User login
Dr. Clyde Yancy: CANTOS wows, opens new therapeutic avenues
BARCELONA – For Clyde Yancy, MD, presentation of the bombshell CANTOS trial results at the annual congress of the European Congress of Cardiology made for “a really good day.”
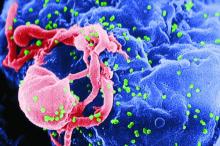
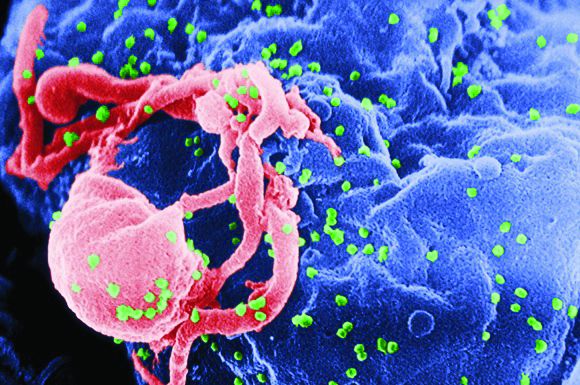
Those results showed that inhibiting the interleukin-1 beta innate immunity pathway with canakinumab reduced recurrent cardiovascular events and lung cancer. But further, they introduced a new way of identifying and treating patients for secondary prevention.
“Here is an alternative way to get to cardiovascular events; here is bringing inflammation right to the front page of what we do as cardiologists to prevent events; here is a brand-new agent that is a monoclonal antibody against interleukin that may be modifying this risk, and … a remarkable advantage that really needs to be replicated,” said Dr. Yancy, chief of medicine-cardiology at Northwestern University in Chicago, in a video interview.
“This is a really good day” because we’ve got new things to think about, new ways to approach our patients, and [we may soon be] entering the realm where we’ll want personalized therapy based on the unique phenotype a patient represents, and think about the pathways to disease through these brand new schemes” that are helping us understand the burden of disease, he declared.
BARCELONA – For Clyde Yancy, MD, presentation of the bombshell CANTOS trial results at the annual congress of the European Congress of Cardiology made for “a really good day.”
Those results showed that inhibiting the interleukin-1 beta innate immunity pathway with canakinumab reduced recurrent cardiovascular events and lung cancer. But further, they introduced a new way of identifying and treating patients for secondary prevention.
“Here is an alternative way to get to cardiovascular events; here is bringing inflammation right to the front page of what we do as cardiologists to prevent events; here is a brand-new agent that is a monoclonal antibody against interleukin that may be modifying this risk, and … a remarkable advantage that really needs to be replicated,” said Dr. Yancy, chief of medicine-cardiology at Northwestern University in Chicago, in a video interview.
“This is a really good day” because we’ve got new things to think about, new ways to approach our patients, and [we may soon be] entering the realm where we’ll want personalized therapy based on the unique phenotype a patient represents, and think about the pathways to disease through these brand new schemes” that are helping us understand the burden of disease, he declared.
BARCELONA – For Clyde Yancy, MD, presentation of the bombshell CANTOS trial results at the annual congress of the European Congress of Cardiology made for “a really good day.”
Those results showed that inhibiting the interleukin-1 beta innate immunity pathway with canakinumab reduced recurrent cardiovascular events and lung cancer. But further, they introduced a new way of identifying and treating patients for secondary prevention.
“Here is an alternative way to get to cardiovascular events; here is bringing inflammation right to the front page of what we do as cardiologists to prevent events; here is a brand-new agent that is a monoclonal antibody against interleukin that may be modifying this risk, and … a remarkable advantage that really needs to be replicated,” said Dr. Yancy, chief of medicine-cardiology at Northwestern University in Chicago, in a video interview.
“This is a really good day” because we’ve got new things to think about, new ways to approach our patients, and [we may soon be] entering the realm where we’ll want personalized therapy based on the unique phenotype a patient represents, and think about the pathways to disease through these brand new schemes” that are helping us understand the burden of disease, he declared.
AT THE ESC CONGRESS 2017
Initiation of ART beneficial in reducing all-cause mortality in AIDS-free patients
Immediate initiation of combined antiretroviral therapy (ART) shows benefits in reducing all-cause mortality in non-AIDS HIV-positive patients aged 50 years or older, according to Sara Lodi, PhD, and her associates.
In a study of 9,596 eligible patients, 2,672 (28%) were U.S. veterans. Results found the 5-year risk of all-cause mortality under immediate ART initiation was 5.3% in the general HIV population and 14.4% in the veterans. The 5-year risk of all-cause mortality was 0.40% lower for the general HIV population and 1.61% lower for veterans compared with immediate initiation versus initiation at CD4 below 350 cells/mm3.
Results also showed rates of all-cause mortality and non-AIDS mortality per 1,000 person-years were 12.3 and 6.3 for the general HIV population and 42.4 and 9.7 for the veterans. In both populations, the observed rates of all-cause and non-AIDS mortality were higher for males and for individuals with lower CD4 count and older age at baseline.
“Immediate initiation of ART appears to be beneficial in reducing all-cause mortality in AIDS-free patients aged 50 years or older, despite their low baseline CD4 count,” the researchers concluded. “More effort should be made into diagnosing HIV earlier, particularly in older patients in order to ensure timely initiation of treatment and follow-up for concomitant comorbidities, thereby maximizing the benefit of early treatment for HIV.”
The study was funded by grants from the NIH and the UK Medical Research Council. Several of the investigators disclosed receiving grants from a number of drug companies.
Read the study in JAIDS (doi: 10.1097/QAI.0000000000001498).
Immediate initiation of combined antiretroviral therapy (ART) shows benefits in reducing all-cause mortality in non-AIDS HIV-positive patients aged 50 years or older, according to Sara Lodi, PhD, and her associates.
In a study of 9,596 eligible patients, 2,672 (28%) were U.S. veterans. Results found the 5-year risk of all-cause mortality under immediate ART initiation was 5.3% in the general HIV population and 14.4% in the veterans. The 5-year risk of all-cause mortality was 0.40% lower for the general HIV population and 1.61% lower for veterans compared with immediate initiation versus initiation at CD4 below 350 cells/mm3.
Results also showed rates of all-cause mortality and non-AIDS mortality per 1,000 person-years were 12.3 and 6.3 for the general HIV population and 42.4 and 9.7 for the veterans. In both populations, the observed rates of all-cause and non-AIDS mortality were higher for males and for individuals with lower CD4 count and older age at baseline.
“Immediate initiation of ART appears to be beneficial in reducing all-cause mortality in AIDS-free patients aged 50 years or older, despite their low baseline CD4 count,” the researchers concluded. “More effort should be made into diagnosing HIV earlier, particularly in older patients in order to ensure timely initiation of treatment and follow-up for concomitant comorbidities, thereby maximizing the benefit of early treatment for HIV.”
The study was funded by grants from the NIH and the UK Medical Research Council. Several of the investigators disclosed receiving grants from a number of drug companies.
Read the study in JAIDS (doi: 10.1097/QAI.0000000000001498).
Immediate initiation of combined antiretroviral therapy (ART) shows benefits in reducing all-cause mortality in non-AIDS HIV-positive patients aged 50 years or older, according to Sara Lodi, PhD, and her associates.
In a study of 9,596 eligible patients, 2,672 (28%) were U.S. veterans. Results found the 5-year risk of all-cause mortality under immediate ART initiation was 5.3% in the general HIV population and 14.4% in the veterans. The 5-year risk of all-cause mortality was 0.40% lower for the general HIV population and 1.61% lower for veterans compared with immediate initiation versus initiation at CD4 below 350 cells/mm3.
Results also showed rates of all-cause mortality and non-AIDS mortality per 1,000 person-years were 12.3 and 6.3 for the general HIV population and 42.4 and 9.7 for the veterans. In both populations, the observed rates of all-cause and non-AIDS mortality were higher for males and for individuals with lower CD4 count and older age at baseline.
“Immediate initiation of ART appears to be beneficial in reducing all-cause mortality in AIDS-free patients aged 50 years or older, despite their low baseline CD4 count,” the researchers concluded. “More effort should be made into diagnosing HIV earlier, particularly in older patients in order to ensure timely initiation of treatment and follow-up for concomitant comorbidities, thereby maximizing the benefit of early treatment for HIV.”
The study was funded by grants from the NIH and the UK Medical Research Council. Several of the investigators disclosed receiving grants from a number of drug companies.
Read the study in JAIDS (doi: 10.1097/QAI.0000000000001498).
FROM THE JOURNAL OF ACQUIRED IMMUNE DEFICIENCY SYNDROMES
Missed opportunities: Opioid overdoses and suicide
The current opioid epidemic in the United States has been universally recognized as one of the most important public health issues to date. This crisis has cost nearly $80 billion in lost productivity, treatment (including emergency, medical, psychiatric, and addiction-specific care), and criminal justice involvement.1 Opioid overdoses have increased by 200% since 2000, with more than 33,000 individuals dying from opioid overdoses in 2015 alone.2,3
Currently, overdoses are considered accidental in origin until proved otherwise, and that assumption has become an acceptable hypothesis for the many parties involved: This hypothesis permits the patient to receive the much-needed overdose treatment, the physicians to discharge the patient from the emergency department after resuscitation and medical stabilization, the hospital to collect reimbursement, the pharmaceutical companies to continue to raise prices – and the health system to ignore recidivism and/or long-term outcomes.
However, while well accepted, the accidental overdose hypothesis might not tell the entire story. A recent, competing etiological hypothesis is that many opioid overdoses may, in fact, be misdiagnosed suicide attempts.7 National suicide prevalence has been increasing since 1999, and both all-cause mortality generally and suicides specifically have been increasing in white, male, and middle-aged patients, which encompass the same demographic groups affected by the opioid epidemic.8,9
Also, more than 50% of patients with opioid use disorder have histories of major depressive disorder,which, when untreated, may further drive suicidal thoughts and behavior.10,11Maria A. Oquendo, MD, PHD, immediate past president of the American Psychiatric Association, wrote in a guest post on the blog of Nora D. Volkow, MD, director of the National Institute on Drug Abuse, about the strong link between opioid use disorders and suicidal thoughts and behavior Furthermore, a 2004 literature review on substance use disorders and suicide found that individuals with opioid use disorders had a 13 times greater risk of completed suicide, compared with the general population.12
Additional associations
A recent study of nearly 5 million veterans enrolled in the Veterans Health Administration demonstrated that, even when adjusted for age and comorbid psychiatric diagnoses, opioid use disorder was associated with an increased risk for suicide; particularly striking was that this risk was doubled in women.13
A survey of 40,000 subjects from the 2014 National Survey on Drug Use and Health demonstrated that prescription opioid misuse was associated with an increased risk of suicidal ideation, and weekly misuse was associated with increased suicide planning and attempts.
The data regarding the prevalence of suicidal ideation in patients who have overdosed are limited, although recent evidence from the National Vital Statistics System on adolescent (aged 15-19 years) drug overdose is concerning, with 772 drug overdoses occurring in this age demographic in 2015 alone. Opioids were involved in the vast majority of fatal drug overdoses among this group, and the prevalence of death from opioid overdoses increased during 1999-2007 (0.8/100,000 to 2.7/100,000), stabilized during 2007-2011, declined during 2012-2014 (down to 2.0/100,000) then increased in 2015 (up to 2.4/100,000). While 80.4% of all drug overdoses in this group (including opioids) were considered unintentional, 13.5% were most likely completed suicides.14
These results suggest that, at the very least, some proportion of opioid overdoses are suicide attempts, and the actual prevalence may be much larger. All of this is difficult to discern as these data come from an epidemiological survey with data input as International Classification of Diseases, 10th revision, codes. Thus, the real-life and real-time quality of the psychiatric and postmortem evaluation that led to the determination of a suicide attempt is unknown. More explicitly, because a thorough evaluation and collateral history may have been lacking, this study may have underestimated the prevalence of overdoses that were actual suicide attempts.
