User login
The etiology of premenstrual dysphoric disorder: 5 interwoven pieces
In an age when psychiatry strives to identify the biologic causes of disease, studying endocrine-related mood disorders is particularly intriguing. DSM-5 defines premenstrual dysphoric disorder (PMDD) as a depressive disorder, with a 12-month prevalence ranging from 1.8% to 5.8% among women who menstruate.1-3 Factors that differentiate PMDD from other affective disorders include etiology, duration, and temporal relationship with the menstrual cycle.
PMDD is a disorder of consistent yet intermittent change in mental health and functionality. Therefore, it may be underdiagnosed and consequently undertreated if a psychiatric evaluation does not coincide with symptom occurrence or if patients do not understand that intermittent symptoms are treatable.
This article summarizes what is known about the etiology of PMDD. Although there are several treatments for PMDD, many women experience adverse effects or incomplete effectiveness. Further understanding of this disorder may lead to more efficacious treatments. Additionally, understanding the pathophysiology of PMDD might shed a light on the etiology of other disorders that are temporally related to reproductive life changes, such as pregnancy-, postpartum-, or menopause-related affective dysregulation.
Making the diagnosis
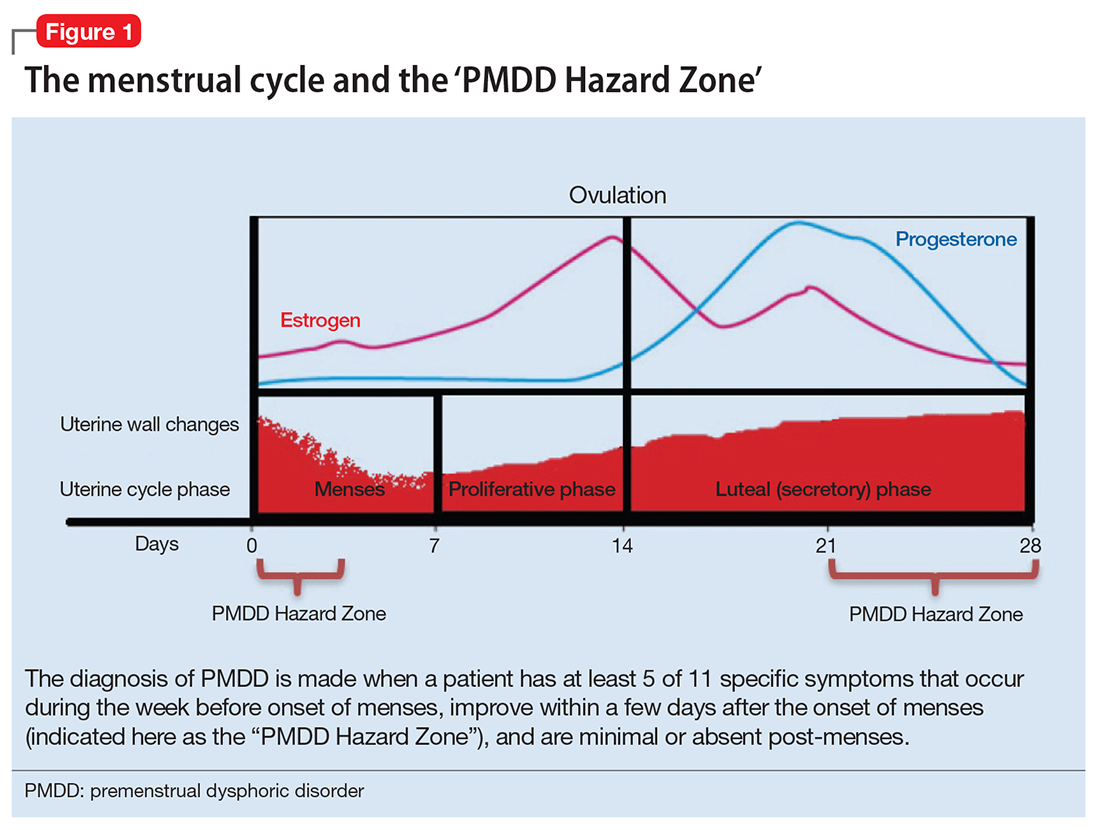
The diagnosis of PMDD is made when a patient has at least 5 of 11 specific symptoms that occur during the week before onset of menses, improve within a few days after the onset of menses (shown as the “PMDD Hazard Zone” in Figure 1), and are minimal or absent post-menses.3 Symptoms should be tracked prospectively for at least 2 menstrual cycles in order to confirm the diagnosis (one must be an affective symptom and another must be a behavioral/cognitive symptom).3
The affective symptoms are:
- lability of affect (eg, sudden sadness, tearfulness, or sensitivity to rejection)
- irritability, anger, or increased interpersonal conflicts
- depressed mood, hopelessness, or self- deprecating thoughts
- anxiety or tension, feeling “keyed up” or “on edge.”
The behavioral/cognitive symptoms are:
- decreased interest in usual activities (eg, work, hobbies, friends, school)
- difficulty concentrating
- lethargy, low energy, easy fatigability
- change in appetite, overeating, food cravings
- hypersomnia or insomnia
- feeling overwhelmed or out of control
- physical symptoms (breast tenderness or swelling, headache, joint or muscle pain, bloating, weight gain).
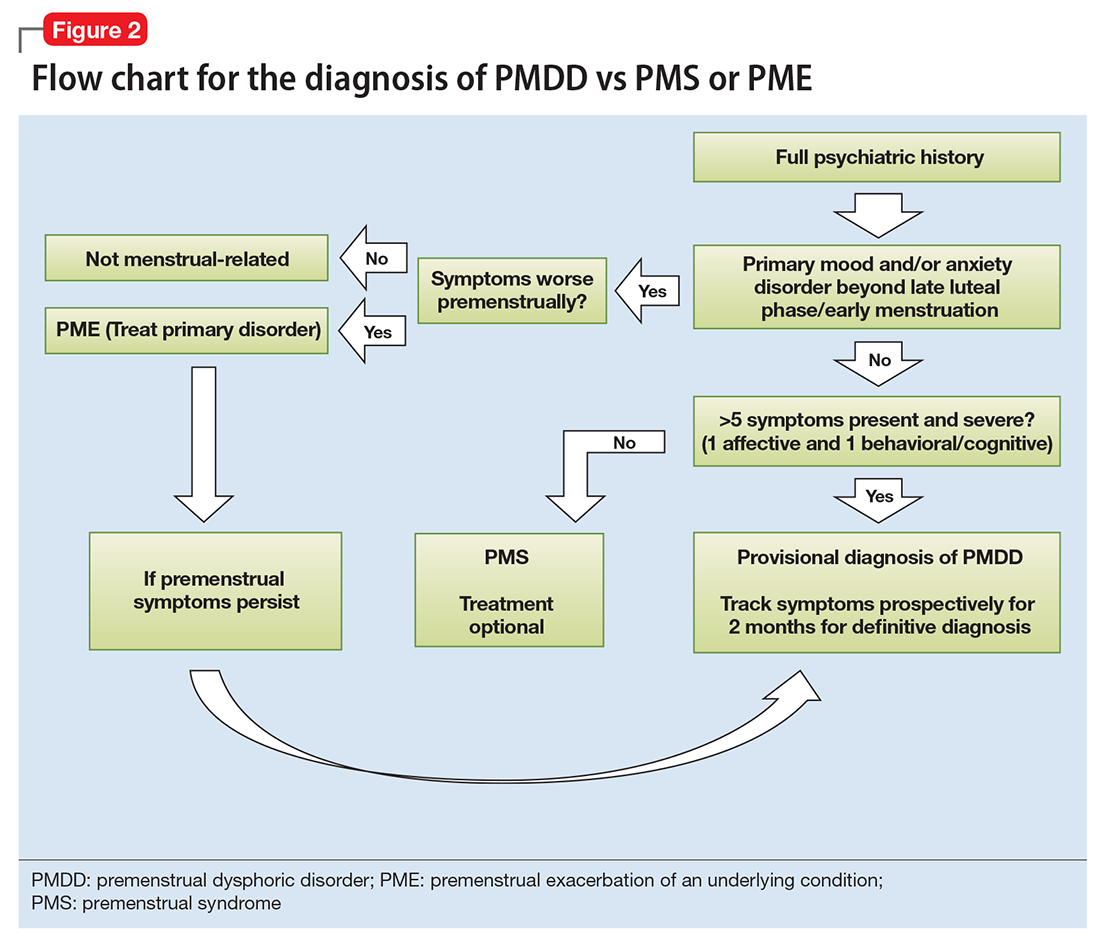
Ruling out premenstrual exacerbation (PME). Perhaps the most common cause for misdiagnosis of PMDD is failing to rule out PME of another underlying or comorbid condition (Figure 2). In many women who have a primary mood or anxiety disorder, the late luteal phase is a vulnerable time. A patient might be coping with untreated anxiety, for example, but the symptoms become unbearable the week before menstruation begins, which is likely when she seeks help. At this stage, a diagnosis of PMDD should be provisional at best. Often, PME is treated by treating the underlying condition. Therefore, a full diagnostic psychiatric interview is important to first rule out other underlying psychiatric disorders. PMDD is diagnosed if the premenstrual symptoms persist for 2 consecutive months after treating the suspected mood or anxiety disorder. Patients can use one of many PMDD daily symptom charts available online. Alternatively, they can use a cycle-tracking mobile phone application to correlate their symptoms with their cycle and share this information with their providers.
Consider these 5 interwoven pieces
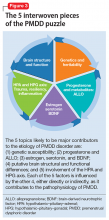
The many variables that contribute to the pathophysiology of PMDD overlap and should be considered connecting pieces in the puzzle that is the etiology of this disorder (Figure 3). In reviewing the literature, we have identified 5 topics likely to be major contributors to this disorder:
- genetic susceptibility
- progesterone and allopregnanolone (ALLO)
- estrogen, serotonin, and brain-derived neurotrophic factor (BDNF)
- putative brain structural and functional differences
- further involvement of the hypothalamic–pituitary–adrenal (HPA) axis and hypothalamic–pituitary–gonadal (HPG) axis: trauma, resiliency, and inflammation.
Genetic susceptibility. PMDD is thought to have a heritability range between 30% to 80%.3 This is demonstrated by family and twin studies4-7 and specific genetic studies.8 The involvement of genetics means an underlying neurobiologic pathophysiology is in place.
Estrogen receptor alpha (ESR1) gene. Huo et al8 found an associated variation in ESR1 in women with PMDD compared with controls. They speculated that because ESR1 is important for arousal, if dysfunctional, this gene could be implicated in somatic as well as affective and cognitive deficits in PMDD patients. In another study, investigators reported a relationship between PMDD and heritable personality traits, as well as a link between these traits and ESR1 polymorphic variants.1 They suggested that personality traits (independent of affective state) might be used to distinguish patients with PMDD from controls.1
Studies on serotonin gene polymorphism and serotonin transporter genotype. Although a study of serotonin gene polymorphism did not find an association between serotonin1A gene polymorphism and PMDD, it did show that the presence of at least 1 C allele was associated with a 2.5-fold increased risk of PMDD.9 Another study did not find an association between the serotonin transporter genotype 5-HTTLPR and PMDD.10 However, it showed lower frontocingulate cortex activation during the luteal phase of PMDD patients compared with controls, suggesting that PMDD is linked to impaired frontocingulate cortex activation induced by emotions during the luteal phase.10
Seasonal affective disorder (SAD) and PMDD have shared clinical features. A polymorphism in the serotonin transporter promoter gene 5-HTTLPR has been associated with SAD. One study found that patients with comorbid SAD and PMDD are genetically more vulnerable to comorbid affective disorders compared with patients who have SAD only.11
Progesterone and ALLO. Chronic exposure to progesterone and ALLO (a main progesterone metabolite) and rapid withdrawal from ovarian hormones may play a role in the etiology of PMDD. Much like alcohol or benzodiazepines, ALLO is a potent positive allosteric modulator of GABAA receptors and has sedative, anesthetic, and anxiolytic properties. In times of acute stress, increased ALLO is known to provide relief.12,13 However, in women with PMDD, this typical ALLO increase might not occur.14
Patients with PMDD have been reported to have decreased levels of ALLO in the luteal phase.15-17 In one study, women with highly symptomatic PMDD had lower levels of ALLO compared with women with less symptomatic PMDD.14 A gonadotropin-releasing hormone challenge study showed the increase in ALLO response was less in PMDD patients compared with controls.17 Luteal-phase ALLO concentrations are reported to be lower in women with premenstrual syndrome (PMS), a milder form of PMDD.14,17
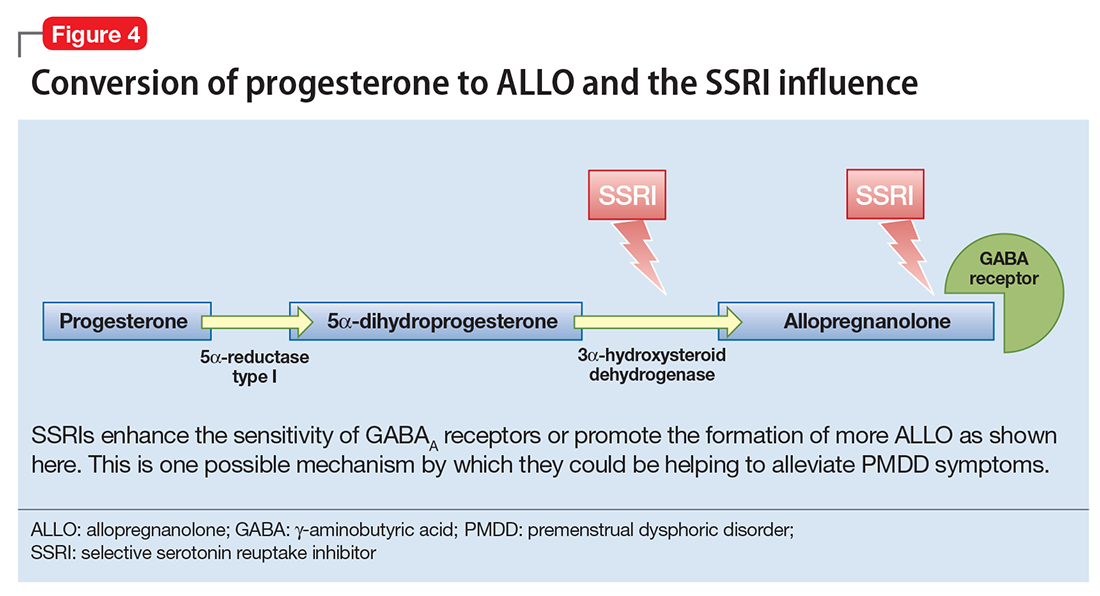
The efficacy of selective serotonin reuptake inhibitors (SSRIs) for treating PMDD could be the result of the interaction of these medications with neuroactive steroids,18 possibly because SSRIs enhance the sensitivity of GABAA receptors or promote the formation of more ALLO (Figure 4).19-21
Estrogen, serotonin, and BDNF. Estrogen affects multiple neurotransmitter systems that regulate mood, cognition, sleep, and eating.22 Studying estrogen in context of PMDD is important because women with PMDD can have low mood, specific food cravings, and impaired cognitive function.
Estrogen–serotonin interactions are thought to be involved in hormone-related mood disorders such as perimenopausal depression and PMDD.23,24 However, the nature of their relationship is not yet fully understood. Ovariectomized animals have shown estrogen-induced changes related to serotonin metabolism, binding, and transmission in the regions of the brain involved in regulation of affect and cognition. Research in menopausal women also has provided some support for this interaction.24
Positron emission tomography studies in humans have found increased cortical serotonin binding modulated by levels of estrogen, similar to those previously seen in rat studies.24-27 One study showed an increased binding potential of serotonin in the cerebral cortex with estrogen treatment. This study further showed an even greater binding potential with estrogen plus progesterone, signaling a synergistic effect of the 2 hormones.28
SSRIs are an effective treatment for the irritability, anxiety, and mood swings of PMDD.29-30 Although the exact mechanism of action is unknown, the serotonergic properties are certainly of primary attention. For some PMDD patients, SSRIs work within hours to days, as opposed to days or weeks for patients with depression or anxiety, which suggests a separate or co-occurring mechanism of action is in place. In a double-blind, placebo-controlled crossover study, researchers administered the serotonin receptor antagonist metergoline to women with PMDD whose symptoms had remitted during treatment with fluoxetine and a group of healthy controls who were not receiving any medication.31 The women with PMDD experienced a return of symptoms 24 hours after treatment with metergoline but not with placebo; the controls experienced no mood changes.31
BDNF is a neurotransmitter linked to estrogen and likely related to PMDD. BDNF is critical for neurogenesis and is expressed in brain regions involved in learning and memory and also affects regulation.32 BDNF levels are increased by serotonergic antidepressants, affected by estradiol, and have cyclicity throughout the menstrual cycle.33-35
Putative brain structural and functional differences. Imaging studies have suggested differences in brain structure in women with PMDD, with a focus on the amygdala and the prefrontal cortex. Women with PMDD have greater gray matter volume in the posterior cerebellum,36 greater gray matter density of hippocampal cortex, and lower gray matter density in the parahippocampal cortex.37
Some studies have shown a functional variability of the amygdala’s response to stress in women with PMDD vs healthy controls.38,39 A proton magnetic resonance spectroscopy (1H-MRS) study of the displays the possibility of an altered GABAergic function in patients with PMDD.40
Patients will PMDD have enhanced dorsolateral prefrontal cortex reactivity when anticipating negative stimuli (but not to the actual exposure) during the luteal phase. A positive correlation between this reactivity and progesterone levels also was observed.41 Some researchers have suggested that prefrontal cortex dysfunction may be a risk factor for PMDD.42
HPA axis and HPG axis: Trauma, resiliency, inflammation. Altered cortisol levels (higher during the luteal phase43 and lower during times of stress14,44) suggest a possibly altered HPA axis in some women with PMDD. However, studies on this topic have been few and inconsistent.
Dysregulation of the HPG axis could cause vasomotor symptoms, sleep dysregulation, and mood symptoms during menopause; women with PMDD can also experience these symptoms. The influence of estrogen and progesterone on mood is also highly dependent on this axis.
Ultimately, the interplay between the HPA axis and the HPG axis is important. One study found that women with PMDD who had high serum ALLO levels (HPG-related) had blunted nocturnal cortisol levels (HPA-related) compared with healthy controls who had low ALLO levels.45
Significant stress and trauma exposure have been associated with PMDD. A study of 3,968 women found a history of trauma and PTSD were independently associated with PMDD.46 Another study of approximately 3,000 women found a strong correlation between abuse and PMS.47 However, a third study found no correlations between PMDD and trauma.48
Patients with a predisposition to PMDD may be more vulnerable to develop a posttraumatic stress-related disorder, perhaps due to decreased biologic resiliency. For example, the startle response (hypervigilance) has been shown to be different in women with PMDD. One study suggested that suboptimal production of premenstrual ALLO may lead to increased arousal and increased stress reactivity to psychosocial or environmental triggers.49
The possible role of inflammation in PMDD deserves further investigation. The luteal phase entails an increase in the production of proinflammatory markers.50,51 A 10-fold increase in progesterone is correlated with a 20% to 23% increase in C-reactive protein levels.52,53 Women with inflammatory diseases (eg, gingivitis or irritable bowel syndrome) show worsening of symptoms prior to menstruation.54-56 One study found increased levels of proinflammatory markers in women with PMDD compared with controls.57
Putting together the 5 pieces of the puzzle
Because PMDD is heritable, it must have an underlying neurobiologic pathophysiology. Brain imaging studies show differences in structure and function in women with PMDD across the menstrual cycle. Conversion of progesterone to ALLO and the GABAergic influence of this metabolite is a topic of interest in current research. Similarly, the role of estrogen and its connection to serotonin and other neurotransmitters such as BDNF have been implicated.
The link between a history of stress, trauma, and PMDD raises the question of biologic resiliency and illness in these patients, as it connects to the HPA and HPG axis and production of inflammatory stress hormones and steroid hormones and their metabolites. PMDD can be conceptualized as variable sensitivity to hormonal response to stress,58 thus contextualizing biochemical and psychological resiliency.
Further research is needed to clarify the possibility of a shared pathophysiology between endocrine-related mood disorders such as postpartum depression (PPD) and PMDD because current research is controversial.59,60 In PPD, women who are exposed to high levels of progesterone and estrogen during pregnancy (just like in the mid-luteal phase) have a sudden drop in these hormones postpartum.
The ‘withdrawal theory.’ The affective symptoms of PMDD resolve almost instantaneously after the start of menstruation. Perhaps this type of immediate relief is akin to substance use disorders and symptoms of withdrawal. It could be that reinstatement of a certain amount of gonadal steroids in the follicular phase of the cycle diminishes a withdrawal-like response to these steroids.
Currently, the main leading theory is that PMDD is a result of “an abnormal response to normal hormonal changes.”61 A new study also has shown that the change in estradiol/progesterone levels (vs the steady state) was associated with PMDD symptoms.62 Thinking of PMDD as a disorder of withdrawal offers an alternative (yet complementary) perspective to the current theory: PMDD may be caused by the absence or diminishing of the above-named hormones and their metabolites in the late luteal phase (in the context of developed “tolerance” during the early- to mid-luteal phase).
Considering the interplay between neurotransmitters and neurosteroids, both a “serotonin withdrawal theory” (caused by a drop in steroid hormones) and a “GABAergic withdrawal theory” (due to the decline in progesterone) could be proposed. This theory would be supported by the fact that SSRIs seem to mitigate symptoms of PMDD as well as the genetic association between PMDD and ESR1. It is more than likely that the “withdrawal” is caused by the interactions between estrogen-serotonin, progesterone-ALLO, and GABA receptors, and the complementary fashion in which progesterone and estrogen influence each other.
1. Miller A, Vo H, Huo L, et al. Estrogen receptor alpha (ESR-1) associations with psychological traits in women with PMDD and controls. J Psychiatr Res. 2010;44(12):788-794.
2. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169(5):465-475.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Wilson CA, Turner CW, Keye WR Jr. Firstborn adolescent daughters and mothers with and without premenstrual syndrome: a comparison. J Adolesc Health. 1991;12(2):130-137.
5. Kendler KS, Silberg JL, Neale MC, et al. Genetic and environmental factors in the aetiology of menstrual, premenstrual and neurotic symptoms: a population-based twin study. Psychol Med. 1992;22(1):85-100.
6. Condon JT. The premenstrual syndrome: a twin study. Br J Psychiatry. 1993;162:481-486.
7. Kendler KS, Karkowski LM, Corey LA, et al. Longitudinal population-based twin study of retrospectively reported premenstrual symptoms and lifetime major depression. Am J Psychiatry. 1998;155(9):1234-1240.
8. Huo L, Straub RE, Roca C, et al. Risk for premenstrual dysphoric disorder is associated with genetic variation in ESR1, the estrogen receptor alpha gene. Biol Psychiatry. 2007;62(8):925-933.
9. Dhingra V, Magnay JL, O’Brien PM, et al. Serotonin receptor 1A C(-1019)G polymorphism associated with premenstrual dysphoric disorder. Obstet Gynecol. 2007;110(4):788-792.
10. Comasco E, Hahn A, Ganger S, et al. Emotional fronto-cingulate cortex activation and brain derived neurotrophic factor polymorphism in premenstrual dysphoric disorder. Hum Brain Mapp. 2014;35(9):4450-4458.
11. Praschak-Rieder N, Willeit M, Winkler D, et al. Role of family history and 5-HTTLPR polymorphism in female seasonal affective disorder patients with and without premenstrual dysphoric disorder. Eur Neuropsychopharmacol. 2002;12(2):129-134.
12. Klatzkin RR, Morrow AL, Light KC, et al. Associations of histories of depression and PMDD diagnosis with allopregnanolone concentrations following the oral administration of micronized progesterone. Psychoneuroendocrinology. 2006;31(10):1208-1219.
13. Crowley SK, Girdler SS. Neurosteroid, GABAergic and hypothalamic pituitary adrenal (HPA) axis regulation: what is the current state of knowledge in humans? Psychopharmacology (Berl). 2014;231(17):3619-3634.
14. Girdler SS, Straneva PA, Light KC, et al. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49(9):788-797.
15. Rapkin AJ, Morgan M, Goldman L, et al. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol. 1997;90(5):709-714.
16. Bicíková M, Dibbelt L, Hill M, et al. Allopregnanolone in women with premenstrual syndrome. Horm Metab Res. 1998;30(4):227-230.
17. Monteleone P, Luisi S, Tonetti A, et al. Allopregnanolone concentrations and premenstrual syndrome. Eur J Endocrinol. 2000;142(3):269-273.
18. Steiner M, Steinberg S, Stewart D, et al. Fluoxetine in the treatment of premenstrual dysphoria. Canadian Fluoxetine/Premenstrual Dysphoria Collaborative Study Group. N Engl J Med. 1995;332(23):1529-1534.
19. Sundström I, Bäckström T. Citalopram increases pregnanolone sensitivity in patients with premenstrual syndrome: an open trial. Psychoneuroendocrinology. 1998;23(1):73-88.
20. Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci U S A. 1999;96(23):13512-13517.
21. Trauger JW, Jiang A, Stearns BA, et al. Kinetics of allopregnanolone formation catalyzed by human 3 alpha-hydroxysteroid dehydrogenase type III (AKR1C2). Biochemistry. 2002;41(45):13451-13459.
22. Shanmugan S, Epperson CN. Estrogen and the prefrontal cortex: towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Hum Brain Mapp. 2014;35(3):847-865.
23. Rubinow DR, Schmidt PJ, Roca CA. Estrogen-serotonin interactions: implications for affective regulation. Biol Psychiatry. 1998;44(9):839-850.
24. Amin Z, Canli T, Epperson CN. Effect of estrogen-serotonin interactions on mood and cognition. Behav Cogn Neurosci Rev. 2005;4(1):43-58.
25. Cyr M, Bossé R, Di Paolo T. Gonadal hormones modulate 5-hydroxytryptamine2A receptors: emphasis on the rat frontal cortex. Neuroscience. 1998;83(3):829-836.
26. Fink G, Sumner BE, Rosie R, et al. Estrogen control of central neurotransmission: effect on mood, mental state, and memory. Cell Mol Neurobiol. 1996;16(3):325-344.
27. Sumner BE, Grant KE, Rosie R, et al. Effects of tamoxifen on serotonin transporter and 5-hydroxytryptamine(2A) receptor binding sites and mRNA levels in the brain of ovariectomized rats with or without acute estradiol replacement. Brain Res Mol Brain Res. 1999;73(1-2):119-128.
28. Moses-Kolko EL, Berga SL, Greer PJ, et al. Widespread increases of cortical serotonin type 2A receptor availability after hormone therapy in euthymic postmenopausal women. Fertil Steril. 2003;80(3):554-559.
29. Su TP, Schmidt PJ, Danaceau MA, et al. Fluoxetine in the treatment of premenstrual dysphoria. Neuropsychopharmacology. 1997;16(5):346-356.
30. Steinberg EM, Cardoso GM, Martinez PE, et al. Rapid response to fluoxetine in women with premenstrual dysphoric disorder. Depress Anxiety. 2012;29(6):531-540.
31. Roca CA, Schmidt PJ, Smith MJ, et al. Effects of metergoline on symptoms in women with premenstrual dysphoric disorder. Am J Psychiatry. 2002;159(11):1876-1881.
32. Gray JD, Milner TA, McEwen BS. Dynamic plasticity: the role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience. 2013;239:214-227.
33. Carbone DL, Handa RJ. Sex and stress hormone influences on the expression and activity of brain-derived neurotrophic factor. Neuroscience. 2013;239:295-303.
34. Pilar-Cuéllar F, Vidal R, Pazos A. Subchronic treatment with fluoxetine and ketanserin increases hippocampal brain-derived neurotrophic factor, β-catenin and antidepressant-like effects. Br J Pharmacol. 2012;165(4b):1046-1057.
35. Deuschle M, Gilles M, Scharnholz B, et al. Changes of serum concentrations of brain-derived neurotrophic factor (BDNF) during treatment with venlafaxine and mirtazapine: role of medication and response to treatment. Pharmacopsychiatry. 2013;46(2):54-58.
36. Berman SM, London ED, Morgan M, et al. Elevated gray matter volume of the emotional cerebellum in women with premenstrual dysphoric disorder. J Affect Disord. 2013;146(2):266-271.
37. Jeong HG, Ham BJ, Yeo HB, et al. Gray matter abnormalities in patients with premenstrual dysphoric disorder: an optimized voxel-based morphometry. J Affect Disord. 2012;140(3):260-267.
38. Protopopescu X, Tuescher O, Pan H, et al. Toward a functional neuroanatomy of premenstrual dysphoric disorder. J Affect Disord. 2008;108(1-2):87-94.
39. Gingnell M, Morell A, Bannbers E, et al. Menstrual cycle effects on amygdala reactivity to emotional stimulation in premenstrual dysphoric disorder. Horm Behav. 2012;62(4):400-406.
40. Epperson CN, Haga K, Mason GF, et al. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2002;59(9):851-858.
41. Gingnell M, Bannbers E, Wikström J, et al. Premenstrual dysphoric disorder and prefrontal reactivity during anticipation of emotional stimuli. Eur Neuropsychopharmacol. 2013;23(11):1474-1483.
42. Baller EB, Wei SM, Kohn PD, et al. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: a multimodal neuroimaging study. Am J Psychiatry. 2013;170(3):305-314.
43. Rasgon N, McGuire M, Tanavoli S, et al. Neuroendocrine response to an intravenous L-tryptophan challenge in women with premenstrual syndrome. Fertil Steril. 2000;73(1):144-149.
44. Huang Y, Zhou R, Wu M, et al. Premenstrual syndrome is associated with blunted cortisol reactivity to the TSST. Stress. 2015;18(2):160-168.
45. Segebladh B, Bannbers E, Moby L, et al. Allopregnanolone serum concentrations and diurnal cortisol secretion in women with premenstrual dysphoric disorder. Arch Womens Ment Health. 2013;16(2):131-137.
