User login
Antiamyloid solanezumab fails to slow decline in mild Alzheimer’s
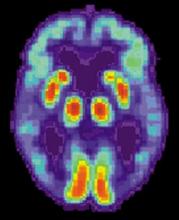
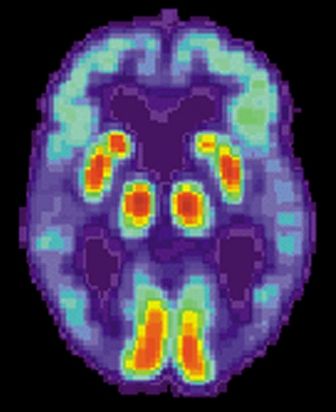
Solanezumab, a monoclonal antibody that targets amyloid plaques, did not slow cognitive decline in patients with mild Alzheimer’s disease.
Although the drug did reduce soluble amyloid beta by 40%, compared with placebo, that change did not translate into a clinically meaningful cognitive benefit, Eric Siemers, MD, said in a press briefing called by Eli Lilly & Co., which manufactures solanezumab. The company did not release specific cognitive data from the highly anticipated Expedition 3 trial, except to say that changes on the ADAS-Cog14 (Alzheimer’s Disease Assessment Scale–cognitive subscale-14), the main cognitive endpoint, had a nonsignificant P value of 0.095 [Corection: Changed from 0.95 in original posting]. Functional data were also withheld.
The entire clinical picture won’t emerge until Dec. 8, when Lilly presents full data at the annual Clinical Trials in Alzheimer’s Disease meeting in San Diego. But, in the meantime, Dr. Siemers did say that Lilly won’t be pursuing a Food and Drug Administration New Drug Application for solanezumab as a disease-modifying therapy for mild Alzheimer’s. However, it’s not the end of the road for solanezumab. Lilly is still investigating it in ExpeditionPro, a trial which will employ the antibody in patients with prodromal AD.
Solanezumab is also part of two very important public/private partnership studies: The Anti-Amyloid Treatment in Asymptomatic Alzheimer’s study (A4 study), investigating its effect in cognitively healthy elders with Alzheimer’s risk factors, and the Dominantly Inherited Alzheimer’s Network (DIAN) study of patients with autosomal dominant mutations in Alzheimer’s genes.
It’s unclear what impact the failed Expedition 3 could have on those, Dr. Siemers said. “These studies are also funded by the National Institute on Aging in collaboration with academic institutions, so we will need to sort through the data very carefully and speak with our academic colleagues before making any decisions on this.”
If solanezumab was successful in either one of those trials – if they continue – Lilly could still apply for approval in those patient groups, Dr. Siemers noted.
The results are disappointing, but not entirely unexpected in the research community. Many felt that Expedition 3 was founded on shaky clinical ground from the start. The study was based on subgroup analyses of Expedition 1 and Expedition 2, both of which failed to meet their primary endpoints in patients with mild-moderate AD. But when researchers pooled both groups of mild patients, they found that solanezumab conferred a 34% slowing of cognitive decline in one cognitive measure, the ADAS-Cog14. This translated to a clinical change of less than 2 points on the scale, however.
Lilly very carefully drafted Expedition 3 to come as close to recreating those findings as possible, said Lon Schneider, MD, of the University of Southern California, Los Angeles.
“By taking the ideal patients from two studies and combining them as if they were from one study, Lilly predicted that they could find a 1.5-2.0 point [Correction: Changed from 1.5 in original posting] difference in one particular outcome, the ADAS-Cog14, [Correction: the ADAS-Cog14 not included in previous posting] in a study of more than 2,000 patients,” Dr. Schneider said in an interview.
This is the research equivalent of Heraclitus’ adage that one can never step in the same river twice: These could never be the same patients, from the same places, at the same time, with the same clinical picture, Dr. Schneider said – especially since Expedition 3 had a purified cohort of only amyloid-positive patients, while close to 25% of patients in the earlier studies probably had no brain amyloid at all.
“This is what happens when we rely on subanalysis to create drug trials. We slice it and dice it until we find some patients for whom the drug is better than placebo, and then say, ‘We have a winner.’ ” Even in the best-case scenario, he said, Expedition 3 would have found only the same modest benefit as its predecessors. “Then we would be in the difficult situation of saying we have a disease-modifying therapy that is associated with only very small clinical changes.”
Dr. Siemers rejected the idea that Expedition 3’s failure is another nail in the coffin of the amyloid hypothesis, or at least in the idea that taming amyloid can prevent dementia or rescue cognition. “You can’t disprove a hypothesis based on one study done in patients with mild dementia,” he said.
Dennis Selkoe, PhD, the Vincent and Stella Coates Professor of Neurologic Diseases at Harvard Medical School, Boston, said Expedition’s failure does not negate the amyloid hypothesis or the appropriateness of amyloid as a therapeutic target.
“Like others, I am disappointed for our patients that solanezumab failed to meet its clinical endpoint by just a little bit. But it is not truly surprising, as this drug was always known to be a ‘weak’ antibody in terms of its ability to mobilize amyloid from the brain,” he said in an interview.
Research into antiamyloid therapies should continue, he said.
“This outcome does nothing to alter the overwhelming genetic, neuropathological and biomarker evidence that amyloid beta protein buildup acts to precipitate the AD process, and it also does not alter the potential promise of current agents in trials: Merck’s beta secretase inhibitor and Biogen’s aducanumab antibody, which appears to be much more effective in mobilizing amyloid beta. Other approaches, in addition to antiamyloid agents, are very much desired and are gradually moving forward, which is good news. But for the time being, antiamyloid treatments will be those most examined in trials, for scientifically and technically sound reasons.”
Dr. Schneider has served as a consultant for Eli Lilly & Co in the past. Dr. Selkoe is a founding scientist and director of Elan Pharmaceuticals.
[email protected]
On Twitter @alz_gal
Solenezumab’s failure in patients with mild-stage Alzheimer’s disease is disappointing, but is it really surprising?
Post hoc analyses of subsets in clinical trials have been notoriously misleading, and this, sadly, is one more example. Overinterpretation of ongoing trials should likewise be kept in check until phase III data are in.
The signals from every putative disease-modifying therapy to date have been weak at best, and one might worry more if such a weak signal actually hit the magical statistical P value of .05. Then we might launch an expensive new phase of AD therapy that not only falls short of halting disease progression, but simply prolongs the course, adds to the cost, further burdens an already overburdened system, and leads family members to ask providers whether the drug is “still working” (a common question I hear in patients taking any one of the current symptomatic medications).
We need a major breakthrough, and reducing disease progression a little bit is not a major breakthrough. It may even be something worse.
Dr. Richard J. Caselli is associate director and clinical core director of the Alzheimer’s Disease Center at Mayo Clinic, Scottsdale, Ariz.
Solenezumab’s failure in patients with mild-stage Alzheimer’s disease is disappointing, but is it really surprising?
Post hoc analyses of subsets in clinical trials have been notoriously misleading, and this, sadly, is one more example. Overinterpretation of ongoing trials should likewise be kept in check until phase III data are in.
The signals from every putative disease-modifying therapy to date have been weak at best, and one might worry more if such a weak signal actually hit the magical statistical P value of .05. Then we might launch an expensive new phase of AD therapy that not only falls short of halting disease progression, but simply prolongs the course, adds to the cost, further burdens an already overburdened system, and leads family members to ask providers whether the drug is “still working” (a common question I hear in patients taking any one of the current symptomatic medications).
We need a major breakthrough, and reducing disease progression a little bit is not a major breakthrough. It may even be something worse.
Dr. Richard J. Caselli is associate director and clinical core director of the Alzheimer’s Disease Center at Mayo Clinic, Scottsdale, Ariz.
Solenezumab’s failure in patients with mild-stage Alzheimer’s disease is disappointing, but is it really surprising?
Post hoc analyses of subsets in clinical trials have been notoriously misleading, and this, sadly, is one more example. Overinterpretation of ongoing trials should likewise be kept in check until phase III data are in.
The signals from every putative disease-modifying therapy to date have been weak at best, and one might worry more if such a weak signal actually hit the magical statistical P value of .05. Then we might launch an expensive new phase of AD therapy that not only falls short of halting disease progression, but simply prolongs the course, adds to the cost, further burdens an already overburdened system, and leads family members to ask providers whether the drug is “still working” (a common question I hear in patients taking any one of the current symptomatic medications).
We need a major breakthrough, and reducing disease progression a little bit is not a major breakthrough. It may even be something worse.
Dr. Richard J. Caselli is associate director and clinical core director of the Alzheimer’s Disease Center at Mayo Clinic, Scottsdale, Ariz.
Solanezumab, a monoclonal antibody that targets amyloid plaques, did not slow cognitive decline in patients with mild Alzheimer’s disease.
Although the drug did reduce soluble amyloid beta by 40%, compared with placebo, that change did not translate into a clinically meaningful cognitive benefit, Eric Siemers, MD, said in a press briefing called by Eli Lilly & Co., which manufactures solanezumab. The company did not release specific cognitive data from the highly anticipated Expedition 3 trial, except to say that changes on the ADAS-Cog14 (Alzheimer’s Disease Assessment Scale–cognitive subscale-14), the main cognitive endpoint, had a nonsignificant P value of 0.095 [Corection: Changed from 0.95 in original posting]. Functional data were also withheld.
The entire clinical picture won’t emerge until Dec. 8, when Lilly presents full data at the annual Clinical Trials in Alzheimer’s Disease meeting in San Diego. But, in the meantime, Dr. Siemers did say that Lilly won’t be pursuing a Food and Drug Administration New Drug Application for solanezumab as a disease-modifying therapy for mild Alzheimer’s. However, it’s not the end of the road for solanezumab. Lilly is still investigating it in ExpeditionPro, a trial which will employ the antibody in patients with prodromal AD.
Solanezumab is also part of two very important public/private partnership studies: The Anti-Amyloid Treatment in Asymptomatic Alzheimer’s study (A4 study), investigating its effect in cognitively healthy elders with Alzheimer’s risk factors, and the Dominantly Inherited Alzheimer’s Network (DIAN) study of patients with autosomal dominant mutations in Alzheimer’s genes.
It’s unclear what impact the failed Expedition 3 could have on those, Dr. Siemers said. “These studies are also funded by the National Institute on Aging in collaboration with academic institutions, so we will need to sort through the data very carefully and speak with our academic colleagues before making any decisions on this.”
If solanezumab was successful in either one of those trials – if they continue – Lilly could still apply for approval in those patient groups, Dr. Siemers noted.
The results are disappointing, but not entirely unexpected in the research community. Many felt that Expedition 3 was founded on shaky clinical ground from the start. The study was based on subgroup analyses of Expedition 1 and Expedition 2, both of which failed to meet their primary endpoints in patients with mild-moderate AD. But when researchers pooled both groups of mild patients, they found that solanezumab conferred a 34% slowing of cognitive decline in one cognitive measure, the ADAS-Cog14. This translated to a clinical change of less than 2 points on the scale, however.
Lilly very carefully drafted Expedition 3 to come as close to recreating those findings as possible, said Lon Schneider, MD, of the University of Southern California, Los Angeles.
“By taking the ideal patients from two studies and combining them as if they were from one study, Lilly predicted that they could find a 1.5-2.0 point [Correction: Changed from 1.5 in original posting] difference in one particular outcome, the ADAS-Cog14, [Correction: the ADAS-Cog14 not included in previous posting] in a study of more than 2,000 patients,” Dr. Schneider said in an interview.
This is the research equivalent of Heraclitus’ adage that one can never step in the same river twice: These could never be the same patients, from the same places, at the same time, with the same clinical picture, Dr. Schneider said – especially since Expedition 3 had a purified cohort of only amyloid-positive patients, while close to 25% of patients in the earlier studies probably had no brain amyloid at all.
“This is what happens when we rely on subanalysis to create drug trials. We slice it and dice it until we find some patients for whom the drug is better than placebo, and then say, ‘We have a winner.’ ” Even in the best-case scenario, he said, Expedition 3 would have found only the same modest benefit as its predecessors. “Then we would be in the difficult situation of saying we have a disease-modifying therapy that is associated with only very small clinical changes.”
Dr. Siemers rejected the idea that Expedition 3’s failure is another nail in the coffin of the amyloid hypothesis, or at least in the idea that taming amyloid can prevent dementia or rescue cognition. “You can’t disprove a hypothesis based on one study done in patients with mild dementia,” he said.
Dennis Selkoe, PhD, the Vincent and Stella Coates Professor of Neurologic Diseases at Harvard Medical School, Boston, said Expedition’s failure does not negate the amyloid hypothesis or the appropriateness of amyloid as a therapeutic target.
“Like others, I am disappointed for our patients that solanezumab failed to meet its clinical endpoint by just a little bit. But it is not truly surprising, as this drug was always known to be a ‘weak’ antibody in terms of its ability to mobilize amyloid from the brain,” he said in an interview.
Research into antiamyloid therapies should continue, he said.
“This outcome does nothing to alter the overwhelming genetic, neuropathological and biomarker evidence that amyloid beta protein buildup acts to precipitate the AD process, and it also does not alter the potential promise of current agents in trials: Merck’s beta secretase inhibitor and Biogen’s aducanumab antibody, which appears to be much more effective in mobilizing amyloid beta. Other approaches, in addition to antiamyloid agents, are very much desired and are gradually moving forward, which is good news. But for the time being, antiamyloid treatments will be those most examined in trials, for scientifically and technically sound reasons.”
Dr. Schneider has served as a consultant for Eli Lilly & Co in the past. Dr. Selkoe is a founding scientist and director of Elan Pharmaceuticals.
[email protected]
On Twitter @alz_gal
Solanezumab, a monoclonal antibody that targets amyloid plaques, did not slow cognitive decline in patients with mild Alzheimer’s disease.
Although the drug did reduce soluble amyloid beta by 40%, compared with placebo, that change did not translate into a clinically meaningful cognitive benefit, Eric Siemers, MD, said in a press briefing called by Eli Lilly & Co., which manufactures solanezumab. The company did not release specific cognitive data from the highly anticipated Expedition 3 trial, except to say that changes on the ADAS-Cog14 (Alzheimer’s Disease Assessment Scale–cognitive subscale-14), the main cognitive endpoint, had a nonsignificant P value of 0.095 [Corection: Changed from 0.95 in original posting]. Functional data were also withheld.
The entire clinical picture won’t emerge until Dec. 8, when Lilly presents full data at the annual Clinical Trials in Alzheimer’s Disease meeting in San Diego. But, in the meantime, Dr. Siemers did say that Lilly won’t be pursuing a Food and Drug Administration New Drug Application for solanezumab as a disease-modifying therapy for mild Alzheimer’s. However, it’s not the end of the road for solanezumab. Lilly is still investigating it in ExpeditionPro, a trial which will employ the antibody in patients with prodromal AD.
Solanezumab is also part of two very important public/private partnership studies: The Anti-Amyloid Treatment in Asymptomatic Alzheimer’s study (A4 study), investigating its effect in cognitively healthy elders with Alzheimer’s risk factors, and the Dominantly Inherited Alzheimer’s Network (DIAN) study of patients with autosomal dominant mutations in Alzheimer’s genes.
It’s unclear what impact the failed Expedition 3 could have on those, Dr. Siemers said. “These studies are also funded by the National Institute on Aging in collaboration with academic institutions, so we will need to sort through the data very carefully and speak with our academic colleagues before making any decisions on this.”
If solanezumab was successful in either one of those trials – if they continue – Lilly could still apply for approval in those patient groups, Dr. Siemers noted.
The results are disappointing, but not entirely unexpected in the research community. Many felt that Expedition 3 was founded on shaky clinical ground from the start. The study was based on subgroup analyses of Expedition 1 and Expedition 2, both of which failed to meet their primary endpoints in patients with mild-moderate AD. But when researchers pooled both groups of mild patients, they found that solanezumab conferred a 34% slowing of cognitive decline in one cognitive measure, the ADAS-Cog14. This translated to a clinical change of less than 2 points on the scale, however.
Lilly very carefully drafted Expedition 3 to come as close to recreating those findings as possible, said Lon Schneider, MD, of the University of Southern California, Los Angeles.
“By taking the ideal patients from two studies and combining them as if they were from one study, Lilly predicted that they could find a 1.5-2.0 point [Correction: Changed from 1.5 in original posting] difference in one particular outcome, the ADAS-Cog14, [Correction: the ADAS-Cog14 not included in previous posting] in a study of more than 2,000 patients,” Dr. Schneider said in an interview.
This is the research equivalent of Heraclitus’ adage that one can never step in the same river twice: These could never be the same patients, from the same places, at the same time, with the same clinical picture, Dr. Schneider said – especially since Expedition 3 had a purified cohort of only amyloid-positive patients, while close to 25% of patients in the earlier studies probably had no brain amyloid at all.
“This is what happens when we rely on subanalysis to create drug trials. We slice it and dice it until we find some patients for whom the drug is better than placebo, and then say, ‘We have a winner.’ ” Even in the best-case scenario, he said, Expedition 3 would have found only the same modest benefit as its predecessors. “Then we would be in the difficult situation of saying we have a disease-modifying therapy that is associated with only very small clinical changes.”
Dr. Siemers rejected the idea that Expedition 3’s failure is another nail in the coffin of the amyloid hypothesis, or at least in the idea that taming amyloid can prevent dementia or rescue cognition. “You can’t disprove a hypothesis based on one study done in patients with mild dementia,” he said.
Dennis Selkoe, PhD, the Vincent and Stella Coates Professor of Neurologic Diseases at Harvard Medical School, Boston, said Expedition’s failure does not negate the amyloid hypothesis or the appropriateness of amyloid as a therapeutic target.
“Like others, I am disappointed for our patients that solanezumab failed to meet its clinical endpoint by just a little bit. But it is not truly surprising, as this drug was always known to be a ‘weak’ antibody in terms of its ability to mobilize amyloid from the brain,” he said in an interview.
Research into antiamyloid therapies should continue, he said.
“This outcome does nothing to alter the overwhelming genetic, neuropathological and biomarker evidence that amyloid beta protein buildup acts to precipitate the AD process, and it also does not alter the potential promise of current agents in trials: Merck’s beta secretase inhibitor and Biogen’s aducanumab antibody, which appears to be much more effective in mobilizing amyloid beta. Other approaches, in addition to antiamyloid agents, are very much desired and are gradually moving forward, which is good news. But for the time being, antiamyloid treatments will be those most examined in trials, for scientifically and technically sound reasons.”
Dr. Schneider has served as a consultant for Eli Lilly & Co in the past. Dr. Selkoe is a founding scientist and director of Elan Pharmaceuticals.
[email protected]
On Twitter @alz_gal
Key clinical point:
Major finding: Specific clinical data were not released.
Data source: The 18-month–long Expedition 3 study randomized 2,100 patients with imaging-proven amyloid plaques to either placebo or 400-mg solanezumab every 4 weeks.
Disclosures: Eli Lilly & Co. manufactures solanezumab. Dr. Siemers is an employee of the company.
Conference News Roundup—American Heart Association
Childhood Adversity Is Linked to Blood Pressure Dysfunction
A difficult childhood may be associated with a risk of poor blood pressure regulation, researchers reported. In some studies, blood pressure variability has been associated with an elevated risk of cardiovascular disease and complications from hypertension.
Researchers at the Augusta University Medical College of Georgia investigated the effect of adverse childhood experiences (eg, childhood abuse or neglect, dysfunctional homes, or low socioeconomic status) during the transition from childhood to adulthood. Earlier research has linked adverse childhood experiences to faster increase of blood pressure in adulthood.
