User login
Study: Two antithrombotic drugs better than 3
BARCELONA—A new study suggests a combination of 2 antithrombotic drugs is as effective as, but safer than, a 3-drug combination for patients with atrial fibrillation who have undergone percutaneous coronary intervention (PCI).
The combinations were similarly effective in preventing thromboembolic events, unplanned revascularization, or death.
But the 2-drug combination—dabigatran plus clopidogrel/ticagrelor—reduced the risk of major or clinically relevant non-major bleeding when compared to the 3-drug combination—warfarin plus aspirin and clopidogrel/ticagrelor.
These results were presented at ESC Congress 2017 (abstract 1920) and published in NEJM. The trial, known as RE-DUAL PCI, was sponsored by Boehringer Ingelheim, makers of dabigatran.
“When we treat patients who have atrial fibrillation and need a stent, we need to strike a difficult balance between risk of clotting and risk of bleeding,” said study author Christopher Cannon, MD, of Brigham and Women’s Hospital in Boston, Massachusetts.
“Our study finds that patients who received 2 anticlotting medications—including one of a newer class of drug—had fewer bleeding events without being more at risk for a stroke or other cardiac events.”
Patients and treatment
The RE-DUAL PCI trial included 2725 patients with atrial fibrillation who had undergone PCI. Patients were randomized to receive the 3-drug combination or the 2-drug combination. The 2-drug combination included 2 different doses of dabigatran—110 mg or 150 mg twice daily.
The researchers compared the 100-mg dual-therapy group (n=981) to the entire triple-therapy group (n=981), and they compared the 150-mg dual-therapy group (n=763) to a corresponding triple-therapy group (n=764).
The corresponding triple-therapy group only included patients who had been eligible for the 150-mg dual-therapy group, meaning this group did not include elderly patients outside the US.
Results
The primary endpoint was the first major or clinically relevant non-major bleeding event.
The incidence of this endpoint was 15.4% in the 110-mg dual-therapy group and 26.9% in the triple-therapy group (hazard ratio [HR]=0.52, P<0.001 for non-inferiority, P<0.001 for superiority).
The incidence was 20.2% in the 150-mg dual-therapy group and 25.7% in the corresponding triple-therapy group (HR=0.72, P<0.001 for non-inferiority).
A main secondary endpoint was a composite efficacy endpoint of thromboembolic events (myocardial infarction, stroke, or systemic embolism), unplanned revascularization (PCI or coronary-artery bypass grafting), or death.
The incidence of this endpoint was 15.2% in the 110-mg dual-therapy group and 13.4% in the triple-therapy group (HR=1.13, P=0.30). And it was 11.8% in the 150-mg dual-therapy group and 12.8% in the corresponding triple-therapy group (HR=0.89, P=0.44).
The incidence was 13.7% in the 2 dual-therapy groups combined and 13.4% in the triple-therapy group (HR=1.04. P=0.005 for non-inferiority).
Serious adverse events during treatment occurred in 42.7% of the patients in the 110-mg dual-therapy group, 39.6% in the 150-mg dual-therapy group, and 41.8% in the triple-therapy group.
Fatal serious adverse events occurred during treatment in 3.9%, 3.2%, and 4.3%, respectively.
“These data are very reassuring,” Dr Cannon said. “We now have new information to help select the right treatment for individual patients, which has been hard to date, and this study can help.” ![]()
BARCELONA—A new study suggests a combination of 2 antithrombotic drugs is as effective as, but safer than, a 3-drug combination for patients with atrial fibrillation who have undergone percutaneous coronary intervention (PCI).
The combinations were similarly effective in preventing thromboembolic events, unplanned revascularization, or death.
But the 2-drug combination—dabigatran plus clopidogrel/ticagrelor—reduced the risk of major or clinically relevant non-major bleeding when compared to the 3-drug combination—warfarin plus aspirin and clopidogrel/ticagrelor.
These results were presented at ESC Congress 2017 (abstract 1920) and published in NEJM. The trial, known as RE-DUAL PCI, was sponsored by Boehringer Ingelheim, makers of dabigatran.
“When we treat patients who have atrial fibrillation and need a stent, we need to strike a difficult balance between risk of clotting and risk of bleeding,” said study author Christopher Cannon, MD, of Brigham and Women’s Hospital in Boston, Massachusetts.
“Our study finds that patients who received 2 anticlotting medications—including one of a newer class of drug—had fewer bleeding events without being more at risk for a stroke or other cardiac events.”
Patients and treatment
The RE-DUAL PCI trial included 2725 patients with atrial fibrillation who had undergone PCI. Patients were randomized to receive the 3-drug combination or the 2-drug combination. The 2-drug combination included 2 different doses of dabigatran—110 mg or 150 mg twice daily.
The researchers compared the 100-mg dual-therapy group (n=981) to the entire triple-therapy group (n=981), and they compared the 150-mg dual-therapy group (n=763) to a corresponding triple-therapy group (n=764).
The corresponding triple-therapy group only included patients who had been eligible for the 150-mg dual-therapy group, meaning this group did not include elderly patients outside the US.
Results
The primary endpoint was the first major or clinically relevant non-major bleeding event.
The incidence of this endpoint was 15.4% in the 110-mg dual-therapy group and 26.9% in the triple-therapy group (hazard ratio [HR]=0.52, P<0.001 for non-inferiority, P<0.001 for superiority).
The incidence was 20.2% in the 150-mg dual-therapy group and 25.7% in the corresponding triple-therapy group (HR=0.72, P<0.001 for non-inferiority).
A main secondary endpoint was a composite efficacy endpoint of thromboembolic events (myocardial infarction, stroke, or systemic embolism), unplanned revascularization (PCI or coronary-artery bypass grafting), or death.
The incidence of this endpoint was 15.2% in the 110-mg dual-therapy group and 13.4% in the triple-therapy group (HR=1.13, P=0.30). And it was 11.8% in the 150-mg dual-therapy group and 12.8% in the corresponding triple-therapy group (HR=0.89, P=0.44).
The incidence was 13.7% in the 2 dual-therapy groups combined and 13.4% in the triple-therapy group (HR=1.04. P=0.005 for non-inferiority).
Serious adverse events during treatment occurred in 42.7% of the patients in the 110-mg dual-therapy group, 39.6% in the 150-mg dual-therapy group, and 41.8% in the triple-therapy group.
Fatal serious adverse events occurred during treatment in 3.9%, 3.2%, and 4.3%, respectively.
“These data are very reassuring,” Dr Cannon said. “We now have new information to help select the right treatment for individual patients, which has been hard to date, and this study can help.” ![]()
BARCELONA—A new study suggests a combination of 2 antithrombotic drugs is as effective as, but safer than, a 3-drug combination for patients with atrial fibrillation who have undergone percutaneous coronary intervention (PCI).
The combinations were similarly effective in preventing thromboembolic events, unplanned revascularization, or death.
But the 2-drug combination—dabigatran plus clopidogrel/ticagrelor—reduced the risk of major or clinically relevant non-major bleeding when compared to the 3-drug combination—warfarin plus aspirin and clopidogrel/ticagrelor.
These results were presented at ESC Congress 2017 (abstract 1920) and published in NEJM. The trial, known as RE-DUAL PCI, was sponsored by Boehringer Ingelheim, makers of dabigatran.
“When we treat patients who have atrial fibrillation and need a stent, we need to strike a difficult balance between risk of clotting and risk of bleeding,” said study author Christopher Cannon, MD, of Brigham and Women’s Hospital in Boston, Massachusetts.
“Our study finds that patients who received 2 anticlotting medications—including one of a newer class of drug—had fewer bleeding events without being more at risk for a stroke or other cardiac events.”
Patients and treatment
The RE-DUAL PCI trial included 2725 patients with atrial fibrillation who had undergone PCI. Patients were randomized to receive the 3-drug combination or the 2-drug combination. The 2-drug combination included 2 different doses of dabigatran—110 mg or 150 mg twice daily.
The researchers compared the 100-mg dual-therapy group (n=981) to the entire triple-therapy group (n=981), and they compared the 150-mg dual-therapy group (n=763) to a corresponding triple-therapy group (n=764).
The corresponding triple-therapy group only included patients who had been eligible for the 150-mg dual-therapy group, meaning this group did not include elderly patients outside the US.
Results
The primary endpoint was the first major or clinically relevant non-major bleeding event.
The incidence of this endpoint was 15.4% in the 110-mg dual-therapy group and 26.9% in the triple-therapy group (hazard ratio [HR]=0.52, P<0.001 for non-inferiority, P<0.001 for superiority).
The incidence was 20.2% in the 150-mg dual-therapy group and 25.7% in the corresponding triple-therapy group (HR=0.72, P<0.001 for non-inferiority).
A main secondary endpoint was a composite efficacy endpoint of thromboembolic events (myocardial infarction, stroke, or systemic embolism), unplanned revascularization (PCI or coronary-artery bypass grafting), or death.
The incidence of this endpoint was 15.2% in the 110-mg dual-therapy group and 13.4% in the triple-therapy group (HR=1.13, P=0.30). And it was 11.8% in the 150-mg dual-therapy group and 12.8% in the corresponding triple-therapy group (HR=0.89, P=0.44).
The incidence was 13.7% in the 2 dual-therapy groups combined and 13.4% in the triple-therapy group (HR=1.04. P=0.005 for non-inferiority).
Serious adverse events during treatment occurred in 42.7% of the patients in the 110-mg dual-therapy group, 39.6% in the 150-mg dual-therapy group, and 41.8% in the triple-therapy group.
Fatal serious adverse events occurred during treatment in 3.9%, 3.2%, and 4.3%, respectively.
“These data are very reassuring,” Dr Cannon said. “We now have new information to help select the right treatment for individual patients, which has been hard to date, and this study can help.” ![]()
CNS lymphoma responds to CAR T-cell therapy
Researchers have reported the first known case of central nervous system (CNS) lymphoma responding to chimeric antigen receptor (CAR) T-cell therapy.
The investigational CAR T-cell therapy JCAR017 induced complete remission of brain metastasis in a patient with refractory diffuse large B-cell lymphoma (DLBCL).
When a subcutaneous tumor began to recur 2 months after the patient received JCAR017 and a surgical biopsy was performed, the CAR T cells spontaneously re-expanded and the tumor again went into remission.
While the patient eventually relapsed and died more than a year after receiving JCAR017, the brain tumor never recurred.
“Brain involvement in DLBCL carries a grave prognosis, and the ability to induce a complete and durable response with conventional therapies is rare,” said Jeremy Abramson, MD, of Massachusetts General Hospital in Boston.
“In addition, all available CAR T-cell trials have excluded patients with central nervous system involvement. This result has implications not only for secondary DLBCL like this case but also for primary central nervous system lymphoma, for which treatment options are similarly limited after relapse and few patents are cured.”
Dr Abramson and his colleagues described this case in a letter to NEJM. The patient was involved in a trial of JCAR017, which was sponsored by Juno Therapeutics.
The patient was a 68-year-old woman with germinal center B-cell-like DLBCL with a BCL2 rearrangement and multiple copies of MYC and BCL6.
The patients’ disease was refractory to conventional chemotherapy and an 8/8 HLA-matched stem cell transplant. After she enrolled in a phase 1 trial of JCAR017, the patient was found to have a new lesion in the right temporal lobe of her brain.
One month after the patient received JCAR017—given after lymphodepletion with fludarabine and cyclophosphamide—imaging showed complete remission of the brain lesion.
The subcutaneous lesion that recurred 2 months later disappeared after the biopsy with no further treatment. Blood testing showed an expansion of CAR T cells that coincided with the tumor’s regression.
While re-expansion of CAR T cells has been reported in response to other immunotherapy drugs, this is the first report of such a response to a biopsy.
“Typically, the drugs we use to fight cancer and other diseases wear off over time,” Dr Abramson said. “This spontaneous re-expansion after biopsy highlights this therapy as something entirely different, a ‘living drug’ that can re-expand and proliferate in response to biologic stimuli.” ![]()
Researchers have reported the first known case of central nervous system (CNS) lymphoma responding to chimeric antigen receptor (CAR) T-cell therapy.
The investigational CAR T-cell therapy JCAR017 induced complete remission of brain metastasis in a patient with refractory diffuse large B-cell lymphoma (DLBCL).
When a subcutaneous tumor began to recur 2 months after the patient received JCAR017 and a surgical biopsy was performed, the CAR T cells spontaneously re-expanded and the tumor again went into remission.
While the patient eventually relapsed and died more than a year after receiving JCAR017, the brain tumor never recurred.
“Brain involvement in DLBCL carries a grave prognosis, and the ability to induce a complete and durable response with conventional therapies is rare,” said Jeremy Abramson, MD, of Massachusetts General Hospital in Boston.
“In addition, all available CAR T-cell trials have excluded patients with central nervous system involvement. This result has implications not only for secondary DLBCL like this case but also for primary central nervous system lymphoma, for which treatment options are similarly limited after relapse and few patents are cured.”
Dr Abramson and his colleagues described this case in a letter to NEJM. The patient was involved in a trial of JCAR017, which was sponsored by Juno Therapeutics.
The patient was a 68-year-old woman with germinal center B-cell-like DLBCL with a BCL2 rearrangement and multiple copies of MYC and BCL6.
The patients’ disease was refractory to conventional chemotherapy and an 8/8 HLA-matched stem cell transplant. After she enrolled in a phase 1 trial of JCAR017, the patient was found to have a new lesion in the right temporal lobe of her brain.
One month after the patient received JCAR017—given after lymphodepletion with fludarabine and cyclophosphamide—imaging showed complete remission of the brain lesion.
The subcutaneous lesion that recurred 2 months later disappeared after the biopsy with no further treatment. Blood testing showed an expansion of CAR T cells that coincided with the tumor’s regression.
While re-expansion of CAR T cells has been reported in response to other immunotherapy drugs, this is the first report of such a response to a biopsy.
“Typically, the drugs we use to fight cancer and other diseases wear off over time,” Dr Abramson said. “This spontaneous re-expansion after biopsy highlights this therapy as something entirely different, a ‘living drug’ that can re-expand and proliferate in response to biologic stimuli.” ![]()
Researchers have reported the first known case of central nervous system (CNS) lymphoma responding to chimeric antigen receptor (CAR) T-cell therapy.
The investigational CAR T-cell therapy JCAR017 induced complete remission of brain metastasis in a patient with refractory diffuse large B-cell lymphoma (DLBCL).
When a subcutaneous tumor began to recur 2 months after the patient received JCAR017 and a surgical biopsy was performed, the CAR T cells spontaneously re-expanded and the tumor again went into remission.
While the patient eventually relapsed and died more than a year after receiving JCAR017, the brain tumor never recurred.
“Brain involvement in DLBCL carries a grave prognosis, and the ability to induce a complete and durable response with conventional therapies is rare,” said Jeremy Abramson, MD, of Massachusetts General Hospital in Boston.
“In addition, all available CAR T-cell trials have excluded patients with central nervous system involvement. This result has implications not only for secondary DLBCL like this case but also for primary central nervous system lymphoma, for which treatment options are similarly limited after relapse and few patents are cured.”
Dr Abramson and his colleagues described this case in a letter to NEJM. The patient was involved in a trial of JCAR017, which was sponsored by Juno Therapeutics.
The patient was a 68-year-old woman with germinal center B-cell-like DLBCL with a BCL2 rearrangement and multiple copies of MYC and BCL6.
The patients’ disease was refractory to conventional chemotherapy and an 8/8 HLA-matched stem cell transplant. After she enrolled in a phase 1 trial of JCAR017, the patient was found to have a new lesion in the right temporal lobe of her brain.
One month after the patient received JCAR017—given after lymphodepletion with fludarabine and cyclophosphamide—imaging showed complete remission of the brain lesion.
The subcutaneous lesion that recurred 2 months later disappeared after the biopsy with no further treatment. Blood testing showed an expansion of CAR T cells that coincided with the tumor’s regression.
While re-expansion of CAR T cells has been reported in response to other immunotherapy drugs, this is the first report of such a response to a biopsy.
“Typically, the drugs we use to fight cancer and other diseases wear off over time,” Dr Abramson said. “This spontaneous re-expansion after biopsy highlights this therapy as something entirely different, a ‘living drug’ that can re-expand and proliferate in response to biologic stimuli.” ![]()
TKI granted priority review for newly diagnosed CML
The US Food and Drug Administration (FDA) has granted priority review to a supplemental new drug application (sNDA) for the tyrosine kinase inhibitor (TKI) bosutinib (Bosulif®).
If approved, the sNDA would expand the use of bosutinib to include patients with newly diagnosed, chronic phase, Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML).
Bosutinib is currently FDA-approved to treat adults with chronic, accelerated, or blast phase Ph+ CML with resistance or intolerance to prior therapy.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA plans to make a decision on the sNDA for bosutinib by the end of this year.
Meanwhile, the European Medicines Agency (EMA) has validated for review a type II variation application for bosutinib in patients with newly diagnosed, chronic phase Ph+ CML.
Bosutinib already has conditional marketing authorization in the European Economic Area for the treatment of adults with Ph+ CML who previously received at least 1 TKI and adults with Ph+ CML for whom imatinib, nilotinib, and dasatinib are not considered appropriate.
Phase 3 trial
The applications submitted to the EMA and FDA are both supported by early results from the phase 3 BFORE trial. Results from this trial were presented at the ASCO Annual Meeting in May.
In this ongoing study, researchers are comparing bosutinib and imatinib as first-line treatment of chronic phase CML.
As of the ASCO presentation, the trial had enrolled 536 patients who were randomized 1:1 to receive bosutinib (n=268) or imatinib (n=268).
The presentation included results in a modified intent-to-treat population of Ph+ patients with e13a2/e14a2 transcripts who had at least 12 months of follow-up. In this group, there were 246 patients in the bosutinib arm and 241 in the imatinib arm.
Most of the patients were still on therapy at the 12-month mark or beyond—78% in the bosutinib arm and 73.2% in the imatinib arm. The median treatment duration was 14.1 months and 13.8 months, respectively.
At 12 months, the rate of major molecular response was 47.2% in the bosutinib arm and 36.9% in the imatinib arm (P= 0.02). The rate of complete cytogenetic response was 77.2% and 66.4%, respectively (P<0.008).
One patient in the bosutinib arm and 4 in the imatinib arm discontinued treatment due to disease progression, while 12.7% and 8.7%, respectively, discontinued treatment due to drug-related toxicity.
Adverse events that were more common in the bosutinib arm than the imatinib arm included grade 3 or higher diarrhea (7.8% vs 0.8%), increased alanine levels (19% vs 1.5%), increased aspartate levels (9.7% vs 1.9%), cardiovascular events (3% vs 0.4%), and peripheral vascular events (1.5% vs 1.1%). Cerebrovascular events were more common with imatinib than bosutinib (0.4% and 0%, respectively). ![]()
The US Food and Drug Administration (FDA) has granted priority review to a supplemental new drug application (sNDA) for the tyrosine kinase inhibitor (TKI) bosutinib (Bosulif®).
If approved, the sNDA would expand the use of bosutinib to include patients with newly diagnosed, chronic phase, Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML).
Bosutinib is currently FDA-approved to treat adults with chronic, accelerated, or blast phase Ph+ CML with resistance or intolerance to prior therapy.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA plans to make a decision on the sNDA for bosutinib by the end of this year.
Meanwhile, the European Medicines Agency (EMA) has validated for review a type II variation application for bosutinib in patients with newly diagnosed, chronic phase Ph+ CML.
Bosutinib already has conditional marketing authorization in the European Economic Area for the treatment of adults with Ph+ CML who previously received at least 1 TKI and adults with Ph+ CML for whom imatinib, nilotinib, and dasatinib are not considered appropriate.
Phase 3 trial
The applications submitted to the EMA and FDA are both supported by early results from the phase 3 BFORE trial. Results from this trial were presented at the ASCO Annual Meeting in May.
In this ongoing study, researchers are comparing bosutinib and imatinib as first-line treatment of chronic phase CML.
As of the ASCO presentation, the trial had enrolled 536 patients who were randomized 1:1 to receive bosutinib (n=268) or imatinib (n=268).
The presentation included results in a modified intent-to-treat population of Ph+ patients with e13a2/e14a2 transcripts who had at least 12 months of follow-up. In this group, there were 246 patients in the bosutinib arm and 241 in the imatinib arm.
Most of the patients were still on therapy at the 12-month mark or beyond—78% in the bosutinib arm and 73.2% in the imatinib arm. The median treatment duration was 14.1 months and 13.8 months, respectively.
At 12 months, the rate of major molecular response was 47.2% in the bosutinib arm and 36.9% in the imatinib arm (P= 0.02). The rate of complete cytogenetic response was 77.2% and 66.4%, respectively (P<0.008).
One patient in the bosutinib arm and 4 in the imatinib arm discontinued treatment due to disease progression, while 12.7% and 8.7%, respectively, discontinued treatment due to drug-related toxicity.
Adverse events that were more common in the bosutinib arm than the imatinib arm included grade 3 or higher diarrhea (7.8% vs 0.8%), increased alanine levels (19% vs 1.5%), increased aspartate levels (9.7% vs 1.9%), cardiovascular events (3% vs 0.4%), and peripheral vascular events (1.5% vs 1.1%). Cerebrovascular events were more common with imatinib than bosutinib (0.4% and 0%, respectively). ![]()
The US Food and Drug Administration (FDA) has granted priority review to a supplemental new drug application (sNDA) for the tyrosine kinase inhibitor (TKI) bosutinib (Bosulif®).
If approved, the sNDA would expand the use of bosutinib to include patients with newly diagnosed, chronic phase, Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML).
Bosutinib is currently FDA-approved to treat adults with chronic, accelerated, or blast phase Ph+ CML with resistance or intolerance to prior therapy.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA plans to make a decision on the sNDA for bosutinib by the end of this year.
Meanwhile, the European Medicines Agency (EMA) has validated for review a type II variation application for bosutinib in patients with newly diagnosed, chronic phase Ph+ CML.
Bosutinib already has conditional marketing authorization in the European Economic Area for the treatment of adults with Ph+ CML who previously received at least 1 TKI and adults with Ph+ CML for whom imatinib, nilotinib, and dasatinib are not considered appropriate.
Phase 3 trial
The applications submitted to the EMA and FDA are both supported by early results from the phase 3 BFORE trial. Results from this trial were presented at the ASCO Annual Meeting in May.
In this ongoing study, researchers are comparing bosutinib and imatinib as first-line treatment of chronic phase CML.
As of the ASCO presentation, the trial had enrolled 536 patients who were randomized 1:1 to receive bosutinib (n=268) or imatinib (n=268).
The presentation included results in a modified intent-to-treat population of Ph+ patients with e13a2/e14a2 transcripts who had at least 12 months of follow-up. In this group, there were 246 patients in the bosutinib arm and 241 in the imatinib arm.
Most of the patients were still on therapy at the 12-month mark or beyond—78% in the bosutinib arm and 73.2% in the imatinib arm. The median treatment duration was 14.1 months and 13.8 months, respectively.
At 12 months, the rate of major molecular response was 47.2% in the bosutinib arm and 36.9% in the imatinib arm (P= 0.02). The rate of complete cytogenetic response was 77.2% and 66.4%, respectively (P<0.008).
One patient in the bosutinib arm and 4 in the imatinib arm discontinued treatment due to disease progression, while 12.7% and 8.7%, respectively, discontinued treatment due to drug-related toxicity.
Adverse events that were more common in the bosutinib arm than the imatinib arm included grade 3 or higher diarrhea (7.8% vs 0.8%), increased alanine levels (19% vs 1.5%), increased aspartate levels (9.7% vs 1.9%), cardiovascular events (3% vs 0.4%), and peripheral vascular events (1.5% vs 1.1%). Cerebrovascular events were more common with imatinib than bosutinib (0.4% and 0%, respectively). ![]()
HERDOO2 may guide duration of treatment for unprovoked VTE
Clinical Question: Can HERDOO2 guide anticoagulation cessation in women with unprovoked venous thromboembolism (VTE)?
Background: Patients with unprovoked VTE have increased recurrence rates after stopping anticoagulation, but no tools have been validated to identify low risk patients.