46. Pilver CE, Levy BR, Libby DJ, et al. Posttraumatic stress disorder and trauma characteristics are correlates of premenstrual dysphoric disorder. Arch Womens Ment Health. 2011;14(5):383-393.
47. Bertone-Johnson ER, Whitcomb BW, Missmer SA, et al. Early life emotional, physical, and sexual abuse and the development of premenstrual syndrome: a longitudinal study. J Womens Health (Larchmt). 2014;23(9):729-739.
48. Segebladh B, Bannbers E, Kask K, et al. Prevalence of violence exposure in women with premenstrual dysphoric disorder in comparison with other gynecological patients and asymptomatic controls. Acta Obstet Gynecol Scand. 2011;90(7):746-752.
49. Kask K, Gulinello M, Bäckström T, et al. Patients with premenstrual dysphoric disorder have increased startle response across both cycle phases and lower levels of prepulse inhibition during the late luteal phase of the menstrual cycle. Neuropsychopharmacology. 2008;33(9):2283-2290.
50. O’Brien SM, Fitzgerald P, Scully P, et al. Impact of gender and menstrual cycle phase on plasma cytokine concentrations. Neuroimmunomodulation. 2007;14(2):84-90.
51. Northoff H, Symons S, Zieker D, et al. Gender- and menstrual phase dependent regulation of inflammatory gene expression in response to aerobic exercise. Exerc Immunol Rev. 2008;14:86-103.
52. Gaskins AJ, Wilchesky M, Mumford SL, et al. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: the BioCycle Study. Am J Epidemiol. 2012;175(5):423-431.
53. Wander K, Brindle E, O’Connor KA. C-reactive protein across the menstrual cycle. Am J Phys Anthropol. 2008;136(2):138-146.
54. Jane ZY, Chang CC, Lin HK, et al. The association between the exacerbation of irritable bowel syndrome and menstrual symptoms in young Taiwanese women. Gastroenterol Nurs. 2011;34(4):277-286.
55. Kane SV, Sable K, Hanauer SB. The menstrual cycle and its effect on inflammatory bowel disease and irritable bowel syndrome: a prevalence study. Am J Gastroenterol. 1998;93(10):1867-1872.
56. Shourie V, Dwarakanath CD, Prashanth GV, et al. The effect of menstrual cycle on periodontal health - a clinical and microbiological study. Oral Health Prev Dent. 2012;10(2):185-192.
57. Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep. 2015;17(11):87.
58. Maeng LY, Milad MR. Sex differences in anxiety disorders: Interactions between fear, stress, and gonadal hormones. Horm Behav. 2015;76:106-117.
59. Lee YJ, Yi SW, Ju DH, et al. Correlation between postpartum depression and premenstrual dysphoric disorder: single center study. Obstet Gynecol Sci. 2015;58(5):353-358.
60. Kepple AL, Lee EE, Haq N, et al. History of postpartum depression in a clinic-based sample of women with premenstrual dysphoric disorder. J Clin Psychiatry. 2016;77(4):e415-e420.
61. Schmidt PJ, Nieman LK, Danaceau MA, et al. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. 1998;338(4):209-216.
62. Schmidt PJ, Martinez PE, Nieman LK, et al. Premenstrual dysphoric disorder symptoms following ovarian suppression: Triggered by change in ovarian steroid levels but not continuous stable levels. Am J Psychiatry. [published online April 21, 2017]. doi: 10.1176/appi.ajp.2017.16101113.
In an age when psychiatry strives to identify the biologic causes of disease, studying endocrine-related mood disorders is particularly intriguing. DSM-5 defines premenstrual dysphoric disorder (PMDD) as a depressive disorder, with a 12-month prevalence ranging from 1.8% to 5.8% among women who menstruate.1-3 Factors that differentiate PMDD from other affective disorders include etiology, duration, and temporal relationship with the menstrual cycle.
PMDD is a disorder of consistent yet intermittent change in mental health and functionality. Therefore, it may be underdiagnosed and consequently undertreated if a psychiatric evaluation does not coincide with symptom occurrence or if patients do not understand that intermittent symptoms are treatable.
This article summarizes what is known about the etiology of PMDD. Although there are several treatments for PMDD, many women experience adverse effects or incomplete effectiveness. Further understanding of this disorder may lead to more efficacious treatments. Additionally, understanding the pathophysiology of PMDD might shed a light on the etiology of other disorders that are temporally related to reproductive life changes, such as pregnancy-, postpartum-, or menopause-related affective dysregulation.
Making the diagnosis
The diagnosis of PMDD is made when a patient has at least 5 of 11 specific symptoms that occur during the week before onset of menses, improve within a few days after the onset of menses (shown as the “PMDD Hazard Zone” in Figure 1), and are minimal or absent post-menses.3 Symptoms should be tracked prospectively for at least 2 menstrual cycles in order to confirm the diagnosis (one must be an affective symptom and another must be a behavioral/cognitive symptom).3

The affective symptoms are:
- lability of affect (eg, sudden sadness, tearfulness, or sensitivity to rejection)
- irritability, anger, or increased interpersonal conflicts
- depressed mood, hopelessness, or self- deprecating thoughts
- anxiety or tension, feeling “keyed up” or “on edge.”
The behavioral/cognitive symptoms are:
- decreased interest in usual activities (eg, work, hobbies, friends, school)
- difficulty concentrating
- lethargy, low energy, easy fatigability
- change in appetite, overeating, food cravings
- hypersomnia or insomnia
- feeling overwhelmed or out of control
- physical symptoms (breast tenderness or swelling, headache, joint or muscle pain, bloating, weight gain).
Ruling out premenstrual exacerbation (PME). Perhaps the most common cause for misdiagnosis of PMDD is failing to rule out PME of another underlying or comorbid condition (Figure 2). In many women who have a primary mood or anxiety disorder, the late luteal phase is a vulnerable time. A patient might be coping with untreated anxiety, for example, but the symptoms become unbearable the week before menstruation begins, which is likely when she seeks help. At this stage, a diagnosis of PMDD should be provisional at best. Often, PME is treated by treating the underlying condition. Therefore, a full diagnostic psychiatric interview is important to first rule out other underlying psychiatric disorders. PMDD is diagnosed if the premenstrual symptoms persist for 2 consecutive months after treating the suspected mood or anxiety disorder. Patients can use one of many PMDD daily symptom charts available online. Alternatively, they can use a cycle-tracking mobile phone application to correlate their symptoms with their cycle and share this information with their providers.

Consider these 5 interwoven pieces
The many variables that contribute to the pathophysiology of PMDD overlap and should be considered connecting pieces in the puzzle that is the etiology of this disorder (Figure 3). In reviewing the literature, we have identified 5 topics likely to be major contributors to this disorder:
- genetic susceptibility
- progesterone and allopregnanolone (ALLO)
- estrogen, serotonin, and brain-derived neurotrophic factor (BDNF)
- putative brain structural and functional differences
- further involvement of the hypothalamic–pituitary–adrenal (HPA) axis and hypothalamic–pituitary–gonadal (HPG) axis: trauma, resiliency, and inflammation.
Genetic susceptibility. PMDD is thought to have a heritability range between 30% to 80%.3 This is demonstrated by family and twin studies4-7 and specific genetic studies.8 The involvement of genetics means an underlying neurobiologic pathophysiology is in place.
Estrogen receptor alpha (ESR1) gene. Huo et al8 found an associated variation in ESR1 in women with PMDD compared with controls. They speculated that because ESR1 is important for arousal, if dysfunctional, this gene could be implicated in somatic as well as affective and cognitive deficits in PMDD patients. In another study, investigators reported a relationship between PMDD and heritable personality traits, as well as a link between these traits and ESR1 polymorphic variants.1 They suggested that personality traits (independent of affective state) might be used to distinguish patients with PMDD from controls.1
Studies on serotonin gene polymorphism and serotonin transporter genotype. Although a study of serotonin gene polymorphism did not find an association between serotonin1A gene polymorphism and PMDD, it did show that the presence of at least 1 C allele was associated with a 2.5-fold increased risk of PMDD.9 Another study did not find an association between the serotonin transporter genotype 5-HTTLPR and PMDD.10 However, it showed lower frontocingulate cortex activation during the luteal phase of PMDD patients compared with controls, suggesting that PMDD is linked to impaired frontocingulate cortex activation induced by emotions during the luteal phase.10
Seasonal affective disorder (SAD) and PMDD have shared clinical features. A polymorphism in the serotonin transporter promoter gene 5-HTTLPR has been associated with SAD. One study found that patients with comorbid SAD and PMDD are genetically more vulnerable to comorbid affective disorders compared with patients who have SAD only.11
Progesterone and ALLO. Chronic exposure to progesterone and ALLO (a main progesterone metabolite) and rapid withdrawal from ovarian hormones may play a role in the etiology of PMDD. Much like alcohol or benzodiazepines, ALLO is a potent positive allosteric modulator of GABAA receptors and has sedative, anesthetic, and anxiolytic properties. In times of acute stress, increased ALLO is known to provide relief.12,13 However, in women with PMDD, this typical ALLO increase might not occur.14
Patients with PMDD have been reported to have decreased levels of ALLO in the luteal phase.15-17 In one study, women with highly symptomatic PMDD had lower levels of ALLO compared with women with less symptomatic PMDD.14 A gonadotropin-releasing hormone challenge study showed the increase in ALLO response was less in PMDD patients compared with controls.17 Luteal-phase ALLO concentrations are reported to be lower in women with premenstrual syndrome (PMS), a milder form of PMDD.14,17
The efficacy of selective serotonin reuptake inhibitors (SSRIs) for treating PMDD could be the result of the interaction of these medications with neuroactive steroids,18 possibly because SSRIs enhance the sensitivity of GABAA receptors or promote the formation of more ALLO (Figure 4).19-21

Estrogen, serotonin, and BDNF. Estrogen affects multiple neurotransmitter systems that regulate mood, cognition, sleep, and eating.22 Studying estrogen in context of PMDD is important because women with PMDD can have low mood, specific food cravings, and impaired cognitive function.
Estrogen–serotonin interactions are thought to be involved in hormone-related mood disorders such as perimenopausal depression and PMDD.23,24 However, the nature of their relationship is not yet fully understood. Ovariectomized animals have shown estrogen-induced changes related to serotonin metabolism, binding, and transmission in the regions of the brain involved in regulation of affect and cognition. Research in menopausal women also has provided some support for this interaction.24
Positron emission tomography studies in humans have found increased cortical serotonin binding modulated by levels of estrogen, similar to those previously seen in rat studies.24-27 One study showed an increased binding potential of serotonin in the cerebral cortex with estrogen treatment. This study further showed an even greater binding potential with estrogen plus progesterone, signaling a synergistic effect of the 2 hormones.28
SSRIs are an effective treatment for the irritability, anxiety, and mood swings of PMDD.29-30 Although the exact mechanism of action is unknown, the serotonergic properties are certainly of primary attention. For some PMDD patients, SSRIs work within hours to days, as opposed to days or weeks for patients with depression or anxiety, which suggests a separate or co-occurring mechanism of action is in place. In a double-blind, placebo-controlled crossover study, researchers administered the serotonin receptor antagonist metergoline to women with PMDD whose symptoms had remitted during treatment with fluoxetine and a group of healthy controls who were not receiving any medication.31 The women with PMDD experienced a return of symptoms 24 hours after treatment with metergoline but not with placebo; the controls experienced no mood changes.31
BDNF is a neurotransmitter linked to estrogen and likely related to PMDD. BDNF is critical for neurogenesis and is expressed in brain regions involved in learning and memory and also affects regulation.32 BDNF levels are increased by serotonergic antidepressants, affected by estradiol, and have cyclicity throughout the menstrual cycle.33-35
Putative brain structural and functional differences. Imaging studies have suggested differences in brain structure in women with PMDD, with a focus on the amygdala and the prefrontal cortex. Women with PMDD have greater gray matter volume in the posterior cerebellum,36 greater gray matter density of hippocampal cortex, and lower gray matter density in the parahippocampal cortex.37
Some studies have shown a functional variability of the amygdala’s response to stress in women with PMDD vs healthy controls.38,39 A proton magnetic resonance spectroscopy (1H-MRS) study of the displays the possibility of an altered GABAergic function in patients with PMDD.40
Patients will PMDD have enhanced dorsolateral prefrontal cortex reactivity when anticipating negative stimuli (but not to the actual exposure) during the luteal phase. A positive correlation between this reactivity and progesterone levels also was observed.41 Some researchers have suggested that prefrontal cortex dysfunction may be a risk factor for PMDD.42
HPA axis and HPG axis: Trauma, resiliency, inflammation. Altered cortisol levels (higher during the luteal phase43 and lower during times of stress14,44) suggest a possibly altered HPA axis in some women with PMDD. However, studies on this topic have been few and inconsistent.
Dysregulation of the HPG axis could cause vasomotor symptoms, sleep dysregulation, and mood symptoms during menopause; women with PMDD can also experience these symptoms. The influence of estrogen and progesterone on mood is also highly dependent on this axis.
Ultimately, the interplay between the HPA axis and the HPG axis is important. One study found that women with PMDD who had high serum ALLO levels (HPG-related) had blunted nocturnal cortisol levels (HPA-related) compared with healthy controls who had low ALLO levels.45
Significant stress and trauma exposure have been associated with PMDD. A study of 3,968 women found a history of trauma and PTSD were independently associated with PMDD.46 Another study of approximately 3,000 women found a strong correlation between abuse and PMS.47 However, a third study found no correlations between PMDD and trauma.48
Patients with a predisposition to PMDD may be more vulnerable to develop a posttraumatic stress-related disorder, perhaps due to decreased biologic resiliency. For example, the startle response (hypervigilance) has been shown to be different in women with PMDD. One study suggested that suboptimal production of premenstrual ALLO may lead to increased arousal and increased stress reactivity to psychosocial or environmental triggers.49
The possible role of inflammation in PMDD deserves further investigation. The luteal phase entails an increase in the production of proinflammatory markers.50,51 A 10-fold increase in progesterone is correlated with a 20% to 23% increase in C-reactive protein levels.52,53 Women with inflammatory diseases (eg, gingivitis or irritable bowel syndrome) show worsening of symptoms prior to menstruation.54-56 One study found increased levels of proinflammatory markers in women with PMDD compared with controls.57
Putting together the 5 pieces of the puzzle
Because PMDD is heritable, it must have an underlying neurobiologic pathophysiology. Brain imaging studies show differences in structure and function in women with PMDD across the menstrual cycle. Conversion of progesterone to ALLO and the GABAergic influence of this metabolite is a topic of interest in current research. Similarly, the role of estrogen and its connection to serotonin and other neurotransmitters such as BDNF have been implicated.
The link between a history of stress, trauma, and PMDD raises the question of biologic resiliency and illness in these patients, as it connects to the HPA and HPG axis and production of inflammatory stress hormones and steroid hormones and their metabolites. PMDD can be conceptualized as variable sensitivity to hormonal response to stress,58 thus contextualizing biochemical and psychological resiliency.
Further research is needed to clarify the possibility of a shared pathophysiology between endocrine-related mood disorders such as postpartum depression (PPD) and PMDD because current research is controversial.59,60 In PPD, women who are exposed to high levels of progesterone and estrogen during pregnancy (just like in the mid-luteal phase) have a sudden drop in these hormones postpartum.
The ‘withdrawal theory.’ The affective symptoms of PMDD resolve almost instantaneously after the start of menstruation. Perhaps this type of immediate relief is akin to substance use disorders and symptoms of withdrawal. It could be that reinstatement of a certain amount of gonadal steroids in the follicular phase of the cycle diminishes a withdrawal-like response to these steroids.
Currently, the main leading theory is that PMDD is a result of “an abnormal response to normal hormonal changes.”61 A new study also has shown that the change in estradiol/progesterone levels (vs the steady state) was associated with PMDD symptoms.62 Thinking of PMDD as a disorder of withdrawal offers an alternative (yet complementary) perspective to the current theory: PMDD may be caused by the absence or diminishing of the above-named hormones and their metabolites in the late luteal phase (in the context of developed “tolerance” during the early- to mid-luteal phase).
Considering the interplay between neurotransmitters and neurosteroids, both a “serotonin withdrawal theory” (caused by a drop in steroid hormones) and a “GABAergic withdrawal theory” (due to the decline in progesterone) could be proposed. This theory would be supported by the fact that SSRIs seem to mitigate symptoms of PMDD as well as the genetic association between PMDD and ESR1. It is more than likely that the “withdrawal” is caused by the interactions between estrogen-serotonin, progesterone-ALLO, and GABA receptors, and the complementary fashion in which progesterone and estrogen influence each other.
In an age when psychiatry strives to identify the biologic causes of disease, studying endocrine-related mood disorders is particularly intriguing. DSM-5 defines premenstrual dysphoric disorder (PMDD) as a depressive disorder, with a 12-month prevalence ranging from 1.8% to 5.8% among women who menstruate.1-3 Factors that differentiate PMDD from other affective disorders include etiology, duration, and temporal relationship with the menstrual cycle.
PMDD is a disorder of consistent yet intermittent change in mental health and functionality. Therefore, it may be underdiagnosed and consequently undertreated if a psychiatric evaluation does not coincide with symptom occurrence or if patients do not understand that intermittent symptoms are treatable.
This article summarizes what is known about the etiology of PMDD. Although there are several treatments for PMDD, many women experience adverse effects or incomplete effectiveness. Further understanding of this disorder may lead to more efficacious treatments. Additionally, understanding the pathophysiology of PMDD might shed a light on the etiology of other disorders that are temporally related to reproductive life changes, such as pregnancy-, postpartum-, or menopause-related affective dysregulation.
Making the diagnosis
The diagnosis of PMDD is made when a patient has at least 5 of 11 specific symptoms that occur during the week before onset of menses, improve within a few days after the onset of menses (shown as the “PMDD Hazard Zone” in Figure 1), and are minimal or absent post-menses.3 Symptoms should be tracked prospectively for at least 2 menstrual cycles in order to confirm the diagnosis (one must be an affective symptom and another must be a behavioral/cognitive symptom).3

The affective symptoms are:
- lability of affect (eg, sudden sadness, tearfulness, or sensitivity to rejection)
- irritability, anger, or increased interpersonal conflicts
- depressed mood, hopelessness, or self- deprecating thoughts
- anxiety or tension, feeling “keyed up” or “on edge.”
The behavioral/cognitive symptoms are:
- decreased interest in usual activities (eg, work, hobbies, friends, school)
- difficulty concentrating
- lethargy, low energy, easy fatigability
- change in appetite, overeating, food cravings
- hypersomnia or insomnia
- feeling overwhelmed or out of control
- physical symptoms (breast tenderness or swelling, headache, joint or muscle pain, bloating, weight gain).
Ruling out premenstrual exacerbation (PME). Perhaps the most common cause for misdiagnosis of PMDD is failing to rule out PME of another underlying or comorbid condition (Figure 2). In many women who have a primary mood or anxiety disorder, the late luteal phase is a vulnerable time. A patient might be coping with untreated anxiety, for example, but the symptoms become unbearable the week before menstruation begins, which is likely when she seeks help. At this stage, a diagnosis of PMDD should be provisional at best. Often, PME is treated by treating the underlying condition. Therefore, a full diagnostic psychiatric interview is important to first rule out other underlying psychiatric disorders. PMDD is diagnosed if the premenstrual symptoms persist for 2 consecutive months after treating the suspected mood or anxiety disorder. Patients can use one of many PMDD daily symptom charts available online. Alternatively, they can use a cycle-tracking mobile phone application to correlate their symptoms with their cycle and share this information with their providers.

Consider these 5 interwoven pieces
The many variables that contribute to the pathophysiology of PMDD overlap and should be considered connecting pieces in the puzzle that is the etiology of this disorder (Figure 3). In reviewing the literature, we have identified 5 topics likely to be major contributors to this disorder:
- genetic susceptibility
- progesterone and allopregnanolone (ALLO)
- estrogen, serotonin, and brain-derived neurotrophic factor (BDNF)
- putative brain structural and functional differences
- further involvement of the hypothalamic–pituitary–adrenal (HPA) axis and hypothalamic–pituitary–gonadal (HPG) axis: trauma, resiliency, and inflammation.
Genetic susceptibility. PMDD is thought to have a heritability range between 30% to 80%.3 This is demonstrated by family and twin studies4-7 and specific genetic studies.8 The involvement of genetics means an underlying neurobiologic pathophysiology is in place.
Estrogen receptor alpha (ESR1) gene. Huo et al8 found an associated variation in ESR1 in women with PMDD compared with controls. They speculated that because ESR1 is important for arousal, if dysfunctional, this gene could be implicated in somatic as well as affective and cognitive deficits in PMDD patients. In another study, investigators reported a relationship between PMDD and heritable personality traits, as well as a link between these traits and ESR1 polymorphic variants.1 They suggested that personality traits (independent of affective state) might be used to distinguish patients with PMDD from controls.1
Studies on serotonin gene polymorphism and serotonin transporter genotype. Although a study of serotonin gene polymorphism did not find an association between serotonin1A gene polymorphism and PMDD, it did show that the presence of at least 1 C allele was associated with a 2.5-fold increased risk of PMDD.9 Another study did not find an association between the serotonin transporter genotype 5-HTTLPR and PMDD.10 However, it showed lower frontocingulate cortex activation during the luteal phase of PMDD patients compared with controls, suggesting that PMDD is linked to impaired frontocingulate cortex activation induced by emotions during the luteal phase.10
Seasonal affective disorder (SAD) and PMDD have shared clinical features. A polymorphism in the serotonin transporter promoter gene 5-HTTLPR has been associated with SAD. One study found that patients with comorbid SAD and PMDD are genetically more vulnerable to comorbid affective disorders compared with patients who have SAD only.11
Progesterone and ALLO. Chronic exposure to progesterone and ALLO (a main progesterone metabolite) and rapid withdrawal from ovarian hormones may play a role in the etiology of PMDD. Much like alcohol or benzodiazepines, ALLO is a potent positive allosteric modulator of GABAA receptors and has sedative, anesthetic, and anxiolytic properties. In times of acute stress, increased ALLO is known to provide relief.12,13 However, in women with PMDD, this typical ALLO increase might not occur.14
Patients with PMDD have been reported to have decreased levels of ALLO in the luteal phase.15-17 In one study, women with highly symptomatic PMDD had lower levels of ALLO compared with women with less symptomatic PMDD.14 A gonadotropin-releasing hormone challenge study showed the increase in ALLO response was less in PMDD patients compared with controls.17 Luteal-phase ALLO concentrations are reported to be lower in women with premenstrual syndrome (PMS), a milder form of PMDD.14,17
The efficacy of selective serotonin reuptake inhibitors (SSRIs) for treating PMDD could be the result of the interaction of these medications with neuroactive steroids,18 possibly because SSRIs enhance the sensitivity of GABAA receptors or promote the formation of more ALLO (Figure 4).19-21

Estrogen, serotonin, and BDNF. Estrogen affects multiple neurotransmitter systems that regulate mood, cognition, sleep, and eating.22 Studying estrogen in context of PMDD is important because women with PMDD can have low mood, specific food cravings, and impaired cognitive function.
Estrogen–serotonin interactions are thought to be involved in hormone-related mood disorders such as perimenopausal depression and PMDD.23,24 However, the nature of their relationship is not yet fully understood. Ovariectomized animals have shown estrogen-induced changes related to serotonin metabolism, binding, and transmission in the regions of the brain involved in regulation of affect and cognition. Research in menopausal women also has provided some support for this interaction.24
Positron emission tomography studies in humans have found increased cortical serotonin binding modulated by levels of estrogen, similar to those previously seen in rat studies.24-27 One study showed an increased binding potential of serotonin in the cerebral cortex with estrogen treatment. This study further showed an even greater binding potential with estrogen plus progesterone, signaling a synergistic effect of the 2 hormones.28
SSRIs are an effective treatment for the irritability, anxiety, and mood swings of PMDD.29-30 Although the exact mechanism of action is unknown, the serotonergic properties are certainly of primary attention. For some PMDD patients, SSRIs work within hours to days, as opposed to days or weeks for patients with depression or anxiety, which suggests a separate or co-occurring mechanism of action is in place. In a double-blind, placebo-controlled crossover study, researchers administered the serotonin receptor antagonist metergoline to women with PMDD whose symptoms had remitted during treatment with fluoxetine and a group of healthy controls who were not receiving any medication.31 The women with PMDD experienced a return of symptoms 24 hours after treatment with metergoline but not with placebo; the controls experienced no mood changes.31
BDNF is a neurotransmitter linked to estrogen and likely related to PMDD. BDNF is critical for neurogenesis and is expressed in brain regions involved in learning and memory and also affects regulation.32 BDNF levels are increased by serotonergic antidepressants, affected by estradiol, and have cyclicity throughout the menstrual cycle.33-35
Putative brain structural and functional differences. Imaging studies have suggested differences in brain structure in women with PMDD, with a focus on the amygdala and the prefrontal cortex. Women with PMDD have greater gray matter volume in the posterior cerebellum,36 greater gray matter density of hippocampal cortex, and lower gray matter density in the parahippocampal cortex.37
Some studies have shown a functional variability of the amygdala’s response to stress in women with PMDD vs healthy controls.38,39 A proton magnetic resonance spectroscopy (1H-MRS) study of the displays the possibility of an altered GABAergic function in patients with PMDD.40
Patients will PMDD have enhanced dorsolateral prefrontal cortex reactivity when anticipating negative stimuli (but not to the actual exposure) during the luteal phase. A positive correlation between this reactivity and progesterone levels also was observed.41 Some researchers have suggested that prefrontal cortex dysfunction may be a risk factor for PMDD.42
HPA axis and HPG axis: Trauma, resiliency, inflammation. Altered cortisol levels (higher during the luteal phase43 and lower during times of stress14,44) suggest a possibly altered HPA axis in some women with PMDD. However, studies on this topic have been few and inconsistent.
Dysregulation of the HPG axis could cause vasomotor symptoms, sleep dysregulation, and mood symptoms during menopause; women with PMDD can also experience these symptoms. The influence of estrogen and progesterone on mood is also highly dependent on this axis.
Ultimately, the interplay between the HPA axis and the HPG axis is important. One study found that women with PMDD who had high serum ALLO levels (HPG-related) had blunted nocturnal cortisol levels (HPA-related) compared with healthy controls who had low ALLO levels.45
Significant stress and trauma exposure have been associated with PMDD. A study of 3,968 women found a history of trauma and PTSD were independently associated with PMDD.46 Another study of approximately 3,000 women found a strong correlation between abuse and PMS.47 However, a third study found no correlations between PMDD and trauma.48
Patients with a predisposition to PMDD may be more vulnerable to develop a posttraumatic stress-related disorder, perhaps due to decreased biologic resiliency. For example, the startle response (hypervigilance) has been shown to be different in women with PMDD. One study suggested that suboptimal production of premenstrual ALLO may lead to increased arousal and increased stress reactivity to psychosocial or environmental triggers.49
The possible role of inflammation in PMDD deserves further investigation. The luteal phase entails an increase in the production of proinflammatory markers.50,51 A 10-fold increase in progesterone is correlated with a 20% to 23% increase in C-reactive protein levels.52,53 Women with inflammatory diseases (eg, gingivitis or irritable bowel syndrome) show worsening of symptoms prior to menstruation.54-56 One study found increased levels of proinflammatory markers in women with PMDD compared with controls.57
Putting together the 5 pieces of the puzzle
Because PMDD is heritable, it must have an underlying neurobiologic pathophysiology. Brain imaging studies show differences in structure and function in women with PMDD across the menstrual cycle. Conversion of progesterone to ALLO and the GABAergic influence of this metabolite is a topic of interest in current research. Similarly, the role of estrogen and its connection to serotonin and other neurotransmitters such as BDNF have been implicated.
The link between a history of stress, trauma, and PMDD raises the question of biologic resiliency and illness in these patients, as it connects to the HPA and HPG axis and production of inflammatory stress hormones and steroid hormones and their metabolites. PMDD can be conceptualized as variable sensitivity to hormonal response to stress,58 thus contextualizing biochemical and psychological resiliency.
Further research is needed to clarify the possibility of a shared pathophysiology between endocrine-related mood disorders such as postpartum depression (PPD) and PMDD because current research is controversial.59,60 In PPD, women who are exposed to high levels of progesterone and estrogen during pregnancy (just like in the mid-luteal phase) have a sudden drop in these hormones postpartum.
The ‘withdrawal theory.’ The affective symptoms of PMDD resolve almost instantaneously after the start of menstruation. Perhaps this type of immediate relief is akin to substance use disorders and symptoms of withdrawal. It could be that reinstatement of a certain amount of gonadal steroids in the follicular phase of the cycle diminishes a withdrawal-like response to these steroids.
Currently, the main leading theory is that PMDD is a result of “an abnormal response to normal hormonal changes.”61 A new study also has shown that the change in estradiol/progesterone levels (vs the steady state) was associated with PMDD symptoms.62 Thinking of PMDD as a disorder of withdrawal offers an alternative (yet complementary) perspective to the current theory: PMDD may be caused by the absence or diminishing of the above-named hormones and their metabolites in the late luteal phase (in the context of developed “tolerance” during the early- to mid-luteal phase).
Considering the interplay between neurotransmitters and neurosteroids, both a “serotonin withdrawal theory” (caused by a drop in steroid hormones) and a “GABAergic withdrawal theory” (due to the decline in progesterone) could be proposed. This theory would be supported by the fact that SSRIs seem to mitigate symptoms of PMDD as well as the genetic association between PMDD and ESR1. It is more than likely that the “withdrawal” is caused by the interactions between estrogen-serotonin, progesterone-ALLO, and GABA receptors, and the complementary fashion in which progesterone and estrogen influence each other.
1. Miller A, Vo H, Huo L, et al. Estrogen receptor alpha (ESR-1) associations with psychological traits in women with PMDD and controls. J Psychiatr Res. 2010;44(12):788-794.
2. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169(5):465-475.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Wilson CA, Turner CW, Keye WR Jr. Firstborn adolescent daughters and mothers with and without premenstrual syndrome: a comparison. J Adolesc Health. 1991;12(2):130-137.
