User login
New members welcomed to Consultant Board
We are pleased to welcome Laura Searcy and Cathy Haut to the Pediatric News Consultant Board.
Laura Searcy, MN, APRN, PPCNP-BC, is a pediatric nurse practitioner with more than 25 years’ experience in clinical practice, health policy, and advocacy with a focus on pediatric primary care, child and adolescent injury prevention, substance abuse prevention, and government affairs. Receiving her bachelor’s degree in nursing from the University of Florida in Gainesville, she earned her master’s degree in child health and pediatric nurse practitioner degrees from Emory University in Atlanta. Currently on the medical staff at WellStar Kennestone Regional Medical Center in Marietta, Ga., delivering care to newborns, Ms. Searcy has served as a preceptor and clinical instructor for all levels of nursing students at many institutions.
President of the National Association of Pediatric Nurse Practitioners (NAPNAP), Ms. Searcy previously served on the NAPNAP executive board as president-elect and health policy committee chair. She is a past Georgia NAPNAP chapter president and legislative chair as well as a cofounder of the Georgia Coalition of Advanced Practice Registered Nurses. A member of the American Association of Nurse Practitioners (AANP), Ms. Searcy received the 2014 AANP Nurse Practitioner State Award for Excellence from Georgia.
Widely recognized for her leadership skills, Ms. Searcy gained much of her governance experience serving three terms, including chair and vice chair, on the Cobb County Georgia Board of Education. The U.S. Secretary of Education appointed her to the Southeast Regional Advisement Committee, and the Georgia School Board Association appointed her to the National School Board Association Federal Relations Network. Ms. Searcy also has more than 12 years of nonprofit board experience, working with children’s advocacy centers and youth alcohol and drug-abuse prevention organizations.
Cathy Haut, DNP, CPNP-AC, CPNP-PC, CCRN, is a pediatric nurse practitioner who is the immediate past president of NAPNAP. She received her Master of Science degree as a neonatal clinical nurse specialist at the University of Maryland in the early 1990s and a post–master’s certificate as a pediatric nurse practitioner in 1997. Dr. Haut completed the Doctor of Nursing Practice program in 2010, focusing on pediatric nurses’ knowledge and attitudes toward palliative care. Board-certified in both primary and acute care, she has worked in pediatrics for more than 30 years, the past 19 years as a pediatric nurse practitioner. A fellow in the American Association of Colleges of Nurse’s Leadership for Academic Nursing Program, she holds a certificate in teaching in nursing education.
Dr. Haut works at Beacon Pediatrics, a large primary care practice in Rehoboth Beach, Del. She also works part time for Pediatrix Medical Group, serving the Pediatric Intensive Care Unit medical team at the Herman & Walter Samuelson Children’s Hospital at Sinai in Baltimore and serves as adjunct faculty at the University of Maryland School of Nursing, also in Baltimore.
The coeditor of a textbook, Lippincott Certification Review: Pediatric Acute Care Nurse Practitioner, Dr. Haut has published and presented on many clinical and professional topics. She has volunteered for different nursing organizations in a variety of positions, and has been an active member and leader of NAPNAP’s Maryland Chesapeake Chapter since 1996, serving as president in 2006-2007.
We are pleased to welcome Laura Searcy and Cathy Haut to the Pediatric News Consultant Board.
Laura Searcy, MN, APRN, PPCNP-BC, is a pediatric nurse practitioner with more than 25 years’ experience in clinical practice, health policy, and advocacy with a focus on pediatric primary care, child and adolescent injury prevention, substance abuse prevention, and government affairs. Receiving her bachelor’s degree in nursing from the University of Florida in Gainesville, she earned her master’s degree in child health and pediatric nurse practitioner degrees from Emory University in Atlanta. Currently on the medical staff at WellStar Kennestone Regional Medical Center in Marietta, Ga., delivering care to newborns, Ms. Searcy has served as a preceptor and clinical instructor for all levels of nursing students at many institutions.
President of the National Association of Pediatric Nurse Practitioners (NAPNAP), Ms. Searcy previously served on the NAPNAP executive board as president-elect and health policy committee chair. She is a past Georgia NAPNAP chapter president and legislative chair as well as a cofounder of the Georgia Coalition of Advanced Practice Registered Nurses. A member of the American Association of Nurse Practitioners (AANP), Ms. Searcy received the 2014 AANP Nurse Practitioner State Award for Excellence from Georgia.
Widely recognized for her leadership skills, Ms. Searcy gained much of her governance experience serving three terms, including chair and vice chair, on the Cobb County Georgia Board of Education. The U.S. Secretary of Education appointed her to the Southeast Regional Advisement Committee, and the Georgia School Board Association appointed her to the National School Board Association Federal Relations Network. Ms. Searcy also has more than 12 years of nonprofit board experience, working with children’s advocacy centers and youth alcohol and drug-abuse prevention organizations.
Cathy Haut, DNP, CPNP-AC, CPNP-PC, CCRN, is a pediatric nurse practitioner who is the immediate past president of NAPNAP. She received her Master of Science degree as a neonatal clinical nurse specialist at the University of Maryland in the early 1990s and a post–master’s certificate as a pediatric nurse practitioner in 1997. Dr. Haut completed the Doctor of Nursing Practice program in 2010, focusing on pediatric nurses’ knowledge and attitudes toward palliative care. Board-certified in both primary and acute care, she has worked in pediatrics for more than 30 years, the past 19 years as a pediatric nurse practitioner. A fellow in the American Association of Colleges of Nurse’s Leadership for Academic Nursing Program, she holds a certificate in teaching in nursing education.
Dr. Haut works at Beacon Pediatrics, a large primary care practice in Rehoboth Beach, Del. She also works part time for Pediatrix Medical Group, serving the Pediatric Intensive Care Unit medical team at the Herman & Walter Samuelson Children’s Hospital at Sinai in Baltimore and serves as adjunct faculty at the University of Maryland School of Nursing, also in Baltimore.
The coeditor of a textbook, Lippincott Certification Review: Pediatric Acute Care Nurse Practitioner, Dr. Haut has published and presented on many clinical and professional topics. She has volunteered for different nursing organizations in a variety of positions, and has been an active member and leader of NAPNAP’s Maryland Chesapeake Chapter since 1996, serving as president in 2006-2007.
We are pleased to welcome Laura Searcy and Cathy Haut to the Pediatric News Consultant Board.
Laura Searcy, MN, APRN, PPCNP-BC, is a pediatric nurse practitioner with more than 25 years’ experience in clinical practice, health policy, and advocacy with a focus on pediatric primary care, child and adolescent injury prevention, substance abuse prevention, and government affairs. Receiving her bachelor’s degree in nursing from the University of Florida in Gainesville, she earned her master’s degree in child health and pediatric nurse practitioner degrees from Emory University in Atlanta. Currently on the medical staff at WellStar Kennestone Regional Medical Center in Marietta, Ga., delivering care to newborns, Ms. Searcy has served as a preceptor and clinical instructor for all levels of nursing students at many institutions.
President of the National Association of Pediatric Nurse Practitioners (NAPNAP), Ms. Searcy previously served on the NAPNAP executive board as president-elect and health policy committee chair. She is a past Georgia NAPNAP chapter president and legislative chair as well as a cofounder of the Georgia Coalition of Advanced Practice Registered Nurses. A member of the American Association of Nurse Practitioners (AANP), Ms. Searcy received the 2014 AANP Nurse Practitioner State Award for Excellence from Georgia.
Widely recognized for her leadership skills, Ms. Searcy gained much of her governance experience serving three terms, including chair and vice chair, on the Cobb County Georgia Board of Education. The U.S. Secretary of Education appointed her to the Southeast Regional Advisement Committee, and the Georgia School Board Association appointed her to the National School Board Association Federal Relations Network. Ms. Searcy also has more than 12 years of nonprofit board experience, working with children’s advocacy centers and youth alcohol and drug-abuse prevention organizations.
Cathy Haut, DNP, CPNP-AC, CPNP-PC, CCRN, is a pediatric nurse practitioner who is the immediate past president of NAPNAP. She received her Master of Science degree as a neonatal clinical nurse specialist at the University of Maryland in the early 1990s and a post–master’s certificate as a pediatric nurse practitioner in 1997. Dr. Haut completed the Doctor of Nursing Practice program in 2010, focusing on pediatric nurses’ knowledge and attitudes toward palliative care. Board-certified in both primary and acute care, she has worked in pediatrics for more than 30 years, the past 19 years as a pediatric nurse practitioner. A fellow in the American Association of Colleges of Nurse’s Leadership for Academic Nursing Program, she holds a certificate in teaching in nursing education.
Dr. Haut works at Beacon Pediatrics, a large primary care practice in Rehoboth Beach, Del. She also works part time for Pediatrix Medical Group, serving the Pediatric Intensive Care Unit medical team at the Herman & Walter Samuelson Children’s Hospital at Sinai in Baltimore and serves as adjunct faculty at the University of Maryland School of Nursing, also in Baltimore.
The coeditor of a textbook, Lippincott Certification Review: Pediatric Acute Care Nurse Practitioner, Dr. Haut has published and presented on many clinical and professional topics. She has volunteered for different nursing organizations in a variety of positions, and has been an active member and leader of NAPNAP’s Maryland Chesapeake Chapter since 1996, serving as president in 2006-2007.
Optimal medical therapy doesn’t affect DAPT efficacy
ROME – Continued dual-antiplatelet therapy beyond 12 months after coronary stenting is neither helped nor hindered by concomitant background optimal medical therapy, according to a secondary analysis of the landmark DAPT trial.
Numerous studies have shown that only 46%-66% of patients with stable ischemic heart disease are adherent to guideline-directed optimal medical therapy (OMT) with statins, beta-blockers, and ACE inhibitors or angiotensin receptor blockers. Yet until this new analysis from DAPT (the Dual Antiplatelet Therapy Study), the impact of OMT on the treatment effect of prolonged DAPT was unstudied, Charles D. Resor, MD, said at the annual congress of the European Society of Cardiology.
It was plausible that the treatment benefit of continued DAPT beyond 12 months after coronary stenting might be stunted by concomitant OMT, or alternatively that OMT and DAPT might exert synergistic effects in reducing the risk of ischemic events. As it turned out, neither of these possibilities turned out to be the case, according to Dr. Resor of Brigham and Women’s Hospital, Boston.
“I think OMT is underutilized, but while we would like physicians to emphasize OMT use in more cases, the decision to use DAPT beyond 12 months should be made irrespective of OMT use,” he said.
“That’s an important message for clinicians,” commented session cochair Keith A.A. Fox, MD, of the University of Edinburgh.
The landmark DAPT trial established that there is a significant benefit of continued dual-antiplatelet therapy beyond 12 months from coronary stenting in patients with stable ischemic heart disease (N Engl J Med. 2014 Dec 4;371:2155-66). Dr. Resor presented a secondary analysis of clinical outcomes in 11,643 study participants stratified by their OMT status as well as by whether they were randomized to dual-antiplatelet therapy or aspirin plus placebo.
Sixty-three percent of subjects were on OMT at enrollment. Adherence to OMT was high: At 30 months post stenting, 62% of participants remained on OMT.
Between 12 and 30 months after percutaneous coronary intervention, continued dual-antiplatelet therapy in patients also on OMT was associated with a 2.1% incidence of acute MI, a 36% reduction in risk, compared with the 3.3% rate in subjects on placebo plus OMT. In patients not on OMT, the MI rate was 2.2% in those on dual-antiplatelet therapy versus 5.2% with placebo, for a 59% relative risk reduction in the active treatment arm.
The rate of the composite endpoint of major adverse cardiovascular and cerebrovascular events in patients on dual-antiplatelet therapy and OMT was 4.2%, compared with a 5.0% rate in patients on placebo and OMT.
Moderate or severe bleeding, as measured by the Global Use of Strategies to Open Occluded Arteries (GUSTO) criteria occurred in 2.2% of patients on dual-antiplatelet therapy and OMT, compared with 1.0% in those on placebo plus OMT. In patients not on OMT, the bleeding rate was 2.8% in the dual-antiplatelet therapy group and 2.2% with placebo, a nonsignificant difference.
Overall, patients on OMT had significantly lower rates of acute MI than those not on OMT (2.7%, compared with 3.7%), as well as fewer major adverse cardiovascular and cerebrovascular events (4.6% versus 5.7%), and less moderate or severe bleeding (1.6%, compared with 2.5% in patients not on OMT). However, rates of stroke, stent thrombosis, and death didn’t differ between patients on OMT and those who were not.
