User login
Toxicity analysis of docetaxel, cisplatin, and 5- fluorouracil neoadjuvant chemotherapy in Indian patients with head and neck cancers
Background There is a lack of data that systematically address toxicity with docetaxel, cisplatin, and 5-fluorouracil (TPF) regimen in routine care.
Objective To detect, profile, and quantify the toxicity in Indian patients with head and neck cancers who received neoadjuvant TPF chemotherapy in a routine clinical practice (non-trial setting).
Methods 58 patients with locally advanced head and neck cancer who received TPF chemotherapy were selected for this analysis. They received 2 cycles of TPF chemotherapy every 21 days. The patients were monitored for the occurrence of adverse drug reactions in accordance with Common Terminology Criteria for Adverse Events (version 4.03) during the hospitalization (median length of stay in cycle 1, 10 days), daily (at least until day 8 after chemotherapy initiation), then at days 15 and 20. Descriptive statistics was done and factors predicting for toxicity were identified using logistic regression analysis.
Results The cumulative rate of grade ¦3 anemia, neutropenia, and thrombocytopenia were 12.1%, 56.9%, and 5.2%, respectively. The cumulative incidence of febrile neutropenia was 20.7% (12 of 58 patients). The cumulative incidences of mucositis and diarrhea were 67.2% and 74.1%, respectively. There was no mortality associated with induction chemotherapy, and all of the patients completed the planned 2 cycles of TPF. None of the tested factors predicted for any of the adverse events considered in the study.
Limitations Small, single-center study
Conclusion The incidence of TPF-related toxicity in Indian patients in routine practice is high, and the toxicities differ substantially from the toxicities seen in trial settings.
Click on the PDF icon at the top of this introduction to read the full article.
Background There is a lack of data that systematically address toxicity with docetaxel, cisplatin, and 5-fluorouracil (TPF) regimen in routine care.
Objective To detect, profile, and quantify the toxicity in Indian patients with head and neck cancers who received neoadjuvant TPF chemotherapy in a routine clinical practice (non-trial setting).
Methods 58 patients with locally advanced head and neck cancer who received TPF chemotherapy were selected for this analysis. They received 2 cycles of TPF chemotherapy every 21 days. The patients were monitored for the occurrence of adverse drug reactions in accordance with Common Terminology Criteria for Adverse Events (version 4.03) during the hospitalization (median length of stay in cycle 1, 10 days), daily (at least until day 8 after chemotherapy initiation), then at days 15 and 20. Descriptive statistics was done and factors predicting for toxicity were identified using logistic regression analysis.
Results The cumulative rate of grade ¦3 anemia, neutropenia, and thrombocytopenia were 12.1%, 56.9%, and 5.2%, respectively. The cumulative incidence of febrile neutropenia was 20.7% (12 of 58 patients). The cumulative incidences of mucositis and diarrhea were 67.2% and 74.1%, respectively. There was no mortality associated with induction chemotherapy, and all of the patients completed the planned 2 cycles of TPF. None of the tested factors predicted for any of the adverse events considered in the study.
Limitations Small, single-center study
Conclusion The incidence of TPF-related toxicity in Indian patients in routine practice is high, and the toxicities differ substantially from the toxicities seen in trial settings.
Click on the PDF icon at the top of this introduction to read the full article.
Background There is a lack of data that systematically address toxicity with docetaxel, cisplatin, and 5-fluorouracil (TPF) regimen in routine care.
Objective To detect, profile, and quantify the toxicity in Indian patients with head and neck cancers who received neoadjuvant TPF chemotherapy in a routine clinical practice (non-trial setting).
Methods 58 patients with locally advanced head and neck cancer who received TPF chemotherapy were selected for this analysis. They received 2 cycles of TPF chemotherapy every 21 days. The patients were monitored for the occurrence of adverse drug reactions in accordance with Common Terminology Criteria for Adverse Events (version 4.03) during the hospitalization (median length of stay in cycle 1, 10 days), daily (at least until day 8 after chemotherapy initiation), then at days 15 and 20. Descriptive statistics was done and factors predicting for toxicity were identified using logistic regression analysis.
Results The cumulative rate of grade ¦3 anemia, neutropenia, and thrombocytopenia were 12.1%, 56.9%, and 5.2%, respectively. The cumulative incidence of febrile neutropenia was 20.7% (12 of 58 patients). The cumulative incidences of mucositis and diarrhea were 67.2% and 74.1%, respectively. There was no mortality associated with induction chemotherapy, and all of the patients completed the planned 2 cycles of TPF. None of the tested factors predicted for any of the adverse events considered in the study.
Limitations Small, single-center study
Conclusion The incidence of TPF-related toxicity in Indian patients in routine practice is high, and the toxicities differ substantially from the toxicities seen in trial settings.
Click on the PDF icon at the top of this introduction to read the full article.
Risk-reducing salpingectomy at benign hysterectomy: Have surgeons embraced this practice?
According to its January 2015 Committee Opinion, the American College of Obstetricians and Gynecologists supported the following recommendations and conclusions regarding salpingectomy for ovarian cancer prevention1:
- The surgeon and patient should discuss the potential benefits of the removal of the fallopian tubes during a hysterectomy in women at population risk of ovarian cancer who are not having an oophorectomy.
- When counseling women about laparoscopic sterilization methods, clinicians can communicate that bilateral salpingectomy can be considered a method that provides effective contraception.
- Prophylactic salpingectomy may offer clinicians the opportunity to prevent ovarian cancer in their patients.
- Randomized controlled trials are needed to support the validity of this approach to reduce the incidence of ovarian cancer.
To determine the change in rate of salpingectomy performed at benign hysterectomy at Michigan hospitals, Sara Till, MD, MPH, and colleagues from the University of Michigan Health System performed a retrospective cross-sectioned study of data from the Michigan Surgical Quality Collaborative. They examined hysterectomies performed for all surgical routes between January 2013 and April 2015. Exclusion criteria included malignancy and obstetric indication. The primary objective was to measure salpingectomy at the time of hysterectomy with ovarian preservation. Measures studied included demographics; comorbidities; perioperative and postoperative results; and hospital/surgeon-related data; including surgeon volume, hospital type (ie, teaching), and hospital size.2
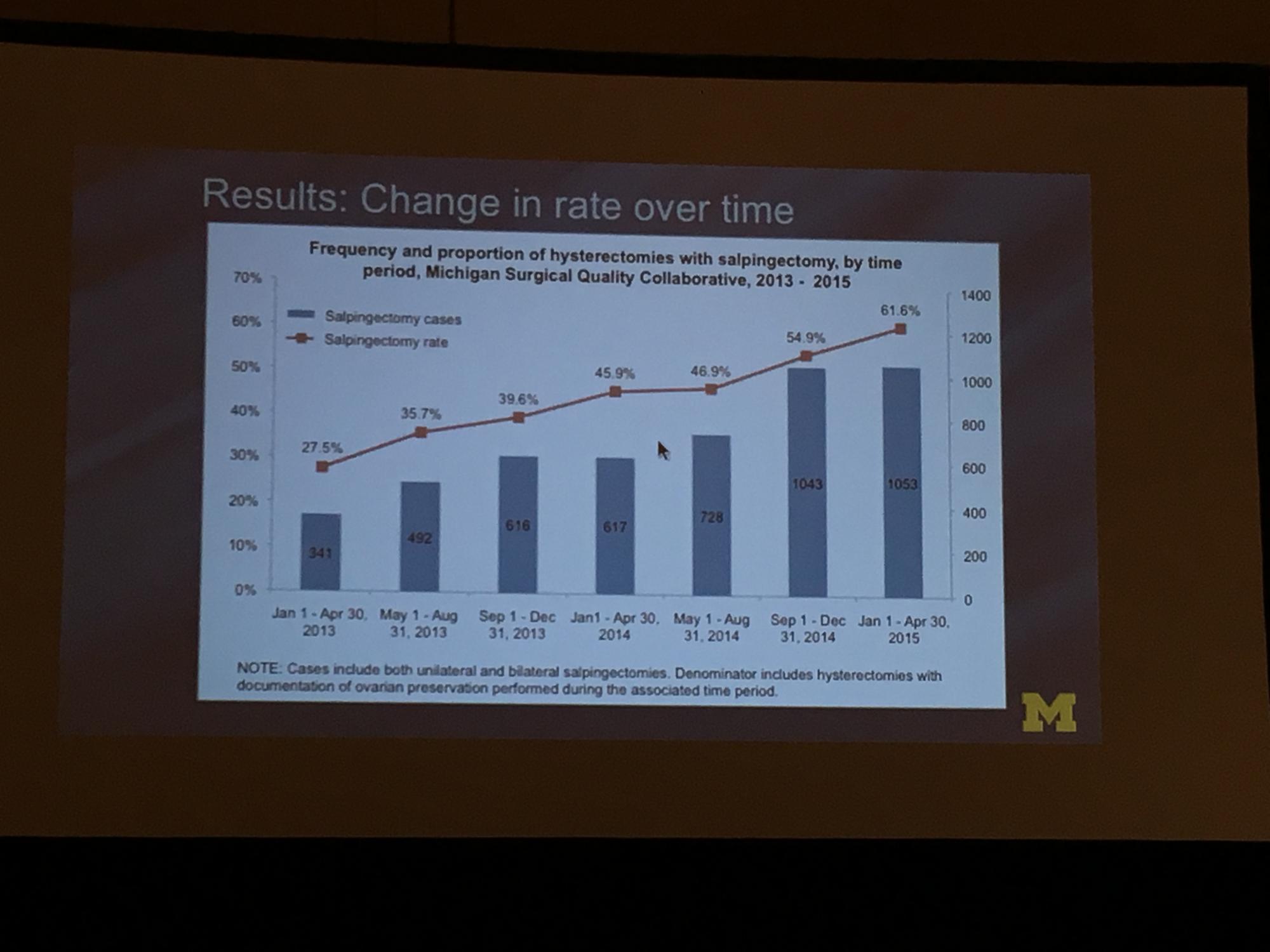
During the study period (January 1, 2013, to April 30, 2015), 18,642 hysterectomies were performed for benign indications, of which 55.7% (n = 10,382) were ovarian conserving. Among patients who underwent ovarian conserving hysterectomy, 44.9% (n = 4,668) had salpingectomy, with rates increasing steadily from 26.4% to 61.1% across the study period (P<.001). Salpingectomy was more likely with a laparoscopic approach (odds ratio [OR], 2.93; 95% confidence interval [CI], 2.69–3.20) and among women aged <60 years (OR, 2.60; 95% CI, 1.42–1.98), but did not vary with surgeon volume. After adjustments for age, body mass index, and surgical approach using a mixed model, the researchers found substantial variation in rates of salpingectomy across hospital sites, ranging from 3.7% to 88.3%. Variation in adjusted salpingectomy rates was not associated with academic affiliation or hospital size.2
Dr. Till and colleagues concluded that there was a substantial rise in risk-reducing salpingectomy from January 1, 2013, to April 30, 2015, and that there is substantial variation in the practice of salpingectomy, which is not accounted for by patient, surgeon, or hospital characteristics.2
- American College of Obstetricians and Gynecologists, Committee on Gynecologic Practice. Salpingectomy for ovarian cancer prevention. Committee Opinion No. 620 [published correction appears in: Obstet Gynecol. 2016;127(2):405]. Obstet Gynecol. 2015;125(1):279–281.
- Till SR, Edwards MG, Kobernik EK, Kamdar NS, As-Sanie S, Morgan DM. Implementation rate of risk-reducing salpingectomy at time of benign hysterectomy. Poster presented at: AAGL Global Congress of Minimally Invasive Gynecology; November 16, 2016; Orlando, Florida. J Minim Invasiv Gynecol. 2016;23(7 suppl):S1.
According to its January 2015 Committee Opinion, the American College of Obstetricians and Gynecologists supported the following recommendations and conclusions regarding salpingectomy for ovarian cancer prevention1:
- The surgeon and patient should discuss the potential benefits of the removal of the fallopian tubes during a hysterectomy in women at population risk of ovarian cancer who are not having an oophorectomy.
- When counseling women about laparoscopic sterilization methods, clinicians can communicate that bilateral salpingectomy can be considered a method that provides effective contraception.
- Prophylactic salpingectomy may offer clinicians the opportunity to prevent ovarian cancer in their patients.
- Randomized controlled trials are needed to support the validity of this approach to reduce the incidence of ovarian cancer.
To determine the change in rate of salpingectomy performed at benign hysterectomy at Michigan hospitals, Sara Till, MD, MPH, and colleagues from the University of Michigan Health System performed a retrospective cross-sectioned study of data from the Michigan Surgical Quality Collaborative. They examined hysterectomies performed for all surgical routes between January 2013 and April 2015. Exclusion criteria included malignancy and obstetric indication. The primary objective was to measure salpingectomy at the time of hysterectomy with ovarian preservation. Measures studied included demographics; comorbidities; perioperative and postoperative results; and hospital/surgeon-related data; including surgeon volume, hospital type (ie, teaching), and hospital size.2
During the study period (January 1, 2013, to April 30, 2015), 18,642 hysterectomies were performed for benign indications, of which 55.7% (n = 10,382) were ovarian conserving. Among patients who underwent ovarian conserving hysterectomy, 44.9% (n = 4,668) had salpingectomy, with rates increasing steadily from 26.4% to 61.1% across the study period (P<.001). Salpingectomy was more likely with a laparoscopic approach (odds ratio [OR], 2.93; 95% confidence interval [CI], 2.69–3.20) and among women aged <60 years (OR, 2.60; 95% CI, 1.42–1.98), but did not vary with surgeon volume. After adjustments for age, body mass index, and surgical approach using a mixed model, the researchers found substantial variation in rates of salpingectomy across hospital sites, ranging from 3.7% to 88.3%. Variation in adjusted salpingectomy rates was not associated with academic affiliation or hospital size.2
Dr. Till and colleagues concluded that there was a substantial rise in risk-reducing salpingectomy from January 1, 2013, to April 30, 2015, and that there is substantial variation in the practice of salpingectomy, which is not accounted for by patient, surgeon, or hospital characteristics.2
According to its January 2015 Committee Opinion, the American College of Obstetricians and Gynecologists supported the following recommendations and conclusions regarding salpingectomy for ovarian cancer prevention1:
- The surgeon and patient should discuss the potential benefits of the removal of the fallopian tubes during a hysterectomy in women at population risk of ovarian cancer who are not having an oophorectomy.
- When counseling women about laparoscopic sterilization methods, clinicians can communicate that bilateral salpingectomy can be considered a method that provides effective contraception.
- Prophylactic salpingectomy may offer clinicians the opportunity to prevent ovarian cancer in their patients.
- Randomized controlled trials are needed to support the validity of this approach to reduce the incidence of ovarian cancer.
To determine the change in rate of salpingectomy performed at benign hysterectomy at Michigan hospitals, Sara Till, MD, MPH, and colleagues from the University of Michigan Health System performed a retrospective cross-sectioned study of data from the Michigan Surgical Quality Collaborative. They examined hysterectomies performed for all surgical routes between January 2013 and April 2015. Exclusion criteria included malignancy and obstetric indication. The primary objective was to measure salpingectomy at the time of hysterectomy with ovarian preservation. Measures studied included demographics; comorbidities; perioperative and postoperative results; and hospital/surgeon-related data; including surgeon volume, hospital type (ie, teaching), and hospital size.2
During the study period (January 1, 2013, to April 30, 2015), 18,642 hysterectomies were performed for benign indications, of which 55.7% (n = 10,382) were ovarian conserving. Among patients who underwent ovarian conserving hysterectomy, 44.9% (n = 4,668) had salpingectomy, with rates increasing steadily from 26.4% to 61.1% across the study period (P<.001). Salpingectomy was more likely with a laparoscopic approach (odds ratio [OR], 2.93; 95% confidence interval [CI], 2.69–3.20) and among women aged <60 years (OR, 2.60; 95% CI, 1.42–1.98), but did not vary with surgeon volume. After adjustments for age, body mass index, and surgical approach using a mixed model, the researchers found substantial variation in rates of salpingectomy across hospital sites, ranging from 3.7% to 88.3%. Variation in adjusted salpingectomy rates was not associated with academic affiliation or hospital size.2
Dr. Till and colleagues concluded that there was a substantial rise in risk-reducing salpingectomy from January 1, 2013, to April 30, 2015, and that there is substantial variation in the practice of salpingectomy, which is not accounted for by patient, surgeon, or hospital characteristics.2
- American College of Obstetricians and Gynecologists, Committee on Gynecologic Practice. Salpingectomy for ovarian cancer prevention. Committee Opinion No. 620 [published correction appears in: Obstet Gynecol. 2016;127(2):405]. Obstet Gynecol. 2015;125(1):279–281.
- Till SR, Edwards MG, Kobernik EK, Kamdar NS, As-Sanie S, Morgan DM. Implementation rate of risk-reducing salpingectomy at time of benign hysterectomy. Poster presented at: AAGL Global Congress of Minimally Invasive Gynecology; November 16, 2016; Orlando, Florida. J Minim Invasiv Gynecol. 2016;23(7 suppl):S1.
- American College of Obstetricians and Gynecologists, Committee on Gynecologic Practice. Salpingectomy for ovarian cancer prevention. Committee Opinion No. 620 [published correction appears in: Obstet Gynecol. 2016;127(2):405]. Obstet Gynecol. 2015;125(1):279–281.
- Till SR, Edwards MG, Kobernik EK, Kamdar NS, As-Sanie S, Morgan DM. Implementation rate of risk-reducing salpingectomy at time of benign hysterectomy. Poster presented at: AAGL Global Congress of Minimally Invasive Gynecology; November 16, 2016; Orlando, Florida. J Minim Invasiv Gynecol. 2016;23(7 suppl):S1.
Perceived Leg-Length Discrepancy After Primary Total Knee Arthroplasty: Does Knee Alignment Play a Role?
Leg-length discrepancy (LLD) is common in the general population1 and particularly in patients with degenerative joint diseases of the hip and knee.2 Common complications of LLD include femoral, sciatic, and peroneal nerve palsy; lower back pain; gait abnormalities3; and general dissatisfaction. LLD is a concern for orthopedic surgeons who perform total knee arthroplasty (TKA) because limb lengthening is common after this procedure.4,5 Surgeons are aware of the limb lengthening that occurs during TKA,4,5 and studies have confirmed that LLD usually decreases after TKA.4,5
Despite surgeons’ best efforts, some patients still perceive LLD after surgery, though the incidence of perceived LLD in patients who have had TKA has not been well documented. Aside from actual, objectively measured LLD, there may be other factors that lead patients to perceive LLD. Study results have suggested that preoperative varus–valgus alignment of the knee joint may correlate with how much an operative leg is lengthened after TKA4,5; however, the outcome investigated was objective LLD measurements, not perceived LLD. Understanding the factors that may influence patients’ ability to perceive LLD would allow surgeons to preoperatively identify patients who are at higher risk for postoperative perceived LLD. This information, along with expected time to resolution of postoperative perceived LLD, would allow surgeons to educate their patients accordingly.
We conducted a study to determine the incidence of perceived LLD before and after primary TKA in patients with unilateral osteoarthritis and to determine the correlation between mechanical axis of the knee and perceived LLD before and after surgery. Given that surgery may correct mechanical axis misalignment, we investigated the correlation between this correction and its ability to change patients’ preoperative and postoperative perceived LLD. We hypothesized that a large correction of mechanical axis would lead patients to perceive LLD after surgery. The relationship of body mass index (BMI) and age to patients’ perceived LLD was also assessed. The incidence and time frame of resolution of postoperative perceived LLD were determined.
Methods
Approval for this study was received from the Institutional Review Board at our institution, Rush University Medical Center in Chicago, Illinois. Seventy-three patients undergoing primary TKA performed by 3 surgeons at 2 institutions between February 2010 and January 2013 were prospectively enrolled. Inclusion criteria were age 18 years to 90 years and primary TKA for unilateral osteoarthritis; exclusion criteria were allergy or intolerance to the study materials, operative treatment of affected joint or its underlying etiology within prior month, previous surgeries (other than arthroscopy) on affected joint, previous surgeries (on unaffected lower extremity) that may influence preoperative and postoperative leg lengths, and any substance abuse or dependence within the past 6 months. Patients provided written informed consent for total knee arthroplasty.
All surgeries were performed by Dr. Levine, Dr. Della Valle, and Dr. Sporer using the medial parapatellar or midvastus approach with tourniquet. Similar standard postoperative rehabilitation protocols with early mobilization were used in all cases.
During clinical evaluation, patient demographic data were collected and LLD surveys administered. Patients were asked, before surgery and 3 to 6 weeks, 3 months, 6 months, and 1 year after surgery, if they perceived LLD. A patient who no longer perceived LLD after surgery was no longer followed for this study.
At the preoperative clinic visit and at the 3-month or 6-week postoperative visit, standing mechanical axis radiographs were viewed by 2 of the authors (not the primary surgeons) using PACS (picture archiving and communication system software). The mechanical axis of the operative leg was measured with ImageJ software by taking the angle from the center of the femur to the middle of the ankle joint, with the vertex assigned to the middle of the knee joint.
We used a 2-tailed unpaired t test to determine the relationship of preoperative mechanical axis to perceived LLD (or lack thereof) before surgery. The data were analyzed for separate varus and valgus deformities. Then we determined the relationship of postoperative mechanical axis to perceived LLD (or lack thereof) after surgery. The McNemar test was used to determine the effect of surgery on patients’ LLD perceptions.
To determine the relationship between preoperative-to-postoperative change in mechanical axis and change in LLD perceptions, we divided patients into 4 groups. Group 1 had both preoperative and postoperative perceived LLD, group 2 had no preoperative or postoperative perceived LLD, group 3 had preoperative perceived LLD but no postoperative perceived LLD, and group 4 had postoperative perceived LLD but no preoperative perceived LLD. The absolute value of the difference between preoperative and postoperative mechanical axis was then determined, relative to 180°, to account for changes in varus to valgus deformity before and after surgery and vice versa. Analysis of variance (ANOVA) was used to detect differences between groups. This analysis was then stratified based on BMI and age.
Results
Of the 73 enrolled patients, 2 were excluded from results analysis because of inadequate data—one did not complete the postoperative LLD survey, and the other did not have postoperative standing mechanical axis radiographs—leaving 71 patients (27 men, 44 women) with adequate data. Mean (SD) age of all patients was 65 (8.4) years (range, 47-89 years). Mean (SD) BMI was 35.1 (9.9; range, 20.2-74.8).