Lessons for physicians
Given the epidemiological evidence linking suicidal thoughts and behavior with opioid use disorders, the frequency of overdoses, demographic factors, and recidivism with naloxone rescue, we should be very concerned that many overdoses are unrecognized suicide attempts. Many physicians can recount giving naloxone to a patient – reversing his or her overdose and simultaneously saving his or her life – only to be confronted with anger and combativeness on the part of the patient. When this response occurs, many physicians may attribute the behavioral dysregulation to the patient’s lack of experience with or tolerance to the drug (especially among naive users) or may disregard the emotional response altogether. The danger in physicians’ reacting like this to such behavior is that substantial ambiguity regarding the patient’s motives still remains: Did the patient intentionally use intravenously thinking he or she would die? Was the patient ambivalent about death? Did the patient wish he or she would die – or not wake up? Or was the patient just was playing a version of “Russian roulette” with needles and lethal quantities of opioids?
When considering logical next steps after naloxone reversal to ensure appropriate diagnosis of and treatment for the patient, a psychiatric consultation and thorough evaluation may be indispensable. This is particularly important given that those who attempt suicide or have active suicidal ideation often are evasive about their behavior and current state of mind.15 Thus, these individuals may be unwilling to disclose active suicidal ideation, intent, and/or plans when interviewed. A psychiatrist, however, has the skill set to evaluate risk and protective factors, assess for other psychiatric comorbidities carefully, and make recommendations for safe disposition and comprehensive treatment. Just as a comprehensive cardiovascular evaluation, formulation of a differential diagnosis, and treatment of chronic cardiovascular disease is the standard of care after a cardiac emergency intervention, we suggest quite similar practice standards for an opioid overdose intervention.
Dr. Srivastava is a fourth-year psychiatry resident at Washington University in St. Louis. Dr. Gold is the 17th Distinguished Alumni Professor at the University of Florida, Gainesville, and professor of psychiatry (adjunct) at Washington University. He also serves as chairman of the scientific advisory boards for RiverMend Health.
References
1. Med Care. 2016 Oct;54:901-6.
2. MMWR Morb Mortal Wkly Rep. 2016 Dec 30;65(5051):1445-52.
3. MMWR Morb Mortal Wkly Rep. 2016 Jan 1;64(50-51):1378-82.
4. N Engl J Med. 2016 Dec 8;375(23):2213-15.
5. Drug Alcohol Depend. 2017 Sep 1;178:176-87.
6. BMJ. 2013 Jan 30;346:f174.
7. Nora’s Blog. 2017 Apr 20. https://www.drugabuse.gov/about-nida/noras-blog/2017/04/opioid-use-disorders-suicide-hidden-tragedy-guest-blog
8. NCHS Data Brief. 2016 Apr;(241):1-8.
9. Proc Natl Acad Sci U S A. 2015 Dec 8;112(49):15078-83.
10. Addict Behav. 2009 Jun-Jul;34(6-7):498-504.
11. J Affect Disord. 2013 May;147(1-3):17-28.
12. Drug Alcohol Depend. 2004 Dec 7;76 Suppl:S11-9.
13. Addiction. 2017 Jul;112(7):1193-1201.
14. NCHS Data Brief. 2017 Aug;282:1-7.
15. Am J Psychiatry. 2003 Nov;160(11 Suppl):1-60.
The current opioid epidemic in the United States has been universally recognized as one of the most important public health issues to date. This crisis has cost nearly $80 billion in lost productivity, treatment (including emergency, medical, psychiatric, and addiction-specific care), and criminal justice involvement.1 Opioid overdoses have increased by 200% since 2000, with more than 33,000 individuals dying from opioid overdoses in 2015 alone.2,3
Currently, overdoses are considered accidental in origin until proved otherwise, and that assumption has become an acceptable hypothesis for the many parties involved: This hypothesis permits the patient to receive the much-needed overdose treatment, the physicians to discharge the patient from the emergency department after resuscitation and medical stabilization, the hospital to collect reimbursement, the pharmaceutical companies to continue to raise prices – and the health system to ignore recidivism and/or long-term outcomes.
However, while well accepted, the accidental overdose hypothesis might not tell the entire story. A recent, competing etiological hypothesis is that many opioid overdoses may, in fact, be misdiagnosed suicide attempts.7 National suicide prevalence has been increasing since 1999, and both all-cause mortality generally and suicides specifically have been increasing in white, male, and middle-aged patients, which encompass the same demographic groups affected by the opioid epidemic.8,9
Also, more than 50% of patients with opioid use disorder have histories of major depressive disorder,which, when untreated, may further drive suicidal thoughts and behavior.10,11Maria A. Oquendo, MD, PHD, immediate past president of the American Psychiatric Association, wrote in a guest post on the blog of Nora D. Volkow, MD, director of the National Institute on Drug Abuse, about the strong link between opioid use disorders and suicidal thoughts and behavior Furthermore, a 2004 literature review on substance use disorders and suicide found that individuals with opioid use disorders had a 13 times greater risk of completed suicide, compared with the general population.12
Additional associations
A recent study of nearly 5 million veterans enrolled in the Veterans Health Administration demonstrated that, even when adjusted for age and comorbid psychiatric diagnoses, opioid use disorder was associated with an increased risk for suicide; particularly striking was that this risk was doubled in women.13
A survey of 40,000 subjects from the 2014 National Survey on Drug Use and Health demonstrated that prescription opioid misuse was associated with an increased risk of suicidal ideation, and weekly misuse was associated with increased suicide planning and attempts.
The data regarding the prevalence of suicidal ideation in patients who have overdosed are limited, although recent evidence from the National Vital Statistics System on adolescent (aged 15-19 years) drug overdose is concerning, with 772 drug overdoses occurring in this age demographic in 2015 alone. Opioids were involved in the vast majority of fatal drug overdoses among this group, and the prevalence of death from opioid overdoses increased during 1999-2007 (0.8/100,000 to 2.7/100,000), stabilized during 2007-2011, declined during 2012-2014 (down to 2.0/100,000) then increased in 2015 (up to 2.4/100,000). While 80.4% of all drug overdoses in this group (including opioids) were considered unintentional, 13.5% were most likely completed suicides.14
These results suggest that, at the very least, some proportion of opioid overdoses are suicide attempts, and the actual prevalence may be much larger. All of this is difficult to discern as these data come from an epidemiological survey with data input as International Classification of Diseases, 10th revision, codes. Thus, the real-life and real-time quality of the psychiatric and postmortem evaluation that led to the determination of a suicide attempt is unknown. More explicitly, because a thorough evaluation and collateral history may have been lacking, this study may have underestimated the prevalence of overdoses that were actual suicide attempts.
Lessons for physicians
Given the epidemiological evidence linking suicidal thoughts and behavior with opioid use disorders, the frequency of overdoses, demographic factors, and recidivism with naloxone rescue, we should be very concerned that many overdoses are unrecognized suicide attempts. Many physicians can recount giving naloxone to a patient – reversing his or her overdose and simultaneously saving his or her life – only to be confronted with anger and combativeness on the part of the patient. When this response occurs, many physicians may attribute the behavioral dysregulation to the patient’s lack of experience with or tolerance to the drug (especially among naive users) or may disregard the emotional response altogether. The danger in physicians’ reacting like this to such behavior is that substantial ambiguity regarding the patient’s motives still remains: Did the patient intentionally use intravenously thinking he or she would die? Was the patient ambivalent about death? Did the patient wish he or she would die – or not wake up? Or was the patient just was playing a version of “Russian roulette” with needles and lethal quantities of opioids?
When considering logical next steps after naloxone reversal to ensure appropriate diagnosis of and treatment for the patient, a psychiatric consultation and thorough evaluation may be indispensable. This is particularly important given that those who attempt suicide or have active suicidal ideation often are evasive about their behavior and current state of mind.15 Thus, these individuals may be unwilling to disclose active suicidal ideation, intent, and/or plans when interviewed. A psychiatrist, however, has the skill set to evaluate risk and protective factors, assess for other psychiatric comorbidities carefully, and make recommendations for safe disposition and comprehensive treatment. Just as a comprehensive cardiovascular evaluation, formulation of a differential diagnosis, and treatment of chronic cardiovascular disease is the standard of care after a cardiac emergency intervention, we suggest quite similar practice standards for an opioid overdose intervention.
Dr. Srivastava is a fourth-year psychiatry resident at Washington University in St. Louis. Dr. Gold is the 17th Distinguished Alumni Professor at the University of Florida, Gainesville, and professor of psychiatry (adjunct) at Washington University. He also serves as chairman of the scientific advisory boards for RiverMend Health.
References
1. Med Care. 2016 Oct;54:901-6.
2. MMWR Morb Mortal Wkly Rep. 2016 Dec 30;65(5051):1445-52.
3. MMWR Morb Mortal Wkly Rep. 2016 Jan 1;64(50-51):1378-82.
4. N Engl J Med. 2016 Dec 8;375(23):2213-15.
5. Drug Alcohol Depend. 2017 Sep 1;178:176-87.
6. BMJ. 2013 Jan 30;346:f174.
7. Nora’s Blog. 2017 Apr 20. https://www.drugabuse.gov/about-nida/noras-blog/2017/04/opioid-use-disorders-suicide-hidden-tragedy-guest-blog
8. NCHS Data Brief. 2016 Apr;(241):1-8.
9. Proc Natl Acad Sci U S A. 2015 Dec 8;112(49):15078-83.
10. Addict Behav. 2009 Jun-Jul;34(6-7):498-504.
11. J Affect Disord. 2013 May;147(1-3):17-28.
12. Drug Alcohol Depend. 2004 Dec 7;76 Suppl:S11-9.
13. Addiction. 2017 Jul;112(7):1193-1201.
14. NCHS Data Brief. 2017 Aug;282:1-7.
15. Am J Psychiatry. 2003 Nov;160(11 Suppl):1-60.
The current opioid epidemic in the United States has been universally recognized as one of the most important public health issues to date. This crisis has cost nearly $80 billion in lost productivity, treatment (including emergency, medical, psychiatric, and addiction-specific care), and criminal justice involvement.1 Opioid overdoses have increased by 200% since 2000, with more than 33,000 individuals dying from opioid overdoses in 2015 alone.2,3
Currently, overdoses are considered accidental in origin until proved otherwise, and that assumption has become an acceptable hypothesis for the many parties involved: This hypothesis permits the patient to receive the much-needed overdose treatment, the physicians to discharge the patient from the emergency department after resuscitation and medical stabilization, the hospital to collect reimbursement, the pharmaceutical companies to continue to raise prices – and the health system to ignore recidivism and/or long-term outcomes.
However, while well accepted, the accidental overdose hypothesis might not tell the entire story. A recent, competing etiological hypothesis is that many opioid overdoses may, in fact, be misdiagnosed suicide attempts.7 National suicide prevalence has been increasing since 1999, and both all-cause mortality generally and suicides specifically have been increasing in white, male, and middle-aged patients, which encompass the same demographic groups affected by the opioid epidemic.8,9
Also, more than 50% of patients with opioid use disorder have histories of major depressive disorder,which, when untreated, may further drive suicidal thoughts and behavior.10,11Maria A. Oquendo, MD, PHD, immediate past president of the American Psychiatric Association, wrote in a guest post on the blog of Nora D. Volkow, MD, director of the National Institute on Drug Abuse, about the strong link between opioid use disorders and suicidal thoughts and behavior Furthermore, a 2004 literature review on substance use disorders and suicide found that individuals with opioid use disorders had a 13 times greater risk of completed suicide, compared with the general population.12
Additional associations
A recent study of nearly 5 million veterans enrolled in the Veterans Health Administration demonstrated that, even when adjusted for age and comorbid psychiatric diagnoses, opioid use disorder was associated with an increased risk for suicide; particularly striking was that this risk was doubled in women.13
A survey of 40,000 subjects from the 2014 National Survey on Drug Use and Health demonstrated that prescription opioid misuse was associated with an increased risk of suicidal ideation, and weekly misuse was associated with increased suicide planning and attempts.
