User login
Postoperative delirium in a 64-year-old woman
A 64-year-old woman undergoes elective T10-S1 nerve decompression with fusion for chronic idiopathic scoliosis. Soon afterward, she develops acute urinary retention attributed to an Escherichia coli urinary tract infection and narcotic medications. She is treated with antibiotics, an indwelling catheter is inserted, and her symptoms resolve. She is transferred to the inpatient physical rehabilitation unit.
On postoperative day 9, she develops an acute change in mental status, suddenly becoming extremely anxious and falsely believing she has a “terminal illness.” A psychiatrist suggests that these symptoms are a manifestation of delirium, given the patient’s recent surgery and exposure to benzodiazepine and narcotic medications. On postoperative day 10, she is awake but is now mute and uncooperative. An internist is consulted for an evaluation for encephalopathy and delirium.
MEDICAL HISTORY
Her medical history, obtained by chart review and interviewing her husband, includes well-controlled bipolar disorder over the last 4 years, with no episodes of frank psychosis or mania. She had a “bout of delirium” 4 years earlier attributed to a catastrophic life event, but the symptoms resolved after adjustment of her anxiolytic and mood-stabilizing drugs. She also has well-controlled hypertension, hypothyroidism, and gastroesophageal reflux. Her only surgery was her recent elective procedure.
She has a family history of dementia (Pick disease in her mother).
She is married, lives with her husband, and has an adult son. She is employed as a media specialist and also teaches English as a second language. Before this hospital admission, she was described as happy and content, though her primary psychiatrist had noted intermittent anxiety. Her husband does not suspect illicit drug use and denies significant alcohol or tobacco abuse.
A thorough review of systems is not possible, given her encephalopathy. But before her acute decline, she had complained of “choking on blood” and a subjective inability to swallow.
Her home medications include dextroamphetamine extended-release, alprazolam as needed for sleep, venlafaxine extended-release, lamotrigine, lisinopril, propranolol, amlodipine, atorvastatin, levothyroxine, omeprazole, iron, and vitamin B12. At the time of the evaluation, she is on her home medications with the addition of olanzapine, vitamin D, polyethylene glycol, and an intravenous infusion of dextrose 5% with 0.45% saline at a rate of 100 mL/hour. She has allergies to latex, penicillin, peanuts, and shellfish.
PHYSICAL EXAMINATION
On physical examination, the patient seems healthy and appears normal for her stated age. She is wearing a spinal brace and is in no apparent distress. She is afebrile, pulse 104 beats per minute, respirations 16 breaths per minute and unlabored, and oxygen saturation good on room air. The skin is normal. No thyromegaly, bruits, or lymphadenopathy is noted. Cardiovascular, respiratory, and abdominal examinations, though limited by the spinal brace, are unremarkable. She has no evidence of peripheral edema or vascular insufficiency. Muscle bulk and tone are adequate and symmetric.
She is awake and alert and able to follow simple commands with some prompting. She does not initiate movements spontaneously. She makes some eye contact but does not track or acknowledge the interviewer consistently and does not respond verbally to questions. Her sclera are nonicteric, the pupils are equally round and reactive to light, and the external ocular muscles are intact. There is no facial asymmetry, and the tongue protrudes at midline. She blinks appropriately to threat bilaterally. Strength is at least 3/5 in the upper extremities and 2/5 in the lower extremities, though the examination is limited by lack of patient cooperation. She shows minimal grimace on noxious stimulation but does not withdraw extremities. Reflexes are present and mildly depressed symmetrically. Plantar reflexes are downgoing bilaterally.
INITIAL LABORATORY EVALUATION
On initial laboratory testing, the serum sodium is 132 mmol/L (reference range 136–144), stable since admission. Point-of-care glucose is 98 mg/dL. Aspartate aminotransferase and alanine aminotransferase levels are mildly elevated at 59 U/L (13–35) and 51 U/L (7–38), respectively, but serum ammonia is undetectable. Vitamin B12, folate, thyroid-stimulating hormone, and free thyroxine are within the normal ranges. Leukocytosis is noted, with 14 × 109 cells/L (3.7–11.0), 86% neutrophils, and a mild left shift. Urinalysis is negative for leukocyte esterase, nitrites, and white blood cells.
APPROACH TO ALTERED MENTAL STATUS
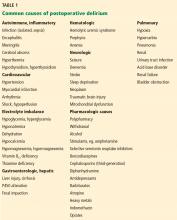
1. Which of the following risk factors predisposes this patient to postoperative delirium?
- Hyponatremia
- Polypharmacy
- Family history of dementia
- Depression
Altered mental status, or encephalopathy, is one of the most common yet challenging conditions in medicine. When a consult is placed for altered mental status, it is important to determine the affected domain that has changed from the patient’s normal state. Changes can include alterations in consciousness, attention, behavior, cognition, language, speech, and praxis and can reflect varying degrees of cerebral dysfunction.
Electrolyte abnormalities
Disorders of sodium homeostasis are common in hospitalized patients and may contribute to the onset of delirium. Hyponatremia is especially frequent and often iatrogenic, with a prevalence significantly higher in women (2.1% vs 1.3%, P = .0044) and in the elderly.2
Neurologic manifestations are often the result of cerebral edema due to osmolar volume shifts.3–6 Acute hyponatremic encephalopathy is most likely to occur when sodium shifts are rapid, usually within 24 hours, and is often seen in postoperative patients requiring significant volume resuscitation with hypotonic fluids.6 Young premenopausal women appear to be at especially high risk of permanent brain damage secondary to hyponatremic encephalopathy,7 a finding that may reflect the limited compliance within the intracranial vault and lack of significant involutional parenchymal changes that occur with aging.8–11
Aging also has important effects on fluid balance, as restoration of body fluid homeostasis is slower in older patients.12
Hormonal effects of estrogen appear to play a synergistic role in the expression of arginine vasopressin in postmenopausal women, further contributing to hyponatremia.
Although our patient has mild hyponatremia, there has been no acute change in her sodium balance since admission to the hospital, and so it is unlikely to be the cause of her acute delirium. Her mild hyponatremia may in part be from hypo-osmolar maintenance fluids with dextrose 5% and 0.45% normal saline.
Mild chronic hyponatremia may affect balance and has been associated with increased mortality risk in certain chronic disease states, but this is unlikely to be the main cause of acute delirium.
Polypharmacy
Patients admitted to the hospital with polypharmacy are at high risk of drug-induced delirium. In approaching delirium, a patient’s medications should be evaluated for interactions, as well as for possible effects of newly prescribed drugs. New medications that affect cytochrome P450 enzymes warrant investigation, as do drugs with narrow therapeutic windows that the patient has been using long-term.
Consultation with a clinical pharmacist is often helpful. Macrolides, protease inhibitors, and nondihydropyridine calcium channel blockers are common P450 inhibitors, while many anticonvulsants are known inducers of the P450 system. Selective serotonin reuptake inhibitors and diuretics can lead to electrolyte imbalances such as hyponatremia, which may further predispose to bouts of delirium, as described above.
The patient’s extensive list of psychoactive medications makes polypharmacy a significant risk factor for delirium. Quetiapine and venlafaxine both cause sedation and increase the risk of serotonin syndrome. However, in this case, the patient does not have marked fever, rigidity, or hyperreflexia to corroborate that diagnosis.
Dementia
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), defines dementia as a disorder involving cognitive impairment in at least 1 cognitive domain, with a significant decline from a previous level of functioning.1 These impairments need not necessarily occur separately from bouts of delirium, but the time course for most forms of dementia tends to be progressive over a subacute to chronic duration.
Dementia increases the risk for acute confusion and delirium in hospitalized patients.13 This is partly reflected by pathophysiologic changes that leave elderly patients susceptible to the effects of anticholinergic drugs.14 Structural changes due to small-vessel ischemia may also predispose patients to seizures in the setting of metabolic derangement or critical illness. Diagnosing dementia thus remains a challenge, as dementia must be clearly distinguished from other disorders such as delirium and depression.
The acute change in this patient’s case makes the isolated diagnosis of dementia much less likely than other causes of altered mental status. Also, her previous level of function does not suggest a clinically significant personal history of impairment.
Mental illness
Several studies have examined the link between preoperative mental health disorders and postoperative delirium.15–17 Depression appears to be a risk factor for postoperative delirium in patients undergoing elective orthopedic surgery,15 and this includes elderly patients.16 While a clear etiologic link has yet to be determined, disruption of circadian rhythm and abnormal cerebral response to stress may play a role. Studies have also suggested an association between schizophrenia and delirium, though this may be related to perioperative suspension of medications.17
Bipolar disorder has not been well studied with regard to postoperative complications. However, this patient has had a previous episode of decompensated mania, therefore making bipolar disorder a plausible condition in the differential diagnosis.
CASE CONTINUED: ACUTE DETERIORATION
Without a clearly identifiable cause for our patient’s acute confusional state, neurology and medical consultants recommend neuroimaging.
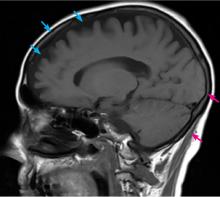
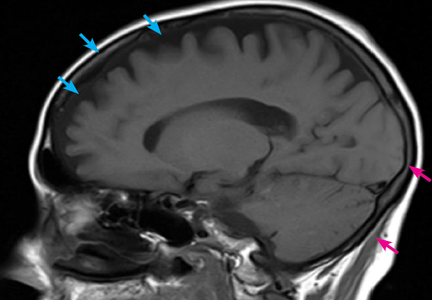
Computed tomography (CT) and magnetic resonance imaging (MRI) without contrast are ordered and performed on postoperative day 11 and demonstrate chronic small-vessel ischemic disease, consistent with our patient’s age, as well as frontotemporal atrophy. There is no evidence of mass effect, bleeding, or acute ischemia.
Overnight, she becomes obtunded, and the rapid response team is called. Her vital signs appear stable, and she is afebrile. Basic laboratory studies, imaging, and electrocardiography are repeated, and the results are unchanged from recent tests. She is transferred to the intensive care unit (ICU) for closer monitoring.
2. What is most likely cause of the patient’s declining mental status, and what is the next appropriate step?
- Acute stroke: repeat MRI with contrast
- Urinary tract infection: order blood and urine cultures, and start empiric antibiotics
- Neuroleptic malignant syndrome: start dantrolene
- Seizures: order electroencephalography (EEG)
Acute stroke
Acute stroke can affect mental status and consciousness through several pathways. Stroke syndromes can vary in presentation depending on the level of cortical and subcortical involvement, with clinical manifestations including confusion, aphasia, neglect, and inattention. Wakefulness and the ability to maintain consciousness is impaired, with disruption of the ascending reticular activating system, often seen in injuries to the brainstem. Large territorial or hemispheric infarcts, with subsequent cerebral edema, can also disrupt this system and lead to cerebral herniation and coma.
MRI without contrast is extremely sensitive for ischemia and can typically detect ischemia in acute stroke within 3 to 30 minutes.18–20 Repeating the study with contrast is unlikely to provide additional benefit.
In our patient’s case, the lack of localizing neurologic symptoms, in addition to her recent negative neuroimaging workup, makes the diagnosis of acute stroke unlikely.
Infection
The role of severe infection in patients with altered mental status is well documented and likely relates to diffuse cerebral dysfunction caused by an inflammatory cascade. Less well understood is the role of occult infection, especially urinary tract infection, in otherwise immunocompetent patients. Urinary tract infection has long been thought to cause delirium in otherwise asymptomatic elderly patients, but few studies have examined this relationship, and those studies have been shown to have significant methodologic errors.21 In the absence of better data, urinary tract infection as the cause of frank delirium in an otherwise well patient should be viewed with skepticism, and alternative causes should be sought.
Although the patient has a nonspecific leukocytosis, her benign urinalysis and lack of corroborating evidence makes urinary tract infection an unlikely cause of her frank delirium.
Neuroleptic malignant syndrome
Neuroleptic malignant syndrome is defined as fever, rigidity, mental status changes, and autonomic instability after exposure to antidopaminergic drugs. It is classically seen after administration of typical antipsychotics, though atypical antipsychotics and antiemetic drugs may be implicated as well.
Patients often exhibit agitation and confusion, which when severe may progress to mutism and catatonia. Likewise, psychotropic drugs such as quetiapine and venlafaxine, used in combination, have the additional risk of serotonin syndrome.
Additional symptoms include hyperreflexia, ataxia, and myoclonus. Withdrawal of the causative agent and supportive care are the mainstays of therapy. Targeted therapies with agents such as dantrolene, bromocriptine, and amantadine have also been reported anecdotally, but their efficacy is unclear, with variable results.22
As noted earlier, the addition of quetiapine to the patient’s already lengthy medication list could conceivably cause neuroleptic malignant syndrome or serotonin syndrome and should be considered. However, additional neurologic findings to confirm this diagnosis are lacking.
Seizures
Nonconvulsive seizure, particularly nonconvulsive status epilepticus (NCSE), is not well recognized and is particularly challenging to diagnose without EEG. In several case series of patients presenting to the emergency room with altered mental status, NCSE was found in 16% to 28% of patients in whom EEG was performed after an initial evaluation failed to show an obvious cause for the delirium.23,24 Historical features are unreliable for ruling out NCSE as a cause of delirium, as up to 41% of patients in whom the condition is ultimately diagnosed have only confusion as the presenting clinical symptom.25
Likewise, alternating ictal and postictal periods may mimic the typical waxing and waning course classically associated with delirium of other causes. Physical findings such as nystagmus, anisocoria, and hippus may be helpful but are often overlooked or absent. EEG is thus an essential requirement for the diagnosis.26
Given the lack of a clear diagnosis, a workup with EEG should be considered in this patient.
CASE CONTINUED: ADDITIONAL SIGNS
In the ICU, our patient is evaluated by the intensivist team. Her vital signs are stable, and while she is now awakening, she is unable to follow commands and remains mute. She does not initiate movement spontaneously but offers slight resistance to passive movements, holding and maintaining postures her extremities are placed in. She keeps her eyes closed, but when opened by the examining physician, dysconjugate gaze and anisocoria are noted.
3. What clinical entity is most consistent with these physical findings, and what is the next step in management?
- Catatonia secondary to bipolar disorder type I: challenge with intravenous lorazepam 2 mg
- Oculomotor nerve palsy due to enlarging intracranial aneurysm: aggressive blood pressure lowering, elevation of the head of the bed
- Toxic leukoencephalopathy: supportive care and withdrawal of the causative agent
- NCSE: challenge with intravenous lorazepam 2 mg and order EEG
Catatonia
The DSM-5 defines catatonia as a behavioral syndrome complicating an underlying psychiatric or medical condition, as opposed to a distinct diagnosis. It is most commonly encountered in psychiatric illnesses including bipolar disorder, major depression, and schizophrenia. Akinesis, stupor, mutism, and “waxy” flexibility often dominate the clinical picture.
The pathophysiology is poorly defined, but likely involves neurotransmitter imbalances particularly with an increase in N-methyl-d-aspartate (NMDA) activity and suppression of gamma-aminobutyric acid (GABA) activity. This hypothesis is supported by the finding that benzodiazepines, electroconvulsive therapy, and NMDA antagonists such as amantadine are all effective in treating catatonia.27,28 Findings of focal neurologic abnormalities warrant further investigation. EEG may be necessary to differentiate catatonia from NCSE, as both may respond to a benzodiazepine challenge.
As pure catatonia is a diagnosis of exclusion, further workup, including EEG, is necessary to confirm the diagnosis.
Oculomotor nerve palsy
Anisocoria together with dysconjugate gaze should prompt consideration of a lesion involving the oculomotor nerve. Loss of tonic muscle activity from the lateral rectus and superior oblique cause a downward and outward gaze. Furthermore, loss of parasympathetic tone occurs with compressive palsies of the oculomotor nerve, clinically manifesting as a mydriatic and unreactive pupil with ptosis. Given its anatomic course and proximity to other vascular and parenchymal structures, the oculomotor nerve is vulnerable to compression from many sources, including aneurysmal dilation (especially of the posterior cerebral artery), uncal herniation, and inflammation of the cavernous sinus.
Noncontrast CT and lumbar puncture are very sensitive for making the diagnosis of sentinel bleeding within the first 24 hours,29 whereas computed tomographic angiography and magnetic resonance angiography can reliably detect unruptured aneurysms as small as 3 mm.30
Conditions that can lead to oculomotor palsy are unlikely to cause an acute gain in appendicular muscle tone, as noted by the catatonia this patient is demonstrating. Also, mass lesions or bleeding associated with oculomotor palsy is likely to cause acute loss of tone. Chronic upper-motor neuron lesions lead to spasticity rather than the waxy flexibility seen in this patient. In our patient, the findings of isolated anisocoria without further clinical evidence of oculomotor nerve compression make this diagnosis unlikely.
Toxic leukoencephalopathy
Toxic leukoencephalopathy—widespread destruction of myelin, particularly in the white matter tracts that support higher cortical functions—can be caused by antineoplastic agents, immunosuppressant agents, and industrial solvents, as well as by abuse of vaporized drugs such as heroin (“chasing the dragon”). In its mild forms it may cause behavioral disturbances or inattention. In severe forms, a neurobehavioral syndrome of akinetic mutism may be present and can mimic catatonia.31
The diagnosis is often based on the clinical history and neuroimaging, particularly MRI, which demonstrates hyperintensity of the white matter tracts in T2-weighted images.32
This patient does not have a clear history of exposure to an agent typically associated with toxic leukoencephalopathy and does not have the corroborating MRI findings to support this diagnosis.
CASE CONTINUED
Because recent neuroimaging revealed no structural brain lesions and no cause for brain herniation, the patient receives a challenge of 2 mg of intravenous lorazepam to treat potential NCSE. Subsequent improvement is noted in her anisocoria, gaze deviation, and encephalopathy. EEG reveals frequent focal seizures arising from mesial frontal regions with bilateral hemisphere propagation, consistent with bifrontal focal NCSE.
As our patient is being transferred to a room for continuous EEG monitoring, her condition begins to deteriorate, and she again becomes more encephalopathic, with anisocoria and dysconjugate gaze. Additional doses of lorazepam are given (to complete a 0.1-mg/kg load), and additional therapy with intravenous fosphenytoin (20-mg/kg load) is given. Intubation is done for airway protection.
Continuous EEG monitoring reveals multiple frequent electrographic seizures arising from the bifrontal territories, concerning for persistent focal NCSE. A midazolam drip is initiated for EEG burst suppression of cerebral activity. Over 24 hours, EEG shows resolution of seizure activity. As the patient is weaned from sedation, she awakens and follows commands consistently, tolerating extubation without complications. Her neurologic status remains stable over the next 48 hours, having returned to her neurologic baseline level of functioning. She is able to be transferred out of the ICU in stable condition while continuing on scheduled antiepileptic therapy with phenytoin.
ALTERED MENTAL STATUS IN INPATIENTS
Altered mental status is one of the most frequently encountered reasons for medical consultation from nonmedical services. The workup and management of metabolic, toxic, psychiatric, and neurologic causes requires a deep appreciation for the broad differential diagnosis and a multidisciplinary approach. Physicians caring for these patients should avoid prematurely drawing conclusions when the patient’s clinical condition fails to respond to typical measures.
Delirium is a challenging adverse event in older patients during hospitalization, with a significant national financial burden of $164 billion per year.33 The prevalence of delirium in adults on hospital admission is estimated as 14% to 24%, with an inpatient hospitalization incidence ranging from 6% to 56% in general hospital patients.34 In addition, postoperative delirium has been reported in 15% to 53% of older patients.35
While delirium is preventable in 30% to 40% of cases,36,37 it remains an important independent prognostic determinant of hospital outcomes.38–40
Delirium in hospitalized patients requires a thorough, individualized workup. In our patient’s case, the clinical findings of hypoactive delirium were found to be manifestations of NCSE, a rare life-threatening and potentially reversible neurologic disease.
While establishing seizures as a diagnosis, careful attention must first be directed towards investigating environmental or metabolic triggers that may be inciting the disease. This often involves a similar workup for metabolic derangements, as seen in the approach to delirium.
The diagnosis of NCSE, while made in this patient’s case, remains challenging. Careful physical examination should assess for automatisms, “negative” symptoms (staring, aphasia, weakness), and “positive” symptoms (hallucinations, psychosis). Cataplexy, mutism, and other acute psychiatric features have been associated with NCSE,44 highlighting the importance of EEG. A trial of a benzodiazepine in conjunction with clinical and EEG monitoring may help guide clinical decision- making.
As there is no current universally accepted definition for NCSE nor an accepted agreement on required EEG diagnostic features at this time,41 accurate diagnosis is most likely to be obtained in facilities with both subspecialty neurologic consultation and EEG capabilities.
Our patient’s family history of Pick disease is interesting, as this is a progressive form of frontotemporal dementia with both sporadic and genetically linked cases. Recent studies have shown evidence that patients with neurodegenerative disease have increased seizure frequency early in the disease course,31 and efforts are under way to establish the incidence of first unprovoked seizure in patients with frontotemporal dementia. In our patient’s case, resolution of seizure activity yielded a return to her baseline level of neurologic function.
Early use of selective serotonin reuptake inhibitors has been shown to help with the behavioral symptoms of frontotemporal dementia,45 but increasing requirements over time may indicate progression of neurodegeneration and should warrant further appropriate investigation.
In our patient’s case, escalating dose requirements may have reflected worsening frontotemporal atrophy. However, the diagnosis of a neurodegenerative disease such as frontotemporal dementia in a patient such as ours is not definitively established at this time and is being investigated on an outpatient basis.
Given the frequency of delirium and its many risk factors in the inpatient setting, verifying a causative diagnosis can be difficult. Detailed consideration of the patient’s individual clinical circumstances, often in concert with appropriate subspecialty consultations, is essential to the evaluation. Although it is time-intensive, multidisciplinary intervention can lead to safer outcomes and shorter hospital stays.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association Publishing; 2013. http://psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596. Accessed July 7, 2017.
- Mohan S, Gu S, Parikh A, Radhakrishnan J. Prevalence of hyponatremia and association with mortality: results from NHANES. Am J Med 2013; 126:1127–1137.e1.
- Sterns RH. Disorders of plasma sodium—causes, consequences, and correction. N Engl J Med 2015; 372:55–65.
- Rose B, Post T. Clinical physiology of acid-base and electrolyte disorders. 5th ed. New York, NY: McGraw-Hill; 2001.
- McManus ML, Churchwell KB, Strange K. Regulation of cell volume in health and disease. N Engl J Med 1995; 333:1260–1266.
- Strange K. Regulation of solute and water balance and cell volume in the central nervous system. J Am Soc Nephrol 1992; 3:12–27.
- Ayus JC, Wheeler JM, Arieff AI. Postoperative hyponatremic encephalopathy in menstruant women. Ann Intern Med 1992; 117:891–897.
- Gur RC, Mozley PD, Resnick SM, et al. Gender differences in age effect on brain atrophy measured by magnetic resonance imaging. Proc Natl Acad Sci USA 1991; 88:2845–2849.
- Rosomoff HL, Zugibe FT. Distribution of intracranial contents in experimental edema. Arch Neurol 1963; 9:26–34.
- Melton JE, Nattie EE. Brain and CSF water and ions during dilutional and isosmotic hyponatremia in the rat. Am J Physiol 1983; 244:R724–R732.
- Nattie EE, Edwards WH. Brain and CSF water and ions in newborn puppies during acute hypo- and hypernatremia. J Appl Physiol Respir Environ Exerc Physiol 1981; 51:1086–1091.
- Stachenfeld NS, DiPietro L, Palter SF, Nadel ER. Estrogen influences osmotic secretion of AVP and body water balance in postmenopausal women. Am J Physiol 1998; 274:R187–R195.
- Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc 2002; 50:1723–1732.
- de Smet Y, Ruberg M, Serdaru M, Dubois B, Lhermitte F, Agid Y. Confusion, dementia and anticholinergics in Parkinson’s disease. J Neurol Neurosurg Psychiatry 1982; 45:1161–1164.
- Mollon B, Mahure SA, Ding DY, Zuckerman JD, Kwon YW. The influence of a history of clinical depression on peri-operative outcomes in elective total shoulder arthroplasty: a ten-year national analysis. Bone Joint J 2016; 98-B:818–824.
- Kosar CM, Tabloski PA, Travison TG, et al. Effect of preoperative pain and depressive symptoms on the development of postoperative delirium. Lancet Psychiatry 2014; 1:431–436.
- Copeland LA, Zeber JE, Pugh MJ, Mortensen EM, Restrepo MI, Lawrence VA. Postoperative complications in the seriously mentally ill: a systematic review of the literature. Ann Surg 2008; 248:31–38.
- Warach S, Gaa J, Siewert B, Wielopolski P, Edelman RR. Acute human stroke studied by whole brain echo planar diffusion-weighted magnetic resonance imaging. Ann Neurol 1995; 37:231–241.
- Sorensen AG, Buonanno FS, Gonzalez RG, et al. Hyperacute stroke: evaluation with combined multisection diffusion-weighted and hemodynamically weighted echo-planar MR imaging. Radiology 1996; 199:391–401.
- Li F, Han S, Tatlisumak T, et al. A new method to improve in-bore middle cerebral artery occlusion in rats: demonstration with diffusion—and perfusion—weighted imaging. Stroke 1998; 29:1715–1720.
- Balogun SA, Philbrick JT. Delirium, a symptom of UTI in the elderly: fact or fable? A systematic review. Can Geriatr J 2013; 17:22–26.
- Reulbach U, Dütsch C, Biermann T, et al. Managing an effective treatment for neuroleptic malignant syndrome. Crit Care 2007; 11:R4.
- Naeije G, Depondt C, Meeus C, Korpak K, Pepersack T, Legros B. EEG patterns compatible with nonconvulsive status epilepticus are common in elderly patients with delirium: a prospective study with continuous EEG monitoring. Epilepsy Behav 2014; 36:18–21.
- Veran O, Kahane P, Thomas P, Hamelin S, Sabourdy C, Vercueil L. De novo epileptic confusion in the elderly: a 1-year prospective study. Epilepsia 2010; 51:1030–1035.
- Sutter R, Rüegg S, Kaplan PW. Epidemiology, diagnosis, and management of nonconvulsive status epilepticus. Opening Pandora’s box. Neurol Clin Pract 2012; 2:275–286.
- Husain AM, Horn GJ, Jacobson MP. Non-convulsive status epilepticus: usefulness of clinical features in selecting patients for urgent EEG. J Neurol Neurosurg Psychiatry 2003; 74:189–191.
- Ungvari GS, Chiu HF, Chow LY, Lau BS, Tang WK. Lorazepam for chronic catatonia: a randomized, double-blind, placebo-controlled cross-over study. Psychopharmacology (Berl) 1999; 142:393–398.
- Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry Clin Neurosci 2007; 19:406– 412.
- Perry JJ, Spacek A, Forbes M, et al. Is the combination of negative computed tomography result and negative lumbar puncture result sufficient to rule out subarachnoid hemorrhage? Ann Emerg Med 2008; 51:707–713.
- Li MH, Cheng YS, Li YD, et al. Large-cohort comparison between three-dimensional time-of-flight magnetic resonance and rotational digital subtraction angiographies in intracranial aneurysm detection. Stroke 2009; 40:3127–3129.
- Filley CM, Kleinschmidt-DeMasters BK. Toxic leukoencephalopathy. N Engl J Med 2001; 345:425–432.
- Magnetic resonance imaging of the central nervous system. Council on Scientific Affairs. Report of the Panel on Magnetic Resonance Imaging. JAMA 1988; 259:1211–1222.
- Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med 2008; 168:27–32.
- Inouye SK. Delirium in hospitalized older patients. Clin Geriatr Med 1998; 14:745–764.
- Agostini JV, Inouye SK, Hazzard W, Blass J. Delirium. In: Principles of Geriatric Medicine and Gerontology. 5th ed. New York, NY: McGraw-Hill; 2003:1503–1515.
- Inouye SK, Bogardus ST Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999; 340:669–676.
- Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc 2001; 49:516–522.
- Inouye SK, Rushing JT, Foreman MD, Palmer RM, Pompei P. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med 1998; 13:234–242.
- Rothschild JM, Bates DW, Leape LL. Preventable medical injuries in older patients. Arch Intern Med 2000; 160:2717–2728.
- Gillick MR, Serrell NA, Gillick LS. Adverse consequences of hospitalization in the elderly. Soc Sci Med 1982; 16:1033–1038.
- Drislane FW. Presentation, evaluation, and treatment of nonconvulsive status epilepticus. Epilepsy Behav 2000; 1:301-314.
- Rosenow F, Hamer HM, Knake S. The epidemiology of convulsive and nonconvulsive status epilepticus. Epilepsia 2007; 48(suppl 8):82–84.
- Woodford HJ, George J, Jackson M. Non-convulsive status epilepticus: a practical approach to diagnosis in confused older people. Postgrad Med J 2015; 91:655–661.
- Kaplan PW. Nonconvulsive status epilepticus in the emergency room. Epilepsia 1996; 37:643–650.
- Swartz JR, Miller BL, Lesser IM, Darby AL. Frontotemporal dementia: treatment response to serotonin selective reuptake inhibitors. J Clin Psychiatry 1997; 58:212–216.
A 64-year-old woman undergoes elective T10-S1 nerve decompression with fusion for chronic idiopathic scoliosis. Soon afterward, she develops acute urinary retention attributed to an Escherichia coli urinary tract infection and narcotic medications. She is treated with antibiotics, an indwelling catheter is inserted, and her symptoms resolve. She is transferred to the inpatient physical rehabilitation unit.
On postoperative day 9, she develops an acute change in mental status, suddenly becoming extremely anxious and falsely believing she has a “terminal illness.” A psychiatrist suggests that these symptoms are a manifestation of delirium, given the patient’s recent surgery and exposure to benzodiazepine and narcotic medications. On postoperative day 10, she is awake but is now mute and uncooperative. An internist is consulted for an evaluation for encephalopathy and delirium.
MEDICAL HISTORY
Her medical history, obtained by chart review and interviewing her husband, includes well-controlled bipolar disorder over the last 4 years, with no episodes of frank psychosis or mania. She had a “bout of delirium” 4 years earlier attributed to a catastrophic life event, but the symptoms resolved after adjustment of her anxiolytic and mood-stabilizing drugs. She also has well-controlled hypertension, hypothyroidism, and gastroesophageal reflux. Her only surgery was her recent elective procedure.
She has a family history of dementia (Pick disease in her mother).
She is married, lives with her husband, and has an adult son. She is employed as a media specialist and also teaches English as a second language. Before this hospital admission, she was described as happy and content, though her primary psychiatrist had noted intermittent anxiety. Her husband does not suspect illicit drug use and denies significant alcohol or tobacco abuse.
A thorough review of systems is not possible, given her encephalopathy. But before her acute decline, she had complained of “choking on blood” and a subjective inability to swallow.
Her home medications include dextroamphetamine extended-release, alprazolam as needed for sleep, venlafaxine extended-release, lamotrigine, lisinopril, propranolol, amlodipine, atorvastatin, levothyroxine, omeprazole, iron, and vitamin B12. At the time of the evaluation, she is on her home medications with the addition of olanzapine, vitamin D, polyethylene glycol, and an intravenous infusion of dextrose 5% with 0.45% saline at a rate of 100 mL/hour. She has allergies to latex, penicillin, peanuts, and shellfish.
PHYSICAL EXAMINATION
On physical examination, the patient seems healthy and appears normal for her stated age. She is wearing a spinal brace and is in no apparent distress. She is afebrile, pulse 104 beats per minute, respirations 16 breaths per minute and unlabored, and oxygen saturation good on room air. The skin is normal. No thyromegaly, bruits, or lymphadenopathy is noted. Cardiovascular, respiratory, and abdominal examinations, though limited by the spinal brace, are unremarkable. She has no evidence of peripheral edema or vascular insufficiency. Muscle bulk and tone are adequate and symmetric.
She is awake and alert and able to follow simple commands with some prompting. She does not initiate movements spontaneously. She makes some eye contact but does not track or acknowledge the interviewer consistently and does not respond verbally to questions. Her sclera are nonicteric, the pupils are equally round and reactive to light, and the external ocular muscles are intact. There is no facial asymmetry, and the tongue protrudes at midline. She blinks appropriately to threat bilaterally. Strength is at least 3/5 in the upper extremities and 2/5 in the lower extremities, though the examination is limited by lack of patient cooperation. She shows minimal grimace on noxious stimulation but does not withdraw extremities. Reflexes are present and mildly depressed symmetrically. Plantar reflexes are downgoing bilaterally.
INITIAL LABORATORY EVALUATION
On initial laboratory testing, the serum sodium is 132 mmol/L (reference range 136–144), stable since admission. Point-of-care glucose is 98 mg/dL. Aspartate aminotransferase and alanine aminotransferase levels are mildly elevated at 59 U/L (13–35) and 51 U/L (7–38), respectively, but serum ammonia is undetectable. Vitamin B12, folate, thyroid-stimulating hormone, and free thyroxine are within the normal ranges. Leukocytosis is noted, with 14 × 109 cells/L (3.7–11.0), 86% neutrophils, and a mild left shift. Urinalysis is negative for leukocyte esterase, nitrites, and white blood cells.
APPROACH TO ALTERED MENTAL STATUS
1. Which of the following risk factors predisposes this patient to postoperative delirium?
- Hyponatremia
- Polypharmacy
- Family history of dementia
- Depression
Altered mental status, or encephalopathy, is one of the most common yet challenging conditions in medicine. When a consult is placed for altered mental status, it is important to determine the affected domain that has changed from the patient’s normal state. Changes can include alterations in consciousness, attention, behavior, cognition, language, speech, and praxis and can reflect varying degrees of cerebral dysfunction.
Electrolyte abnormalities
Disorders of sodium homeostasis are common in hospitalized patients and may contribute to the onset of delirium. Hyponatremia is especially frequent and often iatrogenic, with a prevalence significantly higher in women (2.1% vs 1.3%, P = .0044) and in the elderly.2
Neurologic manifestations are often the result of cerebral edema due to osmolar volume shifts.3–6 Acute hyponatremic encephalopathy is most likely to occur when sodium shifts are rapid, usually within 24 hours, and is often seen in postoperative patients requiring significant volume resuscitation with hypotonic fluids.6 Young premenopausal women appear to be at especially high risk of permanent brain damage secondary to hyponatremic encephalopathy,7 a finding that may reflect the limited compliance within the intracranial vault and lack of significant involutional parenchymal changes that occur with aging.8–11
Aging also has important effects on fluid balance, as restoration of body fluid homeostasis is slower in older patients.12
Hormonal effects of estrogen appear to play a synergistic role in the expression of arginine vasopressin in postmenopausal women, further contributing to hyponatremia.
Although our patient has mild hyponatremia, there has been no acute change in her sodium balance since admission to the hospital, and so it is unlikely to be the cause of her acute delirium. Her mild hyponatremia may in part be from hypo-osmolar maintenance fluids with dextrose 5% and 0.45% normal saline.
Mild chronic hyponatremia may affect balance and has been associated with increased mortality risk in certain chronic disease states, but this is unlikely to be the main cause of acute delirium.
