User login
Practice Makes Perfect?
It is human nature to practice things that we are already good at doing. If you’re a golfer, then you know what I’m talking about. I hit the driver over and over again on the range, but never practice hitting the bad lie in the bunker, or the half-swing wedge from a tight lie. I sink hundreds of 3 footers, but can’t putt into this range from 50 feet. I’ve gotten much better at golf since I started playing, but my scores have hardly gone down.
I think a similar thing happens in our orthopedic practices. I read everything I can on the anterior cruciate ligament, yet I already feel comfortable with my reconstruction technique. I skim, or avoid reading altogether, articles about topics I don’t like to treat, like the hand or spine. Yet, I still see these things every day in my practice and on call. If my depth of knowledge in these areas was as good as it is in sports medicine, I could provide better, more immediate care to my patients, rather than refer them to subspecialists.
A perfect orthopedic example would be the patellofemoral joint. One of the least enjoyable patient encounters for me is the young adult with normal alignment and intractable anterior knee pain that does not respond to nonoperative treatment. I’m concerned any surgical intervention may make them worse and I’m often left without much to offer the patient.
It’s for this reason AJO has partnered with Dr. Jack Farr to produce the patellofemoral issue; to provide a comprehensive guide to the latest thinking in the treatment of patellofemoral disorders (see the March/April 2017 issue). We solicited so much outstanding content, that a single issue could not hold all of the articles. In this issue, our patellofemoral series continues with 3 outstanding articles. Magnussen presents "Patella Alta Sees You, Do You See It?" and Hinckel and colleagues have authored a guide to patellofemoral cartilage restoration. Unal and colleagues follow-up with a review of the lateral retinaculum.
In our "Codes to Know" section, we reexamine diagnostic arthroscopy, a code most of us have billed infrequently. New technologies, however, have made it possible to peer into the joint in the office, and McMillan and colleagues teach us how to make it economically feasible, even for employed physicians.
Finally, we have a number of great articles on difficult problems—the stiff elbow, complex distal radius fractures, and intraoperative acetabular fractures during total hip arthroplasty.
Please enjoy this issue and think about what topics you tend to shy away from. I’m willing to bet you can add the most to your practice by studying up on these topics. As always, please provide your feedback to our editorial team so that we can continue to make improvements to our journal. We envision a change in the way orthopedists utilize a journal in their practice, and are continuously looking for ways to make AJO a more relevant tool for improving your patient care and workflow. We are working hard to give our readers the journal they deserve, but in my spare time, I’ll be brushing up on trochleoplasties and half-swing wedges.
It is human nature to practice things that we are already good at doing. If you’re a golfer, then you know what I’m talking about. I hit the driver over and over again on the range, but never practice hitting the bad lie in the bunker, or the half-swing wedge from a tight lie. I sink hundreds of 3 footers, but can’t putt into this range from 50 feet. I’ve gotten much better at golf since I started playing, but my scores have hardly gone down.
I think a similar thing happens in our orthopedic practices. I read everything I can on the anterior cruciate ligament, yet I already feel comfortable with my reconstruction technique. I skim, or avoid reading altogether, articles about topics I don’t like to treat, like the hand or spine. Yet, I still see these things every day in my practice and on call. If my depth of knowledge in these areas was as good as it is in sports medicine, I could provide better, more immediate care to my patients, rather than refer them to subspecialists.
A perfect orthopedic example would be the patellofemoral joint. One of the least enjoyable patient encounters for me is the young adult with normal alignment and intractable anterior knee pain that does not respond to nonoperative treatment. I’m concerned any surgical intervention may make them worse and I’m often left without much to offer the patient.
It’s for this reason AJO has partnered with Dr. Jack Farr to produce the patellofemoral issue; to provide a comprehensive guide to the latest thinking in the treatment of patellofemoral disorders (see the March/April 2017 issue). We solicited so much outstanding content, that a single issue could not hold all of the articles. In this issue, our patellofemoral series continues with 3 outstanding articles. Magnussen presents "Patella Alta Sees You, Do You See It?" and Hinckel and colleagues have authored a guide to patellofemoral cartilage restoration. Unal and colleagues follow-up with a review of the lateral retinaculum.
In our "Codes to Know" section, we reexamine diagnostic arthroscopy, a code most of us have billed infrequently. New technologies, however, have made it possible to peer into the joint in the office, and McMillan and colleagues teach us how to make it economically feasible, even for employed physicians.
Finally, we have a number of great articles on difficult problems—the stiff elbow, complex distal radius fractures, and intraoperative acetabular fractures during total hip arthroplasty.
Please enjoy this issue and think about what topics you tend to shy away from. I’m willing to bet you can add the most to your practice by studying up on these topics. As always, please provide your feedback to our editorial team so that we can continue to make improvements to our journal. We envision a change in the way orthopedists utilize a journal in their practice, and are continuously looking for ways to make AJO a more relevant tool for improving your patient care and workflow. We are working hard to give our readers the journal they deserve, but in my spare time, I’ll be brushing up on trochleoplasties and half-swing wedges.
It is human nature to practice things that we are already good at doing. If you’re a golfer, then you know what I’m talking about. I hit the driver over and over again on the range, but never practice hitting the bad lie in the bunker, or the half-swing wedge from a tight lie. I sink hundreds of 3 footers, but can’t putt into this range from 50 feet. I’ve gotten much better at golf since I started playing, but my scores have hardly gone down.
I think a similar thing happens in our orthopedic practices. I read everything I can on the anterior cruciate ligament, yet I already feel comfortable with my reconstruction technique. I skim, or avoid reading altogether, articles about topics I don’t like to treat, like the hand or spine. Yet, I still see these things every day in my practice and on call. If my depth of knowledge in these areas was as good as it is in sports medicine, I could provide better, more immediate care to my patients, rather than refer them to subspecialists.
A perfect orthopedic example would be the patellofemoral joint. One of the least enjoyable patient encounters for me is the young adult with normal alignment and intractable anterior knee pain that does not respond to nonoperative treatment. I’m concerned any surgical intervention may make them worse and I’m often left without much to offer the patient.
It’s for this reason AJO has partnered with Dr. Jack Farr to produce the patellofemoral issue; to provide a comprehensive guide to the latest thinking in the treatment of patellofemoral disorders (see the March/April 2017 issue). We solicited so much outstanding content, that a single issue could not hold all of the articles. In this issue, our patellofemoral series continues with 3 outstanding articles. Magnussen presents "Patella Alta Sees You, Do You See It?" and Hinckel and colleagues have authored a guide to patellofemoral cartilage restoration. Unal and colleagues follow-up with a review of the lateral retinaculum.
In our "Codes to Know" section, we reexamine diagnostic arthroscopy, a code most of us have billed infrequently. New technologies, however, have made it possible to peer into the joint in the office, and McMillan and colleagues teach us how to make it economically feasible, even for employed physicians.
Finally, we have a number of great articles on difficult problems—the stiff elbow, complex distal radius fractures, and intraoperative acetabular fractures during total hip arthroplasty.
Please enjoy this issue and think about what topics you tend to shy away from. I’m willing to bet you can add the most to your practice by studying up on these topics. As always, please provide your feedback to our editorial team so that we can continue to make improvements to our journal. We envision a change in the way orthopedists utilize a journal in their practice, and are continuously looking for ways to make AJO a more relevant tool for improving your patient care and workflow. We are working hard to give our readers the journal they deserve, but in my spare time, I’ll be brushing up on trochleoplasties and half-swing wedges.
Prediction tool for mortality after respiratory compromise
Background: Scoring systems exist to predict outcomes following cardiac arrest. There is currently no reliable model to predict outcome of patients who have survived acute respiratory compromise (ARC).
Study Design: A retrospective cohort study.
Setting: Get with the Guidelines Resuscitation (GWTG-R) is an online medical registry that tracks ARC data from more than 300 hospitals.
Synopsis: Using the GWTG-R database of ARC, researchers identified 13,193 cases of ARC to study the variables affecting prognosis. They randomized the group into derivation (75% of patients) and validation (25% of patients) cohorts and used c-statistics to create the prognostic scoring system. The greatest predictors of in-hospital mortality were age greater than 80 years, hypotension in the four hours preceding the ARC event, and the need for intubation.
This scoring system did not take into account any comorbidities (such as organ failure) that occurred shortly after the ARC event, although these likely affect mortality.
Bottom Line: Predicting in-hospital mortality for survivors of ARC events may help clinical prognostication. Such tools could also facilitate comparisons between hospitals and guide quality improvement projects.
Citation: Moskowitz A, Anderson LW, Karlsson M, et. al. Predicting in-hospital mortality for initial survivors of acute respiratory compromise (ARC) events: Development and validation of the ARC score. Resuscitation. 2017 Jun;115:5-10.
Dr. Suman is clinical instructor of medicine in the University of Kentucky division of hospital medicine.
Background: Scoring systems exist to predict outcomes following cardiac arrest. There is currently no reliable model to predict outcome of patients who have survived acute respiratory compromise (ARC).
Study Design: A retrospective cohort study.
Setting: Get with the Guidelines Resuscitation (GWTG-R) is an online medical registry that tracks ARC data from more than 300 hospitals.
Synopsis: Using the GWTG-R database of ARC, researchers identified 13,193 cases of ARC to study the variables affecting prognosis. They randomized the group into derivation (75% of patients) and validation (25% of patients) cohorts and used c-statistics to create the prognostic scoring system. The greatest predictors of in-hospital mortality were age greater than 80 years, hypotension in the four hours preceding the ARC event, and the need for intubation.
This scoring system did not take into account any comorbidities (such as organ failure) that occurred shortly after the ARC event, although these likely affect mortality.
Bottom Line: Predicting in-hospital mortality for survivors of ARC events may help clinical prognostication. Such tools could also facilitate comparisons between hospitals and guide quality improvement projects.
Citation: Moskowitz A, Anderson LW, Karlsson M, et. al. Predicting in-hospital mortality for initial survivors of acute respiratory compromise (ARC) events: Development and validation of the ARC score. Resuscitation. 2017 Jun;115:5-10.
Dr. Suman is clinical instructor of medicine in the University of Kentucky division of hospital medicine.
Background: Scoring systems exist to predict outcomes following cardiac arrest. There is currently no reliable model to predict outcome of patients who have survived acute respiratory compromise (ARC).
Study Design: A retrospective cohort study.
Setting: Get with the Guidelines Resuscitation (GWTG-R) is an online medical registry that tracks ARC data from more than 300 hospitals.
Synopsis: Using the GWTG-R database of ARC, researchers identified 13,193 cases of ARC to study the variables affecting prognosis. They randomized the group into derivation (75% of patients) and validation (25% of patients) cohorts and used c-statistics to create the prognostic scoring system. The greatest predictors of in-hospital mortality were age greater than 80 years, hypotension in the four hours preceding the ARC event, and the need for intubation.
This scoring system did not take into account any comorbidities (such as organ failure) that occurred shortly after the ARC event, although these likely affect mortality.
Bottom Line: Predicting in-hospital mortality for survivors of ARC events may help clinical prognostication. Such tools could also facilitate comparisons between hospitals and guide quality improvement projects.
Citation: Moskowitz A, Anderson LW, Karlsson M, et. al. Predicting in-hospital mortality for initial survivors of acute respiratory compromise (ARC) events: Development and validation of the ARC score. Resuscitation. 2017 Jun;115:5-10.
Dr. Suman is clinical instructor of medicine in the University of Kentucky division of hospital medicine.
External cephalic version: How to increase the chances for success
About 3% to 4% of all fetuses at term are in breech presentation. Since 2000, when Hannah and colleagues reported finding that vaginal delivery of breech-presenting babies was riskier than cesarean delivery,1 most breech-presenting neonates in the United States have been delivered abdominally2—despite subsequent questioning of some of that study’s conclusions.
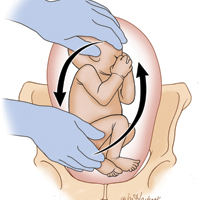
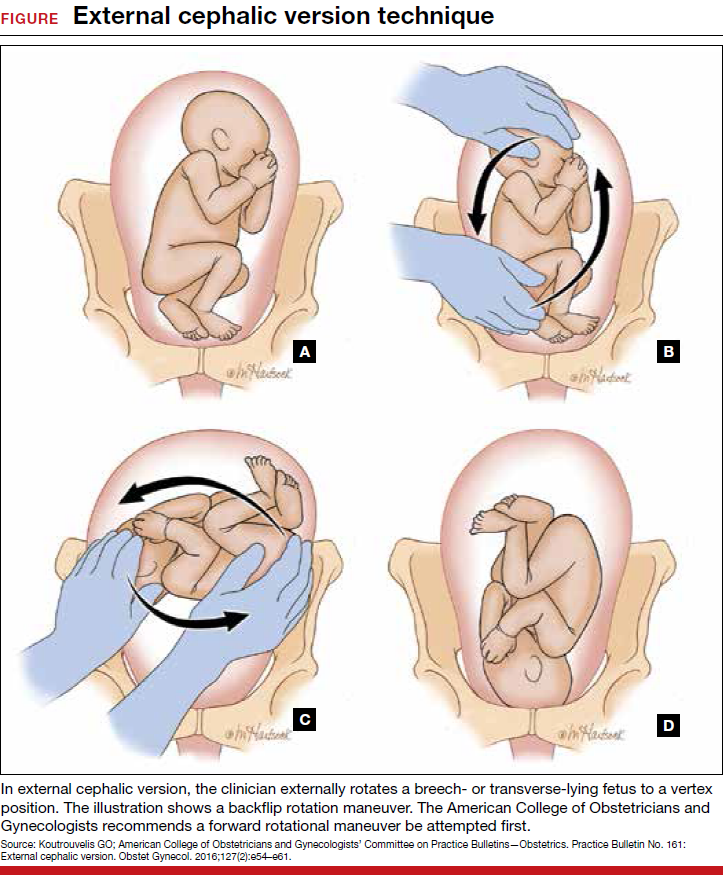
Each year in the United States, approximately 4 million babies are born, and fetal malpresentation accounts for 110,000 to 150,000 cesarean deliveries. In fact, about 15% of all cesarean deliveries in the United States are for breech presentation or transverse lie; in England the percentage is 10%.3 Fortunately, the repopularized technique of external cephalic version (ECV), in which the clinician externally rotates a breech- or transverse-lying fetus to a vertex position (FIGURE), along with the facilitating tools of tocolysis and neuraxial analgesia/anesthesia, is helping to reduce the number of breech presentations in fetuses at term and thus the number of cesarean deliveries and their sequelae—placenta accreta, prolonged recovery, and cesarean deliveries in subsequent pregnancies.
Reluctance to perform ECV is unfounded
In the United States, the practice of offering ECV to women who present with their fetus in breech presentation at term varies tremendously. It is routine at some institutions but not even offered at others.
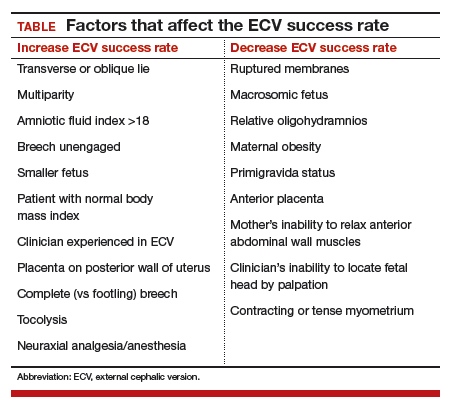
Many ObGyns are reluctant to perform ECV. Cited reasons include the potential for injury to the fetus and mother (and related liability concerns), the ease of elective cesarean delivery, the variable success rate of ECV (35% to 86%),4 and the pain that women often have with the procedure. According to the literature, however, these concerns either are unfounded or can be mitigated with use of current techniques. Multiple studies have found that the risk of ECV to the fetus and mother is minimal, and that tocolysis and neuraxial anesthesia can facilitate the success of ECV and relieve the pain associated with the procedure.
Related article:
2017 Update on obstetrics
Indications for ECV
The indications for ECV include breech, oblique, or transverse lie presentation after 36 weeks’ gestation and the mother’s desire to avoid cesarean delivery. A clinician skilled in ECV and a facility where emergency cesarean delivery is possible are essential.
There are several instances in which ECV should not be attempted.
Contraindications include:
- concerns about fetal status, including nonreactive nonstress test, biophysical profile score <6/8, severe intrauterine growth restriction, decreased end-diastolic umbilical blood flow
- placenta previa
- multifetal gestation before delivery of first twin
- severe oligohydramnios
- severe preeclampsia
- significant fetal anomaly
- known malformation of uterus
- breech with hyperextended head or arms above shoulders, as seen on ultrasonography.
More controversial contraindications include prior uterine incision, maternal obesity (body mass index >40 kg/m2), ruptured membranes, and fetal macrosomia.
Read about timing, success rates, risk factors, alternate approaches for ECV
Optimal timing for the ECV procedure
Current practice is to wait until 36 to 37 weeks to perform ECV, as most fetuses spontaneously move into vertex presentation by 36 weeks’ gestation. This time frame has several advantages: Many unnecessary attempts at ECV are avoided; only 8% of fetuses in breech presentation after 36 weeks spontaneously change to vertex5; many fetuses revert to breech if ECV is performed too early; and prematurity generally is not an issue in the rare case that immediate delivery is required during or just after attempted ECV.
ECV during labor. Performing ECV during labor appears to pose no increased risk to mother or fetus if membranes are intact and there are no other contraindications to the procedure. Some clinicians perform ECV only during labor. The advantages are that the fetus has had every chance to move into vertex presentation on its own, the equipment used to continuously monitor the fetus during ECV is in place, and cesarean delivery and anesthesia are immediately available in the event ECV is unsuccessful.
The major disadvantage of waiting until labor is that the increased size of the fetus makes ECV more difficult. In addition, the membranes may have already ruptured, and the breech may have descended deeply into the pelvis.
Related article:
For the management of labor, patience is a virtue
Success rates in breech-to-vertex conversions
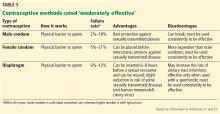
In 2016, the American College of Obstetricians and Gynecologists (ACOG) reported an average ECV success rate of 58% (range, 16% to 100%).6 ACOG noted that, with transverse lie, the success rate was significantly higher. Other studies have found a wide range of rates: 58% in 1,308 patients in a Cochrane review by Hofmeyr and colleagues7; 47% in a study by Beuckens and colleagues8; and 63.1% for primiparas and 82.7% for multiparas in a study by Tong Leung and colleagues.9 These rates were affected by whether ECV was performed with or without tocolysis, with or without intravenous analgesia, and with or without neuraxial analgesia/anesthesia (TABLE).
Likelihood of vaginal delivery after successful ECV
The rate of vaginal delivery after successful ECV is roughly half that of fetuses that were never in breech presentation.10 In successful ECV cases, dystocia and nonreassuring fetal heart rate patterns are the major indications for cesarean delivery. Some experts have speculated that the factors leading to near-term breech presentation—such as an unengaged presenting part or a mother’s smaller pelvis—also may be risk factors for dystocia in labor. Despite this, the rate of vaginal delivery of successfully verted babies has been reported to be as high as 80%.10
As might be expected, post-ECV vaginal deliveries are more common in multiparous than in primiparous women.
Although multiple problems may occur with ECV, generally they are rare and reversible. For instance, Grootscholten and colleagues found a stillbirth and placental abruption rate of only 0.25% in a large group of patients who underwent ECV.11 Similarly, the rate of emergency cesarean delivery was 0.35%. In addition, Hofmeyr and Kulier, in their Cochrane Data Review of 2015, found no significant differences in the Apgar scores and pH’s of babies in the ECV group compared with babies in breech presentation whose mothers did not undergo ECV.7 Results of other studies have confirmed the safety of ECV.12,13
One significant risk of ECV attempts is fetal-to-maternal blood transfer. Boucher and colleagues found that 2.4% of 1,244 women who underwent ECV had a positive Kleihauer-Betke test result, and, in one-third of the positive cases, more than 1 mL of fetal blood was found in maternal circulation.14 This risk can be minimized by administering Rho (D) immune globulin to all Rh-negative mothers after the procedure.
Even these small risks, however, should not be considered in isolation. The infrequent complications of ECV must be compared with what can occur with breech-presenting fetuses during labor or cesarean delivery: complications of breech vaginal delivery, cord prolapse, difficulties with cesarean delivery, and maternal operative complications related to present and future cesarean deliveries.
Alternative approaches to converting breech presentation of unproven efficacy
Over the years, attempts have been made to address breech presentations with measures short of ECV. There is little evidence that these measures work, or work consistently.
- Observation. After 36 weeks’ gestation, only 8% of fetuses in breech presentationspontaneously move into vertex presentation.5
- Maternal positioning. There is no good evidence that such maneuvers are effective in changing fetal presentation.15
- Moxibustion and acupuncture. Moxibustion is inhalation of smoke from burning herbal compounds. In formal studies using controls, these techniques did not consistently increase the rate of movement from breech to vertex presentation.16–18 Likewise, studies with the use of acupuncture have not shown consistent success in changing fetal presentation.19
Read about various methods to facilitate ECV success
Methods to facilitate ECV success
Two techniques that can facilitate ECV success are tocolysis, which relaxes the uterus, and neuraxial analgesia/anesthesia, which relaxes anterior abdominal wall muscles and reduces or relieves ECV-associated pain.
Tocolysis
In tocolysis, a medication is administered to reduce myometrial activity and to relax the uterine muscle so that it stretches more easily around the fetus during repositioning. Tocolytic medications originally were studied for their use in decreasing myometrial tone during preterm labor.
Tocolysis clearly is effective in increasing ECV success rates. Reviewing the results of 4 randomized trials, Cluver showed a 1.38 risk ratio for successful ECV when terbutaline was used versus when there was no tocolysis. The risk ratio for cesarean delivery was 0.82.20 Fernandez, in a study of 103 women divided into terbutaline versus placebo groups, had a 52% success rate for ECV with the terbutaline group versus only a 27% success rate with the placebo group.21
Tocolytic medications include terbutaline, nifedipine, and nitroglycerin.
Tocolysis most often involves the use of β2-adrenergic receptor agonists, particularly terbutaline (despite the boxed safety warning in its prescribing information). A 0.25-mg dose of terbutaline is given subcutaneously 15 to 30 minutes before ECV. Clinicians have successfully used β2-adrenergic receptor agonists in the treatment of patients in preterm labor, and there are more data on this class of medications than on other agents used to facilitate ECV.
Although nifedipine is as effective as terbutaline in the temporary treatment of preterm uterine contractions, several studies have found this calcium channel blocker less effective than terbutaline in facilitating ECV.22,23
The uterus-relaxing effect of nitroglycerin was once thought to make this medication appropriate for facilitating ECV, but multiple studies have found success rates unimproved. In some cases, the drug performed more poorly than placebo.24 Moreover, nitroglycerin is associated with a fairly high rate of adverse effects, such as headaches and blood pressure changes.
Neuraxial analgesia/anesthesia
Over the past 2 decades, there has been a resurgence in the use of neuraxial analgesia/anesthesia in ECV. This technique is more effective than others in improving ECV success rates, it reduces maternal discomfort, and it is very safe. Specifically, it relaxes the maternal abdominal wall muscles and thereby facilitates ECV. Another benefit is that the anesthesia is in place and available for use should emergency cesarean delivery be needed during or after attempted ECV. Neuraxial anesthesia, which includes spinal, epidural, and combined spinal-epidural techniques, is almost always used with tocolysis.
The major complications of neuraxial analgesia/anesthesia are maternal hypotension and fetal bradycardia. Each is dose related and usually transient.
In the past, there was concern that using regional anesthesia to control pain would reduce a patient’s natural warning symptoms and result in a clinician applying excessive force, thus increasing the chances of fetal and maternal injury and even fetal death. However, multiple studies have found that ECV complication rates are not increased with use of neuraxial methods.
Higher doses of neuraxial anesthesia produce higher ECV success rates. This dose-dependent relationship is almost surely attributable to the fact that, although lower dose neuraxial analgesia can relieve the pain associated with ECV, an anesthetic dose is needed to relax the abdominal wall muscles and facilitate fetus repositioning.
The literature is clear: ECV success rates are significantly increased with the use of neuraxial techniques, with anesthesia having higher success rates than analgesia. Reviewing the results of 6 controlled trials in which a total of 508 patients underwent ECV with tocolysis, Goetzinger and colleagues found that the chance of ECV success was almost 60% higher in the 253 patients who received regional anesthesia than in the 255 patients who received intravenous or no analgesia.25 Moreover, only 48.4% of the regional anesthesia patients as compared with 59.3% of patients who did not have regional anesthesia underwent cesarean delivery, roughly a 20% decrease. Pain scores were consistently lower in the regional anesthesia group. Multiple other studies have reported similar results.
Although the use of neuraxial anesthesia increases the ECV success rate, and decreases the cesarean delivery rate for breech presentation by 5% to 15%,25 some groups of obstetrics professionals, noting that the decreased cesarean delivery rate does not meet the formal criterion for statistical significance, have expressed reservations about recommending regional anesthesia for ECV. Thus, despite the positive results obtained with neuraxial anesthesia, neither the literature nor authoritative professional organizations definitively recommend the use of neuraxial anesthesia in facilitating ECV.
This lack of official recommendation, however, overlooks an important point: While the cesarean delivery percentage decrease that occurs with the use of neuraxial anesthesia may not be statistically significant, the promise of a pain-free procedure will encourage more women to undergo ECV. If the procedure population increases, then the average ECV success rate of roughly 60%6 applies to a larger base of patients, reducing the total number of cesarean deliveries for breech presentation. As only a small percentage of the 110,000 to 150,000 women with breech presentation at 36 weeks currently elects to undergo ECV, any increase in the number of women who proceed with attempts at fetal repositioning once procedural pain is no longer an issue will accordingly reduce the number of cesarean deliveries for the indication of malpresentation.
Related article:
Nitrous oxide for labor pain
Overarching goal: Reduce cesarean delivery rate and associated risks
In the United States, increasing the use of ECV in cases of breech-presenting fetuses would reduce the cesarean delivery rate by about 10%, thereby reducing recovery time for cesarean deliveries, minimizing the risks associated with these deliveries (current and future), and providing the health care system with a major cost savings.
Tocolysis and the use of neuraxial anesthesia each increases the ECV success rate and each is remarkably safe within the context of a well-defined protocol. Reducing the pain associated with ECV by administering neuraxial anesthesia will increase the number of women electing to undergo the procedure and ultimately will reduce the number of cesarean deliveries performed for the indication of breech presentation.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Hannah ME, Hannah WJ, Hewson SA, Hodnett ED, Saigal S, Willan AR. Planned cesarean section versus planned vaginal birth for breech presentation at term: a randomised multicentre trial. Term Breech Trial Collaborative Group. Lancet. 2000;356(9239):1375–1383.
- Weiniger CF, Lyell DJ, Tsen LC, et al. Maternal outcomes of term breech presentation delivery: impact of successful external cephalic version in a nationwide sample of delivery admissions in the United States. BMC Pregnancy Childbirth. 2016;16(1):150.
- Eller DP, Van Dorsten JP. Breech presentation. Curr Opin Obstet Gynecol.1993;5(5)664–668.
- Cunningham FG, Leveno KJ, Bloom SL, et al. Williams Obstetrics. 24th ed. New York, NY: McGraw Hill; 2014:570.
- Westgren M, Edvall H, Nordstrom L, Svalenius E, Ranstam J. Spontaneous cephalic version of breech presentation in the last trimester. Br J Obstet Gynaecol. 1985;92(1):19–22.
- External cephalic version. ACOG Practice Bulletin No. 161. American College of Obstetricians and Gynecologists. Washington, DC: ACOG; 2016.
- Hofmeyr GJ, Kulier R, West HM. External cephalic version for breech presentation at term. Cochrane Database Syst Rev. 2015;(4):CD000083.
- Beuckens A, Rijnders M, Verburgt-Doeleman GH, Rijninks-van Driel GC, Thorpe J, Hutton EK. An observational study of the success and complications of 2546 external cephalic versions in low-risk pregnant women performed by trained midwives. BJOG. 2016;123(3):415–423.
- Tong Leung VK, Suen SS, Singh Sahota D, Lau TK, Yeung Leung T. External cephalic version does not increase the risk of intra-uterine death: a 17-year experience and literature review. J Matern Fetal Neonatal Med. 2012;25(9):1774–1778.
- de Hundt M, Velzel J, de Groot CJ, Mol BW, Kok M. Mode of delivery after successful external cephalic version: a systematic review and meta-analysis. Obstet Gynecol. 2014;123(6):1327–1334.
- Grootscholten K, Kok M, Oei SG, Mol BW, van der Post JA. External cephalic version–related risks: a meta-analysis. Obstet Gynecol. 2008;112(5):1143–1151.
- Collaris RJ, Oei SG. External cephalic version: a safe procedure? A systematic review of version-related risk. Acta Obstet Gynecol Scand. 2004;83(6):511–518.
- Khaw KS, Lee SW, Ngan Kee WD, et al. Randomized trial of anesthetic interventions in external cephalic version for breech presentation. Br J Anaesth. 2015;114(6):944–950.
- Boucher M, Marquette GP, Varin J, Champagne J, Bujold E. Fetomaternal hemorrhage during external cephalic version. Obstet Gynecol. 2008;112(1):79–84.
- Hofmeyr GJ, Kulier R. Cephalic version by postural management for breech presentation. Cochrane Database Syst Rev. 2012;(10):CD00051.
- Coulon C, Poleszczuk M, Paty-Montaigne MH, et al. Version of breech fetuses by moxibustion with acupuncture: a randomized controlled trial. Obstet Gynecol. 2014;124(1):32–39.
- Bue L, Lauszus FF. Moxibustion did not have an effect in a randomised clinical trial for version of breech position. Dan Med J. 2016;63(2):pii:A5199.
- Coyle ME, Smith CA, Peat B. Cephalic version by moxibustion for breech presentation. Cochrane Database Syst Rev. 2012;(5):CD003928.
- Sananes N, Roth GE, Aissi GA, et al. Acupuncture version of breech presentation: a randomized sham-controlled single-blinded trial. Eur J Obstet Gynecol Reprod Biol. 2016;204:24–30.
- Cluver C, Gyte GM, Sinclair M, Dowswell T, Hofmeyr G. Interventions for helping to turn breech babies to head first presentation when using external cephalic version. Cochrane Database Syst Rev. 2015;(2):CD000184.
- Fernandez CO, Bloom SL, Smulian JC, Ananth CV, Wendel GD Jr. A randomized placebo-controlled evaluation of terbutaline for external cephalic version. Obstet Gynecol. 1997;90(5):775–779.
- Mohamed Ismail NA, Ibrahim M, Mohd Naim N, Mahdy ZA, Jamil MA, Mohd Razi ZR. Nifedipine versus terbutaline for tocolysis in external cephalic version. Int J Gynaecol Obstet. 2008;102(3):263–266.
- Kok M, Bais J, van Lith J, et al. Nifedipine as a uterine relaxant for external cephalic version: a meta-analysis. Am J Obstet Gynecol. 2008;112(2 pt 1):271–276.
- Bujold E, Boucher M, Rinfred D, Berman S, Ferreira E, Marquette GP. Sublingual nitroglycerin versus placebo as a tocolytic for external cephalic version: a randomized controlled trial in parous women. Am J Obstet Gynecol. 2003;189(4):1070–1073.
- Goetzinger KR, Harper LM, Tuuli MG, Macones GA, Colditz GA. Effect of regional anesthesia on the success of external cephalic version: a systematic review and meta-analysis. Obstet Gynecol. 2011;118(5):1137–1144.
About 3% to 4% of all fetuses at term are in breech presentation. Since 2000, when Hannah and colleagues reported finding that vaginal delivery of breech-presenting babies was riskier than cesarean delivery,1 most breech-presenting neonates in the United States have been delivered abdominally2—despite subsequent questioning of some of that study’s conclusions.
Each year in the United States, approximately 4 million babies are born, and fetal malpresentation accounts for 110,000 to 150,000 cesarean deliveries. In fact, about 15% of all cesarean deliveries in the United States are for breech presentation or transverse lie; in England the percentage is 10%.3 Fortunately, the repopularized technique of external cephalic version (ECV), in which the clinician externally rotates a breech- or transverse-lying fetus to a vertex position (FIGURE), along with the facilitating tools of tocolysis and neuraxial analgesia/anesthesia, is helping to reduce the number of breech presentations in fetuses at term and thus the number of cesarean deliveries and their sequelae—placenta accreta, prolonged recovery, and cesarean deliveries in subsequent pregnancies.

Reluctance to perform ECV is unfounded
In the United States, the practice of offering ECV to women who present with their fetus in breech presentation at term varies tremendously. It is routine at some institutions but not even offered at others.
Many ObGyns are reluctant to perform ECV. Cited reasons include the potential for injury to the fetus and mother (and related liability concerns), the ease of elective cesarean delivery, the variable success rate of ECV (35% to 86%),4 and the pain that women often have with the procedure. According to the literature, however, these concerns either are unfounded or can be mitigated with use of current techniques. Multiple studies have found that the risk of ECV to the fetus and mother is minimal, and that tocolysis and neuraxial anesthesia can facilitate the success of ECV and relieve the pain associated with the procedure.
Related article:
2017 Update on obstetrics
Indications for ECV
The indications for ECV include breech, oblique, or transverse lie presentation after 36 weeks’ gestation and the mother’s desire to avoid cesarean delivery. A clinician skilled in ECV and a facility where emergency cesarean delivery is possible are essential.
There are several instances in which ECV should not be attempted.
Contraindications include:
- concerns about fetal status, including nonreactive nonstress test, biophysical profile score <6/8, severe intrauterine growth restriction, decreased end-diastolic umbilical blood flow
- placenta previa
- multifetal gestation before delivery of first twin
- severe oligohydramnios
- severe preeclampsia
- significant fetal anomaly
- known malformation of uterus
- breech with hyperextended head or arms above shoulders, as seen on ultrasonography.
More controversial contraindications include prior uterine incision, maternal obesity (body mass index >40 kg/m2), ruptured membranes, and fetal macrosomia.
Read about timing, success rates, risk factors, alternate approaches for ECV
Optimal timing for the ECV procedure
Current practice is to wait until 36 to 37 weeks to perform ECV, as most fetuses spontaneously move into vertex presentation by 36 weeks’ gestation. This time frame has several advantages: Many unnecessary attempts at ECV are avoided; only 8% of fetuses in breech presentation after 36 weeks spontaneously change to vertex5; many fetuses revert to breech if ECV is performed too early; and prematurity generally is not an issue in the rare case that immediate delivery is required during or just after attempted ECV.
ECV during labor. Performing ECV during labor appears to pose no increased risk to mother or fetus if membranes are intact and there are no other contraindications to the procedure. Some clinicians perform ECV only during labor. The advantages are that the fetus has had every chance to move into vertex presentation on its own, the equipment used to continuously monitor the fetus during ECV is in place, and cesarean delivery and anesthesia are immediately available in the event ECV is unsuccessful.
The major disadvantage of waiting until labor is that the increased size of the fetus makes ECV more difficult. In addition, the membranes may have already ruptured, and the breech may have descended deeply into the pelvis.
Related article:
For the management of labor, patience is a virtue
Success rates in breech-to-vertex conversions
In 2016, the American College of Obstetricians and Gynecologists (ACOG) reported an average ECV success rate of 58% (range, 16% to 100%).6 ACOG noted that, with transverse lie, the success rate was significantly higher. Other studies have found a wide range of rates: 58% in 1,308 patients in a Cochrane review by Hofmeyr and colleagues7; 47% in a study by Beuckens and colleagues8; and 63.1% for primiparas and 82.7% for multiparas in a study by Tong Leung and colleagues.9 These rates were affected by whether ECV was performed with or without tocolysis, with or without intravenous analgesia, and with or without neuraxial analgesia/anesthesia (TABLE).

Likelihood of vaginal delivery after successful ECV
The rate of vaginal delivery after successful ECV is roughly half that of fetuses that were never in breech presentation.10 In successful ECV cases, dystocia and nonreassuring fetal heart rate patterns are the major indications for cesarean delivery. Some experts have speculated that the factors leading to near-term breech presentation—such as an unengaged presenting part or a mother’s smaller pelvis—also may be risk factors for dystocia in labor. Despite this, the rate of vaginal delivery of successfully verted babies has been reported to be as high as 80%.10
As might be expected, post-ECV vaginal deliveries are more common in multiparous than in primiparous women.
Although multiple problems may occur with ECV, generally they are rare and reversible. For instance, Grootscholten and colleagues found a stillbirth and placental abruption rate of only 0.25% in a large group of patients who underwent ECV.11 Similarly, the rate of emergency cesarean delivery was 0.35%. In addition, Hofmeyr and Kulier, in their Cochrane Data Review of 2015, found no significant differences in the Apgar scores and pH’s of babies in the ECV group compared with babies in breech presentation whose mothers did not undergo ECV.7 Results of other studies have confirmed the safety of ECV.12,13
One significant risk of ECV attempts is fetal-to-maternal blood transfer. Boucher and colleagues found that 2.4% of 1,244 women who underwent ECV had a positive Kleihauer-Betke test result, and, in one-third of the positive cases, more than 1 mL of fetal blood was found in maternal circulation.14 This risk can be minimized by administering Rho (D) immune globulin to all Rh-negative mothers after the procedure.
Even these small risks, however, should not be considered in isolation. The infrequent complications of ECV must be compared with what can occur with breech-presenting fetuses during labor or cesarean delivery: complications of breech vaginal delivery, cord prolapse, difficulties with cesarean delivery, and maternal operative complications related to present and future cesarean deliveries.
Alternative approaches to converting breech presentation of unproven efficacy
Over the years, attempts have been made to address breech presentations with measures short of ECV. There is little evidence that these measures work, or work consistently.
- Observation. After 36 weeks’ gestation, only 8% of fetuses in breech presentationspontaneously move into vertex presentation.5
- Maternal positioning. There is no good evidence that such maneuvers are effective in changing fetal presentation.15
- Moxibustion and acupuncture. Moxibustion is inhalation of smoke from burning herbal compounds. In formal studies using controls, these techniques did not consistently increase the rate of movement from breech to vertex presentation.16–18 Likewise, studies with the use of acupuncture have not shown consistent success in changing fetal presentation.19
Read about various methods to facilitate ECV success
Methods to facilitate ECV success
Two techniques that can facilitate ECV success are tocolysis, which relaxes the uterus, and neuraxial analgesia/anesthesia, which relaxes anterior abdominal wall muscles and reduces or relieves ECV-associated pain.
Tocolysis
In tocolysis, a medication is administered to reduce myometrial activity and to relax the uterine muscle so that it stretches more easily around the fetus during repositioning. Tocolytic medications originally were studied for their use in decreasing myometrial tone during preterm labor.
Tocolysis clearly is effective in increasing ECV success rates. Reviewing the results of 4 randomized trials, Cluver showed a 1.38 risk ratio for successful ECV when terbutaline was used versus when there was no tocolysis. The risk ratio for cesarean delivery was 0.82.20 Fernandez, in a study of 103 women divided into terbutaline versus placebo groups, had a 52% success rate for ECV with the terbutaline group versus only a 27% success rate with the placebo group.21
Tocolytic medications include terbutaline, nifedipine, and nitroglycerin.
Tocolysis most often involves the use of β2-adrenergic receptor agonists, particularly terbutaline (despite the boxed safety warning in its prescribing information). A 0.25-mg dose of terbutaline is given subcutaneously 15 to 30 minutes before ECV. Clinicians have successfully used β2-adrenergic receptor agonists in the treatment of patients in preterm labor, and there are more data on this class of medications than on other agents used to facilitate ECV.
Although nifedipine is as effective as terbutaline in the temporary treatment of preterm uterine contractions, several studies have found this calcium channel blocker less effective than terbutaline in facilitating ECV.22,23
The uterus-relaxing effect of nitroglycerin was once thought to make this medication appropriate for facilitating ECV, but multiple studies have found success rates unimproved. In some cases, the drug performed more poorly than placebo.24 Moreover, nitroglycerin is associated with a fairly high rate of adverse effects, such as headaches and blood pressure changes.
Neuraxial analgesia/anesthesia
Over the past 2 decades, there has been a resurgence in the use of neuraxial analgesia/anesthesia in ECV. This technique is more effective than others in improving ECV success rates, it reduces maternal discomfort, and it is very safe. Specifically, it relaxes the maternal abdominal wall muscles and thereby facilitates ECV. Another benefit is that the anesthesia is in place and available for use should emergency cesarean delivery be needed during or after attempted ECV. Neuraxial anesthesia, which includes spinal, epidural, and combined spinal-epidural techniques, is almost always used with tocolysis.
The major complications of neuraxial analgesia/anesthesia are maternal hypotension and fetal bradycardia. Each is dose related and usually transient.
In the past, there was concern that using regional anesthesia to control pain would reduce a patient’s natural warning symptoms and result in a clinician applying excessive force, thus increasing the chances of fetal and maternal injury and even fetal death. However, multiple studies have found that ECV complication rates are not increased with use of neuraxial methods.
Higher doses of neuraxial anesthesia produce higher ECV success rates. This dose-dependent relationship is almost surely attributable to the fact that, although lower dose neuraxial analgesia can relieve the pain associated with ECV, an anesthetic dose is needed to relax the abdominal wall muscles and facilitate fetus repositioning.
The literature is clear: ECV success rates are significantly increased with the use of neuraxial techniques, with anesthesia having higher success rates than analgesia. Reviewing the results of 6 controlled trials in which a total of 508 patients underwent ECV with tocolysis, Goetzinger and colleagues found that the chance of ECV success was almost 60% higher in the 253 patients who received regional anesthesia than in the 255 patients who received intravenous or no analgesia.25 Moreover, only 48.4% of the regional anesthesia patients as compared with 59.3% of patients who did not have regional anesthesia underwent cesarean delivery, roughly a 20% decrease. Pain scores were consistently lower in the regional anesthesia group. Multiple other studies have reported similar results.
Although the use of neuraxial anesthesia increases the ECV success rate, and decreases the cesarean delivery rate for breech presentation by 5% to 15%,25 some groups of obstetrics professionals, noting that the decreased cesarean delivery rate does not meet the formal criterion for statistical significance, have expressed reservations about recommending regional anesthesia for ECV. Thus, despite the positive results obtained with neuraxial anesthesia, neither the literature nor authoritative professional organizations definitively recommend the use of neuraxial anesthesia in facilitating ECV.
This lack of official recommendation, however, overlooks an important point: While the cesarean delivery percentage decrease that occurs with the use of neuraxial anesthesia may not be statistically significant, the promise of a pain-free procedure will encourage more women to undergo ECV. If the procedure population increases, then the average ECV success rate of roughly 60%6 applies to a larger base of patients, reducing the total number of cesarean deliveries for breech presentation. As only a small percentage of the 110,000 to 150,000 women with breech presentation at 36 weeks currently elects to undergo ECV, any increase in the number of women who proceed with attempts at fetal repositioning once procedural pain is no longer an issue will accordingly reduce the number of cesarean deliveries for the indication of malpresentation.