The data regarding the prevalence of suicidal ideation in patients who have overdosed are limited, although recent evidence from the National Vital Statistics System on adolescent (aged 15-19 years) drug overdose is concerning, with 772 drug overdoses occurring in this age demographic in 2015 alone. Opioids were involved in the vast majority of fatal drug overdoses among this group, and the prevalence of death from opioid overdoses increased during 1999-2007 (0.8/100,000 to 2.7/100,000), stabilized during 2007-2011, declined during 2012-2014 (down to 2.0/100,000) then increased in 2015 (up to 2.4/100,000). While 80.4% of all drug overdoses in this group (including opioids) were considered unintentional, 13.5% were most likely completed suicides.14
These results suggest that, at the very least, some proportion of opioid overdoses are suicide attempts, and the actual prevalence may be much larger. All of this is difficult to discern as these data come from an epidemiological survey with data input as International Classification of Diseases, 10th revision, codes. Thus, the real-life and real-time quality of the psychiatric and postmortem evaluation that led to the determination of a suicide attempt is unknown. More explicitly, because a thorough evaluation and collateral history may have been lacking, this study may have underestimated the prevalence of overdoses that were actual suicide attempts.
Lessons for physicians
Given the epidemiological evidence linking suicidal thoughts and behavior with opioid use disorders, the frequency of overdoses, demographic factors, and recidivism with naloxone rescue, we should be very concerned that many overdoses are unrecognized suicide attempts. Many physicians can recount giving naloxone to a patient – reversing his or her overdose and simultaneously saving his or her life – only to be confronted with anger and combativeness on the part of the patient. When this response occurs, many physicians may attribute the behavioral dysregulation to the patient’s lack of experience with or tolerance to the drug (especially among naive users) or may disregard the emotional response altogether. The danger in physicians’ reacting like this to such behavior is that substantial ambiguity regarding the patient’s motives still remains: Did the patient intentionally use intravenously thinking he or she would die? Was the patient ambivalent about death? Did the patient wish he or she would die – or not wake up? Or was the patient just was playing a version of “Russian roulette” with needles and lethal quantities of opioids?
When considering logical next steps after naloxone reversal to ensure appropriate diagnosis of and treatment for the patient, a psychiatric consultation and thorough evaluation may be indispensable. This is particularly important given that those who attempt suicide or have active suicidal ideation often are evasive about their behavior and current state of mind.15 Thus, these individuals may be unwilling to disclose active suicidal ideation, intent, and/or plans when interviewed. A psychiatrist, however, has the skill set to evaluate risk and protective factors, assess for other psychiatric comorbidities carefully, and make recommendations for safe disposition and comprehensive treatment. Just as a comprehensive cardiovascular evaluation, formulation of a differential diagnosis, and treatment of chronic cardiovascular disease is the standard of care after a cardiac emergency intervention, we suggest quite similar practice standards for an opioid overdose intervention.
Dr. Srivastava is a fourth-year psychiatry resident at Washington University in St. Louis. Dr. Gold is the 17th Distinguished Alumni Professor at the University of Florida, Gainesville, and professor of psychiatry (adjunct) at Washington University. He also serves as chairman of the scientific advisory boards for RiverMend Health.
References
1. Med Care. 2016 Oct;54:901-6.
2. MMWR Morb Mortal Wkly Rep. 2016 Dec 30;65(5051):1445-52.
3. MMWR Morb Mortal Wkly Rep. 2016 Jan 1;64(50-51):1378-82.
4. N Engl J Med. 2016 Dec 8;375(23):2213-15.
5. Drug Alcohol Depend. 2017 Sep 1;178:176-87.
6. BMJ. 2013 Jan 30;346:f174.
7. Nora’s Blog. 2017 Apr 20. https://www.drugabuse.gov/about-nida/noras-blog/2017/04/opioid-use-disorders-suicide-hidden-tragedy-guest-blog
8. NCHS Data Brief. 2016 Apr;(241):1-8.
9. Proc Natl Acad Sci U S A. 2015 Dec 8;112(49):15078-83.
10. Addict Behav. 2009 Jun-Jul;34(6-7):498-504.
11. J Affect Disord. 2013 May;147(1-3):17-28.
12. Drug Alcohol Depend. 2004 Dec 7;76 Suppl:S11-9.
13. Addiction. 2017 Jul;112(7):1193-1201.
14. NCHS Data Brief. 2017 Aug;282:1-7.
15. Am J Psychiatry. 2003 Nov;160(11 Suppl):1-60.
Investigational Drug May Effectively Treat Wilson’s Disease
VANCOUVER—The investigational agent bis-choline tetrathiomolybdate (WTX101) may help treat Wilson’s disease, according to a study presented at the 21st International Congress of Parkinson’s Disease and Movement Disorders. The drug lowered circulating copper, and this effect was associated with reduced disability, improved neurologic status without initial paradoxical worsening, and stable liver function.
“With dose adjustments, WTX101 demonstrated a favorable safety profile, and with a simple regimen (once daily in most patients), WTX101 has the potential to address several unmet needs in Wilson’s disease,” said Danny Bega, MD, Assistant Professor of Neurology (Movement Disorders) at Northwestern University’s Feinberg School of Medicine in Chicago, and colleagues.
Wilson’s disease is a rare genetic disorder of impaired copper transport, in which copper accumulates pathologically in the CNS, liver, and other tissues. Current treatments for this disease, such as zinc and chelators (eg, D-penicillamine and trientine), require multiple doses and may be tolerated poorly. To address these challenges, Wilson Therapeutics developed WTX101, an oral first-in-class copper-modulating agent given once daily as monotherapy for Wilson’s disease.
Unlike other treatments for Wilson’s disease, WTX101 appears to have direct intracellular activity in hepatocytes, where it binds excess copper and promotes biliary copper excretion. In addition, WTX101 rapidly binds nonceruloplasmin-bound copper (NCC), thus creating a stable complex with albumin.
Eligible Participants
To evaluate the clinical efficacy and safety of WTX101, as well as the copper control that it enables, in patients with Wilson’s disease, Dr. Bega and colleagues conducted an open-label, single-arm, phase II study at 11 sites in the United States and Europe. Eligible participants were age 18 or older and had been diagnosed with Wilson’s disease based on a Leipzig score of ≥ 4. They also had had no prior treatment for Wilson’s disease or ≤ 24 months of chelation or zinc therapy. Finally, participants’ NCC levels were within or above the normal reference range (≥ 0.8 µM).
Patients received WTX101 for 24 weeks. Researchers used a response-guided dosing regimen with individualized doses of between 15 and 120 mg/day based on NCC levels and safety criteria. In most patients, dosing occurred once daily.
The primary end point was change from baseline to 24 weeks in NCC levels corrected for bound copper contained in tetrathiomolybdate-copper-albumin complexes. Secondary end points included clinical, neurologic, and hepatic assessments.
Of 28 participants involved in the study, 15 were women. The mean age was 34.1. Nine patients were treatment naïve, an additional nine patients had prior treatment for fewer than 28 days, and 10 patients had prior treatment for 28 days to two years. The mean Unified Wilson’s Disease Rating Scale (UWDRS)
In all, 22 patients completed the 24-week study. Three patients discontinued WTX101 due to liver-related adverse events. Investigators discontinued three patients because of neurologic or psychiatric manifestations. At the end of the study, daily dosages were 15 mg for six patients, 30 mg for 13 patients, and 60 mg for nine patients.
Benefits of Treatment and Adverse Events
WTX101 was associated with rapid improvements in NCC. Mean NCC levels were within the upper limit of normal by week 12. At 24 weeks, 71% of patients met the primary end point of achieving or maintaining normalized levels of NCC or a ≥ 25% reduction in NCC from baseline. The mean NCC at baseline was reduced by 72% at week 24.
At week 24, investigators observed significant improvements in trained-rater-assessed neurologic signs, as measured by UWDRS Part 3. The UWDRS Part 3 score improved by ≥ 4 points in 13 patients, stabilized in six patients, and deteriorated by 5 points in two patients.
No participants had early paradoxical neurologic worsening. Significant improvement in patient-reported disability was observed at week 24. In addition, the UWDRS Part 2 score improved by ≥ 1 point in 12 patients and was unchanged in nine patients. No patient reported deterioration.
The Model for End-Stage Liver Disease (MELD) score remained stable in patients throughout the study, and the mean MELD score was 7.7 ±1.9 at baseline and 7.2 ±1.8 at week 24.
Overall, WTX101 was generally well tolerated. Most adverse events were mild or moderate. Psychiatric and gait disturbance were most likely unrelated to the study treatment. Eleven patients who received 30 mg/day or more had elevated liver function tests. These elevations were mostly mild or moderate and normalized within two weeks after dose adjustments.
“Additional clinical evaluation of WTX101 in a larger controlled trial is warranted to further establish its safety and efficacy for the treatment of Wilson’s disease,” said Dr. Bega and colleagues.
This study was sponsored by Wilson Therapeutics, which is headquartered in Stockholm.
—Erica Tricarico
VANCOUVER—The investigational agent bis-choline tetrathiomolybdate (WTX101) may help treat Wilson’s disease, according to a study presented at the 21st International Congress of Parkinson’s Disease and Movement Disorders. The drug lowered circulating copper, and this effect was associated with reduced disability, improved neurologic status without initial paradoxical worsening, and stable liver function.
“With dose adjustments, WTX101 demonstrated a favorable safety profile, and with a simple regimen (once daily in most patients), WTX101 has the potential to address several unmet needs in Wilson’s disease,” said Danny Bega, MD, Assistant Professor of Neurology (Movement Disorders) at Northwestern University’s Feinberg School of Medicine in Chicago, and colleagues.
Wilson’s disease is a rare genetic disorder of impaired copper transport, in which copper accumulates pathologically in the CNS, liver, and other tissues. Current treatments for this disease, such as zinc and chelators (eg, D-penicillamine and trientine), require multiple doses and may be tolerated poorly. To address these challenges, Wilson Therapeutics developed WTX101, an oral first-in-class copper-modulating agent given once daily as monotherapy for Wilson’s disease.
Unlike other treatments for Wilson’s disease, WTX101 appears to have direct intracellular activity in hepatocytes, where it binds excess copper and promotes biliary copper excretion. In addition, WTX101 rapidly binds nonceruloplasmin-bound copper (NCC), thus creating a stable complex with albumin.
Eligible Participants
To evaluate the clinical efficacy and safety of WTX101, as well as the copper control that it enables, in patients with Wilson’s disease, Dr. Bega and colleagues conducted an open-label, single-arm, phase II study at 11 sites in the United States and Europe. Eligible participants were age 18 or older and had been diagnosed with Wilson’s disease based on a Leipzig score of ≥ 4. They also had had no prior treatment for Wilson’s disease or ≤ 24 months of chelation or zinc therapy. Finally, participants’ NCC levels were within or above the normal reference range (≥ 0.8 µM).
Patients received WTX101 for 24 weeks. Researchers used a response-guided dosing regimen with individualized doses of between 15 and 120 mg/day based on NCC levels and safety criteria. In most patients, dosing occurred once daily.
The primary end point was change from baseline to 24 weeks in NCC levels corrected for bound copper contained in tetrathiomolybdate-copper-albumin complexes. Secondary end points included clinical, neurologic, and hepatic assessments.
Of 28 participants involved in the study, 15 were women. The mean age was 34.1. Nine patients were treatment naïve, an additional nine patients had prior treatment for fewer than 28 days, and 10 patients had prior treatment for 28 days to two years. The mean Unified Wilson’s Disease Rating Scale (UWDRS)
In all, 22 patients completed the 24-week study. Three patients discontinued WTX101 due to liver-related adverse events. Investigators discontinued three patients because of neurologic or psychiatric manifestations. At the end of the study, daily dosages were 15 mg for six patients, 30 mg for 13 patients, and 60 mg for nine patients.
Benefits of Treatment and Adverse Events
WTX101 was associated with rapid improvements in NCC. Mean NCC levels were within the upper limit of normal by week 12. At 24 weeks, 71% of patients met the primary end point of achieving or maintaining normalized levels of NCC or a ≥ 25% reduction in NCC from baseline. The mean NCC at baseline was reduced by 72% at week 24.
At week 24, investigators observed significant improvements in trained-rater-assessed neurologic signs, as measured by UWDRS Part 3. The UWDRS Part 3 score improved by ≥ 4 points in 13 patients, stabilized in six patients, and deteriorated by 5 points in two patients.
No participants had early paradoxical neurologic worsening. Significant improvement in patient-reported disability was observed at week 24. In addition, the UWDRS Part 2 score improved by ≥ 1 point in 12 patients and was unchanged in nine patients. No patient reported deterioration.