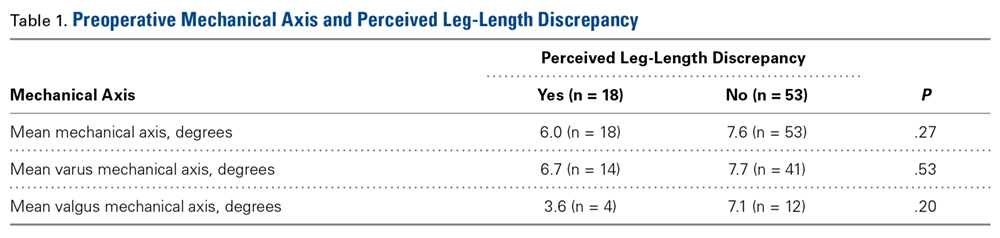
Of the 71 patients with adequate data, 18 had preoperative perceived LLD and 53 did not; in addition, 7 had postoperative perceived LLD and 64 did not. All 7 patients with postoperative perceived LLD noted resolution of LLD, at a mean of 8.5 weeks (range, 3 weeks-3 months). There was a significant difference between the 18 patients with preoperative perceived LLD and the 7 with postoperative perceived LLD (P = .035, analyzed with the McNemar test).
Table 1 lists the mean preoperative mechanical axis measurements for patients with and without preoperative perceived LLD.
Table 2 lists the mean postoperative mechanical axis measurements for patients with and without postoperative perceived LLD.
Table 3 lists the mean absolute values of mechanical axis correction (preoperative to postoperative) for the 4 patient groups described in the Methods section.
Discussion
In this study, 18 patients (25%) had preoperative perceived LLD, proving that perceived LLD is common in patients who undergo TKA for unilateral osteoarthritis. Surgeons should give their patients a preoperative survey on perceived LLD, as survey responses may inform and influence surgical decisions and strategies.
Of the 18 patients with preoperative perceived LLD, only 1 had postoperative perceived LLD. That perceived LLD decreased after surgery makes sense given the widely accepted notion that actual LLD is common before primary TKA but in most cases is corrected during surgery.4,5 As LLD correction during surgery is so successful, surgeons should tell their patients with preoperative perceived LLD that in most cases it will be fixed after TKA.
Although the incidence of perceived LLD decreased after TKA (as mentioned earlier), the decrease seemed to be restricted mostly to patients with preoperative perceived LLD, and the underlying LLD was most probably corrected by the surgery. However, surgery introduced perceived LLD in 6 cases, supporting the notion that it is crucial to understand which patients are at higher risk for postoperative perceived LLD and what if any time frame can be expected for resolution in these cases. In our study, all cases of perceived LLD had resolved by a mean follow-up of 8.5 weeks (range, 3 weeks-3 months). This phenomenon of resolution may be attributed to some of the physical, objective LLD corrections that naturally occur throughout the postoperative course,4 though psychological factors may also be involved. Our study results suggest patients should be counseled that, though about 10% of patients perceive LLD after primary TKA, the vast majority of perceived LLD cases resolve within 3 months.
One study goal was to determine the relationship between the mechanical axis of the knee and perceived LLD both before and after surgery. There were no significant relationships. This was also true when cases of varus and valgus deformity were analyzed separately.
Another study goal was to determine if a surgical change in the mechanical alignment of the knee would influence preoperative-to-postoperative LLD perceptions. In our analysis, patients were divided into 4 groups based on their preoperative and postoperative LLD perceptions (see Methods section). ANOVA revealed no significant differences in absolute values of mechanical axis correction among the 4 groups. Likewise, there were no correlations between BMI and age and mechanical axis correction among the groups, suggesting LLD perception is unrelated to any of these variables. Ideally, if a relationship between a threshold knee alignment value and perceived LLD existed, surgeons would be able to counsel patients at higher risk for perceived LLD about how their knee alignment may contribute to their perception. Unfortunately, our study results did not show any significant statistical relationships in this regard.
The problem of LLD in patients undergoing TKA is not new, and much research is needed to determine the correlation between perceived versus actual discrepancies, and why they occur. Our study results confirmed that TKA corrects most cases of preoperative perceived LLD but introduces perceived LLD in other cases. Whether preoperative or postoperative LLD is merely perceived or is in fact an actual discrepancy remains to be seen.
One limitation of this study was its lack of leg-length measurements. Although we studied knee alignment specifically, it would have been useful to compare perceived LLD with measured leg lengths, either clinically or radiographically, especially since leg lengths obviously play a role in any perceived LLD. We used mechanical alignment as a surrogate for actual LLD because we hypothesized that alignment may contribute to patients’ perceived discrepancies.
Another limitation was the relatively small sample. Only 24 cases of perceived LLD were analyzed. Given our low rates of perceived LLD (25% before surgery, 10% after surgery), it is difficult to study a large enough TKA group to establish a statistically significant number of cases. Nevertheless, investigators may use larger groups to establish more meaningful relationships.
A third limitation was that alignment was measured on the operative side but not the contralateral side. As we were focusing on perceived discrepancy, contralateral knee alignment may play an important role. Our study involved patients with unilateral osteoarthritis, so it would be reasonable to assume the nonoperative knee was almost neutral in alignment in most cases. However, given that varus/valgus misalignment is a known risk factor for osteoarthritis,6 many of our patients with unilateral disease may very well have had preexisting misalignment of both knees. The undetermined alignment of the nonoperative side may be a confounding variable in the relationship between operative knee alignment and perceived LLD.
Fourth, not all patients were surveyed 3 weeks after surgery. Some were first surveyed at 6 weeks, and it is possible there were cases of transient postoperative LLD that resolved before that point. Therefore, our reported incidence of postoperative LLD could have missed some cases. In addition, our mean 8.5-week period for LLD resolution may not have accounted for these resolved cases of transient perceived LLD.
Am J Orthop. 2016;45(7):E429-E433. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. O’Brien S, Kernohan G, Fitzpatrick C, Hill J, Beverland D. Perception of imposed leg length inequality in normal subjects. Hip Int. 2010;20(4):505-511.
2. Noll DR. Leg length discrepancy and osteoarthritic knee pain in the elderly: an observational study. J Am Osteopath Assoc. 2013;113(9):670-678.
3. Clark CR, Huddleston HD, Schoch EP 3rd, Thomas BJ. Leg-length discrepancy after total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(1):38-45.
4. Chang MJ, Kang YG, Chang CB, Seong SC, Kim TK. The patterns of limb length, height, weight and body mass index changes after total knee arthroplasty. J Arthroplasty. 2013;28(10):1856-1861.
5. Lang JE, Scott RD, Lonner JH, Bono JV, Hunter DJ, Li L. Magnitude of limb lengthening after primary total knee arthroplasty. J Arthroplasty. 2012;27(3):341-346.
6. Sharma L, Song J, Dunlop D, et al. Varus and valgus alignment and incident and progressive knee osteoarthritis. Ann Rheum Dis. 2010;69(11):1940-1945.
Leg-length discrepancy (LLD) is common in the general population1 and particularly in patients with degenerative joint diseases of the hip and knee.2 Common complications of LLD include femoral, sciatic, and peroneal nerve palsy; lower back pain; gait abnormalities3; and general dissatisfaction. LLD is a concern for orthopedic surgeons who perform total knee arthroplasty (TKA) because limb lengthening is common after this procedure.4,5 Surgeons are aware of the limb lengthening that occurs during TKA,4,5 and studies have confirmed that LLD usually decreases after TKA.4,5
Despite surgeons’ best efforts, some patients still perceive LLD after surgery, though the incidence of perceived LLD in patients who have had TKA has not been well documented. Aside from actual, objectively measured LLD, there may be other factors that lead patients to perceive LLD. Study results have suggested that preoperative varus–valgus alignment of the knee joint may correlate with how much an operative leg is lengthened after TKA4,5; however, the outcome investigated was objective LLD measurements, not perceived LLD. Understanding the factors that may influence patients’ ability to perceive LLD would allow surgeons to preoperatively identify patients who are at higher risk for postoperative perceived LLD. This information, along with expected time to resolution of postoperative perceived LLD, would allow surgeons to educate their patients accordingly.
We conducted a study to determine the incidence of perceived LLD before and after primary TKA in patients with unilateral osteoarthritis and to determine the correlation between mechanical axis of the knee and perceived LLD before and after surgery. Given that surgery may correct mechanical axis misalignment, we investigated the correlation between this correction and its ability to change patients’ preoperative and postoperative perceived LLD. We hypothesized that a large correction of mechanical axis would lead patients to perceive LLD after surgery. The relationship of body mass index (BMI) and age to patients’ perceived LLD was also assessed. The incidence and time frame of resolution of postoperative perceived LLD were determined.
Methods
Approval for this study was received from the Institutional Review Board at our institution, Rush University Medical Center in Chicago, Illinois. Seventy-three patients undergoing primary TKA performed by 3 surgeons at 2 institutions between February 2010 and January 2013 were prospectively enrolled. Inclusion criteria were age 18 years to 90 years and primary TKA for unilateral osteoarthritis; exclusion criteria were allergy or intolerance to the study materials, operative treatment of affected joint or its underlying etiology within prior month, previous surgeries (other than arthroscopy) on affected joint, previous surgeries (on unaffected lower extremity) that may influence preoperative and postoperative leg lengths, and any substance abuse or dependence within the past 6 months. Patients provided written informed consent for total knee arthroplasty.
All surgeries were performed by Dr. Levine, Dr. Della Valle, and Dr. Sporer using the medial parapatellar or midvastus approach with tourniquet. Similar standard postoperative rehabilitation protocols with early mobilization were used in all cases.
During clinical evaluation, patient demographic data were collected and LLD surveys administered. Patients were asked, before surgery and 3 to 6 weeks, 3 months, 6 months, and 1 year after surgery, if they perceived LLD. A patient who no longer perceived LLD after surgery was no longer followed for this study.
At the preoperative clinic visit and at the 3-month or 6-week postoperative visit, standing mechanical axis radiographs were viewed by 2 of the authors (not the primary surgeons) using PACS (picture archiving and communication system software). The mechanical axis of the operative leg was measured with ImageJ software by taking the angle from the center of the femur to the middle of the ankle joint, with the vertex assigned to the middle of the knee joint.
We used a 2-tailed unpaired t test to determine the relationship of preoperative mechanical axis to perceived LLD (or lack thereof) before surgery. The data were analyzed for separate varus and valgus deformities. Then we determined the relationship of postoperative mechanical axis to perceived LLD (or lack thereof) after surgery. The McNemar test was used to determine the effect of surgery on patients’ LLD perceptions.
To determine the relationship between preoperative-to-postoperative change in mechanical axis and change in LLD perceptions, we divided patients into 4 groups. Group 1 had both preoperative and postoperative perceived LLD, group 2 had no preoperative or postoperative perceived LLD, group 3 had preoperative perceived LLD but no postoperative perceived LLD, and group 4 had postoperative perceived LLD but no preoperative perceived LLD. The absolute value of the difference between preoperative and postoperative mechanical axis was then determined, relative to 180°, to account for changes in varus to valgus deformity before and after surgery and vice versa. Analysis of variance (ANOVA) was used to detect differences between groups. This analysis was then stratified based on BMI and age.
Results
Of the 73 enrolled patients, 2 were excluded from results analysis because of inadequate data—one did not complete the postoperative LLD survey, and the other did not have postoperative standing mechanical axis radiographs—leaving 71 patients (27 men, 44 women) with adequate data. Mean (SD) age of all patients was 65 (8.4) years (range, 47-89 years). Mean (SD) BMI was 35.1 (9.9; range, 20.2-74.8).
Of the 71 patients with adequate data, 18 had preoperative perceived LLD and 53 did not; in addition, 7 had postoperative perceived LLD and 64 did not. All 7 patients with postoperative perceived LLD noted resolution of LLD, at a mean of 8.5 weeks (range, 3 weeks-3 months). There was a significant difference between the 18 patients with preoperative perceived LLD and the 7 with postoperative perceived LLD (P = .035, analyzed with the McNemar test).
Table 1 lists the mean preoperative mechanical axis measurements for patients with and without preoperative perceived LLD.
Table 2 lists the mean postoperative mechanical axis measurements for patients with and without postoperative perceived LLD.
Table 3 lists the mean absolute values of mechanical axis correction (preoperative to postoperative) for the 4 patient groups described in the Methods section.
Discussion
In this study, 18 patients (25%) had preoperative perceived LLD, proving that perceived LLD is common in patients who undergo TKA for unilateral osteoarthritis. Surgeons should give their patients a preoperative survey on perceived LLD, as survey responses may inform and influence surgical decisions and strategies.
Of the 18 patients with preoperative perceived LLD, only 1 had postoperative perceived LLD. That perceived LLD decreased after surgery makes sense given the widely accepted notion that actual LLD is common before primary TKA but in most cases is corrected during surgery.4,5 As LLD correction during surgery is so successful, surgeons should tell their patients with preoperative perceived LLD that in most cases it will be fixed after TKA.
Although the incidence of perceived LLD decreased after TKA (as mentioned earlier), the decrease seemed to be restricted mostly to patients with preoperative perceived LLD, and the underlying LLD was most probably corrected by the surgery. However, surgery introduced perceived LLD in 6 cases, supporting the notion that it is crucial to understand which patients are at higher risk for postoperative perceived LLD and what if any time frame can be expected for resolution in these cases. In our study, all cases of perceived LLD had resolved by a mean follow-up of 8.5 weeks (range, 3 weeks-3 months). This phenomenon of resolution may be attributed to some of the physical, objective LLD corrections that naturally occur throughout the postoperative course,4 though psychological factors may also be involved. Our study results suggest patients should be counseled that, though about 10% of patients perceive LLD after primary TKA, the vast majority of perceived LLD cases resolve within 3 months.
One study goal was to determine the relationship between the mechanical axis of the knee and perceived LLD both before and after surgery. There were no significant relationships. This was also true when cases of varus and valgus deformity were analyzed separately.
Another study goal was to determine if a surgical change in the mechanical alignment of the knee would influence preoperative-to-postoperative LLD perceptions. In our analysis, patients were divided into 4 groups based on their preoperative and postoperative LLD perceptions (see Methods section). ANOVA revealed no significant differences in absolute values of mechanical axis correction among the 4 groups. Likewise, there were no correlations between BMI and age and mechanical axis correction among the groups, suggesting LLD perception is unrelated to any of these variables. Ideally, if a relationship between a threshold knee alignment value and perceived LLD existed, surgeons would be able to counsel patients at higher risk for perceived LLD about how their knee alignment may contribute to their perception. Unfortunately, our study results did not show any significant statistical relationships in this regard.
The problem of LLD in patients undergoing TKA is not new, and much research is needed to determine the correlation between perceived versus actual discrepancies, and why they occur. Our study results confirmed that TKA corrects most cases of preoperative perceived LLD but introduces perceived LLD in other cases. Whether preoperative or postoperative LLD is merely perceived or is in fact an actual discrepancy remains to be seen.
One limitation of this study was its lack of leg-length measurements. Although we studied knee alignment specifically, it would have been useful to compare perceived LLD with measured leg lengths, either clinically or radiographically, especially since leg lengths obviously play a role in any perceived LLD. We used mechanical alignment as a surrogate for actual LLD because we hypothesized that alignment may contribute to patients’ perceived discrepancies.
Another limitation was the relatively small sample. Only 24 cases of perceived LLD were analyzed. Given our low rates of perceived LLD (25% before surgery, 10% after surgery), it is difficult to study a large enough TKA group to establish a statistically significant number of cases. Nevertheless, investigators may use larger groups to establish more meaningful relationships.
A third limitation was that alignment was measured on the operative side but not the contralateral side. As we were focusing on perceived discrepancy, contralateral knee alignment may play an important role. Our study involved patients with unilateral osteoarthritis, so it would be reasonable to assume the nonoperative knee was almost neutral in alignment in most cases. However, given that varus/valgus misalignment is a known risk factor for osteoarthritis,6 many of our patients with unilateral disease may very well have had preexisting misalignment of both knees. The undetermined alignment of the nonoperative side may be a confounding variable in the relationship between operative knee alignment and perceived LLD.
Fourth, not all patients were surveyed 3 weeks after surgery. Some were first surveyed at 6 weeks, and it is possible there were cases of transient postoperative LLD that resolved before that point. Therefore, our reported incidence of postoperative LLD could have missed some cases. In addition, our mean 8.5-week period for LLD resolution may not have accounted for these resolved cases of transient perceived LLD.
Am J Orthop. 2016;45(7):E429-E433. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Leg-length discrepancy (LLD) is common in the general population1 and particularly in patients with degenerative joint diseases of the hip and knee.2 Common complications of LLD include femoral, sciatic, and peroneal nerve palsy; lower back pain; gait abnormalities3; and general dissatisfaction. LLD is a concern for orthopedic surgeons who perform total knee arthroplasty (TKA) because limb lengthening is common after this procedure.4,5 Surgeons are aware of the limb lengthening that occurs during TKA,4,5 and studies have confirmed that LLD usually decreases after TKA.4,5
Despite surgeons’ best efforts, some patients still perceive LLD after surgery, though the incidence of perceived LLD in patients who have had TKA has not been well documented. Aside from actual, objectively measured LLD, there may be other factors that lead patients to perceive LLD. Study results have suggested that preoperative varus–valgus alignment of the knee joint may correlate with how much an operative leg is lengthened after TKA4,5; however, the outcome investigated was objective LLD measurements, not perceived LLD. Understanding the factors that may influence patients’ ability to perceive LLD would allow surgeons to preoperatively identify patients who are at higher risk for postoperative perceived LLD. This information, along with expected time to resolution of postoperative perceived LLD, would allow surgeons to educate their patients accordingly.
We conducted a study to determine the incidence of perceived LLD before and after primary TKA in patients with unilateral osteoarthritis and to determine the correlation between mechanical axis of the knee and perceived LLD before and after surgery. Given that surgery may correct mechanical axis misalignment, we investigated the correlation between this correction and its ability to change patients’ preoperative and postoperative perceived LLD. We hypothesized that a large correction of mechanical axis would lead patients to perceive LLD after surgery. The relationship of body mass index (BMI) and age to patients’ perceived LLD was also assessed. The incidence and time frame of resolution of postoperative perceived LLD were determined.
Methods
Approval for this study was received from the Institutional Review Board at our institution, Rush University Medical Center in Chicago, Illinois. Seventy-three patients undergoing primary TKA performed by 3 surgeons at 2 institutions between February 2010 and January 2013 were prospectively enrolled. Inclusion criteria were age 18 years to 90 years and primary TKA for unilateral osteoarthritis; exclusion criteria were allergy or intolerance to the study materials, operative treatment of affected joint or its underlying etiology within prior month, previous surgeries (other than arthroscopy) on affected joint, previous surgeries (on unaffected lower extremity) that may influence preoperative and postoperative leg lengths, and any substance abuse or dependence within the past 6 months. Patients provided written informed consent for total knee arthroplasty.
All surgeries were performed by Dr. Levine, Dr. Della Valle, and Dr. Sporer using the medial parapatellar or midvastus approach with tourniquet. Similar standard postoperative rehabilitation protocols with early mobilization were used in all cases.
During clinical evaluation, patient demographic data were collected and LLD surveys administered. Patients were asked, before surgery and 3 to 6 weeks, 3 months, 6 months, and 1 year after surgery, if they perceived LLD. A patient who no longer perceived LLD after surgery was no longer followed for this study.
At the preoperative clinic visit and at the 3-month or 6-week postoperative visit, standing mechanical axis radiographs were viewed by 2 of the authors (not the primary surgeons) using PACS (picture archiving and communication system software). The mechanical axis of the operative leg was measured with ImageJ software by taking the angle from the center of the femur to the middle of the ankle joint, with the vertex assigned to the middle of the knee joint.
We used a 2-tailed unpaired t test to determine the relationship of preoperative mechanical axis to perceived LLD (or lack thereof) before surgery. The data were analyzed for separate varus and valgus deformities. Then we determined the relationship of postoperative mechanical axis to perceived LLD (or lack thereof) after surgery. The McNemar test was used to determine the effect of surgery on patients’ LLD perceptions.
To determine the relationship between preoperative-to-postoperative change in mechanical axis and change in LLD perceptions, we divided patients into 4 groups. Group 1 had both preoperative and postoperative perceived LLD, group 2 had no preoperative or postoperative perceived LLD, group 3 had preoperative perceived LLD but no postoperative perceived LLD, and group 4 had postoperative perceived LLD but no preoperative perceived LLD. The absolute value of the difference between preoperative and postoperative mechanical axis was then determined, relative to 180°, to account for changes in varus to valgus deformity before and after surgery and vice versa. Analysis of variance (ANOVA) was used to detect differences between groups. This analysis was then stratified based on BMI and age.
Results
Of the 73 enrolled patients, 2 were excluded from results analysis because of inadequate data—one did not complete the postoperative LLD survey, and the other did not have postoperative standing mechanical axis radiographs—leaving 71 patients (27 men, 44 women) with adequate data. Mean (SD) age of all patients was 65 (8.4) years (range, 47-89 years). Mean (SD) BMI was 35.1 (9.9; range, 20.2-74.8).
Of the 71 patients with adequate data, 18 had preoperative perceived LLD and 53 did not; in addition, 7 had postoperative perceived LLD and 64 did not. All 7 patients with postoperative perceived LLD noted resolution of LLD, at a mean of 8.5 weeks (range, 3 weeks-3 months). There was a significant difference between the 18 patients with preoperative perceived LLD and the 7 with postoperative perceived LLD (P = .035, analyzed with the McNemar test).
Table 1 lists the mean preoperative mechanical axis measurements for patients with and without preoperative perceived LLD.
Table 2 lists the mean postoperative mechanical axis measurements for patients with and without postoperative perceived LLD.
Table 3 lists the mean absolute values of mechanical axis correction (preoperative to postoperative) for the 4 patient groups described in the Methods section.
Discussion
In this study, 18 patients (25%) had preoperative perceived LLD, proving that perceived LLD is common in patients who undergo TKA for unilateral osteoarthritis. Surgeons should give their patients a preoperative survey on perceived LLD, as survey responses may inform and influence surgical decisions and strategies.
Of the 18 patients with preoperative perceived LLD, only 1 had postoperative perceived LLD. That perceived LLD decreased after surgery makes sense given the widely accepted notion that actual LLD is common before primary TKA but in most cases is corrected during surgery.4,5 As LLD correction during surgery is so successful, surgeons should tell their patients with preoperative perceived LLD that in most cases it will be fixed after TKA.
Although the incidence of perceived LLD decreased after TKA (as mentioned earlier), the decrease seemed to be restricted mostly to patients with preoperative perceived LLD, and the underlying LLD was most probably corrected by the surgery. However, surgery introduced perceived LLD in 6 cases, supporting the notion that it is crucial to understand which patients are at higher risk for postoperative perceived LLD and what if any time frame can be expected for resolution in these cases. In our study, all cases of perceived LLD had resolved by a mean follow-up of 8.5 weeks (range, 3 weeks-3 months). This phenomenon of resolution may be attributed to some of the physical, objective LLD corrections that naturally occur throughout the postoperative course,4 though psychological factors may also be involved. Our study results suggest patients should be counseled that, though about 10% of patients perceive LLD after primary TKA, the vast majority of perceived LLD cases resolve within 3 months.
