User login
Perceived Benefits of a Research Fellowship for Dermatology Residency Applicants: Outcomes of a Faculty-Reported Survey
Dermatology residency positions continue to be highly coveted among applicants in the match. In 2019, dermatology proved to be the most competitive specialty, with 36.3% of US medical school seniors and independent applicants going unmatched.1 Prior to the transition to a pass/fail system, the mean US Medical Licensing Examination (USMLE) Step 1 score for matched applicants increased from 247 in 2014 to 251 in 2019. The growing number of scholarly activities reported by applicants has contributed to the competitiveness of the specialty. In 2018, the mean number of abstracts, presentations, and publications reported by matched applicants was 14.71, which was higher than other competitive specialties, including orthopedic surgery and otolaryngology (11.5 and 10.4, respectively). Dermatology applicants who did not match in 2018 reported a mean of 8.6 abstracts, presentations, and publications, which was on par with successful applicants in many other specialties.1 In 2011, Stratman and Ness2 found that publishing manuscripts and listing research experience were factors strongly associated with matching into dermatology for reapplicants. These trends in reported research have added pressure for applicants to increase their publications.
Given that many students do not choose a career in dermatology until later in medical school, some students choose to take a gap year between their third and fourth years of medical school to pursue a research fellowship (RF) and produce publications, in theory to increase the chances of matching in dermatology. A survey of dermatology applicants conducted by Costello et al3 in 2021 found that, of the students who completed a gap year (n=90; 31.25%), 78.7% (n=71) of them completed an RF, and those who completed RFs were more likely to match at top dermatology residency programs (P<.01). The authors also reported that there was no significant difference in overall match rates between gap-year and non–gap-year applicants.3 Another survey of 328 medical students found that the most common reason students take years off for research during medical school is to increase competitiveness for residency application.4 Although it is clear that students completing an RF often find success in the match, there are limited published data on how those involved in selecting dermatology residents view this additional year. We surveyed faculty members participating in the resident selection process to assess their viewpoints on how RFs factored into an applicant’s odds of matching into dermatology residency and performance as a resident.
Materials and Methods
An institutional review board application was submitted through the Geisinger Health System (Danville, Pennsylvania), and an exemption to complete the survey was granted. The survey consisted of 16 questions via REDCap electronic data capture and was sent to a listserve of dermatology program directors who were asked to distribute the survey to program chairs and faculty members within their department. Survey questions evaluated the participants’ involvement in medical student advising and the residency selection process. Questions relating to the respondents’ opinions were based on a 5-point Likert scale on level of agreement (1=strongly agree; 5=strongly disagree) or importance (1=a great deal; 5=not at all). All responses were collected anonymously. Data points were compiled and analyzed using REDCap. Statistical analysis via χ2 tests were conducted when appropriate.
Results
The survey was sent to 142 individuals and distributed to faculty members within those departments between August 16, 2019, and September 24, 2019. The survey elicited a total of 110 respondents. Demographic information is shown in eTable 1. Of these respondents, 35.5% were program directors, 23.6% were program chairs, 3.6% were both program director and program chair, and 37.3% were core faculty members. Although respondents’ roles were varied, 96.4% indicated that they were involved in both advising medical students and in selecting residents.
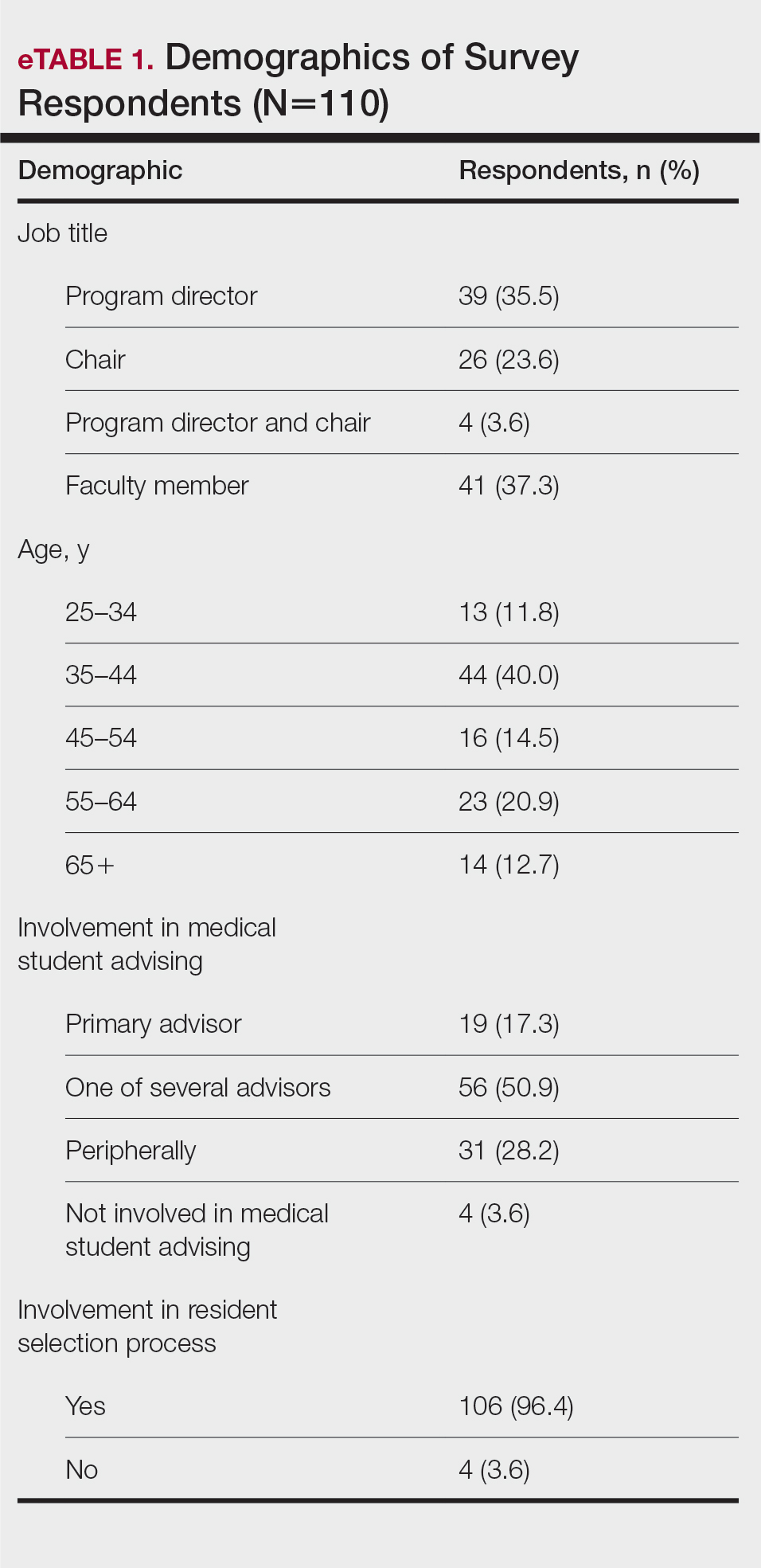
None of the respondents indicated that they always recommend that students complete an RF, and only 4.5% indicated that they usually recommend it; 40% of respondents rarely or never recommend an RF, while 55.5% sometimes recommend it. Although there was a variety of responses to how frequently faculty members recommend an RF, almost all respondents (98.2%) agreed that the reason medical students pursued an RF prior to residency application was to increase the competitiveness of their residency application. However, 20% of respondents believed that students in this cohort were seeking to gain a deeper understanding of the specialty, and 27.3% thought that this cohort had genuine interest in research. Interestingly, despite the medical students’ intentions of choosing an RF, most respondents (67.3%) agreed or strongly agreed that the publications produced by fellows make an impact on the dermatologic scientific community.
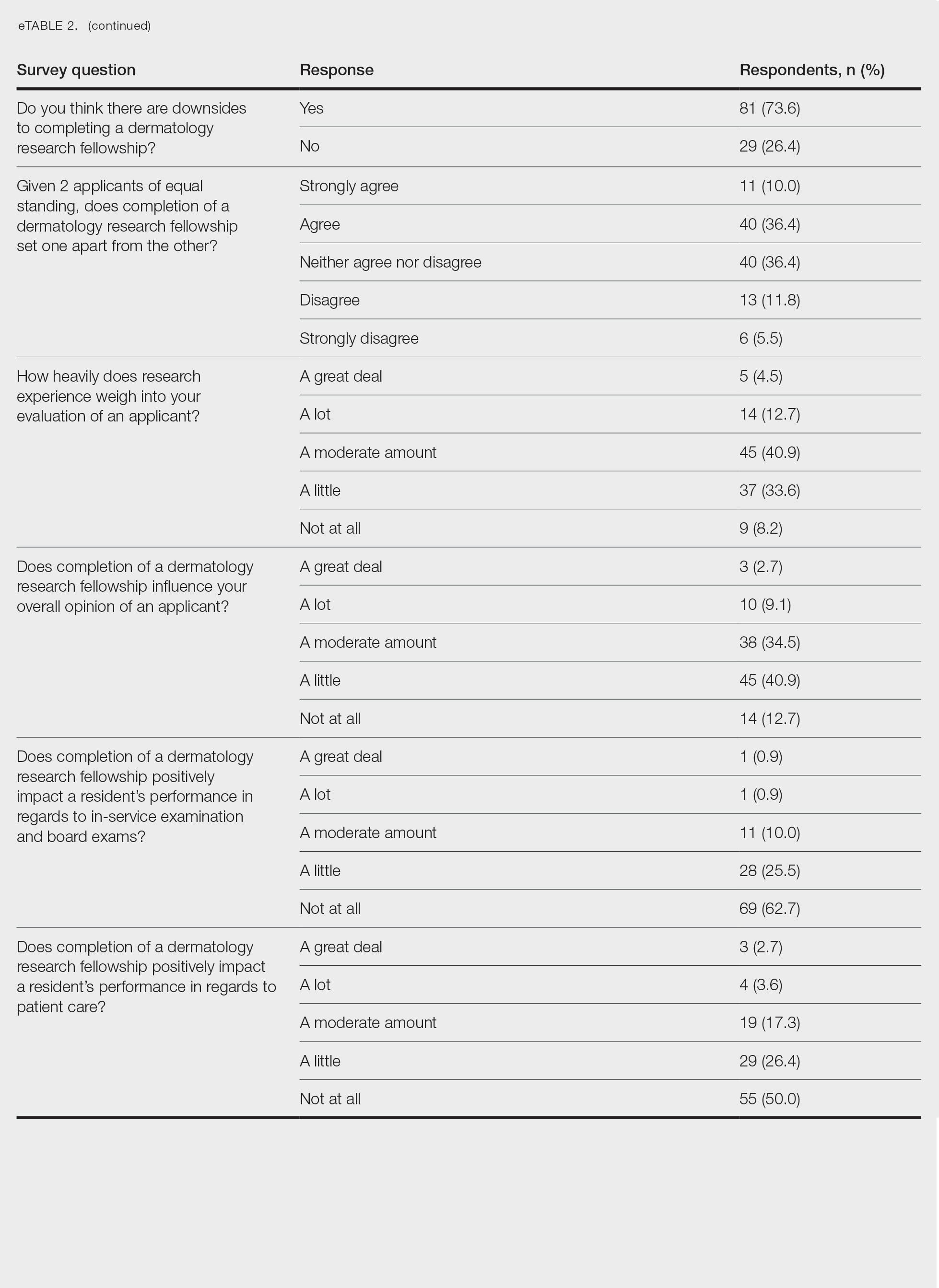
Although some respondents indicated that completion of an RF positively impacts resident performance with regard to patient care, most indicated that the impact was a little (26.4%) or not at all (50%). Additionally, a minority of respondents (11.8%) believed that RFs positively impact resident performance on in-service and board examinations at least a moderate amount, with 62.7% indicating no positive impact at all. Only 12.7% of participants agreed or strongly agreed that completion of an RF led to increased applicant involvement in research throughout their career, and most (73.6%) believed there were downsides to completing an RF. Finally, only 20% agreed or strongly agreed that students who completed an RF were more dedicated to the field of dermatology (eTable 2).
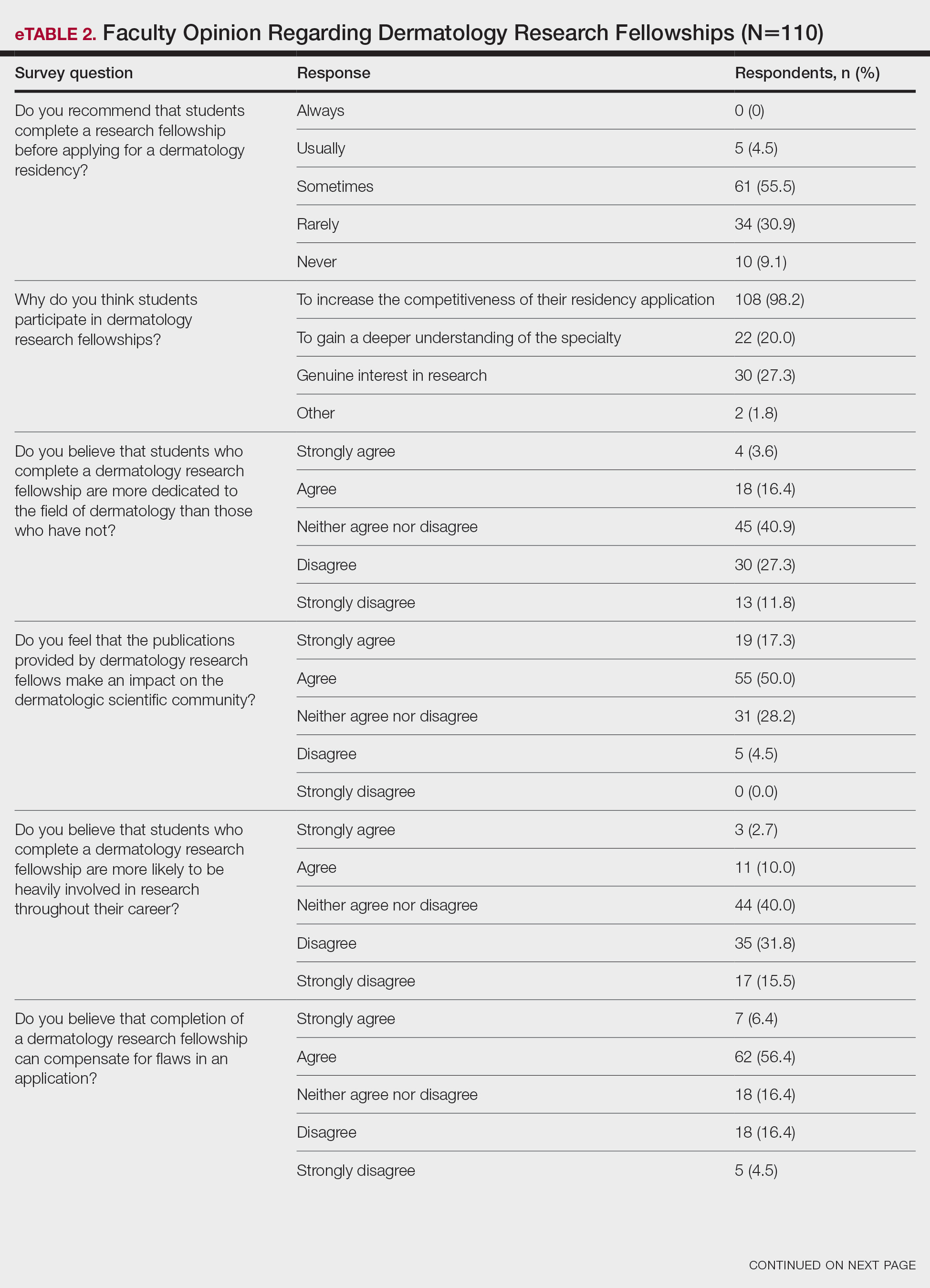
Further evaluation of the data indicated that the perceived utility of RFs did not affect respondents’ recommendation on whether to pursue an RF or not. For example, of the 4.5% of respondents who indicated that they always or usually recommended RFs, only 1 respondent believed that students who completed an RF were more dedicated to the field of dermatology than those who did not. Although 55.5% of respondents answered that they sometimes recommended completion of an RF, less than a quarter of this group believed that students who completed an RF were more likely to be heavily involved in research throughout their career (P=.99).
Overall, 11.8% of respondents indicated that completion of a dermatology RF influenced the evaluation of an applicant a great deal or a lot, while 53.6% of respondents indicated a little or no influence at all. Most respondents (62.8%) agreed or strongly agreed that completion of an RF can compensate for flaws in a residency application. Furthermore, when asked if completion of an RF could set 2 otherwise equivocal applicants apart from one another, 46.4% of respondents agreed or strongly agreed with the statement, while only 17.3% disagreed or strongly disagreed (eTable 2).
Comment
This study characterized how completion of an RF is viewed by those involved in advising medical students and selecting dermatology residents. The growing pressure for applicants to increase the number of publications combined with the competitiveness of applying for a dermatology residency position has led to increased participation in RFs. However, studies have found that students who completed an RF often did so despite a lack of interest.4 Nonetheless, little is known about how this is perceived by those involved in choosing residents.
We found that few respondents always or usually advised applicants to complete an RF, but the majority sometimes recommended them, demonstrating the complexity of this issue. Completion of an RF impacted 11.8% of respondents’ overall opinion of an applicant a lot or a great deal, while most respondents (53.6%) were influenced a little or not at all. However, 46.4% of respondents indicated that completion of a dermatology RF would set apart 2 applicants of otherwise equal standing, and 62.8% agreed or strongly agreed that completion of an RF would compensate for flaws in an application. These responses align with the findings of a study conducted by Kaffenberger et al,5 who surveyed members of the Association of Professors of Dermatology and found that 74.5% (73/98) of mentors almost always or sometimes recommended a research gap year for reasons that included low grades, low USMLE Step scores, and little research. These data suggest that completion of an RF can give a competitive advantage to applicants despite most advisors acknowledging that these applicants are not likely to be involved in research throughout their careers, perform better on standardized examinations, or provide better patient care.
Given the complexity of this issue, respondents may not have been able to accurately answer the question about how much an RF influenced their overall opinion of an applicant because of subconscious bias. Furthermore, respondents likely tailored their recommendations to complete an RF based on individual applicant strengths and weaknesses, and the specific reasons why one may recommend an RF need to be further investigated.
Although there may be other perceived advantages to RFs that were not captured by our survey, completion of a dermatology RF is not without disadvantages. Fellowships often are unfunded and offered in cities with high costs of living. Additionally, students are forced to delay graduation from medical school by a year at minimum and continue to accrue interest on medical school loans during this time. The financial burdens of completing an RF may exclude students of lower socioeconomic status and contribute to a decrease in diversity within the field. Dermatology has been found to be the second least diverse specialty, behind orthopedics.6 Soliman et al7 found that racial minorities and low-income students were more likely to cite socioeconomic barriers as factors involved in their decision not to pursue a career in dermatology. This notion was supported by Rinderknecht et al,8 who found that Black and Latinx dermatology applicants were more likely to come from disadvantaged backgrounds, and Black applicants were more likely to indicate financial concerns as their primary reason for not pursuing an RF. The impact of accumulated student debt and decreased access should be carefully weighed against the potential benefits of an RF. However, as the USMLE transitions their Step 1 score reporting from numerical to a pass/fail system, it also is possible that dermatology programs will place more emphasis on research productivity when evaluating applications for residency. Overall, the decision to recommend an RF represents an extremely complex topic, as indicated by the results of this study.
Limitations—Our survey-based study is limited by response rate and response bias. Despite the large number of responses, the overall response rate cannot be determined because it is unknown how many total faculty members actually received the survey. Moreover, data collected from current dermatology residents who have completed RFs vs those who have not as they pertain to resident performance and preparedness for the rigors of a dermatology residency would be useful.
- National Resident Matching Program. Results and Data: 2019 Main Residency Match. National Resident Matching Program; 2019. Accessed September 13, 2023. https://www.nrmp.org/wp-content/uploads/2021/07/NRMP-Results-and-Data-2019_04112019_final.pdf
- Stratman EJ, Ness RM. Factors associated with successful matching to dermatology residency programs by reapplicants and other applicants who previously graduated from medical school. Arch Dermatol. 2011;147:196-202.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role research gap-years play in a successful dermatology match. J Am Acad Dermatol. 2021;85:AB22.
- Pathipati AS, Taleghani N. Research in medical school: a survey evaluating why medical students take research years. Cureus. 2016;8:E741.
- Kaffenberger J, Lee B, Ahmed AM. How to advise medical students interested in dermatology: a survey of academic dermatology mentors. Cutis. 2023;111:124-127.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254.
- Rinderknecht FA, Brumfiel CM, Jefferson IS, et al. Differences in underrepresented in medicine applicant backgrounds and outcomes in the 2020-2021 dermatology residency match. Cutis. 2022;110:76-79.
Dermatology residency positions continue to be highly coveted among applicants in the match. In 2019, dermatology proved to be the most competitive specialty, with 36.3% of US medical school seniors and independent applicants going unmatched.1 Prior to the transition to a pass/fail system, the mean US Medical Licensing Examination (USMLE) Step 1 score for matched applicants increased from 247 in 2014 to 251 in 2019. The growing number of scholarly activities reported by applicants has contributed to the competitiveness of the specialty. In 2018, the mean number of abstracts, presentations, and publications reported by matched applicants was 14.71, which was higher than other competitive specialties, including orthopedic surgery and otolaryngology (11.5 and 10.4, respectively). Dermatology applicants who did not match in 2018 reported a mean of 8.6 abstracts, presentations, and publications, which was on par with successful applicants in many other specialties.1 In 2011, Stratman and Ness2 found that publishing manuscripts and listing research experience were factors strongly associated with matching into dermatology for reapplicants. These trends in reported research have added pressure for applicants to increase their publications.
Given that many students do not choose a career in dermatology until later in medical school, some students choose to take a gap year between their third and fourth years of medical school to pursue a research fellowship (RF) and produce publications, in theory to increase the chances of matching in dermatology. A survey of dermatology applicants conducted by Costello et al3 in 2021 found that, of the students who completed a gap year (n=90; 31.25%), 78.7% (n=71) of them completed an RF, and those who completed RFs were more likely to match at top dermatology residency programs (P<.01). The authors also reported that there was no significant difference in overall match rates between gap-year and non–gap-year applicants.3 Another survey of 328 medical students found that the most common reason students take years off for research during medical school is to increase competitiveness for residency application.4 Although it is clear that students completing an RF often find success in the match, there are limited published data on how those involved in selecting dermatology residents view this additional year. We surveyed faculty members participating in the resident selection process to assess their viewpoints on how RFs factored into an applicant’s odds of matching into dermatology residency and performance as a resident.
Materials and Methods
An institutional review board application was submitted through the Geisinger Health System (Danville, Pennsylvania), and an exemption to complete the survey was granted. The survey consisted of 16 questions via REDCap electronic data capture and was sent to a listserve of dermatology program directors who were asked to distribute the survey to program chairs and faculty members within their department. Survey questions evaluated the participants’ involvement in medical student advising and the residency selection process. Questions relating to the respondents’ opinions were based on a 5-point Likert scale on level of agreement (1=strongly agree; 5=strongly disagree) or importance (1=a great deal; 5=not at all). All responses were collected anonymously. Data points were compiled and analyzed using REDCap. Statistical analysis via χ2 tests were conducted when appropriate.
Results
The survey was sent to 142 individuals and distributed to faculty members within those departments between August 16, 2019, and September 24, 2019. The survey elicited a total of 110 respondents. Demographic information is shown in eTable 1. Of these respondents, 35.5% were program directors, 23.6% were program chairs, 3.6% were both program director and program chair, and 37.3% were core faculty members. Although respondents’ roles were varied, 96.4% indicated that they were involved in both advising medical students and in selecting residents.

None of the respondents indicated that they always recommend that students complete an RF, and only 4.5% indicated that they usually recommend it; 40% of respondents rarely or never recommend an RF, while 55.5% sometimes recommend it. Although there was a variety of responses to how frequently faculty members recommend an RF, almost all respondents (98.2%) agreed that the reason medical students pursued an RF prior to residency application was to increase the competitiveness of their residency application. However, 20% of respondents believed that students in this cohort were seeking to gain a deeper understanding of the specialty, and 27.3% thought that this cohort had genuine interest in research. Interestingly, despite the medical students’ intentions of choosing an RF, most respondents (67.3%) agreed or strongly agreed that the publications produced by fellows make an impact on the dermatologic scientific community.
Although some respondents indicated that completion of an RF positively impacts resident performance with regard to patient care, most indicated that the impact was a little (26.4%) or not at all (50%). Additionally, a minority of respondents (11.8%) believed that RFs positively impact resident performance on in-service and board examinations at least a moderate amount, with 62.7% indicating no positive impact at all. Only 12.7% of participants agreed or strongly agreed that completion of an RF led to increased applicant involvement in research throughout their career, and most (73.6%) believed there were downsides to completing an RF. Finally, only 20% agreed or strongly agreed that students who completed an RF were more dedicated to the field of dermatology (eTable 2).