The Model for End-Stage Liver Disease (MELD) score remained stable in patients throughout the study, and the mean MELD score was 7.7 ±1.9 at baseline and 7.2 ±1.8 at week 24.
Overall, WTX101 was generally well tolerated. Most adverse events were mild or moderate. Psychiatric and gait disturbance were most likely unrelated to the study treatment. Eleven patients who received 30 mg/day or more had elevated liver function tests. These elevations were mostly mild or moderate and normalized within two weeks after dose adjustments.
“Additional clinical evaluation of WTX101 in a larger controlled trial is warranted to further establish its safety and efficacy for the treatment of Wilson’s disease,” said Dr. Bega and colleagues.
This study was sponsored by Wilson Therapeutics, which is headquartered in Stockholm.
—Erica Tricarico
VANCOUVER—The investigational agent bis-choline tetrathiomolybdate (WTX101) may help treat Wilson’s disease, according to a study presented at the 21st International Congress of Parkinson’s Disease and Movement Disorders. The drug lowered circulating copper, and this effect was associated with reduced disability, improved neurologic status without initial paradoxical worsening, and stable liver function.
“With dose adjustments, WTX101 demonstrated a favorable safety profile, and with a simple regimen (once daily in most patients), WTX101 has the potential to address several unmet needs in Wilson’s disease,” said Danny Bega, MD, Assistant Professor of Neurology (Movement Disorders) at Northwestern University’s Feinberg School of Medicine in Chicago, and colleagues.
Wilson’s disease is a rare genetic disorder of impaired copper transport, in which copper accumulates pathologically in the CNS, liver, and other tissues. Current treatments for this disease, such as zinc and chelators (eg, D-penicillamine and trientine), require multiple doses and may be tolerated poorly. To address these challenges, Wilson Therapeutics developed WTX101, an oral first-in-class copper-modulating agent given once daily as monotherapy for Wilson’s disease.
Unlike other treatments for Wilson’s disease, WTX101 appears to have direct intracellular activity in hepatocytes, where it binds excess copper and promotes biliary copper excretion. In addition, WTX101 rapidly binds nonceruloplasmin-bound copper (NCC), thus creating a stable complex with albumin.
Eligible Participants
To evaluate the clinical efficacy and safety of WTX101, as well as the copper control that it enables, in patients with Wilson’s disease, Dr. Bega and colleagues conducted an open-label, single-arm, phase II study at 11 sites in the United States and Europe. Eligible participants were age 18 or older and had been diagnosed with Wilson’s disease based on a Leipzig score of ≥ 4. They also had had no prior treatment for Wilson’s disease or ≤ 24 months of chelation or zinc therapy. Finally, participants’ NCC levels were within or above the normal reference range (≥ 0.8 µM).
Patients received WTX101 for 24 weeks. Researchers used a response-guided dosing regimen with individualized doses of between 15 and 120 mg/day based on NCC levels and safety criteria. In most patients, dosing occurred once daily.
The primary end point was change from baseline to 24 weeks in NCC levels corrected for bound copper contained in tetrathiomolybdate-copper-albumin complexes. Secondary end points included clinical, neurologic, and hepatic assessments.
Of 28 participants involved in the study, 15 were women. The mean age was 34.1. Nine patients were treatment naïve, an additional nine patients had prior treatment for fewer than 28 days, and 10 patients had prior treatment for 28 days to two years. The mean Unified Wilson’s Disease Rating Scale (UWDRS)
In all, 22 patients completed the 24-week study. Three patients discontinued WTX101 due to liver-related adverse events. Investigators discontinued three patients because of neurologic or psychiatric manifestations. At the end of the study, daily dosages were 15 mg for six patients, 30 mg for 13 patients, and 60 mg for nine patients.
Benefits of Treatment and Adverse Events
WTX101 was associated with rapid improvements in NCC. Mean NCC levels were within the upper limit of normal by week 12. At 24 weeks, 71% of patients met the primary end point of achieving or maintaining normalized levels of NCC or a ≥ 25% reduction in NCC from baseline. The mean NCC at baseline was reduced by 72% at week 24.
At week 24, investigators observed significant improvements in trained-rater-assessed neurologic signs, as measured by UWDRS Part 3. The UWDRS Part 3 score improved by ≥ 4 points in 13 patients, stabilized in six patients, and deteriorated by 5 points in two patients.
No participants had early paradoxical neurologic worsening. Significant improvement in patient-reported disability was observed at week 24. In addition, the UWDRS Part 2 score improved by ≥ 1 point in 12 patients and was unchanged in nine patients. No patient reported deterioration.
The Model for End-Stage Liver Disease (MELD) score remained stable in patients throughout the study, and the mean MELD score was 7.7 ±1.9 at baseline and 7.2 ±1.8 at week 24.
Overall, WTX101 was generally well tolerated. Most adverse events were mild or moderate. Psychiatric and gait disturbance were most likely unrelated to the study treatment. Eleven patients who received 30 mg/day or more had elevated liver function tests. These elevations were mostly mild or moderate and normalized within two weeks after dose adjustments.
“Additional clinical evaluation of WTX101 in a larger controlled trial is warranted to further establish its safety and efficacy for the treatment of Wilson’s disease,” said Dr. Bega and colleagues.
This study was sponsored by Wilson Therapeutics, which is headquartered in Stockholm.
—Erica Tricarico
VIDEO: Anacetrapib doubles HDL, but patients gain from its modest LDL cut
BARCELONA – After years of neutral study results in pivotal trials, a drug that raises patients’ high-density lipoprotein cholesterol finally showed a statistically significant and clinically meaningful benefit in a major trial with more than 30,000 patients run for 4 years.
The only catch? It didn’t seem to work by raising HDL.
Instead, it was the off-target effect of also lowering low density lipoprotein (LDL) cholesterol that seems to have driven a modest but clinically significant benefit from anacetrapib, a member of the class of drugs that inhibit the cholesterol ester transfer protein that includes the trial flame-out agents torcetrapib, dalcetrapib, and evacetrapib.
Daily treatment with 100 mg of anacetrapib on top of intensive therapy with atorvastatin led to a 9% relative risk reduction in major coronary events that didn’t become apparent compared with placebo until patients took the drug for more than 2 years, and was “well tolerated,” with a notably benign safety profile, Martin Landray, MD, said at the annual congress of the European Society of Cardiology.
“This is a drug that would have a role clinically, along the lines of ezetimibe,” said Louise Bowman, MD, a clinical epidemiologist and clinical trialist at Oxford who served with Dr. Landray as coprincipal investigator on the study.
Even at a time when proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitors are now routinely available to produce profound reductions in LDL cholesterol, a drug like anacetrapib that produces a more modest reduction can have a clinically useful role, she said. Having anacetrapib available as another option for safely lowering LDL cholesterol “could be complementary” to the lipid-lowering drug classes already in use, Dr. Bowman stressed in a video interview.
“This was a very well treated population on an intensive statin dosage, but when we added the new drug on top of that we saw a clear additional benefit.”
Despite this now proven potential to make a clinical impact, executives at Merck, the company developing anacetrapib, and a cosponsor of this trial, have not yet decided how to follow up on the results. A statement released by the company just before Dr. Landray’s report said: “Merck continues to review the results of the trial with external experts, and will consider whether to file new drug applications with the [Food and Drug Administration] and other regulatory agencies.”
The results also provided a striking lesson that proving a new drug’s value can require running a very large trial for several years.
“Why was this trial positive” when the earlier trials with torcetrapib, dalcetrapib, and evacetrapib were not? “One reason is that our trial had twice as many patients and twice as many events with much longer follow-up,” Dr. Landray said.
Concurrently with his report, the results appeared in an article published online (N Engl J Med. 2017 Aug 29. doi: 10.1056/NEJMoa1706444).
The Randomized Evaluation of the Effects of Anacetrapib Through Lipid-Modification (REVEAL) trial enrolled 30,449 patients at 431 centers in North America, Europe, and China. The average age of the patients was 67 years. Patients had to have established arterial disease: 88% had coronary artery disease, 22% had cerebrovascular disease, and 8% had peripheral artery disease (numbers total more than 100% because some patients had documented disease in more than one arterial bed). The average level of LDL cholesterol was 61 mg/dL, HDL cholesterol was 40 mg/dL, and non-HDL cholesterol averaged 92 mg/dL. During anacetrapib treatment HDL levels roughly doubled, while levels of non-HDL cholesterol fell by an average of 18%.
After a median treatment time of 4.1 years, the study’s primary endpoint – the combined rate of coronary death, nonfatal MI, or need for coronary revascularization – occurred in 10.8% of the patient on anacetrapib and in 11.8% of those in the placebo-control group, a 9% relative risk reduction that was consistent across all prespecified subgroups of patients in the study.
This level of benefit compared with the degree of non-HDL cholesterol lowering observed was strikingly consistent with the relationships between achieved lipid reductions and the clinical results seen in all the published studies with statins and with ezetimibe.
“Anacetrapib lowers LDL and raises HDL, so we knew it would be difficult to disentangle” which effects led to the clinical benefits seen, said Dr. Bowman. But the magnitude of the non-HDL lowering effect relative to the observed benefit “lined up very nicely” with the effects in the statin and ezetimibe trials. On the other hand, “if you double HDL cholesterol you’d expect to see a substantial contribution from that, and we did not, so if the HDL-lowering has an effect it’s probably small,” she said, cautioning that right now this is just an unproven inference. “Our findings are consistent with an LDL effect.”
REVEAL’s other major finding was anacetrapib’s good safety and tolerance profile, with 85% of patients randomized to receive anacetrapib continuing to take the drug through the end of the study. Treatment with the drug linked with a small but statistically significant 0.6% drop in the incidence of diabetes compared with placebo patients, and a small but statistically significant 0.84% increase in new onset stage 3 chronic kidney disease but with no increase in serious adverse events associated with kidney failure. The drug’s use showed no suggestion of a link with cancer, liver disease, muscle effects, cognitive effects, infections, or other serious or nonserious adverse effects. Patients on anacetrapib had on average a systolic blood pressure that was 0.7 mm Hg higher than that of patients on placebo and a diastolic blood pressure that averaged 0.3 mm Hg higher compared with the placebo group. The rate of hypertension-associated serious adverse events was low and virtually identical in the two study groups.
REVEAL received partial funding from Merck, the company developing anacetrapib. Dr. Landray and Dr. Bowman had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
The REVEAL results show for the first time that targeting the cholesterol ester transfer protein mechanism can result in a decrease in coronary events, even in patients with low baseline levels of cholesterol. The findings hold promise for the strategy of targeting this mechanism. But it’s very difficult to dissect out whether the benefits seen were largely due to increasing HDL cholesterol or reducing LDL cholesterol.
About half the enrolled patients had a baseline HDL cholesterol level of less than 40 mg/dL, the type of patient most likely to benefit from raising HDL levels. Another uncertainty when raising HDL cholesterol is whether the induced HDL has the physical and functional properties of the HDL cholesterol that exists in healthy people with normal HDL levels. We can’t exclude the possibility that the HDL cholesterol induced by anacetrapib doesn’t translate into improved physiologic function and clinical benefit. On the other hand, we cannot exclude a possible contribution from HDL.
It is also worth noting that the potential exists to pair anacetrapib treatment with another lipid-lowering treatment with a complimentary mechanism of action, specifically ezetimibe.
M. John Chapman, PhD , is a professor at the Pierre and Marie Curie University in Paris. He has received honoraria from Merck and also from Amgen, Kowa, Pfizer, Regeneron, Sanofi, Servier, and Unilever. He made these comments as designated discussant for the REVEAL report.
The REVEAL results show for the first time that targeting the cholesterol ester transfer protein mechanism can result in a decrease in coronary events, even in patients with low baseline levels of cholesterol. The findings hold promise for the strategy of targeting this mechanism. But it’s very difficult to dissect out whether the benefits seen were largely due to increasing HDL cholesterol or reducing LDL cholesterol.
About half the enrolled patients had a baseline HDL cholesterol level of less than 40 mg/dL, the type of patient most likely to benefit from raising HDL levels. Another uncertainty when raising HDL cholesterol is whether the induced HDL has the physical and functional properties of the HDL cholesterol that exists in healthy people with normal HDL levels. We can’t exclude the possibility that the HDL cholesterol induced by anacetrapib doesn’t translate into improved physiologic function and clinical benefit. On the other hand, we cannot exclude a possible contribution from HDL.