Setting: Forty-four referral centers in seven countries.
Synopsis: Of patients with unprovoked, symptomatic VTE, 2,747 were evaluated after receiving anticoagulation for 5-12 months. HERDOO2 was used to classify women as low (0-1 points) or high (equal to or greater than 2 points) risk categories. Men were considered high risk. Anticoagulation was stopped for low risk patients. Treatment of high risk patients was left to physician choice.
Overall, high risk patients who continued anticoagulation had a 1.6% recurrence rate. Low risk women who stopped anticoagulation had a 3% recurrence rate per patient year, but postmenopausal women aged 50 years or older had a rate of 5.7%. High risk patients who stopped anticoagulation had a 7.4% recurrence rate. This study included multiple sites, but only 44% of participants were women. HERDOO2 should be used cautiously in postmenopausal women aged 50 years or older and in nonwhite women.
Bottom Line: HERDOO2 may help guide the decision to stop anticoagulation in select low-risk women with unprovoked VTE.
Citation: Rodger MA, Gregoire LG, Anderson DR, et al. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: Multinational prospective cohort management study. BMJ. 2017 March;356:j1065.
Dr. Helfrich is an assistant professor in the University of Kentucky division of hospital medicine.
Clinical Question: Can HERDOO2 guide anticoagulation cessation in women with unprovoked venous thromboembolism (VTE)?
Background: Patients with unprovoked VTE have increased recurrence rates after stopping anticoagulation, but no tools have been validated to identify low risk patients.
Setting: Forty-four referral centers in seven countries.
Synopsis: Of patients with unprovoked, symptomatic VTE, 2,747 were evaluated after receiving anticoagulation for 5-12 months. HERDOO2 was used to classify women as low (0-1 points) or high (equal to or greater than 2 points) risk categories. Men were considered high risk. Anticoagulation was stopped for low risk patients. Treatment of high risk patients was left to physician choice.
Overall, high risk patients who continued anticoagulation had a 1.6% recurrence rate. Low risk women who stopped anticoagulation had a 3% recurrence rate per patient year, but postmenopausal women aged 50 years or older had a rate of 5.7%. High risk patients who stopped anticoagulation had a 7.4% recurrence rate. This study included multiple sites, but only 44% of participants were women. HERDOO2 should be used cautiously in postmenopausal women aged 50 years or older and in nonwhite women.
Bottom Line: HERDOO2 may help guide the decision to stop anticoagulation in select low-risk women with unprovoked VTE.
Citation: Rodger MA, Gregoire LG, Anderson DR, et al. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: Multinational prospective cohort management study. BMJ. 2017 March;356:j1065.
Dr. Helfrich is an assistant professor in the University of Kentucky division of hospital medicine.
Clinical Question: Can HERDOO2 guide anticoagulation cessation in women with unprovoked venous thromboembolism (VTE)?
Background: Patients with unprovoked VTE have increased recurrence rates after stopping anticoagulation, but no tools have been validated to identify low risk patients.
Setting: Forty-four referral centers in seven countries.
Synopsis: Of patients with unprovoked, symptomatic VTE, 2,747 were evaluated after receiving anticoagulation for 5-12 months. HERDOO2 was used to classify women as low (0-1 points) or high (equal to or greater than 2 points) risk categories. Men were considered high risk. Anticoagulation was stopped for low risk patients. Treatment of high risk patients was left to physician choice.
Overall, high risk patients who continued anticoagulation had a 1.6% recurrence rate. Low risk women who stopped anticoagulation had a 3% recurrence rate per patient year, but postmenopausal women aged 50 years or older had a rate of 5.7%. High risk patients who stopped anticoagulation had a 7.4% recurrence rate. This study included multiple sites, but only 44% of participants were women. HERDOO2 should be used cautiously in postmenopausal women aged 50 years or older and in nonwhite women.
Bottom Line: HERDOO2 may help guide the decision to stop anticoagulation in select low-risk women with unprovoked VTE.
Citation: Rodger MA, Gregoire LG, Anderson DR, et al. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: Multinational prospective cohort management study. BMJ. 2017 March;356:j1065.
Dr. Helfrich is an assistant professor in the University of Kentucky division of hospital medicine.
50 years of pediatric dermatology
The world in pediatric dermatology has changed in incredible ways since 1967. In fact, pediatric dermatology was not an organized specialty until years later! This article will look back at some of the history of pediatric dermatology, exploring how different the field was 50 years ago, and how it has evolved into the vibrant field that it is. By looking at some disease states, and differences in practice in relation to the care of dermatologic conditions in children both by pediatricians and dermatologists, we can see the tremendous evolution in our understanding and management of pediatric skin conditions, and perhaps gain insight into the future.
Pediatric dermatology was fairly “neonatal” 50 years ago, with only a few practitioners in the field. Recognizing that up to 30% of pediatric primary care visits include a skin-related problem, and that there was limited training about skin diseases among primary care practitioners and inconsistent training amongst dermatologists, there was a clinical need for establishing the subspecialty of pediatric dermatology. The first international symposium was held in Mexico City in October 1972, and with this meeting the International Society of Pediatric Dermatology was founded. The Society for Pediatric Dermatology (SPD) began in 1973, with Alvin Jacobs, MD, Samuel Weinberg, MD, Nancy Esterly, MD, Sidney Hurwitz, MD, William Weston, MD, and Coleman Jacobson, MD, as some of the initial “founding mothers and fathers.” The journal Pediatric Dermatology released its first issue in 1982 (35 years ago), and the American Academy of Pediatrics did not have a section of dermatology until 1986.
Pediatrics and dermatology: The interface
Many of the first generation of pediatric dermatologists trained as pediatricians prior to pursuing their dermatology work, with some being “assigned” dermatology as pediatric experts, while others did formal residencies in dermatology. This history is important, as pediatric dermatology was, and remains, integrated with pediatrics, even while training in dermatology residencies became standard practice. An important part of the development of the field has been the education of pediatricians and dermatologists by pediatric dermatologists, with a strong sensibility that improved training for both generalists and specialists about pediatric skin disease would yield better care for patients and families.
Initially, there were very few pediatric or dermatology programs in the United States that had pediatric dermatologists. Over the succeeding decades, this is now less common, although even now there are still dermatology and pediatric residency programs that do not have a pediatric dermatologist for either training or to serve their patients. The founding leaders of the SPD set a tone of collaboration nationally and internationally, reaching out to pediatric colleagues and dermatology associates from around the world, and establishing superb educational programs for the exchange of ideas, presentation of challenging cases, and promoting state of the art knowledge of the field. Through annual meetings of the SPD, conferences immediately preceding the American Academy of Dermatology annual meetings, the World Congress of Pediatric Dermatology, and other regional and international meetings, the field developed as the number of practitioners grew, and as the specialized published literature reflected new knowledge in diagnosis and therapy.
Building upon the history of collaboration and reflecting the maturation of the field with a desire to influence the breadth and quantity of research in pediatric dermatology, the Pediatric Dermatology Research Alliance (PeDRA) was formed in 2012. This organization was formed to promote and facilitate high quality collaborative clinical, translational, educational, and basic science research in pediatric dermatology with a vision to create sustainable, collaborative networks to better understand, prevent, treat, and cure dermatologic diseases in children. This network is now composed of over 230 members representing over 68 institutions from the United States and Canada, but including involvement globally from Mexico, Europe, and the Middle East.
Examples of changing perspectives: hemangiomas
A good way to look at evolution of the field is take a look at some of the similarities and differences in clinical practice in relation to common and uncommon disease states.
A great example is hemangiomas. Some of the first natural history studies on hemangiomas were done in the early 1960s, establishing that many lesions had a typical clinical course of fairly rapid growth, plateau, and involution over time. Of course, the identification of hemangiomas of infancy (or “HOI” in the trade), was confused with vascular malformations, and no one had recognized variant tumors that were distinct, such as rapidly involuting and noninvoluting congenital hemangiomas (RICHs or NICHs), tufted angiomas, and hemangioendotheliomas. PHACE syndrome (posterior fossa brain malformations) had yet to be described (that was done in 1996 by Ilona Frieden and her colleagues). For a time period, hemangiomas were treated with X-rays, before the negative impact of such radiation was acknowledged. For many years after that, even deforming and functionally significant lesions were “followed clinically” for natural involution, presumably a backlash from the radiation therapy interventions.
This story also reflects how organized research efforts helped with the evolution of knowledge and clinical care. The Hemangioma of Infancy Group was formed to take a collaborative approach to characterize and study hemangiomas and related tumors. Beginning with energetic, insightful pediatric dermatologists, and little funding, they changed our knowledge base of how hemangiomas present, the risk factors for their development and the characteristics and multiple organ findings associated with PHACE and other syndromic hemangiomas.
Procedural pediatric dermatology: Tremendous revolution in surgery and laser
The first generation of pediatric dermatologists were considered medical dermatologist specialists. And how important this specialty work was! Acne, atopic dermatitis, psoriasis, diaper and seborrheic dermatitis, and rare genetic syndromes, these conditions were a major part of the work of early pediatric dermatologists (and remain so now). What was not common was for pediatric dermatologists to have procedural or surgical practices, while this now is routinely part of the work of specialists in the field. How did this shift occur?
The fundamental shift began to occur with the introduction of the pulsed dye laser in 1989 and the publication of a seminal article in the New England Journal of Medicine (1989 Feb 16;320[7]:416-21) on its utility in treating port-wine stains in children with minimal scarring. Vascular lesions including port-wine stains were common, and pediatric dermatologists managed these patients for both diagnosis and medical management. Also, dermatology residencies at this time offered training in cutaneous surgery, excisions (including Mohs surgery) and repairs, and trainees in pediatric dermatology were “trained up” to high levels of expertise. As lasers were incorporated into dermatology residency work and practices, pediatric dermatologists had the exposure and skill to do this work. An added advantage was having the pediatric knowledge of how to handle children and adolescents in an age appropriate manner, and consideration of methods to minimize the pain and anxiety of procedures. Within a few years, pediatric dermatologists were at the forefront of the use of topical anesthetics (EMLA and liposomal lidocaine) and had general anesthesia privileges for laser and excisional surgery.
So while pediatric dermatologists still do “small procedures” every hour in most practices (cryotherapy for warts, cantharidin for molluscum, shave and punch biopsies), a subset now have extensive procedural practices, which in recent years has extended to pigment lesion lasers (to treat nevus of Ota), hair lasers (to treat perineal areas to prevent pilonidal cyst recurrence or to treat hirsutism), and combinations of lasers to treat hypertrophic, constrictive, and/or deforming scars).
Inflammatory skin disorders: Bread and butter ... and peanut butter?
The care of pediatric inflammatory skin disorders has evolved, but more slowly for some diseases than others. Acne vulgaris now is recognized as much more common under age 12 years than previously, presumably reflecting earlier pubertal changes in our preteens. Over the past 30 years, therapy has evolved with the use of topical retinoids (still underused by pediatricians, considered a “practice gap”), hormonal therapy with combined oral contraceptives, and oral isotretinoin, a powerful but highly effective systemic agent for severe and refractory acne. Specific pediatric guidelines came much later. Pediatric acne expert recommendations were formulated by the American Acne and Rosacea Society and endorsed by the American Academy of Pediatrics in 2013 (Pediatrics. 2013;131:S163-86). Over the past few years, there is a push by experts for more judicious use of antibiotics for acne (oral and topical) to minimize the emergence of bacterial resistance.
Psoriasis has been a condition that has been “behind the revolution,” in that no biologic agent was approved for pediatric psoriasis in the United States until several months ago, lagging behind Europe and elsewhere in the world by almost a decade. Adult psoriasis has been recognized to be associated with a broad set of comorbidities, including obesity and early heart disease, and there is now research on how children are at risk as well, and new recommendations on how to screen children with psoriasis. Moderate to severe psoriasis in adults is now tremendously controllable with biologic agents targeting TNF-alpha, IL 12/23, and IL-17. Etanercept has been approved for children with psoriasis aged 4 years and older, and other biologic agents are under study.
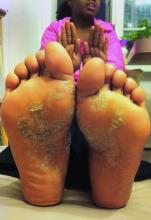
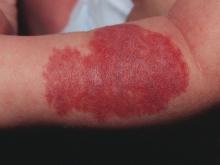
Atopic dermatitis now is ready for its revolution! AD has increased in prevalence from around 5% of the pediatric population 30-plus years ago to 10%-15%. Treatment of most individuals has remained the same over the decades: Good skin care, frequent moisturizers, topical corticosteroids for flares, management of infection if noted. The topical calcineurin inhibitors (TCIs) broadened the therapeutic approach when introduced in 2000 and 2001, but the boxed warning resulted in some practitioners minimizing their utilization of these useful agents.

It has been recognized for years that children with AD have higher risk of developing food allergies than children without AD. A changing understanding of how early food exposure may induce tolerance is changing the world of allergy and influencing the care of children with AD. This is where the peanut butter (or other processed peanut, such as “Bamba”) may be life saving. New guidelines have come from the National Institute of Allergy and Infectious Diseases recommending that infants with severe eczema (or egg allergy, or both) have introduction of age-appropriate peanut-containing food as early as 4-6 months of age to reduce the risk of development of peanut allergy. It is recommended that these infants undergo early evaluation for possible sensitization to peanut protein, with referral to allergists for skin prick tests or serum IgE screens (though if positive, referral to allergists is appropriate), and assess the safety of going ahead with early feeding. It is hoped that following these new guidelines can minimize the development of peanut allergy.
The future
Where will pediatric skin disease, or more importantly, skin health over a lifetime be in 50 years? Can we cure or prevent the consequences of our lethal and life altering genetic diseases such as epidermolysis bullosa or our neurocutaneous disorders? Will our new insights into birthmarks (they are mostly somatic mutations) allow us to form specific, personalized therapies to minimize their impact? Will we be using computers equipped with imaging devices and algorithms to assess our patients’ moles, papules, and nodules? Will our vaccines have wiped out warts, molluscum, and perhaps, acne? Will we have cured our inflammatory skin disorders, or perhaps prevented them by interventions in the neonatal period? No predictions will be offered here, other than that we can look forward to incredible changes for our future generations of health care practitioners, patients, and families.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Eichenfield has served as a consultant for Anacor/Pfizer and Regeneron/Sanofi. Email him at [email protected].
The world in pediatric dermatology has changed in incredible ways since 1967. In fact, pediatric dermatology was not an organized specialty until years later! This article will look back at some of the history of pediatric dermatology, exploring how different the field was 50 years ago, and how it has evolved into the vibrant field that it is. By looking at some disease states, and differences in practice in relation to the care of dermatologic conditions in children both by pediatricians and dermatologists, we can see the tremendous evolution in our understanding and management of pediatric skin conditions, and perhaps gain insight into the future.
Pediatric dermatology was fairly “neonatal” 50 years ago, with only a few practitioners in the field. Recognizing that up to 30% of pediatric primary care visits include a skin-related problem, and that there was limited training about skin diseases among primary care practitioners and inconsistent training amongst dermatologists, there was a clinical need for establishing the subspecialty of pediatric dermatology. The first international symposium was held in Mexico City in October 1972, and with this meeting the International Society of Pediatric Dermatology was founded. The Society for Pediatric Dermatology (SPD) began in 1973, with Alvin Jacobs, MD, Samuel Weinberg, MD, Nancy Esterly, MD, Sidney Hurwitz, MD, William Weston, MD, and Coleman Jacobson, MD, as some of the initial “founding mothers and fathers.” The journal Pediatric Dermatology released its first issue in 1982 (35 years ago), and the American Academy of Pediatrics did not have a section of dermatology until 1986.
Pediatrics and dermatology: The interface
Many of the first generation of pediatric dermatologists trained as pediatricians prior to pursuing their dermatology work, with some being “assigned” dermatology as pediatric experts, while others did formal residencies in dermatology. This history is important, as pediatric dermatology was, and remains, integrated with pediatrics, even while training in dermatology residencies became standard practice. An important part of the development of the field has been the education of pediatricians and dermatologists by pediatric dermatologists, with a strong sensibility that improved training for both generalists and specialists about pediatric skin disease would yield better care for patients and families.
Initially, there were very few pediatric or dermatology programs in the United States that had pediatric dermatologists. Over the succeeding decades, this is now less common, although even now there are still dermatology and pediatric residency programs that do not have a pediatric dermatologist for either training or to serve their patients. The founding leaders of the SPD set a tone of collaboration nationally and internationally, reaching out to pediatric colleagues and dermatology associates from around the world, and establishing superb educational programs for the exchange of ideas, presentation of challenging cases, and promoting state of the art knowledge of the field. Through annual meetings of the SPD, conferences immediately preceding the American Academy of Dermatology annual meetings, the World Congress of Pediatric Dermatology, and other regional and international meetings, the field developed as the number of practitioners grew, and as the specialized published literature reflected new knowledge in diagnosis and therapy.
Building upon the history of collaboration and reflecting the maturation of the field with a desire to influence the breadth and quantity of research in pediatric dermatology, the Pediatric Dermatology Research Alliance (PeDRA) was formed in 2012. This organization was formed to promote and facilitate high quality collaborative clinical, translational, educational, and basic science research in pediatric dermatology with a vision to create sustainable, collaborative networks to better understand, prevent, treat, and cure dermatologic diseases in children. This network is now composed of over 230 members representing over 68 institutions from the United States and Canada, but including involvement globally from Mexico, Europe, and the Middle East.
Examples of changing perspectives: hemangiomas
A good way to look at evolution of the field is take a look at some of the similarities and differences in clinical practice in relation to common and uncommon disease states.
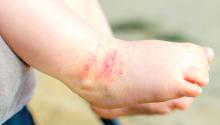
A great example is hemangiomas. Some of the first natural history studies on hemangiomas were done in the early 1960s, establishing that many lesions had a typical clinical course of fairly rapid growth, plateau, and involution over time. Of course, the identification of hemangiomas of infancy (or “HOI” in the trade), was confused with vascular malformations, and no one had recognized variant tumors that were distinct, such as rapidly involuting and noninvoluting congenital hemangiomas (RICHs or NICHs), tufted angiomas, and hemangioendotheliomas. PHACE syndrome (posterior fossa brain malformations) had yet to be described (that was done in 1996 by Ilona Frieden and her colleagues). For a time period, hemangiomas were treated with X-rays, before the negative impact of such radiation was acknowledged. For many years after that, even deforming and functionally significant lesions were “followed clinically” for natural involution, presumably a backlash from the radiation therapy interventions.
This story also reflects how organized research efforts helped with the evolution of knowledge and clinical care. The Hemangioma of Infancy Group was formed to take a collaborative approach to characterize and study hemangiomas and related tumors. Beginning with energetic, insightful pediatric dermatologists, and little funding, they changed our knowledge base of how hemangiomas present, the risk factors for their development and the characteristics and multiple organ findings associated with PHACE and other syndromic hemangiomas.
Procedural pediatric dermatology: Tremendous revolution in surgery and laser
The first generation of pediatric dermatologists were considered medical dermatologist specialists. And how important this specialty work was! Acne, atopic dermatitis, psoriasis, diaper and seborrheic dermatitis, and rare genetic syndromes, these conditions were a major part of the work of early pediatric dermatologists (and remain so now). What was not common was for pediatric dermatologists to have procedural or surgical practices, while this now is routinely part of the work of specialists in the field. How did this shift occur?
The fundamental shift began to occur with the introduction of the pulsed dye laser in 1989 and the publication of a seminal article in the New England Journal of Medicine (1989 Feb 16;320[7]:416-21) on its utility in treating port-wine stains in children with minimal scarring. Vascular lesions including port-wine stains were common, and pediatric dermatologists managed these patients for both diagnosis and medical management. Also, dermatology residencies at this time offered training in cutaneous surgery, excisions (including Mohs surgery) and repairs, and trainees in pediatric dermatology were “trained up” to high levels of expertise. As lasers were incorporated into dermatology residency work and practices, pediatric dermatologists had the exposure and skill to do this work. An added advantage was having the pediatric knowledge of how to handle children and adolescents in an age appropriate manner, and consideration of methods to minimize the pain and anxiety of procedures. Within a few years, pediatric dermatologists were at the forefront of the use of topical anesthetics (EMLA and liposomal lidocaine) and had general anesthesia privileges for laser and excisional surgery.
So while pediatric dermatologists still do “small procedures” every hour in most practices (cryotherapy for warts, cantharidin for molluscum, shave and punch biopsies), a subset now have extensive procedural practices, which in recent years has extended to pigment lesion lasers (to treat nevus of Ota), hair lasers (to treat perineal areas to prevent pilonidal cyst recurrence or to treat hirsutism), and combinations of lasers to treat hypertrophic, constrictive, and/or deforming scars).
Inflammatory skin disorders: Bread and butter ... and peanut butter?
The care of pediatric inflammatory skin disorders has evolved, but more slowly for some diseases than others. Acne vulgaris now is recognized as much more common under age 12 years than previously, presumably reflecting earlier pubertal changes in our preteens. Over the past 30 years, therapy has evolved with the use of topical retinoids (still underused by pediatricians, considered a “practice gap”), hormonal therapy with combined oral contraceptives, and oral isotretinoin, a powerful but highly effective systemic agent for severe and refractory acne. Specific pediatric guidelines came much later. Pediatric acne expert recommendations were formulated by the American Acne and Rosacea Society and endorsed by the American Academy of Pediatrics in 2013 (Pediatrics. 2013;131:S163-86). Over the past few years, there is a push by experts for more judicious use of antibiotics for acne (oral and topical) to minimize the emergence of bacterial resistance.
Psoriasis has been a condition that has been “behind the revolution,” in that no biologic agent was approved for pediatric psoriasis in the United States until several months ago, lagging behind Europe and elsewhere in the world by almost a decade. Adult psoriasis has been recognized to be associated with a broad set of comorbidities, including obesity and early heart disease, and there is now research on how children are at risk as well, and new recommendations on how to screen children with psoriasis. Moderate to severe psoriasis in adults is now tremendously controllable with biologic agents targeting TNF-alpha, IL 12/23, and IL-17. Etanercept has been approved for children with psoriasis aged 4 years and older, and other biologic agents are under study.
Atopic dermatitis now is ready for its revolution! AD has increased in prevalence from around 5% of the pediatric population 30-plus years ago to 10%-15%. Treatment of most individuals has remained the same over the decades: Good skin care, frequent moisturizers, topical corticosteroids for flares, management of infection if noted. The topical calcineurin inhibitors (TCIs) broadened the therapeutic approach when introduced in 2000 and 2001, but the boxed warning resulted in some practitioners minimizing their utilization of these useful agents.

It has been recognized for years that children with AD have higher risk of developing food allergies than children without AD. A changing understanding of how early food exposure may induce tolerance is changing the world of allergy and influencing the care of children with AD. This is where the peanut butter (or other processed peanut, such as “Bamba”) may be life saving. New guidelines have come from the National Institute of Allergy and Infectious Diseases recommending that infants with severe eczema (or egg allergy, or both) have introduction of age-appropriate peanut-containing food as early as 4-6 months of age to reduce the risk of development of peanut allergy. It is recommended that these infants undergo early evaluation for possible sensitization to peanut protein, with referral to allergists for skin prick tests or serum IgE screens (though if positive, referral to allergists is appropriate), and assess the safety of going ahead with early feeding. It is hoped that following these new guidelines can minimize the development of peanut allergy.
The future
Where will pediatric skin disease, or more importantly, skin health over a lifetime be in 50 years? Can we cure or prevent the consequences of our lethal and life altering genetic diseases such as epidermolysis bullosa or our neurocutaneous disorders? Will our new insights into birthmarks (they are mostly somatic mutations) allow us to form specific, personalized therapies to minimize their impact? Will we be using computers equipped with imaging devices and algorithms to assess our patients’ moles, papules, and nodules? Will our vaccines have wiped out warts, molluscum, and perhaps, acne? Will we have cured our inflammatory skin disorders, or perhaps prevented them by interventions in the neonatal period? No predictions will be offered here, other than that we can look forward to incredible changes for our future generations of health care practitioners, patients, and families.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Eichenfield has served as a consultant for Anacor/Pfizer and Regeneron/Sanofi. Email him at [email protected].
