User login
Assessment of goals of care in nursing home reduces hospitalization for patients with dementia
CLINICAL QUESTION: For patients with advanced dementia, does a goals-of-care intervention improve communication and care outcomes?
BACKGROUND: Patients with advanced dementia are frequently admitted from nursing homes for acute conditions. Prior research demonstrates deficits in documentation of advanced directives.
STUDY DESIGN: Single-blind cluster randomized trial.
SETTING: Twenty-two nursing homes in North Carolina.
BOTTOM LINE: Goals of care discussions for patients with advanced dementia appears to reduce hospitalizations.
CITATIONS: Hanson LC, Zimmerman S, Song MK, et al. Effect of the goals of care intervention for advanced dementia: a randomized clinical trial. JAMA Intern Med. 2017 Jan;177:24-31.
Dr. Cumbler is the associate chief of hospital medicine, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
CLINICAL QUESTION: For patients with advanced dementia, does a goals-of-care intervention improve communication and care outcomes?
BACKGROUND: Patients with advanced dementia are frequently admitted from nursing homes for acute conditions. Prior research demonstrates deficits in documentation of advanced directives.
STUDY DESIGN: Single-blind cluster randomized trial.
SETTING: Twenty-two nursing homes in North Carolina.
BOTTOM LINE: Goals of care discussions for patients with advanced dementia appears to reduce hospitalizations.
CITATIONS: Hanson LC, Zimmerman S, Song MK, et al. Effect of the goals of care intervention for advanced dementia: a randomized clinical trial. JAMA Intern Med. 2017 Jan;177:24-31.
Dr. Cumbler is the associate chief of hospital medicine, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
CLINICAL QUESTION: For patients with advanced dementia, does a goals-of-care intervention improve communication and care outcomes?
BACKGROUND: Patients with advanced dementia are frequently admitted from nursing homes for acute conditions. Prior research demonstrates deficits in documentation of advanced directives.
STUDY DESIGN: Single-blind cluster randomized trial.
SETTING: Twenty-two nursing homes in North Carolina.
BOTTOM LINE: Goals of care discussions for patients with advanced dementia appears to reduce hospitalizations.
CITATIONS: Hanson LC, Zimmerman S, Song MK, et al. Effect of the goals of care intervention for advanced dementia: a randomized clinical trial. JAMA Intern Med. 2017 Jan;177:24-31.
Dr. Cumbler is the associate chief of hospital medicine, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
Antipsychotics ineffective for symptoms of delirium in palliative care
CLINICAL QUESTION: Do antipsychotics provide symptomatic benefit for delirium in palliative care?
BACKGROUND: Antipsychotics are frequently used for the treatment of delirium and guideline recommended for delirium-associated distress. However, a 2016 meta-analysis found antipsychotics are not associated with change in delirium duration or severity. Antipsychotics for palliative management of delirium at end of life is not well studied.
STUDY DESIGN: Double-blind randomized controlled trial with placebo, haloperidol, and risperidone arms.
SETTING: Eleven Australian inpatient hospice or palliative care services.
SYNOPSIS: 247 patients (mean age, 74.9 years; 88.3% with cancer) with advanced incurable disease and active delirium were studied. Most had mild-moderate severity delirium. All received nonpharmacological measures and plan to address reversible precipitants. Patients were randomized to placebo (84), haloperidol (81), or risperidone (82) for 72 hours. Dose titration was allowed based on delirium symptoms. In intention to treat analysis the delirium severity scores were statistically higher in haloperidol and risperidone arms, compared with placebo. This reached statistical significance although less than the minimum clinically significant difference. Mortality, use of rescue medicines, and extrapyramidal symptoms were higher in antipsychotic groups.
BOTTOM LINE: Antipsychotics cause side effects without efficacy in palliation of symptoms of delirium.
CITATIONS: Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med. 2017 Jan;177:34-42.
Dr. Cumbler is the associate chief of hospital medicine, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
CLINICAL QUESTION: Do antipsychotics provide symptomatic benefit for delirium in palliative care?
BACKGROUND: Antipsychotics are frequently used for the treatment of delirium and guideline recommended for delirium-associated distress. However, a 2016 meta-analysis found antipsychotics are not associated with change in delirium duration or severity. Antipsychotics for palliative management of delirium at end of life is not well studied.
STUDY DESIGN: Double-blind randomized controlled trial with placebo, haloperidol, and risperidone arms.
SETTING: Eleven Australian inpatient hospice or palliative care services.
SYNOPSIS: 247 patients (mean age, 74.9 years; 88.3% with cancer) with advanced incurable disease and active delirium were studied. Most had mild-moderate severity delirium. All received nonpharmacological measures and plan to address reversible precipitants. Patients were randomized to placebo (84), haloperidol (81), or risperidone (82) for 72 hours. Dose titration was allowed based on delirium symptoms. In intention to treat analysis the delirium severity scores were statistically higher in haloperidol and risperidone arms, compared with placebo. This reached statistical significance although less than the minimum clinically significant difference. Mortality, use of rescue medicines, and extrapyramidal symptoms were higher in antipsychotic groups.
BOTTOM LINE: Antipsychotics cause side effects without efficacy in palliation of symptoms of delirium.
CITATIONS: Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med. 2017 Jan;177:34-42.
Dr. Cumbler is the associate chief of hospital medicine, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
CLINICAL QUESTION: Do antipsychotics provide symptomatic benefit for delirium in palliative care?
BACKGROUND: Antipsychotics are frequently used for the treatment of delirium and guideline recommended for delirium-associated distress. However, a 2016 meta-analysis found antipsychotics are not associated with change in delirium duration or severity. Antipsychotics for palliative management of delirium at end of life is not well studied.
STUDY DESIGN: Double-blind randomized controlled trial with placebo, haloperidol, and risperidone arms.
SETTING: Eleven Australian inpatient hospice or palliative care services.
SYNOPSIS: 247 patients (mean age, 74.9 years; 88.3% with cancer) with advanced incurable disease and active delirium were studied. Most had mild-moderate severity delirium. All received nonpharmacological measures and plan to address reversible precipitants. Patients were randomized to placebo (84), haloperidol (81), or risperidone (82) for 72 hours. Dose titration was allowed based on delirium symptoms. In intention to treat analysis the delirium severity scores were statistically higher in haloperidol and risperidone arms, compared with placebo. This reached statistical significance although less than the minimum clinically significant difference. Mortality, use of rescue medicines, and extrapyramidal symptoms were higher in antipsychotic groups.
BOTTOM LINE: Antipsychotics cause side effects without efficacy in palliation of symptoms of delirium.
CITATIONS: Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med. 2017 Jan;177:34-42.
Dr. Cumbler is the associate chief of hospital medicine, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
Hepatitis Outlook: Late February 2017
If you work on the front lines of medical care, treating patients with hepatitis, you may not have time to review all the hepatitis research that enters the medical literature every month. Here’s a quick look at some notable news items and journal articles published over the past month, which cover a variety of the major hepatitis viruses.
Sequential therapy with pegylated interferon after long-term–nucleoside/nucleotide analog therapy enhances the reduction of hepatitis B surface antigen and may represent a treatment option to promote HBsAg loss, a recent study revealed.
Researchers in Egypt say that combining serum microRNAs with baseline predictors could serve as a new non-invasive algorithm for staging hepatitis C virus (HCV)-associated liver fibrosis.
Patients with acute HBV infection who also have prodromal fever, which is associated with the lack of hepatitis B core antigen due to HBV mutations, are at high risk for acute liver failure, and these patients should be treated with special care, a recent study found.
Iranian researchers found that innate immune response genes are expressed differentially among chronic HBV phases and say this difference may help to develop new precise and noninvasive methods to determine the progression of disease in chronic HBV patients.
A study in Hepatology found that the protein arginine methyltransferase 5 restricts hepatitis B virus replication via epigenetic repression of covalently closed circular DNA transcription and interference with pregenomic RNA encapsidation.
Chronic hepatitis C virus (HCV) patients in China experienced immunosuppression mediated by regulatory T cells that was lower during and after combination therapy, regardless of treatment response. Immunosuppression was higher in patients with sustained viral response than in those without SVR at the end of follow-up.
Hepatoblasts derived from human embryonic stem cells are the optimal hosts for HCV infectivity, according to a study in Hepatology.
A study of U.S. veterans found that HBV reactivation of varying severity, even in the setting of isolated anti–hepatitis B core antigen with or without accompanying hepatitis, can occur. The authors said the occurrence of accompanying severe hepatitis was rare, however.
A study in Italy found that an elevated fibrosis-4 index turned out to be an important predictor of hepatocellular carcinoma occurrence in patients with chronic HCV. The investigators said the assessment of FIB-4 in HCV RNA-positive individuals may help in identifying the highest Hierarchical Condition Categories–risk individuals who need anti-HCV treatment most urgently.
Despite the eradication of HCV transmission by blood products, HCV infection continues to be one of the leading blood-borne infections in Europe, according to an epidemiological study in Infectious Agents and Cancer.
[email protected]
On Twitter @richpizzi
If you work on the front lines of medical care, treating patients with hepatitis, you may not have time to review all the hepatitis research that enters the medical literature every month. Here’s a quick look at some notable news items and journal articles published over the past month, which cover a variety of the major hepatitis viruses.
Sequential therapy with pegylated interferon after long-term–nucleoside/nucleotide analog therapy enhances the reduction of hepatitis B surface antigen and may represent a treatment option to promote HBsAg loss, a recent study revealed.
Researchers in Egypt say that combining serum microRNAs with baseline predictors could serve as a new non-invasive algorithm for staging hepatitis C virus (HCV)-associated liver fibrosis.
Patients with acute HBV infection who also have prodromal fever, which is associated with the lack of hepatitis B core antigen due to HBV mutations, are at high risk for acute liver failure, and these patients should be treated with special care, a recent study found.
Iranian researchers found that innate immune response genes are expressed differentially among chronic HBV phases and say this difference may help to develop new precise and noninvasive methods to determine the progression of disease in chronic HBV patients.
A study in Hepatology found that the protein arginine methyltransferase 5 restricts hepatitis B virus replication via epigenetic repression of covalently closed circular DNA transcription and interference with pregenomic RNA encapsidation.
Chronic hepatitis C virus (HCV) patients in China experienced immunosuppression mediated by regulatory T cells that was lower during and after combination therapy, regardless of treatment response. Immunosuppression was higher in patients with sustained viral response than in those without SVR at the end of follow-up.
Hepatoblasts derived from human embryonic stem cells are the optimal hosts for HCV infectivity, according to a study in Hepatology.
A study of U.S. veterans found that HBV reactivation of varying severity, even in the setting of isolated anti–hepatitis B core antigen with or without accompanying hepatitis, can occur. The authors said the occurrence of accompanying severe hepatitis was rare, however.
A study in Italy found that an elevated fibrosis-4 index turned out to be an important predictor of hepatocellular carcinoma occurrence in patients with chronic HCV. The investigators said the assessment of FIB-4 in HCV RNA-positive individuals may help in identifying the highest Hierarchical Condition Categories–risk individuals who need anti-HCV treatment most urgently.
Despite the eradication of HCV transmission by blood products, HCV infection continues to be one of the leading blood-borne infections in Europe, according to an epidemiological study in Infectious Agents and Cancer.
[email protected]
On Twitter @richpizzi
If you work on the front lines of medical care, treating patients with hepatitis, you may not have time to review all the hepatitis research that enters the medical literature every month. Here’s a quick look at some notable news items and journal articles published over the past month, which cover a variety of the major hepatitis viruses.
Sequential therapy with pegylated interferon after long-term–nucleoside/nucleotide analog therapy enhances the reduction of hepatitis B surface antigen and may represent a treatment option to promote HBsAg loss, a recent study revealed.
Researchers in Egypt say that combining serum microRNAs with baseline predictors could serve as a new non-invasive algorithm for staging hepatitis C virus (HCV)-associated liver fibrosis.
Patients with acute HBV infection who also have prodromal fever, which is associated with the lack of hepatitis B core antigen due to HBV mutations, are at high risk for acute liver failure, and these patients should be treated with special care, a recent study found.
Iranian researchers found that innate immune response genes are expressed differentially among chronic HBV phases and say this difference may help to develop new precise and noninvasive methods to determine the progression of disease in chronic HBV patients.
A study in Hepatology found that the protein arginine methyltransferase 5 restricts hepatitis B virus replication via epigenetic repression of covalently closed circular DNA transcription and interference with pregenomic RNA encapsidation.
Chronic hepatitis C virus (HCV) patients in China experienced immunosuppression mediated by regulatory T cells that was lower during and after combination therapy, regardless of treatment response. Immunosuppression was higher in patients with sustained viral response than in those without SVR at the end of follow-up.
Hepatoblasts derived from human embryonic stem cells are the optimal hosts for HCV infectivity, according to a study in Hepatology.
A study of U.S. veterans found that HBV reactivation of varying severity, even in the setting of isolated anti–hepatitis B core antigen with or without accompanying hepatitis, can occur. The authors said the occurrence of accompanying severe hepatitis was rare, however.
A study in Italy found that an elevated fibrosis-4 index turned out to be an important predictor of hepatocellular carcinoma occurrence in patients with chronic HCV. The investigators said the assessment of FIB-4 in HCV RNA-positive individuals may help in identifying the highest Hierarchical Condition Categories–risk individuals who need anti-HCV treatment most urgently.
Despite the eradication of HCV transmission by blood products, HCV infection continues to be one of the leading blood-borne infections in Europe, according to an epidemiological study in Infectious Agents and Cancer.
[email protected]
On Twitter @richpizzi
Update on malpractice trends
Question: Recent developments in malpractice include the following:
A. Severity and frequency rates continue to rise.
B. Apology laws appear to be very effective in reducing claims.
C. Litigation surrounds whether an assistant may obtain a patient’s informed consent on behalf of the doctor.
D. A and B.
E. A, B, and C.
Answer: C. Over the past decade, malpractice claims have in fact diminished, accompanied by a leveling or reduction in premiums.1 Rates have plummeted to roughly half of previous levels, averaging six claims per 100 doctors in 2016.
According to The Doctors Company, internists paid an average premium of $15,853, compared with an average of $19,900 in 2006, general surgeons $52,905 instead of $68,186, and obstetricians $72,999, a drop from $93,230. Even claims-plagued Florida’s Dade County has seen a dramatic drop in internist premiums by some $27,000, down to $47,707.2
The latest attack on MICRA, in 2015, concerned a wrongful death suit brought by a woman whose mother died from hemorrhagic complications related to Coumadin use following heart surgery.4 Her constitutional challenges included violation of equal protection, due process, and the right to a jury trial. But these were essentially all grounded on an entitlement to recover additional noneconomic damages sufficient to cover attorney fees. The trial court had reduced her $1 million noneconomic damages to $250,000 as required by MICRA. A California appeals court rejected her claim as being “contrary to many well-established legal principles.”