One study goal was to determine the relationship between the mechanical axis of the knee and perceived LLD both before and after surgery. There were no significant relationships. This was also true when cases of varus and valgus deformity were analyzed separately.
Another study goal was to determine if a surgical change in the mechanical alignment of the knee would influence preoperative-to-postoperative LLD perceptions. In our analysis, patients were divided into 4 groups based on their preoperative and postoperative LLD perceptions (see Methods section). ANOVA revealed no significant differences in absolute values of mechanical axis correction among the 4 groups. Likewise, there were no correlations between BMI and age and mechanical axis correction among the groups, suggesting LLD perception is unrelated to any of these variables. Ideally, if a relationship between a threshold knee alignment value and perceived LLD existed, surgeons would be able to counsel patients at higher risk for perceived LLD about how their knee alignment may contribute to their perception. Unfortunately, our study results did not show any significant statistical relationships in this regard.
The problem of LLD in patients undergoing TKA is not new, and much research is needed to determine the correlation between perceived versus actual discrepancies, and why they occur. Our study results confirmed that TKA corrects most cases of preoperative perceived LLD but introduces perceived LLD in other cases. Whether preoperative or postoperative LLD is merely perceived or is in fact an actual discrepancy remains to be seen.
One limitation of this study was its lack of leg-length measurements. Although we studied knee alignment specifically, it would have been useful to compare perceived LLD with measured leg lengths, either clinically or radiographically, especially since leg lengths obviously play a role in any perceived LLD. We used mechanical alignment as a surrogate for actual LLD because we hypothesized that alignment may contribute to patients’ perceived discrepancies.
Another limitation was the relatively small sample. Only 24 cases of perceived LLD were analyzed. Given our low rates of perceived LLD (25% before surgery, 10% after surgery), it is difficult to study a large enough TKA group to establish a statistically significant number of cases. Nevertheless, investigators may use larger groups to establish more meaningful relationships.
A third limitation was that alignment was measured on the operative side but not the contralateral side. As we were focusing on perceived discrepancy, contralateral knee alignment may play an important role. Our study involved patients with unilateral osteoarthritis, so it would be reasonable to assume the nonoperative knee was almost neutral in alignment in most cases. However, given that varus/valgus misalignment is a known risk factor for osteoarthritis,6 many of our patients with unilateral disease may very well have had preexisting misalignment of both knees. The undetermined alignment of the nonoperative side may be a confounding variable in the relationship between operative knee alignment and perceived LLD.
Fourth, not all patients were surveyed 3 weeks after surgery. Some were first surveyed at 6 weeks, and it is possible there were cases of transient postoperative LLD that resolved before that point. Therefore, our reported incidence of postoperative LLD could have missed some cases. In addition, our mean 8.5-week period for LLD resolution may not have accounted for these resolved cases of transient perceived LLD.
Am J Orthop. 2016;45(7):E429-E433. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. O’Brien S, Kernohan G, Fitzpatrick C, Hill J, Beverland D. Perception of imposed leg length inequality in normal subjects. Hip Int. 2010;20(4):505-511.
2. Noll DR. Leg length discrepancy and osteoarthritic knee pain in the elderly: an observational study. J Am Osteopath Assoc. 2013;113(9):670-678.
3. Clark CR, Huddleston HD, Schoch EP 3rd, Thomas BJ. Leg-length discrepancy after total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(1):38-45.
4. Chang MJ, Kang YG, Chang CB, Seong SC, Kim TK. The patterns of limb length, height, weight and body mass index changes after total knee arthroplasty. J Arthroplasty. 2013;28(10):1856-1861.
5. Lang JE, Scott RD, Lonner JH, Bono JV, Hunter DJ, Li L. Magnitude of limb lengthening after primary total knee arthroplasty. J Arthroplasty. 2012;27(3):341-346.
6. Sharma L, Song J, Dunlop D, et al. Varus and valgus alignment and incident and progressive knee osteoarthritis. Ann Rheum Dis. 2010;69(11):1940-1945.
1. O’Brien S, Kernohan G, Fitzpatrick C, Hill J, Beverland D. Perception of imposed leg length inequality in normal subjects. Hip Int. 2010;20(4):505-511.
2. Noll DR. Leg length discrepancy and osteoarthritic knee pain in the elderly: an observational study. J Am Osteopath Assoc. 2013;113(9):670-678.
3. Clark CR, Huddleston HD, Schoch EP 3rd, Thomas BJ. Leg-length discrepancy after total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(1):38-45.
4. Chang MJ, Kang YG, Chang CB, Seong SC, Kim TK. The patterns of limb length, height, weight and body mass index changes after total knee arthroplasty. J Arthroplasty. 2013;28(10):1856-1861.
5. Lang JE, Scott RD, Lonner JH, Bono JV, Hunter DJ, Li L. Magnitude of limb lengthening after primary total knee arthroplasty. J Arthroplasty. 2012;27(3):341-346.
6. Sharma L, Song J, Dunlop D, et al. Varus and valgus alignment and incident and progressive knee osteoarthritis. Ann Rheum Dis. 2010;69(11):1940-1945.
Tumor boards linked to improved survival in hepatocellular carcinoma
BOSTON – Veterans were about 13% less likely to die within 5 years of hepatocellular carcinoma diagnosis when multidisciplinary tumor boards managed their care than if they did not, according to a large, multicenter observational study.
Seeing a hepatologist or surgeon within 30 days of diagnosis also significantly improved 5-year overall survival, even after controlling for age, race, Charlson-Deyo comorbidity index, Barcelona Clinic Liver Cancer (BCLC) stage, academic center and geographic region of care, and the distance patients lived from the nearest Veterans Affairs transplant center, Marina Serper, MD, reported at the annual meeting of the American Association for the Study of Liver Diseases. “More studies are needed to understand how to best use multidisciplinary tumor boards to improve the care of patients with hepatocellular carcinoma,” she said.
Outcomes data for hepatocellular carcinoma mostly come from clinical trials; transplant centers; and Surveillance, Epidemiology, and End Results-Medicare analyses, noted Dr. Serper of the University of Pennsylvania in Philadelphia.
For a better look at veterans, she and her associates combined administrative, laboratory, and death data with medical chart reviews and information from the Organ Procurement and Transplantation Network’s Standard Transplant Analysis and Research file. The initial cohort included more than 6,800 veterans whose ICD-9CM diagnosis code indicated a malignant hepatic neoplasm. Excluding patients with neoplasms such as cholangiocarcinoma and those managed outside the VA left 3,989 VA patients with hepatocellular carcinoma.
In the multivariable analysis, use of multidisciplinary tumor boards was associated with a statistically significant 13% improvement in 5-year overall survival (hazard ratio, 0.87; 95% confidence interval, 0.81-0.94; P less than .001). Improved survival also was linked with seeing certain specialists within 30 days of diagnosis, including hepatologists (HR, 0.77; P less than .001) and surgeons (HR, 0.72; P less than .001). Consulting with a hepatologist within 30 days of diagnosis, however, did not improve the chances of receiving curative therapy, such as liver transplantation, resection, local ablation, transarterial chemoembolization, or Y-90 radioembolization.
Care also varied substantially geographically and by academic affiliation, Dr. Serper noted. “Treatment of hepatocellular carcinoma is complex, as it depends as much on liver function as it does on tumor staging,” she emphasized. “Studies to improve multidisciplinary approaches for hepatocellular carcinoma in the community are needed to increase rates of curative therapy and improve clinical outcomes.”
Patients in this study averaged 62 years of age at diagnosis, 54% were white, 36% were within Milan criteria, and 45% had a Child-Turcotte-Pugh score of B or higher. Nearly 18% had macrovascular invasion at diagnosis, and 7% had metastatic disease. Nearly two-thirds of patients were BCLC stage A or B at diagnosis, and more than a third had underlying alcohol misuse and chronic hepatitis C virus infection.
The work was funded by unrestricted grants from Bayer Healthcare Pharmaceuticals and the VA’s HIV, Hepatitis and Public Health Pathogens Programs. The investigators had no relevant financial disclosures.
BOSTON – Veterans were about 13% less likely to die within 5 years of hepatocellular carcinoma diagnosis when multidisciplinary tumor boards managed their care than if they did not, according to a large, multicenter observational study.
Seeing a hepatologist or surgeon within 30 days of diagnosis also significantly improved 5-year overall survival, even after controlling for age, race, Charlson-Deyo comorbidity index, Barcelona Clinic Liver Cancer (BCLC) stage, academic center and geographic region of care, and the distance patients lived from the nearest Veterans Affairs transplant center, Marina Serper, MD, reported at the annual meeting of the American Association for the Study of Liver Diseases. “More studies are needed to understand how to best use multidisciplinary tumor boards to improve the care of patients with hepatocellular carcinoma,” she said.
Outcomes data for hepatocellular carcinoma mostly come from clinical trials; transplant centers; and Surveillance, Epidemiology, and End Results-Medicare analyses, noted Dr. Serper of the University of Pennsylvania in Philadelphia.
For a better look at veterans, she and her associates combined administrative, laboratory, and death data with medical chart reviews and information from the Organ Procurement and Transplantation Network’s Standard Transplant Analysis and Research file. The initial cohort included more than 6,800 veterans whose ICD-9CM diagnosis code indicated a malignant hepatic neoplasm. Excluding patients with neoplasms such as cholangiocarcinoma and those managed outside the VA left 3,989 VA patients with hepatocellular carcinoma.
In the multivariable analysis, use of multidisciplinary tumor boards was associated with a statistically significant 13% improvement in 5-year overall survival (hazard ratio, 0.87; 95% confidence interval, 0.81-0.94; P less than .001). Improved survival also was linked with seeing certain specialists within 30 days of diagnosis, including hepatologists (HR, 0.77; P less than .001) and surgeons (HR, 0.72; P less than .001). Consulting with a hepatologist within 30 days of diagnosis, however, did not improve the chances of receiving curative therapy, such as liver transplantation, resection, local ablation, transarterial chemoembolization, or Y-90 radioembolization.
Care also varied substantially geographically and by academic affiliation, Dr. Serper noted. “Treatment of hepatocellular carcinoma is complex, as it depends as much on liver function as it does on tumor staging,” she emphasized. “Studies to improve multidisciplinary approaches for hepatocellular carcinoma in the community are needed to increase rates of curative therapy and improve clinical outcomes.”
Patients in this study averaged 62 years of age at diagnosis, 54% were white, 36% were within Milan criteria, and 45% had a Child-Turcotte-Pugh score of B or higher. Nearly 18% had macrovascular invasion at diagnosis, and 7% had metastatic disease. Nearly two-thirds of patients were BCLC stage A or B at diagnosis, and more than a third had underlying alcohol misuse and chronic hepatitis C virus infection.
The work was funded by unrestricted grants from Bayer Healthcare Pharmaceuticals and the VA’s HIV, Hepatitis and Public Health Pathogens Programs. The investigators had no relevant financial disclosures.
BOSTON – Veterans were about 13% less likely to die within 5 years of hepatocellular carcinoma diagnosis when multidisciplinary tumor boards managed their care than if they did not, according to a large, multicenter observational study.
Seeing a hepatologist or surgeon within 30 days of diagnosis also significantly improved 5-year overall survival, even after controlling for age, race, Charlson-Deyo comorbidity index, Barcelona Clinic Liver Cancer (BCLC) stage, academic center and geographic region of care, and the distance patients lived from the nearest Veterans Affairs transplant center, Marina Serper, MD, reported at the annual meeting of the American Association for the Study of Liver Diseases. “More studies are needed to understand how to best use multidisciplinary tumor boards to improve the care of patients with hepatocellular carcinoma,” she said.
Outcomes data for hepatocellular carcinoma mostly come from clinical trials; transplant centers; and Surveillance, Epidemiology, and End Results-Medicare analyses, noted Dr. Serper of the University of Pennsylvania in Philadelphia.
For a better look at veterans, she and her associates combined administrative, laboratory, and death data with medical chart reviews and information from the Organ Procurement and Transplantation Network’s Standard Transplant Analysis and Research file. The initial cohort included more than 6,800 veterans whose ICD-9CM diagnosis code indicated a malignant hepatic neoplasm. Excluding patients with neoplasms such as cholangiocarcinoma and those managed outside the VA left 3,989 VA patients with hepatocellular carcinoma.
In the multivariable analysis, use of multidisciplinary tumor boards was associated with a statistically significant 13% improvement in 5-year overall survival (hazard ratio, 0.87; 95% confidence interval, 0.81-0.94; P less than .001). Improved survival also was linked with seeing certain specialists within 30 days of diagnosis, including hepatologists (HR, 0.77; P less than .001) and surgeons (HR, 0.72; P less than .001). Consulting with a hepatologist within 30 days of diagnosis, however, did not improve the chances of receiving curative therapy, such as liver transplantation, resection, local ablation, transarterial chemoembolization, or Y-90 radioembolization.
Care also varied substantially geographically and by academic affiliation, Dr. Serper noted. “Treatment of hepatocellular carcinoma is complex, as it depends as much on liver function as it does on tumor staging,” she emphasized. “Studies to improve multidisciplinary approaches for hepatocellular carcinoma in the community are needed to increase rates of curative therapy and improve clinical outcomes.”
Patients in this study averaged 62 years of age at diagnosis, 54% were white, 36% were within Milan criteria, and 45% had a Child-Turcotte-Pugh score of B or higher. Nearly 18% had macrovascular invasion at diagnosis, and 7% had metastatic disease. Nearly two-thirds of patients were BCLC stage A or B at diagnosis, and more than a third had underlying alcohol misuse and chronic hepatitis C virus infection.
The work was funded by unrestricted grants from Bayer Healthcare Pharmaceuticals and the VA’s HIV, Hepatitis and Public Health Pathogens Programs. The investigators had no relevant financial disclosures.
AT THE LIVER MEETING 2016
Key clinical point: The use of multidisciplinary tumor boards was associated with significantly improved overall survival in patients with hepatocellular carcinoma.
Major finding: The risk of death within 5 years dropped by about 13% (hazard ratio, 0.87; 95% confidence interval, 0.81-0.94; P less than .001).
Data source: A retrospective study of 3,989 Veterans Affairs patients with hepatocellular carcinoma.
Disclosures: The work was funded by unrestricted grants from Bayer Healthcare Pharmaceuticals and the VA’s HIV, Hepatitis and Public Health Pathogens Programs. The investigators had no relevant financial disclosures.
Breast milk doesn’t contain meaningful levels of certolizumab pegol
WASHINGTON – Certolizumab pegol is not transmitted into human breast milk in any clinically meaningful level, a postmarketing pharmacokinetic study has determined.
While there were individual differences in how much of the TNF inhibitor did cross into milk, none of the 17 women in the study transmitted more than 0.076 mcg/mL in any sample, Megan Clowse, MD, said at the annual meeting of the American College of Rheumatology.
“This is well below even 1% of the expected plasma concentration of a therapeutic dose,” said Dr. Clowse, a rheumatologist and director of the Duke Autoimmunity in Pregnancy Registry at Duke University, Durham, N.C. “Additionally, the mean relative infant dose was 0.125% – also far below the cutoff of less than 10% of the adult dose, the level generally thought to be of little concern for infant well-being.”
The transmission potential, however, has always been assumed to be low. “It’s a protein that would largely be degraded in the gastrointestinal tract of the baby, so there would be low bioavailability. But also CZP has no Fc portion, so it is not pulled across the intestinal lumina by the neonatal Fc receptor.”
Despite those assumptions and the positive – although limited – data, UCB conducted a 4-week postmarketing study to fully determine transmission levels. The CRADLE study enrolled 17 women taking CZP while breastfeeding healthy, full-term infants. Breast milk samples were taken at days 0, 2, 4, 6, 8, 10, 12, and 14 across one dosing period (14 days for those taking 200 mg every 2 weeks; and 28 days for those taking 400 mg every 4 weeks).
In addition to being the first study to estimate the average daily infant dose, CRADLE used a specially created ELISA to measure the drug. “This was a very carefully thought-out measure designed to be 10 times more sensitive than any assay ever used to identify this drug,” Dr. Clowse said. “It had a very high specificity, having to attach to both the TNF portion and the PEG component.”
All the women had a healthy term infant who was exclusively breastfed. Mothers had to be in steady-state dosing with at least three prior doses before the first sample and could not have taken any other biologics within five half-lives of those medications.
The mean age of the 17 women in the analysis was 34 years. Rheumatoid arthritis was the most common diagnosis (7); other conditions were Crohn’s disease (5), psoriatic arthritis (3), and ankylosing spondylitis (2). The majority of the infants (13) were younger than 6 months at the time of the study.
Most of the women (13) had some measurable CZP in at least one sample, and four had measurable CZP in almost every sample. But of the entire 137 samples tested, 77 (56%) came back below the limit of quantification, which was less than 0.032 mcg/mL. Another 52 samples came back as less than twice the lower limit of quantification (less than 0.064 mcg/mL). Among these, though, most were less than 0.050 mcg/mL. Only eight samples approached the level of less than three times the lower limit of quantification (less than 0.096 mcg/mL); of these, the highest level was 0.076 mcg/mL.
There were some strong individual trends, Dr. Clowse noted. Only two women showed the highest levels: Out of seven samples, one had two such readings, and the other had five. In four women, all of the samples were below the lower limit of quantification. The rest of the women had mixed results, which tended to cluster in the middle of their treatment cycle and then go down.
The median maximum concentration in breast milk was 0.04285 mcg/mL, which translated to an average daily infant dose of 0.0035 mg/kg/day. This was an infant dose of 0.125% of the mother’s dose, Dr. Clowse said.
A 5-week safety study followed the breast milk sampling phase. During this time, nine infants had some sort of event. These were mild and not different from that normally seen in breastfed infants. Several events were paired with maternal events, Dr. Clowse said. Two pairs had upper respiratory tract infections, and one mother developed a Candida skin infection while her infant developed oral candidiasis.
UCB sponsored the CRADLE study. Dr. Clowse is a consultant for the company.
[email protected]
On Twitter @alz_gal
WASHINGTON – Certolizumab pegol is not transmitted into human breast milk in any clinically meaningful level, a postmarketing pharmacokinetic study has determined.
While there were individual differences in how much of the TNF inhibitor did cross into milk, none of the 17 women in the study transmitted more than 0.076 mcg/mL in any sample, Megan Clowse, MD, said at the annual meeting of the American College of Rheumatology.
“This is well below even 1% of the expected plasma concentration of a therapeutic dose,” said Dr. Clowse, a rheumatologist and director of the Duke Autoimmunity in Pregnancy Registry at Duke University, Durham, N.C. “Additionally, the mean relative infant dose was 0.125% – also far below the cutoff of less than 10% of the adult dose, the level generally thought to be of little concern for infant well-being.”
The transmission potential, however, has always been assumed to be low. “It’s a protein that would largely be degraded in the gastrointestinal tract of the baby, so there would be low bioavailability. But also CZP has no Fc portion, so it is not pulled across the intestinal lumina by the neonatal Fc receptor.”
Despite those assumptions and the positive – although limited – data, UCB conducted a 4-week postmarketing study to fully determine transmission levels. The CRADLE study enrolled 17 women taking CZP while breastfeeding healthy, full-term infants. Breast milk samples were taken at days 0, 2, 4, 6, 8, 10, 12, and 14 across one dosing period (14 days for those taking 200 mg every 2 weeks; and 28 days for those taking 400 mg every 4 weeks).
In addition to being the first study to estimate the average daily infant dose, CRADLE used a specially created ELISA to measure the drug. “This was a very carefully thought-out measure designed to be 10 times more sensitive than any assay ever used to identify this drug,” Dr. Clowse said. “It had a very high specificity, having to attach to both the TNF portion and the PEG component.”
All the women had a healthy term infant who was exclusively breastfed. Mothers had to be in steady-state dosing with at least three prior doses before the first sample and could not have taken any other biologics within five half-lives of those medications.
The mean age of the 17 women in the analysis was 34 years. Rheumatoid arthritis was the most common diagnosis (7); other conditions were Crohn’s disease (5), psoriatic arthritis (3), and ankylosing spondylitis (2). The majority of the infants (13) were younger than 6 months at the time of the study.
Most of the women (13) had some measurable CZP in at least one sample, and four had measurable CZP in almost every sample. But of the entire 137 samples tested, 77 (56%) came back below the limit of quantification, which was less than 0.032 mcg/mL. Another 52 samples came back as less than twice the lower limit of quantification (less than 0.064 mcg/mL). Among these, though, most were less than 0.050 mcg/mL. Only eight samples approached the level of less than three times the lower limit of quantification (less than 0.096 mcg/mL); of these, the highest level was 0.076 mcg/mL.
There were some strong individual trends, Dr. Clowse noted. Only two women showed the highest levels: Out of seven samples, one had two such readings, and the other had five. In four women, all of the samples were below the lower limit of quantification. The rest of the women had mixed results, which tended to cluster in the middle of their treatment cycle and then go down.
The median maximum concentration in breast milk was 0.04285 mcg/mL, which translated to an average daily infant dose of 0.0035 mg/kg/day. This was an infant dose of 0.125% of the mother’s dose, Dr. Clowse said.
A 5-week safety study followed the breast milk sampling phase. During this time, nine infants had some sort of event. These were mild and not different from that normally seen in breastfed infants. Several events were paired with maternal events, Dr. Clowse said. Two pairs had upper respiratory tract infections, and one mother developed a Candida skin infection while her infant developed oral candidiasis.
UCB sponsored the CRADLE study. Dr. Clowse is a consultant for the company.
[email protected]
On Twitter @alz_gal
WASHINGTON – Certolizumab pegol is not transmitted into human breast milk in any clinically meaningful level, a postmarketing pharmacokinetic study has determined.
While there were individual differences in how much of the TNF inhibitor did cross into milk, none of the 17 women in the study transmitted more than 0.076 mcg/mL in any sample, Megan Clowse, MD, said at the annual meeting of the American College of Rheumatology.
“This is well below even 1% of the expected plasma concentration of a therapeutic dose,” said Dr. Clowse, a rheumatologist and director of the Duke Autoimmunity in Pregnancy Registry at Duke University, Durham, N.C. “Additionally, the mean relative infant dose was 0.125% – also far below the cutoff of less than 10% of the adult dose, the level generally thought to be of little concern for infant well-being.”
The transmission potential, however, has always been assumed to be low. “It’s a protein that would largely be degraded in the gastrointestinal tract of the baby, so there would be low bioavailability. But also CZP has no Fc portion, so it is not pulled across the intestinal lumina by the neonatal Fc receptor.”
