User login
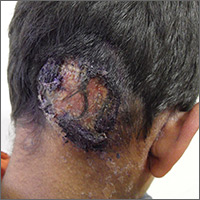
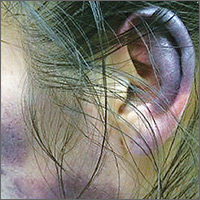
Inflammatory masses on boy’s scalp
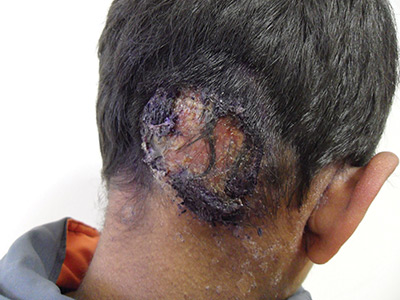
The patient was given a diagnosis of tinea capitis (ringworm of the scalp) based on the clinical presentation. (His brother and sister were told that they had tinea corporis and tinea faciei, which our patient also had on his face.)
Tinea capitis is a fungal infection of the scalp that usually starts as flaky and crusty patches of skin, broken-off hair, erythema, scaling, and pustules on the scalp. This can quickly deteriorate into a boggy and pruritic mass of inflamed tissue known as a kerion, which can become severely inflamed and develop regional lymphadenopathy. Hypersensitive and highly inflammatory reactions that look similar to a bacterial infection may be found when the infection is caused by a zoophilic dermatophyte.
Tinea capitis primarily affects children younger than 10 years of age, with a peak incidence among African American boys. Because US public health agencies no longer require physicians to report cases of tinea capitis, its true incidence in the United States is unknown, but it is believed to be increasing.
Tinea capitis is treated with systemic antifungal medication. Oral antifungal agents, such as griseofulvin, itraconazole, terbinafine, and fluconazole, are effective. Oral fluconazole is typically administered at a dosage of 5 to 6 mg/kg/d for 3 to 6 weeks; an alternative regimen, 8 mg/kg once weekly for 8 to 12 weeks, is safe, effective, and associated with high compliance. Short-duration therapy with fluconazole 6 mg/kg/d for 2 weeks is also effective.
This patient was treated with oral fluconazole 50 mg/d for 2 weeks and showed rapid improvement. Fluconazole was continued at 150 mg weekly for another 2 weeks, and at 6 weeks, his scalp lesions had completely resolved. The patient’s siblings were initially treated with topical itraconazole, without effect. They were switched to oral fluconazole 50 mg/d and improved.
Adapted from: Kim K. Inflammatory masses on boy’s scalp. J Fam Pract. 2015;64:367-369

The patient was given a diagnosis of tinea capitis (ringworm of the scalp) based on the clinical presentation. (His brother and sister were told that they had tinea corporis and tinea faciei, which our patient also had on his face.)
Tinea capitis is a fungal infection of the scalp that usually starts as flaky and crusty patches of skin, broken-off hair, erythema, scaling, and pustules on the scalp. This can quickly deteriorate into a boggy and pruritic mass of inflamed tissue known as a kerion, which can become severely inflamed and develop regional lymphadenopathy. Hypersensitive and highly inflammatory reactions that look similar to a bacterial infection may be found when the infection is caused by a zoophilic dermatophyte.
Tinea capitis primarily affects children younger than 10 years of age, with a peak incidence among African American boys. Because US public health agencies no longer require physicians to report cases of tinea capitis, its true incidence in the United States is unknown, but it is believed to be increasing.
Tinea capitis is treated with systemic antifungal medication. Oral antifungal agents, such as griseofulvin, itraconazole, terbinafine, and fluconazole, are effective. Oral fluconazole is typically administered at a dosage of 5 to 6 mg/kg/d for 3 to 6 weeks; an alternative regimen, 8 mg/kg once weekly for 8 to 12 weeks, is safe, effective, and associated with high compliance. Short-duration therapy with fluconazole 6 mg/kg/d for 2 weeks is also effective.
This patient was treated with oral fluconazole 50 mg/d for 2 weeks and showed rapid improvement. Fluconazole was continued at 150 mg weekly for another 2 weeks, and at 6 weeks, his scalp lesions had completely resolved. The patient’s siblings were initially treated with topical itraconazole, without effect. They were switched to oral fluconazole 50 mg/d and improved.
Adapted from: Kim K. Inflammatory masses on boy’s scalp. J Fam Pract. 2015;64:367-369

The patient was given a diagnosis of tinea capitis (ringworm of the scalp) based on the clinical presentation. (His brother and sister were told that they had tinea corporis and tinea faciei, which our patient also had on his face.)
Tinea capitis is a fungal infection of the scalp that usually starts as flaky and crusty patches of skin, broken-off hair, erythema, scaling, and pustules on the scalp. This can quickly deteriorate into a boggy and pruritic mass of inflamed tissue known as a kerion, which can become severely inflamed and develop regional lymphadenopathy. Hypersensitive and highly inflammatory reactions that look similar to a bacterial infection may be found when the infection is caused by a zoophilic dermatophyte.
Tinea capitis primarily affects children younger than 10 years of age, with a peak incidence among African American boys. Because US public health agencies no longer require physicians to report cases of tinea capitis, its true incidence in the United States is unknown, but it is believed to be increasing.
Tinea capitis is treated with systemic antifungal medication. Oral antifungal agents, such as griseofulvin, itraconazole, terbinafine, and fluconazole, are effective. Oral fluconazole is typically administered at a dosage of 5 to 6 mg/kg/d for 3 to 6 weeks; an alternative regimen, 8 mg/kg once weekly for 8 to 12 weeks, is safe, effective, and associated with high compliance. Short-duration therapy with fluconazole 6 mg/kg/d for 2 weeks is also effective.
This patient was treated with oral fluconazole 50 mg/d for 2 weeks and showed rapid improvement. Fluconazole was continued at 150 mg weekly for another 2 weeks, and at 6 weeks, his scalp lesions had completely resolved. The patient’s siblings were initially treated with topical itraconazole, without effect. They were switched to oral fluconazole 50 mg/d and improved.
Adapted from: Kim K. Inflammatory masses on boy’s scalp. J Fam Pract. 2015;64:367-369
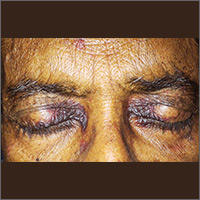
FDA approves drug to treat CRS induced by CAR T-cell therapy
The US Food and Drug Administration (FDA) has approved tocilizumab (Actemra®) for the treatment of patients age 2 and older who have severe or life-threatening cytokine release syndrome (CRS) induced by chimeric antigen receptor (CAR) T-cell therapy.
Tocilizumab is a humanized interleukin-6 receptor antagonist.
The drug is also FDA-approved to treat adults with rheumatoid arthritis or giant cell arteritis and patients age 2 and older with polyarticular juvenile idiopathic arthritis or systemic juvenile idiopathic arthritis.
The full prescribing information for tocilizumab, which includes a boxed warning about the risk of serious infections, is available at http://www.actemra.com. The drug is jointly developed by Genentech (a member of the Roche Group) and Chugai Pharmaceutical Co.
The FDA’s latest approval of tocilizumab coincided with the FDA’s approval of the CAR T-cell therapy tisagenlecleucel (Kymriah, formerly CTL019) to treat pediatric and young adult patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia.
According to Genentech, the FDA’s decision to expand the approval of tocilizumab is based on a retrospective analysis of pooled outcome data from clinical trials of CAR T-cell therapies in patients with hematologic malignancies.
For this analysis, researchers assessed 45 pediatric and adult patients treated with tocilizumab, with or without additional high-dose corticosteroids, for severe or life-threatening CRS.
Thirty-one patients (69%) achieved a response, defined as resolution of CRS within 14 days of the first dose of tocilizumab.
No more than 2 doses of tocilizumab were needed, and no drugs other than tocilizumab and corticosteroids were used for treatment.
No adverse reactions related to tocilizumab were reported. ![]()
The US Food and Drug Administration (FDA) has approved tocilizumab (Actemra®) for the treatment of patients age 2 and older who have severe or life-threatening cytokine release syndrome (CRS) induced by chimeric antigen receptor (CAR) T-cell therapy.
Tocilizumab is a humanized interleukin-6 receptor antagonist.
The drug is also FDA-approved to treat adults with rheumatoid arthritis or giant cell arteritis and patients age 2 and older with polyarticular juvenile idiopathic arthritis or systemic juvenile idiopathic arthritis.
The full prescribing information for tocilizumab, which includes a boxed warning about the risk of serious infections, is available at http://www.actemra.com. The drug is jointly developed by Genentech (a member of the Roche Group) and Chugai Pharmaceutical Co.
The FDA’s latest approval of tocilizumab coincided with the FDA’s approval of the CAR T-cell therapy tisagenlecleucel (Kymriah, formerly CTL019) to treat pediatric and young adult patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia.
According to Genentech, the FDA’s decision to expand the approval of tocilizumab is based on a retrospective analysis of pooled outcome data from clinical trials of CAR T-cell therapies in patients with hematologic malignancies.
For this analysis, researchers assessed 45 pediatric and adult patients treated with tocilizumab, with or without additional high-dose corticosteroids, for severe or life-threatening CRS.
Thirty-one patients (69%) achieved a response, defined as resolution of CRS within 14 days of the first dose of tocilizumab.
No more than 2 doses of tocilizumab were needed, and no drugs other than tocilizumab and corticosteroids were used for treatment.
No adverse reactions related to tocilizumab were reported. ![]()
The US Food and Drug Administration (FDA) has approved tocilizumab (Actemra®) for the treatment of patients age 2 and older who have severe or life-threatening cytokine release syndrome (CRS) induced by chimeric antigen receptor (CAR) T-cell therapy.
Tocilizumab is a humanized interleukin-6 receptor antagonist.
The drug is also FDA-approved to treat adults with rheumatoid arthritis or giant cell arteritis and patients age 2 and older with polyarticular juvenile idiopathic arthritis or systemic juvenile idiopathic arthritis.
The full prescribing information for tocilizumab, which includes a boxed warning about the risk of serious infections, is available at http://www.actemra.com. The drug is jointly developed by Genentech (a member of the Roche Group) and Chugai Pharmaceutical Co.
The FDA’s latest approval of tocilizumab coincided with the FDA’s approval of the CAR T-cell therapy tisagenlecleucel (Kymriah, formerly CTL019) to treat pediatric and young adult patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia.
According to Genentech, the FDA’s decision to expand the approval of tocilizumab is based on a retrospective analysis of pooled outcome data from clinical trials of CAR T-cell therapies in patients with hematologic malignancies.
For this analysis, researchers assessed 45 pediatric and adult patients treated with tocilizumab, with or without additional high-dose corticosteroids, for severe or life-threatening CRS.
Thirty-one patients (69%) achieved a response, defined as resolution of CRS within 14 days of the first dose of tocilizumab.
No more than 2 doses of tocilizumab were needed, and no drugs other than tocilizumab and corticosteroids were used for treatment.
No adverse reactions related to tocilizumab were reported. ![]()
Hypertension treatment strategies for older adults
CASE 1 An 82-year-old black woman comes in for an annual exam. She has no medical concerns. She volunteers at a hospice, walks daily, and maintains a healthy diet. Her past medical history (PMH) includes osteopenia and osteoarthritis, and her medications include acetaminophen as needed and vitamin D. She has no drug allergies. Her exam reveals a blood pressure (BP) of 148/70 mm Hg, a body mass index of 31, and a heart rate (HR) of 71 beats per minute (bpm). Cardiac and pulmonary exams are normal, and she shows no signs of peripheral edema.
CASE 2 An 88-year-old white man presents to the office for a 3-month follow-up of his hypertension. His systolic BP at home has ranged from 140 to 170 mm Hg. He denies chest pain, shortness of breath, or lower extremity edema. He lives with his wife and frequently swims for exercise. His PMH is significant for depression and degenerative disc disease. His medications include hydrochlorothiazide 12.5 mg/d, sertraline 50 mg/d, and naproxen 250 mg bid. His BP is 160/80 mm Hg and his HR is 70 bpm with normal cardiovascular (CV) and pulmonary exams.
CASE 3 An 80-year-old white man with diabetes mellitus (DM), hypertension, and chronic kidney disease (CKD) presents for a 3-month follow-up visit. His home systolic BP has been in the 140s to 150s. He is functional in all of his activities of daily living (ADLs), but is starting to require assistance with medications, finances, and transportation. He takes aspirin 81 mg/d, chlorthalidone 25 mg/d, and atenolol 50 mg/d. Remarkable laboratory test results include a hemoglobin A1c of 8.6%, a serum creatinine of 1.9 mg/dL (normal range: 0.6-1.2 mg/dL), and an albumin-creatinine ratio of 250 mg/g (normal range: <30 mg/g). During the exam, his BP is 143/70 mm Hg, his HR is 70 bpm, he is alert and oriented to person, place, and time, and he has normal CV and pulmonary exams with no signs of peripheral edema. He has decreased sensation in his feet, but normal reflexes.
How would you proceed with the care of these 3 patients?
Hypertension is the most common diagnosis made during physician office visits in the United States.1 Nearly one-third of the population has hypertension, and its prevalence increases with age, such that 67% of men and 79% of women ≥75 years of age have the condition.2
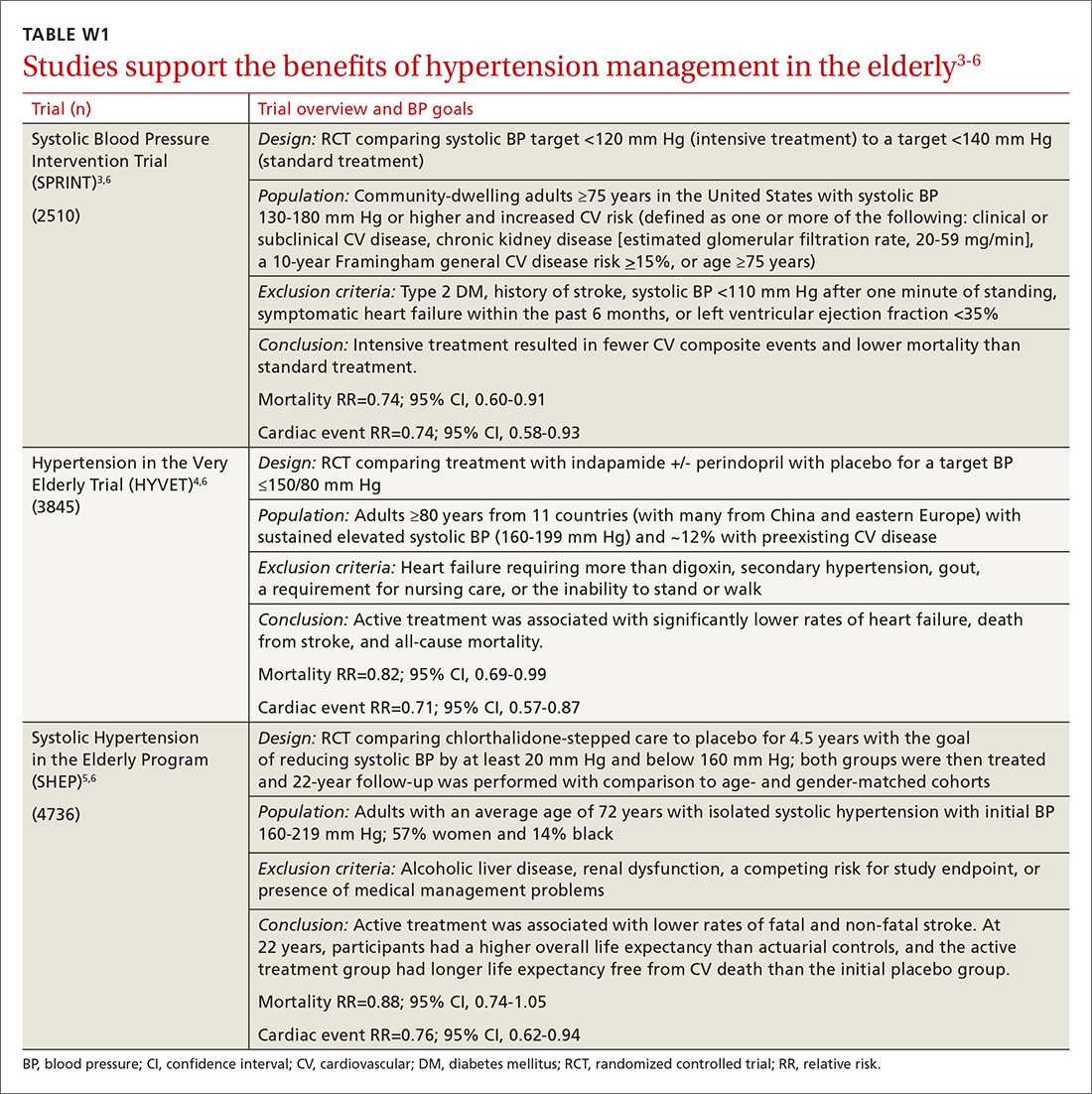
Evidence indicates that hypertension is a modifiable risk factor for CV and all-cause mortality (TABLE W13-6). All adults ≥75 years of age are at increased CV risk based on Framingham criteria,7 making hypertension management paramount. Complicating the situation are findings that indicate nearly half of adults with hypertension have inadequate BP control.2
Clinicians require clear direction about optimal BP targets, how best to adjust antihypertensive medications for comorbidities, and how to incorporate frailty and cognitive impairment into management strategies. This article presents recommendations derived from recent evidence and consensus guidelines regarding the management of hypertension in adults ≥75 years of age.
[polldaddy:9818133]
Diagnosing hypertension
According to the seventh report of the Joint National Committee (JNC 7), hypertension is defined as a systolic BP ≥140 mm Hg and/or a diastolic BP ≥90 mm Hg.8 The JNC’s more recent report (JNC 8), however, does not define hypertension; instead, it sets forth treatment thresholds (eg, that there is strong evidence to support treating individuals ≥60 years of age when BP ≥150/90 mm Hg).9
It starts with an accurate BP measurement. Ensuring the accuracy of a BP measurement requires multiple readings over time. White coat hypertension and masked hypertension can complicate BP measurement. Home measurements better correlate with atherosclerotic cardiovascular disease (ASCVD) risk than do office measurements.10-12 In fact, the US Preventive Services Task Force recommends obtaining measurements outside of the clinic setting prior to initiating treatment for hypertension.13
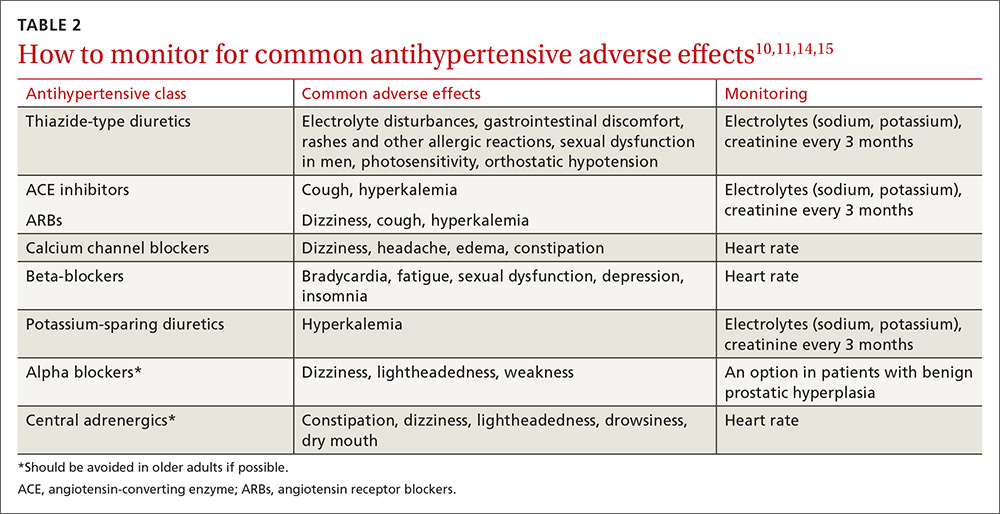
Educate staff on the proper technique for obtaining BP measurements in the office (ie, taking measurements using an appropriately sized cuff when patients have been seated for at least 5 minutes with feet uncrossed and with their arm supported at heart level). Cold temperatures, coffee consumption, talking, and recent tobacco use can transiently raise BP. TABLE 110 outlines the initial work-up after confirming the diagnosis of hypertension. No other routine tests are recommended for the management of hypertension except those associated with medication monitoring (outlined in TABLE 210,11,14,15).
What’s the optimal BP target for older patients? No consensus exists on an optimal BP target for older patients. JNC 8 recommends a target BP <150/90 mm Hg in patients ≥60 years of age.9 The American College of Physicians recommends a systolic BP target <140 mm Hg in patients ≥60 years of age with increased stroke or CV risk.11 A subgroup analysis of patients ≥75 years of age from the Systolic BP Intervention Trial (SPRINT)3 was stopped early because of the clear composite CV and mortality benefits associated with targeting a systolic BP <120 mm Hg as compared with <140 mm Hg (TABLE W13-6). Although a criticism of this trial and its results is that the researchers included only adults with high CV risk, all adults ≥75 years of age are considered to have high CV risk by the SPRINT study.3 Another criticism is that early suspension of the trial may have exaggerated treatment effects.6
Lastly, results were seemingly discrepant from previous trials, most notably, the Action to Control CV Risk in Diabetes (ACCORD) trial.6,16 However, on closer review, the ACCORD trial16 included only patients with DM, while the SPRINT3 trial excluded patients with DM, and ACCORD comprised a younger population than the SPRINT subgroup analysis. Also, the ACCORD trial did demonstrate stroke reduction and non-significant reduction in CV events, albeit, at the cost of increased adverse events, such as hypotension, bradycardia, and hypokalemia, with tighter BP control.16
Population differences presumably explain the discrepancy in results, and a systolic BP target of <120 mm Hg is appropriate in community-dwelling, non-diabetic adults ≥75 years of age. If this target goal cannot be achieved without undue burden (ie, without syncope, hypotension, bradycardia, electrolyte disturbance, renal impairment, or substantial medication burden), a recent meta-analysis found evidence that a systolic BP goal <140 mm Hg also provides benefit.6
Initiate treatment, watch for age-related changes
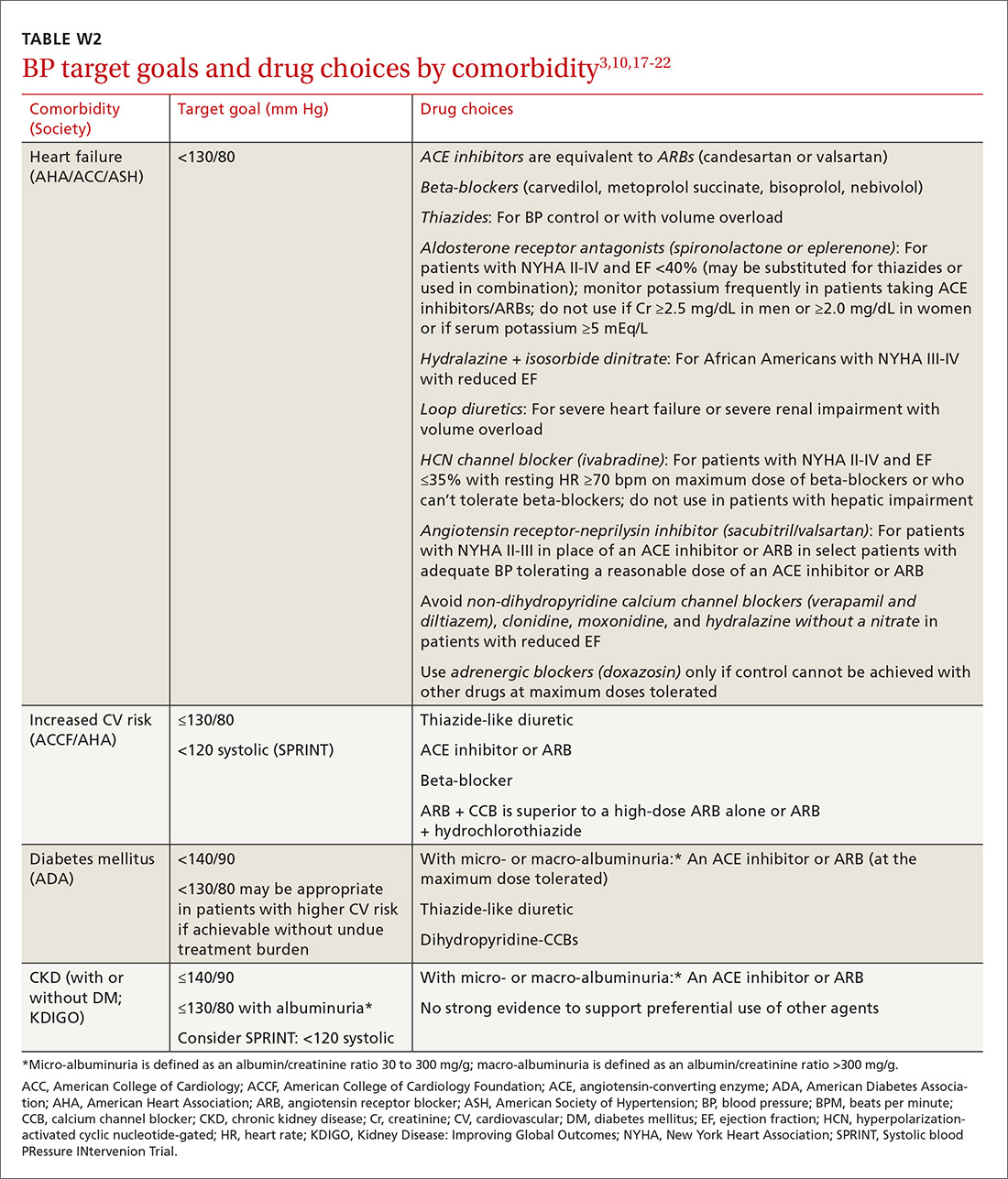
Lifestyle modifications (including appropriate weight loss; reduced caffeine, salt, and alcohol intake; increased physical activity; and smoking cessation) are important in the initial and ongoing management of hypertension.10,11,17,18 JNC 8 recommends initial treatment with a thiazide-type diuretic, calcium channel blocker (CCB), angiotensin converting enzyme (ACE) inhibitor, or angiotensin receptor blocker (ARB) in the nonblack population, and a CCB or thiazide diuretic in the black population.9 Specific initial medication choices for comorbid conditions are outlined in TABLE W23,10,17-22. JNC 8 recommends against the use of a beta-blocker or alpha blocker for initial treatment of hypertension.9
Start a second drug instead of maximizing the dose of the first
If the target BP cannot be achieved within one month of initiating medication, JNC 8 recommends increasing the dose of the initial drug or adding a second drug without preference for one strategy over the other.9 However, a meta-analysis demonstrates that approximately 80% of the antihypertensive effect of a drug can be achieved with half of the standard dose of the medication; this is true for thiazide-type diuretics, ACE inhibitors/ARBs, beta-blockers, and CCBs.23
Furthermore, due to fewer adverse effects and positive synergies, studies show that combining low doses of 2 medications is more beneficial than high-dose monotherapy.19,23,24 Prescribing combination pills can be helpful to limit pill burden. It is appropriate to combine any of the 4 classes of medications recommended as initial therapy by JNC 8 except for an ACE inhibitor combined with an ARB. If the target BP cannot be achieved with 3 drugs in those classes, other medications such as potassium-sparing diuretics or beta-blockers can be added.9
Changes associated with aging
Changes associated with aging include atherosclerosis and stiffening of blood vessels, increased systolic BP, widened pulse pressure, reduced glomerular filtration rate, reduced sodium elimination and volume expansion, sinoatrial node cellular dropout, and decreased sensitivity of baroreceptors.10 Because of these alterations, antihypertensive requirements may change, and resistant hypertension may develop. In addition, older patients may be more susceptible to orthostatic hypotension, heart block, electrolyte derangements, and other antihypertensive adverse effects.
When hypertension is difficult to control. Resistant hypertension is defined as hypertension that cannot be controlled with 3 drugs from 3 different antihypertensive classes, one of which is a diuretic. Cognitive impairment, polypharmacy, and multimorbidity may contribute to difficult-to-control hypertension in older adults and should be assessed prior to work-up for other secondary causes of poorly controlled hypertension.
- Cognitive impairment is often unrecognized and may impact medication adherence, which can masquerade as treatment failure. Assess for cognitive impairment on an ongoing basis with the aging patient, especially when medication adherence appears poor.
- Polypharmacy may also contribute to uncontrolled BP. Common pharmacotherapeutic contributors to uncontrolled BP include nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, high-dose decongestants, and selective norepinephrine reuptake inhibitors.25
- Multimorbidity describes 2 or more chronic medical conditions in one patient. These patients are medically complex. Comorbidities can increase pill burden and make medication adherence difficult for patients. Other poorly controlled disease states can worsen hypertension (eg, renal dysfunction secondary to diabetes). Optimize treatment of comorbid conditions.
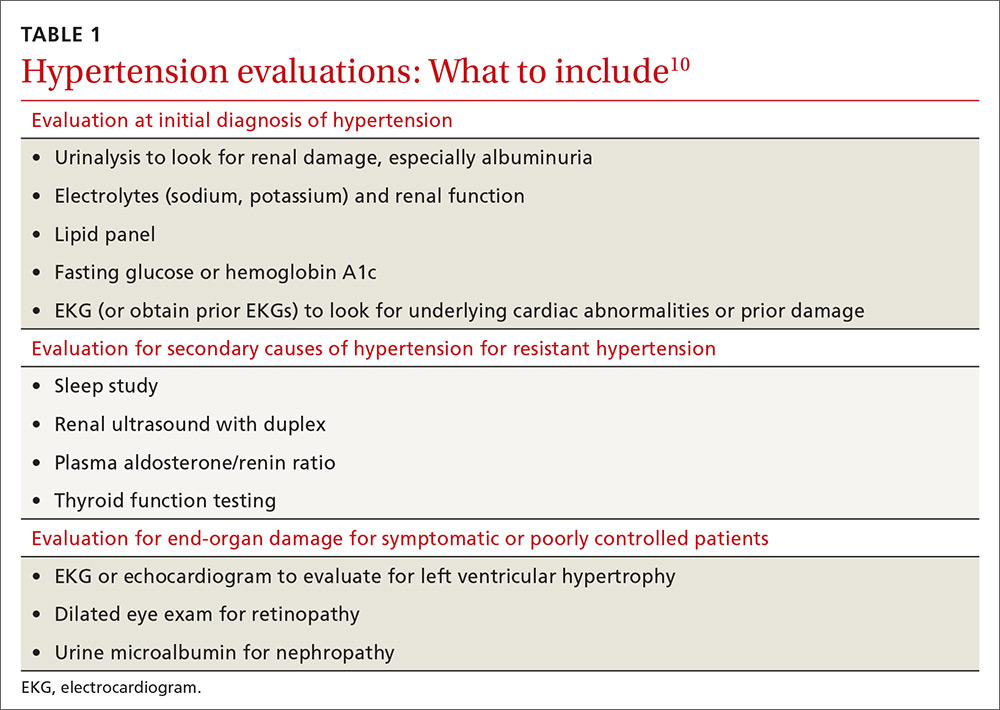
Secondary causes. If resistant hypertension persists despite confirming medication adherence and eliminating offending medications, a work-up should ensue for secondary causes of hypertension, as well as end-organ damage. Causes of secondary hypertension include sleep apnea (see this month's HelpDesk), renal dysfunction (renal artery stenosis), aldosterone-mediated hypertension (often with hypokalemia), and thyroid disease. Evaluation for secondary causes of hypertension and end-organ damage is outlined in TABLE 1.10 Patients with well-controlled hypertension do not require repeated assessments for end-organ damage unless new symptoms—such as chest pain or edema—develop.
Consider comorbidities
Clinical trials implicitly or explicitly exclude patients with multiple comorbidities. JNC 8 provided minimal guidance for adjusting BP targets based on comorbidity with only nondiabetic CKD and DM specifically addressed.9 Guidelines from specialty organizations and recent trials provide some additional guidance in these situations and are outlined in TABLE W23,10,17-22.
Heart failure. Hypertension is a major risk factor for heart failure. Long-term treatment of systolic and diastolic hypertension can reduce the incidence of heart failure by approximately half with increased benefit in patients with prior myocardial infarction.22 Research demonstrates clear mortality benefits of certain antihypertensive drug classes, including diuretics, beta-blockers, ACE inhibitors, ARBs, aldosterone antagonists, combination hydralazine and nitrates, and angiotensin receptor-neprilysin inhibitors.21,22 The overall treatment goal in heart failure is to optimize drugs with mortality benefit, while lowering BP to a goal <130/80 mm Hg in patients ≥75 years of age.22
Increased risk for CV disease. The SPRINT trial3 defined high risk of CV disease as clinical or subclinical CV disease, CKD, 10-year ASCVD risk of ≥15%, or age ≥75 years. SPRINT supports a systolic BP goal <120 mm Hg, but, as a reminder, SPRINT excluded patients with diabetes. The American College of Cardiology Foundation Task Force and the American Heart Association define high CV risk as a 10-year ASCVD risk ≥10% and recommend a BP goal <130/80 mm Hg.10
Diabetes mellitus. A BP >115/75 mm Hg is associated with increased CV events and mortality in patients with DM.18 The American Diabetes Association (ADA) and JNC 8 recommend a BP target <140/90 mm Hg.9,18 ADA suggests a lower target of 130/80 mm Hg in patients with high CV risk if it is achievable without undue burden.18
Studies show increased mortality associated with initiating additional treatment once a systolic goal <140 mm Hg has been achieved in patients with DM.26 The ACCORD trial found increased adverse events with aggressive BP lowering to <120/80 mm Hg.16
For patients with DM requiring more than one antihypertensive agent, there are CV mortality benefits associated with administering at least one antihypertensive drug at night, likely related to the beneficial effect of physiologic nocturnal dipping.27
Chronic kidney disease. JNC 8 specifically recommends an ACE inhibitor or ARB for initial or add-on treatment in patients with CKD and a BP goal <140/90 mm Hg.9 The Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group recommends a BP target ≤140/90 mm Hg in patients without albuminuria and ≤130/80 mm Hg in patients with albuminuria to protect against the progression of nephropathy.17 The SPRINT trial3 included patients with CKD, and KDIGO has not yet updated its guidelines to reflect SPRINT.
Frailty is a clinical syndrome that has been defined as a state of increased vulnerability that is associated with a decline in reserve and function.28 The largest hypertension studies in older adults address frailty, although often the most frail patients are excluded from these studies (TABLE W13-6).
The Hypertension in the Very Elderly Trial (HYVET) categorized patients as frail, pre-frail, or robust and found a consistent benefit of antihypertensive treatment on stroke, CV events, and total mortality—regardless of baseline frailty status.29 The SPRINT trial included only community-dwelling adults.3 Other studies suggest that hypertension actually has a protective effect by lowering overall mortality in frail older adults, especially in the frailest and oldest nursing home populations.30,31
Although there is a paucity of data to direct the management of hypertension in frail older patients, physicians should prioritize the condition and focus on adverse events from antihypertensives and on slow titration of medications. The JNC 8 BP target of <150/90 mm Hg is a reasonable BP goal in this population, given the lack of evidence for lower or higher targets.9 Many frail patients have one or more of the comorbidities described earlier, and it is reasonable to strive for the comorbidity-specific target, provided it can be achieved without undue burden.
Cognitive impairment and dementia. The association between hypertension and dementia/cognitive impairment is evolving. Hypertension may impact various forms of dementia, such as Alzheimer’s disease (AD) or vascular dementia, differently. There is evidence linking hypertension to AD.32 The relationship between BP and brain perfusion is complex with the potential existence of an age-adjusted relationship such that mid-life hypertension may increase the risk of dementia while late-life hypertension may not.33
A number of studies reveal the evolving nature of our understanding of these 2 conditions:
- A recent systematic review and meta-analysis examining intensive BP treatments in older adults demonstrated that lower BP targets did not increase cognitive decline.6
- HYVET’s cognitive function assessment did not find a significant reduction in the incidence of dementia with BP reduction over a short follow-up period, but when results were combined in a meta-analysis with other placebo-controlled, double-blind trials of antihypertensive treatments, there was significant reduction in incident dementia in patients randomized to antihypertensive treatment.34
- The ACCORD Memory in Diabetes trial (ACCORD-MIND) had the unexpected outcome that intensive lowering of systolic BP to a target <120 mm Hg resulted in a greater decline in total brain volume, compared with the standard BP goal <140 mm Hg. This was measured with magnetic resonance imaging in older adults with type 2 DM.35
- Results from the SPRINT sub-analysis Memory and Cognition in Decreased Hypertension trial are forthcoming and aim to determine the effects of BP reduction on dementia.36
The JNC 89 BP target <150/90 mm Hg or a comorbidity-specific target, if achievable without undue burden, is reasonable in patients with dementia. In a systematic review of observational studies in patients with hypertension and dementia, diuretics, CCBs, ACE inhibitors/ARBs, and beta-blockers were commonly used medications with a trend toward prescribing CCBs and ACE inhibitors/ARBs.37
As previously highlighted, cognitive impairment may lead to problems with medication adherence and even inadvertent improper medication use, potentially resulting in adverse events from antihypertensives. If cognitive impairment or dementia is suspected, ensure additional measures (such as medication assistance or supervision) are in place before prescribing antihypertensives.
Certain diseases, such as Parkinson’s-related dementia and multiple system atrophy, can cause autonomic instability, which can increase the risk of falls and complicate hypertension management. Carefully monitor patients for signs of orthostasis.

