User login
Hospitalists Should Endorse Their Team Members
Editor’s note: “Everything We Say and Do” is an informational series developed by SHM’s Patient Experience Committee to provide readers with thoughtful and actionable communication tactics that have great potential to positively impact patients’ experience of care. Each article will focus on how the contributor applies one or more of the “key communication” tactics in practice to maintain provider accountability for “everything we say and do that affects our patients’ thoughts, feelings, and well-being.”
View a chart outlining key communication tactics
What I Say and Do
At every opportunity, I position and endorse my colleagues who are or will be participating in my patient’s care by describing their roles and expressing my confidence in their abilities.
Why I Do It
It is vital that our patients feel assured they are being cared for by a high-functioning team of experts. During any given hospital stay, our patients will meet consulting physicians, nurses, therapists, case managers … The list goes on and on. Each person plays a vital part in patients’ care. But it can be difficult for patients to understand every person’s role and to feel assured that each person is highly skilled and aligned with the care plan.
As hospitalists, we are in a unique position to provide a foundation of assuredness and confidence that is a cornerstone of patient experience before our teammates meet patients. When we miss this opportunity, our patients perceive us as a sea of white coats passing in and out of their rooms rather than a cohesive team with their best interests at heart.
How I Do It
Let’s take the example of an elderly patient admitted for a hip fracture after a fall. Alongside the hospitalist will be the orthopedic surgeon, nurse, physical therapist, and case manager, all working toward an optimal outcome. In each case, the hospitalist can choose to provide no information about these team members or to position them for a positive first impression.
Here are the steps to take when positioning colleagues with patients:
- Identify team members and explain their roles.
- Endorse colleagues by expressing honest confidence in their expertise and ability.
- Describe how communication between you and your team members will work.
- Assure the patient that during handoff, your colleagues will be up-to-date and aligned with the plan.
- Tell your patients they are part of a team dedicated to a safe and effective hospitalization.
Mark Shapiro, MD, is medical director for hospital medicine at St. Joseph Health Medical Group in Santa Rosa, Calif., and producer and host of Explore the Space podcast (explorethespaceshow.com).
Editor’s note: “Everything We Say and Do” is an informational series developed by SHM’s Patient Experience Committee to provide readers with thoughtful and actionable communication tactics that have great potential to positively impact patients’ experience of care. Each article will focus on how the contributor applies one or more of the “key communication” tactics in practice to maintain provider accountability for “everything we say and do that affects our patients’ thoughts, feelings, and well-being.”
View a chart outlining key communication tactics
What I Say and Do
At every opportunity, I position and endorse my colleagues who are or will be participating in my patient’s care by describing their roles and expressing my confidence in their abilities.
Why I Do It
It is vital that our patients feel assured they are being cared for by a high-functioning team of experts. During any given hospital stay, our patients will meet consulting physicians, nurses, therapists, case managers … The list goes on and on. Each person plays a vital part in patients’ care. But it can be difficult for patients to understand every person’s role and to feel assured that each person is highly skilled and aligned with the care plan.
As hospitalists, we are in a unique position to provide a foundation of assuredness and confidence that is a cornerstone of patient experience before our teammates meet patients. When we miss this opportunity, our patients perceive us as a sea of white coats passing in and out of their rooms rather than a cohesive team with their best interests at heart.
How I Do It
Let’s take the example of an elderly patient admitted for a hip fracture after a fall. Alongside the hospitalist will be the orthopedic surgeon, nurse, physical therapist, and case manager, all working toward an optimal outcome. In each case, the hospitalist can choose to provide no information about these team members or to position them for a positive first impression.
Here are the steps to take when positioning colleagues with patients:
- Identify team members and explain their roles.
- Endorse colleagues by expressing honest confidence in their expertise and ability.
- Describe how communication between you and your team members will work.
- Assure the patient that during handoff, your colleagues will be up-to-date and aligned with the plan.
- Tell your patients they are part of a team dedicated to a safe and effective hospitalization.
Mark Shapiro, MD, is medical director for hospital medicine at St. Joseph Health Medical Group in Santa Rosa, Calif., and producer and host of Explore the Space podcast (explorethespaceshow.com).
Editor’s note: “Everything We Say and Do” is an informational series developed by SHM’s Patient Experience Committee to provide readers with thoughtful and actionable communication tactics that have great potential to positively impact patients’ experience of care. Each article will focus on how the contributor applies one or more of the “key communication” tactics in practice to maintain provider accountability for “everything we say and do that affects our patients’ thoughts, feelings, and well-being.”
View a chart outlining key communication tactics
What I Say and Do
At every opportunity, I position and endorse my colleagues who are or will be participating in my patient’s care by describing their roles and expressing my confidence in their abilities.
Why I Do It
It is vital that our patients feel assured they are being cared for by a high-functioning team of experts. During any given hospital stay, our patients will meet consulting physicians, nurses, therapists, case managers … The list goes on and on. Each person plays a vital part in patients’ care. But it can be difficult for patients to understand every person’s role and to feel assured that each person is highly skilled and aligned with the care plan.
As hospitalists, we are in a unique position to provide a foundation of assuredness and confidence that is a cornerstone of patient experience before our teammates meet patients. When we miss this opportunity, our patients perceive us as a sea of white coats passing in and out of their rooms rather than a cohesive team with their best interests at heart.
How I Do It
Let’s take the example of an elderly patient admitted for a hip fracture after a fall. Alongside the hospitalist will be the orthopedic surgeon, nurse, physical therapist, and case manager, all working toward an optimal outcome. In each case, the hospitalist can choose to provide no information about these team members or to position them for a positive first impression.
Here are the steps to take when positioning colleagues with patients:
- Identify team members and explain their roles.
- Endorse colleagues by expressing honest confidence in their expertise and ability.
- Describe how communication between you and your team members will work.
- Assure the patient that during handoff, your colleagues will be up-to-date and aligned with the plan.
- Tell your patients they are part of a team dedicated to a safe and effective hospitalization.
Mark Shapiro, MD, is medical director for hospital medicine at St. Joseph Health Medical Group in Santa Rosa, Calif., and producer and host of Explore the Space podcast (explorethespaceshow.com).
Non-Hodgkin Lymphoma Death Rates Continue to Fall
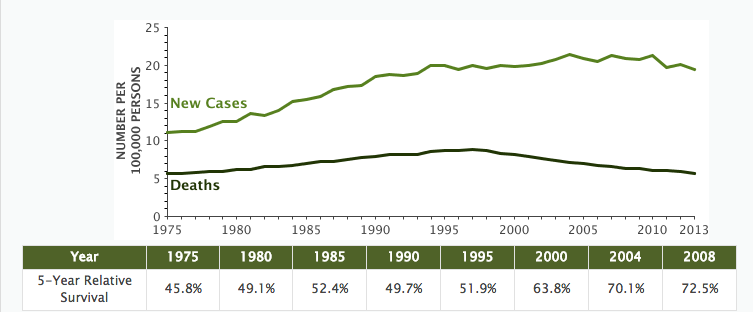
The 5-year relative survival rate for non-Hodgkin lymphoma (NHL) climbed to 72.7% and is as high as 82.6% for localized NHL, according to the most recent SEER data. The number of new cases remains high at 19.1 per 100,000 people (all races) per year; however the number of deaths is relatively low at 5.7 deaths per 100,000 people (all races) per year. Death rates have been falling on average 2.4% each year from 2004 to 2013.
While the new cases represent 4.3% of all new cancer diagnoses, NHL deaths represent 3.4% of all cancer deaths. Based on 2011-2013 SEER data, about 2.1% of men and women will receive a NHL diagnosis at some point during their lifetime.
Patient diagnoses by stage:
- 28% are diagnosed at the local stage
- 15% are diagnosed with spread to regional lymph nodes
- 50% are diagnosed after distant cancer has metastasized
- 8% unknown/unstaged
As of 2013, there were an estimated 569,536 people living with NHL in the U.S.
Using statistical models for analysis, rates for new non-Hodgkin lymphoma cases have not changed significantly over the past 10 years.
The 5-year relative survival rate for non-Hodgkin lymphoma (NHL) climbed to 72.7% and is as high as 82.6% for localized NHL, according to the most recent SEER data. The number of new cases remains high at 19.1 per 100,000 people (all races) per year; however the number of deaths is relatively low at 5.7 deaths per 100,000 people (all races) per year. Death rates have been falling on average 2.4% each year from 2004 to 2013.
While the new cases represent 4.3% of all new cancer diagnoses, NHL deaths represent 3.4% of all cancer deaths. Based on 2011-2013 SEER data, about 2.1% of men and women will receive a NHL diagnosis at some point during their lifetime.
Patient diagnoses by stage:
- 28% are diagnosed at the local stage
- 15% are diagnosed with spread to regional lymph nodes
- 50% are diagnosed after distant cancer has metastasized
- 8% unknown/unstaged
As of 2013, there were an estimated 569,536 people living with NHL in the U.S.
Using statistical models for analysis, rates for new non-Hodgkin lymphoma cases have not changed significantly over the past 10 years.

The 5-year relative survival rate for non-Hodgkin lymphoma (NHL) climbed to 72.7% and is as high as 82.6% for localized NHL, according to the most recent SEER data. The number of new cases remains high at 19.1 per 100,000 people (all races) per year; however the number of deaths is relatively low at 5.7 deaths per 100,000 people (all races) per year. Death rates have been falling on average 2.4% each year from 2004 to 2013.
While the new cases represent 4.3% of all new cancer diagnoses, NHL deaths represent 3.4% of all cancer deaths. Based on 2011-2013 SEER data, about 2.1% of men and women will receive a NHL diagnosis at some point during their lifetime.
Patient diagnoses by stage:
- 28% are diagnosed at the local stage
- 15% are diagnosed with spread to regional lymph nodes
- 50% are diagnosed after distant cancer has metastasized
- 8% unknown/unstaged
As of 2013, there were an estimated 569,536 people living with NHL in the U.S.
Using statistical models for analysis, rates for new non-Hodgkin lymphoma cases have not changed significantly over the past 10 years.

EC grants ixazomib conditional approval to treat MM
The European Commission (EC) has granted conditional marketing authorization for ixazomib (NinlaroTM) to be used in combination with lenalidomide and dexamethasone to treat adults with multiple myeloma (MM) who have received at least 1 prior therapy.
This decision makes ixazomib the first oral proteasome inhibitor approved to treat MM in the European Economic Area.
“With the approval of Ninlaro by the European Commission, physicians across the region will have the option to prescribe an all-oral triplet regimen to treat patients with multiple myeloma who have received at least 1 prior therapy,” said Philippe Moreau, MD, of the University Hospital of Nantes in France.
Conditional marketing authorization represents an expedited path for approval. The EC grants this type of authorization before pivotal registration studies are completed.
Conditional marketing authorization is granted to products whose benefits are thought to outweigh their risks, products that address unmet needs, and products that are expected to provide a significant public health benefit.
The conditional authorization for ixazomib means the company developing the drug, Takeda Pharmaceutical Company Limited, is required to provide post-approval updates on safety and efficacy analyses from ongoing studies to demonstrate the long-term effects of ixazomib.
Phase 3 trial
The EC’s decision to grant ixazomib conditional marketing authorization is based on results from the phase 3 TOURMALINE-MM1 trial, which were presented at the 2015 ASH Annual Meeting.
The trial included 722 patients with relapsed or refractory MM. The patients were randomized to receive ixazomib, lenalidomide, and dexamethasone (IRd, n=360) or placebo, lenalidomide, and dexamethasone (Rd, n=362).
Baseline patient characteristics were similar between the treatment arms. Fifty-nine percent of patients in both arms had received 1 prior line of therapy, and 41% in both arms had 2 or 3 prior lines of therapy.
Seventy-eight percent of patients responded to IRd, and 72% responded to Rd (P=0.035). The rates of complete response were 12% and 7%, respectively (P=0.019).