“While the associations between OMT use and lower rates of MI and MACCE [major adverse cardiovascular and cerebrovascular events] were expected, the association with lower rates of moderate or severe bleeding was not; we suspect the lower bleeding risk is mostly due to some residual confounding,” according to Dr. Resor.
He reported having no financial conflicts of interest regarding the new secondary analysis of DAPT, which was sponsored by the Harvard Clinical Research Institute.
Simultaneously with Dr. Resor’s presentation, the new DAPT study analysis was published online (Circulation. 2016 Aug 30. doi: 10.1161/CIRCULATIONAHA.116.024531.)
ROME – Continued dual-antiplatelet therapy beyond 12 months after coronary stenting is neither helped nor hindered by concomitant background optimal medical therapy, according to a secondary analysis of the landmark DAPT trial.
Numerous studies have shown that only 46%-66% of patients with stable ischemic heart disease are adherent to guideline-directed optimal medical therapy (OMT) with statins, beta-blockers, and ACE inhibitors or angiotensin receptor blockers. Yet until this new analysis from DAPT (the Dual Antiplatelet Therapy Study), the impact of OMT on the treatment effect of prolonged DAPT was unstudied, Charles D. Resor, MD, said at the annual congress of the European Society of Cardiology.
It was plausible that the treatment benefit of continued DAPT beyond 12 months after coronary stenting might be stunted by concomitant OMT, or alternatively that OMT and DAPT might exert synergistic effects in reducing the risk of ischemic events. As it turned out, neither of these possibilities turned out to be the case, according to Dr. Resor of Brigham and Women’s Hospital, Boston.
“I think OMT is underutilized, but while we would like physicians to emphasize OMT use in more cases, the decision to use DAPT beyond 12 months should be made irrespective of OMT use,” he said.
“That’s an important message for clinicians,” commented session cochair Keith A.A. Fox, MD, of the University of Edinburgh.
The landmark DAPT trial established that there is a significant benefit of continued dual-antiplatelet therapy beyond 12 months from coronary stenting in patients with stable ischemic heart disease (N Engl J Med. 2014 Dec 4;371:2155-66). Dr. Resor presented a secondary analysis of clinical outcomes in 11,643 study participants stratified by their OMT status as well as by whether they were randomized to dual-antiplatelet therapy or aspirin plus placebo.
Sixty-three percent of subjects were on OMT at enrollment. Adherence to OMT was high: At 30 months post stenting, 62% of participants remained on OMT.
Between 12 and 30 months after percutaneous coronary intervention, continued dual-antiplatelet therapy in patients also on OMT was associated with a 2.1% incidence of acute MI, a 36% reduction in risk, compared with the 3.3% rate in subjects on placebo plus OMT. In patients not on OMT, the MI rate was 2.2% in those on dual-antiplatelet therapy versus 5.2% with placebo, for a 59% relative risk reduction in the active treatment arm.
The rate of the composite endpoint of major adverse cardiovascular and cerebrovascular events in patients on dual-antiplatelet therapy and OMT was 4.2%, compared with a 5.0% rate in patients on placebo and OMT.
Moderate or severe bleeding, as measured by the Global Use of Strategies to Open Occluded Arteries (GUSTO) criteria occurred in 2.2% of patients on dual-antiplatelet therapy and OMT, compared with 1.0% in those on placebo plus OMT. In patients not on OMT, the bleeding rate was 2.8% in the dual-antiplatelet therapy group and 2.2% with placebo, a nonsignificant difference.
Overall, patients on OMT had significantly lower rates of acute MI than those not on OMT (2.7%, compared with 3.7%), as well as fewer major adverse cardiovascular and cerebrovascular events (4.6% versus 5.7%), and less moderate or severe bleeding (1.6%, compared with 2.5% in patients not on OMT). However, rates of stroke, stent thrombosis, and death didn’t differ between patients on OMT and those who were not.
“While the associations between OMT use and lower rates of MI and MACCE [major adverse cardiovascular and cerebrovascular events] were expected, the association with lower rates of moderate or severe bleeding was not; we suspect the lower bleeding risk is mostly due to some residual confounding,” according to Dr. Resor.
He reported having no financial conflicts of interest regarding the new secondary analysis of DAPT, which was sponsored by the Harvard Clinical Research Institute.
Simultaneously with Dr. Resor’s presentation, the new DAPT study analysis was published online (Circulation. 2016 Aug 30. doi: 10.1161/CIRCULATIONAHA.116.024531.)
ROME – Continued dual-antiplatelet therapy beyond 12 months after coronary stenting is neither helped nor hindered by concomitant background optimal medical therapy, according to a secondary analysis of the landmark DAPT trial.
Numerous studies have shown that only 46%-66% of patients with stable ischemic heart disease are adherent to guideline-directed optimal medical therapy (OMT) with statins, beta-blockers, and ACE inhibitors or angiotensin receptor blockers. Yet until this new analysis from DAPT (the Dual Antiplatelet Therapy Study), the impact of OMT on the treatment effect of prolonged DAPT was unstudied, Charles D. Resor, MD, said at the annual congress of the European Society of Cardiology.
It was plausible that the treatment benefit of continued DAPT beyond 12 months after coronary stenting might be stunted by concomitant OMT, or alternatively that OMT and DAPT might exert synergistic effects in reducing the risk of ischemic events. As it turned out, neither of these possibilities turned out to be the case, according to Dr. Resor of Brigham and Women’s Hospital, Boston.
“I think OMT is underutilized, but while we would like physicians to emphasize OMT use in more cases, the decision to use DAPT beyond 12 months should be made irrespective of OMT use,” he said.
“That’s an important message for clinicians,” commented session cochair Keith A.A. Fox, MD, of the University of Edinburgh.
The landmark DAPT trial established that there is a significant benefit of continued dual-antiplatelet therapy beyond 12 months from coronary stenting in patients with stable ischemic heart disease (N Engl J Med. 2014 Dec 4;371:2155-66). Dr. Resor presented a secondary analysis of clinical outcomes in 11,643 study participants stratified by their OMT status as well as by whether they were randomized to dual-antiplatelet therapy or aspirin plus placebo.
Sixty-three percent of subjects were on OMT at enrollment. Adherence to OMT was high: At 30 months post stenting, 62% of participants remained on OMT.
Between 12 and 30 months after percutaneous coronary intervention, continued dual-antiplatelet therapy in patients also on OMT was associated with a 2.1% incidence of acute MI, a 36% reduction in risk, compared with the 3.3% rate in subjects on placebo plus OMT. In patients not on OMT, the MI rate was 2.2% in those on dual-antiplatelet therapy versus 5.2% with placebo, for a 59% relative risk reduction in the active treatment arm.
The rate of the composite endpoint of major adverse cardiovascular and cerebrovascular events in patients on dual-antiplatelet therapy and OMT was 4.2%, compared with a 5.0% rate in patients on placebo and OMT.
Moderate or severe bleeding, as measured by the Global Use of Strategies to Open Occluded Arteries (GUSTO) criteria occurred in 2.2% of patients on dual-antiplatelet therapy and OMT, compared with 1.0% in those on placebo plus OMT. In patients not on OMT, the bleeding rate was 2.8% in the dual-antiplatelet therapy group and 2.2% with placebo, a nonsignificant difference.
Overall, patients on OMT had significantly lower rates of acute MI than those not on OMT (2.7%, compared with 3.7%), as well as fewer major adverse cardiovascular and cerebrovascular events (4.6% versus 5.7%), and less moderate or severe bleeding (1.6%, compared with 2.5% in patients not on OMT). However, rates of stroke, stent thrombosis, and death didn’t differ between patients on OMT and those who were not.
“While the associations between OMT use and lower rates of MI and MACCE [major adverse cardiovascular and cerebrovascular events] were expected, the association with lower rates of moderate or severe bleeding was not; we suspect the lower bleeding risk is mostly due to some residual confounding,” according to Dr. Resor.
He reported having no financial conflicts of interest regarding the new secondary analysis of DAPT, which was sponsored by the Harvard Clinical Research Institute.
Simultaneously with Dr. Resor’s presentation, the new DAPT study analysis was published online (Circulation. 2016 Aug 30. doi: 10.1161/CIRCULATIONAHA.116.024531.)
AT THE ESC CONGRESS 2016
Key clinical point: The decision to use DAPT beyond 12 months should be made irrespective of optimal medical therapy use.
Major finding: Between 12 and 30 months after coronary stenting, continued dual-antiplatelet therapy was associated with a 2.1% rate of acute MI in patients on optimal medical therapy and a 2.2% rate in those not on optimal medical therapy.
Data source: A secondary analysis of 11,648 participants in the double-blind, randomized Dual Antiplatelet Therapy Study who were followed prospectively for the period of 12-30 months post PCI.
Disclosures: The study presenter reported having no financial conflicts regarding the new secondary analysis of DAPT, which was sponsored by the Harvard Clinical Research Institute.
IHS Takes Serious Steps to Bolster Workforce and Improve Care
IHS director Mary Smith recently issued an update on what’s been happening since the recent launch of “an aggressive strategy to improve the quality of care in the Great Plains area and across the country.”
The past 10 years have seen a new focus on quality improvement, she said, and IHS is working to enhance that with, for example, a system wide mock survey initiative at all 27 IHS hospitals to assess compliance with the Centers for Medicare & Medicaid Services (CMS) Conditions of Participation and readiness for reaccreditation.
Related: IHS and CMS Partner for Patient Safety Improvements
The IHS continues to face significant workforce challenges, including a chronic shortage of quality health care providers (HCPs), according to Smith. To that end, more than 2 dozen Commissioned Corps clinicians have been deployed to temporary placements in the Great Plains area hospitals with CMS findings. To attract more HCPs, the National Institutes of Health has been helping IHS recruit more nurses into its clinical program; IHS is also revising position descriptions, using more comprehensive recruitment plans, raising pay, and instituting relocation pay for GS-12 and lower clinical positions and lower grades. IHS is also implementing a “stronger” search committee process and advertising vacancies more widely.
Another change is the expansion of tribal participation in filling vacant area director positions. Members of a tribe from each area will, for the first time, play a role at the outset of the hiring process, Smith said.
Related: Dangerous Staff Shortages in the IHS
The strategy also emphasizes bringing in health care quality expertise. This initiative began with the launch of a Hospital Engagement Network (HEN 2.0), which allows IHS hospitals to share strategies on how to reduce avoidable readmissions and hospital-acquired conditions.
The last priority area is to strengthen relationships with local and regional partners, like health care systems, colleges, and direct service hospitals. “Some of the most helpful expertise and the most effective leadership is right in the Tribal communities we work with every day,” Smith said. The “government-to-government relationship with Tribes is the foundation of our work at IHS.”
IHS director Mary Smith recently issued an update on what’s been happening since the recent launch of “an aggressive strategy to improve the quality of care in the Great Plains area and across the country.”
The past 10 years have seen a new focus on quality improvement, she said, and IHS is working to enhance that with, for example, a system wide mock survey initiative at all 27 IHS hospitals to assess compliance with the Centers for Medicare & Medicaid Services (CMS) Conditions of Participation and readiness for reaccreditation.
Related: IHS and CMS Partner for Patient Safety Improvements
The IHS continues to face significant workforce challenges, including a chronic shortage of quality health care providers (HCPs), according to Smith. To that end, more than 2 dozen Commissioned Corps clinicians have been deployed to temporary placements in the Great Plains area hospitals with CMS findings. To attract more HCPs, the National Institutes of Health has been helping IHS recruit more nurses into its clinical program; IHS is also revising position descriptions, using more comprehensive recruitment plans, raising pay, and instituting relocation pay for GS-12 and lower clinical positions and lower grades. IHS is also implementing a “stronger” search committee process and advertising vacancies more widely.
Another change is the expansion of tribal participation in filling vacant area director positions. Members of a tribe from each area will, for the first time, play a role at the outset of the hiring process, Smith said.
Related: Dangerous Staff Shortages in the IHS
The strategy also emphasizes bringing in health care quality expertise. This initiative began with the launch of a Hospital Engagement Network (HEN 2.0), which allows IHS hospitals to share strategies on how to reduce avoidable readmissions and hospital-acquired conditions.
The last priority area is to strengthen relationships with local and regional partners, like health care systems, colleges, and direct service hospitals. “Some of the most helpful expertise and the most effective leadership is right in the Tribal communities we work with every day,” Smith said. The “government-to-government relationship with Tribes is the foundation of our work at IHS.”
IHS director Mary Smith recently issued an update on what’s been happening since the recent launch of “an aggressive strategy to improve the quality of care in the Great Plains area and across the country.”
