User login
Get on board with APMs or risk having your practice commoditized
Gastroenterologists need to get on board with assuming risk through the adoption of advanced alternative payment methods under Medicare’s new Quality Payment Program or risk losing money through the commoditization of their services.
That’s the message Lawrence Kosinski, MD, of the Illinois Gastroenterology Group, has for his fellow gastroenterologists.
“To me, MIPS [Merit-based Incentive Payment System] is just an extension of what we were doing with PQRS and meaningful use, value-based modifiers,” Dr. Kosinski, who serves as practice councillor on the AGA Governing Board, said in an interview in advance of his presentation at the 2017 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology.
If gastroenterologists choose to stay on that path rather than accept the two-sided risk of following the APM track of the Quality Payment Program, “you are going to have commoditized services and you are going to just make less money and work for somebody else. But if you want to be in control of your own future, we have to strive to work in [the APM] direction.”
To get there, the culture of gastroenterology will need to shift.
“Our culture right now is one where we get paid for making widgets,” he said. “And the more widgets we make, the more money we make. The more colonoscopies we do, the more money we make. The more we can charge for those colonoscopies and get collected, the better things are for us.”
The future could allow gastroenterologists to charge for more and different services, he added.
“A lot of people are working to replace colonoscopies with much less expensive alternative,” Dr. Kosinski said. “So where does a gastroenterologist have a competitive advantage? It’s in the knowledge of these complex diseases that are running up the cost of health care. Over 80% of the cost of health care is for the management of chronic disease. We happen to have a very expensive set of chronic diseases that we take care of in GI, very complicated illnesses. We need to leverage that. We need to leverage the management of those patients, but we need to be able to show how our work decreases the overall cost of care so that we can get a piece of that risk premium.”
He warned: “If we don’t do that, we’re just going to be commoditized proceduralists.”
And the upside will be better reimbursements for doctors and lower overall costs for health systems.
“We may be able to increase our own reimbursement as long as we are critical in lowering the overall cost of care for the entire population,” he said.
To that end, “well-managed GI practices should be able to develop a competitive advantage in a value space to be able to supply the needed services,” he said, adding that “we’re going to have to provide value by improving quality through a focus on patient outcomes. We have to assure a new level of patient relationships and ultimately focus on lowering the cost of care for a population of patients.”
Gastroenterologists need to get on board with assuming risk through the adoption of advanced alternative payment methods under Medicare’s new Quality Payment Program or risk losing money through the commoditization of their services.
That’s the message Lawrence Kosinski, MD, of the Illinois Gastroenterology Group, has for his fellow gastroenterologists.
“To me, MIPS [Merit-based Incentive Payment System] is just an extension of what we were doing with PQRS and meaningful use, value-based modifiers,” Dr. Kosinski, who serves as practice councillor on the AGA Governing Board, said in an interview in advance of his presentation at the 2017 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology.
If gastroenterologists choose to stay on that path rather than accept the two-sided risk of following the APM track of the Quality Payment Program, “you are going to have commoditized services and you are going to just make less money and work for somebody else. But if you want to be in control of your own future, we have to strive to work in [the APM] direction.”
To get there, the culture of gastroenterology will need to shift.
“Our culture right now is one where we get paid for making widgets,” he said. “And the more widgets we make, the more money we make. The more colonoscopies we do, the more money we make. The more we can charge for those colonoscopies and get collected, the better things are for us.”
The future could allow gastroenterologists to charge for more and different services, he added.
“A lot of people are working to replace colonoscopies with much less expensive alternative,” Dr. Kosinski said. “So where does a gastroenterologist have a competitive advantage? It’s in the knowledge of these complex diseases that are running up the cost of health care. Over 80% of the cost of health care is for the management of chronic disease. We happen to have a very expensive set of chronic diseases that we take care of in GI, very complicated illnesses. We need to leverage that. We need to leverage the management of those patients, but we need to be able to show how our work decreases the overall cost of care so that we can get a piece of that risk premium.”
He warned: “If we don’t do that, we’re just going to be commoditized proceduralists.”
And the upside will be better reimbursements for doctors and lower overall costs for health systems.
“We may be able to increase our own reimbursement as long as we are critical in lowering the overall cost of care for the entire population,” he said.
To that end, “well-managed GI practices should be able to develop a competitive advantage in a value space to be able to supply the needed services,” he said, adding that “we’re going to have to provide value by improving quality through a focus on patient outcomes. We have to assure a new level of patient relationships and ultimately focus on lowering the cost of care for a population of patients.”
Gastroenterologists need to get on board with assuming risk through the adoption of advanced alternative payment methods under Medicare’s new Quality Payment Program or risk losing money through the commoditization of their services.
That’s the message Lawrence Kosinski, MD, of the Illinois Gastroenterology Group, has for his fellow gastroenterologists.
“To me, MIPS [Merit-based Incentive Payment System] is just an extension of what we were doing with PQRS and meaningful use, value-based modifiers,” Dr. Kosinski, who serves as practice councillor on the AGA Governing Board, said in an interview in advance of his presentation at the 2017 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology.
If gastroenterologists choose to stay on that path rather than accept the two-sided risk of following the APM track of the Quality Payment Program, “you are going to have commoditized services and you are going to just make less money and work for somebody else. But if you want to be in control of your own future, we have to strive to work in [the APM] direction.”
To get there, the culture of gastroenterology will need to shift.
“Our culture right now is one where we get paid for making widgets,” he said. “And the more widgets we make, the more money we make. The more colonoscopies we do, the more money we make. The more we can charge for those colonoscopies and get collected, the better things are for us.”
The future could allow gastroenterologists to charge for more and different services, he added.
“A lot of people are working to replace colonoscopies with much less expensive alternative,” Dr. Kosinski said. “So where does a gastroenterologist have a competitive advantage? It’s in the knowledge of these complex diseases that are running up the cost of health care. Over 80% of the cost of health care is for the management of chronic disease. We happen to have a very expensive set of chronic diseases that we take care of in GI, very complicated illnesses. We need to leverage that. We need to leverage the management of those patients, but we need to be able to show how our work decreases the overall cost of care so that we can get a piece of that risk premium.”
He warned: “If we don’t do that, we’re just going to be commoditized proceduralists.”
And the upside will be better reimbursements for doctors and lower overall costs for health systems.
“We may be able to increase our own reimbursement as long as we are critical in lowering the overall cost of care for the entire population,” he said.
To that end, “well-managed GI practices should be able to develop a competitive advantage in a value space to be able to supply the needed services,” he said, adding that “we’re going to have to provide value by improving quality through a focus on patient outcomes. We have to assure a new level of patient relationships and ultimately focus on lowering the cost of care for a population of patients.”
Hard water versus your skin
Observational studies suggest that hard water is associated with the development of atopic dermatitis (AD). Studies of children in the United Kingdom, Spain, and Japan show the prevalence of AD is significantly higher in the highest water hardness categories than that in the lowest. Calcium cations in water can interfere with normal epidermal calcium gradients that are necessary for corneocyte development and proper stratum corneum barrier formation.
Water hardness, determined by the amount of dissolved calcium and magnesium in the water, varies by geography and mineral content of the water supply. The hardest water supply in the United States is mostly localized to the Upper Plains and Rocky Mountain areas. General guidelines for classification of waters are: 0-60 mg/L calcium carbonate (soft); 61-120 mg/L (moderately hard); 121-180 mg/L (hard); and more than 180 mg/L (very hard). In regions where there is hard water, the surfactants in soap, such as sodium dodecyl sulfate, react with the calcium and magnesium ions in hard water, resulting in precipitation of the surfactant – leaving a film of residue on the skin, shower tiles, pipes, glassware, etc.
Atopic dermatitis, xerosis, and pruritus are some of the common skin reactions to hard water. Other less-well-defined effects on the skin include clogged pores and acne from surfactant residue left on the skin and altered sebum production. In addition, more surfactants or cleansers are needed to clean the skin and hair in areas with hard water because the abundant cations require a much heavier lather to dissolve.
Calcium and magnesium cations left on the skin can also form free radicals. Free radicals over time can result in collagen and elastin breakdown and in the increased prevalence of fine lines and wrinkles.
Hard water and geography should be considered a possible factor when assessing patients with recalcitrant eczema, pruritus, or xerosis that cannot otherwise be reversed. Water softening treatments are a simple solution in areas where the mineral content of water is elevated or the water plays a role in clinical skin disease.
Dr. Lily Talakoub and Dr. Naissan O. Wesley and are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
United States Geological Survey Water Quality Information: Water Hardness and Alkalinity.
J Am Acad Dermatol. 1987 Jun;16(6):1263-4.
Contact Dermatitis. 1996 Dec;35(6):337-43.
Lancet. 1998 Aug 15;352(9127):527-31.
Contact Dermatitis. 1999;41(6):311-4.
Environ Res. 2004 Jan;94(1):33-7.
Observational studies suggest that hard water is associated with the development of atopic dermatitis (AD). Studies of children in the United Kingdom, Spain, and Japan show the prevalence of AD is significantly higher in the highest water hardness categories than that in the lowest. Calcium cations in water can interfere with normal epidermal calcium gradients that are necessary for corneocyte development and proper stratum corneum barrier formation.
Water hardness, determined by the amount of dissolved calcium and magnesium in the water, varies by geography and mineral content of the water supply. The hardest water supply in the United States is mostly localized to the Upper Plains and Rocky Mountain areas. General guidelines for classification of waters are: 0-60 mg/L calcium carbonate (soft); 61-120 mg/L (moderately hard); 121-180 mg/L (hard); and more than 180 mg/L (very hard). In regions where there is hard water, the surfactants in soap, such as sodium dodecyl sulfate, react with the calcium and magnesium ions in hard water, resulting in precipitation of the surfactant – leaving a film of residue on the skin, shower tiles, pipes, glassware, etc.
Atopic dermatitis, xerosis, and pruritus are some of the common skin reactions to hard water. Other less-well-defined effects on the skin include clogged pores and acne from surfactant residue left on the skin and altered sebum production. In addition, more surfactants or cleansers are needed to clean the skin and hair in areas with hard water because the abundant cations require a much heavier lather to dissolve.
Calcium and magnesium cations left on the skin can also form free radicals. Free radicals over time can result in collagen and elastin breakdown and in the increased prevalence of fine lines and wrinkles.
Hard water and geography should be considered a possible factor when assessing patients with recalcitrant eczema, pruritus, or xerosis that cannot otherwise be reversed. Water softening treatments are a simple solution in areas where the mineral content of water is elevated or the water plays a role in clinical skin disease.
Dr. Lily Talakoub and Dr. Naissan O. Wesley and are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
United States Geological Survey Water Quality Information: Water Hardness and Alkalinity.
J Am Acad Dermatol. 1987 Jun;16(6):1263-4.
Contact Dermatitis. 1996 Dec;35(6):337-43.
Lancet. 1998 Aug 15;352(9127):527-31.
Contact Dermatitis. 1999;41(6):311-4.
Environ Res. 2004 Jan;94(1):33-7.
Observational studies suggest that hard water is associated with the development of atopic dermatitis (AD). Studies of children in the United Kingdom, Spain, and Japan show the prevalence of AD is significantly higher in the highest water hardness categories than that in the lowest. Calcium cations in water can interfere with normal epidermal calcium gradients that are necessary for corneocyte development and proper stratum corneum barrier formation.
Water hardness, determined by the amount of dissolved calcium and magnesium in the water, varies by geography and mineral content of the water supply. The hardest water supply in the United States is mostly localized to the Upper Plains and Rocky Mountain areas. General guidelines for classification of waters are: 0-60 mg/L calcium carbonate (soft); 61-120 mg/L (moderately hard); 121-180 mg/L (hard); and more than 180 mg/L (very hard). In regions where there is hard water, the surfactants in soap, such as sodium dodecyl sulfate, react with the calcium and magnesium ions in hard water, resulting in precipitation of the surfactant – leaving a film of residue on the skin, shower tiles, pipes, glassware, etc.
Atopic dermatitis, xerosis, and pruritus are some of the common skin reactions to hard water. Other less-well-defined effects on the skin include clogged pores and acne from surfactant residue left on the skin and altered sebum production. In addition, more surfactants or cleansers are needed to clean the skin and hair in areas with hard water because the abundant cations require a much heavier lather to dissolve.
Calcium and magnesium cations left on the skin can also form free radicals. Free radicals over time can result in collagen and elastin breakdown and in the increased prevalence of fine lines and wrinkles.
Hard water and geography should be considered a possible factor when assessing patients with recalcitrant eczema, pruritus, or xerosis that cannot otherwise be reversed. Water softening treatments are a simple solution in areas where the mineral content of water is elevated or the water plays a role in clinical skin disease.
Dr. Lily Talakoub and Dr. Naissan O. Wesley and are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
United States Geological Survey Water Quality Information: Water Hardness and Alkalinity.
J Am Acad Dermatol. 1987 Jun;16(6):1263-4.
Contact Dermatitis. 1996 Dec;35(6):337-43.
Lancet. 1998 Aug 15;352(9127):527-31.
Contact Dermatitis. 1999;41(6):311-4.
Environ Res. 2004 Jan;94(1):33-7.
Real-time data, transparency needed to improve safety
With an increasing number of emerging technological advances in both medical devices and procedures, two factors will help improve safety and outcomes for patients: better real-time data and information transparency.
And the key to getting that data will be broader use of registries, according to Dana Telem, MD, director of the Comprehensive Hernia Program at the University of Michigan, Ann Arbor.
Dr. Telem will discuss the importance of real-time information and registries during her April 13 presentation at the 2017 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
Speaking in advance of the meeting, Dr. Telem noted that understanding value and finding potential safety issues are among the reasons for using data registries.
“I think that’s really why we need to focus on getting real-time data and that’s why I think registry efforts are so important,” she said. “What registries are really great at [is] identifying those isolated events.”
In addition, they help with another key concept she will highlight during her talk: transparency, something that is especially important as new techniques and devices are introduced.
“We understand that there are going to be early adopters, and you need to have the pioneers in order to take those next steps,” she said.
But to get the most out of those new technologies, data need to be recorded not only for short-term outcomes but over the long term as well “so that we can really assess the value of the techniques or the devices that we are putting out into the community.”
And by providing transparency on what is known, as well as unknown, it also helps patients in making their own decisions on whether to proceed with something new.
She believes that, especially when it comes to newer technologies, patients will be willing to be a part of that data collection if doctors are transparent.
“Many people are willing as long as you are honest with them,” Dr. Telem said. “I think particularly that when newer devices and techniques come out, particularly endoscopic procedures that can save them an operation, they are more willing than not, oftentimes, to do it, which is also where it gets a little bit dangerous if you are not transparent.”