At a median follow-up of about 15 months, the median progression-free survival was 20.6 months in the IRd arm and 14.7 months in the Rd arm. The hazard ratio was 0.742 (P=0.012).
At a median follow-up of about 23 months, the median overall survival had not been reached in either treatment arm. Follow-up analyses for overall survival are planned for 2017.
The incidence of adverse events (AEs) was 98% in the IRd arm and 99% in the Rd arm. The incidence of grade 3 or higher AEs was 74% and 69%, respectively. The incidence of serious AEs was 47% and 49%, respectively.
Common AEs in the IRd and Rd arms, respectively, were diarrhea (45% vs 39%), constipation (35% vs 26%), nausea (29% vs 22%), vomiting (23% vs 12%), rash (36% vs 23%), back pain (24% vs 17%), upper respiratory tract infection (23% vs 19%), thrombocytopenia (31% vs 16%), peripheral neuropathy (27% vs 22%), peripheral edema (28% vs 20%), thromboembolism (8% vs 11%), and neutropenia (33% vs 31%). ![]()

The European Commission (EC) has granted conditional marketing authorization for ixazomib (NinlaroTM) to be used in combination with lenalidomide and dexamethasone to treat adults with multiple myeloma (MM) who have received at least 1 prior therapy.
This decision makes ixazomib the first oral proteasome inhibitor approved to treat MM in the European Economic Area.
“With the approval of Ninlaro by the European Commission, physicians across the region will have the option to prescribe an all-oral triplet regimen to treat patients with multiple myeloma who have received at least 1 prior therapy,” said Philippe Moreau, MD, of the University Hospital of Nantes in France.
Conditional marketing authorization represents an expedited path for approval. The EC grants this type of authorization before pivotal registration studies are completed.
Conditional marketing authorization is granted to products whose benefits are thought to outweigh their risks, products that address unmet needs, and products that are expected to provide a significant public health benefit.
The conditional authorization for ixazomib means the company developing the drug, Takeda Pharmaceutical Company Limited, is required to provide post-approval updates on safety and efficacy analyses from ongoing studies to demonstrate the long-term effects of ixazomib.
Phase 3 trial
The EC’s decision to grant ixazomib conditional marketing authorization is based on results from the phase 3 TOURMALINE-MM1 trial, which were presented at the 2015 ASH Annual Meeting.
The trial included 722 patients with relapsed or refractory MM. The patients were randomized to receive ixazomib, lenalidomide, and dexamethasone (IRd, n=360) or placebo, lenalidomide, and dexamethasone (Rd, n=362).
Baseline patient characteristics were similar between the treatment arms. Fifty-nine percent of patients in both arms had received 1 prior line of therapy, and 41% in both arms had 2 or 3 prior lines of therapy.
Seventy-eight percent of patients responded to IRd, and 72% responded to Rd (P=0.035). The rates of complete response were 12% and 7%, respectively (P=0.019).
At a median follow-up of about 15 months, the median progression-free survival was 20.6 months in the IRd arm and 14.7 months in the Rd arm. The hazard ratio was 0.742 (P=0.012).
At a median follow-up of about 23 months, the median overall survival had not been reached in either treatment arm. Follow-up analyses for overall survival are planned for 2017.
The incidence of adverse events (AEs) was 98% in the IRd arm and 99% in the Rd arm. The incidence of grade 3 or higher AEs was 74% and 69%, respectively. The incidence of serious AEs was 47% and 49%, respectively.
Common AEs in the IRd and Rd arms, respectively, were diarrhea (45% vs 39%), constipation (35% vs 26%), nausea (29% vs 22%), vomiting (23% vs 12%), rash (36% vs 23%), back pain (24% vs 17%), upper respiratory tract infection (23% vs 19%), thrombocytopenia (31% vs 16%), peripheral neuropathy (27% vs 22%), peripheral edema (28% vs 20%), thromboembolism (8% vs 11%), and neutropenia (33% vs 31%). ![]()

The European Commission (EC) has granted conditional marketing authorization for ixazomib (NinlaroTM) to be used in combination with lenalidomide and dexamethasone to treat adults with multiple myeloma (MM) who have received at least 1 prior therapy.
This decision makes ixazomib the first oral proteasome inhibitor approved to treat MM in the European Economic Area.
“With the approval of Ninlaro by the European Commission, physicians across the region will have the option to prescribe an all-oral triplet regimen to treat patients with multiple myeloma who have received at least 1 prior therapy,” said Philippe Moreau, MD, of the University Hospital of Nantes in France.
Conditional marketing authorization represents an expedited path for approval. The EC grants this type of authorization before pivotal registration studies are completed.
Conditional marketing authorization is granted to products whose benefits are thought to outweigh their risks, products that address unmet needs, and products that are expected to provide a significant public health benefit.
The conditional authorization for ixazomib means the company developing the drug, Takeda Pharmaceutical Company Limited, is required to provide post-approval updates on safety and efficacy analyses from ongoing studies to demonstrate the long-term effects of ixazomib.
Phase 3 trial
The EC’s decision to grant ixazomib conditional marketing authorization is based on results from the phase 3 TOURMALINE-MM1 trial, which were presented at the 2015 ASH Annual Meeting.
The trial included 722 patients with relapsed or refractory MM. The patients were randomized to receive ixazomib, lenalidomide, and dexamethasone (IRd, n=360) or placebo, lenalidomide, and dexamethasone (Rd, n=362).
Baseline patient characteristics were similar between the treatment arms. Fifty-nine percent of patients in both arms had received 1 prior line of therapy, and 41% in both arms had 2 or 3 prior lines of therapy.
Seventy-eight percent of patients responded to IRd, and 72% responded to Rd (P=0.035). The rates of complete response were 12% and 7%, respectively (P=0.019).
At a median follow-up of about 15 months, the median progression-free survival was 20.6 months in the IRd arm and 14.7 months in the Rd arm. The hazard ratio was 0.742 (P=0.012).
At a median follow-up of about 23 months, the median overall survival had not been reached in either treatment arm. Follow-up analyses for overall survival are planned for 2017.
The incidence of adverse events (AEs) was 98% in the IRd arm and 99% in the Rd arm. The incidence of grade 3 or higher AEs was 74% and 69%, respectively. The incidence of serious AEs was 47% and 49%, respectively.
Common AEs in the IRd and Rd arms, respectively, were diarrhea (45% vs 39%), constipation (35% vs 26%), nausea (29% vs 22%), vomiting (23% vs 12%), rash (36% vs 23%), back pain (24% vs 17%), upper respiratory tract infection (23% vs 19%), thrombocytopenia (31% vs 16%), peripheral neuropathy (27% vs 22%), peripheral edema (28% vs 20%), thromboembolism (8% vs 11%), and neutropenia (33% vs 31%). ![]()
Decitabine produces responses in high-risk MDS, AML

receiving chemotherapy
Photo by Rhoda Baer
Patients with TP53-mutated myelodysplastic syndromes (MDS) or acute myeloid leukemia (AML) may benefit from treatment with decitabine, according to a study published in NEJM.
All patients in this study who had TP53 mutations responded to decitabine.
Although these responses were not durable, the patients’ median overall survival was similar to that of patients with lower-risk disease who received decitabine.
“The findings need to be validated in a larger trial, but they do suggest that TP53 mutations can reliably predict responses to decitabine, potentially prolonging survival in this ultra-high-risk group of patients and providing a bridge to transplantation in some patients who might not otherwise be candidates,” said study author Timothy J. Ley, MD, of Washington University School of Medicine in St. Louis, Missouri.
For this study, Dr Ley and his colleagues analyzed 116 patients—54 with AML, 36 with relapsed AML, and 26 with MDS.
Eighty-four of the patients were enrolled in a prospective trial and received decitabine at a dose of 20 mg/m2/day for 10 consecutive days in monthly cycles. Thirty-two additional patients received decitabine on different protocols.
To determine whether genetic mutations could be used to predict responses to decitabine, the researchers performed enhanced exome or gene-panel sequencing in 67 of the patients. The team also performed sequencing at multiple time points to evaluate patterns of mutation clearance in 54 patients.
Response
Thirteen percent of patients (n=15) achieved a complete response (CR), 21% (n=24) had a CR with incomplete count recovery, 5% (n=6) had a morphologic CR with hematologic improvement, and 7% (n=8) had a morphologic CR without hematologic improvement.
Eight percent of patients (n=9) had a partial response, 20% (n=23) had stable disease, and 16% (n=19) had progressive disease.
There were 21 patients with TP53 mutations, and all of them achieved bone marrow blast clearance with less than 5% blasts.
Nineteen percent (n=4) had a CR, 43% (n=9) had a CR with incomplete count recovery, 24% (n=5) had morphologic CR with hematologic improvement, and 14% (n=3) had morphologic CR without hematologic improvement.
“What’s really unique here is that all the patients in the study with TP53 mutations had a response to decitabine and achieved an initial remission,” Dr Ley said.
“With standard aggressive chemotherapy, we only see about 20% to 30% of these patients achieving remission, which is the critical first step to have a chance to cure patients with additional therapies.”
Dr Ley and his colleagues also found that patients in this study were likely to respond to decitabine if they were considered “unfavorable risk” based on extensive chromosomal rearrangements. (Many of these patients also had TP53 mutations.)
Indeed, 67% (29/43) of patients with an unfavorable risk had less than 5% blasts after treatment with decitabine, compared with 34% (24/71) of patients with intermediate or favorable risk.
“The challenge with using decitabine has been knowing which patients are most likely to respond,” said study author Amanda Cashen, MD, of Washington University School of Medicine.
“The value of this study is the comprehensive mutational analysis that helps us figure out which patients are likely to benefit. This information opens the door to using decitabine in a more targeted fashion to treat not just older patients, but also younger patients who carry TP53 mutations.”
Survival and next steps
The researchers found that responses to decitabine were usually short-lived. The drug did not provide complete mutation clearance, which led to relapse.
“Remissions with decitabine typically don’t last long, and no one was cured with this drug,” Dr Ley noted. “But patients who responded to decitabine live longer than what you would expect with aggressive chemotherapy, and that can mean something. Some people live a year or 2 and with a good quality of life because the chemotherapy is not too toxic.”
The median overall survival was 11.6 months among patients with unfavorable risk and 10 months among patients with favorable or intermediate risk (P=0.29).
The median overall survival was 12.7 months among patients with TP53 mutations and 15.4 months among patients with wild-type TP53 (P=0.79).
“It’s important to note that patients with an extremely poor prognosis in this relatively small study had the same survival outcomes as patients facing a better prognosis, which is encouraging,” said study author John Welch, MD, PhD, of Washington University School of Medicine.
“We don’t yet understand why patients with TP53 mutations consistently respond to decitabine, and more work is needed to understand that phenomenon. We’re now planning a larger trial to evaluate decitabine in AML patients of all ages who carry TP53 mutations. It’s exciting to think we may have a therapy that has the potential to improve response rates in this group of high-risk patients.” ![]()

receiving chemotherapy
Photo by Rhoda Baer
Patients with TP53-mutated myelodysplastic syndromes (MDS) or acute myeloid leukemia (AML) may benefit from treatment with decitabine, according to a study published in NEJM.
All patients in this study who had TP53 mutations responded to decitabine.
Although these responses were not durable, the patients’ median overall survival was similar to that of patients with lower-risk disease who received decitabine.
“The findings need to be validated in a larger trial, but they do suggest that TP53 mutations can reliably predict responses to decitabine, potentially prolonging survival in this ultra-high-risk group of patients and providing a bridge to transplantation in some patients who might not otherwise be candidates,” said study author Timothy J. Ley, MD, of Washington University School of Medicine in St. Louis, Missouri.
For this study, Dr Ley and his colleagues analyzed 116 patients—54 with AML, 36 with relapsed AML, and 26 with MDS.
Eighty-four of the patients were enrolled in a prospective trial and received decitabine at a dose of 20 mg/m2/day for 10 consecutive days in monthly cycles. Thirty-two additional patients received decitabine on different protocols.