The investigators conducted periodic around-the-clock blood pressure monitoring to capture daytime and nighttime readings in 373 participants between the ages of 7 and 38 during a 23-year period. Participants who reported childhood adversity were 17% more likely to have blood pressure that was higher than the clinical definition of hypertension during the daytime.
"Adverse environments in early life have been consistently associated with the increased risk of hypertension in later life," said Shaoyong Su, PhD, lead author and Associate Professor of Pediatrics at Augusta University Medical College of Georgia. "We found that children who experienced childhood abuse or neglect, dysfunctional homes, and low socioeconomic status were far more likely to have higher blood pressure at night, as well as blood pressure variability over 24 hours, in addition to more rapid onset of hypertension at an earlier age."
Twenty-four-hour ambulatory blood pressure is considered a better predictor of organ damage and cardiovascular events because it can assess not only nighttime blood pressure levels, but also the blood pressure variability in real life. Blood pressure was monitored as many as 15 times during the study.
Researchers said that there was no difference in blood pressure regulation at various ages, thus suggesting that the patterns of adverse events in childhood are similar through young adulthood.
Most physicians focus on average blood pressure readings, but the new findings suggest that they should also ask younger patients about childhood adversity and watch for high blood pressure variability, noted Dr. Su. "This is not something most clinicians currently address, but it is a simple step that could identify many individuals at risk of adult hypertension and help them achieve control at an earlier age. This could avoid problems as they age," he added.
Blood pressure variability has been linked to various problems in adults, including decreased brain function in older adults, increased risk of stroke, and poorer post-stroke recovery. Likewise, early-onset hypertension and prehypertension have been linked to adverse preclinical cardiovascular disease, including left ventricular hypertrophy and evidence of increased arterial stiffness.
The current study was funded by the NIH and the National Heart Lung and Blood Institute.
Poor Sleep May Increase Risk for Irregular Heart Rhythms
Disruptions in sleep may increase the risk of atrial fibrillation, according to preliminary research. Obstructive sleep apnea, sleep interrupted by pauses in breathing, is a known risk factor for atrial fibrillation, which can lead to stroke, heart failure, and other heart-related complications. But whether there is a relationship between disrupted sleep and atrial fibrillation in the absence of sleep apnea is unclear.
Researchers at the University of California, San Francisco examined three sources of data, each using a different approach, to isolate and confirm the effects of poor sleep on atrial fibrillation. Their analyses of these studies showed that disrupted sleep, including insomnia, may be independently associated with atrial fibrillation. People who reported frequent nighttime awakening had an approximately 26% higher risk of developing atrial fibrillation, compared with those who did not wake up many times. In addition, people diagnosed with insomnia had a 29% higher risk of developing atrial fibrillation, compared with those without insomnia.
"The idea that these three studies gave us consistent results was exciting," said lead study author Matt Christensen, a fourth-year medical student at the University of Michigan in Ann Arbor. Past research has shown a link between poor sleep among people with atrial fibrillation. This study, however, focused on people whose pre-existing sleep disruptions were associated with developing atrial fibrillation later in life.
The data sources included the Health eHeart Study, an Internet-based cross-sectional study of more than 4,600 people; the Cardiovascular Health Study, an 11-year longitudinal study of more than 5,700 people, of whom almost 1,600 (28%) developed atrial fibrillation; and the California Healthcare Cost and Utilization Project, a hospital-based database spanning five years and covering almost 14 million patients.
In all three studies, researchers adjusted for the effects of obstructive sleep apnea and atrial fibrillation risk factors that might also be related to sleep. Some of those factors were age, sex, race, diabetes, high blood pressure, heart failure, and smoking.
In a separate analysis, the same researchers reviewed a subset of participants in the Cardiovascular Health Study to understand the effect of sleep disruptions during different sleep phases on the risk of atrial fibrillation in patients without obstructive sleep apnea. The analysis showed that having less REM sleep than other sleep phases during the night is linked to a higher likelihood of developing atrial fibrillation.
"By examining the actual characteristics of sleep, such as how much REM sleep you get, it points us toward a more plausible mechanism. There could be something particular about how sleep impacts the autonomic nervous system," said Mr. Christensen.
Another possible explanation for the link between sleep disruptions and atrial fibrillation is that frequent waking puts extra stress on the heart's chambers, said Mr. Christensen. Participants in this analysis were also enrolled in the Sleep Heart Health Study. They had a formal sleep study to objectively measure sleep quality. That element strengthened the study's conclusions because it did not rely on self-reported data, said Mr. Christensen.
In this analysis, 1,131 people (average age, 77) participated in a study with almost 10 years of follow-up. Researchers measured how long participants slept, how well they slept, how long it took to fall asleep, and the patterns of sleep (ie, how much time was spent in REM sleep vs non-REM sleep). Then they analyzed the sleep disruptions' effects to control the effects of age, sex, race, smoking, diabetes, high blood pressure, and other risk factors.
The exact link between sleep and the development of atrial fibrillation is still a mystery, but we are getting closer to a clear picture, said the study authors. "Ultimately, even without a clear understanding of the responsible mechanisms, we believe these findings suggest that strategies to enhance sleep quality, such as incorporating known techniques to improve sleep hygiene, may help prevent this important arrhythmia," said senior author Gregory Marcus, MD, MAS, a cardiologist at the University of California, San Francisco.
Poor sleep is a known contributor to other heart disease risk factors such as high blood pressure, obesity, and stroke. Getting enough physical activity, avoiding too much caffeine, and having an evening routine are good starting tips for sound sleep, said the researchers.
This study was funded in part by the Sarnoff Cardiovascular Research Foundation, the NIH, and the Agency for Healthcare Research and Quality.
Popular Heartburn Medication May Increase Ischemic Stroke Risk
Proton pump inhibitors (PPIs), which are used to reduce stomach acid and treat heartburn, may increase the risk of ischemic stroke, according to preliminary research. "PPIs have been associated with unhealthy vascular function, including heart attacks, kidney disease and dementia," said Thomas Sehested, MD, lead author and a researcher at the Danish Heart Foundation in Copenhagen. "We wanted to see if PPIs also posed a risk for ischemic stroke, especially given their increasing use in the general population."
Researchers analyzed the records of 244,679 Danish patients (average age, 57) who had an endoscopy. During nearly six years of follow up, 9,489 patients had an ischemic stroke for the first time in their lives. Researchers determined whether the stroke occurred while patients were using one of the following four PPIs: omeprazole, pantoprazole, lansoprazole, and esomeprazole.
For ischemic stroke, researchers found that overall stroke risk increased by 21% when patients were taking a PPI. At the lowest doses of the PPIs, the researchers found slight or no increased stroke risk. At the highest dose of these four PPIs, stroke risk increased by amounts ranging from 30% percent for lansoprazole to 94% for pantoprazole. There was no increased risk of stroke associated with another group of acid-reducing medications known as H2 blockers, which includes famotidine and ranitidine.
In comparison with nonusers, users of PPI were older and had more health conditions, including atrial fibrillation, at baseline (3.4% vs 3.8%). The study accounted for age, gender, and medical factors, including high blood pressure, atrial fibrillation, heart failure, and the use of certain pain relievers that have been linked to heart attack and stroke.
The authors believe that their findings, along with those of previous studies, should encourage more cautious use of PPIs. Most PPIs in the United States are now available over the counter, noted Dr. Sehested. "At one time, PPIs were thought to be safe, without major side effects. This study further questions the cardiovascular safety of these drugs."
Although their study did not find a link between H2 blockers and stroke, the authors could not say that this group of drugs would be better for patients than PPIs. Doctors prescribing PPIs should carefully consider whether their use is warranted and for how long, said Dr. Sehested. "We know from prior studies that a lot of individuals are using PPIs for a much longer time than indicated, which is especially true for elderly patients."
Study limitations include an observational design, which cannot establish cause and effect, and the fact that nearly all the participants were white. Authors believe that a randomized controlled trial of PPIs and cardiovascular disease is warranted. This study was funded by the Danish Heart Foundation.
Catheter Ablations Reduce Risks of Recurrent Stroke
Patients with atrial fibrillation and a prior history of stroke who undergo catheter ablation to treat the abnormal heart rhythm lower their long-term risk of a recurrent stroke by 50%, according to research from the Intermountain Medical Center Heart Institute.
When medications are not successful in treating the arrhythmia, catheter ablations are used to create scar tissue in the upper left atrium of the heart that prevents rapid, chaotic electric currents, often from the pulmonary veins, from causing the abnormal rhythm.
"One of the most devastating complications of atrial fibrillation is a stroke, and its prevention is the treatment cornerstone of the abnormal heart rhythm," said Jared Bunch, MD, lead author of the study and Director of Electrophysiology at the Intermountain Medical Center Heart Institute in Salt Lake City. "Patients that have a prior history of stroke have a much greater risk of recurrent strokes. Our new research shows [that] the more successful we are in treating the abnormal rhythm through the process required with catheter ablation, the better chance we have of lowering a patient's long-term risk of stroke."
The Intermountain study compared a group of 140 patients, who had a prior history of stroke and underwent their first catheter ablation, with two other patient groups, both of which also had a prior history of stroke, including 416 patients with atrial fibrillation who did not receive a catheter ablation, and 416 patients with stroke who did not have atrial fibrillation. The patients were followed for five years and observed for recurrent outcomes of stroke, heart failure, and death.
The five-year risk of patients having another stroke was decreased in the patients who had a catheter ablation to treat atrial fibrillation, compared with the group that had no catheter ablation to treat their abnormal heart rhythm. The stroke rates of patients with atrial fibrillation who underwent an ablation procedure were similar to those of patients with no history of atrial fibrillation.
"One of the most important findings of this study was that stroke rates in patients that underwent an ablation were similar to [those of] patients with no history of atrial fibrillation. This suggests that our management approach can alter some of the negative effects and natural history of atrial fibrillation. As physicians, we spend a lot of our time and energy trying to prevent stroke. This study helps us understand better how our management approaches can alter stroke risk," said Dr. Bunch. "Our research shows that more aggressive treatment of atrial fibrillation by using catheter ablations will reduce the chances [that] a person will have a life-threatening stroke."
Migraine Increases Cardiovascular Risk in Women With Symptoms of Ischemic Heart Disease
Among women being evaluated for ischemic heart disease, those who reported a history of migraine headaches have an increased risk of future cardiovascular (CV) event. This finding is primarily driven by the more than twofold increased risk of stroke.
Data regarding the association between migraine headaches and CV events in women have been inconsistent. Cecil A. Rambarat, MD, a resident at the University of Florida in Gainesville, and colleagues conducted a study to determine the long-term risk of CV events among women with and without migraine headaches who were evaluated for suspected ischemic heart disease in the Women's Ischemia Syndrome Evaluation (WISE) Study.
Women reporting a history of migraine headache were identified from the WISE cohort. Extended follow-up data were available, for a median follow-up of six years. Cox proportional adjusted hazard ratios (HR) were constructed for time to first adverse CV event (ie, CV death, nonfatal myocardial infarction [MI], heart failure hospitalization, or nonfatal stroke) among women with and without migraine headaches. In addition, HRs were determined for each event separately, as well as for all-cause death, angina hospitalization, death or MI, and CV death or MI.
Data on self-reported migraine headaches were available for 917 women. A total of 224 (24.4%) women reported a history of migraine headaches. Compared with those who did not report a history of migraines, women with a history of migraine headaches had an increased adjusted risk of CV events (HR, 1.83) at a median follow-up of six years. This result was mainly due to an increase in the risk of stroke (HR, 2.33).
The variables for which Dr. Rambarat and colleagues adjusted the data included age, race, BMI, history of diabetes, hypertension, dyslipidemia, smoking, family history of coronary artery disease, WISE coronary artery disease severity score, and aspirin use.
Childhood Adversity Is Linked to Blood Pressure Dysfunction
A difficult childhood may be associated with a risk of poor blood pressure regulation, researchers reported. In some studies, blood pressure variability has been associated with an elevated risk of cardiovascular disease and complications from hypertension.
Researchers at the Augusta University Medical College of Georgia investigated the effect of adverse childhood experiences (eg, childhood abuse or neglect, dysfunctional homes, or low socioeconomic status) during the transition from childhood to adulthood. Earlier research has linked adverse childhood experiences to faster increase of blood pressure in adulthood.
The investigators conducted periodic around-the-clock blood pressure monitoring to capture daytime and nighttime readings in 373 participants between the ages of 7 and 38 during a 23-year period. Participants who reported childhood adversity were 17% more likely to have blood pressure that was higher than the clinical definition of hypertension during the daytime.
"Adverse environments in early life have been consistently associated with the increased risk of hypertension in later life," said Shaoyong Su, PhD, lead author and Associate Professor of Pediatrics at Augusta University Medical College of Georgia. "We found that children who experienced childhood abuse or neglect, dysfunctional homes, and low socioeconomic status were far more likely to have higher blood pressure at night, as well as blood pressure variability over 24 hours, in addition to more rapid onset of hypertension at an earlier age."
Twenty-four-hour ambulatory blood pressure is considered a better predictor of organ damage and cardiovascular events because it can assess not only nighttime blood pressure levels, but also the blood pressure variability in real life. Blood pressure was monitored as many as 15 times during the study.
Researchers said that there was no difference in blood pressure regulation at various ages, thus suggesting that the patterns of adverse events in childhood are similar through young adulthood.
Most physicians focus on average blood pressure readings, but the new findings suggest that they should also ask younger patients about childhood adversity and watch for high blood pressure variability, noted Dr. Su. "This is not something most clinicians currently address, but it is a simple step that could identify many individuals at risk of adult hypertension and help them achieve control at an earlier age. This could avoid problems as they age," he added.
Blood pressure variability has been linked to various problems in adults, including decreased brain function in older adults, increased risk of stroke, and poorer post-stroke recovery. Likewise, early-onset hypertension and prehypertension have been linked to adverse preclinical cardiovascular disease, including left ventricular hypertrophy and evidence of increased arterial stiffness.
The current study was funded by the NIH and the National Heart Lung and Blood Institute.
Poor Sleep May Increase Risk for Irregular Heart Rhythms
Disruptions in sleep may increase the risk of atrial fibrillation, according to preliminary research. Obstructive sleep apnea, sleep interrupted by pauses in breathing, is a known risk factor for atrial fibrillation, which can lead to stroke, heart failure, and other heart-related complications. But whether there is a relationship between disrupted sleep and atrial fibrillation in the absence of sleep apnea is unclear.
Researchers at the University of California, San Francisco examined three sources of data, each using a different approach, to isolate and confirm the effects of poor sleep on atrial fibrillation. Their analyses of these studies showed that disrupted sleep, including insomnia, may be independently associated with atrial fibrillation. People who reported frequent nighttime awakening had an approximately 26% higher risk of developing atrial fibrillation, compared with those who did not wake up many times. In addition, people diagnosed with insomnia had a 29% higher risk of developing atrial fibrillation, compared with those without insomnia.
"The idea that these three studies gave us consistent results was exciting," said lead study author Matt Christensen, a fourth-year medical student at the University of Michigan in Ann Arbor. Past research has shown a link between poor sleep among people with atrial fibrillation. This study, however, focused on people whose pre-existing sleep disruptions were associated with developing atrial fibrillation later in life.
The data sources included the Health eHeart Study, an Internet-based cross-sectional study of more than 4,600 people; the Cardiovascular Health Study, an 11-year longitudinal study of more than 5,700 people, of whom almost 1,600 (28%) developed atrial fibrillation; and the California Healthcare Cost and Utilization Project, a hospital-based database spanning five years and covering almost 14 million patients.
In all three studies, researchers adjusted for the effects of obstructive sleep apnea and atrial fibrillation risk factors that might also be related to sleep. Some of those factors were age, sex, race, diabetes, high blood pressure, heart failure, and smoking.
In a separate analysis, the same researchers reviewed a subset of participants in the Cardiovascular Health Study to understand the effect of sleep disruptions during different sleep phases on the risk of atrial fibrillation in patients without obstructive sleep apnea. The analysis showed that having less REM sleep than other sleep phases during the night is linked to a higher likelihood of developing atrial fibrillation.
"By examining the actual characteristics of sleep, such as how much REM sleep you get, it points us toward a more plausible mechanism. There could be something particular about how sleep impacts the autonomic nervous system," said Mr. Christensen.
Another possible explanation for the link between sleep disruptions and atrial fibrillation is that frequent waking puts extra stress on the heart's chambers, said Mr. Christensen. Participants in this analysis were also enrolled in the Sleep Heart Health Study. They had a formal sleep study to objectively measure sleep quality. That element strengthened the study's conclusions because it did not rely on self-reported data, said Mr. Christensen.
In this analysis, 1,131 people (average age, 77) participated in a study with almost 10 years of follow-up. Researchers measured how long participants slept, how well they slept, how long it took to fall asleep, and the patterns of sleep (ie, how much time was spent in REM sleep vs non-REM sleep). Then they analyzed the sleep disruptions' effects to control the effects of age, sex, race, smoking, diabetes, high blood pressure, and other risk factors.
The exact link between sleep and the development of atrial fibrillation is still a mystery, but we are getting closer to a clear picture, said the study authors. "Ultimately, even without a clear understanding of the responsible mechanisms, we believe these findings suggest that strategies to enhance sleep quality, such as incorporating known techniques to improve sleep hygiene, may help prevent this important arrhythmia," said senior author Gregory Marcus, MD, MAS, a cardiologist at the University of California, San Francisco.
Poor sleep is a known contributor to other heart disease risk factors such as high blood pressure, obesity, and stroke. Getting enough physical activity, avoiding too much caffeine, and having an evening routine are good starting tips for sound sleep, said the researchers.
This study was funded in part by the Sarnoff Cardiovascular Research Foundation, the NIH, and the Agency for Healthcare Research and Quality.
Popular Heartburn Medication May Increase Ischemic Stroke Risk
Proton pump inhibitors (PPIs), which are used to reduce stomach acid and treat heartburn, may increase the risk of ischemic stroke, according to preliminary research. "PPIs have been associated with unhealthy vascular function, including heart attacks, kidney disease and dementia," said Thomas Sehested, MD, lead author and a researcher at the Danish Heart Foundation in Copenhagen. "We wanted to see if PPIs also posed a risk for ischemic stroke, especially given their increasing use in the general population."
Researchers analyzed the records of 244,679 Danish patients (average age, 57) who had an endoscopy. During nearly six years of follow up, 9,489 patients had an ischemic stroke for the first time in their lives. Researchers determined whether the stroke occurred while patients were using one of the following four PPIs: omeprazole, pantoprazole, lansoprazole, and esomeprazole.
For ischemic stroke, researchers found that overall stroke risk increased by 21% when patients were taking a PPI. At the lowest doses of the PPIs, the researchers found slight or no increased stroke risk. At the highest dose of these four PPIs, stroke risk increased by amounts ranging from 30% percent for lansoprazole to 94% for pantoprazole. There was no increased risk of stroke associated with another group of acid-reducing medications known as H2 blockers, which includes famotidine and ranitidine.
In comparison with nonusers, users of PPI were older and had more health conditions, including atrial fibrillation, at baseline (3.4% vs 3.8%). The study accounted for age, gender, and medical factors, including high blood pressure, atrial fibrillation, heart failure, and the use of certain pain relievers that have been linked to heart attack and stroke.
The authors believe that their findings, along with those of previous studies, should encourage more cautious use of PPIs. Most PPIs in the United States are now available over the counter, noted Dr. Sehested. "At one time, PPIs were thought to be safe, without major side effects. This study further questions the cardiovascular safety of these drugs."