But even being transparent can be a challenge.
“I think a lot of the issue that we have in health care is what type of information we are providing and how are we providing it, and is the receiver of information actually understanding what we are saying?” she pondered.
“I don’t think we know the answers to that, but I think part of the solution at least is to be as open about what we know as well as what we don’t know at the same time and let the patient make a decision based on their value system,” she continued. “A lot of times with these newer technologies or techniques, you really have to sit down with the patient and ask them about their long-term goals, what are acceptable and unacceptable outcomes.”
Recognizing the importance of data registries, in 2014 AGA launched a new initiative working as a neutral, objective broker to establish clinical research studies. Through this program, AGA collects important data to assess the value of new technologies and procedures on patient care. Ultimately, the data AGA collects will support the approval, coverage, and adoption of new technologies that demonstrate promise and merit. To learn more about AGA’s registry initiative or get involved, please contact Sonya Serra, AGA’s senior director of registry development and integrity, at [email protected].
With an increasing number of emerging technological advances in both medical devices and procedures, two factors will help improve safety and outcomes for patients: better real-time data and information transparency.
And the key to getting that data will be broader use of registries, according to Dana Telem, MD, director of the Comprehensive Hernia Program at the University of Michigan, Ann Arbor.
Dr. Telem will discuss the importance of real-time information and registries during her April 13 presentation at the 2017 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
Speaking in advance of the meeting, Dr. Telem noted that understanding value and finding potential safety issues are among the reasons for using data registries.
“I think that’s really why we need to focus on getting real-time data and that’s why I think registry efforts are so important,” she said. “What registries are really great at [is] identifying those isolated events.”
In addition, they help with another key concept she will highlight during her talk: transparency, something that is especially important as new techniques and devices are introduced.
“We understand that there are going to be early adopters, and you need to have the pioneers in order to take those next steps,” she said.
But to get the most out of those new technologies, data need to be recorded not only for short-term outcomes but over the long term as well “so that we can really assess the value of the techniques or the devices that we are putting out into the community.”
And by providing transparency on what is known, as well as unknown, it also helps patients in making their own decisions on whether to proceed with something new.
She believes that, especially when it comes to newer technologies, patients will be willing to be a part of that data collection if doctors are transparent.
“Many people are willing as long as you are honest with them,” Dr. Telem said. “I think particularly that when newer devices and techniques come out, particularly endoscopic procedures that can save them an operation, they are more willing than not, oftentimes, to do it, which is also where it gets a little bit dangerous if you are not transparent.”
But even being transparent can be a challenge.
“I think a lot of the issue that we have in health care is what type of information we are providing and how are we providing it, and is the receiver of information actually understanding what we are saying?” she pondered.
“I don’t think we know the answers to that, but I think part of the solution at least is to be as open about what we know as well as what we don’t know at the same time and let the patient make a decision based on their value system,” she continued. “A lot of times with these newer technologies or techniques, you really have to sit down with the patient and ask them about their long-term goals, what are acceptable and unacceptable outcomes.”
Recognizing the importance of data registries, in 2014 AGA launched a new initiative working as a neutral, objective broker to establish clinical research studies. Through this program, AGA collects important data to assess the value of new technologies and procedures on patient care. Ultimately, the data AGA collects will support the approval, coverage, and adoption of new technologies that demonstrate promise and merit. To learn more about AGA’s registry initiative or get involved, please contact Sonya Serra, AGA’s senior director of registry development and integrity, at [email protected].
With an increasing number of emerging technological advances in both medical devices and procedures, two factors will help improve safety and outcomes for patients: better real-time data and information transparency.
And the key to getting that data will be broader use of registries, according to Dana Telem, MD, director of the Comprehensive Hernia Program at the University of Michigan, Ann Arbor.
Dr. Telem will discuss the importance of real-time information and registries during her April 13 presentation at the 2017 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
Speaking in advance of the meeting, Dr. Telem noted that understanding value and finding potential safety issues are among the reasons for using data registries.
“I think that’s really why we need to focus on getting real-time data and that’s why I think registry efforts are so important,” she said. “What registries are really great at [is] identifying those isolated events.”
In addition, they help with another key concept she will highlight during her talk: transparency, something that is especially important as new techniques and devices are introduced.
“We understand that there are going to be early adopters, and you need to have the pioneers in order to take those next steps,” she said.
But to get the most out of those new technologies, data need to be recorded not only for short-term outcomes but over the long term as well “so that we can really assess the value of the techniques or the devices that we are putting out into the community.”
And by providing transparency on what is known, as well as unknown, it also helps patients in making their own decisions on whether to proceed with something new.
She believes that, especially when it comes to newer technologies, patients will be willing to be a part of that data collection if doctors are transparent.
“Many people are willing as long as you are honest with them,” Dr. Telem said. “I think particularly that when newer devices and techniques come out, particularly endoscopic procedures that can save them an operation, they are more willing than not, oftentimes, to do it, which is also where it gets a little bit dangerous if you are not transparent.”
But even being transparent can be a challenge.
“I think a lot of the issue that we have in health care is what type of information we are providing and how are we providing it, and is the receiver of information actually understanding what we are saying?” she pondered.
“I don’t think we know the answers to that, but I think part of the solution at least is to be as open about what we know as well as what we don’t know at the same time and let the patient make a decision based on their value system,” she continued. “A lot of times with these newer technologies or techniques, you really have to sit down with the patient and ask them about their long-term goals, what are acceptable and unacceptable outcomes.”
Recognizing the importance of data registries, in 2014 AGA launched a new initiative working as a neutral, objective broker to establish clinical research studies. Through this program, AGA collects important data to assess the value of new technologies and procedures on patient care. Ultimately, the data AGA collects will support the approval, coverage, and adoption of new technologies that demonstrate promise and merit. To learn more about AGA’s registry initiative or get involved, please contact Sonya Serra, AGA’s senior director of registry development and integrity, at [email protected].
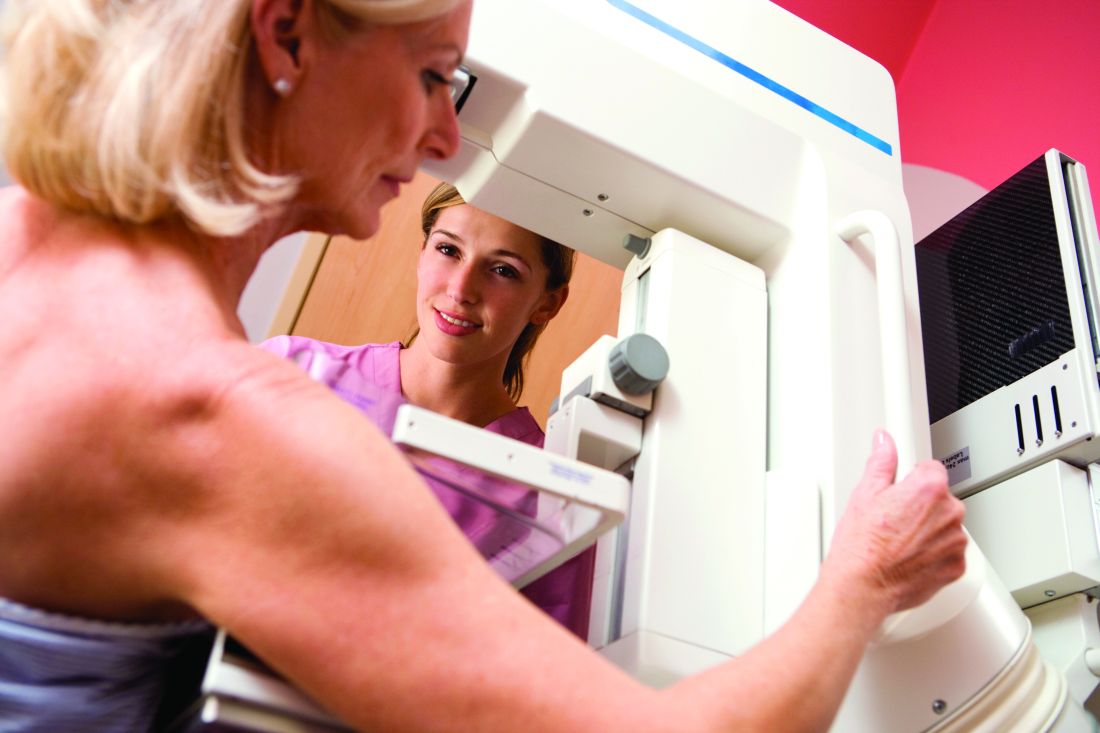
Physicians favor ACOG mammography recommendations
Primary care physicians tend to follow breast cancer screening recommendations from the American Congress of Obstetricians and Gynecologists, according to findings from a recent survey.
The American Congress of Obstetricians and Gynecologists (ACOG), the American Cancer Society (ACS), and the U.S. Preventive Services Task Force (USPSTF) offer conflicting guidelines on the optimal time to initiate and discontinue screening mammography, as well as the optimal screening interval. ACOG recommends annual screening for women aged 40 and older, while the ACS advises annual screening at age 45, and the USPSTF calls for biennial mammograms starting at age 50, though all three organizations stress individualized management.
There were 871 respondents, including family medicine/general practice physicians (44.2%), internists (29.7%), and gynecologists (26.1%). The average age of the respondents was 53 years, and more than half of them had been in practice for more than 20 years. A slight majority (55%) were men, and most (71%) were white.
A total of 26.0% of the respondents said they trusted ACOG screening guidelines the most, 23.8% said they trusted ACS guidelines, and 22.9% said they trusted USPSTF guidelines the most.
In total, 81% of physicians recommended screening to women aged 40-44 years, 88% recommended screening to women aged 45-49 years, and 67% recommended screening for women 75 years or older. Among physicians who recommended screening, most recommended annual exams.
These findings show that physicians differ sharply in their adherence to practice guidelines. The results also “provide an important benchmark as guidelines continue evolving, and underscore the need to delineate barriers and facilitators to implementing guidelines in clinical practice,” the researchers wrote.
Primary care physicians tend to follow breast cancer screening recommendations from the American Congress of Obstetricians and Gynecologists, according to findings from a recent survey.
The American Congress of Obstetricians and Gynecologists (ACOG), the American Cancer Society (ACS), and the U.S. Preventive Services Task Force (USPSTF) offer conflicting guidelines on the optimal time to initiate and discontinue screening mammography, as well as the optimal screening interval. ACOG recommends annual screening for women aged 40 and older, while the ACS advises annual screening at age 45, and the USPSTF calls for biennial mammograms starting at age 50, though all three organizations stress individualized management.
There were 871 respondents, including family medicine/general practice physicians (44.2%), internists (29.7%), and gynecologists (26.1%). The average age of the respondents was 53 years, and more than half of them had been in practice for more than 20 years. A slight majority (55%) were men, and most (71%) were white.
A total of 26.0% of the respondents said they trusted ACOG screening guidelines the most, 23.8% said they trusted ACS guidelines, and 22.9% said they trusted USPSTF guidelines the most.
In total, 81% of physicians recommended screening to women aged 40-44 years, 88% recommended screening to women aged 45-49 years, and 67% recommended screening for women 75 years or older. Among physicians who recommended screening, most recommended annual exams.
These findings show that physicians differ sharply in their adherence to practice guidelines. The results also “provide an important benchmark as guidelines continue evolving, and underscore the need to delineate barriers and facilitators to implementing guidelines in clinical practice,” the researchers wrote.
Primary care physicians tend to follow breast cancer screening recommendations from the American Congress of Obstetricians and Gynecologists, according to findings from a recent survey.
The American Congress of Obstetricians and Gynecologists (ACOG), the American Cancer Society (ACS), and the U.S. Preventive Services Task Force (USPSTF) offer conflicting guidelines on the optimal time to initiate and discontinue screening mammography, as well as the optimal screening interval. ACOG recommends annual screening for women aged 40 and older, while the ACS advises annual screening at age 45, and the USPSTF calls for biennial mammograms starting at age 50, though all three organizations stress individualized management.
There were 871 respondents, including family medicine/general practice physicians (44.2%), internists (29.7%), and gynecologists (26.1%). The average age of the respondents was 53 years, and more than half of them had been in practice for more than 20 years. A slight majority (55%) were men, and most (71%) were white.
A total of 26.0% of the respondents said they trusted ACOG screening guidelines the most, 23.8% said they trusted ACS guidelines, and 22.9% said they trusted USPSTF guidelines the most.
In total, 81% of physicians recommended screening to women aged 40-44 years, 88% recommended screening to women aged 45-49 years, and 67% recommended screening for women 75 years or older. Among physicians who recommended screening, most recommended annual exams.
These findings show that physicians differ sharply in their adherence to practice guidelines. The results also “provide an important benchmark as guidelines continue evolving, and underscore the need to delineate barriers and facilitators to implementing guidelines in clinical practice,” the researchers wrote.
FROM JAMA INTERNAL MEDICINE
Key clinical point:
Major finding: Across physicians groups, 81% of respondents recommended screening women aged 40-44 years.
Data source: An analysis of survey responses regarding breast cancer care from a nationally representative sample of 871 family/general medicine physicians, internists, and gynecologists.
Disclosures: The researchers reported having no conflicts of interest.
Obesity in women with RA greatly influences CRP levels
Obesity is associated with elevated C-reactive protein levels among women with rheumatoid arthritis, but this elevation reflects higher fat mass rather than RA disease activity, according to a report published online April 10 in Arthritis Care & Research.
The study findings suggest that C-reactive protein (CRP) results should be interpreted with caution among obese women with RA, noted Michael D. George, MD, of the division of rheumatology at the University of Pennsylvania, Philadelphia, and his associates.
To examine possible associations between body mass index and inflammatory markers in RA, the investigators performed a secondary analysis of data from 451 adult RA patients in three cross-sectional cohorts and 1,652 RA patients in the longitudinal Veterans Affairs Rheumatoid Arthritis Registry cohort. The investigators compared these findings with those of about 21,000 control subjects from the general population who were assessed in the 2007-2010 and 1971-1974 National Health and Nutrition Examination Survey programs.
Among women with RA, obesity was associated with elevated CRP independently of other measures of disease activity, including swollen joint count, tender joint count, and global scores of inflammation. A “strikingly similar association” was seen in the control population. This indicates that the high CRP values in obese women with RA “are not reflective of greater RA activity but rather are an expected phenomenon related to adiposity,” Dr. George and his associates said (Arthritis Care Res. 2017 Apr 10. doi: 10.1002/acr.23229).