To determine whether genetic mutations could be used to predict responses to decitabine, the researchers performed enhanced exome or gene-panel sequencing in 67 of the patients. The team also performed sequencing at multiple time points to evaluate patterns of mutation clearance in 54 patients.
Response
Thirteen percent of patients (n=15) achieved a complete response (CR), 21% (n=24) had a CR with incomplete count recovery, 5% (n=6) had a morphologic CR with hematologic improvement, and 7% (n=8) had a morphologic CR without hematologic improvement.
Eight percent of patients (n=9) had a partial response, 20% (n=23) had stable disease, and 16% (n=19) had progressive disease.
There were 21 patients with TP53 mutations, and all of them achieved bone marrow blast clearance with less than 5% blasts.
Nineteen percent (n=4) had a CR, 43% (n=9) had a CR with incomplete count recovery, 24% (n=5) had morphologic CR with hematologic improvement, and 14% (n=3) had morphologic CR without hematologic improvement.
“What’s really unique here is that all the patients in the study with TP53 mutations had a response to decitabine and achieved an initial remission,” Dr Ley said.
“With standard aggressive chemotherapy, we only see about 20% to 30% of these patients achieving remission, which is the critical first step to have a chance to cure patients with additional therapies.”
Dr Ley and his colleagues also found that patients in this study were likely to respond to decitabine if they were considered “unfavorable risk” based on extensive chromosomal rearrangements. (Many of these patients also had TP53 mutations.)
Indeed, 67% (29/43) of patients with an unfavorable risk had less than 5% blasts after treatment with decitabine, compared with 34% (24/71) of patients with intermediate or favorable risk.
“The challenge with using decitabine has been knowing which patients are most likely to respond,” said study author Amanda Cashen, MD, of Washington University School of Medicine.
“The value of this study is the comprehensive mutational analysis that helps us figure out which patients are likely to benefit. This information opens the door to using decitabine in a more targeted fashion to treat not just older patients, but also younger patients who carry TP53 mutations.”
Survival and next steps
The researchers found that responses to decitabine were usually short-lived. The drug did not provide complete mutation clearance, which led to relapse.
“Remissions with decitabine typically don’t last long, and no one was cured with this drug,” Dr Ley noted. “But patients who responded to decitabine live longer than what you would expect with aggressive chemotherapy, and that can mean something. Some people live a year or 2 and with a good quality of life because the chemotherapy is not too toxic.”
The median overall survival was 11.6 months among patients with unfavorable risk and 10 months among patients with favorable or intermediate risk (P=0.29).
The median overall survival was 12.7 months among patients with TP53 mutations and 15.4 months among patients with wild-type TP53 (P=0.79).
“It’s important to note that patients with an extremely poor prognosis in this relatively small study had the same survival outcomes as patients facing a better prognosis, which is encouraging,” said study author John Welch, MD, PhD, of Washington University School of Medicine.
“We don’t yet understand why patients with TP53 mutations consistently respond to decitabine, and more work is needed to understand that phenomenon. We’re now planning a larger trial to evaluate decitabine in AML patients of all ages who carry TP53 mutations. It’s exciting to think we may have a therapy that has the potential to improve response rates in this group of high-risk patients.” ![]()

receiving chemotherapy
Photo by Rhoda Baer
Patients with TP53-mutated myelodysplastic syndromes (MDS) or acute myeloid leukemia (AML) may benefit from treatment with decitabine, according to a study published in NEJM.
All patients in this study who had TP53 mutations responded to decitabine.
Although these responses were not durable, the patients’ median overall survival was similar to that of patients with lower-risk disease who received decitabine.
“The findings need to be validated in a larger trial, but they do suggest that TP53 mutations can reliably predict responses to decitabine, potentially prolonging survival in this ultra-high-risk group of patients and providing a bridge to transplantation in some patients who might not otherwise be candidates,” said study author Timothy J. Ley, MD, of Washington University School of Medicine in St. Louis, Missouri.
For this study, Dr Ley and his colleagues analyzed 116 patients—54 with AML, 36 with relapsed AML, and 26 with MDS.
Eighty-four of the patients were enrolled in a prospective trial and received decitabine at a dose of 20 mg/m2/day for 10 consecutive days in monthly cycles. Thirty-two additional patients received decitabine on different protocols.
To determine whether genetic mutations could be used to predict responses to decitabine, the researchers performed enhanced exome or gene-panel sequencing in 67 of the patients. The team also performed sequencing at multiple time points to evaluate patterns of mutation clearance in 54 patients.
Response
Thirteen percent of patients (n=15) achieved a complete response (CR), 21% (n=24) had a CR with incomplete count recovery, 5% (n=6) had a morphologic CR with hematologic improvement, and 7% (n=8) had a morphologic CR without hematologic improvement.
Eight percent of patients (n=9) had a partial response, 20% (n=23) had stable disease, and 16% (n=19) had progressive disease.
There were 21 patients with TP53 mutations, and all of them achieved bone marrow blast clearance with less than 5% blasts.
Nineteen percent (n=4) had a CR, 43% (n=9) had a CR with incomplete count recovery, 24% (n=5) had morphologic CR with hematologic improvement, and 14% (n=3) had morphologic CR without hematologic improvement.
“What’s really unique here is that all the patients in the study with TP53 mutations had a response to decitabine and achieved an initial remission,” Dr Ley said.
“With standard aggressive chemotherapy, we only see about 20% to 30% of these patients achieving remission, which is the critical first step to have a chance to cure patients with additional therapies.”
Dr Ley and his colleagues also found that patients in this study were likely to respond to decitabine if they were considered “unfavorable risk” based on extensive chromosomal rearrangements. (Many of these patients also had TP53 mutations.)
Indeed, 67% (29/43) of patients with an unfavorable risk had less than 5% blasts after treatment with decitabine, compared with 34% (24/71) of patients with intermediate or favorable risk.
“The challenge with using decitabine has been knowing which patients are most likely to respond,” said study author Amanda Cashen, MD, of Washington University School of Medicine.
“The value of this study is the comprehensive mutational analysis that helps us figure out which patients are likely to benefit. This information opens the door to using decitabine in a more targeted fashion to treat not just older patients, but also younger patients who carry TP53 mutations.”
Survival and next steps
The researchers found that responses to decitabine were usually short-lived. The drug did not provide complete mutation clearance, which led to relapse.
“Remissions with decitabine typically don’t last long, and no one was cured with this drug,” Dr Ley noted. “But patients who responded to decitabine live longer than what you would expect with aggressive chemotherapy, and that can mean something. Some people live a year or 2 and with a good quality of life because the chemotherapy is not too toxic.”
The median overall survival was 11.6 months among patients with unfavorable risk and 10 months among patients with favorable or intermediate risk (P=0.29).
The median overall survival was 12.7 months among patients with TP53 mutations and 15.4 months among patients with wild-type TP53 (P=0.79).
“It’s important to note that patients with an extremely poor prognosis in this relatively small study had the same survival outcomes as patients facing a better prognosis, which is encouraging,” said study author John Welch, MD, PhD, of Washington University School of Medicine.
“We don’t yet understand why patients with TP53 mutations consistently respond to decitabine, and more work is needed to understand that phenomenon. We’re now planning a larger trial to evaluate decitabine in AML patients of all ages who carry TP53 mutations. It’s exciting to think we may have a therapy that has the potential to improve response rates in this group of high-risk patients.” ![]()
Tazemetostat receives fast track designation for DLBCL

The US Food and Drug Administration (FDA) has granted fast track designation for tazemetostat as a treatment for patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) with EZH2 activating mutations.
Tazemetostat inhibits EZH2, a histone methyltransferase that appears to play a role in the growth and proliferation of a number of cancers, including DLBCL.
Tazemetostat is being developed by Epizyme, Inc.
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the FDA’s fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the biologic license application or new drug application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
Tazemetostat trials
Tazemetostat is under investigation as monotherapy and in combination with other agents as a treatment for multiple cancers.
Results from a phase 1 study suggested tazemetostat monotherapy can produce durable responses in patients with advanced non-Hodgkin lymphomas, including DLBCL. The study was presented at the 2015 ASH Annual Meeting.
Now, Epizyme is conducting a phase 2 study of tazemetostat monotherapy in adults with relapsed or refractory DLBCL or follicular lymphoma.
Tazemetostat is also being evaluated in 2 combination studies in patients with DLBCL.
In a phase 1b/2 trial, researchers are investigating tazemetostat in combination with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) as a front-line treatment for patients with DLBCL.
In a phase 1b study, researchers are evaluating tazemetostat in combination with atezolizumab, an anti-PD-L1 immunotherapy, in patients with relapsed and refractory DLBCL. ![]()

The US Food and Drug Administration (FDA) has granted fast track designation for tazemetostat as a treatment for patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) with EZH2 activating mutations.
Tazemetostat inhibits EZH2, a histone methyltransferase that appears to play a role in the growth and proliferation of a number of cancers, including DLBCL.
Tazemetostat is being developed by Epizyme, Inc.
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the FDA’s fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the biologic license application or new drug application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
Tazemetostat trials
Tazemetostat is under investigation as monotherapy and in combination with other agents as a treatment for multiple cancers.
Results from a phase 1 study suggested tazemetostat monotherapy can produce durable responses in patients with advanced non-Hodgkin lymphomas, including DLBCL. The study was presented at the 2015 ASH Annual Meeting.
Now, Epizyme is conducting a phase 2 study of tazemetostat monotherapy in adults with relapsed or refractory DLBCL or follicular lymphoma.
Tazemetostat is also being evaluated in 2 combination studies in patients with DLBCL.
In a phase 1b/2 trial, researchers are investigating tazemetostat in combination with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) as a front-line treatment for patients with DLBCL.
In a phase 1b study, researchers are evaluating tazemetostat in combination with atezolizumab, an anti-PD-L1 immunotherapy, in patients with relapsed and refractory DLBCL. ![]()

The US Food and Drug Administration (FDA) has granted fast track designation for tazemetostat as a treatment for patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) with EZH2 activating mutations.
Tazemetostat inhibits EZH2, a histone methyltransferase that appears to play a role in the growth and proliferation of a number of cancers, including DLBCL.
Tazemetostat is being developed by Epizyme, Inc.
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the FDA’s fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the biologic license application or new drug application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
Tazemetostat trials
Tazemetostat is under investigation as monotherapy and in combination with other agents as a treatment for multiple cancers.
Results from a phase 1 study suggested tazemetostat monotherapy can produce durable responses in patients with advanced non-Hodgkin lymphomas, including DLBCL. The study was presented at the 2015 ASH Annual Meeting.
Now, Epizyme is conducting a phase 2 study of tazemetostat monotherapy in adults with relapsed or refractory DLBCL or follicular lymphoma.
Tazemetostat is also being evaluated in 2 combination studies in patients with DLBCL.
In a phase 1b/2 trial, researchers are investigating tazemetostat in combination with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) as a front-line treatment for patients with DLBCL.
In a phase 1b study, researchers are evaluating tazemetostat in combination with atezolizumab, an anti-PD-L1 immunotherapy, in patients with relapsed and refractory DLBCL. ![]()
Increased death rate with platelets for aspirin/clopidogrel GI bleed
Patients with normal platelet counts who have a GI bleed while on antiplatelets were almost six times more likely to die in the hospital if they had a platelet transfusion in a retrospective cohort study from the Yale University in New Haven, Conn.
Ten of the 14 deaths in the 204 transfused patients – versus none of the 3 deaths in the 204 nontransfused patients - were due to bleeding, so it’s possible that the mortality difference was simply because patients with worse bleeding were more likely to get transfused. “On the other hand, the adjusted [odds ratios] for mortality (4.5-6.8 with different sensitivity analyses) [were] large, increasing the likelihood of a cause-and-effect relationship,” said investigators led by gastroenterologist Liam Zakko, MD, now at the Mayo Clinic in Rochester, Minn. (Clin Gastroenterol Hepatol. 2016 Jul 25. doi: 10.1016/j.cgh.2016.07.017).