The past 10 years have seen a new focus on quality improvement, she said, and IHS is working to enhance that with, for example, a system wide mock survey initiative at all 27 IHS hospitals to assess compliance with the Centers for Medicare & Medicaid Services (CMS) Conditions of Participation and readiness for reaccreditation.
Related: IHS and CMS Partner for Patient Safety Improvements
The IHS continues to face significant workforce challenges, including a chronic shortage of quality health care providers (HCPs), according to Smith. To that end, more than 2 dozen Commissioned Corps clinicians have been deployed to temporary placements in the Great Plains area hospitals with CMS findings. To attract more HCPs, the National Institutes of Health has been helping IHS recruit more nurses into its clinical program; IHS is also revising position descriptions, using more comprehensive recruitment plans, raising pay, and instituting relocation pay for GS-12 and lower clinical positions and lower grades. IHS is also implementing a “stronger” search committee process and advertising vacancies more widely.
Another change is the expansion of tribal participation in filling vacant area director positions. Members of a tribe from each area will, for the first time, play a role at the outset of the hiring process, Smith said.
Related: Dangerous Staff Shortages in the IHS
The strategy also emphasizes bringing in health care quality expertise. This initiative began with the launch of a Hospital Engagement Network (HEN 2.0), which allows IHS hospitals to share strategies on how to reduce avoidable readmissions and hospital-acquired conditions.
The last priority area is to strengthen relationships with local and regional partners, like health care systems, colleges, and direct service hospitals. “Some of the most helpful expertise and the most effective leadership is right in the Tribal communities we work with every day,” Smith said. The “government-to-government relationship with Tribes is the foundation of our work at IHS.”
PAFC provides early embolus detection in mice
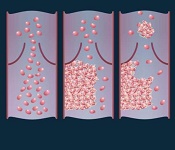
vein (left), thrombus formation
(middle), and a thrombus (right)
detaching from a vessel wall,
leading to thromboembolism.
Image by Moscow Institute
of Physics and Technology
Photoacoustic flow cytometry (PAFC) can provide real-time, non-invasive detection of emboli, according to research published in PLOS ONE.
Experiments in mice showed that PAFC can reveal emboli triggered by melanoma and medical procedures by detecting transient changes in blood absorption.
Investigators believe that by providing early embolus detection, PAFC could enable well-timed anticoagulant therapy and potentially prevent lethal complications.
For this study, the team used PAFC to monitor embolus formation in 4 groups of mice.
Two of the groups had melanoma. In one, the implanted tumor underwent compression. In the other, the investigators performed a surgical excision of the tumor.
The remaining 2 groups consisted of healthy mice. In one of these groups, mice had their limbs clamped to produce vessel stasis. In the other, the mice underwent surgery.
The investigators found that PAFC could detect a single embolus, and the method allowed them to distinguish between erythrocyte-rich and leukocyte/platelet-rich emboli.
They also observed a correlation between the presence of white emboli and melanoma.
The level of circulating emboli was significantly higher in the melanoma-bearing mice than in the healthy ones (P=0.0013).
However, the number of circulating emboli temporarily increased in the healthy mice during vessel stasis (P=0.033) and after surgical excisions (P=0.031).
The melanoma-bearing mice also experienced increases in the number of circulating emboli during tumor compression (P=0.013) and after tumor excision (P=0.012).
“We showed that it is possible to detect emboli in the bloodstream using photoacoustic flow cytometry,” said study author Alexander Melerzanov, PhD, of the Moscow Institute of Physics and Technology in Russia.
“PAFC may also be used to destroy blood clots, and we hope to work on this in our next experiments.” ![]()

vein (left), thrombus formation
(middle), and a thrombus (right)
detaching from a vessel wall,
leading to thromboembolism.
Image by Moscow Institute
of Physics and Technology
Photoacoustic flow cytometry (PAFC) can provide real-time, non-invasive detection of emboli, according to research published in PLOS ONE.
Experiments in mice showed that PAFC can reveal emboli triggered by melanoma and medical procedures by detecting transient changes in blood absorption.
Investigators believe that by providing early embolus detection, PAFC could enable well-timed anticoagulant therapy and potentially prevent lethal complications.
For this study, the team used PAFC to monitor embolus formation in 4 groups of mice.
Two of the groups had melanoma. In one, the implanted tumor underwent compression. In the other, the investigators performed a surgical excision of the tumor.
The remaining 2 groups consisted of healthy mice. In one of these groups, mice had their limbs clamped to produce vessel stasis. In the other, the mice underwent surgery.
The investigators found that PAFC could detect a single embolus, and the method allowed them to distinguish between erythrocyte-rich and leukocyte/platelet-rich emboli.
They also observed a correlation between the presence of white emboli and melanoma.
The level of circulating emboli was significantly higher in the melanoma-bearing mice than in the healthy ones (P=0.0013).
However, the number of circulating emboli temporarily increased in the healthy mice during vessel stasis (P=0.033) and after surgical excisions (P=0.031).
The melanoma-bearing mice also experienced increases in the number of circulating emboli during tumor compression (P=0.013) and after tumor excision (P=0.012).
“We showed that it is possible to detect emboli in the bloodstream using photoacoustic flow cytometry,” said study author Alexander Melerzanov, PhD, of the Moscow Institute of Physics and Technology in Russia.
“PAFC may also be used to destroy blood clots, and we hope to work on this in our next experiments.” ![]()

vein (left), thrombus formation
(middle), and a thrombus (right)
detaching from a vessel wall,
leading to thromboembolism.
Image by Moscow Institute
of Physics and Technology
Photoacoustic flow cytometry (PAFC) can provide real-time, non-invasive detection of emboli, according to research published in PLOS ONE.
Experiments in mice showed that PAFC can reveal emboli triggered by melanoma and medical procedures by detecting transient changes in blood absorption.
Investigators believe that by providing early embolus detection, PAFC could enable well-timed anticoagulant therapy and potentially prevent lethal complications.
For this study, the team used PAFC to monitor embolus formation in 4 groups of mice.
Two of the groups had melanoma. In one, the implanted tumor underwent compression. In the other, the investigators performed a surgical excision of the tumor.
The remaining 2 groups consisted of healthy mice. In one of these groups, mice had their limbs clamped to produce vessel stasis. In the other, the mice underwent surgery.
The investigators found that PAFC could detect a single embolus, and the method allowed them to distinguish between erythrocyte-rich and leukocyte/platelet-rich emboli.
They also observed a correlation between the presence of white emboli and melanoma.
The level of circulating emboli was significantly higher in the melanoma-bearing mice than in the healthy ones (P=0.0013).
However, the number of circulating emboli temporarily increased in the healthy mice during vessel stasis (P=0.033) and after surgical excisions (P=0.031).
The melanoma-bearing mice also experienced increases in the number of circulating emboli during tumor compression (P=0.013) and after tumor excision (P=0.012).
“We showed that it is possible to detect emboli in the bloodstream using photoacoustic flow cytometry,” said study author Alexander Melerzanov, PhD, of the Moscow Institute of Physics and Technology in Russia.
“PAFC may also be used to destroy blood clots, and we hope to work on this in our next experiments.” ![]()
Team creates method for predicting drug toxicity

for a clinical trial
Photo by Esther Dyson
Researchers have devised a method for predicting whether experimental drugs will fail clinical trials due to excessive toxicity.
They say the method, known as PrOCTOR, upends conventional wisdom about the criteria on which to evaluate new drugs’ safety.
Rather than exclusively examining molecular structure to determine viability, PrOCTOR combines a host of structural features and features related to how the drug binds to molecules in the body.
“We looked more broadly at drug molecule features that drug developers thought were unimportant in predicting drug safety in the past. Then, we let the data speak for itself,” said study author Olivier Elemento, PhD, of Weill Cornell Medicine in New York, New York.
Dr Elemento and his colleagues described PrOCTOR in Cell Chemical Biology.
PrOCTOR was inspired by an approach that baseball statisticians adopted to better predict which players would be successful offensively. Instead of relying on collective wisdom from baseball insiders, statisticians decided to use an objective numbers analysis to measure in-game productivity, a strategy known as “Moneyball.”
Similarly, Dr Elemento and his colleagues developed a computational method that analyzes data from 48 different features of a drug—from molecular weight to details about its target—to determine whether it would be safe for clinical use.
Using machine learning, the researchers trained PrOCTOR on drugs approved by the US Food and Drug Administration (FDA) as well as drugs that failed clinical trials due to toxicity problems.
Based on this information, the researchers created “PrOCTOR scores” that could help distinguish drugs approved by the FDA from those that failed for toxicity.
They tested PrOCTOR on hundreds of additional drugs approved in Europe and Japan and using side effect data on approved drugs collected by the FDA.
The researchers said PrOCTOR was able to accurately recognize toxic side effects that were a consequence of a drug’s chemical features and its target. Records revealing that many of these drugs had failed clinical trials supported PrOCTOR’s accuracy.
“We were able to find several features that led to a very predictive model,” Dr Elemento said. “Hopefully, this approach could be used to determine whether it’s worth pursuing a drug prior to starting human trials.” ![]()

for a clinical trial
Photo by Esther Dyson
Researchers have devised a method for predicting whether experimental drugs will fail clinical trials due to excessive toxicity.
They say the method, known as PrOCTOR, upends conventional wisdom about the criteria on which to evaluate new drugs’ safety.
Rather than exclusively examining molecular structure to determine viability, PrOCTOR combines a host of structural features and features related to how the drug binds to molecules in the body.
“We looked more broadly at drug molecule features that drug developers thought were unimportant in predicting drug safety in the past. Then, we let the data speak for itself,” said study author Olivier Elemento, PhD, of Weill Cornell Medicine in New York, New York.
Dr Elemento and his colleagues described PrOCTOR in Cell Chemical Biology.
PrOCTOR was inspired by an approach that baseball statisticians adopted to better predict which players would be successful offensively. Instead of relying on collective wisdom from baseball insiders, statisticians decided to use an objective numbers analysis to measure in-game productivity, a strategy known as “Moneyball.”
Similarly, Dr Elemento and his colleagues developed a computational method that analyzes data from 48 different features of a drug—from molecular weight to details about its target—to determine whether it would be safe for clinical use.
Using machine learning, the researchers trained PrOCTOR on drugs approved by the US Food and Drug Administration (FDA) as well as drugs that failed clinical trials due to toxicity problems.
Based on this information, the researchers created “PrOCTOR scores” that could help distinguish drugs approved by the FDA from those that failed for toxicity.
They tested PrOCTOR on hundreds of additional drugs approved in Europe and Japan and using side effect data on approved drugs collected by the FDA.
The researchers said PrOCTOR was able to accurately recognize toxic side effects that were a consequence of a drug’s chemical features and its target. Records revealing that many of these drugs had failed clinical trials supported PrOCTOR’s accuracy.
“We were able to find several features that led to a very predictive model,” Dr Elemento said. “Hopefully, this approach could be used to determine whether it’s worth pursuing a drug prior to starting human trials.” ![]()

for a clinical trial
Photo by Esther Dyson
Researchers have devised a method for predicting whether experimental drugs will fail clinical trials due to excessive toxicity.
They say the method, known as PrOCTOR, upends conventional wisdom about the criteria on which to evaluate new drugs’ safety.
Rather than exclusively examining molecular structure to determine viability, PrOCTOR combines a host of structural features and features related to how the drug binds to molecules in the body.
“We looked more broadly at drug molecule features that drug developers thought were unimportant in predicting drug safety in the past. Then, we let the data speak for itself,” said study author Olivier Elemento, PhD, of Weill Cornell Medicine in New York, New York.
Dr Elemento and his colleagues described PrOCTOR in Cell Chemical Biology.
PrOCTOR was inspired by an approach that baseball statisticians adopted to better predict which players would be successful offensively. Instead of relying on collective wisdom from baseball insiders, statisticians decided to use an objective numbers analysis to measure in-game productivity, a strategy known as “Moneyball.”
Similarly, Dr Elemento and his colleagues developed a computational method that analyzes data from 48 different features of a drug—from molecular weight to details about its target—to determine whether it would be safe for clinical use.
Using machine learning, the researchers trained PrOCTOR on drugs approved by the US Food and Drug Administration (FDA) as well as drugs that failed clinical trials due to toxicity problems.
Based on this information, the researchers created “PrOCTOR scores” that could help distinguish drugs approved by the FDA from those that failed for toxicity.
They tested PrOCTOR on hundreds of additional drugs approved in Europe and Japan and using side effect data on approved drugs collected by the FDA.