In contrast, obesity in men with RA did not correlate with elevated CRP levels. In fact, underweight men with RA tended to have significantly higher CRP than that of normal-weight and obese men. “It was beyond the scope of this study to fully evaluate the complex relationship between RA disease activity, disease severity, weight loss, frailty, comorbidities, advancing age, and other factors that might contribute to higher levels of systemic inflammation in low-BMI men,” the investigators noted.
The National Institutes of Health, the Rheumatology Research Foundation, the U.S. Department of Veterans Affairs, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the National Institute of General Medical Sciences supported the work. Dr. George reported having no relevant disclosures; his associates reported ties to Pfizer, Novartis, and Amgen.
Obesity is associated with elevated C-reactive protein levels among women with rheumatoid arthritis, but this elevation reflects higher fat mass rather than RA disease activity, according to a report published online April 10 in Arthritis Care & Research.
The study findings suggest that C-reactive protein (CRP) results should be interpreted with caution among obese women with RA, noted Michael D. George, MD, of the division of rheumatology at the University of Pennsylvania, Philadelphia, and his associates.
To examine possible associations between body mass index and inflammatory markers in RA, the investigators performed a secondary analysis of data from 451 adult RA patients in three cross-sectional cohorts and 1,652 RA patients in the longitudinal Veterans Affairs Rheumatoid Arthritis Registry cohort. The investigators compared these findings with those of about 21,000 control subjects from the general population who were assessed in the 2007-2010 and 1971-1974 National Health and Nutrition Examination Survey programs.
Among women with RA, obesity was associated with elevated CRP independently of other measures of disease activity, including swollen joint count, tender joint count, and global scores of inflammation. A “strikingly similar association” was seen in the control population. This indicates that the high CRP values in obese women with RA “are not reflective of greater RA activity but rather are an expected phenomenon related to adiposity,” Dr. George and his associates said (Arthritis Care Res. 2017 Apr 10. doi: 10.1002/acr.23229).
In contrast, obesity in men with RA did not correlate with elevated CRP levels. In fact, underweight men with RA tended to have significantly higher CRP than that of normal-weight and obese men. “It was beyond the scope of this study to fully evaluate the complex relationship between RA disease activity, disease severity, weight loss, frailty, comorbidities, advancing age, and other factors that might contribute to higher levels of systemic inflammation in low-BMI men,” the investigators noted.
The National Institutes of Health, the Rheumatology Research Foundation, the U.S. Department of Veterans Affairs, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the National Institute of General Medical Sciences supported the work. Dr. George reported having no relevant disclosures; his associates reported ties to Pfizer, Novartis, and Amgen.
Obesity is associated with elevated C-reactive protein levels among women with rheumatoid arthritis, but this elevation reflects higher fat mass rather than RA disease activity, according to a report published online April 10 in Arthritis Care & Research.
The study findings suggest that C-reactive protein (CRP) results should be interpreted with caution among obese women with RA, noted Michael D. George, MD, of the division of rheumatology at the University of Pennsylvania, Philadelphia, and his associates.
To examine possible associations between body mass index and inflammatory markers in RA, the investigators performed a secondary analysis of data from 451 adult RA patients in three cross-sectional cohorts and 1,652 RA patients in the longitudinal Veterans Affairs Rheumatoid Arthritis Registry cohort. The investigators compared these findings with those of about 21,000 control subjects from the general population who were assessed in the 2007-2010 and 1971-1974 National Health and Nutrition Examination Survey programs.
Among women with RA, obesity was associated with elevated CRP independently of other measures of disease activity, including swollen joint count, tender joint count, and global scores of inflammation. A “strikingly similar association” was seen in the control population. This indicates that the high CRP values in obese women with RA “are not reflective of greater RA activity but rather are an expected phenomenon related to adiposity,” Dr. George and his associates said (Arthritis Care Res. 2017 Apr 10. doi: 10.1002/acr.23229).
In contrast, obesity in men with RA did not correlate with elevated CRP levels. In fact, underweight men with RA tended to have significantly higher CRP than that of normal-weight and obese men. “It was beyond the scope of this study to fully evaluate the complex relationship between RA disease activity, disease severity, weight loss, frailty, comorbidities, advancing age, and other factors that might contribute to higher levels of systemic inflammation in low-BMI men,” the investigators noted.
The National Institutes of Health, the Rheumatology Research Foundation, the U.S. Department of Veterans Affairs, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the National Institute of General Medical Sciences supported the work. Dr. George reported having no relevant disclosures; his associates reported ties to Pfizer, Novartis, and Amgen.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point:
Major finding: Among women with RA, obesity was associated with elevated CRP independently of other measures of disease activity, including swollen joint count, tender joint count, and global scores of inflammation.
Data source: A secondary analysis of data from cross-sectional and longitudinal cohort studies involving 2,103 adults with RA.
Disclosures: The National Institutes of Health, the Rheumatology Research Foundation, the U.S. Department of Veterans Affairs, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the National Institute of General Medical Sciences supported the work. Dr. George reported having no relevant disclosures; his associates reported ties to Pfizer, Novartis, and Amgen.
DNRs affect residents’ patient care decisions
Internal medicine residents reported being less likely to consider certain aggressive interventions outside of CPR on patients with do not resuscitate (DNR) and do not intubate (DNI) orders, according to a study.
These findings have researchers worried about a trend of doctors ignoring patient preferences, especially those who may have DNRs but do not want to ignore other treatment options, according to Elizabeth K. Stevenson, MD, of the Division of Pulmonary and Critical Care Medicine, North Shore Medical Center, Salem, Mass., and her colleagues.
“DNR/DNI patients were less likely to receive many invasive procedures, surgical consultations, or transfer to the ICU,” wrote Dr. Stevenson and her colleagues. “[D]ecisions to withhold many types of care not specified in DNR/DNI orders is concerning, given that the majority of patients with a DNR/DNI status in registry studies indicated they would accept other interventions beyond CPR and intubation.”
Researchers surveyed 553 internal medicine residents in the United States using an Internet survey that presented four vignettes describing clinical situations. Participants were asked to rank how likely they would be to employ listed intervention methods, from “strongly agree” to “strongly disagree,” in each scenario (Ann Am Thorac Soc. 2017, Apr;14[4]:536-42).
Two different versions of the survey were randomly assigned, varying only in terms of which vignettes included patients with a DNR/DNI order.
Of the interventions listed for each scenario, decisions to transfer patients to the intensive care unit and suggest surgery consultations showed the strongest association with code status.
“Residents were significantly less likely to indicate they would provide invasive procedures (including central venous catheter placement, esophagogastroduodenoscopy, colonoscopy, bronchoscopy, dialysis, and surgery consultation) to patients who had a status of DNR/DNI compared with Full Code,” the investigators noted. “In contrast, decisions to pursue noninvasive diagnostic or therapeutic interventions (CT scans, administration of oxygen or intravenous fluids, blood cultures, and initiation of anticoagulation) did not significantly differ by patient code status, with high levels of use across all vignettes.”
In one vignette involving surgical consultation for an 80-year-old woman with septic shock secondary to Clostridium difficile infection, 89.1% of residents recommended a consult for full-care patients, while 77.7% recommended one for a patient with a DNR/DNI (P = .0008).
Despite these findings, 94%-96% of participants reported willingness to consult with patients on their preferences before treatment decisions, which Dr. Stevenson and her peers found somewhat comforting, although it did not completely assuage them.
“Although the ideal approach would be to have more comprehensive discussion and documentation of patients’ goals of care in the outpatient setting, realistically, many patients will neither have had such discussions nor [have] completed advance directives before hospitalization,” investigators wrote.
The study was limited by the size of the sample, which numbered approximately 2% of the active internal medicine residents in the United States. The researchers recognized that these scenarios were theoretical, and that practicing physicians may act differently when faced with a medical situation in real life. The study also was limited by the concentration of respondents within a single program, as shared experiences or teachers may cause similar responses to theoretical situations, they wrote.
One of the study’s authors reports grants from the National Institutes of Health. The other investigators report no relevant financial disclosures.
[email protected]
On Twitter @EAZTweets[polldaddy:9722880]
End-of-life treatment usually should be based on the preferences of the patients and how aggressive they want their physicians to be. Yet the study by Dr. Stevenson et al. shows that decisions in types of care are more often being based on the preferences of the doctors, which is very concerning. Engaging patients in a high-quality discussion of options and care preferences is an essential part of end-of-life treatment, and this trend of physician-attributable variation shows a level of paternalism that has no place in this type of care, and could lead to dire results for patients. For example, 72% of residents in one of the theoretical situations chose to intervene with dialysis in a full-code patient, while only 38% chose to do so for patients with a DNR. While the situations are theoretical, these findings uncover a disregard for patients’ autonomy in decisions about their own care. Since patients are unable to choose their own residents and many residents will not have the opportunity to consult with every patient, DNR patients are certainly vulnerable to the possibility of being assessed for treatment based on their code status. Residents are the future of medicine, and must be trained out of this habit so that patients’ preferences are not overlooked.
Joanna L. Hart, MD, is a research fellow in the Pulmonary, Allergy, and Critical Care Division, and the Palliative and Advanced Illness Research Center, University of Pennsylvania, Philadelphia. Meeta Prasad Kerlin, MD, MSCE, is the associate program director at the same institution. They had not disclosures. Their comments are in an editorial (Ann Am Thorac Soc. 2017 Apr;14[4]:491-2).
End-of-life treatment usually should be based on the preferences of the patients and how aggressive they want their physicians to be. Yet the study by Dr. Stevenson et al. shows that decisions in types of care are more often being based on the preferences of the doctors, which is very concerning. Engaging patients in a high-quality discussion of options and care preferences is an essential part of end-of-life treatment, and this trend of physician-attributable variation shows a level of paternalism that has no place in this type of care, and could lead to dire results for patients. For example, 72% of residents in one of the theoretical situations chose to intervene with dialysis in a full-code patient, while only 38% chose to do so for patients with a DNR. While the situations are theoretical, these findings uncover a disregard for patients’ autonomy in decisions about their own care. Since patients are unable to choose their own residents and many residents will not have the opportunity to consult with every patient, DNR patients are certainly vulnerable to the possibility of being assessed for treatment based on their code status. Residents are the future of medicine, and must be trained out of this habit so that patients’ preferences are not overlooked.
Joanna L. Hart, MD, is a research fellow in the Pulmonary, Allergy, and Critical Care Division, and the Palliative and Advanced Illness Research Center, University of Pennsylvania, Philadelphia. Meeta Prasad Kerlin, MD, MSCE, is the associate program director at the same institution. They had not disclosures. Their comments are in an editorial (Ann Am Thorac Soc. 2017 Apr;14[4]:491-2).
End-of-life treatment usually should be based on the preferences of the patients and how aggressive they want their physicians to be. Yet the study by Dr. Stevenson et al. shows that decisions in types of care are more often being based on the preferences of the doctors, which is very concerning. Engaging patients in a high-quality discussion of options and care preferences is an essential part of end-of-life treatment, and this trend of physician-attributable variation shows a level of paternalism that has no place in this type of care, and could lead to dire results for patients. For example, 72% of residents in one of the theoretical situations chose to intervene with dialysis in a full-code patient, while only 38% chose to do so for patients with a DNR. While the situations are theoretical, these findings uncover a disregard for patients’ autonomy in decisions about their own care. Since patients are unable to choose their own residents and many residents will not have the opportunity to consult with every patient, DNR patients are certainly vulnerable to the possibility of being assessed for treatment based on their code status. Residents are the future of medicine, and must be trained out of this habit so that patients’ preferences are not overlooked.
Joanna L. Hart, MD, is a research fellow in the Pulmonary, Allergy, and Critical Care Division, and the Palliative and Advanced Illness Research Center, University of Pennsylvania, Philadelphia. Meeta Prasad Kerlin, MD, MSCE, is the associate program director at the same institution. They had not disclosures. Their comments are in an editorial (Ann Am Thorac Soc. 2017 Apr;14[4]:491-2).
Internal medicine residents reported being less likely to consider certain aggressive interventions outside of CPR on patients with do not resuscitate (DNR) and do not intubate (DNI) orders, according to a study.
These findings have researchers worried about a trend of doctors ignoring patient preferences, especially those who may have DNRs but do not want to ignore other treatment options, according to Elizabeth K. Stevenson, MD, of the Division of Pulmonary and Critical Care Medicine, North Shore Medical Center, Salem, Mass., and her colleagues.
“DNR/DNI patients were less likely to receive many invasive procedures, surgical consultations, or transfer to the ICU,” wrote Dr. Stevenson and her colleagues. “[D]ecisions to withhold many types of care not specified in DNR/DNI orders is concerning, given that the majority of patients with a DNR/DNI status in registry studies indicated they would accept other interventions beyond CPR and intubation.”
Researchers surveyed 553 internal medicine residents in the United States using an Internet survey that presented four vignettes describing clinical situations. Participants were asked to rank how likely they would be to employ listed intervention methods, from “strongly agree” to “strongly disagree,” in each scenario (Ann Am Thorac Soc. 2017, Apr;14[4]:536-42).
Two different versions of the survey were randomly assigned, varying only in terms of which vignettes included patients with a DNR/DNI order.
Of the interventions listed for each scenario, decisions to transfer patients to the intensive care unit and suggest surgery consultations showed the strongest association with code status.
“Residents were significantly less likely to indicate they would provide invasive procedures (including central venous catheter placement, esophagogastroduodenoscopy, colonoscopy, bronchoscopy, dialysis, and surgery consultation) to patients who had a status of DNR/DNI compared with Full Code,” the investigators noted. “In contrast, decisions to pursue noninvasive diagnostic or therapeutic interventions (CT scans, administration of oxygen or intravenous fluids, blood cultures, and initiation of anticoagulation) did not significantly differ by patient code status, with high levels of use across all vignettes.”
In one vignette involving surgical consultation for an 80-year-old woman with septic shock secondary to Clostridium difficile infection, 89.1% of residents recommended a consult for full-care patients, while 77.7% recommended one for a patient with a DNR/DNI (P = .0008).
Despite these findings, 94%-96% of participants reported willingness to consult with patients on their preferences before treatment decisions, which Dr. Stevenson and her peers found somewhat comforting, although it did not completely assuage them.
“Although the ideal approach would be to have more comprehensive discussion and documentation of patients’ goals of care in the outpatient setting, realistically, many patients will neither have had such discussions nor [have] completed advance directives before hospitalization,” investigators wrote.
The study was limited by the size of the sample, which numbered approximately 2% of the active internal medicine residents in the United States. The researchers recognized that these scenarios were theoretical, and that practicing physicians may act differently when faced with a medical situation in real life. The study also was limited by the concentration of respondents within a single program, as shared experiences or teachers may cause similar responses to theoretical situations, they wrote.