Related article:
Nitrous oxide for labor pain
Overarching goal: Reduce cesarean delivery rate and associated risks
In the United States, increasing the use of ECV in cases of breech-presenting fetuses would reduce the cesarean delivery rate by about 10%, thereby reducing recovery time for cesarean deliveries, minimizing the risks associated with these deliveries (current and future), and providing the health care system with a major cost savings.
Tocolysis and the use of neuraxial anesthesia each increases the ECV success rate and each is remarkably safe within the context of a well-defined protocol. Reducing the pain associated with ECV by administering neuraxial anesthesia will increase the number of women electing to undergo the procedure and ultimately will reduce the number of cesarean deliveries performed for the indication of breech presentation.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
About 3% to 4% of all fetuses at term are in breech presentation. Since 2000, when Hannah and colleagues reported finding that vaginal delivery of breech-presenting babies was riskier than cesarean delivery,1 most breech-presenting neonates in the United States have been delivered abdominally2—despite subsequent questioning of some of that study’s conclusions.
Each year in the United States, approximately 4 million babies are born, and fetal malpresentation accounts for 110,000 to 150,000 cesarean deliveries. In fact, about 15% of all cesarean deliveries in the United States are for breech presentation or transverse lie; in England the percentage is 10%.3 Fortunately, the repopularized technique of external cephalic version (ECV), in which the clinician externally rotates a breech- or transverse-lying fetus to a vertex position (FIGURE), along with the facilitating tools of tocolysis and neuraxial analgesia/anesthesia, is helping to reduce the number of breech presentations in fetuses at term and thus the number of cesarean deliveries and their sequelae—placenta accreta, prolonged recovery, and cesarean deliveries in subsequent pregnancies.

Reluctance to perform ECV is unfounded
In the United States, the practice of offering ECV to women who present with their fetus in breech presentation at term varies tremendously. It is routine at some institutions but not even offered at others.
Many ObGyns are reluctant to perform ECV. Cited reasons include the potential for injury to the fetus and mother (and related liability concerns), the ease of elective cesarean delivery, the variable success rate of ECV (35% to 86%),4 and the pain that women often have with the procedure. According to the literature, however, these concerns either are unfounded or can be mitigated with use of current techniques. Multiple studies have found that the risk of ECV to the fetus and mother is minimal, and that tocolysis and neuraxial anesthesia can facilitate the success of ECV and relieve the pain associated with the procedure.
Related article:
2017 Update on obstetrics
Indications for ECV
The indications for ECV include breech, oblique, or transverse lie presentation after 36 weeks’ gestation and the mother’s desire to avoid cesarean delivery. A clinician skilled in ECV and a facility where emergency cesarean delivery is possible are essential.
There are several instances in which ECV should not be attempted.
Contraindications include:
- concerns about fetal status, including nonreactive nonstress test, biophysical profile score <6/8, severe intrauterine growth restriction, decreased end-diastolic umbilical blood flow
- placenta previa
- multifetal gestation before delivery of first twin
- severe oligohydramnios
- severe preeclampsia
- significant fetal anomaly
- known malformation of uterus
- breech with hyperextended head or arms above shoulders, as seen on ultrasonography.
More controversial contraindications include prior uterine incision, maternal obesity (body mass index >40 kg/m2), ruptured membranes, and fetal macrosomia.
Read about timing, success rates, risk factors, alternate approaches for ECV
Optimal timing for the ECV procedure
Current practice is to wait until 36 to 37 weeks to perform ECV, as most fetuses spontaneously move into vertex presentation by 36 weeks’ gestation. This time frame has several advantages: Many unnecessary attempts at ECV are avoided; only 8% of fetuses in breech presentation after 36 weeks spontaneously change to vertex5; many fetuses revert to breech if ECV is performed too early; and prematurity generally is not an issue in the rare case that immediate delivery is required during or just after attempted ECV.
ECV during labor. Performing ECV during labor appears to pose no increased risk to mother or fetus if membranes are intact and there are no other contraindications to the procedure. Some clinicians perform ECV only during labor. The advantages are that the fetus has had every chance to move into vertex presentation on its own, the equipment used to continuously monitor the fetus during ECV is in place, and cesarean delivery and anesthesia are immediately available in the event ECV is unsuccessful.
The major disadvantage of waiting until labor is that the increased size of the fetus makes ECV more difficult. In addition, the membranes may have already ruptured, and the breech may have descended deeply into the pelvis.
Related article:
For the management of labor, patience is a virtue
Success rates in breech-to-vertex conversions
In 2016, the American College of Obstetricians and Gynecologists (ACOG) reported an average ECV success rate of 58% (range, 16% to 100%).6 ACOG noted that, with transverse lie, the success rate was significantly higher. Other studies have found a wide range of rates: 58% in 1,308 patients in a Cochrane review by Hofmeyr and colleagues7; 47% in a study by Beuckens and colleagues8; and 63.1% for primiparas and 82.7% for multiparas in a study by Tong Leung and colleagues.9 These rates were affected by whether ECV was performed with or without tocolysis, with or without intravenous analgesia, and with or without neuraxial analgesia/anesthesia (TABLE).

Likelihood of vaginal delivery after successful ECV
The rate of vaginal delivery after successful ECV is roughly half that of fetuses that were never in breech presentation.10 In successful ECV cases, dystocia and nonreassuring fetal heart rate patterns are the major indications for cesarean delivery. Some experts have speculated that the factors leading to near-term breech presentation—such as an unengaged presenting part or a mother’s smaller pelvis—also may be risk factors for dystocia in labor. Despite this, the rate of vaginal delivery of successfully verted babies has been reported to be as high as 80%.10
As might be expected, post-ECV vaginal deliveries are more common in multiparous than in primiparous women.
Although multiple problems may occur with ECV, generally they are rare and reversible. For instance, Grootscholten and colleagues found a stillbirth and placental abruption rate of only 0.25% in a large group of patients who underwent ECV.11 Similarly, the rate of emergency cesarean delivery was 0.35%. In addition, Hofmeyr and Kulier, in their Cochrane Data Review of 2015, found no significant differences in the Apgar scores and pH’s of babies in the ECV group compared with babies in breech presentation whose mothers did not undergo ECV.7 Results of other studies have confirmed the safety of ECV.12,13
One significant risk of ECV attempts is fetal-to-maternal blood transfer. Boucher and colleagues found that 2.4% of 1,244 women who underwent ECV had a positive Kleihauer-Betke test result, and, in one-third of the positive cases, more than 1 mL of fetal blood was found in maternal circulation.14 This risk can be minimized by administering Rho (D) immune globulin to all Rh-negative mothers after the procedure.
Even these small risks, however, should not be considered in isolation. The infrequent complications of ECV must be compared with what can occur with breech-presenting fetuses during labor or cesarean delivery: complications of breech vaginal delivery, cord prolapse, difficulties with cesarean delivery, and maternal operative complications related to present and future cesarean deliveries.
Alternative approaches to converting breech presentation of unproven efficacy
Over the years, attempts have been made to address breech presentations with measures short of ECV. There is little evidence that these measures work, or work consistently.
- Observation. After 36 weeks’ gestation, only 8% of fetuses in breech presentationspontaneously move into vertex presentation.5
- Maternal positioning. There is no good evidence that such maneuvers are effective in changing fetal presentation.15
- Moxibustion and acupuncture. Moxibustion is inhalation of smoke from burning herbal compounds. In formal studies using controls, these techniques did not consistently increase the rate of movement from breech to vertex presentation.16–18 Likewise, studies with the use of acupuncture have not shown consistent success in changing fetal presentation.19
Read about various methods to facilitate ECV success
Methods to facilitate ECV success
Two techniques that can facilitate ECV success are tocolysis, which relaxes the uterus, and neuraxial analgesia/anesthesia, which relaxes anterior abdominal wall muscles and reduces or relieves ECV-associated pain.
Tocolysis
In tocolysis, a medication is administered to reduce myometrial activity and to relax the uterine muscle so that it stretches more easily around the fetus during repositioning. Tocolytic medications originally were studied for their use in decreasing myometrial tone during preterm labor.
Tocolysis clearly is effective in increasing ECV success rates. Reviewing the results of 4 randomized trials, Cluver showed a 1.38 risk ratio for successful ECV when terbutaline was used versus when there was no tocolysis. The risk ratio for cesarean delivery was 0.82.20 Fernandez, in a study of 103 women divided into terbutaline versus placebo groups, had a 52% success rate for ECV with the terbutaline group versus only a 27% success rate with the placebo group.21
Tocolytic medications include terbutaline, nifedipine, and nitroglycerin.
Tocolysis most often involves the use of β2-adrenergic receptor agonists, particularly terbutaline (despite the boxed safety warning in its prescribing information). A 0.25-mg dose of terbutaline is given subcutaneously 15 to 30 minutes before ECV. Clinicians have successfully used β2-adrenergic receptor agonists in the treatment of patients in preterm labor, and there are more data on this class of medications than on other agents used to facilitate ECV.
Although nifedipine is as effective as terbutaline in the temporary treatment of preterm uterine contractions, several studies have found this calcium channel blocker less effective than terbutaline in facilitating ECV.22,23
The uterus-relaxing effect of nitroglycerin was once thought to make this medication appropriate for facilitating ECV, but multiple studies have found success rates unimproved. In some cases, the drug performed more poorly than placebo.24 Moreover, nitroglycerin is associated with a fairly high rate of adverse effects, such as headaches and blood pressure changes.
Neuraxial analgesia/anesthesia
Over the past 2 decades, there has been a resurgence in the use of neuraxial analgesia/anesthesia in ECV. This technique is more effective than others in improving ECV success rates, it reduces maternal discomfort, and it is very safe. Specifically, it relaxes the maternal abdominal wall muscles and thereby facilitates ECV. Another benefit is that the anesthesia is in place and available for use should emergency cesarean delivery be needed during or after attempted ECV. Neuraxial anesthesia, which includes spinal, epidural, and combined spinal-epidural techniques, is almost always used with tocolysis.
The major complications of neuraxial analgesia/anesthesia are maternal hypotension and fetal bradycardia. Each is dose related and usually transient.
In the past, there was concern that using regional anesthesia to control pain would reduce a patient’s natural warning symptoms and result in a clinician applying excessive force, thus increasing the chances of fetal and maternal injury and even fetal death. However, multiple studies have found that ECV complication rates are not increased with use of neuraxial methods.
Higher doses of neuraxial anesthesia produce higher ECV success rates. This dose-dependent relationship is almost surely attributable to the fact that, although lower dose neuraxial analgesia can relieve the pain associated with ECV, an anesthetic dose is needed to relax the abdominal wall muscles and facilitate fetus repositioning.
The literature is clear: ECV success rates are significantly increased with the use of neuraxial techniques, with anesthesia having higher success rates than analgesia. Reviewing the results of 6 controlled trials in which a total of 508 patients underwent ECV with tocolysis, Goetzinger and colleagues found that the chance of ECV success was almost 60% higher in the 253 patients who received regional anesthesia than in the 255 patients who received intravenous or no analgesia.25 Moreover, only 48.4% of the regional anesthesia patients as compared with 59.3% of patients who did not have regional anesthesia underwent cesarean delivery, roughly a 20% decrease. Pain scores were consistently lower in the regional anesthesia group. Multiple other studies have reported similar results.
Although the use of neuraxial anesthesia increases the ECV success rate, and decreases the cesarean delivery rate for breech presentation by 5% to 15%,25 some groups of obstetrics professionals, noting that the decreased cesarean delivery rate does not meet the formal criterion for statistical significance, have expressed reservations about recommending regional anesthesia for ECV. Thus, despite the positive results obtained with neuraxial anesthesia, neither the literature nor authoritative professional organizations definitively recommend the use of neuraxial anesthesia in facilitating ECV.
This lack of official recommendation, however, overlooks an important point: While the cesarean delivery percentage decrease that occurs with the use of neuraxial anesthesia may not be statistically significant, the promise of a pain-free procedure will encourage more women to undergo ECV. If the procedure population increases, then the average ECV success rate of roughly 60%6 applies to a larger base of patients, reducing the total number of cesarean deliveries for breech presentation. As only a small percentage of the 110,000 to 150,000 women with breech presentation at 36 weeks currently elects to undergo ECV, any increase in the number of women who proceed with attempts at fetal repositioning once procedural pain is no longer an issue will accordingly reduce the number of cesarean deliveries for the indication of malpresentation.
Related article:
Nitrous oxide for labor pain
Overarching goal: Reduce cesarean delivery rate and associated risks
In the United States, increasing the use of ECV in cases of breech-presenting fetuses would reduce the cesarean delivery rate by about 10%, thereby reducing recovery time for cesarean deliveries, minimizing the risks associated with these deliveries (current and future), and providing the health care system with a major cost savings.
Tocolysis and the use of neuraxial anesthesia each increases the ECV success rate and each is remarkably safe within the context of a well-defined protocol. Reducing the pain associated with ECV by administering neuraxial anesthesia will increase the number of women electing to undergo the procedure and ultimately will reduce the number of cesarean deliveries performed for the indication of breech presentation.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Hannah ME, Hannah WJ, Hewson SA, Hodnett ED, Saigal S, Willan AR. Planned cesarean section versus planned vaginal birth for breech presentation at term: a randomised multicentre trial. Term Breech Trial Collaborative Group. Lancet. 2000;356(9239):1375–1383.
- Weiniger CF, Lyell DJ, Tsen LC, et al. Maternal outcomes of term breech presentation delivery: impact of successful external cephalic version in a nationwide sample of delivery admissions in the United States. BMC Pregnancy Childbirth. 2016;16(1):150.
- Eller DP, Van Dorsten JP. Breech presentation. Curr Opin Obstet Gynecol.1993;5(5)664–668.
- Cunningham FG, Leveno KJ, Bloom SL, et al. Williams Obstetrics. 24th ed. New York, NY: McGraw Hill; 2014:570.
- Westgren M, Edvall H, Nordstrom L, Svalenius E, Ranstam J. Spontaneous cephalic version of breech presentation in the last trimester. Br J Obstet Gynaecol. 1985;92(1):19–22.
- External cephalic version. ACOG Practice Bulletin No. 161. American College of Obstetricians and Gynecologists. Washington, DC: ACOG; 2016.
- Hofmeyr GJ, Kulier R, West HM. External cephalic version for breech presentation at term. Cochrane Database Syst Rev. 2015;(4):CD000083.
- Beuckens A, Rijnders M, Verburgt-Doeleman GH, Rijninks-van Driel GC, Thorpe J, Hutton EK. An observational study of the success and complications of 2546 external cephalic versions in low-risk pregnant women performed by trained midwives. BJOG. 2016;123(3):415–423.
- Tong Leung VK, Suen SS, Singh Sahota D, Lau TK, Yeung Leung T. External cephalic version does not increase the risk of intra-uterine death: a 17-year experience and literature review. J Matern Fetal Neonatal Med. 2012;25(9):1774–1778.
- de Hundt M, Velzel J, de Groot CJ, Mol BW, Kok M. Mode of delivery after successful external cephalic version: a systematic review and meta-analysis. Obstet Gynecol. 2014;123(6):1327–1334.
- Grootscholten K, Kok M, Oei SG, Mol BW, van der Post JA. External cephalic version–related risks: a meta-analysis. Obstet Gynecol. 2008;112(5):1143–1151.
- Collaris RJ, Oei SG. External cephalic version: a safe procedure? A systematic review of version-related risk. Acta Obstet Gynecol Scand. 2004;83(6):511–518.
- Khaw KS, Lee SW, Ngan Kee WD, et al. Randomized trial of anesthetic interventions in external cephalic version for breech presentation. Br J Anaesth. 2015;114(6):944–950.
- Boucher M, Marquette GP, Varin J, Champagne J, Bujold E. Fetomaternal hemorrhage during external cephalic version. Obstet Gynecol. 2008;112(1):79–84.
- Hofmeyr GJ, Kulier R. Cephalic version by postural management for breech presentation. Cochrane Database Syst Rev. 2012;(10):CD00051.
- Coulon C, Poleszczuk M, Paty-Montaigne MH, et al. Version of breech fetuses by moxibustion with acupuncture: a randomized controlled trial. Obstet Gynecol. 2014;124(1):32–39.
- Bue L, Lauszus FF. Moxibustion did not have an effect in a randomised clinical trial for version of breech position. Dan Med J. 2016;63(2):pii:A5199.
- Coyle ME, Smith CA, Peat B. Cephalic version by moxibustion for breech presentation. Cochrane Database Syst Rev. 2012;(5):CD003928.
- Sananes N, Roth GE, Aissi GA, et al. Acupuncture version of breech presentation: a randomized sham-controlled single-blinded trial. Eur J Obstet Gynecol Reprod Biol. 2016;204:24–30.
- Cluver C, Gyte GM, Sinclair M, Dowswell T, Hofmeyr G. Interventions for helping to turn breech babies to head first presentation when using external cephalic version. Cochrane Database Syst Rev. 2015;(2):CD000184.
- Fernandez CO, Bloom SL, Smulian JC, Ananth CV, Wendel GD Jr. A randomized placebo-controlled evaluation of terbutaline for external cephalic version. Obstet Gynecol. 1997;90(5):775–779.
- Mohamed Ismail NA, Ibrahim M, Mohd Naim N, Mahdy ZA, Jamil MA, Mohd Razi ZR. Nifedipine versus terbutaline for tocolysis in external cephalic version. Int J Gynaecol Obstet. 2008;102(3):263–266.
- Kok M, Bais J, van Lith J, et al. Nifedipine as a uterine relaxant for external cephalic version: a meta-analysis. Am J Obstet Gynecol. 2008;112(2 pt 1):271–276.
- Bujold E, Boucher M, Rinfred D, Berman S, Ferreira E, Marquette GP. Sublingual nitroglycerin versus placebo as a tocolytic for external cephalic version: a randomized controlled trial in parous women. Am J Obstet Gynecol. 2003;189(4):1070–1073.
- Goetzinger KR, Harper LM, Tuuli MG, Macones GA, Colditz GA. Effect of regional anesthesia on the success of external cephalic version: a systematic review and meta-analysis. Obstet Gynecol. 2011;118(5):1137–1144.
- Hannah ME, Hannah WJ, Hewson SA, Hodnett ED, Saigal S, Willan AR. Planned cesarean section versus planned vaginal birth for breech presentation at term: a randomised multicentre trial. Term Breech Trial Collaborative Group. Lancet. 2000;356(9239):1375–1383.
- Weiniger CF, Lyell DJ, Tsen LC, et al. Maternal outcomes of term breech presentation delivery: impact of successful external cephalic version in a nationwide sample of delivery admissions in the United States. BMC Pregnancy Childbirth. 2016;16(1):150.
- Eller DP, Van Dorsten JP. Breech presentation. Curr Opin Obstet Gynecol.1993;5(5)664–668.
- Cunningham FG, Leveno KJ, Bloom SL, et al. Williams Obstetrics. 24th ed. New York, NY: McGraw Hill; 2014:570.
- Westgren M, Edvall H, Nordstrom L, Svalenius E, Ranstam J. Spontaneous cephalic version of breech presentation in the last trimester. Br J Obstet Gynaecol. 1985;92(1):19–22.
- External cephalic version. ACOG Practice Bulletin No. 161. American College of Obstetricians and Gynecologists. Washington, DC: ACOG; 2016.
- Hofmeyr GJ, Kulier R, West HM. External cephalic version for breech presentation at term. Cochrane Database Syst Rev. 2015;(4):CD000083.
- Beuckens A, Rijnders M, Verburgt-Doeleman GH, Rijninks-van Driel GC, Thorpe J, Hutton EK. An observational study of the success and complications of 2546 external cephalic versions in low-risk pregnant women performed by trained midwives. BJOG. 2016;123(3):415–423.
- Tong Leung VK, Suen SS, Singh Sahota D, Lau TK, Yeung Leung T. External cephalic version does not increase the risk of intra-uterine death: a 17-year experience and literature review. J Matern Fetal Neonatal Med. 2012;25(9):1774–1778.
- de Hundt M, Velzel J, de Groot CJ, Mol BW, Kok M. Mode of delivery after successful external cephalic version: a systematic review and meta-analysis. Obstet Gynecol. 2014;123(6):1327–1334.
- Grootscholten K, Kok M, Oei SG, Mol BW, van der Post JA. External cephalic version–related risks: a meta-analysis. Obstet Gynecol. 2008;112(5):1143–1151.
- Collaris RJ, Oei SG. External cephalic version: a safe procedure? A systematic review of version-related risk. Acta Obstet Gynecol Scand. 2004;83(6):511–518.
- Khaw KS, Lee SW, Ngan Kee WD, et al. Randomized trial of anesthetic interventions in external cephalic version for breech presentation. Br J Anaesth. 2015;114(6):944–950.
- Boucher M, Marquette GP, Varin J, Champagne J, Bujold E. Fetomaternal hemorrhage during external cephalic version. Obstet Gynecol. 2008;112(1):79–84.
- Hofmeyr GJ, Kulier R. Cephalic version by postural management for breech presentation. Cochrane Database Syst Rev. 2012;(10):CD00051.
- Coulon C, Poleszczuk M, Paty-Montaigne MH, et al. Version of breech fetuses by moxibustion with acupuncture: a randomized controlled trial. Obstet Gynecol. 2014;124(1):32–39.
- Bue L, Lauszus FF. Moxibustion did not have an effect in a randomised clinical trial for version of breech position. Dan Med J. 2016;63(2):pii:A5199.
- Coyle ME, Smith CA, Peat B. Cephalic version by moxibustion for breech presentation. Cochrane Database Syst Rev. 2012;(5):CD003928.
- Sananes N, Roth GE, Aissi GA, et al. Acupuncture version of breech presentation: a randomized sham-controlled single-blinded trial. Eur J Obstet Gynecol Reprod Biol. 2016;204:24–30.
- Cluver C, Gyte GM, Sinclair M, Dowswell T, Hofmeyr G. Interventions for helping to turn breech babies to head first presentation when using external cephalic version. Cochrane Database Syst Rev. 2015;(2):CD000184.
- Fernandez CO, Bloom SL, Smulian JC, Ananth CV, Wendel GD Jr. A randomized placebo-controlled evaluation of terbutaline for external cephalic version. Obstet Gynecol. 1997;90(5):775–779.
- Mohamed Ismail NA, Ibrahim M, Mohd Naim N, Mahdy ZA, Jamil MA, Mohd Razi ZR. Nifedipine versus terbutaline for tocolysis in external cephalic version. Int J Gynaecol Obstet. 2008;102(3):263–266.
- Kok M, Bais J, van Lith J, et al. Nifedipine as a uterine relaxant for external cephalic version: a meta-analysis. Am J Obstet Gynecol. 2008;112(2 pt 1):271–276.
- Bujold E, Boucher M, Rinfred D, Berman S, Ferreira E, Marquette GP. Sublingual nitroglycerin versus placebo as a tocolytic for external cephalic version: a randomized controlled trial in parous women. Am J Obstet Gynecol. 2003;189(4):1070–1073.
- Goetzinger KR, Harper LM, Tuuli MG, Macones GA, Colditz GA. Effect of regional anesthesia on the success of external cephalic version: a systematic review and meta-analysis. Obstet Gynecol. 2011;118(5):1137–1144.
Fast Tracks
- Current practice is to wait until 36 to 37 weeks of gestation to perform ECV, since most fetuses spontaneously move into vertex presentation by 36 weeks
- Tocolysis, which relaxes the uterus, and neuraxial analgesia/anesthesia, which relaxes anterior abdominal wall muscles and reduces ECV-associated pain, can facilitate ECV success
- Several studies have found that nifedipine is less effective than terbutaline in facilitating ECV
- Higher doses of neuraxial anesthesia produce higher ECV success rates, possibly because the higher anesthetic dose relaxes the abdominal wall muscles and facilitates fetus repositioning
A minimally invasive treatment for early GI cancers
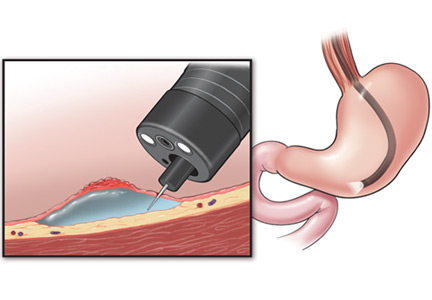
The treatment of early esophageal, gastric, and colorectal cancer is changing.1 For many years, surgery was the mainstay of treatment for early-stage gastrointestinal cancer. Unfortunately, surgery leads to significant loss of function of the organ, resulting in increased morbidity and decreased quality of life.2
Endoscopic techniques, particularly endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), have been developed and are widely used in Japan, where gastrointestinal cancer is more common than in the West. This article reviews the indications, complications, and outcomes of ESD for early gastrointestinal neoplasms, so that readers will recognize the subset of patients who would benefit from ESD in a Western setting.
ENDOSCOPIC MUCOSAL RESECTION AND SUBMUCOSAL DISSECTION
Since the first therapeutic polypectomy was performed in Japan in 1974, several endoscopic techniques for tumor resection have been developed.3
EMR, one of the most successful and widely used techniques, involves elevating the lesion either with submucosal injection of a solution or with cap suction, and then removing it with a snare.4 Most lesions smaller than 20 mm can be removed in one piece (en bloc).5 Larger lesions are removed in multiple pieces (ie, piecemeal). Unfortunately, some fibrotic lesions, which are usually difficult to lift, cannot be completely removed by EMR.
ESD was first performed in the late 1990s with the aim of overcoming the limitations of EMR in resecting large or fibrotic tumors en bloc.6,7 Since then, ESD technique has been standardized and training centers have been created, especially in Asia, where it is widely used for treatment of early gastric cancer.3,8–10 Since 2012 it has been covered by the Japanese National Health Insurance for treatment of early gastric cancer, and since 2014 for treatment of colorectal malignant tumors measuring 2 to 5 cm.11
Adoption of ESD has been slow in Western countries, where many patients are still referred for surgery or undergo EMR for removal of superficial neoplasms. Reasons for this slow adoption are that gastric cancer is much less common in Western countries, and also that ESD demands a high level of technical skill, is difficult to learn, and is expensive.3,12,13 However, small groups of Western endoscopists have become interested and are advocating it, first studying it on their own and then training in a Japanese center and learning from experts performing the procedure.
Therefore, in a Western setting, ESD should be performed in specialized endoscopy centers and offered to selected patients.1
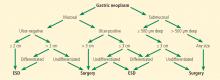
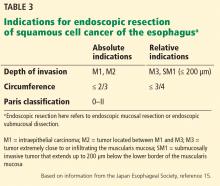
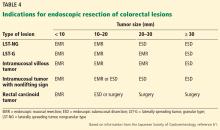
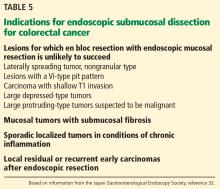
CANDIDATES SHOULD HAVE EARLY-STAGE, SUPERFICIAL TUMORS
Ideal candidates for endoscopic resection are patients who have early cancer with a negligible risk of lymph node metastasis, such as cancer limited to the mucosa (stage T1a).7 Therefore, to determine the best treatment for a patient with a newly diagnosed gastrointestinal neoplasm, it is mandatory to estimate the depth of invasion.
The depth of invasion is directly correlated with lymph node involvement, which is ultimately the main predictive factor for long-term adverse outcomes of gastrointestinal tumors.4,14–17 Accurate multidisciplinary preprocedure estimations are mandatory, as incorrect evaluations may result in inappropriate therapy and residual cancer.18
Other factors that have been used to predict lymph node involvement include tumor size, macroscopic appearance, histologic differentiation, and lymphatic and vascular involvement.19 Some of these factors can be assessed by special endoscopic techniques (chromoendoscopy and narrow-band imaging with magnifying endoscopy) that allow accurate real-time estimation of the depth of invasion of the lesion.5,17,20–27 Evaluation of microsurface and microvascular arrangements is especially useful for determining the feasibility of ESD in gastric tumors, evaluation of intracapillary loops is useful in esophageal lesions, and assessment of mucosal pit patterns is useful for colorectal lesions.21–29
Endoscopic ultrasonography is another tool that has been used to estimate the depth of the tumor. Although it can differentiate between definite intramucosal and definite submucosal invasive cancers, its ability to confirm minute submucosal invasion is limited. Its use as the sole tumor staging modality is not encouraged, and it should always be used in conjunction with endoscopic evaluation.18
Though the aforementioned factors help stratify patients, pathologic staging is the best predictor of lymph node metastasis. ESD provides adequate specimens for accurate pathologic evaluation, as it removes lesions en bloc.30
All patients found to have risk factors for lymph node metastasis on endoscopic, ultrasonographic, or pathologic analysis should be referred for surgical evaluation.9,19,31,32
ENDOSCOPIC SUBMUCOSAL DISSECTION
Before the procedure, the patient’s physicians need to do the following:
Determine the best type of intervention (EMR, ESD, ablation, surgery) for the specific lesion.3 A multidisciplinary approach is encouraged, with involvement of the internist, gastroenterologist, and surgeon.
Plan for anesthesia, additional consultations, pre- and postprocedural hospital admission, and need for special equipment.33
During the procedure
Define the lateral extent of the lesion using magnification chromoendoscopy or narrow-band imaging. In the stomach, a biopsy sample should be taken from the worst-looking segment and from normal-looking mucosa. Multiple biopsies should be avoided to prevent subsequent fibrosis.33 In the colon, biopsy should be avoided.34
Identify and circumferentially mark the target lesion. Cautery or argon plasma coagulation can be used for making markings at a distance of 5 to 10 mm from the edges.33 This is done to recognize the borders of the lesion, because they can become distorted after submucosal injection.14 This step is unnecessary in colorectal cases, as tumor margins can be adequately visualized after chromoendoscopy.16,35
Lift the lesion by injecting saline, 0.5% hyaluronate, or glycerin to create a submucosal fluid cushion.19,33
Perform a circumferential incision lateral to the mucosal margins to allow for a normal tissue margin.33 Partial incision is performed for esophageal and colorectal ESD to avoid fluid leakage from the submucosal layer, achieving a sustained submucosal lift and safer dissection.16
Submucosal dissection. The submucosal layer is dissected with an electrocautery knife until the lesion is completely removed. Dissection should be done carefully to keep the submucosal plane.33 Hemoclips or hemostat forceps can be used to control visible bleeding. The resected specimen is then stretched and fixed to a board using small pins for further histopathologic evaluation.35
Postprocedural monitoring. All patients should be admitted for overnight observation. Those who undergo gastric ESD should receive high-dose acid suppression, and the next day they can be started on a liquid diet.19
STOMACH CANCER
Indications for ESD for stomach cancer in the East
The incidence of gastric cancer is higher in Japan and Korea, where widespread screening programs have led to early identification and early treatment of this disease.36
Pathology studies37 of samples from patients with gastric cancer identified the following as risk factors for lymph node metastasis, which would make ESD unsuitable:
- Undifferentiated type
- Tumors larger than 2 cm
- Lymphatic or venous involvement
- Submucosal invasion
- Ulcerative change.
Based on these findings, the situations in which there was no risk of lymph node involvement (ie, when none of the above factors are present) were accepted as absolute indications for endoscopic resection of early gastric cancer.38 Further histologic studies identified a subset of patients with lesions with very low risk of lymph node metastasis, which outweighed the risk of surgery. Based on these findings, expanded criteria for gastric ESD were proposed,39,40 and the Japanese gastric cancer treatment guidelines now include these expanded preoperative indications9,17 (Table 1).
The Japanese Gastric Cancer Association has proposed a treatment algorithm based on the histopathologic evaluation after resection (Figure 2).9
Outcomes
In the largest series of patients who underwent curative ESD for early gastric cancer, the 5-year survival rate was 92.6%, the 5-year disease-specific survival rate was 99.9%, and the 5-year relative survival rate was 105%.41
Similarly, in a Japanese population-based survival analysis, the relative 5-year survival rate for localized gastric cancer was 94.4%.42 Rates of en bloc resection and complete resection with ESD are higher than those with EMR, resulting in a lower risk of local recurrence in selected patients who undergo ESD.8,43,44
Although rare, local recurrence after curative gastric ESD has been reported.45 The annual incidence of local recurrence has been estimated to be 0.84%.46
ESD entails a shorter hospital stay and requires fewer resources than surgery, resulting in lower medical costs (Table 2).44 Additionally, as endoscopic resection is associated with less morbidity, fewer procedure-related adverse events, and fewer complications, ESD could be used as the standard treatment for early gastric cancer.47,48
The Western perspective on endoscopic submucosal dissection for gastric cancer
Since the prevalence of gastric cancer in Western countries is significantly lower than in Japan and Korea, local data and experience are scarce. However, experts performing ESD in the West have adopted the indications of the Japan Gastroenterological Endoscopy Society. The European Society of Gastrointestinal Endoscopy recommends ESD for excision of most superficial gastric neoplasms, with EMR being preferred only in lesions smaller than 15 mm, Paris classification 0 or IIA.5,32
Patients with gastric lesions measuring 15 mm or larger should undergo high-quality endoscopy, preferably chromoendoscopy, to evaluate the mucosal patterns and determine the depth of invasion. If superficial involvement is confirmed, other imaging techniques are not routinely recommended.5 A surgery consult is also recommended.
ESOPHAGEAL CANCER
Indications for ESD for esophageal cancer in the East
Due to the success of ESD for early gastric cancer, this technique is now also used for superficial esophageal neoplasms.19,49 It should be done in a specialized center, as it is more technically difficult than gastric ESD: the esophageal lumen is narrow, the wall is thin, and the esophagus moves with respiration and heartbeat.50 A multidisciplinary approach including an endoscopist, a surgeon, and a pathologist is highly recommended for evaluation and treatment.
EMR is preferred for removal of mucosal cancer, in view of its safety profile and success rates. ESD can be considered in cases of lesions larger than 15 mm, poorly lifting tumors, and those with the possibility of submucosal invasion (Table 3).5,45,49,51
Circumference involvement is critical when determining eligible candidates, as a defect involving more than three-fourths of the esophageal circumference can lead to esophageal strictures.52 Controlled prospective studies have shown promising results from giving intralesional and oral steroids to prevent stricture after ESD, which could potentially overcome this size limitation.53,54
Outcomes for esophageal cancer
ESD has been shown to be safe and effective, achieving en bloc resection in 85% to 100% of patients.19,51 Its advantages over EMR include en bloc resection, complete resection, and high curative rates, resulting in higher recurrence-free survival.2,55,56 Although the incidence of complications such as bleeding, perforation, and stricture formation are higher with ESD, patients usually recover uneventfully.2,19,20
ESD in the esophagus: The Western perspective
As data on the efficacy of EMR vs ESD for the treatment of Barrett esophagus with adenocarcinoma are limited, EMR is the gold standard endoscopic technique for removal of visible esophageal dysplastic lesions.5,51,57 ESD can be considered for tumors larger than 15 mm, for poorly lifting lesions, and if there is suspicion of submucosal invasion.5
Patients should be evaluated by an experienced endoscopist, using an advanced imaging technique such as narrow-band imaging or chromoendoscopy. If suspicious features are found, endoscopic ultrasonography should be considered to confirm submucosal invasion or lymph node involvement.5
COLORECTAL CANCER
Indications for ESD for colorectal cancer in the East
Colon cancer is one of the leading causes of cancer-related deaths worldwide.58 Since ESD has been found to be effective and safe in treating gastric cancer, it has also been used to remove large colorectal tumors.59 However, ESD is not universally accepted in the treatment of colorectal neoplasms due to its greater technical difficulty, longer procedural time, and higher risk of perforating the thinner colonic wall compared with EMR.21,60
Outcomes for colorectal cancer
Tumor size of 50 mm or larger is a risk factor for complications, while a high procedure volume at the center is a protective factor.60
Endoscopic treatment of colorectal cancer: The Western perspective
EMR is the gold standard for removal of superficial colorectal lesions. However, ESD can be considered if there is suspicion of superficial submucosal invasion, especially for lesions larger than 20 mm that cannot be resected en bloc by EMR.32 ESD can also be used for fibrotic lesions not amenable to complete EMR removal, or as a salvage procedure after recurrence after EMR.67 Proper selection of cases is critical.1
Patients who have a superficial colonic lesion should be evaluated by means of high-definition endoscopy and chromoendoscopy to assess the mucosal pattern and establish feasibility of endoscopic resection. If submucosal invasion is suspected, staging with endoscopic ultrasonography or magnetic resonance imaging should be considered.5
FOLLOW-UP AFTER ESD
Endoscopic surveillance after the procedure is recommended, given the persistent risk of metachronous cancer after curative ESD due to its organ-sparing quality.68 Surveillance endoscopy aims to achieve early detection and subsequent endoscopic resection of metachronous lesions.
Histopathologic evaluation assessing the presence of malignant cells in the margins of a resected sample is mandatory for determining the next step in treatment. If margins are negative, follow-up endoscopy can be done every 6 to 12 months. If margins are positive, the approach includes surgery, reattempting ESD or endoscopic surveillance in 3 or 6 months.3,32 Although the surveillance strategy varies according to individual risk of metachronous cancer, it should be continued indefinitely.68
COMPLICATIONS OF ESD
The most common procedure-related complications of ESD are bleeding, perforation, and stricture. Most intraprocedural adverse events can be managed endoscopically.69
Bleeding
Most bleeding occurs during the procedure or early after it and can be controlled with electrocautery.49,69 No episodes of massive bleeding, defined as causing clinical symptoms and requiring transfusion or surgery, have been reported.20,43,55
In gastric ESD, delayed bleeding rates have ranged from 0 to 15.6%.69 Bleeding may be prevented with endoscopic coagulation of visible vessels after dissection has been completed and by proton pump inhibitor therapy.70,71 Excessive coagulation should be avoided to lower the risk of perforation.33
In colorectal ESD the bleeding rate has been reported to be 2.2%; applying coagulation to an area where a blood vessel is suspected before cutting (precoagulation) may prevent subsequent bleeding.21
Perforation
For gastric ESD, perforation rates range from 1.2% to 5.2%.69 Esophageal perforation rates can be up to 4%.49 In colorectal ESD, perforation rates have been reported to be 1.6% to 6.6%.60,72
Although most of the cases were successfully managed with conservative treatment, some required emergency surgery.60,73
Strictures
In a case series of 532 patients undergoing gastric ESD, stricture was reported in 5 patients, all of whom presented with obstructive symptoms.74 Risk factors for post-ESD gastric stenosis are a mucosal defect with a circumferential extent of more than three-fourths or a longitudinal extent of more than 5 cm.75
Strictures are common after esophageal ESD, with rates ranging from 2% to 26%. The risk is higher when longer segments are removed or circumferential resection is performed. As previously mentioned, this complication may be reduced with ingestion or injection of steroids after the procedure.53,54
Surprisingly, ESD of large colorectal lesions involving more than three-fourths of the circumference of the rectum is rarely complicated by stenosis.76
LIMITATIONS OF ESD
ESD requires a high level of technical skill, is time-consuming, and has a higher rate of complications than conventional endoscopic resection. A standardized ESD training system is needed, as the procedure is more difficult than EMR. Training in porcine models has been shown to confer competency in ESD in a Western setting.13,16,33
Colorectal ESD is an even more challenging procedure, given the potential for complications related to its anatomy. Training centers in Japan usually have their trainees first master gastric ESD, then assist in more than 20 colorectal ESDs conducted by experienced endoscopists, and accomplish 30 cases before performing the procedure safely and independently.
As the incidence of gastric cancer is low in Western countries, trainees may also begin with lower rectal lesions, which are easier to remove.77 Incorporation of ESD in the West would require a clear treatment algorithm. It is a complex procedure, with higher rates of complications, a prolonged learning curve, and prolonged procedure time. Therefore, it should be performed in specialized centers and under the special situations discussed here to ensure that the benefits for the patients outweigh the risks.
VALUE OF ENDOSCOPIC SUBMUCOSAL DISSECTION
The optimal method for resecting gastrointestinal neoplasms should be safe, cost-effective, and quick and should also completely remove the lesion. The best treatment strategy takes into account the characteristics of the lesion and the comorbidities and wishes of the patient. Internists should be aware of the multiple options available to achieve the best outcome for the patient.1
Endoscopic resection of superficial gastrointestinal neoplasms, including EMR and ESD, has been a subject of increasing interest due to its minimally invasive and potentially curative character. However, cancer can recur after endoscopic resection because the procedure is organ-sparing.
ESD allows resection of early gastrointestinal tumors with a minimally invasive technique. It can achieve higher curative resection rates and lower recurrence rates compared with EMR. Compared with surgery, ESD leads to less morbidity, fewer procedure-related complications, and lower medical costs. Indications should be rigorously followed to achieve successful treatments in selected patients.
Multiple variables have to be taken into account when deciding which treatment is best, such as tumor characteristics, the patient’s baseline condition, physician expertise, and hospital resources.48 Less-invasive treatments may improve the prognosis of patients. No matter the approach, patients should be treated in specialized treatment centers.
Internal medicine physicians should be aware of the advances in treatments for early gastrointestinal cancer so appropriate options can be considered.
- Burgess NG, Bourke MJ. Endoscopic resection of colorectal lesions: the narrowing divide between East and West. Dig Endosc 2016; 28:296–305.
- Kim DH, Jung HY, Gong EJ, et al. Endoscopic and oncologic outcomes of endoscopic resection for superficial esophageal neoplasm. Gut Liver 2015; 9:470–477.
- Draganov PV, Gotoda T, Chavalitdhamrong D, Wallace MB. Techniques of endoscopic submucosal dissection: application for the Western endoscopist? Gastrointest Endosc 2013; 78:677–688.
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14:101–112.
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2015; 47:829–854.
- Farhat S, Chaussade S, Ponchon T, et al; SFED ESD Study Group. Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy 2011; 43:664–670.
- Gotoda T, Jung H. Endoscopic resection (endoscopic mucosal resection/endoscopic submucosal dissection) for early gastric cancer. Dig Endosc 2013; 25(suppl 1):55–63.
- Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc 2009; 69:1228–1235.
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011; 14:113–123.
- Ono H. Endoscopic submucosal dissection for early gastric cancer. Chin J Dig Dis 2005; 6:119–121.
- Watanabe T, Itabashi M, Shimada Y, et al; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 2015; 20:207–239.
- Oyama T, Yahagi N, Ponchon T, Kiesslich T, Berr F. How to establish endoscopic submucosal dissection in Western countries. World J Gastroenterol 2015; 21:11209–11220.
- Bhatt A, Abe S, Kumaravel A, et al. SU1575 Western skill training in endoscopic submucosal dissection (ESD)—an international remote video based study—the WEST ESD Study. Gastrointest Endosc 2015; 81(suppl):AB335–AB336.
- Sano T, Sasako M, Kinoshita T, Maruyama K. Recurrence of early gastric cancer follow-up of 1475 patients and review of the Japanese literature. Cancer 1993; 72:3174–3178.
- Japan Esophageal Society. Japanese classification of esophageal cancer, tenth edition: part I. Esophagus 2009; 6:1–25.
- Bhatt A, Abe S, Kumaravel A, Vargo J, Saito Y. Indications and techniques for endoscopic submucosal dissection. Am J Gastroenterol 2015; 110:784–791.
- Eleftheriadis N, Inoue H, Ikeda H, et al. Definition and staging of early esophageal, gastric and colorectal cancer. J Tumor 2014; 2:161–178.