Polypharmacy
Patients admitted to the hospital with polypharmacy are at high risk of drug-induced delirium. In approaching delirium, a patient’s medications should be evaluated for interactions, as well as for possible effects of newly prescribed drugs. New medications that affect cytochrome P450 enzymes warrant investigation, as do drugs with narrow therapeutic windows that the patient has been using long-term.
Consultation with a clinical pharmacist is often helpful. Macrolides, protease inhibitors, and nondihydropyridine calcium channel blockers are common P450 inhibitors, while many anticonvulsants are known inducers of the P450 system. Selective serotonin reuptake inhibitors and diuretics can lead to electrolyte imbalances such as hyponatremia, which may further predispose to bouts of delirium, as described above.
The patient’s extensive list of psychoactive medications makes polypharmacy a significant risk factor for delirium. Quetiapine and venlafaxine both cause sedation and increase the risk of serotonin syndrome. However, in this case, the patient does not have marked fever, rigidity, or hyperreflexia to corroborate that diagnosis.
Dementia
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), defines dementia as a disorder involving cognitive impairment in at least 1 cognitive domain, with a significant decline from a previous level of functioning.1 These impairments need not necessarily occur separately from bouts of delirium, but the time course for most forms of dementia tends to be progressive over a subacute to chronic duration.
Dementia increases the risk for acute confusion and delirium in hospitalized patients.13 This is partly reflected by pathophysiologic changes that leave elderly patients susceptible to the effects of anticholinergic drugs.14 Structural changes due to small-vessel ischemia may also predispose patients to seizures in the setting of metabolic derangement or critical illness. Diagnosing dementia thus remains a challenge, as dementia must be clearly distinguished from other disorders such as delirium and depression.
The acute change in this patient’s case makes the isolated diagnosis of dementia much less likely than other causes of altered mental status. Also, her previous level of function does not suggest a clinically significant personal history of impairment.
Mental illness
Several studies have examined the link between preoperative mental health disorders and postoperative delirium.15–17 Depression appears to be a risk factor for postoperative delirium in patients undergoing elective orthopedic surgery,15 and this includes elderly patients.16 While a clear etiologic link has yet to be determined, disruption of circadian rhythm and abnormal cerebral response to stress may play a role. Studies have also suggested an association between schizophrenia and delirium, though this may be related to perioperative suspension of medications.17
Bipolar disorder has not been well studied with regard to postoperative complications. However, this patient has had a previous episode of decompensated mania, therefore making bipolar disorder a plausible condition in the differential diagnosis.
CASE CONTINUED: ACUTE DETERIORATION
Without a clearly identifiable cause for our patient’s acute confusional state, neurology and medical consultants recommend neuroimaging.
Computed tomography (CT) and magnetic resonance imaging (MRI) without contrast are ordered and performed on postoperative day 11 and demonstrate chronic small-vessel ischemic disease, consistent with our patient’s age, as well as frontotemporal atrophy. There is no evidence of mass effect, bleeding, or acute ischemia.
Overnight, she becomes obtunded, and the rapid response team is called. Her vital signs appear stable, and she is afebrile. Basic laboratory studies, imaging, and electrocardiography are repeated, and the results are unchanged from recent tests. She is transferred to the intensive care unit (ICU) for closer monitoring.
2. What is most likely cause of the patient’s declining mental status, and what is the next appropriate step?
- Acute stroke: repeat MRI with contrast
- Urinary tract infection: order blood and urine cultures, and start empiric antibiotics
- Neuroleptic malignant syndrome: start dantrolene
- Seizures: order electroencephalography (EEG)
Acute stroke
Acute stroke can affect mental status and consciousness through several pathways. Stroke syndromes can vary in presentation depending on the level of cortical and subcortical involvement, with clinical manifestations including confusion, aphasia, neglect, and inattention. Wakefulness and the ability to maintain consciousness is impaired, with disruption of the ascending reticular activating system, often seen in injuries to the brainstem. Large territorial or hemispheric infarcts, with subsequent cerebral edema, can also disrupt this system and lead to cerebral herniation and coma.
MRI without contrast is extremely sensitive for ischemia and can typically detect ischemia in acute stroke within 3 to 30 minutes.18–20 Repeating the study with contrast is unlikely to provide additional benefit.
In our patient’s case, the lack of localizing neurologic symptoms, in addition to her recent negative neuroimaging workup, makes the diagnosis of acute stroke unlikely.
Infection
The role of severe infection in patients with altered mental status is well documented and likely relates to diffuse cerebral dysfunction caused by an inflammatory cascade. Less well understood is the role of occult infection, especially urinary tract infection, in otherwise immunocompetent patients. Urinary tract infection has long been thought to cause delirium in otherwise asymptomatic elderly patients, but few studies have examined this relationship, and those studies have been shown to have significant methodologic errors.21 In the absence of better data, urinary tract infection as the cause of frank delirium in an otherwise well patient should be viewed with skepticism, and alternative causes should be sought.
Although the patient has a nonspecific leukocytosis, her benign urinalysis and lack of corroborating evidence makes urinary tract infection an unlikely cause of her frank delirium.
Neuroleptic malignant syndrome
Neuroleptic malignant syndrome is defined as fever, rigidity, mental status changes, and autonomic instability after exposure to antidopaminergic drugs. It is classically seen after administration of typical antipsychotics, though atypical antipsychotics and antiemetic drugs may be implicated as well.
Patients often exhibit agitation and confusion, which when severe may progress to mutism and catatonia. Likewise, psychotropic drugs such as quetiapine and venlafaxine, used in combination, have the additional risk of serotonin syndrome.
Additional symptoms include hyperreflexia, ataxia, and myoclonus. Withdrawal of the causative agent and supportive care are the mainstays of therapy. Targeted therapies with agents such as dantrolene, bromocriptine, and amantadine have also been reported anecdotally, but their efficacy is unclear, with variable results.22
As noted earlier, the addition of quetiapine to the patient’s already lengthy medication list could conceivably cause neuroleptic malignant syndrome or serotonin syndrome and should be considered. However, additional neurologic findings to confirm this diagnosis are lacking.
Seizures
Nonconvulsive seizure, particularly nonconvulsive status epilepticus (NCSE), is not well recognized and is particularly challenging to diagnose without EEG. In several case series of patients presenting to the emergency room with altered mental status, NCSE was found in 16% to 28% of patients in whom EEG was performed after an initial evaluation failed to show an obvious cause for the delirium.23,24 Historical features are unreliable for ruling out NCSE as a cause of delirium, as up to 41% of patients in whom the condition is ultimately diagnosed have only confusion as the presenting clinical symptom.25
Likewise, alternating ictal and postictal periods may mimic the typical waxing and waning course classically associated with delirium of other causes. Physical findings such as nystagmus, anisocoria, and hippus may be helpful but are often overlooked or absent. EEG is thus an essential requirement for the diagnosis.26
Given the lack of a clear diagnosis, a workup with EEG should be considered in this patient.
CASE CONTINUED: ADDITIONAL SIGNS
In the ICU, our patient is evaluated by the intensivist team. Her vital signs are stable, and while she is now awakening, she is unable to follow commands and remains mute. She does not initiate movement spontaneously but offers slight resistance to passive movements, holding and maintaining postures her extremities are placed in. She keeps her eyes closed, but when opened by the examining physician, dysconjugate gaze and anisocoria are noted.
3. What clinical entity is most consistent with these physical findings, and what is the next step in management?
- Catatonia secondary to bipolar disorder type I: challenge with intravenous lorazepam 2 mg
- Oculomotor nerve palsy due to enlarging intracranial aneurysm: aggressive blood pressure lowering, elevation of the head of the bed
- Toxic leukoencephalopathy: supportive care and withdrawal of the causative agent
- NCSE: challenge with intravenous lorazepam 2 mg and order EEG
Catatonia
The DSM-5 defines catatonia as a behavioral syndrome complicating an underlying psychiatric or medical condition, as opposed to a distinct diagnosis. It is most commonly encountered in psychiatric illnesses including bipolar disorder, major depression, and schizophrenia. Akinesis, stupor, mutism, and “waxy” flexibility often dominate the clinical picture.
The pathophysiology is poorly defined, but likely involves neurotransmitter imbalances particularly with an increase in N-methyl-d-aspartate (NMDA) activity and suppression of gamma-aminobutyric acid (GABA) activity. This hypothesis is supported by the finding that benzodiazepines, electroconvulsive therapy, and NMDA antagonists such as amantadine are all effective in treating catatonia.27,28 Findings of focal neurologic abnormalities warrant further investigation. EEG may be necessary to differentiate catatonia from NCSE, as both may respond to a benzodiazepine challenge.
As pure catatonia is a diagnosis of exclusion, further workup, including EEG, is necessary to confirm the diagnosis.
Oculomotor nerve palsy
Anisocoria together with dysconjugate gaze should prompt consideration of a lesion involving the oculomotor nerve. Loss of tonic muscle activity from the lateral rectus and superior oblique cause a downward and outward gaze. Furthermore, loss of parasympathetic tone occurs with compressive palsies of the oculomotor nerve, clinically manifesting as a mydriatic and unreactive pupil with ptosis. Given its anatomic course and proximity to other vascular and parenchymal structures, the oculomotor nerve is vulnerable to compression from many sources, including aneurysmal dilation (especially of the posterior cerebral artery), uncal herniation, and inflammation of the cavernous sinus.
Noncontrast CT and lumbar puncture are very sensitive for making the diagnosis of sentinel bleeding within the first 24 hours,29 whereas computed tomographic angiography and magnetic resonance angiography can reliably detect unruptured aneurysms as small as 3 mm.30
Conditions that can lead to oculomotor palsy are unlikely to cause an acute gain in appendicular muscle tone, as noted by the catatonia this patient is demonstrating. Also, mass lesions or bleeding associated with oculomotor palsy is likely to cause acute loss of tone. Chronic upper-motor neuron lesions lead to spasticity rather than the waxy flexibility seen in this patient. In our patient, the findings of isolated anisocoria without further clinical evidence of oculomotor nerve compression make this diagnosis unlikely.
Toxic leukoencephalopathy
Toxic leukoencephalopathy—widespread destruction of myelin, particularly in the white matter tracts that support higher cortical functions—can be caused by antineoplastic agents, immunosuppressant agents, and industrial solvents, as well as by abuse of vaporized drugs such as heroin (“chasing the dragon”). In its mild forms it may cause behavioral disturbances or inattention. In severe forms, a neurobehavioral syndrome of akinetic mutism may be present and can mimic catatonia.31
The diagnosis is often based on the clinical history and neuroimaging, particularly MRI, which demonstrates hyperintensity of the white matter tracts in T2-weighted images.32
This patient does not have a clear history of exposure to an agent typically associated with toxic leukoencephalopathy and does not have the corroborating MRI findings to support this diagnosis.
CASE CONTINUED
Because recent neuroimaging revealed no structural brain lesions and no cause for brain herniation, the patient receives a challenge of 2 mg of intravenous lorazepam to treat potential NCSE. Subsequent improvement is noted in her anisocoria, gaze deviation, and encephalopathy. EEG reveals frequent focal seizures arising from mesial frontal regions with bilateral hemisphere propagation, consistent with bifrontal focal NCSE.
As our patient is being transferred to a room for continuous EEG monitoring, her condition begins to deteriorate, and she again becomes more encephalopathic, with anisocoria and dysconjugate gaze. Additional doses of lorazepam are given (to complete a 0.1-mg/kg load), and additional therapy with intravenous fosphenytoin (20-mg/kg load) is given. Intubation is done for airway protection.
Continuous EEG monitoring reveals multiple frequent electrographic seizures arising from the bifrontal territories, concerning for persistent focal NCSE. A midazolam drip is initiated for EEG burst suppression of cerebral activity. Over 24 hours, EEG shows resolution of seizure activity. As the patient is weaned from sedation, she awakens and follows commands consistently, tolerating extubation without complications. Her neurologic status remains stable over the next 48 hours, having returned to her neurologic baseline level of functioning. She is able to be transferred out of the ICU in stable condition while continuing on scheduled antiepileptic therapy with phenytoin.
ALTERED MENTAL STATUS IN INPATIENTS
Altered mental status is one of the most frequently encountered reasons for medical consultation from nonmedical services. The workup and management of metabolic, toxic, psychiatric, and neurologic causes requires a deep appreciation for the broad differential diagnosis and a multidisciplinary approach. Physicians caring for these patients should avoid prematurely drawing conclusions when the patient’s clinical condition fails to respond to typical measures.
Delirium is a challenging adverse event in older patients during hospitalization, with a significant national financial burden of $164 billion per year.33 The prevalence of delirium in adults on hospital admission is estimated as 14% to 24%, with an inpatient hospitalization incidence ranging from 6% to 56% in general hospital patients.34 In addition, postoperative delirium has been reported in 15% to 53% of older patients.35
While delirium is preventable in 30% to 40% of cases,36,37 it remains an important independent prognostic determinant of hospital outcomes.38–40
Delirium in hospitalized patients requires a thorough, individualized workup. In our patient’s case, the clinical findings of hypoactive delirium were found to be manifestations of NCSE, a rare life-threatening and potentially reversible neurologic disease.
While establishing seizures as a diagnosis, careful attention must first be directed towards investigating environmental or metabolic triggers that may be inciting the disease. This often involves a similar workup for metabolic derangements, as seen in the approach to delirium.
The diagnosis of NCSE, while made in this patient’s case, remains challenging. Careful physical examination should assess for automatisms, “negative” symptoms (staring, aphasia, weakness), and “positive” symptoms (hallucinations, psychosis). Cataplexy, mutism, and other acute psychiatric features have been associated with NCSE,44 highlighting the importance of EEG. A trial of a benzodiazepine in conjunction with clinical and EEG monitoring may help guide clinical decision- making.
As there is no current universally accepted definition for NCSE nor an accepted agreement on required EEG diagnostic features at this time,41 accurate diagnosis is most likely to be obtained in facilities with both subspecialty neurologic consultation and EEG capabilities.
Our patient’s family history of Pick disease is interesting, as this is a progressive form of frontotemporal dementia with both sporadic and genetically linked cases. Recent studies have shown evidence that patients with neurodegenerative disease have increased seizure frequency early in the disease course,31 and efforts are under way to establish the incidence of first unprovoked seizure in patients with frontotemporal dementia. In our patient’s case, resolution of seizure activity yielded a return to her baseline level of neurologic function.
Early use of selective serotonin reuptake inhibitors has been shown to help with the behavioral symptoms of frontotemporal dementia,45 but increasing requirements over time may indicate progression of neurodegeneration and should warrant further appropriate investigation.
In our patient’s case, escalating dose requirements may have reflected worsening frontotemporal atrophy. However, the diagnosis of a neurodegenerative disease such as frontotemporal dementia in a patient such as ours is not definitively established at this time and is being investigated on an outpatient basis.
Given the frequency of delirium and its many risk factors in the inpatient setting, verifying a causative diagnosis can be difficult. Detailed consideration of the patient’s individual clinical circumstances, often in concert with appropriate subspecialty consultations, is essential to the evaluation. Although it is time-intensive, multidisciplinary intervention can lead to safer outcomes and shorter hospital stays.
A 64-year-old woman undergoes elective T10-S1 nerve decompression with fusion for chronic idiopathic scoliosis. Soon afterward, she develops acute urinary retention attributed to an Escherichia coli urinary tract infection and narcotic medications. She is treated with antibiotics, an indwelling catheter is inserted, and her symptoms resolve. She is transferred to the inpatient physical rehabilitation unit.
On postoperative day 9, she develops an acute change in mental status, suddenly becoming extremely anxious and falsely believing she has a “terminal illness.” A psychiatrist suggests that these symptoms are a manifestation of delirium, given the patient’s recent surgery and exposure to benzodiazepine and narcotic medications. On postoperative day 10, she is awake but is now mute and uncooperative. An internist is consulted for an evaluation for encephalopathy and delirium.
MEDICAL HISTORY
Her medical history, obtained by chart review and interviewing her husband, includes well-controlled bipolar disorder over the last 4 years, with no episodes of frank psychosis or mania. She had a “bout of delirium” 4 years earlier attributed to a catastrophic life event, but the symptoms resolved after adjustment of her anxiolytic and mood-stabilizing drugs. She also has well-controlled hypertension, hypothyroidism, and gastroesophageal reflux. Her only surgery was her recent elective procedure.
She has a family history of dementia (Pick disease in her mother).
She is married, lives with her husband, and has an adult son. She is employed as a media specialist and also teaches English as a second language. Before this hospital admission, she was described as happy and content, though her primary psychiatrist had noted intermittent anxiety. Her husband does not suspect illicit drug use and denies significant alcohol or tobacco abuse.
A thorough review of systems is not possible, given her encephalopathy. But before her acute decline, she had complained of “choking on blood” and a subjective inability to swallow.
Her home medications include dextroamphetamine extended-release, alprazolam as needed for sleep, venlafaxine extended-release, lamotrigine, lisinopril, propranolol, amlodipine, atorvastatin, levothyroxine, omeprazole, iron, and vitamin B12. At the time of the evaluation, she is on her home medications with the addition of olanzapine, vitamin D, polyethylene glycol, and an intravenous infusion of dextrose 5% with 0.45% saline at a rate of 100 mL/hour. She has allergies to latex, penicillin, peanuts, and shellfish.
PHYSICAL EXAMINATION
On physical examination, the patient seems healthy and appears normal for her stated age. She is wearing a spinal brace and is in no apparent distress. She is afebrile, pulse 104 beats per minute, respirations 16 breaths per minute and unlabored, and oxygen saturation good on room air. The skin is normal. No thyromegaly, bruits, or lymphadenopathy is noted. Cardiovascular, respiratory, and abdominal examinations, though limited by the spinal brace, are unremarkable. She has no evidence of peripheral edema or vascular insufficiency. Muscle bulk and tone are adequate and symmetric.
She is awake and alert and able to follow simple commands with some prompting. She does not initiate movements spontaneously. She makes some eye contact but does not track or acknowledge the interviewer consistently and does not respond verbally to questions. Her sclera are nonicteric, the pupils are equally round and reactive to light, and the external ocular muscles are intact. There is no facial asymmetry, and the tongue protrudes at midline. She blinks appropriately to threat bilaterally. Strength is at least 3/5 in the upper extremities and 2/5 in the lower extremities, though the examination is limited by lack of patient cooperation. She shows minimal grimace on noxious stimulation but does not withdraw extremities. Reflexes are present and mildly depressed symmetrically. Plantar reflexes are downgoing bilaterally.
INITIAL LABORATORY EVALUATION
On initial laboratory testing, the serum sodium is 132 mmol/L (reference range 136–144), stable since admission. Point-of-care glucose is 98 mg/dL. Aspartate aminotransferase and alanine aminotransferase levels are mildly elevated at 59 U/L (13–35) and 51 U/L (7–38), respectively, but serum ammonia is undetectable. Vitamin B12, folate, thyroid-stimulating hormone, and free thyroxine are within the normal ranges. Leukocytosis is noted, with 14 × 109 cells/L (3.7–11.0), 86% neutrophils, and a mild left shift. Urinalysis is negative for leukocyte esterase, nitrites, and white blood cells.
APPROACH TO ALTERED MENTAL STATUS
1. Which of the following risk factors predisposes this patient to postoperative delirium?
- Hyponatremia
- Polypharmacy
- Family history of dementia
- Depression
Altered mental status, or encephalopathy, is one of the most common yet challenging conditions in medicine. When a consult is placed for altered mental status, it is important to determine the affected domain that has changed from the patient’s normal state. Changes can include alterations in consciousness, attention, behavior, cognition, language, speech, and praxis and can reflect varying degrees of cerebral dysfunction.
Electrolyte abnormalities
Disorders of sodium homeostasis are common in hospitalized patients and may contribute to the onset of delirium. Hyponatremia is especially frequent and often iatrogenic, with a prevalence significantly higher in women (2.1% vs 1.3%, P = .0044) and in the elderly.2
Neurologic manifestations are often the result of cerebral edema due to osmolar volume shifts.3–6 Acute hyponatremic encephalopathy is most likely to occur when sodium shifts are rapid, usually within 24 hours, and is often seen in postoperative patients requiring significant volume resuscitation with hypotonic fluids.6 Young premenopausal women appear to be at especially high risk of permanent brain damage secondary to hyponatremic encephalopathy,7 a finding that may reflect the limited compliance within the intracranial vault and lack of significant involutional parenchymal changes that occur with aging.8–11
Aging also has important effects on fluid balance, as restoration of body fluid homeostasis is slower in older patients.12
Hormonal effects of estrogen appear to play a synergistic role in the expression of arginine vasopressin in postmenopausal women, further contributing to hyponatremia.
Although our patient has mild hyponatremia, there has been no acute change in her sodium balance since admission to the hospital, and so it is unlikely to be the cause of her acute delirium. Her mild hyponatremia may in part be from hypo-osmolar maintenance fluids with dextrose 5% and 0.45% normal saline.
Mild chronic hyponatremia may affect balance and has been associated with increased mortality risk in certain chronic disease states, but this is unlikely to be the main cause of acute delirium.
Polypharmacy
Patients admitted to the hospital with polypharmacy are at high risk of drug-induced delirium. In approaching delirium, a patient’s medications should be evaluated for interactions, as well as for possible effects of newly prescribed drugs. New medications that affect cytochrome P450 enzymes warrant investigation, as do drugs with narrow therapeutic windows that the patient has been using long-term.
Consultation with a clinical pharmacist is often helpful. Macrolides, protease inhibitors, and nondihydropyridine calcium channel blockers are common P450 inhibitors, while many anticonvulsants are known inducers of the P450 system. Selective serotonin reuptake inhibitors and diuretics can lead to electrolyte imbalances such as hyponatremia, which may further predispose to bouts of delirium, as described above.
The patient’s extensive list of psychoactive medications makes polypharmacy a significant risk factor for delirium. Quetiapine and venlafaxine both cause sedation and increase the risk of serotonin syndrome. However, in this case, the patient does not have marked fever, rigidity, or hyperreflexia to corroborate that diagnosis.
Dementia
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), defines dementia as a disorder involving cognitive impairment in at least 1 cognitive domain, with a significant decline from a previous level of functioning.1 These impairments need not necessarily occur separately from bouts of delirium, but the time course for most forms of dementia tends to be progressive over a subacute to chronic duration.
Dementia increases the risk for acute confusion and delirium in hospitalized patients.13 This is partly reflected by pathophysiologic changes that leave elderly patients susceptible to the effects of anticholinergic drugs.14 Structural changes due to small-vessel ischemia may also predispose patients to seizures in the setting of metabolic derangement or critical illness. Diagnosing dementia thus remains a challenge, as dementia must be clearly distinguished from other disorders such as delirium and depression.
The acute change in this patient’s case makes the isolated diagnosis of dementia much less likely than other causes of altered mental status. Also, her previous level of function does not suggest a clinically significant personal history of impairment.
Mental illness
Several studies have examined the link between preoperative mental health disorders and postoperative delirium.15–17 Depression appears to be a risk factor for postoperative delirium in patients undergoing elective orthopedic surgery,15 and this includes elderly patients.16 While a clear etiologic link has yet to be determined, disruption of circadian rhythm and abnormal cerebral response to stress may play a role. Studies have also suggested an association between schizophrenia and delirium, though this may be related to perioperative suspension of medications.17
Bipolar disorder has not been well studied with regard to postoperative complications. However, this patient has had a previous episode of decompensated mania, therefore making bipolar disorder a plausible condition in the differential diagnosis.
CASE CONTINUED: ACUTE DETERIORATION
Without a clearly identifiable cause for our patient’s acute confusional state, neurology and medical consultants recommend neuroimaging.
Computed tomography (CT) and magnetic resonance imaging (MRI) without contrast are ordered and performed on postoperative day 11 and demonstrate chronic small-vessel ischemic disease, consistent with our patient’s age, as well as frontotemporal atrophy. There is no evidence of mass effect, bleeding, or acute ischemia.
Overnight, she becomes obtunded, and the rapid response team is called. Her vital signs appear stable, and she is afebrile. Basic laboratory studies, imaging, and electrocardiography are repeated, and the results are unchanged from recent tests. She is transferred to the intensive care unit (ICU) for closer monitoring.
2. What is most likely cause of the patient’s declining mental status, and what is the next appropriate step?
- Acute stroke: repeat MRI with contrast
- Urinary tract infection: order blood and urine cultures, and start empiric antibiotics
- Neuroleptic malignant syndrome: start dantrolene
- Seizures: order electroencephalography (EEG)
Acute stroke
Acute stroke can affect mental status and consciousness through several pathways. Stroke syndromes can vary in presentation depending on the level of cortical and subcortical involvement, with clinical manifestations including confusion, aphasia, neglect, and inattention. Wakefulness and the ability to maintain consciousness is impaired, with disruption of the ascending reticular activating system, often seen in injuries to the brainstem. Large territorial or hemispheric infarcts, with subsequent cerebral edema, can also disrupt this system and lead to cerebral herniation and coma.
MRI without contrast is extremely sensitive for ischemia and can typically detect ischemia in acute stroke within 3 to 30 minutes.18–20 Repeating the study with contrast is unlikely to provide additional benefit.
In our patient’s case, the lack of localizing neurologic symptoms, in addition to her recent negative neuroimaging workup, makes the diagnosis of acute stroke unlikely.
Infection
The role of severe infection in patients with altered mental status is well documented and likely relates to diffuse cerebral dysfunction caused by an inflammatory cascade. Less well understood is the role of occult infection, especially urinary tract infection, in otherwise immunocompetent patients. Urinary tract infection has long been thought to cause delirium in otherwise asymptomatic elderly patients, but few studies have examined this relationship, and those studies have been shown to have significant methodologic errors.21 In the absence of better data, urinary tract infection as the cause of frank delirium in an otherwise well patient should be viewed with skepticism, and alternative causes should be sought.
Although the patient has a nonspecific leukocytosis, her benign urinalysis and lack of corroborating evidence makes urinary tract infection an unlikely cause of her frank delirium.
Neuroleptic malignant syndrome
Neuroleptic malignant syndrome is defined as fever, rigidity, mental status changes, and autonomic instability after exposure to antidopaminergic drugs. It is classically seen after administration of typical antipsychotics, though atypical antipsychotics and antiemetic drugs may be implicated as well.
Patients often exhibit agitation and confusion, which when severe may progress to mutism and catatonia. Likewise, psychotropic drugs such as quetiapine and venlafaxine, used in combination, have the additional risk of serotonin syndrome.
Additional symptoms include hyperreflexia, ataxia, and myoclonus. Withdrawal of the causative agent and supportive care are the mainstays of therapy. Targeted therapies with agents such as dantrolene, bromocriptine, and amantadine have also been reported anecdotally, but their efficacy is unclear, with variable results.22
As noted earlier, the addition of quetiapine to the patient’s already lengthy medication list could conceivably cause neuroleptic malignant syndrome or serotonin syndrome and should be considered. However, additional neurologic findings to confirm this diagnosis are lacking.
Seizures
Nonconvulsive seizure, particularly nonconvulsive status epilepticus (NCSE), is not well recognized and is particularly challenging to diagnose without EEG. In several case series of patients presenting to the emergency room with altered mental status, NCSE was found in 16% to 28% of patients in whom EEG was performed after an initial evaluation failed to show an obvious cause for the delirium.23,24 Historical features are unreliable for ruling out NCSE as a cause of delirium, as up to 41% of patients in whom the condition is ultimately diagnosed have only confusion as the presenting clinical symptom.25
Likewise, alternating ictal and postictal periods may mimic the typical waxing and waning course classically associated with delirium of other causes. Physical findings such as nystagmus, anisocoria, and hippus may be helpful but are often overlooked or absent. EEG is thus an essential requirement for the diagnosis.26
Given the lack of a clear diagnosis, a workup with EEG should be considered in this patient.
CASE CONTINUED: ADDITIONAL SIGNS
In the ICU, our patient is evaluated by the intensivist team. Her vital signs are stable, and while she is now awakening, she is unable to follow commands and remains mute. She does not initiate movement spontaneously but offers slight resistance to passive movements, holding and maintaining postures her extremities are placed in. She keeps her eyes closed, but when opened by the examining physician, dysconjugate gaze and anisocoria are noted.
3. What clinical entity is most consistent with these physical findings, and what is the next step in management?
- Catatonia secondary to bipolar disorder type I: challenge with intravenous lorazepam 2 mg
- Oculomotor nerve palsy due to enlarging intracranial aneurysm: aggressive blood pressure lowering, elevation of the head of the bed
- Toxic leukoencephalopathy: supportive care and withdrawal of the causative agent
- NCSE: challenge with intravenous lorazepam 2 mg and order EEG
Catatonia
The DSM-5 defines catatonia as a behavioral syndrome complicating an underlying psychiatric or medical condition, as opposed to a distinct diagnosis. It is most commonly encountered in psychiatric illnesses including bipolar disorder, major depression, and schizophrenia. Akinesis, stupor, mutism, and “waxy” flexibility often dominate the clinical picture.
The pathophysiology is poorly defined, but likely involves neurotransmitter imbalances particularly with an increase in N-methyl-d-aspartate (NMDA) activity and suppression of gamma-aminobutyric acid (GABA) activity. This hypothesis is supported by the finding that benzodiazepines, electroconvulsive therapy, and NMDA antagonists such as amantadine are all effective in treating catatonia.27,28 Findings of focal neurologic abnormalities warrant further investigation. EEG may be necessary to differentiate catatonia from NCSE, as both may respond to a benzodiazepine challenge.
As pure catatonia is a diagnosis of exclusion, further workup, including EEG, is necessary to confirm the diagnosis.
Oculomotor nerve palsy
Anisocoria together with dysconjugate gaze should prompt consideration of a lesion involving the oculomotor nerve. Loss of tonic muscle activity from the lateral rectus and superior oblique cause a downward and outward gaze. Furthermore, loss of parasympathetic tone occurs with compressive palsies of the oculomotor nerve, clinically manifesting as a mydriatic and unreactive pupil with ptosis. Given its anatomic course and proximity to other vascular and parenchymal structures, the oculomotor nerve is vulnerable to compression from many sources, including aneurysmal dilation (especially of the posterior cerebral artery), uncal herniation, and inflammation of the cavernous sinus.
Noncontrast CT and lumbar puncture are very sensitive for making the diagnosis of sentinel bleeding within the first 24 hours,29 whereas computed tomographic angiography and magnetic resonance angiography can reliably detect unruptured aneurysms as small as 3 mm.30
Conditions that can lead to oculomotor palsy are unlikely to cause an acute gain in appendicular muscle tone, as noted by the catatonia this patient is demonstrating. Also, mass lesions or bleeding associated with oculomotor palsy is likely to cause acute loss of tone. Chronic upper-motor neuron lesions lead to spasticity rather than the waxy flexibility seen in this patient. In our patient, the findings of isolated anisocoria without further clinical evidence of oculomotor nerve compression make this diagnosis unlikely.
Toxic leukoencephalopathy
Toxic leukoencephalopathy—widespread destruction of myelin, particularly in the white matter tracts that support higher cortical functions—can be caused by antineoplastic agents, immunosuppressant agents, and industrial solvents, as well as by abuse of vaporized drugs such as heroin (“chasing the dragon”). In its mild forms it may cause behavioral disturbances or inattention. In severe forms, a neurobehavioral syndrome of akinetic mutism may be present and can mimic catatonia.31
The diagnosis is often based on the clinical history and neuroimaging, particularly MRI, which demonstrates hyperintensity of the white matter tracts in T2-weighted images.32
This patient does not have a clear history of exposure to an agent typically associated with toxic leukoencephalopathy and does not have the corroborating MRI findings to support this diagnosis.
CASE CONTINUED
Because recent neuroimaging revealed no structural brain lesions and no cause for brain herniation, the patient receives a challenge of 2 mg of intravenous lorazepam to treat potential NCSE. Subsequent improvement is noted in her anisocoria, gaze deviation, and encephalopathy. EEG reveals frequent focal seizures arising from mesial frontal regions with bilateral hemisphere propagation, consistent with bifrontal focal NCSE.
As our patient is being transferred to a room for continuous EEG monitoring, her condition begins to deteriorate, and she again becomes more encephalopathic, with anisocoria and dysconjugate gaze. Additional doses of lorazepam are given (to complete a 0.1-mg/kg load), and additional therapy with intravenous fosphenytoin (20-mg/kg load) is given. Intubation is done for airway protection.
Continuous EEG monitoring reveals multiple frequent electrographic seizures arising from the bifrontal territories, concerning for persistent focal NCSE. A midazolam drip is initiated for EEG burst suppression of cerebral activity. Over 24 hours, EEG shows resolution of seizure activity. As the patient is weaned from sedation, she awakens and follows commands consistently, tolerating extubation without complications. Her neurologic status remains stable over the next 48 hours, having returned to her neurologic baseline level of functioning. She is able to be transferred out of the ICU in stable condition while continuing on scheduled antiepileptic therapy with phenytoin.
ALTERED MENTAL STATUS IN INPATIENTS
Altered mental status is one of the most frequently encountered reasons for medical consultation from nonmedical services. The workup and management of metabolic, toxic, psychiatric, and neurologic causes requires a deep appreciation for the broad differential diagnosis and a multidisciplinary approach. Physicians caring for these patients should avoid prematurely drawing conclusions when the patient’s clinical condition fails to respond to typical measures.
Delirium is a challenging adverse event in older patients during hospitalization, with a significant national financial burden of $164 billion per year.33 The prevalence of delirium in adults on hospital admission is estimated as 14% to 24%, with an inpatient hospitalization incidence ranging from 6% to 56% in general hospital patients.34 In addition, postoperative delirium has been reported in 15% to 53% of older patients.35
While delirium is preventable in 30% to 40% of cases,36,37 it remains an important independent prognostic determinant of hospital outcomes.38–40
Delirium in hospitalized patients requires a thorough, individualized workup. In our patient’s case, the clinical findings of hypoactive delirium were found to be manifestations of NCSE, a rare life-threatening and potentially reversible neurologic disease.
While establishing seizures as a diagnosis, careful attention must first be directed towards investigating environmental or metabolic triggers that may be inciting the disease. This often involves a similar workup for metabolic derangements, as seen in the approach to delirium.
The diagnosis of NCSE, while made in this patient’s case, remains challenging. Careful physical examination should assess for automatisms, “negative” symptoms (staring, aphasia, weakness), and “positive” symptoms (hallucinations, psychosis). Cataplexy, mutism, and other acute psychiatric features have been associated with NCSE,44 highlighting the importance of EEG. A trial of a benzodiazepine in conjunction with clinical and EEG monitoring may help guide clinical decision- making.
As there is no current universally accepted definition for NCSE nor an accepted agreement on required EEG diagnostic features at this time,41 accurate diagnosis is most likely to be obtained in facilities with both subspecialty neurologic consultation and EEG capabilities.