The researchers said PrOCTOR was able to accurately recognize toxic side effects that were a consequence of a drug’s chemical features and its target. Records revealing that many of these drugs had failed clinical trials supported PrOCTOR’s accuracy.
“We were able to find several features that led to a very predictive model,” Dr Elemento said. “Hopefully, this approach could be used to determine whether it’s worth pursuing a drug prior to starting human trials.” ![]()
Targeting AML’s dependence on fat

New research has revealed a potential treatment strategy for acute myeloid leukemia (AML) and other malignancies that show a preference for metabolizing fat over sugar.
The study suggests the protein prolyl hydroxylase 3 (PHD3) is a key regulator of fatty acid oxidation, and PHD3 expression is low in certain malignancies—particularly AML—but overexpressing PHD3 can have anticancer effects.
Researchers believe this finding might aid the development of therapies that work by starving tumors of their fuel.
“This really represents a new frontier in looking at the metabolism of cancer,” said study author Marcia Haigis, PhD, of Harvard Medical School in Boston, Massachusetts.
“Understanding the molecular handle of this pathway is the first step toward translating the basic work into therapy.”
Dr Haigis and her colleagues described the work in Molecular Cell.
The researchers knew that when cells run low on nutrients, they switch from sugar to fat as their fuel source to sustain function.
When cells have low energy, the protein AMPK targets the enzyme ACC to activate fat oxidation, which helps cells burn fats to make energy. However, when cells have enough resources, they seek to maintain energy balance.
Dr Haigis and her colleagues were searching for precisely how cells turn off fat oxidation and homed in on PHD3. Recent studies had suggested that PHD3 plays a part in cell metabolism, but,
until now, its precise role has remained unclear.
In a series of experiments, Dr Haigis’s team showed that PHD3 suppressed fat-burning by chemically modifying and activating ACC2—a version of the same enzyme responsible for keeping cellular fat-burning in check.
To determine PHD3’s role in cancer, the researchers combed through databases of all human cancers. The team theorized that sugar-craving malignancies would have high levels of PDH3, and cancers that relied on fat for their energy would have low levels.
The researchers found the lowest levels of PHD3 in AML, so they decided to examine the effects of restoring PHD3 in AML cells and a mouse model of the disease.
The experiments showed that restoring PHD3 expression reduces AML potency, and ACC2 is required for the inhibitory effects of PHD3. Overexpressing PHD3 limited fatty acid oxidation via regulation of ACC2, which decreased leukemia cell proliferation and enhanced survival in the mouse model of AML.
Dr Haigis noted that more basic research is needed before this work can move ahead to the clinic, as it’s still unclear why certain cancers depend on fat.
“What do fats provide to tumors that other fuels don’t?” Dr Haigis asked. “That’s one open question, and this is only the first chapter in the story.” ![]()

New research has revealed a potential treatment strategy for acute myeloid leukemia (AML) and other malignancies that show a preference for metabolizing fat over sugar.
The study suggests the protein prolyl hydroxylase 3 (PHD3) is a key regulator of fatty acid oxidation, and PHD3 expression is low in certain malignancies—particularly AML—but overexpressing PHD3 can have anticancer effects.
Researchers believe this finding might aid the development of therapies that work by starving tumors of their fuel.
“This really represents a new frontier in looking at the metabolism of cancer,” said study author Marcia Haigis, PhD, of Harvard Medical School in Boston, Massachusetts.
“Understanding the molecular handle of this pathway is the first step toward translating the basic work into therapy.”
Dr Haigis and her colleagues described the work in Molecular Cell.
The researchers knew that when cells run low on nutrients, they switch from sugar to fat as their fuel source to sustain function.
When cells have low energy, the protein AMPK targets the enzyme ACC to activate fat oxidation, which helps cells burn fats to make energy. However, when cells have enough resources, they seek to maintain energy balance.
Dr Haigis and her colleagues were searching for precisely how cells turn off fat oxidation and homed in on PHD3. Recent studies had suggested that PHD3 plays a part in cell metabolism, but,
until now, its precise role has remained unclear.
In a series of experiments, Dr Haigis’s team showed that PHD3 suppressed fat-burning by chemically modifying and activating ACC2—a version of the same enzyme responsible for keeping cellular fat-burning in check.
To determine PHD3’s role in cancer, the researchers combed through databases of all human cancers. The team theorized that sugar-craving malignancies would have high levels of PDH3, and cancers that relied on fat for their energy would have low levels.
The researchers found the lowest levels of PHD3 in AML, so they decided to examine the effects of restoring PHD3 in AML cells and a mouse model of the disease.
The experiments showed that restoring PHD3 expression reduces AML potency, and ACC2 is required for the inhibitory effects of PHD3. Overexpressing PHD3 limited fatty acid oxidation via regulation of ACC2, which decreased leukemia cell proliferation and enhanced survival in the mouse model of AML.
Dr Haigis noted that more basic research is needed before this work can move ahead to the clinic, as it’s still unclear why certain cancers depend on fat.
“What do fats provide to tumors that other fuels don’t?” Dr Haigis asked. “That’s one open question, and this is only the first chapter in the story.” ![]()

New research has revealed a potential treatment strategy for acute myeloid leukemia (AML) and other malignancies that show a preference for metabolizing fat over sugar.
The study suggests the protein prolyl hydroxylase 3 (PHD3) is a key regulator of fatty acid oxidation, and PHD3 expression is low in certain malignancies—particularly AML—but overexpressing PHD3 can have anticancer effects.
Researchers believe this finding might aid the development of therapies that work by starving tumors of their fuel.
“This really represents a new frontier in looking at the metabolism of cancer,” said study author Marcia Haigis, PhD, of Harvard Medical School in Boston, Massachusetts.
“Understanding the molecular handle of this pathway is the first step toward translating the basic work into therapy.”
Dr Haigis and her colleagues described the work in Molecular Cell.
The researchers knew that when cells run low on nutrients, they switch from sugar to fat as their fuel source to sustain function.
When cells have low energy, the protein AMPK targets the enzyme ACC to activate fat oxidation, which helps cells burn fats to make energy. However, when cells have enough resources, they seek to maintain energy balance.
Dr Haigis and her colleagues were searching for precisely how cells turn off fat oxidation and homed in on PHD3. Recent studies had suggested that PHD3 plays a part in cell metabolism, but,
until now, its precise role has remained unclear.
In a series of experiments, Dr Haigis’s team showed that PHD3 suppressed fat-burning by chemically modifying and activating ACC2—a version of the same enzyme responsible for keeping cellular fat-burning in check.
To determine PHD3’s role in cancer, the researchers combed through databases of all human cancers. The team theorized that sugar-craving malignancies would have high levels of PDH3, and cancers that relied on fat for their energy would have low levels.
The researchers found the lowest levels of PHD3 in AML, so they decided to examine the effects of restoring PHD3 in AML cells and a mouse model of the disease.
The experiments showed that restoring PHD3 expression reduces AML potency, and ACC2 is required for the inhibitory effects of PHD3. Overexpressing PHD3 limited fatty acid oxidation via regulation of ACC2, which decreased leukemia cell proliferation and enhanced survival in the mouse model of AML.
Dr Haigis noted that more basic research is needed before this work can move ahead to the clinic, as it’s still unclear why certain cancers depend on fat.
“What do fats provide to tumors that other fuels don’t?” Dr Haigis asked. “That’s one open question, and this is only the first chapter in the story.” ![]()
Leukemia no longer deadliest childhood cancer in US

Photo by Bill Branson
Brain cancer has overtaken leukemia to become the deadliest childhood cancer in the US, according to a report from the US Centers for Disease Control and Prevention’s National Center for Health Statistics.
The report includes cancer mortality statistics from 1999 to 2014 pertaining to children and adolescents (ages 1 to 19).
In both 1999 and 2014, more than half of all cancer deaths in this population were attributable to leukemias or brain cancers.
In 1999, a greater percentage of deaths were attributed to leukemias than to brain cancers—29.7% and 23.7%, respectively.
But in 2014, brain cancer deaths exceeded leukemia deaths—29.9% and 24.9%, respectively.
The data also showed that, overall, cancer mortality has decreased among children and adolescents in the US.
The cancer death rate was 20% lower in 2014 than in 1999—2.28 deaths per 100,000 persons and 2.85 deaths per 100,000 persons, respectively.
Cancer death rates declined from 1999 to 2014 for all of the age groups studied (divided by 5-year increments) and for both sexes. However, cancer death rates were consistently higher for males than females.
In 2014, 54.8% of cancer deaths among children and adolescents were attributable to either leukemias or brain cancers.
The 2014 death rates by cancer site, from most common to least, were as follows:
- Brain cancers (29.9%)
- Leukemias (24.9%)
- Bone and articular cartilage cancers (10.1%)
- Cancers of the thyroid and other endocrine glands (9.0%)
- Mesothelial and soft tissue cancers (7.7%)
- Non-Hodgkin lymphomas (3.9%)
- Cancers of the liver and intrahepatic bile ducts (2.0%)
- Cancers of the kidney and renal pelvis (1.8%).
Data on the remaining cancer sites were not shown separately.
For more information, see the full report, “Declines in Cancer Death Rates Among Children and Adolescents in the United States, 1999–2014.” ![]()

Photo by Bill Branson
Brain cancer has overtaken leukemia to become the deadliest childhood cancer in the US, according to a report from the US Centers for Disease Control and Prevention’s National Center for Health Statistics.
The report includes cancer mortality statistics from 1999 to 2014 pertaining to children and adolescents (ages 1 to 19).
In both 1999 and 2014, more than half of all cancer deaths in this population were attributable to leukemias or brain cancers.
In 1999, a greater percentage of deaths were attributed to leukemias than to brain cancers—29.7% and 23.7%, respectively.
But in 2014, brain cancer deaths exceeded leukemia deaths—29.9% and 24.9%, respectively.
The data also showed that, overall, cancer mortality has decreased among children and adolescents in the US.
The cancer death rate was 20% lower in 2014 than in 1999—2.28 deaths per 100,000 persons and 2.85 deaths per 100,000 persons, respectively.
Cancer death rates declined from 1999 to 2014 for all of the age groups studied (divided by 5-year increments) and for both sexes. However, cancer death rates were consistently higher for males than females.
In 2014, 54.8% of cancer deaths among children and adolescents were attributable to either leukemias or brain cancers.
The 2014 death rates by cancer site, from most common to least, were as follows:
- Brain cancers (29.9%)
- Leukemias (24.9%)
- Bone and articular cartilage cancers (10.1%)
- Cancers of the thyroid and other endocrine glands (9.0%)
- Mesothelial and soft tissue cancers (7.7%)
- Non-Hodgkin lymphomas (3.9%)
- Cancers of the liver and intrahepatic bile ducts (2.0%)
- Cancers of the kidney and renal pelvis (1.8%).
Data on the remaining cancer sites were not shown separately.
For more information, see the full report, “Declines in Cancer Death Rates Among Children and Adolescents in the United States, 1999–2014.” ![]()

Photo by Bill Branson
Brain cancer has overtaken leukemia to become the deadliest childhood cancer in the US, according to a report from the US Centers for Disease Control and Prevention’s National Center for Health Statistics.
The report includes cancer mortality statistics from 1999 to 2014 pertaining to children and adolescents (ages 1 to 19).
In both 1999 and 2014, more than half of all cancer deaths in this population were attributable to leukemias or brain cancers.
In 1999, a greater percentage of deaths were attributed to leukemias than to brain cancers—29.7% and 23.7%, respectively.
But in 2014, brain cancer deaths exceeded leukemia deaths—29.9% and 24.9%, respectively.
The data also showed that, overall, cancer mortality has decreased among children and adolescents in the US.
The cancer death rate was 20% lower in 2014 than in 1999—2.28 deaths per 100,000 persons and 2.85 deaths per 100,000 persons, respectively.
Cancer death rates declined from 1999 to 2014 for all of the age groups studied (divided by 5-year increments) and for both sexes. However, cancer death rates were consistently higher for males than females.
In 2014, 54.8% of cancer deaths among children and adolescents were attributable to either leukemias or brain cancers.
The 2014 death rates by cancer site, from most common to least, were as follows:
- Brain cancers (29.9%)
- Leukemias (24.9%)
- Bone and articular cartilage cancers (10.1%)
- Cancers of the thyroid and other endocrine glands (9.0%)
- Mesothelial and soft tissue cancers (7.7%)
- Non-Hodgkin lymphomas (3.9%)
- Cancers of the liver and intrahepatic bile ducts (2.0%)
- Cancers of the kidney and renal pelvis (1.8%).