5. Kendler KS, Silberg JL, Neale MC, et al. Genetic and environmental factors in the aetiology of menstrual, premenstrual and neurotic symptoms: a population-based twin study. Psychol Med. 1992;22(1):85-100.
6. Condon JT. The premenstrual syndrome: a twin study. Br J Psychiatry. 1993;162:481-486.
7. Kendler KS, Karkowski LM, Corey LA, et al. Longitudinal population-based twin study of retrospectively reported premenstrual symptoms and lifetime major depression. Am J Psychiatry. 1998;155(9):1234-1240.
8. Huo L, Straub RE, Roca C, et al. Risk for premenstrual dysphoric disorder is associated with genetic variation in ESR1, the estrogen receptor alpha gene. Biol Psychiatry. 2007;62(8):925-933.
9. Dhingra V, Magnay JL, O’Brien PM, et al. Serotonin receptor 1A C(-1019)G polymorphism associated with premenstrual dysphoric disorder. Obstet Gynecol. 2007;110(4):788-792.
10. Comasco E, Hahn A, Ganger S, et al. Emotional fronto-cingulate cortex activation and brain derived neurotrophic factor polymorphism in premenstrual dysphoric disorder. Hum Brain Mapp. 2014;35(9):4450-4458.
11. Praschak-Rieder N, Willeit M, Winkler D, et al. Role of family history and 5-HTTLPR polymorphism in female seasonal affective disorder patients with and without premenstrual dysphoric disorder. Eur Neuropsychopharmacol. 2002;12(2):129-134.
12. Klatzkin RR, Morrow AL, Light KC, et al. Associations of histories of depression and PMDD diagnosis with allopregnanolone concentrations following the oral administration of micronized progesterone. Psychoneuroendocrinology. 2006;31(10):1208-1219.
13. Crowley SK, Girdler SS. Neurosteroid, GABAergic and hypothalamic pituitary adrenal (HPA) axis regulation: what is the current state of knowledge in humans? Psychopharmacology (Berl). 2014;231(17):3619-3634.
14. Girdler SS, Straneva PA, Light KC, et al. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49(9):788-797.
15. Rapkin AJ, Morgan M, Goldman L, et al. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol. 1997;90(5):709-714.
16. Bicíková M, Dibbelt L, Hill M, et al. Allopregnanolone in women with premenstrual syndrome. Horm Metab Res. 1998;30(4):227-230.
17. Monteleone P, Luisi S, Tonetti A, et al. Allopregnanolone concentrations and premenstrual syndrome. Eur J Endocrinol. 2000;142(3):269-273.
18. Steiner M, Steinberg S, Stewart D, et al. Fluoxetine in the treatment of premenstrual dysphoria. Canadian Fluoxetine/Premenstrual Dysphoria Collaborative Study Group. N Engl J Med. 1995;332(23):1529-1534.
19. Sundström I, Bäckström T. Citalopram increases pregnanolone sensitivity in patients with premenstrual syndrome: an open trial. Psychoneuroendocrinology. 1998;23(1):73-88.
20. Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci U S A. 1999;96(23):13512-13517.
21. Trauger JW, Jiang A, Stearns BA, et al. Kinetics of allopregnanolone formation catalyzed by human 3 alpha-hydroxysteroid dehydrogenase type III (AKR1C2). Biochemistry. 2002;41(45):13451-13459.
22. Shanmugan S, Epperson CN. Estrogen and the prefrontal cortex: towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Hum Brain Mapp. 2014;35(3):847-865.
23. Rubinow DR, Schmidt PJ, Roca CA. Estrogen-serotonin interactions: implications for affective regulation. Biol Psychiatry. 1998;44(9):839-850.
24. Amin Z, Canli T, Epperson CN. Effect of estrogen-serotonin interactions on mood and cognition. Behav Cogn Neurosci Rev. 2005;4(1):43-58.
25. Cyr M, Bossé R, Di Paolo T. Gonadal hormones modulate 5-hydroxytryptamine2A receptors: emphasis on the rat frontal cortex. Neuroscience. 1998;83(3):829-836.
26. Fink G, Sumner BE, Rosie R, et al. Estrogen control of central neurotransmission: effect on mood, mental state, and memory. Cell Mol Neurobiol. 1996;16(3):325-344.
27. Sumner BE, Grant KE, Rosie R, et al. Effects of tamoxifen on serotonin transporter and 5-hydroxytryptamine(2A) receptor binding sites and mRNA levels in the brain of ovariectomized rats with or without acute estradiol replacement. Brain Res Mol Brain Res. 1999;73(1-2):119-128.
28. Moses-Kolko EL, Berga SL, Greer PJ, et al. Widespread increases of cortical serotonin type 2A receptor availability after hormone therapy in euthymic postmenopausal women. Fertil Steril. 2003;80(3):554-559.
29. Su TP, Schmidt PJ, Danaceau MA, et al. Fluoxetine in the treatment of premenstrual dysphoria. Neuropsychopharmacology. 1997;16(5):346-356.
30. Steinberg EM, Cardoso GM, Martinez PE, et al. Rapid response to fluoxetine in women with premenstrual dysphoric disorder. Depress Anxiety. 2012;29(6):531-540.
31. Roca CA, Schmidt PJ, Smith MJ, et al. Effects of metergoline on symptoms in women with premenstrual dysphoric disorder. Am J Psychiatry. 2002;159(11):1876-1881.
32. Gray JD, Milner TA, McEwen BS. Dynamic plasticity: the role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience. 2013;239:214-227.
33. Carbone DL, Handa RJ. Sex and stress hormone influences on the expression and activity of brain-derived neurotrophic factor. Neuroscience. 2013;239:295-303.
34. Pilar-Cuéllar F, Vidal R, Pazos A. Subchronic treatment with fluoxetine and ketanserin increases hippocampal brain-derived neurotrophic factor, β-catenin and antidepressant-like effects. Br J Pharmacol. 2012;165(4b):1046-1057.
35. Deuschle M, Gilles M, Scharnholz B, et al. Changes of serum concentrations of brain-derived neurotrophic factor (BDNF) during treatment with venlafaxine and mirtazapine: role of medication and response to treatment. Pharmacopsychiatry. 2013;46(2):54-58.
36. Berman SM, London ED, Morgan M, et al. Elevated gray matter volume of the emotional cerebellum in women with premenstrual dysphoric disorder. J Affect Disord. 2013;146(2):266-271.
37. Jeong HG, Ham BJ, Yeo HB, et al. Gray matter abnormalities in patients with premenstrual dysphoric disorder: an optimized voxel-based morphometry. J Affect Disord. 2012;140(3):260-267.
38. Protopopescu X, Tuescher O, Pan H, et al. Toward a functional neuroanatomy of premenstrual dysphoric disorder. J Affect Disord. 2008;108(1-2):87-94.
39. Gingnell M, Morell A, Bannbers E, et al. Menstrual cycle effects on amygdala reactivity to emotional stimulation in premenstrual dysphoric disorder. Horm Behav. 2012;62(4):400-406.
40. Epperson CN, Haga K, Mason GF, et al. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2002;59(9):851-858.
41. Gingnell M, Bannbers E, Wikström J, et al. Premenstrual dysphoric disorder and prefrontal reactivity during anticipation of emotional stimuli. Eur Neuropsychopharmacol. 2013;23(11):1474-1483.
42. Baller EB, Wei SM, Kohn PD, et al. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: a multimodal neuroimaging study. Am J Psychiatry. 2013;170(3):305-314.
43. Rasgon N, McGuire M, Tanavoli S, et al. Neuroendocrine response to an intravenous L-tryptophan challenge in women with premenstrual syndrome. Fertil Steril. 2000;73(1):144-149.
44. Huang Y, Zhou R, Wu M, et al. Premenstrual syndrome is associated with blunted cortisol reactivity to the TSST. Stress. 2015;18(2):160-168.
45. Segebladh B, Bannbers E, Moby L, et al. Allopregnanolone serum concentrations and diurnal cortisol secretion in women with premenstrual dysphoric disorder. Arch Womens Ment Health. 2013;16(2):131-137.
46. Pilver CE, Levy BR, Libby DJ, et al. Posttraumatic stress disorder and trauma characteristics are correlates of premenstrual dysphoric disorder. Arch Womens Ment Health. 2011;14(5):383-393.
47. Bertone-Johnson ER, Whitcomb BW, Missmer SA, et al. Early life emotional, physical, and sexual abuse and the development of premenstrual syndrome: a longitudinal study. J Womens Health (Larchmt). 2014;23(9):729-739.
48. Segebladh B, Bannbers E, Kask K, et al. Prevalence of violence exposure in women with premenstrual dysphoric disorder in comparison with other gynecological patients and asymptomatic controls. Acta Obstet Gynecol Scand. 2011;90(7):746-752.
49. Kask K, Gulinello M, Bäckström T, et al. Patients with premenstrual dysphoric disorder have increased startle response across both cycle phases and lower levels of prepulse inhibition during the late luteal phase of the menstrual cycle. Neuropsychopharmacology. 2008;33(9):2283-2290.
50. O’Brien SM, Fitzgerald P, Scully P, et al. Impact of gender and menstrual cycle phase on plasma cytokine concentrations. Neuroimmunomodulation. 2007;14(2):84-90.
51. Northoff H, Symons S, Zieker D, et al. Gender- and menstrual phase dependent regulation of inflammatory gene expression in response to aerobic exercise. Exerc Immunol Rev. 2008;14:86-103.
52. Gaskins AJ, Wilchesky M, Mumford SL, et al. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: the BioCycle Study. Am J Epidemiol. 2012;175(5):423-431.
53. Wander K, Brindle E, O’Connor KA. C-reactive protein across the menstrual cycle. Am J Phys Anthropol. 2008;136(2):138-146.
54. Jane ZY, Chang CC, Lin HK, et al. The association between the exacerbation of irritable bowel syndrome and menstrual symptoms in young Taiwanese women. Gastroenterol Nurs. 2011;34(4):277-286.
55. Kane SV, Sable K, Hanauer SB. The menstrual cycle and its effect on inflammatory bowel disease and irritable bowel syndrome: a prevalence study. Am J Gastroenterol. 1998;93(10):1867-1872.
56. Shourie V, Dwarakanath CD, Prashanth GV, et al. The effect of menstrual cycle on periodontal health - a clinical and microbiological study. Oral Health Prev Dent. 2012;10(2):185-192.
57. Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep. 2015;17(11):87.
58. Maeng LY, Milad MR. Sex differences in anxiety disorders: Interactions between fear, stress, and gonadal hormones. Horm Behav. 2015;76:106-117.
59. Lee YJ, Yi SW, Ju DH, et al. Correlation between postpartum depression and premenstrual dysphoric disorder: single center study. Obstet Gynecol Sci. 2015;58(5):353-358.
60. Kepple AL, Lee EE, Haq N, et al. History of postpartum depression in a clinic-based sample of women with premenstrual dysphoric disorder. J Clin Psychiatry. 2016;77(4):e415-e420.
61. Schmidt PJ, Nieman LK, Danaceau MA, et al. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. 1998;338(4):209-216.
62. Schmidt PJ, Martinez PE, Nieman LK, et al. Premenstrual dysphoric disorder symptoms following ovarian suppression: Triggered by change in ovarian steroid levels but not continuous stable levels. Am J Psychiatry. [published online April 21, 2017]. doi: 10.1176/appi.ajp.2017.16101113.
1. Miller A, Vo H, Huo L, et al. Estrogen receptor alpha (ESR-1) associations with psychological traits in women with PMDD and controls. J Psychiatr Res. 2010;44(12):788-794.
2. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169(5):465-475.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Wilson CA, Turner CW, Keye WR Jr. Firstborn adolescent daughters and mothers with and without premenstrual syndrome: a comparison. J Adolesc Health. 1991;12(2):130-137.
5. Kendler KS, Silberg JL, Neale MC, et al. Genetic and environmental factors in the aetiology of menstrual, premenstrual and neurotic symptoms: a population-based twin study. Psychol Med. 1992;22(1):85-100.
6. Condon JT. The premenstrual syndrome: a twin study. Br J Psychiatry. 1993;162:481-486.
7. Kendler KS, Karkowski LM, Corey LA, et al. Longitudinal population-based twin study of retrospectively reported premenstrual symptoms and lifetime major depression. Am J Psychiatry. 1998;155(9):1234-1240.
8. Huo L, Straub RE, Roca C, et al. Risk for premenstrual dysphoric disorder is associated with genetic variation in ESR1, the estrogen receptor alpha gene. Biol Psychiatry. 2007;62(8):925-933.
9. Dhingra V, Magnay JL, O’Brien PM, et al. Serotonin receptor 1A C(-1019)G polymorphism associated with premenstrual dysphoric disorder. Obstet Gynecol. 2007;110(4):788-792.
10. Comasco E, Hahn A, Ganger S, et al. Emotional fronto-cingulate cortex activation and brain derived neurotrophic factor polymorphism in premenstrual dysphoric disorder. Hum Brain Mapp. 2014;35(9):4450-4458.
11. Praschak-Rieder N, Willeit M, Winkler D, et al. Role of family history and 5-HTTLPR polymorphism in female seasonal affective disorder patients with and without premenstrual dysphoric disorder. Eur Neuropsychopharmacol. 2002;12(2):129-134.
12. Klatzkin RR, Morrow AL, Light KC, et al. Associations of histories of depression and PMDD diagnosis with allopregnanolone concentrations following the oral administration of micronized progesterone. Psychoneuroendocrinology. 2006;31(10):1208-1219.
13. Crowley SK, Girdler SS. Neurosteroid, GABAergic and hypothalamic pituitary adrenal (HPA) axis regulation: what is the current state of knowledge in humans? Psychopharmacology (Berl). 2014;231(17):3619-3634.
14. Girdler SS, Straneva PA, Light KC, et al. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49(9):788-797.
15. Rapkin AJ, Morgan M, Goldman L, et al. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol. 1997;90(5):709-714.
16. Bicíková M, Dibbelt L, Hill M, et al. Allopregnanolone in women with premenstrual syndrome. Horm Metab Res. 1998;30(4):227-230.
17. Monteleone P, Luisi S, Tonetti A, et al. Allopregnanolone concentrations and premenstrual syndrome. Eur J Endocrinol. 2000;142(3):269-273.
18. Steiner M, Steinberg S, Stewart D, et al. Fluoxetine in the treatment of premenstrual dysphoria. Canadian Fluoxetine/Premenstrual Dysphoria Collaborative Study Group. N Engl J Med. 1995;332(23):1529-1534.
19. Sundström I, Bäckström T. Citalopram increases pregnanolone sensitivity in patients with premenstrual syndrome: an open trial. Psychoneuroendocrinology. 1998;23(1):73-88.
20. Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci U S A. 1999;96(23):13512-13517.
21. Trauger JW, Jiang A, Stearns BA, et al. Kinetics of allopregnanolone formation catalyzed by human 3 alpha-hydroxysteroid dehydrogenase type III (AKR1C2). Biochemistry. 2002;41(45):13451-13459.
22. Shanmugan S, Epperson CN. Estrogen and the prefrontal cortex: towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Hum Brain Mapp. 2014;35(3):847-865.
23. Rubinow DR, Schmidt PJ, Roca CA. Estrogen-serotonin interactions: implications for affective regulation. Biol Psychiatry. 1998;44(9):839-850.
24. Amin Z, Canli T, Epperson CN. Effect of estrogen-serotonin interactions on mood and cognition. Behav Cogn Neurosci Rev. 2005;4(1):43-58.
25. Cyr M, Bossé R, Di Paolo T. Gonadal hormones modulate 5-hydroxytryptamine2A receptors: emphasis on the rat frontal cortex. Neuroscience. 1998;83(3):829-836.
26. Fink G, Sumner BE, Rosie R, et al. Estrogen control of central neurotransmission: effect on mood, mental state, and memory. Cell Mol Neurobiol. 1996;16(3):325-344.
27. Sumner BE, Grant KE, Rosie R, et al. Effects of tamoxifen on serotonin transporter and 5-hydroxytryptamine(2A) receptor binding sites and mRNA levels in the brain of ovariectomized rats with or without acute estradiol replacement. Brain Res Mol Brain Res. 1999;73(1-2):119-128.
28. Moses-Kolko EL, Berga SL, Greer PJ, et al. Widespread increases of cortical serotonin type 2A receptor availability after hormone therapy in euthymic postmenopausal women. Fertil Steril. 2003;80(3):554-559.
29. Su TP, Schmidt PJ, Danaceau MA, et al. Fluoxetine in the treatment of premenstrual dysphoria. Neuropsychopharmacology. 1997;16(5):346-356.
30. Steinberg EM, Cardoso GM, Martinez PE, et al. Rapid response to fluoxetine in women with premenstrual dysphoric disorder. Depress Anxiety. 2012;29(6):531-540.
31. Roca CA, Schmidt PJ, Smith MJ, et al. Effects of metergoline on symptoms in women with premenstrual dysphoric disorder. Am J Psychiatry. 2002;159(11):1876-1881.
32. Gray JD, Milner TA, McEwen BS. Dynamic plasticity: the role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience. 2013;239:214-227.
33. Carbone DL, Handa RJ. Sex and stress hormone influences on the expression and activity of brain-derived neurotrophic factor. Neuroscience. 2013;239:295-303.
34. Pilar-Cuéllar F, Vidal R, Pazos A. Subchronic treatment with fluoxetine and ketanserin increases hippocampal brain-derived neurotrophic factor, β-catenin and antidepressant-like effects. Br J Pharmacol. 2012;165(4b):1046-1057.
35. Deuschle M, Gilles M, Scharnholz B, et al. Changes of serum concentrations of brain-derived neurotrophic factor (BDNF) during treatment with venlafaxine and mirtazapine: role of medication and response to treatment. Pharmacopsychiatry. 2013;46(2):54-58.
36. Berman SM, London ED, Morgan M, et al. Elevated gray matter volume of the emotional cerebellum in women with premenstrual dysphoric disorder. J Affect Disord. 2013;146(2):266-271.
37. Jeong HG, Ham BJ, Yeo HB, et al. Gray matter abnormalities in patients with premenstrual dysphoric disorder: an optimized voxel-based morphometry. J Affect Disord. 2012;140(3):260-267.
38. Protopopescu X, Tuescher O, Pan H, et al. Toward a functional neuroanatomy of premenstrual dysphoric disorder. J Affect Disord. 2008;108(1-2):87-94.
39. Gingnell M, Morell A, Bannbers E, et al. Menstrual cycle effects on amygdala reactivity to emotional stimulation in premenstrual dysphoric disorder. Horm Behav. 2012;62(4):400-406.
40. Epperson CN, Haga K, Mason GF, et al. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2002;59(9):851-858.
41. Gingnell M, Bannbers E, Wikström J, et al. Premenstrual dysphoric disorder and prefrontal reactivity during anticipation of emotional stimuli. Eur Neuropsychopharmacol. 2013;23(11):1474-1483.
42. Baller EB, Wei SM, Kohn PD, et al. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: a multimodal neuroimaging study. Am J Psychiatry. 2013;170(3):305-314.
43. Rasgon N, McGuire M, Tanavoli S, et al. Neuroendocrine response to an intravenous L-tryptophan challenge in women with premenstrual syndrome. Fertil Steril. 2000;73(1):144-149.
44. Huang Y, Zhou R, Wu M, et al. Premenstrual syndrome is associated with blunted cortisol reactivity to the TSST. Stress. 2015;18(2):160-168.
45. Segebladh B, Bannbers E, Moby L, et al. Allopregnanolone serum concentrations and diurnal cortisol secretion in women with premenstrual dysphoric disorder. Arch Womens Ment Health. 2013;16(2):131-137.
46. Pilver CE, Levy BR, Libby DJ, et al. Posttraumatic stress disorder and trauma characteristics are correlates of premenstrual dysphoric disorder. Arch Womens Ment Health. 2011;14(5):383-393.
47. Bertone-Johnson ER, Whitcomb BW, Missmer SA, et al. Early life emotional, physical, and sexual abuse and the development of premenstrual syndrome: a longitudinal study. J Womens Health (Larchmt). 2014;23(9):729-739.
48. Segebladh B, Bannbers E, Kask K, et al. Prevalence of violence exposure in women with premenstrual dysphoric disorder in comparison with other gynecological patients and asymptomatic controls. Acta Obstet Gynecol Scand. 2011;90(7):746-752.
49. Kask K, Gulinello M, Bäckström T, et al. Patients with premenstrual dysphoric disorder have increased startle response across both cycle phases and lower levels of prepulse inhibition during the late luteal phase of the menstrual cycle. Neuropsychopharmacology. 2008;33(9):2283-2290.
50. O’Brien SM, Fitzgerald P, Scully P, et al. Impact of gender and menstrual cycle phase on plasma cytokine concentrations. Neuroimmunomodulation. 2007;14(2):84-90.
51. Northoff H, Symons S, Zieker D, et al. Gender- and menstrual phase dependent regulation of inflammatory gene expression in response to aerobic exercise. Exerc Immunol Rev. 2008;14:86-103.
52. Gaskins AJ, Wilchesky M, Mumford SL, et al. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: the BioCycle Study. Am J Epidemiol. 2012;175(5):423-431.
53. Wander K, Brindle E, O’Connor KA. C-reactive protein across the menstrual cycle. Am J Phys Anthropol. 2008;136(2):138-146.
54. Jane ZY, Chang CC, Lin HK, et al. The association between the exacerbation of irritable bowel syndrome and menstrual symptoms in young Taiwanese women. Gastroenterol Nurs. 2011;34(4):277-286.
55. Kane SV, Sable K, Hanauer SB. The menstrual cycle and its effect on inflammatory bowel disease and irritable bowel syndrome: a prevalence study. Am J Gastroenterol. 1998;93(10):1867-1872.
56. Shourie V, Dwarakanath CD, Prashanth GV, et al. The effect of menstrual cycle on periodontal health - a clinical and microbiological study. Oral Health Prev Dent. 2012;10(2):185-192.
57. Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep. 2015;17(11):87.
58. Maeng LY, Milad MR. Sex differences in anxiety disorders: Interactions between fear, stress, and gonadal hormones. Horm Behav. 2015;76:106-117.
59. Lee YJ, Yi SW, Ju DH, et al. Correlation between postpartum depression and premenstrual dysphoric disorder: single center study. Obstet Gynecol Sci. 2015;58(5):353-358.
60. Kepple AL, Lee EE, Haq N, et al. History of postpartum depression in a clinic-based sample of women with premenstrual dysphoric disorder. J Clin Psychiatry. 2016;77(4):e415-e420.
61. Schmidt PJ, Nieman LK, Danaceau MA, et al. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. 1998;338(4):209-216.
62. Schmidt PJ, Martinez PE, Nieman LK, et al. Premenstrual dysphoric disorder symptoms following ovarian suppression: Triggered by change in ovarian steroid levels but not continuous stable levels. Am J Psychiatry. [published online April 21, 2017]. doi: 10.1176/appi.ajp.2017.16101113.
Premenstrual dysphoric disorder
Anxiety disorders in children and adolescents
How to preserve your own well-being in a challenging medical environment
Like all physicians, psychiatrists practice in an increasingly complex health care environment, with escalating demands for productivity, rising threats of malpractice, expanding clinical oversight, and growing concerns about income. Additionally, psychiatric practice presents its own challenges, including limited resources and concerns about patient violence and suicide. These concerns can make it difficult to establish a healthy work–life balance.
Physicians, including psychiatrists, are at risk for alcohol or substance abuse/dependency, burnout, and suicide. As psychiatrists, we need to attend to our own personal and professional health so that we can best help our patients. This review focuses on the challenges psychiatrists face that can adversely affect their well-being and offers strategies to reduce the risk of burnout and enhance wellness.
The challenges of medicine and their impact on psychiatrists
The practice of medicine is inherently challenging. It requires hard work, discipline, dedication, and faithfulness to high ethical standards. Additional challenges include declining autonomy and opportunities for social support, increasing accountability, and a growing interest in reducing the cost of care by employing more non-physician health professionals—which in psychiatry typically include psychologists, nurse practitioners, and social workers. The uncertainty of the Affordable Care Act, declining income, and concerns about the nature of future medical practice are also stressors.1,2
Factors that contribute to psychiatrists’ stress include:
- limited resources
- concerns about patient violence and suicide
- crowded inpatient units
- changing culture in mental health services
- high work demands
- poorly defined roles of consultants
- declining authority
- frustration with the inability to impact systemic change
- conflict between responsibility toward employers vs the patient
- isolation.3
Concern about patient suicide is a significant stressor.4,5 Some evidence suggests that the impact of a patient’s suicide on a physician is more severe when it occurs during training than after graduation and is inversely correlated with the clinician’s perceived social integration into their professional network.5
Impediments to a physician’s well-being
Alcohol abuse/dependence. Approximately 13% of male physicians and 21% of female physicians meet Alcohol Use Disorders Identification Test Version C criteria for alcohol abuse or dependence, according to a study of approximately 7,300 U.S. physicians from all specialties.6 (In this study, prescription drug abuse and use of illicit drugs were rare.) Age, hours worked, male sex, being married or partnered, having children, and being in a specialty other than internal medicine were independently associated with alcohol abuse or dependence.
Fortunately, psychiatrists were among the specialties with below average likelihood to meet diagnostic criteria for alcohol abuse/dependency.6 However, alcohol abuse or dependency was associated with burnout, depression, suicidal ideation, lower quality of life, lower career satisfaction, and medical errors.
Burnout is a long-term stress reaction consisting of:
- physical and emotional exhaustion (feeling depleted)
- depersonalization (cynicism, lack of engagement with or negative attitudes toward patients)
- reduced sense of personal accomplishment (lack of a sense of purpose).7
In a 2017 survey of >14,000 U.S. physicians from 27 specialties, 42% of psychiatrists reported burnout.8 In another survey of approximately 300 resident physicians across all specialties in a tertiary academic hospital, 69% met criteria for burnout.9 This condition affects resident physicians as well as those in practice. Residents and program directors cited a lack of work–life balance and feeling unappreciated as factors contributing to burnout.
Among physicians, factors that contribute to burnout include loss of autonomy, diminished status as physicians, and increased work pressures. Burnout has a negative impact on both patients and health care systems. It is associated with an increased risk of depression and can contribute to:
- broken relationships
- alcohol abuse
- physician suicide
- decreased quality of care, including patient safety and satisfaction
- increased risk of malpractice suits
- reduced patient adherence to medical recommendations.5,10-12
Physicians who embrace medicine as a calling (ie, committing one’s life to personally meaningful work that serves a prosocial purpose) experience less burnout. According to a survey of approximately 900 primary care physicians and 300 psychiatrists, 42% of psychiatrists strongly agreed that medicine is a calling.13 Overall, physicians with a high sense of calling reported less burnout than those with a lower sense of calling (17% vs 31%, respectively).13
Depression and suicide. Gold et al12 analyzed a database that included information on approximately 31,600 adult suicide victims, and 203 of these victims were physicians. Compared with others, physicians were more likely to have a diagnosed mental illness or an occupation-related problem that contributed to suicide. Toxicology results also showed that physician suicide victims were significantly more likely than non-physician victims to test positive for benzodiazepines and barbiturates, but not antidepressants, which suggests that physicians with depression may not have been receiving adequate treatment.12
Although occupation-related stress and inadequate mental health treatment may be modifiable risk factors to reduce suicide deaths among physicians, stigma and fear of medical staff and licensure issues may deter physicians from seeking treatment.14
Steps to avoid burnout
Evidence-based interventions. There is limited evidence-based data regarding specific interventions for preventing burnout and reducing stress among physicians, particularly among psychiatrists.4
A randomized controlled trial of 74 practicing physicians at the Mayo Clinic in Rochester, Minnesota, evaluated the effectiveness of 19 biweekly physician-facilitated discussion groups.15 The groups covered topics such as elements of mindfulness, reflection, shared experience, and small-group learning. The institution provided 1 hour of paid time every other week for physicians to participate in this program. Physicians in the control group could schedule and use this time as they chose. Researchers also collected data on 350 non-trial participants.
The proportion of participants who strongly agreed that their work was meaningful increased 6.3% in the intervention group but decreased 6.3% in the control group and 13.4% among non-trial participants (P = .04).15 Rates of depersonalization, emotional exhaustion, and overall burnout decreased substantially in the intervention group, decreased slightly in the control group, and increased in the non-trial cohort. Results were sustained at 12 months after the study. There were no statistically significant differences in stress, symptoms of depression, overall quality of life, or job satisfaction.15
Preliminary evidence suggests that residents and fellows would find a wellness or suicide prevention program helpful. One study found that the use of one such program, which provided individual counseling, psychiatric evaluation, and wellness workshops for residents, fellows, and faculty in an academic health center, increased from 5% to 25% of eligible participants, and participants reported high levels of satisfaction with the program.16 Such programs would require institutional support for space and clinical staff.15
Empathy. As psychiatrists, we are taught to be empathetic. Yet, with the numerous challenges we face, it is not always easy. Stressors such as an increased workload or burnout can adversely affect a psychiatrist’s ability to provide empathetic care.17 However, empathetic treatment has clear benefits for both physicians and patients. Empathic skills can lead to more professional satisfaction and outcomes, which are important components of accountability, and can:
- promote patient satisfaction
- establish trust
- reduce anxiety
- increase adherence to treatment regimens
- improve health outcomes
- decrease the likelihood of malpractice suits.17
Mindfulness is a “flexible state of mind in which we are actively engaged in the present, noticing new things and sensitive to context.”18,19 It may sound mundane to cling to phrases such as “living in the present,” but mindfulness can be a valuable tool for psychiatrists who struggle to maintain well-being in medicine’s challenging milieu. The process of mindfulness—actively drawing distinctions and noticing new things, “seeing the familiar in the novel and the novel in the familiar”—can ensure that we have active minds, that we are involved, and that we are capturing the joy of living in the stimulating present.18
Focus on issues you can control
Many of the factors that negatively influence professional satisfaction and well-being, such as loss of autonomy, demand for increased patient care volume, and increasing scrutiny on the quality of care, are beyond a psychiatrist’s control. Medical administrators can help reduce some of these issues by increasing physician autonomy, offering physicians the opportunity to work part-time, offering medical staff workshops to enhance positive communication, or addressing leadership problems. However, psychiatrists may benefit most by identifying modifiable issues under their own control, such as prioritizing a work–life balance, applying the fundamentals of a health prevention strategy to their own lives (Box20,21), approaching medicine as a calling, embracing an empathetic approach to patient care, and bringing mindfulness to medical practice.