Further evaluation of the data indicated that the perceived utility of RFs did not affect respondents’ recommendation on whether to pursue an RF or not. For example, of the 4.5% of respondents who indicated that they always or usually recommended RFs, only 1 respondent believed that students who completed an RF were more dedicated to the field of dermatology than those who did not. Although 55.5% of respondents answered that they sometimes recommended completion of an RF, less than a quarter of this group believed that students who completed an RF were more likely to be heavily involved in research throughout their career (P=.99).
Overall, 11.8% of respondents indicated that completion of a dermatology RF influenced the evaluation of an applicant a great deal or a lot, while 53.6% of respondents indicated a little or no influence at all. Most respondents (62.8%) agreed or strongly agreed that completion of an RF can compensate for flaws in a residency application. Furthermore, when asked if completion of an RF could set 2 otherwise equivocal applicants apart from one another, 46.4% of respondents agreed or strongly agreed with the statement, while only 17.3% disagreed or strongly disagreed (eTable 2).
Comment
This study characterized how completion of an RF is viewed by those involved in advising medical students and selecting dermatology residents. The growing pressure for applicants to increase the number of publications combined with the competitiveness of applying for a dermatology residency position has led to increased participation in RFs. However, studies have found that students who completed an RF often did so despite a lack of interest.4 Nonetheless, little is known about how this is perceived by those involved in choosing residents.
We found that few respondents always or usually advised applicants to complete an RF, but the majority sometimes recommended them, demonstrating the complexity of this issue. Completion of an RF impacted 11.8% of respondents’ overall opinion of an applicant a lot or a great deal, while most respondents (53.6%) were influenced a little or not at all. However, 46.4% of respondents indicated that completion of a dermatology RF would set apart 2 applicants of otherwise equal standing, and 62.8% agreed or strongly agreed that completion of an RF would compensate for flaws in an application. These responses align with the findings of a study conducted by Kaffenberger et al,5 who surveyed members of the Association of Professors of Dermatology and found that 74.5% (73/98) of mentors almost always or sometimes recommended a research gap year for reasons that included low grades, low USMLE Step scores, and little research. These data suggest that completion of an RF can give a competitive advantage to applicants despite most advisors acknowledging that these applicants are not likely to be involved in research throughout their careers, perform better on standardized examinations, or provide better patient care.
Given the complexity of this issue, respondents may not have been able to accurately answer the question about how much an RF influenced their overall opinion of an applicant because of subconscious bias. Furthermore, respondents likely tailored their recommendations to complete an RF based on individual applicant strengths and weaknesses, and the specific reasons why one may recommend an RF need to be further investigated.
Although there may be other perceived advantages to RFs that were not captured by our survey, completion of a dermatology RF is not without disadvantages. Fellowships often are unfunded and offered in cities with high costs of living. Additionally, students are forced to delay graduation from medical school by a year at minimum and continue to accrue interest on medical school loans during this time. The financial burdens of completing an RF may exclude students of lower socioeconomic status and contribute to a decrease in diversity within the field. Dermatology has been found to be the second least diverse specialty, behind orthopedics.6 Soliman et al7 found that racial minorities and low-income students were more likely to cite socioeconomic barriers as factors involved in their decision not to pursue a career in dermatology. This notion was supported by Rinderknecht et al,8 who found that Black and Latinx dermatology applicants were more likely to come from disadvantaged backgrounds, and Black applicants were more likely to indicate financial concerns as their primary reason for not pursuing an RF. The impact of accumulated student debt and decreased access should be carefully weighed against the potential benefits of an RF. However, as the USMLE transitions their Step 1 score reporting from numerical to a pass/fail system, it also is possible that dermatology programs will place more emphasis on research productivity when evaluating applications for residency. Overall, the decision to recommend an RF represents an extremely complex topic, as indicated by the results of this study.
Limitations—Our survey-based study is limited by response rate and response bias. Despite the large number of responses, the overall response rate cannot be determined because it is unknown how many total faculty members actually received the survey. Moreover, data collected from current dermatology residents who have completed RFs vs those who have not as they pertain to resident performance and preparedness for the rigors of a dermatology residency would be useful.
Dermatology residency positions continue to be highly coveted among applicants in the match. In 2019, dermatology proved to be the most competitive specialty, with 36.3% of US medical school seniors and independent applicants going unmatched.1 Prior to the transition to a pass/fail system, the mean US Medical Licensing Examination (USMLE) Step 1 score for matched applicants increased from 247 in 2014 to 251 in 2019. The growing number of scholarly activities reported by applicants has contributed to the competitiveness of the specialty. In 2018, the mean number of abstracts, presentations, and publications reported by matched applicants was 14.71, which was higher than other competitive specialties, including orthopedic surgery and otolaryngology (11.5 and 10.4, respectively). Dermatology applicants who did not match in 2018 reported a mean of 8.6 abstracts, presentations, and publications, which was on par with successful applicants in many other specialties.1 In 2011, Stratman and Ness2 found that publishing manuscripts and listing research experience were factors strongly associated with matching into dermatology for reapplicants. These trends in reported research have added pressure for applicants to increase their publications.
Given that many students do not choose a career in dermatology until later in medical school, some students choose to take a gap year between their third and fourth years of medical school to pursue a research fellowship (RF) and produce publications, in theory to increase the chances of matching in dermatology. A survey of dermatology applicants conducted by Costello et al3 in 2021 found that, of the students who completed a gap year (n=90; 31.25%), 78.7% (n=71) of them completed an RF, and those who completed RFs were more likely to match at top dermatology residency programs (P<.01). The authors also reported that there was no significant difference in overall match rates between gap-year and non–gap-year applicants.3 Another survey of 328 medical students found that the most common reason students take years off for research during medical school is to increase competitiveness for residency application.4 Although it is clear that students completing an RF often find success in the match, there are limited published data on how those involved in selecting dermatology residents view this additional year. We surveyed faculty members participating in the resident selection process to assess their viewpoints on how RFs factored into an applicant’s odds of matching into dermatology residency and performance as a resident.
Materials and Methods
An institutional review board application was submitted through the Geisinger Health System (Danville, Pennsylvania), and an exemption to complete the survey was granted. The survey consisted of 16 questions via REDCap electronic data capture and was sent to a listserve of dermatology program directors who were asked to distribute the survey to program chairs and faculty members within their department. Survey questions evaluated the participants’ involvement in medical student advising and the residency selection process. Questions relating to the respondents’ opinions were based on a 5-point Likert scale on level of agreement (1=strongly agree; 5=strongly disagree) or importance (1=a great deal; 5=not at all). All responses were collected anonymously. Data points were compiled and analyzed using REDCap. Statistical analysis via χ2 tests were conducted when appropriate.
Results
The survey was sent to 142 individuals and distributed to faculty members within those departments between August 16, 2019, and September 24, 2019. The survey elicited a total of 110 respondents. Demographic information is shown in eTable 1. Of these respondents, 35.5% were program directors, 23.6% were program chairs, 3.6% were both program director and program chair, and 37.3% were core faculty members. Although respondents’ roles were varied, 96.4% indicated that they were involved in both advising medical students and in selecting residents.

None of the respondents indicated that they always recommend that students complete an RF, and only 4.5% indicated that they usually recommend it; 40% of respondents rarely or never recommend an RF, while 55.5% sometimes recommend it. Although there was a variety of responses to how frequently faculty members recommend an RF, almost all respondents (98.2%) agreed that the reason medical students pursued an RF prior to residency application was to increase the competitiveness of their residency application. However, 20% of respondents believed that students in this cohort were seeking to gain a deeper understanding of the specialty, and 27.3% thought that this cohort had genuine interest in research. Interestingly, despite the medical students’ intentions of choosing an RF, most respondents (67.3%) agreed or strongly agreed that the publications produced by fellows make an impact on the dermatologic scientific community.
Although some respondents indicated that completion of an RF positively impacts resident performance with regard to patient care, most indicated that the impact was a little (26.4%) or not at all (50%). Additionally, a minority of respondents (11.8%) believed that RFs positively impact resident performance on in-service and board examinations at least a moderate amount, with 62.7% indicating no positive impact at all. Only 12.7% of participants agreed or strongly agreed that completion of an RF led to increased applicant involvement in research throughout their career, and most (73.6%) believed there were downsides to completing an RF. Finally, only 20% agreed or strongly agreed that students who completed an RF were more dedicated to the field of dermatology (eTable 2).


Further evaluation of the data indicated that the perceived utility of RFs did not affect respondents’ recommendation on whether to pursue an RF or not. For example, of the 4.5% of respondents who indicated that they always or usually recommended RFs, only 1 respondent believed that students who completed an RF were more dedicated to the field of dermatology than those who did not. Although 55.5% of respondents answered that they sometimes recommended completion of an RF, less than a quarter of this group believed that students who completed an RF were more likely to be heavily involved in research throughout their career (P=.99).
Overall, 11.8% of respondents indicated that completion of a dermatology RF influenced the evaluation of an applicant a great deal or a lot, while 53.6% of respondents indicated a little or no influence at all. Most respondents (62.8%) agreed or strongly agreed that completion of an RF can compensate for flaws in a residency application. Furthermore, when asked if completion of an RF could set 2 otherwise equivocal applicants apart from one another, 46.4% of respondents agreed or strongly agreed with the statement, while only 17.3% disagreed or strongly disagreed (eTable 2).
Comment
This study characterized how completion of an RF is viewed by those involved in advising medical students and selecting dermatology residents. The growing pressure for applicants to increase the number of publications combined with the competitiveness of applying for a dermatology residency position has led to increased participation in RFs. However, studies have found that students who completed an RF often did so despite a lack of interest.4 Nonetheless, little is known about how this is perceived by those involved in choosing residents.
We found that few respondents always or usually advised applicants to complete an RF, but the majority sometimes recommended them, demonstrating the complexity of this issue. Completion of an RF impacted 11.8% of respondents’ overall opinion of an applicant a lot or a great deal, while most respondents (53.6%) were influenced a little or not at all. However, 46.4% of respondents indicated that completion of a dermatology RF would set apart 2 applicants of otherwise equal standing, and 62.8% agreed or strongly agreed that completion of an RF would compensate for flaws in an application. These responses align with the findings of a study conducted by Kaffenberger et al,5 who surveyed members of the Association of Professors of Dermatology and found that 74.5% (73/98) of mentors almost always or sometimes recommended a research gap year for reasons that included low grades, low USMLE Step scores, and little research. These data suggest that completion of an RF can give a competitive advantage to applicants despite most advisors acknowledging that these applicants are not likely to be involved in research throughout their careers, perform better on standardized examinations, or provide better patient care.
Given the complexity of this issue, respondents may not have been able to accurately answer the question about how much an RF influenced their overall opinion of an applicant because of subconscious bias. Furthermore, respondents likely tailored their recommendations to complete an RF based on individual applicant strengths and weaknesses, and the specific reasons why one may recommend an RF need to be further investigated.
Although there may be other perceived advantages to RFs that were not captured by our survey, completion of a dermatology RF is not without disadvantages. Fellowships often are unfunded and offered in cities with high costs of living. Additionally, students are forced to delay graduation from medical school by a year at minimum and continue to accrue interest on medical school loans during this time. The financial burdens of completing an RF may exclude students of lower socioeconomic status and contribute to a decrease in diversity within the field. Dermatology has been found to be the second least diverse specialty, behind orthopedics.6 Soliman et al7 found that racial minorities and low-income students were more likely to cite socioeconomic barriers as factors involved in their decision not to pursue a career in dermatology. This notion was supported by Rinderknecht et al,8 who found that Black and Latinx dermatology applicants were more likely to come from disadvantaged backgrounds, and Black applicants were more likely to indicate financial concerns as their primary reason for not pursuing an RF. The impact of accumulated student debt and decreased access should be carefully weighed against the potential benefits of an RF. However, as the USMLE transitions their Step 1 score reporting from numerical to a pass/fail system, it also is possible that dermatology programs will place more emphasis on research productivity when evaluating applications for residency. Overall, the decision to recommend an RF represents an extremely complex topic, as indicated by the results of this study.
Limitations—Our survey-based study is limited by response rate and response bias. Despite the large number of responses, the overall response rate cannot be determined because it is unknown how many total faculty members actually received the survey. Moreover, data collected from current dermatology residents who have completed RFs vs those who have not as they pertain to resident performance and preparedness for the rigors of a dermatology residency would be useful.
- National Resident Matching Program. Results and Data: 2019 Main Residency Match. National Resident Matching Program; 2019. Accessed September 13, 2023. https://www.nrmp.org/wp-content/uploads/2021/07/NRMP-Results-and-Data-2019_04112019_final.pdf
- Stratman EJ, Ness RM. Factors associated with successful matching to dermatology residency programs by reapplicants and other applicants who previously graduated from medical school. Arch Dermatol. 2011;147:196-202.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role research gap-years play in a successful dermatology match. J Am Acad Dermatol. 2021;85:AB22.
- Pathipati AS, Taleghani N. Research in medical school: a survey evaluating why medical students take research years. Cureus. 2016;8:E741.
- Kaffenberger J, Lee B, Ahmed AM. How to advise medical students interested in dermatology: a survey of academic dermatology mentors. Cutis. 2023;111:124-127.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254.
- Rinderknecht FA, Brumfiel CM, Jefferson IS, et al. Differences in underrepresented in medicine applicant backgrounds and outcomes in the 2020-2021 dermatology residency match. Cutis. 2022;110:76-79.
- National Resident Matching Program. Results and Data: 2019 Main Residency Match. National Resident Matching Program; 2019. Accessed September 13, 2023. https://www.nrmp.org/wp-content/uploads/2021/07/NRMP-Results-and-Data-2019_04112019_final.pdf
- Stratman EJ, Ness RM. Factors associated with successful matching to dermatology residency programs by reapplicants and other applicants who previously graduated from medical school. Arch Dermatol. 2011;147:196-202.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role research gap-years play in a successful dermatology match. J Am Acad Dermatol. 2021;85:AB22.
- Pathipati AS, Taleghani N. Research in medical school: a survey evaluating why medical students take research years. Cureus. 2016;8:E741.
- Kaffenberger J, Lee B, Ahmed AM. How to advise medical students interested in dermatology: a survey of academic dermatology mentors. Cutis. 2023;111:124-127.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254.
- Rinderknecht FA, Brumfiel CM, Jefferson IS, et al. Differences in underrepresented in medicine applicant backgrounds and outcomes in the 2020-2021 dermatology residency match. Cutis. 2022;110:76-79.
PRACTICE POINTS
- Many medical students seeking to match into a dermatology residency program complete a research fellowship (RF).
- Completion of an RF can give a competitive advantage to applicants even though most advisors acknowledge that these applicants are not likely to be involved in research throughout their career, perform better on standardized examinations, or provide better patient care.
- The decision to recommend an RF represents an extremely complex topic and should be tailored to each individual applicant.
Assessment of the Efficacy of Tranexamic Acid Solution 5% in the Treatment of Melasma in Patients of South Asian Descent
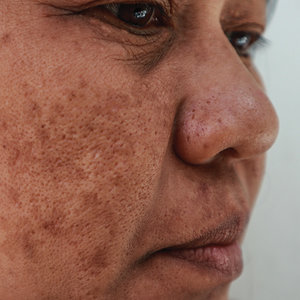
Melasma is a complex, long-lasting, acquired dermatologic pigmentation disorder resulting in grey-brown patches that last for more than 3 months. Sun-exposed areas including the nose, cheeks, forehead, and forearms are most likely to be affected.1 In Southeast Asia, 0.25% to 4% of the population affected by melasma is aged 30 to 40 years.2 In particular, melasma is a concern among pregnant women due to increased levels of melanocyte-stimulating hormones (MSHs) and is impacted by genetics, hormonal influence, and exposure to UV light.3,4 In Pakistan, approximately 46% of women are affected by melasma during pregnancy.2,5 Although few studies have focused on the clinical approaches to melasma in darker skin types, it continues to disproportionately affect the skin of color population.4
The areas of hyperpigmentation seen in melasma exhibit increased deposition of melanin in the epidermis and dermis, but melanocytes are not elevated. However, in areas of hyperpigmentation, the melanocytes are larger and more dendritic and demonstrate an increased level of melanogenesis.6 During pregnancy, especially in the third trimester, elevated levels of estrogen, progesterone, and MSH often are found in association with melasma.7 Tyrosinase (TYR) activity increases and cellular proliferation is reduced after treatment of melanocytes in culture with β-estradiol.8 Sex steroids increase transcription of genes encoding melanogenic enzymes in normal human melanocytes, especially TYR.9 These results are consistent with the notable increases in melanin synthesis and TYR activity reported for normal human melanocytes under similar conditions in culture.10 Because melanocytes contain both cytosolic and nuclear estrogen receptors, melanocytes in patients with melasma may be inherently more sensitive to the stimulatory effects of estrogens and possibly other steroid hormones.11
The current treatment options for melasma have varying levels of success and include topical depigmenting agents such as hydroquinone, tretinoin, azelaic acid, kojic acid, and corticosteroids; dermabrasion; and chemical peels.12-14 Chemical peels with glycolic acid, salicylic acid, lactic acid, trichloroacetic acid, and phenol, as well as laser therapy, are reliable management options.13,14 Traditionally, melasma has been treated with a combination of modalities along with photoprotection and trigger avoidance.12
The efficacy and safety of the available therapies for melasma are still controversial and require further exploration. In recent years, off-label tranexamic acid (TA) has emerged as a potential therapy for melasma. Although the mechanism of action remains unclear, TA may inhibit melanin synthesis by blocking the interaction between melanocytes and keratinocytes.15 Tranexamic acid also may reverse the abnormal dermal changes associated with melasma by inhibiting melanogenesis and angiogenesis.16
Although various therapeutic options exist for melasma, the search for a reliable option in patients with darker skin types continues.13 We sought to evaluate the efficacy of TA solution 5% in reducing the severity of melasma in South Asian patients, thereby improving patient outcomes and maximizing patient satisfaction. Topical TA is inexpensive and readily accessible and does not cause systemic side effects. These qualities make it a promising treatment compared to traditional therapies.
Methods
We conducted a randomized controlled trial at Rawalpindi Medical Institute (Punjab, Pakistan). The researchers obtained informed consent for all enrolled patients. Cases were sampled from the original patient population seen at the office using nonprobability consecutive sampling. The sample size was calculated with a 95% CI, margin of error of 9%, and expected percentage of efficacy of 86.1% by using TA solution 5%. South Asian male and female patients aged 20 to 45 years with melasma were included in the analysis. Patients were excluded if they were already taking TA, oral contraceptive pills, or photosensitizing drugs (eg, nonsteroidal anti-inflammatory drugs, tetracyclines, phenytoin, carbamazepine); were pregnant; had chronic kidney disease (creatinine >2.0 mg/dL); had cardiac abnormalities (abnormal electrocardiogram); had hematologic disorders (international normalized ratio >2); or had received another melasma treatment within the last 3 to 6 months.
All enrolled patients underwent a detailed history and physical examination. Patient demographics were subsequently noted, including age, sex, history of diabetes mellitus or hypertension, and duration of melasma. The melasma area and severity index (MASI) score of each patient was calculated at baseline, and a corresponding photograph was taken.
The topical solution was prepared with 5 g of TA dissolved in 10 cc of ethanol at 96 °F, 10 cc of 1,3-butanediol, and distilled water up to 100 cc. The TA solution was applied to the affected areas once daily by the patient for 12 weeks. Each application covered the affected areas completely. Patients were instructed to apply sunscreen with sun protection factor 60 to those same areas for UV protection after 15 minutes of TA application. Biweekly follow-ups were scheduled during the trial, and the MASI score was recorded at these visits. If the mean MASI score was reduced by half after 12 weeks of treatment, then the treatment was considered efficacious with a 95% CI.
The percentage reduction from baseline was calculated as follows: percentage reduction=(baseline score– follow-up score)/baseline score×100.
Statistical Analysis—Data were analyzed in SPSS Statistics 25 (IBM). The quantitative variables of age, duration of melasma, and body mass index were presented as mean (SD). Qualitative variables such as sex, history of diabetes mellitus or hypertension, site of melasma, and efficacy were presented as frequencies and percentages. Mean MASI scores at baseline and 12 weeks posttreatment were compared using a paired t test (P≤.05). Data were stratified for age, sex, history of diabetes mellitus or hypertension, site of melasma, and duration of melasma, and a χ2 test was applied to compare efficacy in stratified groups (P≤.05).
Results
Sixty patients were enrolled in the study. Of them, 17 (28.33%) were male, and 43 (71.67%) were female (2:5 ratio). They ranged in age from 20 to 45 years (mean [SD], 31.93 [6.26] years). Thirty-seven patients (61.67%) were aged 31 to 45 years of age (Table 1). The mean (SD) duration of disease was 10.18 (2.10) months. The response to TA was recorded based on patient distribution according to the site of melasma as well as history of diabetes mellitus and hypertension.