- Yoshinaga S, Oda I, Nonaka S, Kushima R, Saito Y. Endoscopic ultrasound using ultrasound probes for the diagnosis of early esophageal and gastric cancers. World J Gastrointest Endosc 2012; 4:218–226.
- Stahl M, Mariette C, Haustermans K, Cervantes A, Arnold D; ESMO Guidelines Working Group. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi51–vi56.
- Higuchi K, Tanabe S, Azuma M, et al. A phase II study of endoscopic submucosal dissection for superficial esophageal neoplasms (KDOG 0901). Gastrointest Endosc 2013; 78:704–710.
- Sakamoto T, Mori G, Yamada M, et al. Endoscopic submucosal dissection for colorectal neoplasms: a review. World J Gastroenterol 2014; 20:16153–16158.
- Ohta A, Tominaga K, Sakai Y. Efficacy of magnifying colonoscopy for the diagnosis of colorectal neoplasia: comparison with histopathological findings. Dig Endosc 2004; 16:308–314.
- Katagiri A, Fu K, Sano Y, et al. Narrow band imaging with magnifying colonoscopy as diagnostic tool for predicting histology of early colorectal neoplasia. Aliment Pharmacol Ther 2008; 27:1269–1274.
- Fu KI, Kato S, Sano Y, et al. Staging of early colorectal cancers: magnifying colonoscopy versus endoscopic ultrasonography for estimation of depth of invasion. Dig Dis Sci 2008; 53:1886–1892.
- Uraoka T, Saito Y, Ikematsu H, Yamamoto K, Sano Y. Sano’s capillary pattern classification for narrow-band imaging of early colorectal lesions. Dig Endosc 2011; 23(suppl 1):112–115.
- Ikematsu H, Matsuda T, Emura F, et al. Efficacy of capillary pattern type IIIA/IIIB by magnifying narrow band imaging for estimating depth of invasion of early colorectal neoplasms. BMC Gastroenterol 2010;10:33.
- Matsuda T, Fujii T, Saito Y, et al. Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol 2008; 103:2700–2706.
- Sato H, Inoue H, Ikeda H, et al. Utility of intrapapillary capillary loops seen on magnifying narrow-band imaging in estimating invasive depth of esophageal squamous cell carcinoma. Endoscopy 2015; 8:122–128.
- Muto M, Yao K, Kaise M, et al. Magnifying endoscopy simple diagnostic algorithm for early gastric cancer (MESDA-G). Dig Endosc 2016; 28:379–393.
- Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; European Society for Medical Oncology (ESMO); European Society of Surgical Oncology (ESSO); European Society of Radiotherapy and Oncology (ESTRO). Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi57–vi63.
- Kuwano H, Nishimura Y, Ohtsu A, et al. Guidelines for diagnosis and treatment of carcinoma of the esophagus. April 2007 edition: part I - Edited by the Japan Esophageal Society. Esophagus 2008; 5:61–73.
- Tanaka S, Kashida H, Saito Y, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc 2015; 27:417–434.
- Gotoda T, Ho KY, Soetikno R, Kaltenbach T, Draganov P. Gastric ESD: current status and future directions of devices and training. Gastrointest Endosc Clin North Am 2014; 24:213–233.
- Saito Y, Sakamoto T, Nakajima T, Matsuda T. Colorectal ESD: current indications and latest technical advances. Gastrointest Endosc Clin N Am 2014; 24:245–255.
- Saito Y, Otake Y, Sakamoto T, et al. Indications for and technical aspects of colorectal endoscopic submucosal dissection. Gut Liver 2013; 7:263–269.
- Saragoni L. Upgrading the definition of early gastric cancer: better staging means more appropriate treatment. Cancer Biol Med 2015; 12:355–361.
- Tsujitani S, Oka S, Saito H, et al. Less invasive surgery for early gastric cancer based on the low probability of lymph node metastasis. Surgery 1999; 125:148–154.
- Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc 2003; 57:567–579.
- Hirasawa T, Gotoda T, Miyata S, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 2009; 12:148–152.
- Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000; 3:219–225.
- Suzuki H, Oda I, Abe S, et al. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer 2016; 19:198–205.
- Matsuda T, Ajiki W, Marugame T, Ioka A, Tsukuma H, Sobue T; Research Group of Population-Based Cancer Registries of Japan. Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: a chronological and international comparative study. Jpn J Clin Oncol 2011; 41:40–51.
- Ahn JY, Jung HY, Choi KD, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc 2011; 74:485–493.
- Kim Y, Kim YW, Choi IJ, et al. Cost comparison between surgical treatments and endoscopic submucosal dissection in patients with early gastric cancer in Korea. Gut Liver 2015; 9:174–180.
- Abe S, Oda I, Nakajima T, et al. A case of local recurrence and distant metastasis following curative endoscopic submucosal dissection of early gastric cancer. Gastric Cancer 2015; 18:188–192.
- Hahn KY, Park JC, Kim EH, et al. Incidence and impact of scheduled endoscopic surveillance on recurrence after curative endoscopic resection for early gastric cancer. Gastrointest Endosc 2016; 84:628–638.e1.
- Wang S, Zhang Z, Liu M, Li S, Jiang C. Endoscopic resection compared with gastrectomy to treat early gastric cancer: a systematic review and meta-analysis. PLoS One 2015; 10:e0144774.
- Kondo A, de Moura EG, Bernardo WM, et al. Endoscopy vs surgery in the treatment of early gastric cancer: systematic review. World J Gastroenterol 2015; 21:13177–13187.
- Kothari S, Kaul V. Endoscopic mucosal resection and endoscopic submucosal dissection for endoscopic therapy of Barrett’s esophagus-related neoplasia. Gastroenterol Clin North Am 2015; 44:317–335.
- Yamashita T, Zeniya A, Ishii H, et al. Endoscopic mucosal resection using a cap-fitted panendoscope and endoscopic submucosal dissection as optimal endoscopic procedures for superficial esophageal carcinoma. Surg Endosc 2011; 25:2541–2546.
- Kagemoto K, Oka S, Tanaka S, et al. Clinical outcomes of endoscopic submucosal dissection for superficial Barrett’s adenocarcinoma. Gastrointest Endosc 2014; 80:239–245.
- Katada C, Muto M, Manabe T, Boku N, Ohtsu A, Yoshida S. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc 2003; 57:165–169.
- Hanaoka N, Ishihara R, Takeuchi Y, et al. 1139: A single session of intralesional steroid injection to prevent esophageal stricture after endoscopic submucosal dissection for esophageal squamous cell carcinoma. Gastrointest Endosc 2012; 75(suppl):AB175.
- Yamaguchi N, Isomoto H, Nakayama T, et al. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc 2011; 73:1115–1121.
- Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 2009; 70:860–866.
- Katada C, Muto M, Manabe T, Ohtsu A, Yoshida S. Local recurrence of squamous-cell carcinoma of the esophagus after EMR. Gastrointest Endosc 2005; 61:219–225.
- Hirasawa K, Kokawa A, Oka H, et al. Superficial adenocarcinoma of the esophagogastric junction: long-term results of endoscopic submucosal dissection. Gastrointest Endosc 2010; 72:960–966.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
- Nakajima T, Saito Y, Tanaka S, et al. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc 2013; 27:3262–3770.
- Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 2010; 72:1217–1225.
- Tanaka S, Saitoh Y, Matsuda T, et al; Japanese Society of Gastroenterology. Evidence-based clinical practice guidelines for management of colorectal polyps. J Gastroenterol 2015; 50:252–260.
- Oka S, Tanaka S, Saito Y, et al; Colorectal Endoscopic Resection Standardization Implementation Working Group of the Japanese Society for Cancer of the Colon and Rectum, Tokyo, Japan. Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol 2015; 110:697–707.
- Saito Y, Fukuzawa M, Matsuda T, et al. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc 2010; 24:343–352.
- Makazu M, Sakamoto T, So E, et al. Relationship between indeterminate or positive lateral margin and local recurrence after endoscopic resection of colorectal polyps. Endosc Int Open 2015; 3:E252–E257.
- Belderbos TD, Leenders M, Moons LM, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy 2014; 46:388–402.
- Fujiya M, Tanaka K, Dokoshi T, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc 2015; 81:583–595.
- Rahmi G, Tanaka S, Ohara Y, et al. Efficacy of endoscopic submucosal dissection for residual or recurrent superficial colorectal tumors after endoscopic mucosal resection. J Dig Dis 2015; 16:14–21.
- Abe S, Oda I, Suzuki H, et al. Long-term surveillance and treatment outcomes of metachronous gastric cancer occurring after curative endoscopic submucosal dissection. Endoscopy 2015; 47:1113–1118.
- Oda I, Suzuki H, Nonaka S, Yoshinaga S. Complications of gastric endoscopic submucosal dissection. Dig Endosc 2013; 25(suppl 1):71–78.
- Takizawa K, Oda I, Gotoda T, et al. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection—an analysis of risk factors. Endoscopy 2008; 40:179–183.
- Uedo N, Takeuchi Y, Yamada T, et al. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol 2007; 102:1610–1616.
- Hayashi N, Tanaka S, Nishiyama S, et al. Predictors of incomplete resection and perforation associated with endoscopic submucosal dissection for colorectal tumors. Gastrointest Endosc 2014; 79:427–435.
- Suzuki H, Oda I, Sekiguchi M, et al. Management and associated factors of delayed perforation after gastric endoscopic submucosal dissection. World J Gastroenterol 2015; 21:12635–12643.
- Tsunada S, Ogata S, Mannen K, et al. Case series of endoscopic balloon dilation to treat a stricture caused by circumferential resection of the gastric antrum by endoscopic submucosal dissection. Gastrointest Endosc 2008; 67:979–983.
- Coda S, Oda I, Gotoda T, Yokoi C, Kikuchi T, Ono H. Risk factors for cardiac and pyloric stenosis after endoscopic submucosal dissection, and efficacy of endoscopic balloon dilation treatment. Endoscopy 2009; 41:421–426.
- Abe S, Sakamoto T, Takamaru H, et al. Stenosis rates after endoscopic submucosal dissection of large rectal tumors involving greater than three quarters of the luminal circumference. Surg Endosc 2016; 30:5459–5464.
- Sakamoto T, Saito Y, Fukunaga S, Nakajima T, Matsuda T. Learning curve associated with colorectal endoscopic submucosal dissection for endoscopists experienced in gastric endoscopic submucosal dissection. Dis Colon Rectum 2011; 54:1307–1312.
The treatment of early esophageal, gastric, and colorectal cancer is changing.1 For many years, surgery was the mainstay of treatment for early-stage gastrointestinal cancer. Unfortunately, surgery leads to significant loss of function of the organ, resulting in increased morbidity and decreased quality of life.2
Endoscopic techniques, particularly endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), have been developed and are widely used in Japan, where gastrointestinal cancer is more common than in the West. This article reviews the indications, complications, and outcomes of ESD for early gastrointestinal neoplasms, so that readers will recognize the subset of patients who would benefit from ESD in a Western setting.
ENDOSCOPIC MUCOSAL RESECTION AND SUBMUCOSAL DISSECTION
Since the first therapeutic polypectomy was performed in Japan in 1974, several endoscopic techniques for tumor resection have been developed.3
EMR, one of the most successful and widely used techniques, involves elevating the lesion either with submucosal injection of a solution or with cap suction, and then removing it with a snare.4 Most lesions smaller than 20 mm can be removed in one piece (en bloc).5 Larger lesions are removed in multiple pieces (ie, piecemeal). Unfortunately, some fibrotic lesions, which are usually difficult to lift, cannot be completely removed by EMR.
ESD was first performed in the late 1990s with the aim of overcoming the limitations of EMR in resecting large or fibrotic tumors en bloc.6,7 Since then, ESD technique has been standardized and training centers have been created, especially in Asia, where it is widely used for treatment of early gastric cancer.3,8–10 Since 2012 it has been covered by the Japanese National Health Insurance for treatment of early gastric cancer, and since 2014 for treatment of colorectal malignant tumors measuring 2 to 5 cm.11
Adoption of ESD has been slow in Western countries, where many patients are still referred for surgery or undergo EMR for removal of superficial neoplasms. Reasons for this slow adoption are that gastric cancer is much less common in Western countries, and also that ESD demands a high level of technical skill, is difficult to learn, and is expensive.3,12,13 However, small groups of Western endoscopists have become interested and are advocating it, first studying it on their own and then training in a Japanese center and learning from experts performing the procedure.
Therefore, in a Western setting, ESD should be performed in specialized endoscopy centers and offered to selected patients.1
CANDIDATES SHOULD HAVE EARLY-STAGE, SUPERFICIAL TUMORS
Ideal candidates for endoscopic resection are patients who have early cancer with a negligible risk of lymph node metastasis, such as cancer limited to the mucosa (stage T1a).7 Therefore, to determine the best treatment for a patient with a newly diagnosed gastrointestinal neoplasm, it is mandatory to estimate the depth of invasion.
The depth of invasion is directly correlated with lymph node involvement, which is ultimately the main predictive factor for long-term adverse outcomes of gastrointestinal tumors.4,14–17 Accurate multidisciplinary preprocedure estimations are mandatory, as incorrect evaluations may result in inappropriate therapy and residual cancer.18
Other factors that have been used to predict lymph node involvement include tumor size, macroscopic appearance, histologic differentiation, and lymphatic and vascular involvement.19 Some of these factors can be assessed by special endoscopic techniques (chromoendoscopy and narrow-band imaging with magnifying endoscopy) that allow accurate real-time estimation of the depth of invasion of the lesion.5,17,20–27 Evaluation of microsurface and microvascular arrangements is especially useful for determining the feasibility of ESD in gastric tumors, evaluation of intracapillary loops is useful in esophageal lesions, and assessment of mucosal pit patterns is useful for colorectal lesions.21–29
Endoscopic ultrasonography is another tool that has been used to estimate the depth of the tumor. Although it can differentiate between definite intramucosal and definite submucosal invasive cancers, its ability to confirm minute submucosal invasion is limited. Its use as the sole tumor staging modality is not encouraged, and it should always be used in conjunction with endoscopic evaluation.18
Though the aforementioned factors help stratify patients, pathologic staging is the best predictor of lymph node metastasis. ESD provides adequate specimens for accurate pathologic evaluation, as it removes lesions en bloc.30
All patients found to have risk factors for lymph node metastasis on endoscopic, ultrasonographic, or pathologic analysis should be referred for surgical evaluation.9,19,31,32
ENDOSCOPIC SUBMUCOSAL DISSECTION
Before the procedure, the patient’s physicians need to do the following:
Determine the best type of intervention (EMR, ESD, ablation, surgery) for the specific lesion.3 A multidisciplinary approach is encouraged, with involvement of the internist, gastroenterologist, and surgeon.
Plan for anesthesia, additional consultations, pre- and postprocedural hospital admission, and need for special equipment.33
During the procedure
Define the lateral extent of the lesion using magnification chromoendoscopy or narrow-band imaging. In the stomach, a biopsy sample should be taken from the worst-looking segment and from normal-looking mucosa. Multiple biopsies should be avoided to prevent subsequent fibrosis.33 In the colon, biopsy should be avoided.34
Identify and circumferentially mark the target lesion. Cautery or argon plasma coagulation can be used for making markings at a distance of 5 to 10 mm from the edges.33 This is done to recognize the borders of the lesion, because they can become distorted after submucosal injection.14 This step is unnecessary in colorectal cases, as tumor margins can be adequately visualized after chromoendoscopy.16,35
Lift the lesion by injecting saline, 0.5% hyaluronate, or glycerin to create a submucosal fluid cushion.19,33
Perform a circumferential incision lateral to the mucosal margins to allow for a normal tissue margin.33 Partial incision is performed for esophageal and colorectal ESD to avoid fluid leakage from the submucosal layer, achieving a sustained submucosal lift and safer dissection.16
Submucosal dissection. The submucosal layer is dissected with an electrocautery knife until the lesion is completely removed. Dissection should be done carefully to keep the submucosal plane.33 Hemoclips or hemostat forceps can be used to control visible bleeding. The resected specimen is then stretched and fixed to a board using small pins for further histopathologic evaluation.35
Postprocedural monitoring. All patients should be admitted for overnight observation. Those who undergo gastric ESD should receive high-dose acid suppression, and the next day they can be started on a liquid diet.19
STOMACH CANCER
Indications for ESD for stomach cancer in the East
The incidence of gastric cancer is higher in Japan and Korea, where widespread screening programs have led to early identification and early treatment of this disease.36
Pathology studies37 of samples from patients with gastric cancer identified the following as risk factors for lymph node metastasis, which would make ESD unsuitable:
- Undifferentiated type
- Tumors larger than 2 cm
- Lymphatic or venous involvement
- Submucosal invasion
- Ulcerative change.
Based on these findings, the situations in which there was no risk of lymph node involvement (ie, when none of the above factors are present) were accepted as absolute indications for endoscopic resection of early gastric cancer.38 Further histologic studies identified a subset of patients with lesions with very low risk of lymph node metastasis, which outweighed the risk of surgery. Based on these findings, expanded criteria for gastric ESD were proposed,39,40 and the Japanese gastric cancer treatment guidelines now include these expanded preoperative indications9,17 (Table 1).
The Japanese Gastric Cancer Association has proposed a treatment algorithm based on the histopathologic evaluation after resection (Figure 2).9
Outcomes
In the largest series of patients who underwent curative ESD for early gastric cancer, the 5-year survival rate was 92.6%, the 5-year disease-specific survival rate was 99.9%, and the 5-year relative survival rate was 105%.41
Similarly, in a Japanese population-based survival analysis, the relative 5-year survival rate for localized gastric cancer was 94.4%.42 Rates of en bloc resection and complete resection with ESD are higher than those with EMR, resulting in a lower risk of local recurrence in selected patients who undergo ESD.8,43,44
Although rare, local recurrence after curative gastric ESD has been reported.45 The annual incidence of local recurrence has been estimated to be 0.84%.46
ESD entails a shorter hospital stay and requires fewer resources than surgery, resulting in lower medical costs (Table 2).44 Additionally, as endoscopic resection is associated with less morbidity, fewer procedure-related adverse events, and fewer complications, ESD could be used as the standard treatment for early gastric cancer.47,48
The Western perspective on endoscopic submucosal dissection for gastric cancer
Since the prevalence of gastric cancer in Western countries is significantly lower than in Japan and Korea, local data and experience are scarce. However, experts performing ESD in the West have adopted the indications of the Japan Gastroenterological Endoscopy Society. The European Society of Gastrointestinal Endoscopy recommends ESD for excision of most superficial gastric neoplasms, with EMR being preferred only in lesions smaller than 15 mm, Paris classification 0 or IIA.5,32
Patients with gastric lesions measuring 15 mm or larger should undergo high-quality endoscopy, preferably chromoendoscopy, to evaluate the mucosal patterns and determine the depth of invasion. If superficial involvement is confirmed, other imaging techniques are not routinely recommended.5 A surgery consult is also recommended.
ESOPHAGEAL CANCER
Indications for ESD for esophageal cancer in the East
Due to the success of ESD for early gastric cancer, this technique is now also used for superficial esophageal neoplasms.19,49 It should be done in a specialized center, as it is more technically difficult than gastric ESD: the esophageal lumen is narrow, the wall is thin, and the esophagus moves with respiration and heartbeat.50 A multidisciplinary approach including an endoscopist, a surgeon, and a pathologist is highly recommended for evaluation and treatment.
EMR is preferred for removal of mucosal cancer, in view of its safety profile and success rates. ESD can be considered in cases of lesions larger than 15 mm, poorly lifting tumors, and those with the possibility of submucosal invasion (Table 3).5,45,49,51
Circumference involvement is critical when determining eligible candidates, as a defect involving more than three-fourths of the esophageal circumference can lead to esophageal strictures.52 Controlled prospective studies have shown promising results from giving intralesional and oral steroids to prevent stricture after ESD, which could potentially overcome this size limitation.53,54
Outcomes for esophageal cancer
ESD has been shown to be safe and effective, achieving en bloc resection in 85% to 100% of patients.19,51 Its advantages over EMR include en bloc resection, complete resection, and high curative rates, resulting in higher recurrence-free survival.2,55,56 Although the incidence of complications such as bleeding, perforation, and stricture formation are higher with ESD, patients usually recover uneventfully.2,19,20
ESD in the esophagus: The Western perspective
As data on the efficacy of EMR vs ESD for the treatment of Barrett esophagus with adenocarcinoma are limited, EMR is the gold standard endoscopic technique for removal of visible esophageal dysplastic lesions.5,51,57 ESD can be considered for tumors larger than 15 mm, for poorly lifting lesions, and if there is suspicion of submucosal invasion.5
Patients should be evaluated by an experienced endoscopist, using an advanced imaging technique such as narrow-band imaging or chromoendoscopy. If suspicious features are found, endoscopic ultrasonography should be considered to confirm submucosal invasion or lymph node involvement.5
COLORECTAL CANCER
Indications for ESD for colorectal cancer in the East
Colon cancer is one of the leading causes of cancer-related deaths worldwide.58 Since ESD has been found to be effective and safe in treating gastric cancer, it has also been used to remove large colorectal tumors.59 However, ESD is not universally accepted in the treatment of colorectal neoplasms due to its greater technical difficulty, longer procedural time, and higher risk of perforating the thinner colonic wall compared with EMR.21,60
Outcomes for colorectal cancer
Tumor size of 50 mm or larger is a risk factor for complications, while a high procedure volume at the center is a protective factor.60
Endoscopic treatment of colorectal cancer: The Western perspective
EMR is the gold standard for removal of superficial colorectal lesions. However, ESD can be considered if there is suspicion of superficial submucosal invasion, especially for lesions larger than 20 mm that cannot be resected en bloc by EMR.32 ESD can also be used for fibrotic lesions not amenable to complete EMR removal, or as a salvage procedure after recurrence after EMR.67 Proper selection of cases is critical.1
Patients who have a superficial colonic lesion should be evaluated by means of high-definition endoscopy and chromoendoscopy to assess the mucosal pattern and establish feasibility of endoscopic resection. If submucosal invasion is suspected, staging with endoscopic ultrasonography or magnetic resonance imaging should be considered.5
FOLLOW-UP AFTER ESD
Endoscopic surveillance after the procedure is recommended, given the persistent risk of metachronous cancer after curative ESD due to its organ-sparing quality.68 Surveillance endoscopy aims to achieve early detection and subsequent endoscopic resection of metachronous lesions.
Histopathologic evaluation assessing the presence of malignant cells in the margins of a resected sample is mandatory for determining the next step in treatment. If margins are negative, follow-up endoscopy can be done every 6 to 12 months. If margins are positive, the approach includes surgery, reattempting ESD or endoscopic surveillance in 3 or 6 months.3,32 Although the surveillance strategy varies according to individual risk of metachronous cancer, it should be continued indefinitely.68
COMPLICATIONS OF ESD
The most common procedure-related complications of ESD are bleeding, perforation, and stricture. Most intraprocedural adverse events can be managed endoscopically.69
Bleeding
Most bleeding occurs during the procedure or early after it and can be controlled with electrocautery.49,69 No episodes of massive bleeding, defined as causing clinical symptoms and requiring transfusion or surgery, have been reported.20,43,55
In gastric ESD, delayed bleeding rates have ranged from 0 to 15.6%.69 Bleeding may be prevented with endoscopic coagulation of visible vessels after dissection has been completed and by proton pump inhibitor therapy.70,71 Excessive coagulation should be avoided to lower the risk of perforation.33
In colorectal ESD the bleeding rate has been reported to be 2.2%; applying coagulation to an area where a blood vessel is suspected before cutting (precoagulation) may prevent subsequent bleeding.21
Perforation
For gastric ESD, perforation rates range from 1.2% to 5.2%.69 Esophageal perforation rates can be up to 4%.49 In colorectal ESD, perforation rates have been reported to be 1.6% to 6.6%.60,72
Although most of the cases were successfully managed with conservative treatment, some required emergency surgery.60,73
Strictures
In a case series of 532 patients undergoing gastric ESD, stricture was reported in 5 patients, all of whom presented with obstructive symptoms.74 Risk factors for post-ESD gastric stenosis are a mucosal defect with a circumferential extent of more than three-fourths or a longitudinal extent of more than 5 cm.75
Strictures are common after esophageal ESD, with rates ranging from 2% to 26%. The risk is higher when longer segments are removed or circumferential resection is performed. As previously mentioned, this complication may be reduced with ingestion or injection of steroids after the procedure.53,54
Surprisingly, ESD of large colorectal lesions involving more than three-fourths of the circumference of the rectum is rarely complicated by stenosis.76
LIMITATIONS OF ESD
ESD requires a high level of technical skill, is time-consuming, and has a higher rate of complications than conventional endoscopic resection. A standardized ESD training system is needed, as the procedure is more difficult than EMR. Training in porcine models has been shown to confer competency in ESD in a Western setting.13,16,33
Colorectal ESD is an even more challenging procedure, given the potential for complications related to its anatomy. Training centers in Japan usually have their trainees first master gastric ESD, then assist in more than 20 colorectal ESDs conducted by experienced endoscopists, and accomplish 30 cases before performing the procedure safely and independently.
As the incidence of gastric cancer is low in Western countries, trainees may also begin with lower rectal lesions, which are easier to remove.77 Incorporation of ESD in the West would require a clear treatment algorithm. It is a complex procedure, with higher rates of complications, a prolonged learning curve, and prolonged procedure time. Therefore, it should be performed in specialized centers and under the special situations discussed here to ensure that the benefits for the patients outweigh the risks.
VALUE OF ENDOSCOPIC SUBMUCOSAL DISSECTION
The optimal method for resecting gastrointestinal neoplasms should be safe, cost-effective, and quick and should also completely remove the lesion. The best treatment strategy takes into account the characteristics of the lesion and the comorbidities and wishes of the patient. Internists should be aware of the multiple options available to achieve the best outcome for the patient.1
Endoscopic resection of superficial gastrointestinal neoplasms, including EMR and ESD, has been a subject of increasing interest due to its minimally invasive and potentially curative character. However, cancer can recur after endoscopic resection because the procedure is organ-sparing.
ESD allows resection of early gastrointestinal tumors with a minimally invasive technique. It can achieve higher curative resection rates and lower recurrence rates compared with EMR. Compared with surgery, ESD leads to less morbidity, fewer procedure-related complications, and lower medical costs. Indications should be rigorously followed to achieve successful treatments in selected patients.
Multiple variables have to be taken into account when deciding which treatment is best, such as tumor characteristics, the patient’s baseline condition, physician expertise, and hospital resources.48 Less-invasive treatments may improve the prognosis of patients. No matter the approach, patients should be treated in specialized treatment centers.
Internal medicine physicians should be aware of the advances in treatments for early gastrointestinal cancer so appropriate options can be considered.
The treatment of early esophageal, gastric, and colorectal cancer is changing.1 For many years, surgery was the mainstay of treatment for early-stage gastrointestinal cancer. Unfortunately, surgery leads to significant loss of function of the organ, resulting in increased morbidity and decreased quality of life.2
Endoscopic techniques, particularly endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), have been developed and are widely used in Japan, where gastrointestinal cancer is more common than in the West. This article reviews the indications, complications, and outcomes of ESD for early gastrointestinal neoplasms, so that readers will recognize the subset of patients who would benefit from ESD in a Western setting.
ENDOSCOPIC MUCOSAL RESECTION AND SUBMUCOSAL DISSECTION
Since the first therapeutic polypectomy was performed in Japan in 1974, several endoscopic techniques for tumor resection have been developed.3
EMR, one of the most successful and widely used techniques, involves elevating the lesion either with submucosal injection of a solution or with cap suction, and then removing it with a snare.4 Most lesions smaller than 20 mm can be removed in one piece (en bloc).5 Larger lesions are removed in multiple pieces (ie, piecemeal). Unfortunately, some fibrotic lesions, which are usually difficult to lift, cannot be completely removed by EMR.
ESD was first performed in the late 1990s with the aim of overcoming the limitations of EMR in resecting large or fibrotic tumors en bloc.6,7 Since then, ESD technique has been standardized and training centers have been created, especially in Asia, where it is widely used for treatment of early gastric cancer.3,8–10 Since 2012 it has been covered by the Japanese National Health Insurance for treatment of early gastric cancer, and since 2014 for treatment of colorectal malignant tumors measuring 2 to 5 cm.11
Adoption of ESD has been slow in Western countries, where many patients are still referred for surgery or undergo EMR for removal of superficial neoplasms. Reasons for this slow adoption are that gastric cancer is much less common in Western countries, and also that ESD demands a high level of technical skill, is difficult to learn, and is expensive.3,12,13 However, small groups of Western endoscopists have become interested and are advocating it, first studying it on their own and then training in a Japanese center and learning from experts performing the procedure.
Therefore, in a Western setting, ESD should be performed in specialized endoscopy centers and offered to selected patients.1
CANDIDATES SHOULD HAVE EARLY-STAGE, SUPERFICIAL TUMORS
Ideal candidates for endoscopic resection are patients who have early cancer with a negligible risk of lymph node metastasis, such as cancer limited to the mucosa (stage T1a).7 Therefore, to determine the best treatment for a patient with a newly diagnosed gastrointestinal neoplasm, it is mandatory to estimate the depth of invasion.
The depth of invasion is directly correlated with lymph node involvement, which is ultimately the main predictive factor for long-term adverse outcomes of gastrointestinal tumors.4,14–17 Accurate multidisciplinary preprocedure estimations are mandatory, as incorrect evaluations may result in inappropriate therapy and residual cancer.18
Other factors that have been used to predict lymph node involvement include tumor size, macroscopic appearance, histologic differentiation, and lymphatic and vascular involvement.19 Some of these factors can be assessed by special endoscopic techniques (chromoendoscopy and narrow-band imaging with magnifying endoscopy) that allow accurate real-time estimation of the depth of invasion of the lesion.5,17,20–27 Evaluation of microsurface and microvascular arrangements is especially useful for determining the feasibility of ESD in gastric tumors, evaluation of intracapillary loops is useful in esophageal lesions, and assessment of mucosal pit patterns is useful for colorectal lesions.21–29
Endoscopic ultrasonography is another tool that has been used to estimate the depth of the tumor. Although it can differentiate between definite intramucosal and definite submucosal invasive cancers, its ability to confirm minute submucosal invasion is limited. Its use as the sole tumor staging modality is not encouraged, and it should always be used in conjunction with endoscopic evaluation.18
Though the aforementioned factors help stratify patients, pathologic staging is the best predictor of lymph node metastasis. ESD provides adequate specimens for accurate pathologic evaluation, as it removes lesions en bloc.30
All patients found to have risk factors for lymph node metastasis on endoscopic, ultrasonographic, or pathologic analysis should be referred for surgical evaluation.9,19,31,32
ENDOSCOPIC SUBMUCOSAL DISSECTION
Before the procedure, the patient’s physicians need to do the following:
Determine the best type of intervention (EMR, ESD, ablation, surgery) for the specific lesion.3 A multidisciplinary approach is encouraged, with involvement of the internist, gastroenterologist, and surgeon.
Plan for anesthesia, additional consultations, pre- and postprocedural hospital admission, and need for special equipment.33
During the procedure
Define the lateral extent of the lesion using magnification chromoendoscopy or narrow-band imaging. In the stomach, a biopsy sample should be taken from the worst-looking segment and from normal-looking mucosa. Multiple biopsies should be avoided to prevent subsequent fibrosis.33 In the colon, biopsy should be avoided.34
Identify and circumferentially mark the target lesion. Cautery or argon plasma coagulation can be used for making markings at a distance of 5 to 10 mm from the edges.33 This is done to recognize the borders of the lesion, because they can become distorted after submucosal injection.14 This step is unnecessary in colorectal cases, as tumor margins can be adequately visualized after chromoendoscopy.16,35
Lift the lesion by injecting saline, 0.5% hyaluronate, or glycerin to create a submucosal fluid cushion.19,33
Perform a circumferential incision lateral to the mucosal margins to allow for a normal tissue margin.33 Partial incision is performed for esophageal and colorectal ESD to avoid fluid leakage from the submucosal layer, achieving a sustained submucosal lift and safer dissection.16
Submucosal dissection. The submucosal layer is dissected with an electrocautery knife until the lesion is completely removed. Dissection should be done carefully to keep the submucosal plane.33 Hemoclips or hemostat forceps can be used to control visible bleeding. The resected specimen is then stretched and fixed to a board using small pins for further histopathologic evaluation.35
Postprocedural monitoring. All patients should be admitted for overnight observation. Those who undergo gastric ESD should receive high-dose acid suppression, and the next day they can be started on a liquid diet.19
STOMACH CANCER
Indications for ESD for stomach cancer in the East
The incidence of gastric cancer is higher in Japan and Korea, where widespread screening programs have led to early identification and early treatment of this disease.36
Pathology studies37 of samples from patients with gastric cancer identified the following as risk factors for lymph node metastasis, which would make ESD unsuitable:
- Undifferentiated type
- Tumors larger than 2 cm
- Lymphatic or venous involvement
- Submucosal invasion
- Ulcerative change.
Based on these findings, the situations in which there was no risk of lymph node involvement (ie, when none of the above factors are present) were accepted as absolute indications for endoscopic resection of early gastric cancer.38 Further histologic studies identified a subset of patients with lesions with very low risk of lymph node metastasis, which outweighed the risk of surgery. Based on these findings, expanded criteria for gastric ESD were proposed,39,40 and the Japanese gastric cancer treatment guidelines now include these expanded preoperative indications9,17 (Table 1).
The Japanese Gastric Cancer Association has proposed a treatment algorithm based on the histopathologic evaluation after resection (Figure 2).9
Outcomes
In the largest series of patients who underwent curative ESD for early gastric cancer, the 5-year survival rate was 92.6%, the 5-year disease-specific survival rate was 99.9%, and the 5-year relative survival rate was 105%.41
Similarly, in a Japanese population-based survival analysis, the relative 5-year survival rate for localized gastric cancer was 94.4%.42 Rates of en bloc resection and complete resection with ESD are higher than those with EMR, resulting in a lower risk of local recurrence in selected patients who undergo ESD.8,43,44
Although rare, local recurrence after curative gastric ESD has been reported.45 The annual incidence of local recurrence has been estimated to be 0.84%.46
ESD entails a shorter hospital stay and requires fewer resources than surgery, resulting in lower medical costs (Table 2).44 Additionally, as endoscopic resection is associated with less morbidity, fewer procedure-related adverse events, and fewer complications, ESD could be used as the standard treatment for early gastric cancer.47,48
The Western perspective on endoscopic submucosal dissection for gastric cancer
Since the prevalence of gastric cancer in Western countries is significantly lower than in Japan and Korea, local data and experience are scarce. However, experts performing ESD in the West have adopted the indications of the Japan Gastroenterological Endoscopy Society. The European Society of Gastrointestinal Endoscopy recommends ESD for excision of most superficial gastric neoplasms, with EMR being preferred only in lesions smaller than 15 mm, Paris classification 0 or IIA.5,32
Patients with gastric lesions measuring 15 mm or larger should undergo high-quality endoscopy, preferably chromoendoscopy, to evaluate the mucosal patterns and determine the depth of invasion. If superficial involvement is confirmed, other imaging techniques are not routinely recommended.5 A surgery consult is also recommended.
ESOPHAGEAL CANCER
Indications for ESD for esophageal cancer in the East
Due to the success of ESD for early gastric cancer, this technique is now also used for superficial esophageal neoplasms.19,49 It should be done in a specialized center, as it is more technically difficult than gastric ESD: the esophageal lumen is narrow, the wall is thin, and the esophagus moves with respiration and heartbeat.50 A multidisciplinary approach including an endoscopist, a surgeon, and a pathologist is highly recommended for evaluation and treatment.
EMR is preferred for removal of mucosal cancer, in view of its safety profile and success rates. ESD can be considered in cases of lesions larger than 15 mm, poorly lifting tumors, and those with the possibility of submucosal invasion (Table 3).5,45,49,51
Circumference involvement is critical when determining eligible candidates, as a defect involving more than three-fourths of the esophageal circumference can lead to esophageal strictures.52 Controlled prospective studies have shown promising results from giving intralesional and oral steroids to prevent stricture after ESD, which could potentially overcome this size limitation.53,54
Outcomes for esophageal cancer
ESD has been shown to be safe and effective, achieving en bloc resection in 85% to 100% of patients.19,51 Its advantages over EMR include en bloc resection, complete resection, and high curative rates, resulting in higher recurrence-free survival.2,55,56 Although the incidence of complications such as bleeding, perforation, and stricture formation are higher with ESD, patients usually recover uneventfully.2,19,20
ESD in the esophagus: The Western perspective
As data on the efficacy of EMR vs ESD for the treatment of Barrett esophagus with adenocarcinoma are limited, EMR is the gold standard endoscopic technique for removal of visible esophageal dysplastic lesions.5,51,57 ESD can be considered for tumors larger than 15 mm, for poorly lifting lesions, and if there is suspicion of submucosal invasion.5
Patients should be evaluated by an experienced endoscopist, using an advanced imaging technique such as narrow-band imaging or chromoendoscopy. If suspicious features are found, endoscopic ultrasonography should be considered to confirm submucosal invasion or lymph node involvement.5
COLORECTAL CANCER
Indications for ESD for colorectal cancer in the East
Colon cancer is one of the leading causes of cancer-related deaths worldwide.58 Since ESD has been found to be effective and safe in treating gastric cancer, it has also been used to remove large colorectal tumors.59 However, ESD is not universally accepted in the treatment of colorectal neoplasms due to its greater technical difficulty, longer procedural time, and higher risk of perforating the thinner colonic wall compared with EMR.21,60
Outcomes for colorectal cancer
Tumor size of 50 mm or larger is a risk factor for complications, while a high procedure volume at the center is a protective factor.60
Endoscopic treatment of colorectal cancer: The Western perspective
EMR is the gold standard for removal of superficial colorectal lesions. However, ESD can be considered if there is suspicion of superficial submucosal invasion, especially for lesions larger than 20 mm that cannot be resected en bloc by EMR.32 ESD can also be used for fibrotic lesions not amenable to complete EMR removal, or as a salvage procedure after recurrence after EMR.67 Proper selection of cases is critical.1
Patients who have a superficial colonic lesion should be evaluated by means of high-definition endoscopy and chromoendoscopy to assess the mucosal pattern and establish feasibility of endoscopic resection. If submucosal invasion is suspected, staging with endoscopic ultrasonography or magnetic resonance imaging should be considered.5
FOLLOW-UP AFTER ESD
Endoscopic surveillance after the procedure is recommended, given the persistent risk of metachronous cancer after curative ESD due to its organ-sparing quality.68 Surveillance endoscopy aims to achieve early detection and subsequent endoscopic resection of metachronous lesions.
Histopathologic evaluation assessing the presence of malignant cells in the margins of a resected sample is mandatory for determining the next step in treatment. If margins are negative, follow-up endoscopy can be done every 6 to 12 months. If margins are positive, the approach includes surgery, reattempting ESD or endoscopic surveillance in 3 or 6 months.3,32 Although the surveillance strategy varies according to individual risk of metachronous cancer, it should be continued indefinitely.68
COMPLICATIONS OF ESD
The most common procedure-related complications of ESD are bleeding, perforation, and stricture. Most intraprocedural adverse events can be managed endoscopically.69
Bleeding
Most bleeding occurs during the procedure or early after it and can be controlled with electrocautery.49,69 No episodes of massive bleeding, defined as causing clinical symptoms and requiring transfusion or surgery, have been reported.20,43,55
In gastric ESD, delayed bleeding rates have ranged from 0 to 15.6%.69 Bleeding may be prevented with endoscopic coagulation of visible vessels after dissection has been completed and by proton pump inhibitor therapy.70,71 Excessive coagulation should be avoided to lower the risk of perforation.33
In colorectal ESD the bleeding rate has been reported to be 2.2%; applying coagulation to an area where a blood vessel is suspected before cutting (precoagulation) may prevent subsequent bleeding.21
Perforation
For gastric ESD, perforation rates range from 1.2% to 5.2%.69 Esophageal perforation rates can be up to 4%.49 In colorectal ESD, perforation rates have been reported to be 1.6% to 6.6%.60,72
Although most of the cases were successfully managed with conservative treatment, some required emergency surgery.60,73
Strictures
In a case series of 532 patients undergoing gastric ESD, stricture was reported in 5 patients, all of whom presented with obstructive symptoms.74 Risk factors for post-ESD gastric stenosis are a mucosal defect with a circumferential extent of more than three-fourths or a longitudinal extent of more than 5 cm.75
Strictures are common after esophageal ESD, with rates ranging from 2% to 26%. The risk is higher when longer segments are removed or circumferential resection is performed. As previously mentioned, this complication may be reduced with ingestion or injection of steroids after the procedure.53,54
Surprisingly, ESD of large colorectal lesions involving more than three-fourths of the circumference of the rectum is rarely complicated by stenosis.76
LIMITATIONS OF ESD
ESD requires a high level of technical skill, is time-consuming, and has a higher rate of complications than conventional endoscopic resection. A standardized ESD training system is needed, as the procedure is more difficult than EMR. Training in porcine models has been shown to confer competency in ESD in a Western setting.13,16,33
Colorectal ESD is an even more challenging procedure, given the potential for complications related to its anatomy. Training centers in Japan usually have their trainees first master gastric ESD, then assist in more than 20 colorectal ESDs conducted by experienced endoscopists, and accomplish 30 cases before performing the procedure safely and independently.
As the incidence of gastric cancer is low in Western countries, trainees may also begin with lower rectal lesions, which are easier to remove.77 Incorporation of ESD in the West would require a clear treatment algorithm. It is a complex procedure, with higher rates of complications, a prolonged learning curve, and prolonged procedure time. Therefore, it should be performed in specialized centers and under the special situations discussed here to ensure that the benefits for the patients outweigh the risks.
VALUE OF ENDOSCOPIC SUBMUCOSAL DISSECTION
The optimal method for resecting gastrointestinal neoplasms should be safe, cost-effective, and quick and should also completely remove the lesion. The best treatment strategy takes into account the characteristics of the lesion and the comorbidities and wishes of the patient. Internists should be aware of the multiple options available to achieve the best outcome for the patient.1
Endoscopic resection of superficial gastrointestinal neoplasms, including EMR and ESD, has been a subject of increasing interest due to its minimally invasive and potentially curative character. However, cancer can recur after endoscopic resection because the procedure is organ-sparing.
ESD allows resection of early gastrointestinal tumors with a minimally invasive technique. It can achieve higher curative resection rates and lower recurrence rates compared with EMR. Compared with surgery, ESD leads to less morbidity, fewer procedure-related complications, and lower medical costs. Indications should be rigorously followed to achieve successful treatments in selected patients.
Multiple variables have to be taken into account when deciding which treatment is best, such as tumor characteristics, the patient’s baseline condition, physician expertise, and hospital resources.48 Less-invasive treatments may improve the prognosis of patients. No matter the approach, patients should be treated in specialized treatment centers.
Internal medicine physicians should be aware of the advances in treatments for early gastrointestinal cancer so appropriate options can be considered.
- Burgess NG, Bourke MJ. Endoscopic resection of colorectal lesions: the narrowing divide between East and West. Dig Endosc 2016; 28:296–305.