It is also worth noting that the potential exists to pair anacetrapib treatment with another lipid-lowering treatment with a complimentary mechanism of action, specifically ezetimibe.
M. John Chapman, PhD , is a professor at the Pierre and Marie Curie University in Paris. He has received honoraria from Merck and also from Amgen, Kowa, Pfizer, Regeneron, Sanofi, Servier, and Unilever. He made these comments as designated discussant for the REVEAL report.
The REVEAL results show for the first time that targeting the cholesterol ester transfer protein mechanism can result in a decrease in coronary events, even in patients with low baseline levels of cholesterol. The findings hold promise for the strategy of targeting this mechanism. But it’s very difficult to dissect out whether the benefits seen were largely due to increasing HDL cholesterol or reducing LDL cholesterol.
About half the enrolled patients had a baseline HDL cholesterol level of less than 40 mg/dL, the type of patient most likely to benefit from raising HDL levels. Another uncertainty when raising HDL cholesterol is whether the induced HDL has the physical and functional properties of the HDL cholesterol that exists in healthy people with normal HDL levels. We can’t exclude the possibility that the HDL cholesterol induced by anacetrapib doesn’t translate into improved physiologic function and clinical benefit. On the other hand, we cannot exclude a possible contribution from HDL.
It is also worth noting that the potential exists to pair anacetrapib treatment with another lipid-lowering treatment with a complimentary mechanism of action, specifically ezetimibe.
M. John Chapman, PhD , is a professor at the Pierre and Marie Curie University in Paris. He has received honoraria from Merck and also from Amgen, Kowa, Pfizer, Regeneron, Sanofi, Servier, and Unilever. He made these comments as designated discussant for the REVEAL report.
BARCELONA – After years of neutral study results in pivotal trials, a drug that raises patients’ high-density lipoprotein cholesterol finally showed a statistically significant and clinically meaningful benefit in a major trial with more than 30,000 patients run for 4 years.
The only catch? It didn’t seem to work by raising HDL.
Instead, it was the off-target effect of also lowering low density lipoprotein (LDL) cholesterol that seems to have driven a modest but clinically significant benefit from anacetrapib, a member of the class of drugs that inhibit the cholesterol ester transfer protein that includes the trial flame-out agents torcetrapib, dalcetrapib, and evacetrapib.
Daily treatment with 100 mg of anacetrapib on top of intensive therapy with atorvastatin led to a 9% relative risk reduction in major coronary events that didn’t become apparent compared with placebo until patients took the drug for more than 2 years, and was “well tolerated,” with a notably benign safety profile, Martin Landray, MD, said at the annual congress of the European Society of Cardiology.
“This is a drug that would have a role clinically, along the lines of ezetimibe,” said Louise Bowman, MD, a clinical epidemiologist and clinical trialist at Oxford who served with Dr. Landray as coprincipal investigator on the study.
Even at a time when proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitors are now routinely available to produce profound reductions in LDL cholesterol, a drug like anacetrapib that produces a more modest reduction can have a clinically useful role, she said. Having anacetrapib available as another option for safely lowering LDL cholesterol “could be complementary” to the lipid-lowering drug classes already in use, Dr. Bowman stressed in a video interview.
“This was a very well treated population on an intensive statin dosage, but when we added the new drug on top of that we saw a clear additional benefit.”
Despite this now proven potential to make a clinical impact, executives at Merck, the company developing anacetrapib, and a cosponsor of this trial, have not yet decided how to follow up on the results. A statement released by the company just before Dr. Landray’s report said: “Merck continues to review the results of the trial with external experts, and will consider whether to file new drug applications with the [Food and Drug Administration] and other regulatory agencies.”
The results also provided a striking lesson that proving a new drug’s value can require running a very large trial for several years.
“Why was this trial positive” when the earlier trials with torcetrapib, dalcetrapib, and evacetrapib were not? “One reason is that our trial had twice as many patients and twice as many events with much longer follow-up,” Dr. Landray said.
Concurrently with his report, the results appeared in an article published online (N Engl J Med. 2017 Aug 29. doi: 10.1056/NEJMoa1706444).
The Randomized Evaluation of the Effects of Anacetrapib Through Lipid-Modification (REVEAL) trial enrolled 30,449 patients at 431 centers in North America, Europe, and China. The average age of the patients was 67 years. Patients had to have established arterial disease: 88% had coronary artery disease, 22% had cerebrovascular disease, and 8% had peripheral artery disease (numbers total more than 100% because some patients had documented disease in more than one arterial bed). The average level of LDL cholesterol was 61 mg/dL, HDL cholesterol was 40 mg/dL, and non-HDL cholesterol averaged 92 mg/dL. During anacetrapib treatment HDL levels roughly doubled, while levels of non-HDL cholesterol fell by an average of 18%.
After a median treatment time of 4.1 years, the study’s primary endpoint – the combined rate of coronary death, nonfatal MI, or need for coronary revascularization – occurred in 10.8% of the patient on anacetrapib and in 11.8% of those in the placebo-control group, a 9% relative risk reduction that was consistent across all prespecified subgroups of patients in the study.
This level of benefit compared with the degree of non-HDL cholesterol lowering observed was strikingly consistent with the relationships between achieved lipid reductions and the clinical results seen in all the published studies with statins and with ezetimibe.
“Anacetrapib lowers LDL and raises HDL, so we knew it would be difficult to disentangle” which effects led to the clinical benefits seen, said Dr. Bowman. But the magnitude of the non-HDL lowering effect relative to the observed benefit “lined up very nicely” with the effects in the statin and ezetimibe trials. On the other hand, “if you double HDL cholesterol you’d expect to see a substantial contribution from that, and we did not, so if the HDL-lowering has an effect it’s probably small,” she said, cautioning that right now this is just an unproven inference. “Our findings are consistent with an LDL effect.”
REVEAL’s other major finding was anacetrapib’s good safety and tolerance profile, with 85% of patients randomized to receive anacetrapib continuing to take the drug through the end of the study. Treatment with the drug linked with a small but statistically significant 0.6% drop in the incidence of diabetes compared with placebo patients, and a small but statistically significant 0.84% increase in new onset stage 3 chronic kidney disease but with no increase in serious adverse events associated with kidney failure. The drug’s use showed no suggestion of a link with cancer, liver disease, muscle effects, cognitive effects, infections, or other serious or nonserious adverse effects. Patients on anacetrapib had on average a systolic blood pressure that was 0.7 mm Hg higher than that of patients on placebo and a diastolic blood pressure that averaged 0.3 mm Hg higher compared with the placebo group. The rate of hypertension-associated serious adverse events was low and virtually identical in the two study groups.
REVEAL received partial funding from Merck, the company developing anacetrapib. Dr. Landray and Dr. Bowman had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
BARCELONA – After years of neutral study results in pivotal trials, a drug that raises patients’ high-density lipoprotein cholesterol finally showed a statistically significant and clinically meaningful benefit in a major trial with more than 30,000 patients run for 4 years.
The only catch? It didn’t seem to work by raising HDL.
Instead, it was the off-target effect of also lowering low density lipoprotein (LDL) cholesterol that seems to have driven a modest but clinically significant benefit from anacetrapib, a member of the class of drugs that inhibit the cholesterol ester transfer protein that includes the trial flame-out agents torcetrapib, dalcetrapib, and evacetrapib.
Daily treatment with 100 mg of anacetrapib on top of intensive therapy with atorvastatin led to a 9% relative risk reduction in major coronary events that didn’t become apparent compared with placebo until patients took the drug for more than 2 years, and was “well tolerated,” with a notably benign safety profile, Martin Landray, MD, said at the annual congress of the European Society of Cardiology.
“This is a drug that would have a role clinically, along the lines of ezetimibe,” said Louise Bowman, MD, a clinical epidemiologist and clinical trialist at Oxford who served with Dr. Landray as coprincipal investigator on the study.
Even at a time when proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitors are now routinely available to produce profound reductions in LDL cholesterol, a drug like anacetrapib that produces a more modest reduction can have a clinically useful role, she said. Having anacetrapib available as another option for safely lowering LDL cholesterol “could be complementary” to the lipid-lowering drug classes already in use, Dr. Bowman stressed in a video interview.
“This was a very well treated population on an intensive statin dosage, but when we added the new drug on top of that we saw a clear additional benefit.”
Despite this now proven potential to make a clinical impact, executives at Merck, the company developing anacetrapib, and a cosponsor of this trial, have not yet decided how to follow up on the results. A statement released by the company just before Dr. Landray’s report said: “Merck continues to review the results of the trial with external experts, and will consider whether to file new drug applications with the [Food and Drug Administration] and other regulatory agencies.”
The results also provided a striking lesson that proving a new drug’s value can require running a very large trial for several years.
“Why was this trial positive” when the earlier trials with torcetrapib, dalcetrapib, and evacetrapib were not? “One reason is that our trial had twice as many patients and twice as many events with much longer follow-up,” Dr. Landray said.
Concurrently with his report, the results appeared in an article published online (N Engl J Med. 2017 Aug 29. doi: 10.1056/NEJMoa1706444).
The Randomized Evaluation of the Effects of Anacetrapib Through Lipid-Modification (REVEAL) trial enrolled 30,449 patients at 431 centers in North America, Europe, and China. The average age of the patients was 67 years. Patients had to have established arterial disease: 88% had coronary artery disease, 22% had cerebrovascular disease, and 8% had peripheral artery disease (numbers total more than 100% because some patients had documented disease in more than one arterial bed). The average level of LDL cholesterol was 61 mg/dL, HDL cholesterol was 40 mg/dL, and non-HDL cholesterol averaged 92 mg/dL. During anacetrapib treatment HDL levels roughly doubled, while levels of non-HDL cholesterol fell by an average of 18%.
After a median treatment time of 4.1 years, the study’s primary endpoint – the combined rate of coronary death, nonfatal MI, or need for coronary revascularization – occurred in 10.8% of the patient on anacetrapib and in 11.8% of those in the placebo-control group, a 9% relative risk reduction that was consistent across all prespecified subgroups of patients in the study.
This level of benefit compared with the degree of non-HDL cholesterol lowering observed was strikingly consistent with the relationships between achieved lipid reductions and the clinical results seen in all the published studies with statins and with ezetimibe.
“Anacetrapib lowers LDL and raises HDL, so we knew it would be difficult to disentangle” which effects led to the clinical benefits seen, said Dr. Bowman. But the magnitude of the non-HDL lowering effect relative to the observed benefit “lined up very nicely” with the effects in the statin and ezetimibe trials. On the other hand, “if you double HDL cholesterol you’d expect to see a substantial contribution from that, and we did not, so if the HDL-lowering has an effect it’s probably small,” she said, cautioning that right now this is just an unproven inference. “Our findings are consistent with an LDL effect.”
REVEAL’s other major finding was anacetrapib’s good safety and tolerance profile, with 85% of patients randomized to receive anacetrapib continuing to take the drug through the end of the study. Treatment with the drug linked with a small but statistically significant 0.6% drop in the incidence of diabetes compared with placebo patients, and a small but statistically significant 0.84% increase in new onset stage 3 chronic kidney disease but with no increase in serious adverse events associated with kidney failure. The drug’s use showed no suggestion of a link with cancer, liver disease, muscle effects, cognitive effects, infections, or other serious or nonserious adverse effects. Patients on anacetrapib had on average a systolic blood pressure that was 0.7 mm Hg higher than that of patients on placebo and a diastolic blood pressure that averaged 0.3 mm Hg higher compared with the placebo group. The rate of hypertension-associated serious adverse events was low and virtually identical in the two study groups.
REVEAL received partial funding from Merck, the company developing anacetrapib. Dr. Landray and Dr. Bowman had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
AT THE ESC CONGRESS 2017
Key clinical point:
Major finding: Patients treated with anacetrapib had a statistically significant 9% decrease in major coronary events, compared with placebo-treated controls.
Data source: REVEAL, a multicenter, pivotal trial with 30,449 patients treated for about 4 years.
Disclosures: REVEAL received partial funding from Merck, the company developing anacetrapib. Dr. Landray and Dr. Bowman had no disclosures.
Can Today’s Stress Level Predict Tomorrow’s Migraine Attack?
