User login
Atrial Fibrillation
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Initial outcomes of PERT at Cleveland Clinic
LOS ANGELES – Initial outcomes measures are beginning to emerge from Pulmonary Embolism Response Teams.
Members of the Cleveland Clinic’s PERT, which was established in 2014, presented some of their preliminary data during a presentation at the CHEST annual meeting.
The concept behind the PERT is to rapidly mobilize a team with varied expertise helpful for treating patients with pulmonary embolisms (PEs). While the PERT “can be activated by any (clinician) for any patient, even low-risk patients ... those with submassive and massive PEs [intermediate- and high-risk patients]” are the target patients, said Dr. Mahar of the Cleveland Clinic.
The first PERT was created at Massachusetts General Hospital in Boston in 2012, according to the National Consortium of Pulmonary Embolism Response Team’s website. As of May 2015, the PERT model has been adopted by physicians and health care professionals from more than 40 institutions.
Dr. Mahar reported that the Cleveland Clinic’s PERT is activated through a single pager that resides with a vascular medicine fellow during the day and a critical care fellow at night. When paged, the fellow promptly evaluates the patient and ensures a complete basic work-up, which includes an ECG, cardiac enzymes, N-terminal pro b-type natriuretic peptide, lower-extremity deep vein thrombosis scans, transthoracic echocardiogram, and confirmatory CT/PE protocol or ventilation/perfusion scan.
Based on the simplified Pulmonary Embolism Severity Index and Bova scores, the patient is risk stratified and the patient’s indications, and relative and absolute contraindications to advanced therapies are reviewed. The fellow next sends a group notification to the PERT via email and text message. The team then convenes online for a virtual meeting and case presentation that includes sharing of lab and test results and images.
The process sounds complex, but the surgeon, interventional radiologist, vascular medicine specialist, and cardiologist are on call and simultaneously get the message and respond, Dr. Mahar said. With a team approach, the decision to use advanced therapies – systemic lytics, surgery, catheter-directed lysis and extracorporeal membrane oxygenation – is expedited. “For example, over the last 2 years, four out of four patients who underwent surgical embolectomies had good outcomes without any deaths,” he said.
Based on a retrospective chart review from October 2014 through August 2016, Cleveland Clinic’s PERT had been activated for 134 patients, 112 of whom were found to have PEs, Dr. Mahar said during his presentation at the annual meeting of the American College of Chest Physicians (CHEST).
The number of low risk, submassive, and massive PEs were 14 (12%), 76 (68%), and 22 (20%), respectively. Just over half of the PE patients, 55% (60 patients), were treated with anticoagulation therapy alone. Inferior vena cava filters were placed in 32 patients (29%); 14 patients received catheter-directed thrombolysis, 3 received a suction thrombectomy, and 4 received a surgical embolectomy.
The 30-day all-cause mortality rate was 9%; the deaths occurred in six patients who had massive PEs, three patients with submassive PEs, and one patient with a low-risk PE. Six of the patients who died had been treated with anticoagulation, two had received catheter-directed thrombolysis, and one had received a full dose of systemic thrombolysis.
Bleeding complications occurred in 10 patients, 6 of whom were treated with anticoagulation alone and 4 of whom underwent catheter-directed thrombolysis.
Cleveland Clinic is a large entity with multiple resources, but the principles of PERT can be applied in smaller facilities, as well, according to Gustavo A. Heresi-Davila, MD, medical director of the Cleveland Clinic’s pulmonary thromboendarterectomy program and the lead researcher for the PERT project at the clinic. “I would emphasize the notion that a PERT has to be multidisciplinary, as people with different backgrounds and expertise bring complementary talent to the discussion of each case. I would not minimize the challenges of assembling such a team,” he said during an interview following the meeting.
The moderator of the meeting session, Robert Schilz, DO, PhD, noted, that the goal of PERT is to determine the best approach for an individual patient based on available resources. To establish a PERT, “you don’t have to be able to put a patient on ECMO [extracorporeal membrane oxygenation] in 15 minutes, and you don’t have to be able to do endarterectomies, embolectomies, and all the catheter-drive techniques emergently. But you do need to have the disposition to have efficient and standardized care, and the solutions may need to be very geographic. What hospital A may do may be very different from hospital B.”
Small hospitals can draw on their available resources, added Dr. Schilz, director of pulmonary vascular disease and lung transplantation at Case Western Reserve University, Cleveland. “Most hospitals have cardiologists on call 24/7, and many have some flavor of interventional radiology; others have clear referral and transfer schemes. Emergency department personnel at small rural hospitals can rapidly identify patients appropriate for transfer.”
Dr. Mahar added that PERTs are already being utilized in smaller hospitals and that he thinks that, in the next 5 years, having a PERT will be the standard protocol.
Dr. Mahar reported no disclosures.
Mary Jo Dales contributed to this report.
LOS ANGELES – Initial outcomes measures are beginning to emerge from Pulmonary Embolism Response Teams.
Members of the Cleveland Clinic’s PERT, which was established in 2014, presented some of their preliminary data during a presentation at the CHEST annual meeting.
The concept behind the PERT is to rapidly mobilize a team with varied expertise helpful for treating patients with pulmonary embolisms (PEs). While the PERT “can be activated by any (clinician) for any patient, even low-risk patients ... those with submassive and massive PEs [intermediate- and high-risk patients]” are the target patients, said Dr. Mahar of the Cleveland Clinic.
The first PERT was created at Massachusetts General Hospital in Boston in 2012, according to the National Consortium of Pulmonary Embolism Response Team’s website. As of May 2015, the PERT model has been adopted by physicians and health care professionals from more than 40 institutions.
Dr. Mahar reported that the Cleveland Clinic’s PERT is activated through a single pager that resides with a vascular medicine fellow during the day and a critical care fellow at night. When paged, the fellow promptly evaluates the patient and ensures a complete basic work-up, which includes an ECG, cardiac enzymes, N-terminal pro b-type natriuretic peptide, lower-extremity deep vein thrombosis scans, transthoracic echocardiogram, and confirmatory CT/PE protocol or ventilation/perfusion scan.
Based on the simplified Pulmonary Embolism Severity Index and Bova scores, the patient is risk stratified and the patient’s indications, and relative and absolute contraindications to advanced therapies are reviewed. The fellow next sends a group notification to the PERT via email and text message. The team then convenes online for a virtual meeting and case presentation that includes sharing of lab and test results and images.
The process sounds complex, but the surgeon, interventional radiologist, vascular medicine specialist, and cardiologist are on call and simultaneously get the message and respond, Dr. Mahar said. With a team approach, the decision to use advanced therapies – systemic lytics, surgery, catheter-directed lysis and extracorporeal membrane oxygenation – is expedited. “For example, over the last 2 years, four out of four patients who underwent surgical embolectomies had good outcomes without any deaths,” he said.
Based on a retrospective chart review from October 2014 through August 2016, Cleveland Clinic’s PERT had been activated for 134 patients, 112 of whom were found to have PEs, Dr. Mahar said during his presentation at the annual meeting of the American College of Chest Physicians (CHEST).
The number of low risk, submassive, and massive PEs were 14 (12%), 76 (68%), and 22 (20%), respectively. Just over half of the PE patients, 55% (60 patients), were treated with anticoagulation therapy alone. Inferior vena cava filters were placed in 32 patients (29%); 14 patients received catheter-directed thrombolysis, 3 received a suction thrombectomy, and 4 received a surgical embolectomy.
The 30-day all-cause mortality rate was 9%; the deaths occurred in six patients who had massive PEs, three patients with submassive PEs, and one patient with a low-risk PE. Six of the patients who died had been treated with anticoagulation, two had received catheter-directed thrombolysis, and one had received a full dose of systemic thrombolysis.
Bleeding complications occurred in 10 patients, 6 of whom were treated with anticoagulation alone and 4 of whom underwent catheter-directed thrombolysis.
Cleveland Clinic is a large entity with multiple resources, but the principles of PERT can be applied in smaller facilities, as well, according to Gustavo A. Heresi-Davila, MD, medical director of the Cleveland Clinic’s pulmonary thromboendarterectomy program and the lead researcher for the PERT project at the clinic. “I would emphasize the notion that a PERT has to be multidisciplinary, as people with different backgrounds and expertise bring complementary talent to the discussion of each case. I would not minimize the challenges of assembling such a team,” he said during an interview following the meeting.
The moderator of the meeting session, Robert Schilz, DO, PhD, noted, that the goal of PERT is to determine the best approach for an individual patient based on available resources. To establish a PERT, “you don’t have to be able to put a patient on ECMO [extracorporeal membrane oxygenation] in 15 minutes, and you don’t have to be able to do endarterectomies, embolectomies, and all the catheter-drive techniques emergently. But you do need to have the disposition to have efficient and standardized care, and the solutions may need to be very geographic. What hospital A may do may be very different from hospital B.”
Small hospitals can draw on their available resources, added Dr. Schilz, director of pulmonary vascular disease and lung transplantation at Case Western Reserve University, Cleveland. “Most hospitals have cardiologists on call 24/7, and many have some flavor of interventional radiology; others have clear referral and transfer schemes. Emergency department personnel at small rural hospitals can rapidly identify patients appropriate for transfer.”
Dr. Mahar added that PERTs are already being utilized in smaller hospitals and that he thinks that, in the next 5 years, having a PERT will be the standard protocol.
Dr. Mahar reported no disclosures.
Mary Jo Dales contributed to this report.
LOS ANGELES – Initial outcomes measures are beginning to emerge from Pulmonary Embolism Response Teams.
Members of the Cleveland Clinic’s PERT, which was established in 2014, presented some of their preliminary data during a presentation at the CHEST annual meeting.
The concept behind the PERT is to rapidly mobilize a team with varied expertise helpful for treating patients with pulmonary embolisms (PEs). While the PERT “can be activated by any (clinician) for any patient, even low-risk patients ... those with submassive and massive PEs [intermediate- and high-risk patients]” are the target patients, said Dr. Mahar of the Cleveland Clinic.
The first PERT was created at Massachusetts General Hospital in Boston in 2012, according to the National Consortium of Pulmonary Embolism Response Team’s website. As of May 2015, the PERT model has been adopted by physicians and health care professionals from more than 40 institutions.
Dr. Mahar reported that the Cleveland Clinic’s PERT is activated through a single pager that resides with a vascular medicine fellow during the day and a critical care fellow at night. When paged, the fellow promptly evaluates the patient and ensures a complete basic work-up, which includes an ECG, cardiac enzymes, N-terminal pro b-type natriuretic peptide, lower-extremity deep vein thrombosis scans, transthoracic echocardiogram, and confirmatory CT/PE protocol or ventilation/perfusion scan.
Based on the simplified Pulmonary Embolism Severity Index and Bova scores, the patient is risk stratified and the patient’s indications, and relative and absolute contraindications to advanced therapies are reviewed. The fellow next sends a group notification to the PERT via email and text message. The team then convenes online for a virtual meeting and case presentation that includes sharing of lab and test results and images.
The process sounds complex, but the surgeon, interventional radiologist, vascular medicine specialist, and cardiologist are on call and simultaneously get the message and respond, Dr. Mahar said. With a team approach, the decision to use advanced therapies – systemic lytics, surgery, catheter-directed lysis and extracorporeal membrane oxygenation – is expedited. “For example, over the last 2 years, four out of four patients who underwent surgical embolectomies had good outcomes without any deaths,” he said.
Based on a retrospective chart review from October 2014 through August 2016, Cleveland Clinic’s PERT had been activated for 134 patients, 112 of whom were found to have PEs, Dr. Mahar said during his presentation at the annual meeting of the American College of Chest Physicians (CHEST).
The number of low risk, submassive, and massive PEs were 14 (12%), 76 (68%), and 22 (20%), respectively. Just over half of the PE patients, 55% (60 patients), were treated with anticoagulation therapy alone. Inferior vena cava filters were placed in 32 patients (29%); 14 patients received catheter-directed thrombolysis, 3 received a suction thrombectomy, and 4 received a surgical embolectomy.
The 30-day all-cause mortality rate was 9%; the deaths occurred in six patients who had massive PEs, three patients with submassive PEs, and one patient with a low-risk PE. Six of the patients who died had been treated with anticoagulation, two had received catheter-directed thrombolysis, and one had received a full dose of systemic thrombolysis.
Bleeding complications occurred in 10 patients, 6 of whom were treated with anticoagulation alone and 4 of whom underwent catheter-directed thrombolysis.
Cleveland Clinic is a large entity with multiple resources, but the principles of PERT can be applied in smaller facilities, as well, according to Gustavo A. Heresi-Davila, MD, medical director of the Cleveland Clinic’s pulmonary thromboendarterectomy program and the lead researcher for the PERT project at the clinic. “I would emphasize the notion that a PERT has to be multidisciplinary, as people with different backgrounds and expertise bring complementary talent to the discussion of each case. I would not minimize the challenges of assembling such a team,” he said during an interview following the meeting.
The moderator of the meeting session, Robert Schilz, DO, PhD, noted, that the goal of PERT is to determine the best approach for an individual patient based on available resources. To establish a PERT, “you don’t have to be able to put a patient on ECMO [extracorporeal membrane oxygenation] in 15 minutes, and you don’t have to be able to do endarterectomies, embolectomies, and all the catheter-drive techniques emergently. But you do need to have the disposition to have efficient and standardized care, and the solutions may need to be very geographic. What hospital A may do may be very different from hospital B.”
Small hospitals can draw on their available resources, added Dr. Schilz, director of pulmonary vascular disease and lung transplantation at Case Western Reserve University, Cleveland. “Most hospitals have cardiologists on call 24/7, and many have some flavor of interventional radiology; others have clear referral and transfer schemes. Emergency department personnel at small rural hospitals can rapidly identify patients appropriate for transfer.”
Dr. Mahar added that PERTs are already being utilized in smaller hospitals and that he thinks that, in the next 5 years, having a PERT will be the standard protocol.
