenoid erosion in the coronal plane was classified (E0, E1, E2, E3) according to Sirveaux and colleagues.21 Hamada grades and glenoid erosion types are listed in Table 1. The overall trend in classification by BMI group was statistically significant for Hamada grade (P = .004) but not glenoid erosion type (P = .153).
Before surgery, the surgeon also evaluated rotator cuff status using CTA or MRI. All patients had full-thickness tears of the supraspinatus and infraspinatus. The subscapularis was variably present, and subscapularis repair was performed when the subscapularis was intact. Rotator cuff status is listed in Table 2. There were no significant differences in the distribution of intact subscapularis (P = .402) or teres minor (P = .188) among the normal-weight, overweight, and obese groups. No patient had a latissimus dorsi transfer at time of RSA.
Statistical Analysis
Independent-samples t tests assuming unequal variances were used to compare the 3 BMI groups on age, follow-up duration, preoperative shoulder function scores, and mobility. Chi-square tests were used to identify any significant group differences in comorbidities (eg, complications, arm dominance, prevalence of depression, prevalence of diabetes) and patient satisfaction. Repeated-measures analysis of variance was used to evaluate main effects, changes from before surgery to final follow-up, and BMI group differences, as well as differences in changes from before surgery to final follow-up among the 3 BMI groups.
Results
Among BMI groups (<25 kg/m2, 25-30 kg/m2, >30 kg/m2), there were no statistically significant preoperative differences in age, sex, follow-up duration, complications, arm dominance, prevalence of depression, or prevalence of diabetes (P >. 05) (Table 3). Table 4 lists the groups’ preoperative and final follow-up data (Constant score, ASES score, WOOS index, SANE, mobility). There were no statistically significant preoperative group differences in Constant score, ASES score, WOOS index, SANE, mobility, or patient satisfaction (P > .05) (Tables 5, 6).
All groups’ shoulder function scores and mobility improved significantly from before surgery to final follow-up (P < .001) (Table 5). The groups’ magnitudes of change (improvement) from before surgery to final follow-up were almost identical, with no significant differences in shoulder function scores or mobility (Table 5). The only significant difference was in Constant–Strength, which was higher in the obese group (P = .017) (Table 5). Patient satisfaction ratings improved after surgery, with 79% of the normal-weight group reporting being satisfied or very satisfied with their shoulders (Table 6). The overweight and obese groups gave similar satisfied (81%) and very satisfied (82%) ratings. The small differences between group satisfaction scores were nonsignificant (P = .967).
Complications
The normal-weight group had 4 complications: periprosthetic infection (2 cases), intraoperative humeral fracture (1), and scapular spine stress fracture (1). The overweight group had 1 complication, an acromial stress fracture that was successfully treated with conservative measures. The obese group had 1 patient with 2 postoperative dislocations. The first dislocation was treated with closed reduction and bracing, and the second required revision surgery. There was no statistical difference in complications among the groups (P = .680).
Discussion
To our knowledge, this is the first study of the effects of varying BMI on functional outcomes of RSA with minimum 2-year follow-up. There appears to be minimal impact on shoulder function scores, complications, and patient satisfaction among normal-weight, overweight, and obese patients with RCTA treated by the same surgeon using similar techniques.
The relationship between obesity and increased perioperative risks or poorer surgical outcomes has been well established in orthopedic surgery. In a systematic review, Falagas and Kompoti22 found increased risk for perioperative and nosocomial infections in obese patients. Schoenfeld and colleagues23 and Jiang and colleagues24 reported increased risk for complications in spinal surgery. The total joint arthroplasty literature is rife with evidence suggesting higher BMI leads to increased risk for complications, including infection and deep venous thrombosis, as well as decreased functional outcome scores.25-29 Recent studies on shoulder surgery have shown worse outcomes in rotator cuff repair30 and a higher revision rate in hemiarthroplasy.31
Other RSA studies have examined short-term complications or perioperative risk factors associated with BMI. In a study using slightly different BMI groupings, Gupta and colleagues12 reported significantly higher complication rates for RSA patients with BMI higher than 35 kg/m2 compared to patients with BMI of 25 to 35 kg/m2 and compared to patients with BMI lower than 25 kg/m2. Their study highlighted medical and surgical complications and used a short follow-up period (minimum, 90 days). It did not assess shoulder function scores, and included multiple indications for RSA (eg, RCTA, proximal humerus fracture, inflammatory arthropathy). In another study, higher BMI was reported as a risk factor for early dislocation after RSA, but only 11 patients with a history of dislocation were assessed, and minimum follow-up was 6 months.32 We know of only one study that addressed RSA outcomes in obese patients and used minimum 2-year follow-up, but its primary endpoint was rate of complications, and it did not report shoulder function scores.11 Li and colleagues33 conducted a study similar to ours, but with primary total shoulder arthroplasty (TSA) patients, and reported similar results. Relative to normal BMI, higher BMI did not have a detrimental effect on short-term improvement in shoulder function after TSA.
Given the US obesity epidemic, our study results are encouraging. Depending on many factors, obesity remains a risk factor for poor outcomes in patients who undergo orthopedic surgery. As our results show, however, patients with higher BMI can obtain functional outcomes similar to those experienced by patients with normal-weight BMI after RSA for RCTA.
The primary limitation of this study was its retrospective design. Strengths of the study included its having a single surgeon and a single diagnosis: RCTA. In addition, each group was robust in size, a standard operative/postoperative protocol was used, and clinical results were measured with multiple validated shoulder function scores.
Conclusion
Improved shoulder function scores, mobility, and patient satisfaction can be expected after RSA for RCTA in patients with BMI higher than 30 kg/m2. These patients did not exhibit an increase in complications at short-term follow-up.
1. World Health Organization. Obesity and overweight [factsheet 311]. Updated January 2015. http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed March 27, 2016.
2. National Center for Health Statistics, Centers for Disease Control and Prevention. Obesity and overweight. Updated February 25, 2016. http://www.cdc.gov/nchs/fastats/obesity-overweight.htm. Accessed March 27, 2016.
3. Boileau P, Gonzalez JF, Chuinard C, Bicknell R, Walch G. Reverse total shoulder arthroplasty after failed rotator cuff surgery. J Shoulder Elbow Surg. 2009;18(4):600-606.
4. Drake GN, O’Connor DP, Edwards TB. Indications for reverse total shoulder arthroplasty in rotator cuff disease. Clin Orthop Relat Res. 2010;468(6):1526-1533.
5. Gerber C, Pennington SD, Nyffeler RW. Reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2009;17(5):284-289.
6. Lenarz C, Shishani Y, McCrum C, Nowinski RJ, Edwards TB, Gobezie R. Is reverse shoulder arthroplasty appropriate for the treatment of fractures in the older patient? Early observations. Clin Orthop Relat Res. 2011;469(12):3324-3331.
7. Muh SJ, Streit JJ, Wanner JP, et al. Early follow-up of reverse total shoulder arthroplasty in patients sixty years of age or younger. J Bone Joint Surg Am. 2013;95(20):1877-1883.
8. Wall B, Nové-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485.
9. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):1915-1923.
10. Boileau P, Melis B, Duperron D, Moineau G, Rumian AP, Han Y. Revision surgery of reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(10):1359-1370.
11. Beck JD, Irgit KS, Andreychik CM, Maloney PJ, Tang X, Harter GD. Reverse total shoulder arthroplasty in obese patients. J Hand Surg Am. 2013;38(5):965-970.
12. Gupta AK, Chalmers PN, Rahman Z, et al. Reverse total shoulder arthroplasty in patients of varying body mass index. J Shoulder Elbow Surg. 2014;23(1):35-42.
13. Hamada K, Fukuda H, Mikasa M, Kobayashi Y. Roentgenographic findings in massive rotator cuff tears. A long-term observation. Clin Orthop Relat Res. 1990;(254):92-96.
14. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
15. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons standardized shoulder assessment form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
16. Lo IK, Griffin S, Kirkley A. The development of a disease-specific quality of life measurement tool for osteoarthritis of the shoulder: the Western Ontario Osteoarthritis of the Shoulder (WOOS) index. Osteoarthritis Cartilage. 2001;9(8):771-778.
17. Williams GN, Gangel TJ, Arciero RA, Uhorchak JM, Taylor DC. Comparison of the Single Assessment Numeric Evaluation method and two shoulder rating scales. Outcomes measures after shoulder surgery. Am J Sports Med. 1999;27(2):214-221.
18. Gartsman GM, Edwards TB. Shoulder Arthroplasty. Philadelpia, PA: Saunders Elsevier; 2008.
19. Liotard JP, Edwards TB, Padey A, Walch G, Boulahia A. Hydrotherapy rehabilitation after shoulder surgery. Tech Shoulder Elbow Surg. 2003;4:44-49.
20. Trappey GJ 4th, O’Connor DP, Edwards TB. What are the instability and infection rates after reverse shoulder arthroplasty? Clin Orthop Relat Res. 2011;469(9):2505-2511.
21. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
22. Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis. 2006;6(7):438-446.
23. Schoenfeld AJ, Carey PA, Cleveland AW 3rd, Bader JO, Bono CM. Patient factors, comorbidities, and surgical characteristics that increase mortality and complication risk after spinal arthrodesis: a prognostic study based on 5,887 patients. Spine J. 2013;13(10):1171-1179.
24. Jiang J, Teng Y, Fan Z, Khan S, Xia Y. Does obesity affect the surgical outcome and complication rates of spinal surgery? A meta-analysis. Clin Orthop Relat Res. 2014;472(3):968-975.
25. Bozic KJ, Lau E, Kurtz S, et al. Patient-related risk factors for periprosthetic joint infection and postoperative mortality following total hip arthroplasty in Medicare patients. J Bone Joint Surg Am. 2012;94(9):794-800.
26. Franklin PD, Li W, Ayers DC. The Chitranjan Ranawat Award: functional outcome after total knee replacement varies with patient attributes. Clin Orthop Relat Res. 2008;466(11):2597-2604.
27. Huddleston JI, Wang Y, Uquillas C, Herndon JH, Maloney WJ. Age and obesity are risk factors for adverse events after total hip arthroplasty. Clin Orthop Relat Res. 2012;470(2):490-496.
28. Jämsen E, Nevalainen P, Eskelinen A, Huotari K, Kalliovalkama J, Moilanen T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am. 2012;94(14):e101.
29. Naziri Q, Issa K, Malkani AL, Bonutti PM, Harwin SF, Mont MA. Bariatric orthopaedics: total knee arthroplasty in super-obese patients (BMI > 50 kg/m2). Survivorship and complications. Clin Orthop Relat Res. 2013;471(11):3523-3530.
30. Warrender WJ, Brown OL, Abboud JA. Outcomes of arthroscopic rotator cuff repairs in obese patients. J Shoulder Elbow Surg. 2011;20(6):961-967.
31. Singh JA, Sperling JW, Cofield RH. Risk factors for revision surgery after humeral head replacement: 1,431 shoulders over 3 decades. J Shoulder Elbow Surg. 2012;21(8):1039-1044.
32. Chalmers PN, Rahman Z, Romeo AA, Nicholson GP. Early dislocation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):737-744.
33. Li X, Williams PN, Nguyen JT, Craig EV, Warren RF, Gulotta LV. Functional outcomes after total shoulder arthroplasty in obese patients. J Bone Joint Surg Am. 2013;95(21):e160.
1. World Health Organization. Obesity and overweight [factsheet 311]. Updated January 2015. http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed March 27, 2016.
2. National Center for Health Statistics, Centers for Disease Control and Prevention. Obesity and overweight. Updated February 25, 2016. http://www.cdc.gov/nchs/fastats/obesity-overweight.htm. Accessed March 27, 2016.
3. Boileau P, Gonzalez JF, Chuinard C, Bicknell R, Walch G. Reverse total shoulder arthroplasty after failed rotator cuff surgery. J Shoulder Elbow Surg. 2009;18(4):600-606.
4. Drake GN, O’Connor DP, Edwards TB. Indications for reverse total shoulder arthroplasty in rotator cuff disease. Clin Orthop Relat Res. 2010;468(6):1526-1533.
5. Gerber C, Pennington SD, Nyffeler RW. Reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2009;17(5):284-289.
6. Lenarz C, Shishani Y, McCrum C, Nowinski RJ, Edwards TB, Gobezie R. Is reverse shoulder arthroplasty appropriate for the treatment of fractures in the older patient? Early observations. Clin Orthop Relat Res. 2011;469(12):3324-3331.
7. Muh SJ, Streit JJ, Wanner JP, et al. Early follow-up of reverse total shoulder arthroplasty in patients sixty years of age or younger. J Bone Joint Surg Am. 2013;95(20):1877-1883.
8. Wall B, Nové-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485.
9. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):1915-1923.
10. Boileau P, Melis B, Duperron D, Moineau G, Rumian AP, Han Y. Revision surgery of reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(10):1359-1370.
11. Beck JD, Irgit KS, Andreychik CM, Maloney PJ, Tang X, Harter GD. Reverse total shoulder arthroplasty in obese patients. J Hand Surg Am. 2013;38(5):965-970.
12. Gupta AK, Chalmers PN, Rahman Z, et al. Reverse total shoulder arthroplasty in patients of varying body mass index. J Shoulder Elbow Surg. 2014;23(1):35-42.
13. Hamada K, Fukuda H, Mikasa M, Kobayashi Y. Roentgenographic findings in massive rotator cuff tears. A long-term observation. Clin Orthop Relat Res. 1990;(254):92-96.
14. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
15. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons standardized shoulder assessment form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
16. Lo IK, Griffin S, Kirkley A. The development of a disease-specific quality of life measurement tool for osteoarthritis of the shoulder: the Western Ontario Osteoarthritis of the Shoulder (WOOS) index. Osteoarthritis Cartilage. 2001;9(8):771-778.
17. Williams GN, Gangel TJ, Arciero RA, Uhorchak JM, Taylor DC. Comparison of the Single Assessment Numeric Evaluation method and two shoulder rating scales. Outcomes measures after shoulder surgery. Am J Sports Med. 1999;27(2):214-221.
18. Gartsman GM, Edwards TB. Shoulder Arthroplasty. Philadelpia, PA: Saunders Elsevier; 2008.
19. Liotard JP, Edwards TB, Padey A, Walch G, Boulahia A. Hydrotherapy rehabilitation after shoulder surgery. Tech Shoulder Elbow Surg. 2003;4:44-49.
20. Trappey GJ 4th, O’Connor DP, Edwards TB. What are the instability and infection rates after reverse shoulder arthroplasty? Clin Orthop Relat Res. 2011;469(9):2505-2511.
21. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
22. Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis. 2006;6(7):438-446.
23. Schoenfeld AJ, Carey PA, Cleveland AW 3rd, Bader JO, Bono CM. Patient factors, comorbidities, and surgical characteristics that increase mortality and complication risk after spinal arthrodesis: a prognostic study based on 5,887 patients. Spine J. 2013;13(10):1171-1179.
24. Jiang J, Teng Y, Fan Z, Khan S, Xia Y. Does obesity affect the surgical outcome and complication rates of spinal surgery? A meta-analysis. Clin Orthop Relat Res. 2014;472(3):968-975.
25. Bozic KJ, Lau E, Kurtz S, et al. Patient-related risk factors for periprosthetic joint infection and postoperative mortality following total hip arthroplasty in Medicare patients. J Bone Joint Surg Am. 2012;94(9):794-800.
26. Franklin PD, Li W, Ayers DC. The Chitranjan Ranawat Award: functional outcome after total knee replacement varies with patient attributes. Clin Orthop Relat Res. 2008;466(11):2597-2604.
27. Huddleston JI, Wang Y, Uquillas C, Herndon JH, Maloney WJ. Age and obesity are risk factors for adverse events after total hip arthroplasty. Clin Orthop Relat Res. 2012;470(2):490-496.
28. Jämsen E, Nevalainen P, Eskelinen A, Huotari K, Kalliovalkama J, Moilanen T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am. 2012;94(14):e101.
29. Naziri Q, Issa K, Malkani AL, Bonutti PM, Harwin SF, Mont MA. Bariatric orthopaedics: total knee arthroplasty in super-obese patients (BMI > 50 kg/m2). Survivorship and complications. Clin Orthop Relat Res. 2013;471(11):3523-3530.
30. Warrender WJ, Brown OL, Abboud JA. Outcomes of arthroscopic rotator cuff repairs in obese patients. J Shoulder Elbow Surg. 2011;20(6):961-967.
31. Singh JA, Sperling JW, Cofield RH. Risk factors for revision surgery after humeral head replacement: 1,431 shoulders over 3 decades. J Shoulder Elbow Surg. 2012;21(8):1039-1044.
32. Chalmers PN, Rahman Z, Romeo AA, Nicholson GP. Early dislocation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):737-744.
33. Li X, Williams PN, Nguyen JT, Craig EV, Warren RF, Gulotta LV. Functional outcomes after total shoulder arthroplasty in obese patients. J Bone Joint Surg Am. 2013;95(21):e160.
Use of an Anti-Gravity Treadmill for Early Postoperative Rehabilitation After Total Knee Replacement: A Pilot Study to Determine Safety and Feasibility
Patients undergoing total knee arthroplasty (TKA) may benefit from focused postoperative rehabilitation. Although there is limited research comparing different rehabilitation protocols after TKA,1 any type of rehabilitation often helps to optimize range of motion (ROM), strength, balance, and ambulation.2 Early mobilization and rehabilitation after TKA reduces pain, fear, anxiety, and risk of postoperative venous thromboembolic disease.3 Earlier discharge to home or community settings deceases time for inpatient rehabilitation, patient and family education, and gait training, which places a greater emphasis on outpatient rehabilitation.4
Although rapid rehabilitation protocols have gained wide acceptance, concern remains that a higher intensity intervention initiated immediately after hospital discharge could lead to an increased incidence of pain and swelling, and to poorer ROM and functional outcomes.5 Progressive weight-bearing activities, such as walking, are routinely recommended during rehabilitation to facilitate return to normal function. Not all patients are capable of full weight-bearing activity in the early postoperative period and assistive devices (ADs), such as walkers, crutches, and canes, are routinely employed. An opportunity to enhance early TKA rehabilitation exists with devices that allow functional gait training while modifying weight-bearing forces across the joint. Assistive devices, hydrotherapy (walking in water),6,7 and lower body positive-pressure chambers8 can reduce the forces at the knee during weight-bearing exercise.
Lower body positive-pressure devices have been extensively studied in physiological response of healthy humans;9-12 in disease states such as cerebral palsy13 and obesity;14 and in other postoperative orthopedic conditions, such as anterior cruciate ligament reconstruction, meniscectomy,8 microfracture,15 TKA,16 and Achilles tendon repair.17 These studies demonstrate that a lower body positive-pressure treadmill is associated with minimal cardiovascular effect while producing a significant decrease in ground reaction forces without altering gait kinematics.
We postulated that an anti-gravity treadmill may be safe and effective for gait training during rehabilitation following TKA. The primary objective was to determine the safety and feasibility of using the AlterG® Anti-Gravity Treadmill® device for postoperative gait training during rehabilitation following TKA. The secondary objective was to determine the effects of gait training (land-based vs anti-gravity) during postoperative rehabilitation on subjective patient outcomes assessed by Knee Injury and Osteoarthritis Outcome Score (KOOS), mobility assessed by the Timed Up and Go test (TUG), and pain assessed by a Numerical Rating Scale (NRS) to conduct a power analysis to determine sample sizes for efficacy studies based on these preliminary findings.
MethodsParticipants/Patient Enrollment and Study Overview
After signing an Institutional Review Board-approved consent, 30 patients were enrolled, and TKA surgeries were performed by 1 of 5 surgeons at 1 hospital. To be enrolled in the study, subjects must have (1) had a unilateral primary TKA, (2) been discharged from the hospital to home (not to a skilled nursing facility), (3) had only 3 to 4 home physical therapy (PT) sessions, (4) agreed to further outpatient PT at a single site, and (5) agreed to complete patient questionnaires. Exclusion criteria included (1) inability to meet inclusion criteria, (2) gross musculoskeletal deformity, (3) uncontrolled chronic or systemic disease, and (4) inability to follow instructions because of mental impairment, substance abuse, or addiction. Home PT was conducted for 3 to 4 sessions after surgery, and outpatient PT was continued at the study site per protocol for 4 weeks; subjects were asked to return for follow-up 3 months postoperatively. Patients were randomized on the first day of their outpatient PT to either a land-based (control) or an anti-gravity-based group using the AlterG Anti-Gravity Treadmill (AlterG group) gait training during outpatient PT sessions. Patients attended outpatient PT 2 days per week for 4 weeks for a total of 8 sessions. Therapy sessions lasted 45 to 60 minutes and included manual therapy, gait training, and therapeutic exercises/activities. The KOOS18,19 and TUG20 scores were evaluated at baseline (ie, first therapy session), end of physical therapy (EOPT) (ie, at final therapy session), and end of study (EOS) (ie, 3 months postoperatively). The NRS for pain was evaluated at baseline and at EOPT. Physical therapists were questioned for satisfaction with the anti-gravity rehabilitation protocol at EOPT.
Physical Therapy Protocols
All patients were treated consistently by 1 of 5 physical therapists at 1 outpatient setting; physical therapists averaged 11 years of experience in treating orthopedic conditions. Care was delivered in accordance with professional standards and the therapist’s assessment of medical necessity. Considerations included, but were not limited to, overall general health, any medical comorbidity, support system, and an ongoing assessment of ROM, strength, pain, and functional status. Each PT session started with a 5- to 10-minute warm-up on a standard cycle ergometer and was followed by manual therapy, gait training (land-based vs anti-gravity), therapeutic exercises/activities, and treatment modalities.
The time spent, activities selected, and modalities or physical agents chosen during the PT session were based on the patient’s needs and progress toward his/her functional goals. Manual therapy techniques consisted of soft-tissue mobilization, passive ROM, joint mobilization, passive stretching, scar mobilization, manual resistive exercises, and proprioceptive neuromuscular facilitation techniques. Therapeutic exercises/activities consisted of lower extremity resistance exercises (weight bearing and non-weight bearing), ROM exercises, stretching, balance, stair training, agility, activities of daily life (ADL) training, and a comprehensive home exercise program. Modalities or physical agents used during this study included moist hot packs, cold packs, ultrasound, electrical stimulation, and Kinesio Tape. Physical agents were incorporated into the individual’s plan of care based on medical necessity when deemed appropriate by the treating therapist. The exercise prescription was based on an individual’s status and tolerance and the number of sets and repetitions were based on fatigue.
Gait Training
The patients were randomized (1:1) to either land-based or anti-gravity gait training. For the control group, land-based gait training was performed with or without an appropriate AD and appropriate assistance, tactile cueing, and verbal cueing from a physical therapist. Duration (minutes) and gait-training progression were dependent on the participant’s functional goals, pain level (assessed throughout treatment), and level of fatigue. For the AlterG group, gait training was performed in the AlterG Anti-Gravity Treadmill, M320 (Alter-G; Figure 1). On day 1, the AlterG pressure chamber was set to allow only 50% of the patient’s body weight to be transmitted to the treadmill floor, and speed was controlled by the patient according to his/her comfort level. The percentage of body weight was adjusted to allow for a safe and normalized gait pattern with a pain level no greater than 5 (0 to 10 scale) throughout the PT session. A report card was recorded at each PT session, including body-weight setting (%), speed (miles per hour), incline (%), and duration (minutes) (Figure 2). For subsequent visits, the body-weight setting was started from the end point of the previous session.
Data Collection and Analysis
SPSS version 12.0 (SPSS Inc.) was used for all analyses, and an alpha level of .05 determined statistical significance when comparing group differences. The safety and feasibility of the anti-gravity (AlterG) vs land-based (control) gait training was assessed by the presence (or absence) of adverse events (AEs) and complications, and the date the patient discontinued use of his/her AD. A chi-square test was used to assess differences between control and AlterG groups regarding patient discontinuance of an AD. Additionally, for patients randomized to AlterG, a report card summarized means and frequencies for body weight, speed, incline, and duration. At EOPT, the frequency of therapists who were satisfied with the AlterG Anti-Gravity Treadmill as part of the rehabilitation protocol was reported. The preliminary effects of gait training (land-based vs anti-gravity) during postoperative rehabilitation on functional outcomes (subjective patient outcomes assessed by KOOS, mobility assessed by the TUG test, and pain assessed by a NRS) were evaluated by independent sample t tests. Paired sample t tests were used to compare each of the functional outcomes at EOPT or EOS to the baseline value.
Results
Of the 30 patients enrolled, 29 (96.7%; 29/30) patients completed the study; 1 patient, who could not complete all PT sessions because of medical and transportation issues, was excluded. The remaining 29 patients comprised the study population (control = 15; AlterG = 14). All patients were compliant with PT protocols.