Our patient’s family history of Pick disease is interesting, as this is a progressive form of frontotemporal dementia with both sporadic and genetically linked cases. Recent studies have shown evidence that patients with neurodegenerative disease have increased seizure frequency early in the disease course,31 and efforts are under way to establish the incidence of first unprovoked seizure in patients with frontotemporal dementia. In our patient’s case, resolution of seizure activity yielded a return to her baseline level of neurologic function.
Early use of selective serotonin reuptake inhibitors has been shown to help with the behavioral symptoms of frontotemporal dementia,45 but increasing requirements over time may indicate progression of neurodegeneration and should warrant further appropriate investigation.
In our patient’s case, escalating dose requirements may have reflected worsening frontotemporal atrophy. However, the diagnosis of a neurodegenerative disease such as frontotemporal dementia in a patient such as ours is not definitively established at this time and is being investigated on an outpatient basis.
Given the frequency of delirium and its many risk factors in the inpatient setting, verifying a causative diagnosis can be difficult. Detailed consideration of the patient’s individual clinical circumstances, often in concert with appropriate subspecialty consultations, is essential to the evaluation. Although it is time-intensive, multidisciplinary intervention can lead to safer outcomes and shorter hospital stays.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association Publishing; 2013. http://psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596. Accessed July 7, 2017.
- Mohan S, Gu S, Parikh A, Radhakrishnan J. Prevalence of hyponatremia and association with mortality: results from NHANES. Am J Med 2013; 126:1127–1137.e1.
- Sterns RH. Disorders of plasma sodium—causes, consequences, and correction. N Engl J Med 2015; 372:55–65.
- Rose B, Post T. Clinical physiology of acid-base and electrolyte disorders. 5th ed. New York, NY: McGraw-Hill; 2001.
- McManus ML, Churchwell KB, Strange K. Regulation of cell volume in health and disease. N Engl J Med 1995; 333:1260–1266.
- Strange K. Regulation of solute and water balance and cell volume in the central nervous system. J Am Soc Nephrol 1992; 3:12–27.
- Ayus JC, Wheeler JM, Arieff AI. Postoperative hyponatremic encephalopathy in menstruant women. Ann Intern Med 1992; 117:891–897.
- Gur RC, Mozley PD, Resnick SM, et al. Gender differences in age effect on brain atrophy measured by magnetic resonance imaging. Proc Natl Acad Sci USA 1991; 88:2845–2849.
- Rosomoff HL, Zugibe FT. Distribution of intracranial contents in experimental edema. Arch Neurol 1963; 9:26–34.
- Melton JE, Nattie EE. Brain and CSF water and ions during dilutional and isosmotic hyponatremia in the rat. Am J Physiol 1983; 244:R724–R732.
- Nattie EE, Edwards WH. Brain and CSF water and ions in newborn puppies during acute hypo- and hypernatremia. J Appl Physiol Respir Environ Exerc Physiol 1981; 51:1086–1091.
- Stachenfeld NS, DiPietro L, Palter SF, Nadel ER. Estrogen influences osmotic secretion of AVP and body water balance in postmenopausal women. Am J Physiol 1998; 274:R187–R195.
- Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc 2002; 50:1723–1732.
- de Smet Y, Ruberg M, Serdaru M, Dubois B, Lhermitte F, Agid Y. Confusion, dementia and anticholinergics in Parkinson’s disease. J Neurol Neurosurg Psychiatry 1982; 45:1161–1164.
- Mollon B, Mahure SA, Ding DY, Zuckerman JD, Kwon YW. The influence of a history of clinical depression on peri-operative outcomes in elective total shoulder arthroplasty: a ten-year national analysis. Bone Joint J 2016; 98-B:818–824.
- Kosar CM, Tabloski PA, Travison TG, et al. Effect of preoperative pain and depressive symptoms on the development of postoperative delirium. Lancet Psychiatry 2014; 1:431–436.
- Copeland LA, Zeber JE, Pugh MJ, Mortensen EM, Restrepo MI, Lawrence VA. Postoperative complications in the seriously mentally ill: a systematic review of the literature. Ann Surg 2008; 248:31–38.
- Warach S, Gaa J, Siewert B, Wielopolski P, Edelman RR. Acute human stroke studied by whole brain echo planar diffusion-weighted magnetic resonance imaging. Ann Neurol 1995; 37:231–241.
- Sorensen AG, Buonanno FS, Gonzalez RG, et al. Hyperacute stroke: evaluation with combined multisection diffusion-weighted and hemodynamically weighted echo-planar MR imaging. Radiology 1996; 199:391–401.
- Li F, Han S, Tatlisumak T, et al. A new method to improve in-bore middle cerebral artery occlusion in rats: demonstration with diffusion—and perfusion—weighted imaging. Stroke 1998; 29:1715–1720.
- Balogun SA, Philbrick JT. Delirium, a symptom of UTI in the elderly: fact or fable? A systematic review. Can Geriatr J 2013; 17:22–26.
- Reulbach U, Dütsch C, Biermann T, et al. Managing an effective treatment for neuroleptic malignant syndrome. Crit Care 2007; 11:R4.
- Naeije G, Depondt C, Meeus C, Korpak K, Pepersack T, Legros B. EEG patterns compatible with nonconvulsive status epilepticus are common in elderly patients with delirium: a prospective study with continuous EEG monitoring. Epilepsy Behav 2014; 36:18–21.
- Veran O, Kahane P, Thomas P, Hamelin S, Sabourdy C, Vercueil L. De novo epileptic confusion in the elderly: a 1-year prospective study. Epilepsia 2010; 51:1030–1035.
- Sutter R, Rüegg S, Kaplan PW. Epidemiology, diagnosis, and management of nonconvulsive status epilepticus. Opening Pandora’s box. Neurol Clin Pract 2012; 2:275–286.
- Husain AM, Horn GJ, Jacobson MP. Non-convulsive status epilepticus: usefulness of clinical features in selecting patients for urgent EEG. J Neurol Neurosurg Psychiatry 2003; 74:189–191.
- Ungvari GS, Chiu HF, Chow LY, Lau BS, Tang WK. Lorazepam for chronic catatonia: a randomized, double-blind, placebo-controlled cross-over study. Psychopharmacology (Berl) 1999; 142:393–398.
- Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry Clin Neurosci 2007; 19:406– 412.
- Perry JJ, Spacek A, Forbes M, et al. Is the combination of negative computed tomography result and negative lumbar puncture result sufficient to rule out subarachnoid hemorrhage? Ann Emerg Med 2008; 51:707–713.
- Li MH, Cheng YS, Li YD, et al. Large-cohort comparison between three-dimensional time-of-flight magnetic resonance and rotational digital subtraction angiographies in intracranial aneurysm detection. Stroke 2009; 40:3127–3129.
- Filley CM, Kleinschmidt-DeMasters BK. Toxic leukoencephalopathy. N Engl J Med 2001; 345:425–432.
- Magnetic resonance imaging of the central nervous system. Council on Scientific Affairs. Report of the Panel on Magnetic Resonance Imaging. JAMA 1988; 259:1211–1222.
- Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med 2008; 168:27–32.
- Inouye SK. Delirium in hospitalized older patients. Clin Geriatr Med 1998; 14:745–764.
- Agostini JV, Inouye SK, Hazzard W, Blass J. Delirium. In: Principles of Geriatric Medicine and Gerontology. 5th ed. New York, NY: McGraw-Hill; 2003:1503–1515.
- Inouye SK, Bogardus ST Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999; 340:669–676.
- Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc 2001; 49:516–522.
- Inouye SK, Rushing JT, Foreman MD, Palmer RM, Pompei P. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med 1998; 13:234–242.
- Rothschild JM, Bates DW, Leape LL. Preventable medical injuries in older patients. Arch Intern Med 2000; 160:2717–2728.
- Gillick MR, Serrell NA, Gillick LS. Adverse consequences of hospitalization in the elderly. Soc Sci Med 1982; 16:1033–1038.
- Drislane FW. Presentation, evaluation, and treatment of nonconvulsive status epilepticus. Epilepsy Behav 2000; 1:301-314.
- Rosenow F, Hamer HM, Knake S. The epidemiology of convulsive and nonconvulsive status epilepticus. Epilepsia 2007; 48(suppl 8):82–84.
- Woodford HJ, George J, Jackson M. Non-convulsive status epilepticus: a practical approach to diagnosis in confused older people. Postgrad Med J 2015; 91:655–661.
- Kaplan PW. Nonconvulsive status epilepticus in the emergency room. Epilepsia 1996; 37:643–650.
- Swartz JR, Miller BL, Lesser IM, Darby AL. Frontotemporal dementia: treatment response to serotonin selective reuptake inhibitors. J Clin Psychiatry 1997; 58:212–216.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association Publishing; 2013. http://psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596. Accessed July 7, 2017.
- Mohan S, Gu S, Parikh A, Radhakrishnan J. Prevalence of hyponatremia and association with mortality: results from NHANES. Am J Med 2013; 126:1127–1137.e1.
- Sterns RH. Disorders of plasma sodium—causes, consequences, and correction. N Engl J Med 2015; 372:55–65.
- Rose B, Post T. Clinical physiology of acid-base and electrolyte disorders. 5th ed. New York, NY: McGraw-Hill; 2001.
- McManus ML, Churchwell KB, Strange K. Regulation of cell volume in health and disease. N Engl J Med 1995; 333:1260–1266.
- Strange K. Regulation of solute and water balance and cell volume in the central nervous system. J Am Soc Nephrol 1992; 3:12–27.
- Ayus JC, Wheeler JM, Arieff AI. Postoperative hyponatremic encephalopathy in menstruant women. Ann Intern Med 1992; 117:891–897.
- Gur RC, Mozley PD, Resnick SM, et al. Gender differences in age effect on brain atrophy measured by magnetic resonance imaging. Proc Natl Acad Sci USA 1991; 88:2845–2849.
- Rosomoff HL, Zugibe FT. Distribution of intracranial contents in experimental edema. Arch Neurol 1963; 9:26–34.
- Melton JE, Nattie EE. Brain and CSF water and ions during dilutional and isosmotic hyponatremia in the rat. Am J Physiol 1983; 244:R724–R732.
- Nattie EE, Edwards WH. Brain and CSF water and ions in newborn puppies during acute hypo- and hypernatremia. J Appl Physiol Respir Environ Exerc Physiol 1981; 51:1086–1091.
- Stachenfeld NS, DiPietro L, Palter SF, Nadel ER. Estrogen influences osmotic secretion of AVP and body water balance in postmenopausal women. Am J Physiol 1998; 274:R187–R195.
- Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc 2002; 50:1723–1732.
- de Smet Y, Ruberg M, Serdaru M, Dubois B, Lhermitte F, Agid Y. Confusion, dementia and anticholinergics in Parkinson’s disease. J Neurol Neurosurg Psychiatry 1982; 45:1161–1164.
- Mollon B, Mahure SA, Ding DY, Zuckerman JD, Kwon YW. The influence of a history of clinical depression on peri-operative outcomes in elective total shoulder arthroplasty: a ten-year national analysis. Bone Joint J 2016; 98-B:818–824.
- Kosar CM, Tabloski PA, Travison TG, et al. Effect of preoperative pain and depressive symptoms on the development of postoperative delirium. Lancet Psychiatry 2014; 1:431–436.
- Copeland LA, Zeber JE, Pugh MJ, Mortensen EM, Restrepo MI, Lawrence VA. Postoperative complications in the seriously mentally ill: a systematic review of the literature. Ann Surg 2008; 248:31–38.
- Warach S, Gaa J, Siewert B, Wielopolski P, Edelman RR. Acute human stroke studied by whole brain echo planar diffusion-weighted magnetic resonance imaging. Ann Neurol 1995; 37:231–241.
- Sorensen AG, Buonanno FS, Gonzalez RG, et al. Hyperacute stroke: evaluation with combined multisection diffusion-weighted and hemodynamically weighted echo-planar MR imaging. Radiology 1996; 199:391–401.
- Li F, Han S, Tatlisumak T, et al. A new method to improve in-bore middle cerebral artery occlusion in rats: demonstration with diffusion—and perfusion—weighted imaging. Stroke 1998; 29:1715–1720.
- Balogun SA, Philbrick JT. Delirium, a symptom of UTI in the elderly: fact or fable? A systematic review. Can Geriatr J 2013; 17:22–26.
- Reulbach U, Dütsch C, Biermann T, et al. Managing an effective treatment for neuroleptic malignant syndrome. Crit Care 2007; 11:R4.
- Naeije G, Depondt C, Meeus C, Korpak K, Pepersack T, Legros B. EEG patterns compatible with nonconvulsive status epilepticus are common in elderly patients with delirium: a prospective study with continuous EEG monitoring. Epilepsy Behav 2014; 36:18–21.
- Veran O, Kahane P, Thomas P, Hamelin S, Sabourdy C, Vercueil L. De novo epileptic confusion in the elderly: a 1-year prospective study. Epilepsia 2010; 51:1030–1035.
- Sutter R, Rüegg S, Kaplan PW. Epidemiology, diagnosis, and management of nonconvulsive status epilepticus. Opening Pandora’s box. Neurol Clin Pract 2012; 2:275–286.
- Husain AM, Horn GJ, Jacobson MP. Non-convulsive status epilepticus: usefulness of clinical features in selecting patients for urgent EEG. J Neurol Neurosurg Psychiatry 2003; 74:189–191.
- Ungvari GS, Chiu HF, Chow LY, Lau BS, Tang WK. Lorazepam for chronic catatonia: a randomized, double-blind, placebo-controlled cross-over study. Psychopharmacology (Berl) 1999; 142:393–398.
- Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry Clin Neurosci 2007; 19:406– 412.
- Perry JJ, Spacek A, Forbes M, et al. Is the combination of negative computed tomography result and negative lumbar puncture result sufficient to rule out subarachnoid hemorrhage? Ann Emerg Med 2008; 51:707–713.
- Li MH, Cheng YS, Li YD, et al. Large-cohort comparison between three-dimensional time-of-flight magnetic resonance and rotational digital subtraction angiographies in intracranial aneurysm detection. Stroke 2009; 40:3127–3129.
- Filley CM, Kleinschmidt-DeMasters BK. Toxic leukoencephalopathy. N Engl J Med 2001; 345:425–432.
- Magnetic resonance imaging of the central nervous system. Council on Scientific Affairs. Report of the Panel on Magnetic Resonance Imaging. JAMA 1988; 259:1211–1222.
- Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med 2008; 168:27–32.
- Inouye SK. Delirium in hospitalized older patients. Clin Geriatr Med 1998; 14:745–764.
- Agostini JV, Inouye SK, Hazzard W, Blass J. Delirium. In: Principles of Geriatric Medicine and Gerontology. 5th ed. New York, NY: McGraw-Hill; 2003:1503–1515.
- Inouye SK, Bogardus ST Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999; 340:669–676.
- Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc 2001; 49:516–522.
- Inouye SK, Rushing JT, Foreman MD, Palmer RM, Pompei P. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med 1998; 13:234–242.
- Rothschild JM, Bates DW, Leape LL. Preventable medical injuries in older patients. Arch Intern Med 2000; 160:2717–2728.
- Gillick MR, Serrell NA, Gillick LS. Adverse consequences of hospitalization in the elderly. Soc Sci Med 1982; 16:1033–1038.
- Drislane FW. Presentation, evaluation, and treatment of nonconvulsive status epilepticus. Epilepsy Behav 2000; 1:301-314.
- Rosenow F, Hamer HM, Knake S. The epidemiology of convulsive and nonconvulsive status epilepticus. Epilepsia 2007; 48(suppl 8):82–84.
- Woodford HJ, George J, Jackson M. Non-convulsive status epilepticus: a practical approach to diagnosis in confused older people. Postgrad Med J 2015; 91:655–661.
- Kaplan PW. Nonconvulsive status epilepticus in the emergency room. Epilepsia 1996; 37:643–650.
- Swartz JR, Miller BL, Lesser IM, Darby AL. Frontotemporal dementia: treatment response to serotonin selective reuptake inhibitors. J Clin Psychiatry 1997; 58:212–216.
Bedside manners: How to deal with delirium
During my training in Leiden, Netherlands, I was infused with the lessons of Herman Boerhaave (1668–1738), the professor who is considered the pioneer of bedside teaching.1 This practice had begun in Padua and was then brought to Leiden, where Boerhaave transformed it into an art form. At the Caecilia hospital, the municipal clerics provided Boerhaave with 2 wards for teaching; 1 with 6 beds for men and the other with 6 beds for women. Medical historian Henry Sigerist2 has commented that half the physicians of Europe were trained on these 12 beds.
Boerhaave made daily rounds with his students, examining the patients, reviewing their histories, and inspecting their urine. He considered postmortem examinations essential and made his students attend the autopsies of patients who died: “In spite of the most detailed description of all disease phenomena one does not know anything of the cause until one has opened the body.”2
What was once the basis of clinical medicine is now fading, with both clinical rounds and autopsies being replaced by imaging techniques of body parts and automated analysis of Vacutainer samples. These novelties provide us with far more diagnostic accuracy than Boerhaave had, and randomized controlled trials provide us with an evidence base. But bedside observation and case reports are still relevant,3 and autopsies still reveal important, clinically missed diagnoses.4
In this issue of the Journal, Imm et al5 describe a case of presumed postoperative delirium in a 64-year-old hospitalized patient. They remind us that crucial signs and symptoms can guide how to use our modern diagnostic tools.
DELIRIUM IS OFTEN OVERLOOKED
Delirium is often overlooked by physicians. But why? The characteristic disturbances in attention and cognition are easy to interpret, while the various observation scales have high sensitivity and should signal the need for further interrogation. Perhaps the reason we often overlook the signs and symptoms is that we assume that delirium is just normal sickness behavior.
Another reason we may fail to recognize the syndrome is more fundamental and closely related to how we practice medicine. These days, we place such trust in high-tech diagnostics that we feel the modern procedures supersede the classic examination of patients. But mental disturbances can only be detected by history-taking and clinical observation.
Moreover, the actual mental state is less important than the subtle changes in it. A continuously disturbed mind does not pose a problem, but a casual remark by a family member or informal caregiver that “his mood has changed” should seize our attention.6
Here, the fragmented and disconnected practice of modern medicine takes its toll. Shorter working hours have helped to preserve our own mental performance, but at the cost of being less able to follow the patient’s mental status over time and to recognize a change of behavior. Applying repeated, standardized assessments of these vital signs may help solve the problem, but repeated observations are easily neglected, as are body temperature, blood pressure, and others.
DELIRIUM IS SERIOUS
Imm et al also remind us that delirium is serious. The case-fatality rate in delirium equals that in acute cardiovascular events or metastatic cancer, even though its impact is often not thought to be as severe. Far too often the mental symptoms are dismissed and judged to be something to be handled in the outpatient clinic after the acute problems are addressed.
In part, this may be because no professional society or advocacy group is promoting the recognition, diagnosis, and treatment of delirium or pushing for incentives to do so. We have cardiologists and oncologists but not deliriologists. But in a way, it may be a good thing that no specialist group “owns” delirium, as the syndrome is elicited by various underlying disease mechanisms, and every physician should be vigilant to recognize it.
DELIRIUM REQUIRES PROMPT MANAGEMENT
If delirium is a life-threatening condition, it necessitates a prompt and coordinated series of diagnostic actions, judgments, and decisions.7 Although most delirious patients are not admitted to an intensive care unit, they should be considered critically ill and must be provided a corresponding level of care. Here, the old clinical aphorism holds: action should be taken before the sun sets or rises. Attention should be on worsening of the underlying disease, unexpected comorbid conditions, and side effects of our interventions.
As the case reported by Imm et al shows, the causative factors may be recognized only after in-depth examination.4 The pathogenesis of delirium is poorly understood, and there is no specific therapy for it. There is not even conclusive evidence that the standard use of antipsychotics is beneficial, whereas their side effects cannot be overestimated.7 Our interventions are aimed at eliminating the underlying pathologies that have triggered the delirious state, as well as on preventing complications of the mental disturbance.
Many of us have had the experience of watching one of our children develop fever and confusion. When our older patients become delirious, it should raise the same level of alarm and activity as when it happens in a child.
- Koehler U, Hildebrandt O, Koehler J, Nell C. The pioneer of bedside teaching—Herman Boerhaave (1668–1738). Dtsch Med Wochenschr 2014; 139:2655–2659.
- Sigerist HE. A History of Medicine. New York: Oxford University Press 1951;1. [According to Walker HK. Chapter 1. The origins of the history and physical examination. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston: Buterworths, 1990.] www.ncbi.nlm.nih.gov/books/NBK458. Accessed August 7, 2017.
- Vandenbroucke JP. In defense of case reports and case series. Ann Intern Med 2001; 134:330–334.
- Shojania KG, Burton EC, McDonald KM, Goldman M. Changes in rates of autopsy-detected diagnostic errors over time: a systematic review. JAMA 2003; 289:2849–2856.
- Imm M, Torres LF, Kottapally M. Postoperative delirium in a 64-year-old woman. Cleve Clinic J Med 2017; 84:690–698.
- Steis MR, Evans L, Hirschman KB, et al. Screening for delirium using family caregivers: convergent validity of the family confusion assessment method and interviewer-rated confusion assessment method. J Am Geriatr Soc 2012; 60:2121–2126.
- Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people Lancet 2014; 383:911–922.
During my training in Leiden, Netherlands, I was infused with the lessons of Herman Boerhaave (1668–1738), the professor who is considered the pioneer of bedside teaching.1 This practice had begun in Padua and was then brought to Leiden, where Boerhaave transformed it into an art form. At the Caecilia hospital, the municipal clerics provided Boerhaave with 2 wards for teaching; 1 with 6 beds for men and the other with 6 beds for women. Medical historian Henry Sigerist2 has commented that half the physicians of Europe were trained on these 12 beds.
Boerhaave made daily rounds with his students, examining the patients, reviewing their histories, and inspecting their urine. He considered postmortem examinations essential and made his students attend the autopsies of patients who died: “In spite of the most detailed description of all disease phenomena one does not know anything of the cause until one has opened the body.”2
What was once the basis of clinical medicine is now fading, with both clinical rounds and autopsies being replaced by imaging techniques of body parts and automated analysis of Vacutainer samples. These novelties provide us with far more diagnostic accuracy than Boerhaave had, and randomized controlled trials provide us with an evidence base. But bedside observation and case reports are still relevant,3 and autopsies still reveal important, clinically missed diagnoses.4
In this issue of the Journal, Imm et al5 describe a case of presumed postoperative delirium in a 64-year-old hospitalized patient. They remind us that crucial signs and symptoms can guide how to use our modern diagnostic tools.
DELIRIUM IS OFTEN OVERLOOKED
Delirium is often overlooked by physicians. But why? The characteristic disturbances in attention and cognition are easy to interpret, while the various observation scales have high sensitivity and should signal the need for further interrogation. Perhaps the reason we often overlook the signs and symptoms is that we assume that delirium is just normal sickness behavior.
Another reason we may fail to recognize the syndrome is more fundamental and closely related to how we practice medicine. These days, we place such trust in high-tech diagnostics that we feel the modern procedures supersede the classic examination of patients. But mental disturbances can only be detected by history-taking and clinical observation.
Moreover, the actual mental state is less important than the subtle changes in it. A continuously disturbed mind does not pose a problem, but a casual remark by a family member or informal caregiver that “his mood has changed” should seize our attention.6
Here, the fragmented and disconnected practice of modern medicine takes its toll. Shorter working hours have helped to preserve our own mental performance, but at the cost of being less able to follow the patient’s mental status over time and to recognize a change of behavior. Applying repeated, standardized assessments of these vital signs may help solve the problem, but repeated observations are easily neglected, as are body temperature, blood pressure, and others.
DELIRIUM IS SERIOUS
Imm et al also remind us that delirium is serious. The case-fatality rate in delirium equals that in acute cardiovascular events or metastatic cancer, even though its impact is often not thought to be as severe. Far too often the mental symptoms are dismissed and judged to be something to be handled in the outpatient clinic after the acute problems are addressed.
In part, this may be because no professional society or advocacy group is promoting the recognition, diagnosis, and treatment of delirium or pushing for incentives to do so. We have cardiologists and oncologists but not deliriologists. But in a way, it may be a good thing that no specialist group “owns” delirium, as the syndrome is elicited by various underlying disease mechanisms, and every physician should be vigilant to recognize it.
DELIRIUM REQUIRES PROMPT MANAGEMENT
If delirium is a life-threatening condition, it necessitates a prompt and coordinated series of diagnostic actions, judgments, and decisions.7 Although most delirious patients are not admitted to an intensive care unit, they should be considered critically ill and must be provided a corresponding level of care. Here, the old clinical aphorism holds: action should be taken before the sun sets or rises. Attention should be on worsening of the underlying disease, unexpected comorbid conditions, and side effects of our interventions.
As the case reported by Imm et al shows, the causative factors may be recognized only after in-depth examination.4 The pathogenesis of delirium is poorly understood, and there is no specific therapy for it. There is not even conclusive evidence that the standard use of antipsychotics is beneficial, whereas their side effects cannot be overestimated.7 Our interventions are aimed at eliminating the underlying pathologies that have triggered the delirious state, as well as on preventing complications of the mental disturbance.
Many of us have had the experience of watching one of our children develop fever and confusion. When our older patients become delirious, it should raise the same level of alarm and activity as when it happens in a child.
During my training in Leiden, Netherlands, I was infused with the lessons of Herman Boerhaave (1668–1738), the professor who is considered the pioneer of bedside teaching.1 This practice had begun in Padua and was then brought to Leiden, where Boerhaave transformed it into an art form. At the Caecilia hospital, the municipal clerics provided Boerhaave with 2 wards for teaching; 1 with 6 beds for men and the other with 6 beds for women. Medical historian Henry Sigerist2 has commented that half the physicians of Europe were trained on these 12 beds.
Boerhaave made daily rounds with his students, examining the patients, reviewing their histories, and inspecting their urine. He considered postmortem examinations essential and made his students attend the autopsies of patients who died: “In spite of the most detailed description of all disease phenomena one does not know anything of the cause until one has opened the body.”2
What was once the basis of clinical medicine is now fading, with both clinical rounds and autopsies being replaced by imaging techniques of body parts and automated analysis of Vacutainer samples. These novelties provide us with far more diagnostic accuracy than Boerhaave had, and randomized controlled trials provide us with an evidence base. But bedside observation and case reports are still relevant,3 and autopsies still reveal important, clinically missed diagnoses.4
In this issue of the Journal, Imm et al5 describe a case of presumed postoperative delirium in a 64-year-old hospitalized patient. They remind us that crucial signs and symptoms can guide how to use our modern diagnostic tools.
DELIRIUM IS OFTEN OVERLOOKED
Delirium is often overlooked by physicians. But why? The characteristic disturbances in attention and cognition are easy to interpret, while the various observation scales have high sensitivity and should signal the need for further interrogation. Perhaps the reason we often overlook the signs and symptoms is that we assume that delirium is just normal sickness behavior.
Another reason we may fail to recognize the syndrome is more fundamental and closely related to how we practice medicine. These days, we place such trust in high-tech diagnostics that we feel the modern procedures supersede the classic examination of patients. But mental disturbances can only be detected by history-taking and clinical observation.
Moreover, the actual mental state is less important than the subtle changes in it. A continuously disturbed mind does not pose a problem, but a casual remark by a family member or informal caregiver that “his mood has changed” should seize our attention.6
Here, the fragmented and disconnected practice of modern medicine takes its toll. Shorter working hours have helped to preserve our own mental performance, but at the cost of being less able to follow the patient’s mental status over time and to recognize a change of behavior. Applying repeated, standardized assessments of these vital signs may help solve the problem, but repeated observations are easily neglected, as are body temperature, blood pressure, and others.
DELIRIUM IS SERIOUS
Imm et al also remind us that delirium is serious. The case-fatality rate in delirium equals that in acute cardiovascular events or metastatic cancer, even though its impact is often not thought to be as severe. Far too often the mental symptoms are dismissed and judged to be something to be handled in the outpatient clinic after the acute problems are addressed.
In part, this may be because no professional society or advocacy group is promoting the recognition, diagnosis, and treatment of delirium or pushing for incentives to do so. We have cardiologists and oncologists but not deliriologists. But in a way, it may be a good thing that no specialist group “owns” delirium, as the syndrome is elicited by various underlying disease mechanisms, and every physician should be vigilant to recognize it.
DELIRIUM REQUIRES PROMPT MANAGEMENT
If delirium is a life-threatening condition, it necessitates a prompt and coordinated series of diagnostic actions, judgments, and decisions.7 Although most delirious patients are not admitted to an intensive care unit, they should be considered critically ill and must be provided a corresponding level of care. Here, the old clinical aphorism holds: action should be taken before the sun sets or rises. Attention should be on worsening of the underlying disease, unexpected comorbid conditions, and side effects of our interventions.
As the case reported by Imm et al shows, the causative factors may be recognized only after in-depth examination.4 The pathogenesis of delirium is poorly understood, and there is no specific therapy for it. There is not even conclusive evidence that the standard use of antipsychotics is beneficial, whereas their side effects cannot be overestimated.7 Our interventions are aimed at eliminating the underlying pathologies that have triggered the delirious state, as well as on preventing complications of the mental disturbance.
Many of us have had the experience of watching one of our children develop fever and confusion. When our older patients become delirious, it should raise the same level of alarm and activity as when it happens in a child.
- Koehler U, Hildebrandt O, Koehler J, Nell C. The pioneer of bedside teaching—Herman Boerhaave (1668–1738). Dtsch Med Wochenschr 2014; 139:2655–2659.
- Sigerist HE. A History of Medicine. New York: Oxford University Press 1951;1. [According to Walker HK. Chapter 1. The origins of the history and physical examination. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston: Buterworths, 1990.] www.ncbi.nlm.nih.gov/books/NBK458. Accessed August 7, 2017.
- Vandenbroucke JP. In defense of case reports and case series. Ann Intern Med 2001; 134:330–334.
- Shojania KG, Burton EC, McDonald KM, Goldman M. Changes in rates of autopsy-detected diagnostic errors over time: a systematic review. JAMA 2003; 289:2849–2856.
- Imm M, Torres LF, Kottapally M. Postoperative delirium in a 64-year-old woman. Cleve Clinic J Med 2017; 84:690–698.
- Steis MR, Evans L, Hirschman KB, et al. Screening for delirium using family caregivers: convergent validity of the family confusion assessment method and interviewer-rated confusion assessment method. J Am Geriatr Soc 2012; 60:2121–2126.
- Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people Lancet 2014; 383:911–922.
- Koehler U, Hildebrandt O, Koehler J, Nell C. The pioneer of bedside teaching—Herman Boerhaave (1668–1738). Dtsch Med Wochenschr 2014; 139:2655–2659.
- Sigerist HE. A History of Medicine. New York: Oxford University Press 1951;1. [According to Walker HK. Chapter 1. The origins of the history and physical examination. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston: Buterworths, 1990.] www.ncbi.nlm.nih.gov/books/NBK458. Accessed August 7, 2017.
- Vandenbroucke JP. In defense of case reports and case series. Ann Intern Med 2001; 134:330–334.
- Shojania KG, Burton EC, McDonald KM, Goldman M. Changes in rates of autopsy-detected diagnostic errors over time: a systematic review. JAMA 2003; 289:2849–2856.
- Imm M, Torres LF, Kottapally M. Postoperative delirium in a 64-year-old woman. Cleve Clinic J Med 2017; 84:690–698.
- Steis MR, Evans L, Hirschman KB, et al. Screening for delirium using family caregivers: convergent validity of the family confusion assessment method and interviewer-rated confusion assessment method. J Am Geriatr Soc 2012; 60:2121–2126.
- Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people Lancet 2014; 383:911–922.
Watson, the game is a foot…or a palm
What common message do a 64-year-old woman with postoperative cognitive changes and an 83-year-old man with red palms have for us as physicians? As I read their clinical scenarios and the editorial by Westendorp, I was struck by the value and significance of informed clinical observation, an activity that I fear is going the way of the music CD and handwritten letters.
As I read the descriptions of these patients I was reminded of the internal satisfaction that I feel when I pick up a clinical or historical finding that directs me to a specific diagnosis and therapeutic recommendation. Sherlock Holmes I am not. Those satisfying pickups are infrequent, and I have no idea how many clues I have missed. I do know that most come from taking the time to perform a methodical physical examination, directed and informed by the patient’s recounted history. Some, like red palms or anisocoria, may be readily apparent and diagnostically useful—if the observer recognizes their potential significance. The 2 patients described in this issue of the Journal highlight the value of both observation and the knowledge and experience to place what we observe into a clinical context. Watson (the computer) can provide data regarding the potential significance of a physical finding, but only if someone first detects its existence.
Once it is recognized (or pointed out), we can all pull out our smartphones and Google “palmar erythema and disease,” and on our screen up pops liver disease, pregnancy, and assorted other conditions, including malignancies. But how many of us in our clinic, as opposed to the artificial scenario of reading it in the Journal or attending a clinicopathologic conference, will spontaneously recognize palmar erythema as a potentially relevant clinical finding?
For many physicians, the sense of professional satisfaction in making these observations is diminished. The professional joy gleaned from these moments has been diluted. We are in jeopardy of losing the passion for the professional work that we do as well as the intellectual and emotional satisfaction that accompanies a nuanced professional job well done, while focusing instead on our contracted jobs, frequently evaluated by our ability to meet commercial needs. The absence of emotional and intellectual satisfaction that should come from these collected moments of patient interaction and reflection undoubtedly contributes to the rising rate of physician burnout.
There are so many pressures on us in the office. Did I record that my new patient with known rheumatoid arthritis (who has had a recent MI and pneumonia and who has tried several biologic therapies without success and is in need of a creative change in her medication) has a cousin with hypothyroidism so I could include family history in my electronic medical record note and thus bill at a “desired” level of complexity? Did I use the appropriate catchphrase stating that over 50% of my time was spent in education of the patient (after collecting and reading for 30 minutes the stack of prior records, preparing to do battle with her insurance company to get the next therapy approved for coverage)?
There is little wonder that an observation of red palms gets missed or, if it is noted, that the Google search is never actually done. And when we do recognize the finding and its clinical significance, we often don’t take a moment to reflect and bask in the glow of a job well done, the satisfaction of successfully applying both our knowledge and experience to help resolve a clinical problem.
As Westendorp points out, bedside observation is still relevant. And I will add that there still should be joy in the intellectual pursuit of the job well done as well as the patient well managed. It takes more than a smartphone to know when and how to look at the palms and the eyes before typing in a Google search or consulting the digital (not the doctor) Watson. Those are skills to be proud of.
What common message do a 64-year-old woman with postoperative cognitive changes and an 83-year-old man with red palms have for us as physicians? As I read their clinical scenarios and the editorial by Westendorp, I was struck by the value and significance of informed clinical observation, an activity that I fear is going the way of the music CD and handwritten letters.