Despite those assumptions and the positive – although limited – data, UCB conducted a 4-week postmarketing study to fully determine transmission levels. The CRADLE study enrolled 17 women taking CZP while breastfeeding healthy, full-term infants. Breast milk samples were taken at days 0, 2, 4, 6, 8, 10, 12, and 14 across one dosing period (14 days for those taking 200 mg every 2 weeks; and 28 days for those taking 400 mg every 4 weeks).
In addition to being the first study to estimate the average daily infant dose, CRADLE used a specially created ELISA to measure the drug. “This was a very carefully thought-out measure designed to be 10 times more sensitive than any assay ever used to identify this drug,” Dr. Clowse said. “It had a very high specificity, having to attach to both the TNF portion and the PEG component.”
All the women had a healthy term infant who was exclusively breastfed. Mothers had to be in steady-state dosing with at least three prior doses before the first sample and could not have taken any other biologics within five half-lives of those medications.
The mean age of the 17 women in the analysis was 34 years. Rheumatoid arthritis was the most common diagnosis (7); other conditions were Crohn’s disease (5), psoriatic arthritis (3), and ankylosing spondylitis (2). The majority of the infants (13) were younger than 6 months at the time of the study.
Most of the women (13) had some measurable CZP in at least one sample, and four had measurable CZP in almost every sample. But of the entire 137 samples tested, 77 (56%) came back below the limit of quantification, which was less than 0.032 mcg/mL. Another 52 samples came back as less than twice the lower limit of quantification (less than 0.064 mcg/mL). Among these, though, most were less than 0.050 mcg/mL. Only eight samples approached the level of less than three times the lower limit of quantification (less than 0.096 mcg/mL); of these, the highest level was 0.076 mcg/mL.
There were some strong individual trends, Dr. Clowse noted. Only two women showed the highest levels: Out of seven samples, one had two such readings, and the other had five. In four women, all of the samples were below the lower limit of quantification. The rest of the women had mixed results, which tended to cluster in the middle of their treatment cycle and then go down.
The median maximum concentration in breast milk was 0.04285 mcg/mL, which translated to an average daily infant dose of 0.0035 mg/kg/day. This was an infant dose of 0.125% of the mother’s dose, Dr. Clowse said.
A 5-week safety study followed the breast milk sampling phase. During this time, nine infants had some sort of event. These were mild and not different from that normally seen in breastfed infants. Several events were paired with maternal events, Dr. Clowse said. Two pairs had upper respiratory tract infections, and one mother developed a Candida skin infection while her infant developed oral candidiasis.
UCB sponsored the CRADLE study. Dr. Clowse is a consultant for the company.
[email protected]
On Twitter @alz_gal
AT THE ACR ANNUAL MEETING
Key clinical point:
Major finding: None of the 137 samples contained more than 0.076 mcg/mL of the drug.
Data source: The 4-week postmarketing study comprised 17 breastfeeding women.
Disclosures: UCB sponsored the study. Dr. Clowse is a consultant for the company.
Posttraumatic Stress Disorder, Depression, and Other Comorbidities: Clinical and Systems Approaches to Diagnostic Uncertainties
Over the past decade, nationwide attention has focused on mental health conditions associated with military service. Recent legal mandates have led to changes in the DoD, VA, and HHS health systems aimed at increasing access to care, decreasing barriers to care, and expanding research on mental health conditions commonly seen in service members and veterans. On August 31, 2012, President Barack Obama signed the Improving Access to Mental Health Services for Veterans, Service Members, and Military Families executive order, establishing an interagency task force from the VA, DoD, and HHS.1 The task force was charged with addressing quality of care and provider training in the management of commonly comorbid conditions, including (among other conditions) posttraumatic stress disorder (PTSD) and depression.
Depression and PTSD present major health burdens in both military and veteran cohorts. Overlap in clinical presentation and significant rates of comorbidity complicate effective management of these conditions. This article offers a brief review of the diagnostic and epidemiologic complexities associated with PTSD and depression, a summary of research relevant to these issues, and a description of recent system-level developments within the Military Health System (MHS) designed to improve care through better approaches in identification, management, and research of these conditions.
Diagnostic Uncertainty
Both PTSD and major depressive disorder (MDD) have been recognized as mental health disorders since the American Psychiatric Association’s Diagnostic and Statistical Manual (DSM) discarded its previous etiologically based approach to diagnostic classification in 1980 in favor of a system in which diagnosis is based on observable symptoms.2,3 With the release of DSM-5 in 2013, the diagnostic criteria for PTSD underwent a substantial transformation.4 Previously, PTSD was described as an anxiety disorder, and some of its manifestations overlapped descriptively (and in many cases, etiologically) with anxiety and depressive illnesses.5
Clinicians also often described shorter-lived, developmental, formes fruste, or otherwise subsyndromal manifestations of trauma associated with PTSD. In DSM-5, PTSD was removed from the anxiety disorders section and placed in a new category of disorders labeled Trauma and Stressor-Related Disorders. This new category also included reactive attachment disorder (in children), acute stress disorder, adjustment disorders, and unspecified or other trauma and stressor-related disorders. Other major changes to the PTSD diagnostic criteria included modification to the DSM-IV-TR (text revision) trauma definition (making the construct more specific), removal of the requirement for explicit subjective emotional reaction to a traumatic event, and greater emphasis on negative cognitions and mood. Debate surrounds the updated symptom criteria with critics questioning whether there is any improvement in the clinical utility of the diagnosis, especially in light of the substantial policy and practice implications the change engenders.6
Recently, Hoge and colleagues examined the psychometric implications of the diagnostic changes (between DSM-IV-TR and DSM-5) in the PTSD definition.6 The authors found that although the 2 definitions showed nearly identical association with other psychiatric disorders (including depression) and functional impairment, 30% of soldiers who met DSM-IV-TR criteria for PTSD failed to meet criteria in DSM-5, and another 20% met only DSM-5 criteria. Recognizing discordance in PTSD and associated diagnoses, the U.S. Army Medical Command mandated that its clinicians familiarize themselves with the controversies surrounding the discordant diagnoses and coding of subthreshold PTSD.7
Adding to the problem of diagnostic uncertainty, the clinical presentation of MDD includes significant overlap with that of PTSD. Specifically, symptoms of guilt, diminished interests, problems with concentration, and sleep disturbances are descriptive of both disorders. Furthermore, the criteria set for several subthreshold forms of MDD evidence considerable overlap with PTSD symptoms. For example, diagnostic criteria for disruptive mood dysregulation disorder include behavioral outbursts and irritability, and diagnostic criteria for dysthymia include sleep disturbances and concentration problems.
Adjustment disorders are categorized as trauma and stressor-related disorders in DSM-5 and hold many emotional and behavioral symptoms in common with PTSD. The “acute” and “chronic” adjustment disorder specifiers contribute to problems in diagnostic certainty for PTSD. In general, issues pertaining to diagnostic uncertainty and overlap likely reflect the limits of using a diagnostic classification system that relies exclusively on observational and subjective reports of psychological symptoms.8,9
In a treatment environment where a veteran or active-duty patient has presented for care, in the face of these shared symptom sets, clinicians frequently offer initial diagnoses. These diagnoses are often based on perceived etiologic factors derived from patients’ descriptions of stressors encountered during military service. This tendency likely contributes to considerable inconsistencies and potential inaccuracies in diagnoses, and much of the variance can be attributed to the clinicians’ degree of familiarity with military exposures, perceptions of what constitutes trauma, and outside pressure to assign or avoid specific diagnoses.
Importantly, the phenomenologic differences between PTSD and depressive disorders increase the likelihood of poorly aligned and inconsistent treatment plans, and this lack of clarity may, in turn, compromise effective patient care. To address some of these diagnostic challenges, the VA and DoD incorporate military culture training into clinicians’ curriculum to increase provider familiarity with the common stressors and challenges of military life, mandate the use of validated measures to support diagnostic decision making, and regularly review policies that influence diagnostic practices.
Epidemiology
The prevalence rates for PTSD are increasing in the military, possibly stemming from the demands on service members engaged in years’ long wars. Despite the increased attention on this phenomenon, research has demonstrated that the majority of service members who deploy do not develop PTSD or significant trauma-related functional impairment.10 Furthermore, many cases of PTSD diagnosed in the MHS stem from traumatic experiences other than combat exposure, including childhood abuse and neglect, sexual and other assaults, accidents and health care exposures, domestic abuse, and bullying. Depression arguably has received less attention despite comparable prevalence rates in military populations, high co-occurrence of PTSD and depression, and depression being associated with a greater odds ratio for mortality that includes death by suicide in military service members.11
Estimates of the prevalence of PTSD from the U.S. Army suggest that it exists in 3% to 6% of military members who have not deployed and in 6% to 25% of service members with combat deployment histories. The frequency and intensity of combat are strong predictors of risk.7 A recent epidemiologic study using inpatient and outpatient encounter records showed that the prevalence of PTSD in the active military component was 2.0% in the middle of calendar year (CY) 2010; a two-thirds increase from 1.2% in CY 2007.12 The incidence of PTSD
Epidemiologic studies and prevalence/incidence rates derived from administrative data rely on strict case definitions. Consequently, such administrative investigations include data only from service members
PTSD and Depression Treatment
Despite the high rates of PTSD and MDD comorbidity, few treatments have been developed for and tested on an exclusively comorbid sample of patients.13 However, psychopharmacologic agents targeting depression have been applied to the treatment of PTSD, and PTSD psychotherapy trials typically include depression response as a secondary outcome. The generalizability of findings to a truly comorbid population may be limited based on study sampling frames and the unique characteristics of patients with comorbid PTSD and depression.14-16 Several psychopharmacologic treatments for depression have been evaluated as frontline treatments for PTSD. The 3 pharmacologic treatments that demonstrate efficacy in treating PTSD include fluoxetine, paroxetine, and venlafaxine.17
Although these pharmacologic agents represent good candidate treatments for comorbid patients, the effect size of pharmacologic treatments are generally smaller than those of psychotherapeutic treatments for PTSD.17,18 This observation, however, is based on indirect comparisons, and a recent systematic review concluded that the evidence was insufficient to determine the comparative effectiveness between psychotherapy and pharmacotherapy for PTSD.19 Evidence indicates that trauma-focused cognitive behavioral therapies consistently demonstrate efficacy and effectiveness in treating PTSD.19,20 These treatments also have been shown to significantly reduce depressive symptoms among PTSD samples.21
Based on strong bodies of evidence, these pharmacologic and psychological treatments have received the highest level of recommendation in the VA and DoD.22,23 Accordingly, both agencies have invested considerable resources in large-scale efforts to improve patient access to these particular treatments. Despite these impressive implementation efforts, however, the limitations of relying exclusively on these treatments as frontline approaches within large health care systems have become evident.24-26
Penetration of Therapies
Penetration of these evidence-based treatments (EBTs) within the DoD and VHA remains limited. For instance, one study showed that VA clinicians in mental health specialty care clinics may provide only about 4 hours of EBT per week.27
Other reports suggest that only about 60% of treatment-seeking patients in PTSD clinics receive any type of evidence-based therapy and that within-session care quality is questionable based on a systematic review of chart notes.28,29 Attrition in trauma-focused therapy is a recognized limitation, with 1 out of 3 treatment-seeking patients not completing a full dose of evidence-based treatment.30-33 Large-scale analyses of VHA and DoD utilization data suggest that the majority of PTSD patients do not receive a sufficient number of sessions to be characterized as an adequate dose of EBT, with a majority of dropouts occur- ring after just a few sessions.34-37
Hoge and colleagues found that < 50% of soldiers meeting criteria for PTSD received any mental health care within the prior 6 months with one-quarter of those patients dropping out of care prematurely.38 Among a large cohort of soldiers engaged in care for the treatment of PTSD, only about 40% received a number of EBT treatment sessions that could qualify as an adequate dose.38 Thus, although major advancements in the development and implementation of effective treatments for PTSD and depression have occurred, the penetration of these treatments is limited, and the majority of patients in need of treatment potentially receive inadequate care.39
System level approaches that integrate behavioral health services into the primary care system have been proposed to address these care gaps for service members and veterans.40-42 Fundamentally, system-level approaches seek to improve the reach and effectiveness of care through large-scale screening efforts, a greater emphasis on the quality of patient care, and enhanced care continuity across episodes of treatment.
Primary Care
With the primary care setting considered the de facto mental health system, integrated approaches enhance the reach of care by incorporating uniform mental health screening and referral for patients coming through primary care. Specific evidence-based treatments can be integrated into this approach within a stepped-care framework that aims to match patients strategically to the right type of care and leverage specialty care resources as needed. Integrated care approaches for the treatment of PTSD and depression have been developed and evaluated inside and outside of the MHS. Findings indicate that integrated treatment approaches can improve care access, care continuity, patient satisfaction, quality of care,and in several trials, PTSD and depression outcomes.43-47
Recently, an integrated care approach targeting U.S. Army soldiers who screened positive for PTSD or depression in primary care was evaluated in a multisite effectiveness trial.48 Patients randomized to the treatment approach experienced significant improvements in both PTSD and depression symptoms relative to patients in usual care.43 In addition, patients treated in this care model received significantly more mental health services; the patterns of care indicated that patients with comorbid PTSD and depression were more likely to be triaged to specialty care, whereas patients with a single diagnosis were more likely to be managed in primary care.49 This trial suggests that integrated care models feasibly can be implemented in the U.S. Army care system, yielding increased uptake of mental health care, more efficiently matched care based on patient comorbidities, and improved PTSD and depression outcomes.
Treatment Research
The MHS supports a large portfolio of research in PTSD and depression through DoD/VA research consortia (eg, the Congressionally Directed Medical Research Program, the Consortium to Alleviate PTSD, the Injury and Traumatic Stress Clinical Consortium). The U.S. Army Medical Research and Materiel Command (USAMRMC) executes and manages the portfolio of research, relying on a joint program committee of DoD and non-DoD experts to make funding recommendations based on identified research priorities, policy guidance, and knowledge translation needs.
Health systems research on PTSD and MDD in federal health care settings is expanding. For example, the RAND Corporation recently evaluated a candidate set of quality measures for PTSD and MDD, using an operational definition of an episode of care.37 This work is intended to inform efforts to measure and improve the quality of care for PTSD and depression across the enterprise.
The DoD Defense Centers of Excellence for Psychological Health and Traumatic Brain Injury is simultaneously completing an inferential assessment of adjunctive mental health care services, many focused on PTSD and depression, throughout the health care enterprise. Along with the substantial resources devoted to research on PTSD and depression, the MHS is implementing strategies to improve the system of care for service members with mental health conditions.
Army Care System Innovations
The U.S. Army is engaged in a variety of strategies to improve the identification of patients with mental health conditions, increase access to mental health services, and enhance the quality of care that soldiers receive for PTSD and depression. To improve the coordination of mental health care, the U.S. Army Medical Command implemented a wide-scale innovative transformation of its mental health care system through the establishment of the Behavioral Health Service Line program management office.
This move eliminated separate departments of psychiatry, psychology, and social work in favor of integrated behavioral health departments that are now responsible for all mental health care delivered to soldiers, including inpatient, outpatient, partial hospitalization, residential, embedded care in garrison, and primary care settings. This transformation ensured coordination of care for soldiers, eliminating potential miscommunication with patients, commands, and other clinicians while clearly defining performance indicators in process (eg, productivity, scheduling, access to care, and patient satisfaction) and outcome measures.49 In conjunction with the development of its service line, the U.S. Army created a Behavioral Health Data Portal (BHDP), an electronic and standardized means to assess clinical outcomes for common conditions.
To promote higher quality mental health care, the Office of the Surgeon General of the U.S. Army provided direct guidance on the treatment of PTSD and depression. U.S. Army policy mandates that providers treating mental health conditions adhere to the VA/DoD clinical practice guidelines (CPGs) and that soldiers with PTSD and depression be offered treatments with the highest level of scientific support and that outcome measures be routinely administered. In line with the CPGs, U.S. Army policy also recommends the use of both integrated and embedded mental health care approaches to address PTSD, depression, and other common physical and psychological health conditions.
To reduce stigma and improve mental health care access, the U.S. Army began implementing integrated care approaches in 2007 with its Re-Engineering Systems of Primary Care Treatment in the Military (RESPECT-Mil) program, an evidence-based collaborative care model.51-55 This approach included structured screening and diagnostic procedures, predictable follow-up schedules for patients, and the coordination of the divisions of responsibility among and between primary care providers, paraprofessionals, and behavioral health care providers. From 2007 to 2013, this collaborative care model was rolled out across 96 clinics worldwide and provided PTSD and depression screening to more than 1 million encounters per year.52,53
More recently, the U.S. Army led DoD in integrating behavioral health personnel in patient centered medical homes (PCMH) in compliance with DoD Instruction 6490.15.56 This hybrid integrated care model combines collaborative care elements developed in the RESPECT-Mil program with elements of the U.S. Air Force Behavioral Health Optimization project colocating behavioral health providers in primary care settings to provide brief consultative services.
MHS Care Enhancements
Many of the innovations deployed throughout the U.S. Army system of behavioral health care have driven changes across the MHS as a whole. The DoD and the VA have made substantive systemwide policy and practice changes to improve care for beneficiaries with PTSD, depression, and comorbid PTSD and depression. In particular, significant implementation efforts have addressed population screening strategies, outcome monitoring to support measurement-based care, increased access to effective care, and revision of the disability evaluation system.
To improve the identification and referral of soldiers with deployment-related mental health concerns, the DoD implemented a comprehensive program that screens service members prior to deployment, immediately on redeployment, and then again 6 months after returning from deployment. Additionally, annual primary care- based screening requirements have been instituted as part of the DoD PCMH initiative. Both deployment-related and primary care-based screenings include an instrumentation to detect symptoms of PTSD and depression and extend the reach of mental health screening to the entire MHS population.
Building on the success of BHDP, former Assistant Secretary of Defense for Health Affairs Jonathan Woodson mandated BHDP use across the MHS for all patients in DoD behavioral health clinics and the use of outcome measures for the treatment of PTSD, anxiety, depression, and alcohol use disorders.57 A DoD-wide requirement to use the PTSD checklist and patient health questionnaire to monitor PTSD and depression symptoms at mental health intakes and regularly at follow-up visits is being implemented. The Defense Centers of Excellence for Psychological Health and Traumatic Brain Injury, through its Practice-Based Implementation Network (underwritten by a Joint Incentive Fund managed between DoD and VA), has worked across the MHS and the VA to facilitate the implementation, uptake, and adoption of this initiative.
The DoD established the Center for Deployment Psychology (CDP) in 2006 to promote clinician training in EBTs with the aim of increasing service members’ access to effective psychological treatments. Since its inception, the CDP has provided EBT training to more than 40,000 behavioral health providers. Although the impact of these and other efforts on improving the quality of care that patients receive is unknown, a recent study documented widespread self-reported usage of EBT components in U.S. Army clinics and that providers formally trained in EBTs were more likely to deliver EBTs.58
Finally, systemwide changes to the VA Schedule of Ratings for Disability (VASRD) and integration of DoD and VA disability evaluation systems have led to shifts in diagnosis toward PTSD that usually merit a minimum 50% disability rating. Mandates in law require military clinicians to evaluate patients who have deployed for PTSD and TBI prior to taking any actions associated with administrative separation. The practice of attributing PTSD symptoms to character pathology or personality disorders, even when these symptoms did not clearly manifest or worsen with military service, has likely been eliminated from practice in military and veteran populations.
Robust policy changes to limit personality disorder discharges started in fiscal year 2007, when there were 4,127 personality disorder separations across DoD. This number was reduced to 300 within 5 years. Policy changes regarding separation not only seem to have affected discharges, but also may have shaped diagnostic practice. The incidence rate of personality disorder diagnoses declined from 513 per 100,000 person-years in 2007 to 284 per 100,000 person-years by 2011.59 The VASRD recognizes chronic adjustment disorder as a disability, and the National Defense Authorization Act of 2008 mandated that DoD follow disability guidelines promulgated by VA.
As stated in the memorandum Clinical Policy Guidance for Assessment and Treatment of Post-Traumatic Stress Disorders (August 24, 2012), DoD recognizes chronic adjustment disorder as an unfitting condition that merits referral to its disability evaluation system.60 Acute adjustment disorders may still lead to administrative separations, as many service members manifest emotional symptoms stemming from the failure to adjust to the routine vicissitudes of military life. Finally, many court jurisdictions, including veteran’s courts, military courts, and commanders empowered to adjudicate nonjudicial infractions under the Uniform Code of Military Justice, have recognized PTSD as grounds for the mitigation of penalties associated with a wide array of criminal and administrative infractions.
Conclusion
In response to the increased mental health burden following a decade of war and the associated pressures stemming from federal mandates, the MHS has invested unprecedented resources into improving care for military service members. The U.S. Army has played a prominent role in this endeavor by investing in clinical research efforts to accelerate discovery on the causes and cures for these conditions, enacting policies that mandate best practices, and implementing evidence-based care approaches across the system of care. Despite this progress, however, understanding and effectively treating the most prevalent mental health conditions remain a challenge across the DoD and VHA health care systems. Many service members and veterans still do not receive timely, high-quality care for PTSD, depression, and other common comorbidities associated with military experience, and controversies in diagnostic clarification abound.
In short, great strides have been made, yet there is still a large distance to go. The vision of an effective, efficient, comprehensive care system for mental health conditions will continue to be pursued and achieved through collaborations across key agencies and the scientific community, implementation of health system approaches that support population care, and the sustained efforts of dedicated clinicians, staff, and clinic leaders who deliver the care to our service members and veterans.
1. The White House, Office of the Press Secretary. Executive Order 13625: Improving Access to Mental Health Services for Veterans, Service Members, and Military Families. https://www.whitehouse.gov/the-press-office/2012/08/31/executive-order-improving-access-mental-health-services-veterans-service. Published August 31, 2012. Accessed September 20, 2016.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. Arlington, VA: American Psychiatric Association Press; 1980.
3. Mayes R, Horwitz AV. DSM-III and the revolution in the classification of mental illness. J Hist Behav Sci. 2005;41(3):249-267.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association Press; 2013.
5. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed., text rev. Arlington, VA: American Psychiatric Association Press; 2000.
6. Hoge CW, Riviere LA, Wilk JE, Herrell RK, Weathers FW. The prevalence of post-traumatic stress disorder (PTSD) in US combat soldiers: a head-to-head comparison of DSM-5 versus DSM-IV-TR symptom criteria with the PTSD checklist. Lancet Psychiatry. 2014;1(4):269-277.
7. OTSG-MEDCOM. Policy Memo 14-094: Policy Guidance on the Assessment and Treatment of Posttraumatic Stress Disorder (PTSD). Published December 18, 2014.
8. Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry, 2010;167(7):748-751.
9. National Institute of Mental Health. NIMH strategic plan for research. http://www.nimh.nih.gov/about/strategic-planning-reports/index.shtml. Revised 2015. Accessed September 20, 2016.
10. Colston M, Hocter W. Forensic aspects of posttraumatic stress disorder. In: Ritchie EC, ed. Forensic and Ethical Issues in Military Behavioral Health. Washington, DC: U.S. Department of the Army; 2015:97-110.
11. Defense Centers of Excellence for Psychological Health and Traumatic Brain Injury. National Center for Telehealth and Technology. Department of Defense suicide event report: calendar year 2013 annual report. http://t2health.dcoe.mil/programs/dodser. Published January 13, 2015. Accessed September 20, 2016.
12. Otto JL, O’Donnell FL, Ford SA, Ritschard HV. Selected mental health disorders among active component members, US Armed Forces, 2007-2010. MSMR. 2010;17(11):2-5.
13. Gutner CA, Galovski T, Bovin MJ, Schnurr PP. Emergence of transdiagnostic treatments for PTSD and posttraumatic distress. Curr Psychiatry Rep. 2016;18(10):95-101.
14. Campbell DG, Felker BL, Liu CF, et al. Prevalence of depression-PTSD comorbidity: implications for clinical practice guidelines and primary care-based interventions. J Gen Intern Med. 2007;22(6):711-718.
15. Chan D, Cheadle AD, Reiber G, Unützer J, Chaney EF. Health care utilization and its costs for depressed veterans with and without comorbid PTSD symptoms. Psychiatr Serv. 2009;60(12):1612-1617.
16. Maguen S, Cohen B, Cohen G, Madden E, Bertenthal D, Seal K. Gender differences in health service utilization among Iraq and Afghanistan veterans with posttraumatic stress disorder. J Womens Health (Larchmt). 2012;21(6):666-673.
17. Hoskins M, Pearce J, Bethell A, et al. Pharmacotherapy for post-traumatic stress disorder: systematic review and meta-analysis. Br J Psychiatry. 2015;206(2):93-100.
18. Puetz TW, Youngstedt SD, Herring MP. Effects of pharmacotherapy on combat-related PTSD, anxiety, and depression: a systematic review and meta-regression analysis. PLoS One. 2015;10(5):e0126529.
19. Jonas DE, Cusack K, Forneris CA, et al. Psychological and pharmacological treatments for adults with posttraumatic stress disorder (PTSD). Comparative effectiveness review no. 92. https://effectivehealthcare.ahrq.gov/ehc/products/347/1435/PTSD-adult-treatment-report-130403.pdf. Published April 3, 2013. Accessed September 20, 2016.
20. Haagen JFG, Smid GE, Knipscheer JW, Kleber RJ. The efficacy of recommended treatments for veterans with PTSD: a metaregression analysis. Clin Psychol Rev. 2015;40:184-194.
21. Tran K, Moulton K, Santesso N, Rabb D. Cognitive processing therapy for post-traumatic stress disorder: a systematic review and meta-analysis. https://www.cadth.ca/cognitive-processing-therapy-post-traumatic-stress-disorder-systematic-review-and-meta-analysis. Published August 11, 2015. Accessed September 20, 2016.
22. VA/DoD Management of Post-Traumatic Stress Working Group. VA/DoD Clinical Practice Guideline for Management of Post-Traumatic Stress. Version 2. http://www.healthquality.va.gov/guidelines/MH/ptsd/. Published October, 2010. Accessed September 20, 2016.
23. VA/DoD Management of Major Depressive Disorder Working Group. VA/DoD Clinical Practice Guideline for the Management of Major Depressive Disorder. Version 3. http://www.healthquality.va.gov/guidelines/mh/mdd/index.asp. Published April 2016. Accessed September 20, 2016.
24. Zatzick DF, Galea S. An epidemiologic approach to the development of early trauma focused intervention. J Trauma Stress. 2007;20(4):401-412.
25. Zatzick DF, Koepsell T, Rivara FP. Using target population specification, effect size, and reach to estimate and compare the population impact of two PTSD preventive interventions. Psychiatry. 2009;72(4):346-359.
26. Glasgow RE, Nelson CC, Strycker LA, King DK. Using RE-AIM metrics to evaluate diabetes self-management support interventions. Am J Prev Med. 2006;30(1):67-73.
27. Finley EP, Garcia HA, Ketchum NS, et al. Utilization of evidence-based psychotherapies in Veterans Affairs posttraumatic stress disorder outpatient clinics. Psychol Serv. 2015;12(1):73-82.
28. Mott JM, Mondragon S, Hundt NE, Beason-Smith M, Grady RH, Teng EJ. Characteristics of U.S. veterans who begin and complete prolonged exposure and cognitive processing therapy for PTSD. J Trauma Stress. 2014;27(3):265-273.
29. Shiner B, D’Avolio LW, Nguyen TM, et al. Measuring use of evidence based psychotherapy for PTSD. Adm Policy Ment Health. 2013;40(4):311-318.
30. Schnurr PP, Friedman MJ, Engel CC, et al. Cognitive behavioral therapy for posttraumatic stress disorder in women: a randomized controlled trial. JAMA. 2007;297(8):820-830.
31. Tuerk PW, Yoder M, Grubaugh A, Myrick H, Hamner M, Acierno R. Prolonged exposure therapy for combat-related posttraumatic stress disorder: an examination of treatment effectiveness for veterans of the wars in Afghanistan and Iraq. J Anxiety Disord. 2011;25(3):397-403.
32. Chard KM, Schumm JA, Owens GP, Cottingham SM. A comparison of OEF and OIF veterans and Vietnam veterans receiving cognitive processing therapy. J Trauma Stress. 2010;23(1):25-32.
33. Monson CM, Schnurr PP, Resick PA, Friedman MJ, Young-Xu Y, Stevens SP. Cognitive processing therapy for veterans with military-related posttraumatic stress disorder. J Consult Clin Psychol. 2006;74(5):898-907.
34. Mott JM, Hundt NE, Sansgiry S, Mignogna J, Cully JA. Changes in psychotherapy utilization among veterans with depression, anxiety, and PTSD. Psychiatr Serv. 2014;65(1):106-112.
35. Seal KH, Maguen S, Cohen B, et al. VA mental health services utilization in Iraq and Afghanistan veterans in the first year of receiving new mental health diagnoses. J Trauma Stress. 2010;23(1):5-16.
36. Russell M, Silver SM. Training needs for the treatment of combat-related posttraumatic stress disorder: a survey of Department of Defense clinicians. Traumatology. 2007;13(3):4-10.
37. Schell TL, Marshall GN. Survey of individuals previously deployed for OEF/OIF. In: Tanielian T, Jaycox LH, eds. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: RAND Corporation; 2008:87-118.
38. Hoge CW, Grossman SH, Auchterlonie JL, Riviere LA, Milliken CS, >Wilk JE. PTSD treatment for soldiers after combat deployment: low utilization of mental health care and reasons for dropout. Psychiatr Serv. 2014;65(8):997-1004.
39. Committee on the Assessment of Ongoing Efforts in the Treatment of Posttraumatic Stress Disorder, Board on the Health of Select Populations, Institute of Medicine. Treatment for Posttraumatic Stress Disorder in Military and Veteran Populations: Final Assessment. Washington, DC: National Academies Press; 2014.
40. Schnurr PP. Extending collaborative care for posttraumatic mental health. JAMA Intern Med. 2016;176(7):956-957.
41. Hoge CW. Interventions for war-related posttraumatic stress disorder: meeting veterans where they are. JAMA. 2011;306(5):549-551.
42. Engel CC. Improving primary care for military personnel and veterans with posttraumatic stress disorder: the road ahead. Gen Hosp Psychiatry. 2005;27(3):158-160.
43. Engel CC, Jaycox LH, Freed MC, et al. Centrally assisted collaborative telecare management for posttraumatic stress disorder and depression in military primary care: a randomized controlled trial. JAMA Intern Med. 2016;176(7):948-956.
44. Fortney JC, Pyne JM, Kimbrell TA, et al. Telemedicine-based collaborative care for posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(1):58-67.
45. Schnurr PP, Friedman MJ, Oxman TE, et al. RESPECT-PTSD: re-engineering systems for the primary care treatment of PTSD, a randomized controlled trial. J Gen Intern Med. 2013;28(1):32-40.
46. Zatzick D, Roy-Byrne P, Russo J, et al. A randomized effectiveness trial of stepped collaborative care for acutely injured trauma survivors. Arch Gen Psychiatry. 2004;61(5):498-506.
47. Zatzick D, O’Connor SS, Russo J, et al. Technology-enhanced stepped collaborative care targeting posttraumatic stress disorder and comorbidity after injury: a randomized controlled trial. J Trauma Stress. 2015;28(5):391-400.
48. Engel CC, Bray RM, Jaycox LH, et al. Implementing collaborative primary care for depression and posttraumatic stress disorder: design and sample for a randomized trial in the U.S. Military Health System. Contemp Clin Trials. 2014;39(2):310-319.
49. Belsher BE, Jaycox LH, Freed MC, et al. Mental health utilization patterns during a stepped, collaborative care effectiveness trial for PTSD and depression in the military health system. Med Care. 2016;54(7):706-713.
50. Hepner KA, Roth CP, Farris C, et al. Measuring the Quality of Care for Psychological Health Conditions in the Military Health System: Candidate Quality Measures for Posttraumatic Stress Disorder and Major Depressive Disorder. Santa Monica, CA: RAND Corporation; 2015.
51. Engel C, Oxman T, Yamamoto C, et al. RESPECT-Mil: feasibility of a systems-level collaborative care approach to depression and post-traumatic stress disorder in military primary care. Mil Med. 2008;173(10):935-940.
52. Belsher BE, Curry J, McCutchan P, et al. Implementation of a collaborative care initiative for PTSD and depression in the Army primary care system. Soc Work Ment Health. 2014;12(5-6):500-522.
53. Wong EC, Jaycox LH, Ayer L, et al. Evaluating the Implementation of the Re-Engineering Systems of Primary Care Treatment in the Military (RESPECT-Mil). Santa Monica, CA: RAND Corporation; 2015.
54. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10:CD006525.
55. Woltmann E, Grogan-Kaylor A, Perron B, Georges H, Kilbourne AM, Bauer MS. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis. Am J Psychiatry. 2012;169(8):790-804.
56. Wright JL. DoD Directive 6490.15. www.dtic.mil/whs/directives/corres/pdf/649015p.pdf.Revised November 20, 2014. Accessed October 3, 2016. 57. Woodson J. Military treatment facility mental health clinical outcomes guidance. http://dcoe.mil/Libraries/Documents/MentalHealthClinicalOutcomesGuidance_Woodson.pdf. Published September 9, 2013. Accessed October 4, 2016.
58. Wilk JE, West JC, Duffy FF, Herrell RK, Rae DS, Hoge CW. Use of evidence-based treatment for posttraumatic stress disorder in Army behavioral healthcare. Psychiatry. 2013;76(4):336-348.
59. Stockton PN, Olsen ET, Hayford S, et al. Security from within: independent review of the Washington Navy Yard shooting. http://archive.defense.gov/pubs/Independent-Review-of-the-WNY-Shooting-14-Nov-2013.pdf. Published November, 2013. Accessed September 20, 2016.
60. Woodson J. ASD(HA) Memorandum: Clinical Policy Guidance for Assessment and Treatment of Posttraumatic Stress Disorder. August 24, 2012.
Over the past decade, nationwide attention has focused on mental health conditions associated with military service. Recent legal mandates have led to changes in the DoD, VA, and HHS health systems aimed at increasing access to care, decreasing barriers to care, and expanding research on mental health conditions commonly seen in service members and veterans. On August 31, 2012, President Barack Obama signed the Improving Access to Mental Health Services for Veterans, Service Members, and Military Families executive order, establishing an interagency task force from the VA, DoD, and HHS.1 The task force was charged with addressing quality of care and provider training in the management of commonly comorbid conditions, including (among other conditions) posttraumatic stress disorder (PTSD) and depression.
Depression and PTSD present major health burdens in both military and veteran cohorts. Overlap in clinical presentation and significant rates of comorbidity complicate effective management of these conditions. This article offers a brief review of the diagnostic and epidemiologic complexities associated with PTSD and depression, a summary of research relevant to these issues, and a description of recent system-level developments within the Military Health System (MHS) designed to improve care through better approaches in identification, management, and research of these conditions.
Diagnostic Uncertainty
Both PTSD and major depressive disorder (MDD) have been recognized as mental health disorders since the American Psychiatric Association’s Diagnostic and Statistical Manual (DSM) discarded its previous etiologically based approach to diagnostic classification in 1980 in favor of a system in which diagnosis is based on observable symptoms.2,3 With the release of DSM-5 in 2013, the diagnostic criteria for PTSD underwent a substantial transformation.4 Previously, PTSD was described as an anxiety disorder, and some of its manifestations overlapped descriptively (and in many cases, etiologically) with anxiety and depressive illnesses.5
Clinicians also often described shorter-lived, developmental, formes fruste, or otherwise subsyndromal manifestations of trauma associated with PTSD. In DSM-5, PTSD was removed from the anxiety disorders section and placed in a new category of disorders labeled Trauma and Stressor-Related Disorders. This new category also included reactive attachment disorder (in children), acute stress disorder, adjustment disorders, and unspecified or other trauma and stressor-related disorders. Other major changes to the PTSD diagnostic criteria included modification to the DSM-IV-TR (text revision) trauma definition (making the construct more specific), removal of the requirement for explicit subjective emotional reaction to a traumatic event, and greater emphasis on negative cognitions and mood. Debate surrounds the updated symptom criteria with critics questioning whether there is any improvement in the clinical utility of the diagnosis, especially in light of the substantial policy and practice implications the change engenders.6
Recently, Hoge and colleagues examined the psychometric implications of the diagnostic changes (between DSM-IV-TR and DSM-5) in the PTSD definition.6 The authors found that although the 2 definitions showed nearly identical association with other psychiatric disorders (including depression) and functional impairment, 30% of soldiers who met DSM-IV-TR criteria for PTSD failed to meet criteria in DSM-5, and another 20% met only DSM-5 criteria. Recognizing discordance in PTSD and associated diagnoses, the U.S. Army Medical Command mandated that its clinicians familiarize themselves with the controversies surrounding the discordant diagnoses and coding of subthreshold PTSD.7
Adding to the problem of diagnostic uncertainty, the clinical presentation of MDD includes significant overlap with that of PTSD. Specifically, symptoms of guilt, diminished interests, problems with concentration, and sleep disturbances are descriptive of both disorders. Furthermore, the criteria set for several subthreshold forms of MDD evidence considerable overlap with PTSD symptoms. For example, diagnostic criteria for disruptive mood dysregulation disorder include behavioral outbursts and irritability, and diagnostic criteria for dysthymia include sleep disturbances and concentration problems.
Adjustment disorders are categorized as trauma and stressor-related disorders in DSM-5 and hold many emotional and behavioral symptoms in common with PTSD. The “acute” and “chronic” adjustment disorder specifiers contribute to problems in diagnostic certainty for PTSD. In general, issues pertaining to diagnostic uncertainty and overlap likely reflect the limits of using a diagnostic classification system that relies exclusively on observational and subjective reports of psychological symptoms.8,9
In a treatment environment where a veteran or active-duty patient has presented for care, in the face of these shared symptom sets, clinicians frequently offer initial diagnoses. These diagnoses are often based on perceived etiologic factors derived from patients’ descriptions of stressors encountered during military service. This tendency likely contributes to considerable inconsistencies and potential inaccuracies in diagnoses, and much of the variance can be attributed to the clinicians’ degree of familiarity with military exposures, perceptions of what constitutes trauma, and outside pressure to assign or avoid specific diagnoses.
Importantly, the phenomenologic differences between PTSD and depressive disorders increase the likelihood of poorly aligned and inconsistent treatment plans, and this lack of clarity may, in turn, compromise effective patient care. To address some of these diagnostic challenges, the VA and DoD incorporate military culture training into clinicians’ curriculum to increase provider familiarity with the common stressors and challenges of military life, mandate the use of validated measures to support diagnostic decision making, and regularly review policies that influence diagnostic practices.
Epidemiology
The prevalence rates for PTSD are increasing in the military, possibly stemming from the demands on service members engaged in years’ long wars. Despite the increased attention on this phenomenon, research has demonstrated that the majority of service members who deploy do not develop PTSD or significant trauma-related functional impairment.10 Furthermore, many cases of PTSD diagnosed in the MHS stem from traumatic experiences other than combat exposure, including childhood abuse and neglect, sexual and other assaults, accidents and health care exposures, domestic abuse, and bullying. Depression arguably has received less attention despite comparable prevalence rates in military populations, high co-occurrence of PTSD and depression, and depression being associated with a greater odds ratio for mortality that includes death by suicide in military service members.11
Estimates of the prevalence of PTSD from the U.S. Army suggest that it exists in 3% to 6% of military members who have not deployed and in 6% to 25% of service members with combat deployment histories. The frequency and intensity of combat are strong predictors of risk.7 A recent epidemiologic study using inpatient and outpatient encounter records showed that the prevalence of PTSD in the active military component was 2.0% in the middle of calendar year (CY) 2010; a two-thirds increase from 1.2% in CY 2007.12 The incidence of PTSD
Epidemiologic studies and prevalence/incidence rates derived from administrative data rely on strict case definitions. Consequently, such administrative investigations include data only from service members
PTSD and Depression Treatment
Despite the high rates of PTSD and MDD comorbidity, few treatments have been developed for and tested on an exclusively comorbid sample of patients.13 However, psychopharmacologic agents targeting depression have been applied to the treatment of PTSD, and PTSD psychotherapy trials typically include depression response as a secondary outcome. The generalizability of findings to a truly comorbid population may be limited based on study sampling frames and the unique characteristics of patients with comorbid PTSD and depression.14-16 Several psychopharmacologic treatments for depression have been evaluated as frontline treatments for PTSD. The 3 pharmacologic treatments that demonstrate efficacy in treating PTSD include fluoxetine, paroxetine, and venlafaxine.17
Although these pharmacologic agents represent good candidate treatments for comorbid patients, the effect size of pharmacologic treatments are generally smaller than those of psychotherapeutic treatments for PTSD.17,18 This observation, however, is based on indirect comparisons, and a recent systematic review concluded that the evidence was insufficient to determine the comparative effectiveness between psychotherapy and pharmacotherapy for PTSD.19 Evidence indicates that trauma-focused cognitive behavioral therapies consistently demonstrate efficacy and effectiveness in treating PTSD.19,20 These treatments also have been shown to significantly reduce depressive symptoms among PTSD samples.21
Based on strong bodies of evidence, these pharmacologic and psychological treatments have received the highest level of recommendation in the VA and DoD.22,23 Accordingly, both agencies have invested considerable resources in large-scale efforts to improve patient access to these particular treatments. Despite these impressive implementation efforts, however, the limitations of relying exclusively on these treatments as frontline approaches within large health care systems have become evident.24-26
Penetration of Therapies
Penetration of these evidence-based treatments (EBTs) within the DoD and VHA remains limited. For instance, one study showed that VA clinicians in mental health specialty care clinics may provide only about 4 hours of EBT per week.27
Other reports suggest that only about 60% of treatment-seeking patients in PTSD clinics receive any type of evidence-based therapy and that within-session care quality is questionable based on a systematic review of chart notes.28,29 Attrition in trauma-focused therapy is a recognized limitation, with 1 out of 3 treatment-seeking patients not completing a full dose of evidence-based treatment.30-33 Large-scale analyses of VHA and DoD utilization data suggest that the majority of PTSD patients do not receive a sufficient number of sessions to be characterized as an adequate dose of EBT, with a majority of dropouts occur- ring after just a few sessions.34-37
Hoge and colleagues found that < 50% of soldiers meeting criteria for PTSD received any mental health care within the prior 6 months with one-quarter of those patients dropping out of care prematurely.38 Among a large cohort of soldiers engaged in care for the treatment of PTSD, only about 40% received a number of EBT treatment sessions that could qualify as an adequate dose.38 Thus, although major advancements in the development and implementation of effective treatments for PTSD and depression have occurred, the penetration of these treatments is limited, and the majority of patients in need of treatment potentially receive inadequate care.39
System level approaches that integrate behavioral health services into the primary care system have been proposed to address these care gaps for service members and veterans.40-42 Fundamentally, system-level approaches seek to improve the reach and effectiveness of care through large-scale screening efforts, a greater emphasis on the quality of patient care, and enhanced care continuity across episodes of treatment.
Primary Care
With the primary care setting considered the de facto mental health system, integrated approaches enhance the reach of care by incorporating uniform mental health screening and referral for patients coming through primary care. Specific evidence-based treatments can be integrated into this approach within a stepped-care framework that aims to match patients strategically to the right type of care and leverage specialty care resources as needed. Integrated care approaches for the treatment of PTSD and depression have been developed and evaluated inside and outside of the MHS. Findings indicate that integrated treatment approaches can improve care access, care continuity, patient satisfaction, quality of care,and in several trials, PTSD and depression outcomes.43-47
Recently, an integrated care approach targeting U.S. Army soldiers who screened positive for PTSD or depression in primary care was evaluated in a multisite effectiveness trial.48 Patients randomized to the treatment approach experienced significant improvements in both PTSD and depression symptoms relative to patients in usual care.43 In addition, patients treated in this care model received significantly more mental health services; the patterns of care indicated that patients with comorbid PTSD and depression were more likely to be triaged to specialty care, whereas patients with a single diagnosis were more likely to be managed in primary care.49 This trial suggests that integrated care models feasibly can be implemented in the U.S. Army care system, yielding increased uptake of mental health care, more efficiently matched care based on patient comorbidities, and improved PTSD and depression outcomes.
Treatment Research
The MHS supports a large portfolio of research in PTSD and depression through DoD/VA research consortia (eg, the Congressionally Directed Medical Research Program, the Consortium to Alleviate PTSD, the Injury and Traumatic Stress Clinical Consortium). The U.S. Army Medical Research and Materiel Command (USAMRMC) executes and manages the portfolio of research, relying on a joint program committee of DoD and non-DoD experts to make funding recommendations based on identified research priorities, policy guidance, and knowledge translation needs.
Health systems research on PTSD and MDD in federal health care settings is expanding. For example, the RAND Corporation recently evaluated a candidate set of quality measures for PTSD and MDD, using an operational definition of an episode of care.37 This work is intended to inform efforts to measure and improve the quality of care for PTSD and depression across the enterprise.
The DoD Defense Centers of Excellence for Psychological Health and Traumatic Brain Injury is simultaneously completing an inferential assessment of adjunctive mental health care services, many focused on PTSD and depression, throughout the health care enterprise. Along with the substantial resources devoted to research on PTSD and depression, the MHS is implementing strategies to improve the system of care for service members with mental health conditions.