Neurologists and patients may be able to predict migraine attacks using a model based on the level of stress from daily hassles, according to research published in the July issue of Headache. The model was well calibrated, but its forecasts were based on participants’ base rates of headache, said the authors. With additional adjustments, the model could enable patients to treat migraine attacks pre-emptively.
Although headache disorders are common, it remains unclear what triggers a migraine attack. Patients have identified many possible triggers, including perceived stress. In people with episodic migraine and chronic migraine, perceived stress is associated with the onset of headache. Researchers previously had not provided evidence that any of the potential triggers could predict a migraine attack, however.
Electronic Diaries Captured Headache Frequency
Timothy T. Houle, PhD, Associate Professor of Anesthesia at Massachusetts General Hospital in Boston, and colleagues conducted the prospective Headache Prediction Study to examine precipitating factors of migraine headache. They recruited participants with episodic migraine who had more than two headache attacks per month and had between four and 14 headache days per month. Secondary headache disorder and change in the nature of headache symptoms in the previous six weeks were among the exclusion criteria.
Participants completed morning and evening diary entries daily using electronic systems. In the entries, the participants recorded headaches, headache characteristics, and abortive medications used since the last entry. Participants used the Daily Stress Inventory to assess stress in their evening diary entries. Using these assessments, the investigators examined the frequency of stressors, the sum of the stress impact ratings, and the average stress impact ratings. The primary analysis was the prediction of a future headache attack based on current levels of stress and headache.
Potential for New Treatment Strategies
Dr. Houle and colleagues enrolled 100 participants between September 2009 and May 2014. Five participants dropped out. Approximately 91% of participants were female, and 87% were Caucasian. Mean age was 40. The 95 participants contributed 4,626 days of diary data. In all, 431 diary entries were missing or unavailable for analysis. Participants had a headache attack on approximately 39% of days. Days that preceded a headache were associated with greater stress than days that did not precede a headache.
After estimating a series of models, the researchers found that a generalized linear mixed-effects model using either the frequency of stressful events or the perceived intensity of stressful events fit the data well. The forecasting model had “promising predictive utility” in the training sample and in a validation sample, said the authors. The model had good calibration between forecast probabilities and observed headache frequencies, but had low levels of resolution, meaning that “the forecast probabilities are close to the individual’s long-run average,” said Dr. Houle.
“This appears to be the first evidence that individual headache attacks can be forecast within an individual sufferer, and this finding creates substantial opportunities for additional treatment strategies if the forecasting model can be refined,” said Dr. Houle. “A forecasting model could be used to enhance pharmacologic treatment opportunities, reduce anxiety about the unpredictability of attacks, increase locus-of-control beliefs, and lead to increased self-efficacy assessments about the self-management of migraine attacks.” Neurologists should consider the investigators’ stress model a first step toward headache prediction, and not a final model for widespread clinical use, he added.
Complexities Need Consideration
These data are “fascinating,” but neurologists should consider several complexities as they develop methods for the short-term prevention of predictable migraine, said Richard B. Lipton, MD, Edwin S. Lowe Chair in Neurology at Albert Einstein College of Medicine in New York and Director of the Montefiore Headache Center, and colleagues in an accompanying editorial. First, they must distinguish group-level and within-person analyses of attack predictors. Trigger factors vary from person to person, and within-person analysis may be crucial to prediction and prevention, said Dr. Lipton. Second, in addition to stress, other trigger factors such as premonitory features, self-prediction, and biomarkers also may aid in forecasting attacks. Finally, researchers can measure and model predictors of impending attacks in various ways (eg, lead–lag effects and cumulative effects).
“Houle et al have set the stage for short-term prediction of headaches in persons with migraine as a potential foundation for short-term preventive therapies,” said Dr. Lipton. “To realize the potential of these approaches, we must refine the art of headache forecasting and then test targeted interventions in carefully selected patients.”
—Erik Greb
Suggested Reading
Houle TT, Turner DP, Golding AN, et al. Forecasting individual headache attacks using perceived stress: Development of a multivariable prediction model for persons with episodic migraine. Headache. 2017;57(7):1041-1050.
Lipton RB, Pavlovic JM, Buse DC. Why migraine forecasting matters. Headache. 2017;57(7):1023-1025.
Neurologists and patients may be able to predict migraine attacks using a model based on the level of stress from daily hassles, according to research published in the July issue of Headache. The model was well calibrated, but its forecasts were based on participants’ base rates of headache, said the authors. With additional adjustments, the model could enable patients to treat migraine attacks pre-emptively.
Although headache disorders are common, it remains unclear what triggers a migraine attack. Patients have identified many possible triggers, including perceived stress. In people with episodic migraine and chronic migraine, perceived stress is associated with the onset of headache. Researchers previously had not provided evidence that any of the potential triggers could predict a migraine attack, however.
Electronic Diaries Captured Headache Frequency
Timothy T. Houle, PhD, Associate Professor of Anesthesia at Massachusetts General Hospital in Boston, and colleagues conducted the prospective Headache Prediction Study to examine precipitating factors of migraine headache. They recruited participants with episodic migraine who had more than two headache attacks per month and had between four and 14 headache days per month. Secondary headache disorder and change in the nature of headache symptoms in the previous six weeks were among the exclusion criteria.
Participants completed morning and evening diary entries daily using electronic systems. In the entries, the participants recorded headaches, headache characteristics, and abortive medications used since the last entry. Participants used the Daily Stress Inventory to assess stress in their evening diary entries. Using these assessments, the investigators examined the frequency of stressors, the sum of the stress impact ratings, and the average stress impact ratings. The primary analysis was the prediction of a future headache attack based on current levels of stress and headache.
Potential for New Treatment Strategies
Dr. Houle and colleagues enrolled 100 participants between September 2009 and May 2014. Five participants dropped out. Approximately 91% of participants were female, and 87% were Caucasian. Mean age was 40. The 95 participants contributed 4,626 days of diary data. In all, 431 diary entries were missing or unavailable for analysis. Participants had a headache attack on approximately 39% of days. Days that preceded a headache were associated with greater stress than days that did not precede a headache.
After estimating a series of models, the researchers found that a generalized linear mixed-effects model using either the frequency of stressful events or the perceived intensity of stressful events fit the data well. The forecasting model had “promising predictive utility” in the training sample and in a validation sample, said the authors. The model had good calibration between forecast probabilities and observed headache frequencies, but had low levels of resolution, meaning that “the forecast probabilities are close to the individual’s long-run average,” said Dr. Houle.
“This appears to be the first evidence that individual headache attacks can be forecast within an individual sufferer, and this finding creates substantial opportunities for additional treatment strategies if the forecasting model can be refined,” said Dr. Houle. “A forecasting model could be used to enhance pharmacologic treatment opportunities, reduce anxiety about the unpredictability of attacks, increase locus-of-control beliefs, and lead to increased self-efficacy assessments about the self-management of migraine attacks.” Neurologists should consider the investigators’ stress model a first step toward headache prediction, and not a final model for widespread clinical use, he added.
Complexities Need Consideration
These data are “fascinating,” but neurologists should consider several complexities as they develop methods for the short-term prevention of predictable migraine, said Richard B. Lipton, MD, Edwin S. Lowe Chair in Neurology at Albert Einstein College of Medicine in New York and Director of the Montefiore Headache Center, and colleagues in an accompanying editorial. First, they must distinguish group-level and within-person analyses of attack predictors. Trigger factors vary from person to person, and within-person analysis may be crucial to prediction and prevention, said Dr. Lipton. Second, in addition to stress, other trigger factors such as premonitory features, self-prediction, and biomarkers also may aid in forecasting attacks. Finally, researchers can measure and model predictors of impending attacks in various ways (eg, lead–lag effects and cumulative effects).
“Houle et al have set the stage for short-term prediction of headaches in persons with migraine as a potential foundation for short-term preventive therapies,” said Dr. Lipton. “To realize the potential of these approaches, we must refine the art of headache forecasting and then test targeted interventions in carefully selected patients.”
—Erik Greb
Suggested Reading
Houle TT, Turner DP, Golding AN, et al. Forecasting individual headache attacks using perceived stress: Development of a multivariable prediction model for persons with episodic migraine. Headache. 2017;57(7):1041-1050.
Lipton RB, Pavlovic JM, Buse DC. Why migraine forecasting matters. Headache. 2017;57(7):1023-1025.
Neurologists and patients may be able to predict migraine attacks using a model based on the level of stress from daily hassles, according to research published in the July issue of Headache. The model was well calibrated, but its forecasts were based on participants’ base rates of headache, said the authors. With additional adjustments, the model could enable patients to treat migraine attacks pre-emptively.
Although headache disorders are common, it remains unclear what triggers a migraine attack. Patients have identified many possible triggers, including perceived stress. In people with episodic migraine and chronic migraine, perceived stress is associated with the onset of headache. Researchers previously had not provided evidence that any of the potential triggers could predict a migraine attack, however.
Electronic Diaries Captured Headache Frequency
Timothy T. Houle, PhD, Associate Professor of Anesthesia at Massachusetts General Hospital in Boston, and colleagues conducted the prospective Headache Prediction Study to examine precipitating factors of migraine headache. They recruited participants with episodic migraine who had more than two headache attacks per month and had between four and 14 headache days per month. Secondary headache disorder and change in the nature of headache symptoms in the previous six weeks were among the exclusion criteria.
Participants completed morning and evening diary entries daily using electronic systems. In the entries, the participants recorded headaches, headache characteristics, and abortive medications used since the last entry. Participants used the Daily Stress Inventory to assess stress in their evening diary entries. Using these assessments, the investigators examined the frequency of stressors, the sum of the stress impact ratings, and the average stress impact ratings. The primary analysis was the prediction of a future headache attack based on current levels of stress and headache.
Potential for New Treatment Strategies
Dr. Houle and colleagues enrolled 100 participants between September 2009 and May 2014. Five participants dropped out. Approximately 91% of participants were female, and 87% were Caucasian. Mean age was 40. The 95 participants contributed 4,626 days of diary data. In all, 431 diary entries were missing or unavailable for analysis. Participants had a headache attack on approximately 39% of days. Days that preceded a headache were associated with greater stress than days that did not precede a headache.
After estimating a series of models, the researchers found that a generalized linear mixed-effects model using either the frequency of stressful events or the perceived intensity of stressful events fit the data well. The forecasting model had “promising predictive utility” in the training sample and in a validation sample, said the authors. The model had good calibration between forecast probabilities and observed headache frequencies, but had low levels of resolution, meaning that “the forecast probabilities are close to the individual’s long-run average,” said Dr. Houle.
“This appears to be the first evidence that individual headache attacks can be forecast within an individual sufferer, and this finding creates substantial opportunities for additional treatment strategies if the forecasting model can be refined,” said Dr. Houle. “A forecasting model could be used to enhance pharmacologic treatment opportunities, reduce anxiety about the unpredictability of attacks, increase locus-of-control beliefs, and lead to increased self-efficacy assessments about the self-management of migraine attacks.” Neurologists should consider the investigators’ stress model a first step toward headache prediction, and not a final model for widespread clinical use, he added.
Complexities Need Consideration
These data are “fascinating,” but neurologists should consider several complexities as they develop methods for the short-term prevention of predictable migraine, said Richard B. Lipton, MD, Edwin S. Lowe Chair in Neurology at Albert Einstein College of Medicine in New York and Director of the Montefiore Headache Center, and colleagues in an accompanying editorial. First, they must distinguish group-level and within-person analyses of attack predictors. Trigger factors vary from person to person, and within-person analysis may be crucial to prediction and prevention, said Dr. Lipton. Second, in addition to stress, other trigger factors such as premonitory features, self-prediction, and biomarkers also may aid in forecasting attacks. Finally, researchers can measure and model predictors of impending attacks in various ways (eg, lead–lag effects and cumulative effects).
“Houle et al have set the stage for short-term prediction of headaches in persons with migraine as a potential foundation for short-term preventive therapies,” said Dr. Lipton. “To realize the potential of these approaches, we must refine the art of headache forecasting and then test targeted interventions in carefully selected patients.”
—Erik Greb
Suggested Reading
Houle TT, Turner DP, Golding AN, et al. Forecasting individual headache attacks using perceived stress: Development of a multivariable prediction model for persons with episodic migraine. Headache. 2017;57(7):1041-1050.