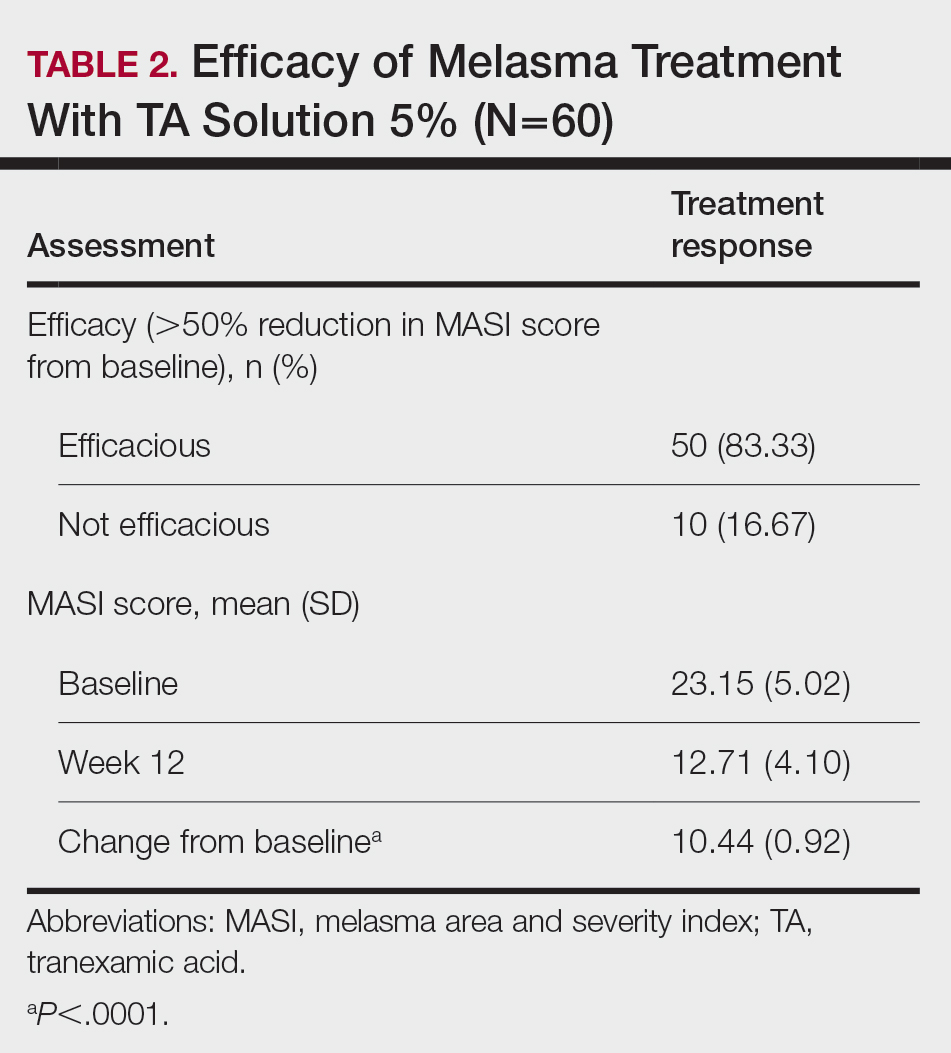
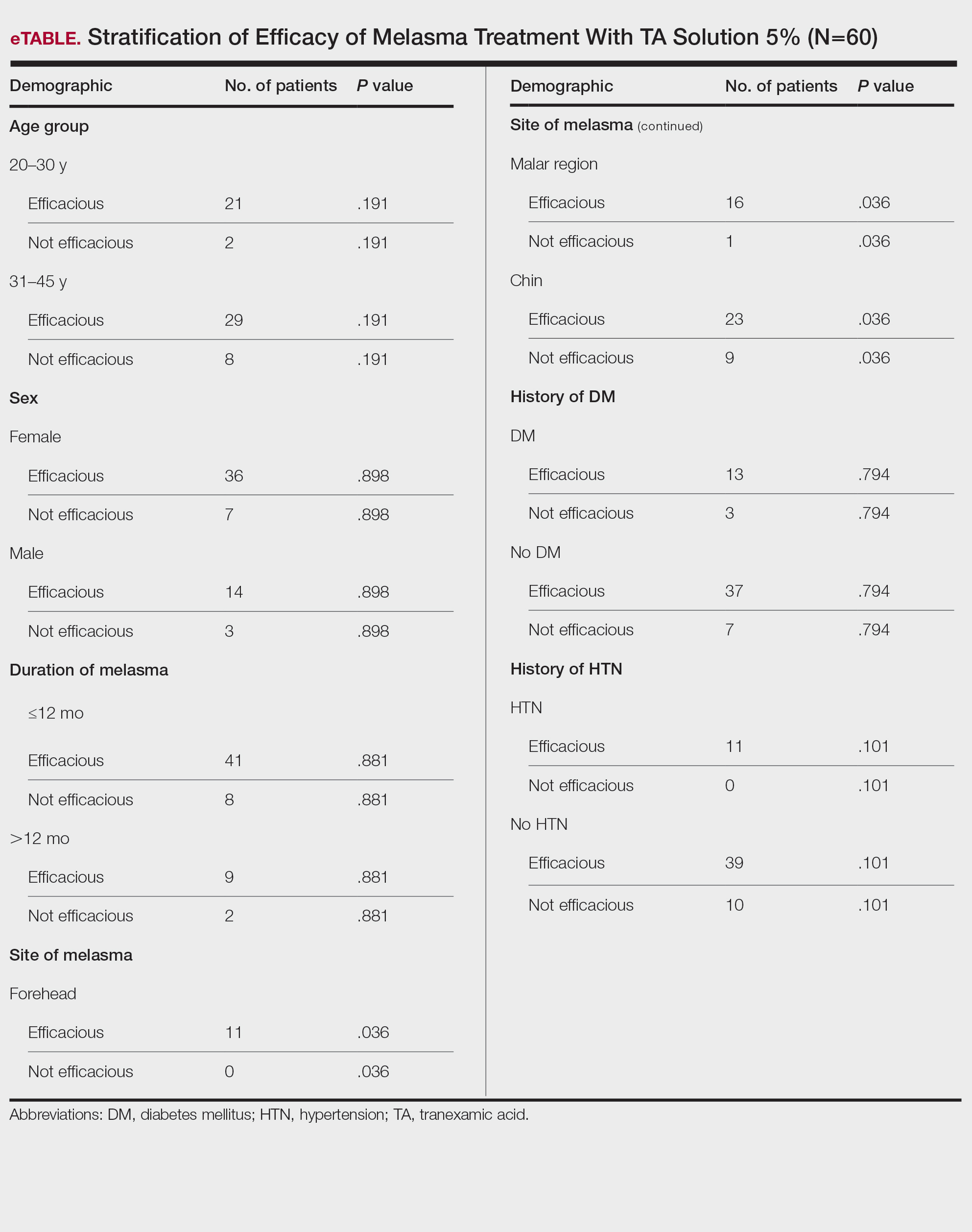
Topical TA was found to be efficacious for melasma in 50 (83.33%) patients. The mean (SD) baseline and week 12 MASI scores were 23.15 (5.02) and 12.71 (4.10)(P<.0001), respectively (Table 2). The stratification of efficacy with respect to age, sex, duration of melasma, site of melasma, and history of diabetes mellitus or hypertension is shown in the eTable. The site of melasma was significant with respect to stratification of efficacy. On the forehead, TA was found to be efficacious in 11 patients and nonefficacious in 0 patients (P=.036). In the malar region, it was efficacious in 16 patients and nonefficacious in 1 patient (P=.036). Finally, on the chin, it was efficacious in 23 patients and nonefficacious in 9 patients (P=.036).
Comment
Melasma Presentation and Development—Melasma is a chronic skin condition that more often affects patients with darker skin types. This condition is characterized by hyperpigmentation of skin that is directly exposed to the sun, such as the cheek, nose, forehead, and above the upper lip.17 Although the mechanism behind how melasma develops is unknown, one theory suggests that UV light can lead to increased plasmin in keratinocytes.18 This increased plasmin will thereby increase the arachidonic acid and α-MSH, leading to the observed uneven hyperpigmentation that is notable in melasma. Melasma is common in patients using oral contraceptives or expired cosmetic drugs; in those who are pregnant; and in those with liver dysfunction.18 Melasma has a negative impact on patients’ quality of life because of substantial psychological and social distress. Thus, finding an accessible treatment is imperative.19
Melasma Management—The most common treatments for melasma have been topical bleaching agents and photoprotection. Combination therapy options include chemical peels, dermabrasion, and laser treatments, though they present with limited efficacy.17,20 Because melasma focuses on pigmentation correction, topical treatments work to disturb melanocyte pigment production at the enzymatic level.21 Tyrosinase is rate limiting in melanin production, as it converts L-tyrosinase to L-3,4-dihydroxyphenylalanine, using copper to interact with L-3,4-dihydroxyphenylalanine as a cofactor in the active site.22 Therefore, tyrosine is a major target for many drugs that have been developed for melasma to decrease melaninization.21
Recently, research has focused on the effects of topical, intradermal, and oral TA for melasma.17 Tranexamic acid most commonly has been used in medicine as a fibrinolytic agent because of its antiplasmin properties. It has been hypothesized that TA can inhibit the release of paracrine melanogenic factors that normally act to stimulate melanocytes.17 Although studies have supported the safety and efficacy of TA, there remains a lack of clinical studies that are sufficiently powered. No definitive consensus on the use of TA for melasma currently exists, which indicates the need for large-scale, randomized, controlled trials.23
One trial (N=25) found that TA solution 5% achieved efficacy (>50% reduction in MASI score from baseline) in 86.1% of patients with melasma.24 In another study (N=18), topical TA 5% achieved efficacy (>50% reduction in MASI score) in 86% of patients with melasma.25
Melasma Comorbidities—To determine if certain comorbidities, such as diabetes mellitus or hypertension, influenced the progression of melasma, we stratified the efficacy results for patients with these 2 comorbidities, which showed no significant difference (P=.794 and P=.101, respectively). Thus, the relatively higher prevalence of diabetes mellitus (16 patients) and hypertension (11 patients) did not contribute to the efficacy of TA in lowering MASI scores over the 12-week period, which supports the findings of Doolan and Gupta,26 who investigated the endocrinologic conditions associated with melasma and found no such association with diabetes mellitus or hypertension.
TA Formulations for Melasma—The efficacy of topical TA has been explored in several studies. Six studies with sample sizes of 13 to 50 patients each showed statistically significant differences in MASI scores between baseline and following TA treatment (P<.001).27-32 Several formulations and regimens were utilized, including TA cream 3% for 12 weeks, TA gel 5% for 12 weeks, TA solution 3% for 12 weeks, TA liposome 5% for 12 weeks, and TA solution 2% for 12 weeks.18 Additionally, these studies found TA to be effective in limiting dyschromia and decreasing MASI scores. There were no statistically significant differences between formulations and method of application. Topical TA has been found to be just as effective as other treatments for melasma, including intradermal TA injections, topical hydroquinone, and a combination of topical hydroquinone and dexamethasone.18
Further study of the efficacy of intradermal TA is necessary because many human trials have lacked statistical significance or a control group. Lee et al32 conducted a trial of 100 female patients who received weekly intradermal TA microinjections for 12 weeks. After 8 and 12 weeks, MASI scores decreased significantly (P<.01).32 Similarly, Badran et al33 observed 60 female patients in 3 trial groups: group A received TA (4 mg/mL) intradermal injections every 2 weeks, group B received TA (10 mg/mL) intradermal injections every 2 weeks, and group C received TA cream 10% twice daily. Although all groups showed improvement in MASI, group B, which had the highest intradermal TA concentration, exhibited the most improvement. Thus, it was determined that intradermal application led to better results, but the cream was still effective.33
Saki et al34 conducted a randomized, split-face trial of 37 patients comparing the efficacy of intradermal TA and topical hydroquinone. Each group was treated with either monthly intradermal TA injections or nightly hydroquinone for 3 months. After 4 weeks of treatment, TA initially had a greater improvement. However, after 20 weeks, the overall changes were not significant between the 2 groups.34 Pazyar et al35 conducted a randomized, split-face trial of 49 patients comparing the efficacy of intradermal TA and hydroquinone cream. After 24 weeks of biweekly TA injections or twice-daily hydroquinone, there were no statistically significant differences in the decreased MASI scores between treatments.35 Additional large, double-blind, controlled trials are needed to thoroughly assess the role of intradermal TA in comparison to its treatment counterpart of hydroquinone.
Ebrahimi and Naeini29 conducted a 12-week, double-blind, split-phase trial of 50 Iranian melasma patients, which showed that 27.3% of patients rated the improvement in melasma as excellent, 42.4% as good, and 30.3% as fair after using TA solution 3%. Wu et al36 also showed a total melasma improvement rate of 80.9% in 256 patients with long-term oral use of TA. In a study by Kim et al31 (N=245), the mean MASI score considerably decreased after topical TA use, with a total response rate of 95.6%. In another study, Atefi et al37 presented significantly increased levels of satisfaction in patients treated with topical TA 5% vs hydroquinone (P=.015).
Melasma in Patients With Darker Skin Types—Special attention must be given to choosing the appropriate medication in melasma patients with darker skin types, as there is an increased risk for postinflammatory hyperpigmentation. Currently, few randomized controlled trials exist that fulfill the criteria of evaluating pharmacologic options for patients with melasma, and even fewer studies solely focus on patients with darker skin types.38 In addition to treatment advances, patients must be educated on the need to avoid sun exposure when possible or to use photoprotection, especially in the South Asian region, where these practices rarely are taught. Our study provided a unique analysis regarding the efficacy of TA solution 5% for the treatment of melasma in patients of South Asian descent. Clinicians can use these findings as a foundation for treating all patients with melasma but particularly those with darker skin types.
Study Limitations—Our study consisted of 60 patients; although our study had more patients than similar trials, larger studies are needed. Additionally, other variables were excluded from our analysis, such as comorbidities beyond diabetes mellitus and hypertension.
Conclusion
This study contributes to the growing field of melasma therapeutics by evaluating the efficacy of using TA solution 5% for the treatment of melasma in South Asian patients with darker skin types. Clinicians may use our study to broaden their treatment options for a common condition while also addressing the lack of clinical options for patients with darker skin types. Further studies investigating the effectiveness of TA in large clinical trials in humans are warranted to understand the efficacy and the risk for any complications.
- Espósito ACC, Brianezi G, De Souza NP, et al. Exploratory study of epidermis, basement membrane zone, upper dermis alterations and Wnt pathway activation in melasma compared to adjacent and retroauricular skin. Ann Dermatol. 2020;32:101-108.
- Janney MS, Subramaniyan R, Dabas R, et al. A randomized controlled study comparing the efficacy of topical 5% tranexamic acid solution versus 3% hydroquinone cream in melasma. J Cutan Aesthet Surg. 2019;12:63-67.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasmaJ Cosmet Laser Ther. 2018;20:134-139.
- Grimes PE, Ijaz S, Nashawati R, et al. New oral and topical approaches for the treatment of melasma. Int J Womens Dermatol. 2019;5:30-36.
- Handel AC, Miot LDB, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Barankin B, Silver SG, Carruthers A. The skin in pregnancy. J Cutan Med Surg. 2002;6:236-240.
- Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
- Smith AG, Shuster S, Thody AJ, et al. Chloasma, oral contraceptives, and plasma immunoreactive beta-melanocyte-stimulating hormone. J Invest Dermatol. 1977;68:169-170.
- Ranson M, Posen S, Mason RS. Human melanocytes as a target tissue for hormones: in vitro studies with 1 alpha-25, dihydroxyvitamin D3, alpha-melanocyte stimulating hormone, and beta-estradiol. J Invest Dermatol. 1988;91:593-598.
- Kippenberger S, Loitsch S, Solano F, et al. Quantification of tyrosinase, TRP-1, and Trp-2 transcripts in human melanocytes by reverse transcriptase-competitive multiplex PCR—regulation by steroid hormones. J Invest Dermatol. 1998;110:364-367.
- McLeod SD, Ranson M, Mason RS. Effects of estrogens on human melanocytes in vitro. J Steroid Biochem Mol Biol. 1994;49:9-14.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasma. J Cosmet Laser Ther. 2018;20:134-139.
- Sheu SL. Treatment of melasma using tranexamic acid: what’s known and what’s next. Cutis. 2018;101:E7-E8.
- Tian B. The Asian problem of frequent laser toning for melasma. J Clin Aesthet Dermatol. 2017;10:40-42.
- Zhang L, Tan WQ, Fang QQ, et al. Tranexamic acid for adults with melasma: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:1683414.
- Zhu JW, Ni YJ, Tong XY, et al. Tranexamic acid inhibits angiogenesis and melanogenesis in vitro by targeting VEGF receptors. Int J Med Sci. 2020;17:903-911.
- Colferai MMT, Miquelin GM, Steiner D. Evaluation of oral tranexamic acid in the treatment of melasma. J Cosmet Dermatol. 2019;18:1495-1501.
- Taraz M, Niknam S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatol Ther. 2017;30:19-26.
- Yalamanchili R, Shastry V, Betkerur J. Clinico-epidemiological study and quality of life assessment in melasma. Indian J Dermatol. 2015;60:519.
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776-781.
- Kim YJ, Kim MJ, Kweon DK, et al. Quantification of hypopigmentation activity in vitro. J Vis Exp. 2019;145:20-25.
- Cardoso R, Valente R, Souza da Costa CH, et al. Analysis of kojic acid derivatives as competitive inhibitors of tyrosinase: a molecular modeling approach. Molecules. 2021;26:2875.
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
- Khuraiya S, Kachhawa D, Chouhan B, et al. A comparative study of topical 5% tranexamic acid and triple combination therapy for the treatment of melasma in Indian population. Pigment International. 2019;6:18-23.
- Steiner D, Feola C, Bialeski N, et al. Study evaluating the efficacy of topical and injected tranexamic acid in treatment of melasma. Surg Cosmet Dermatol. 2009;1:174-177.
- Doolan B, Gupta M. Melasma. Aust J Gen Pract. 2021;50:880-885.
- Banihashemi M, Zabolinejad N, Jaafari MR, et al. Comparison of therapeutic effects of liposomal tranexamic acid and conventional hydroquinone on melasma. J Cosmet Dermatol. 2015;14:174-177.
- Chung JY, Lee JH, Lee JH. Topical tranexamic acid as an adjuvant treatment in melasma: side-by-side comparison clinical study. J Dermatolog Treat. 2016;27:373-377.
- Ebrahimi B, Naeini FF. Topical tranexamic acid as a promising treatment for melasma. J Res Med Sci. 2014;19:753-757.
- Kanechorn Na Ayuthaya P, Niumphradit N, Manosroi A, et al. Topical 5% tranexamic acid for the treatment of melasma in Asians: a double-blind randomized controlled clinical trial. J Cosmet Laser Ther. 2012;14:150-154.
- Kim SJ, Park JY, Shibata T, et al. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin Exp Dermatol. 2016;41:480-485.
- Lee JH, Park JG, Lim SH, et al. Localized intradermal microinjection of tranexamic acid for treatment of melasma in Asian patients: a preliminary clinical trial. Dermatol Surg. 2006;32:626-631.
- Badran AY, Ali AU, Gomaa AS. Efficacy of topical versus intradermal injection of tranexamic acid in Egyptian melasma patients: a randomised clinical trial. Australas J Dermatol. 2021;62:E373-E379.
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial. J Dermatolog Treat. 2018;29:405-410.
- Pazyar N, Yaghoobi R, Zeynalie M, et al. Comparison of the efficacy of intradermal injected tranexamic acid vs hydroquinone cream in the treatment of melasma. Clin Cosmet Investig Dermatol. 2019;12:115-122.
- Wu S, Shi H, Wu H, et al. Treatment of melasma with oral administration of tranexamic acid. Aesthetic Plast Surg. 2012;36:964-970.
- Atefi N, Dalvand B, Ghassemi M, et al. Therapeutic effects of topical tranexamic acid in comparison with hydroquinone in treatment of women with melasma. Dermatol Ther (Heidelb). 2017;7:417-424.
- Cestari T, Arellano I, Hexsel D, et al. Melasma in Latin America: options for therapy and treatment algorithm. J Eur Acad Dermatol Venereol. 2009;23:760-772.
Melasma is a complex, long-lasting, acquired dermatologic pigmentation disorder resulting in grey-brown patches that last for more than 3 months. Sun-exposed areas including the nose, cheeks, forehead, and forearms are most likely to be affected.1 In Southeast Asia, 0.25% to 4% of the population affected by melasma is aged 30 to 40 years.2 In particular, melasma is a concern among pregnant women due to increased levels of melanocyte-stimulating hormones (MSHs) and is impacted by genetics, hormonal influence, and exposure to UV light.3,4 In Pakistan, approximately 46% of women are affected by melasma during pregnancy.2,5 Although few studies have focused on the clinical approaches to melasma in darker skin types, it continues to disproportionately affect the skin of color population.4
The areas of hyperpigmentation seen in melasma exhibit increased deposition of melanin in the epidermis and dermis, but melanocytes are not elevated. However, in areas of hyperpigmentation, the melanocytes are larger and more dendritic and demonstrate an increased level of melanogenesis.6 During pregnancy, especially in the third trimester, elevated levels of estrogen, progesterone, and MSH often are found in association with melasma.7 Tyrosinase (TYR) activity increases and cellular proliferation is reduced after treatment of melanocytes in culture with β-estradiol.8 Sex steroids increase transcription of genes encoding melanogenic enzymes in normal human melanocytes, especially TYR.9 These results are consistent with the notable increases in melanin synthesis and TYR activity reported for normal human melanocytes under similar conditions in culture.10 Because melanocytes contain both cytosolic and nuclear estrogen receptors, melanocytes in patients with melasma may be inherently more sensitive to the stimulatory effects of estrogens and possibly other steroid hormones.11
The current treatment options for melasma have varying levels of success and include topical depigmenting agents such as hydroquinone, tretinoin, azelaic acid, kojic acid, and corticosteroids; dermabrasion; and chemical peels.12-14 Chemical peels with glycolic acid, salicylic acid, lactic acid, trichloroacetic acid, and phenol, as well as laser therapy, are reliable management options.13,14 Traditionally, melasma has been treated with a combination of modalities along with photoprotection and trigger avoidance.12
The efficacy and safety of the available therapies for melasma are still controversial and require further exploration. In recent years, off-label tranexamic acid (TA) has emerged as a potential therapy for melasma. Although the mechanism of action remains unclear, TA may inhibit melanin synthesis by blocking the interaction between melanocytes and keratinocytes.15 Tranexamic acid also may reverse the abnormal dermal changes associated with melasma by inhibiting melanogenesis and angiogenesis.16
Although various therapeutic options exist for melasma, the search for a reliable option in patients with darker skin types continues.13 We sought to evaluate the efficacy of TA solution 5% in reducing the severity of melasma in South Asian patients, thereby improving patient outcomes and maximizing patient satisfaction. Topical TA is inexpensive and readily accessible and does not cause systemic side effects. These qualities make it a promising treatment compared to traditional therapies.
Methods
We conducted a randomized controlled trial at Rawalpindi Medical Institute (Punjab, Pakistan). The researchers obtained informed consent for all enrolled patients. Cases were sampled from the original patient population seen at the office using nonprobability consecutive sampling. The sample size was calculated with a 95% CI, margin of error of 9%, and expected percentage of efficacy of 86.1% by using TA solution 5%. South Asian male and female patients aged 20 to 45 years with melasma were included in the analysis. Patients were excluded if they were already taking TA, oral contraceptive pills, or photosensitizing drugs (eg, nonsteroidal anti-inflammatory drugs, tetracyclines, phenytoin, carbamazepine); were pregnant; had chronic kidney disease (creatinine >2.0 mg/dL); had cardiac abnormalities (abnormal electrocardiogram); had hematologic disorders (international normalized ratio >2); or had received another melasma treatment within the last 3 to 6 months.
All enrolled patients underwent a detailed history and physical examination. Patient demographics were subsequently noted, including age, sex, history of diabetes mellitus or hypertension, and duration of melasma. The melasma area and severity index (MASI) score of each patient was calculated at baseline, and a corresponding photograph was taken.
The topical solution was prepared with 5 g of TA dissolved in 10 cc of ethanol at 96 °F, 10 cc of 1,3-butanediol, and distilled water up to 100 cc. The TA solution was applied to the affected areas once daily by the patient for 12 weeks. Each application covered the affected areas completely. Patients were instructed to apply sunscreen with sun protection factor 60 to those same areas for UV protection after 15 minutes of TA application. Biweekly follow-ups were scheduled during the trial, and the MASI score was recorded at these visits. If the mean MASI score was reduced by half after 12 weeks of treatment, then the treatment was considered efficacious with a 95% CI.
The percentage reduction from baseline was calculated as follows: percentage reduction=(baseline score– follow-up score)/baseline score×100.
Statistical Analysis—Data were analyzed in SPSS Statistics 25 (IBM). The quantitative variables of age, duration of melasma, and body mass index were presented as mean (SD). Qualitative variables such as sex, history of diabetes mellitus or hypertension, site of melasma, and efficacy were presented as frequencies and percentages. Mean MASI scores at baseline and 12 weeks posttreatment were compared using a paired t test (P≤.05). Data were stratified for age, sex, history of diabetes mellitus or hypertension, site of melasma, and duration of melasma, and a χ2 test was applied to compare efficacy in stratified groups (P≤.05).
Results
Sixty patients were enrolled in the study. Of them, 17 (28.33%) were male, and 43 (71.67%) were female (2:5 ratio). They ranged in age from 20 to 45 years (mean [SD], 31.93 [6.26] years). Thirty-seven patients (61.67%) were aged 31 to 45 years of age (Table 1). The mean (SD) duration of disease was 10.18 (2.10) months. The response to TA was recorded based on patient distribution according to the site of melasma as well as history of diabetes mellitus and hypertension.

Topical TA was found to be efficacious for melasma in 50 (83.33%) patients. The mean (SD) baseline and week 12 MASI scores were 23.15 (5.02) and 12.71 (4.10)(P<.0001), respectively (Table 2). The stratification of efficacy with respect to age, sex, duration of melasma, site of melasma, and history of diabetes mellitus or hypertension is shown in the eTable. The site of melasma was significant with respect to stratification of efficacy. On the forehead, TA was found to be efficacious in 11 patients and nonefficacious in 0 patients (P=.036). In the malar region, it was efficacious in 16 patients and nonefficacious in 1 patient (P=.036). Finally, on the chin, it was efficacious in 23 patients and nonefficacious in 9 patients (P=.036).

Comment
Melasma Presentation and Development—Melasma is a chronic skin condition that more often affects patients with darker skin types. This condition is characterized by hyperpigmentation of skin that is directly exposed to the sun, such as the cheek, nose, forehead, and above the upper lip.17 Although the mechanism behind how melasma develops is unknown, one theory suggests that UV light can lead to increased plasmin in keratinocytes.18 This increased plasmin will thereby increase the arachidonic acid and α-MSH, leading to the observed uneven hyperpigmentation that is notable in melasma. Melasma is common in patients using oral contraceptives or expired cosmetic drugs; in those who are pregnant; and in those with liver dysfunction.18 Melasma has a negative impact on patients’ quality of life because of substantial psychological and social distress. Thus, finding an accessible treatment is imperative.19