Dr. Mahar reported no disclosures.
Mary Jo Dales contributed to this report.
FROM CHEST 2016
Carotid Stenting Tied to Cardiovascular Events in Real-World Study
ROME—Carotid stenting was associated with a roughly 30% higher risk of cardiovascular events, compared with carotid endarterectomy, during 12 years of follow-up in a large, real-world, population-based cohort study, Mohamad A. Hussain, MD, reported at the Annual Congress of the European Society of Cardiology. “Our data raise concerns about the external validity of randomized controlled trials of carotid endarterectomy versus stenting and question the potential interchangeability of carotid endarterectomy and stenting as stated in clinical practice guidelines,” said Dr. Hussain, a vascular surgeon at the University of Toronto.
Major practice guidelines cite randomized trial evidence in suggesting that carotid endarterectomy and stenting can be used interchangeably in treating low- or average-risk patients with significant carotid artery disease. Dr. Hussain and his coinvestigators, suspecting that the generalizability of the randomized trial findings might be limited because of operator and institutional selection bias, decided to conduct a retrospective cohort study of all patients older than 40 who underwent carotid endarterectomy or carotid stenting in the province of Ontario from April 2002 through March 2013.
Using validated chart abstraction software, they identified 12,529 patients who had carotid endarterectomy and 1,935 who had carotid stenting. The two groups were similar in terms of most baseline characteristics. Notably, however, stent recipients were significantly more likely to have symptomatic carotid disease and also had more comorbid conditions, as reflected in a higher Charlson Comorbidity Index score.
The primary outcome in the study was the 12-year rate of a composite comprising ischemic stroke, transient ischemic attack (TIA), myocardial infarction, or death. The rate was 35.4% in the carotid endarterectomy group and 44.5% in the stent group. After adjustment for the baseline differences, the stent group still had a statistically significant 28% greater risk of the primary outcome.
“We found the difference remained significant in all of our subgroup analyses, regardless of age, sex, year of procedure, symptomatic or asymptomatic carotid artery disease, CAD [coronary artery disease] or no CAD, diabetes (type 1 or 2) or no diabetes. Outcomes with endarterectomy were always significantly better,” said Dr. Hussain.
“I think our study shows that in clinical practice, we’re not quite seeing the outcomes reported in the clinical trials,” he added.
A Closer Look at the Data
As for the individual components of the composite end point, the 12-year rate of ischemic stroke or TIA was 9% in the carotid endarterectomy group and 14% with stenting, for an adjusted 40% increased risk in the stent group. The 12-year all-cause mortality rate was 26% in the carotid endarterectomy group and 34% with stenting, for an adjusted 28% increased risk. The incidence of myoca
The investigators next conducted a confirmatory propensity-matched analysis in which 1,927 of the stented patients were closely matched to 3,844 surgical patients, eliminating baseline differences in the prevalence of symptomatic carotid artery disease and other disparities. In this matched cohort, the primary outcome occurred in 37.4% of the carotid endarterectomy group and 44.3% of stent patients, for an adjusted 32% increase in risk in the stented group.
The differences in outcome were driven by sharply higher periprocedural risk in the stented group. After the periprocedural period, the outcome curves remained parallel in the two treatment groups.
In the first 30 days post procedure, the primary composite outcome occurred in 5.4% of the carotid endarterectomy group and 10% of stented patients, for an adjusted 40% increase in relative risk in percutaneously treated patients. The 30-day rate of ischemic stroke or TIA was 3.4% in the surgical group, compared with 6.4% in stented patients. Thirty-day mortality was 0.9% with carotid endarterectomy versus 3.3% with stenting.
Possible Explanations for the Disparity
Asked for his thoughts on the disparity between the results of his real-world study and the major randomized trials of carotid endarterectomy versus stenting, Dr. Hussain replied, “It may be because the trials had high-volume operators at high-volume centers who are experts in carotid stenting, while in the real world, many physicians may not be selecting the right people for carotid stenting.”
Differences in sample size may also figure in the disparity, he continued. He noted that in the recent 10-year report from the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST), the composite end point of stroke, myocardial infarction, or death occurred in 9.9% of the carotid endarterectomy group, compared with 11.8% of the stenting group, but this difference in favor of carotid endarterectomy did not achieve statistical significance because of the wide confidence intervals resulting from a smaller sample size than in the Ontario study.
Looking to the future, Dr. Hussain said he thinks the ongoing CREST-2 trial is “important.” It is randomizing patients with asymptomatic high-grade carotid stenosis to uniform intensive medical management either alone or in combination with carotid endarterectomy or stenting with embolic protection. “That study might end up showing us that medical therapy is as good as or even better than stenting or carotid endarterectomy, especially in asymptomatic patients,” he said.
Dr. Hussain reported having no financial conflicts regarding his academically funded study.
—Bruce Jancin
Suggested Reading
Brott TG, Howard G, Roubin GS, et al. Long-term results of stenting versus endarterectomy for carotid-artery stenosis. N Engl J Med. 2016;374(11):1021-1031.
ROME—Carotid stenting was associated with a roughly 30% higher risk of cardiovascular events, compared with carotid endarterectomy, during 12 years of follow-up in a large, real-world, population-based cohort study, Mohamad A. Hussain, MD, reported at the Annual Congress of the European Society of Cardiology. “Our data raise concerns about the external validity of randomized controlled trials of carotid endarterectomy versus stenting and question the potential interchangeability of carotid endarterectomy and stenting as stated in clinical practice guidelines,” said Dr. Hussain, a vascular surgeon at the University of Toronto.
Major practice guidelines cite randomized trial evidence in suggesting that carotid endarterectomy and stenting can be used interchangeably in treating low- or average-risk patients with significant carotid artery disease. Dr. Hussain and his coinvestigators, suspecting that the generalizability of the randomized trial findings might be limited because of operator and institutional selection bias, decided to conduct a retrospective cohort study of all patients older than 40 who underwent carotid endarterectomy or carotid stenting in the province of Ontario from April 2002 through March 2013.
Using validated chart abstraction software, they identified 12,529 patients who had carotid endarterectomy and 1,935 who had carotid stenting. The two groups were similar in terms of most baseline characteristics. Notably, however, stent recipients were significantly more likely to have symptomatic carotid disease and also had more comorbid conditions, as reflected in a higher Charlson Comorbidity Index score.
The primary outcome in the study was the 12-year rate of a composite comprising ischemic stroke, transient ischemic attack (TIA), myocardial infarction, or death. The rate was 35.4% in the carotid endarterectomy group and 44.5% in the stent group. After adjustment for the baseline differences, the stent group still had a statistically significant 28% greater risk of the primary outcome.
“We found the difference remained significant in all of our subgroup analyses, regardless of age, sex, year of procedure, symptomatic or asymptomatic carotid artery disease, CAD [coronary artery disease] or no CAD, diabetes (type 1 or 2) or no diabetes. Outcomes with endarterectomy were always significantly better,” said Dr. Hussain.
“I think our study shows that in clinical practice, we’re not quite seeing the outcomes reported in the clinical trials,” he added.
A Closer Look at the Data
As for the individual components of the composite end point, the 12-year rate of ischemic stroke or TIA was 9% in the carotid endarterectomy group and 14% with stenting, for an adjusted 40% increased risk in the stent group. The 12-year all-cause mortality rate was 26% in the carotid endarterectomy group and 34% with stenting, for an adjusted 28% increased risk. The incidence of myoca
The investigators next conducted a confirmatory propensity-matched analysis in which 1,927 of the stented patients were closely matched to 3,844 surgical patients, eliminating baseline differences in the prevalence of symptomatic carotid artery disease and other disparities. In this matched cohort, the primary outcome occurred in 37.4% of the carotid endarterectomy group and 44.3% of stent patients, for an adjusted 32% increase in risk in the stented group.
The differences in outcome were driven by sharply higher periprocedural risk in the stented group. After the periprocedural period, the outcome curves remained parallel in the two treatment groups.
In the first 30 days post procedure, the primary composite outcome occurred in 5.4% of the carotid endarterectomy group and 10% of stented patients, for an adjusted 40% increase in relative risk in percutaneously treated patients. The 30-day rate of ischemic stroke or TIA was 3.4% in the surgical group, compared with 6.4% in stented patients. Thirty-day mortality was 0.9% with carotid endarterectomy versus 3.3% with stenting.
Possible Explanations for the Disparity
Asked for his thoughts on the disparity between the results of his real-world study and the major randomized trials of carotid endarterectomy versus stenting, Dr. Hussain replied, “It may be because the trials had high-volume operators at high-volume centers who are experts in carotid stenting, while in the real world, many physicians may not be selecting the right people for carotid stenting.”
Differences in sample size may also figure in the disparity, he continued. He noted that in the recent 10-year report from the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST), the composite end point of stroke, myocardial infarction, or death occurred in 9.9% of the carotid endarterectomy group, compared with 11.8% of the stenting group, but this difference in favor of carotid endarterectomy did not achieve statistical significance because of the wide confidence intervals resulting from a smaller sample size than in the Ontario study.
Looking to the future, Dr. Hussain said he thinks the ongoing CREST-2 trial is “important.” It is randomizing patients with asymptomatic high-grade carotid stenosis to uniform intensive medical management either alone or in combination with carotid endarterectomy or stenting with embolic protection. “That study might end up showing us that medical therapy is as good as or even better than stenting or carotid endarterectomy, especially in asymptomatic patients,” he said.
Dr. Hussain reported having no financial conflicts regarding his academically funded study.
—Bruce Jancin
Suggested Reading
Brott TG, Howard G, Roubin GS, et al. Long-term results of stenting versus endarterectomy for carotid-artery stenosis. N Engl J Med. 2016;374(11):1021-1031.
ROME—Carotid stenting was associated with a roughly 30% higher risk of cardiovascular events, compared with carotid endarterectomy, during 12 years of follow-up in a large, real-world, population-based cohort study, Mohamad A. Hussain, MD, reported at the Annual Congress of the European Society of Cardiology. “Our data raise concerns about the external validity of randomized controlled trials of carotid endarterectomy versus stenting and question the potential interchangeability of carotid endarterectomy and stenting as stated in clinical practice guidelines,” said Dr. Hussain, a vascular surgeon at the University of Toronto.
Major practice guidelines cite randomized trial evidence in suggesting that carotid endarterectomy and stenting can be used interchangeably in treating low- or average-risk patients with significant carotid artery disease. Dr. Hussain and his coinvestigators, suspecting that the generalizability of the randomized trial findings might be limited because of operator and institutional selection bias, decided to conduct a retrospective cohort study of all patients older than 40 who underwent carotid endarterectomy or carotid stenting in the province of Ontario from April 2002 through March 2013.
Using validated chart abstraction software, they identified 12,529 patients who had carotid endarterectomy and 1,935 who had carotid stenting. The two groups were similar in terms of most baseline characteristics. Notably, however, stent recipients were significantly more likely to have symptomatic carotid disease and also had more comorbid conditions, as reflected in a higher Charlson Comorbidity Index score.
The primary outcome in the study was the 12-year rate of a composite comprising ischemic stroke, transient ischemic attack (TIA), myocardial infarction, or death. The rate was 35.4% in the carotid endarterectomy group and 44.5% in the stent group. After adjustment for the baseline differences, the stent group still had a statistically significant 28% greater risk of the primary outcome.
“We found the difference remained significant in all of our subgroup analyses, regardless of age, sex, year of procedure, symptomatic or asymptomatic carotid artery disease, CAD [coronary artery disease] or no CAD, diabetes (type 1 or 2) or no diabetes. Outcomes with endarterectomy were always significantly better,” said Dr. Hussain.
“I think our study shows that in clinical practice, we’re not quite seeing the outcomes reported in the clinical trials,” he added.
A Closer Look at the Data
As for the individual components of the composite end point, the 12-year rate of ischemic stroke or TIA was 9% in the carotid endarterectomy group and 14% with stenting, for an adjusted 40% increased risk in the stent group. The 12-year all-cause mortality rate was 26% in the carotid endarterectomy group and 34% with stenting, for an adjusted 28% increased risk. The incidence of myoca
The investigators next conducted a confirmatory propensity-matched analysis in which 1,927 of the stented patients were closely matched to 3,844 surgical patients, eliminating baseline differences in the prevalence of symptomatic carotid artery disease and other disparities. In this matched cohort, the primary outcome occurred in 37.4% of the carotid endarterectomy group and 44.3% of stent patients, for an adjusted 32% increase in risk in the stented group.
The differences in outcome were driven by sharply higher periprocedural risk in the stented group. After the periprocedural period, the outcome curves remained parallel in the two treatment groups.
In the first 30 days post procedure, the primary composite outcome occurred in 5.4% of the carotid endarterectomy group and 10% of stented patients, for an adjusted 40% increase in relative risk in percutaneously treated patients. The 30-day rate of ischemic stroke or TIA was 3.4% in the surgical group, compared with 6.4% in stented patients. Thirty-day mortality was 0.9% with carotid endarterectomy versus 3.3% with stenting.
Possible Explanations for the Disparity
Asked for his thoughts on the disparity between the results of his real-world study and the major randomized trials of carotid endarterectomy versus stenting, Dr. Hussain replied, “It may be because the trials had high-volume operators at high-volume centers who are experts in carotid stenting, while in the real world, many physicians may not be selecting the right people for carotid stenting.”
Differences in sample size may also figure in the disparity, he continued. He noted that in the recent 10-year report from the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST), the composite end point of stroke, myocardial infarction, or death occurred in 9.9% of the carotid endarterectomy group, compared with 11.8% of the stenting group, but this difference in favor of carotid endarterectomy did not achieve statistical significance because of the wide confidence intervals resulting from a smaller sample size than in the Ontario study.