One of the study’s authors reports grants from the National Institutes of Health. The other investigators report no relevant financial disclosures.
[email protected]
On Twitter @EAZTweets[polldaddy:9722880]
Internal medicine residents reported being less likely to consider certain aggressive interventions outside of CPR on patients with do not resuscitate (DNR) and do not intubate (DNI) orders, according to a study.
These findings have researchers worried about a trend of doctors ignoring patient preferences, especially those who may have DNRs but do not want to ignore other treatment options, according to Elizabeth K. Stevenson, MD, of the Division of Pulmonary and Critical Care Medicine, North Shore Medical Center, Salem, Mass., and her colleagues.
“DNR/DNI patients were less likely to receive many invasive procedures, surgical consultations, or transfer to the ICU,” wrote Dr. Stevenson and her colleagues. “[D]ecisions to withhold many types of care not specified in DNR/DNI orders is concerning, given that the majority of patients with a DNR/DNI status in registry studies indicated they would accept other interventions beyond CPR and intubation.”
Researchers surveyed 553 internal medicine residents in the United States using an Internet survey that presented four vignettes describing clinical situations. Participants were asked to rank how likely they would be to employ listed intervention methods, from “strongly agree” to “strongly disagree,” in each scenario (Ann Am Thorac Soc. 2017, Apr;14[4]:536-42).
Two different versions of the survey were randomly assigned, varying only in terms of which vignettes included patients with a DNR/DNI order.
Of the interventions listed for each scenario, decisions to transfer patients to the intensive care unit and suggest surgery consultations showed the strongest association with code status.
“Residents were significantly less likely to indicate they would provide invasive procedures (including central venous catheter placement, esophagogastroduodenoscopy, colonoscopy, bronchoscopy, dialysis, and surgery consultation) to patients who had a status of DNR/DNI compared with Full Code,” the investigators noted. “In contrast, decisions to pursue noninvasive diagnostic or therapeutic interventions (CT scans, administration of oxygen or intravenous fluids, blood cultures, and initiation of anticoagulation) did not significantly differ by patient code status, with high levels of use across all vignettes.”
In one vignette involving surgical consultation for an 80-year-old woman with septic shock secondary to Clostridium difficile infection, 89.1% of residents recommended a consult for full-care patients, while 77.7% recommended one for a patient with a DNR/DNI (P = .0008).
Despite these findings, 94%-96% of participants reported willingness to consult with patients on their preferences before treatment decisions, which Dr. Stevenson and her peers found somewhat comforting, although it did not completely assuage them.
“Although the ideal approach would be to have more comprehensive discussion and documentation of patients’ goals of care in the outpatient setting, realistically, many patients will neither have had such discussions nor [have] completed advance directives before hospitalization,” investigators wrote.
The study was limited by the size of the sample, which numbered approximately 2% of the active internal medicine residents in the United States. The researchers recognized that these scenarios were theoretical, and that practicing physicians may act differently when faced with a medical situation in real life. The study also was limited by the concentration of respondents within a single program, as shared experiences or teachers may cause similar responses to theoretical situations, they wrote.
One of the study’s authors reports grants from the National Institutes of Health. The other investigators report no relevant financial disclosures.
[email protected]
On Twitter @EAZTweets[polldaddy:9722880]
FROM ANNALS OF THE AMERICAN THORACIC SOCIETY
Key clinical point:
Major finding: Among 553 residents, patient code status was associated with invasive care decisions beyond intubation and CPR, especially transfers to the intensive care unit.
Data source: Randomized, cross-sectional Internet survey containing four clinical situations disseminated among internal medicine residents across the United States.
Disclosures: One of the study’s authors reports grants from the National Institutes of Health. The other investigators report no relevant financial disclosures.
Compounding rules challenge practice norms
As new rules about drug compounding get shaped, rheumatologists seek to protect their ability to combine injectable drugs – most commonly a steroid and a local anesthetic – in their own offices.
In a position statement sent to government agencies and members of Congress in February, the American College of Rheumatology voiced concerns that the practice, which it called “critical,” could become a casualty of drug-compounding regulations under revision by the United States Pharmacopeial Convention (USP), a nonprofit group whose standards are enforceable by state and federal regulators.
In the same position statement on compounding, the ACR said it also seeks a change to a Food and Drug Administration rule limiting practitioners’ access to quinacrine, a drug only available through compounding pharmacies that is sometimes used to treat lupus patients. Quinacrine is not on the FDA’s current list of bulk substances approved for compounding, except by special permission. The ACR has asked the agency to add quinacrine to the list, but no one knows when this will happen.
Rheumatologists may also be more restricted than before in terms of which compounding pharmacies they can turn to, as new federal standards divide them into two types – those that can provide medicines in larger quantities and those that can’t.
Steroid fiasco sparked rule revisions
The ACR’s concerns follow a tighter focus by state and federal agencies on drug compounding after a fungal meningitis outbreak in 2012 was traced to contaminated steroids produced in bulk by a compounding pharmacy.
More than 800 infections, 64 of them fatal, occurred after the New England Compounding Center in Framingham, Mass., sold contaminated methylprednisolone acetate that was used in epidural and intra-articular joint injections.
The following year Congress passed the Drug Quality and Security Act, which aims, in part, to prevent compounding pharmacies from engaging in what amounts to unregulated manufacturing.
As part of the law, the FDA created a list of drugs appropriate for compounding and a process by which larger compounding pharmacies must register with the FDA, and agree to inspections. The USP standards, meanwhile, address detailed technical and safety aspects of compounding and are enforceable by the FDA and state agencies.
“USP and FDA have had the ability to regulate compounding for over a decade, but only recently have the rules become actively enforced,” said Donald Miller, PharmD, of North Dakota State University, Fargo, who helped shape the ACR’s position statement on compounding with the help of rheumatologists in private practice.
“When you make guidelines for safety, they make sense, but then you can’t anticipate the way it’s going to affect individuals’ practice. And that’s where rheumatology got caught up,” said Dr. Miller, who was a member of the FDA Arthritis Advisory Committee in 2014-2016.
In-office mixing a top concern
Other specialties, including dermatology and immunology, also stand to be affected by various changes to compounding law and practice – and their societies have been active in voicing concerns.
Though the latest revisions of USP chapter 797, which impacts in-office mixing, are still being sorted out, it’s the No. 1 compounding-related concern for rheumatologists, Dr. Miller said.
Rheumatologists routinely mix an analgesic and a steroid for injection. The analgesic makes the steroids less viscous, and offers patients hours of immediate relief. They also add analgesics to hyaluronic acid injected for viscosupplementation. The mixing is usually conducted bedside, and the injections are administered right away.
Technically, combining these products amounts to sterile compounding, Dr. Miller explained. “And theoretically, under these rules, a physician could still do this, but they’d have to do it under a sterile hood like you find in a pharmacy, and that’s just not practical. It also becomes a matter of interpretation.”
USP chapter 797 sanctions in-office mixing for “immediate use” with individual patients – which is nearly always the case for the steroid injections used in rheumatology. But it’s unclear whether “immediate use” means emergency use only, or allows for routine use, as rheumatologists hope.
“One reason this came to rheumatology’s attention is that some state boards of medicine were inspecting and saying ‘Hey, you can’t do that,’ ” Dr. Miller said.
“There’s that law of unintended consequences where you snare things in a net that you really don’t want to,” Dr. Huffstutter said.
Marcus Snow, MD, a rheumatologist at the University of Nebraska, Omaha, who also worked on the statement, said that most rheumatologists are likely unaware that their ability to mix drugs in-office has been called into question.
“I brought it up at our division meeting with a group of 10 rheumatologists, and no one was aware that this was coming down the pike,” Dr. Snow said in an interview.
Pediatric issues
Pediatric rheumatologists, and adult rheumatologists who see children occasionally, use compounding pharmacies to create palatable oral medicines and adjusted doses of adult treatments.
They also use injections combining steroids with analgesics, and consider the addition of the analgesic a key aid to compliance.
“The biggest barrier we have is patient and parent anxiety about doing the procedure and the associated pain. We always administer our steroids mixed with lidocaine to help with the postprocedural discomfort,” said Adam Reinhardt, MD, chief of pediatric rheumatology at the University of Nebraska and Children’s Hospital and Medical Center in Omaha.
Steroid injections can mean avoiding or delaying systemic treatment in children with oligoarticular arthritis, he said. “Most of us consider them a first-line therapy. The hope is that you can get by without having to use meds like methotrexate if you can get a prolonged response in the one or two joints that are active in that patient.”
But Dr. Reinhardt said that, while he mixed his own injections during his fellowship training, Children’s of Omaha now insists that they be prepared by in-house pharmacists, working under sterile hoods. The delay to receiving them in the clinic or procedure room is 40 minutes to an hour, he said, which the clinicians accommodate through careful scheduling.
The change from mixing in-clinic to relying on the central pharmacy came about in recent years, Dr. Reinhardt said, because of broader concerns related to medication storage in the clinics. While ordering from the central pharmacy works for his practice, he said, “I probably only inject maybe 50-70 joints a year, while adult rheumatologists are injecting far more than that. For a busy private practice, I can see that being a huge time constraint,” he said.
Relevance of rules
None of the rheumatologists interviewed questioned the need for tightened state and federal oversight of compounding practices overall – just the applicability of certain rules to their own practice.
Dr. Snow and Dr. Huffstutter noted that reports of infected joints – a potential result of a contaminated injection – are sporadic and rare. “There’s very little research in this, but [these types of injections] have been standard practice for decades,” Dr. Snow said.
Srikanth Mukkera, MD, a rheumatologist in Tupelo, Miss., agreed that “sporadic cases of joint infection do happen following injection, but it can be hard to show if an injection was the cause.”
Assuring that medicines are mixed only immediately prior to injection, and not stored, reduces the likelihood of contamination, Dr. Mukkera said. Moreover, he noted, epidural injections such as those that resulted in the 2012 meningitis outbreak carry different risks than those seen in intra-articular injections.
Dr. Miller, the lead author of the ACR statement, said that the rheumatologists on our committee “don’t know of anyone that’s had a knee or other joint infection from a contaminated injection. They feel that unless somebody finds some evidence of that, they should be allowed to continue” with their usual practice.
He said that he feels that the USP will ultimately heed the concerns of rheumatologists and hopefully provide a more relaxed interpretation of in-office compounding. “We’re hoping they’ll make some exceptions when they revise 797 standards or at least maybe leave room for organizations to create a best practice statement. We’ll see,” Dr. Miller said.
But this is in no way guaranteed. Dr. Huffstutter said he fears that, if the rules come to be interpreted more narrowly, even standard practices like reconstituting biologic drugs for infusion – something that’s also a routine part of in-office practice – could fall under the rubric of sterile compounding and come into question.
The quinacrine problem
A separate compounding-related issue in rheumatology is clinicians’ access to quinacrine, an antimalarial rheumatology drug that, while infrequently used, represents the only alternative to hydroxychloroquine for some lupus patients.
“There are no alternatives out there for hydroxychloroquine, so we need it as a backup,” Dr. Snow said. “If hydroxychloroquine isn’t an option, there’s nothing out there that we can use. There’s no easy replacement.”
Dr. Huffstutter said he currently had no patients on quinacrine. “It’s not very often that we use it, but in those patients that really need it, it can make a huge difference in how they do.”
Quinacrine is no longer manufactured commercially as a finished drug product but is available in a powder that compounding physicians put into 100-mg capsules. It is not on the FDA’s current list of drugs available for compounding except with special permission.
While the ACR has requested that the FDA add it the list of bulk drug substances that can be used in compounding, quinacrine remains off the list for now – and, providers say, hard to find.
Moreover, while rheumatologists may have previously been able to order and store quantities of quinacrine and other compounded nonsterile medications to dispense to their patients, they can no longer easily do so, as only the FDA-approved compounding “outsourcing facilities” are allowed to process larger orders; the rest can only respond to prescriptions for individual patients.
Dr. Miller said it’s likely that quinacrine will make it onto the FDA’s next list of bulk drugs available for compounding. “The FDA has kind of said, ‘Don’t worry about it,’ ” he said.
As new rules about drug compounding get shaped, rheumatologists seek to protect their ability to combine injectable drugs – most commonly a steroid and a local anesthetic – in their own offices.
In a position statement sent to government agencies and members of Congress in February, the American College of Rheumatology voiced concerns that the practice, which it called “critical,” could become a casualty of drug-compounding regulations under revision by the United States Pharmacopeial Convention (USP), a nonprofit group whose standards are enforceable by state and federal regulators.
In the same position statement on compounding, the ACR said it also seeks a change to a Food and Drug Administration rule limiting practitioners’ access to quinacrine, a drug only available through compounding pharmacies that is sometimes used to treat lupus patients. Quinacrine is not on the FDA’s current list of bulk substances approved for compounding, except by special permission. The ACR has asked the agency to add quinacrine to the list, but no one knows when this will happen.
Rheumatologists may also be more restricted than before in terms of which compounding pharmacies they can turn to, as new federal standards divide them into two types – those that can provide medicines in larger quantities and those that can’t.
Steroid fiasco sparked rule revisions
The ACR’s concerns follow a tighter focus by state and federal agencies on drug compounding after a fungal meningitis outbreak in 2012 was traced to contaminated steroids produced in bulk by a compounding pharmacy.
More than 800 infections, 64 of them fatal, occurred after the New England Compounding Center in Framingham, Mass., sold contaminated methylprednisolone acetate that was used in epidural and intra-articular joint injections.
The following year Congress passed the Drug Quality and Security Act, which aims, in part, to prevent compounding pharmacies from engaging in what amounts to unregulated manufacturing.
As part of the law, the FDA created a list of drugs appropriate for compounding and a process by which larger compounding pharmacies must register with the FDA, and agree to inspections. The USP standards, meanwhile, address detailed technical and safety aspects of compounding and are enforceable by the FDA and state agencies.
“USP and FDA have had the ability to regulate compounding for over a decade, but only recently have the rules become actively enforced,” said Donald Miller, PharmD, of North Dakota State University, Fargo, who helped shape the ACR’s position statement on compounding with the help of rheumatologists in private practice.
“When you make guidelines for safety, they make sense, but then you can’t anticipate the way it’s going to affect individuals’ practice. And that’s where rheumatology got caught up,” said Dr. Miller, who was a member of the FDA Arthritis Advisory Committee in 2014-2016.
In-office mixing a top concern
Other specialties, including dermatology and immunology, also stand to be affected by various changes to compounding law and practice – and their societies have been active in voicing concerns.
Though the latest revisions of USP chapter 797, which impacts in-office mixing, are still being sorted out, it’s the No. 1 compounding-related concern for rheumatologists, Dr. Miller said.