Data on the remaining cancer sites were not shown separately.
For more information, see the full report, “Declines in Cancer Death Rates Among Children and Adolescents in the United States, 1999–2014.” ![]()
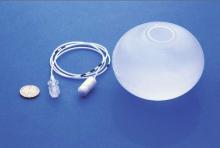
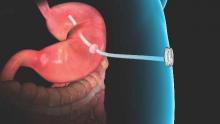
Endobariatrics: Coming to a clinic near you
SAN DIEGO – Device companies are working hard to bring obesity management to the endoscopy suite.
The field is called endobariatrics, and its goal is to fill the gap between surgery and pharmacotherapy. Drugs and lifestyle counseling don’t work for all patients, but many patients resist having surgery. These devices are meant to fill the need for nonsurgical, “noninvasive” solutions to obesity management.
Endobariatrics has the potential to be a boon for both obese patients and gastroenterology practices. Surgeons, however, approach these innovations with caution, due in part to past experiences of rescuing patients with failed devices. “Some innovations get disseminated without adequate studies to demonstrate whether they are superior, or even equal in efficacy and safety to, well-established procedures for the same condition. Even when they have been conducted, negative studies showing no difference may end up being rejected by journals and not published,” noted Karen Deveney, MD, FACS, professor of surgery, vice-chair for education in the department of surgery at Oregon Health & Science University, Portland, and co-Editor of ACS Surgery News.
Several new investigational devices and approaches were showcased at the annual Digestive Disease Week; some “are beginning to approach the kind of results we see with surgical techniques,” said Steven Edmundowicz, MD, medical director of the University of Colorado Digestive Health Center, Aurora.
“We are seeing a tremendous amount of development in this space, but it’s early, and we have to be cautious,” he said. There have already been a few disappointments, including the EndoBarrier, a fluoropolymer liner anchored in the duodenal bulb and unfurled down the duodenum to block food absorption. A key U.S. trial was recently halted due to liver abscesses.
Dr. Edmundowicz reviewed the latest developments presented at DDW.Self-assembling magnets for dual-path enteral anastomoses
The goal of the GI Windows system is to create a partial jejunoileal, side to side bypass without surgery. A 28-mm magnet ring is introduced to the ileum by colonoscopy, and a second ring to the jejunum by endoscopy. The rings snap together and tissue caught between them dies from pressure necrosis, leaving patients with a jejunoileal communication. Once food reaches that point, it either diverts through the anastomosis or continues past it down the digestive track. The magnets pass after the anastomosis forms in a week or so.
In a 6-month feasibility study from the Czech Republic, 10 obese patients lost 28.3% of their excess weight without diet restrictions. Those with diabetes had a mean hemoglobin A1c drop of 1.8%, and normalization of fasting blood glucose levels. The procedure took just over an hour and a half after the first five cases.
“I am very excited about [this]; I really want to see where the data are going,” Dr. Edmundowicz said.
Duodenal mucosal resurfacing
The idea of the Revita System (Fractyl) is to ablate “diabetic mucosa” in the duodenum so that normal mucosa can replace it. Saline is injected endoscopically under a portion of the duodenal mucosa to lift it off the muscularis; once isolated, the mucosa is destroyed – some in the audience thought “cooked” was a better word – by exposure to a hot water balloon catheter threaded into the lumen.
Thirty-nine overweight or obese type 2 diabetics had a 1.2% improvement at 6 months from a baseline hemoglobin A1c of 9.6% in a series from Santiago, Chile. Weight loss was modest in the trial; the system is being developed for type 2 diabetics.
There is some histologic support for the notion of a diabetic mucosa with both structural and hormonal aberrations, but it’s unclear if it’s a sign or cause of sickness. Even so, “the mucosa regenerates” and won’t be diabetic “for a while” after the procedure, said investigator Manoel Galvao Neto, MD, of the Gastro Obeso Center, São Paulo.
Gastric balloons
Inflating a balloon in the stomach to make people feel full isn’t new, but the notion of putting the balloon into a capsule that patients can swallow and inflating it through a tether is a more recent notion.
The Obalon Therapeutics is one such device. In an unblinded, sham-controlled trial with 336 obese patients, subjects who got the 250-mL, nitrogen-filled Obalons – most received three – lost about 3% more of their total body weight at 24 weeks than those who did not. Although swallowed, Obalon is removed endoscopically. Meanwhile, 34 obese patients who swallowed the 550-mL, fluid-filled Elipse balloon (Allurion) had a total body weight loss of 9.5% and mean excess weight loss of 37.2% at 4 months, by which time Elipse deflates on its own and passes without endoscopic retrieval.
“This is a very promising approach. I am very excited about digested balloons,” said Dr. Edmundowicz, an investigator in the Obalon study.
Endoscopic sleeve gastroplasty
Endoscopic sleeve gastroplasty duplicates sleeve gastrectomy with stitches placed endoscopically to seal off the greater curvature of the stomach; functionally, patients are left with a narrow sleeve of a stomach. In a multicenter series presented at DDW, 242 patients had a mean total body weight loss of 19.8% at 18 months, with a low incidence of complications. “Weight loss appears to be continuing,” Dr. Edmundowicz said. Investigators used the Apollo OverStitch (Apollo Endosurgery) to place the sutures.
Aspiration therapy
With Food and Drug Administration approval on June 14, AspireAssist (Aspire Bariatrics) is probably the best known of the newer approaches. Patients drain a portion of their meals through an endoscopically placed percutaneous gastrostomy tube a half hour or so after eating. It takes 5-10 minutes. The agency is eager to keep it out of the hands of bulimics.
One-year data were reported at DDW; 111 obese AspireAssist subjects lost a mean of 37.2% of their excess weight versus 13% in 60 patients randomized to lifestyle counseling alone.
“It may not be aesthetically pleasing, but it certainly works. It’s a viable technology,” said Dr. Edmundowicz, who was an investigator.
The studies were funded by companies developing the devices and techniques. Dr. Edmundowicz has stock options, or is a consultant or researcher, Aspire, Obalon, GI Dynamics, Elira, and other firms.
Caveat emptor, all over again
Although many of these innovations will eventually achieve acceptable clinical results in trials and be adopted by both gastroenterologists and surgeons, a note of caution is in order, argues Tyler G. Hughes, MD, FACS, is clinical professor in the department of surgery and director of medical education at the Kansas University School of Medicine, Salina Campus, coeditor of ACS Surgery News.
“The recent uptick in ‘non-surgical’ obesity treatment reverberates as history gets ready to repeat itself. If surgeons don’t take the lead, two things are likely to happen. First, ‘interventionists,’ ignorant of the lessons of yesterday will introduce foreign bodies into the soft GI tract only to see these ‘safe’ devices create surgical havoc or malabsorptive disasters. Second, instead of doing a complete spectrum of obesity procedures, surgeons will be left with the worst cases and fixing complications while the ‘interventionist’ (and I do not limit that to gastroenterologists) do the easy cases at prices three times the cost of the old way. Will this really benefit the patients?
Rather than permitting a race to the newest unproven device to satisfy public clamor, while conveniently making a tidy profit for anyone able to claim the title ‘interventionist,’ bariatric surgeons need to grab the endoscope with both hands and do the research and reporting for these new procedures. It starts with the need for every surgical resident to be proficient with the GI endoscopes and ends with surgeons seeing surgery in a wider view than through an incision,” Dr. Hughes said.
SAN DIEGO – Device companies are working hard to bring obesity management to the endoscopy suite.
The field is called endobariatrics, and its goal is to fill the gap between surgery and pharmacotherapy. Drugs and lifestyle counseling don’t work for all patients, but many patients resist having surgery. These devices are meant to fill the need for nonsurgical, “noninvasive” solutions to obesity management.
Endobariatrics has the potential to be a boon for both obese patients and gastroenterology practices. Surgeons, however, approach these innovations with caution, due in part to past experiences of rescuing patients with failed devices. “Some innovations get disseminated without adequate studies to demonstrate whether they are superior, or even equal in efficacy and safety to, well-established procedures for the same condition. Even when they have been conducted, negative studies showing no difference may end up being rejected by journals and not published,” noted Karen Deveney, MD, FACS, professor of surgery, vice-chair for education in the department of surgery at Oregon Health & Science University, Portland, and co-Editor of ACS Surgery News.
Several new investigational devices and approaches were showcased at the annual Digestive Disease Week; some “are beginning to approach the kind of results we see with surgical techniques,” said Steven Edmundowicz, MD, medical director of the University of Colorado Digestive Health Center, Aurora.
“We are seeing a tremendous amount of development in this space, but it’s early, and we have to be cautious,” he said. There have already been a few disappointments, including the EndoBarrier, a fluoropolymer liner anchored in the duodenal bulb and unfurled down the duodenum to block food absorption. A key U.S. trial was recently halted due to liver abscesses.
Dr. Edmundowicz reviewed the latest developments presented at DDW.Self-assembling magnets for dual-path enteral anastomoses
The goal of the GI Windows system is to create a partial jejunoileal, side to side bypass without surgery. A 28-mm magnet ring is introduced to the ileum by colonoscopy, and a second ring to the jejunum by endoscopy. The rings snap together and tissue caught between them dies from pressure necrosis, leaving patients with a jejunoileal communication. Once food reaches that point, it either diverts through the anastomosis or continues past it down the digestive track. The magnets pass after the anastomosis forms in a week or so.
In a 6-month feasibility study from the Czech Republic, 10 obese patients lost 28.3% of their excess weight without diet restrictions. Those with diabetes had a mean hemoglobin A1c drop of 1.8%, and normalization of fasting blood glucose levels. The procedure took just over an hour and a half after the first five cases.
“I am very excited about [this]; I really want to see where the data are going,” Dr. Edmundowicz said.
Duodenal mucosal resurfacing
The idea of the Revita System (Fractyl) is to ablate “diabetic mucosa” in the duodenum so that normal mucosa can replace it. Saline is injected endoscopically under a portion of the duodenal mucosa to lift it off the muscularis; once isolated, the mucosa is destroyed – some in the audience thought “cooked” was a better word – by exposure to a hot water balloon catheter threaded into the lumen.
Thirty-nine overweight or obese type 2 diabetics had a 1.2% improvement at 6 months from a baseline hemoglobin A1c of 9.6% in a series from Santiago, Chile. Weight loss was modest in the trial; the system is being developed for type 2 diabetics.
There is some histologic support for the notion of a diabetic mucosa with both structural and hormonal aberrations, but it’s unclear if it’s a sign or cause of sickness. Even so, “the mucosa regenerates” and won’t be diabetic “for a while” after the procedure, said investigator Manoel Galvao Neto, MD, of the Gastro Obeso Center, São Paulo.
Gastric balloons
Inflating a balloon in the stomach to make people feel full isn’t new, but the notion of putting the balloon into a capsule that patients can swallow and inflating it through a tether is a more recent notion.
The Obalon Therapeutics is one such device. In an unblinded, sham-controlled trial with 336 obese patients, subjects who got the 250-mL, nitrogen-filled Obalons – most received three – lost about 3% more of their total body weight at 24 weeks than those who did not. Although swallowed, Obalon is removed endoscopically. Meanwhile, 34 obese patients who swallowed the 550-mL, fluid-filled Elipse balloon (Allurion) had a total body weight loss of 9.5% and mean excess weight loss of 37.2% at 4 months, by which time Elipse deflates on its own and passes without endoscopic retrieval.
“This is a very promising approach. I am very excited about digested balloons,” said Dr. Edmundowicz, an investigator in the Obalon study.
Endoscopic sleeve gastroplasty
Endoscopic sleeve gastroplasty duplicates sleeve gastrectomy with stitches placed endoscopically to seal off the greater curvature of the stomach; functionally, patients are left with a narrow sleeve of a stomach. In a multicenter series presented at DDW, 242 patients had a mean total body weight loss of 19.8% at 18 months, with a low incidence of complications. “Weight loss appears to be continuing,” Dr. Edmundowicz said. Investigators used the Apollo OverStitch (Apollo Endosurgery) to place the sutures.
Aspiration therapy
With Food and Drug Administration approval on June 14, AspireAssist (Aspire Bariatrics) is probably the best known of the newer approaches. Patients drain a portion of their meals through an endoscopically placed percutaneous gastrostomy tube a half hour or so after eating. It takes 5-10 minutes. The agency is eager to keep it out of the hands of bulimics.
One-year data were reported at DDW; 111 obese AspireAssist subjects lost a mean of 37.2% of their excess weight versus 13% in 60 patients randomized to lifestyle counseling alone.