Current guidelines suggest platelet transfusions are an option for antiplatelet patients with serious GI bleeds, but the Yale team found that they did not reduce rebleeding. “The observation of increased mortality without documentation of clinical benefit suggests a very cautious approach to the use of platelet transfusion. ... We do not support the use of platelet transfusions in patients with GI [bleeds] who are taking antiplatelet agents,” the investigators wrote.
Subjects in the two groups were matched for sex, age, and GI bleed location, and all had platelet counts above 100 × 109/L. Almost everyone was on aspirin for cardiovascular protection, and 30% were on also on clopidogrel.
Just over half in both groups had upper GI bleeds, and about 40% in each group had colonic bleeds. Transfused patients had more-severe bleeding, with overall lower blood pressure and lower hemoglobin; a larger proportion was admitted to the ICU.
On univariate analyses, platelet patients had more cardiovascular events (23% vs. 13%) while in the hospital. They were also more likely to stay in the hospital for more than 4 days (47% vs. 33%) and more likely to die while there (7% vs. 1%). On multivariable analysis, only the greater risk for death during admission remained statistically significant (odds ratio, 5.57; 95% confidence interval, 1.52-27.1). The adjusted odds ratio for recurrent bleeding was not significant.
Four patients in the platelet group died from cardiovascular causes. One patient in the control group had a fatal cardiovascular event.
Although counterintuitive, the authors said that it’s possible that platelet transfusions might actually increase the risk of severe and fatal GI bleeding. “Mechanisms by which platelet transfusion would increase mortality or [GI bleeding]–related mortality are not clear,” but “platelet transfusions are reported to be proinflammatory and alter recipient immunity,” they said.
At least for now, “the most prudent way to manage patients on antiplatelet agents with [GI bleeding] is to follow current evidence-based recommendations,” including early endoscopy, endoscopic hemostatic therapy for high-risk lesions, and intensive proton pump inhibitor therapy in patients with ulcers and high-risk endoscopic features.
“Although not based on high-quality evidence, we believe that hemostatic techniques that do not cause significant tissue damage (e.g., clips rather than thermal devices or sclerosants) should be used in patients on antiplatelet agents, especially if patients are expected to remain on these agents in the future,” they said.
The mean age in the study was 74 years, and about two-thirds of the subjects were men.
The authors had no disclosures.
The management of patients with gastrointestinal bleeding on antithrombotic drugs is a major challenge for gastroenterologists. Unfortunately, the use of aspirin alone has been shown to increase the risk of GI bleed twofold, and the addition of a thienopyridine additionally increases the risk of bleeding twofold. Furthermore, there is no available agent to reverse antiplatelet affects of these drugs, which irreversibly block platelet function for the life of the platelet (8-10 days). Current recommendations for the management of severe GI bleeding in patients receiving antithrombotic therapy include platelet transfusion, including those with a normal platelet count. However, this comes with a price as reversal of platelet function may increase the rate of cardiovascular events.
Zakko et al. performed a retrospective case-control study evaluating the role of platelet transfusion in patients presenting with GI bleeding. Patients were matched by age, sex, and the location of the GI bleed. Most patients included in the study were on low-dose aspirin and almost a third of the patients were taking both aspirin and a thienopyridine. Patients receiving platelet transfusions appeared to have more severe GI bleeding compared with matched controls, as patients receiving transfusion were more likely to have been hypotensive, tachycardic, have a low hemoglobin level, and require treatment in the intensive care unit (72% vs. 28%, P less than .0001). Patients receiving platelet transfusions were also more likely than matched controls to have recurrent GI bleeding as well as major cardiovascular adverse events, including myocardial infarction and inpatient death. After adjusting for patient characteristics, patients receiving platelet transfusions were more likely to have an increased risk of death (adjusted OR, 5.57; 95% CI, 1.52-27.1). The authors conclude that “the use of platelet transfusions in patients with GI bleeding who are taking antiplatelet agents without thrombocytopenia did not reduce rebleeding but was associated with higher mortality.”
Currently, there is no convincing evidence to support platelet transfusion in patients with bleeding on aspirin and/or a thienopyridine. Because the majority of the deaths were due to GI bleeding and not cardiovascular events, the observed increase in adverse events in patients receiving platelet transfusions likely reflects more severe GI bleeding in patients receiving platelet transfusions than in controls. We should avoid platelet transfusions and focus our management on achieving adequate resuscitation, use of proton pump inhibitors for patients with high-risk ulcers, and early endoscopy with endoscopic therapy for high-risk lesions.
John R. Saltzman, MD, AGAF, is director of endoscopy, Brigham and Women’s Hospital, professor of medicine, Harvard Medical School, Boston. He has no conflicts of interest.
The management of patients with gastrointestinal bleeding on antithrombotic drugs is a major challenge for gastroenterologists. Unfortunately, the use of aspirin alone has been shown to increase the risk of GI bleed twofold, and the addition of a thienopyridine additionally increases the risk of bleeding twofold. Furthermore, there is no available agent to reverse antiplatelet affects of these drugs, which irreversibly block platelet function for the life of the platelet (8-10 days). Current recommendations for the management of severe GI bleeding in patients receiving antithrombotic therapy include platelet transfusion, including those with a normal platelet count. However, this comes with a price as reversal of platelet function may increase the rate of cardiovascular events.
Zakko et al. performed a retrospective case-control study evaluating the role of platelet transfusion in patients presenting with GI bleeding. Patients were matched by age, sex, and the location of the GI bleed. Most patients included in the study were on low-dose aspirin and almost a third of the patients were taking both aspirin and a thienopyridine. Patients receiving platelet transfusions appeared to have more severe GI bleeding compared with matched controls, as patients receiving transfusion were more likely to have been hypotensive, tachycardic, have a low hemoglobin level, and require treatment in the intensive care unit (72% vs. 28%, P less than .0001). Patients receiving platelet transfusions were also more likely than matched controls to have recurrent GI bleeding as well as major cardiovascular adverse events, including myocardial infarction and inpatient death. After adjusting for patient characteristics, patients receiving platelet transfusions were more likely to have an increased risk of death (adjusted OR, 5.57; 95% CI, 1.52-27.1). The authors conclude that “the use of platelet transfusions in patients with GI bleeding who are taking antiplatelet agents without thrombocytopenia did not reduce rebleeding but was associated with higher mortality.”
Currently, there is no convincing evidence to support platelet transfusion in patients with bleeding on aspirin and/or a thienopyridine. Because the majority of the deaths were due to GI bleeding and not cardiovascular events, the observed increase in adverse events in patients receiving platelet transfusions likely reflects more severe GI bleeding in patients receiving platelet transfusions than in controls. We should avoid platelet transfusions and focus our management on achieving adequate resuscitation, use of proton pump inhibitors for patients with high-risk ulcers, and early endoscopy with endoscopic therapy for high-risk lesions.
John R. Saltzman, MD, AGAF, is director of endoscopy, Brigham and Women’s Hospital, professor of medicine, Harvard Medical School, Boston. He has no conflicts of interest.
The management of patients with gastrointestinal bleeding on antithrombotic drugs is a major challenge for gastroenterologists. Unfortunately, the use of aspirin alone has been shown to increase the risk of GI bleed twofold, and the addition of a thienopyridine additionally increases the risk of bleeding twofold. Furthermore, there is no available agent to reverse antiplatelet affects of these drugs, which irreversibly block platelet function for the life of the platelet (8-10 days). Current recommendations for the management of severe GI bleeding in patients receiving antithrombotic therapy include platelet transfusion, including those with a normal platelet count. However, this comes with a price as reversal of platelet function may increase the rate of cardiovascular events.
Zakko et al. performed a retrospective case-control study evaluating the role of platelet transfusion in patients presenting with GI bleeding. Patients were matched by age, sex, and the location of the GI bleed. Most patients included in the study were on low-dose aspirin and almost a third of the patients were taking both aspirin and a thienopyridine. Patients receiving platelet transfusions appeared to have more severe GI bleeding compared with matched controls, as patients receiving transfusion were more likely to have been hypotensive, tachycardic, have a low hemoglobin level, and require treatment in the intensive care unit (72% vs. 28%, P less than .0001). Patients receiving platelet transfusions were also more likely than matched controls to have recurrent GI bleeding as well as major cardiovascular adverse events, including myocardial infarction and inpatient death. After adjusting for patient characteristics, patients receiving platelet transfusions were more likely to have an increased risk of death (adjusted OR, 5.57; 95% CI, 1.52-27.1). The authors conclude that “the use of platelet transfusions in patients with GI bleeding who are taking antiplatelet agents without thrombocytopenia did not reduce rebleeding but was associated with higher mortality.”
Currently, there is no convincing evidence to support platelet transfusion in patients with bleeding on aspirin and/or a thienopyridine. Because the majority of the deaths were due to GI bleeding and not cardiovascular events, the observed increase in adverse events in patients receiving platelet transfusions likely reflects more severe GI bleeding in patients receiving platelet transfusions than in controls. We should avoid platelet transfusions and focus our management on achieving adequate resuscitation, use of proton pump inhibitors for patients with high-risk ulcers, and early endoscopy with endoscopic therapy for high-risk lesions.
John R. Saltzman, MD, AGAF, is director of endoscopy, Brigham and Women’s Hospital, professor of medicine, Harvard Medical School, Boston. He has no conflicts of interest.
Patients with normal platelet counts who have a GI bleed while on antiplatelets were almost six times more likely to die in the hospital if they had a platelet transfusion in a retrospective cohort study from the Yale University in New Haven, Conn.
Ten of the 14 deaths in the 204 transfused patients – versus none of the 3 deaths in the 204 nontransfused patients - were due to bleeding, so it’s possible that the mortality difference was simply because patients with worse bleeding were more likely to get transfused. “On the other hand, the adjusted [odds ratios] for mortality (4.5-6.8 with different sensitivity analyses) [were] large, increasing the likelihood of a cause-and-effect relationship,” said investigators led by gastroenterologist Liam Zakko, MD, now at the Mayo Clinic in Rochester, Minn. (Clin Gastroenterol Hepatol. 2016 Jul 25. doi: 10.1016/j.cgh.2016.07.017).
Current guidelines suggest platelet transfusions are an option for antiplatelet patients with serious GI bleeds, but the Yale team found that they did not reduce rebleeding. “The observation of increased mortality without documentation of clinical benefit suggests a very cautious approach to the use of platelet transfusion. ... We do not support the use of platelet transfusions in patients with GI [bleeds] who are taking antiplatelet agents,” the investigators wrote.
Subjects in the two groups were matched for sex, age, and GI bleed location, and all had platelet counts above 100 × 109/L. Almost everyone was on aspirin for cardiovascular protection, and 30% were on also on clopidogrel.
Just over half in both groups had upper GI bleeds, and about 40% in each group had colonic bleeds. Transfused patients had more-severe bleeding, with overall lower blood pressure and lower hemoglobin; a larger proportion was admitted to the ICU.
On univariate analyses, platelet patients had more cardiovascular events (23% vs. 13%) while in the hospital. They were also more likely to stay in the hospital for more than 4 days (47% vs. 33%) and more likely to die while there (7% vs. 1%). On multivariable analysis, only the greater risk for death during admission remained statistically significant (odds ratio, 5.57; 95% confidence interval, 1.52-27.1). The adjusted odds ratio for recurrent bleeding was not significant.
Four patients in the platelet group died from cardiovascular causes. One patient in the control group had a fatal cardiovascular event.
Although counterintuitive, the authors said that it’s possible that platelet transfusions might actually increase the risk of severe and fatal GI bleeding. “Mechanisms by which platelet transfusion would increase mortality or [GI bleeding]–related mortality are not clear,” but “platelet transfusions are reported to be proinflammatory and alter recipient immunity,” they said.