Although their study did not find a link between H2 blockers and stroke, the authors could not say that this group of drugs would be better for patients than PPIs. Doctors prescribing PPIs should carefully consider whether their use is warranted and for how long, said Dr. Sehested. "We know from prior studies that a lot of individuals are using PPIs for a much longer time than indicated, which is especially true for elderly patients."
Study limitations include an observational design, which cannot establish cause and effect, and the fact that nearly all the participants were white. Authors believe that a randomized controlled trial of PPIs and cardiovascular disease is warranted. This study was funded by the Danish Heart Foundation.
Catheter Ablations Reduce Risks of Recurrent Stroke
Patients with atrial fibrillation and a prior history of stroke who undergo catheter ablation to treat the abnormal heart rhythm lower their long-term risk of a recurrent stroke by 50%, according to research from the Intermountain Medical Center Heart Institute.
When medications are not successful in treating the arrhythmia, catheter ablations are used to create scar tissue in the upper left atrium of the heart that prevents rapid, chaotic electric currents, often from the pulmonary veins, from causing the abnormal rhythm.
"One of the most devastating complications of atrial fibrillation is a stroke, and its prevention is the treatment cornerstone of the abnormal heart rhythm," said Jared Bunch, MD, lead author of the study and Director of Electrophysiology at the Intermountain Medical Center Heart Institute in Salt Lake City. "Patients that have a prior history of stroke have a much greater risk of recurrent strokes. Our new research shows [that] the more successful we are in treating the abnormal rhythm through the process required with catheter ablation, the better chance we have of lowering a patient's long-term risk of stroke."
The Intermountain study compared a group of 140 patients, who had a prior history of stroke and underwent their first catheter ablation, with two other patient groups, both of which also had a prior history of stroke, including 416 patients with atrial fibrillation who did not receive a catheter ablation, and 416 patients with stroke who did not have atrial fibrillation. The patients were followed for five years and observed for recurrent outcomes of stroke, heart failure, and death.
The five-year risk of patients having another stroke was decreased in the patients who had a catheter ablation to treat atrial fibrillation, compared with the group that had no catheter ablation to treat their abnormal heart rhythm. The stroke rates of patients with atrial fibrillation who underwent an ablation procedure were similar to those of patients with no history of atrial fibrillation.
"One of the most important findings of this study was that stroke rates in patients that underwent an ablation were similar to [those of] patients with no history of atrial fibrillation. This suggests that our management approach can alter some of the negative effects and natural history of atrial fibrillation. As physicians, we spend a lot of our time and energy trying to prevent stroke. This study helps us understand better how our management approaches can alter stroke risk," said Dr. Bunch. "Our research shows that more aggressive treatment of atrial fibrillation by using catheter ablations will reduce the chances [that] a person will have a life-threatening stroke."
Migraine Increases Cardiovascular Risk in Women With Symptoms of Ischemic Heart Disease
Among women being evaluated for ischemic heart disease, those who reported a history of migraine headaches have an increased risk of future cardiovascular (CV) event. This finding is primarily driven by the more than twofold increased risk of stroke.
Data regarding the association between migraine headaches and CV events in women have been inconsistent. Cecil A. Rambarat, MD, a resident at the University of Florida in Gainesville, and colleagues conducted a study to determine the long-term risk of CV events among women with and without migraine headaches who were evaluated for suspected ischemic heart disease in the Women's Ischemia Syndrome Evaluation (WISE) Study.
Women reporting a history of migraine headache were identified from the WISE cohort. Extended follow-up data were available, for a median follow-up of six years. Cox proportional adjusted hazard ratios (HR) were constructed for time to first adverse CV event (ie, CV death, nonfatal myocardial infarction [MI], heart failure hospitalization, or nonfatal stroke) among women with and without migraine headaches. In addition, HRs were determined for each event separately, as well as for all-cause death, angina hospitalization, death or MI, and CV death or MI.
Data on self-reported migraine headaches were available for 917 women. A total of 224 (24.4%) women reported a history of migraine headaches. Compared with those who did not report a history of migraines, women with a history of migraine headaches had an increased adjusted risk of CV events (HR, 1.83) at a median follow-up of six years. This result was mainly due to an increase in the risk of stroke (HR, 2.33).
The variables for which Dr. Rambarat and colleagues adjusted the data included age, race, BMI, history of diabetes, hypertension, dyslipidemia, smoking, family history of coronary artery disease, WISE coronary artery disease severity score, and aspirin use.
Childhood Adversity Is Linked to Blood Pressure Dysfunction
A difficult childhood may be associated with a risk of poor blood pressure regulation, researchers reported. In some studies, blood pressure variability has been associated with an elevated risk of cardiovascular disease and complications from hypertension.
Researchers at the Augusta University Medical College of Georgia investigated the effect of adverse childhood experiences (eg, childhood abuse or neglect, dysfunctional homes, or low socioeconomic status) during the transition from childhood to adulthood. Earlier research has linked adverse childhood experiences to faster increase of blood pressure in adulthood.
The investigators conducted periodic around-the-clock blood pressure monitoring to capture daytime and nighttime readings in 373 participants between the ages of 7 and 38 during a 23-year period. Participants who reported childhood adversity were 17% more likely to have blood pressure that was higher than the clinical definition of hypertension during the daytime.
"Adverse environments in early life have been consistently associated with the increased risk of hypertension in later life," said Shaoyong Su, PhD, lead author and Associate Professor of Pediatrics at Augusta University Medical College of Georgia. "We found that children who experienced childhood abuse or neglect, dysfunctional homes, and low socioeconomic status were far more likely to have higher blood pressure at night, as well as blood pressure variability over 24 hours, in addition to more rapid onset of hypertension at an earlier age."
Twenty-four-hour ambulatory blood pressure is considered a better predictor of organ damage and cardiovascular events because it can assess not only nighttime blood pressure levels, but also the blood pressure variability in real life. Blood pressure was monitored as many as 15 times during the study.
Researchers said that there was no difference in blood pressure regulation at various ages, thus suggesting that the patterns of adverse events in childhood are similar through young adulthood.
Most physicians focus on average blood pressure readings, but the new findings suggest that they should also ask younger patients about childhood adversity and watch for high blood pressure variability, noted Dr. Su. "This is not something most clinicians currently address, but it is a simple step that could identify many individuals at risk of adult hypertension and help them achieve control at an earlier age. This could avoid problems as they age," he added.
Blood pressure variability has been linked to various problems in adults, including decreased brain function in older adults, increased risk of stroke, and poorer post-stroke recovery. Likewise, early-onset hypertension and prehypertension have been linked to adverse preclinical cardiovascular disease, including left ventricular hypertrophy and evidence of increased arterial stiffness.
The current study was funded by the NIH and the National Heart Lung and Blood Institute.
Poor Sleep May Increase Risk for Irregular Heart Rhythms
Disruptions in sleep may increase the risk of atrial fibrillation, according to preliminary research. Obstructive sleep apnea, sleep interrupted by pauses in breathing, is a known risk factor for atrial fibrillation, which can lead to stroke, heart failure, and other heart-related complications. But whether there is a relationship between disrupted sleep and atrial fibrillation in the absence of sleep apnea is unclear.
Researchers at the University of California, San Francisco examined three sources of data, each using a different approach, to isolate and confirm the effects of poor sleep on atrial fibrillation. Their analyses of these studies showed that disrupted sleep, including insomnia, may be independently associated with atrial fibrillation. People who reported frequent nighttime awakening had an approximately 26% higher risk of developing atrial fibrillation, compared with those who did not wake up many times. In addition, people diagnosed with insomnia had a 29% higher risk of developing atrial fibrillation, compared with those without insomnia.
"The idea that these three studies gave us consistent results was exciting," said lead study author Matt Christensen, a fourth-year medical student at the University of Michigan in Ann Arbor. Past research has shown a link between poor sleep among people with atrial fibrillation. This study, however, focused on people whose pre-existing sleep disruptions were associated with developing atrial fibrillation later in life.
The data sources included the Health eHeart Study, an Internet-based cross-sectional study of more than 4,600 people; the Cardiovascular Health Study, an 11-year longitudinal study of more than 5,700 people, of whom almost 1,600 (28%) developed atrial fibrillation; and the California Healthcare Cost and Utilization Project, a hospital-based database spanning five years and covering almost 14 million patients.
In all three studies, researchers adjusted for the effects of obstructive sleep apnea and atrial fibrillation risk factors that might also be related to sleep. Some of those factors were age, sex, race, diabetes, high blood pressure, heart failure, and smoking.
In a separate analysis, the same researchers reviewed a subset of participants in the Cardiovascular Health Study to understand the effect of sleep disruptions during different sleep phases on the risk of atrial fibrillation in patients without obstructive sleep apnea. The analysis showed that having less REM sleep than other sleep phases during the night is linked to a higher likelihood of developing atrial fibrillation.
"By examining the actual characteristics of sleep, such as how much REM sleep you get, it points us toward a more plausible mechanism. There could be something particular about how sleep impacts the autonomic nervous system," said Mr. Christensen.
Another possible explanation for the link between sleep disruptions and atrial fibrillation is that frequent waking puts extra stress on the heart's chambers, said Mr. Christensen. Participants in this analysis were also enrolled in the Sleep Heart Health Study. They had a formal sleep study to objectively measure sleep quality. That element strengthened the study's conclusions because it did not rely on self-reported data, said Mr. Christensen.
In this analysis, 1,131 people (average age, 77) participated in a study with almost 10 years of follow-up. Researchers measured how long participants slept, how well they slept, how long it took to fall asleep, and the patterns of sleep (ie, how much time was spent in REM sleep vs non-REM sleep). Then they analyzed the sleep disruptions' effects to control the effects of age, sex, race, smoking, diabetes, high blood pressure, and other risk factors.
The exact link between sleep and the development of atrial fibrillation is still a mystery, but we are getting closer to a clear picture, said the study authors. "Ultimately, even without a clear understanding of the responsible mechanisms, we believe these findings suggest that strategies to enhance sleep quality, such as incorporating known techniques to improve sleep hygiene, may help prevent this important arrhythmia," said senior author Gregory Marcus, MD, MAS, a cardiologist at the University of California, San Francisco.
Poor sleep is a known contributor to other heart disease risk factors such as high blood pressure, obesity, and stroke. Getting enough physical activity, avoiding too much caffeine, and having an evening routine are good starting tips for sound sleep, said the researchers.
This study was funded in part by the Sarnoff Cardiovascular Research Foundation, the NIH, and the Agency for Healthcare Research and Quality.
Popular Heartburn Medication May Increase Ischemic Stroke Risk
Proton pump inhibitors (PPIs), which are used to reduce stomach acid and treat heartburn, may increase the risk of ischemic stroke, according to preliminary research. "PPIs have been associated with unhealthy vascular function, including heart attacks, kidney disease and dementia," said Thomas Sehested, MD, lead author and a researcher at the Danish Heart Foundation in Copenhagen. "We wanted to see if PPIs also posed a risk for ischemic stroke, especially given their increasing use in the general population."
Researchers analyzed the records of 244,679 Danish patients (average age, 57) who had an endoscopy. During nearly six years of follow up, 9,489 patients had an ischemic stroke for the first time in their lives. Researchers determined whether the stroke occurred while patients were using one of the following four PPIs: omeprazole, pantoprazole, lansoprazole, and esomeprazole.
For ischemic stroke, researchers found that overall stroke risk increased by 21% when patients were taking a PPI. At the lowest doses of the PPIs, the researchers found slight or no increased stroke risk. At the highest dose of these four PPIs, stroke risk increased by amounts ranging from 30% percent for lansoprazole to 94% for pantoprazole. There was no increased risk of stroke associated with another group of acid-reducing medications known as H2 blockers, which includes famotidine and ranitidine.
In comparison with nonusers, users of PPI were older and had more health conditions, including atrial fibrillation, at baseline (3.4% vs 3.8%). The study accounted for age, gender, and medical factors, including high blood pressure, atrial fibrillation, heart failure, and the use of certain pain relievers that have been linked to heart attack and stroke.
The authors believe that their findings, along with those of previous studies, should encourage more cautious use of PPIs. Most PPIs in the United States are now available over the counter, noted Dr. Sehested. "At one time, PPIs were thought to be safe, without major side effects. This study further questions the cardiovascular safety of these drugs."
Although their study did not find a link between H2 blockers and stroke, the authors could not say that this group of drugs would be better for patients than PPIs. Doctors prescribing PPIs should carefully consider whether their use is warranted and for how long, said Dr. Sehested. "We know from prior studies that a lot of individuals are using PPIs for a much longer time than indicated, which is especially true for elderly patients."
Study limitations include an observational design, which cannot establish cause and effect, and the fact that nearly all the participants were white. Authors believe that a randomized controlled trial of PPIs and cardiovascular disease is warranted. This study was funded by the Danish Heart Foundation.
Catheter Ablations Reduce Risks of Recurrent Stroke
Patients with atrial fibrillation and a prior history of stroke who undergo catheter ablation to treat the abnormal heart rhythm lower their long-term risk of a recurrent stroke by 50%, according to research from the Intermountain Medical Center Heart Institute.
When medications are not successful in treating the arrhythmia, catheter ablations are used to create scar tissue in the upper left atrium of the heart that prevents rapid, chaotic electric currents, often from the pulmonary veins, from causing the abnormal rhythm.
"One of the most devastating complications of atrial fibrillation is a stroke, and its prevention is the treatment cornerstone of the abnormal heart rhythm," said Jared Bunch, MD, lead author of the study and Director of Electrophysiology at the Intermountain Medical Center Heart Institute in Salt Lake City. "Patients that have a prior history of stroke have a much greater risk of recurrent strokes. Our new research shows [that] the more successful we are in treating the abnormal rhythm through the process required with catheter ablation, the better chance we have of lowering a patient's long-term risk of stroke."
The Intermountain study compared a group of 140 patients, who had a prior history of stroke and underwent their first catheter ablation, with two other patient groups, both of which also had a prior history of stroke, including 416 patients with atrial fibrillation who did not receive a catheter ablation, and 416 patients with stroke who did not have atrial fibrillation. The patients were followed for five years and observed for recurrent outcomes of stroke, heart failure, and death.
The five-year risk of patients having another stroke was decreased in the patients who had a catheter ablation to treat atrial fibrillation, compared with the group that had no catheter ablation to treat their abnormal heart rhythm. The stroke rates of patients with atrial fibrillation who underwent an ablation procedure were similar to those of patients with no history of atrial fibrillation.
"One of the most important findings of this study was that stroke rates in patients that underwent an ablation were similar to [those of] patients with no history of atrial fibrillation. This suggests that our management approach can alter some of the negative effects and natural history of atrial fibrillation. As physicians, we spend a lot of our time and energy trying to prevent stroke. This study helps us understand better how our management approaches can alter stroke risk," said Dr. Bunch. "Our research shows that more aggressive treatment of atrial fibrillation by using catheter ablations will reduce the chances [that] a person will have a life-threatening stroke."
Migraine Increases Cardiovascular Risk in Women With Symptoms of Ischemic Heart Disease
Among women being evaluated for ischemic heart disease, those who reported a history of migraine headaches have an increased risk of future cardiovascular (CV) event. This finding is primarily driven by the more than twofold increased risk of stroke.
Data regarding the association between migraine headaches and CV events in women have been inconsistent. Cecil A. Rambarat, MD, a resident at the University of Florida in Gainesville, and colleagues conducted a study to determine the long-term risk of CV events among women with and without migraine headaches who were evaluated for suspected ischemic heart disease in the Women's Ischemia Syndrome Evaluation (WISE) Study.
Women reporting a history of migraine headache were identified from the WISE cohort. Extended follow-up data were available, for a median follow-up of six years. Cox proportional adjusted hazard ratios (HR) were constructed for time to first adverse CV event (ie, CV death, nonfatal myocardial infarction [MI], heart failure hospitalization, or nonfatal stroke) among women with and without migraine headaches. In addition, HRs were determined for each event separately, as well as for all-cause death, angina hospitalization, death or MI, and CV death or MI.
Data on self-reported migraine headaches were available for 917 women. A total of 224 (24.4%) women reported a history of migraine headaches. Compared with those who did not report a history of migraines, women with a history of migraine headaches had an increased adjusted risk of CV events (HR, 1.83) at a median follow-up of six years. This result was mainly due to an increase in the risk of stroke (HR, 2.33).
The variables for which Dr. Rambarat and colleagues adjusted the data included age, race, BMI, history of diabetes, hypertension, dyslipidemia, smoking, family history of coronary artery disease, WISE coronary artery disease severity score, and aspirin use.
PPIs may boost ischemic stroke risk
NEW ORLEANS – The use of proton pump inhibitors (PPIs) was associated with significantly increased risk of having a first ischemic stroke in a large nationwide Danish cohort study, Thomas S. Sehested, MD, reported at the American Heart Association scientific sessions.
The relationship was dose dependent. At the lowest available dose of each of the four PPIs studied there was no significantly increased risk. At the intermediate doses of three of the four PPIs studied, the increased risk of ischemic stroke became statistically significant. And the highest dose of each drug was associated with the greatest ischemic stroke risk.
In Denmark, for instance, where most PPIs are prescription only and use is easily trackable, it’s estimated that, at any given time, 7% of the adult population is taking a PPI, often not as directed in the labeling.
The impetus for this study, Dr. Sehested explained, was the mounting evidence that PPIs may constitute an independent risk factor for acute MI and other cardiovascular events. For example, a recent meta-analysis of 17 randomized controlled trials totaling 7,540 participants published through mid-2015 concluded that the use of PPIs was associated with a 70% increase in cardiovascular risk (Neurogastroenterol Motil. 2016 Aug 30. doi: 10.1111/nmo.12926).
He reported on 245,676 Danes above age 30 who were free of prior MI or stroke when they underwent elective GI endoscopy during 1997-2012. After a 30-day postendoscopy grace period during which 1,476 patients had a first MI, stroke, or died of any cause, the final study population was 244,200, of whom 43.7% were PPI users during the grace period and beyond.
During a median 5.8 years of follow-up, 9,489 subjects (3.9%) had a first ischemic stroke. Because of the comprehensive nature of Denmark’s interlocking birth to death registries, there was virtually no loss to follow-up in this study.
The unadjusted incidence of ischemic stroke in PPI nonusers was 55.7 per 10,000 person-years, compared with 88.9 per 10,000 in PPI users.
The PPI users were slightly older than nonusers by roughly 3 years. They were also an absolute 5% more likely to be hypertensive and an absolute 1.7% more likely to be regular users of NSAIDs. All of these differences, while modest, were statistically significant because of the large patient numbers involved.
In a multivariate analysis adjusted for age, sex, calendar year, comorbid diabetes, hypertension, alcohol use disorder, heart failure, peptic ulcer, peripheral artery disease, kidney disease, aspirin, oral anticoagulants and other medications, and socioeconomic status, current users of PPIs were 19% more likely to have a first ischemic stroke than nonusers. That difference is statistically significant and clinically meaningful, Dr. Sehested said.
In contrast, when the same sort of nationwide analysis was repeated, comparing current users of histamine-2 receptor antagonists to nonusers of those drugs or PPIs, there was no difference in ischemic stroke risk between the two groups.
The message, according to Dr. Sehested, is that physicians should encourage more cautious use of PPIs. And especially in the United States, where most PPIs are available over the counter, it’s prudent during office visits to ask what nonprescription drugs a patient is taking.
Dr. Sehested presented his study findings in a session devoted to original research in cardiovascular epidemiology. Many top American epidemiologists were present in the audience, and several rose to congratulate him on his presentation of the latest elegant epidemiologic study to come out of Denmark, the only place in the world where this sort of nationwide comprehensive research is possible.