- Kim DH, Jung HY, Gong EJ, et al. Endoscopic and oncologic outcomes of endoscopic resection for superficial esophageal neoplasm. Gut Liver 2015; 9:470–477.
- Draganov PV, Gotoda T, Chavalitdhamrong D, Wallace MB. Techniques of endoscopic submucosal dissection: application for the Western endoscopist? Gastrointest Endosc 2013; 78:677–688.
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14:101–112.
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2015; 47:829–854.
- Farhat S, Chaussade S, Ponchon T, et al; SFED ESD Study Group. Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy 2011; 43:664–670.
- Gotoda T, Jung H. Endoscopic resection (endoscopic mucosal resection/endoscopic submucosal dissection) for early gastric cancer. Dig Endosc 2013; 25(suppl 1):55–63.
- Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc 2009; 69:1228–1235.
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011; 14:113–123.
- Ono H. Endoscopic submucosal dissection for early gastric cancer. Chin J Dig Dis 2005; 6:119–121.
- Watanabe T, Itabashi M, Shimada Y, et al; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 2015; 20:207–239.
- Oyama T, Yahagi N, Ponchon T, Kiesslich T, Berr F. How to establish endoscopic submucosal dissection in Western countries. World J Gastroenterol 2015; 21:11209–11220.
- Bhatt A, Abe S, Kumaravel A, et al. SU1575 Western skill training in endoscopic submucosal dissection (ESD)—an international remote video based study—the WEST ESD Study. Gastrointest Endosc 2015; 81(suppl):AB335–AB336.
- Sano T, Sasako M, Kinoshita T, Maruyama K. Recurrence of early gastric cancer follow-up of 1475 patients and review of the Japanese literature. Cancer 1993; 72:3174–3178.
- Japan Esophageal Society. Japanese classification of esophageal cancer, tenth edition: part I. Esophagus 2009; 6:1–25.
- Bhatt A, Abe S, Kumaravel A, Vargo J, Saito Y. Indications and techniques for endoscopic submucosal dissection. Am J Gastroenterol 2015; 110:784–791.
- Eleftheriadis N, Inoue H, Ikeda H, et al. Definition and staging of early esophageal, gastric and colorectal cancer. J Tumor 2014; 2:161–178.
- Yoshinaga S, Oda I, Nonaka S, Kushima R, Saito Y. Endoscopic ultrasound using ultrasound probes for the diagnosis of early esophageal and gastric cancers. World J Gastrointest Endosc 2012; 4:218–226.
- Stahl M, Mariette C, Haustermans K, Cervantes A, Arnold D; ESMO Guidelines Working Group. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi51–vi56.
- Higuchi K, Tanabe S, Azuma M, et al. A phase II study of endoscopic submucosal dissection for superficial esophageal neoplasms (KDOG 0901). Gastrointest Endosc 2013; 78:704–710.
- Sakamoto T, Mori G, Yamada M, et al. Endoscopic submucosal dissection for colorectal neoplasms: a review. World J Gastroenterol 2014; 20:16153–16158.
- Ohta A, Tominaga K, Sakai Y. Efficacy of magnifying colonoscopy for the diagnosis of colorectal neoplasia: comparison with histopathological findings. Dig Endosc 2004; 16:308–314.
- Katagiri A, Fu K, Sano Y, et al. Narrow band imaging with magnifying colonoscopy as diagnostic tool for predicting histology of early colorectal neoplasia. Aliment Pharmacol Ther 2008; 27:1269–1274.
- Fu KI, Kato S, Sano Y, et al. Staging of early colorectal cancers: magnifying colonoscopy versus endoscopic ultrasonography for estimation of depth of invasion. Dig Dis Sci 2008; 53:1886–1892.
- Uraoka T, Saito Y, Ikematsu H, Yamamoto K, Sano Y. Sano’s capillary pattern classification for narrow-band imaging of early colorectal lesions. Dig Endosc 2011; 23(suppl 1):112–115.
- Ikematsu H, Matsuda T, Emura F, et al. Efficacy of capillary pattern type IIIA/IIIB by magnifying narrow band imaging for estimating depth of invasion of early colorectal neoplasms. BMC Gastroenterol 2010;10:33.
- Matsuda T, Fujii T, Saito Y, et al. Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol 2008; 103:2700–2706.
- Sato H, Inoue H, Ikeda H, et al. Utility of intrapapillary capillary loops seen on magnifying narrow-band imaging in estimating invasive depth of esophageal squamous cell carcinoma. Endoscopy 2015; 8:122–128.
- Muto M, Yao K, Kaise M, et al. Magnifying endoscopy simple diagnostic algorithm for early gastric cancer (MESDA-G). Dig Endosc 2016; 28:379–393.
- Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; European Society for Medical Oncology (ESMO); European Society of Surgical Oncology (ESSO); European Society of Radiotherapy and Oncology (ESTRO). Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi57–vi63.
- Kuwano H, Nishimura Y, Ohtsu A, et al. Guidelines for diagnosis and treatment of carcinoma of the esophagus. April 2007 edition: part I - Edited by the Japan Esophageal Society. Esophagus 2008; 5:61–73.
- Tanaka S, Kashida H, Saito Y, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc 2015; 27:417–434.
- Gotoda T, Ho KY, Soetikno R, Kaltenbach T, Draganov P. Gastric ESD: current status and future directions of devices and training. Gastrointest Endosc Clin North Am 2014; 24:213–233.
- Saito Y, Sakamoto T, Nakajima T, Matsuda T. Colorectal ESD: current indications and latest technical advances. Gastrointest Endosc Clin N Am 2014; 24:245–255.
- Saito Y, Otake Y, Sakamoto T, et al. Indications for and technical aspects of colorectal endoscopic submucosal dissection. Gut Liver 2013; 7:263–269.
- Saragoni L. Upgrading the definition of early gastric cancer: better staging means more appropriate treatment. Cancer Biol Med 2015; 12:355–361.
- Tsujitani S, Oka S, Saito H, et al. Less invasive surgery for early gastric cancer based on the low probability of lymph node metastasis. Surgery 1999; 125:148–154.
- Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc 2003; 57:567–579.
- Hirasawa T, Gotoda T, Miyata S, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 2009; 12:148–152.
- Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000; 3:219–225.
- Suzuki H, Oda I, Abe S, et al. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer 2016; 19:198–205.
- Matsuda T, Ajiki W, Marugame T, Ioka A, Tsukuma H, Sobue T; Research Group of Population-Based Cancer Registries of Japan. Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: a chronological and international comparative study. Jpn J Clin Oncol 2011; 41:40–51.
- Ahn JY, Jung HY, Choi KD, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc 2011; 74:485–493.
- Kim Y, Kim YW, Choi IJ, et al. Cost comparison between surgical treatments and endoscopic submucosal dissection in patients with early gastric cancer in Korea. Gut Liver 2015; 9:174–180.
- Abe S, Oda I, Nakajima T, et al. A case of local recurrence and distant metastasis following curative endoscopic submucosal dissection of early gastric cancer. Gastric Cancer 2015; 18:188–192.
- Hahn KY, Park JC, Kim EH, et al. Incidence and impact of scheduled endoscopic surveillance on recurrence after curative endoscopic resection for early gastric cancer. Gastrointest Endosc 2016; 84:628–638.e1.
- Wang S, Zhang Z, Liu M, Li S, Jiang C. Endoscopic resection compared with gastrectomy to treat early gastric cancer: a systematic review and meta-analysis. PLoS One 2015; 10:e0144774.
- Kondo A, de Moura EG, Bernardo WM, et al. Endoscopy vs surgery in the treatment of early gastric cancer: systematic review. World J Gastroenterol 2015; 21:13177–13187.
- Kothari S, Kaul V. Endoscopic mucosal resection and endoscopic submucosal dissection for endoscopic therapy of Barrett’s esophagus-related neoplasia. Gastroenterol Clin North Am 2015; 44:317–335.
- Yamashita T, Zeniya A, Ishii H, et al. Endoscopic mucosal resection using a cap-fitted panendoscope and endoscopic submucosal dissection as optimal endoscopic procedures for superficial esophageal carcinoma. Surg Endosc 2011; 25:2541–2546.
- Kagemoto K, Oka S, Tanaka S, et al. Clinical outcomes of endoscopic submucosal dissection for superficial Barrett’s adenocarcinoma. Gastrointest Endosc 2014; 80:239–245.
- Katada C, Muto M, Manabe T, Boku N, Ohtsu A, Yoshida S. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc 2003; 57:165–169.
- Hanaoka N, Ishihara R, Takeuchi Y, et al. 1139: A single session of intralesional steroid injection to prevent esophageal stricture after endoscopic submucosal dissection for esophageal squamous cell carcinoma. Gastrointest Endosc 2012; 75(suppl):AB175.
- Yamaguchi N, Isomoto H, Nakayama T, et al. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc 2011; 73:1115–1121.
- Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 2009; 70:860–866.
- Katada C, Muto M, Manabe T, Ohtsu A, Yoshida S. Local recurrence of squamous-cell carcinoma of the esophagus after EMR. Gastrointest Endosc 2005; 61:219–225.
- Hirasawa K, Kokawa A, Oka H, et al. Superficial adenocarcinoma of the esophagogastric junction: long-term results of endoscopic submucosal dissection. Gastrointest Endosc 2010; 72:960–966.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
- Nakajima T, Saito Y, Tanaka S, et al. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc 2013; 27:3262–3770.
- Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 2010; 72:1217–1225.
- Tanaka S, Saitoh Y, Matsuda T, et al; Japanese Society of Gastroenterology. Evidence-based clinical practice guidelines for management of colorectal polyps. J Gastroenterol 2015; 50:252–260.
- Oka S, Tanaka S, Saito Y, et al; Colorectal Endoscopic Resection Standardization Implementation Working Group of the Japanese Society for Cancer of the Colon and Rectum, Tokyo, Japan. Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol 2015; 110:697–707.
- Saito Y, Fukuzawa M, Matsuda T, et al. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc 2010; 24:343–352.
- Makazu M, Sakamoto T, So E, et al. Relationship between indeterminate or positive lateral margin and local recurrence after endoscopic resection of colorectal polyps. Endosc Int Open 2015; 3:E252–E257.
- Belderbos TD, Leenders M, Moons LM, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy 2014; 46:388–402.
- Fujiya M, Tanaka K, Dokoshi T, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc 2015; 81:583–595.
- Rahmi G, Tanaka S, Ohara Y, et al. Efficacy of endoscopic submucosal dissection for residual or recurrent superficial colorectal tumors after endoscopic mucosal resection. J Dig Dis 2015; 16:14–21.
- Abe S, Oda I, Suzuki H, et al. Long-term surveillance and treatment outcomes of metachronous gastric cancer occurring after curative endoscopic submucosal dissection. Endoscopy 2015; 47:1113–1118.
- Oda I, Suzuki H, Nonaka S, Yoshinaga S. Complications of gastric endoscopic submucosal dissection. Dig Endosc 2013; 25(suppl 1):71–78.
- Takizawa K, Oda I, Gotoda T, et al. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection—an analysis of risk factors. Endoscopy 2008; 40:179–183.
- Uedo N, Takeuchi Y, Yamada T, et al. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol 2007; 102:1610–1616.
- Hayashi N, Tanaka S, Nishiyama S, et al. Predictors of incomplete resection and perforation associated with endoscopic submucosal dissection for colorectal tumors. Gastrointest Endosc 2014; 79:427–435.
- Suzuki H, Oda I, Sekiguchi M, et al. Management and associated factors of delayed perforation after gastric endoscopic submucosal dissection. World J Gastroenterol 2015; 21:12635–12643.
- Tsunada S, Ogata S, Mannen K, et al. Case series of endoscopic balloon dilation to treat a stricture caused by circumferential resection of the gastric antrum by endoscopic submucosal dissection. Gastrointest Endosc 2008; 67:979–983.
- Coda S, Oda I, Gotoda T, Yokoi C, Kikuchi T, Ono H. Risk factors for cardiac and pyloric stenosis after endoscopic submucosal dissection, and efficacy of endoscopic balloon dilation treatment. Endoscopy 2009; 41:421–426.
- Abe S, Sakamoto T, Takamaru H, et al. Stenosis rates after endoscopic submucosal dissection of large rectal tumors involving greater than three quarters of the luminal circumference. Surg Endosc 2016; 30:5459–5464.
- Sakamoto T, Saito Y, Fukunaga S, Nakajima T, Matsuda T. Learning curve associated with colorectal endoscopic submucosal dissection for endoscopists experienced in gastric endoscopic submucosal dissection. Dis Colon Rectum 2011; 54:1307–1312.
- Burgess NG, Bourke MJ. Endoscopic resection of colorectal lesions: the narrowing divide between East and West. Dig Endosc 2016; 28:296–305.
- Kim DH, Jung HY, Gong EJ, et al. Endoscopic and oncologic outcomes of endoscopic resection for superficial esophageal neoplasm. Gut Liver 2015; 9:470–477.
- Draganov PV, Gotoda T, Chavalitdhamrong D, Wallace MB. Techniques of endoscopic submucosal dissection: application for the Western endoscopist? Gastrointest Endosc 2013; 78:677–688.
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14:101–112.
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2015; 47:829–854.
- Farhat S, Chaussade S, Ponchon T, et al; SFED ESD Study Group. Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy 2011; 43:664–670.
- Gotoda T, Jung H. Endoscopic resection (endoscopic mucosal resection/endoscopic submucosal dissection) for early gastric cancer. Dig Endosc 2013; 25(suppl 1):55–63.
- Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc 2009; 69:1228–1235.
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011; 14:113–123.
- Ono H. Endoscopic submucosal dissection for early gastric cancer. Chin J Dig Dis 2005; 6:119–121.
- Watanabe T, Itabashi M, Shimada Y, et al; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 2015; 20:207–239.
- Oyama T, Yahagi N, Ponchon T, Kiesslich T, Berr F. How to establish endoscopic submucosal dissection in Western countries. World J Gastroenterol 2015; 21:11209–11220.
- Bhatt A, Abe S, Kumaravel A, et al. SU1575 Western skill training in endoscopic submucosal dissection (ESD)—an international remote video based study—the WEST ESD Study. Gastrointest Endosc 2015; 81(suppl):AB335–AB336.
- Sano T, Sasako M, Kinoshita T, Maruyama K. Recurrence of early gastric cancer follow-up of 1475 patients and review of the Japanese literature. Cancer 1993; 72:3174–3178.
- Japan Esophageal Society. Japanese classification of esophageal cancer, tenth edition: part I. Esophagus 2009; 6:1–25.
- Bhatt A, Abe S, Kumaravel A, Vargo J, Saito Y. Indications and techniques for endoscopic submucosal dissection. Am J Gastroenterol 2015; 110:784–791.
- Eleftheriadis N, Inoue H, Ikeda H, et al. Definition and staging of early esophageal, gastric and colorectal cancer. J Tumor 2014; 2:161–178.
- Yoshinaga S, Oda I, Nonaka S, Kushima R, Saito Y. Endoscopic ultrasound using ultrasound probes for the diagnosis of early esophageal and gastric cancers. World J Gastrointest Endosc 2012; 4:218–226.
- Stahl M, Mariette C, Haustermans K, Cervantes A, Arnold D; ESMO Guidelines Working Group. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi51–vi56.
- Higuchi K, Tanabe S, Azuma M, et al. A phase II study of endoscopic submucosal dissection for superficial esophageal neoplasms (KDOG 0901). Gastrointest Endosc 2013; 78:704–710.
- Sakamoto T, Mori G, Yamada M, et al. Endoscopic submucosal dissection for colorectal neoplasms: a review. World J Gastroenterol 2014; 20:16153–16158.
- Ohta A, Tominaga K, Sakai Y. Efficacy of magnifying colonoscopy for the diagnosis of colorectal neoplasia: comparison with histopathological findings. Dig Endosc 2004; 16:308–314.
- Katagiri A, Fu K, Sano Y, et al. Narrow band imaging with magnifying colonoscopy as diagnostic tool for predicting histology of early colorectal neoplasia. Aliment Pharmacol Ther 2008; 27:1269–1274.
- Fu KI, Kato S, Sano Y, et al. Staging of early colorectal cancers: magnifying colonoscopy versus endoscopic ultrasonography for estimation of depth of invasion. Dig Dis Sci 2008; 53:1886–1892.
- Uraoka T, Saito Y, Ikematsu H, Yamamoto K, Sano Y. Sano’s capillary pattern classification for narrow-band imaging of early colorectal lesions. Dig Endosc 2011; 23(suppl 1):112–115.
- Ikematsu H, Matsuda T, Emura F, et al. Efficacy of capillary pattern type IIIA/IIIB by magnifying narrow band imaging for estimating depth of invasion of early colorectal neoplasms. BMC Gastroenterol 2010;10:33.
- Matsuda T, Fujii T, Saito Y, et al. Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol 2008; 103:2700–2706.
- Sato H, Inoue H, Ikeda H, et al. Utility of intrapapillary capillary loops seen on magnifying narrow-band imaging in estimating invasive depth of esophageal squamous cell carcinoma. Endoscopy 2015; 8:122–128.
- Muto M, Yao K, Kaise M, et al. Magnifying endoscopy simple diagnostic algorithm for early gastric cancer (MESDA-G). Dig Endosc 2016; 28:379–393.
- Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; European Society for Medical Oncology (ESMO); European Society of Surgical Oncology (ESSO); European Society of Radiotherapy and Oncology (ESTRO). Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(suppl 6):vi57–vi63.
- Kuwano H, Nishimura Y, Ohtsu A, et al. Guidelines for diagnosis and treatment of carcinoma of the esophagus. April 2007 edition: part I - Edited by the Japan Esophageal Society. Esophagus 2008; 5:61–73.
- Tanaka S, Kashida H, Saito Y, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc 2015; 27:417–434.
- Gotoda T, Ho KY, Soetikno R, Kaltenbach T, Draganov P. Gastric ESD: current status and future directions of devices and training. Gastrointest Endosc Clin North Am 2014; 24:213–233.
- Saito Y, Sakamoto T, Nakajima T, Matsuda T. Colorectal ESD: current indications and latest technical advances. Gastrointest Endosc Clin N Am 2014; 24:245–255.
- Saito Y, Otake Y, Sakamoto T, et al. Indications for and technical aspects of colorectal endoscopic submucosal dissection. Gut Liver 2013; 7:263–269.
- Saragoni L. Upgrading the definition of early gastric cancer: better staging means more appropriate treatment. Cancer Biol Med 2015; 12:355–361.
- Tsujitani S, Oka S, Saito H, et al. Less invasive surgery for early gastric cancer based on the low probability of lymph node metastasis. Surgery 1999; 125:148–154.
- Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc 2003; 57:567–579.
- Hirasawa T, Gotoda T, Miyata S, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 2009; 12:148–152.
- Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000; 3:219–225.
- Suzuki H, Oda I, Abe S, et al. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer 2016; 19:198–205.
- Matsuda T, Ajiki W, Marugame T, Ioka A, Tsukuma H, Sobue T; Research Group of Population-Based Cancer Registries of Japan. Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: a chronological and international comparative study. Jpn J Clin Oncol 2011; 41:40–51.
- Ahn JY, Jung HY, Choi KD, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc 2011; 74:485–493.
- Kim Y, Kim YW, Choi IJ, et al. Cost comparison between surgical treatments and endoscopic submucosal dissection in patients with early gastric cancer in Korea. Gut Liver 2015; 9:174–180.
- Abe S, Oda I, Nakajima T, et al. A case of local recurrence and distant metastasis following curative endoscopic submucosal dissection of early gastric cancer. Gastric Cancer 2015; 18:188–192.
- Hahn KY, Park JC, Kim EH, et al. Incidence and impact of scheduled endoscopic surveillance on recurrence after curative endoscopic resection for early gastric cancer. Gastrointest Endosc 2016; 84:628–638.e1.
- Wang S, Zhang Z, Liu M, Li S, Jiang C. Endoscopic resection compared with gastrectomy to treat early gastric cancer: a systematic review and meta-analysis. PLoS One 2015; 10:e0144774.
- Kondo A, de Moura EG, Bernardo WM, et al. Endoscopy vs surgery in the treatment of early gastric cancer: systematic review. World J Gastroenterol 2015; 21:13177–13187.
- Kothari S, Kaul V. Endoscopic mucosal resection and endoscopic submucosal dissection for endoscopic therapy of Barrett’s esophagus-related neoplasia. Gastroenterol Clin North Am 2015; 44:317–335.
- Yamashita T, Zeniya A, Ishii H, et al. Endoscopic mucosal resection using a cap-fitted panendoscope and endoscopic submucosal dissection as optimal endoscopic procedures for superficial esophageal carcinoma. Surg Endosc 2011; 25:2541–2546.
- Kagemoto K, Oka S, Tanaka S, et al. Clinical outcomes of endoscopic submucosal dissection for superficial Barrett’s adenocarcinoma. Gastrointest Endosc 2014; 80:239–245.
- Katada C, Muto M, Manabe T, Boku N, Ohtsu A, Yoshida S. Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc 2003; 57:165–169.
- Hanaoka N, Ishihara R, Takeuchi Y, et al. 1139: A single session of intralesional steroid injection to prevent esophageal stricture after endoscopic submucosal dissection for esophageal squamous cell carcinoma. Gastrointest Endosc 2012; 75(suppl):AB175.
- Yamaguchi N, Isomoto H, Nakayama T, et al. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc 2011; 73:1115–1121.
- Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 2009; 70:860–866.
- Katada C, Muto M, Manabe T, Ohtsu A, Yoshida S. Local recurrence of squamous-cell carcinoma of the esophagus after EMR. Gastrointest Endosc 2005; 61:219–225.
- Hirasawa K, Kokawa A, Oka H, et al. Superficial adenocarcinoma of the esophagogastric junction: long-term results of endoscopic submucosal dissection. Gastrointest Endosc 2010; 72:960–966.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
- Nakajima T, Saito Y, Tanaka S, et al. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc 2013; 27:3262–3770.
- Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 2010; 72:1217–1225.
- Tanaka S, Saitoh Y, Matsuda T, et al; Japanese Society of Gastroenterology. Evidence-based clinical practice guidelines for management of colorectal polyps. J Gastroenterol 2015; 50:252–260.
- Oka S, Tanaka S, Saito Y, et al; Colorectal Endoscopic Resection Standardization Implementation Working Group of the Japanese Society for Cancer of the Colon and Rectum, Tokyo, Japan. Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol 2015; 110:697–707.
- Saito Y, Fukuzawa M, Matsuda T, et al. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc 2010; 24:343–352.
- Makazu M, Sakamoto T, So E, et al. Relationship between indeterminate or positive lateral margin and local recurrence after endoscopic resection of colorectal polyps. Endosc Int Open 2015; 3:E252–E257.
- Belderbos TD, Leenders M, Moons LM, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy 2014; 46:388–402.
- Fujiya M, Tanaka K, Dokoshi T, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc 2015; 81:583–595.
- Rahmi G, Tanaka S, Ohara Y, et al. Efficacy of endoscopic submucosal dissection for residual or recurrent superficial colorectal tumors after endoscopic mucosal resection. J Dig Dis 2015; 16:14–21.
- Abe S, Oda I, Suzuki H, et al. Long-term surveillance and treatment outcomes of metachronous gastric cancer occurring after curative endoscopic submucosal dissection. Endoscopy 2015; 47:1113–1118.
- Oda I, Suzuki H, Nonaka S, Yoshinaga S. Complications of gastric endoscopic submucosal dissection. Dig Endosc 2013; 25(suppl 1):71–78.
- Takizawa K, Oda I, Gotoda T, et al. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection—an analysis of risk factors. Endoscopy 2008; 40:179–183.
- Uedo N, Takeuchi Y, Yamada T, et al. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol 2007; 102:1610–1616.
- Hayashi N, Tanaka S, Nishiyama S, et al. Predictors of incomplete resection and perforation associated with endoscopic submucosal dissection for colorectal tumors. Gastrointest Endosc 2014; 79:427–435.
- Suzuki H, Oda I, Sekiguchi M, et al. Management and associated factors of delayed perforation after gastric endoscopic submucosal dissection. World J Gastroenterol 2015; 21:12635–12643.
- Tsunada S, Ogata S, Mannen K, et al. Case series of endoscopic balloon dilation to treat a stricture caused by circumferential resection of the gastric antrum by endoscopic submucosal dissection. Gastrointest Endosc 2008; 67:979–983.
- Coda S, Oda I, Gotoda T, Yokoi C, Kikuchi T, Ono H. Risk factors for cardiac and pyloric stenosis after endoscopic submucosal dissection, and efficacy of endoscopic balloon dilation treatment. Endoscopy 2009; 41:421–426.
- Abe S, Sakamoto T, Takamaru H, et al. Stenosis rates after endoscopic submucosal dissection of large rectal tumors involving greater than three quarters of the luminal circumference. Surg Endosc 2016; 30:5459–5464.
- Sakamoto T, Saito Y, Fukunaga S, Nakajima T, Matsuda T. Learning curve associated with colorectal endoscopic submucosal dissection for endoscopists experienced in gastric endoscopic submucosal dissection. Dis Colon Rectum 2011; 54:1307–1312.
KEY POINTS
- ESD is a minimally invasive endoscopic technique with curative potential for patients with superficial GI neoplasia.
- ESD preserves the integrity of the organ while achieving curative resection of large neoplasms.
- ESD is indicated rather than surgery in patients with early GI lesions with a negligible risk of lymph node metastasis.
- Complications of the procedure include bleeding, perforation, and stenosis. Most of these respond to endoscopic treatment.
- Successful ESD requires supportive teamwork among internists, gastroenterologists, pathologists, and surgeons.
Heartburn or heart attack? A mimic of MI
A 71-year-old man with a history of hypertension, 4 prior myocardial infarctions (MIs), and well-compensated ischemic cardiomyopathy presented to the emergency department after 2 episodes of sharp pain in the left upper abdomen and chest. The episodes lasted 1 to 2 minutes and were not relieved by rest. Their location was similar to that of the pain he experienced with his MIs. He could not identify any exacerbating or ameliorating factors. The pain had resolved without specific therapy before he arrived.
He reported polydipsia and constipation over the past 2 weeks and generalized muscle weakness and acute exacerbations of chronic back pain in the past 2 days. Neither he nor a friend who accompanied him noticed any confusion. He had been taking as many as 15 calcium carbonate tablets a day for 6 weeks to self-treat dyspepsia refractory to once-daily ranitidine, and hydrochlorothiazide for his hypertension for 3 weeks.
FURTHER EVALUATION, CARDIOLOGY CONSULT
On physical examination, he had diffuse weakness, dry mucous membranes, and an irregular heart rhythm.
Laboratory testing showed the following:
- Troponin I 0.11 ng/mL (reference range ≤ 0.04); repeated, it was 0.12 ng/mL
- Serum creatinine 3.4 mg/dL (0.44–1.27) (9 months earlier it had been 0.99 mg/dL)
- Serum calcium 17.3 mg/dL (8.6–10.5)
- Parathyroid hormone 9 pg/mL (12–88)
- Serum bicarbonate 33 mmol/L (24–32); 2 weeks earlier, it had been 27 mmol/L.
DIAGNOSIS: MILK-ALKALI SYNDROME
The diagnosis, based on the presentation and the results of the workup, was milk-alkali syndrome complicated by recent hydrochlorothiazide use. This syndrome consists of the triad of hypercalcemia, metabolic alkalosis, and acute kidney injury, all due to excessive ingestion of calcium and alkali, usually calcium carbonate.
His hydrochlorothiazide and calcium carbonate were discontinued. He was given intravenous normal saline and subcutaneous calcitonin, and his serum calcium level came down to 11.5 mg/dL within the next 24 hours. His dyspepsia was treated with pantoprazole.
The patient had no further episodes of chest pain, and the cardiology consult team again recommended against coronary angiography. Repeat ECG after the hypercalcemia resolved showed results identical to those 4 months before his admission. Two months later, his serum calcium level was 9.4 mg/dL and his creatinine level was 1.24 mg/dL.
A MIMIC OF STEMI
In numerous reported cases, these electrocardiographic findings coupled with chest pain led to misdiagnosis of STEMI.1–3 While STEMI and occasionally hypercalcemia can cause ST elevation, hypercalcemia causes a significant shortening of the corrected QT interval that is not associated with STEMI.4,5
Ultimately, the diagnosis of MI involves clinical, laboratory, and ECG findings, and if a strong clinical suspicion for myocardial ischemia exists, STEMI cannot reliably be distinguished from hypercalcemia by ECG alone. It is nonetheless important to be aware of this complication of hypercalcemia to avoid unnecessary cardiac interventions.
- Ashizawa N, Arakawa S, Koide Y, Toda G, Seto S, Yano K. Hypercalcemia due to vitamin D intoxication with clinical features mimicking acute myocardial infarction. Intern Med 2003; 42:340–344.
- Nishi SP, Barbagelata NA, Atar S, Birnbaum Y, Tuero E. Hypercalcemia-induced ST-segment elevation mimicking acute myocardial infarction. J Electrocardiol 2006; 39:298–300.
- Turnham S, Kilickap M, Kilinc S. ST segment elevation mimicking acute myocardial infarction in hypercalcemia. Heart 2005; 91:999.
- Nierenberg DW, Ransil BJ. Q-aTc interval as a clinical indicator of hypercalcemia. Am J Cardiol 1979; 44:243–248.
- Ahmed R, Hashiba K. Reliability of QT intervals as indicators of clinical hypercalcemia. Clin Cardiol 1988; 11:395–400.
A 71-year-old man with a history of hypertension, 4 prior myocardial infarctions (MIs), and well-compensated ischemic cardiomyopathy presented to the emergency department after 2 episodes of sharp pain in the left upper abdomen and chest. The episodes lasted 1 to 2 minutes and were not relieved by rest. Their location was similar to that of the pain he experienced with his MIs. He could not identify any exacerbating or ameliorating factors. The pain had resolved without specific therapy before he arrived.
He reported polydipsia and constipation over the past 2 weeks and generalized muscle weakness and acute exacerbations of chronic back pain in the past 2 days. Neither he nor a friend who accompanied him noticed any confusion. He had been taking as many as 15 calcium carbonate tablets a day for 6 weeks to self-treat dyspepsia refractory to once-daily ranitidine, and hydrochlorothiazide for his hypertension for 3 weeks.
FURTHER EVALUATION, CARDIOLOGY CONSULT
On physical examination, he had diffuse weakness, dry mucous membranes, and an irregular heart rhythm.
Laboratory testing showed the following:
- Troponin I 0.11 ng/mL (reference range ≤ 0.04); repeated, it was 0.12 ng/mL
- Serum creatinine 3.4 mg/dL (0.44–1.27) (9 months earlier it had been 0.99 mg/dL)
- Serum calcium 17.3 mg/dL (8.6–10.5)
- Parathyroid hormone 9 pg/mL (12–88)
- Serum bicarbonate 33 mmol/L (24–32); 2 weeks earlier, it had been 27 mmol/L.
DIAGNOSIS: MILK-ALKALI SYNDROME
The diagnosis, based on the presentation and the results of the workup, was milk-alkali syndrome complicated by recent hydrochlorothiazide use. This syndrome consists of the triad of hypercalcemia, metabolic alkalosis, and acute kidney injury, all due to excessive ingestion of calcium and alkali, usually calcium carbonate.
His hydrochlorothiazide and calcium carbonate were discontinued. He was given intravenous normal saline and subcutaneous calcitonin, and his serum calcium level came down to 11.5 mg/dL within the next 24 hours. His dyspepsia was treated with pantoprazole.
The patient had no further episodes of chest pain, and the cardiology consult team again recommended against coronary angiography. Repeat ECG after the hypercalcemia resolved showed results identical to those 4 months before his admission. Two months later, his serum calcium level was 9.4 mg/dL and his creatinine level was 1.24 mg/dL.
A MIMIC OF STEMI
In numerous reported cases, these electrocardiographic findings coupled with chest pain led to misdiagnosis of STEMI.1–3 While STEMI and occasionally hypercalcemia can cause ST elevation, hypercalcemia causes a significant shortening of the corrected QT interval that is not associated with STEMI.4,5
Ultimately, the diagnosis of MI involves clinical, laboratory, and ECG findings, and if a strong clinical suspicion for myocardial ischemia exists, STEMI cannot reliably be distinguished from hypercalcemia by ECG alone. It is nonetheless important to be aware of this complication of hypercalcemia to avoid unnecessary cardiac interventions.
A 71-year-old man with a history of hypertension, 4 prior myocardial infarctions (MIs), and well-compensated ischemic cardiomyopathy presented to the emergency department after 2 episodes of sharp pain in the left upper abdomen and chest. The episodes lasted 1 to 2 minutes and were not relieved by rest. Their location was similar to that of the pain he experienced with his MIs. He could not identify any exacerbating or ameliorating factors. The pain had resolved without specific therapy before he arrived.
He reported polydipsia and constipation over the past 2 weeks and generalized muscle weakness and acute exacerbations of chronic back pain in the past 2 days. Neither he nor a friend who accompanied him noticed any confusion. He had been taking as many as 15 calcium carbonate tablets a day for 6 weeks to self-treat dyspepsia refractory to once-daily ranitidine, and hydrochlorothiazide for his hypertension for 3 weeks.
FURTHER EVALUATION, CARDIOLOGY CONSULT
On physical examination, he had diffuse weakness, dry mucous membranes, and an irregular heart rhythm.
Laboratory testing showed the following:
- Troponin I 0.11 ng/mL (reference range ≤ 0.04); repeated, it was 0.12 ng/mL
- Serum creatinine 3.4 mg/dL (0.44–1.27) (9 months earlier it had been 0.99 mg/dL)
- Serum calcium 17.3 mg/dL (8.6–10.5)
- Parathyroid hormone 9 pg/mL (12–88)
- Serum bicarbonate 33 mmol/L (24–32); 2 weeks earlier, it had been 27 mmol/L.
DIAGNOSIS: MILK-ALKALI SYNDROME
The diagnosis, based on the presentation and the results of the workup, was milk-alkali syndrome complicated by recent hydrochlorothiazide use. This syndrome consists of the triad of hypercalcemia, metabolic alkalosis, and acute kidney injury, all due to excessive ingestion of calcium and alkali, usually calcium carbonate.
His hydrochlorothiazide and calcium carbonate were discontinued. He was given intravenous normal saline and subcutaneous calcitonin, and his serum calcium level came down to 11.5 mg/dL within the next 24 hours. His dyspepsia was treated with pantoprazole.
The patient had no further episodes of chest pain, and the cardiology consult team again recommended against coronary angiography. Repeat ECG after the hypercalcemia resolved showed results identical to those 4 months before his admission. Two months later, his serum calcium level was 9.4 mg/dL and his creatinine level was 1.24 mg/dL.
A MIMIC OF STEMI
In numerous reported cases, these electrocardiographic findings coupled with chest pain led to misdiagnosis of STEMI.1–3 While STEMI and occasionally hypercalcemia can cause ST elevation, hypercalcemia causes a significant shortening of the corrected QT interval that is not associated with STEMI.4,5
Ultimately, the diagnosis of MI involves clinical, laboratory, and ECG findings, and if a strong clinical suspicion for myocardial ischemia exists, STEMI cannot reliably be distinguished from hypercalcemia by ECG alone. It is nonetheless important to be aware of this complication of hypercalcemia to avoid unnecessary cardiac interventions.
- Ashizawa N, Arakawa S, Koide Y, Toda G, Seto S, Yano K. Hypercalcemia due to vitamin D intoxication with clinical features mimicking acute myocardial infarction. Intern Med 2003; 42:340–344.
- Nishi SP, Barbagelata NA, Atar S, Birnbaum Y, Tuero E. Hypercalcemia-induced ST-segment elevation mimicking acute myocardial infarction. J Electrocardiol 2006; 39:298–300.
- Turnham S, Kilickap M, Kilinc S. ST segment elevation mimicking acute myocardial infarction in hypercalcemia. Heart 2005; 91:999.
- Nierenberg DW, Ransil BJ. Q-aTc interval as a clinical indicator of hypercalcemia. Am J Cardiol 1979; 44:243–248.
- Ahmed R, Hashiba K. Reliability of QT intervals as indicators of clinical hypercalcemia. Clin Cardiol 1988; 11:395–400.
- Ashizawa N, Arakawa S, Koide Y, Toda G, Seto S, Yano K. Hypercalcemia due to vitamin D intoxication with clinical features mimicking acute myocardial infarction. Intern Med 2003; 42:340–344.
- Nishi SP, Barbagelata NA, Atar S, Birnbaum Y, Tuero E. Hypercalcemia-induced ST-segment elevation mimicking acute myocardial infarction. J Electrocardiol 2006; 39:298–300.
- Turnham S, Kilickap M, Kilinc S. ST segment elevation mimicking acute myocardial infarction in hypercalcemia. Heart 2005; 91:999.
- Nierenberg DW, Ransil BJ. Q-aTc interval as a clinical indicator of hypercalcemia. Am J Cardiol 1979; 44:243–248.
- Ahmed R, Hashiba K. Reliability of QT intervals as indicators of clinical hypercalcemia. Clin Cardiol 1988; 11:395–400.
Measuring both serum amylase and lipase for acute pancreatitis lowers quality and raises cost
A 43-year-old, previously healthy woman was admitted to the hospital after 1 day of severe epigastric abdominal pain, nausea, and vomiting. She denied alcohol or tobacco use.
Her physical examination revealed normal vital signs and epigastric tenderness without rebound tenderness.
Notable laboratory results:
- Aspartate aminotransferase 149 U/L (reference range 10–35)
- Alanine aminotransferase 140 U/L (10–35)
- Alkaline phosphatase 178 IU/L (35–104)
- Total bilirubin 1.8 mg/dL (0.2–1.3)
- Amylase 1,244 U/L (28–100)
- Lipase 14,628 U/L (7–59).
Abdominal ultrasonography showed a dilated bile duct and gallstones.
The patient was diagnosed with biliary pancreatitis and was treated by placing her on nothing-by-mouth (NPO) status and giving intravenous fluids and analgesics. All symptoms had resolved by hospital day 3. She underwent laparoscopic cholecystectomy and was discharged the following day.
This is a typical case of biliary pancreatitis that was diagnosed and treated appropriately with a positive outcome. But was it necessary or beneficial to measure both the serum amylase and serum lipase to make the correct diagnosis and treat the patient appropriately?
IS MEASURING SERUM AMYLASE NECESSARY?
The American College of Gastroenterology practice guidelines suggest that measuring both serum amylase and serum lipase is not necessary.1 Serum lipase alone is the preferred test for diagnosing acute pancreatitis, since it is more sensitive than serum amylase, just as specific, rises more quickly, and remains elevated longer.
In a retrospective study of 151 patients with acute pancreatitis,2 the sensitivity of lipase was 96.6% and the specificity was 99.4%.2 In contrast, the sensitivity of amylase was 78.6% and the specificity was 99.1%.
In another study,3 in 476 patients with acute pancreatitis, lipase had a sensitivity of 91% vs 62% for amylase. Again, specificity was similar between the two tests (92% for lipase and 93% for amylase). The authors concluded that lipase should replace amylase as the first-line laboratory investigation for suspected acute pancreatitis.
Smith et al4 reviewed 1,825 patients with acute pancreatitis and similarly concluded that pancreatic lipase is a more accurate biomarker of acute pancreatitis than serum amylase.
PRACTICE AT OUR HOSPITAL
Despite this guideline and evidence, concurrent ordering of serum amylase and lipase is common at many institutions.
We evaluated the practice of ordering both serum amylase and lipase for diagnosis of acute pancreatitis at our 300-bed academic medical hospital. From January 2011 through August 2014, our institution completed 26,254 orders for serum amylase and lipase measurement in 13,198 patients. In 9,938 (75%) of the patients, amylase and lipase were ordered concurrently. Of these, 482 patients (4.8%) had either amylase or lipase elevated above the diagnostic threshold, ie, 3 times the upper limit of normal, and 63 of the 482 patients had an elevation in serum amylase greater than 3 times the upper limit of normal without an elevation in serum lipase.
None of the patients had acute pancreatitis clinically (eg, typical abdominal pain, nausea, vomiting) or on imaging (pancreatic edema). The definitive cause of nonpancreatic hyperamylasemia could not be determined in these patients; they did not have evidence of salivary disorder, malignancy, or tubo-ovarian disease, and the hyperamylasemia was believed to be related to renal disease, diabetic ketoacidosis, infection, or medications, or to be idiopathic.
In 12 patients, the discrepancy between an elevated amylase and normal lipase resulted in additional imaging with computed tomography. Four patients were also unnecessarily kept NPO for 1 to 3 days, depriving them of nutrition and prolonging their hospital stay.
To minimize concurrent ordering of serum amylase and lipase, we introduced a best-practice alert in the computerized physician order entry systems. The alert mentioned that “ordering both serum amylase and lipase in cases of suspected pancreatitis is unnecessary. Serum lipase alone is sufficient.” However, ordering providers could still order both tests if they wanted to.
In the 3 months after the alert was implemented, serum lipase was ordered 1,780 times with 532 (30%) concurrent orders of amylase. Before the alert was instituted, amylase testing was ordered a mean of 450 times per month; afterward, this decreased by about 60%.
We are now considering eliminating serum amylase testing, as suggested by prior studies5 and the American Society of Clinical Pathology.6
ELIMINATING NEEDLESS EXPENSES
The relentless and unsustainable rise in healthcare costs has prompted physician-led groups such as the American Board of Internal Medicine Foundation and the American College of Physicians to focus on ways to cut waste and incorporate high-value, cost-conscious care into clinical practice.
In 2009 alone, waste in total healthcare expenditures was estimated at $765 billion. More than half of this astronomical figure was attributed to unnecessary and inefficiently delivered services, expenditures that physicians can directly avoid with changes to their practice.7,8 Unnecessary laboratory tests such as serum amylase are just one of many wasteful practices.
Hospitals have much to lose when unnecessary tests are ordered. For inpatient hospital admissions in the United States, payment is based on the diagnosis-related group system, in which hospitals are paid a fixed amount per diagnosis. There is no additional reimbursement for laboratory tests. An unnecessary test such as serum amylase in suspected cases of acute pancreatitis thus becomes an expense with no corresponding benefit.
The cost of performing a serum amylase test for a typical laboratory is around $4 to $6. Serum amylase testing at our hospital resulted in unnecessary expense of about $35,000 annually. If we add the costs of additional imaging and prolonged hospitalization, the expenses are substantially more.
Despite this, most hospitals have been unwilling or unable to tackle the problem. This may be due to respect for physician autonomy, seemingly small financial loss, or organizational inertia. For the entire healthcare system, these seemingly minor costs add up. For example, from 2011 to 2014, Medicare Part B alone spent $19.4 million on serum amylase testing.
Ordering unnecessary laboratory tests is not a problem specific to our hospital, but rather a common problem encountered at many hospitals. Recognizing the widespread practice of ordering amylase, the Choosing Wisely initiative shared new recommendations from the American Society for Clinical Pathology supporting the use of lipase instead of amylase in suspected acute pancreatitis.7
Physicians who continue to order these tests show a disregard for evidence-based medicine, patient care, and healthcare costs.
CLINICAL BOTTOM LINE
Concurrent use of amylase and lipase testing to diagnose acute pancreatitis is an unnecessary expense for the hospital and can negatively affect patient care as it can lead to additional tests and prolonged hospitalization. Steps should be taken to minimize ordering of amylase by educating physicians and instituting best-practice alerts, or by eliminating the test altogether.