Melasma Management—The most common treatments for melasma have been topical bleaching agents and photoprotection. Combination therapy options include chemical peels, dermabrasion, and laser treatments, though they present with limited efficacy.17,20 Because melasma focuses on pigmentation correction, topical treatments work to disturb melanocyte pigment production at the enzymatic level.21 Tyrosinase is rate limiting in melanin production, as it converts L-tyrosinase to L-3,4-dihydroxyphenylalanine, using copper to interact with L-3,4-dihydroxyphenylalanine as a cofactor in the active site.22 Therefore, tyrosine is a major target for many drugs that have been developed for melasma to decrease melaninization.21
Recently, research has focused on the effects of topical, intradermal, and oral TA for melasma.17 Tranexamic acid most commonly has been used in medicine as a fibrinolytic agent because of its antiplasmin properties. It has been hypothesized that TA can inhibit the release of paracrine melanogenic factors that normally act to stimulate melanocytes.17 Although studies have supported the safety and efficacy of TA, there remains a lack of clinical studies that are sufficiently powered. No definitive consensus on the use of TA for melasma currently exists, which indicates the need for large-scale, randomized, controlled trials.23
One trial (N=25) found that TA solution 5% achieved efficacy (>50% reduction in MASI score from baseline) in 86.1% of patients with melasma.24 In another study (N=18), topical TA 5% achieved efficacy (>50% reduction in MASI score) in 86% of patients with melasma.25
Melasma Comorbidities—To determine if certain comorbidities, such as diabetes mellitus or hypertension, influenced the progression of melasma, we stratified the efficacy results for patients with these 2 comorbidities, which showed no significant difference (P=.794 and P=.101, respectively). Thus, the relatively higher prevalence of diabetes mellitus (16 patients) and hypertension (11 patients) did not contribute to the efficacy of TA in lowering MASI scores over the 12-week period, which supports the findings of Doolan and Gupta,26 who investigated the endocrinologic conditions associated with melasma and found no such association with diabetes mellitus or hypertension.
TA Formulations for Melasma—The efficacy of topical TA has been explored in several studies. Six studies with sample sizes of 13 to 50 patients each showed statistically significant differences in MASI scores between baseline and following TA treatment (P<.001).27-32 Several formulations and regimens were utilized, including TA cream 3% for 12 weeks, TA gel 5% for 12 weeks, TA solution 3% for 12 weeks, TA liposome 5% for 12 weeks, and TA solution 2% for 12 weeks.18 Additionally, these studies found TA to be effective in limiting dyschromia and decreasing MASI scores. There were no statistically significant differences between formulations and method of application. Topical TA has been found to be just as effective as other treatments for melasma, including intradermal TA injections, topical hydroquinone, and a combination of topical hydroquinone and dexamethasone.18
Further study of the efficacy of intradermal TA is necessary because many human trials have lacked statistical significance or a control group. Lee et al32 conducted a trial of 100 female patients who received weekly intradermal TA microinjections for 12 weeks. After 8 and 12 weeks, MASI scores decreased significantly (P<.01).32 Similarly, Badran et al33 observed 60 female patients in 3 trial groups: group A received TA (4 mg/mL) intradermal injections every 2 weeks, group B received TA (10 mg/mL) intradermal injections every 2 weeks, and group C received TA cream 10% twice daily. Although all groups showed improvement in MASI, group B, which had the highest intradermal TA concentration, exhibited the most improvement. Thus, it was determined that intradermal application led to better results, but the cream was still effective.33
Saki et al34 conducted a randomized, split-face trial of 37 patients comparing the efficacy of intradermal TA and topical hydroquinone. Each group was treated with either monthly intradermal TA injections or nightly hydroquinone for 3 months. After 4 weeks of treatment, TA initially had a greater improvement. However, after 20 weeks, the overall changes were not significant between the 2 groups.34 Pazyar et al35 conducted a randomized, split-face trial of 49 patients comparing the efficacy of intradermal TA and hydroquinone cream. After 24 weeks of biweekly TA injections or twice-daily hydroquinone, there were no statistically significant differences in the decreased MASI scores between treatments.35 Additional large, double-blind, controlled trials are needed to thoroughly assess the role of intradermal TA in comparison to its treatment counterpart of hydroquinone.
Ebrahimi and Naeini29 conducted a 12-week, double-blind, split-phase trial of 50 Iranian melasma patients, which showed that 27.3% of patients rated the improvement in melasma as excellent, 42.4% as good, and 30.3% as fair after using TA solution 3%. Wu et al36 also showed a total melasma improvement rate of 80.9% in 256 patients with long-term oral use of TA. In a study by Kim et al31 (N=245), the mean MASI score considerably decreased after topical TA use, with a total response rate of 95.6%. In another study, Atefi et al37 presented significantly increased levels of satisfaction in patients treated with topical TA 5% vs hydroquinone (P=.015).
Melasma in Patients With Darker Skin Types—Special attention must be given to choosing the appropriate medication in melasma patients with darker skin types, as there is an increased risk for postinflammatory hyperpigmentation. Currently, few randomized controlled trials exist that fulfill the criteria of evaluating pharmacologic options for patients with melasma, and even fewer studies solely focus on patients with darker skin types.38 In addition to treatment advances, patients must be educated on the need to avoid sun exposure when possible or to use photoprotection, especially in the South Asian region, where these practices rarely are taught. Our study provided a unique analysis regarding the efficacy of TA solution 5% for the treatment of melasma in patients of South Asian descent. Clinicians can use these findings as a foundation for treating all patients with melasma but particularly those with darker skin types.
Study Limitations—Our study consisted of 60 patients; although our study had more patients than similar trials, larger studies are needed. Additionally, other variables were excluded from our analysis, such as comorbidities beyond diabetes mellitus and hypertension.
Conclusion
This study contributes to the growing field of melasma therapeutics by evaluating the efficacy of using TA solution 5% for the treatment of melasma in South Asian patients with darker skin types. Clinicians may use our study to broaden their treatment options for a common condition while also addressing the lack of clinical options for patients with darker skin types. Further studies investigating the effectiveness of TA in large clinical trials in humans are warranted to understand the efficacy and the risk for any complications.
Melasma is a complex, long-lasting, acquired dermatologic pigmentation disorder resulting in grey-brown patches that last for more than 3 months. Sun-exposed areas including the nose, cheeks, forehead, and forearms are most likely to be affected.1 In Southeast Asia, 0.25% to 4% of the population affected by melasma is aged 30 to 40 years.2 In particular, melasma is a concern among pregnant women due to increased levels of melanocyte-stimulating hormones (MSHs) and is impacted by genetics, hormonal influence, and exposure to UV light.3,4 In Pakistan, approximately 46% of women are affected by melasma during pregnancy.2,5 Although few studies have focused on the clinical approaches to melasma in darker skin types, it continues to disproportionately affect the skin of color population.4
The areas of hyperpigmentation seen in melasma exhibit increased deposition of melanin in the epidermis and dermis, but melanocytes are not elevated. However, in areas of hyperpigmentation, the melanocytes are larger and more dendritic and demonstrate an increased level of melanogenesis.6 During pregnancy, especially in the third trimester, elevated levels of estrogen, progesterone, and MSH often are found in association with melasma.7 Tyrosinase (TYR) activity increases and cellular proliferation is reduced after treatment of melanocytes in culture with β-estradiol.8 Sex steroids increase transcription of genes encoding melanogenic enzymes in normal human melanocytes, especially TYR.9 These results are consistent with the notable increases in melanin synthesis and TYR activity reported for normal human melanocytes under similar conditions in culture.10 Because melanocytes contain both cytosolic and nuclear estrogen receptors, melanocytes in patients with melasma may be inherently more sensitive to the stimulatory effects of estrogens and possibly other steroid hormones.11
The current treatment options for melasma have varying levels of success and include topical depigmenting agents such as hydroquinone, tretinoin, azelaic acid, kojic acid, and corticosteroids; dermabrasion; and chemical peels.12-14 Chemical peels with glycolic acid, salicylic acid, lactic acid, trichloroacetic acid, and phenol, as well as laser therapy, are reliable management options.13,14 Traditionally, melasma has been treated with a combination of modalities along with photoprotection and trigger avoidance.12
The efficacy and safety of the available therapies for melasma are still controversial and require further exploration. In recent years, off-label tranexamic acid (TA) has emerged as a potential therapy for melasma. Although the mechanism of action remains unclear, TA may inhibit melanin synthesis by blocking the interaction between melanocytes and keratinocytes.15 Tranexamic acid also may reverse the abnormal dermal changes associated with melasma by inhibiting melanogenesis and angiogenesis.16
Although various therapeutic options exist for melasma, the search for a reliable option in patients with darker skin types continues.13 We sought to evaluate the efficacy of TA solution 5% in reducing the severity of melasma in South Asian patients, thereby improving patient outcomes and maximizing patient satisfaction. Topical TA is inexpensive and readily accessible and does not cause systemic side effects. These qualities make it a promising treatment compared to traditional therapies.
Methods
We conducted a randomized controlled trial at Rawalpindi Medical Institute (Punjab, Pakistan). The researchers obtained informed consent for all enrolled patients. Cases were sampled from the original patient population seen at the office using nonprobability consecutive sampling. The sample size was calculated with a 95% CI, margin of error of 9%, and expected percentage of efficacy of 86.1% by using TA solution 5%. South Asian male and female patients aged 20 to 45 years with melasma were included in the analysis. Patients were excluded if they were already taking TA, oral contraceptive pills, or photosensitizing drugs (eg, nonsteroidal anti-inflammatory drugs, tetracyclines, phenytoin, carbamazepine); were pregnant; had chronic kidney disease (creatinine >2.0 mg/dL); had cardiac abnormalities (abnormal electrocardiogram); had hematologic disorders (international normalized ratio >2); or had received another melasma treatment within the last 3 to 6 months.
All enrolled patients underwent a detailed history and physical examination. Patient demographics were subsequently noted, including age, sex, history of diabetes mellitus or hypertension, and duration of melasma. The melasma area and severity index (MASI) score of each patient was calculated at baseline, and a corresponding photograph was taken.
The topical solution was prepared with 5 g of TA dissolved in 10 cc of ethanol at 96 °F, 10 cc of 1,3-butanediol, and distilled water up to 100 cc. The TA solution was applied to the affected areas once daily by the patient for 12 weeks. Each application covered the affected areas completely. Patients were instructed to apply sunscreen with sun protection factor 60 to those same areas for UV protection after 15 minutes of TA application. Biweekly follow-ups were scheduled during the trial, and the MASI score was recorded at these visits. If the mean MASI score was reduced by half after 12 weeks of treatment, then the treatment was considered efficacious with a 95% CI.
The percentage reduction from baseline was calculated as follows: percentage reduction=(baseline score– follow-up score)/baseline score×100.
Statistical Analysis—Data were analyzed in SPSS Statistics 25 (IBM). The quantitative variables of age, duration of melasma, and body mass index were presented as mean (SD). Qualitative variables such as sex, history of diabetes mellitus or hypertension, site of melasma, and efficacy were presented as frequencies and percentages. Mean MASI scores at baseline and 12 weeks posttreatment were compared using a paired t test (P≤.05). Data were stratified for age, sex, history of diabetes mellitus or hypertension, site of melasma, and duration of melasma, and a χ2 test was applied to compare efficacy in stratified groups (P≤.05).
Results
Sixty patients were enrolled in the study. Of them, 17 (28.33%) were male, and 43 (71.67%) were female (2:5 ratio). They ranged in age from 20 to 45 years (mean [SD], 31.93 [6.26] years). Thirty-seven patients (61.67%) were aged 31 to 45 years of age (Table 1). The mean (SD) duration of disease was 10.18 (2.10) months. The response to TA was recorded based on patient distribution according to the site of melasma as well as history of diabetes mellitus and hypertension.

Topical TA was found to be efficacious for melasma in 50 (83.33%) patients. The mean (SD) baseline and week 12 MASI scores were 23.15 (5.02) and 12.71 (4.10)(P<.0001), respectively (Table 2). The stratification of efficacy with respect to age, sex, duration of melasma, site of melasma, and history of diabetes mellitus or hypertension is shown in the eTable. The site of melasma was significant with respect to stratification of efficacy. On the forehead, TA was found to be efficacious in 11 patients and nonefficacious in 0 patients (P=.036). In the malar region, it was efficacious in 16 patients and nonefficacious in 1 patient (P=.036). Finally, on the chin, it was efficacious in 23 patients and nonefficacious in 9 patients (P=.036).

Comment
Melasma Presentation and Development—Melasma is a chronic skin condition that more often affects patients with darker skin types. This condition is characterized by hyperpigmentation of skin that is directly exposed to the sun, such as the cheek, nose, forehead, and above the upper lip.17 Although the mechanism behind how melasma develops is unknown, one theory suggests that UV light can lead to increased plasmin in keratinocytes.18 This increased plasmin will thereby increase the arachidonic acid and α-MSH, leading to the observed uneven hyperpigmentation that is notable in melasma. Melasma is common in patients using oral contraceptives or expired cosmetic drugs; in those who are pregnant; and in those with liver dysfunction.18 Melasma has a negative impact on patients’ quality of life because of substantial psychological and social distress. Thus, finding an accessible treatment is imperative.19

Melasma Management—The most common treatments for melasma have been topical bleaching agents and photoprotection. Combination therapy options include chemical peels, dermabrasion, and laser treatments, though they present with limited efficacy.17,20 Because melasma focuses on pigmentation correction, topical treatments work to disturb melanocyte pigment production at the enzymatic level.21 Tyrosinase is rate limiting in melanin production, as it converts L-tyrosinase to L-3,4-dihydroxyphenylalanine, using copper to interact with L-3,4-dihydroxyphenylalanine as a cofactor in the active site.22 Therefore, tyrosine is a major target for many drugs that have been developed for melasma to decrease melaninization.21
Recently, research has focused on the effects of topical, intradermal, and oral TA for melasma.17 Tranexamic acid most commonly has been used in medicine as a fibrinolytic agent because of its antiplasmin properties. It has been hypothesized that TA can inhibit the release of paracrine melanogenic factors that normally act to stimulate melanocytes.17 Although studies have supported the safety and efficacy of TA, there remains a lack of clinical studies that are sufficiently powered. No definitive consensus on the use of TA for melasma currently exists, which indicates the need for large-scale, randomized, controlled trials.23
One trial (N=25) found that TA solution 5% achieved efficacy (>50% reduction in MASI score from baseline) in 86.1% of patients with melasma.24 In another study (N=18), topical TA 5% achieved efficacy (>50% reduction in MASI score) in 86% of patients with melasma.25
Melasma Comorbidities—To determine if certain comorbidities, such as diabetes mellitus or hypertension, influenced the progression of melasma, we stratified the efficacy results for patients with these 2 comorbidities, which showed no significant difference (P=.794 and P=.101, respectively). Thus, the relatively higher prevalence of diabetes mellitus (16 patients) and hypertension (11 patients) did not contribute to the efficacy of TA in lowering MASI scores over the 12-week period, which supports the findings of Doolan and Gupta,26 who investigated the endocrinologic conditions associated with melasma and found no such association with diabetes mellitus or hypertension.
TA Formulations for Melasma—The efficacy of topical TA has been explored in several studies. Six studies with sample sizes of 13 to 50 patients each showed statistically significant differences in MASI scores between baseline and following TA treatment (P<.001).27-32 Several formulations and regimens were utilized, including TA cream 3% for 12 weeks, TA gel 5% for 12 weeks, TA solution 3% for 12 weeks, TA liposome 5% for 12 weeks, and TA solution 2% for 12 weeks.18 Additionally, these studies found TA to be effective in limiting dyschromia and decreasing MASI scores. There were no statistically significant differences between formulations and method of application. Topical TA has been found to be just as effective as other treatments for melasma, including intradermal TA injections, topical hydroquinone, and a combination of topical hydroquinone and dexamethasone.18
Further study of the efficacy of intradermal TA is necessary because many human trials have lacked statistical significance or a control group. Lee et al32 conducted a trial of 100 female patients who received weekly intradermal TA microinjections for 12 weeks. After 8 and 12 weeks, MASI scores decreased significantly (P<.01).32 Similarly, Badran et al33 observed 60 female patients in 3 trial groups: group A received TA (4 mg/mL) intradermal injections every 2 weeks, group B received TA (10 mg/mL) intradermal injections every 2 weeks, and group C received TA cream 10% twice daily. Although all groups showed improvement in MASI, group B, which had the highest intradermal TA concentration, exhibited the most improvement. Thus, it was determined that intradermal application led to better results, but the cream was still effective.33
Saki et al34 conducted a randomized, split-face trial of 37 patients comparing the efficacy of intradermal TA and topical hydroquinone. Each group was treated with either monthly intradermal TA injections or nightly hydroquinone for 3 months. After 4 weeks of treatment, TA initially had a greater improvement. However, after 20 weeks, the overall changes were not significant between the 2 groups.34 Pazyar et al35 conducted a randomized, split-face trial of 49 patients comparing the efficacy of intradermal TA and hydroquinone cream. After 24 weeks of biweekly TA injections or twice-daily hydroquinone, there were no statistically significant differences in the decreased MASI scores between treatments.35 Additional large, double-blind, controlled trials are needed to thoroughly assess the role of intradermal TA in comparison to its treatment counterpart of hydroquinone.
Ebrahimi and Naeini29 conducted a 12-week, double-blind, split-phase trial of 50 Iranian melasma patients, which showed that 27.3% of patients rated the improvement in melasma as excellent, 42.4% as good, and 30.3% as fair after using TA solution 3%. Wu et al36 also showed a total melasma improvement rate of 80.9% in 256 patients with long-term oral use of TA. In a study by Kim et al31 (N=245), the mean MASI score considerably decreased after topical TA use, with a total response rate of 95.6%. In another study, Atefi et al37 presented significantly increased levels of satisfaction in patients treated with topical TA 5% vs hydroquinone (P=.015).
Melasma in Patients With Darker Skin Types—Special attention must be given to choosing the appropriate medication in melasma patients with darker skin types, as there is an increased risk for postinflammatory hyperpigmentation. Currently, few randomized controlled trials exist that fulfill the criteria of evaluating pharmacologic options for patients with melasma, and even fewer studies solely focus on patients with darker skin types.38 In addition to treatment advances, patients must be educated on the need to avoid sun exposure when possible or to use photoprotection, especially in the South Asian region, where these practices rarely are taught. Our study provided a unique analysis regarding the efficacy of TA solution 5% for the treatment of melasma in patients of South Asian descent. Clinicians can use these findings as a foundation for treating all patients with melasma but particularly those with darker skin types.
Study Limitations—Our study consisted of 60 patients; although our study had more patients than similar trials, larger studies are needed. Additionally, other variables were excluded from our analysis, such as comorbidities beyond diabetes mellitus and hypertension.
Conclusion
This study contributes to the growing field of melasma therapeutics by evaluating the efficacy of using TA solution 5% for the treatment of melasma in South Asian patients with darker skin types. Clinicians may use our study to broaden their treatment options for a common condition while also addressing the lack of clinical options for patients with darker skin types. Further studies investigating the effectiveness of TA in large clinical trials in humans are warranted to understand the efficacy and the risk for any complications.
- Espósito ACC, Brianezi G, De Souza NP, et al. Exploratory study of epidermis, basement membrane zone, upper dermis alterations and Wnt pathway activation in melasma compared to adjacent and retroauricular skin. Ann Dermatol. 2020;32:101-108.
- Janney MS, Subramaniyan R, Dabas R, et al. A randomized controlled study comparing the efficacy of topical 5% tranexamic acid solution versus 3% hydroquinone cream in melasma. J Cutan Aesthet Surg. 2019;12:63-67.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasmaJ Cosmet Laser Ther. 2018;20:134-139.
- Grimes PE, Ijaz S, Nashawati R, et al. New oral and topical approaches for the treatment of melasma. Int J Womens Dermatol. 2019;5:30-36.
- Handel AC, Miot LDB, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Barankin B, Silver SG, Carruthers A. The skin in pregnancy. J Cutan Med Surg. 2002;6:236-240.
- Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
- Smith AG, Shuster S, Thody AJ, et al. Chloasma, oral contraceptives, and plasma immunoreactive beta-melanocyte-stimulating hormone. J Invest Dermatol. 1977;68:169-170.
- Ranson M, Posen S, Mason RS. Human melanocytes as a target tissue for hormones: in vitro studies with 1 alpha-25, dihydroxyvitamin D3, alpha-melanocyte stimulating hormone, and beta-estradiol. J Invest Dermatol. 1988;91:593-598.
- Kippenberger S, Loitsch S, Solano F, et al. Quantification of tyrosinase, TRP-1, and Trp-2 transcripts in human melanocytes by reverse transcriptase-competitive multiplex PCR—regulation by steroid hormones. J Invest Dermatol. 1998;110:364-367.
- McLeod SD, Ranson M, Mason RS. Effects of estrogens on human melanocytes in vitro. J Steroid Biochem Mol Biol. 1994;49:9-14.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasma. J Cosmet Laser Ther. 2018;20:134-139.
- Sheu SL. Treatment of melasma using tranexamic acid: what’s known and what’s next. Cutis. 2018;101:E7-E8.
- Tian B. The Asian problem of frequent laser toning for melasma. J Clin Aesthet Dermatol. 2017;10:40-42.
- Zhang L, Tan WQ, Fang QQ, et al. Tranexamic acid for adults with melasma: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:1683414.
- Zhu JW, Ni YJ, Tong XY, et al. Tranexamic acid inhibits angiogenesis and melanogenesis in vitro by targeting VEGF receptors. Int J Med Sci. 2020;17:903-911.
- Colferai MMT, Miquelin GM, Steiner D. Evaluation of oral tranexamic acid in the treatment of melasma. J Cosmet Dermatol. 2019;18:1495-1501.
- Taraz M, Niknam S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatol Ther. 2017;30:19-26.
- Yalamanchili R, Shastry V, Betkerur J. Clinico-epidemiological study and quality of life assessment in melasma. Indian J Dermatol. 2015;60:519.
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776-781.
- Kim YJ, Kim MJ, Kweon DK, et al. Quantification of hypopigmentation activity in vitro. J Vis Exp. 2019;145:20-25.
- Cardoso R, Valente R, Souza da Costa CH, et al. Analysis of kojic acid derivatives as competitive inhibitors of tyrosinase: a molecular modeling approach. Molecules. 2021;26:2875.
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
- Khuraiya S, Kachhawa D, Chouhan B, et al. A comparative study of topical 5% tranexamic acid and triple combination therapy for the treatment of melasma in Indian population. Pigment International. 2019;6:18-23.
- Steiner D, Feola C, Bialeski N, et al. Study evaluating the efficacy of topical and injected tranexamic acid in treatment of melasma. Surg Cosmet Dermatol. 2009;1:174-177.
- Doolan B, Gupta M. Melasma. Aust J Gen Pract. 2021;50:880-885.
- Banihashemi M, Zabolinejad N, Jaafari MR, et al. Comparison of therapeutic effects of liposomal tranexamic acid and conventional hydroquinone on melasma. J Cosmet Dermatol. 2015;14:174-177.
- Chung JY, Lee JH, Lee JH. Topical tranexamic acid as an adjuvant treatment in melasma: side-by-side comparison clinical study. J Dermatolog Treat. 2016;27:373-377.
- Ebrahimi B, Naeini FF. Topical tranexamic acid as a promising treatment for melasma. J Res Med Sci. 2014;19:753-757.
- Kanechorn Na Ayuthaya P, Niumphradit N, Manosroi A, et al. Topical 5% tranexamic acid for the treatment of melasma in Asians: a double-blind randomized controlled clinical trial. J Cosmet Laser Ther. 2012;14:150-154.
- Kim SJ, Park JY, Shibata T, et al. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin Exp Dermatol. 2016;41:480-485.
- Lee JH, Park JG, Lim SH, et al. Localized intradermal microinjection of tranexamic acid for treatment of melasma in Asian patients: a preliminary clinical trial. Dermatol Surg. 2006;32:626-631.
- Badran AY, Ali AU, Gomaa AS. Efficacy of topical versus intradermal injection of tranexamic acid in Egyptian melasma patients: a randomised clinical trial. Australas J Dermatol. 2021;62:E373-E379.
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial. J Dermatolog Treat. 2018;29:405-410.
- Pazyar N, Yaghoobi R, Zeynalie M, et al. Comparison of the efficacy of intradermal injected tranexamic acid vs hydroquinone cream in the treatment of melasma. Clin Cosmet Investig Dermatol. 2019;12:115-122.
- Wu S, Shi H, Wu H, et al. Treatment of melasma with oral administration of tranexamic acid. Aesthetic Plast Surg. 2012;36:964-970.
- Atefi N, Dalvand B, Ghassemi M, et al. Therapeutic effects of topical tranexamic acid in comparison with hydroquinone in treatment of women with melasma. Dermatol Ther (Heidelb). 2017;7:417-424.
- Cestari T, Arellano I, Hexsel D, et al. Melasma in Latin America: options for therapy and treatment algorithm. J Eur Acad Dermatol Venereol. 2009;23:760-772.
- Espósito ACC, Brianezi G, De Souza NP, et al. Exploratory study of epidermis, basement membrane zone, upper dermis alterations and Wnt pathway activation in melasma compared to adjacent and retroauricular skin. Ann Dermatol. 2020;32:101-108.
- Janney MS, Subramaniyan R, Dabas R, et al. A randomized controlled study comparing the efficacy of topical 5% tranexamic acid solution versus 3% hydroquinone cream in melasma. J Cutan Aesthet Surg. 2019;12:63-67.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasmaJ Cosmet Laser Ther. 2018;20:134-139.
- Grimes PE, Ijaz S, Nashawati R, et al. New oral and topical approaches for the treatment of melasma. Int J Womens Dermatol. 2019;5:30-36.
- Handel AC, Miot LDB, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Barankin B, Silver SG, Carruthers A. The skin in pregnancy. J Cutan Med Surg. 2002;6:236-240.
- Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
- Smith AG, Shuster S, Thody AJ, et al. Chloasma, oral contraceptives, and plasma immunoreactive beta-melanocyte-stimulating hormone. J Invest Dermatol. 1977;68:169-170.
- Ranson M, Posen S, Mason RS. Human melanocytes as a target tissue for hormones: in vitro studies with 1 alpha-25, dihydroxyvitamin D3, alpha-melanocyte stimulating hormone, and beta-estradiol. J Invest Dermatol. 1988;91:593-598.
- Kippenberger S, Loitsch S, Solano F, et al. Quantification of tyrosinase, TRP-1, and Trp-2 transcripts in human melanocytes by reverse transcriptase-competitive multiplex PCR—regulation by steroid hormones. J Invest Dermatol. 1998;110:364-367.
- McLeod SD, Ranson M, Mason RS. Effects of estrogens on human melanocytes in vitro. J Steroid Biochem Mol Biol. 1994;49:9-14.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasma. J Cosmet Laser Ther. 2018;20:134-139.
- Sheu SL. Treatment of melasma using tranexamic acid: what’s known and what’s next. Cutis. 2018;101:E7-E8.
- Tian B. The Asian problem of frequent laser toning for melasma. J Clin Aesthet Dermatol. 2017;10:40-42.
- Zhang L, Tan WQ, Fang QQ, et al. Tranexamic acid for adults with melasma: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:1683414.
- Zhu JW, Ni YJ, Tong XY, et al. Tranexamic acid inhibits angiogenesis and melanogenesis in vitro by targeting VEGF receptors. Int J Med Sci. 2020;17:903-911.
- Colferai MMT, Miquelin GM, Steiner D. Evaluation of oral tranexamic acid in the treatment of melasma. J Cosmet Dermatol. 2019;18:1495-1501.
- Taraz M, Niknam S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatol Ther. 2017;30:19-26.
- Yalamanchili R, Shastry V, Betkerur J. Clinico-epidemiological study and quality of life assessment in melasma. Indian J Dermatol. 2015;60:519.
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776-781.
- Kim YJ, Kim MJ, Kweon DK, et al. Quantification of hypopigmentation activity in vitro. J Vis Exp. 2019;145:20-25.
- Cardoso R, Valente R, Souza da Costa CH, et al. Analysis of kojic acid derivatives as competitive inhibitors of tyrosinase: a molecular modeling approach. Molecules. 2021;26:2875.
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
- Khuraiya S, Kachhawa D, Chouhan B, et al. A comparative study of topical 5% tranexamic acid and triple combination therapy for the treatment of melasma in Indian population. Pigment International. 2019;6:18-23.
- Steiner D, Feola C, Bialeski N, et al. Study evaluating the efficacy of topical and injected tranexamic acid in treatment of melasma. Surg Cosmet Dermatol. 2009;1:174-177.
- Doolan B, Gupta M. Melasma. Aust J Gen Pract. 2021;50:880-885.
- Banihashemi M, Zabolinejad N, Jaafari MR, et al. Comparison of therapeutic effects of liposomal tranexamic acid and conventional hydroquinone on melasma. J Cosmet Dermatol. 2015;14:174-177.
- Chung JY, Lee JH, Lee JH. Topical tranexamic acid as an adjuvant treatment in melasma: side-by-side comparison clinical study. J Dermatolog Treat. 2016;27:373-377.
- Ebrahimi B, Naeini FF. Topical tranexamic acid as a promising treatment for melasma. J Res Med Sci. 2014;19:753-757.
- Kanechorn Na Ayuthaya P, Niumphradit N, Manosroi A, et al. Topical 5% tranexamic acid for the treatment of melasma in Asians: a double-blind randomized controlled clinical trial. J Cosmet Laser Ther. 2012;14:150-154.
- Kim SJ, Park JY, Shibata T, et al. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin Exp Dermatol. 2016;41:480-485.
- Lee JH, Park JG, Lim SH, et al. Localized intradermal microinjection of tranexamic acid for treatment of melasma in Asian patients: a preliminary clinical trial. Dermatol Surg. 2006;32:626-631.
- Badran AY, Ali AU, Gomaa AS. Efficacy of topical versus intradermal injection of tranexamic acid in Egyptian melasma patients: a randomised clinical trial. Australas J Dermatol. 2021;62:E373-E379.
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial. J Dermatolog Treat. 2018;29:405-410.
- Pazyar N, Yaghoobi R, Zeynalie M, et al. Comparison of the efficacy of intradermal injected tranexamic acid vs hydroquinone cream in the treatment of melasma. Clin Cosmet Investig Dermatol. 2019;12:115-122.
- Wu S, Shi H, Wu H, et al. Treatment of melasma with oral administration of tranexamic acid. Aesthetic Plast Surg. 2012;36:964-970.
- Atefi N, Dalvand B, Ghassemi M, et al. Therapeutic effects of topical tranexamic acid in comparison with hydroquinone in treatment of women with melasma. Dermatol Ther (Heidelb). 2017;7:417-424.
- Cestari T, Arellano I, Hexsel D, et al. Melasma in Latin America: options for therapy and treatment algorithm. J Eur Acad Dermatol Venereol. 2009;23:760-772.
PRATICE POINTS
- Tranexamic acid (TA) solution 5% is an efficacious treatment for skin of color patients with melasma.
- Topical TA is a treatment alternative for patients who may not be able to tolerate oral TA.
- Our study revealed the greatest efficacy for TA solution 5% was seen on the forehead and malar region, with less efficacy on the chin.
Verrucous Plaque on the Foot
The Diagnosis: Eccrine Poroma
Histopathology demonstrated epidermal thickening, epidermal protrusions, a well-defined mass of tumor cells that extended from the epidermis down to the dermis, and luminal structures. Poroid cells and ovoid nuclei with basophilic cytoplasm also were evident (Figure 1). Dermoscopy showed papillomatous growth, milky-red areas, and dotted vessels (Figure 2). Reflectance confocal microscopy (RCM) at the spinous layer showed hyporefractile, dark, roundish lumina surrounded by keratinocytes (Figure 3). Based on the histologic, dermoscopic, and RCM findings, our patient was diagnosed with eccrine poroma.
Goldman et al1 first described poroma in 1956. Poromas, which include eccrine poroma, are a group of benign cutaneous neoplasms arising from the terminal eccrine or apocrine sweat gland ducts.2 Histologically, poroid cells appear as cuboidal keratinocytes with monomorphous ovoid nuclei and discrete nucleoli.3 They usually appear as nodules or plaques with colors varying from flesh colored to red, brown, or bluish, and they clinically mimic several benign and malignant skin tumors. The differential diagnosis may include keratoacanthoma, plantar wart, verrucous carcinoma, basal cell carcinoma, and squamous cell carcinoma. Poromas can be of eccrine or apocrine origin.4 They also belong to a broad group of neoplasms, including nodular hidradenomas, clear cell hidradenomas, hidroacanthoma simplex, dermal duct tumors, and hidradenomas.5 Four subtypes—poroma, poroid hidradenoma, hidroacanthoma simplex, and dermal duct tumor—have been documented.6 Because poromas have nonspecific and variable clinical presentations, they often are misdiagnosed as other skin neoplasms, and differentiation may be difficult. For example, some cases of poroma present with follicular, sebaceous, and/or apocrine differentiation, leading to difficulty in diagnosis.
Characteristic features of eccrine poroma seen on dermoscopy and RCM have the potential to aid in the diagnosis compared to histopathology. Marchetti et al7 proposed 4 patterns of characteristic dermoscopic findings. Pattern 1 refers to the classic description with bleeding spots, a structureless yellow appearance, milkyred globules, and branched vessels. Patterns 2 and 3 simulate basal cell carcinoma, dermal nevus, or vascular tumors. Pattern 4 refers to tumors that are large in size and resemble keratinizing neoplasms.7 Brugués et al8 described poromas with the following RCM findings: an atypical honeycomb shape that was well separated from the normal epithelium, hyporefractile nests with atypical cells, lack of palisading, and dark holes. One study described RCM parameters as cords without palisading, dark holes, prominent vascularization, and abundant stroma—findings that were positively associated with poroma in a univariate analysis. These findings assist in distinguishing poromas from other conditions in the differential diagnosis.9
There is a substantial overlap in clinical appearance with malignant conditions, including basal cell carcinoma, squamous cell carcinoma, cutaneous metastases, and Paget disease; therefore, the use of dermoscopy and RCM may be helpful in the diagnosis and recognition of specific features, as well as the corresponding patterns of poroma. Poromas commonly display vascularized features due to the variability of dermoscopic patterns of eccrine poroma, and further studies are required to establish the specificity of vascularized features.
Acral lesions are more likely to show the classic clinical features of erythema and exophytic growth. A case of a collision tumor with the verrucous changes of poroma, seborrheic keratosis, and viral wart has been described.10 The verrucous changes may lead to misdiagnosis as plantar warts or other neoplasms. Clinicians also should consider conditions that are induced by friction or trauma. In our patient, dermoscopy and RCM aided in the diagnosis of eccrine poroma due to the interference of prominent overlying verrucous changes.
Treatment of poroma is optional. Deeper lesions can be treated with surgical excision, and superficial lesions may be treated with electrosurgical destruction. Our patient was treated with surgical excision followed by repair of the surgical defect with a double V-Y flap.
- Goldman P, Pinkus H, Rogin JR. Eccrine poroma; tumors exhibiting features of the epidermal sweat duct unit. AMA Arch Derm. 1956; 74:511-521.
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma [published online January 28, 2022]. Dermatopathology (Basel). 2022;9:36-47. doi:10.3390/dermatopathology9010007
- Ahmed Jan N, Masood S. Poroma. StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK560909/
- Casper DJ, Glass LF, Shenefelt PD. An unusually large eccrine poroma: a case report and review of the literature. Cutis. 2011; 88:227-229.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053-1061.
- Betti R, Bombonato C, Cerri A, et al. Unusual sites for poromas are not very unusual: a survey of 101 cases. Clin Exp Dermatol. 2014; 39:119-122.
- Marchetti MA, Marino ML, Virmani P, et al. Dermoscopic features and patterns of poromas: a multicenter observational case-control study conducted by the International Dermoscopy Society (IDS). J Eur Acad Dermatol Venereol. 2018;32:1263-1271.
- Brugués A, Gamboa M, Alós L, et al. The challenging diagnosis of eccrine poromas. J Am Acad Dermatol. 2016;74:E113-E115.
- Di Tullio F, Mandel VD, Ignazio S, et al. The role of reflectance confocal microscopy in the diagnosis of eccrine poroma: a retrospective casecontrol study. Exp Dermatol. 2022;31:1779-1790.
- Bloom BS, Kamino H, Hale CS, et al. Collision tumor of eccrine poroma, seborrheic keratosis, and a viral wart. Dermatol Online J. 2014;20:13030/qt8tm0r9b9.
The Diagnosis: Eccrine Poroma
Histopathology demonstrated epidermal thickening, epidermal protrusions, a well-defined mass of tumor cells that extended from the epidermis down to the dermis, and luminal structures. Poroid cells and ovoid nuclei with basophilic cytoplasm also were evident (Figure 1). Dermoscopy showed papillomatous growth, milky-red areas, and dotted vessels (Figure 2). Reflectance confocal microscopy (RCM) at the spinous layer showed hyporefractile, dark, roundish lumina surrounded by keratinocytes (Figure 3). Based on the histologic, dermoscopic, and RCM findings, our patient was diagnosed with eccrine poroma.