The world in pediatric dermatology has changed in incredible ways since 1967. In fact, pediatric dermatology was not an organized specialty until years later! This article will look back at some of the history of pediatric dermatology, exploring how different the field was 50 years ago, and how it has evolved into the vibrant field that it is. By looking at some disease states, and differences in practice in relation to the care of dermatologic conditions in children both by pediatricians and dermatologists, we can see the tremendous evolution in our understanding and management of pediatric skin conditions, and perhaps gain insight into the future.
Pediatric dermatology was fairly “neonatal” 50 years ago, with only a few practitioners in the field. Recognizing that up to 30% of pediatric primary care visits include a skin-related problem, and that there was limited training about skin diseases among primary care practitioners and inconsistent training amongst dermatologists, there was a clinical need for establishing the subspecialty of pediatric dermatology. The first international symposium was held in Mexico City in October 1972, and with this meeting the International Society of Pediatric Dermatology was founded. The Society for Pediatric Dermatology (SPD) began in 1973, with Alvin Jacobs, MD, Samuel Weinberg, MD, Nancy Esterly, MD, Sidney Hurwitz, MD, William Weston, MD, and Coleman Jacobson, MD, as some of the initial “founding mothers and fathers.” The journal Pediatric Dermatology released its first issue in 1982 (35 years ago), and the American Academy of Pediatrics did not have a section of dermatology until 1986.
Pediatrics and dermatology: The interface
Many of the first generation of pediatric dermatologists trained as pediatricians prior to pursuing their dermatology work, with some being “assigned” dermatology as pediatric experts, while others did formal residencies in dermatology. This history is important, as pediatric dermatology was, and remains, integrated with pediatrics, even while training in dermatology residencies became standard practice. An important part of the development of the field has been the education of pediatricians and dermatologists by pediatric dermatologists, with a strong sensibility that improved training for both generalists and specialists about pediatric skin disease would yield better care for patients and families.
Initially, there were very few pediatric or dermatology programs in the United States that had pediatric dermatologists. Over the succeeding decades, this is now less common, although even now there are still dermatology and pediatric residency programs that do not have a pediatric dermatologist for either training or to serve their patients. The founding leaders of the SPD set a tone of collaboration nationally and internationally, reaching out to pediatric colleagues and dermatology associates from around the world, and establishing superb educational programs for the exchange of ideas, presentation of challenging cases, and promoting state of the art knowledge of the field. Through annual meetings of the SPD, conferences immediately preceding the American Academy of Dermatology annual meetings, the World Congress of Pediatric Dermatology, and other regional and international meetings, the field developed as the number of practitioners grew, and as the specialized published literature reflected new knowledge in diagnosis and therapy.
Building upon the history of collaboration and reflecting the maturation of the field with a desire to influence the breadth and quantity of research in pediatric dermatology, the Pediatric Dermatology Research Alliance (PeDRA) was formed in 2012. This organization was formed to promote and facilitate high quality collaborative clinical, translational, educational, and basic science research in pediatric dermatology with a vision to create sustainable, collaborative networks to better understand, prevent, treat, and cure dermatologic diseases in children. This network is now composed of over 230 members representing over 68 institutions from the United States and Canada, but including involvement globally from Mexico, Europe, and the Middle East.
Examples of changing perspectives: hemangiomas
A good way to look at evolution of the field is take a look at some of the similarities and differences in clinical practice in relation to common and uncommon disease states.
A great example is hemangiomas. Some of the first natural history studies on hemangiomas were done in the early 1960s, establishing that many lesions had a typical clinical course of fairly rapid growth, plateau, and involution over time. Of course, the identification of hemangiomas of infancy (or “HOI” in the trade), was confused with vascular malformations, and no one had recognized variant tumors that were distinct, such as rapidly involuting and noninvoluting congenital hemangiomas (RICHs or NICHs), tufted angiomas, and hemangioendotheliomas. PHACE syndrome (posterior fossa brain malformations) had yet to be described (that was done in 1996 by Ilona Frieden and her colleagues). For a time period, hemangiomas were treated with X-rays, before the negative impact of such radiation was acknowledged. For many years after that, even deforming and functionally significant lesions were “followed clinically” for natural involution, presumably a backlash from the radiation therapy interventions.
This story also reflects how organized research efforts helped with the evolution of knowledge and clinical care. The Hemangioma of Infancy Group was formed to take a collaborative approach to characterize and study hemangiomas and related tumors. Beginning with energetic, insightful pediatric dermatologists, and little funding, they changed our knowledge base of how hemangiomas present, the risk factors for their development and the characteristics and multiple organ findings associated with PHACE and other syndromic hemangiomas.
Procedural pediatric dermatology: Tremendous revolution in surgery and laser
The first generation of pediatric dermatologists were considered medical dermatologist specialists. And how important this specialty work was! Acne, atopic dermatitis, psoriasis, diaper and seborrheic dermatitis, and rare genetic syndromes, these conditions were a major part of the work of early pediatric dermatologists (and remain so now). What was not common was for pediatric dermatologists to have procedural or surgical practices, while this now is routinely part of the work of specialists in the field. How did this shift occur?
The fundamental shift began to occur with the introduction of the pulsed dye laser in 1989 and the publication of a seminal article in the New England Journal of Medicine (1989 Feb 16;320[7]:416-21) on its utility in treating port-wine stains in children with minimal scarring. Vascular lesions including port-wine stains were common, and pediatric dermatologists managed these patients for both diagnosis and medical management. Also, dermatology residencies at this time offered training in cutaneous surgery, excisions (including Mohs surgery) and repairs, and trainees in pediatric dermatology were “trained up” to high levels of expertise. As lasers were incorporated into dermatology residency work and practices, pediatric dermatologists had the exposure and skill to do this work. An added advantage was having the pediatric knowledge of how to handle children and adolescents in an age appropriate manner, and consideration of methods to minimize the pain and anxiety of procedures. Within a few years, pediatric dermatologists were at the forefront of the use of topical anesthetics (EMLA and liposomal lidocaine) and had general anesthesia privileges for laser and excisional surgery.
So while pediatric dermatologists still do “small procedures” every hour in most practices (cryotherapy for warts, cantharidin for molluscum, shave and punch biopsies), a subset now have extensive procedural practices, which in recent years has extended to pigment lesion lasers (to treat nevus of Ota), hair lasers (to treat perineal areas to prevent pilonidal cyst recurrence or to treat hirsutism), and combinations of lasers to treat hypertrophic, constrictive, and/or deforming scars).
Inflammatory skin disorders: Bread and butter ... and peanut butter?
The care of pediatric inflammatory skin disorders has evolved, but more slowly for some diseases than others. Acne vulgaris now is recognized as much more common under age 12 years than previously, presumably reflecting earlier pubertal changes in our preteens. Over the past 30 years, therapy has evolved with the use of topical retinoids (still underused by pediatricians, considered a “practice gap”), hormonal therapy with combined oral contraceptives, and oral isotretinoin, a powerful but highly effective systemic agent for severe and refractory acne. Specific pediatric guidelines came much later. Pediatric acne expert recommendations were formulated by the American Acne and Rosacea Society and endorsed by the American Academy of Pediatrics in 2013 (Pediatrics. 2013;131:S163-86). Over the past few years, there is a push by experts for more judicious use of antibiotics for acne (oral and topical) to minimize the emergence of bacterial resistance.
Psoriasis has been a condition that has been “behind the revolution,” in that no biologic agent was approved for pediatric psoriasis in the United States until several months ago, lagging behind Europe and elsewhere in the world by almost a decade. Adult psoriasis has been recognized to be associated with a broad set of comorbidities, including obesity and early heart disease, and there is now research on how children are at risk as well, and new recommendations on how to screen children with psoriasis. Moderate to severe psoriasis in adults is now tremendously controllable with biologic agents targeting TNF-alpha, IL 12/23, and IL-17. Etanercept has been approved for children with psoriasis aged 4 years and older, and other biologic agents are under study.
Atopic dermatitis now is ready for its revolution! AD has increased in prevalence from around 5% of the pediatric population 30-plus years ago to 10%-15%. Treatment of most individuals has remained the same over the decades: Good skin care, frequent moisturizers, topical corticosteroids for flares, management of infection if noted. The topical calcineurin inhibitors (TCIs) broadened the therapeutic approach when introduced in 2000 and 2001, but the boxed warning resulted in some practitioners minimizing their utilization of these useful agents.

It has been recognized for years that children with AD have higher risk of developing food allergies than children without AD. A changing understanding of how early food exposure may induce tolerance is changing the world of allergy and influencing the care of children with AD. This is where the peanut butter (or other processed peanut, such as “Bamba”) may be life saving. New guidelines have come from the National Institute of Allergy and Infectious Diseases recommending that infants with severe eczema (or egg allergy, or both) have introduction of age-appropriate peanut-containing food as early as 4-6 months of age to reduce the risk of development of peanut allergy. It is recommended that these infants undergo early evaluation for possible sensitization to peanut protein, with referral to allergists for skin prick tests or serum IgE screens (though if positive, referral to allergists is appropriate), and assess the safety of going ahead with early feeding. It is hoped that following these new guidelines can minimize the development of peanut allergy.
The future
Where will pediatric skin disease, or more importantly, skin health over a lifetime be in 50 years? Can we cure or prevent the consequences of our lethal and life altering genetic diseases such as epidermolysis bullosa or our neurocutaneous disorders? Will our new insights into birthmarks (they are mostly somatic mutations) allow us to form specific, personalized therapies to minimize their impact? Will we be using computers equipped with imaging devices and algorithms to assess our patients’ moles, papules, and nodules? Will our vaccines have wiped out warts, molluscum, and perhaps, acne? Will we have cured our inflammatory skin disorders, or perhaps prevented them by interventions in the neonatal period? No predictions will be offered here, other than that we can look forward to incredible changes for our future generations of health care practitioners, patients, and families.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Eichenfield has served as a consultant for Anacor/Pfizer and Regeneron/Sanofi. Email him at [email protected].
Suture found in bladder after hysterectomy
Suture found in bladder after hysterectomy
A 40-year-old woman underwent a hysterectomy due to dysmenorrhea. Despite the presence of blood in the catheter bag after the procedure, the surgeon did not consult a urologist or perform a cystoscopy. Later, when the patient reported urinary retention, urinary leakage, and dyspareunia, a urologist performed a cystoscopy and discovered a suture in the bladder wall and a vesicovaginal fistula.
PATIENTS' CLAIM:
During the procedure, the gynecologic surgeon inadvertently placed a suture in the bladder wall. The presence of blood in the Foley catheter required an immediate urology consult and cystoscopy, during which the presence of the errant suture would have been discovered. Repair surgery then would have prevented subsequent injuries.
PHYSICIANS' DEFENSE:
The surgeon used reasonable judgment, as there were explanations for the blood in the catheter due to a difficult catheter placement and lysis of bladder adhesions.
VERDICT:
A Michigan defense verdict was returned.
Related article:
How to avoid intestinal and urinary tract injuries during gynecologic laparoscopy
Bowel injury during tubal ligation
A 40-year-old woman underwent laparoscopic tubal ligation using cauterization at an outpatient surgery center. Two hours after the procedure, her BP began to drop. She was promptly transferred to a hospital and underwent emergency surgery that revealed a bowel injury. Part of the patient’s small intestine was resected.
PATIENTS' CLAIM:
The gynecologic surgeon committed a medical error when she injured the bowel during trocar insertion.
DEFENDANTS' DEFENSE:
The bowel injury was a known complication of the surgery.
VERDICT:
A Louisiana defense verdict was returned.
Related article:
How to avoid major vessel injury during gynecologic laparoscopy
Colon injured twice: $1M settlement
A 59-year-old woman underwent laparoscopic total hysterectomy and salpingectomy. Her history included an umbilical hernia repair.
Two days after surgery, the patient experienced abdominal pain, chills, abdominal distention, and a foul-smelling discharge from her umbilical suture site. She went to the emergency department where a computed tomography scan revealed 2 injuries in the bowel. Emergency laparotomy included transverse colon resection and right colon colostomy with Hartmann’s pouch. She wore an ostomy bag for 8 months. She developed an infection because of the colostomy and also required operations to resolve a bowel obstruction and repair incisional hernias.
PATIENTS' CLAIM:
The gynecologic surgeon was negligent when performing the surgery. When he inserted the Veress needle and trocar through the patient’s umbilicus, the transverse colon was injured twice with a 3-cm anterior tear and a 1-cm posterior laceration. The injuries were not discovered during the procedure. He should have been more careful knowing that she had undergone prior umbilical hernia surgery.
PHYSICIANS' DEFENSE:
The case was settled before the trial began.
VERDICT:
A $1 million Virginia settlement was reached.
Chronic pain after sling procedure: $2M verdict
A 63-year-old woman reported urinary incontinence to her gynecologist, who performed a transobturator midurethral sling procedure. After surgery, the patient experienced pelvic pain, urinary urgency, intermittent incontinence, and dyspareunia. She returned to the gynecologist twice. He performed a cystoscopy after the second visit but found nothing wrong.
The patient sought a second opinion. A gynecologic surgeon found a large mass in the patient’s bladder consisting of a crystallized piece of tape that had been used to secure the sling supporting the bladder. The mass was removed and the patient reported that, although surgery alleviated many symptoms, she was not pain-free.
PATIENTS' CLAIM:
The gynecologist negligently inserted the end of the sling through one wall of her bladder and failed to detect the malpositioning during surgery or later. He failed to diagnose and treat bladder stones that resulted from the sling’s malpositioning. He failed to perform a cystoscopy when she first reported symptoms and improperly performed cystoscopy at the second visit.
DEFENDANTS' DEFENSE:
There was no negligence on the part of the gynecologist. The patient did not report ongoing symptoms until 1 year after sling insertion.
VERDICT:
A $2 million Pennsylvania verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Suture found in bladder after hysterectomy
A 40-year-old woman underwent a hysterectomy due to dysmenorrhea. Despite the presence of blood in the catheter bag after the procedure, the surgeon did not consult a urologist or perform a cystoscopy. Later, when the patient reported urinary retention, urinary leakage, and dyspareunia, a urologist performed a cystoscopy and discovered a suture in the bladder wall and a vesicovaginal fistula.
PATIENTS' CLAIM:
During the procedure, the gynecologic surgeon inadvertently placed a suture in the bladder wall. The presence of blood in the Foley catheter required an immediate urology consult and cystoscopy, during which the presence of the errant suture would have been discovered. Repair surgery then would have prevented subsequent injuries.
PHYSICIANS' DEFENSE:
The surgeon used reasonable judgment, as there were explanations for the blood in the catheter due to a difficult catheter placement and lysis of bladder adhesions.
VERDICT:
A Michigan defense verdict was returned.
Related article:
How to avoid intestinal and urinary tract injuries during gynecologic laparoscopy
Bowel injury during tubal ligation
A 40-year-old woman underwent laparoscopic tubal ligation using cauterization at an outpatient surgery center. Two hours after the procedure, her BP began to drop. She was promptly transferred to a hospital and underwent emergency surgery that revealed a bowel injury. Part of the patient’s small intestine was resected.
PATIENTS' CLAIM:
The gynecologic surgeon committed a medical error when she injured the bowel during trocar insertion.
DEFENDANTS' DEFENSE:
The bowel injury was a known complication of the surgery.
VERDICT:
A Louisiana defense verdict was returned.
Related article:
How to avoid major vessel injury during gynecologic laparoscopy
Colon injured twice: $1M settlement
A 59-year-old woman underwent laparoscopic total hysterectomy and salpingectomy. Her history included an umbilical hernia repair.
Two days after surgery, the patient experienced abdominal pain, chills, abdominal distention, and a foul-smelling discharge from her umbilical suture site. She went to the emergency department where a computed tomography scan revealed 2 injuries in the bowel. Emergency laparotomy included transverse colon resection and right colon colostomy with Hartmann’s pouch. She wore an ostomy bag for 8 months. She developed an infection because of the colostomy and also required operations to resolve a bowel obstruction and repair incisional hernias.
PATIENTS' CLAIM:
The gynecologic surgeon was negligent when performing the surgery. When he inserted the Veress needle and trocar through the patient’s umbilicus, the transverse colon was injured twice with a 3-cm anterior tear and a 1-cm posterior laceration. The injuries were not discovered during the procedure. He should have been more careful knowing that she had undergone prior umbilical hernia surgery.
PHYSICIANS' DEFENSE:
The case was settled before the trial began.
VERDICT:
A $1 million Virginia settlement was reached.
Chronic pain after sling procedure: $2M verdict
A 63-year-old woman reported urinary incontinence to her gynecologist, who performed a transobturator midurethral sling procedure. After surgery, the patient experienced pelvic pain, urinary urgency, intermittent incontinence, and dyspareunia. She returned to the gynecologist twice. He performed a cystoscopy after the second visit but found nothing wrong.
The patient sought a second opinion. A gynecologic surgeon found a large mass in the patient’s bladder consisting of a crystallized piece of tape that had been used to secure the sling supporting the bladder. The mass was removed and the patient reported that, although surgery alleviated many symptoms, she was not pain-free.
PATIENTS' CLAIM:
The gynecologist negligently inserted the end of the sling through one wall of her bladder and failed to detect the malpositioning during surgery or later. He failed to diagnose and treat bladder stones that resulted from the sling’s malpositioning. He failed to perform a cystoscopy when she first reported symptoms and improperly performed cystoscopy at the second visit.
DEFENDANTS' DEFENSE:
There was no negligence on the part of the gynecologist. The patient did not report ongoing symptoms until 1 year after sling insertion.
VERDICT:
A $2 million Pennsylvania verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Suture found in bladder after hysterectomy
A 40-year-old woman underwent a hysterectomy due to dysmenorrhea. Despite the presence of blood in the catheter bag after the procedure, the surgeon did not consult a urologist or perform a cystoscopy. Later, when the patient reported urinary retention, urinary leakage, and dyspareunia, a urologist performed a cystoscopy and discovered a suture in the bladder wall and a vesicovaginal fistula.
PATIENTS' CLAIM:
During the procedure, the gynecologic surgeon inadvertently placed a suture in the bladder wall. The presence of blood in the Foley catheter required an immediate urology consult and cystoscopy, during which the presence of the errant suture would have been discovered. Repair surgery then would have prevented subsequent injuries.
PHYSICIANS' DEFENSE:
The surgeon used reasonable judgment, as there were explanations for the blood in the catheter due to a difficult catheter placement and lysis of bladder adhesions.
VERDICT:
A Michigan defense verdict was returned.
Related article:
How to avoid intestinal and urinary tract injuries during gynecologic laparoscopy
Bowel injury during tubal ligation
A 40-year-old woman underwent laparoscopic tubal ligation using cauterization at an outpatient surgery center. Two hours after the procedure, her BP began to drop. She was promptly transferred to a hospital and underwent emergency surgery that revealed a bowel injury. Part of the patient’s small intestine was resected.
PATIENTS' CLAIM:
The gynecologic surgeon committed a medical error when she injured the bowel during trocar insertion.
DEFENDANTS' DEFENSE:
The bowel injury was a known complication of the surgery.
VERDICT:
A Louisiana defense verdict was returned.
Related article:
How to avoid major vessel injury during gynecologic laparoscopy
Colon injured twice: $1M settlement
A 59-year-old woman underwent laparoscopic total hysterectomy and salpingectomy. Her history included an umbilical hernia repair.
Two days after surgery, the patient experienced abdominal pain, chills, abdominal distention, and a foul-smelling discharge from her umbilical suture site. She went to the emergency department where a computed tomography scan revealed 2 injuries in the bowel. Emergency laparotomy included transverse colon resection and right colon colostomy with Hartmann’s pouch. She wore an ostomy bag for 8 months. She developed an infection because of the colostomy and also required operations to resolve a bowel obstruction and repair incisional hernias.
PATIENTS' CLAIM:
The gynecologic surgeon was negligent when performing the surgery. When he inserted the Veress needle and trocar through the patient’s umbilicus, the transverse colon was injured twice with a 3-cm anterior tear and a 1-cm posterior laceration. The injuries were not discovered during the procedure. He should have been more careful knowing that she had undergone prior umbilical hernia surgery.
PHYSICIANS' DEFENSE:
The case was settled before the trial began.
VERDICT:
A $1 million Virginia settlement was reached.
Chronic pain after sling procedure: $2M verdict
A 63-year-old woman reported urinary incontinence to her gynecologist, who performed a transobturator midurethral sling procedure. After surgery, the patient experienced pelvic pain, urinary urgency, intermittent incontinence, and dyspareunia. She returned to the gynecologist twice. He performed a cystoscopy after the second visit but found nothing wrong.
The patient sought a second opinion. A gynecologic surgeon found a large mass in the patient’s bladder consisting of a crystallized piece of tape that had been used to secure the sling supporting the bladder. The mass was removed and the patient reported that, although surgery alleviated many symptoms, she was not pain-free.
PATIENTS' CLAIM:
The gynecologist negligently inserted the end of the sling through one wall of her bladder and failed to detect the malpositioning during surgery or later. He failed to diagnose and treat bladder stones that resulted from the sling’s malpositioning. He failed to perform a cystoscopy when she first reported symptoms and improperly performed cystoscopy at the second visit.
DEFENDANTS' DEFENSE:
There was no negligence on the part of the gynecologist. The patient did not report ongoing symptoms until 1 year after sling insertion.
VERDICT:
A $2 million Pennsylvania verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Premature birth after preeclampsia: $23.1M verdict
Premature birth after preeclampsia: $23.1M verdict
When a woman saw her ObGyn on August 16 at 24 weeks’ gestation, test results showed proteinuria and high blood pressure (BP). The following day, she was hospitalized for a 24-hour urine test and BP evaluation supervised by an on-call ObGyn and her ObGyn. Test results confirmed preeclampsia. She was released from the hospital. A few days later, she was found to have continued high BP and increased proteinuria, and restricted fetal growth was detected. On August 29 at 26 weeks’ gestation, the baby girl was born with severe cystic periventricular leukomalacia by emergency cesarean delivery. She cannot perform basic tasks and will need 24-hour care for the rest of her life.
PARENTS' CLAIM:
The hospital staff and 2 ObGyns failed to timely diagnose and treat preeclampsia. The treating ObGyn neither prescribed medication to treat preeclampsia nor administered antenatal corticosteroids to enhance fetal lung and brain development, both of which should have been started on August 17. Hospital health care providers failed to transfer her to a Level III facility equipped to handle a premature birth of less than 33 weeks’ gestation.
DEFENDANTS' DEFENSE:
The hospital and ObGyn denied negligence.
VERDICT:
Prior to trial, the mother settled with the on-call ObGyn for an undisclosed amount. A $23.15 million Florida verdict was returned, apportioning 70% liability to the treating ObGyn and 30% to the hospital.
Related article:
For the management of labor, patience is a virtue
Shoulder dystocia, paralysis: $950,000 settlement
During delivery, shoulder dystocia was encountered. The ObGyn used maneuvers to release the shoulder and completed the delivery. The child has a brachial plexus injury. Despite nerve graft surgery, her right arm, shoulder, and hand are paralyzed.
PARENTS' CLAIM:
The ObGyn failed to properly manage the delivery. Shoulder dystocia had been encountered during the delivery of a sibling, but the ObGyn failed to communicate the need for cesarean delivery in future pregnancies.
DEFENDANTS' DEFENSE:
There was no negligence. The case settled during trial.
VERDICT:
A $950,000 California settlement was reached with the hospital and ObGyn.
Related article:
Shoulder dystocia: Taking the fear out of management
Child has brachial plexus injury
A mother was admitted to the hospital shortly after her membranes broke. Meconium was detected but the fetal heart-rate (FHR) monitor results were normal. About 15 minutes after admission, she was seen by an attending ObGyn, who started oxytocin to induce labor. FHR monitoring results were acceptable throughout the day, and by midafternoon, the mother was ready to deliver. A fetal baseline heart rate of less than 110 bpm was detected as staff prepared for the delivery. Less than an hour later, the baby’s head crowned and the ObGyn quickly identified shoulder dystocia. Nurses repositioned the mother, the baby rotated, and was delivered. Apgar scores were normal despite a shoulder injury.