“Wow! I just love the work you do in Denmark. It’s really inspiring,” commented David Siscovick, MD, senior vice president for research at the New York Academy of Medicine and professor emeritus of medicine and epidemiology at the University of Washington in Seattle.
He had a question: “Did you deal with PPI starters and stoppers and compliance in any way?”
Dr. Sehested replied that he and his coinvestigators were able to see who was on a PPI at any given point in the study, and they accounted for that. One issue the researchers plan to examine but haven’t yet had a chance to, however, is the relationship between duration of PPI therapy and ischemic stroke risk. It’s likely that some patients had already been on a PPI for a lengthy time at elective endoscopy, which is when the study in its current form began.
“I think that would strengthen the study,” he said.
Comoderator Jorge Kizer, MD, of Albert Einstein College of Medicine in New York, commented, “Confounding by indication is clearly the elephant in the room. The guidelines actually recommend adding a PPI if a patient is on dual-antiplatelet therapy and has an NSAID added. Did you adjust for that? It would boost confidence that the results are actually due to the PPI.”
Dr. Sehested answered that the great majority of individuals with cardiovascular disease at baseline were excluded from the analysis.
“I don’t think we had that many on dual-antiplatelet therapy,” he added.
Preclinical studies suggest a possible mechanism by which PPIs may harm cardiovascular health. The drugs reduce nitric oxide synthase levels, with resultant endothelial dysfunction, he said.
Dr. Sehested is employed at the Danish Heart Foundation, which funded the study.
A growing number of retrospective studies have associated proton pump inhibitors with a host of serious adverse effects. These include chronic kidney disease, dementia, osteoporosis, cardiovascular events, pneumonia, enteric infections, and others. The authors of this large, retrospective Danish study have now added ischemic stroke to the list.
Nonetheless, this study should serve as wake-up call to closely examine the risks and benefits of ongoing PPI use for each individual patient. For example, guidelines clearly advocate the use of PPIs in patients at high risk for peptic ulcer disease (for example, use of aspirin and warfarin together), and these patients should continue PPIs unless more convincing evidence of serious side effects emerge. On the other hand, several studies have shown that many patients with uncomplicated gastroesophageal reflux disease symptoms can achieve symptom control with substitution of histamine2 blockers, p.r.n. dosing of PPIs, or without acid-reducing medications entirely. Still, many patients with confirmed pathologic acid reflux are likely to require ongoing PPIs. For patients who continue PPIs, they should use the lowest effective dose.
Surely, physicians will be discussing PPI adverse effects with increasing numbers of patients. Until higher-quality evidence in the form of a randomized controlled trial emerges, physicians should get used to explaining the principles of epidemiology.
Jacob Kurlander, MD, is a clinical lecturer in the division of gastroenterology, University of Michigan, Ann Arbor. He has received research funding from Ironwood Pharmaceuticals.
A growing number of retrospective studies have associated proton pump inhibitors with a host of serious adverse effects. These include chronic kidney disease, dementia, osteoporosis, cardiovascular events, pneumonia, enteric infections, and others. The authors of this large, retrospective Danish study have now added ischemic stroke to the list.
Nonetheless, this study should serve as wake-up call to closely examine the risks and benefits of ongoing PPI use for each individual patient. For example, guidelines clearly advocate the use of PPIs in patients at high risk for peptic ulcer disease (for example, use of aspirin and warfarin together), and these patients should continue PPIs unless more convincing evidence of serious side effects emerge. On the other hand, several studies have shown that many patients with uncomplicated gastroesophageal reflux disease symptoms can achieve symptom control with substitution of histamine2 blockers, p.r.n. dosing of PPIs, or without acid-reducing medications entirely. Still, many patients with confirmed pathologic acid reflux are likely to require ongoing PPIs. For patients who continue PPIs, they should use the lowest effective dose.
Surely, physicians will be discussing PPI adverse effects with increasing numbers of patients. Until higher-quality evidence in the form of a randomized controlled trial emerges, physicians should get used to explaining the principles of epidemiology.
Jacob Kurlander, MD, is a clinical lecturer in the division of gastroenterology, University of Michigan, Ann Arbor. He has received research funding from Ironwood Pharmaceuticals.
A growing number of retrospective studies have associated proton pump inhibitors with a host of serious adverse effects. These include chronic kidney disease, dementia, osteoporosis, cardiovascular events, pneumonia, enteric infections, and others. The authors of this large, retrospective Danish study have now added ischemic stroke to the list.
Nonetheless, this study should serve as wake-up call to closely examine the risks and benefits of ongoing PPI use for each individual patient. For example, guidelines clearly advocate the use of PPIs in patients at high risk for peptic ulcer disease (for example, use of aspirin and warfarin together), and these patients should continue PPIs unless more convincing evidence of serious side effects emerge. On the other hand, several studies have shown that many patients with uncomplicated gastroesophageal reflux disease symptoms can achieve symptom control with substitution of histamine2 blockers, p.r.n. dosing of PPIs, or without acid-reducing medications entirely. Still, many patients with confirmed pathologic acid reflux are likely to require ongoing PPIs. For patients who continue PPIs, they should use the lowest effective dose.
Surely, physicians will be discussing PPI adverse effects with increasing numbers of patients. Until higher-quality evidence in the form of a randomized controlled trial emerges, physicians should get used to explaining the principles of epidemiology.
Jacob Kurlander, MD, is a clinical lecturer in the division of gastroenterology, University of Michigan, Ann Arbor. He has received research funding from Ironwood Pharmaceuticals.
NEW ORLEANS – The use of proton pump inhibitors (PPIs) was associated with significantly increased risk of having a first ischemic stroke in a large nationwide Danish cohort study, Thomas S. Sehested, MD, reported at the American Heart Association scientific sessions.
The relationship was dose dependent. At the lowest available dose of each of the four PPIs studied there was no significantly increased risk. At the intermediate doses of three of the four PPIs studied, the increased risk of ischemic stroke became statistically significant. And the highest dose of each drug was associated with the greatest ischemic stroke risk.
In Denmark, for instance, where most PPIs are prescription only and use is easily trackable, it’s estimated that, at any given time, 7% of the adult population is taking a PPI, often not as directed in the labeling.
The impetus for this study, Dr. Sehested explained, was the mounting evidence that PPIs may constitute an independent risk factor for acute MI and other cardiovascular events. For example, a recent meta-analysis of 17 randomized controlled trials totaling 7,540 participants published through mid-2015 concluded that the use of PPIs was associated with a 70% increase in cardiovascular risk (Neurogastroenterol Motil. 2016 Aug 30. doi: 10.1111/nmo.12926).
He reported on 245,676 Danes above age 30 who were free of prior MI or stroke when they underwent elective GI endoscopy during 1997-2012. After a 30-day postendoscopy grace period during which 1,476 patients had a first MI, stroke, or died of any cause, the final study population was 244,200, of whom 43.7% were PPI users during the grace period and beyond.
During a median 5.8 years of follow-up, 9,489 subjects (3.9%) had a first ischemic stroke. Because of the comprehensive nature of Denmark’s interlocking birth to death registries, there was virtually no loss to follow-up in this study.
The unadjusted incidence of ischemic stroke in PPI nonusers was 55.7 per 10,000 person-years, compared with 88.9 per 10,000 in PPI users.
The PPI users were slightly older than nonusers by roughly 3 years. They were also an absolute 5% more likely to be hypertensive and an absolute 1.7% more likely to be regular users of NSAIDs. All of these differences, while modest, were statistically significant because of the large patient numbers involved.
In a multivariate analysis adjusted for age, sex, calendar year, comorbid diabetes, hypertension, alcohol use disorder, heart failure, peptic ulcer, peripheral artery disease, kidney disease, aspirin, oral anticoagulants and other medications, and socioeconomic status, current users of PPIs were 19% more likely to have a first ischemic stroke than nonusers. That difference is statistically significant and clinically meaningful, Dr. Sehested said.
In contrast, when the same sort of nationwide analysis was repeated, comparing current users of histamine-2 receptor antagonists to nonusers of those drugs or PPIs, there was no difference in ischemic stroke risk between the two groups.
The message, according to Dr. Sehested, is that physicians should encourage more cautious use of PPIs. And especially in the United States, where most PPIs are available over the counter, it’s prudent during office visits to ask what nonprescription drugs a patient is taking.
Dr. Sehested presented his study findings in a session devoted to original research in cardiovascular epidemiology. Many top American epidemiologists were present in the audience, and several rose to congratulate him on his presentation of the latest elegant epidemiologic study to come out of Denmark, the only place in the world where this sort of nationwide comprehensive research is possible.
“Wow! I just love the work you do in Denmark. It’s really inspiring,” commented David Siscovick, MD, senior vice president for research at the New York Academy of Medicine and professor emeritus of medicine and epidemiology at the University of Washington in Seattle.
He had a question: “Did you deal with PPI starters and stoppers and compliance in any way?”
Dr. Sehested replied that he and his coinvestigators were able to see who was on a PPI at any given point in the study, and they accounted for that. One issue the researchers plan to examine but haven’t yet had a chance to, however, is the relationship between duration of PPI therapy and ischemic stroke risk. It’s likely that some patients had already been on a PPI for a lengthy time at elective endoscopy, which is when the study in its current form began.
“I think that would strengthen the study,” he said.
Comoderator Jorge Kizer, MD, of Albert Einstein College of Medicine in New York, commented, “Confounding by indication is clearly the elephant in the room. The guidelines actually recommend adding a PPI if a patient is on dual-antiplatelet therapy and has an NSAID added. Did you adjust for that? It would boost confidence that the results are actually due to the PPI.”
Dr. Sehested answered that the great majority of individuals with cardiovascular disease at baseline were excluded from the analysis.
“I don’t think we had that many on dual-antiplatelet therapy,” he added.
Preclinical studies suggest a possible mechanism by which PPIs may harm cardiovascular health. The drugs reduce nitric oxide synthase levels, with resultant endothelial dysfunction, he said.
Dr. Sehested is employed at the Danish Heart Foundation, which funded the study.
NEW ORLEANS – The use of proton pump inhibitors (PPIs) was associated with significantly increased risk of having a first ischemic stroke in a large nationwide Danish cohort study, Thomas S. Sehested, MD, reported at the American Heart Association scientific sessions.
The relationship was dose dependent. At the lowest available dose of each of the four PPIs studied there was no significantly increased risk. At the intermediate doses of three of the four PPIs studied, the increased risk of ischemic stroke became statistically significant. And the highest dose of each drug was associated with the greatest ischemic stroke risk.
In Denmark, for instance, where most PPIs are prescription only and use is easily trackable, it’s estimated that, at any given time, 7% of the adult population is taking a PPI, often not as directed in the labeling.
The impetus for this study, Dr. Sehested explained, was the mounting evidence that PPIs may constitute an independent risk factor for acute MI and other cardiovascular events. For example, a recent meta-analysis of 17 randomized controlled trials totaling 7,540 participants published through mid-2015 concluded that the use of PPIs was associated with a 70% increase in cardiovascular risk (Neurogastroenterol Motil. 2016 Aug 30. doi: 10.1111/nmo.12926).
He reported on 245,676 Danes above age 30 who were free of prior MI or stroke when they underwent elective GI endoscopy during 1997-2012. After a 30-day postendoscopy grace period during which 1,476 patients had a first MI, stroke, or died of any cause, the final study population was 244,200, of whom 43.7% were PPI users during the grace period and beyond.
During a median 5.8 years of follow-up, 9,489 subjects (3.9%) had a first ischemic stroke. Because of the comprehensive nature of Denmark’s interlocking birth to death registries, there was virtually no loss to follow-up in this study.
The unadjusted incidence of ischemic stroke in PPI nonusers was 55.7 per 10,000 person-years, compared with 88.9 per 10,000 in PPI users.
The PPI users were slightly older than nonusers by roughly 3 years. They were also an absolute 5% more likely to be hypertensive and an absolute 1.7% more likely to be regular users of NSAIDs. All of these differences, while modest, were statistically significant because of the large patient numbers involved.
In a multivariate analysis adjusted for age, sex, calendar year, comorbid diabetes, hypertension, alcohol use disorder, heart failure, peptic ulcer, peripheral artery disease, kidney disease, aspirin, oral anticoagulants and other medications, and socioeconomic status, current users of PPIs were 19% more likely to have a first ischemic stroke than nonusers. That difference is statistically significant and clinically meaningful, Dr. Sehested said.
In contrast, when the same sort of nationwide analysis was repeated, comparing current users of histamine-2 receptor antagonists to nonusers of those drugs or PPIs, there was no difference in ischemic stroke risk between the two groups.
The message, according to Dr. Sehested, is that physicians should encourage more cautious use of PPIs. And especially in the United States, where most PPIs are available over the counter, it’s prudent during office visits to ask what nonprescription drugs a patient is taking.
Dr. Sehested presented his study findings in a session devoted to original research in cardiovascular epidemiology. Many top American epidemiologists were present in the audience, and several rose to congratulate him on his presentation of the latest elegant epidemiologic study to come out of Denmark, the only place in the world where this sort of nationwide comprehensive research is possible.
“Wow! I just love the work you do in Denmark. It’s really inspiring,” commented David Siscovick, MD, senior vice president for research at the New York Academy of Medicine and professor emeritus of medicine and epidemiology at the University of Washington in Seattle.
He had a question: “Did you deal with PPI starters and stoppers and compliance in any way?”
Dr. Sehested replied that he and his coinvestigators were able to see who was on a PPI at any given point in the study, and they accounted for that. One issue the researchers plan to examine but haven’t yet had a chance to, however, is the relationship between duration of PPI therapy and ischemic stroke risk. It’s likely that some patients had already been on a PPI for a lengthy time at elective endoscopy, which is when the study in its current form began.
“I think that would strengthen the study,” he said.
Comoderator Jorge Kizer, MD, of Albert Einstein College of Medicine in New York, commented, “Confounding by indication is clearly the elephant in the room. The guidelines actually recommend adding a PPI if a patient is on dual-antiplatelet therapy and has an NSAID added. Did you adjust for that? It would boost confidence that the results are actually due to the PPI.”
Dr. Sehested answered that the great majority of individuals with cardiovascular disease at baseline were excluded from the analysis.
“I don’t think we had that many on dual-antiplatelet therapy,” he added.
Preclinical studies suggest a possible mechanism by which PPIs may harm cardiovascular health. The drugs reduce nitric oxide synthase levels, with resultant endothelial dysfunction, he said.
Dr. Sehested is employed at the Danish Heart Foundation, which funded the study.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Current use of a proton pump inhibitor was independently associated with a 19% increased risk of a first ischemic stroke, and the risk was greater at the top approved doses.
Data source: This retrospective nationwide Danish study involved 244,200 adults age 30 or older followed for a median of nearly 6 years following elective GI endoscopy.
Disclosures: The presenter is employed at the Danish Heart Foundation, which funded the study.
Make HIV testing of adolescents routine
Nearly 2 decades ago, I was a pediatric infectious diseases fellow fielding a call from a community pediatrician seeking advice on patient management. The patient in question was a 15-year-old male with fever, rash, and cervical adenopathy – a good clinical story for Epstein-Barr virus infection. A heterophile antibody test was negative, however, as were EBV titers.
We talked for a couple of minutes about the vagaries of EBV testing, as well as other organisms that could cause a mononucleosis-like illness. “Cytomegalovirus is a possibility, along with toxoplasmosis,” I told him. “I’d also test for HIV.”
There was a moment of silence and little throat-clearing. “I don’t think we need to that,” he finally responded. “I’ve known this boy since he was a baby, and I’m sure HIV’s not an issue. He’s not that kind of kid.”
Bear in mind that we lived in a Midwestern city with low rates of HIV, and I suspect this seasoned pediatrician had never seen a case. I argued (as only an impassioned trainee can) that every kid is the kind that could be at risk for HIV, and testing was ultimately done (and was negative).
A lot has changed in the intervening years. HIV infection, at least in adolescents and adults, can be controlled with a single pill taken once a day. Children infected perinatally can grow up and have (uninfected) children of their own. We have reasonably effective pre- and postexposure prophylaxis.
One thing that hasn’t changed, however, is the reluctance of some of us to test our patients for HIV. So what’s up with that?
It’s not because the virus has gone away. On Oct. 14, 2016, amid little fanfare, the Centers for Disease Control and Prevention released the United States Summary of Notifiable Infectious Diseases and Conditions for 2014. A total of 35,606 cases of HIV infection were diagnosed in the United States and reported to the CDC, and 7,723 were in individuals aged 15-24 years.
It is possible that the number of cases in adolescents is even higher. The CDC estimates as many as 60% of youth with HIV don’t know that they are infected, likely because they’ve never been tested. According to the 2015 Youth Risk Behavior Survey (YRBS), only 10% of United States high school students had ever been tested for HIV, and the number of teens tested has been dropping over time. In 2013, for example, the prevalence of having ever been tested for HIV was 13%.
It’s not because today’s teenagers lack risk factors, including sexual activity and drug use. Just over 30% of the U.S. students surveyed for the YRBS reported sexual intercourse with at least one person in the preceding 3 months, and more than 11% had had four or more lifetime partners. Among sexually active teenagers in the United States, only 57% reported that they or their partner used a condom during last sexual intercourse. Overall, 2% of those surveyed admitted a history of injecting an illegal drug.
It’s not because public health experts haven’t deemed testing a priority. The CDC recommends that everyone aged 13-64 years should get tested at least once. Annual testing is recommended for some individuals, including sexually active gay and bisexual males, those who have had more than one sexual partner since their last HIV test, and those who have another sexually transmitted disease. A 2011 American Academy of Pediatrics policy statement affirms the need for routine testing, calling for all adolescents living in geographic areas with an HIV prevalence greater than 0.1% to be offered routine HIV screening at least once by age 16-18 years. In communities with a lower prevalence, the AAP recommends routine HIV testing for sexually active adolescents as well as those with other risk factors, including substance use. Annual HIV testing is recommended for high-risk teenagers, and whenever testing for other sexually transmitted infections (STIs) is performed.
It’s probably not that most teenagers are being offered HIV tests and they’re declining. In 2008, the emergency department at Le Bonheur Children’s Hospital in Memphis, Tenn., implemented a protocol for routine, opt-out HIV screening for medically stable patients aged 13-18 years (Pediatrics. 2009 Oct;124:1076-84). Of the 2,002 patients approached for screening over an approximately 7-month period, only 267 (13%) opted out and of those, 73 had already been tested.
Yet many of us still are not testing. More recently, investigators in Philadelphia performed a retrospective, cross-sectional study of 1,000 randomly selected 13- to 19-year-old patients attending routine well visits conducted at 29 pediatric primary care practices to assess clinician documentation of sexual history and screening for STIs and HIV (J Pediatr. 2014 Aug;165[2]:343-7). Only 212 visits (21.2%) had a documented sexual history, and only 16 patients were tested for HIV (1.6%). HIV testing was more likely to be performed on older adolescents, those of non-Hispanic black race/ethnicity, and those with nonprivate insurance. Study authors called the results “concerning” and advocated for standardized protocols, documentation templates, and electronic decision support to facilitate improved sexual health assessments and screening.
I suspect we all can do better. I’m not a primary care provider, but I do see adolescents with a variety of complaints. I’m pretty diligent about testing teenagers admitted with unexplained fever, vague constitutional symptoms, and those with symptoms that suggest another STI. I’m less effective at discussing HIV testing with those being treated for a postop wound infection, or a routine community-acquired pneumonia.