Patient demographics were similar between the control and AlterG groups (Table 1). The control group comprised 9 women (60%; 9/15) and 6 men (40%; 6/15), age 69.9 ± 7.8 years and a body mass index of 28.8 ± 4.2. Similarly, the AlterG group comprised 7 women (50%; 7/14) and 7 men (50%; 7/14), age 66.5 ± 7.8 years and a body mass index of 28.4 ± 5.2.
At the baseline PT visit, patients in the control and AlterG groups had similar KOOS, TUG, and NRS scores. At baseline, mean KOOS for symptoms, pain, sports/recreation, ADL, and quality of life were 52.7, 52.9, 22.7, 64, and 31.8, respectively, although 50% of patients did not complete the sports/recreation subset of the KOOS. In addition, the mean time to complete the TUG test was 14.5 seconds, which was within the normal limits for disabled patients. This was slightly longer than normal mobility (TUG <10 seconds),20 but patients had relatively low levels of pain (mean NRS = 2.5, on a scale of 0-11).
All patients completed the PT protocols without indication of injury or AEs related to their operative knee. Three patients (10.3%; 3/29) experienced a deep venous thromboembolism (DVT), 2 in the control group (13.3%; 2/15), and 1 in the AlterG group (7.1%; 1/14). Venous thromboembolism protocol of enoxaparin 30 mg twice daily while in the hospital and enoxaparin 40 mg once daily for 10 days after discharge was followed for all patients.
Overall, more than half of patients (55.2%; 16/29) discontinued their AD during the 4-week PT period, with the remaining discontinuing prior to EOPT (24.1%; 7/29) or after EOPT (20.7%; 6/29). No statistically significant differences were found between the control and AlterG groups regarding discontinuance of AD.
Among those randomized to the AlterG group, all patients performed within the protocol established for the device for body-weight setting, treadmill speed, and duration of walking. The average body-weight treadmill setting increased by ~30% over the treatment period, from 55% at baseline to 84% at EOPT. The average speed increased by ~70%, from 1.6 mph at baseline to 2.7 mph at EOPT. The mean duration of AlterG use increased by ~75%, from 7.2 minutes at baseline to 12.7 minutes at EOPT. All physical therapists (100%) reported satisfaction with the AlterG for use in early postoperative rehabilitation and reported that patients’ treatment progressed positively.
While functional outcomes (KOOS, TUG, or NRS) did not vary with the type of gait training (P > .2 for land-based vs anti-gravity), functional outcomes improved over time (all P < .01 from baseline to EOPT and all P < .01 from baseline to EOS).
The KOOS scores improved from baseline to EOPT and from baseline to EOS (ie, 3-month follow-up visit) for both treatment groups (Figure 3). More patients completed the sports/recreation portion of the KOOS scores at EOPT and EOS compared to baseline. Forty-three percent and 25% of patients did not complete KOOS sports/recreation questions at EOPT and EOS, respectively, compared to 50% at baseline. This suggests that patients were improving to a level where sports/recreation scores were more applicable than directly after TKA surgery. The TUG scores had the greatest improvement from baseline to EOPT, with a decrease in time of 5 seconds and 7 seconds for the control and AlterG groups, respectively, and slight improvement from EOPT to EOS, with a decrease in time of 1 second and 2 seconds for the control and AlterG groups, respectively (Table 2). By the EOS, the values for the TUG tests for both treatment groups were within normal (<10 seconds) range.20 The NRS scores improved from baseline to EOPT with a score of 1 ± 1 in both control and AlterG groups.
Using these preliminary efficacy results, a post-hoc power analysis (α = .05 and 1β = 80%) was performed with the ADL domain of KOOS as the primary endpoint. Based on a standard deviation of 20 points and an effect size of 5 points, the sample size was estimated to be N = 250 per treatment group.
Discussion
We conducted a pilot study to assess, primarily, the feasibility and safety, and, secondarily, the efficacy, of a lower body positive-pressure treadmill for rehabilitation of patients after TKA. This small study showed that use of the AlterG Anti-Gravity Treadmill was not only safe and feasible during postoperative TKA rehabilitation, but also was well tolerated by patients and was rated highly satisfactory by physical therapists. Patients who used AlterG during gait training improved functionally (in terms of KOOS, TUG, and NRS) after 8 treatment sessions compared to baseline. However, there were no statistical differences between groups (control vs AlterG). Thus, these results suggest that an anti-gravity device for gait training may be a useful adjunct for postoperative TKA rehabilitation, but further studies are needed to determine the efficacy of anti-gravity compared to traditional land-based gait training.
The study of rehabilitation protocols during postoperative PT involved consideration of a number of issues. First, differences in functional outcomes compared to traditional rehabilitation could not be detected in this study because of the small number of patients, but the patients treated with anti-gravity gait training showed improvement in functional outcomes over time and did not report any added complications. Given that the primary outcome of this study was safety and feasibility, these added efficacy results are supplemental and useful in helping to plan studies. Second, the functional outcomes used to measure the efficacy of the anti-gravity treadmill may not be sensitive enough to detect differences between rehabilitation protocols. Use of a treadmill to measure speed improvement, endurance, and tolerance in both groups could be valuable in future studies. More studies may need to refine characteristics that are important to postoperative rehabilitation success, and quantitative and subjective measures that must be defined.
The results reported here using an anti-gravity treadmill for postoperative TKA rehabilitation support the safety and feasibility that has been reported in other orthopedic rehabilitation settings. Anti-gravity treadmills, which have been used to study patients after meniscectomy or anterior cruciate ligament reconstruction8 and Achilles repair,17 have demonstrated predictable decreases in ground reaction forces with increasing positive-pressure unweighting, reductions in pain with ambulation, and allowance of earlier institution of walking and jogging during rehabilitation.17
Patient safety is an important attribute for any postoperative rehabilitation protocol, especially in an elderly population undergoing major surgery. One of our important goals was to assess the safety of AlterG. We noted no AEs attributable to the device, which was supported by work indicating no adverse impact on systemic cardiovascular parameters in a similar lower body positive-pressure environment.9 Although 3 patients (10%) developed symptomatic DVT, there were no differences between the groups in the incidence of DVT. Use of an anti-gravity treadmill has also been examined for cardiovascular responses in TKA patients. In a study of 24 adults with TKA, researchers found that anti-gravity support allowed TKA patients to walk at faster speeds and tolerate greater inclines with lower heart rate, blood pressure, and oxygen consumption.21 With respect to efficacy of the rehabilitation intervention, we demonstrated significant improvements in all functional outcomes in both groups but no differences between the study groups. We concluded that AlterG was at least as effective as standard therapy in this small cohort. TKA is a very successful procedure, and the improvement in pain and function after surgery is fairly dramatic in most patients, regardless of specific rehabilitation protocols. Therefore, the substantial improvement in clinical outcomes may overshadow any enhanced benefits of the anti-gravity treadmill. Further investigations into the efficacy of AlterG are needed in a larger cohort to determine if this type of treatment is more beneficial than traditional land-based gait training.
Standard scoring systems such as KOOS, TUG, and NRS may not be sensitive enough to detect differences between treatment groups with small sample sizes. Given the results of the post hoc power analysis, a large number of patients (N = 250/group) would be necessary to detect any potential difference in clinical outcomes between the 2 groups. Larger studies are required to answer relevant questions, and additional outcome measures may be needed to detect differences between treatment groups. Relevant questions include whether earlier institution of the anti-gravity device during the immediate TKA postoperative period would be beneficial compared to standard postoperative PT, and whether PT enhanced with the anti-gravity device has incremental benefit in functional outcomes and in time to reach those goals. Finally, given the present attention to healthcare expenses, a cost-benefit analysis of anti-gravity device treatment vs traditional PT would be useful. Once the patient has become familiar with the function of an anti-gravity treadmill, gait therapy could proceed without the direct intervention of the therapist, potentially improving efficient delivery of rehabilitation services.
Studying the effect of different postoperative rehabilitation protocols after orthopedic surgeries can be challenging. In a large (N > 350) randomized controlled trial to study the effect of ergometer cycling after hip and knee replacement, patients who used the cycle ergometer had a higher Western Ontario and McMaster Universities Arthritis Index and greater satisfaction than those who did not after hip arthroplasty, but not after TKA.22 Improvements in muscular coordination and proprioception with the cycle ergometer may have been offset by increases in edema, joint effusion, and pain from the loading of the joint and the relatively fast rate of cycling compared to passive motion or ambulation. While many therapists and surgeons advocate cycling for rehabilitation after knee surgery, the need remains for a better definition of an optimal TKA rehabilitation program. A study of 82 patients comparing early progressive strength training to no early strength training showed no difference in the 6-minute walk test at 8 weeks.23 A systematic review of progressive resistance training (PRT) found that although postoperative PRT is safe and feasible, the methodological quality of existing studies is too low to allow conclusions regarding its efficacy.24 Gait training in an environment where weight-bearing loads can be closely controlled, monitored, and individualized may be an ideal methodology to enhance rehabilitation and return to function for knee replacement surgery.
This current study showed that the use of AlterG as an adjunct for postoperative rehabilitation is safe, accepted by patients and therapists, and leads to clinical functional outcomes that are at least as good as traditional postoperative TKA rehabilitation. We conclude that AlterG demonstrates utility and a potential for innovation in TKA rehabilitation.
1. NIH Consensus Statement on total knee arthroplasty. NIH Consensus State Sci Statements. 2003;20(1):1-34.
2. Jones CA, Voaklander DC, Suarez-Almazor ME. Determinants of function after total knee arthroplasty. Phys Ther. 2003;83(8):696-706.
3. Pearse EO, Caldwell BF, Lockwood RJ, Hollard J. Early mobilisation after conventional knee replacement may reduce the risk of post-operative venous thromboembolism. J Bone Joint Surg Br. 2007;89(3):316-322.
4. Westby MD, Kennedy D, Jones D, Jones A, Doyle-Waters MM, Backman C. Post-acute physiotherapy for primary total knee arthroplasty. Cochrane Database Syst Rev. 2008. doi.10.1002/14651858.CD007099
5. Bade MJ, Stevens-Lapsley JE. Early high-intensity rehabilitation following total knee arthroplasty improves outcomes. J Orthop Sports Phys Ther. 2011;41(12):932-941.
6. Ivanenko YP, Grasso R, Macellari V, Lacquaniti F. Control of foot trajectory in human locomotion: role of ground contact forces in simulated reduced gravity. J Neurophysiol. 2002;87(6):3070-3089.
7. Pöyhönen T, Keskinen KL, Kyröläinen H, Hautala A, Savolainen J, Mälkiä E. Neuromuscular function during therapeutic knee exercise under water and on dry land. Arch Phys Med Rehabil. 2001;82(10):1446-1452.
8. Eastlack RK, Hargens AR, Groppo ER, Steinbach GC, White KK, Pedowitz RA. Lower body positive-pressure exercise after knee surgery. Clin Orthop Rel Res. 2005;431:213-219.
9. Cutuk A, Groppo ER, Quigley EJ, White KW, Pedowitz RA, Hargens AR. Ambulation in simulated fractional gravity using lower body positive pressure: cardiovascular safety and gait analyses. J Appl Physiol. 2006;101(3):771-777.
10. Gojanovic B, Cutti P, Shultz R, Matheson GO. Maximal physiological parameters during partial body-weight support treadmill testing. Med Sci Sports Exerc. 2012;44(10):1935-1941.
11. Figueroa MA, Manning J, Escamilla P. Physiological responses to the AlterG Anti-Gravity Treadmill. Int J Applied Sci Tech. 2011;1:92-97.
12. Hoffman MD, Donaghe HE. Physiological responses to body weight-supported treadmill exercise in healthy adults. Arch Phys Med Rehabil. 2011;92(6):960-966.
13. Kurz MJ, Corr B, Stuberg W, Volkman KG, Smith N. Evaluation of lower body positive pressure supported treadmill training for children with cerebral palsy. Pediatr Phys Ther. 2011;23(3):232-239.
14. Christian M. Managing knee osteoarthritis: the effects of anti-gravity treadmill exercise on joint pain and physical function. Available at: http://mspace.lib.umanitoba.ca/handle/1993/8580. Accessed March 31, 2016.
15. Wilk KE, Macrina LC, Reinhold MM. Rehabilitation following microfracture of the knee. Cartilage. 2010;1(2):96-107.
16. Patil SS, Branovacki G, Martin MR, Pulido PA, Levy YD, Colwell CW Jr. 14-year median follow-up using the press-fit condylar sigma design for total knee arthroplasty. J Arthroplasty. 2013;28(8):1286-1290.
17. Saxena A, Granot A. Use of an anti-gravity treadmill in the rehabilitation of the operated achilles tendon: a pilot study. J Foot Ankle Surg. 2011;50(5):558-561.
18. Roos EM, Roos HP, Ekdahl C, Lohmander LS. Knee injury and Osteoarthritis Outcome Score (KOOS) - validation of a Swedish version. Scand J Med Sci Sports. 1998;8(6):439-448.
19. Roos EM, Toksvig-Larsen S. Knee injury and Osteoarthritis Outcome Score (KOOS) - validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. 2003;1:17.
20. Timed Up and Go (TUG). Available at: http://www.rheumatology.org/I-Am-A/Rheumatologist/Research/Clinician-Researchers/Timed-Up-Go-TUG Accessed: March 15, 2016.
21. Webber SC, Horvey KJ, Yurach Pikaluk MT, Butcher SJ. Cardiovascular responses in older adults with total knee arthroplasty at rest and with exercise on a positive pressure treadmill. Eur J Appl Physiol. 2014;114(3):653-662.
22. Liebs TR, Herzberg W, Ruther W, Haasters J, Russlies M, Hassenpflug J. Ergometer cycling after hip and knee replacement surgery: a randomized control trial. J Bone Joint Surg Am. 2010;92(4):814-822.
23. Jakobsen TL, Kehlet H, Husted H, Petersen J, Bandholm T. Early progressive strength training to enhance recovery after fast-track total knee arthroplasty: a randomized controlled trial. Arthritis Care Res. 2014;66(12):1856-1866.
24. Skoffer B, Dalgas U, Mechlenburg I. Progressive resistance training before and after total hip and knee arthroplasty: a systematic review. Clin Rehabil. 2015;29(1):14-29.
Patients undergoing total knee arthroplasty (TKA) may benefit from focused postoperative rehabilitation. Although there is limited research comparing different rehabilitation protocols after TKA,1 any type of rehabilitation often helps to optimize range of motion (ROM), strength, balance, and ambulation.2 Early mobilization and rehabilitation after TKA reduces pain, fear, anxiety, and risk of postoperative venous thromboembolic disease.3 Earlier discharge to home or community settings deceases time for inpatient rehabilitation, patient and family education, and gait training, which places a greater emphasis on outpatient rehabilitation.4
Although rapid rehabilitation protocols have gained wide acceptance, concern remains that a higher intensity intervention initiated immediately after hospital discharge could lead to an increased incidence of pain and swelling, and to poorer ROM and functional outcomes.5 Progressive weight-bearing activities, such as walking, are routinely recommended during rehabilitation to facilitate return to normal function. Not all patients are capable of full weight-bearing activity in the early postoperative period and assistive devices (ADs), such as walkers, crutches, and canes, are routinely employed. An opportunity to enhance early TKA rehabilitation exists with devices that allow functional gait training while modifying weight-bearing forces across the joint. Assistive devices, hydrotherapy (walking in water),6,7 and lower body positive-pressure chambers8 can reduce the forces at the knee during weight-bearing exercise.
Lower body positive-pressure devices have been extensively studied in physiological response of healthy humans;9-12 in disease states such as cerebral palsy13 and obesity;14 and in other postoperative orthopedic conditions, such as anterior cruciate ligament reconstruction, meniscectomy,8 microfracture,15 TKA,16 and Achilles tendon repair.17 These studies demonstrate that a lower body positive-pressure treadmill is associated with minimal cardiovascular effect while producing a significant decrease in ground reaction forces without altering gait kinematics.
We postulated that an anti-gravity treadmill may be safe and effective for gait training during rehabilitation following TKA. The primary objective was to determine the safety and feasibility of using the AlterG® Anti-Gravity Treadmill® device for postoperative gait training during rehabilitation following TKA. The secondary objective was to determine the effects of gait training (land-based vs anti-gravity) during postoperative rehabilitation on subjective patient outcomes assessed by Knee Injury and Osteoarthritis Outcome Score (KOOS), mobility assessed by the Timed Up and Go test (TUG), and pain assessed by a Numerical Rating Scale (NRS) to conduct a power analysis to determine sample sizes for efficacy studies based on these preliminary findings.
MethodsParticipants/Patient Enrollment and Study Overview
After signing an Institutional Review Board-approved consent, 30 patients were enrolled, and TKA surgeries were performed by 1 of 5 surgeons at 1 hospital. To be enrolled in the study, subjects must have (1) had a unilateral primary TKA, (2) been discharged from the hospital to home (not to a skilled nursing facility), (3) had only 3 to 4 home physical therapy (PT) sessions, (4) agreed to further outpatient PT at a single site, and (5) agreed to complete patient questionnaires. Exclusion criteria included (1) inability to meet inclusion criteria, (2) gross musculoskeletal deformity, (3) uncontrolled chronic or systemic disease, and (4) inability to follow instructions because of mental impairment, substance abuse, or addiction. Home PT was conducted for 3 to 4 sessions after surgery, and outpatient PT was continued at the study site per protocol for 4 weeks; subjects were asked to return for follow-up 3 months postoperatively. Patients were randomized on the first day of their outpatient PT to either a land-based (control) or an anti-gravity-based group using the AlterG Anti-Gravity Treadmill (AlterG group) gait training during outpatient PT sessions. Patients attended outpatient PT 2 days per week for 4 weeks for a total of 8 sessions. Therapy sessions lasted 45 to 60 minutes and included manual therapy, gait training, and therapeutic exercises/activities. The KOOS18,19 and TUG20 scores were evaluated at baseline (ie, first therapy session), end of physical therapy (EOPT) (ie, at final therapy session), and end of study (EOS) (ie, 3 months postoperatively). The NRS for pain was evaluated at baseline and at EOPT. Physical therapists were questioned for satisfaction with the anti-gravity rehabilitation protocol at EOPT.
Physical Therapy Protocols
All patients were treated consistently by 1 of 5 physical therapists at 1 outpatient setting; physical therapists averaged 11 years of experience in treating orthopedic conditions. Care was delivered in accordance with professional standards and the therapist’s assessment of medical necessity. Considerations included, but were not limited to, overall general health, any medical comorbidity, support system, and an ongoing assessment of ROM, strength, pain, and functional status. Each PT session started with a 5- to 10-minute warm-up on a standard cycle ergometer and was followed by manual therapy, gait training (land-based vs anti-gravity), therapeutic exercises/activities, and treatment modalities.
The time spent, activities selected, and modalities or physical agents chosen during the PT session were based on the patient’s needs and progress toward his/her functional goals. Manual therapy techniques consisted of soft-tissue mobilization, passive ROM, joint mobilization, passive stretching, scar mobilization, manual resistive exercises, and proprioceptive neuromuscular facilitation techniques. Therapeutic exercises/activities consisted of lower extremity resistance exercises (weight bearing and non-weight bearing), ROM exercises, stretching, balance, stair training, agility, activities of daily life (ADL) training, and a comprehensive home exercise program. Modalities or physical agents used during this study included moist hot packs, cold packs, ultrasound, electrical stimulation, and Kinesio Tape. Physical agents were incorporated into the individual’s plan of care based on medical necessity when deemed appropriate by the treating therapist. The exercise prescription was based on an individual’s status and tolerance and the number of sets and repetitions were based on fatigue.
Gait Training
The patients were randomized (1:1) to either land-based or anti-gravity gait training. For the control group, land-based gait training was performed with or without an appropriate AD and appropriate assistance, tactile cueing, and verbal cueing from a physical therapist. Duration (minutes) and gait-training progression were dependent on the participant’s functional goals, pain level (assessed throughout treatment), and level of fatigue. For the AlterG group, gait training was performed in the AlterG Anti-Gravity Treadmill, M320 (Alter-G; Figure 1). On day 1, the AlterG pressure chamber was set to allow only 50% of the patient’s body weight to be transmitted to the treadmill floor, and speed was controlled by the patient according to his/her comfort level. The percentage of body weight was adjusted to allow for a safe and normalized gait pattern with a pain level no greater than 5 (0 to 10 scale) throughout the PT session. A report card was recorded at each PT session, including body-weight setting (%), speed (miles per hour), incline (%), and duration (minutes) (Figure 2). For subsequent visits, the body-weight setting was started from the end point of the previous session.
Data Collection and Analysis
SPSS version 12.0 (SPSS Inc.) was used for all analyses, and an alpha level of .05 determined statistical significance when comparing group differences. The safety and feasibility of the anti-gravity (AlterG) vs land-based (control) gait training was assessed by the presence (or absence) of adverse events (AEs) and complications, and the date the patient discontinued use of his/her AD. A chi-square test was used to assess differences between control and AlterG groups regarding patient discontinuance of an AD. Additionally, for patients randomized to AlterG, a report card summarized means and frequencies for body weight, speed, incline, and duration. At EOPT, the frequency of therapists who were satisfied with the AlterG Anti-Gravity Treadmill as part of the rehabilitation protocol was reported. The preliminary effects of gait training (land-based vs anti-gravity) during postoperative rehabilitation on functional outcomes (subjective patient outcomes assessed by KOOS, mobility assessed by the TUG test, and pain assessed by a NRS) were evaluated by independent sample t tests. Paired sample t tests were used to compare each of the functional outcomes at EOPT or EOS to the baseline value.
Results
Of the 30 patients enrolled, 29 (96.7%; 29/30) patients completed the study; 1 patient, who could not complete all PT sessions because of medical and transportation issues, was excluded. The remaining 29 patients comprised the study population (control = 15; AlterG = 14). All patients were compliant with PT protocols.
Patient demographics were similar between the control and AlterG groups (Table 1). The control group comprised 9 women (60%; 9/15) and 6 men (40%; 6/15), age 69.9 ± 7.8 years and a body mass index of 28.8 ± 4.2. Similarly, the AlterG group comprised 7 women (50%; 7/14) and 7 men (50%; 7/14), age 66.5 ± 7.8 years and a body mass index of 28.4 ± 5.2.
At the baseline PT visit, patients in the control and AlterG groups had similar KOOS, TUG, and NRS scores. At baseline, mean KOOS for symptoms, pain, sports/recreation, ADL, and quality of life were 52.7, 52.9, 22.7, 64, and 31.8, respectively, although 50% of patients did not complete the sports/recreation subset of the KOOS. In addition, the mean time to complete the TUG test was 14.5 seconds, which was within the normal limits for disabled patients. This was slightly longer than normal mobility (TUG <10 seconds),20 but patients had relatively low levels of pain (mean NRS = 2.5, on a scale of 0-11).
All patients completed the PT protocols without indication of injury or AEs related to their operative knee. Three patients (10.3%; 3/29) experienced a deep venous thromboembolism (DVT), 2 in the control group (13.3%; 2/15), and 1 in the AlterG group (7.1%; 1/14). Venous thromboembolism protocol of enoxaparin 30 mg twice daily while in the hospital and enoxaparin 40 mg once daily for 10 days after discharge was followed for all patients.
Overall, more than half of patients (55.2%; 16/29) discontinued their AD during the 4-week PT period, with the remaining discontinuing prior to EOPT (24.1%; 7/29) or after EOPT (20.7%; 6/29). No statistically significant differences were found between the control and AlterG groups regarding discontinuance of AD.
Among those randomized to the AlterG group, all patients performed within the protocol established for the device for body-weight setting, treadmill speed, and duration of walking. The average body-weight treadmill setting increased by ~30% over the treatment period, from 55% at baseline to 84% at EOPT. The average speed increased by ~70%, from 1.6 mph at baseline to 2.7 mph at EOPT. The mean duration of AlterG use increased by ~75%, from 7.2 minutes at baseline to 12.7 minutes at EOPT. All physical therapists (100%) reported satisfaction with the AlterG for use in early postoperative rehabilitation and reported that patients’ treatment progressed positively.
While functional outcomes (KOOS, TUG, or NRS) did not vary with the type of gait training (P > .2 for land-based vs anti-gravity), functional outcomes improved over time (all P < .01 from baseline to EOPT and all P < .01 from baseline to EOS).