“It may not be aesthetically pleasing, but it certainly works. It’s a viable technology,” said Dr. Edmundowicz, who was an investigator.
The studies were funded by companies developing the devices and techniques. Dr. Edmundowicz has stock options, or is a consultant or researcher, Aspire, Obalon, GI Dynamics, Elira, and other firms.
Caveat emptor, all over again
Although many of these innovations will eventually achieve acceptable clinical results in trials and be adopted by both gastroenterologists and surgeons, a note of caution is in order, argues Tyler G. Hughes, MD, FACS, is clinical professor in the department of surgery and director of medical education at the Kansas University School of Medicine, Salina Campus, coeditor of ACS Surgery News.
“The recent uptick in ‘non-surgical’ obesity treatment reverberates as history gets ready to repeat itself. If surgeons don’t take the lead, two things are likely to happen. First, ‘interventionists,’ ignorant of the lessons of yesterday will introduce foreign bodies into the soft GI tract only to see these ‘safe’ devices create surgical havoc or malabsorptive disasters. Second, instead of doing a complete spectrum of obesity procedures, surgeons will be left with the worst cases and fixing complications while the ‘interventionist’ (and I do not limit that to gastroenterologists) do the easy cases at prices three times the cost of the old way. Will this really benefit the patients?
Rather than permitting a race to the newest unproven device to satisfy public clamor, while conveniently making a tidy profit for anyone able to claim the title ‘interventionist,’ bariatric surgeons need to grab the endoscope with both hands and do the research and reporting for these new procedures. It starts with the need for every surgical resident to be proficient with the GI endoscopes and ends with surgeons seeing surgery in a wider view than through an incision,” Dr. Hughes said.
SAN DIEGO – Device companies are working hard to bring obesity management to the endoscopy suite.
The field is called endobariatrics, and its goal is to fill the gap between surgery and pharmacotherapy. Drugs and lifestyle counseling don’t work for all patients, but many patients resist having surgery. These devices are meant to fill the need for nonsurgical, “noninvasive” solutions to obesity management.
Endobariatrics has the potential to be a boon for both obese patients and gastroenterology practices. Surgeons, however, approach these innovations with caution, due in part to past experiences of rescuing patients with failed devices. “Some innovations get disseminated without adequate studies to demonstrate whether they are superior, or even equal in efficacy and safety to, well-established procedures for the same condition. Even when they have been conducted, negative studies showing no difference may end up being rejected by journals and not published,” noted Karen Deveney, MD, FACS, professor of surgery, vice-chair for education in the department of surgery at Oregon Health & Science University, Portland, and co-Editor of ACS Surgery News.
Several new investigational devices and approaches were showcased at the annual Digestive Disease Week; some “are beginning to approach the kind of results we see with surgical techniques,” said Steven Edmundowicz, MD, medical director of the University of Colorado Digestive Health Center, Aurora.
“We are seeing a tremendous amount of development in this space, but it’s early, and we have to be cautious,” he said. There have already been a few disappointments, including the EndoBarrier, a fluoropolymer liner anchored in the duodenal bulb and unfurled down the duodenum to block food absorption. A key U.S. trial was recently halted due to liver abscesses.
Dr. Edmundowicz reviewed the latest developments presented at DDW.Self-assembling magnets for dual-path enteral anastomoses
The goal of the GI Windows system is to create a partial jejunoileal, side to side bypass without surgery. A 28-mm magnet ring is introduced to the ileum by colonoscopy, and a second ring to the jejunum by endoscopy. The rings snap together and tissue caught between them dies from pressure necrosis, leaving patients with a jejunoileal communication. Once food reaches that point, it either diverts through the anastomosis or continues past it down the digestive track. The magnets pass after the anastomosis forms in a week or so.
In a 6-month feasibility study from the Czech Republic, 10 obese patients lost 28.3% of their excess weight without diet restrictions. Those with diabetes had a mean hemoglobin A1c drop of 1.8%, and normalization of fasting blood glucose levels. The procedure took just over an hour and a half after the first five cases.
“I am very excited about [this]; I really want to see where the data are going,” Dr. Edmundowicz said.
Duodenal mucosal resurfacing
The idea of the Revita System (Fractyl) is to ablate “diabetic mucosa” in the duodenum so that normal mucosa can replace it. Saline is injected endoscopically under a portion of the duodenal mucosa to lift it off the muscularis; once isolated, the mucosa is destroyed – some in the audience thought “cooked” was a better word – by exposure to a hot water balloon catheter threaded into the lumen.
Thirty-nine overweight or obese type 2 diabetics had a 1.2% improvement at 6 months from a baseline hemoglobin A1c of 9.6% in a series from Santiago, Chile. Weight loss was modest in the trial; the system is being developed for type 2 diabetics.
There is some histologic support for the notion of a diabetic mucosa with both structural and hormonal aberrations, but it’s unclear if it’s a sign or cause of sickness. Even so, “the mucosa regenerates” and won’t be diabetic “for a while” after the procedure, said investigator Manoel Galvao Neto, MD, of the Gastro Obeso Center, São Paulo.
Gastric balloons
Inflating a balloon in the stomach to make people feel full isn’t new, but the notion of putting the balloon into a capsule that patients can swallow and inflating it through a tether is a more recent notion.
The Obalon Therapeutics is one such device. In an unblinded, sham-controlled trial with 336 obese patients, subjects who got the 250-mL, nitrogen-filled Obalons – most received three – lost about 3% more of their total body weight at 24 weeks than those who did not. Although swallowed, Obalon is removed endoscopically. Meanwhile, 34 obese patients who swallowed the 550-mL, fluid-filled Elipse balloon (Allurion) had a total body weight loss of 9.5% and mean excess weight loss of 37.2% at 4 months, by which time Elipse deflates on its own and passes without endoscopic retrieval.
“This is a very promising approach. I am very excited about digested balloons,” said Dr. Edmundowicz, an investigator in the Obalon study.
Endoscopic sleeve gastroplasty
Endoscopic sleeve gastroplasty duplicates sleeve gastrectomy with stitches placed endoscopically to seal off the greater curvature of the stomach; functionally, patients are left with a narrow sleeve of a stomach. In a multicenter series presented at DDW, 242 patients had a mean total body weight loss of 19.8% at 18 months, with a low incidence of complications. “Weight loss appears to be continuing,” Dr. Edmundowicz said. Investigators used the Apollo OverStitch (Apollo Endosurgery) to place the sutures.
Aspiration therapy
With Food and Drug Administration approval on June 14, AspireAssist (Aspire Bariatrics) is probably the best known of the newer approaches. Patients drain a portion of their meals through an endoscopically placed percutaneous gastrostomy tube a half hour or so after eating. It takes 5-10 minutes. The agency is eager to keep it out of the hands of bulimics.
One-year data were reported at DDW; 111 obese AspireAssist subjects lost a mean of 37.2% of their excess weight versus 13% in 60 patients randomized to lifestyle counseling alone.
“It may not be aesthetically pleasing, but it certainly works. It’s a viable technology,” said Dr. Edmundowicz, who was an investigator.
The studies were funded by companies developing the devices and techniques. Dr. Edmundowicz has stock options, or is a consultant or researcher, Aspire, Obalon, GI Dynamics, Elira, and other firms.
Caveat emptor, all over again
Although many of these innovations will eventually achieve acceptable clinical results in trials and be adopted by both gastroenterologists and surgeons, a note of caution is in order, argues Tyler G. Hughes, MD, FACS, is clinical professor in the department of surgery and director of medical education at the Kansas University School of Medicine, Salina Campus, coeditor of ACS Surgery News.
“The recent uptick in ‘non-surgical’ obesity treatment reverberates as history gets ready to repeat itself. If surgeons don’t take the lead, two things are likely to happen. First, ‘interventionists,’ ignorant of the lessons of yesterday will introduce foreign bodies into the soft GI tract only to see these ‘safe’ devices create surgical havoc or malabsorptive disasters. Second, instead of doing a complete spectrum of obesity procedures, surgeons will be left with the worst cases and fixing complications while the ‘interventionist’ (and I do not limit that to gastroenterologists) do the easy cases at prices three times the cost of the old way. Will this really benefit the patients?
Rather than permitting a race to the newest unproven device to satisfy public clamor, while conveniently making a tidy profit for anyone able to claim the title ‘interventionist,’ bariatric surgeons need to grab the endoscope with both hands and do the research and reporting for these new procedures. It starts with the need for every surgical resident to be proficient with the GI endoscopes and ends with surgeons seeing surgery in a wider view than through an incision,” Dr. Hughes said.
AT DDW 2016
Commentary: New treatments for new patients – A call to action for bariatric surgeons
How many common bile duct operations have you done lately? How about laparotomies for bleeding duodenal ulcers? How about central venous access catheters? How many procedures that you as a general surgeon may have done a few decades ago are now done primarily by endoscopists, radiologists, or others using minimal access for their procedures?
Now hold that thought, as we turn to the field of bariatric surgery.
Twenty years ago, in 1996, there were approximately 12,000 bariatric operations performed in the United States. From 1998 to 2004, that number increased to almost 136,000 per year (J Am Coll Surg. 2011;213:261-6). What was the reason? While other factors likely had some influence, the overwhelming factor was that laparoscopic bariatric surgery became available. Patients perceived this approach as less invasive. Primary care referring physicians did as well.
The rapid rise in laparoscopy boosted the popularity of bariatric surgery but since that bump, the bariatric surgery numbers have remained flat. Currently, less than 2% of eligible patients who are morbidly obese opt for surgical treatment each year – despite the fact that the safety of bariatric surgery has dramatically improved over the past 15 years. Currently the mortality rate for laparoscopic gastric bypass is at 0.15% (Ann Surg. 2014;259:123-30) and sleeve gastrectomy mortality is lower than that. Only appendectomy has a lower mortality rate among major abdominal operations. Despite the safety record, and despite over a decade of publications demonstrating the effectiveness of bariatric surgery in prolonging life, improving or eliminating comorbid medical problems of obesity, improving the quality of life of patients, and decreasing the cost of their medical care, there still has been no major new shift toward surgery by patients who would benefit from it.
What we are offering is not what these patients want. We as bariatric and metabolic surgeons must face the reality of that fact.
While endoscopic bariatric procedures are not new, their use to date has been limited to modifying existing operations, such as narrowing the anastomosis after gastric bypass for patients who are regaining weight. Such procedures have enjoyed at best mild to moderate short-term success, but poor long-term success.
The performance of a successful endoscopic sleeve gastrectomy, however, is a different issue. The sleeve gastrectomy has rapidly become the most popular bariatric operation. Its successful performance endoscopically (Endoscopy. 2015;47:164-6) should serve notice to bariatric surgeons that the time has come to learn to do endoscopic bariatric surgery. If effective, it almost certainly will be what patients seek in the future.
Societal stigmas, patient expectations, and our culture all drive the perception that obesity is a problem that individuals should be able to solve on their own. It is this firmly entrenched belief that is the foundation of the multi-billion dollar diet products industry. Yet the concept of having surgery to treat severe obesity is one that most severely obese patients do not easily embrace. A first-hand successful experience of a friend or relative is often needed for these individuals to consider a surgical procedure. While most patients with ultrasound-proven gallstones who are symptomatic will be referred to a surgeon by their primary care physician, how often is this true for the patient with a body mass index over 35?
The appeal of endoscopic procedures for patients and referring physicians is, of course, that these procedures are perceived as not really surgery. They are minimally invasive endoscopic procedures. The risk profile is very low. Why not consider it? patients may ask themselves. After all, you are not really having surgery.
Many of my bariatric colleagues will likely disagree with this recommendation to embrace endoscopic procedures. After all, the track record to date of many of these procedures and devices has not been impressive. All devices to date that have involved endoscopic treatment of obesity have either failed and been removed from the market, or are in their infancy still looking to establish efficacy (Clin Gastroenterol Hepatol. 2016;14:507-15). I have been vocally critical at symposia and national meetings of the concept of using an endoscopic suturing device to narrow the anastomosis of patients with a gastric bypass who are regaining weight. I have argued that the procedure is doomed to long-term minimal success or more likely flat-out failure. However, such a conservative approach that demands proof of long-term efficacy could predictably place us conservative curmudgeons on the sidelines of treating obesity when successful endoscopic procedures are available and become the sought-after option, just as laparoscopy became the sought-after option 17 years ago. How many surgeons offered primarily open bariatric operations after about 2005?