December is a good time to reflect on practice and make resolutions for the new year. I resolve to talk to more of my adolescent patients about HIV. Who’s with me?
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville, Ky., and Kosair Children’s Hospital, also in Louisville. Email her at [email protected].
Nearly 2 decades ago, I was a pediatric infectious diseases fellow fielding a call from a community pediatrician seeking advice on patient management. The patient in question was a 15-year-old male with fever, rash, and cervical adenopathy – a good clinical story for Epstein-Barr virus infection. A heterophile antibody test was negative, however, as were EBV titers.
We talked for a couple of minutes about the vagaries of EBV testing, as well as other organisms that could cause a mononucleosis-like illness. “Cytomegalovirus is a possibility, along with toxoplasmosis,” I told him. “I’d also test for HIV.”
There was a moment of silence and little throat-clearing. “I don’t think we need to that,” he finally responded. “I’ve known this boy since he was a baby, and I’m sure HIV’s not an issue. He’s not that kind of kid.”
Bear in mind that we lived in a Midwestern city with low rates of HIV, and I suspect this seasoned pediatrician had never seen a case. I argued (as only an impassioned trainee can) that every kid is the kind that could be at risk for HIV, and testing was ultimately done (and was negative).
A lot has changed in the intervening years. HIV infection, at least in adolescents and adults, can be controlled with a single pill taken once a day. Children infected perinatally can grow up and have (uninfected) children of their own. We have reasonably effective pre- and postexposure prophylaxis.
One thing that hasn’t changed, however, is the reluctance of some of us to test our patients for HIV. So what’s up with that?
It’s not because the virus has gone away. On Oct. 14, 2016, amid little fanfare, the Centers for Disease Control and Prevention released the United States Summary of Notifiable Infectious Diseases and Conditions for 2014. A total of 35,606 cases of HIV infection were diagnosed in the United States and reported to the CDC, and 7,723 were in individuals aged 15-24 years.
It is possible that the number of cases in adolescents is even higher. The CDC estimates as many as 60% of youth with HIV don’t know that they are infected, likely because they’ve never been tested. According to the 2015 Youth Risk Behavior Survey (YRBS), only 10% of United States high school students had ever been tested for HIV, and the number of teens tested has been dropping over time. In 2013, for example, the prevalence of having ever been tested for HIV was 13%.
It’s not because today’s teenagers lack risk factors, including sexual activity and drug use. Just over 30% of the U.S. students surveyed for the YRBS reported sexual intercourse with at least one person in the preceding 3 months, and more than 11% had had four or more lifetime partners. Among sexually active teenagers in the United States, only 57% reported that they or their partner used a condom during last sexual intercourse. Overall, 2% of those surveyed admitted a history of injecting an illegal drug.
It’s not because public health experts haven’t deemed testing a priority. The CDC recommends that everyone aged 13-64 years should get tested at least once. Annual testing is recommended for some individuals, including sexually active gay and bisexual males, those who have had more than one sexual partner since their last HIV test, and those who have another sexually transmitted disease. A 2011 American Academy of Pediatrics policy statement affirms the need for routine testing, calling for all adolescents living in geographic areas with an HIV prevalence greater than 0.1% to be offered routine HIV screening at least once by age 16-18 years. In communities with a lower prevalence, the AAP recommends routine HIV testing for sexually active adolescents as well as those with other risk factors, including substance use. Annual HIV testing is recommended for high-risk teenagers, and whenever testing for other sexually transmitted infections (STIs) is performed.
It’s probably not that most teenagers are being offered HIV tests and they’re declining. In 2008, the emergency department at Le Bonheur Children’s Hospital in Memphis, Tenn., implemented a protocol for routine, opt-out HIV screening for medically stable patients aged 13-18 years (Pediatrics. 2009 Oct;124:1076-84). Of the 2,002 patients approached for screening over an approximately 7-month period, only 267 (13%) opted out and of those, 73 had already been tested.
Yet many of us still are not testing. More recently, investigators in Philadelphia performed a retrospective, cross-sectional study of 1,000 randomly selected 13- to 19-year-old patients attending routine well visits conducted at 29 pediatric primary care practices to assess clinician documentation of sexual history and screening for STIs and HIV (J Pediatr. 2014 Aug;165[2]:343-7). Only 212 visits (21.2%) had a documented sexual history, and only 16 patients were tested for HIV (1.6%). HIV testing was more likely to be performed on older adolescents, those of non-Hispanic black race/ethnicity, and those with nonprivate insurance. Study authors called the results “concerning” and advocated for standardized protocols, documentation templates, and electronic decision support to facilitate improved sexual health assessments and screening.
I suspect we all can do better. I’m not a primary care provider, but I do see adolescents with a variety of complaints. I’m pretty diligent about testing teenagers admitted with unexplained fever, vague constitutional symptoms, and those with symptoms that suggest another STI. I’m less effective at discussing HIV testing with those being treated for a postop wound infection, or a routine community-acquired pneumonia.
December is a good time to reflect on practice and make resolutions for the new year. I resolve to talk to more of my adolescent patients about HIV. Who’s with me?
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville, Ky., and Kosair Children’s Hospital, also in Louisville. Email her at [email protected].
Nearly 2 decades ago, I was a pediatric infectious diseases fellow fielding a call from a community pediatrician seeking advice on patient management. The patient in question was a 15-year-old male with fever, rash, and cervical adenopathy – a good clinical story for Epstein-Barr virus infection. A heterophile antibody test was negative, however, as were EBV titers.
We talked for a couple of minutes about the vagaries of EBV testing, as well as other organisms that could cause a mononucleosis-like illness. “Cytomegalovirus is a possibility, along with toxoplasmosis,” I told him. “I’d also test for HIV.”
There was a moment of silence and little throat-clearing. “I don’t think we need to that,” he finally responded. “I’ve known this boy since he was a baby, and I’m sure HIV’s not an issue. He’s not that kind of kid.”
Bear in mind that we lived in a Midwestern city with low rates of HIV, and I suspect this seasoned pediatrician had never seen a case. I argued (as only an impassioned trainee can) that every kid is the kind that could be at risk for HIV, and testing was ultimately done (and was negative).
A lot has changed in the intervening years. HIV infection, at least in adolescents and adults, can be controlled with a single pill taken once a day. Children infected perinatally can grow up and have (uninfected) children of their own. We have reasonably effective pre- and postexposure prophylaxis.
One thing that hasn’t changed, however, is the reluctance of some of us to test our patients for HIV. So what’s up with that?
It’s not because the virus has gone away. On Oct. 14, 2016, amid little fanfare, the Centers for Disease Control and Prevention released the United States Summary of Notifiable Infectious Diseases and Conditions for 2014. A total of 35,606 cases of HIV infection were diagnosed in the United States and reported to the CDC, and 7,723 were in individuals aged 15-24 years.
It is possible that the number of cases in adolescents is even higher. The CDC estimates as many as 60% of youth with HIV don’t know that they are infected, likely because they’ve never been tested. According to the 2015 Youth Risk Behavior Survey (YRBS), only 10% of United States high school students had ever been tested for HIV, and the number of teens tested has been dropping over time. In 2013, for example, the prevalence of having ever been tested for HIV was 13%.
It’s not because today’s teenagers lack risk factors, including sexual activity and drug use. Just over 30% of the U.S. students surveyed for the YRBS reported sexual intercourse with at least one person in the preceding 3 months, and more than 11% had had four or more lifetime partners. Among sexually active teenagers in the United States, only 57% reported that they or their partner used a condom during last sexual intercourse. Overall, 2% of those surveyed admitted a history of injecting an illegal drug.
It’s not because public health experts haven’t deemed testing a priority. The CDC recommends that everyone aged 13-64 years should get tested at least once. Annual testing is recommended for some individuals, including sexually active gay and bisexual males, those who have had more than one sexual partner since their last HIV test, and those who have another sexually transmitted disease. A 2011 American Academy of Pediatrics policy statement affirms the need for routine testing, calling for all adolescents living in geographic areas with an HIV prevalence greater than 0.1% to be offered routine HIV screening at least once by age 16-18 years. In communities with a lower prevalence, the AAP recommends routine HIV testing for sexually active adolescents as well as those with other risk factors, including substance use. Annual HIV testing is recommended for high-risk teenagers, and whenever testing for other sexually transmitted infections (STIs) is performed.
It’s probably not that most teenagers are being offered HIV tests and they’re declining. In 2008, the emergency department at Le Bonheur Children’s Hospital in Memphis, Tenn., implemented a protocol for routine, opt-out HIV screening for medically stable patients aged 13-18 years (Pediatrics. 2009 Oct;124:1076-84). Of the 2,002 patients approached for screening over an approximately 7-month period, only 267 (13%) opted out and of those, 73 had already been tested.
Yet many of us still are not testing. More recently, investigators in Philadelphia performed a retrospective, cross-sectional study of 1,000 randomly selected 13- to 19-year-old patients attending routine well visits conducted at 29 pediatric primary care practices to assess clinician documentation of sexual history and screening for STIs and HIV (J Pediatr. 2014 Aug;165[2]:343-7). Only 212 visits (21.2%) had a documented sexual history, and only 16 patients were tested for HIV (1.6%). HIV testing was more likely to be performed on older adolescents, those of non-Hispanic black race/ethnicity, and those with nonprivate insurance. Study authors called the results “concerning” and advocated for standardized protocols, documentation templates, and electronic decision support to facilitate improved sexual health assessments and screening.
I suspect we all can do better. I’m not a primary care provider, but I do see adolescents with a variety of complaints. I’m pretty diligent about testing teenagers admitted with unexplained fever, vague constitutional symptoms, and those with symptoms that suggest another STI. I’m less effective at discussing HIV testing with those being treated for a postop wound infection, or a routine community-acquired pneumonia.
December is a good time to reflect on practice and make resolutions for the new year. I resolve to talk to more of my adolescent patients about HIV. Who’s with me?
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville, Ky., and Kosair Children’s Hospital, also in Louisville. Email her at [email protected].
Fighting back against psoriasis bullies
Dallas dermatologist Alan Menter, MD, doesn’t boast bullying-prevention superpowers, but what he does have is close enough: An eagerness to get the word out to anyone – parent or principal, psychologist or pediatrician – who can help prevent a child with psoriasis from being bullied.
Over his long career, Dr. Menter has made many calls to adults in positions of influence over children. “I’ve talked to pediatricians, and I’ve even called up schools and talked to principals to try get the bullying situation reduced to an extent where the kids can live happy, normal lives without kids taunting them.”
Dr. Menter, chief of dermatology at Baylor University in Dallas, has plenty of company. Other dermatologists are paying close attention to their youngest patients with psoriasis as researchers work to get a better handle on the bullying problem.
“We really want to identify this early on and do whatever is required to turn it around,” said Amy Paller, MD, professor of dermatology and pediatrics and chair of the department of dermatology at Northwestern University, Chicago. “These visible skin lesions can have a very significant effect on how children feel about themselves and others. When this is going on early in life, during childhood or teen years, there’s really a risk for lifelong issues.”
Dr. Menter’s interest in psoriasis and bullying began during his childhood in South Africa when he watched children bully his brother, who had the condition. “I’ve always had a great desire to improve the quality of life in psoriasis patients,” he said, and that passion grew as he worked in a day care center for children with psoriasis. “I had an opportunity to talk to children and recognize the impact that psoriasis has on them.”
Research from across the world reveals that children with psoriasis face an extraordinary burden from bullying. “They’re teased incessantly and bullied because they’ve got such a visible disease,” he said.
The introspective and depressed nature of many children with psoriasis makes the situation even more difficult, he noted, since their emotional makeup prevents them from responding easily to taunting.
The extent of the bullying problem, however, isn’t fully understood. Research into bullying and skin disorders is “very limited,” said Kelly Cordoro, MD, of the departments of dermatology and pediatrics at the University of California, San Francisco. “What little evidence does exist suggests that kids with visible skin disease, including psoriasis, are often bullied, and this can impact them significantly,” she said, pointing to a 2013 study that suggested those with acne, psoriasis, and atopic dermatitis are especially vulnerable (Clin Dermatol. 2013;31[1]:66-71).
Sports are a special area of concern. “They don’t want to get into gym shorts, and they don’t want to engage in sports because they get hot and itchy,” Dr. Paller said. “Or people stay away from them because they think there’s something they can catch, so they’re not chosen for sports activities.”
Indeed, children with psoriasis may be left out of games like tag and contact sports because other children are afraid of touching them, Dr. Cordoro observed. “Other kids do not want to be near them. It is truly heartbreaking and derives largely from ignorance.”
What can dermatologists do? Dr. Cordoro recommends that they take time to ask their youngest patients about their lives: “Is your psoriasis affecting your friendships?” “How are things going at school?” “Do kids ask you about your psoriasis? What do you say?”
“We can identify at-risk kids this way and work with parents, schools, coaches, and counselors towards productive interventions like educational programs,” Dr. Cordoro said. “Education is the key. As kids, parents, and adults become educated, the psoriatic child is less likely to be teased and excluded. Kids with psoriasis may lack the confidence to defend themselves, and arming them with one-liners and basic educational points about their condition empowers them to address it directly.”
Dr. Paller, who is also director of the Northwestern University Skin Disease Research Center, said it’s a good idea to add questions to the usual list of queries about subjects like sleep and itching. In cases when a child is bullied, it may help to reach out to teachers and principals, and to counselors and social workers if needed, she noted.
Parents play an important role, too, Dr. Menter said, although they may be in the dark about bullying. “What I’ve learned is that kids will seldom come home and tell their parent they’ve been bullied.”
He urges both children and their parents to understand the nature of psoriasis and be open about it. “Don’t hide it,” he suggested. “Tell people that ‘I’ve got psoriasis, and it’s not contagious.’ ” And then, hopefully, the healing can begin.
Dr. Paller and Dr. Cordoro reported no relevant disclosures. Dr. Menter disclosed relationships with many pharmaceutical companies, including AbbVie, Allergan, Amgen, Boehringer Ingelheim, Eli Lilly, Merck, Novartis and Pfizer.
Dallas dermatologist Alan Menter, MD, doesn’t boast bullying-prevention superpowers, but what he does have is close enough: An eagerness to get the word out to anyone – parent or principal, psychologist or pediatrician – who can help prevent a child with psoriasis from being bullied.
Over his long career, Dr. Menter has made many calls to adults in positions of influence over children. “I’ve talked to pediatricians, and I’ve even called up schools and talked to principals to try get the bullying situation reduced to an extent where the kids can live happy, normal lives without kids taunting them.”
Dr. Menter, chief of dermatology at Baylor University in Dallas, has plenty of company. Other dermatologists are paying close attention to their youngest patients with psoriasis as researchers work to get a better handle on the bullying problem.
“We really want to identify this early on and do whatever is required to turn it around,” said Amy Paller, MD, professor of dermatology and pediatrics and chair of the department of dermatology at Northwestern University, Chicago. “These visible skin lesions can have a very significant effect on how children feel about themselves and others. When this is going on early in life, during childhood or teen years, there’s really a risk for lifelong issues.”
Dr. Menter’s interest in psoriasis and bullying began during his childhood in South Africa when he watched children bully his brother, who had the condition. “I’ve always had a great desire to improve the quality of life in psoriasis patients,” he said, and that passion grew as he worked in a day care center for children with psoriasis. “I had an opportunity to talk to children and recognize the impact that psoriasis has on them.”
Research from across the world reveals that children with psoriasis face an extraordinary burden from bullying. “They’re teased incessantly and bullied because they’ve got such a visible disease,” he said.
The introspective and depressed nature of many children with psoriasis makes the situation even more difficult, he noted, since their emotional makeup prevents them from responding easily to taunting.
The extent of the bullying problem, however, isn’t fully understood. Research into bullying and skin disorders is “very limited,” said Kelly Cordoro, MD, of the departments of dermatology and pediatrics at the University of California, San Francisco. “What little evidence does exist suggests that kids with visible skin disease, including psoriasis, are often bullied, and this can impact them significantly,” she said, pointing to a 2013 study that suggested those with acne, psoriasis, and atopic dermatitis are especially vulnerable (Clin Dermatol. 2013;31[1]:66-71).
Sports are a special area of concern. “They don’t want to get into gym shorts, and they don’t want to engage in sports because they get hot and itchy,” Dr. Paller said. “Or people stay away from them because they think there’s something they can catch, so they’re not chosen for sports activities.”
Indeed, children with psoriasis may be left out of games like tag and contact sports because other children are afraid of touching them, Dr. Cordoro observed. “Other kids do not want to be near them. It is truly heartbreaking and derives largely from ignorance.”
What can dermatologists do? Dr. Cordoro recommends that they take time to ask their youngest patients about their lives: “Is your psoriasis affecting your friendships?” “How are things going at school?” “Do kids ask you about your psoriasis? What do you say?”
“We can identify at-risk kids this way and work with parents, schools, coaches, and counselors towards productive interventions like educational programs,” Dr. Cordoro said. “Education is the key. As kids, parents, and adults become educated, the psoriatic child is less likely to be teased and excluded. Kids with psoriasis may lack the confidence to defend themselves, and arming them with one-liners and basic educational points about their condition empowers them to address it directly.”
Dr. Paller, who is also director of the Northwestern University Skin Disease Research Center, said it’s a good idea to add questions to the usual list of queries about subjects like sleep and itching. In cases when a child is bullied, it may help to reach out to teachers and principals, and to counselors and social workers if needed, she noted.
Parents play an important role, too, Dr. Menter said, although they may be in the dark about bullying. “What I’ve learned is that kids will seldom come home and tell their parent they’ve been bullied.”
He urges both children and their parents to understand the nature of psoriasis and be open about it. “Don’t hide it,” he suggested. “Tell people that ‘I’ve got psoriasis, and it’s not contagious.’ ” And then, hopefully, the healing can begin.
Dr. Paller and Dr. Cordoro reported no relevant disclosures. Dr. Menter disclosed relationships with many pharmaceutical companies, including AbbVie, Allergan, Amgen, Boehringer Ingelheim, Eli Lilly, Merck, Novartis and Pfizer.
Dallas dermatologist Alan Menter, MD, doesn’t boast bullying-prevention superpowers, but what he does have is close enough: An eagerness to get the word out to anyone – parent or principal, psychologist or pediatrician – who can help prevent a child with psoriasis from being bullied.
Over his long career, Dr. Menter has made many calls to adults in positions of influence over children. “I’ve talked to pediatricians, and I’ve even called up schools and talked to principals to try get the bullying situation reduced to an extent where the kids can live happy, normal lives without kids taunting them.”
Dr. Menter, chief of dermatology at Baylor University in Dallas, has plenty of company. Other dermatologists are paying close attention to their youngest patients with psoriasis as researchers work to get a better handle on the bullying problem.
“We really want to identify this early on and do whatever is required to turn it around,” said Amy Paller, MD, professor of dermatology and pediatrics and chair of the department of dermatology at Northwestern University, Chicago. “These visible skin lesions can have a very significant effect on how children feel about themselves and others. When this is going on early in life, during childhood or teen years, there’s really a risk for lifelong issues.”
Dr. Menter’s interest in psoriasis and bullying began during his childhood in South Africa when he watched children bully his brother, who had the condition. “I’ve always had a great desire to improve the quality of life in psoriasis patients,” he said, and that passion grew as he worked in a day care center for children with psoriasis. “I had an opportunity to talk to children and recognize the impact that psoriasis has on them.”
Research from across the world reveals that children with psoriasis face an extraordinary burden from bullying. “They’re teased incessantly and bullied because they’ve got such a visible disease,” he said.
The introspective and depressed nature of many children with psoriasis makes the situation even more difficult, he noted, since their emotional makeup prevents them from responding easily to taunting.