Goldman et al1 first described poroma in 1956. Poromas, which include eccrine poroma, are a group of benign cutaneous neoplasms arising from the terminal eccrine or apocrine sweat gland ducts.2 Histologically, poroid cells appear as cuboidal keratinocytes with monomorphous ovoid nuclei and discrete nucleoli.3 They usually appear as nodules or plaques with colors varying from flesh colored to red, brown, or bluish, and they clinically mimic several benign and malignant skin tumors. The differential diagnosis may include keratoacanthoma, plantar wart, verrucous carcinoma, basal cell carcinoma, and squamous cell carcinoma. Poromas can be of eccrine or apocrine origin.4 They also belong to a broad group of neoplasms, including nodular hidradenomas, clear cell hidradenomas, hidroacanthoma simplex, dermal duct tumors, and hidradenomas.5 Four subtypes—poroma, poroid hidradenoma, hidroacanthoma simplex, and dermal duct tumor—have been documented.6 Because poromas have nonspecific and variable clinical presentations, they often are misdiagnosed as other skin neoplasms, and differentiation may be difficult. For example, some cases of poroma present with follicular, sebaceous, and/or apocrine differentiation, leading to difficulty in diagnosis.

Characteristic features of eccrine poroma seen on dermoscopy and RCM have the potential to aid in the diagnosis compared to histopathology. Marchetti et al7 proposed 4 patterns of characteristic dermoscopic findings. Pattern 1 refers to the classic description with bleeding spots, a structureless yellow appearance, milkyred globules, and branched vessels. Patterns 2 and 3 simulate basal cell carcinoma, dermal nevus, or vascular tumors. Pattern 4 refers to tumors that are large in size and resemble keratinizing neoplasms.7 Brugués et al8 described poromas with the following RCM findings: an atypical honeycomb shape that was well separated from the normal epithelium, hyporefractile nests with atypical cells, lack of palisading, and dark holes. One study described RCM parameters as cords without palisading, dark holes, prominent vascularization, and abundant stroma—findings that were positively associated with poroma in a univariate analysis. These findings assist in distinguishing poromas from other conditions in the differential diagnosis.9

There is a substantial overlap in clinical appearance with malignant conditions, including basal cell carcinoma, squamous cell carcinoma, cutaneous metastases, and Paget disease; therefore, the use of dermoscopy and RCM may be helpful in the diagnosis and recognition of specific features, as well as the corresponding patterns of poroma. Poromas commonly display vascularized features due to the variability of dermoscopic patterns of eccrine poroma, and further studies are required to establish the specificity of vascularized features.
Acral lesions are more likely to show the classic clinical features of erythema and exophytic growth. A case of a collision tumor with the verrucous changes of poroma, seborrheic keratosis, and viral wart has been described.10 The verrucous changes may lead to misdiagnosis as plantar warts or other neoplasms. Clinicians also should consider conditions that are induced by friction or trauma. In our patient, dermoscopy and RCM aided in the diagnosis of eccrine poroma due to the interference of prominent overlying verrucous changes.
Treatment of poroma is optional. Deeper lesions can be treated with surgical excision, and superficial lesions may be treated with electrosurgical destruction. Our patient was treated with surgical excision followed by repair of the surgical defect with a double V-Y flap.
The Diagnosis: Eccrine Poroma
Histopathology demonstrated epidermal thickening, epidermal protrusions, a well-defined mass of tumor cells that extended from the epidermis down to the dermis, and luminal structures. Poroid cells and ovoid nuclei with basophilic cytoplasm also were evident (Figure 1). Dermoscopy showed papillomatous growth, milky-red areas, and dotted vessels (Figure 2). Reflectance confocal microscopy (RCM) at the spinous layer showed hyporefractile, dark, roundish lumina surrounded by keratinocytes (Figure 3). Based on the histologic, dermoscopic, and RCM findings, our patient was diagnosed with eccrine poroma.

Goldman et al1 first described poroma in 1956. Poromas, which include eccrine poroma, are a group of benign cutaneous neoplasms arising from the terminal eccrine or apocrine sweat gland ducts.2 Histologically, poroid cells appear as cuboidal keratinocytes with monomorphous ovoid nuclei and discrete nucleoli.3 They usually appear as nodules or plaques with colors varying from flesh colored to red, brown, or bluish, and they clinically mimic several benign and malignant skin tumors. The differential diagnosis may include keratoacanthoma, plantar wart, verrucous carcinoma, basal cell carcinoma, and squamous cell carcinoma. Poromas can be of eccrine or apocrine origin.4 They also belong to a broad group of neoplasms, including nodular hidradenomas, clear cell hidradenomas, hidroacanthoma simplex, dermal duct tumors, and hidradenomas.5 Four subtypes—poroma, poroid hidradenoma, hidroacanthoma simplex, and dermal duct tumor—have been documented.6 Because poromas have nonspecific and variable clinical presentations, they often are misdiagnosed as other skin neoplasms, and differentiation may be difficult. For example, some cases of poroma present with follicular, sebaceous, and/or apocrine differentiation, leading to difficulty in diagnosis.

Characteristic features of eccrine poroma seen on dermoscopy and RCM have the potential to aid in the diagnosis compared to histopathology. Marchetti et al7 proposed 4 patterns of characteristic dermoscopic findings. Pattern 1 refers to the classic description with bleeding spots, a structureless yellow appearance, milkyred globules, and branched vessels. Patterns 2 and 3 simulate basal cell carcinoma, dermal nevus, or vascular tumors. Pattern 4 refers to tumors that are large in size and resemble keratinizing neoplasms.7 Brugués et al8 described poromas with the following RCM findings: an atypical honeycomb shape that was well separated from the normal epithelium, hyporefractile nests with atypical cells, lack of palisading, and dark holes. One study described RCM parameters as cords without palisading, dark holes, prominent vascularization, and abundant stroma—findings that were positively associated with poroma in a univariate analysis. These findings assist in distinguishing poromas from other conditions in the differential diagnosis.9