At least for now, “the most prudent way to manage patients on antiplatelet agents with [GI bleeding] is to follow current evidence-based recommendations,” including early endoscopy, endoscopic hemostatic therapy for high-risk lesions, and intensive proton pump inhibitor therapy in patients with ulcers and high-risk endoscopic features.
“Although not based on high-quality evidence, we believe that hemostatic techniques that do not cause significant tissue damage (e.g., clips rather than thermal devices or sclerosants) should be used in patients on antiplatelet agents, especially if patients are expected to remain on these agents in the future,” they said.
The mean age in the study was 74 years, and about two-thirds of the subjects were men.
The authors had no disclosures.
Patients with normal platelet counts who have a GI bleed while on antiplatelets were almost six times more likely to die in the hospital if they had a platelet transfusion in a retrospective cohort study from the Yale University in New Haven, Conn.
Ten of the 14 deaths in the 204 transfused patients – versus none of the 3 deaths in the 204 nontransfused patients - were due to bleeding, so it’s possible that the mortality difference was simply because patients with worse bleeding were more likely to get transfused. “On the other hand, the adjusted [odds ratios] for mortality (4.5-6.8 with different sensitivity analyses) [were] large, increasing the likelihood of a cause-and-effect relationship,” said investigators led by gastroenterologist Liam Zakko, MD, now at the Mayo Clinic in Rochester, Minn. (Clin Gastroenterol Hepatol. 2016 Jul 25. doi: 10.1016/j.cgh.2016.07.017).
Current guidelines suggest platelet transfusions are an option for antiplatelet patients with serious GI bleeds, but the Yale team found that they did not reduce rebleeding. “The observation of increased mortality without documentation of clinical benefit suggests a very cautious approach to the use of platelet transfusion. ... We do not support the use of platelet transfusions in patients with GI [bleeds] who are taking antiplatelet agents,” the investigators wrote.
Subjects in the two groups were matched for sex, age, and GI bleed location, and all had platelet counts above 100 × 109/L. Almost everyone was on aspirin for cardiovascular protection, and 30% were on also on clopidogrel.
Just over half in both groups had upper GI bleeds, and about 40% in each group had colonic bleeds. Transfused patients had more-severe bleeding, with overall lower blood pressure and lower hemoglobin; a larger proportion was admitted to the ICU.
On univariate analyses, platelet patients had more cardiovascular events (23% vs. 13%) while in the hospital. They were also more likely to stay in the hospital for more than 4 days (47% vs. 33%) and more likely to die while there (7% vs. 1%). On multivariable analysis, only the greater risk for death during admission remained statistically significant (odds ratio, 5.57; 95% confidence interval, 1.52-27.1). The adjusted odds ratio for recurrent bleeding was not significant.
Four patients in the platelet group died from cardiovascular causes. One patient in the control group had a fatal cardiovascular event.
Although counterintuitive, the authors said that it’s possible that platelet transfusions might actually increase the risk of severe and fatal GI bleeding. “Mechanisms by which platelet transfusion would increase mortality or [GI bleeding]–related mortality are not clear,” but “platelet transfusions are reported to be proinflammatory and alter recipient immunity,” they said.
At least for now, “the most prudent way to manage patients on antiplatelet agents with [GI bleeding] is to follow current evidence-based recommendations,” including early endoscopy, endoscopic hemostatic therapy for high-risk lesions, and intensive proton pump inhibitor therapy in patients with ulcers and high-risk endoscopic features.
“Although not based on high-quality evidence, we believe that hemostatic techniques that do not cause significant tissue damage (e.g., clips rather than thermal devices or sclerosants) should be used in patients on antiplatelet agents, especially if patients are expected to remain on these agents in the future,” they said.
The mean age in the study was 74 years, and about two-thirds of the subjects were men.
The authors had no disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point:
Major finding: Compared with those not transfused, the risk for death during admission remained statistically significant on multivariate analysis (OR, 5.57; 95% CI, 1.52-27.1).
Data source: Retrospective cohort study of 408 GI bleed patients
Disclosures: The authors had no disclosures.
SPG Stimulation May Enhance Delivery of Drugs to the Brain
BALTIMORE—Stimulation of the sphenopalatine ganglion (SPG) may be a safe and effective method of temporarily disrupting the blood–brain barrier to deliver therapeutics to the brain. In an animal model of stroke, SPG stimulation enhances the delivery of mesenchymal stem cells and improves functional outcomes, according to research presented at the 141st Annual Meeting of the American Neurological Association. The technique does not entail unwanted systemic effects and potentially could be applied in the treatment of other neurologic disorders.
Although it would be undesirable to deliver bone-marrow stem cells to the human brain, SPG stimulation could aid the delivery of neural stem cells, viral vectors, antibody infusions, and gene therapies, said Lorraine Iacovitti, PhD, Director of the Jefferson Stem Cell and Regenerative Neuroscience Center at Thomas Jefferson University in Philadelphia. She and her colleagues plan to investigate the mechanisms responsible for the response to SPG stimulation. In addition, they will examine various stimulation frequencies and determine the size of therapies that can be delivered to the brain.
Disruption of the Blood–Brain Barrier
Modifying the blood–brain barrier has been a longstanding goal of medicine. Achieving this goal would “improve treatments for many neurologic diseases and disorders, particularly if you could combine it with a focused endovascular delivery system so that these reagents get to the appropriate regions,” said Dr. Iacovitti. In 2004, Yarnitsky et al found that stimulating the SPG caused a transient, reversible increase in blood–brain barrier permeability in animals. The technique enabled Evans blue to penetrate nearly the entire side of the brain that received stimulation.
Michael Lang, MD, a fifth-year neurosurgical resident, led Dr. Iacovitti’s group in a study of SPG stimulation in rats with middle cerebral artery (MCA) occlusion. The researchers previously had found that injection of exogenous bone-marrow mesenchymal stem cells reduced infarct size, improved behavioral deficits, and decreased proinflammatory factors in this model of stroke. Although some stem cells reached the brain, most collected in the lungs, the kidneys, and the liver. Dr. Iacovitti’s group hypothesized that SPG stimulation would increase mesenchymal stem cell engraftment following intra-arterial delivery.
SPG Stimulation in a Stroke Model
The investigators studied three groups of rats. One group received MCA occlusion. The second group received MCA occlusion and an intra-arterial infusion of mesenchymal stem cells at one day post stroke. The third group underwent MCA occlusion, intra-arterial infusion of mesenchymal stem cells, and SPG stimulation at one day post stroke. The stimulation frequency was 10 Hz, and the potential was 5 V. Stimulation continuously alternated between 90-s on and 60-s off for a total of 20 minutes.
In the absence of SPG stimulation, few, if any, stem cells reached the parenchyma. The cells did reach the parenchyma, however, in rats that received SPG stimulation. In addition, SPG stimulation was associated with an improvement in functional outcome. At day 7 and at day 14, the researchers observed a difference in function between animals that received mesenchymal stem cells alone and those that received mesenchymal stem cells plus SPG stimulation. At day 14, the Modified Neurologic Severity score was approximately 50% lower in rats that received stem cells and SPG stimulation, compared with untreated rats.
Electron microscopy revealed that most tight junctions in the rats’ brains appeared normal after SPG stimulation, although tight junction discontinuity was common. The effect was similar to that of a mannitol infusion, said Dr. Iacovitti. “It is possible that stem cells are moving out of circulation into the brain in a fashion similar to what you would see after tumor-necrosis-factor-alpha-stimulated inflammation, where you would get immune cells to move out of the blood vessels and into the damaged brain area through a process of diapedesis.” Unlike mannitol administration, which causes dangerous systemic side effects, SPG stimulation has no observed adverse side effects.
“The combination of endovascular selectivity with SPG stimulation is potentially an extremely powerful tool to deliver [therapies] across the blood–brain barrier into the brain,” she continued. “We have just started to look at getting viruses across…. This work has really just begun.”
Dr. Iacovitti’s research was funded by grants awarded by the NIH, the Joseph and Marie Field Family Foundation, and the Mary E. Groff Charitable Trust.
—Erik Greb
BALTIMORE—Stimulation of the sphenopalatine ganglion (SPG) may be a safe and effective method of temporarily disrupting the blood–brain barrier to deliver therapeutics to the brain. In an animal model of stroke, SPG stimulation enhances the delivery of mesenchymal stem cells and improves functional outcomes, according to research presented at the 141st Annual Meeting of the American Neurological Association. The technique does not entail unwanted systemic effects and potentially could be applied in the treatment of other neurologic disorders.
Although it would be undesirable to deliver bone-marrow stem cells to the human brain, SPG stimulation could aid the delivery of neural stem cells, viral vectors, antibody infusions, and gene therapies, said Lorraine Iacovitti, PhD, Director of the Jefferson Stem Cell and Regenerative Neuroscience Center at Thomas Jefferson University in Philadelphia. She and her colleagues plan to investigate the mechanisms responsible for the response to SPG stimulation. In addition, they will examine various stimulation frequencies and determine the size of therapies that can be delivered to the brain.
Disruption of the Blood–Brain Barrier
Modifying the blood–brain barrier has been a longstanding goal of medicine. Achieving this goal would “improve treatments for many neurologic diseases and disorders, particularly if you could combine it with a focused endovascular delivery system so that these reagents get to the appropriate regions,” said Dr. Iacovitti. In 2004, Yarnitsky et al found that stimulating the SPG caused a transient, reversible increase in blood–brain barrier permeability in animals. The technique enabled Evans blue to penetrate nearly the entire side of the brain that received stimulation.
Michael Lang, MD, a fifth-year neurosurgical resident, led Dr. Iacovitti’s group in a study of SPG stimulation in rats with middle cerebral artery (MCA) occlusion. The researchers previously had found that injection of exogenous bone-marrow mesenchymal stem cells reduced infarct size, improved behavioral deficits, and decreased proinflammatory factors in this model of stroke. Although some stem cells reached the brain, most collected in the lungs, the kidneys, and the liver. Dr. Iacovitti’s group hypothesized that SPG stimulation would increase mesenchymal stem cell engraftment following intra-arterial delivery.
SPG Stimulation in a Stroke Model
The investigators studied three groups of rats. One group received MCA occlusion. The second group received MCA occlusion and an intra-arterial infusion of mesenchymal stem cells at one day post stroke. The third group underwent MCA occlusion, intra-arterial infusion of mesenchymal stem cells, and SPG stimulation at one day post stroke. The stimulation frequency was 10 Hz, and the potential was 5 V. Stimulation continuously alternated between 90-s on and 60-s off for a total of 20 minutes.
In the absence of SPG stimulation, few, if any, stem cells reached the parenchyma. The cells did reach the parenchyma, however, in rats that received SPG stimulation. In addition, SPG stimulation was associated with an improvement in functional outcome. At day 7 and at day 14, the researchers observed a difference in function between animals that received mesenchymal stem cells alone and those that received mesenchymal stem cells plus SPG stimulation. At day 14, the Modified Neurologic Severity score was approximately 50% lower in rats that received stem cells and SPG stimulation, compared with untreated rats.
Electron microscopy revealed that most tight junctions in the rats’ brains appeared normal after SPG stimulation, although tight junction discontinuity was common. The effect was similar to that of a mannitol infusion, said Dr. Iacovitti. “It is possible that stem cells are moving out of circulation into the brain in a fashion similar to what you would see after tumor-necrosis-factor-alpha-stimulated inflammation, where you would get immune cells to move out of the blood vessels and into the damaged brain area through a process of diapedesis.” Unlike mannitol administration, which causes dangerous systemic side effects, SPG stimulation has no observed adverse side effects.
“The combination of endovascular selectivity with SPG stimulation is potentially an extremely powerful tool to deliver [therapies] across the blood–brain barrier into the brain,” she continued. “We have just started to look at getting viruses across…. This work has really just begun.”
Dr. Iacovitti’s research was funded by grants awarded by the NIH, the Joseph and Marie Field Family Foundation, and the Mary E. Groff Charitable Trust.