The extent of the bullying problem, however, isn’t fully understood. Research into bullying and skin disorders is “very limited,” said Kelly Cordoro, MD, of the departments of dermatology and pediatrics at the University of California, San Francisco. “What little evidence does exist suggests that kids with visible skin disease, including psoriasis, are often bullied, and this can impact them significantly,” she said, pointing to a 2013 study that suggested those with acne, psoriasis, and atopic dermatitis are especially vulnerable (Clin Dermatol. 2013;31[1]:66-71).
Sports are a special area of concern. “They don’t want to get into gym shorts, and they don’t want to engage in sports because they get hot and itchy,” Dr. Paller said. “Or people stay away from them because they think there’s something they can catch, so they’re not chosen for sports activities.”
Indeed, children with psoriasis may be left out of games like tag and contact sports because other children are afraid of touching them, Dr. Cordoro observed. “Other kids do not want to be near them. It is truly heartbreaking and derives largely from ignorance.”
What can dermatologists do? Dr. Cordoro recommends that they take time to ask their youngest patients about their lives: “Is your psoriasis affecting your friendships?” “How are things going at school?” “Do kids ask you about your psoriasis? What do you say?”
“We can identify at-risk kids this way and work with parents, schools, coaches, and counselors towards productive interventions like educational programs,” Dr. Cordoro said. “Education is the key. As kids, parents, and adults become educated, the psoriatic child is less likely to be teased and excluded. Kids with psoriasis may lack the confidence to defend themselves, and arming them with one-liners and basic educational points about their condition empowers them to address it directly.”
Dr. Paller, who is also director of the Northwestern University Skin Disease Research Center, said it’s a good idea to add questions to the usual list of queries about subjects like sleep and itching. In cases when a child is bullied, it may help to reach out to teachers and principals, and to counselors and social workers if needed, she noted.
Parents play an important role, too, Dr. Menter said, although they may be in the dark about bullying. “What I’ve learned is that kids will seldom come home and tell their parent they’ve been bullied.”
He urges both children and their parents to understand the nature of psoriasis and be open about it. “Don’t hide it,” he suggested. “Tell people that ‘I’ve got psoriasis, and it’s not contagious.’ ” And then, hopefully, the healing can begin.
Dr. Paller and Dr. Cordoro reported no relevant disclosures. Dr. Menter disclosed relationships with many pharmaceutical companies, including AbbVie, Allergan, Amgen, Boehringer Ingelheim, Eli Lilly, Merck, Novartis and Pfizer.
Worse outcomes for double-hit lymphomas after ASCT
Patients with double-hit lymphomas (DHLs) and double-expressor lymphomas (DELs) have inferior outcomes after undergoing autologous stem cell transplantation (ASCT), according to a new study published in the Journal of Clinical Oncology.
The worst outcomes were observed in patients with concurrent DELs and DHLs, and this “supports the concept that the double-hit/double-expressor biology appears to render DLBCL resistant to and less likely to be cured by chemotherapy,” write Alex Herrera, MD, an oncologist at the City of Hope, Duarte, Calif., and his colleagues.
But that said, a significant proportion of patients with relapsed/refractory DEL did experience durable remissions following ASCT, particularly those with isolated DEL without DHL.
This suggests that “the presence of DEL alone should not be considered a contraindication to ASCT,” the authors wrote (J Clin Oncol. 2016 Oct. 24. doi: 10.1200/JCO.2016.68.2740).
DHLs and DELs are subtypes of diffuse large B-cell lymphoma (DLBCL), and while they are associated with poor outcomes after standard chemoimmunotherapy, data remain limited as to outcomes of patients with relapsed or refractory disease who undergo ASCT.
The retrospective multicenter study included 117 patients with chemotherapy-sensitive relapsed/refractory DLBCL who underwent ASCT and had archival tumor material available. DEL with MYC/BCL2 coexpression was observed in 52 patients (44%) while 15 patients expressed MYC-R (13%), of whom 12 (10%) had DHL.
The median follow-up time was 45 months for survivors, and the 4-year progression-free survival (PFS) and overall survival (OS) were 54% for the entire cohort.
The 4-year PFS and OS in patients with DHL was worse as compared to those without DHL; 28% vs. 57% (P = .013), and 25% vs. 66% (P less than .001), respectively.
Those with DHL had poorer PFS (28%) and OS (25%), compared with patients with DEL but not DHL (PFS, 53% and OS, 61%) as well as patients with neither DEL nor DHL (PFS, 60% and OS, 70%; three-way P value for PFS, P = .013; OS, P = .002).
Patients with concurrent DEL and DHL had the poorest outcome, with a 4-year PFS of 0%.
After researchers adjusted for clinical characteristics, the only factors that remained significantly associated with PFS were DEL (hazard ratio, 1.8; P = .035) and DHL (HR, 2.9; P = .009). Factors that were significantly associated with OS were DHL (HR, 3.4; P = .004) and remission status at ASCT (HR for partial response, 2.4; P = .007).
Overall, patients with DHL were less likely to achieve a complete response following salvage therapy, and those with DEL and patients with DHL had a shorter time to relapse after induction therapy.
“Although some patients with relapsed/refractory DHL had long-term remission after ASCT (isolated DHL without DEL), the low survival rate in this group argues that alternative transplantation strategies, including allogeneic hematopoietic stem cell transplantation or peri-ASCT relapse prevention strategies should be studied,” they concluded.
Recognizing that the majority of patients with double-hit or double-expressor lymphoma will relapse after R-CHOP, this study evaluates the efficacy of autologous stem cell transplantation as a salvage modality. This is a carefully conducted, albeit retrospective, analysis of patients with relapsed or refractory double-hit or double-expressor lymphoma undergoing autologous hematopoietic stem cell transplantation (auto-HCT) at two high-volume institutions.
Although it is perhaps not surprising that double-hit and double-expressor phenotypes confer inferior outcomes, it is worth examining these issues in some detail. The first issue is that the authors have defined categories that are not recognized by the World Health Organization, but are routinely seen in clinical practice.
For example, it might be assumed that all patients with double-hit lymphoma will have MYC/BCL2 protein expression and, therefore, also have double-expressor lymphoma, but some patients with double-hit lymphoma do not have protein expression of MYC/BCL2 and these patients may have better outcomes than patients whose tumors display both double-hit and double-expressor characteristics.
A second caveat to interpreting the results is that the study population does not reflect the true denominator of all patients with relapsed diffuse large B-cell lymphoma, because only chemotherapy-sensitive patients undergoing auto-HCT were included.
So, should patients with relapsed double-hit and double-expressor lymphoma be offered auto-HCT? What are the alternatives to auto-HCT? Unfortunately, there are no clear answers to these questions, although the surprisingly excellent outcomes for patients without either of these features (70% long-term survival) suggest that there is a group of patients for whom auto-HCT remains an effective and standard tool. For double-hit and double-expressor lymphoma, a clinical trial based on specific biologic changes in individual patients is the ideal but is far from reality at this point.
Overall, despite being a retrospective series with a high attrition rate based on tissue availability, the central review of pathology, uniform assessment of double-hit and double-expressor features, and mature follow-up of 45 months makes this a thought-provoking and timely paper.
Sonali M. Smith, MD, is from the University of Chicago, and has disclosed a consulting or advisory role with Genentech, Seattle Genetics, TG Therapeutics, Gilead Sciences, Immunogenix, Pharmacyclics, NanoString Technologies, Genmab, Juno Therapeutics, Abbvie, and Portola Pharmaceuticals. These remarks were taken from the editorial accompanying Dr. Herrara’s report (J Clin Oncol. 2016 Oct 17. doi: 10.1200/JCO.2016.70.0625).
Recognizing that the majority of patients with double-hit or double-expressor lymphoma will relapse after R-CHOP, this study evaluates the efficacy of autologous stem cell transplantation as a salvage modality. This is a carefully conducted, albeit retrospective, analysis of patients with relapsed or refractory double-hit or double-expressor lymphoma undergoing autologous hematopoietic stem cell transplantation (auto-HCT) at two high-volume institutions.
Although it is perhaps not surprising that double-hit and double-expressor phenotypes confer inferior outcomes, it is worth examining these issues in some detail. The first issue is that the authors have defined categories that are not recognized by the World Health Organization, but are routinely seen in clinical practice.
For example, it might be assumed that all patients with double-hit lymphoma will have MYC/BCL2 protein expression and, therefore, also have double-expressor lymphoma, but some patients with double-hit lymphoma do not have protein expression of MYC/BCL2 and these patients may have better outcomes than patients whose tumors display both double-hit and double-expressor characteristics.
A second caveat to interpreting the results is that the study population does not reflect the true denominator of all patients with relapsed diffuse large B-cell lymphoma, because only chemotherapy-sensitive patients undergoing auto-HCT were included.
So, should patients with relapsed double-hit and double-expressor lymphoma be offered auto-HCT? What are the alternatives to auto-HCT? Unfortunately, there are no clear answers to these questions, although the surprisingly excellent outcomes for patients without either of these features (70% long-term survival) suggest that there is a group of patients for whom auto-HCT remains an effective and standard tool. For double-hit and double-expressor lymphoma, a clinical trial based on specific biologic changes in individual patients is the ideal but is far from reality at this point.
Overall, despite being a retrospective series with a high attrition rate based on tissue availability, the central review of pathology, uniform assessment of double-hit and double-expressor features, and mature follow-up of 45 months makes this a thought-provoking and timely paper.
Sonali M. Smith, MD, is from the University of Chicago, and has disclosed a consulting or advisory role with Genentech, Seattle Genetics, TG Therapeutics, Gilead Sciences, Immunogenix, Pharmacyclics, NanoString Technologies, Genmab, Juno Therapeutics, Abbvie, and Portola Pharmaceuticals. These remarks were taken from the editorial accompanying Dr. Herrara’s report (J Clin Oncol. 2016 Oct 17. doi: 10.1200/JCO.2016.70.0625).
Recognizing that the majority of patients with double-hit or double-expressor lymphoma will relapse after R-CHOP, this study evaluates the efficacy of autologous stem cell transplantation as a salvage modality. This is a carefully conducted, albeit retrospective, analysis of patients with relapsed or refractory double-hit or double-expressor lymphoma undergoing autologous hematopoietic stem cell transplantation (auto-HCT) at two high-volume institutions.
Although it is perhaps not surprising that double-hit and double-expressor phenotypes confer inferior outcomes, it is worth examining these issues in some detail. The first issue is that the authors have defined categories that are not recognized by the World Health Organization, but are routinely seen in clinical practice.
For example, it might be assumed that all patients with double-hit lymphoma will have MYC/BCL2 protein expression and, therefore, also have double-expressor lymphoma, but some patients with double-hit lymphoma do not have protein expression of MYC/BCL2 and these patients may have better outcomes than patients whose tumors display both double-hit and double-expressor characteristics.
A second caveat to interpreting the results is that the study population does not reflect the true denominator of all patients with relapsed diffuse large B-cell lymphoma, because only chemotherapy-sensitive patients undergoing auto-HCT were included.
So, should patients with relapsed double-hit and double-expressor lymphoma be offered auto-HCT? What are the alternatives to auto-HCT? Unfortunately, there are no clear answers to these questions, although the surprisingly excellent outcomes for patients without either of these features (70% long-term survival) suggest that there is a group of patients for whom auto-HCT remains an effective and standard tool. For double-hit and double-expressor lymphoma, a clinical trial based on specific biologic changes in individual patients is the ideal but is far from reality at this point.
Overall, despite being a retrospective series with a high attrition rate based on tissue availability, the central review of pathology, uniform assessment of double-hit and double-expressor features, and mature follow-up of 45 months makes this a thought-provoking and timely paper.
Sonali M. Smith, MD, is from the University of Chicago, and has disclosed a consulting or advisory role with Genentech, Seattle Genetics, TG Therapeutics, Gilead Sciences, Immunogenix, Pharmacyclics, NanoString Technologies, Genmab, Juno Therapeutics, Abbvie, and Portola Pharmaceuticals. These remarks were taken from the editorial accompanying Dr. Herrara’s report (J Clin Oncol. 2016 Oct 17. doi: 10.1200/JCO.2016.70.0625).
Patients with double-hit lymphomas (DHLs) and double-expressor lymphomas (DELs) have inferior outcomes after undergoing autologous stem cell transplantation (ASCT), according to a new study published in the Journal of Clinical Oncology.
The worst outcomes were observed in patients with concurrent DELs and DHLs, and this “supports the concept that the double-hit/double-expressor biology appears to render DLBCL resistant to and less likely to be cured by chemotherapy,” write Alex Herrera, MD, an oncologist at the City of Hope, Duarte, Calif., and his colleagues.
But that said, a significant proportion of patients with relapsed/refractory DEL did experience durable remissions following ASCT, particularly those with isolated DEL without DHL.
This suggests that “the presence of DEL alone should not be considered a contraindication to ASCT,” the authors wrote (J Clin Oncol. 2016 Oct. 24. doi: 10.1200/JCO.2016.68.2740).
DHLs and DELs are subtypes of diffuse large B-cell lymphoma (DLBCL), and while they are associated with poor outcomes after standard chemoimmunotherapy, data remain limited as to outcomes of patients with relapsed or refractory disease who undergo ASCT.
The retrospective multicenter study included 117 patients with chemotherapy-sensitive relapsed/refractory DLBCL who underwent ASCT and had archival tumor material available. DEL with MYC/BCL2 coexpression was observed in 52 patients (44%) while 15 patients expressed MYC-R (13%), of whom 12 (10%) had DHL.
The median follow-up time was 45 months for survivors, and the 4-year progression-free survival (PFS) and overall survival (OS) were 54% for the entire cohort.
The 4-year PFS and OS in patients with DHL was worse as compared to those without DHL; 28% vs. 57% (P = .013), and 25% vs. 66% (P less than .001), respectively.
Those with DHL had poorer PFS (28%) and OS (25%), compared with patients with DEL but not DHL (PFS, 53% and OS, 61%) as well as patients with neither DEL nor DHL (PFS, 60% and OS, 70%; three-way P value for PFS, P = .013; OS, P = .002).
Patients with concurrent DEL and DHL had the poorest outcome, with a 4-year PFS of 0%.
After researchers adjusted for clinical characteristics, the only factors that remained significantly associated with PFS were DEL (hazard ratio, 1.8; P = .035) and DHL (HR, 2.9; P = .009). Factors that were significantly associated with OS were DHL (HR, 3.4; P = .004) and remission status at ASCT (HR for partial response, 2.4; P = .007).
Overall, patients with DHL were less likely to achieve a complete response following salvage therapy, and those with DEL and patients with DHL had a shorter time to relapse after induction therapy.
“Although some patients with relapsed/refractory DHL had long-term remission after ASCT (isolated DHL without DEL), the low survival rate in this group argues that alternative transplantation strategies, including allogeneic hematopoietic stem cell transplantation or peri-ASCT relapse prevention strategies should be studied,” they concluded.
Patients with double-hit lymphomas (DHLs) and double-expressor lymphomas (DELs) have inferior outcomes after undergoing autologous stem cell transplantation (ASCT), according to a new study published in the Journal of Clinical Oncology.
The worst outcomes were observed in patients with concurrent DELs and DHLs, and this “supports the concept that the double-hit/double-expressor biology appears to render DLBCL resistant to and less likely to be cured by chemotherapy,” write Alex Herrera, MD, an oncologist at the City of Hope, Duarte, Calif., and his colleagues.
But that said, a significant proportion of patients with relapsed/refractory DEL did experience durable remissions following ASCT, particularly those with isolated DEL without DHL.
This suggests that “the presence of DEL alone should not be considered a contraindication to ASCT,” the authors wrote (J Clin Oncol. 2016 Oct. 24. doi: 10.1200/JCO.2016.68.2740).
DHLs and DELs are subtypes of diffuse large B-cell lymphoma (DLBCL), and while they are associated with poor outcomes after standard chemoimmunotherapy, data remain limited as to outcomes of patients with relapsed or refractory disease who undergo ASCT.
The retrospective multicenter study included 117 patients with chemotherapy-sensitive relapsed/refractory DLBCL who underwent ASCT and had archival tumor material available. DEL with MYC/BCL2 coexpression was observed in 52 patients (44%) while 15 patients expressed MYC-R (13%), of whom 12 (10%) had DHL.
The median follow-up time was 45 months for survivors, and the 4-year progression-free survival (PFS) and overall survival (OS) were 54% for the entire cohort.
The 4-year PFS and OS in patients with DHL was worse as compared to those without DHL; 28% vs. 57% (P = .013), and 25% vs. 66% (P less than .001), respectively.
Those with DHL had poorer PFS (28%) and OS (25%), compared with patients with DEL but not DHL (PFS, 53% and OS, 61%) as well as patients with neither DEL nor DHL (PFS, 60% and OS, 70%; three-way P value for PFS, P = .013; OS, P = .002).
Patients with concurrent DEL and DHL had the poorest outcome, with a 4-year PFS of 0%.
After researchers adjusted for clinical characteristics, the only factors that remained significantly associated with PFS were DEL (hazard ratio, 1.8; P = .035) and DHL (HR, 2.9; P = .009). Factors that were significantly associated with OS were DHL (HR, 3.4; P = .004) and remission status at ASCT (HR for partial response, 2.4; P = .007).
Overall, patients with DHL were less likely to achieve a complete response following salvage therapy, and those with DEL and patients with DHL had a shorter time to relapse after induction therapy.
“Although some patients with relapsed/refractory DHL had long-term remission after ASCT (isolated DHL without DEL), the low survival rate in this group argues that alternative transplantation strategies, including allogeneic hematopoietic stem cell transplantation or peri-ASCT relapse prevention strategies should be studied,” they concluded.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:
Major finding: The 4-year progression-free survival in patients with DEL vs. non-DEL was 48% versus 59% (P = .049), and the 4-year OS was 56% vs. 67% (P = .10).
Data source: Retrospective, multicenter study that included 117 patients with relapsed/refractory aggressive B-cell non-Hodgkin lymphoma who underwent ASCT.
Disclosures: The study was funded by a Conquer Cancer Foundation/ASCO Young Investigator Award and National Cancer Institute Grants; the Dana-Farber Cancer Institute Award Fund for Collaborative Research Initiatives in Hematologic Oncology; the Harold and Virginia Lash/David Lash Fund for Lymphoma Research; and NCI Grant No. P30CA033572 for work performed in the COH Pathology Core. Dr. Herrera reports receiving research funding from Seattle Genetics, Pharmacyclics, Genentech, Immune Design, and Sequenta, and received travel, accommodations, and expenses from Bristol-Myers Squibb. Several coauthors also report relationships with industry.
Cystic fibrosis–related diabetes requires unique treatment
SAN DIEGO – according to Katie Larson Ode, MD.
Medical advances have dramatically boosted life expectancy for people with cystic fibrosis, allowing some to survive into middle age. “As more of our patients live longer, [cystic fibrosis–related diabetes (CFRD)] will become a much more common problem for endocrinologists, educators, nurses, and nutritionists to address,” said Dr. Ode, a pediatrician at the University of Iowa in Iowa City. “Proper management of cystic fibrosis-related diabetes is lifesaving for our CF patients.”
“CF is caused by a defect in an important chloride channel that regulates the salt and water content of secretions,” said Antoinette Moran, MD, professor and division chief of pediatric endocrinology and diabetes at the University of Minnesota, Minneapolis, while speaking at the annual meeting of the American Association of Diabetes Educators. “This affects many organs, but death is generally due to chronic obstructive lung disease.”