There is a substantial overlap in clinical appearance with malignant conditions, including basal cell carcinoma, squamous cell carcinoma, cutaneous metastases, and Paget disease; therefore, the use of dermoscopy and RCM may be helpful in the diagnosis and recognition of specific features, as well as the corresponding patterns of poroma. Poromas commonly display vascularized features due to the variability of dermoscopic patterns of eccrine poroma, and further studies are required to establish the specificity of vascularized features.
Acral lesions are more likely to show the classic clinical features of erythema and exophytic growth. A case of a collision tumor with the verrucous changes of poroma, seborrheic keratosis, and viral wart has been described.10 The verrucous changes may lead to misdiagnosis as plantar warts or other neoplasms. Clinicians also should consider conditions that are induced by friction or trauma. In our patient, dermoscopy and RCM aided in the diagnosis of eccrine poroma due to the interference of prominent overlying verrucous changes.
Treatment of poroma is optional. Deeper lesions can be treated with surgical excision, and superficial lesions may be treated with electrosurgical destruction. Our patient was treated with surgical excision followed by repair of the surgical defect with a double V-Y flap.
- Goldman P, Pinkus H, Rogin JR. Eccrine poroma; tumors exhibiting features of the epidermal sweat duct unit. AMA Arch Derm. 1956; 74:511-521.
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma [published online January 28, 2022]. Dermatopathology (Basel). 2022;9:36-47. doi:10.3390/dermatopathology9010007
- Ahmed Jan N, Masood S. Poroma. StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK560909/
- Casper DJ, Glass LF, Shenefelt PD. An unusually large eccrine poroma: a case report and review of the literature. Cutis. 2011; 88:227-229.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053-1061.
- Betti R, Bombonato C, Cerri A, et al. Unusual sites for poromas are not very unusual: a survey of 101 cases. Clin Exp Dermatol. 2014; 39:119-122.
- Marchetti MA, Marino ML, Virmani P, et al. Dermoscopic features and patterns of poromas: a multicenter observational case-control study conducted by the International Dermoscopy Society (IDS). J Eur Acad Dermatol Venereol. 2018;32:1263-1271.
- Brugués A, Gamboa M, Alós L, et al. The challenging diagnosis of eccrine poromas. J Am Acad Dermatol. 2016;74:E113-E115.
- Di Tullio F, Mandel VD, Ignazio S, et al. The role of reflectance confocal microscopy in the diagnosis of eccrine poroma: a retrospective casecontrol study. Exp Dermatol. 2022;31:1779-1790.
- Bloom BS, Kamino H, Hale CS, et al. Collision tumor of eccrine poroma, seborrheic keratosis, and a viral wart. Dermatol Online J. 2014;20:13030/qt8tm0r9b9.
- Goldman P, Pinkus H, Rogin JR. Eccrine poroma; tumors exhibiting features of the epidermal sweat duct unit. AMA Arch Derm. 1956; 74:511-521.
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma [published online January 28, 2022]. Dermatopathology (Basel). 2022;9:36-47. doi:10.3390/dermatopathology9010007
- Ahmed Jan N, Masood S. Poroma. StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK560909/
- Casper DJ, Glass LF, Shenefelt PD. An unusually large eccrine poroma: a case report and review of the literature. Cutis. 2011; 88:227-229.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053-1061.
- Betti R, Bombonato C, Cerri A, et al. Unusual sites for poromas are not very unusual: a survey of 101 cases. Clin Exp Dermatol. 2014; 39:119-122.
- Marchetti MA, Marino ML, Virmani P, et al. Dermoscopic features and patterns of poromas: a multicenter observational case-control study conducted by the International Dermoscopy Society (IDS). J Eur Acad Dermatol Venereol. 2018;32:1263-1271.
- Brugués A, Gamboa M, Alós L, et al. The challenging diagnosis of eccrine poromas. J Am Acad Dermatol. 2016;74:E113-E115.
- Di Tullio F, Mandel VD, Ignazio S, et al. The role of reflectance confocal microscopy in the diagnosis of eccrine poroma: a retrospective casecontrol study. Exp Dermatol. 2022;31:1779-1790.
- Bloom BS, Kamino H, Hale CS, et al. Collision tumor of eccrine poroma, seborrheic keratosis, and a viral wart. Dermatol Online J. 2014;20:13030/qt8tm0r9b9.
A 62-year-old man presented with an enlarging plaque on the foot of 3 years’ duration. He experienced minor pain while walking but reported no other symptoms. His family history was negative for similar anomalies, and his medical history was negative for the presence of malignant tumors. Physical examination revealed a 2-mm erythematous plaque on the plantar aspect of the right foot with prominent overlying verrucous changes and no ulceration or regional lymphadenopathy. Dermoscopy and reflectance confocal microscopy of the lesion were performed along with a histopathologic examination after complete surgical excision.
Elnahal to AVAHO: PACT Act Can Transform, Expand Veteran Care
CHICAGO – The US Department of Veterans Affairs (VA) top medical officer told the Association of VA Hematology/Oncology (AVAHO) oncology members that they are at the forefront of the agency’s push to expand care for veterans who may have been injured by exposure to hazardous materials on the job.
“All of you are playing a critical role in implementing the PACT Act, the signature legislative achievement of the Biden administration,” said Shereef Elnahal, MD, MBA, the VA Under Secretary for Health, in a keynote address at the 2023 annual meeting of AVAHO. “But more importantly, if we do our jobs right, it could be the largest expansion of veterans’ benefits in the history of this country. That requires us to have the capacity to deliver care to so many more individuals.”
The VA has provided more than 4.1 million free toxic exposure screenings to veterans since President Biden signed the PACT Act (The Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics Act) in August 2022. The legislation prioritizes claims for cancer, terminal illnesses, and homelessness, and the White House says it has allowed the Veterans Health Administration and the Veterans Benefits Administration to grow at the fastest rates in 2 decades.
“Almost every type of solid tumor is now considered a presumptive condition associated with burden of exposure to veterans deployed anywhere in Central Command, either in the Persian Gulf War or the post-9/11 conflicts,” said Under Secretary Elnahal, who was confirmed in his job by the Senate in July 2022.
Implementing the PACT Act “requires all of us to make investments and further strengthen our system’s care for oncology,” he said. It is also crucial, to reduce “leakage into the community,” referring to veterans who leave the VA for private care. “I know for a fact that the care that veterans get when they have oncology services available in our direct-care system within VA is better. That's not a contention. That is proven by different peer-reviewed studies over the years. And I think that comparison is only intensifying when it comes to how much better evidence-based care our veterans receive at the hands of all of you across the country.”
Elnahal highlighted the development of a “2-way” cancer registry that will allow the National Institute and the VA to exchange cancer diagnosis and treatment data with state registries. “This will give the VA access to critical data in a complete way—to what veterans have experienced, especially veterans who are getting parts of their care in one place and parts of their care in a different place.”
On the data front, he also noted that “the PACT Act also requires us to research the future and determine the next set of presumptive conditions that are related to the hazards of serving our country. It requires that we have robust data sets to be able to gain those insights.”
More globally, Elnahal said the VA can play a crucial role in the Cancer Moonshot Program: “We can win the race, and VA can contribute asymmetrically to that race, to make cancer a chronic condition.”
He highlighted efforts within the VA to battle cancer such as programs to reduce disparities, boost cancer screening, treat rural veterans via a national teleoncology service, and implement the Close to Me program to bring infusion services to veterans in isolated regions.
But Elnahal’s presentation was not entirely rosy. He warned that 40% of veterans are being served outside the VA. “That's sort of a rule-of-thumb threshold when you start looking more like a payer than a provider.”
He also noted that while the VA hired 54,000 people in just the past year—6.2% growth—it takes a long time to bring workers on board. “That’s why I'm holding every single leader in our system accountable for reducing onboarding times by at least a month,” he said. The AVAHO audience enthusiastically applauded.
CHICAGO – The US Department of Veterans Affairs (VA) top medical officer told the Association of VA Hematology/Oncology (AVAHO) oncology members that they are at the forefront of the agency’s push to expand care for veterans who may have been injured by exposure to hazardous materials on the job.
“All of you are playing a critical role in implementing the PACT Act, the signature legislative achievement of the Biden administration,” said Shereef Elnahal, MD, MBA, the VA Under Secretary for Health, in a keynote address at the 2023 annual meeting of AVAHO. “But more importantly, if we do our jobs right, it could be the largest expansion of veterans’ benefits in the history of this country. That requires us to have the capacity to deliver care to so many more individuals.”
The VA has provided more than 4.1 million free toxic exposure screenings to veterans since President Biden signed the PACT Act (The Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics Act) in August 2022. The legislation prioritizes claims for cancer, terminal illnesses, and homelessness, and the White House says it has allowed the Veterans Health Administration and the Veterans Benefits Administration to grow at the fastest rates in 2 decades.
“Almost every type of solid tumor is now considered a presumptive condition associated with burden of exposure to veterans deployed anywhere in Central Command, either in the Persian Gulf War or the post-9/11 conflicts,” said Under Secretary Elnahal, who was confirmed in his job by the Senate in July 2022.
Implementing the PACT Act “requires all of us to make investments and further strengthen our system’s care for oncology,” he said. It is also crucial, to reduce “leakage into the community,” referring to veterans who leave the VA for private care. “I know for a fact that the care that veterans get when they have oncology services available in our direct-care system within VA is better. That's not a contention. That is proven by different peer-reviewed studies over the years. And I think that comparison is only intensifying when it comes to how much better evidence-based care our veterans receive at the hands of all of you across the country.”
Elnahal highlighted the development of a “2-way” cancer registry that will allow the National Institute and the VA to exchange cancer diagnosis and treatment data with state registries. “This will give the VA access to critical data in a complete way—to what veterans have experienced, especially veterans who are getting parts of their care in one place and parts of their care in a different place.”
On the data front, he also noted that “the PACT Act also requires us to research the future and determine the next set of presumptive conditions that are related to the hazards of serving our country. It requires that we have robust data sets to be able to gain those insights.”
More globally, Elnahal said the VA can play a crucial role in the Cancer Moonshot Program: “We can win the race, and VA can contribute asymmetrically to that race, to make cancer a chronic condition.”
He highlighted efforts within the VA to battle cancer such as programs to reduce disparities, boost cancer screening, treat rural veterans via a national teleoncology service, and implement the Close to Me program to bring infusion services to veterans in isolated regions.
But Elnahal’s presentation was not entirely rosy. He warned that 40% of veterans are being served outside the VA. “That's sort of a rule-of-thumb threshold when you start looking more like a payer than a provider.”
He also noted that while the VA hired 54,000 people in just the past year—6.2% growth—it takes a long time to bring workers on board. “That’s why I'm holding every single leader in our system accountable for reducing onboarding times by at least a month,” he said. The AVAHO audience enthusiastically applauded.
CHICAGO – The US Department of Veterans Affairs (VA) top medical officer told the Association of VA Hematology/Oncology (AVAHO) oncology members that they are at the forefront of the agency’s push to expand care for veterans who may have been injured by exposure to hazardous materials on the job.
“All of you are playing a critical role in implementing the PACT Act, the signature legislative achievement of the Biden administration,” said Shereef Elnahal, MD, MBA, the VA Under Secretary for Health, in a keynote address at the 2023 annual meeting of AVAHO. “But more importantly, if we do our jobs right, it could be the largest expansion of veterans’ benefits in the history of this country. That requires us to have the capacity to deliver care to so many more individuals.”
The VA has provided more than 4.1 million free toxic exposure screenings to veterans since President Biden signed the PACT Act (The Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics Act) in August 2022. The legislation prioritizes claims for cancer, terminal illnesses, and homelessness, and the White House says it has allowed the Veterans Health Administration and the Veterans Benefits Administration to grow at the fastest rates in 2 decades.
“Almost every type of solid tumor is now considered a presumptive condition associated with burden of exposure to veterans deployed anywhere in Central Command, either in the Persian Gulf War or the post-9/11 conflicts,” said Under Secretary Elnahal, who was confirmed in his job by the Senate in July 2022.
Implementing the PACT Act “requires all of us to make investments and further strengthen our system’s care for oncology,” he said. It is also crucial, to reduce “leakage into the community,” referring to veterans who leave the VA for private care. “I know for a fact that the care that veterans get when they have oncology services available in our direct-care system within VA is better. That's not a contention. That is proven by different peer-reviewed studies over the years. And I think that comparison is only intensifying when it comes to how much better evidence-based care our veterans receive at the hands of all of you across the country.”
Elnahal highlighted the development of a “2-way” cancer registry that will allow the National Institute and the VA to exchange cancer diagnosis and treatment data with state registries. “This will give the VA access to critical data in a complete way—to what veterans have experienced, especially veterans who are getting parts of their care in one place and parts of their care in a different place.”
On the data front, he also noted that “the PACT Act also requires us to research the future and determine the next set of presumptive conditions that are related to the hazards of serving our country. It requires that we have robust data sets to be able to gain those insights.”
More globally, Elnahal said the VA can play a crucial role in the Cancer Moonshot Program: “We can win the race, and VA can contribute asymmetrically to that race, to make cancer a chronic condition.”
He highlighted efforts within the VA to battle cancer such as programs to reduce disparities, boost cancer screening, treat rural veterans via a national teleoncology service, and implement the Close to Me program to bring infusion services to veterans in isolated regions.
But Elnahal’s presentation was not entirely rosy. He warned that 40% of veterans are being served outside the VA. “That's sort of a rule-of-thumb threshold when you start looking more like a payer than a provider.”
He also noted that while the VA hired 54,000 people in just the past year—6.2% growth—it takes a long time to bring workers on board. “That’s why I'm holding every single leader in our system accountable for reducing onboarding times by at least a month,” he said. The AVAHO audience enthusiastically applauded.
Tapering lupus drugs in stable patients: Large study outlines risks, benefits
The question looms large for patients with stable systemic lupus erythematosus (SLE): to taper or not to taper corticosteroids or immunosuppressive therapy? For patients and the physicians treating them, the evidence points in both directions. Flares are exacerbated by tapering, but simultaneously organ damage is tempered. Where is the balance? What competing factors together inform decision-making?
A recent multinational, observational cohort study conducted by Jiacai Cho, MBBS, of National University Hospital, Singapore, and colleagues, and published in The Lancet Rheumatology concluded that, given the odds of excess flares associated with tapering of corticosteroids and immunosuppressive therapy in patients with stable SLE, drug tapering warrants careful consideration of risks and benefits and is best reserved for those in complete clinical and serological remission with stable disease for at least 6 months. However, in an accompanying editorial, Yann Nguyen, MD, MPH, and Nathalie Costedoat-Chalumeau, MD, PhD, of the National Referral Center for Rare Autoimmune and Systemic Diseases at Cochin Hospital, Paris, and the Center for Research in Epidemiology and Statistics at Paris City University, argued for tipping the scale back from some of those expressed cautions.
In interviews, experts in the field expressed both strong appreciation for the cohort study and, like the editorialists, cognizance of its limitations.
Dr. Cho and colleagues recruited 3,002 adult patients with SLE (92.2% female, median age 39.5 years), from 25 sites across 13 Asia-Pacific countries. They were receiving routine clinical care and had achieved stable disease in at least one of two or more visits. Stable disease was defined by meeting criteria for Lupus Low Disease Activity State (LLDAS; SLE Disease Activity Index 2000 [SLEDAI-2K] score ≤ 4, Physician Global Assessment [PGA] ≤ 1, and prednisolone ≤ 7.5 mg/day), the 2021 DORIS definition of remission (clinical SLEDAI-2K score 0, PGA score < 0.5, and prednisolone dose ≤ 5 mg/day), or DORIS complete remission on therapy (SLEDAI-2K score 0, PGA score < 0.5, and prednisolone dose ≤ 5 mg/day). Any decrease in dose of corticosteroids or immunosuppressive therapy (mycophenolate mofetil, calcineurin inhibitors, azathioprine, leflunomide, or methotrexate) defined tapering. The investigators compared the odds of disease flares (SELENA-SLEDAI Flare Index) at the visit following tapering among those with tapering versus those who had continued the same drug doses.
Higher odds of flare with tapering
Tapering, compared with continuing with the same dose, was clearly associated with higher odds of flare at the next visit (11.4% with continuing vs. 17.0% with tapering; odds ratio, 1.24; 95% confidence interval, 1.10-1.39; P = .0005). Flares among patients who tapered were also slightly more often severe than with continuing the same dose (21.5% of flares vs. 19.7%). The level of remission at the time of tapering also mattered. Of 2,095 continuous tapering attempts, 860 (41.1%) were initiated in LLDAS, 596 (28.4%) in remission, and 639 (30.5%) in complete remission. Tapering when in LLDAS or remission, compared with complete remission, was associated with a higher likelihood of flare by 1 year (LLDAS: OR, 1.37; 95% CI, 1.03-1.81; P = .029; and remission: OR, 1.45; 95% CI, 1.08-1.94; P = .013). Time to first flare followed the same pattern. Also, sustained LLDAS, remission, or complete remission for at least 6 months just before the time of taper was associated with lower odds of flare at next visit and flares in 1 year, and longer time to flare.
Take baseline disease status, hydroxychloroquine’s effect into account
Dr. Nguyen and Dr. Costedoat-Chalumeau underscored several factors that may soften the risk for flares seen with tapering. They pointed to higher baseline doses of prednisone and immunosuppressants (and thus likely more severe disease that is more likely to flare) in the patients with tapering. Also, the SELENA-SLEDAI Flare Index used in the study classifies some clinically insignificant flares as mild to moderate and ignores the benefit of tapering. (It classifies patients as having a severe flare even when starting a new immunosuppressant prescription, such as azathioprine, methotrexate, or both, in an effort to reduce corticosteroid use.) They wrote that the study did not assess the rate of clinically meaningful flares (“essentially renal flares”), nor did it highlight that the “tiny” increase in absolute risk of severe flares (from 2.2% to 3.7%) could be further contextualized by the offset of the smaller, unmeasured rate of clinically significant flares and the “extremely relevant” risk of concomitant damage from prolonged treatment.
Dr. Nguyen and Dr. Costedoat-Chalumeau urged hydroxychloroquine use for all patients unless clearly contraindicated. In their own research, they have detailed hydroxychloroquine benefits in reducing not only flare risk, but also comorbidities, damage, and mortality. In the current study, the prevalence of hydroxychloroquine use in all the patient visits was only 63.3%. “We can assume that if more patients had been treated with hydroxychloroquine, both the number of flares and the difference between the two strategies would have been lower,” they wrote. They cited findings from a study of patients in remission for 2 years or longer in the Toronto Lupus Cohort in which a gradual taper of corticosteroids over 1 year was safe and feasible and resulted in less damage accrual at 24 months than not tapering. Optimizing tapering can minimize flare risk, they concluded.
Tapering SLE medications always involves some chance of flare and has to be considered a calculated risk, Sasha Bernatsky, MD, the James McGill professor of medicine in the division of rheumatology at McGill University, Montreal, said in an interview. “Long-term prednisone is not good for patients. I have heard it called ‘the miracle drug from hell’ – meaning that, yes, it controls disease, but at a cost of long-term complications. So we must be conscientious about tapering prednisone.” She observed that in the short-term, there may not be a huge risk to keeping a patient on an antimalarial and counseling patients to stay on it because their risk of flare is higher if they taper. Rheumatologists usually agree, however, that after 10 years or more, there is a real chance of retinal toxicity. “In our Montreal cohort, the risk of retinal toxicity was 5% after an average of 12.8 years of antimalarial use. My concern is that if a patient develops SLE in their 20s, how do we decide if we should keep them on an antimalarial for the next 60 or 70 years? If we keep them on the drug from age 25 to 45, and they then get retinal toxicity, they would essentially never be able to be on the drug again. So I do try to keep patients on the lowest dose of an antimalarial that is possible.”
Dr. Bernatsky pointed out further, “We think about tapering other immunosuppressants (such as methotrexate or mycophenolate or azathioprine) quite differently than prednisone tapering. We take our time a bit more, since many patients will tolerate being on standard doses of these drugs fairly well. If or when we do consider tapering these drugs, both our intuition and the literature suggests that someone with worse baseline disease activity or severity, who has needed a lot of steroids and multiple combinations of drugs to control disease, has a higher chance of flaring than someone with milder disease. As the editorial points out, lupus physicians (and their patients) need to think carefully about the patient’s risk profile, and be sure to tailor follow-up based on flare risk.”
Frank discussions with patients about the risks of tapering are needed, she said. “On one hand, there is consensus about how some aspects of lupus should be managed (for example, aggressive treatment of severe nephritis), but on the other hand, when it comes to long-term management and especially discussing tapering, we must have good discussions with patients. When a patient asks if they can taper a drug – many just lower or stop their drugs without asking – I am as honest as I can be, but ultimately have to admit any taper could be associated with a flare. It’s helpful to have actual figures to discuss with patients.”
No surprises
“This is an interesting study, which did not produce any surprises,” Dafna D. Gladman, MD, professor of medicine at University of Toronto and senior scientist at the university’s Schroeder Arthritis Institute, said when asked to comment. “We already knew from previous studies that abrupt withdrawal is not a good idea, and that if you taper when a patient is under conditions of remission, the rate of flare is actually lower than the usual rate of flare that occurs in people who continue on these medications. But the major limitation is that they did not specifically look at those who we would taper in clinical practice. In addition, they do not specify that the patients had to be on low-dose glucocorticoids before tapering, and they combined both immunosuppressive and steroids. It is not clear from the study what the excess flare rate was, or whether the flares were mild or severe. Most flares in patients with SLE are mild, consisting of skin and joint manifestations, while only a few patients have flares in kidney or neurologic manifestations.”
Dr. Gladman described her approach to tapering: “We aim for our patients to be taking no more than 5 mg of prednisone and to be in at least clinical remission with a SLEDAI-2K of 0 for at least 2 years before we would taper to glucocorticoids withdrawal. We always withdraw glucocorticoids first and immunosuppressives later, and keep patients on antimalarials the longest, unless there are specific side effects to the immunosuppressive or antimalarials which require their cessation earlier.”
Uncertainty persists
Other SLE experts weighing in confirmed the view that future research should aim to achieve clarity about the relative risks and benefits of tapering SLE drug regimens to maintain disease remission while minimizing potential for organ damage.
“Steroids are our friend and our enemy,” Joan T. Merrill, MD, professor of medicine at the University of Oklahoma Health Sciences Center, Oklahoma City, said in an interview. “If a person with lupus is in a lot of trouble, corticosteroids are almost universally a good option to get them out. But for too many decades, for too many patients, despite all the improvements we have made in better understanding the disease and developing some promising new treatments, we have yet to shed the inexorable toxicity in multiple organs of steroid dependence.” She continued, “Corticosteroids, even at low dose, may have broad-spectrum effects. But, in fact, so do many of the more ‘targeted’ agents. If all patients were lined up at the beginning of a study while being given azathioprine or a calcineurin inhibitor or belimumab at a stable, tolerable dose, you might see the same data if you tapered that agent down. What we really need is improved individualized guidance about when and how fast to remove immune modulators from stable patients with lupus without disturbing the balance that had been achieved in such a quiescent patient.”
That enduring uncertainty was echoed by Daniel J. Wallace, MD, professor of medicine at Cedars-Sinai Medical Center, Los Angeles: “The take-home message from this interesting paper,” he commented, “is that current lupus biomarkers are not adequate. They do not guide the practitioner well enough, so that all too often medication regimens are tapered even though the risks are not really well known. Also, there is evidence in the literature that fibrosis and ‘damage’ progress even if acute phase reactants such as sedimentation rate, [C-reactive protein], complement 3 and 4, and anti-dsDNA are normal. We don’t have a good metric to detect them.”
Dr. Cho and colleagues’ study was funded by AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen, Merck Serono, GlaxoSmithKline, and UCB. Dr. Gladman disclosed consulting and/or research support from AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly, Janssen, Novartis, Pfizer, and UCB.
The question looms large for patients with stable systemic lupus erythematosus (SLE): to taper or not to taper corticosteroids or immunosuppressive therapy? For patients and the physicians treating them, the evidence points in both directions. Flares are exacerbated by tapering, but simultaneously organ damage is tempered. Where is the balance? What competing factors together inform decision-making?
A recent multinational, observational cohort study conducted by Jiacai Cho, MBBS, of National University Hospital, Singapore, and colleagues, and published in The Lancet Rheumatology concluded that, given the odds of excess flares associated with tapering of corticosteroids and immunosuppressive therapy in patients with stable SLE, drug tapering warrants careful consideration of risks and benefits and is best reserved for those in complete clinical and serological remission with stable disease for at least 6 months. However, in an accompanying editorial, Yann Nguyen, MD, MPH, and Nathalie Costedoat-Chalumeau, MD, PhD, of the National Referral Center for Rare Autoimmune and Systemic Diseases at Cochin Hospital, Paris, and the Center for Research in Epidemiology and Statistics at Paris City University, argued for tipping the scale back from some of those expressed cautions.
In interviews, experts in the field expressed both strong appreciation for the cohort study and, like the editorialists, cognizance of its limitations.
Dr. Cho and colleagues recruited 3,002 adult patients with SLE (92.2% female, median age 39.5 years), from 25 sites across 13 Asia-Pacific countries. They were receiving routine clinical care and had achieved stable disease in at least one of two or more visits. Stable disease was defined by meeting criteria for Lupus Low Disease Activity State (LLDAS; SLE Disease Activity Index 2000 [SLEDAI-2K] score ≤ 4, Physician Global Assessment [PGA] ≤ 1, and prednisolone ≤ 7.5 mg/day), the 2021 DORIS definition of remission (clinical SLEDAI-2K score 0, PGA score < 0.5, and prednisolone dose ≤ 5 mg/day), or DORIS complete remission on therapy (SLEDAI-2K score 0, PGA score < 0.5, and prednisolone dose ≤ 5 mg/day). Any decrease in dose of corticosteroids or immunosuppressive therapy (mycophenolate mofetil, calcineurin inhibitors, azathioprine, leflunomide, or methotrexate) defined tapering. The investigators compared the odds of disease flares (SELENA-SLEDAI Flare Index) at the visit following tapering among those with tapering versus those who had continued the same drug doses.
Higher odds of flare with tapering
Tapering, compared with continuing with the same dose, was clearly associated with higher odds of flare at the next visit (11.4% with continuing vs. 17.0% with tapering; odds ratio, 1.24; 95% confidence interval, 1.10-1.39; P = .0005). Flares among patients who tapered were also slightly more often severe than with continuing the same dose (21.5% of flares vs. 19.7%). The level of remission at the time of tapering also mattered. Of 2,095 continuous tapering attempts, 860 (41.1%) were initiated in LLDAS, 596 (28.4%) in remission, and 639 (30.5%) in complete remission. Tapering when in LLDAS or remission, compared with complete remission, was associated with a higher likelihood of flare by 1 year (LLDAS: OR, 1.37; 95% CI, 1.03-1.81; P = .029; and remission: OR, 1.45; 95% CI, 1.08-1.94; P = .013). Time to first flare followed the same pattern. Also, sustained LLDAS, remission, or complete remission for at least 6 months just before the time of taper was associated with lower odds of flare at next visit and flares in 1 year, and longer time to flare.
Take baseline disease status, hydroxychloroquine’s effect into account
Dr. Nguyen and Dr. Costedoat-Chalumeau underscored several factors that may soften the risk for flares seen with tapering. They pointed to higher baseline doses of prednisone and immunosuppressants (and thus likely more severe disease that is more likely to flare) in the patients with tapering. Also, the SELENA-SLEDAI Flare Index used in the study classifies some clinically insignificant flares as mild to moderate and ignores the benefit of tapering. (It classifies patients as having a severe flare even when starting a new immunosuppressant prescription, such as azathioprine, methotrexate, or both, in an effort to reduce corticosteroid use.) They wrote that the study did not assess the rate of clinically meaningful flares (“essentially renal flares”), nor did it highlight that the “tiny” increase in absolute risk of severe flares (from 2.2% to 3.7%) could be further contextualized by the offset of the smaller, unmeasured rate of clinically significant flares and the “extremely relevant” risk of concomitant damage from prolonged treatment.
Dr. Nguyen and Dr. Costedoat-Chalumeau urged hydroxychloroquine use for all patients unless clearly contraindicated. In their own research, they have detailed hydroxychloroquine benefits in reducing not only flare risk, but also comorbidities, damage, and mortality. In the current study, the prevalence of hydroxychloroquine use in all the patient visits was only 63.3%. “We can assume that if more patients had been treated with hydroxychloroquine, both the number of flares and the difference between the two strategies would have been lower,” they wrote. They cited findings from a study of patients in remission for 2 years or longer in the Toronto Lupus Cohort in which a gradual taper of corticosteroids over 1 year was safe and feasible and resulted in less damage accrual at 24 months than not tapering. Optimizing tapering can minimize flare risk, they concluded.
Tapering SLE medications always involves some chance of flare and has to be considered a calculated risk, Sasha Bernatsky, MD, the James McGill professor of medicine in the division of rheumatology at McGill University, Montreal, said in an interview. “Long-term prednisone is not good for patients. I have heard it called ‘the miracle drug from hell’ – meaning that, yes, it controls disease, but at a cost of long-term complications. So we must be conscientious about tapering prednisone.” She observed that in the short-term, there may not be a huge risk to keeping a patient on an antimalarial and counseling patients to stay on it because their risk of flare is higher if they taper. Rheumatologists usually agree, however, that after 10 years or more, there is a real chance of retinal toxicity. “In our Montreal cohort, the risk of retinal toxicity was 5% after an average of 12.8 years of antimalarial use. My concern is that if a patient develops SLE in their 20s, how do we decide if we should keep them on an antimalarial for the next 60 or 70 years? If we keep them on the drug from age 25 to 45, and they then get retinal toxicity, they would essentially never be able to be on the drug again. So I do try to keep patients on the lowest dose of an antimalarial that is possible.”
Dr. Bernatsky pointed out further, “We think about tapering other immunosuppressants (such as methotrexate or mycophenolate or azathioprine) quite differently than prednisone tapering. We take our time a bit more, since many patients will tolerate being on standard doses of these drugs fairly well. If or when we do consider tapering these drugs, both our intuition and the literature suggests that someone with worse baseline disease activity or severity, who has needed a lot of steroids and multiple combinations of drugs to control disease, has a higher chance of flaring than someone with milder disease. As the editorial points out, lupus physicians (and their patients) need to think carefully about the patient’s risk profile, and be sure to tailor follow-up based on flare risk.”