—Erik Greb
BALTIMORE—Stimulation of the sphenopalatine ganglion (SPG) may be a safe and effective method of temporarily disrupting the blood–brain barrier to deliver therapeutics to the brain. In an animal model of stroke, SPG stimulation enhances the delivery of mesenchymal stem cells and improves functional outcomes, according to research presented at the 141st Annual Meeting of the American Neurological Association. The technique does not entail unwanted systemic effects and potentially could be applied in the treatment of other neurologic disorders.
Although it would be undesirable to deliver bone-marrow stem cells to the human brain, SPG stimulation could aid the delivery of neural stem cells, viral vectors, antibody infusions, and gene therapies, said Lorraine Iacovitti, PhD, Director of the Jefferson Stem Cell and Regenerative Neuroscience Center at Thomas Jefferson University in Philadelphia. She and her colleagues plan to investigate the mechanisms responsible for the response to SPG stimulation. In addition, they will examine various stimulation frequencies and determine the size of therapies that can be delivered to the brain.
Disruption of the Blood–Brain Barrier
Modifying the blood–brain barrier has been a longstanding goal of medicine. Achieving this goal would “improve treatments for many neurologic diseases and disorders, particularly if you could combine it with a focused endovascular delivery system so that these reagents get to the appropriate regions,” said Dr. Iacovitti. In 2004, Yarnitsky et al found that stimulating the SPG caused a transient, reversible increase in blood–brain barrier permeability in animals. The technique enabled Evans blue to penetrate nearly the entire side of the brain that received stimulation.
Michael Lang, MD, a fifth-year neurosurgical resident, led Dr. Iacovitti’s group in a study of SPG stimulation in rats with middle cerebral artery (MCA) occlusion. The researchers previously had found that injection of exogenous bone-marrow mesenchymal stem cells reduced infarct size, improved behavioral deficits, and decreased proinflammatory factors in this model of stroke. Although some stem cells reached the brain, most collected in the lungs, the kidneys, and the liver. Dr. Iacovitti’s group hypothesized that SPG stimulation would increase mesenchymal stem cell engraftment following intra-arterial delivery.
SPG Stimulation in a Stroke Model
The investigators studied three groups of rats. One group received MCA occlusion. The second group received MCA occlusion and an intra-arterial infusion of mesenchymal stem cells at one day post stroke. The third group underwent MCA occlusion, intra-arterial infusion of mesenchymal stem cells, and SPG stimulation at one day post stroke. The stimulation frequency was 10 Hz, and the potential was 5 V. Stimulation continuously alternated between 90-s on and 60-s off for a total of 20 minutes.
In the absence of SPG stimulation, few, if any, stem cells reached the parenchyma. The cells did reach the parenchyma, however, in rats that received SPG stimulation. In addition, SPG stimulation was associated with an improvement in functional outcome. At day 7 and at day 14, the researchers observed a difference in function between animals that received mesenchymal stem cells alone and those that received mesenchymal stem cells plus SPG stimulation. At day 14, the Modified Neurologic Severity score was approximately 50% lower in rats that received stem cells and SPG stimulation, compared with untreated rats.
Electron microscopy revealed that most tight junctions in the rats’ brains appeared normal after SPG stimulation, although tight junction discontinuity was common. The effect was similar to that of a mannitol infusion, said Dr. Iacovitti. “It is possible that stem cells are moving out of circulation into the brain in a fashion similar to what you would see after tumor-necrosis-factor-alpha-stimulated inflammation, where you would get immune cells to move out of the blood vessels and into the damaged brain area through a process of diapedesis.” Unlike mannitol administration, which causes dangerous systemic side effects, SPG stimulation has no observed adverse side effects.
“The combination of endovascular selectivity with SPG stimulation is potentially an extremely powerful tool to deliver [therapies] across the blood–brain barrier into the brain,” she continued. “We have just started to look at getting viruses across…. This work has really just begun.”
Dr. Iacovitti’s research was funded by grants awarded by the NIH, the Joseph and Marie Field Family Foundation, and the Mary E. Groff Charitable Trust.
—Erik Greb
Metabolomics of liquid biopsies offer a comprehensive look at NAFLD
BOSTON – Metabolomics of liquid biopsies noninvasively identified nonalcoholic fatty liver disease (NAFLD) with and without steatosis, and assessed the severity of both steatosis and fibrosis, Puneet Puri, MD, reported at the annual meeting of the American Association for the Study of Liver Diseases.
“These data provide proof of concept that liquid biopsy metabolomics can be used to resolve diagnostic questions in NAFLD management,” said Dr. Puri of Virginia Commonwealth University Medical Center in Richmond.
The researchers first developed a model that distinguished NAFLD patients from controls based on body mass index and the relative plasma concentrations of 11 triglycerides. This model correctly classified patients and controls 90% of the time (area under the receiver operating characteristic curve [AUROC], 0.90; standard deviation, 0.02) in the discovery cohort, and 93% of the time in the validation cohort (AUROC, 0.93; SD, 0.03). The sensitivity of the model was 98% in the discovery cohort and 97% in the validation cohort, and its specificity was 78% in the discovery cohort and 82% in the validation cohort.
The investigators then developed a lipodomic signature to assess the severity of steatosis in NAFLD patients, using magnetic resonance (MR) hepatic fat fraction data as the standard. This lipodomic signature correlated with MR with an r value of 0.81 (P less than .0001).
Next, they evaluated metabolomics for diagnosing nonalcoholic steatohepatitis (NASH). A model that accounted for body mass index (BMI) and the relative concentrations of 20 triglycerides distinguished biopsy-confirmed nonalcoholic fatty liver without steatosis from NASH with an AUROC of 0.95, a sensitivity of 0.83, and a specificity of 0.94 in the discovery cohort. In the validation cohort, the AUROC was 0.84, sensitivity was 79%, and specificity was 92%.
Finally, the researchers developed a way to use metabolomics to evaluate the severity of fibrosis. An algorithm that incorporated 16 variables for phospholipids, triacylglycerols, and nonesterified fatty acids distinguished F0 from F1 through F4 fibrosis with an AUROC of 0.92. Its sensitivity was 90%, and its specificity was 77%. A separate algorithm that incorporated five variables for phospholipids, triacylglycerols, acylcarnitines, sphingolipids, and sterols distinguished F1/F2 fibrosis from F3/F4 fibrosis with an AUROC of 0.89. Its sensitivity was only 62%, but its specificity was 93%.
This proof-of-concept study supports the idea that NAFLD and NASH cause metabolic changes, which in turn alter the circulating metabolome and can be noninvasively measured for diagnostic purposes, Dr. Puri concluded.
Dr. Puri did not list funding sources. He reported having no relevant financial conflicts of interest.
BOSTON – Metabolomics of liquid biopsies noninvasively identified nonalcoholic fatty liver disease (NAFLD) with and without steatosis, and assessed the severity of both steatosis and fibrosis, Puneet Puri, MD, reported at the annual meeting of the American Association for the Study of Liver Diseases.
“These data provide proof of concept that liquid biopsy metabolomics can be used to resolve diagnostic questions in NAFLD management,” said Dr. Puri of Virginia Commonwealth University Medical Center in Richmond.
The researchers first developed a model that distinguished NAFLD patients from controls based on body mass index and the relative plasma concentrations of 11 triglycerides. This model correctly classified patients and controls 90% of the time (area under the receiver operating characteristic curve [AUROC], 0.90; standard deviation, 0.02) in the discovery cohort, and 93% of the time in the validation cohort (AUROC, 0.93; SD, 0.03). The sensitivity of the model was 98% in the discovery cohort and 97% in the validation cohort, and its specificity was 78% in the discovery cohort and 82% in the validation cohort.
The investigators then developed a lipodomic signature to assess the severity of steatosis in NAFLD patients, using magnetic resonance (MR) hepatic fat fraction data as the standard. This lipodomic signature correlated with MR with an r value of 0.81 (P less than .0001).
Next, they evaluated metabolomics for diagnosing nonalcoholic steatohepatitis (NASH). A model that accounted for body mass index (BMI) and the relative concentrations of 20 triglycerides distinguished biopsy-confirmed nonalcoholic fatty liver without steatosis from NASH with an AUROC of 0.95, a sensitivity of 0.83, and a specificity of 0.94 in the discovery cohort. In the validation cohort, the AUROC was 0.84, sensitivity was 79%, and specificity was 92%.
Finally, the researchers developed a way to use metabolomics to evaluate the severity of fibrosis. An algorithm that incorporated 16 variables for phospholipids, triacylglycerols, and nonesterified fatty acids distinguished F0 from F1 through F4 fibrosis with an AUROC of 0.92. Its sensitivity was 90%, and its specificity was 77%. A separate algorithm that incorporated five variables for phospholipids, triacylglycerols, acylcarnitines, sphingolipids, and sterols distinguished F1/F2 fibrosis from F3/F4 fibrosis with an AUROC of 0.89. Its sensitivity was only 62%, but its specificity was 93%.
This proof-of-concept study supports the idea that NAFLD and NASH cause metabolic changes, which in turn alter the circulating metabolome and can be noninvasively measured for diagnostic purposes, Dr. Puri concluded.
Dr. Puri did not list funding sources. He reported having no relevant financial conflicts of interest.
BOSTON – Metabolomics of liquid biopsies noninvasively identified nonalcoholic fatty liver disease (NAFLD) with and without steatosis, and assessed the severity of both steatosis and fibrosis, Puneet Puri, MD, reported at the annual meeting of the American Association for the Study of Liver Diseases.
“These data provide proof of concept that liquid biopsy metabolomics can be used to resolve diagnostic questions in NAFLD management,” said Dr. Puri of Virginia Commonwealth University Medical Center in Richmond.
The researchers first developed a model that distinguished NAFLD patients from controls based on body mass index and the relative plasma concentrations of 11 triglycerides. This model correctly classified patients and controls 90% of the time (area under the receiver operating characteristic curve [AUROC], 0.90; standard deviation, 0.02) in the discovery cohort, and 93% of the time in the validation cohort (AUROC, 0.93; SD, 0.03). The sensitivity of the model was 98% in the discovery cohort and 97% in the validation cohort, and its specificity was 78% in the discovery cohort and 82% in the validation cohort.
The investigators then developed a lipodomic signature to assess the severity of steatosis in NAFLD patients, using magnetic resonance (MR) hepatic fat fraction data as the standard. This lipodomic signature correlated with MR with an r value of 0.81 (P less than .0001).
Next, they evaluated metabolomics for diagnosing nonalcoholic steatohepatitis (NASH). A model that accounted for body mass index (BMI) and the relative concentrations of 20 triglycerides distinguished biopsy-confirmed nonalcoholic fatty liver without steatosis from NASH with an AUROC of 0.95, a sensitivity of 0.83, and a specificity of 0.94 in the discovery cohort. In the validation cohort, the AUROC was 0.84, sensitivity was 79%, and specificity was 92%.
Finally, the researchers developed a way to use metabolomics to evaluate the severity of fibrosis. An algorithm that incorporated 16 variables for phospholipids, triacylglycerols, and nonesterified fatty acids distinguished F0 from F1 through F4 fibrosis with an AUROC of 0.92. Its sensitivity was 90%, and its specificity was 77%. A separate algorithm that incorporated five variables for phospholipids, triacylglycerols, acylcarnitines, sphingolipids, and sterols distinguished F1/F2 fibrosis from F3/F4 fibrosis with an AUROC of 0.89. Its sensitivity was only 62%, but its specificity was 93%.
This proof-of-concept study supports the idea that NAFLD and NASH cause metabolic changes, which in turn alter the circulating metabolome and can be noninvasively measured for diagnostic purposes, Dr. Puri concluded.
Dr. Puri did not list funding sources. He reported having no relevant financial conflicts of interest.
AT THE LIVER MEETING 2016
Key clinical point: Metabolomics of liquid biopsies identified and characterized nonalcoholic fatty liver disease in a proof-of-concept study.