1. Goitein L. Physician well-being: addressing downstream effects, but looking upstream. JAMA Intern Med. 2014;174(4):533-534.
2. Dunn PM, Arnetz BB, Christensen JF, et al. Meeting the imperative to improve physician well-being: assessment of an innovative program. J Gen Intern Med. 2007;22(11):1544-1552.
3. Kumar S. Burnout in psychiatrists. World Psychiatry. 2007;6(3):186-189.
4. Fothergill A, Edwards D, Burnard P. Stress, burnout, coping and stress management in psychiatrists: findings from a systematic review. Int J Soc Psychiatry. 2004;50(1):54-65.
5. Ruskin R, Sakinofsky I, Bagby RM, et al. Impact of patient suicide on psychiatrists and psychiatric trainees. Acad Psychiatry. 2004;28(2):104-110.
6. Oreskovich MR, Shanafelt T, Dyrbye LN, et al. The prevalence of substance use disorders in American physicians. Am J Addict. 2015;24(1):30-38.
7. Maslach C, Jackson SE. The measurement of experienced burnout. J Occup Behav. 1981;2:99-113.
8. Peckham C. Medscape Psychiatrist Lifestyle Report 2017: race and ethnicity, bias and burnout. http://www.medscape.com/features/slideshow/lifestyle/2017/psychiatry#page=1. Published January 11, 2017. Accessed July 25, 2017.
9. Holmes EG, Connolly A, Putnam KT, et al. Taking care of our own: a multispecialty study of resident and program director perspectives on contributors to burnout and potential interventions. Acad Psychiatry. 2017;41(2):159-166.
10. Shanafelt TD, Noseworthy JH. Executive leadership and physician well-being: nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92(1):129-146.
11. Gold KJ, Sen A, Schwenk TL. Details on suicide among US physicians: data from the National Violent Death Reporting System. Gen Hosp Psychiatry. 2013;35(1):45-49.
12. Gold MS, Frost-Pineda K, Melker RJ. Physician suicide and drug abuse. Am J Psychiatry. 2005;162:1390; author reply 1390.
13. Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41(2):167-173.
14. Gold KJ, Andrew LB, Goldman EB, et al. “I would never want to have a mental health diagnosis on my record”: a survey of female physicians on mental health diagnosis, treatment, and reporting. Gen Hosp Psychiatry. 2016;43:51-57.
15. West CP, Dyrbye LN, Rabatin JT, et al. Intervention to promote physician well-being, job satisfaction, and professionalism: a randomized clinical trial. JAMA Intern Med. 2014;174(4):527-533.
16. Ey S, Moffit M, Kinzie JM, et al. Feasibility of a comprehensive wellness and suicide prevention program: a decade of caring for physicians in training and practice. J Grad Med Educ. 2016;8(5):747-753.
17. Newton BW. Walking a fine line: is it possible to remain an empathic physician and have a hardened heart? Front Hum Neurosci. 2013;7:233.
18. Langer EJ. Mindful learning: current directions in psychological science. Am Psychological Society. 2000(6);9:220-223.
19. Crum AJ, Langer EJ. Mind-set matters: exercise and the placebo effect. Psychol Sci. 2007;18(2):165-171.
20. U.S. Department of Health & Human Services, Office of the Surgeon General. National Prevention Strategy. https://www.surgeongeneral.gov/priorities/prevention/strategy/report.pdf. Published June 2011. Accessed July 26, 2017.
21. Benjamin RM. The national prevention strategy: shifting the nation’s health-care system. Public Health Rep. 2011;126(6):774-776.
Like all physicians, psychiatrists practice in an increasingly complex health care environment, with escalating demands for productivity, rising threats of malpractice, expanding clinical oversight, and growing concerns about income. Additionally, psychiatric practice presents its own challenges, including limited resources and concerns about patient violence and suicide. These concerns can make it difficult to establish a healthy work–life balance.
Physicians, including psychiatrists, are at risk for alcohol or substance abuse/dependency, burnout, and suicide. As psychiatrists, we need to attend to our own personal and professional health so that we can best help our patients. This review focuses on the challenges psychiatrists face that can adversely affect their well-being and offers strategies to reduce the risk of burnout and enhance wellness.
The challenges of medicine and their impact on psychiatrists
The practice of medicine is inherently challenging. It requires hard work, discipline, dedication, and faithfulness to high ethical standards. Additional challenges include declining autonomy and opportunities for social support, increasing accountability, and a growing interest in reducing the cost of care by employing more non-physician health professionals—which in psychiatry typically include psychologists, nurse practitioners, and social workers. The uncertainty of the Affordable Care Act, declining income, and concerns about the nature of future medical practice are also stressors.1,2
Factors that contribute to psychiatrists’ stress include:
- limited resources
- concerns about patient violence and suicide
- crowded inpatient units
- changing culture in mental health services
- high work demands
- poorly defined roles of consultants
- declining authority
- frustration with the inability to impact systemic change
- conflict between responsibility toward employers vs the patient
- isolation.3
Concern about patient suicide is a significant stressor.4,5 Some evidence suggests that the impact of a patient’s suicide on a physician is more severe when it occurs during training than after graduation and is inversely correlated with the clinician’s perceived social integration into their professional network.5
Impediments to a physician’s well-being
Alcohol abuse/dependence. Approximately 13% of male physicians and 21% of female physicians meet Alcohol Use Disorders Identification Test Version C criteria for alcohol abuse or dependence, according to a study of approximately 7,300 U.S. physicians from all specialties.6 (In this study, prescription drug abuse and use of illicit drugs were rare.) Age, hours worked, male sex, being married or partnered, having children, and being in a specialty other than internal medicine were independently associated with alcohol abuse or dependence.
Fortunately, psychiatrists were among the specialties with below average likelihood to meet diagnostic criteria for alcohol abuse/dependency.6 However, alcohol abuse or dependency was associated with burnout, depression, suicidal ideation, lower quality of life, lower career satisfaction, and medical errors.
Burnout is a long-term stress reaction consisting of:
- physical and emotional exhaustion (feeling depleted)
- depersonalization (cynicism, lack of engagement with or negative attitudes toward patients)
- reduced sense of personal accomplishment (lack of a sense of purpose).7
In a 2017 survey of >14,000 U.S. physicians from 27 specialties, 42% of psychiatrists reported burnout.8 In another survey of approximately 300 resident physicians across all specialties in a tertiary academic hospital, 69% met criteria for burnout.9 This condition affects resident physicians as well as those in practice. Residents and program directors cited a lack of work–life balance and feeling unappreciated as factors contributing to burnout.
Among physicians, factors that contribute to burnout include loss of autonomy, diminished status as physicians, and increased work pressures. Burnout has a negative impact on both patients and health care systems. It is associated with an increased risk of depression and can contribute to:
- broken relationships
- alcohol abuse
- physician suicide
- decreased quality of care, including patient safety and satisfaction
- increased risk of malpractice suits
- reduced patient adherence to medical recommendations.5,10-12
Physicians who embrace medicine as a calling (ie, committing one’s life to personally meaningful work that serves a prosocial purpose) experience less burnout. According to a survey of approximately 900 primary care physicians and 300 psychiatrists, 42% of psychiatrists strongly agreed that medicine is a calling.13 Overall, physicians with a high sense of calling reported less burnout than those with a lower sense of calling (17% vs 31%, respectively).13
Depression and suicide. Gold et al12 analyzed a database that included information on approximately 31,600 adult suicide victims, and 203 of these victims were physicians. Compared with others, physicians were more likely to have a diagnosed mental illness or an occupation-related problem that contributed to suicide. Toxicology results also showed that physician suicide victims were significantly more likely than non-physician victims to test positive for benzodiazepines and barbiturates, but not antidepressants, which suggests that physicians with depression may not have been receiving adequate treatment.12
Although occupation-related stress and inadequate mental health treatment may be modifiable risk factors to reduce suicide deaths among physicians, stigma and fear of medical staff and licensure issues may deter physicians from seeking treatment.14
Steps to avoid burnout
Evidence-based interventions. There is limited evidence-based data regarding specific interventions for preventing burnout and reducing stress among physicians, particularly among psychiatrists.4
A randomized controlled trial of 74 practicing physicians at the Mayo Clinic in Rochester, Minnesota, evaluated the effectiveness of 19 biweekly physician-facilitated discussion groups.15 The groups covered topics such as elements of mindfulness, reflection, shared experience, and small-group learning. The institution provided 1 hour of paid time every other week for physicians to participate in this program. Physicians in the control group could schedule and use this time as they chose. Researchers also collected data on 350 non-trial participants.
The proportion of participants who strongly agreed that their work was meaningful increased 6.3% in the intervention group but decreased 6.3% in the control group and 13.4% among non-trial participants (P = .04).15 Rates of depersonalization, emotional exhaustion, and overall burnout decreased substantially in the intervention group, decreased slightly in the control group, and increased in the non-trial cohort. Results were sustained at 12 months after the study. There were no statistically significant differences in stress, symptoms of depression, overall quality of life, or job satisfaction.15
Preliminary evidence suggests that residents and fellows would find a wellness or suicide prevention program helpful. One study found that the use of one such program, which provided individual counseling, psychiatric evaluation, and wellness workshops for residents, fellows, and faculty in an academic health center, increased from 5% to 25% of eligible participants, and participants reported high levels of satisfaction with the program.16 Such programs would require institutional support for space and clinical staff.15
Empathy. As psychiatrists, we are taught to be empathetic. Yet, with the numerous challenges we face, it is not always easy. Stressors such as an increased workload or burnout can adversely affect a psychiatrist’s ability to provide empathetic care.17 However, empathetic treatment has clear benefits for both physicians and patients. Empathic skills can lead to more professional satisfaction and outcomes, which are important components of accountability, and can:
- promote patient satisfaction
- establish trust
- reduce anxiety
- increase adherence to treatment regimens
- improve health outcomes
- decrease the likelihood of malpractice suits.17
Mindfulness is a “flexible state of mind in which we are actively engaged in the present, noticing new things and sensitive to context.”18,19 It may sound mundane to cling to phrases such as “living in the present,” but mindfulness can be a valuable tool for psychiatrists who struggle to maintain well-being in medicine’s challenging milieu. The process of mindfulness—actively drawing distinctions and noticing new things, “seeing the familiar in the novel and the novel in the familiar”—can ensure that we have active minds, that we are involved, and that we are capturing the joy of living in the stimulating present.18
Focus on issues you can control
Many of the factors that negatively influence professional satisfaction and well-being, such as loss of autonomy, demand for increased patient care volume, and increasing scrutiny on the quality of care, are beyond a psychiatrist’s control. Medical administrators can help reduce some of these issues by increasing physician autonomy, offering physicians the opportunity to work part-time, offering medical staff workshops to enhance positive communication, or addressing leadership problems. However, psychiatrists may benefit most by identifying modifiable issues under their own control, such as prioritizing a work–life balance, applying the fundamentals of a health prevention strategy to their own lives (Box20,21), approaching medicine as a calling, embracing an empathetic approach to patient care, and bringing mindfulness to medical practice.

Like all physicians, psychiatrists practice in an increasingly complex health care environment, with escalating demands for productivity, rising threats of malpractice, expanding clinical oversight, and growing concerns about income. Additionally, psychiatric practice presents its own challenges, including limited resources and concerns about patient violence and suicide. These concerns can make it difficult to establish a healthy work–life balance.
Physicians, including psychiatrists, are at risk for alcohol or substance abuse/dependency, burnout, and suicide. As psychiatrists, we need to attend to our own personal and professional health so that we can best help our patients. This review focuses on the challenges psychiatrists face that can adversely affect their well-being and offers strategies to reduce the risk of burnout and enhance wellness.
The challenges of medicine and their impact on psychiatrists
The practice of medicine is inherently challenging. It requires hard work, discipline, dedication, and faithfulness to high ethical standards. Additional challenges include declining autonomy and opportunities for social support, increasing accountability, and a growing interest in reducing the cost of care by employing more non-physician health professionals—which in psychiatry typically include psychologists, nurse practitioners, and social workers. The uncertainty of the Affordable Care Act, declining income, and concerns about the nature of future medical practice are also stressors.1,2
Factors that contribute to psychiatrists’ stress include:
- limited resources
- concerns about patient violence and suicide
- crowded inpatient units
- changing culture in mental health services
- high work demands
- poorly defined roles of consultants
- declining authority
- frustration with the inability to impact systemic change
- conflict between responsibility toward employers vs the patient
- isolation.3
Concern about patient suicide is a significant stressor.4,5 Some evidence suggests that the impact of a patient’s suicide on a physician is more severe when it occurs during training than after graduation and is inversely correlated with the clinician’s perceived social integration into their professional network.5
Impediments to a physician’s well-being
Alcohol abuse/dependence. Approximately 13% of male physicians and 21% of female physicians meet Alcohol Use Disorders Identification Test Version C criteria for alcohol abuse or dependence, according to a study of approximately 7,300 U.S. physicians from all specialties.6 (In this study, prescription drug abuse and use of illicit drugs were rare.) Age, hours worked, male sex, being married or partnered, having children, and being in a specialty other than internal medicine were independently associated with alcohol abuse or dependence.
Fortunately, psychiatrists were among the specialties with below average likelihood to meet diagnostic criteria for alcohol abuse/dependency.6 However, alcohol abuse or dependency was associated with burnout, depression, suicidal ideation, lower quality of life, lower career satisfaction, and medical errors.
Burnout is a long-term stress reaction consisting of:
- physical and emotional exhaustion (feeling depleted)
- depersonalization (cynicism, lack of engagement with or negative attitudes toward patients)
- reduced sense of personal accomplishment (lack of a sense of purpose).7
In a 2017 survey of >14,000 U.S. physicians from 27 specialties, 42% of psychiatrists reported burnout.8 In another survey of approximately 300 resident physicians across all specialties in a tertiary academic hospital, 69% met criteria for burnout.9 This condition affects resident physicians as well as those in practice. Residents and program directors cited a lack of work–life balance and feeling unappreciated as factors contributing to burnout.
Among physicians, factors that contribute to burnout include loss of autonomy, diminished status as physicians, and increased work pressures. Burnout has a negative impact on both patients and health care systems. It is associated with an increased risk of depression and can contribute to:
- broken relationships
- alcohol abuse
- physician suicide
- decreased quality of care, including patient safety and satisfaction
- increased risk of malpractice suits
- reduced patient adherence to medical recommendations.5,10-12
Physicians who embrace medicine as a calling (ie, committing one’s life to personally meaningful work that serves a prosocial purpose) experience less burnout. According to a survey of approximately 900 primary care physicians and 300 psychiatrists, 42% of psychiatrists strongly agreed that medicine is a calling.13 Overall, physicians with a high sense of calling reported less burnout than those with a lower sense of calling (17% vs 31%, respectively).13
Depression and suicide. Gold et al12 analyzed a database that included information on approximately 31,600 adult suicide victims, and 203 of these victims were physicians. Compared with others, physicians were more likely to have a diagnosed mental illness or an occupation-related problem that contributed to suicide. Toxicology results also showed that physician suicide victims were significantly more likely than non-physician victims to test positive for benzodiazepines and barbiturates, but not antidepressants, which suggests that physicians with depression may not have been receiving adequate treatment.12
Although occupation-related stress and inadequate mental health treatment may be modifiable risk factors to reduce suicide deaths among physicians, stigma and fear of medical staff and licensure issues may deter physicians from seeking treatment.14
Steps to avoid burnout
Evidence-based interventions. There is limited evidence-based data regarding specific interventions for preventing burnout and reducing stress among physicians, particularly among psychiatrists.4
A randomized controlled trial of 74 practicing physicians at the Mayo Clinic in Rochester, Minnesota, evaluated the effectiveness of 19 biweekly physician-facilitated discussion groups.15 The groups covered topics such as elements of mindfulness, reflection, shared experience, and small-group learning. The institution provided 1 hour of paid time every other week for physicians to participate in this program. Physicians in the control group could schedule and use this time as they chose. Researchers also collected data on 350 non-trial participants.
The proportion of participants who strongly agreed that their work was meaningful increased 6.3% in the intervention group but decreased 6.3% in the control group and 13.4% among non-trial participants (P = .04).15 Rates of depersonalization, emotional exhaustion, and overall burnout decreased substantially in the intervention group, decreased slightly in the control group, and increased in the non-trial cohort. Results were sustained at 12 months after the study. There were no statistically significant differences in stress, symptoms of depression, overall quality of life, or job satisfaction.15
Preliminary evidence suggests that residents and fellows would find a wellness or suicide prevention program helpful. One study found that the use of one such program, which provided individual counseling, psychiatric evaluation, and wellness workshops for residents, fellows, and faculty in an academic health center, increased from 5% to 25% of eligible participants, and participants reported high levels of satisfaction with the program.16 Such programs would require institutional support for space and clinical staff.15
Empathy. As psychiatrists, we are taught to be empathetic. Yet, with the numerous challenges we face, it is not always easy. Stressors such as an increased workload or burnout can adversely affect a psychiatrist’s ability to provide empathetic care.17 However, empathetic treatment has clear benefits for both physicians and patients. Empathic skills can lead to more professional satisfaction and outcomes, which are important components of accountability, and can:
- promote patient satisfaction
- establish trust
- reduce anxiety
- increase adherence to treatment regimens
- improve health outcomes
- decrease the likelihood of malpractice suits.17
Mindfulness is a “flexible state of mind in which we are actively engaged in the present, noticing new things and sensitive to context.”18,19 It may sound mundane to cling to phrases such as “living in the present,” but mindfulness can be a valuable tool for psychiatrists who struggle to maintain well-being in medicine’s challenging milieu. The process of mindfulness—actively drawing distinctions and noticing new things, “seeing the familiar in the novel and the novel in the familiar”—can ensure that we have active minds, that we are involved, and that we are capturing the joy of living in the stimulating present.18
Focus on issues you can control
Many of the factors that negatively influence professional satisfaction and well-being, such as loss of autonomy, demand for increased patient care volume, and increasing scrutiny on the quality of care, are beyond a psychiatrist’s control. Medical administrators can help reduce some of these issues by increasing physician autonomy, offering physicians the opportunity to work part-time, offering medical staff workshops to enhance positive communication, or addressing leadership problems. However, psychiatrists may benefit most by identifying modifiable issues under their own control, such as prioritizing a work–life balance, applying the fundamentals of a health prevention strategy to their own lives (Box20,21), approaching medicine as a calling, embracing an empathetic approach to patient care, and bringing mindfulness to medical practice.

1. Goitein L. Physician well-being: addressing downstream effects, but looking upstream. JAMA Intern Med. 2014;174(4):533-534.
2. Dunn PM, Arnetz BB, Christensen JF, et al. Meeting the imperative to improve physician well-being: assessment of an innovative program. J Gen Intern Med. 2007;22(11):1544-1552.
3. Kumar S. Burnout in psychiatrists. World Psychiatry. 2007;6(3):186-189.
4. Fothergill A, Edwards D, Burnard P. Stress, burnout, coping and stress management in psychiatrists: findings from a systematic review. Int J Soc Psychiatry. 2004;50(1):54-65.
5. Ruskin R, Sakinofsky I, Bagby RM, et al. Impact of patient suicide on psychiatrists and psychiatric trainees. Acad Psychiatry. 2004;28(2):104-110.
6. Oreskovich MR, Shanafelt T, Dyrbye LN, et al. The prevalence of substance use disorders in American physicians. Am J Addict. 2015;24(1):30-38.
7. Maslach C, Jackson SE. The measurement of experienced burnout. J Occup Behav. 1981;2:99-113.
8. Peckham C. Medscape Psychiatrist Lifestyle Report 2017: race and ethnicity, bias and burnout. http://www.medscape.com/features/slideshow/lifestyle/2017/psychiatry#page=1. Published January 11, 2017. Accessed July 25, 2017.
9. Holmes EG, Connolly A, Putnam KT, et al. Taking care of our own: a multispecialty study of resident and program director perspectives on contributors to burnout and potential interventions. Acad Psychiatry. 2017;41(2):159-166.
10. Shanafelt TD, Noseworthy JH. Executive leadership and physician well-being: nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92(1):129-146.
11. Gold KJ, Sen A, Schwenk TL. Details on suicide among US physicians: data from the National Violent Death Reporting System. Gen Hosp Psychiatry. 2013;35(1):45-49.
12. Gold MS, Frost-Pineda K, Melker RJ. Physician suicide and drug abuse. Am J Psychiatry. 2005;162:1390; author reply 1390.
13. Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41(2):167-173.
14. Gold KJ, Andrew LB, Goldman EB, et al. “I would never want to have a mental health diagnosis on my record”: a survey of female physicians on mental health diagnosis, treatment, and reporting. Gen Hosp Psychiatry. 2016;43:51-57.
15. West CP, Dyrbye LN, Rabatin JT, et al. Intervention to promote physician well-being, job satisfaction, and professionalism: a randomized clinical trial. JAMA Intern Med. 2014;174(4):527-533.
16. Ey S, Moffit M, Kinzie JM, et al. Feasibility of a comprehensive wellness and suicide prevention program: a decade of caring for physicians in training and practice. J Grad Med Educ. 2016;8(5):747-753.
17. Newton BW. Walking a fine line: is it possible to remain an empathic physician and have a hardened heart? Front Hum Neurosci. 2013;7:233.
18. Langer EJ. Mindful learning: current directions in psychological science. Am Psychological Society. 2000(6);9:220-223.
19. Crum AJ, Langer EJ. Mind-set matters: exercise and the placebo effect. Psychol Sci. 2007;18(2):165-171.
20. U.S. Department of Health & Human Services, Office of the Surgeon General. National Prevention Strategy. https://www.surgeongeneral.gov/priorities/prevention/strategy/report.pdf. Published June 2011. Accessed July 26, 2017.
21. Benjamin RM. The national prevention strategy: shifting the nation’s health-care system. Public Health Rep. 2011;126(6):774-776.
1. Goitein L. Physician well-being: addressing downstream effects, but looking upstream. JAMA Intern Med. 2014;174(4):533-534.
2. Dunn PM, Arnetz BB, Christensen JF, et al. Meeting the imperative to improve physician well-being: assessment of an innovative program. J Gen Intern Med. 2007;22(11):1544-1552.
3. Kumar S. Burnout in psychiatrists. World Psychiatry. 2007;6(3):186-189.
4. Fothergill A, Edwards D, Burnard P. Stress, burnout, coping and stress management in psychiatrists: findings from a systematic review. Int J Soc Psychiatry. 2004;50(1):54-65.
5. Ruskin R, Sakinofsky I, Bagby RM, et al. Impact of patient suicide on psychiatrists and psychiatric trainees. Acad Psychiatry. 2004;28(2):104-110.
6. Oreskovich MR, Shanafelt T, Dyrbye LN, et al. The prevalence of substance use disorders in American physicians. Am J Addict. 2015;24(1):30-38.
7. Maslach C, Jackson SE. The measurement of experienced burnout. J Occup Behav. 1981;2:99-113.
8. Peckham C. Medscape Psychiatrist Lifestyle Report 2017: race and ethnicity, bias and burnout. http://www.medscape.com/features/slideshow/lifestyle/2017/psychiatry#page=1. Published January 11, 2017. Accessed July 25, 2017.
9. Holmes EG, Connolly A, Putnam KT, et al. Taking care of our own: a multispecialty study of resident and program director perspectives on contributors to burnout and potential interventions. Acad Psychiatry. 2017;41(2):159-166.
10. Shanafelt TD, Noseworthy JH. Executive leadership and physician well-being: nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92(1):129-146.
11. Gold KJ, Sen A, Schwenk TL. Details on suicide among US physicians: data from the National Violent Death Reporting System. Gen Hosp Psychiatry. 2013;35(1):45-49.
12. Gold MS, Frost-Pineda K, Melker RJ. Physician suicide and drug abuse. Am J Psychiatry. 2005;162:1390; author reply 1390.
13. Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41(2):167-173.
14. Gold KJ, Andrew LB, Goldman EB, et al. “I would never want to have a mental health diagnosis on my record”: a survey of female physicians on mental health diagnosis, treatment, and reporting. Gen Hosp Psychiatry. 2016;43:51-57.
15. West CP, Dyrbye LN, Rabatin JT, et al. Intervention to promote physician well-being, job satisfaction, and professionalism: a randomized clinical trial. JAMA Intern Med. 2014;174(4):527-533.
16. Ey S, Moffit M, Kinzie JM, et al. Feasibility of a comprehensive wellness and suicide prevention program: a decade of caring for physicians in training and practice. J Grad Med Educ. 2016;8(5):747-753.
17. Newton BW. Walking a fine line: is it possible to remain an empathic physician and have a hardened heart? Front Hum Neurosci. 2013;7:233.
18. Langer EJ. Mindful learning: current directions in psychological science. Am Psychological Society. 2000(6);9:220-223.
19. Crum AJ, Langer EJ. Mind-set matters: exercise and the placebo effect. Psychol Sci. 2007;18(2):165-171.
20. U.S. Department of Health & Human Services, Office of the Surgeon General. National Prevention Strategy. https://www.surgeongeneral.gov/priorities/prevention/strategy/report.pdf. Published June 2011. Accessed July 26, 2017.
21. Benjamin RM. The national prevention strategy: shifting the nation’s health-care system. Public Health Rep. 2011;126(6):774-776.
Advancing clinical neuroscience literacy among psychiatric practitioners
An abundance of recent neuroscience advances is directly related to psychiatric disorders, because the primary mission of the brain is to generate a mind, and every new discovery provides another piece of the psychiatric disorders puzzle. The time also is ripe to incorporate clinical neuroscience concepts and language in our clinical practice and terminology. The neuroscientification of clinical psychiatry must start with clinical neuroscience literacy.
Although the traditional training of psychiatrists has evolved, it continues to perpetuate the old-fashioned model of care exemplified by the mental status examination, which documents the patient’s appearance, speech, mood, affect, thoughts, perceptions, behavior, cognition, insight, and judgement. Evaluations and progress notes have been constrained by this decades-old formula of observing, interviewing, and documenting signs and symptoms, and arriving at a working diagnosis, followed by a treatment plan comprised of a cluster of drug names, psychotherapeutic modalities, and social or rehabilitation interventions. This widely accepted procedure is important because it focuses on the mind. But where are the details about the brain, whose structural and functional aberrations generate the anomalies of the mind and are the scientific foundations of psychiatric care?
All psychiatrists are fully aware that brain pathology is the source of every psychiatric disorder they evaluate, diagnose, and treat. But it is time to formulate every patient’s care using neuroscience data and include neural mechanisms of the psychiatric disorder in the chart. Our clinical language must be integrated with the rapidly growing neuroscience of abnormalities in brain–behavior links.
Psychiatry is lagging behind neurology, its sister brain specialty, where neural pathways and processes are front and center in describing symptoms. According to Eisenberg,1 psychiatry training in the 1980s was, for the most part, “brainless.” But it should not remain so, because neuroscience advances have skyrocketed since he made that provocative statement 3 decades ago. Yet, the psychiatric residency training curriculum in many programs is lagging behind the rapid evolution of psychiatry as a clinical neuroscience.2
To its credit, the Accreditation Council for Graduate Medical Education, which oversees and accredits residency training programs in all specialties, including psychiatry, recently announced that psychiatric residency training must emphasize neuroscience competence side-by-side with clinical competence. Psychiatric residents must increasingly incorporate neurobiology in their formulation of clinical care and determine how the selected pharmacologic therapy addresses the dysregulated neural circuitry underlying the clinical manifestation. A good example of this method is a recently published case of posttraumatic stress disorder (PTSD),3 which discussed the clinical components and treatment of this brain disorder through the prism of clinical neuroscience research data. PTSD “trauma” is not only psychological, but also neurobiological, and both must be incorporated in formulating a clinical case.
Another important step has emerged to focus on infusing neuroscience facts and concepts within the clinical training of psychiatric residents. The National Neuroscience Curriculum Initiative (www.nncionline.org) is a timely and welcome initiative that will aggressively promulgate a clinical neuroscientification of psychiatric training, triggering a roadmap for modern, cutting-edge psychiatric practice.4 This will help consolidate psychiatry’s rightful place as a clinical neuroscience, without relinquishing its biopsychosocial roots.
As research continues to elucidate the neural mechanisms of key psychiatric symptoms, such as anxiety, depression, mania, impulsiveness, compulsions, delusions, or hallucinations, the transformation of psychiatry into an authentic clinical neuroscience is inevitable. But contemporary psychiatric practitioners must retool and start their journey toward neuroscience literacy by attending relevant continuing medical education presentations and regularly reading journals that focus on clinical psychiatric neuroscience, such as Molecular Psychiatry, JAMA Psychiatry, Biological Psychiatry, Neuropsychopharmacology, and Progress in Neuro-psychopharmacology and Biological Psychiatry.
It is my sincere hope that my fellow clinical psychiatrists will steadily grow their clinical neuroscience literacy and apply it to daily patient care. By formulating psychiatric signs and symptoms in evidence-based, neurobiolo
1. Eisenberg L. Mindlessness and brainlessness in psychiatry. Br J Psychiatry. 1986;148:497-508.
2. Reynolds CF 3rd, Lewis DA, Detre T, et al. The future of psychiatry as clinical neuroscience. Acad Med. 2009;84(4):446-450.
3. Ross DA, Arbuckle MR, Travis MJ, et al. An integrated neuroscience perspective on formulation and treatment planning for posttraumatic stress disorder: an educational review. JAMA Psychiatry. 2017;74(4):407-415.
4. Insel TR, Quirion R. Psychiatry as a clinical neuroscience discipline. JAMA. 2005;294(17):2221-2224.
5. Stahl SM. Neuroscience-based Nomenclature: classifying psychotropics by mechanism of action rather than indication. Current Psychiatry. 2017;16(5):15-16.