Frank discussions with patients about the risks of tapering are needed, she said. “On one hand, there is consensus about how some aspects of lupus should be managed (for example, aggressive treatment of severe nephritis), but on the other hand, when it comes to long-term management and especially discussing tapering, we must have good discussions with patients. When a patient asks if they can taper a drug – many just lower or stop their drugs without asking – I am as honest as I can be, but ultimately have to admit any taper could be associated with a flare. It’s helpful to have actual figures to discuss with patients.”
No surprises
“This is an interesting study, which did not produce any surprises,” Dafna D. Gladman, MD, professor of medicine at University of Toronto and senior scientist at the university’s Schroeder Arthritis Institute, said when asked to comment. “We already knew from previous studies that abrupt withdrawal is not a good idea, and that if you taper when a patient is under conditions of remission, the rate of flare is actually lower than the usual rate of flare that occurs in people who continue on these medications. But the major limitation is that they did not specifically look at those who we would taper in clinical practice. In addition, they do not specify that the patients had to be on low-dose glucocorticoids before tapering, and they combined both immunosuppressive and steroids. It is not clear from the study what the excess flare rate was, or whether the flares were mild or severe. Most flares in patients with SLE are mild, consisting of skin and joint manifestations, while only a few patients have flares in kidney or neurologic manifestations.”
Dr. Gladman described her approach to tapering: “We aim for our patients to be taking no more than 5 mg of prednisone and to be in at least clinical remission with a SLEDAI-2K of 0 for at least 2 years before we would taper to glucocorticoids withdrawal. We always withdraw glucocorticoids first and immunosuppressives later, and keep patients on antimalarials the longest, unless there are specific side effects to the immunosuppressive or antimalarials which require their cessation earlier.”
Uncertainty persists
Other SLE experts weighing in confirmed the view that future research should aim to achieve clarity about the relative risks and benefits of tapering SLE drug regimens to maintain disease remission while minimizing potential for organ damage.
“Steroids are our friend and our enemy,” Joan T. Merrill, MD, professor of medicine at the University of Oklahoma Health Sciences Center, Oklahoma City, said in an interview. “If a person with lupus is in a lot of trouble, corticosteroids are almost universally a good option to get them out. But for too many decades, for too many patients, despite all the improvements we have made in better understanding the disease and developing some promising new treatments, we have yet to shed the inexorable toxicity in multiple organs of steroid dependence.” She continued, “Corticosteroids, even at low dose, may have broad-spectrum effects. But, in fact, so do many of the more ‘targeted’ agents. If all patients were lined up at the beginning of a study while being given azathioprine or a calcineurin inhibitor or belimumab at a stable, tolerable dose, you might see the same data if you tapered that agent down. What we really need is improved individualized guidance about when and how fast to remove immune modulators from stable patients with lupus without disturbing the balance that had been achieved in such a quiescent patient.”
That enduring uncertainty was echoed by Daniel J. Wallace, MD, professor of medicine at Cedars-Sinai Medical Center, Los Angeles: “The take-home message from this interesting paper,” he commented, “is that current lupus biomarkers are not adequate. They do not guide the practitioner well enough, so that all too often medication regimens are tapered even though the risks are not really well known. Also, there is evidence in the literature that fibrosis and ‘damage’ progress even if acute phase reactants such as sedimentation rate, [C-reactive protein], complement 3 and 4, and anti-dsDNA are normal. We don’t have a good metric to detect them.”
Dr. Cho and colleagues’ study was funded by AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen, Merck Serono, GlaxoSmithKline, and UCB. Dr. Gladman disclosed consulting and/or research support from AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly, Janssen, Novartis, Pfizer, and UCB.
The question looms large for patients with stable systemic lupus erythematosus (SLE): to taper or not to taper corticosteroids or immunosuppressive therapy? For patients and the physicians treating them, the evidence points in both directions. Flares are exacerbated by tapering, but simultaneously organ damage is tempered. Where is the balance? What competing factors together inform decision-making?
A recent multinational, observational cohort study conducted by Jiacai Cho, MBBS, of National University Hospital, Singapore, and colleagues, and published in The Lancet Rheumatology concluded that, given the odds of excess flares associated with tapering of corticosteroids and immunosuppressive therapy in patients with stable SLE, drug tapering warrants careful consideration of risks and benefits and is best reserved for those in complete clinical and serological remission with stable disease for at least 6 months. However, in an accompanying editorial, Yann Nguyen, MD, MPH, and Nathalie Costedoat-Chalumeau, MD, PhD, of the National Referral Center for Rare Autoimmune and Systemic Diseases at Cochin Hospital, Paris, and the Center for Research in Epidemiology and Statistics at Paris City University, argued for tipping the scale back from some of those expressed cautions.
In interviews, experts in the field expressed both strong appreciation for the cohort study and, like the editorialists, cognizance of its limitations.
Dr. Cho and colleagues recruited 3,002 adult patients with SLE (92.2% female, median age 39.5 years), from 25 sites across 13 Asia-Pacific countries. They were receiving routine clinical care and had achieved stable disease in at least one of two or more visits. Stable disease was defined by meeting criteria for Lupus Low Disease Activity State (LLDAS; SLE Disease Activity Index 2000 [SLEDAI-2K] score ≤ 4, Physician Global Assessment [PGA] ≤ 1, and prednisolone ≤ 7.5 mg/day), the 2021 DORIS definition of remission (clinical SLEDAI-2K score 0, PGA score < 0.5, and prednisolone dose ≤ 5 mg/day), or DORIS complete remission on therapy (SLEDAI-2K score 0, PGA score < 0.5, and prednisolone dose ≤ 5 mg/day). Any decrease in dose of corticosteroids or immunosuppressive therapy (mycophenolate mofetil, calcineurin inhibitors, azathioprine, leflunomide, or methotrexate) defined tapering. The investigators compared the odds of disease flares (SELENA-SLEDAI Flare Index) at the visit following tapering among those with tapering versus those who had continued the same drug doses.
Higher odds of flare with tapering
Tapering, compared with continuing with the same dose, was clearly associated with higher odds of flare at the next visit (11.4% with continuing vs. 17.0% with tapering; odds ratio, 1.24; 95% confidence interval, 1.10-1.39; P = .0005). Flares among patients who tapered were also slightly more often severe than with continuing the same dose (21.5% of flares vs. 19.7%). The level of remission at the time of tapering also mattered. Of 2,095 continuous tapering attempts, 860 (41.1%) were initiated in LLDAS, 596 (28.4%) in remission, and 639 (30.5%) in complete remission. Tapering when in LLDAS or remission, compared with complete remission, was associated with a higher likelihood of flare by 1 year (LLDAS: OR, 1.37; 95% CI, 1.03-1.81; P = .029; and remission: OR, 1.45; 95% CI, 1.08-1.94; P = .013). Time to first flare followed the same pattern. Also, sustained LLDAS, remission, or complete remission for at least 6 months just before the time of taper was associated with lower odds of flare at next visit and flares in 1 year, and longer time to flare.
Take baseline disease status, hydroxychloroquine’s effect into account
Dr. Nguyen and Dr. Costedoat-Chalumeau underscored several factors that may soften the risk for flares seen with tapering. They pointed to higher baseline doses of prednisone and immunosuppressants (and thus likely more severe disease that is more likely to flare) in the patients with tapering. Also, the SELENA-SLEDAI Flare Index used in the study classifies some clinically insignificant flares as mild to moderate and ignores the benefit of tapering. (It classifies patients as having a severe flare even when starting a new immunosuppressant prescription, such as azathioprine, methotrexate, or both, in an effort to reduce corticosteroid use.) They wrote that the study did not assess the rate of clinically meaningful flares (“essentially renal flares”), nor did it highlight that the “tiny” increase in absolute risk of severe flares (from 2.2% to 3.7%) could be further contextualized by the offset of the smaller, unmeasured rate of clinically significant flares and the “extremely relevant” risk of concomitant damage from prolonged treatment.
Dr. Nguyen and Dr. Costedoat-Chalumeau urged hydroxychloroquine use for all patients unless clearly contraindicated. In their own research, they have detailed hydroxychloroquine benefits in reducing not only flare risk, but also comorbidities, damage, and mortality. In the current study, the prevalence of hydroxychloroquine use in all the patient visits was only 63.3%. “We can assume that if more patients had been treated with hydroxychloroquine, both the number of flares and the difference between the two strategies would have been lower,” they wrote. They cited findings from a study of patients in remission for 2 years or longer in the Toronto Lupus Cohort in which a gradual taper of corticosteroids over 1 year was safe and feasible and resulted in less damage accrual at 24 months than not tapering. Optimizing tapering can minimize flare risk, they concluded.
Tapering SLE medications always involves some chance of flare and has to be considered a calculated risk, Sasha Bernatsky, MD, the James McGill professor of medicine in the division of rheumatology at McGill University, Montreal, said in an interview. “Long-term prednisone is not good for patients. I have heard it called ‘the miracle drug from hell’ – meaning that, yes, it controls disease, but at a cost of long-term complications. So we must be conscientious about tapering prednisone.” She observed that in the short-term, there may not be a huge risk to keeping a patient on an antimalarial and counseling patients to stay on it because their risk of flare is higher if they taper. Rheumatologists usually agree, however, that after 10 years or more, there is a real chance of retinal toxicity. “In our Montreal cohort, the risk of retinal toxicity was 5% after an average of 12.8 years of antimalarial use. My concern is that if a patient develops SLE in their 20s, how do we decide if we should keep them on an antimalarial for the next 60 or 70 years? If we keep them on the drug from age 25 to 45, and they then get retinal toxicity, they would essentially never be able to be on the drug again. So I do try to keep patients on the lowest dose of an antimalarial that is possible.”
Dr. Bernatsky pointed out further, “We think about tapering other immunosuppressants (such as methotrexate or mycophenolate or azathioprine) quite differently than prednisone tapering. We take our time a bit more, since many patients will tolerate being on standard doses of these drugs fairly well. If or when we do consider tapering these drugs, both our intuition and the literature suggests that someone with worse baseline disease activity or severity, who has needed a lot of steroids and multiple combinations of drugs to control disease, has a higher chance of flaring than someone with milder disease. As the editorial points out, lupus physicians (and their patients) need to think carefully about the patient’s risk profile, and be sure to tailor follow-up based on flare risk.”
Frank discussions with patients about the risks of tapering are needed, she said. “On one hand, there is consensus about how some aspects of lupus should be managed (for example, aggressive treatment of severe nephritis), but on the other hand, when it comes to long-term management and especially discussing tapering, we must have good discussions with patients. When a patient asks if they can taper a drug – many just lower or stop their drugs without asking – I am as honest as I can be, but ultimately have to admit any taper could be associated with a flare. It’s helpful to have actual figures to discuss with patients.”
No surprises
“This is an interesting study, which did not produce any surprises,” Dafna D. Gladman, MD, professor of medicine at University of Toronto and senior scientist at the university’s Schroeder Arthritis Institute, said when asked to comment. “We already knew from previous studies that abrupt withdrawal is not a good idea, and that if you taper when a patient is under conditions of remission, the rate of flare is actually lower than the usual rate of flare that occurs in people who continue on these medications. But the major limitation is that they did not specifically look at those who we would taper in clinical practice. In addition, they do not specify that the patients had to be on low-dose glucocorticoids before tapering, and they combined both immunosuppressive and steroids. It is not clear from the study what the excess flare rate was, or whether the flares were mild or severe. Most flares in patients with SLE are mild, consisting of skin and joint manifestations, while only a few patients have flares in kidney or neurologic manifestations.”
Dr. Gladman described her approach to tapering: “We aim for our patients to be taking no more than 5 mg of prednisone and to be in at least clinical remission with a SLEDAI-2K of 0 for at least 2 years before we would taper to glucocorticoids withdrawal. We always withdraw glucocorticoids first and immunosuppressives later, and keep patients on antimalarials the longest, unless there are specific side effects to the immunosuppressive or antimalarials which require their cessation earlier.”
Uncertainty persists
Other SLE experts weighing in confirmed the view that future research should aim to achieve clarity about the relative risks and benefits of tapering SLE drug regimens to maintain disease remission while minimizing potential for organ damage.
“Steroids are our friend and our enemy,” Joan T. Merrill, MD, professor of medicine at the University of Oklahoma Health Sciences Center, Oklahoma City, said in an interview. “If a person with lupus is in a lot of trouble, corticosteroids are almost universally a good option to get them out. But for too many decades, for too many patients, despite all the improvements we have made in better understanding the disease and developing some promising new treatments, we have yet to shed the inexorable toxicity in multiple organs of steroid dependence.” She continued, “Corticosteroids, even at low dose, may have broad-spectrum effects. But, in fact, so do many of the more ‘targeted’ agents. If all patients were lined up at the beginning of a study while being given azathioprine or a calcineurin inhibitor or belimumab at a stable, tolerable dose, you might see the same data if you tapered that agent down. What we really need is improved individualized guidance about when and how fast to remove immune modulators from stable patients with lupus without disturbing the balance that had been achieved in such a quiescent patient.”
That enduring uncertainty was echoed by Daniel J. Wallace, MD, professor of medicine at Cedars-Sinai Medical Center, Los Angeles: “The take-home message from this interesting paper,” he commented, “is that current lupus biomarkers are not adequate. They do not guide the practitioner well enough, so that all too often medication regimens are tapered even though the risks are not really well known. Also, there is evidence in the literature that fibrosis and ‘damage’ progress even if acute phase reactants such as sedimentation rate, [C-reactive protein], complement 3 and 4, and anti-dsDNA are normal. We don’t have a good metric to detect them.”
Dr. Cho and colleagues’ study was funded by AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen, Merck Serono, GlaxoSmithKline, and UCB. Dr. Gladman disclosed consulting and/or research support from AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly, Janssen, Novartis, Pfizer, and UCB.
FROM THE LANCET RHEUMATOLOGY
Menstruation linked to underdiagnosis of type 2 diabetes?
The analysis estimates that an additional 17% of undiagnosed women younger than 50 years could be reclassified as having T2D, and that women under 50 had an A1c distribution that was markedly lower than that of men under 50, by a mean of 1.6 mmol/mol.
In a study that will be presented at this year’s annual meeting of the European Association for the Study of Diabetes (EASD), the researchers wanted to investigate whether a contributing factor to late diagnosis of T2D in women under 50 may be the difference in A1c levels due to hemoglobin replacement linked to menstrual blood loss.
The study was published online in Diabetes Therapy. “If the threshold for diagnosis of diabetes ... was lowered by 2 mmol/mol in women under the age of 50, an additional 17% of these women (approximately equivalent to 35,000 women in England and Wales) would be diagnosed with diabetes ... which may contribute to up to 64% of the difference in mortality rates between men/women with diabetes mellitus aged 16-50 years,” the researchers noted.
They added that A1c levels in women under 50 years were found to be consistently lower than those in men, and with A1c levels in women reaching the equivalent of those in men up to 10 years later, this “may result in delayed diagnosis of diabetes mellitus in premenopausal women.”
Noting that the study was observational, senior author Adrian Heald, MD, consultant endocrinologist, Salford (England) Royal NHS Foundation Trust, said that it “may be the case that prediabetes and type 2 diabetes in women are not being spotted because the set point needs to be slightly lower, but a systematic study sampling from the population of at-risk individuals is needed further to our findings.
“We also need to refer back to use of the glucose tolerance test, because A1c has been used for the past 15 years but it is not the gold standard,” added Dr. Heald. “Clinicians have often wondered if patients might be missed with A1c measurement, or even overdiagnosed.”
Lucy Chambers, PhD, from Diabetes UK, acknowledged that the research was valuable but added: “More research on sex differences in thresholds for a type 2 diagnosis is needed to inform any changes to clinical practice. In the meantime, we encourage clinicians to follow the current guidance of not ruling out type 2 diabetes based on a one-off A1c below the diagnostic threshold.”
But in support of greater understanding around the sex differences in A1c diagnostic thresholds, Dr. Chambers added: “Receiving an accurate and timely diagnosis ensures that women get the treatment and support needed to manage their type 2 diabetes and avoid long-term complications, including heart disease, where sex-based inequalities in care already contribute to poorer outcomes for women.”
Effect of A1c reference range on T2D diagnosis and associated CVD
Compared with men, women with T2D have poorer glycemic control; a higher risk for cardiovascular (CV) complications; reduced life expectancy (5.3 years shorter vs. 4.5 years shorter); and a higher risk factor burden, such as obesity and hypertension at diagnosis.
In addition, T2D is a stronger risk factor for CV disease (CVD) in women than in men, and those aged 35-59 years who receive a diagnosis have the highest relative CV death risk across all age and sex groups.
The researchers pointed out that previous studies have observed differences in A1c relative to menopause, and they too found that “A1c levels rose after the age of 50 in women.”
However, they noted that the implication of differing A1c reference ranges on delayed diabetes diagnosis with worsening CV risk profile had not been previously recognized and that their study “[h]ighlights for the first time that, while 1.6 mmol/mol may appear only a small difference in terms of laboratory measurement, at population level this has implications for significant number of premenopausal women.”
The researchers initially observed the trend in local data in Salford, in the northwest of England. “These ... data highlighted that women seemed to be diagnosed with type 2 diabetes at an older age, so we wanted to examine what the source of that might be,” study author Mike Stedman, BSc, director, Res Consortium, Andover, England, said in an interview.
Dr. Stedman and his colleagues assessed the sex and age differences of A1c in individuals who had not been diagnosed with diabetes (A1c ≤ 48 mmol/mol [≤ 6.5%]). “We looked at data from other labs [in addition to those in Salford, totaling 938,678 people] to see if this was a local phenomenon. They could only provide more recent data, but these also showed a similar pattern,” he added.
Finally, Dr. Stedman, Dr. Heald, and their colleagues estimated the possible national impact by extrapolating findings based on population data from the UK Office of National Statistics and on National Diabetes Audit data for type 2 diabetes prevalence and related excess mortality. This brought them to the conclusion that T2D would be diagnosed in an additional 17% of women if the threshold were lowered by 2 mmol/mol, to 46 mmol/mol, in women under 50 years.
Lower A1c in women under 50 may delay T2D diagnosis by up to 10 years
The analysis found that the median A1c increased with age, with values in women younger than 50 years consistently being 1 mmol/mol lower than values in men. In contrast, A1c values in women over 50 years were equivalent to those in men.
However, at age 50 years, compared with men, A1c in women was found to lag by approximately 5 years. Women under 50 had an A1c distribution that was lower than that of men by an average of 1.6 mmol/mol (4.7% of mean; P < .0001), whereas this difference in individuals aged 50 years or older was less pronounced (P < .0001).
The authors wrote that “an undermeasurement of approximately 1.6 mmol/mol A1c in women may delay their diabetes ... diagnosis by up to 10 years.”
Further analysis showed that, at an A1c of 48 mmol/mol, 50% fewer women than men under the age of 50 could be diagnosed with T2D, whereas only 20% fewer women than men aged 50 years or older could be diagnosed with T2D.
Lowering the A1c threshold for diagnosis of T2D from 48 mmol/mol to 46 mmol/mol in women under 50 led to an estimate that an additional 35,345 undiagnosed women in England could be reclassified as having a T2D diagnosis.
The authors pointed out that “gender difference in adverse cardiovascular risk factors are known to be present prior to the development of [type 2] diabetes” and that “once diagnosed, atherosclerotic CVD prevalence is twice as high in patients with diabetes ... compared to those without a diagnosis.”
Dr. Heald added that there is always the possibility that other factors might be at play and that the work posed questions rather than presented answers.
Taking a pragmatic view, the researchers suggested that “one alternative approach may be to offer further assessment using fasting plasma glucose or oral glucose tolerance testing in those with A1c values of 46 or 47 mmol/mol.”
“In anyone with an early diagnosis of type 2 diabetes, in addition to dietary modification and especially if there is cardiovascular risk, then one might start them on metformin due to the cardiovascular benefits as well as the sugar-lowering effects,” said Dr. Heald, adding that “we certainly don’t want women missing out on metformin that could have huge benefits in the longer term.”
Dr. Stedman and Dr. Heald declared no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work. Dr. Chambers has declared no conflicts.
A version of this article appeared on Medscape.com.
The analysis estimates that an additional 17% of undiagnosed women younger than 50 years could be reclassified as having T2D, and that women under 50 had an A1c distribution that was markedly lower than that of men under 50, by a mean of 1.6 mmol/mol.
In a study that will be presented at this year’s annual meeting of the European Association for the Study of Diabetes (EASD), the researchers wanted to investigate whether a contributing factor to late diagnosis of T2D in women under 50 may be the difference in A1c levels due to hemoglobin replacement linked to menstrual blood loss.
The study was published online in Diabetes Therapy. “If the threshold for diagnosis of diabetes ... was lowered by 2 mmol/mol in women under the age of 50, an additional 17% of these women (approximately equivalent to 35,000 women in England and Wales) would be diagnosed with diabetes ... which may contribute to up to 64% of the difference in mortality rates between men/women with diabetes mellitus aged 16-50 years,” the researchers noted.
They added that A1c levels in women under 50 years were found to be consistently lower than those in men, and with A1c levels in women reaching the equivalent of those in men up to 10 years later, this “may result in delayed diagnosis of diabetes mellitus in premenopausal women.”
Noting that the study was observational, senior author Adrian Heald, MD, consultant endocrinologist, Salford (England) Royal NHS Foundation Trust, said that it “may be the case that prediabetes and type 2 diabetes in women are not being spotted because the set point needs to be slightly lower, but a systematic study sampling from the population of at-risk individuals is needed further to our findings.
“We also need to refer back to use of the glucose tolerance test, because A1c has been used for the past 15 years but it is not the gold standard,” added Dr. Heald. “Clinicians have often wondered if patients might be missed with A1c measurement, or even overdiagnosed.”
Lucy Chambers, PhD, from Diabetes UK, acknowledged that the research was valuable but added: “More research on sex differences in thresholds for a type 2 diagnosis is needed to inform any changes to clinical practice. In the meantime, we encourage clinicians to follow the current guidance of not ruling out type 2 diabetes based on a one-off A1c below the diagnostic threshold.”
But in support of greater understanding around the sex differences in A1c diagnostic thresholds, Dr. Chambers added: “Receiving an accurate and timely diagnosis ensures that women get the treatment and support needed to manage their type 2 diabetes and avoid long-term complications, including heart disease, where sex-based inequalities in care already contribute to poorer outcomes for women.”
Effect of A1c reference range on T2D diagnosis and associated CVD
Compared with men, women with T2D have poorer glycemic control; a higher risk for cardiovascular (CV) complications; reduced life expectancy (5.3 years shorter vs. 4.5 years shorter); and a higher risk factor burden, such as obesity and hypertension at diagnosis.
In addition, T2D is a stronger risk factor for CV disease (CVD) in women than in men, and those aged 35-59 years who receive a diagnosis have the highest relative CV death risk across all age and sex groups.
The researchers pointed out that previous studies have observed differences in A1c relative to menopause, and they too found that “A1c levels rose after the age of 50 in women.”
However, they noted that the implication of differing A1c reference ranges on delayed diabetes diagnosis with worsening CV risk profile had not been previously recognized and that their study “[h]ighlights for the first time that, while 1.6 mmol/mol may appear only a small difference in terms of laboratory measurement, at population level this has implications for significant number of premenopausal women.”
The researchers initially observed the trend in local data in Salford, in the northwest of England. “These ... data highlighted that women seemed to be diagnosed with type 2 diabetes at an older age, so we wanted to examine what the source of that might be,” study author Mike Stedman, BSc, director, Res Consortium, Andover, England, said in an interview.
Dr. Stedman and his colleagues assessed the sex and age differences of A1c in individuals who had not been diagnosed with diabetes (A1c ≤ 48 mmol/mol [≤ 6.5%]). “We looked at data from other labs [in addition to those in Salford, totaling 938,678 people] to see if this was a local phenomenon. They could only provide more recent data, but these also showed a similar pattern,” he added.
Finally, Dr. Stedman, Dr. Heald, and their colleagues estimated the possible national impact by extrapolating findings based on population data from the UK Office of National Statistics and on National Diabetes Audit data for type 2 diabetes prevalence and related excess mortality. This brought them to the conclusion that T2D would be diagnosed in an additional 17% of women if the threshold were lowered by 2 mmol/mol, to 46 mmol/mol, in women under 50 years.
Lower A1c in women under 50 may delay T2D diagnosis by up to 10 years
The analysis found that the median A1c increased with age, with values in women younger than 50 years consistently being 1 mmol/mol lower than values in men. In contrast, A1c values in women over 50 years were equivalent to those in men.
However, at age 50 years, compared with men, A1c in women was found to lag by approximately 5 years. Women under 50 had an A1c distribution that was lower than that of men by an average of 1.6 mmol/mol (4.7% of mean; P < .0001), whereas this difference in individuals aged 50 years or older was less pronounced (P < .0001).
The authors wrote that “an undermeasurement of approximately 1.6 mmol/mol A1c in women may delay their diabetes ... diagnosis by up to 10 years.”
Further analysis showed that, at an A1c of 48 mmol/mol, 50% fewer women than men under the age of 50 could be diagnosed with T2D, whereas only 20% fewer women than men aged 50 years or older could be diagnosed with T2D.
Lowering the A1c threshold for diagnosis of T2D from 48 mmol/mol to 46 mmol/mol in women under 50 led to an estimate that an additional 35,345 undiagnosed women in England could be reclassified as having a T2D diagnosis.
The authors pointed out that “gender difference in adverse cardiovascular risk factors are known to be present prior to the development of [type 2] diabetes” and that “once diagnosed, atherosclerotic CVD prevalence is twice as high in patients with diabetes ... compared to those without a diagnosis.”
Dr. Heald added that there is always the possibility that other factors might be at play and that the work posed questions rather than presented answers.
Taking a pragmatic view, the researchers suggested that “one alternative approach may be to offer further assessment using fasting plasma glucose or oral glucose tolerance testing in those with A1c values of 46 or 47 mmol/mol.”
“In anyone with an early diagnosis of type 2 diabetes, in addition to dietary modification and especially if there is cardiovascular risk, then one might start them on metformin due to the cardiovascular benefits as well as the sugar-lowering effects,” said Dr. Heald, adding that “we certainly don’t want women missing out on metformin that could have huge benefits in the longer term.”
Dr. Stedman and Dr. Heald declared no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work. Dr. Chambers has declared no conflicts.
A version of this article appeared on Medscape.com.
The analysis estimates that an additional 17% of undiagnosed women younger than 50 years could be reclassified as having T2D, and that women under 50 had an A1c distribution that was markedly lower than that of men under 50, by a mean of 1.6 mmol/mol.
In a study that will be presented at this year’s annual meeting of the European Association for the Study of Diabetes (EASD), the researchers wanted to investigate whether a contributing factor to late diagnosis of T2D in women under 50 may be the difference in A1c levels due to hemoglobin replacement linked to menstrual blood loss.
The study was published online in Diabetes Therapy. “If the threshold for diagnosis of diabetes ... was lowered by 2 mmol/mol in women under the age of 50, an additional 17% of these women (approximately equivalent to 35,000 women in England and Wales) would be diagnosed with diabetes ... which may contribute to up to 64% of the difference in mortality rates between men/women with diabetes mellitus aged 16-50 years,” the researchers noted.
They added that A1c levels in women under 50 years were found to be consistently lower than those in men, and with A1c levels in women reaching the equivalent of those in men up to 10 years later, this “may result in delayed diagnosis of diabetes mellitus in premenopausal women.”
Noting that the study was observational, senior author Adrian Heald, MD, consultant endocrinologist, Salford (England) Royal NHS Foundation Trust, said that it “may be the case that prediabetes and type 2 diabetes in women are not being spotted because the set point needs to be slightly lower, but a systematic study sampling from the population of at-risk individuals is needed further to our findings.
“We also need to refer back to use of the glucose tolerance test, because A1c has been used for the past 15 years but it is not the gold standard,” added Dr. Heald. “Clinicians have often wondered if patients might be missed with A1c measurement, or even overdiagnosed.”
Lucy Chambers, PhD, from Diabetes UK, acknowledged that the research was valuable but added: “More research on sex differences in thresholds for a type 2 diagnosis is needed to inform any changes to clinical practice. In the meantime, we encourage clinicians to follow the current guidance of not ruling out type 2 diabetes based on a one-off A1c below the diagnostic threshold.”
But in support of greater understanding around the sex differences in A1c diagnostic thresholds, Dr. Chambers added: “Receiving an accurate and timely diagnosis ensures that women get the treatment and support needed to manage their type 2 diabetes and avoid long-term complications, including heart disease, where sex-based inequalities in care already contribute to poorer outcomes for women.”
Effect of A1c reference range on T2D diagnosis and associated CVD
Compared with men, women with T2D have poorer glycemic control; a higher risk for cardiovascular (CV) complications; reduced life expectancy (5.3 years shorter vs. 4.5 years shorter); and a higher risk factor burden, such as obesity and hypertension at diagnosis.
In addition, T2D is a stronger risk factor for CV disease (CVD) in women than in men, and those aged 35-59 years who receive a diagnosis have the highest relative CV death risk across all age and sex groups.
The researchers pointed out that previous studies have observed differences in A1c relative to menopause, and they too found that “A1c levels rose after the age of 50 in women.”
However, they noted that the implication of differing A1c reference ranges on delayed diabetes diagnosis with worsening CV risk profile had not been previously recognized and that their study “[h]ighlights for the first time that, while 1.6 mmol/mol may appear only a small difference in terms of laboratory measurement, at population level this has implications for significant number of premenopausal women.”
The researchers initially observed the trend in local data in Salford, in the northwest of England. “These ... data highlighted that women seemed to be diagnosed with type 2 diabetes at an older age, so we wanted to examine what the source of that might be,” study author Mike Stedman, BSc, director, Res Consortium, Andover, England, said in an interview.
Dr. Stedman and his colleagues assessed the sex and age differences of A1c in individuals who had not been diagnosed with diabetes (A1c ≤ 48 mmol/mol [≤ 6.5%]). “We looked at data from other labs [in addition to those in Salford, totaling 938,678 people] to see if this was a local phenomenon. They could only provide more recent data, but these also showed a similar pattern,” he added.
Finally, Dr. Stedman, Dr. Heald, and their colleagues estimated the possible national impact by extrapolating findings based on population data from the UK Office of National Statistics and on National Diabetes Audit data for type 2 diabetes prevalence and related excess mortality. This brought them to the conclusion that T2D would be diagnosed in an additional 17% of women if the threshold were lowered by 2 mmol/mol, to 46 mmol/mol, in women under 50 years.