In the 1950s, few children with CF lived past elementary school age. Now, researchers estimate that the median life span for babies born and diagnosed in 2010 could reach more than 50 years (Ann Intern Med. 2014 Aug 19;161[4]:233-41), according to the Cystic Fibrosis Foundation.
But as they age, CF patients’ risk for CFRD also increases.
A 2009 study led by Dr. Moran (Diabetes Care 2009 Sep;32[9]: 1626-31) tracked 872 CF patients from three periods in the 1990s and 2000s and found CFRD in 2% of children, 19% of adolescents, and 40%-50% of adults. “For a typical CF patient, the chances of developing CFRD by age 40 are roughly 80%,” said Andrew Norris, MD, PhD, of the departments of pediatrics and biochemistry at the University of Iowa.
CF patients aren’t limited to one form of diabetes. “Occasionally a patient may be unlucky enough to have more than one type,” Dr. Moran said, since the diseases have separate pathophysiologies. While rare, type 1 appears to be more common than type 2, which is often linked to obesity; CF patients struggle to put on weight. “It would be highly unusual for a CF patient to develop type 2 diabetes,” Dr. Norris said.
The specialists said patients with CFRD are unique among diabetics for several reasons:
• Microvascular complications are rare. “After many years of diabetes, CFRD patients are at risk for microvascular complications, but they tend to be less common and less severe than in other forms of diabetes, likely because CF patients still make some of their own insulin,” Dr. Moran said.
• Macrovascular complications are not seen. “This is important because recommendations given to people with other forms of the diabetes to reduce risk of macrovascular complications – weight loss, low-fat diet, low-salt diet, etc.– do no apply in CF and can actually be harmful,” Dr. Moran said.
• Insulin is especially crucial. “Maintaining weight and lean body mass is critical for survival in CF, so the chief clinical concern with CFRD is nutrition and the potential impact of insulin insufficiency on mortality,” Dr. Moran said. “Insulin replacement is the only approved therapy for CFRD. Treatment is very similar to that of type 1 diabetic patients in a honeymoon state. Very early on, patients may receive a single dose of basal insulin only or of premeal rapid-acting insulin only. Eventually most CF patients require basal-bolus insulin therapy.”
Doses tend to be lower than in other diabetics, she said, “except for when CF patients are acutely ill. During acute illness, CF patients become insulin resistant and require high insulin doses.”
Still, “once I start insulin, my goal is to deliver as large a dose as the patient can safely tolerate, to maximize anabolic impact,” she said.
The physicians offered these tips about tracking and treating CF patients:
• Screen CF patients via oral glucose tolerance testing on an annual basis, starting no later than age 10. Don’t trust hemoglobin A1c levels or fasting glucose levels, Dr. Norris said, because they are not sensitive enough. In fact, Dr. Moran said, “HbA1cis spuriously low in CF. “Even mild diabetes can be immediately detrimental to a CF patient’s lung health and mortality risk,” Dr. Norris said. “If you wait until the onset of classic diabetes symptoms or until the fasting glucose or hemoglobin A1c is elevated, you will have done a great disservice to the CF patient, as preventable potentially life-threatening lung damage may have already occurred.”
• Check into your center’s testing protocol. “Unfortunately, current screening rates are markedly insufficient at many centers,” Dr. Norris said. “I encourage endocrine teams to reach out to their affiliated CF treatment center to discuss and help implement screening.”
What’s next? The University of Iowa’s Dr. Larson said he is hopeful about advances in medical care. “Currently, patients must follow an extremely complex regimen of airway clearance, inhaled antibiotics, and oral medications, typically with recurrent hospitalization with increasing age and eventual death from lung disease or lung transplant,” she said. “However, in the past few years, we have had a dramatic change for a percentage of our patients with the development of small molecules that can actually fix the chloride-channel defect itself.”
The treatment seems to arrest and even improve lung disease in eligible patients, she said. “So the future seems very bright for CF.”
The Cystic Fibrosis Foundation has developed a 3-year Envision program to train adult and pediatric endocrinologists in the care of CFRD and endocrine issues.
Dr. Moran had no relevant disclosures. Dr. Norris has consulted for Vertex Pharmaceuticals, which makes cystic fibrosis therapeutics, in the past year. Dr. Larson Ode reports that her research is funded by the National Institutes of Health and the Cystic Fibrosis Foundation.
SAN DIEGO – according to Katie Larson Ode, MD.
Medical advances have dramatically boosted life expectancy for people with cystic fibrosis, allowing some to survive into middle age. “As more of our patients live longer, [cystic fibrosis–related diabetes (CFRD)] will become a much more common problem for endocrinologists, educators, nurses, and nutritionists to address,” said Dr. Ode, a pediatrician at the University of Iowa in Iowa City. “Proper management of cystic fibrosis-related diabetes is lifesaving for our CF patients.”
“CF is caused by a defect in an important chloride channel that regulates the salt and water content of secretions,” said Antoinette Moran, MD, professor and division chief of pediatric endocrinology and diabetes at the University of Minnesota, Minneapolis, while speaking at the annual meeting of the American Association of Diabetes Educators. “This affects many organs, but death is generally due to chronic obstructive lung disease.”
In the 1950s, few children with CF lived past elementary school age. Now, researchers estimate that the median life span for babies born and diagnosed in 2010 could reach more than 50 years (Ann Intern Med. 2014 Aug 19;161[4]:233-41), according to the Cystic Fibrosis Foundation.
But as they age, CF patients’ risk for CFRD also increases.
A 2009 study led by Dr. Moran (Diabetes Care 2009 Sep;32[9]: 1626-31) tracked 872 CF patients from three periods in the 1990s and 2000s and found CFRD in 2% of children, 19% of adolescents, and 40%-50% of adults. “For a typical CF patient, the chances of developing CFRD by age 40 are roughly 80%,” said Andrew Norris, MD, PhD, of the departments of pediatrics and biochemistry at the University of Iowa.
CF patients aren’t limited to one form of diabetes. “Occasionally a patient may be unlucky enough to have more than one type,” Dr. Moran said, since the diseases have separate pathophysiologies. While rare, type 1 appears to be more common than type 2, which is often linked to obesity; CF patients struggle to put on weight. “It would be highly unusual for a CF patient to develop type 2 diabetes,” Dr. Norris said.
The specialists said patients with CFRD are unique among diabetics for several reasons:
• Microvascular complications are rare. “After many years of diabetes, CFRD patients are at risk for microvascular complications, but they tend to be less common and less severe than in other forms of diabetes, likely because CF patients still make some of their own insulin,” Dr. Moran said.
• Macrovascular complications are not seen. “This is important because recommendations given to people with other forms of the diabetes to reduce risk of macrovascular complications – weight loss, low-fat diet, low-salt diet, etc.– do no apply in CF and can actually be harmful,” Dr. Moran said.
• Insulin is especially crucial. “Maintaining weight and lean body mass is critical for survival in CF, so the chief clinical concern with CFRD is nutrition and the potential impact of insulin insufficiency on mortality,” Dr. Moran said. “Insulin replacement is the only approved therapy for CFRD. Treatment is very similar to that of type 1 diabetic patients in a honeymoon state. Very early on, patients may receive a single dose of basal insulin only or of premeal rapid-acting insulin only. Eventually most CF patients require basal-bolus insulin therapy.”
Doses tend to be lower than in other diabetics, she said, “except for when CF patients are acutely ill. During acute illness, CF patients become insulin resistant and require high insulin doses.”
Still, “once I start insulin, my goal is to deliver as large a dose as the patient can safely tolerate, to maximize anabolic impact,” she said.
The physicians offered these tips about tracking and treating CF patients:
• Screen CF patients via oral glucose tolerance testing on an annual basis, starting no later than age 10. Don’t trust hemoglobin A1c levels or fasting glucose levels, Dr. Norris said, because they are not sensitive enough. In fact, Dr. Moran said, “HbA1cis spuriously low in CF. “Even mild diabetes can be immediately detrimental to a CF patient’s lung health and mortality risk,” Dr. Norris said. “If you wait until the onset of classic diabetes symptoms or until the fasting glucose or hemoglobin A1c is elevated, you will have done a great disservice to the CF patient, as preventable potentially life-threatening lung damage may have already occurred.”
• Check into your center’s testing protocol. “Unfortunately, current screening rates are markedly insufficient at many centers,” Dr. Norris said. “I encourage endocrine teams to reach out to their affiliated CF treatment center to discuss and help implement screening.”
What’s next? The University of Iowa’s Dr. Larson said he is hopeful about advances in medical care. “Currently, patients must follow an extremely complex regimen of airway clearance, inhaled antibiotics, and oral medications, typically with recurrent hospitalization with increasing age and eventual death from lung disease or lung transplant,” she said. “However, in the past few years, we have had a dramatic change for a percentage of our patients with the development of small molecules that can actually fix the chloride-channel defect itself.”
The treatment seems to arrest and even improve lung disease in eligible patients, she said. “So the future seems very bright for CF.”
The Cystic Fibrosis Foundation has developed a 3-year Envision program to train adult and pediatric endocrinologists in the care of CFRD and endocrine issues.
Dr. Moran had no relevant disclosures. Dr. Norris has consulted for Vertex Pharmaceuticals, which makes cystic fibrosis therapeutics, in the past year. Dr. Larson Ode reports that her research is funded by the National Institutes of Health and the Cystic Fibrosis Foundation.
SAN DIEGO – according to Katie Larson Ode, MD.
Medical advances have dramatically boosted life expectancy for people with cystic fibrosis, allowing some to survive into middle age. “As more of our patients live longer, [cystic fibrosis–related diabetes (CFRD)] will become a much more common problem for endocrinologists, educators, nurses, and nutritionists to address,” said Dr. Ode, a pediatrician at the University of Iowa in Iowa City. “Proper management of cystic fibrosis-related diabetes is lifesaving for our CF patients.”
“CF is caused by a defect in an important chloride channel that regulates the salt and water content of secretions,” said Antoinette Moran, MD, professor and division chief of pediatric endocrinology and diabetes at the University of Minnesota, Minneapolis, while speaking at the annual meeting of the American Association of Diabetes Educators. “This affects many organs, but death is generally due to chronic obstructive lung disease.”
In the 1950s, few children with CF lived past elementary school age. Now, researchers estimate that the median life span for babies born and diagnosed in 2010 could reach more than 50 years (Ann Intern Med. 2014 Aug 19;161[4]:233-41), according to the Cystic Fibrosis Foundation.
But as they age, CF patients’ risk for CFRD also increases.
A 2009 study led by Dr. Moran (Diabetes Care 2009 Sep;32[9]: 1626-31) tracked 872 CF patients from three periods in the 1990s and 2000s and found CFRD in 2% of children, 19% of adolescents, and 40%-50% of adults. “For a typical CF patient, the chances of developing CFRD by age 40 are roughly 80%,” said Andrew Norris, MD, PhD, of the departments of pediatrics and biochemistry at the University of Iowa.
CF patients aren’t limited to one form of diabetes. “Occasionally a patient may be unlucky enough to have more than one type,” Dr. Moran said, since the diseases have separate pathophysiologies. While rare, type 1 appears to be more common than type 2, which is often linked to obesity; CF patients struggle to put on weight. “It would be highly unusual for a CF patient to develop type 2 diabetes,” Dr. Norris said.
The specialists said patients with CFRD are unique among diabetics for several reasons:
• Microvascular complications are rare. “After many years of diabetes, CFRD patients are at risk for microvascular complications, but they tend to be less common and less severe than in other forms of diabetes, likely because CF patients still make some of their own insulin,” Dr. Moran said.
• Macrovascular complications are not seen. “This is important because recommendations given to people with other forms of the diabetes to reduce risk of macrovascular complications – weight loss, low-fat diet, low-salt diet, etc.– do no apply in CF and can actually be harmful,” Dr. Moran said.
• Insulin is especially crucial. “Maintaining weight and lean body mass is critical for survival in CF, so the chief clinical concern with CFRD is nutrition and the potential impact of insulin insufficiency on mortality,” Dr. Moran said. “Insulin replacement is the only approved therapy for CFRD. Treatment is very similar to that of type 1 diabetic patients in a honeymoon state. Very early on, patients may receive a single dose of basal insulin only or of premeal rapid-acting insulin only. Eventually most CF patients require basal-bolus insulin therapy.”
Doses tend to be lower than in other diabetics, she said, “except for when CF patients are acutely ill. During acute illness, CF patients become insulin resistant and require high insulin doses.”
Still, “once I start insulin, my goal is to deliver as large a dose as the patient can safely tolerate, to maximize anabolic impact,” she said.
The physicians offered these tips about tracking and treating CF patients:
• Screen CF patients via oral glucose tolerance testing on an annual basis, starting no later than age 10. Don’t trust hemoglobin A1c levels or fasting glucose levels, Dr. Norris said, because they are not sensitive enough. In fact, Dr. Moran said, “HbA1cis spuriously low in CF. “Even mild diabetes can be immediately detrimental to a CF patient’s lung health and mortality risk,” Dr. Norris said. “If you wait until the onset of classic diabetes symptoms or until the fasting glucose or hemoglobin A1c is elevated, you will have done a great disservice to the CF patient, as preventable potentially life-threatening lung damage may have already occurred.”
• Check into your center’s testing protocol. “Unfortunately, current screening rates are markedly insufficient at many centers,” Dr. Norris said. “I encourage endocrine teams to reach out to their affiliated CF treatment center to discuss and help implement screening.”
What’s next? The University of Iowa’s Dr. Larson said he is hopeful about advances in medical care. “Currently, patients must follow an extremely complex regimen of airway clearance, inhaled antibiotics, and oral medications, typically with recurrent hospitalization with increasing age and eventual death from lung disease or lung transplant,” she said. “However, in the past few years, we have had a dramatic change for a percentage of our patients with the development of small molecules that can actually fix the chloride-channel defect itself.”
The treatment seems to arrest and even improve lung disease in eligible patients, she said. “So the future seems very bright for CF.”
The Cystic Fibrosis Foundation has developed a 3-year Envision program to train adult and pediatric endocrinologists in the care of CFRD and endocrine issues.
Dr. Moran had no relevant disclosures. Dr. Norris has consulted for Vertex Pharmaceuticals, which makes cystic fibrosis therapeutics, in the past year. Dr. Larson Ode reports that her research is funded by the National Institutes of Health and the Cystic Fibrosis Foundation.
EXPERT ANALYSIS FROM AADE 16
Chief resident service increased trainees’ confidence and independence
CORONADO, CALIF. – Creation of a surgical chief resident service meant to increase resident autonomy and provide continuity of patient care with appropriate faculty supervision has been successful, results from a small single-center study showed.
“Providing opportunities for autonomy to bolster the development of independence and confidence during surgery residency remains among the most pronounced challenges of the current training paradigm,” Benjamin T. Jarman, MD, said at the annual meeting of the Western Surgical Association. “Prior to 2011, our graduating surgery residents reported a lack of perceived autonomy during their training and a need to improve practice management skills. To be clear, they consistently felt confident in their surgical abilities, but they did not sense that they were routinely engaged in directing all phases of care.”
In an effort to provide chief surgery residents with increased autonomy and full-spectrum continuity of patient care, Dr. Jarman and his associates initiated a chief resident service (CRS) in January of 2011. It was designed as an independent service with call responsibilities, office hours, operative scheduling, procedural coding, and endoscopy time. “We constructed a weekly schedule to be consistent with the practice of a general surgeon in the first year after residency,” Dr. Jarman explained. “We also added administrative time for research, patient coordination, and completion of records. Each class of chief residents was educated about these responsibilities as a group, and individual sit-down sessions occurred before they started the rotation. Expectations were made clear, and the importance of clear communication was stressed. The service was geared to provide excellent exposure to practice management skills.” Members of teaching faculty were assigned to each episode of patient care to meet all supervision guidelines and patients were educated accordingly. “The primary difference of and key to this service is that of patient continuity with the chief resident from preoperative assessment to postoperative care,” he said. “So our faculty had to adapt to the transient role that our residents are accustomed to.”
Dr. Jarman presented results from a study of nine surgeons who completed the CRS between January 2011 and June 2014. Total operative volume during residency was assessed in addition to select procedures for the chief service experience versus the residents’ first year of clinical practice. Residents who pursued fellowship training submitted their operative logs from their first year postfellowship. Graduates were surveyed to assess their current clinical practice, satisfaction with the chief service, and whether they perceived a correlation of the CRS with their clinical practice. Patient evaluations were reviewed as well. The researchers focused on the following procedures for comparison: laparoscopic appendectomy, laparoscopic cholecystectomy, colectomy, ventral/incisional hernia repair, inguinal hernia repair, upper endoscopy, and lower endoscopy.
All nine chief surgery residents completed the chief service and completed case logs. “The first three residents to graduate after implementation of the service spent 2 months each on the rotation, while subsequent graduates spent between 4 and 6 months, depending on how many chiefs we had in a given year,” Dr. Jarman said. The median total case volume was 1,101 during the 5-year residency, 92 during the CRS, and 299 during the first year of practice. When the researchers evaluated overall median case volumes, lower endoscopy volumes were higher during the first year of practice, compared with during the CRS (a median of 71 vs. 10 cases, respectively); otherwise there were similar case volumes across the other procedures selected for evaluation. Next, they determined the mean case volumes by month for the selected general surgical procedures and found similar case volumes with the exception of colectomy, which was more commonly performed during the CRS, compared with during the first year of practice (a mean of 1 vs. 0.4 cases; P=0.016).
All nine graduates completed an electronic survey relaying details about their current practice and degree of satisfaction with the CRS; 100% reported being “very satisfied” with their CRS, and 100% found it “very beneficial” to their practice. In addition, 56% said that their cases on the CRS were “somewhat similar” to their current practice, while 44% said that their cases were “very similar” to their current practice.
Since the inception of the CRS, Dr. Jarman and his associates have made several adjustments to the CRS, including incorporation of endoscopy time, adjusted office hours, the required presence of surgery assistants in the OR, and requiring fourth-year residents to attend the ACS Leadership Conference in preparation for the CRS role. He acknowledged certain limitations of the study, including its small sample size and the fact that its participants had variable clinical experience. “But we’re on the ground running,” Dr. Jarman said of the CRS. “The chief residents are wide-eyed and very engaged in this process, and the impact on their development and respect for all the caveats of independent practice has been significant. The strengths of the service include exposure to practice management skills, whole-spectrum clinical care for a single resident at a time, and operative experience which correlates to that experience of a first-year surgeon.” He reported having no financial disclosures.
CORONADO, CALIF. – Creation of a surgical chief resident service meant to increase resident autonomy and provide continuity of patient care with appropriate faculty supervision has been successful, results from a small single-center study showed.
“Providing opportunities for autonomy to bolster the development of independence and confidence during surgery residency remains among the most pronounced challenges of the current training paradigm,” Benjamin T. Jarman, MD, said at the annual meeting of the Western Surgical Association. “Prior to 2011, our graduating surgery residents reported a lack of perceived autonomy during their training and a need to improve practice management skills. To be clear, they consistently felt confident in their surgical abilities, but they did not sense that they were routinely engaged in directing all phases of care.”
In an effort to provide chief surgery residents with increased autonomy and full-spectrum continuity of patient care, Dr. Jarman and his associates initiated a chief resident service (CRS) in January of 2011. It was designed as an independent service with call responsibilities, office hours, operative scheduling, procedural coding, and endoscopy time. “We constructed a weekly schedule to be consistent with the practice of a general surgeon in the first year after residency,” Dr. Jarman explained. “We also added administrative time for research, patient coordination, and completion of records. Each class of chief residents was educated about these responsibilities as a group, and individual sit-down sessions occurred before they started the rotation. Expectations were made clear, and the importance of clear communication was stressed. The service was geared to provide excellent exposure to practice management skills.” Members of teaching faculty were assigned to each episode of patient care to meet all supervision guidelines and patients were educated accordingly. “The primary difference of and key to this service is that of patient continuity with the chief resident from preoperative assessment to postoperative care,” he said. “So our faculty had to adapt to the transient role that our residents are accustomed to.”
