User login
What Do We Know About Pediatric MS?
BALTIMORE—Lack of evidence for disease-modifying therapies (DMTs) in children presents a significant challenge in pediatric multiple sclerosis (MS). Forty percent of children with pediatric MS discontinue DMTs for inefficacy or side effects, according to an overview presented at the 141st Annual Meeting of the American Neurological Association.
“We need to keep working on clinical trials in kids and not shy away from investigating the stronger agents,” said Jennifer Graves, MD, PhD, Assistant Professor of Neurology at the University of California, San Francisco School of Medicine. Future studies should incorporate comprehensive outcomes that include measures of cognition, brain volume, and retinal integrity, she added. National and international collaborations currently under way may help achieve sample sizes that could provide conclusive evidence.
Pediatric MS Has Distinctive Features
Nearly 5% of patients with MS have pediatric onset of symptoms; 20%–30% of these patients have onset before age 11. The mean age of onset for pediatric MS is 13. In several ways, the course of MS is different in children than in adults. Children typically have higher relapse rates than adults and are less likely to develop primary or secondary progressive MS in their childhood. Neurologists are concerned, however, about the possibility that these children will develop secondary progression in their 20s or 30s.
“A lot of these kids drop off the map between seeing us in pediatric centers and moving to adult MS centers. Often, these are the people that show up at [age] 30 with significant disability and high lesion burdens because they dropped out of care when they left their parents’ homes,” said Dr. Graves.
Unlike in adult MS, the gender ratio is approximately equal in prepubertal pediatric MS. There is a near 1:1 ratio of females to males in children who present before age 11, but the ratio increases over time to 2:1 or 3:1 in adolescents and adults. Postpubertal and adult patients with MS have similar features, but prepubertal patients have features distinct from those of adult MS. For example, prepubertal patients have a lower prevalence of oligoclonal bands. Their MRI lesions tend to be larger and less distinct and sometimes resolve completely. In addition, children tend to have better motor, visual, and cerebellar recovery from relapses than adults.
A study published in the July issue of Pediatrics described the demographic and clinical characteristics of patients with pediatric MS in the United States. Of the 490 children and adolescents enrolled, 28% developed symptoms before age 12. Sixty-seven percent of participants identified as white, 21% as African-American, and 70% as non-Hispanic. Thirty-nine percent of patients had one or two foreign-born parents. Approximately 31% of patients had a prodrome (often infectious), which occurred mostly in children under age 12. Researchers observed a difference in the type of relapses in children under 12, compared with adolescents and adults. Young children tended to have more cerebellar symptoms, encephalopathy, and greater lesion burden in the posterior fossa.
Perinatal Risk Factors in Pediatric MS
In work from the US Network of Pediatric MS Centers presented at the 68th Annual Meeting of the American Academy of Neurology, Cesarean section (C-section) was associated with a 60% reduced odds of having pediatric-onset MS. The association remained statistically significant after the researchers adjusted for socioeconomic status and other maternal variables such as BMI, age, gestational diabetes, pre-eclampsia, and birth complications. While the mechanism of the association remains unknown, it is of interest in light of the new insights regarding the microbiome and MS. Children born by C-section typically have different gut flora for at least the first year of life, said Dr. Graves. When the data were adjusted for C-section result, maternal illness was independently associated with a twofold increase in the risk of pediatric MS.
The mechanism of maternal illness effects on MS risk have yet to be elucidated, but in animal models of MS, exposure during pregnancy to certain bacterial antigens can result in a proinflammatory Th17 phenotype and may cause long-term effects in offspring. An exploratory analysis in this perinatal risk factor study revealed that variables associated with agricultural work by the parent and home use of pesticides and insecticides were associated with a twofold increased risk of pediatric MS.
Diet, BMI, and Exercise
Diets high in salt have been associated with high relapse rate in adults. This association has not been found in pediatric MS, however. High fat intake is associated with high relapse rate in children with MS, according to research presented at the 32nd Congress of the European Committee for Treatment and Research in MS. Diets rich in fruits and vegetables are linked to a lower relapse rate, even after adjusting for fat intake.
In addition, data indicate that BMI is directly proportional to the risk for pediatric MS. Vigorous activity, however, is associated with decreased risk of pediatric MS, a decreased number of T2 lesions, and a decreased relapse rate, according to a Canadian study.
Role of Risk Genes and Genetic Ancestry in Pediatric MS
Genetics may play a key role in the development of early-onset pediatric MS. Researchers are analyzing data in the United States for people of multiple genetic ancestries. Of 110 nonhuman leukocyte antigen (HLA) risk variants for adult MS, 36 may increase the risk of pediatric MS, either at the level of single-nucleotide polymorphisms (SNPs) or as an aggregate genetic risk factor. The effect size of each SNP is greater in children than in adults; each SNP may increase the risk of MS by twofold or more.
Imaging Efforts
Imaging could improve understanding of pediatric MS. National and international efforts to standardize protocols for 3-T imaging, volumetric scans, and diffusion tensor imaging (DTI) are under way. DTI already has revealed abnormal fractional anisotropy in pediatric MS, and this abnormality is associated with cognitive difficulty. MRI and optical coherence tomography (OCT) help to distinguish between MS, neuromyelitis optica (NMO), and acute disseminated encephalomyelitis (ADEM).
OCT imaging in children indicates similar levels of neuronal and axonal injury as in adults, despite better visual recovery in children. In a study published in the Multiple Sclerosis Journal, boys with pediatric MS were found to have greater axonal loss than girls with pediatric MS.
Testing DMTs’ Efficacy
Several studies of DMTs in pediatric MS are under way. PARADIGMS is an ongoing, 24-month, double-blind, double-randomized trial investigating the effect of fingolimod on relapse rate in pediatric MS. The control group is receiving
Observational registries are studying the safety and clinical experience with disease modifying agents in children. Investigators recently published data for 100 children in an Italian registry of patients with MS treated with natalizumab. The treatment decreased relapse rate to 0.1. Approximately 28% of the population had no evidence of disease activity. The drug appeared to be well tolerated in children and to be efficacious.
—Erica Tricarico
Suggested Reading
Belman AL, Krupp LB, Olsen CS, et al. Characteristics of children and adolescents with multiple sclerosis. Pediatrics. 2016;138(1).
BALTIMORE—Lack of evidence for disease-modifying therapies (DMTs) in children presents a significant challenge in pediatric multiple sclerosis (MS). Forty percent of children with pediatric MS discontinue DMTs for inefficacy or side effects, according to an overview presented at the 141st Annual Meeting of the American Neurological Association.
“We need to keep working on clinical trials in kids and not shy away from investigating the stronger agents,” said Jennifer Graves, MD, PhD, Assistant Professor of Neurology at the University of California, San Francisco School of Medicine. Future studies should incorporate comprehensive outcomes that include measures of cognition, brain volume, and retinal integrity, she added. National and international collaborations currently under way may help achieve sample sizes that could provide conclusive evidence.
Pediatric MS Has Distinctive Features
Nearly 5% of patients with MS have pediatric onset of symptoms; 20%–30% of these patients have onset before age 11. The mean age of onset for pediatric MS is 13. In several ways, the course of MS is different in children than in adults. Children typically have higher relapse rates than adults and are less likely to develop primary or secondary progressive MS in their childhood. Neurologists are concerned, however, about the possibility that these children will develop secondary progression in their 20s or 30s.
“A lot of these kids drop off the map between seeing us in pediatric centers and moving to adult MS centers. Often, these are the people that show up at [age] 30 with significant disability and high lesion burdens because they dropped out of care when they left their parents’ homes,” said Dr. Graves.
Unlike in adult MS, the gender ratio is approximately equal in prepubertal pediatric MS. There is a near 1:1 ratio of females to males in children who present before age 11, but the ratio increases over time to 2:1 or 3:1 in adolescents and adults. Postpubertal and adult patients with MS have similar features, but prepubertal patients have features distinct from those of adult MS. For example, prepubertal patients have a lower prevalence of oligoclonal bands. Their MRI lesions tend to be larger and less distinct and sometimes resolve completely. In addition, children tend to have better motor, visual, and cerebellar recovery from relapses than adults.
A study published in the July issue of Pediatrics described the demographic and clinical characteristics of patients with pediatric MS in the United States. Of the 490 children and adolescents enrolled, 28% developed symptoms before age 12. Sixty-seven percent of participants identified as white, 21% as African-American, and 70% as non-Hispanic. Thirty-nine percent of patients had one or two foreign-born parents. Approximately 31% of patients had a prodrome (often infectious), which occurred mostly in children under age 12. Researchers observed a difference in the type of relapses in children under 12, compared with adolescents and adults. Young children tended to have more cerebellar symptoms, encephalopathy, and greater lesion burden in the posterior fossa.
Perinatal Risk Factors in Pediatric MS
In work from the US Network of Pediatric MS Centers presented at the 68th Annual Meeting of the American Academy of Neurology, Cesarean section (C-section) was associated with a 60% reduced odds of having pediatric-onset MS. The association remained statistically significant after the researchers adjusted for socioeconomic status and other maternal variables such as BMI, age, gestational diabetes, pre-eclampsia, and birth complications. While the mechanism of the association remains unknown, it is of interest in light of the new insights regarding the microbiome and MS. Children born by C-section typically have different gut flora for at least the first year of life, said Dr. Graves. When the data were adjusted for C-section result, maternal illness was independently associated with a twofold increase in the risk of pediatric MS.
The mechanism of maternal illness effects on MS risk have yet to be elucidated, but in animal models of MS, exposure during pregnancy to certain bacterial antigens can result in a proinflammatory Th17 phenotype and may cause long-term effects in offspring. An exploratory analysis in this perinatal risk factor study revealed that variables associated with agricultural work by the parent and home use of pesticides and insecticides were associated with a twofold increased risk of pediatric MS.
Diet, BMI, and Exercise
Diets high in salt have been associated with high relapse rate in adults. This association has not been found in pediatric MS, however. High fat intake is associated with high relapse rate in children with MS, according to research presented at the 32nd Congress of the European Committee for Treatment and Research in MS. Diets rich in fruits and vegetables are linked to a lower relapse rate, even after adjusting for fat intake.
In addition, data indicate that BMI is directly proportional to the risk for pediatric MS. Vigorous activity, however, is associated with decreased risk of pediatric MS, a decreased number of T2 lesions, and a decreased relapse rate, according to a Canadian study.
Role of Risk Genes and Genetic Ancestry in Pediatric MS
Genetics may play a key role in the development of early-onset pediatric MS. Researchers are analyzing data in the United States for people of multiple genetic ancestries. Of 110 nonhuman leukocyte antigen (HLA) risk variants for adult MS, 36 may increase the risk of pediatric MS, either at the level of single-nucleotide polymorphisms (SNPs) or as an aggregate genetic risk factor. The effect size of each SNP is greater in children than in adults; each SNP may increase the risk of MS by twofold or more.
Imaging Efforts
Imaging could improve understanding of pediatric MS. National and international efforts to standardize protocols for 3-T imaging, volumetric scans, and diffusion tensor imaging (DTI) are under way. DTI already has revealed abnormal fractional anisotropy in pediatric MS, and this abnormality is associated with cognitive difficulty. MRI and optical coherence tomography (OCT) help to distinguish between MS, neuromyelitis optica (NMO), and acute disseminated encephalomyelitis (ADEM).
OCT imaging in children indicates similar levels of neuronal and axonal injury as in adults, despite better visual recovery in children. In a study published in the Multiple Sclerosis Journal, boys with pediatric MS were found to have greater axonal loss than girls with pediatric MS.
Testing DMTs’ Efficacy
Several studies of DMTs in pediatric MS are under way. PARADIGMS is an ongoing, 24-month, double-blind, double-randomized trial investigating the effect of fingolimod on relapse rate in pediatric MS. The control group is receiving
Observational registries are studying the safety and clinical experience with disease modifying agents in children. Investigators recently published data for 100 children in an Italian registry of patients with MS treated with natalizumab. The treatment decreased relapse rate to 0.1. Approximately 28% of the population had no evidence of disease activity. The drug appeared to be well tolerated in children and to be efficacious.
—Erica Tricarico
Suggested Reading
Belman AL, Krupp LB, Olsen CS, et al. Characteristics of children and adolescents with multiple sclerosis. Pediatrics. 2016;138(1).
BALTIMORE—Lack of evidence for disease-modifying therapies (DMTs) in children presents a significant challenge in pediatric multiple sclerosis (MS). Forty percent of children with pediatric MS discontinue DMTs for inefficacy or side effects, according to an overview presented at the 141st Annual Meeting of the American Neurological Association.
“We need to keep working on clinical trials in kids and not shy away from investigating the stronger agents,” said Jennifer Graves, MD, PhD, Assistant Professor of Neurology at the University of California, San Francisco School of Medicine. Future studies should incorporate comprehensive outcomes that include measures of cognition, brain volume, and retinal integrity, she added. National and international collaborations currently under way may help achieve sample sizes that could provide conclusive evidence.
Pediatric MS Has Distinctive Features
Nearly 5% of patients with MS have pediatric onset of symptoms; 20%–30% of these patients have onset before age 11. The mean age of onset for pediatric MS is 13. In several ways, the course of MS is different in children than in adults. Children typically have higher relapse rates than adults and are less likely to develop primary or secondary progressive MS in their childhood. Neurologists are concerned, however, about the possibility that these children will develop secondary progression in their 20s or 30s.
“A lot of these kids drop off the map between seeing us in pediatric centers and moving to adult MS centers. Often, these are the people that show up at [age] 30 with significant disability and high lesion burdens because they dropped out of care when they left their parents’ homes,” said Dr. Graves.
Unlike in adult MS, the gender ratio is approximately equal in prepubertal pediatric MS. There is a near 1:1 ratio of females to males in children who present before age 11, but the ratio increases over time to 2:1 or 3:1 in adolescents and adults. Postpubertal and adult patients with MS have similar features, but prepubertal patients have features distinct from those of adult MS. For example, prepubertal patients have a lower prevalence of oligoclonal bands. Their MRI lesions tend to be larger and less distinct and sometimes resolve completely. In addition, children tend to have better motor, visual, and cerebellar recovery from relapses than adults.
A study published in the July issue of Pediatrics described the demographic and clinical characteristics of patients with pediatric MS in the United States. Of the 490 children and adolescents enrolled, 28% developed symptoms before age 12. Sixty-seven percent of participants identified as white, 21% as African-American, and 70% as non-Hispanic. Thirty-nine percent of patients had one or two foreign-born parents. Approximately 31% of patients had a prodrome (often infectious), which occurred mostly in children under age 12. Researchers observed a difference in the type of relapses in children under 12, compared with adolescents and adults. Young children tended to have more cerebellar symptoms, encephalopathy, and greater lesion burden in the posterior fossa.
Perinatal Risk Factors in Pediatric MS
In work from the US Network of Pediatric MS Centers presented at the 68th Annual Meeting of the American Academy of Neurology, Cesarean section (C-section) was associated with a 60% reduced odds of having pediatric-onset MS. The association remained statistically significant after the researchers adjusted for socioeconomic status and other maternal variables such as BMI, age, gestational diabetes, pre-eclampsia, and birth complications. While the mechanism of the association remains unknown, it is of interest in light of the new insights regarding the microbiome and MS. Children born by C-section typically have different gut flora for at least the first year of life, said Dr. Graves. When the data were adjusted for C-section result, maternal illness was independently associated with a twofold increase in the risk of pediatric MS.
The mechanism of maternal illness effects on MS risk have yet to be elucidated, but in animal models of MS, exposure during pregnancy to certain bacterial antigens can result in a proinflammatory Th17 phenotype and may cause long-term effects in offspring. An exploratory analysis in this perinatal risk factor study revealed that variables associated with agricultural work by the parent and home use of pesticides and insecticides were associated with a twofold increased risk of pediatric MS.
Diet, BMI, and Exercise
Diets high in salt have been associated with high relapse rate in adults. This association has not been found in pediatric MS, however. High fat intake is associated with high relapse rate in children with MS, according to research presented at the 32nd Congress of the European Committee for Treatment and Research in MS. Diets rich in fruits and vegetables are linked to a lower relapse rate, even after adjusting for fat intake.
In addition, data indicate that BMI is directly proportional to the risk for pediatric MS. Vigorous activity, however, is associated with decreased risk of pediatric MS, a decreased number of T2 lesions, and a decreased relapse rate, according to a Canadian study.
Role of Risk Genes and Genetic Ancestry in Pediatric MS
Genetics may play a key role in the development of early-onset pediatric MS. Researchers are analyzing data in the United States for people of multiple genetic ancestries. Of 110 nonhuman leukocyte antigen (HLA) risk variants for adult MS, 36 may increase the risk of pediatric MS, either at the level of single-nucleotide polymorphisms (SNPs) or as an aggregate genetic risk factor. The effect size of each SNP is greater in children than in adults; each SNP may increase the risk of MS by twofold or more.
Imaging Efforts
Imaging could improve understanding of pediatric MS. National and international efforts to standardize protocols for 3-T imaging, volumetric scans, and diffusion tensor imaging (DTI) are under way. DTI already has revealed abnormal fractional anisotropy in pediatric MS, and this abnormality is associated with cognitive difficulty. MRI and optical coherence tomography (OCT) help to distinguish between MS, neuromyelitis optica (NMO), and acute disseminated encephalomyelitis (ADEM).
OCT imaging in children indicates similar levels of neuronal and axonal injury as in adults, despite better visual recovery in children. In a study published in the Multiple Sclerosis Journal, boys with pediatric MS were found to have greater axonal loss than girls with pediatric MS.
Testing DMTs’ Efficacy
Several studies of DMTs in pediatric MS are under way. PARADIGMS is an ongoing, 24-month, double-blind, double-randomized trial investigating the effect of fingolimod on relapse rate in pediatric MS. The control group is receiving
Observational registries are studying the safety and clinical experience with disease modifying agents in children. Investigators recently published data for 100 children in an Italian registry of patients with MS treated with natalizumab. The treatment decreased relapse rate to 0.1. Approximately 28% of the population had no evidence of disease activity. The drug appeared to be well tolerated in children and to be efficacious.
—Erica Tricarico
Suggested Reading
Belman AL, Krupp LB, Olsen CS, et al. Characteristics of children and adolescents with multiple sclerosis. Pediatrics. 2016;138(1).
Myeloablative HSCT bests IV CYC for systemic scleroderma
WASHINGTON – Myeloablative autologous hematopoietic stem cell transplantation was superior to monthly intravenous cyclophosphamide in patients with severe scleroderma with internal organ involvement in the randomized, multicenter Scleroderma: Cyclophosphamide or Transplantation (SCOT) trial.
At both 54 and 48 months, a comparison of global rank composite scores favored myeloablation followed by CD34+-selected autologous hematopoietic stem cell transplantation (HSCT) over 12 monthly pulses of 750 mg/m2 cyclophosphamide in the intention-to-treat population of 36 and 39 subjects with diffuse cutaneous systemic sclerosis and high-risk lung and/or renal involvement who were randomized to the groups, respectively (P = .013 at 54 months and P = .008 at 48 months). In subjects who actually underwent HSCT or received at least nine cyclophosphamide doses, the effect was even stronger (P = .004 at 54 months and P = .003 at 48 months), Keith M. Sullivan, MD, reported at the annual meeting of the American College of Rheumatology.
The global rank composite scores were derived based on a hierarchy of outcomes including – in rank order – death, event-free survival, forced vital capacity, scleroderma health assessment questionnaire score, and modified Rodnan skin score (mRSS); each patient was compared, based on the hierarchy, with every other patient in the study.
HSCT was superior overall and within each of the components of the score, Dr. Sullivan said.
The findings were supported by secondary analyses showing that at 54 months, event-free survival was 79% in the HSCT group vs. 50% in the cyclophosphamide group, and overall survival was 91% vs. 77% in the groups, respectively; the differences between the groups were statistically significant, said Dr. Sullivan of Duke University, Durham, N.C.
“In advanced scleroderma, that is a remarkable finding,” he said of the nearly 30-point difference in event-free survival rates between the groups.
Further, disease-modifying antirheumatic drugs were initiated by 54 months in 9% of the HSCT recipients vs. 44% of those in the cyclophosphamide group, he noted.
The rate of serious adverse events was similar in the two groups, although grade 3 or greater adverse events, including treatment-related cytopenias, were more common in the HSCT group, as was herpes zoster. Remarkably, most serious adverse events and adverse events occurred in the first 14 months, Dr. Sullivan noted.
Treatment-related mortality at 54 months was 3% in the HSCT group, and 0% in the cyclophosphamide group, he said.
Study subjects were adults aged 18-69 years with mean baseline modified Rodnan skin score of 30, mean baseline forced vital capacity of 74%, and mean diffusing capacity of the lung for carbon monoxide (DLCO) of 53% of predicted. All but two had lung involvement.
“So [there was] significant skin and pulmonary impairment,” he said.
There are, unquestionably, risks with transplant, but the SCOT findings support myeloablative autologous HSCT as a significant advance in the care of diffuse cutaneous systemic scleroderma with internal organ involvement, as this approach provided superior long-term outcomes, compared with 12 months of IV cyclophosphamide, he said, adding that the global rank composite score developed for this study was a useful measure of scleroderma outcomes.
“Therefore it would be prudent to consider early referral to the transplant center for consultation, which would allow patients, earlier in their disease – before the mRSS hit 30 and the DLCOs hit 50 – to make informed decisions,” he concluded.
The SCOT trial was funded by the National Institute of Allergy and Infectious Diseases. The authors reported having no disclosures.
WASHINGTON – Myeloablative autologous hematopoietic stem cell transplantation was superior to monthly intravenous cyclophosphamide in patients with severe scleroderma with internal organ involvement in the randomized, multicenter Scleroderma: Cyclophosphamide or Transplantation (SCOT) trial.
At both 54 and 48 months, a comparison of global rank composite scores favored myeloablation followed by CD34+-selected autologous hematopoietic stem cell transplantation (HSCT) over 12 monthly pulses of 750 mg/m2 cyclophosphamide in the intention-to-treat population of 36 and 39 subjects with diffuse cutaneous systemic sclerosis and high-risk lung and/or renal involvement who were randomized to the groups, respectively (P = .013 at 54 months and P = .008 at 48 months). In subjects who actually underwent HSCT or received at least nine cyclophosphamide doses, the effect was even stronger (P = .004 at 54 months and P = .003 at 48 months), Keith M. Sullivan, MD, reported at the annual meeting of the American College of Rheumatology.
The global rank composite scores were derived based on a hierarchy of outcomes including – in rank order – death, event-free survival, forced vital capacity, scleroderma health assessment questionnaire score, and modified Rodnan skin score (mRSS); each patient was compared, based on the hierarchy, with every other patient in the study.
HSCT was superior overall and within each of the components of the score, Dr. Sullivan said.
The findings were supported by secondary analyses showing that at 54 months, event-free survival was 79% in the HSCT group vs. 50% in the cyclophosphamide group, and overall survival was 91% vs. 77% in the groups, respectively; the differences between the groups were statistically significant, said Dr. Sullivan of Duke University, Durham, N.C.
“In advanced scleroderma, that is a remarkable finding,” he said of the nearly 30-point difference in event-free survival rates between the groups.
Further, disease-modifying antirheumatic drugs were initiated by 54 months in 9% of the HSCT recipients vs. 44% of those in the cyclophosphamide group, he noted.
The rate of serious adverse events was similar in the two groups, although grade 3 or greater adverse events, including treatment-related cytopenias, were more common in the HSCT group, as was herpes zoster. Remarkably, most serious adverse events and adverse events occurred in the first 14 months, Dr. Sullivan noted.
Treatment-related mortality at 54 months was 3% in the HSCT group, and 0% in the cyclophosphamide group, he said.
Study subjects were adults aged 18-69 years with mean baseline modified Rodnan skin score of 30, mean baseline forced vital capacity of 74%, and mean diffusing capacity of the lung for carbon monoxide (DLCO) of 53% of predicted. All but two had lung involvement.
“So [there was] significant skin and pulmonary impairment,” he said.
There are, unquestionably, risks with transplant, but the SCOT findings support myeloablative autologous HSCT as a significant advance in the care of diffuse cutaneous systemic scleroderma with internal organ involvement, as this approach provided superior long-term outcomes, compared with 12 months of IV cyclophosphamide, he said, adding that the global rank composite score developed for this study was a useful measure of scleroderma outcomes.
“Therefore it would be prudent to consider early referral to the transplant center for consultation, which would allow patients, earlier in their disease – before the mRSS hit 30 and the DLCOs hit 50 – to make informed decisions,” he concluded.
The SCOT trial was funded by the National Institute of Allergy and Infectious Diseases. The authors reported having no disclosures.
WASHINGTON – Myeloablative autologous hematopoietic stem cell transplantation was superior to monthly intravenous cyclophosphamide in patients with severe scleroderma with internal organ involvement in the randomized, multicenter Scleroderma: Cyclophosphamide or Transplantation (SCOT) trial.
At both 54 and 48 months, a comparison of global rank composite scores favored myeloablation followed by CD34+-selected autologous hematopoietic stem cell transplantation (HSCT) over 12 monthly pulses of 750 mg/m2 cyclophosphamide in the intention-to-treat population of 36 and 39 subjects with diffuse cutaneous systemic sclerosis and high-risk lung and/or renal involvement who were randomized to the groups, respectively (P = .013 at 54 months and P = .008 at 48 months). In subjects who actually underwent HSCT or received at least nine cyclophosphamide doses, the effect was even stronger (P = .004 at 54 months and P = .003 at 48 months), Keith M. Sullivan, MD, reported at the annual meeting of the American College of Rheumatology.