If we are to reach more than 1%-2% of the eligible patients who would benefit from treatment for morbid obesity and its related medical problems, then we need to take a different approach. We have about maximized how well we can do the surgical approach we now offer. It isn’t gaining in popularity among those who need it most. While we should not abandon the procedures that have been proven so effective, we should embrace new options for our patients.
It is certainly possible that the stigma of having surgery will resolve if a patient has an endoscopic procedure that is successful but only transiently. A more definitive operative procedure then may follow. Is that not ultimately better for that patient than having him or her never pursue a treatment for obesity? While this argument seems appropriate for the long-term overall patient good, the short-term increase in cost may make it a difficult sell to payers. But insurance companies are already doing their very best not to pay for highly medically effective and proven cost-effective bariatric surgery now. Only public pressure will force them to change – perhaps the public pressure of demand for endoscopic procedures.
Bariatric surgeons: It’s time to become bariatric endoscopists. If we want new patients, we need to adopt new treatments.
Dr. Schirmer is the Stephen H. Watts Professor of Surgery at the University of Virginia Health Sciences Center, Charlottesville.
How many common bile duct operations have you done lately? How about laparotomies for bleeding duodenal ulcers? How about central venous access catheters? How many procedures that you as a general surgeon may have done a few decades ago are now done primarily by endoscopists, radiologists, or others using minimal access for their procedures?
Now hold that thought, as we turn to the field of bariatric surgery.
Twenty years ago, in 1996, there were approximately 12,000 bariatric operations performed in the United States. From 1998 to 2004, that number increased to almost 136,000 per year (J Am Coll Surg. 2011;213:261-6). What was the reason? While other factors likely had some influence, the overwhelming factor was that laparoscopic bariatric surgery became available. Patients perceived this approach as less invasive. Primary care referring physicians did as well.
The rapid rise in laparoscopy boosted the popularity of bariatric surgery but since that bump, the bariatric surgery numbers have remained flat. Currently, less than 2% of eligible patients who are morbidly obese opt for surgical treatment each year – despite the fact that the safety of bariatric surgery has dramatically improved over the past 15 years. Currently the mortality rate for laparoscopic gastric bypass is at 0.15% (Ann Surg. 2014;259:123-30) and sleeve gastrectomy mortality is lower than that. Only appendectomy has a lower mortality rate among major abdominal operations. Despite the safety record, and despite over a decade of publications demonstrating the effectiveness of bariatric surgery in prolonging life, improving or eliminating comorbid medical problems of obesity, improving the quality of life of patients, and decreasing the cost of their medical care, there still has been no major new shift toward surgery by patients who would benefit from it.
What we are offering is not what these patients want. We as bariatric and metabolic surgeons must face the reality of that fact.
While endoscopic bariatric procedures are not new, their use to date has been limited to modifying existing operations, such as narrowing the anastomosis after gastric bypass for patients who are regaining weight. Such procedures have enjoyed at best mild to moderate short-term success, but poor long-term success.
The performance of a successful endoscopic sleeve gastrectomy, however, is a different issue. The sleeve gastrectomy has rapidly become the most popular bariatric operation. Its successful performance endoscopically (Endoscopy. 2015;47:164-6) should serve notice to bariatric surgeons that the time has come to learn to do endoscopic bariatric surgery. If effective, it almost certainly will be what patients seek in the future.
Societal stigmas, patient expectations, and our culture all drive the perception that obesity is a problem that individuals should be able to solve on their own. It is this firmly entrenched belief that is the foundation of the multi-billion dollar diet products industry. Yet the concept of having surgery to treat severe obesity is one that most severely obese patients do not easily embrace. A first-hand successful experience of a friend or relative is often needed for these individuals to consider a surgical procedure. While most patients with ultrasound-proven gallstones who are symptomatic will be referred to a surgeon by their primary care physician, how often is this true for the patient with a body mass index over 35?
The appeal of endoscopic procedures for patients and referring physicians is, of course, that these procedures are perceived as not really surgery. They are minimally invasive endoscopic procedures. The risk profile is very low. Why not consider it? patients may ask themselves. After all, you are not really having surgery.
Many of my bariatric colleagues will likely disagree with this recommendation to embrace endoscopic procedures. After all, the track record to date of many of these procedures and devices has not been impressive. All devices to date that have involved endoscopic treatment of obesity have either failed and been removed from the market, or are in their infancy still looking to establish efficacy (Clin Gastroenterol Hepatol. 2016;14:507-15). I have been vocally critical at symposia and national meetings of the concept of using an endoscopic suturing device to narrow the anastomosis of patients with a gastric bypass who are regaining weight. I have argued that the procedure is doomed to long-term minimal success or more likely flat-out failure. However, such a conservative approach that demands proof of long-term efficacy could predictably place us conservative curmudgeons on the sidelines of treating obesity when successful endoscopic procedures are available and become the sought-after option, just as laparoscopy became the sought-after option 17 years ago. How many surgeons offered primarily open bariatric operations after about 2005?
If we are to reach more than 1%-2% of the eligible patients who would benefit from treatment for morbid obesity and its related medical problems, then we need to take a different approach. We have about maximized how well we can do the surgical approach we now offer. It isn’t gaining in popularity among those who need it most. While we should not abandon the procedures that have been proven so effective, we should embrace new options for our patients.
It is certainly possible that the stigma of having surgery will resolve if a patient has an endoscopic procedure that is successful but only transiently. A more definitive operative procedure then may follow. Is that not ultimately better for that patient than having him or her never pursue a treatment for obesity? While this argument seems appropriate for the long-term overall patient good, the short-term increase in cost may make it a difficult sell to payers. But insurance companies are already doing their very best not to pay for highly medically effective and proven cost-effective bariatric surgery now. Only public pressure will force them to change – perhaps the public pressure of demand for endoscopic procedures.
Bariatric surgeons: It’s time to become bariatric endoscopists. If we want new patients, we need to adopt new treatments.
Dr. Schirmer is the Stephen H. Watts Professor of Surgery at the University of Virginia Health Sciences Center, Charlottesville.
How many common bile duct operations have you done lately? How about laparotomies for bleeding duodenal ulcers? How about central venous access catheters? How many procedures that you as a general surgeon may have done a few decades ago are now done primarily by endoscopists, radiologists, or others using minimal access for their procedures?
Now hold that thought, as we turn to the field of bariatric surgery.
Twenty years ago, in 1996, there were approximately 12,000 bariatric operations performed in the United States. From 1998 to 2004, that number increased to almost 136,000 per year (J Am Coll Surg. 2011;213:261-6). What was the reason? While other factors likely had some influence, the overwhelming factor was that laparoscopic bariatric surgery became available. Patients perceived this approach as less invasive. Primary care referring physicians did as well.
The rapid rise in laparoscopy boosted the popularity of bariatric surgery but since that bump, the bariatric surgery numbers have remained flat. Currently, less than 2% of eligible patients who are morbidly obese opt for surgical treatment each year – despite the fact that the safety of bariatric surgery has dramatically improved over the past 15 years. Currently the mortality rate for laparoscopic gastric bypass is at 0.15% (Ann Surg. 2014;259:123-30) and sleeve gastrectomy mortality is lower than that. Only appendectomy has a lower mortality rate among major abdominal operations. Despite the safety record, and despite over a decade of publications demonstrating the effectiveness of bariatric surgery in prolonging life, improving or eliminating comorbid medical problems of obesity, improving the quality of life of patients, and decreasing the cost of their medical care, there still has been no major new shift toward surgery by patients who would benefit from it.
What we are offering is not what these patients want. We as bariatric and metabolic surgeons must face the reality of that fact.
While endoscopic bariatric procedures are not new, their use to date has been limited to modifying existing operations, such as narrowing the anastomosis after gastric bypass for patients who are regaining weight. Such procedures have enjoyed at best mild to moderate short-term success, but poor long-term success.
The performance of a successful endoscopic sleeve gastrectomy, however, is a different issue. The sleeve gastrectomy has rapidly become the most popular bariatric operation. Its successful performance endoscopically (Endoscopy. 2015;47:164-6) should serve notice to bariatric surgeons that the time has come to learn to do endoscopic bariatric surgery. If effective, it almost certainly will be what patients seek in the future.
Societal stigmas, patient expectations, and our culture all drive the perception that obesity is a problem that individuals should be able to solve on their own. It is this firmly entrenched belief that is the foundation of the multi-billion dollar diet products industry. Yet the concept of having surgery to treat severe obesity is one that most severely obese patients do not easily embrace. A first-hand successful experience of a friend or relative is often needed for these individuals to consider a surgical procedure. While most patients with ultrasound-proven gallstones who are symptomatic will be referred to a surgeon by their primary care physician, how often is this true for the patient with a body mass index over 35?
The appeal of endoscopic procedures for patients and referring physicians is, of course, that these procedures are perceived as not really surgery. They are minimally invasive endoscopic procedures. The risk profile is very low. Why not consider it? patients may ask themselves. After all, you are not really having surgery.
Many of my bariatric colleagues will likely disagree with this recommendation to embrace endoscopic procedures. After all, the track record to date of many of these procedures and devices has not been impressive. All devices to date that have involved endoscopic treatment of obesity have either failed and been removed from the market, or are in their infancy still looking to establish efficacy (Clin Gastroenterol Hepatol. 2016;14:507-15). I have been vocally critical at symposia and national meetings of the concept of using an endoscopic suturing device to narrow the anastomosis of patients with a gastric bypass who are regaining weight. I have argued that the procedure is doomed to long-term minimal success or more likely flat-out failure. However, such a conservative approach that demands proof of long-term efficacy could predictably place us conservative curmudgeons on the sidelines of treating obesity when successful endoscopic procedures are available and become the sought-after option, just as laparoscopy became the sought-after option 17 years ago. How many surgeons offered primarily open bariatric operations after about 2005?
If we are to reach more than 1%-2% of the eligible patients who would benefit from treatment for morbid obesity and its related medical problems, then we need to take a different approach. We have about maximized how well we can do the surgical approach we now offer. It isn’t gaining in popularity among those who need it most. While we should not abandon the procedures that have been proven so effective, we should embrace new options for our patients.
It is certainly possible that the stigma of having surgery will resolve if a patient has an endoscopic procedure that is successful but only transiently. A more definitive operative procedure then may follow. Is that not ultimately better for that patient than having him or her never pursue a treatment for obesity? While this argument seems appropriate for the long-term overall patient good, the short-term increase in cost may make it a difficult sell to payers. But insurance companies are already doing their very best not to pay for highly medically effective and proven cost-effective bariatric surgery now. Only public pressure will force them to change – perhaps the public pressure of demand for endoscopic procedures.
Bariatric surgeons: It’s time to become bariatric endoscopists. If we want new patients, we need to adopt new treatments.
Dr. Schirmer is the Stephen H. Watts Professor of Surgery at the University of Virginia Health Sciences Center, Charlottesville.
The future of surgery
Predicting the future has been a favorite topic of surgeons through the ages for addresses to august surgical societies.
Confident predictions of the future of surgery, however, have not always stood the test of time. Speaking at the University of Manchester’s Centenary celebration in 1973 at an international symposium on “Medicine in the 21st Century,” the noted surgeon J. Englebert Dunphy correctly predicted the prominence of joint-replacement procedures, but incorrectly asserted that medical advances would virtually eliminate the need for cholecystectomy through dissolution of gallstones and the need for surgical approaches to atherosclerosis through plaque prevention and dissolution. He accurately predicted that infections and sepsis would remain serious problems. But he missed the mark when he predicted that surgical pain would be eliminated by a pill that would block somatic nerve impulses without any respiratory or circulatory effects (Surgery. 1974 Mar;75:332-7). Technologic advances such as laparoscopic cholecystectomy, which emerged less than 20 years into the future, were not on his radar screen.
Change and disruptive technology
To be sure, the surgical procedures and methods have changed markedly since the time I trained in surgery in the 1970s. The most obvious change is the shift from large incisions to small ones, with the commensurate quick recovery and short hospital stays. This change is primarily because of the emergence of disruptive technology, a concept that has pervaded every avenue of our current lives, not just surgery: Think Uber, robotics in industry, the Internet, smartphones, and the miniaturization of just about every communication means. In medicine, these disruptive technologies have led to the emergence of the electronic health record, new imaging modalities, percutaneous interventional techniques, fiber optic endoscopy, laparoscopy, and endovascular surgery.