Rheumatologists routinely mix an analgesic and a steroid for injection. The analgesic makes the steroids less viscous, and offers patients hours of immediate relief. They also add analgesics to hyaluronic acid injected for viscosupplementation. The mixing is usually conducted bedside, and the injections are administered right away.
Technically, combining these products amounts to sterile compounding, Dr. Miller explained. “And theoretically, under these rules, a physician could still do this, but they’d have to do it under a sterile hood like you find in a pharmacy, and that’s just not practical. It also becomes a matter of interpretation.”
USP chapter 797 sanctions in-office mixing for “immediate use” with individual patients – which is nearly always the case for the steroid injections used in rheumatology. But it’s unclear whether “immediate use” means emergency use only, or allows for routine use, as rheumatologists hope.
“One reason this came to rheumatology’s attention is that some state boards of medicine were inspecting and saying ‘Hey, you can’t do that,’ ” Dr. Miller said.
“There’s that law of unintended consequences where you snare things in a net that you really don’t want to,” Dr. Huffstutter said.
Marcus Snow, MD, a rheumatologist at the University of Nebraska, Omaha, who also worked on the statement, said that most rheumatologists are likely unaware that their ability to mix drugs in-office has been called into question.
“I brought it up at our division meeting with a group of 10 rheumatologists, and no one was aware that this was coming down the pike,” Dr. Snow said in an interview.
Pediatric issues
Pediatric rheumatologists, and adult rheumatologists who see children occasionally, use compounding pharmacies to create palatable oral medicines and adjusted doses of adult treatments.
They also use injections combining steroids with analgesics, and consider the addition of the analgesic a key aid to compliance.
“The biggest barrier we have is patient and parent anxiety about doing the procedure and the associated pain. We always administer our steroids mixed with lidocaine to help with the postprocedural discomfort,” said Adam Reinhardt, MD, chief of pediatric rheumatology at the University of Nebraska and Children’s Hospital and Medical Center in Omaha.
Steroid injections can mean avoiding or delaying systemic treatment in children with oligoarticular arthritis, he said. “Most of us consider them a first-line therapy. The hope is that you can get by without having to use meds like methotrexate if you can get a prolonged response in the one or two joints that are active in that patient.”
But Dr. Reinhardt said that, while he mixed his own injections during his fellowship training, Children’s of Omaha now insists that they be prepared by in-house pharmacists, working under sterile hoods. The delay to receiving them in the clinic or procedure room is 40 minutes to an hour, he said, which the clinicians accommodate through careful scheduling.
The change from mixing in-clinic to relying on the central pharmacy came about in recent years, Dr. Reinhardt said, because of broader concerns related to medication storage in the clinics. While ordering from the central pharmacy works for his practice, he said, “I probably only inject maybe 50-70 joints a year, while adult rheumatologists are injecting far more than that. For a busy private practice, I can see that being a huge time constraint,” he said.
Relevance of rules
None of the rheumatologists interviewed questioned the need for tightened state and federal oversight of compounding practices overall – just the applicability of certain rules to their own practice.
Dr. Snow and Dr. Huffstutter noted that reports of infected joints – a potential result of a contaminated injection – are sporadic and rare. “There’s very little research in this, but [these types of injections] have been standard practice for decades,” Dr. Snow said.
Srikanth Mukkera, MD, a rheumatologist in Tupelo, Miss., agreed that “sporadic cases of joint infection do happen following injection, but it can be hard to show if an injection was the cause.”
Assuring that medicines are mixed only immediately prior to injection, and not stored, reduces the likelihood of contamination, Dr. Mukkera said. Moreover, he noted, epidural injections such as those that resulted in the 2012 meningitis outbreak carry different risks than those seen in intra-articular injections.
Dr. Miller, the lead author of the ACR statement, said that the rheumatologists on our committee “don’t know of anyone that’s had a knee or other joint infection from a contaminated injection. They feel that unless somebody finds some evidence of that, they should be allowed to continue” with their usual practice.
He said that he feels that the USP will ultimately heed the concerns of rheumatologists and hopefully provide a more relaxed interpretation of in-office compounding. “We’re hoping they’ll make some exceptions when they revise 797 standards or at least maybe leave room for organizations to create a best practice statement. We’ll see,” Dr. Miller said.
But this is in no way guaranteed. Dr. Huffstutter said he fears that, if the rules come to be interpreted more narrowly, even standard practices like reconstituting biologic drugs for infusion – something that’s also a routine part of in-office practice – could fall under the rubric of sterile compounding and come into question.
The quinacrine problem
A separate compounding-related issue in rheumatology is clinicians’ access to quinacrine, an antimalarial rheumatology drug that, while infrequently used, represents the only alternative to hydroxychloroquine for some lupus patients.
“There are no alternatives out there for hydroxychloroquine, so we need it as a backup,” Dr. Snow said. “If hydroxychloroquine isn’t an option, there’s nothing out there that we can use. There’s no easy replacement.”
Dr. Huffstutter said he currently had no patients on quinacrine. “It’s not very often that we use it, but in those patients that really need it, it can make a huge difference in how they do.”
Quinacrine is no longer manufactured commercially as a finished drug product but is available in a powder that compounding physicians put into 100-mg capsules. It is not on the FDA’s current list of drugs available for compounding except with special permission.
While the ACR has requested that the FDA add it the list of bulk drug substances that can be used in compounding, quinacrine remains off the list for now – and, providers say, hard to find.
Moreover, while rheumatologists may have previously been able to order and store quantities of quinacrine and other compounded nonsterile medications to dispense to their patients, they can no longer easily do so, as only the FDA-approved compounding “outsourcing facilities” are allowed to process larger orders; the rest can only respond to prescriptions for individual patients.
Dr. Miller said it’s likely that quinacrine will make it onto the FDA’s next list of bulk drugs available for compounding. “The FDA has kind of said, ‘Don’t worry about it,’ ” he said.
As new rules about drug compounding get shaped, rheumatologists seek to protect their ability to combine injectable drugs – most commonly a steroid and a local anesthetic – in their own offices.
In a position statement sent to government agencies and members of Congress in February, the American College of Rheumatology voiced concerns that the practice, which it called “critical,” could become a casualty of drug-compounding regulations under revision by the United States Pharmacopeial Convention (USP), a nonprofit group whose standards are enforceable by state and federal regulators.
In the same position statement on compounding, the ACR said it also seeks a change to a Food and Drug Administration rule limiting practitioners’ access to quinacrine, a drug only available through compounding pharmacies that is sometimes used to treat lupus patients. Quinacrine is not on the FDA’s current list of bulk substances approved for compounding, except by special permission. The ACR has asked the agency to add quinacrine to the list, but no one knows when this will happen.
Rheumatologists may also be more restricted than before in terms of which compounding pharmacies they can turn to, as new federal standards divide them into two types – those that can provide medicines in larger quantities and those that can’t.
Steroid fiasco sparked rule revisions
The ACR’s concerns follow a tighter focus by state and federal agencies on drug compounding after a fungal meningitis outbreak in 2012 was traced to contaminated steroids produced in bulk by a compounding pharmacy.
More than 800 infections, 64 of them fatal, occurred after the New England Compounding Center in Framingham, Mass., sold contaminated methylprednisolone acetate that was used in epidural and intra-articular joint injections.
The following year Congress passed the Drug Quality and Security Act, which aims, in part, to prevent compounding pharmacies from engaging in what amounts to unregulated manufacturing.
As part of the law, the FDA created a list of drugs appropriate for compounding and a process by which larger compounding pharmacies must register with the FDA, and agree to inspections. The USP standards, meanwhile, address detailed technical and safety aspects of compounding and are enforceable by the FDA and state agencies.
“USP and FDA have had the ability to regulate compounding for over a decade, but only recently have the rules become actively enforced,” said Donald Miller, PharmD, of North Dakota State University, Fargo, who helped shape the ACR’s position statement on compounding with the help of rheumatologists in private practice.
“When you make guidelines for safety, they make sense, but then you can’t anticipate the way it’s going to affect individuals’ practice. And that’s where rheumatology got caught up,” said Dr. Miller, who was a member of the FDA Arthritis Advisory Committee in 2014-2016.
In-office mixing a top concern
Other specialties, including dermatology and immunology, also stand to be affected by various changes to compounding law and practice – and their societies have been active in voicing concerns.
Though the latest revisions of USP chapter 797, which impacts in-office mixing, are still being sorted out, it’s the No. 1 compounding-related concern for rheumatologists, Dr. Miller said.
Rheumatologists routinely mix an analgesic and a steroid for injection. The analgesic makes the steroids less viscous, and offers patients hours of immediate relief. They also add analgesics to hyaluronic acid injected for viscosupplementation. The mixing is usually conducted bedside, and the injections are administered right away.
Technically, combining these products amounts to sterile compounding, Dr. Miller explained. “And theoretically, under these rules, a physician could still do this, but they’d have to do it under a sterile hood like you find in a pharmacy, and that’s just not practical. It also becomes a matter of interpretation.”
USP chapter 797 sanctions in-office mixing for “immediate use” with individual patients – which is nearly always the case for the steroid injections used in rheumatology. But it’s unclear whether “immediate use” means emergency use only, or allows for routine use, as rheumatologists hope.
“One reason this came to rheumatology’s attention is that some state boards of medicine were inspecting and saying ‘Hey, you can’t do that,’ ” Dr. Miller said.
“There’s that law of unintended consequences where you snare things in a net that you really don’t want to,” Dr. Huffstutter said.
Marcus Snow, MD, a rheumatologist at the University of Nebraska, Omaha, who also worked on the statement, said that most rheumatologists are likely unaware that their ability to mix drugs in-office has been called into question.
“I brought it up at our division meeting with a group of 10 rheumatologists, and no one was aware that this was coming down the pike,” Dr. Snow said in an interview.
Pediatric issues
Pediatric rheumatologists, and adult rheumatologists who see children occasionally, use compounding pharmacies to create palatable oral medicines and adjusted doses of adult treatments.
They also use injections combining steroids with analgesics, and consider the addition of the analgesic a key aid to compliance.
“The biggest barrier we have is patient and parent anxiety about doing the procedure and the associated pain. We always administer our steroids mixed with lidocaine to help with the postprocedural discomfort,” said Adam Reinhardt, MD, chief of pediatric rheumatology at the University of Nebraska and Children’s Hospital and Medical Center in Omaha.
Steroid injections can mean avoiding or delaying systemic treatment in children with oligoarticular arthritis, he said. “Most of us consider them a first-line therapy. The hope is that you can get by without having to use meds like methotrexate if you can get a prolonged response in the one or two joints that are active in that patient.”
But Dr. Reinhardt said that, while he mixed his own injections during his fellowship training, Children’s of Omaha now insists that they be prepared by in-house pharmacists, working under sterile hoods. The delay to receiving them in the clinic or procedure room is 40 minutes to an hour, he said, which the clinicians accommodate through careful scheduling.
The change from mixing in-clinic to relying on the central pharmacy came about in recent years, Dr. Reinhardt said, because of broader concerns related to medication storage in the clinics. While ordering from the central pharmacy works for his practice, he said, “I probably only inject maybe 50-70 joints a year, while adult rheumatologists are injecting far more than that. For a busy private practice, I can see that being a huge time constraint,” he said.
Relevance of rules
None of the rheumatologists interviewed questioned the need for tightened state and federal oversight of compounding practices overall – just the applicability of certain rules to their own practice.
Dr. Snow and Dr. Huffstutter noted that reports of infected joints – a potential result of a contaminated injection – are sporadic and rare. “There’s very little research in this, but [these types of injections] have been standard practice for decades,” Dr. Snow said.
Srikanth Mukkera, MD, a rheumatologist in Tupelo, Miss., agreed that “sporadic cases of joint infection do happen following injection, but it can be hard to show if an injection was the cause.”
Assuring that medicines are mixed only immediately prior to injection, and not stored, reduces the likelihood of contamination, Dr. Mukkera said. Moreover, he noted, epidural injections such as those that resulted in the 2012 meningitis outbreak carry different risks than those seen in intra-articular injections.
Dr. Miller, the lead author of the ACR statement, said that the rheumatologists on our committee “don’t know of anyone that’s had a knee or other joint infection from a contaminated injection. They feel that unless somebody finds some evidence of that, they should be allowed to continue” with their usual practice.
He said that he feels that the USP will ultimately heed the concerns of rheumatologists and hopefully provide a more relaxed interpretation of in-office compounding. “We’re hoping they’ll make some exceptions when they revise 797 standards or at least maybe leave room for organizations to create a best practice statement. We’ll see,” Dr. Miller said.
But this is in no way guaranteed. Dr. Huffstutter said he fears that, if the rules come to be interpreted more narrowly, even standard practices like reconstituting biologic drugs for infusion – something that’s also a routine part of in-office practice – could fall under the rubric of sterile compounding and come into question.
The quinacrine problem
A separate compounding-related issue in rheumatology is clinicians’ access to quinacrine, an antimalarial rheumatology drug that, while infrequently used, represents the only alternative to hydroxychloroquine for some lupus patients.
“There are no alternatives out there for hydroxychloroquine, so we need it as a backup,” Dr. Snow said. “If hydroxychloroquine isn’t an option, there’s nothing out there that we can use. There’s no easy replacement.”
Dr. Huffstutter said he currently had no patients on quinacrine. “It’s not very often that we use it, but in those patients that really need it, it can make a huge difference in how they do.”
Quinacrine is no longer manufactured commercially as a finished drug product but is available in a powder that compounding physicians put into 100-mg capsules. It is not on the FDA’s current list of drugs available for compounding except with special permission.
While the ACR has requested that the FDA add it the list of bulk drug substances that can be used in compounding, quinacrine remains off the list for now – and, providers say, hard to find.
Moreover, while rheumatologists may have previously been able to order and store quantities of quinacrine and other compounded nonsterile medications to dispense to their patients, they can no longer easily do so, as only the FDA-approved compounding “outsourcing facilities” are allowed to process larger orders; the rest can only respond to prescriptions for individual patients.
Dr. Miller said it’s likely that quinacrine will make it onto the FDA’s next list of bulk drugs available for compounding. “The FDA has kind of said, ‘Don’t worry about it,’ ” he said.
Military Sexual Trauma and Sexual Health: Practice and Future Research for Mental Health Professionals
About 24% of women and 1% of men will experience military sexual trauma (MST) during their service.1 Despite the higher percentage of women reporting MST, the estimated number of men (55,491) and women (72,497) who endorse MST is relatively similar. Military sexual trauma is associated with negative psychosocial (eg, decreased quality of life) and psychiatric (eg, posttraumatic stress disorder [PTSD], depression) sequelae. Surís and colleagues provided a full review of sequelae, with PTSD being the most discussed consequence of MST.2 However, sexually transmitted infections (STIs) during or after MST are a consequence of growing concern.