An abundance of recent neuroscience advances is directly related to psychiatric disorders, because the primary mission of the brain is to generate a mind, and every new discovery provides another piece of the psychiatric disorders puzzle. The time also is ripe to incorporate clinical neuroscience concepts and language in our clinical practice and terminology. The neuroscientification of clinical psychiatry must start with clinical neuroscience literacy.
Although the traditional training of psychiatrists has evolved, it continues to perpetuate the old-fashioned model of care exemplified by the mental status examination, which documents the patient’s appearance, speech, mood, affect, thoughts, perceptions, behavior, cognition, insight, and judgement. Evaluations and progress notes have been constrained by this decades-old formula of observing, interviewing, and documenting signs and symptoms, and arriving at a working diagnosis, followed by a treatment plan comprised of a cluster of drug names, psychotherapeutic modalities, and social or rehabilitation interventions. This widely accepted procedure is important because it focuses on the mind. But where are the details about the brain, whose structural and functional aberrations generate the anomalies of the mind and are the scientific foundations of psychiatric care?
All psychiatrists are fully aware that brain pathology is the source of every psychiatric disorder they evaluate, diagnose, and treat. But it is time to formulate every patient’s care using neuroscience data and include neural mechanisms of the psychiatric disorder in the chart. Our clinical language must be integrated with the rapidly growing neuroscience of abnormalities in brain–behavior links.
Psychiatry is lagging behind neurology, its sister brain specialty, where neural pathways and processes are front and center in describing symptoms. According to Eisenberg,1 psychiatry training in the 1980s was, for the most part, “brainless.” But it should not remain so, because neuroscience advances have skyrocketed since he made that provocative statement 3 decades ago. Yet, the psychiatric residency training curriculum in many programs is lagging behind the rapid evolution of psychiatry as a clinical neuroscience.2
To its credit, the Accreditation Council for Graduate Medical Education, which oversees and accredits residency training programs in all specialties, including psychiatry, recently announced that psychiatric residency training must emphasize neuroscience competence side-by-side with clinical competence. Psychiatric residents must increasingly incorporate neurobiology in their formulation of clinical care and determine how the selected pharmacologic therapy addresses the dysregulated neural circuitry underlying the clinical manifestation. A good example of this method is a recently published case of posttraumatic stress disorder (PTSD),3 which discussed the clinical components and treatment of this brain disorder through the prism of clinical neuroscience research data. PTSD “trauma” is not only psychological, but also neurobiological, and both must be incorporated in formulating a clinical case.
Another important step has emerged to focus on infusing neuroscience facts and concepts within the clinical training of psychiatric residents. The National Neuroscience Curriculum Initiative (www.nncionline.org) is a timely and welcome initiative that will aggressively promulgate a clinical neuroscientification of psychiatric training, triggering a roadmap for modern, cutting-edge psychiatric practice.4 This will help consolidate psychiatry’s rightful place as a clinical neuroscience, without relinquishing its biopsychosocial roots.
As research continues to elucidate the neural mechanisms of key psychiatric symptoms, such as anxiety, depression, mania, impulsiveness, compulsions, delusions, or hallucinations, the transformation of psychiatry into an authentic clinical neuroscience is inevitable. But contemporary psychiatric practitioners must retool and start their journey toward neuroscience literacy by attending relevant continuing medical education presentations and regularly reading journals that focus on clinical psychiatric neuroscience, such as Molecular Psychiatry, JAMA Psychiatry, Biological Psychiatry, Neuropsychopharmacology, and Progress in Neuro-psychopharmacology and Biological Psychiatry.
It is my sincere hope that my fellow clinical psychiatrists will steadily grow their clinical neuroscience literacy and apply it to daily patient care. By formulating psychiatric signs and symptoms in evidence-based, neurobiolo
An abundance of recent neuroscience advances is directly related to psychiatric disorders, because the primary mission of the brain is to generate a mind, and every new discovery provides another piece of the psychiatric disorders puzzle. The time also is ripe to incorporate clinical neuroscience concepts and language in our clinical practice and terminology. The neuroscientification of clinical psychiatry must start with clinical neuroscience literacy.
Although the traditional training of psychiatrists has evolved, it continues to perpetuate the old-fashioned model of care exemplified by the mental status examination, which documents the patient’s appearance, speech, mood, affect, thoughts, perceptions, behavior, cognition, insight, and judgement. Evaluations and progress notes have been constrained by this decades-old formula of observing, interviewing, and documenting signs and symptoms, and arriving at a working diagnosis, followed by a treatment plan comprised of a cluster of drug names, psychotherapeutic modalities, and social or rehabilitation interventions. This widely accepted procedure is important because it focuses on the mind. But where are the details about the brain, whose structural and functional aberrations generate the anomalies of the mind and are the scientific foundations of psychiatric care?
All psychiatrists are fully aware that brain pathology is the source of every psychiatric disorder they evaluate, diagnose, and treat. But it is time to formulate every patient’s care using neuroscience data and include neural mechanisms of the psychiatric disorder in the chart. Our clinical language must be integrated with the rapidly growing neuroscience of abnormalities in brain–behavior links.
Psychiatry is lagging behind neurology, its sister brain specialty, where neural pathways and processes are front and center in describing symptoms. According to Eisenberg,1 psychiatry training in the 1980s was, for the most part, “brainless.” But it should not remain so, because neuroscience advances have skyrocketed since he made that provocative statement 3 decades ago. Yet, the psychiatric residency training curriculum in many programs is lagging behind the rapid evolution of psychiatry as a clinical neuroscience.2
To its credit, the Accreditation Council for Graduate Medical Education, which oversees and accredits residency training programs in all specialties, including psychiatry, recently announced that psychiatric residency training must emphasize neuroscience competence side-by-side with clinical competence. Psychiatric residents must increasingly incorporate neurobiology in their formulation of clinical care and determine how the selected pharmacologic therapy addresses the dysregulated neural circuitry underlying the clinical manifestation. A good example of this method is a recently published case of posttraumatic stress disorder (PTSD),3 which discussed the clinical components and treatment of this brain disorder through the prism of clinical neuroscience research data. PTSD “trauma” is not only psychological, but also neurobiological, and both must be incorporated in formulating a clinical case.
Another important step has emerged to focus on infusing neuroscience facts and concepts within the clinical training of psychiatric residents. The National Neuroscience Curriculum Initiative (www.nncionline.org) is a timely and welcome initiative that will aggressively promulgate a clinical neuroscientification of psychiatric training, triggering a roadmap for modern, cutting-edge psychiatric practice.4 This will help consolidate psychiatry’s rightful place as a clinical neuroscience, without relinquishing its biopsychosocial roots.
As research continues to elucidate the neural mechanisms of key psychiatric symptoms, such as anxiety, depression, mania, impulsiveness, compulsions, delusions, or hallucinations, the transformation of psychiatry into an authentic clinical neuroscience is inevitable. But contemporary psychiatric practitioners must retool and start their journey toward neuroscience literacy by attending relevant continuing medical education presentations and regularly reading journals that focus on clinical psychiatric neuroscience, such as Molecular Psychiatry, JAMA Psychiatry, Biological Psychiatry, Neuropsychopharmacology, and Progress in Neuro-psychopharmacology and Biological Psychiatry.
It is my sincere hope that my fellow clinical psychiatrists will steadily grow their clinical neuroscience literacy and apply it to daily patient care. By formulating psychiatric signs and symptoms in evidence-based, neurobiolo
1. Eisenberg L. Mindlessness and brainlessness in psychiatry. Br J Psychiatry. 1986;148:497-508.
2. Reynolds CF 3rd, Lewis DA, Detre T, et al. The future of psychiatry as clinical neuroscience. Acad Med. 2009;84(4):446-450.
3. Ross DA, Arbuckle MR, Travis MJ, et al. An integrated neuroscience perspective on formulation and treatment planning for posttraumatic stress disorder: an educational review. JAMA Psychiatry. 2017;74(4):407-415.
4. Insel TR, Quirion R. Psychiatry as a clinical neuroscience discipline. JAMA. 2005;294(17):2221-2224.
5. Stahl SM. Neuroscience-based Nomenclature: classifying psychotropics by mechanism of action rather than indication. Current Psychiatry. 2017;16(5):15-16.
1. Eisenberg L. Mindlessness and brainlessness in psychiatry. Br J Psychiatry. 1986;148:497-508.
2. Reynolds CF 3rd, Lewis DA, Detre T, et al. The future of psychiatry as clinical neuroscience. Acad Med. 2009;84(4):446-450.
3. Ross DA, Arbuckle MR, Travis MJ, et al. An integrated neuroscience perspective on formulation and treatment planning for posttraumatic stress disorder: an educational review. JAMA Psychiatry. 2017;74(4):407-415.
4. Insel TR, Quirion R. Psychiatry as a clinical neuroscience discipline. JAMA. 2005;294(17):2221-2224.
5. Stahl SM. Neuroscience-based Nomenclature: classifying psychotropics by mechanism of action rather than indication. Current Psychiatry. 2017;16(5):15-16.
Considering work as an expert witness? Look before you leap!
Dear Dr. Mossman,
I am retired, but an attorney friend of mine has asked me to help out by performing forensic evaluations. I’m tempted to try it because the work sounds meaningful and interesting. I won’t have a doctor–patient relationship with the attorney’s clients, and I expect the work will take <10 hours a week. Do I need malpractice coverage? Should I consider any other medicolegal issues before I start?
Submitted by “Dr. B”
One of the great things about being a psychiatrist is the variety of available practice options. Like Dr. B, many psychiatrists contemplate using their clinical know-how to perform forensic evaluations. For some psychiatrists, part-time work as an expert witness may provide an appealing change of pace from their other clinical duties1 and a way to supplement their income.2
But as would be true for other kinds of medical practice, Dr. B is wise to consider the possible risks before jumping into forensic work. To help Dr. B decide about getting insurance coverage, we will:
- explain briefly the subspecialty of forensic psychiatry
- review the theory of malpractice and negligence torts
- discuss whether forensic evaluations can create doctor–patient relationships
- explore the availability and limitations of immunity for forensic work
- describe other types of liability with forensic work
- summarize steps to avoid liability.
Introduction to forensic psychiatry
Some psychiatrists—and many people who are not psychiatrists—have a vague or incorrect understanding of forensic psychiatry. Put succinctly, “Forensic Psychiatry is a subspecialty of psychiatry in which scientific and clinical expertise is applied in legal contexts….”3 To practice forensic psychiatry well, a psychiatrist must have some understanding of the law and how to apply and translate clinical concepts to fit legal criteria.4 Psychiatrists who offer to serve as expert witnesses should be familiar with how the courtroom functions, the nuances of how expert testimony is used, and possible sources of bias.4,5
Forensic work can create role conflicts. For most types of forensic assessments, psychiatrists should not provide forensic opinions or testimony about their own patients.3 Even psychiatrists who only work as expert witnesses must balance duties of assisting the trier of fact, fulfilling the consultation role to the retaining party, upholding the standards and ethics of the profession, and striving to provide truthful, objective testimony.2
Special training usually is required
The most important qualification for being a good psychiatric expert witness is being a good psychiatrist, and courts do not require psychiatrists to have specialty training in forensic psychiatry to perform forensic psychiatric evaluations. Yet, the field of forensic psychiatry has developed over the past 50 years to the point that psychiatrists need special training to properly perform many, if not most, types of forensic evaluations.6 Much of forensic psychiatry involves writing specialized reports for lawyers and the court,7 and experts are supposed to meet professional standards, regardless of their training.8-10 Psychiatrists who perform forensic work are obligated to claim expertise only in areas where their knowledge, skills, training, and experience justify such claims. These considerations explain why, since 1999, the American Board of Psychiatry and Neurology has limited eligibility for board certification in forensic psychiatry to psychiatrists who have completed accredited forensic fellowships.11
Malpractice: A short review
To address Dr. B’s question about malpractice coverage, we first review what malpractice is.
“Tort” is a legal term for injury, and tort claims arise when one party harms another and the harmed party seeks money as compensation.9 In a tort claim alleging negligence, the plaintiff (ie, the person bringing the suit) asserts that the defendant had a legally recognized duty, that the defendant breached that duty, and that breach of duty harmed the plaintiff.8
Physicians have a legal duty to “possess the requisite knowledge and skill such as is possessed by the average member of the medical profession; … exercise ordinary and reasonable care in the application of such knowledge and skill; and … use best judgment in such application.”10 A medical malpractice lawsuit asserts that a doctor breached this duty and caused injury in the course of the medical practice.
Malpractice in forensic cases
Practicing medicine typically occurs within the context of treatment relationships. One might think, as Dr. B did, that because forensic evaluations do not involve treating patients, they do not create the kind of doctor–patient relationship that could lead to malpractice liability. This is incorrect, however, for several reasons.
Certain well-intended actions during a forensic evaluation, such as explaining the implications of a diagnosis, giving specific advice about a medication, or making a recommendation about where or how to obtain treatment, may create a doctor–patient relationship.12,13 Many states’ laws on what constitutes the practice of medicine include performing examinations, diagnosing, or referring to oneself as “Dr.” or as a medical practitioner.14-17 State courts have interpreted these laws to further define what constitutes medical practice and the creation of a doctor–patient relationship during a forensic examination.18,19 Some legal scholars20 and the American Medical Association (AMA)9 regard provision of expert testimony as practicing medicine because such testimony requires the application of medical science and rendering of diagnoses.
Immunity and shifts away from it
For many years, courts granted civil immunity to expert witnesses for several policy reasons.8,9,13,20-22 Courts recognized that losing parties might want to blame whomever they could, and immunity could provide legal protection for expert witnesses. Without such protection, witnesses might feel more pressured to give testimony favorable to their side at the loss of objectivity,23,24 or experts might be discouraged from testifying at all. This would be true especially for academic psychiatrists who testify infrequently or for retired doctors, such as Dr. B, who might not want to carry insurance for just one case.21 According to this argument, rather than using the threat of litigation to keep out improper testimony, courts should rely on both admissibility standards25,26 and the adversarial nature of proceedings.21
Those who oppose granting immunity to experts argue that admissibility rules and cross-examination do too little to prevent bad testimony; the threat of liability, however, motivates experts to be more cautious and scientifically rigorous in their approach.21 Opponents also have argued that the threat of liability might reduce improper testimony, which they believe was partly responsible for rising malpractice premiums.20
Courts vary in how they consider granting immunity and to what extent. For example:
- Some courts will not grant immunity to so-called “friendly experts,” while others have limited immunity for adversarial experts.20-22
- Some courts have applied immunity to general fact witnesses but not to professional experts.21,24,27
- When immunity is considered, it is usually regarding actual testimony. Yet, some courts have included pretrial services.21,28-30
- Some courts have considered the testimonial issue at hand when deciding whether to extend immunity. For example, immunity may not apply if the issue is loss of profits21,31 or if an experiment is conducted to demonstrate the extent of a physical injury.21,32
If you plan to serve as an expert witness, find out what, if any, immunity is available in the jurisdiction where you expect to testify. If you do not have immunity, you may be subject to various malpractice claims, including alleged physical or emotional harm resulting from the evaluation1 (perhaps caused by misuse of empathic statements33), an accusation of negligent misdiagnosis of an evaluee,8 or failing to act upon a duty to warn or protect that arises during an assessment.34
Other liability
Dr. B also asked about medicolegal issues other than malpractice. Although negligence is the claim that forensic psychiatrists most commonly encounter,10 other types of claims arise in practice-related legal actions. Potential causes of action include failure to obtain or attempt to obtain informed consent, breach of confidentiality, or not responding to a psychiatric emergency during evaluation. The plaintiff usually must show that the expert’s conduct was the cause-in-fact of injury.8
Besides civil lawsuits, forensic work may generate complaints to state medical boards.10 Occasionally, state medical boards have revoked psychiatrists’ licenses for improper testimony.20 Aggrieved parties may allege violations of the Health Insurance Portability and Accountability Act of 1996, such as mishandling protected health information. Psychiatrists also may face sanction by professional societies—for example, censure by the American Psychiatric Association9,10 or the AMA13 for ethics violations—if their improper testimony is considered unprofessional conduct. The theory behind this is that judges and jurors cannot be technical experts in every field, so the field must have a mechanism to police itself.20,35,36 Finally, forensic experts can face criminal charges for perjury if they lie under oath.8
How to protect yourself
Even when legal claims against psychiatrists turn out to be baseless, legal costs of defending oneself can mount quickly. Knowing this, Dr. B may conclude that obtaining malpractice insurance would be wise. But a malpractice policy alone may not meet all Dr. B’s needs, because some policies do not cover ordinary negligence or other potential causes of legal action against a psychiatrist.13 Some companies offer these extra types of coverage for work as an expert witness at no additional cost, and some offer access to risk management services with specialized knowledge about forensic psychiatric practice.
1. Appelbaum PS. Law and psychiatry: liability for forensic evaluations: a word of caution. Psychiatr Serv. 2001;52(7):885-886.
2. Shuman DW, Greenberg SA. The expert witness, the adversary system, and the voice of reason: reconciling impartiality and advocacy. Professional Psychology: Research and Practice. 2003;34(3):219-224.
3. American Academy of Psychiatry and the Law. Ethics guidelines for the practice of forensic psychiatry. http://www.aapl.org/ethics.htm. Published May 2005. Accessed July 11, 2017.
4. Gutheil TG. Forensic psychiatry as a specialty. Psychiatric Times. http://www.psychiatrictimes.com/articles/forensic-psychiatry-sp
5. Knoll J, Gerbasi J. Psychiatric malpractice case analysis: striving for objectivity. J Am Acad Psychiatry Law. 2006;34(2):215-223.
6. Sadoff RL. The practice of forensic psychiatry: perils, problems, and pitfalls. J Am Acad Psychiatry Law. 1998;26(2):305-314.
7. Simon RI. Authorship in forensic psychiatry: a perspective. J Am Acad Psychiatry Law. 2007;35(1):18-26.
8. Masterson LR. Witness immunity or malpractice liability for professionals hired as experts? Rev Litig. 1998;17(2):393-418.
9. Binder RL. Liability for the psychiatrist expert witness. Am J Psychiatry. 2002;159(11):1819-1825.
10. Gold LH, Davidson JE. Do you understand your risk? Liability and third-party evaluations in civil litigation. J Am Acad Psychiatry Law. 2007;35(2):200-210.
11. American Academy of Psychiatry and the Law. ABPN certification in the subspecialty of forensic psychiatry. http://www.aapl.org/abpn-certification. Accessed July 9, 2017.
12. Marett CP, Mossman D. What are your responsibilities after a screening call? Current Psychiatry. 2014;13(9):54-57.
13. Weinstock R, Garrick T. Is liability possible for forensic psychiatrists? Bull Am Acad Psychiatry Law. 1995;23(2):183-193.
14. Ohio Revised Code §4731.34.
15. Kentucky Revised Statutes §311.550(10) (2017).
16. California Business & Professions Code §2052.5 (through 2012 Leg Sess).
17. Oregon Revised Statutes §677.085 (2013).
18. Blake V. When is a patient-physician relationship established? Virtual Mentor. 2012;14(5):403-406.
19. Zettler PJ. Toward coherent federal oversight of medicine. San Diego Law Review. 2015;52:427-500.
20. Turner JA. Going after the ‘hired guns’: is improper expert witness testimony unprofessional conduct or the negligent practice of medicine? Spec Law Dig Health Care Law. 2006;328:9-43.
21. Weiss LS, Orrick H. Expert witness malpractice actions: emerging trend or aberration? Practical Litigator. 2004;15(2):27-38.
22. McAbee GN. Improper expert medical testimony. Existing and proposed mechanisms of oversight. J Leg Med. 1998;19(2):257-272.
23. Panitz v Behrend, 632 A 2d 562 (Pa Super Ct 1993).
24. Murphy v A.A. Mathews, 841 S.W. 2d 671 (Mo 1992).
25. Daubert v Merrell Dow Pharmaceuticals, 509 U.S. 579 (1993).
26. Rule 702. Testimony by expert witnesses. In: Michigan Legal Publishing Ltd. Federal Rules of evidence. Grand Rapids, MI: Michigan Legal Publishing Ltd; 2017:21.
27. Committee on Medical Liability and Risk Management. Policy statement—expert witness participation in civil and criminal proceedings. Pediatrics. 2009;124(1):428-438.
28. Mattco Forge, Inc., v Arthur Young & Co., 6 Cal Rptr 2d 781 (Cal Ct App 1992).
29. Marrogi v Howard, 248 F 3d 382 (5th Cir 2001).
30. Boyes-Bogie v Horvitz, 2001 WL 1771989 (Mass Super 2001).
31. LLMD of Michigan, Inc., v Jackson-Cross Co., 740 A. 2d 186 (Pa 1999).
32. Pollock v Panjabi, 781 A 2d 518 (Conn Super Ct 2000).
33. Brodsky SL, Wilson JK. Empathy in forensic evaluations: a systematic reconsideration. Behav Sci Law. 2013;31(2):192-202.
34. Heilbrun K, DeMatteo D, Marczyk G, et al. Standards of practice and care in forensic mental health assessment: legal, professional, and principles-based consideration. Psych Pub Pol L. 2008;14(1):1-26.
35. Appelbaum PS. Law & psychiatry: policing expert testimony: the role of professional organizations. Psychiatr Serv. 2002;53(4):389-390,399.
36. Austin v American Association of Neurological Surgeons, 253 F 3d 967 (7th Cir 2001).
37. Gutheil TG, Simon RI. Attorneys’ pressures on the expert witness: early warning signs of endangered honesty, objectivity, and fair compensation. J Am Acad Psychiatry Law. 1999;27(4):546-553; discussion 554-562.
38. Gold LH, Anfang SA, Drukteinis AM, et al. AAPL practice guideline for the forensic evaluation of psychiatric disability. J Am Acad Psychiatry Law. 2008;36(suppl 4):S3-S50.
39. Knoll JL IV, Resnick PJ. Deposition dos and don’ts: how to answer 8 tricky questions. Current Psychiatry. 2008;7(3):25-28,36,39-40.
40. Hoge MA, Tebes JK, Davidson L, et al. The roles of behavioral health professionals in class action litigation. J Am Acad Psychiatry Law. 2002;30(1):49-58; discussion 59-64.
41. Simon RI, Shuman DW. Conducting forensic examinations on the road: are you practicing your profession without a license? Licensure requirements for out-of-state forensic examinations. J Am Acad Psychiatry Law. 2001;29(1):75-82.
42. Reid WH. Licensure requirements for out-of-state forensic examinations. J Am Acad Psychiatry Law. 2000;28(4):433-437.
43. Collins B, ed. When in doubt, tell the truth: and other quotations from Mark Twain. New York, NY: Columbia University Press; 1997.
Dear Dr. Mossman,
I am retired, but an attorney friend of mine has asked me to help out by performing forensic evaluations. I’m tempted to try it because the work sounds meaningful and interesting. I won’t have a doctor–patient relationship with the attorney’s clients, and I expect the work will take <10 hours a week. Do I need malpractice coverage? Should I consider any other medicolegal issues before I start?
Submitted by “Dr. B”
One of the great things about being a psychiatrist is the variety of available practice options. Like Dr. B, many psychiatrists contemplate using their clinical know-how to perform forensic evaluations. For some psychiatrists, part-time work as an expert witness may provide an appealing change of pace from their other clinical duties1 and a way to supplement their income.2
But as would be true for other kinds of medical practice, Dr. B is wise to consider the possible risks before jumping into forensic work. To help Dr. B decide about getting insurance coverage, we will:
- explain briefly the subspecialty of forensic psychiatry
- review the theory of malpractice and negligence torts
- discuss whether forensic evaluations can create doctor–patient relationships
- explore the availability and limitations of immunity for forensic work
- describe other types of liability with forensic work
- summarize steps to avoid liability.
Introduction to forensic psychiatry
Some psychiatrists—and many people who are not psychiatrists—have a vague or incorrect understanding of forensic psychiatry. Put succinctly, “Forensic Psychiatry is a subspecialty of psychiatry in which scientific and clinical expertise is applied in legal contexts….”3 To practice forensic psychiatry well, a psychiatrist must have some understanding of the law and how to apply and translate clinical concepts to fit legal criteria.4 Psychiatrists who offer to serve as expert witnesses should be familiar with how the courtroom functions, the nuances of how expert testimony is used, and possible sources of bias.4,5
Forensic work can create role conflicts. For most types of forensic assessments, psychiatrists should not provide forensic opinions or testimony about their own patients.3 Even psychiatrists who only work as expert witnesses must balance duties of assisting the trier of fact, fulfilling the consultation role to the retaining party, upholding the standards and ethics of the profession, and striving to provide truthful, objective testimony.2
Special training usually is required
The most important qualification for being a good psychiatric expert witness is being a good psychiatrist, and courts do not require psychiatrists to have specialty training in forensic psychiatry to perform forensic psychiatric evaluations. Yet, the field of forensic psychiatry has developed over the past 50 years to the point that psychiatrists need special training to properly perform many, if not most, types of forensic evaluations.6 Much of forensic psychiatry involves writing specialized reports for lawyers and the court,7 and experts are supposed to meet professional standards, regardless of their training.8-10 Psychiatrists who perform forensic work are obligated to claim expertise only in areas where their knowledge, skills, training, and experience justify such claims. These considerations explain why, since 1999, the American Board of Psychiatry and Neurology has limited eligibility for board certification in forensic psychiatry to psychiatrists who have completed accredited forensic fellowships.11
Malpractice: A short review
To address Dr. B’s question about malpractice coverage, we first review what malpractice is.
“Tort” is a legal term for injury, and tort claims arise when one party harms another and the harmed party seeks money as compensation.9 In a tort claim alleging negligence, the plaintiff (ie, the person bringing the suit) asserts that the defendant had a legally recognized duty, that the defendant breached that duty, and that breach of duty harmed the plaintiff.8
Physicians have a legal duty to “possess the requisite knowledge and skill such as is possessed by the average member of the medical profession; … exercise ordinary and reasonable care in the application of such knowledge and skill; and … use best judgment in such application.”10 A medical malpractice lawsuit asserts that a doctor breached this duty and caused injury in the course of the medical practice.
Malpractice in forensic cases
Practicing medicine typically occurs within the context of treatment relationships. One might think, as Dr. B did, that because forensic evaluations do not involve treating patients, they do not create the kind of doctor–patient relationship that could lead to malpractice liability. This is incorrect, however, for several reasons.
Certain well-intended actions during a forensic evaluation, such as explaining the implications of a diagnosis, giving specific advice about a medication, or making a recommendation about where or how to obtain treatment, may create a doctor–patient relationship.12,13 Many states’ laws on what constitutes the practice of medicine include performing examinations, diagnosing, or referring to oneself as “Dr.” or as a medical practitioner.14-17 State courts have interpreted these laws to further define what constitutes medical practice and the creation of a doctor–patient relationship during a forensic examination.18,19 Some legal scholars20 and the American Medical Association (AMA)9 regard provision of expert testimony as practicing medicine because such testimony requires the application of medical science and rendering of diagnoses.
Immunity and shifts away from it
For many years, courts granted civil immunity to expert witnesses for several policy reasons.8,9,13,20-22 Courts recognized that losing parties might want to blame whomever they could, and immunity could provide legal protection for expert witnesses. Without such protection, witnesses might feel more pressured to give testimony favorable to their side at the loss of objectivity,23,24 or experts might be discouraged from testifying at all. This would be true especially for academic psychiatrists who testify infrequently or for retired doctors, such as Dr. B, who might not want to carry insurance for just one case.21 According to this argument, rather than using the threat of litigation to keep out improper testimony, courts should rely on both admissibility standards25,26 and the adversarial nature of proceedings.21
Those who oppose granting immunity to experts argue that admissibility rules and cross-examination do too little to prevent bad testimony; the threat of liability, however, motivates experts to be more cautious and scientifically rigorous in their approach.21 Opponents also have argued that the threat of liability might reduce improper testimony, which they believe was partly responsible for rising malpractice premiums.20
Courts vary in how they consider granting immunity and to what extent. For example:
- Some courts will not grant immunity to so-called “friendly experts,” while others have limited immunity for adversarial experts.20-22
- Some courts have applied immunity to general fact witnesses but not to professional experts.21,24,27
- When immunity is considered, it is usually regarding actual testimony. Yet, some courts have included pretrial services.21,28-30
- Some courts have considered the testimonial issue at hand when deciding whether to extend immunity. For example, immunity may not apply if the issue is loss of profits21,31 or if an experiment is conducted to demonstrate the extent of a physical injury.21,32
If you plan to serve as an expert witness, find out what, if any, immunity is available in the jurisdiction where you expect to testify. If you do not have immunity, you may be subject to various malpractice claims, including alleged physical or emotional harm resulting from the evaluation1 (perhaps caused by misuse of empathic statements33), an accusation of negligent misdiagnosis of an evaluee,8 or failing to act upon a duty to warn or protect that arises during an assessment.34
Other liability
Dr. B also asked about medicolegal issues other than malpractice. Although negligence is the claim that forensic psychiatrists most commonly encounter,10 other types of claims arise in practice-related legal actions. Potential causes of action include failure to obtain or attempt to obtain informed consent, breach of confidentiality, or not responding to a psychiatric emergency during evaluation. The plaintiff usually must show that the expert’s conduct was the cause-in-fact of injury.8
Besides civil lawsuits, forensic work may generate complaints to state medical boards.10 Occasionally, state medical boards have revoked psychiatrists’ licenses for improper testimony.20 Aggrieved parties may allege violations of the Health Insurance Portability and Accountability Act of 1996, such as mishandling protected health information. Psychiatrists also may face sanction by professional societies—for example, censure by the American Psychiatric Association9,10 or the AMA13 for ethics violations—if their improper testimony is considered unprofessional conduct. The theory behind this is that judges and jurors cannot be technical experts in every field, so the field must have a mechanism to police itself.20,35,36 Finally, forensic experts can face criminal charges for perjury if they lie under oath.8
How to protect yourself
Even when legal claims against psychiatrists turn out to be baseless, legal costs of defending oneself can mount quickly. Knowing this, Dr. B may conclude that obtaining malpractice insurance would be wise. But a malpractice policy alone may not meet all Dr. B’s needs, because some policies do not cover ordinary negligence or other potential causes of legal action against a psychiatrist.13 Some companies offer these extra types of coverage for work as an expert witness at no additional cost, and some offer access to risk management services with specialized knowledge about forensic psychiatric practice.