Disclosure of medical errors is a relative newcomer as an ethical and effective way of thwarting potential malpractice claims. Many states have enacted so-called “apology laws,” an outgrowth of the communication and resolution programs popularized by the Lexington (Ky.) VA Medical Center, University of Michigan Health System, Harvard’s affiliated institutions, and Colorado’s COPIC Insurance.
Apology laws disallow statements of sympathy from being admitted into evidence. In some cases, these laws may assist the physician. For example, the Ohio Supreme Court has ruled that a surgeon’s comments and alleged admission of guilt (“I take full responsibility for this” regarding accidentally sectioning the common bile duct) were properly shielded from discovery by the state’s apology statute, even though the incident took place before the law went into effect.5
However, apology laws do vary from state to state, and some do not shield admissions regarding causation of error or fault.
A recent study suggests that apology laws don’t work. A Vanderbilt University study published online used a unique dataset covering all malpractice claims for 90% of physicians practicing in a single specialty across the country.6 The findings revealed that, for physicians who do not regularly perform surgery, apology laws actually increased the probability of facing a lawsuit. For surgeons, apology laws do not have a substantial effect on the probability of facing a claim or the average payment made to resolve a claim.
The study’s authors concluded that “apology laws are not substitutes for specific physician disclosure programs, and that the experiences of these types of programs are not generalizable to the physician population at large. In other words, simply being allowed to apologize is not enough to reduce malpractice risk.”
In the informed consent arena, the latest development in the law revolves around whether a physician assistant, in lieu of the surgeon himself, can obtain informed consent from a patient.
In Shinal v. Toms,7 Megan Shinal underwent surgery to remove a craniopharyngioma, but it regrew and required re-exploration by Dr. Steven Toms. Dr. Toms testified that Ms. Shinal had agreed that he would determine during the surgery whether he should remove the entire tumor or perform a partial resection. The operation was complicated by a carotid artery perforation, which left the patient with impaired vision and ambulation.
The complaint asserted that Dr. Toms’s physician assistant, not Dr. Toms himself, had provided the actual discussion during the informed consent process, and thus the patient’s consent was invalid.
The jury was allowed to consider the information provided by the doctor’s support staff, and the Superior Court of Pennsylvania affirmed the validity of the patient’s consent, holding that consent is based on the scope of information relayed rather than the identity of the individual communicating the information. This carefully watched case is now on final appeal before the Supreme Court of Pennsylvania.
At a personal level, physicians dread the stress surrounding medical malpractice litigation. The process frontally attacks their competence and consumes much time and energy, notwithstanding there being little or no exposure of personal assets because of insurance protection. Virtually all doctors practice defensive medicine, which has been defined as “deviation from sound medical practice that is induced primarily by a threat of liability.”
At a societal level, defensive medicine is reported to add substantially to the nation’s medical bill. The figure tossed around is $12 billion to $50 billion a year, based mostly on estimates by the American Medical Association and an older study extrapolating potential Medicare savings from litigation over heart disease.8
A more recent report continues to emphasize the high cost of defensive medicine.9 Jackson Healthcare invited 138,686 physicians to participate in a confidential online survey to quantify the costs and impact of defensive medicine. More than 3,000 physicians spanning all states and medical specialties completed the survey; however, this represented only a 2.21% response rate.
The authors concluded that defensive medicine is a significant force driving the high cost of health care in the United States, and that physicians estimate the cost of defensive medicine to be in the $650 billion to $850 billion range, or between 26% and 34% of annual health care costs.
Skeptics question the way the profession defines defensive medicine, pointing out that malpractice concerns may not be the primary reason, as most interventions add some marginal value to patient care. There may also be conflicting motivations of physicians, such as financial or other personal rewards.
Above all, there is no acceptable method for measuring the extent and use of defensive medicine, and survey reports are apt to be misleading because of bias and the lack of controls and baseline data.
Looking ahead, what can we expect for malpractice law under the Trump administration? Tom Price, MD, a former Republican congressman from Georgia, is an orthopedic surgeon and the new secretary of the Department of Health & Human Services. He has previously spoken passionately about tort reforms such as defensive medicine, damage caps, health tribunals, and practice guidelines. Many states have already incorporated some of these measures into their own tort reforms – with salutary results. It remains to be seen whether HHS will deem any omnibus federal legislation necessary at this point.
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the materials have been taken from my earlier columns in Internal Medicine News. For additional information, readers may contact the author at [email protected].
References
1. JAMA. 2014 Nov 26;312(20):2146-55.
2. “Malpractice 2017: Do We Need Reform?” Internal Medicine News, March 1, 2017, page 1.
3. Fein v. Permanente, 38 Cal.3d 137 (1985).
4. Chan v. Curran, 237 CA 4th 601 (2015).
5. Estate of Johnson v. Randall Smith, Inc., 135 Ohio St.3d 440 (2013).
6. Available at https://papers.ssrn.com/sol3/papers2.cfm?abstract_id=2883693.
7. Shinal v. Toms, 122 A. 3d 1066 (Pa. Super. Ct. 2015).
8. Q J Econ. (1996) 111 (2): 353-390.
9. Available at www.jacksonhealthcare.com/media/8968/defensivemedicine_ebook_final.pdf.
Question: Recent developments in malpractice include the following:
A. Severity and frequency rates continue to rise.
B. Apology laws appear to be very effective in reducing claims.
C. Litigation surrounds whether an assistant may obtain a patient’s informed consent on behalf of the doctor.
D. A and B.
E. A, B, and C.
Answer: C. Over the past decade, malpractice claims have in fact diminished, accompanied by a leveling or reduction in premiums.1 Rates have plummeted to roughly half of previous levels, averaging six claims per 100 doctors in 2016.
According to The Doctors Company, internists paid an average premium of $15,853, compared with an average of $19,900 in 2006, general surgeons $52,905 instead of $68,186, and obstetricians $72,999, a drop from $93,230. Even claims-plagued Florida’s Dade County has seen a dramatic drop in internist premiums by some $27,000, down to $47,707.2
The latest attack on MICRA, in 2015, concerned a wrongful death suit brought by a woman whose mother died from hemorrhagic complications related to Coumadin use following heart surgery.4 Her constitutional challenges included violation of equal protection, due process, and the right to a jury trial. But these were essentially all grounded on an entitlement to recover additional noneconomic damages sufficient to cover attorney fees. The trial court had reduced her $1 million noneconomic damages to $250,000 as required by MICRA. A California appeals court rejected her claim as being “contrary to many well-established legal principles.”
Disclosure of medical errors is a relative newcomer as an ethical and effective way of thwarting potential malpractice claims. Many states have enacted so-called “apology laws,” an outgrowth of the communication and resolution programs popularized by the Lexington (Ky.) VA Medical Center, University of Michigan Health System, Harvard’s affiliated institutions, and Colorado’s COPIC Insurance.
Apology laws disallow statements of sympathy from being admitted into evidence. In some cases, these laws may assist the physician. For example, the Ohio Supreme Court has ruled that a surgeon’s comments and alleged admission of guilt (“I take full responsibility for this” regarding accidentally sectioning the common bile duct) were properly shielded from discovery by the state’s apology statute, even though the incident took place before the law went into effect.5
However, apology laws do vary from state to state, and some do not shield admissions regarding causation of error or fault.
A recent study suggests that apology laws don’t work. A Vanderbilt University study published online used a unique dataset covering all malpractice claims for 90% of physicians practicing in a single specialty across the country.6 The findings revealed that, for physicians who do not regularly perform surgery, apology laws actually increased the probability of facing a lawsuit. For surgeons, apology laws do not have a substantial effect on the probability of facing a claim or the average payment made to resolve a claim.
The study’s authors concluded that “apology laws are not substitutes for specific physician disclosure programs, and that the experiences of these types of programs are not generalizable to the physician population at large. In other words, simply being allowed to apologize is not enough to reduce malpractice risk.”
In the informed consent arena, the latest development in the law revolves around whether a physician assistant, in lieu of the surgeon himself, can obtain informed consent from a patient.
In Shinal v. Toms,7 Megan Shinal underwent surgery to remove a craniopharyngioma, but it regrew and required re-exploration by Dr. Steven Toms. Dr. Toms testified that Ms. Shinal had agreed that he would determine during the surgery whether he should remove the entire tumor or perform a partial resection. The operation was complicated by a carotid artery perforation, which left the patient with impaired vision and ambulation.
The complaint asserted that Dr. Toms’s physician assistant, not Dr. Toms himself, had provided the actual discussion during the informed consent process, and thus the patient’s consent was invalid.
The jury was allowed to consider the information provided by the doctor’s support staff, and the Superior Court of Pennsylvania affirmed the validity of the patient’s consent, holding that consent is based on the scope of information relayed rather than the identity of the individual communicating the information. This carefully watched case is now on final appeal before the Supreme Court of Pennsylvania.
At a personal level, physicians dread the stress surrounding medical malpractice litigation. The process frontally attacks their competence and consumes much time and energy, notwithstanding there being little or no exposure of personal assets because of insurance protection. Virtually all doctors practice defensive medicine, which has been defined as “deviation from sound medical practice that is induced primarily by a threat of liability.”
At a societal level, defensive medicine is reported to add substantially to the nation’s medical bill. The figure tossed around is $12 billion to $50 billion a year, based mostly on estimates by the American Medical Association and an older study extrapolating potential Medicare savings from litigation over heart disease.8
A more recent report continues to emphasize the high cost of defensive medicine.9 Jackson Healthcare invited 138,686 physicians to participate in a confidential online survey to quantify the costs and impact of defensive medicine. More than 3,000 physicians spanning all states and medical specialties completed the survey; however, this represented only a 2.21% response rate.
The authors concluded that defensive medicine is a significant force driving the high cost of health care in the United States, and that physicians estimate the cost of defensive medicine to be in the $650 billion to $850 billion range, or between 26% and 34% of annual health care costs.
Skeptics question the way the profession defines defensive medicine, pointing out that malpractice concerns may not be the primary reason, as most interventions add some marginal value to patient care. There may also be conflicting motivations of physicians, such as financial or other personal rewards.
Above all, there is no acceptable method for measuring the extent and use of defensive medicine, and survey reports are apt to be misleading because of bias and the lack of controls and baseline data.
Looking ahead, what can we expect for malpractice law under the Trump administration? Tom Price, MD, a former Republican congressman from Georgia, is an orthopedic surgeon and the new secretary of the Department of Health & Human Services. He has previously spoken passionately about tort reforms such as defensive medicine, damage caps, health tribunals, and practice guidelines. Many states have already incorporated some of these measures into their own tort reforms – with salutary results. It remains to be seen whether HHS will deem any omnibus federal legislation necessary at this point.
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the materials have been taken from my earlier columns in Internal Medicine News. For additional information, readers may contact the author at [email protected].
References
1. JAMA. 2014 Nov 26;312(20):2146-55.
2. “Malpractice 2017: Do We Need Reform?” Internal Medicine News, March 1, 2017, page 1.
3. Fein v. Permanente, 38 Cal.3d 137 (1985).
4. Chan v. Curran, 237 CA 4th 601 (2015).
5. Estate of Johnson v. Randall Smith, Inc., 135 Ohio St.3d 440 (2013).
6. Available at https://papers.ssrn.com/sol3/papers2.cfm?abstract_id=2883693.
7. Shinal v. Toms, 122 A. 3d 1066 (Pa. Super. Ct. 2015).
8. Q J Econ. (1996) 111 (2): 353-390.
9. Available at www.jacksonhealthcare.com/media/8968/defensivemedicine_ebook_final.pdf.
Question: Recent developments in malpractice include the following:
A. Severity and frequency rates continue to rise.
B. Apology laws appear to be very effective in reducing claims.
C. Litigation surrounds whether an assistant may obtain a patient’s informed consent on behalf of the doctor.
D. A and B.
E. A, B, and C.
Answer: C. Over the past decade, malpractice claims have in fact diminished, accompanied by a leveling or reduction in premiums.1 Rates have plummeted to roughly half of previous levels, averaging six claims per 100 doctors in 2016.
According to The Doctors Company, internists paid an average premium of $15,853, compared with an average of $19,900 in 2006, general surgeons $52,905 instead of $68,186, and obstetricians $72,999, a drop from $93,230. Even claims-plagued Florida’s Dade County has seen a dramatic drop in internist premiums by some $27,000, down to $47,707.2
The latest attack on MICRA, in 2015, concerned a wrongful death suit brought by a woman whose mother died from hemorrhagic complications related to Coumadin use following heart surgery.4 Her constitutional challenges included violation of equal protection, due process, and the right to a jury trial. But these were essentially all grounded on an entitlement to recover additional noneconomic damages sufficient to cover attorney fees. The trial court had reduced her $1 million noneconomic damages to $250,000 as required by MICRA. A California appeals court rejected her claim as being “contrary to many well-established legal principles.”
Disclosure of medical errors is a relative newcomer as an ethical and effective way of thwarting potential malpractice claims. Many states have enacted so-called “apology laws,” an outgrowth of the communication and resolution programs popularized by the Lexington (Ky.) VA Medical Center, University of Michigan Health System, Harvard’s affiliated institutions, and Colorado’s COPIC Insurance.
Apology laws disallow statements of sympathy from being admitted into evidence. In some cases, these laws may assist the physician. For example, the Ohio Supreme Court has ruled that a surgeon’s comments and alleged admission of guilt (“I take full responsibility for this” regarding accidentally sectioning the common bile duct) were properly shielded from discovery by the state’s apology statute, even though the incident took place before the law went into effect.5
However, apology laws do vary from state to state, and some do not shield admissions regarding causation of error or fault.
A recent study suggests that apology laws don’t work. A Vanderbilt University study published online used a unique dataset covering all malpractice claims for 90% of physicians practicing in a single specialty across the country.6 The findings revealed that, for physicians who do not regularly perform surgery, apology laws actually increased the probability of facing a lawsuit. For surgeons, apology laws do not have a substantial effect on the probability of facing a claim or the average payment made to resolve a claim.
The study’s authors concluded that “apology laws are not substitutes for specific physician disclosure programs, and that the experiences of these types of programs are not generalizable to the physician population at large. In other words, simply being allowed to apologize is not enough to reduce malpractice risk.”
In the informed consent arena, the latest development in the law revolves around whether a physician assistant, in lieu of the surgeon himself, can obtain informed consent from a patient.
In Shinal v. Toms,7 Megan Shinal underwent surgery to remove a craniopharyngioma, but it regrew and required re-exploration by Dr. Steven Toms. Dr. Toms testified that Ms. Shinal had agreed that he would determine during the surgery whether he should remove the entire tumor or perform a partial resection. The operation was complicated by a carotid artery perforation, which left the patient with impaired vision and ambulation.