Looking to the future, Dr. Hussain said he thinks the ongoing CREST-2 trial is “important.” It is randomizing patients with asymptomatic high-grade carotid stenosis to uniform intensive medical management either alone or in combination with carotid endarterectomy or stenting with embolic protection. “That study might end up showing us that medical therapy is as good as or even better than stenting or carotid endarterectomy, especially in asymptomatic patients,” he said.
Dr. Hussain reported having no financial conflicts regarding his academically funded study.
—Bruce Jancin
Suggested Reading
Brott TG, Howard G, Roubin GS, et al. Long-term results of stenting versus endarterectomy for carotid-artery stenosis. N Engl J Med. 2016;374(11):1021-1031.
Debunking Melanoma Myths: Do Sunscreens Cause Cancer?
Myth: Sunscreens cause cancer
Regular sunscreen use is recommended by the American Academy of Dermatology as a primary method of sun protection to reduce the risk of melanoma and other nonmelanoma skin cancers. However, due to reports in the media, patients often inquire if sunscreen ingredients, specifically oxybenzone and retinyl palmitate as well as nanoparticles, are toxic and actually cause malignant melanoma and other skin cancers rather than prevent them.
Overall, the known benefits of sunscreen use to minimize short-term and long-term damage to the skin from UV radiation outweigh any unproven claims of toxicity or human health hazard. Active ingredients in sunscreens, such as oxybenzone and retinyl palmitate, are regulated as over-the-counter drugs by the US Food and Drug Administration and have a long-standing history of providing effective broad-spectrum protection from UV radiation. Despite concerns that oxybenzone can penetrate the skin and effect hormone levels, there is no evidence supporting this claim. Although oxybenzone is absorbed by the body, it is subsequently excreted and has no potential for harmful buildup. It also has been suggested that retinyl palmitate generates free radicals that can lead to cancer formation; however, the risk has only been linked to UV exposure in isolation, and antioxidants in the body can theoretically neutralize these free radicals before they lead to cancer development.
Sunscreens containing nanoparticles of inorganic filters such as zinc oxide and titanium dioxide also have been scrutinized. These formulations have largely proven effective in protecting against UVA and UVB radiation, and claims that nanoparticles are small enough to penetrate the epidermis and be absorbed in the human bloodstream have been refuted.
The positive association between sunscreen use and risk of developing malignant melanoma may be due to selection bias and uncontrolled confounding in studies rather than proven toxicity of sunscreen ingredients. Results from a meta-analysis of 11 case-control studies indicated that there is no association and the researchers discussed the role of selection bias in contributing to the positive association between sunscreen use and melanoma development. For instance, some studies failed to control for factors that commonly are linked with increased melanoma risk (eg, red or fair hair color, blue eye color, presence of nevi, freckling). Also, increased sun exposure among patients who use sunscreens may have impacted study results.
Dermatologists should emphasize to concerned patients that long-term sunscreen use has been proven to reduce the incidence of melanoma. A 2011 Australian study evaluated the effects of long-term application of sunscreen on the risk of cutaneous melanoma in 1621 randomly selected participants who applied sunscreen in combination with 30 mg of beta-carotene or placebo supplements for 4 years and were observed for 10 more years. They observed a reduction in primary melanomas and invasive melanomas in the sunscreen group, concluding that melanoma may be preventable with regular sunscreen use in adults.
For patients who are still concerned, dermatologists can recommend sunscreens containing organic UV filters only. Education about factors that contribute to the increased rate of melanoma also is necessary. Longer lifespans, the thinning ozone layer, increased popularity of outdoor activities, exposed skin due to clothing style, use of tanning beds, earlier detection of skin cancer, and other factors may be responsible. Greater exposure to UV radiation rather than commercial sunscreens is the likely cause of skin cancer.
Ask the expert: does sunscreen cause cancer? Skin Cancer Foundation website. http://www.skincancer.org/skin-cancer-information/ask-the-experts/does-sunscreen-cause-cancer. Published Fall 2008. Accessed November 17, 2016.
Green AC, Williams GM, Logan V, et al. Reduced melanoma after regular sunscreen use: randomized trial follow-up [published online December 6, 2010]. J Clin Oncol. 2011;29:257-263.
Huncharek M, Kupelnick B. Use of topical sunscreens and the risk of malignant melanoma: a meta-analysis of 9067 patients from 11 case-control studies. Am J Public Health. 2002;92:1173-1177.
Morrison WL, Wang SQ. Sunscreens: safe and effective? Skin Cancer Foundation website. http://www.skincancer.org/prevention/sun-protection/sunscreen/sunscreens-safe-and-effective. Published November 17, 2011. Accessed November 17, 2016.
Sunscreen remains a safe, effective form of sun protection [press release]. Schaumburg, IL: American Academy of Dermatology; May 16, 2012. https://www.aad.org/media/news-releases/sunscreen-remains-a-safe-effective-form-of-sun-protection. Accessed November 17, 2016.
Myth: Sunscreens cause cancer
Regular sunscreen use is recommended by the American Academy of Dermatology as a primary method of sun protection to reduce the risk of melanoma and other nonmelanoma skin cancers. However, due to reports in the media, patients often inquire if sunscreen ingredients, specifically oxybenzone and retinyl palmitate as well as nanoparticles, are toxic and actually cause malignant melanoma and other skin cancers rather than prevent them.
Overall, the known benefits of sunscreen use to minimize short-term and long-term damage to the skin from UV radiation outweigh any unproven claims of toxicity or human health hazard. Active ingredients in sunscreens, such as oxybenzone and retinyl palmitate, are regulated as over-the-counter drugs by the US Food and Drug Administration and have a long-standing history of providing effective broad-spectrum protection from UV radiation. Despite concerns that oxybenzone can penetrate the skin and effect hormone levels, there is no evidence supporting this claim. Although oxybenzone is absorbed by the body, it is subsequently excreted and has no potential for harmful buildup. It also has been suggested that retinyl palmitate generates free radicals that can lead to cancer formation; however, the risk has only been linked to UV exposure in isolation, and antioxidants in the body can theoretically neutralize these free radicals before they lead to cancer development.
Sunscreens containing nanoparticles of inorganic filters such as zinc oxide and titanium dioxide also have been scrutinized. These formulations have largely proven effective in protecting against UVA and UVB radiation, and claims that nanoparticles are small enough to penetrate the epidermis and be absorbed in the human bloodstream have been refuted.
The positive association between sunscreen use and risk of developing malignant melanoma may be due to selection bias and uncontrolled confounding in studies rather than proven toxicity of sunscreen ingredients. Results from a meta-analysis of 11 case-control studies indicated that there is no association and the researchers discussed the role of selection bias in contributing to the positive association between sunscreen use and melanoma development. For instance, some studies failed to control for factors that commonly are linked with increased melanoma risk (eg, red or fair hair color, blue eye color, presence of nevi, freckling). Also, increased sun exposure among patients who use sunscreens may have impacted study results.
Dermatologists should emphasize to concerned patients that long-term sunscreen use has been proven to reduce the incidence of melanoma. A 2011 Australian study evaluated the effects of long-term application of sunscreen on the risk of cutaneous melanoma in 1621 randomly selected participants who applied sunscreen in combination with 30 mg of beta-carotene or placebo supplements for 4 years and were observed for 10 more years. They observed a reduction in primary melanomas and invasive melanomas in the sunscreen group, concluding that melanoma may be preventable with regular sunscreen use in adults.
For patients who are still concerned, dermatologists can recommend sunscreens containing organic UV filters only. Education about factors that contribute to the increased rate of melanoma also is necessary. Longer lifespans, the thinning ozone layer, increased popularity of outdoor activities, exposed skin due to clothing style, use of tanning beds, earlier detection of skin cancer, and other factors may be responsible. Greater exposure to UV radiation rather than commercial sunscreens is the likely cause of skin cancer.
Myth: Sunscreens cause cancer
Regular sunscreen use is recommended by the American Academy of Dermatology as a primary method of sun protection to reduce the risk of melanoma and other nonmelanoma skin cancers. However, due to reports in the media, patients often inquire if sunscreen ingredients, specifically oxybenzone and retinyl palmitate as well as nanoparticles, are toxic and actually cause malignant melanoma and other skin cancers rather than prevent them.
Overall, the known benefits of sunscreen use to minimize short-term and long-term damage to the skin from UV radiation outweigh any unproven claims of toxicity or human health hazard. Active ingredients in sunscreens, such as oxybenzone and retinyl palmitate, are regulated as over-the-counter drugs by the US Food and Drug Administration and have a long-standing history of providing effective broad-spectrum protection from UV radiation. Despite concerns that oxybenzone can penetrate the skin and effect hormone levels, there is no evidence supporting this claim. Although oxybenzone is absorbed by the body, it is subsequently excreted and has no potential for harmful buildup. It also has been suggested that retinyl palmitate generates free radicals that can lead to cancer formation; however, the risk has only been linked to UV exposure in isolation, and antioxidants in the body can theoretically neutralize these free radicals before they lead to cancer development.
Sunscreens containing nanoparticles of inorganic filters such as zinc oxide and titanium dioxide also have been scrutinized. These formulations have largely proven effective in protecting against UVA and UVB radiation, and claims that nanoparticles are small enough to penetrate the epidermis and be absorbed in the human bloodstream have been refuted.
The positive association between sunscreen use and risk of developing malignant melanoma may be due to selection bias and uncontrolled confounding in studies rather than proven toxicity of sunscreen ingredients. Results from a meta-analysis of 11 case-control studies indicated that there is no association and the researchers discussed the role of selection bias in contributing to the positive association between sunscreen use and melanoma development. For instance, some studies failed to control for factors that commonly are linked with increased melanoma risk (eg, red or fair hair color, blue eye color, presence of nevi, freckling). Also, increased sun exposure among patients who use sunscreens may have impacted study results.
Dermatologists should emphasize to concerned patients that long-term sunscreen use has been proven to reduce the incidence of melanoma. A 2011 Australian study evaluated the effects of long-term application of sunscreen on the risk of cutaneous melanoma in 1621 randomly selected participants who applied sunscreen in combination with 30 mg of beta-carotene or placebo supplements for 4 years and were observed for 10 more years. They observed a reduction in primary melanomas and invasive melanomas in the sunscreen group, concluding that melanoma may be preventable with regular sunscreen use in adults.
For patients who are still concerned, dermatologists can recommend sunscreens containing organic UV filters only. Education about factors that contribute to the increased rate of melanoma also is necessary. Longer lifespans, the thinning ozone layer, increased popularity of outdoor activities, exposed skin due to clothing style, use of tanning beds, earlier detection of skin cancer, and other factors may be responsible. Greater exposure to UV radiation rather than commercial sunscreens is the likely cause of skin cancer.
Ask the expert: does sunscreen cause cancer? Skin Cancer Foundation website. http://www.skincancer.org/skin-cancer-information/ask-the-experts/does-sunscreen-cause-cancer. Published Fall 2008. Accessed November 17, 2016.
Green AC, Williams GM, Logan V, et al. Reduced melanoma after regular sunscreen use: randomized trial follow-up [published online December 6, 2010]. J Clin Oncol. 2011;29:257-263.
Huncharek M, Kupelnick B. Use of topical sunscreens and the risk of malignant melanoma: a meta-analysis of 9067 patients from 11 case-control studies. Am J Public Health. 2002;92:1173-1177.
Morrison WL, Wang SQ. Sunscreens: safe and effective? Skin Cancer Foundation website. http://www.skincancer.org/prevention/sun-protection/sunscreen/sunscreens-safe-and-effective. Published November 17, 2011. Accessed November 17, 2016.
Sunscreen remains a safe, effective form of sun protection [press release]. Schaumburg, IL: American Academy of Dermatology; May 16, 2012. https://www.aad.org/media/news-releases/sunscreen-remains-a-safe-effective-form-of-sun-protection. Accessed November 17, 2016.
Ask the expert: does sunscreen cause cancer? Skin Cancer Foundation website. http://www.skincancer.org/skin-cancer-information/ask-the-experts/does-sunscreen-cause-cancer. Published Fall 2008. Accessed November 17, 2016.
Green AC, Williams GM, Logan V, et al. Reduced melanoma after regular sunscreen use: randomized trial follow-up [published online December 6, 2010]. J Clin Oncol. 2011;29:257-263.
Huncharek M, Kupelnick B. Use of topical sunscreens and the risk of malignant melanoma: a meta-analysis of 9067 patients from 11 case-control studies. Am J Public Health. 2002;92:1173-1177.
Morrison WL, Wang SQ. Sunscreens: safe and effective? Skin Cancer Foundation website. http://www.skincancer.org/prevention/sun-protection/sunscreen/sunscreens-safe-and-effective. Published November 17, 2011. Accessed November 17, 2016.
Sunscreen remains a safe, effective form of sun protection [press release]. Schaumburg, IL: American Academy of Dermatology; May 16, 2012. https://www.aad.org/media/news-releases/sunscreen-remains-a-safe-effective-form-of-sun-protection. Accessed November 17, 2016.
Interrupting Oral Anticoagulation in AF Carries High Thromboembolic Cost
ROME—Temporary interruption of oral anticoagulation for stroke prevention in patients with atrial fibrillation (AF) occurs often and is associated with substantially increased risk of cardioembolic events and all-cause mortality, according to a new prespecified secondary analysis of the ENGAGE-AF TIMI 48 trial.
Results of the analysis, which were presented at the 2016 Annual Congress of the European Society of Cardiology, showed that many of these treatment interruptions occur in response to nonserious adverse events such as minor bleeding, planned dental procedures, or simply because of patient wishes. The new ENGAGE-AF TIMI 48 findings should encourage physicians and patients to think twice before interrupting anticoagulant therapy for such reasons, which seem inadequate in light of the new evidence of the potentially serious consequences, said Ilaria Cavallari, MD, a research fellow with the TIMI Study Group.
“Interruption of oral anticoagulation in patients with AF should be avoided or as brief as possible and under medical control, especially following nonserious adverse events,” said Dr. Cavallari.