CASE 1 Repeat the BP measurement in the office once the patient has been seated for ≥5 minutes, and have the patient monitor her BP at home; schedule a follow-up visit in 2 weeks. If hypertension is confirmed with home measurements, then, in addition to lifestyle modifications, initiate treatment with a CCB or thiazide diuretic to achieve a systolic BP goal <120 mm Hg. Titrate medications slowly while monitoring for adverse effects.
CASE 2 Consistent with the office measurement, the patient has home BP readings that are above the BP target (<120 mm Hg systolic). He has been taking a single antihypertensive for longer than one month. Discontinue his NSAID prior to adding any new medications. If his BP is still above target without NSAIDs, then add a second agent, such as a low dose of an ACE inhibitor, ARB, or CCB, rather than maximizing the dose of hydrochlorothiazide.
CASE 3 Given the patient’s diabetes, CKD, and albuminuria, a target BP goal <130/80 mm Hg is reasonable. An ACE inhibitor or ARB is a better medication choice than atenolol in this patient with albuminuria. Because of the deterioration in his ADLs, careful assessment of mobility, functionality, comorbidities, frailty, and cognitive function should take place at each office visit and inform adjustments to the patient’s BP target. Employ cautious medication titration with monitoring for adverse effects, especially hypotension and syncope. If his functional status declines, adverse effects develop, or the medication regimen becomes burdensome, relax the target BP goal to 150/90 mm Hg.
CORRESPONDENCE
Julienne K. Kirk, PharmD, Family and Community Medicine, Wake Forest School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157-1084; [email protected].
1. National Ambulatory Medical Care Survey: 2013 State and National Summary Tables. Available at: https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2013_namcs_web_tables.pdf. Accessed May 29, 2017.
2. Centers for Disease Control and Prevention. High blood pressure facts. Available at: https://cdc.gov/bloodpressure/facts.htm. Accessed May 29, 2017.
3. Williamson JD, Suplano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673-2682.
4. Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887-1898.
5. Kostis WJ, Cabrera J, Messerli FH, et al. Competing cardiovascular and noncardiovascular risks and longevity in the systolic hypertension in the elderly program. Am J Cardiol. 2014;113:676-681.
6. Weiss J, Freeman M, Low A, et al. Benefits and harms of intensive blood pressure treatment in adults aged 60 years or older: a systematic review and meta-analysis. Ann Intern Med. 2017;166:419-429.
7. Framingham Heart Study. Available at: https://www.framinghamheartstudy.org/risk-functions/cardiovascular-disease/10-year-risk.php. Accessed May 29, 2017.
8. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
9. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
10. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on clinical expert consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57:2037-2114.
11. Qaseem A, Wilt TJ, Rich R, et al. Pharmacological treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166:430-437.
12. Sega R, Facchetti R, Bombelli M, et al. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111:1777-1783.
13. US Preventive Services Task Force. Final recommendation statement: high blood pressure in adults: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/high-blood-pressure-in-adults-screening. Accessed May 29, 2017.
14. Steinman MA, Miao Y, Boscardin WJ, et al. Prescribing quality in older veterans: a multifocal approach. J Gen Intern Med. 2014;29:1379-1386.
15. Schwartz JB. Primary prevention: do the very elderly require a different approach. Trends Cardiovasc Med. 2015:25:228-239.
16. Accord Study Group, Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
17. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2012;2:337-414.
18. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(suppl 1):S1-S135.
19. Ogawa H, Kim-Mitsuyama S, Matsui K, et al, OSCAR Study Group. Angiotensin II receptor blocker-based therapy in Japanese elderly, high-risk, hypertensive patients. Am J Med. 2012;125:981-990.
20. Rosendorff C, Lackland DT, Allison M, et al. AHA/ACC/ASH Scientific Statement. Treatment of hypertension in patients with coronary heart disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. Hypertension. 2015;65:1372-1407.
21. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327.
22. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137-e161.
23. Law MR, Wald MJ, Morris JK, et al. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326:1427.
24. Wald DS, Law M, Morris JK, et al. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290-300.
25. Mukete BN, Ferdinand KC. Polypharmacy in older adults with hypertension: a comprehensive review. J Clin Hypertens (Greenwich). 2016;18:10-18.
26. Brunstrom M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
27. Hermida RC, Ayala DE, Mojón A, et al. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diab Care. 2011;34:1270-1276.
28. Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27:1-15.
29. Warwick J, Falashcetti E, Rockwood K, et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the Hypertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med. 2015;13:78.
30. Zhang XE, Cheng B, Wang Q. Relationship between high blood pressure and cardiovascular outcomes in elderly frail patients: a systematic review and meta-analysis. Geriatric Nurs. 2016;37:385-392.
31. Benetos A, Rossignol P, Cherbuini A, et al. Polypharmacy in the aging patient: management of hypertension in octogenarians. JAMA. 2015;314:170-180.
32. de Bruijn R, Ikram MA. Cardiovascular risk factors and future risk of Alzheimer’s disease. BMC Med. 2014;12:130.
33. Joas E, Bäckman K, Gustafson D, et al. Blood pressure trajectories from midlife to late life in relation to dementia in women followed for 37 years. Hypertension. 2012;59:796-801.
34. Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lanc Neurol. 2008;7:683-689.
35. Williamson JD, Launer LJ, Bryan RN, et al. Cognitive function and brain structure in persons with type 2 diabetes mellitus after intensive lowering of blood pressure and lipid levels: a randomized clinical trial. JAMA Intern Med. 2014;174:324-333.
36. Tom Wade, MD. Methods of the SPRINT MIND Trial—how they did it + why it matters to primary care physicians. Available at: /www.tomwademd.net/methods-of-the-sprint-mind-trial-how-they-did-it-why-it-matters-to-primary-care-physicians/. Accessed August 11, 2017.
37. Welsh TJ, Gladman JR, Gordon AL. The treatment of hypertension in people with dementia: a systematic review of observational studies. BMC Geriatr. 2014;14:19.
CASE 1 An 82-year-old black woman comes in for an annual exam. She has no medical concerns. She volunteers at a hospice, walks daily, and maintains a healthy diet. Her past medical history (PMH) includes osteopenia and osteoarthritis, and her medications include acetaminophen as needed and vitamin D. She has no drug allergies. Her exam reveals a blood pressure (BP) of 148/70 mm Hg, a body mass index of 31, and a heart rate (HR) of 71 beats per minute (bpm). Cardiac and pulmonary exams are normal, and she shows no signs of peripheral edema.
CASE 2 An 88-year-old white man presents to the office for a 3-month follow-up of his hypertension. His systolic BP at home has ranged from 140 to 170 mm Hg. He denies chest pain, shortness of breath, or lower extremity edema. He lives with his wife and frequently swims for exercise. His PMH is significant for depression and degenerative disc disease. His medications include hydrochlorothiazide 12.5 mg/d, sertraline 50 mg/d, and naproxen 250 mg bid. His BP is 160/80 mm Hg and his HR is 70 bpm with normal cardiovascular (CV) and pulmonary exams.
CASE 3 An 80-year-old white man with diabetes mellitus (DM), hypertension, and chronic kidney disease (CKD) presents for a 3-month follow-up visit. His home systolic BP has been in the 140s to 150s. He is functional in all of his activities of daily living (ADLs), but is starting to require assistance with medications, finances, and transportation. He takes aspirin 81 mg/d, chlorthalidone 25 mg/d, and atenolol 50 mg/d. Remarkable laboratory test results include a hemoglobin A1c of 8.6%, a serum creatinine of 1.9 mg/dL (normal range: 0.6-1.2 mg/dL), and an albumin-creatinine ratio of 250 mg/g (normal range: <30 mg/g). During the exam, his BP is 143/70 mm Hg, his HR is 70 bpm, he is alert and oriented to person, place, and time, and he has normal CV and pulmonary exams with no signs of peripheral edema. He has decreased sensation in his feet, but normal reflexes.
How would you proceed with the care of these 3 patients?
Hypertension is the most common diagnosis made during physician office visits in the United States.1 Nearly one-third of the population has hypertension, and its prevalence increases with age, such that 67% of men and 79% of women ≥75 years of age have the condition.2
Evidence indicates that hypertension is a modifiable risk factor for CV and all-cause mortality (TABLE W13-6). All adults ≥75 years of age are at increased CV risk based on Framingham criteria,7 making hypertension management paramount. Complicating the situation are findings that indicate nearly half of adults with hypertension have inadequate BP control.2
Clinicians require clear direction about optimal BP targets, how best to adjust antihypertensive medications for comorbidities, and how to incorporate frailty and cognitive impairment into management strategies. This article presents recommendations derived from recent evidence and consensus guidelines regarding the management of hypertension in adults ≥75 years of age.
[polldaddy:9818133]
Diagnosing hypertension
According to the seventh report of the Joint National Committee (JNC 7), hypertension is defined as a systolic BP ≥140 mm Hg and/or a diastolic BP ≥90 mm Hg.8 The JNC’s more recent report (JNC 8), however, does not define hypertension; instead, it sets forth treatment thresholds (eg, that there is strong evidence to support treating individuals ≥60 years of age when BP ≥150/90 mm Hg).9
It starts with an accurate BP measurement. Ensuring the accuracy of a BP measurement requires multiple readings over time. White coat hypertension and masked hypertension can complicate BP measurement. Home measurements better correlate with atherosclerotic cardiovascular disease (ASCVD) risk than do office measurements.10-12 In fact, the US Preventive Services Task Force recommends obtaining measurements outside of the clinic setting prior to initiating treatment for hypertension.13

Educate staff on the proper technique for obtaining BP measurements in the office (ie, taking measurements using an appropriately sized cuff when patients have been seated for at least 5 minutes with feet uncrossed and with their arm supported at heart level). Cold temperatures, coffee consumption, talking, and recent tobacco use can transiently raise BP. TABLE 110 outlines the initial work-up after confirming the diagnosis of hypertension. No other routine tests are recommended for the management of hypertension except those associated with medication monitoring (outlined in TABLE 210,11,14,15).

What’s the optimal BP target for older patients? No consensus exists on an optimal BP target for older patients. JNC 8 recommends a target BP <150/90 mm Hg in patients ≥60 years of age.9 The American College of Physicians recommends a systolic BP target <140 mm Hg in patients ≥60 years of age with increased stroke or CV risk.11 A subgroup analysis of patients ≥75 years of age from the Systolic BP Intervention Trial (SPRINT)3 was stopped early because of the clear composite CV and mortality benefits associated with targeting a systolic BP <120 mm Hg as compared with <140 mm Hg (TABLE W13-6). Although a criticism of this trial and its results is that the researchers included only adults with high CV risk, all adults ≥75 years of age are considered to have high CV risk by the SPRINT study.3 Another criticism is that early suspension of the trial may have exaggerated treatment effects.6

Lastly, results were seemingly discrepant from previous trials, most notably, the Action to Control CV Risk in Diabetes (ACCORD) trial.6,16 However, on closer review, the ACCORD trial16 included only patients with DM, while the SPRINT3 trial excluded patients with DM, and ACCORD comprised a younger population than the SPRINT subgroup analysis. Also, the ACCORD trial did demonstrate stroke reduction and non-significant reduction in CV events, albeit, at the cost of increased adverse events, such as hypotension, bradycardia, and hypokalemia, with tighter BP control.16
Population differences presumably explain the discrepancy in results, and a systolic BP target of <120 mm Hg is appropriate in community-dwelling, non-diabetic adults ≥75 years of age. If this target goal cannot be achieved without undue burden (ie, without syncope, hypotension, bradycardia, electrolyte disturbance, renal impairment, or substantial medication burden), a recent meta-analysis found evidence that a systolic BP goal <140 mm Hg also provides benefit.6
Initiate treatment, watch for age-related changes
Lifestyle modifications (including appropriate weight loss; reduced caffeine, salt, and alcohol intake; increased physical activity; and smoking cessation) are important in the initial and ongoing management of hypertension.10,11,17,18 JNC 8 recommends initial treatment with a thiazide-type diuretic, calcium channel blocker (CCB), angiotensin converting enzyme (ACE) inhibitor, or angiotensin receptor blocker (ARB) in the nonblack population, and a CCB or thiazide diuretic in the black population.9 Specific initial medication choices for comorbid conditions are outlined in TABLE W23,10,17-22. JNC 8 recommends against the use of a beta-blocker or alpha blocker for initial treatment of hypertension.9