Dear Dr. Mossman,
I am retired, but an attorney friend of mine has asked me to help out by performing forensic evaluations. I’m tempted to try it because the work sounds meaningful and interesting. I won’t have a doctor–patient relationship with the attorney’s clients, and I expect the work will take <10 hours a week. Do I need malpractice coverage? Should I consider any other medicolegal issues before I start?
Submitted by “Dr. B”
One of the great things about being a psychiatrist is the variety of available practice options. Like Dr. B, many psychiatrists contemplate using their clinical know-how to perform forensic evaluations. For some psychiatrists, part-time work as an expert witness may provide an appealing change of pace from their other clinical duties1 and a way to supplement their income.2
But as would be true for other kinds of medical practice, Dr. B is wise to consider the possible risks before jumping into forensic work. To help Dr. B decide about getting insurance coverage, we will:
- explain briefly the subspecialty of forensic psychiatry
- review the theory of malpractice and negligence torts
- discuss whether forensic evaluations can create doctor–patient relationships
- explore the availability and limitations of immunity for forensic work
- describe other types of liability with forensic work
- summarize steps to avoid liability.
Introduction to forensic psychiatry
Some psychiatrists—and many people who are not psychiatrists—have a vague or incorrect understanding of forensic psychiatry. Put succinctly, “Forensic Psychiatry is a subspecialty of psychiatry in which scientific and clinical expertise is applied in legal contexts….”3 To practice forensic psychiatry well, a psychiatrist must have some understanding of the law and how to apply and translate clinical concepts to fit legal criteria.4 Psychiatrists who offer to serve as expert witnesses should be familiar with how the courtroom functions, the nuances of how expert testimony is used, and possible sources of bias.4,5
Forensic work can create role conflicts. For most types of forensic assessments, psychiatrists should not provide forensic opinions or testimony about their own patients.3 Even psychiatrists who only work as expert witnesses must balance duties of assisting the trier of fact, fulfilling the consultation role to the retaining party, upholding the standards and ethics of the profession, and striving to provide truthful, objective testimony.2
Special training usually is required
The most important qualification for being a good psychiatric expert witness is being a good psychiatrist, and courts do not require psychiatrists to have specialty training in forensic psychiatry to perform forensic psychiatric evaluations. Yet, the field of forensic psychiatry has developed over the past 50 years to the point that psychiatrists need special training to properly perform many, if not most, types of forensic evaluations.6 Much of forensic psychiatry involves writing specialized reports for lawyers and the court,7 and experts are supposed to meet professional standards, regardless of their training.8-10 Psychiatrists who perform forensic work are obligated to claim expertise only in areas where their knowledge, skills, training, and experience justify such claims. These considerations explain why, since 1999, the American Board of Psychiatry and Neurology has limited eligibility for board certification in forensic psychiatry to psychiatrists who have completed accredited forensic fellowships.11
Malpractice: A short review
To address Dr. B’s question about malpractice coverage, we first review what malpractice is.
“Tort” is a legal term for injury, and tort claims arise when one party harms another and the harmed party seeks money as compensation.9 In a tort claim alleging negligence, the plaintiff (ie, the person bringing the suit) asserts that the defendant had a legally recognized duty, that the defendant breached that duty, and that breach of duty harmed the plaintiff.8
Physicians have a legal duty to “possess the requisite knowledge and skill such as is possessed by the average member of the medical profession; … exercise ordinary and reasonable care in the application of such knowledge and skill; and … use best judgment in such application.”10 A medical malpractice lawsuit asserts that a doctor breached this duty and caused injury in the course of the medical practice.
Malpractice in forensic cases
Practicing medicine typically occurs within the context of treatment relationships. One might think, as Dr. B did, that because forensic evaluations do not involve treating patients, they do not create the kind of doctor–patient relationship that could lead to malpractice liability. This is incorrect, however, for several reasons.
Certain well-intended actions during a forensic evaluation, such as explaining the implications of a diagnosis, giving specific advice about a medication, or making a recommendation about where or how to obtain treatment, may create a doctor–patient relationship.12,13 Many states’ laws on what constitutes the practice of medicine include performing examinations, diagnosing, or referring to oneself as “Dr.” or as a medical practitioner.14-17 State courts have interpreted these laws to further define what constitutes medical practice and the creation of a doctor–patient relationship during a forensic examination.18,19 Some legal scholars20 and the American Medical Association (AMA)9 regard provision of expert testimony as practicing medicine because such testimony requires the application of medical science and rendering of diagnoses.
Immunity and shifts away from it
For many years, courts granted civil immunity to expert witnesses for several policy reasons.8,9,13,20-22 Courts recognized that losing parties might want to blame whomever they could, and immunity could provide legal protection for expert witnesses. Without such protection, witnesses might feel more pressured to give testimony favorable to their side at the loss of objectivity,23,24 or experts might be discouraged from testifying at all. This would be true especially for academic psychiatrists who testify infrequently or for retired doctors, such as Dr. B, who might not want to carry insurance for just one case.21 According to this argument, rather than using the threat of litigation to keep out improper testimony, courts should rely on both admissibility standards25,26 and the adversarial nature of proceedings.21
Those who oppose granting immunity to experts argue that admissibility rules and cross-examination do too little to prevent bad testimony; the threat of liability, however, motivates experts to be more cautious and scientifically rigorous in their approach.21 Opponents also have argued that the threat of liability might reduce improper testimony, which they believe was partly responsible for rising malpractice premiums.20
Courts vary in how they consider granting immunity and to what extent. For example:
- Some courts will not grant immunity to so-called “friendly experts,” while others have limited immunity for adversarial experts.20-22
- Some courts have applied immunity to general fact witnesses but not to professional experts.21,24,27
- When immunity is considered, it is usually regarding actual testimony. Yet, some courts have included pretrial services.21,28-30
- Some courts have considered the testimonial issue at hand when deciding whether to extend immunity. For example, immunity may not apply if the issue is loss of profits21,31 or if an experiment is conducted to demonstrate the extent of a physical injury.21,32
If you plan to serve as an expert witness, find out what, if any, immunity is available in the jurisdiction where you expect to testify. If you do not have immunity, you may be subject to various malpractice claims, including alleged physical or emotional harm resulting from the evaluation1 (perhaps caused by misuse of empathic statements33), an accusation of negligent misdiagnosis of an evaluee,8 or failing to act upon a duty to warn or protect that arises during an assessment.34
Other liability
Dr. B also asked about medicolegal issues other than malpractice. Although negligence is the claim that forensic psychiatrists most commonly encounter,10 other types of claims arise in practice-related legal actions. Potential causes of action include failure to obtain or attempt to obtain informed consent, breach of confidentiality, or not responding to a psychiatric emergency during evaluation. The plaintiff usually must show that the expert’s conduct was the cause-in-fact of injury.8
Besides civil lawsuits, forensic work may generate complaints to state medical boards.10 Occasionally, state medical boards have revoked psychiatrists’ licenses for improper testimony.20 Aggrieved parties may allege violations of the Health Insurance Portability and Accountability Act of 1996, such as mishandling protected health information. Psychiatrists also may face sanction by professional societies—for example, censure by the American Psychiatric Association9,10 or the AMA13 for ethics violations—if their improper testimony is considered unprofessional conduct. The theory behind this is that judges and jurors cannot be technical experts in every field, so the field must have a mechanism to police itself.20,35,36 Finally, forensic experts can face criminal charges for perjury if they lie under oath.8
How to protect yourself
Even when legal claims against psychiatrists turn out to be baseless, legal costs of defending oneself can mount quickly. Knowing this, Dr. B may conclude that obtaining malpractice insurance would be wise. But a malpractice policy alone may not meet all Dr. B’s needs, because some policies do not cover ordinary negligence or other potential causes of legal action against a psychiatrist.13 Some companies offer these extra types of coverage for work as an expert witness at no additional cost, and some offer access to risk management services with specialized knowledge about forensic psychiatric practice.
1. Appelbaum PS. Law and psychiatry: liability for forensic evaluations: a word of caution. Psychiatr Serv. 2001;52(7):885-886.
2. Shuman DW, Greenberg SA. The expert witness, the adversary system, and the voice of reason: reconciling impartiality and advocacy. Professional Psychology: Research and Practice. 2003;34(3):219-224.
3. American Academy of Psychiatry and the Law. Ethics guidelines for the practice of forensic psychiatry. http://www.aapl.org/ethics.htm. Published May 2005. Accessed July 11, 2017.
4. Gutheil TG. Forensic psychiatry as a specialty. Psychiatric Times. http://www.psychiatrictimes.com/articles/forensic-psychiatry-sp
5. Knoll J, Gerbasi J. Psychiatric malpractice case analysis: striving for objectivity. J Am Acad Psychiatry Law. 2006;34(2):215-223.
6. Sadoff RL. The practice of forensic psychiatry: perils, problems, and pitfalls. J Am Acad Psychiatry Law. 1998;26(2):305-314.
7. Simon RI. Authorship in forensic psychiatry: a perspective. J Am Acad Psychiatry Law. 2007;35(1):18-26.
8. Masterson LR. Witness immunity or malpractice liability for professionals hired as experts? Rev Litig. 1998;17(2):393-418.
9. Binder RL. Liability for the psychiatrist expert witness. Am J Psychiatry. 2002;159(11):1819-1825.
10. Gold LH, Davidson JE. Do you understand your risk? Liability and third-party evaluations in civil litigation. J Am Acad Psychiatry Law. 2007;35(2):200-210.
11. American Academy of Psychiatry and the Law. ABPN certification in the subspecialty of forensic psychiatry. http://www.aapl.org/abpn-certification. Accessed July 9, 2017.
12. Marett CP, Mossman D. What are your responsibilities after a screening call? Current Psychiatry. 2014;13(9):54-57.
13. Weinstock R, Garrick T. Is liability possible for forensic psychiatrists? Bull Am Acad Psychiatry Law. 1995;23(2):183-193.
14. Ohio Revised Code §4731.34.
15. Kentucky Revised Statutes §311.550(10) (2017).
16. California Business & Professions Code §2052.5 (through 2012 Leg Sess).
17. Oregon Revised Statutes §677.085 (2013).
18. Blake V. When is a patient-physician relationship established? Virtual Mentor. 2012;14(5):403-406.
19. Zettler PJ. Toward coherent federal oversight of medicine. San Diego Law Review. 2015;52:427-500.
20. Turner JA. Going after the ‘hired guns’: is improper expert witness testimony unprofessional conduct or the negligent practice of medicine? Spec Law Dig Health Care Law. 2006;328:9-43.
21. Weiss LS, Orrick H. Expert witness malpractice actions: emerging trend or aberration? Practical Litigator. 2004;15(2):27-38.
22. McAbee GN. Improper expert medical testimony. Existing and proposed mechanisms of oversight. J Leg Med. 1998;19(2):257-272.
23. Panitz v Behrend, 632 A 2d 562 (Pa Super Ct 1993).
24. Murphy v A.A. Mathews, 841 S.W. 2d 671 (Mo 1992).
25. Daubert v Merrell Dow Pharmaceuticals, 509 U.S. 579 (1993).
26. Rule 702. Testimony by expert witnesses. In: Michigan Legal Publishing Ltd. Federal Rules of evidence. Grand Rapids, MI: Michigan Legal Publishing Ltd; 2017:21.
27. Committee on Medical Liability and Risk Management. Policy statement—expert witness participation in civil and criminal proceedings. Pediatrics. 2009;124(1):428-438.
28. Mattco Forge, Inc., v Arthur Young & Co., 6 Cal Rptr 2d 781 (Cal Ct App 1992).
29. Marrogi v Howard, 248 F 3d 382 (5th Cir 2001).
30. Boyes-Bogie v Horvitz, 2001 WL 1771989 (Mass Super 2001).
31. LLMD of Michigan, Inc., v Jackson-Cross Co., 740 A. 2d 186 (Pa 1999).
32. Pollock v Panjabi, 781 A 2d 518 (Conn Super Ct 2000).
33. Brodsky SL, Wilson JK. Empathy in forensic evaluations: a systematic reconsideration. Behav Sci Law. 2013;31(2):192-202.
34. Heilbrun K, DeMatteo D, Marczyk G, et al. Standards of practice and care in forensic mental health assessment: legal, professional, and principles-based consideration. Psych Pub Pol L. 2008;14(1):1-26.
35. Appelbaum PS. Law & psychiatry: policing expert testimony: the role of professional organizations. Psychiatr Serv. 2002;53(4):389-390,399.
36. Austin v American Association of Neurological Surgeons, 253 F 3d 967 (7th Cir 2001).
37. Gutheil TG, Simon RI. Attorneys’ pressures on the expert witness: early warning signs of endangered honesty, objectivity, and fair compensation. J Am Acad Psychiatry Law. 1999;27(4):546-553; discussion 554-562.
38. Gold LH, Anfang SA, Drukteinis AM, et al. AAPL practice guideline for the forensic evaluation of psychiatric disability. J Am Acad Psychiatry Law. 2008;36(suppl 4):S3-S50.
39. Knoll JL IV, Resnick PJ. Deposition dos and don’ts: how to answer 8 tricky questions. Current Psychiatry. 2008;7(3):25-28,36,39-40.
40. Hoge MA, Tebes JK, Davidson L, et al. The roles of behavioral health professionals in class action litigation. J Am Acad Psychiatry Law. 2002;30(1):49-58; discussion 59-64.
41. Simon RI, Shuman DW. Conducting forensic examinations on the road: are you practicing your profession without a license? Licensure requirements for out-of-state forensic examinations. J Am Acad Psychiatry Law. 2001;29(1):75-82.
42. Reid WH. Licensure requirements for out-of-state forensic examinations. J Am Acad Psychiatry Law. 2000;28(4):433-437.
43. Collins B, ed. When in doubt, tell the truth: and other quotations from Mark Twain. New York, NY: Columbia University Press; 1997.
1. Appelbaum PS. Law and psychiatry: liability for forensic evaluations: a word of caution. Psychiatr Serv. 2001;52(7):885-886.
2. Shuman DW, Greenberg SA. The expert witness, the adversary system, and the voice of reason: reconciling impartiality and advocacy. Professional Psychology: Research and Practice. 2003;34(3):219-224.
3. American Academy of Psychiatry and the Law. Ethics guidelines for the practice of forensic psychiatry. http://www.aapl.org/ethics.htm. Published May 2005. Accessed July 11, 2017.
4. Gutheil TG. Forensic psychiatry as a specialty. Psychiatric Times. http://www.psychiatrictimes.com/articles/forensic-psychiatry-sp
5. Knoll J, Gerbasi J. Psychiatric malpractice case analysis: striving for objectivity. J Am Acad Psychiatry Law. 2006;34(2):215-223.
6. Sadoff RL. The practice of forensic psychiatry: perils, problems, and pitfalls. J Am Acad Psychiatry Law. 1998;26(2):305-314.
7. Simon RI. Authorship in forensic psychiatry: a perspective. J Am Acad Psychiatry Law. 2007;35(1):18-26.
8. Masterson LR. Witness immunity or malpractice liability for professionals hired as experts? Rev Litig. 1998;17(2):393-418.
9. Binder RL. Liability for the psychiatrist expert witness. Am J Psychiatry. 2002;159(11):1819-1825.
10. Gold LH, Davidson JE. Do you understand your risk? Liability and third-party evaluations in civil litigation. J Am Acad Psychiatry Law. 2007;35(2):200-210.
11. American Academy of Psychiatry and the Law. ABPN certification in the subspecialty of forensic psychiatry. http://www.aapl.org/abpn-certification. Accessed July 9, 2017.
12. Marett CP, Mossman D. What are your responsibilities after a screening call? Current Psychiatry. 2014;13(9):54-57.
13. Weinstock R, Garrick T. Is liability possible for forensic psychiatrists? Bull Am Acad Psychiatry Law. 1995;23(2):183-193.
14. Ohio Revised Code §4731.34.
15. Kentucky Revised Statutes §311.550(10) (2017).
16. California Business & Professions Code §2052.5 (through 2012 Leg Sess).
17. Oregon Revised Statutes §677.085 (2013).
18. Blake V. When is a patient-physician relationship established? Virtual Mentor. 2012;14(5):403-406.
19. Zettler PJ. Toward coherent federal oversight of medicine. San Diego Law Review. 2015;52:427-500.
20. Turner JA. Going after the ‘hired guns’: is improper expert witness testimony unprofessional conduct or the negligent practice of medicine? Spec Law Dig Health Care Law. 2006;328:9-43.
21. Weiss LS, Orrick H. Expert witness malpractice actions: emerging trend or aberration? Practical Litigator. 2004;15(2):27-38.
22. McAbee GN. Improper expert medical testimony. Existing and proposed mechanisms of oversight. J Leg Med. 1998;19(2):257-272.
23. Panitz v Behrend, 632 A 2d 562 (Pa Super Ct 1993).
24. Murphy v A.A. Mathews, 841 S.W. 2d 671 (Mo 1992).
25. Daubert v Merrell Dow Pharmaceuticals, 509 U.S. 579 (1993).
26. Rule 702. Testimony by expert witnesses. In: Michigan Legal Publishing Ltd. Federal Rules of evidence. Grand Rapids, MI: Michigan Legal Publishing Ltd; 2017:21.
27. Committee on Medical Liability and Risk Management. Policy statement—expert witness participation in civil and criminal proceedings. Pediatrics. 2009;124(1):428-438.
28. Mattco Forge, Inc., v Arthur Young & Co., 6 Cal Rptr 2d 781 (Cal Ct App 1992).
29. Marrogi v Howard, 248 F 3d 382 (5th Cir 2001).
30. Boyes-Bogie v Horvitz, 2001 WL 1771989 (Mass Super 2001).
31. LLMD of Michigan, Inc., v Jackson-Cross Co., 740 A. 2d 186 (Pa 1999).
32. Pollock v Panjabi, 781 A 2d 518 (Conn Super Ct 2000).
33. Brodsky SL, Wilson JK. Empathy in forensic evaluations: a systematic reconsideration. Behav Sci Law. 2013;31(2):192-202.
34. Heilbrun K, DeMatteo D, Marczyk G, et al. Standards of practice and care in forensic mental health assessment: legal, professional, and principles-based consideration. Psych Pub Pol L. 2008;14(1):1-26.
35. Appelbaum PS. Law & psychiatry: policing expert testimony: the role of professional organizations. Psychiatr Serv. 2002;53(4):389-390,399.
36. Austin v American Association of Neurological Surgeons, 253 F 3d 967 (7th Cir 2001).
37. Gutheil TG, Simon RI. Attorneys’ pressures on the expert witness: early warning signs of endangered honesty, objectivity, and fair compensation. J Am Acad Psychiatry Law. 1999;27(4):546-553; discussion 554-562.
38. Gold LH, Anfang SA, Drukteinis AM, et al. AAPL practice guideline for the forensic evaluation of psychiatric disability. J Am Acad Psychiatry Law. 2008;36(suppl 4):S3-S50.
39. Knoll JL IV, Resnick PJ. Deposition dos and don’ts: how to answer 8 tricky questions. Current Psychiatry. 2008;7(3):25-28,36,39-40.
40. Hoge MA, Tebes JK, Davidson L, et al. The roles of behavioral health professionals in class action litigation. J Am Acad Psychiatry Law. 2002;30(1):49-58; discussion 59-64.
41. Simon RI, Shuman DW. Conducting forensic examinations on the road: are you practicing your profession without a license? Licensure requirements for out-of-state forensic examinations. J Am Acad Psychiatry Law. 2001;29(1):75-82.
42. Reid WH. Licensure requirements for out-of-state forensic examinations. J Am Acad Psychiatry Law. 2000;28(4):433-437.
43. Collins B, ed. When in doubt, tell the truth: and other quotations from Mark Twain. New York, NY: Columbia University Press; 1997.
Suspicious, sleepless, and smoking
CASE Sleepless, hallucinating
Mr. F, age 30, is brought to the emergency department (ED) by his brother, with whom he has been living for the last 2 days; his brother says that Mr. F’s wife is afraid of her husband and concerned about her children’s safety. Mr. F has been talking to himself, saying “odd things,” and has an unpredictable temper. He claims that his long-deceased father is alive and telling him “to move to a land that he brought [sic] for him.” In order to follow his father’s instructions, Mr. F says he wants to “see the ambassador so he can get his passport ready.” He also believes his wife and children are intruders in his home. Although he had never smoked before, Mr. F has started smoking ≥2 packs of cigarettes per day, sometimes smoking a pack in 30 minutes. He has not eaten or slept for the last 2 days and lies awake in bed all night staring at the ceiling and smiling to himself.
On examination, Mr. F is short with a slight build and has large, dark eyes, disheveled, short, brown hair, and a scraggly beard. English is not his first language, and he speaks with a thick Eastern European accent. His speech is latent, monotonous, tangential, and illogical. He is alert, oriented only to his person, and says he is 21 or 27 years old and at the hospital for “smoking medication and that’s it.” Despite immigrating to the United States 8 years ago, Mr. F claims he has spent his whole life “here,” although he is unsure of exactly where that is. Cognition and memory are impaired. Regarding his wife and 5 children, he says, “I am a virgin. How then can I have children? That woman is abusing me by forcefully entering my house with 5 kids.” He is fidgety, appears anxious, and does not make eye contact with the examiner during the interview. He is suspicious and irritable. Initial medical workup in the ED is negative.
[polldaddy:9813268]
EVALUATION Labs and observation
Because Mr. F had delusions and hallucinations for the past 2 days and the initial medical workup was negative, brief psychotic disorder is suspected.1 He is admitted to a secure psychiatric floor for further evaluation. He has no documented medical history. A thorough medical workup for a cause of his hallucinations and delusions, including EEG and brain MRI, is negative. Additional collateral interviews with Mr. F’s wife and brother at a family meeting indicate Mr. F had a slow onset of symptoms that began 4 to 5 years ago. Initially, he became isolated, withdrawn, inactive, and had poor sleep. Recently, he also had become suspicious, irritable, delusional, and hallucinatory. Mr. F used to work full-time in construction, then began working intermittently in a warehouse as a day laborer, but has not worked for the last few months. He used to be an involved father and reliable partner, helping with household chores and caring for the children. However, for the last few months, he had become increasingly apathetic and isolated.
During the comprehensive workup for psychosis, Mr. F’s symptoms continue. He is disoriented; although it is 2015, he states it is “2007… I carry a cell phone so I don’t need to know.” On July 31, he is told the date, and for several days after that, he states that it is July 31. When asked his birth date, he looks at his hospital wrist ID. His affect is flat, but he states he feels “fine” and smiles at inappropriate times. He answers open-ended questions briefly, with irrelevant or illogical answers after long pauses, or not at all. His eye contact is poor; he seems preoccupied with internal stimuli, and it is difficult to keep his attention.
Mr. F says he is a “natural-born Bosnian gypsy translator,” and that he needs to finish “building the warehouse” with his father and grandfather (both are deceased). The nurses note that he is withdrawn, inactive, and suspicious; he spends most of the day lying in bed awake, and in the evening he paces in the hallway. Mr. F does not interact with other patients, is guarded when questioned, and does not eat much. He has minimal insight into his condition and says that he is at the hospital for “fevers and a cold,” “ESL treatment,” or because his “right side is thicker” than his left. It is unclear what Mr. F means by “ESL.” It may refer to English as a Second Language, given his apparent perseveration regarding his immigration status and language ability, but this is speculation.
[polldaddy:9813271]
TREATMENT Residual symptoms
With the additional collateral history and a negative medical workup, Mr. F meets DSM-5 criteria for acute, first-episode schizophrenia1 and is started on risperidone, 2 mg/d, titrated up to 2 mg twice daily, and trazodone, 50 mg, as needed, as a sleep aid. He shows significant improvement in his symptoms early in his treatment course. During visiting hours and at family meetings, he recognizes his wife, and during interviews he denies any continuing hallucinations. He initially says that he never failed to recognize his wife and kids, but later explains that he “woke up different…from a dream, and she was a different woman.” When asked specifically about hearing his father’s voice, he is uncertain, saying “No,” “I don’t know,” “I didn’t hear,” or “Not anymore.”
Despite his improvement, Mr. F continues to be disoriented and suspicious, and has minimal insight into his illness. He also continues to exhibit significant negative symptoms and cognitive impairment. Mr. F is withdrawn and has a flat affect, poverty of speech, delayed processing, and poor focus and attention.
On hospital Day 6, Mr. F reports feeling depressed. He misses his children and wants to go home. He has lost several pounds because he had a poor appetite and is now underweight. He is apathetic; interactions with staff and patients are minimal, he declines to attend group therapy sessions, and he still spends most of his time lying in bed awake or pacing the hallway. He also expresses a desire to quit smoking.
[polldaddy:9813273]
The authors’ observations
Despite its lack of specific inclusion in the DSM-5 criteria,1 cognitive impairment is a distinct, core, and nearly universal feature of schizophrenia. As demonstrated by Mr. F’s case, the severity of cognitive impairment in schizophrenia has no association with the positive symptoms of schizophrenia; it is a patient’s neurocognitive abilities—not the severity of his (her) psychotic symptoms—that most strongly predict functional outcomes.2
Neurocognitive impairment is a strong contributor to and predictor of disability in schizophrenia.3,4 Treatment of the cognitive symptoms of schizophrenia with antipsychotics has been largely ineffective.2 Effective drug therapy regimens are still being developed, and although there are some promising novel targets, no drug is FDA-approved to treat the cognitive symptoms of schizophrenia.2,4 However, it is known that additional treatment modalities, including social skills training and/or vocational rehabilitation, as well as treatment of comorbid conditions, may lead to improved cognitive status and, as a result, improved functional outcomes in schizophrenia.2-4
It is well documented that persons with schizophrenia in households with high expressed emotion (EE) have higher rates of relapse, independent of demographics and pharmacotherapy.5 EE is a measure of the family environment that evaluates how the relatives of a psychiatric patient spontaneously talk about the patient. Relatives are considered to have high EE if they show hostility or marked emotional overinvolvement, or if they make a certain number of critical comments. The tool used to measure EE is the Camberwell Family Interview Schedule.6,7 Rates of first-year relapse in high EE homes when family treatment is employed drop significantly, especially when combined with social skills training.8 The patient’s family members are educated about EE and its potential negative effects on the patient.
Cognitive remediation therapy (CRT) uses therapist-led, computer-based techniques to preserve intact neuroplasticity and has been shown to improve cognition and functional status, especially when paired with vocational rehabilitation or social skills training.2,3 Many trials confirm that CRT produces meaningful, durable improvements in cognition and functioning.3 One systematic review that focused on trials in early schizophrenia found that CRT had a significant effect on functioning and symptoms, and that these effects were larger when CRT was combined with adjunctive psychiatric rehabilitation and small group interventions.3
OUTCOME Gradual improvement
Mr. F is started on nicotine gum, 2 mg/d, for smoking cessation and fluoxetine, 20 mg/d, for depression, and a dietary consult is made for his poor appetite and weight loss. His psychotic symptoms continue to improve, and by hospital Day 10, his depressive symptoms begin to improve as well: his affect brightens, he has increased appetite, and he wants to shave. He also exhibits mildly increased insight into his illness.
Mr. F is discharged with risperidone, 2 mg twice daily, for schizophrenia, fluoxetine, 20 mg/d, for depression, and trazodone, 50 mg, as needed, for sleep, and is referred to a community mental health center for comprehensive follow-up, including vocational rehabilitation and social skills training.
The authors’ observations
A major goal of the National Institute of Mental Health’s Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) initiative was to develop a consensus cognitive battery for clinical trials of cognition-enhancing treatments for schizophrenia. The MATRICS Consensus Cognitive Battery (MCCB) is a comprehensive cognitive assessment designed for use in patients with schizophrenia (Table 39). Although the MCCB was developed to be the standard tool for assessing cognitive change in clinical trials of cognition-enhancing drugs for schizophrenia, it also may aid evaluation of cognitive remediation strategies.9
In Mr. F’s case, such testing was not performed, in part because of his improvement. The MoCA was chosen because it is a universally accepted brief cognitive assessment tool used for screening. More robust testing can be administered by the neuropsychiatry team if indicated and if resources are available.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Nasrallah HA, Keefe RS, Javitt DC. Cognitive deficits and poor functional outcomes in schizophrenia: clinical and neurobiological progress. Current Psychiatry. 2014;13(6):S1-S11.
3. Revell ER, Neill JC, Harte M, et al. A systematic review and meta-analysis of cognitive remediation in early schizophrenia. Schizophr Res. 2015;168(1-2):213-222.
4. Goff DC, Hill M, Barch D. The treatment of cognitive impairment in schizophrenia. Pharmacol Biochem Behav. 2011;99(2):245-253.
5. Bebbington P, Kuipers L. The predictive utility of expressed emotion in schizophrenia: an aggregate analysis. Psychol Med. 1994;24(3):707-718.
6. Butzlaff RL, Hooley JM. Expressed emotion and psychiatric relapse: a meta-analysis. Arch Gen Psychiatry. 1998;55(6):547-552.
7. Vaughn C, Leff J. The measurement of expressed emotion in the families of psychiatric patients. Br J Soc Clin Psychol. 1976;15(2):157-165.
8. Hogarty GE, Anderson CM, Reiss DJ, et al. Family psychoeducation, social skills training, and maintenance chemotherapy in the aftercare treatment of schizophrenia. I. One-year effects of a controlled study on relapse and expressed emotion. Arch Gen Psychiatry. 1986;43(7):633-642.
9. Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203-213.
CASE Sleepless, hallucinating
Mr. F, age 30, is brought to the emergency department (ED) by his brother, with whom he has been living for the last 2 days; his brother says that Mr. F’s wife is afraid of her husband and concerned about her children’s safety. Mr. F has been talking to himself, saying “odd things,” and has an unpredictable temper. He claims that his long-deceased father is alive and telling him “to move to a land that he brought [sic] for him.” In order to follow his father’s instructions, Mr. F says he wants to “see the ambassador so he can get his passport ready.” He also believes his wife and children are intruders in his home. Although he had never smoked before, Mr. F has started smoking ≥2 packs of cigarettes per day, sometimes smoking a pack in 30 minutes. He has not eaten or slept for the last 2 days and lies awake in bed all night staring at the ceiling and smiling to himself.