The KOOS scores improved from baseline to EOPT and from baseline to EOS (ie, 3-month follow-up visit) for both treatment groups (Figure 3). More patients completed the sports/recreation portion of the KOOS scores at EOPT and EOS compared to baseline. Forty-three percent and 25% of patients did not complete KOOS sports/recreation questions at EOPT and EOS, respectively, compared to 50% at baseline. This suggests that patients were improving to a level where sports/recreation scores were more applicable than directly after TKA surgery. The TUG scores had the greatest improvement from baseline to EOPT, with a decrease in time of 5 seconds and 7 seconds for the control and AlterG groups, respectively, and slight improvement from EOPT to EOS, with a decrease in time of 1 second and 2 seconds for the control and AlterG groups, respectively (Table 2). By the EOS, the values for the TUG tests for both treatment groups were within normal (<10 seconds) range.20 The NRS scores improved from baseline to EOPT with a score of 1 ± 1 in both control and AlterG groups.
Using these preliminary efficacy results, a post-hoc power analysis (α = .05 and 1β = 80%) was performed with the ADL domain of KOOS as the primary endpoint. Based on a standard deviation of 20 points and an effect size of 5 points, the sample size was estimated to be N = 250 per treatment group.
Discussion
We conducted a pilot study to assess, primarily, the feasibility and safety, and, secondarily, the efficacy, of a lower body positive-pressure treadmill for rehabilitation of patients after TKA. This small study showed that use of the AlterG Anti-Gravity Treadmill was not only safe and feasible during postoperative TKA rehabilitation, but also was well tolerated by patients and was rated highly satisfactory by physical therapists. Patients who used AlterG during gait training improved functionally (in terms of KOOS, TUG, and NRS) after 8 treatment sessions compared to baseline. However, there were no statistical differences between groups (control vs AlterG). Thus, these results suggest that an anti-gravity device for gait training may be a useful adjunct for postoperative TKA rehabilitation, but further studies are needed to determine the efficacy of anti-gravity compared to traditional land-based gait training.
The study of rehabilitation protocols during postoperative PT involved consideration of a number of issues. First, differences in functional outcomes compared to traditional rehabilitation could not be detected in this study because of the small number of patients, but the patients treated with anti-gravity gait training showed improvement in functional outcomes over time and did not report any added complications. Given that the primary outcome of this study was safety and feasibility, these added efficacy results are supplemental and useful in helping to plan studies. Second, the functional outcomes used to measure the efficacy of the anti-gravity treadmill may not be sensitive enough to detect differences between rehabilitation protocols. Use of a treadmill to measure speed improvement, endurance, and tolerance in both groups could be valuable in future studies. More studies may need to refine characteristics that are important to postoperative rehabilitation success, and quantitative and subjective measures that must be defined.
The results reported here using an anti-gravity treadmill for postoperative TKA rehabilitation support the safety and feasibility that has been reported in other orthopedic rehabilitation settings. Anti-gravity treadmills, which have been used to study patients after meniscectomy or anterior cruciate ligament reconstruction8 and Achilles repair,17 have demonstrated predictable decreases in ground reaction forces with increasing positive-pressure unweighting, reductions in pain with ambulation, and allowance of earlier institution of walking and jogging during rehabilitation.17
Patient safety is an important attribute for any postoperative rehabilitation protocol, especially in an elderly population undergoing major surgery. One of our important goals was to assess the safety of AlterG. We noted no AEs attributable to the device, which was supported by work indicating no adverse impact on systemic cardiovascular parameters in a similar lower body positive-pressure environment.9 Although 3 patients (10%) developed symptomatic DVT, there were no differences between the groups in the incidence of DVT. Use of an anti-gravity treadmill has also been examined for cardiovascular responses in TKA patients. In a study of 24 adults with TKA, researchers found that anti-gravity support allowed TKA patients to walk at faster speeds and tolerate greater inclines with lower heart rate, blood pressure, and oxygen consumption.21 With respect to efficacy of the rehabilitation intervention, we demonstrated significant improvements in all functional outcomes in both groups but no differences between the study groups. We concluded that AlterG was at least as effective as standard therapy in this small cohort. TKA is a very successful procedure, and the improvement in pain and function after surgery is fairly dramatic in most patients, regardless of specific rehabilitation protocols. Therefore, the substantial improvement in clinical outcomes may overshadow any enhanced benefits of the anti-gravity treadmill. Further investigations into the efficacy of AlterG are needed in a larger cohort to determine if this type of treatment is more beneficial than traditional land-based gait training.
Standard scoring systems such as KOOS, TUG, and NRS may not be sensitive enough to detect differences between treatment groups with small sample sizes. Given the results of the post hoc power analysis, a large number of patients (N = 250/group) would be necessary to detect any potential difference in clinical outcomes between the 2 groups. Larger studies are required to answer relevant questions, and additional outcome measures may be needed to detect differences between treatment groups. Relevant questions include whether earlier institution of the anti-gravity device during the immediate TKA postoperative period would be beneficial compared to standard postoperative PT, and whether PT enhanced with the anti-gravity device has incremental benefit in functional outcomes and in time to reach those goals. Finally, given the present attention to healthcare expenses, a cost-benefit analysis of anti-gravity device treatment vs traditional PT would be useful. Once the patient has become familiar with the function of an anti-gravity treadmill, gait therapy could proceed without the direct intervention of the therapist, potentially improving efficient delivery of rehabilitation services.
Studying the effect of different postoperative rehabilitation protocols after orthopedic surgeries can be challenging. In a large (N > 350) randomized controlled trial to study the effect of ergometer cycling after hip and knee replacement, patients who used the cycle ergometer had a higher Western Ontario and McMaster Universities Arthritis Index and greater satisfaction than those who did not after hip arthroplasty, but not after TKA.22 Improvements in muscular coordination and proprioception with the cycle ergometer may have been offset by increases in edema, joint effusion, and pain from the loading of the joint and the relatively fast rate of cycling compared to passive motion or ambulation. While many therapists and surgeons advocate cycling for rehabilitation after knee surgery, the need remains for a better definition of an optimal TKA rehabilitation program. A study of 82 patients comparing early progressive strength training to no early strength training showed no difference in the 6-minute walk test at 8 weeks.23 A systematic review of progressive resistance training (PRT) found that although postoperative PRT is safe and feasible, the methodological quality of existing studies is too low to allow conclusions regarding its efficacy.24 Gait training in an environment where weight-bearing loads can be closely controlled, monitored, and individualized may be an ideal methodology to enhance rehabilitation and return to function for knee replacement surgery.
This current study showed that the use of AlterG as an adjunct for postoperative rehabilitation is safe, accepted by patients and therapists, and leads to clinical functional outcomes that are at least as good as traditional postoperative TKA rehabilitation. We conclude that AlterG demonstrates utility and a potential for innovation in TKA rehabilitation.
Patients undergoing total knee arthroplasty (TKA) may benefit from focused postoperative rehabilitation. Although there is limited research comparing different rehabilitation protocols after TKA,1 any type of rehabilitation often helps to optimize range of motion (ROM), strength, balance, and ambulation.2 Early mobilization and rehabilitation after TKA reduces pain, fear, anxiety, and risk of postoperative venous thromboembolic disease.3 Earlier discharge to home or community settings deceases time for inpatient rehabilitation, patient and family education, and gait training, which places a greater emphasis on outpatient rehabilitation.4
Although rapid rehabilitation protocols have gained wide acceptance, concern remains that a higher intensity intervention initiated immediately after hospital discharge could lead to an increased incidence of pain and swelling, and to poorer ROM and functional outcomes.5 Progressive weight-bearing activities, such as walking, are routinely recommended during rehabilitation to facilitate return to normal function. Not all patients are capable of full weight-bearing activity in the early postoperative period and assistive devices (ADs), such as walkers, crutches, and canes, are routinely employed. An opportunity to enhance early TKA rehabilitation exists with devices that allow functional gait training while modifying weight-bearing forces across the joint. Assistive devices, hydrotherapy (walking in water),6,7 and lower body positive-pressure chambers8 can reduce the forces at the knee during weight-bearing exercise.
Lower body positive-pressure devices have been extensively studied in physiological response of healthy humans;9-12 in disease states such as cerebral palsy13 and obesity;14 and in other postoperative orthopedic conditions, such as anterior cruciate ligament reconstruction, meniscectomy,8 microfracture,15 TKA,16 and Achilles tendon repair.17 These studies demonstrate that a lower body positive-pressure treadmill is associated with minimal cardiovascular effect while producing a significant decrease in ground reaction forces without altering gait kinematics.
We postulated that an anti-gravity treadmill may be safe and effective for gait training during rehabilitation following TKA. The primary objective was to determine the safety and feasibility of using the AlterG® Anti-Gravity Treadmill® device for postoperative gait training during rehabilitation following TKA. The secondary objective was to determine the effects of gait training (land-based vs anti-gravity) during postoperative rehabilitation on subjective patient outcomes assessed by Knee Injury and Osteoarthritis Outcome Score (KOOS), mobility assessed by the Timed Up and Go test (TUG), and pain assessed by a Numerical Rating Scale (NRS) to conduct a power analysis to determine sample sizes for efficacy studies based on these preliminary findings.
MethodsParticipants/Patient Enrollment and Study Overview
After signing an Institutional Review Board-approved consent, 30 patients were enrolled, and TKA surgeries were performed by 1 of 5 surgeons at 1 hospital. To be enrolled in the study, subjects must have (1) had a unilateral primary TKA, (2) been discharged from the hospital to home (not to a skilled nursing facility), (3) had only 3 to 4 home physical therapy (PT) sessions, (4) agreed to further outpatient PT at a single site, and (5) agreed to complete patient questionnaires. Exclusion criteria included (1) inability to meet inclusion criteria, (2) gross musculoskeletal deformity, (3) uncontrolled chronic or systemic disease, and (4) inability to follow instructions because of mental impairment, substance abuse, or addiction. Home PT was conducted for 3 to 4 sessions after surgery, and outpatient PT was continued at the study site per protocol for 4 weeks; subjects were asked to return for follow-up 3 months postoperatively. Patients were randomized on the first day of their outpatient PT to either a land-based (control) or an anti-gravity-based group using the AlterG Anti-Gravity Treadmill (AlterG group) gait training during outpatient PT sessions. Patients attended outpatient PT 2 days per week for 4 weeks for a total of 8 sessions. Therapy sessions lasted 45 to 60 minutes and included manual therapy, gait training, and therapeutic exercises/activities. The KOOS18,19 and TUG20 scores were evaluated at baseline (ie, first therapy session), end of physical therapy (EOPT) (ie, at final therapy session), and end of study (EOS) (ie, 3 months postoperatively). The NRS for pain was evaluated at baseline and at EOPT. Physical therapists were questioned for satisfaction with the anti-gravity rehabilitation protocol at EOPT.
Physical Therapy Protocols
All patients were treated consistently by 1 of 5 physical therapists at 1 outpatient setting; physical therapists averaged 11 years of experience in treating orthopedic conditions. Care was delivered in accordance with professional standards and the therapist’s assessment of medical necessity. Considerations included, but were not limited to, overall general health, any medical comorbidity, support system, and an ongoing assessment of ROM, strength, pain, and functional status. Each PT session started with a 5- to 10-minute warm-up on a standard cycle ergometer and was followed by manual therapy, gait training (land-based vs anti-gravity), therapeutic exercises/activities, and treatment modalities.
The time spent, activities selected, and modalities or physical agents chosen during the PT session were based on the patient’s needs and progress toward his/her functional goals. Manual therapy techniques consisted of soft-tissue mobilization, passive ROM, joint mobilization, passive stretching, scar mobilization, manual resistive exercises, and proprioceptive neuromuscular facilitation techniques. Therapeutic exercises/activities consisted of lower extremity resistance exercises (weight bearing and non-weight bearing), ROM exercises, stretching, balance, stair training, agility, activities of daily life (ADL) training, and a comprehensive home exercise program. Modalities or physical agents used during this study included moist hot packs, cold packs, ultrasound, electrical stimulation, and Kinesio Tape. Physical agents were incorporated into the individual’s plan of care based on medical necessity when deemed appropriate by the treating therapist. The exercise prescription was based on an individual’s status and tolerance and the number of sets and repetitions were based on fatigue.
Gait Training
The patients were randomized (1:1) to either land-based or anti-gravity gait training. For the control group, land-based gait training was performed with or without an appropriate AD and appropriate assistance, tactile cueing, and verbal cueing from a physical therapist. Duration (minutes) and gait-training progression were dependent on the participant’s functional goals, pain level (assessed throughout treatment), and level of fatigue. For the AlterG group, gait training was performed in the AlterG Anti-Gravity Treadmill, M320 (Alter-G; Figure 1). On day 1, the AlterG pressure chamber was set to allow only 50% of the patient’s body weight to be transmitted to the treadmill floor, and speed was controlled by the patient according to his/her comfort level. The percentage of body weight was adjusted to allow for a safe and normalized gait pattern with a pain level no greater than 5 (0 to 10 scale) throughout the PT session. A report card was recorded at each PT session, including body-weight setting (%), speed (miles per hour), incline (%), and duration (minutes) (Figure 2). For subsequent visits, the body-weight setting was started from the end point of the previous session.
Data Collection and Analysis
SPSS version 12.0 (SPSS Inc.) was used for all analyses, and an alpha level of .05 determined statistical significance when comparing group differences. The safety and feasibility of the anti-gravity (AlterG) vs land-based (control) gait training was assessed by the presence (or absence) of adverse events (AEs) and complications, and the date the patient discontinued use of his/her AD. A chi-square test was used to assess differences between control and AlterG groups regarding patient discontinuance of an AD. Additionally, for patients randomized to AlterG, a report card summarized means and frequencies for body weight, speed, incline, and duration. At EOPT, the frequency of therapists who were satisfied with the AlterG Anti-Gravity Treadmill as part of the rehabilitation protocol was reported. The preliminary effects of gait training (land-based vs anti-gravity) during postoperative rehabilitation on functional outcomes (subjective patient outcomes assessed by KOOS, mobility assessed by the TUG test, and pain assessed by a NRS) were evaluated by independent sample t tests. Paired sample t tests were used to compare each of the functional outcomes at EOPT or EOS to the baseline value.
Results
Of the 30 patients enrolled, 29 (96.7%; 29/30) patients completed the study; 1 patient, who could not complete all PT sessions because of medical and transportation issues, was excluded. The remaining 29 patients comprised the study population (control = 15; AlterG = 14). All patients were compliant with PT protocols.
Patient demographics were similar between the control and AlterG groups (Table 1). The control group comprised 9 women (60%; 9/15) and 6 men (40%; 6/15), age 69.9 ± 7.8 years and a body mass index of 28.8 ± 4.2. Similarly, the AlterG group comprised 7 women (50%; 7/14) and 7 men (50%; 7/14), age 66.5 ± 7.8 years and a body mass index of 28.4 ± 5.2.
At the baseline PT visit, patients in the control and AlterG groups had similar KOOS, TUG, and NRS scores. At baseline, mean KOOS for symptoms, pain, sports/recreation, ADL, and quality of life were 52.7, 52.9, 22.7, 64, and 31.8, respectively, although 50% of patients did not complete the sports/recreation subset of the KOOS. In addition, the mean time to complete the TUG test was 14.5 seconds, which was within the normal limits for disabled patients. This was slightly longer than normal mobility (TUG <10 seconds),20 but patients had relatively low levels of pain (mean NRS = 2.5, on a scale of 0-11).
All patients completed the PT protocols without indication of injury or AEs related to their operative knee. Three patients (10.3%; 3/29) experienced a deep venous thromboembolism (DVT), 2 in the control group (13.3%; 2/15), and 1 in the AlterG group (7.1%; 1/14). Venous thromboembolism protocol of enoxaparin 30 mg twice daily while in the hospital and enoxaparin 40 mg once daily for 10 days after discharge was followed for all patients.
Overall, more than half of patients (55.2%; 16/29) discontinued their AD during the 4-week PT period, with the remaining discontinuing prior to EOPT (24.1%; 7/29) or after EOPT (20.7%; 6/29). No statistically significant differences were found between the control and AlterG groups regarding discontinuance of AD.
Among those randomized to the AlterG group, all patients performed within the protocol established for the device for body-weight setting, treadmill speed, and duration of walking. The average body-weight treadmill setting increased by ~30% over the treatment period, from 55% at baseline to 84% at EOPT. The average speed increased by ~70%, from 1.6 mph at baseline to 2.7 mph at EOPT. The mean duration of AlterG use increased by ~75%, from 7.2 minutes at baseline to 12.7 minutes at EOPT. All physical therapists (100%) reported satisfaction with the AlterG for use in early postoperative rehabilitation and reported that patients’ treatment progressed positively.
While functional outcomes (KOOS, TUG, or NRS) did not vary with the type of gait training (P > .2 for land-based vs anti-gravity), functional outcomes improved over time (all P < .01 from baseline to EOPT and all P < .01 from baseline to EOS).
The KOOS scores improved from baseline to EOPT and from baseline to EOS (ie, 3-month follow-up visit) for both treatment groups (Figure 3). More patients completed the sports/recreation portion of the KOOS scores at EOPT and EOS compared to baseline. Forty-three percent and 25% of patients did not complete KOOS sports/recreation questions at EOPT and EOS, respectively, compared to 50% at baseline. This suggests that patients were improving to a level where sports/recreation scores were more applicable than directly after TKA surgery. The TUG scores had the greatest improvement from baseline to EOPT, with a decrease in time of 5 seconds and 7 seconds for the control and AlterG groups, respectively, and slight improvement from EOPT to EOS, with a decrease in time of 1 second and 2 seconds for the control and AlterG groups, respectively (Table 2). By the EOS, the values for the TUG tests for both treatment groups were within normal (<10 seconds) range.20 The NRS scores improved from baseline to EOPT with a score of 1 ± 1 in both control and AlterG groups.
Using these preliminary efficacy results, a post-hoc power analysis (α = .05 and 1β = 80%) was performed with the ADL domain of KOOS as the primary endpoint. Based on a standard deviation of 20 points and an effect size of 5 points, the sample size was estimated to be N = 250 per treatment group.
Discussion
We conducted a pilot study to assess, primarily, the feasibility and safety, and, secondarily, the efficacy, of a lower body positive-pressure treadmill for rehabilitation of patients after TKA. This small study showed that use of the AlterG Anti-Gravity Treadmill was not only safe and feasible during postoperative TKA rehabilitation, but also was well tolerated by patients and was rated highly satisfactory by physical therapists. Patients who used AlterG during gait training improved functionally (in terms of KOOS, TUG, and NRS) after 8 treatment sessions compared to baseline. However, there were no statistical differences between groups (control vs AlterG). Thus, these results suggest that an anti-gravity device for gait training may be a useful adjunct for postoperative TKA rehabilitation, but further studies are needed to determine the efficacy of anti-gravity compared to traditional land-based gait training.
The study of rehabilitation protocols during postoperative PT involved consideration of a number of issues. First, differences in functional outcomes compared to traditional rehabilitation could not be detected in this study because of the small number of patients, but the patients treated with anti-gravity gait training showed improvement in functional outcomes over time and did not report any added complications. Given that the primary outcome of this study was safety and feasibility, these added efficacy results are supplemental and useful in helping to plan studies. Second, the functional outcomes used to measure the efficacy of the anti-gravity treadmill may not be sensitive enough to detect differences between rehabilitation protocols. Use of a treadmill to measure speed improvement, endurance, and tolerance in both groups could be valuable in future studies. More studies may need to refine characteristics that are important to postoperative rehabilitation success, and quantitative and subjective measures that must be defined.
The results reported here using an anti-gravity treadmill for postoperative TKA rehabilitation support the safety and feasibility that has been reported in other orthopedic rehabilitation settings. Anti-gravity treadmills, which have been used to study patients after meniscectomy or anterior cruciate ligament reconstruction8 and Achilles repair,17 have demonstrated predictable decreases in ground reaction forces with increasing positive-pressure unweighting, reductions in pain with ambulation, and allowance of earlier institution of walking and jogging during rehabilitation.17
Patient safety is an important attribute for any postoperative rehabilitation protocol, especially in an elderly population undergoing major surgery. One of our important goals was to assess the safety of AlterG. We noted no AEs attributable to the device, which was supported by work indicating no adverse impact on systemic cardiovascular parameters in a similar lower body positive-pressure environment.9 Although 3 patients (10%) developed symptomatic DVT, there were no differences between the groups in the incidence of DVT. Use of an anti-gravity treadmill has also been examined for cardiovascular responses in TKA patients. In a study of 24 adults with TKA, researchers found that anti-gravity support allowed TKA patients to walk at faster speeds and tolerate greater inclines with lower heart rate, blood pressure, and oxygen consumption.21 With respect to efficacy of the rehabilitation intervention, we demonstrated significant improvements in all functional outcomes in both groups but no differences between the study groups. We concluded that AlterG was at least as effective as standard therapy in this small cohort. TKA is a very successful procedure, and the improvement in pain and function after surgery is fairly dramatic in most patients, regardless of specific rehabilitation protocols. Therefore, the substantial improvement in clinical outcomes may overshadow any enhanced benefits of the anti-gravity treadmill. Further investigations into the efficacy of AlterG are needed in a larger cohort to determine if this type of treatment is more beneficial than traditional land-based gait training.
Standard scoring systems such as KOOS, TUG, and NRS may not be sensitive enough to detect differences between treatment groups with small sample sizes. Given the results of the post hoc power analysis, a large number of patients (N = 250/group) would be necessary to detect any potential difference in clinical outcomes between the 2 groups. Larger studies are required to answer relevant questions, and additional outcome measures may be needed to detect differences between treatment groups. Relevant questions include whether earlier institution of the anti-gravity device during the immediate TKA postoperative period would be beneficial compared to standard postoperative PT, and whether PT enhanced with the anti-gravity device has incremental benefit in functional outcomes and in time to reach those goals. Finally, given the present attention to healthcare expenses, a cost-benefit analysis of anti-gravity device treatment vs traditional PT would be useful. Once the patient has become familiar with the function of an anti-gravity treadmill, gait therapy could proceed without the direct intervention of the therapist, potentially improving efficient delivery of rehabilitation services.
Studying the effect of different postoperative rehabilitation protocols after orthopedic surgeries can be challenging. In a large (N > 350) randomized controlled trial to study the effect of ergometer cycling after hip and knee replacement, patients who used the cycle ergometer had a higher Western Ontario and McMaster Universities Arthritis Index and greater satisfaction than those who did not after hip arthroplasty, but not after TKA.22 Improvements in muscular coordination and proprioception with the cycle ergometer may have been offset by increases in edema, joint effusion, and pain from the loading of the joint and the relatively fast rate of cycling compared to passive motion or ambulation. While many therapists and surgeons advocate cycling for rehabilitation after knee surgery, the need remains for a better definition of an optimal TKA rehabilitation program. A study of 82 patients comparing early progressive strength training to no early strength training showed no difference in the 6-minute walk test at 8 weeks.23 A systematic review of progressive resistance training (PRT) found that although postoperative PRT is safe and feasible, the methodological quality of existing studies is too low to allow conclusions regarding its efficacy.24 Gait training in an environment where weight-bearing loads can be closely controlled, monitored, and individualized may be an ideal methodology to enhance rehabilitation and return to function for knee replacement surgery.
This current study showed that the use of AlterG as an adjunct for postoperative rehabilitation is safe, accepted by patients and therapists, and leads to clinical functional outcomes that are at least as good as traditional postoperative TKA rehabilitation. We conclude that AlterG demonstrates utility and a potential for innovation in TKA rehabilitation.
1. NIH Consensus Statement on total knee arthroplasty. NIH Consensus State Sci Statements. 2003;20(1):1-34.
2. Jones CA, Voaklander DC, Suarez-Almazor ME. Determinants of function after total knee arthroplasty. Phys Ther. 2003;83(8):696-706.
3. Pearse EO, Caldwell BF, Lockwood RJ, Hollard J. Early mobilisation after conventional knee replacement may reduce the risk of post-operative venous thromboembolism. J Bone Joint Surg Br. 2007;89(3):316-322.
4. Westby MD, Kennedy D, Jones D, Jones A, Doyle-Waters MM, Backman C. Post-acute physiotherapy for primary total knee arthroplasty. Cochrane Database Syst Rev. 2008. doi.10.1002/14651858.CD007099
5. Bade MJ, Stevens-Lapsley JE. Early high-intensity rehabilitation following total knee arthroplasty improves outcomes. J Orthop Sports Phys Ther. 2011;41(12):932-941.
6. Ivanenko YP, Grasso R, Macellari V, Lacquaniti F. Control of foot trajectory in human locomotion: role of ground contact forces in simulated reduced gravity. J Neurophysiol. 2002;87(6):3070-3089.
7. Pöyhönen T, Keskinen KL, Kyröläinen H, Hautala A, Savolainen J, Mälkiä E. Neuromuscular function during therapeutic knee exercise under water and on dry land. Arch Phys Med Rehabil. 2001;82(10):1446-1452.
8. Eastlack RK, Hargens AR, Groppo ER, Steinbach GC, White KK, Pedowitz RA. Lower body positive-pressure exercise after knee surgery. Clin Orthop Rel Res. 2005;431:213-219.