PARENTS' CLAIM:
The ObGyn caused the injury by using excessive force during delivery. After attempting gentle traction, the ObGyn should have changed strategies.
DEFENDANTS' DEFENSE:
The ObGyn asserted that she used gentle traction that prevented twisting or stretching the baby’s nerves. The birth was normal and she followed all protocols, resulting in the birth of a cognitively intact baby, as evidenced by the child’s Apgar scores. The baby was large and labor and delivery went very quickly, both factors that could have led to the baby’s injuries. The ObGyn’s actions did not cause the injuries.
VERDICT:
A Pennsylvania defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Premature birth after preeclampsia: $23.1M verdict
When a woman saw her ObGyn on August 16 at 24 weeks’ gestation, test results showed proteinuria and high blood pressure (BP). The following day, she was hospitalized for a 24-hour urine test and BP evaluation supervised by an on-call ObGyn and her ObGyn. Test results confirmed preeclampsia. She was released from the hospital. A few days later, she was found to have continued high BP and increased proteinuria, and restricted fetal growth was detected. On August 29 at 26 weeks’ gestation, the baby girl was born with severe cystic periventricular leukomalacia by emergency cesarean delivery. She cannot perform basic tasks and will need 24-hour care for the rest of her life.
PARENTS' CLAIM:
The hospital staff and 2 ObGyns failed to timely diagnose and treat preeclampsia. The treating ObGyn neither prescribed medication to treat preeclampsia nor administered antenatal corticosteroids to enhance fetal lung and brain development, both of which should have been started on August 17. Hospital health care providers failed to transfer her to a Level III facility equipped to handle a premature birth of less than 33 weeks’ gestation.
DEFENDANTS' DEFENSE:
The hospital and ObGyn denied negligence.
VERDICT:
Prior to trial, the mother settled with the on-call ObGyn for an undisclosed amount. A $23.15 million Florida verdict was returned, apportioning 70% liability to the treating ObGyn and 30% to the hospital.
Related article:
For the management of labor, patience is a virtue
Shoulder dystocia, paralysis: $950,000 settlement
During delivery, shoulder dystocia was encountered. The ObGyn used maneuvers to release the shoulder and completed the delivery. The child has a brachial plexus injury. Despite nerve graft surgery, her right arm, shoulder, and hand are paralyzed.
PARENTS' CLAIM:
The ObGyn failed to properly manage the delivery. Shoulder dystocia had been encountered during the delivery of a sibling, but the ObGyn failed to communicate the need for cesarean delivery in future pregnancies.
DEFENDANTS' DEFENSE:
There was no negligence. The case settled during trial.
VERDICT:
A $950,000 California settlement was reached with the hospital and ObGyn.
Related article:
Shoulder dystocia: Taking the fear out of management
Child has brachial plexus injury
A mother was admitted to the hospital shortly after her membranes broke. Meconium was detected but the fetal heart-rate (FHR) monitor results were normal. About 15 minutes after admission, she was seen by an attending ObGyn, who started oxytocin to induce labor. FHR monitoring results were acceptable throughout the day, and by midafternoon, the mother was ready to deliver. A fetal baseline heart rate of less than 110 bpm was detected as staff prepared for the delivery. Less than an hour later, the baby’s head crowned and the ObGyn quickly identified shoulder dystocia. Nurses repositioned the mother, the baby rotated, and was delivered. Apgar scores were normal despite a shoulder injury.
PARENTS' CLAIM:
The ObGyn caused the injury by using excessive force during delivery. After attempting gentle traction, the ObGyn should have changed strategies.
DEFENDANTS' DEFENSE:
The ObGyn asserted that she used gentle traction that prevented twisting or stretching the baby’s nerves. The birth was normal and she followed all protocols, resulting in the birth of a cognitively intact baby, as evidenced by the child’s Apgar scores. The baby was large and labor and delivery went very quickly, both factors that could have led to the baby’s injuries. The ObGyn’s actions did not cause the injuries.
VERDICT:
A Pennsylvania defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Premature birth after preeclampsia: $23.1M verdict
When a woman saw her ObGyn on August 16 at 24 weeks’ gestation, test results showed proteinuria and high blood pressure (BP). The following day, she was hospitalized for a 24-hour urine test and BP evaluation supervised by an on-call ObGyn and her ObGyn. Test results confirmed preeclampsia. She was released from the hospital. A few days later, she was found to have continued high BP and increased proteinuria, and restricted fetal growth was detected. On August 29 at 26 weeks’ gestation, the baby girl was born with severe cystic periventricular leukomalacia by emergency cesarean delivery. She cannot perform basic tasks and will need 24-hour care for the rest of her life.
PARENTS' CLAIM:
The hospital staff and 2 ObGyns failed to timely diagnose and treat preeclampsia. The treating ObGyn neither prescribed medication to treat preeclampsia nor administered antenatal corticosteroids to enhance fetal lung and brain development, both of which should have been started on August 17. Hospital health care providers failed to transfer her to a Level III facility equipped to handle a premature birth of less than 33 weeks’ gestation.
DEFENDANTS' DEFENSE:
The hospital and ObGyn denied negligence.
VERDICT:
Prior to trial, the mother settled with the on-call ObGyn for an undisclosed amount. A $23.15 million Florida verdict was returned, apportioning 70% liability to the treating ObGyn and 30% to the hospital.
Related article:
For the management of labor, patience is a virtue
Shoulder dystocia, paralysis: $950,000 settlement
During delivery, shoulder dystocia was encountered. The ObGyn used maneuvers to release the shoulder and completed the delivery. The child has a brachial plexus injury. Despite nerve graft surgery, her right arm, shoulder, and hand are paralyzed.
PARENTS' CLAIM:
The ObGyn failed to properly manage the delivery. Shoulder dystocia had been encountered during the delivery of a sibling, but the ObGyn failed to communicate the need for cesarean delivery in future pregnancies.
DEFENDANTS' DEFENSE:
There was no negligence. The case settled during trial.
VERDICT:
A $950,000 California settlement was reached with the hospital and ObGyn.
Related article:
Shoulder dystocia: Taking the fear out of management
Child has brachial plexus injury
A mother was admitted to the hospital shortly after her membranes broke. Meconium was detected but the fetal heart-rate (FHR) monitor results were normal. About 15 minutes after admission, she was seen by an attending ObGyn, who started oxytocin to induce labor. FHR monitoring results were acceptable throughout the day, and by midafternoon, the mother was ready to deliver. A fetal baseline heart rate of less than 110 bpm was detected as staff prepared for the delivery. Less than an hour later, the baby’s head crowned and the ObGyn quickly identified shoulder dystocia. Nurses repositioned the mother, the baby rotated, and was delivered. Apgar scores were normal despite a shoulder injury.
PARENTS' CLAIM:
The ObGyn caused the injury by using excessive force during delivery. After attempting gentle traction, the ObGyn should have changed strategies.
DEFENDANTS' DEFENSE:
The ObGyn asserted that she used gentle traction that prevented twisting or stretching the baby’s nerves. The birth was normal and she followed all protocols, resulting in the birth of a cognitively intact baby, as evidenced by the child’s Apgar scores. The baby was large and labor and delivery went very quickly, both factors that could have led to the baby’s injuries. The ObGyn’s actions did not cause the injuries.
VERDICT:
A Pennsylvania defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Percutaneous Release of Trigger Digits
Take-Home Points
- The author had a 90% success rate with no complications in treating almost 600 trigger digits.
- All digits can be safely treated, including multiple fingers on one hand, all in an office setting.
- Percutaneous trigger release appears to be a safe and reliable alternative to open surgery.
- Success rate, discomfort, and cost may make a percutaneous trigger release preferable to even a trial of corticosteroid injection.
- A failed percutaneous release can be successfully treated with an open release, if needed.
Trigger finger, or stenosing flexor tenosynovitis, is a condition characterized by clicking or locking during finger movement, sometimes resulting in the freezing of a digit in flexion or extension1 (Figure 1). [[{"fid":"202300","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":""}}}]]Tendon inflammation is thought to cause constriction of the tendon sheath and bunching of the fibrous bundles of the first annular (A1) pulley, often creating a palpable nodule at the base of the digit.2,3 Many patients experience intermittent joint pain and swelling, which may progress to triggering or complete locking of the digit.1 One of the most common conditions treated by hand surgeons, trigger finger is most often reported in the dominant hand of women in their sixth decade of life and has been associated with several conditions, including diabetes and rheumatoid arthritis.4-6 Other researchers have indicated the thumb and ring finger are most commonly affected, though all fingers can potentially trigger.7,8
Initial treatment often involves injecting corticosteroid into the flexor tendon sheath, at or proximal to the annular pulley system, to reduce inflammation and the fibrous nodule.3 Another injection study found an initial success rate of 57% with a single injection, and 86% with a second injection, but patients were monitored for only 6 months, a period that may have been too short for symptom recurrence.7
On failure of steroid injections, patients typically are treated with open tendon sheath incision.9 This procedure, usually performed in a hospital or outpatient surgery setting, requires postoperative wound care, including dressing changes, suture removal, possible hand therapy, and follow-up physician visits. Operative treatment involves making a 1-cm to 2-cm incision, releasing the A1 pulley, and skin suturing.7,8,10 The most common postoperative complaint is incisional tenderness, though long-term scar pain, infection, nerve injury, and disease recurrence have been reported.8 Overall, the procedure is very successful, providing up to 100% symptom relief.7,8,10
Endoscopic release of trigger finger has also been described as an effective operative treatment. This technique involves passing a small cannula through a palmar incision—using an endoscope and retrograde knife within this 2.7-mm tunnel.10 With this treatment, reduced visibility may increase the risk of nerve injury.10 Although generally successful, endoscopic release requires anesthesia and expensive instruments and has a significant learning curve.8,10
More recently, percutaneous release of trigger finger has been described as a definitive, in-office treatment.5,6,11,12 Percutaneous release has the obvious advantages of no open incision, less scarring, less discomfort, and shorter recovery. Several studies have found comparable success rates for open and percutaneous procedures but consistently shorter recovery with the percutaneous technique.7,8,12 Given its lower recurrence rate (vs steroid injections) and shorter recovery and lower cost (vs a surgical procedure), percutaneous treatment of stenosing tenosynovitis appears to be a safe, highly successful, and minimally invasive treatment method.8 This study represents a single surgeon’s experience with percutaneous tendon sheath incision over a 10-year period.
Methods
Patients presented with symptoms of stenosing flexor tenosynovitis with severity ranging from intermittent triggering to frank locking of the digit. Most patients underwent prior conservative treatment, including corticosteroid injections and hand therapy. With each patient, the senior author discussed the pathophysiology of trigger digit; treatment options, including observation, hand therapy, corticosteroid injection, percutaneous release, and open release; and potential risks and complications. The treatment path—initial corticosteroid injection, percutaneous release, or open release—was left up to the patient. The only exclusion criterion was prior surgery to the involved digit, and there was no discrimination by finger, symptomatic period, or severity. Each released digit was recorded independently. In no case was anticoagulant therapy discontinued.
A complete medical history was obtained for each patient.
Over a 10-year period (March 2003-December 2013), percutaneous release was performed on 596 trigger fingers in 429 patients, 18 years old or older. Of these patients, 279 were female. Mean age was 62 years (range, 26-97 years). Of the 531 releases with handedness recorded, 56.3% were performed on trigger digits on dominant hands (Table 1). [[{"fid":"202302","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"2"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"2":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table 1.","field_file_image_credit[und][0][value]":""}}}]]Mean duration of symptoms before percutaneous release was 9.7 months (range, 0.5-132 months). Of the 596 digits, 69 were reported to have previously sustained trauma, and 161 had been unsuccessfully treated with one or more cortisone injections before undergoing release. Of the suspected comorbidities examined, carpal tunnel syndrome was previously diagnosed in 79 patients and diabetes in 56 patients.1
Of the 429 patients, 313 had a single digit released and 116 had multiple digits released. Of the 116 patients in the multiple-release group, 80 had 2 fingers released, 24 had 3 released, 7 had 4 released, and 5 had 5 released. The 596 released trigger fingers consisted of 188 thumbs, 41 index fingers, 185 middle fingers, 140 ring fingers, and 42 small fingers.
Surgical Technique
In-office percutaneous trigger finger releases were performed with a local anesthetic. One milliliter of lidocaine 1% injection was used to anesthetize the skin, the subcutaneous tissues, and the flexor tendon sheath at the level of the A1 pulley. As described by Pandey and colleagues,6 the proper location of the pulley was confirmed using specific surface landmarks on each digit. After waiting several minutes to allow the anesthetic to take effect, the surgeon inserted an 18-gauge needle into the center of the pulley with the digit held in extension (Figure 2). [[{"fid":"202303","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"3"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"3":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":""}}}]]The needle was carefully moved longitudinally along the length of the pulley with the bevel of the needle parallel to the tendon. A grating sensation was felt as the fibers of the pulley were cut. Several needle passes were made until the pulley was felt to have been released. Complete release was determined by loss of the grating sensation, along with complete relief of any further symptoms of triggering. The puncture site was cleaned and covered with a light sterile dressing (watch the Video online). There was no postoperative immobilization, and patients were encouraged to immediately return to normal use of the digit. Hand therapy was not prescribed, and pain medications were not dispensed. A 1-week follow-up appointment was scheduled, and patients were advised to return for evaluation in the event of any recurring symptoms (eg, triggering, swelling, stiffness, pain).
Results
were successfully released with 1 percutaneous procedure (recurrence or failure rate, 9.9%). The thumb was the digit most reliably released (success rate, 94.7%) (Table 2). [[{"fid":"202306","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"4"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"4":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table 2.","field_file_image_credit[und][0][value]":""}}}]]Patients with recurrent or unresolved symptoms were given the options of a second percutaneous release or an open surgical procedure. Of the 59 digits unsuccessfully released, as identified by persistent triggering or locking of the digit, 17 were treated with a second percutaneous release (15 were successful), and 40 underwent open tendon sheath incision as a second procedure (success rate, 100%); triggering persisted in the remaining 2 digits, and these were considered failures (the 2 patients did not pursue further treatment).
There were no complications: infection; nerve, artery, or tendon injury; or chronic pain. Some patients had mild stiffness, swelling, or pain for a few days after the procedure, and these effects typically resolved without treatment. In 29 digits, persistent pain or swelling without triggering was successfully treated with a corticosteroid injection.
Discussion
[[{"fid":"202307","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"5"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Table 3.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"5":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Table 3.","field_file_image_credit[und][0][value]":""}}}]]Over a 10-year period, 596 percutaneous trigger finger releases were sequentially performed by a single surgeon. The 90% success rate compares favorably with rates found in other studies (Table 3).5-9,12-14 The surgeon’s success rates for individual years vary and demonstrate no clear trend or learning curve with the procedure (Figure 3). There were no significant complications. Patient satisfaction with the procedure was high.[[{"fid":"202308","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"6"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"6":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":""}}}]]
There were no injuries to digital nerves, arteries, or flexor tendons, either early or late, and no reports of infections or long-term pain or loss of motion. Although it is quite probable that in some procedures the longitudinal passes of the 18-gauge needle may have also slightly cut into the flexor tendon after passing through the A1 pulley, the direction of the needle passes was in line with the direction of the collagen fibers of the tendon, and thus any inadvertent superficial abrasion would not have structurally weakened the tendon. Of the 40 digits that underwent open release after incomplete or failed percutaneous release, none showed significant longitudinal lacerations of the superficialis tendon. During these revision surgeries, the typical intraoperative finding was incomplete release of the A1 pulley, usually at the distal end. Although loss of the grating sensation or relief of further triggering symptoms was considered adequate evidence of a successful release in this study, small tendon attachments could remain and potentially could lead to recurrent triggering. Given the high success rate achieved with the large sample, however, these 2 factors are considered appropriate indicators of successful release.
It is unclear why there was a relatively consistent 10% failure rate and why it did not decrease over the 10-year study period. Although the technique used does not have a significant learning curve, it appears that digits are not actively triggering at time of procedure have a higher failure rate. When a patient’s digit is actively triggering, assessment of the success of the procedure is relatively straightforward, whereas when a digit intermittently triggers and locks and is not symptomatic in the office, success cannot be immediately determined.
No specific digit was significantly more prone to failed releases, though the small finger had the lowest success rate (85.7%). Given that only 56.4% of patients experienced triggering on the dominant hand, there is not enough evidence to suggest a significant relationship between likelihood of a trigger digit and a patient’s hand dominance. Similarly, there was no correlation between the duration of symptoms and the success of the percutaneous procedure.
Investigation of the relationship between the previously suggested comorbidities of carpal tunnel syndrome and diabetes was also inconclusive. Only 79 (18%) of 429 patients reported having carpal tunnel syndrome, and even fewer, 56 (13.0%), reported having diabetes. Only 69 of the 596 treated digits reportedly had sustained trauma before developing triggering symptoms, and only 12 of the 69 were unsuccessfully released. In addition, of the 161 digits in which one or more steroid injections failed to resolve triggering symptoms, 158 (87.3%) were successfully released with 1 percutaneous procedure. Collectively, these data show percutaneous release can effectively eliminate triggering symptoms in a digit that has sustained injury or that has been unsuccessfully treated with nonoperative methods. Failed percutaneous release subsequently can be reliably treated with an open procedure, and results are excellent.
This study had several limitations. It was retrospective, nonblinded, and did not compare outcomes of percutaneous release with those of an open procedure. Data are presented to support the efficacy and safety of percutaneous release as a treatment option. Another limitation is that pre-release treatment was not controlled. Patients had been treated with a variety of nonoperative methods, including use of anti-inflammatory medication, hand therapy, splinting, and one or more corticosteroid injections, both at our office and elsewhere.
Percutaneous release appears to have an advantage in terms of pain relief, but the study did not evaluate or control for procedure discomfort. However, patients who had been treated with a corticosteroid injection before percutaneous release consistently refused corticosteroid injections for subsequent trigger digits, citing the dramatic pain reduction achieved with release relative to injection. Similarly, all patients who had a trigger digit treated with open tendon sheath incision in the past indicated a strong preference for the percutaneous release.
Follow-up on this patient population was inconsistent and incomplete. Many patients did not return, presumably because they considered the procedure a success and thought follow-up was unnecessary. However, some patients may have had a recurrence or an incomplete release and gone elsewhere for treatment.
The results of this study, to date the largest study on percutaneous release of trigger finger, provide more evidence of the safety and efficacy of this procedure as a treatment option. The success rate of percutaneous release is high, surpasses that of nonoperative treatments such as steroid injections, and approaches that of open and endoscopic surgical alternatives. Some of the obvious advantages of percutaneous release are less visible scarring, fewer incision-related complications, and shorter rehabilitation.10 In addition, post-procedure pain is possibly reduced, symptom relief is comparable, operative time is significantly shorter,8 and percutaneous release is easily performed in the office setting.
Percutaneous release is a viable treatment option for stenosing flexor tenosynovitis, regardless of previously used nonoperative treatment methods, duration or severity of symptoms, or trigger digit treated.
1. Makkouk AH, Oetgen ME, Swigart CR, Dodds SD. Trigger finger: etiology, evaluation, and treatment. Curr Rev Musculoskelet Med. 2008;1(2):92-96.
2. Fahey JJ, Bollinger JA. Trigger-finger in adults and children. J Bone Joint Surg Am. 1954;36(6):1200-1218.
3. Marks MR, Gunther SF. Efficacy of cortisone injection in treatment of trigger fingers and thumbs. J Hand Surg Am. 1989;14(4):722-727.
4. Chammas M, Bousquet P, Renard E, Poirier JL, Jaffiol C, Allieu Y. Dupuytren’s disease, carpal tunnel syndrome, trigger finger, and diabetes mellitus. J Hand Surg Am. 1995;20(1):109-114.
5. Habbu R, Putman MD, Adams JE. Percutaneous release of the A1 pulley: a cadaver study. J Hand Surg Am. 2012;37(11):2273-2277.
6. Pandey BK, Sharma S, Manandhar RR, Pradhan RL, Lakhey S, Rijal KP. Percutaneous trigger finger release. Nepal Orthop Assoc J. 2010;1(1):1-5.
7. Sato ES, Gomes dos Santos JB, Belloti JC, Albertoni WM, Faloppa F. Treatment of trigger finger: randomized clinical trial comparing the methods of corticosteroid injection, percutaneous release and open surgery. Rheumatology. 2012;51(1):93-99.
8. Dierks U, Hoffmann R, Meek MF. Open versus percutaneous release of the A1-pulley for stenosing tendovaginitis: a prospective randomized trial. Tech Hand Up Extrem Surg. 2008;12(3):183-187.
9. Tanaka J. Percutaneous trigger finger release. Tech Hand Up Extrem Surg. 1999;3(1):52-57.
10. Pegoli L, Cavalli E, Cortese P, Parolo C, Pajardi G. A comparison of endoscopic and open trigger finger release. Hand Surg. 2008;13(3):147-151.
11. Ryzewicz M, Wolf JM. Trigger digits: principles, management, and complications. J Hand Surg Am. 2006;31(1):135-146.
12. Schramm JM, Nguyen M, Wongworawat MD. The safety of percutaneous trigger finger release. Hand. 2008;3(1):44-46.
13. Paulius KL, Maguina P. Ultrasound-assisted percutaneous trigger finger release: is it safe? Hand. 2009;4(1):35-37.
14. Cihantimur B, Akin S, Ozcan M. Percutaneous treatment of trigger finger. 34 fingers followed 0.5-2 years. Acta Orthop Scand. 1998;69(2):167-168.
Take-Home Points
- The author had a 90% success rate with no complications in treating almost 600 trigger digits.
- All digits can be safely treated, including multiple fingers on one hand, all in an office setting.
- Percutaneous trigger release appears to be a safe and reliable alternative to open surgery.
- Success rate, discomfort, and cost may make a percutaneous trigger release preferable to even a trial of corticosteroid injection.
- A failed percutaneous release can be successfully treated with an open release, if needed.
Trigger finger, or stenosing flexor tenosynovitis, is a condition characterized by clicking or locking during finger movement, sometimes resulting in the freezing of a digit in flexion or extension1 (Figure 1). [[{"fid":"202300","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":""}}}]]Tendon inflammation is thought to cause constriction of the tendon sheath and bunching of the fibrous bundles of the first annular (A1) pulley, often creating a palpable nodule at the base of the digit.2,3 Many patients experience intermittent joint pain and swelling, which may progress to triggering or complete locking of the digit.1 One of the most common conditions treated by hand surgeons, trigger finger is most often reported in the dominant hand of women in their sixth decade of life and has been associated with several conditions, including diabetes and rheumatoid arthritis.4-6 Other researchers have indicated the thumb and ring finger are most commonly affected, though all fingers can potentially trigger.7,8
Initial treatment often involves injecting corticosteroid into the flexor tendon sheath, at or proximal to the annular pulley system, to reduce inflammation and the fibrous nodule.3 Another injection study found an initial success rate of 57% with a single injection, and 86% with a second injection, but patients were monitored for only 6 months, a period that may have been too short for symptom recurrence.7
On failure of steroid injections, patients typically are treated with open tendon sheath incision.9 This procedure, usually performed in a hospital or outpatient surgery setting, requires postoperative wound care, including dressing changes, suture removal, possible hand therapy, and follow-up physician visits. Operative treatment involves making a 1-cm to 2-cm incision, releasing the A1 pulley, and skin suturing.7,8,10 The most common postoperative complaint is incisional tenderness, though long-term scar pain, infection, nerve injury, and disease recurrence have been reported.8 Overall, the procedure is very successful, providing up to 100% symptom relief.7,8,10
Endoscopic release of trigger finger has also been described as an effective operative treatment. This technique involves passing a small cannula through a palmar incision—using an endoscope and retrograde knife within this 2.7-mm tunnel.10 With this treatment, reduced visibility may increase the risk of nerve injury.10 Although generally successful, endoscopic release requires anesthesia and expensive instruments and has a significant learning curve.8,10
More recently, percutaneous release of trigger finger has been described as a definitive, in-office treatment.5,6,11,12 Percutaneous release has the obvious advantages of no open incision, less scarring, less discomfort, and shorter recovery. Several studies have found comparable success rates for open and percutaneous procedures but consistently shorter recovery with the percutaneous technique.7,8,12 Given its lower recurrence rate (vs steroid injections) and shorter recovery and lower cost (vs a surgical procedure), percutaneous treatment of stenosing tenosynovitis appears to be a safe, highly successful, and minimally invasive treatment method.8 This study represents a single surgeon’s experience with percutaneous tendon sheath incision over a 10-year period.