Lipton RB, Pavlovic JM, Buse DC. Why migraine forecasting matters. Headache. 2017;57(7):1023-1025.
Patients With Dementia With Lewy Bodies May Have Unfavorable Hospital Outcomes
VANCOUVER—Patients with dementia with Lewy bodies have a high rate of hospitalization for hallucinations or confusion, falls, and infection, according to a study presented at the 21st International Congress of Parkinson’s Disease and Movement Disorders. Once these patients are hospitalized, they have a high rate of complications. Many patients require transition to a higher level of care.
“These results bring to light a need to educate caregivers on the hazards of hospitalization in this patient population,” said C. Chauncey Spears, MD, Senior Movement Disorders Fellow at the Center for Movement Disorders and Neurorestoration at the University of Florida in Gainesville. Hospitalization is associated with increased mortality in Parkinson’s disease and Alzheimer’s disease, but there are limited published data regarding hospitalization of patients with dementia with Lewy bodies.
To identify causes, complications, and outcomes of hospitalization in patients with dementia with Lewy bodies, the researchers conducted a cross-sectional chart review of patients who were hospitalized at the University of Florida Health Shands Hospital in 2014 and 2015.
The researchers included patients with a diagnosis of dementia with Lewy bodies by discharge who were older than 18. Patients with a diagnosis of other forms of parkinsonism were excluded.
The investigators reviewed 178 patient encounters with 117 patients. The mean age was 78, and 48 participants were women. In addition, there were 134 hospital complications. Patients with dementia with Lewy bodies were most commonly hospitalized for hallucinations or confusion, falls, and infection. This finding was independent of age, premorbid dementia, and home use of antipsychotic or anticholinergic agents. Further statistical analysis suggested a trend toward hospitalization for falls or hallucinations in older patients, said the researchers.
The median length of hospital stay was five days, with a trend toward longer admissions for patients admitted with hallucinations, confusion, or failure to cope. Prior to hospitalization, about 64% of patients were living at home, and 41% of patients were taking antipsychotics. During hospitalization, nearly half of patients experienced new or worsened confusion. “We found that haloperidol and risperidone are commonly used to treat these patients in the hospital, possibly due to their parenteral administration. This raises the concern of whether these medications could contribute to worse outcomes, as patients with dementia with Lewy bodies have a known risk of neuroleptic sensitivity,” said Dr. Spears.
Overall, patients with dementia with Lewy bodies had unfavorable hospital outcomes; 32.6% of patients transitioned to a higher level of care at discharge, relative to their baseline, and 15.2% of patients transitioned to hospice or died. “It will be important to identify variables to limit hospitalization and poor outcomes,” said Dr. Spears. “Further studies with large sample sizes are needed to evaluate for independent predictors of hospitalization, complications, and poorer outcomes, which may include ensuring optimal outpatient treatments for hallucinations [and] confusion, caregiver–provider awareness, and evaluating the risk versus benefit of antipsychotic and anticholinergic agents.”
—Erica Tricarico
VANCOUVER—Patients with dementia with Lewy bodies have a high rate of hospitalization for hallucinations or confusion, falls, and infection, according to a study presented at the 21st International Congress of Parkinson’s Disease and Movement Disorders. Once these patients are hospitalized, they have a high rate of complications. Many patients require transition to a higher level of care.
“These results bring to light a need to educate caregivers on the hazards of hospitalization in this patient population,” said C. Chauncey Spears, MD, Senior Movement Disorders Fellow at the Center for Movement Disorders and Neurorestoration at the University of Florida in Gainesville. Hospitalization is associated with increased mortality in Parkinson’s disease and Alzheimer’s disease, but there are limited published data regarding hospitalization of patients with dementia with Lewy bodies.
To identify causes, complications, and outcomes of hospitalization in patients with dementia with Lewy bodies, the researchers conducted a cross-sectional chart review of patients who were hospitalized at the University of Florida Health Shands Hospital in 2014 and 2015.
The researchers included patients with a diagnosis of dementia with Lewy bodies by discharge who were older than 18. Patients with a diagnosis of other forms of parkinsonism were excluded.
The investigators reviewed 178 patient encounters with 117 patients. The mean age was 78, and 48 participants were women. In addition, there were 134 hospital complications. Patients with dementia with Lewy bodies were most commonly hospitalized for hallucinations or confusion, falls, and infection. This finding was independent of age, premorbid dementia, and home use of antipsychotic or anticholinergic agents. Further statistical analysis suggested a trend toward hospitalization for falls or hallucinations in older patients, said the researchers.
The median length of hospital stay was five days, with a trend toward longer admissions for patients admitted with hallucinations, confusion, or failure to cope. Prior to hospitalization, about 64% of patients were living at home, and 41% of patients were taking antipsychotics. During hospitalization, nearly half of patients experienced new or worsened confusion. “We found that haloperidol and risperidone are commonly used to treat these patients in the hospital, possibly due to their parenteral administration. This raises the concern of whether these medications could contribute to worse outcomes, as patients with dementia with Lewy bodies have a known risk of neuroleptic sensitivity,” said Dr. Spears.
Overall, patients with dementia with Lewy bodies had unfavorable hospital outcomes; 32.6% of patients transitioned to a higher level of care at discharge, relative to their baseline, and 15.2% of patients transitioned to hospice or died. “It will be important to identify variables to limit hospitalization and poor outcomes,” said Dr. Spears. “Further studies with large sample sizes are needed to evaluate for independent predictors of hospitalization, complications, and poorer outcomes, which may include ensuring optimal outpatient treatments for hallucinations [and] confusion, caregiver–provider awareness, and evaluating the risk versus benefit of antipsychotic and anticholinergic agents.”
—Erica Tricarico
VANCOUVER—Patients with dementia with Lewy bodies have a high rate of hospitalization for hallucinations or confusion, falls, and infection, according to a study presented at the 21st International Congress of Parkinson’s Disease and Movement Disorders. Once these patients are hospitalized, they have a high rate of complications. Many patients require transition to a higher level of care.
“These results bring to light a need to educate caregivers on the hazards of hospitalization in this patient population,” said C. Chauncey Spears, MD, Senior Movement Disorders Fellow at the Center for Movement Disorders and Neurorestoration at the University of Florida in Gainesville. Hospitalization is associated with increased mortality in Parkinson’s disease and Alzheimer’s disease, but there are limited published data regarding hospitalization of patients with dementia with Lewy bodies.
To identify causes, complications, and outcomes of hospitalization in patients with dementia with Lewy bodies, the researchers conducted a cross-sectional chart review of patients who were hospitalized at the University of Florida Health Shands Hospital in 2014 and 2015.
The researchers included patients with a diagnosis of dementia with Lewy bodies by discharge who were older than 18. Patients with a diagnosis of other forms of parkinsonism were excluded.
The investigators reviewed 178 patient encounters with 117 patients. The mean age was 78, and 48 participants were women. In addition, there were 134 hospital complications. Patients with dementia with Lewy bodies were most commonly hospitalized for hallucinations or confusion, falls, and infection. This finding was independent of age, premorbid dementia, and home use of antipsychotic or anticholinergic agents. Further statistical analysis suggested a trend toward hospitalization for falls or hallucinations in older patients, said the researchers.
The median length of hospital stay was five days, with a trend toward longer admissions for patients admitted with hallucinations, confusion, or failure to cope. Prior to hospitalization, about 64% of patients were living at home, and 41% of patients were taking antipsychotics. During hospitalization, nearly half of patients experienced new or worsened confusion. “We found that haloperidol and risperidone are commonly used to treat these patients in the hospital, possibly due to their parenteral administration. This raises the concern of whether these medications could contribute to worse outcomes, as patients with dementia with Lewy bodies have a known risk of neuroleptic sensitivity,” said Dr. Spears.
Overall, patients with dementia with Lewy bodies had unfavorable hospital outcomes; 32.6% of patients transitioned to a higher level of care at discharge, relative to their baseline, and 15.2% of patients transitioned to hospice or died. “It will be important to identify variables to limit hospitalization and poor outcomes,” said Dr. Spears. “Further studies with large sample sizes are needed to evaluate for independent predictors of hospitalization, complications, and poorer outcomes, which may include ensuring optimal outpatient treatments for hallucinations [and] confusion, caregiver–provider awareness, and evaluating the risk versus benefit of antipsychotic and anticholinergic agents.”
—Erica Tricarico
Product Update: Histologics curettes, SculpSure, drchrono, Vuva Magnetic Dilators
COLPOSCOPY BRUSHES SHOW BETTER TISSUE YIELD

A new study from the University of California–Riverside and Aurora Diagnostics in Palm Beach, Florida, evaluated 10,000 colposcopy workups and found that the disposable Histologics Soft-ECC and SoftBiopsy curettes were superior in tissue yield and offered a lower risk of cross contamination than metal curettes.
The Soft-ECC Endocervical Curette Tissue Collection System is used to biopsy the endocervical canal during colposcopy or evaluation for abnormal uterine bleeding. It gently frictionally abrades and collects transepithelial tissue samples into the Kylon fabric. The Soft-ECC-S has a smaller pad for the short, shallow, or stenotic cervix.
During exocervical biopsy with the SoftBiopsy Gynecological Biopsy Device, the Kylon fabric hooks rake across the tissue plane dislodging and shearing tissue strips and fragments from just below the basement membrane to collect in the fabric.
Both products have tips that are snapped off at the scored handle and placed in a fixative vial for transport to the laboratory.
FOR MORE INFORMATION, VISIT: https://www.histologics.com/
NONINVASIVE BODY CONTOURING

The Cynosure division of Hologic has just received US Food and Drug Administration (FDA) expanded clearance for SculpSure for noninvasive body contouring (lipolysis) of back, inner, and outer thighs. SculpSure is clinically proven to permanently eliminate fat cells and is already FDA-cleared to treat the abdomen and love handles (flanks).
According to Hologic, SculpSure uses a selective wavelength laser that precisely targets fat cells under the skin without affecting the skin’s surface. The laser raises the temperature of body fat to disrupt and destroy these cells, which are naturally eliminated over time and do not return or regenerate. Hologic says that patients are able to achieve desired results, without downtime or surgery, through customized treatment plans, each lasting only 25 minutes. Results can be seen as quickly as 6 weeks; optimal results typically are seen at 12 weeks, they say.
FOR MORE INFORMATION, VISIT: http://www.sculpsure.com
SOFTWARE PLATFORM FOR OBGYNS

drchrono offers software platforms for medical offices, including electronic health records (EHR), practice management, medical billing, revenue cycle management (RCM), and health care application programming interface (API) tools for iPad, iPhone, and web. Products are available for small and large practice groups, urgent care centers, and specialties, including ObGyns. A patient can complete forms and schedule visits through the Patient Portal as well as receive educational materials through HIPAA-compliant messaging and face-to-face telemedicine.
drchrono says its cloud-based platform allows ObGyns to chart in seconds using customizable medical forms, medical speech-to-text, and photo/drawing tools. ObGyn-specific ICD10, Current Procedural Terminology, and E&M codes can autopopulate into forms. Prenatal flow sheets and other clinical forms can be sent to L&D, other providers, or billing departments using drchrono’s direct messaging integration with the local hospital EHR or e-Fax. Prenatal and blood testing, ultrasounds, paper charts, and photographs can be ordered, tracked, and shared with patients and providers.
FOR MORE INFORMATION, VISIT: https://www.drchrono.com/
MAGNETIC DILATOR THERAPY FOR DYSPAREUNIA

The VuVa Magnetic Dilator Therapy is a nonsurgical, nonprescription program that VuVatech announces has been proven to relieve pelvic pain and dyspareunia caused by vaginismus, vulvodynia, and vaginal atrophy.
The VuVa Vaginal Dilator Set contains more than 60 neodymium magnets. With iron a component of blood that carries oxygen, when the magnet is placed next to a painful area, it draws fresh oxygenated blood to the nerves and surrounding muscles for increased blood circulation that accelerates healing while reducing pain. When a VuVa Magnetic Vaginal Dilator is used 20 minutes before intercourse, soft tissue lengthens, relaxing and calming muscles, ligaments, and nerves.