Start a second drug instead of maximizing the dose of the first
If the target BP cannot be achieved within one month of initiating medication, JNC 8 recommends increasing the dose of the initial drug or adding a second drug without preference for one strategy over the other.9 However, a meta-analysis demonstrates that approximately 80% of the antihypertensive effect of a drug can be achieved with half of the standard dose of the medication; this is true for thiazide-type diuretics, ACE inhibitors/ARBs, beta-blockers, and CCBs.23
Furthermore, due to fewer adverse effects and positive synergies, studies show that combining low doses of 2 medications is more beneficial than high-dose monotherapy.19,23,24 Prescribing combination pills can be helpful to limit pill burden. It is appropriate to combine any of the 4 classes of medications recommended as initial therapy by JNC 8 except for an ACE inhibitor combined with an ARB. If the target BP cannot be achieved with 3 drugs in those classes, other medications such as potassium-sparing diuretics or beta-blockers can be added.9
Changes associated with aging
Changes associated with aging include atherosclerosis and stiffening of blood vessels, increased systolic BP, widened pulse pressure, reduced glomerular filtration rate, reduced sodium elimination and volume expansion, sinoatrial node cellular dropout, and decreased sensitivity of baroreceptors.10 Because of these alterations, antihypertensive requirements may change, and resistant hypertension may develop. In addition, older patients may be more susceptible to orthostatic hypotension, heart block, electrolyte derangements, and other antihypertensive adverse effects.
When hypertension is difficult to control. Resistant hypertension is defined as hypertension that cannot be controlled with 3 drugs from 3 different antihypertensive classes, one of which is a diuretic. Cognitive impairment, polypharmacy, and multimorbidity may contribute to difficult-to-control hypertension in older adults and should be assessed prior to work-up for other secondary causes of poorly controlled hypertension.
- Cognitive impairment is often unrecognized and may impact medication adherence, which can masquerade as treatment failure. Assess for cognitive impairment on an ongoing basis with the aging patient, especially when medication adherence appears poor.
- Polypharmacy may also contribute to uncontrolled BP. Common pharmacotherapeutic contributors to uncontrolled BP include nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, high-dose decongestants, and selective norepinephrine reuptake inhibitors.25
- Multimorbidity describes 2 or more chronic medical conditions in one patient. These patients are medically complex. Comorbidities can increase pill burden and make medication adherence difficult for patients. Other poorly controlled disease states can worsen hypertension (eg, renal dysfunction secondary to diabetes). Optimize treatment of comorbid conditions.
Secondary causes. If resistant hypertension persists despite confirming medication adherence and eliminating offending medications, a work-up should ensue for secondary causes of hypertension, as well as end-organ damage. Causes of secondary hypertension include sleep apnea (see this month's HelpDesk), renal dysfunction (renal artery stenosis), aldosterone-mediated hypertension (often with hypokalemia), and thyroid disease. Evaluation for secondary causes of hypertension and end-organ damage is outlined in TABLE 1.10 Patients with well-controlled hypertension do not require repeated assessments for end-organ damage unless new symptoms—such as chest pain or edema—develop.
Consider comorbidities
Clinical trials implicitly or explicitly exclude patients with multiple comorbidities. JNC 8 provided minimal guidance for adjusting BP targets based on comorbidity with only nondiabetic CKD and DM specifically addressed.9 Guidelines from specialty organizations and recent trials provide some additional guidance in these situations and are outlined in TABLE W23,10,17-22.
Heart failure. Hypertension is a major risk factor for heart failure. Long-term treatment of systolic and diastolic hypertension can reduce the incidence of heart failure by approximately half with increased benefit in patients with prior myocardial infarction.22 Research demonstrates clear mortality benefits of certain antihypertensive drug classes, including diuretics, beta-blockers, ACE inhibitors, ARBs, aldosterone antagonists, combination hydralazine and nitrates, and angiotensin receptor-neprilysin inhibitors.21,22 The overall treatment goal in heart failure is to optimize drugs with mortality benefit, while lowering BP to a goal <130/80 mm Hg in patients ≥75 years of age.22
Increased risk for CV disease. The SPRINT trial3 defined high risk of CV disease as clinical or subclinical CV disease, CKD, 10-year ASCVD risk of ≥15%, or age ≥75 years. SPRINT supports a systolic BP goal <120 mm Hg, but, as a reminder, SPRINT excluded patients with diabetes. The American College of Cardiology Foundation Task Force and the American Heart Association define high CV risk as a 10-year ASCVD risk ≥10% and recommend a BP goal <130/80 mm Hg.10
Diabetes mellitus. A BP >115/75 mm Hg is associated with increased CV events and mortality in patients with DM.18 The American Diabetes Association (ADA) and JNC 8 recommend a BP target <140/90 mm Hg.9,18 ADA suggests a lower target of 130/80 mm Hg in patients with high CV risk if it is achievable without undue burden.18
Studies show increased mortality associated with initiating additional treatment once a systolic goal <140 mm Hg has been achieved in patients with DM.26 The ACCORD trial found increased adverse events with aggressive BP lowering to <120/80 mm Hg.16
For patients with DM requiring more than one antihypertensive agent, there are CV mortality benefits associated with administering at least one antihypertensive drug at night, likely related to the beneficial effect of physiologic nocturnal dipping.27
Chronic kidney disease. JNC 8 specifically recommends an ACE inhibitor or ARB for initial or add-on treatment in patients with CKD and a BP goal <140/90 mm Hg.9 The Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group recommends a BP target ≤140/90 mm Hg in patients without albuminuria and ≤130/80 mm Hg in patients with albuminuria to protect against the progression of nephropathy.17 The SPRINT trial3 included patients with CKD, and KDIGO has not yet updated its guidelines to reflect SPRINT.
Frailty is a clinical syndrome that has been defined as a state of increased vulnerability that is associated with a decline in reserve and function.28 The largest hypertension studies in older adults address frailty, although often the most frail patients are excluded from these studies (TABLE W13-6).
The Hypertension in the Very Elderly Trial (HYVET) categorized patients as frail, pre-frail, or robust and found a consistent benefit of antihypertensive treatment on stroke, CV events, and total mortality—regardless of baseline frailty status.29 The SPRINT trial included only community-dwelling adults.3 Other studies suggest that hypertension actually has a protective effect by lowering overall mortality in frail older adults, especially in the frailest and oldest nursing home populations.30,31
Although there is a paucity of data to direct the management of hypertension in frail older patients, physicians should prioritize the condition and focus on adverse events from antihypertensives and on slow titration of medications. The JNC 8 BP target of <150/90 mm Hg is a reasonable BP goal in this population, given the lack of evidence for lower or higher targets.9 Many frail patients have one or more of the comorbidities described earlier, and it is reasonable to strive for the comorbidity-specific target, provided it can be achieved without undue burden.
Cognitive impairment and dementia. The association between hypertension and dementia/cognitive impairment is evolving. Hypertension may impact various forms of dementia, such as Alzheimer’s disease (AD) or vascular dementia, differently. There is evidence linking hypertension to AD.32 The relationship between BP and brain perfusion is complex with the potential existence of an age-adjusted relationship such that mid-life hypertension may increase the risk of dementia while late-life hypertension may not.33
A number of studies reveal the evolving nature of our understanding of these 2 conditions:
- A recent systematic review and meta-analysis examining intensive BP treatments in older adults demonstrated that lower BP targets did not increase cognitive decline.6
- HYVET’s cognitive function assessment did not find a significant reduction in the incidence of dementia with BP reduction over a short follow-up period, but when results were combined in a meta-analysis with other placebo-controlled, double-blind trials of antihypertensive treatments, there was significant reduction in incident dementia in patients randomized to antihypertensive treatment.34
- The ACCORD Memory in Diabetes trial (ACCORD-MIND) had the unexpected outcome that intensive lowering of systolic BP to a target <120 mm Hg resulted in a greater decline in total brain volume, compared with the standard BP goal <140 mm Hg. This was measured with magnetic resonance imaging in older adults with type 2 DM.35
- Results from the SPRINT sub-analysis Memory and Cognition in Decreased Hypertension trial are forthcoming and aim to determine the effects of BP reduction on dementia.36
The JNC 89 BP target <150/90 mm Hg or a comorbidity-specific target, if achievable without undue burden, is reasonable in patients with dementia. In a systematic review of observational studies in patients with hypertension and dementia, diuretics, CCBs, ACE inhibitors/ARBs, and beta-blockers were commonly used medications with a trend toward prescribing CCBs and ACE inhibitors/ARBs.37
As previously highlighted, cognitive impairment may lead to problems with medication adherence and even inadvertent improper medication use, potentially resulting in adverse events from antihypertensives. If cognitive impairment or dementia is suspected, ensure additional measures (such as medication assistance or supervision) are in place before prescribing antihypertensives.
Certain diseases, such as Parkinson’s-related dementia and multiple system atrophy, can cause autonomic instability, which can increase the risk of falls and complicate hypertension management. Carefully monitor patients for signs of orthostasis.

CASE 1 Repeat the BP measurement in the office once the patient has been seated for ≥5 minutes, and have the patient monitor her BP at home; schedule a follow-up visit in 2 weeks. If hypertension is confirmed with home measurements, then, in addition to lifestyle modifications, initiate treatment with a CCB or thiazide diuretic to achieve a systolic BP goal <120 mm Hg. Titrate medications slowly while monitoring for adverse effects.
CASE 2 Consistent with the office measurement, the patient has home BP readings that are above the BP target (<120 mm Hg systolic). He has been taking a single antihypertensive for longer than one month. Discontinue his NSAID prior to adding any new medications. If his BP is still above target without NSAIDs, then add a second agent, such as a low dose of an ACE inhibitor, ARB, or CCB, rather than maximizing the dose of hydrochlorothiazide.
CASE 3 Given the patient’s diabetes, CKD, and albuminuria, a target BP goal <130/80 mm Hg is reasonable. An ACE inhibitor or ARB is a better medication choice than atenolol in this patient with albuminuria. Because of the deterioration in his ADLs, careful assessment of mobility, functionality, comorbidities, frailty, and cognitive function should take place at each office visit and inform adjustments to the patient’s BP target. Employ cautious medication titration with monitoring for adverse effects, especially hypotension and syncope. If his functional status declines, adverse effects develop, or the medication regimen becomes burdensome, relax the target BP goal to 150/90 mm Hg.
CORRESPONDENCE
Julienne K. Kirk, PharmD, Family and Community Medicine, Wake Forest School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157-1084; [email protected].
CASE 1 An 82-year-old black woman comes in for an annual exam. She has no medical concerns. She volunteers at a hospice, walks daily, and maintains a healthy diet. Her past medical history (PMH) includes osteopenia and osteoarthritis, and her medications include acetaminophen as needed and vitamin D. She has no drug allergies. Her exam reveals a blood pressure (BP) of 148/70 mm Hg, a body mass index of 31, and a heart rate (HR) of 71 beats per minute (bpm). Cardiac and pulmonary exams are normal, and she shows no signs of peripheral edema.
CASE 2 An 88-year-old white man presents to the office for a 3-month follow-up of his hypertension. His systolic BP at home has ranged from 140 to 170 mm Hg. He denies chest pain, shortness of breath, or lower extremity edema. He lives with his wife and frequently swims for exercise. His PMH is significant for depression and degenerative disc disease. His medications include hydrochlorothiazide 12.5 mg/d, sertraline 50 mg/d, and naproxen 250 mg bid. His BP is 160/80 mm Hg and his HR is 70 bpm with normal cardiovascular (CV) and pulmonary exams.
CASE 3 An 80-year-old white man with diabetes mellitus (DM), hypertension, and chronic kidney disease (CKD) presents for a 3-month follow-up visit. His home systolic BP has been in the 140s to 150s. He is functional in all of his activities of daily living (ADLs), but is starting to require assistance with medications, finances, and transportation. He takes aspirin 81 mg/d, chlorthalidone 25 mg/d, and atenolol 50 mg/d. Remarkable laboratory test results include a hemoglobin A1c of 8.6%, a serum creatinine of 1.9 mg/dL (normal range: 0.6-1.2 mg/dL), and an albumin-creatinine ratio of 250 mg/g (normal range: <30 mg/g). During the exam, his BP is 143/70 mm Hg, his HR is 70 bpm, he is alert and oriented to person, place, and time, and he has normal CV and pulmonary exams with no signs of peripheral edema. He has decreased sensation in his feet, but normal reflexes.
How would you proceed with the care of these 3 patients?
Hypertension is the most common diagnosis made during physician office visits in the United States.1 Nearly one-third of the population has hypertension, and its prevalence increases with age, such that 67% of men and 79% of women ≥75 years of age have the condition.2
Evidence indicates that hypertension is a modifiable risk factor for CV and all-cause mortality (TABLE W13-6). All adults ≥75 years of age are at increased CV risk based on Framingham criteria,7 making hypertension management paramount. Complicating the situation are findings that indicate nearly half of adults with hypertension have inadequate BP control.2
Clinicians require clear direction about optimal BP targets, how best to adjust antihypertensive medications for comorbidities, and how to incorporate frailty and cognitive impairment into management strategies. This article presents recommendations derived from recent evidence and consensus guidelines regarding the management of hypertension in adults ≥75 years of age.
[polldaddy:9818133]
Diagnosing hypertension
According to the seventh report of the Joint National Committee (JNC 7), hypertension is defined as a systolic BP ≥140 mm Hg and/or a diastolic BP ≥90 mm Hg.8 The JNC’s more recent report (JNC 8), however, does not define hypertension; instead, it sets forth treatment thresholds (eg, that there is strong evidence to support treating individuals ≥60 years of age when BP ≥150/90 mm Hg).9
It starts with an accurate BP measurement. Ensuring the accuracy of a BP measurement requires multiple readings over time. White coat hypertension and masked hypertension can complicate BP measurement. Home measurements better correlate with atherosclerotic cardiovascular disease (ASCVD) risk than do office measurements.10-12 In fact, the US Preventive Services Task Force recommends obtaining measurements outside of the clinic setting prior to initiating treatment for hypertension.13

Educate staff on the proper technique for obtaining BP measurements in the office (ie, taking measurements using an appropriately sized cuff when patients have been seated for at least 5 minutes with feet uncrossed and with their arm supported at heart level). Cold temperatures, coffee consumption, talking, and recent tobacco use can transiently raise BP. TABLE 110 outlines the initial work-up after confirming the diagnosis of hypertension. No other routine tests are recommended for the management of hypertension except those associated with medication monitoring (outlined in TABLE 210,11,14,15).

What’s the optimal BP target for older patients? No consensus exists on an optimal BP target for older patients. JNC 8 recommends a target BP <150/90 mm Hg in patients ≥60 years of age.9 The American College of Physicians recommends a systolic BP target <140 mm Hg in patients ≥60 years of age with increased stroke or CV risk.11 A subgroup analysis of patients ≥75 years of age from the Systolic BP Intervention Trial (SPRINT)3 was stopped early because of the clear composite CV and mortality benefits associated with targeting a systolic BP <120 mm Hg as compared with <140 mm Hg (TABLE W13-6). Although a criticism of this trial and its results is that the researchers included only adults with high CV risk, all adults ≥75 years of age are considered to have high CV risk by the SPRINT study.3 Another criticism is that early suspension of the trial may have exaggerated treatment effects.6

Lastly, results were seemingly discrepant from previous trials, most notably, the Action to Control CV Risk in Diabetes (ACCORD) trial.6,16 However, on closer review, the ACCORD trial16 included only patients with DM, while the SPRINT3 trial excluded patients with DM, and ACCORD comprised a younger population than the SPRINT subgroup analysis. Also, the ACCORD trial did demonstrate stroke reduction and non-significant reduction in CV events, albeit, at the cost of increased adverse events, such as hypotension, bradycardia, and hypokalemia, with tighter BP control.16
Population differences presumably explain the discrepancy in results, and a systolic BP target of <120 mm Hg is appropriate in community-dwelling, non-diabetic adults ≥75 years of age. If this target goal cannot be achieved without undue burden (ie, without syncope, hypotension, bradycardia, electrolyte disturbance, renal impairment, or substantial medication burden), a recent meta-analysis found evidence that a systolic BP goal <140 mm Hg also provides benefit.6
Initiate treatment, watch for age-related changes
Lifestyle modifications (including appropriate weight loss; reduced caffeine, salt, and alcohol intake; increased physical activity; and smoking cessation) are important in the initial and ongoing management of hypertension.10,11,17,18 JNC 8 recommends initial treatment with a thiazide-type diuretic, calcium channel blocker (CCB), angiotensin converting enzyme (ACE) inhibitor, or angiotensin receptor blocker (ARB) in the nonblack population, and a CCB or thiazide diuretic in the black population.9 Specific initial medication choices for comorbid conditions are outlined in TABLE W23,10,17-22. JNC 8 recommends against the use of a beta-blocker or alpha blocker for initial treatment of hypertension.9

Start a second drug instead of maximizing the dose of the first
If the target BP cannot be achieved within one month of initiating medication, JNC 8 recommends increasing the dose of the initial drug or adding a second drug without preference for one strategy over the other.9 However, a meta-analysis demonstrates that approximately 80% of the antihypertensive effect of a drug can be achieved with half of the standard dose of the medication; this is true for thiazide-type diuretics, ACE inhibitors/ARBs, beta-blockers, and CCBs.23
Furthermore, due to fewer adverse effects and positive synergies, studies show that combining low doses of 2 medications is more beneficial than high-dose monotherapy.19,23,24 Prescribing combination pills can be helpful to limit pill burden. It is appropriate to combine any of the 4 classes of medications recommended as initial therapy by JNC 8 except for an ACE inhibitor combined with an ARB. If the target BP cannot be achieved with 3 drugs in those classes, other medications such as potassium-sparing diuretics or beta-blockers can be added.9
Changes associated with aging
Changes associated with aging include atherosclerosis and stiffening of blood vessels, increased systolic BP, widened pulse pressure, reduced glomerular filtration rate, reduced sodium elimination and volume expansion, sinoatrial node cellular dropout, and decreased sensitivity of baroreceptors.10 Because of these alterations, antihypertensive requirements may change, and resistant hypertension may develop. In addition, older patients may be more susceptible to orthostatic hypotension, heart block, electrolyte derangements, and other antihypertensive adverse effects.
When hypertension is difficult to control. Resistant hypertension is defined as hypertension that cannot be controlled with 3 drugs from 3 different antihypertensive classes, one of which is a diuretic. Cognitive impairment, polypharmacy, and multimorbidity may contribute to difficult-to-control hypertension in older adults and should be assessed prior to work-up for other secondary causes of poorly controlled hypertension.
- Cognitive impairment is often unrecognized and may impact medication adherence, which can masquerade as treatment failure. Assess for cognitive impairment on an ongoing basis with the aging patient, especially when medication adherence appears poor.
- Polypharmacy may also contribute to uncontrolled BP. Common pharmacotherapeutic contributors to uncontrolled BP include nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, high-dose decongestants, and selective norepinephrine reuptake inhibitors.25
- Multimorbidity describes 2 or more chronic medical conditions in one patient. These patients are medically complex. Comorbidities can increase pill burden and make medication adherence difficult for patients. Other poorly controlled disease states can worsen hypertension (eg, renal dysfunction secondary to diabetes). Optimize treatment of comorbid conditions.
Secondary causes. If resistant hypertension persists despite confirming medication adherence and eliminating offending medications, a work-up should ensue for secondary causes of hypertension, as well as end-organ damage. Causes of secondary hypertension include sleep apnea (see this month's HelpDesk), renal dysfunction (renal artery stenosis), aldosterone-mediated hypertension (often with hypokalemia), and thyroid disease. Evaluation for secondary causes of hypertension and end-organ damage is outlined in TABLE 1.10 Patients with well-controlled hypertension do not require repeated assessments for end-organ damage unless new symptoms—such as chest pain or edema—develop.
Consider comorbidities
Clinical trials implicitly or explicitly exclude patients with multiple comorbidities. JNC 8 provided minimal guidance for adjusting BP targets based on comorbidity with only nondiabetic CKD and DM specifically addressed.9 Guidelines from specialty organizations and recent trials provide some additional guidance in these situations and are outlined in TABLE W23,10,17-22.
Heart failure. Hypertension is a major risk factor for heart failure. Long-term treatment of systolic and diastolic hypertension can reduce the incidence of heart failure by approximately half with increased benefit in patients with prior myocardial infarction.22 Research demonstrates clear mortality benefits of certain antihypertensive drug classes, including diuretics, beta-blockers, ACE inhibitors, ARBs, aldosterone antagonists, combination hydralazine and nitrates, and angiotensin receptor-neprilysin inhibitors.21,22 The overall treatment goal in heart failure is to optimize drugs with mortality benefit, while lowering BP to a goal <130/80 mm Hg in patients ≥75 years of age.22
Increased risk for CV disease. The SPRINT trial3 defined high risk of CV disease as clinical or subclinical CV disease, CKD, 10-year ASCVD risk of ≥15%, or age ≥75 years. SPRINT supports a systolic BP goal <120 mm Hg, but, as a reminder, SPRINT excluded patients with diabetes. The American College of Cardiology Foundation Task Force and the American Heart Association define high CV risk as a 10-year ASCVD risk ≥10% and recommend a BP goal <130/80 mm Hg.10
Diabetes mellitus. A BP >115/75 mm Hg is associated with increased CV events and mortality in patients with DM.18 The American Diabetes Association (ADA) and JNC 8 recommend a BP target <140/90 mm Hg.9,18 ADA suggests a lower target of 130/80 mm Hg in patients with high CV risk if it is achievable without undue burden.18
Studies show increased mortality associated with initiating additional treatment once a systolic goal <140 mm Hg has been achieved in patients with DM.26 The ACCORD trial found increased adverse events with aggressive BP lowering to <120/80 mm Hg.16
For patients with DM requiring more than one antihypertensive agent, there are CV mortality benefits associated with administering at least one antihypertensive drug at night, likely related to the beneficial effect of physiologic nocturnal dipping.27
Chronic kidney disease. JNC 8 specifically recommends an ACE inhibitor or ARB for initial or add-on treatment in patients with CKD and a BP goal <140/90 mm Hg.9 The Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group recommends a BP target ≤140/90 mm Hg in patients without albuminuria and ≤130/80 mm Hg in patients with albuminuria to protect against the progression of nephropathy.17 The SPRINT trial3 included patients with CKD, and KDIGO has not yet updated its guidelines to reflect SPRINT.
Frailty is a clinical syndrome that has been defined as a state of increased vulnerability that is associated with a decline in reserve and function.28 The largest hypertension studies in older adults address frailty, although often the most frail patients are excluded from these studies (TABLE W13-6).
The Hypertension in the Very Elderly Trial (HYVET) categorized patients as frail, pre-frail, or robust and found a consistent benefit of antihypertensive treatment on stroke, CV events, and total mortality—regardless of baseline frailty status.29 The SPRINT trial included only community-dwelling adults.3 Other studies suggest that hypertension actually has a protective effect by lowering overall mortality in frail older adults, especially in the frailest and oldest nursing home populations.30,31
Although there is a paucity of data to direct the management of hypertension in frail older patients, physicians should prioritize the condition and focus on adverse events from antihypertensives and on slow titration of medications. The JNC 8 BP target of <150/90 mm Hg is a reasonable BP goal in this population, given the lack of evidence for lower or higher targets.9 Many frail patients have one or more of the comorbidities described earlier, and it is reasonable to strive for the comorbidity-specific target, provided it can be achieved without undue burden.
Cognitive impairment and dementia. The association between hypertension and dementia/cognitive impairment is evolving. Hypertension may impact various forms of dementia, such as Alzheimer’s disease (AD) or vascular dementia, differently. There is evidence linking hypertension to AD.32 The relationship between BP and brain perfusion is complex with the potential existence of an age-adjusted relationship such that mid-life hypertension may increase the risk of dementia while late-life hypertension may not.33
A number of studies reveal the evolving nature of our understanding of these 2 conditions:
- A recent systematic review and meta-analysis examining intensive BP treatments in older adults demonstrated that lower BP targets did not increase cognitive decline.6
- HYVET’s cognitive function assessment did not find a significant reduction in the incidence of dementia with BP reduction over a short follow-up period, but when results were combined in a meta-analysis with other placebo-controlled, double-blind trials of antihypertensive treatments, there was significant reduction in incident dementia in patients randomized to antihypertensive treatment.34
- The ACCORD Memory in Diabetes trial (ACCORD-MIND) had the unexpected outcome that intensive lowering of systolic BP to a target <120 mm Hg resulted in a greater decline in total brain volume, compared with the standard BP goal <140 mm Hg. This was measured with magnetic resonance imaging in older adults with type 2 DM.35
- Results from the SPRINT sub-analysis Memory and Cognition in Decreased Hypertension trial are forthcoming and aim to determine the effects of BP reduction on dementia.36
The JNC 89 BP target <150/90 mm Hg or a comorbidity-specific target, if achievable without undue burden, is reasonable in patients with dementia. In a systematic review of observational studies in patients with hypertension and dementia, diuretics, CCBs, ACE inhibitors/ARBs, and beta-blockers were commonly used medications with a trend toward prescribing CCBs and ACE inhibitors/ARBs.37
As previously highlighted, cognitive impairment may lead to problems with medication adherence and even inadvertent improper medication use, potentially resulting in adverse events from antihypertensives. If cognitive impairment or dementia is suspected, ensure additional measures (such as medication assistance or supervision) are in place before prescribing antihypertensives.
Certain diseases, such as Parkinson’s-related dementia and multiple system atrophy, can cause autonomic instability, which can increase the risk of falls and complicate hypertension management. Carefully monitor patients for signs of orthostasis.