Army Care System Innovations
The U.S. Army is engaged in a variety of strategies to improve the identification of patients with mental health conditions, increase access to mental health services, and enhance the quality of care that soldiers receive for PTSD and depression. To improve the coordination of mental health care, the U.S. Army Medical Command implemented a wide-scale innovative transformation of its mental health care system through the establishment of the Behavioral Health Service Line program management office.
This move eliminated separate departments of psychiatry, psychology, and social work in favor of integrated behavioral health departments that are now responsible for all mental health care delivered to soldiers, including inpatient, outpatient, partial hospitalization, residential, embedded care in garrison, and primary care settings. This transformation ensured coordination of care for soldiers, eliminating potential miscommunication with patients, commands, and other clinicians while clearly defining performance indicators in process (eg, productivity, scheduling, access to care, and patient satisfaction) and outcome measures.49 In conjunction with the development of its service line, the U.S. Army created a Behavioral Health Data Portal (BHDP), an electronic and standardized means to assess clinical outcomes for common conditions.
To promote higher quality mental health care, the Office of the Surgeon General of the U.S. Army provided direct guidance on the treatment of PTSD and depression. U.S. Army policy mandates that providers treating mental health conditions adhere to the VA/DoD clinical practice guidelines (CPGs) and that soldiers with PTSD and depression be offered treatments with the highest level of scientific support and that outcome measures be routinely administered. In line with the CPGs, U.S. Army policy also recommends the use of both integrated and embedded mental health care approaches to address PTSD, depression, and other common physical and psychological health conditions.
To reduce stigma and improve mental health care access, the U.S. Army began implementing integrated care approaches in 2007 with its Re-Engineering Systems of Primary Care Treatment in the Military (RESPECT-Mil) program, an evidence-based collaborative care model.51-55 This approach included structured screening and diagnostic procedures, predictable follow-up schedules for patients, and the coordination of the divisions of responsibility among and between primary care providers, paraprofessionals, and behavioral health care providers. From 2007 to 2013, this collaborative care model was rolled out across 96 clinics worldwide and provided PTSD and depression screening to more than 1 million encounters per year.52,53
More recently, the U.S. Army led DoD in integrating behavioral health personnel in patient centered medical homes (PCMH) in compliance with DoD Instruction 6490.15.56 This hybrid integrated care model combines collaborative care elements developed in the RESPECT-Mil program with elements of the U.S. Air Force Behavioral Health Optimization project colocating behavioral health providers in primary care settings to provide brief consultative services.
MHS Care Enhancements
Many of the innovations deployed throughout the U.S. Army system of behavioral health care have driven changes across the MHS as a whole. The DoD and the VA have made substantive systemwide policy and practice changes to improve care for beneficiaries with PTSD, depression, and comorbid PTSD and depression. In particular, significant implementation efforts have addressed population screening strategies, outcome monitoring to support measurement-based care, increased access to effective care, and revision of the disability evaluation system.
To improve the identification and referral of soldiers with deployment-related mental health concerns, the DoD implemented a comprehensive program that screens service members prior to deployment, immediately on redeployment, and then again 6 months after returning from deployment. Additionally, annual primary care- based screening requirements have been instituted as part of the DoD PCMH initiative. Both deployment-related and primary care-based screenings include an instrumentation to detect symptoms of PTSD and depression and extend the reach of mental health screening to the entire MHS population.
Building on the success of BHDP, former Assistant Secretary of Defense for Health Affairs Jonathan Woodson mandated BHDP use across the MHS for all patients in DoD behavioral health clinics and the use of outcome measures for the treatment of PTSD, anxiety, depression, and alcohol use disorders.57 A DoD-wide requirement to use the PTSD checklist and patient health questionnaire to monitor PTSD and depression symptoms at mental health intakes and regularly at follow-up visits is being implemented. The Defense Centers of Excellence for Psychological Health and Traumatic Brain Injury, through its Practice-Based Implementation Network (underwritten by a Joint Incentive Fund managed between DoD and VA), has worked across the MHS and the VA to facilitate the implementation, uptake, and adoption of this initiative.
The DoD established the Center for Deployment Psychology (CDP) in 2006 to promote clinician training in EBTs with the aim of increasing service members’ access to effective psychological treatments. Since its inception, the CDP has provided EBT training to more than 40,000 behavioral health providers. Although the impact of these and other efforts on improving the quality of care that patients receive is unknown, a recent study documented widespread self-reported usage of EBT components in U.S. Army clinics and that providers formally trained in EBTs were more likely to deliver EBTs.58
Finally, systemwide changes to the VA Schedule of Ratings for Disability (VASRD) and integration of DoD and VA disability evaluation systems have led to shifts in diagnosis toward PTSD that usually merit a minimum 50% disability rating. Mandates in law require military clinicians to evaluate patients who have deployed for PTSD and TBI prior to taking any actions associated with administrative separation. The practice of attributing PTSD symptoms to character pathology or personality disorders, even when these symptoms did not clearly manifest or worsen with military service, has likely been eliminated from practice in military and veteran populations.
Robust policy changes to limit personality disorder discharges started in fiscal year 2007, when there were 4,127 personality disorder separations across DoD. This number was reduced to 300 within 5 years. Policy changes regarding separation not only seem to have affected discharges, but also may have shaped diagnostic practice. The incidence rate of personality disorder diagnoses declined from 513 per 100,000 person-years in 2007 to 284 per 100,000 person-years by 2011.59 The VASRD recognizes chronic adjustment disorder as a disability, and the National Defense Authorization Act of 2008 mandated that DoD follow disability guidelines promulgated by VA.
As stated in the memorandum Clinical Policy Guidance for Assessment and Treatment of Post-Traumatic Stress Disorders (August 24, 2012), DoD recognizes chronic adjustment disorder as an unfitting condition that merits referral to its disability evaluation system.60 Acute adjustment disorders may still lead to administrative separations, as many service members manifest emotional symptoms stemming from the failure to adjust to the routine vicissitudes of military life. Finally, many court jurisdictions, including veteran’s courts, military courts, and commanders empowered to adjudicate nonjudicial infractions under the Uniform Code of Military Justice, have recognized PTSD as grounds for the mitigation of penalties associated with a wide array of criminal and administrative infractions.
Conclusion
In response to the increased mental health burden following a decade of war and the associated pressures stemming from federal mandates, the MHS has invested unprecedented resources into improving care for military service members. The U.S. Army has played a prominent role in this endeavor by investing in clinical research efforts to accelerate discovery on the causes and cures for these conditions, enacting policies that mandate best practices, and implementing evidence-based care approaches across the system of care. Despite this progress, however, understanding and effectively treating the most prevalent mental health conditions remain a challenge across the DoD and VHA health care systems. Many service members and veterans still do not receive timely, high-quality care for PTSD, depression, and other common comorbidities associated with military experience, and controversies in diagnostic clarification abound.
In short, great strides have been made, yet there is still a large distance to go. The vision of an effective, efficient, comprehensive care system for mental health conditions will continue to be pursued and achieved through collaborations across key agencies and the scientific community, implementation of health system approaches that support population care, and the sustained efforts of dedicated clinicians, staff, and clinic leaders who deliver the care to our service members and veterans.
Over the past decade, nationwide attention has focused on mental health conditions associated with military service. Recent legal mandates have led to changes in the DoD, VA, and HHS health systems aimed at increasing access to care, decreasing barriers to care, and expanding research on mental health conditions commonly seen in service members and veterans. On August 31, 2012, President Barack Obama signed the Improving Access to Mental Health Services for Veterans, Service Members, and Military Families executive order, establishing an interagency task force from the VA, DoD, and HHS.1 The task force was charged with addressing quality of care and provider training in the management of commonly comorbid conditions, including (among other conditions) posttraumatic stress disorder (PTSD) and depression.
Depression and PTSD present major health burdens in both military and veteran cohorts. Overlap in clinical presentation and significant rates of comorbidity complicate effective management of these conditions. This article offers a brief review of the diagnostic and epidemiologic complexities associated with PTSD and depression, a summary of research relevant to these issues, and a description of recent system-level developments within the Military Health System (MHS) designed to improve care through better approaches in identification, management, and research of these conditions.
Diagnostic Uncertainty
Both PTSD and major depressive disorder (MDD) have been recognized as mental health disorders since the American Psychiatric Association’s Diagnostic and Statistical Manual (DSM) discarded its previous etiologically based approach to diagnostic classification in 1980 in favor of a system in which diagnosis is based on observable symptoms.2,3 With the release of DSM-5 in 2013, the diagnostic criteria for PTSD underwent a substantial transformation.4 Previously, PTSD was described as an anxiety disorder, and some of its manifestations overlapped descriptively (and in many cases, etiologically) with anxiety and depressive illnesses.5
Clinicians also often described shorter-lived, developmental, formes fruste, or otherwise subsyndromal manifestations of trauma associated with PTSD. In DSM-5, PTSD was removed from the anxiety disorders section and placed in a new category of disorders labeled Trauma and Stressor-Related Disorders. This new category also included reactive attachment disorder (in children), acute stress disorder, adjustment disorders, and unspecified or other trauma and stressor-related disorders. Other major changes to the PTSD diagnostic criteria included modification to the DSM-IV-TR (text revision) trauma definition (making the construct more specific), removal of the requirement for explicit subjective emotional reaction to a traumatic event, and greater emphasis on negative cognitions and mood. Debate surrounds the updated symptom criteria with critics questioning whether there is any improvement in the clinical utility of the diagnosis, especially in light of the substantial policy and practice implications the change engenders.6
Recently, Hoge and colleagues examined the psychometric implications of the diagnostic changes (between DSM-IV-TR and DSM-5) in the PTSD definition.6 The authors found that although the 2 definitions showed nearly identical association with other psychiatric disorders (including depression) and functional impairment, 30% of soldiers who met DSM-IV-TR criteria for PTSD failed to meet criteria in DSM-5, and another 20% met only DSM-5 criteria. Recognizing discordance in PTSD and associated diagnoses, the U.S. Army Medical Command mandated that its clinicians familiarize themselves with the controversies surrounding the discordant diagnoses and coding of subthreshold PTSD.7
Adding to the problem of diagnostic uncertainty, the clinical presentation of MDD includes significant overlap with that of PTSD. Specifically, symptoms of guilt, diminished interests, problems with concentration, and sleep disturbances are descriptive of both disorders. Furthermore, the criteria set for several subthreshold forms of MDD evidence considerable overlap with PTSD symptoms. For example, diagnostic criteria for disruptive mood dysregulation disorder include behavioral outbursts and irritability, and diagnostic criteria for dysthymia include sleep disturbances and concentration problems.
Adjustment disorders are categorized as trauma and stressor-related disorders in DSM-5 and hold many emotional and behavioral symptoms in common with PTSD. The “acute” and “chronic” adjustment disorder specifiers contribute to problems in diagnostic certainty for PTSD. In general, issues pertaining to diagnostic uncertainty and overlap likely reflect the limits of using a diagnostic classification system that relies exclusively on observational and subjective reports of psychological symptoms.8,9
In a treatment environment where a veteran or active-duty patient has presented for care, in the face of these shared symptom sets, clinicians frequently offer initial diagnoses. These diagnoses are often based on perceived etiologic factors derived from patients’ descriptions of stressors encountered during military service. This tendency likely contributes to considerable inconsistencies and potential inaccuracies in diagnoses, and much of the variance can be attributed to the clinicians’ degree of familiarity with military exposures, perceptions of what constitutes trauma, and outside pressure to assign or avoid specific diagnoses.
Importantly, the phenomenologic differences between PTSD and depressive disorders increase the likelihood of poorly aligned and inconsistent treatment plans, and this lack of clarity may, in turn, compromise effective patient care. To address some of these diagnostic challenges, the VA and DoD incorporate military culture training into clinicians’ curriculum to increase provider familiarity with the common stressors and challenges of military life, mandate the use of validated measures to support diagnostic decision making, and regularly review policies that influence diagnostic practices.
Epidemiology
The prevalence rates for PTSD are increasing in the military, possibly stemming from the demands on service members engaged in years’ long wars. Despite the increased attention on this phenomenon, research has demonstrated that the majority of service members who deploy do not develop PTSD or significant trauma-related functional impairment.10 Furthermore, many cases of PTSD diagnosed in the MHS stem from traumatic experiences other than combat exposure, including childhood abuse and neglect, sexual and other assaults, accidents and health care exposures, domestic abuse, and bullying. Depression arguably has received less attention despite comparable prevalence rates in military populations, high co-occurrence of PTSD and depression, and depression being associated with a greater odds ratio for mortality that includes death by suicide in military service members.11
Estimates of the prevalence of PTSD from the U.S. Army suggest that it exists in 3% to 6% of military members who have not deployed and in 6% to 25% of service members with combat deployment histories. The frequency and intensity of combat are strong predictors of risk.7 A recent epidemiologic study using inpatient and outpatient encounter records showed that the prevalence of PTSD in the active military component was 2.0% in the middle of calendar year (CY) 2010; a two-thirds increase from 1.2% in CY 2007.12 The incidence of PTSD
Epidemiologic studies and prevalence/incidence rates derived from administrative data rely on strict case definitions. Consequently, such administrative investigations include data only from service members
PTSD and Depression Treatment
Despite the high rates of PTSD and MDD comorbidity, few treatments have been developed for and tested on an exclusively comorbid sample of patients.13 However, psychopharmacologic agents targeting depression have been applied to the treatment of PTSD, and PTSD psychotherapy trials typically include depression response as a secondary outcome. The generalizability of findings to a truly comorbid population may be limited based on study sampling frames and the unique characteristics of patients with comorbid PTSD and depression.14-16 Several psychopharmacologic treatments for depression have been evaluated as frontline treatments for PTSD. The 3 pharmacologic treatments that demonstrate efficacy in treating PTSD include fluoxetine, paroxetine, and venlafaxine.17
Although these pharmacologic agents represent good candidate treatments for comorbid patients, the effect size of pharmacologic treatments are generally smaller than those of psychotherapeutic treatments for PTSD.17,18 This observation, however, is based on indirect comparisons, and a recent systematic review concluded that the evidence was insufficient to determine the comparative effectiveness between psychotherapy and pharmacotherapy for PTSD.19 Evidence indicates that trauma-focused cognitive behavioral therapies consistently demonstrate efficacy and effectiveness in treating PTSD.19,20 These treatments also have been shown to significantly reduce depressive symptoms among PTSD samples.21
Based on strong bodies of evidence, these pharmacologic and psychological treatments have received the highest level of recommendation in the VA and DoD.22,23 Accordingly, both agencies have invested considerable resources in large-scale efforts to improve patient access to these particular treatments. Despite these impressive implementation efforts, however, the limitations of relying exclusively on these treatments as frontline approaches within large health care systems have become evident.24-26
Penetration of Therapies
Penetration of these evidence-based treatments (EBTs) within the DoD and VHA remains limited. For instance, one study showed that VA clinicians in mental health specialty care clinics may provide only about 4 hours of EBT per week.27
Other reports suggest that only about 60% of treatment-seeking patients in PTSD clinics receive any type of evidence-based therapy and that within-session care quality is questionable based on a systematic review of chart notes.28,29 Attrition in trauma-focused therapy is a recognized limitation, with 1 out of 3 treatment-seeking patients not completing a full dose of evidence-based treatment.30-33 Large-scale analyses of VHA and DoD utilization data suggest that the majority of PTSD patients do not receive a sufficient number of sessions to be characterized as an adequate dose of EBT, with a majority of dropouts occur- ring after just a few sessions.34-37
Hoge and colleagues found that < 50% of soldiers meeting criteria for PTSD received any mental health care within the prior 6 months with one-quarter of those patients dropping out of care prematurely.38 Among a large cohort of soldiers engaged in care for the treatment of PTSD, only about 40% received a number of EBT treatment sessions that could qualify as an adequate dose.38 Thus, although major advancements in the development and implementation of effective treatments for PTSD and depression have occurred, the penetration of these treatments is limited, and the majority of patients in need of treatment potentially receive inadequate care.39
System level approaches that integrate behavioral health services into the primary care system have been proposed to address these care gaps for service members and veterans.40-42 Fundamentally, system-level approaches seek to improve the reach and effectiveness of care through large-scale screening efforts, a greater emphasis on the quality of patient care, and enhanced care continuity across episodes of treatment.
Primary Care
With the primary care setting considered the de facto mental health system, integrated approaches enhance the reach of care by incorporating uniform mental health screening and referral for patients coming through primary care. Specific evidence-based treatments can be integrated into this approach within a stepped-care framework that aims to match patients strategically to the right type of care and leverage specialty care resources as needed. Integrated care approaches for the treatment of PTSD and depression have been developed and evaluated inside and outside of the MHS. Findings indicate that integrated treatment approaches can improve care access, care continuity, patient satisfaction, quality of care,and in several trials, PTSD and depression outcomes.43-47
Recently, an integrated care approach targeting U.S. Army soldiers who screened positive for PTSD or depression in primary care was evaluated in a multisite effectiveness trial.48 Patients randomized to the treatment approach experienced significant improvements in both PTSD and depression symptoms relative to patients in usual care.43 In addition, patients treated in this care model received significantly more mental health services; the patterns of care indicated that patients with comorbid PTSD and depression were more likely to be triaged to specialty care, whereas patients with a single diagnosis were more likely to be managed in primary care.49 This trial suggests that integrated care models feasibly can be implemented in the U.S. Army care system, yielding increased uptake of mental health care, more efficiently matched care based on patient comorbidities, and improved PTSD and depression outcomes.
Treatment Research
The MHS supports a large portfolio of research in PTSD and depression through DoD/VA research consortia (eg, the Congressionally Directed Medical Research Program, the Consortium to Alleviate PTSD, the Injury and Traumatic Stress Clinical Consortium). The U.S. Army Medical Research and Materiel Command (USAMRMC) executes and manages the portfolio of research, relying on a joint program committee of DoD and non-DoD experts to make funding recommendations based on identified research priorities, policy guidance, and knowledge translation needs.
Health systems research on PTSD and MDD in federal health care settings is expanding. For example, the RAND Corporation recently evaluated a candidate set of quality measures for PTSD and MDD, using an operational definition of an episode of care.37 This work is intended to inform efforts to measure and improve the quality of care for PTSD and depression across the enterprise.
The DoD Defense Centers of Excellence for Psychological Health and Traumatic Brain Injury is simultaneously completing an inferential assessment of adjunctive mental health care services, many focused on PTSD and depression, throughout the health care enterprise. Along with the substantial resources devoted to research on PTSD and depression, the MHS is implementing strategies to improve the system of care for service members with mental health conditions.
Army Care System Innovations
The U.S. Army is engaged in a variety of strategies to improve the identification of patients with mental health conditions, increase access to mental health services, and enhance the quality of care that soldiers receive for PTSD and depression. To improve the coordination of mental health care, the U.S. Army Medical Command implemented a wide-scale innovative transformation of its mental health care system through the establishment of the Behavioral Health Service Line program management office.
This move eliminated separate departments of psychiatry, psychology, and social work in favor of integrated behavioral health departments that are now responsible for all mental health care delivered to soldiers, including inpatient, outpatient, partial hospitalization, residential, embedded care in garrison, and primary care settings. This transformation ensured coordination of care for soldiers, eliminating potential miscommunication with patients, commands, and other clinicians while clearly defining performance indicators in process (eg, productivity, scheduling, access to care, and patient satisfaction) and outcome measures.49 In conjunction with the development of its service line, the U.S. Army created a Behavioral Health Data Portal (BHDP), an electronic and standardized means to assess clinical outcomes for common conditions.
To promote higher quality mental health care, the Office of the Surgeon General of the U.S. Army provided direct guidance on the treatment of PTSD and depression. U.S. Army policy mandates that providers treating mental health conditions adhere to the VA/DoD clinical practice guidelines (CPGs) and that soldiers with PTSD and depression be offered treatments with the highest level of scientific support and that outcome measures be routinely administered. In line with the CPGs, U.S. Army policy also recommends the use of both integrated and embedded mental health care approaches to address PTSD, depression, and other common physical and psychological health conditions.
To reduce stigma and improve mental health care access, the U.S. Army began implementing integrated care approaches in 2007 with its Re-Engineering Systems of Primary Care Treatment in the Military (RESPECT-Mil) program, an evidence-based collaborative care model.51-55 This approach included structured screening and diagnostic procedures, predictable follow-up schedules for patients, and the coordination of the divisions of responsibility among and between primary care providers, paraprofessionals, and behavioral health care providers. From 2007 to 2013, this collaborative care model was rolled out across 96 clinics worldwide and provided PTSD and depression screening to more than 1 million encounters per year.52,53
More recently, the U.S. Army led DoD in integrating behavioral health personnel in patient centered medical homes (PCMH) in compliance with DoD Instruction 6490.15.56 This hybrid integrated care model combines collaborative care elements developed in the RESPECT-Mil program with elements of the U.S. Air Force Behavioral Health Optimization project colocating behavioral health providers in primary care settings to provide brief consultative services.
MHS Care Enhancements
Many of the innovations deployed throughout the U.S. Army system of behavioral health care have driven changes across the MHS as a whole. The DoD and the VA have made substantive systemwide policy and practice changes to improve care for beneficiaries with PTSD, depression, and comorbid PTSD and depression. In particular, significant implementation efforts have addressed population screening strategies, outcome monitoring to support measurement-based care, increased access to effective care, and revision of the disability evaluation system.
To improve the identification and referral of soldiers with deployment-related mental health concerns, the DoD implemented a comprehensive program that screens service members prior to deployment, immediately on redeployment, and then again 6 months after returning from deployment. Additionally, annual primary care- based screening requirements have been instituted as part of the DoD PCMH initiative. Both deployment-related and primary care-based screenings include an instrumentation to detect symptoms of PTSD and depression and extend the reach of mental health screening to the entire MHS population.
Building on the success of BHDP, former Assistant Secretary of Defense for Health Affairs Jonathan Woodson mandated BHDP use across the MHS for all patients in DoD behavioral health clinics and the use of outcome measures for the treatment of PTSD, anxiety, depression, and alcohol use disorders.57 A DoD-wide requirement to use the PTSD checklist and patient health questionnaire to monitor PTSD and depression symptoms at mental health intakes and regularly at follow-up visits is being implemented. The Defense Centers of Excellence for Psychological Health and Traumatic Brain Injury, through its Practice-Based Implementation Network (underwritten by a Joint Incentive Fund managed between DoD and VA), has worked across the MHS and the VA to facilitate the implementation, uptake, and adoption of this initiative.