As I read the descriptions of these patients I was reminded of the internal satisfaction that I feel when I pick up a clinical or historical finding that directs me to a specific diagnosis and therapeutic recommendation. Sherlock Holmes I am not. Those satisfying pickups are infrequent, and I have no idea how many clues I have missed. I do know that most come from taking the time to perform a methodical physical examination, directed and informed by the patient’s recounted history. Some, like red palms or anisocoria, may be readily apparent and diagnostically useful—if the observer recognizes their potential significance. The 2 patients described in this issue of the Journal highlight the value of both observation and the knowledge and experience to place what we observe into a clinical context. Watson (the computer) can provide data regarding the potential significance of a physical finding, but only if someone first detects its existence.
Once it is recognized (or pointed out), we can all pull out our smartphones and Google “palmar erythema and disease,” and on our screen up pops liver disease, pregnancy, and assorted other conditions, including malignancies. But how many of us in our clinic, as opposed to the artificial scenario of reading it in the Journal or attending a clinicopathologic conference, will spontaneously recognize palmar erythema as a potentially relevant clinical finding?
For many physicians, the sense of professional satisfaction in making these observations is diminished. The professional joy gleaned from these moments has been diluted. We are in jeopardy of losing the passion for the professional work that we do as well as the intellectual and emotional satisfaction that accompanies a nuanced professional job well done, while focusing instead on our contracted jobs, frequently evaluated by our ability to meet commercial needs. The absence of emotional and intellectual satisfaction that should come from these collected moments of patient interaction and reflection undoubtedly contributes to the rising rate of physician burnout.
There are so many pressures on us in the office. Did I record that my new patient with known rheumatoid arthritis (who has had a recent MI and pneumonia and who has tried several biologic therapies without success and is in need of a creative change in her medication) has a cousin with hypothyroidism so I could include family history in my electronic medical record note and thus bill at a “desired” level of complexity? Did I use the appropriate catchphrase stating that over 50% of my time was spent in education of the patient (after collecting and reading for 30 minutes the stack of prior records, preparing to do battle with her insurance company to get the next therapy approved for coverage)?
There is little wonder that an observation of red palms gets missed or, if it is noted, that the Google search is never actually done. And when we do recognize the finding and its clinical significance, we often don’t take a moment to reflect and bask in the glow of a job well done, the satisfaction of successfully applying both our knowledge and experience to help resolve a clinical problem.
As Westendorp points out, bedside observation is still relevant. And I will add that there still should be joy in the intellectual pursuit of the job well done as well as the patient well managed. It takes more than a smartphone to know when and how to look at the palms and the eyes before typing in a Google search or consulting the digital (not the doctor) Watson. Those are skills to be proud of.
What common message do a 64-year-old woman with postoperative cognitive changes and an 83-year-old man with red palms have for us as physicians? As I read their clinical scenarios and the editorial by Westendorp, I was struck by the value and significance of informed clinical observation, an activity that I fear is going the way of the music CD and handwritten letters.
As I read the descriptions of these patients I was reminded of the internal satisfaction that I feel when I pick up a clinical or historical finding that directs me to a specific diagnosis and therapeutic recommendation. Sherlock Holmes I am not. Those satisfying pickups are infrequent, and I have no idea how many clues I have missed. I do know that most come from taking the time to perform a methodical physical examination, directed and informed by the patient’s recounted history. Some, like red palms or anisocoria, may be readily apparent and diagnostically useful—if the observer recognizes their potential significance. The 2 patients described in this issue of the Journal highlight the value of both observation and the knowledge and experience to place what we observe into a clinical context. Watson (the computer) can provide data regarding the potential significance of a physical finding, but only if someone first detects its existence.
Once it is recognized (or pointed out), we can all pull out our smartphones and Google “palmar erythema and disease,” and on our screen up pops liver disease, pregnancy, and assorted other conditions, including malignancies. But how many of us in our clinic, as opposed to the artificial scenario of reading it in the Journal or attending a clinicopathologic conference, will spontaneously recognize palmar erythema as a potentially relevant clinical finding?
For many physicians, the sense of professional satisfaction in making these observations is diminished. The professional joy gleaned from these moments has been diluted. We are in jeopardy of losing the passion for the professional work that we do as well as the intellectual and emotional satisfaction that accompanies a nuanced professional job well done, while focusing instead on our contracted jobs, frequently evaluated by our ability to meet commercial needs. The absence of emotional and intellectual satisfaction that should come from these collected moments of patient interaction and reflection undoubtedly contributes to the rising rate of physician burnout.
There are so many pressures on us in the office. Did I record that my new patient with known rheumatoid arthritis (who has had a recent MI and pneumonia and who has tried several biologic therapies without success and is in need of a creative change in her medication) has a cousin with hypothyroidism so I could include family history in my electronic medical record note and thus bill at a “desired” level of complexity? Did I use the appropriate catchphrase stating that over 50% of my time was spent in education of the patient (after collecting and reading for 30 minutes the stack of prior records, preparing to do battle with her insurance company to get the next therapy approved for coverage)?
There is little wonder that an observation of red palms gets missed or, if it is noted, that the Google search is never actually done. And when we do recognize the finding and its clinical significance, we often don’t take a moment to reflect and bask in the glow of a job well done, the satisfaction of successfully applying both our knowledge and experience to help resolve a clinical problem.
As Westendorp points out, bedside observation is still relevant. And I will add that there still should be joy in the intellectual pursuit of the job well done as well as the patient well managed. It takes more than a smartphone to know when and how to look at the palms and the eyes before typing in a Google search or consulting the digital (not the doctor) Watson. Those are skills to be proud of.
Another complication of cirrhosis
A 53-year-old Native American woman with a history of liver cirrhosis secondary to alcohol abuse presents to the emergency department after 2 days of diffuse abdominal pain and weakness. The pain was sudden in onset and has progressed relentlessly over the last day, reaching 9 on a scale of 10 in severity. Family members say that her oral intake has been decreased for the last 2 days, but she has had no fever, vomiting, change in bowel habit, blood in stool, or black stool. She has never undergone surgery, and has had one uncomplicated pregnancy.
Physical examination
Vital signs:
- Blood pressure 82/57 mm Hg
- Heart rate 96 beats per minute
- Temperature 37.3°C (99.1°F)
- Respiratory rate 16 per minute
- Oxygen saturation 92% while receiving oxygen at 2 L/minute.
The patient is somnolent and has scleral icterus. Her cardiopulmonary examination is normal. Her abdomen is tense, distended, and diffusely tender. She has bilateral +2 pitting edema in her lower extremities. She is oriented to person only and is noted to have asterixis. Her baseline Model for End-stage Liver Disease score is 18 points on a scale of 6 (less ill) to 40 (gravely ill).
Laboratory studies:
- Hemoglobin 9.8 g/dL (reference range 11.5–15.5)
- Platelet count 100 × 109/L (150–400)
- White blood cell count 9.9 × 109/L (3.7–11.0)
- Serum creatinine 1.06 mg/dL (0.58–0.96)
- Bilirubin 6.3 mg/dL (0.2–1.3)
- International normalized ratio of the prothrombin time 2.15 (0.8–1.2)
- Blood urea nitrogen 13 mg/dL (7–21)
- Serum albumin 2.7 g/dL (3.9–4.9).
Intravenous fluid resuscitation is initiated but the patient remains hypotensive, and on repeat laboratory testing 4 hours later her hemoglobin level has dropped to 7.3 mg/dL.
DIFFERENTIAL DIAGNOSIS
1. Which of the following are likely causes of this patient’s presentation?
- Splenic arterial aneurysm rupture
- Spontaneous bacterial peritonitis
- Variceal hemorrhage
- Portal vein thrombosis
- Abdominal aortic aneurysm rupture
Ruptured splenic artery aneurysm
Splenic artery aneurysms are the third most common intra-abdominal aneurysm, after those of the abdominal aorta and iliac artery.1 They are often asymptomatic and are being detected more frequently because of increased use of computed tomography (CT).2 Symptomatic splenic artery aneurysms may present with abdominal pain and have the potential to rupture, which can be life-threatening.3,4
This patient may have a ruptured splenic artery aneurysm, given her hemodynamic shock.
Spontaneous bacterial peritonitis
Ten percent to 20% of hospitalized patients with cirrhosis and ascites develop spontaneous bacterial peritonitis. Patients may present with ascites and abdominal pain, tenderness to palpation, fever, encephalopathy, or worsening liver and renal function.
Diagnostic paracentesis is paramount to delineate the cause of ascites; one should calculate the serum-ascites albumin gradient and obtain a cell count and culture of the ascitic fluid. The diagnosis of spontaneous bacterial peritonitis can be made if the ascitic fluid polymorphonuclear cell count is 0.25 × 109/L or higher, even if the ascitic fluid culture is negative.5,6 Simultaneous blood cultures should also be collected, as 50% of cases are associated with bacteremia.
The in-hospital mortality rate of an episode of spontaneous bacterial peritonitis has been reduced to 10% to 20% thanks to prompt diagnosis and empiric treatment with third-generation cephalosporins.7
Five percent of cases of infected ascites fluid are due to secondary bacterial peritonitis from a perforated viscus or a loculated abscess, which cannot be differentiated clinically from spontaneous bacterial peritonitis but can be diagnosed with CT.8
This patient may be presenting with septic shock secondary to either of these causes.
Variceal hemorrhage
Half of patients with cirrhosis have gastroesophageal varices due to portal hypertension. Endoscopic surveillance is warranted, as the risk of hemorrhage is 12% to 15% per year, and the mortality rate approaches 15% to 20% with each episode. Prompt resuscitation, diagnosis, and control of bleeding is paramount.
Esophagogastroduodenoscopy is used for both diagnosis and intervention. Short-term prophylactic use of antibiotics improves survival by preventing infections in the event bleeding recurs.9–11
Our patient may be presenting with hemodynamic shock from bleeding esophageal varices.
Portal vein thrombosis
Portal vein thrombosis is a common complication of cirrhosis, occurring in 5% to 28% of patients. The risk increases with the severity of liver disease and in association with hepatocellular carcinoma.12 Forty-three percent of cases are discovered incidentally in asymptomatic patients during ultrasonography, 39% present with upper gastrointestinal bleeding, and 18% present with abdominal pain.13,14
Portal vein thrombosis is the complete or partial obstruction of blood flow due to a thrombus in the lumen of the portal vein. Contrast ultrasonography and CT can be used to establish the diagnosis.15
Anticoagulation is recommended in cases of complete thrombosis in candidates for living-donor liver transplant and for those at risk of mesenteric ischemia because of the thrombus extending into the mesenteric veins. In symptomatic patients, the decision to initiate anticoagulation should be made on a case-by-case basis after appropriate screening and management of varices.16–18
Our patient’s thrombocytopenia reflects the severity of portal hypertension and increases her risk of portal vein thrombosis, but this is unlikely to be the sole cause of the hemodynamic compromise in this patient.
Ruptured abdominal aortic aneurysm
Rupture of an abdominal aortic aneurysm is a medical emergency, with a mortality rate approaching 90%. Risk factors for abdominal aortic aneurysms are smoking, male sex, age over 65, history of cardiovascular disease, hypertension, and a family history of abdominal aortic aneurysm, especially if a first-degree relative is affected.19 Endovascular repair is associated with lower rates of death and complications compared with open repair.20
The patient does not have any of those risk factors, making this diagnosis less likely.
CASE CONTINUED: RUPTURED SPLENIC ARTERY ANEURYSM
Emergency CT of the abdomen and pelvis with contrast enhancement shows a large left intraperitoneal hematoma with active extravasation from a ruptured splenic artery aneurysm (Figure 1). The patient receives packed red blood cells and fresh-frozen plasma before being transferred to our hospital.
2. Which of the following is false regarding splenic artery aneurysms?
- They are the most common type of splanchnic arterial aneurysm
- True aneurysms are more common than pseudoaneurysms
- Asymptomatic aneurysms are discovered incidentally during assessment for other radiographic indications
- Splenic artery aneurysm in portal hypertension is the result of athero-sclerotic changes to the vascular intima
Splenic artery aneurysm in portal hypertension is not the result of atherosclerotic change to the vascular intima.
Splenic artery aneurysms are the most common type of splanchnic artery aneurysm.1 True aneurysms involve all 3 layers of the arterial wall, ie, intima, media, and adventitia. Cirrhosis and portal hypertension are associated with true aneurysm formation. The proposed mechanism of aneurysm formation is increased splenic blood flow in response to portal congestion with resultant hemodynamic stress that disrupts arterial wall structure, leading to aneurysmal dilation.21
In earlier reports, the incidence of true splenic artery aneurysm in portal hypertension varied from 2.9% to 50%, the latter representing autopsy findings of small aneurysms that were found in the splenic hilum of patients with cirrhosis.22–25 The incidence of clinically significant aneurysms in cirrhosis is unknown but incidental asymptomatic aneurysm is being detected more frequently on imaging studies pursued for screening purposes.26
The risk of rupture is low, only 2% to 10% in older studies and likely even lower now due to increased incidental detection in asymptomatic patients.27 However, emergent management of rupture at a tertiary care facility is paramount, as the mortality rate of ruptured splenic artery aneurysm is 29% to 36%.1,26,28
Splenic artery pseudoaneurysm is rarer and has a different pathophysiologic process than true aneurysm. It usually arises in the setting of trauma, pancreatitis, or postsurgery.29,30 Pseudoaneurysm is more likely to rupture, owing to compromise in the vascular wall integrity.4,21,28 As a result, treatment is indicated for every pseudoaneurysm regardless of size.
RISK FACTORS FOR SPLENIC ARTERY ANEURYSM
3. Which of the following is true regarding our patient’s risk of splenic artery aneurysm?
- Liver cirrhosis and portal hypertension are her greatest risk factors for it
- Female sex and prior pregnancy are her greatest risk factors for it
- Being Native American makes it more likely that the patient has splenic artery aneurysm secondary to collagen vascular disease
- Her risk of rupture would diminish after receiving a liver transplant
Liver cirrhosis and portal hypertension are her greatest risk factors for splenic artery aneurysm.
Risk factors for true aneurysm include hypertension, atherosclerosis, portal hypertension with or without liver cirrhosis, liver transplant, third trimester of pregnancy, and multiparity.1,4,26,28,31 Splenic artery aneurysm is usually diagnosed in the sixth decade. It may be 4 times as common in women, given a hormonal influence.32 Cirrhosis is also associated with massive splenic artery aneurysm (≥ 5 cm). Although rare, massive splenic artery aneurysm is more frequent in men (the male-to-female ratio is 1.78:1) and has a heightened risk of rupture.28 The incidence of rupture increases to around 3% to 4% after liver transplant.33 Rare causes of true aneurysm include fibrodysplasia, collagen vascular disease (eg, Loeys-Dietz and type IV Ehler-Danlos syndromes), vasculitis (eg, polyarteritis nodosa due to amphetamine abuse), and mycotic aneurysms.24,25,28,29
This patient’s age, sex, and history of cirrhosis puts her at increased risk of splenic artery aneurysm. The risk of rupture is highest in the peripartum period and in patients with cirrhosis who become pregnant. Although being Native American portends an increased risk for collagen vascular disease, the latter is unlikely to be a contributing factor.
TREATMENT OF SPLENIC ARTERY ANEURYSM
4. Which of the following is false regarding treatment of splenic artery aneurysms?
- Aneurysms larger than 2 cm and those that are expanding require repair
- Treatment should be offered if the patient has symptoms attributable to the aneurysm
- Asymptomatic aneurysms in pregnant women can be followed with watchful waiting
- Minimally invasive therapies such as percutaneous embolization may be a good option in poor operative candidates
Asymptomatic aneurysms in pregnant women should not be followed with watchful waiting—they should be repaired, as rupture carries a maternal mortality rate of 75% and a fetal mortality rate of 95%.34
Complications of splenic artery aneurysm depend on the type of aneurysm and its predisposing factors. Indications for treatment of true aneurysms include:
- Symptoms attributable to the aneurysm (hence, the second answer choice above is true)
- Diameter 2 cm or greater or enlarging diameter (hence, the first answer choice is true)
- Women of childbearing age in anticipation of pregnancy
- Need for surgical intervention such as portocaval shunt and liver transplant.
Conservative management is associated with a late mortality risk of 4.9%.2 Interventional options include percutaneous embolization or stenting; or laparotomy with splenic artery ligation or excision with or without splenectomy.1,28,35–37
Endovascular and open surgical repair have both been used to treat splenic artery aneurysms. The method used depends on the patient’s surgical history and aneurysm anatomy such as splenic artery tortuosity hindering passage of a catheter. Open surgery is associated with longer intraoperative time and length of hospital stay and higher rates of 30-day mortality and perioperative morbidity.38–41 With endovascular repair, the complication of persistent or recurrent flow occurs in 3% to 5% of cases by 30 days; hence, postprocedural surveillance is recommended.42–44 Endovascular repair has a higher reintervention rate but may still be more cost-effective than open surgical repair.
Because patients with cirrhosis have a higher risk of surgical complications,45 elective endovascular treatment may be an option for patients with aneurysms at high risk of rupturing. Endovascular treatment of visceral aneurysms is associated with complications such as postembolization syndrome (fever, abdominal pain, pleural effusion, and pancreatitis), access site hematoma, splenic infarction, and persistent abdominal pain.42
Patients with cirrhosis as the cause of splenic artery aneurysm tend to need longer hospitalization after endovascular treatment, but there is insufficient evidence to suggest that they are at higher risk of other complications.37
CASE CONTINUED: SPLENIC ARTERY EMBOLIZATION
The patient undergoes emergency splenic artery embolization, performed by an interventional radiology team (Figure 2 and Figure 3). Over the next few days, her mental status improves and her abdominal pain resolves. Her hemoglobin level remains stable after the procedure.
The surgical and interventional radiology teams discuss the risk of repeat intervention with the patient and her family, who prefer a nonoperative approach. She is managed supportively in the intensive care unit and is finally discharged home in stable condition and is scheduled for outpatient follow-up.
SUSPECT THIS FATAL CONDITION
The low prevalence of ruptured splenic artery aneurysm may lead physicians to attribute septic shock to spontaneous bacterial peritonitis or hemorrhagic shock from gastroesophageal varices in patients with cirrhosis, but a high index of suspicion and early recognition of this rare disease can lead to timely diagnosis and treatment of this highly fatal complication.
KEY POINTS
- Splenic artery aneurysm is a common complication of cirrhosis, often diagnosed incidentally.
- Elective embolization should be considered for asymptomatic splenic artery aneurysms larger than 2 cm in diameter, clinically symptomatic aneurysms, women of childbearing age, and patients who are candidates for liver transplant.
- Although splenic artery aneurysm rupture is rare, it has a high mortality rate and warrants a high index of suspicion to institute prompt specialized intervention.
- We recommend that physicians consider splenic artery aneurysm rupture in their differential diagnoses in patients with liver cirrhosis presenting with abdominal pain, altered mental status, and hemodynamic shock.
- Bakhos CT, McIntosh BC, Nukta FA, et al. Staged arterial embolization and surgical resection of a giant splenic artery aneurysm. Ann Vasc Surg 2007; 21:208–210.
- Hogendoorn W, Lavida A, Hunink MG, et al. Open repair, endovascular repair, and conservative management of true splenic artery aneurysms. J Vasc Surg 2014; 60:1667–1676.e1.
- Algudkar A. Unruptured splenic artery aneurysm presenting as epigastric pain. JRSM Short Rep 2010; 1:24.
- Abbas MA, Stone WM, Fowl RJ, et al. Splenic artery aneurysms: two decades experience at Mayo Clinic. Ann Vasc Surg 2002; 16:442–449.
- Hoefs JC, Canawati HN, Sapico FL, Hopkins RR, Weiner J, Montgomerie JZ. Spontaneous bacterial peritonitis. Hepatology 1982; 2:399–407.
- Runyon BA, Hoefs JC. Culture-negative neutrocytic ascites: a variant of spontaneous bacterial peritonitis. Hepatology 1984; 4:1209–1211.
- Garcia-Tsao G. Spontaneous bacterial peritonitis: a historical perspective. J Hepatol 2004; 41:522–527.
- Soriano G, Castellote J, Alvarez C, et al. Secondary bacterial peritonitis in cirrhosis: a retrospective study of clinical and analytical characteristics, diagnosis and management. J Hepatol 2010; 52:39–44.
- D’Amico G, De Franchis R; Cooperative Study Group. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology 2003; 38:599–612.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD; Practice Guidelines Committee of American Association for Study of Liver Diseases; Practice Parameters Committee of American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol 2007; 102:2086–2102.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007; 46:922–938.
- Tsochatzis EA, Senzolo M, Germani G, Gatt A, Burroughs AK. Systematic review: portal vein thrombosis in cirrhosis. Aliment Pharmacol Ther 2010; 31:366–374.
- Kobori L, van der Kolk MJ, de Jong KP, et al. Splenic artery aneurysms in liver transplant patients. Liver Transplant Group. J Hepatol 1997; 27:890–893.
- Manzano-Robleda Mdel C, Barranco-Fragoso B, Uribe M, Mendez-Sanchez N. Portal vein thrombosis: what is new? Ann Hepatol 2015; 14:20–27.
- Sarin SK, Philips CA, Kamath PS, et al. Toward a comprehensive new classification of portal vein thrombosis in patients with cirrhosis. Gastroenterology 2016; 151:574–577.e3.
- DeLeve LD, Valla DC, Garcia-Tsao G; American Association for the Study of Liver Diseases. Vascular disorders of the liver. Hepatology 2009; 49:1729–1764.
- Manzanet G, Sanjuan F, Orbis P, et al. Liver transplantation in patients with portal vein thrombosis. Liver Transpl 2001; 7:125–131.
- John BV, Konjeti R, Aggarwal A, et al. Impact of untreated portal vein thrombosis on pre and post liver transplant outcomes in cirrhosis. Ann Hepatol 2013; 12:952–958.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al; American Association for Vascular Surgery/Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)—summary of recommendations. J Vasc Interv Radiol 2006; 17:1383–1397.
- Schermerhorn ML, O’Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, Landon BE. Endovascular vs open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med 2008; 358:464–474.
- Ohta M, Hashizume M, Ueno K, Tanoue K, Sugimachi K, Hasuo K. Hemodynamic study of splenic artery aneurysm in portal hypertension. Hepatogastroenterology 1994; 41:181–184.
- Sunagozaka H, Tsuji H, Mizukoshi E, et al. The development and clinical features of splenic aneurysm associated with liver cirrhosis. Liver Int 2006; 26:291–297.
- Manenti F, Williams R. Injection studies of the splenic vasculature in portal hypertension. Gut 1966; 7:175–180.
- Stanley JC, Fry WJ. Pathogenesis and clinical significance of splenic artery aneurysms. Surgery 1974; 76:898–909.
- Lee PC, Rhee RY, Gordon RY, Fung JJ, Webster MW. Management of splenic artery aneurysms: the significance of portal and essential hypertension. J Am Coll Surg 1999; 189:483–490.
- Al-Habbal Y, Christophi C, Muralidharan V. Aneurysms of the splenic artery—a review. Surgeon 2010; 8:223–231.
- Mattar SG, Lumsden AB. The management of splenic artery aneurysms: experience with 23 cases. Am J Surg 1995; 169:580–584.
- Akbulut S, Otan E. Management of giant splenic artery aneurysm: comprehensive literature review. Medicine (Baltimore) 2015; 94:e1016.
- Agrawal GA, Johnson PT, Fishman EK. Splenic artery aneurysms and pseudoaneurysms: clinical distinctions and CT appearances. AJR Am J Roentgenol 2007; 188:992–999.
- Tessier DJ, Stone WM, Fowl RJ, et al. Clinical features and management of splenic artery pseudoaneurysm: case series and cumulative review of literature. J Vasc Surg 2003; 38:969–974.
- Dave SP, Reis ED, Hossain A, Taub PJ, Kerstein MD, Hollier LH. Splenic artery aneurysm in the 1990s. Ann Vasc Surg 2000; 14:223–229.
- Parrish J, Maxwell C, Beecroft JR. Splenic artery aneurysm in pregnancy. J Obstet Gynaecol Can 2015; 37:816–818.
- Moon DB, Lee SG, Hwang S, et al. Characteristics and management of splenic artery aneurysms in adult living donor liver transplant recipients. Liver Transpl 2009; 15:1535–1541.
- Sadat U, Dar O, Walsh S, Varty K. Splenic artery aneurysms in pregnancy—a systematic review. Int J Surg 2008; 6:261–265.
- Geoghegan T, McAuley G, Snow A, Torreggiani WC. Emergency embolization of multiple splenic artery pseudoaneurysms associated with portal hypertension complicating cystic fibrosis. Australas Radiol 2007; 51(suppl):B337–B339.
- Jiang R, Ding X, Jian W, Jiang J, Hu S, Zhang Z. Combined endovascular embolization and open surgery for splenic artery aneurysm with arteriovenous fistula. Ann Vasc Surg 2016; 30:311.e1–311.e4.
- Naganuma M, Matsui H, Koizumi J, Fushimi K, Yasunaga H. Short-term outcomes following elective transcatheter arterial embolization for splenic artery aneurysms: data from a nationwide administrative database. Acta Radiol Open 2015; 4:2047981615574354.
- Batagini NC, El-Arousy H, Clair DG, Kirksey L. Open versus endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Ann Vasc Surg 2016; 35:1–8.
- Marone EM, Mascia D, Kahlberg A, Brioschi C, Tshomba Y, Chiesa R. Is open repair still the gold standard in visceral artery aneurysm management? Ann Vasc Surg 2011; 25:936–946.
- Sticco A, Aggarwal A, Shapiro M, Pratt A, Rissuci D, D'Ayala M. A comparison of open and endovascular treatment strategies for the management of splenic artery aneurysms. Vascular 2016; 24:487–491.
- Hogendoorn W, Lavida A, Hunink MG, et al. Cost-effectiveness of endovascular repair, open repair, and conservative management of splenic artery aneurysms. J Vasc Surg 2015; 61:1432–1440.
- Fankhauser GT, Stone WM, Naidu SG, et al; Mayo Vascular Research Center Consortium. The minimally invasive management of visceral artery aneurysms and pseudoaneurysms. J Vasc Surg 2011; 53:966–970.
- Lagana D, Carrafiello G, Mangini M, et al. Multimodal approach to endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Eur J Radiol 2006; 59:104–111.
- Guillon R, Garcier JM, Abergel A, et al. Management of splenic artery aneurysms and false aneurysms with endovascular treatment in 12 patients. Cardiovasc Intervent Radiol 2003; 26:256–260.
- Northup PG, Wanamaker RC, Lee VD, Adams RB, Berg CL. Model for end-stage liver disease (MELD) predicts nontransplant surgical mortality in patients with cirrhosis. Ann Surg 2005; 242:244–251.
A 53-year-old Native American woman with a history of liver cirrhosis secondary to alcohol abuse presents to the emergency department after 2 days of diffuse abdominal pain and weakness. The pain was sudden in onset and has progressed relentlessly over the last day, reaching 9 on a scale of 10 in severity. Family members say that her oral intake has been decreased for the last 2 days, but she has had no fever, vomiting, change in bowel habit, blood in stool, or black stool. She has never undergone surgery, and has had one uncomplicated pregnancy.
Physical examination
Vital signs:
- Blood pressure 82/57 mm Hg
- Heart rate 96 beats per minute
- Temperature 37.3°C (99.1°F)
- Respiratory rate 16 per minute
- Oxygen saturation 92% while receiving oxygen at 2 L/minute.
The patient is somnolent and has scleral icterus. Her cardiopulmonary examination is normal. Her abdomen is tense, distended, and diffusely tender. She has bilateral +2 pitting edema in her lower extremities. She is oriented to person only and is noted to have asterixis. Her baseline Model for End-stage Liver Disease score is 18 points on a scale of 6 (less ill) to 40 (gravely ill).
Laboratory studies:
- Hemoglobin 9.8 g/dL (reference range 11.5–15.5)
- Platelet count 100 × 109/L (150–400)
- White blood cell count 9.9 × 109/L (3.7–11.0)
- Serum creatinine 1.06 mg/dL (0.58–0.96)
- Bilirubin 6.3 mg/dL (0.2–1.3)
- International normalized ratio of the prothrombin time 2.15 (0.8–1.2)
- Blood urea nitrogen 13 mg/dL (7–21)
- Serum albumin 2.7 g/dL (3.9–4.9).
Intravenous fluid resuscitation is initiated but the patient remains hypotensive, and on repeat laboratory testing 4 hours later her hemoglobin level has dropped to 7.3 mg/dL.
DIFFERENTIAL DIAGNOSIS
1. Which of the following are likely causes of this patient’s presentation?
- Splenic arterial aneurysm rupture
- Spontaneous bacterial peritonitis
- Variceal hemorrhage
- Portal vein thrombosis
- Abdominal aortic aneurysm rupture
Ruptured splenic artery aneurysm
Splenic artery aneurysms are the third most common intra-abdominal aneurysm, after those of the abdominal aorta and iliac artery.1 They are often asymptomatic and are being detected more frequently because of increased use of computed tomography (CT).2 Symptomatic splenic artery aneurysms may present with abdominal pain and have the potential to rupture, which can be life-threatening.3,4
This patient may have a ruptured splenic artery aneurysm, given her hemodynamic shock.
Spontaneous bacterial peritonitis
Ten percent to 20% of hospitalized patients with cirrhosis and ascites develop spontaneous bacterial peritonitis. Patients may present with ascites and abdominal pain, tenderness to palpation, fever, encephalopathy, or worsening liver and renal function.
Diagnostic paracentesis is paramount to delineate the cause of ascites; one should calculate the serum-ascites albumin gradient and obtain a cell count and culture of the ascitic fluid. The diagnosis of spontaneous bacterial peritonitis can be made if the ascitic fluid polymorphonuclear cell count is 0.25 × 109/L or higher, even if the ascitic fluid culture is negative.5,6 Simultaneous blood cultures should also be collected, as 50% of cases are associated with bacteremia.
The in-hospital mortality rate of an episode of spontaneous bacterial peritonitis has been reduced to 10% to 20% thanks to prompt diagnosis and empiric treatment with third-generation cephalosporins.7
Five percent of cases of infected ascites fluid are due to secondary bacterial peritonitis from a perforated viscus or a loculated abscess, which cannot be differentiated clinically from spontaneous bacterial peritonitis but can be diagnosed with CT.8
This patient may be presenting with septic shock secondary to either of these causes.
Variceal hemorrhage
Half of patients with cirrhosis have gastroesophageal varices due to portal hypertension. Endoscopic surveillance is warranted, as the risk of hemorrhage is 12% to 15% per year, and the mortality rate approaches 15% to 20% with each episode. Prompt resuscitation, diagnosis, and control of bleeding is paramount.
Esophagogastroduodenoscopy is used for both diagnosis and intervention. Short-term prophylactic use of antibiotics improves survival by preventing infections in the event bleeding recurs.9–11
Our patient may be presenting with hemodynamic shock from bleeding esophageal varices.
Portal vein thrombosis
Portal vein thrombosis is a common complication of cirrhosis, occurring in 5% to 28% of patients. The risk increases with the severity of liver disease and in association with hepatocellular carcinoma.12 Forty-three percent of cases are discovered incidentally in asymptomatic patients during ultrasonography, 39% present with upper gastrointestinal bleeding, and 18% present with abdominal pain.13,14
Portal vein thrombosis is the complete or partial obstruction of blood flow due to a thrombus in the lumen of the portal vein. Contrast ultrasonography and CT can be used to establish the diagnosis.15
Anticoagulation is recommended in cases of complete thrombosis in candidates for living-donor liver transplant and for those at risk of mesenteric ischemia because of the thrombus extending into the mesenteric veins. In symptomatic patients, the decision to initiate anticoagulation should be made on a case-by-case basis after appropriate screening and management of varices.16–18
Our patient’s thrombocytopenia reflects the severity of portal hypertension and increases her risk of portal vein thrombosis, but this is unlikely to be the sole cause of the hemodynamic compromise in this patient.
Ruptured abdominal aortic aneurysm
Rupture of an abdominal aortic aneurysm is a medical emergency, with a mortality rate approaching 90%. Risk factors for abdominal aortic aneurysms are smoking, male sex, age over 65, history of cardiovascular disease, hypertension, and a family history of abdominal aortic aneurysm, especially if a first-degree relative is affected.19 Endovascular repair is associated with lower rates of death and complications compared with open repair.20
The patient does not have any of those risk factors, making this diagnosis less likely.
CASE CONTINUED: RUPTURED SPLENIC ARTERY ANEURYSM
Emergency CT of the abdomen and pelvis with contrast enhancement shows a large left intraperitoneal hematoma with active extravasation from a ruptured splenic artery aneurysm (Figure 1). The patient receives packed red blood cells and fresh-frozen plasma before being transferred to our hospital.
2. Which of the following is false regarding splenic artery aneurysms?
- They are the most common type of splanchnic arterial aneurysm
- True aneurysms are more common than pseudoaneurysms
- Asymptomatic aneurysms are discovered incidentally during assessment for other radiographic indications
- Splenic artery aneurysm in portal hypertension is the result of athero-sclerotic changes to the vascular intima
Splenic artery aneurysm in portal hypertension is not the result of atherosclerotic change to the vascular intima.
Splenic artery aneurysms are the most common type of splanchnic artery aneurysm.1 True aneurysms involve all 3 layers of the arterial wall, ie, intima, media, and adventitia. Cirrhosis and portal hypertension are associated with true aneurysm formation. The proposed mechanism of aneurysm formation is increased splenic blood flow in response to portal congestion with resultant hemodynamic stress that disrupts arterial wall structure, leading to aneurysmal dilation.21
In earlier reports, the incidence of true splenic artery aneurysm in portal hypertension varied from 2.9% to 50%, the latter representing autopsy findings of small aneurysms that were found in the splenic hilum of patients with cirrhosis.22–25 The incidence of clinically significant aneurysms in cirrhosis is unknown but incidental asymptomatic aneurysm is being detected more frequently on imaging studies pursued for screening purposes.26
The risk of rupture is low, only 2% to 10% in older studies and likely even lower now due to increased incidental detection in asymptomatic patients.27 However, emergent management of rupture at a tertiary care facility is paramount, as the mortality rate of ruptured splenic artery aneurysm is 29% to 36%.1,26,28
Splenic artery pseudoaneurysm is rarer and has a different pathophysiologic process than true aneurysm. It usually arises in the setting of trauma, pancreatitis, or postsurgery.29,30 Pseudoaneurysm is more likely to rupture, owing to compromise in the vascular wall integrity.4,21,28 As a result, treatment is indicated for every pseudoaneurysm regardless of size.
RISK FACTORS FOR SPLENIC ARTERY ANEURYSM
3. Which of the following is true regarding our patient’s risk of splenic artery aneurysm?
- Liver cirrhosis and portal hypertension are her greatest risk factors for it
- Female sex and prior pregnancy are her greatest risk factors for it
- Being Native American makes it more likely that the patient has splenic artery aneurysm secondary to collagen vascular disease
- Her risk of rupture would diminish after receiving a liver transplant
Liver cirrhosis and portal hypertension are her greatest risk factors for splenic artery aneurysm.