The complaint asserted that Dr. Toms’s physician assistant, not Dr. Toms himself, had provided the actual discussion during the informed consent process, and thus the patient’s consent was invalid.
The jury was allowed to consider the information provided by the doctor’s support staff, and the Superior Court of Pennsylvania affirmed the validity of the patient’s consent, holding that consent is based on the scope of information relayed rather than the identity of the individual communicating the information. This carefully watched case is now on final appeal before the Supreme Court of Pennsylvania.
At a personal level, physicians dread the stress surrounding medical malpractice litigation. The process frontally attacks their competence and consumes much time and energy, notwithstanding there being little or no exposure of personal assets because of insurance protection. Virtually all doctors practice defensive medicine, which has been defined as “deviation from sound medical practice that is induced primarily by a threat of liability.”
At a societal level, defensive medicine is reported to add substantially to the nation’s medical bill. The figure tossed around is $12 billion to $50 billion a year, based mostly on estimates by the American Medical Association and an older study extrapolating potential Medicare savings from litigation over heart disease.8
A more recent report continues to emphasize the high cost of defensive medicine.9 Jackson Healthcare invited 138,686 physicians to participate in a confidential online survey to quantify the costs and impact of defensive medicine. More than 3,000 physicians spanning all states and medical specialties completed the survey; however, this represented only a 2.21% response rate.
The authors concluded that defensive medicine is a significant force driving the high cost of health care in the United States, and that physicians estimate the cost of defensive medicine to be in the $650 billion to $850 billion range, or between 26% and 34% of annual health care costs.
Skeptics question the way the profession defines defensive medicine, pointing out that malpractice concerns may not be the primary reason, as most interventions add some marginal value to patient care. There may also be conflicting motivations of physicians, such as financial or other personal rewards.
Above all, there is no acceptable method for measuring the extent and use of defensive medicine, and survey reports are apt to be misleading because of bias and the lack of controls and baseline data.
Looking ahead, what can we expect for malpractice law under the Trump administration? Tom Price, MD, a former Republican congressman from Georgia, is an orthopedic surgeon and the new secretary of the Department of Health & Human Services. He has previously spoken passionately about tort reforms such as defensive medicine, damage caps, health tribunals, and practice guidelines. Many states have already incorporated some of these measures into their own tort reforms – with salutary results. It remains to be seen whether HHS will deem any omnibus federal legislation necessary at this point.
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the materials have been taken from my earlier columns in Internal Medicine News. For additional information, readers may contact the author at [email protected].
References
1. JAMA. 2014 Nov 26;312(20):2146-55.
2. “Malpractice 2017: Do We Need Reform?” Internal Medicine News, March 1, 2017, page 1.
3. Fein v. Permanente, 38 Cal.3d 137 (1985).
4. Chan v. Curran, 237 CA 4th 601 (2015).
5. Estate of Johnson v. Randall Smith, Inc., 135 Ohio St.3d 440 (2013).
6. Available at https://papers.ssrn.com/sol3/papers2.cfm?abstract_id=2883693.
7. Shinal v. Toms, 122 A. 3d 1066 (Pa. Super. Ct. 2015).
8. Q J Econ. (1996) 111 (2): 353-390.
9. Available at www.jacksonhealthcare.com/media/8968/defensivemedicine_ebook_final.pdf.
Antiphospholipid Syndrome in a Patient With Rheumatoid Arthritis
Case Report
A 39-year-old woman with a 20-year history of rheumatoid arthritis (RA) presented to a university-affiliated tertiary care hospital with painful ulcerations on the bilateral dorsal feet that started as bullae 16 weeks prior to presentation. Initial skin biopsy performed by an outside dermatologist 8 weeks prior to presentation showed vasculitis and culture was positive for methicillin-sensitive Staphylococcus aureus. She was started on a prednisone taper and cephalexin, which did not improve the lower extremity ulcerations and the pain became progressively worse. At the time of presentation to our dermatology department, the patient was taking prednisone, hydroxychloroquine, hydrocodone-acetaminophen, and gabapentin. Prior therapy with sulfasalazine failed; etanercept and methotrexate were discontinued years prior due to side effects. The patient had no history of deep vein thrombosis, pulmonary embolism, or miscarriage.
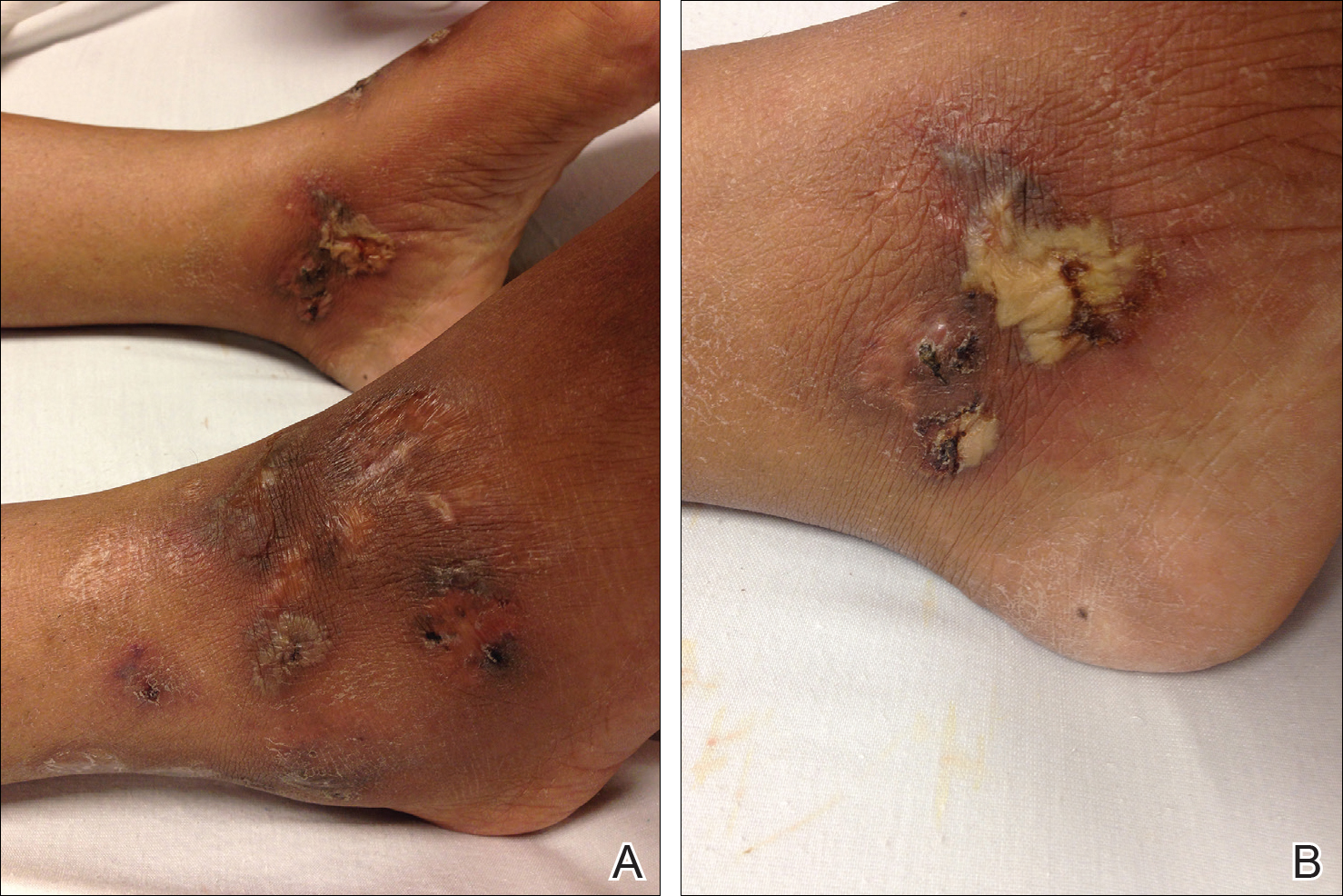
At presentation, the patient was afebrile and her vital signs were stable. Physical examination showed multiple ulcers and erosions on the bilateral dorsal feet with a few scattered retiform red-purple patches (Figure). One bulla was present on the right dorsal foot. All lesions were tender to the touch and edema was present on the bilateral feet. No oral ulcerations were present and no focal neuropathies or palpable cords were appreciated in the lower extremities. There were no other cutaneous abnormalities.
Laboratory studies showed a white blood cell count of 9.54×103/µL (reference range, 4.16-9.95×103/µL), hemoglobin count of 12.4 g/dL (reference range, 11.6-15.2 g/dL), and a platelet count of 175×103/µL (reference range, 143-398×103/µL). A basic metabolic panel was normal except for an elevated glucose level of 185 mg/dL (reference range, 65-100 mg/dL). Urinalysis was normal. Erythrocyte sedimentation rate and C-reactive protein level were not elevated. Antinuclear antibodies and double-stranded DNA antibodies were normal. Prothrombin time was 10.4 seconds (reference range, 9.2-11.5 seconds) and dilute viper's venom time was negative. Rheumatoid factor level was elevated at 76 IU/mL (reference range, <25 IU/mL) and anti-citrullinated peptide antibody was moderately elevated at 42 U/mL (negative, <20 U/mL; weak positive, 20-39 U/mL; moderate positive, 40-59 U/mL; strong positive, >59 U/mL). The cardiolipin antibodies IgG, IgM, and IgA were within reference range. Results of β2-glycoprotein I IgG and IgM antibody tests were normal, but IgA was elevated at 34 µg/mL (reference range, <20 µg/mL). Wound cultures grew moderate Enterobacter cloacae and Staphylococcus lugdunensis.
Slides from 2 prior punch biopsies obtained by an outside hospital approximately 8 weeks prior from the right and left dorsal foot lesions were reviewed. Both biopsies were histologically similar. Postcapillary venules showed extensive vasculitis with numerous fibrin thrombi in the lumens in both biopsy specimens. The biopsy from the right foot showed prominent ulceration of the epidermis, with a few of the affected vessels showing minimal accompanying nuclear dust; however, the predominant pattern was not that of leukocytoclastic vasculitis. Biopsy from the left foot showed prominent epidermal necrosis with focal reepithelialization and scattered eosinophils. The pathologist felt that a vasculitis secondary to coagulopathy was most likely but that a drug reaction and rheumatoid vasculitis would be other entities to consider in the differential. A review of the laboratory findings from the outside hospital from approximately 12 weeks prior to presentation showed IgM was normal but IgG was elevated at 28 U/mL (reference range, 0-15 U/mL) and IgA was elevated at 8 U/mL (reference range, 0-7 U/mL); β2-glycoprotein I IgG antibodies were elevated at 37 mg/dL (reference range, 0-25.0 mg/dL) and β2-glycoprotein I IgA antibodies were elevated at 5 mg/dL (reference range, 0-4.0 mg/dL).
The clinical suspicion of a thrombotic event on the dorsal feet, which was confirmed histologically, and the persistently positive antiphospholipid (aPL) antibody titers helped to establish the diagnosis of antiphospholipid syndrome (APS) in the setting of RA. The dose of prednisone was increased from 10 mg daily on admission to 40 mg daily. The patient was started on enoxaparin 60 mg subcutaneously twice daily at initial presentation and was bridged to oral warfarin 2 mg daily after the diagnosis of APS was established. Oral doxycycline 100 mg twice daily was started for wound infection. The ulcerations gradually improved over the course of her 7-day hospitalization. She was continued on prednisone, hydroxychloroquine, and warfarin as an outpatient and has had no recurrence of lesions after 3 years of follow-up on this regimen.
Comment
Antiphospholipid syndrome is an autoimmune condition defined by a venous and/or arterial thrombotic event and/or pregnancy morbidity in the presence of persistently elevated aPL antibody titers. The most frequently detected subgroups of aPL are anticardiolipin (aCL) antibodies, anti-β2-glycoprotein I antibodies, and lupus anticoagulants.1 Primary APS occurs as an isolated entity, whereas secondary APS occurs in the setting of a preexisting autoimmune disease, infection, malignancy, or medication.2 The diagnostic criteria for APS requires positive aPL titers at least 12 weeks apart and a clinically confirmed thrombotic event or pregnancy morbidity.3
About one-third to half of patients with APS exhibit cutaneous manifestations.4,5 Livedo reticularis is most commonly observed and represents the first clinical sign of APS in 17.5% of cases.6 Cutaneous findings of APS also include anetoderma, cutaneous ulceration and necrosis, necrotizing vasculitis, livedoid vasculitis, thrombophlebitis, purpura, ecchymoses, painful skin nodules, and subungual hemorrhages.7 The various cutaneous manifestations of APS are associated with a range of histopathologic findings, but noninflammatory thrombosis in small arteries and/or veins in the dermis and subcutaneous fat tissue is the most common histologic feature.4 Our patient exhibited cutaneous ulceration and necrosis, and biopsy clearly showed the presence of vasculitis and fibrin thrombi within postcapillary venules. These findings along with the persistently elevated β2-glycoprotein I IgA solidified the diagnosis of APS.
The most common cutaneous manifestations of RA are nodules (32%), Raynaud phenomenon (10%), and vasculitis (3%).8 The mean prevalence of aPL antibodies in patients with RA is 28%, though reports range from 5% to 75%.1 The presence of aPL or aCL does not predict the development of thrombosis and/or thrombocytopenia in RA patients9,10; however, aCL antibodies in RA patients are associated with a higher risk for developing rheumatoid nodules. It is hypothesized that the majority of aCL antibodies identified in RA patients have different specificities than those identified in other diseases that are associated with thrombotic events.1
Anticoagulation has been proven to decrease the risk for recurrent thrombotic events in patients with APS.11 Patients should discontinue the use of estrogen-containing oral contraceptives; avoid smoking cigarettes; and treat hypertension, hyperlipidemia, and diabetes mellitus, if present. The type and duration of anticoagulation therapy, especially for the treatment of the cutaneous manifestations of APS, is less well defined. Antiplatelet therapies such as low-dose aspirin or dipyridamole often are used for less severe cutaneous manifestations such as livedoid vasculopathy. Warfarin with a target international normalized ratio of 2.0 to 3.0 is most commonly used following major thrombotic events, including cutaneous necrosis and digital gangrene. The role of corticosteroids and immunosuppressants is unclear; one study showed that these therapies did not prevent further thrombotic events in patients with systemic lupus erythematosus.4
Conclusion
Although aPL antibodies are most prevalent in patients with systemic lupus erythematosus, an estimated 28% of patients with RA have elevated aPL titers. The aPL antibodies recognized in RA patients are thought to have a different specificity than those recognized in other APS-associated diseases because elevated aPL antibody titers are not associated with an increased incidence of thrombotic events in RA patients; however, larger studies are needed to clarify this phenomenon. It remains to be determined if this case of APS and RA represents a coincidence or a true disease association, but the recognition of the cutaneous and histological features of APS is crucial for establishing a diagnosis and initiating anticoagulation therapy to prevent further morbidity and mortality.