Sexually Transmitted Infections
The link between sexual trauma and increased incidence of STIs is well established. Survivors of rape are at a higher risk of exposure to STIs due to unprotected sexual contact that may occur during the assault(s).3 Numerous studies have demonstrated that sexual trauma is directly related to greater engagement in risky sexual behaviors (eg, more sexual partners, unprotected sex, and “sex trading”).2,4
This relationship is particularly concerning given that individuals in the military tend to report sexual trauma with greater propensity than that reported in civilian populations.4 Additionally, military personnel and veteran populations tend to engage in high-risk behaviors (eg, alcohol and drug use) more often than their civilian counterparts, increasing their potential susceptibility to predatory sexual trauma(s) and victimization.2,5 Taken in aggregate, military personnel and veterans may be at increased risk for STIs compared with the civilian population due to the increased incidence of risky sexual behavior and sexual traumatization during military service.
Sexually transmitted infections potentially lead to immediate-term (eg, physical discomfort, sexual dysfunction) and long-term (eg, cancer, infertility) adverse health consequences.6 Early detection is crucial in the treatment of STIs because it can aid in preventing STI transmission and allow for early intervention. See Table for a list of common STIs and their prevalence, testing method, method of sexual transmission, and treatment. Early detection is also important because many STIs may be asymptomatic (eg, HIV, human papillomavirus [HPV]), which decreases the likelihood of seeking testing or treatment as well as increases the likelihood of transmission.7
Current Research
To date, only 1 study has explicitly examined the relationship between MST and STIs. In 2011, researchers analyzed a large national database of 420,725 male and female Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans.6 In the study, both male and female OEF/OIF veterans who endorsed MST were significantly more likely than those who did not endorse MST to have a STI diagnosis. The researchers noted that this finding underscored the necessity for sexual health assessment in survivors of MST, to facilitate early detection and treatment.
STI Risk Assessment
Because military personnel and veterans often initially disclose their MST to a mental health provider (MHP), these providers operate in a unique circumstance where they may be the individual’s first point of contact for determining STI risk. In these circumstances, the MHP should consider the utility of briefly assessing the patient’s sexual health and making subsequent medical referrals as necessary.
To accurately assess a patient’s STI risk, the MHP should gather information regarding both current/acute risk (eg, “Have you had unprotected sexual contact, for instance, genital contact without a condom or oral sex without a dental dam, in the past month?”) as well as longer standing (eg, “Have you had unprotected sexual contact... in the past year?”) STI risk. Providers also should consider oral, anal, and genital modes of sexual contact as well as common STI symptoms (eg, warts, sores, genital discharge, and/or pain or burning sensation when peeing or during sex). Additionally, psychoeducation should be provided, especially information regarding the asymptomatic nature of certain STIs, such as HIV and HPV, and the risk of transmission in all forms of sexual contact, including nongenital anal contact. Appropriate referrals to sexual health education, including safe sex practices, also should be considered in order to minimize risk of future STI transmission.
Mental health providers also should determine the date of patient’s most recent STI test. Not all STIs can be detected via a blood test or routine yearly Papanicolaou (Pap test) physicals. Additionally, MHPs should be aware that risky practices, including substance misuse, are more common in survivors of MST and that there is an association between substance misuse and STIs.2,6 If testing has not occurred recently, MHPs should strongly encourage the individual to access STI testing and provide resources as necessary, such as access to low-cost STI testing. Further, if the MHP has reason to suspect the presence of an STI after the brief assessment (eg, individual endorses unprotected sexual contact or risky behaviors, including substance misuse, sex trading, and STI symptomatology; a positive STI test but the patient has not accessed treatment), an appropriate sexual health referral should be made.
During the assessment and psychoeducation processes, terminology and language plays an integral role. If a MHP assumes an individual has sexual contact only with opposite sex partners (eg, asking a male “How many women have you had sexual contact with in the past 30 days?” vs “How many partners have you had sexual contact with in the past 30 days?”), the MHP will not accurately assess the individual’s level of current risk. Additionally, it is important to remember that sexual behavior does not always align with sexual identity: A man who identifies as heterosexual may still have sexual contact with men. Due to the sensitive nature of sexual health, MHPs should be careful to use nonjudgmental language, such as using the term sex work rather than the more pejorative term prostitution, to avoid offending patients and to increase their likelihood to disclose sexual health information.
Nonjudgmental language is especially relevant when working with gender-minority veterans (eg, transgender, gender nonconforming, gender transitioning), because this clinical population has a higher risk of victimization and lower rates of help-seeking health behavior.7 In particular, a sizable portion of individuals who identify as transgender do not seek services out of fear that they will be discriminated against, humiliated, or misunderstood.7 To assuage these concerns, MHPs should ensure they refer to the veteran with the veteran’s preferred pronouns. For example, a MHP could ask “I would like to be respectful, how would you like to be addressed?” or “What name and pronoun would you like me/us to use?” Providers also should consider nonbinary pronouns when appropriate (eg, singular: ze/hir/hirs; plural: they/them/theirs). Providers also should recognize that making a mistake is not uncommon, and they should apologize to maintain rapport and maximize the patient’s comfort during this distressing process. Further, MHPs should consider additional training, education, and/or consultation if they feel uncomfortable or ill prepared when working with gender-minority veterans.
Future Research
Research has attempted to understand the consequences of MST on sexual health; however, despite these efforts, more research is necessary. The majority of published studies have focused on females even though a similar number of males have reported MST.2 This dearth of published studies likely is due to hesitation by male active-duty personnel and veterans to disclose or seek treatment for MST and because the percentage of females reporting MST is much higher. Males are less likely to report or seek treatment for MST because of stigma-based concerns (eg, shame, self-blame, privacy concerns).8,9 Therefore, it is difficult to acquire a sizable research sample to study. As previously noted, a single study has specifically examined MST and STI risk. Although this study included a sizable population of male OEF/OIF veterans, results have yet to be replicated in other clinical populations of interest, such as male military personnel and male veterans of other service eras.
Research is even more limited regarding lesbian, gay, bisexual, transgender, and other gender-minority military personnel and veterans. Although researchers propose that these populations may experience a similar, or even heightened, likelihood of MST during their service, no empirical research yet exists to fully examine this hypothesis.10,11 It is important to note that the paucity of research attention may be related to the Don’t Ask, Don’t Tell (DADT) policy, which obstructed the open discussion and empirical examination of sexual and gender minorities within military populations.11 The DADT policy led to limited awareness and greater stigmatization among sexual- and gender-minority personnel, resulting in poorer sexual health outcomes in these populations.10,11 With the end of DADT in 2011, it is now imperative for future research to examine the prevalence and associated consequences of MST in sexual- and gender-minority military personnel and veterans.
Conclusion
The DoD and VA should be commended for their continued focus on understanding the health consequences of MST. These efforts have yielded substantial information regarding the negative effects of MST on sexual health; in particular, increased risk for STIs. These findings suggest that MHPs may, at times, be the first point of contact for MST-related sexual health concerns. These providers should be aware of their ability to assess for STI risk and make appropriate referrals to facilitate early detection and access to treatment. Despite the presence of MST-related sexual health research, continued research remains necessary. In particular, a broader focus that includes other genders (eg, male, transgender) and sexual minorities would further inform research and clinical practice.
1. Military Sexual Trauma Support Team. Military sexual trauma (MST) screening report fiscal year 2012. Washington DC: U.S. Department of Veterans Affairs, Office of Patient Care Services, Mental Health Services; 2013.
2. Surís A, Holliday R, Weitlauf JC, North CS; the Veteran Safety Initiative Writing Collaborative. Military sexual trauma in the context of veterans’ life experiences. Fed Pract. 2013;30(suppl 3):16S-20S.
3. Jenny C, Hooton TM, Bowers A, et al. Sexually transmitted diseases in victims of rape. N Eng J Med. 1990;322(11):713-716.
4. Senn TE, Carey MP, Vanable PA, Coury-Doniger P, Urban MA. Childhood sexual abuse and sexual risk behavior among men and women attending a sexually transmitted disease clinic. J Consult Clin Psychol. 2006;74(4):720-731.
5. Schultz JR, Bell KM, Naugle AE, Polusny MA. Child sexual abuse and adult sexual assault among military veteran and civilian women. Mil Med. 2006;171(8):723-728.
6. Turchik JA, Pavao J, Nazarian D, Iqbal S, McLean C, Kimerling R. Sexually transmitted infections and sexual dysfunctions among newly returned veterans with and without military sexual trauma. Int J Sex Health. 2012;24(1):45-59.
7. National LGBT Health Education Center. Affirmative care for transgender and gender non-conforming people: best practices for front-line health care staff. https://www.lgbthealth education.org/wp-content/uploads/2016/12/Affirmative-Care-for-Transgender -and-Gender-Non-conforming-People-Best-Practices -for-Frontline-Health-Care-Staff.pdf. Published 2016. Accessed February 16, 2017.
8. Morris EE, Smith JC, Farooqui SY, Surís AM. Unseen battles: the recognition, assessment, and treatment issues of men with military sexual trauma (MST). Trauma Violence Abuse. 2014;15(2):94-101.
9. Turchik JA, Edwards KM. Myths about male rape: a literature review. Psychol Men Masc. 2012;13(2):211-226.
10. Mattocks KM, Kauth MR, Sandfort T, Matza AR, Sullivan JC, Shipherd J. Understanding health-care needs of sexual and gender minority veterans: how targeted research and policy can improve health. LGBT Health. 2014;1(1):50-57.
11. Burks DJ. Lesbian, gay, and bisexual victimization in the military: an unintended consequence of “Don’t Ask, Don’t Tell”? Am Psychol. 2011;66(7):604-613.
About 24% of women and 1% of men will experience military sexual trauma (MST) during their service.1 Despite the higher percentage of women reporting MST, the estimated number of men (55,491) and women (72,497) who endorse MST is relatively similar. Military sexual trauma is associated with negative psychosocial (eg, decreased quality of life) and psychiatric (eg, posttraumatic stress disorder [PTSD], depression) sequelae. Surís and colleagues provided a full review of sequelae, with PTSD being the most discussed consequence of MST.2 However, sexually transmitted infections (STIs) during or after MST are a consequence of growing concern.
Sexually Transmitted Infections
The link between sexual trauma and increased incidence of STIs is well established. Survivors of rape are at a higher risk of exposure to STIs due to unprotected sexual contact that may occur during the assault(s).3 Numerous studies have demonstrated that sexual trauma is directly related to greater engagement in risky sexual behaviors (eg, more sexual partners, unprotected sex, and “sex trading”).2,4
This relationship is particularly concerning given that individuals in the military tend to report sexual trauma with greater propensity than that reported in civilian populations.4 Additionally, military personnel and veteran populations tend to engage in high-risk behaviors (eg, alcohol and drug use) more often than their civilian counterparts, increasing their potential susceptibility to predatory sexual trauma(s) and victimization.2,5 Taken in aggregate, military personnel and veterans may be at increased risk for STIs compared with the civilian population due to the increased incidence of risky sexual behavior and sexual traumatization during military service.
Sexually transmitted infections potentially lead to immediate-term (eg, physical discomfort, sexual dysfunction) and long-term (eg, cancer, infertility) adverse health consequences.6 Early detection is crucial in the treatment of STIs because it can aid in preventing STI transmission and allow for early intervention. See Table for a list of common STIs and their prevalence, testing method, method of sexual transmission, and treatment. Early detection is also important because many STIs may be asymptomatic (eg, HIV, human papillomavirus [HPV]), which decreases the likelihood of seeking testing or treatment as well as increases the likelihood of transmission.7
Current Research
To date, only 1 study has explicitly examined the relationship between MST and STIs. In 2011, researchers analyzed a large national database of 420,725 male and female Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans.6 In the study, both male and female OEF/OIF veterans who endorsed MST were significantly more likely than those who did not endorse MST to have a STI diagnosis. The researchers noted that this finding underscored the necessity for sexual health assessment in survivors of MST, to facilitate early detection and treatment.
STI Risk Assessment
Because military personnel and veterans often initially disclose their MST to a mental health provider (MHP), these providers operate in a unique circumstance where they may be the individual’s first point of contact for determining STI risk. In these circumstances, the MHP should consider the utility of briefly assessing the patient’s sexual health and making subsequent medical referrals as necessary.
To accurately assess a patient’s STI risk, the MHP should gather information regarding both current/acute risk (eg, “Have you had unprotected sexual contact, for instance, genital contact without a condom or oral sex without a dental dam, in the past month?”) as well as longer standing (eg, “Have you had unprotected sexual contact... in the past year?”) STI risk. Providers also should consider oral, anal, and genital modes of sexual contact as well as common STI symptoms (eg, warts, sores, genital discharge, and/or pain or burning sensation when peeing or during sex). Additionally, psychoeducation should be provided, especially information regarding the asymptomatic nature of certain STIs, such as HIV and HPV, and the risk of transmission in all forms of sexual contact, including nongenital anal contact. Appropriate referrals to sexual health education, including safe sex practices, also should be considered in order to minimize risk of future STI transmission.
Mental health providers also should determine the date of patient’s most recent STI test. Not all STIs can be detected via a blood test or routine yearly Papanicolaou (Pap test) physicals. Additionally, MHPs should be aware that risky practices, including substance misuse, are more common in survivors of MST and that there is an association between substance misuse and STIs.2,6 If testing has not occurred recently, MHPs should strongly encourage the individual to access STI testing and provide resources as necessary, such as access to low-cost STI testing. Further, if the MHP has reason to suspect the presence of an STI after the brief assessment (eg, individual endorses unprotected sexual contact or risky behaviors, including substance misuse, sex trading, and STI symptomatology; a positive STI test but the patient has not accessed treatment), an appropriate sexual health referral should be made.
During the assessment and psychoeducation processes, terminology and language plays an integral role. If a MHP assumes an individual has sexual contact only with opposite sex partners (eg, asking a male “How many women have you had sexual contact with in the past 30 days?” vs “How many partners have you had sexual contact with in the past 30 days?”), the MHP will not accurately assess the individual’s level of current risk. Additionally, it is important to remember that sexual behavior does not always align with sexual identity: A man who identifies as heterosexual may still have sexual contact with men. Due to the sensitive nature of sexual health, MHPs should be careful to use nonjudgmental language, such as using the term sex work rather than the more pejorative term prostitution, to avoid offending patients and to increase their likelihood to disclose sexual health information.