CASE 1 Repeat the BP measurement in the office once the patient has been seated for ≥5 minutes, and have the patient monitor her BP at home; schedule a follow-up visit in 2 weeks. If hypertension is confirmed with home measurements, then, in addition to lifestyle modifications, initiate treatment with a CCB or thiazide diuretic to achieve a systolic BP goal <120 mm Hg. Titrate medications slowly while monitoring for adverse effects.
CASE 2 Consistent with the office measurement, the patient has home BP readings that are above the BP target (<120 mm Hg systolic). He has been taking a single antihypertensive for longer than one month. Discontinue his NSAID prior to adding any new medications. If his BP is still above target without NSAIDs, then add a second agent, such as a low dose of an ACE inhibitor, ARB, or CCB, rather than maximizing the dose of hydrochlorothiazide.
CASE 3 Given the patient’s diabetes, CKD, and albuminuria, a target BP goal <130/80 mm Hg is reasonable. An ACE inhibitor or ARB is a better medication choice than atenolol in this patient with albuminuria. Because of the deterioration in his ADLs, careful assessment of mobility, functionality, comorbidities, frailty, and cognitive function should take place at each office visit and inform adjustments to the patient’s BP target. Employ cautious medication titration with monitoring for adverse effects, especially hypotension and syncope. If his functional status declines, adverse effects develop, or the medication regimen becomes burdensome, relax the target BP goal to 150/90 mm Hg.
CORRESPONDENCE
Julienne K. Kirk, PharmD, Family and Community Medicine, Wake Forest School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157-1084; [email protected].
1. National Ambulatory Medical Care Survey: 2013 State and National Summary Tables. Available at: https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2013_namcs_web_tables.pdf. Accessed May 29, 2017.
2. Centers for Disease Control and Prevention. High blood pressure facts. Available at: https://cdc.gov/bloodpressure/facts.htm. Accessed May 29, 2017.
3. Williamson JD, Suplano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673-2682.
4. Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887-1898.
5. Kostis WJ, Cabrera J, Messerli FH, et al. Competing cardiovascular and noncardiovascular risks and longevity in the systolic hypertension in the elderly program. Am J Cardiol. 2014;113:676-681.
6. Weiss J, Freeman M, Low A, et al. Benefits and harms of intensive blood pressure treatment in adults aged 60 years or older: a systematic review and meta-analysis. Ann Intern Med. 2017;166:419-429.
7. Framingham Heart Study. Available at: https://www.framinghamheartstudy.org/risk-functions/cardiovascular-disease/10-year-risk.php. Accessed May 29, 2017.
8. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
9. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
10. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on clinical expert consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57:2037-2114.
11. Qaseem A, Wilt TJ, Rich R, et al. Pharmacological treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166:430-437.
12. Sega R, Facchetti R, Bombelli M, et al. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111:1777-1783.
13. US Preventive Services Task Force. Final recommendation statement: high blood pressure in adults: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/high-blood-pressure-in-adults-screening. Accessed May 29, 2017.
14. Steinman MA, Miao Y, Boscardin WJ, et al. Prescribing quality in older veterans: a multifocal approach. J Gen Intern Med. 2014;29:1379-1386.
15. Schwartz JB. Primary prevention: do the very elderly require a different approach. Trends Cardiovasc Med. 2015:25:228-239.
16. Accord Study Group, Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
17. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2012;2:337-414.
18. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(suppl 1):S1-S135.
19. Ogawa H, Kim-Mitsuyama S, Matsui K, et al, OSCAR Study Group. Angiotensin II receptor blocker-based therapy in Japanese elderly, high-risk, hypertensive patients. Am J Med. 2012;125:981-990.
20. Rosendorff C, Lackland DT, Allison M, et al. AHA/ACC/ASH Scientific Statement. Treatment of hypertension in patients with coronary heart disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. Hypertension. 2015;65:1372-1407.
21. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327.
22. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137-e161.
23. Law MR, Wald MJ, Morris JK, et al. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326:1427.
24. Wald DS, Law M, Morris JK, et al. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290-300.
25. Mukete BN, Ferdinand KC. Polypharmacy in older adults with hypertension: a comprehensive review. J Clin Hypertens (Greenwich). 2016;18:10-18.
26. Brunstrom M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
27. Hermida RC, Ayala DE, Mojón A, et al. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diab Care. 2011;34:1270-1276.
28. Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27:1-15.
29. Warwick J, Falashcetti E, Rockwood K, et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the Hypertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med. 2015;13:78.
30. Zhang XE, Cheng B, Wang Q. Relationship between high blood pressure and cardiovascular outcomes in elderly frail patients: a systematic review and meta-analysis. Geriatric Nurs. 2016;37:385-392.
31. Benetos A, Rossignol P, Cherbuini A, et al. Polypharmacy in the aging patient: management of hypertension in octogenarians. JAMA. 2015;314:170-180.
32. de Bruijn R, Ikram MA. Cardiovascular risk factors and future risk of Alzheimer’s disease. BMC Med. 2014;12:130.
33. Joas E, Bäckman K, Gustafson D, et al. Blood pressure trajectories from midlife to late life in relation to dementia in women followed for 37 years. Hypertension. 2012;59:796-801.
34. Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lanc Neurol. 2008;7:683-689.
35. Williamson JD, Launer LJ, Bryan RN, et al. Cognitive function and brain structure in persons with type 2 diabetes mellitus after intensive lowering of blood pressure and lipid levels: a randomized clinical trial. JAMA Intern Med. 2014;174:324-333.
36. Tom Wade, MD. Methods of the SPRINT MIND Trial—how they did it + why it matters to primary care physicians. Available at: /www.tomwademd.net/methods-of-the-sprint-mind-trial-how-they-did-it-why-it-matters-to-primary-care-physicians/. Accessed August 11, 2017.
37. Welsh TJ, Gladman JR, Gordon AL. The treatment of hypertension in people with dementia: a systematic review of observational studies. BMC Geriatr. 2014;14:19.
1. National Ambulatory Medical Care Survey: 2013 State and National Summary Tables. Available at: https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2013_namcs_web_tables.pdf. Accessed May 29, 2017.
2. Centers for Disease Control and Prevention. High blood pressure facts. Available at: https://cdc.gov/bloodpressure/facts.htm. Accessed May 29, 2017.
3. Williamson JD, Suplano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673-2682.
4. Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887-1898.
5. Kostis WJ, Cabrera J, Messerli FH, et al. Competing cardiovascular and noncardiovascular risks and longevity in the systolic hypertension in the elderly program. Am J Cardiol. 2014;113:676-681.
6. Weiss J, Freeman M, Low A, et al. Benefits and harms of intensive blood pressure treatment in adults aged 60 years or older: a systematic review and meta-analysis. Ann Intern Med. 2017;166:419-429.
7. Framingham Heart Study. Available at: https://www.framinghamheartstudy.org/risk-functions/cardiovascular-disease/10-year-risk.php. Accessed May 29, 2017.
8. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
9. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
10. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on clinical expert consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57:2037-2114.
11. Qaseem A, Wilt TJ, Rich R, et al. Pharmacological treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166:430-437.
12. Sega R, Facchetti R, Bombelli M, et al. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111:1777-1783.
13. US Preventive Services Task Force. Final recommendation statement: high blood pressure in adults: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/high-blood-pressure-in-adults-screening. Accessed May 29, 2017.
14. Steinman MA, Miao Y, Boscardin WJ, et al. Prescribing quality in older veterans: a multifocal approach. J Gen Intern Med. 2014;29:1379-1386.
15. Schwartz JB. Primary prevention: do the very elderly require a different approach. Trends Cardiovasc Med. 2015:25:228-239.
16. Accord Study Group, Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
17. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2012;2:337-414.
18. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(suppl 1):S1-S135.
19. Ogawa H, Kim-Mitsuyama S, Matsui K, et al, OSCAR Study Group. Angiotensin II receptor blocker-based therapy in Japanese elderly, high-risk, hypertensive patients. Am J Med. 2012;125:981-990.
20. Rosendorff C, Lackland DT, Allison M, et al. AHA/ACC/ASH Scientific Statement. Treatment of hypertension in patients with coronary heart disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. Hypertension. 2015;65:1372-1407.
21. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327.
22. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137-e161.
23. Law MR, Wald MJ, Morris JK, et al. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326:1427.
24. Wald DS, Law M, Morris JK, et al. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290-300.
25. Mukete BN, Ferdinand KC. Polypharmacy in older adults with hypertension: a comprehensive review. J Clin Hypertens (Greenwich). 2016;18:10-18.
26. Brunstrom M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
27. Hermida RC, Ayala DE, Mojón A, et al. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diab Care. 2011;34:1270-1276.
28. Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27:1-15.
29. Warwick J, Falashcetti E, Rockwood K, et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the Hypertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med. 2015;13:78.
30. Zhang XE, Cheng B, Wang Q. Relationship between high blood pressure and cardiovascular outcomes in elderly frail patients: a systematic review and meta-analysis. Geriatric Nurs. 2016;37:385-392.
31. Benetos A, Rossignol P, Cherbuini A, et al. Polypharmacy in the aging patient: management of hypertension in octogenarians. JAMA. 2015;314:170-180.
32. de Bruijn R, Ikram MA. Cardiovascular risk factors and future risk of Alzheimer’s disease. BMC Med. 2014;12:130.
33. Joas E, Bäckman K, Gustafson D, et al. Blood pressure trajectories from midlife to late life in relation to dementia in women followed for 37 years. Hypertension. 2012;59:796-801.
34. Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lanc Neurol. 2008;7:683-689.
35. Williamson JD, Launer LJ, Bryan RN, et al. Cognitive function and brain structure in persons with type 2 diabetes mellitus after intensive lowering of blood pressure and lipid levels: a randomized clinical trial. JAMA Intern Med. 2014;174:324-333.
36. Tom Wade, MD. Methods of the SPRINT MIND Trial—how they did it + why it matters to primary care physicians. Available at: /www.tomwademd.net/methods-of-the-sprint-mind-trial-how-they-did-it-why-it-matters-to-primary-care-physicians/. Accessed August 11, 2017.
37. Welsh TJ, Gladman JR, Gordon AL. The treatment of hypertension in people with dementia: a systematic review of observational studies. BMC Geriatr. 2014;14:19.
From The Journal of Family Practice | 2017;66(9):546-548,550-554.
PRACTICE RECOMMENDATIONS
› Target a systolic blood pressure (BP) <120 mm Hg in community-dwelling, non-diabetic patients ≥75 years of age if it is achievable without undue burden. A
› Combine low doses of 2 medications, rather than increase the dose of a single agent, to achieve the desired BP target. A
› Consider cognitive function, polypharmacy, multimorbidity, and frailty when assessing and treating hypertension in older patients. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Protein may be target for improving HSCT
New research published in The Journal of Clinical Investigation suggests the protein Del-1 regulates the hematopoietic stem cell (HSC) niche.
Researchers therefore believe that targeting Del-1 could be an effective way to improve HSC transplants (HSCTs) for donors and recipients.
There may also be ways to modulate levels of Del-1 to enhance immune cell production in patients with certain hematologic malignancies.
“Because the hematopoietic stem cell niche is so important for the creation of bone marrow and blood cells and because Del-1 is a soluble protein and is easily manipulated, one can see that it could be a target in many potential applications,” said study author George Hajishengallis, DDS, PhD, of the University of Pennsylvania School of Dental Medicine in Philadelphia.
“I think that Del-1 represents a major regulator of the hematopoietic stem cell niche,” added study author Triantafyllos Chavakis, MD, PhD, of the Technical University of Dresden in Germany. “It will be worthwhile to study its expression in the context of hematopoietic malignancy.”
This research began when Drs Hajishengallis and Chavakis identified Del-1 as a potential drug target for gum disease. They found the protein prevents inflammatory cells from moving into the gums.
Both researchers and their labs also discovered that Del-1 was expressed in the bone marrow as well. So the researchers began following up to determine the protein’s function there.
“In the beginning, I thought it would have a simple function, like regulating the exit of mature leukocytes from the marrow into the periphery, something analogous to what it was doing in the gingiva,” Dr Hajishengallis said. “But it turned out it had a much more important and global role than what I had imagined.”
The researchers’ investigations revealed that Del-1 was expressed by at least 3 cell types that support HSCs: arteriolar endothelial cells, CXCL12-abundant reticular cells, and cells of the osteoblastic lineage.
Using mice deficient in Del-1, the researchers found the protein promotes proliferation and differentiation of HSCs, sending more progenitor cells down a path toward becoming myeloid cells rather than lymphocytes.
In HSCT experiments, the team discovered the presence of Del-1 in recipient bone marrow is required for the transplanted HSCs to engraft in the recipient and to facilitate the process of myelopoiesis.
When the researchers mimicked a systemic infection in mice, animals deficient in Del-1 were slower to begin making myeloid cells again compared to mice with normal Del-1 levels.
“We saw roles for Del-1 in both steady-state and emergency conditions,” Dr Hajishengallis said.
He and his colleagues also identified the protein with which Del-1 interacts, the ß3 integrin, perhaps pointing to a target for therapeutic interventions down the line.
The researchers see potential applications in HSCTs, for both donors and recipients.
In donors, blocking the interaction between Del-1 and HSCs could enhance the mobilization of those progenitors into the bloodstream. This could be helpful for increasing donor cell numbers for transplantation.
HSCT recipients, on the other hand, may need enhanced Del-1 interaction to ensure the transplanted cells engraft and begin making new blood cells more rapidly.
In addition, people undergoing chemotherapy who develop febrile neutropenia might benefit from the role of Del-1 in supporting the production of immune-related blood cells such as neutrophils.
“It’s easy to think of practical applications for these findings,” Dr Hajishengallis said. “Now, we need to find out whether it works in practice, so our studies continue.” ![]()
New research published in The Journal of Clinical Investigation suggests the protein Del-1 regulates the hematopoietic stem cell (HSC) niche.
Researchers therefore believe that targeting Del-1 could be an effective way to improve HSC transplants (HSCTs) for donors and recipients.
There may also be ways to modulate levels of Del-1 to enhance immune cell production in patients with certain hematologic malignancies.
“Because the hematopoietic stem cell niche is so important for the creation of bone marrow and blood cells and because Del-1 is a soluble protein and is easily manipulated, one can see that it could be a target in many potential applications,” said study author George Hajishengallis, DDS, PhD, of the University of Pennsylvania School of Dental Medicine in Philadelphia.
“I think that Del-1 represents a major regulator of the hematopoietic stem cell niche,” added study author Triantafyllos Chavakis, MD, PhD, of the Technical University of Dresden in Germany. “It will be worthwhile to study its expression in the context of hematopoietic malignancy.”
This research began when Drs Hajishengallis and Chavakis identified Del-1 as a potential drug target for gum disease. They found the protein prevents inflammatory cells from moving into the gums.
Both researchers and their labs also discovered that Del-1 was expressed in the bone marrow as well. So the researchers began following up to determine the protein’s function there.
“In the beginning, I thought it would have a simple function, like regulating the exit of mature leukocytes from the marrow into the periphery, something analogous to what it was doing in the gingiva,” Dr Hajishengallis said. “But it turned out it had a much more important and global role than what I had imagined.”
The researchers’ investigations revealed that Del-1 was expressed by at least 3 cell types that support HSCs: arteriolar endothelial cells, CXCL12-abundant reticular cells, and cells of the osteoblastic lineage.
Using mice deficient in Del-1, the researchers found the protein promotes proliferation and differentiation of HSCs, sending more progenitor cells down a path toward becoming myeloid cells rather than lymphocytes.
In HSCT experiments, the team discovered the presence of Del-1 in recipient bone marrow is required for the transplanted HSCs to engraft in the recipient and to facilitate the process of myelopoiesis.
When the researchers mimicked a systemic infection in mice, animals deficient in Del-1 were slower to begin making myeloid cells again compared to mice with normal Del-1 levels.
“We saw roles for Del-1 in both steady-state and emergency conditions,” Dr Hajishengallis said.
He and his colleagues also identified the protein with which Del-1 interacts, the ß3 integrin, perhaps pointing to a target for therapeutic interventions down the line.
The researchers see potential applications in HSCTs, for both donors and recipients.
In donors, blocking the interaction between Del-1 and HSCs could enhance the mobilization of those progenitors into the bloodstream. This could be helpful for increasing donor cell numbers for transplantation.
HSCT recipients, on the other hand, may need enhanced Del-1 interaction to ensure the transplanted cells engraft and begin making new blood cells more rapidly.
In addition, people undergoing chemotherapy who develop febrile neutropenia might benefit from the role of Del-1 in supporting the production of immune-related blood cells such as neutrophils.
“It’s easy to think of practical applications for these findings,” Dr Hajishengallis said. “Now, we need to find out whether it works in practice, so our studies continue.” ![]()
New research published in The Journal of Clinical Investigation suggests the protein Del-1 regulates the hematopoietic stem cell (HSC) niche.
Researchers therefore believe that targeting Del-1 could be an effective way to improve HSC transplants (HSCTs) for donors and recipients.
There may also be ways to modulate levels of Del-1 to enhance immune cell production in patients with certain hematologic malignancies.
“Because the hematopoietic stem cell niche is so important for the creation of bone marrow and blood cells and because Del-1 is a soluble protein and is easily manipulated, one can see that it could be a target in many potential applications,” said study author George Hajishengallis, DDS, PhD, of the University of Pennsylvania School of Dental Medicine in Philadelphia.
“I think that Del-1 represents a major regulator of the hematopoietic stem cell niche,” added study author Triantafyllos Chavakis, MD, PhD, of the Technical University of Dresden in Germany. “It will be worthwhile to study its expression in the context of hematopoietic malignancy.”
This research began when Drs Hajishengallis and Chavakis identified Del-1 as a potential drug target for gum disease. They found the protein prevents inflammatory cells from moving into the gums.
Both researchers and their labs also discovered that Del-1 was expressed in the bone marrow as well. So the researchers began following up to determine the protein’s function there.
“In the beginning, I thought it would have a simple function, like regulating the exit of mature leukocytes from the marrow into the periphery, something analogous to what it was doing in the gingiva,” Dr Hajishengallis said. “But it turned out it had a much more important and global role than what I had imagined.”
The researchers’ investigations revealed that Del-1 was expressed by at least 3 cell types that support HSCs: arteriolar endothelial cells, CXCL12-abundant reticular cells, and cells of the osteoblastic lineage.
Using mice deficient in Del-1, the researchers found the protein promotes proliferation and differentiation of HSCs, sending more progenitor cells down a path toward becoming myeloid cells rather than lymphocytes.
In HSCT experiments, the team discovered the presence of Del-1 in recipient bone marrow is required for the transplanted HSCs to engraft in the recipient and to facilitate the process of myelopoiesis.
When the researchers mimicked a systemic infection in mice, animals deficient in Del-1 were slower to begin making myeloid cells again compared to mice with normal Del-1 levels.
“We saw roles for Del-1 in both steady-state and emergency conditions,” Dr Hajishengallis said.
He and his colleagues also identified the protein with which Del-1 interacts, the ß3 integrin, perhaps pointing to a target for therapeutic interventions down the line.
The researchers see potential applications in HSCTs, for both donors and recipients.
In donors, blocking the interaction between Del-1 and HSCs could enhance the mobilization of those progenitors into the bloodstream. This could be helpful for increasing donor cell numbers for transplantation.
HSCT recipients, on the other hand, may need enhanced Del-1 interaction to ensure the transplanted cells engraft and begin making new blood cells more rapidly.
In addition, people undergoing chemotherapy who develop febrile neutropenia might benefit from the role of Del-1 in supporting the production of immune-related blood cells such as neutrophils.
“It’s easy to think of practical applications for these findings,” Dr Hajishengallis said. “Now, we need to find out whether it works in practice, so our studies continue.” ![]()
Immigrant with stomach pain, distension, nausea, and fever • Dx?
THE CASE
A 34-year-old Eritrean man presented to the emergency department with complaints of diffuse abdominal pain and distention. He had emigrated to the United States 3 months earlier, following 5 years in a refugee camp in Ethiopia. Two weeks earlier, the patient sought care at his primary care clinic and was diagnosed with post-operative urinary retention and constipation following a recent hemorrhoidectomy. A Foley catheter was inserted and provided a short period of relief.
Following the visit, however, his abdominal pain worsened. He also experienced increasing abdominal distention, a declining appetite, and persistent nausea. The patient said that he was unable to urinate and had not had a bowel movement in 6 days. He also described fevers, drenching night sweats, chills, and a 4-kg weight loss over 2 months.
On physical examination, the patient had a wasted appearance. He was afebrile, alert, and oriented, but anxious and writhing in pain. An abdominal examination revealed some distention, generalized guarding, and tenderness. There was dullness to percussion in all regions without rebound, and no caput medusa was noted. The remainder of the physical examination was unremarkable.
Pertinent laboratory values included negative screens for human immunodeficiency virus (HIV) 1 and 2, and a purified protein derivative test that produced 10 mm of induration at 48 hours. An interferon-gamma release assay was not performed following these results. A computerized tomography (CT) scan of the abdomen and pelvis with intravenous and oral contrast revealed thickening of the peritoneal lining with infiltration of the mesenteric fat and large loculated fluid collections in the abdominal cavity (FIGURE). A CT scan of the patient’s lungs showed some mild atelectasis with left-sided effusion.
After hospital admission, the patient spiked fevers as high as 103.3° F and developed progressively worsening ascites. An ultrasound-guided paracentesis was performed, during which almost 2 liters of yellow, hazy fluid was removed. Fluid and blood cultures were negative.
THE DIAGNOSIS
With a high clinical suspicion for tuberculosis (TB) peritonitis, we requested a surgical consultation and a peritoneal biopsy was performed. The patient was started on ethambutol, isoniazid, pyrazinamide, pyridoxine, and rifampin while the biopsy results were pending.
Pathology subsequently confirmed a diagnosis of TB peritonitis, reporting dense fibroconnective tissue with areas of chronic inflammation and occasional accumulations of histiocytes with multinucleated giant cells showing granulomatous inflammation. An acid-fast (AF) bacilli stain for Mycobacteria showed a single curved bacillus compatible with Mycobacterium tuberculosis.
The patient was discharged following a 3-week hospital stay. At his follow-up visit several weeks later, the patient reported marked improvement and increasing exercise tolerance. He had gained weight, and the abdominal distention and tenderness had resolved.
DISCUSSION
Worldwide, TB is one of the top 10 causes of death. The World Health Organization estimates that there were 1.4 million TB deaths globally in 2015.1 And while rates of TB are decreasing in the United States, there was a resurgence from 1985 to 1992.2 This was attributable to the HIV/acquired immunodeficiency syndrome epidemic, increased immigration from countries endemic for TB, and deterioration of the TB public health infrastructure.3
Transmission. M tuberculosis is a rod-shaped, nonspore-forming AF bacillus that typically infects the lungs, but may infect other areas of the body. Transmission typically occurs via airborne spread of droplets from an infected individual. Possible other methods of disease dissemination include ingestion of infected sputum, hematogenous spread from active pulmonary TB, or ingestion of contaminated milk or food.
M tuberculosis elicits a proinflammatory phase, which facilitates the formation of a granuloma within the host tissues. The host’s immune response to M tuberculosis plays a role in the risk of developing this type of TB.3
TB presentation is classified as pulmonary, extrapulmonary, or both. Clinicians are generally attentive to the classic symptoms of pulmonary TB: cough, weight loss, night sweats, and fever. Presentation of extrapulmonary TB, however, may vary.4
According to one study, the most common presenting symptoms for peritoneal TB are weight loss, abdominal pain, and/or fever, all of which our patient experienced.5 In addition, our patient was an immigrant from Africa, and black patients have been shown to have a significantly higher incidence of extrapulmonary TB than their nonblack counterparts.6 Although our patient was HIV-negative, a recent meta-analysis confirmed the strong association between extrapulmonary TB and HIV, emphasizing the importance of including HIV screens in the standard work-up for TB.7
Other symptoms may include microcytosis, anemia, thrombocytosis, and an elevated erythrocyte sedimentation rate. Although a chest x-ray is often negative, advanced imaging, such as CT or magnetic resonance imaging, is often abnormal and may point to the diagnosis.5
Treatment of extrapulmonary TB is generally the same as that for pulmonary TB and, interestingly, the incidence of multi-drug resistant extrapulmonary TB is not necessarily higher than it is for pulmonary TB (<1% vs 1.6%).3,7 In light of this, a standard regimen—like the one our patient received—is generally utilized for 6 to 9 months. Nonetheless, resistance testing should still be performed.3,4
THE TAKEAWAY
While considered uncommon, more than 20% of TB cases in the United States are extrapulmonary (the most common form is TB lymphadenitis).7,8 It is imperative to identify appropriate risk factors, including associated comorbidities, patient characteristics, and population/endemic differences in immigrant populations.
In this case, although the symptom combination of persistent abdominal pain, fever, and weight loss may not trigger suspicion of a TB diagnosis in isolation, combining the symptoms with knowledge of the patient’s immigration status should at least raise an eyebrow. Given their nonpulmonary symptoms, many of these patients will not present to pulmonologists, making diagnosis particularly relevant to primary care.
1. World Health Organization. Global tuberculosis report 2016. Available at: http://www.who.int/tb/publications/global_report/gtbr2016_executive_summary.pdf?ua=1. Accessed August 22, 2017.
2. Peto HM, Pratt RH, Harrington TA, et al. Epidemiology of extrapulmonary tuberculosis in the United States, 1993-2006. Clin Infect Dis. 2009;49:1350-1357.
3. Centers for Disease Control and Prevention. Reported Tuberculosis in the United States, 2006. Available at: http://digitallibrary.utah.gov/awweb/awarchive?type=file&item=56908. Accessed August 3, 2017.
4. World Health Organization. Global tuberculosis report 2012. Available at: http://apps.who.int/medicinedocs/documents/s19908en/s19908en.pdf. Accessed July 27, 2017.
5. Ramesh J, Banait GS, Ormerod LP. Abdominal tuberculosis in a district general hospital: a retrospective review of 86 cases. QJM. 2008;101:189-195.
6. Fiske CT, Griffin MR, Erin H, et al. Black race, sex and extrapulmonary tuberculosis risk: an observational study. BMC Infect Dis. 2010;10:16.
7. Naing C, Mak JW, Maung M, et al. Meta-analysis: the association between HIV infection and extrapulmonary tuberculosis. Lung. 2013;191:27-34.
8. Neelakantan S, Nair PP, Emmanuel RV, et al. Diversities in presentations of extrapulmonary tuberculosis. BMJ Case Rep. 2013.
THE CASE
A 34-year-old Eritrean man presented to the emergency department with complaints of diffuse abdominal pain and distention. He had emigrated to the United States 3 months earlier, following 5 years in a refugee camp in Ethiopia. Two weeks earlier, the patient sought care at his primary care clinic and was diagnosed with post-operative urinary retention and constipation following a recent hemorrhoidectomy. A Foley catheter was inserted and provided a short period of relief.
Following the visit, however, his abdominal pain worsened. He also experienced increasing abdominal distention, a declining appetite, and persistent nausea. The patient said that he was unable to urinate and had not had a bowel movement in 6 days. He also described fevers, drenching night sweats, chills, and a 4-kg weight loss over 2 months.
On physical examination, the patient had a wasted appearance. He was afebrile, alert, and oriented, but anxious and writhing in pain. An abdominal examination revealed some distention, generalized guarding, and tenderness. There was dullness to percussion in all regions without rebound, and no caput medusa was noted. The remainder of the physical examination was unremarkable.
Pertinent laboratory values included negative screens for human immunodeficiency virus (HIV) 1 and 2, and a purified protein derivative test that produced 10 mm of induration at 48 hours. An interferon-gamma release assay was not performed following these results. A computerized tomography (CT) scan of the abdomen and pelvis with intravenous and oral contrast revealed thickening of the peritoneal lining with infiltration of the mesenteric fat and large loculated fluid collections in the abdominal cavity (FIGURE). A CT scan of the patient’s lungs showed some mild atelectasis with left-sided effusion.