9. Cutuk A, Groppo ER, Quigley EJ, White KW, Pedowitz RA, Hargens AR. Ambulation in simulated fractional gravity using lower body positive pressure: cardiovascular safety and gait analyses. J Appl Physiol. 2006;101(3):771-777.
10. Gojanovic B, Cutti P, Shultz R, Matheson GO. Maximal physiological parameters during partial body-weight support treadmill testing. Med Sci Sports Exerc. 2012;44(10):1935-1941.
11. Figueroa MA, Manning J, Escamilla P. Physiological responses to the AlterG Anti-Gravity Treadmill. Int J Applied Sci Tech. 2011;1:92-97.
12. Hoffman MD, Donaghe HE. Physiological responses to body weight-supported treadmill exercise in healthy adults. Arch Phys Med Rehabil. 2011;92(6):960-966.
13. Kurz MJ, Corr B, Stuberg W, Volkman KG, Smith N. Evaluation of lower body positive pressure supported treadmill training for children with cerebral palsy. Pediatr Phys Ther. 2011;23(3):232-239.
14. Christian M. Managing knee osteoarthritis: the effects of anti-gravity treadmill exercise on joint pain and physical function. Available at: http://mspace.lib.umanitoba.ca/handle/1993/8580. Accessed March 31, 2016.
15. Wilk KE, Macrina LC, Reinhold MM. Rehabilitation following microfracture of the knee. Cartilage. 2010;1(2):96-107.
16. Patil SS, Branovacki G, Martin MR, Pulido PA, Levy YD, Colwell CW Jr. 14-year median follow-up using the press-fit condylar sigma design for total knee arthroplasty. J Arthroplasty. 2013;28(8):1286-1290.
17. Saxena A, Granot A. Use of an anti-gravity treadmill in the rehabilitation of the operated achilles tendon: a pilot study. J Foot Ankle Surg. 2011;50(5):558-561.
18. Roos EM, Roos HP, Ekdahl C, Lohmander LS. Knee injury and Osteoarthritis Outcome Score (KOOS) - validation of a Swedish version. Scand J Med Sci Sports. 1998;8(6):439-448.
19. Roos EM, Toksvig-Larsen S. Knee injury and Osteoarthritis Outcome Score (KOOS) - validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. 2003;1:17.
20. Timed Up and Go (TUG). Available at: http://www.rheumatology.org/I-Am-A/Rheumatologist/Research/Clinician-Researchers/Timed-Up-Go-TUG Accessed: March 15, 2016.
21. Webber SC, Horvey KJ, Yurach Pikaluk MT, Butcher SJ. Cardiovascular responses in older adults with total knee arthroplasty at rest and with exercise on a positive pressure treadmill. Eur J Appl Physiol. 2014;114(3):653-662.
22. Liebs TR, Herzberg W, Ruther W, Haasters J, Russlies M, Hassenpflug J. Ergometer cycling after hip and knee replacement surgery: a randomized control trial. J Bone Joint Surg Am. 2010;92(4):814-822.
23. Jakobsen TL, Kehlet H, Husted H, Petersen J, Bandholm T. Early progressive strength training to enhance recovery after fast-track total knee arthroplasty: a randomized controlled trial. Arthritis Care Res. 2014;66(12):1856-1866.
24. Skoffer B, Dalgas U, Mechlenburg I. Progressive resistance training before and after total hip and knee arthroplasty: a systematic review. Clin Rehabil. 2015;29(1):14-29.
1. NIH Consensus Statement on total knee arthroplasty. NIH Consensus State Sci Statements. 2003;20(1):1-34.
2. Jones CA, Voaklander DC, Suarez-Almazor ME. Determinants of function after total knee arthroplasty. Phys Ther. 2003;83(8):696-706.
3. Pearse EO, Caldwell BF, Lockwood RJ, Hollard J. Early mobilisation after conventional knee replacement may reduce the risk of post-operative venous thromboembolism. J Bone Joint Surg Br. 2007;89(3):316-322.
4. Westby MD, Kennedy D, Jones D, Jones A, Doyle-Waters MM, Backman C. Post-acute physiotherapy for primary total knee arthroplasty. Cochrane Database Syst Rev. 2008. doi.10.1002/14651858.CD007099
5. Bade MJ, Stevens-Lapsley JE. Early high-intensity rehabilitation following total knee arthroplasty improves outcomes. J Orthop Sports Phys Ther. 2011;41(12):932-941.
6. Ivanenko YP, Grasso R, Macellari V, Lacquaniti F. Control of foot trajectory in human locomotion: role of ground contact forces in simulated reduced gravity. J Neurophysiol. 2002;87(6):3070-3089.
7. Pöyhönen T, Keskinen KL, Kyröläinen H, Hautala A, Savolainen J, Mälkiä E. Neuromuscular function during therapeutic knee exercise under water and on dry land. Arch Phys Med Rehabil. 2001;82(10):1446-1452.
8. Eastlack RK, Hargens AR, Groppo ER, Steinbach GC, White KK, Pedowitz RA. Lower body positive-pressure exercise after knee surgery. Clin Orthop Rel Res. 2005;431:213-219.
9. Cutuk A, Groppo ER, Quigley EJ, White KW, Pedowitz RA, Hargens AR. Ambulation in simulated fractional gravity using lower body positive pressure: cardiovascular safety and gait analyses. J Appl Physiol. 2006;101(3):771-777.
10. Gojanovic B, Cutti P, Shultz R, Matheson GO. Maximal physiological parameters during partial body-weight support treadmill testing. Med Sci Sports Exerc. 2012;44(10):1935-1941.
11. Figueroa MA, Manning J, Escamilla P. Physiological responses to the AlterG Anti-Gravity Treadmill. Int J Applied Sci Tech. 2011;1:92-97.
12. Hoffman MD, Donaghe HE. Physiological responses to body weight-supported treadmill exercise in healthy adults. Arch Phys Med Rehabil. 2011;92(6):960-966.
13. Kurz MJ, Corr B, Stuberg W, Volkman KG, Smith N. Evaluation of lower body positive pressure supported treadmill training for children with cerebral palsy. Pediatr Phys Ther. 2011;23(3):232-239.
14. Christian M. Managing knee osteoarthritis: the effects of anti-gravity treadmill exercise on joint pain and physical function. Available at: http://mspace.lib.umanitoba.ca/handle/1993/8580. Accessed March 31, 2016.
15. Wilk KE, Macrina LC, Reinhold MM. Rehabilitation following microfracture of the knee. Cartilage. 2010;1(2):96-107.
16. Patil SS, Branovacki G, Martin MR, Pulido PA, Levy YD, Colwell CW Jr. 14-year median follow-up using the press-fit condylar sigma design for total knee arthroplasty. J Arthroplasty. 2013;28(8):1286-1290.
17. Saxena A, Granot A. Use of an anti-gravity treadmill in the rehabilitation of the operated achilles tendon: a pilot study. J Foot Ankle Surg. 2011;50(5):558-561.
18. Roos EM, Roos HP, Ekdahl C, Lohmander LS. Knee injury and Osteoarthritis Outcome Score (KOOS) - validation of a Swedish version. Scand J Med Sci Sports. 1998;8(6):439-448.
19. Roos EM, Toksvig-Larsen S. Knee injury and Osteoarthritis Outcome Score (KOOS) - validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. 2003;1:17.
20. Timed Up and Go (TUG). Available at: http://www.rheumatology.org/I-Am-A/Rheumatologist/Research/Clinician-Researchers/Timed-Up-Go-TUG Accessed: March 15, 2016.
21. Webber SC, Horvey KJ, Yurach Pikaluk MT, Butcher SJ. Cardiovascular responses in older adults with total knee arthroplasty at rest and with exercise on a positive pressure treadmill. Eur J Appl Physiol. 2014;114(3):653-662.
22. Liebs TR, Herzberg W, Ruther W, Haasters J, Russlies M, Hassenpflug J. Ergometer cycling after hip and knee replacement surgery: a randomized control trial. J Bone Joint Surg Am. 2010;92(4):814-822.
23. Jakobsen TL, Kehlet H, Husted H, Petersen J, Bandholm T. Early progressive strength training to enhance recovery after fast-track total knee arthroplasty: a randomized controlled trial. Arthritis Care Res. 2014;66(12):1856-1866.
24. Skoffer B, Dalgas U, Mechlenburg I. Progressive resistance training before and after total hip and knee arthroplasty: a systematic review. Clin Rehabil. 2015;29(1):14-29.
FDA grants priority review of olaratumab for advanced sarcoma
The Food and Drug Administration has granted priority review of olaratumab, in combination with doxorubicin, for the treatment of patients with advanced soft tissue sarcoma who unsuccessfully underwent prior radiotherapy or surgery for their cancer.
Olaratumab is a human IgG1 monoclonal antibody that directly targets tumor cells by disrupting the platelet-derived growth factor receptor alpha, a receptor that is believed to play a role in tumor growth and progression.
“We are hopeful that, if approved, olaratumab will provide a meaningful addition to the limited treatment options for this rare and difficult-to-treat disease,” Dr. Richard Gaynor, senior vice president of product development and medical affairs for Lilly Oncology, the maker of the drug, said in a written statement issued by company.
The biologics license application submission for olaratumab was based on a phase II clinical trial of 129 patients with metastatic or unresectable soft tissue sarcoma. Sixty-four patients were assigned to the treatment group and received both doxorubicin and olaratumab. Patients in this group continued to receive olaratumab through observed disease progression. Sixty-five patients were assigned to the control group and received only doxorubicin until disease progression was first observed. Patients who received olaratumab in addition to doxorubicin experienced a longer median progression-free survival, compared with patients who only received doxorubicin (6.6 months vs. 4.1 months; stratified hazard ratio, 0.672; 95% confidence interval, 0.442 to 1.021; P = .0615). The results of the phase II clinical trial were reported at the 2015 American Society of Clinical Oncology annual meeting and the 2015 Connective Tissue Oncology Society annual meeting.
The FDA previously granted olaratumab breakthrough therapy, fast track, and orphan drug designation.
On Twitter @jess_craig94
The Food and Drug Administration has granted priority review of olaratumab, in combination with doxorubicin, for the treatment of patients with advanced soft tissue sarcoma who unsuccessfully underwent prior radiotherapy or surgery for their cancer.
Olaratumab is a human IgG1 monoclonal antibody that directly targets tumor cells by disrupting the platelet-derived growth factor receptor alpha, a receptor that is believed to play a role in tumor growth and progression.
“We are hopeful that, if approved, olaratumab will provide a meaningful addition to the limited treatment options for this rare and difficult-to-treat disease,” Dr. Richard Gaynor, senior vice president of product development and medical affairs for Lilly Oncology, the maker of the drug, said in a written statement issued by company.
The biologics license application submission for olaratumab was based on a phase II clinical trial of 129 patients with metastatic or unresectable soft tissue sarcoma. Sixty-four patients were assigned to the treatment group and received both doxorubicin and olaratumab. Patients in this group continued to receive olaratumab through observed disease progression. Sixty-five patients were assigned to the control group and received only doxorubicin until disease progression was first observed. Patients who received olaratumab in addition to doxorubicin experienced a longer median progression-free survival, compared with patients who only received doxorubicin (6.6 months vs. 4.1 months; stratified hazard ratio, 0.672; 95% confidence interval, 0.442 to 1.021; P = .0615). The results of the phase II clinical trial were reported at the 2015 American Society of Clinical Oncology annual meeting and the 2015 Connective Tissue Oncology Society annual meeting.
The FDA previously granted olaratumab breakthrough therapy, fast track, and orphan drug designation.
On Twitter @jess_craig94
The Food and Drug Administration has granted priority review of olaratumab, in combination with doxorubicin, for the treatment of patients with advanced soft tissue sarcoma who unsuccessfully underwent prior radiotherapy or surgery for their cancer.
Olaratumab is a human IgG1 monoclonal antibody that directly targets tumor cells by disrupting the platelet-derived growth factor receptor alpha, a receptor that is believed to play a role in tumor growth and progression.
“We are hopeful that, if approved, olaratumab will provide a meaningful addition to the limited treatment options for this rare and difficult-to-treat disease,” Dr. Richard Gaynor, senior vice president of product development and medical affairs for Lilly Oncology, the maker of the drug, said in a written statement issued by company.
The biologics license application submission for olaratumab was based on a phase II clinical trial of 129 patients with metastatic or unresectable soft tissue sarcoma. Sixty-four patients were assigned to the treatment group and received both doxorubicin and olaratumab. Patients in this group continued to receive olaratumab through observed disease progression. Sixty-five patients were assigned to the control group and received only doxorubicin until disease progression was first observed. Patients who received olaratumab in addition to doxorubicin experienced a longer median progression-free survival, compared with patients who only received doxorubicin (6.6 months vs. 4.1 months; stratified hazard ratio, 0.672; 95% confidence interval, 0.442 to 1.021; P = .0615). The results of the phase II clinical trial were reported at the 2015 American Society of Clinical Oncology annual meeting and the 2015 Connective Tissue Oncology Society annual meeting.
The FDA previously granted olaratumab breakthrough therapy, fast track, and orphan drug designation.
On Twitter @jess_craig94
The Cruciate Ligaments in Total Knee Arthroplasty
Hinge knee arthroplasty was introduced in the 1950s.1 All 4 major ligaments were replaced by the hinge, which provided stabilization while allowing sagittal plane motion. Its goal was stability, not replication of normal kinematics. The addition of methyl methacrylate cement improved fixation and allowed surface design modifications that addressed normal articular motion. Implants such as the Gunston Polycentric,2 the Duocondylar,3 and the Geometric4 resurfaced the medial and lateral compartments of the knee while preserving the cruciate ligaments. The implants were subject to greater translational forces without the hinge and loosening became a major problem despite the advances in cementing. It became evident in the 1970s that preservation of the cruciates complicated the procedure. Cruciate resection simplified the operation and allowed improved fixation. The ICLH prosthesis resected the cruciates and used the articular surface design to give stability to the knee.5,6 The total condylar prosthesis had a “tibial” imminence that mimicked the shape of the tibial surface but also sacrificed both of the cruciate ligaments (Figure 1).
Designers recognized that the cruciate ligaments affected knee kinematics; however, they elected to sacrifice the anterior cruciate ligament (ACL) for surgical simplicity and implant longevity.6 In the early 1980s, both the cruciate-retaining (CR) total knee arthroplasty (TKA) (Figure 2) and posterior-stabilized (PS) TKA (Figure 3) designs addressed the posterior cruciate ligament (PCL) function. The PCL was preserved in the “cruciate-retaining” TKA, substituted in the “posterior-stabilized” TKA using a cam-post mechanism. The CR TKA designers believed that PCL preservation produced a more balanced knee with a more anatomical result, a more normal joint line, and better function, especially on stair climbing. The PS TKA designers admitted the value of posterior stabilization but argued that it was too difficult to consistently save the PCL in all cases, and that the PS knee was easier for surgeons to implant with more reliable roll back.7
The Geometric knee was developed in the 1970s to retain both cruciate ligaments.4 Unfortunately, it created a kinematic conflict by using a constrained articular surface design that prevented the motion required by the cruciate ligaments. This conflict resulted in tibial loosening and early failures. The compromised results decreased interest in the bicruciate-retaining (BCR) TKA designs, allowing the CR TKA and PS TKA designs to flourish for the next 20 years with little or no attempts to retain the ACL.
In the 1980s the BCR TKA design was pursued by Townley8 and Cartier.9 Townley8 believed that cruciate resection was a concession to “improper joint synchronization”8 and Cartier9 thought that cruciate preservation permitted more normal proprioception.9 Unlike prior BCR TKA designs, the mid-term clinical results were equal to or better than the standard CR TKA or PS TKA of the time, and 9- to 11-year follow-up demonstrated comparable outcomes.8 While these results highlighted the possibility of a BCR TKA, the surgical technique and failures of the Geometric knee discouraged surgeons from pursuing the BCR TKA.
Interest in cruciate-preserving knee arthroplasty returned with partial knee replacements, with patients reporting more normal proprioception and motion.10 The techniques became more popular with the introduction of the minimally invasive surgeries in the early 2000s and cruciate ligament preservation became a more interesting concept.11,12 Some surgeons preserved the cruciates by using separate implants for the medial, lateral, and patellofemoral surfaces.10 These results were acceptable for the time but required considerable surgical talent and did not report 20-year results similar to the CR and PS knees.
Most prosthetic designs attempt to copy the normal knee anatomy. Using fluoroscopic studies and computer analysis, designers began to investigate the motion (or kinematics) of the normal knee and realized that despite the fact the TKA looked like the human knee, the designs were not kinematically correct.13
Although TKA successfully treats pain secondary to degenerative joint disease, many patients are unable to return to their prior level of function, with up to 20% reporting dissatisfaction with their level of activity.14 The observed differences in kinematics between a normal knee and a TKA may explain part of this discrepancy.
Normal Knee Motion
The tibiofemoral articulation in a normal knee follows a reproducible pattern of motion as the knee moves from extension to flexion. The lateral femoral condyle (LFC) translates posteriorly with a combination of rolling and sliding motion, while the medial femoral condyle (MFC) has minimal posterior translation and thus acts as a pivot for knee motion. The MFC is larger, less curved, and has a biphasic shape with 2 distinct radiuses of curvature that correspond to an “extension” and “flexion” facet. The transition between the MFC facets occurs at approximately 30° of flexion, whereby the contact point transfers posteriorly with little condylar translation.15-17 In contrast, the LFC is smaller, has a single radius of curvature, and gradually translates posteriorly throughout flexion. Static magnetic resonance imaging of the knee from 0° to 120° shows an average of 19 mm posterior translation for the LFC and 2 mm for the MFC.15-20
In deep flexion, beyond 130°, posterior translation continues for both condyles. The LFC experiences enough excursion to cause loss of joint congruity and partial posterior subluxation.19,20 The MFC shows little additional posterior translation, yet it too loses joint congruity through condylar lift-off. Contact between the posterior horn of the medial meniscus and the posterior femoral condyle limits further flexion.16,21
The difference in motion between the condyles leads to internal tibial rotation during flexion. The initial 10° of knee flexion produces 5° of internal rotation, and an additional 15° of internal tibial rotation occurs throughout the remainder of knee flexion.
Fluoroscopic imaging with computed tomography (CT)- or magnetic resonance (MR)-based modeling has shown the dynamic in vivo relationship of the tibiofemoral joint. Studies have confirmed significantly greater LFC posterior translation as compared to the MFC;22 however, in vivo studies have also shown notable variability in articular rotation and translation based on activity. This highlights the role of ligamentous tension and muscle contraction in kinematics.21-23
The ACL in TKA
The majority of current TKA designs sacrifice the ACL without substituting for its function. The loss of the ACL has significant effects upon the kinematics of the knee.
The ACL is composed of 2 bundles, the anteromedial and posterolateral bundles, which originate on the LFC and insert broadly onto the tibial intercondylar eminence. Its primary role is to resist anterior tibial translation, particularly from 0° to 30° of flexion, which corresponds to the peak quadriceps force that pulls the tibia anteriorly.24 ACL deficiency causes anterior tibial translation during early flexion and abnormal internal tibial rotation.25-27 ACL deficient knees demonstrate a posterior femoral position in full extension, and increased MFC translation during knee flexion.28-32
The role of the ACL in knee arthroplasty has been evaluated by comparing unicompartmental knee arthroplasty (UKA) with TKA, as a reflection of ACL preserving vs sacrificing procedures.33-35 Sagittal plane translation is similar between UKA and normal knees,33,34 while the CR TKA and PS TKA designs show anterior tibia subluxation in full extension.33-35 The difference between UKA and TKA is greatest in extension, corresponding to the ACL functional range. These findings highlight kinematic similarities between TKA designs and the ACL deficient knee.
The majority of UKAs demonstrate near-normal kinematics. A small percentage of the study group demonstrated aberrant anterior tibial motion, highlighting a concern over ACL attenuation with time. Additionally, studies that evaluate the ACL in osteoarthritic knees have questioned the baseline integrity of the ACL.36 Yet the long-term outcomes in UKA design have shown preservation of kinematics due to intact cruciates.37
The PCL in TKA
Because the majority of TKA designs sacrifice the ACL, the classic debate has focused on the utility of the native PCL. Both the CR and PS TKA are designed to offer posterior stabilization; however, kinematic studies have demonstrated notable differences.38,39
The CR TKA design relies on the PCL to resist posterior sag and to prevent the hamstring musculature from pulling the tibia posteriorly during flexion. Studies have shown paradoxical anterior translation of both femoral condyles during flexion, particularly on the medial side of the knee.40 There is also increased variability in femoral rollback. It is unclear whether the PCL can function normally in the absence of the ACL, which causes the PCL to adapt a less anatomic vertical position. The PCL may also be unable to function significantly without the ACL because of pre-existing degenerative histological changes.41
The PS TKA utilizes a cam-post mechanism for posterior stabilization. In contrast to normal knee kinematics, this mechanism creates equal MFC and LFC posterior translation, 8 mm on average at 90° flexion.40 The equivalent translation in PS designs contributes to decreased internal tibial rotation and an increased polyethylene wear at the post.
Role of Surface Geometry
The articular geometry of the knee plays an important role in normal knee kinematics. Initial TKA designs used a femoral component with a single radius of curvature for both femoral condyles.42Current TKA designs that match the femoral component to the native femoral anatomy, by differing the medial and lateral condyle geometry, have demonstrated kinematics that better resemble a native knee.43 Additional changes to the radius of curvature along the posterior facet of the femoral condyles may reduce impingement during deep flexion. These “high flex” designs have demonstrated equivalent range of motion in some studies44 and improved weight-bearing motion in others.45 Surface geometry is important but is not the entire answer to kinematics.
Advances in TKA Design
Knee motion is guided by multiple factors, including the tibiofemoral articular geometry, the surrounding soft tissue tension, and muscle tone. Bicruciate-substituting (BCS) TKA and BCR TKA are forms of evolution from the CR and PS TKA and attempt to respect the function of both cruciate ligaments and provide better kinematics.
The BCS TKA utilizes a modified cam-post articulation to provide both anterior and posterior stabilization (Figure 4).46 The surgical approach remains the same and the implant geometry affects the motion. The BCS TKA design demonstrates femoral rollback at 90° with an average of 14 mm for the MFC and 23 mm for the LFC, and 10° internal tibial rotation.46,47 Additionally, it provides increased sagittal stability during early flexion and an improved pivot shift (indicating improved anterior stabilization).
The BCR designs preserve both cruciates and provide anterior and posterior stabilization. Fluoroscopic imaging has demonstrated contact points in full extension, and posterior rollback at 90° flexion that more closely replicates the normal knee.48
Design and Surgical Techniques for Bicruciate Knee Replacements
If all of the ligaments are preserved, the TKA surfaces must allow motion to be driven by the ligaments in combination with the surfaces alone. The femur can be designed anatomically with asymmetric condyles. The femoral box must allow for preservation of the tibial bone island without impinging upon the cruciate ligaments. The tibial surface must be minimally constrained with concavity medially and convexity laterally.
The bone island preservation does not permit a single-piece tibial polyethylene insert. Therefore, the inserts will replicate the UKA designs (Figure 5). The knee should allow greater range of motion with the possibility of heel to buttocks contact. This increased motion will lead to greater roll back of the femur on the tibia and can lead to subluxation of the femoral runner off of the tibial surface on the lateral side, mimicking the normal knee. This subluxation is desirable but may lead to increased wear of the polyethylene on the lateral side of the knee.
The instruments should be specific for the design but must also be user-friendly. The 2 major issues with the surgery are balancing the knee in full extension and flexion, and preservation of the tibial bone island. The preexisting knee deformity should be <10° in all planes to limit the amount of collateral ligament releases. The collaterals must be balanced in a similar fashion to the standard TKA. Flexion contracture can be treated with posterior capsular release around the cruciates or with an increased distal femoral resection (2 mm at the maximum).
It is important to size the femur correctly because it will be difficult to adjust the flexion gap on the tibial side. A 9-mm posterior medial femoral condyle resection is a reasonable guide if the condyle is not atrophic. However, the exact resection thickness will be implant-specific and should be correlated with the dimensions of the prosthesis being implanted. The tibial bone island must be properly rotated with respect to the center line (Akagi’s line)49 and must not be undercut. The tibial instrument should include pins or blocks to prevent the sawblades from undercutting the island (Figure 6), as undermining leads to fracture in full extension. If undermining occurs, it may be possible to place a cancellous screw through the island and still preserve the ligaments. The integrity of the island is best tested by bringing the knee to full extension and checking for liftoff of the bone. If there is significant compromise of the island, the bone should be resected and either a CR or PS TKA can be implanted. Della Valle and colleagues50 reported a 9.2% incidence (11 of 119 cases) of bone island fracture in their early experience with a BCR TKA and improved this to 1.9% (5/258 cases) after reassessing their technique.