Lower A1c in women under 50 may delay T2D diagnosis by up to 10 years
The analysis found that the median A1c increased with age, with values in women younger than 50 years consistently being 1 mmol/mol lower than values in men. In contrast, A1c values in women over 50 years were equivalent to those in men.
However, at age 50 years, compared with men, A1c in women was found to lag by approximately 5 years. Women under 50 had an A1c distribution that was lower than that of men by an average of 1.6 mmol/mol (4.7% of mean; P < .0001), whereas this difference in individuals aged 50 years or older was less pronounced (P < .0001).
The authors wrote that “an undermeasurement of approximately 1.6 mmol/mol A1c in women may delay their diabetes ... diagnosis by up to 10 years.”
Further analysis showed that, at an A1c of 48 mmol/mol, 50% fewer women than men under the age of 50 could be diagnosed with T2D, whereas only 20% fewer women than men aged 50 years or older could be diagnosed with T2D.
Lowering the A1c threshold for diagnosis of T2D from 48 mmol/mol to 46 mmol/mol in women under 50 led to an estimate that an additional 35,345 undiagnosed women in England could be reclassified as having a T2D diagnosis.
The authors pointed out that “gender difference in adverse cardiovascular risk factors are known to be present prior to the development of [type 2] diabetes” and that “once diagnosed, atherosclerotic CVD prevalence is twice as high in patients with diabetes ... compared to those without a diagnosis.”
Dr. Heald added that there is always the possibility that other factors might be at play and that the work posed questions rather than presented answers.
Taking a pragmatic view, the researchers suggested that “one alternative approach may be to offer further assessment using fasting plasma glucose or oral glucose tolerance testing in those with A1c values of 46 or 47 mmol/mol.”
“In anyone with an early diagnosis of type 2 diabetes, in addition to dietary modification and especially if there is cardiovascular risk, then one might start them on metformin due to the cardiovascular benefits as well as the sugar-lowering effects,” said Dr. Heald, adding that “we certainly don’t want women missing out on metformin that could have huge benefits in the longer term.”
Dr. Stedman and Dr. Heald declared no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work. Dr. Chambers has declared no conflicts.
A version of this article appeared on Medscape.com.
FROM EASD 2023
Can zoo poo help manage diabetic foot ulcers?
In a striking convergence of veterinary biology and medical science, researchers from the University of Sheffield (England) have unveiled findings that could potentially advance the treatment of diabetic foot ulcers, a condition affecting an estimated 18.6 million people worldwide. The unexpected ingredient in this potentially transformative therapy? Feces from endangered species, sourced from Yorkshire Wildlife Park, Doncaster, England.
The scourge of antibiotic resistance
Diabetic foot ulcers are a significant challenge in health care, not only because of their prevalence but also because of the alarming rise of antibiotic-resistant bacterial infections. Current antibiotic treatments frequently fail, leading to life-altering consequences like amputations and significant health care costs – estimated at one-third of the total direct costs of diabetes care. The critical need for alternative therapies has propelled scientists into a pressing search for novel antimicrobial agents.
A pioneering approach: zoo poo as bioactive goldmine
Led by Professor Graham Stafford, chair of molecular microbiology at the University of Sheffield, the research team began to explore a rather unorthodox resource: the fecal matter of endangered animals like Guinea baboons, lemurs, and Visayan pigs. While such a source might seem surprising at first glance, the rationale becomes clear when considering the nature of bacteriophages.
What are bacteriophages?
Bacteriophages, commonly known as phages, are viruses that selectively target and kill bacteria. Despite being the most prevalent biological entities on Earth, their therapeutic potential has remained largely untapped. What makes bacteriophages particularly interesting is their ability to kill antibiotic-resistant bacteria – a feature making them prime candidates for treating otherwise unmanageable diabetic foot ulcers. (Armstrong DG, et al; Fish R, et al).
Findings and future directions
Professor Stafford and his team discovered that the feces of several endangered animals harbored bacteriophages capable of killing bacterial strains resistant to antibiotics. The findings not only hold promise for a groundbreaking treatment but also provide another compelling reason to conserve endangered species: Their inherent biodiversity might contain cures for a range of infectious diseases.
While research is ongoing and clinical trials have not yet begun, the preliminary results are overwhelmingly promising.
We often look to complex technologies and synthetic materials for medical science breakthroughs, yet sometimes the most innovative solutions can be found in the most overlooked places. In this case, the feces of endangered species could turn out to be a vital asset in battling antibiotic resistance, thus affecting diabetic foot care in ways we never imagined possible.
The research conducted at the University of Sheffield also serves as a powerful argument for a One Health approach – a multidisciplinary field focusing on the interconnectedness of human, animal, and environmental health.
This intriguing work reaffirms the need for an interdisciplinary approach in tackling the world’s pressing health care challenges. The collaborative efforts between the University of Sheffield and Yorkshire Wildlife Park exemplify how academic research and conservation can come together to yield solutions for some of the most devastating and costly health conditions, while also underscoring the invaluable role that biodiversity plays in our collective well-being. Here’s to teaming up to act against amputation worldwide.
Dr. Armstrong is professor of surgery and director of limb preservation at University of Southern California, Los Angeles. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
In a striking convergence of veterinary biology and medical science, researchers from the University of Sheffield (England) have unveiled findings that could potentially advance the treatment of diabetic foot ulcers, a condition affecting an estimated 18.6 million people worldwide. The unexpected ingredient in this potentially transformative therapy? Feces from endangered species, sourced from Yorkshire Wildlife Park, Doncaster, England.
The scourge of antibiotic resistance
Diabetic foot ulcers are a significant challenge in health care, not only because of their prevalence but also because of the alarming rise of antibiotic-resistant bacterial infections. Current antibiotic treatments frequently fail, leading to life-altering consequences like amputations and significant health care costs – estimated at one-third of the total direct costs of diabetes care. The critical need for alternative therapies has propelled scientists into a pressing search for novel antimicrobial agents.
A pioneering approach: zoo poo as bioactive goldmine
Led by Professor Graham Stafford, chair of molecular microbiology at the University of Sheffield, the research team began to explore a rather unorthodox resource: the fecal matter of endangered animals like Guinea baboons, lemurs, and Visayan pigs. While such a source might seem surprising at first glance, the rationale becomes clear when considering the nature of bacteriophages.
What are bacteriophages?
Bacteriophages, commonly known as phages, are viruses that selectively target and kill bacteria. Despite being the most prevalent biological entities on Earth, their therapeutic potential has remained largely untapped. What makes bacteriophages particularly interesting is their ability to kill antibiotic-resistant bacteria – a feature making them prime candidates for treating otherwise unmanageable diabetic foot ulcers. (Armstrong DG, et al; Fish R, et al).
Findings and future directions
Professor Stafford and his team discovered that the feces of several endangered animals harbored bacteriophages capable of killing bacterial strains resistant to antibiotics. The findings not only hold promise for a groundbreaking treatment but also provide another compelling reason to conserve endangered species: Their inherent biodiversity might contain cures for a range of infectious diseases.
While research is ongoing and clinical trials have not yet begun, the preliminary results are overwhelmingly promising.
We often look to complex technologies and synthetic materials for medical science breakthroughs, yet sometimes the most innovative solutions can be found in the most overlooked places. In this case, the feces of endangered species could turn out to be a vital asset in battling antibiotic resistance, thus affecting diabetic foot care in ways we never imagined possible.
The research conducted at the University of Sheffield also serves as a powerful argument for a One Health approach – a multidisciplinary field focusing on the interconnectedness of human, animal, and environmental health.
This intriguing work reaffirms the need for an interdisciplinary approach in tackling the world’s pressing health care challenges. The collaborative efforts between the University of Sheffield and Yorkshire Wildlife Park exemplify how academic research and conservation can come together to yield solutions for some of the most devastating and costly health conditions, while also underscoring the invaluable role that biodiversity plays in our collective well-being. Here’s to teaming up to act against amputation worldwide.
Dr. Armstrong is professor of surgery and director of limb preservation at University of Southern California, Los Angeles. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
In a striking convergence of veterinary biology and medical science, researchers from the University of Sheffield (England) have unveiled findings that could potentially advance the treatment of diabetic foot ulcers, a condition affecting an estimated 18.6 million people worldwide. The unexpected ingredient in this potentially transformative therapy? Feces from endangered species, sourced from Yorkshire Wildlife Park, Doncaster, England.
The scourge of antibiotic resistance
Diabetic foot ulcers are a significant challenge in health care, not only because of their prevalence but also because of the alarming rise of antibiotic-resistant bacterial infections. Current antibiotic treatments frequently fail, leading to life-altering consequences like amputations and significant health care costs – estimated at one-third of the total direct costs of diabetes care. The critical need for alternative therapies has propelled scientists into a pressing search for novel antimicrobial agents.
A pioneering approach: zoo poo as bioactive goldmine
Led by Professor Graham Stafford, chair of molecular microbiology at the University of Sheffield, the research team began to explore a rather unorthodox resource: the fecal matter of endangered animals like Guinea baboons, lemurs, and Visayan pigs. While such a source might seem surprising at first glance, the rationale becomes clear when considering the nature of bacteriophages.
What are bacteriophages?
Bacteriophages, commonly known as phages, are viruses that selectively target and kill bacteria. Despite being the most prevalent biological entities on Earth, their therapeutic potential has remained largely untapped. What makes bacteriophages particularly interesting is their ability to kill antibiotic-resistant bacteria – a feature making them prime candidates for treating otherwise unmanageable diabetic foot ulcers. (Armstrong DG, et al; Fish R, et al).
Findings and future directions
Professor Stafford and his team discovered that the feces of several endangered animals harbored bacteriophages capable of killing bacterial strains resistant to antibiotics. The findings not only hold promise for a groundbreaking treatment but also provide another compelling reason to conserve endangered species: Their inherent biodiversity might contain cures for a range of infectious diseases.
While research is ongoing and clinical trials have not yet begun, the preliminary results are overwhelmingly promising.
We often look to complex technologies and synthetic materials for medical science breakthroughs, yet sometimes the most innovative solutions can be found in the most overlooked places. In this case, the feces of endangered species could turn out to be a vital asset in battling antibiotic resistance, thus affecting diabetic foot care in ways we never imagined possible.
The research conducted at the University of Sheffield also serves as a powerful argument for a One Health approach – a multidisciplinary field focusing on the interconnectedness of human, animal, and environmental health.
This intriguing work reaffirms the need for an interdisciplinary approach in tackling the world’s pressing health care challenges. The collaborative efforts between the University of Sheffield and Yorkshire Wildlife Park exemplify how academic research and conservation can come together to yield solutions for some of the most devastating and costly health conditions, while also underscoring the invaluable role that biodiversity plays in our collective well-being. Here’s to teaming up to act against amputation worldwide.
Dr. Armstrong is professor of surgery and director of limb preservation at University of Southern California, Los Angeles. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
FDA issues letter regarding lebrikizumab review for atopic dermatitis
The Food and Drug Administration has issued a complete response letter regarding lebrikizumab, an investigational biologic for the treatment of adult and adolescent patients with moderate to severe atopic dermatitis, describing concerns about findings made during an inspection of a third-party contract manufacturer that included the “monoclonal antibody drug substance” for lebrikizumab, Eli Lilly announced in an Oct. 2 press release.
Lebrikizumab is under FDA review for treating atopic dermatitis; a complete response letter indicates that the review has been completed, and highlights issues that need to be addressed before a final decision on approval is made.
The press release noted that the agency did not raise any concerns about the clinical data package, safety, or label for lebrikizumab, an investigational, monoclonal antibody that binds to the cytokine interleukin (IL)-13, and is designed to be administered once per month.
In the press release, the company said it would work with the third-party manufacturer and the FDA to address the feedback “in order to make lebrikizumab available to patients.”
The Food and Drug Administration has issued a complete response letter regarding lebrikizumab, an investigational biologic for the treatment of adult and adolescent patients with moderate to severe atopic dermatitis, describing concerns about findings made during an inspection of a third-party contract manufacturer that included the “monoclonal antibody drug substance” for lebrikizumab, Eli Lilly announced in an Oct. 2 press release.
Lebrikizumab is under FDA review for treating atopic dermatitis; a complete response letter indicates that the review has been completed, and highlights issues that need to be addressed before a final decision on approval is made.
The press release noted that the agency did not raise any concerns about the clinical data package, safety, or label for lebrikizumab, an investigational, monoclonal antibody that binds to the cytokine interleukin (IL)-13, and is designed to be administered once per month.
In the press release, the company said it would work with the third-party manufacturer and the FDA to address the feedback “in order to make lebrikizumab available to patients.”
The Food and Drug Administration has issued a complete response letter regarding lebrikizumab, an investigational biologic for the treatment of adult and adolescent patients with moderate to severe atopic dermatitis, describing concerns about findings made during an inspection of a third-party contract manufacturer that included the “monoclonal antibody drug substance” for lebrikizumab, Eli Lilly announced in an Oct. 2 press release.
Lebrikizumab is under FDA review for treating atopic dermatitis; a complete response letter indicates that the review has been completed, and highlights issues that need to be addressed before a final decision on approval is made.
The press release noted that the agency did not raise any concerns about the clinical data package, safety, or label for lebrikizumab, an investigational, monoclonal antibody that binds to the cytokine interleukin (IL)-13, and is designed to be administered once per month.
In the press release, the company said it would work with the third-party manufacturer and the FDA to address the feedback “in order to make lebrikizumab available to patients.”
Measures of PTH predict postthyroidectomy hypocalcemia
according to the results of a prospective study of 60 patients.
Postthyroidectomy hypocalcemia remains a major complication in patients who have undergone total thyroidectomy, and early identification can reduce disease burden and improve outcomes, according to Ahmed Sobhy Youssef, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, and colleagues.
In a presentation at the annual meeting of the American Academy of Otolaryngology-Head and Neck Surgery, Dr. Youssef presented results of the study, which looked at early postoperative parathyroid hormone as a predictor of postthyroidectomy hypocalcemia.
During his fellowship in Oklahoma in the wake of the COVID-19 pandemic, Dr. Youssef observed a wide variation in follow-up for calcium levels after thyroidectomy. “Some surgeons will order PTH and ionized calcium 4 hours after surgery, others would order later, at 6-8 hours,” he said in an interview. However, “all patients would be admitted for 1-2 nights [before being] discharged home, which meant more restrictions on the number of beds allowed for our head and neck cancer service.”
Discussion with his department chair led to a literature review seeking strategies to discharge patients earlier, and Dr. Youssef developed the idea for early PTH testing.
The study population included 60 adults who underwent thyroidectomy for benign or malignant disease at a single center between January 2022 and January 2023. The researchers measured PTH at 1 hour after surgery and compared it to results of a standard postoperative measure at 4 hours after surgery.
The researchers found a significant positive correlation between PTH measured 1 hour after surgery and ionized calcium (Ca) at 4 hours. The sensitivity of the early PTH assay, defined as “measured below 14 pg/ml,” was 100% to detect hypocalcemia, with an area under the curve of 0.797.
“The results were amazing,” said Dr. Youssef. “We found that when we measure PTH as early as 1 hour after total thyroidectomy, while patients are still in recovery, PTH was very sensitive to predict hypocalcemia.” The correlation was strong with measures at 4 hours.
“Our takeaway message is the 1-hour level PTH is very reliable in predicting hypocalcemia,” he added. This measure can serve as a guide for discharging patients the same day, with instructions to return if they develop any symptoms of hypocalcemia.
The use of early PTH also helped to reduce hospital admissions and identified patients who were eligible for same-day discharge with no need for additional replacement medications, Dr. Youssef said.
So far, “we have had no readmissions for thyroidectomy patients since we started to follow this protocol at our institution,” he noted.
The findings were limited by the relatively small sample size, and more research is needed. However, the results suggest that early measurement of PTH at 1 hour after surgery is an accurate predictor of hypocalcemia in total thyroidectomy patients.
“I strongly recommend high thyroidectomy volume institutions apply the same protocol and publish their data about that so we can come up with a consensus/guideline for management of calcium following thyroidectomy,” Dr. Youssef said.
More proof of PTH’s predictive power
“The utility of postoperative PTH for predicting symptomatic hypocalcemia is beneficial for guiding postoperative management of patients following total thyroidectomy,” said Larissa Sweeny, MD, of the University of Miami, who served as a moderator for the session in which the study was presented.
“Proper identification of patients that require supplemental medications following surgery reduces administration of medications to patients that do not require supplemental medications,” Dr. Sweeny said in an interview.
In addition, better identification not only ensures that the patients who do require supplemental medications receive them but also reduces postoperative complications and readmissions, she said.
For clinical practice, the current study “reinforces the utility of postoperative PTH lab values for guiding medication administration following total thyroidectomy,” said Dr. Sweeny. “I have been using postoperative PTH lab values following total thyroidectomy to guide my postoperative management of these patients for over 6 years.”
However, looking ahead to additional research, “Correlation with dosage of supplemental calcium and duration to return of normal PTH would be helpful information,” Dr. Sweeny said.
The study received no outside funding. The researchers and Dr. Sweeny report no relevant financial relationships.
A version of this article appeared on Medscape.com.
according to the results of a prospective study of 60 patients.
Postthyroidectomy hypocalcemia remains a major complication in patients who have undergone total thyroidectomy, and early identification can reduce disease burden and improve outcomes, according to Ahmed Sobhy Youssef, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, and colleagues.
In a presentation at the annual meeting of the American Academy of Otolaryngology-Head and Neck Surgery, Dr. Youssef presented results of the study, which looked at early postoperative parathyroid hormone as a predictor of postthyroidectomy hypocalcemia.
During his fellowship in Oklahoma in the wake of the COVID-19 pandemic, Dr. Youssef observed a wide variation in follow-up for calcium levels after thyroidectomy. “Some surgeons will order PTH and ionized calcium 4 hours after surgery, others would order later, at 6-8 hours,” he said in an interview. However, “all patients would be admitted for 1-2 nights [before being] discharged home, which meant more restrictions on the number of beds allowed for our head and neck cancer service.”
Discussion with his department chair led to a literature review seeking strategies to discharge patients earlier, and Dr. Youssef developed the idea for early PTH testing.
The study population included 60 adults who underwent thyroidectomy for benign or malignant disease at a single center between January 2022 and January 2023. The researchers measured PTH at 1 hour after surgery and compared it to results of a standard postoperative measure at 4 hours after surgery.
The researchers found a significant positive correlation between PTH measured 1 hour after surgery and ionized calcium (Ca) at 4 hours. The sensitivity of the early PTH assay, defined as “measured below 14 pg/ml,” was 100% to detect hypocalcemia, with an area under the curve of 0.797.
“The results were amazing,” said Dr. Youssef. “We found that when we measure PTH as early as 1 hour after total thyroidectomy, while patients are still in recovery, PTH was very sensitive to predict hypocalcemia.” The correlation was strong with measures at 4 hours.
“Our takeaway message is the 1-hour level PTH is very reliable in predicting hypocalcemia,” he added. This measure can serve as a guide for discharging patients the same day, with instructions to return if they develop any symptoms of hypocalcemia.
The use of early PTH also helped to reduce hospital admissions and identified patients who were eligible for same-day discharge with no need for additional replacement medications, Dr. Youssef said.
So far, “we have had no readmissions for thyroidectomy patients since we started to follow this protocol at our institution,” he noted.
The findings were limited by the relatively small sample size, and more research is needed. However, the results suggest that early measurement of PTH at 1 hour after surgery is an accurate predictor of hypocalcemia in total thyroidectomy patients.
“I strongly recommend high thyroidectomy volume institutions apply the same protocol and publish their data about that so we can come up with a consensus/guideline for management of calcium following thyroidectomy,” Dr. Youssef said.
More proof of PTH’s predictive power
“The utility of postoperative PTH for predicting symptomatic hypocalcemia is beneficial for guiding postoperative management of patients following total thyroidectomy,” said Larissa Sweeny, MD, of the University of Miami, who served as a moderator for the session in which the study was presented.
“Proper identification of patients that require supplemental medications following surgery reduces administration of medications to patients that do not require supplemental medications,” Dr. Sweeny said in an interview.
In addition, better identification not only ensures that the patients who do require supplemental medications receive them but also reduces postoperative complications and readmissions, she said.
For clinical practice, the current study “reinforces the utility of postoperative PTH lab values for guiding medication administration following total thyroidectomy,” said Dr. Sweeny. “I have been using postoperative PTH lab values following total thyroidectomy to guide my postoperative management of these patients for over 6 years.”
However, looking ahead to additional research, “Correlation with dosage of supplemental calcium and duration to return of normal PTH would be helpful information,” Dr. Sweeny said.
The study received no outside funding. The researchers and Dr. Sweeny report no relevant financial relationships.
A version of this article appeared on Medscape.com.
according to the results of a prospective study of 60 patients.
Postthyroidectomy hypocalcemia remains a major complication in patients who have undergone total thyroidectomy, and early identification can reduce disease burden and improve outcomes, according to Ahmed Sobhy Youssef, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, and colleagues.
In a presentation at the annual meeting of the American Academy of Otolaryngology-Head and Neck Surgery, Dr. Youssef presented results of the study, which looked at early postoperative parathyroid hormone as a predictor of postthyroidectomy hypocalcemia.
During his fellowship in Oklahoma in the wake of the COVID-19 pandemic, Dr. Youssef observed a wide variation in follow-up for calcium levels after thyroidectomy. “Some surgeons will order PTH and ionized calcium 4 hours after surgery, others would order later, at 6-8 hours,” he said in an interview. However, “all patients would be admitted for 1-2 nights [before being] discharged home, which meant more restrictions on the number of beds allowed for our head and neck cancer service.”
Discussion with his department chair led to a literature review seeking strategies to discharge patients earlier, and Dr. Youssef developed the idea for early PTH testing.
The study population included 60 adults who underwent thyroidectomy for benign or malignant disease at a single center between January 2022 and January 2023. The researchers measured PTH at 1 hour after surgery and compared it to results of a standard postoperative measure at 4 hours after surgery.
The researchers found a significant positive correlation between PTH measured 1 hour after surgery and ionized calcium (Ca) at 4 hours. The sensitivity of the early PTH assay, defined as “measured below 14 pg/ml,” was 100% to detect hypocalcemia, with an area under the curve of 0.797.
“The results were amazing,” said Dr. Youssef. “We found that when we measure PTH as early as 1 hour after total thyroidectomy, while patients are still in recovery, PTH was very sensitive to predict hypocalcemia.” The correlation was strong with measures at 4 hours.
“Our takeaway message is the 1-hour level PTH is very reliable in predicting hypocalcemia,” he added. This measure can serve as a guide for discharging patients the same day, with instructions to return if they develop any symptoms of hypocalcemia.
The use of early PTH also helped to reduce hospital admissions and identified patients who were eligible for same-day discharge with no need for additional replacement medications, Dr. Youssef said.
So far, “we have had no readmissions for thyroidectomy patients since we started to follow this protocol at our institution,” he noted.
The findings were limited by the relatively small sample size, and more research is needed. However, the results suggest that early measurement of PTH at 1 hour after surgery is an accurate predictor of hypocalcemia in total thyroidectomy patients.
“I strongly recommend high thyroidectomy volume institutions apply the same protocol and publish their data about that so we can come up with a consensus/guideline for management of calcium following thyroidectomy,” Dr. Youssef said.
More proof of PTH’s predictive power
“The utility of postoperative PTH for predicting symptomatic hypocalcemia is beneficial for guiding postoperative management of patients following total thyroidectomy,” said Larissa Sweeny, MD, of the University of Miami, who served as a moderator for the session in which the study was presented.
“Proper identification of patients that require supplemental medications following surgery reduces administration of medications to patients that do not require supplemental medications,” Dr. Sweeny said in an interview.
In addition, better identification not only ensures that the patients who do require supplemental medications receive them but also reduces postoperative complications and readmissions, she said.
For clinical practice, the current study “reinforces the utility of postoperative PTH lab values for guiding medication administration following total thyroidectomy,” said Dr. Sweeny. “I have been using postoperative PTH lab values following total thyroidectomy to guide my postoperative management of these patients for over 6 years.”
However, looking ahead to additional research, “Correlation with dosage of supplemental calcium and duration to return of normal PTH would be helpful information,” Dr. Sweeny said.
The study received no outside funding. The researchers and Dr. Sweeny report no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM AAO-HNS ANNUAL MEETING
Hyaluronic acid suppository improves menopause symptoms
TOPLINE:
Among women with genitourinary syndrome of menopause, 12 weeks of treatment with vaginal suppositories containing hyaluronic acid (HLA) reduces vulvovaginal symptoms, according to trial results presented at the annual Menopause Meeting. HLA may be a promising nonhormonal therapy for this condition, the researchers said.
METHODOLOGY:
- Investigators randomly assigned 49 women to receive treatment with a vaginal suppository containing 5 mg of HLA or standard-of-care treatment with vaginal estrogen cream (0.01%).
- The trial was conducted between September 2021 and August 2022.
TAKEAWAY:
- Patients in both treatment arms experienced improvements on the Vulvovaginal Symptom Questionnaire (VSQ), the study’s primary outcome.
- The VSQ assesses vulvovaginal symptoms associated with menopause such as itching, burning, and dryness, as well as the emotional toll of symptoms and their effect on sexual activity.
- Change in VSQ score did not significantly differ between the treatment groups. The measure improved from 5.2 to 1.7 in the group that received estrogen, and from 5.8 to 2.5 in those who received HLA (P = .81).
- No treatment-related severe adverse events were reported.
IN PRACTICE:
“Women often need to decide between different therapies for genitourinary syndrome of menopause,” study author Benjamin Brucker, MD, of New York University said in an interview. “Now we can help counsel them about this formulation of HLA.”
SOURCE:
Poster P-1 was presented at the 2023 meeting of the Menopause Society, held Sept. 27-30 in Philadelphia.
DISCLOSURES:
The study was funded by Bonafide Health, a company that sells supplements to treat menopause symptoms, including vaginal suppositories containing HLA.
A version of this article appeared on Medscape.com.
TOPLINE:
Among women with genitourinary syndrome of menopause, 12 weeks of treatment with vaginal suppositories containing hyaluronic acid (HLA) reduces vulvovaginal symptoms, according to trial results presented at the annual Menopause Meeting. HLA may be a promising nonhormonal therapy for this condition, the researchers said.
METHODOLOGY:
- Investigators randomly assigned 49 women to receive treatment with a vaginal suppository containing 5 mg of HLA or standard-of-care treatment with vaginal estrogen cream (0.01%).
- The trial was conducted between September 2021 and August 2022.
TAKEAWAY:
- Patients in both treatment arms experienced improvements on the Vulvovaginal Symptom Questionnaire (VSQ), the study’s primary outcome.
- The VSQ assesses vulvovaginal symptoms associated with menopause such as itching, burning, and dryness, as well as the emotional toll of symptoms and their effect on sexual activity.
- Change in VSQ score did not significantly differ between the treatment groups. The measure improved from 5.2 to 1.7 in the group that received estrogen, and from 5.8 to 2.5 in those who received HLA (P = .81).
- No treatment-related severe adverse events were reported.
IN PRACTICE:
“Women often need to decide between different therapies for genitourinary syndrome of menopause,” study author Benjamin Brucker, MD, of New York University said in an interview. “Now we can help counsel them about this formulation of HLA.”
SOURCE:
Poster P-1 was presented at the 2023 meeting of the Menopause Society, held Sept. 27-30 in Philadelphia.
DISCLOSURES:
The study was funded by Bonafide Health, a company that sells supplements to treat menopause symptoms, including vaginal suppositories containing HLA.
A version of this article appeared on Medscape.com.
TOPLINE:
Among women with genitourinary syndrome of menopause, 12 weeks of treatment with vaginal suppositories containing hyaluronic acid (HLA) reduces vulvovaginal symptoms, according to trial results presented at the annual Menopause Meeting. HLA may be a promising nonhormonal therapy for this condition, the researchers said.
METHODOLOGY:
- Investigators randomly assigned 49 women to receive treatment with a vaginal suppository containing 5 mg of HLA or standard-of-care treatment with vaginal estrogen cream (0.01%).
- The trial was conducted between September 2021 and August 2022.
TAKEAWAY:
- Patients in both treatment arms experienced improvements on the Vulvovaginal Symptom Questionnaire (VSQ), the study’s primary outcome.
- The VSQ assesses vulvovaginal symptoms associated with menopause such as itching, burning, and dryness, as well as the emotional toll of symptoms and their effect on sexual activity.
- Change in VSQ score did not significantly differ between the treatment groups. The measure improved from 5.2 to 1.7 in the group that received estrogen, and from 5.8 to 2.5 in those who received HLA (P = .81).
- No treatment-related severe adverse events were reported.
IN PRACTICE:
“Women often need to decide between different therapies for genitourinary syndrome of menopause,” study author Benjamin Brucker, MD, of New York University said in an interview. “Now we can help counsel them about this formulation of HLA.”
SOURCE:
Poster P-1 was presented at the 2023 meeting of the Menopause Society, held Sept. 27-30 in Philadelphia.
DISCLOSURES:
The study was funded by Bonafide Health, a company that sells supplements to treat menopause symptoms, including vaginal suppositories containing HLA.
A version of this article appeared on Medscape.com.