- Olech E, Merrill JT. The prevalence and clinical significance of antiphospholipid antibodies in rheumatoid arthritis. Curr Rheumatol Rep. 2006;8:100-108.
- Thornsberry LA, LoSicco KI, English JC. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450-462.
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306.
- Asherson A, Francès C, Iaccarino FL, et al. Theantiphospholipid antibody syndrome: diagnosis, skin manifestations and current therapy. Clin Exp Rheumatol. 2006;24(1 suppl 40):S46-S51.
- Cervera R, Piette JC, Font J, et al; Euro-Phospholipid Project Group. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002;46:1019-1027.
- Francès C, Niang S, Laffitte E, et al. Dermatologic manifestations of antiphospholipid syndrome. two hundred consecutive cases. Arthritis Rheum. 2005;52:1785-1793.
- Gibson GE, Su WP, Pittelkow MR. Antiphospholipid syndrome and the skin. J Am Acad Dermatol. 1997;36(6, pt 1):970-982.
- Young A. Extra-articular manifestations and complications of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21:907-927.
- Palomo I, Pinochet C, Alarcón M, et al. Prevalence of antiphospholipid antibodies in Chilean patients with rheumatoid arthritis. J Clin Lab Anal. 2006;20:190-194.
- Wolf P, Gretler J, Aglas F, et al. Anticardiolipin antibodies in rheumatoid arthritis: their relation to rheumatoid nodules and cutaneous vascular manifestations. Br J Dermatol. 1994;131:48-51.
- Lim W, Crowther MA, Eikelboom JW. Management of antiphospholipid antibody syndrome: a systematic review. JAMA. 2006;295:1050-1057.
Case Report
A 39-year-old woman with a 20-year history of rheumatoid arthritis (RA) presented to a university-affiliated tertiary care hospital with painful ulcerations on the bilateral dorsal feet that started as bullae 16 weeks prior to presentation. Initial skin biopsy performed by an outside dermatologist 8 weeks prior to presentation showed vasculitis and culture was positive for methicillin-sensitive Staphylococcus aureus. She was started on a prednisone taper and cephalexin, which did not improve the lower extremity ulcerations and the pain became progressively worse. At the time of presentation to our dermatology department, the patient was taking prednisone, hydroxychloroquine, hydrocodone-acetaminophen, and gabapentin. Prior therapy with sulfasalazine failed; etanercept and methotrexate were discontinued years prior due to side effects. The patient had no history of deep vein thrombosis, pulmonary embolism, or miscarriage.
At presentation, the patient was afebrile and her vital signs were stable. Physical examination showed multiple ulcers and erosions on the bilateral dorsal feet with a few scattered retiform red-purple patches (Figure). One bulla was present on the right dorsal foot. All lesions were tender to the touch and edema was present on the bilateral feet. No oral ulcerations were present and no focal neuropathies or palpable cords were appreciated in the lower extremities. There were no other cutaneous abnormalities.

Laboratory studies showed a white blood cell count of 9.54×103/µL (reference range, 4.16-9.95×103/µL), hemoglobin count of 12.4 g/dL (reference range, 11.6-15.2 g/dL), and a platelet count of 175×103/µL (reference range, 143-398×103/µL). A basic metabolic panel was normal except for an elevated glucose level of 185 mg/dL (reference range, 65-100 mg/dL). Urinalysis was normal. Erythrocyte sedimentation rate and C-reactive protein level were not elevated. Antinuclear antibodies and double-stranded DNA antibodies were normal. Prothrombin time was 10.4 seconds (reference range, 9.2-11.5 seconds) and dilute viper's venom time was negative. Rheumatoid factor level was elevated at 76 IU/mL (reference range, <25 IU/mL) and anti-citrullinated peptide antibody was moderately elevated at 42 U/mL (negative, <20 U/mL; weak positive, 20-39 U/mL; moderate positive, 40-59 U/mL; strong positive, >59 U/mL). The cardiolipin antibodies IgG, IgM, and IgA were within reference range. Results of β2-glycoprotein I IgG and IgM antibody tests were normal, but IgA was elevated at 34 µg/mL (reference range, <20 µg/mL). Wound cultures grew moderate Enterobacter cloacae and Staphylococcus lugdunensis.
Slides from 2 prior punch biopsies obtained by an outside hospital approximately 8 weeks prior from the right and left dorsal foot lesions were reviewed. Both biopsies were histologically similar. Postcapillary venules showed extensive vasculitis with numerous fibrin thrombi in the lumens in both biopsy specimens. The biopsy from the right foot showed prominent ulceration of the epidermis, with a few of the affected vessels showing minimal accompanying nuclear dust; however, the predominant pattern was not that of leukocytoclastic vasculitis. Biopsy from the left foot showed prominent epidermal necrosis with focal reepithelialization and scattered eosinophils. The pathologist felt that a vasculitis secondary to coagulopathy was most likely but that a drug reaction and rheumatoid vasculitis would be other entities to consider in the differential. A review of the laboratory findings from the outside hospital from approximately 12 weeks prior to presentation showed IgM was normal but IgG was elevated at 28 U/mL (reference range, 0-15 U/mL) and IgA was elevated at 8 U/mL (reference range, 0-7 U/mL); β2-glycoprotein I IgG antibodies were elevated at 37 mg/dL (reference range, 0-25.0 mg/dL) and β2-glycoprotein I IgA antibodies were elevated at 5 mg/dL (reference range, 0-4.0 mg/dL).
The clinical suspicion of a thrombotic event on the dorsal feet, which was confirmed histologically, and the persistently positive antiphospholipid (aPL) antibody titers helped to establish the diagnosis of antiphospholipid syndrome (APS) in the setting of RA. The dose of prednisone was increased from 10 mg daily on admission to 40 mg daily. The patient was started on enoxaparin 60 mg subcutaneously twice daily at initial presentation and was bridged to oral warfarin 2 mg daily after the diagnosis of APS was established. Oral doxycycline 100 mg twice daily was started for wound infection. The ulcerations gradually improved over the course of her 7-day hospitalization. She was continued on prednisone, hydroxychloroquine, and warfarin as an outpatient and has had no recurrence of lesions after 3 years of follow-up on this regimen.
Comment
Antiphospholipid syndrome is an autoimmune condition defined by a venous and/or arterial thrombotic event and/or pregnancy morbidity in the presence of persistently elevated aPL antibody titers. The most frequently detected subgroups of aPL are anticardiolipin (aCL) antibodies, anti-β2-glycoprotein I antibodies, and lupus anticoagulants.1 Primary APS occurs as an isolated entity, whereas secondary APS occurs in the setting of a preexisting autoimmune disease, infection, malignancy, or medication.2 The diagnostic criteria for APS requires positive aPL titers at least 12 weeks apart and a clinically confirmed thrombotic event or pregnancy morbidity.3
About one-third to half of patients with APS exhibit cutaneous manifestations.4,5 Livedo reticularis is most commonly observed and represents the first clinical sign of APS in 17.5% of cases.6 Cutaneous findings of APS also include anetoderma, cutaneous ulceration and necrosis, necrotizing vasculitis, livedoid vasculitis, thrombophlebitis, purpura, ecchymoses, painful skin nodules, and subungual hemorrhages.7 The various cutaneous manifestations of APS are associated with a range of histopathologic findings, but noninflammatory thrombosis in small arteries and/or veins in the dermis and subcutaneous fat tissue is the most common histologic feature.4 Our patient exhibited cutaneous ulceration and necrosis, and biopsy clearly showed the presence of vasculitis and fibrin thrombi within postcapillary venules. These findings along with the persistently elevated β2-glycoprotein I IgA solidified the diagnosis of APS.
The most common cutaneous manifestations of RA are nodules (32%), Raynaud phenomenon (10%), and vasculitis (3%).8 The mean prevalence of aPL antibodies in patients with RA is 28%, though reports range from 5% to 75%.1 The presence of aPL or aCL does not predict the development of thrombosis and/or thrombocytopenia in RA patients9,10; however, aCL antibodies in RA patients are associated with a higher risk for developing rheumatoid nodules. It is hypothesized that the majority of aCL antibodies identified in RA patients have different specificities than those identified in other diseases that are associated with thrombotic events.1
Anticoagulation has been proven to decrease the risk for recurrent thrombotic events in patients with APS.11 Patients should discontinue the use of estrogen-containing oral contraceptives; avoid smoking cigarettes; and treat hypertension, hyperlipidemia, and diabetes mellitus, if present. The type and duration of anticoagulation therapy, especially for the treatment of the cutaneous manifestations of APS, is less well defined. Antiplatelet therapies such as low-dose aspirin or dipyridamole often are used for less severe cutaneous manifestations such as livedoid vasculopathy. Warfarin with a target international normalized ratio of 2.0 to 3.0 is most commonly used following major thrombotic events, including cutaneous necrosis and digital gangrene. The role of corticosteroids and immunosuppressants is unclear; one study showed that these therapies did not prevent further thrombotic events in patients with systemic lupus erythematosus.4
Conclusion
Although aPL antibodies are most prevalent in patients with systemic lupus erythematosus, an estimated 28% of patients with RA have elevated aPL titers. The aPL antibodies recognized in RA patients are thought to have a different specificity than those recognized in other APS-associated diseases because elevated aPL antibody titers are not associated with an increased incidence of thrombotic events in RA patients; however, larger studies are needed to clarify this phenomenon. It remains to be determined if this case of APS and RA represents a coincidence or a true disease association, but the recognition of the cutaneous and histological features of APS is crucial for establishing a diagnosis and initiating anticoagulation therapy to prevent further morbidity and mortality.
Case Report
A 39-year-old woman with a 20-year history of rheumatoid arthritis (RA) presented to a university-affiliated tertiary care hospital with painful ulcerations on the bilateral dorsal feet that started as bullae 16 weeks prior to presentation. Initial skin biopsy performed by an outside dermatologist 8 weeks prior to presentation showed vasculitis and culture was positive for methicillin-sensitive Staphylococcus aureus. She was started on a prednisone taper and cephalexin, which did not improve the lower extremity ulcerations and the pain became progressively worse. At the time of presentation to our dermatology department, the patient was taking prednisone, hydroxychloroquine, hydrocodone-acetaminophen, and gabapentin. Prior therapy with sulfasalazine failed; etanercept and methotrexate were discontinued years prior due to side effects. The patient had no history of deep vein thrombosis, pulmonary embolism, or miscarriage.
At presentation, the patient was afebrile and her vital signs were stable. Physical examination showed multiple ulcers and erosions on the bilateral dorsal feet with a few scattered retiform red-purple patches (Figure). One bulla was present on the right dorsal foot. All lesions were tender to the touch and edema was present on the bilateral feet. No oral ulcerations were present and no focal neuropathies or palpable cords were appreciated in the lower extremities. There were no other cutaneous abnormalities.

Laboratory studies showed a white blood cell count of 9.54×103/µL (reference range, 4.16-9.95×103/µL), hemoglobin count of 12.4 g/dL (reference range, 11.6-15.2 g/dL), and a platelet count of 175×103/µL (reference range, 143-398×103/µL). A basic metabolic panel was normal except for an elevated glucose level of 185 mg/dL (reference range, 65-100 mg/dL). Urinalysis was normal. Erythrocyte sedimentation rate and C-reactive protein level were not elevated. Antinuclear antibodies and double-stranded DNA antibodies were normal. Prothrombin time was 10.4 seconds (reference range, 9.2-11.5 seconds) and dilute viper's venom time was negative. Rheumatoid factor level was elevated at 76 IU/mL (reference range, <25 IU/mL) and anti-citrullinated peptide antibody was moderately elevated at 42 U/mL (negative, <20 U/mL; weak positive, 20-39 U/mL; moderate positive, 40-59 U/mL; strong positive, >59 U/mL). The cardiolipin antibodies IgG, IgM, and IgA were within reference range. Results of β2-glycoprotein I IgG and IgM antibody tests were normal, but IgA was elevated at 34 µg/mL (reference range, <20 µg/mL). Wound cultures grew moderate Enterobacter cloacae and Staphylococcus lugdunensis.
Slides from 2 prior punch biopsies obtained by an outside hospital approximately 8 weeks prior from the right and left dorsal foot lesions were reviewed. Both biopsies were histologically similar. Postcapillary venules showed extensive vasculitis with numerous fibrin thrombi in the lumens in both biopsy specimens. The biopsy from the right foot showed prominent ulceration of the epidermis, with a few of the affected vessels showing minimal accompanying nuclear dust; however, the predominant pattern was not that of leukocytoclastic vasculitis. Biopsy from the left foot showed prominent epidermal necrosis with focal reepithelialization and scattered eosinophils. The pathologist felt that a vasculitis secondary to coagulopathy was most likely but that a drug reaction and rheumatoid vasculitis would be other entities to consider in the differential. A review of the laboratory findings from the outside hospital from approximately 12 weeks prior to presentation showed IgM was normal but IgG was elevated at 28 U/mL (reference range, 0-15 U/mL) and IgA was elevated at 8 U/mL (reference range, 0-7 U/mL); β2-glycoprotein I IgG antibodies were elevated at 37 mg/dL (reference range, 0-25.0 mg/dL) and β2-glycoprotein I IgA antibodies were elevated at 5 mg/dL (reference range, 0-4.0 mg/dL).
The clinical suspicion of a thrombotic event on the dorsal feet, which was confirmed histologically, and the persistently positive antiphospholipid (aPL) antibody titers helped to establish the diagnosis of antiphospholipid syndrome (APS) in the setting of RA. The dose of prednisone was increased from 10 mg daily on admission to 40 mg daily. The patient was started on enoxaparin 60 mg subcutaneously twice daily at initial presentation and was bridged to oral warfarin 2 mg daily after the diagnosis of APS was established. Oral doxycycline 100 mg twice daily was started for wound infection. The ulcerations gradually improved over the course of her 7-day hospitalization. She was continued on prednisone, hydroxychloroquine, and warfarin as an outpatient and has had no recurrence of lesions after 3 years of follow-up on this regimen.
Comment
Antiphospholipid syndrome is an autoimmune condition defined by a venous and/or arterial thrombotic event and/or pregnancy morbidity in the presence of persistently elevated aPL antibody titers. The most frequently detected subgroups of aPL are anticardiolipin (aCL) antibodies, anti-β2-glycoprotein I antibodies, and lupus anticoagulants.1 Primary APS occurs as an isolated entity, whereas secondary APS occurs in the setting of a preexisting autoimmune disease, infection, malignancy, or medication.2 The diagnostic criteria for APS requires positive aPL titers at least 12 weeks apart and a clinically confirmed thrombotic event or pregnancy morbidity.3
About one-third to half of patients with APS exhibit cutaneous manifestations.4,5 Livedo reticularis is most commonly observed and represents the first clinical sign of APS in 17.5% of cases.6 Cutaneous findings of APS also include anetoderma, cutaneous ulceration and necrosis, necrotizing vasculitis, livedoid vasculitis, thrombophlebitis, purpura, ecchymoses, painful skin nodules, and subungual hemorrhages.7 The various cutaneous manifestations of APS are associated with a range of histopathologic findings, but noninflammatory thrombosis in small arteries and/or veins in the dermis and subcutaneous fat tissue is the most common histologic feature.4 Our patient exhibited cutaneous ulceration and necrosis, and biopsy clearly showed the presence of vasculitis and fibrin thrombi within postcapillary venules. These findings along with the persistently elevated β2-glycoprotein I IgA solidified the diagnosis of APS.