- Banks PA, Freeman ML, Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol 2006; 101:2379–2400.
- Gomez D, Addison A, De Rosa A, Brooks A, Cameron IC. Retrospective study of patients with acute pancreatitis: is serum amylase still required? BMJ Open 2012; 2. pii:e001471.
- Hofmeyr S, Meyer C, Warren BL. Serum lipase should be the laboratory test of choice for suspected acute pancreatitis. S Afr J Surg 2014; 52:72–75.
- Smith RC, Southwell-Keely J, Chesher D. Should serum pancreatic lipase replace serum amylase as a biomarker of acute pancreatitis? ANZ J Surg 2005; 75:399–404.
- Volz KA, McGillicuddy DC, Horowitz GL, Wolfe RE, Joyce N, Sanchez LD. Eliminating amylase testing from the evaluation of pancreatitis in the emergency department. West J Emerg Med 2010; 11:344–347.
- American Society for Clinical Pathology. Do not test for amylase in cases of suspected pancreatitis. Instead, test for lipase. Choosing Wisely; 2016. www.choosingwisely.org/clinician-lists/american-society-clinical-pathology-testing-for-amylase. Accessed August 3, 2017.
- Smith M, Saunders R, Stuckhardt L, McGinnis JM; Committee on the Learning Health Care System in America, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: The National Academies Press; 2013. www.hep.fsu.edu/~wahl/artic/NAP/HealthCare13444.pdf. Accessed August 3, 2017.
- American College of Physicians. Eliminating healthcare waste and overordering of tests. www.acponline.org/clinical-information/high-value-care/medical-educators-resources/curriculum-for-educators-and-residents/curriculum-version-3. Accessed August 3, 2017.
A 43-year-old, previously healthy woman was admitted to the hospital after 1 day of severe epigastric abdominal pain, nausea, and vomiting. She denied alcohol or tobacco use.
Her physical examination revealed normal vital signs and epigastric tenderness without rebound tenderness.
Notable laboratory results:
- Aspartate aminotransferase 149 U/L (reference range 10–35)
- Alanine aminotransferase 140 U/L (10–35)
- Alkaline phosphatase 178 IU/L (35–104)
- Total bilirubin 1.8 mg/dL (0.2–1.3)
- Amylase 1,244 U/L (28–100)
- Lipase 14,628 U/L (7–59).
Abdominal ultrasonography showed a dilated bile duct and gallstones.
The patient was diagnosed with biliary pancreatitis and was treated by placing her on nothing-by-mouth (NPO) status and giving intravenous fluids and analgesics. All symptoms had resolved by hospital day 3. She underwent laparoscopic cholecystectomy and was discharged the following day.
This is a typical case of biliary pancreatitis that was diagnosed and treated appropriately with a positive outcome. But was it necessary or beneficial to measure both the serum amylase and serum lipase to make the correct diagnosis and treat the patient appropriately?
IS MEASURING SERUM AMYLASE NECESSARY?
The American College of Gastroenterology practice guidelines suggest that measuring both serum amylase and serum lipase is not necessary.1 Serum lipase alone is the preferred test for diagnosing acute pancreatitis, since it is more sensitive than serum amylase, just as specific, rises more quickly, and remains elevated longer.
In a retrospective study of 151 patients with acute pancreatitis,2 the sensitivity of lipase was 96.6% and the specificity was 99.4%.2 In contrast, the sensitivity of amylase was 78.6% and the specificity was 99.1%.
In another study,3 in 476 patients with acute pancreatitis, lipase had a sensitivity of 91% vs 62% for amylase. Again, specificity was similar between the two tests (92% for lipase and 93% for amylase). The authors concluded that lipase should replace amylase as the first-line laboratory investigation for suspected acute pancreatitis.
Smith et al4 reviewed 1,825 patients with acute pancreatitis and similarly concluded that pancreatic lipase is a more accurate biomarker of acute pancreatitis than serum amylase.
PRACTICE AT OUR HOSPITAL
Despite this guideline and evidence, concurrent ordering of serum amylase and lipase is common at many institutions.
We evaluated the practice of ordering both serum amylase and lipase for diagnosis of acute pancreatitis at our 300-bed academic medical hospital. From January 2011 through August 2014, our institution completed 26,254 orders for serum amylase and lipase measurement in 13,198 patients. In 9,938 (75%) of the patients, amylase and lipase were ordered concurrently. Of these, 482 patients (4.8%) had either amylase or lipase elevated above the diagnostic threshold, ie, 3 times the upper limit of normal, and 63 of the 482 patients had an elevation in serum amylase greater than 3 times the upper limit of normal without an elevation in serum lipase.
None of the patients had acute pancreatitis clinically (eg, typical abdominal pain, nausea, vomiting) or on imaging (pancreatic edema). The definitive cause of nonpancreatic hyperamylasemia could not be determined in these patients; they did not have evidence of salivary disorder, malignancy, or tubo-ovarian disease, and the hyperamylasemia was believed to be related to renal disease, diabetic ketoacidosis, infection, or medications, or to be idiopathic.
In 12 patients, the discrepancy between an elevated amylase and normal lipase resulted in additional imaging with computed tomography. Four patients were also unnecessarily kept NPO for 1 to 3 days, depriving them of nutrition and prolonging their hospital stay.
To minimize concurrent ordering of serum amylase and lipase, we introduced a best-practice alert in the computerized physician order entry systems. The alert mentioned that “ordering both serum amylase and lipase in cases of suspected pancreatitis is unnecessary. Serum lipase alone is sufficient.” However, ordering providers could still order both tests if they wanted to.
In the 3 months after the alert was implemented, serum lipase was ordered 1,780 times with 532 (30%) concurrent orders of amylase. Before the alert was instituted, amylase testing was ordered a mean of 450 times per month; afterward, this decreased by about 60%.
We are now considering eliminating serum amylase testing, as suggested by prior studies5 and the American Society of Clinical Pathology.6
ELIMINATING NEEDLESS EXPENSES
The relentless and unsustainable rise in healthcare costs has prompted physician-led groups such as the American Board of Internal Medicine Foundation and the American College of Physicians to focus on ways to cut waste and incorporate high-value, cost-conscious care into clinical practice.
In 2009 alone, waste in total healthcare expenditures was estimated at $765 billion. More than half of this astronomical figure was attributed to unnecessary and inefficiently delivered services, expenditures that physicians can directly avoid with changes to their practice.7,8 Unnecessary laboratory tests such as serum amylase are just one of many wasteful practices.
Hospitals have much to lose when unnecessary tests are ordered. For inpatient hospital admissions in the United States, payment is based on the diagnosis-related group system, in which hospitals are paid a fixed amount per diagnosis. There is no additional reimbursement for laboratory tests. An unnecessary test such as serum amylase in suspected cases of acute pancreatitis thus becomes an expense with no corresponding benefit.
The cost of performing a serum amylase test for a typical laboratory is around $4 to $6. Serum amylase testing at our hospital resulted in unnecessary expense of about $35,000 annually. If we add the costs of additional imaging and prolonged hospitalization, the expenses are substantially more.
Despite this, most hospitals have been unwilling or unable to tackle the problem. This may be due to respect for physician autonomy, seemingly small financial loss, or organizational inertia. For the entire healthcare system, these seemingly minor costs add up. For example, from 2011 to 2014, Medicare Part B alone spent $19.4 million on serum amylase testing.
Ordering unnecessary laboratory tests is not a problem specific to our hospital, but rather a common problem encountered at many hospitals. Recognizing the widespread practice of ordering amylase, the Choosing Wisely initiative shared new recommendations from the American Society for Clinical Pathology supporting the use of lipase instead of amylase in suspected acute pancreatitis.7
Physicians who continue to order these tests show a disregard for evidence-based medicine, patient care, and healthcare costs.
CLINICAL BOTTOM LINE
Concurrent use of amylase and lipase testing to diagnose acute pancreatitis is an unnecessary expense for the hospital and can negatively affect patient care as it can lead to additional tests and prolonged hospitalization. Steps should be taken to minimize ordering of amylase by educating physicians and instituting best-practice alerts, or by eliminating the test altogether.
A 43-year-old, previously healthy woman was admitted to the hospital after 1 day of severe epigastric abdominal pain, nausea, and vomiting. She denied alcohol or tobacco use.
Her physical examination revealed normal vital signs and epigastric tenderness without rebound tenderness.
Notable laboratory results:
- Aspartate aminotransferase 149 U/L (reference range 10–35)
- Alanine aminotransferase 140 U/L (10–35)
- Alkaline phosphatase 178 IU/L (35–104)
- Total bilirubin 1.8 mg/dL (0.2–1.3)
- Amylase 1,244 U/L (28–100)
- Lipase 14,628 U/L (7–59).
Abdominal ultrasonography showed a dilated bile duct and gallstones.
The patient was diagnosed with biliary pancreatitis and was treated by placing her on nothing-by-mouth (NPO) status and giving intravenous fluids and analgesics. All symptoms had resolved by hospital day 3. She underwent laparoscopic cholecystectomy and was discharged the following day.
This is a typical case of biliary pancreatitis that was diagnosed and treated appropriately with a positive outcome. But was it necessary or beneficial to measure both the serum amylase and serum lipase to make the correct diagnosis and treat the patient appropriately?
IS MEASURING SERUM AMYLASE NECESSARY?
The American College of Gastroenterology practice guidelines suggest that measuring both serum amylase and serum lipase is not necessary.1 Serum lipase alone is the preferred test for diagnosing acute pancreatitis, since it is more sensitive than serum amylase, just as specific, rises more quickly, and remains elevated longer.
In a retrospective study of 151 patients with acute pancreatitis,2 the sensitivity of lipase was 96.6% and the specificity was 99.4%.2 In contrast, the sensitivity of amylase was 78.6% and the specificity was 99.1%.
In another study,3 in 476 patients with acute pancreatitis, lipase had a sensitivity of 91% vs 62% for amylase. Again, specificity was similar between the two tests (92% for lipase and 93% for amylase). The authors concluded that lipase should replace amylase as the first-line laboratory investigation for suspected acute pancreatitis.
Smith et al4 reviewed 1,825 patients with acute pancreatitis and similarly concluded that pancreatic lipase is a more accurate biomarker of acute pancreatitis than serum amylase.
PRACTICE AT OUR HOSPITAL
Despite this guideline and evidence, concurrent ordering of serum amylase and lipase is common at many institutions.
We evaluated the practice of ordering both serum amylase and lipase for diagnosis of acute pancreatitis at our 300-bed academic medical hospital. From January 2011 through August 2014, our institution completed 26,254 orders for serum amylase and lipase measurement in 13,198 patients. In 9,938 (75%) of the patients, amylase and lipase were ordered concurrently. Of these, 482 patients (4.8%) had either amylase or lipase elevated above the diagnostic threshold, ie, 3 times the upper limit of normal, and 63 of the 482 patients had an elevation in serum amylase greater than 3 times the upper limit of normal without an elevation in serum lipase.
None of the patients had acute pancreatitis clinically (eg, typical abdominal pain, nausea, vomiting) or on imaging (pancreatic edema). The definitive cause of nonpancreatic hyperamylasemia could not be determined in these patients; they did not have evidence of salivary disorder, malignancy, or tubo-ovarian disease, and the hyperamylasemia was believed to be related to renal disease, diabetic ketoacidosis, infection, or medications, or to be idiopathic.
In 12 patients, the discrepancy between an elevated amylase and normal lipase resulted in additional imaging with computed tomography. Four patients were also unnecessarily kept NPO for 1 to 3 days, depriving them of nutrition and prolonging their hospital stay.
To minimize concurrent ordering of serum amylase and lipase, we introduced a best-practice alert in the computerized physician order entry systems. The alert mentioned that “ordering both serum amylase and lipase in cases of suspected pancreatitis is unnecessary. Serum lipase alone is sufficient.” However, ordering providers could still order both tests if they wanted to.
In the 3 months after the alert was implemented, serum lipase was ordered 1,780 times with 532 (30%) concurrent orders of amylase. Before the alert was instituted, amylase testing was ordered a mean of 450 times per month; afterward, this decreased by about 60%.
We are now considering eliminating serum amylase testing, as suggested by prior studies5 and the American Society of Clinical Pathology.6
ELIMINATING NEEDLESS EXPENSES
The relentless and unsustainable rise in healthcare costs has prompted physician-led groups such as the American Board of Internal Medicine Foundation and the American College of Physicians to focus on ways to cut waste and incorporate high-value, cost-conscious care into clinical practice.
In 2009 alone, waste in total healthcare expenditures was estimated at $765 billion. More than half of this astronomical figure was attributed to unnecessary and inefficiently delivered services, expenditures that physicians can directly avoid with changes to their practice.7,8 Unnecessary laboratory tests such as serum amylase are just one of many wasteful practices.
Hospitals have much to lose when unnecessary tests are ordered. For inpatient hospital admissions in the United States, payment is based on the diagnosis-related group system, in which hospitals are paid a fixed amount per diagnosis. There is no additional reimbursement for laboratory tests. An unnecessary test such as serum amylase in suspected cases of acute pancreatitis thus becomes an expense with no corresponding benefit.
The cost of performing a serum amylase test for a typical laboratory is around $4 to $6. Serum amylase testing at our hospital resulted in unnecessary expense of about $35,000 annually. If we add the costs of additional imaging and prolonged hospitalization, the expenses are substantially more.
Despite this, most hospitals have been unwilling or unable to tackle the problem. This may be due to respect for physician autonomy, seemingly small financial loss, or organizational inertia. For the entire healthcare system, these seemingly minor costs add up. For example, from 2011 to 2014, Medicare Part B alone spent $19.4 million on serum amylase testing.
Ordering unnecessary laboratory tests is not a problem specific to our hospital, but rather a common problem encountered at many hospitals. Recognizing the widespread practice of ordering amylase, the Choosing Wisely initiative shared new recommendations from the American Society for Clinical Pathology supporting the use of lipase instead of amylase in suspected acute pancreatitis.7
Physicians who continue to order these tests show a disregard for evidence-based medicine, patient care, and healthcare costs.
CLINICAL BOTTOM LINE
Concurrent use of amylase and lipase testing to diagnose acute pancreatitis is an unnecessary expense for the hospital and can negatively affect patient care as it can lead to additional tests and prolonged hospitalization. Steps should be taken to minimize ordering of amylase by educating physicians and instituting best-practice alerts, or by eliminating the test altogether.
- Banks PA, Freeman ML, Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol 2006; 101:2379–2400.
- Gomez D, Addison A, De Rosa A, Brooks A, Cameron IC. Retrospective study of patients with acute pancreatitis: is serum amylase still required? BMJ Open 2012; 2. pii:e001471.
- Hofmeyr S, Meyer C, Warren BL. Serum lipase should be the laboratory test of choice for suspected acute pancreatitis. S Afr J Surg 2014; 52:72–75.
- Smith RC, Southwell-Keely J, Chesher D. Should serum pancreatic lipase replace serum amylase as a biomarker of acute pancreatitis? ANZ J Surg 2005; 75:399–404.
- Volz KA, McGillicuddy DC, Horowitz GL, Wolfe RE, Joyce N, Sanchez LD. Eliminating amylase testing from the evaluation of pancreatitis in the emergency department. West J Emerg Med 2010; 11:344–347.
- American Society for Clinical Pathology. Do not test for amylase in cases of suspected pancreatitis. Instead, test for lipase. Choosing Wisely; 2016. www.choosingwisely.org/clinician-lists/american-society-clinical-pathology-testing-for-amylase. Accessed August 3, 2017.
- Smith M, Saunders R, Stuckhardt L, McGinnis JM; Committee on the Learning Health Care System in America, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: The National Academies Press; 2013. www.hep.fsu.edu/~wahl/artic/NAP/HealthCare13444.pdf. Accessed August 3, 2017.
- American College of Physicians. Eliminating healthcare waste and overordering of tests. www.acponline.org/clinical-information/high-value-care/medical-educators-resources/curriculum-for-educators-and-residents/curriculum-version-3. Accessed August 3, 2017.
- Banks PA, Freeman ML, Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol 2006; 101:2379–2400.
- Gomez D, Addison A, De Rosa A, Brooks A, Cameron IC. Retrospective study of patients with acute pancreatitis: is serum amylase still required? BMJ Open 2012; 2. pii:e001471.
- Hofmeyr S, Meyer C, Warren BL. Serum lipase should be the laboratory test of choice for suspected acute pancreatitis. S Afr J Surg 2014; 52:72–75.
- Smith RC, Southwell-Keely J, Chesher D. Should serum pancreatic lipase replace serum amylase as a biomarker of acute pancreatitis? ANZ J Surg 2005; 75:399–404.
- Volz KA, McGillicuddy DC, Horowitz GL, Wolfe RE, Joyce N, Sanchez LD. Eliminating amylase testing from the evaluation of pancreatitis in the emergency department. West J Emerg Med 2010; 11:344–347.
- American Society for Clinical Pathology. Do not test for amylase in cases of suspected pancreatitis. Instead, test for lipase. Choosing Wisely; 2016. www.choosingwisely.org/clinician-lists/american-society-clinical-pathology-testing-for-amylase. Accessed August 3, 2017.
- Smith M, Saunders R, Stuckhardt L, McGinnis JM; Committee on the Learning Health Care System in America, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: The National Academies Press; 2013. www.hep.fsu.edu/~wahl/artic/NAP/HealthCare13444.pdf. Accessed August 3, 2017.
- American College of Physicians. Eliminating healthcare waste and overordering of tests. www.acponline.org/clinical-information/high-value-care/medical-educators-resources/curriculum-for-educators-and-residents/curriculum-version-3. Accessed August 3, 2017.
Antibiotic stewardship: Why we must, how we can
Antibiotic stewardship has always been a good idea. Now it is also required by the Joint Commission and the Center for Medicare and Medicaid Services (CMS). This article reviews the state of antibiotic use in the United States and efforts to improve antibiotic stewardship in practice.
ANTIBIOTICS ARE DIFFERENT FROM OTHER DRUGS
Their efficacy wanes over time. Antibiotics are the only medications that become less useful over time even if used correctly. Although other types of drugs are continuously being improved, the old ones work as well today as they did when they first came out. But antibiotics that were in use 50 years ago are no longer as effective.
They are a shared resource. Antibiotics are regularly used by many specialties to deliver routine and advanced medical care. Surgeries, transplantation, and immunosuppressive therapy would be unsafe without antibiotics to treat infections. Some patients awaiting lung transplant are not considered good candidates if they have evidence of colonization by antibiotic-resistant organisms.
Individual use may harm others. Even people who are not exposed to an antibiotic can suffer the consequences of how others use them.
In a retrospective cohort study, Freedberg et al1 analyzed the risk of hospitalized patients developing Clostridium difficile infection and found that the risk was higher if the previous occupant of the bed had received antibiotics. The putative mechanism is that a patient receiving antibiotics develops altered gut flora, leading to C difficile spores released into the environment and not eradicated by normal cleaning. The next patient using the bed is then exposed and infected.
ANTIBIOTIC USE IS HIGH
The US Centers for Disease Control (CDC) monitors antibiotic prescriptions throughout the United States. In the outpatient setting, enough antibiotics are prescribed nationwide for 5 out of every 6 people to get 1 course of antibiotics annually (835 prescriptions per 1,000 people). Rates vary widely among states, with the lowest rate in Alaska (501 prescriptions per 1,000 people) and the highest in West Virginia (1,285 prescriptions per 1,000 people).2 In comparison, Scandinavian countries prescribe about 400 courses per 1,000 people, about 20% less than our lowest-prescribing state.3
Antibiotics are probably the most frequently prescribed drugs in US hospitals. Data from 2006 to 2012 showed that 55% of hospitalized patients received at least 1 dose of an antibiotic and that overall about 75% of all hospital days involved an antibiotic.4 Rates did not vary by hospital size, but nonteaching hospitals tended to use antibiotics more than teaching hospitals. Antibiotic use is much more common in intensive care units than in hospital wards (1,092 and 720 days of antibiotic treatment per 1,000 patient-days, respectively).
Although overall antibiotic use did not change significantly over the years of the survey, use patterns did: fluoroquinolone use dropped by 20%, possibly reflecting rising resistance or increased attention to associated side effects (although fluoroquinolones remain the most widely prescribed inpatient antibiotic class), and use of first-generation cephalosporins fell by 7%. A cause for concern is that the use of broad-spectrum and “last-resort” antibiotics increased: carbapenem use by 37%, vancomycin use by 32%, beta-lactam/beta-lactamase inhibitor use by 26%, and third- and fourth-generation cephalosporin use by 12%.4
About one-third of use is unnecessary
Many studies have tried to measure the extent of inappropriate or unnecessary antibiotic use. The results have been remarkably consistent at 20% to 40% for both inpatient and outpatient studies. One study of hospitalized patients not in the intensive care unit found that 30% of 1,941 days of prescribed antimicrobial therapy were unnecessary, mostly because patients received antibiotics for longer than needed or because antibiotics were used to treat noninfectious syndromes or colonizing microorganisms.5
ANTIBIOTIC EXPOSURE HAS NEGATIVE CONSEQUENCES
Any exposure to a medication involves the potential for side effects; this is true for antibiotics whether or not their use is appropriate. An estimated 140,000 visits to emergency departments occur annually for adverse reactions to antibiotics.6 In hospitalized patients, these reactions can be severe, including renal and bone marrow toxicity. As with any medications, the risks and benefits of antibiotic therapy must be weighed patient by patient.
Disturbance of gut microbiome
Antibiotics’ disruptive effects on normal gut flora are becoming better understood and are even believed to increase the risk of obesity and asthma.7,8
Animal models provide evidence that altered flora is associated with sepsis, which is attributed to the gut microbiome’s role in containing dissemination of bacteria in the body.9 An ecological study provides further evidence. Baggs et al10 retrospectively studied more than 9 million patients discharged without sepsis from 473 US hospitals, of whom 0.6% were readmitted for sepsis within 90 days. Exposure to a broad-spectrum antibiotic was associated with a 50% increased risk of readmission within 90 days of discharge because of sepsis (odds ratio 1.50, 95% confidence interval 1.47–1.53).
Increase of C difficile infections
Antibiotics exert selective pressure, killing susceptible bacteria and allowing resistant bacteria to thrive.
The risk of C difficile infection is 7 to 10 times higher than at baseline for 1 month after antibiotic use and 3 times higher than baseline in the 2 months after that.11 Multiple studies have found that stewardship efforts to reduce antibiotic use have resulted in fewer C difficile infections.
A nationwide effort in England over the past decade to reduce C difficile infections has resulted in 50% less use of fluoroquinolones and third-generation cephalosporins in patients over age 65. During that time, the incidence of C difficile infection in that age group fell by about 70%, with concomitant reductions in mortality and colectomy associated with infection. No increase in rates of hospital admissions, infection complications, or death were observed.12–14
GOAL: BETTER CARE (NOT CHEAPER CARE OR LESS ANTIBIOTIC USE)
The primary goal of antibiotic stewardship is better patient care. The goal is not reduced antibiotic use or cost savings, although these could be viewed as favorable side effects. Sometimes, better patient care involves using more antibiotics: eg, a patient with presumed sepsis should be started quickly on broad-spectrum antibiotics, an action that also falls under antibiotic stewardship. The focus for stewardship efforts should be on optimizing appropriate use, ie, promoting the use of the right agent at the correct dosage and for the proper duration.
Stewardship improves clinical outcomes
Antibiotic stewardship is important not only to society but to individual patients.
Singh et al15 randomized patients suspected of having ventilator-associated pneumonia (but with a low likelihood of pneumonia) to either a 3-day course of ciprofloxacin or standard care (antibiotics for 10 to 21 days, with the drug and duration chosen by the treating physician). After 3 days, the patients in the experimental group were reevaluated, and antibiotics were stopped if the likelihood of pneumonia was still deemed low. In patients who received only the short course of antibiotics, mean length of stay in the intensive care unit was 9 days and the risk of acquiring an antibiotic-resistant superinfection during hospitalization was 14%, compared with a 15-day length of stay and 38% risk of antibiotic-resistant superinfection in patients in the standard treatment group.
Fishman16 reported a study at a single hospital that randomized patients to either receive standard care according to physician choice or be treated according to an antibiotic stewardship program. Patients in the antibiotic stewardship group were almost 3 times more likely than controls to receive appropriate therapy according to guidelines. More important, the antibiotic stewardship patients were almost twice as likely to be cured of their infection and were more than 80% less likely to have treatment failure.
DEVELOPING EFFECTIVE ANTIBIOTIC STEWARDSHIP PROGRAMS
A good model for improving antibiotic use is a recent nationwide program designed to reduce central line-associated bloodstream infections.17 Rates of these infections have dropped by about 50% over the past 5 years. The program included:
- Research to better understand the problem and how to fight it
- Well-defined programs and interventions
- Education to implement interventions, eg, deploying teams to teach better techniques of inserting and maintaining central lines
- A strong national measurement system (the CDC’s National Healthcare Safety Network) to track infections.
What constitutes an antibiotic stewardship program?
The CDC examined successful stewardship programs in a variety of hospital types, including large academic hospitals and smaller hospitals, and identified 7 common core elements that could serve as general principles that were common to successful antibiotic stewardship programs18:
- Leadership commitment from administration
- A single leader responsible for outcomes
- A single pharmacy leader
- Tracking of antibiotic use
- Regular reporting of antibiotic use and resistance
- Educating providers on use and resistance
- Specific improvement interventions.
Stewardship is harder in some settings
In reply to a CDC survey in 2014, 41% of more than 4,000 hospitals reported that they had antibiotic stewardship programs with all 7 core elements. The single element that predicted whether a complete program was in place was leadership support.19 The following year, 48% of respondents reported that they had a complete program in place. Percentages varied among states, with highs in Utah (77%) and California (70%) and lows in North Dakota (12%) and Vermont (7%). Large hospitals and major teaching hospitals were more likely to have a program with all 7 elements: 31% of hospitals with 50 or fewer beds had a complete program vs 66% of hospitals with at least 200 beds.20
Short-stay, critical-access hospitals pose a special challenge, as only 26% reported having all core elements.19,20 These facilities have fewer than 25 beds, and many patient stays are less than 3 days. Some do not employ full-time pharmacists or full-time clinicians. The CDC is collaborating with the American Hospital Association and the Pew Charitable Trusts to focus efforts on helping these hospitals, which requires a more flexible approach. About 100 critical-access hospitals nationwide have reported implementing all of the core elements and can serve as models for the others.
MEASURING IMPROVEMENT
The CDC has adopted a 3-pronged approach to measuring improvements in hospital antibiotic use:
- Estimate national aggregate antibiotic use described above
- Acquire information on antibiotic use at facility, practice, and provider levels
- Assess appropriate antibiotic use.
In hospitals, the CDC has concentrated on facility-level measurement. Hospitals need a system to track their own use and compare it with that of similar facilities. The CDC’s monitoring program, the Antibiotic Use Option of the National Healthcare Safety Network, captures electronic data on antibiotic use in a facility, enabling monitoring of use in each unit. Data can also be aggregated at regional, state, and national levels. This information can be used to develop benchmarks for antibiotic use, so that similar hospitals can be compared.
What is the ‘right’ amount of antibiotic use? Enter SAAR
Creating benchmarks for antibiotic use poses a number of challenges compared with most other areas in healthcare. Most public health measures are binary—eg, people either get an infection, a vaccination, or a smoking cessation intervention or not—and the direction of progress is clear. Antibiotics are different: not everybody needs them, but some people do. Usage should be reduced, but by exactly how much is unclear and varies between hospitals. In addition, being an outlier does not necessarily indicate a problem: a hospital unit for organ transplants will have high rates of antibiotic use, which is likely appropriate.
The CDC has taken initial steps to develop a risk-adjusted benchmark measure for hospital antibiotic use, the Standardized Antimicrobial Administration Ratio (SAAR). It compares a hospital’s observed antibiotic use with a calculation of predicted use based on its facility characteristics. Although still at an early stage, SAAR has been released and has been endorsed by the National Quality Forum. About 200 hospitals are submitting data to the CDC and collaborating with the CDC to evaluate the SAAR’s utility in driving improved antibiotic use.
Problems in measuring appropriate use
Measuring appropriate antibiotic use is easier in the outpatient setting, where detailed data have been collected for many years.
Fleming-Dutra et al21 compared medications prescribed during outpatient visits and the diagnoses coded for the visits. They found that about 13% of all outpatient visits resulted in an antibiotic prescription, 30% of which had no listed diagnosis that would justify an antibiotic (eg, viral upper respiratory infection). This kind of information provides a target for stewardship programs.
It is more difficult to conduct such a study in a hospital setting. Simply comparing discharge diagnoses to antibiotics prescribed is not useful: often antibiotics are started presumptively on admission for a patient with signs and symptoms of an infection, then stopped if the diagnosis does not warrant antibiotics, which is a reasonable strategy.
Also, many times, a patient with asymptomatic bacteriuria, which does not warrant antibiotics, is misdiagnosed as having a urinary tract infection, which does. So simply looking at the discharge code may not reveal whether therapy was appropriate.
Some studies have provided useful information. Fridkin et al22 studied 36 hospitals for the use of vancomycin, which is an especially good candidate drug for study because guidelines exist for appropriate use. Data were collected only from patients given vancomycin for more than 3 days, which should have eliminated empiric use of the drug and included only pathogen-driven therapy. Cases where therapy was for skin and soft-tissue infections were excluded because cultures are not usually obtained for these cases. Of patients given vancomycin, 9% had no diagnostic culture obtained at antibiotic initiation, 22% had diagnostic culture but results showed no gram-positive bacterial growth, and 5% had culture results revealing only oxacillin-susceptible Staphylococcus aureus. In 36% of cases, opportunities existed for improved prescribing.
Such data could be collected from the electronic medical record, and the CDC is focusing efforts in this direction.
NATIONAL ACTIVITIES IN ANTIBIOTIC STEWARDSHIP
In 2014, the White House launched a national strategy to combat antibiotic resistance,23 followed by an action plan in 2015.24 As a result, new investments have been made to improve antibiotic use, including funding for state health departments to begin stewardship efforts and to expand public awareness of the problems of antibiotic overuse. Research efforts are also being funded to improve implementation of existing stewardship practices and to develop new ones.
CMS is also exploring how to drive improved antibiotic use. In October 2016, it started requiring all US nursing homes to have antibiotic stewardship programs, and a similar requirement for hospitals has been proposed.
The Joint Commission issued a standard requiring that all their accredited facilities, starting with hospitals, have an antibiotic stewardship program by January 2017. This standard requires implementation of all the CDC’s core elements.
PROVEN INTERVENTIONS
Focusing on key interventions that are likely to be effective and well received by providers is a useful strategy for antibiotic stewardship efforts. A number of such interventions have been supported by research.
Postprescription antibiotic reviews or antibiotic ‘time-outs’
Antibiotics are often started empirically to treat hospitalized patients suspected of having an infection. The need for the antibiotic should be assessed a few days later, when culture results and more clinical information are available.
Elligsen et al25 evaluated the effects of providing a formal review and suggestions for antimicrobial optimization to critical care teams of 3 intensive care units in a single hospital after 3 and 10 days of antibiotic therapy. Mean monthly antibiotic use decreased from 644 days of therapy per 1,000 patient-days in the preintervention period to 503 days of therapy per 1,000 patient-days (P < .0001). C difficile infections were reduced from 11 cases to 6. Overall gram-negative susceptibility to meropenem increased in the critical care units.
Targeting specific infections
Some infections are especially important to target with improvement efforts.
In 2011, Magill et al26 conducted 1-day prevalence surveys in 183 hospitals in 10 states to examine patterns of antibiotic use. They found that lower respiratory tract infections and urinary tract infections accounted for more than half of all antibiotic use (35% and 22%, respectively), making them good candidates for improved use.
Community-acquired pneumonia can be targeted at multiple fronts. One study showed that almost 30% of patients diagnosed with community-acquired pneumonia in the emergency department did not actually have pneumonia.27 Duration of antibiotic therapy could also be targeted. Guidelines recommend that most patients with uncomplicated community-acquired pneumonia receive 5 to 7 days of antibiotic therapy. Avdic et al28 performed a simple intervention involving education and feedback to teams in 1 hospital regarding antibiotic choice and duration. This resulted in reducing the duration of therapy for community-acquired pneumonia from a median of 10 to 7 days.
Asymptomatic bacteriuria is often misdiagnosed as a urinary tract infection and treated unnecessarily.29–31
Trautner et al32 addressed this problem by targeting urine cultures rather than antibiotics, using a simple algorithm: if a patient did not have symptoms of urinary tract infection (fever, acute hematuria, delirium, rigors, flank pain, pelvic discomfort, urgency, frequency, dysuria, suprapubic pain), a urine culture was not recommended. If a patient did have symptoms but a problem other than urinary tract infection was deemed likely, evaluation of other sources of infection was recommended. Use of the algorithm resulted in fewer urine cultures and less antibiotic overtreatment of asymptomatic bacteriuria. Reductions persisted after the intervention ended.
Antibiotic time-out at hospital discharge
Another study evaluated an intervention that required a pharmacist consultation for the critical care team when a patient was to be discharged with intravenous antibiotics (most often for pneumonia). In 28% of cases, chart review revealed that the infection had been completely treated at the time of discharge, so further antibiotic treatment was not indicated. No patients who avoided antibiotics at discharge were readmitted or subsequently visited the emergency department.33
Targeting outpatient settings
A number of studies have evaluated simple interventions to improve outpatient antibiotic prescribing. Meeker et al34 had providers place a poster in their examination rooms with a picture of the physician and a signed letter committing to the appropriate use of antibiotics. Inappropriate antibiotic use decreased 20% in the intervention group vs controls (P = .02).
In a subsequent study,35 the same group required providers to include a justification note in the electronic medical record every time an antibiotic was prescribed for an indication when guidelines do not recommend one. Inappropriate prescribing dropped from 23% to 5% (P < .001).
Another intervention in this study35 provided physicians with periodic feedback according to whether their therapy was concordant with guidelines. They received an email with a subject line of either “You are a top performer” or “You are not a top performer.” The contents of the email provided data on how many antibiotic prescriptions they wrote for conditions that did not warrant them and how their prescribing habits compared with those of their top-performing peers. Mean inappropriate antibiotic prescribing fell from 20% to 4%.35
This is a critical time for antibiotic stewardship efforts in the United States. The need has never been more urgent and, fortunately, the opportunities have never been more abundant. Requirements for stewardship programs will drive implementation, but hospitals will need support and guidance to help ensure that stewardship programs are as effective as possible. Ultimately, improving antibiotic use will require collaboration among all stakeholders. CDC is eager to partner with providers and others in their efforts to improve antibiotic use.
- Freedberg DE, Salmasian H, Cohen B, Abrams JA, Larson EL. Receipt of antibiotics in hospitalized patients and risk for Clostridium difficile infection in subsequent patients who occupy the same bed. JAMA Intern Med 2016; 176:1801–1808.
- Centers for Disease Control and Prevention. Get smart: know when antibiotics work. Measuring outpatient antibiotic prescribing. https://www.cdc.gov/getsmart/community/programs-measurement/measuring-antibiotic-prescribing.html. Accessed February 5, 2017.
- Ternhag A, Hellman J. More on U.S. outpatient antibiotic prescribing, 2010. N Engl J Med 2013; 369:1175–1176.
- Baggs J, Fridkin SK, Pollack LA, Srinivasan A, Jernigan JA. Estimating national trends in inpatient antibiotic use among US hospitals from 2006 to 2012. JAMA Intern Med 2016; 176:1639–1648.
- Hecker MT, Aron DC, Patel NP, Lehmann MK, Donskey CJ. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med 2003; 163:972–978.
- Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis 2008; 47:735–743.
- Korpela K, de Vos WM. Antibiotic use in childhood alters the gut microbiota and predisposes to overweight. Microb Cell 2016; 3:296–298.
- Gray LE, O’Hely M, Ranganathan S, Sly PD, Vuillermin P. The maternal diet, gut bacteria, and bacterial metabolites during pregnancy influence offspring asthma. Front Immunol 2017; 8:365
- Haak BW, Wiersinga WJ. The role of the gut microbiota in sepsis. Lancet Gastroenterol Hepatol 2017; 2:135–143.
- Baggs J, Jernigan J, Mccormick K, Epstein L, Laufer-Halpin AS, Mcdonald C. Increased risk of sepsis during hospital readmission following exposure to certain antibiotics during hospitalization. Abstract presented at IDWeek, October 26-30, 2016, New Orleans, LA. https://idsa.confex.com/idsa/2016/webprogram/Paper58587.html. Accessed August 8, 2017.
- Hensgens MP, Goorhuis A, Dekkers OM, Kuijper EJ. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother 2012; 67:742–748.
- Ashiru-Oredope D, Sharland M, Charani E, McNulty C, Cooke J; ARHAI Antimicrobial Stewardship Group. Improving the quality of antibiotic prescribing in the NHS by developing a new antimicrobial stewardship programme: Start Smart—Then Focus. J Antimicrob Chemother 2012; 67(suppl 1):i51–i63.
- Wilcox MH, Shetty N, Fawley WN, et al. Changing epidemiology of Clostridium difficile infection following the introduction of a national ribotyping-based surveillance scheme in England. Clin Infect Dis 2012; 55:1056-1063.
- Public Health England. Clostridium difficile infection: monthly data by NHS acute trust. https://www.gov.uk/government/statistics/clostridium-difficile-infection-monthly-data-by-nhs-acute-trust. Accessed August 4, 2017.
- Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med 2000; 162:505–511.
- Fishman N. Antimicrobial stewardship. Am J Med 2006; 119:S53–S61.
- US Centers for Disease Control and Prevention. Healthcare-associated infections (HAI) progress report. https://www.cdc.gov/hai/surveillance/progress-report/index.html. Accessed August 4, 2017.
- US Centers for Disease Control and Prevention. Get Smart for Healthcare. Core elements of hospital antibiotic stewardship programs. https://www.cdc.gov/getsmart/healthcare/implementation/core-elements.html. Accessed August 8, 2017.
- Pollack LA, van Santen KL, Weiner LM, Dudeck MA, Edwards JR, Srinivasan A. Antibiotic stewardship programs in U.S. acute care hospitals: findings from the 2014 National Healthcare Safety Network Annual Hospital Survey. Clin Infect Dis 2016; 63:443–449.
- US Centers for Disease Control and Prevention. Antibiotic stewardship in acute care hospitals by state 2014. https://gis.cdc.gov/grasp/PSA/STMapView.html. Accessed August 4, 2017.
- Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016; 315:1864-1873.
- Fridkin S, Baggs J, Fagan R, et al; Centers for Disease Control and Prevention (CDC). Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep 2014; 63:194–200.
- National strategy for combating antibiotic-resistant bacteria. September 2014. https://www.whitehouse.gov/sites/default/files/docs/carb_national_strategy.pdf. Accessed August 9, 2017.
- National action plan for combating antibiotic-resistant Bacteria. March 2015. https://www.whitehouse.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf
- Elligsen M, Walker SA, Pinto R, et al. Audit and feedback to reduce broad-spectrum antibiotic use among intensive care unit patients: a controlled interrupted time series analysis. Infect Control Hosp Epidemiol 2012; 33:354–361.
- Magill SS, Edwards JR, Beldavs ZG, et al; Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA 2014; 312:1438-1446.
- Chandra A, Nicks B, Maniago E, Nouh A, Limkakeng A. A multicenter analysis of the ED diagnosis of pneumonia. Am J Emerg Med 2010;28:862–865.
- Avdic E, Cushinotto LA, Hughes AH, et al. Impact of an antimicrobial stewardship intervention on shortening the duration of therapy for community-acquired pneumonia. Clin Infect Dis 2012; 54:1581–1587.
- Dalen DM, Zvonar RK, Jessamine PG, et al. An evaluation of the management of asymptomatic catheter-associated bacteriuria and candiduria at the Ottawa Hospital. Can J Infect Dis Med Micribiol 2005; 16:166–170.
- Gandhi T, Flanders SA, Markovitz E, Saint S, Kaul DR. Importance of urinary tract infection to antibiotic use among hospitalized patients. Infect Control Hosp Epidemiol 2009; 30:193–195.
- Cope M, Cevallos ME, Cadle RM, Darouiche RO, Musher DM, Trautner BW. Inappropriate treatment of catheter-associated asymptomatic bacteriuria in a tertiary care hospital. Clin Infect Dis 2009; 48:1182–1188.
- Trautner BW, Grigoryan L, Petersen NJ, et al. Effectiveness of an antimicrobial stewardship approach for urinary catheter-associated asymptomatic bacteriuria. JAMA Intern Med 2015; 175:1120–1127.
- Shrestha NK, Bhaskaran A, Scalera NM, Schmitt SK, Rehm SJ, Gordon SM. Antimicrobial stewardship at transition of care from hospital to community. Infect Control Hosp Epidemiol 2012; 33:401–404.
- Meeker D, Knight TK, Friedberg MW, et al. Nudging guideline-concordant antibiotic prescribing: a randomized clinical trial. JAMA Intern Med 2014; 174:425–431.
- Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA 2016; 315:562–572.
Antibiotic stewardship has always been a good idea. Now it is also required by the Joint Commission and the Center for Medicare and Medicaid Services (CMS). This article reviews the state of antibiotic use in the United States and efforts to improve antibiotic stewardship in practice.
ANTIBIOTICS ARE DIFFERENT FROM OTHER DRUGS
Their efficacy wanes over time. Antibiotics are the only medications that become less useful over time even if used correctly. Although other types of drugs are continuously being improved, the old ones work as well today as they did when they first came out. But antibiotics that were in use 50 years ago are no longer as effective.
They are a shared resource. Antibiotics are regularly used by many specialties to deliver routine and advanced medical care. Surgeries, transplantation, and immunosuppressive therapy would be unsafe without antibiotics to treat infections. Some patients awaiting lung transplant are not considered good candidates if they have evidence of colonization by antibiotic-resistant organisms.
Individual use may harm others. Even people who are not exposed to an antibiotic can suffer the consequences of how others use them.
In a retrospective cohort study, Freedberg et al1 analyzed the risk of hospitalized patients developing Clostridium difficile infection and found that the risk was higher if the previous occupant of the bed had received antibiotics. The putative mechanism is that a patient receiving antibiotics develops altered gut flora, leading to C difficile spores released into the environment and not eradicated by normal cleaning. The next patient using the bed is then exposed and infected.
ANTIBIOTIC USE IS HIGH
The US Centers for Disease Control (CDC) monitors antibiotic prescriptions throughout the United States. In the outpatient setting, enough antibiotics are prescribed nationwide for 5 out of every 6 people to get 1 course of antibiotics annually (835 prescriptions per 1,000 people). Rates vary widely among states, with the lowest rate in Alaska (501 prescriptions per 1,000 people) and the highest in West Virginia (1,285 prescriptions per 1,000 people).2 In comparison, Scandinavian countries prescribe about 400 courses per 1,000 people, about 20% less than our lowest-prescribing state.3
Antibiotics are probably the most frequently prescribed drugs in US hospitals. Data from 2006 to 2012 showed that 55% of hospitalized patients received at least 1 dose of an antibiotic and that overall about 75% of all hospital days involved an antibiotic.4 Rates did not vary by hospital size, but nonteaching hospitals tended to use antibiotics more than teaching hospitals. Antibiotic use is much more common in intensive care units than in hospital wards (1,092 and 720 days of antibiotic treatment per 1,000 patient-days, respectively).