The global rank composite scores were derived based on a hierarchy of outcomes including – in rank order – death, event-free survival, forced vital capacity, scleroderma health assessment questionnaire score, and modified Rodnan skin score (mRSS); each patient was compared, based on the hierarchy, with every other patient in the study.
HSCT was superior overall and within each of the components of the score, Dr. Sullivan said.
The findings were supported by secondary analyses showing that at 54 months, event-free survival was 79% in the HSCT group vs. 50% in the cyclophosphamide group, and overall survival was 91% vs. 77% in the groups, respectively; the differences between the groups were statistically significant, said Dr. Sullivan of Duke University, Durham, N.C.
“In advanced scleroderma, that is a remarkable finding,” he said of the nearly 30-point difference in event-free survival rates between the groups.
Further, disease-modifying antirheumatic drugs were initiated by 54 months in 9% of the HSCT recipients vs. 44% of those in the cyclophosphamide group, he noted.
The rate of serious adverse events was similar in the two groups, although grade 3 or greater adverse events, including treatment-related cytopenias, were more common in the HSCT group, as was herpes zoster. Remarkably, most serious adverse events and adverse events occurred in the first 14 months, Dr. Sullivan noted.
Treatment-related mortality at 54 months was 3% in the HSCT group, and 0% in the cyclophosphamide group, he said.
Study subjects were adults aged 18-69 years with mean baseline modified Rodnan skin score of 30, mean baseline forced vital capacity of 74%, and mean diffusing capacity of the lung for carbon monoxide (DLCO) of 53% of predicted. All but two had lung involvement.
“So [there was] significant skin and pulmonary impairment,” he said.
There are, unquestionably, risks with transplant, but the SCOT findings support myeloablative autologous HSCT as a significant advance in the care of diffuse cutaneous systemic scleroderma with internal organ involvement, as this approach provided superior long-term outcomes, compared with 12 months of IV cyclophosphamide, he said, adding that the global rank composite score developed for this study was a useful measure of scleroderma outcomes.
“Therefore it would be prudent to consider early referral to the transplant center for consultation, which would allow patients, earlier in their disease – before the mRSS hit 30 and the DLCOs hit 50 – to make informed decisions,” he concluded.
The SCOT trial was funded by the National Institute of Allergy and Infectious Diseases. The authors reported having no disclosures.
AT THE ACR ANNUAL MEETING
Key clinical point:
Major finding: Event-free survival was 79% in the HSCT group vs. 50% in the cyclophosphamide group, and overall survival was 91% vs. 77% in the groups, respectively.
Data source: The randomized, multicenter SCOT trial of 75 systemic scleroderma patients.
Disclosures: The SCOT trial was funded by the National Institute of Allergy and Infectious Diseases. The authors reported having no disclosures.
Survey: Primary care needs opioid alternatives
Almost a third of doctors blamed overprescribing for the opioid crisis, according to a survey of 225 U.S. primary care, emergency department, and pain management physicians by InCrowd, an online physician survey company.
Respondents said their own and other physicians’ overprescribing is the single biggest factor fueling the leap in opioid abuse over the past 5 years.
In short, the survey pointed out what front-line doctors think needs to be fixed as the nation combats prescription opioid abuse and the subsequent heroin epidemic. Their insights “should be a rallying cry” for changes in 2017, said epidemiologist Diane Hayes, PhD, president and cofounder of InCrowd.
Making pain the “fifth vital sign” and allowing patients to downgrade doctors on surveys if they don’t refill narcotic prescriptions compounded the situation. Lengthy waits for specialists with better pain options, many of whom are not covered by Medicaid or the Affordable Care Act, also added to the problem, survey respondents said.
“We’re caught in the middle” between the Joint Commission on Accreditation of Healthcare Organization’s fifth vital sign and overprescribing, a primary care doctor said.
Seventy-three percent of survey respondents said that they want opioid alternatives. They’re tired of trying to get the job done with NSAIDs, physical therapy, and exercise. About half recommend behavioral health interventions, while 20% recommend vitamin and herbal supplements. Only 10% recommend medical marijuana, probably because most U.S. patients can’t get it.
Meanwhile, the respondents said they want opioid prescribing hemmed in. Almost two-thirds wanted refill limits and more frequent refill evaluations, and many agreed that there needs to be a weaning protocol before the drugs are even started. Some wanted to limit advertising.
Easton Jackson, MD, a primary care physician in West Valley City, Utah, who answered the survey, helped make the answers real by sharing his thoughts.
“We need to recognize that ... people don’t set out to get addicted to opioids ... We need to educate [patients] and assist them with their expectations. They need to understand that they’re going to have pain from surgery and injuries. Our goal isn’t to make them pain free. It’s to manage their pain,” he said.
“We as physicians need to write for fewer pills and in lower doses. We need to see our patients back sooner. If it’s not working, stop increasing the dose and instead taper the patient off the medication. We need to be familiar with the adjuvant therapies. As easy as it is to say, ‘send them all to the pain specialist,’ there simply aren’t enough of them around,” Dr. Easton said.
Physician respondents to InCrowd’s opioid survey have practiced an average of 25 years, and were scattered around the United States. They filled out the four-question survey during Oct. 27-28, 2016. They signed up to receive and answer InCrowd’s questions, and were paid nominally for their time.
Half (50%) of respondents estimated that they prescribed opioids to fewer than 10% of their patients, while 38% said they prescribed to less than half of their patients, and 12% estimated they prescribed opioids to more than half of their patients.
Almost a third of doctors blamed overprescribing for the opioid crisis, according to a survey of 225 U.S. primary care, emergency department, and pain management physicians by InCrowd, an online physician survey company.
Respondents said their own and other physicians’ overprescribing is the single biggest factor fueling the leap in opioid abuse over the past 5 years.
In short, the survey pointed out what front-line doctors think needs to be fixed as the nation combats prescription opioid abuse and the subsequent heroin epidemic. Their insights “should be a rallying cry” for changes in 2017, said epidemiologist Diane Hayes, PhD, president and cofounder of InCrowd.
Making pain the “fifth vital sign” and allowing patients to downgrade doctors on surveys if they don’t refill narcotic prescriptions compounded the situation. Lengthy waits for specialists with better pain options, many of whom are not covered by Medicaid or the Affordable Care Act, also added to the problem, survey respondents said.
“We’re caught in the middle” between the Joint Commission on Accreditation of Healthcare Organization’s fifth vital sign and overprescribing, a primary care doctor said.
Seventy-three percent of survey respondents said that they want opioid alternatives. They’re tired of trying to get the job done with NSAIDs, physical therapy, and exercise. About half recommend behavioral health interventions, while 20% recommend vitamin and herbal supplements. Only 10% recommend medical marijuana, probably because most U.S. patients can’t get it.
Meanwhile, the respondents said they want opioid prescribing hemmed in. Almost two-thirds wanted refill limits and more frequent refill evaluations, and many agreed that there needs to be a weaning protocol before the drugs are even started. Some wanted to limit advertising.
Easton Jackson, MD, a primary care physician in West Valley City, Utah, who answered the survey, helped make the answers real by sharing his thoughts.
“We need to recognize that ... people don’t set out to get addicted to opioids ... We need to educate [patients] and assist them with their expectations. They need to understand that they’re going to have pain from surgery and injuries. Our goal isn’t to make them pain free. It’s to manage their pain,” he said.
“We as physicians need to write for fewer pills and in lower doses. We need to see our patients back sooner. If it’s not working, stop increasing the dose and instead taper the patient off the medication. We need to be familiar with the adjuvant therapies. As easy as it is to say, ‘send them all to the pain specialist,’ there simply aren’t enough of them around,” Dr. Easton said.
Physician respondents to InCrowd’s opioid survey have practiced an average of 25 years, and were scattered around the United States. They filled out the four-question survey during Oct. 27-28, 2016. They signed up to receive and answer InCrowd’s questions, and were paid nominally for their time.
Half (50%) of respondents estimated that they prescribed opioids to fewer than 10% of their patients, while 38% said they prescribed to less than half of their patients, and 12% estimated they prescribed opioids to more than half of their patients.
Almost a third of doctors blamed overprescribing for the opioid crisis, according to a survey of 225 U.S. primary care, emergency department, and pain management physicians by InCrowd, an online physician survey company.
Respondents said their own and other physicians’ overprescribing is the single biggest factor fueling the leap in opioid abuse over the past 5 years.
In short, the survey pointed out what front-line doctors think needs to be fixed as the nation combats prescription opioid abuse and the subsequent heroin epidemic. Their insights “should be a rallying cry” for changes in 2017, said epidemiologist Diane Hayes, PhD, president and cofounder of InCrowd.
Making pain the “fifth vital sign” and allowing patients to downgrade doctors on surveys if they don’t refill narcotic prescriptions compounded the situation. Lengthy waits for specialists with better pain options, many of whom are not covered by Medicaid or the Affordable Care Act, also added to the problem, survey respondents said.
“We’re caught in the middle” between the Joint Commission on Accreditation of Healthcare Organization’s fifth vital sign and overprescribing, a primary care doctor said.
Seventy-three percent of survey respondents said that they want opioid alternatives. They’re tired of trying to get the job done with NSAIDs, physical therapy, and exercise. About half recommend behavioral health interventions, while 20% recommend vitamin and herbal supplements. Only 10% recommend medical marijuana, probably because most U.S. patients can’t get it.
Meanwhile, the respondents said they want opioid prescribing hemmed in. Almost two-thirds wanted refill limits and more frequent refill evaluations, and many agreed that there needs to be a weaning protocol before the drugs are even started. Some wanted to limit advertising.
Easton Jackson, MD, a primary care physician in West Valley City, Utah, who answered the survey, helped make the answers real by sharing his thoughts.
“We need to recognize that ... people don’t set out to get addicted to opioids ... We need to educate [patients] and assist them with their expectations. They need to understand that they’re going to have pain from surgery and injuries. Our goal isn’t to make them pain free. It’s to manage their pain,” he said.
“We as physicians need to write for fewer pills and in lower doses. We need to see our patients back sooner. If it’s not working, stop increasing the dose and instead taper the patient off the medication. We need to be familiar with the adjuvant therapies. As easy as it is to say, ‘send them all to the pain specialist,’ there simply aren’t enough of them around,” Dr. Easton said.
Physician respondents to InCrowd’s opioid survey have practiced an average of 25 years, and were scattered around the United States. They filled out the four-question survey during Oct. 27-28, 2016. They signed up to receive and answer InCrowd’s questions, and were paid nominally for their time.
Half (50%) of respondents estimated that they prescribed opioids to fewer than 10% of their patients, while 38% said they prescribed to less than half of their patients, and 12% estimated they prescribed opioids to more than half of their patients.
Safety and Efficacy of Five Years of Levodopa–Carbidopa Intestinal Gel Treatment
PORTLAND, OR—Most patients with advanced Parkinson’s disease who received levodopa–carbidopa intestinal gel in an open-label, continued-access-to-treatment study had, at five years, transitioned to using the therapy outside of the study when it became commercially available or continued to participate in the extension study, according to data presented at the Fourth World Parkinson Congress.
Forty-two percent of the patients in the phase III extension study transitioned to a commercially available drug (designated as carbidopa–levodopa enteral suspension in the United States), and 24% of patients remained in the multinational study, said Hubert H. Fernandez, MD, Head of Movement Disorders at the Center for Neurological Restoration at the Cleveland Clinic, and colleagues. Patients may remain in the extension study until a commercially available product is available in the country where they live.
Levodopa–carbidopa intestinal gel is infused continuously directly to the jejunum using a portable pump during approximately 16 hours of wakefulness. The therapy is designed to overcome some of the limitations of oral levodopa, which may lose effectiveness as Parkinson’s disease progresses.
Although investigators observed a high incidence of adverse events, long-term use of levodopa–carbidopa intestinal gel was well tolerated, the researchers said. The average discontinuation rate of 9.6% per year, including all causes of death, was relatively low, Dr. Fernandez and colleagues said.
The most frequently reported adverse events were associated with complications related to percutaneous gastrojejunostomy, such as stoma site maintenance. Other adverse events were associated with advanced Parkinson’s disease, aging, or levodopa. Most patients experienced device malfunctions and required pump replacement during the study.
The extension study began in November 2009. Data through September 30, 2015, were used in the present study. The study enrolled 262 patients with advanced Parkinson’s disease from 11 countries who had completed a 12-week double-blind study and its 52-week open-label extension or who had completed a separate 54-week open-label study. Participants attended scheduled study visits every six months.
Patients had a mean age of 64, 62% were male, and mean disease duration was 11.4 years. Mean exposure to levodopa–carbidopa intestinal gel was 3.1 years in the present study. Patients’ mean total exposure to the treatment was 4.1 years. Fifty-six percent of patients were exposed to the treatment for at least five years.
Adverse events led to discontinuation in 62 patients (24%). Device complaints occurred in 244 patients (93%). The most common device complaints included device malfunction (85%), device occlusion (57%), and device dislocation (56%). Thirty-eight patients died during the study. Two patients died as a result of intestinal dilatation and cardiac arrest, which an investigator considered possibly related to the treatment.
As part of an amended study protocol, patients in the United States began completing efficacy assessments in December 2013 (ie, Parkinson’s disease diary, Unified Parkinson’s Disease Rating Scale, and the Parkinson’s Disease Questionnaire). Patients in the United States showed sustained and clinically meaningful benefits of treatment that were demonstrated by decreased off time and increased on time without troublesome dyskinesia, the researchers concluded.
—Jake Remaly
PORTLAND, OR—Most patients with advanced Parkinson’s disease who received levodopa–carbidopa intestinal gel in an open-label, continued-access-to-treatment study had, at five years, transitioned to using the therapy outside of the study when it became commercially available or continued to participate in the extension study, according to data presented at the Fourth World Parkinson Congress.
Forty-two percent of the patients in the phase III extension study transitioned to a commercially available drug (designated as carbidopa–levodopa enteral suspension in the United States), and 24% of patients remained in the multinational study, said Hubert H. Fernandez, MD, Head of Movement Disorders at the Center for Neurological Restoration at the Cleveland Clinic, and colleagues. Patients may remain in the extension study until a commercially available product is available in the country where they live.
Levodopa–carbidopa intestinal gel is infused continuously directly to the jejunum using a portable pump during approximately 16 hours of wakefulness. The therapy is designed to overcome some of the limitations of oral levodopa, which may lose effectiveness as Parkinson’s disease progresses.
Although investigators observed a high incidence of adverse events, long-term use of levodopa–carbidopa intestinal gel was well tolerated, the researchers said. The average discontinuation rate of 9.6% per year, including all causes of death, was relatively low, Dr. Fernandez and colleagues said.
The most frequently reported adverse events were associated with complications related to percutaneous gastrojejunostomy, such as stoma site maintenance. Other adverse events were associated with advanced Parkinson’s disease, aging, or levodopa. Most patients experienced device malfunctions and required pump replacement during the study.
The extension study began in November 2009. Data through September 30, 2015, were used in the present study. The study enrolled 262 patients with advanced Parkinson’s disease from 11 countries who had completed a 12-week double-blind study and its 52-week open-label extension or who had completed a separate 54-week open-label study. Participants attended scheduled study visits every six months.
Patients had a mean age of 64, 62% were male, and mean disease duration was 11.4 years. Mean exposure to levodopa–carbidopa intestinal gel was 3.1 years in the present study. Patients’ mean total exposure to the treatment was 4.1 years. Fifty-six percent of patients were exposed to the treatment for at least five years.
Adverse events led to discontinuation in 62 patients (24%). Device complaints occurred in 244 patients (93%). The most common device complaints included device malfunction (85%), device occlusion (57%), and device dislocation (56%). Thirty-eight patients died during the study. Two patients died as a result of intestinal dilatation and cardiac arrest, which an investigator considered possibly related to the treatment.
As part of an amended study protocol, patients in the United States began completing efficacy assessments in December 2013 (ie, Parkinson’s disease diary, Unified Parkinson’s Disease Rating Scale, and the Parkinson’s Disease Questionnaire). Patients in the United States showed sustained and clinically meaningful benefits of treatment that were demonstrated by decreased off time and increased on time without troublesome dyskinesia, the researchers concluded.
—Jake Remaly
PORTLAND, OR—Most patients with advanced Parkinson’s disease who received levodopa–carbidopa intestinal gel in an open-label, continued-access-to-treatment study had, at five years, transitioned to using the therapy outside of the study when it became commercially available or continued to participate in the extension study, according to data presented at the Fourth World Parkinson Congress.
Forty-two percent of the patients in the phase III extension study transitioned to a commercially available drug (designated as carbidopa–levodopa enteral suspension in the United States), and 24% of patients remained in the multinational study, said Hubert H. Fernandez, MD, Head of Movement Disorders at the Center for Neurological Restoration at the Cleveland Clinic, and colleagues. Patients may remain in the extension study until a commercially available product is available in the country where they live.
Levodopa–carbidopa intestinal gel is infused continuously directly to the jejunum using a portable pump during approximately 16 hours of wakefulness. The therapy is designed to overcome some of the limitations of oral levodopa, which may lose effectiveness as Parkinson’s disease progresses.
Although investigators observed a high incidence of adverse events, long-term use of levodopa–carbidopa intestinal gel was well tolerated, the researchers said. The average discontinuation rate of 9.6% per year, including all causes of death, was relatively low, Dr. Fernandez and colleagues said.
The most frequently reported adverse events were associated with complications related to percutaneous gastrojejunostomy, such as stoma site maintenance. Other adverse events were associated with advanced Parkinson’s disease, aging, or levodopa. Most patients experienced device malfunctions and required pump replacement during the study.
The extension study began in November 2009. Data through September 30, 2015, were used in the present study. The study enrolled 262 patients with advanced Parkinson’s disease from 11 countries who had completed a 12-week double-blind study and its 52-week open-label extension or who had completed a separate 54-week open-label study. Participants attended scheduled study visits every six months.
Patients had a mean age of 64, 62% were male, and mean disease duration was 11.4 years. Mean exposure to levodopa–carbidopa intestinal gel was 3.1 years in the present study. Patients’ mean total exposure to the treatment was 4.1 years. Fifty-six percent of patients were exposed to the treatment for at least five years.
Adverse events led to discontinuation in 62 patients (24%). Device complaints occurred in 244 patients (93%). The most common device complaints included device malfunction (85%), device occlusion (57%), and device dislocation (56%). Thirty-eight patients died during the study. Two patients died as a result of intestinal dilatation and cardiac arrest, which an investigator considered possibly related to the treatment.
As part of an amended study protocol, patients in the United States began completing efficacy assessments in December 2013 (ie, Parkinson’s disease diary, Unified Parkinson’s Disease Rating Scale, and the Parkinson’s Disease Questionnaire). Patients in the United States showed sustained and clinically meaningful benefits of treatment that were demonstrated by decreased off time and increased on time without troublesome dyskinesia, the researchers concluded.
—Jake Remaly
ADDRESS II study: Atacicept shows promise for SLE
WASHINGTON – The recombinant fusion protein atacicept, which targets B-cell stimulating factors BLyS and APRIL, had a favorable safety profile and showed some evidence of efficacy in systemic lupus erythematosus patients – particularly those with high disease activity and serologically active disease – in the randomized, phase IIb ADDRESS II study.
Although the primary endpoint of the study – percentage of patients achieving SLE responder index-4 (SRI-4) response at 24 weeks – was not met, a reduction in both disease activity and severe flares was observed in patients in the multicenter study who received atacicept vs. placebo, Joan T. Merrill, MD, of the Oklahoma Medical Research Foundation, Oklahoma City, reported in a late-breaking poster at the annual meeting of the American College of Rheumatology.
However, in a prespecified sensitivity analysis using study day 1 rather than screening visit as baseline, significantly more patients in the atacicept groups achieved SRI-4 response at week 24. For example, compared with a 41% SRI-4 response rate in the placebo group, the rate was 55.9% for the 75-mg atacicept group (odds ratio, 1.88) and 55.8% for the 150-mg atacicept group (odds ratio, 1.96), Dr. Merrill said.
Further, enhanced improvements in both SRI-4 and SRI-6 (a composite measure including reduction of at least 6 points on the SLEDAI-2K) were seen in the atacicept groups vs. the placebo group in 158 patients with high disease activity (defined as SLEDAI-2K of 10 or greater), in 84 patients with serologically active disease (those with dsDNA antibody-positive disease and low complement), and in 69 patients with both.
For example, SRI-4 response rates in patients with high disease activity were significantly greater with atacicept 150 mg vs. placebo at week 24 (62.7% vs. 42.3%; odds ratio, 2.43), and the corresponding SRI-6 rates were 54.9% vs. 28.8% with an odds ratio of 3.30.
The SRI-4 rates at week 24 in those with both high disease activity and serologically active disease who received 150 mg atacicept vs. placebo were 65% vs. 25% (odds ratio, 7.48), and the SRI-6 rates in those patients were 55% vs. 16.7% (odds ratio, 7.13).
In patients with high disease activity, severe flare was reduced with both the 75-mg and 150-mg doses of atacicept, Dr. Merrill said, noting that in the intent-to-treat population, BILAG A flare was significantly reduced with atacicept 75 mg vs. placebo, and severe flares (as measured with the SLEDAI flare index) was reduced with atacicept 150 mg.
Atacicept was associated with increased serum complement C3/C4, and with decreased IgG, IgM, IgA, and anti-dsDNA antibodies over time, she said.
Treatment-emergent adverse events occurred in similar percentages of patients in the groups (71% with placebo, 81.4% with 75 mg atacicept, and 80.8% with 150 mg atacicept). Serious treatment-emergent adverse events occurred in 11%, 8.8%, and 5.8%, respectively.
In a press statement, Dr. Merrill said the results are impressive, particularly for a small, 24-week study.
“If confirmed in future studies, this could hold exciting possibilities for our patients,” she said.
ADDRESS II was sponsored by EMD Serono. Dr. Merrill reported financial relationships with Anthera Pharmaceuticals and Lilly. Other authors reported financial relationships with EMD Serono/Merck Serono.
WASHINGTON – The recombinant fusion protein atacicept, which targets B-cell stimulating factors BLyS and APRIL, had a favorable safety profile and showed some evidence of efficacy in systemic lupus erythematosus patients – particularly those with high disease activity and serologically active disease – in the randomized, phase IIb ADDRESS II study.
Although the primary endpoint of the study – percentage of patients achieving SLE responder index-4 (SRI-4) response at 24 weeks – was not met, a reduction in both disease activity and severe flares was observed in patients in the multicenter study who received atacicept vs. placebo, Joan T. Merrill, MD, of the Oklahoma Medical Research Foundation, Oklahoma City, reported in a late-breaking poster at the annual meeting of the American College of Rheumatology.
However, in a prespecified sensitivity analysis using study day 1 rather than screening visit as baseline, significantly more patients in the atacicept groups achieved SRI-4 response at week 24. For example, compared with a 41% SRI-4 response rate in the placebo group, the rate was 55.9% for the 75-mg atacicept group (odds ratio, 1.88) and 55.8% for the 150-mg atacicept group (odds ratio, 1.96), Dr. Merrill said.
Further, enhanced improvements in both SRI-4 and SRI-6 (a composite measure including reduction of at least 6 points on the SLEDAI-2K) were seen in the atacicept groups vs. the placebo group in 158 patients with high disease activity (defined as SLEDAI-2K of 10 or greater), in 84 patients with serologically active disease (those with dsDNA antibody-positive disease and low complement), and in 69 patients with both.
For example, SRI-4 response rates in patients with high disease activity were significantly greater with atacicept 150 mg vs. placebo at week 24 (62.7% vs. 42.3%; odds ratio, 2.43), and the corresponding SRI-6 rates were 54.9% vs. 28.8% with an odds ratio of 3.30.
The SRI-4 rates at week 24 in those with both high disease activity and serologically active disease who received 150 mg atacicept vs. placebo were 65% vs. 25% (odds ratio, 7.48), and the SRI-6 rates in those patients were 55% vs. 16.7% (odds ratio, 7.13).
In patients with high disease activity, severe flare was reduced with both the 75-mg and 150-mg doses of atacicept, Dr. Merrill said, noting that in the intent-to-treat population, BILAG A flare was significantly reduced with atacicept 75 mg vs. placebo, and severe flares (as measured with the SLEDAI flare index) was reduced with atacicept 150 mg.
Atacicept was associated with increased serum complement C3/C4, and with decreased IgG, IgM, IgA, and anti-dsDNA antibodies over time, she said.
Treatment-emergent adverse events occurred in similar percentages of patients in the groups (71% with placebo, 81.4% with 75 mg atacicept, and 80.8% with 150 mg atacicept). Serious treatment-emergent adverse events occurred in 11%, 8.8%, and 5.8%, respectively.
In a press statement, Dr. Merrill said the results are impressive, particularly for a small, 24-week study.
“If confirmed in future studies, this could hold exciting possibilities for our patients,” she said.
ADDRESS II was sponsored by EMD Serono. Dr. Merrill reported financial relationships with Anthera Pharmaceuticals and Lilly. Other authors reported financial relationships with EMD Serono/Merck Serono.