Risk factors for true aneurysm include hypertension, atherosclerosis, portal hypertension with or without liver cirrhosis, liver transplant, third trimester of pregnancy, and multiparity.1,4,26,28,31 Splenic artery aneurysm is usually diagnosed in the sixth decade. It may be 4 times as common in women, given a hormonal influence.32 Cirrhosis is also associated with massive splenic artery aneurysm (≥ 5 cm). Although rare, massive splenic artery aneurysm is more frequent in men (the male-to-female ratio is 1.78:1) and has a heightened risk of rupture.28 The incidence of rupture increases to around 3% to 4% after liver transplant.33 Rare causes of true aneurysm include fibrodysplasia, collagen vascular disease (eg, Loeys-Dietz and type IV Ehler-Danlos syndromes), vasculitis (eg, polyarteritis nodosa due to amphetamine abuse), and mycotic aneurysms.24,25,28,29
This patient’s age, sex, and history of cirrhosis puts her at increased risk of splenic artery aneurysm. The risk of rupture is highest in the peripartum period and in patients with cirrhosis who become pregnant. Although being Native American portends an increased risk for collagen vascular disease, the latter is unlikely to be a contributing factor.
TREATMENT OF SPLENIC ARTERY ANEURYSM
4. Which of the following is false regarding treatment of splenic artery aneurysms?
- Aneurysms larger than 2 cm and those that are expanding require repair
- Treatment should be offered if the patient has symptoms attributable to the aneurysm
- Asymptomatic aneurysms in pregnant women can be followed with watchful waiting
- Minimally invasive therapies such as percutaneous embolization may be a good option in poor operative candidates
Asymptomatic aneurysms in pregnant women should not be followed with watchful waiting—they should be repaired, as rupture carries a maternal mortality rate of 75% and a fetal mortality rate of 95%.34
Complications of splenic artery aneurysm depend on the type of aneurysm and its predisposing factors. Indications for treatment of true aneurysms include:
- Symptoms attributable to the aneurysm (hence, the second answer choice above is true)
- Diameter 2 cm or greater or enlarging diameter (hence, the first answer choice is true)
- Women of childbearing age in anticipation of pregnancy
- Need for surgical intervention such as portocaval shunt and liver transplant.
Conservative management is associated with a late mortality risk of 4.9%.2 Interventional options include percutaneous embolization or stenting; or laparotomy with splenic artery ligation or excision with or without splenectomy.1,28,35–37
Endovascular and open surgical repair have both been used to treat splenic artery aneurysms. The method used depends on the patient’s surgical history and aneurysm anatomy such as splenic artery tortuosity hindering passage of a catheter. Open surgery is associated with longer intraoperative time and length of hospital stay and higher rates of 30-day mortality and perioperative morbidity.38–41 With endovascular repair, the complication of persistent or recurrent flow occurs in 3% to 5% of cases by 30 days; hence, postprocedural surveillance is recommended.42–44 Endovascular repair has a higher reintervention rate but may still be more cost-effective than open surgical repair.
Because patients with cirrhosis have a higher risk of surgical complications,45 elective endovascular treatment may be an option for patients with aneurysms at high risk of rupturing. Endovascular treatment of visceral aneurysms is associated with complications such as postembolization syndrome (fever, abdominal pain, pleural effusion, and pancreatitis), access site hematoma, splenic infarction, and persistent abdominal pain.42
Patients with cirrhosis as the cause of splenic artery aneurysm tend to need longer hospitalization after endovascular treatment, but there is insufficient evidence to suggest that they are at higher risk of other complications.37
CASE CONTINUED: SPLENIC ARTERY EMBOLIZATION
The patient undergoes emergency splenic artery embolization, performed by an interventional radiology team (Figure 2 and Figure 3). Over the next few days, her mental status improves and her abdominal pain resolves. Her hemoglobin level remains stable after the procedure.
The surgical and interventional radiology teams discuss the risk of repeat intervention with the patient and her family, who prefer a nonoperative approach. She is managed supportively in the intensive care unit and is finally discharged home in stable condition and is scheduled for outpatient follow-up.
SUSPECT THIS FATAL CONDITION
The low prevalence of ruptured splenic artery aneurysm may lead physicians to attribute septic shock to spontaneous bacterial peritonitis or hemorrhagic shock from gastroesophageal varices in patients with cirrhosis, but a high index of suspicion and early recognition of this rare disease can lead to timely diagnosis and treatment of this highly fatal complication.
KEY POINTS
- Splenic artery aneurysm is a common complication of cirrhosis, often diagnosed incidentally.
- Elective embolization should be considered for asymptomatic splenic artery aneurysms larger than 2 cm in diameter, clinically symptomatic aneurysms, women of childbearing age, and patients who are candidates for liver transplant.
- Although splenic artery aneurysm rupture is rare, it has a high mortality rate and warrants a high index of suspicion to institute prompt specialized intervention.
- We recommend that physicians consider splenic artery aneurysm rupture in their differential diagnoses in patients with liver cirrhosis presenting with abdominal pain, altered mental status, and hemodynamic shock.
A 53-year-old Native American woman with a history of liver cirrhosis secondary to alcohol abuse presents to the emergency department after 2 days of diffuse abdominal pain and weakness. The pain was sudden in onset and has progressed relentlessly over the last day, reaching 9 on a scale of 10 in severity. Family members say that her oral intake has been decreased for the last 2 days, but she has had no fever, vomiting, change in bowel habit, blood in stool, or black stool. She has never undergone surgery, and has had one uncomplicated pregnancy.
Physical examination
Vital signs:
- Blood pressure 82/57 mm Hg
- Heart rate 96 beats per minute
- Temperature 37.3°C (99.1°F)
- Respiratory rate 16 per minute
- Oxygen saturation 92% while receiving oxygen at 2 L/minute.
The patient is somnolent and has scleral icterus. Her cardiopulmonary examination is normal. Her abdomen is tense, distended, and diffusely tender. She has bilateral +2 pitting edema in her lower extremities. She is oriented to person only and is noted to have asterixis. Her baseline Model for End-stage Liver Disease score is 18 points on a scale of 6 (less ill) to 40 (gravely ill).
Laboratory studies:
- Hemoglobin 9.8 g/dL (reference range 11.5–15.5)
- Platelet count 100 × 109/L (150–400)
- White blood cell count 9.9 × 109/L (3.7–11.0)
- Serum creatinine 1.06 mg/dL (0.58–0.96)
- Bilirubin 6.3 mg/dL (0.2–1.3)
- International normalized ratio of the prothrombin time 2.15 (0.8–1.2)
- Blood urea nitrogen 13 mg/dL (7–21)
- Serum albumin 2.7 g/dL (3.9–4.9).
Intravenous fluid resuscitation is initiated but the patient remains hypotensive, and on repeat laboratory testing 4 hours later her hemoglobin level has dropped to 7.3 mg/dL.
DIFFERENTIAL DIAGNOSIS
1. Which of the following are likely causes of this patient’s presentation?
- Splenic arterial aneurysm rupture
- Spontaneous bacterial peritonitis
- Variceal hemorrhage
- Portal vein thrombosis
- Abdominal aortic aneurysm rupture
Ruptured splenic artery aneurysm
Splenic artery aneurysms are the third most common intra-abdominal aneurysm, after those of the abdominal aorta and iliac artery.1 They are often asymptomatic and are being detected more frequently because of increased use of computed tomography (CT).2 Symptomatic splenic artery aneurysms may present with abdominal pain and have the potential to rupture, which can be life-threatening.3,4
This patient may have a ruptured splenic artery aneurysm, given her hemodynamic shock.
Spontaneous bacterial peritonitis
Ten percent to 20% of hospitalized patients with cirrhosis and ascites develop spontaneous bacterial peritonitis. Patients may present with ascites and abdominal pain, tenderness to palpation, fever, encephalopathy, or worsening liver and renal function.
Diagnostic paracentesis is paramount to delineate the cause of ascites; one should calculate the serum-ascites albumin gradient and obtain a cell count and culture of the ascitic fluid. The diagnosis of spontaneous bacterial peritonitis can be made if the ascitic fluid polymorphonuclear cell count is 0.25 × 109/L or higher, even if the ascitic fluid culture is negative.5,6 Simultaneous blood cultures should also be collected, as 50% of cases are associated with bacteremia.
The in-hospital mortality rate of an episode of spontaneous bacterial peritonitis has been reduced to 10% to 20% thanks to prompt diagnosis and empiric treatment with third-generation cephalosporins.7
Five percent of cases of infected ascites fluid are due to secondary bacterial peritonitis from a perforated viscus or a loculated abscess, which cannot be differentiated clinically from spontaneous bacterial peritonitis but can be diagnosed with CT.8
This patient may be presenting with septic shock secondary to either of these causes.
Variceal hemorrhage
Half of patients with cirrhosis have gastroesophageal varices due to portal hypertension. Endoscopic surveillance is warranted, as the risk of hemorrhage is 12% to 15% per year, and the mortality rate approaches 15% to 20% with each episode. Prompt resuscitation, diagnosis, and control of bleeding is paramount.
Esophagogastroduodenoscopy is used for both diagnosis and intervention. Short-term prophylactic use of antibiotics improves survival by preventing infections in the event bleeding recurs.9–11
Our patient may be presenting with hemodynamic shock from bleeding esophageal varices.
Portal vein thrombosis
Portal vein thrombosis is a common complication of cirrhosis, occurring in 5% to 28% of patients. The risk increases with the severity of liver disease and in association with hepatocellular carcinoma.12 Forty-three percent of cases are discovered incidentally in asymptomatic patients during ultrasonography, 39% present with upper gastrointestinal bleeding, and 18% present with abdominal pain.13,14
Portal vein thrombosis is the complete or partial obstruction of blood flow due to a thrombus in the lumen of the portal vein. Contrast ultrasonography and CT can be used to establish the diagnosis.15
Anticoagulation is recommended in cases of complete thrombosis in candidates for living-donor liver transplant and for those at risk of mesenteric ischemia because of the thrombus extending into the mesenteric veins. In symptomatic patients, the decision to initiate anticoagulation should be made on a case-by-case basis after appropriate screening and management of varices.16–18
Our patient’s thrombocytopenia reflects the severity of portal hypertension and increases her risk of portal vein thrombosis, but this is unlikely to be the sole cause of the hemodynamic compromise in this patient.
Ruptured abdominal aortic aneurysm
Rupture of an abdominal aortic aneurysm is a medical emergency, with a mortality rate approaching 90%. Risk factors for abdominal aortic aneurysms are smoking, male sex, age over 65, history of cardiovascular disease, hypertension, and a family history of abdominal aortic aneurysm, especially if a first-degree relative is affected.19 Endovascular repair is associated with lower rates of death and complications compared with open repair.20
The patient does not have any of those risk factors, making this diagnosis less likely.
CASE CONTINUED: RUPTURED SPLENIC ARTERY ANEURYSM
Emergency CT of the abdomen and pelvis with contrast enhancement shows a large left intraperitoneal hematoma with active extravasation from a ruptured splenic artery aneurysm (Figure 1). The patient receives packed red blood cells and fresh-frozen plasma before being transferred to our hospital.
2. Which of the following is false regarding splenic artery aneurysms?
- They are the most common type of splanchnic arterial aneurysm
- True aneurysms are more common than pseudoaneurysms
- Asymptomatic aneurysms are discovered incidentally during assessment for other radiographic indications
- Splenic artery aneurysm in portal hypertension is the result of athero-sclerotic changes to the vascular intima
Splenic artery aneurysm in portal hypertension is not the result of atherosclerotic change to the vascular intima.
Splenic artery aneurysms are the most common type of splanchnic artery aneurysm.1 True aneurysms involve all 3 layers of the arterial wall, ie, intima, media, and adventitia. Cirrhosis and portal hypertension are associated with true aneurysm formation. The proposed mechanism of aneurysm formation is increased splenic blood flow in response to portal congestion with resultant hemodynamic stress that disrupts arterial wall structure, leading to aneurysmal dilation.21
In earlier reports, the incidence of true splenic artery aneurysm in portal hypertension varied from 2.9% to 50%, the latter representing autopsy findings of small aneurysms that were found in the splenic hilum of patients with cirrhosis.22–25 The incidence of clinically significant aneurysms in cirrhosis is unknown but incidental asymptomatic aneurysm is being detected more frequently on imaging studies pursued for screening purposes.26
The risk of rupture is low, only 2% to 10% in older studies and likely even lower now due to increased incidental detection in asymptomatic patients.27 However, emergent management of rupture at a tertiary care facility is paramount, as the mortality rate of ruptured splenic artery aneurysm is 29% to 36%.1,26,28
Splenic artery pseudoaneurysm is rarer and has a different pathophysiologic process than true aneurysm. It usually arises in the setting of trauma, pancreatitis, or postsurgery.29,30 Pseudoaneurysm is more likely to rupture, owing to compromise in the vascular wall integrity.4,21,28 As a result, treatment is indicated for every pseudoaneurysm regardless of size.
RISK FACTORS FOR SPLENIC ARTERY ANEURYSM
3. Which of the following is true regarding our patient’s risk of splenic artery aneurysm?
- Liver cirrhosis and portal hypertension are her greatest risk factors for it
- Female sex and prior pregnancy are her greatest risk factors for it
- Being Native American makes it more likely that the patient has splenic artery aneurysm secondary to collagen vascular disease
- Her risk of rupture would diminish after receiving a liver transplant
Liver cirrhosis and portal hypertension are her greatest risk factors for splenic artery aneurysm.
Risk factors for true aneurysm include hypertension, atherosclerosis, portal hypertension with or without liver cirrhosis, liver transplant, third trimester of pregnancy, and multiparity.1,4,26,28,31 Splenic artery aneurysm is usually diagnosed in the sixth decade. It may be 4 times as common in women, given a hormonal influence.32 Cirrhosis is also associated with massive splenic artery aneurysm (≥ 5 cm). Although rare, massive splenic artery aneurysm is more frequent in men (the male-to-female ratio is 1.78:1) and has a heightened risk of rupture.28 The incidence of rupture increases to around 3% to 4% after liver transplant.33 Rare causes of true aneurysm include fibrodysplasia, collagen vascular disease (eg, Loeys-Dietz and type IV Ehler-Danlos syndromes), vasculitis (eg, polyarteritis nodosa due to amphetamine abuse), and mycotic aneurysms.24,25,28,29
This patient’s age, sex, and history of cirrhosis puts her at increased risk of splenic artery aneurysm. The risk of rupture is highest in the peripartum period and in patients with cirrhosis who become pregnant. Although being Native American portends an increased risk for collagen vascular disease, the latter is unlikely to be a contributing factor.
TREATMENT OF SPLENIC ARTERY ANEURYSM
4. Which of the following is false regarding treatment of splenic artery aneurysms?
- Aneurysms larger than 2 cm and those that are expanding require repair
- Treatment should be offered if the patient has symptoms attributable to the aneurysm
- Asymptomatic aneurysms in pregnant women can be followed with watchful waiting
- Minimally invasive therapies such as percutaneous embolization may be a good option in poor operative candidates
Asymptomatic aneurysms in pregnant women should not be followed with watchful waiting—they should be repaired, as rupture carries a maternal mortality rate of 75% and a fetal mortality rate of 95%.34
Complications of splenic artery aneurysm depend on the type of aneurysm and its predisposing factors. Indications for treatment of true aneurysms include:
- Symptoms attributable to the aneurysm (hence, the second answer choice above is true)
- Diameter 2 cm or greater or enlarging diameter (hence, the first answer choice is true)
- Women of childbearing age in anticipation of pregnancy
- Need for surgical intervention such as portocaval shunt and liver transplant.
Conservative management is associated with a late mortality risk of 4.9%.2 Interventional options include percutaneous embolization or stenting; or laparotomy with splenic artery ligation or excision with or without splenectomy.1,28,35–37
Endovascular and open surgical repair have both been used to treat splenic artery aneurysms. The method used depends on the patient’s surgical history and aneurysm anatomy such as splenic artery tortuosity hindering passage of a catheter. Open surgery is associated with longer intraoperative time and length of hospital stay and higher rates of 30-day mortality and perioperative morbidity.38–41 With endovascular repair, the complication of persistent or recurrent flow occurs in 3% to 5% of cases by 30 days; hence, postprocedural surveillance is recommended.42–44 Endovascular repair has a higher reintervention rate but may still be more cost-effective than open surgical repair.
Because patients with cirrhosis have a higher risk of surgical complications,45 elective endovascular treatment may be an option for patients with aneurysms at high risk of rupturing. Endovascular treatment of visceral aneurysms is associated with complications such as postembolization syndrome (fever, abdominal pain, pleural effusion, and pancreatitis), access site hematoma, splenic infarction, and persistent abdominal pain.42
Patients with cirrhosis as the cause of splenic artery aneurysm tend to need longer hospitalization after endovascular treatment, but there is insufficient evidence to suggest that they are at higher risk of other complications.37
CASE CONTINUED: SPLENIC ARTERY EMBOLIZATION
The patient undergoes emergency splenic artery embolization, performed by an interventional radiology team (Figure 2 and Figure 3). Over the next few days, her mental status improves and her abdominal pain resolves. Her hemoglobin level remains stable after the procedure.
The surgical and interventional radiology teams discuss the risk of repeat intervention with the patient and her family, who prefer a nonoperative approach. She is managed supportively in the intensive care unit and is finally discharged home in stable condition and is scheduled for outpatient follow-up.
SUSPECT THIS FATAL CONDITION
The low prevalence of ruptured splenic artery aneurysm may lead physicians to attribute septic shock to spontaneous bacterial peritonitis or hemorrhagic shock from gastroesophageal varices in patients with cirrhosis, but a high index of suspicion and early recognition of this rare disease can lead to timely diagnosis and treatment of this highly fatal complication.
KEY POINTS
- Splenic artery aneurysm is a common complication of cirrhosis, often diagnosed incidentally.
- Elective embolization should be considered for asymptomatic splenic artery aneurysms larger than 2 cm in diameter, clinically symptomatic aneurysms, women of childbearing age, and patients who are candidates for liver transplant.
- Although splenic artery aneurysm rupture is rare, it has a high mortality rate and warrants a high index of suspicion to institute prompt specialized intervention.
- We recommend that physicians consider splenic artery aneurysm rupture in their differential diagnoses in patients with liver cirrhosis presenting with abdominal pain, altered mental status, and hemodynamic shock.
- Bakhos CT, McIntosh BC, Nukta FA, et al. Staged arterial embolization and surgical resection of a giant splenic artery aneurysm. Ann Vasc Surg 2007; 21:208–210.
- Hogendoorn W, Lavida A, Hunink MG, et al. Open repair, endovascular repair, and conservative management of true splenic artery aneurysms. J Vasc Surg 2014; 60:1667–1676.e1.
- Algudkar A. Unruptured splenic artery aneurysm presenting as epigastric pain. JRSM Short Rep 2010; 1:24.
- Abbas MA, Stone WM, Fowl RJ, et al. Splenic artery aneurysms: two decades experience at Mayo Clinic. Ann Vasc Surg 2002; 16:442–449.
- Hoefs JC, Canawati HN, Sapico FL, Hopkins RR, Weiner J, Montgomerie JZ. Spontaneous bacterial peritonitis. Hepatology 1982; 2:399–407.
- Runyon BA, Hoefs JC. Culture-negative neutrocytic ascites: a variant of spontaneous bacterial peritonitis. Hepatology 1984; 4:1209–1211.
- Garcia-Tsao G. Spontaneous bacterial peritonitis: a historical perspective. J Hepatol 2004; 41:522–527.
- Soriano G, Castellote J, Alvarez C, et al. Secondary bacterial peritonitis in cirrhosis: a retrospective study of clinical and analytical characteristics, diagnosis and management. J Hepatol 2010; 52:39–44.
- D’Amico G, De Franchis R; Cooperative Study Group. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology 2003; 38:599–612.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD; Practice Guidelines Committee of American Association for Study of Liver Diseases; Practice Parameters Committee of American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol 2007; 102:2086–2102.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007; 46:922–938.
- Tsochatzis EA, Senzolo M, Germani G, Gatt A, Burroughs AK. Systematic review: portal vein thrombosis in cirrhosis. Aliment Pharmacol Ther 2010; 31:366–374.
- Kobori L, van der Kolk MJ, de Jong KP, et al. Splenic artery aneurysms in liver transplant patients. Liver Transplant Group. J Hepatol 1997; 27:890–893.
- Manzano-Robleda Mdel C, Barranco-Fragoso B, Uribe M, Mendez-Sanchez N. Portal vein thrombosis: what is new? Ann Hepatol 2015; 14:20–27.
- Sarin SK, Philips CA, Kamath PS, et al. Toward a comprehensive new classification of portal vein thrombosis in patients with cirrhosis. Gastroenterology 2016; 151:574–577.e3.
- DeLeve LD, Valla DC, Garcia-Tsao G; American Association for the Study of Liver Diseases. Vascular disorders of the liver. Hepatology 2009; 49:1729–1764.
- Manzanet G, Sanjuan F, Orbis P, et al. Liver transplantation in patients with portal vein thrombosis. Liver Transpl 2001; 7:125–131.
- John BV, Konjeti R, Aggarwal A, et al. Impact of untreated portal vein thrombosis on pre and post liver transplant outcomes in cirrhosis. Ann Hepatol 2013; 12:952–958.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al; American Association for Vascular Surgery/Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)—summary of recommendations. J Vasc Interv Radiol 2006; 17:1383–1397.
- Schermerhorn ML, O’Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, Landon BE. Endovascular vs open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med 2008; 358:464–474.
- Ohta M, Hashizume M, Ueno K, Tanoue K, Sugimachi K, Hasuo K. Hemodynamic study of splenic artery aneurysm in portal hypertension. Hepatogastroenterology 1994; 41:181–184.
- Sunagozaka H, Tsuji H, Mizukoshi E, et al. The development and clinical features of splenic aneurysm associated with liver cirrhosis. Liver Int 2006; 26:291–297.
- Manenti F, Williams R. Injection studies of the splenic vasculature in portal hypertension. Gut 1966; 7:175–180.
- Stanley JC, Fry WJ. Pathogenesis and clinical significance of splenic artery aneurysms. Surgery 1974; 76:898–909.
- Lee PC, Rhee RY, Gordon RY, Fung JJ, Webster MW. Management of splenic artery aneurysms: the significance of portal and essential hypertension. J Am Coll Surg 1999; 189:483–490.
- Al-Habbal Y, Christophi C, Muralidharan V. Aneurysms of the splenic artery—a review. Surgeon 2010; 8:223–231.
- Mattar SG, Lumsden AB. The management of splenic artery aneurysms: experience with 23 cases. Am J Surg 1995; 169:580–584.
- Akbulut S, Otan E. Management of giant splenic artery aneurysm: comprehensive literature review. Medicine (Baltimore) 2015; 94:e1016.
- Agrawal GA, Johnson PT, Fishman EK. Splenic artery aneurysms and pseudoaneurysms: clinical distinctions and CT appearances. AJR Am J Roentgenol 2007; 188:992–999.
- Tessier DJ, Stone WM, Fowl RJ, et al. Clinical features and management of splenic artery pseudoaneurysm: case series and cumulative review of literature. J Vasc Surg 2003; 38:969–974.
- Dave SP, Reis ED, Hossain A, Taub PJ, Kerstein MD, Hollier LH. Splenic artery aneurysm in the 1990s. Ann Vasc Surg 2000; 14:223–229.
- Parrish J, Maxwell C, Beecroft JR. Splenic artery aneurysm in pregnancy. J Obstet Gynaecol Can 2015; 37:816–818.
- Moon DB, Lee SG, Hwang S, et al. Characteristics and management of splenic artery aneurysms in adult living donor liver transplant recipients. Liver Transpl 2009; 15:1535–1541.
- Sadat U, Dar O, Walsh S, Varty K. Splenic artery aneurysms in pregnancy—a systematic review. Int J Surg 2008; 6:261–265.
- Geoghegan T, McAuley G, Snow A, Torreggiani WC. Emergency embolization of multiple splenic artery pseudoaneurysms associated with portal hypertension complicating cystic fibrosis. Australas Radiol 2007; 51(suppl):B337–B339.
- Jiang R, Ding X, Jian W, Jiang J, Hu S, Zhang Z. Combined endovascular embolization and open surgery for splenic artery aneurysm with arteriovenous fistula. Ann Vasc Surg 2016; 30:311.e1–311.e4.
- Naganuma M, Matsui H, Koizumi J, Fushimi K, Yasunaga H. Short-term outcomes following elective transcatheter arterial embolization for splenic artery aneurysms: data from a nationwide administrative database. Acta Radiol Open 2015; 4:2047981615574354.
- Batagini NC, El-Arousy H, Clair DG, Kirksey L. Open versus endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Ann Vasc Surg 2016; 35:1–8.
- Marone EM, Mascia D, Kahlberg A, Brioschi C, Tshomba Y, Chiesa R. Is open repair still the gold standard in visceral artery aneurysm management? Ann Vasc Surg 2011; 25:936–946.
- Sticco A, Aggarwal A, Shapiro M, Pratt A, Rissuci D, D'Ayala M. A comparison of open and endovascular treatment strategies for the management of splenic artery aneurysms. Vascular 2016; 24:487–491.
- Hogendoorn W, Lavida A, Hunink MG, et al. Cost-effectiveness of endovascular repair, open repair, and conservative management of splenic artery aneurysms. J Vasc Surg 2015; 61:1432–1440.
- Fankhauser GT, Stone WM, Naidu SG, et al; Mayo Vascular Research Center Consortium. The minimally invasive management of visceral artery aneurysms and pseudoaneurysms. J Vasc Surg 2011; 53:966–970.
- Lagana D, Carrafiello G, Mangini M, et al. Multimodal approach to endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Eur J Radiol 2006; 59:104–111.
- Guillon R, Garcier JM, Abergel A, et al. Management of splenic artery aneurysms and false aneurysms with endovascular treatment in 12 patients. Cardiovasc Intervent Radiol 2003; 26:256–260.
- Northup PG, Wanamaker RC, Lee VD, Adams RB, Berg CL. Model for end-stage liver disease (MELD) predicts nontransplant surgical mortality in patients with cirrhosis. Ann Surg 2005; 242:244–251.
- Bakhos CT, McIntosh BC, Nukta FA, et al. Staged arterial embolization and surgical resection of a giant splenic artery aneurysm. Ann Vasc Surg 2007; 21:208–210.
- Hogendoorn W, Lavida A, Hunink MG, et al. Open repair, endovascular repair, and conservative management of true splenic artery aneurysms. J Vasc Surg 2014; 60:1667–1676.e1.
- Algudkar A. Unruptured splenic artery aneurysm presenting as epigastric pain. JRSM Short Rep 2010; 1:24.
- Abbas MA, Stone WM, Fowl RJ, et al. Splenic artery aneurysms: two decades experience at Mayo Clinic. Ann Vasc Surg 2002; 16:442–449.
- Hoefs JC, Canawati HN, Sapico FL, Hopkins RR, Weiner J, Montgomerie JZ. Spontaneous bacterial peritonitis. Hepatology 1982; 2:399–407.
- Runyon BA, Hoefs JC. Culture-negative neutrocytic ascites: a variant of spontaneous bacterial peritonitis. Hepatology 1984; 4:1209–1211.
- Garcia-Tsao G. Spontaneous bacterial peritonitis: a historical perspective. J Hepatol 2004; 41:522–527.
- Soriano G, Castellote J, Alvarez C, et al. Secondary bacterial peritonitis in cirrhosis: a retrospective study of clinical and analytical characteristics, diagnosis and management. J Hepatol 2010; 52:39–44.
- D’Amico G, De Franchis R; Cooperative Study Group. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology 2003; 38:599–612.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD; Practice Guidelines Committee of American Association for Study of Liver Diseases; Practice Parameters Committee of American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol 2007; 102:2086–2102.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007; 46:922–938.
- Tsochatzis EA, Senzolo M, Germani G, Gatt A, Burroughs AK. Systematic review: portal vein thrombosis in cirrhosis. Aliment Pharmacol Ther 2010; 31:366–374.
- Kobori L, van der Kolk MJ, de Jong KP, et al. Splenic artery aneurysms in liver transplant patients. Liver Transplant Group. J Hepatol 1997; 27:890–893.
- Manzano-Robleda Mdel C, Barranco-Fragoso B, Uribe M, Mendez-Sanchez N. Portal vein thrombosis: what is new? Ann Hepatol 2015; 14:20–27.
- Sarin SK, Philips CA, Kamath PS, et al. Toward a comprehensive new classification of portal vein thrombosis in patients with cirrhosis. Gastroenterology 2016; 151:574–577.e3.
- DeLeve LD, Valla DC, Garcia-Tsao G; American Association for the Study of Liver Diseases. Vascular disorders of the liver. Hepatology 2009; 49:1729–1764.
- Manzanet G, Sanjuan F, Orbis P, et al. Liver transplantation in patients with portal vein thrombosis. Liver Transpl 2001; 7:125–131.
- John BV, Konjeti R, Aggarwal A, et al. Impact of untreated portal vein thrombosis on pre and post liver transplant outcomes in cirrhosis. Ann Hepatol 2013; 12:952–958.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al; American Association for Vascular Surgery/Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)—summary of recommendations. J Vasc Interv Radiol 2006; 17:1383–1397.
- Schermerhorn ML, O’Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, Landon BE. Endovascular vs open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med 2008; 358:464–474.
- Ohta M, Hashizume M, Ueno K, Tanoue K, Sugimachi K, Hasuo K. Hemodynamic study of splenic artery aneurysm in portal hypertension. Hepatogastroenterology 1994; 41:181–184.
- Sunagozaka H, Tsuji H, Mizukoshi E, et al. The development and clinical features of splenic aneurysm associated with liver cirrhosis. Liver Int 2006; 26:291–297.
- Manenti F, Williams R. Injection studies of the splenic vasculature in portal hypertension. Gut 1966; 7:175–180.
- Stanley JC, Fry WJ. Pathogenesis and clinical significance of splenic artery aneurysms. Surgery 1974; 76:898–909.
- Lee PC, Rhee RY, Gordon RY, Fung JJ, Webster MW. Management of splenic artery aneurysms: the significance of portal and essential hypertension. J Am Coll Surg 1999; 189:483–490.
- Al-Habbal Y, Christophi C, Muralidharan V. Aneurysms of the splenic artery—a review. Surgeon 2010; 8:223–231.
- Mattar SG, Lumsden AB. The management of splenic artery aneurysms: experience with 23 cases. Am J Surg 1995; 169:580–584.
- Akbulut S, Otan E. Management of giant splenic artery aneurysm: comprehensive literature review. Medicine (Baltimore) 2015; 94:e1016.
- Agrawal GA, Johnson PT, Fishman EK. Splenic artery aneurysms and pseudoaneurysms: clinical distinctions and CT appearances. AJR Am J Roentgenol 2007; 188:992–999.
- Tessier DJ, Stone WM, Fowl RJ, et al. Clinical features and management of splenic artery pseudoaneurysm: case series and cumulative review of literature. J Vasc Surg 2003; 38:969–974.
- Dave SP, Reis ED, Hossain A, Taub PJ, Kerstein MD, Hollier LH. Splenic artery aneurysm in the 1990s. Ann Vasc Surg 2000; 14:223–229.
- Parrish J, Maxwell C, Beecroft JR. Splenic artery aneurysm in pregnancy. J Obstet Gynaecol Can 2015; 37:816–818.
- Moon DB, Lee SG, Hwang S, et al. Characteristics and management of splenic artery aneurysms in adult living donor liver transplant recipients. Liver Transpl 2009; 15:1535–1541.
- Sadat U, Dar O, Walsh S, Varty K. Splenic artery aneurysms in pregnancy—a systematic review. Int J Surg 2008; 6:261–265.
- Geoghegan T, McAuley G, Snow A, Torreggiani WC. Emergency embolization of multiple splenic artery pseudoaneurysms associated with portal hypertension complicating cystic fibrosis. Australas Radiol 2007; 51(suppl):B337–B339.
- Jiang R, Ding X, Jian W, Jiang J, Hu S, Zhang Z. Combined endovascular embolization and open surgery for splenic artery aneurysm with arteriovenous fistula. Ann Vasc Surg 2016; 30:311.e1–311.e4.
- Naganuma M, Matsui H, Koizumi J, Fushimi K, Yasunaga H. Short-term outcomes following elective transcatheter arterial embolization for splenic artery aneurysms: data from a nationwide administrative database. Acta Radiol Open 2015; 4:2047981615574354.
- Batagini NC, El-Arousy H, Clair DG, Kirksey L. Open versus endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Ann Vasc Surg 2016; 35:1–8.
- Marone EM, Mascia D, Kahlberg A, Brioschi C, Tshomba Y, Chiesa R. Is open repair still the gold standard in visceral artery aneurysm management? Ann Vasc Surg 2011; 25:936–946.
- Sticco A, Aggarwal A, Shapiro M, Pratt A, Rissuci D, D'Ayala M. A comparison of open and endovascular treatment strategies for the management of splenic artery aneurysms. Vascular 2016; 24:487–491.
- Hogendoorn W, Lavida A, Hunink MG, et al. Cost-effectiveness of endovascular repair, open repair, and conservative management of splenic artery aneurysms. J Vasc Surg 2015; 61:1432–1440.
- Fankhauser GT, Stone WM, Naidu SG, et al; Mayo Vascular Research Center Consortium. The minimally invasive management of visceral artery aneurysms and pseudoaneurysms. J Vasc Surg 2011; 53:966–970.
- Lagana D, Carrafiello G, Mangini M, et al. Multimodal approach to endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Eur J Radiol 2006; 59:104–111.
- Guillon R, Garcier JM, Abergel A, et al. Management of splenic artery aneurysms and false aneurysms with endovascular treatment in 12 patients. Cardiovasc Intervent Radiol 2003; 26:256–260.
- Northup PG, Wanamaker RC, Lee VD, Adams RB, Berg CL. Model for end-stage liver disease (MELD) predicts nontransplant surgical mortality in patients with cirrhosis. Ann Surg 2005; 242:244–251.
Metastatic pulmonary calcification and end-stage renal disease
A 64-year-old man with end-stage renal disease was evaluated in the pulmonary clinic for persistent abnormalities on axial computed tomography (CT) of the chest. He was a lifelong nonsmoker and had no history of exposure to occupational dust or fumes. His oxygen saturation was 100% on room air, and he denied any respiratory symptoms.
WHEN TO CONSIDER METASTATIC PULMONARY CALCIFICATION
The differential diagnosis for chronic upper-lobe-predominant ground-glass nodules is broad and includes atypical infections, recurrent alveolar hemorrhage, hypersensitivity pneumonitis, vasculitis, sarcoidosis, chronic eosinophilic pneumonia, occupational lung disease, and pulmonary alveolar microlithiasis. However, several aspects of our patient’s case suggested an often overlooked diagnosis, metastatic pulmonary calcification.
Metastatic pulmonary calcification is caused by deposition of calcium salts in lung tissue and is most commonly seen in patients on dialysis,1,2 and our patient had been dependent on dialysis for many years. The chronically elevated calcium-phosphorus product and secondary hyperparathyroidism often seen with end-stage renal disease may explain this association.