Although overall antibiotic use did not change significantly over the years of the survey, use patterns did: fluoroquinolone use dropped by 20%, possibly reflecting rising resistance or increased attention to associated side effects (although fluoroquinolones remain the most widely prescribed inpatient antibiotic class), and use of first-generation cephalosporins fell by 7%. A cause for concern is that the use of broad-spectrum and “last-resort” antibiotics increased: carbapenem use by 37%, vancomycin use by 32%, beta-lactam/beta-lactamase inhibitor use by 26%, and third- and fourth-generation cephalosporin use by 12%.4
About one-third of use is unnecessary
Many studies have tried to measure the extent of inappropriate or unnecessary antibiotic use. The results have been remarkably consistent at 20% to 40% for both inpatient and outpatient studies. One study of hospitalized patients not in the intensive care unit found that 30% of 1,941 days of prescribed antimicrobial therapy were unnecessary, mostly because patients received antibiotics for longer than needed or because antibiotics were used to treat noninfectious syndromes or colonizing microorganisms.5
ANTIBIOTIC EXPOSURE HAS NEGATIVE CONSEQUENCES
Any exposure to a medication involves the potential for side effects; this is true for antibiotics whether or not their use is appropriate. An estimated 140,000 visits to emergency departments occur annually for adverse reactions to antibiotics.6 In hospitalized patients, these reactions can be severe, including renal and bone marrow toxicity. As with any medications, the risks and benefits of antibiotic therapy must be weighed patient by patient.
Disturbance of gut microbiome
Antibiotics’ disruptive effects on normal gut flora are becoming better understood and are even believed to increase the risk of obesity and asthma.7,8
Animal models provide evidence that altered flora is associated with sepsis, which is attributed to the gut microbiome’s role in containing dissemination of bacteria in the body.9 An ecological study provides further evidence. Baggs et al10 retrospectively studied more than 9 million patients discharged without sepsis from 473 US hospitals, of whom 0.6% were readmitted for sepsis within 90 days. Exposure to a broad-spectrum antibiotic was associated with a 50% increased risk of readmission within 90 days of discharge because of sepsis (odds ratio 1.50, 95% confidence interval 1.47–1.53).
Increase of C difficile infections
Antibiotics exert selective pressure, killing susceptible bacteria and allowing resistant bacteria to thrive.
The risk of C difficile infection is 7 to 10 times higher than at baseline for 1 month after antibiotic use and 3 times higher than baseline in the 2 months after that.11 Multiple studies have found that stewardship efforts to reduce antibiotic use have resulted in fewer C difficile infections.
A nationwide effort in England over the past decade to reduce C difficile infections has resulted in 50% less use of fluoroquinolones and third-generation cephalosporins in patients over age 65. During that time, the incidence of C difficile infection in that age group fell by about 70%, with concomitant reductions in mortality and colectomy associated with infection. No increase in rates of hospital admissions, infection complications, or death were observed.12–14
GOAL: BETTER CARE (NOT CHEAPER CARE OR LESS ANTIBIOTIC USE)
The primary goal of antibiotic stewardship is better patient care. The goal is not reduced antibiotic use or cost savings, although these could be viewed as favorable side effects. Sometimes, better patient care involves using more antibiotics: eg, a patient with presumed sepsis should be started quickly on broad-spectrum antibiotics, an action that also falls under antibiotic stewardship. The focus for stewardship efforts should be on optimizing appropriate use, ie, promoting the use of the right agent at the correct dosage and for the proper duration.
Stewardship improves clinical outcomes
Antibiotic stewardship is important not only to society but to individual patients.
Singh et al15 randomized patients suspected of having ventilator-associated pneumonia (but with a low likelihood of pneumonia) to either a 3-day course of ciprofloxacin or standard care (antibiotics for 10 to 21 days, with the drug and duration chosen by the treating physician). After 3 days, the patients in the experimental group were reevaluated, and antibiotics were stopped if the likelihood of pneumonia was still deemed low. In patients who received only the short course of antibiotics, mean length of stay in the intensive care unit was 9 days and the risk of acquiring an antibiotic-resistant superinfection during hospitalization was 14%, compared with a 15-day length of stay and 38% risk of antibiotic-resistant superinfection in patients in the standard treatment group.
Fishman16 reported a study at a single hospital that randomized patients to either receive standard care according to physician choice or be treated according to an antibiotic stewardship program. Patients in the antibiotic stewardship group were almost 3 times more likely than controls to receive appropriate therapy according to guidelines. More important, the antibiotic stewardship patients were almost twice as likely to be cured of their infection and were more than 80% less likely to have treatment failure.
DEVELOPING EFFECTIVE ANTIBIOTIC STEWARDSHIP PROGRAMS
A good model for improving antibiotic use is a recent nationwide program designed to reduce central line-associated bloodstream infections.17 Rates of these infections have dropped by about 50% over the past 5 years. The program included:
- Research to better understand the problem and how to fight it
- Well-defined programs and interventions
- Education to implement interventions, eg, deploying teams to teach better techniques of inserting and maintaining central lines
- A strong national measurement system (the CDC’s National Healthcare Safety Network) to track infections.
What constitutes an antibiotic stewardship program?
The CDC examined successful stewardship programs in a variety of hospital types, including large academic hospitals and smaller hospitals, and identified 7 common core elements that could serve as general principles that were common to successful antibiotic stewardship programs18:
- Leadership commitment from administration
- A single leader responsible for outcomes
- A single pharmacy leader
- Tracking of antibiotic use
- Regular reporting of antibiotic use and resistance
- Educating providers on use and resistance
- Specific improvement interventions.
Stewardship is harder in some settings
In reply to a CDC survey in 2014, 41% of more than 4,000 hospitals reported that they had antibiotic stewardship programs with all 7 core elements. The single element that predicted whether a complete program was in place was leadership support.19 The following year, 48% of respondents reported that they had a complete program in place. Percentages varied among states, with highs in Utah (77%) and California (70%) and lows in North Dakota (12%) and Vermont (7%). Large hospitals and major teaching hospitals were more likely to have a program with all 7 elements: 31% of hospitals with 50 or fewer beds had a complete program vs 66% of hospitals with at least 200 beds.20
Short-stay, critical-access hospitals pose a special challenge, as only 26% reported having all core elements.19,20 These facilities have fewer than 25 beds, and many patient stays are less than 3 days. Some do not employ full-time pharmacists or full-time clinicians. The CDC is collaborating with the American Hospital Association and the Pew Charitable Trusts to focus efforts on helping these hospitals, which requires a more flexible approach. About 100 critical-access hospitals nationwide have reported implementing all of the core elements and can serve as models for the others.
MEASURING IMPROVEMENT
The CDC has adopted a 3-pronged approach to measuring improvements in hospital antibiotic use:
- Estimate national aggregate antibiotic use described above
- Acquire information on antibiotic use at facility, practice, and provider levels
- Assess appropriate antibiotic use.
In hospitals, the CDC has concentrated on facility-level measurement. Hospitals need a system to track their own use and compare it with that of similar facilities. The CDC’s monitoring program, the Antibiotic Use Option of the National Healthcare Safety Network, captures electronic data on antibiotic use in a facility, enabling monitoring of use in each unit. Data can also be aggregated at regional, state, and national levels. This information can be used to develop benchmarks for antibiotic use, so that similar hospitals can be compared.
What is the ‘right’ amount of antibiotic use? Enter SAAR
Creating benchmarks for antibiotic use poses a number of challenges compared with most other areas in healthcare. Most public health measures are binary—eg, people either get an infection, a vaccination, or a smoking cessation intervention or not—and the direction of progress is clear. Antibiotics are different: not everybody needs them, but some people do. Usage should be reduced, but by exactly how much is unclear and varies between hospitals. In addition, being an outlier does not necessarily indicate a problem: a hospital unit for organ transplants will have high rates of antibiotic use, which is likely appropriate.
The CDC has taken initial steps to develop a risk-adjusted benchmark measure for hospital antibiotic use, the Standardized Antimicrobial Administration Ratio (SAAR). It compares a hospital’s observed antibiotic use with a calculation of predicted use based on its facility characteristics. Although still at an early stage, SAAR has been released and has been endorsed by the National Quality Forum. About 200 hospitals are submitting data to the CDC and collaborating with the CDC to evaluate the SAAR’s utility in driving improved antibiotic use.
Problems in measuring appropriate use
Measuring appropriate antibiotic use is easier in the outpatient setting, where detailed data have been collected for many years.
Fleming-Dutra et al21 compared medications prescribed during outpatient visits and the diagnoses coded for the visits. They found that about 13% of all outpatient visits resulted in an antibiotic prescription, 30% of which had no listed diagnosis that would justify an antibiotic (eg, viral upper respiratory infection). This kind of information provides a target for stewardship programs.
It is more difficult to conduct such a study in a hospital setting. Simply comparing discharge diagnoses to antibiotics prescribed is not useful: often antibiotics are started presumptively on admission for a patient with signs and symptoms of an infection, then stopped if the diagnosis does not warrant antibiotics, which is a reasonable strategy.
Also, many times, a patient with asymptomatic bacteriuria, which does not warrant antibiotics, is misdiagnosed as having a urinary tract infection, which does. So simply looking at the discharge code may not reveal whether therapy was appropriate.
Some studies have provided useful information. Fridkin et al22 studied 36 hospitals for the use of vancomycin, which is an especially good candidate drug for study because guidelines exist for appropriate use. Data were collected only from patients given vancomycin for more than 3 days, which should have eliminated empiric use of the drug and included only pathogen-driven therapy. Cases where therapy was for skin and soft-tissue infections were excluded because cultures are not usually obtained for these cases. Of patients given vancomycin, 9% had no diagnostic culture obtained at antibiotic initiation, 22% had diagnostic culture but results showed no gram-positive bacterial growth, and 5% had culture results revealing only oxacillin-susceptible Staphylococcus aureus. In 36% of cases, opportunities existed for improved prescribing.
Such data could be collected from the electronic medical record, and the CDC is focusing efforts in this direction.
NATIONAL ACTIVITIES IN ANTIBIOTIC STEWARDSHIP
In 2014, the White House launched a national strategy to combat antibiotic resistance,23 followed by an action plan in 2015.24 As a result, new investments have been made to improve antibiotic use, including funding for state health departments to begin stewardship efforts and to expand public awareness of the problems of antibiotic overuse. Research efforts are also being funded to improve implementation of existing stewardship practices and to develop new ones.
CMS is also exploring how to drive improved antibiotic use. In October 2016, it started requiring all US nursing homes to have antibiotic stewardship programs, and a similar requirement for hospitals has been proposed.
The Joint Commission issued a standard requiring that all their accredited facilities, starting with hospitals, have an antibiotic stewardship program by January 2017. This standard requires implementation of all the CDC’s core elements.
PROVEN INTERVENTIONS
Focusing on key interventions that are likely to be effective and well received by providers is a useful strategy for antibiotic stewardship efforts. A number of such interventions have been supported by research.
Postprescription antibiotic reviews or antibiotic ‘time-outs’
Antibiotics are often started empirically to treat hospitalized patients suspected of having an infection. The need for the antibiotic should be assessed a few days later, when culture results and more clinical information are available.
Elligsen et al25 evaluated the effects of providing a formal review and suggestions for antimicrobial optimization to critical care teams of 3 intensive care units in a single hospital after 3 and 10 days of antibiotic therapy. Mean monthly antibiotic use decreased from 644 days of therapy per 1,000 patient-days in the preintervention period to 503 days of therapy per 1,000 patient-days (P < .0001). C difficile infections were reduced from 11 cases to 6. Overall gram-negative susceptibility to meropenem increased in the critical care units.
Targeting specific infections
Some infections are especially important to target with improvement efforts.
In 2011, Magill et al26 conducted 1-day prevalence surveys in 183 hospitals in 10 states to examine patterns of antibiotic use. They found that lower respiratory tract infections and urinary tract infections accounted for more than half of all antibiotic use (35% and 22%, respectively), making them good candidates for improved use.
Community-acquired pneumonia can be targeted at multiple fronts. One study showed that almost 30% of patients diagnosed with community-acquired pneumonia in the emergency department did not actually have pneumonia.27 Duration of antibiotic therapy could also be targeted. Guidelines recommend that most patients with uncomplicated community-acquired pneumonia receive 5 to 7 days of antibiotic therapy. Avdic et al28 performed a simple intervention involving education and feedback to teams in 1 hospital regarding antibiotic choice and duration. This resulted in reducing the duration of therapy for community-acquired pneumonia from a median of 10 to 7 days.
Asymptomatic bacteriuria is often misdiagnosed as a urinary tract infection and treated unnecessarily.29–31
Trautner et al32 addressed this problem by targeting urine cultures rather than antibiotics, using a simple algorithm: if a patient did not have symptoms of urinary tract infection (fever, acute hematuria, delirium, rigors, flank pain, pelvic discomfort, urgency, frequency, dysuria, suprapubic pain), a urine culture was not recommended. If a patient did have symptoms but a problem other than urinary tract infection was deemed likely, evaluation of other sources of infection was recommended. Use of the algorithm resulted in fewer urine cultures and less antibiotic overtreatment of asymptomatic bacteriuria. Reductions persisted after the intervention ended.
Antibiotic time-out at hospital discharge
Another study evaluated an intervention that required a pharmacist consultation for the critical care team when a patient was to be discharged with intravenous antibiotics (most often for pneumonia). In 28% of cases, chart review revealed that the infection had been completely treated at the time of discharge, so further antibiotic treatment was not indicated. No patients who avoided antibiotics at discharge were readmitted or subsequently visited the emergency department.33
Targeting outpatient settings
A number of studies have evaluated simple interventions to improve outpatient antibiotic prescribing. Meeker et al34 had providers place a poster in their examination rooms with a picture of the physician and a signed letter committing to the appropriate use of antibiotics. Inappropriate antibiotic use decreased 20% in the intervention group vs controls (P = .02).
In a subsequent study,35 the same group required providers to include a justification note in the electronic medical record every time an antibiotic was prescribed for an indication when guidelines do not recommend one. Inappropriate prescribing dropped from 23% to 5% (P < .001).
Another intervention in this study35 provided physicians with periodic feedback according to whether their therapy was concordant with guidelines. They received an email with a subject line of either “You are a top performer” or “You are not a top performer.” The contents of the email provided data on how many antibiotic prescriptions they wrote for conditions that did not warrant them and how their prescribing habits compared with those of their top-performing peers. Mean inappropriate antibiotic prescribing fell from 20% to 4%.35
This is a critical time for antibiotic stewardship efforts in the United States. The need has never been more urgent and, fortunately, the opportunities have never been more abundant. Requirements for stewardship programs will drive implementation, but hospitals will need support and guidance to help ensure that stewardship programs are as effective as possible. Ultimately, improving antibiotic use will require collaboration among all stakeholders. CDC is eager to partner with providers and others in their efforts to improve antibiotic use.
Antibiotic stewardship has always been a good idea. Now it is also required by the Joint Commission and the Center for Medicare and Medicaid Services (CMS). This article reviews the state of antibiotic use in the United States and efforts to improve antibiotic stewardship in practice.
ANTIBIOTICS ARE DIFFERENT FROM OTHER DRUGS
Their efficacy wanes over time. Antibiotics are the only medications that become less useful over time even if used correctly. Although other types of drugs are continuously being improved, the old ones work as well today as they did when they first came out. But antibiotics that were in use 50 years ago are no longer as effective.
They are a shared resource. Antibiotics are regularly used by many specialties to deliver routine and advanced medical care. Surgeries, transplantation, and immunosuppressive therapy would be unsafe without antibiotics to treat infections. Some patients awaiting lung transplant are not considered good candidates if they have evidence of colonization by antibiotic-resistant organisms.
Individual use may harm others. Even people who are not exposed to an antibiotic can suffer the consequences of how others use them.
In a retrospective cohort study, Freedberg et al1 analyzed the risk of hospitalized patients developing Clostridium difficile infection and found that the risk was higher if the previous occupant of the bed had received antibiotics. The putative mechanism is that a patient receiving antibiotics develops altered gut flora, leading to C difficile spores released into the environment and not eradicated by normal cleaning. The next patient using the bed is then exposed and infected.
ANTIBIOTIC USE IS HIGH
The US Centers for Disease Control (CDC) monitors antibiotic prescriptions throughout the United States. In the outpatient setting, enough antibiotics are prescribed nationwide for 5 out of every 6 people to get 1 course of antibiotics annually (835 prescriptions per 1,000 people). Rates vary widely among states, with the lowest rate in Alaska (501 prescriptions per 1,000 people) and the highest in West Virginia (1,285 prescriptions per 1,000 people).2 In comparison, Scandinavian countries prescribe about 400 courses per 1,000 people, about 20% less than our lowest-prescribing state.3
Antibiotics are probably the most frequently prescribed drugs in US hospitals. Data from 2006 to 2012 showed that 55% of hospitalized patients received at least 1 dose of an antibiotic and that overall about 75% of all hospital days involved an antibiotic.4 Rates did not vary by hospital size, but nonteaching hospitals tended to use antibiotics more than teaching hospitals. Antibiotic use is much more common in intensive care units than in hospital wards (1,092 and 720 days of antibiotic treatment per 1,000 patient-days, respectively).
Although overall antibiotic use did not change significantly over the years of the survey, use patterns did: fluoroquinolone use dropped by 20%, possibly reflecting rising resistance or increased attention to associated side effects (although fluoroquinolones remain the most widely prescribed inpatient antibiotic class), and use of first-generation cephalosporins fell by 7%. A cause for concern is that the use of broad-spectrum and “last-resort” antibiotics increased: carbapenem use by 37%, vancomycin use by 32%, beta-lactam/beta-lactamase inhibitor use by 26%, and third- and fourth-generation cephalosporin use by 12%.4
About one-third of use is unnecessary
Many studies have tried to measure the extent of inappropriate or unnecessary antibiotic use. The results have been remarkably consistent at 20% to 40% for both inpatient and outpatient studies. One study of hospitalized patients not in the intensive care unit found that 30% of 1,941 days of prescribed antimicrobial therapy were unnecessary, mostly because patients received antibiotics for longer than needed or because antibiotics were used to treat noninfectious syndromes or colonizing microorganisms.5
ANTIBIOTIC EXPOSURE HAS NEGATIVE CONSEQUENCES
Any exposure to a medication involves the potential for side effects; this is true for antibiotics whether or not their use is appropriate. An estimated 140,000 visits to emergency departments occur annually for adverse reactions to antibiotics.6 In hospitalized patients, these reactions can be severe, including renal and bone marrow toxicity. As with any medications, the risks and benefits of antibiotic therapy must be weighed patient by patient.
Disturbance of gut microbiome
Antibiotics’ disruptive effects on normal gut flora are becoming better understood and are even believed to increase the risk of obesity and asthma.7,8
Animal models provide evidence that altered flora is associated with sepsis, which is attributed to the gut microbiome’s role in containing dissemination of bacteria in the body.9 An ecological study provides further evidence. Baggs et al10 retrospectively studied more than 9 million patients discharged without sepsis from 473 US hospitals, of whom 0.6% were readmitted for sepsis within 90 days. Exposure to a broad-spectrum antibiotic was associated with a 50% increased risk of readmission within 90 days of discharge because of sepsis (odds ratio 1.50, 95% confidence interval 1.47–1.53).
Increase of C difficile infections
Antibiotics exert selective pressure, killing susceptible bacteria and allowing resistant bacteria to thrive.
The risk of C difficile infection is 7 to 10 times higher than at baseline for 1 month after antibiotic use and 3 times higher than baseline in the 2 months after that.11 Multiple studies have found that stewardship efforts to reduce antibiotic use have resulted in fewer C difficile infections.
A nationwide effort in England over the past decade to reduce C difficile infections has resulted in 50% less use of fluoroquinolones and third-generation cephalosporins in patients over age 65. During that time, the incidence of C difficile infection in that age group fell by about 70%, with concomitant reductions in mortality and colectomy associated with infection. No increase in rates of hospital admissions, infection complications, or death were observed.12–14
GOAL: BETTER CARE (NOT CHEAPER CARE OR LESS ANTIBIOTIC USE)
The primary goal of antibiotic stewardship is better patient care. The goal is not reduced antibiotic use or cost savings, although these could be viewed as favorable side effects. Sometimes, better patient care involves using more antibiotics: eg, a patient with presumed sepsis should be started quickly on broad-spectrum antibiotics, an action that also falls under antibiotic stewardship. The focus for stewardship efforts should be on optimizing appropriate use, ie, promoting the use of the right agent at the correct dosage and for the proper duration.
Stewardship improves clinical outcomes
Antibiotic stewardship is important not only to society but to individual patients.
Singh et al15 randomized patients suspected of having ventilator-associated pneumonia (but with a low likelihood of pneumonia) to either a 3-day course of ciprofloxacin or standard care (antibiotics for 10 to 21 days, with the drug and duration chosen by the treating physician). After 3 days, the patients in the experimental group were reevaluated, and antibiotics were stopped if the likelihood of pneumonia was still deemed low. In patients who received only the short course of antibiotics, mean length of stay in the intensive care unit was 9 days and the risk of acquiring an antibiotic-resistant superinfection during hospitalization was 14%, compared with a 15-day length of stay and 38% risk of antibiotic-resistant superinfection in patients in the standard treatment group.
Fishman16 reported a study at a single hospital that randomized patients to either receive standard care according to physician choice or be treated according to an antibiotic stewardship program. Patients in the antibiotic stewardship group were almost 3 times more likely than controls to receive appropriate therapy according to guidelines. More important, the antibiotic stewardship patients were almost twice as likely to be cured of their infection and were more than 80% less likely to have treatment failure.
DEVELOPING EFFECTIVE ANTIBIOTIC STEWARDSHIP PROGRAMS
A good model for improving antibiotic use is a recent nationwide program designed to reduce central line-associated bloodstream infections.17 Rates of these infections have dropped by about 50% over the past 5 years. The program included:
- Research to better understand the problem and how to fight it
- Well-defined programs and interventions
- Education to implement interventions, eg, deploying teams to teach better techniques of inserting and maintaining central lines
- A strong national measurement system (the CDC’s National Healthcare Safety Network) to track infections.
What constitutes an antibiotic stewardship program?
The CDC examined successful stewardship programs in a variety of hospital types, including large academic hospitals and smaller hospitals, and identified 7 common core elements that could serve as general principles that were common to successful antibiotic stewardship programs18:
- Leadership commitment from administration
- A single leader responsible for outcomes
- A single pharmacy leader
- Tracking of antibiotic use
- Regular reporting of antibiotic use and resistance
- Educating providers on use and resistance
- Specific improvement interventions.
Stewardship is harder in some settings
In reply to a CDC survey in 2014, 41% of more than 4,000 hospitals reported that they had antibiotic stewardship programs with all 7 core elements. The single element that predicted whether a complete program was in place was leadership support.19 The following year, 48% of respondents reported that they had a complete program in place. Percentages varied among states, with highs in Utah (77%) and California (70%) and lows in North Dakota (12%) and Vermont (7%). Large hospitals and major teaching hospitals were more likely to have a program with all 7 elements: 31% of hospitals with 50 or fewer beds had a complete program vs 66% of hospitals with at least 200 beds.20
Short-stay, critical-access hospitals pose a special challenge, as only 26% reported having all core elements.19,20 These facilities have fewer than 25 beds, and many patient stays are less than 3 days. Some do not employ full-time pharmacists or full-time clinicians. The CDC is collaborating with the American Hospital Association and the Pew Charitable Trusts to focus efforts on helping these hospitals, which requires a more flexible approach. About 100 critical-access hospitals nationwide have reported implementing all of the core elements and can serve as models for the others.
MEASURING IMPROVEMENT
The CDC has adopted a 3-pronged approach to measuring improvements in hospital antibiotic use:
- Estimate national aggregate antibiotic use described above
- Acquire information on antibiotic use at facility, practice, and provider levels
- Assess appropriate antibiotic use.
In hospitals, the CDC has concentrated on facility-level measurement. Hospitals need a system to track their own use and compare it with that of similar facilities. The CDC’s monitoring program, the Antibiotic Use Option of the National Healthcare Safety Network, captures electronic data on antibiotic use in a facility, enabling monitoring of use in each unit. Data can also be aggregated at regional, state, and national levels. This information can be used to develop benchmarks for antibiotic use, so that similar hospitals can be compared.
What is the ‘right’ amount of antibiotic use? Enter SAAR
Creating benchmarks for antibiotic use poses a number of challenges compared with most other areas in healthcare. Most public health measures are binary—eg, people either get an infection, a vaccination, or a smoking cessation intervention or not—and the direction of progress is clear. Antibiotics are different: not everybody needs them, but some people do. Usage should be reduced, but by exactly how much is unclear and varies between hospitals. In addition, being an outlier does not necessarily indicate a problem: a hospital unit for organ transplants will have high rates of antibiotic use, which is likely appropriate.
The CDC has taken initial steps to develop a risk-adjusted benchmark measure for hospital antibiotic use, the Standardized Antimicrobial Administration Ratio (SAAR). It compares a hospital’s observed antibiotic use with a calculation of predicted use based on its facility characteristics. Although still at an early stage, SAAR has been released and has been endorsed by the National Quality Forum. About 200 hospitals are submitting data to the CDC and collaborating with the CDC to evaluate the SAAR’s utility in driving improved antibiotic use.
Problems in measuring appropriate use
Measuring appropriate antibiotic use is easier in the outpatient setting, where detailed data have been collected for many years.
Fleming-Dutra et al21 compared medications prescribed during outpatient visits and the diagnoses coded for the visits. They found that about 13% of all outpatient visits resulted in an antibiotic prescription, 30% of which had no listed diagnosis that would justify an antibiotic (eg, viral upper respiratory infection). This kind of information provides a target for stewardship programs.
It is more difficult to conduct such a study in a hospital setting. Simply comparing discharge diagnoses to antibiotics prescribed is not useful: often antibiotics are started presumptively on admission for a patient with signs and symptoms of an infection, then stopped if the diagnosis does not warrant antibiotics, which is a reasonable strategy.
Also, many times, a patient with asymptomatic bacteriuria, which does not warrant antibiotics, is misdiagnosed as having a urinary tract infection, which does. So simply looking at the discharge code may not reveal whether therapy was appropriate.
Some studies have provided useful information. Fridkin et al22 studied 36 hospitals for the use of vancomycin, which is an especially good candidate drug for study because guidelines exist for appropriate use. Data were collected only from patients given vancomycin for more than 3 days, which should have eliminated empiric use of the drug and included only pathogen-driven therapy. Cases where therapy was for skin and soft-tissue infections were excluded because cultures are not usually obtained for these cases. Of patients given vancomycin, 9% had no diagnostic culture obtained at antibiotic initiation, 22% had diagnostic culture but results showed no gram-positive bacterial growth, and 5% had culture results revealing only oxacillin-susceptible Staphylococcus aureus. In 36% of cases, opportunities existed for improved prescribing.
Such data could be collected from the electronic medical record, and the CDC is focusing efforts in this direction.
NATIONAL ACTIVITIES IN ANTIBIOTIC STEWARDSHIP
In 2014, the White House launched a national strategy to combat antibiotic resistance,23 followed by an action plan in 2015.24 As a result, new investments have been made to improve antibiotic use, including funding for state health departments to begin stewardship efforts and to expand public awareness of the problems of antibiotic overuse. Research efforts are also being funded to improve implementation of existing stewardship practices and to develop new ones.
CMS is also exploring how to drive improved antibiotic use. In October 2016, it started requiring all US nursing homes to have antibiotic stewardship programs, and a similar requirement for hospitals has been proposed.
The Joint Commission issued a standard requiring that all their accredited facilities, starting with hospitals, have an antibiotic stewardship program by January 2017. This standard requires implementation of all the CDC’s core elements.
PROVEN INTERVENTIONS
Focusing on key interventions that are likely to be effective and well received by providers is a useful strategy for antibiotic stewardship efforts. A number of such interventions have been supported by research.
Postprescription antibiotic reviews or antibiotic ‘time-outs’
Antibiotics are often started empirically to treat hospitalized patients suspected of having an infection. The need for the antibiotic should be assessed a few days later, when culture results and more clinical information are available.
Elligsen et al25 evaluated the effects of providing a formal review and suggestions for antimicrobial optimization to critical care teams of 3 intensive care units in a single hospital after 3 and 10 days of antibiotic therapy. Mean monthly antibiotic use decreased from 644 days of therapy per 1,000 patient-days in the preintervention period to 503 days of therapy per 1,000 patient-days (P < .0001). C difficile infections were reduced from 11 cases to 6. Overall gram-negative susceptibility to meropenem increased in the critical care units.
Targeting specific infections
Some infections are especially important to target with improvement efforts.
In 2011, Magill et al26 conducted 1-day prevalence surveys in 183 hospitals in 10 states to examine patterns of antibiotic use. They found that lower respiratory tract infections and urinary tract infections accounted for more than half of all antibiotic use (35% and 22%, respectively), making them good candidates for improved use.
Community-acquired pneumonia can be targeted at multiple fronts. One study showed that almost 30% of patients diagnosed with community-acquired pneumonia in the emergency department did not actually have pneumonia.27 Duration of antibiotic therapy could also be targeted. Guidelines recommend that most patients with uncomplicated community-acquired pneumonia receive 5 to 7 days of antibiotic therapy. Avdic et al28 performed a simple intervention involving education and feedback to teams in 1 hospital regarding antibiotic choice and duration. This resulted in reducing the duration of therapy for community-acquired pneumonia from a median of 10 to 7 days.
Asymptomatic bacteriuria is often misdiagnosed as a urinary tract infection and treated unnecessarily.29–31
Trautner et al32 addressed this problem by targeting urine cultures rather than antibiotics, using a simple algorithm: if a patient did not have symptoms of urinary tract infection (fever, acute hematuria, delirium, rigors, flank pain, pelvic discomfort, urgency, frequency, dysuria, suprapubic pain), a urine culture was not recommended. If a patient did have symptoms but a problem other than urinary tract infection was deemed likely, evaluation of other sources of infection was recommended. Use of the algorithm resulted in fewer urine cultures and less antibiotic overtreatment of asymptomatic bacteriuria. Reductions persisted after the intervention ended.
Antibiotic time-out at hospital discharge
Another study evaluated an intervention that required a pharmacist consultation for the critical care team when a patient was to be discharged with intravenous antibiotics (most often for pneumonia). In 28% of cases, chart review revealed that the infection had been completely treated at the time of discharge, so further antibiotic treatment was not indicated. No patients who avoided antibiotics at discharge were readmitted or subsequently visited the emergency department.33
Targeting outpatient settings
A number of studies have evaluated simple interventions to improve outpatient antibiotic prescribing. Meeker et al34 had providers place a poster in their examination rooms with a picture of the physician and a signed letter committing to the appropriate use of antibiotics. Inappropriate antibiotic use decreased 20% in the intervention group vs controls (P = .02).
In a subsequent study,35 the same group required providers to include a justification note in the electronic medical record every time an antibiotic was prescribed for an indication when guidelines do not recommend one. Inappropriate prescribing dropped from 23% to 5% (P < .001).
Another intervention in this study35 provided physicians with periodic feedback according to whether their therapy was concordant with guidelines. They received an email with a subject line of either “You are a top performer” or “You are not a top performer.” The contents of the email provided data on how many antibiotic prescriptions they wrote for conditions that did not warrant them and how their prescribing habits compared with those of their top-performing peers. Mean inappropriate antibiotic prescribing fell from 20% to 4%.35
This is a critical time for antibiotic stewardship efforts in the United States. The need has never been more urgent and, fortunately, the opportunities have never been more abundant. Requirements for stewardship programs will drive implementation, but hospitals will need support and guidance to help ensure that stewardship programs are as effective as possible. Ultimately, improving antibiotic use will require collaboration among all stakeholders. CDC is eager to partner with providers and others in their efforts to improve antibiotic use.
- Freedberg DE, Salmasian H, Cohen B, Abrams JA, Larson EL. Receipt of antibiotics in hospitalized patients and risk for Clostridium difficile infection in subsequent patients who occupy the same bed. JAMA Intern Med 2016; 176:1801–1808.
- Centers for Disease Control and Prevention. Get smart: know when antibiotics work. Measuring outpatient antibiotic prescribing. https://www.cdc.gov/getsmart/community/programs-measurement/measuring-antibiotic-prescribing.html. Accessed February 5, 2017.
- Ternhag A, Hellman J. More on U.S. outpatient antibiotic prescribing, 2010. N Engl J Med 2013; 369:1175–1176.
- Baggs J, Fridkin SK, Pollack LA, Srinivasan A, Jernigan JA. Estimating national trends in inpatient antibiotic use among US hospitals from 2006 to 2012. JAMA Intern Med 2016; 176:1639–1648.
- Hecker MT, Aron DC, Patel NP, Lehmann MK, Donskey CJ. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med 2003; 163:972–978.
- Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis 2008; 47:735–743.
- Korpela K, de Vos WM. Antibiotic use in childhood alters the gut microbiota and predisposes to overweight. Microb Cell 2016; 3:296–298.
- Gray LE, O’Hely M, Ranganathan S, Sly PD, Vuillermin P. The maternal diet, gut bacteria, and bacterial metabolites during pregnancy influence offspring asthma. Front Immunol 2017; 8:365
- Haak BW, Wiersinga WJ. The role of the gut microbiota in sepsis. Lancet Gastroenterol Hepatol 2017; 2:135–143.
- Baggs J, Jernigan J, Mccormick K, Epstein L, Laufer-Halpin AS, Mcdonald C. Increased risk of sepsis during hospital readmission following exposure to certain antibiotics during hospitalization. Abstract presented at IDWeek, October 26-30, 2016, New Orleans, LA. https://idsa.confex.com/idsa/2016/webprogram/Paper58587.html. Accessed August 8, 2017.
- Hensgens MP, Goorhuis A, Dekkers OM, Kuijper EJ. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother 2012; 67:742–748.
- Ashiru-Oredope D, Sharland M, Charani E, McNulty C, Cooke J; ARHAI Antimicrobial Stewardship Group. Improving the quality of antibiotic prescribing in the NHS by developing a new antimicrobial stewardship programme: Start Smart—Then Focus. J Antimicrob Chemother 2012; 67(suppl 1):i51–i63.
- Wilcox MH, Shetty N, Fawley WN, et al. Changing epidemiology of Clostridium difficile infection following the introduction of a national ribotyping-based surveillance scheme in England. Clin Infect Dis 2012; 55:1056-1063.
- Public Health England. Clostridium difficile infection: monthly data by NHS acute trust. https://www.gov.uk/government/statistics/clostridium-difficile-infection-monthly-data-by-nhs-acute-trust. Accessed August 4, 2017.
- Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med 2000; 162:505–511.
- Fishman N. Antimicrobial stewardship. Am J Med 2006; 119:S53–S61.
- US Centers for Disease Control and Prevention. Healthcare-associated infections (HAI) progress report. https://www.cdc.gov/hai/surveillance/progress-report/index.html. Accessed August 4, 2017.
- US Centers for Disease Control and Prevention. Get Smart for Healthcare. Core elements of hospital antibiotic stewardship programs. https://www.cdc.gov/getsmart/healthcare/implementation/core-elements.html. Accessed August 8, 2017.
- Pollack LA, van Santen KL, Weiner LM, Dudeck MA, Edwards JR, Srinivasan A. Antibiotic stewardship programs in U.S. acute care hospitals: findings from the 2014 National Healthcare Safety Network Annual Hospital Survey. Clin Infect Dis 2016; 63:443–449.
- US Centers for Disease Control and Prevention. Antibiotic stewardship in acute care hospitals by state 2014. https://gis.cdc.gov/grasp/PSA/STMapView.html. Accessed August 4, 2017.
- Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016; 315:1864-1873.
- Fridkin S, Baggs J, Fagan R, et al; Centers for Disease Control and Prevention (CDC). Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep 2014; 63:194–200.
- National strategy for combating antibiotic-resistant bacteria. September 2014. https://www.whitehouse.gov/sites/default/files/docs/carb_national_strategy.pdf. Accessed August 9, 2017.
- National action plan for combating antibiotic-resistant Bacteria. March 2015. https://www.whitehouse.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf
- Elligsen M, Walker SA, Pinto R, et al. Audit and feedback to reduce broad-spectrum antibiotic use among intensive care unit patients: a controlled interrupted time series analysis. Infect Control Hosp Epidemiol 2012; 33:354–361.
- Magill SS, Edwards JR, Beldavs ZG, et al; Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA 2014; 312:1438-1446.
- Chandra A, Nicks B, Maniago E, Nouh A, Limkakeng A. A multicenter analysis of the ED diagnosis of pneumonia. Am J Emerg Med 2010;28:862–865.
- Avdic E, Cushinotto LA, Hughes AH, et al. Impact of an antimicrobial stewardship intervention on shortening the duration of therapy for community-acquired pneumonia. Clin Infect Dis 2012; 54:1581–1587.
- Dalen DM, Zvonar RK, Jessamine PG, et al. An evaluation of the management of asymptomatic catheter-associated bacteriuria and candiduria at the Ottawa Hospital. Can J Infect Dis Med Micribiol 2005; 16:166–170.
- Gandhi T, Flanders SA, Markovitz E, Saint S, Kaul DR. Importance of urinary tract infection to antibiotic use among hospitalized patients. Infect Control Hosp Epidemiol 2009; 30:193–195.
- Cope M, Cevallos ME, Cadle RM, Darouiche RO, Musher DM, Trautner BW. Inappropriate treatment of catheter-associated asymptomatic bacteriuria in a tertiary care hospital. Clin Infect Dis 2009; 48:1182–1188.
- Trautner BW, Grigoryan L, Petersen NJ, et al. Effectiveness of an antimicrobial stewardship approach for urinary catheter-associated asymptomatic bacteriuria. JAMA Intern Med 2015; 175:1120–1127.
- Shrestha NK, Bhaskaran A, Scalera NM, Schmitt SK, Rehm SJ, Gordon SM. Antimicrobial stewardship at transition of care from hospital to community. Infect Control Hosp Epidemiol 2012; 33:401–404.
- Meeker D, Knight TK, Friedberg MW, et al. Nudging guideline-concordant antibiotic prescribing: a randomized clinical trial. JAMA Intern Med 2014; 174:425–431.
- Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA 2016; 315:562–572.
- Freedberg DE, Salmasian H, Cohen B, Abrams JA, Larson EL. Receipt of antibiotics in hospitalized patients and risk for Clostridium difficile infection in subsequent patients who occupy the same bed. JAMA Intern Med 2016; 176:1801–1808.
- Centers for Disease Control and Prevention. Get smart: know when antibiotics work. Measuring outpatient antibiotic prescribing. https://www.cdc.gov/getsmart/community/programs-measurement/measuring-antibiotic-prescribing.html. Accessed February 5, 2017.
- Ternhag A, Hellman J. More on U.S. outpatient antibiotic prescribing, 2010. N Engl J Med 2013; 369:1175–1176.
- Baggs J, Fridkin SK, Pollack LA, Srinivasan A, Jernigan JA. Estimating national trends in inpatient antibiotic use among US hospitals from 2006 to 2012. JAMA Intern Med 2016; 176:1639–1648.
- Hecker MT, Aron DC, Patel NP, Lehmann MK, Donskey CJ. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med 2003; 163:972–978.
- Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis 2008; 47:735–743.
- Korpela K, de Vos WM. Antibiotic use in childhood alters the gut microbiota and predisposes to overweight. Microb Cell 2016; 3:296–298.
- Gray LE, O’Hely M, Ranganathan S, Sly PD, Vuillermin P. The maternal diet, gut bacteria, and bacterial metabolites during pregnancy influence offspring asthma. Front Immunol 2017; 8:365
- Haak BW, Wiersinga WJ. The role of the gut microbiota in sepsis. Lancet Gastroenterol Hepatol 2017; 2:135–143.
- Baggs J, Jernigan J, Mccormick K, Epstein L, Laufer-Halpin AS, Mcdonald C. Increased risk of sepsis during hospital readmission following exposure to certain antibiotics during hospitalization. Abstract presented at IDWeek, October 26-30, 2016, New Orleans, LA. https://idsa.confex.com/idsa/2016/webprogram/Paper58587.html. Accessed August 8, 2017.
- Hensgens MP, Goorhuis A, Dekkers OM, Kuijper EJ. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother 2012; 67:742–748.
- Ashiru-Oredope D, Sharland M, Charani E, McNulty C, Cooke J; ARHAI Antimicrobial Stewardship Group. Improving the quality of antibiotic prescribing in the NHS by developing a new antimicrobial stewardship programme: Start Smart—Then Focus. J Antimicrob Chemother 2012; 67(suppl 1):i51–i63.
- Wilcox MH, Shetty N, Fawley WN, et al. Changing epidemiology of Clostridium difficile infection following the introduction of a national ribotyping-based surveillance scheme in England. Clin Infect Dis 2012; 55:1056-1063.
- Public Health England. Clostridium difficile infection: monthly data by NHS acute trust. https://www.gov.uk/government/statistics/clostridium-difficile-infection-monthly-data-by-nhs-acute-trust. Accessed August 4, 2017.
- Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med 2000; 162:505–511.
- Fishman N. Antimicrobial stewardship. Am J Med 2006; 119:S53–S61.
- US Centers for Disease Control and Prevention. Healthcare-associated infections (HAI) progress report. https://www.cdc.gov/hai/surveillance/progress-report/index.html. Accessed August 4, 2017.
- US Centers for Disease Control and Prevention. Get Smart for Healthcare. Core elements of hospital antibiotic stewardship programs. https://www.cdc.gov/getsmart/healthcare/implementation/core-elements.html. Accessed August 8, 2017.
- Pollack LA, van Santen KL, Weiner LM, Dudeck MA, Edwards JR, Srinivasan A. Antibiotic stewardship programs in U.S. acute care hospitals: findings from the 2014 National Healthcare Safety Network Annual Hospital Survey. Clin Infect Dis 2016; 63:443–449.
- US Centers for Disease Control and Prevention. Antibiotic stewardship in acute care hospitals by state 2014. https://gis.cdc.gov/grasp/PSA/STMapView.html. Accessed August 4, 2017.
- Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016; 315:1864-1873.
- Fridkin S, Baggs J, Fagan R, et al; Centers for Disease Control and Prevention (CDC). Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep 2014; 63:194–200.
- National strategy for combating antibiotic-resistant bacteria. September 2014. https://www.whitehouse.gov/sites/default/files/docs/carb_national_strategy.pdf. Accessed August 9, 2017.
- National action plan for combating antibiotic-resistant Bacteria. March 2015. https://www.whitehouse.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf
- Elligsen M, Walker SA, Pinto R, et al. Audit and feedback to reduce broad-spectrum antibiotic use among intensive care unit patients: a controlled interrupted time series analysis. Infect Control Hosp Epidemiol 2012; 33:354–361.
- Magill SS, Edwards JR, Beldavs ZG, et al; Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA 2014; 312:1438-1446.