Nonjudgmental language is especially relevant when working with gender-minority veterans (eg, transgender, gender nonconforming, gender transitioning), because this clinical population has a higher risk of victimization and lower rates of help-seeking health behavior.7 In particular, a sizable portion of individuals who identify as transgender do not seek services out of fear that they will be discriminated against, humiliated, or misunderstood.7 To assuage these concerns, MHPs should ensure they refer to the veteran with the veteran’s preferred pronouns. For example, a MHP could ask “I would like to be respectful, how would you like to be addressed?” or “What name and pronoun would you like me/us to use?” Providers also should consider nonbinary pronouns when appropriate (eg, singular: ze/hir/hirs; plural: they/them/theirs). Providers also should recognize that making a mistake is not uncommon, and they should apologize to maintain rapport and maximize the patient’s comfort during this distressing process. Further, MHPs should consider additional training, education, and/or consultation if they feel uncomfortable or ill prepared when working with gender-minority veterans.
Future Research
Research has attempted to understand the consequences of MST on sexual health; however, despite these efforts, more research is necessary. The majority of published studies have focused on females even though a similar number of males have reported MST.2 This dearth of published studies likely is due to hesitation by male active-duty personnel and veterans to disclose or seek treatment for MST and because the percentage of females reporting MST is much higher. Males are less likely to report or seek treatment for MST because of stigma-based concerns (eg, shame, self-blame, privacy concerns).8,9 Therefore, it is difficult to acquire a sizable research sample to study. As previously noted, a single study has specifically examined MST and STI risk. Although this study included a sizable population of male OEF/OIF veterans, results have yet to be replicated in other clinical populations of interest, such as male military personnel and male veterans of other service eras.
Research is even more limited regarding lesbian, gay, bisexual, transgender, and other gender-minority military personnel and veterans. Although researchers propose that these populations may experience a similar, or even heightened, likelihood of MST during their service, no empirical research yet exists to fully examine this hypothesis.10,11 It is important to note that the paucity of research attention may be related to the Don’t Ask, Don’t Tell (DADT) policy, which obstructed the open discussion and empirical examination of sexual and gender minorities within military populations.11 The DADT policy led to limited awareness and greater stigmatization among sexual- and gender-minority personnel, resulting in poorer sexual health outcomes in these populations.10,11 With the end of DADT in 2011, it is now imperative for future research to examine the prevalence and associated consequences of MST in sexual- and gender-minority military personnel and veterans.
Conclusion
The DoD and VA should be commended for their continued focus on understanding the health consequences of MST. These efforts have yielded substantial information regarding the negative effects of MST on sexual health; in particular, increased risk for STIs. These findings suggest that MHPs may, at times, be the first point of contact for MST-related sexual health concerns. These providers should be aware of their ability to assess for STI risk and make appropriate referrals to facilitate early detection and access to treatment. Despite the presence of MST-related sexual health research, continued research remains necessary. In particular, a broader focus that includes other genders (eg, male, transgender) and sexual minorities would further inform research and clinical practice.
About 24% of women and 1% of men will experience military sexual trauma (MST) during their service.1 Despite the higher percentage of women reporting MST, the estimated number of men (55,491) and women (72,497) who endorse MST is relatively similar. Military sexual trauma is associated with negative psychosocial (eg, decreased quality of life) and psychiatric (eg, posttraumatic stress disorder [PTSD], depression) sequelae. Surís and colleagues provided a full review of sequelae, with PTSD being the most discussed consequence of MST.2 However, sexually transmitted infections (STIs) during or after MST are a consequence of growing concern.
Sexually Transmitted Infections
The link between sexual trauma and increased incidence of STIs is well established. Survivors of rape are at a higher risk of exposure to STIs due to unprotected sexual contact that may occur during the assault(s).3 Numerous studies have demonstrated that sexual trauma is directly related to greater engagement in risky sexual behaviors (eg, more sexual partners, unprotected sex, and “sex trading”).2,4
This relationship is particularly concerning given that individuals in the military tend to report sexual trauma with greater propensity than that reported in civilian populations.4 Additionally, military personnel and veteran populations tend to engage in high-risk behaviors (eg, alcohol and drug use) more often than their civilian counterparts, increasing their potential susceptibility to predatory sexual trauma(s) and victimization.2,5 Taken in aggregate, military personnel and veterans may be at increased risk for STIs compared with the civilian population due to the increased incidence of risky sexual behavior and sexual traumatization during military service.
Sexually transmitted infections potentially lead to immediate-term (eg, physical discomfort, sexual dysfunction) and long-term (eg, cancer, infertility) adverse health consequences.6 Early detection is crucial in the treatment of STIs because it can aid in preventing STI transmission and allow for early intervention. See Table for a list of common STIs and their prevalence, testing method, method of sexual transmission, and treatment. Early detection is also important because many STIs may be asymptomatic (eg, HIV, human papillomavirus [HPV]), which decreases the likelihood of seeking testing or treatment as well as increases the likelihood of transmission.7
Current Research
To date, only 1 study has explicitly examined the relationship between MST and STIs. In 2011, researchers analyzed a large national database of 420,725 male and female Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans.6 In the study, both male and female OEF/OIF veterans who endorsed MST were significantly more likely than those who did not endorse MST to have a STI diagnosis. The researchers noted that this finding underscored the necessity for sexual health assessment in survivors of MST, to facilitate early detection and treatment.
STI Risk Assessment
Because military personnel and veterans often initially disclose their MST to a mental health provider (MHP), these providers operate in a unique circumstance where they may be the individual’s first point of contact for determining STI risk. In these circumstances, the MHP should consider the utility of briefly assessing the patient’s sexual health and making subsequent medical referrals as necessary.
To accurately assess a patient’s STI risk, the MHP should gather information regarding both current/acute risk (eg, “Have you had unprotected sexual contact, for instance, genital contact without a condom or oral sex without a dental dam, in the past month?”) as well as longer standing (eg, “Have you had unprotected sexual contact... in the past year?”) STI risk. Providers also should consider oral, anal, and genital modes of sexual contact as well as common STI symptoms (eg, warts, sores, genital discharge, and/or pain or burning sensation when peeing or during sex). Additionally, psychoeducation should be provided, especially information regarding the asymptomatic nature of certain STIs, such as HIV and HPV, and the risk of transmission in all forms of sexual contact, including nongenital anal contact. Appropriate referrals to sexual health education, including safe sex practices, also should be considered in order to minimize risk of future STI transmission.
Mental health providers also should determine the date of patient’s most recent STI test. Not all STIs can be detected via a blood test or routine yearly Papanicolaou (Pap test) physicals. Additionally, MHPs should be aware that risky practices, including substance misuse, are more common in survivors of MST and that there is an association between substance misuse and STIs.2,6 If testing has not occurred recently, MHPs should strongly encourage the individual to access STI testing and provide resources as necessary, such as access to low-cost STI testing. Further, if the MHP has reason to suspect the presence of an STI after the brief assessment (eg, individual endorses unprotected sexual contact or risky behaviors, including substance misuse, sex trading, and STI symptomatology; a positive STI test but the patient has not accessed treatment), an appropriate sexual health referral should be made.
During the assessment and psychoeducation processes, terminology and language plays an integral role. If a MHP assumes an individual has sexual contact only with opposite sex partners (eg, asking a male “How many women have you had sexual contact with in the past 30 days?” vs “How many partners have you had sexual contact with in the past 30 days?”), the MHP will not accurately assess the individual’s level of current risk. Additionally, it is important to remember that sexual behavior does not always align with sexual identity: A man who identifies as heterosexual may still have sexual contact with men. Due to the sensitive nature of sexual health, MHPs should be careful to use nonjudgmental language, such as using the term sex work rather than the more pejorative term prostitution, to avoid offending patients and to increase their likelihood to disclose sexual health information.
Nonjudgmental language is especially relevant when working with gender-minority veterans (eg, transgender, gender nonconforming, gender transitioning), because this clinical population has a higher risk of victimization and lower rates of help-seeking health behavior.7 In particular, a sizable portion of individuals who identify as transgender do not seek services out of fear that they will be discriminated against, humiliated, or misunderstood.7 To assuage these concerns, MHPs should ensure they refer to the veteran with the veteran’s preferred pronouns. For example, a MHP could ask “I would like to be respectful, how would you like to be addressed?” or “What name and pronoun would you like me/us to use?” Providers also should consider nonbinary pronouns when appropriate (eg, singular: ze/hir/hirs; plural: they/them/theirs). Providers also should recognize that making a mistake is not uncommon, and they should apologize to maintain rapport and maximize the patient’s comfort during this distressing process. Further, MHPs should consider additional training, education, and/or consultation if they feel uncomfortable or ill prepared when working with gender-minority veterans.
Future Research
Research has attempted to understand the consequences of MST on sexual health; however, despite these efforts, more research is necessary. The majority of published studies have focused on females even though a similar number of males have reported MST.2 This dearth of published studies likely is due to hesitation by male active-duty personnel and veterans to disclose or seek treatment for MST and because the percentage of females reporting MST is much higher. Males are less likely to report or seek treatment for MST because of stigma-based concerns (eg, shame, self-blame, privacy concerns).8,9 Therefore, it is difficult to acquire a sizable research sample to study. As previously noted, a single study has specifically examined MST and STI risk. Although this study included a sizable population of male OEF/OIF veterans, results have yet to be replicated in other clinical populations of interest, such as male military personnel and male veterans of other service eras.
Research is even more limited regarding lesbian, gay, bisexual, transgender, and other gender-minority military personnel and veterans. Although researchers propose that these populations may experience a similar, or even heightened, likelihood of MST during their service, no empirical research yet exists to fully examine this hypothesis.10,11 It is important to note that the paucity of research attention may be related to the Don’t Ask, Don’t Tell (DADT) policy, which obstructed the open discussion and empirical examination of sexual and gender minorities within military populations.11 The DADT policy led to limited awareness and greater stigmatization among sexual- and gender-minority personnel, resulting in poorer sexual health outcomes in these populations.10,11 With the end of DADT in 2011, it is now imperative for future research to examine the prevalence and associated consequences of MST in sexual- and gender-minority military personnel and veterans.
Conclusion
The DoD and VA should be commended for their continued focus on understanding the health consequences of MST. These efforts have yielded substantial information regarding the negative effects of MST on sexual health; in particular, increased risk for STIs. These findings suggest that MHPs may, at times, be the first point of contact for MST-related sexual health concerns. These providers should be aware of their ability to assess for STI risk and make appropriate referrals to facilitate early detection and access to treatment. Despite the presence of MST-related sexual health research, continued research remains necessary. In particular, a broader focus that includes other genders (eg, male, transgender) and sexual minorities would further inform research and clinical practice.
1. Military Sexual Trauma Support Team. Military sexual trauma (MST) screening report fiscal year 2012. Washington DC: U.S. Department of Veterans Affairs, Office of Patient Care Services, Mental Health Services; 2013.
2. Surís A, Holliday R, Weitlauf JC, North CS; the Veteran Safety Initiative Writing Collaborative. Military sexual trauma in the context of veterans’ life experiences. Fed Pract. 2013;30(suppl 3):16S-20S.
3. Jenny C, Hooton TM, Bowers A, et al. Sexually transmitted diseases in victims of rape. N Eng J Med. 1990;322(11):713-716.
4. Senn TE, Carey MP, Vanable PA, Coury-Doniger P, Urban MA. Childhood sexual abuse and sexual risk behavior among men and women attending a sexually transmitted disease clinic. J Consult Clin Psychol. 2006;74(4):720-731.
5. Schultz JR, Bell KM, Naugle AE, Polusny MA. Child sexual abuse and adult sexual assault among military veteran and civilian women. Mil Med. 2006;171(8):723-728.
6. Turchik JA, Pavao J, Nazarian D, Iqbal S, McLean C, Kimerling R. Sexually transmitted infections and sexual dysfunctions among newly returned veterans with and without military sexual trauma. Int J Sex Health. 2012;24(1):45-59.
7. National LGBT Health Education Center. Affirmative care for transgender and gender non-conforming people: best practices for front-line health care staff. https://www.lgbthealth education.org/wp-content/uploads/2016/12/Affirmative-Care-for-Transgender -and-Gender-Non-conforming-People-Best-Practices -for-Frontline-Health-Care-Staff.pdf. Published 2016. Accessed February 16, 2017.
8. Morris EE, Smith JC, Farooqui SY, Surís AM. Unseen battles: the recognition, assessment, and treatment issues of men with military sexual trauma (MST). Trauma Violence Abuse. 2014;15(2):94-101.
9. Turchik JA, Edwards KM. Myths about male rape: a literature review. Psychol Men Masc. 2012;13(2):211-226.
10. Mattocks KM, Kauth MR, Sandfort T, Matza AR, Sullivan JC, Shipherd J. Understanding health-care needs of sexual and gender minority veterans: how targeted research and policy can improve health. LGBT Health. 2014;1(1):50-57.
11. Burks DJ. Lesbian, gay, and bisexual victimization in the military: an unintended consequence of “Don’t Ask, Don’t Tell”? Am Psychol. 2011;66(7):604-613.
1. Military Sexual Trauma Support Team. Military sexual trauma (MST) screening report fiscal year 2012. Washington DC: U.S. Department of Veterans Affairs, Office of Patient Care Services, Mental Health Services; 2013.
2. Surís A, Holliday R, Weitlauf JC, North CS; the Veteran Safety Initiative Writing Collaborative. Military sexual trauma in the context of veterans’ life experiences. Fed Pract. 2013;30(suppl 3):16S-20S.
3. Jenny C, Hooton TM, Bowers A, et al. Sexually transmitted diseases in victims of rape. N Eng J Med. 1990;322(11):713-716.
4. Senn TE, Carey MP, Vanable PA, Coury-Doniger P, Urban MA. Childhood sexual abuse and sexual risk behavior among men and women attending a sexually transmitted disease clinic. J Consult Clin Psychol. 2006;74(4):720-731.
5. Schultz JR, Bell KM, Naugle AE, Polusny MA. Child sexual abuse and adult sexual assault among military veteran and civilian women. Mil Med. 2006;171(8):723-728.
6. Turchik JA, Pavao J, Nazarian D, Iqbal S, McLean C, Kimerling R. Sexually transmitted infections and sexual dysfunctions among newly returned veterans with and without military sexual trauma. Int J Sex Health. 2012;24(1):45-59.
7. National LGBT Health Education Center. Affirmative care for transgender and gender non-conforming people: best practices for front-line health care staff. https://www.lgbthealth education.org/wp-content/uploads/2016/12/Affirmative-Care-for-Transgender -and-Gender-Non-conforming-People-Best-Practices -for-Frontline-Health-Care-Staff.pdf. Published 2016. Accessed February 16, 2017.