Our patient’s lack of symptoms is also an important diagnostic clue. Unlike many other causes of chronic upper-lobe-predominant ground-glass nodules, metastatic pulmonary calcification does not usually cause symptoms and is often identified only at autopsy.3 Results of pulmonary function testing are often normal.4
Metastatic pulmonary calcification can appear as diffusely calcified nodules or high-attenuation areas of consolidation on CT. However, as in our patient’s case, CT may demonstrate fluffy, centrilobular ground-glass nodules due to the microscopic size of the deposited calcium crystals.1 Identifying calcified vessels on imaging supports the diagnosis.4
Treatment of metastatic pulmonary calcification in a patient with end-stage renal disease is focused on correcting underlying metabolic abnormalities with phosphate binders, vitamin D supplementation, and dialysis.
- Chan ED, Morales DV, Welsh CH, McDermott MT, Schwarz MI. Calcium deposition with or without bone formation in the lung. Am J Respir Crit Care Med 2002; 165:1654–1669.
- Beyzaei A, Francis J, Knight H, Simon DB, Finkelstein FO. Metabolic lung disease: diffuse metastatic pulmonary calcifications with progression to calciphylaxis in end-stage renal disease. Adv Perit Dial 2007; 23:112–117.
- Conger JD, Hammond WS, Alfrey AC, Contiguglia SR, Stanford RE, Huffer WE. Pulmonary calcification in chronic dialysis patients. Clinical and pathologic studies. Ann Intern Med 1975; 83:330–336.
- Belem LC, Zanetti G, Souza AS Jr, et al. Metastatic pulmonary calcification: state-of-the-art review focused on imaging findings. Respir Med 2014; 108:668–676.
A 64-year-old man with end-stage renal disease was evaluated in the pulmonary clinic for persistent abnormalities on axial computed tomography (CT) of the chest. He was a lifelong nonsmoker and had no history of exposure to occupational dust or fumes. His oxygen saturation was 100% on room air, and he denied any respiratory symptoms.
WHEN TO CONSIDER METASTATIC PULMONARY CALCIFICATION
The differential diagnosis for chronic upper-lobe-predominant ground-glass nodules is broad and includes atypical infections, recurrent alveolar hemorrhage, hypersensitivity pneumonitis, vasculitis, sarcoidosis, chronic eosinophilic pneumonia, occupational lung disease, and pulmonary alveolar microlithiasis. However, several aspects of our patient’s case suggested an often overlooked diagnosis, metastatic pulmonary calcification.
Metastatic pulmonary calcification is caused by deposition of calcium salts in lung tissue and is most commonly seen in patients on dialysis,1,2 and our patient had been dependent on dialysis for many years. The chronically elevated calcium-phosphorus product and secondary hyperparathyroidism often seen with end-stage renal disease may explain this association.
Our patient’s lack of symptoms is also an important diagnostic clue. Unlike many other causes of chronic upper-lobe-predominant ground-glass nodules, metastatic pulmonary calcification does not usually cause symptoms and is often identified only at autopsy.3 Results of pulmonary function testing are often normal.4
Metastatic pulmonary calcification can appear as diffusely calcified nodules or high-attenuation areas of consolidation on CT. However, as in our patient’s case, CT may demonstrate fluffy, centrilobular ground-glass nodules due to the microscopic size of the deposited calcium crystals.1 Identifying calcified vessels on imaging supports the diagnosis.4
Treatment of metastatic pulmonary calcification in a patient with end-stage renal disease is focused on correcting underlying metabolic abnormalities with phosphate binders, vitamin D supplementation, and dialysis.
A 64-year-old man with end-stage renal disease was evaluated in the pulmonary clinic for persistent abnormalities on axial computed tomography (CT) of the chest. He was a lifelong nonsmoker and had no history of exposure to occupational dust or fumes. His oxygen saturation was 100% on room air, and he denied any respiratory symptoms.
WHEN TO CONSIDER METASTATIC PULMONARY CALCIFICATION
The differential diagnosis for chronic upper-lobe-predominant ground-glass nodules is broad and includes atypical infections, recurrent alveolar hemorrhage, hypersensitivity pneumonitis, vasculitis, sarcoidosis, chronic eosinophilic pneumonia, occupational lung disease, and pulmonary alveolar microlithiasis. However, several aspects of our patient’s case suggested an often overlooked diagnosis, metastatic pulmonary calcification.
Metastatic pulmonary calcification is caused by deposition of calcium salts in lung tissue and is most commonly seen in patients on dialysis,1,2 and our patient had been dependent on dialysis for many years. The chronically elevated calcium-phosphorus product and secondary hyperparathyroidism often seen with end-stage renal disease may explain this association.
Our patient’s lack of symptoms is also an important diagnostic clue. Unlike many other causes of chronic upper-lobe-predominant ground-glass nodules, metastatic pulmonary calcification does not usually cause symptoms and is often identified only at autopsy.3 Results of pulmonary function testing are often normal.4
Metastatic pulmonary calcification can appear as diffusely calcified nodules or high-attenuation areas of consolidation on CT. However, as in our patient’s case, CT may demonstrate fluffy, centrilobular ground-glass nodules due to the microscopic size of the deposited calcium crystals.1 Identifying calcified vessels on imaging supports the diagnosis.4
Treatment of metastatic pulmonary calcification in a patient with end-stage renal disease is focused on correcting underlying metabolic abnormalities with phosphate binders, vitamin D supplementation, and dialysis.
- Chan ED, Morales DV, Welsh CH, McDermott MT, Schwarz MI. Calcium deposition with or without bone formation in the lung. Am J Respir Crit Care Med 2002; 165:1654–1669.
- Beyzaei A, Francis J, Knight H, Simon DB, Finkelstein FO. Metabolic lung disease: diffuse metastatic pulmonary calcifications with progression to calciphylaxis in end-stage renal disease. Adv Perit Dial 2007; 23:112–117.
- Conger JD, Hammond WS, Alfrey AC, Contiguglia SR, Stanford RE, Huffer WE. Pulmonary calcification in chronic dialysis patients. Clinical and pathologic studies. Ann Intern Med 1975; 83:330–336.
- Belem LC, Zanetti G, Souza AS Jr, et al. Metastatic pulmonary calcification: state-of-the-art review focused on imaging findings. Respir Med 2014; 108:668–676.
- Chan ED, Morales DV, Welsh CH, McDermott MT, Schwarz MI. Calcium deposition with or without bone formation in the lung. Am J Respir Crit Care Med 2002; 165:1654–1669.
- Beyzaei A, Francis J, Knight H, Simon DB, Finkelstein FO. Metabolic lung disease: diffuse metastatic pulmonary calcifications with progression to calciphylaxis in end-stage renal disease. Adv Perit Dial 2007; 23:112–117.
- Conger JD, Hammond WS, Alfrey AC, Contiguglia SR, Stanford RE, Huffer WE. Pulmonary calcification in chronic dialysis patients. Clinical and pathologic studies. Ann Intern Med 1975; 83:330–336.
- Belem LC, Zanetti G, Souza AS Jr, et al. Metastatic pulmonary calcification: state-of-the-art review focused on imaging findings. Respir Med 2014; 108:668–676.
Cardiac mass: Tumor or thrombus?
To the Editor: We read with great interest the article by Patnaik et al1 about a patient who had a cardiac metastasis of ovarian cancer, and we would like to raise a few points.
It is important to clarify that metastatic cardiac tumors are not necessary malignant. Intravenous leiomyomatosis is a benign small-muscle tumor that can spread to the heart, causing various cardiac symptoms.2 Even with extensive disease, patients with intravenous leiomyomatosis may remain asymptomatic until cardiac involvement occurs. The most common cardiac symptoms are dyspnea, syncope, and lower-extremity edema.
Cardiac involvement in intravenous leiomyomatosis may occur via direct invasion or hematogenous or lymphatic spread of the tumor. In leiomyoma and leiomyosarcoma, cardiac invasion usually occurs via direct spread through the inferior vena cava into the right atrium and ventricle. Thus, cardiac involvement with these tumors (except for nephroma) was reported to exclusively involve the right side of the heart.
In 2014, we reported a unique case of intravenous leiomyomatosis that extended from the right side into the left side of the heart and the aorta via an atrial septal defect.2 Intracardiac extension of intravenous leiomyomatosis may result in pulmonary embolism, systemic embolization if involving the left side, and, rarely, sudden death.2
In patients with malignancy, differentiating between thrombosis and tumor is critical. These patients have a hypercoagulable state and a fourfold increase in thrombosis risk, and chemotherapy increases this risk even more.3 Although tissue pathology examination is important for differentiating thrombosis from tumor, visualization of the direct extension of the mass from the primary source into the heart through the inferior vena cava by ultrasonography, computed tomography, or magnetic resonance imaging may help in making this distinction.2
- Patnaik S, Shah M, Sharma S, Ram P, Rammohan HS, Rubin A. A large mass in the right ventricle: tumor or thrombus? Cleve Clin J Med 2017; 84:517–519.
- Abdelghany M, Sodagam A, Patel P, Goldblatt C, Patel R. Intracardiac atypical leiomyoma involving all four cardiac chambers and the aorta. Rev Cardiovasc Med 2014; 15:271–275.
- Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008; 111:4902–4907.
To the Editor: We read with great interest the article by Patnaik et al1 about a patient who had a cardiac metastasis of ovarian cancer, and we would like to raise a few points.
It is important to clarify that metastatic cardiac tumors are not necessary malignant. Intravenous leiomyomatosis is a benign small-muscle tumor that can spread to the heart, causing various cardiac symptoms.2 Even with extensive disease, patients with intravenous leiomyomatosis may remain asymptomatic until cardiac involvement occurs. The most common cardiac symptoms are dyspnea, syncope, and lower-extremity edema.
Cardiac involvement in intravenous leiomyomatosis may occur via direct invasion or hematogenous or lymphatic spread of the tumor. In leiomyoma and leiomyosarcoma, cardiac invasion usually occurs via direct spread through the inferior vena cava into the right atrium and ventricle. Thus, cardiac involvement with these tumors (except for nephroma) was reported to exclusively involve the right side of the heart.
In 2014, we reported a unique case of intravenous leiomyomatosis that extended from the right side into the left side of the heart and the aorta via an atrial septal defect.2 Intracardiac extension of intravenous leiomyomatosis may result in pulmonary embolism, systemic embolization if involving the left side, and, rarely, sudden death.2
In patients with malignancy, differentiating between thrombosis and tumor is critical. These patients have a hypercoagulable state and a fourfold increase in thrombosis risk, and chemotherapy increases this risk even more.3 Although tissue pathology examination is important for differentiating thrombosis from tumor, visualization of the direct extension of the mass from the primary source into the heart through the inferior vena cava by ultrasonography, computed tomography, or magnetic resonance imaging may help in making this distinction.2
To the Editor: We read with great interest the article by Patnaik et al1 about a patient who had a cardiac metastasis of ovarian cancer, and we would like to raise a few points.
It is important to clarify that metastatic cardiac tumors are not necessary malignant. Intravenous leiomyomatosis is a benign small-muscle tumor that can spread to the heart, causing various cardiac symptoms.2 Even with extensive disease, patients with intravenous leiomyomatosis may remain asymptomatic until cardiac involvement occurs. The most common cardiac symptoms are dyspnea, syncope, and lower-extremity edema.
Cardiac involvement in intravenous leiomyomatosis may occur via direct invasion or hematogenous or lymphatic spread of the tumor. In leiomyoma and leiomyosarcoma, cardiac invasion usually occurs via direct spread through the inferior vena cava into the right atrium and ventricle. Thus, cardiac involvement with these tumors (except for nephroma) was reported to exclusively involve the right side of the heart.
In 2014, we reported a unique case of intravenous leiomyomatosis that extended from the right side into the left side of the heart and the aorta via an atrial septal defect.2 Intracardiac extension of intravenous leiomyomatosis may result in pulmonary embolism, systemic embolization if involving the left side, and, rarely, sudden death.2
In patients with malignancy, differentiating between thrombosis and tumor is critical. These patients have a hypercoagulable state and a fourfold increase in thrombosis risk, and chemotherapy increases this risk even more.3 Although tissue pathology examination is important for differentiating thrombosis from tumor, visualization of the direct extension of the mass from the primary source into the heart through the inferior vena cava by ultrasonography, computed tomography, or magnetic resonance imaging may help in making this distinction.2
- Patnaik S, Shah M, Sharma S, Ram P, Rammohan HS, Rubin A. A large mass in the right ventricle: tumor or thrombus? Cleve Clin J Med 2017; 84:517–519.
- Abdelghany M, Sodagam A, Patel P, Goldblatt C, Patel R. Intracardiac atypical leiomyoma involving all four cardiac chambers and the aorta. Rev Cardiovasc Med 2014; 15:271–275.
- Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008; 111:4902–4907.
- Patnaik S, Shah M, Sharma S, Ram P, Rammohan HS, Rubin A. A large mass in the right ventricle: tumor or thrombus? Cleve Clin J Med 2017; 84:517–519.
- Abdelghany M, Sodagam A, Patel P, Goldblatt C, Patel R. Intracardiac atypical leiomyoma involving all four cardiac chambers and the aorta. Rev Cardiovasc Med 2014; 15:271–275.
- Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008; 111:4902–4907.
Anticoagulation for atrial fibrillation
To the Editor: As a geriatric medicine fellow, I eagerly read Hagerty and Rich’s review “Fall risk and anticoagulation for atrial fibrillation in the elderly: A delicate balance”1 and Suh’s editorial, “Whether to anticoagulate: Toward a more reasoned approach”2 in the January 2017 issue. Both pieces were helpful and informative.
I appreciate that Dr. Suh encourages shared decision-making between physicians and patients that balances patient preferences and risk stratification to inform whether to anticoagulate. He states, “Unfortunately, there is no similar screening tool to predict bleeding risk from anticoagulation with greater precision in the middle to lower part of the risk spectrum...The patient’s life expectancy and personal preferences are important independent factors that affect the decision of whether to anticoagulate or not.”
Dr. Mark Eckman’s Atrial Fibrillation Decision Support Tool (AFDST) incorporates patients’ CHA2DS2-VASc and HAS-BLED scores to determine their quality-adjusted life expectancy on or off anticoagulation. The tool helps guide physicians and patients to make shared decisions about anticoagulation.3–5 The AFDST informs clinicians if a patient is undertreated or being treated unnecessarily. Eckman and his colleagues have demonstrated the AFDST’s effective application in clinical practice, including for older adults. I invite readers to learn more about Eckman’s work!
- Hagerty T, Rich MW. Fall risk and anticoagulation for atrial fibrillation in the elderly: a delicate balance. Cleve Clin J Med 2017; 84:35–40.
- Suh TT. Whether to anticoagulate: toward a more reasoned approach. Cleve Clin J Med 2017; 84:41–42.
- Eckman MH, Lip GYH, Wise RE, et al. Impact of an atrial fibrillation decision support tool on thromboprophylaxis for atrial fibrillation. Am Heart J 2016; 176:17–27.
- Eckman MH, Wise RE, Speer B, et al. Integrating real-time clinical information to provide estimates of net clinical benefit antithrombotic therapy for patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes 2014; 7:680–686.
- Eckman MH, Lip TYH, Wise RE, et al. Using an atrial fibrillation decision support tool for thromboprophylaxis in atrial fibrillation: effect of sex and age. J Am Geriatr Soc 2016; 64:1054–1060.
To the Editor: As a geriatric medicine fellow, I eagerly read Hagerty and Rich’s review “Fall risk and anticoagulation for atrial fibrillation in the elderly: A delicate balance”1 and Suh’s editorial, “Whether to anticoagulate: Toward a more reasoned approach”2 in the January 2017 issue. Both pieces were helpful and informative.
I appreciate that Dr. Suh encourages shared decision-making between physicians and patients that balances patient preferences and risk stratification to inform whether to anticoagulate. He states, “Unfortunately, there is no similar screening tool to predict bleeding risk from anticoagulation with greater precision in the middle to lower part of the risk spectrum...The patient’s life expectancy and personal preferences are important independent factors that affect the decision of whether to anticoagulate or not.”
Dr. Mark Eckman’s Atrial Fibrillation Decision Support Tool (AFDST) incorporates patients’ CHA2DS2-VASc and HAS-BLED scores to determine their quality-adjusted life expectancy on or off anticoagulation. The tool helps guide physicians and patients to make shared decisions about anticoagulation.3–5 The AFDST informs clinicians if a patient is undertreated or being treated unnecessarily. Eckman and his colleagues have demonstrated the AFDST’s effective application in clinical practice, including for older adults. I invite readers to learn more about Eckman’s work!
To the Editor: As a geriatric medicine fellow, I eagerly read Hagerty and Rich’s review “Fall risk and anticoagulation for atrial fibrillation in the elderly: A delicate balance”1 and Suh’s editorial, “Whether to anticoagulate: Toward a more reasoned approach”2 in the January 2017 issue. Both pieces were helpful and informative.
I appreciate that Dr. Suh encourages shared decision-making between physicians and patients that balances patient preferences and risk stratification to inform whether to anticoagulate. He states, “Unfortunately, there is no similar screening tool to predict bleeding risk from anticoagulation with greater precision in the middle to lower part of the risk spectrum...The patient’s life expectancy and personal preferences are important independent factors that affect the decision of whether to anticoagulate or not.”
Dr. Mark Eckman’s Atrial Fibrillation Decision Support Tool (AFDST) incorporates patients’ CHA2DS2-VASc and HAS-BLED scores to determine their quality-adjusted life expectancy on or off anticoagulation. The tool helps guide physicians and patients to make shared decisions about anticoagulation.3–5 The AFDST informs clinicians if a patient is undertreated or being treated unnecessarily. Eckman and his colleagues have demonstrated the AFDST’s effective application in clinical practice, including for older adults. I invite readers to learn more about Eckman’s work!
- Hagerty T, Rich MW. Fall risk and anticoagulation for atrial fibrillation in the elderly: a delicate balance. Cleve Clin J Med 2017; 84:35–40.
- Suh TT. Whether to anticoagulate: toward a more reasoned approach. Cleve Clin J Med 2017; 84:41–42.
- Eckman MH, Lip GYH, Wise RE, et al. Impact of an atrial fibrillation decision support tool on thromboprophylaxis for atrial fibrillation. Am Heart J 2016; 176:17–27.
- Eckman MH, Wise RE, Speer B, et al. Integrating real-time clinical information to provide estimates of net clinical benefit antithrombotic therapy for patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes 2014; 7:680–686.
- Eckman MH, Lip TYH, Wise RE, et al. Using an atrial fibrillation decision support tool for thromboprophylaxis in atrial fibrillation: effect of sex and age. J Am Geriatr Soc 2016; 64:1054–1060.
- Hagerty T, Rich MW. Fall risk and anticoagulation for atrial fibrillation in the elderly: a delicate balance. Cleve Clin J Med 2017; 84:35–40.
- Suh TT. Whether to anticoagulate: toward a more reasoned approach. Cleve Clin J Med 2017; 84:41–42.
- Eckman MH, Lip GYH, Wise RE, et al. Impact of an atrial fibrillation decision support tool on thromboprophylaxis for atrial fibrillation. Am Heart J 2016; 176:17–27.
- Eckman MH, Wise RE, Speer B, et al. Integrating real-time clinical information to provide estimates of net clinical benefit antithrombotic therapy for patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes 2014; 7:680–686.
- Eckman MH, Lip TYH, Wise RE, et al. Using an atrial fibrillation decision support tool for thromboprophylaxis in atrial fibrillation: effect of sex and age. J Am Geriatr Soc 2016; 64:1054–1060.
In reply: Anticoagulation for atrial fibrillation
In Reply: I appreciate Dr. Henning’s letter in response to my editorial.1 Indeed, Dr. Eckman’s Atrial Fibrillation Decision Support Tool (AFDST) is useful for determining quality-adjusted life expectancy on or off anticoagulation, and could possibly help with shared decision-making in regard to anticoagulation.2–4
However, the AFDST does not incorporate personal preferences regarding anticoagulant or medication use in general. Many older adults are on too many medications (ie, polypharmacy) and wish to reduce their overall pill count.
A number of potential barriers to shared decision-making regarding medication use have been identified, including poor physician communication skills, the growing number of available medications, multiple prescribers for the same patient, lack of trust in the prescribing physician, and patients feeling that their preferences are not valued or important.5 Until communication and acceptance between prescribers and patients regarding possible medication choices improves, shared decision-making for medication use in general and anticoagulant use in particular will be an unfulfilled ideal.
- Suh TT. Whether to anticoagulate: toward a more reasoned approach. Cleve Clin J Med 2017; 84:41–42.
- Eckman MH, Lip GYH, Wise RE, et al. Impact of an atrial fibrillation decision support tool on thromboprophylaxis for atrial fibrillation. Am Heart J 2016; 176:17–27.
- Eckman MH, Wise RE, Speer B, et al. Integrating real-time clinical information to provide estimates of net clinical benefit antithrombotic therapy for patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes 2014; 7:680–686.
- Eckman MH, Lip TYH, Wise RE, et al. Using an atrial fibrillation decision support tool for thromboprophylaxis in atrial fibrillation: effect of sex and age. J Am Geriatr Soc 2016; 64:1054–1060.
- Belcher VN, Fried TR, Agostini JV, Tinetti ME. Views of older adults on patient participation in medication-related decision making. J Gen Intern Med 2006; 21:298–303.
In Reply: I appreciate Dr. Henning’s letter in response to my editorial.1 Indeed, Dr. Eckman’s Atrial Fibrillation Decision Support Tool (AFDST) is useful for determining quality-adjusted life expectancy on or off anticoagulation, and could possibly help with shared decision-making in regard to anticoagulation.2–4
However, the AFDST does not incorporate personal preferences regarding anticoagulant or medication use in general. Many older adults are on too many medications (ie, polypharmacy) and wish to reduce their overall pill count.
A number of potential barriers to shared decision-making regarding medication use have been identified, including poor physician communication skills, the growing number of available medications, multiple prescribers for the same patient, lack of trust in the prescribing physician, and patients feeling that their preferences are not valued or important.5 Until communication and acceptance between prescribers and patients regarding possible medication choices improves, shared decision-making for medication use in general and anticoagulant use in particular will be an unfulfilled ideal.
In Reply: I appreciate Dr. Henning’s letter in response to my editorial.1 Indeed, Dr. Eckman’s Atrial Fibrillation Decision Support Tool (AFDST) is useful for determining quality-adjusted life expectancy on or off anticoagulation, and could possibly help with shared decision-making in regard to anticoagulation.2–4
However, the AFDST does not incorporate personal preferences regarding anticoagulant or medication use in general. Many older adults are on too many medications (ie, polypharmacy) and wish to reduce their overall pill count.
A number of potential barriers to shared decision-making regarding medication use have been identified, including poor physician communication skills, the growing number of available medications, multiple prescribers for the same patient, lack of trust in the prescribing physician, and patients feeling that their preferences are not valued or important.5 Until communication and acceptance between prescribers and patients regarding possible medication choices improves, shared decision-making for medication use in general and anticoagulant use in particular will be an unfulfilled ideal.
- Suh TT. Whether to anticoagulate: toward a more reasoned approach. Cleve Clin J Med 2017; 84:41–42.
- Eckman MH, Lip GYH, Wise RE, et al. Impact of an atrial fibrillation decision support tool on thromboprophylaxis for atrial fibrillation. Am Heart J 2016; 176:17–27.
- Eckman MH, Wise RE, Speer B, et al. Integrating real-time clinical information to provide estimates of net clinical benefit antithrombotic therapy for patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes 2014; 7:680–686.
- Eckman MH, Lip TYH, Wise RE, et al. Using an atrial fibrillation decision support tool for thromboprophylaxis in atrial fibrillation: effect of sex and age. J Am Geriatr Soc 2016; 64:1054–1060.
- Belcher VN, Fried TR, Agostini JV, Tinetti ME. Views of older adults on patient participation in medication-related decision making. J Gen Intern Med 2006; 21:298–303.
- Suh TT. Whether to anticoagulate: toward a more reasoned approach. Cleve Clin J Med 2017; 84:41–42.
- Eckman MH, Lip GYH, Wise RE, et al. Impact of an atrial fibrillation decision support tool on thromboprophylaxis for atrial fibrillation. Am Heart J 2016; 176:17–27.
- Eckman MH, Wise RE, Speer B, et al. Integrating real-time clinical information to provide estimates of net clinical benefit antithrombotic therapy for patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes 2014; 7:680–686.
- Eckman MH, Lip TYH, Wise RE, et al. Using an atrial fibrillation decision support tool for thromboprophylaxis in atrial fibrillation: effect of sex and age. J Am Geriatr Soc 2016; 64:1054–1060.
- Belcher VN, Fried TR, Agostini JV, Tinetti ME. Views of older adults on patient participation in medication-related decision making. J Gen Intern Med 2006; 21:298–303.
Renal denervation: What happened, and why?
Many patients, clinicians, and researchers had hoped that renal denervation would help control resistant hypertension. However, in the SYMPLICITY HTN-3 trial,1 named for the catheter-based system used in the study (Symplicity RDN, Medtronic, Dublin, Ireland), this endovascular procedure failed to meet its primary and secondary efficacy end points, although it was found to be safe. These results were surprising, especially given the results of an earlier randomized trial (SYMPLICITY HTN-2),2 which showed larger reductions in blood pressures 6 months after denervation than in the current trial.
Here, we discuss the results of the SYMPLICITY HTN-3 trial and offer possible explanations for its negative outcomes.
LEAD-UP TO SYMPLICITY HTN-3
Renal denervation consists of passing a catheter through the femoral artery into the renal arteries and ablating their sympathetic nerves using radiofrequency energy. In theory, this should interrupt efferent sympathetic communication between the brain and renal arteries, reducing muscular contraction of these arteries, increasing renal blood flow, reducing activation of the renin-angiotensin-adosterone system, thus reducing sodium retention, reducing afferent sympathetic communication between the kidneys and brain, and in turn reducing further sympathetic activity elsewhere in the body, such as in the heart. Blood pressure should fall.3
The results of the SYMPLICITY HTN-1 and 2 trials were discussed in an earlier article in this Journal,3 and the Medtronic-Ardian renal denervation system has been available in Europe and Australia for clinical use for over 2 years.4 Indeed, after the SYMPLICITY HTN-2 results were published in 2010, Boston Scientific’s Vessix, St. Jude Medical’s EnligHTN, and Covidien’s OneShot radiofrequency renal denervation devices—albeit each with some modifications—received a Conformité Européene (CE) mark and became available in Europe and Australia for clinical use. These devices are not available for clinical use or research in the United States.3,5
Therefore, SYMPLICITY HTN-3, sponsored by Medtronic, was designed to obtain US Food and Drug Administration approval in the United States.6
SYMPLICITY HTN-3 DESIGN
Inclusion criteria were similar to those in the earlier SYMPLICITY trials. Patients had to have resistant hypertension, defined as a systolic blood pressure ≥ 160 mm Hg despite taking at least 3 blood pressure medications at maximum tolerated doses. Patients were excluded if they had a glomerular filtration rate of less than 45 mL/min/1.73 m2, renal artery stenosis, or known secondary hypertension.
A total of 1,441 patients were enrolled, of whom 364 were eventually randomized to undergo renal denervation, and 171 were randomized to undergo a sham procedure. The mean systolic blood pressure at baseline was 188 mm Hg in each group. Most patients were taking maximum doses of blood pressure medications, and almost one-fourth were taking an aldosterone antagonist. Patients in both groups were taking an average of 5 medications.
The 2 groups were well matched for important covariates, including obstructive sleep apnea, diabetes mellitus, and renal insufficiency. Most of the patients were white; 25% of the renal denervation group and 29% of the sham procedure group were black.
The physicians conducting the follow-up appointments did not know which procedure the patients underwent, and neither did the patients. Medications were closely monitored, and patients had close follow-up. The catheter (Symplicity RDS, Medtronic) was of the same design that was used in the earlier SYMPLICITY trials and in clinical practice in countries where renal denervation was available.
Researchers expected that the systolic blood pressure, as measured in the office, would fall in both groups, but they hoped it would fall farther in the denervation group—at least 5 mm Hg farther, the primary end point of the trial. The secondary effectiveness end point was a 2-mm Hg greater reduction in 24-hour ambulatory systolic blood pressure.
SYMPLICITY HTN-3 RESULTS
No statistically significant difference in safety was observed between the denervation and control groups. However, the procedure was associated with 1 embolic event and 1 case of renal artery stenosis.
Blood pressure fell in both groups. However, at 6 months, office systolic pressure had fallen by a mean of 14.13 mm Hg in the denervation group and 11.74 mm Hg in the sham procedure group, a difference of only 2.39 mm Hg. The mean ambulatory systolic blood pressure had fallen by 6.75 vs 4.79 mm Hg, a difference of only 1.96 mm Hg. Neither difference was statistically significant.
A number of prespecified subgroup analyses were conducted, but the benefit of the procedure was statistically significant in only 3 subgroups: patients who were not black (P = .01), patients who were less than 65 years old (P = .04), and patients who had an estimated glomerular filtration rate of 60 mL/min/1.73 m2 or higher (P = .05).
WHAT WENT WRONG?
The results of SYMPLICITY HTN-3 were disappointing and led companies that were developing renal denervation devices to discontinue or reevaluate their programs.
Although the results were surprising, many observers (including our group) raised concerns about the initial enthusiasm surrounding renal denervation.3–7 Indeed, in 2010, we had concerns about the discrepancy between office-based blood pressure measurements (the primary end point of all renal denervation trials) and ambulatory blood pressure measurements in SYMPLICITY HTN-2.7
The enthusiasm surrounding this procedure led to the publication of 2 consensus documents on this novel therapy based on only 1 small randomized controlled study (SYMPLICITY HTN-2).8,9 Renal denervation was even reported to be useful in other conditions involving the sympathorenal axis, including diabetes mellitus, metabolic syndrome, and obstructive sleep apnea, and also as a potential treatment adjunct in atrial fibrillation and other arrhythmias.5
What went wrong?
Shortcomings in trial design?
The trial was well designed. Both patients and operators were blinded to the procedure, and 24-hour ambulatory blood pressure monitoring was used. We presume that appropriate patients with resistant hypertension were enrolled—the mean baseline systolic blood pressure was 188 mm Hg, and patients in each group were taking an average of 5 medications.
On the other hand, true medication adherence is difficult to ascertain. Further, the term maximal “tolerated” doses of medications is vague, and we cannot rule out the possibility that some patients were enrolled who did not truly have resistant hypertension—they simply did not want to take medications.
Patients were required to be on a stable medication regimen before enrollment and, ideally, to not have any medication changes during the course of the study, but at least 40% of patients did require medication changes during the study. Additionally, it is unclear whether all patients underwent specific testing to rule out secondary hypertension, as this was done at the discretion of the treating physician.
First-generation catheters?
The same type of catheter was used as in the earlier SYMPLICITY trials, and it had been used in many patients in clinical practice in countries where the catheter is routinely available. It is unknown, however, whether newer multisite denervation devices would yield better results than the first-generation devices used in SYMPLICITY HTN-3. But even this would not explain the discrepancies in data between earlier trials and this trial.
Operator inexperience?
It has been suggested that operator inexperience may have played a role, but an analysis of operator volume did not find any association between this variable and the outcomes. Each procedure was supervised by at least 1 and in most cases 2 certified Medtronic representatives, who made certain that meticulous attention was paid to procedure details and that no shortcuts were taken during the procedure.
Inadequate ablation?
While we can assume that the correct technique was followed in most cases, renal denervation is still a “blind” procedure, and there is no nerve mapping to ascertain the degree of ablation achieved. Notably, patients who had the most ablations reportedly had a greater average drop in systolic ambulatory blood pressure than those who received fewer ablations. Sympathetic nervous system activity is a potential marker of adequacy of ablation, but it was not routinely assessed in the SYMPLICITY HTN-3 trial. Techniques to assess sympathetic nerve activity such as norepinephrine spillover and muscle sympathetic nerve activity are highly specialized and available only at a few research centers, and are not available for routine clinical use.
While these points may explain the negative findings of this trial, they fail to account for the discrepant results between this study and previous trials that used exactly the same definitions and techniques.
Patient demographics?
Is it possible that renal denervation has a differential effect according to race? All previous renal denervation studies were conducted in Europe or Australia; therefore, few data are available on the efficacy of the procedure in other racial groups, such as black Americans. Most of the patients in this trial were white, but approximately 25% were black—a good representation. There was a statistically significant benefit favoring renal denervation in nonblack (mostly white) patients, but not in black patients. This may be related to racial differences in the pathophysiology of hypertension or possibly due to chance alone.
A Hawthorne effect?
A Hawthorne effect (patients being more compliant because physicians are paying more attention to them) is unlikely, since the renal denervation arm did not have any reduction in blood pressure medications. At 6 months, both the sham group and the procedure group were still on an average of 5 medications.
Additionally, while the blood pressure reduction in both treatment groups was significant, the systolic blood pressure at 6 months was still 166 mm Hg in the denervation group and 168 mm Hg in the sham group. If denervation was effective, one would have expected a greater reduction in blood pressure or at least a decrease in the number of medications needed, eg, 1 to 2 fewer medications in the denervation group compared with the sham procedure group.
Regression to the mean?
It is unknown whether the results represent a statistical error such as regression to the mean. But given the run-in period and the confirmatory data from 24-hour ambulatory blood pressure, this would be unlikely.
WHAT NOW?
Is renal denervation dead? SYMPLICITY HTN-3 is only a single trial with multiple shortcomings and lessons to learn from. Since its publication, there have been updates from 2 prospective, randomized, open-label trials concerning the efficacy of catheter-based renal denervation in lowering blood pressure.10,11
DENERHTN (Renal Denervation for Hypertension)10 studied patients with ambulatory systolic blood pressure higher than 135 mm Hg, diastolic blood pressure higher than 80 mm Hg, or both (after excluding secondary etiologies), despite 4 weeks of standardized triple-drug treatment including a diuretic. Patients were randomized to standardized stepped-care antihypertensive treatment alone (control group) or standard care plus renal denervation. The latter resulted in a significant further reduction in ambulatory blood pressure at 6 months.
The Prague-15 trial11 studied patients with resistant hypertension. Secondary etiologies were excluded and adherence to therapy was confirmed by measuring plasma medication levels. It showed that renal denervation along with optimal antihypertensive medical therapy (unchanged after randomization) resulted in a significant reduction in ambulatory blood pressure that was comparable to the effect of intensified antihypertensive medical therapy including spironolactone. (Studies have shown that spironolactone is effective when added on as a fourth-line medication in resistant hypertension.12) At 6 months, patients in the intensive medical therapy group were using an average of 0.3 more antihypertensive medications than those in the procedure group.
These two trials addressed some of the drawbacks of the SYMPLICITY HTN-3 trial. However, both have many limitations including and not limited to being open-label and nonblinded, lacking a sham procedure, using a lower blood pressure threshold than SYMPLICITY HTN-3 did to define resistant hypertension, and using the same catheter as in the SYMPLICITY trials.