The ENGAGE-AF TIMI 48 study was the pivotal phase III, double-blind, 21,105-patient clinical trial that led to FDA and European approval of edoxaban, a direct oral factor Xa inhibitor, for stroke prevention in moderate- to high-risk patients with AF. The study showed that edoxaban at what later became the approved dose of 60 mg/day, or at 30 mg/day in patients with impaired renal function, body weight of 60 kg or less, or on concomitant therapy with a platelet glycoprotein inhibitor, resulted in a 21% reduction in the risk of stroke or systemic embolism and a 20% reduction in major bleeding, compared with warfarin, over 2.8 years of follow-up.
Dr. Cavallari presented a prespecified secondary retrospective analysis that focused on treatment interruptions: the reasons and the price paid in terms of thromboembolic events.
One or more treatment interruptions lasting for longer than three days occurred in 63% of patients during a median 2.8 years of follow-up. Since these were participants in a clinical trial with relatively close patient contact, it is likely that the true interruption rate in real-world clinical practice is even higher, Dr. Cavallari said.
Interruptions were significantly more frequent in patients assigned to warfarin than among the groups assigned to edoxaban. The median duration of treatment interruptions was nine days. After excluding patients who were on any other anticoagulant during their interruption—low-molecular-weight heparin being the most common—investigators were left with 9,148 patients.
The end points of interest in this analysis were the major adverse events occurring during a time window lasting from four days after their last dose of oral anticoagulant until day 34 or when they resumed their study drug. The 30-day incidence of ischemic stroke or systemic embolism was 1.27%. The rate of a composite including cardiovascular death, myocardial infarction, and ischemic stroke or systemic embolism was 4.99%. The 30-day rate of an end point Dr. Cavallari termed primary net clinical outcome—a composite of stroke or systemic embolism, major bleeding, and all-cause mortality—was 7.16%.
These 30-day event rates among treatment interrupters are notably high, compared with the one-year rates in patients who did not interrupt oral anticoagulant therapy: 0.26% for ischemic stroke or systemic embolism; 0.36% for the composite of cardiovascular death, myocardial infarction, and ischemic stroke; and 0.56% for the primary net clinical outcome, she continued.
The most common reason for treatment interruptions was adverse events, which accounted for 41% of the interruptions.
Drilling deeper into the types of adverse events that triggered treatment interruption, 1.5% of interrupters did so because of an on-treatment ischemic stroke or systemic embolism, 4.7% did so because of major bleeding, 8% had minor and clinically relevant nonmajor bleeding, and 30% interrupted treatment for other serious or nonserious adverse events.
Interrupting therapy because of an adverse event often had serious consequences, as reflected in an adjusted 3.94-fold increased risk of 30-day all-cause mortality, compared with patients who stopped for other reasons. Patients who stopped treatment because of a stroke, transient ischemic attack, or systemic embolism had a 30-day all-cause mortality rate of 29.3%. Those who interrupted treatment because of a major bleeding event had an 8.8% 30-day mortality. When minor or clinically relevant nonmajor bleeding was the impetus for a treatment interruption, the associated 30-day mortality was 3.4%.
Almost a third (29%) of treatment interruptions were the result of physician decisions in response to an upcoming invasive procedure, most often dental work.
The 30-day rates of ischemic stroke or systemic embolism and primary net clinical outcome did not differ significantly between patients who interrupted warfarin versus edoxaban at the approved dose. Nonetheless, this new secondary analysis from ENGAGE-AF TIMI 48 supports the parent study’s conclusion that edoxaban is preferable to warfarin in patients with AF, according to Dr. Cavallari.
“In light of the increased risk of ischemic events after interruption of oral anticoagulation, new oral anticoagulants represent an attractive alternative to vitamin K antagonists, given their faster onset of action, better adherence rates, safety, and tolerability profiles,” she concluded.
ENGAGE-AF TIMI 48 was funded by Daiichi Sankyo. Dr. Cavallari reported no financial conflicts of interest regarding her presentation.
—Bruce Jancin
Suggested Reading
Giugliano RP, Ruff CT, Braumwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093-2104.
ROME—Temporary interruption of oral anticoagulation for stroke prevention in patients with atrial fibrillation (AF) occurs often and is associated with substantially increased risk of cardioembolic events and all-cause mortality, according to a new prespecified secondary analysis of the ENGAGE-AF TIMI 48 trial.
Results of the analysis, which were presented at the 2016 Annual Congress of the European Society of Cardiology, showed that many of these treatment interruptions occur in response to nonserious adverse events such as minor bleeding, planned dental procedures, or simply because of patient wishes. The new ENGAGE-AF TIMI 48 findings should encourage physicians and patients to think twice before interrupting anticoagulant therapy for such reasons, which seem inadequate in light of the new evidence of the potentially serious consequences, said Ilaria Cavallari, MD, a research fellow with the TIMI Study Group.
“Interruption of oral anticoagulation in patients with AF should be avoided or as brief as possible and under medical control, especially following nonserious adverse events,” said Dr. Cavallari.
The ENGAGE-AF TIMI 48 study was the pivotal phase III, double-blind, 21,105-patient clinical trial that led to FDA and European approval of edoxaban, a direct oral factor Xa inhibitor, for stroke prevention in moderate- to high-risk patients with AF. The study showed that edoxaban at what later became the approved dose of 60 mg/day, or at 30 mg/day in patients with impaired renal function, body weight of 60 kg or less, or on concomitant therapy with a platelet glycoprotein inhibitor, resulted in a 21% reduction in the risk of stroke or systemic embolism and a 20% reduction in major bleeding, compared with warfarin, over 2.8 years of follow-up.
Dr. Cavallari presented a prespecified secondary retrospective analysis that focused on treatment interruptions: the reasons and the price paid in terms of thromboembolic events.
One or more treatment interruptions lasting for longer than three days occurred in 63% of patients during a median 2.8 years of follow-up. Since these were participants in a clinical trial with relatively close patient contact, it is likely that the true interruption rate in real-world clinical practice is even higher, Dr. Cavallari said.
Interruptions were significantly more frequent in patients assigned to warfarin than among the groups assigned to edoxaban. The median duration of treatment interruptions was nine days. After excluding patients who were on any other anticoagulant during their interruption—low-molecular-weight heparin being the most common—investigators were left with 9,148 patients.
The end points of interest in this analysis were the major adverse events occurring during a time window lasting from four days after their last dose of oral anticoagulant until day 34 or when they resumed their study drug. The 30-day incidence of ischemic stroke or systemic embolism was 1.27%. The rate of a composite including cardiovascular death, myocardial infarction, and ischemic stroke or systemic embolism was 4.99%. The 30-day rate of an end point Dr. Cavallari termed primary net clinical outcome—a composite of stroke or systemic embolism, major bleeding, and all-cause mortality—was 7.16%.
These 30-day event rates among treatment interrupters are notably high, compared with the one-year rates in patients who did not interrupt oral anticoagulant therapy: 0.26% for ischemic stroke or systemic embolism; 0.36% for the composite of cardiovascular death, myocardial infarction, and ischemic stroke; and 0.56% for the primary net clinical outcome, she continued.
The most common reason for treatment interruptions was adverse events, which accounted for 41% of the interruptions.
Drilling deeper into the types of adverse events that triggered treatment interruption, 1.5% of interrupters did so because of an on-treatment ischemic stroke or systemic embolism, 4.7% did so because of major bleeding, 8% had minor and clinically relevant nonmajor bleeding, and 30% interrupted treatment for other serious or nonserious adverse events.
Interrupting therapy because of an adverse event often had serious consequences, as reflected in an adjusted 3.94-fold increased risk of 30-day all-cause mortality, compared with patients who stopped for other reasons. Patients who stopped treatment because of a stroke, transient ischemic attack, or systemic embolism had a 30-day all-cause mortality rate of 29.3%. Those who interrupted treatment because of a major bleeding event had an 8.8% 30-day mortality. When minor or clinically relevant nonmajor bleeding was the impetus for a treatment interruption, the associated 30-day mortality was 3.4%.
Almost a third (29%) of treatment interruptions were the result of physician decisions in response to an upcoming invasive procedure, most often dental work.
The 30-day rates of ischemic stroke or systemic embolism and primary net clinical outcome did not differ significantly between patients who interrupted warfarin versus edoxaban at the approved dose. Nonetheless, this new secondary analysis from ENGAGE-AF TIMI 48 supports the parent study’s conclusion that edoxaban is preferable to warfarin in patients with AF, according to Dr. Cavallari.
“In light of the increased risk of ischemic events after interruption of oral anticoagulation, new oral anticoagulants represent an attractive alternative to vitamin K antagonists, given their faster onset of action, better adherence rates, safety, and tolerability profiles,” she concluded.
ENGAGE-AF TIMI 48 was funded by Daiichi Sankyo. Dr. Cavallari reported no financial conflicts of interest regarding her presentation.
—Bruce Jancin
Suggested Reading
Giugliano RP, Ruff CT, Braumwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093-2104.
ROME—Temporary interruption of oral anticoagulation for stroke prevention in patients with atrial fibrillation (AF) occurs often and is associated with substantially increased risk of cardioembolic events and all-cause mortality, according to a new prespecified secondary analysis of the ENGAGE-AF TIMI 48 trial.
Results of the analysis, which were presented at the 2016 Annual Congress of the European Society of Cardiology, showed that many of these treatment interruptions occur in response to nonserious adverse events such as minor bleeding, planned dental procedures, or simply because of patient wishes. The new ENGAGE-AF TIMI 48 findings should encourage physicians and patients to think twice before interrupting anticoagulant therapy for such reasons, which seem inadequate in light of the new evidence of the potentially serious consequences, said Ilaria Cavallari, MD, a research fellow with the TIMI Study Group.
“Interruption of oral anticoagulation in patients with AF should be avoided or as brief as possible and under medical control, especially following nonserious adverse events,” said Dr. Cavallari.
The ENGAGE-AF TIMI 48 study was the pivotal phase III, double-blind, 21,105-patient clinical trial that led to FDA and European approval of edoxaban, a direct oral factor Xa inhibitor, for stroke prevention in moderate- to high-risk patients with AF. The study showed that edoxaban at what later became the approved dose of 60 mg/day, or at 30 mg/day in patients with impaired renal function, body weight of 60 kg or less, or on concomitant therapy with a platelet glycoprotein inhibitor, resulted in a 21% reduction in the risk of stroke or systemic embolism and a 20% reduction in major bleeding, compared with warfarin, over 2.8 years of follow-up.
Dr. Cavallari presented a prespecified secondary retrospective analysis that focused on treatment interruptions: the reasons and the price paid in terms of thromboembolic events.
One or more treatment interruptions lasting for longer than three days occurred in 63% of patients during a median 2.8 years of follow-up. Since these were participants in a clinical trial with relatively close patient contact, it is likely that the true interruption rate in real-world clinical practice is even higher, Dr. Cavallari said.
Interruptions were significantly more frequent in patients assigned to warfarin than among the groups assigned to edoxaban. The median duration of treatment interruptions was nine days. After excluding patients who were on any other anticoagulant during their interruption—low-molecular-weight heparin being the most common—investigators were left with 9,148 patients.
The end points of interest in this analysis were the major adverse events occurring during a time window lasting from four days after their last dose of oral anticoagulant until day 34 or when they resumed their study drug. The 30-day incidence of ischemic stroke or systemic embolism was 1.27%. The rate of a composite including cardiovascular death, myocardial infarction, and ischemic stroke or systemic embolism was 4.99%. The 30-day rate of an end point Dr. Cavallari termed primary net clinical outcome—a composite of stroke or systemic embolism, major bleeding, and all-cause mortality—was 7.16%.
These 30-day event rates among treatment interrupters are notably high, compared with the one-year rates in patients who did not interrupt oral anticoagulant therapy: 0.26% for ischemic stroke or systemic embolism; 0.36% for the composite of cardiovascular death, myocardial infarction, and ischemic stroke; and 0.56% for the primary net clinical outcome, she continued.
The most common reason for treatment interruptions was adverse events, which accounted for 41% of the interruptions.
Drilling deeper into the types of adverse events that triggered treatment interruption, 1.5% of interrupters did so because of an on-treatment ischemic stroke or systemic embolism, 4.7% did so because of major bleeding, 8% had minor and clinically relevant nonmajor bleeding, and 30% interrupted treatment for other serious or nonserious adverse events.
Interrupting therapy because of an adverse event often had serious consequences, as reflected in an adjusted 3.94-fold increased risk of 30-day all-cause mortality, compared with patients who stopped for other reasons. Patients who stopped treatment because of a stroke, transient ischemic attack, or systemic embolism had a 30-day all-cause mortality rate of 29.3%. Those who interrupted treatment because of a major bleeding event had an 8.8% 30-day mortality. When minor or clinically relevant nonmajor bleeding was the impetus for a treatment interruption, the associated 30-day mortality was 3.4%.
Almost a third (29%) of treatment interruptions were the result of physician decisions in response to an upcoming invasive procedure, most often dental work.
The 30-day rates of ischemic stroke or systemic embolism and primary net clinical outcome did not differ significantly between patients who interrupted warfarin versus edoxaban at the approved dose. Nonetheless, this new secondary analysis from ENGAGE-AF TIMI 48 supports the parent study’s conclusion that edoxaban is preferable to warfarin in patients with AF, according to Dr. Cavallari.
“In light of the increased risk of ischemic events after interruption of oral anticoagulation, new oral anticoagulants represent an attractive alternative to vitamin K antagonists, given their faster onset of action, better adherence rates, safety, and tolerability profiles,” she concluded.
ENGAGE-AF TIMI 48 was funded by Daiichi Sankyo. Dr. Cavallari reported no financial conflicts of interest regarding her presentation.
—Bruce Jancin
Suggested Reading
Giugliano RP, Ruff CT, Braumwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093-2104.