The most common cutaneous manifestations of RA are nodules (32%), Raynaud phenomenon (10%), and vasculitis (3%).8 The mean prevalence of aPL antibodies in patients with RA is 28%, though reports range from 5% to 75%.1 The presence of aPL or aCL does not predict the development of thrombosis and/or thrombocytopenia in RA patients9,10; however, aCL antibodies in RA patients are associated with a higher risk for developing rheumatoid nodules. It is hypothesized that the majority of aCL antibodies identified in RA patients have different specificities than those identified in other diseases that are associated with thrombotic events.1
Anticoagulation has been proven to decrease the risk for recurrent thrombotic events in patients with APS.11 Patients should discontinue the use of estrogen-containing oral contraceptives; avoid smoking cigarettes; and treat hypertension, hyperlipidemia, and diabetes mellitus, if present. The type and duration of anticoagulation therapy, especially for the treatment of the cutaneous manifestations of APS, is less well defined. Antiplatelet therapies such as low-dose aspirin or dipyridamole often are used for less severe cutaneous manifestations such as livedoid vasculopathy. Warfarin with a target international normalized ratio of 2.0 to 3.0 is most commonly used following major thrombotic events, including cutaneous necrosis and digital gangrene. The role of corticosteroids and immunosuppressants is unclear; one study showed that these therapies did not prevent further thrombotic events in patients with systemic lupus erythematosus.4
Conclusion
Although aPL antibodies are most prevalent in patients with systemic lupus erythematosus, an estimated 28% of patients with RA have elevated aPL titers. The aPL antibodies recognized in RA patients are thought to have a different specificity than those recognized in other APS-associated diseases because elevated aPL antibody titers are not associated with an increased incidence of thrombotic events in RA patients; however, larger studies are needed to clarify this phenomenon. It remains to be determined if this case of APS and RA represents a coincidence or a true disease association, but the recognition of the cutaneous and histological features of APS is crucial for establishing a diagnosis and initiating anticoagulation therapy to prevent further morbidity and mortality.
- Olech E, Merrill JT. The prevalence and clinical significance of antiphospholipid antibodies in rheumatoid arthritis. Curr Rheumatol Rep. 2006;8:100-108.
- Thornsberry LA, LoSicco KI, English JC. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450-462.
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306.
- Asherson A, Francès C, Iaccarino FL, et al. Theantiphospholipid antibody syndrome: diagnosis, skin manifestations and current therapy. Clin Exp Rheumatol. 2006;24(1 suppl 40):S46-S51.
- Cervera R, Piette JC, Font J, et al; Euro-Phospholipid Project Group. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002;46:1019-1027.
- Francès C, Niang S, Laffitte E, et al. Dermatologic manifestations of antiphospholipid syndrome. two hundred consecutive cases. Arthritis Rheum. 2005;52:1785-1793.
- Gibson GE, Su WP, Pittelkow MR. Antiphospholipid syndrome and the skin. J Am Acad Dermatol. 1997;36(6, pt 1):970-982.
- Young A. Extra-articular manifestations and complications of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21:907-927.
- Palomo I, Pinochet C, Alarcón M, et al. Prevalence of antiphospholipid antibodies in Chilean patients with rheumatoid arthritis. J Clin Lab Anal. 2006;20:190-194.
- Wolf P, Gretler J, Aglas F, et al. Anticardiolipin antibodies in rheumatoid arthritis: their relation to rheumatoid nodules and cutaneous vascular manifestations. Br J Dermatol. 1994;131:48-51.
- Lim W, Crowther MA, Eikelboom JW. Management of antiphospholipid antibody syndrome: a systematic review. JAMA. 2006;295:1050-1057.
- Olech E, Merrill JT. The prevalence and clinical significance of antiphospholipid antibodies in rheumatoid arthritis. Curr Rheumatol Rep. 2006;8:100-108.
- Thornsberry LA, LoSicco KI, English JC. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450-462.
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306.
- Asherson A, Francès C, Iaccarino FL, et al. Theantiphospholipid antibody syndrome: diagnosis, skin manifestations and current therapy. Clin Exp Rheumatol. 2006;24(1 suppl 40):S46-S51.
- Cervera R, Piette JC, Font J, et al; Euro-Phospholipid Project Group. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002;46:1019-1027.
- Francès C, Niang S, Laffitte E, et al. Dermatologic manifestations of antiphospholipid syndrome. two hundred consecutive cases. Arthritis Rheum. 2005;52:1785-1793.
- Gibson GE, Su WP, Pittelkow MR. Antiphospholipid syndrome and the skin. J Am Acad Dermatol. 1997;36(6, pt 1):970-982.
- Young A. Extra-articular manifestations and complications of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21:907-927.
- Palomo I, Pinochet C, Alarcón M, et al. Prevalence of antiphospholipid antibodies in Chilean patients with rheumatoid arthritis. J Clin Lab Anal. 2006;20:190-194.
- Wolf P, Gretler J, Aglas F, et al. Anticardiolipin antibodies in rheumatoid arthritis: their relation to rheumatoid nodules and cutaneous vascular manifestations. Br J Dermatol. 1994;131:48-51.
- Lim W, Crowther MA, Eikelboom JW. Management of antiphospholipid antibody syndrome: a systematic review. JAMA. 2006;295:1050-1057.
Practice Points
- Antiphospholipid syndrome (APS) is an autoimmune condition defined by a venous and/or arterial thrombotic event and/or pregnancy morbidity in the presence of persistently elevated antiphospholipid antibody titers.
- Cutaneous findings of APS include livedo reticularis most commonly but also anetoderma, cutaneous ulceration and necrosis, necrotizing vasculitis, livedoid vasculitis, thrombophlebitis, purpura, ecchymoses, painful skin nodules, and subungual hemorrhages.
- The various cutaneous manifestations of APS are associated with a range of histopathologic findings, but noninflammatory thrombosis in small arteries and/or veins in the dermis and subcutaneous fat tissue is the most common histologic feature.
Etanercept found not optimal for reducing anterior uveitis in ankylosing spondylitis
Two anti–tumor necrosis factor monoclonal antibodies, adalimumab and infliximab, showed evidence of being markedly more effective than the anti-TNF–receptor inhibitor etanercept at reducing the rate of anterior uveitis in patients with ankylosing spondylitis in a retrospective Swedish cohort study.
To compare the efficacy of the three TNF inhibitors, researchers analyzed data in nationwide Swedish population-based registries for 1,365 ankylosing spondylitis (AS) patients who initiated treatment during a 7-year period. Treatment began with adalimumab in 406 patients, infliximab in 605, and etanercept in 354, said Elisabeth Lie, MD, of the department of rheumatology and inflammation research at the University of Gothenburg (Sweden), and her associates.
“Compared with the rates [of anterior uveitis] pretreatment, the rates increased when initiating treatment with etanercept, but decreased when starting adalimumab or infliximab,” the investigators wrote (Ann Rheum Dis. 2017 Mar 2. doi: 10.1136/annrheumdis-2016-210931).
The biological explanation for this discrepancy is unclear. It is possible that etanercept simply isn’t as protective as the other two agents, but it also appears possible that etanercept may act paradoxically to induce anterior uveitis in some patients. However, it should be noted that “previous studies have indicated that etanercept still reduces the number of uveitis flares more effectively than placebo,” Dr. Lie and her associates noted.
Regardless of the underlying reason, these findings, taken together with those of previous studies, “support the choice of another TNF inhibitor than etanercept in patients with AS with a history of anterior uveitis,” they said.
Dr. Lie also reported the results at the 2015 American College of Rheumatology annual meeting.
This study was supported by the Swedish Research Council, Gothenburg University, the Stockholm County Council, the Swedish National Rheumatism Association, the Swedish COMBINE public-private research program, the Swedish Cancer Society, the EU-IMI BT Cure project, and the Swedish Foundation for Strategic Research. Dr. Lie reported receiving personal fees from AbbVie, Bristol-Myers Squibb, Hospira, Pfizer, and UCB; her associates reported ties to numerous industry sources.
Two anti–tumor necrosis factor monoclonal antibodies, adalimumab and infliximab, showed evidence of being markedly more effective than the anti-TNF–receptor inhibitor etanercept at reducing the rate of anterior uveitis in patients with ankylosing spondylitis in a retrospective Swedish cohort study.
To compare the efficacy of the three TNF inhibitors, researchers analyzed data in nationwide Swedish population-based registries for 1,365 ankylosing spondylitis (AS) patients who initiated treatment during a 7-year period. Treatment began with adalimumab in 406 patients, infliximab in 605, and etanercept in 354, said Elisabeth Lie, MD, of the department of rheumatology and inflammation research at the University of Gothenburg (Sweden), and her associates.
“Compared with the rates [of anterior uveitis] pretreatment, the rates increased when initiating treatment with etanercept, but decreased when starting adalimumab or infliximab,” the investigators wrote (Ann Rheum Dis. 2017 Mar 2. doi: 10.1136/annrheumdis-2016-210931).
The biological explanation for this discrepancy is unclear. It is possible that etanercept simply isn’t as protective as the other two agents, but it also appears possible that etanercept may act paradoxically to induce anterior uveitis in some patients. However, it should be noted that “previous studies have indicated that etanercept still reduces the number of uveitis flares more effectively than placebo,” Dr. Lie and her associates noted.
Regardless of the underlying reason, these findings, taken together with those of previous studies, “support the choice of another TNF inhibitor than etanercept in patients with AS with a history of anterior uveitis,” they said.
Dr. Lie also reported the results at the 2015 American College of Rheumatology annual meeting.
This study was supported by the Swedish Research Council, Gothenburg University, the Stockholm County Council, the Swedish National Rheumatism Association, the Swedish COMBINE public-private research program, the Swedish Cancer Society, the EU-IMI BT Cure project, and the Swedish Foundation for Strategic Research. Dr. Lie reported receiving personal fees from AbbVie, Bristol-Myers Squibb, Hospira, Pfizer, and UCB; her associates reported ties to numerous industry sources.
Two anti–tumor necrosis factor monoclonal antibodies, adalimumab and infliximab, showed evidence of being markedly more effective than the anti-TNF–receptor inhibitor etanercept at reducing the rate of anterior uveitis in patients with ankylosing spondylitis in a retrospective Swedish cohort study.
To compare the efficacy of the three TNF inhibitors, researchers analyzed data in nationwide Swedish population-based registries for 1,365 ankylosing spondylitis (AS) patients who initiated treatment during a 7-year period. Treatment began with adalimumab in 406 patients, infliximab in 605, and etanercept in 354, said Elisabeth Lie, MD, of the department of rheumatology and inflammation research at the University of Gothenburg (Sweden), and her associates.
“Compared with the rates [of anterior uveitis] pretreatment, the rates increased when initiating treatment with etanercept, but decreased when starting adalimumab or infliximab,” the investigators wrote (Ann Rheum Dis. 2017 Mar 2. doi: 10.1136/annrheumdis-2016-210931).
The biological explanation for this discrepancy is unclear. It is possible that etanercept simply isn’t as protective as the other two agents, but it also appears possible that etanercept may act paradoxically to induce anterior uveitis in some patients. However, it should be noted that “previous studies have indicated that etanercept still reduces the number of uveitis flares more effectively than placebo,” Dr. Lie and her associates noted.
Regardless of the underlying reason, these findings, taken together with those of previous studies, “support the choice of another TNF inhibitor than etanercept in patients with AS with a history of anterior uveitis,” they said.
Dr. Lie also reported the results at the 2015 American College of Rheumatology annual meeting.
This study was supported by the Swedish Research Council, Gothenburg University, the Stockholm County Council, the Swedish National Rheumatism Association, the Swedish COMBINE public-private research program, the Swedish Cancer Society, the EU-IMI BT Cure project, and the Swedish Foundation for Strategic Research. Dr. Lie reported receiving personal fees from AbbVie, Bristol-Myers Squibb, Hospira, Pfizer, and UCB; her associates reported ties to numerous industry sources.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: Etanercept was associated with nearly a fourfold higher risk of developing uveitis than was adalimumab (HR, 3.86) and a twofold higher risk than was infliximab (HR, 1.99), but there was no difference in risk between adalimumab and infliximab.
Data source: A retrospective cohort study involving 1,365 AS patients enrolled in nationwide Swedish registries during a 7-year period.
Disclosures: This study was supported by the Swedish Research Council, Gothenburg University, the Stockholm County Council, the Swedish National Rheumatism Association, the Swedish COMBINE public-private research program, the Swedish Cancer Society, the EU-IMI BT Cure project, and the Swedish Foundation for Strategic Research. Dr. Lie reported receiving personal fees from AbbVie, Bristol-Myers Squibb, Hospira, Pfizer, and UCB; her associates reported ties to numerous industry sources.
VIDEO: Postop troponin T spike flags high mortality risk
WASHINGTON – A rise in the blood level of troponin T immediately after patients underwent noncardiac surgery identified a high risk group with a 30-day mortality rate nearly fivefold higher than that of patients who did not have a postoperative troponin T spike, according to a prospective study of more than 21,000 patients.
For 93% of the patients who have these perioperative spikes in troponin T, a marker of myocardial ischemia, the increased level was the only indicator of a heart problem, P.J. Devereaux, MD, said at the annual meeting of the American College of Cardiology. The painkillers that patients receive following surgery generally mask the chest discomfort they might otherwise feel from their heart damage, he explained. The clinical condition is called myocardial injury after noncardiac surgery.
“We’re seeing more older patients with a high burden of vascular disease undergoing surgery, and surgery is a very significant stress, so a large proportion of these patients will have [myocardial injury after noncardiac surgery] and that affects 30-day survival,” said Dr. Devereaux, professor and director of cardiology at McMaster University in Hamilton, Ont.
Based on the new findings, he recommended performing a baseline assessment of troponin T levels in patients scheduled for noncardiac surgery if they are at least 65 years of age, or if they are age 45-64 years with known vascular disease, followed by repeat testing 1 and 2 days after surgery to check whether a spike in the measure had occurred. The high sensitivity troponin T (hsTnT) test he used in the study is relatively costly (and received Food and Drug Administration approval for U.S. marketing in January 2017), but Dr. Devereaux believed that, used in this way, the cost for testing would be reasonable, given its powerful ability to identify high-risk patients and relative to the cost of other screening tools routinely used in U.S. medical practice.