On examination, Mr. F is short with a slight build and has large, dark eyes, disheveled, short, brown hair, and a scraggly beard. English is not his first language, and he speaks with a thick Eastern European accent. His speech is latent, monotonous, tangential, and illogical. He is alert, oriented only to his person, and says he is 21 or 27 years old and at the hospital for “smoking medication and that’s it.” Despite immigrating to the United States 8 years ago, Mr. F claims he has spent his whole life “here,” although he is unsure of exactly where that is. Cognition and memory are impaired. Regarding his wife and 5 children, he says, “I am a virgin. How then can I have children? That woman is abusing me by forcefully entering my house with 5 kids.” He is fidgety, appears anxious, and does not make eye contact with the examiner during the interview. He is suspicious and irritable. Initial medical workup in the ED is negative.
[polldaddy:9813268]
EVALUATION Labs and observation
Because Mr. F had delusions and hallucinations for the past 2 days and the initial medical workup was negative, brief psychotic disorder is suspected.1 He is admitted to a secure psychiatric floor for further evaluation. He has no documented medical history. A thorough medical workup for a cause of his hallucinations and delusions, including EEG and brain MRI, is negative. Additional collateral interviews with Mr. F’s wife and brother at a family meeting indicate Mr. F had a slow onset of symptoms that began 4 to 5 years ago. Initially, he became isolated, withdrawn, inactive, and had poor sleep. Recently, he also had become suspicious, irritable, delusional, and hallucinatory. Mr. F used to work full-time in construction, then began working intermittently in a warehouse as a day laborer, but has not worked for the last few months. He used to be an involved father and reliable partner, helping with household chores and caring for the children. However, for the last few months, he had become increasingly apathetic and isolated.
During the comprehensive workup for psychosis, Mr. F’s symptoms continue. He is disoriented; although it is 2015, he states it is “2007… I carry a cell phone so I don’t need to know.” On July 31, he is told the date, and for several days after that, he states that it is July 31. When asked his birth date, he looks at his hospital wrist ID. His affect is flat, but he states he feels “fine” and smiles at inappropriate times. He answers open-ended questions briefly, with irrelevant or illogical answers after long pauses, or not at all. His eye contact is poor; he seems preoccupied with internal stimuli, and it is difficult to keep his attention.
Mr. F says he is a “natural-born Bosnian gypsy translator,” and that he needs to finish “building the warehouse” with his father and grandfather (both are deceased). The nurses note that he is withdrawn, inactive, and suspicious; he spends most of the day lying in bed awake, and in the evening he paces in the hallway. Mr. F does not interact with other patients, is guarded when questioned, and does not eat much. He has minimal insight into his condition and says that he is at the hospital for “fevers and a cold,” “ESL treatment,” or because his “right side is thicker” than his left. It is unclear what Mr. F means by “ESL.” It may refer to English as a Second Language, given his apparent perseveration regarding his immigration status and language ability, but this is speculation.
[polldaddy:9813271]
TREATMENT Residual symptoms
With the additional collateral history and a negative medical workup, Mr. F meets DSM-5 criteria for acute, first-episode schizophrenia1 and is started on risperidone, 2 mg/d, titrated up to 2 mg twice daily, and trazodone, 50 mg, as needed, as a sleep aid. He shows significant improvement in his symptoms early in his treatment course. During visiting hours and at family meetings, he recognizes his wife, and during interviews he denies any continuing hallucinations. He initially says that he never failed to recognize his wife and kids, but later explains that he “woke up different…from a dream, and she was a different woman.” When asked specifically about hearing his father’s voice, he is uncertain, saying “No,” “I don’t know,” “I didn’t hear,” or “Not anymore.”
Despite his improvement, Mr. F continues to be disoriented and suspicious, and has minimal insight into his illness. He also continues to exhibit significant negative symptoms and cognitive impairment. Mr. F is withdrawn and has a flat affect, poverty of speech, delayed processing, and poor focus and attention.
On hospital Day 6, Mr. F reports feeling depressed. He misses his children and wants to go home. He has lost several pounds because he had a poor appetite and is now underweight. He is apathetic; interactions with staff and patients are minimal, he declines to attend group therapy sessions, and he still spends most of his time lying in bed awake or pacing the hallway. He also expresses a desire to quit smoking.
[polldaddy:9813273]
The authors’ observations
Despite its lack of specific inclusion in the DSM-5 criteria,1 cognitive impairment is a distinct, core, and nearly universal feature of schizophrenia. As demonstrated by Mr. F’s case, the severity of cognitive impairment in schizophrenia has no association with the positive symptoms of schizophrenia; it is a patient’s neurocognitive abilities—not the severity of his (her) psychotic symptoms—that most strongly predict functional outcomes.2
Neurocognitive impairment is a strong contributor to and predictor of disability in schizophrenia.3,4 Treatment of the cognitive symptoms of schizophrenia with antipsychotics has been largely ineffective.2 Effective drug therapy regimens are still being developed, and although there are some promising novel targets, no drug is FDA-approved to treat the cognitive symptoms of schizophrenia.2,4 However, it is known that additional treatment modalities, including social skills training and/or vocational rehabilitation, as well as treatment of comorbid conditions, may lead to improved cognitive status and, as a result, improved functional outcomes in schizophrenia.2-4
It is well documented that persons with schizophrenia in households with high expressed emotion (EE) have higher rates of relapse, independent of demographics and pharmacotherapy.5 EE is a measure of the family environment that evaluates how the relatives of a psychiatric patient spontaneously talk about the patient. Relatives are considered to have high EE if they show hostility or marked emotional overinvolvement, or if they make a certain number of critical comments. The tool used to measure EE is the Camberwell Family Interview Schedule.6,7 Rates of first-year relapse in high EE homes when family treatment is employed drop significantly, especially when combined with social skills training.8 The patient’s family members are educated about EE and its potential negative effects on the patient.
Cognitive remediation therapy (CRT) uses therapist-led, computer-based techniques to preserve intact neuroplasticity and has been shown to improve cognition and functional status, especially when paired with vocational rehabilitation or social skills training.2,3 Many trials confirm that CRT produces meaningful, durable improvements in cognition and functioning.3 One systematic review that focused on trials in early schizophrenia found that CRT had a significant effect on functioning and symptoms, and that these effects were larger when CRT was combined with adjunctive psychiatric rehabilitation and small group interventions.3
OUTCOME Gradual improvement
Mr. F is started on nicotine gum, 2 mg/d, for smoking cessation and fluoxetine, 20 mg/d, for depression, and a dietary consult is made for his poor appetite and weight loss. His psychotic symptoms continue to improve, and by hospital Day 10, his depressive symptoms begin to improve as well: his affect brightens, he has increased appetite, and he wants to shave. He also exhibits mildly increased insight into his illness.
Mr. F is discharged with risperidone, 2 mg twice daily, for schizophrenia, fluoxetine, 20 mg/d, for depression, and trazodone, 50 mg, as needed, for sleep, and is referred to a community mental health center for comprehensive follow-up, including vocational rehabilitation and social skills training.
The authors’ observations
A major goal of the National Institute of Mental Health’s Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) initiative was to develop a consensus cognitive battery for clinical trials of cognition-enhancing treatments for schizophrenia. The MATRICS Consensus Cognitive Battery (MCCB) is a comprehensive cognitive assessment designed for use in patients with schizophrenia (Table 39). Although the MCCB was developed to be the standard tool for assessing cognitive change in clinical trials of cognition-enhancing drugs for schizophrenia, it also may aid evaluation of cognitive remediation strategies.9

In Mr. F’s case, such testing was not performed, in part because of his improvement. The MoCA was chosen because it is a universally accepted brief cognitive assessment tool used for screening. More robust testing can be administered by the neuropsychiatry team if indicated and if resources are available.
CASE Sleepless, hallucinating
Mr. F, age 30, is brought to the emergency department (ED) by his brother, with whom he has been living for the last 2 days; his brother says that Mr. F’s wife is afraid of her husband and concerned about her children’s safety. Mr. F has been talking to himself, saying “odd things,” and has an unpredictable temper. He claims that his long-deceased father is alive and telling him “to move to a land that he brought [sic] for him.” In order to follow his father’s instructions, Mr. F says he wants to “see the ambassador so he can get his passport ready.” He also believes his wife and children are intruders in his home. Although he had never smoked before, Mr. F has started smoking ≥2 packs of cigarettes per day, sometimes smoking a pack in 30 minutes. He has not eaten or slept for the last 2 days and lies awake in bed all night staring at the ceiling and smiling to himself.
On examination, Mr. F is short with a slight build and has large, dark eyes, disheveled, short, brown hair, and a scraggly beard. English is not his first language, and he speaks with a thick Eastern European accent. His speech is latent, monotonous, tangential, and illogical. He is alert, oriented only to his person, and says he is 21 or 27 years old and at the hospital for “smoking medication and that’s it.” Despite immigrating to the United States 8 years ago, Mr. F claims he has spent his whole life “here,” although he is unsure of exactly where that is. Cognition and memory are impaired. Regarding his wife and 5 children, he says, “I am a virgin. How then can I have children? That woman is abusing me by forcefully entering my house with 5 kids.” He is fidgety, appears anxious, and does not make eye contact with the examiner during the interview. He is suspicious and irritable. Initial medical workup in the ED is negative.
[polldaddy:9813268]
EVALUATION Labs and observation
Because Mr. F had delusions and hallucinations for the past 2 days and the initial medical workup was negative, brief psychotic disorder is suspected.1 He is admitted to a secure psychiatric floor for further evaluation. He has no documented medical history. A thorough medical workup for a cause of his hallucinations and delusions, including EEG and brain MRI, is negative. Additional collateral interviews with Mr. F’s wife and brother at a family meeting indicate Mr. F had a slow onset of symptoms that began 4 to 5 years ago. Initially, he became isolated, withdrawn, inactive, and had poor sleep. Recently, he also had become suspicious, irritable, delusional, and hallucinatory. Mr. F used to work full-time in construction, then began working intermittently in a warehouse as a day laborer, but has not worked for the last few months. He used to be an involved father and reliable partner, helping with household chores and caring for the children. However, for the last few months, he had become increasingly apathetic and isolated.
During the comprehensive workup for psychosis, Mr. F’s symptoms continue. He is disoriented; although it is 2015, he states it is “2007… I carry a cell phone so I don’t need to know.” On July 31, he is told the date, and for several days after that, he states that it is July 31. When asked his birth date, he looks at his hospital wrist ID. His affect is flat, but he states he feels “fine” and smiles at inappropriate times. He answers open-ended questions briefly, with irrelevant or illogical answers after long pauses, or not at all. His eye contact is poor; he seems preoccupied with internal stimuli, and it is difficult to keep his attention.
Mr. F says he is a “natural-born Bosnian gypsy translator,” and that he needs to finish “building the warehouse” with his father and grandfather (both are deceased). The nurses note that he is withdrawn, inactive, and suspicious; he spends most of the day lying in bed awake, and in the evening he paces in the hallway. Mr. F does not interact with other patients, is guarded when questioned, and does not eat much. He has minimal insight into his condition and says that he is at the hospital for “fevers and a cold,” “ESL treatment,” or because his “right side is thicker” than his left. It is unclear what Mr. F means by “ESL.” It may refer to English as a Second Language, given his apparent perseveration regarding his immigration status and language ability, but this is speculation.
[polldaddy:9813271]
TREATMENT Residual symptoms
With the additional collateral history and a negative medical workup, Mr. F meets DSM-5 criteria for acute, first-episode schizophrenia1 and is started on risperidone, 2 mg/d, titrated up to 2 mg twice daily, and trazodone, 50 mg, as needed, as a sleep aid. He shows significant improvement in his symptoms early in his treatment course. During visiting hours and at family meetings, he recognizes his wife, and during interviews he denies any continuing hallucinations. He initially says that he never failed to recognize his wife and kids, but later explains that he “woke up different…from a dream, and she was a different woman.” When asked specifically about hearing his father’s voice, he is uncertain, saying “No,” “I don’t know,” “I didn’t hear,” or “Not anymore.”
Despite his improvement, Mr. F continues to be disoriented and suspicious, and has minimal insight into his illness. He also continues to exhibit significant negative symptoms and cognitive impairment. Mr. F is withdrawn and has a flat affect, poverty of speech, delayed processing, and poor focus and attention.
On hospital Day 6, Mr. F reports feeling depressed. He misses his children and wants to go home. He has lost several pounds because he had a poor appetite and is now underweight. He is apathetic; interactions with staff and patients are minimal, he declines to attend group therapy sessions, and he still spends most of his time lying in bed awake or pacing the hallway. He also expresses a desire to quit smoking.
[polldaddy:9813273]
The authors’ observations
Despite its lack of specific inclusion in the DSM-5 criteria,1 cognitive impairment is a distinct, core, and nearly universal feature of schizophrenia. As demonstrated by Mr. F’s case, the severity of cognitive impairment in schizophrenia has no association with the positive symptoms of schizophrenia; it is a patient’s neurocognitive abilities—not the severity of his (her) psychotic symptoms—that most strongly predict functional outcomes.2
Neurocognitive impairment is a strong contributor to and predictor of disability in schizophrenia.3,4 Treatment of the cognitive symptoms of schizophrenia with antipsychotics has been largely ineffective.2 Effective drug therapy regimens are still being developed, and although there are some promising novel targets, no drug is FDA-approved to treat the cognitive symptoms of schizophrenia.2,4 However, it is known that additional treatment modalities, including social skills training and/or vocational rehabilitation, as well as treatment of comorbid conditions, may lead to improved cognitive status and, as a result, improved functional outcomes in schizophrenia.2-4
It is well documented that persons with schizophrenia in households with high expressed emotion (EE) have higher rates of relapse, independent of demographics and pharmacotherapy.5 EE is a measure of the family environment that evaluates how the relatives of a psychiatric patient spontaneously talk about the patient. Relatives are considered to have high EE if they show hostility or marked emotional overinvolvement, or if they make a certain number of critical comments. The tool used to measure EE is the Camberwell Family Interview Schedule.6,7 Rates of first-year relapse in high EE homes when family treatment is employed drop significantly, especially when combined with social skills training.8 The patient’s family members are educated about EE and its potential negative effects on the patient.
Cognitive remediation therapy (CRT) uses therapist-led, computer-based techniques to preserve intact neuroplasticity and has been shown to improve cognition and functional status, especially when paired with vocational rehabilitation or social skills training.2,3 Many trials confirm that CRT produces meaningful, durable improvements in cognition and functioning.3 One systematic review that focused on trials in early schizophrenia found that CRT had a significant effect on functioning and symptoms, and that these effects were larger when CRT was combined with adjunctive psychiatric rehabilitation and small group interventions.3
OUTCOME Gradual improvement
Mr. F is started on nicotine gum, 2 mg/d, for smoking cessation and fluoxetine, 20 mg/d, for depression, and a dietary consult is made for his poor appetite and weight loss. His psychotic symptoms continue to improve, and by hospital Day 10, his depressive symptoms begin to improve as well: his affect brightens, he has increased appetite, and he wants to shave. He also exhibits mildly increased insight into his illness.
Mr. F is discharged with risperidone, 2 mg twice daily, for schizophrenia, fluoxetine, 20 mg/d, for depression, and trazodone, 50 mg, as needed, for sleep, and is referred to a community mental health center for comprehensive follow-up, including vocational rehabilitation and social skills training.
The authors’ observations
A major goal of the National Institute of Mental Health’s Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) initiative was to develop a consensus cognitive battery for clinical trials of cognition-enhancing treatments for schizophrenia. The MATRICS Consensus Cognitive Battery (MCCB) is a comprehensive cognitive assessment designed for use in patients with schizophrenia (Table 39). Although the MCCB was developed to be the standard tool for assessing cognitive change in clinical trials of cognition-enhancing drugs for schizophrenia, it also may aid evaluation of cognitive remediation strategies.9

In Mr. F’s case, such testing was not performed, in part because of his improvement. The MoCA was chosen because it is a universally accepted brief cognitive assessment tool used for screening. More robust testing can be administered by the neuropsychiatry team if indicated and if resources are available.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Nasrallah HA, Keefe RS, Javitt DC. Cognitive deficits and poor functional outcomes in schizophrenia: clinical and neurobiological progress. Current Psychiatry. 2014;13(6):S1-S11.
3. Revell ER, Neill JC, Harte M, et al. A systematic review and meta-analysis of cognitive remediation in early schizophrenia. Schizophr Res. 2015;168(1-2):213-222.
4. Goff DC, Hill M, Barch D. The treatment of cognitive impairment in schizophrenia. Pharmacol Biochem Behav. 2011;99(2):245-253.
5. Bebbington P, Kuipers L. The predictive utility of expressed emotion in schizophrenia: an aggregate analysis. Psychol Med. 1994;24(3):707-718.
6. Butzlaff RL, Hooley JM. Expressed emotion and psychiatric relapse: a meta-analysis. Arch Gen Psychiatry. 1998;55(6):547-552.
7. Vaughn C, Leff J. The measurement of expressed emotion in the families of psychiatric patients. Br J Soc Clin Psychol. 1976;15(2):157-165.
8. Hogarty GE, Anderson CM, Reiss DJ, et al. Family psychoeducation, social skills training, and maintenance chemotherapy in the aftercare treatment of schizophrenia. I. One-year effects of a controlled study on relapse and expressed emotion. Arch Gen Psychiatry. 1986;43(7):633-642.
9. Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203-213.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Nasrallah HA, Keefe RS, Javitt DC. Cognitive deficits and poor functional outcomes in schizophrenia: clinical and neurobiological progress. Current Psychiatry. 2014;13(6):S1-S11.
3. Revell ER, Neill JC, Harte M, et al. A systematic review and meta-analysis of cognitive remediation in early schizophrenia. Schizophr Res. 2015;168(1-2):213-222.
4. Goff DC, Hill M, Barch D. The treatment of cognitive impairment in schizophrenia. Pharmacol Biochem Behav. 2011;99(2):245-253.
5. Bebbington P, Kuipers L. The predictive utility of expressed emotion in schizophrenia: an aggregate analysis. Psychol Med. 1994;24(3):707-718.
6. Butzlaff RL, Hooley JM. Expressed emotion and psychiatric relapse: a meta-analysis. Arch Gen Psychiatry. 1998;55(6):547-552.
7. Vaughn C, Leff J. The measurement of expressed emotion in the families of psychiatric patients. Br J Soc Clin Psychol. 1976;15(2):157-165.
8. Hogarty GE, Anderson CM, Reiss DJ, et al. Family psychoeducation, social skills training, and maintenance chemotherapy in the aftercare treatment of schizophrenia. I. One-year effects of a controlled study on relapse and expressed emotion. Arch Gen Psychiatry. 1986;43(7):633-642.
9. Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203-213.
Should psychologists be allowed to prescribe?
In response to Dr. Nasrallah’s editorial “Prescribing is the culmination of extensive medical training and psychologists don’t qualify” (From the Editor,
Tedd Judd, PhD, ABPP-CN
Diplomate in Clinical Neuropsychology
Certified Hispanic Mental Health Specialist
Cross-Cultural Specialist
Bellingham, Washington
I read Dr. Nasrallah’s editorial with a critical eye. As a psychologist in private clinical and forensic practice for more than 30 years, it is disheartening that you toe the politico-economic line proffered over the decades that establishes and buoys a clash between our helping professions in the hoary guise of protecting the consuming public.
It is disingenuous and misleading for you to cite “28,000 hours of training… 8 years of medical school” as a prerequisite for having adequate “psychopharmacological skills.”
Psychologists and psychiatrists can learn the same necessary and comprehensive skills to perform competent and equivalent prescription duties in succinct, operational ways.
It is about time the welfare of the consuming public be served instead of territorial profiteering. Perhaps you should focus more on the dwindling numbers of psychiatrists who perform psychotherapy in conjunction with psychopharmacology than on limiting the pool of providers who are qualified by training to do both. How many of those 28,000 hours are dedicated to training your psychiatrists in psychotherapy?
Norman R. Klein, PhD
Licensed Psychologist
Westport, Connecticut
Dr. Nasrallah wrote an unsurprisingly eloquent and passionate editorial and argues a cogent case for restricting prescription privileges to medically trained professionals. I wonder, though, if public health statistics of outcomes among mental health patients in states where clinical psychologists have been licensed to prescribe, such as New Mexico and Hawaii, bear out any of Dr. Nasrallah’s concerns.
Ole J. Thienhaus, MD, MBA
Department Head and Professor of Psychiatry
University of Arizona
College of Medicine-Tucson
Tucson, Arizona
Dr. Nasrallah responds
I am not surprised by Dr. Judd’s or Dr. Klein’s disagreement with my editorial asserting that psychologists do not receive the medical training that qualifies them to prescribe. They side with their fellow psychologists, just as psychiatrists agree with me. After all, those of us who have had the extensive training of psychiatric physicians know the abundance of medical skills needed for competent prescribing and find it preposterous that psychologists, who have a PhD and are acknowledged for their psychotherapy and psychometric skills, can take a drastic shortcut by getting politicians to give them the right to prescribe. Dr. Klein has no idea how much training it takes to become a competent prescriber, so his comments that both psychiatrists and psychologists can be similarly trained cannot be taken seriously. Even after 4 years of psychiatric residency with daily psychopharmacology teaching and training psychiatrists still feel they have much more to learn. It is dangerous hubris to think that even without the vital medical school foundation prior to psychiatric training that psychologists can
Here, I provide a description of one state’s proposed the training that psychologists would receive. I hope that Drs. Judd and Klein will recognize the dangerously inadequate training recently proposed for psychologists to become “prescribers.”
Proposed curriculum for psychologists
1. Online instruction, not face-to-face classroom experience
2. Many courses are prerecorded
3. Instructors are psychologists, not psychiatrists
4. Psychologists can complete the program at their own pace, which can be done in a few weeks
5. Hours of instruction range between 306 to 468 hours, compared with 500 hours required for massage therapists
6. A minimum of 40 hours of “basic training on clinical assessment” is required, compared with 60 hours for electrologists
7. The “graduate” must pass a test prepared by the American Psychological Association, which advocates for prescriptive authority and is not an independent testing organization
8. There is no minimum of requirements of an undergraduate biomedical prerequisite course—the work that is required for all medical students, physician assistants, and nursing students—which includes chemistry or biochemistry (with laboratory experience), human anatomy, physiology, general biology, microbiology (with laboratory experience), cell biology, and molecular biology
9. Recommended number of patient encounters is anemic: 600 encounters, which can be 10 encounters with 60 patients or 15 encounters with 40 patients. This is far below what is required of psychiatric residents
10. The proposed training requires treating a minimum of 75 patients over 2 years. A typical third-year psychiatric resident sees 75 patients every month. Each first- and second-year resident works up and treats >600 inpatients in <1 year
11. At the end of the practicum, applicants must demonstrate competency in 9 milestones, but competency is not defined. In contrast, psychiatric residency programs have mandates from the Accreditation Council for Graduate Medical Education requiring that residents be graded every 6 months on 23 milestones, with specific anchor points provided
12. Only 25% of the practicum occurs on psychiatric inpatient wards or outpatient clinics. One wonders where the patients who need psychopharmacology would be
13. Supervision is inadequate. There is no requirement for supervision by psychiatrists, whose training and experience make them qualified psychopharmacologists
14. There is no guidance on the frequency or intensity of supervision. In psychiatry, residents are supervised with each patient encounter over 4 years. Should psychologists without medical training be held to a lesser standard?
15. There are no specifications of continuing medical education, ongoing supervision, or outcomes
16. The potential dangers of psychotropics are not emphasized. For example:
• permanent or life-threatening adverse effects, such as tardive dyskinesia or agranulocytosis
• addiction potential, such as with stimulants or benzodiazepines
• potentially fatal drug interactions with monoamine oxidase inhibitors and meperidine or serotonin syndrome, or cardiac arrests with overdoses of tricyclic antidepressants
17. Many medications require ongoing monitoring. Some involve physical examination (extrapyramidal side effects, metabolic syndrome) or laboratory tests (lithium, carbamazepine, clozapine, valproate, renal and hepatic functions, metabolic profile for all antipsychotics). Failure to monitor may lead to fatal outcomes. Some medications are considered unsafe during pregnancy or breast-feeding.
Psychologists do a great service for patients with mental illness by providing evidence-based psychotherapies, such as cognitive-behavioral, dialectical-behavioral, interpersonal, and behavioral therapy. They complement what psychiatrists and nurse practitioners do with pharmacotherapy. Many patients with mild or moderate psychiatric disorders improve significantly with psychotherapy without the use of psychotropics. Psychologists should focus on what they were trained to do because they can benefit numerous patients. That is much better than trying to become prescribers and practice mediocre psychopharmacology without the requisite medical training. Patients with mental illness deserve no less.
Henry A. Nasrallah, MD
Professor and Chair
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
In response to Dr. Nasrallah’s editorial “Prescribing is the culmination of extensive medical training and psychologists don’t qualify” (From the Editor,
Tedd Judd, PhD, ABPP-CN
Diplomate in Clinical Neuropsychology
Certified Hispanic Mental Health Specialist
Cross-Cultural Specialist
Bellingham, Washington
I read Dr. Nasrallah’s editorial with a critical eye. As a psychologist in private clinical and forensic practice for more than 30 years, it is disheartening that you toe the politico-economic line proffered over the decades that establishes and buoys a clash between our helping professions in the hoary guise of protecting the consuming public.
It is disingenuous and misleading for you to cite “28,000 hours of training… 8 years of medical school” as a prerequisite for having adequate “psychopharmacological skills.”
Psychologists and psychiatrists can learn the same necessary and comprehensive skills to perform competent and equivalent prescription duties in succinct, operational ways.
It is about time the welfare of the consuming public be served instead of territorial profiteering. Perhaps you should focus more on the dwindling numbers of psychiatrists who perform psychotherapy in conjunction with psychopharmacology than on limiting the pool of providers who are qualified by training to do both. How many of those 28,000 hours are dedicated to training your psychiatrists in psychotherapy?
Norman R. Klein, PhD
Licensed Psychologist
Westport, Connecticut
Dr. Nasrallah wrote an unsurprisingly eloquent and passionate editorial and argues a cogent case for restricting prescription privileges to medically trained professionals. I wonder, though, if public health statistics of outcomes among mental health patients in states where clinical psychologists have been licensed to prescribe, such as New Mexico and Hawaii, bear out any of Dr. Nasrallah’s concerns.
Ole J. Thienhaus, MD, MBA
Department Head and Professor of Psychiatry
University of Arizona
College of Medicine-Tucson
Tucson, Arizona
Dr. Nasrallah responds
I am not surprised by Dr. Judd’s or Dr. Klein’s disagreement with my editorial asserting that psychologists do not receive the medical training that qualifies them to prescribe. They side with their fellow psychologists, just as psychiatrists agree with me. After all, those of us who have had the extensive training of psychiatric physicians know the abundance of medical skills needed for competent prescribing and find it preposterous that psychologists, who have a PhD and are acknowledged for their psychotherapy and psychometric skills, can take a drastic shortcut by getting politicians to give them the right to prescribe. Dr. Klein has no idea how much training it takes to become a competent prescriber, so his comments that both psychiatrists and psychologists can be similarly trained cannot be taken seriously. Even after 4 years of psychiatric residency with daily psychopharmacology teaching and training psychiatrists still feel they have much more to learn. It is dangerous hubris to think that even without the vital medical school foundation prior to psychiatric training that psychologists can
Here, I provide a description of one state’s proposed the training that psychologists would receive. I hope that Drs. Judd and Klein will recognize the dangerously inadequate training recently proposed for psychologists to become “prescribers.”
Proposed curriculum for psychologists
1. Online instruction, not face-to-face classroom experience
2. Many courses are prerecorded
3. Instructors are psychologists, not psychiatrists
4. Psychologists can complete the program at their own pace, which can be done in a few weeks
5. Hours of instruction range between 306 to 468 hours, compared with 500 hours required for massage therapists
6. A minimum of 40 hours of “basic training on clinical assessment” is required, compared with 60 hours for electrologists
7. The “graduate” must pass a test prepared by the American Psychological Association, which advocates for prescriptive authority and is not an independent testing organization
8. There is no minimum of requirements of an undergraduate biomedical prerequisite course—the work that is required for all medical students, physician assistants, and nursing students—which includes chemistry or biochemistry (with laboratory experience), human anatomy, physiology, general biology, microbiology (with laboratory experience), cell biology, and molecular biology
9. Recommended number of patient encounters is anemic: 600 encounters, which can be 10 encounters with 60 patients or 15 encounters with 40 patients. This is far below what is required of psychiatric residents
10. The proposed training requires treating a minimum of 75 patients over 2 years. A typical third-year psychiatric resident sees 75 patients every month. Each first- and second-year resident works up and treats >600 inpatients in <1 year
11. At the end of the practicum, applicants must demonstrate competency in 9 milestones, but competency is not defined. In contrast, psychiatric residency programs have mandates from the Accreditation Council for Graduate Medical Education requiring that residents be graded every 6 months on 23 milestones, with specific anchor points provided
12. Only 25% of the practicum occurs on psychiatric inpatient wards or outpatient clinics. One wonders where the patients who need psychopharmacology would be
13. Supervision is inadequate. There is no requirement for supervision by psychiatrists, whose training and experience make them qualified psychopharmacologists
14. There is no guidance on the frequency or intensity of supervision. In psychiatry, residents are supervised with each patient encounter over 4 years. Should psychologists without medical training be held to a lesser standard?
15. There are no specifications of continuing medical education, ongoing supervision, or outcomes
16. The potential dangers of psychotropics are not emphasized. For example:
• permanent or life-threatening adverse effects, such as tardive dyskinesia or agranulocytosis
• addiction potential, such as with stimulants or benzodiazepines
• potentially fatal drug interactions with monoamine oxidase inhibitors and meperidine or serotonin syndrome, or cardiac arrests with overdoses of tricyclic antidepressants
17. Many medications require ongoing monitoring. Some involve physical examination (extrapyramidal side effects, metabolic syndrome) or laboratory tests (lithium, carbamazepine, clozapine, valproate, renal and hepatic functions, metabolic profile for all antipsychotics). Failure to monitor may lead to fatal outcomes. Some medications are considered unsafe during pregnancy or breast-feeding.