Methods
Patients presented with symptoms of stenosing flexor tenosynovitis with severity ranging from intermittent triggering to frank locking of the digit. Most patients underwent prior conservative treatment, including corticosteroid injections and hand therapy. With each patient, the senior author discussed the pathophysiology of trigger digit; treatment options, including observation, hand therapy, corticosteroid injection, percutaneous release, and open release; and potential risks and complications. The treatment path—initial corticosteroid injection, percutaneous release, or open release—was left up to the patient. The only exclusion criterion was prior surgery to the involved digit, and there was no discrimination by finger, symptomatic period, or severity. Each released digit was recorded independently. In no case was anticoagulant therapy discontinued.
A complete medical history was obtained for each patient.
Over a 10-year period (March 2003-December 2013), percutaneous release was performed on 596 trigger fingers in 429 patients, 18 years old or older. Of these patients, 279 were female. Mean age was 62 years (range, 26-97 years). Of the 531 releases with handedness recorded, 56.3% were performed on trigger digits on dominant hands (Table 1). [[{"fid":"202302","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"2"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"2":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table 1.","field_file_image_credit[und][0][value]":""}}}]]Mean duration of symptoms before percutaneous release was 9.7 months (range, 0.5-132 months). Of the 596 digits, 69 were reported to have previously sustained trauma, and 161 had been unsuccessfully treated with one or more cortisone injections before undergoing release. Of the suspected comorbidities examined, carpal tunnel syndrome was previously diagnosed in 79 patients and diabetes in 56 patients.1
Of the 429 patients, 313 had a single digit released and 116 had multiple digits released. Of the 116 patients in the multiple-release group, 80 had 2 fingers released, 24 had 3 released, 7 had 4 released, and 5 had 5 released. The 596 released trigger fingers consisted of 188 thumbs, 41 index fingers, 185 middle fingers, 140 ring fingers, and 42 small fingers.
Surgical Technique
In-office percutaneous trigger finger releases were performed with a local anesthetic. One milliliter of lidocaine 1% injection was used to anesthetize the skin, the subcutaneous tissues, and the flexor tendon sheath at the level of the A1 pulley. As described by Pandey and colleagues,6 the proper location of the pulley was confirmed using specific surface landmarks on each digit. After waiting several minutes to allow the anesthetic to take effect, the surgeon inserted an 18-gauge needle into the center of the pulley with the digit held in extension (Figure 2). [[{"fid":"202303","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"3"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"3":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":""}}}]]The needle was carefully moved longitudinally along the length of the pulley with the bevel of the needle parallel to the tendon. A grating sensation was felt as the fibers of the pulley were cut. Several needle passes were made until the pulley was felt to have been released. Complete release was determined by loss of the grating sensation, along with complete relief of any further symptoms of triggering. The puncture site was cleaned and covered with a light sterile dressing (watch the Video online). There was no postoperative immobilization, and patients were encouraged to immediately return to normal use of the digit. Hand therapy was not prescribed, and pain medications were not dispensed. A 1-week follow-up appointment was scheduled, and patients were advised to return for evaluation in the event of any recurring symptoms (eg, triggering, swelling, stiffness, pain).
Results
were successfully released with 1 percutaneous procedure (recurrence or failure rate, 9.9%). The thumb was the digit most reliably released (success rate, 94.7%) (Table 2). [[{"fid":"202306","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"4"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"4":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table 2.","field_file_image_credit[und][0][value]":""}}}]]Patients with recurrent or unresolved symptoms were given the options of a second percutaneous release or an open surgical procedure. Of the 59 digits unsuccessfully released, as identified by persistent triggering or locking of the digit, 17 were treated with a second percutaneous release (15 were successful), and 40 underwent open tendon sheath incision as a second procedure (success rate, 100%); triggering persisted in the remaining 2 digits, and these were considered failures (the 2 patients did not pursue further treatment).
There were no complications: infection; nerve, artery, or tendon injury; or chronic pain. Some patients had mild stiffness, swelling, or pain for a few days after the procedure, and these effects typically resolved without treatment. In 29 digits, persistent pain or swelling without triggering was successfully treated with a corticosteroid injection.
Discussion
[[{"fid":"202307","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"5"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Table 3.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"5":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Table 3.","field_file_image_credit[und][0][value]":""}}}]]Over a 10-year period, 596 percutaneous trigger finger releases were sequentially performed by a single surgeon. The 90% success rate compares favorably with rates found in other studies (Table 3).5-9,12-14 The surgeon’s success rates for individual years vary and demonstrate no clear trend or learning curve with the procedure (Figure 3). There were no significant complications. Patient satisfaction with the procedure was high.[[{"fid":"202308","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"6"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"6":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":""}}}]]
There were no injuries to digital nerves, arteries, or flexor tendons, either early or late, and no reports of infections or long-term pain or loss of motion. Although it is quite probable that in some procedures the longitudinal passes of the 18-gauge needle may have also slightly cut into the flexor tendon after passing through the A1 pulley, the direction of the needle passes was in line with the direction of the collagen fibers of the tendon, and thus any inadvertent superficial abrasion would not have structurally weakened the tendon. Of the 40 digits that underwent open release after incomplete or failed percutaneous release, none showed significant longitudinal lacerations of the superficialis tendon. During these revision surgeries, the typical intraoperative finding was incomplete release of the A1 pulley, usually at the distal end. Although loss of the grating sensation or relief of further triggering symptoms was considered adequate evidence of a successful release in this study, small tendon attachments could remain and potentially could lead to recurrent triggering. Given the high success rate achieved with the large sample, however, these 2 factors are considered appropriate indicators of successful release.
It is unclear why there was a relatively consistent 10% failure rate and why it did not decrease over the 10-year study period. Although the technique used does not have a significant learning curve, it appears that digits are not actively triggering at time of procedure have a higher failure rate. When a patient’s digit is actively triggering, assessment of the success of the procedure is relatively straightforward, whereas when a digit intermittently triggers and locks and is not symptomatic in the office, success cannot be immediately determined.
No specific digit was significantly more prone to failed releases, though the small finger had the lowest success rate (85.7%). Given that only 56.4% of patients experienced triggering on the dominant hand, there is not enough evidence to suggest a significant relationship between likelihood of a trigger digit and a patient’s hand dominance. Similarly, there was no correlation between the duration of symptoms and the success of the percutaneous procedure.
Investigation of the relationship between the previously suggested comorbidities of carpal tunnel syndrome and diabetes was also inconclusive. Only 79 (18%) of 429 patients reported having carpal tunnel syndrome, and even fewer, 56 (13.0%), reported having diabetes. Only 69 of the 596 treated digits reportedly had sustained trauma before developing triggering symptoms, and only 12 of the 69 were unsuccessfully released. In addition, of the 161 digits in which one or more steroid injections failed to resolve triggering symptoms, 158 (87.3%) were successfully released with 1 percutaneous procedure. Collectively, these data show percutaneous release can effectively eliminate triggering symptoms in a digit that has sustained injury or that has been unsuccessfully treated with nonoperative methods. Failed percutaneous release subsequently can be reliably treated with an open procedure, and results are excellent.
This study had several limitations. It was retrospective, nonblinded, and did not compare outcomes of percutaneous release with those of an open procedure. Data are presented to support the efficacy and safety of percutaneous release as a treatment option. Another limitation is that pre-release treatment was not controlled. Patients had been treated with a variety of nonoperative methods, including use of anti-inflammatory medication, hand therapy, splinting, and one or more corticosteroid injections, both at our office and elsewhere.
Percutaneous release appears to have an advantage in terms of pain relief, but the study did not evaluate or control for procedure discomfort. However, patients who had been treated with a corticosteroid injection before percutaneous release consistently refused corticosteroid injections for subsequent trigger digits, citing the dramatic pain reduction achieved with release relative to injection. Similarly, all patients who had a trigger digit treated with open tendon sheath incision in the past indicated a strong preference for the percutaneous release.
Follow-up on this patient population was inconsistent and incomplete. Many patients did not return, presumably because they considered the procedure a success and thought follow-up was unnecessary. However, some patients may have had a recurrence or an incomplete release and gone elsewhere for treatment.
The results of this study, to date the largest study on percutaneous release of trigger finger, provide more evidence of the safety and efficacy of this procedure as a treatment option. The success rate of percutaneous release is high, surpasses that of nonoperative treatments such as steroid injections, and approaches that of open and endoscopic surgical alternatives. Some of the obvious advantages of percutaneous release are less visible scarring, fewer incision-related complications, and shorter rehabilitation.10 In addition, post-procedure pain is possibly reduced, symptom relief is comparable, operative time is significantly shorter,8 and percutaneous release is easily performed in the office setting.
Percutaneous release is a viable treatment option for stenosing flexor tenosynovitis, regardless of previously used nonoperative treatment methods, duration or severity of symptoms, or trigger digit treated.
Take-Home Points
- The author had a 90% success rate with no complications in treating almost 600 trigger digits.
- All digits can be safely treated, including multiple fingers on one hand, all in an office setting.
- Percutaneous trigger release appears to be a safe and reliable alternative to open surgery.
- Success rate, discomfort, and cost may make a percutaneous trigger release preferable to even a trial of corticosteroid injection.
- A failed percutaneous release can be successfully treated with an open release, if needed.
Trigger finger, or stenosing flexor tenosynovitis, is a condition characterized by clicking or locking during finger movement, sometimes resulting in the freezing of a digit in flexion or extension1 (Figure 1). [[{"fid":"202300","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":""}}}]]Tendon inflammation is thought to cause constriction of the tendon sheath and bunching of the fibrous bundles of the first annular (A1) pulley, often creating a palpable nodule at the base of the digit.2,3 Many patients experience intermittent joint pain and swelling, which may progress to triggering or complete locking of the digit.1 One of the most common conditions treated by hand surgeons, trigger finger is most often reported in the dominant hand of women in their sixth decade of life and has been associated with several conditions, including diabetes and rheumatoid arthritis.4-6 Other researchers have indicated the thumb and ring finger are most commonly affected, though all fingers can potentially trigger.7,8
Initial treatment often involves injecting corticosteroid into the flexor tendon sheath, at or proximal to the annular pulley system, to reduce inflammation and the fibrous nodule.3 Another injection study found an initial success rate of 57% with a single injection, and 86% with a second injection, but patients were monitored for only 6 months, a period that may have been too short for symptom recurrence.7
On failure of steroid injections, patients typically are treated with open tendon sheath incision.9 This procedure, usually performed in a hospital or outpatient surgery setting, requires postoperative wound care, including dressing changes, suture removal, possible hand therapy, and follow-up physician visits. Operative treatment involves making a 1-cm to 2-cm incision, releasing the A1 pulley, and skin suturing.7,8,10 The most common postoperative complaint is incisional tenderness, though long-term scar pain, infection, nerve injury, and disease recurrence have been reported.8 Overall, the procedure is very successful, providing up to 100% symptom relief.7,8,10
Endoscopic release of trigger finger has also been described as an effective operative treatment. This technique involves passing a small cannula through a palmar incision—using an endoscope and retrograde knife within this 2.7-mm tunnel.10 With this treatment, reduced visibility may increase the risk of nerve injury.10 Although generally successful, endoscopic release requires anesthesia and expensive instruments and has a significant learning curve.8,10
More recently, percutaneous release of trigger finger has been described as a definitive, in-office treatment.5,6,11,12 Percutaneous release has the obvious advantages of no open incision, less scarring, less discomfort, and shorter recovery. Several studies have found comparable success rates for open and percutaneous procedures but consistently shorter recovery with the percutaneous technique.7,8,12 Given its lower recurrence rate (vs steroid injections) and shorter recovery and lower cost (vs a surgical procedure), percutaneous treatment of stenosing tenosynovitis appears to be a safe, highly successful, and minimally invasive treatment method.8 This study represents a single surgeon’s experience with percutaneous tendon sheath incision over a 10-year period.
Methods
Patients presented with symptoms of stenosing flexor tenosynovitis with severity ranging from intermittent triggering to frank locking of the digit. Most patients underwent prior conservative treatment, including corticosteroid injections and hand therapy. With each patient, the senior author discussed the pathophysiology of trigger digit; treatment options, including observation, hand therapy, corticosteroid injection, percutaneous release, and open release; and potential risks and complications. The treatment path—initial corticosteroid injection, percutaneous release, or open release—was left up to the patient. The only exclusion criterion was prior surgery to the involved digit, and there was no discrimination by finger, symptomatic period, or severity. Each released digit was recorded independently. In no case was anticoagulant therapy discontinued.
A complete medical history was obtained for each patient.
Over a 10-year period (March 2003-December 2013), percutaneous release was performed on 596 trigger fingers in 429 patients, 18 years old or older. Of these patients, 279 were female. Mean age was 62 years (range, 26-97 years). Of the 531 releases with handedness recorded, 56.3% were performed on trigger digits on dominant hands (Table 1). [[{"fid":"202302","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"2"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"2":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table 1.","field_file_image_credit[und][0][value]":""}}}]]Mean duration of symptoms before percutaneous release was 9.7 months (range, 0.5-132 months). Of the 596 digits, 69 were reported to have previously sustained trauma, and 161 had been unsuccessfully treated with one or more cortisone injections before undergoing release. Of the suspected comorbidities examined, carpal tunnel syndrome was previously diagnosed in 79 patients and diabetes in 56 patients.1
Of the 429 patients, 313 had a single digit released and 116 had multiple digits released. Of the 116 patients in the multiple-release group, 80 had 2 fingers released, 24 had 3 released, 7 had 4 released, and 5 had 5 released. The 596 released trigger fingers consisted of 188 thumbs, 41 index fingers, 185 middle fingers, 140 ring fingers, and 42 small fingers.
Surgical Technique
In-office percutaneous trigger finger releases were performed with a local anesthetic. One milliliter of lidocaine 1% injection was used to anesthetize the skin, the subcutaneous tissues, and the flexor tendon sheath at the level of the A1 pulley. As described by Pandey and colleagues,6 the proper location of the pulley was confirmed using specific surface landmarks on each digit. After waiting several minutes to allow the anesthetic to take effect, the surgeon inserted an 18-gauge needle into the center of the pulley with the digit held in extension (Figure 2). [[{"fid":"202303","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"3"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"3":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":""}}}]]The needle was carefully moved longitudinally along the length of the pulley with the bevel of the needle parallel to the tendon. A grating sensation was felt as the fibers of the pulley were cut. Several needle passes were made until the pulley was felt to have been released. Complete release was determined by loss of the grating sensation, along with complete relief of any further symptoms of triggering. The puncture site was cleaned and covered with a light sterile dressing (watch the Video online). There was no postoperative immobilization, and patients were encouraged to immediately return to normal use of the digit. Hand therapy was not prescribed, and pain medications were not dispensed. A 1-week follow-up appointment was scheduled, and patients were advised to return for evaluation in the event of any recurring symptoms (eg, triggering, swelling, stiffness, pain).
Results
were successfully released with 1 percutaneous procedure (recurrence or failure rate, 9.9%). The thumb was the digit most reliably released (success rate, 94.7%) (Table 2). [[{"fid":"202306","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"4"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"4":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table 2.","field_file_image_credit[und][0][value]":""}}}]]Patients with recurrent or unresolved symptoms were given the options of a second percutaneous release or an open surgical procedure. Of the 59 digits unsuccessfully released, as identified by persistent triggering or locking of the digit, 17 were treated with a second percutaneous release (15 were successful), and 40 underwent open tendon sheath incision as a second procedure (success rate, 100%); triggering persisted in the remaining 2 digits, and these were considered failures (the 2 patients did not pursue further treatment).
There were no complications: infection; nerve, artery, or tendon injury; or chronic pain. Some patients had mild stiffness, swelling, or pain for a few days after the procedure, and these effects typically resolved without treatment. In 29 digits, persistent pain or swelling without triggering was successfully treated with a corticosteroid injection.
Discussion
[[{"fid":"202307","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"5"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Table 3.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"5":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Table 3.","field_file_image_credit[und][0][value]":""}}}]]Over a 10-year period, 596 percutaneous trigger finger releases were sequentially performed by a single surgeon. The 90% success rate compares favorably with rates found in other studies (Table 3).5-9,12-14 The surgeon’s success rates for individual years vary and demonstrate no clear trend or learning curve with the procedure (Figure 3). There were no significant complications. Patient satisfaction with the procedure was high.[[{"fid":"202308","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"6"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"6":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":""}}}]]
There were no injuries to digital nerves, arteries, or flexor tendons, either early or late, and no reports of infections or long-term pain or loss of motion. Although it is quite probable that in some procedures the longitudinal passes of the 18-gauge needle may have also slightly cut into the flexor tendon after passing through the A1 pulley, the direction of the needle passes was in line with the direction of the collagen fibers of the tendon, and thus any inadvertent superficial abrasion would not have structurally weakened the tendon. Of the 40 digits that underwent open release after incomplete or failed percutaneous release, none showed significant longitudinal lacerations of the superficialis tendon. During these revision surgeries, the typical intraoperative finding was incomplete release of the A1 pulley, usually at the distal end. Although loss of the grating sensation or relief of further triggering symptoms was considered adequate evidence of a successful release in this study, small tendon attachments could remain and potentially could lead to recurrent triggering. Given the high success rate achieved with the large sample, however, these 2 factors are considered appropriate indicators of successful release.
It is unclear why there was a relatively consistent 10% failure rate and why it did not decrease over the 10-year study period. Although the technique used does not have a significant learning curve, it appears that digits are not actively triggering at time of procedure have a higher failure rate. When a patient’s digit is actively triggering, assessment of the success of the procedure is relatively straightforward, whereas when a digit intermittently triggers and locks and is not symptomatic in the office, success cannot be immediately determined.
No specific digit was significantly more prone to failed releases, though the small finger had the lowest success rate (85.7%). Given that only 56.4% of patients experienced triggering on the dominant hand, there is not enough evidence to suggest a significant relationship between likelihood of a trigger digit and a patient’s hand dominance. Similarly, there was no correlation between the duration of symptoms and the success of the percutaneous procedure.
Investigation of the relationship between the previously suggested comorbidities of carpal tunnel syndrome and diabetes was also inconclusive. Only 79 (18%) of 429 patients reported having carpal tunnel syndrome, and even fewer, 56 (13.0%), reported having diabetes. Only 69 of the 596 treated digits reportedly had sustained trauma before developing triggering symptoms, and only 12 of the 69 were unsuccessfully released. In addition, of the 161 digits in which one or more steroid injections failed to resolve triggering symptoms, 158 (87.3%) were successfully released with 1 percutaneous procedure. Collectively, these data show percutaneous release can effectively eliminate triggering symptoms in a digit that has sustained injury or that has been unsuccessfully treated with nonoperative methods. Failed percutaneous release subsequently can be reliably treated with an open procedure, and results are excellent.
This study had several limitations. It was retrospective, nonblinded, and did not compare outcomes of percutaneous release with those of an open procedure. Data are presented to support the efficacy and safety of percutaneous release as a treatment option. Another limitation is that pre-release treatment was not controlled. Patients had been treated with a variety of nonoperative methods, including use of anti-inflammatory medication, hand therapy, splinting, and one or more corticosteroid injections, both at our office and elsewhere.
Percutaneous release appears to have an advantage in terms of pain relief, but the study did not evaluate or control for procedure discomfort. However, patients who had been treated with a corticosteroid injection before percutaneous release consistently refused corticosteroid injections for subsequent trigger digits, citing the dramatic pain reduction achieved with release relative to injection. Similarly, all patients who had a trigger digit treated with open tendon sheath incision in the past indicated a strong preference for the percutaneous release.
Follow-up on this patient population was inconsistent and incomplete. Many patients did not return, presumably because they considered the procedure a success and thought follow-up was unnecessary. However, some patients may have had a recurrence or an incomplete release and gone elsewhere for treatment.
The results of this study, to date the largest study on percutaneous release of trigger finger, provide more evidence of the safety and efficacy of this procedure as a treatment option. The success rate of percutaneous release is high, surpasses that of nonoperative treatments such as steroid injections, and approaches that of open and endoscopic surgical alternatives. Some of the obvious advantages of percutaneous release are less visible scarring, fewer incision-related complications, and shorter rehabilitation.10 In addition, post-procedure pain is possibly reduced, symptom relief is comparable, operative time is significantly shorter,8 and percutaneous release is easily performed in the office setting.
Percutaneous release is a viable treatment option for stenosing flexor tenosynovitis, regardless of previously used nonoperative treatment methods, duration or severity of symptoms, or trigger digit treated.
1. Makkouk AH, Oetgen ME, Swigart CR, Dodds SD. Trigger finger: etiology, evaluation, and treatment. Curr Rev Musculoskelet Med. 2008;1(2):92-96.
2. Fahey JJ, Bollinger JA. Trigger-finger in adults and children. J Bone Joint Surg Am. 1954;36(6):1200-1218.
3. Marks MR, Gunther SF. Efficacy of cortisone injection in treatment of trigger fingers and thumbs. J Hand Surg Am. 1989;14(4):722-727.
4. Chammas M, Bousquet P, Renard E, Poirier JL, Jaffiol C, Allieu Y. Dupuytren’s disease, carpal tunnel syndrome, trigger finger, and diabetes mellitus. J Hand Surg Am. 1995;20(1):109-114.
5. Habbu R, Putman MD, Adams JE. Percutaneous release of the A1 pulley: a cadaver study. J Hand Surg Am. 2012;37(11):2273-2277.
6. Pandey BK, Sharma S, Manandhar RR, Pradhan RL, Lakhey S, Rijal KP. Percutaneous trigger finger release. Nepal Orthop Assoc J. 2010;1(1):1-5.
7. Sato ES, Gomes dos Santos JB, Belloti JC, Albertoni WM, Faloppa F. Treatment of trigger finger: randomized clinical trial comparing the methods of corticosteroid injection, percutaneous release and open surgery. Rheumatology. 2012;51(1):93-99.
8. Dierks U, Hoffmann R, Meek MF. Open versus percutaneous release of the A1-pulley for stenosing tendovaginitis: a prospective randomized trial. Tech Hand Up Extrem Surg. 2008;12(3):183-187.
9. Tanaka J. Percutaneous trigger finger release. Tech Hand Up Extrem Surg. 1999;3(1):52-57.
10. Pegoli L, Cavalli E, Cortese P, Parolo C, Pajardi G. A comparison of endoscopic and open trigger finger release. Hand Surg. 2008;13(3):147-151.
11. Ryzewicz M, Wolf JM. Trigger digits: principles, management, and complications. J Hand Surg Am. 2006;31(1):135-146.
12. Schramm JM, Nguyen M, Wongworawat MD. The safety of percutaneous trigger finger release. Hand. 2008;3(1):44-46.
13. Paulius KL, Maguina P. Ultrasound-assisted percutaneous trigger finger release: is it safe? Hand. 2009;4(1):35-37.
14. Cihantimur B, Akin S, Ozcan M. Percutaneous treatment of trigger finger. 34 fingers followed 0.5-2 years. Acta Orthop Scand. 1998;69(2):167-168.
1. Makkouk AH, Oetgen ME, Swigart CR, Dodds SD. Trigger finger: etiology, evaluation, and treatment. Curr Rev Musculoskelet Med. 2008;1(2):92-96.
2. Fahey JJ, Bollinger JA. Trigger-finger in adults and children. J Bone Joint Surg Am. 1954;36(6):1200-1218.
3. Marks MR, Gunther SF. Efficacy of cortisone injection in treatment of trigger fingers and thumbs. J Hand Surg Am. 1989;14(4):722-727.
4. Chammas M, Bousquet P, Renard E, Poirier JL, Jaffiol C, Allieu Y. Dupuytren’s disease, carpal tunnel syndrome, trigger finger, and diabetes mellitus. J Hand Surg Am. 1995;20(1):109-114.