The gap tension should be evaluated either with traditional spacer blocks or with tensioning devices on the medial and lateral side of the knee after the tibial resections are completed. The polyethylene inserts are anatomically different. It may be possible to vary the thickness from medial to lateral, but not in excess of 2 mm.
As the BCR surgical techniques evolve, the balancing and tibial resection may be refined through specialized instrumentation. Such “smart instruments” that incorporate gyros may expedite tibial alignment, and sensor devices may assist with gap balancing. Haptic surgical robotic guides may assist in the tibial resection, facilitating bone island preservation by avoiding any possibility of undermining. At present these assistive aides are not necessary for the operation but may play a future role.
Clinical Results of Knee Arthroplasties
The results of knee replacements improved steadily from the 1970s through the 1990s. The scoring systems were somewhat limited and there was little data on the perception of the patients. The prosthetic designs stabilized at the end of the 1990s with only minor modifications since the year 2000. The 20-year results show similar findings for both the CR and the PS designs. There is little evidence to suggest a clinical correlation with the observed kinematic differences between CR and PS TKA designs.40,51-58 Multiple studies have demonstrated equivalent range of motion38,39,59 and subjective outcome measures (Table 1).60 A randomized prospective trial that compared kinematics and functional scores between the 2 designs failed to observe significant differences in function despite differences in kinematics.46 Equivalence in clinical outcome was further supported by a Cochrane Review meta-analysis that evaluated 1810 patients in 17 selected studies.61 The Knee Society scores have all been in the 92% to 95% ratings with survivals between 90% and 95%.
However, only 80% to 90% of patients are fully satisfied with their implants. The reasons for the dissatisfaction include unexplained anterior knee pain, stiffness, unexplained swelling, loss of range of motion, changes in proprioception, and loss of preoperative functions.14
The mid-term results of the BCR knees that were performed in the 1980s showed similar results to the CR and PS knees. Townley8 reported excellent clinical results with only 2% loosening at 2 to 11 years after surgery. Cloutier and colleagues9 reported 95% survival with improved proprioception at 9 to 11 years after surgery(Table 2).62,63
Studies comparing traditional TKA designs with cruciate preserving designs, both UKA and BCR, have found differences in subjective outcomes.62,64 Comparison of UKA and TKA in the same patient demonstrated significant preference for UKA, particularly with stair-climbing.65 Similarly, comparison between BCR and PS TKA or CR TKA demonstrated preference for BCR in 85% of patients.62
The new BCR knee designs have just started to come to the market.50 The surgical techniques are much improved over the 1980s and cruciate preservation is certainly much easier now. The new designs can produce full range of motion with kinematics that are almost identical to the normal knee in the cadaver laboratory and in computer analyses. These designs certainly should have a similar 20-year survival to the original BCR knees. However, the critical evaluation will be the patient satisfaction scores. With greater motion, better kinematics, and more precise balancing the scores would improve with these designs.
Conclusion
The cruciate ligaments of the knee are central to control of the motion of the normal knee. TKA is a successful operation with at least a 40- to 50-year history. The techniques have continued to develop but 15% to 20% of patients are dissatisfied with the results.14 Evaluations of the prostheses are more sophisticated and kinematics appears to have a central position in the evaluation. If the knee is to move more anatomically correctly, all of the ligaments must be preserved. Proprioception certainly plays a role in the patient’s judgment of the result. History has shown that a BCR knee can be implanted with good mid-term results and it should certainly be possible to build on these results and design a knee that will incorporate all of the ligaments with full range of motion and increased levels of activity.
1. Walldius B. Arthroplasty of the knee with an endoprosthesis. Acta Chir Scand. 1957;113(6):445-446.
2. Gunston FH. Polycentric knee arthroplasty. Prosthetic simulation of normal knee movement. J Bone Joint Surg Br. 1971;53(2):272-277.
3. Insall JN, Ranawat CS, Aglietti P, Shine J. A comparison of four models of total knee-replacement prostheses. J Bone Joint Surg Am. 1976;58(6):754-765.
4. Coventry MB, Finerman GA, Riley LH, Turner RH, Upshaw JE. A new geometric knee for total knee arthroplasty. Clin Orthop Relat Res.1972;83:157-162.
5. Freeman MA, Sculco T, Todd RC. Replacement of the severely damaged arthritic knee by the ICLH (Freeman-Swanson) arthroplasty. J Bone Joint Surg Br. 1977;59(1):64-71.
6. Freeman MA, Insall JN, Besser W, Walker PS, Hallel T. Excision of the cruciate ligaments in total knee replacement. Clin Orthop Relat Res. 1977(126):209-212.
7. Pagnano MW, Cushner FD, Scott WN. Role of the posterior cruciate ligament in total knee arthroplasty. J Am Acad Orthop Surg. 1998;6(3):176-187.
8. Townley CO. The anatomic total knee resurfacing arthroplasty. Clin Orthop Relat Res. 1985(192):82-96.
9. Cloutier JM, Sabouret P, Deghrar A. Total knee arthroplasty with retention of both cruciate ligaments. A nine to eleven-year follow-up study. J Bone Joint Surg Am. 1999; 81(5):697-702.
10. Banks SA, Fregly BJ, Boniforti F, Reinschmidt C, Romagnoli S. Comparing in vivo kinematics of unicondylar and bi-unicondylar knee replacements. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):551-556.
11. Repicci JA, Eberle RW. Minimally invasive surgical technique for unicondylar knee arthroplasty. J South Orthop Assoc. 1999;8(1):20-27; discussion 27.
12. Romanowski MR, Repicci JA. Minimally invasive unicondylar arthroplasty: eight-year follow-up. J Knee Surg. 2002;15(1):17-22.
13. Banks SA, Markovich GD, Hodge WA. In vivo kinematics of cruciate-retaining and -substituting knee arthroplasties. J Arthroplasty. 1997;12(3):297-304.
14. Nam D, Nunley RM, Barrack RL. Patient dissatisfaction following total knee replacement: a growing concern? Bone Joint J. 2014;96-B(11 Supple A):96-100.
15. Iwaki H, Pinskerova V, Freeman MA. Tibiofemoral movement 1: the shapes and relative movements of the femur and tibia in the unloaded cadaver knee. J Bone Joint Surg Br. 2000;82(8):1189-1195.
16. Johal P, Williams A, Wragg P, Hunt D, Gedroyc W. Tibio-femoral movement in the living knee. A study of weight bearing and non-weight bearing knee kinematics using ‘interventional’ MRI. J Biomech. 2005;38(2):269-276.
17. Pinskerova V, Johal P, Nakagawa S, et al. Does the femur roll-back with flexion? J Bone Joint Surg Br. 2004;86(6):925-931.
18. Hill PF, Vedi V, Williams A, Pinskerova V, Freeman MA. Tibiofemoral movement 2: the loaded and unloaded living knee studied by MRI. J Bone Joint Surg Br. 2000;82(8):1196-1198.
19. Nakagawa S, Kadoya Y, Todo S, et al. Tibiofemoral movement 3: full flexion in the living knee studied by MRI. J Bone Joint Surg Br. 2000;82(8):1199-1200.
20. Freeman MA, Pinskerova V. The movement of the knee studied by magnetic resonance imaging. Clin Orthop Relat Res. 2003(410):35-43.
21. Moro-oka TA, Hamai S, Miura H, et al. Dynamic activity dependence of in vivo normal knee kinematics. J Orthop Res. 2008;26(4):428-434.
22. Komistek RD, Dennis DA, Mahfouz M. In vivo fluoroscopic analysis of the normal human knee. Clin Orthop Relat Res. 2003(410):69-81.
23. Li G, DeFrate LE, Park SE, Gill TJ, Rubash HE. In vivo articular cartilage contact kinematics of the knee: an investigation using dual-orthogonal fluoroscopy and magnetic resonance image-based computer models. Am J Sports Med. 2005;33(1):102-107.
24. Grood ES, Suntay WJ, Noyes FR, Butler DL. Biomechanics of the knee-extension exercise. Effect of cutting the anterior cruciate ligament. J Bone Joint Surg Am. 1984;66(5):725-734.
25. Noyes FR, Jetter AW, Grood ES, Harms SP, Gardner EJ, Levy MS. Anterior cruciate ligament function in providing rotational stability assessed by medial and lateral tibiofemoral compartment translations and subluxations. Am J Sports Med. 2015;43(3):683-692.
26. Good L, Askew MJ, Boom A, Melby A 3rd. Kinematic in-vitro comparison between the normal knee and two techniques for reconstruction of the anterior cruciate ligament. Clin Biomech (Bristol, Avon). 1993;8(5):243-249.
27. Beard DJ, Murray DW, Gill HS. Reconstruction does not reduce tibial translation in the cruciate-deficient knee an in vivo study. J Bone Joint Surg Br. 2001;83(8):1098-1103.
28. Dennis DA, Mahfouz MR, Komistek RD, Hoff W. In vivo determination of normal and anterior cruciate ligament-deficient knee kinematics. J Biomech. 2005;38(2):241-253.
29. Beynnon BD, Fleming BC, Labovitch R, Parsons B. Chronic anterior cruciate ligament deficiency is associated with increased anterior translation of the tibia during the transition from non-weightbearing to weightbearing. J Orthop Res. 2002;20(2):332-337.
30. Brandsson S, Karlsson J, Eriksson BI, Kärrholm J. Kinematics after tear in the anterior cruciate ligament: dynamic bilateral radiostereometric studies in 11 patients. Acta Orthop Scand. 2001;72(4):372-378.
31. Andriacchi TP, Briant PL, Bevill SL, Koo S. Rotational changes at the knee after ACL injury cause cartilage thinning. Clin Orthop Relat Res. 2006;442:39-44.
32. Scarvell JM, Smith PN, Refshauge KM, Galloway HR, Woods KR. Comparison of kinematic analysis by mapping tibiofemoral contact with movement of the femoral condylar centres in healthy and anterior cruciate ligament injured knees. J Orthop Res. 2004;22(5):955-962.
33. Miller RK, Goodfellow JW, Murray DW, O’Connor JJ. In vitro measurement of patellofemoral force after three types of knee replacement. J Bone Joint Surg Br. 1998;80(5):900-906.
34. Price AJ, Rees JL, Beard DL, Gill RH, Dodd CA, Murray DM. Sagittal plane kinematics of a mobile-bearing unicompartmental knee arthroplasty at 10 years: a comparative in vivo fluoroscopic analysis. J Arthroplasty. 2004;19(5):590-597.
35. Dennis D, Komistek R, Scuderi G, et al. In vivo three-dimensional determination of kinematics for subjects with a normal knee or a unicompartmental or total knee replacement. J Bone Joint Surg Am. 2001;83-A Suppl 2 Pt 2:104-115.
36. Arbuthnot JE, Brink RB. Assessment of the antero-posterior and rotational stability of the anterior cruciate ligament analogue in a guided motion bi-cruciate stabilized total knee arthroplasty. J Med Eng Technol. 2009;33(8):610-615.
37. Hollinghurst D, Stoney J, Ward T, et al. No deterioration of kinematics and cruciate function 10 years after medial unicompartmental arthroplasty. Knee. 2006;13(6):440-444.
38. Dennis DA, Komistek RD, Colwell CE Jr, et al. In vivo anteroposterior femorotibial translation of total knee arthroplasty: a multicenter analysis. Clin Orthop Relat Res. 1998(356):47-57.
39. Dennis DA, Komistek RD, Hoff WA, Gabriel SM. In vivo knee kinematics derived using an inverse perspective technique. Clin Orthop Relat Res. 1996;(331):107-117.
40. Yoshiya S, Matsui N, Komistek RD, Dennis DA, Mahfouz M, Kurosaka M. In vivo kinematic comparison of posterior cruciate-retaining and posterior stabilized total knee arthroplasties under passive and weight-bearing conditions. J Arthroplasty. 2005;20(6):777-783.
41. Kleinbart FA, Bryk E, Evangelista J, Scott WN, Vigorita VJ. Histologic comparison of posterior cruciate ligaments from arthritic and age-matched knee specimens. J Arthroplasty. 1996;11(6):726-731.
42. Bull AM, Kessler O, Alam M, Amis AA. Changes in knee kinematics reflect the articular geometry after arthroplasty. Clin Orthop Relat Res. 2008;466(10):2491-2499.
43. Komistek RD, Mahfouz MR, Bertin KC, Rosenberg A, Kennedy W. In vivo determination of total knee arthroplasty kinematics: a multicenter analysis of an asymmetrical posterior cruciate retaining total knee arthroplasty. J Arthroplasty. 2008;23(1):41-50.
44. Mehin R, Burnett RS, Brasher PM. Does the new generation of high-flex knee prostheses improve the post-operative range of movement?: a meta-analysis. J Bone Joint Surg Br. 2010;92(10):1429-1434.
45. Dennis DA, Heekin RD, Clark CR, Murphy JA, O’Dell TL, Dwyer KA. Effect of implant design on knee flexion. J Arthroplasty. 2013;28(3):429-438.
46. Victor J, Mueller JK, Komistek RD, Sharma A, Nadaud MC, Bellemans J. In vivo kinematics after a cruciate-substituting TKA. Clin Orthop Relat Res. 2010;468(3):807-814.
47. Catani F, Ensini A, Belvedere C, et al. In vivo kinematics and kinetics of a bi-cruciate substituting total knee arthroplasty: a combined fluoroscopic and gait analysis study. J Orthop Res. 2009;27(12):1569-1575.
48. Stiehl JB, Komistek RD, Cloutier JM, Dennis DA. The cruciate ligaments in total knee arthroplasty: a kinematic analysis of 2 total knee arthroplasties. J Arthroplasty. 2000;15(5):545-550.
49. Akagi M, Oh M, Nonaka T, Tsujimoto H, Asano T, Hamanishi C. An anteroposterior axis of the tibia for total knee arthroplasty. Clin Orthop Relat Res. 2004;(420):213-219.
50. Della Valle CJ, Andriacchi TP, Berend KR, DeClaire JH, Lombardi AV Jr, Peters CL. Early experience with bi-cruciate retaining TKA. Poster presented at: American Academy of Orthopaedic Surgeons 2015 Annual Meeting; March 24-28, 2015; Las Vegas, NV.
51. Udomkiat P, Meng BJ, Dorr LD, Wan Z. Functional comparison of posterior cruciate retention and substitution knee replacement. Clin Orthop Relat Res. 2000;(378):192-201.
52. Tanzer M, Smith K, Burnett S. Posterior-stabilized versus cruciate-retaining total knee arthroplasty: balancing the gap. J Arthroplasty. 2002;17(7):813-819.
53. Maruyama S, Yoshiya S, Matsui N, Kuroda R, Kurosaka M. Functional comparison of posterior cruciate-retaining versus posterior stabilized total knee arthroplasty. J Arthroplasty. 2004;19(3):349-53.
54. Clark CR, Rorabeck CH, MacDonald S, MacDonald D, Swafford J, Cleland D. Posterior-stabilized and cruciate-retaining total knee replacement: a randomized study. Clin Orthop Relat Res. 2001;(392):208-212.
55. Swanik CB, Lephart SM, Rubash HE. Proprioception, kinesthesia, and balance after total knee arthroplasty with cruciate-retaining and posterior stabilized prostheses. J Bone Joint Surg Am. 2004;86-A(2):328-334.
56. Harato K, Bourne RB, Victor J, Snyder M, Hart J, Ries MD. Midterm comparison of posterior cruciate-retaining versus -substituting total knee arthroplasty using the Genesis II prosthesis. A multicenter prospective randomized clinical trial. Knee. 2008;15(3):217-221.
57. Catani F, Leardini A, Ensini A, et al. The stability of the cemented tibial component of total knee arthroplasty: posterior cruciate-retaining versus posterior-stabilized design. J Arthroplasty. 2004;19(6):775-782.
58. Dennis DA, Komistek RD, Stiehl JB, Walker SA, Dennis KN. Range of motion after total knee arthroplasty: the effect of implant design and weight-bearing conditions. J Arthroplasty. 1998;13(7):748-752.
59. Becker MW, Insall JN, Faris PM. Bilateral total knee arthroplasty. One cruciate retaining and one cruciate substituting. Clin Orthop Relat Res. 1991;(271):122-124.
60. Kim YH, Choi Y, Kwon OR, Kim JS. Functional outcome and range of motion of high-flexion posterior cruciate-retaining and high-flexion posterior cruciate-substituting total knee prostheses. A prospective, randomized study. J Bone Joint Surg Am. 2009;91(4):753-760.
61. Verra WC, van den Boom LG, Jacobs W, Clement DJ, Wymenga AA, Nelissen RG. Retention versus sacrifice of the posterior cruciate ligament in total knee arthroplasty for treating osteoarthritis. Cochrane Database Syst Rev. 2013;10:CD004803.
62. Pritchett JW. Patients prefer a bicruciate-retaining or the medial pivot total knee prosthesis. J Arthroplasty. 2011;26(2):224-228.
63. Sabouret P, Lavoie F, Cloutier JM. Total knee replacement with retention of both cruciate ligaments: a 22-year follow-up study. Bone Joint J. 2013;95-B(7):917-922.
64. Andriacchi TP, Galante JO, Fermier RW. The influence of total knee-replacement design on walking and stair-climbing. J Bone Joint Surg Am. 1982;64(9):1328-1335.
65. Laurencin CT, Zelicof SB, Scott RD, Ewald FC. Unicompartmental versus total knee arthroplasty in the same patient. A comparative study. Clin Orthop Relat Res. 1991;(273):151-156.
66. Victor J, Banks S, Bellemans J. Kinematics of posterior cruciate ligament-retaining and -substituting total knee arthroplasty: a prospective randomised outcome study. J Bone Joint Surg Br. 2005;87(5):646-655.
Hinge knee arthroplasty was introduced in the 1950s.1 All 4 major ligaments were replaced by the hinge, which provided stabilization while allowing sagittal plane motion. Its goal was stability, not replication of normal kinematics. The addition of methyl methacrylate cement improved fixation and allowed surface design modifications that addressed normal articular motion. Implants such as the Gunston Polycentric,2 the Duocondylar,3 and the Geometric4 resurfaced the medial and lateral compartments of the knee while preserving the cruciate ligaments. The implants were subject to greater translational forces without the hinge and loosening became a major problem despite the advances in cementing. It became evident in the 1970s that preservation of the cruciates complicated the procedure. Cruciate resection simplified the operation and allowed improved fixation. The ICLH prosthesis resected the cruciates and used the articular surface design to give stability to the knee.5,6 The total condylar prosthesis had a “tibial” imminence that mimicked the shape of the tibial surface but also sacrificed both of the cruciate ligaments (Figure 1).
Designers recognized that the cruciate ligaments affected knee kinematics; however, they elected to sacrifice the anterior cruciate ligament (ACL) for surgical simplicity and implant longevity.6 In the early 1980s, both the cruciate-retaining (CR) total knee arthroplasty (TKA) (Figure 2) and posterior-stabilized (PS) TKA (Figure 3) designs addressed the posterior cruciate ligament (PCL) function. The PCL was preserved in the “cruciate-retaining” TKA, substituted in the “posterior-stabilized” TKA using a cam-post mechanism. The CR TKA designers believed that PCL preservation produced a more balanced knee with a more anatomical result, a more normal joint line, and better function, especially on stair climbing. The PS TKA designers admitted the value of posterior stabilization but argued that it was too difficult to consistently save the PCL in all cases, and that the PS knee was easier for surgeons to implant with more reliable roll back.7
The Geometric knee was developed in the 1970s to retain both cruciate ligaments.4 Unfortunately, it created a kinematic conflict by using a constrained articular surface design that prevented the motion required by the cruciate ligaments. This conflict resulted in tibial loosening and early failures. The compromised results decreased interest in the bicruciate-retaining (BCR) TKA designs, allowing the CR TKA and PS TKA designs to flourish for the next 20 years with little or no attempts to retain the ACL.
In the 1980s the BCR TKA design was pursued by Townley8 and Cartier.9 Townley8 believed that cruciate resection was a concession to “improper joint synchronization”8 and Cartier9 thought that cruciate preservation permitted more normal proprioception.9 Unlike prior BCR TKA designs, the mid-term clinical results were equal to or better than the standard CR TKA or PS TKA of the time, and 9- to 11-year follow-up demonstrated comparable outcomes.8 While these results highlighted the possibility of a BCR TKA, the surgical technique and failures of the Geometric knee discouraged surgeons from pursuing the BCR TKA.
Interest in cruciate-preserving knee arthroplasty returned with partial knee replacements, with patients reporting more normal proprioception and motion.10 The techniques became more popular with the introduction of the minimally invasive surgeries in the early 2000s and cruciate ligament preservation became a more interesting concept.11,12 Some surgeons preserved the cruciates by using separate implants for the medial, lateral, and patellofemoral surfaces.10 These results were acceptable for the time but required considerable surgical talent and did not report 20-year results similar to the CR and PS knees.
Most prosthetic designs attempt to copy the normal knee anatomy. Using fluoroscopic studies and computer analysis, designers began to investigate the motion (or kinematics) of the normal knee and realized that despite the fact the TKA looked like the human knee, the designs were not kinematically correct.13
Although TKA successfully treats pain secondary to degenerative joint disease, many patients are unable to return to their prior level of function, with up to 20% reporting dissatisfaction with their level of activity.14 The observed differences in kinematics between a normal knee and a TKA may explain part of this discrepancy.
Normal Knee Motion
The tibiofemoral articulation in a normal knee follows a reproducible pattern of motion as the knee moves from extension to flexion. The lateral femoral condyle (LFC) translates posteriorly with a combination of rolling and sliding motion, while the medial femoral condyle (MFC) has minimal posterior translation and thus acts as a pivot for knee motion. The MFC is larger, less curved, and has a biphasic shape with 2 distinct radiuses of curvature that correspond to an “extension” and “flexion” facet. The transition between the MFC facets occurs at approximately 30° of flexion, whereby the contact point transfers posteriorly with little condylar translation.15-17 In contrast, the LFC is smaller, has a single radius of curvature, and gradually translates posteriorly throughout flexion. Static magnetic resonance imaging of the knee from 0° to 120° shows an average of 19 mm posterior translation for the LFC and 2 mm for the MFC.15-20
In deep flexion, beyond 130°, posterior translation continues for both condyles. The LFC experiences enough excursion to cause loss of joint congruity and partial posterior subluxation.19,20 The MFC shows little additional posterior translation, yet it too loses joint congruity through condylar lift-off. Contact between the posterior horn of the medial meniscus and the posterior femoral condyle limits further flexion.16,21
The difference in motion between the condyles leads to internal tibial rotation during flexion. The initial 10° of knee flexion produces 5° of internal rotation, and an additional 15° of internal tibial rotation occurs throughout the remainder of knee flexion.
Fluoroscopic imaging with computed tomography (CT)- or magnetic resonance (MR)-based modeling has shown the dynamic in vivo relationship of the tibiofemoral joint. Studies have confirmed significantly greater LFC posterior translation as compared to the MFC;22 however, in vivo studies have also shown notable variability in articular rotation and translation based on activity. This highlights the role of ligamentous tension and muscle contraction in kinematics.21-23
The ACL in TKA
The majority of current TKA designs sacrifice the ACL without substituting for its function. The loss of the ACL has significant effects upon the kinematics of the knee.
The ACL is composed of 2 bundles, the anteromedial and posterolateral bundles, which originate on the LFC and insert broadly onto the tibial intercondylar eminence. Its primary role is to resist anterior tibial translation, particularly from 0° to 30° of flexion, which corresponds to the peak quadriceps force that pulls the tibia anteriorly.24 ACL deficiency causes anterior tibial translation during early flexion and abnormal internal tibial rotation.25-27 ACL deficient knees demonstrate a posterior femoral position in full extension, and increased MFC translation during knee flexion.28-32
The role of the ACL in knee arthroplasty has been evaluated by comparing unicompartmental knee arthroplasty (UKA) with TKA, as a reflection of ACL preserving vs sacrificing procedures.33-35 Sagittal plane translation is similar between UKA and normal knees,33,34 while the CR TKA and PS TKA designs show anterior tibia subluxation in full extension.33-35 The difference between UKA and TKA is greatest in extension, corresponding to the ACL functional range. These findings highlight kinematic similarities between TKA designs and the ACL deficient knee.