Allopregnanolone May Treat Superrefractory Status Epilepticus Effectively
BALTIMORE—The investigational agent allopregnanolone may effectively treat superrefractory status epilepticus, according to research presented at the 141st Annual Meeting of the American Neurological Association. The drug appears to end seizures in patients for whom all other treatment options have failed. Unlike other current treatments, allopregnanolone does not entail risks of respiratory depression or hypotension.
“As we gain more experience with [allopregnanolone] in these superrefractory patients, I’m hoping that there’s going to be opportunities for using it in different settings in status [epilepticus],” said Michael Rogawski, MD, PhD, Professor of Neurology and Pharmacology at the University of California, Davis. The drug may be appropriate for refractory status epilepticus, for example. Dr. Rogawski and his colleagues are studying allopregnanolone in combination with diazepam and midazolam in models of status epilepticus, and results so far indicate that the medications work well in combination. “Our hope is that allopregnanolone could be used earlier on in status [epilepticus], potentially as a first-line agent, either as a replacement for benzodiazepines … or in combination with the benzodiazepine.”
An Analog of an Anesthetic
Allopregnanolone is an analog of alphaxalone, a surgical anesthetic used commonly in the 1970s. Alphaxalone was withdrawn from the market because it was difficult and dangerous to formulate. In the mid-1980s, researchers recognized that allopregnanolone, which has a similar chemical structure to that of alphaxalone, was endogenous to the body. Allopregnanolone is a positive allosteric modulator of GABAA receptors, and the body produces it by metabolism of progesterone.
Unlike benzodiazepines, neurosteroids such as allopregnanolone act not only on synaptic GABAA receptors, but also on extrasynaptic GABAA receptors, which bias neurons to be less excitable. Studies in seizure models and epilepsy models have indicated that neurosteroids are effective antiseizure agents. Dr. Rogawski and colleagues found that allopregnanolone effectively treats various models of status epilepticus.
Like other neurosteroids, allopregnanolone is highly lipophilic and easily crosses the blood–brain barrier. After intramuscular administration to mice, levels of allopregnanolone in the brain were much higher than levels in the plasma.
Results in Human Patients
After prolonged seizure activity, synaptic GABAA receptors are internalized and become unavailable as targets for benzodiazepines. Because allopregnanolone acts on extrasynaptic GABAA receptors, Dr. Rogawski and colleagues hypothesized that the medicine might be more effective than benzodiazepines in benzodiazepine-refractory status epilepticus. They found that when diazepam or allopregnanolone was administered to rats at 10 minutes after the onset of status epilepticus, the seizures stopped. When the drugs were administered at 40 minutes after the onset of status epilepticus, diazepam was ineffective, but allopregnanolone stopped the seizures.
To evaluate allopregnanolone in humans, Dr. Rogawski and colleagues created an IV formulation of the therapy by solubilizing it with cyclodextrins. They then gained FDA approval for clinical studies. When epilepsy centers caring for patients with superrefractory status epilepticus contacted Dr. Rogawski, they were able to obtain emergency FDA authorization to treat these patients with allopregnanolone supplied by Dr. Rogawski.
One patient was a 23-year-old man receiving barbiturate anesthesia. Every time his doctors withdrew the barbiturate anesthesia, his seizures recurred. After the patient received allopregnanolone, he was able to be weaned from the barbiturates without rebound seizure activity. The drug produced similar results in a 28-year-old man, an 11-year-old girl, and a 2-year-old girl. The investigators did not identify any treatment-related adverse events.
These patients were all critically ill, and withdrawal of life support was being considered, said Dr. Rogawski. Some did not tolerate the barbiturate anesthesia, including the 2-year-old girl, who was a patient at the UC Davis Medical Center. She developed hypotension, an ileus, and persistent urinary retention. Following allopregnanolone treatment, the dose of pentobarbital was reduced so that her blood pressure recovered and the ileus resolved. In this and the other cases, allopregnanolone seemed to be life-saving, but its efficacy will need to be validated in controlled studies, said Dr. Rogawski.
Allopregnanolone has been licensed to SAGE Therapeutics, which is studying the drug under the name SAGE-547.
—Erik Greb
Suggested Reading
Broomall E, Natale JE, Grimason M, et al. Pediatric super-refractory status epilepticus treated with allopregnanolone. Ann Neurol. 2014;76(6):911-915.
Kokate TG, Cohen AL, Karp E, Rogawski MA. Neuroactive steroids protect against pilocarpine- and kainic acid-induced limbic seizures and status epilepticus in mice. Neuropharmacology. 1996;35(8):1049-1056.
Rogawski MA, Loya CM, Reddy K, et al. Neuroactive steroids for the treatment of status epilepticus. Epilepsia. 2013;54 Suppl 6:93-98.
Rohracher A, Höfler J, Kalss G, et al. Perampanel in patients with refractory and super-refractory status epilepticus in a neurological intensive care unit. Epilepsy Behav. 2015;49:354-358.
Sabharwal V, Ramsay E, Martinez R, et al. Propofol-ketamine combination therapy for effective control of super-refractory status epilepticus. Epilepsy Behav. 2015;52(Pt A):264-266.
BALTIMORE—The investigational agent allopregnanolone may effectively treat superrefractory status epilepticus, according to research presented at the 141st Annual Meeting of the American Neurological Association. The drug appears to end seizures in patients for whom all other treatment options have failed. Unlike other current treatments, allopregnanolone does not entail risks of respiratory depression or hypotension.
“As we gain more experience with [allopregnanolone] in these superrefractory patients, I’m hoping that there’s going to be opportunities for using it in different settings in status [epilepticus],” said Michael Rogawski, MD, PhD, Professor of Neurology and Pharmacology at the University of California, Davis. The drug may be appropriate for refractory status epilepticus, for example. Dr. Rogawski and his colleagues are studying allopregnanolone in combination with diazepam and midazolam in models of status epilepticus, and results so far indicate that the medications work well in combination. “Our hope is that allopregnanolone could be used earlier on in status [epilepticus], potentially as a first-line agent, either as a replacement for benzodiazepines … or in combination with the benzodiazepine.”
An Analog of an Anesthetic
Allopregnanolone is an analog of alphaxalone, a surgical anesthetic used commonly in the 1970s. Alphaxalone was withdrawn from the market because it was difficult and dangerous to formulate. In the mid-1980s, researchers recognized that allopregnanolone, which has a similar chemical structure to that of alphaxalone, was endogenous to the body. Allopregnanolone is a positive allosteric modulator of GABAA receptors, and the body produces it by metabolism of progesterone.
Unlike benzodiazepines, neurosteroids such as allopregnanolone act not only on synaptic GABAA receptors, but also on extrasynaptic GABAA receptors, which bias neurons to be less excitable. Studies in seizure models and epilepsy models have indicated that neurosteroids are effective antiseizure agents. Dr. Rogawski and colleagues found that allopregnanolone effectively treats various models of status epilepticus.
Like other neurosteroids, allopregnanolone is highly lipophilic and easily crosses the blood–brain barrier. After intramuscular administration to mice, levels of allopregnanolone in the brain were much higher than levels in the plasma.
Results in Human Patients
After prolonged seizure activity, synaptic GABAA receptors are internalized and become unavailable as targets for benzodiazepines. Because allopregnanolone acts on extrasynaptic GABAA receptors, Dr. Rogawski and colleagues hypothesized that the medicine might be more effective than benzodiazepines in benzodiazepine-refractory status epilepticus. They found that when diazepam or allopregnanolone was administered to rats at 10 minutes after the onset of status epilepticus, the seizures stopped. When the drugs were administered at 40 minutes after the onset of status epilepticus, diazepam was ineffective, but allopregnanolone stopped the seizures.
To evaluate allopregnanolone in humans, Dr. Rogawski and colleagues created an IV formulation of the therapy by solubilizing it with cyclodextrins. They then gained FDA approval for clinical studies. When epilepsy centers caring for patients with superrefractory status epilepticus contacted Dr. Rogawski, they were able to obtain emergency FDA authorization to treat these patients with allopregnanolone supplied by Dr. Rogawski.
One patient was a 23-year-old man receiving barbiturate anesthesia. Every time his doctors withdrew the barbiturate anesthesia, his seizures recurred. After the patient received allopregnanolone, he was able to be weaned from the barbiturates without rebound seizure activity. The drug produced similar results in a 28-year-old man, an 11-year-old girl, and a 2-year-old girl. The investigators did not identify any treatment-related adverse events.
These patients were all critically ill, and withdrawal of life support was being considered, said Dr. Rogawski. Some did not tolerate the barbiturate anesthesia, including the 2-year-old girl, who was a patient at the UC Davis Medical Center. She developed hypotension, an ileus, and persistent urinary retention. Following allopregnanolone treatment, the dose of pentobarbital was reduced so that her blood pressure recovered and the ileus resolved. In this and the other cases, allopregnanolone seemed to be life-saving, but its efficacy will need to be validated in controlled studies, said Dr. Rogawski.
Allopregnanolone has been licensed to SAGE Therapeutics, which is studying the drug under the name SAGE-547.
—Erik Greb
Suggested Reading
Broomall E, Natale JE, Grimason M, et al. Pediatric super-refractory status epilepticus treated with allopregnanolone. Ann Neurol. 2014;76(6):911-915.
Kokate TG, Cohen AL, Karp E, Rogawski MA. Neuroactive steroids protect against pilocarpine- and kainic acid-induced limbic seizures and status epilepticus in mice. Neuropharmacology. 1996;35(8):1049-1056.
Rogawski MA, Loya CM, Reddy K, et al. Neuroactive steroids for the treatment of status epilepticus. Epilepsia. 2013;54 Suppl 6:93-98.
Rohracher A, Höfler J, Kalss G, et al. Perampanel in patients with refractory and super-refractory status epilepticus in a neurological intensive care unit. Epilepsy Behav. 2015;49:354-358.
Sabharwal V, Ramsay E, Martinez R, et al. Propofol-ketamine combination therapy for effective control of super-refractory status epilepticus. Epilepsy Behav. 2015;52(Pt A):264-266.
BALTIMORE—The investigational agent allopregnanolone may effectively treat superrefractory status epilepticus, according to research presented at the 141st Annual Meeting of the American Neurological Association. The drug appears to end seizures in patients for whom all other treatment options have failed. Unlike other current treatments, allopregnanolone does not entail risks of respiratory depression or hypotension.
“As we gain more experience with [allopregnanolone] in these superrefractory patients, I’m hoping that there’s going to be opportunities for using it in different settings in status [epilepticus],” said Michael Rogawski, MD, PhD, Professor of Neurology and Pharmacology at the University of California, Davis. The drug may be appropriate for refractory status epilepticus, for example. Dr. Rogawski and his colleagues are studying allopregnanolone in combination with diazepam and midazolam in models of status epilepticus, and results so far indicate that the medications work well in combination. “Our hope is that allopregnanolone could be used earlier on in status [epilepticus], potentially as a first-line agent, either as a replacement for benzodiazepines … or in combination with the benzodiazepine.”
An Analog of an Anesthetic
Allopregnanolone is an analog of alphaxalone, a surgical anesthetic used commonly in the 1970s. Alphaxalone was withdrawn from the market because it was difficult and dangerous to formulate. In the mid-1980s, researchers recognized that allopregnanolone, which has a similar chemical structure to that of alphaxalone, was endogenous to the body. Allopregnanolone is a positive allosteric modulator of GABAA receptors, and the body produces it by metabolism of progesterone.
Unlike benzodiazepines, neurosteroids such as allopregnanolone act not only on synaptic GABAA receptors, but also on extrasynaptic GABAA receptors, which bias neurons to be less excitable. Studies in seizure models and epilepsy models have indicated that neurosteroids are effective antiseizure agents. Dr. Rogawski and colleagues found that allopregnanolone effectively treats various models of status epilepticus.
Like other neurosteroids, allopregnanolone is highly lipophilic and easily crosses the blood–brain barrier. After intramuscular administration to mice, levels of allopregnanolone in the brain were much higher than levels in the plasma.
Results in Human Patients
After prolonged seizure activity, synaptic GABAA receptors are internalized and become unavailable as targets for benzodiazepines. Because allopregnanolone acts on extrasynaptic GABAA receptors, Dr. Rogawski and colleagues hypothesized that the medicine might be more effective than benzodiazepines in benzodiazepine-refractory status epilepticus. They found that when diazepam or allopregnanolone was administered to rats at 10 minutes after the onset of status epilepticus, the seizures stopped. When the drugs were administered at 40 minutes after the onset of status epilepticus, diazepam was ineffective, but allopregnanolone stopped the seizures.
To evaluate allopregnanolone in humans, Dr. Rogawski and colleagues created an IV formulation of the therapy by solubilizing it with cyclodextrins. They then gained FDA approval for clinical studies. When epilepsy centers caring for patients with superrefractory status epilepticus contacted Dr. Rogawski, they were able to obtain emergency FDA authorization to treat these patients with allopregnanolone supplied by Dr. Rogawski.
One patient was a 23-year-old man receiving barbiturate anesthesia. Every time his doctors withdrew the barbiturate anesthesia, his seizures recurred. After the patient received allopregnanolone, he was able to be weaned from the barbiturates without rebound seizure activity. The drug produced similar results in a 28-year-old man, an 11-year-old girl, and a 2-year-old girl. The investigators did not identify any treatment-related adverse events.
These patients were all critically ill, and withdrawal of life support was being considered, said Dr. Rogawski. Some did not tolerate the barbiturate anesthesia, including the 2-year-old girl, who was a patient at the UC Davis Medical Center. She developed hypotension, an ileus, and persistent urinary retention. Following allopregnanolone treatment, the dose of pentobarbital was reduced so that her blood pressure recovered and the ileus resolved. In this and the other cases, allopregnanolone seemed to be life-saving, but its efficacy will need to be validated in controlled studies, said Dr. Rogawski.