The DoD established the Center for Deployment Psychology (CDP) in 2006 to promote clinician training in EBTs with the aim of increasing service members’ access to effective psychological treatments. Since its inception, the CDP has provided EBT training to more than 40,000 behavioral health providers. Although the impact of these and other efforts on improving the quality of care that patients receive is unknown, a recent study documented widespread self-reported usage of EBT components in U.S. Army clinics and that providers formally trained in EBTs were more likely to deliver EBTs.58
Finally, systemwide changes to the VA Schedule of Ratings for Disability (VASRD) and integration of DoD and VA disability evaluation systems have led to shifts in diagnosis toward PTSD that usually merit a minimum 50% disability rating. Mandates in law require military clinicians to evaluate patients who have deployed for PTSD and TBI prior to taking any actions associated with administrative separation. The practice of attributing PTSD symptoms to character pathology or personality disorders, even when these symptoms did not clearly manifest or worsen with military service, has likely been eliminated from practice in military and veteran populations.
Robust policy changes to limit personality disorder discharges started in fiscal year 2007, when there were 4,127 personality disorder separations across DoD. This number was reduced to 300 within 5 years. Policy changes regarding separation not only seem to have affected discharges, but also may have shaped diagnostic practice. The incidence rate of personality disorder diagnoses declined from 513 per 100,000 person-years in 2007 to 284 per 100,000 person-years by 2011.59 The VASRD recognizes chronic adjustment disorder as a disability, and the National Defense Authorization Act of 2008 mandated that DoD follow disability guidelines promulgated by VA.
As stated in the memorandum Clinical Policy Guidance for Assessment and Treatment of Post-Traumatic Stress Disorders (August 24, 2012), DoD recognizes chronic adjustment disorder as an unfitting condition that merits referral to its disability evaluation system.60 Acute adjustment disorders may still lead to administrative separations, as many service members manifest emotional symptoms stemming from the failure to adjust to the routine vicissitudes of military life. Finally, many court jurisdictions, including veteran’s courts, military courts, and commanders empowered to adjudicate nonjudicial infractions under the Uniform Code of Military Justice, have recognized PTSD as grounds for the mitigation of penalties associated with a wide array of criminal and administrative infractions.
Conclusion
In response to the increased mental health burden following a decade of war and the associated pressures stemming from federal mandates, the MHS has invested unprecedented resources into improving care for military service members. The U.S. Army has played a prominent role in this endeavor by investing in clinical research efforts to accelerate discovery on the causes and cures for these conditions, enacting policies that mandate best practices, and implementing evidence-based care approaches across the system of care. Despite this progress, however, understanding and effectively treating the most prevalent mental health conditions remain a challenge across the DoD and VHA health care systems. Many service members and veterans still do not receive timely, high-quality care for PTSD, depression, and other common comorbidities associated with military experience, and controversies in diagnostic clarification abound.
In short, great strides have been made, yet there is still a large distance to go. The vision of an effective, efficient, comprehensive care system for mental health conditions will continue to be pursued and achieved through collaborations across key agencies and the scientific community, implementation of health system approaches that support population care, and the sustained efforts of dedicated clinicians, staff, and clinic leaders who deliver the care to our service members and veterans.
1. The White House, Office of the Press Secretary. Executive Order 13625: Improving Access to Mental Health Services for Veterans, Service Members, and Military Families. https://www.whitehouse.gov/the-press-office/2012/08/31/executive-order-improving-access-mental-health-services-veterans-service. Published August 31, 2012. Accessed September 20, 2016.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. Arlington, VA: American Psychiatric Association Press; 1980.
3. Mayes R, Horwitz AV. DSM-III and the revolution in the classification of mental illness. J Hist Behav Sci. 2005;41(3):249-267.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association Press; 2013.
5. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed., text rev. Arlington, VA: American Psychiatric Association Press; 2000.
6. Hoge CW, Riviere LA, Wilk JE, Herrell RK, Weathers FW. The prevalence of post-traumatic stress disorder (PTSD) in US combat soldiers: a head-to-head comparison of DSM-5 versus DSM-IV-TR symptom criteria with the PTSD checklist. Lancet Psychiatry. 2014;1(4):269-277.
7. OTSG-MEDCOM. Policy Memo 14-094: Policy Guidance on the Assessment and Treatment of Posttraumatic Stress Disorder (PTSD). Published December 18, 2014.
8. Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry, 2010;167(7):748-751.
9. National Institute of Mental Health. NIMH strategic plan for research. http://www.nimh.nih.gov/about/strategic-planning-reports/index.shtml. Revised 2015. Accessed September 20, 2016.
10. Colston M, Hocter W. Forensic aspects of posttraumatic stress disorder. In: Ritchie EC, ed. Forensic and Ethical Issues in Military Behavioral Health. Washington, DC: U.S. Department of the Army; 2015:97-110.
11. Defense Centers of Excellence for Psychological Health and Traumatic Brain Injury. National Center for Telehealth and Technology. Department of Defense suicide event report: calendar year 2013 annual report. http://t2health.dcoe.mil/programs/dodser. Published January 13, 2015. Accessed September 20, 2016.
12. Otto JL, O’Donnell FL, Ford SA, Ritschard HV. Selected mental health disorders among active component members, US Armed Forces, 2007-2010. MSMR. 2010;17(11):2-5.
13. Gutner CA, Galovski T, Bovin MJ, Schnurr PP. Emergence of transdiagnostic treatments for PTSD and posttraumatic distress. Curr Psychiatry Rep. 2016;18(10):95-101.
14. Campbell DG, Felker BL, Liu CF, et al. Prevalence of depression-PTSD comorbidity: implications for clinical practice guidelines and primary care-based interventions. J Gen Intern Med. 2007;22(6):711-718.
15. Chan D, Cheadle AD, Reiber G, Unützer J, Chaney EF. Health care utilization and its costs for depressed veterans with and without comorbid PTSD symptoms. Psychiatr Serv. 2009;60(12):1612-1617.
16. Maguen S, Cohen B, Cohen G, Madden E, Bertenthal D, Seal K. Gender differences in health service utilization among Iraq and Afghanistan veterans with posttraumatic stress disorder. J Womens Health (Larchmt). 2012;21(6):666-673.
17. Hoskins M, Pearce J, Bethell A, et al. Pharmacotherapy for post-traumatic stress disorder: systematic review and meta-analysis. Br J Psychiatry. 2015;206(2):93-100.
18. Puetz TW, Youngstedt SD, Herring MP. Effects of pharmacotherapy on combat-related PTSD, anxiety, and depression: a systematic review and meta-regression analysis. PLoS One. 2015;10(5):e0126529.
19. Jonas DE, Cusack K, Forneris CA, et al. Psychological and pharmacological treatments for adults with posttraumatic stress disorder (PTSD). Comparative effectiveness review no. 92. https://effectivehealthcare.ahrq.gov/ehc/products/347/1435/PTSD-adult-treatment-report-130403.pdf. Published April 3, 2013. Accessed September 20, 2016.
20. Haagen JFG, Smid GE, Knipscheer JW, Kleber RJ. The efficacy of recommended treatments for veterans with PTSD: a metaregression analysis. Clin Psychol Rev. 2015;40:184-194.
21. Tran K, Moulton K, Santesso N, Rabb D. Cognitive processing therapy for post-traumatic stress disorder: a systematic review and meta-analysis. https://www.cadth.ca/cognitive-processing-therapy-post-traumatic-stress-disorder-systematic-review-and-meta-analysis. Published August 11, 2015. Accessed September 20, 2016.
22. VA/DoD Management of Post-Traumatic Stress Working Group. VA/DoD Clinical Practice Guideline for Management of Post-Traumatic Stress. Version 2. http://www.healthquality.va.gov/guidelines/MH/ptsd/. Published October, 2010. Accessed September 20, 2016.
23. VA/DoD Management of Major Depressive Disorder Working Group. VA/DoD Clinical Practice Guideline for the Management of Major Depressive Disorder. Version 3. http://www.healthquality.va.gov/guidelines/mh/mdd/index.asp. Published April 2016. Accessed September 20, 2016.
24. Zatzick DF, Galea S. An epidemiologic approach to the development of early trauma focused intervention. J Trauma Stress. 2007;20(4):401-412.
25. Zatzick DF, Koepsell T, Rivara FP. Using target population specification, effect size, and reach to estimate and compare the population impact of two PTSD preventive interventions. Psychiatry. 2009;72(4):346-359.
26. Glasgow RE, Nelson CC, Strycker LA, King DK. Using RE-AIM metrics to evaluate diabetes self-management support interventions. Am J Prev Med. 2006;30(1):67-73.
27. Finley EP, Garcia HA, Ketchum NS, et al. Utilization of evidence-based psychotherapies in Veterans Affairs posttraumatic stress disorder outpatient clinics. Psychol Serv. 2015;12(1):73-82.
28. Mott JM, Mondragon S, Hundt NE, Beason-Smith M, Grady RH, Teng EJ. Characteristics of U.S. veterans who begin and complete prolonged exposure and cognitive processing therapy for PTSD. J Trauma Stress. 2014;27(3):265-273.
29. Shiner B, D’Avolio LW, Nguyen TM, et al. Measuring use of evidence based psychotherapy for PTSD. Adm Policy Ment Health. 2013;40(4):311-318.
30. Schnurr PP, Friedman MJ, Engel CC, et al. Cognitive behavioral therapy for posttraumatic stress disorder in women: a randomized controlled trial. JAMA. 2007;297(8):820-830.
31. Tuerk PW, Yoder M, Grubaugh A, Myrick H, Hamner M, Acierno R. Prolonged exposure therapy for combat-related posttraumatic stress disorder: an examination of treatment effectiveness for veterans of the wars in Afghanistan and Iraq. J Anxiety Disord. 2011;25(3):397-403.
32. Chard KM, Schumm JA, Owens GP, Cottingham SM. A comparison of OEF and OIF veterans and Vietnam veterans receiving cognitive processing therapy. J Trauma Stress. 2010;23(1):25-32.
33. Monson CM, Schnurr PP, Resick PA, Friedman MJ, Young-Xu Y, Stevens SP. Cognitive processing therapy for veterans with military-related posttraumatic stress disorder. J Consult Clin Psychol. 2006;74(5):898-907.
34. Mott JM, Hundt NE, Sansgiry S, Mignogna J, Cully JA. Changes in psychotherapy utilization among veterans with depression, anxiety, and PTSD. Psychiatr Serv. 2014;65(1):106-112.
35. Seal KH, Maguen S, Cohen B, et al. VA mental health services utilization in Iraq and Afghanistan veterans in the first year of receiving new mental health diagnoses. J Trauma Stress. 2010;23(1):5-16.
36. Russell M, Silver SM. Training needs for the treatment of combat-related posttraumatic stress disorder: a survey of Department of Defense clinicians. Traumatology. 2007;13(3):4-10.
37. Schell TL, Marshall GN. Survey of individuals previously deployed for OEF/OIF. In: Tanielian T, Jaycox LH, eds. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: RAND Corporation; 2008:87-118.
38. Hoge CW, Grossman SH, Auchterlonie JL, Riviere LA, Milliken CS, >Wilk JE. PTSD treatment for soldiers after combat deployment: low utilization of mental health care and reasons for dropout. Psychiatr Serv. 2014;65(8):997-1004.
39. Committee on the Assessment of Ongoing Efforts in the Treatment of Posttraumatic Stress Disorder, Board on the Health of Select Populations, Institute of Medicine. Treatment for Posttraumatic Stress Disorder in Military and Veteran Populations: Final Assessment. Washington, DC: National Academies Press; 2014.
40. Schnurr PP. Extending collaborative care for posttraumatic mental health. JAMA Intern Med. 2016;176(7):956-957.
41. Hoge CW. Interventions for war-related posttraumatic stress disorder: meeting veterans where they are. JAMA. 2011;306(5):549-551.
42. Engel CC. Improving primary care for military personnel and veterans with posttraumatic stress disorder: the road ahead. Gen Hosp Psychiatry. 2005;27(3):158-160.
43. Engel CC, Jaycox LH, Freed MC, et al. Centrally assisted collaborative telecare management for posttraumatic stress disorder and depression in military primary care: a randomized controlled trial. JAMA Intern Med. 2016;176(7):948-956.
44. Fortney JC, Pyne JM, Kimbrell TA, et al. Telemedicine-based collaborative care for posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(1):58-67.
45. Schnurr PP, Friedman MJ, Oxman TE, et al. RESPECT-PTSD: re-engineering systems for the primary care treatment of PTSD, a randomized controlled trial. J Gen Intern Med. 2013;28(1):32-40.
46. Zatzick D, Roy-Byrne P, Russo J, et al. A randomized effectiveness trial of stepped collaborative care for acutely injured trauma survivors. Arch Gen Psychiatry. 2004;61(5):498-506.
47. Zatzick D, O’Connor SS, Russo J, et al. Technology-enhanced stepped collaborative care targeting posttraumatic stress disorder and comorbidity after injury: a randomized controlled trial. J Trauma Stress. 2015;28(5):391-400.
48. Engel CC, Bray RM, Jaycox LH, et al. Implementing collaborative primary care for depression and posttraumatic stress disorder: design and sample for a randomized trial in the U.S. Military Health System. Contemp Clin Trials. 2014;39(2):310-319.
49. Belsher BE, Jaycox LH, Freed MC, et al. Mental health utilization patterns during a stepped, collaborative care effectiveness trial for PTSD and depression in the military health system. Med Care. 2016;54(7):706-713.
50. Hepner KA, Roth CP, Farris C, et al. Measuring the Quality of Care for Psychological Health Conditions in the Military Health System: Candidate Quality Measures for Posttraumatic Stress Disorder and Major Depressive Disorder. Santa Monica, CA: RAND Corporation; 2015.
51. Engel C, Oxman T, Yamamoto C, et al. RESPECT-Mil: feasibility of a systems-level collaborative care approach to depression and post-traumatic stress disorder in military primary care. Mil Med. 2008;173(10):935-940.
52. Belsher BE, Curry J, McCutchan P, et al. Implementation of a collaborative care initiative for PTSD and depression in the Army primary care system. Soc Work Ment Health. 2014;12(5-6):500-522.
53. Wong EC, Jaycox LH, Ayer L, et al. Evaluating the Implementation of the Re-Engineering Systems of Primary Care Treatment in the Military (RESPECT-Mil). Santa Monica, CA: RAND Corporation; 2015.
54. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10:CD006525.
55. Woltmann E, Grogan-Kaylor A, Perron B, Georges H, Kilbourne AM, Bauer MS. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis. Am J Psychiatry. 2012;169(8):790-804.
56. Wright JL. DoD Directive 6490.15. www.dtic.mil/whs/directives/corres/pdf/649015p.pdf.Revised November 20, 2014. Accessed October 3, 2016. 57. Woodson J. Military treatment facility mental health clinical outcomes guidance. http://dcoe.mil/Libraries/Documents/MentalHealthClinicalOutcomesGuidance_Woodson.pdf. Published September 9, 2013. Accessed October 4, 2016.
58. Wilk JE, West JC, Duffy FF, Herrell RK, Rae DS, Hoge CW. Use of evidence-based treatment for posttraumatic stress disorder in Army behavioral healthcare. Psychiatry. 2013;76(4):336-348.
59. Stockton PN, Olsen ET, Hayford S, et al. Security from within: independent review of the Washington Navy Yard shooting. http://archive.defense.gov/pubs/Independent-Review-of-the-WNY-Shooting-14-Nov-2013.pdf. Published November, 2013. Accessed September 20, 2016.
60. Woodson J. ASD(HA) Memorandum: Clinical Policy Guidance for Assessment and Treatment of Posttraumatic Stress Disorder. August 24, 2012.
1. The White House, Office of the Press Secretary. Executive Order 13625: Improving Access to Mental Health Services for Veterans, Service Members, and Military Families. https://www.whitehouse.gov/the-press-office/2012/08/31/executive-order-improving-access-mental-health-services-veterans-service. Published August 31, 2012. Accessed September 20, 2016.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. Arlington, VA: American Psychiatric Association Press; 1980.
3. Mayes R, Horwitz AV. DSM-III and the revolution in the classification of mental illness. J Hist Behav Sci. 2005;41(3):249-267.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association Press; 2013.
5. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed., text rev. Arlington, VA: American Psychiatric Association Press; 2000.
6. Hoge CW, Riviere LA, Wilk JE, Herrell RK, Weathers FW. The prevalence of post-traumatic stress disorder (PTSD) in US combat soldiers: a head-to-head comparison of DSM-5 versus DSM-IV-TR symptom criteria with the PTSD checklist. Lancet Psychiatry. 2014;1(4):269-277.
7. OTSG-MEDCOM. Policy Memo 14-094: Policy Guidance on the Assessment and Treatment of Posttraumatic Stress Disorder (PTSD). Published December 18, 2014.
8. Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry, 2010;167(7):748-751.
9. National Institute of Mental Health. NIMH strategic plan for research. http://www.nimh.nih.gov/about/strategic-planning-reports/index.shtml. Revised 2015. Accessed September 20, 2016.
10. Colston M, Hocter W. Forensic aspects of posttraumatic stress disorder. In: Ritchie EC, ed. Forensic and Ethical Issues in Military Behavioral Health. Washington, DC: U.S. Department of the Army; 2015:97-110.
11. Defense Centers of Excellence for Psychological Health and Traumatic Brain Injury. National Center for Telehealth and Technology. Department of Defense suicide event report: calendar year 2013 annual report. http://t2health.dcoe.mil/programs/dodser. Published January 13, 2015. Accessed September 20, 2016.
12. Otto JL, O’Donnell FL, Ford SA, Ritschard HV. Selected mental health disorders among active component members, US Armed Forces, 2007-2010. MSMR. 2010;17(11):2-5.
13. Gutner CA, Galovski T, Bovin MJ, Schnurr PP. Emergence of transdiagnostic treatments for PTSD and posttraumatic distress. Curr Psychiatry Rep. 2016;18(10):95-101.
14. Campbell DG, Felker BL, Liu CF, et al. Prevalence of depression-PTSD comorbidity: implications for clinical practice guidelines and primary care-based interventions. J Gen Intern Med. 2007;22(6):711-718.
15. Chan D, Cheadle AD, Reiber G, Unützer J, Chaney EF. Health care utilization and its costs for depressed veterans with and without comorbid PTSD symptoms. Psychiatr Serv. 2009;60(12):1612-1617.
16. Maguen S, Cohen B, Cohen G, Madden E, Bertenthal D, Seal K. Gender differences in health service utilization among Iraq and Afghanistan veterans with posttraumatic stress disorder. J Womens Health (Larchmt). 2012;21(6):666-673.
17. Hoskins M, Pearce J, Bethell A, et al. Pharmacotherapy for post-traumatic stress disorder: systematic review and meta-analysis. Br J Psychiatry. 2015;206(2):93-100.
18. Puetz TW, Youngstedt SD, Herring MP. Effects of pharmacotherapy on combat-related PTSD, anxiety, and depression: a systematic review and meta-regression analysis. PLoS One. 2015;10(5):e0126529.
19. Jonas DE, Cusack K, Forneris CA, et al. Psychological and pharmacological treatments for adults with posttraumatic stress disorder (PTSD). Comparative effectiveness review no. 92. https://effectivehealthcare.ahrq.gov/ehc/products/347/1435/PTSD-adult-treatment-report-130403.pdf. Published April 3, 2013. Accessed September 20, 2016.
20. Haagen JFG, Smid GE, Knipscheer JW, Kleber RJ. The efficacy of recommended treatments for veterans with PTSD: a metaregression analysis. Clin Psychol Rev. 2015;40:184-194.
21. Tran K, Moulton K, Santesso N, Rabb D. Cognitive processing therapy for post-traumatic stress disorder: a systematic review and meta-analysis. https://www.cadth.ca/cognitive-processing-therapy-post-traumatic-stress-disorder-systematic-review-and-meta-analysis. Published August 11, 2015. Accessed September 20, 2016.
22. VA/DoD Management of Post-Traumatic Stress Working Group. VA/DoD Clinical Practice Guideline for Management of Post-Traumatic Stress. Version 2. http://www.healthquality.va.gov/guidelines/MH/ptsd/. Published October, 2010. Accessed September 20, 2016.
23. VA/DoD Management of Major Depressive Disorder Working Group. VA/DoD Clinical Practice Guideline for the Management of Major Depressive Disorder. Version 3. http://www.healthquality.va.gov/guidelines/mh/mdd/index.asp. Published April 2016. Accessed September 20, 2016.
24. Zatzick DF, Galea S. An epidemiologic approach to the development of early trauma focused intervention. J Trauma Stress. 2007;20(4):401-412.
25. Zatzick DF, Koepsell T, Rivara FP. Using target population specification, effect size, and reach to estimate and compare the population impact of two PTSD preventive interventions. Psychiatry. 2009;72(4):346-359.
26. Glasgow RE, Nelson CC, Strycker LA, King DK. Using RE-AIM metrics to evaluate diabetes self-management support interventions. Am J Prev Med. 2006;30(1):67-73.
27. Finley EP, Garcia HA, Ketchum NS, et al. Utilization of evidence-based psychotherapies in Veterans Affairs posttraumatic stress disorder outpatient clinics. Psychol Serv. 2015;12(1):73-82.
28. Mott JM, Mondragon S, Hundt NE, Beason-Smith M, Grady RH, Teng EJ. Characteristics of U.S. veterans who begin and complete prolonged exposure and cognitive processing therapy for PTSD. J Trauma Stress. 2014;27(3):265-273.
29. Shiner B, D’Avolio LW, Nguyen TM, et al. Measuring use of evidence based psychotherapy for PTSD. Adm Policy Ment Health. 2013;40(4):311-318.
30. Schnurr PP, Friedman MJ, Engel CC, et al. Cognitive behavioral therapy for posttraumatic stress disorder in women: a randomized controlled trial. JAMA. 2007;297(8):820-830.
31. Tuerk PW, Yoder M, Grubaugh A, Myrick H, Hamner M, Acierno R. Prolonged exposure therapy for combat-related posttraumatic stress disorder: an examination of treatment effectiveness for veterans of the wars in Afghanistan and Iraq. J Anxiety Disord. 2011;25(3):397-403.
32. Chard KM, Schumm JA, Owens GP, Cottingham SM. A comparison of OEF and OIF veterans and Vietnam veterans receiving cognitive processing therapy. J Trauma Stress. 2010;23(1):25-32.
33. Monson CM, Schnurr PP, Resick PA, Friedman MJ, Young-Xu Y, Stevens SP. Cognitive processing therapy for veterans with military-related posttraumatic stress disorder. J Consult Clin Psychol. 2006;74(5):898-907.
34. Mott JM, Hundt NE, Sansgiry S, Mignogna J, Cully JA. Changes in psychotherapy utilization among veterans with depression, anxiety, and PTSD. Psychiatr Serv. 2014;65(1):106-112.