Major finding: Four distinct models diagnosed NAFLD, diagnosed NASH, and characterized the severity of steatosis and fibrosis.
Data source: A multicenter study of 817 patients with biopsy-confirmed NAFLD and 130 biopsy-confirmed controls.
Disclosures: Dr. Puri did not list funding sources. He reported having no relevant financial conflicts of interest.
VIDEO: Denosumab trumps risedronate in bone building for glucocorticoid-induced osteoporosis
WASHINGTON – Denosumab built significantly more bone at the hip and lumbar spine than did risedronate when given for 1 year to patients with glucocorticoid-induced osteoporosis in an ongoing 2-year, head-to-head, randomized trial.
Denosumab (Prolia) is currently approved for the treatment of postmenopausal osteoporosis, and it performed so well in the trial that it could be put forward for the indication of glucocorticoid-induced osteoporosis as well, Kenneth Saag, MD, said at the annual meeting of the American College of Rheumatology.
“I would say there is definitely potential for this as a new therapeutic option for these patients,” he said in a video interview about the trial’s primary outcome of denosumab’s noninferiority to risedronate in percentage change in bone mineral density (BMD) at the lumbar spine after 1 year and secondary outcomes of the superiority of denosumab over risedronate in total hip and lumbar spine BMD at 1 year.
Denosumab is a particularly intriguing treatment option for patients with glucocorticoid-induced osteoporosis. They experience a double hit on bone health: increased RANKL, a protein that stimulates osteoclast development, and decreased osteoprotegerin, a protein that inhibits osteoclasts. Denosumab is a RANKL-inhibitor and, as such, tamps down on osteoclastic bone remodeling, said Dr. Saag, vice chair of the department of medicine and director of the Center for Education and Research on Therapeutics at the University of Alabama at Birmingham.
The phase III trial comprised 795 patients who were taking corticosteroids for a variety of rheumatic diseases, including rheumatoid arthritis, polymyalgia rheumatica, and systemic lupus erythematosus, and randomized them to denosumab or risedronate, which is already FDA approved for glucocorticoid-induced bone loss. Patients were randomized to 24 months of subcutaneous denosumab 60 mg given every 6 months or oral risedronate 5-mg daily. The study is still ongoing to test secondary outcomes at 24 months.
The patients were split into those who were continuing glucocorticoid therapy (505) and those who were just initiating it (290). Patients’ mean age ranged from 61 to 67 years, with the glucocorticoid-initiating group (GC-I) being somewhat older. The mean daily prednisone-equivalent dose was 16 mg in that group and 12 mg in the glucocorticoid-continuing group (GC-C). The mean BMD T-scores in the GC-C group were –1.96 at the lumbar spine and –1.56 at the total hip. In the GC-I group, BMD T-scores were –1.06 at the lumbar spine and –0.98 at the total hip.
In the GC-C group, denosumab increased BMD significantly more than risedronate at both spine and hip. At the lumbar spine, denosumab was associated with a mean increase of 4.4% over baseline, compared with a 2.3% increase with risedronate. Total hip BMD increased 2.1% with denosumab and 0.6% with risedronate.
The results were similar in the GC-I group. Denosumab increased lumbar spine BMD by 3.8% over baseline, compared with an increase of 0.8% with risedronate. Total hip BMD increased 1.7% with denosumab and 0.2% with risedronate.
Denosumab was also associated with significantly greater increases in femoral neck BMD in both groups, Dr. Saag noted. There were no significant differences in markers of bone turnover between the treatment groups. Adverse events, including pneumonia, diverticulitis, and bronchitis, were similar.
Amgen, manufacturer of denosumab, is sponsoring the 24-month study. Dr. Saag has been a consultant for Amgen. One coauthor is an employee of Amgen, and others disclosed financial relationships with Amgen and other pharmaceutical companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @alz_gal
WASHINGTON – Denosumab built significantly more bone at the hip and lumbar spine than did risedronate when given for 1 year to patients with glucocorticoid-induced osteoporosis in an ongoing 2-year, head-to-head, randomized trial.
Denosumab (Prolia) is currently approved for the treatment of postmenopausal osteoporosis, and it performed so well in the trial that it could be put forward for the indication of glucocorticoid-induced osteoporosis as well, Kenneth Saag, MD, said at the annual meeting of the American College of Rheumatology.
“I would say there is definitely potential for this as a new therapeutic option for these patients,” he said in a video interview about the trial’s primary outcome of denosumab’s noninferiority to risedronate in percentage change in bone mineral density (BMD) at the lumbar spine after 1 year and secondary outcomes of the superiority of denosumab over risedronate in total hip and lumbar spine BMD at 1 year.
Denosumab is a particularly intriguing treatment option for patients with glucocorticoid-induced osteoporosis. They experience a double hit on bone health: increased RANKL, a protein that stimulates osteoclast development, and decreased osteoprotegerin, a protein that inhibits osteoclasts. Denosumab is a RANKL-inhibitor and, as such, tamps down on osteoclastic bone remodeling, said Dr. Saag, vice chair of the department of medicine and director of the Center for Education and Research on Therapeutics at the University of Alabama at Birmingham.
The phase III trial comprised 795 patients who were taking corticosteroids for a variety of rheumatic diseases, including rheumatoid arthritis, polymyalgia rheumatica, and systemic lupus erythematosus, and randomized them to denosumab or risedronate, which is already FDA approved for glucocorticoid-induced bone loss. Patients were randomized to 24 months of subcutaneous denosumab 60 mg given every 6 months or oral risedronate 5-mg daily. The study is still ongoing to test secondary outcomes at 24 months.
The patients were split into those who were continuing glucocorticoid therapy (505) and those who were just initiating it (290). Patients’ mean age ranged from 61 to 67 years, with the glucocorticoid-initiating group (GC-I) being somewhat older. The mean daily prednisone-equivalent dose was 16 mg in that group and 12 mg in the glucocorticoid-continuing group (GC-C). The mean BMD T-scores in the GC-C group were –1.96 at the lumbar spine and –1.56 at the total hip. In the GC-I group, BMD T-scores were –1.06 at the lumbar spine and –0.98 at the total hip.
In the GC-C group, denosumab increased BMD significantly more than risedronate at both spine and hip. At the lumbar spine, denosumab was associated with a mean increase of 4.4% over baseline, compared with a 2.3% increase with risedronate. Total hip BMD increased 2.1% with denosumab and 0.6% with risedronate.
The results were similar in the GC-I group. Denosumab increased lumbar spine BMD by 3.8% over baseline, compared with an increase of 0.8% with risedronate. Total hip BMD increased 1.7% with denosumab and 0.2% with risedronate.
Denosumab was also associated with significantly greater increases in femoral neck BMD in both groups, Dr. Saag noted. There were no significant differences in markers of bone turnover between the treatment groups. Adverse events, including pneumonia, diverticulitis, and bronchitis, were similar.
Amgen, manufacturer of denosumab, is sponsoring the 24-month study. Dr. Saag has been a consultant for Amgen. One coauthor is an employee of Amgen, and others disclosed financial relationships with Amgen and other pharmaceutical companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @alz_gal
WASHINGTON – Denosumab built significantly more bone at the hip and lumbar spine than did risedronate when given for 1 year to patients with glucocorticoid-induced osteoporosis in an ongoing 2-year, head-to-head, randomized trial.
Denosumab (Prolia) is currently approved for the treatment of postmenopausal osteoporosis, and it performed so well in the trial that it could be put forward for the indication of glucocorticoid-induced osteoporosis as well, Kenneth Saag, MD, said at the annual meeting of the American College of Rheumatology.
“I would say there is definitely potential for this as a new therapeutic option for these patients,” he said in a video interview about the trial’s primary outcome of denosumab’s noninferiority to risedronate in percentage change in bone mineral density (BMD) at the lumbar spine after 1 year and secondary outcomes of the superiority of denosumab over risedronate in total hip and lumbar spine BMD at 1 year.
Denosumab is a particularly intriguing treatment option for patients with glucocorticoid-induced osteoporosis. They experience a double hit on bone health: increased RANKL, a protein that stimulates osteoclast development, and decreased osteoprotegerin, a protein that inhibits osteoclasts. Denosumab is a RANKL-inhibitor and, as such, tamps down on osteoclastic bone remodeling, said Dr. Saag, vice chair of the department of medicine and director of the Center for Education and Research on Therapeutics at the University of Alabama at Birmingham.
The phase III trial comprised 795 patients who were taking corticosteroids for a variety of rheumatic diseases, including rheumatoid arthritis, polymyalgia rheumatica, and systemic lupus erythematosus, and randomized them to denosumab or risedronate, which is already FDA approved for glucocorticoid-induced bone loss. Patients were randomized to 24 months of subcutaneous denosumab 60 mg given every 6 months or oral risedronate 5-mg daily. The study is still ongoing to test secondary outcomes at 24 months.
The patients were split into those who were continuing glucocorticoid therapy (505) and those who were just initiating it (290). Patients’ mean age ranged from 61 to 67 years, with the glucocorticoid-initiating group (GC-I) being somewhat older. The mean daily prednisone-equivalent dose was 16 mg in that group and 12 mg in the glucocorticoid-continuing group (GC-C). The mean BMD T-scores in the GC-C group were –1.96 at the lumbar spine and –1.56 at the total hip. In the GC-I group, BMD T-scores were –1.06 at the lumbar spine and –0.98 at the total hip.
In the GC-C group, denosumab increased BMD significantly more than risedronate at both spine and hip. At the lumbar spine, denosumab was associated with a mean increase of 4.4% over baseline, compared with a 2.3% increase with risedronate. Total hip BMD increased 2.1% with denosumab and 0.6% with risedronate.
The results were similar in the GC-I group. Denosumab increased lumbar spine BMD by 3.8% over baseline, compared with an increase of 0.8% with risedronate. Total hip BMD increased 1.7% with denosumab and 0.2% with risedronate.
Denosumab was also associated with significantly greater increases in femoral neck BMD in both groups, Dr. Saag noted. There were no significant differences in markers of bone turnover between the treatment groups. Adverse events, including pneumonia, diverticulitis, and bronchitis, were similar.
Amgen, manufacturer of denosumab, is sponsoring the 24-month study. Dr. Saag has been a consultant for Amgen. One coauthor is an employee of Amgen, and others disclosed financial relationships with Amgen and other pharmaceutical companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @alz_gal
AT THE ACR ANNUAL MEETING
Key clinical point:
Major finding: In patients on continuous glucocorticoid therapy, denosumab increased BMD by 4.4% at the lumbar spine and 2.1% at the total hip, compared with increases of 2.3% and 0.6% with risedronate.
Data source: 12-month results of the 24-month, phase III study of 795 patients.
Disclosures: Amgen sponsored the study. Dr. Saag has been a consultant for the company. One coauthor is an employee of Amgen, and others disclosed financial relationships with Amgen and other pharmaceutical companies.
Study shows NJ tube and PEG-J on par for enteral nutrition, but each has complications
CORONADO, CALIF. – Percutaneous gastrostomy with jejunal extension (PEG-J) is an appealing and effective method for delivery of enteral nutrition in necrotizing pancreatitis patients, without the mechanical issues and discomfort associated with nasojejunal (NJ) tube, results from a single-center retrospective study showed.
“The advantages of PEG-J route for enteral nutrition in necrotizing pancreatitis patients must be weighed carefully against the potentially severe complication profile,” study author Alexandra M. Roch, MD, said at the annual meeting of the Western Surgical Association.
Historically, the preferred way to manage patients with necrotizing pancreatitis was via parenteral nutrition with a lack of pancreatic stimulation, said Dr. Roch, of the department of surgery at Indiana University, Indianapolis. However, parenteral nutrition is associated with increased permeability, a lack of peristaltic stimulation, changes in intestinal flora, and an increased risk of infection.