Better technology is coming
Advanced renal denervation catheters are needed that are multielectrode, smaller, easier to manipulate, and capable of providing simultaneous, circumferential, more-intense, and deeper ablations. The ongoing Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug-Resistant Hypertension (INSPIRED)16 and Renal Denervation Using the Vessix Renal Denervation System for the Treatment of Hypertension (REDUCE-HTN: REINFORCE)17 trials are using contemporary innovative ablation catheters to address the limitations of the first-generation Symplicity catheter.
Further, Fischell et al18 reported encouraging results of renal denervation performed by injecting ethanol into the adventitial space of the renal arteries. This is still an invasive procedure; however, ethanol can spread out in all directions and reach all targeted nerves, potentially resulting in a more complete renal artery sympathetic ablation.
As technology advances, the WAVE IV trial19 is examining renal denervation performed from the outside through the skin using high-intensity focused ultrasound, which eliminates the need for femoral arterial catheterization, a promising noninvasive approach.
Proposals for future trials
The European Clinical Consensus Conference for Renal Denervation20 proposed that future trials of renal denervation include patients with moderate rather than resistant hypertension, reflecting the pathogenic importance of sympathetic activity in earlier stages of hypertension. The conference also proposed excluding patients with stiff large arteries, a cause of isolated systolic hypertension. Other proposals included standardizing concomitant antihypertensive therapy, preferably treating all patients with the combination of a renin-angiotensin system blocker, calcium channel blocker, and diuretic in the run-in period; monitoring drug adherence through the use of pill counts, electronic pill dispensers, and drug blood tests; and using change in ambulatory blood pressure as the primary efficacy end point and change in office blood pressure as a secondary end point.
Trials ongoing
To possibly address the limitations posed by the SYMPLICITY HTN-3 trial and to answer other important questions, several sham-controlled clinical trials of renal denervation are currently being conducted:
- INSPiRED16
- REDUCE-HTN: REINFORCE17
- Spyral HTN-Off Med21
- Spyral HTN-On Med21
- Study of the ReCor Medical Paradise System in Clinical Hypertension (RADIANCE-HTN).22
We hope these new studies can more clearly identify subsets of patients who would benefit from this technology, determine predictors of blood pressure reduction in such patients, and lead to newer devices that may provide more complete ablation.
Obviously, we also need better ways to identify the exact location of these sympathetic nerves within the renal artery and have a clearer sense of procedural success.
Until then, our colleagues in Europe and Australia continue to treat patients with this technology as we appropriately and patiently wait for level 1 clinical evidence of its efficacy.
Acknowledgments: We thank Kathryn Brock, BA, Editorial Services Manager, Heart and Vascular Institute, Cleveland Clinic, for her assistance in the preparation of this paper.
- Bhatt DL, Kandzari DE, O’Neill WW, et al, for the SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014; 370:1393–1401.
- Symplicity HTN-2 Investigators, Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (the Symplicity HTN-2 trial): a randomised controlled trial. Lancet 2010; 376:1903–1909.
- Bunte MC, Infante de Oliveira E, Shishehbor MH. Endovascular treatment of resistant and uncontrolled hypertension: therapies on the horizon. JACC Cardiovasc Interv 2013; 6:1–9.
- Thomas G, Shishehbor MH, Bravo EL, Nally JV. Renal denervation to treat resistant hypertension: guarded optimism. Cleve Clin J Med 2012; 79:501–510.
- Shishehbor MH, Bunte MC. Anatomical exclusion for renal denervation: are we putting the cart before the horse? JACC Cardiovasc Interv 2014; 7:193–194.
- Bhatt DL, Bakris GL. The promise of renal denervation. Cleve Clin J Med 2012; 79:498–500.
- Bunte MC. Renal sympathetic denervation for refractory hypertension. Lancet 2011; 377:1074; author reply 1075.
- Mahfoud F, Luscher TF, Andersson B, et al; European Society of Cardiology. Expert consensus document from the European Society of Cardiology on catheter-based renal denervation. Eur Heart J 2013; 34:2149–2157.
- Schlaich MP, Schmieder RE, Bakris G, et al. International expert consensus statement: percutaneous transluminal renal denervation for the treatment of resistant hypertension. J Am Coll Cardiol 2013; 62:2031–2045.
- Azizi M, Sapoval M, Gosse P, et al; Renal Denervation for Hypertension (DENERHTN) investigators. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet 2015; 385:1957–1965.
- Rosa J, Widimsky P, Tousek P, et al. Randomized comparison of renal denervation versus intensified pharmacotherapy including spironolactone in true-resistant hypertension: six-month results from the Prague-15 study. Hypertension 2015; 65:407–413.
- Williams B, MacDonald TM, Morant S, et al; British Hypertension Society’s PATHWAY Studies Group. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet 2015; 386:2059–2068.
- Sakakura K, Ladich E, Cheng Q, et al. Anatomic assessment of sympathetic peri-arterial renal nerves in man. J Am Coll Cardiol 2014; 64:635–643.
- Mahfoud F, Edelman ER, Bohm M. Catheter-based renal denervation is no simple matter: lessons to be learned from our anatomy? J Am Coll Cardiol 2014; 64:644–646.
- Id D, Kaltenbach B, Bertog SC, et al. Does the presence of accessory renal arteries affect the efficacy of renal denervation? JACC Cardiovasc Interv 2013; 6:1085–1091.
- Jin Y, Jacobs L, Baelen M, et al; Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug-Resistant Hypertension (Inspired) Investigators. Rationale and design of the Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug-Resistant Hypertension (INSPiRED) trial. Blood Press 2014; 23:138–146.
- ClinicalTrialsgov. Renal Denervation Using the Vessix Renal Denervation System for the Treatment of Hypertension (REDUCE HTN: REINFORCE). https://clinicaltrials.gov/ct2/show/NCT02392351?term=REDUCE-HTN%3A+REINFORCE&rank=1. Accessed August 3, 2017.
- Fischell TA, Ebner A, Gallo S, et al. Transcatheter alcohol-mediated perivascular renal denervation with the peregrine system: first-in-human experience. JACC Cardiovasc Interv 2016; 9:589–598.
- ClinicalTrialsgov. Sham controlled study of renal denervation for subjects with uncontrolled hypertension (WAVE_IV) (NCT02029885). https://clinicaltrials.gov/ct2/show/results/NCT02029885. Accessed August 3, 2017.
- Mahfoud F, Bohm M, Azizi M, et al. Proceedings from the European clinical consensus conference for renal denervation: considerations on future clinical trial design. Eur Heart J 2015; 36:2219–2227.
- Kandzari DE, Kario K, Mahfoud F, et al. The SPYRAL HTN Global Clinical Trial Program: rationale and design for studies of renal denervation in the absence (SPYRAL HTN OFF-MED) and presence (SPYRAL HTN ON-MED) of antihypertensive medications. Am Heart J 2016; 171:82–91.
- ClinicalTrialsgov. A Study of the ReCor Medical Paradise System in Clinical Hypertension (RADIANCE-HTN). https://clinicaltrials.gov/ct2/show/NCT02649426?term=RADIANCE&rank=3. Accessed August 3, 2017.
Many patients, clinicians, and researchers had hoped that renal denervation would help control resistant hypertension. However, in the SYMPLICITY HTN-3 trial,1 named for the catheter-based system used in the study (Symplicity RDN, Medtronic, Dublin, Ireland), this endovascular procedure failed to meet its primary and secondary efficacy end points, although it was found to be safe. These results were surprising, especially given the results of an earlier randomized trial (SYMPLICITY HTN-2),2 which showed larger reductions in blood pressures 6 months after denervation than in the current trial.
Here, we discuss the results of the SYMPLICITY HTN-3 trial and offer possible explanations for its negative outcomes.
LEAD-UP TO SYMPLICITY HTN-3
Renal denervation consists of passing a catheter through the femoral artery into the renal arteries and ablating their sympathetic nerves using radiofrequency energy. In theory, this should interrupt efferent sympathetic communication between the brain and renal arteries, reducing muscular contraction of these arteries, increasing renal blood flow, reducing activation of the renin-angiotensin-adosterone system, thus reducing sodium retention, reducing afferent sympathetic communication between the kidneys and brain, and in turn reducing further sympathetic activity elsewhere in the body, such as in the heart. Blood pressure should fall.3
The results of the SYMPLICITY HTN-1 and 2 trials were discussed in an earlier article in this Journal,3 and the Medtronic-Ardian renal denervation system has been available in Europe and Australia for clinical use for over 2 years.4 Indeed, after the SYMPLICITY HTN-2 results were published in 2010, Boston Scientific’s Vessix, St. Jude Medical’s EnligHTN, and Covidien’s OneShot radiofrequency renal denervation devices—albeit each with some modifications—received a Conformité Européene (CE) mark and became available in Europe and Australia for clinical use. These devices are not available for clinical use or research in the United States.3,5
Therefore, SYMPLICITY HTN-3, sponsored by Medtronic, was designed to obtain US Food and Drug Administration approval in the United States.6
SYMPLICITY HTN-3 DESIGN
Inclusion criteria were similar to those in the earlier SYMPLICITY trials. Patients had to have resistant hypertension, defined as a systolic blood pressure ≥ 160 mm Hg despite taking at least 3 blood pressure medications at maximum tolerated doses. Patients were excluded if they had a glomerular filtration rate of less than 45 mL/min/1.73 m2, renal artery stenosis, or known secondary hypertension.
A total of 1,441 patients were enrolled, of whom 364 were eventually randomized to undergo renal denervation, and 171 were randomized to undergo a sham procedure. The mean systolic blood pressure at baseline was 188 mm Hg in each group. Most patients were taking maximum doses of blood pressure medications, and almost one-fourth were taking an aldosterone antagonist. Patients in both groups were taking an average of 5 medications.
The 2 groups were well matched for important covariates, including obstructive sleep apnea, diabetes mellitus, and renal insufficiency. Most of the patients were white; 25% of the renal denervation group and 29% of the sham procedure group were black.
The physicians conducting the follow-up appointments did not know which procedure the patients underwent, and neither did the patients. Medications were closely monitored, and patients had close follow-up. The catheter (Symplicity RDS, Medtronic) was of the same design that was used in the earlier SYMPLICITY trials and in clinical practice in countries where renal denervation was available.
Researchers expected that the systolic blood pressure, as measured in the office, would fall in both groups, but they hoped it would fall farther in the denervation group—at least 5 mm Hg farther, the primary end point of the trial. The secondary effectiveness end point was a 2-mm Hg greater reduction in 24-hour ambulatory systolic blood pressure.
SYMPLICITY HTN-3 RESULTS
No statistically significant difference in safety was observed between the denervation and control groups. However, the procedure was associated with 1 embolic event and 1 case of renal artery stenosis.
Blood pressure fell in both groups. However, at 6 months, office systolic pressure had fallen by a mean of 14.13 mm Hg in the denervation group and 11.74 mm Hg in the sham procedure group, a difference of only 2.39 mm Hg. The mean ambulatory systolic blood pressure had fallen by 6.75 vs 4.79 mm Hg, a difference of only 1.96 mm Hg. Neither difference was statistically significant.
A number of prespecified subgroup analyses were conducted, but the benefit of the procedure was statistically significant in only 3 subgroups: patients who were not black (P = .01), patients who were less than 65 years old (P = .04), and patients who had an estimated glomerular filtration rate of 60 mL/min/1.73 m2 or higher (P = .05).
WHAT WENT WRONG?
The results of SYMPLICITY HTN-3 were disappointing and led companies that were developing renal denervation devices to discontinue or reevaluate their programs.
Although the results were surprising, many observers (including our group) raised concerns about the initial enthusiasm surrounding renal denervation.3–7 Indeed, in 2010, we had concerns about the discrepancy between office-based blood pressure measurements (the primary end point of all renal denervation trials) and ambulatory blood pressure measurements in SYMPLICITY HTN-2.7
The enthusiasm surrounding this procedure led to the publication of 2 consensus documents on this novel therapy based on only 1 small randomized controlled study (SYMPLICITY HTN-2).8,9 Renal denervation was even reported to be useful in other conditions involving the sympathorenal axis, including diabetes mellitus, metabolic syndrome, and obstructive sleep apnea, and also as a potential treatment adjunct in atrial fibrillation and other arrhythmias.5
What went wrong?
Shortcomings in trial design?
The trial was well designed. Both patients and operators were blinded to the procedure, and 24-hour ambulatory blood pressure monitoring was used. We presume that appropriate patients with resistant hypertension were enrolled—the mean baseline systolic blood pressure was 188 mm Hg, and patients in each group were taking an average of 5 medications.
On the other hand, true medication adherence is difficult to ascertain. Further, the term maximal “tolerated” doses of medications is vague, and we cannot rule out the possibility that some patients were enrolled who did not truly have resistant hypertension—they simply did not want to take medications.
Patients were required to be on a stable medication regimen before enrollment and, ideally, to not have any medication changes during the course of the study, but at least 40% of patients did require medication changes during the study. Additionally, it is unclear whether all patients underwent specific testing to rule out secondary hypertension, as this was done at the discretion of the treating physician.
First-generation catheters?
The same type of catheter was used as in the earlier SYMPLICITY trials, and it had been used in many patients in clinical practice in countries where the catheter is routinely available. It is unknown, however, whether newer multisite denervation devices would yield better results than the first-generation devices used in SYMPLICITY HTN-3. But even this would not explain the discrepancies in data between earlier trials and this trial.
Operator inexperience?
It has been suggested that operator inexperience may have played a role, but an analysis of operator volume did not find any association between this variable and the outcomes. Each procedure was supervised by at least 1 and in most cases 2 certified Medtronic representatives, who made certain that meticulous attention was paid to procedure details and that no shortcuts were taken during the procedure.
Inadequate ablation?
While we can assume that the correct technique was followed in most cases, renal denervation is still a “blind” procedure, and there is no nerve mapping to ascertain the degree of ablation achieved. Notably, patients who had the most ablations reportedly had a greater average drop in systolic ambulatory blood pressure than those who received fewer ablations. Sympathetic nervous system activity is a potential marker of adequacy of ablation, but it was not routinely assessed in the SYMPLICITY HTN-3 trial. Techniques to assess sympathetic nerve activity such as norepinephrine spillover and muscle sympathetic nerve activity are highly specialized and available only at a few research centers, and are not available for routine clinical use.
While these points may explain the negative findings of this trial, they fail to account for the discrepant results between this study and previous trials that used exactly the same definitions and techniques.
Patient demographics?
Is it possible that renal denervation has a differential effect according to race? All previous renal denervation studies were conducted in Europe or Australia; therefore, few data are available on the efficacy of the procedure in other racial groups, such as black Americans. Most of the patients in this trial were white, but approximately 25% were black—a good representation. There was a statistically significant benefit favoring renal denervation in nonblack (mostly white) patients, but not in black patients. This may be related to racial differences in the pathophysiology of hypertension or possibly due to chance alone.
A Hawthorne effect?
A Hawthorne effect (patients being more compliant because physicians are paying more attention to them) is unlikely, since the renal denervation arm did not have any reduction in blood pressure medications. At 6 months, both the sham group and the procedure group were still on an average of 5 medications.
Additionally, while the blood pressure reduction in both treatment groups was significant, the systolic blood pressure at 6 months was still 166 mm Hg in the denervation group and 168 mm Hg in the sham group. If denervation was effective, one would have expected a greater reduction in blood pressure or at least a decrease in the number of medications needed, eg, 1 to 2 fewer medications in the denervation group compared with the sham procedure group.
Regression to the mean?
It is unknown whether the results represent a statistical error such as regression to the mean. But given the run-in period and the confirmatory data from 24-hour ambulatory blood pressure, this would be unlikely.
WHAT NOW?
Is renal denervation dead? SYMPLICITY HTN-3 is only a single trial with multiple shortcomings and lessons to learn from. Since its publication, there have been updates from 2 prospective, randomized, open-label trials concerning the efficacy of catheter-based renal denervation in lowering blood pressure.10,11
DENERHTN (Renal Denervation for Hypertension)10 studied patients with ambulatory systolic blood pressure higher than 135 mm Hg, diastolic blood pressure higher than 80 mm Hg, or both (after excluding secondary etiologies), despite 4 weeks of standardized triple-drug treatment including a diuretic. Patients were randomized to standardized stepped-care antihypertensive treatment alone (control group) or standard care plus renal denervation. The latter resulted in a significant further reduction in ambulatory blood pressure at 6 months.
The Prague-15 trial11 studied patients with resistant hypertension. Secondary etiologies were excluded and adherence to therapy was confirmed by measuring plasma medication levels. It showed that renal denervation along with optimal antihypertensive medical therapy (unchanged after randomization) resulted in a significant reduction in ambulatory blood pressure that was comparable to the effect of intensified antihypertensive medical therapy including spironolactone. (Studies have shown that spironolactone is effective when added on as a fourth-line medication in resistant hypertension.12) At 6 months, patients in the intensive medical therapy group were using an average of 0.3 more antihypertensive medications than those in the procedure group.
These two trials addressed some of the drawbacks of the SYMPLICITY HTN-3 trial. However, both have many limitations including and not limited to being open-label and nonblinded, lacking a sham procedure, using a lower blood pressure threshold than SYMPLICITY HTN-3 did to define resistant hypertension, and using the same catheter as in the SYMPLICITY trials.
Better technology is coming
Advanced renal denervation catheters are needed that are multielectrode, smaller, easier to manipulate, and capable of providing simultaneous, circumferential, more-intense, and deeper ablations. The ongoing Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug-Resistant Hypertension (INSPIRED)16 and Renal Denervation Using the Vessix Renal Denervation System for the Treatment of Hypertension (REDUCE-HTN: REINFORCE)17 trials are using contemporary innovative ablation catheters to address the limitations of the first-generation Symplicity catheter.
Further, Fischell et al18 reported encouraging results of renal denervation performed by injecting ethanol into the adventitial space of the renal arteries. This is still an invasive procedure; however, ethanol can spread out in all directions and reach all targeted nerves, potentially resulting in a more complete renal artery sympathetic ablation.
As technology advances, the WAVE IV trial19 is examining renal denervation performed from the outside through the skin using high-intensity focused ultrasound, which eliminates the need for femoral arterial catheterization, a promising noninvasive approach.
Proposals for future trials
The European Clinical Consensus Conference for Renal Denervation20 proposed that future trials of renal denervation include patients with moderate rather than resistant hypertension, reflecting the pathogenic importance of sympathetic activity in earlier stages of hypertension. The conference also proposed excluding patients with stiff large arteries, a cause of isolated systolic hypertension. Other proposals included standardizing concomitant antihypertensive therapy, preferably treating all patients with the combination of a renin-angiotensin system blocker, calcium channel blocker, and diuretic in the run-in period; monitoring drug adherence through the use of pill counts, electronic pill dispensers, and drug blood tests; and using change in ambulatory blood pressure as the primary efficacy end point and change in office blood pressure as a secondary end point.
Trials ongoing
To possibly address the limitations posed by the SYMPLICITY HTN-3 trial and to answer other important questions, several sham-controlled clinical trials of renal denervation are currently being conducted:
- INSPiRED16
- REDUCE-HTN: REINFORCE17
- Spyral HTN-Off Med21
- Spyral HTN-On Med21
- Study of the ReCor Medical Paradise System in Clinical Hypertension (RADIANCE-HTN).22
We hope these new studies can more clearly identify subsets of patients who would benefit from this technology, determine predictors of blood pressure reduction in such patients, and lead to newer devices that may provide more complete ablation.
Obviously, we also need better ways to identify the exact location of these sympathetic nerves within the renal artery and have a clearer sense of procedural success.
Until then, our colleagues in Europe and Australia continue to treat patients with this technology as we appropriately and patiently wait for level 1 clinical evidence of its efficacy.
Acknowledgments: We thank Kathryn Brock, BA, Editorial Services Manager, Heart and Vascular Institute, Cleveland Clinic, for her assistance in the preparation of this paper.
Many patients, clinicians, and researchers had hoped that renal denervation would help control resistant hypertension. However, in the SYMPLICITY HTN-3 trial,1 named for the catheter-based system used in the study (Symplicity RDN, Medtronic, Dublin, Ireland), this endovascular procedure failed to meet its primary and secondary efficacy end points, although it was found to be safe. These results were surprising, especially given the results of an earlier randomized trial (SYMPLICITY HTN-2),2 which showed larger reductions in blood pressures 6 months after denervation than in the current trial.
Here, we discuss the results of the SYMPLICITY HTN-3 trial and offer possible explanations for its negative outcomes.
LEAD-UP TO SYMPLICITY HTN-3
Renal denervation consists of passing a catheter through the femoral artery into the renal arteries and ablating their sympathetic nerves using radiofrequency energy. In theory, this should interrupt efferent sympathetic communication between the brain and renal arteries, reducing muscular contraction of these arteries, increasing renal blood flow, reducing activation of the renin-angiotensin-adosterone system, thus reducing sodium retention, reducing afferent sympathetic communication between the kidneys and brain, and in turn reducing further sympathetic activity elsewhere in the body, such as in the heart. Blood pressure should fall.3
The results of the SYMPLICITY HTN-1 and 2 trials were discussed in an earlier article in this Journal,3 and the Medtronic-Ardian renal denervation system has been available in Europe and Australia for clinical use for over 2 years.4 Indeed, after the SYMPLICITY HTN-2 results were published in 2010, Boston Scientific’s Vessix, St. Jude Medical’s EnligHTN, and Covidien’s OneShot radiofrequency renal denervation devices—albeit each with some modifications—received a Conformité Européene (CE) mark and became available in Europe and Australia for clinical use. These devices are not available for clinical use or research in the United States.3,5
Therefore, SYMPLICITY HTN-3, sponsored by Medtronic, was designed to obtain US Food and Drug Administration approval in the United States.6
SYMPLICITY HTN-3 DESIGN
Inclusion criteria were similar to those in the earlier SYMPLICITY trials. Patients had to have resistant hypertension, defined as a systolic blood pressure ≥ 160 mm Hg despite taking at least 3 blood pressure medications at maximum tolerated doses. Patients were excluded if they had a glomerular filtration rate of less than 45 mL/min/1.73 m2, renal artery stenosis, or known secondary hypertension.
A total of 1,441 patients were enrolled, of whom 364 were eventually randomized to undergo renal denervation, and 171 were randomized to undergo a sham procedure. The mean systolic blood pressure at baseline was 188 mm Hg in each group. Most patients were taking maximum doses of blood pressure medications, and almost one-fourth were taking an aldosterone antagonist. Patients in both groups were taking an average of 5 medications.
The 2 groups were well matched for important covariates, including obstructive sleep apnea, diabetes mellitus, and renal insufficiency. Most of the patients were white; 25% of the renal denervation group and 29% of the sham procedure group were black.
The physicians conducting the follow-up appointments did not know which procedure the patients underwent, and neither did the patients. Medications were closely monitored, and patients had close follow-up. The catheter (Symplicity RDS, Medtronic) was of the same design that was used in the earlier SYMPLICITY trials and in clinical practice in countries where renal denervation was available.
Researchers expected that the systolic blood pressure, as measured in the office, would fall in both groups, but they hoped it would fall farther in the denervation group—at least 5 mm Hg farther, the primary end point of the trial. The secondary effectiveness end point was a 2-mm Hg greater reduction in 24-hour ambulatory systolic blood pressure.
SYMPLICITY HTN-3 RESULTS
No statistically significant difference in safety was observed between the denervation and control groups. However, the procedure was associated with 1 embolic event and 1 case of renal artery stenosis.
Blood pressure fell in both groups. However, at 6 months, office systolic pressure had fallen by a mean of 14.13 mm Hg in the denervation group and 11.74 mm Hg in the sham procedure group, a difference of only 2.39 mm Hg. The mean ambulatory systolic blood pressure had fallen by 6.75 vs 4.79 mm Hg, a difference of only 1.96 mm Hg. Neither difference was statistically significant.
A number of prespecified subgroup analyses were conducted, but the benefit of the procedure was statistically significant in only 3 subgroups: patients who were not black (P = .01), patients who were less than 65 years old (P = .04), and patients who had an estimated glomerular filtration rate of 60 mL/min/1.73 m2 or higher (P = .05).
WHAT WENT WRONG?
The results of SYMPLICITY HTN-3 were disappointing and led companies that were developing renal denervation devices to discontinue or reevaluate their programs.
Although the results were surprising, many observers (including our group) raised concerns about the initial enthusiasm surrounding renal denervation.3–7 Indeed, in 2010, we had concerns about the discrepancy between office-based blood pressure measurements (the primary end point of all renal denervation trials) and ambulatory blood pressure measurements in SYMPLICITY HTN-2.7
The enthusiasm surrounding this procedure led to the publication of 2 consensus documents on this novel therapy based on only 1 small randomized controlled study (SYMPLICITY HTN-2).8,9 Renal denervation was even reported to be useful in other conditions involving the sympathorenal axis, including diabetes mellitus, metabolic syndrome, and obstructive sleep apnea, and also as a potential treatment adjunct in atrial fibrillation and other arrhythmias.5
What went wrong?
Shortcomings in trial design?
The trial was well designed. Both patients and operators were blinded to the procedure, and 24-hour ambulatory blood pressure monitoring was used. We presume that appropriate patients with resistant hypertension were enrolled—the mean baseline systolic blood pressure was 188 mm Hg, and patients in each group were taking an average of 5 medications.
On the other hand, true medication adherence is difficult to ascertain. Further, the term maximal “tolerated” doses of medications is vague, and we cannot rule out the possibility that some patients were enrolled who did not truly have resistant hypertension—they simply did not want to take medications.
Patients were required to be on a stable medication regimen before enrollment and, ideally, to not have any medication changes during the course of the study, but at least 40% of patients did require medication changes during the study. Additionally, it is unclear whether all patients underwent specific testing to rule out secondary hypertension, as this was done at the discretion of the treating physician.
First-generation catheters?
The same type of catheter was used as in the earlier SYMPLICITY trials, and it had been used in many patients in clinical practice in countries where the catheter is routinely available. It is unknown, however, whether newer multisite denervation devices would yield better results than the first-generation devices used in SYMPLICITY HTN-3. But even this would not explain the discrepancies in data between earlier trials and this trial.
Operator inexperience?
It has been suggested that operator inexperience may have played a role, but an analysis of operator volume did not find any association between this variable and the outcomes. Each procedure was supervised by at least 1 and in most cases 2 certified Medtronic representatives, who made certain that meticulous attention was paid to procedure details and that no shortcuts were taken during the procedure.
Inadequate ablation?
While we can assume that the correct technique was followed in most cases, renal denervation is still a “blind” procedure, and there is no nerve mapping to ascertain the degree of ablation achieved. Notably, patients who had the most ablations reportedly had a greater average drop in systolic ambulatory blood pressure than those who received fewer ablations. Sympathetic nervous system activity is a potential marker of adequacy of ablation, but it was not routinely assessed in the SYMPLICITY HTN-3 trial. Techniques to assess sympathetic nerve activity such as norepinephrine spillover and muscle sympathetic nerve activity are highly specialized and available only at a few research centers, and are not available for routine clinical use.
While these points may explain the negative findings of this trial, they fail to account for the discrepant results between this study and previous trials that used exactly the same definitions and techniques.
Patient demographics?
Is it possible that renal denervation has a differential effect according to race? All previous renal denervation studies were conducted in Europe or Australia; therefore, few data are available on the efficacy of the procedure in other racial groups, such as black Americans. Most of the patients in this trial were white, but approximately 25% were black—a good representation. There was a statistically significant benefit favoring renal denervation in nonblack (mostly white) patients, but not in black patients. This may be related to racial differences in the pathophysiology of hypertension or possibly due to chance alone.
A Hawthorne effect?
A Hawthorne effect (patients being more compliant because physicians are paying more attention to them) is unlikely, since the renal denervation arm did not have any reduction in blood pressure medications. At 6 months, both the sham group and the procedure group were still on an average of 5 medications.
Additionally, while the blood pressure reduction in both treatment groups was significant, the systolic blood pressure at 6 months was still 166 mm Hg in the denervation group and 168 mm Hg in the sham group. If denervation was effective, one would have expected a greater reduction in blood pressure or at least a decrease in the number of medications needed, eg, 1 to 2 fewer medications in the denervation group compared with the sham procedure group.
Regression to the mean?
It is unknown whether the results represent a statistical error such as regression to the mean. But given the run-in period and the confirmatory data from 24-hour ambulatory blood pressure, this would be unlikely.
WHAT NOW?
Is renal denervation dead? SYMPLICITY HTN-3 is only a single trial with multiple shortcomings and lessons to learn from. Since its publication, there have been updates from 2 prospective, randomized, open-label trials concerning the efficacy of catheter-based renal denervation in lowering blood pressure.10,11
DENERHTN (Renal Denervation for Hypertension)10 studied patients with ambulatory systolic blood pressure higher than 135 mm Hg, diastolic blood pressure higher than 80 mm Hg, or both (after excluding secondary etiologies), despite 4 weeks of standardized triple-drug treatment including a diuretic. Patients were randomized to standardized stepped-care antihypertensive treatment alone (control group) or standard care plus renal denervation. The latter resulted in a significant further reduction in ambulatory blood pressure at 6 months.
The Prague-15 trial11 studied patients with resistant hypertension. Secondary etiologies were excluded and adherence to therapy was confirmed by measuring plasma medication levels. It showed that renal denervation along with optimal antihypertensive medical therapy (unchanged after randomization) resulted in a significant reduction in ambulatory blood pressure that was comparable to the effect of intensified antihypertensive medical therapy including spironolactone. (Studies have shown that spironolactone is effective when added on as a fourth-line medication in resistant hypertension.12) At 6 months, patients in the intensive medical therapy group were using an average of 0.3 more antihypertensive medications than those in the procedure group.
These two trials addressed some of the drawbacks of the SYMPLICITY HTN-3 trial. However, both have many limitations including and not limited to being open-label and nonblinded, lacking a sham procedure, using a lower blood pressure threshold than SYMPLICITY HTN-3 did to define resistant hypertension, and using the same catheter as in the SYMPLICITY trials.
Better technology is coming
Advanced renal denervation catheters are needed that are multielectrode, smaller, easier to manipulate, and capable of providing simultaneous, circumferential, more-intense, and deeper ablations. The ongoing Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug-Resistant Hypertension (INSPIRED)16 and Renal Denervation Using the Vessix Renal Denervation System for the Treatment of Hypertension (REDUCE-HTN: REINFORCE)17 trials are using contemporary innovative ablation catheters to address the limitations of the first-generation Symplicity catheter.
Further, Fischell et al18 reported encouraging results of renal denervation performed by injecting ethanol into the adventitial space of the renal arteries. This is still an invasive procedure; however, ethanol can spread out in all directions and reach all targeted nerves, potentially resulting in a more complete renal artery sympathetic ablation.
As technology advances, the WAVE IV trial19 is examining renal denervation performed from the outside through the skin using high-intensity focused ultrasound, which eliminates the need for femoral arterial catheterization, a promising noninvasive approach.
Proposals for future trials
The European Clinical Consensus Conference for Renal Denervation20 proposed that future trials of renal denervation include patients with moderate rather than resistant hypertension, reflecting the pathogenic importance of sympathetic activity in earlier stages of hypertension. The conference also proposed excluding patients with stiff large arteries, a cause of isolated systolic hypertension. Other proposals included standardizing concomitant antihypertensive therapy, preferably treating all patients with the combination of a renin-angiotensin system blocker, calcium channel blocker, and diuretic in the run-in period; monitoring drug adherence through the use of pill counts, electronic pill dispensers, and drug blood tests; and using change in ambulatory blood pressure as the primary efficacy end point and change in office blood pressure as a secondary end point.
Trials ongoing
To possibly address the limitations posed by the SYMPLICITY HTN-3 trial and to answer other important questions, several sham-controlled clinical trials of renal denervation are currently being conducted:
- INSPiRED16
- REDUCE-HTN: REINFORCE17
- Spyral HTN-Off Med21
- Spyral HTN-On Med21
- Study of the ReCor Medical Paradise System in Clinical Hypertension (RADIANCE-HTN).22
We hope these new studies can more clearly identify subsets of patients who would benefit from this technology, determine predictors of blood pressure reduction in such patients, and lead to newer devices that may provide more complete ablation.
Obviously, we also need better ways to identify the exact location of these sympathetic nerves within the renal artery and have a clearer sense of procedural success.
Until then, our colleagues in Europe and Australia continue to treat patients with this technology as we appropriately and patiently wait for level 1 clinical evidence of its efficacy.
Acknowledgments: We thank Kathryn Brock, BA, Editorial Services Manager, Heart and Vascular Institute, Cleveland Clinic, for her assistance in the preparation of this paper.
- Bhatt DL, Kandzari DE, O’Neill WW, et al, for the SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014; 370:1393–1401.
- Symplicity HTN-2 Investigators, Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (the Symplicity HTN-2 trial): a randomised controlled trial. Lancet 2010; 376:1903–1909.
- Bunte MC, Infante de Oliveira E, Shishehbor MH. Endovascular treatment of resistant and uncontrolled hypertension: therapies on the horizon. JACC Cardiovasc Interv 2013; 6:1–9.
- Thomas G, Shishehbor MH, Bravo EL, Nally JV. Renal denervation to treat resistant hypertension: guarded optimism. Cleve Clin J Med 2012; 79:501–510.
- Shishehbor MH, Bunte MC. Anatomical exclusion for renal denervation: are we putting the cart before the horse? JACC Cardiovasc Interv 2014; 7:193–194.
- Bhatt DL, Bakris GL. The promise of renal denervation. Cleve Clin J Med 2012; 79:498–500.
- Bunte MC. Renal sympathetic denervation for refractory hypertension. Lancet 2011; 377:1074; author reply 1075.
- Mahfoud F, Luscher TF, Andersson B, et al; European Society of Cardiology. Expert consensus document from the European Society of Cardiology on catheter-based renal denervation. Eur Heart J 2013; 34:2149–2157.
- Schlaich MP, Schmieder RE, Bakris G, et al. International expert consensus statement: percutaneous transluminal renal denervation for the treatment of resistant hypertension. J Am Coll Cardiol 2013; 62:2031–2045.
- Azizi M, Sapoval M, Gosse P, et al; Renal Denervation for Hypertension (DENERHTN) investigators. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet 2015; 385:1957–1965.
- Rosa J, Widimsky P, Tousek P, et al. Randomized comparison of renal denervation versus intensified pharmacotherapy including spironolactone in true-resistant hypertension: six-month results from the Prague-15 study. Hypertension 2015; 65:407–413.
- Williams B, MacDonald TM, Morant S, et al; British Hypertension Society’s PATHWAY Studies Group. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet 2015; 386:2059–2068.
- Sakakura K, Ladich E, Cheng Q, et al. Anatomic assessment of sympathetic peri-arterial renal nerves in man. J Am Coll Cardiol 2014; 64:635–643.
- Mahfoud F, Edelman ER, Bohm M. Catheter-based renal denervation is no simple matter: lessons to be learned from our anatomy? J Am Coll Cardiol 2014; 64:644–646.
- Id D, Kaltenbach B, Bertog SC, et al. Does the presence of accessory renal arteries affect the efficacy of renal denervation? JACC Cardiovasc Interv 2013; 6:1085–1091.