WASHINGTON – The recombinant fusion protein atacicept, which targets B-cell stimulating factors BLyS and APRIL, had a favorable safety profile and showed some evidence of efficacy in systemic lupus erythematosus patients – particularly those with high disease activity and serologically active disease – in the randomized, phase IIb ADDRESS II study.
Although the primary endpoint of the study – percentage of patients achieving SLE responder index-4 (SRI-4) response at 24 weeks – was not met, a reduction in both disease activity and severe flares was observed in patients in the multicenter study who received atacicept vs. placebo, Joan T. Merrill, MD, of the Oklahoma Medical Research Foundation, Oklahoma City, reported in a late-breaking poster at the annual meeting of the American College of Rheumatology.
However, in a prespecified sensitivity analysis using study day 1 rather than screening visit as baseline, significantly more patients in the atacicept groups achieved SRI-4 response at week 24. For example, compared with a 41% SRI-4 response rate in the placebo group, the rate was 55.9% for the 75-mg atacicept group (odds ratio, 1.88) and 55.8% for the 150-mg atacicept group (odds ratio, 1.96), Dr. Merrill said.
Further, enhanced improvements in both SRI-4 and SRI-6 (a composite measure including reduction of at least 6 points on the SLEDAI-2K) were seen in the atacicept groups vs. the placebo group in 158 patients with high disease activity (defined as SLEDAI-2K of 10 or greater), in 84 patients with serologically active disease (those with dsDNA antibody-positive disease and low complement), and in 69 patients with both.
For example, SRI-4 response rates in patients with high disease activity were significantly greater with atacicept 150 mg vs. placebo at week 24 (62.7% vs. 42.3%; odds ratio, 2.43), and the corresponding SRI-6 rates were 54.9% vs. 28.8% with an odds ratio of 3.30.
The SRI-4 rates at week 24 in those with both high disease activity and serologically active disease who received 150 mg atacicept vs. placebo were 65% vs. 25% (odds ratio, 7.48), and the SRI-6 rates in those patients were 55% vs. 16.7% (odds ratio, 7.13).
In patients with high disease activity, severe flare was reduced with both the 75-mg and 150-mg doses of atacicept, Dr. Merrill said, noting that in the intent-to-treat population, BILAG A flare was significantly reduced with atacicept 75 mg vs. placebo, and severe flares (as measured with the SLEDAI flare index) was reduced with atacicept 150 mg.
Atacicept was associated with increased serum complement C3/C4, and with decreased IgG, IgM, IgA, and anti-dsDNA antibodies over time, she said.
Treatment-emergent adverse events occurred in similar percentages of patients in the groups (71% with placebo, 81.4% with 75 mg atacicept, and 80.8% with 150 mg atacicept). Serious treatment-emergent adverse events occurred in 11%, 8.8%, and 5.8%, respectively.
In a press statement, Dr. Merrill said the results are impressive, particularly for a small, 24-week study.
“If confirmed in future studies, this could hold exciting possibilities for our patients,” she said.
ADDRESS II was sponsored by EMD Serono. Dr. Merrill reported financial relationships with Anthera Pharmaceuticals and Lilly. Other authors reported financial relationships with EMD Serono/Merck Serono.
AT THE ACR ANNUAL MEETING
Key clinical point:
Major finding: In a prespecified sensitivity analysis, SLI-4 response was 41% with placebo, 55.9% with atacicept 75 mg (odds ratio, 1.88), and 55.8% with atacicept 150 mg (odds ratio 1.96).
Data source: The multicenter, randomized, phase IIb ADDRESS II study of 306 patients.
Disclosures: ADDRESS II was sponsored by EMD Serono. Dr. Merrill reported financial relationships with Anthera Pharmaceuticals and Lilly. Other authors reported financial relationships with EMD Serono/Merck Serono.
Higher Latitude Is Associated With Earlier Age of MS Onset
Among patients of European descent, higher latitude regions are associated with an earlier age at onset of multiple sclerosis (MS), according to data published online ahead of print November 3 in the Journal of Neurology, Neurosurgery and Psychiatry. Age at MS onset also is lower among people with less exposure to ultraviolet radiation.
Bruce V. Taylor, MBBS, Professorial Research Fellow at the University of Tasmania, Australia, and his collaborators examined data for 22,162 eligible patients participating in the international MSBase Registry. Eligible participants had MS, were age 16 or older, and came from centers of largely European descent. The investigators defined age at onset as the year of the first symptom suggestive of inflammatory CNS demyelination. They evaluated predictors of age at onset using linear regression.
Most patients in the sample were women (70.4%) and had relapsing-remitting MS (91.5%). In addition, most participants were from the northern hemisphere (81.4%), particularly Europe (67.2%), and a large proportion (15.7%) were from Australia. The sample’s mean age at MS onset was 32.3.
In the univariable analysis, latitude as a continuous linear factor was significantly negatively associated with age at onset. Every 10° increment of latitude was associated with a 0.3-year earlier onset. After adjustment for relevant covariates, patients of the highest latitude stratum had MS onset nearly 1.9 years earlier than patients of the lowest latitude stratum. In an evaluation of latitude as a continuous variable in the multivariable analysis, a 10° increase in latitude was associated with a 0.82-year earlier onset.
A similar pattern emerged for exposure to ultraviolet light. After adjustment for confounders, the investigators found a dose-dependent association between ultraviolet B and age at MS onset. In the multivariable analysis, people with the lowest ultraviolet B exposure had MS onset nearly two years earlier than people with the highest ultraviolet B exposure.
After adjustment for variables, age at MS onset was 0.43 years lower among women than among men. The latitudinal gradient of age at onset was not significantly different between men and women. Birth dates were evenly distributed in all four seasons, and the researchers found no association between season of birth and age at onset.
—Erik Greb
Suggested Reading
Tao C, Simpson S Jr, van der Mei I, et al. Higher latitude is significantly associated with an earlier age of disease onset in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2016 Nov 3 [Epub ahead of print].
Among patients of European descent, higher latitude regions are associated with an earlier age at onset of multiple sclerosis (MS), according to data published online ahead of print November 3 in the Journal of Neurology, Neurosurgery and Psychiatry. Age at MS onset also is lower among people with less exposure to ultraviolet radiation.
Bruce V. Taylor, MBBS, Professorial Research Fellow at the University of Tasmania, Australia, and his collaborators examined data for 22,162 eligible patients participating in the international MSBase Registry. Eligible participants had MS, were age 16 or older, and came from centers of largely European descent. The investigators defined age at onset as the year of the first symptom suggestive of inflammatory CNS demyelination. They evaluated predictors of age at onset using linear regression.
Most patients in the sample were women (70.4%) and had relapsing-remitting MS (91.5%). In addition, most participants were from the northern hemisphere (81.4%), particularly Europe (67.2%), and a large proportion (15.7%) were from Australia. The sample’s mean age at MS onset was 32.3.
In the univariable analysis, latitude as a continuous linear factor was significantly negatively associated with age at onset. Every 10° increment of latitude was associated with a 0.3-year earlier onset. After adjustment for relevant covariates, patients of the highest latitude stratum had MS onset nearly 1.9 years earlier than patients of the lowest latitude stratum. In an evaluation of latitude as a continuous variable in the multivariable analysis, a 10° increase in latitude was associated with a 0.82-year earlier onset.
A similar pattern emerged for exposure to ultraviolet light. After adjustment for confounders, the investigators found a dose-dependent association between ultraviolet B and age at MS onset. In the multivariable analysis, people with the lowest ultraviolet B exposure had MS onset nearly two years earlier than people with the highest ultraviolet B exposure.
After adjustment for variables, age at MS onset was 0.43 years lower among women than among men. The latitudinal gradient of age at onset was not significantly different between men and women. Birth dates were evenly distributed in all four seasons, and the researchers found no association between season of birth and age at onset.
—Erik Greb
Suggested Reading
Tao C, Simpson S Jr, van der Mei I, et al. Higher latitude is significantly associated with an earlier age of disease onset in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2016 Nov 3 [Epub ahead of print].
Among patients of European descent, higher latitude regions are associated with an earlier age at onset of multiple sclerosis (MS), according to data published online ahead of print November 3 in the Journal of Neurology, Neurosurgery and Psychiatry. Age at MS onset also is lower among people with less exposure to ultraviolet radiation.
Bruce V. Taylor, MBBS, Professorial Research Fellow at the University of Tasmania, Australia, and his collaborators examined data for 22,162 eligible patients participating in the international MSBase Registry. Eligible participants had MS, were age 16 or older, and came from centers of largely European descent. The investigators defined age at onset as the year of the first symptom suggestive of inflammatory CNS demyelination. They evaluated predictors of age at onset using linear regression.
Most patients in the sample were women (70.4%) and had relapsing-remitting MS (91.5%). In addition, most participants were from the northern hemisphere (81.4%), particularly Europe (67.2%), and a large proportion (15.7%) were from Australia. The sample’s mean age at MS onset was 32.3.
In the univariable analysis, latitude as a continuous linear factor was significantly negatively associated with age at onset. Every 10° increment of latitude was associated with a 0.3-year earlier onset. After adjustment for relevant covariates, patients of the highest latitude stratum had MS onset nearly 1.9 years earlier than patients of the lowest latitude stratum. In an evaluation of latitude as a continuous variable in the multivariable analysis, a 10° increase in latitude was associated with a 0.82-year earlier onset.
A similar pattern emerged for exposure to ultraviolet light. After adjustment for confounders, the investigators found a dose-dependent association between ultraviolet B and age at MS onset. In the multivariable analysis, people with the lowest ultraviolet B exposure had MS onset nearly two years earlier than people with the highest ultraviolet B exposure.
After adjustment for variables, age at MS onset was 0.43 years lower among women than among men. The latitudinal gradient of age at onset was not significantly different between men and women. Birth dates were evenly distributed in all four seasons, and the researchers found no association between season of birth and age at onset.
—Erik Greb
Suggested Reading
Tao C, Simpson S Jr, van der Mei I, et al. Higher latitude is significantly associated with an earlier age of disease onset in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2016 Nov 3 [Epub ahead of print].
Total Knee Arthroplasty With Retained Tibial Implants: The Role of Minimally Invasive Hardware Removal
Technique
The patient is positioned on a radiolucent table, and a mobile fluoroscopy unit is available. A tourniquet is applied to the upper thigh but typically is not inflated during the percutaneous hardware removal portion of the operation. It is crucial to have information on retained implants so the correct screwdrivers for screw removal can be selected. In addition, provisions for stripped screws should be made. In each of the 3 cases we managed, the Synthes Screw Removal Set was available. Presence of an implant system known to have problems with cold welding of screws (eg, Less Invasive Stabilization System; Synthes) may necessitate additional preparations, such as making conical extraction devices available.1
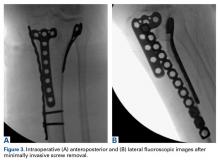
After preoperative administration of antibiotics, the surgeon typically removes only those proximal tibia screws that are preventing insertion of the tibial base plate. Fluoroscopic guidance is used to locate these screws and then remove them with percutaneous stab incisions. (Retained plates are not removed.) The exact method of localizing and removing the screws percutaneously is crucial. A small stab incision is made in the dermal layer. The number of stab incisions to be made depends on the number of screws to be removed. One small incision is needed for each screw hole. Occasionally mobilizing the skin and redirecting the screwdriver in the deep tissues can allow 2 screws to be removed through a single skin wound. The screwdriver head can be inserted through the muscle and fascial layers without the need for deep dissection. The plate is then felt with the screwdriver and the screw head located. It is very important that the screw head be adequately engaged to prevent stripping. The surgeon should not rush this step. The C-arm can be helpful here. Fluoroscopy not only can guide the screwdriver to the screw hole but can confirm the screwdriver is at right angles to the plate, not oblique. Only when the surgeon is completely satisfied that the screw head is well engaged should the attempt to back out the screw be made. If the screw strips, the screwdriver can be removed, and an attempt can be made to insert a percutaneous stripped screw removal device.1 If this fails, then the technique must be abandoned for a more traditional approach.
Plating complex tibial plateau fractures through a separate posteromedial approach is now popular.2 The deep location and screw orientation of posteromedial hardware make percutaneous removal unfeasible. In these cases, a separate posteromedial incision may be needed—usually posterior enough so it minimally compromises the anterior soft tissues. The incision typically uses the old posteromedial surgical scar but may not need to be as large as the original approach, as only selected screws need be removed. The saphenous neurovascular bundle may still be at risk, depending on the location of these incisions. The plate is not removed.
After the necessary screws are removed, the tourniquet can be inflated, if desired. The total knee arthroplasty (TKA) then proceeds in usual fashion through a single incision and a medial parapatellar arthrotomy.
Results
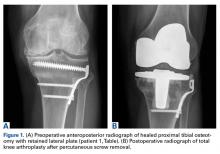
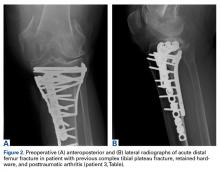
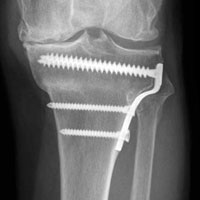
Between January 2009 and February 2014, Dr. Georgiadis converted 3 cases of retained tibial hardware and severe knee arthrosis to a TKA in a single operation. These cases were reviewed after Institutional Review Board approval was obtained. One patient underwent a closing-wedge high tibial osteotomy 14 years earlier, and the other 2 sustained tibial plateau fractures. Clinical details of the 3 cases are presented in the Table.
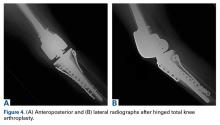
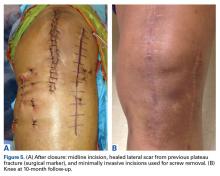
In 2 of the cases, anterolateral surgical scars were present. New, separate percutaneous stab incisions were used to remove screws, which meant less of the original skin incision could be used for the TKA (Figures 1A, 1B).
In the third case, involving multiple plates, a similar strategy was used, but an additional small posteromedial incision was required (Figures 2-5). The TKA then proceeded through a new midline incision. This case was performed for tibiofemoral arthrosis in the setting of an acute distal femur fracture, but this had no bearing on the technique.
Tibial base plates were inserted in the usual manner. Length and type of tibial stem were left to the discretion of the surgeon. There were no changes from the usual surgical technique. All patients went on to routine, uneventful wound healing. Follow-up ranged from 10 months to 59 months.
Discussion
If the decision is made to proceed with TKA after previous knee surgery, careful preoperative planning is needed.
For young patients with knee arthrosis and angular deformity, it has been recommended that proximal tibial osteotomy be performed to delay the need for joint replacement.3,4 Although a wide variety of osteotomy techniques is available, plates and screws are often used. With long-term follow-up, knee arthrosis can be expected to progress, and some of these cases will be converted to knee arthroplasty.3,4Displaced tibial plateau fractures are intra-articular injuries. Treatment requires surgery.
Blood work for inflammatory markers (erythrocyte sedimentation rate, C-reactive protein level) should be performed before surgery. In the event of an elevated laboratory value or clinical suspicion (joint effusion), the joint should be aspirated before any arthroplasty procedure.
Preoperative planning for hardware removal is essential.22 The correct screwdriver and a metal cutting burr (for stripped screws) should be available. These needs may be anticipated with certain types of locking plates.1
Surgical incision planning is also crucial in preventing wound problems that can lead to deep prosthetic infection.23,24 Blood supply to the skin of the anterior knee is primarily medially derived; incisions that are more medial put lateral skin flaps at risk.25 Use of the most recently healed or previous lateral-based scars has been recommended. In cases of adherent skin or poor soft-tissue envelope, plastic surgery (eg, soft-tissue expansion, gastrocnemius muscle, fasciocutaneous flaps) may be necessary.26-28Surgeons must decide to perform either a single operation or a multiple-stage operation. Naturally, most patients prefer a single procedure. All previous hardware can be removed, or only the hardware that is preventing insertion of the tibial base plate. Removing the least amount of hardware is advantageous in that surgical stripping and soft-tissue damage are reduced.
In this initial series, we successfully converted 3 tibial implants to TKAs (each as a single operation) by removing only screws in percutaneous or minimally invasive fashion—the prosthetic joint approach did not involve additional soft-tissue stripping. We did not specifically record the time needed for implant removal separately from the time needed for TKA. As the Table shows, this technique can lengthen surgery. Operative time and blood loss can be more variable because of numerous factors, including scar tissue and an altered surgical field from previous surgery, in addition to hardware removal difficulties. Therefore, surgeons should budget more operative time for these procedures. Although longer operations theoretically may increase infection rates, we think the risk is mitigated by the percutaneous aspects of the described technique.
We do not think that most orthopedic surgeons addressing retained plate–screw constructs consider minimally invasive screw removal and plate retention. To our knowledge, the literature includes only 1 case report of a similar technique.29This technique has many potential drawbacks, the foremost being use of intraoperative fluoroscopy. For more complex fractures, fluoroscopy time can be significant if the surgeon is committed to a true percutaneous approach (Table). In addition, use of a mobile fluoroscopy unit adds personnel to the operating theater, which potentially increases the infection rate. There may be cases in which tibial hardware interferes with tibial cuts, necessitating plate removal, but we did not encounter this in our series. This technique is potentially time-consuming. Operating room time can be expected to increase relative to wide exposures that allow quick access to existing implants. For this reason, some surgeons may decide to forgo this technique. Most modern proximal tibial fracture plates are contoured to fit well over the bone. However, some may still be prominent, and surgeons may choose to perform an open approach to remove them. Last, the clinical impact of plates retained without screws in the proximal tibia is not known. Theoretically, they may still act as a nidus for occult infection, and may act as a stress riser for peri-implant fracture. Therefore, for each patient, the surgeon must decide if the extra surgical time, fluoroscopy exposure, and plate retention are worthwhile.
In this 3-case series, screws were removed percutaneously over the proximal tibia. There were no neurovascular injuries in these cases, though there is potential for nerve and artery injuries with percutaneous screw removal, as in the anterolateral area of the distal third of the tibia.30,31 Thus, our technique may not be applicable in such cases. Most patients with plates and screws retained after proximal tibial surgery do not need to have the screws removed from the distal tibia. There also is the potential for saphenous nerve injury if a small medial or posteromedial incision is made. No such injury occurred in our small series.
Surgeons must consider many factors when deciding whether to proceed with TKA in the setting of existing tibial hardware. If staged reconstruction is not planned, consideration can be given to percutaneous screw removal without plate removal in an attempt to minimize further soft-tissue stripping. This has the theoretical advantage of decreasing wound complications. We have been pleased with our initial patient experience and continue to use this technique.
Am J Orthop. 2016;45(7):E481-E486. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Georgiadis GM, Gove NK, Smith AD, Rodway IP. Removal of the Less Invasive Stabilization System. J Orthop Trauma. 2004;18(8):562-564.
2. Georgiadis GM. Combined anterior and posterior approaches for complex tibial plateau fractures. J Bone Joint Surg Br. 1994;76(2):285-289.
3. Insall JN, Joseph DM, Msika C. High tibial osteotomy for varus gonarthrosis. A long-term follow-up study. J Bone Joint Surg Am. 1984;66(7):1040-1048.
4. Sprenger TR, Doerzbacher JF. Tibial osteotomy for the treatment of varus gonarthrosis. Survival and failure analysis to twenty-two years. J Bone Joint Surg Am. 2003;85(3):469-474.
5. Moore TM, Patzakis MJ, Harvey JP. Tibial plateau fractures: definition, demographics, treatment rationale, and long-term results of closed traction management or operative reduction. J Orthop Trauma. 1987;1(2):97-119.
6. Shah SN, Karunakar MA. Early wound complications after operative treatment of high energy tibial plateau fractures through two incisions. Bull NYU Hosp Joint Dis. 2007;65(2):115-119.
7. Yang EC, Weiner L, Strauss E, Sedin E, Kelley M, Raphael J. Metaphyseal dissociation fractures of the proximal tibia. An analysis of treatment and complications. Am J Orthop. 1995;24(9):695-704.
8. Young MJ, Barrack RL. Complications of internal fixation of tibial plateau fractures. Orthop Rev. 1994;23(2):149-154.
9. Luo CF, Sun H, Zhang B, Zeng BF. Three-column fixation for complex tibial plateau fractures. J Orthop Trauma. 2010;24(11):683-692.
10. Barei DP, Nork SE, Mills WJ, Henley MB, Benirschke SK. Complications associated with internal fixation of high-energy bicondylar tibial plateau fractures utilizing a two-incision technique. J Orthop Trauma. 2004;18(10):649-657.
11. Ruffolo MR, Gettys FK, Montijo HE, Seymour RB, Karunakar MA. Complications of high-energy bicondylar tibial plateau fractures treated with dual plating through 2 incisions. J Orthop Trauma. 2015;29(2):85-90.
12. Honkonen SE. Degenerative arthritis after tibial plateau fractures. J Orthop Trauma. 1995;9(4):273-277.
13. Volpin G, Dowd GS, Stein H, Bentley G. Degenerative arthritis after intra-articular fractures of the knee. Long-term results. J Bone Joint Surg Br. 1990;72(4):634-638.
14. Mehin R, O’Brien P, Broekhuyse H, Blachut P, Guy P. Endstage arthritis following tibia plateau fractures: average 10-year follow-up. Can J Surg. 2012;55(2):87-94.
15. Wasserstein D, Henry P, Paterson JM, Kreder HJ, Jenkinson R. Risk of total knee arthroplasty after operatively treated tibial plateau fracture: a matched-population-based cohort study. J Bone Joint Surg Am. 2014;96(2):144-150.
16. Meding JB, Keating EM, Ritter MA, Faris PM. Total knee arthroplasty after high tibial osteotomy. A comparison study in patients who had bilateral total knee replacement. J Bone Joint Surg Am. 2000;82(9):1252-1259.
17. Parvizi J, Hanssen AD, Spangheli MJ. Total knee arthroplasty following proximal tibial osteotomy: risk factors for failure. J Bone Joint Surg Am. 2004;86(3):474-479.
18. Windsor RE, Insall JN, Vince KG. Technical considerations of total knee arthroplasty after proximal tibial osteotomy. J Bone Joint Surg Am. 1988;70(4):547-555.
19. Civinini R, Carulli C, Matassi F, Villano M, Innocenti M. Total knee arthroplasty after complex tibial plateau fractures. Chir Organi Mov. 2009;93(3):143-147.
20. Saleh KJ, Sherman P, Katkin P, et al. Total knee arthroplasty after open reduction and internal fixation of fractures of the tibial plateau: a minimum five-year follow-up study. J Bone Joint Surg Am. 2001;83(8):1144-1148.
21. Weiss NG, Parvizi J, Trousdale RT, Bryce RD, Lewallen DG. Total knee arthroplasty in patients with a prior fracture of the tibial plateau. J Bone Joint Surg Am. 2003;85(2):218-221.
22. Hak DJ, McElvany M. Removal of broken hardware. J Am Acad Orthop Surg. 2008:16(2):113-120.
23. Della Valle CJ, Berger RA, Rosenberg AG. Surgical exposures in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;(446):59-68.
24. Vince KG, Abdeen A. Wound problems in total knee arthroplasty. Clin Orthop Relat Res. 2006;(452):88-90.
25. Colombel M, Mariz Y, Dahhan P, Kénési C. Arterial and lymphatic supply of the knee integuments. Surg Radiol Anat. 1998;20(1):35-40.
26. Namba RS, Diao E. Tissue expansion for staged reimplantation of infected total knee arthroplasty. J Arthroplasty. 1997;12(4):471-474.
27. Markovich GD, Dorr LD, Klein NE, McPherson EJ, Vince KG. Muscle flaps in total knee arthroplasty. Clin Orthop Relat Res. 1995;(321):122-130.
28. Hallock GG. Salvage of total knee arthroplasty with local fasciocutaneous flaps. J Bone Joint Surg Am. 1990;72(8):1236-1239.
29. Roswell M, Gale D. Total knee arthroplasty following internal fixation of a lateral tibial plateau fracture. Injury Extra. 2005;36(8):352-354.
30. Deangelis JP, Deangelis NA, Anderson R. Anatomy of the superficial peroneal nerve in relation to fixation of tibia fractures with the Less Invasive Stabilization System. J Orthop Trauma. 2004;18(8):536-539.
31. Pichler W, Grechenig W, Tesch NP, Weinberg AM, Heidari N, Clement H. The risk of iatrogenic injury to the deep peroneal nerve in minimally invasive osteosynthesis of the tibia with the Less Invasive Stabilisation System: a cadaver study. J Bone Joint Surg Br. 2009;91(3):385-387.