- Chandra A, Nicks B, Maniago E, Nouh A, Limkakeng A. A multicenter analysis of the ED diagnosis of pneumonia. Am J Emerg Med 2010;28:862–865.
- Avdic E, Cushinotto LA, Hughes AH, et al. Impact of an antimicrobial stewardship intervention on shortening the duration of therapy for community-acquired pneumonia. Clin Infect Dis 2012; 54:1581–1587.
- Dalen DM, Zvonar RK, Jessamine PG, et al. An evaluation of the management of asymptomatic catheter-associated bacteriuria and candiduria at the Ottawa Hospital. Can J Infect Dis Med Micribiol 2005; 16:166–170.
- Gandhi T, Flanders SA, Markovitz E, Saint S, Kaul DR. Importance of urinary tract infection to antibiotic use among hospitalized patients. Infect Control Hosp Epidemiol 2009; 30:193–195.
- Cope M, Cevallos ME, Cadle RM, Darouiche RO, Musher DM, Trautner BW. Inappropriate treatment of catheter-associated asymptomatic bacteriuria in a tertiary care hospital. Clin Infect Dis 2009; 48:1182–1188.
- Trautner BW, Grigoryan L, Petersen NJ, et al. Effectiveness of an antimicrobial stewardship approach for urinary catheter-associated asymptomatic bacteriuria. JAMA Intern Med 2015; 175:1120–1127.
- Shrestha NK, Bhaskaran A, Scalera NM, Schmitt SK, Rehm SJ, Gordon SM. Antimicrobial stewardship at transition of care from hospital to community. Infect Control Hosp Epidemiol 2012; 33:401–404.
- Meeker D, Knight TK, Friedberg MW, et al. Nudging guideline-concordant antibiotic prescribing: a randomized clinical trial. JAMA Intern Med 2014; 174:425–431.
- Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA 2016; 315:562–572.
KEY POINTS
- Antibiotics are fundamentally different from other medications, posing special challenges and needs for improving their use.
- Antibiotic usage in the United States varies widely among healthcare settings.
- Antibiotic stewardship efforts should focus on optimizing appropriate use rather than simply reducing use.
- Effective interventions include timely consultation on appropriate prescribing, targeting specific infections, and providing feedback to physicians.
Palmar erythema as a sign of cancer
An 83-year-old man presented with fatigue and anorexia. Two years earlier, a small lung nodule had been found that was suspected to be primary lung cancer; however, he had refused surgical treatment or chemotherapy because of his age.
Aminotransferase and alkaline phosphatase levels had been normal, and results of serologic testing for hepatitis viruses were negative, making chronic liver disease unlikely. He was not a habitual drinker and was not taking any medications.
Laboratory and imaging studies revealed lung cancer with hepatic metastasis complicated by severe hypercalcemia. The estradiol level was normal, but the serum vascular endothelial growth factor (VEGF) concentration was high.
PALMAR ERYTHEMA AND SYSTEMIC DISEASE
Palmar erythema syndrome is characterized by reddening of the palmar skin, especially in the thenar and hypothenar areas, the distal portion of the palm, and the fingertips; the dorsal surface of the hand is rarely affected.1 The affected areas are typically not pruritic or painful.
Conditions in the differential diagnosis
The differential diagnosis of palmar erythema includes allergic drug eruptions, contact dermatitis, erythema multiforme, cellulitis, dermatomyositis, and palmoplantar pustulosis. Hand-foot syndrome and hand-foot skin reaction are other important conditions to consider in cancer patients undergoing chemotherapy.2 Hand-foot syndrome and hand-foot skin reaction can present as palmoplantar erythema and are usually accompanied by dysesthesia and swelling.
Palmar erythema can develop in either primary or secondary forms as a result of an underlying systemic disease.2 Although the pathogenesis is not fully understood, the impaired degradation or increased production of angiogenic factors appears to be essential. The hormone estrogen can induce vascularization3 and is known to cause palmar erythema in pregnant women and patients with cirrhosis. Because estradiol is metabolized in the liver, increased levels of estrogen are associated with hepatic decompensation in cirrhosis.4
Neoplasm can cause palmar erythema.2,5 In a clinicopathologic study of brain tumors, palmar erythema was recognized in 27 (25%) of 107 patients.5 Histologic examination in that study demonstrated that the intensity of erythema correlated with both cutaneous vessel dilation and prominent vascularization in the patients’ brain tumors, suggesting the role of circulating angiogenic factors such as VEGF. VEGF is a potent mediator of angiogenesis, which is critical for tumor development and growth.6
Our patient had a metastatic hepatic tumor, a normal estradiol level, and an increased level of VEGF, suggesting that the VEGF produced by the neoplasm promoted the development of palmar erythema.
The presence of palmar erythema in cancer patients is likely underestimated2 and may suggest the presence of malignancy when it develops in the elderly.
In our patient, intensive hydration and intravenous bisphosphonate administration rapidly corrected the malignancy-associated hypercalcemia. However, he was severely debilitated during the hospital stay and developed aspiration pneumonia repeatedly. Thereafter, he was transferred to hospice care. The palmar erythema remained after the electrolyte disorder was corrected.
- Perera GA. A note on palmar erythema (so-called liver palms). JAMA 1942; 119:1417–1418.
- Serrao R, Zirwas M, English JC. Palmar erythema. Am J Clin Dermatol 2007; 8:347–356.
- Losordo DW, Isner JM. Estrogen and angiogenesis: a review. Arterioscler Thromb Vasc Biol 2001; 21:6–12.
- Maruyama Y, Adachi Y, Aoki N, Suzuki Y, Shinohara H, Yamamoto T. Mechanism of feminization in male patients with non-alcoholic liver cirrhosis: role of sex hormone-binding globulin. Gastroenterol Jpn 1991; 26: 435–439.
- Noble JP, Boisnic S, Branchet-Gumila MC, Poisson M. Palmar erythema: cutaneous marker of neoplasms. Dermatology 2002; 204:209–213.
- Zheng CL, Qiu C, Shen MX, et al. Prognostic impact of elevation of vascular endothelial growth factor family expression in patients with non-small cell lung cancer: an updated meta-analysis. Asian Pac J Cancer Prev 2015; 16:1881–1895.
An 83-year-old man presented with fatigue and anorexia. Two years earlier, a small lung nodule had been found that was suspected to be primary lung cancer; however, he had refused surgical treatment or chemotherapy because of his age.
Aminotransferase and alkaline phosphatase levels had been normal, and results of serologic testing for hepatitis viruses were negative, making chronic liver disease unlikely. He was not a habitual drinker and was not taking any medications.
Laboratory and imaging studies revealed lung cancer with hepatic metastasis complicated by severe hypercalcemia. The estradiol level was normal, but the serum vascular endothelial growth factor (VEGF) concentration was high.
PALMAR ERYTHEMA AND SYSTEMIC DISEASE
Palmar erythema syndrome is characterized by reddening of the palmar skin, especially in the thenar and hypothenar areas, the distal portion of the palm, and the fingertips; the dorsal surface of the hand is rarely affected.1 The affected areas are typically not pruritic or painful.
Conditions in the differential diagnosis
The differential diagnosis of palmar erythema includes allergic drug eruptions, contact dermatitis, erythema multiforme, cellulitis, dermatomyositis, and palmoplantar pustulosis. Hand-foot syndrome and hand-foot skin reaction are other important conditions to consider in cancer patients undergoing chemotherapy.2 Hand-foot syndrome and hand-foot skin reaction can present as palmoplantar erythema and are usually accompanied by dysesthesia and swelling.
Palmar erythema can develop in either primary or secondary forms as a result of an underlying systemic disease.2 Although the pathogenesis is not fully understood, the impaired degradation or increased production of angiogenic factors appears to be essential. The hormone estrogen can induce vascularization3 and is known to cause palmar erythema in pregnant women and patients with cirrhosis. Because estradiol is metabolized in the liver, increased levels of estrogen are associated with hepatic decompensation in cirrhosis.4
Neoplasm can cause palmar erythema.2,5 In a clinicopathologic study of brain tumors, palmar erythema was recognized in 27 (25%) of 107 patients.5 Histologic examination in that study demonstrated that the intensity of erythema correlated with both cutaneous vessel dilation and prominent vascularization in the patients’ brain tumors, suggesting the role of circulating angiogenic factors such as VEGF. VEGF is a potent mediator of angiogenesis, which is critical for tumor development and growth.6
Our patient had a metastatic hepatic tumor, a normal estradiol level, and an increased level of VEGF, suggesting that the VEGF produced by the neoplasm promoted the development of palmar erythema.
The presence of palmar erythema in cancer patients is likely underestimated2 and may suggest the presence of malignancy when it develops in the elderly.
In our patient, intensive hydration and intravenous bisphosphonate administration rapidly corrected the malignancy-associated hypercalcemia. However, he was severely debilitated during the hospital stay and developed aspiration pneumonia repeatedly. Thereafter, he was transferred to hospice care. The palmar erythema remained after the electrolyte disorder was corrected.
An 83-year-old man presented with fatigue and anorexia. Two years earlier, a small lung nodule had been found that was suspected to be primary lung cancer; however, he had refused surgical treatment or chemotherapy because of his age.
Aminotransferase and alkaline phosphatase levels had been normal, and results of serologic testing for hepatitis viruses were negative, making chronic liver disease unlikely. He was not a habitual drinker and was not taking any medications.
Laboratory and imaging studies revealed lung cancer with hepatic metastasis complicated by severe hypercalcemia. The estradiol level was normal, but the serum vascular endothelial growth factor (VEGF) concentration was high.
PALMAR ERYTHEMA AND SYSTEMIC DISEASE
Palmar erythema syndrome is characterized by reddening of the palmar skin, especially in the thenar and hypothenar areas, the distal portion of the palm, and the fingertips; the dorsal surface of the hand is rarely affected.1 The affected areas are typically not pruritic or painful.
Conditions in the differential diagnosis
The differential diagnosis of palmar erythema includes allergic drug eruptions, contact dermatitis, erythema multiforme, cellulitis, dermatomyositis, and palmoplantar pustulosis. Hand-foot syndrome and hand-foot skin reaction are other important conditions to consider in cancer patients undergoing chemotherapy.2 Hand-foot syndrome and hand-foot skin reaction can present as palmoplantar erythema and are usually accompanied by dysesthesia and swelling.
Palmar erythema can develop in either primary or secondary forms as a result of an underlying systemic disease.2 Although the pathogenesis is not fully understood, the impaired degradation or increased production of angiogenic factors appears to be essential. The hormone estrogen can induce vascularization3 and is known to cause palmar erythema in pregnant women and patients with cirrhosis. Because estradiol is metabolized in the liver, increased levels of estrogen are associated with hepatic decompensation in cirrhosis.4
Neoplasm can cause palmar erythema.2,5 In a clinicopathologic study of brain tumors, palmar erythema was recognized in 27 (25%) of 107 patients.5 Histologic examination in that study demonstrated that the intensity of erythema correlated with both cutaneous vessel dilation and prominent vascularization in the patients’ brain tumors, suggesting the role of circulating angiogenic factors such as VEGF. VEGF is a potent mediator of angiogenesis, which is critical for tumor development and growth.6
Our patient had a metastatic hepatic tumor, a normal estradiol level, and an increased level of VEGF, suggesting that the VEGF produced by the neoplasm promoted the development of palmar erythema.
The presence of palmar erythema in cancer patients is likely underestimated2 and may suggest the presence of malignancy when it develops in the elderly.
In our patient, intensive hydration and intravenous bisphosphonate administration rapidly corrected the malignancy-associated hypercalcemia. However, he was severely debilitated during the hospital stay and developed aspiration pneumonia repeatedly. Thereafter, he was transferred to hospice care. The palmar erythema remained after the electrolyte disorder was corrected.
- Perera GA. A note on palmar erythema (so-called liver palms). JAMA 1942; 119:1417–1418.
- Serrao R, Zirwas M, English JC. Palmar erythema. Am J Clin Dermatol 2007; 8:347–356.
- Losordo DW, Isner JM. Estrogen and angiogenesis: a review. Arterioscler Thromb Vasc Biol 2001; 21:6–12.
- Maruyama Y, Adachi Y, Aoki N, Suzuki Y, Shinohara H, Yamamoto T. Mechanism of feminization in male patients with non-alcoholic liver cirrhosis: role of sex hormone-binding globulin. Gastroenterol Jpn 1991; 26: 435–439.
- Noble JP, Boisnic S, Branchet-Gumila MC, Poisson M. Palmar erythema: cutaneous marker of neoplasms. Dermatology 2002; 204:209–213.
- Zheng CL, Qiu C, Shen MX, et al. Prognostic impact of elevation of vascular endothelial growth factor family expression in patients with non-small cell lung cancer: an updated meta-analysis. Asian Pac J Cancer Prev 2015; 16:1881–1895.
- Perera GA. A note on palmar erythema (so-called liver palms). JAMA 1942; 119:1417–1418.
- Serrao R, Zirwas M, English JC. Palmar erythema. Am J Clin Dermatol 2007; 8:347–356.
- Losordo DW, Isner JM. Estrogen and angiogenesis: a review. Arterioscler Thromb Vasc Biol 2001; 21:6–12.
- Maruyama Y, Adachi Y, Aoki N, Suzuki Y, Shinohara H, Yamamoto T. Mechanism of feminization in male patients with non-alcoholic liver cirrhosis: role of sex hormone-binding globulin. Gastroenterol Jpn 1991; 26: 435–439.
- Noble JP, Boisnic S, Branchet-Gumila MC, Poisson M. Palmar erythema: cutaneous marker of neoplasms. Dermatology 2002; 204:209–213.
- Zheng CL, Qiu C, Shen MX, et al. Prognostic impact of elevation of vascular endothelial growth factor family expression in patients with non-small cell lung cancer: an updated meta-analysis. Asian Pac J Cancer Prev 2015; 16:1881–1895.
Reproductive planning for women after solid-organ transplant
Increasing numbers of women of childbearing age are receiving solid-organ transplants. All need counseling on how to prevent pregnancy while they are taking immunosuppressive agents. Some want to become pregnant after their transplant and thus require counseling and follow-up to maintain good health during pregnancy (Table 1).1
Primary care physicians can assist with basic contraception counseling and pregnancy planning for their patients who have had solid-organ transplants. In this review, we describe contraceptive options and pregnancy planning for these women.
TRANSPLANTS IN WOMEN ARE INCREASING
Over the past 20 years, the number of solid-organ transplants in US women has increased steadily. Since 1988, 38% of the 634,000 transplants performed were in women, and 47% of these women were of childbearing age (ages 18 to 49).2 Kidneys accounted for 60% of solid-organ transplants,2 and kidney transplant is now commonly performed in women of childbearing age. In 2012, of 176,000 patients with a functioning renal graft, 40.5% were women, and recipients between ages 20 and 44 composed the second-largest age group.3
FERTILITY IN WOMEN WITH END-STAGE RENAL DISEASE
Women in their reproductive years who have end-stage renal disease have lower fertility rates than women in the general population. In women undergoing peritoneal dialysis or hemodialysis, conception rates decrease to around 0.5% per year.4 This lower rate is most likely related to hypothalamic-pituitary-gonadal dysfunction, leading to reduced or total impairment of ovulation, menstrual irregularities, and infertility.5
Fertility often returns within a few months after transplant,1,6 and reported posttransplant pregnancy rates range from 3.3% to 18%,7–9 with up to one-third of pregnancies being unintended.6,10 These numbers are likely an underestimate because they do not reflect all pregnancies that are terminated, as many women do not voluntarily report having had an abortion.
Fertility is also severely diminished in women with end-stage liver disease. After liver transplant, sex hormone levels return to normal for many women, and menses soon resume.11
In 2005, the National Transplantation Pregnancy Registry reported 1,418 pregnancies in 919 female recipients of solid-organ transplants. In 2010, this number had increased to 1,940 pregnancies in 1,185 recipients, of whom 75% were kidney transplant recipients.12
A successful pregnancy outcome is most likely when a minimum of 1 year intervenes between transplant and conception.12,13
TERATOGENICITY OF IMMUNOSUPPRESSANTS
Immunosuppressant drugs commonly used for maintenance therapy after solid-organ transplant include the following:
- Calcineurin inhibitors (eg, cyclosporine, tacrolimus)
- Antiproliferative and antimetabolite agents (eg, mycophenolate mofetil, azathioprine)
- Corticosteroids
- Mammalian target of rapamycin inhibitors (eg, sirolimus, everolimus)
- T-cell costimulation blockers (eg, belatacept).14
The US Food and Drug Administration (FDA) previously classified mycophenolate mofetil and azathioprine in pregnancy risk category D (positive evidence of human fetal risk). The teratogenic risk of mycophenolate mofetil is well established in studies documenting specific congenital malformations and fetal loss in the first trimester.13,15 The teratogenic risk of azathioprine, on the other hand, is estimated to be minimal to small.16 Many of the associated fetal abnormalities may be related to the complexity of the underlying medical condition of the mother rather than to the medication.16
In June 2015, the FDA’s new Pregnancy and Lactation Labeling Rule went into effect, which removes the pregnancy letter categories A, B, C, D, and X from labeling.17 This rule was designed to help providers counsel their patients regarding the specific risks and benefits of a drug when used by pregnant or nursing women. However, the ABCDX categories are still commonly used. Table 2 shows information about the risks during pregnancy and lactation posed by the immunosuppressive drugs commonly used by posttransplant patients.18
CRITERIA FOR A SUCCESSFUL PREGNANCY
To ensure a safe and successful pregnancy with the fewest fetal and maternal complications, women are generally advised to avoid pregnancy for at least 1 year after transplant.19,20
In addition, women should meet certain clinical prerequisites after transplant before they conceive, as outlined by the American Society of Transplantation.19,20 These include:
- No rejection within the previous year
- Adequate and stable graft function (eg, serum creatinine < 1.5 mg/dL and urinary protein excretion < 500 mg/24 hours)
- No acute infection that might affect the fetus
- Maintenance immunosuppression at stable dosages.
Other circumstances to consider include episodes of rejection in the first year after transplant (as evidenced by biopsy results or glomerular filtration rate), the woman’s age (advanced maternal age is unfavorable), or any history of noncompliance.
Every pregnancy in a transplant recipient must be carefully planned. Primary care providers should encourage patients to meet with their transplant team and obstetricians early and often to allow time for the care team to adjust the type and dosing of immunosuppressant drugs, to ensure stable graft function, and to optimize any current chronic medical conditions such as diabetes mellitus or hypertension before conception.
CONTRACEPTIVE COUNSELING AFTER TRANSPLANT
Pregnancy should be avoided while transplant patients are taking FDA category D immunosuppressant drugs and, as already mentioned, during the first year after transplant. Unintended pregnancy can have serious health consequences for the mother and the fetus, as well as poor pregnancy outcomes. The US Centers for Disease Control and Prevention (CDC) lists solid-organ transplant within the past 2 years as a condition that can lead to adverse events as a result of pregnancy.21 After a transplant, a woman’s risks from an unintended pregnancy are always greater than the risks from any contraceptive, and this is important to reinforce in counseling.
Two forms of reliable contraception should be used at all times, and consistent condom use should be encouraged as one of the methods. Condoms are not reliable when used as the sole contraceptive method because they have an 18% typical-use failure rate. However, they are an excellent adjunct to other contraceptive methods because they have the additional benefit of protecting against sexually transmitted disease.
Choosing the appropriate contraceptive method for recipients of solid-organ transplants can be challenging because of several factors, including the recipient’s preexisting medical problems and drug interactions of immunosuppressant medications.
CDC criteria and categories for contraceptive use
In 2010, the CDC released the US version of the Medical Eligibility Criteria (US MEC) for contraceptive use, which was based on the 2009 World Health Organization Medical Eligibility Criteria (WHO MEC); these criteria were revised in August 2016.21
- Category 1: A condition for which there is no restriction for the use of the contraceptive method
- Category 2: A condition for which the advantages of using the method generally outweigh the theoretical or proven risks
- Category 3: A condition for which the theoretical or proven risks usually outweigh the advantages of using the method
- Category 4: A condition that represents an unacceptable health risk if the contraceptive method is used.
These recommendations aimed to improve family planning options by clarifying the possible safe and effective contraceptive options available while considering the patient’s medical condition. The CDC added solid-organ transplant recipients to this document because of the prevalence of this group in the United States.
The CDC categorizes a patient’s medical condition after transplant as either complicated or uncomplicated. Complicated conditions include acute or chronic graft failure, graft rejection, and cardiac allograft vasculopathy.21
Effectiveness of contraceptive methods
Contraceptive methods can be divided into 4 categories based on estimated effectiveness, ie, the pregnancy rate with “typical use” of that particular method in 1 year21–23:
- Very effective (0%–0.9%)
- Effective (1%–9%)
- Moderately effective (10%–25%)
- Less effective (26%–32%).
Typical use refers to failure rates for women and men whose use is not consistent nor always correct. Correct use, also described in the sections that follow, refers to failure rates for those whose use is consistent and always correct.
Women should be counseled regarding all available contraceptive options that are medically suitable for them, so they can choose the method that best fits their needs and lifestyle. They should receive counseling on emergency contraception, barrier protection against sexually transmitted disease, and the correct use of the contraceptive method they choose. They should be advised that if their chosen contraceptive method is unsatisfactory for any reason, they can switch to another method. Most importantly, providers need to impress on their patients that the risks associated with unintended pregnancy are far greater than the risks from any of the contraceptive methods.
VERY EFFECTIVE CONTRACEPTIVES (UNINTENDED PREGNANCY RATE 0%–0.9%)
This tier of contraception is the most effective regardless of the patient’s adherence; it includes long-acting, reversible contraceptives and permanent sterilization (both male and female) (Table 3).21–23
Long-acting reversible contraceptives include intrauterine devices (IUDs) and the subdermal etonogestrel implant. Given their efficacy and favorable safety profile, long-acting reversible contraceptives are being promoted for use in women who have chronic medical conditions, such as transplants.24
Intrauterine devices
IUDs are long-acting and reversible. They can be used by women who are nulliparous and those of all ages, including adolescents.22
Two types of IUDs are available in the United States: nonhormonal (copper) and hormonal (levonorgestrel). The copper IUD is effective for at least 10 years, whereas the levonorgestrel IUDs last for 3 to 5 years.22
Four levonorgestrel IUDs are currently available in the United States. Their sizes and doses vary: Mirena (52 µg), Skyla (13.5 µg), Liletta (52 µg), and Kyleena (19.5 µg).
Fewer than 1% of women become pregnant in the first year of IUD use.22,23 IUDs are an ideal option for women with solid-organ transplants because they are so effective and because the patient does not have to do anything once the IUD is in.22–24 The levonorgestrel IUD Mirena has the additional advantage of reducing heavy menstrual bleeding and is currently the only hormonal IUD with FDA approval for the management of menorrhagia.
About 12% of women in the general population use IUDs as their contraceptive method of choice,25 whereas after solid-organ transplantation about 15% to 20% of women do.26
Two historic concerns regarding IUDs may explain their low rate of use in transplant recipients.
First, IUDs were believed to be less effective in women on immunosuppressive drugs because IUDs act by inducing a local inflammatory reaction. However, IUDs involve macrophage activation, which is independent of the immune processes modified by immunosuppressants (primarily T-cell function).27 A recent pilot study showed a strong inflammatory reaction in the endometrium of transplant recipients after levonorgestrel IUD insertion.28
Second, there was concern about the increased risk of pelvic inflammatory disease with IUDs, but studies have shown levonorgestrel IUDs to be safe in transplant patients.29,30
The CDC21 lists copper and levonorgestrel IUDs in MEC category 3 (the risks generally outweigh the advantages) for initiation in patients with complicated transplants and in category 2 (advantages generally outweigh the risks) in patients with uncomplicated organ transplants. The devices are in category 2 for both complicated and uncomplicated cases if the IUD is already in place.
Subdermal implant
A subdermal implant consisting of a single rod containing 68 mg of etonogestrel is commercially available in the United States. It is one of the most effective contraceptive methods, with the lowest rates of pregnancy—less than 1% per year, with protection lasting at least 3 years.22,23 This low risk makes the subdermal implant a suitable method of contraception after transplant. Daily compliance is not required, and there are no hepatic first-pass effects, which results in higher bioavailability and less chance of drug interactions.
The main disadvantage of the subdermal implant and IUDs is unscheduled bleeding. An important benefit is prolonged amenorrhea, not only for patient convenience, but for reduction of endometrial cancer risk. Insertion and removal of the implant are considered minor office procedures. The implants are classified as US MEC category 2 in uncomplicated cases; initiation in complicated cases is considered category 3 but continuation is considered category 2.21
Permanent sterilization
Permanent sterilization is another option for women and men. In women, the fallopian tubes can be occluded with a coil system implanted vaginally through a hysteroscope, or they can be severed, tied, or clamped in a laparoscopic procedure or during cesarean delivery. Pregnancy rates after tubal ligation are less than 1%,23,31 although concern exists for high failure rates with the hysteroscopic method.
Because younger patients are more likely than older patients to subsequently regret having the procedure done, all available contraceptive options should be discussed with them.31
For men, permanent sterilization is done by vasectomy, which has less associated risk and cost compared with sterilization for women.
EFFECTIVE CONTRACEPTIVE METHODS (UNINTENDED PREGNANCY RATE 1%–9%)
Effective contraceptive methods, the next tier down from very effective methods, include injectable contraceptives, combined hormonal contraceptives, and progestin-only contraceptives (Table 4).
Injectable contraceptives
Depot medroxyprogesterone acetate is an injectable progestin-only contraceptive that carries a pregnancy risk of 6% with typical use and less than 1% with correct use.23 Thus, some failures are due to patients not returning for follow-up, but in some patients this method is not effective. Injections are given intramuscularly once every 3 months, avoiding the need for daily use.
A valid concern for transplant patients is that medroxyprogesterone acetate reduces bone mineral density. Although the bone effects are reversible in healthy adult women, caution is needed when prescribing this option to transplant patients who are already at increased risk of bone disease attributable to renal osteodystrophy and chronic corticosteroid use. 32,33
Recently, a subcutaneous formulation of depot medroxyprogesterone acetate (104 mg)was added to the WHO MEC for contraceptive use.34,35 The recommendations for the subcutaneous form are similar to those for the intramuscular form. In healthy women, the subcutaneous formulation is as safe and effective as the intramuscular form,36 but its efficacy after solid-organ transplant has not been determined. Both forms of depot medroxyprogesterone acetate are category 2 in the US MEC for both complicated and uncomplicated transplant cases.21
Combined hormonal contraceptives
Combined hormonal contraceptives contain both estrogen and progesterone and are available as pills, patches, or rings. Each product has an unintended pregnancy risk of 9% with typical use and less than 1% with correct use.23 They require strict patient adherence to regular daily use, which likely explains their high failure rate with typical use.
Combined hormonal contraceptives reduce mortality risk in women in the general population,37 but their effect on mortality risk after transplant is unknown and needs further study. In women who received liver transplants, low-dose combined hormonal contraceptives have been found to be effective and well tolerated, but initiation should be delayed at least 6 months until postoperative organ stability is demonstrated.11
Combined oral contraceptives are the most widely prescribed because they are convenient and familiar and have an acceptable safety profile in transplant patients,11,33,37 despite their high failure rate with typical use. They regulate the menstrual cycle and reduce anemia associated with menstruation.
The transdermal contraceptive patch has a mechanism of action similar to that of the combined oral contraceptives, but it delivers estrogen and progesterone transdermally through the abdominal wall, thus avoiding first-pass metabolism in the liver and enzymatic degradation in the gut. It delivers 35 µg of ethinyl estradiol and 150 µg of norelgestromin (an active metabolite of norgestimate) daily.38 It may cause higher circulating levels of estrogen than a combined oral contraceptive and may be associated with a higher risk of venous thromboembolism, but the evidence is conflicting.39–42
The vaginal ring, made of Silastic, delivers ethinyl estradiol in a low dose (15 µg/day) and etonorgestrel 0.12 mg/day. Like the patch, it has the advantage of bypassing first-pass metabolism in the liver, making it a good option for transplant patients who are taking antirejection drugs, thus avoiding drug interactions.41
Both the transdermal patch and vaginal ring were studied in transplant patients and had favorable results.24,43 The combined hormonal oral contraceptive pills, patch, and ring are in category 4 (unacceptable health risk) in the US MEC in patients with complicated cases, but they are in category 2 in uncomplicated cases.21
Combined hormonal contraceptives should not be considered first-line options by themselves for transplant patients because of their high failure rate with typical use.24
Progestin-only pills
Although progestin-only pills have not been studied specifically in transplant patients, they can be considered for women who have contraindications to estrogen use. Estrogen use is contraindicated in women with a history of venous thromboembolism, thrombogenic mutations, estrogen-dependent neoplasia, hepatocellular adenoma, severe hypertension, vascular disease, and Budd-Chiari syndrome.
Progestin-only pills inhibit ovulation in only about half of a woman’s cycles, but they prevent conception by other mechanisms as well, such as causing thickening of the cervical mucus. They also alter the endometrium to make it unfavorable for implantation and reduce the ciliary activity of the fallopian tube.
Strict adherence is important for effectiveness because progestin-only pills have a shorter half-life than combined hormonal contraceptives and also suppress ovulation less effectively.22 Failure rates are similar or somewhat higher than with combined hormonal contraceptives; with typical use, about 9 in 100 women can become pregnant in the first year.23 According to the US MEC,21 progestin-only pills are classified as category 2 for patients after both complicated and uncomplicated transplants.
MODERATELY EFFECTIVE METHODS (PREGNANCY RATE 10%–25%)
This tier of contraceptives includes all barrier methods, ie, male and female condoms, vaginal diaphragms, cervical caps, and sponges (Table 5).
Condoms (male and female)
When male condoms are used as the only birth control method, pregnancy occurs less often (18% with typical use and 2% with correct use) than with female condoms (21% with typical use and 5% with correct use).23 Male and female condoms are the only contraceptive methods that also prevent transmission of sexually transmitted disease.24
Caps, sponges, diaphragms
Cervical caps, vaginal sponges, and vaginal diaphragms are other forms of barrier contraceptives. All barrier methods should be combined with another contraceptive method to provide reliable protection against pregnancy. These methods are considered category 1 according to the US MEC.
LESS-EFFECTIVE METHODS
Fertility awareness-based methods such as the rhythm method have an associated pregnancy rate of about 25% with typical use and 3% to 5% with correct use23 and cannot be relied on for use by transplant recipients.24
Withdrawal and spermicides are considered least effective and unreliable for pregnancy prevention.
KNOW YOUR OPTIONS
With the growing number of women in their reproductive years receiving solid-organ transplants in the United States, it is increasingly important for healthcare providers to be aware of contraceptive options and reproductive life planning for this high-risk population.
Safe and effective forms of contraception are available, and additional information to guide the choice can be found in the Summary Chart of US MEC for Contraceptive Use, which is also available in a free smart phone app through the CDC.44
Pregnancy after transplant carries high risks, requiring these patients to have special counseling and monitoring. Fortunately, planned pregnancy at least 1 year after transplant can lead to successful outcomes in these women.
- McKay DB, Josephson MA. Pregnancy in recipients of solid organs: effects on mother and child. N Engl J Med 2006; 354:1281–1293.
- US Department of Health and Human Services. Organ procurement and transplantation network. https://optn.transplant.hrsa.gov/. Accessed July 17, 2017.
- United States Renal Data System. 2014 annual data report. https://www.usrds.org/2014/view/Default.aspx. Accessed July 17, 2017.
- Hou S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis 1999; 33:235–252.
- Josephson MA, McKay DB. Women and transplantation: fertility, sexuality, pregnancy, contraception. Adv Chronic Kidney Dis 2013; 20:433–440.
- Gill JS, Zalunardo N, Rose C, Tonelli M. The pregnancy rate and live birth rate in kidney transplant recipients. Am J Transplant 2009; 9:1541–1549.
- Mohapatra A, Basu G. Pregnancy in kidney disease. Health Sciences 2012; 1(2). http://healthsciences.ac.in/july-sep-12/downloads/pregnancy_in_kidney_disease.pdf. Accessed July 25, 2017.
- Potluri K, Moldenhauer J, Karlman R, Hou S. Beta HCG levels in a pregnant dialysis patient: a cautionary tale. NDT Plus 2011; 4:42–43.
- Kennedy C, Hussein W, Spencer S, et al. Reproductive health in Irish female renal transplant recipients. Ir J Med Sci 2012; 181:59–63.
- Ghazizadeh S, Lessan-Pezeshki M, Khatami M, et al. Unwanted pregnancy among kidney transplant recipients in Iran. Transplant Proc 2005; 37:3085–3086.
- Jabiry-Zieniewicz Z, Bobrowska K, Kaminski P, Wielgos M, Zieniewicz K, Krawczyk M. Low-dose hormonal contraception after liver transplantation. Transplant Proc 2007; 39:1530–1532.
- Coscia LA, Constantinescu S, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl 2010: 24:65–85.
- Mohamed-Ahmed O, Nelson-Piercy C, Bramham K, et al. Pregnancy outcomes in liver and cardiothoracic transplant recipients: a UK national cohort study. PLoS One 2014; 9:e89151.
- Enderby C, Keller CA. An overview of immunosuppression in solid organ transplantation. Am J Manag Care 2015; 21(suppl 1):s12–s23.
- Hoeltzenbein M, Elefant E, Vial T, et al. Teratogenicity of mycophenolate confirmed in a prospective study of the European Network of Teratology Information Services. Am J Med Genet A 2012; 158A:588–596.
- Polifka JE, Friedman JM. Teratogen update: azathioprine and 6-mercaptopurine. Teratology 2002; 65:240–261.
- Dinatale M. The pregnancy and lactation labeling rule (PLLR). US Food and Drug Administration, 2016. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM520454.pdf. Accessed July 25, 2017.
- Lexicomp. http://online.lexi.com/lco/action/api/find/globalid/6612?utd=1. Accessed July 27, 2017.
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009; 9(suppl 3):S1–S155.
- Deshpande NA, Coscia LA, Gomez-Lobo V, Moritz MJ, Armenti VT. Pregnancy after solid organ transplantation: a guide for obstetric management. Rev Obstet Gynecol 2013; 6:116–125.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016; 65:1–103.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 121: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2011; 118:184–196.
- Trussell J. Contraceptive failure in the United States. Contraception 2011; 83:397–404.
- Krajewski CM, Geetha D, Gomez-Lobo V. Contraceptive options for women with a history of solid-organ transplantation. Transplantation 2013; 95:1183–1186.
- Stern LF, Simons HR, Kohn JE, Debevec EJ, Morfesis JM, Patel AA. Differences in contraceptive use between family planning providers and the U.S. population: results of a nationwide survey. Contraception 2015; 91:464–469.
- Rafie S, Lai S, Garcia JE, Mody SK. Contraceptive use in female recipients of a solid-organ transplant. Prog Transplant 2014; 24:344–348.
- Labied S, Galant C, Nisolle M, et al. Differential elevation of matrix metalloproteinase expression in women exposed to levonorgestrel-releasing intrauterine system for a short or prolonged period of time. Hum Reprod 2009; 24:113–121.
- Kim CR, Martinez-Maza O, Magpantay L, et al. Immunologic evaluation of the endometrium with a levonorgestrel intrauterine device in solid organ transplant women and healthy controls. Contraception 2016; 94:534–540.
- Ramhendar T, Byrne P. Use of the levonorgestrel-releasing intrauterine system in renal transplant recipients: a retrospective case review. Contraception 2012; 86:288–289.
- Huguelet PS, Sheehan C, Spitzer RF, Scott S. Use of the levonorgestrel 52-mg intrauterine system in adolescent and young adult solid organ transplant recipients: a case series. Contraception 2017; 95:378–381.
- Peterson HB, Xia Z, Hughes JM, Wilcox LS, Tylor LR, Trussell J. The risk of pregnancy after tubal sterilization: findings from the US Collaborative Review of Sterilization. Am J Obstet Gynecol 1996; 174:1161–1168.
- Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 2007; 18:1319–1328.
- Krajewski C, Sucato G. Reproductive health care after transplantation. Best Pract Res Clin Obstet Gynaecol 2014; 28:1222–1234.
- World Health Organization. Medical eligibility criteria for contraceptive use. Fifth edition 2015. http://apps.who.int/iris/bitstream/10665/172915/1/WHO_RHR_15.07_eng.pdf. Accessed July 27, 2017.
- Pietrzak B, Bobrowska K, Jabiry-Zieniewicz Z, et al. Oral and transdermal hormonal contraception in women after kidney transplantation. Transplant Proc 2007; 39:2759–2762.
- Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA-SC. Contraception 2004; 70:269–275.
- Vessey M, Painter R, Yeates D. Mortality in relation to oral contraceptive use and cigarette smoking. Lancet 2003; 362:185–191.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, Kaptein MC. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception 2005; 72:168–174.
- Jick SS, Kaye JA, Russmann S, Jick H. Risk of nonfatal venous thromboembolism in women using a contraceptive transdermal patch and oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2006; 73:223–228.
- Jick S, Kaye JA, Li L, Jick H. Further results on the risk of nonfatal venous thromboembolism in users of the contraceptive transdermal patch compared to users of oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2007; 76:4–7.
- Estes CM, Westhoff C. Contraception for the transplant patient. Semin Perinatol 2007; 31:372–377.
- Cole JA, Norman H, Doherty M, Walker AM. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol 2007; 109:339–346.
- Paternoster DM, Riboni F, Bertolino M, et al. The contraceptive vaginal ring in women with renal and liver transplantation: analysis of preliminary results. Transplant Proc 2010; 42:1162–1165.
- Centers for Disease Control and Prevention (CDC). Summary chart of US medical eligibility criteria for contraceptive use. https://www.cdc.gov/reproductivehealth/unintendedpregnancy/pdf/legal_summary-chart_english_final_tag508.pdf. Accessed July 17, 2017.
Increasing numbers of women of childbearing age are receiving solid-organ transplants. All need counseling on how to prevent pregnancy while they are taking immunosuppressive agents. Some want to become pregnant after their transplant and thus require counseling and follow-up to maintain good health during pregnancy (Table 1).1
Primary care physicians can assist with basic contraception counseling and pregnancy planning for their patients who have had solid-organ transplants. In this review, we describe contraceptive options and pregnancy planning for these women.
TRANSPLANTS IN WOMEN ARE INCREASING
Over the past 20 years, the number of solid-organ transplants in US women has increased steadily. Since 1988, 38% of the 634,000 transplants performed were in women, and 47% of these women were of childbearing age (ages 18 to 49).2 Kidneys accounted for 60% of solid-organ transplants,2 and kidney transplant is now commonly performed in women of childbearing age. In 2012, of 176,000 patients with a functioning renal graft, 40.5% were women, and recipients between ages 20 and 44 composed the second-largest age group.3
FERTILITY IN WOMEN WITH END-STAGE RENAL DISEASE
Women in their reproductive years who have end-stage renal disease have lower fertility rates than women in the general population. In women undergoing peritoneal dialysis or hemodialysis, conception rates decrease to around 0.5% per year.4 This lower rate is most likely related to hypothalamic-pituitary-gonadal dysfunction, leading to reduced or total impairment of ovulation, menstrual irregularities, and infertility.5
Fertility often returns within a few months after transplant,1,6 and reported posttransplant pregnancy rates range from 3.3% to 18%,7–9 with up to one-third of pregnancies being unintended.6,10 These numbers are likely an underestimate because they do not reflect all pregnancies that are terminated, as many women do not voluntarily report having had an abortion.
Fertility is also severely diminished in women with end-stage liver disease. After liver transplant, sex hormone levels return to normal for many women, and menses soon resume.11
In 2005, the National Transplantation Pregnancy Registry reported 1,418 pregnancies in 919 female recipients of solid-organ transplants. In 2010, this number had increased to 1,940 pregnancies in 1,185 recipients, of whom 75% were kidney transplant recipients.12
A successful pregnancy outcome is most likely when a minimum of 1 year intervenes between transplant and conception.12,13
TERATOGENICITY OF IMMUNOSUPPRESSANTS
Immunosuppressant drugs commonly used for maintenance therapy after solid-organ transplant include the following:
- Calcineurin inhibitors (eg, cyclosporine, tacrolimus)
- Antiproliferative and antimetabolite agents (eg, mycophenolate mofetil, azathioprine)
- Corticosteroids
- Mammalian target of rapamycin inhibitors (eg, sirolimus, everolimus)
- T-cell costimulation blockers (eg, belatacept).14
The US Food and Drug Administration (FDA) previously classified mycophenolate mofetil and azathioprine in pregnancy risk category D (positive evidence of human fetal risk). The teratogenic risk of mycophenolate mofetil is well established in studies documenting specific congenital malformations and fetal loss in the first trimester.13,15 The teratogenic risk of azathioprine, on the other hand, is estimated to be minimal to small.16 Many of the associated fetal abnormalities may be related to the complexity of the underlying medical condition of the mother rather than to the medication.16
In June 2015, the FDA’s new Pregnancy and Lactation Labeling Rule went into effect, which removes the pregnancy letter categories A, B, C, D, and X from labeling.17 This rule was designed to help providers counsel their patients regarding the specific risks and benefits of a drug when used by pregnant or nursing women. However, the ABCDX categories are still commonly used. Table 2 shows information about the risks during pregnancy and lactation posed by the immunosuppressive drugs commonly used by posttransplant patients.18
CRITERIA FOR A SUCCESSFUL PREGNANCY
To ensure a safe and successful pregnancy with the fewest fetal and maternal complications, women are generally advised to avoid pregnancy for at least 1 year after transplant.19,20
In addition, women should meet certain clinical prerequisites after transplant before they conceive, as outlined by the American Society of Transplantation.19,20 These include:
- No rejection within the previous year
- Adequate and stable graft function (eg, serum creatinine < 1.5 mg/dL and urinary protein excretion < 500 mg/24 hours)
- No acute infection that might affect the fetus
- Maintenance immunosuppression at stable dosages.
Other circumstances to consider include episodes of rejection in the first year after transplant (as evidenced by biopsy results or glomerular filtration rate), the woman’s age (advanced maternal age is unfavorable), or any history of noncompliance.
Every pregnancy in a transplant recipient must be carefully planned. Primary care providers should encourage patients to meet with their transplant team and obstetricians early and often to allow time for the care team to adjust the type and dosing of immunosuppressant drugs, to ensure stable graft function, and to optimize any current chronic medical conditions such as diabetes mellitus or hypertension before conception.
CONTRACEPTIVE COUNSELING AFTER TRANSPLANT
Pregnancy should be avoided while transplant patients are taking FDA category D immunosuppressant drugs and, as already mentioned, during the first year after transplant. Unintended pregnancy can have serious health consequences for the mother and the fetus, as well as poor pregnancy outcomes. The US Centers for Disease Control and Prevention (CDC) lists solid-organ transplant within the past 2 years as a condition that can lead to adverse events as a result of pregnancy.21 After a transplant, a woman’s risks from an unintended pregnancy are always greater than the risks from any contraceptive, and this is important to reinforce in counseling.
Two forms of reliable contraception should be used at all times, and consistent condom use should be encouraged as one of the methods. Condoms are not reliable when used as the sole contraceptive method because they have an 18% typical-use failure rate. However, they are an excellent adjunct to other contraceptive methods because they have the additional benefit of protecting against sexually transmitted disease.