The majority of UKAs demonstrate near-normal kinematics. A small percentage of the study group demonstrated aberrant anterior tibial motion, highlighting a concern over ACL attenuation with time. Additionally, studies that evaluate the ACL in osteoarthritic knees have questioned the baseline integrity of the ACL.36 Yet the long-term outcomes in UKA design have shown preservation of kinematics due to intact cruciates.37
The PCL in TKA
Because the majority of TKA designs sacrifice the ACL, the classic debate has focused on the utility of the native PCL. Both the CR and PS TKA are designed to offer posterior stabilization; however, kinematic studies have demonstrated notable differences.38,39
The CR TKA design relies on the PCL to resist posterior sag and to prevent the hamstring musculature from pulling the tibia posteriorly during flexion. Studies have shown paradoxical anterior translation of both femoral condyles during flexion, particularly on the medial side of the knee.40 There is also increased variability in femoral rollback. It is unclear whether the PCL can function normally in the absence of the ACL, which causes the PCL to adapt a less anatomic vertical position. The PCL may also be unable to function significantly without the ACL because of pre-existing degenerative histological changes.41
The PS TKA utilizes a cam-post mechanism for posterior stabilization. In contrast to normal knee kinematics, this mechanism creates equal MFC and LFC posterior translation, 8 mm on average at 90° flexion.40 The equivalent translation in PS designs contributes to decreased internal tibial rotation and an increased polyethylene wear at the post.
Role of Surface Geometry
The articular geometry of the knee plays an important role in normal knee kinematics. Initial TKA designs used a femoral component with a single radius of curvature for both femoral condyles.42Current TKA designs that match the femoral component to the native femoral anatomy, by differing the medial and lateral condyle geometry, have demonstrated kinematics that better resemble a native knee.43 Additional changes to the radius of curvature along the posterior facet of the femoral condyles may reduce impingement during deep flexion. These “high flex” designs have demonstrated equivalent range of motion in some studies44 and improved weight-bearing motion in others.45 Surface geometry is important but is not the entire answer to kinematics.
Advances in TKA Design
Knee motion is guided by multiple factors, including the tibiofemoral articular geometry, the surrounding soft tissue tension, and muscle tone. Bicruciate-substituting (BCS) TKA and BCR TKA are forms of evolution from the CR and PS TKA and attempt to respect the function of both cruciate ligaments and provide better kinematics.
The BCS TKA utilizes a modified cam-post articulation to provide both anterior and posterior stabilization (Figure 4).46 The surgical approach remains the same and the implant geometry affects the motion. The BCS TKA design demonstrates femoral rollback at 90° with an average of 14 mm for the MFC and 23 mm for the LFC, and 10° internal tibial rotation.46,47 Additionally, it provides increased sagittal stability during early flexion and an improved pivot shift (indicating improved anterior stabilization).
The BCR designs preserve both cruciates and provide anterior and posterior stabilization. Fluoroscopic imaging has demonstrated contact points in full extension, and posterior rollback at 90° flexion that more closely replicates the normal knee.48
Design and Surgical Techniques for Bicruciate Knee Replacements
If all of the ligaments are preserved, the TKA surfaces must allow motion to be driven by the ligaments in combination with the surfaces alone. The femur can be designed anatomically with asymmetric condyles. The femoral box must allow for preservation of the tibial bone island without impinging upon the cruciate ligaments. The tibial surface must be minimally constrained with concavity medially and convexity laterally.
The bone island preservation does not permit a single-piece tibial polyethylene insert. Therefore, the inserts will replicate the UKA designs (Figure 5). The knee should allow greater range of motion with the possibility of heel to buttocks contact. This increased motion will lead to greater roll back of the femur on the tibia and can lead to subluxation of the femoral runner off of the tibial surface on the lateral side, mimicking the normal knee. This subluxation is desirable but may lead to increased wear of the polyethylene on the lateral side of the knee.
The instruments should be specific for the design but must also be user-friendly. The 2 major issues with the surgery are balancing the knee in full extension and flexion, and preservation of the tibial bone island. The preexisting knee deformity should be <10° in all planes to limit the amount of collateral ligament releases. The collaterals must be balanced in a similar fashion to the standard TKA. Flexion contracture can be treated with posterior capsular release around the cruciates or with an increased distal femoral resection (2 mm at the maximum).
It is important to size the femur correctly because it will be difficult to adjust the flexion gap on the tibial side. A 9-mm posterior medial femoral condyle resection is a reasonable guide if the condyle is not atrophic. However, the exact resection thickness will be implant-specific and should be correlated with the dimensions of the prosthesis being implanted. The tibial bone island must be properly rotated with respect to the center line (Akagi’s line)49 and must not be undercut. The tibial instrument should include pins or blocks to prevent the sawblades from undercutting the island (Figure 6), as undermining leads to fracture in full extension. If undermining occurs, it may be possible to place a cancellous screw through the island and still preserve the ligaments. The integrity of the island is best tested by bringing the knee to full extension and checking for liftoff of the bone. If there is significant compromise of the island, the bone should be resected and either a CR or PS TKA can be implanted. Della Valle and colleagues50 reported a 9.2% incidence (11 of 119 cases) of bone island fracture in their early experience with a BCR TKA and improved this to 1.9% (5/258 cases) after reassessing their technique.
The gap tension should be evaluated either with traditional spacer blocks or with tensioning devices on the medial and lateral side of the knee after the tibial resections are completed. The polyethylene inserts are anatomically different. It may be possible to vary the thickness from medial to lateral, but not in excess of 2 mm.
As the BCR surgical techniques evolve, the balancing and tibial resection may be refined through specialized instrumentation. Such “smart instruments” that incorporate gyros may expedite tibial alignment, and sensor devices may assist with gap balancing. Haptic surgical robotic guides may assist in the tibial resection, facilitating bone island preservation by avoiding any possibility of undermining. At present these assistive aides are not necessary for the operation but may play a future role.
Clinical Results of Knee Arthroplasties
The results of knee replacements improved steadily from the 1970s through the 1990s. The scoring systems were somewhat limited and there was little data on the perception of the patients. The prosthetic designs stabilized at the end of the 1990s with only minor modifications since the year 2000. The 20-year results show similar findings for both the CR and the PS designs. There is little evidence to suggest a clinical correlation with the observed kinematic differences between CR and PS TKA designs.40,51-58 Multiple studies have demonstrated equivalent range of motion38,39,59 and subjective outcome measures (Table 1).60 A randomized prospective trial that compared kinematics and functional scores between the 2 designs failed to observe significant differences in function despite differences in kinematics.46 Equivalence in clinical outcome was further supported by a Cochrane Review meta-analysis that evaluated 1810 patients in 17 selected studies.61 The Knee Society scores have all been in the 92% to 95% ratings with survivals between 90% and 95%.
However, only 80% to 90% of patients are fully satisfied with their implants. The reasons for the dissatisfaction include unexplained anterior knee pain, stiffness, unexplained swelling, loss of range of motion, changes in proprioception, and loss of preoperative functions.14
The mid-term results of the BCR knees that were performed in the 1980s showed similar results to the CR and PS knees. Townley8 reported excellent clinical results with only 2% loosening at 2 to 11 years after surgery. Cloutier and colleagues9 reported 95% survival with improved proprioception at 9 to 11 years after surgery(Table 2).62,63
Studies comparing traditional TKA designs with cruciate preserving designs, both UKA and BCR, have found differences in subjective outcomes.62,64 Comparison of UKA and TKA in the same patient demonstrated significant preference for UKA, particularly with stair-climbing.65 Similarly, comparison between BCR and PS TKA or CR TKA demonstrated preference for BCR in 85% of patients.62
The new BCR knee designs have just started to come to the market.50 The surgical techniques are much improved over the 1980s and cruciate preservation is certainly much easier now. The new designs can produce full range of motion with kinematics that are almost identical to the normal knee in the cadaver laboratory and in computer analyses. These designs certainly should have a similar 20-year survival to the original BCR knees. However, the critical evaluation will be the patient satisfaction scores. With greater motion, better kinematics, and more precise balancing the scores would improve with these designs.
Conclusion
The cruciate ligaments of the knee are central to control of the motion of the normal knee. TKA is a successful operation with at least a 40- to 50-year history. The techniques have continued to develop but 15% to 20% of patients are dissatisfied with the results.14 Evaluations of the prostheses are more sophisticated and kinematics appears to have a central position in the evaluation. If the knee is to move more anatomically correctly, all of the ligaments must be preserved. Proprioception certainly plays a role in the patient’s judgment of the result. History has shown that a BCR knee can be implanted with good mid-term results and it should certainly be possible to build on these results and design a knee that will incorporate all of the ligaments with full range of motion and increased levels of activity.
Hinge knee arthroplasty was introduced in the 1950s.1 All 4 major ligaments were replaced by the hinge, which provided stabilization while allowing sagittal plane motion. Its goal was stability, not replication of normal kinematics. The addition of methyl methacrylate cement improved fixation and allowed surface design modifications that addressed normal articular motion. Implants such as the Gunston Polycentric,2 the Duocondylar,3 and the Geometric4 resurfaced the medial and lateral compartments of the knee while preserving the cruciate ligaments. The implants were subject to greater translational forces without the hinge and loosening became a major problem despite the advances in cementing. It became evident in the 1970s that preservation of the cruciates complicated the procedure. Cruciate resection simplified the operation and allowed improved fixation. The ICLH prosthesis resected the cruciates and used the articular surface design to give stability to the knee.5,6 The total condylar prosthesis had a “tibial” imminence that mimicked the shape of the tibial surface but also sacrificed both of the cruciate ligaments (Figure 1).
Designers recognized that the cruciate ligaments affected knee kinematics; however, they elected to sacrifice the anterior cruciate ligament (ACL) for surgical simplicity and implant longevity.6 In the early 1980s, both the cruciate-retaining (CR) total knee arthroplasty (TKA) (Figure 2) and posterior-stabilized (PS) TKA (Figure 3) designs addressed the posterior cruciate ligament (PCL) function. The PCL was preserved in the “cruciate-retaining” TKA, substituted in the “posterior-stabilized” TKA using a cam-post mechanism. The CR TKA designers believed that PCL preservation produced a more balanced knee with a more anatomical result, a more normal joint line, and better function, especially on stair climbing. The PS TKA designers admitted the value of posterior stabilization but argued that it was too difficult to consistently save the PCL in all cases, and that the PS knee was easier for surgeons to implant with more reliable roll back.7
The Geometric knee was developed in the 1970s to retain both cruciate ligaments.4 Unfortunately, it created a kinematic conflict by using a constrained articular surface design that prevented the motion required by the cruciate ligaments. This conflict resulted in tibial loosening and early failures. The compromised results decreased interest in the bicruciate-retaining (BCR) TKA designs, allowing the CR TKA and PS TKA designs to flourish for the next 20 years with little or no attempts to retain the ACL.
In the 1980s the BCR TKA design was pursued by Townley8 and Cartier.9 Townley8 believed that cruciate resection was a concession to “improper joint synchronization”8 and Cartier9 thought that cruciate preservation permitted more normal proprioception.9 Unlike prior BCR TKA designs, the mid-term clinical results were equal to or better than the standard CR TKA or PS TKA of the time, and 9- to 11-year follow-up demonstrated comparable outcomes.8 While these results highlighted the possibility of a BCR TKA, the surgical technique and failures of the Geometric knee discouraged surgeons from pursuing the BCR TKA.
Interest in cruciate-preserving knee arthroplasty returned with partial knee replacements, with patients reporting more normal proprioception and motion.10 The techniques became more popular with the introduction of the minimally invasive surgeries in the early 2000s and cruciate ligament preservation became a more interesting concept.11,12 Some surgeons preserved the cruciates by using separate implants for the medial, lateral, and patellofemoral surfaces.10 These results were acceptable for the time but required considerable surgical talent and did not report 20-year results similar to the CR and PS knees.
Most prosthetic designs attempt to copy the normal knee anatomy. Using fluoroscopic studies and computer analysis, designers began to investigate the motion (or kinematics) of the normal knee and realized that despite the fact the TKA looked like the human knee, the designs were not kinematically correct.13
Although TKA successfully treats pain secondary to degenerative joint disease, many patients are unable to return to their prior level of function, with up to 20% reporting dissatisfaction with their level of activity.14 The observed differences in kinematics between a normal knee and a TKA may explain part of this discrepancy.
Normal Knee Motion
The tibiofemoral articulation in a normal knee follows a reproducible pattern of motion as the knee moves from extension to flexion. The lateral femoral condyle (LFC) translates posteriorly with a combination of rolling and sliding motion, while the medial femoral condyle (MFC) has minimal posterior translation and thus acts as a pivot for knee motion. The MFC is larger, less curved, and has a biphasic shape with 2 distinct radiuses of curvature that correspond to an “extension” and “flexion” facet. The transition between the MFC facets occurs at approximately 30° of flexion, whereby the contact point transfers posteriorly with little condylar translation.15-17 In contrast, the LFC is smaller, has a single radius of curvature, and gradually translates posteriorly throughout flexion. Static magnetic resonance imaging of the knee from 0° to 120° shows an average of 19 mm posterior translation for the LFC and 2 mm for the MFC.15-20
In deep flexion, beyond 130°, posterior translation continues for both condyles. The LFC experiences enough excursion to cause loss of joint congruity and partial posterior subluxation.19,20 The MFC shows little additional posterior translation, yet it too loses joint congruity through condylar lift-off. Contact between the posterior horn of the medial meniscus and the posterior femoral condyle limits further flexion.16,21
The difference in motion between the condyles leads to internal tibial rotation during flexion. The initial 10° of knee flexion produces 5° of internal rotation, and an additional 15° of internal tibial rotation occurs throughout the remainder of knee flexion.
Fluoroscopic imaging with computed tomography (CT)- or magnetic resonance (MR)-based modeling has shown the dynamic in vivo relationship of the tibiofemoral joint. Studies have confirmed significantly greater LFC posterior translation as compared to the MFC;22 however, in vivo studies have also shown notable variability in articular rotation and translation based on activity. This highlights the role of ligamentous tension and muscle contraction in kinematics.21-23
The ACL in TKA
The majority of current TKA designs sacrifice the ACL without substituting for its function. The loss of the ACL has significant effects upon the kinematics of the knee.
The ACL is composed of 2 bundles, the anteromedial and posterolateral bundles, which originate on the LFC and insert broadly onto the tibial intercondylar eminence. Its primary role is to resist anterior tibial translation, particularly from 0° to 30° of flexion, which corresponds to the peak quadriceps force that pulls the tibia anteriorly.24 ACL deficiency causes anterior tibial translation during early flexion and abnormal internal tibial rotation.25-27 ACL deficient knees demonstrate a posterior femoral position in full extension, and increased MFC translation during knee flexion.28-32
The role of the ACL in knee arthroplasty has been evaluated by comparing unicompartmental knee arthroplasty (UKA) with TKA, as a reflection of ACL preserving vs sacrificing procedures.33-35 Sagittal plane translation is similar between UKA and normal knees,33,34 while the CR TKA and PS TKA designs show anterior tibia subluxation in full extension.33-35 The difference between UKA and TKA is greatest in extension, corresponding to the ACL functional range. These findings highlight kinematic similarities between TKA designs and the ACL deficient knee.
The majority of UKAs demonstrate near-normal kinematics. A small percentage of the study group demonstrated aberrant anterior tibial motion, highlighting a concern over ACL attenuation with time. Additionally, studies that evaluate the ACL in osteoarthritic knees have questioned the baseline integrity of the ACL.36 Yet the long-term outcomes in UKA design have shown preservation of kinematics due to intact cruciates.37
The PCL in TKA
Because the majority of TKA designs sacrifice the ACL, the classic debate has focused on the utility of the native PCL. Both the CR and PS TKA are designed to offer posterior stabilization; however, kinematic studies have demonstrated notable differences.38,39
The CR TKA design relies on the PCL to resist posterior sag and to prevent the hamstring musculature from pulling the tibia posteriorly during flexion. Studies have shown paradoxical anterior translation of both femoral condyles during flexion, particularly on the medial side of the knee.40 There is also increased variability in femoral rollback. It is unclear whether the PCL can function normally in the absence of the ACL, which causes the PCL to adapt a less anatomic vertical position. The PCL may also be unable to function significantly without the ACL because of pre-existing degenerative histological changes.41
The PS TKA utilizes a cam-post mechanism for posterior stabilization. In contrast to normal knee kinematics, this mechanism creates equal MFC and LFC posterior translation, 8 mm on average at 90° flexion.40 The equivalent translation in PS designs contributes to decreased internal tibial rotation and an increased polyethylene wear at the post.
Role of Surface Geometry
The articular geometry of the knee plays an important role in normal knee kinematics. Initial TKA designs used a femoral component with a single radius of curvature for both femoral condyles.42Current TKA designs that match the femoral component to the native femoral anatomy, by differing the medial and lateral condyle geometry, have demonstrated kinematics that better resemble a native knee.43 Additional changes to the radius of curvature along the posterior facet of the femoral condyles may reduce impingement during deep flexion. These “high flex” designs have demonstrated equivalent range of motion in some studies44 and improved weight-bearing motion in others.45 Surface geometry is important but is not the entire answer to kinematics.
Advances in TKA Design
Knee motion is guided by multiple factors, including the tibiofemoral articular geometry, the surrounding soft tissue tension, and muscle tone. Bicruciate-substituting (BCS) TKA and BCR TKA are forms of evolution from the CR and PS TKA and attempt to respect the function of both cruciate ligaments and provide better kinematics.
The BCS TKA utilizes a modified cam-post articulation to provide both anterior and posterior stabilization (Figure 4).46 The surgical approach remains the same and the implant geometry affects the motion. The BCS TKA design demonstrates femoral rollback at 90° with an average of 14 mm for the MFC and 23 mm for the LFC, and 10° internal tibial rotation.46,47 Additionally, it provides increased sagittal stability during early flexion and an improved pivot shift (indicating improved anterior stabilization).
The BCR designs preserve both cruciates and provide anterior and posterior stabilization. Fluoroscopic imaging has demonstrated contact points in full extension, and posterior rollback at 90° flexion that more closely replicates the normal knee.48
Design and Surgical Techniques for Bicruciate Knee Replacements
If all of the ligaments are preserved, the TKA surfaces must allow motion to be driven by the ligaments in combination with the surfaces alone. The femur can be designed anatomically with asymmetric condyles. The femoral box must allow for preservation of the tibial bone island without impinging upon the cruciate ligaments. The tibial surface must be minimally constrained with concavity medially and convexity laterally.
The bone island preservation does not permit a single-piece tibial polyethylene insert. Therefore, the inserts will replicate the UKA designs (Figure 5). The knee should allow greater range of motion with the possibility of heel to buttocks contact. This increased motion will lead to greater roll back of the femur on the tibia and can lead to subluxation of the femoral runner off of the tibial surface on the lateral side, mimicking the normal knee. This subluxation is desirable but may lead to increased wear of the polyethylene on the lateral side of the knee.
The instruments should be specific for the design but must also be user-friendly. The 2 major issues with the surgery are balancing the knee in full extension and flexion, and preservation of the tibial bone island. The preexisting knee deformity should be <10° in all planes to limit the amount of collateral ligament releases. The collaterals must be balanced in a similar fashion to the standard TKA. Flexion contracture can be treated with posterior capsular release around the cruciates or with an increased distal femoral resection (2 mm at the maximum).
It is important to size the femur correctly because it will be difficult to adjust the flexion gap on the tibial side. A 9-mm posterior medial femoral condyle resection is a reasonable guide if the condyle is not atrophic. However, the exact resection thickness will be implant-specific and should be correlated with the dimensions of the prosthesis being implanted. The tibial bone island must be properly rotated with respect to the center line (Akagi’s line)49 and must not be undercut. The tibial instrument should include pins or blocks to prevent the sawblades from undercutting the island (Figure 6), as undermining leads to fracture in full extension. If undermining occurs, it may be possible to place a cancellous screw through the island and still preserve the ligaments. The integrity of the island is best tested by bringing the knee to full extension and checking for liftoff of the bone. If there is significant compromise of the island, the bone should be resected and either a CR or PS TKA can be implanted. Della Valle and colleagues50 reported a 9.2% incidence (11 of 119 cases) of bone island fracture in their early experience with a BCR TKA and improved this to 1.9% (5/258 cases) after reassessing their technique.
The gap tension should be evaluated either with traditional spacer blocks or with tensioning devices on the medial and lateral side of the knee after the tibial resections are completed. The polyethylene inserts are anatomically different. It may be possible to vary the thickness from medial to lateral, but not in excess of 2 mm.
As the BCR surgical techniques evolve, the balancing and tibial resection may be refined through specialized instrumentation. Such “smart instruments” that incorporate gyros may expedite tibial alignment, and sensor devices may assist with gap balancing. Haptic surgical robotic guides may assist in the tibial resection, facilitating bone island preservation by avoiding any possibility of undermining. At present these assistive aides are not necessary for the operation but may play a future role.
Clinical Results of Knee Arthroplasties
The results of knee replacements improved steadily from the 1970s through the 1990s. The scoring systems were somewhat limited and there was little data on the perception of the patients. The prosthetic designs stabilized at the end of the 1990s with only minor modifications since the year 2000. The 20-year results show similar findings for both the CR and the PS designs. There is little evidence to suggest a clinical correlation with the observed kinematic differences between CR and PS TKA designs.40,51-58 Multiple studies have demonstrated equivalent range of motion38,39,59 and subjective outcome measures (Table 1).60 A randomized prospective trial that compared kinematics and functional scores between the 2 designs failed to observe significant differences in function despite differences in kinematics.46 Equivalence in clinical outcome was further supported by a Cochrane Review meta-analysis that evaluated 1810 patients in 17 selected studies.61 The Knee Society scores have all been in the 92% to 95% ratings with survivals between 90% and 95%.
However, only 80% to 90% of patients are fully satisfied with their implants. The reasons for the dissatisfaction include unexplained anterior knee pain, stiffness, unexplained swelling, loss of range of motion, changes in proprioception, and loss of preoperative functions.14
The mid-term results of the BCR knees that were performed in the 1980s showed similar results to the CR and PS knees. Townley8 reported excellent clinical results with only 2% loosening at 2 to 11 years after surgery. Cloutier and colleagues9 reported 95% survival with improved proprioception at 9 to 11 years after surgery(Table 2).62,63
Studies comparing traditional TKA designs with cruciate preserving designs, both UKA and BCR, have found differences in subjective outcomes.62,64 Comparison of UKA and TKA in the same patient demonstrated significant preference for UKA, particularly with stair-climbing.65 Similarly, comparison between BCR and PS TKA or CR TKA demonstrated preference for BCR in 85% of patients.62
The new BCR knee designs have just started to come to the market.50 The surgical techniques are much improved over the 1980s and cruciate preservation is certainly much easier now. The new designs can produce full range of motion with kinematics that are almost identical to the normal knee in the cadaver laboratory and in computer analyses. These designs certainly should have a similar 20-year survival to the original BCR knees. However, the critical evaluation will be the patient satisfaction scores. With greater motion, better kinematics, and more precise balancing the scores would improve with these designs.
Conclusion
The cruciate ligaments of the knee are central to control of the motion of the normal knee. TKA is a successful operation with at least a 40- to 50-year history. The techniques have continued to develop but 15% to 20% of patients are dissatisfied with the results.14 Evaluations of the prostheses are more sophisticated and kinematics appears to have a central position in the evaluation. If the knee is to move more anatomically correctly, all of the ligaments must be preserved. Proprioception certainly plays a role in the patient’s judgment of the result. History has shown that a BCR knee can be implanted with good mid-term results and it should certainly be possible to build on these results and design a knee that will incorporate all of the ligaments with full range of motion and increased levels of activity.
1. Walldius B. Arthroplasty of the knee with an endoprosthesis. Acta Chir Scand. 1957;113(6):445-446.
2. Gunston FH. Polycentric knee arthroplasty. Prosthetic simulation of normal knee movement. J Bone Joint Surg Br. 1971;53(2):272-277.
3. Insall JN, Ranawat CS, Aglietti P, Shine J. A comparison of four models of total knee-replacement prostheses. J Bone Joint Surg Am. 1976;58(6):754-765.
4. Coventry MB, Finerman GA, Riley LH, Turner RH, Upshaw JE. A new geometric knee for total knee arthroplasty. Clin Orthop Relat Res.1972;83:157-162.
5. Freeman MA, Sculco T, Todd RC. Replacement of the severely damaged arthritic knee by the ICLH (Freeman-Swanson) arthroplasty. J Bone Joint Surg Br. 1977;59(1):64-71.
6. Freeman MA, Insall JN, Besser W, Walker PS, Hallel T. Excision of the cruciate ligaments in total knee replacement. Clin Orthop Relat Res. 1977(126):209-212.
7. Pagnano MW, Cushner FD, Scott WN. Role of the posterior cruciate ligament in total knee arthroplasty. J Am Acad Orthop Surg. 1998;6(3):176-187.
8. Townley CO. The anatomic total knee resurfacing arthroplasty. Clin Orthop Relat Res. 1985(192):82-96.
9. Cloutier JM, Sabouret P, Deghrar A. Total knee arthroplasty with retention of both cruciate ligaments. A nine to eleven-year follow-up study. J Bone Joint Surg Am. 1999; 81(5):697-702.