Technique
The patient is positioned on a radiolucent table, and a mobile fluoroscopy unit is available. A tourniquet is applied to the upper thigh but typically is not inflated during the percutaneous hardware removal portion of the operation. It is crucial to have information on retained implants so the correct screwdrivers for screw removal can be selected. In addition, provisions for stripped screws should be made. In each of the 3 cases we managed, the Synthes Screw Removal Set was available. Presence of an implant system known to have problems with cold welding of screws (eg, Less Invasive Stabilization System; Synthes) may necessitate additional preparations, such as making conical extraction devices available.1
After preoperative administration of antibiotics, the surgeon typically removes only those proximal tibia screws that are preventing insertion of the tibial base plate. Fluoroscopic guidance is used to locate these screws and then remove them with percutaneous stab incisions. (Retained plates are not removed.) The exact method of localizing and removing the screws percutaneously is crucial. A small stab incision is made in the dermal layer. The number of stab incisions to be made depends on the number of screws to be removed. One small incision is needed for each screw hole. Occasionally mobilizing the skin and redirecting the screwdriver in the deep tissues can allow 2 screws to be removed through a single skin wound. The screwdriver head can be inserted through the muscle and fascial layers without the need for deep dissection. The plate is then felt with the screwdriver and the screw head located. It is very important that the screw head be adequately engaged to prevent stripping. The surgeon should not rush this step. The C-arm can be helpful here. Fluoroscopy not only can guide the screwdriver to the screw hole but can confirm the screwdriver is at right angles to the plate, not oblique. Only when the surgeon is completely satisfied that the screw head is well engaged should the attempt to back out the screw be made. If the screw strips, the screwdriver can be removed, and an attempt can be made to insert a percutaneous stripped screw removal device.1 If this fails, then the technique must be abandoned for a more traditional approach.
Plating complex tibial plateau fractures through a separate posteromedial approach is now popular.2 The deep location and screw orientation of posteromedial hardware make percutaneous removal unfeasible. In these cases, a separate posteromedial incision may be needed—usually posterior enough so it minimally compromises the anterior soft tissues. The incision typically uses the old posteromedial surgical scar but may not need to be as large as the original approach, as only selected screws need be removed. The saphenous neurovascular bundle may still be at risk, depending on the location of these incisions. The plate is not removed.
After the necessary screws are removed, the tourniquet can be inflated, if desired. The total knee arthroplasty (TKA) then proceeds in usual fashion through a single incision and a medial parapatellar arthrotomy.
Results
Between January 2009 and February 2014, Dr. Georgiadis converted 3 cases of retained tibial hardware and severe knee arthrosis to a TKA in a single operation. These cases were reviewed after Institutional Review Board approval was obtained. One patient underwent a closing-wedge high tibial osteotomy 14 years earlier, and the other 2 sustained tibial plateau fractures. Clinical details of the 3 cases are presented in the Table.
In 2 of the cases, anterolateral surgical scars were present. New, separate percutaneous stab incisions were used to remove screws, which meant less of the original skin incision could be used for the TKA (Figures 1A, 1B).
In the third case, involving multiple plates, a similar strategy was used, but an additional small posteromedial incision was required (Figures 2-5). The TKA then proceeded through a new midline incision. This case was performed for tibiofemoral arthrosis in the setting of an acute distal femur fracture, but this had no bearing on the technique.
Tibial base plates were inserted in the usual manner. Length and type of tibial stem were left to the discretion of the surgeon. There were no changes from the usual surgical technique. All patients went on to routine, uneventful wound healing. Follow-up ranged from 10 months to 59 months.
Discussion
If the decision is made to proceed with TKA after previous knee surgery, careful preoperative planning is needed.
For young patients with knee arthrosis and angular deformity, it has been recommended that proximal tibial osteotomy be performed to delay the need for joint replacement.3,4 Although a wide variety of osteotomy techniques is available, plates and screws are often used. With long-term follow-up, knee arthrosis can be expected to progress, and some of these cases will be converted to knee arthroplasty.3,4Displaced tibial plateau fractures are intra-articular injuries. Treatment requires surgery.
Blood work for inflammatory markers (erythrocyte sedimentation rate, C-reactive protein level) should be performed before surgery. In the event of an elevated laboratory value or clinical suspicion (joint effusion), the joint should be aspirated before any arthroplasty procedure.
Preoperative planning for hardware removal is essential.22 The correct screwdriver and a metal cutting burr (for stripped screws) should be available. These needs may be anticipated with certain types of locking plates.1
Surgical incision planning is also crucial in preventing wound problems that can lead to deep prosthetic infection.23,24 Blood supply to the skin of the anterior knee is primarily medially derived; incisions that are more medial put lateral skin flaps at risk.25 Use of the most recently healed or previous lateral-based scars has been recommended. In cases of adherent skin or poor soft-tissue envelope, plastic surgery (eg, soft-tissue expansion, gastrocnemius muscle, fasciocutaneous flaps) may be necessary.26-28Surgeons must decide to perform either a single operation or a multiple-stage operation. Naturally, most patients prefer a single procedure. All previous hardware can be removed, or only the hardware that is preventing insertion of the tibial base plate. Removing the least amount of hardware is advantageous in that surgical stripping and soft-tissue damage are reduced.
In this initial series, we successfully converted 3 tibial implants to TKAs (each as a single operation) by removing only screws in percutaneous or minimally invasive fashion—the prosthetic joint approach did not involve additional soft-tissue stripping. We did not specifically record the time needed for implant removal separately from the time needed for TKA. As the Table shows, this technique can lengthen surgery. Operative time and blood loss can be more variable because of numerous factors, including scar tissue and an altered surgical field from previous surgery, in addition to hardware removal difficulties. Therefore, surgeons should budget more operative time for these procedures. Although longer operations theoretically may increase infection rates, we think the risk is mitigated by the percutaneous aspects of the described technique.
We do not think that most orthopedic surgeons addressing retained plate–screw constructs consider minimally invasive screw removal and plate retention. To our knowledge, the literature includes only 1 case report of a similar technique.29This technique has many potential drawbacks, the foremost being use of intraoperative fluoroscopy. For more complex fractures, fluoroscopy time can be significant if the surgeon is committed to a true percutaneous approach (Table). In addition, use of a mobile fluoroscopy unit adds personnel to the operating theater, which potentially increases the infection rate. There may be cases in which tibial hardware interferes with tibial cuts, necessitating plate removal, but we did not encounter this in our series. This technique is potentially time-consuming. Operating room time can be expected to increase relative to wide exposures that allow quick access to existing implants. For this reason, some surgeons may decide to forgo this technique. Most modern proximal tibial fracture plates are contoured to fit well over the bone. However, some may still be prominent, and surgeons may choose to perform an open approach to remove them. Last, the clinical impact of plates retained without screws in the proximal tibia is not known. Theoretically, they may still act as a nidus for occult infection, and may act as a stress riser for peri-implant fracture. Therefore, for each patient, the surgeon must decide if the extra surgical time, fluoroscopy exposure, and plate retention are worthwhile.
In this 3-case series, screws were removed percutaneously over the proximal tibia. There were no neurovascular injuries in these cases, though there is potential for nerve and artery injuries with percutaneous screw removal, as in the anterolateral area of the distal third of the tibia.30,31 Thus, our technique may not be applicable in such cases. Most patients with plates and screws retained after proximal tibial surgery do not need to have the screws removed from the distal tibia. There also is the potential for saphenous nerve injury if a small medial or posteromedial incision is made. No such injury occurred in our small series.
Surgeons must consider many factors when deciding whether to proceed with TKA in the setting of existing tibial hardware. If staged reconstruction is not planned, consideration can be given to percutaneous screw removal without plate removal in an attempt to minimize further soft-tissue stripping. This has the theoretical advantage of decreasing wound complications. We have been pleased with our initial patient experience and continue to use this technique.
Am J Orthop. 2016;45(7):E481-E486. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Technique
The patient is positioned on a radiolucent table, and a mobile fluoroscopy unit is available. A tourniquet is applied to the upper thigh but typically is not inflated during the percutaneous hardware removal portion of the operation. It is crucial to have information on retained implants so the correct screwdrivers for screw removal can be selected. In addition, provisions for stripped screws should be made. In each of the 3 cases we managed, the Synthes Screw Removal Set was available. Presence of an implant system known to have problems with cold welding of screws (eg, Less Invasive Stabilization System; Synthes) may necessitate additional preparations, such as making conical extraction devices available.1
After preoperative administration of antibiotics, the surgeon typically removes only those proximal tibia screws that are preventing insertion of the tibial base plate. Fluoroscopic guidance is used to locate these screws and then remove them with percutaneous stab incisions. (Retained plates are not removed.) The exact method of localizing and removing the screws percutaneously is crucial. A small stab incision is made in the dermal layer. The number of stab incisions to be made depends on the number of screws to be removed. One small incision is needed for each screw hole. Occasionally mobilizing the skin and redirecting the screwdriver in the deep tissues can allow 2 screws to be removed through a single skin wound. The screwdriver head can be inserted through the muscle and fascial layers without the need for deep dissection. The plate is then felt with the screwdriver and the screw head located. It is very important that the screw head be adequately engaged to prevent stripping. The surgeon should not rush this step. The C-arm can be helpful here. Fluoroscopy not only can guide the screwdriver to the screw hole but can confirm the screwdriver is at right angles to the plate, not oblique. Only when the surgeon is completely satisfied that the screw head is well engaged should the attempt to back out the screw be made. If the screw strips, the screwdriver can be removed, and an attempt can be made to insert a percutaneous stripped screw removal device.1 If this fails, then the technique must be abandoned for a more traditional approach.
Plating complex tibial plateau fractures through a separate posteromedial approach is now popular.2 The deep location and screw orientation of posteromedial hardware make percutaneous removal unfeasible. In these cases, a separate posteromedial incision may be needed—usually posterior enough so it minimally compromises the anterior soft tissues. The incision typically uses the old posteromedial surgical scar but may not need to be as large as the original approach, as only selected screws need be removed. The saphenous neurovascular bundle may still be at risk, depending on the location of these incisions. The plate is not removed.
After the necessary screws are removed, the tourniquet can be inflated, if desired. The total knee arthroplasty (TKA) then proceeds in usual fashion through a single incision and a medial parapatellar arthrotomy.
Results
Between January 2009 and February 2014, Dr. Georgiadis converted 3 cases of retained tibial hardware and severe knee arthrosis to a TKA in a single operation. These cases were reviewed after Institutional Review Board approval was obtained. One patient underwent a closing-wedge high tibial osteotomy 14 years earlier, and the other 2 sustained tibial plateau fractures. Clinical details of the 3 cases are presented in the Table.
In 2 of the cases, anterolateral surgical scars were present. New, separate percutaneous stab incisions were used to remove screws, which meant less of the original skin incision could be used for the TKA (Figures 1A, 1B).
In the third case, involving multiple plates, a similar strategy was used, but an additional small posteromedial incision was required (Figures 2-5). The TKA then proceeded through a new midline incision. This case was performed for tibiofemoral arthrosis in the setting of an acute distal femur fracture, but this had no bearing on the technique.
Tibial base plates were inserted in the usual manner. Length and type of tibial stem were left to the discretion of the surgeon. There were no changes from the usual surgical technique. All patients went on to routine, uneventful wound healing. Follow-up ranged from 10 months to 59 months.
Discussion
If the decision is made to proceed with TKA after previous knee surgery, careful preoperative planning is needed.
For young patients with knee arthrosis and angular deformity, it has been recommended that proximal tibial osteotomy be performed to delay the need for joint replacement.3,4 Although a wide variety of osteotomy techniques is available, plates and screws are often used. With long-term follow-up, knee arthrosis can be expected to progress, and some of these cases will be converted to knee arthroplasty.3,4Displaced tibial plateau fractures are intra-articular injuries. Treatment requires surgery.
Blood work for inflammatory markers (erythrocyte sedimentation rate, C-reactive protein level) should be performed before surgery. In the event of an elevated laboratory value or clinical suspicion (joint effusion), the joint should be aspirated before any arthroplasty procedure.
Preoperative planning for hardware removal is essential.22 The correct screwdriver and a metal cutting burr (for stripped screws) should be available. These needs may be anticipated with certain types of locking plates.1
Surgical incision planning is also crucial in preventing wound problems that can lead to deep prosthetic infection.23,24 Blood supply to the skin of the anterior knee is primarily medially derived; incisions that are more medial put lateral skin flaps at risk.25 Use of the most recently healed or previous lateral-based scars has been recommended. In cases of adherent skin or poor soft-tissue envelope, plastic surgery (eg, soft-tissue expansion, gastrocnemius muscle, fasciocutaneous flaps) may be necessary.26-28Surgeons must decide to perform either a single operation or a multiple-stage operation. Naturally, most patients prefer a single procedure. All previous hardware can be removed, or only the hardware that is preventing insertion of the tibial base plate. Removing the least amount of hardware is advantageous in that surgical stripping and soft-tissue damage are reduced.
In this initial series, we successfully converted 3 tibial implants to TKAs (each as a single operation) by removing only screws in percutaneous or minimally invasive fashion—the prosthetic joint approach did not involve additional soft-tissue stripping. We did not specifically record the time needed for implant removal separately from the time needed for TKA. As the Table shows, this technique can lengthen surgery. Operative time and blood loss can be more variable because of numerous factors, including scar tissue and an altered surgical field from previous surgery, in addition to hardware removal difficulties. Therefore, surgeons should budget more operative time for these procedures. Although longer operations theoretically may increase infection rates, we think the risk is mitigated by the percutaneous aspects of the described technique.
We do not think that most orthopedic surgeons addressing retained plate–screw constructs consider minimally invasive screw removal and plate retention. To our knowledge, the literature includes only 1 case report of a similar technique.29This technique has many potential drawbacks, the foremost being use of intraoperative fluoroscopy. For more complex fractures, fluoroscopy time can be significant if the surgeon is committed to a true percutaneous approach (Table). In addition, use of a mobile fluoroscopy unit adds personnel to the operating theater, which potentially increases the infection rate. There may be cases in which tibial hardware interferes with tibial cuts, necessitating plate removal, but we did not encounter this in our series. This technique is potentially time-consuming. Operating room time can be expected to increase relative to wide exposures that allow quick access to existing implants. For this reason, some surgeons may decide to forgo this technique. Most modern proximal tibial fracture plates are contoured to fit well over the bone. However, some may still be prominent, and surgeons may choose to perform an open approach to remove them. Last, the clinical impact of plates retained without screws in the proximal tibia is not known. Theoretically, they may still act as a nidus for occult infection, and may act as a stress riser for peri-implant fracture. Therefore, for each patient, the surgeon must decide if the extra surgical time, fluoroscopy exposure, and plate retention are worthwhile.
In this 3-case series, screws were removed percutaneously over the proximal tibia. There were no neurovascular injuries in these cases, though there is potential for nerve and artery injuries with percutaneous screw removal, as in the anterolateral area of the distal third of the tibia.30,31 Thus, our technique may not be applicable in such cases. Most patients with plates and screws retained after proximal tibial surgery do not need to have the screws removed from the distal tibia. There also is the potential for saphenous nerve injury if a small medial or posteromedial incision is made. No such injury occurred in our small series.
Surgeons must consider many factors when deciding whether to proceed with TKA in the setting of existing tibial hardware. If staged reconstruction is not planned, consideration can be given to percutaneous screw removal without plate removal in an attempt to minimize further soft-tissue stripping. This has the theoretical advantage of decreasing wound complications. We have been pleased with our initial patient experience and continue to use this technique.
Am J Orthop. 2016;45(7):E481-E486. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Georgiadis GM, Gove NK, Smith AD, Rodway IP. Removal of the Less Invasive Stabilization System. J Orthop Trauma. 2004;18(8):562-564.
2. Georgiadis GM. Combined anterior and posterior approaches for complex tibial plateau fractures. J Bone Joint Surg Br. 1994;76(2):285-289.
3. Insall JN, Joseph DM, Msika C. High tibial osteotomy for varus gonarthrosis. A long-term follow-up study. J Bone Joint Surg Am. 1984;66(7):1040-1048.
4. Sprenger TR, Doerzbacher JF. Tibial osteotomy for the treatment of varus gonarthrosis. Survival and failure analysis to twenty-two years. J Bone Joint Surg Am. 2003;85(3):469-474.
5. Moore TM, Patzakis MJ, Harvey JP. Tibial plateau fractures: definition, demographics, treatment rationale, and long-term results of closed traction management or operative reduction. J Orthop Trauma. 1987;1(2):97-119.
6. Shah SN, Karunakar MA. Early wound complications after operative treatment of high energy tibial plateau fractures through two incisions. Bull NYU Hosp Joint Dis. 2007;65(2):115-119.
7. Yang EC, Weiner L, Strauss E, Sedin E, Kelley M, Raphael J. Metaphyseal dissociation fractures of the proximal tibia. An analysis of treatment and complications. Am J Orthop. 1995;24(9):695-704.
8. Young MJ, Barrack RL. Complications of internal fixation of tibial plateau fractures. Orthop Rev. 1994;23(2):149-154.
9. Luo CF, Sun H, Zhang B, Zeng BF. Three-column fixation for complex tibial plateau fractures. J Orthop Trauma. 2010;24(11):683-692.
10. Barei DP, Nork SE, Mills WJ, Henley MB, Benirschke SK. Complications associated with internal fixation of high-energy bicondylar tibial plateau fractures utilizing a two-incision technique. J Orthop Trauma. 2004;18(10):649-657.
11. Ruffolo MR, Gettys FK, Montijo HE, Seymour RB, Karunakar MA. Complications of high-energy bicondylar tibial plateau fractures treated with dual plating through 2 incisions. J Orthop Trauma. 2015;29(2):85-90.
12. Honkonen SE. Degenerative arthritis after tibial plateau fractures. J Orthop Trauma. 1995;9(4):273-277.
13. Volpin G, Dowd GS, Stein H, Bentley G. Degenerative arthritis after intra-articular fractures of the knee. Long-term results. J Bone Joint Surg Br. 1990;72(4):634-638.
14. Mehin R, O’Brien P, Broekhuyse H, Blachut P, Guy P. Endstage arthritis following tibia plateau fractures: average 10-year follow-up. Can J Surg. 2012;55(2):87-94.
15. Wasserstein D, Henry P, Paterson JM, Kreder HJ, Jenkinson R. Risk of total knee arthroplasty after operatively treated tibial plateau fracture: a matched-population-based cohort study. J Bone Joint Surg Am. 2014;96(2):144-150.
16. Meding JB, Keating EM, Ritter MA, Faris PM. Total knee arthroplasty after high tibial osteotomy. A comparison study in patients who had bilateral total knee replacement. J Bone Joint Surg Am. 2000;82(9):1252-1259.
17. Parvizi J, Hanssen AD, Spangheli MJ. Total knee arthroplasty following proximal tibial osteotomy: risk factors for failure. J Bone Joint Surg Am. 2004;86(3):474-479.
18. Windsor RE, Insall JN, Vince KG. Technical considerations of total knee arthroplasty after proximal tibial osteotomy. J Bone Joint Surg Am. 1988;70(4):547-555.
19. Civinini R, Carulli C, Matassi F, Villano M, Innocenti M. Total knee arthroplasty after complex tibial plateau fractures. Chir Organi Mov. 2009;93(3):143-147.
20. Saleh KJ, Sherman P, Katkin P, et al. Total knee arthroplasty after open reduction and internal fixation of fractures of the tibial plateau: a minimum five-year follow-up study. J Bone Joint Surg Am. 2001;83(8):1144-1148.
21. Weiss NG, Parvizi J, Trousdale RT, Bryce RD, Lewallen DG. Total knee arthroplasty in patients with a prior fracture of the tibial plateau. J Bone Joint Surg Am. 2003;85(2):218-221.
22. Hak DJ, McElvany M. Removal of broken hardware. J Am Acad Orthop Surg. 2008:16(2):113-120.
23. Della Valle CJ, Berger RA, Rosenberg AG. Surgical exposures in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;(446):59-68.
24. Vince KG, Abdeen A. Wound problems in total knee arthroplasty. Clin Orthop Relat Res. 2006;(452):88-90.
25. Colombel M, Mariz Y, Dahhan P, Kénési C. Arterial and lymphatic supply of the knee integuments. Surg Radiol Anat. 1998;20(1):35-40.
26. Namba RS, Diao E. Tissue expansion for staged reimplantation of infected total knee arthroplasty. J Arthroplasty. 1997;12(4):471-474.
27. Markovich GD, Dorr LD, Klein NE, McPherson EJ, Vince KG. Muscle flaps in total knee arthroplasty. Clin Orthop Relat Res. 1995;(321):122-130.
28. Hallock GG. Salvage of total knee arthroplasty with local fasciocutaneous flaps. J Bone Joint Surg Am. 1990;72(8):1236-1239.
29. Roswell M, Gale D. Total knee arthroplasty following internal fixation of a lateral tibial plateau fracture. Injury Extra. 2005;36(8):352-354.
30. Deangelis JP, Deangelis NA, Anderson R. Anatomy of the superficial peroneal nerve in relation to fixation of tibia fractures with the Less Invasive Stabilization System. J Orthop Trauma. 2004;18(8):536-539.
31. Pichler W, Grechenig W, Tesch NP, Weinberg AM, Heidari N, Clement H. The risk of iatrogenic injury to the deep peroneal nerve in minimally invasive osteosynthesis of the tibia with the Less Invasive Stabilisation System: a cadaver study. J Bone Joint Surg Br. 2009;91(3):385-387.
1. Georgiadis GM, Gove NK, Smith AD, Rodway IP. Removal of the Less Invasive Stabilization System. J Orthop Trauma. 2004;18(8):562-564.
2. Georgiadis GM. Combined anterior and posterior approaches for complex tibial plateau fractures. J Bone Joint Surg Br. 1994;76(2):285-289.
3. Insall JN, Joseph DM, Msika C. High tibial osteotomy for varus gonarthrosis. A long-term follow-up study. J Bone Joint Surg Am. 1984;66(7):1040-1048.
4. Sprenger TR, Doerzbacher JF. Tibial osteotomy for the treatment of varus gonarthrosis. Survival and failure analysis to twenty-two years. J Bone Joint Surg Am. 2003;85(3):469-474.
5. Moore TM, Patzakis MJ, Harvey JP. Tibial plateau fractures: definition, demographics, treatment rationale, and long-term results of closed traction management or operative reduction. J Orthop Trauma. 1987;1(2):97-119.
6. Shah SN, Karunakar MA. Early wound complications after operative treatment of high energy tibial plateau fractures through two incisions. Bull NYU Hosp Joint Dis. 2007;65(2):115-119.
7. Yang EC, Weiner L, Strauss E, Sedin E, Kelley M, Raphael J. Metaphyseal dissociation fractures of the proximal tibia. An analysis of treatment and complications. Am J Orthop. 1995;24(9):695-704.
8. Young MJ, Barrack RL. Complications of internal fixation of tibial plateau fractures. Orthop Rev. 1994;23(2):149-154.
9. Luo CF, Sun H, Zhang B, Zeng BF. Three-column fixation for complex tibial plateau fractures. J Orthop Trauma. 2010;24(11):683-692.
10. Barei DP, Nork SE, Mills WJ, Henley MB, Benirschke SK. Complications associated with internal fixation of high-energy bicondylar tibial plateau fractures utilizing a two-incision technique. J Orthop Trauma. 2004;18(10):649-657.
11. Ruffolo MR, Gettys FK, Montijo HE, Seymour RB, Karunakar MA. Complications of high-energy bicondylar tibial plateau fractures treated with dual plating through 2 incisions. J Orthop Trauma. 2015;29(2):85-90.
12. Honkonen SE. Degenerative arthritis after tibial plateau fractures. J Orthop Trauma. 1995;9(4):273-277.
13. Volpin G, Dowd GS, Stein H, Bentley G. Degenerative arthritis after intra-articular fractures of the knee. Long-term results. J Bone Joint Surg Br. 1990;72(4):634-638.
14. Mehin R, O’Brien P, Broekhuyse H, Blachut P, Guy P. Endstage arthritis following tibia plateau fractures: average 10-year follow-up. Can J Surg. 2012;55(2):87-94.
15. Wasserstein D, Henry P, Paterson JM, Kreder HJ, Jenkinson R. Risk of total knee arthroplasty after operatively treated tibial plateau fracture: a matched-population-based cohort study. J Bone Joint Surg Am. 2014;96(2):144-150.
16. Meding JB, Keating EM, Ritter MA, Faris PM. Total knee arthroplasty after high tibial osteotomy. A comparison study in patients who had bilateral total knee replacement. J Bone Joint Surg Am. 2000;82(9):1252-1259.
17. Parvizi J, Hanssen AD, Spangheli MJ. Total knee arthroplasty following proximal tibial osteotomy: risk factors for failure. J Bone Joint Surg Am. 2004;86(3):474-479.
18. Windsor RE, Insall JN, Vince KG. Technical considerations of total knee arthroplasty after proximal tibial osteotomy. J Bone Joint Surg Am. 1988;70(4):547-555.
19. Civinini R, Carulli C, Matassi F, Villano M, Innocenti M. Total knee arthroplasty after complex tibial plateau fractures. Chir Organi Mov. 2009;93(3):143-147.
20. Saleh KJ, Sherman P, Katkin P, et al. Total knee arthroplasty after open reduction and internal fixation of fractures of the tibial plateau: a minimum five-year follow-up study. J Bone Joint Surg Am. 2001;83(8):1144-1148.
21. Weiss NG, Parvizi J, Trousdale RT, Bryce RD, Lewallen DG. Total knee arthroplasty in patients with a prior fracture of the tibial plateau. J Bone Joint Surg Am. 2003;85(2):218-221.
22. Hak DJ, McElvany M. Removal of broken hardware. J Am Acad Orthop Surg. 2008:16(2):113-120.
23. Della Valle CJ, Berger RA, Rosenberg AG. Surgical exposures in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;(446):59-68.
24. Vince KG, Abdeen A. Wound problems in total knee arthroplasty. Clin Orthop Relat Res. 2006;(452):88-90.
25. Colombel M, Mariz Y, Dahhan P, Kénési C. Arterial and lymphatic supply of the knee integuments. Surg Radiol Anat. 1998;20(1):35-40.