Dr. Jarman presented results from a study of nine surgeons who completed the CRS between January 2011 and June 2014. Total operative volume during residency was assessed in addition to select procedures for the chief service experience versus the residents’ first year of clinical practice. Residents who pursued fellowship training submitted their operative logs from their first year postfellowship. Graduates were surveyed to assess their current clinical practice, satisfaction with the chief service, and whether they perceived a correlation of the CRS with their clinical practice. Patient evaluations were reviewed as well. The researchers focused on the following procedures for comparison: laparoscopic appendectomy, laparoscopic cholecystectomy, colectomy, ventral/incisional hernia repair, inguinal hernia repair, upper endoscopy, and lower endoscopy.
All nine chief surgery residents completed the chief service and completed case logs. “The first three residents to graduate after implementation of the service spent 2 months each on the rotation, while subsequent graduates spent between 4 and 6 months, depending on how many chiefs we had in a given year,” Dr. Jarman said. The median total case volume was 1,101 during the 5-year residency, 92 during the CRS, and 299 during the first year of practice. When the researchers evaluated overall median case volumes, lower endoscopy volumes were higher during the first year of practice, compared with during the CRS (a median of 71 vs. 10 cases, respectively); otherwise there were similar case volumes across the other procedures selected for evaluation. Next, they determined the mean case volumes by month for the selected general surgical procedures and found similar case volumes with the exception of colectomy, which was more commonly performed during the CRS, compared with during the first year of practice (a mean of 1 vs. 0.4 cases; P=0.016).
All nine graduates completed an electronic survey relaying details about their current practice and degree of satisfaction with the CRS; 100% reported being “very satisfied” with their CRS, and 100% found it “very beneficial” to their practice. In addition, 56% said that their cases on the CRS were “somewhat similar” to their current practice, while 44% said that their cases were “very similar” to their current practice.
Since the inception of the CRS, Dr. Jarman and his associates have made several adjustments to the CRS, including incorporation of endoscopy time, adjusted office hours, the required presence of surgery assistants in the OR, and requiring fourth-year residents to attend the ACS Leadership Conference in preparation for the CRS role. He acknowledged certain limitations of the study, including its small sample size and the fact that its participants had variable clinical experience. “But we’re on the ground running,” Dr. Jarman said of the CRS. “The chief residents are wide-eyed and very engaged in this process, and the impact on their development and respect for all the caveats of independent practice has been significant. The strengths of the service include exposure to practice management skills, whole-spectrum clinical care for a single resident at a time, and operative experience which correlates to that experience of a first-year surgeon.” He reported having no financial disclosures.
CORONADO, CALIF. – Creation of a surgical chief resident service meant to increase resident autonomy and provide continuity of patient care with appropriate faculty supervision has been successful, results from a small single-center study showed.
“Providing opportunities for autonomy to bolster the development of independence and confidence during surgery residency remains among the most pronounced challenges of the current training paradigm,” Benjamin T. Jarman, MD, said at the annual meeting of the Western Surgical Association. “Prior to 2011, our graduating surgery residents reported a lack of perceived autonomy during their training and a need to improve practice management skills. To be clear, they consistently felt confident in their surgical abilities, but they did not sense that they were routinely engaged in directing all phases of care.”
In an effort to provide chief surgery residents with increased autonomy and full-spectrum continuity of patient care, Dr. Jarman and his associates initiated a chief resident service (CRS) in January of 2011. It was designed as an independent service with call responsibilities, office hours, operative scheduling, procedural coding, and endoscopy time. “We constructed a weekly schedule to be consistent with the practice of a general surgeon in the first year after residency,” Dr. Jarman explained. “We also added administrative time for research, patient coordination, and completion of records. Each class of chief residents was educated about these responsibilities as a group, and individual sit-down sessions occurred before they started the rotation. Expectations were made clear, and the importance of clear communication was stressed. The service was geared to provide excellent exposure to practice management skills.” Members of teaching faculty were assigned to each episode of patient care to meet all supervision guidelines and patients were educated accordingly. “The primary difference of and key to this service is that of patient continuity with the chief resident from preoperative assessment to postoperative care,” he said. “So our faculty had to adapt to the transient role that our residents are accustomed to.”
Dr. Jarman presented results from a study of nine surgeons who completed the CRS between January 2011 and June 2014. Total operative volume during residency was assessed in addition to select procedures for the chief service experience versus the residents’ first year of clinical practice. Residents who pursued fellowship training submitted their operative logs from their first year postfellowship. Graduates were surveyed to assess their current clinical practice, satisfaction with the chief service, and whether they perceived a correlation of the CRS with their clinical practice. Patient evaluations were reviewed as well. The researchers focused on the following procedures for comparison: laparoscopic appendectomy, laparoscopic cholecystectomy, colectomy, ventral/incisional hernia repair, inguinal hernia repair, upper endoscopy, and lower endoscopy.
All nine chief surgery residents completed the chief service and completed case logs. “The first three residents to graduate after implementation of the service spent 2 months each on the rotation, while subsequent graduates spent between 4 and 6 months, depending on how many chiefs we had in a given year,” Dr. Jarman said. The median total case volume was 1,101 during the 5-year residency, 92 during the CRS, and 299 during the first year of practice. When the researchers evaluated overall median case volumes, lower endoscopy volumes were higher during the first year of practice, compared with during the CRS (a median of 71 vs. 10 cases, respectively); otherwise there were similar case volumes across the other procedures selected for evaluation. Next, they determined the mean case volumes by month for the selected general surgical procedures and found similar case volumes with the exception of colectomy, which was more commonly performed during the CRS, compared with during the first year of practice (a mean of 1 vs. 0.4 cases; P=0.016).
All nine graduates completed an electronic survey relaying details about their current practice and degree of satisfaction with the CRS; 100% reported being “very satisfied” with their CRS, and 100% found it “very beneficial” to their practice. In addition, 56% said that their cases on the CRS were “somewhat similar” to their current practice, while 44% said that their cases were “very similar” to their current practice.
Since the inception of the CRS, Dr. Jarman and his associates have made several adjustments to the CRS, including incorporation of endoscopy time, adjusted office hours, the required presence of surgery assistants in the OR, and requiring fourth-year residents to attend the ACS Leadership Conference in preparation for the CRS role. He acknowledged certain limitations of the study, including its small sample size and the fact that its participants had variable clinical experience. “But we’re on the ground running,” Dr. Jarman said of the CRS. “The chief residents are wide-eyed and very engaged in this process, and the impact on their development and respect for all the caveats of independent practice has been significant. The strengths of the service include exposure to practice management skills, whole-spectrum clinical care for a single resident at a time, and operative experience which correlates to that experience of a first-year surgeon.” He reported having no financial disclosures.
AT WSA 2016
Key clinical point:
Major finding: More than half of general surgery residency graduates (56%) said that their cases on the chief resident service were “somewhat similar” to their current practice, while 44% said that their cases were “very similar” to their current practice.
Data source: An study of nine surgeons who completed the chief resident service between January 2011 and June 2014.
Disclosures: Dr. Jarman reported having no financial disclosures.
Idelalisib held unlikely to become frontline therapy for CLL
NEW YORK – Safety issues with idelalisib, a first-in-class PI3K delta inhibitor, are an important concern in patients with chronic lymphocytic leukemia – particularly those aged 65 years and younger, according to Steven Coutre, MD.
Findings from the second interim analysis of the pivotal trial for idelalisib as reported at the 2014 annual meeting of the American Society of Hematology (Sharman J., et al. Abstract 330) and a recent update from an open-label extension represent the longest reported follow-up of idelalisib-treated patients to date. Those analyses showed substantial progression-free and overall survival advantages with idelalisib plus rituximab vs. placebo plus rituximab (progression-free survival in the latest update was 19.4 vs. 7.3 months, respectively; hazard ratio 0.25), said Dr. Coutre, professor of medicine at Stanford (Calif.) University.
“Still, even with that report, the follow-up on that initial study was relatively short, and I think it really underreports some of the safety issues of the toxicities that we see,” he said at an international congress on hematologic malignancies.
To further investigate those safety issues, Dr. Coutre and his colleagues performed an integrated analysis of eight clinical trials, including the pivotal trial. That analysis found idelalisib to be efficacious, but significant toxicities were noted, “particularly in younger less heavily-pretreated patients. [Idelalisib] should not be used as frontline therapy ... and I don’t think it will be developed in the future as frontline therapy,” Dr. Coutre said.
“At least in the relapse setting, idelalisib plus rituximab is a choice for all of our patients, but it has to be a considered choice; you have to put it in the context of other therapies that the patients have available to them and make a decision based on your individual patient.”
In the analysis, Dr. Coutre and his colleagues found numerous adverse events occurring in 15% or more of idelalisib-treated patients. These included diarrhea/colitis – with median onset at 7.1 months – in 37% who received monotherapy and 40% who received combination therapy (grade 3 or greater diarrhea/colitis in 11% and 17%, respectively), and pneumonia in 13% and 18% of patients (grade 3 or greater in 11% and 14%, respectively), Dr. Coutre said.
Transaminitis – which was observed early in the studies, usually within the first 8 weeks of treatment – also was common, occurring in 50% and 47% of those with monotherapy and combination therapy, respectively (grade 3 or greater, 16% and 13%, respectively).
Pneumonitis occurred in about 3% of patients, and usually within the first 6 months of therapy.
“So that’s sort of where things were until earlier this year when we learned from several ongoing studies, very important studies in both previously treated patients with low-grade lymphomas and previously untreated patients, that there was a further problem – and that is a difference in mortality,” he said.
Patients in many of these studies, including all of those where it was being used for previously untreated patients, were taken off the drug because of this finding.
“As a result of further analysis of these studies, there’s a new safety label indication,” he said.
Of note, in another idelalisib study reported at ASH 2015 an age-related toxicity concern emerged.
“Idelalisib was used for previously untreated patients. But the distinction here is that for the first time it was being used in younger patients – patients under the age of 65,” he said, noting that severe transaminitis occurred in a significant number of the younger patients.
More than half (52%) of patients had grade 3 or greater hepatotoxicity and all 7 subjects aged 65 years and younger required systemic steroids for toxicities.
Age was found in the study to be a significant risk factor for early hepatotoxicity.
“We’re finally starting to understand the mechanisms behind this, or at least the likely mechanisms, and this seems to be immune mediated,” he said, adding, “you see a lymphocytic infiltrate on liver biopsies, you see a lymphocytic colitis in patients who receive idelalisib.”
Typically, toxicities in these patients can be managed with corticosteroids, but in some cases of severe toxicity even steroids seem to be insufficient, he said.
Further, rapid recurrence is often seen upon re-exposure to the drug, especially with respect to hepatotoxicity, he added.
Preclinical data from mouse models supports this mechanism.
“And importantly [those models] demonstrated a decrease in regulatory T cells in patients treated with idelalisib,” he said.
Dr. Coutre reported consulting for Abbvie, Celgene, Gilead, Janssen, and Pharmacyclics.
NEW YORK – Safety issues with idelalisib, a first-in-class PI3K delta inhibitor, are an important concern in patients with chronic lymphocytic leukemia – particularly those aged 65 years and younger, according to Steven Coutre, MD.
Findings from the second interim analysis of the pivotal trial for idelalisib as reported at the 2014 annual meeting of the American Society of Hematology (Sharman J., et al. Abstract 330) and a recent update from an open-label extension represent the longest reported follow-up of idelalisib-treated patients to date. Those analyses showed substantial progression-free and overall survival advantages with idelalisib plus rituximab vs. placebo plus rituximab (progression-free survival in the latest update was 19.4 vs. 7.3 months, respectively; hazard ratio 0.25), said Dr. Coutre, professor of medicine at Stanford (Calif.) University.
“Still, even with that report, the follow-up on that initial study was relatively short, and I think it really underreports some of the safety issues of the toxicities that we see,” he said at an international congress on hematologic malignancies.
To further investigate those safety issues, Dr. Coutre and his colleagues performed an integrated analysis of eight clinical trials, including the pivotal trial. That analysis found idelalisib to be efficacious, but significant toxicities were noted, “particularly in younger less heavily-pretreated patients. [Idelalisib] should not be used as frontline therapy ... and I don’t think it will be developed in the future as frontline therapy,” Dr. Coutre said.
“At least in the relapse setting, idelalisib plus rituximab is a choice for all of our patients, but it has to be a considered choice; you have to put it in the context of other therapies that the patients have available to them and make a decision based on your individual patient.”
In the analysis, Dr. Coutre and his colleagues found numerous adverse events occurring in 15% or more of idelalisib-treated patients. These included diarrhea/colitis – with median onset at 7.1 months – in 37% who received monotherapy and 40% who received combination therapy (grade 3 or greater diarrhea/colitis in 11% and 17%, respectively), and pneumonia in 13% and 18% of patients (grade 3 or greater in 11% and 14%, respectively), Dr. Coutre said.
Transaminitis – which was observed early in the studies, usually within the first 8 weeks of treatment – also was common, occurring in 50% and 47% of those with monotherapy and combination therapy, respectively (grade 3 or greater, 16% and 13%, respectively).
Pneumonitis occurred in about 3% of patients, and usually within the first 6 months of therapy.
“So that’s sort of where things were until earlier this year when we learned from several ongoing studies, very important studies in both previously treated patients with low-grade lymphomas and previously untreated patients, that there was a further problem – and that is a difference in mortality,” he said.
Patients in many of these studies, including all of those where it was being used for previously untreated patients, were taken off the drug because of this finding.
“As a result of further analysis of these studies, there’s a new safety label indication,” he said.
Of note, in another idelalisib study reported at ASH 2015 an age-related toxicity concern emerged.
“Idelalisib was used for previously untreated patients. But the distinction here is that for the first time it was being used in younger patients – patients under the age of 65,” he said, noting that severe transaminitis occurred in a significant number of the younger patients.
More than half (52%) of patients had grade 3 or greater hepatotoxicity and all 7 subjects aged 65 years and younger required systemic steroids for toxicities.
Age was found in the study to be a significant risk factor for early hepatotoxicity.
“We’re finally starting to understand the mechanisms behind this, or at least the likely mechanisms, and this seems to be immune mediated,” he said, adding, “you see a lymphocytic infiltrate on liver biopsies, you see a lymphocytic colitis in patients who receive idelalisib.”
Typically, toxicities in these patients can be managed with corticosteroids, but in some cases of severe toxicity even steroids seem to be insufficient, he said.
Further, rapid recurrence is often seen upon re-exposure to the drug, especially with respect to hepatotoxicity, he added.
Preclinical data from mouse models supports this mechanism.
“And importantly [those models] demonstrated a decrease in regulatory T cells in patients treated with idelalisib,” he said.
Dr. Coutre reported consulting for Abbvie, Celgene, Gilead, Janssen, and Pharmacyclics.
NEW YORK – Safety issues with idelalisib, a first-in-class PI3K delta inhibitor, are an important concern in patients with chronic lymphocytic leukemia – particularly those aged 65 years and younger, according to Steven Coutre, MD.
Findings from the second interim analysis of the pivotal trial for idelalisib as reported at the 2014 annual meeting of the American Society of Hematology (Sharman J., et al. Abstract 330) and a recent update from an open-label extension represent the longest reported follow-up of idelalisib-treated patients to date. Those analyses showed substantial progression-free and overall survival advantages with idelalisib plus rituximab vs. placebo plus rituximab (progression-free survival in the latest update was 19.4 vs. 7.3 months, respectively; hazard ratio 0.25), said Dr. Coutre, professor of medicine at Stanford (Calif.) University.
“Still, even with that report, the follow-up on that initial study was relatively short, and I think it really underreports some of the safety issues of the toxicities that we see,” he said at an international congress on hematologic malignancies.
To further investigate those safety issues, Dr. Coutre and his colleagues performed an integrated analysis of eight clinical trials, including the pivotal trial. That analysis found idelalisib to be efficacious, but significant toxicities were noted, “particularly in younger less heavily-pretreated patients. [Idelalisib] should not be used as frontline therapy ... and I don’t think it will be developed in the future as frontline therapy,” Dr. Coutre said.
“At least in the relapse setting, idelalisib plus rituximab is a choice for all of our patients, but it has to be a considered choice; you have to put it in the context of other therapies that the patients have available to them and make a decision based on your individual patient.”
In the analysis, Dr. Coutre and his colleagues found numerous adverse events occurring in 15% or more of idelalisib-treated patients. These included diarrhea/colitis – with median onset at 7.1 months – in 37% who received monotherapy and 40% who received combination therapy (grade 3 or greater diarrhea/colitis in 11% and 17%, respectively), and pneumonia in 13% and 18% of patients (grade 3 or greater in 11% and 14%, respectively), Dr. Coutre said.
Transaminitis – which was observed early in the studies, usually within the first 8 weeks of treatment – also was common, occurring in 50% and 47% of those with monotherapy and combination therapy, respectively (grade 3 or greater, 16% and 13%, respectively).
Pneumonitis occurred in about 3% of patients, and usually within the first 6 months of therapy.
“So that’s sort of where things were until earlier this year when we learned from several ongoing studies, very important studies in both previously treated patients with low-grade lymphomas and previously untreated patients, that there was a further problem – and that is a difference in mortality,” he said.
Patients in many of these studies, including all of those where it was being used for previously untreated patients, were taken off the drug because of this finding.
“As a result of further analysis of these studies, there’s a new safety label indication,” he said.
Of note, in another idelalisib study reported at ASH 2015 an age-related toxicity concern emerged.
“Idelalisib was used for previously untreated patients. But the distinction here is that for the first time it was being used in younger patients – patients under the age of 65,” he said, noting that severe transaminitis occurred in a significant number of the younger patients.
More than half (52%) of patients had grade 3 or greater hepatotoxicity and all 7 subjects aged 65 years and younger required systemic steroids for toxicities.
Age was found in the study to be a significant risk factor for early hepatotoxicity.
“We’re finally starting to understand the mechanisms behind this, or at least the likely mechanisms, and this seems to be immune mediated,” he said, adding, “you see a lymphocytic infiltrate on liver biopsies, you see a lymphocytic colitis in patients who receive idelalisib.”
Typically, toxicities in these patients can be managed with corticosteroids, but in some cases of severe toxicity even steroids seem to be insufficient, he said.
Further, rapid recurrence is often seen upon re-exposure to the drug, especially with respect to hepatotoxicity, he added.
Preclinical data from mouse models supports this mechanism.
“And importantly [those models] demonstrated a decrease in regulatory T cells in patients treated with idelalisib,” he said.
Dr. Coutre reported consulting for Abbvie, Celgene, Gilead, Janssen, and Pharmacyclics.
EXPERT ANALYSIS FROM LYMPHOMA & MYELOMA
Complete colpectomy & colpocleisis: Model for simulation

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Related articles:
- Natural orifice sacral colpopexy
- Alternative options for visualizing ureteral patency during intraoperative cystoscopy
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Related articles:
- Natural orifice sacral colpopexy
- Alternative options for visualizing ureteral patency during intraoperative cystoscopy
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Related articles:
- Natural orifice sacral colpopexy
- Alternative options for visualizing ureteral patency during intraoperative cystoscopy
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist
This video is brought to you by
View more videos from SGS here