5. Habbu R, Putman MD, Adams JE. Percutaneous release of the A1 pulley: a cadaver study. J Hand Surg Am. 2012;37(11):2273-2277.
6. Pandey BK, Sharma S, Manandhar RR, Pradhan RL, Lakhey S, Rijal KP. Percutaneous trigger finger release. Nepal Orthop Assoc J. 2010;1(1):1-5.
7. Sato ES, Gomes dos Santos JB, Belloti JC, Albertoni WM, Faloppa F. Treatment of trigger finger: randomized clinical trial comparing the methods of corticosteroid injection, percutaneous release and open surgery. Rheumatology. 2012;51(1):93-99.
8. Dierks U, Hoffmann R, Meek MF. Open versus percutaneous release of the A1-pulley for stenosing tendovaginitis: a prospective randomized trial. Tech Hand Up Extrem Surg. 2008;12(3):183-187.
9. Tanaka J. Percutaneous trigger finger release. Tech Hand Up Extrem Surg. 1999;3(1):52-57.
10. Pegoli L, Cavalli E, Cortese P, Parolo C, Pajardi G. A comparison of endoscopic and open trigger finger release. Hand Surg. 2008;13(3):147-151.
11. Ryzewicz M, Wolf JM. Trigger digits: principles, management, and complications. J Hand Surg Am. 2006;31(1):135-146.
12. Schramm JM, Nguyen M, Wongworawat MD. The safety of percutaneous trigger finger release. Hand. 2008;3(1):44-46.
13. Paulius KL, Maguina P. Ultrasound-assisted percutaneous trigger finger release: is it safe? Hand. 2009;4(1):35-37.
14. Cihantimur B, Akin S, Ozcan M. Percutaneous treatment of trigger finger. 34 fingers followed 0.5-2 years. Acta Orthop Scand. 1998;69(2):167-168.
Postpartum Treatment of Metastatic Recurrent Giant Cell Tumor of Capitate Bone of Wrist
Take-Home Points
- GCT of bones of the wrist is rare. This article is the only report of a wrist GCT during pregnancy that we could identify.
- Routine treatment usually consists of surgical excision with local adjuvant, and in the wrist, often results in reduced wrist motion.
- GCT of the wrist is more aggressive than the more common locations in long bones, with higher local recurrence rates if treated with surgery alone.
- Diagnosis is often delayed for GCT of the wrist, due to insufficient imaging, which should include CT or MRI.
- For pregnant women with GCT, local adjuvant treatments can be used in addition to surgery. Following pregnancy, denosumab can be used systemically, and can be effective with metastatic or unresectable disease.
Giant cell tumor (GCT) of bone accounts for about 5% of primary bone tumors.1-3 Only 3% to 5% of GCTs occur in the hand.4,5 Wrist involvement, which is rare, most often involves the hamate bone.5-7 Capitate bone involvement is exceedingly rare.8-11 Although histologically benign, GCT can recur locally after treatment with curettage alone, and lung metastases are found in 2% to 5% of cases.2,12-14 Therefore, en bloc tumor excision is preferred in the setting of cortical erosion or soft-tissue involvement.1,4,8 Wrist joint motion is inevitably reduced, and bone graft donor-site morbidity is significant.6-8
In the unusual case reported here, GCT presented in the capitate bone and, after the patient became pregnant, recurred in the hamate and trapezoid bones with soft-tissue extension and lung metastases. The capitate was excised en bloc and reconstructed with an interposition of polymethylmethacrylate bone cement. Pulmonary metastases developed, and the GCT expanded to involve multiple carpal bones and the bases of the second through fourth metacarpals. A 10-month course of systemic chemotherapy with the RANK ligand (RANKL) inhibitor denosumab was started after the pregnancy. After this treatment, the patient underwent both tumor resection and reconstruction with autogenous bicortical iliac crest bone graft (ICBG) carefully designed to preserve range of motion and maintain the fingers in anatomical position. Treatment with denosumab was continued after surgery. Although this case offers no endpoint for postoperative chemotherapy with denosumab, preoperative treatment dramatically reduced the GCT and permitted limb-sparing reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 19-year-old right-handed woman with atraumatic swelling of the left wrist presented to an orthopedic surgeon at an outside facility. Physical examination revealed tender fullness on the dorsum of the wrist, slightly reduced range of motion and grip strength, and a neurovascularly intact wrist. The diagnosis was periarticular cyst, and the patient underwent physical therapy. Two years later, the swelling returned, tenderness was increasing, and symptoms did not resolve with cast immobilization. A radiograph showed a lytic lesion in the capitate bone (Figure 1).[[{"fid":"202332","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":""}}}]]
GCT was diagnosed with percutaneous needle biopsy. A preoperative chest radiograph was reported normal. For initial treatment, the capitate and trapezoid bones were resected en bloc through a dorsal approach. Reconstruction consisted of limited arthrodesis using bone cement without additional fixation.
At 6-month follow-up, the patient was pregnant, and there was a recurrence of the wrist lesion. During the first 2 months of pregnancy, swelling and pain rapidly progressed, and a palpable mass formed. Radiographs showed a lytic lesion extending into the hamate bone (Figure 2), and magnetic resonance imaging (MRI) showed articular extension of the lesion with involvement of the base of the fourth metacarpal. [[{"fid":"202334","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"2"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"2":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":""}}}]]Targeted anti-RANKL therapy was not recommended (and was not available at the patient’s home hospital). The patient deferred surgical treatment because of the pregnancy, which proved otherwise uneventful and ended with a full-term delivery.
After the pregnancy, radiographs of the wrist showed complete destruction of the hamate and trapezium bones, with erosion of the bases of the second through fourth metacarpals (Figure 3A). [[{"fid":"202335","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"3"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"3":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":""}}}]]The patient presented at our institution 4 years after initial diagnosis. Computed tomography (CT) of the chest showed numerous bilateral pulmonary nodular opacities. Wrist imaging showed soft-tissue extension (Figure 3B). The diagnosis of recurrent metastatic GCT was confirmed with needle biopsies of the wrist mass and the right lung nodule.
Systemic chemotherapy was initiated with 120 mg of denosumab, given subcutaneously on days 1, 8, and 15 and then monthly during the 10 months leading up to surgery. Serum calcium was monitored during treatment and remained within the normal range the entire time, except for once at the start of therapy, when it dropped to 6.8 mg/dL. After 8 months, the soft-tissue mass, originally 8 cm × 8 cm × 6 cm, shrunk and stabilized at 5 cm × 4 cm × 4 cm (Figure 3B), and a bony shell reformed around it. Nodules in both lung fields showed response to denosumab.
Histologic examination revealed scattered osteoclast-like, multinucleated giant cells, consistent with a recurrent lesion (Figure 4). [[{"fid":"202336","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"4"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 4.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"4":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 4.","field_file_image_credit[und][0][value]":""}}}]]After 10 months of treatment with denosumab, the patient underwent resection (dorsal approach) of the residual cement, the soft-tissue mass, the affected carpal bones, half of the third metacarpal, and the second and fourth metacarpal bases. The proximal carpal row was preserved after no intra-articular involvement was verified. The closet margin was marginal; the tumor mass abutted without encompassing the flexor tendons and median nerve. The tumor was meticulously elevated from the neurovascular and tendinous structures, which were not sacrificed. Hydrogen peroxide was used for local adjuvant treatment. Bicortical autogenous ICBG was placed between the remaining scaphoid, lunate, and metacarpal bones. The second, third, and fourth metacarpal bases were stabilized on the overlapping outer table of ICBG with 2.0-mm plates and miniscrews (Figure 5A). Kirschner wires were used to stabilize the proximal bone graft and the scapholunate fossa. Cancellous bone graft was packed between the structural bone graft and neighboring unaffected carpal bones (Figure 5A). Immobilization with a short-arm thumb spica cast was maintained for 6 weeks after surgery and was followed by a 12-week rehabilitation program. The patient returned to normal activities when plain radiographs showed solid bony union (Figure 5B). Fourteen months after initial surgery, tenolysis was performed to free the extensor tendons (index, middle, and ring fingers on dorsum of left hand) from adhesions to the bone graft. At 37-month follow-up (Figure 5C), there was no clinical or radiographic evidence of progression in the wrist.[[{"fid":"202337","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"5"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 5.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"5":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 5.","field_file_image_credit[und][0][value]":""}}}]]
The patient had bilateral pulmonary metastases (Figures 6A, 6B). Treatment with denosumab produced an initial response (smaller pulmonary lesions) and subsequent stability. After 12 months of treatment with denosumab, the patient underwent left thoracotomy and wedge resection of pulmonary metastases on the left. Pathologic evaluation revealed pulmonary parenchyma with calcification and ossification and limited viable tumor. Given the dramatic effects on the left pulmonary metastases, denosumab was continued, and surgical intervention on the right was not attempted. Pulmonary metastases were stable afterward (Figure 6C).[[{"fid":"202338","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"6"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 6.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"6":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 6.","field_file_image_credit[und][0][value]":""}}}]]
At 54-month follow-up, systemic treatment with denosumab was continued. The patient had no pain in the wrist or hand and was able to use the left hand normally. There was some fissuring of the third and fourth digits over each other. However, the patient had good grip strength and was using eating utensils, picking up water bottles, and engaging in other activities without difficulty.
Discussion
GCT isolated to the carpus is rare. However, compared with GCT in the more common locations in long bones, it is also more aggressive, and its local recurrence rates are higher, probably 60% or more if treated with curettage alone.15 Therefore, excision augmented with adjuvant treatment is recommended.2,7 Use of bone cement in the hand is relatively uncommon.4,5,7-10
The diagnosis of GCT in the carpus is difficult and often delayed. The initial complaint is usually mild wrist pain after relatively mild trauma.5 The first reported case of GCT in the lunate bone was mistakenly thought to be Kienbock disease.5 Similarly, our patient was initially given a nononcologic diagnosis, which prompted conservative management.
Whether the biological behavior of GCT in the carpus differs from that of GCT in other sites is unclear. The high recurrence rates might be attributable in part to suboptimal curettage.5,6 En bloc resections of involved bone inevitably result in carpal instability or loss of wrist motion if arthrodesis is performed.4-7,11 In the present case, resection was followed by limited arthrodesis to mitigate motion losses.
Multifocal GCT in the carpal bones often affects younger patients and has a high rate of recurrence.7,16 In the present case, the patient’s pregnancy delayed treatment and allowed tumor extension into soft tissues and metacarpal bones. Given her young age, en bloc tumor resection was performed, with the proximal carpal row spared to preserve wrist motion. ICBG was carefully shaped to match the defect that remained after tumor resection.7 Supporting wrist height to prevent carpal collapse provided a stable base for remaining distal segments of the second through fourth metacarpals. After short-arm thumb spica casting and early rehabilitation, the patient recovered wrist motion and use of the involved fingers distal to the carpometacarpal joints.
In pregnant women, GCTs have been found primarily in the long bones and spine but are rare.17-21 A review of the literature (1950-present) revealed that the present article is the first report of GCT in the hand or wrist bones of a pregnant woman.18,20,21 There is no consensus as to whether surgical excision should be performed during pregnancy.18,20,21 In 1 unusual case, at 18 weeks’ gestation GCT in the distal femur was resected with curettage and bone grafting, and there were no complications.21 Therefore, pregnancy termination is not indicated for GCT.
The relationship between tumorigenesis and pregnancy is unclear.18,20,21 Empirically, pregnancy is thought to promote tumor growth.18,20 Estrogen and progesterone levels are elevated during pregnancy, potentially influencing tumor cells that are hormonally sensitive.18,20 An early report in which reverse transcription–polymerase chain reaction showed estrogen receptor expression in GCT osteoclast-like cells was followed by several studies that failed to find estrogen receptors at the protein level.19 In contrast, progesterone receptors were found in 50% of GCTs in a study.22 However, the etiopathogenic significance of this is unclear. In pregnant women, vascular endothelial growth factor, placental growth factor, and other growth factors induce osteoclast formation.23 ß-Human chorionic gonadotropin expression (ß-hCG) has been found in 58% of cases, with some showing ß-hCG elevation in the serum.24 Other studies have focused on an immunologic explanation for occurrence of GCT during pregnancy.18 Oncofetal antigens, which are similar to fetal antigens, have been found in fibrosarcoma and in an osteosarcoma cell line but not in GCT.18-20 Thus, though occurrence during pregnancy may be coincidental given the frequency of GCT in women of childbearing age, it is plausible that tumor growth may be enhanced by pregnancy. More studies are needed to understand the relationship between giant cell proliferation and pregnancy-related growth factors and hormones.
With GCT, the rate of pulmonary metastases ranges from 0% to 4%; these metastases are usually diagnosed at time of local recurrence, or 2 years to 3 years after initial GCT diagnosis.2,3,12,14,25 Lung metastases secondary to GCT in the hand or foot bones are rare; our literature review identified only 4 cases.12,14 Risk factors for lung metastasis include local recurrence, aggressive appearance (Enneking grade 3) on radiograph, Ki-67 antigen expression, and distal radius location.14 The mechanism of metastasis is unknown.12,14
Lung metastases are usually excised, but they may spontaneously evolve toward necrosis and ossification.12 In cases in which surgery is unfeasible, chemotherapy (eg, with doxorubicin) has been used to control progression.12,14 Radiation can cause sarcomatous transformation and is contraindicated. Interferon26-28 and other antiangiogenic strategies have been successfully used in systemic therapy for GCT of bone. More recently, bisphosphonates29-32 and denosumab33 have been investigated.29,32-36 The limited toxicity of denosumab makes the drug a very attractive treatment option for recurrent or unresectable GCT of bone.33 Reported rates of mortality from lung metastases have ranged from 0% to 40%.14 There is evidence that control of lung metastases during the first 3 years after diagnosis is important for favorable outcomes.2,3
Malignant stromal cells of GCT of bone have been known to secrete RANKL, which recruits osteoclasts and osteoclast precursor cells, which in turn generate aggressive osteolytic activity.33,37 Denosumab, a monoclonal antibody that inhibits RANKL, is effective in stopping osteoclastic activity. In a phase 2 trial of denosumab in the treatment of GCT of bone, 96% of treated patients with unresectable disease showed no progression at 13 months.38 In addition, 74% of treated patients who had resectable disease but were likely to have morbid surgery did not require surgery, and 62% of treated patients who underwent surgery were able to have a less morbid procedure. Forty-one percent to 58% of treated patients had a reduction in tumor size.
Denosumab is very well tolerated. The phase 2 trial found serious adverse events in 9% of patients, and in 5% of cases the drug was discontinued because of toxicity.38 Serious adverse events include osteonecrosis of jaw, hypocalcemia, and hypophosphatemia.37 Electrolyte changes with denosumab are easy to monitor and manage. Although the favorable toxicity profile of denosumab allows for long-term therapy, the data on therapy duration in patients with unresectable disease are unclear. Patients who discontinue therapy should be closely monitored, as disease can progress in this setting.37
In contrast to GCT of larger bones, GCT of the wrist is rare and typically more aggressive, and has higher local recurrence rates. In many cases, diagnosis is delayed by insufficient imaging, which optimally should include either CT or MRI (Table). [[{"fid":"202341","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"7"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"7":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table.","field_file_image_credit[und][0][value]":""}}}]]For pregnant women with GCT, options include surgical resection with curettage and local adjuvant treatment. After pregnancy, denosumab can be used systemically, and can be effective with metastatic or unresectable disease. Surgical treatment in the wrist can be challenging when partial or complete resections of carpal bones are required. Occupational therapy is recommended for optimization of hand function after surgery.
1. Balke M, Ahrens H, Streitbuerger A, et al. Treatment options for recurrent giant cell tumors of bone. J Cancer Res Clin Oncol. 2009;135(1):149-158.
2. Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Giant cell tumor of bone risk factors for recurrence. Clin Orthop Relat Res. 2011;469(2):591-599.
3. Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Recurrent giant cell tumor of long bones: analysis of surgical management. Clin Orthop Relat Res. 2011;469(4):1181-1187.
4. Averill RM, Smith RJ, Campbell CJ. Giant-cell tumors of the bones of the hand. J Hand Surg Am. 1980;5(1):39-50.
5. Shigematsu K, Kobata Y, Yajima H, Kawamura K, Maegawa N, Takakura Y. Giant-cell tumors of the carpus. J Hand Surg Am. 2006;31(7):1214-1219.
6. Gupta GG, Lucas GL, Pirela-Cruz M. Multifocal giant cell tumor of the capitate, hamate, and triquetrum: a case report. J Hand Surg Am. 1995;20(6):1003-1006.
7. Tarng YW, Yang SW, Hsu CJ. Surgical treatment of multifocal giant cell tumor of carpal bones with preservation of wrist function: case report. J Hand Surg Am. 2009;34(2):262-265.
8. Angelini A, Mavrogenis AF, Ruggieri P. Giant cell tumor of the capitate. Musculoskelet Surg. 2011;95(1):45-48.
9. Howard FM, Lassen K. Giant cell tumor of the capitate. J Hand Surg Am. 1984;9(2):272-274.
10. McDonald DJ, Schajowicz F. Giant cell tumor of the capitate. A case report. Clin Orthop Relat Res. 1992(279):264-268.
11. Wilson SC, Cascio BM, Plauche HR. Giant-cell tumor of the capitate. Orthopedics. 2001;24(11):1085-1086.
12. Combalia-Aleu A, Sastre S, Fernández-de-Retana P, Tomás X, Palacin A. Giant cell tumor of the talus with pulmonary metastasis: seven years follow up. Foot. 2006;16(2):107-111.
13. Donthineni R, Boriani L, Ofluoglu O, Bandiera S. Metastatic behaviour of giant cell tumour of the spine. Int Orthop. 2009;33(2):497-501.
14. Jacopin S, Viehweger E, Glard Y, et al. Fatal lung metastasis secondary to index finger giant cell tumor in an 8-year-old child. Orthop Traumatol Surg Res. 2010;96(3):310-313.
15. Plate AM, Lee SJ, Steiner G, Posner MA. Tumor-like lesions and benign tumors of the hand and wrist. J Am Acad Orthop Surg. 2003;11(2):129-141.
16. Moreel P, Le Viet D. Failure of initial surgical treatment of a giant cell tumor of the capitate and its salvage: a case report [in French]. Chir Main. 2006;25(6):315-318.
17. Caillouette JC, Mattar N. Massive peripheral giant-cell reparative granuloma of the jaw: a pregnancy dependent tumor. Trans Pac Coast Obstet Gynecol Soc. 1978;45:78-81.
18. Kathiresan AS, Johnson JN, Hood BJ, Montoya SP, Vanni S, Gonzalez-Quintero VH. Giant cell bone tumor of the thoracic spine presenting in late pregnancy. Obstet Gynecol. 2011;118(2 pt 2):428-431.
19. Komiya S, Zenmyo M, Inoue A. Bone tumors in the pelvis presenting growth during pregnancy. Arch Orthop Trauma Surg. 1999;119(1-2):22-29.
20. Ross AE, Bojescul JA, Kuklo TR. Giant cell tumor: a case report of recurrence during pregnancy. Spine. 2005;30(12):E332-3E35.
21. Sharma JB, Chanana C, Rastogi, et al. Successful pregnancy outcome with elective caesarean section following two attempts of surgical excision of large giant cell tumor of the lower limb during pregnancy. Arch Gynecol Obstet. 2006;274(5):313-315.
22. Demertzis N, Kotsiandri F, Giotis I, Apostolikas N. Giant-cell tumors of bone and progesterone receptors. Orthopedics. 2003;26(12):1209-1212.
23. Taylor RM, Kashima TG, Knowles HJ, Athanasou NA. VEGF, FLT3 ligand, PlGF and HGF can substitute for M-CSF to induce human osteoclast formation: implications for giant cell tumour pathobiology. Lab Invest. 2012;92(10):1398-1406.
24. Lawless ME, Jour G, Hoch BL, Rendi MH. Beta-human chorionic gonadotropin expression in recurrent and metastatic giant cell tumors of bone: a potential mimicker of germ cell tumor. Int J Surg Pathol. 2014;22(7):617-622.
25. Viswanathan S, Jambhekar NA. Metastatic giant cell tumor of bone: are there associated factors and best treatment modalities? Clin Orthop Relat Res. 2010;468(3):827-833.
26. Kaban LB, Troulis MJ, Ebb D, August M, Hornicek FJ, Dodson TB. Antiangiogenic therapy with interferon alpha for giant cell lesions of the jaws. J Oral Maxillofac Surg. 2002;60(10):1103-1111.
27. Kaiser U, Neumann K, Havemann K. Generalised giant-cell tumour of bone: successful treatment of pulmonary metastases with interferon alpha, a case report. J Cancer Res Clin Oncol. 1993;119(5):301-303.
28. Dickerman JD. Interferon and giant cell tumors. Pediatrics. 1999;103(6 pt 1):1282-1283.
29. Balke M, Campanacci L, Gebert C, et al. Bisphosphonate treatment of aggressive primary, recurrent and metastatic giant cell tumour of bone. BMC Cancer. 2010;10:462.
30. Gille O, Oliveira Bde A, Guerin P, Lepreux S, Richez C, Vital JM. Regression of giant cell tumor of the cervical spine with bisphosphonate as single therapy. Spine. 2012;37(6):E396-E399.
31. Moriceau G, Ory B, Gobin B, et al. Therapeutic approach of primary bone tumours by bisphosphonates. Curr Pharm Des. 2010;16(27):2981-2987.
32. Tse LF, Wong KC, Kumta SM, Huang L, Chow TC, Griffith JF. Bisphosphonates reduce local recurrence in extremity giant cell tumor of bone: a case–control study. Bone. 2008;42(1):68-73.
33. Thomas D, Henshaw R, Skubitz K, et al. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol. 2010;11(3):275-280.
34. Balke M, Hardes J. Denosumab: a breakthrough in treatment of giant-cell tumour of bone? Lancet Oncol. 2010;11(3):218-219.
35. Kyrgidis A, Toulis K. Safety and efficacy of denosumab in giant-cell tumour of bone. Lancet Oncol. 2010;11(6):513-514.
36. Thomas D, Carriere P, Jacobs I. Safety of denosumab in giant-cell tumour of bone. Lancet Oncol. 2010;11(9):815.
37. Skubitz KM. Giant cell tumor of bone: current treatment options. Curr Treat Options Oncol. 2014;15(3):507-518.
38. Chawla S, Henshaw R, Seeger L, et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol. 2013;14(9):901-908.
Take-Home Points
- GCT of bones of the wrist is rare. This article is the only report of a wrist GCT during pregnancy that we could identify.
- Routine treatment usually consists of surgical excision with local adjuvant, and in the wrist, often results in reduced wrist motion.
- GCT of the wrist is more aggressive than the more common locations in long bones, with higher local recurrence rates if treated with surgery alone.
- Diagnosis is often delayed for GCT of the wrist, due to insufficient imaging, which should include CT or MRI.
- For pregnant women with GCT, local adjuvant treatments can be used in addition to surgery. Following pregnancy, denosumab can be used systemically, and can be effective with metastatic or unresectable disease.