Psychologists do a great service for patients with mental illness by providing evidence-based psychotherapies, such as cognitive-behavioral, dialectical-behavioral, interpersonal, and behavioral therapy. They complement what psychiatrists and nurse practitioners do with pharmacotherapy. Many patients with mild or moderate psychiatric disorders improve significantly with psychotherapy without the use of psychotropics. Psychologists should focus on what they were trained to do because they can benefit numerous patients. That is much better than trying to become prescribers and practice mediocre psychopharmacology without the requisite medical training. Patients with mental illness deserve no less.
Henry A. Nasrallah, MD
Professor and Chair
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
In response to Dr. Nasrallah’s editorial “Prescribing is the culmination of extensive medical training and psychologists don’t qualify” (From the Editor,
Tedd Judd, PhD, ABPP-CN
Diplomate in Clinical Neuropsychology
Certified Hispanic Mental Health Specialist
Cross-Cultural Specialist
Bellingham, Washington
I read Dr. Nasrallah’s editorial with a critical eye. As a psychologist in private clinical and forensic practice for more than 30 years, it is disheartening that you toe the politico-economic line proffered over the decades that establishes and buoys a clash between our helping professions in the hoary guise of protecting the consuming public.
It is disingenuous and misleading for you to cite “28,000 hours of training… 8 years of medical school” as a prerequisite for having adequate “psychopharmacological skills.”
Psychologists and psychiatrists can learn the same necessary and comprehensive skills to perform competent and equivalent prescription duties in succinct, operational ways.
It is about time the welfare of the consuming public be served instead of territorial profiteering. Perhaps you should focus more on the dwindling numbers of psychiatrists who perform psychotherapy in conjunction with psychopharmacology than on limiting the pool of providers who are qualified by training to do both. How many of those 28,000 hours are dedicated to training your psychiatrists in psychotherapy?
Norman R. Klein, PhD
Licensed Psychologist
Westport, Connecticut
Dr. Nasrallah wrote an unsurprisingly eloquent and passionate editorial and argues a cogent case for restricting prescription privileges to medically trained professionals. I wonder, though, if public health statistics of outcomes among mental health patients in states where clinical psychologists have been licensed to prescribe, such as New Mexico and Hawaii, bear out any of Dr. Nasrallah’s concerns.
Ole J. Thienhaus, MD, MBA
Department Head and Professor of Psychiatry
University of Arizona
College of Medicine-Tucson
Tucson, Arizona
Dr. Nasrallah responds
I am not surprised by Dr. Judd’s or Dr. Klein’s disagreement with my editorial asserting that psychologists do not receive the medical training that qualifies them to prescribe. They side with their fellow psychologists, just as psychiatrists agree with me. After all, those of us who have had the extensive training of psychiatric physicians know the abundance of medical skills needed for competent prescribing and find it preposterous that psychologists, who have a PhD and are acknowledged for their psychotherapy and psychometric skills, can take a drastic shortcut by getting politicians to give them the right to prescribe. Dr. Klein has no idea how much training it takes to become a competent prescriber, so his comments that both psychiatrists and psychologists can be similarly trained cannot be taken seriously. Even after 4 years of psychiatric residency with daily psychopharmacology teaching and training psychiatrists still feel they have much more to learn. It is dangerous hubris to think that even without the vital medical school foundation prior to psychiatric training that psychologists can
Here, I provide a description of one state’s proposed the training that psychologists would receive. I hope that Drs. Judd and Klein will recognize the dangerously inadequate training recently proposed for psychologists to become “prescribers.”
Proposed curriculum for psychologists
1. Online instruction, not face-to-face classroom experience
2. Many courses are prerecorded
3. Instructors are psychologists, not psychiatrists
4. Psychologists can complete the program at their own pace, which can be done in a few weeks
5. Hours of instruction range between 306 to 468 hours, compared with 500 hours required for massage therapists
6. A minimum of 40 hours of “basic training on clinical assessment” is required, compared with 60 hours for electrologists
7. The “graduate” must pass a test prepared by the American Psychological Association, which advocates for prescriptive authority and is not an independent testing organization
8. There is no minimum of requirements of an undergraduate biomedical prerequisite course—the work that is required for all medical students, physician assistants, and nursing students—which includes chemistry or biochemistry (with laboratory experience), human anatomy, physiology, general biology, microbiology (with laboratory experience), cell biology, and molecular biology
9. Recommended number of patient encounters is anemic: 600 encounters, which can be 10 encounters with 60 patients or 15 encounters with 40 patients. This is far below what is required of psychiatric residents
10. The proposed training requires treating a minimum of 75 patients over 2 years. A typical third-year psychiatric resident sees 75 patients every month. Each first- and second-year resident works up and treats >600 inpatients in <1 year
11. At the end of the practicum, applicants must demonstrate competency in 9 milestones, but competency is not defined. In contrast, psychiatric residency programs have mandates from the Accreditation Council for Graduate Medical Education requiring that residents be graded every 6 months on 23 milestones, with specific anchor points provided
12. Only 25% of the practicum occurs on psychiatric inpatient wards or outpatient clinics. One wonders where the patients who need psychopharmacology would be
13. Supervision is inadequate. There is no requirement for supervision by psychiatrists, whose training and experience make them qualified psychopharmacologists
14. There is no guidance on the frequency or intensity of supervision. In psychiatry, residents are supervised with each patient encounter over 4 years. Should psychologists without medical training be held to a lesser standard?
15. There are no specifications of continuing medical education, ongoing supervision, or outcomes
16. The potential dangers of psychotropics are not emphasized. For example:
• permanent or life-threatening adverse effects, such as tardive dyskinesia or agranulocytosis
• addiction potential, such as with stimulants or benzodiazepines
• potentially fatal drug interactions with monoamine oxidase inhibitors and meperidine or serotonin syndrome, or cardiac arrests with overdoses of tricyclic antidepressants
17. Many medications require ongoing monitoring. Some involve physical examination (extrapyramidal side effects, metabolic syndrome) or laboratory tests (lithium, carbamazepine, clozapine, valproate, renal and hepatic functions, metabolic profile for all antipsychotics). Failure to monitor may lead to fatal outcomes. Some medications are considered unsafe during pregnancy or breast-feeding.
Psychologists do a great service for patients with mental illness by providing evidence-based psychotherapies, such as cognitive-behavioral, dialectical-behavioral, interpersonal, and behavioral therapy. They complement what psychiatrists and nurse practitioners do with pharmacotherapy. Many patients with mild or moderate psychiatric disorders improve significantly with psychotherapy without the use of psychotropics. Psychologists should focus on what they were trained to do because they can benefit numerous patients. That is much better than trying to become prescribers and practice mediocre psychopharmacology without the requisite medical training. Patients with mental illness deserve no less.
Henry A. Nasrallah, MD
Professor and Chair
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
Can melatonin alleviate antipsychotic-induced weight gain?
Second-generation antipsychotics (SGAs) have been a remarkably effective innovation in psychotropic therapy. Unfortunately, the metabolic effects of these medications
Modabbernia et al1 demonstrated positive results from melatonin augmentation in an 8-week, randomized, double-blind, placebo-controlled study of 48 patients with first-episode schizophrenia. Compared with patients who received olanzapine and placebo, those taking olanzapine and melatonin, 3 mg/d, had significantly less weight gain, smaller increases in abdominal obesity, and lower triglycerides. Patients who were given melatonin also had a significantly greater reduction on the Positive and Negative Symptom Scale score.1
Romo-Nava et al2 had similar findings in an 8-week, randomized, double-blind, placebo-controlled trial. Forty-four patients (24 with schizophrenia, 20 with bipolar disorder) who were taking clozapine, quetiapine, risperidone, or olanzapine received adjunctive melatonin, 5 mg/d, or placebo. Patients receiving melatonin had significantly less weight gain (P = .04) and significantly reduced diastolic blood pressure (5.1 vs 1.1 mm Hg; P = .03).
In both studies, researchers hypothesized that melatonin exerted its effect through the suprachiasmatic nucleus—the part of the hypothalamus that regulates body weight, energy balance, and metabolism. Exogenous melatonin suppresses intra-abdominal fat and restores serum leptin and insulin levels in middle-aged rats, partly due to correcting the age-related decline in melatonin production.3
Wang et al4 conducted a systematic review of using melatonin in patients taking SGAs. In addition to preventing metabolic adverse effects of antipsychotics, melatonin also reduced weight gain from lithium.
Early evidence suggests that this inexpensive and relatively safe augmenting agent can minimize metabolic effects of SGAs. It is surprising that scheduled melatonin has eluded popular use in psychiatry.
1. Modabbernia A, Heidari P, Soleimani R, et al. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2014;53:133-140.
2. Romo-Nava F, Alvarez-Icaza González D, Fresán-Orellana A, et al. Melatonin attenuates antipsychotic metabolic effects: an eight-week randomized, double-blind, parallel-group, placebo-controlled clinical trial. Bipolar Disord. 2014;16(4):410-421.
3. Rasmussen DD, Marck BT, Boldt BM, et al. Suppression of hypothalamic pro-opiomelanocortin (POMC) gene expression by daily melatonin supplementation in aging rats. J Pineal Res. 2003;34(2):127-133.
4. Wang HR, Woo YS, Bahk WM. The role of melatonin and melatonin agonists in counteracting antipsychotic-induced metabolic side effects: a systematic review. Int Clin Psychopharmacol. 2016;31(6):301-306.
Second-generation antipsychotics (SGAs) have been a remarkably effective innovation in psychotropic therapy. Unfortunately, the metabolic effects of these medications
Modabbernia et al1 demonstrated positive results from melatonin augmentation in an 8-week, randomized, double-blind, placebo-controlled study of 48 patients with first-episode schizophrenia. Compared with patients who received olanzapine and placebo, those taking olanzapine and melatonin, 3 mg/d, had significantly less weight gain, smaller increases in abdominal obesity, and lower triglycerides. Patients who were given melatonin also had a significantly greater reduction on the Positive and Negative Symptom Scale score.1
Romo-Nava et al2 had similar findings in an 8-week, randomized, double-blind, placebo-controlled trial. Forty-four patients (24 with schizophrenia, 20 with bipolar disorder) who were taking clozapine, quetiapine, risperidone, or olanzapine received adjunctive melatonin, 5 mg/d, or placebo. Patients receiving melatonin had significantly less weight gain (P = .04) and significantly reduced diastolic blood pressure (5.1 vs 1.1 mm Hg; P = .03).
In both studies, researchers hypothesized that melatonin exerted its effect through the suprachiasmatic nucleus—the part of the hypothalamus that regulates body weight, energy balance, and metabolism. Exogenous melatonin suppresses intra-abdominal fat and restores serum leptin and insulin levels in middle-aged rats, partly due to correcting the age-related decline in melatonin production.3
Wang et al4 conducted a systematic review of using melatonin in patients taking SGAs. In addition to preventing metabolic adverse effects of antipsychotics, melatonin also reduced weight gain from lithium.
Early evidence suggests that this inexpensive and relatively safe augmenting agent can minimize metabolic effects of SGAs. It is surprising that scheduled melatonin has eluded popular use in psychiatry.
Second-generation antipsychotics (SGAs) have been a remarkably effective innovation in psychotropic therapy. Unfortunately, the metabolic effects of these medications
Modabbernia et al1 demonstrated positive results from melatonin augmentation in an 8-week, randomized, double-blind, placebo-controlled study of 48 patients with first-episode schizophrenia. Compared with patients who received olanzapine and placebo, those taking olanzapine and melatonin, 3 mg/d, had significantly less weight gain, smaller increases in abdominal obesity, and lower triglycerides. Patients who were given melatonin also had a significantly greater reduction on the Positive and Negative Symptom Scale score.1
Romo-Nava et al2 had similar findings in an 8-week, randomized, double-blind, placebo-controlled trial. Forty-four patients (24 with schizophrenia, 20 with bipolar disorder) who were taking clozapine, quetiapine, risperidone, or olanzapine received adjunctive melatonin, 5 mg/d, or placebo. Patients receiving melatonin had significantly less weight gain (P = .04) and significantly reduced diastolic blood pressure (5.1 vs 1.1 mm Hg; P = .03).
In both studies, researchers hypothesized that melatonin exerted its effect through the suprachiasmatic nucleus—the part of the hypothalamus that regulates body weight, energy balance, and metabolism. Exogenous melatonin suppresses intra-abdominal fat and restores serum leptin and insulin levels in middle-aged rats, partly due to correcting the age-related decline in melatonin production.3
Wang et al4 conducted a systematic review of using melatonin in patients taking SGAs. In addition to preventing metabolic adverse effects of antipsychotics, melatonin also reduced weight gain from lithium.
Early evidence suggests that this inexpensive and relatively safe augmenting agent can minimize metabolic effects of SGAs. It is surprising that scheduled melatonin has eluded popular use in psychiatry.
1. Modabbernia A, Heidari P, Soleimani R, et al. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2014;53:133-140.
2. Romo-Nava F, Alvarez-Icaza González D, Fresán-Orellana A, et al. Melatonin attenuates antipsychotic metabolic effects: an eight-week randomized, double-blind, parallel-group, placebo-controlled clinical trial. Bipolar Disord. 2014;16(4):410-421.
3. Rasmussen DD, Marck BT, Boldt BM, et al. Suppression of hypothalamic pro-opiomelanocortin (POMC) gene expression by daily melatonin supplementation in aging rats. J Pineal Res. 2003;34(2):127-133.
4. Wang HR, Woo YS, Bahk WM. The role of melatonin and melatonin agonists in counteracting antipsychotic-induced metabolic side effects: a systematic review. Int Clin Psychopharmacol. 2016;31(6):301-306.
1. Modabbernia A, Heidari P, Soleimani R, et al. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2014;53:133-140.
2. Romo-Nava F, Alvarez-Icaza González D, Fresán-Orellana A, et al. Melatonin attenuates antipsychotic metabolic effects: an eight-week randomized, double-blind, parallel-group, placebo-controlled clinical trial. Bipolar Disord. 2014;16(4):410-421.
3. Rasmussen DD, Marck BT, Boldt BM, et al. Suppression of hypothalamic pro-opiomelanocortin (POMC) gene expression by daily melatonin supplementation in aging rats. J Pineal Res. 2003;34(2):127-133.
4. Wang HR, Woo YS, Bahk WM. The role of melatonin and melatonin agonists in counteracting antipsychotic-induced metabolic side effects: a systematic review. Int Clin Psychopharmacol. 2016;31(6):301-306.
Understanding Social Media in GI Practice: Influence, Learn, and Prosper
Gone are the days when social media was primarily used by millennials and those early adopters on the diffusion-of-innovation curve. Now, baby boomers and laggards alike are using social media to communicate with the world around them. Furthermore, health and healthcare issues are common topics in the social media universe. Eight in 10 Internet users seek health information online and 74% of these health information seekers use social media.1,2 Additionally, when they look online, they are more likely to trust information from doctors (61%) than from hospitals (55%), insurers (42%), or pharmaceutical companies (37%).3 Therefore, there is tremendous opportunity for physicians to engage patients, policy makers, advocacy groups, and other health care influencers in order to share reliable information. Yet, we must do so responsibly. There is a considerable degree of misinformation circulated in social media and we believe that physicians should help combat this by providing accurate information.
On a more individual level, social media can help you stay up-to-date on best practices, breakthroughs, and controversies in medicine. It can help you take control of your online reputation rather than letting it be the default Google search results. Social media can also be a vehicle through which you build your offline network of potential colleagues, collaborators, and supporters as well as facilitate speaking, consulting, research, and other professional opportunities.
We that we have convinced you to actively participate in social media professionally. Next, we would like to share our top six best practices for responsible use.
1. Understand and define your goals. We have broadly laid out our rationale but that is different from your specific, desired outcomes. If you do not know what you are trying to accomplish you will have no idea if you are successful or if what you are doing is working versus whether you should try different strategies. Social media does take time; therefore, you should be strategic and goal oriented.
3. Share reliable/vetted information in your area of expertise and interest. Do not try to be all things to all people. Focus on content that distinguishes you and meets your goals. On the other hand, this should not be all about you; this can be boring, difficult, and give the impression that all you care about is self-promotion. No more than a quarter to a third of the content should be about you and the rest should be curated content from other reliable sources. Sharing with attribution helps you build your community. Also, people appreciate vetted content in the great web of misinformation available. You can facilitate audience engagement by including graphics, photos, and videos and by engaging and responding to other posts. Importantly, having a disclaimer on your account (e.g., retweets are not endorsements, posts are not medical advice) is never a substitute for knowing/vetting what you are sharing.
4. Exercise caution when responding to medical questions on social media and/or sharing patient information. While we encourage engagement, you should never answer specific medical questions. This develops a doctor-patient relationship and creates legal “duty.” It could even constitute practicing without a license, if the person asking the question lives in another state. Instead, provide general information about a condition, especially as a link to a reliable site (www.gastro.org/patient-care/patientinfo-center) and suggest seeking care from their local medical professional. Along these lines, do not share any potentially identifiable patient information without documented permission. In addition to the obvious (e.g., patient name, photos, medical documents with identifiers), avoid stories of care, complications, rare conditions, or identifiable specimens. With an approximate date and the location of your practice, it may be very easy for someone to determine the patient’s identity.
6. Know and adhere to the social media policies of your practice, institution, organization, or employer. Most academic institutions have social media policies and they are becoming more widely adapted to other settings. While you may just get a metaphorical slap on the wrist for not following the rules, I think we all would agree that it would be a tragedy to get fired over a social media post.
However, none of the above best practices are a substitute for being intentional and mindful when sharing information on social media, whether it be Facebook, Twitter, Instagram, Youtube, or another platform. What does being intentional and mindful on social media mean? Absolutely avoid commenting/posting about patients, colleagues, or your workplace in any way that could be perceived to be negative. Declare conflicts of interest where applicable (i.e., if you’re a consultant for a pharma company, avoid endorsing a drug without declaring your conflict). Above all else, don’t post anything that you wouldn’t mind being on a billboard or mainstream news.
Participation is an investment of your most valuable resource: time. Therefore, know your goals and revisit these goals and your success in reaching them regularly. Start small and expand as your time and interest allows. Finally, minimize your exposure to risk by keeping our guidance and your institutional policies in mind and always pause before you post.
You can find out more about the AGA, its programs, and publications via our social media outlets, including:
Twitter:
@amergastroassn
@AGA_CGH
@AGA_CMGH
@AGA_Gastro
Facebook:
@AmerGastroAssn
@cghjournal
@cmghjournal
@gastrojournal
Dr. Fisher is associate professor in the department of medicine, division of gastroenterology, Duke University, Durham, N.C. VA Medical Center. Twitter: @DrDeborahFisher. Dr. Gray is assistant professor, department of medicine, division of gastroenterology, hepatology and nutrition, Ohio State University College of Medicine. Twitter: @DMGrayMD.
This information was presented at a Meet-the-Professor Luncheon at DDW® 2017. More for in-depth details than are described in this article, refer to this session on DDW on Demand (http://www.ddw.org/education/session-recordings).
References
1 Von Muhlen M., Ohno-Machado L. J Am Med Inform Assoc. 2012;19(5):777-81.
2 Childs L.M., Martin C.Y. Am J Health System Pharm. 2012;69(23):2044-50.
3. Social media “likes” healthcare. From marketing to social business – 2012 Report
https://www.pwc.com/us/en/health-industries/health-research-institute/publications/health-care-social-media.html.
4. www.martinsights.com/social-media-marketing/social-media-strategy/new-global-social-media-research/.
5. Online Medical Professionalism: Patient and Public Relationships: Policy Statement From the American College of Physicians and the Federation of State Medical Boards. Ann Intern Med. 2013;158(8):620-7.
Gone are the days when social media was primarily used by millennials and those early adopters on the diffusion-of-innovation curve. Now, baby boomers and laggards alike are using social media to communicate with the world around them. Furthermore, health and healthcare issues are common topics in the social media universe. Eight in 10 Internet users seek health information online and 74% of these health information seekers use social media.1,2 Additionally, when they look online, they are more likely to trust information from doctors (61%) than from hospitals (55%), insurers (42%), or pharmaceutical companies (37%).3 Therefore, there is tremendous opportunity for physicians to engage patients, policy makers, advocacy groups, and other health care influencers in order to share reliable information. Yet, we must do so responsibly. There is a considerable degree of misinformation circulated in social media and we believe that physicians should help combat this by providing accurate information.
On a more individual level, social media can help you stay up-to-date on best practices, breakthroughs, and controversies in medicine. It can help you take control of your online reputation rather than letting it be the default Google search results. Social media can also be a vehicle through which you build your offline network of potential colleagues, collaborators, and supporters as well as facilitate speaking, consulting, research, and other professional opportunities.
We that we have convinced you to actively participate in social media professionally. Next, we would like to share our top six best practices for responsible use.
1. Understand and define your goals. We have broadly laid out our rationale but that is different from your specific, desired outcomes. If you do not know what you are trying to accomplish you will have no idea if you are successful or if what you are doing is working versus whether you should try different strategies. Social media does take time; therefore, you should be strategic and goal oriented.
3. Share reliable/vetted information in your area of expertise and interest. Do not try to be all things to all people. Focus on content that distinguishes you and meets your goals. On the other hand, this should not be all about you; this can be boring, difficult, and give the impression that all you care about is self-promotion. No more than a quarter to a third of the content should be about you and the rest should be curated content from other reliable sources. Sharing with attribution helps you build your community. Also, people appreciate vetted content in the great web of misinformation available. You can facilitate audience engagement by including graphics, photos, and videos and by engaging and responding to other posts. Importantly, having a disclaimer on your account (e.g., retweets are not endorsements, posts are not medical advice) is never a substitute for knowing/vetting what you are sharing.
4. Exercise caution when responding to medical questions on social media and/or sharing patient information. While we encourage engagement, you should never answer specific medical questions. This develops a doctor-patient relationship and creates legal “duty.” It could even constitute practicing without a license, if the person asking the question lives in another state. Instead, provide general information about a condition, especially as a link to a reliable site (www.gastro.org/patient-care/patientinfo-center) and suggest seeking care from their local medical professional. Along these lines, do not share any potentially identifiable patient information without documented permission. In addition to the obvious (e.g., patient name, photos, medical documents with identifiers), avoid stories of care, complications, rare conditions, or identifiable specimens. With an approximate date and the location of your practice, it may be very easy for someone to determine the patient’s identity.
6. Know and adhere to the social media policies of your practice, institution, organization, or employer. Most academic institutions have social media policies and they are becoming more widely adapted to other settings. While you may just get a metaphorical slap on the wrist for not following the rules, I think we all would agree that it would be a tragedy to get fired over a social media post.
However, none of the above best practices are a substitute for being intentional and mindful when sharing information on social media, whether it be Facebook, Twitter, Instagram, Youtube, or another platform. What does being intentional and mindful on social media mean? Absolutely avoid commenting/posting about patients, colleagues, or your workplace in any way that could be perceived to be negative. Declare conflicts of interest where applicable (i.e., if you’re a consultant for a pharma company, avoid endorsing a drug without declaring your conflict). Above all else, don’t post anything that you wouldn’t mind being on a billboard or mainstream news.
Participation is an investment of your most valuable resource: time. Therefore, know your goals and revisit these goals and your success in reaching them regularly. Start small and expand as your time and interest allows. Finally, minimize your exposure to risk by keeping our guidance and your institutional policies in mind and always pause before you post.
You can find out more about the AGA, its programs, and publications via our social media outlets, including:
Twitter:
@amergastroassn
@AGA_CGH
@AGA_CMGH
@AGA_Gastro
Facebook:
@AmerGastroAssn
@cghjournal
@cmghjournal
@gastrojournal
Dr. Fisher is associate professor in the department of medicine, division of gastroenterology, Duke University, Durham, N.C. VA Medical Center. Twitter: @DrDeborahFisher. Dr. Gray is assistant professor, department of medicine, division of gastroenterology, hepatology and nutrition, Ohio State University College of Medicine. Twitter: @DMGrayMD.
This information was presented at a Meet-the-Professor Luncheon at DDW® 2017. More for in-depth details than are described in this article, refer to this session on DDW on Demand (http://www.ddw.org/education/session-recordings).
References
1 Von Muhlen M., Ohno-Machado L. J Am Med Inform Assoc. 2012;19(5):777-81.
2 Childs L.M., Martin C.Y. Am J Health System Pharm. 2012;69(23):2044-50.
3. Social media “likes” healthcare. From marketing to social business – 2012 Report
https://www.pwc.com/us/en/health-industries/health-research-institute/publications/health-care-social-media.html.
4. www.martinsights.com/social-media-marketing/social-media-strategy/new-global-social-media-research/.
5. Online Medical Professionalism: Patient and Public Relationships: Policy Statement From the American College of Physicians and the Federation of State Medical Boards. Ann Intern Med. 2013;158(8):620-7.
Gone are the days when social media was primarily used by millennials and those early adopters on the diffusion-of-innovation curve. Now, baby boomers and laggards alike are using social media to communicate with the world around them. Furthermore, health and healthcare issues are common topics in the social media universe. Eight in 10 Internet users seek health information online and 74% of these health information seekers use social media.1,2 Additionally, when they look online, they are more likely to trust information from doctors (61%) than from hospitals (55%), insurers (42%), or pharmaceutical companies (37%).3 Therefore, there is tremendous opportunity for physicians to engage patients, policy makers, advocacy groups, and other health care influencers in order to share reliable information. Yet, we must do so responsibly. There is a considerable degree of misinformation circulated in social media and we believe that physicians should help combat this by providing accurate information.
On a more individual level, social media can help you stay up-to-date on best practices, breakthroughs, and controversies in medicine. It can help you take control of your online reputation rather than letting it be the default Google search results. Social media can also be a vehicle through which you build your offline network of potential colleagues, collaborators, and supporters as well as facilitate speaking, consulting, research, and other professional opportunities.
We that we have convinced you to actively participate in social media professionally. Next, we would like to share our top six best practices for responsible use.
1. Understand and define your goals. We have broadly laid out our rationale but that is different from your specific, desired outcomes. If you do not know what you are trying to accomplish you will have no idea if you are successful or if what you are doing is working versus whether you should try different strategies. Social media does take time; therefore, you should be strategic and goal oriented.
3. Share reliable/vetted information in your area of expertise and interest. Do not try to be all things to all people. Focus on content that distinguishes you and meets your goals. On the other hand, this should not be all about you; this can be boring, difficult, and give the impression that all you care about is self-promotion. No more than a quarter to a third of the content should be about you and the rest should be curated content from other reliable sources. Sharing with attribution helps you build your community. Also, people appreciate vetted content in the great web of misinformation available. You can facilitate audience engagement by including graphics, photos, and videos and by engaging and responding to other posts. Importantly, having a disclaimer on your account (e.g., retweets are not endorsements, posts are not medical advice) is never a substitute for knowing/vetting what you are sharing.
4. Exercise caution when responding to medical questions on social media and/or sharing patient information. While we encourage engagement, you should never answer specific medical questions. This develops a doctor-patient relationship and creates legal “duty.” It could even constitute practicing without a license, if the person asking the question lives in another state. Instead, provide general information about a condition, especially as a link to a reliable site (www.gastro.org/patient-care/patientinfo-center) and suggest seeking care from their local medical professional. Along these lines, do not share any potentially identifiable patient information without documented permission. In addition to the obvious (e.g., patient name, photos, medical documents with identifiers), avoid stories of care, complications, rare conditions, or identifiable specimens. With an approximate date and the location of your practice, it may be very easy for someone to determine the patient’s identity.
6. Know and adhere to the social media policies of your practice, institution, organization, or employer. Most academic institutions have social media policies and they are becoming more widely adapted to other settings. While you may just get a metaphorical slap on the wrist for not following the rules, I think we all would agree that it would be a tragedy to get fired over a social media post.
However, none of the above best practices are a substitute for being intentional and mindful when sharing information on social media, whether it be Facebook, Twitter, Instagram, Youtube, or another platform. What does being intentional and mindful on social media mean? Absolutely avoid commenting/posting about patients, colleagues, or your workplace in any way that could be perceived to be negative. Declare conflicts of interest where applicable (i.e., if you’re a consultant for a pharma company, avoid endorsing a drug without declaring your conflict). Above all else, don’t post anything that you wouldn’t mind being on a billboard or mainstream news.
Participation is an investment of your most valuable resource: time. Therefore, know your goals and revisit these goals and your success in reaching them regularly. Start small and expand as your time and interest allows. Finally, minimize your exposure to risk by keeping our guidance and your institutional policies in mind and always pause before you post.
You can find out more about the AGA, its programs, and publications via our social media outlets, including:
Twitter:
@amergastroassn
@AGA_CGH
@AGA_CMGH
@AGA_Gastro
Facebook:
@AmerGastroAssn
@cghjournal
@cmghjournal
@gastrojournal
Dr. Fisher is associate professor in the department of medicine, division of gastroenterology, Duke University, Durham, N.C. VA Medical Center. Twitter: @DrDeborahFisher. Dr. Gray is assistant professor, department of medicine, division of gastroenterology, hepatology and nutrition, Ohio State University College of Medicine. Twitter: @DMGrayMD.
This information was presented at a Meet-the-Professor Luncheon at DDW® 2017. More for in-depth details than are described in this article, refer to this session on DDW on Demand (http://www.ddw.org/education/session-recordings).
References
1 Von Muhlen M., Ohno-Machado L. J Am Med Inform Assoc. 2012;19(5):777-81.
2 Childs L.M., Martin C.Y. Am J Health System Pharm. 2012;69(23):2044-50.
3. Social media “likes” healthcare. From marketing to social business – 2012 Report
https://www.pwc.com/us/en/health-industries/health-research-institute/publications/health-care-social-media.html.
4. www.martinsights.com/social-media-marketing/social-media-strategy/new-global-social-media-research/.
5. Online Medical Professionalism: Patient and Public Relationships: Policy Statement From the American College of Physicians and the Federation of State Medical Boards. Ann Intern Med. 2013;158(8):620-7.