After hospital admission, the patient spiked fevers as high as 103.3° F and developed progressively worsening ascites. An ultrasound-guided paracentesis was performed, during which almost 2 liters of yellow, hazy fluid was removed. Fluid and blood cultures were negative.
THE DIAGNOSIS
With a high clinical suspicion for tuberculosis (TB) peritonitis, we requested a surgical consultation and a peritoneal biopsy was performed. The patient was started on ethambutol, isoniazid, pyrazinamide, pyridoxine, and rifampin while the biopsy results were pending.
Pathology subsequently confirmed a diagnosis of TB peritonitis, reporting dense fibroconnective tissue with areas of chronic inflammation and occasional accumulations of histiocytes with multinucleated giant cells showing granulomatous inflammation. An acid-fast (AF) bacilli stain for Mycobacteria showed a single curved bacillus compatible with Mycobacterium tuberculosis.
The patient was discharged following a 3-week hospital stay. At his follow-up visit several weeks later, the patient reported marked improvement and increasing exercise tolerance. He had gained weight, and the abdominal distention and tenderness had resolved.
DISCUSSION
Worldwide, TB is one of the top 10 causes of death. The World Health Organization estimates that there were 1.4 million TB deaths globally in 2015.1 And while rates of TB are decreasing in the United States, there was a resurgence from 1985 to 1992.2 This was attributable to the HIV/acquired immunodeficiency syndrome epidemic, increased immigration from countries endemic for TB, and deterioration of the TB public health infrastructure.3
Transmission. M tuberculosis is a rod-shaped, nonspore-forming AF bacillus that typically infects the lungs, but may infect other areas of the body. Transmission typically occurs via airborne spread of droplets from an infected individual. Possible other methods of disease dissemination include ingestion of infected sputum, hematogenous spread from active pulmonary TB, or ingestion of contaminated milk or food.
M tuberculosis elicits a proinflammatory phase, which facilitates the formation of a granuloma within the host tissues. The host’s immune response to M tuberculosis plays a role in the risk of developing this type of TB.3
TB presentation is classified as pulmonary, extrapulmonary, or both. Clinicians are generally attentive to the classic symptoms of pulmonary TB: cough, weight loss, night sweats, and fever. Presentation of extrapulmonary TB, however, may vary.4
According to one study, the most common presenting symptoms for peritoneal TB are weight loss, abdominal pain, and/or fever, all of which our patient experienced.5 In addition, our patient was an immigrant from Africa, and black patients have been shown to have a significantly higher incidence of extrapulmonary TB than their nonblack counterparts.6 Although our patient was HIV-negative, a recent meta-analysis confirmed the strong association between extrapulmonary TB and HIV, emphasizing the importance of including HIV screens in the standard work-up for TB.7
Other symptoms may include microcytosis, anemia, thrombocytosis, and an elevated erythrocyte sedimentation rate. Although a chest x-ray is often negative, advanced imaging, such as CT or magnetic resonance imaging, is often abnormal and may point to the diagnosis.5
Treatment of extrapulmonary TB is generally the same as that for pulmonary TB and, interestingly, the incidence of multi-drug resistant extrapulmonary TB is not necessarily higher than it is for pulmonary TB (<1% vs 1.6%).3,7 In light of this, a standard regimen—like the one our patient received—is generally utilized for 6 to 9 months. Nonetheless, resistance testing should still be performed.3,4
THE TAKEAWAY
While considered uncommon, more than 20% of TB cases in the United States are extrapulmonary (the most common form is TB lymphadenitis).7,8 It is imperative to identify appropriate risk factors, including associated comorbidities, patient characteristics, and population/endemic differences in immigrant populations.
In this case, although the symptom combination of persistent abdominal pain, fever, and weight loss may not trigger suspicion of a TB diagnosis in isolation, combining the symptoms with knowledge of the patient’s immigration status should at least raise an eyebrow. Given their nonpulmonary symptoms, many of these patients will not present to pulmonologists, making diagnosis particularly relevant to primary care.
THE CASE
A 34-year-old Eritrean man presented to the emergency department with complaints of diffuse abdominal pain and distention. He had emigrated to the United States 3 months earlier, following 5 years in a refugee camp in Ethiopia. Two weeks earlier, the patient sought care at his primary care clinic and was diagnosed with post-operative urinary retention and constipation following a recent hemorrhoidectomy. A Foley catheter was inserted and provided a short period of relief.
Following the visit, however, his abdominal pain worsened. He also experienced increasing abdominal distention, a declining appetite, and persistent nausea. The patient said that he was unable to urinate and had not had a bowel movement in 6 days. He also described fevers, drenching night sweats, chills, and a 4-kg weight loss over 2 months.
On physical examination, the patient had a wasted appearance. He was afebrile, alert, and oriented, but anxious and writhing in pain. An abdominal examination revealed some distention, generalized guarding, and tenderness. There was dullness to percussion in all regions without rebound, and no caput medusa was noted. The remainder of the physical examination was unremarkable.
Pertinent laboratory values included negative screens for human immunodeficiency virus (HIV) 1 and 2, and a purified protein derivative test that produced 10 mm of induration at 48 hours. An interferon-gamma release assay was not performed following these results. A computerized tomography (CT) scan of the abdomen and pelvis with intravenous and oral contrast revealed thickening of the peritoneal lining with infiltration of the mesenteric fat and large loculated fluid collections in the abdominal cavity (FIGURE). A CT scan of the patient’s lungs showed some mild atelectasis with left-sided effusion.