26. Namba RS, Diao E. Tissue expansion for staged reimplantation of infected total knee arthroplasty. J Arthroplasty. 1997;12(4):471-474.
27. Markovich GD, Dorr LD, Klein NE, McPherson EJ, Vince KG. Muscle flaps in total knee arthroplasty. Clin Orthop Relat Res. 1995;(321):122-130.
28. Hallock GG. Salvage of total knee arthroplasty with local fasciocutaneous flaps. J Bone Joint Surg Am. 1990;72(8):1236-1239.
29. Roswell M, Gale D. Total knee arthroplasty following internal fixation of a lateral tibial plateau fracture. Injury Extra. 2005;36(8):352-354.
30. Deangelis JP, Deangelis NA, Anderson R. Anatomy of the superficial peroneal nerve in relation to fixation of tibia fractures with the Less Invasive Stabilization System. J Orthop Trauma. 2004;18(8):536-539.
31. Pichler W, Grechenig W, Tesch NP, Weinberg AM, Heidari N, Clement H. The risk of iatrogenic injury to the deep peroneal nerve in minimally invasive osteosynthesis of the tibia with the Less Invasive Stabilisation System: a cadaver study. J Bone Joint Surg Br. 2009;91(3):385-387.
Updated ACCP Guideline for Antithrombotic Therapy for VTE Disease
Venous thromboembolism (VTE), which includes deep venous thrombosis (DVT) and pulmonary embolism (PE), continues to be a major cause of morbidity and mortality among hospitalized patients. Although it is well-known that anticoagulation therapy is effective in the prevention and treatment of VTE events, these agents are some of the highest-risk medications a hospitalist will prescribe given the danger of major bleeding. With the recent approval of several newer anticoagulants, it is important for the practicing hospitalist to be comfortable initiating, maintaining, and stopping these agents in a wide variety of patient populations.
Guideline Updates
In February 2016, an update to the ninth edition of the antithrombotic guideline from the American College of Chest Physician (ACCP) was published and included updated recommendations on 12 topics in addition to three new topics. This 10th-edition guideline update is referred to as AT10.1
One of the most notable changes in the updated guideline is the recommended choice of anticoagulant in patients with acute DVT or PE without cancer. Now, the direct oral anticoagulants (DOACs) dabigatran, rivaroxaban, apixaban, or edoxaban are recommended over warfarin. Although this is a weak recommendation based on moderate-quality evidence (grade 2B), this is the first time that warfarin is not considered first-line therapy. It should be emphasized that none of the four FDA-approved DOACs are preferred over another, and they should be avoided in patients who are pregnant or have severe renal disease. In patients with DVT or PE and cancer, low-molecular-weight heparin (LMWH) is still the preferred medication. If LMWH is not prescribed, AT10 does not have a preference for either a DOAC or warfarin for patients with cancer.
When it comes to duration of anticoagulation following a VTE event, the updated guideline continues to recommend three months for a provoked VTE event, with consideration for lifelong anticoagulation for an unprovoked event for patients at low or moderate bleeding risk. However, it now suggests that the recurrence risk factors of male sex and a positive D-dimer measured one month after stopping anticoagulant therapy should be taken into consideration when deciding whether extended anticoagulation is indicated.
AT10 also includes new recommendations concerning the role of aspirin for extended VTE treatment. Interestingly, the 2008 ACCP guideline gave a strong recommendation against the use of aspirin for VTE management in any patient population. In the 2012 guideline, the role of aspirin was not addressed for VTE treatment. Now, AT10 states that low-dose aspirin can be used in patients who stop anticoagulant therapy for treatment of an unprovoked proximal DVT or PE as an extended therapy (grade 2B). The significant change in this recommendation stems from two recent randomized trials that compared aspirin with placebo for the prevention of VTE recurrence in patients who have completed a course of anticoagulation for a first unprovoked proximal DVT or PE.2,3 Although the guideline doesn’t consider aspirin to be a reasonable alternative to anticoagulation for patients who require extended therapy and are agreeable to continue, for patients who have decided to stop anticoagulation, aspirin appears to reduce recurrent VTE by approximately one-third, with no significant increased risk of bleeding.
Another significant change in AT10 is the recommendation against the routine use of compression stockings to prevent postthrombotic syndrome (PTS). This change was influenced by a recent multicenter randomized trial showing that elastic compression stockings did not prevent PTS after an acute proximal DVT.4 The guideline authors remark that this recommendation focuses on the prevention of the chronic complications of PTS rather than treatment of the symptoms. Thus, for patients with acute or chronic leg pain or swelling from DVT, compression stockings may be justified.
A topic that was not addressed in the previous guideline was whether patients with a subsegmental PE should be treated. The guideline now suggests that patients with only subsegmental PE and no ultrasound-proven proximal DVT of the legs should undergo “clinical surveillance” rather than anticoagulation (grade 2C). Exceptions include patients at high risk for recurrent VTE (e.g., hospitalization, reduced mobility, active cancer, or irreversible VTE risk factors) and those with a low cardiopulmonary reserve or marked symptoms thought to be from PE. AT10 also states that patient preferences regarding anticoagulation treatment as well as the patient’s risk of bleeding should be taken into consideration. If the decision is made to not prescribe anticoagulation for subsegmental PE, patients should be advised to seek reevaluation if their symptoms persist or worsen.
The 2012 guideline included a new recommendation that patients with low-risk PE (typically defined by a low Pulmonary Embolism Severity Index [PESI] score) could be discharged “early” from the hospital. This recommendation has now been modified to state that patients with low-risk PE may be treated entirely at home. It is worth noting that outpatient management of low-risk PE has become much less complicated if using a DOAC, particularly rivaroxaban and apixaban as neither require initial treatment with parenteral anticoagulation.
AT10 has not changed the recommendation for which patients should receive thrombolytic therapy for treatment of PE. It recommends systemic thrombolytic therapy for patients with acute PE associated with hypotension (defined as systolic blood pressure less than 90 mmHg for 15 minutes) who are not at high risk for bleeding (grade 2B). Likewise, for patients with acute PE not associated with hypotension, the guideline recommends against systemic thrombolytics (grade 1B). If thrombolytics are implemented, AT10 favors systemic administration over catheter-directed thrombolysis (CDT) due to the higher-quality evidence available. However, the authors state that CDT may be preferred for patients at higher risk of bleeding and when local expertise is available. Lastly, catheter-assisted thrombus removal should be considered in patients with acute PE and hypotension who have a high bleeding risk, who have failed systemic thrombolytics, or who are in shock and likely to die before systemic thrombolytics become therapeutic.
Although no prospective trials have evaluated the management of patients with recurrent VTE events while on anticoagulation therapy, AT10 offers some guidance. After ensuring the patient truly had a recurrent VTE event while on therapeutic warfarin or compliant with a DOAC, the authors suggest switching to LMWH for at least one month (grade 2C). Furthermore, for patients who have a recurrent VTE event while compliant on long-term LMWH, the guideline suggests increasing the dose of LMWH by about one-quarter to one-third (grade 2C).
Guideline Analysis
It is important to note that of the 54 recommendations included in the complete guideline update, only 20 were strong recommendations (grade 1), and none were based on high-quality evidence (level A). It is obvious that more research is needed in this field. Regardless, the ACCP antithrombotic guideline remains the authoritative source in VTE management and has a strong influence on practice behavior. With the recent addition of several newer anticoagulants, AT10 is particularly useful in helping providers understand when and when not to use them. The authors indicate that future iterations will be continually updated, describing them as “living guidelines.” The format of AT10 was designed to facilitate this method with the goal of having discrete topics discussed as new evidence becomes available.
Hospital Medicine Takeaways
Despite the lack of randomized and prospective clinical trials, the updated recommendations from AT10 provide important information on challenging VTE issues that the hospitalist can apply to most patients most of the time. Important updates include:
- Prescribe DOACs as first-line agents for the treatment of acute VTE in patients without cancer.
- Use aspirin for the prevention of recurrent VTE in patients who stop anticoagulation for treatment of an unprovoked DVT or PE.
- Avoid compression stockings for the sole purpose of preventing postthrombotic syndrome.
- Do not admit patients with low-risk PE (as determined by the PESI score) to the hospital but rather treat them entirely at home.
Lastly, it is important to remember that VTE treatment decisions need to be individualized based on the clinical, imaging, and biochemical features of your patient.
Paul J. Grant, MD, SFHM, is assistant professor of medicine and director of perioperative and consultative medicine within the Department of Internal Medicine at the University of Michigan Health System in Ann Arbor.
References
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352.
- Brighton TA, Eikelboom JW, Mann K, et al. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med. 2012;367(21):1979-1987.
- Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366(21):1959-1967.
- Kahn SR, Shapiro S, Wells PS, et al. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo controlled trial. Lancet. 2014;383(9920):880-888.
Venous thromboembolism (VTE), which includes deep venous thrombosis (DVT) and pulmonary embolism (PE), continues to be a major cause of morbidity and mortality among hospitalized patients. Although it is well-known that anticoagulation therapy is effective in the prevention and treatment of VTE events, these agents are some of the highest-risk medications a hospitalist will prescribe given the danger of major bleeding. With the recent approval of several newer anticoagulants, it is important for the practicing hospitalist to be comfortable initiating, maintaining, and stopping these agents in a wide variety of patient populations.
Guideline Updates
In February 2016, an update to the ninth edition of the antithrombotic guideline from the American College of Chest Physician (ACCP) was published and included updated recommendations on 12 topics in addition to three new topics. This 10th-edition guideline update is referred to as AT10.1
One of the most notable changes in the updated guideline is the recommended choice of anticoagulant in patients with acute DVT or PE without cancer. Now, the direct oral anticoagulants (DOACs) dabigatran, rivaroxaban, apixaban, or edoxaban are recommended over warfarin. Although this is a weak recommendation based on moderate-quality evidence (grade 2B), this is the first time that warfarin is not considered first-line therapy. It should be emphasized that none of the four FDA-approved DOACs are preferred over another, and they should be avoided in patients who are pregnant or have severe renal disease. In patients with DVT or PE and cancer, low-molecular-weight heparin (LMWH) is still the preferred medication. If LMWH is not prescribed, AT10 does not have a preference for either a DOAC or warfarin for patients with cancer.
When it comes to duration of anticoagulation following a VTE event, the updated guideline continues to recommend three months for a provoked VTE event, with consideration for lifelong anticoagulation for an unprovoked event for patients at low or moderate bleeding risk. However, it now suggests that the recurrence risk factors of male sex and a positive D-dimer measured one month after stopping anticoagulant therapy should be taken into consideration when deciding whether extended anticoagulation is indicated.
AT10 also includes new recommendations concerning the role of aspirin for extended VTE treatment. Interestingly, the 2008 ACCP guideline gave a strong recommendation against the use of aspirin for VTE management in any patient population. In the 2012 guideline, the role of aspirin was not addressed for VTE treatment. Now, AT10 states that low-dose aspirin can be used in patients who stop anticoagulant therapy for treatment of an unprovoked proximal DVT or PE as an extended therapy (grade 2B). The significant change in this recommendation stems from two recent randomized trials that compared aspirin with placebo for the prevention of VTE recurrence in patients who have completed a course of anticoagulation for a first unprovoked proximal DVT or PE.2,3 Although the guideline doesn’t consider aspirin to be a reasonable alternative to anticoagulation for patients who require extended therapy and are agreeable to continue, for patients who have decided to stop anticoagulation, aspirin appears to reduce recurrent VTE by approximately one-third, with no significant increased risk of bleeding.
Another significant change in AT10 is the recommendation against the routine use of compression stockings to prevent postthrombotic syndrome (PTS). This change was influenced by a recent multicenter randomized trial showing that elastic compression stockings did not prevent PTS after an acute proximal DVT.4 The guideline authors remark that this recommendation focuses on the prevention of the chronic complications of PTS rather than treatment of the symptoms. Thus, for patients with acute or chronic leg pain or swelling from DVT, compression stockings may be justified.
A topic that was not addressed in the previous guideline was whether patients with a subsegmental PE should be treated. The guideline now suggests that patients with only subsegmental PE and no ultrasound-proven proximal DVT of the legs should undergo “clinical surveillance” rather than anticoagulation (grade 2C). Exceptions include patients at high risk for recurrent VTE (e.g., hospitalization, reduced mobility, active cancer, or irreversible VTE risk factors) and those with a low cardiopulmonary reserve or marked symptoms thought to be from PE. AT10 also states that patient preferences regarding anticoagulation treatment as well as the patient’s risk of bleeding should be taken into consideration. If the decision is made to not prescribe anticoagulation for subsegmental PE, patients should be advised to seek reevaluation if their symptoms persist or worsen.
The 2012 guideline included a new recommendation that patients with low-risk PE (typically defined by a low Pulmonary Embolism Severity Index [PESI] score) could be discharged “early” from the hospital. This recommendation has now been modified to state that patients with low-risk PE may be treated entirely at home. It is worth noting that outpatient management of low-risk PE has become much less complicated if using a DOAC, particularly rivaroxaban and apixaban as neither require initial treatment with parenteral anticoagulation.
AT10 has not changed the recommendation for which patients should receive thrombolytic therapy for treatment of PE. It recommends systemic thrombolytic therapy for patients with acute PE associated with hypotension (defined as systolic blood pressure less than 90 mmHg for 15 minutes) who are not at high risk for bleeding (grade 2B). Likewise, for patients with acute PE not associated with hypotension, the guideline recommends against systemic thrombolytics (grade 1B). If thrombolytics are implemented, AT10 favors systemic administration over catheter-directed thrombolysis (CDT) due to the higher-quality evidence available. However, the authors state that CDT may be preferred for patients at higher risk of bleeding and when local expertise is available. Lastly, catheter-assisted thrombus removal should be considered in patients with acute PE and hypotension who have a high bleeding risk, who have failed systemic thrombolytics, or who are in shock and likely to die before systemic thrombolytics become therapeutic.
Although no prospective trials have evaluated the management of patients with recurrent VTE events while on anticoagulation therapy, AT10 offers some guidance. After ensuring the patient truly had a recurrent VTE event while on therapeutic warfarin or compliant with a DOAC, the authors suggest switching to LMWH for at least one month (grade 2C). Furthermore, for patients who have a recurrent VTE event while compliant on long-term LMWH, the guideline suggests increasing the dose of LMWH by about one-quarter to one-third (grade 2C).
Guideline Analysis
It is important to note that of the 54 recommendations included in the complete guideline update, only 20 were strong recommendations (grade 1), and none were based on high-quality evidence (level A). It is obvious that more research is needed in this field. Regardless, the ACCP antithrombotic guideline remains the authoritative source in VTE management and has a strong influence on practice behavior. With the recent addition of several newer anticoagulants, AT10 is particularly useful in helping providers understand when and when not to use them. The authors indicate that future iterations will be continually updated, describing them as “living guidelines.” The format of AT10 was designed to facilitate this method with the goal of having discrete topics discussed as new evidence becomes available.
Hospital Medicine Takeaways
Despite the lack of randomized and prospective clinical trials, the updated recommendations from AT10 provide important information on challenging VTE issues that the hospitalist can apply to most patients most of the time. Important updates include:
- Prescribe DOACs as first-line agents for the treatment of acute VTE in patients without cancer.
- Use aspirin for the prevention of recurrent VTE in patients who stop anticoagulation for treatment of an unprovoked DVT or PE.
- Avoid compression stockings for the sole purpose of preventing postthrombotic syndrome.
- Do not admit patients with low-risk PE (as determined by the PESI score) to the hospital but rather treat them entirely at home.
Lastly, it is important to remember that VTE treatment decisions need to be individualized based on the clinical, imaging, and biochemical features of your patient.
Paul J. Grant, MD, SFHM, is assistant professor of medicine and director of perioperative and consultative medicine within the Department of Internal Medicine at the University of Michigan Health System in Ann Arbor.
References
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352.
- Brighton TA, Eikelboom JW, Mann K, et al. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med. 2012;367(21):1979-1987.
- Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366(21):1959-1967.
- Kahn SR, Shapiro S, Wells PS, et al. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo controlled trial. Lancet. 2014;383(9920):880-888.
Venous thromboembolism (VTE), which includes deep venous thrombosis (DVT) and pulmonary embolism (PE), continues to be a major cause of morbidity and mortality among hospitalized patients. Although it is well-known that anticoagulation therapy is effective in the prevention and treatment of VTE events, these agents are some of the highest-risk medications a hospitalist will prescribe given the danger of major bleeding. With the recent approval of several newer anticoagulants, it is important for the practicing hospitalist to be comfortable initiating, maintaining, and stopping these agents in a wide variety of patient populations.
Guideline Updates
In February 2016, an update to the ninth edition of the antithrombotic guideline from the American College of Chest Physician (ACCP) was published and included updated recommendations on 12 topics in addition to three new topics. This 10th-edition guideline update is referred to as AT10.1
One of the most notable changes in the updated guideline is the recommended choice of anticoagulant in patients with acute DVT or PE without cancer. Now, the direct oral anticoagulants (DOACs) dabigatran, rivaroxaban, apixaban, or edoxaban are recommended over warfarin. Although this is a weak recommendation based on moderate-quality evidence (grade 2B), this is the first time that warfarin is not considered first-line therapy. It should be emphasized that none of the four FDA-approved DOACs are preferred over another, and they should be avoided in patients who are pregnant or have severe renal disease. In patients with DVT or PE and cancer, low-molecular-weight heparin (LMWH) is still the preferred medication. If LMWH is not prescribed, AT10 does not have a preference for either a DOAC or warfarin for patients with cancer.
When it comes to duration of anticoagulation following a VTE event, the updated guideline continues to recommend three months for a provoked VTE event, with consideration for lifelong anticoagulation for an unprovoked event for patients at low or moderate bleeding risk. However, it now suggests that the recurrence risk factors of male sex and a positive D-dimer measured one month after stopping anticoagulant therapy should be taken into consideration when deciding whether extended anticoagulation is indicated.
AT10 also includes new recommendations concerning the role of aspirin for extended VTE treatment. Interestingly, the 2008 ACCP guideline gave a strong recommendation against the use of aspirin for VTE management in any patient population. In the 2012 guideline, the role of aspirin was not addressed for VTE treatment. Now, AT10 states that low-dose aspirin can be used in patients who stop anticoagulant therapy for treatment of an unprovoked proximal DVT or PE as an extended therapy (grade 2B). The significant change in this recommendation stems from two recent randomized trials that compared aspirin with placebo for the prevention of VTE recurrence in patients who have completed a course of anticoagulation for a first unprovoked proximal DVT or PE.2,3 Although the guideline doesn’t consider aspirin to be a reasonable alternative to anticoagulation for patients who require extended therapy and are agreeable to continue, for patients who have decided to stop anticoagulation, aspirin appears to reduce recurrent VTE by approximately one-third, with no significant increased risk of bleeding.
Another significant change in AT10 is the recommendation against the routine use of compression stockings to prevent postthrombotic syndrome (PTS). This change was influenced by a recent multicenter randomized trial showing that elastic compression stockings did not prevent PTS after an acute proximal DVT.4 The guideline authors remark that this recommendation focuses on the prevention of the chronic complications of PTS rather than treatment of the symptoms. Thus, for patients with acute or chronic leg pain or swelling from DVT, compression stockings may be justified.
A topic that was not addressed in the previous guideline was whether patients with a subsegmental PE should be treated. The guideline now suggests that patients with only subsegmental PE and no ultrasound-proven proximal DVT of the legs should undergo “clinical surveillance” rather than anticoagulation (grade 2C). Exceptions include patients at high risk for recurrent VTE (e.g., hospitalization, reduced mobility, active cancer, or irreversible VTE risk factors) and those with a low cardiopulmonary reserve or marked symptoms thought to be from PE. AT10 also states that patient preferences regarding anticoagulation treatment as well as the patient’s risk of bleeding should be taken into consideration. If the decision is made to not prescribe anticoagulation for subsegmental PE, patients should be advised to seek reevaluation if their symptoms persist or worsen.
The 2012 guideline included a new recommendation that patients with low-risk PE (typically defined by a low Pulmonary Embolism Severity Index [PESI] score) could be discharged “early” from the hospital. This recommendation has now been modified to state that patients with low-risk PE may be treated entirely at home. It is worth noting that outpatient management of low-risk PE has become much less complicated if using a DOAC, particularly rivaroxaban and apixaban as neither require initial treatment with parenteral anticoagulation.
AT10 has not changed the recommendation for which patients should receive thrombolytic therapy for treatment of PE. It recommends systemic thrombolytic therapy for patients with acute PE associated with hypotension (defined as systolic blood pressure less than 90 mmHg for 15 minutes) who are not at high risk for bleeding (grade 2B). Likewise, for patients with acute PE not associated with hypotension, the guideline recommends against systemic thrombolytics (grade 1B). If thrombolytics are implemented, AT10 favors systemic administration over catheter-directed thrombolysis (CDT) due to the higher-quality evidence available. However, the authors state that CDT may be preferred for patients at higher risk of bleeding and when local expertise is available. Lastly, catheter-assisted thrombus removal should be considered in patients with acute PE and hypotension who have a high bleeding risk, who have failed systemic thrombolytics, or who are in shock and likely to die before systemic thrombolytics become therapeutic.
Although no prospective trials have evaluated the management of patients with recurrent VTE events while on anticoagulation therapy, AT10 offers some guidance. After ensuring the patient truly had a recurrent VTE event while on therapeutic warfarin or compliant with a DOAC, the authors suggest switching to LMWH for at least one month (grade 2C). Furthermore, for patients who have a recurrent VTE event while compliant on long-term LMWH, the guideline suggests increasing the dose of LMWH by about one-quarter to one-third (grade 2C).
Guideline Analysis
It is important to note that of the 54 recommendations included in the complete guideline update, only 20 were strong recommendations (grade 1), and none were based on high-quality evidence (level A). It is obvious that more research is needed in this field. Regardless, the ACCP antithrombotic guideline remains the authoritative source in VTE management and has a strong influence on practice behavior. With the recent addition of several newer anticoagulants, AT10 is particularly useful in helping providers understand when and when not to use them. The authors indicate that future iterations will be continually updated, describing them as “living guidelines.” The format of AT10 was designed to facilitate this method with the goal of having discrete topics discussed as new evidence becomes available.
Hospital Medicine Takeaways
Despite the lack of randomized and prospective clinical trials, the updated recommendations from AT10 provide important information on challenging VTE issues that the hospitalist can apply to most patients most of the time. Important updates include:
- Prescribe DOACs as first-line agents for the treatment of acute VTE in patients without cancer.
- Use aspirin for the prevention of recurrent VTE in patients who stop anticoagulation for treatment of an unprovoked DVT or PE.
- Avoid compression stockings for the sole purpose of preventing postthrombotic syndrome.
- Do not admit patients with low-risk PE (as determined by the PESI score) to the hospital but rather treat them entirely at home.
Lastly, it is important to remember that VTE treatment decisions need to be individualized based on the clinical, imaging, and biochemical features of your patient.
Paul J. Grant, MD, SFHM, is assistant professor of medicine and director of perioperative and consultative medicine within the Department of Internal Medicine at the University of Michigan Health System in Ann Arbor.
References
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352.
- Brighton TA, Eikelboom JW, Mann K, et al. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med. 2012;367(21):1979-1987.
- Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366(21):1959-1967.
- Kahn SR, Shapiro S, Wells PS, et al. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo controlled trial. Lancet. 2014;383(9920):880-888.
AEs from anticoagulants common cause of ED visits
caring for a patient
Photo by Tom Watanabe
A new study has revealed which drugs most commonly caused adverse events (AEs) leading to emergency department (ED) visits in the US in 2013 and 2014.
The drug class most often implicated in ED visits was anticoagulants.
Other common drug classes were antibiotics, diabetes agents, and opioid analgesics.
Nadine Shehab, PharmD, of the US Centers for Disease Control and Prevention in Atlanta, Georgia, and her colleagues reported these findings in JAMA.
The researchers examined characteristics of ED visits for drug-related AEs in the US in 2013-2014 and changes in ED visits for drug-related AEs since 2005-2006.
The team analyzed nationally representative data from 58 EDs participating in the National Electronic Injury Surveillance System-Cooperative Adverse Drug Event Surveillance project.
Based on data from 42,585 cases, the researchers estimated that 4 ED visits for drug-related AEs occurred per 1000 individuals annually in 2013 and 2014. And 27% of ED visits for drug-related AEs resulted in hospitalization.
Results by drug class
The most commonly implicated drug classes were anticoagulants (18%), antibiotics (16%), diabetes agents (13%), opioid analgesics (7%), antiplatelet agents (7%), renin-angiotensin system inhibitors (4%), antineoplastic agents (3%), and sedative or hypnotic agents (3%).
Four anticoagulants (warfarin, rivaroxaban, dabigatran, and enoxaparin) and 5 diabetes agents (insulin and 4 oral agents) were among the 15 most common drugs implicated.
Results by age
Antibiotics were the most common drug class implicated in ED visits for drug-related AEs among children age 5 or younger (56%) and among children and adolescents ages 6 to 19 (32%).
Drugs not belonging to the most common classes (overall) were most commonly implicated in ED visits for adults ages 20 to 34 (26%), 35 to 49 (26%), and 50 to 64 (23%).