Giant cell tumor (GCT) of bone accounts for about 5% of primary bone tumors.1-3 Only 3% to 5% of GCTs occur in the hand.4,5 Wrist involvement, which is rare, most often involves the hamate bone.5-7 Capitate bone involvement is exceedingly rare.8-11 Although histologically benign, GCT can recur locally after treatment with curettage alone, and lung metastases are found in 2% to 5% of cases.2,12-14 Therefore, en bloc tumor excision is preferred in the setting of cortical erosion or soft-tissue involvement.1,4,8 Wrist joint motion is inevitably reduced, and bone graft donor-site morbidity is significant.6-8
In the unusual case reported here, GCT presented in the capitate bone and, after the patient became pregnant, recurred in the hamate and trapezoid bones with soft-tissue extension and lung metastases. The capitate was excised en bloc and reconstructed with an interposition of polymethylmethacrylate bone cement. Pulmonary metastases developed, and the GCT expanded to involve multiple carpal bones and the bases of the second through fourth metacarpals. A 10-month course of systemic chemotherapy with the RANK ligand (RANKL) inhibitor denosumab was started after the pregnancy. After this treatment, the patient underwent both tumor resection and reconstruction with autogenous bicortical iliac crest bone graft (ICBG) carefully designed to preserve range of motion and maintain the fingers in anatomical position. Treatment with denosumab was continued after surgery. Although this case offers no endpoint for postoperative chemotherapy with denosumab, preoperative treatment dramatically reduced the GCT and permitted limb-sparing reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 19-year-old right-handed woman with atraumatic swelling of the left wrist presented to an orthopedic surgeon at an outside facility. Physical examination revealed tender fullness on the dorsum of the wrist, slightly reduced range of motion and grip strength, and a neurovascularly intact wrist. The diagnosis was periarticular cyst, and the patient underwent physical therapy. Two years later, the swelling returned, tenderness was increasing, and symptoms did not resolve with cast immobilization. A radiograph showed a lytic lesion in the capitate bone (Figure 1).[[{"fid":"202332","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":""}}}]]
GCT was diagnosed with percutaneous needle biopsy. A preoperative chest radiograph was reported normal. For initial treatment, the capitate and trapezoid bones were resected en bloc through a dorsal approach. Reconstruction consisted of limited arthrodesis using bone cement without additional fixation.
At 6-month follow-up, the patient was pregnant, and there was a recurrence of the wrist lesion. During the first 2 months of pregnancy, swelling and pain rapidly progressed, and a palpable mass formed. Radiographs showed a lytic lesion extending into the hamate bone (Figure 2), and magnetic resonance imaging (MRI) showed articular extension of the lesion with involvement of the base of the fourth metacarpal. [[{"fid":"202334","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"2"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"2":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":""}}}]]Targeted anti-RANKL therapy was not recommended (and was not available at the patient’s home hospital). The patient deferred surgical treatment because of the pregnancy, which proved otherwise uneventful and ended with a full-term delivery.
After the pregnancy, radiographs of the wrist showed complete destruction of the hamate and trapezium bones, with erosion of the bases of the second through fourth metacarpals (Figure 3A). [[{"fid":"202335","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"3"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"3":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":""}}}]]The patient presented at our institution 4 years after initial diagnosis. Computed tomography (CT) of the chest showed numerous bilateral pulmonary nodular opacities. Wrist imaging showed soft-tissue extension (Figure 3B). The diagnosis of recurrent metastatic GCT was confirmed with needle biopsies of the wrist mass and the right lung nodule.
Systemic chemotherapy was initiated with 120 mg of denosumab, given subcutaneously on days 1, 8, and 15 and then monthly during the 10 months leading up to surgery. Serum calcium was monitored during treatment and remained within the normal range the entire time, except for once at the start of therapy, when it dropped to 6.8 mg/dL. After 8 months, the soft-tissue mass, originally 8 cm × 8 cm × 6 cm, shrunk and stabilized at 5 cm × 4 cm × 4 cm (Figure 3B), and a bony shell reformed around it. Nodules in both lung fields showed response to denosumab.
Histologic examination revealed scattered osteoclast-like, multinucleated giant cells, consistent with a recurrent lesion (Figure 4). [[{"fid":"202336","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"4"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 4.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"4":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 4.","field_file_image_credit[und][0][value]":""}}}]]After 10 months of treatment with denosumab, the patient underwent resection (dorsal approach) of the residual cement, the soft-tissue mass, the affected carpal bones, half of the third metacarpal, and the second and fourth metacarpal bases. The proximal carpal row was preserved after no intra-articular involvement was verified. The closet margin was marginal; the tumor mass abutted without encompassing the flexor tendons and median nerve. The tumor was meticulously elevated from the neurovascular and tendinous structures, which were not sacrificed. Hydrogen peroxide was used for local adjuvant treatment. Bicortical autogenous ICBG was placed between the remaining scaphoid, lunate, and metacarpal bones. The second, third, and fourth metacarpal bases were stabilized on the overlapping outer table of ICBG with 2.0-mm plates and miniscrews (Figure 5A). Kirschner wires were used to stabilize the proximal bone graft and the scapholunate fossa. Cancellous bone graft was packed between the structural bone graft and neighboring unaffected carpal bones (Figure 5A). Immobilization with a short-arm thumb spica cast was maintained for 6 weeks after surgery and was followed by a 12-week rehabilitation program. The patient returned to normal activities when plain radiographs showed solid bony union (Figure 5B). Fourteen months after initial surgery, tenolysis was performed to free the extensor tendons (index, middle, and ring fingers on dorsum of left hand) from adhesions to the bone graft. At 37-month follow-up (Figure 5C), there was no clinical or radiographic evidence of progression in the wrist.[[{"fid":"202337","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"5"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 5.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"5":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 5.","field_file_image_credit[und][0][value]":""}}}]]
The patient had bilateral pulmonary metastases (Figures 6A, 6B). Treatment with denosumab produced an initial response (smaller pulmonary lesions) and subsequent stability. After 12 months of treatment with denosumab, the patient underwent left thoracotomy and wedge resection of pulmonary metastases on the left. Pathologic evaluation revealed pulmonary parenchyma with calcification and ossification and limited viable tumor. Given the dramatic effects on the left pulmonary metastases, denosumab was continued, and surgical intervention on the right was not attempted. Pulmonary metastases were stable afterward (Figure 6C).[[{"fid":"202338","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"6"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 6.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"6":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 6.","field_file_image_credit[und][0][value]":""}}}]]
At 54-month follow-up, systemic treatment with denosumab was continued. The patient had no pain in the wrist or hand and was able to use the left hand normally. There was some fissuring of the third and fourth digits over each other. However, the patient had good grip strength and was using eating utensils, picking up water bottles, and engaging in other activities without difficulty.
Discussion
GCT isolated to the carpus is rare. However, compared with GCT in the more common locations in long bones, it is also more aggressive, and its local recurrence rates are higher, probably 60% or more if treated with curettage alone.15 Therefore, excision augmented with adjuvant treatment is recommended.2,7 Use of bone cement in the hand is relatively uncommon.4,5,7-10
The diagnosis of GCT in the carpus is difficult and often delayed. The initial complaint is usually mild wrist pain after relatively mild trauma.5 The first reported case of GCT in the lunate bone was mistakenly thought to be Kienbock disease.5 Similarly, our patient was initially given a nononcologic diagnosis, which prompted conservative management.
Whether the biological behavior of GCT in the carpus differs from that of GCT in other sites is unclear. The high recurrence rates might be attributable in part to suboptimal curettage.5,6 En bloc resections of involved bone inevitably result in carpal instability or loss of wrist motion if arthrodesis is performed.4-7,11 In the present case, resection was followed by limited arthrodesis to mitigate motion losses.
Multifocal GCT in the carpal bones often affects younger patients and has a high rate of recurrence.7,16 In the present case, the patient’s pregnancy delayed treatment and allowed tumor extension into soft tissues and metacarpal bones. Given her young age, en bloc tumor resection was performed, with the proximal carpal row spared to preserve wrist motion. ICBG was carefully shaped to match the defect that remained after tumor resection.7 Supporting wrist height to prevent carpal collapse provided a stable base for remaining distal segments of the second through fourth metacarpals. After short-arm thumb spica casting and early rehabilitation, the patient recovered wrist motion and use of the involved fingers distal to the carpometacarpal joints.
In pregnant women, GCTs have been found primarily in the long bones and spine but are rare.17-21 A review of the literature (1950-present) revealed that the present article is the first report of GCT in the hand or wrist bones of a pregnant woman.18,20,21 There is no consensus as to whether surgical excision should be performed during pregnancy.18,20,21 In 1 unusual case, at 18 weeks’ gestation GCT in the distal femur was resected with curettage and bone grafting, and there were no complications.21 Therefore, pregnancy termination is not indicated for GCT.
The relationship between tumorigenesis and pregnancy is unclear.18,20,21 Empirically, pregnancy is thought to promote tumor growth.18,20 Estrogen and progesterone levels are elevated during pregnancy, potentially influencing tumor cells that are hormonally sensitive.18,20 An early report in which reverse transcription–polymerase chain reaction showed estrogen receptor expression in GCT osteoclast-like cells was followed by several studies that failed to find estrogen receptors at the protein level.19 In contrast, progesterone receptors were found in 50% of GCTs in a study.22 However, the etiopathogenic significance of this is unclear. In pregnant women, vascular endothelial growth factor, placental growth factor, and other growth factors induce osteoclast formation.23 ß-Human chorionic gonadotropin expression (ß-hCG) has been found in 58% of cases, with some showing ß-hCG elevation in the serum.24 Other studies have focused on an immunologic explanation for occurrence of GCT during pregnancy.18 Oncofetal antigens, which are similar to fetal antigens, have been found in fibrosarcoma and in an osteosarcoma cell line but not in GCT.18-20 Thus, though occurrence during pregnancy may be coincidental given the frequency of GCT in women of childbearing age, it is plausible that tumor growth may be enhanced by pregnancy. More studies are needed to understand the relationship between giant cell proliferation and pregnancy-related growth factors and hormones.
With GCT, the rate of pulmonary metastases ranges from 0% to 4%; these metastases are usually diagnosed at time of local recurrence, or 2 years to 3 years after initial GCT diagnosis.2,3,12,14,25 Lung metastases secondary to GCT in the hand or foot bones are rare; our literature review identified only 4 cases.12,14 Risk factors for lung metastasis include local recurrence, aggressive appearance (Enneking grade 3) on radiograph, Ki-67 antigen expression, and distal radius location.14 The mechanism of metastasis is unknown.12,14
Lung metastases are usually excised, but they may spontaneously evolve toward necrosis and ossification.12 In cases in which surgery is unfeasible, chemotherapy (eg, with doxorubicin) has been used to control progression.12,14 Radiation can cause sarcomatous transformation and is contraindicated. Interferon26-28 and other antiangiogenic strategies have been successfully used in systemic therapy for GCT of bone. More recently, bisphosphonates29-32 and denosumab33 have been investigated.29,32-36 The limited toxicity of denosumab makes the drug a very attractive treatment option for recurrent or unresectable GCT of bone.33 Reported rates of mortality from lung metastases have ranged from 0% to 40%.14 There is evidence that control of lung metastases during the first 3 years after diagnosis is important for favorable outcomes.2,3
Malignant stromal cells of GCT of bone have been known to secrete RANKL, which recruits osteoclasts and osteoclast precursor cells, which in turn generate aggressive osteolytic activity.33,37 Denosumab, a monoclonal antibody that inhibits RANKL, is effective in stopping osteoclastic activity. In a phase 2 trial of denosumab in the treatment of GCT of bone, 96% of treated patients with unresectable disease showed no progression at 13 months.38 In addition, 74% of treated patients who had resectable disease but were likely to have morbid surgery did not require surgery, and 62% of treated patients who underwent surgery were able to have a less morbid procedure. Forty-one percent to 58% of treated patients had a reduction in tumor size.
Denosumab is very well tolerated. The phase 2 trial found serious adverse events in 9% of patients, and in 5% of cases the drug was discontinued because of toxicity.38 Serious adverse events include osteonecrosis of jaw, hypocalcemia, and hypophosphatemia.37 Electrolyte changes with denosumab are easy to monitor and manage. Although the favorable toxicity profile of denosumab allows for long-term therapy, the data on therapy duration in patients with unresectable disease are unclear. Patients who discontinue therapy should be closely monitored, as disease can progress in this setting.37
In contrast to GCT of larger bones, GCT of the wrist is rare and typically more aggressive, and has higher local recurrence rates. In many cases, diagnosis is delayed by insufficient imaging, which optimally should include either CT or MRI (Table). [[{"fid":"202341","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"7"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"7":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table.","field_file_image_credit[und][0][value]":""}}}]]For pregnant women with GCT, options include surgical resection with curettage and local adjuvant treatment. After pregnancy, denosumab can be used systemically, and can be effective with metastatic or unresectable disease. Surgical treatment in the wrist can be challenging when partial or complete resections of carpal bones are required. Occupational therapy is recommended for optimization of hand function after surgery.
Take-Home Points
- GCT of bones of the wrist is rare. This article is the only report of a wrist GCT during pregnancy that we could identify.
- Routine treatment usually consists of surgical excision with local adjuvant, and in the wrist, often results in reduced wrist motion.
- GCT of the wrist is more aggressive than the more common locations in long bones, with higher local recurrence rates if treated with surgery alone.
- Diagnosis is often delayed for GCT of the wrist, due to insufficient imaging, which should include CT or MRI.
- For pregnant women with GCT, local adjuvant treatments can be used in addition to surgery. Following pregnancy, denosumab can be used systemically, and can be effective with metastatic or unresectable disease.
Giant cell tumor (GCT) of bone accounts for about 5% of primary bone tumors.1-3 Only 3% to 5% of GCTs occur in the hand.4,5 Wrist involvement, which is rare, most often involves the hamate bone.5-7 Capitate bone involvement is exceedingly rare.8-11 Although histologically benign, GCT can recur locally after treatment with curettage alone, and lung metastases are found in 2% to 5% of cases.2,12-14 Therefore, en bloc tumor excision is preferred in the setting of cortical erosion or soft-tissue involvement.1,4,8 Wrist joint motion is inevitably reduced, and bone graft donor-site morbidity is significant.6-8
In the unusual case reported here, GCT presented in the capitate bone and, after the patient became pregnant, recurred in the hamate and trapezoid bones with soft-tissue extension and lung metastases. The capitate was excised en bloc and reconstructed with an interposition of polymethylmethacrylate bone cement. Pulmonary metastases developed, and the GCT expanded to involve multiple carpal bones and the bases of the second through fourth metacarpals. A 10-month course of systemic chemotherapy with the RANK ligand (RANKL) inhibitor denosumab was started after the pregnancy. After this treatment, the patient underwent both tumor resection and reconstruction with autogenous bicortical iliac crest bone graft (ICBG) carefully designed to preserve range of motion and maintain the fingers in anatomical position. Treatment with denosumab was continued after surgery. Although this case offers no endpoint for postoperative chemotherapy with denosumab, preoperative treatment dramatically reduced the GCT and permitted limb-sparing reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 19-year-old right-handed woman with atraumatic swelling of the left wrist presented to an orthopedic surgeon at an outside facility. Physical examination revealed tender fullness on the dorsum of the wrist, slightly reduced range of motion and grip strength, and a neurovascularly intact wrist. The diagnosis was periarticular cyst, and the patient underwent physical therapy. Two years later, the swelling returned, tenderness was increasing, and symptoms did not resolve with cast immobilization. A radiograph showed a lytic lesion in the capitate bone (Figure 1).[[{"fid":"202332","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":""}}}]]
GCT was diagnosed with percutaneous needle biopsy. A preoperative chest radiograph was reported normal. For initial treatment, the capitate and trapezoid bones were resected en bloc through a dorsal approach. Reconstruction consisted of limited arthrodesis using bone cement without additional fixation.
At 6-month follow-up, the patient was pregnant, and there was a recurrence of the wrist lesion. During the first 2 months of pregnancy, swelling and pain rapidly progressed, and a palpable mass formed. Radiographs showed a lytic lesion extending into the hamate bone (Figure 2), and magnetic resonance imaging (MRI) showed articular extension of the lesion with involvement of the base of the fourth metacarpal. [[{"fid":"202334","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"2"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"2":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":""}}}]]Targeted anti-RANKL therapy was not recommended (and was not available at the patient’s home hospital). The patient deferred surgical treatment because of the pregnancy, which proved otherwise uneventful and ended with a full-term delivery.
After the pregnancy, radiographs of the wrist showed complete destruction of the hamate and trapezium bones, with erosion of the bases of the second through fourth metacarpals (Figure 3A). [[{"fid":"202335","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"3"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"3":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":""}}}]]The patient presented at our institution 4 years after initial diagnosis. Computed tomography (CT) of the chest showed numerous bilateral pulmonary nodular opacities. Wrist imaging showed soft-tissue extension (Figure 3B). The diagnosis of recurrent metastatic GCT was confirmed with needle biopsies of the wrist mass and the right lung nodule.
Systemic chemotherapy was initiated with 120 mg of denosumab, given subcutaneously on days 1, 8, and 15 and then monthly during the 10 months leading up to surgery. Serum calcium was monitored during treatment and remained within the normal range the entire time, except for once at the start of therapy, when it dropped to 6.8 mg/dL. After 8 months, the soft-tissue mass, originally 8 cm × 8 cm × 6 cm, shrunk and stabilized at 5 cm × 4 cm × 4 cm (Figure 3B), and a bony shell reformed around it. Nodules in both lung fields showed response to denosumab.
Histologic examination revealed scattered osteoclast-like, multinucleated giant cells, consistent with a recurrent lesion (Figure 4). [[{"fid":"202336","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"4"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 4.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"4":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 4.","field_file_image_credit[und][0][value]":""}}}]]After 10 months of treatment with denosumab, the patient underwent resection (dorsal approach) of the residual cement, the soft-tissue mass, the affected carpal bones, half of the third metacarpal, and the second and fourth metacarpal bases. The proximal carpal row was preserved after no intra-articular involvement was verified. The closet margin was marginal; the tumor mass abutted without encompassing the flexor tendons and median nerve. The tumor was meticulously elevated from the neurovascular and tendinous structures, which were not sacrificed. Hydrogen peroxide was used for local adjuvant treatment. Bicortical autogenous ICBG was placed between the remaining scaphoid, lunate, and metacarpal bones. The second, third, and fourth metacarpal bases were stabilized on the overlapping outer table of ICBG with 2.0-mm plates and miniscrews (Figure 5A). Kirschner wires were used to stabilize the proximal bone graft and the scapholunate fossa. Cancellous bone graft was packed between the structural bone graft and neighboring unaffected carpal bones (Figure 5A). Immobilization with a short-arm thumb spica cast was maintained for 6 weeks after surgery and was followed by a 12-week rehabilitation program. The patient returned to normal activities when plain radiographs showed solid bony union (Figure 5B). Fourteen months after initial surgery, tenolysis was performed to free the extensor tendons (index, middle, and ring fingers on dorsum of left hand) from adhesions to the bone graft. At 37-month follow-up (Figure 5C), there was no clinical or radiographic evidence of progression in the wrist.[[{"fid":"202337","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"5"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 5.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"5":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 5.","field_file_image_credit[und][0][value]":""}}}]]
The patient had bilateral pulmonary metastases (Figures 6A, 6B). Treatment with denosumab produced an initial response (smaller pulmonary lesions) and subsequent stability. After 12 months of treatment with denosumab, the patient underwent left thoracotomy and wedge resection of pulmonary metastases on the left. Pathologic evaluation revealed pulmonary parenchyma with calcification and ossification and limited viable tumor. Given the dramatic effects on the left pulmonary metastases, denosumab was continued, and surgical intervention on the right was not attempted. Pulmonary metastases were stable afterward (Figure 6C).[[{"fid":"202338","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"6"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 6.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"6":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 6.","field_file_image_credit[und][0][value]":""}}}]]
At 54-month follow-up, systemic treatment with denosumab was continued. The patient had no pain in the wrist or hand and was able to use the left hand normally. There was some fissuring of the third and fourth digits over each other. However, the patient had good grip strength and was using eating utensils, picking up water bottles, and engaging in other activities without difficulty.
Discussion
GCT isolated to the carpus is rare. However, compared with GCT in the more common locations in long bones, it is also more aggressive, and its local recurrence rates are higher, probably 60% or more if treated with curettage alone.15 Therefore, excision augmented with adjuvant treatment is recommended.2,7 Use of bone cement in the hand is relatively uncommon.4,5,7-10
The diagnosis of GCT in the carpus is difficult and often delayed. The initial complaint is usually mild wrist pain after relatively mild trauma.5 The first reported case of GCT in the lunate bone was mistakenly thought to be Kienbock disease.5 Similarly, our patient was initially given a nononcologic diagnosis, which prompted conservative management.
Whether the biological behavior of GCT in the carpus differs from that of GCT in other sites is unclear. The high recurrence rates might be attributable in part to suboptimal curettage.5,6 En bloc resections of involved bone inevitably result in carpal instability or loss of wrist motion if arthrodesis is performed.4-7,11 In the present case, resection was followed by limited arthrodesis to mitigate motion losses.
Multifocal GCT in the carpal bones often affects younger patients and has a high rate of recurrence.7,16 In the present case, the patient’s pregnancy delayed treatment and allowed tumor extension into soft tissues and metacarpal bones. Given her young age, en bloc tumor resection was performed, with the proximal carpal row spared to preserve wrist motion. ICBG was carefully shaped to match the defect that remained after tumor resection.7 Supporting wrist height to prevent carpal collapse provided a stable base for remaining distal segments of the second through fourth metacarpals. After short-arm thumb spica casting and early rehabilitation, the patient recovered wrist motion and use of the involved fingers distal to the carpometacarpal joints.
In pregnant women, GCTs have been found primarily in the long bones and spine but are rare.17-21 A review of the literature (1950-present) revealed that the present article is the first report of GCT in the hand or wrist bones of a pregnant woman.18,20,21 There is no consensus as to whether surgical excision should be performed during pregnancy.18,20,21 In 1 unusual case, at 18 weeks’ gestation GCT in the distal femur was resected with curettage and bone grafting, and there were no complications.21 Therefore, pregnancy termination is not indicated for GCT.
The relationship between tumorigenesis and pregnancy is unclear.18,20,21 Empirically, pregnancy is thought to promote tumor growth.18,20 Estrogen and progesterone levels are elevated during pregnancy, potentially influencing tumor cells that are hormonally sensitive.18,20 An early report in which reverse transcription–polymerase chain reaction showed estrogen receptor expression in GCT osteoclast-like cells was followed by several studies that failed to find estrogen receptors at the protein level.19 In contrast, progesterone receptors were found in 50% of GCTs in a study.22 However, the etiopathogenic significance of this is unclear. In pregnant women, vascular endothelial growth factor, placental growth factor, and other growth factors induce osteoclast formation.23 ß-Human chorionic gonadotropin expression (ß-hCG) has been found in 58% of cases, with some showing ß-hCG elevation in the serum.24 Other studies have focused on an immunologic explanation for occurrence of GCT during pregnancy.18 Oncofetal antigens, which are similar to fetal antigens, have been found in fibrosarcoma and in an osteosarcoma cell line but not in GCT.18-20 Thus, though occurrence during pregnancy may be coincidental given the frequency of GCT in women of childbearing age, it is plausible that tumor growth may be enhanced by pregnancy. More studies are needed to understand the relationship between giant cell proliferation and pregnancy-related growth factors and hormones.
With GCT, the rate of pulmonary metastases ranges from 0% to 4%; these metastases are usually diagnosed at time of local recurrence, or 2 years to 3 years after initial GCT diagnosis.2,3,12,14,25 Lung metastases secondary to GCT in the hand or foot bones are rare; our literature review identified only 4 cases.12,14 Risk factors for lung metastasis include local recurrence, aggressive appearance (Enneking grade 3) on radiograph, Ki-67 antigen expression, and distal radius location.14 The mechanism of metastasis is unknown.12,14
Lung metastases are usually excised, but they may spontaneously evolve toward necrosis and ossification.12 In cases in which surgery is unfeasible, chemotherapy (eg, with doxorubicin) has been used to control progression.12,14 Radiation can cause sarcomatous transformation and is contraindicated. Interferon26-28 and other antiangiogenic strategies have been successfully used in systemic therapy for GCT of bone. More recently, bisphosphonates29-32 and denosumab33 have been investigated.29,32-36 The limited toxicity of denosumab makes the drug a very attractive treatment option for recurrent or unresectable GCT of bone.33 Reported rates of mortality from lung metastases have ranged from 0% to 40%.14 There is evidence that control of lung metastases during the first 3 years after diagnosis is important for favorable outcomes.2,3
Malignant stromal cells of GCT of bone have been known to secrete RANKL, which recruits osteoclasts and osteoclast precursor cells, which in turn generate aggressive osteolytic activity.33,37 Denosumab, a monoclonal antibody that inhibits RANKL, is effective in stopping osteoclastic activity. In a phase 2 trial of denosumab in the treatment of GCT of bone, 96% of treated patients with unresectable disease showed no progression at 13 months.38 In addition, 74% of treated patients who had resectable disease but were likely to have morbid surgery did not require surgery, and 62% of treated patients who underwent surgery were able to have a less morbid procedure. Forty-one percent to 58% of treated patients had a reduction in tumor size.