After hospital admission, the patient spiked fevers as high as 103.3° F and developed progressively worsening ascites. An ultrasound-guided paracentesis was performed, during which almost 2 liters of yellow, hazy fluid was removed. Fluid and blood cultures were negative.
THE DIAGNOSIS
With a high clinical suspicion for tuberculosis (TB) peritonitis, we requested a surgical consultation and a peritoneal biopsy was performed. The patient was started on ethambutol, isoniazid, pyrazinamide, pyridoxine, and rifampin while the biopsy results were pending.
Pathology subsequently confirmed a diagnosis of TB peritonitis, reporting dense fibroconnective tissue with areas of chronic inflammation and occasional accumulations of histiocytes with multinucleated giant cells showing granulomatous inflammation. An acid-fast (AF) bacilli stain for Mycobacteria showed a single curved bacillus compatible with Mycobacterium tuberculosis.
The patient was discharged following a 3-week hospital stay. At his follow-up visit several weeks later, the patient reported marked improvement and increasing exercise tolerance. He had gained weight, and the abdominal distention and tenderness had resolved.
DISCUSSION
Worldwide, TB is one of the top 10 causes of death. The World Health Organization estimates that there were 1.4 million TB deaths globally in 2015.1 And while rates of TB are decreasing in the United States, there was a resurgence from 1985 to 1992.2 This was attributable to the HIV/acquired immunodeficiency syndrome epidemic, increased immigration from countries endemic for TB, and deterioration of the TB public health infrastructure.3
Transmission. M tuberculosis is a rod-shaped, nonspore-forming AF bacillus that typically infects the lungs, but may infect other areas of the body. Transmission typically occurs via airborne spread of droplets from an infected individual. Possible other methods of disease dissemination include ingestion of infected sputum, hematogenous spread from active pulmonary TB, or ingestion of contaminated milk or food.
M tuberculosis elicits a proinflammatory phase, which facilitates the formation of a granuloma within the host tissues. The host’s immune response to M tuberculosis plays a role in the risk of developing this type of TB.3
TB presentation is classified as pulmonary, extrapulmonary, or both. Clinicians are generally attentive to the classic symptoms of pulmonary TB: cough, weight loss, night sweats, and fever. Presentation of extrapulmonary TB, however, may vary.4
According to one study, the most common presenting symptoms for peritoneal TB are weight loss, abdominal pain, and/or fever, all of which our patient experienced.5 In addition, our patient was an immigrant from Africa, and black patients have been shown to have a significantly higher incidence of extrapulmonary TB than their nonblack counterparts.6 Although our patient was HIV-negative, a recent meta-analysis confirmed the strong association between extrapulmonary TB and HIV, emphasizing the importance of including HIV screens in the standard work-up for TB.7
Other symptoms may include microcytosis, anemia, thrombocytosis, and an elevated erythrocyte sedimentation rate. Although a chest x-ray is often negative, advanced imaging, such as CT or magnetic resonance imaging, is often abnormal and may point to the diagnosis.5
Treatment of extrapulmonary TB is generally the same as that for pulmonary TB and, interestingly, the incidence of multi-drug resistant extrapulmonary TB is not necessarily higher than it is for pulmonary TB (<1% vs 1.6%).3,7 In light of this, a standard regimen—like the one our patient received—is generally utilized for 6 to 9 months. Nonetheless, resistance testing should still be performed.3,4
THE TAKEAWAY
While considered uncommon, more than 20% of TB cases in the United States are extrapulmonary (the most common form is TB lymphadenitis).7,8 It is imperative to identify appropriate risk factors, including associated comorbidities, patient characteristics, and population/endemic differences in immigrant populations.
In this case, although the symptom combination of persistent abdominal pain, fever, and weight loss may not trigger suspicion of a TB diagnosis in isolation, combining the symptoms with knowledge of the patient’s immigration status should at least raise an eyebrow. Given their nonpulmonary symptoms, many of these patients will not present to pulmonologists, making diagnosis particularly relevant to primary care.
1. World Health Organization. Global tuberculosis report 2016. Available at: http://www.who.int/tb/publications/global_report/gtbr2016_executive_summary.pdf?ua=1. Accessed August 22, 2017.
2. Peto HM, Pratt RH, Harrington TA, et al. Epidemiology of extrapulmonary tuberculosis in the United States, 1993-2006. Clin Infect Dis. 2009;49:1350-1357.
3. Centers for Disease Control and Prevention. Reported Tuberculosis in the United States, 2006. Available at: http://digitallibrary.utah.gov/awweb/awarchive?type=file&item=56908. Accessed August 3, 2017.
4. World Health Organization. Global tuberculosis report 2012. Available at: http://apps.who.int/medicinedocs/documents/s19908en/s19908en.pdf. Accessed July 27, 2017.
5. Ramesh J, Banait GS, Ormerod LP. Abdominal tuberculosis in a district general hospital: a retrospective review of 86 cases. QJM. 2008;101:189-195.
6. Fiske CT, Griffin MR, Erin H, et al. Black race, sex and extrapulmonary tuberculosis risk: an observational study. BMC Infect Dis. 2010;10:16.
7. Naing C, Mak JW, Maung M, et al. Meta-analysis: the association between HIV infection and extrapulmonary tuberculosis. Lung. 2013;191:27-34.
8. Neelakantan S, Nair PP, Emmanuel RV, et al. Diversities in presentations of extrapulmonary tuberculosis. BMJ Case Rep. 2013.
1. World Health Organization. Global tuberculosis report 2016. Available at: http://www.who.int/tb/publications/global_report/gtbr2016_executive_summary.pdf?ua=1. Accessed August 22, 2017.
2. Peto HM, Pratt RH, Harrington TA, et al. Epidemiology of extrapulmonary tuberculosis in the United States, 1993-2006. Clin Infect Dis. 2009;49:1350-1357.
3. Centers for Disease Control and Prevention. Reported Tuberculosis in the United States, 2006. Available at: http://digitallibrary.utah.gov/awweb/awarchive?type=file&item=56908. Accessed August 3, 2017.
4. World Health Organization. Global tuberculosis report 2012. Available at: http://apps.who.int/medicinedocs/documents/s19908en/s19908en.pdf. Accessed July 27, 2017.
5. Ramesh J, Banait GS, Ormerod LP. Abdominal tuberculosis in a district general hospital: a retrospective review of 86 cases. QJM. 2008;101:189-195.
6. Fiske CT, Griffin MR, Erin H, et al. Black race, sex and extrapulmonary tuberculosis risk: an observational study. BMC Infect Dis. 2010;10:16.
7. Naing C, Mak JW, Maung M, et al. Meta-analysis: the association between HIV infection and extrapulmonary tuberculosis. Lung. 2013;191:27-34.
8. Neelakantan S, Nair PP, Emmanuel RV, et al. Diversities in presentations of extrapulmonary tuberculosis. BMJ Case Rep. 2013.
Bruises on the ears and body
Over the course of a month, this 34-year-old woman had sought care at our facility—and another—on 3 separate occasions for painful bruises (visits #1 and #3) and deep vein thrombosis (DVT; visit #2). The bruises first appeared acutely on her arms (FIGURE 1A), prompting her first visit to our ED and leading to a hospital stay. Several weeks later, the patient developed new bruise-like lesions on her earlobes (FIGURE 1B), face, trunk, and lower extremities. In between these 2 visits, the patient was seen in another ED (and admitted) for right upper extremity DVT and was started on enoxaparin, followed by warfarin.
The patient had no history of trauma, but did have a 7-year history of cocaine abuse. The initial bruises appeared one week after using cocaine from a different dealer.
On her most recent visit, her vitals and physical examination were unremarkable, apart from the skin findings. Her complete blood count, complete metabolic panel, and urinalysis were unremarkable. On her previous admissions, the patient’s urine drug test had been positive for cocaine. She’d also tested positive for cytoplasmic antineutrophil cytoplasmic antibodies (c-ANCA), antinuclear antibodies (ANA), anti-double stranded DNA (anti-dsDNA), and anticardiolipin IgM.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Levamisole-induced cutaneous vasculopathy
The patient was given a diagnosis of levamisole-induced cutaneous vasculopathy based on her history of cocaine use; the typical, painful palpable purpura with angulated borders and a necrotic center (retiform purpura); and positive immunologic markers.1-5 DVT has also been reported in association with levamisole-induced vasculopathy.6
Intended for livestock. Levamisole is a pharmaceutical agent typically used as an anthelmintic in livestock, but, since 2007, it has increasingly been found as an adulterant in cocaine.7 According to the Drug Enforcement Administration, 71% of cocaine tested in 2009 contained levamisole.7
Experts speculate that levamisole is used in the production process of cocaine to increase its volume and enhance its psychoactive effects.1-4 In humans, levamisole’s immunomodulatory properties were once used to treat various cancers and immunologic conditions, but it was withdrawn from the US market in 2000 due to adverse effects.1,2,4 Severe adverse reactions associated with levamisole include agranulocytosis, vascular occlusive disease, and thrombotic vasculopathy with or without vasculitis.1,2
The incidence of levamisole-induced cutaneous vasculopathy is unknown. That said, it’s important to suspect the condition in cocaine users who present with retiform purpura. Earlobe lesions are characteristic, while involvement of other areas is variable.2-4
Differential includes other causes of purpura
A number of conditions make up the differential. While each of these presents with areas of skin necrosis or purpura, a thorough history can be revealing. Factors such as substance abuse, recent treatment with a vitamin K antagonist, or even a recent infection can be the key to making the diagnosis.
Warfarin-induced skin necrosis presents with large, irregular bullae that eventually become necrotic. It appears one to 10 days after treatment with a vitamin K antagonist and typically affects areas of the body with greater subcutaneous adipose tissue, including the breasts, thighs, buttocks, and penis. Microscopic examination reveals bland thrombi with no inflammation of the vessel wall.5
ANCA-associated small-vessel vasculitis includes microscopic polyangiitis, Wegner’s granulomatosis, and Churg-Strauss syndrome. With these conditions, palpable purpuras are more commonly seen in areas that are dependent on, or affected by, venous stasis. Microscopic evaluation reveals leukocytoclastic vasculitis. Perinuclear ANCA is more often positive than c-ANCA.5
Purpura fulminans is a medical emergency characterized by skin necrosis and disseminated intravascular coagulation. It can rapidly lead to multi-organ failure. Microscopic evaluation reveals thrombotic occlusion of small- and medium-sized vessels. It affects neonates and children, and is associated with severe sepsis or an autoimmune response to an otherwise benign childhood infection. It may also be a symptom of hereditary protein C or protein S deficiency.8
Cholesterol emboli are more common in men ages 50 and older with atherosclerotic disease, hypertension, or tobacco use. Abrupt onset of livedo reticularis may be followed by retiform purpura, ulcers, nodules, and gangrene. Lesions most often appear on the distal lower extremities and buttocks. Systemic symptoms may include fever, weight loss, myalgia, and altered mental status. Multiple organ systems may be involved. Frozen sections reveal needle-shaped clefts and doubly-refractile crystals.5
Evolving skin lesions, relevant lab findings
Initially, patients with levamisole-induced cutaneous vasculopathy will develop painful, palpable purpura with angulated borders and a necrotic center. The lesions can progress, though, to bullae, necrosis, or eschar formation.1,3,4
Patients with this condition frequently test positive for ANCA and, even more frequently, for c-ANCA, ANA, antiphospholipid antibodies, leukopenia, and neutropenia. Other immunologic markers for which patients may test positive include anti-cardiolipin antibodies, lupus anticoagulant, anti-dsDNA, myeloperoxidase, and anti-Sjögren’s-syndrome-related antigen A (also known as anti-Ro) antibodies.1-4
Natural progression of levamisole-induced cutaneous vasculopathy is generally benign. Most clinical signs and symptoms—as well as serologic manifestations—resolve without intervention after cessation of cocaine use. However, signs and symptoms may recur with subsequent exposure.
There is no specific therapy for levamisole-induced cutaneous vasculopathy, but prednisone and other immunosuppressive agents can be used in patients with severe or systemic symptoms.1-4 Necrotic lesions and eschar formation may be complicated by infection and require debridement and/or skin grafts.
Our patient was discharged after a brief hospital stay, as she had no indication of systemic involvement and no new or worsening skin lesions. She was given wound care instructions and advised to stop using cocaine. The patient was counseled at bedside by a physician and given information on community resources (outpatient treatment, support groups, etc) by social services. However, the patient continued to use the substance and had several readmissions with worsening skin lesions complicated by secondary bacterial infection. She did not have systemic complications, but required antibiotics, multiple wound debridement sessions, and subsequent skin grafts.
CORRESPONDENCE
Yu Wah, MD, ABIHM, University of Texas Health Science Center at Houston, 6431 Fannin Street, Suite JJL 308, Houston, TX 77030; [email protected].
1. Gaertner EM, Switlyk SA. Dermatologic complications from levamisole-contaminated cocaine: a case report and review of the literature. Cutis. 2014;93:102-106.
2. Strazzula L, Brown KK, Brieva JC, et al. Levamisole toxicity mimicking autoimmune disease. J Am Acad Dermatol. 2013;69:954-959.
3. Espinoza LR, Perez Alamino R. Cocaine-induced vasculitis: clinical and immunological spectrum. Curr Rheumatol Rep. 2012;14:532-538.
4. Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia–a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol. 2011;65:722-725.
5. Wysong A, Venkatesan P. An approach to the patient with retiform purpura. Dermatol Ther. 2011;24:151-172.
6. Wilson L, Hull C, Petersen M, et al. End organ damage in levamisole adulterated cocaine: More than just purpura and agranulocytosis. J Am Acad Dermatol. 2013;68:AB9.
7. US Department of Justice. National Drug Threat Assessment 2010. Impact of drugs on society. Available at: http://www.justice.gov/archive/ndic/pubs38/38661/drugImpact.htm. Accessed July 26, 2017.
8. Chalmers E, Cooper P, Forman K, et al. Purpura fulminans: recognition, diagnosis and management. Arch Dis Child. 2011;96:1066-1071.
Over the course of a month, this 34-year-old woman had sought care at our facility—and another—on 3 separate occasions for painful bruises (visits #1 and #3) and deep vein thrombosis (DVT; visit #2). The bruises first appeared acutely on her arms (FIGURE 1A), prompting her first visit to our ED and leading to a hospital stay. Several weeks later, the patient developed new bruise-like lesions on her earlobes (FIGURE 1B), face, trunk, and lower extremities. In between these 2 visits, the patient was seen in another ED (and admitted) for right upper extremity DVT and was started on enoxaparin, followed by warfarin.
The patient had no history of trauma, but did have a 7-year history of cocaine abuse. The initial bruises appeared one week after using cocaine from a different dealer.
On her most recent visit, her vitals and physical examination were unremarkable, apart from the skin findings. Her complete blood count, complete metabolic panel, and urinalysis were unremarkable. On her previous admissions, the patient’s urine drug test had been positive for cocaine. She’d also tested positive for cytoplasmic antineutrophil cytoplasmic antibodies (c-ANCA), antinuclear antibodies (ANA), anti-double stranded DNA (anti-dsDNA), and anticardiolipin IgM.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Levamisole-induced cutaneous vasculopathy
The patient was given a diagnosis of levamisole-induced cutaneous vasculopathy based on her history of cocaine use; the typical, painful palpable purpura with angulated borders and a necrotic center (retiform purpura); and positive immunologic markers.1-5 DVT has also been reported in association with levamisole-induced vasculopathy.6
Intended for livestock. Levamisole is a pharmaceutical agent typically used as an anthelmintic in livestock, but, since 2007, it has increasingly been found as an adulterant in cocaine.7 According to the Drug Enforcement Administration, 71% of cocaine tested in 2009 contained levamisole.7
Experts speculate that levamisole is used in the production process of cocaine to increase its volume and enhance its psychoactive effects.1-4 In humans, levamisole’s immunomodulatory properties were once used to treat various cancers and immunologic conditions, but it was withdrawn from the US market in 2000 due to adverse effects.1,2,4 Severe adverse reactions associated with levamisole include agranulocytosis, vascular occlusive disease, and thrombotic vasculopathy with or without vasculitis.1,2
The incidence of levamisole-induced cutaneous vasculopathy is unknown. That said, it’s important to suspect the condition in cocaine users who present with retiform purpura. Earlobe lesions are characteristic, while involvement of other areas is variable.2-4
Differential includes other causes of purpura
A number of conditions make up the differential. While each of these presents with areas of skin necrosis or purpura, a thorough history can be revealing. Factors such as substance abuse, recent treatment with a vitamin K antagonist, or even a recent infection can be the key to making the diagnosis.
Warfarin-induced skin necrosis presents with large, irregular bullae that eventually become necrotic. It appears one to 10 days after treatment with a vitamin K antagonist and typically affects areas of the body with greater subcutaneous adipose tissue, including the breasts, thighs, buttocks, and penis. Microscopic examination reveals bland thrombi with no inflammation of the vessel wall.5
ANCA-associated small-vessel vasculitis includes microscopic polyangiitis, Wegner’s granulomatosis, and Churg-Strauss syndrome. With these conditions, palpable purpuras are more commonly seen in areas that are dependent on, or affected by, venous stasis. Microscopic evaluation reveals leukocytoclastic vasculitis. Perinuclear ANCA is more often positive than c-ANCA.5
Purpura fulminans is a medical emergency characterized by skin necrosis and disseminated intravascular coagulation. It can rapidly lead to multi-organ failure. Microscopic evaluation reveals thrombotic occlusion of small- and medium-sized vessels. It affects neonates and children, and is associated with severe sepsis or an autoimmune response to an otherwise benign childhood infection. It may also be a symptom of hereditary protein C or protein S deficiency.8
Cholesterol emboli are more common in men ages 50 and older with atherosclerotic disease, hypertension, or tobacco use. Abrupt onset of livedo reticularis may be followed by retiform purpura, ulcers, nodules, and gangrene. Lesions most often appear on the distal lower extremities and buttocks. Systemic symptoms may include fever, weight loss, myalgia, and altered mental status. Multiple organ systems may be involved. Frozen sections reveal needle-shaped clefts and doubly-refractile crystals.5
Evolving skin lesions, relevant lab findings
Initially, patients with levamisole-induced cutaneous vasculopathy will develop painful, palpable purpura with angulated borders and a necrotic center. The lesions can progress, though, to bullae, necrosis, or eschar formation.1,3,4
Patients with this condition frequently test positive for ANCA and, even more frequently, for c-ANCA, ANA, antiphospholipid antibodies, leukopenia, and neutropenia. Other immunologic markers for which patients may test positive include anti-cardiolipin antibodies, lupus anticoagulant, anti-dsDNA, myeloperoxidase, and anti-Sjögren’s-syndrome-related antigen A (also known as anti-Ro) antibodies.1-4
Natural progression of levamisole-induced cutaneous vasculopathy is generally benign. Most clinical signs and symptoms—as well as serologic manifestations—resolve without intervention after cessation of cocaine use. However, signs and symptoms may recur with subsequent exposure.
There is no specific therapy for levamisole-induced cutaneous vasculopathy, but prednisone and other immunosuppressive agents can be used in patients with severe or systemic symptoms.1-4 Necrotic lesions and eschar formation may be complicated by infection and require debridement and/or skin grafts.
Our patient was discharged after a brief hospital stay, as she had no indication of systemic involvement and no new or worsening skin lesions. She was given wound care instructions and advised to stop using cocaine. The patient was counseled at bedside by a physician and given information on community resources (outpatient treatment, support groups, etc) by social services. However, the patient continued to use the substance and had several readmissions with worsening skin lesions complicated by secondary bacterial infection. She did not have systemic complications, but required antibiotics, multiple wound debridement sessions, and subsequent skin grafts.
CORRESPONDENCE
Yu Wah, MD, ABIHM, University of Texas Health Science Center at Houston, 6431 Fannin Street, Suite JJL 308, Houston, TX 77030; [email protected].
Over the course of a month, this 34-year-old woman had sought care at our facility—and another—on 3 separate occasions for painful bruises (visits #1 and #3) and deep vein thrombosis (DVT; visit #2). The bruises first appeared acutely on her arms (FIGURE 1A), prompting her first visit to our ED and leading to a hospital stay. Several weeks later, the patient developed new bruise-like lesions on her earlobes (FIGURE 1B), face, trunk, and lower extremities. In between these 2 visits, the patient was seen in another ED (and admitted) for right upper extremity DVT and was started on enoxaparin, followed by warfarin.
The patient had no history of trauma, but did have a 7-year history of cocaine abuse. The initial bruises appeared one week after using cocaine from a different dealer.
On her most recent visit, her vitals and physical examination were unremarkable, apart from the skin findings. Her complete blood count, complete metabolic panel, and urinalysis were unremarkable. On her previous admissions, the patient’s urine drug test had been positive for cocaine. She’d also tested positive for cytoplasmic antineutrophil cytoplasmic antibodies (c-ANCA), antinuclear antibodies (ANA), anti-double stranded DNA (anti-dsDNA), and anticardiolipin IgM.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Levamisole-induced cutaneous vasculopathy
The patient was given a diagnosis of levamisole-induced cutaneous vasculopathy based on her history of cocaine use; the typical, painful palpable purpura with angulated borders and a necrotic center (retiform purpura); and positive immunologic markers.1-5 DVT has also been reported in association with levamisole-induced vasculopathy.6
Intended for livestock. Levamisole is a pharmaceutical agent typically used as an anthelmintic in livestock, but, since 2007, it has increasingly been found as an adulterant in cocaine.7 According to the Drug Enforcement Administration, 71% of cocaine tested in 2009 contained levamisole.7
Experts speculate that levamisole is used in the production process of cocaine to increase its volume and enhance its psychoactive effects.1-4 In humans, levamisole’s immunomodulatory properties were once used to treat various cancers and immunologic conditions, but it was withdrawn from the US market in 2000 due to adverse effects.1,2,4 Severe adverse reactions associated with levamisole include agranulocytosis, vascular occlusive disease, and thrombotic vasculopathy with or without vasculitis.1,2
The incidence of levamisole-induced cutaneous vasculopathy is unknown. That said, it’s important to suspect the condition in cocaine users who present with retiform purpura. Earlobe lesions are characteristic, while involvement of other areas is variable.2-4
Differential includes other causes of purpura
A number of conditions make up the differential. While each of these presents with areas of skin necrosis or purpura, a thorough history can be revealing. Factors such as substance abuse, recent treatment with a vitamin K antagonist, or even a recent infection can be the key to making the diagnosis.
Warfarin-induced skin necrosis presents with large, irregular bullae that eventually become necrotic. It appears one to 10 days after treatment with a vitamin K antagonist and typically affects areas of the body with greater subcutaneous adipose tissue, including the breasts, thighs, buttocks, and penis. Microscopic examination reveals bland thrombi with no inflammation of the vessel wall.5
ANCA-associated small-vessel vasculitis includes microscopic polyangiitis, Wegner’s granulomatosis, and Churg-Strauss syndrome. With these conditions, palpable purpuras are more commonly seen in areas that are dependent on, or affected by, venous stasis. Microscopic evaluation reveals leukocytoclastic vasculitis. Perinuclear ANCA is more often positive than c-ANCA.5
Purpura fulminans is a medical emergency characterized by skin necrosis and disseminated intravascular coagulation. It can rapidly lead to multi-organ failure. Microscopic evaluation reveals thrombotic occlusion of small- and medium-sized vessels. It affects neonates and children, and is associated with severe sepsis or an autoimmune response to an otherwise benign childhood infection. It may also be a symptom of hereditary protein C or protein S deficiency.8
Cholesterol emboli are more common in men ages 50 and older with atherosclerotic disease, hypertension, or tobacco use. Abrupt onset of livedo reticularis may be followed by retiform purpura, ulcers, nodules, and gangrene. Lesions most often appear on the distal lower extremities and buttocks. Systemic symptoms may include fever, weight loss, myalgia, and altered mental status. Multiple organ systems may be involved. Frozen sections reveal needle-shaped clefts and doubly-refractile crystals.5
Evolving skin lesions, relevant lab findings
Initially, patients with levamisole-induced cutaneous vasculopathy will develop painful, palpable purpura with angulated borders and a necrotic center. The lesions can progress, though, to bullae, necrosis, or eschar formation.1,3,4
Patients with this condition frequently test positive for ANCA and, even more frequently, for c-ANCA, ANA, antiphospholipid antibodies, leukopenia, and neutropenia. Other immunologic markers for which patients may test positive include anti-cardiolipin antibodies, lupus anticoagulant, anti-dsDNA, myeloperoxidase, and anti-Sjögren’s-syndrome-related antigen A (also known as anti-Ro) antibodies.1-4
Natural progression of levamisole-induced cutaneous vasculopathy is generally benign. Most clinical signs and symptoms—as well as serologic manifestations—resolve without intervention after cessation of cocaine use. However, signs and symptoms may recur with subsequent exposure.
There is no specific therapy for levamisole-induced cutaneous vasculopathy, but prednisone and other immunosuppressive agents can be used in patients with severe or systemic symptoms.1-4 Necrotic lesions and eschar formation may be complicated by infection and require debridement and/or skin grafts.
Our patient was discharged after a brief hospital stay, as she had no indication of systemic involvement and no new or worsening skin lesions. She was given wound care instructions and advised to stop using cocaine. The patient was counseled at bedside by a physician and given information on community resources (outpatient treatment, support groups, etc) by social services. However, the patient continued to use the substance and had several readmissions with worsening skin lesions complicated by secondary bacterial infection. She did not have systemic complications, but required antibiotics, multiple wound debridement sessions, and subsequent skin grafts.
CORRESPONDENCE
Yu Wah, MD, ABIHM, University of Texas Health Science Center at Houston, 6431 Fannin Street, Suite JJL 308, Houston, TX 77030; [email protected].
1. Gaertner EM, Switlyk SA. Dermatologic complications from levamisole-contaminated cocaine: a case report and review of the literature. Cutis. 2014;93:102-106.
2. Strazzula L, Brown KK, Brieva JC, et al. Levamisole toxicity mimicking autoimmune disease. J Am Acad Dermatol. 2013;69:954-959.
3. Espinoza LR, Perez Alamino R. Cocaine-induced vasculitis: clinical and immunological spectrum. Curr Rheumatol Rep. 2012;14:532-538.
4. Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia–a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol. 2011;65:722-725.
5. Wysong A, Venkatesan P. An approach to the patient with retiform purpura. Dermatol Ther. 2011;24:151-172.
6. Wilson L, Hull C, Petersen M, et al. End organ damage in levamisole adulterated cocaine: More than just purpura and agranulocytosis. J Am Acad Dermatol. 2013;68:AB9.
7. US Department of Justice. National Drug Threat Assessment 2010. Impact of drugs on society. Available at: http://www.justice.gov/archive/ndic/pubs38/38661/drugImpact.htm. Accessed July 26, 2017.
8. Chalmers E, Cooper P, Forman K, et al. Purpura fulminans: recognition, diagnosis and management. Arch Dis Child. 2011;96:1066-1071.
1. Gaertner EM, Switlyk SA. Dermatologic complications from levamisole-contaminated cocaine: a case report and review of the literature. Cutis. 2014;93:102-106.
2. Strazzula L, Brown KK, Brieva JC, et al. Levamisole toxicity mimicking autoimmune disease. J Am Acad Dermatol. 2013;69:954-959.
3. Espinoza LR, Perez Alamino R. Cocaine-induced vasculitis: clinical and immunological spectrum. Curr Rheumatol Rep. 2012;14:532-538.
4. Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia–a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol. 2011;65:722-725.
5. Wysong A, Venkatesan P. An approach to the patient with retiform purpura. Dermatol Ther. 2011;24:151-172.
6. Wilson L, Hull C, Petersen M, et al. End organ damage in levamisole adulterated cocaine: More than just purpura and agranulocytosis. J Am Acad Dermatol. 2013;68:AB9.
7. US Department of Justice. National Drug Threat Assessment 2010. Impact of drugs on society. Available at: http://www.justice.gov/archive/ndic/pubs38/38661/drugImpact.htm. Accessed July 26, 2017.
8. Chalmers E, Cooper P, Forman K, et al. Purpura fulminans: recognition, diagnosis and management. Arch Dis Child. 2011;96:1066-1071.
Skip the antidepressant when the patient has chronic disease?
It makes sense to think that treating patients who have congestive heart failure (CHF) and depression with an antidepressant would be effective. But common sense is not always supported by empiric observation or evidence.
In this month’s PURL, the authors summarize the MOOD-HF study,1 a randomized controlled trial (RCT) of escitalopram for the treatment of patients with CHF and depression. After 2 years, no outcomes—including depression scores—were better in the treatment vs the placebo group. One can only speculate as to why this antidepressant was not effective in this population. Clearly, this group differs somehow from subjects enrolled in traditional depression trials; notably, their depression was diagnosed after the onset of CHF, suggesting the depression was a reaction to their illness.
Not the first time. This is the second large trial to find no benefit to using a selective serotonin reuptake inhibitor (SSRI) to treat depression in patients with CHF; the previous trial to do so looked at sertraline.2 In fact, when it comes to patients with chronic diseases, such as diabetes and coronary artery disease, there is scant evidence to support the common belief that screening them for depression and treating them with SSRIs improves patient outcomes.3 On the other hand, there are no definitive clinical trials investigating other antidepressants in the treatment of depressed patients with chronic illness, so it is possible that other drugs could be effective. There is evidence, however, from a recent RCT that cognitive behavioral therapy—compared with usual care—improves depression, anxiety, fatigue, and social functioning in patients with CHF.4
Where does that leave us? In our practice, we annually screen all adults, including those with chronic illness, for depression with the 2-question Patient Health Questionnaire. As a matter of course, we should acknowledge and explore all patients’ depressed mood, offer emotional support, and refer for psychotherapy when appropriate. And since collaborative care has been shown to improve outcomes in patients with depression and, for that matter, diabetes (see this month’s audiocast), consider this model of care if it is available.5
I believe it’s worthwhile to discuss the use of antidepressants with patients who have CHF. It’s reasonable to be optimistic with them and to expect that their depression will improve with time, as noted in the placebo groups of the randomized trials mentioned above.1,2 And giving patients hope is always good medicine.
1. Angermann CE, Gelbrich G, Störk S, et al, for the MOOD-HF Study Investigators and Committee Members. Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression. The MOOD-HF randomized clinical trial. JAMA. 2016;315:2683-2693.
2. O’Connor CM, Jiang W, Kuchibhatla M, et al, SADHART-CHF Investigators. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56:692-699.
3. Health Quality Ontario. Screening and management of depression for adults with chronic diseases: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13:1-45.
4. Freedland KE, Carney RM, Rich MW, et al. Cognitive behavior therapy for depression and self-care in heart failure patients: a randomized clinical trial. JAMA Intern Med. 2015;175:1773-1782.
5. Huang Y, Wei X, Wu T, et al. Collaborative care for patients with depression and diabetes mellitus: a systematic review and meta-analysis. BMC Psychiatry. 2013;13:260.
It makes sense to think that treating patients who have congestive heart failure (CHF) and depression with an antidepressant would be effective. But common sense is not always supported by empiric observation or evidence.
In this month’s PURL, the authors summarize the MOOD-HF study,1 a randomized controlled trial (RCT) of escitalopram for the treatment of patients with CHF and depression. After 2 years, no outcomes—including depression scores—were better in the treatment vs the placebo group. One can only speculate as to why this antidepressant was not effective in this population. Clearly, this group differs somehow from subjects enrolled in traditional depression trials; notably, their depression was diagnosed after the onset of CHF, suggesting the depression was a reaction to their illness.
Not the first time. This is the second large trial to find no benefit to using a selective serotonin reuptake inhibitor (SSRI) to treat depression in patients with CHF; the previous trial to do so looked at sertraline.2 In fact, when it comes to patients with chronic diseases, such as diabetes and coronary artery disease, there is scant evidence to support the common belief that screening them for depression and treating them with SSRIs improves patient outcomes.3 On the other hand, there are no definitive clinical trials investigating other antidepressants in the treatment of depressed patients with chronic illness, so it is possible that other drugs could be effective. There is evidence, however, from a recent RCT that cognitive behavioral therapy—compared with usual care—improves depression, anxiety, fatigue, and social functioning in patients with CHF.4
Where does that leave us? In our practice, we annually screen all adults, including those with chronic illness, for depression with the 2-question Patient Health Questionnaire. As a matter of course, we should acknowledge and explore all patients’ depressed mood, offer emotional support, and refer for psychotherapy when appropriate. And since collaborative care has been shown to improve outcomes in patients with depression and, for that matter, diabetes (see this month’s audiocast), consider this model of care if it is available.5
I believe it’s worthwhile to discuss the use of antidepressants with patients who have CHF. It’s reasonable to be optimistic with them and to expect that their depression will improve with time, as noted in the placebo groups of the randomized trials mentioned above.1,2 And giving patients hope is always good medicine.
It makes sense to think that treating patients who have congestive heart failure (CHF) and depression with an antidepressant would be effective. But common sense is not always supported by empiric observation or evidence.
In this month’s PURL, the authors summarize the MOOD-HF study,1 a randomized controlled trial (RCT) of escitalopram for the treatment of patients with CHF and depression. After 2 years, no outcomes—including depression scores—were better in the treatment vs the placebo group. One can only speculate as to why this antidepressant was not effective in this population. Clearly, this group differs somehow from subjects enrolled in traditional depression trials; notably, their depression was diagnosed after the onset of CHF, suggesting the depression was a reaction to their illness.
Not the first time. This is the second large trial to find no benefit to using a selective serotonin reuptake inhibitor (SSRI) to treat depression in patients with CHF; the previous trial to do so looked at sertraline.2 In fact, when it comes to patients with chronic diseases, such as diabetes and coronary artery disease, there is scant evidence to support the common belief that screening them for depression and treating them with SSRIs improves patient outcomes.3 On the other hand, there are no definitive clinical trials investigating other antidepressants in the treatment of depressed patients with chronic illness, so it is possible that other drugs could be effective. There is evidence, however, from a recent RCT that cognitive behavioral therapy—compared with usual care—improves depression, anxiety, fatigue, and social functioning in patients with CHF.4
Where does that leave us? In our practice, we annually screen all adults, including those with chronic illness, for depression with the 2-question Patient Health Questionnaire. As a matter of course, we should acknowledge and explore all patients’ depressed mood, offer emotional support, and refer for psychotherapy when appropriate. And since collaborative care has been shown to improve outcomes in patients with depression and, for that matter, diabetes (see this month’s audiocast), consider this model of care if it is available.5
I believe it’s worthwhile to discuss the use of antidepressants with patients who have CHF. It’s reasonable to be optimistic with them and to expect that their depression will improve with time, as noted in the placebo groups of the randomized trials mentioned above.1,2 And giving patients hope is always good medicine.
1. Angermann CE, Gelbrich G, Störk S, et al, for the MOOD-HF Study Investigators and Committee Members. Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression. The MOOD-HF randomized clinical trial. JAMA. 2016;315:2683-2693.
2. O’Connor CM, Jiang W, Kuchibhatla M, et al, SADHART-CHF Investigators. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56:692-699.
3. Health Quality Ontario. Screening and management of depression for adults with chronic diseases: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13:1-45.
4. Freedland KE, Carney RM, Rich MW, et al. Cognitive behavior therapy for depression and self-care in heart failure patients: a randomized clinical trial. JAMA Intern Med. 2015;175:1773-1782.
5. Huang Y, Wei X, Wu T, et al. Collaborative care for patients with depression and diabetes mellitus: a systematic review and meta-analysis. BMC Psychiatry. 2013;13:260.
1. Angermann CE, Gelbrich G, Störk S, et al, for the MOOD-HF Study Investigators and Committee Members. Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression. The MOOD-HF randomized clinical trial. JAMA. 2016;315:2683-2693.
2. O’Connor CM, Jiang W, Kuchibhatla M, et al, SADHART-CHF Investigators. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56:692-699.
3. Health Quality Ontario. Screening and management of depression for adults with chronic diseases: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13:1-45.
4. Freedland KE, Carney RM, Rich MW, et al. Cognitive behavior therapy for depression and self-care in heart failure patients: a randomized clinical trial. JAMA Intern Med. 2015;175:1773-1782.
5. Huang Y, Wei X, Wu T, et al. Collaborative care for patients with depression and diabetes mellitus: a systematic review and meta-analysis. BMC Psychiatry. 2013;13:260.
Periorbital ecchymoses and breathlessness
A 54-year-old man presented at our facility with a 3-month history of exertional breathlessness and purple blotches around his eyes. Examination revealed bilateral periorbital and perioral ecchymosis, purpuric spots along his waist, and waxy papules on his eyelids (FIGURE 1). In addition, the patient had macroglossia with nodular infiltration and irregular indentations at the lateral margin of his tongue (FIGURE 2).
The patient also had a raised jugular venous pressure and prominent atrial and ventricular waves. Further examination revealed a fourth heart sound over the left ventricular apex, as well as bilateral basal rales. All other systems were normal except for mild hepatomegaly.
Routine hematologic and biochemical lab work was unremarkable. X-rays of the spine and skull were normal, but a chest x-ray showed mild cardiomegaly. An electrocardiogram (EKG) showed a QS complex from leads V1 to V4 (a pseudo-infarction pattern; FIGURE 3A). An echocardiogram showed biatrial enlargement, left ventricular hypertrophy with a left ventricular ejection fraction of 48%, a speckled pattern on the myocardium, a thickened interatrial septum, and mild pericardial effusion (FIGURE 3B).
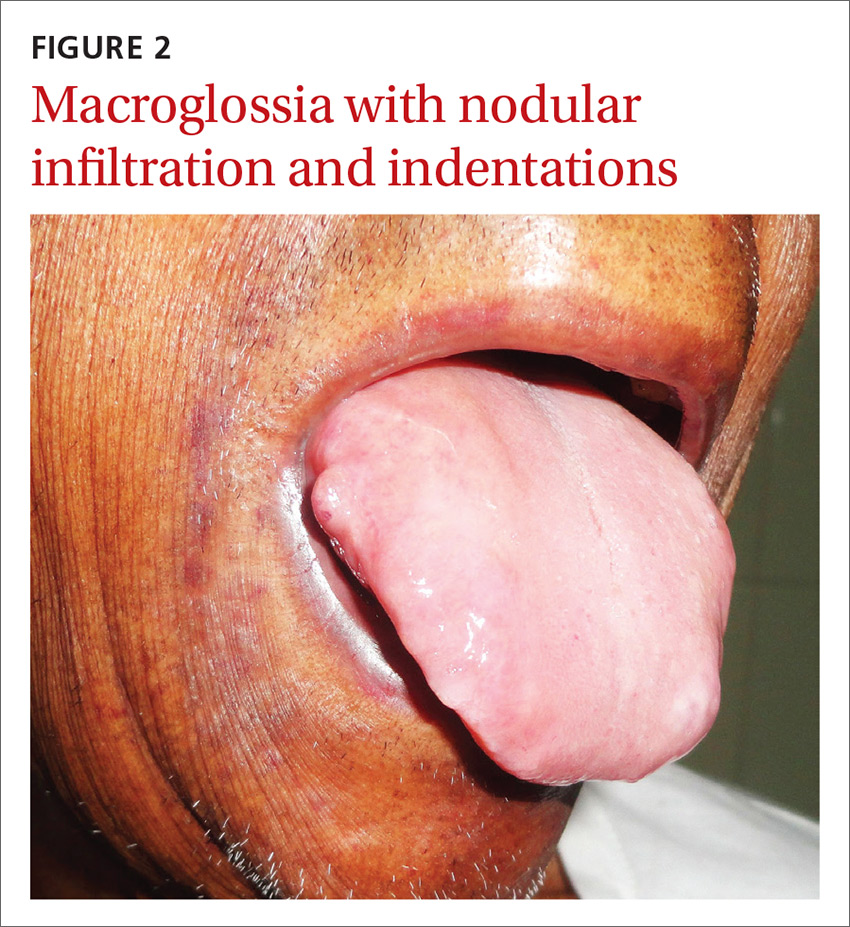
A color Doppler revealed mild mitral and tricuspid regurgitation with a restrictive pattern of mitral valve flow. Serum protein electrophoresis was normal.
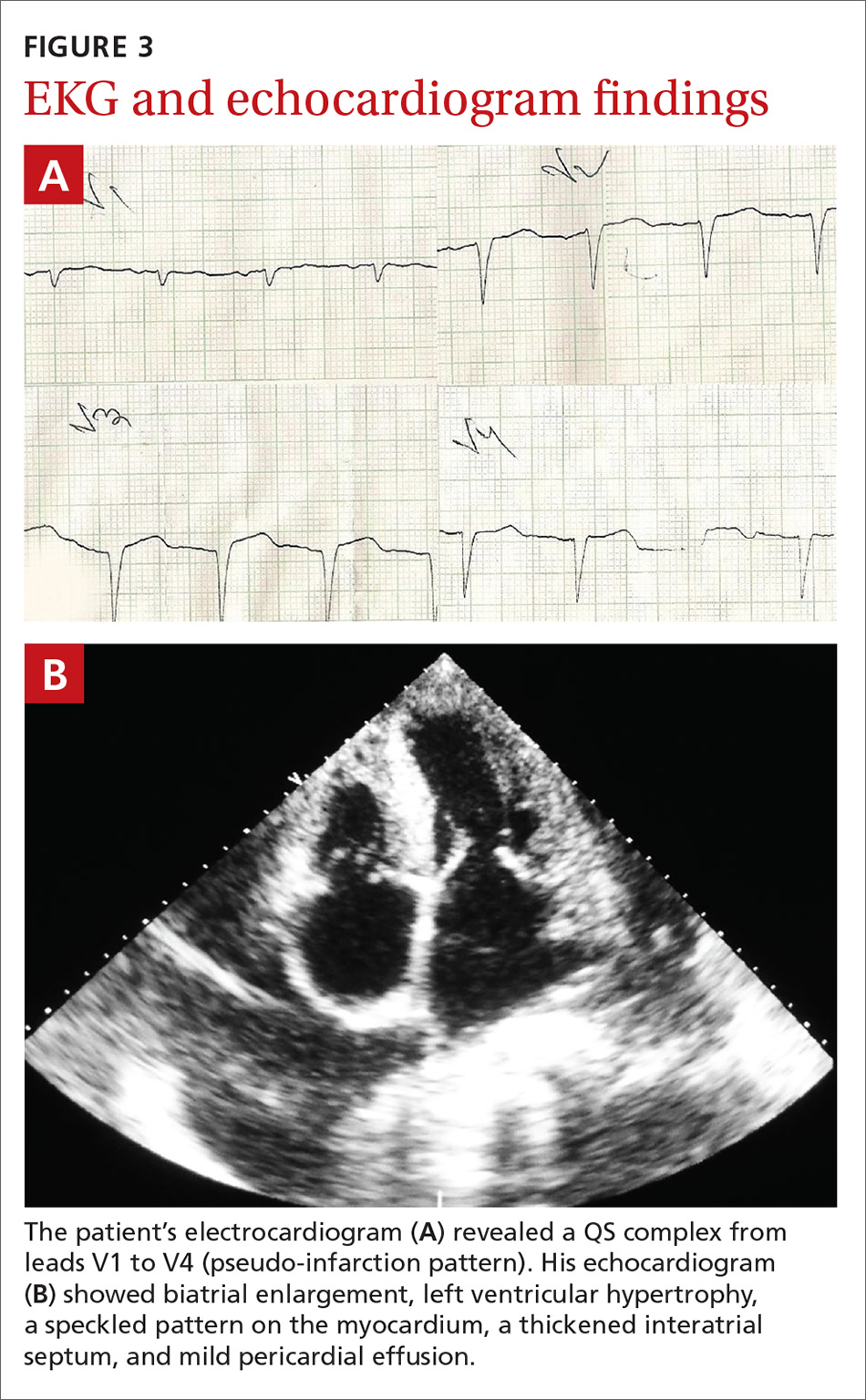
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Primary systemic amyloidosis
A diagnosis of primary systemic amyloidosis was confirmed with histopathologic examination of the abdominal fat pad using Congo red stain. Clinical, imaging, and laboratory features supported this diagnosis.
Primary systemic amyloidosis (also known as light-chain amyloidosis) is the most common type of systemic amyloidosis, affecting an estimated 5 to 12 million people per year.1,2 It occurs when there is a buildup of the abnormal protein amyloid. Organs that may be affected include the heart, kidneys, skin, nerves, and liver. There are no clear environmental, racial, or genetic risk factors for this condition.
With primary systemic amyloidosis, the ecchymosis present around the eyes may also appear elsewhere on the body (pinch purpura). Other symptoms may include macroglossia; sensory and autonomic neuropathy; and concomitant renal, cardiac, and hepatic involvement. In elderly patients with these symptoms, myeloma-associated systemic amyloidosis should be ruled out.2 Histopathologic examination of the abdominal fat pad or rectum is usually diagnostic.
Systemic amyloidosis and the heart
In patients with symptoms of congestive heart failure, a finding of thick heart walls on echocardiogram may indicate cardiac amyloidosis, particularly if there is no other underlying heart disease that could explain such findings. An even stronger indicator is the additional finding of low-voltage complexes on EKG.3
Periorbital ecchymosis can be a sign of many conditions
Bilateral periorbital ecchymosis, also known as “raccoon eyes,” was an important clinical clue to the diagnosis in our patient, but multiple conditions should be considered when raccoon eyes are present.
Basal skull fracture occurs with a history of trauma. Clinical and radiologic signs of injuries can usually be found in other areas of the body.6
Periorbital cellulitis presents with unilateral erythematous periorbital swelling. A rapid increase in the patient’s temperature and swelling of tissue may occur. Movement of the extraocular muscles and visual acuity are usually normal.7
Blood dyscrasias usually involve a history of external bleeding.7 A thorough laboratory evaluation, including a complete blood count, platelet function tests, and a blood coagulation profile, is usually sufficient to exclude these cases.
A variety of treatment options
Clinicians have used angiotensin-converting enzyme inhibitors, long-acting nitrates, vasodilators, and diuretics to treat cardiac amyloidosis with varying results. For patients with atrial fibrillation (AF), ibutilide and amiodarone are useful antiarrhythmic drugs.3,8 In addition, experts recommend anticoagulation therapy with warfarin, dabigatran, or rivaroxaban for patients with AF because of the high risk of stroke.3,8 Symptomatic bradycardia and high-grade conduction-system disease usually require pacemaker implantation.
A guarded prognosis. The prognosis for patients with primary systemic amyloidosis is usually poor. Cardiac failure and renal failure are the major causes of death. The median survival time is 13 months, and only 5% of patients survive longer than 10 years.4,5
Our patient was prescribed furosemide 40 mg/d, ramipril 1.25 mg/d, and spironolactone 25 mg/d. Within a couple weeks, his symptoms improved. However, 3 months after being diagnosed, the patient succumbed to heart failure.
CORRESPONDENCE
Sudip Kumar Ghosh, MD, DNB, Department of Dermatology, Venereology, and Leprosy, R. G. Kar Medical College, 1, Khudiram Bose Sarani, Kolkata, West Bengal 700004, India; [email protected].
1. Gertz MA. The classification and typing of amyloid deposits. Am J Clin Pathol. 2004;121:787-789.
2. Sanchorawala V. Light-chain (AL) amyloidosis: diagnosis and treatment. Clin J Am Soc Nephrol. 2006;1:1331-1341.
3. Quarta CC, Kruger JL, Falk RH. Cardiac amyloidosis. Circulation. 2012;126:e178-e182.
4. Kyle RA, Gertz MA, Greipp PR, et al. A trial of three regimens for primary amyloidosis: colchicine alone, melphalan and prednisone, and melphalan, prednisone, and colchicine. N Engl J Med. 1997;336:1202-1207.
5. Kyle RA, Gertz MA, Greipp PR, et al. Long-term survival (10 years or more) in 30 patients with primary amyloidosis. Blood. 1999;93:1062-1066.
6. Somasundaram A, Laxton AW, Perrin RG. The clinical features of periorbital ecchymosis in a series of trauma patients. Injury. 2014;45:203-205.
7. Ghosh SK, Dutta A, Basu M. Raccoon eyes in a case of metastatic neuroblastoma. Indian J Dermatol Venereol Leprol. 2012;78:740-741.
8. Hassan W, Al-Sergani H, Mourad W, et al. Amyloid heart disease. New frontiers and insights in pathophysiology, diagnosis, and management. Tex Heart Inst J. 2005;32:178-184.
A 54-year-old man presented at our facility with a 3-month history of exertional breathlessness and purple blotches around his eyes. Examination revealed bilateral periorbital and perioral ecchymosis, purpuric spots along his waist, and waxy papules on his eyelids (FIGURE 1). In addition, the patient had macroglossia with nodular infiltration and irregular indentations at the lateral margin of his tongue (FIGURE 2).
The patient also had a raised jugular venous pressure and prominent atrial and ventricular waves. Further examination revealed a fourth heart sound over the left ventricular apex, as well as bilateral basal rales. All other systems were normal except for mild hepatomegaly.