“It looks very cost-effective,” he said in a video interview.
If the baseline and two follow-up measures of hsTnT showed a postoperative level of at least 20 ng/L that rose above the baseline level by at least 5 ng/L, or if the postoperative level was at least 65 ng/L, Dr. Devereaux recommended starting daily treatment with aspirin and a statin to try to contain any perioperative myocardial damage the patient may have, and follow with comprehensive assessment of the patient by a cardiologist or other internal medicine physician.
“Given the risks associated with a rise in hsTnT in this study, Dr. Devereaux’s recommendations are very reasonable until we collect more data on this,” said Frank W. Sellke, MD, professor of surgery and chief of cardiothoracic surgery at Brown Medical School and the Lifespan Hospitals in Providence, R.I. “What was surprising was how few patients had symptoms” of myocardial ischemia. “You can’t do hsTnT measurements on every patient who goes in for a hernia operation; it’s not practical. But his findings are fairly compelling, and hopefully the cost of this testing will come down,” Dr. Sellke said in an interview.
In the multicenter study of 21,842 patients, 24% had a postoperative hsTnT level of 20 ng/L or greater, including 5% with a level of 65 ng/L or greater. The 30-day mortality rate was 3% among those with a perioperative level of 20-64 ng/L, 9% among patients with perioperative hsTnT levels of 65-999 ng/L, and 30% among the 54 patients (0.2% of the study group) with perioperative levels that reached 1,000 ng/L or greater. Dr. Devereaux reported.
This iteration of the Vascular Events In Noncardiac Surgery Patients Cohort Evaluation Study (VISION) enrolled patients who were at least 45 years of age and underwent noncardiac surgery at 23 centers in 13 countries, including the United States and Canada. All patients underwent hsTnT testing 6-12 hours after surgery and 1, 2, and 3 days after surgery, but only 40% also had a baseline measurement before their surgery began. Full 30-day follow-up occurred for 21,050. The patients’ average age was 63 years. The most common surgery was “low-risk,” in 35%, followed by “major” general surgery in 20%, and “major” orthopedic surgery in 16%. At 30 days, 266 patients (1.2%) had died.
These findings “help define a cutoff for hsTnT that will be clinically useful to change practice,” said Athena Poppas, MD, a cardiologist and director of the Cardiovascular Institute at Rhode Island Hospital in Providence. Dr. Poppas was a designated discussant for Dr. Devereaux’s report at the meeting.
In January 2017, the Canadian Cardiovascular Society issued guidelines for perioperative cardiac risk assessment and management for patients undergoing noncardiac surgery (Can J Cardiol. 2017 Jan;33[1]:17-32). Dr. Devereaux was a member of the writing panel for these guidelines. This is what the guidelines said about using troponin T measurements:
“We recommend obtaining daily troponin measurements for 48-72 hours after noncardiac surgery in patients with a baseline risk greater than 5% for cardiovascular death or nonfatal myocardial infarction at 30 days after surgery (i.e., patients with an elevated NT-proBNP/BNP measurement before surgery or, if there is no NT-proBNP/BNP measurement before surgery, in those who have an RCRI [revised cardiac risk index] score of 1 or greater, age 45-64 years with significant cardiovascular disease, or age 65 years or older).”
The VISION study is sponsored by Roche Diagnostics, which markets the high sensitivity troponin T assay used in the study. Dr. Devereaux has received research funding from Roche Diagnostics and from Abbott Diagnostics and Boehringer Ingelheim. Dr. Sellke and Dr. Poppas had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
WASHINGTON – A rise in the blood level of troponin T immediately after patients underwent noncardiac surgery identified a high risk group with a 30-day mortality rate nearly fivefold higher than that of patients who did not have a postoperative troponin T spike, according to a prospective study of more than 21,000 patients.
For 93% of the patients who have these perioperative spikes in troponin T, a marker of myocardial ischemia, the increased level was the only indicator of a heart problem, P.J. Devereaux, MD, said at the annual meeting of the American College of Cardiology. The painkillers that patients receive following surgery generally mask the chest discomfort they might otherwise feel from their heart damage, he explained. The clinical condition is called myocardial injury after noncardiac surgery.
“We’re seeing more older patients with a high burden of vascular disease undergoing surgery, and surgery is a very significant stress, so a large proportion of these patients will have [myocardial injury after noncardiac surgery] and that affects 30-day survival,” said Dr. Devereaux, professor and director of cardiology at McMaster University in Hamilton, Ont.
Based on the new findings, he recommended performing a baseline assessment of troponin T levels in patients scheduled for noncardiac surgery if they are at least 65 years of age, or if they are age 45-64 years with known vascular disease, followed by repeat testing 1 and 2 days after surgery to check whether a spike in the measure had occurred. The high sensitivity troponin T (hsTnT) test he used in the study is relatively costly (and received Food and Drug Administration approval for U.S. marketing in January 2017), but Dr. Devereaux believed that, used in this way, the cost for testing would be reasonable, given its powerful ability to identify high-risk patients and relative to the cost of other screening tools routinely used in U.S. medical practice.
“It looks very cost-effective,” he said in a video interview.
If the baseline and two follow-up measures of hsTnT showed a postoperative level of at least 20 ng/L that rose above the baseline level by at least 5 ng/L, or if the postoperative level was at least 65 ng/L, Dr. Devereaux recommended starting daily treatment with aspirin and a statin to try to contain any perioperative myocardial damage the patient may have, and follow with comprehensive assessment of the patient by a cardiologist or other internal medicine physician.
“Given the risks associated with a rise in hsTnT in this study, Dr. Devereaux’s recommendations are very reasonable until we collect more data on this,” said Frank W. Sellke, MD, professor of surgery and chief of cardiothoracic surgery at Brown Medical School and the Lifespan Hospitals in Providence, R.I. “What was surprising was how few patients had symptoms” of myocardial ischemia. “You can’t do hsTnT measurements on every patient who goes in for a hernia operation; it’s not practical. But his findings are fairly compelling, and hopefully the cost of this testing will come down,” Dr. Sellke said in an interview.
In the multicenter study of 21,842 patients, 24% had a postoperative hsTnT level of 20 ng/L or greater, including 5% with a level of 65 ng/L or greater. The 30-day mortality rate was 3% among those with a perioperative level of 20-64 ng/L, 9% among patients with perioperative hsTnT levels of 65-999 ng/L, and 30% among the 54 patients (0.2% of the study group) with perioperative levels that reached 1,000 ng/L or greater. Dr. Devereaux reported.
This iteration of the Vascular Events In Noncardiac Surgery Patients Cohort Evaluation Study (VISION) enrolled patients who were at least 45 years of age and underwent noncardiac surgery at 23 centers in 13 countries, including the United States and Canada. All patients underwent hsTnT testing 6-12 hours after surgery and 1, 2, and 3 days after surgery, but only 40% also had a baseline measurement before their surgery began. Full 30-day follow-up occurred for 21,050. The patients’ average age was 63 years. The most common surgery was “low-risk,” in 35%, followed by “major” general surgery in 20%, and “major” orthopedic surgery in 16%. At 30 days, 266 patients (1.2%) had died.
These findings “help define a cutoff for hsTnT that will be clinically useful to change practice,” said Athena Poppas, MD, a cardiologist and director of the Cardiovascular Institute at Rhode Island Hospital in Providence. Dr. Poppas was a designated discussant for Dr. Devereaux’s report at the meeting.
In January 2017, the Canadian Cardiovascular Society issued guidelines for perioperative cardiac risk assessment and management for patients undergoing noncardiac surgery (Can J Cardiol. 2017 Jan;33[1]:17-32). Dr. Devereaux was a member of the writing panel for these guidelines. This is what the guidelines said about using troponin T measurements:
“We recommend obtaining daily troponin measurements for 48-72 hours after noncardiac surgery in patients with a baseline risk greater than 5% for cardiovascular death or nonfatal myocardial infarction at 30 days after surgery (i.e., patients with an elevated NT-proBNP/BNP measurement before surgery or, if there is no NT-proBNP/BNP measurement before surgery, in those who have an RCRI [revised cardiac risk index] score of 1 or greater, age 45-64 years with significant cardiovascular disease, or age 65 years or older).”
The VISION study is sponsored by Roche Diagnostics, which markets the high sensitivity troponin T assay used in the study. Dr. Devereaux has received research funding from Roche Diagnostics and from Abbott Diagnostics and Boehringer Ingelheim. Dr. Sellke and Dr. Poppas had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
WASHINGTON – A rise in the blood level of troponin T immediately after patients underwent noncardiac surgery identified a high risk group with a 30-day mortality rate nearly fivefold higher than that of patients who did not have a postoperative troponin T spike, according to a prospective study of more than 21,000 patients.
For 93% of the patients who have these perioperative spikes in troponin T, a marker of myocardial ischemia, the increased level was the only indicator of a heart problem, P.J. Devereaux, MD, said at the annual meeting of the American College of Cardiology. The painkillers that patients receive following surgery generally mask the chest discomfort they might otherwise feel from their heart damage, he explained. The clinical condition is called myocardial injury after noncardiac surgery.
“We’re seeing more older patients with a high burden of vascular disease undergoing surgery, and surgery is a very significant stress, so a large proportion of these patients will have [myocardial injury after noncardiac surgery] and that affects 30-day survival,” said Dr. Devereaux, professor and director of cardiology at McMaster University in Hamilton, Ont.
Based on the new findings, he recommended performing a baseline assessment of troponin T levels in patients scheduled for noncardiac surgery if they are at least 65 years of age, or if they are age 45-64 years with known vascular disease, followed by repeat testing 1 and 2 days after surgery to check whether a spike in the measure had occurred. The high sensitivity troponin T (hsTnT) test he used in the study is relatively costly (and received Food and Drug Administration approval for U.S. marketing in January 2017), but Dr. Devereaux believed that, used in this way, the cost for testing would be reasonable, given its powerful ability to identify high-risk patients and relative to the cost of other screening tools routinely used in U.S. medical practice.
“It looks very cost-effective,” he said in a video interview.
If the baseline and two follow-up measures of hsTnT showed a postoperative level of at least 20 ng/L that rose above the baseline level by at least 5 ng/L, or if the postoperative level was at least 65 ng/L, Dr. Devereaux recommended starting daily treatment with aspirin and a statin to try to contain any perioperative myocardial damage the patient may have, and follow with comprehensive assessment of the patient by a cardiologist or other internal medicine physician.
“Given the risks associated with a rise in hsTnT in this study, Dr. Devereaux’s recommendations are very reasonable until we collect more data on this,” said Frank W. Sellke, MD, professor of surgery and chief of cardiothoracic surgery at Brown Medical School and the Lifespan Hospitals in Providence, R.I. “What was surprising was how few patients had symptoms” of myocardial ischemia. “You can’t do hsTnT measurements on every patient who goes in for a hernia operation; it’s not practical. But his findings are fairly compelling, and hopefully the cost of this testing will come down,” Dr. Sellke said in an interview.
In the multicenter study of 21,842 patients, 24% had a postoperative hsTnT level of 20 ng/L or greater, including 5% with a level of 65 ng/L or greater. The 30-day mortality rate was 3% among those with a perioperative level of 20-64 ng/L, 9% among patients with perioperative hsTnT levels of 65-999 ng/L, and 30% among the 54 patients (0.2% of the study group) with perioperative levels that reached 1,000 ng/L or greater. Dr. Devereaux reported.
This iteration of the Vascular Events In Noncardiac Surgery Patients Cohort Evaluation Study (VISION) enrolled patients who were at least 45 years of age and underwent noncardiac surgery at 23 centers in 13 countries, including the United States and Canada. All patients underwent hsTnT testing 6-12 hours after surgery and 1, 2, and 3 days after surgery, but only 40% also had a baseline measurement before their surgery began. Full 30-day follow-up occurred for 21,050. The patients’ average age was 63 years. The most common surgery was “low-risk,” in 35%, followed by “major” general surgery in 20%, and “major” orthopedic surgery in 16%. At 30 days, 266 patients (1.2%) had died.
These findings “help define a cutoff for hsTnT that will be clinically useful to change practice,” said Athena Poppas, MD, a cardiologist and director of the Cardiovascular Institute at Rhode Island Hospital in Providence. Dr. Poppas was a designated discussant for Dr. Devereaux’s report at the meeting.
In January 2017, the Canadian Cardiovascular Society issued guidelines for perioperative cardiac risk assessment and management for patients undergoing noncardiac surgery (Can J Cardiol. 2017 Jan;33[1]:17-32). Dr. Devereaux was a member of the writing panel for these guidelines. This is what the guidelines said about using troponin T measurements:
“We recommend obtaining daily troponin measurements for 48-72 hours after noncardiac surgery in patients with a baseline risk greater than 5% for cardiovascular death or nonfatal myocardial infarction at 30 days after surgery (i.e., patients with an elevated NT-proBNP/BNP measurement before surgery or, if there is no NT-proBNP/BNP measurement before surgery, in those who have an RCRI [revised cardiac risk index] score of 1 or greater, age 45-64 years with significant cardiovascular disease, or age 65 years or older).”
The VISION study is sponsored by Roche Diagnostics, which markets the high sensitivity troponin T assay used in the study. Dr. Devereaux has received research funding from Roche Diagnostics and from Abbott Diagnostics and Boehringer Ingelheim. Dr. Sellke and Dr. Poppas had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
AT ACC 17
Key clinical point:
Major finding: A postoperative high sensitivity troponin T rise of 5 ng/L or more linked with a 4.7-fold increase in 30-day mortality.
Data source: VISION, a prospective, multicenter observational study of 21,842 patients undergoing noncardiac surgery.
Disclosures: The VISION study is sponsored by Roche Diagnostics, which markets the high sensitivity troponin T assay used in the study. Dr. Devereaux has received research funding from Roche Diagnostics and from Abbott Diagnostics and Boehringer Ingelheim.
Breast cancer info on centers’ sites leaves room for improvement
ORLANDO – Women with breast cancer who look for well-rounded information about treatment on the websites of prominent U.S. cancer centers are likely to come up short, suggests a study presented at a symposium on quality care sponsored by the American Society of Clinical Oncology.
Research has shown that nearly all women with breast cancer search the Internet for information about its treatment, and two-thirds report that what they find has a strong influence on their decision-making process (J Cancer Educ. 2013;28[4]:662-8).
But the analysis of content on the websites of 63 National Cancer Institute–designated comprehensive cancer centers or clinical cancer centers found that, on average, they addressed only 21% of a set of key concepts that women need to understand to make informed decisions about surgery, radiation therapy, chemotherapy, hormone therapy, and breast reconstruction. This contrasted starkly with 85% for the National Cancer Institute’s own website (cancer.gov) and 88% for the Susan G. Komen Foundation’s website (komen.org).