10. Banks SA, Fregly BJ, Boniforti F, Reinschmidt C, Romagnoli S. Comparing in vivo kinematics of unicondylar and bi-unicondylar knee replacements. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):551-556.
11. Repicci JA, Eberle RW. Minimally invasive surgical technique for unicondylar knee arthroplasty. J South Orthop Assoc. 1999;8(1):20-27; discussion 27.
12. Romanowski MR, Repicci JA. Minimally invasive unicondylar arthroplasty: eight-year follow-up. J Knee Surg. 2002;15(1):17-22.
13. Banks SA, Markovich GD, Hodge WA. In vivo kinematics of cruciate-retaining and -substituting knee arthroplasties. J Arthroplasty. 1997;12(3):297-304.
14. Nam D, Nunley RM, Barrack RL. Patient dissatisfaction following total knee replacement: a growing concern? Bone Joint J. 2014;96-B(11 Supple A):96-100.
15. Iwaki H, Pinskerova V, Freeman MA. Tibiofemoral movement 1: the shapes and relative movements of the femur and tibia in the unloaded cadaver knee. J Bone Joint Surg Br. 2000;82(8):1189-1195.
16. Johal P, Williams A, Wragg P, Hunt D, Gedroyc W. Tibio-femoral movement in the living knee. A study of weight bearing and non-weight bearing knee kinematics using ‘interventional’ MRI. J Biomech. 2005;38(2):269-276.
17. Pinskerova V, Johal P, Nakagawa S, et al. Does the femur roll-back with flexion? J Bone Joint Surg Br. 2004;86(6):925-931.
18. Hill PF, Vedi V, Williams A, Pinskerova V, Freeman MA. Tibiofemoral movement 2: the loaded and unloaded living knee studied by MRI. J Bone Joint Surg Br. 2000;82(8):1196-1198.
19. Nakagawa S, Kadoya Y, Todo S, et al. Tibiofemoral movement 3: full flexion in the living knee studied by MRI. J Bone Joint Surg Br. 2000;82(8):1199-1200.
20. Freeman MA, Pinskerova V. The movement of the knee studied by magnetic resonance imaging. Clin Orthop Relat Res. 2003(410):35-43.
21. Moro-oka TA, Hamai S, Miura H, et al. Dynamic activity dependence of in vivo normal knee kinematics. J Orthop Res. 2008;26(4):428-434.
22. Komistek RD, Dennis DA, Mahfouz M. In vivo fluoroscopic analysis of the normal human knee. Clin Orthop Relat Res. 2003(410):69-81.
23. Li G, DeFrate LE, Park SE, Gill TJ, Rubash HE. In vivo articular cartilage contact kinematics of the knee: an investigation using dual-orthogonal fluoroscopy and magnetic resonance image-based computer models. Am J Sports Med. 2005;33(1):102-107.
24. Grood ES, Suntay WJ, Noyes FR, Butler DL. Biomechanics of the knee-extension exercise. Effect of cutting the anterior cruciate ligament. J Bone Joint Surg Am. 1984;66(5):725-734.
25. Noyes FR, Jetter AW, Grood ES, Harms SP, Gardner EJ, Levy MS. Anterior cruciate ligament function in providing rotational stability assessed by medial and lateral tibiofemoral compartment translations and subluxations. Am J Sports Med. 2015;43(3):683-692.
26. Good L, Askew MJ, Boom A, Melby A 3rd. Kinematic in-vitro comparison between the normal knee and two techniques for reconstruction of the anterior cruciate ligament. Clin Biomech (Bristol, Avon). 1993;8(5):243-249.
27. Beard DJ, Murray DW, Gill HS. Reconstruction does not reduce tibial translation in the cruciate-deficient knee an in vivo study. J Bone Joint Surg Br. 2001;83(8):1098-1103.
28. Dennis DA, Mahfouz MR, Komistek RD, Hoff W. In vivo determination of normal and anterior cruciate ligament-deficient knee kinematics. J Biomech. 2005;38(2):241-253.
29. Beynnon BD, Fleming BC, Labovitch R, Parsons B. Chronic anterior cruciate ligament deficiency is associated with increased anterior translation of the tibia during the transition from non-weightbearing to weightbearing. J Orthop Res. 2002;20(2):332-337.
30. Brandsson S, Karlsson J, Eriksson BI, Kärrholm J. Kinematics after tear in the anterior cruciate ligament: dynamic bilateral radiostereometric studies in 11 patients. Acta Orthop Scand. 2001;72(4):372-378.
31. Andriacchi TP, Briant PL, Bevill SL, Koo S. Rotational changes at the knee after ACL injury cause cartilage thinning. Clin Orthop Relat Res. 2006;442:39-44.
32. Scarvell JM, Smith PN, Refshauge KM, Galloway HR, Woods KR. Comparison of kinematic analysis by mapping tibiofemoral contact with movement of the femoral condylar centres in healthy and anterior cruciate ligament injured knees. J Orthop Res. 2004;22(5):955-962.
33. Miller RK, Goodfellow JW, Murray DW, O’Connor JJ. In vitro measurement of patellofemoral force after three types of knee replacement. J Bone Joint Surg Br. 1998;80(5):900-906.
34. Price AJ, Rees JL, Beard DL, Gill RH, Dodd CA, Murray DM. Sagittal plane kinematics of a mobile-bearing unicompartmental knee arthroplasty at 10 years: a comparative in vivo fluoroscopic analysis. J Arthroplasty. 2004;19(5):590-597.
35. Dennis D, Komistek R, Scuderi G, et al. In vivo three-dimensional determination of kinematics for subjects with a normal knee or a unicompartmental or total knee replacement. J Bone Joint Surg Am. 2001;83-A Suppl 2 Pt 2:104-115.
36. Arbuthnot JE, Brink RB. Assessment of the antero-posterior and rotational stability of the anterior cruciate ligament analogue in a guided motion bi-cruciate stabilized total knee arthroplasty. J Med Eng Technol. 2009;33(8):610-615.
37. Hollinghurst D, Stoney J, Ward T, et al. No deterioration of kinematics and cruciate function 10 years after medial unicompartmental arthroplasty. Knee. 2006;13(6):440-444.
38. Dennis DA, Komistek RD, Colwell CE Jr, et al. In vivo anteroposterior femorotibial translation of total knee arthroplasty: a multicenter analysis. Clin Orthop Relat Res. 1998(356):47-57.
39. Dennis DA, Komistek RD, Hoff WA, Gabriel SM. In vivo knee kinematics derived using an inverse perspective technique. Clin Orthop Relat Res. 1996;(331):107-117.
40. Yoshiya S, Matsui N, Komistek RD, Dennis DA, Mahfouz M, Kurosaka M. In vivo kinematic comparison of posterior cruciate-retaining and posterior stabilized total knee arthroplasties under passive and weight-bearing conditions. J Arthroplasty. 2005;20(6):777-783.
41. Kleinbart FA, Bryk E, Evangelista J, Scott WN, Vigorita VJ. Histologic comparison of posterior cruciate ligaments from arthritic and age-matched knee specimens. J Arthroplasty. 1996;11(6):726-731.
42. Bull AM, Kessler O, Alam M, Amis AA. Changes in knee kinematics reflect the articular geometry after arthroplasty. Clin Orthop Relat Res. 2008;466(10):2491-2499.
43. Komistek RD, Mahfouz MR, Bertin KC, Rosenberg A, Kennedy W. In vivo determination of total knee arthroplasty kinematics: a multicenter analysis of an asymmetrical posterior cruciate retaining total knee arthroplasty. J Arthroplasty. 2008;23(1):41-50.
44. Mehin R, Burnett RS, Brasher PM. Does the new generation of high-flex knee prostheses improve the post-operative range of movement?: a meta-analysis. J Bone Joint Surg Br. 2010;92(10):1429-1434.
45. Dennis DA, Heekin RD, Clark CR, Murphy JA, O’Dell TL, Dwyer KA. Effect of implant design on knee flexion. J Arthroplasty. 2013;28(3):429-438.
46. Victor J, Mueller JK, Komistek RD, Sharma A, Nadaud MC, Bellemans J. In vivo kinematics after a cruciate-substituting TKA. Clin Orthop Relat Res. 2010;468(3):807-814.
47. Catani F, Ensini A, Belvedere C, et al. In vivo kinematics and kinetics of a bi-cruciate substituting total knee arthroplasty: a combined fluoroscopic and gait analysis study. J Orthop Res. 2009;27(12):1569-1575.
48. Stiehl JB, Komistek RD, Cloutier JM, Dennis DA. The cruciate ligaments in total knee arthroplasty: a kinematic analysis of 2 total knee arthroplasties. J Arthroplasty. 2000;15(5):545-550.
49. Akagi M, Oh M, Nonaka T, Tsujimoto H, Asano T, Hamanishi C. An anteroposterior axis of the tibia for total knee arthroplasty. Clin Orthop Relat Res. 2004;(420):213-219.
50. Della Valle CJ, Andriacchi TP, Berend KR, DeClaire JH, Lombardi AV Jr, Peters CL. Early experience with bi-cruciate retaining TKA. Poster presented at: American Academy of Orthopaedic Surgeons 2015 Annual Meeting; March 24-28, 2015; Las Vegas, NV.
51. Udomkiat P, Meng BJ, Dorr LD, Wan Z. Functional comparison of posterior cruciate retention and substitution knee replacement. Clin Orthop Relat Res. 2000;(378):192-201.
52. Tanzer M, Smith K, Burnett S. Posterior-stabilized versus cruciate-retaining total knee arthroplasty: balancing the gap. J Arthroplasty. 2002;17(7):813-819.
53. Maruyama S, Yoshiya S, Matsui N, Kuroda R, Kurosaka M. Functional comparison of posterior cruciate-retaining versus posterior stabilized total knee arthroplasty. J Arthroplasty. 2004;19(3):349-53.
54. Clark CR, Rorabeck CH, MacDonald S, MacDonald D, Swafford J, Cleland D. Posterior-stabilized and cruciate-retaining total knee replacement: a randomized study. Clin Orthop Relat Res. 2001;(392):208-212.
55. Swanik CB, Lephart SM, Rubash HE. Proprioception, kinesthesia, and balance after total knee arthroplasty with cruciate-retaining and posterior stabilized prostheses. J Bone Joint Surg Am. 2004;86-A(2):328-334.
56. Harato K, Bourne RB, Victor J, Snyder M, Hart J, Ries MD. Midterm comparison of posterior cruciate-retaining versus -substituting total knee arthroplasty using the Genesis II prosthesis. A multicenter prospective randomized clinical trial. Knee. 2008;15(3):217-221.
57. Catani F, Leardini A, Ensini A, et al. The stability of the cemented tibial component of total knee arthroplasty: posterior cruciate-retaining versus posterior-stabilized design. J Arthroplasty. 2004;19(6):775-782.
58. Dennis DA, Komistek RD, Stiehl JB, Walker SA, Dennis KN. Range of motion after total knee arthroplasty: the effect of implant design and weight-bearing conditions. J Arthroplasty. 1998;13(7):748-752.
59. Becker MW, Insall JN, Faris PM. Bilateral total knee arthroplasty. One cruciate retaining and one cruciate substituting. Clin Orthop Relat Res. 1991;(271):122-124.
60. Kim YH, Choi Y, Kwon OR, Kim JS. Functional outcome and range of motion of high-flexion posterior cruciate-retaining and high-flexion posterior cruciate-substituting total knee prostheses. A prospective, randomized study. J Bone Joint Surg Am. 2009;91(4):753-760.
61. Verra WC, van den Boom LG, Jacobs W, Clement DJ, Wymenga AA, Nelissen RG. Retention versus sacrifice of the posterior cruciate ligament in total knee arthroplasty for treating osteoarthritis. Cochrane Database Syst Rev. 2013;10:CD004803.
62. Pritchett JW. Patients prefer a bicruciate-retaining or the medial pivot total knee prosthesis. J Arthroplasty. 2011;26(2):224-228.
63. Sabouret P, Lavoie F, Cloutier JM. Total knee replacement with retention of both cruciate ligaments: a 22-year follow-up study. Bone Joint J. 2013;95-B(7):917-922.
64. Andriacchi TP, Galante JO, Fermier RW. The influence of total knee-replacement design on walking and stair-climbing. J Bone Joint Surg Am. 1982;64(9):1328-1335.
65. Laurencin CT, Zelicof SB, Scott RD, Ewald FC. Unicompartmental versus total knee arthroplasty in the same patient. A comparative study. Clin Orthop Relat Res. 1991;(273):151-156.
66. Victor J, Banks S, Bellemans J. Kinematics of posterior cruciate ligament-retaining and -substituting total knee arthroplasty: a prospective randomised outcome study. J Bone Joint Surg Br. 2005;87(5):646-655.
1. Walldius B. Arthroplasty of the knee with an endoprosthesis. Acta Chir Scand. 1957;113(6):445-446.
2. Gunston FH. Polycentric knee arthroplasty. Prosthetic simulation of normal knee movement. J Bone Joint Surg Br. 1971;53(2):272-277.
3. Insall JN, Ranawat CS, Aglietti P, Shine J. A comparison of four models of total knee-replacement prostheses. J Bone Joint Surg Am. 1976;58(6):754-765.
4. Coventry MB, Finerman GA, Riley LH, Turner RH, Upshaw JE. A new geometric knee for total knee arthroplasty. Clin Orthop Relat Res.1972;83:157-162.
5. Freeman MA, Sculco T, Todd RC. Replacement of the severely damaged arthritic knee by the ICLH (Freeman-Swanson) arthroplasty. J Bone Joint Surg Br. 1977;59(1):64-71.
6. Freeman MA, Insall JN, Besser W, Walker PS, Hallel T. Excision of the cruciate ligaments in total knee replacement. Clin Orthop Relat Res. 1977(126):209-212.
7. Pagnano MW, Cushner FD, Scott WN. Role of the posterior cruciate ligament in total knee arthroplasty. J Am Acad Orthop Surg. 1998;6(3):176-187.
8. Townley CO. The anatomic total knee resurfacing arthroplasty. Clin Orthop Relat Res. 1985(192):82-96.
9. Cloutier JM, Sabouret P, Deghrar A. Total knee arthroplasty with retention of both cruciate ligaments. A nine to eleven-year follow-up study. J Bone Joint Surg Am. 1999; 81(5):697-702.
10. Banks SA, Fregly BJ, Boniforti F, Reinschmidt C, Romagnoli S. Comparing in vivo kinematics of unicondylar and bi-unicondylar knee replacements. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):551-556.
11. Repicci JA, Eberle RW. Minimally invasive surgical technique for unicondylar knee arthroplasty. J South Orthop Assoc. 1999;8(1):20-27; discussion 27.
12. Romanowski MR, Repicci JA. Minimally invasive unicondylar arthroplasty: eight-year follow-up. J Knee Surg. 2002;15(1):17-22.
13. Banks SA, Markovich GD, Hodge WA. In vivo kinematics of cruciate-retaining and -substituting knee arthroplasties. J Arthroplasty. 1997;12(3):297-304.
14. Nam D, Nunley RM, Barrack RL. Patient dissatisfaction following total knee replacement: a growing concern? Bone Joint J. 2014;96-B(11 Supple A):96-100.
15. Iwaki H, Pinskerova V, Freeman MA. Tibiofemoral movement 1: the shapes and relative movements of the femur and tibia in the unloaded cadaver knee. J Bone Joint Surg Br. 2000;82(8):1189-1195.
16. Johal P, Williams A, Wragg P, Hunt D, Gedroyc W. Tibio-femoral movement in the living knee. A study of weight bearing and non-weight bearing knee kinematics using ‘interventional’ MRI. J Biomech. 2005;38(2):269-276.
17. Pinskerova V, Johal P, Nakagawa S, et al. Does the femur roll-back with flexion? J Bone Joint Surg Br. 2004;86(6):925-931.
18. Hill PF, Vedi V, Williams A, Pinskerova V, Freeman MA. Tibiofemoral movement 2: the loaded and unloaded living knee studied by MRI. J Bone Joint Surg Br. 2000;82(8):1196-1198.
19. Nakagawa S, Kadoya Y, Todo S, et al. Tibiofemoral movement 3: full flexion in the living knee studied by MRI. J Bone Joint Surg Br. 2000;82(8):1199-1200.
20. Freeman MA, Pinskerova V. The movement of the knee studied by magnetic resonance imaging. Clin Orthop Relat Res. 2003(410):35-43.
21. Moro-oka TA, Hamai S, Miura H, et al. Dynamic activity dependence of in vivo normal knee kinematics. J Orthop Res. 2008;26(4):428-434.
22. Komistek RD, Dennis DA, Mahfouz M. In vivo fluoroscopic analysis of the normal human knee. Clin Orthop Relat Res. 2003(410):69-81.
23. Li G, DeFrate LE, Park SE, Gill TJ, Rubash HE. In vivo articular cartilage contact kinematics of the knee: an investigation using dual-orthogonal fluoroscopy and magnetic resonance image-based computer models. Am J Sports Med. 2005;33(1):102-107.
24. Grood ES, Suntay WJ, Noyes FR, Butler DL. Biomechanics of the knee-extension exercise. Effect of cutting the anterior cruciate ligament. J Bone Joint Surg Am. 1984;66(5):725-734.
25. Noyes FR, Jetter AW, Grood ES, Harms SP, Gardner EJ, Levy MS. Anterior cruciate ligament function in providing rotational stability assessed by medial and lateral tibiofemoral compartment translations and subluxations. Am J Sports Med. 2015;43(3):683-692.
26. Good L, Askew MJ, Boom A, Melby A 3rd. Kinematic in-vitro comparison between the normal knee and two techniques for reconstruction of the anterior cruciate ligament. Clin Biomech (Bristol, Avon). 1993;8(5):243-249.
27. Beard DJ, Murray DW, Gill HS. Reconstruction does not reduce tibial translation in the cruciate-deficient knee an in vivo study. J Bone Joint Surg Br. 2001;83(8):1098-1103.
28. Dennis DA, Mahfouz MR, Komistek RD, Hoff W. In vivo determination of normal and anterior cruciate ligament-deficient knee kinematics. J Biomech. 2005;38(2):241-253.
29. Beynnon BD, Fleming BC, Labovitch R, Parsons B. Chronic anterior cruciate ligament deficiency is associated with increased anterior translation of the tibia during the transition from non-weightbearing to weightbearing. J Orthop Res. 2002;20(2):332-337.
30. Brandsson S, Karlsson J, Eriksson BI, Kärrholm J. Kinematics after tear in the anterior cruciate ligament: dynamic bilateral radiostereometric studies in 11 patients. Acta Orthop Scand. 2001;72(4):372-378.
31. Andriacchi TP, Briant PL, Bevill SL, Koo S. Rotational changes at the knee after ACL injury cause cartilage thinning. Clin Orthop Relat Res. 2006;442:39-44.
32. Scarvell JM, Smith PN, Refshauge KM, Galloway HR, Woods KR. Comparison of kinematic analysis by mapping tibiofemoral contact with movement of the femoral condylar centres in healthy and anterior cruciate ligament injured knees. J Orthop Res. 2004;22(5):955-962.
33. Miller RK, Goodfellow JW, Murray DW, O’Connor JJ. In vitro measurement of patellofemoral force after three types of knee replacement. J Bone Joint Surg Br. 1998;80(5):900-906.
34. Price AJ, Rees JL, Beard DL, Gill RH, Dodd CA, Murray DM. Sagittal plane kinematics of a mobile-bearing unicompartmental knee arthroplasty at 10 years: a comparative in vivo fluoroscopic analysis. J Arthroplasty. 2004;19(5):590-597.
35. Dennis D, Komistek R, Scuderi G, et al. In vivo three-dimensional determination of kinematics for subjects with a normal knee or a unicompartmental or total knee replacement. J Bone Joint Surg Am. 2001;83-A Suppl 2 Pt 2:104-115.
36. Arbuthnot JE, Brink RB. Assessment of the antero-posterior and rotational stability of the anterior cruciate ligament analogue in a guided motion bi-cruciate stabilized total knee arthroplasty. J Med Eng Technol. 2009;33(8):610-615.
37. Hollinghurst D, Stoney J, Ward T, et al. No deterioration of kinematics and cruciate function 10 years after medial unicompartmental arthroplasty. Knee. 2006;13(6):440-444.
38. Dennis DA, Komistek RD, Colwell CE Jr, et al. In vivo anteroposterior femorotibial translation of total knee arthroplasty: a multicenter analysis. Clin Orthop Relat Res. 1998(356):47-57.
39. Dennis DA, Komistek RD, Hoff WA, Gabriel SM. In vivo knee kinematics derived using an inverse perspective technique. Clin Orthop Relat Res. 1996;(331):107-117.
40. Yoshiya S, Matsui N, Komistek RD, Dennis DA, Mahfouz M, Kurosaka M. In vivo kinematic comparison of posterior cruciate-retaining and posterior stabilized total knee arthroplasties under passive and weight-bearing conditions. J Arthroplasty. 2005;20(6):777-783.
41. Kleinbart FA, Bryk E, Evangelista J, Scott WN, Vigorita VJ. Histologic comparison of posterior cruciate ligaments from arthritic and age-matched knee specimens. J Arthroplasty. 1996;11(6):726-731.
42. Bull AM, Kessler O, Alam M, Amis AA. Changes in knee kinematics reflect the articular geometry after arthroplasty. Clin Orthop Relat Res. 2008;466(10):2491-2499.
43. Komistek RD, Mahfouz MR, Bertin KC, Rosenberg A, Kennedy W. In vivo determination of total knee arthroplasty kinematics: a multicenter analysis of an asymmetrical posterior cruciate retaining total knee arthroplasty. J Arthroplasty. 2008;23(1):41-50.
44. Mehin R, Burnett RS, Brasher PM. Does the new generation of high-flex knee prostheses improve the post-operative range of movement?: a meta-analysis. J Bone Joint Surg Br. 2010;92(10):1429-1434.
45. Dennis DA, Heekin RD, Clark CR, Murphy JA, O’Dell TL, Dwyer KA. Effect of implant design on knee flexion. J Arthroplasty. 2013;28(3):429-438.
46. Victor J, Mueller JK, Komistek RD, Sharma A, Nadaud MC, Bellemans J. In vivo kinematics after a cruciate-substituting TKA. Clin Orthop Relat Res. 2010;468(3):807-814.
47. Catani F, Ensini A, Belvedere C, et al. In vivo kinematics and kinetics of a bi-cruciate substituting total knee arthroplasty: a combined fluoroscopic and gait analysis study. J Orthop Res. 2009;27(12):1569-1575.
48. Stiehl JB, Komistek RD, Cloutier JM, Dennis DA. The cruciate ligaments in total knee arthroplasty: a kinematic analysis of 2 total knee arthroplasties. J Arthroplasty. 2000;15(5):545-550.
49. Akagi M, Oh M, Nonaka T, Tsujimoto H, Asano T, Hamanishi C. An anteroposterior axis of the tibia for total knee arthroplasty. Clin Orthop Relat Res. 2004;(420):213-219.
50. Della Valle CJ, Andriacchi TP, Berend KR, DeClaire JH, Lombardi AV Jr, Peters CL. Early experience with bi-cruciate retaining TKA. Poster presented at: American Academy of Orthopaedic Surgeons 2015 Annual Meeting; March 24-28, 2015; Las Vegas, NV.
51. Udomkiat P, Meng BJ, Dorr LD, Wan Z. Functional comparison of posterior cruciate retention and substitution knee replacement. Clin Orthop Relat Res. 2000;(378):192-201.
52. Tanzer M, Smith K, Burnett S. Posterior-stabilized versus cruciate-retaining total knee arthroplasty: balancing the gap. J Arthroplasty. 2002;17(7):813-819.
53. Maruyama S, Yoshiya S, Matsui N, Kuroda R, Kurosaka M. Functional comparison of posterior cruciate-retaining versus posterior stabilized total knee arthroplasty. J Arthroplasty. 2004;19(3):349-53.
54. Clark CR, Rorabeck CH, MacDonald S, MacDonald D, Swafford J, Cleland D. Posterior-stabilized and cruciate-retaining total knee replacement: a randomized study. Clin Orthop Relat Res. 2001;(392):208-212.
55. Swanik CB, Lephart SM, Rubash HE. Proprioception, kinesthesia, and balance after total knee arthroplasty with cruciate-retaining and posterior stabilized prostheses. J Bone Joint Surg Am. 2004;86-A(2):328-334.
56. Harato K, Bourne RB, Victor J, Snyder M, Hart J, Ries MD. Midterm comparison of posterior cruciate-retaining versus -substituting total knee arthroplasty using the Genesis II prosthesis. A multicenter prospective randomized clinical trial. Knee. 2008;15(3):217-221.