Routine hematologic and biochemical lab work was unremarkable. X-rays of the spine and skull were normal, but a chest x-ray showed mild cardiomegaly. An electrocardiogram (EKG) showed a QS complex from leads V1 to V4 (a pseudo-infarction pattern; FIGURE 3A). An echocardiogram showed biatrial enlargement, left ventricular hypertrophy with a left ventricular ejection fraction of 48%, a speckled pattern on the myocardium, a thickened interatrial septum, and mild pericardial effusion (FIGURE 3B).

A color Doppler revealed mild mitral and tricuspid regurgitation with a restrictive pattern of mitral valve flow. Serum protein electrophoresis was normal.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Primary systemic amyloidosis
A diagnosis of primary systemic amyloidosis was confirmed with histopathologic examination of the abdominal fat pad using Congo red stain. Clinical, imaging, and laboratory features supported this diagnosis.
Primary systemic amyloidosis (also known as light-chain amyloidosis) is the most common type of systemic amyloidosis, affecting an estimated 5 to 12 million people per year.1,2 It occurs when there is a buildup of the abnormal protein amyloid. Organs that may be affected include the heart, kidneys, skin, nerves, and liver. There are no clear environmental, racial, or genetic risk factors for this condition.
With primary systemic amyloidosis, the ecchymosis present around the eyes may also appear elsewhere on the body (pinch purpura). Other symptoms may include macroglossia; sensory and autonomic neuropathy; and concomitant renal, cardiac, and hepatic involvement. In elderly patients with these symptoms, myeloma-associated systemic amyloidosis should be ruled out.2 Histopathologic examination of the abdominal fat pad or rectum is usually diagnostic.
Systemic amyloidosis and the heart
In patients with symptoms of congestive heart failure, a finding of thick heart walls on echocardiogram may indicate cardiac amyloidosis, particularly if there is no other underlying heart disease that could explain such findings. An even stronger indicator is the additional finding of low-voltage complexes on EKG.3
Periorbital ecchymosis can be a sign of many conditions
Bilateral periorbital ecchymosis, also known as “raccoon eyes,” was an important clinical clue to the diagnosis in our patient, but multiple conditions should be considered when raccoon eyes are present.
Basal skull fracture occurs with a history of trauma. Clinical and radiologic signs of injuries can usually be found in other areas of the body.6
Periorbital cellulitis presents with unilateral erythematous periorbital swelling. A rapid increase in the patient’s temperature and swelling of tissue may occur. Movement of the extraocular muscles and visual acuity are usually normal.7
Blood dyscrasias usually involve a history of external bleeding.7 A thorough laboratory evaluation, including a complete blood count, platelet function tests, and a blood coagulation profile, is usually sufficient to exclude these cases.
A variety of treatment options
Clinicians have used angiotensin-converting enzyme inhibitors, long-acting nitrates, vasodilators, and diuretics to treat cardiac amyloidosis with varying results. For patients with atrial fibrillation (AF), ibutilide and amiodarone are useful antiarrhythmic drugs.3,8 In addition, experts recommend anticoagulation therapy with warfarin, dabigatran, or rivaroxaban for patients with AF because of the high risk of stroke.3,8 Symptomatic bradycardia and high-grade conduction-system disease usually require pacemaker implantation.
A guarded prognosis. The prognosis for patients with primary systemic amyloidosis is usually poor. Cardiac failure and renal failure are the major causes of death. The median survival time is 13 months, and only 5% of patients survive longer than 10 years.4,5
Our patient was prescribed furosemide 40 mg/d, ramipril 1.25 mg/d, and spironolactone 25 mg/d. Within a couple weeks, his symptoms improved. However, 3 months after being diagnosed, the patient succumbed to heart failure.
CORRESPONDENCE
Sudip Kumar Ghosh, MD, DNB, Department of Dermatology, Venereology, and Leprosy, R. G. Kar Medical College, 1, Khudiram Bose Sarani, Kolkata, West Bengal 700004, India; [email protected].
A 54-year-old man presented at our facility with a 3-month history of exertional breathlessness and purple blotches around his eyes. Examination revealed bilateral periorbital and perioral ecchymosis, purpuric spots along his waist, and waxy papules on his eyelids (FIGURE 1). In addition, the patient had macroglossia with nodular infiltration and irregular indentations at the lateral margin of his tongue (FIGURE 2).
The patient also had a raised jugular venous pressure and prominent atrial and ventricular waves. Further examination revealed a fourth heart sound over the left ventricular apex, as well as bilateral basal rales. All other systems were normal except for mild hepatomegaly.

Routine hematologic and biochemical lab work was unremarkable. X-rays of the spine and skull were normal, but a chest x-ray showed mild cardiomegaly. An electrocardiogram (EKG) showed a QS complex from leads V1 to V4 (a pseudo-infarction pattern; FIGURE 3A). An echocardiogram showed biatrial enlargement, left ventricular hypertrophy with a left ventricular ejection fraction of 48%, a speckled pattern on the myocardium, a thickened interatrial septum, and mild pericardial effusion (FIGURE 3B).