Denosumab is very well tolerated. The phase 2 trial found serious adverse events in 9% of patients, and in 5% of cases the drug was discontinued because of toxicity.38 Serious adverse events include osteonecrosis of jaw, hypocalcemia, and hypophosphatemia.37 Electrolyte changes with denosumab are easy to monitor and manage. Although the favorable toxicity profile of denosumab allows for long-term therapy, the data on therapy duration in patients with unresectable disease are unclear. Patients who discontinue therapy should be closely monitored, as disease can progress in this setting.37
In contrast to GCT of larger bones, GCT of the wrist is rare and typically more aggressive, and has higher local recurrence rates. In many cases, diagnosis is delayed by insufficient imaging, which optimally should include either CT or MRI (Table). [[{"fid":"202341","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"7"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"7":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table.","field_file_image_credit[und][0][value]":""}}}]]For pregnant women with GCT, options include surgical resection with curettage and local adjuvant treatment. After pregnancy, denosumab can be used systemically, and can be effective with metastatic or unresectable disease. Surgical treatment in the wrist can be challenging when partial or complete resections of carpal bones are required. Occupational therapy is recommended for optimization of hand function after surgery.
1. Balke M, Ahrens H, Streitbuerger A, et al. Treatment options for recurrent giant cell tumors of bone. J Cancer Res Clin Oncol. 2009;135(1):149-158.
2. Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Giant cell tumor of bone risk factors for recurrence. Clin Orthop Relat Res. 2011;469(2):591-599.
3. Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Recurrent giant cell tumor of long bones: analysis of surgical management. Clin Orthop Relat Res. 2011;469(4):1181-1187.
4. Averill RM, Smith RJ, Campbell CJ. Giant-cell tumors of the bones of the hand. J Hand Surg Am. 1980;5(1):39-50.
5. Shigematsu K, Kobata Y, Yajima H, Kawamura K, Maegawa N, Takakura Y. Giant-cell tumors of the carpus. J Hand Surg Am. 2006;31(7):1214-1219.
6. Gupta GG, Lucas GL, Pirela-Cruz M. Multifocal giant cell tumor of the capitate, hamate, and triquetrum: a case report. J Hand Surg Am. 1995;20(6):1003-1006.
7. Tarng YW, Yang SW, Hsu CJ. Surgical treatment of multifocal giant cell tumor of carpal bones with preservation of wrist function: case report. J Hand Surg Am. 2009;34(2):262-265.
8. Angelini A, Mavrogenis AF, Ruggieri P. Giant cell tumor of the capitate. Musculoskelet Surg. 2011;95(1):45-48.
9. Howard FM, Lassen K. Giant cell tumor of the capitate. J Hand Surg Am. 1984;9(2):272-274.
10. McDonald DJ, Schajowicz F. Giant cell tumor of the capitate. A case report. Clin Orthop Relat Res. 1992(279):264-268.
11. Wilson SC, Cascio BM, Plauche HR. Giant-cell tumor of the capitate. Orthopedics. 2001;24(11):1085-1086.
12. Combalia-Aleu A, Sastre S, Fernández-de-Retana P, Tomás X, Palacin A. Giant cell tumor of the talus with pulmonary metastasis: seven years follow up. Foot. 2006;16(2):107-111.
13. Donthineni R, Boriani L, Ofluoglu O, Bandiera S. Metastatic behaviour of giant cell tumour of the spine. Int Orthop. 2009;33(2):497-501.
14. Jacopin S, Viehweger E, Glard Y, et al. Fatal lung metastasis secondary to index finger giant cell tumor in an 8-year-old child. Orthop Traumatol Surg Res. 2010;96(3):310-313.
15. Plate AM, Lee SJ, Steiner G, Posner MA. Tumor-like lesions and benign tumors of the hand and wrist. J Am Acad Orthop Surg. 2003;11(2):129-141.
16. Moreel P, Le Viet D. Failure of initial surgical treatment of a giant cell tumor of the capitate and its salvage: a case report [in French]. Chir Main. 2006;25(6):315-318.
17. Caillouette JC, Mattar N. Massive peripheral giant-cell reparative granuloma of the jaw: a pregnancy dependent tumor. Trans Pac Coast Obstet Gynecol Soc. 1978;45:78-81.
18. Kathiresan AS, Johnson JN, Hood BJ, Montoya SP, Vanni S, Gonzalez-Quintero VH. Giant cell bone tumor of the thoracic spine presenting in late pregnancy. Obstet Gynecol. 2011;118(2 pt 2):428-431.
19. Komiya S, Zenmyo M, Inoue A. Bone tumors in the pelvis presenting growth during pregnancy. Arch Orthop Trauma Surg. 1999;119(1-2):22-29.
20. Ross AE, Bojescul JA, Kuklo TR. Giant cell tumor: a case report of recurrence during pregnancy. Spine. 2005;30(12):E332-3E35.
21. Sharma JB, Chanana C, Rastogi, et al. Successful pregnancy outcome with elective caesarean section following two attempts of surgical excision of large giant cell tumor of the lower limb during pregnancy. Arch Gynecol Obstet. 2006;274(5):313-315.
22. Demertzis N, Kotsiandri F, Giotis I, Apostolikas N. Giant-cell tumors of bone and progesterone receptors. Orthopedics. 2003;26(12):1209-1212.
23. Taylor RM, Kashima TG, Knowles HJ, Athanasou NA. VEGF, FLT3 ligand, PlGF and HGF can substitute for M-CSF to induce human osteoclast formation: implications for giant cell tumour pathobiology. Lab Invest. 2012;92(10):1398-1406.
24. Lawless ME, Jour G, Hoch BL, Rendi MH. Beta-human chorionic gonadotropin expression in recurrent and metastatic giant cell tumors of bone: a potential mimicker of germ cell tumor. Int J Surg Pathol. 2014;22(7):617-622.
25. Viswanathan S, Jambhekar NA. Metastatic giant cell tumor of bone: are there associated factors and best treatment modalities? Clin Orthop Relat Res. 2010;468(3):827-833.
26. Kaban LB, Troulis MJ, Ebb D, August M, Hornicek FJ, Dodson TB. Antiangiogenic therapy with interferon alpha for giant cell lesions of the jaws. J Oral Maxillofac Surg. 2002;60(10):1103-1111.
27. Kaiser U, Neumann K, Havemann K. Generalised giant-cell tumour of bone: successful treatment of pulmonary metastases with interferon alpha, a case report. J Cancer Res Clin Oncol. 1993;119(5):301-303.
28. Dickerman JD. Interferon and giant cell tumors. Pediatrics. 1999;103(6 pt 1):1282-1283.
29. Balke M, Campanacci L, Gebert C, et al. Bisphosphonate treatment of aggressive primary, recurrent and metastatic giant cell tumour of bone. BMC Cancer. 2010;10:462.
30. Gille O, Oliveira Bde A, Guerin P, Lepreux S, Richez C, Vital JM. Regression of giant cell tumor of the cervical spine with bisphosphonate as single therapy. Spine. 2012;37(6):E396-E399.
31. Moriceau G, Ory B, Gobin B, et al. Therapeutic approach of primary bone tumours by bisphosphonates. Curr Pharm Des. 2010;16(27):2981-2987.
32. Tse LF, Wong KC, Kumta SM, Huang L, Chow TC, Griffith JF. Bisphosphonates reduce local recurrence in extremity giant cell tumor of bone: a case–control study. Bone. 2008;42(1):68-73.
33. Thomas D, Henshaw R, Skubitz K, et al. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol. 2010;11(3):275-280.
34. Balke M, Hardes J. Denosumab: a breakthrough in treatment of giant-cell tumour of bone? Lancet Oncol. 2010;11(3):218-219.
35. Kyrgidis A, Toulis K. Safety and efficacy of denosumab in giant-cell tumour of bone. Lancet Oncol. 2010;11(6):513-514.
36. Thomas D, Carriere P, Jacobs I. Safety of denosumab in giant-cell tumour of bone. Lancet Oncol. 2010;11(9):815.
37. Skubitz KM. Giant cell tumor of bone: current treatment options. Curr Treat Options Oncol. 2014;15(3):507-518.
38. Chawla S, Henshaw R, Seeger L, et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol. 2013;14(9):901-908.
1. Balke M, Ahrens H, Streitbuerger A, et al. Treatment options for recurrent giant cell tumors of bone. J Cancer Res Clin Oncol. 2009;135(1):149-158.
2. Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Giant cell tumor of bone risk factors for recurrence. Clin Orthop Relat Res. 2011;469(2):591-599.
3. Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Recurrent giant cell tumor of long bones: analysis of surgical management. Clin Orthop Relat Res. 2011;469(4):1181-1187.
4. Averill RM, Smith RJ, Campbell CJ. Giant-cell tumors of the bones of the hand. J Hand Surg Am. 1980;5(1):39-50.
5. Shigematsu K, Kobata Y, Yajima H, Kawamura K, Maegawa N, Takakura Y. Giant-cell tumors of the carpus. J Hand Surg Am. 2006;31(7):1214-1219.
6. Gupta GG, Lucas GL, Pirela-Cruz M. Multifocal giant cell tumor of the capitate, hamate, and triquetrum: a case report. J Hand Surg Am. 1995;20(6):1003-1006.
7. Tarng YW, Yang SW, Hsu CJ. Surgical treatment of multifocal giant cell tumor of carpal bones with preservation of wrist function: case report. J Hand Surg Am. 2009;34(2):262-265.
8. Angelini A, Mavrogenis AF, Ruggieri P. Giant cell tumor of the capitate. Musculoskelet Surg. 2011;95(1):45-48.
9. Howard FM, Lassen K. Giant cell tumor of the capitate. J Hand Surg Am. 1984;9(2):272-274.
10. McDonald DJ, Schajowicz F. Giant cell tumor of the capitate. A case report. Clin Orthop Relat Res. 1992(279):264-268.
11. Wilson SC, Cascio BM, Plauche HR. Giant-cell tumor of the capitate. Orthopedics. 2001;24(11):1085-1086.
12. Combalia-Aleu A, Sastre S, Fernández-de-Retana P, Tomás X, Palacin A. Giant cell tumor of the talus with pulmonary metastasis: seven years follow up. Foot. 2006;16(2):107-111.
13. Donthineni R, Boriani L, Ofluoglu O, Bandiera S. Metastatic behaviour of giant cell tumour of the spine. Int Orthop. 2009;33(2):497-501.
14. Jacopin S, Viehweger E, Glard Y, et al. Fatal lung metastasis secondary to index finger giant cell tumor in an 8-year-old child. Orthop Traumatol Surg Res. 2010;96(3):310-313.
15. Plate AM, Lee SJ, Steiner G, Posner MA. Tumor-like lesions and benign tumors of the hand and wrist. J Am Acad Orthop Surg. 2003;11(2):129-141.
16. Moreel P, Le Viet D. Failure of initial surgical treatment of a giant cell tumor of the capitate and its salvage: a case report [in French]. Chir Main. 2006;25(6):315-318.
17. Caillouette JC, Mattar N. Massive peripheral giant-cell reparative granuloma of the jaw: a pregnancy dependent tumor. Trans Pac Coast Obstet Gynecol Soc. 1978;45:78-81.
18. Kathiresan AS, Johnson JN, Hood BJ, Montoya SP, Vanni S, Gonzalez-Quintero VH. Giant cell bone tumor of the thoracic spine presenting in late pregnancy. Obstet Gynecol. 2011;118(2 pt 2):428-431.
19. Komiya S, Zenmyo M, Inoue A. Bone tumors in the pelvis presenting growth during pregnancy. Arch Orthop Trauma Surg. 1999;119(1-2):22-29.
20. Ross AE, Bojescul JA, Kuklo TR. Giant cell tumor: a case report of recurrence during pregnancy. Spine. 2005;30(12):E332-3E35.
21. Sharma JB, Chanana C, Rastogi, et al. Successful pregnancy outcome with elective caesarean section following two attempts of surgical excision of large giant cell tumor of the lower limb during pregnancy. Arch Gynecol Obstet. 2006;274(5):313-315.
22. Demertzis N, Kotsiandri F, Giotis I, Apostolikas N. Giant-cell tumors of bone and progesterone receptors. Orthopedics. 2003;26(12):1209-1212.
23. Taylor RM, Kashima TG, Knowles HJ, Athanasou NA. VEGF, FLT3 ligand, PlGF and HGF can substitute for M-CSF to induce human osteoclast formation: implications for giant cell tumour pathobiology. Lab Invest. 2012;92(10):1398-1406.
24. Lawless ME, Jour G, Hoch BL, Rendi MH. Beta-human chorionic gonadotropin expression in recurrent and metastatic giant cell tumors of bone: a potential mimicker of germ cell tumor. Int J Surg Pathol. 2014;22(7):617-622.
25. Viswanathan S, Jambhekar NA. Metastatic giant cell tumor of bone: are there associated factors and best treatment modalities? Clin Orthop Relat Res. 2010;468(3):827-833.
26. Kaban LB, Troulis MJ, Ebb D, August M, Hornicek FJ, Dodson TB. Antiangiogenic therapy with interferon alpha for giant cell lesions of the jaws. J Oral Maxillofac Surg. 2002;60(10):1103-1111.
27. Kaiser U, Neumann K, Havemann K. Generalised giant-cell tumour of bone: successful treatment of pulmonary metastases with interferon alpha, a case report. J Cancer Res Clin Oncol. 1993;119(5):301-303.
28. Dickerman JD. Interferon and giant cell tumors. Pediatrics. 1999;103(6 pt 1):1282-1283.
29. Balke M, Campanacci L, Gebert C, et al. Bisphosphonate treatment of aggressive primary, recurrent and metastatic giant cell tumour of bone. BMC Cancer. 2010;10:462.
30. Gille O, Oliveira Bde A, Guerin P, Lepreux S, Richez C, Vital JM. Regression of giant cell tumor of the cervical spine with bisphosphonate as single therapy. Spine. 2012;37(6):E396-E399.
31. Moriceau G, Ory B, Gobin B, et al. Therapeutic approach of primary bone tumours by bisphosphonates. Curr Pharm Des. 2010;16(27):2981-2987.
32. Tse LF, Wong KC, Kumta SM, Huang L, Chow TC, Griffith JF. Bisphosphonates reduce local recurrence in extremity giant cell tumor of bone: a case–control study. Bone. 2008;42(1):68-73.
33. Thomas D, Henshaw R, Skubitz K, et al. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol. 2010;11(3):275-280.
34. Balke M, Hardes J. Denosumab: a breakthrough in treatment of giant-cell tumour of bone? Lancet Oncol. 2010;11(3):218-219.
35. Kyrgidis A, Toulis K. Safety and efficacy of denosumab in giant-cell tumour of bone. Lancet Oncol. 2010;11(6):513-514.
36. Thomas D, Carriere P, Jacobs I. Safety of denosumab in giant-cell tumour of bone. Lancet Oncol. 2010;11(9):815.
37. Skubitz KM. Giant cell tumor of bone: current treatment options. Curr Treat Options Oncol. 2014;15(3):507-518.
38. Chawla S, Henshaw R, Seeger L, et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol. 2013;14(9):901-908.
Can Targeting Risk Factors Avert Dementia?
LONDON—Nine modifiable risk factors may account for about a third of dementia cases, according to an estimate by the Lancet International Commission on Dementia Prevention, Intervention, and Care. The estimate is part of a commission report that was presented at the 2017 Alzheimer’s Association International Conference and published in Lancet.
The report reviews the evidence for pharmacologic, psychologic, environmental, and social interventions for patients with dementia. It includes algorithms for the management of psychosis, agitation, and depression. And it emphasizes the importance of assessing risks to patients (eg, abuse and nutritional deficiencies), caring for family caregivers, and future planning.
“There has been a great deal of focus on developing medicines to prevent dementia,” said Lon S. Schneider, MD, Professor of Psychiatry and Behavioral Sciences at the Keck School of Medicine of University of Southern California in Los Angeles and one of the report’s authors. “But we cannot lose sight of the … advances we have already made in treating dementia, including preventive approaches.”
Nine Risk Factors
The authors reviewed the literature on dementia risk factors and estimated the potential percentage reduction in new cases of dementia if a risk factor were eliminated (ie, the population attributable fraction). The results suggest that approximately 35% of cases of dementia are attributable to a combination of low education level in childhood, hearing loss, hypertension, obesity, smoking, depression, physical inactivity, social isolation, and diabetes. In comparison, eliminating risk from the ApoE ε4 allele would be expected to reduce the incidence of dementia by 7%.
The model assumes a causal association between a risk factor and dementia. Although randomized controlled trials to establish causality are not possible for many dementia risk factors, causal relations are plausible, the authors said. Potential mechanisms include effects on cognitive reserve, brain damage, and brain inflammation. Other potentially modifiable risk factors that were not included in the analysis due to insufficient data include diet, visual impairment, sleep disorders, and particulate air pollution.
“While public health interventions will not delay, prevent, or cure all potentially modifiable dementia, the management of metabolic, mental health, hearing, and cerebrovascular risk factors might push back the onset of many cases for some years,” the report says.
Treatment Approaches
Patients with Alzheimer’s disease or dementia with Lewy bodies should be offered cholinesterase inhibitors at all stages of disease, or memantine for severe dementia, the commission recommends. Because effects on cognition and function are small, clinicians often cannot determine treatment response in individual patients. Side effects of cholinesterase inhibitors (eg, nausea, vomiting, diarrhea, vivid dreams, and cramps) often in
Evidence indicates that nonpharmacologic interventions are superior to antipsychotic medications for the treatment of dementia-related agitation and aggression. Antipsychotic drugs increase the risk of death, cardiovascular adverse events, infection, and excessive sedation and should only be used when symptoms cause distress or increase risk, the report says. Physicians should discuss with the patient, his or her family, and care staff whether the possible benefits of treatment with an antipsychotic drug are likely to outweigh the risks, and they should document the discussion.
“The most effective psychosocial treatments are usually multimodal, individualized care, and train carers in skills, including optimizing communication, coping, and environmental adaptations,” according to the commission report.
—Jake Remaly
Suggested Reading
Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017 Jul 19 [Epub ahead of print].
LONDON—Nine modifiable risk factors may account for about a third of dementia cases, according to an estimate by the Lancet International Commission on Dementia Prevention, Intervention, and Care. The estimate is part of a commission report that was presented at the 2017 Alzheimer’s Association International Conference and published in Lancet.
The report reviews the evidence for pharmacologic, psychologic, environmental, and social interventions for patients with dementia. It includes algorithms for the management of psychosis, agitation, and depression. And it emphasizes the importance of assessing risks to patients (eg, abuse and nutritional deficiencies), caring for family caregivers, and future planning.
“There has been a great deal of focus on developing medicines to prevent dementia,” said Lon S. Schneider, MD, Professor of Psychiatry and Behavioral Sciences at the Keck School of Medicine of University of Southern California in Los Angeles and one of the report’s authors. “But we cannot lose sight of the … advances we have already made in treating dementia, including preventive approaches.”
Nine Risk Factors
The authors reviewed the literature on dementia risk factors and estimated the potential percentage reduction in new cases of dementia if a risk factor were eliminated (ie, the population attributable fraction). The results suggest that approximately 35% of cases of dementia are attributable to a combination of low education level in childhood, hearing loss, hypertension, obesity, smoking, depression, physical inactivity, social isolation, and diabetes. In comparison, eliminating risk from the ApoE ε4 allele would be expected to reduce the incidence of dementia by 7%.
The model assumes a causal association between a risk factor and dementia. Although randomized controlled trials to establish causality are not possible for many dementia risk factors, causal relations are plausible, the authors said. Potential mechanisms include effects on cognitive reserve, brain damage, and brain inflammation. Other potentially modifiable risk factors that were not included in the analysis due to insufficient data include diet, visual impairment, sleep disorders, and particulate air pollution.
“While public health interventions will not delay, prevent, or cure all potentially modifiable dementia, the management of metabolic, mental health, hearing, and cerebrovascular risk factors might push back the onset of many cases for some years,” the report says.
Treatment Approaches
Patients with Alzheimer’s disease or dementia with Lewy bodies should be offered cholinesterase inhibitors at all stages of disease, or memantine for severe dementia, the commission recommends. Because effects on cognition and function are small, clinicians often cannot determine treatment response in individual patients. Side effects of cholinesterase inhibitors (eg, nausea, vomiting, diarrhea, vivid dreams, and cramps) often in
Evidence indicates that nonpharmacologic interventions are superior to antipsychotic medications for the treatment of dementia-related agitation and aggression. Antipsychotic drugs increase the risk of death, cardiovascular adverse events, infection, and excessive sedation and should only be used when symptoms cause distress or increase risk, the report says. Physicians should discuss with the patient, his or her family, and care staff whether the possible benefits of treatment with an antipsychotic drug are likely to outweigh the risks, and they should document the discussion.
“The most effective psychosocial treatments are usually multimodal, individualized care, and train carers in skills, including optimizing communication, coping, and environmental adaptations,” according to the commission report.
—Jake Remaly
Suggested Reading
Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017 Jul 19 [Epub ahead of print].
LONDON—Nine modifiable risk factors may account for about a third of dementia cases, according to an estimate by the Lancet International Commission on Dementia Prevention, Intervention, and Care. The estimate is part of a commission report that was presented at the 2017 Alzheimer’s Association International Conference and published in Lancet.
The report reviews the evidence for pharmacologic, psychologic, environmental, and social interventions for patients with dementia. It includes algorithms for the management of psychosis, agitation, and depression. And it emphasizes the importance of assessing risks to patients (eg, abuse and nutritional deficiencies), caring for family caregivers, and future planning.
“There has been a great deal of focus on developing medicines to prevent dementia,” said Lon S. Schneider, MD, Professor of Psychiatry and Behavioral Sciences at the Keck School of Medicine of University of Southern California in Los Angeles and one of the report’s authors. “But we cannot lose sight of the … advances we have already made in treating dementia, including preventive approaches.”
Nine Risk Factors
The authors reviewed the literature on dementia risk factors and estimated the potential percentage reduction in new cases of dementia if a risk factor were eliminated (ie, the population attributable fraction). The results suggest that approximately 35% of cases of dementia are attributable to a combination of low education level in childhood, hearing loss, hypertension, obesity, smoking, depression, physical inactivity, social isolation, and diabetes. In comparison, eliminating risk from the ApoE ε4 allele would be expected to reduce the incidence of dementia by 7%.
The model assumes a causal association between a risk factor and dementia. Although randomized controlled trials to establish causality are not possible for many dementia risk factors, causal relations are plausible, the authors said. Potential mechanisms include effects on cognitive reserve, brain damage, and brain inflammation. Other potentially modifiable risk factors that were not included in the analysis due to insufficient data include diet, visual impairment, sleep disorders, and particulate air pollution.
“While public health interventions will not delay, prevent, or cure all potentially modifiable dementia, the management of metabolic, mental health, hearing, and cerebrovascular risk factors might push back the onset of many cases for some years,” the report says.
Treatment Approaches
Patients with Alzheimer’s disease or dementia with Lewy bodies should be offered cholinesterase inhibitors at all stages of disease, or memantine for severe dementia, the commission recommends. Because effects on cognition and function are small, clinicians often cannot determine treatment response in individual patients. Side effects of cholinesterase inhibitors (eg, nausea, vomiting, diarrhea, vivid dreams, and cramps) often in
Evidence indicates that nonpharmacologic interventions are superior to antipsychotic medications for the treatment of dementia-related agitation and aggression. Antipsychotic drugs increase the risk of death, cardiovascular adverse events, infection, and excessive sedation and should only be used when symptoms cause distress or increase risk, the report says. Physicians should discuss with the patient, his or her family, and care staff whether the possible benefits of treatment with an antipsychotic drug are likely to outweigh the risks, and they should document the discussion.
“The most effective psychosocial treatments are usually multimodal, individualized care, and train carers in skills, including optimizing communication, coping, and environmental adaptations,” according to the commission report.
—Jake Remaly
Suggested Reading
Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017 Jul 19 [Epub ahead of print].