Anticoagulants were the most common drug class implicated in ED visits for adults ages 65 to 79 (28%) and adults age 80 or older (39%).
Changes over time
Since 2005-2006, the proportions of ED visits for drug-related AEs from anticoagulants and diabetes agents have increased, whereas the proportion from antibiotics has decreased.
Population rates of ED visits for drug-related AEs increased from 2005-2006 to 2013-2014 among adults age 65 and older—5.2 visits per 1000 individuals to 9.7 visits per 1000 individuals, respectively.
An increase was also observed for adults ages 50 to 64—2.5 visits per 1000 individuals in 2005-2006, compared to 4.3 visits per 1000 individuals in 2013-2014.
However, the population rates for other age groups were similar for both time periods.
Anticoagulants and antiplatelet agents
Overall, anticoagulants were implicated in 18% of ED visits for drug-related AEs, and 49% of anticoagulant-related AEs led to hospitalization.
Anticoagulant-related ED visits were most commonly related to vitamin K antagonists (15%), followed by factor Xa inhibitors, unfractionated and low-molecular-weight heparins, and oral direct thrombin inhibitors (about 1% each).
Antiplatelet agents were implicated in 7% of ED visits for drug-related AEs, and 44% of antiplatelet agent-related AEs led to hospitalization.
Antiplatelet-related ED visits were most commonly related to platelet P2Y12 receptor antagonists (5%) and aspirin with or without dipyridamole (4%).
Warfarin was implicated in 15% of ED visits for drug-related AEs, clopidogrel and aspirin were each implicated in 4%, and rivaroxaban was implicated in 1%. ![]()

caring for a patient
Photo by Tom Watanabe
A new study has revealed which drugs most commonly caused adverse events (AEs) leading to emergency department (ED) visits in the US in 2013 and 2014.
The drug class most often implicated in ED visits was anticoagulants.
Other common drug classes were antibiotics, diabetes agents, and opioid analgesics.
Nadine Shehab, PharmD, of the US Centers for Disease Control and Prevention in Atlanta, Georgia, and her colleagues reported these findings in JAMA.
The researchers examined characteristics of ED visits for drug-related AEs in the US in 2013-2014 and changes in ED visits for drug-related AEs since 2005-2006.
The team analyzed nationally representative data from 58 EDs participating in the National Electronic Injury Surveillance System-Cooperative Adverse Drug Event Surveillance project.
Based on data from 42,585 cases, the researchers estimated that 4 ED visits for drug-related AEs occurred per 1000 individuals annually in 2013 and 2014. And 27% of ED visits for drug-related AEs resulted in hospitalization.
Results by drug class
The most commonly implicated drug classes were anticoagulants (18%), antibiotics (16%), diabetes agents (13%), opioid analgesics (7%), antiplatelet agents (7%), renin-angiotensin system inhibitors (4%), antineoplastic agents (3%), and sedative or hypnotic agents (3%).
Four anticoagulants (warfarin, rivaroxaban, dabigatran, and enoxaparin) and 5 diabetes agents (insulin and 4 oral agents) were among the 15 most common drugs implicated.
Results by age
Antibiotics were the most common drug class implicated in ED visits for drug-related AEs among children age 5 or younger (56%) and among children and adolescents ages 6 to 19 (32%).
Drugs not belonging to the most common classes (overall) were most commonly implicated in ED visits for adults ages 20 to 34 (26%), 35 to 49 (26%), and 50 to 64 (23%).
Anticoagulants were the most common drug class implicated in ED visits for adults ages 65 to 79 (28%) and adults age 80 or older (39%).
Changes over time
Since 2005-2006, the proportions of ED visits for drug-related AEs from anticoagulants and diabetes agents have increased, whereas the proportion from antibiotics has decreased.
Population rates of ED visits for drug-related AEs increased from 2005-2006 to 2013-2014 among adults age 65 and older—5.2 visits per 1000 individuals to 9.7 visits per 1000 individuals, respectively.
An increase was also observed for adults ages 50 to 64—2.5 visits per 1000 individuals in 2005-2006, compared to 4.3 visits per 1000 individuals in 2013-2014.
However, the population rates for other age groups were similar for both time periods.
Anticoagulants and antiplatelet agents
Overall, anticoagulants were implicated in 18% of ED visits for drug-related AEs, and 49% of anticoagulant-related AEs led to hospitalization.
Anticoagulant-related ED visits were most commonly related to vitamin K antagonists (15%), followed by factor Xa inhibitors, unfractionated and low-molecular-weight heparins, and oral direct thrombin inhibitors (about 1% each).
Antiplatelet agents were implicated in 7% of ED visits for drug-related AEs, and 44% of antiplatelet agent-related AEs led to hospitalization.
Antiplatelet-related ED visits were most commonly related to platelet P2Y12 receptor antagonists (5%) and aspirin with or without dipyridamole (4%).
Warfarin was implicated in 15% of ED visits for drug-related AEs, clopidogrel and aspirin were each implicated in 4%, and rivaroxaban was implicated in 1%. ![]()

caring for a patient
Photo by Tom Watanabe
A new study has revealed which drugs most commonly caused adverse events (AEs) leading to emergency department (ED) visits in the US in 2013 and 2014.
The drug class most often implicated in ED visits was anticoagulants.
Other common drug classes were antibiotics, diabetes agents, and opioid analgesics.
Nadine Shehab, PharmD, of the US Centers for Disease Control and Prevention in Atlanta, Georgia, and her colleagues reported these findings in JAMA.
The researchers examined characteristics of ED visits for drug-related AEs in the US in 2013-2014 and changes in ED visits for drug-related AEs since 2005-2006.
The team analyzed nationally representative data from 58 EDs participating in the National Electronic Injury Surveillance System-Cooperative Adverse Drug Event Surveillance project.
Based on data from 42,585 cases, the researchers estimated that 4 ED visits for drug-related AEs occurred per 1000 individuals annually in 2013 and 2014. And 27% of ED visits for drug-related AEs resulted in hospitalization.
Results by drug class
The most commonly implicated drug classes were anticoagulants (18%), antibiotics (16%), diabetes agents (13%), opioid analgesics (7%), antiplatelet agents (7%), renin-angiotensin system inhibitors (4%), antineoplastic agents (3%), and sedative or hypnotic agents (3%).
Four anticoagulants (warfarin, rivaroxaban, dabigatran, and enoxaparin) and 5 diabetes agents (insulin and 4 oral agents) were among the 15 most common drugs implicated.
Results by age
Antibiotics were the most common drug class implicated in ED visits for drug-related AEs among children age 5 or younger (56%) and among children and adolescents ages 6 to 19 (32%).
Drugs not belonging to the most common classes (overall) were most commonly implicated in ED visits for adults ages 20 to 34 (26%), 35 to 49 (26%), and 50 to 64 (23%).
Anticoagulants were the most common drug class implicated in ED visits for adults ages 65 to 79 (28%) and adults age 80 or older (39%).
Changes over time
Since 2005-2006, the proportions of ED visits for drug-related AEs from anticoagulants and diabetes agents have increased, whereas the proportion from antibiotics has decreased.
Population rates of ED visits for drug-related AEs increased from 2005-2006 to 2013-2014 among adults age 65 and older—5.2 visits per 1000 individuals to 9.7 visits per 1000 individuals, respectively.
An increase was also observed for adults ages 50 to 64—2.5 visits per 1000 individuals in 2005-2006, compared to 4.3 visits per 1000 individuals in 2013-2014.
However, the population rates for other age groups were similar for both time periods.
Anticoagulants and antiplatelet agents
Overall, anticoagulants were implicated in 18% of ED visits for drug-related AEs, and 49% of anticoagulant-related AEs led to hospitalization.
Anticoagulant-related ED visits were most commonly related to vitamin K antagonists (15%), followed by factor Xa inhibitors, unfractionated and low-molecular-weight heparins, and oral direct thrombin inhibitors (about 1% each).
Antiplatelet agents were implicated in 7% of ED visits for drug-related AEs, and 44% of antiplatelet agent-related AEs led to hospitalization.
Antiplatelet-related ED visits were most commonly related to platelet P2Y12 receptor antagonists (5%) and aspirin with or without dipyridamole (4%).
Warfarin was implicated in 15% of ED visits for drug-related AEs, clopidogrel and aspirin were each implicated in 4%, and rivaroxaban was implicated in 1%. ![]()
Who Overdoses at a VA Emergency Department?
Overdose deaths remain epidemic throughout the U.S. The rates of unintentional overdose deaths, increasing by 137% between 2000 and 2014, have been driven by a 4-fold increase in prescription opioid overdoses during that period.1-3
Veterans died of accidental overdose at a rate of 19.85 deaths/ 100,000 people compared with a rate of 10.49 deaths in the general population, based on 2005 data.4 There is wide state-by-state variation with the lowest age-adjusted opioid overdose death rate of 1.9 deaths/100,000 person-years among veterans in Mississippi and the highest rate in Utah of 33.9 deaths/100,000 person-years, using 2001 to 2009 data.5 These data can be compared with a crude general population overdose death rate of 10.6 deaths per 100,000 person-years in Mississippi and 18.4 deaths per 100,000 person-years in the general Utah population during that same period.6
Overdose deaths in the U.S. occur most often in persons aged 25 to 54 years.7 Older age has been associated with iatrogenic opioid overdose in hospitalized patients.8 Pulmonary, cardiovascular, and psychiatric disorders, including past or present substance use, have been associated with an increased risk of opioid overdose.9 However, veterans with substance use disorders are less likely to be prescribed opioids than are nonveterans with substance use disorders.10 Also, concomitant use of sedating medications, such as benzodiazepines (BZDs), can increase mortality from opioid overdose.11 Patients prescribed opioids for chronic pain conditions often take BZDs for various reasons.12 Veterans seem more likely to receive opioids to treat chronic pain but at lower average daily doses than the doses that nonveterans receive.10
Emergency management of life-threatening opioid overdose includes prompt administration of naloxone.13 Naloxone is FDA approved for complete or partial reversal of opioid-induced clinical effects, most critically respiratory depression.14,15 Naloxone administration in the emergency department (ED) may serve as a surrogate for an overdose event. During the study period, naloxone take-home kits were not available in the VA setting.
A 2010 ED study described demographic information and comorbidities in opioid overdose, but the study did not include veterans.16 The clinical characteristics of veterans treated for opioid overdose have not been published. Because identifying characteristics of veterans who overdose may help tailor overdose prevention efforts, the objective of this study is to describe clinical characteristics of veterans treated for opioid overdose.
Methods
A retrospective chart review and archived data study was approved by the University of Utah and VA institutional review boards, and conducted at the George E. Wahlen VAMC in Salt Lake City, Utah. This chart review included veterans who were admitted to the ED and treated with naloxone between January 1, 2009 and January 1, 2013.
The authors used the Pharmacy Benefits Management Data Manager to extract data from the VA Data Warehouse and verified the data by open chart review (Table). The following data were collected: ED visit date (overdose date); demographic information, including age, gender, and race; evidence of next-of-kin or other contact at the same address as the veteran; diagnoses based on ICD-9 codes, including sleep apnea, obesitycardiac disease, pulmonary disease, mental health diagnoses (ICD-9 codes 290-302 [wild card characters (*) included many subdiagnoses]),
cancer, and substance use disorders and/or dependencies (SUDD); tobacco use; VA-issued prescription opioid and BZD availability, including dose, fill dates, quantities dispensed, and day supplies; specialty of opioid prescriber; urine drug screening (UDS) results; and outcome of the overdose.
No standardized research criteria identify overdose in medical chart review.17 For each identified patient, the authors reviewed provider and nursing notes charted during an ED visit that included naloxone administration. The event was included as an opioid overdose when notes indicated that the veteran was unresponsive and given naloxone, which resulted in increased respirations or increased responsiveness. Cases were excluded if the reason for naloxone administration was anything other than opioid overdose.
Medical, mental health, and SUDD diagnoses were included only if the veteran had more than 3 patient care encounters (PCE) with ICD-9 codes for a specific diagnosis entered by providers. A PCE used in the electronic medical record (EMR) helps collect, manage, and display outpatient encounter data, including providers, procedure codes, and diagnostic codes. Tobacco use was extracted from health factors documented during primary care visit screenings. (Health factors help capture data entered in note templates in the EMR and can be used to query trends.) A diagnosis of obesity was based on a calculated body mass index of > 30 kg/m2 on the day of the ED visit date or the most recently charted height and weight. The type of SUDD was stratified into opioids (ICD-9 codes 304.0*), sedatives (ICD-9 code 304.1*), alcohol (ICD-9 code 303.*), and other (ICD-9 codes 304.2-305.9).
The dosage of opioids and BZDs available to a veteran was determined by using methods similar to those described by Gomes and colleagues: the dose of opioids and BZDs available based on prescriptions dispensed during the 120 days prior to the ED visit date and the dose available on the day of the ED visit date if prescription instructions were being followed.18 Prescription opioids and BZDs were converted to daily morphine equivalent dose (MED) and daily lorazepam-equivalent dose (LED), using established methods.19,20
Veterans were stratified into 4 groups based on prescribed medication availability: opioids only, BZDs only, opioids and BZDs, and neither opioids nor BZDs. The specialty of the opioid prescribers was categorized as primary care, pain specialist, surgeon, emergency specialist, or hospitalist (discharge prescription). Veteran EMRs contain a list of medications obtained outside the VA facility, referred to as non-VA prescriptions. These medications werenot included in the analysis because accuracy could not be verified.
A study author reviewed the results of any UDS performed up to 120 days before the ED visit date to determine whether the result reflected the currently prescribed prescription medications. If the UDS was positive for the prescribed opioids and/or BZDs and for any nonprescribed drug, including alcohol, the UDS was classified as not reflective. If the prescribed BZD was alprazolam, clonazepam, or lorazepam, a BZD-positive UDS was not required for the UDS to be considered reflective because of the sensitivity of the UDS BZD immunoassay
used at the George E. Wahlen VAMC clinical laboratory.21
Outcomes of the overdose were categorized as discharged, hospitalized, or deceased. Descriptive statistical analyses were performed using Microsoft Excel. Group comparisons were performed using Pearson chi-square or Student t test.
Results
The ED at the George E. Wahlen VAMC averages 64 visits per day, almost 94,000 visits within the study period. One hundred seventy ED visits between January 1, 2009 and January 1, 2013, involved naloxone administration. Ninety-two visits met the inclusion criteria of opioid overdose, representing about 0.002% of all ED visits at this facility (Figure 1). Six veterans had multiple ED visits within the study period, including 4 veterans who were in the opioid-only group.
The majority of veterans in this study were non-Hispanic white (n = 83, 90%), male (n = 88, 96%), with a mean age of 63 years. Less than 40% listed a next-of-kin or contact person living at their address.
Based on prescriptions available within 120 days before the overdose, 67 veterans (73%) possessed opioid and/or BZD prescriptions. In this group, the MED available on the day of the ED visit ranged from 7.5 mg to 830 mg. The MED was ≤ 200 mg in 71.6% and ≤ 50 mg in 34.3% of these cases. Veterans prescribed both opioids and BZDs had higher MED (average, 259 mg) available within 120 days of the ED visit than did those prescribed opioids only (average, 118 mg) (P = .015; SD, 132.9). The LED ranged from 1 mg to 12 mg for veterans with available BZDs.
Based on prescriptions available on the day of opioid overdose, 53 veterans (58%) had opioid prescriptions. The ranges of MED and LED available on the day of overdose were the same as the 120-day availability period. The average MED was 183 mg in veterans prescribed both opioids and BZDs and 126 mg in those prescribed opioids only (P = .283; SD, 168.65; Figure 2). The time between the last opioid fill date and the overdose visit date averaged 20 days (range, 0 to 28 days) in veterans prescribed opioids.
All veterans had at least 1 diagnosis that in previous studies was associated with increased risk of overdose.9,15 The most common diagnoses included cardiovascular diseases, mental health disorders, pulmonary diseases, and cancer. Other SUDDs not including tobacco use were documented in at least half the veterans with prescribed opioids and/or BZDs. No veteran in the sample had a documented history of opioid SUDD.
Hydrocodone products were available in > 50% of cases. None of the veterans were prescribed buprenorphine products; other opioids, including tramadol, comprised the remainder (Figure 3). Primary care providers prescribed 72% of opioid prescriptions, with pain specialists, discharge physicians, ED providers, and surgeons prescribing the rest. When both opioids and BZDs were available, combinations of a hydrocodone product plus clonazepam or lorazepam were most common. The time between the last opioid fill date and the overdose visit date averaged 20 days (range, 0 to 28 days) in veterans prescribed opioids.
Overall, 64% of the sample had UDS results prior to the ED visit. Of veterans prescribed opioids and/or BZDs, 53% of UDSs reflected prescribed regimens.
On the day of the ED visit, 1 death occurred. Ninety-one veterans (99%) survived the overdose; 79 veterans (86%) were hospitalized, most for < 24 hours.
Discussion
This retrospective review identified 92 veterans who were treated with naloxone in the ED for opioid overdose during a 4-year period at the George E. Wahlen VAMC. Seventy-eight cases were excluded because the reason entered in charts for naloxone administration was itching, constipation, altered mental status, or unclear documentation.
Veterans in this study were older on average than the overdose fatalities in the U.S. Opioid overdose deaths in the U.S. and in Utah occur most frequently in non-Hispanic white men aged between 35 and 54 years.7,22,23 In the 2010 Nationwide Emergency Department Sample of 136,000 opioid overdoses, of which 98% survived, most were aged 18 to 54 years.16 The older age in this study most likely reflects the age range of veterans served in the VHA; however, as more young veterans enter the VHA, the age range of overdose victims may more closely resemble the age ranges found in previous studies. Post hoc analysis showed 8 veterans (9%) with probable intentional opioid overdose based on chart review, whereas the incidence of intentional prescription drug overdose in the U.S. is 17.1%.24
In Utah, almost 93% of fatal overdoses occur at a residential location.22 Less than half the veterans in this study had a contact or next-of-kin listed as living at the same address. Although veterans may not have identified someone living with them, in many cases, it is likely another person witnessed the overdose. Relying on EMRs to identify who should receive prevention education, in addition to the veteran, may result in missed opportunities to include another person likely to witness an overdose.25 Prescribers should make a conscious effort to ask veterans to identify someone who may be able to assist with rescue efforts in the event of an overdose.
Diagnoses associated with increased risk of opioid overdose death include sleep apnea, morbid obesity, pulmonary or cardiovascular diseases, and/or a history of psychiatric disorders and SUDD.8,9,16 In a large sample of older veterans, only 64% had at least 1 medical or psychiatric diagnosis.26 Less than half the 18,000 VA primary care patients in 5 VA centers had any psychiatric condition, and < 65% had cardiovascular disease, pulmonary disease, or cancer.27 All veterans in this study had medical and psychiatric comorbidity.
In contrast, a large ED sample described by Yokell and colleagues found chronic mental conditions in 33.9%, circulatory disorders in 29.1%, and respiratory conditions in 25.6% of their sample.16 Bohnert and associates found a significantly elevated hazard ratio (HR) for any psychiatric disorder in a sample of nearly 4,500 veterans. There was variation in the HR when individual psychiatric diagnoses were broken out, with bipolar disorder having the largest HR and schizophrenia having the lowest but still elevated HR.9 In this study, individual diagnoses were not broken out because the smaller sample size could diminish the clinical significance of any apparent differences.
Edlund and colleagues found that < 8% of veterans treated with opioids for chronic noncancer pain had nonopioid SUDD.10 Bohnert and colleagues found an HR of 21.95 for overdose death among those with opioid-use disorders.9 The sample in this study had a much higher incidence of nonopioid SUDD compared with that ub the study by Edlund and colleagues, but none of the veterans in this study had a documented history of opioid use disorder. The absence of opioid use disorders in this sample is unexpected and points to a need for providers to screen for opioid use disorder whenever opioids are prescribed or renewed. If prevention practices were directed only to those with opioid SUDDs, none of the veterans in this study would have been included in those efforts. Non-SUDD providers should address the risks of opioid overdose in veterans with sleep apnea, morbid obesity, pulmonary or cardiovascular diseases, and/or a history of psychiatric disorders.
Gomes and colleagues found that > 100 mg MED available on the day of overdose doubled the risk of opioid-related mortality.18 The VA/DoD Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain identifies 200 mg MED as a threshold to define high-dose opioid therapy.28 Fulton-Kehoe and colleagues found that 28% of overdose victims were prescribed < 50 mg MED.29 In this study, the average dose available to veterans was > 100 mg MED; however, one-third of all study veterans had < 50 mg MED available. Using a threshold dose of 50 mg MED to target prevention efforts would capture only two-thirds of those who experienced overdose; a 200 mg MED threshold would exclude the majority, based on the average MED in each group in this study. Overdose education should be provided to veterans with access to any dose of opioids.
Use of BZDs with opioids may result in greater central nervous system (CNS) depression, pharmacokinetic interactions, or pharmacodynamic interactions at the µ opioid receptor.30-32 About one-third of veterans in this study were prescribed opioids and BZDs concurrently, a combination noted in about 33% of opioid overdose deaths reported by the CDC.24 Individuals taking methadone combined with BZDs have been found to have severe medical outcomes.33 If preventive efforts are targeted to those receiving opioids and other CNS depressants, such as BZDs, about half (42%) the veterans in this study would not receive a potentially life-saving message about preventing overdoses. All veterans with opioids should be educated about the additional risk of overdose posed by drug interactions with other CNS depressants.
The time since the last fill of opioid prescription ranged from 0 to 28 days. This time frame indicates that some overdoses may have occurred on the day an opioid was filled but most occurred near the end of the expected days’ supply. Because information about adherence or use of the opioid was not studied, it cannot be assumed that medication misuse is the primary reason for the overdose. Delivering prevention efforts only at the time of medication dispensing would be insufficient. Clinicians should review local and remote prescription data, including via their states’ prescription drug monitoring program when discussing the risk of overdose with veterans.
Most veterans had at least 1 UDS result in the chart. Although half the UDSs obtained reflected prescribed medications, the possibility of aberrant behaviors, which increases the risk of overdose, cannot be ruled out with the methods used in this study.34 Medication management agreements that require UDSs for veterans with chronic pain were not mandatory at the George E. Wahlen VAMC during the study period, and those used did not mandate discontinuation of opioid therapy if suspected aberrant behaviors were present.
A Utah study based on interviews of overdose victims’ next-of-kin found that 76% were concerned about victims’ aberrant behaviors, such as medication misuse, prior to the death.22 In contrast, a study of commercial and Medicaid recipients estimated medication misuse rates in
≤ 30% of the sample.35 Urine drug screening results not reflective of the prescribed regimens have been found in up to 50% of patients receiving chronic opioid therapy.
The UDS findings in this study were determined by the authors and did not capture clinical decisions or interpretations made after results were available or whether these decisions resulted in overdose prevention strategies, such as targeted education or changes in prescription availability. Targeting preventive efforts toward veterans only with UDS results suggesting medication misuse would have missed more than half the veterans in this study. Urine drug screening should be used as a clinical monitoring tool whenever opioids, BZDs, or other substances are used or prescribed.
The VA now has a nationwide program, Opioid Overdose Education and Naloxone Distribution (OEND) promoting overdose education and take-home naloxone distribution for providers and patients to prevent opioid-related overdose deaths. A national SharePoint site has been created within the VA that lists resources to support this effort.
Almost all veterans in this review survived the overdose and were hospitalized following the ED visit. Other investigators also have found that the majority (51% to 98%) of overdose victims reaching the ED survived, but fewer patients (3% to 51%) in those studies were hospitalized.16,36 It is unknown whether there are differences in risk factors associated with survived or fatal overdoses.
Limitations
Although Utah ranked third for drug overdose death rates in 2008 and had the highest death rate among veterans from 2001 to 2009, this review captured only overdoses among veterans treated during the study period at the George E. Wahlen VAMC ED.5,6 The number and characteristics of veterans during this same period who were treated for overdose in other community EDs or urgent care centers throughout Utah is unknown.
The definition of opioid and BZD dose available in this study may not represent actual use of opioids or BZDs because it was based on chart review of prescription dispensing information and UDS procedures at the George E. Wahlen VAMC, and medication misuse cannot be ruled out. This study did not evaluate specific aberrant behaviors.
Conclusion
Current overdose prevention screening efforts primarily identify patients on high-dose opioids and those with SUDD. Many veterans in this study were older than the average U.S. victims’ age, did not have SUDD, were prescribed opioid doses not considered high risk by current guidelines, were nearer the end of their medication supply, and had UDS reflective of prescribed medications. This study suggests that any veteran with access to opioids, whether prescribed or not, is at risk for an opioid overdose. Established risk factors may aid in developing overdose prevention programs, but prevention should not be limited to veterans with prescribed opioids and known risk factors. Prescribers should screen for opioid use disorder whenever opioids are prescribed and continue to screen throughout therapy. Broader screening for overdose risk is needed to avoid missing important opportunities for overdose prevention.
Acknowledgments
Gale Anderson, VISN 19 PBM Data Manager, performed initial data query for the study.
References
1. Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR. 2015;64(50):1-5.
2. Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374(2):154-163.
3. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981-1985.
4. Bohnert AS, Ilgen MA, Galea S, McCarthy JF, Blow FC. Accidental poisoning mortality among patients in the Department of Veterans Affairs Health System. Med Care. 2011;49(4):393-396.
5. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-612.