Choosing the appropriate contraceptive method for recipients of solid-organ transplants can be challenging because of several factors, including the recipient’s preexisting medical problems and drug interactions of immunosuppressant medications.
CDC criteria and categories for contraceptive use
In 2010, the CDC released the US version of the Medical Eligibility Criteria (US MEC) for contraceptive use, which was based on the 2009 World Health Organization Medical Eligibility Criteria (WHO MEC); these criteria were revised in August 2016.21
- Category 1: A condition for which there is no restriction for the use of the contraceptive method
- Category 2: A condition for which the advantages of using the method generally outweigh the theoretical or proven risks
- Category 3: A condition for which the theoretical or proven risks usually outweigh the advantages of using the method
- Category 4: A condition that represents an unacceptable health risk if the contraceptive method is used.
These recommendations aimed to improve family planning options by clarifying the possible safe and effective contraceptive options available while considering the patient’s medical condition. The CDC added solid-organ transplant recipients to this document because of the prevalence of this group in the United States.
The CDC categorizes a patient’s medical condition after transplant as either complicated or uncomplicated. Complicated conditions include acute or chronic graft failure, graft rejection, and cardiac allograft vasculopathy.21
Effectiveness of contraceptive methods
Contraceptive methods can be divided into 4 categories based on estimated effectiveness, ie, the pregnancy rate with “typical use” of that particular method in 1 year21–23:
- Very effective (0%–0.9%)
- Effective (1%–9%)
- Moderately effective (10%–25%)
- Less effective (26%–32%).
Typical use refers to failure rates for women and men whose use is not consistent nor always correct. Correct use, also described in the sections that follow, refers to failure rates for those whose use is consistent and always correct.
Women should be counseled regarding all available contraceptive options that are medically suitable for them, so they can choose the method that best fits their needs and lifestyle. They should receive counseling on emergency contraception, barrier protection against sexually transmitted disease, and the correct use of the contraceptive method they choose. They should be advised that if their chosen contraceptive method is unsatisfactory for any reason, they can switch to another method. Most importantly, providers need to impress on their patients that the risks associated with unintended pregnancy are far greater than the risks from any of the contraceptive methods.
VERY EFFECTIVE CONTRACEPTIVES (UNINTENDED PREGNANCY RATE 0%–0.9%)
This tier of contraception is the most effective regardless of the patient’s adherence; it includes long-acting, reversible contraceptives and permanent sterilization (both male and female) (Table 3).21–23
Long-acting reversible contraceptives include intrauterine devices (IUDs) and the subdermal etonogestrel implant. Given their efficacy and favorable safety profile, long-acting reversible contraceptives are being promoted for use in women who have chronic medical conditions, such as transplants.24
Intrauterine devices
IUDs are long-acting and reversible. They can be used by women who are nulliparous and those of all ages, including adolescents.22
Two types of IUDs are available in the United States: nonhormonal (copper) and hormonal (levonorgestrel). The copper IUD is effective for at least 10 years, whereas the levonorgestrel IUDs last for 3 to 5 years.22
Four levonorgestrel IUDs are currently available in the United States. Their sizes and doses vary: Mirena (52 µg), Skyla (13.5 µg), Liletta (52 µg), and Kyleena (19.5 µg).
Fewer than 1% of women become pregnant in the first year of IUD use.22,23 IUDs are an ideal option for women with solid-organ transplants because they are so effective and because the patient does not have to do anything once the IUD is in.22–24 The levonorgestrel IUD Mirena has the additional advantage of reducing heavy menstrual bleeding and is currently the only hormonal IUD with FDA approval for the management of menorrhagia.
About 12% of women in the general population use IUDs as their contraceptive method of choice,25 whereas after solid-organ transplantation about 15% to 20% of women do.26
Two historic concerns regarding IUDs may explain their low rate of use in transplant recipients.
First, IUDs were believed to be less effective in women on immunosuppressive drugs because IUDs act by inducing a local inflammatory reaction. However, IUDs involve macrophage activation, which is independent of the immune processes modified by immunosuppressants (primarily T-cell function).27 A recent pilot study showed a strong inflammatory reaction in the endometrium of transplant recipients after levonorgestrel IUD insertion.28
Second, there was concern about the increased risk of pelvic inflammatory disease with IUDs, but studies have shown levonorgestrel IUDs to be safe in transplant patients.29,30
The CDC21 lists copper and levonorgestrel IUDs in MEC category 3 (the risks generally outweigh the advantages) for initiation in patients with complicated transplants and in category 2 (advantages generally outweigh the risks) in patients with uncomplicated organ transplants. The devices are in category 2 for both complicated and uncomplicated cases if the IUD is already in place.
Subdermal implant
A subdermal implant consisting of a single rod containing 68 mg of etonogestrel is commercially available in the United States. It is one of the most effective contraceptive methods, with the lowest rates of pregnancy—less than 1% per year, with protection lasting at least 3 years.22,23 This low risk makes the subdermal implant a suitable method of contraception after transplant. Daily compliance is not required, and there are no hepatic first-pass effects, which results in higher bioavailability and less chance of drug interactions.
The main disadvantage of the subdermal implant and IUDs is unscheduled bleeding. An important benefit is prolonged amenorrhea, not only for patient convenience, but for reduction of endometrial cancer risk. Insertion and removal of the implant are considered minor office procedures. The implants are classified as US MEC category 2 in uncomplicated cases; initiation in complicated cases is considered category 3 but continuation is considered category 2.21
Permanent sterilization
Permanent sterilization is another option for women and men. In women, the fallopian tubes can be occluded with a coil system implanted vaginally through a hysteroscope, or they can be severed, tied, or clamped in a laparoscopic procedure or during cesarean delivery. Pregnancy rates after tubal ligation are less than 1%,23,31 although concern exists for high failure rates with the hysteroscopic method.
Because younger patients are more likely than older patients to subsequently regret having the procedure done, all available contraceptive options should be discussed with them.31
For men, permanent sterilization is done by vasectomy, which has less associated risk and cost compared with sterilization for women.
EFFECTIVE CONTRACEPTIVE METHODS (UNINTENDED PREGNANCY RATE 1%–9%)
Effective contraceptive methods, the next tier down from very effective methods, include injectable contraceptives, combined hormonal contraceptives, and progestin-only contraceptives (Table 4).
Injectable contraceptives
Depot medroxyprogesterone acetate is an injectable progestin-only contraceptive that carries a pregnancy risk of 6% with typical use and less than 1% with correct use.23 Thus, some failures are due to patients not returning for follow-up, but in some patients this method is not effective. Injections are given intramuscularly once every 3 months, avoiding the need for daily use.
A valid concern for transplant patients is that medroxyprogesterone acetate reduces bone mineral density. Although the bone effects are reversible in healthy adult women, caution is needed when prescribing this option to transplant patients who are already at increased risk of bone disease attributable to renal osteodystrophy and chronic corticosteroid use. 32,33
Recently, a subcutaneous formulation of depot medroxyprogesterone acetate (104 mg)was added to the WHO MEC for contraceptive use.34,35 The recommendations for the subcutaneous form are similar to those for the intramuscular form. In healthy women, the subcutaneous formulation is as safe and effective as the intramuscular form,36 but its efficacy after solid-organ transplant has not been determined. Both forms of depot medroxyprogesterone acetate are category 2 in the US MEC for both complicated and uncomplicated transplant cases.21
Combined hormonal contraceptives
Combined hormonal contraceptives contain both estrogen and progesterone and are available as pills, patches, or rings. Each product has an unintended pregnancy risk of 9% with typical use and less than 1% with correct use.23 They require strict patient adherence to regular daily use, which likely explains their high failure rate with typical use.
Combined hormonal contraceptives reduce mortality risk in women in the general population,37 but their effect on mortality risk after transplant is unknown and needs further study. In women who received liver transplants, low-dose combined hormonal contraceptives have been found to be effective and well tolerated, but initiation should be delayed at least 6 months until postoperative organ stability is demonstrated.11
Combined oral contraceptives are the most widely prescribed because they are convenient and familiar and have an acceptable safety profile in transplant patients,11,33,37 despite their high failure rate with typical use. They regulate the menstrual cycle and reduce anemia associated with menstruation.
The transdermal contraceptive patch has a mechanism of action similar to that of the combined oral contraceptives, but it delivers estrogen and progesterone transdermally through the abdominal wall, thus avoiding first-pass metabolism in the liver and enzymatic degradation in the gut. It delivers 35 µg of ethinyl estradiol and 150 µg of norelgestromin (an active metabolite of norgestimate) daily.38 It may cause higher circulating levels of estrogen than a combined oral contraceptive and may be associated with a higher risk of venous thromboembolism, but the evidence is conflicting.39–42
The vaginal ring, made of Silastic, delivers ethinyl estradiol in a low dose (15 µg/day) and etonorgestrel 0.12 mg/day. Like the patch, it has the advantage of bypassing first-pass metabolism in the liver, making it a good option for transplant patients who are taking antirejection drugs, thus avoiding drug interactions.41
Both the transdermal patch and vaginal ring were studied in transplant patients and had favorable results.24,43 The combined hormonal oral contraceptive pills, patch, and ring are in category 4 (unacceptable health risk) in the US MEC in patients with complicated cases, but they are in category 2 in uncomplicated cases.21
Combined hormonal contraceptives should not be considered first-line options by themselves for transplant patients because of their high failure rate with typical use.24
Progestin-only pills
Although progestin-only pills have not been studied specifically in transplant patients, they can be considered for women who have contraindications to estrogen use. Estrogen use is contraindicated in women with a history of venous thromboembolism, thrombogenic mutations, estrogen-dependent neoplasia, hepatocellular adenoma, severe hypertension, vascular disease, and Budd-Chiari syndrome.
Progestin-only pills inhibit ovulation in only about half of a woman’s cycles, but they prevent conception by other mechanisms as well, such as causing thickening of the cervical mucus. They also alter the endometrium to make it unfavorable for implantation and reduce the ciliary activity of the fallopian tube.
Strict adherence is important for effectiveness because progestin-only pills have a shorter half-life than combined hormonal contraceptives and also suppress ovulation less effectively.22 Failure rates are similar or somewhat higher than with combined hormonal contraceptives; with typical use, about 9 in 100 women can become pregnant in the first year.23 According to the US MEC,21 progestin-only pills are classified as category 2 for patients after both complicated and uncomplicated transplants.
MODERATELY EFFECTIVE METHODS (PREGNANCY RATE 10%–25%)
This tier of contraceptives includes all barrier methods, ie, male and female condoms, vaginal diaphragms, cervical caps, and sponges (Table 5).
Condoms (male and female)
When male condoms are used as the only birth control method, pregnancy occurs less often (18% with typical use and 2% with correct use) than with female condoms (21% with typical use and 5% with correct use).23 Male and female condoms are the only contraceptive methods that also prevent transmission of sexually transmitted disease.24
Caps, sponges, diaphragms
Cervical caps, vaginal sponges, and vaginal diaphragms are other forms of barrier contraceptives. All barrier methods should be combined with another contraceptive method to provide reliable protection against pregnancy. These methods are considered category 1 according to the US MEC.
LESS-EFFECTIVE METHODS
Fertility awareness-based methods such as the rhythm method have an associated pregnancy rate of about 25% with typical use and 3% to 5% with correct use23 and cannot be relied on for use by transplant recipients.24
Withdrawal and spermicides are considered least effective and unreliable for pregnancy prevention.
KNOW YOUR OPTIONS
With the growing number of women in their reproductive years receiving solid-organ transplants in the United States, it is increasingly important for healthcare providers to be aware of contraceptive options and reproductive life planning for this high-risk population.
Safe and effective forms of contraception are available, and additional information to guide the choice can be found in the Summary Chart of US MEC for Contraceptive Use, which is also available in a free smart phone app through the CDC.44
Pregnancy after transplant carries high risks, requiring these patients to have special counseling and monitoring. Fortunately, planned pregnancy at least 1 year after transplant can lead to successful outcomes in these women.
Increasing numbers of women of childbearing age are receiving solid-organ transplants. All need counseling on how to prevent pregnancy while they are taking immunosuppressive agents. Some want to become pregnant after their transplant and thus require counseling and follow-up to maintain good health during pregnancy (Table 1).1
Primary care physicians can assist with basic contraception counseling and pregnancy planning for their patients who have had solid-organ transplants. In this review, we describe contraceptive options and pregnancy planning for these women.
TRANSPLANTS IN WOMEN ARE INCREASING
Over the past 20 years, the number of solid-organ transplants in US women has increased steadily. Since 1988, 38% of the 634,000 transplants performed were in women, and 47% of these women were of childbearing age (ages 18 to 49).2 Kidneys accounted for 60% of solid-organ transplants,2 and kidney transplant is now commonly performed in women of childbearing age. In 2012, of 176,000 patients with a functioning renal graft, 40.5% were women, and recipients between ages 20 and 44 composed the second-largest age group.3
FERTILITY IN WOMEN WITH END-STAGE RENAL DISEASE
Women in their reproductive years who have end-stage renal disease have lower fertility rates than women in the general population. In women undergoing peritoneal dialysis or hemodialysis, conception rates decrease to around 0.5% per year.4 This lower rate is most likely related to hypothalamic-pituitary-gonadal dysfunction, leading to reduced or total impairment of ovulation, menstrual irregularities, and infertility.5
Fertility often returns within a few months after transplant,1,6 and reported posttransplant pregnancy rates range from 3.3% to 18%,7–9 with up to one-third of pregnancies being unintended.6,10 These numbers are likely an underestimate because they do not reflect all pregnancies that are terminated, as many women do not voluntarily report having had an abortion.
Fertility is also severely diminished in women with end-stage liver disease. After liver transplant, sex hormone levels return to normal for many women, and menses soon resume.11
In 2005, the National Transplantation Pregnancy Registry reported 1,418 pregnancies in 919 female recipients of solid-organ transplants. In 2010, this number had increased to 1,940 pregnancies in 1,185 recipients, of whom 75% were kidney transplant recipients.12
A successful pregnancy outcome is most likely when a minimum of 1 year intervenes between transplant and conception.12,13
TERATOGENICITY OF IMMUNOSUPPRESSANTS
Immunosuppressant drugs commonly used for maintenance therapy after solid-organ transplant include the following:
- Calcineurin inhibitors (eg, cyclosporine, tacrolimus)
- Antiproliferative and antimetabolite agents (eg, mycophenolate mofetil, azathioprine)
- Corticosteroids
- Mammalian target of rapamycin inhibitors (eg, sirolimus, everolimus)
- T-cell costimulation blockers (eg, belatacept).14
The US Food and Drug Administration (FDA) previously classified mycophenolate mofetil and azathioprine in pregnancy risk category D (positive evidence of human fetal risk). The teratogenic risk of mycophenolate mofetil is well established in studies documenting specific congenital malformations and fetal loss in the first trimester.13,15 The teratogenic risk of azathioprine, on the other hand, is estimated to be minimal to small.16 Many of the associated fetal abnormalities may be related to the complexity of the underlying medical condition of the mother rather than to the medication.16
In June 2015, the FDA’s new Pregnancy and Lactation Labeling Rule went into effect, which removes the pregnancy letter categories A, B, C, D, and X from labeling.17 This rule was designed to help providers counsel their patients regarding the specific risks and benefits of a drug when used by pregnant or nursing women. However, the ABCDX categories are still commonly used. Table 2 shows information about the risks during pregnancy and lactation posed by the immunosuppressive drugs commonly used by posttransplant patients.18
CRITERIA FOR A SUCCESSFUL PREGNANCY
To ensure a safe and successful pregnancy with the fewest fetal and maternal complications, women are generally advised to avoid pregnancy for at least 1 year after transplant.19,20
In addition, women should meet certain clinical prerequisites after transplant before they conceive, as outlined by the American Society of Transplantation.19,20 These include:
- No rejection within the previous year
- Adequate and stable graft function (eg, serum creatinine < 1.5 mg/dL and urinary protein excretion < 500 mg/24 hours)
- No acute infection that might affect the fetus
- Maintenance immunosuppression at stable dosages.
Other circumstances to consider include episodes of rejection in the first year after transplant (as evidenced by biopsy results or glomerular filtration rate), the woman’s age (advanced maternal age is unfavorable), or any history of noncompliance.
Every pregnancy in a transplant recipient must be carefully planned. Primary care providers should encourage patients to meet with their transplant team and obstetricians early and often to allow time for the care team to adjust the type and dosing of immunosuppressant drugs, to ensure stable graft function, and to optimize any current chronic medical conditions such as diabetes mellitus or hypertension before conception.
CONTRACEPTIVE COUNSELING AFTER TRANSPLANT
Pregnancy should be avoided while transplant patients are taking FDA category D immunosuppressant drugs and, as already mentioned, during the first year after transplant. Unintended pregnancy can have serious health consequences for the mother and the fetus, as well as poor pregnancy outcomes. The US Centers for Disease Control and Prevention (CDC) lists solid-organ transplant within the past 2 years as a condition that can lead to adverse events as a result of pregnancy.21 After a transplant, a woman’s risks from an unintended pregnancy are always greater than the risks from any contraceptive, and this is important to reinforce in counseling.
Two forms of reliable contraception should be used at all times, and consistent condom use should be encouraged as one of the methods. Condoms are not reliable when used as the sole contraceptive method because they have an 18% typical-use failure rate. However, they are an excellent adjunct to other contraceptive methods because they have the additional benefit of protecting against sexually transmitted disease.
Choosing the appropriate contraceptive method for recipients of solid-organ transplants can be challenging because of several factors, including the recipient’s preexisting medical problems and drug interactions of immunosuppressant medications.
CDC criteria and categories for contraceptive use
In 2010, the CDC released the US version of the Medical Eligibility Criteria (US MEC) for contraceptive use, which was based on the 2009 World Health Organization Medical Eligibility Criteria (WHO MEC); these criteria were revised in August 2016.21
- Category 1: A condition for which there is no restriction for the use of the contraceptive method
- Category 2: A condition for which the advantages of using the method generally outweigh the theoretical or proven risks
- Category 3: A condition for which the theoretical or proven risks usually outweigh the advantages of using the method
- Category 4: A condition that represents an unacceptable health risk if the contraceptive method is used.
These recommendations aimed to improve family planning options by clarifying the possible safe and effective contraceptive options available while considering the patient’s medical condition. The CDC added solid-organ transplant recipients to this document because of the prevalence of this group in the United States.
The CDC categorizes a patient’s medical condition after transplant as either complicated or uncomplicated. Complicated conditions include acute or chronic graft failure, graft rejection, and cardiac allograft vasculopathy.21
Effectiveness of contraceptive methods
Contraceptive methods can be divided into 4 categories based on estimated effectiveness, ie, the pregnancy rate with “typical use” of that particular method in 1 year21–23:
- Very effective (0%–0.9%)
- Effective (1%–9%)
- Moderately effective (10%–25%)
- Less effective (26%–32%).
Typical use refers to failure rates for women and men whose use is not consistent nor always correct. Correct use, also described in the sections that follow, refers to failure rates for those whose use is consistent and always correct.
Women should be counseled regarding all available contraceptive options that are medically suitable for them, so they can choose the method that best fits their needs and lifestyle. They should receive counseling on emergency contraception, barrier protection against sexually transmitted disease, and the correct use of the contraceptive method they choose. They should be advised that if their chosen contraceptive method is unsatisfactory for any reason, they can switch to another method. Most importantly, providers need to impress on their patients that the risks associated with unintended pregnancy are far greater than the risks from any of the contraceptive methods.
VERY EFFECTIVE CONTRACEPTIVES (UNINTENDED PREGNANCY RATE 0%–0.9%)
This tier of contraception is the most effective regardless of the patient’s adherence; it includes long-acting, reversible contraceptives and permanent sterilization (both male and female) (Table 3).21–23
Long-acting reversible contraceptives include intrauterine devices (IUDs) and the subdermal etonogestrel implant. Given their efficacy and favorable safety profile, long-acting reversible contraceptives are being promoted for use in women who have chronic medical conditions, such as transplants.24
Intrauterine devices
IUDs are long-acting and reversible. They can be used by women who are nulliparous and those of all ages, including adolescents.22
Two types of IUDs are available in the United States: nonhormonal (copper) and hormonal (levonorgestrel). The copper IUD is effective for at least 10 years, whereas the levonorgestrel IUDs last for 3 to 5 years.22
Four levonorgestrel IUDs are currently available in the United States. Their sizes and doses vary: Mirena (52 µg), Skyla (13.5 µg), Liletta (52 µg), and Kyleena (19.5 µg).
Fewer than 1% of women become pregnant in the first year of IUD use.22,23 IUDs are an ideal option for women with solid-organ transplants because they are so effective and because the patient does not have to do anything once the IUD is in.22–24 The levonorgestrel IUD Mirena has the additional advantage of reducing heavy menstrual bleeding and is currently the only hormonal IUD with FDA approval for the management of menorrhagia.
About 12% of women in the general population use IUDs as their contraceptive method of choice,25 whereas after solid-organ transplantation about 15% to 20% of women do.26
Two historic concerns regarding IUDs may explain their low rate of use in transplant recipients.
First, IUDs were believed to be less effective in women on immunosuppressive drugs because IUDs act by inducing a local inflammatory reaction. However, IUDs involve macrophage activation, which is independent of the immune processes modified by immunosuppressants (primarily T-cell function).27 A recent pilot study showed a strong inflammatory reaction in the endometrium of transplant recipients after levonorgestrel IUD insertion.28
Second, there was concern about the increased risk of pelvic inflammatory disease with IUDs, but studies have shown levonorgestrel IUDs to be safe in transplant patients.29,30
The CDC21 lists copper and levonorgestrel IUDs in MEC category 3 (the risks generally outweigh the advantages) for initiation in patients with complicated transplants and in category 2 (advantages generally outweigh the risks) in patients with uncomplicated organ transplants. The devices are in category 2 for both complicated and uncomplicated cases if the IUD is already in place.
Subdermal implant
A subdermal implant consisting of a single rod containing 68 mg of etonogestrel is commercially available in the United States. It is one of the most effective contraceptive methods, with the lowest rates of pregnancy—less than 1% per year, with protection lasting at least 3 years.22,23 This low risk makes the subdermal implant a suitable method of contraception after transplant. Daily compliance is not required, and there are no hepatic first-pass effects, which results in higher bioavailability and less chance of drug interactions.
The main disadvantage of the subdermal implant and IUDs is unscheduled bleeding. An important benefit is prolonged amenorrhea, not only for patient convenience, but for reduction of endometrial cancer risk. Insertion and removal of the implant are considered minor office procedures. The implants are classified as US MEC category 2 in uncomplicated cases; initiation in complicated cases is considered category 3 but continuation is considered category 2.21
Permanent sterilization
Permanent sterilization is another option for women and men. In women, the fallopian tubes can be occluded with a coil system implanted vaginally through a hysteroscope, or they can be severed, tied, or clamped in a laparoscopic procedure or during cesarean delivery. Pregnancy rates after tubal ligation are less than 1%,23,31 although concern exists for high failure rates with the hysteroscopic method.
Because younger patients are more likely than older patients to subsequently regret having the procedure done, all available contraceptive options should be discussed with them.31
For men, permanent sterilization is done by vasectomy, which has less associated risk and cost compared with sterilization for women.
EFFECTIVE CONTRACEPTIVE METHODS (UNINTENDED PREGNANCY RATE 1%–9%)
Effective contraceptive methods, the next tier down from very effective methods, include injectable contraceptives, combined hormonal contraceptives, and progestin-only contraceptives (Table 4).
Injectable contraceptives
Depot medroxyprogesterone acetate is an injectable progestin-only contraceptive that carries a pregnancy risk of 6% with typical use and less than 1% with correct use.23 Thus, some failures are due to patients not returning for follow-up, but in some patients this method is not effective. Injections are given intramuscularly once every 3 months, avoiding the need for daily use.
A valid concern for transplant patients is that medroxyprogesterone acetate reduces bone mineral density. Although the bone effects are reversible in healthy adult women, caution is needed when prescribing this option to transplant patients who are already at increased risk of bone disease attributable to renal osteodystrophy and chronic corticosteroid use. 32,33
Recently, a subcutaneous formulation of depot medroxyprogesterone acetate (104 mg)was added to the WHO MEC for contraceptive use.34,35 The recommendations for the subcutaneous form are similar to those for the intramuscular form. In healthy women, the subcutaneous formulation is as safe and effective as the intramuscular form,36 but its efficacy after solid-organ transplant has not been determined. Both forms of depot medroxyprogesterone acetate are category 2 in the US MEC for both complicated and uncomplicated transplant cases.21
Combined hormonal contraceptives
Combined hormonal contraceptives contain both estrogen and progesterone and are available as pills, patches, or rings. Each product has an unintended pregnancy risk of 9% with typical use and less than 1% with correct use.23 They require strict patient adherence to regular daily use, which likely explains their high failure rate with typical use.
Combined hormonal contraceptives reduce mortality risk in women in the general population,37 but their effect on mortality risk after transplant is unknown and needs further study. In women who received liver transplants, low-dose combined hormonal contraceptives have been found to be effective and well tolerated, but initiation should be delayed at least 6 months until postoperative organ stability is demonstrated.11
Combined oral contraceptives are the most widely prescribed because they are convenient and familiar and have an acceptable safety profile in transplant patients,11,33,37 despite their high failure rate with typical use. They regulate the menstrual cycle and reduce anemia associated with menstruation.
The transdermal contraceptive patch has a mechanism of action similar to that of the combined oral contraceptives, but it delivers estrogen and progesterone transdermally through the abdominal wall, thus avoiding first-pass metabolism in the liver and enzymatic degradation in the gut. It delivers 35 µg of ethinyl estradiol and 150 µg of norelgestromin (an active metabolite of norgestimate) daily.38 It may cause higher circulating levels of estrogen than a combined oral contraceptive and may be associated with a higher risk of venous thromboembolism, but the evidence is conflicting.39–42
The vaginal ring, made of Silastic, delivers ethinyl estradiol in a low dose (15 µg/day) and etonorgestrel 0.12 mg/day. Like the patch, it has the advantage of bypassing first-pass metabolism in the liver, making it a good option for transplant patients who are taking antirejection drugs, thus avoiding drug interactions.41
Both the transdermal patch and vaginal ring were studied in transplant patients and had favorable results.24,43 The combined hormonal oral contraceptive pills, patch, and ring are in category 4 (unacceptable health risk) in the US MEC in patients with complicated cases, but they are in category 2 in uncomplicated cases.21
Combined hormonal contraceptives should not be considered first-line options by themselves for transplant patients because of their high failure rate with typical use.24
Progestin-only pills
Although progestin-only pills have not been studied specifically in transplant patients, they can be considered for women who have contraindications to estrogen use. Estrogen use is contraindicated in women with a history of venous thromboembolism, thrombogenic mutations, estrogen-dependent neoplasia, hepatocellular adenoma, severe hypertension, vascular disease, and Budd-Chiari syndrome.
Progestin-only pills inhibit ovulation in only about half of a woman’s cycles, but they prevent conception by other mechanisms as well, such as causing thickening of the cervical mucus. They also alter the endometrium to make it unfavorable for implantation and reduce the ciliary activity of the fallopian tube.
Strict adherence is important for effectiveness because progestin-only pills have a shorter half-life than combined hormonal contraceptives and also suppress ovulation less effectively.22 Failure rates are similar or somewhat higher than with combined hormonal contraceptives; with typical use, about 9 in 100 women can become pregnant in the first year.23 According to the US MEC,21 progestin-only pills are classified as category 2 for patients after both complicated and uncomplicated transplants.
MODERATELY EFFECTIVE METHODS (PREGNANCY RATE 10%–25%)
This tier of contraceptives includes all barrier methods, ie, male and female condoms, vaginal diaphragms, cervical caps, and sponges (Table 5).
Condoms (male and female)
When male condoms are used as the only birth control method, pregnancy occurs less often (18% with typical use and 2% with correct use) than with female condoms (21% with typical use and 5% with correct use).23 Male and female condoms are the only contraceptive methods that also prevent transmission of sexually transmitted disease.24
Caps, sponges, diaphragms
Cervical caps, vaginal sponges, and vaginal diaphragms are other forms of barrier contraceptives. All barrier methods should be combined with another contraceptive method to provide reliable protection against pregnancy. These methods are considered category 1 according to the US MEC.
LESS-EFFECTIVE METHODS
Fertility awareness-based methods such as the rhythm method have an associated pregnancy rate of about 25% with typical use and 3% to 5% with correct use23 and cannot be relied on for use by transplant recipients.24
Withdrawal and spermicides are considered least effective and unreliable for pregnancy prevention.
KNOW YOUR OPTIONS
With the growing number of women in their reproductive years receiving solid-organ transplants in the United States, it is increasingly important for healthcare providers to be aware of contraceptive options and reproductive life planning for this high-risk population.
Safe and effective forms of contraception are available, and additional information to guide the choice can be found in the Summary Chart of US MEC for Contraceptive Use, which is also available in a free smart phone app through the CDC.44
Pregnancy after transplant carries high risks, requiring these patients to have special counseling and monitoring. Fortunately, planned pregnancy at least 1 year after transplant can lead to successful outcomes in these women.
- McKay DB, Josephson MA. Pregnancy in recipients of solid organs: effects on mother and child. N Engl J Med 2006; 354:1281–1293.
- US Department of Health and Human Services. Organ procurement and transplantation network. https://optn.transplant.hrsa.gov/. Accessed July 17, 2017.
- United States Renal Data System. 2014 annual data report. https://www.usrds.org/2014/view/Default.aspx. Accessed July 17, 2017.
- Hou S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis 1999; 33:235–252.
- Josephson MA, McKay DB. Women and transplantation: fertility, sexuality, pregnancy, contraception. Adv Chronic Kidney Dis 2013; 20:433–440.
- Gill JS, Zalunardo N, Rose C, Tonelli M. The pregnancy rate and live birth rate in kidney transplant recipients. Am J Transplant 2009; 9:1541–1549.
- Mohapatra A, Basu G. Pregnancy in kidney disease. Health Sciences 2012; 1(2). http://healthsciences.ac.in/july-sep-12/downloads/pregnancy_in_kidney_disease.pdf. Accessed July 25, 2017.
- Potluri K, Moldenhauer J, Karlman R, Hou S. Beta HCG levels in a pregnant dialysis patient: a cautionary tale. NDT Plus 2011; 4:42–43.
- Kennedy C, Hussein W, Spencer S, et al. Reproductive health in Irish female renal transplant recipients. Ir J Med Sci 2012; 181:59–63.
- Ghazizadeh S, Lessan-Pezeshki M, Khatami M, et al. Unwanted pregnancy among kidney transplant recipients in Iran. Transplant Proc 2005; 37:3085–3086.
- Jabiry-Zieniewicz Z, Bobrowska K, Kaminski P, Wielgos M, Zieniewicz K, Krawczyk M. Low-dose hormonal contraception after liver transplantation. Transplant Proc 2007; 39:1530–1532.
- Coscia LA, Constantinescu S, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl 2010: 24:65–85.
- Mohamed-Ahmed O, Nelson-Piercy C, Bramham K, et al. Pregnancy outcomes in liver and cardiothoracic transplant recipients: a UK national cohort study. PLoS One 2014; 9:e89151.
- Enderby C, Keller CA. An overview of immunosuppression in solid organ transplantation. Am J Manag Care 2015; 21(suppl 1):s12–s23.
- Hoeltzenbein M, Elefant E, Vial T, et al. Teratogenicity of mycophenolate confirmed in a prospective study of the European Network of Teratology Information Services. Am J Med Genet A 2012; 158A:588–596.
- Polifka JE, Friedman JM. Teratogen update: azathioprine and 6-mercaptopurine. Teratology 2002; 65:240–261.
- Dinatale M. The pregnancy and lactation labeling rule (PLLR). US Food and Drug Administration, 2016. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM520454.pdf. Accessed July 25, 2017.
- Lexicomp. http://online.lexi.com/lco/action/api/find/globalid/6612?utd=1. Accessed July 27, 2017.
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009; 9(suppl 3):S1–S155.
- Deshpande NA, Coscia LA, Gomez-Lobo V, Moritz MJ, Armenti VT. Pregnancy after solid organ transplantation: a guide for obstetric management. Rev Obstet Gynecol 2013; 6:116–125.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016; 65:1–103.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 121: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2011; 118:184–196.
- Trussell J. Contraceptive failure in the United States. Contraception 2011; 83:397–404.
- Krajewski CM, Geetha D, Gomez-Lobo V. Contraceptive options for women with a history of solid-organ transplantation. Transplantation 2013; 95:1183–1186.
- Stern LF, Simons HR, Kohn JE, Debevec EJ, Morfesis JM, Patel AA. Differences in contraceptive use between family planning providers and the U.S. population: results of a nationwide survey. Contraception 2015; 91:464–469.
- Rafie S, Lai S, Garcia JE, Mody SK. Contraceptive use in female recipients of a solid-organ transplant. Prog Transplant 2014; 24:344–348.
- Labied S, Galant C, Nisolle M, et al. Differential elevation of matrix metalloproteinase expression in women exposed to levonorgestrel-releasing intrauterine system for a short or prolonged period of time. Hum Reprod 2009; 24:113–121.
- Kim CR, Martinez-Maza O, Magpantay L, et al. Immunologic evaluation of the endometrium with a levonorgestrel intrauterine device in solid organ transplant women and healthy controls. Contraception 2016; 94:534–540.
- Ramhendar T, Byrne P. Use of the levonorgestrel-releasing intrauterine system in renal transplant recipients: a retrospective case review. Contraception 2012; 86:288–289.
- Huguelet PS, Sheehan C, Spitzer RF, Scott S. Use of the levonorgestrel 52-mg intrauterine system in adolescent and young adult solid organ transplant recipients: a case series. Contraception 2017; 95:378–381.
- Peterson HB, Xia Z, Hughes JM, Wilcox LS, Tylor LR, Trussell J. The risk of pregnancy after tubal sterilization: findings from the US Collaborative Review of Sterilization. Am J Obstet Gynecol 1996; 174:1161–1168.
- Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 2007; 18:1319–1328.
- Krajewski C, Sucato G. Reproductive health care after transplantation. Best Pract Res Clin Obstet Gynaecol 2014; 28:1222–1234.
- World Health Organization. Medical eligibility criteria for contraceptive use. Fifth edition 2015. http://apps.who.int/iris/bitstream/10665/172915/1/WHO_RHR_15.07_eng.pdf. Accessed July 27, 2017.
- Pietrzak B, Bobrowska K, Jabiry-Zieniewicz Z, et al. Oral and transdermal hormonal contraception in women after kidney transplantation. Transplant Proc 2007; 39:2759–2762.
- Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA-SC. Contraception 2004; 70:269–275.
- Vessey M, Painter R, Yeates D. Mortality in relation to oral contraceptive use and cigarette smoking. Lancet 2003; 362:185–191.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, Kaptein MC. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception 2005; 72:168–174.
- Jick SS, Kaye JA, Russmann S, Jick H. Risk of nonfatal venous thromboembolism in women using a contraceptive transdermal patch and oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2006; 73:223–228.
- Jick S, Kaye JA, Li L, Jick H. Further results on the risk of nonfatal venous thromboembolism in users of the contraceptive transdermal patch compared to users of oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2007; 76:4–7.
- Estes CM, Westhoff C. Contraception for the transplant patient. Semin Perinatol 2007; 31:372–377.
- Cole JA, Norman H, Doherty M, Walker AM. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol 2007; 109:339–346.
- Paternoster DM, Riboni F, Bertolino M, et al. The contraceptive vaginal ring in women with renal and liver transplantation: analysis of preliminary results. Transplant Proc 2010; 42:1162–1165.
- Centers for Disease Control and Prevention (CDC). Summary chart of US medical eligibility criteria for contraceptive use. https://www.cdc.gov/reproductivehealth/unintendedpregnancy/pdf/legal_summary-chart_english_final_tag508.pdf. Accessed July 17, 2017.
- McKay DB, Josephson MA. Pregnancy in recipients of solid organs: effects on mother and child. N Engl J Med 2006; 354:1281–1293.
- US Department of Health and Human Services. Organ procurement and transplantation network. https://optn.transplant.hrsa.gov/. Accessed July 17, 2017.
- United States Renal Data System. 2014 annual data report. https://www.usrds.org/2014/view/Default.aspx. Accessed July 17, 2017.
- Hou S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis 1999; 33:235–252.
- Josephson MA, McKay DB. Women and transplantation: fertility, sexuality, pregnancy, contraception. Adv Chronic Kidney Dis 2013; 20:433–440.
- Gill JS, Zalunardo N, Rose C, Tonelli M. The pregnancy rate and live birth rate in kidney transplant recipients. Am J Transplant 2009; 9:1541–1549.
- Mohapatra A, Basu G. Pregnancy in kidney disease. Health Sciences 2012; 1(2). http://healthsciences.ac.in/july-sep-12/downloads/pregnancy_in_kidney_disease.pdf. Accessed July 25, 2017.
- Potluri K, Moldenhauer J, Karlman R, Hou S. Beta HCG levels in a pregnant dialysis patient: a cautionary tale. NDT Plus 2011; 4:42–43.
- Kennedy C, Hussein W, Spencer S, et al. Reproductive health in Irish female renal transplant recipients. Ir J Med Sci 2012; 181:59–63.
- Ghazizadeh S, Lessan-Pezeshki M, Khatami M, et al. Unwanted pregnancy among kidney transplant recipients in Iran. Transplant Proc 2005; 37:3085–3086.
- Jabiry-Zieniewicz Z, Bobrowska K, Kaminski P, Wielgos M, Zieniewicz K, Krawczyk M. Low-dose hormonal contraception after liver transplantation. Transplant Proc 2007; 39:1530–1532.
- Coscia LA, Constantinescu S, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl 2010: 24:65–85.
- Mohamed-Ahmed O, Nelson-Piercy C, Bramham K, et al. Pregnancy outcomes in liver and cardiothoracic transplant recipients: a UK national cohort study. PLoS One 2014; 9:e89151.
- Enderby C, Keller CA. An overview of immunosuppression in solid organ transplantation. Am J Manag Care 2015; 21(suppl 1):s12–s23.
- Hoeltzenbein M, Elefant E, Vial T, et al. Teratogenicity of mycophenolate confirmed in a prospective study of the European Network of Teratology Information Services. Am J Med Genet A 2012; 158A:588–596.
- Polifka JE, Friedman JM. Teratogen update: azathioprine and 6-mercaptopurine. Teratology 2002; 65:240–261.
- Dinatale M. The pregnancy and lactation labeling rule (PLLR). US Food and Drug Administration, 2016. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM520454.pdf. Accessed July 25, 2017.
- Lexicomp. http://online.lexi.com/lco/action/api/find/globalid/6612?utd=1. Accessed July 27, 2017.
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009; 9(suppl 3):S1–S155.
- Deshpande NA, Coscia LA, Gomez-Lobo V, Moritz MJ, Armenti VT. Pregnancy after solid organ transplantation: a guide for obstetric management. Rev Obstet Gynecol 2013; 6:116–125.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016; 65:1–103.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 121: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2011; 118:184–196.
- Trussell J. Contraceptive failure in the United States. Contraception 2011; 83:397–404.
- Krajewski CM, Geetha D, Gomez-Lobo V. Contraceptive options for women with a history of solid-organ transplantation. Transplantation 2013; 95:1183–1186.
- Stern LF, Simons HR, Kohn JE, Debevec EJ, Morfesis JM, Patel AA. Differences in contraceptive use between family planning providers and the U.S. population: results of a nationwide survey. Contraception 2015; 91:464–469.
- Rafie S, Lai S, Garcia JE, Mody SK. Contraceptive use in female recipients of a solid-organ transplant. Prog Transplant 2014; 24:344–348.
- Labied S, Galant C, Nisolle M, et al. Differential elevation of matrix metalloproteinase expression in women exposed to levonorgestrel-releasing intrauterine system for a short or prolonged period of time. Hum Reprod 2009; 24:113–121.
- Kim CR, Martinez-Maza O, Magpantay L, et al. Immunologic evaluation of the endometrium with a levonorgestrel intrauterine device in solid organ transplant women and healthy controls. Contraception 2016; 94:534–540.
- Ramhendar T, Byrne P. Use of the levonorgestrel-releasing intrauterine system in renal transplant recipients: a retrospective case review. Contraception 2012; 86:288–289.
- Huguelet PS, Sheehan C, Spitzer RF, Scott S. Use of the levonorgestrel 52-mg intrauterine system in adolescent and young adult solid organ transplant recipients: a case series. Contraception 2017; 95:378–381.
- Peterson HB, Xia Z, Hughes JM, Wilcox LS, Tylor LR, Trussell J. The risk of pregnancy after tubal sterilization: findings from the US Collaborative Review of Sterilization. Am J Obstet Gynecol 1996; 174:1161–1168.
- Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 2007; 18:1319–1328.
- Krajewski C, Sucato G. Reproductive health care after transplantation. Best Pract Res Clin Obstet Gynaecol 2014; 28:1222–1234.
- World Health Organization. Medical eligibility criteria for contraceptive use. Fifth edition 2015. http://apps.who.int/iris/bitstream/10665/172915/1/WHO_RHR_15.07_eng.pdf. Accessed July 27, 2017.
- Pietrzak B, Bobrowska K, Jabiry-Zieniewicz Z, et al. Oral and transdermal hormonal contraception in women after kidney transplantation. Transplant Proc 2007; 39:2759–2762.
- Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA-SC. Contraception 2004; 70:269–275.
- Vessey M, Painter R, Yeates D. Mortality in relation to oral contraceptive use and cigarette smoking. Lancet 2003; 362:185–191.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, Kaptein MC. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception 2005; 72:168–174.
- Jick SS, Kaye JA, Russmann S, Jick H. Risk of nonfatal venous thromboembolism in women using a contraceptive transdermal patch and oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2006; 73:223–228.
- Jick S, Kaye JA, Li L, Jick H. Further results on the risk of nonfatal venous thromboembolism in users of the contraceptive transdermal patch compared to users of oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2007; 76:4–7.
- Estes CM, Westhoff C. Contraception for the transplant patient. Semin Perinatol 2007; 31:372–377.
- Cole JA, Norman H, Doherty M, Walker AM. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol 2007; 109:339–346.
- Paternoster DM, Riboni F, Bertolino M, et al. The contraceptive vaginal ring in women with renal and liver transplantation: analysis of preliminary results. Transplant Proc 2010; 42:1162–1165.
- Centers for Disease Control and Prevention (CDC). Summary chart of US medical eligibility criteria for contraceptive use. https://www.cdc.gov/reproductivehealth/unintendedpregnancy/pdf/legal_summary-chart_english_final_tag508.pdf. Accessed July 17, 2017.
KEY POINTS
- The number of solid-organ transplants in US women of childbearing age has increased over the past 20 years.
- Women should wait at least 1 year after receiving a solid-organ transplant before attempting to become pregnant, and then should do so only when cleared by the transplant team and obstetrician, with close monitoring.
- The various types of contraception can be grouped by their effectiveness and by the medical eligibility criteria set by the US Centers for Disease Control and Prevention.
- Transplant recipients of childbearing age should use 2 contraceptive methods concurrently, one of which should be condoms.