35. Seal KH, Maguen S, Cohen B, et al. VA mental health services utilization in Iraq and Afghanistan veterans in the first year of receiving new mental health diagnoses. J Trauma Stress. 2010;23(1):5-16.
36. Russell M, Silver SM. Training needs for the treatment of combat-related posttraumatic stress disorder: a survey of Department of Defense clinicians. Traumatology. 2007;13(3):4-10.
37. Schell TL, Marshall GN. Survey of individuals previously deployed for OEF/OIF. In: Tanielian T, Jaycox LH, eds. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: RAND Corporation; 2008:87-118.
38. Hoge CW, Grossman SH, Auchterlonie JL, Riviere LA, Milliken CS, >Wilk JE. PTSD treatment for soldiers after combat deployment: low utilization of mental health care and reasons for dropout. Psychiatr Serv. 2014;65(8):997-1004.
39. Committee on the Assessment of Ongoing Efforts in the Treatment of Posttraumatic Stress Disorder, Board on the Health of Select Populations, Institute of Medicine. Treatment for Posttraumatic Stress Disorder in Military and Veteran Populations: Final Assessment. Washington, DC: National Academies Press; 2014.
40. Schnurr PP. Extending collaborative care for posttraumatic mental health. JAMA Intern Med. 2016;176(7):956-957.
41. Hoge CW. Interventions for war-related posttraumatic stress disorder: meeting veterans where they are. JAMA. 2011;306(5):549-551.
42. Engel CC. Improving primary care for military personnel and veterans with posttraumatic stress disorder: the road ahead. Gen Hosp Psychiatry. 2005;27(3):158-160.
43. Engel CC, Jaycox LH, Freed MC, et al. Centrally assisted collaborative telecare management for posttraumatic stress disorder and depression in military primary care: a randomized controlled trial. JAMA Intern Med. 2016;176(7):948-956.
44. Fortney JC, Pyne JM, Kimbrell TA, et al. Telemedicine-based collaborative care for posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(1):58-67.
45. Schnurr PP, Friedman MJ, Oxman TE, et al. RESPECT-PTSD: re-engineering systems for the primary care treatment of PTSD, a randomized controlled trial. J Gen Intern Med. 2013;28(1):32-40.
46. Zatzick D, Roy-Byrne P, Russo J, et al. A randomized effectiveness trial of stepped collaborative care for acutely injured trauma survivors. Arch Gen Psychiatry. 2004;61(5):498-506.
47. Zatzick D, O’Connor SS, Russo J, et al. Technology-enhanced stepped collaborative care targeting posttraumatic stress disorder and comorbidity after injury: a randomized controlled trial. J Trauma Stress. 2015;28(5):391-400.
48. Engel CC, Bray RM, Jaycox LH, et al. Implementing collaborative primary care for depression and posttraumatic stress disorder: design and sample for a randomized trial in the U.S. Military Health System. Contemp Clin Trials. 2014;39(2):310-319.
49. Belsher BE, Jaycox LH, Freed MC, et al. Mental health utilization patterns during a stepped, collaborative care effectiveness trial for PTSD and depression in the military health system. Med Care. 2016;54(7):706-713.
50. Hepner KA, Roth CP, Farris C, et al. Measuring the Quality of Care for Psychological Health Conditions in the Military Health System: Candidate Quality Measures for Posttraumatic Stress Disorder and Major Depressive Disorder. Santa Monica, CA: RAND Corporation; 2015.
51. Engel C, Oxman T, Yamamoto C, et al. RESPECT-Mil: feasibility of a systems-level collaborative care approach to depression and post-traumatic stress disorder in military primary care. Mil Med. 2008;173(10):935-940.
52. Belsher BE, Curry J, McCutchan P, et al. Implementation of a collaborative care initiative for PTSD and depression in the Army primary care system. Soc Work Ment Health. 2014;12(5-6):500-522.
53. Wong EC, Jaycox LH, Ayer L, et al. Evaluating the Implementation of the Re-Engineering Systems of Primary Care Treatment in the Military (RESPECT-Mil). Santa Monica, CA: RAND Corporation; 2015.
54. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10:CD006525.
55. Woltmann E, Grogan-Kaylor A, Perron B, Georges H, Kilbourne AM, Bauer MS. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis. Am J Psychiatry. 2012;169(8):790-804.
56. Wright JL. DoD Directive 6490.15. www.dtic.mil/whs/directives/corres/pdf/649015p.pdf.Revised November 20, 2014. Accessed October 3, 2016. 57. Woodson J. Military treatment facility mental health clinical outcomes guidance. http://dcoe.mil/Libraries/Documents/MentalHealthClinicalOutcomesGuidance_Woodson.pdf. Published September 9, 2013. Accessed October 4, 2016.
58. Wilk JE, West JC, Duffy FF, Herrell RK, Rae DS, Hoge CW. Use of evidence-based treatment for posttraumatic stress disorder in Army behavioral healthcare. Psychiatry. 2013;76(4):336-348.
59. Stockton PN, Olsen ET, Hayford S, et al. Security from within: independent review of the Washington Navy Yard shooting. http://archive.defense.gov/pubs/Independent-Review-of-the-WNY-Shooting-14-Nov-2013.pdf. Published November, 2013. Accessed September 20, 2016.
60. Woodson J. ASD(HA) Memorandum: Clinical Policy Guidance for Assessment and Treatment of Posttraumatic Stress Disorder. August 24, 2012.
NCCN guidelines on MM now include MRD testing

The National Comprehensive Cancer Network (NCCN) has revised its clinical practice guidelines on multiple myeloma (MM) to include response criteria developed by the International Myeloma Working Group (IMWG) and testing for minimal residual disease (MRD).
The NCCN develops practice guidelines to help physicians in making informed treatment decisions.
Its recommendations can facilitate reimbursement for testing or treatment.
“The NCCN’s action represents a further step toward broad use of MRD testing,” said Brian Durie, MD, chairman of the International Myeloma Foundation (IMF).
The importance of first identifying and then eliminating MRD is the key principle of the IMF’s Black Swan Research Initiative®, a collaborative effort launched in 2012 to cure MM.
“We’ve long believed early intervention with highly effective treatments is the pathway to curing myeloma, and we are currently testing this in clinical trials,” Dr Durie said.
Through the Black Swan Research Initiative, the IMF helped develop next-generation flow cytometry, 1 of 2 tests recommended by the NCCN to assess the presence of MRD in MM patients. The second test is next-generation sequencing.
The new MM response criteria, on which the NCCN based its most recent revision to the guidelines, were developed and agreed upon by the more than 200 members of the IMWG.
The new response criteria spell out exact definitions of “MRD negative” by next-generation flow cytometry or next-generation sequencing.
“We are pleased that the 2016 IMWG response criteria were adopted in full in the new NCCN recommendations,” said Shaji Kumar, MD, of the Mayo Clinic in Rochester, Minnesota.
The 2016 IMWG response criteria were published in The Lancet Oncology in August. ![]()

The National Comprehensive Cancer Network (NCCN) has revised its clinical practice guidelines on multiple myeloma (MM) to include response criteria developed by the International Myeloma Working Group (IMWG) and testing for minimal residual disease (MRD).
The NCCN develops practice guidelines to help physicians in making informed treatment decisions.
Its recommendations can facilitate reimbursement for testing or treatment.
“The NCCN’s action represents a further step toward broad use of MRD testing,” said Brian Durie, MD, chairman of the International Myeloma Foundation (IMF).
The importance of first identifying and then eliminating MRD is the key principle of the IMF’s Black Swan Research Initiative®, a collaborative effort launched in 2012 to cure MM.
“We’ve long believed early intervention with highly effective treatments is the pathway to curing myeloma, and we are currently testing this in clinical trials,” Dr Durie said.
Through the Black Swan Research Initiative, the IMF helped develop next-generation flow cytometry, 1 of 2 tests recommended by the NCCN to assess the presence of MRD in MM patients. The second test is next-generation sequencing.
The new MM response criteria, on which the NCCN based its most recent revision to the guidelines, were developed and agreed upon by the more than 200 members of the IMWG.
The new response criteria spell out exact definitions of “MRD negative” by next-generation flow cytometry or next-generation sequencing.
“We are pleased that the 2016 IMWG response criteria were adopted in full in the new NCCN recommendations,” said Shaji Kumar, MD, of the Mayo Clinic in Rochester, Minnesota.
The 2016 IMWG response criteria were published in The Lancet Oncology in August. ![]()

The National Comprehensive Cancer Network (NCCN) has revised its clinical practice guidelines on multiple myeloma (MM) to include response criteria developed by the International Myeloma Working Group (IMWG) and testing for minimal residual disease (MRD).
The NCCN develops practice guidelines to help physicians in making informed treatment decisions.
Its recommendations can facilitate reimbursement for testing or treatment.
“The NCCN’s action represents a further step toward broad use of MRD testing,” said Brian Durie, MD, chairman of the International Myeloma Foundation (IMF).
The importance of first identifying and then eliminating MRD is the key principle of the IMF’s Black Swan Research Initiative®, a collaborative effort launched in 2012 to cure MM.
“We’ve long believed early intervention with highly effective treatments is the pathway to curing myeloma, and we are currently testing this in clinical trials,” Dr Durie said.
Through the Black Swan Research Initiative, the IMF helped develop next-generation flow cytometry, 1 of 2 tests recommended by the NCCN to assess the presence of MRD in MM patients. The second test is next-generation sequencing.
The new MM response criteria, on which the NCCN based its most recent revision to the guidelines, were developed and agreed upon by the more than 200 members of the IMWG.
The new response criteria spell out exact definitions of “MRD negative” by next-generation flow cytometry or next-generation sequencing.
“We are pleased that the 2016 IMWG response criteria were adopted in full in the new NCCN recommendations,” said Shaji Kumar, MD, of the Mayo Clinic in Rochester, Minnesota.
The 2016 IMWG response criteria were published in The Lancet Oncology in August. ![]()
Primary Prevention of Coronary Artery Disease
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Sex differences in T-cell profiles may drive anti–PD-L1 responses
NATIONAL HARBOR, MD. – Sex difference in immune regulatory responses may drive the poorer responses to treatment with immune checkpoint inhibitors targeted against programmed death–1 (PD-1 inhibitors) seen in women with advanced melanoma, investigators report.
Among patients with advanced melanoma treated with either pembrolizumab (Keytruda) or nivolumab (Opdivo) monotherapy in four clinical trials, the median objective response rate (ORR) among women was 33.1%, compared with 54.6% among men. Median progression-free survival (PFS), respectively, was 5.5 months vs. 18 months, reported Katy K. Tsai, MD, a clinical instructor in cutaneous oncology at the University of California, San Francisco.
“There has been a lot of interesting data coming out recently about the influence of sex hormones on the immune regulatory response in general, so I do think that is something that needs to be explored further,” Dr. Tsai said at the annual meeting of the Society for Immunotherapy of Cancer.
“There are some interesting data to suggest that perhaps women, and in particular pregnant women or perhaps even women who have higher parity than those who are nulliparous, may have higher circulating levels of T-regs that may contribute to dampening this immune response,” she said.
Response prediction model
Dr. Tsai and her colleagues had previously reported on a validated clinical scoring model for predicting response to anti–PD-1 therapy. In that study, they found that female sex was associated with a lower response rate with an odds ratio of 0.36 (95% confidence interval, 0.19-0.67).
In a separate study, they reported that relative abundance in tumors of a partially exhausted T-cell phenotype (PD-1high/CTLA-4–positive CD8 cells) was predictive of response to anti–PD-1 therapy.
In the current study, they looked at data on 118 women and 218 men who had advanced cutaneous melanoma and were treated in one of four clinical trials of pembrolizumab or nivolumab as monotherapy or in combination with an anti-CTLA4 agent such as ipilimumab (Yervoy) (NCT01295827, NCT01704287, NCT01721746, and NCT02156804).
On flow-cytometry analysis of pre-treatment tumor samples, women had a significantly lower proportion of PD-1high/CTLA-4–positive CD8 cells as compared with men (mean, 16.9% vs. 26%; P = .008).
“The mechanisms of this [discrepancy] may have an immunologic basis given the difference in pre-treatment T-cell profiles between women and men. Sex-related differences in tumor immunity and immunotherapy responses warrant further investigation,” the investigators wrote in a poster presentation.
The study was internally funded. Dr. Tsai and her colleagues reported no relevant disclosures.
NATIONAL HARBOR, MD. – Sex difference in immune regulatory responses may drive the poorer responses to treatment with immune checkpoint inhibitors targeted against programmed death–1 (PD-1 inhibitors) seen in women with advanced melanoma, investigators report.
Among patients with advanced melanoma treated with either pembrolizumab (Keytruda) or nivolumab (Opdivo) monotherapy in four clinical trials, the median objective response rate (ORR) among women was 33.1%, compared with 54.6% among men. Median progression-free survival (PFS), respectively, was 5.5 months vs. 18 months, reported Katy K. Tsai, MD, a clinical instructor in cutaneous oncology at the University of California, San Francisco.
“There has been a lot of interesting data coming out recently about the influence of sex hormones on the immune regulatory response in general, so I do think that is something that needs to be explored further,” Dr. Tsai said at the annual meeting of the Society for Immunotherapy of Cancer.
“There are some interesting data to suggest that perhaps women, and in particular pregnant women or perhaps even women who have higher parity than those who are nulliparous, may have higher circulating levels of T-regs that may contribute to dampening this immune response,” she said.
Response prediction model
Dr. Tsai and her colleagues had previously reported on a validated clinical scoring model for predicting response to anti–PD-1 therapy. In that study, they found that female sex was associated with a lower response rate with an odds ratio of 0.36 (95% confidence interval, 0.19-0.67).
In a separate study, they reported that relative abundance in tumors of a partially exhausted T-cell phenotype (PD-1high/CTLA-4–positive CD8 cells) was predictive of response to anti–PD-1 therapy.
In the current study, they looked at data on 118 women and 218 men who had advanced cutaneous melanoma and were treated in one of four clinical trials of pembrolizumab or nivolumab as monotherapy or in combination with an anti-CTLA4 agent such as ipilimumab (Yervoy) (NCT01295827, NCT01704287, NCT01721746, and NCT02156804).
On flow-cytometry analysis of pre-treatment tumor samples, women had a significantly lower proportion of PD-1high/CTLA-4–positive CD8 cells as compared with men (mean, 16.9% vs. 26%; P = .008).
“The mechanisms of this [discrepancy] may have an immunologic basis given the difference in pre-treatment T-cell profiles between women and men. Sex-related differences in tumor immunity and immunotherapy responses warrant further investigation,” the investigators wrote in a poster presentation.
The study was internally funded. Dr. Tsai and her colleagues reported no relevant disclosures.
NATIONAL HARBOR, MD. – Sex difference in immune regulatory responses may drive the poorer responses to treatment with immune checkpoint inhibitors targeted against programmed death–1 (PD-1 inhibitors) seen in women with advanced melanoma, investigators report.
Among patients with advanced melanoma treated with either pembrolizumab (Keytruda) or nivolumab (Opdivo) monotherapy in four clinical trials, the median objective response rate (ORR) among women was 33.1%, compared with 54.6% among men. Median progression-free survival (PFS), respectively, was 5.5 months vs. 18 months, reported Katy K. Tsai, MD, a clinical instructor in cutaneous oncology at the University of California, San Francisco.
“There has been a lot of interesting data coming out recently about the influence of sex hormones on the immune regulatory response in general, so I do think that is something that needs to be explored further,” Dr. Tsai said at the annual meeting of the Society for Immunotherapy of Cancer.
“There are some interesting data to suggest that perhaps women, and in particular pregnant women or perhaps even women who have higher parity than those who are nulliparous, may have higher circulating levels of T-regs that may contribute to dampening this immune response,” she said.
Response prediction model
Dr. Tsai and her colleagues had previously reported on a validated clinical scoring model for predicting response to anti–PD-1 therapy. In that study, they found that female sex was associated with a lower response rate with an odds ratio of 0.36 (95% confidence interval, 0.19-0.67).
In a separate study, they reported that relative abundance in tumors of a partially exhausted T-cell phenotype (PD-1high/CTLA-4–positive CD8 cells) was predictive of response to anti–PD-1 therapy.
In the current study, they looked at data on 118 women and 218 men who had advanced cutaneous melanoma and were treated in one of four clinical trials of pembrolizumab or nivolumab as monotherapy or in combination with an anti-CTLA4 agent such as ipilimumab (Yervoy) (NCT01295827, NCT01704287, NCT01721746, and NCT02156804).
On flow-cytometry analysis of pre-treatment tumor samples, women had a significantly lower proportion of PD-1high/CTLA-4–positive CD8 cells as compared with men (mean, 16.9% vs. 26%; P = .008).
“The mechanisms of this [discrepancy] may have an immunologic basis given the difference in pre-treatment T-cell profiles between women and men. Sex-related differences in tumor immunity and immunotherapy responses warrant further investigation,” the investigators wrote in a poster presentation.
The study was internally funded. Dr. Tsai and her colleagues reported no relevant disclosures.
AT SITC 2016
Key clinical point: Sex differences in response to PD-1 inhibitors may be caused by differences in immune regulation.
Major finding: On flow-cytometry analysis of pre-treatment tumor samples, women had a significantly lower proportion of PD-1high/CTLA-4–positive CD8 cells as compared with men (mean, 16.9% vs. 26%; P = .008).
Data source: Analysis of data on 336 patients enrolled in four clinical trials of the PD-1 inhibitors pembrolizumab and nivolumab.
Disclosures: The study was internally funded. Dr. Tsai and her colleagues reported no relevant disclosures.
Adjustment for fluid balance improved detection of AKI in critically ill children
CHICAGO – Adjustment for fluid balance increased the detection rate of acute kidney injury, more accurately staged the kidney damage, and distinguished false-positive cases in critically ill children, based on a secondary analysis of the Study of the Prediction of Acute Kidney Injury in Children Using Risk Stratification and Biomarkers (AKI-CHERUB).
Fluid overload can mask acute kidney injury (AKI) in critically ill children. “The failure to correct serum creatinine measure for fluid overload dilutes the impact of AKI on outcomes,” David T. Selewski, MD, of the University of Michigan, Ann Arbor, said at the annual meeting sponsored by the American Society for Nephrology.
The primary outcome was ICU mortality. Secondary outcomes were length of mechanical ventilation, and length of stay in the ICU and the hospital.
The original study documented an ICU mortality rate of 7.1%. AKI was identified in 77 (41.8%) of the 184 patients. The median peak fluid overload during ICU admission was 12.9 (interquartile range, 7.4-20.8).
The serum creatinine data were corrected for fluid balance and these rates were reassessed. Following the adjustment, the rate of AKI increased from 41.8% to 53.4%, with 30 new cases identified according to standard defined criteria. The mean fluid overload was now 11.2 (interquartile range, 5.7-17.7).
In the original cohort, there were 40 cases of severe AKI (stage 2 and 3). Following the creatinine correction, 13 more cases were judged to be severe. Of these, five cases were associated with a worse outcome in terms of ICU mortality. Additionally, 10 cases that had been diagnosed as AKI were found to be false positives.
The results need to be studied in larger studies and in other populations, such as neonates, Dr. Selewski said.
CHICAGO – Adjustment for fluid balance increased the detection rate of acute kidney injury, more accurately staged the kidney damage, and distinguished false-positive cases in critically ill children, based on a secondary analysis of the Study of the Prediction of Acute Kidney Injury in Children Using Risk Stratification and Biomarkers (AKI-CHERUB).
Fluid overload can mask acute kidney injury (AKI) in critically ill children. “The failure to correct serum creatinine measure for fluid overload dilutes the impact of AKI on outcomes,” David T. Selewski, MD, of the University of Michigan, Ann Arbor, said at the annual meeting sponsored by the American Society for Nephrology.
The primary outcome was ICU mortality. Secondary outcomes were length of mechanical ventilation, and length of stay in the ICU and the hospital.
The original study documented an ICU mortality rate of 7.1%. AKI was identified in 77 (41.8%) of the 184 patients. The median peak fluid overload during ICU admission was 12.9 (interquartile range, 7.4-20.8).
The serum creatinine data were corrected for fluid balance and these rates were reassessed. Following the adjustment, the rate of AKI increased from 41.8% to 53.4%, with 30 new cases identified according to standard defined criteria. The mean fluid overload was now 11.2 (interquartile range, 5.7-17.7).
In the original cohort, there were 40 cases of severe AKI (stage 2 and 3). Following the creatinine correction, 13 more cases were judged to be severe. Of these, five cases were associated with a worse outcome in terms of ICU mortality. Additionally, 10 cases that had been diagnosed as AKI were found to be false positives.
The results need to be studied in larger studies and in other populations, such as neonates, Dr. Selewski said.
CHICAGO – Adjustment for fluid balance increased the detection rate of acute kidney injury, more accurately staged the kidney damage, and distinguished false-positive cases in critically ill children, based on a secondary analysis of the Study of the Prediction of Acute Kidney Injury in Children Using Risk Stratification and Biomarkers (AKI-CHERUB).
Fluid overload can mask acute kidney injury (AKI) in critically ill children. “The failure to correct serum creatinine measure for fluid overload dilutes the impact of AKI on outcomes,” David T. Selewski, MD, of the University of Michigan, Ann Arbor, said at the annual meeting sponsored by the American Society for Nephrology.
The primary outcome was ICU mortality. Secondary outcomes were length of mechanical ventilation, and length of stay in the ICU and the hospital.
The original study documented an ICU mortality rate of 7.1%. AKI was identified in 77 (41.8%) of the 184 patients. The median peak fluid overload during ICU admission was 12.9 (interquartile range, 7.4-20.8).
The serum creatinine data were corrected for fluid balance and these rates were reassessed. Following the adjustment, the rate of AKI increased from 41.8% to 53.4%, with 30 new cases identified according to standard defined criteria. The mean fluid overload was now 11.2 (interquartile range, 5.7-17.7).
In the original cohort, there were 40 cases of severe AKI (stage 2 and 3). Following the creatinine correction, 13 more cases were judged to be severe. Of these, five cases were associated with a worse outcome in terms of ICU mortality. Additionally, 10 cases that had been diagnosed as AKI were found to be false positives.
The results need to be studied in larger studies and in other populations, such as neonates, Dr. Selewski said.
AT KIDNEY WEEK 2016
Key clinical point: Acute kidney injury can be detected more accurately in critically ill children by correcting for fluid overload.
Major finding: Fluid overload masked diagnosis of over 40% of patients with acute kidney injury in an observational study.
Data source: Secondary analysis of AKI-CHERUB single-center observational study involving 181 critically ill children.
Disclosures: The AKI-CHERUB study was funded by NIH. Dr. Selewski reported having no financial disclosures.