“These are websites that we think are reliable, cancer center websites, and these are the most prominent cancer centers in the country,” first author Caleb Dulaney, MD, a resident in radiation oncology at the University of Alabama at Birmingham, said in an interview. “This is where a lot of people receive their care, so they should be very reliable as to the information they provide.
“A lot of websites just had information from the NCI basically integrated into their website or a link to the NCI website,” he acknowledged. “Is it really the goal of the cancer center’s website to provide information? It may not be. But you have to take responsibility for being a trusted source of information. So if you are not going to provide it, you should at least direct people to very accurate, reliable information, and it can also kind of inform what you talk about in clinic.”
All of the investigators evaluating sites in the study were medical professionals, so the team has initiated a new study in which patients will instead perform the evaluations.
“We found that for a few websites, one person found a lot of information and another found no information. So the information may technically be there, but is it transmitted to the patient? Can they find it, and do they understand it?” Dr. Dulaney said. “So it will be interesting when we use patients to evaluate these websites. I’ll be curious to see how many questions they are able to find answers to.”
Study details
For the study, the investigators developed a list of 33 decision-specific knowledge questions about breast cancer treatment by drawing on decision quality instruments that assess how informed a woman’s decision-making process is. The primary outcome was whether the website provided sufficient information to answer each question. The researchers assessed seven measures of accessibility as secondary outcomes.
Results showed that websites contained sufficient content to address only 21% of the decision-specific knowledge questions, Dr. Dulaney reported in a poster session. The value was 17% for questions pertaining to breast surgery and radiation therapy, 18% for those pertaining to chemotherapy and hormone therapy, and 21% for those pertaining to breast reconstruction.
In addition, “a lot of websites put the information in silos,” he noted. “You can read about mastectomy, you can read about lumpectomy, you can read about chemo. But you can’t really get the big picture, which is how do these compare to each other, and which treatment is best for me.”
Even the most commonly addressed single question – what type of reconstruction is most likely to require more than one surgery or procedure – was addressed by only 51% of sites. Proportions were similar for questions pertaining to the type of tumors against which hormone therapy works best (48%) and the schedule for radiation therapy after lumpectomy (47%).
At the other extreme, however, very small proportions of sites addressed questions pertaining to how many women with treated early breast cancer will die from the disease (7%), how many undergoing breast reconstruction will experience complications requiring hospitalization or an unplanned procedure (4%), how skipping chemotherapy and hormone therapy influences risk of death (2%), and whether waiting several weeks to decide about those therapies affects survival (2%). These topics are more negative, Dr. Dulaney observed, “but these are things women need to know.”
None of the websites provided sufficient information to answer all 33 knowledge questions. But perhaps more worrisome, 16% did not provide sufficient information to answer any of them, he said.
When it came to accessibility of information, 94% of sites clearly had a breast cancer–specific page, 87% had information about breast cancer–specific trials, and 86% showed members of the center’s breast cancer team. But only 59% were mobile device friendly as assessed with a Google tool, and merely 24% had obvious links to view information in Spanish.
“A lot of minorities and people of lower socioeconomic status exclusively access the Internet via mobile devices, so they may not have a computer or [other] access to the Internet. But they have a cell phone that is probably a smartphone, and they can get online and search for information that way,” Dr. Dulaney said.
Many oncologists may not have had any say regarding the content and accessibility features of their institution’s website, he acknowledged.
“So we should maybe, number one, try to have more involvement in what information goes on to the website, and two, take a look at our own websites to see what’s on there, because patients are going to look for you, and they are going to associate this information with you,” he said. “If you are at a big institution and you really can’t make a change on your website, you can use alternatives such as social media platforms, things like that, to try and get information out to people.”
From a larger perspective, oncologists have often simply counseled patients that they can’t rely on information they have found online, according to Dr. Dulaney.
“But in this day and age, that can’t really be an answer,” he concluded. “Information on the web is ubiquitous, and there is good information out there. We need to do a better job of speaking up in the conversation. We have the answers to a lot of these questions, we just need to make our voices heard and also direct patients to reliable sources of information.”
ORLANDO – Women with breast cancer who look for well-rounded information about treatment on the websites of prominent U.S. cancer centers are likely to come up short, suggests a study presented at a symposium on quality care sponsored by the American Society of Clinical Oncology.
Research has shown that nearly all women with breast cancer search the Internet for information about its treatment, and two-thirds report that what they find has a strong influence on their decision-making process (J Cancer Educ. 2013;28[4]:662-8).
But the analysis of content on the websites of 63 National Cancer Institute–designated comprehensive cancer centers or clinical cancer centers found that, on average, they addressed only 21% of a set of key concepts that women need to understand to make informed decisions about surgery, radiation therapy, chemotherapy, hormone therapy, and breast reconstruction. This contrasted starkly with 85% for the National Cancer Institute’s own website (cancer.gov) and 88% for the Susan G. Komen Foundation’s website (komen.org).
“These are websites that we think are reliable, cancer center websites, and these are the most prominent cancer centers in the country,” first author Caleb Dulaney, MD, a resident in radiation oncology at the University of Alabama at Birmingham, said in an interview. “This is where a lot of people receive their care, so they should be very reliable as to the information they provide.
“A lot of websites just had information from the NCI basically integrated into their website or a link to the NCI website,” he acknowledged. “Is it really the goal of the cancer center’s website to provide information? It may not be. But you have to take responsibility for being a trusted source of information. So if you are not going to provide it, you should at least direct people to very accurate, reliable information, and it can also kind of inform what you talk about in clinic.”
All of the investigators evaluating sites in the study were medical professionals, so the team has initiated a new study in which patients will instead perform the evaluations.
“We found that for a few websites, one person found a lot of information and another found no information. So the information may technically be there, but is it transmitted to the patient? Can they find it, and do they understand it?” Dr. Dulaney said. “So it will be interesting when we use patients to evaluate these websites. I’ll be curious to see how many questions they are able to find answers to.”
Study details
For the study, the investigators developed a list of 33 decision-specific knowledge questions about breast cancer treatment by drawing on decision quality instruments that assess how informed a woman’s decision-making process is. The primary outcome was whether the website provided sufficient information to answer each question. The researchers assessed seven measures of accessibility as secondary outcomes.
Results showed that websites contained sufficient content to address only 21% of the decision-specific knowledge questions, Dr. Dulaney reported in a poster session. The value was 17% for questions pertaining to breast surgery and radiation therapy, 18% for those pertaining to chemotherapy and hormone therapy, and 21% for those pertaining to breast reconstruction.
In addition, “a lot of websites put the information in silos,” he noted. “You can read about mastectomy, you can read about lumpectomy, you can read about chemo. But you can’t really get the big picture, which is how do these compare to each other, and which treatment is best for me.”
Even the most commonly addressed single question – what type of reconstruction is most likely to require more than one surgery or procedure – was addressed by only 51% of sites. Proportions were similar for questions pertaining to the type of tumors against which hormone therapy works best (48%) and the schedule for radiation therapy after lumpectomy (47%).
At the other extreme, however, very small proportions of sites addressed questions pertaining to how many women with treated early breast cancer will die from the disease (7%), how many undergoing breast reconstruction will experience complications requiring hospitalization or an unplanned procedure (4%), how skipping chemotherapy and hormone therapy influences risk of death (2%), and whether waiting several weeks to decide about those therapies affects survival (2%). These topics are more negative, Dr. Dulaney observed, “but these are things women need to know.”
None of the websites provided sufficient information to answer all 33 knowledge questions. But perhaps more worrisome, 16% did not provide sufficient information to answer any of them, he said.
When it came to accessibility of information, 94% of sites clearly had a breast cancer–specific page, 87% had information about breast cancer–specific trials, and 86% showed members of the center’s breast cancer team. But only 59% were mobile device friendly as assessed with a Google tool, and merely 24% had obvious links to view information in Spanish.
“A lot of minorities and people of lower socioeconomic status exclusively access the Internet via mobile devices, so they may not have a computer or [other] access to the Internet. But they have a cell phone that is probably a smartphone, and they can get online and search for information that way,” Dr. Dulaney said.
Many oncologists may not have had any say regarding the content and accessibility features of their institution’s website, he acknowledged.
“So we should maybe, number one, try to have more involvement in what information goes on to the website, and two, take a look at our own websites to see what’s on there, because patients are going to look for you, and they are going to associate this information with you,” he said. “If you are at a big institution and you really can’t make a change on your website, you can use alternatives such as social media platforms, things like that, to try and get information out to people.”
From a larger perspective, oncologists have often simply counseled patients that they can’t rely on information they have found online, according to Dr. Dulaney.
“But in this day and age, that can’t really be an answer,” he concluded. “Information on the web is ubiquitous, and there is good information out there. We need to do a better job of speaking up in the conversation. We have the answers to a lot of these questions, we just need to make our voices heard and also direct patients to reliable sources of information.”
ORLANDO – Women with breast cancer who look for well-rounded information about treatment on the websites of prominent U.S. cancer centers are likely to come up short, suggests a study presented at a symposium on quality care sponsored by the American Society of Clinical Oncology.
Research has shown that nearly all women with breast cancer search the Internet for information about its treatment, and two-thirds report that what they find has a strong influence on their decision-making process (J Cancer Educ. 2013;28[4]:662-8).
But the analysis of content on the websites of 63 National Cancer Institute–designated comprehensive cancer centers or clinical cancer centers found that, on average, they addressed only 21% of a set of key concepts that women need to understand to make informed decisions about surgery, radiation therapy, chemotherapy, hormone therapy, and breast reconstruction. This contrasted starkly with 85% for the National Cancer Institute’s own website (cancer.gov) and 88% for the Susan G. Komen Foundation’s website (komen.org).
“These are websites that we think are reliable, cancer center websites, and these are the most prominent cancer centers in the country,” first author Caleb Dulaney, MD, a resident in radiation oncology at the University of Alabama at Birmingham, said in an interview. “This is where a lot of people receive their care, so they should be very reliable as to the information they provide.
“A lot of websites just had information from the NCI basically integrated into their website or a link to the NCI website,” he acknowledged. “Is it really the goal of the cancer center’s website to provide information? It may not be. But you have to take responsibility for being a trusted source of information. So if you are not going to provide it, you should at least direct people to very accurate, reliable information, and it can also kind of inform what you talk about in clinic.”
All of the investigators evaluating sites in the study were medical professionals, so the team has initiated a new study in which patients will instead perform the evaluations.
“We found that for a few websites, one person found a lot of information and another found no information. So the information may technically be there, but is it transmitted to the patient? Can they find it, and do they understand it?” Dr. Dulaney said. “So it will be interesting when we use patients to evaluate these websites. I’ll be curious to see how many questions they are able to find answers to.”
Study details
For the study, the investigators developed a list of 33 decision-specific knowledge questions about breast cancer treatment by drawing on decision quality instruments that assess how informed a woman’s decision-making process is. The primary outcome was whether the website provided sufficient information to answer each question. The researchers assessed seven measures of accessibility as secondary outcomes.
Results showed that websites contained sufficient content to address only 21% of the decision-specific knowledge questions, Dr. Dulaney reported in a poster session. The value was 17% for questions pertaining to breast surgery and radiation therapy, 18% for those pertaining to chemotherapy and hormone therapy, and 21% for those pertaining to breast reconstruction.
In addition, “a lot of websites put the information in silos,” he noted. “You can read about mastectomy, you can read about lumpectomy, you can read about chemo. But you can’t really get the big picture, which is how do these compare to each other, and which treatment is best for me.”
Even the most commonly addressed single question – what type of reconstruction is most likely to require more than one surgery or procedure – was addressed by only 51% of sites. Proportions were similar for questions pertaining to the type of tumors against which hormone therapy works best (48%) and the schedule for radiation therapy after lumpectomy (47%).
At the other extreme, however, very small proportions of sites addressed questions pertaining to how many women with treated early breast cancer will die from the disease (7%), how many undergoing breast reconstruction will experience complications requiring hospitalization or an unplanned procedure (4%), how skipping chemotherapy and hormone therapy influences risk of death (2%), and whether waiting several weeks to decide about those therapies affects survival (2%). These topics are more negative, Dr. Dulaney observed, “but these are things women need to know.”
None of the websites provided sufficient information to answer all 33 knowledge questions. But perhaps more worrisome, 16% did not provide sufficient information to answer any of them, he said.
When it came to accessibility of information, 94% of sites clearly had a breast cancer–specific page, 87% had information about breast cancer–specific trials, and 86% showed members of the center’s breast cancer team. But only 59% were mobile device friendly as assessed with a Google tool, and merely 24% had obvious links to view information in Spanish.
“A lot of minorities and people of lower socioeconomic status exclusively access the Internet via mobile devices, so they may not have a computer or [other] access to the Internet. But they have a cell phone that is probably a smartphone, and they can get online and search for information that way,” Dr. Dulaney said.
Many oncologists may not have had any say regarding the content and accessibility features of their institution’s website, he acknowledged.
“So we should maybe, number one, try to have more involvement in what information goes on to the website, and two, take a look at our own websites to see what’s on there, because patients are going to look for you, and they are going to associate this information with you,” he said. “If you are at a big institution and you really can’t make a change on your website, you can use alternatives such as social media platforms, things like that, to try and get information out to people.”
From a larger perspective, oncologists have often simply counseled patients that they can’t rely on information they have found online, according to Dr. Dulaney.
“But in this day and age, that can’t really be an answer,” he concluded. “Information on the web is ubiquitous, and there is good information out there. We need to do a better job of speaking up in the conversation. We have the answers to a lot of these questions, we just need to make our voices heard and also direct patients to reliable sources of information.”
AT THE QUALITY CARE SYMPOSIUM
Key clinical point:
Major finding: On average, the sites addressed 21% of 33 key concepts needed to make informed decisions about treatment.
Data source: An analysis of breast cancer information on the websites of 63 NCI-designated comprehensive cancer centers or clinical cancer centers.
Disclosures: Dr. Dulaney disclosed that he had no relevant conflicts of interest.
Shingles vaccine deemed effective in people with autoimmune disease
The herpes zoster vaccine reduces the risk of shingles in older adults with autoimmune disease, even if they are taking immunosuppressants for their condition, but the protection begins to wane after about 5 years, a recent retrospective study found.
“There has been some concern that patients with autoimmune conditions might have a lower immunogenic response to herpes zoster vaccination, especially when treated with immunosuppressive medications such as glucocorticoids,” wrote Huifeng Yun, PhD, of the University of Alabama at Birmingham, and her colleagues.
The researchers used 2006-2013 Medicare data to calculate the risk of shingles among Medicare recipients who had an autoimmune disease and either did or did not receive the herpes zoster vaccine. All the patients had been enrolled in Medicare for at least 12 continuous months and had a diagnosis of ankylosing spondylitis, inflammatory bowel disease, psoriasis, psoriatic arthritis, or rheumatoid arthritis.