- Jin Y, Jacobs L, Baelen M, et al; Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug-Resistant Hypertension (Inspired) Investigators. Rationale and design of the Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug-Resistant Hypertension (INSPiRED) trial. Blood Press 2014; 23:138–146.
- ClinicalTrialsgov. Renal Denervation Using the Vessix Renal Denervation System for the Treatment of Hypertension (REDUCE HTN: REINFORCE). https://clinicaltrials.gov/ct2/show/NCT02392351?term=REDUCE-HTN%3A+REINFORCE&rank=1. Accessed August 3, 2017.
- Fischell TA, Ebner A, Gallo S, et al. Transcatheter alcohol-mediated perivascular renal denervation with the peregrine system: first-in-human experience. JACC Cardiovasc Interv 2016; 9:589–598.
- ClinicalTrialsgov. Sham controlled study of renal denervation for subjects with uncontrolled hypertension (WAVE_IV) (NCT02029885). https://clinicaltrials.gov/ct2/show/results/NCT02029885. Accessed August 3, 2017.
- Mahfoud F, Bohm M, Azizi M, et al. Proceedings from the European clinical consensus conference for renal denervation: considerations on future clinical trial design. Eur Heart J 2015; 36:2219–2227.
- Kandzari DE, Kario K, Mahfoud F, et al. The SPYRAL HTN Global Clinical Trial Program: rationale and design for studies of renal denervation in the absence (SPYRAL HTN OFF-MED) and presence (SPYRAL HTN ON-MED) of antihypertensive medications. Am Heart J 2016; 171:82–91.
- ClinicalTrialsgov. A Study of the ReCor Medical Paradise System in Clinical Hypertension (RADIANCE-HTN). https://clinicaltrials.gov/ct2/show/NCT02649426?term=RADIANCE&rank=3. Accessed August 3, 2017.
- Bhatt DL, Kandzari DE, O’Neill WW, et al, for the SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014; 370:1393–1401.
- Symplicity HTN-2 Investigators, Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (the Symplicity HTN-2 trial): a randomised controlled trial. Lancet 2010; 376:1903–1909.
- Bunte MC, Infante de Oliveira E, Shishehbor MH. Endovascular treatment of resistant and uncontrolled hypertension: therapies on the horizon. JACC Cardiovasc Interv 2013; 6:1–9.
- Thomas G, Shishehbor MH, Bravo EL, Nally JV. Renal denervation to treat resistant hypertension: guarded optimism. Cleve Clin J Med 2012; 79:501–510.
- Shishehbor MH, Bunte MC. Anatomical exclusion for renal denervation: are we putting the cart before the horse? JACC Cardiovasc Interv 2014; 7:193–194.
- Bhatt DL, Bakris GL. The promise of renal denervation. Cleve Clin J Med 2012; 79:498–500.
- Bunte MC. Renal sympathetic denervation for refractory hypertension. Lancet 2011; 377:1074; author reply 1075.
- Mahfoud F, Luscher TF, Andersson B, et al; European Society of Cardiology. Expert consensus document from the European Society of Cardiology on catheter-based renal denervation. Eur Heart J 2013; 34:2149–2157.
- Schlaich MP, Schmieder RE, Bakris G, et al. International expert consensus statement: percutaneous transluminal renal denervation for the treatment of resistant hypertension. J Am Coll Cardiol 2013; 62:2031–2045.
- Azizi M, Sapoval M, Gosse P, et al; Renal Denervation for Hypertension (DENERHTN) investigators. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet 2015; 385:1957–1965.
- Rosa J, Widimsky P, Tousek P, et al. Randomized comparison of renal denervation versus intensified pharmacotherapy including spironolactone in true-resistant hypertension: six-month results from the Prague-15 study. Hypertension 2015; 65:407–413.
- Williams B, MacDonald TM, Morant S, et al; British Hypertension Society’s PATHWAY Studies Group. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet 2015; 386:2059–2068.
- Sakakura K, Ladich E, Cheng Q, et al. Anatomic assessment of sympathetic peri-arterial renal nerves in man. J Am Coll Cardiol 2014; 64:635–643.
- Mahfoud F, Edelman ER, Bohm M. Catheter-based renal denervation is no simple matter: lessons to be learned from our anatomy? J Am Coll Cardiol 2014; 64:644–646.
- Id D, Kaltenbach B, Bertog SC, et al. Does the presence of accessory renal arteries affect the efficacy of renal denervation? JACC Cardiovasc Interv 2013; 6:1085–1091.
- Jin Y, Jacobs L, Baelen M, et al; Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug-Resistant Hypertension (Inspired) Investigators. Rationale and design of the Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug-Resistant Hypertension (INSPiRED) trial. Blood Press 2014; 23:138–146.
- ClinicalTrialsgov. Renal Denervation Using the Vessix Renal Denervation System for the Treatment of Hypertension (REDUCE HTN: REINFORCE). https://clinicaltrials.gov/ct2/show/NCT02392351?term=REDUCE-HTN%3A+REINFORCE&rank=1. Accessed August 3, 2017.
- Fischell TA, Ebner A, Gallo S, et al. Transcatheter alcohol-mediated perivascular renal denervation with the peregrine system: first-in-human experience. JACC Cardiovasc Interv 2016; 9:589–598.
- ClinicalTrialsgov. Sham controlled study of renal denervation for subjects with uncontrolled hypertension (WAVE_IV) (NCT02029885). https://clinicaltrials.gov/ct2/show/results/NCT02029885. Accessed August 3, 2017.
- Mahfoud F, Bohm M, Azizi M, et al. Proceedings from the European clinical consensus conference for renal denervation: considerations on future clinical trial design. Eur Heart J 2015; 36:2219–2227.
- Kandzari DE, Kario K, Mahfoud F, et al. The SPYRAL HTN Global Clinical Trial Program: rationale and design for studies of renal denervation in the absence (SPYRAL HTN OFF-MED) and presence (SPYRAL HTN ON-MED) of antihypertensive medications. Am Heart J 2016; 171:82–91.
- ClinicalTrialsgov. A Study of the ReCor Medical Paradise System in Clinical Hypertension (RADIANCE-HTN). https://clinicaltrials.gov/ct2/show/NCT02649426?term=RADIANCE&rank=3. Accessed August 3, 2017.
KEY POINTS
- Renal denervation consists of passing a catheter into the renal arteries and ablating their sympathetic nerves using radiofrequency energy. In theory, it should lower blood pressure and be an attractive option for treating resistant hypertension.
- SYMPLICITY HTN-3 was a blinded trial in which patients with resistant hypertension were randomized to undergo real or sham renal denervation.
- At 6 months, office systolic blood pressure had failed to fall more in the renal denervation group than in the sham denervation group by a margin of at least 5 mm Hg, the primary efficacy end point of the trial.
- Methodologic and technical shortcomings may explain the negative results of the SYMPLICITY HTN-3 trial, but most device manufacturers have put the brakes on future research into this novel therapy.
- Today, renal denervation is not available in the United States but is available for routine care in Europe and Australia.
Renal denervation: Are we on the right path?
When renal sympathetic denervation, an endovascular procedure designed to treat resistant hypertension, failed to meet its efficacy goal in the SYMPLICITY HTN-3 trial,1 the news was disappointing.
In this issue of the Cleveland Clinic Journal of Medicine, Shishehbor et al2 provide a critical review of the findings of that trial and summarize its intricacies, as well as the results of other important trials of renal denervation therapy for hypertension. To their excellent observations, we would like to add some of our own.
HYPERTENSION: COMMON, OFTEN RESISTANT
The worldwide prevalence of hypertension is increasing. In the year 2000, about 26% of the adult world population had hypertension; by the year 2025, the number is projected to rise to 29%—1.56 billion people.3
Only about 50% of patients with hypertension are treated for it and, of those, about half have it adequately controlled. In one report, about 30% of US patients with hypertension had adequate blood pressure control.4
Patients who have uncontrolled hypertension are usually older and more obese, have higher baseline blood pressure and excessive salt intake, and are more likely to have chronic kidney disease, diabetes, obstructive sleep apnea, and aldosterone excess.5 Many of these conditions are also associated with increased sympathetic nervous system activity.6
Resistance and pseudoresistance
But lack of control of blood pressure is not the same as resistant hypertension. It is important to differentiate resistant hypertension from pseudoresistant hypertension, ie, hypertension that only seems to be resistant.7 Resistant hypertension affects 12.8% of all drug-treated hypertensive patients in the United States, according to data from the National Health and Nutrition Examination Survey.8
Factors that can cause pseudoresistant hypertension include:
Suboptimal antihypertensive regimens (truly resistant hypertension means blood pressure that remains high despite concurrent treatment with 3 antihypertensive drugs of different classes, 1 of which is a diuretic, in maximal doses)
The white coat effect (higher blood pressure in the office than at home, presumably due to the stress of an office visit)
- Suboptimal blood pressure measurement techniques (eg, use of a cuff that is too small, causing falsely high readings)
- Physician inertia (eg, failure to change a regimen that is not working)
- Lifestyle factors (eg, excessive sodium intake)
- Medications that interfere with blood pressure control (eg, nonsteroidal anti-inflammatory drugs)
- Poor adherence to prescribed medications.
Causes of secondary hypertension such as obstructive sleep apnea, primary aldosteronism, and renal artery stenosis should also be ruled out before concluding that a patient has resistant hypertension.
Treatment prevents complications
Hypertension causes a myriad of medical diseases, including accelerated atherosclerosis, myocardial ischemia and infarction, both systolic and diastolic heart failure, rhythm problems (eg, atrial fibrillation), and stroke.
Most patients with resistant hypertension have no identifiable reversible causes of it, exhibit increased sympathetic nervous system activity, and have increased risk of cardiovascular events. The risk can be reduced by treatment.9,10
Adequate and sustained treatment of hypertension prevents and mitigates its complications. The classic Veterans Administration Cooperative Study in the 1960s demonstrated a 96% reduction in cardiovascular events over 18 months with the use of 3 antihypertensive medications in patients with severe hypertension.11 A reduction of as little as 2 mm Hg in the mean blood pressure has been associated with a 10% reduction in the risk of stroke mortality and a 7% decrease in ischemic heart disease mortality.12 This is an important consideration when evaluating the clinical end points of hypertension trials.
SYMPLICITY HTN-3 TRIAL: WHAT DID WE LEARN?
As controlling blood pressure is paramount in reducing cardiovascular complications, it is only natural to look for innovative strategies to supplement the medical treatments of hypertension.
The multicenter SYMPLICITY HTN-3 trial1 was undertaken to establish the efficacy of renal-artery denervation using radiofrequency energy delivered by a catheter-based system (Symplicity RDN, Medtronic, Dublin, Ireland). This randomized, sham-controlled, blinded study did not show a benefit from this procedure with respect to either of its efficacy end points—at 6 months, a reduction in office systolic blood pressure of at least 5 mm Hg more than with medical therapy alone, or a reduction in mean ambulatory systolic pressure of at least 2 mm Hg more than with medical therapy alone.
Despite the negative results, this medium-size (N = 535) randomized clinical trial still represents the highest-level evidence in the field, and we ought to learn something from it.
Limitations of SYMPLICITY HTN-3
Several factors may have contributed to the negative results of the trial.
Patient selection. For the most part, patients enrolled in renal denervation trials, including SYMPLICITY HTN-3, were not selected on the basis of heightened sympathetic nervous system activity. Assessment of sympathetic nervous system activity may identify the population most likely to achieve an adequate response.
Of note, the baseline blood pressure readings of patients in this trial were higher in the office than on ambulatory monitoring. Patients with white coat hypertension have increased sympathetic nervous system activity and thus might actually be good candidates for renal denervation therapy.
Adequacy of ablation was not measured. Many argue that an objective measure of the adequacy of the denervation procedure (qualitative or quantitative) should have been implemented and, if it had been, the results might have been different. For example, when ablation is performed in the main renal artery as well as the branches, the efficacy in reducing levels of norepinephrine is improved.13
Blood pressure fell in both groups. In SYMPLICITY HTN-3 and many other renal denervation trials, patients were assessed using both office and ambulatory blood pressure measurements. The primary end point was the office blood pressure measurement, with a 5-mm Hg difference in reduction chosen to define the superiority margin. This margin was chosen because even small reductions in blood pressure are known to decrease adverse events caused by hypertension. Notably, blood pressure fell significantly in both the control and intervention groups, with an intergroup difference of 2.39 mm Hg (not statistically significant) in favor of denervation.
Medication questions. The SYMPLICITY HTN-3 patients were supposed to be on stable medical regimens with maximal tolerated doses before the procedure. However, it was difficult to assess patients’ adherence to and tolerance of medical therapies. Many (about 40%) of the patients had their medications changed during the study.1
Therefore, a critical look at the study enrollment criteria may shed more light on the reasons for the negative findings. Did these patients truly have resistant hypertension? Before they underwent the treatment, was their prestudy pharmacologic regimen adequately intensified?
ONGOING STUDIES
After the findings of the SYMPLICITY HTN-3 study were released, several other trials—such as the Renal Denervation for Hypertension (DENERHTN)14 and Prague-15 trials15—reported conflicting results. Notably, these were not sham-controlled trials.
Newer studies with robust trial designs are ongoing. A quick search of www.clinicaltrials.gov reveals that at least 89 active clinical trials of renal denervation are registered as of the date of this writing. Excluding those with unknown status, there are 63 trials open or ongoing.
Clinical trials are also ongoing to determine the effects of renal denervation in patients with heart failure, atrial fibrillation, sleep apnea, and chronic kidney disease, all of which are known to involve heightened sympathetic nervous system activity.
NOT READY FOR CLINICAL USE
Although nonpharmacologic treatments of hypertension continue to be studied and are supported by an avalanche of trials in animals and small, mostly nonrandomized trials in humans, one should not forget that the SYMPLICITY HTN-3 trial simply did not meet its primary efficacy end points. We need definitive clinical evidence showing that renal denervation reduces either blood pressure or clinical events before it becomes a mainstream therapy in humans.
Additional trials are being conducted that were designed in accordance with the recommendations of the European Clinical Consensus Conference for Renal Denervation16 in terms of study population, design, and end points. Well-designed studies that conform to those recommendations are critical.
Finally, although our enthusiasm for renal denervation as a treatment of hypertension is tempered, there have been no noteworthy safety concerns related to the procedure, which certainly helps maintain the research momentum in this field.
- Bhatt DL, Kandzari DE, O’Neill WW, et al; SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014; 370:1393–1401.
- Shishehbor MH, Hammad TA, Thomas G. Renal denervation: what happened, and why? Cleve Clin J Med 2017; 84:681–686.
- Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 2005; 365:217–223.
- Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Worldwide prevalence of hypertension: a systematic review. J Hypertens 2004; 22:11–19.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117:e510–e526.
- Tsioufis C, Papademetriou V, Thomopoulos C, Stefanadis C. Renal denervation for sleep apnea and resistant hypertension: alternative or complementary to effective continuous positive airway pressure treatment? Hypertension 2011; 58:e191–e192.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research.Hypertension 2008; 51:1403–1419.
- Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension 2011; 57:1076–1080.
- Papademetriou V, Doumas M, Tsioufis K. Renal sympathetic denervation for the treatment of difficult-to-control or resistant hypertension. Int J Hypertens 2011; 2011:196518.
- Doumas M, Faselis C, Papademetriou V. Renal sympathetic denervation in hypertension. Curr Opin Nephrol Hypertens 2011; 20:647–653.
- Veterans Administration Cooperative Study Group on Antihypertensive Agents. Effect of treatment on morbidity in hypertension: results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA 1967; 202:1028–1034.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Henegar JR, Zhang Y, Hata C, Narciso I, Hall ME, Hall JE. Catheter-based radiofrequency renal denervation: location effects on renal norepinephrine. Am J Hypertens 2015; 28:909–914.
- Azizi M, Sapoval M, Gosse P, et al; Renal Denervation for Hypertension (DENERHTN) investigators. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet 2015; 385:1957–1965.
- Rosa J, Widimsky P, Waldauf P, et al. Role of adding spironolactone and renal denervation in true resistant hypertension: one-year outcomes of randomized PRAGUE-15 study. Hypertension 2016; 67:397–403.
- Mahfoud F, Bohm M, Azizi M, et al. Proceedings from the European Clinical Consensus Conference for Renal Denervation: Considerations on Future Clinical Trial Design. Eur Heart J 2015; 6:2219–2227.
When renal sympathetic denervation, an endovascular procedure designed to treat resistant hypertension, failed to meet its efficacy goal in the SYMPLICITY HTN-3 trial,1 the news was disappointing.
In this issue of the Cleveland Clinic Journal of Medicine, Shishehbor et al2 provide a critical review of the findings of that trial and summarize its intricacies, as well as the results of other important trials of renal denervation therapy for hypertension. To their excellent observations, we would like to add some of our own.
HYPERTENSION: COMMON, OFTEN RESISTANT
The worldwide prevalence of hypertension is increasing. In the year 2000, about 26% of the adult world population had hypertension; by the year 2025, the number is projected to rise to 29%—1.56 billion people.3
Only about 50% of patients with hypertension are treated for it and, of those, about half have it adequately controlled. In one report, about 30% of US patients with hypertension had adequate blood pressure control.4
Patients who have uncontrolled hypertension are usually older and more obese, have higher baseline blood pressure and excessive salt intake, and are more likely to have chronic kidney disease, diabetes, obstructive sleep apnea, and aldosterone excess.5 Many of these conditions are also associated with increased sympathetic nervous system activity.6
Resistance and pseudoresistance
But lack of control of blood pressure is not the same as resistant hypertension. It is important to differentiate resistant hypertension from pseudoresistant hypertension, ie, hypertension that only seems to be resistant.7 Resistant hypertension affects 12.8% of all drug-treated hypertensive patients in the United States, according to data from the National Health and Nutrition Examination Survey.8
Factors that can cause pseudoresistant hypertension include:
Suboptimal antihypertensive regimens (truly resistant hypertension means blood pressure that remains high despite concurrent treatment with 3 antihypertensive drugs of different classes, 1 of which is a diuretic, in maximal doses)
The white coat effect (higher blood pressure in the office than at home, presumably due to the stress of an office visit)
- Suboptimal blood pressure measurement techniques (eg, use of a cuff that is too small, causing falsely high readings)
- Physician inertia (eg, failure to change a regimen that is not working)
- Lifestyle factors (eg, excessive sodium intake)
- Medications that interfere with blood pressure control (eg, nonsteroidal anti-inflammatory drugs)
- Poor adherence to prescribed medications.
Causes of secondary hypertension such as obstructive sleep apnea, primary aldosteronism, and renal artery stenosis should also be ruled out before concluding that a patient has resistant hypertension.
Treatment prevents complications
Hypertension causes a myriad of medical diseases, including accelerated atherosclerosis, myocardial ischemia and infarction, both systolic and diastolic heart failure, rhythm problems (eg, atrial fibrillation), and stroke.
Most patients with resistant hypertension have no identifiable reversible causes of it, exhibit increased sympathetic nervous system activity, and have increased risk of cardiovascular events. The risk can be reduced by treatment.9,10
Adequate and sustained treatment of hypertension prevents and mitigates its complications. The classic Veterans Administration Cooperative Study in the 1960s demonstrated a 96% reduction in cardiovascular events over 18 months with the use of 3 antihypertensive medications in patients with severe hypertension.11 A reduction of as little as 2 mm Hg in the mean blood pressure has been associated with a 10% reduction in the risk of stroke mortality and a 7% decrease in ischemic heart disease mortality.12 This is an important consideration when evaluating the clinical end points of hypertension trials.
SYMPLICITY HTN-3 TRIAL: WHAT DID WE LEARN?
As controlling blood pressure is paramount in reducing cardiovascular complications, it is only natural to look for innovative strategies to supplement the medical treatments of hypertension.
The multicenter SYMPLICITY HTN-3 trial1 was undertaken to establish the efficacy of renal-artery denervation using radiofrequency energy delivered by a catheter-based system (Symplicity RDN, Medtronic, Dublin, Ireland). This randomized, sham-controlled, blinded study did not show a benefit from this procedure with respect to either of its efficacy end points—at 6 months, a reduction in office systolic blood pressure of at least 5 mm Hg more than with medical therapy alone, or a reduction in mean ambulatory systolic pressure of at least 2 mm Hg more than with medical therapy alone.
Despite the negative results, this medium-size (N = 535) randomized clinical trial still represents the highest-level evidence in the field, and we ought to learn something from it.
Limitations of SYMPLICITY HTN-3
Several factors may have contributed to the negative results of the trial.
Patient selection. For the most part, patients enrolled in renal denervation trials, including SYMPLICITY HTN-3, were not selected on the basis of heightened sympathetic nervous system activity. Assessment of sympathetic nervous system activity may identify the population most likely to achieve an adequate response.
Of note, the baseline blood pressure readings of patients in this trial were higher in the office than on ambulatory monitoring. Patients with white coat hypertension have increased sympathetic nervous system activity and thus might actually be good candidates for renal denervation therapy.
Adequacy of ablation was not measured. Many argue that an objective measure of the adequacy of the denervation procedure (qualitative or quantitative) should have been implemented and, if it had been, the results might have been different. For example, when ablation is performed in the main renal artery as well as the branches, the efficacy in reducing levels of norepinephrine is improved.13
Blood pressure fell in both groups. In SYMPLICITY HTN-3 and many other renal denervation trials, patients were assessed using both office and ambulatory blood pressure measurements. The primary end point was the office blood pressure measurement, with a 5-mm Hg difference in reduction chosen to define the superiority margin. This margin was chosen because even small reductions in blood pressure are known to decrease adverse events caused by hypertension. Notably, blood pressure fell significantly in both the control and intervention groups, with an intergroup difference of 2.39 mm Hg (not statistically significant) in favor of denervation.
Medication questions. The SYMPLICITY HTN-3 patients were supposed to be on stable medical regimens with maximal tolerated doses before the procedure. However, it was difficult to assess patients’ adherence to and tolerance of medical therapies. Many (about 40%) of the patients had their medications changed during the study.1
Therefore, a critical look at the study enrollment criteria may shed more light on the reasons for the negative findings. Did these patients truly have resistant hypertension? Before they underwent the treatment, was their prestudy pharmacologic regimen adequately intensified?
ONGOING STUDIES
After the findings of the SYMPLICITY HTN-3 study were released, several other trials—such as the Renal Denervation for Hypertension (DENERHTN)14 and Prague-15 trials15—reported conflicting results. Notably, these were not sham-controlled trials.
Newer studies with robust trial designs are ongoing. A quick search of www.clinicaltrials.gov reveals that at least 89 active clinical trials of renal denervation are registered as of the date of this writing. Excluding those with unknown status, there are 63 trials open or ongoing.
Clinical trials are also ongoing to determine the effects of renal denervation in patients with heart failure, atrial fibrillation, sleep apnea, and chronic kidney disease, all of which are known to involve heightened sympathetic nervous system activity.
NOT READY FOR CLINICAL USE
Although nonpharmacologic treatments of hypertension continue to be studied and are supported by an avalanche of trials in animals and small, mostly nonrandomized trials in humans, one should not forget that the SYMPLICITY HTN-3 trial simply did not meet its primary efficacy end points. We need definitive clinical evidence showing that renal denervation reduces either blood pressure or clinical events before it becomes a mainstream therapy in humans.
Additional trials are being conducted that were designed in accordance with the recommendations of the European Clinical Consensus Conference for Renal Denervation16 in terms of study population, design, and end points. Well-designed studies that conform to those recommendations are critical.
Finally, although our enthusiasm for renal denervation as a treatment of hypertension is tempered, there have been no noteworthy safety concerns related to the procedure, which certainly helps maintain the research momentum in this field.
When renal sympathetic denervation, an endovascular procedure designed to treat resistant hypertension, failed to meet its efficacy goal in the SYMPLICITY HTN-3 trial,1 the news was disappointing.
In this issue of the Cleveland Clinic Journal of Medicine, Shishehbor et al2 provide a critical review of the findings of that trial and summarize its intricacies, as well as the results of other important trials of renal denervation therapy for hypertension. To their excellent observations, we would like to add some of our own.
HYPERTENSION: COMMON, OFTEN RESISTANT
The worldwide prevalence of hypertension is increasing. In the year 2000, about 26% of the adult world population had hypertension; by the year 2025, the number is projected to rise to 29%—1.56 billion people.3
Only about 50% of patients with hypertension are treated for it and, of those, about half have it adequately controlled. In one report, about 30% of US patients with hypertension had adequate blood pressure control.4
Patients who have uncontrolled hypertension are usually older and more obese, have higher baseline blood pressure and excessive salt intake, and are more likely to have chronic kidney disease, diabetes, obstructive sleep apnea, and aldosterone excess.5 Many of these conditions are also associated with increased sympathetic nervous system activity.6
Resistance and pseudoresistance
But lack of control of blood pressure is not the same as resistant hypertension. It is important to differentiate resistant hypertension from pseudoresistant hypertension, ie, hypertension that only seems to be resistant.7 Resistant hypertension affects 12.8% of all drug-treated hypertensive patients in the United States, according to data from the National Health and Nutrition Examination Survey.8
Factors that can cause pseudoresistant hypertension include:
Suboptimal antihypertensive regimens (truly resistant hypertension means blood pressure that remains high despite concurrent treatment with 3 antihypertensive drugs of different classes, 1 of which is a diuretic, in maximal doses)
The white coat effect (higher blood pressure in the office than at home, presumably due to the stress of an office visit)
- Suboptimal blood pressure measurement techniques (eg, use of a cuff that is too small, causing falsely high readings)
- Physician inertia (eg, failure to change a regimen that is not working)
- Lifestyle factors (eg, excessive sodium intake)
- Medications that interfere with blood pressure control (eg, nonsteroidal anti-inflammatory drugs)
- Poor adherence to prescribed medications.
Causes of secondary hypertension such as obstructive sleep apnea, primary aldosteronism, and renal artery stenosis should also be ruled out before concluding that a patient has resistant hypertension.
Treatment prevents complications
Hypertension causes a myriad of medical diseases, including accelerated atherosclerosis, myocardial ischemia and infarction, both systolic and diastolic heart failure, rhythm problems (eg, atrial fibrillation), and stroke.
Most patients with resistant hypertension have no identifiable reversible causes of it, exhibit increased sympathetic nervous system activity, and have increased risk of cardiovascular events. The risk can be reduced by treatment.9,10
Adequate and sustained treatment of hypertension prevents and mitigates its complications. The classic Veterans Administration Cooperative Study in the 1960s demonstrated a 96% reduction in cardiovascular events over 18 months with the use of 3 antihypertensive medications in patients with severe hypertension.11 A reduction of as little as 2 mm Hg in the mean blood pressure has been associated with a 10% reduction in the risk of stroke mortality and a 7% decrease in ischemic heart disease mortality.12 This is an important consideration when evaluating the clinical end points of hypertension trials.
SYMPLICITY HTN-3 TRIAL: WHAT DID WE LEARN?
As controlling blood pressure is paramount in reducing cardiovascular complications, it is only natural to look for innovative strategies to supplement the medical treatments of hypertension.
The multicenter SYMPLICITY HTN-3 trial1 was undertaken to establish the efficacy of renal-artery denervation using radiofrequency energy delivered by a catheter-based system (Symplicity RDN, Medtronic, Dublin, Ireland). This randomized, sham-controlled, blinded study did not show a benefit from this procedure with respect to either of its efficacy end points—at 6 months, a reduction in office systolic blood pressure of at least 5 mm Hg more than with medical therapy alone, or a reduction in mean ambulatory systolic pressure of at least 2 mm Hg more than with medical therapy alone.
Despite the negative results, this medium-size (N = 535) randomized clinical trial still represents the highest-level evidence in the field, and we ought to learn something from it.
Limitations of SYMPLICITY HTN-3
Several factors may have contributed to the negative results of the trial.
Patient selection. For the most part, patients enrolled in renal denervation trials, including SYMPLICITY HTN-3, were not selected on the basis of heightened sympathetic nervous system activity. Assessment of sympathetic nervous system activity may identify the population most likely to achieve an adequate response.
Of note, the baseline blood pressure readings of patients in this trial were higher in the office than on ambulatory monitoring. Patients with white coat hypertension have increased sympathetic nervous system activity and thus might actually be good candidates for renal denervation therapy.
Adequacy of ablation was not measured. Many argue that an objective measure of the adequacy of the denervation procedure (qualitative or quantitative) should have been implemented and, if it had been, the results might have been different. For example, when ablation is performed in the main renal artery as well as the branches, the efficacy in reducing levels of norepinephrine is improved.13
Blood pressure fell in both groups. In SYMPLICITY HTN-3 and many other renal denervation trials, patients were assessed using both office and ambulatory blood pressure measurements. The primary end point was the office blood pressure measurement, with a 5-mm Hg difference in reduction chosen to define the superiority margin. This margin was chosen because even small reductions in blood pressure are known to decrease adverse events caused by hypertension. Notably, blood pressure fell significantly in both the control and intervention groups, with an intergroup difference of 2.39 mm Hg (not statistically significant) in favor of denervation.
Medication questions. The SYMPLICITY HTN-3 patients were supposed to be on stable medical regimens with maximal tolerated doses before the procedure. However, it was difficult to assess patients’ adherence to and tolerance of medical therapies. Many (about 40%) of the patients had their medications changed during the study.1
Therefore, a critical look at the study enrollment criteria may shed more light on the reasons for the negative findings. Did these patients truly have resistant hypertension? Before they underwent the treatment, was their prestudy pharmacologic regimen adequately intensified?
ONGOING STUDIES
After the findings of the SYMPLICITY HTN-3 study were released, several other trials—such as the Renal Denervation for Hypertension (DENERHTN)14 and Prague-15 trials15—reported conflicting results. Notably, these were not sham-controlled trials.
Newer studies with robust trial designs are ongoing. A quick search of www.clinicaltrials.gov reveals that at least 89 active clinical trials of renal denervation are registered as of the date of this writing. Excluding those with unknown status, there are 63 trials open or ongoing.
Clinical trials are also ongoing to determine the effects of renal denervation in patients with heart failure, atrial fibrillation, sleep apnea, and chronic kidney disease, all of which are known to involve heightened sympathetic nervous system activity.
NOT READY FOR CLINICAL USE
Although nonpharmacologic treatments of hypertension continue to be studied and are supported by an avalanche of trials in animals and small, mostly nonrandomized trials in humans, one should not forget that the SYMPLICITY HTN-3 trial simply did not meet its primary efficacy end points. We need definitive clinical evidence showing that renal denervation reduces either blood pressure or clinical events before it becomes a mainstream therapy in humans.
Additional trials are being conducted that were designed in accordance with the recommendations of the European Clinical Consensus Conference for Renal Denervation16 in terms of study population, design, and end points. Well-designed studies that conform to those recommendations are critical.
Finally, although our enthusiasm for renal denervation as a treatment of hypertension is tempered, there have been no noteworthy safety concerns related to the procedure, which certainly helps maintain the research momentum in this field.
- Bhatt DL, Kandzari DE, O’Neill WW, et al; SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014; 370:1393–1401.
- Shishehbor MH, Hammad TA, Thomas G. Renal denervation: what happened, and why? Cleve Clin J Med 2017; 84:681–686.
- Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 2005; 365:217–223.
- Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Worldwide prevalence of hypertension: a systematic review. J Hypertens 2004; 22:11–19.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117:e510–e526.
- Tsioufis C, Papademetriou V, Thomopoulos C, Stefanadis C. Renal denervation for sleep apnea and resistant hypertension: alternative or complementary to effective continuous positive airway pressure treatment? Hypertension 2011; 58:e191–e192.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research.Hypertension 2008; 51:1403–1419.
- Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension 2011; 57:1076–1080.
- Papademetriou V, Doumas M, Tsioufis K. Renal sympathetic denervation for the treatment of difficult-to-control or resistant hypertension. Int J Hypertens 2011; 2011:196518.
- Doumas M, Faselis C, Papademetriou V. Renal sympathetic denervation in hypertension. Curr Opin Nephrol Hypertens 2011; 20:647–653.
- Veterans Administration Cooperative Study Group on Antihypertensive Agents. Effect of treatment on morbidity in hypertension: results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA 1967; 202:1028–1034.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Henegar JR, Zhang Y, Hata C, Narciso I, Hall ME, Hall JE. Catheter-based radiofrequency renal denervation: location effects on renal norepinephrine. Am J Hypertens 2015; 28:909–914.
- Azizi M, Sapoval M, Gosse P, et al; Renal Denervation for Hypertension (DENERHTN) investigators. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet 2015; 385:1957–1965.
- Rosa J, Widimsky P, Waldauf P, et al. Role of adding spironolactone and renal denervation in true resistant hypertension: one-year outcomes of randomized PRAGUE-15 study. Hypertension 2016; 67:397–403.
- Mahfoud F, Bohm M, Azizi M, et al. Proceedings from the European Clinical Consensus Conference for Renal Denervation: Considerations on Future Clinical Trial Design. Eur Heart J 2015; 6:2219–2227.
- Bhatt DL, Kandzari DE, O’Neill WW, et al; SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014; 370:1393–1401.
- Shishehbor MH, Hammad TA, Thomas G. Renal denervation: what happened, and why? Cleve Clin J Med 2017; 84:681–686.
- Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 2005; 365:217–223.
- Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Worldwide prevalence of hypertension: a systematic review. J Hypertens 2004; 22:11–19.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117:e510–e526.
- Tsioufis C, Papademetriou V, Thomopoulos C, Stefanadis C. Renal denervation for sleep apnea and resistant hypertension: alternative or complementary to effective continuous positive airway pressure treatment? Hypertension 2011; 58:e191–e192.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research.Hypertension 2008; 51:1403–1419.
- Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension 2011; 57:1076–1080.
- Papademetriou V, Doumas M, Tsioufis K. Renal sympathetic denervation for the treatment of difficult-to-control or resistant hypertension. Int J Hypertens 2011; 2011:196518.
- Doumas M, Faselis C, Papademetriou V. Renal sympathetic denervation in hypertension. Curr Opin Nephrol Hypertens 2011; 20:647–653.
- Veterans Administration Cooperative Study Group on Antihypertensive Agents. Effect of treatment on morbidity in hypertension: results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA 1967; 202:1028–1034.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Henegar JR, Zhang Y, Hata C, Narciso I, Hall ME, Hall JE. Catheter-based radiofrequency renal denervation: location effects on renal norepinephrine. Am J Hypertens 2015; 28:909–914.
- Azizi M, Sapoval M, Gosse P, et al; Renal Denervation for Hypertension (DENERHTN) investigators. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet 2015; 385:1957–1965.
- Rosa J, Widimsky P, Waldauf P, et al. Role of adding spironolactone and renal denervation in true resistant hypertension: one-year outcomes of randomized PRAGUE-15 study. Hypertension 2016; 67:397–403.
- Mahfoud F, Bohm M, Azizi M, et al. Proceedings from the European Clinical Consensus Conference for Renal Denervation: Considerations on Future Clinical Trial Design. Eur Heart J 2015; 6:2219–2227.