“More recently, enteral nutrition has been used, despite a potential for pancreatic stimulation,” she said. “From 16 randomized, controlled trials with 847 patients, it was associated with decreased mortality, decreased infectious complications, decreased length of hospital stay, and a trend toward decreased rate of organ failure. Based on those findings, enteral nutrition has become the standard of care in acute pancreatitis. The optimal enteral nutrition route, however, is still debated. The traditional route is the nasojejunal [NJ] tube. Its placement is noninvasive, but it is associated with discomfort for the patient, dislodgement in 16%-63% of cases, and potentially sinusitis. Conversely, percutaneous gastrostomy with jejunal extension [PEG-J] is beneficial for patient comfort but has the drawbacks of being an invasive procedure with the risk of cellulitis and more severe complications.”
The aim of the current study was to compare the safety and efficacy of NJ tube and PEG-J enteral nutrition delivery before surgical debridement in patients with necrotizing pancreatitis. Dr. Roch and her associates hypothesized that NJ tube and PEG-J would have a similar complication profile. They retrospectively reviewed the medical records of all patients who underwent surgical debridement for necrotizing pancreatitis at Indiana University Medical Center between 2005 and 2015. Patients with exclusive total parenteral nutrition were excluded from the study, as were those who had incomplete data.
Dr. Roch reported results from 242 patients with a mean age of 54 years. More than half (64%) were men and the main etiology was biliary (47%), followed by alcohol (16%). The median duration of preoperative enteral nutrition was 29 days. Of the 242 patients, 187 had an NJ tube only, 25 had PEG-J only, and 30 patients had an NJ tube followed by PEG-J. More than half of PEG-Js were placed under fluoroscopic guidance, while the remaining 41% were placed endoscopically.
In terms of safety, patients in the NJ tube group had a significantly higher rate of all complications, compared with those in the PEG-J group (52% vs. 27%, respectively; P = .0015). Conversely, there was a significantly higher rate of serious complications among patients in the PEG-J group, compared with the NJ group (11% vs. 0%; P less than .0001). The researchers also found that compared with patients in the PEG-J group, those in the NJ group were more prone to mechanical complications such as difficulty to place (5% vs. 0%, respectively), replacement (30% vs. 5.5%), and repositioning (30% vs. 2%), while PEG-J patients were more prone to infectious complications such as skin infections/cellulitis (4% vs. 0%) and perforation/leakage/peritonitis (11% vs. 0%). When they limited the analysis to grade III or IV complications, the mechanism was always the same: early dislodgement from the GI tract. “The presentation ranged from asymptomatic patients to severe peritonitis,” Dr. Roch said. “Two patients out of the six with severe complications required emergent laparotomy.”
In terms of efficacy, the NJ and PEG-J groups were equivalent in achieving enteral nutrition (67% vs. 68%, respectively). There were also no differences between the two groups in nutritional status when assessed by an increase of serum albumin (38% vs. 36%; P = .87), normalization of serum albumin (9% vs. 16%; P = .14), or in the prevalence of infected necrosis (53% vs. 49%; P = .64).
Dr. Roch acknowledged certain limitations of the study, including its single-center, retrospective design. “Furthermore, we are a tertiary care center, and most patients are referred to us late in the course of their disease,” she said. “Finally, no PEG-Js were placed outside of our institution, raising the question of a selection bias. She reported having no financial disclosures.
CORONADO, CALIF. – Percutaneous gastrostomy with jejunal extension (PEG-J) is an appealing and effective method for delivery of enteral nutrition in necrotizing pancreatitis patients, without the mechanical issues and discomfort associated with nasojejunal (NJ) tube, results from a single-center retrospective study showed.
“The advantages of PEG-J route for enteral nutrition in necrotizing pancreatitis patients must be weighed carefully against the potentially severe complication profile,” study author Alexandra M. Roch, MD, said at the annual meeting of the Western Surgical Association.
Historically, the preferred way to manage patients with necrotizing pancreatitis was via parenteral nutrition with a lack of pancreatic stimulation, said Dr. Roch, of the department of surgery at Indiana University, Indianapolis. However, parenteral nutrition is associated with increased permeability, a lack of peristaltic stimulation, changes in intestinal flora, and an increased risk of infection.
“More recently, enteral nutrition has been used, despite a potential for pancreatic stimulation,” she said. “From 16 randomized, controlled trials with 847 patients, it was associated with decreased mortality, decreased infectious complications, decreased length of hospital stay, and a trend toward decreased rate of organ failure. Based on those findings, enteral nutrition has become the standard of care in acute pancreatitis. The optimal enteral nutrition route, however, is still debated. The traditional route is the nasojejunal [NJ] tube. Its placement is noninvasive, but it is associated with discomfort for the patient, dislodgement in 16%-63% of cases, and potentially sinusitis. Conversely, percutaneous gastrostomy with jejunal extension [PEG-J] is beneficial for patient comfort but has the drawbacks of being an invasive procedure with the risk of cellulitis and more severe complications.”
The aim of the current study was to compare the safety and efficacy of NJ tube and PEG-J enteral nutrition delivery before surgical debridement in patients with necrotizing pancreatitis. Dr. Roch and her associates hypothesized that NJ tube and PEG-J would have a similar complication profile. They retrospectively reviewed the medical records of all patients who underwent surgical debridement for necrotizing pancreatitis at Indiana University Medical Center between 2005 and 2015. Patients with exclusive total parenteral nutrition were excluded from the study, as were those who had incomplete data.
Dr. Roch reported results from 242 patients with a mean age of 54 years. More than half (64%) were men and the main etiology was biliary (47%), followed by alcohol (16%). The median duration of preoperative enteral nutrition was 29 days. Of the 242 patients, 187 had an NJ tube only, 25 had PEG-J only, and 30 patients had an NJ tube followed by PEG-J. More than half of PEG-Js were placed under fluoroscopic guidance, while the remaining 41% were placed endoscopically.
In terms of safety, patients in the NJ tube group had a significantly higher rate of all complications, compared with those in the PEG-J group (52% vs. 27%, respectively; P = .0015). Conversely, there was a significantly higher rate of serious complications among patients in the PEG-J group, compared with the NJ group (11% vs. 0%; P less than .0001). The researchers also found that compared with patients in the PEG-J group, those in the NJ group were more prone to mechanical complications such as difficulty to place (5% vs. 0%, respectively), replacement (30% vs. 5.5%), and repositioning (30% vs. 2%), while PEG-J patients were more prone to infectious complications such as skin infections/cellulitis (4% vs. 0%) and perforation/leakage/peritonitis (11% vs. 0%). When they limited the analysis to grade III or IV complications, the mechanism was always the same: early dislodgement from the GI tract. “The presentation ranged from asymptomatic patients to severe peritonitis,” Dr. Roch said. “Two patients out of the six with severe complications required emergent laparotomy.”
In terms of efficacy, the NJ and PEG-J groups were equivalent in achieving enteral nutrition (67% vs. 68%, respectively). There were also no differences between the two groups in nutritional status when assessed by an increase of serum albumin (38% vs. 36%; P = .87), normalization of serum albumin (9% vs. 16%; P = .14), or in the prevalence of infected necrosis (53% vs. 49%; P = .64).
Dr. Roch acknowledged certain limitations of the study, including its single-center, retrospective design. “Furthermore, we are a tertiary care center, and most patients are referred to us late in the course of their disease,” she said. “Finally, no PEG-Js were placed outside of our institution, raising the question of a selection bias. She reported having no financial disclosures.
CORONADO, CALIF. – Percutaneous gastrostomy with jejunal extension (PEG-J) is an appealing and effective method for delivery of enteral nutrition in necrotizing pancreatitis patients, without the mechanical issues and discomfort associated with nasojejunal (NJ) tube, results from a single-center retrospective study showed.
“The advantages of PEG-J route for enteral nutrition in necrotizing pancreatitis patients must be weighed carefully against the potentially severe complication profile,” study author Alexandra M. Roch, MD, said at the annual meeting of the Western Surgical Association.
Historically, the preferred way to manage patients with necrotizing pancreatitis was via parenteral nutrition with a lack of pancreatic stimulation, said Dr. Roch, of the department of surgery at Indiana University, Indianapolis. However, parenteral nutrition is associated with increased permeability, a lack of peristaltic stimulation, changes in intestinal flora, and an increased risk of infection.
“More recently, enteral nutrition has been used, despite a potential for pancreatic stimulation,” she said. “From 16 randomized, controlled trials with 847 patients, it was associated with decreased mortality, decreased infectious complications, decreased length of hospital stay, and a trend toward decreased rate of organ failure. Based on those findings, enteral nutrition has become the standard of care in acute pancreatitis. The optimal enteral nutrition route, however, is still debated. The traditional route is the nasojejunal [NJ] tube. Its placement is noninvasive, but it is associated with discomfort for the patient, dislodgement in 16%-63% of cases, and potentially sinusitis. Conversely, percutaneous gastrostomy with jejunal extension [PEG-J] is beneficial for patient comfort but has the drawbacks of being an invasive procedure with the risk of cellulitis and more severe complications.”
The aim of the current study was to compare the safety and efficacy of NJ tube and PEG-J enteral nutrition delivery before surgical debridement in patients with necrotizing pancreatitis. Dr. Roch and her associates hypothesized that NJ tube and PEG-J would have a similar complication profile. They retrospectively reviewed the medical records of all patients who underwent surgical debridement for necrotizing pancreatitis at Indiana University Medical Center between 2005 and 2015. Patients with exclusive total parenteral nutrition were excluded from the study, as were those who had incomplete data.
Dr. Roch reported results from 242 patients with a mean age of 54 years. More than half (64%) were men and the main etiology was biliary (47%), followed by alcohol (16%). The median duration of preoperative enteral nutrition was 29 days. Of the 242 patients, 187 had an NJ tube only, 25 had PEG-J only, and 30 patients had an NJ tube followed by PEG-J. More than half of PEG-Js were placed under fluoroscopic guidance, while the remaining 41% were placed endoscopically.
In terms of safety, patients in the NJ tube group had a significantly higher rate of all complications, compared with those in the PEG-J group (52% vs. 27%, respectively; P = .0015). Conversely, there was a significantly higher rate of serious complications among patients in the PEG-J group, compared with the NJ group (11% vs. 0%; P less than .0001). The researchers also found that compared with patients in the PEG-J group, those in the NJ group were more prone to mechanical complications such as difficulty to place (5% vs. 0%, respectively), replacement (30% vs. 5.5%), and repositioning (30% vs. 2%), while PEG-J patients were more prone to infectious complications such as skin infections/cellulitis (4% vs. 0%) and perforation/leakage/peritonitis (11% vs. 0%). When they limited the analysis to grade III or IV complications, the mechanism was always the same: early dislodgement from the GI tract. “The presentation ranged from asymptomatic patients to severe peritonitis,” Dr. Roch said. “Two patients out of the six with severe complications required emergent laparotomy.”
In terms of efficacy, the NJ and PEG-J groups were equivalent in achieving enteral nutrition (67% vs. 68%, respectively). There were also no differences between the two groups in nutritional status when assessed by an increase of serum albumin (38% vs. 36%; P = .87), normalization of serum albumin (9% vs. 16%; P = .14), or in the prevalence of infected necrosis (53% vs. 49%; P = .64).
Dr. Roch acknowledged certain limitations of the study, including its single-center, retrospective design. “Furthermore, we are a tertiary care center, and most patients are referred to us late in the course of their disease,” she said. “Finally, no PEG-Js were placed outside of our institution, raising the question of a selection bias. She reported having no financial disclosures.
AT WSA 2016
Key clinical point:
Major finding: In terms of efficacy, the NJ and PEG-J groups were equivalent in achieving enteral nutrition (67% vs. 68%, respectively).
Data source: A retrospective review of 242 patients who underwent surgical debridement for necrotizing pancreatitis at Indiana University Medical Center between 2005 and 2015.
Disclosures: Dr. Roch reported having no financial disclosures.