6. Centers for Disease Control and Prevention. Policy impact: prescription, painkiller, overdoses. http://www.cdc.gov/drugoverdose/pdf/policyimpact-prescriptionpainkillerod-a.pdf. Published November 2011. Accessed August 25, 2016.
7. Xu J, Murphy SL, Kochanek KD, Bastian BA; Division of Vital Statistics. Deaths: final data for 2013. http://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_02.pdf. Published February 16, 2016. Accessed August 25, 2016.
8. The Joint Commission. Sentinel event alert issue 49: safe use of opioids in the hospital. https://www.jointcommission.org/assets/1/18/SEA_49_opioids_8_2_12_final.pdf. Published August 8, 2012. Accessed April 25, 2015.
9. Bohnert AS, Ilgen MA, Ignacio RV, McCarthy JF, Valenstein M, Blow FC. Risk of death from accidental overdose associated with psychiatric and substance use disorders. Am J Psychiatry. 2012;169(1):64-70.
10. Edlund MJ, Austen MA, Sullivan MD, et al. Patterns of opioid use for chronic noncancer pain in the Veterans Health Administration from 2009 to 2011. Pain. 2014;155:2337-2343.
11. Jann M, Kennedy WK, Lopez G. Benzodiazepines: a major component in unintentional prescription drug overdoses with opioid analgesics. J Pharm Pract. 2014;27(1):5-16.
12. McMillin G, Kusukawa N, Nelson G. Benzodiazepines.Salt Lake City, UT: ARUP Laboratories; 2012.
13. Naloxone hydrochloride [package insert].Lake Forest, IL: Hospira Inc; 2007.
14. Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012;367(2):146-155.
15. Hoffman JR, Schriger DL, Luo JS. The empiric use of naloxone in patients with altered mental status: a reappraisal. Ann Emerg Med. 1991;20(3):246-252.
16. Yokell MA, Delgado MK, Zaller ND, Wang NE, McGowan SK, Green TC. Presentation of prescription and nonprescription opioid overdoses to US emergency departments. JAMA Intern Med. 2014;174(12):2034-2037.
17. Binswanger I, Gardner E, Gabella B, Broderick K, Glanz K. Development of case criteria to define pharmaceutical opioid and heroin overdoses in clinical records. Platform presented at: Association for Medical Education and Research in Substance Abuse 38th Annual National Conference; November 7, 2014; San Francisco, CA.
18. Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171(7):686-691.
19. Jaeger TM, Lohr RH, Pankratz VS. Symptom-triggered therapy for alcohol withdrawal syndrome in medical inpatients. Mayo Clin Proc. 2001;76(7):695-701.20. Washington State Agency Medical Directors’ Group. Opioid dose clculator. http://www
.agencymeddirectors.wa.gov/Calculator/DoseCalcula tor.htm. Accessed October 10, 2016.
21. EMIT II Plus Benzodiazepine Assay [package insert]. Brea, CA: Beckman Coulter, Inc; 2010.
22. Johnson EM, Lanier WA, Merrill RM, et al. Unintentional prescription opioid-related overdose deaths: description of decedents by next of kin or best contact, Utah, 2008-2009. J Gen Intern Med. 2013;28(4):522-529.
23. Utah Department of Health. Fact sheet: prescription pain medication deaths in Utah, 2012. https://www.health.utah.gov/vipp/pdf/FactSheets/2012RxOpioidDeaths.pdf. Updated October 2013. Accessed October 10, 2016.
24. Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657-659.
25. Bohnert AS, Tracy M, Galea S. Characteristics of drug users who witness many overdoses: implications for overdose prevention. Drug Alcohol Depend. 2012;120(1-3):168-173.
26. Yoon J, Zulman D, Scott JY, Maciejewski ML. Costs associated with multimorbidity among VA patients. Med Care. 2014;52(suppl 3):S31-S36.
27. Yoon J, Yano EM, Altman L, et al. Reducing costs of acute care for ambulatory care-sensitive medical conditions: the central roles of comorbid mental illness. Med Care. 2012;50(8):705-713.
28. Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain. Guideline summary. http://www.va.gov/painmanagement/docs/cpg_opioidtherapy_summary.pdf. Published May 2010. Accessed August 25, 2016.
29. Fulton-Kehoe D, Sullivan MD, Turner JA, et al. Opioid poisonings in Washington state Medicaid: trends, dosing, and guidelines. Med Care. 2015;53(8):679-685.
30. Gudin JA, Mogali S, Jones JD, Comer SD. Risks, management, and monitoring of combination opioid, benzodiazepines, and/or alcohol use. Postgrad Med. 2013;125(4):115-130.
31. Poisnel G, Dhilly M, Le Boisselier R, Barre L, Debruyne D. Comparison of five benzodiazepine-receptor agonists on buprenorphine-induced mu-opioid receptor regulation. J Pharmacol Sci. 2009;110(1):36-46.
32. Webster LR, Cochella S, Dasgupta N, et al. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med. 2011;12(suppl 2):S26-S35.
33. Lee SC, Klein-Schwartz W, Doyon S, Welsh C. Comparison of toxicity associated with nonmedical use of benzodiazepines with buprenorphine or methadone. Drug Alcohol Depend. 2014;138:118-123.
34. Owen GT, Burton AW, Schade CM, Passik S. Urine drug testing: current recommendations and best practices. Pain Physician. 2012;15(suppl 3):ES119–ES133.
35. Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan Braden J, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: the TROUP study. Pain. 2010;150(2):332-339.
36. Sporer KA, Firestone J, Isaacs SM. Out-of-hospital treatment of opioid overdoses in an urban setting. Acad Emerg Med. 1996;3(7):660-667.
Overdose deaths remain epidemic throughout the U.S. The rates of unintentional overdose deaths, increasing by 137% between 2000 and 2014, have been driven by a 4-fold increase in prescription opioid overdoses during that period.1-3
Veterans died of accidental overdose at a rate of 19.85 deaths/ 100,000 people compared with a rate of 10.49 deaths in the general population, based on 2005 data.4 There is wide state-by-state variation with the lowest age-adjusted opioid overdose death rate of 1.9 deaths/100,000 person-years among veterans in Mississippi and the highest rate in Utah of 33.9 deaths/100,000 person-years, using 2001 to 2009 data.5 These data can be compared with a crude general population overdose death rate of 10.6 deaths per 100,000 person-years in Mississippi and 18.4 deaths per 100,000 person-years in the general Utah population during that same period.6
Overdose deaths in the U.S. occur most often in persons aged 25 to 54 years.7 Older age has been associated with iatrogenic opioid overdose in hospitalized patients.8 Pulmonary, cardiovascular, and psychiatric disorders, including past or present substance use, have been associated with an increased risk of opioid overdose.9 However, veterans with substance use disorders are less likely to be prescribed opioids than are nonveterans with substance use disorders.10 Also, concomitant use of sedating medications, such as benzodiazepines (BZDs), can increase mortality from opioid overdose.11 Patients prescribed opioids for chronic pain conditions often take BZDs for various reasons.12 Veterans seem more likely to receive opioids to treat chronic pain but at lower average daily doses than the doses that nonveterans receive.10
Emergency management of life-threatening opioid overdose includes prompt administration of naloxone.13 Naloxone is FDA approved for complete or partial reversal of opioid-induced clinical effects, most critically respiratory depression.14,15 Naloxone administration in the emergency department (ED) may serve as a surrogate for an overdose event. During the study period, naloxone take-home kits were not available in the VA setting.
A 2010 ED study described demographic information and comorbidities in opioid overdose, but the study did not include veterans.16 The clinical characteristics of veterans treated for opioid overdose have not been published. Because identifying characteristics of veterans who overdose may help tailor overdose prevention efforts, the objective of this study is to describe clinical characteristics of veterans treated for opioid overdose.
Methods
A retrospective chart review and archived data study was approved by the University of Utah and VA institutional review boards, and conducted at the George E. Wahlen VAMC in Salt Lake City, Utah. This chart review included veterans who were admitted to the ED and treated with naloxone between January 1, 2009 and January 1, 2013.
The authors used the Pharmacy Benefits Management Data Manager to extract data from the VA Data Warehouse and verified the data by open chart review (Table). The following data were collected: ED visit date (overdose date); demographic information, including age, gender, and race; evidence of next-of-kin or other contact at the same address as the veteran; diagnoses based on ICD-9 codes, including sleep apnea, obesitycardiac disease, pulmonary disease, mental health diagnoses (ICD-9 codes 290-302 [wild card characters (*) included many subdiagnoses]),
cancer, and substance use disorders and/or dependencies (SUDD); tobacco use; VA-issued prescription opioid and BZD availability, including dose, fill dates, quantities dispensed, and day supplies; specialty of opioid prescriber; urine drug screening (UDS) results; and outcome of the overdose.
No standardized research criteria identify overdose in medical chart review.17 For each identified patient, the authors reviewed provider and nursing notes charted during an ED visit that included naloxone administration. The event was included as an opioid overdose when notes indicated that the veteran was unresponsive and given naloxone, which resulted in increased respirations or increased responsiveness. Cases were excluded if the reason for naloxone administration was anything other than opioid overdose.
Medical, mental health, and SUDD diagnoses were included only if the veteran had more than 3 patient care encounters (PCE) with ICD-9 codes for a specific diagnosis entered by providers. A PCE used in the electronic medical record (EMR) helps collect, manage, and display outpatient encounter data, including providers, procedure codes, and diagnostic codes. Tobacco use was extracted from health factors documented during primary care visit screenings. (Health factors help capture data entered in note templates in the EMR and can be used to query trends.) A diagnosis of obesity was based on a calculated body mass index of > 30 kg/m2 on the day of the ED visit date or the most recently charted height and weight. The type of SUDD was stratified into opioids (ICD-9 codes 304.0*), sedatives (ICD-9 code 304.1*), alcohol (ICD-9 code 303.*), and other (ICD-9 codes 304.2-305.9).
The dosage of opioids and BZDs available to a veteran was determined by using methods similar to those described by Gomes and colleagues: the dose of opioids and BZDs available based on prescriptions dispensed during the 120 days prior to the ED visit date and the dose available on the day of the ED visit date if prescription instructions were being followed.18 Prescription opioids and BZDs were converted to daily morphine equivalent dose (MED) and daily lorazepam-equivalent dose (LED), using established methods.19,20
Veterans were stratified into 4 groups based on prescribed medication availability: opioids only, BZDs only, opioids and BZDs, and neither opioids nor BZDs. The specialty of the opioid prescribers was categorized as primary care, pain specialist, surgeon, emergency specialist, or hospitalist (discharge prescription). Veteran EMRs contain a list of medications obtained outside the VA facility, referred to as non-VA prescriptions. These medications werenot included in the analysis because accuracy could not be verified.
A study author reviewed the results of any UDS performed up to 120 days before the ED visit date to determine whether the result reflected the currently prescribed prescription medications. If the UDS was positive for the prescribed opioids and/or BZDs and for any nonprescribed drug, including alcohol, the UDS was classified as not reflective. If the prescribed BZD was alprazolam, clonazepam, or lorazepam, a BZD-positive UDS was not required for the UDS to be considered reflective because of the sensitivity of the UDS BZD immunoassay
used at the George E. Wahlen VAMC clinical laboratory.21
Outcomes of the overdose were categorized as discharged, hospitalized, or deceased. Descriptive statistical analyses were performed using Microsoft Excel. Group comparisons were performed using Pearson chi-square or Student t test.
Results
The ED at the George E. Wahlen VAMC averages 64 visits per day, almost 94,000 visits within the study period. One hundred seventy ED visits between January 1, 2009 and January 1, 2013, involved naloxone administration. Ninety-two visits met the inclusion criteria of opioid overdose, representing about 0.002% of all ED visits at this facility (Figure 1). Six veterans had multiple ED visits within the study period, including 4 veterans who were in the opioid-only group.
The majority of veterans in this study were non-Hispanic white (n = 83, 90%), male (n = 88, 96%), with a mean age of 63 years. Less than 40% listed a next-of-kin or contact person living at their address.
Based on prescriptions available within 120 days before the overdose, 67 veterans (73%) possessed opioid and/or BZD prescriptions. In this group, the MED available on the day of the ED visit ranged from 7.5 mg to 830 mg. The MED was ≤ 200 mg in 71.6% and ≤ 50 mg in 34.3% of these cases. Veterans prescribed both opioids and BZDs had higher MED (average, 259 mg) available within 120 days of the ED visit than did those prescribed opioids only (average, 118 mg) (P = .015; SD, 132.9). The LED ranged from 1 mg to 12 mg for veterans with available BZDs.
Based on prescriptions available on the day of opioid overdose, 53 veterans (58%) had opioid prescriptions. The ranges of MED and LED available on the day of overdose were the same as the 120-day availability period. The average MED was 183 mg in veterans prescribed both opioids and BZDs and 126 mg in those prescribed opioids only (P = .283; SD, 168.65; Figure 2). The time between the last opioid fill date and the overdose visit date averaged 20 days (range, 0 to 28 days) in veterans prescribed opioids.
All veterans had at least 1 diagnosis that in previous studies was associated with increased risk of overdose.9,15 The most common diagnoses included cardiovascular diseases, mental health disorders, pulmonary diseases, and cancer. Other SUDDs not including tobacco use were documented in at least half the veterans with prescribed opioids and/or BZDs. No veteran in the sample had a documented history of opioid SUDD.
Hydrocodone products were available in > 50% of cases. None of the veterans were prescribed buprenorphine products; other opioids, including tramadol, comprised the remainder (Figure 3). Primary care providers prescribed 72% of opioid prescriptions, with pain specialists, discharge physicians, ED providers, and surgeons prescribing the rest. When both opioids and BZDs were available, combinations of a hydrocodone product plus clonazepam or lorazepam were most common. The time between the last opioid fill date and the overdose visit date averaged 20 days (range, 0 to 28 days) in veterans prescribed opioids.
Overall, 64% of the sample had UDS results prior to the ED visit. Of veterans prescribed opioids and/or BZDs, 53% of UDSs reflected prescribed regimens.
On the day of the ED visit, 1 death occurred. Ninety-one veterans (99%) survived the overdose; 79 veterans (86%) were hospitalized, most for < 24 hours.
Discussion
This retrospective review identified 92 veterans who were treated with naloxone in the ED for opioid overdose during a 4-year period at the George E. Wahlen VAMC. Seventy-eight cases were excluded because the reason entered in charts for naloxone administration was itching, constipation, altered mental status, or unclear documentation.
Veterans in this study were older on average than the overdose fatalities in the U.S. Opioid overdose deaths in the U.S. and in Utah occur most frequently in non-Hispanic white men aged between 35 and 54 years.7,22,23 In the 2010 Nationwide Emergency Department Sample of 136,000 opioid overdoses, of which 98% survived, most were aged 18 to 54 years.16 The older age in this study most likely reflects the age range of veterans served in the VHA; however, as more young veterans enter the VHA, the age range of overdose victims may more closely resemble the age ranges found in previous studies. Post hoc analysis showed 8 veterans (9%) with probable intentional opioid overdose based on chart review, whereas the incidence of intentional prescription drug overdose in the U.S. is 17.1%.24
In Utah, almost 93% of fatal overdoses occur at a residential location.22 Less than half the veterans in this study had a contact or next-of-kin listed as living at the same address. Although veterans may not have identified someone living with them, in many cases, it is likely another person witnessed the overdose. Relying on EMRs to identify who should receive prevention education, in addition to the veteran, may result in missed opportunities to include another person likely to witness an overdose.25 Prescribers should make a conscious effort to ask veterans to identify someone who may be able to assist with rescue efforts in the event of an overdose.
Diagnoses associated with increased risk of opioid overdose death include sleep apnea, morbid obesity, pulmonary or cardiovascular diseases, and/or a history of psychiatric disorders and SUDD.8,9,16 In a large sample of older veterans, only 64% had at least 1 medical or psychiatric diagnosis.26 Less than half the 18,000 VA primary care patients in 5 VA centers had any psychiatric condition, and < 65% had cardiovascular disease, pulmonary disease, or cancer.27 All veterans in this study had medical and psychiatric comorbidity.
In contrast, a large ED sample described by Yokell and colleagues found chronic mental conditions in 33.9%, circulatory disorders in 29.1%, and respiratory conditions in 25.6% of their sample.16 Bohnert and associates found a significantly elevated hazard ratio (HR) for any psychiatric disorder in a sample of nearly 4,500 veterans. There was variation in the HR when individual psychiatric diagnoses were broken out, with bipolar disorder having the largest HR and schizophrenia having the lowest but still elevated HR.9 In this study, individual diagnoses were not broken out because the smaller sample size could diminish the clinical significance of any apparent differences.
Edlund and colleagues found that < 8% of veterans treated with opioids for chronic noncancer pain had nonopioid SUDD.10 Bohnert and colleagues found an HR of 21.95 for overdose death among those with opioid-use disorders.9 The sample in this study had a much higher incidence of nonopioid SUDD compared with that ub the study by Edlund and colleagues, but none of the veterans in this study had a documented history of opioid use disorder. The absence of opioid use disorders in this sample is unexpected and points to a need for providers to screen for opioid use disorder whenever opioids are prescribed or renewed. If prevention practices were directed only to those with opioid SUDDs, none of the veterans in this study would have been included in those efforts. Non-SUDD providers should address the risks of opioid overdose in veterans with sleep apnea, morbid obesity, pulmonary or cardiovascular diseases, and/or a history of psychiatric disorders.
Gomes and colleagues found that > 100 mg MED available on the day of overdose doubled the risk of opioid-related mortality.18 The VA/DoD Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain identifies 200 mg MED as a threshold to define high-dose opioid therapy.28 Fulton-Kehoe and colleagues found that 28% of overdose victims were prescribed < 50 mg MED.29 In this study, the average dose available to veterans was > 100 mg MED; however, one-third of all study veterans had < 50 mg MED available. Using a threshold dose of 50 mg MED to target prevention efforts would capture only two-thirds of those who experienced overdose; a 200 mg MED threshold would exclude the majority, based on the average MED in each group in this study. Overdose education should be provided to veterans with access to any dose of opioids.
Use of BZDs with opioids may result in greater central nervous system (CNS) depression, pharmacokinetic interactions, or pharmacodynamic interactions at the µ opioid receptor.30-32 About one-third of veterans in this study were prescribed opioids and BZDs concurrently, a combination noted in about 33% of opioid overdose deaths reported by the CDC.24 Individuals taking methadone combined with BZDs have been found to have severe medical outcomes.33 If preventive efforts are targeted to those receiving opioids and other CNS depressants, such as BZDs, about half (42%) the veterans in this study would not receive a potentially life-saving message about preventing overdoses. All veterans with opioids should be educated about the additional risk of overdose posed by drug interactions with other CNS depressants.
The time since the last fill of opioid prescription ranged from 0 to 28 days. This time frame indicates that some overdoses may have occurred on the day an opioid was filled but most occurred near the end of the expected days’ supply. Because information about adherence or use of the opioid was not studied, it cannot be assumed that medication misuse is the primary reason for the overdose. Delivering prevention efforts only at the time of medication dispensing would be insufficient. Clinicians should review local and remote prescription data, including via their states’ prescription drug monitoring program when discussing the risk of overdose with veterans.
Most veterans had at least 1 UDS result in the chart. Although half the UDSs obtained reflected prescribed medications, the possibility of aberrant behaviors, which increases the risk of overdose, cannot be ruled out with the methods used in this study.34 Medication management agreements that require UDSs for veterans with chronic pain were not mandatory at the George E. Wahlen VAMC during the study period, and those used did not mandate discontinuation of opioid therapy if suspected aberrant behaviors were present.
A Utah study based on interviews of overdose victims’ next-of-kin found that 76% were concerned about victims’ aberrant behaviors, such as medication misuse, prior to the death.22 In contrast, a study of commercial and Medicaid recipients estimated medication misuse rates in
≤ 30% of the sample.35 Urine drug screening results not reflective of the prescribed regimens have been found in up to 50% of patients receiving chronic opioid therapy.
The UDS findings in this study were determined by the authors and did not capture clinical decisions or interpretations made after results were available or whether these decisions resulted in overdose prevention strategies, such as targeted education or changes in prescription availability. Targeting preventive efforts toward veterans only with UDS results suggesting medication misuse would have missed more than half the veterans in this study. Urine drug screening should be used as a clinical monitoring tool whenever opioids, BZDs, or other substances are used or prescribed.
The VA now has a nationwide program, Opioid Overdose Education and Naloxone Distribution (OEND) promoting overdose education and take-home naloxone distribution for providers and patients to prevent opioid-related overdose deaths. A national SharePoint site has been created within the VA that lists resources to support this effort.
Almost all veterans in this review survived the overdose and were hospitalized following the ED visit. Other investigators also have found that the majority (51% to 98%) of overdose victims reaching the ED survived, but fewer patients (3% to 51%) in those studies were hospitalized.16,36 It is unknown whether there are differences in risk factors associated with survived or fatal overdoses.
Limitations
Although Utah ranked third for drug overdose death rates in 2008 and had the highest death rate among veterans from 2001 to 2009, this review captured only overdoses among veterans treated during the study period at the George E. Wahlen VAMC ED.5,6 The number and characteristics of veterans during this same period who were treated for overdose in other community EDs or urgent care centers throughout Utah is unknown.
The definition of opioid and BZD dose available in this study may not represent actual use of opioids or BZDs because it was based on chart review of prescription dispensing information and UDS procedures at the George E. Wahlen VAMC, and medication misuse cannot be ruled out. This study did not evaluate specific aberrant behaviors.
Conclusion
Current overdose prevention screening efforts primarily identify patients on high-dose opioids and those with SUDD. Many veterans in this study were older than the average U.S. victims’ age, did not have SUDD, were prescribed opioid doses not considered high risk by current guidelines, were nearer the end of their medication supply, and had UDS reflective of prescribed medications. This study suggests that any veteran with access to opioids, whether prescribed or not, is at risk for an opioid overdose. Established risk factors may aid in developing overdose prevention programs, but prevention should not be limited to veterans with prescribed opioids and known risk factors. Prescribers should screen for opioid use disorder whenever opioids are prescribed and continue to screen throughout therapy. Broader screening for overdose risk is needed to avoid missing important opportunities for overdose prevention.
Acknowledgments
Gale Anderson, VISN 19 PBM Data Manager, performed initial data query for the study.
Overdose deaths remain epidemic throughout the U.S. The rates of unintentional overdose deaths, increasing by 137% between 2000 and 2014, have been driven by a 4-fold increase in prescription opioid overdoses during that period.1-3
Veterans died of accidental overdose at a rate of 19.85 deaths/ 100,000 people compared with a rate of 10.49 deaths in the general population, based on 2005 data.4 There is wide state-by-state variation with the lowest age-adjusted opioid overdose death rate of 1.9 deaths/100,000 person-years among veterans in Mississippi and the highest rate in Utah of 33.9 deaths/100,000 person-years, using 2001 to 2009 data.5 These data can be compared with a crude general population overdose death rate of 10.6 deaths per 100,000 person-years in Mississippi and 18.4 deaths per 100,000 person-years in the general Utah population during that same period.6
Overdose deaths in the U.S. occur most often in persons aged 25 to 54 years.7 Older age has been associated with iatrogenic opioid overdose in hospitalized patients.8 Pulmonary, cardiovascular, and psychiatric disorders, including past or present substance use, have been associated with an increased risk of opioid overdose.9 However, veterans with substance use disorders are less likely to be prescribed opioids than are nonveterans with substance use disorders.10 Also, concomitant use of sedating medications, such as benzodiazepines (BZDs), can increase mortality from opioid overdose.11 Patients prescribed opioids for chronic pain conditions often take BZDs for various reasons.12 Veterans seem more likely to receive opioids to treat chronic pain but at lower average daily doses than the doses that nonveterans receive.10
Emergency management of life-threatening opioid overdose includes prompt administration of naloxone.13 Naloxone is FDA approved for complete or partial reversal of opioid-induced clinical effects, most critically respiratory depression.14,15 Naloxone administration in the emergency department (ED) may serve as a surrogate for an overdose event. During the study period, naloxone take-home kits were not available in the VA setting.
A 2010 ED study described demographic information and comorbidities in opioid overdose, but the study did not include veterans.16 The clinical characteristics of veterans treated for opioid overdose have not been published. Because identifying characteristics of veterans who overdose may help tailor overdose prevention efforts, the objective of this study is to describe clinical characteristics of veterans treated for opioid overdose.
Methods
A retrospective chart review and archived data study was approved by the University of Utah and VA institutional review boards, and conducted at the George E. Wahlen VAMC in Salt Lake City, Utah. This chart review included veterans who were admitted to the ED and treated with naloxone between January 1, 2009 and January 1, 2013.
The authors used the Pharmacy Benefits Management Data Manager to extract data from the VA Data Warehouse and verified the data by open chart review (Table). The following data were collected: ED visit date (overdose date); demographic information, including age, gender, and race; evidence of next-of-kin or other contact at the same address as the veteran; diagnoses based on ICD-9 codes, including sleep apnea, obesitycardiac disease, pulmonary disease, mental health diagnoses (ICD-9 codes 290-302 [wild card characters (*) included many subdiagnoses]),
cancer, and substance use disorders and/or dependencies (SUDD); tobacco use; VA-issued prescription opioid and BZD availability, including dose, fill dates, quantities dispensed, and day supplies; specialty of opioid prescriber; urine drug screening (UDS) results; and outcome of the overdose.