57. Catani F, Leardini A, Ensini A, et al. The stability of the cemented tibial component of total knee arthroplasty: posterior cruciate-retaining versus posterior-stabilized design. J Arthroplasty. 2004;19(6):775-782.
58. Dennis DA, Komistek RD, Stiehl JB, Walker SA, Dennis KN. Range of motion after total knee arthroplasty: the effect of implant design and weight-bearing conditions. J Arthroplasty. 1998;13(7):748-752.
59. Becker MW, Insall JN, Faris PM. Bilateral total knee arthroplasty. One cruciate retaining and one cruciate substituting. Clin Orthop Relat Res. 1991;(271):122-124.
60. Kim YH, Choi Y, Kwon OR, Kim JS. Functional outcome and range of motion of high-flexion posterior cruciate-retaining and high-flexion posterior cruciate-substituting total knee prostheses. A prospective, randomized study. J Bone Joint Surg Am. 2009;91(4):753-760.
61. Verra WC, van den Boom LG, Jacobs W, Clement DJ, Wymenga AA, Nelissen RG. Retention versus sacrifice of the posterior cruciate ligament in total knee arthroplasty for treating osteoarthritis. Cochrane Database Syst Rev. 2013;10:CD004803.
62. Pritchett JW. Patients prefer a bicruciate-retaining or the medial pivot total knee prosthesis. J Arthroplasty. 2011;26(2):224-228.
63. Sabouret P, Lavoie F, Cloutier JM. Total knee replacement with retention of both cruciate ligaments: a 22-year follow-up study. Bone Joint J. 2013;95-B(7):917-922.
64. Andriacchi TP, Galante JO, Fermier RW. The influence of total knee-replacement design on walking and stair-climbing. J Bone Joint Surg Am. 1982;64(9):1328-1335.
65. Laurencin CT, Zelicof SB, Scott RD, Ewald FC. Unicompartmental versus total knee arthroplasty in the same patient. A comparative study. Clin Orthop Relat Res. 1991;(273):151-156.
66. Victor J, Banks S, Bellemans J. Kinematics of posterior cruciate ligament-retaining and -substituting total knee arthroplasty: a prospective randomised outcome study. J Bone Joint Surg Br. 2005;87(5):646-655.
Benign Lesion on the Posterior Aspect of the Neck
Nuchal-Type Fibroma
Nuchal-type fibroma (NTF) is a rare benign proliferation of the dermis and subcutis associated with diabetes mellitus and Gardner syndrome.1,2 Forty-four percent of patients with NTF have diabetes mellitus.2 The posterior aspect of the neck is the most frequently affected site, but lesions also may present on the upper back, lumbosacral area, buttocks, and face. Physical examination generally reveals an indurated, asymptomatic, ill-defined, 3-cm or smaller nodule that is hard and white, unencapsulated, and poorly circumscribed.
Histopathologic examination of NTF typically reveals a nodular paucicellular proliferation of thick collagen bundles with inconspicuous fibroblasts, radiation of collagenous septa into the subcutaneous fat, and entrapment of mature adipose tissue and small nerves (quiz image A). Collagen bundles are thickened with entrapment of adipose tissue without increased cellularity (quiz image B). S-100 staining can show the entrapped nerves.
Similar to NTF, sclerotic fibroma is a firm dermal nodule with histologic examination usually demonstrating a paucicellular collagenous tumor. In sclerotic fibromas, the collagen pattern resembles Vincent van Gogh’s painting “The Starry Night” and may be a marker for Cowden disease (Figure 1).3 Solitary fibrous tumors are distinguished by more hypercellular areas, patternless pattern, and staghorn-shaped blood vessels (Figure 2).4 Spindle cell lipoma classically demonstrates a mixture of mature adipocytes and bland spindle cells in a mucinous or fibrous background with thick collagen bundles with no storiform pattern (Figure 3). Some variants of spindle cell lipoma have minimal or no fat.5 All of these conditions have positive immunohistochemical staining for CD34.
However, dermatofibroma is CD34‒. Dermatofibroma is characterized by an interstitial spindle cell proliferation with a loose storiform pattern, collagen trapping at the outer edges of the tumor, overlying platelike acanthosis, and sometimes follicular induction (Figure 4).

Nuchal-type fibroma also can resemble scleredema. Both lesions can show increased and thickened collagen bundles without notable fibroblast proliferation; the difference is the occurrence of mucin in scleredema. However, incases of late-stage scleredema, mucin is not always demonstrated. Therefore, one can conclude that histologically NTF is closely associated with late-stage scleredema.6
- Dawes LC, La Hei ER, Tobias V, et al. Nuchal fibroma should be recognized as a new extracolonic manifestation of Gardner-variant familial adenomatous polyposis. Aust N Z J Surg. 2000;70:824-826.
- Michal M, Fetsch JF, Hes O, et al. Nuchal-type fibroma: a clinicopathologic study of 52 cases. Cancer. 1999;85:156-163.
- Pernet C, Durand L, Bessis D, et al. Solitary sclerotic fibroma of the skin: a possible clue for Cowden syndrome. Eur J Dermatol. 2012;22:278-279.
- Omori Y, Saeki H, Ito K, et al. Solitary fibrous tumour of the scalp. Clin Exp Dermatol. 2014;39:539-541.
- Billings SD, Folpe AL. Diagnostically challenging spindle cell lipomas: a report of 34 “low-fat” and “fat-free” variants. Am J Dermatopathol. 2007;29:437-442.
- Banney LA, Weedon D, Muir JB. Nuchal fibroma associated with scleredema, diabetes mellitus and organic solvent exposure. Australas J Dermatol. 2000;41:39-41.
Nuchal-Type Fibroma
Nuchal-type fibroma (NTF) is a rare benign proliferation of the dermis and subcutis associated with diabetes mellitus and Gardner syndrome.1,2 Forty-four percent of patients with NTF have diabetes mellitus.2 The posterior aspect of the neck is the most frequently affected site, but lesions also may present on the upper back, lumbosacral area, buttocks, and face. Physical examination generally reveals an indurated, asymptomatic, ill-defined, 3-cm or smaller nodule that is hard and white, unencapsulated, and poorly circumscribed.
Histopathologic examination of NTF typically reveals a nodular paucicellular proliferation of thick collagen bundles with inconspicuous fibroblasts, radiation of collagenous septa into the subcutaneous fat, and entrapment of mature adipose tissue and small nerves (quiz image A). Collagen bundles are thickened with entrapment of adipose tissue without increased cellularity (quiz image B). S-100 staining can show the entrapped nerves.
Similar to NTF, sclerotic fibroma is a firm dermal nodule with histologic examination usually demonstrating a paucicellular collagenous tumor. In sclerotic fibromas, the collagen pattern resembles Vincent van Gogh’s painting “The Starry Night” and may be a marker for Cowden disease (Figure 1).3 Solitary fibrous tumors are distinguished by more hypercellular areas, patternless pattern, and staghorn-shaped blood vessels (Figure 2).4 Spindle cell lipoma classically demonstrates a mixture of mature adipocytes and bland spindle cells in a mucinous or fibrous background with thick collagen bundles with no storiform pattern (Figure 3). Some variants of spindle cell lipoma have minimal or no fat.5 All of these conditions have positive immunohistochemical staining for CD34.



However, dermatofibroma is CD34‒. Dermatofibroma is characterized by an interstitial spindle cell proliferation with a loose storiform pattern, collagen trapping at the outer edges of the tumor, overlying platelike acanthosis, and sometimes follicular induction (Figure 4).

Nuchal-type fibroma also can resemble scleredema. Both lesions can show increased and thickened collagen bundles without notable fibroblast proliferation; the difference is the occurrence of mucin in scleredema. However, incases of late-stage scleredema, mucin is not always demonstrated. Therefore, one can conclude that histologically NTF is closely associated with late-stage scleredema.6
Nuchal-Type Fibroma
Nuchal-type fibroma (NTF) is a rare benign proliferation of the dermis and subcutis associated with diabetes mellitus and Gardner syndrome.1,2 Forty-four percent of patients with NTF have diabetes mellitus.2 The posterior aspect of the neck is the most frequently affected site, but lesions also may present on the upper back, lumbosacral area, buttocks, and face. Physical examination generally reveals an indurated, asymptomatic, ill-defined, 3-cm or smaller nodule that is hard and white, unencapsulated, and poorly circumscribed.
Histopathologic examination of NTF typically reveals a nodular paucicellular proliferation of thick collagen bundles with inconspicuous fibroblasts, radiation of collagenous septa into the subcutaneous fat, and entrapment of mature adipose tissue and small nerves (quiz image A). Collagen bundles are thickened with entrapment of adipose tissue without increased cellularity (quiz image B). S-100 staining can show the entrapped nerves.
Similar to NTF, sclerotic fibroma is a firm dermal nodule with histologic examination usually demonstrating a paucicellular collagenous tumor. In sclerotic fibromas, the collagen pattern resembles Vincent van Gogh’s painting “The Starry Night” and may be a marker for Cowden disease (Figure 1).3 Solitary fibrous tumors are distinguished by more hypercellular areas, patternless pattern, and staghorn-shaped blood vessels (Figure 2).4 Spindle cell lipoma classically demonstrates a mixture of mature adipocytes and bland spindle cells in a mucinous or fibrous background with thick collagen bundles with no storiform pattern (Figure 3). Some variants of spindle cell lipoma have minimal or no fat.5 All of these conditions have positive immunohistochemical staining for CD34.



However, dermatofibroma is CD34‒. Dermatofibroma is characterized by an interstitial spindle cell proliferation with a loose storiform pattern, collagen trapping at the outer edges of the tumor, overlying platelike acanthosis, and sometimes follicular induction (Figure 4).

Nuchal-type fibroma also can resemble scleredema. Both lesions can show increased and thickened collagen bundles without notable fibroblast proliferation; the difference is the occurrence of mucin in scleredema. However, incases of late-stage scleredema, mucin is not always demonstrated. Therefore, one can conclude that histologically NTF is closely associated with late-stage scleredema.6
- Dawes LC, La Hei ER, Tobias V, et al. Nuchal fibroma should be recognized as a new extracolonic manifestation of Gardner-variant familial adenomatous polyposis. Aust N Z J Surg. 2000;70:824-826.
- Michal M, Fetsch JF, Hes O, et al. Nuchal-type fibroma: a clinicopathologic study of 52 cases. Cancer. 1999;85:156-163.
- Pernet C, Durand L, Bessis D, et al. Solitary sclerotic fibroma of the skin: a possible clue for Cowden syndrome. Eur J Dermatol. 2012;22:278-279.
- Omori Y, Saeki H, Ito K, et al. Solitary fibrous tumour of the scalp. Clin Exp Dermatol. 2014;39:539-541.
- Billings SD, Folpe AL. Diagnostically challenging spindle cell lipomas: a report of 34 “low-fat” and “fat-free” variants. Am J Dermatopathol. 2007;29:437-442.
- Banney LA, Weedon D, Muir JB. Nuchal fibroma associated with scleredema, diabetes mellitus and organic solvent exposure. Australas J Dermatol. 2000;41:39-41.
- Dawes LC, La Hei ER, Tobias V, et al. Nuchal fibroma should be recognized as a new extracolonic manifestation of Gardner-variant familial adenomatous polyposis. Aust N Z J Surg. 2000;70:824-826.
- Michal M, Fetsch JF, Hes O, et al. Nuchal-type fibroma: a clinicopathologic study of 52 cases. Cancer. 1999;85:156-163.
- Pernet C, Durand L, Bessis D, et al. Solitary sclerotic fibroma of the skin: a possible clue for Cowden syndrome. Eur J Dermatol. 2012;22:278-279.
- Omori Y, Saeki H, Ito K, et al. Solitary fibrous tumour of the scalp. Clin Exp Dermatol. 2014;39:539-541.
- Billings SD, Folpe AL. Diagnostically challenging spindle cell lipomas: a report of 34 “low-fat” and “fat-free” variants. Am J Dermatopathol. 2007;29:437-442.
- Banney LA, Weedon D, Muir JB. Nuchal fibroma associated with scleredema, diabetes mellitus and organic solvent exposure. Australas J Dermatol. 2000;41:39-41.
The best diagnosis is:
a. dermatofibroma
b. nuchal-type fibroma
c. sclerotic fibroma
d. solitary fibrous tumor
e. spindle cell lipoma
Continue to the next page for the diagnosis >>
One man’s quandary over Fistula First
[Editor's Note: Dr. Sales presents us with both points of view]
POINT: Fistula First for everyone? Sounds good to me
While “do no harm” may be a motto, the truth is, we strive to deliver the best care possible at every moment we interact with patients. There is no argument that an autogenous Arteriovenous Fisutla (AVF) is the best access available for patients—in terms of patency, freedom from intervention and resistance to infection. Thus, providing any other type of access—excluding the bridge catheter—is clearly a “second best” operation.
Other countries boast AVF rates in the upper 80% range and ESRD Network 16 (Alaska, Idaho, Montana, Oregon and Washington) in the U. S. has rates approaching 70%.
If that is the case, why then, does the U. S. still have an overall AVF rate of 63%? It is unlikely that the technical ability of surgeons outside the U. S. or in the upper left corner of our country differs from that of the remainder of the country. Fistula First embarked on a journey to bring this issue to the forefront. Following Drs. Larry Spergel and Tip Jennings advice, many surgeons took to the operating room in an effort to improve the AVF rates in the U. S. In fact, the numbers improved dramatically—but not to the 80+% mark.
Dr. Larry Scher assures us that every arm can have a fistula created and if you are a good interventionalist (or have a good one with whom to work) you can get all of these to mature.
Therefore, all of those 2-2.5mm veins that might not seem “usable,” should be anastomosed carefully to the radial or brachial artery—much as one would perform a distal anastomosis—and coax them into maturing to functional AVFs. If balloon assistance is needed for maturation, make certain the “balloon operator” has a good working knowledge of AV access, lest a nice, big hematoma will greet you, destroying your nicely created AVF. Those of us who cannot achieve the near 100% AVF rate must be technically challenged!
COUNTERPOINT: Fistula First is a bit overdone
Great idea, good execution, too much!
As an early ideologue in the Fistula First movement, I am proud of what we accomplished—education. We got the word out that AVFs were a better option than Arteriovenous Grafts! At the introductory meeting, I did have a rather animated “discussion” with a social worker who was convinced that the reason AVGs outnumbered AVFs was because AVGs reimbursed higher and therefore the surgeons were choosing the AVG for financial reasons (Sometimes it is really hard to maintain your composure!) While it may be true in some quarters, I would venture that was not the real reason affecting the inability of us, as a profession, to deliver the AVF rate that we should.
My alter ego above mentions Drs. Larry Spergel and Tip Jennings—both of whom should be given great credit for opening our eyes to the need for reform. They lead us and we improved. However, the number of AVFs that are being done that are NOT maturing has increased. Just as in any “cult phenomena,” when adherence to a concept supersedes rational thought, the endpoint is blurred. This is what has occurred in the Fistula First.
There are some patients who will never mature an AVF—irrespective of the competence of the vascular surgeon! Many of the veins are simply phlebitic from years of intravenous or venipuncture attacks—now that the PICC teams have learned where the basillic vein is, that vein is often problematic. Additionally, one factor that has never been adequately measured or characterized is skin turgor. Many of our older patients have awful skin turgor and any access (AVF or AVG) will be accompanied by ongoing post-dialysis hematomas with problems.
Our experience has been that octogenarians tend to do better with AVGs than AVFs—heresy to the Fistula First pundits. The number of catheter days is markedly reduced and the long-term outcomes do not vary much in this age group. And guess what, there are some patients who actually do well with a catheter!
Perhaps, two topics are worth mentioning in closing this self-debating article! Fistula First has, appropriately, been renamed Fistula First, Catheter Last! This recognizes the fact that maybe not everyone is a candidate for an AVF—but at the least we should avoid the catheter! The second, much larger issue is whether everyone that is dialyzed should, indeed, be dialyzed! I suspect all readers have been pressured into placing a catheter (or worse) in a patient who has no real chance for a meaningful survival. Additionally, maybe some patients would benefit from nutritional therapy that could delay the onset of dialysis by 6-18 months—imagine how much that would save to an already overburdened system!
Dr. Sales is President, The Cardiovascular Care Group, Westfield , N.J., chief, division of vascular surgery, Overlook Medical Center, Summit, N.J., and clinical assistant professor of Surgery, Mount Sinai School of Medicine, New York, N.Y. Both versions of Dr. Sales are an associate medical editor for Vascular Specialist.
[Editor's Note: Dr. Sales presents us with both points of view]
POINT: Fistula First for everyone? Sounds good to me
While “do no harm” may be a motto, the truth is, we strive to deliver the best care possible at every moment we interact with patients. There is no argument that an autogenous Arteriovenous Fisutla (AVF) is the best access available for patients—in terms of patency, freedom from intervention and resistance to infection. Thus, providing any other type of access—excluding the bridge catheter—is clearly a “second best” operation.
Other countries boast AVF rates in the upper 80% range and ESRD Network 16 (Alaska, Idaho, Montana, Oregon and Washington) in the U. S. has rates approaching 70%.
If that is the case, why then, does the U. S. still have an overall AVF rate of 63%? It is unlikely that the technical ability of surgeons outside the U. S. or in the upper left corner of our country differs from that of the remainder of the country. Fistula First embarked on a journey to bring this issue to the forefront. Following Drs. Larry Spergel and Tip Jennings advice, many surgeons took to the operating room in an effort to improve the AVF rates in the U. S. In fact, the numbers improved dramatically—but not to the 80+% mark.
Dr. Larry Scher assures us that every arm can have a fistula created and if you are a good interventionalist (or have a good one with whom to work) you can get all of these to mature.
Therefore, all of those 2-2.5mm veins that might not seem “usable,” should be anastomosed carefully to the radial or brachial artery—much as one would perform a distal anastomosis—and coax them into maturing to functional AVFs. If balloon assistance is needed for maturation, make certain the “balloon operator” has a good working knowledge of AV access, lest a nice, big hematoma will greet you, destroying your nicely created AVF. Those of us who cannot achieve the near 100% AVF rate must be technically challenged!
COUNTERPOINT: Fistula First is a bit overdone
Great idea, good execution, too much!
As an early ideologue in the Fistula First movement, I am proud of what we accomplished—education. We got the word out that AVFs were a better option than Arteriovenous Grafts! At the introductory meeting, I did have a rather animated “discussion” with a social worker who was convinced that the reason AVGs outnumbered AVFs was because AVGs reimbursed higher and therefore the surgeons were choosing the AVG for financial reasons (Sometimes it is really hard to maintain your composure!) While it may be true in some quarters, I would venture that was not the real reason affecting the inability of us, as a profession, to deliver the AVF rate that we should.
My alter ego above mentions Drs. Larry Spergel and Tip Jennings—both of whom should be given great credit for opening our eyes to the need for reform. They lead us and we improved. However, the number of AVFs that are being done that are NOT maturing has increased. Just as in any “cult phenomena,” when adherence to a concept supersedes rational thought, the endpoint is blurred. This is what has occurred in the Fistula First.
There are some patients who will never mature an AVF—irrespective of the competence of the vascular surgeon! Many of the veins are simply phlebitic from years of intravenous or venipuncture attacks—now that the PICC teams have learned where the basillic vein is, that vein is often problematic. Additionally, one factor that has never been adequately measured or characterized is skin turgor. Many of our older patients have awful skin turgor and any access (AVF or AVG) will be accompanied by ongoing post-dialysis hematomas with problems.
Our experience has been that octogenarians tend to do better with AVGs than AVFs—heresy to the Fistula First pundits. The number of catheter days is markedly reduced and the long-term outcomes do not vary much in this age group. And guess what, there are some patients who actually do well with a catheter!
Perhaps, two topics are worth mentioning in closing this self-debating article! Fistula First has, appropriately, been renamed Fistula First, Catheter Last! This recognizes the fact that maybe not everyone is a candidate for an AVF—but at the least we should avoid the catheter! The second, much larger issue is whether everyone that is dialyzed should, indeed, be dialyzed! I suspect all readers have been pressured into placing a catheter (or worse) in a patient who has no real chance for a meaningful survival. Additionally, maybe some patients would benefit from nutritional therapy that could delay the onset of dialysis by 6-18 months—imagine how much that would save to an already overburdened system!
Dr. Sales is President, The Cardiovascular Care Group, Westfield , N.J., chief, division of vascular surgery, Overlook Medical Center, Summit, N.J., and clinical assistant professor of Surgery, Mount Sinai School of Medicine, New York, N.Y. Both versions of Dr. Sales are an associate medical editor for Vascular Specialist.
[Editor's Note: Dr. Sales presents us with both points of view]
POINT: Fistula First for everyone? Sounds good to me
While “do no harm” may be a motto, the truth is, we strive to deliver the best care possible at every moment we interact with patients. There is no argument that an autogenous Arteriovenous Fisutla (AVF) is the best access available for patients—in terms of patency, freedom from intervention and resistance to infection. Thus, providing any other type of access—excluding the bridge catheter—is clearly a “second best” operation.
Other countries boast AVF rates in the upper 80% range and ESRD Network 16 (Alaska, Idaho, Montana, Oregon and Washington) in the U. S. has rates approaching 70%.
If that is the case, why then, does the U. S. still have an overall AVF rate of 63%? It is unlikely that the technical ability of surgeons outside the U. S. or in the upper left corner of our country differs from that of the remainder of the country. Fistula First embarked on a journey to bring this issue to the forefront. Following Drs. Larry Spergel and Tip Jennings advice, many surgeons took to the operating room in an effort to improve the AVF rates in the U. S. In fact, the numbers improved dramatically—but not to the 80+% mark.
Dr. Larry Scher assures us that every arm can have a fistula created and if you are a good interventionalist (or have a good one with whom to work) you can get all of these to mature.
Therefore, all of those 2-2.5mm veins that might not seem “usable,” should be anastomosed carefully to the radial or brachial artery—much as one would perform a distal anastomosis—and coax them into maturing to functional AVFs. If balloon assistance is needed for maturation, make certain the “balloon operator” has a good working knowledge of AV access, lest a nice, big hematoma will greet you, destroying your nicely created AVF. Those of us who cannot achieve the near 100% AVF rate must be technically challenged!
COUNTERPOINT: Fistula First is a bit overdone
Great idea, good execution, too much!
As an early ideologue in the Fistula First movement, I am proud of what we accomplished—education. We got the word out that AVFs were a better option than Arteriovenous Grafts! At the introductory meeting, I did have a rather animated “discussion” with a social worker who was convinced that the reason AVGs outnumbered AVFs was because AVGs reimbursed higher and therefore the surgeons were choosing the AVG for financial reasons (Sometimes it is really hard to maintain your composure!) While it may be true in some quarters, I would venture that was not the real reason affecting the inability of us, as a profession, to deliver the AVF rate that we should.
My alter ego above mentions Drs. Larry Spergel and Tip Jennings—both of whom should be given great credit for opening our eyes to the need for reform. They lead us and we improved. However, the number of AVFs that are being done that are NOT maturing has increased. Just as in any “cult phenomena,” when adherence to a concept supersedes rational thought, the endpoint is blurred. This is what has occurred in the Fistula First.
There are some patients who will never mature an AVF—irrespective of the competence of the vascular surgeon! Many of the veins are simply phlebitic from years of intravenous or venipuncture attacks—now that the PICC teams have learned where the basillic vein is, that vein is often problematic. Additionally, one factor that has never been adequately measured or characterized is skin turgor. Many of our older patients have awful skin turgor and any access (AVF or AVG) will be accompanied by ongoing post-dialysis hematomas with problems.
Our experience has been that octogenarians tend to do better with AVGs than AVFs—heresy to the Fistula First pundits. The number of catheter days is markedly reduced and the long-term outcomes do not vary much in this age group. And guess what, there are some patients who actually do well with a catheter!
Perhaps, two topics are worth mentioning in closing this self-debating article! Fistula First has, appropriately, been renamed Fistula First, Catheter Last! This recognizes the fact that maybe not everyone is a candidate for an AVF—but at the least we should avoid the catheter! The second, much larger issue is whether everyone that is dialyzed should, indeed, be dialyzed! I suspect all readers have been pressured into placing a catheter (or worse) in a patient who has no real chance for a meaningful survival. Additionally, maybe some patients would benefit from nutritional therapy that could delay the onset of dialysis by 6-18 months—imagine how much that would save to an already overburdened system!
Dr. Sales is President, The Cardiovascular Care Group, Westfield , N.J., chief, division of vascular surgery, Overlook Medical Center, Summit, N.J., and clinical assistant professor of Surgery, Mount Sinai School of Medicine, New York, N.Y. Both versions of Dr. Sales are an associate medical editor for Vascular Specialist.