Allopregnanolone has been licensed to SAGE Therapeutics, which is studying the drug under the name SAGE-547.
—Erik Greb
Suggested Reading
Broomall E, Natale JE, Grimason M, et al. Pediatric super-refractory status epilepticus treated with allopregnanolone. Ann Neurol. 2014;76(6):911-915.
Kokate TG, Cohen AL, Karp E, Rogawski MA. Neuroactive steroids protect against pilocarpine- and kainic acid-induced limbic seizures and status epilepticus in mice. Neuropharmacology. 1996;35(8):1049-1056.
Rogawski MA, Loya CM, Reddy K, et al. Neuroactive steroids for the treatment of status epilepticus. Epilepsia. 2013;54 Suppl 6:93-98.
Rohracher A, Höfler J, Kalss G, et al. Perampanel in patients with refractory and super-refractory status epilepticus in a neurological intensive care unit. Epilepsy Behav. 2015;49:354-358.
Sabharwal V, Ramsay E, Martinez R, et al. Propofol-ketamine combination therapy for effective control of super-refractory status epilepticus. Epilepsy Behav. 2015;52(Pt A):264-266.
Low rate of occult uterine malignancy with vaginal morcellation
ORLANDO – The Food and Drug Administration’s 2014 warning that laparoscopic power morcellation during hysterectomy or myomectomy could spread unsuspected cancerous tissue had a chilling effect across the specialty, but what about the risks associated with morcellation during vaginal hysterectomy?
“There is only one case of morcellation during vaginal hysterectomy with a leiomyosarcoma recorded in the literature,” Megan N. Wasson, DO, a fellow in minimally invasive gynecologic surgery at the Mayo Clinic in Phoenix said at the meeting sponsored by AAGL. “It is really unclear if vaginal and electromechanical morcellation carry the same inherent risk.”
To find out more, Dr. Wasson and her colleagues identified 2,296 patients who underwent total vaginal hysterectomy at one of three academic medical centers. A total of 611 of these women had uterine removal with uncontained morcellation via cold-knife wedge resection. The investigators assessed this group for incidence of occult malignancy, perioperative outcomes, and long-term survival in a retrospective cohort study.
Of the 611 women who underwent morcellation during the study, five patients had an occult malignancy, for a rate less than one percent, 0.82%. Three patients had a stage IA, grade I endometrial adenocarcinoma, and two patients had a low-grade stromal sarcoma. No patients had a leiomyosarcoma.
This group of five patients had a mean age of 49 years, a mean BMI of 32 kg/m2 and a median parity of two. Abnormal uterine bleeding was the indication for surgery for all five patients with a malignancy. The mean uterine weight was elevated at 231 g. One patient with endometrial adenocarcinoma later underwent pelvic lymphadenectomy and vaginal brachytherapy.
“So far, thankfully, all of these patients show no evidence of disease recurrence,” Dr. Wasson said. All five patients are alive, with a mean disease-free survival of 43 months among those with endometrial adenocarcinoma and 37 months for the low-grade stroma sarcoma patients.
“Overall, the incidence of occult uterine carcinoma at the time of vaginal hysterectomy is less than 1%,” Dr. Wasson said. “Thankfully, it does not appear to have a negative effect on patient outcomes when it occurs.”
More research is needed, however. “The risk is very limited in terms of what we know,” she said. “We investigated cancer in this study, but there is also a risk of dissemination of benign conditions.”
All patients underwent a preoperative evaluation that included sampling of the lining of the uterus and imaging. “Out of the five patients with carcinomas, two of the adenocarcinomas had completely benign preoperative sampling and one had hyperplasia, which unfortunately did develop into occult disease,” Dr. Wasson said. “We wouldn’t recommend morcellating any patient with hyperplasia. In the two patients with low-grade stromal sarcoma, neither had any hyperplasia on preoperative sampling.”
Following the 2014 FDA Safety Communication on power morcellation, the AAGL released its own guidance on morcellation during uterine tissue extraction. The AAGL recommended that clinicians avoid morcellation for any patient who had a premalignant or malignant condition or who was at risk for malignancy, and use caution when considering morcellation. “This was for all types of morcellation, including electromechanical and vaginal morcellation,” Dr. Wasson said.
“This was in response to studies and awareness of increased risk of disease with morcellation – specifically leiomyosarcomas – for dissemination of disease in the abdomen and pelvis, but also for an increased risk of recurrence,” she said. “This means, in turn, that patients can have decreased overall survival and disease-free survival, so this is very important when we are talking to our patients.”
Dr. Wasson reported having no relevant financial disclosures.
ORLANDO – The Food and Drug Administration’s 2014 warning that laparoscopic power morcellation during hysterectomy or myomectomy could spread unsuspected cancerous tissue had a chilling effect across the specialty, but what about the risks associated with morcellation during vaginal hysterectomy?
“There is only one case of morcellation during vaginal hysterectomy with a leiomyosarcoma recorded in the literature,” Megan N. Wasson, DO, a fellow in minimally invasive gynecologic surgery at the Mayo Clinic in Phoenix said at the meeting sponsored by AAGL. “It is really unclear if vaginal and electromechanical morcellation carry the same inherent risk.”
To find out more, Dr. Wasson and her colleagues identified 2,296 patients who underwent total vaginal hysterectomy at one of three academic medical centers. A total of 611 of these women had uterine removal with uncontained morcellation via cold-knife wedge resection. The investigators assessed this group for incidence of occult malignancy, perioperative outcomes, and long-term survival in a retrospective cohort study.
Of the 611 women who underwent morcellation during the study, five patients had an occult malignancy, for a rate less than one percent, 0.82%. Three patients had a stage IA, grade I endometrial adenocarcinoma, and two patients had a low-grade stromal sarcoma. No patients had a leiomyosarcoma.
This group of five patients had a mean age of 49 years, a mean BMI of 32 kg/m2 and a median parity of two. Abnormal uterine bleeding was the indication for surgery for all five patients with a malignancy. The mean uterine weight was elevated at 231 g. One patient with endometrial adenocarcinoma later underwent pelvic lymphadenectomy and vaginal brachytherapy.
“So far, thankfully, all of these patients show no evidence of disease recurrence,” Dr. Wasson said. All five patients are alive, with a mean disease-free survival of 43 months among those with endometrial adenocarcinoma and 37 months for the low-grade stroma sarcoma patients.
“Overall, the incidence of occult uterine carcinoma at the time of vaginal hysterectomy is less than 1%,” Dr. Wasson said. “Thankfully, it does not appear to have a negative effect on patient outcomes when it occurs.”
More research is needed, however. “The risk is very limited in terms of what we know,” she said. “We investigated cancer in this study, but there is also a risk of dissemination of benign conditions.”
All patients underwent a preoperative evaluation that included sampling of the lining of the uterus and imaging. “Out of the five patients with carcinomas, two of the adenocarcinomas had completely benign preoperative sampling and one had hyperplasia, which unfortunately did develop into occult disease,” Dr. Wasson said. “We wouldn’t recommend morcellating any patient with hyperplasia. In the two patients with low-grade stromal sarcoma, neither had any hyperplasia on preoperative sampling.”
Following the 2014 FDA Safety Communication on power morcellation, the AAGL released its own guidance on morcellation during uterine tissue extraction. The AAGL recommended that clinicians avoid morcellation for any patient who had a premalignant or malignant condition or who was at risk for malignancy, and use caution when considering morcellation. “This was for all types of morcellation, including electromechanical and vaginal morcellation,” Dr. Wasson said.
“This was in response to studies and awareness of increased risk of disease with morcellation – specifically leiomyosarcomas – for dissemination of disease in the abdomen and pelvis, but also for an increased risk of recurrence,” she said. “This means, in turn, that patients can have decreased overall survival and disease-free survival, so this is very important when we are talking to our patients.”
Dr. Wasson reported having no relevant financial disclosures.
ORLANDO – The Food and Drug Administration’s 2014 warning that laparoscopic power morcellation during hysterectomy or myomectomy could spread unsuspected cancerous tissue had a chilling effect across the specialty, but what about the risks associated with morcellation during vaginal hysterectomy?
“There is only one case of morcellation during vaginal hysterectomy with a leiomyosarcoma recorded in the literature,” Megan N. Wasson, DO, a fellow in minimally invasive gynecologic surgery at the Mayo Clinic in Phoenix said at the meeting sponsored by AAGL. “It is really unclear if vaginal and electromechanical morcellation carry the same inherent risk.”
To find out more, Dr. Wasson and her colleagues identified 2,296 patients who underwent total vaginal hysterectomy at one of three academic medical centers. A total of 611 of these women had uterine removal with uncontained morcellation via cold-knife wedge resection. The investigators assessed this group for incidence of occult malignancy, perioperative outcomes, and long-term survival in a retrospective cohort study.
Of the 611 women who underwent morcellation during the study, five patients had an occult malignancy, for a rate less than one percent, 0.82%. Three patients had a stage IA, grade I endometrial adenocarcinoma, and two patients had a low-grade stromal sarcoma. No patients had a leiomyosarcoma.
This group of five patients had a mean age of 49 years, a mean BMI of 32 kg/m2 and a median parity of two. Abnormal uterine bleeding was the indication for surgery for all five patients with a malignancy. The mean uterine weight was elevated at 231 g. One patient with endometrial adenocarcinoma later underwent pelvic lymphadenectomy and vaginal brachytherapy.
“So far, thankfully, all of these patients show no evidence of disease recurrence,” Dr. Wasson said. All five patients are alive, with a mean disease-free survival of 43 months among those with endometrial adenocarcinoma and 37 months for the low-grade stroma sarcoma patients.
“Overall, the incidence of occult uterine carcinoma at the time of vaginal hysterectomy is less than 1%,” Dr. Wasson said. “Thankfully, it does not appear to have a negative effect on patient outcomes when it occurs.”
More research is needed, however. “The risk is very limited in terms of what we know,” she said. “We investigated cancer in this study, but there is also a risk of dissemination of benign conditions.”
All patients underwent a preoperative evaluation that included sampling of the lining of the uterus and imaging. “Out of the five patients with carcinomas, two of the adenocarcinomas had completely benign preoperative sampling and one had hyperplasia, which unfortunately did develop into occult disease,” Dr. Wasson said. “We wouldn’t recommend morcellating any patient with hyperplasia. In the two patients with low-grade stromal sarcoma, neither had any hyperplasia on preoperative sampling.”
Following the 2014 FDA Safety Communication on power morcellation, the AAGL released its own guidance on morcellation during uterine tissue extraction. The AAGL recommended that clinicians avoid morcellation for any patient who had a premalignant or malignant condition or who was at risk for malignancy, and use caution when considering morcellation. “This was for all types of morcellation, including electromechanical and vaginal morcellation,” Dr. Wasson said.
“This was in response to studies and awareness of increased risk of disease with morcellation – specifically leiomyosarcomas – for dissemination of disease in the abdomen and pelvis, but also for an increased risk of recurrence,” she said. “This means, in turn, that patients can have decreased overall survival and disease-free survival, so this is very important when we are talking to our patients.”
Dr. Wasson reported having no relevant financial disclosures.
AT THE AAGL GLOBAL CONGRESS
Key clinical point:
Major finding: Of 611 patients who underwent morcellation during total vaginal hysterectomy, five patients (0.82%) had occult uterine carcinoma.
Data source: A retrospective cohort study of 611 women who had uterine removal with uncontained morcellation.
Disclosures: Dr. Wasson reported having no relevant financial disclosures.
Scoring Formula Consolidates Stroke and Bleeding Risk in Patients With Atrial Fibrillation
ROME—A new risk-stratification formula for patients with atrial fibrillation starting oral anticoagulant therapy helps sort out their potential net benefit on edoxaban, compared with warfarin. This risk score “could help guide selection of treatment” with a vitamin K antagonist such as warfarin or a new oral anticoagulant (NOAC) such as edoxaban, said Christina L. Fanola, MD, at the 2016 European Society of Cardiology Congress.
“It is a great time to think about this type of score because so many more patients are being diagnosed with atrial fibrillation and there is a lot of clinical equipoise” over which anticoagulant to start patients on, said Dr. Fanola, a cardiologist at Brigham and Women’s Hospital in Boston. She and her associates hope to externally validate the score and test it in cohorts that received other NOACs, such as apixaban (Eliquis), dabigatran (Pradaxa), or rivaroxaban (Xarelto). “Each NOAC may need its own scoring formula,” Dr. Fanola said.
The concept behind the TIMI AF Risk Score is that patients with nonvalvular atrial fibrillation who receive anticoagulant treatment may have fewer disabling ischemic strokes, but also may face the potential risk of life-threatening bleeding events. To create a risk-prediction model that takes into account both of these outcomes, Dr. Fanola and her research associates used data collected in the ENGAGE AF-TIMI 48 (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48) trial, a study that randomized more than 21,000 patients with atrial fibrillation, and on no prior oral anticoagulant regimen, to treatment with edoxaban (Savaysa) or warfarin. This was the pivotal trial for edoxaban’s approval for this indication. All patients enrolled in the study had a CHADS2 score of at least 2, identifying a significant ischemic stroke risk.
A Cox proportional hazards model identified 10 demographic, clinical, and laboratory features that had significant, independent correlations to a primary outcome of disabling stroke, life-threatening bleeding, or death. After weighing the point allocation for each item by the strength of its association, the researchers developed a scoring formula in a model that could account for about 69% of the three combined adverse outcomes.
An analysis that applied the scoring formula to the ENGAGE AF-TIMI 48 database showed that a low-risk score of 0 to 6 correlated with a 4% per year rate of disabling stroke, life-threatening bleed, or death; an intermediate-risk score of 7 to 9 correlated with a 10% per year incidence of this combined outcome; and a high-risk score of 10 or greater correlated with a 21% annual event rate.