No standardized research criteria identify overdose in medical chart review.17 For each identified patient, the authors reviewed provider and nursing notes charted during an ED visit that included naloxone administration. The event was included as an opioid overdose when notes indicated that the veteran was unresponsive and given naloxone, which resulted in increased respirations or increased responsiveness. Cases were excluded if the reason for naloxone administration was anything other than opioid overdose.
Medical, mental health, and SUDD diagnoses were included only if the veteran had more than 3 patient care encounters (PCE) with ICD-9 codes for a specific diagnosis entered by providers. A PCE used in the electronic medical record (EMR) helps collect, manage, and display outpatient encounter data, including providers, procedure codes, and diagnostic codes. Tobacco use was extracted from health factors documented during primary care visit screenings. (Health factors help capture data entered in note templates in the EMR and can be used to query trends.) A diagnosis of obesity was based on a calculated body mass index of > 30 kg/m2 on the day of the ED visit date or the most recently charted height and weight. The type of SUDD was stratified into opioids (ICD-9 codes 304.0*), sedatives (ICD-9 code 304.1*), alcohol (ICD-9 code 303.*), and other (ICD-9 codes 304.2-305.9).
The dosage of opioids and BZDs available to a veteran was determined by using methods similar to those described by Gomes and colleagues: the dose of opioids and BZDs available based on prescriptions dispensed during the 120 days prior to the ED visit date and the dose available on the day of the ED visit date if prescription instructions were being followed.18 Prescription opioids and BZDs were converted to daily morphine equivalent dose (MED) and daily lorazepam-equivalent dose (LED), using established methods.19,20
Veterans were stratified into 4 groups based on prescribed medication availability: opioids only, BZDs only, opioids and BZDs, and neither opioids nor BZDs. The specialty of the opioid prescribers was categorized as primary care, pain specialist, surgeon, emergency specialist, or hospitalist (discharge prescription). Veteran EMRs contain a list of medications obtained outside the VA facility, referred to as non-VA prescriptions. These medications werenot included in the analysis because accuracy could not be verified.
A study author reviewed the results of any UDS performed up to 120 days before the ED visit date to determine whether the result reflected the currently prescribed prescription medications. If the UDS was positive for the prescribed opioids and/or BZDs and for any nonprescribed drug, including alcohol, the UDS was classified as not reflective. If the prescribed BZD was alprazolam, clonazepam, or lorazepam, a BZD-positive UDS was not required for the UDS to be considered reflective because of the sensitivity of the UDS BZD immunoassay
used at the George E. Wahlen VAMC clinical laboratory.21
Outcomes of the overdose were categorized as discharged, hospitalized, or deceased. Descriptive statistical analyses were performed using Microsoft Excel. Group comparisons were performed using Pearson chi-square or Student t test.
Results
The ED at the George E. Wahlen VAMC averages 64 visits per day, almost 94,000 visits within the study period. One hundred seventy ED visits between January 1, 2009 and January 1, 2013, involved naloxone administration. Ninety-two visits met the inclusion criteria of opioid overdose, representing about 0.002% of all ED visits at this facility (Figure 1). Six veterans had multiple ED visits within the study period, including 4 veterans who were in the opioid-only group.
The majority of veterans in this study were non-Hispanic white (n = 83, 90%), male (n = 88, 96%), with a mean age of 63 years. Less than 40% listed a next-of-kin or contact person living at their address.
Based on prescriptions available within 120 days before the overdose, 67 veterans (73%) possessed opioid and/or BZD prescriptions. In this group, the MED available on the day of the ED visit ranged from 7.5 mg to 830 mg. The MED was ≤ 200 mg in 71.6% and ≤ 50 mg in 34.3% of these cases. Veterans prescribed both opioids and BZDs had higher MED (average, 259 mg) available within 120 days of the ED visit than did those prescribed opioids only (average, 118 mg) (P = .015; SD, 132.9). The LED ranged from 1 mg to 12 mg for veterans with available BZDs.
Based on prescriptions available on the day of opioid overdose, 53 veterans (58%) had opioid prescriptions. The ranges of MED and LED available on the day of overdose were the same as the 120-day availability period. The average MED was 183 mg in veterans prescribed both opioids and BZDs and 126 mg in those prescribed opioids only (P = .283; SD, 168.65; Figure 2). The time between the last opioid fill date and the overdose visit date averaged 20 days (range, 0 to 28 days) in veterans prescribed opioids.
All veterans had at least 1 diagnosis that in previous studies was associated with increased risk of overdose.9,15 The most common diagnoses included cardiovascular diseases, mental health disorders, pulmonary diseases, and cancer. Other SUDDs not including tobacco use were documented in at least half the veterans with prescribed opioids and/or BZDs. No veteran in the sample had a documented history of opioid SUDD.
Hydrocodone products were available in > 50% of cases. None of the veterans were prescribed buprenorphine products; other opioids, including tramadol, comprised the remainder (Figure 3). Primary care providers prescribed 72% of opioid prescriptions, with pain specialists, discharge physicians, ED providers, and surgeons prescribing the rest. When both opioids and BZDs were available, combinations of a hydrocodone product plus clonazepam or lorazepam were most common. The time between the last opioid fill date and the overdose visit date averaged 20 days (range, 0 to 28 days) in veterans prescribed opioids.
Overall, 64% of the sample had UDS results prior to the ED visit. Of veterans prescribed opioids and/or BZDs, 53% of UDSs reflected prescribed regimens.
On the day of the ED visit, 1 death occurred. Ninety-one veterans (99%) survived the overdose; 79 veterans (86%) were hospitalized, most for < 24 hours.
Discussion
This retrospective review identified 92 veterans who were treated with naloxone in the ED for opioid overdose during a 4-year period at the George E. Wahlen VAMC. Seventy-eight cases were excluded because the reason entered in charts for naloxone administration was itching, constipation, altered mental status, or unclear documentation.
Veterans in this study were older on average than the overdose fatalities in the U.S. Opioid overdose deaths in the U.S. and in Utah occur most frequently in non-Hispanic white men aged between 35 and 54 years.7,22,23 In the 2010 Nationwide Emergency Department Sample of 136,000 opioid overdoses, of which 98% survived, most were aged 18 to 54 years.16 The older age in this study most likely reflects the age range of veterans served in the VHA; however, as more young veterans enter the VHA, the age range of overdose victims may more closely resemble the age ranges found in previous studies. Post hoc analysis showed 8 veterans (9%) with probable intentional opioid overdose based on chart review, whereas the incidence of intentional prescription drug overdose in the U.S. is 17.1%.24
In Utah, almost 93% of fatal overdoses occur at a residential location.22 Less than half the veterans in this study had a contact or next-of-kin listed as living at the same address. Although veterans may not have identified someone living with them, in many cases, it is likely another person witnessed the overdose. Relying on EMRs to identify who should receive prevention education, in addition to the veteran, may result in missed opportunities to include another person likely to witness an overdose.25 Prescribers should make a conscious effort to ask veterans to identify someone who may be able to assist with rescue efforts in the event of an overdose.
Diagnoses associated with increased risk of opioid overdose death include sleep apnea, morbid obesity, pulmonary or cardiovascular diseases, and/or a history of psychiatric disorders and SUDD.8,9,16 In a large sample of older veterans, only 64% had at least 1 medical or psychiatric diagnosis.26 Less than half the 18,000 VA primary care patients in 5 VA centers had any psychiatric condition, and < 65% had cardiovascular disease, pulmonary disease, or cancer.27 All veterans in this study had medical and psychiatric comorbidity.
In contrast, a large ED sample described by Yokell and colleagues found chronic mental conditions in 33.9%, circulatory disorders in 29.1%, and respiratory conditions in 25.6% of their sample.16 Bohnert and associates found a significantly elevated hazard ratio (HR) for any psychiatric disorder in a sample of nearly 4,500 veterans. There was variation in the HR when individual psychiatric diagnoses were broken out, with bipolar disorder having the largest HR and schizophrenia having the lowest but still elevated HR.9 In this study, individual diagnoses were not broken out because the smaller sample size could diminish the clinical significance of any apparent differences.
Edlund and colleagues found that < 8% of veterans treated with opioids for chronic noncancer pain had nonopioid SUDD.10 Bohnert and colleagues found an HR of 21.95 for overdose death among those with opioid-use disorders.9 The sample in this study had a much higher incidence of nonopioid SUDD compared with that ub the study by Edlund and colleagues, but none of the veterans in this study had a documented history of opioid use disorder. The absence of opioid use disorders in this sample is unexpected and points to a need for providers to screen for opioid use disorder whenever opioids are prescribed or renewed. If prevention practices were directed only to those with opioid SUDDs, none of the veterans in this study would have been included in those efforts. Non-SUDD providers should address the risks of opioid overdose in veterans with sleep apnea, morbid obesity, pulmonary or cardiovascular diseases, and/or a history of psychiatric disorders.
Gomes and colleagues found that > 100 mg MED available on the day of overdose doubled the risk of opioid-related mortality.18 The VA/DoD Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain identifies 200 mg MED as a threshold to define high-dose opioid therapy.28 Fulton-Kehoe and colleagues found that 28% of overdose victims were prescribed < 50 mg MED.29 In this study, the average dose available to veterans was > 100 mg MED; however, one-third of all study veterans had < 50 mg MED available. Using a threshold dose of 50 mg MED to target prevention efforts would capture only two-thirds of those who experienced overdose; a 200 mg MED threshold would exclude the majority, based on the average MED in each group in this study. Overdose education should be provided to veterans with access to any dose of opioids.
Use of BZDs with opioids may result in greater central nervous system (CNS) depression, pharmacokinetic interactions, or pharmacodynamic interactions at the µ opioid receptor.30-32 About one-third of veterans in this study were prescribed opioids and BZDs concurrently, a combination noted in about 33% of opioid overdose deaths reported by the CDC.24 Individuals taking methadone combined with BZDs have been found to have severe medical outcomes.33 If preventive efforts are targeted to those receiving opioids and other CNS depressants, such as BZDs, about half (42%) the veterans in this study would not receive a potentially life-saving message about preventing overdoses. All veterans with opioids should be educated about the additional risk of overdose posed by drug interactions with other CNS depressants.
The time since the last fill of opioid prescription ranged from 0 to 28 days. This time frame indicates that some overdoses may have occurred on the day an opioid was filled but most occurred near the end of the expected days’ supply. Because information about adherence or use of the opioid was not studied, it cannot be assumed that medication misuse is the primary reason for the overdose. Delivering prevention efforts only at the time of medication dispensing would be insufficient. Clinicians should review local and remote prescription data, including via their states’ prescription drug monitoring program when discussing the risk of overdose with veterans.
Most veterans had at least 1 UDS result in the chart. Although half the UDSs obtained reflected prescribed medications, the possibility of aberrant behaviors, which increases the risk of overdose, cannot be ruled out with the methods used in this study.34 Medication management agreements that require UDSs for veterans with chronic pain were not mandatory at the George E. Wahlen VAMC during the study period, and those used did not mandate discontinuation of opioid therapy if suspected aberrant behaviors were present.
A Utah study based on interviews of overdose victims’ next-of-kin found that 76% were concerned about victims’ aberrant behaviors, such as medication misuse, prior to the death.22 In contrast, a study of commercial and Medicaid recipients estimated medication misuse rates in
≤ 30% of the sample.35 Urine drug screening results not reflective of the prescribed regimens have been found in up to 50% of patients receiving chronic opioid therapy.
The UDS findings in this study were determined by the authors and did not capture clinical decisions or interpretations made after results were available or whether these decisions resulted in overdose prevention strategies, such as targeted education or changes in prescription availability. Targeting preventive efforts toward veterans only with UDS results suggesting medication misuse would have missed more than half the veterans in this study. Urine drug screening should be used as a clinical monitoring tool whenever opioids, BZDs, or other substances are used or prescribed.
The VA now has a nationwide program, Opioid Overdose Education and Naloxone Distribution (OEND) promoting overdose education and take-home naloxone distribution for providers and patients to prevent opioid-related overdose deaths. A national SharePoint site has been created within the VA that lists resources to support this effort.
Almost all veterans in this review survived the overdose and were hospitalized following the ED visit. Other investigators also have found that the majority (51% to 98%) of overdose victims reaching the ED survived, but fewer patients (3% to 51%) in those studies were hospitalized.16,36 It is unknown whether there are differences in risk factors associated with survived or fatal overdoses.
Limitations
Although Utah ranked third for drug overdose death rates in 2008 and had the highest death rate among veterans from 2001 to 2009, this review captured only overdoses among veterans treated during the study period at the George E. Wahlen VAMC ED.5,6 The number and characteristics of veterans during this same period who were treated for overdose in other community EDs or urgent care centers throughout Utah is unknown.
The definition of opioid and BZD dose available in this study may not represent actual use of opioids or BZDs because it was based on chart review of prescription dispensing information and UDS procedures at the George E. Wahlen VAMC, and medication misuse cannot be ruled out. This study did not evaluate specific aberrant behaviors.
Conclusion
Current overdose prevention screening efforts primarily identify patients on high-dose opioids and those with SUDD. Many veterans in this study were older than the average U.S. victims’ age, did not have SUDD, were prescribed opioid doses not considered high risk by current guidelines, were nearer the end of their medication supply, and had UDS reflective of prescribed medications. This study suggests that any veteran with access to opioids, whether prescribed or not, is at risk for an opioid overdose. Established risk factors may aid in developing overdose prevention programs, but prevention should not be limited to veterans with prescribed opioids and known risk factors. Prescribers should screen for opioid use disorder whenever opioids are prescribed and continue to screen throughout therapy. Broader screening for overdose risk is needed to avoid missing important opportunities for overdose prevention.
Acknowledgments
Gale Anderson, VISN 19 PBM Data Manager, performed initial data query for the study.
References
1. Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR. 2015;64(50):1-5.
2. Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374(2):154-163.
3. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981-1985.
4. Bohnert AS, Ilgen MA, Galea S, McCarthy JF, Blow FC. Accidental poisoning mortality among patients in the Department of Veterans Affairs Health System. Med Care. 2011;49(4):393-396.
5. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-612.
6. Centers for Disease Control and Prevention. Policy impact: prescription, painkiller, overdoses. http://www.cdc.gov/drugoverdose/pdf/policyimpact-prescriptionpainkillerod-a.pdf. Published November 2011. Accessed August 25, 2016.
7. Xu J, Murphy SL, Kochanek KD, Bastian BA; Division of Vital Statistics. Deaths: final data for 2013. http://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_02.pdf. Published February 16, 2016. Accessed August 25, 2016.
8. The Joint Commission. Sentinel event alert issue 49: safe use of opioids in the hospital. https://www.jointcommission.org/assets/1/18/SEA_49_opioids_8_2_12_final.pdf. Published August 8, 2012. Accessed April 25, 2015.
9. Bohnert AS, Ilgen MA, Ignacio RV, McCarthy JF, Valenstein M, Blow FC. Risk of death from accidental overdose associated with psychiatric and substance use disorders. Am J Psychiatry. 2012;169(1):64-70.
10. Edlund MJ, Austen MA, Sullivan MD, et al. Patterns of opioid use for chronic noncancer pain in the Veterans Health Administration from 2009 to 2011. Pain. 2014;155:2337-2343.
11. Jann M, Kennedy WK, Lopez G. Benzodiazepines: a major component in unintentional prescription drug overdoses with opioid analgesics. J Pharm Pract. 2014;27(1):5-16.
12. McMillin G, Kusukawa N, Nelson G. Benzodiazepines.Salt Lake City, UT: ARUP Laboratories; 2012.
13. Naloxone hydrochloride [package insert].Lake Forest, IL: Hospira Inc; 2007.
14. Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012;367(2):146-155.
15. Hoffman JR, Schriger DL, Luo JS. The empiric use of naloxone in patients with altered mental status: a reappraisal. Ann Emerg Med. 1991;20(3):246-252.
16. Yokell MA, Delgado MK, Zaller ND, Wang NE, McGowan SK, Green TC. Presentation of prescription and nonprescription opioid overdoses to US emergency departments. JAMA Intern Med. 2014;174(12):2034-2037.
17. Binswanger I, Gardner E, Gabella B, Broderick K, Glanz K. Development of case criteria to define pharmaceutical opioid and heroin overdoses in clinical records. Platform presented at: Association for Medical Education and Research in Substance Abuse 38th Annual National Conference; November 7, 2014; San Francisco, CA.
18. Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171(7):686-691.
19. Jaeger TM, Lohr RH, Pankratz VS. Symptom-triggered therapy for alcohol withdrawal syndrome in medical inpatients. Mayo Clin Proc. 2001;76(7):695-701.20. Washington State Agency Medical Directors’ Group. Opioid dose clculator. http://www
.agencymeddirectors.wa.gov/Calculator/DoseCalcula tor.htm. Accessed October 10, 2016.
21. EMIT II Plus Benzodiazepine Assay [package insert]. Brea, CA: Beckman Coulter, Inc; 2010.
22. Johnson EM, Lanier WA, Merrill RM, et al. Unintentional prescription opioid-related overdose deaths: description of decedents by next of kin or best contact, Utah, 2008-2009. J Gen Intern Med. 2013;28(4):522-529.
23. Utah Department of Health. Fact sheet: prescription pain medication deaths in Utah, 2012. https://www.health.utah.gov/vipp/pdf/FactSheets/2012RxOpioidDeaths.pdf. Updated October 2013. Accessed October 10, 2016.
24. Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657-659.
25. Bohnert AS, Tracy M, Galea S. Characteristics of drug users who witness many overdoses: implications for overdose prevention. Drug Alcohol Depend. 2012;120(1-3):168-173.
26. Yoon J, Zulman D, Scott JY, Maciejewski ML. Costs associated with multimorbidity among VA patients. Med Care. 2014;52(suppl 3):S31-S36.
27. Yoon J, Yano EM, Altman L, et al. Reducing costs of acute care for ambulatory care-sensitive medical conditions: the central roles of comorbid mental illness. Med Care. 2012;50(8):705-713.
28. Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain. Guideline summary. http://www.va.gov/painmanagement/docs/cpg_opioidtherapy_summary.pdf. Published May 2010. Accessed August 25, 2016.
29. Fulton-Kehoe D, Sullivan MD, Turner JA, et al. Opioid poisonings in Washington state Medicaid: trends, dosing, and guidelines. Med Care. 2015;53(8):679-685.
30. Gudin JA, Mogali S, Jones JD, Comer SD. Risks, management, and monitoring of combination opioid, benzodiazepines, and/or alcohol use. Postgrad Med. 2013;125(4):115-130.
31. Poisnel G, Dhilly M, Le Boisselier R, Barre L, Debruyne D. Comparison of five benzodiazepine-receptor agonists on buprenorphine-induced mu-opioid receptor regulation. J Pharmacol Sci. 2009;110(1):36-46.
32. Webster LR, Cochella S, Dasgupta N, et al. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med. 2011;12(suppl 2):S26-S35.
33. Lee SC, Klein-Schwartz W, Doyon S, Welsh C. Comparison of toxicity associated with nonmedical use of benzodiazepines with buprenorphine or methadone. Drug Alcohol Depend. 2014;138:118-123.
34. Owen GT, Burton AW, Schade CM, Passik S. Urine drug testing: current recommendations and best practices. Pain Physician. 2012;15(suppl 3):ES119–ES133.
35. Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan Braden J, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: the TROUP study. Pain. 2010;150(2):332-339.
36. Sporer KA, Firestone J, Isaacs SM. Out-of-hospital treatment of opioid overdoses in an urban setting. Acad Emerg Med. 1996;3(7):660-667.
References
1. Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR. 2015;64(50):1-5.
2. Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374(2):154-163.
3. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981-1985.
4. Bohnert AS, Ilgen MA, Galea S, McCarthy JF, Blow FC. Accidental poisoning mortality among patients in the Department of Veterans Affairs Health System. Med Care. 2011;49(4):393-396.
5. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-612.
6. Centers for Disease Control and Prevention. Policy impact: prescription, painkiller, overdoses. http://www.cdc.gov/drugoverdose/pdf/policyimpact-prescriptionpainkillerod-a.pdf. Published November 2011. Accessed August 25, 2016.
7. Xu J, Murphy SL, Kochanek KD, Bastian BA; Division of Vital Statistics. Deaths: final data for 2013. http://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_02.pdf. Published February 16, 2016. Accessed August 25, 2016.
8. The Joint Commission. Sentinel event alert issue 49: safe use of opioids in the hospital. https://www.jointcommission.org/assets/1/18/SEA_49_opioids_8_2_12_final.pdf. Published August 8, 2012. Accessed April 25, 2015.
9. Bohnert AS, Ilgen MA, Ignacio RV, McCarthy JF, Valenstein M, Blow FC. Risk of death from accidental overdose associated with psychiatric and substance use disorders. Am J Psychiatry. 2012;169(1):64-70.
10. Edlund MJ, Austen MA, Sullivan MD, et al. Patterns of opioid use for chronic noncancer pain in the Veterans Health Administration from 2009 to 2011. Pain. 2014;155:2337-2343.
11. Jann M, Kennedy WK, Lopez G. Benzodiazepines: a major component in unintentional prescription drug overdoses with opioid analgesics. J Pharm Pract. 2014;27(1):5-16.
12. McMillin G, Kusukawa N, Nelson G. Benzodiazepines.Salt Lake City, UT: ARUP Laboratories; 2012.
13. Naloxone hydrochloride [package insert].Lake Forest, IL: Hospira Inc; 2007.
14. Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012;367(2):146-155.
15. Hoffman JR, Schriger DL, Luo JS. The empiric use of naloxone in patients with altered mental status: a reappraisal. Ann Emerg Med. 1991;20(3):246-252.
16. Yokell MA, Delgado MK, Zaller ND, Wang NE, McGowan SK, Green TC. Presentation of prescription and nonprescription opioid overdoses to US emergency departments. JAMA Intern Med. 2014;174(12):2034-2037.
17. Binswanger I, Gardner E, Gabella B, Broderick K, Glanz K. Development of case criteria to define pharmaceutical opioid and heroin overdoses in clinical records. Platform presented at: Association for Medical Education and Research in Substance Abuse 38th Annual National Conference; November 7, 2014; San Francisco, CA.
18. Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171(7):686-691.
19. Jaeger TM, Lohr RH, Pankratz VS. Symptom-triggered therapy for alcohol withdrawal syndrome in medical inpatients. Mayo Clin Proc. 2001;76(7):695-701.20. Washington State Agency Medical Directors’ Group. Opioid dose clculator. http://www
.agencymeddirectors.wa.gov/Calculator/DoseCalcula tor.htm. Accessed October 10, 2016.
21. EMIT II Plus Benzodiazepine Assay [package insert]. Brea, CA: Beckman Coulter, Inc; 2010.
22. Johnson EM, Lanier WA, Merrill RM, et al. Unintentional prescription opioid-related overdose deaths: description of decedents by next of kin or best contact, Utah, 2008-2009. J Gen Intern Med. 2013;28(4):522-529.
23. Utah Department of Health. Fact sheet: prescription pain medication deaths in Utah, 2012. https://www.health.utah.gov/vipp/pdf/FactSheets/2012RxOpioidDeaths.pdf. Updated October 2013. Accessed October 10, 2016.
24. Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657-659.
25. Bohnert AS, Tracy M, Galea S. Characteristics of drug users who witness many overdoses: implications for overdose prevention. Drug Alcohol Depend. 2012;120(1-3):168-173.
26. Yoon J, Zulman D, Scott JY, Maciejewski ML. Costs associated with multimorbidity among VA patients. Med Care. 2014;52(suppl 3):S31-S36.
27. Yoon J, Yano EM, Altman L, et al. Reducing costs of acute care for ambulatory care-sensitive medical conditions: the central roles of comorbid mental illness. Med Care. 2012;50(8):705-713.
28. Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain. Guideline summary. http://www.va.gov/painmanagement/docs/cpg_opioidtherapy_summary.pdf. Published May 2010. Accessed August 25, 2016.
29. Fulton-Kehoe D, Sullivan MD, Turner JA, et al. Opioid poisonings in Washington state Medicaid: trends, dosing, and guidelines. Med Care. 2015;53(8):679-685.
30. Gudin JA, Mogali S, Jones JD, Comer SD. Risks, management, and monitoring of combination opioid, benzodiazepines, and/or alcohol use. Postgrad Med. 2013;125(4):115-130.
31. Poisnel G, Dhilly M, Le Boisselier R, Barre L, Debruyne D. Comparison of five benzodiazepine-receptor agonists on buprenorphine-induced mu-opioid receptor regulation. J Pharmacol Sci. 2009;110(1):36-46.
32. Webster LR, Cochella S, Dasgupta N, et al. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med. 2011;12(suppl 2):S26-S35.
33. Lee SC, Klein-Schwartz W, Doyon S, Welsh C. Comparison of toxicity associated with nonmedical use of benzodiazepines with buprenorphine or methadone. Drug Alcohol Depend. 2014;138:118-123.
34. Owen GT, Burton AW, Schade CM, Passik S. Urine drug testing: current recommendations and best practices. Pain Physician. 2012;15(suppl 3):ES119–ES133.
35. Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan Braden J, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: the TROUP study. Pain. 2010;150(2):332-339.
36. Sporer KA, Firestone J, Isaacs SM. Out-of-hospital treatment of opioid overdoses in an urban setting. Acad Emerg Med. 1996;3(7):660-667.