The researchers matched 59,627 patients who received the herpes zoster vaccine with 119,254 unvaccinated patients, based on age, sex, race, calendar year, autoimmune disease type, and use of autoimmune drugs (biologics, disease-modifying antirheumatic drugs, and glucocorticoids). During a follow-up of up to 7 years, the researchers additionally accounted for comorbid medical conditions and concurrent medications each year.
The cohort, with an average age of 73.5 years in both groups, included 53.1% of adults with rheumatoid arthritis, 31.6% with psoriasis, 20.9% with inflammatory bowel disease, 4.7% with psoriatic arthritis, and 1.4% with ankylosing spondylitis.
Those who received the vaccine had a rate of 0.75 herpes zoster cases per 100 people during the first year, which rose to 1.25 cases per 100 people per year at the seventh year after vaccination. The rate among unvaccinated individuals stayed steady at approximately 1.3-1.7 cases per 100 people per year throughout the study period. These rates, as expected, were approximately 50% higher than in the general population over age 70 without autoimmune disease.
Compared with unvaccinated individuals, vaccinated individuals had a reduced relative risk for shingles of 0.74-0.77 after adjustment for confounders, but the risk reduction only remained statistically significant for the first 5 years after vaccination.
The waning seen with the vaccine’s effectiveness “raises the possibility that patients might benefit from a booster vaccine at some point after initial vaccination, although no recommendation currently exists that would support such a practice,” the authors wrote.
Dr. Yun has received research funding from Amgen. Other authors disclosed ties to Amgen, AstraZeneca, Bristol-Myers Squibb, Crescendo Bioscience, Janssen, and Pfizer. One author has received research support and consulting fees from Corrona. The study did not note an external source of funding.
The herpes zoster vaccine reduces the risk of shingles in older adults with autoimmune disease, even if they are taking immunosuppressants for their condition, but the protection begins to wane after about 5 years, a recent retrospective study found.
“There has been some concern that patients with autoimmune conditions might have a lower immunogenic response to herpes zoster vaccination, especially when treated with immunosuppressive medications such as glucocorticoids,” wrote Huifeng Yun, PhD, of the University of Alabama at Birmingham, and her colleagues.
The researchers used 2006-2013 Medicare data to calculate the risk of shingles among Medicare recipients who had an autoimmune disease and either did or did not receive the herpes zoster vaccine. All the patients had been enrolled in Medicare for at least 12 continuous months and had a diagnosis of ankylosing spondylitis, inflammatory bowel disease, psoriasis, psoriatic arthritis, or rheumatoid arthritis.
The researchers matched 59,627 patients who received the herpes zoster vaccine with 119,254 unvaccinated patients, based on age, sex, race, calendar year, autoimmune disease type, and use of autoimmune drugs (biologics, disease-modifying antirheumatic drugs, and glucocorticoids). During a follow-up of up to 7 years, the researchers additionally accounted for comorbid medical conditions and concurrent medications each year.
The cohort, with an average age of 73.5 years in both groups, included 53.1% of adults with rheumatoid arthritis, 31.6% with psoriasis, 20.9% with inflammatory bowel disease, 4.7% with psoriatic arthritis, and 1.4% with ankylosing spondylitis.
Those who received the vaccine had a rate of 0.75 herpes zoster cases per 100 people during the first year, which rose to 1.25 cases per 100 people per year at the seventh year after vaccination. The rate among unvaccinated individuals stayed steady at approximately 1.3-1.7 cases per 100 people per year throughout the study period. These rates, as expected, were approximately 50% higher than in the general population over age 70 without autoimmune disease.
Compared with unvaccinated individuals, vaccinated individuals had a reduced relative risk for shingles of 0.74-0.77 after adjustment for confounders, but the risk reduction only remained statistically significant for the first 5 years after vaccination.
The waning seen with the vaccine’s effectiveness “raises the possibility that patients might benefit from a booster vaccine at some point after initial vaccination, although no recommendation currently exists that would support such a practice,” the authors wrote.
Dr. Yun has received research funding from Amgen. Other authors disclosed ties to Amgen, AstraZeneca, Bristol-Myers Squibb, Crescendo Bioscience, Janssen, and Pfizer. One author has received research support and consulting fees from Corrona. The study did not note an external source of funding.
The herpes zoster vaccine reduces the risk of shingles in older adults with autoimmune disease, even if they are taking immunosuppressants for their condition, but the protection begins to wane after about 5 years, a recent retrospective study found.
“There has been some concern that patients with autoimmune conditions might have a lower immunogenic response to herpes zoster vaccination, especially when treated with immunosuppressive medications such as glucocorticoids,” wrote Huifeng Yun, PhD, of the University of Alabama at Birmingham, and her colleagues.
The researchers used 2006-2013 Medicare data to calculate the risk of shingles among Medicare recipients who had an autoimmune disease and either did or did not receive the herpes zoster vaccine. All the patients had been enrolled in Medicare for at least 12 continuous months and had a diagnosis of ankylosing spondylitis, inflammatory bowel disease, psoriasis, psoriatic arthritis, or rheumatoid arthritis.
The researchers matched 59,627 patients who received the herpes zoster vaccine with 119,254 unvaccinated patients, based on age, sex, race, calendar year, autoimmune disease type, and use of autoimmune drugs (biologics, disease-modifying antirheumatic drugs, and glucocorticoids). During a follow-up of up to 7 years, the researchers additionally accounted for comorbid medical conditions and concurrent medications each year.
The cohort, with an average age of 73.5 years in both groups, included 53.1% of adults with rheumatoid arthritis, 31.6% with psoriasis, 20.9% with inflammatory bowel disease, 4.7% with psoriatic arthritis, and 1.4% with ankylosing spondylitis.
Those who received the vaccine had a rate of 0.75 herpes zoster cases per 100 people during the first year, which rose to 1.25 cases per 100 people per year at the seventh year after vaccination. The rate among unvaccinated individuals stayed steady at approximately 1.3-1.7 cases per 100 people per year throughout the study period. These rates, as expected, were approximately 50% higher than in the general population over age 70 without autoimmune disease.
Compared with unvaccinated individuals, vaccinated individuals had a reduced relative risk for shingles of 0.74-0.77 after adjustment for confounders, but the risk reduction only remained statistically significant for the first 5 years after vaccination.
The waning seen with the vaccine’s effectiveness “raises the possibility that patients might benefit from a booster vaccine at some point after initial vaccination, although no recommendation currently exists that would support such a practice,” the authors wrote.
Dr. Yun has received research funding from Amgen. Other authors disclosed ties to Amgen, AstraZeneca, Bristol-Myers Squibb, Crescendo Bioscience, Janssen, and Pfizer. One author has received research support and consulting fees from Corrona. The study did not note an external source of funding.
Key clinical point:
Major finding: Medicare patients with autoimmune disease had a 23%-26% reduced risk of shingles for 5 years after receiving the herpes zoster vaccine.
Data source: The findings are based on analysis of 2006-2013 Medicare data on 59,627 patients who received the herpes zoster vaccine and 119,254 patients who didn’t.
Disclosures: Dr. Yun has received research funding from Amgen. Other authors disclosed ties to Amgen, AstraZeneca, Bristol-Myers Squibb, Crescendo Bioscience, Janssen, and Pfizer. One author has received research support and consulting fees from Corrona. The study did not note an external source of funding.
Trump administration floats 18% budget cut to HHS
The Department of Health & Human Services would see an 18% funding cut under the first budget proposal from the Trump administration.
The proposal, submitted to Congress March 16, would cut $15.1 billion from fiscal 2017 levels, funding the agency at $69 billion for fiscal year 2018. More than a third of the cuts come from the National Institutes of Health.
The NIH’s overall budget would drop to $25.9 billion in FY 2018, down $5.8 billion from this year (fiscal 2017). The proposal includes “a major reorganization of NIH’s institutes and centers to help focus resources on the highest priority research and training activities, including: eliminating the Fogarty International Center, consolidating the Agency for Healthcare Research and Quality within the NIH, and other consolidations and structural changes across NIH organizations and activities,” according to summary documents from the Office of Management and Budget.
The proposed cuts also account for the funds that are to be appropriated for the 21st Century Cures Act, which was supposed to add $4.8 billion in new appropriated funding, including funds dedicated to the Cancer Moonshot and the BRAIN Initiative.
The Centers for Disease Control and Prevention also would be reformed, getting a new $500 million block grant “to increase state flexibility and focus on the leading public health challenges specific to each state.” It also creates a new Federal Emergency Response Fund to respond to public health outbreaks such as the Zika virus.
Another area receiving a boost under the proposal is the funding for the Health Care Fraud and Abuse Control program at the CMS, which would receive $751 million in fiscal 2018, about 10% more than it did in fiscal 2017. The budget document notes that the “return on investment for the HCFAC account was $5 returned for every $1 expended from 2014-2016.”
The five-to-one return on fraud and abuse investigation in this program is controversial, because of the incentives for those conducting the investigations. Health care providers should not fear that an auditor trying to make his/her quota is being overly severe in reviewing their charts. There is heightened concern about the effects of the program given the changes coming with MACRA.
Other cuts highlighted by the proposal include elimination of $403 million in health professions and nursing training programs, “which lack evidence that they significantly improve the nation’s health workforce,” and a $4.2 billion cut from the elimination of discretionary programs within the Office of Community Services.
The budget process is a long one and the President’s recommendations are just the beginning. AGA is on Capitol Hill daily coordinating with our champions in Congress to ensure that the concerns of GIs are being heard regarding issues that affect our members and our patients. We will continue to work diligently as an organization as well as in coordination with other organizations and as a member of many coalitions to reach out to Capitol Hill, regulators, and payors to ensure that the future
of GI practice and research remains viable. Contact AGA to learn more about how to get involved in your community at [email protected], and learn more about AGA PAC at www.gastro.org/agapac.
The Department of Health & Human Services would see an 18% funding cut under the first budget proposal from the Trump administration.
The proposal, submitted to Congress March 16, would cut $15.1 billion from fiscal 2017 levels, funding the agency at $69 billion for fiscal year 2018. More than a third of the cuts come from the National Institutes of Health.
The NIH’s overall budget would drop to $25.9 billion in FY 2018, down $5.8 billion from this year (fiscal 2017). The proposal includes “a major reorganization of NIH’s institutes and centers to help focus resources on the highest priority research and training activities, including: eliminating the Fogarty International Center, consolidating the Agency for Healthcare Research and Quality within the NIH, and other consolidations and structural changes across NIH organizations and activities,” according to summary documents from the Office of Management and Budget.
The proposed cuts also account for the funds that are to be appropriated for the 21st Century Cures Act, which was supposed to add $4.8 billion in new appropriated funding, including funds dedicated to the Cancer Moonshot and the BRAIN Initiative.
The Centers for Disease Control and Prevention also would be reformed, getting a new $500 million block grant “to increase state flexibility and focus on the leading public health challenges specific to each state.” It also creates a new Federal Emergency Response Fund to respond to public health outbreaks such as the Zika virus.
Another area receiving a boost under the proposal is the funding for the Health Care Fraud and Abuse Control program at the CMS, which would receive $751 million in fiscal 2018, about 10% more than it did in fiscal 2017. The budget document notes that the “return on investment for the HCFAC account was $5 returned for every $1 expended from 2014-2016.”
The five-to-one return on fraud and abuse investigation in this program is controversial, because of the incentives for those conducting the investigations. Health care providers should not fear that an auditor trying to make his/her quota is being overly severe in reviewing their charts. There is heightened concern about the effects of the program given the changes coming with MACRA.
Other cuts highlighted by the proposal include elimination of $403 million in health professions and nursing training programs, “which lack evidence that they significantly improve the nation’s health workforce,” and a $4.2 billion cut from the elimination of discretionary programs within the Office of Community Services.
The budget process is a long one and the President’s recommendations are just the beginning. AGA is on Capitol Hill daily coordinating with our champions in Congress to ensure that the concerns of GIs are being heard regarding issues that affect our members and our patients. We will continue to work diligently as an organization as well as in coordination with other organizations and as a member of many coalitions to reach out to Capitol Hill, regulators, and payors to ensure that the future
of GI practice and research remains viable. Contact AGA to learn more about how to get involved in your community at [email protected], and learn more about AGA PAC at www.gastro.org/agapac.
The Department of Health & Human Services would see an 18% funding cut under the first budget proposal from the Trump administration.
The proposal, submitted to Congress March 16, would cut $15.1 billion from fiscal 2017 levels, funding the agency at $69 billion for fiscal year 2018. More than a third of the cuts come from the National Institutes of Health.
The NIH’s overall budget would drop to $25.9 billion in FY 2018, down $5.8 billion from this year (fiscal 2017). The proposal includes “a major reorganization of NIH’s institutes and centers to help focus resources on the highest priority research and training activities, including: eliminating the Fogarty International Center, consolidating the Agency for Healthcare Research and Quality within the NIH, and other consolidations and structural changes across NIH organizations and activities,” according to summary documents from the Office of Management and Budget.
The proposed cuts also account for the funds that are to be appropriated for the 21st Century Cures Act, which was supposed to add $4.8 billion in new appropriated funding, including funds dedicated to the Cancer Moonshot and the BRAIN Initiative.
The Centers for Disease Control and Prevention also would be reformed, getting a new $500 million block grant “to increase state flexibility and focus on the leading public health challenges specific to each state.” It also creates a new Federal Emergency Response Fund to respond to public health outbreaks such as the Zika virus.
Another area receiving a boost under the proposal is the funding for the Health Care Fraud and Abuse Control program at the CMS, which would receive $751 million in fiscal 2018, about 10% more than it did in fiscal 2017. The budget document notes that the “return on investment for the HCFAC account was $5 returned for every $1 expended from 2014-2016.”
The five-to-one return on fraud and abuse investigation in this program is controversial, because of the incentives for those conducting the investigations. Health care providers should not fear that an auditor trying to make his/her quota is being overly severe in reviewing their charts. There is heightened concern about the effects of the program given the changes coming with MACRA.
Other cuts highlighted by the proposal include elimination of $403 million in health professions and nursing training programs, “which lack evidence that they significantly improve the nation’s health workforce,” and a $4.2 billion cut from the elimination of discretionary programs within the Office of Community Services.
The budget process is a long one and the President’s recommendations are just the beginning. AGA is on Capitol Hill daily coordinating with our champions in Congress to ensure that the concerns of GIs are being heard regarding issues that affect our members and our patients. We will continue to work diligently as an organization as well as in coordination with other organizations and as a member of many coalitions to reach out to Capitol Hill, regulators, and payors to ensure that the future
of GI practice and research remains viable. Contact AGA to learn more about how to get involved in your community at [email protected], and learn more about AGA PAC at www.gastro.org/agapac.