A color Doppler revealed mild mitral and tricuspid regurgitation with a restrictive pattern of mitral valve flow. Serum protein electrophoresis was normal.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Primary systemic amyloidosis
A diagnosis of primary systemic amyloidosis was confirmed with histopathologic examination of the abdominal fat pad using Congo red stain. Clinical, imaging, and laboratory features supported this diagnosis.
Primary systemic amyloidosis (also known as light-chain amyloidosis) is the most common type of systemic amyloidosis, affecting an estimated 5 to 12 million people per year.1,2 It occurs when there is a buildup of the abnormal protein amyloid. Organs that may be affected include the heart, kidneys, skin, nerves, and liver. There are no clear environmental, racial, or genetic risk factors for this condition.
With primary systemic amyloidosis, the ecchymosis present around the eyes may also appear elsewhere on the body (pinch purpura). Other symptoms may include macroglossia; sensory and autonomic neuropathy; and concomitant renal, cardiac, and hepatic involvement. In elderly patients with these symptoms, myeloma-associated systemic amyloidosis should be ruled out.2 Histopathologic examination of the abdominal fat pad or rectum is usually diagnostic.
Systemic amyloidosis and the heart
In patients with symptoms of congestive heart failure, a finding of thick heart walls on echocardiogram may indicate cardiac amyloidosis, particularly if there is no other underlying heart disease that could explain such findings. An even stronger indicator is the additional finding of low-voltage complexes on EKG.3
Periorbital ecchymosis can be a sign of many conditions
Bilateral periorbital ecchymosis, also known as “raccoon eyes,” was an important clinical clue to the diagnosis in our patient, but multiple conditions should be considered when raccoon eyes are present.
Basal skull fracture occurs with a history of trauma. Clinical and radiologic signs of injuries can usually be found in other areas of the body.6
Periorbital cellulitis presents with unilateral erythematous periorbital swelling. A rapid increase in the patient’s temperature and swelling of tissue may occur. Movement of the extraocular muscles and visual acuity are usually normal.7
Blood dyscrasias usually involve a history of external bleeding.7 A thorough laboratory evaluation, including a complete blood count, platelet function tests, and a blood coagulation profile, is usually sufficient to exclude these cases.
A variety of treatment options
Clinicians have used angiotensin-converting enzyme inhibitors, long-acting nitrates, vasodilators, and diuretics to treat cardiac amyloidosis with varying results. For patients with atrial fibrillation (AF), ibutilide and amiodarone are useful antiarrhythmic drugs.3,8 In addition, experts recommend anticoagulation therapy with warfarin, dabigatran, or rivaroxaban for patients with AF because of the high risk of stroke.3,8 Symptomatic bradycardia and high-grade conduction-system disease usually require pacemaker implantation.
A guarded prognosis. The prognosis for patients with primary systemic amyloidosis is usually poor. Cardiac failure and renal failure are the major causes of death. The median survival time is 13 months, and only 5% of patients survive longer than 10 years.4,5
Our patient was prescribed furosemide 40 mg/d, ramipril 1.25 mg/d, and spironolactone 25 mg/d. Within a couple weeks, his symptoms improved. However, 3 months after being diagnosed, the patient succumbed to heart failure.
CORRESPONDENCE
Sudip Kumar Ghosh, MD, DNB, Department of Dermatology, Venereology, and Leprosy, R. G. Kar Medical College, 1, Khudiram Bose Sarani, Kolkata, West Bengal 700004, India; [email protected].
1. Gertz MA. The classification and typing of amyloid deposits. Am J Clin Pathol. 2004;121:787-789.
2. Sanchorawala V. Light-chain (AL) amyloidosis: diagnosis and treatment. Clin J Am Soc Nephrol. 2006;1:1331-1341.
3. Quarta CC, Kruger JL, Falk RH. Cardiac amyloidosis. Circulation. 2012;126:e178-e182.
4. Kyle RA, Gertz MA, Greipp PR, et al. A trial of three regimens for primary amyloidosis: colchicine alone, melphalan and prednisone, and melphalan, prednisone, and colchicine. N Engl J Med. 1997;336:1202-1207.
5. Kyle RA, Gertz MA, Greipp PR, et al. Long-term survival (10 years or more) in 30 patients with primary amyloidosis. Blood. 1999;93:1062-1066.
6. Somasundaram A, Laxton AW, Perrin RG. The clinical features of periorbital ecchymosis in a series of trauma patients. Injury. 2014;45:203-205.
7. Ghosh SK, Dutta A, Basu M. Raccoon eyes in a case of metastatic neuroblastoma. Indian J Dermatol Venereol Leprol. 2012;78:740-741.
8. Hassan W, Al-Sergani H, Mourad W, et al. Amyloid heart disease. New frontiers and insights in pathophysiology, diagnosis, and management. Tex Heart Inst J. 2005;32:178-184.
1. Gertz MA. The classification and typing of amyloid deposits. Am J Clin Pathol. 2004;121:787-789.
2. Sanchorawala V. Light-chain (AL) amyloidosis: diagnosis and treatment. Clin J Am Soc Nephrol. 2006;1:1331-1341.
3. Quarta CC, Kruger JL, Falk RH. Cardiac amyloidosis. Circulation. 2012;126:e178-e182.
4. Kyle RA, Gertz MA, Greipp PR, et al. A trial of three regimens for primary amyloidosis: colchicine alone, melphalan and prednisone, and melphalan, prednisone, and colchicine. N Engl J Med. 1997;336:1202-1207.
5. Kyle RA, Gertz MA, Greipp PR, et al. Long-term survival (10 years or more) in 30 patients with primary amyloidosis. Blood. 1999;93:1062-1066.
6. Somasundaram A, Laxton AW, Perrin RG. The clinical features of periorbital ecchymosis in a series of trauma patients. Injury. 2014;45:203-205.
7. Ghosh SK, Dutta A, Basu M. Raccoon eyes in a case of metastatic neuroblastoma. Indian J Dermatol Venereol Leprol. 2012;78:740-741.
8. Hassan W, Al-Sergani H, Mourad W, et al. Amyloid heart disease. New frontiers and insights in pathophysiology, diagnosis, and management. Tex Heart Inst J. 2005;32:178-184.
SSRIs for depression/heart failure patients? Not so fast
ILLUSTRATIVE CASE
A 60-year-old man comes to your office for a follow-up visit to talk about his congestive heart failure. He has New York Heart Association Class III heart failure with a left ventricular ejection fraction of 30%. You notice that he is downcast, and after evaluation, including a score of 17 on a self-administered 9-item Patient Health Questionnaire (PHQ-9), you determine that he is having a concomitant major depressive episode. Should you start him on a selective serotonin reuptake inhibitor (SSRI)?
Depression is widely recognized as an independent risk factor for both the development of cardiovascular disease (CVD), as well as adverse outcomes in patients with known CVD.2-5 Previous studies have identified poor health behaviors as the primary underlying mechanisms linking depression and the risk of CVD.2,6 Conversely, a recent systematic review suggests that positive constructs—mediated primarily through lifestyle behaviors—may have a protective effect on CVD outcomes.7
As a result, researchers have focused on the treatment of depression to improve CVD outcomes in recent years, including in patients with heart failure. While some randomized studies have shown that SSRIs are a safe and effective treatment for depression in patients with coronary disease, they have not demonstrated improvement in CVD outcomes.8,9 However, a post hoc analysis of the ENRICHD (Enhancing Recovery in Coronary Heart Disease) trial did suggest that SSRI treatment may improve mortality and morbidity post-myocardial infarction.10
The prevalence of depression among patients with heart failure ranges from 10% to 40%, depending on disease severity.11 Depression is associated with worse quality of life, poorer treatment adherence, and higher rates of rehospitalization among patients with heart failure, and is an independent predictor of mortality in this patient population.1 Until recently, only one randomized controlled trial (RCT), the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) study, looked at treatment with SSRIs in patients with heart failure and depression.12 In this trial, sertraline, when compared with placebo, did not improve depression or CVD outcomes over 12 weeks, but the study period may have been insufficiently long to capture the impact on long-term outcomes.
STUDY SUMMARY
SADHART-CHF, but better
In the MOOD-HF (The effects of selective serotonin re-uptake inhibition on morbidity, mortality, and mood in depressed heart failure patients) study, investigators sought to determine whether SSRI treatment for depression in patients with heart failure could improve CVD outcomes over a longer study period (up to 24 months).1 Specifically, this RCT assessed whether treatment with escitalopram vs placebo could reduce the increased morbidity and mortality risk in patients with comorbid chronic systolic heart failure and depression.
This double-blind, placebo-controlled trial was conducted at 16 tertiary medical centers in Germany between 2009 and 2014. Adult patients established at heart failure clinics with New York Heart Association class II to IV heart failure and left ventricular ejection fractions <45% were screened for depression using the PHQ-9. Individuals with PHQ-9 scores ≥12 underwent a structured psychiatric interview with a psychiatrist or psychosomatic specialist. Those who received a diagnosis of major depression were invited to participate in the trial. Patients with recent SSRI use and/or psychotherapy were excluded from participation.
Eligible participants were randomized to receive either escitalopram (10-20 mg/d) or placebo for up to 24 months in addition to standard heart failure care. The starting dose of 5 mg was increased to 10 to 20 mg as tolerated until week 12 of the study; the dose at 12 weeks was considered the maintenance dose. Psychiatric and medical assessments were performed every 6 months during the study period. Depression severity was assessed using the 10-item Montgomery-Åsberg Depression Rating Scale (MADRS).
Outcomes. The primary study outcome was time to a first event of the composite of all-cause death or hospitalization. Secondary outcomes included MADRS score at 12 weeks, anxiety as assessed by the Generalized Anxiety Disorder 7-item scale (GAD-7), and health-related quality of life (QoL) as assessed by the Kansas City Cardiomyopathy Questionnaire (KCCQ). The sample size was calculated to achieve 80% power for the primary outcome. Baseline characteristics between the intervention and placebo groups were balanced after randomization, and the modified intention-to-treat study population included participants who took at least one dose of the study medication.1
Results. Ultimately, 372 participants were included in the analysis (185 in the escitalopram group and 187 in the placebo group). A primary endpoint event occurred in 116 participants (63%) in the escitalopram group and in 119 participants (64%) in the placebo group (hazard ratio [HR]=0.99; 95% confidence interval [CI], 0.76 to 1.27]; P=.92).1 No differences were found between treatment groups for the primary endpoints in either adjusted or unadjusted analyses.
The mean (SD) MADRS score changed from 20.2 (8.6) at baseline to 11.2 (8.1) at 12 weeks with escitalopram and from 21.4 (8.8) to 12.5 (7.6) in the placebo group (between-group difference = -0.9; 95% CI, -2.6 to 0.7; P =.26).10 Overall, participants in the 2 treatment groups had comparable daily doses of study medications, as well as mean treatment duration (18 months), and both groups demonstrated partial remission of depression symptoms over the study period, as well as improved health status and QoL as measured by KCCQ.
Interestingly, QoL as assessed by the KCCQ symptom score was significantly improved in the placebo group at 12 months.1 There were no between-group differences in adverse events or safety measures.1 The trial was discontinued prematurely on February 28, 2014, based on futility after a recommendation from the data and safety monitoring committee.
WHAT’S NEW
Longer study period/different SSRI doesn’t change earlier finding
The MOOD-HF trial directly addresses the major criticism of the SADHART-CHF trial by looking at SSRI treatment of patients with heart failure and depression over a much longer study duration (up to 24 months vs 12 weeks). Also, in contrast to SADHART-CHF, this trial studied escitalopram, rather than sertraline, because some evidence indicates that escitalopram is superior at treating primary depression.13 Despite these differences, the results of MOOD-HF are consistent with the findings of SADHART-CHF: treating patients with both heart failure and depression with an SSRI did not improve the elevated morbidity and mortality risk seen with these comorbid conditions.
Also consistent with SADHART-CHF findings, participants in both groups in the MOOD-HF trial had partial remission of depressive symptoms over the study period, with no significant difference between those treated with escitalopram vs placebo. Given that this high-quality trial, with a much longer treatment period and a possibly more effective SSRI, replicated the findings of SADHART-CHF, the results of MOOD-HF should put to rest the practice of initiating SSRI treatment in depressed patients with heart failure in an attempt to affect CVD outcomes.
CAVEATS
There are other SSRI fish in the sea
There are other SSRIs, besides escitalopram and sertraline, available for use. However, it is likely that this is a class effect.
Additionally, none of the patients in this trial had severe depression, as their PHQ-9 scores were all below 19. Therefore, it remains to be determined if treating patients with severe depression has an impact on cardiovascular outcomes.
Lastly, and most importantly, this study only looked at screening patients for depression and initiating SSRIs in the setting of heart failure. The trial did not include patients already taking SSRIs for pre-existing depression. Thus, the results do not imply evidence for discontinuing SSRIs in patients with heart failure.
Treating comorbid depression and CVD to improve the elevated risk for adverse clinical outcomes remains nuanced and elusive. In fact, the same can be said of non-CVD chronic conditions—such as diabetes—based on recent systematic reviews.13 The summation of these studies suggests that a traditional screen-and-treat approach utilizing SSRIs for depression treatment to affect chronic disease outcomes (that are likely lifestyle-related) may not be cost-effective or patient-centered.
The publication of a recent study showing that cognitive behavioral therapy did improve depression—but not heart failure—among patients with both conditions14 reaffirms that teasing out the impact of depression on lifestyle behaviors and chronic disease outcomes among multimorbid patients is more complex than previously thought. Nevertheless, this is an area of research that should continue to be explored, given the obvious increased risk for poorer chronic disease outcomes in the presence of depression.
CHALLENGES TO IMPLEMENTATION
Changing the tide can be difficult
As with any behavior change among providers, we expect that it will be a challenge to convince providers to stop screening for depression and initiating treatment with an SSRI to affect CV outcomes in patients with heart failure. This is especially so given the body of evidence for depression as a risk factor for increased morbidity and mortality in this population.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Angermann CE, Gelbrich G, Störk S, et al, for the MOOD-HF Study Investigators and Committee Members. Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression. The MOOD-HF randomized clinical trial. JAMA. 2016;315:2683-2693.
2. Sin NL, Kumar AD, Gehi AK, et al. Direction of association between depression and lifestyle behaviors in patients with coronary heart disease: the heart and soul study. Ann Behav Med. 2016;50:523-532.
3. Lett HS, Blumenthal JA, Babyak MA, et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66:305-315.
4. Whooley MA, Wong JM. Depression and cardiovascular disorders. Annu Rev Clin Psychol. 2013;9:327-354.
5. Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66:802-813.
6. Whooley MA, de Jonge P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300:2379-2388.
7. DuBois CM, Lopez OV, Beale EE, et al. Relationships between positive psychological constructs and health outcomes in patients with cardiovascular disease: a systematic review. Int J Cardiol. 2015;195:265-280.
8. Glassman AH, O’Connor CM, Califf RM, et al, for the Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) Group. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288:701-709.
9. Writing Committee for the ENRICHD Investigators. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA. 2003;289:3106-3116.
10. Taylor CB, Youngblood ME, Catellier D, et al, for the ENRICHD Investigators. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005;62:792-798.
11. Rutledge T, Reis VA, Linke SE, et al. Depression in heart failure. A meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48;1527-1537.
12. O’Connor CM, Jiang W, Kuchibhatla M, et al, SADHART-CHF Investigators. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56:692-699.
13. Health Quality Ontario. Screening and management of depression for adults with chronic diseases: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13:1-45.
14. Freedland KE, Carney RM, Rich MW, et al. Cognitive behavior therapy for depression and self-care in heart failure patients: a randomized clinical trial. JAMA Intern Med. 2015;175:1773-1782.
ILLUSTRATIVE CASE
A 60-year-old man comes to your office for a follow-up visit to talk about his congestive heart failure. He has New York Heart Association Class III heart failure with a left ventricular ejection fraction of 30%. You notice that he is downcast, and after evaluation, including a score of 17 on a self-administered 9-item Patient Health Questionnaire (PHQ-9), you determine that he is having a concomitant major depressive episode. Should you start him on a selective serotonin reuptake inhibitor (SSRI)?
Depression is widely recognized as an independent risk factor for both the development of cardiovascular disease (CVD), as well as adverse outcomes in patients with known CVD.2-5 Previous studies have identified poor health behaviors as the primary underlying mechanisms linking depression and the risk of CVD.2,6 Conversely, a recent systematic review suggests that positive constructs—mediated primarily through lifestyle behaviors—may have a protective effect on CVD outcomes.7
As a result, researchers have focused on the treatment of depression to improve CVD outcomes in recent years, including in patients with heart failure. While some randomized studies have shown that SSRIs are a safe and effective treatment for depression in patients with coronary disease, they have not demonstrated improvement in CVD outcomes.8,9 However, a post hoc analysis of the ENRICHD (Enhancing Recovery in Coronary Heart Disease) trial did suggest that SSRI treatment may improve mortality and morbidity post-myocardial infarction.10
The prevalence of depression among patients with heart failure ranges from 10% to 40%, depending on disease severity.11 Depression is associated with worse quality of life, poorer treatment adherence, and higher rates of rehospitalization among patients with heart failure, and is an independent predictor of mortality in this patient population.1 Until recently, only one randomized controlled trial (RCT), the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) study, looked at treatment with SSRIs in patients with heart failure and depression.12 In this trial, sertraline, when compared with placebo, did not improve depression or CVD outcomes over 12 weeks, but the study period may have been insufficiently long to capture the impact on long-term outcomes.
STUDY SUMMARY
SADHART-CHF, but better
In the MOOD-HF (The effects of selective serotonin re-uptake inhibition on morbidity, mortality, and mood in depressed heart failure patients) study, investigators sought to determine whether SSRI treatment for depression in patients with heart failure could improve CVD outcomes over a longer study period (up to 24 months).1 Specifically, this RCT assessed whether treatment with escitalopram vs placebo could reduce the increased morbidity and mortality risk in patients with comorbid chronic systolic heart failure and depression.
This double-blind, placebo-controlled trial was conducted at 16 tertiary medical centers in Germany between 2009 and 2014. Adult patients established at heart failure clinics with New York Heart Association class II to IV heart failure and left ventricular ejection fractions <45% were screened for depression using the PHQ-9. Individuals with PHQ-9 scores ≥12 underwent a structured psychiatric interview with a psychiatrist or psychosomatic specialist. Those who received a diagnosis of major depression were invited to participate in the trial. Patients with recent SSRI use and/or psychotherapy were excluded from participation.
Eligible participants were randomized to receive either escitalopram (10-20 mg/d) or placebo for up to 24 months in addition to standard heart failure care. The starting dose of 5 mg was increased to 10 to 20 mg as tolerated until week 12 of the study; the dose at 12 weeks was considered the maintenance dose. Psychiatric and medical assessments were performed every 6 months during the study period. Depression severity was assessed using the 10-item Montgomery-Åsberg Depression Rating Scale (MADRS).
Outcomes. The primary study outcome was time to a first event of the composite of all-cause death or hospitalization. Secondary outcomes included MADRS score at 12 weeks, anxiety as assessed by the Generalized Anxiety Disorder 7-item scale (GAD-7), and health-related quality of life (QoL) as assessed by the Kansas City Cardiomyopathy Questionnaire (KCCQ). The sample size was calculated to achieve 80% power for the primary outcome. Baseline characteristics between the intervention and placebo groups were balanced after randomization, and the modified intention-to-treat study population included participants who took at least one dose of the study medication.1
Results. Ultimately, 372 participants were included in the analysis (185 in the escitalopram group and 187 in the placebo group). A primary endpoint event occurred in 116 participants (63%) in the escitalopram group and in 119 participants (64%) in the placebo group (hazard ratio [HR]=0.99; 95% confidence interval [CI], 0.76 to 1.27]; P=.92).1 No differences were found between treatment groups for the primary endpoints in either adjusted or unadjusted analyses.
The mean (SD) MADRS score changed from 20.2 (8.6) at baseline to 11.2 (8.1) at 12 weeks with escitalopram and from 21.4 (8.8) to 12.5 (7.6) in the placebo group (between-group difference = -0.9; 95% CI, -2.6 to 0.7; P =.26).10 Overall, participants in the 2 treatment groups had comparable daily doses of study medications, as well as mean treatment duration (18 months), and both groups demonstrated partial remission of depression symptoms over the study period, as well as improved health status and QoL as measured by KCCQ.
Interestingly, QoL as assessed by the KCCQ symptom score was significantly improved in the placebo group at 12 months.1 There were no between-group differences in adverse events or safety measures.1 The trial was discontinued prematurely on February 28, 2014, based on futility after a recommendation from the data and safety monitoring committee.
WHAT’S NEW
Longer study period/different SSRI doesn’t change earlier finding
The MOOD-HF trial directly addresses the major criticism of the SADHART-CHF trial by looking at SSRI treatment of patients with heart failure and depression over a much longer study duration (up to 24 months vs 12 weeks). Also, in contrast to SADHART-CHF, this trial studied escitalopram, rather than sertraline, because some evidence indicates that escitalopram is superior at treating primary depression.13 Despite these differences, the results of MOOD-HF are consistent with the findings of SADHART-CHF: treating patients with both heart failure and depression with an SSRI did not improve the elevated morbidity and mortality risk seen with these comorbid conditions.
Also consistent with SADHART-CHF findings, participants in both groups in the MOOD-HF trial had partial remission of depressive symptoms over the study period, with no significant difference between those treated with escitalopram vs placebo. Given that this high-quality trial, with a much longer treatment period and a possibly more effective SSRI, replicated the findings of SADHART-CHF, the results of MOOD-HF should put to rest the practice of initiating SSRI treatment in depressed patients with heart failure in an attempt to affect CVD outcomes.
CAVEATS
There are other SSRI fish in the sea
There are other SSRIs, besides escitalopram and sertraline, available for use. However, it is likely that this is a class effect.
Additionally, none of the patients in this trial had severe depression, as their PHQ-9 scores were all below 19. Therefore, it remains to be determined if treating patients with severe depression has an impact on cardiovascular outcomes.
Lastly, and most importantly, this study only looked at screening patients for depression and initiating SSRIs in the setting of heart failure. The trial did not include patients already taking SSRIs for pre-existing depression. Thus, the results do not imply evidence for discontinuing SSRIs in patients with heart failure.
Treating comorbid depression and CVD to improve the elevated risk for adverse clinical outcomes remains nuanced and elusive. In fact, the same can be said of non-CVD chronic conditions—such as diabetes—based on recent systematic reviews.13 The summation of these studies suggests that a traditional screen-and-treat approach utilizing SSRIs for depression treatment to affect chronic disease outcomes (that are likely lifestyle-related) may not be cost-effective or patient-centered.
The publication of a recent study showing that cognitive behavioral therapy did improve depression—but not heart failure—among patients with both conditions14 reaffirms that teasing out the impact of depression on lifestyle behaviors and chronic disease outcomes among multimorbid patients is more complex than previously thought. Nevertheless, this is an area of research that should continue to be explored, given the obvious increased risk for poorer chronic disease outcomes in the presence of depression.
CHALLENGES TO IMPLEMENTATION
Changing the tide can be difficult
As with any behavior change among providers, we expect that it will be a challenge to convince providers to stop screening for depression and initiating treatment with an SSRI to affect CV outcomes in patients with heart failure. This is especially so given the body of evidence for depression as a risk factor for increased morbidity and mortality in this population.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 60-year-old man comes to your office for a follow-up visit to talk about his congestive heart failure. He has New York Heart Association Class III heart failure with a left ventricular ejection fraction of 30%. You notice that he is downcast, and after evaluation, including a score of 17 on a self-administered 9-item Patient Health Questionnaire (PHQ-9), you determine that he is having a concomitant major depressive episode. Should you start him on a selective serotonin reuptake inhibitor (SSRI)?
Depression is widely recognized as an independent risk factor for both the development of cardiovascular disease (CVD), as well as adverse outcomes in patients with known CVD.2-5 Previous studies have identified poor health behaviors as the primary underlying mechanisms linking depression and the risk of CVD.2,6 Conversely, a recent systematic review suggests that positive constructs—mediated primarily through lifestyle behaviors—may have a protective effect on CVD outcomes.7
As a result, researchers have focused on the treatment of depression to improve CVD outcomes in recent years, including in patients with heart failure. While some randomized studies have shown that SSRIs are a safe and effective treatment for depression in patients with coronary disease, they have not demonstrated improvement in CVD outcomes.8,9 However, a post hoc analysis of the ENRICHD (Enhancing Recovery in Coronary Heart Disease) trial did suggest that SSRI treatment may improve mortality and morbidity post-myocardial infarction.10
The prevalence of depression among patients with heart failure ranges from 10% to 40%, depending on disease severity.11 Depression is associated with worse quality of life, poorer treatment adherence, and higher rates of rehospitalization among patients with heart failure, and is an independent predictor of mortality in this patient population.1 Until recently, only one randomized controlled trial (RCT), the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) study, looked at treatment with SSRIs in patients with heart failure and depression.12 In this trial, sertraline, when compared with placebo, did not improve depression or CVD outcomes over 12 weeks, but the study period may have been insufficiently long to capture the impact on long-term outcomes.
STUDY SUMMARY
SADHART-CHF, but better
In the MOOD-HF (The effects of selective serotonin re-uptake inhibition on morbidity, mortality, and mood in depressed heart failure patients) study, investigators sought to determine whether SSRI treatment for depression in patients with heart failure could improve CVD outcomes over a longer study period (up to 24 months).1 Specifically, this RCT assessed whether treatment with escitalopram vs placebo could reduce the increased morbidity and mortality risk in patients with comorbid chronic systolic heart failure and depression.
This double-blind, placebo-controlled trial was conducted at 16 tertiary medical centers in Germany between 2009 and 2014. Adult patients established at heart failure clinics with New York Heart Association class II to IV heart failure and left ventricular ejection fractions <45% were screened for depression using the PHQ-9. Individuals with PHQ-9 scores ≥12 underwent a structured psychiatric interview with a psychiatrist or psychosomatic specialist. Those who received a diagnosis of major depression were invited to participate in the trial. Patients with recent SSRI use and/or psychotherapy were excluded from participation.
Eligible participants were randomized to receive either escitalopram (10-20 mg/d) or placebo for up to 24 months in addition to standard heart failure care. The starting dose of 5 mg was increased to 10 to 20 mg as tolerated until week 12 of the study; the dose at 12 weeks was considered the maintenance dose. Psychiatric and medical assessments were performed every 6 months during the study period. Depression severity was assessed using the 10-item Montgomery-Åsberg Depression Rating Scale (MADRS).
Outcomes. The primary study outcome was time to a first event of the composite of all-cause death or hospitalization. Secondary outcomes included MADRS score at 12 weeks, anxiety as assessed by the Generalized Anxiety Disorder 7-item scale (GAD-7), and health-related quality of life (QoL) as assessed by the Kansas City Cardiomyopathy Questionnaire (KCCQ). The sample size was calculated to achieve 80% power for the primary outcome. Baseline characteristics between the intervention and placebo groups were balanced after randomization, and the modified intention-to-treat study population included participants who took at least one dose of the study medication.1
Results. Ultimately, 372 participants were included in the analysis (185 in the escitalopram group and 187 in the placebo group). A primary endpoint event occurred in 116 participants (63%) in the escitalopram group and in 119 participants (64%) in the placebo group (hazard ratio [HR]=0.99; 95% confidence interval [CI], 0.76 to 1.27]; P=.92).1 No differences were found between treatment groups for the primary endpoints in either adjusted or unadjusted analyses.
The mean (SD) MADRS score changed from 20.2 (8.6) at baseline to 11.2 (8.1) at 12 weeks with escitalopram and from 21.4 (8.8) to 12.5 (7.6) in the placebo group (between-group difference = -0.9; 95% CI, -2.6 to 0.7; P =.26).10 Overall, participants in the 2 treatment groups had comparable daily doses of study medications, as well as mean treatment duration (18 months), and both groups demonstrated partial remission of depression symptoms over the study period, as well as improved health status and QoL as measured by KCCQ.
Interestingly, QoL as assessed by the KCCQ symptom score was significantly improved in the placebo group at 12 months.1 There were no between-group differences in adverse events or safety measures.1 The trial was discontinued prematurely on February 28, 2014, based on futility after a recommendation from the data and safety monitoring committee.
WHAT’S NEW
Longer study period/different SSRI doesn’t change earlier finding
The MOOD-HF trial directly addresses the major criticism of the SADHART-CHF trial by looking at SSRI treatment of patients with heart failure and depression over a much longer study duration (up to 24 months vs 12 weeks). Also, in contrast to SADHART-CHF, this trial studied escitalopram, rather than sertraline, because some evidence indicates that escitalopram is superior at treating primary depression.13 Despite these differences, the results of MOOD-HF are consistent with the findings of SADHART-CHF: treating patients with both heart failure and depression with an SSRI did not improve the elevated morbidity and mortality risk seen with these comorbid conditions.
Also consistent with SADHART-CHF findings, participants in both groups in the MOOD-HF trial had partial remission of depressive symptoms over the study period, with no significant difference between those treated with escitalopram vs placebo. Given that this high-quality trial, with a much longer treatment period and a possibly more effective SSRI, replicated the findings of SADHART-CHF, the results of MOOD-HF should put to rest the practice of initiating SSRI treatment in depressed patients with heart failure in an attempt to affect CVD outcomes.
CAVEATS
There are other SSRI fish in the sea
There are other SSRIs, besides escitalopram and sertraline, available for use. However, it is likely that this is a class effect.
Additionally, none of the patients in this trial had severe depression, as their PHQ-9 scores were all below 19. Therefore, it remains to be determined if treating patients with severe depression has an impact on cardiovascular outcomes.
Lastly, and most importantly, this study only looked at screening patients for depression and initiating SSRIs in the setting of heart failure. The trial did not include patients already taking SSRIs for pre-existing depression. Thus, the results do not imply evidence for discontinuing SSRIs in patients with heart failure.
Treating comorbid depression and CVD to improve the elevated risk for adverse clinical outcomes remains nuanced and elusive. In fact, the same can be said of non-CVD chronic conditions—such as diabetes—based on recent systematic reviews.13 The summation of these studies suggests that a traditional screen-and-treat approach utilizing SSRIs for depression treatment to affect chronic disease outcomes (that are likely lifestyle-related) may not be cost-effective or patient-centered.
The publication of a recent study showing that cognitive behavioral therapy did improve depression—but not heart failure—among patients with both conditions14 reaffirms that teasing out the impact of depression on lifestyle behaviors and chronic disease outcomes among multimorbid patients is more complex than previously thought. Nevertheless, this is an area of research that should continue to be explored, given the obvious increased risk for poorer chronic disease outcomes in the presence of depression.
CHALLENGES TO IMPLEMENTATION
Changing the tide can be difficult
As with any behavior change among providers, we expect that it will be a challenge to convince providers to stop screening for depression and initiating treatment with an SSRI to affect CV outcomes in patients with heart failure. This is especially so given the body of evidence for depression as a risk factor for increased morbidity and mortality in this population.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Angermann CE, Gelbrich G, Störk S, et al, for the MOOD-HF Study Investigators and Committee Members. Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression. The MOOD-HF randomized clinical trial. JAMA. 2016;315:2683-2693.
2. Sin NL, Kumar AD, Gehi AK, et al. Direction of association between depression and lifestyle behaviors in patients with coronary heart disease: the heart and soul study. Ann Behav Med. 2016;50:523-532.
3. Lett HS, Blumenthal JA, Babyak MA, et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66:305-315.
4. Whooley MA, Wong JM. Depression and cardiovascular disorders. Annu Rev Clin Psychol. 2013;9:327-354.
5. Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66:802-813.
6. Whooley MA, de Jonge P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300:2379-2388.
7. DuBois CM, Lopez OV, Beale EE, et al. Relationships between positive psychological constructs and health outcomes in patients with cardiovascular disease: a systematic review. Int J Cardiol. 2015;195:265-280.
8. Glassman AH, O’Connor CM, Califf RM, et al, for the Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) Group. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288:701-709.
9. Writing Committee for the ENRICHD Investigators. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA. 2003;289:3106-3116.
10. Taylor CB, Youngblood ME, Catellier D, et al, for the ENRICHD Investigators. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005;62:792-798.
11. Rutledge T, Reis VA, Linke SE, et al. Depression in heart failure. A meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48;1527-1537.
12. O’Connor CM, Jiang W, Kuchibhatla M, et al, SADHART-CHF Investigators. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56:692-699.
13. Health Quality Ontario. Screening and management of depression for adults with chronic diseases: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13:1-45.
14. Freedland KE, Carney RM, Rich MW, et al. Cognitive behavior therapy for depression and self-care in heart failure patients: a randomized clinical trial. JAMA Intern Med. 2015;175:1773-1782.
1. Angermann CE, Gelbrich G, Störk S, et al, for the MOOD-HF Study Investigators and Committee Members. Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression. The MOOD-HF randomized clinical trial. JAMA. 2016;315:2683-2693.
2. Sin NL, Kumar AD, Gehi AK, et al. Direction of association between depression and lifestyle behaviors in patients with coronary heart disease: the heart and soul study. Ann Behav Med. 2016;50:523-532.
3. Lett HS, Blumenthal JA, Babyak MA, et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66:305-315.
4. Whooley MA, Wong JM. Depression and cardiovascular disorders. Annu Rev Clin Psychol. 2013;9:327-354.
5. Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66:802-813.
6. Whooley MA, de Jonge P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300:2379-2388.
7. DuBois CM, Lopez OV, Beale EE, et al. Relationships between positive psychological constructs and health outcomes in patients with cardiovascular disease: a systematic review. Int J Cardiol. 2015;195:265-280.
8. Glassman AH, O’Connor CM, Califf RM, et al, for the Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) Group. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288:701-709.
9. Writing Committee for the ENRICHD Investigators. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA. 2003;289:3106-3116.
10. Taylor CB, Youngblood ME, Catellier D, et al, for the ENRICHD Investigators. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005;62:792-798.
11. Rutledge T, Reis VA, Linke SE, et al. Depression in heart failure. A meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48;1527-1537.
12. O’Connor CM, Jiang W, Kuchibhatla M, et al, SADHART-CHF Investigators. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56:692-699.
13. Health Quality Ontario. Screening and management of depression for adults with chronic diseases: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13:1-45.
14. Freedland KE, Carney RM, Rich MW, et al. Cognitive behavior therapy for depression and self-care in heart failure patients: a randomized clinical trial. JAMA Intern Med. 2015;175:1773-1782.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Do not prescribe selective serotonin reuptake inhibitors to improve depression and reduce cardiovascular risk in patients with congestive heart failure.
STRENGTH OF RECOMMENDATION
B: Based on one large randomized controlled trial.
Angermann CE, Gelbrick G, Störk S, et al, for the MOOD-HF Study Investigators and Committee Members. Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression. The MOOD-HF randomized clinical trial. JAMA. 2016;315:2683-2693.1
Does treating obstructive sleep apnea improve control of Tx-resistant hypertension?
EVIDENCE SUMMARY
A 2015 meta-analysis by Liu et al of 5 RCTs from 2010 to 2015 (446 patients, range 35 to 194 per trial) evaluated the effect of CPAP on 24-hour ambulatory BP in adults with OSA and moderate to severe resistant hypertension.1 Resistance was defined as hypertension despite optimally dosed 3-drug regimens or adequate control of BP achieved with a 4-drug regimen.
Treatment groups received at least 4 hours of CPAP nightly in addition to previously prescribed pharmacotherapy; control groups received either sham CPAP or no CPAP in addition to their regimen of antihypertensive medications. Reported drug regimens included primarily diuretics, angiotensin converting enzyme inhibitors, calcium channel blockers, and aldosterone receptor blockers.
Prestudy systolic BP ranged from 129 mm Hg to 148 mm Hg; diastolic BP ranged from 75 mm Hg to 88 mm Hg. Participants were followed from 2 to 6 months. Pooled data from all 5 studies indicated an overall decrease in mean 24-hour ambulatory systolic BP of 4.8 mm Hg (95% confidence interval [CI], −8.0 to −1.6) and an overall decrease in diastolic BP of 3 mm Hg (95% CI, −5.4 to −0.5) in CPAP-treated patients compared with controls.
An earlier meta-analysis also shows BP reductions with CPAP
Another 2015 meta-analysis by Hu et al of 7 RCTs from 2006 to 2014 evaluated the effect of CPAP on hypertension in 794 patients with OSA.2 Subgroup analysis of 4 of these studies (351 patients, range 35 to 194 per trial) evaluated outcomes in patients with a previous diagnosis of resistant hypertension. This subgroup had 3 trials in common with the Liu et al meta-analysis and one not included in that study. Two other studies in the Liu et al meta-analysis were published after the search dates of this meta-analysis.
Baseline BP wasn’t reported, and treatment resistance was not explicitly defined. Treatment groups received CPAP for 3 to 6 months in addition to their pharmacotherapy regimen, but duration of nightly use was not reported; control groups received only pharmacotherapy. The number and type of antihypertensive medications used was not reported.
Pooled data from the subgroup noted a significant difference in 24-hour mean ambulatory BPs in the CPAP group compared with controls. Systolic BP decreased by 3.9 mm Hg (95% CI, −6.6 to −1.2) and diastolic BP decreased by 3.7 mm Hg (95% CI, −5.2 to −2.1).
1. Liu L, Cao Q, Guo Z, et al. Continuous positive airway pressure in patients with obstructive sleep apnea and resistant hypertension: a meta-analysis of randomized controlled trials. J Clin Hypertens (Greenwich). 2016;18:153-158. (Epub 2015 Aug 17).
2. Hu X, Fan J, Chen S, et al. The role of continuous positive airway pressure in blood pressure control with obstructive sleep apnea and hypertension: a meta-analysis of randomized controlled trials. J Clin Hypertens (Greenwich). 2015;17: 215-222.
EVIDENCE SUMMARY
A 2015 meta-analysis by Liu et al of 5 RCTs from 2010 to 2015 (446 patients, range 35 to 194 per trial) evaluated the effect of CPAP on 24-hour ambulatory BP in adults with OSA and moderate to severe resistant hypertension.1 Resistance was defined as hypertension despite optimally dosed 3-drug regimens or adequate control of BP achieved with a 4-drug regimen.
Treatment groups received at least 4 hours of CPAP nightly in addition to previously prescribed pharmacotherapy; control groups received either sham CPAP or no CPAP in addition to their regimen of antihypertensive medications. Reported drug regimens included primarily diuretics, angiotensin converting enzyme inhibitors, calcium channel blockers, and aldosterone receptor blockers.
Prestudy systolic BP ranged from 129 mm Hg to 148 mm Hg; diastolic BP ranged from 75 mm Hg to 88 mm Hg. Participants were followed from 2 to 6 months. Pooled data from all 5 studies indicated an overall decrease in mean 24-hour ambulatory systolic BP of 4.8 mm Hg (95% confidence interval [CI], −8.0 to −1.6) and an overall decrease in diastolic BP of 3 mm Hg (95% CI, −5.4 to −0.5) in CPAP-treated patients compared with controls.
An earlier meta-analysis also shows BP reductions with CPAP
Another 2015 meta-analysis by Hu et al of 7 RCTs from 2006 to 2014 evaluated the effect of CPAP on hypertension in 794 patients with OSA.2 Subgroup analysis of 4 of these studies (351 patients, range 35 to 194 per trial) evaluated outcomes in patients with a previous diagnosis of resistant hypertension. This subgroup had 3 trials in common with the Liu et al meta-analysis and one not included in that study. Two other studies in the Liu et al meta-analysis were published after the search dates of this meta-analysis.
Baseline BP wasn’t reported, and treatment resistance was not explicitly defined. Treatment groups received CPAP for 3 to 6 months in addition to their pharmacotherapy regimen, but duration of nightly use was not reported; control groups received only pharmacotherapy. The number and type of antihypertensive medications used was not reported.
Pooled data from the subgroup noted a significant difference in 24-hour mean ambulatory BPs in the CPAP group compared with controls. Systolic BP decreased by 3.9 mm Hg (95% CI, −6.6 to −1.2) and diastolic BP decreased by 3.7 mm Hg (95% CI, −5.2 to −2.1).
EVIDENCE SUMMARY
A 2015 meta-analysis by Liu et al of 5 RCTs from 2010 to 2015 (446 patients, range 35 to 194 per trial) evaluated the effect of CPAP on 24-hour ambulatory BP in adults with OSA and moderate to severe resistant hypertension.1 Resistance was defined as hypertension despite optimally dosed 3-drug regimens or adequate control of BP achieved with a 4-drug regimen.
Treatment groups received at least 4 hours of CPAP nightly in addition to previously prescribed pharmacotherapy; control groups received either sham CPAP or no CPAP in addition to their regimen of antihypertensive medications. Reported drug regimens included primarily diuretics, angiotensin converting enzyme inhibitors, calcium channel blockers, and aldosterone receptor blockers.
Prestudy systolic BP ranged from 129 mm Hg to 148 mm Hg; diastolic BP ranged from 75 mm Hg to 88 mm Hg. Participants were followed from 2 to 6 months. Pooled data from all 5 studies indicated an overall decrease in mean 24-hour ambulatory systolic BP of 4.8 mm Hg (95% confidence interval [CI], −8.0 to −1.6) and an overall decrease in diastolic BP of 3 mm Hg (95% CI, −5.4 to −0.5) in CPAP-treated patients compared with controls.
An earlier meta-analysis also shows BP reductions with CPAP
Another 2015 meta-analysis by Hu et al of 7 RCTs from 2006 to 2014 evaluated the effect of CPAP on hypertension in 794 patients with OSA.2 Subgroup analysis of 4 of these studies (351 patients, range 35 to 194 per trial) evaluated outcomes in patients with a previous diagnosis of resistant hypertension. This subgroup had 3 trials in common with the Liu et al meta-analysis and one not included in that study. Two other studies in the Liu et al meta-analysis were published after the search dates of this meta-analysis.
Baseline BP wasn’t reported, and treatment resistance was not explicitly defined. Treatment groups received CPAP for 3 to 6 months in addition to their pharmacotherapy regimen, but duration of nightly use was not reported; control groups received only pharmacotherapy. The number and type of antihypertensive medications used was not reported.
Pooled data from the subgroup noted a significant difference in 24-hour mean ambulatory BPs in the CPAP group compared with controls. Systolic BP decreased by 3.9 mm Hg (95% CI, −6.6 to −1.2) and diastolic BP decreased by 3.7 mm Hg (95% CI, −5.2 to −2.1).
1. Liu L, Cao Q, Guo Z, et al. Continuous positive airway pressure in patients with obstructive sleep apnea and resistant hypertension: a meta-analysis of randomized controlled trials. J Clin Hypertens (Greenwich). 2016;18:153-158. (Epub 2015 Aug 17).
2. Hu X, Fan J, Chen S, et al. The role of continuous positive airway pressure in blood pressure control with obstructive sleep apnea and hypertension: a meta-analysis of randomized controlled trials. J Clin Hypertens (Greenwich). 2015;17: 215-222.
1. Liu L, Cao Q, Guo Z, et al. Continuous positive airway pressure in patients with obstructive sleep apnea and resistant hypertension: a meta-analysis of randomized controlled trials. J Clin Hypertens (Greenwich). 2016;18:153-158. (Epub 2015 Aug 17).
2. Hu X, Fan J, Chen S, et al. The role of continuous positive airway pressure in blood pressure control with obstructive sleep apnea and hypertension: a meta-analysis of randomized controlled trials. J Clin Hypertens (Greenwich). 2015;17: 215-222.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
Maybe. Treating obstructive sleep apnea (OSA) with continuous positive airway pressure (CPAP) is associated with decreases in both systolic and diastolic blood pressure (BP) of 3 to 5 mm Hg in patients with treatment-resistant hypertension. The clinical significance of this small decrease hasn’t been evaluated, however (strength of recommendation [SOR]: C, meta-analyses of randomized controlled trials [RCTs] with disease-oriented outcomes).