Maintaining capacity to do infrequent operations
During my training, H2 receptor antagonists and Helicobacter pylori came on the scene, with the result that ulcer operations almost disappeared from our surgical armamentarium; one of my most frequent operations as a senior and chief resident has become a rarity 40 years later for our trainees. And yet, a general surgeon must know how to perform an ulcer operation and which type is the best for a given circumstance, since perforated and bleeding ulcers are still seen, if infrequently. The perforated ulcer is seen most often in patients with multiple comorbidities who can least tolerate a complication and need one effective and well-executed operation if they are going to survive. How do we continue to teach residents these procedures when they have become infrequent?
There is perhaps some utility in keeping some aging surgeons around to teach residents on fresh cadavers, or to call them out of their assisted living facilities when needed for consultation! Now that the majority of operations in most places are being performed by minimally invasive surgical (MIS) methods, we may not need MIS fellowships or training much longer for trainees to become proficient in these skills, because it is the open operations that are less frequent. Our chief residents are much less secure about performing an open cholecystectomy, of which they have performed perhaps 5, than they are about performing laparoscopic cholecystectomy, of which they have performed 105.
Technologies on the verge of disrupting
Mark Aeder, MD, FACS, recently asked an important question in the ACS Communities thread on the future of surgery: What will the future disruptive innovations be? Which areas of surgery will bloom next?
There are many game-changing emerging technologies that could well turn out to be future “disruptors” of surgery as we know it. Cancer surgery is on track to be transformed by the development of genomics and personalized medicine and immunotherapy for melanoma and lung cancer.
The areas most likely to remain in the surgical realm are trauma, infections, and inflammation. Though safer cars, seatbelt laws, and helmet laws for motorcyclists have already decreased motor vehicle accidents and injury severity, we have still not produced a cure for stupidity or bad luck. Traumatic injuries will always be with us, and surgeons well trained in trauma management will continue to be needed. Appendicitis, cholecystitis, and diverticulitis will continue to require operations, even though inroads have begun with the studies showing success of antibiotic treatment for appendicitis and diverticulitis.
Keep current, avoid bandwagons
The key lesson, not only for our young surgeons-in-training but also for our seasoned surgeons, is to keep learning, keep networking, and keep adopting new techniques as soon as they show true evidence of success.
The best way for surgeons to remain prepared for whatever the future will bring is to stay current with innovations coming on the scene but not jump on the bandwagon too early and adopt new fads without substantial evidence of their soundness. A few retrospective case series reporting success with a new operation is insufficient evidence to try it out on the unsuspecting public. Although the completion of well-designed randomized trials with adequate follow-up takes time, it is better to stick with well-established and evidence-based techniques than rush to embrace an inadequately vetted procedure that may end up harming a patient.
Not all innovative devices or procedures rise to the level of being so truly “experimental” that they would require an institutional review board, and this ought to prompt an extra measure of caution by surgeons. Some “innovations,” such as the endobariatric devices reported elsewhere in this issue, represent novel variations of previous procedures but are as yet unvalidated by long-term studies. Responsible adoption of these novel procedures requires a full disclosure to the patient that the procedure or device is novel, without long-term evidence of success, and may entail unknown risks. Don’t let your enthusiasm to be an early adopter overcome your scientific and historic obligation to prevent harm to the patient.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the co-Editor of ACS Surgery News.
Predicting the future has been a favorite topic of surgeons through the ages for addresses to august surgical societies.
Confident predictions of the future of surgery, however, have not always stood the test of time. Speaking at the University of Manchester’s Centenary celebration in 1973 at an international symposium on “Medicine in the 21st Century,” the noted surgeon J. Englebert Dunphy correctly predicted the prominence of joint-replacement procedures, but incorrectly asserted that medical advances would virtually eliminate the need for cholecystectomy through dissolution of gallstones and the need for surgical approaches to atherosclerosis through plaque prevention and dissolution. He accurately predicted that infections and sepsis would remain serious problems. But he missed the mark when he predicted that surgical pain would be eliminated by a pill that would block somatic nerve impulses without any respiratory or circulatory effects (Surgery. 1974 Mar;75:332-7). Technologic advances such as laparoscopic cholecystectomy, which emerged less than 20 years into the future, were not on his radar screen.
Change and disruptive technology
To be sure, the surgical procedures and methods have changed markedly since the time I trained in surgery in the 1970s. The most obvious change is the shift from large incisions to small ones, with the commensurate quick recovery and short hospital stays. This change is primarily because of the emergence of disruptive technology, a concept that has pervaded every avenue of our current lives, not just surgery: Think Uber, robotics in industry, the Internet, smartphones, and the miniaturization of just about every communication means. In medicine, these disruptive technologies have led to the emergence of the electronic health record, new imaging modalities, percutaneous interventional techniques, fiber optic endoscopy, laparoscopy, and endovascular surgery.
Maintaining capacity to do infrequent operations
During my training, H2 receptor antagonists and Helicobacter pylori came on the scene, with the result that ulcer operations almost disappeared from our surgical armamentarium; one of my most frequent operations as a senior and chief resident has become a rarity 40 years later for our trainees. And yet, a general surgeon must know how to perform an ulcer operation and which type is the best for a given circumstance, since perforated and bleeding ulcers are still seen, if infrequently. The perforated ulcer is seen most often in patients with multiple comorbidities who can least tolerate a complication and need one effective and well-executed operation if they are going to survive. How do we continue to teach residents these procedures when they have become infrequent?
There is perhaps some utility in keeping some aging surgeons around to teach residents on fresh cadavers, or to call them out of their assisted living facilities when needed for consultation! Now that the majority of operations in most places are being performed by minimally invasive surgical (MIS) methods, we may not need MIS fellowships or training much longer for trainees to become proficient in these skills, because it is the open operations that are less frequent. Our chief residents are much less secure about performing an open cholecystectomy, of which they have performed perhaps 5, than they are about performing laparoscopic cholecystectomy, of which they have performed 105.
Technologies on the verge of disrupting
Mark Aeder, MD, FACS, recently asked an important question in the ACS Communities thread on the future of surgery: What will the future disruptive innovations be? Which areas of surgery will bloom next?
There are many game-changing emerging technologies that could well turn out to be future “disruptors” of surgery as we know it. Cancer surgery is on track to be transformed by the development of genomics and personalized medicine and immunotherapy for melanoma and lung cancer.
The areas most likely to remain in the surgical realm are trauma, infections, and inflammation. Though safer cars, seatbelt laws, and helmet laws for motorcyclists have already decreased motor vehicle accidents and injury severity, we have still not produced a cure for stupidity or bad luck. Traumatic injuries will always be with us, and surgeons well trained in trauma management will continue to be needed. Appendicitis, cholecystitis, and diverticulitis will continue to require operations, even though inroads have begun with the studies showing success of antibiotic treatment for appendicitis and diverticulitis.
Keep current, avoid bandwagons
The key lesson, not only for our young surgeons-in-training but also for our seasoned surgeons, is to keep learning, keep networking, and keep adopting new techniques as soon as they show true evidence of success.
The best way for surgeons to remain prepared for whatever the future will bring is to stay current with innovations coming on the scene but not jump on the bandwagon too early and adopt new fads without substantial evidence of their soundness. A few retrospective case series reporting success with a new operation is insufficient evidence to try it out on the unsuspecting public. Although the completion of well-designed randomized trials with adequate follow-up takes time, it is better to stick with well-established and evidence-based techniques than rush to embrace an inadequately vetted procedure that may end up harming a patient.
Not all innovative devices or procedures rise to the level of being so truly “experimental” that they would require an institutional review board, and this ought to prompt an extra measure of caution by surgeons. Some “innovations,” such as the endobariatric devices reported elsewhere in this issue, represent novel variations of previous procedures but are as yet unvalidated by long-term studies. Responsible adoption of these novel procedures requires a full disclosure to the patient that the procedure or device is novel, without long-term evidence of success, and may entail unknown risks. Don’t let your enthusiasm to be an early adopter overcome your scientific and historic obligation to prevent harm to the patient.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the co-Editor of ACS Surgery News.
Predicting the future has been a favorite topic of surgeons through the ages for addresses to august surgical societies.
Confident predictions of the future of surgery, however, have not always stood the test of time. Speaking at the University of Manchester’s Centenary celebration in 1973 at an international symposium on “Medicine in the 21st Century,” the noted surgeon J. Englebert Dunphy correctly predicted the prominence of joint-replacement procedures, but incorrectly asserted that medical advances would virtually eliminate the need for cholecystectomy through dissolution of gallstones and the need for surgical approaches to atherosclerosis through plaque prevention and dissolution. He accurately predicted that infections and sepsis would remain serious problems. But he missed the mark when he predicted that surgical pain would be eliminated by a pill that would block somatic nerve impulses without any respiratory or circulatory effects (Surgery. 1974 Mar;75:332-7). Technologic advances such as laparoscopic cholecystectomy, which emerged less than 20 years into the future, were not on his radar screen.
Change and disruptive technology
To be sure, the surgical procedures and methods have changed markedly since the time I trained in surgery in the 1970s. The most obvious change is the shift from large incisions to small ones, with the commensurate quick recovery and short hospital stays. This change is primarily because of the emergence of disruptive technology, a concept that has pervaded every avenue of our current lives, not just surgery: Think Uber, robotics in industry, the Internet, smartphones, and the miniaturization of just about every communication means. In medicine, these disruptive technologies have led to the emergence of the electronic health record, new imaging modalities, percutaneous interventional techniques, fiber optic endoscopy, laparoscopy, and endovascular surgery.
Maintaining capacity to do infrequent operations
During my training, H2 receptor antagonists and Helicobacter pylori came on the scene, with the result that ulcer operations almost disappeared from our surgical armamentarium; one of my most frequent operations as a senior and chief resident has become a rarity 40 years later for our trainees. And yet, a general surgeon must know how to perform an ulcer operation and which type is the best for a given circumstance, since perforated and bleeding ulcers are still seen, if infrequently. The perforated ulcer is seen most often in patients with multiple comorbidities who can least tolerate a complication and need one effective and well-executed operation if they are going to survive. How do we continue to teach residents these procedures when they have become infrequent?
There is perhaps some utility in keeping some aging surgeons around to teach residents on fresh cadavers, or to call them out of their assisted living facilities when needed for consultation! Now that the majority of operations in most places are being performed by minimally invasive surgical (MIS) methods, we may not need MIS fellowships or training much longer for trainees to become proficient in these skills, because it is the open operations that are less frequent. Our chief residents are much less secure about performing an open cholecystectomy, of which they have performed perhaps 5, than they are about performing laparoscopic cholecystectomy, of which they have performed 105.
Technologies on the verge of disrupting
Mark Aeder, MD, FACS, recently asked an important question in the ACS Communities thread on the future of surgery: What will the future disruptive innovations be? Which areas of surgery will bloom next?
There are many game-changing emerging technologies that could well turn out to be future “disruptors” of surgery as we know it. Cancer surgery is on track to be transformed by the development of genomics and personalized medicine and immunotherapy for melanoma and lung cancer.
The areas most likely to remain in the surgical realm are trauma, infections, and inflammation. Though safer cars, seatbelt laws, and helmet laws for motorcyclists have already decreased motor vehicle accidents and injury severity, we have still not produced a cure for stupidity or bad luck. Traumatic injuries will always be with us, and surgeons well trained in trauma management will continue to be needed. Appendicitis, cholecystitis, and diverticulitis will continue to require operations, even though inroads have begun with the studies showing success of antibiotic treatment for appendicitis and diverticulitis.
Keep current, avoid bandwagons
The key lesson, not only for our young surgeons-in-training but also for our seasoned surgeons, is to keep learning, keep networking, and keep adopting new techniques as soon as they show true evidence of success.
The best way for surgeons to remain prepared for whatever the future will bring is to stay current with innovations coming on the scene but not jump on the bandwagon too early and adopt new fads without substantial evidence of their soundness. A few retrospective case series reporting success with a new operation is insufficient evidence to try it out on the unsuspecting public. Although the completion of well-designed randomized trials with adequate follow-up takes time, it is better to stick with well-established and evidence-based techniques than rush to embrace an inadequately vetted procedure that may end up harming a patient.
Not all innovative devices or procedures rise to the level of being so truly “experimental” that they would require an institutional review board, and this ought to prompt an extra measure of caution by surgeons. Some “innovations,” such as the endobariatric devices reported elsewhere in this issue, represent novel variations of previous procedures but are as yet unvalidated by long-term studies. Responsible adoption of these novel procedures requires a full disclosure to the patient that the procedure or device is novel, without long-term evidence of success, and may entail unknown risks. Don’t let your enthusiasm to be an early adopter overcome your scientific and historic obligation to prevent harm to the patient.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the co-Editor of ACS Surgery News.