8. Morris EE, Smith JC, Farooqui SY, Surís AM. Unseen battles: the recognition, assessment, and treatment issues of men with military sexual trauma (MST). Trauma Violence Abuse. 2014;15(2):94-101.
9. Turchik JA, Edwards KM. Myths about male rape: a literature review. Psychol Men Masc. 2012;13(2):211-226.
10. Mattocks KM, Kauth MR, Sandfort T, Matza AR, Sullivan JC, Shipherd J. Understanding health-care needs of sexual and gender minority veterans: how targeted research and policy can improve health. LGBT Health. 2014;1(1):50-57.
11. Burks DJ. Lesbian, gay, and bisexual victimization in the military: an unintended consequence of “Don’t Ask, Don’t Tell”? Am Psychol. 2011;66(7):604-613.
Weighing risks, benefits of autologous HSCT in MM
The use of autologous transplant after a 3-drug induction regimen for multiple myeloma (MM) prolongs progression-free survival (PFS) but not overall survival (OS), according to new research.
In a phase 3 trial, newly diagnosed MM patients who received lenalidomide, bortezomib, and dexamethasone (RVD) followed by an autologous hematopoietic stem cell transplant (HSCT) had significantly better PFS but similar OS when compared to patients who only received RVD.
In addition, HSCT recipients had significantly higher rates of high-grade blood and lymphatic system disorders, gastrointestinal events, and infections.
The study also showed that OS outcomes were similar for patients who received HSCT after completing treatment with RVD and patients who were in the RVD-only treatment arm but underwent HSCT later, as salvage therapy.
Researchers believe this suggests MM patients can potentially choose when to undergo a transplant.
The team reported their findings in NEJM. The study was supported by grants from Celgene and Janssen and by funds from the French Ministry of Health Programme Hospitalier de Recherche Clinique and from the French National Research Agency.
“Over the past decade, drugs that modulate the immune system and agents known as proteasome inhibitors have shown a great deal of promise in patients with multiple myeloma, when used in combination with chemotherapy,” said study author Paul Richardson, MD, of the Dana-Farber Cancer Institute in Boston, Massachusetts.
“This led us to propose that these combinations could be used selectively with established modalities such as transplant for patients with newly diagnosed myeloma, and this, in turn, raised questions about where and how transplant should be fit into the therapeutic paradigm. Our trial sought to comprehensively address these issues in a prospective fashion, and provide a foundation for future studies as the next generation of agents, such as monoclonal antibodies, impact the field.”
The study enrolled 700 adult patients under the age of 65 who were newly diagnosed with MM. They were treated at 69 centers in France, Belgium, and Switzerland.
The patients were randomized to 2 treatment arms. Both groups received 3 initial cycles of RVD.
One group then received 5 more cycles of RVD. The other received high-dose chemotherapy (melphalan) followed by an autologous HSCT and 2 additional cycles of RVD.
Patients in both groups then received lenalidomide as maintenance therapy for 1 year or until they progressed, experienced unacceptable adverse events (AEs), or withdrew consent.
Response and survival
The complete response rate was significantly higher in the HSCT arm than the RVD-alone arm—59% and 48%, respectively (P=0.03).
And there was a significantly higher percentage of patients who were negative for minimal residual disease in the HSCT arm than in the RVD arm—79% and 65%, respectively (P<0.001).
The median PFS was significantly longer in the HSCT arm than the RVD arm—50 months and 36 months, respectively (P<0.001). The researchers said this benefit was observed across all patient subgroups, including those stratified according to International Staging System stage and cytogenetic risk.
There was no significant between-group difference in the rate of OS at 4 years, which was 81% in the HSCT arm and 82% in the RVD arm.
Subsequent therapy
In the RVD arm, 207 patients progressed, and 172 received second-line therapy, which was followed by salvage HSCT in 136 patients (79%).
In the HSCT arm, 149 patients progressed, and 123 received second-line therapy, which was followed by a second HSCT in 21 patients (17%).
Safety
Nine percent of patients in the RVD arm and 11% in the HSCT arm discontinued treatment due to AEs. There were 2 treatment-related deaths in the RVD arm and 6 in the HSCT arm.
Grade 3/4 AEs with a significantly higher incidence in the HSCT arm than the RVD arm were blood and lymphatic system disorders (95% and 64%, respectively, P<0.001), gastrointestinal disorders (28% and 7%, respectively, P<0.001), and infections (20% and 9%, respectively, P<0.001).
Thirteen patients in the RVD arm and 17 in the HSCT arm had at least 1 invasive second primary malignancy (P=0.36). Acute myeloid leukemia occurred in 1 patient in the RVD arm and 4 in the HSCT arm (P=0.21).
The researchers said these results suggest the benefits of HSCT plus RVD must be weighed against the increased risk of toxicity associated with high-dose chemotherapy plus HSCT, particularly since HSCT after progression might be as effective as early HSCT for ensuring long-term OS. ![]()
The use of autologous transplant after a 3-drug induction regimen for multiple myeloma (MM) prolongs progression-free survival (PFS) but not overall survival (OS), according to new research.
In a phase 3 trial, newly diagnosed MM patients who received lenalidomide, bortezomib, and dexamethasone (RVD) followed by an autologous hematopoietic stem cell transplant (HSCT) had significantly better PFS but similar OS when compared to patients who only received RVD.
In addition, HSCT recipients had significantly higher rates of high-grade blood and lymphatic system disorders, gastrointestinal events, and infections.
The study also showed that OS outcomes were similar for patients who received HSCT after completing treatment with RVD and patients who were in the RVD-only treatment arm but underwent HSCT later, as salvage therapy.
Researchers believe this suggests MM patients can potentially choose when to undergo a transplant.
The team reported their findings in NEJM. The study was supported by grants from Celgene and Janssen and by funds from the French Ministry of Health Programme Hospitalier de Recherche Clinique and from the French National Research Agency.
“Over the past decade, drugs that modulate the immune system and agents known as proteasome inhibitors have shown a great deal of promise in patients with multiple myeloma, when used in combination with chemotherapy,” said study author Paul Richardson, MD, of the Dana-Farber Cancer Institute in Boston, Massachusetts.
“This led us to propose that these combinations could be used selectively with established modalities such as transplant for patients with newly diagnosed myeloma, and this, in turn, raised questions about where and how transplant should be fit into the therapeutic paradigm. Our trial sought to comprehensively address these issues in a prospective fashion, and provide a foundation for future studies as the next generation of agents, such as monoclonal antibodies, impact the field.”
The study enrolled 700 adult patients under the age of 65 who were newly diagnosed with MM. They were treated at 69 centers in France, Belgium, and Switzerland.
The patients were randomized to 2 treatment arms. Both groups received 3 initial cycles of RVD.
One group then received 5 more cycles of RVD. The other received high-dose chemotherapy (melphalan) followed by an autologous HSCT and 2 additional cycles of RVD.
Patients in both groups then received lenalidomide as maintenance therapy for 1 year or until they progressed, experienced unacceptable adverse events (AEs), or withdrew consent.
Response and survival
The complete response rate was significantly higher in the HSCT arm than the RVD-alone arm—59% and 48%, respectively (P=0.03).
And there was a significantly higher percentage of patients who were negative for minimal residual disease in the HSCT arm than in the RVD arm—79% and 65%, respectively (P<0.001).
The median PFS was significantly longer in the HSCT arm than the RVD arm—50 months and 36 months, respectively (P<0.001). The researchers said this benefit was observed across all patient subgroups, including those stratified according to International Staging System stage and cytogenetic risk.
There was no significant between-group difference in the rate of OS at 4 years, which was 81% in the HSCT arm and 82% in the RVD arm.
Subsequent therapy
In the RVD arm, 207 patients progressed, and 172 received second-line therapy, which was followed by salvage HSCT in 136 patients (79%).
In the HSCT arm, 149 patients progressed, and 123 received second-line therapy, which was followed by a second HSCT in 21 patients (17%).
Safety
Nine percent of patients in the RVD arm and 11% in the HSCT arm discontinued treatment due to AEs. There were 2 treatment-related deaths in the RVD arm and 6 in the HSCT arm.
Grade 3/4 AEs with a significantly higher incidence in the HSCT arm than the RVD arm were blood and lymphatic system disorders (95% and 64%, respectively, P<0.001), gastrointestinal disorders (28% and 7%, respectively, P<0.001), and infections (20% and 9%, respectively, P<0.001).
Thirteen patients in the RVD arm and 17 in the HSCT arm had at least 1 invasive second primary malignancy (P=0.36). Acute myeloid leukemia occurred in 1 patient in the RVD arm and 4 in the HSCT arm (P=0.21).
The researchers said these results suggest the benefits of HSCT plus RVD must be weighed against the increased risk of toxicity associated with high-dose chemotherapy plus HSCT, particularly since HSCT after progression might be as effective as early HSCT for ensuring long-term OS. ![]()
The use of autologous transplant after a 3-drug induction regimen for multiple myeloma (MM) prolongs progression-free survival (PFS) but not overall survival (OS), according to new research.
In a phase 3 trial, newly diagnosed MM patients who received lenalidomide, bortezomib, and dexamethasone (RVD) followed by an autologous hematopoietic stem cell transplant (HSCT) had significantly better PFS but similar OS when compared to patients who only received RVD.
In addition, HSCT recipients had significantly higher rates of high-grade blood and lymphatic system disorders, gastrointestinal events, and infections.
The study also showed that OS outcomes were similar for patients who received HSCT after completing treatment with RVD and patients who were in the RVD-only treatment arm but underwent HSCT later, as salvage therapy.
Researchers believe this suggests MM patients can potentially choose when to undergo a transplant.
The team reported their findings in NEJM. The study was supported by grants from Celgene and Janssen and by funds from the French Ministry of Health Programme Hospitalier de Recherche Clinique and from the French National Research Agency.
“Over the past decade, drugs that modulate the immune system and agents known as proteasome inhibitors have shown a great deal of promise in patients with multiple myeloma, when used in combination with chemotherapy,” said study author Paul Richardson, MD, of the Dana-Farber Cancer Institute in Boston, Massachusetts.
“This led us to propose that these combinations could be used selectively with established modalities such as transplant for patients with newly diagnosed myeloma, and this, in turn, raised questions about where and how transplant should be fit into the therapeutic paradigm. Our trial sought to comprehensively address these issues in a prospective fashion, and provide a foundation for future studies as the next generation of agents, such as monoclonal antibodies, impact the field.”
The study enrolled 700 adult patients under the age of 65 who were newly diagnosed with MM. They were treated at 69 centers in France, Belgium, and Switzerland.
The patients were randomized to 2 treatment arms. Both groups received 3 initial cycles of RVD.
One group then received 5 more cycles of RVD. The other received high-dose chemotherapy (melphalan) followed by an autologous HSCT and 2 additional cycles of RVD.
Patients in both groups then received lenalidomide as maintenance therapy for 1 year or until they progressed, experienced unacceptable adverse events (AEs), or withdrew consent.
Response and survival
The complete response rate was significantly higher in the HSCT arm than the RVD-alone arm—59% and 48%, respectively (P=0.03).
And there was a significantly higher percentage of patients who were negative for minimal residual disease in the HSCT arm than in the RVD arm—79% and 65%, respectively (P<0.001).
The median PFS was significantly longer in the HSCT arm than the RVD arm—50 months and 36 months, respectively (P<0.001). The researchers said this benefit was observed across all patient subgroups, including those stratified according to International Staging System stage and cytogenetic risk.
There was no significant between-group difference in the rate of OS at 4 years, which was 81% in the HSCT arm and 82% in the RVD arm.
Subsequent therapy
In the RVD arm, 207 patients progressed, and 172 received second-line therapy, which was followed by salvage HSCT in 136 patients (79%).
In the HSCT arm, 149 patients progressed, and 123 received second-line therapy, which was followed by a second HSCT in 21 patients (17%).
Safety
Nine percent of patients in the RVD arm and 11% in the HSCT arm discontinued treatment due to AEs. There were 2 treatment-related deaths in the RVD arm and 6 in the HSCT arm.
Grade 3/4 AEs with a significantly higher incidence in the HSCT arm than the RVD arm were blood and lymphatic system disorders (95% and 64%, respectively, P<0.001), gastrointestinal disorders (28% and 7%, respectively, P<0.001), and infections (20% and 9%, respectively, P<0.001).
Thirteen patients in the RVD arm and 17 in the HSCT arm had at least 1 invasive second primary malignancy (P=0.36). Acute myeloid leukemia occurred in 1 patient in the RVD arm and 4 in the HSCT arm (P=0.21).
The researchers said these results suggest the benefits of HSCT plus RVD must be weighed against the increased risk of toxicity associated with high-dose chemotherapy plus HSCT, particularly since HSCT after progression might be as effective as early HSCT for ensuring long-term OS. ![]()
The Signs That Can’t Be Ignored
ANSWER
The radiograph shows a large, round hyperdensity within the right lower lobe. This lesion is highly concerning for malignancy and warrants further work-up.
With his gastrointestinal bleed and hypercoagulability from warfarin toxicity, the patient required admission anyway. Subsequent biopsy confirmed the presence of a primary lung carcinoma.

ANSWER
The radiograph shows a large, round hyperdensity within the right lower lobe. This lesion is highly concerning for malignancy and warrants further work-up.
With his gastrointestinal bleed and hypercoagulability from warfarin toxicity, the patient required admission anyway. Subsequent biopsy confirmed the presence of a primary lung carcinoma.

ANSWER
The radiograph shows a large, round hyperdensity within the right lower lobe. This lesion is highly concerning for malignancy and warrants further work-up.
With his gastrointestinal bleed and hypercoagulability from warfarin toxicity, the patient required admission anyway. Subsequent biopsy confirmed the presence of a primary lung carcinoma.
For several days, a 60-year-old man has been feeling weak. He has also noticed that he bruises easily, and he’s experienced black, tarry stools and episodic hemoptysis. He presents to the emergency department, where the triage team sends him for further evaluation.
The patient’s history is significant for a remote diagnosis of a deep venous thrombosis in one of his lower extremities, for which he takes warfarin. He does not recall his most recent INR level. He reports smoking up to one pack of cigarettes per day and consuming alcohol regularly.
Examination reveals an older appearing male in no obvious distress. His blood pressure is 90/60 mm Hg, and his heart rate is 110 beats/min. You note bruises on both arms. The rest of his physical exam is normal. Lung sounds are clear.
Labwork ordered by the triage team indicates a hemoglobin level of 8 g/dL and an INR of 9. In addition, his stool guaiac test came back positive.
You obtain a portable chest radiograph (shown). What is your impression?