Dr. Fanola and her associates conducted a further analysis that evaluated the efficacy of edoxaban, compared with warfarin, among the patients in each of these risk strata. Patients at high risk received a major benefit from edoxaban, with a 30% overall incidence of the combined end point during three years of follow-up, compared with a 51% rate among patients on warfarin. Patients at intermediate risk also received a significant benefit, with a 26% event rate on warfarin and an 18% rate on edoxaban. But patients at low risk had identical 10% event rates with either treatment.
These findings suggest that patients with atrial fibrillation with a TIMI AF score that is high or intermediate would have a better chance for a good outcome on edoxaban, or perhaps a different NOAC, than on warfarin. Patients at low risk seem to have similar outcomes on edoxaban or warfarin, so other considerations can come into play for choosing between these drug options, such as the cost of treatment and the inconvenience of regular warfarin monitoring, Dr. Fanola said.
ENGAGE AF-TIMI 48 was sponsored by Daiichi Sankyo, the company that markets edoxaban. Dr. Fanola had no relevant financial disclosures.
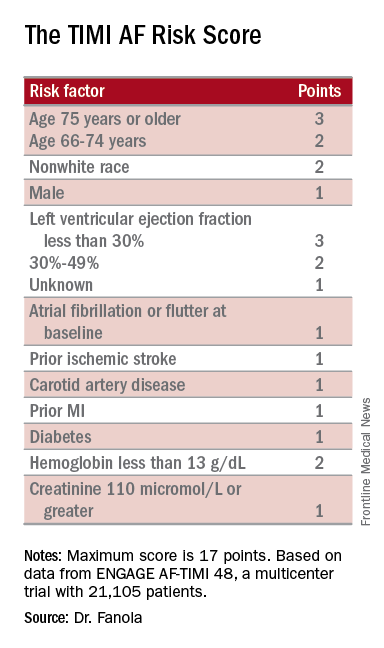
—Mitchel L. Zoler
ROME—A new risk-stratification formula for patients with atrial fibrillation starting oral anticoagulant therapy helps sort out their potential net benefit on edoxaban, compared with warfarin. This risk score “could help guide selection of treatment” with a vitamin K antagonist such as warfarin or a new oral anticoagulant (NOAC) such as edoxaban, said Christina L. Fanola, MD, at the 2016 European Society of Cardiology Congress.
“It is a great time to think about this type of score because so many more patients are being diagnosed with atrial fibrillation and there is a lot of clinical equipoise” over which anticoagulant to start patients on, said Dr. Fanola, a cardiologist at Brigham and Women’s Hospital in Boston. She and her associates hope to externally validate the score and test it in cohorts that received other NOACs, such as apixaban (Eliquis), dabigatran (Pradaxa), or rivaroxaban (Xarelto). “Each NOAC may need its own scoring formula,” Dr. Fanola said.
The concept behind the TIMI AF Risk Score is that patients with nonvalvular atrial fibrillation who receive anticoagulant treatment may have fewer disabling ischemic strokes, but also may face the potential risk of life-threatening bleeding events. To create a risk-prediction model that takes into account both of these outcomes, Dr. Fanola and her research associates used data collected in the ENGAGE AF-TIMI 48 (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48) trial, a study that randomized more than 21,000 patients with atrial fibrillation, and on no prior oral anticoagulant regimen, to treatment with edoxaban (Savaysa) or warfarin. This was the pivotal trial for edoxaban’s approval for this indication. All patients enrolled in the study had a CHADS2 score of at least 2, identifying a significant ischemic stroke risk.
A Cox proportional hazards model identified 10 demographic, clinical, and laboratory features that had significant, independent correlations to a primary outcome of disabling stroke, life-threatening bleeding, or death. After weighing the point allocation for each item by the strength of its association, the researchers developed a scoring formula in a model that could account for about 69% of the three combined adverse outcomes.
An analysis that applied the scoring formula to the ENGAGE AF-TIMI 48 database showed that a low-risk score of 0 to 6 correlated with a 4% per year rate of disabling stroke, life-threatening bleed, or death; an intermediate-risk score of 7 to 9 correlated with a 10% per year incidence of this combined outcome; and a high-risk score of 10 or greater correlated with a 21% annual event rate.
Dr. Fanola and her associates conducted a further analysis that evaluated the efficacy of edoxaban, compared with warfarin, among the patients in each of these risk strata. Patients at high risk received a major benefit from edoxaban, with a 30% overall incidence of the combined end point during three years of follow-up, compared with a 51% rate among patients on warfarin. Patients at intermediate risk also received a significant benefit, with a 26% event rate on warfarin and an 18% rate on edoxaban. But patients at low risk had identical 10% event rates with either treatment.
These findings suggest that patients with atrial fibrillation with a TIMI AF score that is high or intermediate would have a better chance for a good outcome on edoxaban, or perhaps a different NOAC, than on warfarin. Patients at low risk seem to have similar outcomes on edoxaban or warfarin, so other considerations can come into play for choosing between these drug options, such as the cost of treatment and the inconvenience of regular warfarin monitoring, Dr. Fanola said.
ENGAGE AF-TIMI 48 was sponsored by Daiichi Sankyo, the company that markets edoxaban. Dr. Fanola had no relevant financial disclosures.

—Mitchel L. Zoler
ROME—A new risk-stratification formula for patients with atrial fibrillation starting oral anticoagulant therapy helps sort out their potential net benefit on edoxaban, compared with warfarin. This risk score “could help guide selection of treatment” with a vitamin K antagonist such as warfarin or a new oral anticoagulant (NOAC) such as edoxaban, said Christina L. Fanola, MD, at the 2016 European Society of Cardiology Congress.
“It is a great time to think about this type of score because so many more patients are being diagnosed with atrial fibrillation and there is a lot of clinical equipoise” over which anticoagulant to start patients on, said Dr. Fanola, a cardiologist at Brigham and Women’s Hospital in Boston. She and her associates hope to externally validate the score and test it in cohorts that received other NOACs, such as apixaban (Eliquis), dabigatran (Pradaxa), or rivaroxaban (Xarelto). “Each NOAC may need its own scoring formula,” Dr. Fanola said.
The concept behind the TIMI AF Risk Score is that patients with nonvalvular atrial fibrillation who receive anticoagulant treatment may have fewer disabling ischemic strokes, but also may face the potential risk of life-threatening bleeding events. To create a risk-prediction model that takes into account both of these outcomes, Dr. Fanola and her research associates used data collected in the ENGAGE AF-TIMI 48 (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48) trial, a study that randomized more than 21,000 patients with atrial fibrillation, and on no prior oral anticoagulant regimen, to treatment with edoxaban (Savaysa) or warfarin. This was the pivotal trial for edoxaban’s approval for this indication. All patients enrolled in the study had a CHADS2 score of at least 2, identifying a significant ischemic stroke risk.
A Cox proportional hazards model identified 10 demographic, clinical, and laboratory features that had significant, independent correlations to a primary outcome of disabling stroke, life-threatening bleeding, or death. After weighing the point allocation for each item by the strength of its association, the researchers developed a scoring formula in a model that could account for about 69% of the three combined adverse outcomes.
An analysis that applied the scoring formula to the ENGAGE AF-TIMI 48 database showed that a low-risk score of 0 to 6 correlated with a 4% per year rate of disabling stroke, life-threatening bleed, or death; an intermediate-risk score of 7 to 9 correlated with a 10% per year incidence of this combined outcome; and a high-risk score of 10 or greater correlated with a 21% annual event rate.
Dr. Fanola and her associates conducted a further analysis that evaluated the efficacy of edoxaban, compared with warfarin, among the patients in each of these risk strata. Patients at high risk received a major benefit from edoxaban, with a 30% overall incidence of the combined end point during three years of follow-up, compared with a 51% rate among patients on warfarin. Patients at intermediate risk also received a significant benefit, with a 26% event rate on warfarin and an 18% rate on edoxaban. But patients at low risk had identical 10% event rates with either treatment.
These findings suggest that patients with atrial fibrillation with a TIMI AF score that is high or intermediate would have a better chance for a good outcome on edoxaban, or perhaps a different NOAC, than on warfarin. Patients at low risk seem to have similar outcomes on edoxaban or warfarin, so other considerations can come into play for choosing between these drug options, such as the cost of treatment and the inconvenience of regular warfarin monitoring, Dr. Fanola said.
ENGAGE AF-TIMI 48 was sponsored by Daiichi Sankyo, the company that markets edoxaban. Dr. Fanola had no relevant financial disclosures.

—Mitchel L. Zoler
Prognostic Models Fail to Predict Posttraumatic Seizures
Investigators have developed prognostic models that discriminate between patients with and without posttraumatic seizures (PTS) at year 1 and year 2 after traumatic brain injury (TBI), but perform little better than chance at predicting PTS, according to research published in the September issue of Epilepsia. The models do, however, identify potentially important predictors that may help to identify populations at risk for PTS.
“Individuals with characteristics identified in prognostic models as predictors of PTS represent subpopulations that may benefit from tailored seizure prophylaxis guidelines addressing unique premorbid characteristics, pathologies, and procedures,” said Anne Ritter, MPH, PhD, of Uniformed Services University of the Health Sciences in Bethesda, Maryland.
PTS is commonly recognized as a complication of TBI that may be acute or chronic. Although risk factors for PTS have been identified, predicting who will develop PTS remains difficult. Current PTS prognostic models are not widely accepted for clinical use and do not reflect current trends in injury, diagnosis, or care.
“Accurate PTS risk prediction could help define high-risk populations in support of clinical intervention trials. Predictive models could also inform clinical algorithms to identify individuals likely to benefit from tailored seizure prophylaxis or treatment,” said Dr. Ritter.
Dr. Ritter and colleagues conducted a study to develop and internally validate preliminary prognostic regression models that predict PTS during acute care hospitalization and at year 1 and year 2 post injury.
Study Population
Eligible participants had moderate or severe TBI, were admitted to a participating hospital emergency department within 72 hours of injury, were age 16 or older, and received acute care and inpatient rehabilitation within a TBI Model System (TBIMS) designated hospital system. Moderate or severe TBI was defined as posttraumatic amnesia lasting longer than 24 h, loss of consciousness lasting longer than 30 minutes, an emergency department Glasgow Coma Scale score less than 13, or positive neuroimaging findings.
People injured between October 1, 2011, and August 31, 2014, were included in the study. Patients injured during this time period, but not eligible for year 1 follow-up were excluded, however. Data for all participants were selected from the TBIMS National Database.
Researchers limited data to those collected at enrollment, year 1, or year 2 post injury. Enrollment data were collected using chart review and interview and included demographic, social, and injury characteristics, as well as preinjury personal and medical history and acute hospitalization outcome. PTS status, defined as the presence or absence of seizure activity, was the main outcome. It was determined during acute hospitalization, at year 1, and at year 2. In addition, investigators used multivariable logistic regression to generate prognostic models for PTS during acute hospitalization, at year 1, and at year 2. They internally validated models with resampling.
PTS Predictors of Interest
Of 2,136 participants, 2,042 had data available on all predictors identified in simple logistic regression for seizure during acute hospitalization. The sample’s demographic and clinical variables were similar in this investigation to those in previous TBI studies.
The final year 1 prognostic model identified injury severity, subdural hematoma, contusion load, craniotomy, craniectomy, seizure during acute hospitalization, preinjury condition limiting physical activity, preinjury mental health treatment or psychiatric hospitalization, and incarceration as risk factors for PTS. Craniectomy was the most statistically significant predictor in the final model.
At year 2, following validation, predictor variables included subdural hematoma, intraparenchymal fragment, craniotomy, craniectomy, seizure during acute hospitalization, and preinjury incarceration. Acute hospitalization seizure and craniectomy were the most statistically significant predictors of PTS at year 2.
Overall, the models displayed poor discrimination ability for PTS; however, these models may have added benefit, compared with prior models that were not being used clinically, said Dr. Ritter.
“These models must be examined in independent study populations to determine discriminability and validity outside the TBIMS population,” she added.
—Erica Tricarico
Suggested Reading
Ritter AC, Wagner AK, Szaflarski, et al. Prognostic models for predicting posttraumatic seizures during acute hospitalization, and 1 and 2 years following traumatic brain injury. Epilepsia. 2016;57(9):1503-1514.
Investigators have developed prognostic models that discriminate between patients with and without posttraumatic seizures (PTS) at year 1 and year 2 after traumatic brain injury (TBI), but perform little better than chance at predicting PTS, according to research published in the September issue of Epilepsia. The models do, however, identify potentially important predictors that may help to identify populations at risk for PTS.
“Individuals with characteristics identified in prognostic models as predictors of PTS represent subpopulations that may benefit from tailored seizure prophylaxis guidelines addressing unique premorbid characteristics, pathologies, and procedures,” said Anne Ritter, MPH, PhD, of Uniformed Services University of the Health Sciences in Bethesda, Maryland.
PTS is commonly recognized as a complication of TBI that may be acute or chronic. Although risk factors for PTS have been identified, predicting who will develop PTS remains difficult. Current PTS prognostic models are not widely accepted for clinical use and do not reflect current trends in injury, diagnosis, or care.
“Accurate PTS risk prediction could help define high-risk populations in support of clinical intervention trials. Predictive models could also inform clinical algorithms to identify individuals likely to benefit from tailored seizure prophylaxis or treatment,” said Dr. Ritter.
Dr. Ritter and colleagues conducted a study to develop and internally validate preliminary prognostic regression models that predict PTS during acute care hospitalization and at year 1 and year 2 post injury.
Study Population
Eligible participants had moderate or severe TBI, were admitted to a participating hospital emergency department within 72 hours of injury, were age 16 or older, and received acute care and inpatient rehabilitation within a TBI Model System (TBIMS) designated hospital system. Moderate or severe TBI was defined as posttraumatic amnesia lasting longer than 24 h, loss of consciousness lasting longer than 30 minutes, an emergency department Glasgow Coma Scale score less than 13, or positive neuroimaging findings.
People injured between October 1, 2011, and August 31, 2014, were included in the study. Patients injured during this time period, but not eligible for year 1 follow-up were excluded, however. Data for all participants were selected from the TBIMS National Database.
Researchers limited data to those collected at enrollment, year 1, or year 2 post injury. Enrollment data were collected using chart review and interview and included demographic, social, and injury characteristics, as well as preinjury personal and medical history and acute hospitalization outcome. PTS status, defined as the presence or absence of seizure activity, was the main outcome. It was determined during acute hospitalization, at year 1, and at year 2. In addition, investigators used multivariable logistic regression to generate prognostic models for PTS during acute hospitalization, at year 1, and at year 2. They internally validated models with resampling.
PTS Predictors of Interest
Of 2,136 participants, 2,042 had data available on all predictors identified in simple logistic regression for seizure during acute hospitalization. The sample’s demographic and clinical variables were similar in this investigation to those in previous TBI studies.
The final year 1 prognostic model identified injury severity, subdural hematoma, contusion load, craniotomy, craniectomy, seizure during acute hospitalization, preinjury condition limiting physical activity, preinjury mental health treatment or psychiatric hospitalization, and incarceration as risk factors for PTS. Craniectomy was the most statistically significant predictor in the final model.
At year 2, following validation, predictor variables included subdural hematoma, intraparenchymal fragment, craniotomy, craniectomy, seizure during acute hospitalization, and preinjury incarceration. Acute hospitalization seizure and craniectomy were the most statistically significant predictors of PTS at year 2.
Overall, the models displayed poor discrimination ability for PTS; however, these models may have added benefit, compared with prior models that were not being used clinically, said Dr. Ritter.
“These models must be examined in independent study populations to determine discriminability and validity outside the TBIMS population,” she added.
—Erica Tricarico
Suggested Reading
Ritter AC, Wagner AK, Szaflarski, et al. Prognostic models for predicting posttraumatic seizures during acute hospitalization, and 1 and 2 years following traumatic brain injury. Epilepsia. 2016;57(9):1503-1514.
Investigators have developed prognostic models that discriminate between patients with and without posttraumatic seizures (PTS) at year 1 and year 2 after traumatic brain injury (TBI), but perform little better than chance at predicting PTS, according to research published in the September issue of Epilepsia. The models do, however, identify potentially important predictors that may help to identify populations at risk for PTS.
“Individuals with characteristics identified in prognostic models as predictors of PTS represent subpopulations that may benefit from tailored seizure prophylaxis guidelines addressing unique premorbid characteristics, pathologies, and procedures,” said Anne Ritter, MPH, PhD, of Uniformed Services University of the Health Sciences in Bethesda, Maryland.
PTS is commonly recognized as a complication of TBI that may be acute or chronic. Although risk factors for PTS have been identified, predicting who will develop PTS remains difficult. Current PTS prognostic models are not widely accepted for clinical use and do not reflect current trends in injury, diagnosis, or care.
“Accurate PTS risk prediction could help define high-risk populations in support of clinical intervention trials. Predictive models could also inform clinical algorithms to identify individuals likely to benefit from tailored seizure prophylaxis or treatment,” said Dr. Ritter.
Dr. Ritter and colleagues conducted a study to develop and internally validate preliminary prognostic regression models that predict PTS during acute care hospitalization and at year 1 and year 2 post injury.
Study Population
Eligible participants had moderate or severe TBI, were admitted to a participating hospital emergency department within 72 hours of injury, were age 16 or older, and received acute care and inpatient rehabilitation within a TBI Model System (TBIMS) designated hospital system. Moderate or severe TBI was defined as posttraumatic amnesia lasting longer than 24 h, loss of consciousness lasting longer than 30 minutes, an emergency department Glasgow Coma Scale score less than 13, or positive neuroimaging findings.
People injured between October 1, 2011, and August 31, 2014, were included in the study. Patients injured during this time period, but not eligible for year 1 follow-up were excluded, however. Data for all participants were selected from the TBIMS National Database.
Researchers limited data to those collected at enrollment, year 1, or year 2 post injury. Enrollment data were collected using chart review and interview and included demographic, social, and injury characteristics, as well as preinjury personal and medical history and acute hospitalization outcome. PTS status, defined as the presence or absence of seizure activity, was the main outcome. It was determined during acute hospitalization, at year 1, and at year 2. In addition, investigators used multivariable logistic regression to generate prognostic models for PTS during acute hospitalization, at year 1, and at year 2. They internally validated models with resampling.
PTS Predictors of Interest
Of 2,136 participants, 2,042 had data available on all predictors identified in simple logistic regression for seizure during acute hospitalization. The sample’s demographic and clinical variables were similar in this investigation to those in previous TBI studies.
The final year 1 prognostic model identified injury severity, subdural hematoma, contusion load, craniotomy, craniectomy, seizure during acute hospitalization, preinjury condition limiting physical activity, preinjury mental health treatment or psychiatric hospitalization, and incarceration as risk factors for PTS. Craniectomy was the most statistically significant predictor in the final model.
At year 2, following validation, predictor variables included subdural hematoma, intraparenchymal fragment, craniotomy, craniectomy, seizure during acute hospitalization, and preinjury incarceration. Acute hospitalization seizure and craniectomy were the most statistically significant predictors of PTS at year 2.
Overall, the models displayed poor discrimination ability for PTS; however, these models may have added benefit, compared with prior models that were not being used clinically, said Dr. Ritter.
“These models must be examined in independent study populations to determine discriminability and validity outside the TBIMS population,” she added.
—Erica Tricarico
Suggested Reading
Ritter AC, Wagner AK, Szaflarski, et al. Prognostic models for predicting posttraumatic seizures during acute hospitalization, and 1 and 2 years following traumatic brain injury. Epilepsia. 2016;57(9):1503-1514.
HCT survivors experience high rates of late respiratory and infectious complications
Cancer survivors who underwent hematopoietic cell transplantation (HCT) face a greater risk for hospitalizations and mortality, compared with survivors who did not have HCT.
New findings show that disparities in infectious and respiratory complications were marked between the two groups, but differences in circulatory disease, mental health diagnoses, and second cancers were insignificant.
“Clinicians who care for long-term survivors of HCT should be aware of comprehensive surveillance guidelines available for this high-risk population,” wrote Eric J. Chow, MD, of the Fred Hutchinson Cancer Research Center, Seattle, and his colleagues (J Clin Oncol. 2016 Nov 21. doi: 10.1200/JCO.2016.68.8457).
There have only been a few comprehensive analyses that have compared HCT with non-HCT cancer survivors. Thus, the authors noted that it is unclear if HCT survivors are at a greater risk of late complications, compared with other cancer survivors.
To address this issue, Dr. Chow and his team matched 2-year cancer survivors who had undergone HCT (n = 1,792; 52% allogeneic and 90% hematologic malignancies) to non-HCT 2-year cancer survivors, using a state cancer registry (n = 5,455), and the general population (n = 16,340), using driver’s license files.
The investigators found that the 10-year cumulative incidence of any hospitalization or death related to all major organ-system outcomes was significantly different (P less than .05) between the HCT survivors and general population.
Patients with a history of HCT had a 30.6% cumulative incidence of infectious complications (difference vs. non-HCT: 8.7%) and a 26.8% incidence of any respiratory complications (difference vs. non-HCT: 6.9%), the investigators reported.
In contrast, the 10-year cumulative incidences of nervous system, circulatory, and genitourinary complications; mental health outcomes; and the development of new cancers did not differ between the HCT and non-HCT groups.
The incidence of pregnancy-related hospitalization among women of childbearing age was lower in the HCT group, compared with non-HCT patients (group difference, 24.4%).
At the 2-year endpoint, Dr. Chow and his associates noted that certain risks were “notably higher” in patients who had undergone HCT, including primary infections (hazard ratio, 1.4), respiratory complications (HR, 1.3), and death from any cause (HR, 1.1).
A significantly greater hospitalization rate also was observed in the HCT group versus the non-HCT group (280 episodes per 1,000 person-years vs. 173 episodes per 1,000 person years; P less than .001).
“Future work to identify more specific risk factors associated with late infections and respiratory complications may help to further refine these guidelines and identify new prevention strategies,” the authors concluded.
The study was funded by grants from the National Institutes of Health. Dr. Chow had no disclosures and several coauthors report relationships with industry.
Cancer survivors who underwent hematopoietic cell transplantation (HCT) face a greater risk for hospitalizations and mortality, compared with survivors who did not have HCT.
New findings show that disparities in infectious and respiratory complications were marked between the two groups, but differences in circulatory disease, mental health diagnoses, and second cancers were insignificant.
“Clinicians who care for long-term survivors of HCT should be aware of comprehensive surveillance guidelines available for this high-risk population,” wrote Eric J. Chow, MD, of the Fred Hutchinson Cancer Research Center, Seattle, and his colleagues (J Clin Oncol. 2016 Nov 21. doi: 10.1200/JCO.2016.68.8457).
There have only been a few comprehensive analyses that have compared HCT with non-HCT cancer survivors. Thus, the authors noted that it is unclear if HCT survivors are at a greater risk of late complications, compared with other cancer survivors.
To address this issue, Dr. Chow and his team matched 2-year cancer survivors who had undergone HCT (n = 1,792; 52% allogeneic and 90% hematologic malignancies) to non-HCT 2-year cancer survivors, using a state cancer registry (n = 5,455), and the general population (n = 16,340), using driver’s license files.
The investigators found that the 10-year cumulative incidence of any hospitalization or death related to all major organ-system outcomes was significantly different (P less than .05) between the HCT survivors and general population.
Patients with a history of HCT had a 30.6% cumulative incidence of infectious complications (difference vs. non-HCT: 8.7%) and a 26.8% incidence of any respiratory complications (difference vs. non-HCT: 6.9%), the investigators reported.
In contrast, the 10-year cumulative incidences of nervous system, circulatory, and genitourinary complications; mental health outcomes; and the development of new cancers did not differ between the HCT and non-HCT groups.
The incidence of pregnancy-related hospitalization among women of childbearing age was lower in the HCT group, compared with non-HCT patients (group difference, 24.4%).
At the 2-year endpoint, Dr. Chow and his associates noted that certain risks were “notably higher” in patients who had undergone HCT, including primary infections (hazard ratio, 1.4), respiratory complications (HR, 1.3), and death from any cause (HR, 1.1).
A significantly greater hospitalization rate also was observed in the HCT group versus the non-HCT group (280 episodes per 1,000 person-years vs. 173 episodes per 1,000 person years; P less than .001).
“Future work to identify more specific risk factors associated with late infections and respiratory complications may help to further refine these guidelines and identify new prevention strategies,” the authors concluded.
The study was funded by grants from the National Institutes of Health. Dr. Chow had no disclosures and several coauthors report relationships with industry.
Cancer survivors who underwent hematopoietic cell transplantation (HCT) face a greater risk for hospitalizations and mortality, compared with survivors who did not have HCT.
New findings show that disparities in infectious and respiratory complications were marked between the two groups, but differences in circulatory disease, mental health diagnoses, and second cancers were insignificant.
“Clinicians who care for long-term survivors of HCT should be aware of comprehensive surveillance guidelines available for this high-risk population,” wrote Eric J. Chow, MD, of the Fred Hutchinson Cancer Research Center, Seattle, and his colleagues (J Clin Oncol. 2016 Nov 21. doi: 10.1200/JCO.2016.68.8457).
There have only been a few comprehensive analyses that have compared HCT with non-HCT cancer survivors. Thus, the authors noted that it is unclear if HCT survivors are at a greater risk of late complications, compared with other cancer survivors.
To address this issue, Dr. Chow and his team matched 2-year cancer survivors who had undergone HCT (n = 1,792; 52% allogeneic and 90% hematologic malignancies) to non-HCT 2-year cancer survivors, using a state cancer registry (n = 5,455), and the general population (n = 16,340), using driver’s license files.
The investigators found that the 10-year cumulative incidence of any hospitalization or death related to all major organ-system outcomes was significantly different (P less than .05) between the HCT survivors and general population.
Patients with a history of HCT had a 30.6% cumulative incidence of infectious complications (difference vs. non-HCT: 8.7%) and a 26.8% incidence of any respiratory complications (difference vs. non-HCT: 6.9%), the investigators reported.
In contrast, the 10-year cumulative incidences of nervous system, circulatory, and genitourinary complications; mental health outcomes; and the development of new cancers did not differ between the HCT and non-HCT groups.
The incidence of pregnancy-related hospitalization among women of childbearing age was lower in the HCT group, compared with non-HCT patients (group difference, 24.4%).
At the 2-year endpoint, Dr. Chow and his associates noted that certain risks were “notably higher” in patients who had undergone HCT, including primary infections (hazard ratio, 1.4), respiratory complications (HR, 1.3), and death from any cause (HR, 1.1).
A significantly greater hospitalization rate also was observed in the HCT group versus the non-HCT group (280 episodes per 1,000 person-years vs. 173 episodes per 1,000 person years; P less than .001).
“Future work to identify more specific risk factors associated with late infections and respiratory complications may help to further refine these guidelines and identify new prevention strategies,” the authors concluded.
The study was funded by grants from the National Institutes of Health. Dr. Chow had no disclosures and several coauthors report relationships with industry.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Clinicians who care for HCT survivors should be aware of their high rates of late respiratory and infectious complications.
Major finding: Patients with a history of HCT had a 30.6% cumulative incidence of infectious complications (difference vs. non-HCT: 8.7%) and a 26.8% incidence of any respiratory complications (difference vs. non-HCT: 6.9%).
Data source: Retrospective population study using databases to match outcomes between two patient groups and the general population.
Disclosures: The study was funded by grants from the National Institutes of Health. Dr. Chow has no disclosures and several coauthors report relationships with industry.