A nonmagnetic full dilator set is available for women who are trying to get pregnant and for women with pacemakers or defibrillators.
This product is not FDA approved, is not sold as a medical device, and is not intended to diagnose, treat, cure, or prevent any disease. Effectiveness varies, according to the manufacturer.
FOR MORE INFORMATION, VISIT: https://www.vuvatech.com/
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
COLPOSCOPY BRUSHES SHOW BETTER TISSUE YIELD

A new study from the University of California–Riverside and Aurora Diagnostics in Palm Beach, Florida, evaluated 10,000 colposcopy workups and found that the disposable Histologics Soft-ECC and SoftBiopsy curettes were superior in tissue yield and offered a lower risk of cross contamination than metal curettes.
The Soft-ECC Endocervical Curette Tissue Collection System is used to biopsy the endocervical canal during colposcopy or evaluation for abnormal uterine bleeding. It gently frictionally abrades and collects transepithelial tissue samples into the Kylon fabric. The Soft-ECC-S has a smaller pad for the short, shallow, or stenotic cervix.
During exocervical biopsy with the SoftBiopsy Gynecological Biopsy Device, the Kylon fabric hooks rake across the tissue plane dislodging and shearing tissue strips and fragments from just below the basement membrane to collect in the fabric.
Both products have tips that are snapped off at the scored handle and placed in a fixative vial for transport to the laboratory.
FOR MORE INFORMATION, VISIT: https://www.histologics.com/
NONINVASIVE BODY CONTOURING

The Cynosure division of Hologic has just received US Food and Drug Administration (FDA) expanded clearance for SculpSure for noninvasive body contouring (lipolysis) of back, inner, and outer thighs. SculpSure is clinically proven to permanently eliminate fat cells and is already FDA-cleared to treat the abdomen and love handles (flanks).
According to Hologic, SculpSure uses a selective wavelength laser that precisely targets fat cells under the skin without affecting the skin’s surface. The laser raises the temperature of body fat to disrupt and destroy these cells, which are naturally eliminated over time and do not return or regenerate. Hologic says that patients are able to achieve desired results, without downtime or surgery, through customized treatment plans, each lasting only 25 minutes. Results can be seen as quickly as 6 weeks; optimal results typically are seen at 12 weeks, they say.
FOR MORE INFORMATION, VISIT: http://www.sculpsure.com
SOFTWARE PLATFORM FOR OBGYNS

drchrono offers software platforms for medical offices, including electronic health records (EHR), practice management, medical billing, revenue cycle management (RCM), and health care application programming interface (API) tools for iPad, iPhone, and web. Products are available for small and large practice groups, urgent care centers, and specialties, including ObGyns. A patient can complete forms and schedule visits through the Patient Portal as well as receive educational materials through HIPAA-compliant messaging and face-to-face telemedicine.
drchrono says its cloud-based platform allows ObGyns to chart in seconds using customizable medical forms, medical speech-to-text, and photo/drawing tools. ObGyn-specific ICD10, Current Procedural Terminology, and E&M codes can autopopulate into forms. Prenatal flow sheets and other clinical forms can be sent to L&D, other providers, or billing departments using drchrono’s direct messaging integration with the local hospital EHR or e-Fax. Prenatal and blood testing, ultrasounds, paper charts, and photographs can be ordered, tracked, and shared with patients and providers.
FOR MORE INFORMATION, VISIT: https://www.drchrono.com/
MAGNETIC DILATOR THERAPY FOR DYSPAREUNIA

The VuVa Magnetic Dilator Therapy is a nonsurgical, nonprescription program that VuVatech announces has been proven to relieve pelvic pain and dyspareunia caused by vaginismus, vulvodynia, and vaginal atrophy.
The VuVa Vaginal Dilator Set contains more than 60 neodymium magnets. With iron a component of blood that carries oxygen, when the magnet is placed next to a painful area, it draws fresh oxygenated blood to the nerves and surrounding muscles for increased blood circulation that accelerates healing while reducing pain. When a VuVa Magnetic Vaginal Dilator is used 20 minutes before intercourse, soft tissue lengthens, relaxing and calming muscles, ligaments, and nerves.
A nonmagnetic full dilator set is available for women who are trying to get pregnant and for women with pacemakers or defibrillators.
This product is not FDA approved, is not sold as a medical device, and is not intended to diagnose, treat, cure, or prevent any disease. Effectiveness varies, according to the manufacturer.
FOR MORE INFORMATION, VISIT: https://www.vuvatech.com/
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
COLPOSCOPY BRUSHES SHOW BETTER TISSUE YIELD

A new study from the University of California–Riverside and Aurora Diagnostics in Palm Beach, Florida, evaluated 10,000 colposcopy workups and found that the disposable Histologics Soft-ECC and SoftBiopsy curettes were superior in tissue yield and offered a lower risk of cross contamination than metal curettes.
The Soft-ECC Endocervical Curette Tissue Collection System is used to biopsy the endocervical canal during colposcopy or evaluation for abnormal uterine bleeding. It gently frictionally abrades and collects transepithelial tissue samples into the Kylon fabric. The Soft-ECC-S has a smaller pad for the short, shallow, or stenotic cervix.
During exocervical biopsy with the SoftBiopsy Gynecological Biopsy Device, the Kylon fabric hooks rake across the tissue plane dislodging and shearing tissue strips and fragments from just below the basement membrane to collect in the fabric.
Both products have tips that are snapped off at the scored handle and placed in a fixative vial for transport to the laboratory.
FOR MORE INFORMATION, VISIT: https://www.histologics.com/
NONINVASIVE BODY CONTOURING

The Cynosure division of Hologic has just received US Food and Drug Administration (FDA) expanded clearance for SculpSure for noninvasive body contouring (lipolysis) of back, inner, and outer thighs. SculpSure is clinically proven to permanently eliminate fat cells and is already FDA-cleared to treat the abdomen and love handles (flanks).
According to Hologic, SculpSure uses a selective wavelength laser that precisely targets fat cells under the skin without affecting the skin’s surface. The laser raises the temperature of body fat to disrupt and destroy these cells, which are naturally eliminated over time and do not return or regenerate. Hologic says that patients are able to achieve desired results, without downtime or surgery, through customized treatment plans, each lasting only 25 minutes. Results can be seen as quickly as 6 weeks; optimal results typically are seen at 12 weeks, they say.
FOR MORE INFORMATION, VISIT: http://www.sculpsure.com
SOFTWARE PLATFORM FOR OBGYNS

drchrono offers software platforms for medical offices, including electronic health records (EHR), practice management, medical billing, revenue cycle management (RCM), and health care application programming interface (API) tools for iPad, iPhone, and web. Products are available for small and large practice groups, urgent care centers, and specialties, including ObGyns. A patient can complete forms and schedule visits through the Patient Portal as well as receive educational materials through HIPAA-compliant messaging and face-to-face telemedicine.
drchrono says its cloud-based platform allows ObGyns to chart in seconds using customizable medical forms, medical speech-to-text, and photo/drawing tools. ObGyn-specific ICD10, Current Procedural Terminology, and E&M codes can autopopulate into forms. Prenatal flow sheets and other clinical forms can be sent to L&D, other providers, or billing departments using drchrono’s direct messaging integration with the local hospital EHR or e-Fax. Prenatal and blood testing, ultrasounds, paper charts, and photographs can be ordered, tracked, and shared with patients and providers.
FOR MORE INFORMATION, VISIT: https://www.drchrono.com/
MAGNETIC DILATOR THERAPY FOR DYSPAREUNIA

The VuVa Magnetic Dilator Therapy is a nonsurgical, nonprescription program that VuVatech announces has been proven to relieve pelvic pain and dyspareunia caused by vaginismus, vulvodynia, and vaginal atrophy.
The VuVa Vaginal Dilator Set contains more than 60 neodymium magnets. With iron a component of blood that carries oxygen, when the magnet is placed next to a painful area, it draws fresh oxygenated blood to the nerves and surrounding muscles for increased blood circulation that accelerates healing while reducing pain. When a VuVa Magnetic Vaginal Dilator is used 20 minutes before intercourse, soft tissue lengthens, relaxing and calming muscles, ligaments, and nerves.
A nonmagnetic full dilator set is available for women who are trying to get pregnant and for women with pacemakers or defibrillators.
This product is not FDA approved, is not sold as a medical device, and is not intended to diagnose, treat, cure, or prevent any disease. Effectiveness varies, according to the manufacturer.
FOR MORE INFORMATION, VISIT: https://www.vuvatech.com/
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CDC: Flu vaccine recommendations broaden for pregnant women and children
, according to new recommendations from the Centers for Disease Control and Prevention.
This change from the CDC’s previous guidance that pregnant women receive a seasonal inactivated influenza vaccine (IIV) was recommended by the Advisory Committee on Immunization Practices after some heated debate among committee members over evidence presented to support the change in wording (MMWR. 2017 Aug 25;66[(RR-2]:1-20).
The new update gives women the ability to choose between receiving an IIV and FluBlok, a recombinant influenza vaccine (RIV) that is not egg based and can be manufactured more quickly, making it ideal in cases of pandemic or supply shortages, according to the CDC.
Although pregnant women may choose to receive a vaccination during the first trimester, the CDC warns there may be some risk involved.
“Although experience with the use of IIVs is substantial, and data from observational studies are available to support the safety of these vaccines in pregnancy, data are more limited for vaccination during the first trimester,” according to the CDC. “Moreover, there is substantially less experience with more recently licensed IIV products (e.g., quadrivalent, cell culture-based, and adjuvanted vaccines) during pregnancy in general.”
Data also are limited regarding RIVs, the CDC said, with the data used to determine safety among pregnant women “limited to reports of pregnancies occurring incidentally during clinical trials, Vaccine Adverse Event Reporting System (VAERS) reports, and pregnancy registry reports.”
Changes for children
The CDC chose to accept ACIP recommendations regarding Afluria (IIV3), expanding the age of children who can receive the vaccine from 9 years and older to 5 years and older.
Similar labeling changes were accepted for FluLaval Quadrivalent (IIV4), which had previously been given to children 3 years and older but now but will be available for children starting at 6 months of age.
New products
Recent product licensures included in the MMWR report are Afluria Quadrivalent (IIV4) and Flublok Quadrivalent (RIV4), both for persons over 18 years.
According to the CDC, Flublok Quadrivalent (an RIV) met noninferiority measures, compared with a similar IIV quadrivalent vaccine, for the A(H3H2) and B/Yamagata viruses but not for A(H1N1) or B/Victoria viruses.
Vaccine composition for 2017-2018
Approved viruses for the 2017-2018 season trivalent vaccines are an A/Michigan/45/2015 (H1N1) pdm09–like virus, an A/Hong Kong/4801/2014 (H3N2)-like virus, and a B/Brisbane/60/2008–like virus (Victoria lineage), according to the MMWR. Quadrivalent vaccines will include those viruses, with the addition of an B/Phuket/3073/2013–like virus (Yamagata lineage).
The CDC continues to recommend that the quadrivalent live attenuated influenza vaccine FluMist not be used by anyone for the 2017-2018 season, a decision that was made after evidence showed poor effectiveness against influenza A(H1N1)pdm09 viruses in the 2013-2014 and 2015-2016 seasons.
Vaccine updates published in this report were recommended by ACIP during meetings held in October 2016 and February and June 2017.
[email protected]
On Twitter @eaztweets
, according to new recommendations from the Centers for Disease Control and Prevention.
This change from the CDC’s previous guidance that pregnant women receive a seasonal inactivated influenza vaccine (IIV) was recommended by the Advisory Committee on Immunization Practices after some heated debate among committee members over evidence presented to support the change in wording (MMWR. 2017 Aug 25;66[(RR-2]:1-20).
The new update gives women the ability to choose between receiving an IIV and FluBlok, a recombinant influenza vaccine (RIV) that is not egg based and can be manufactured more quickly, making it ideal in cases of pandemic or supply shortages, according to the CDC.
Although pregnant women may choose to receive a vaccination during the first trimester, the CDC warns there may be some risk involved.
“Although experience with the use of IIVs is substantial, and data from observational studies are available to support the safety of these vaccines in pregnancy, data are more limited for vaccination during the first trimester,” according to the CDC. “Moreover, there is substantially less experience with more recently licensed IIV products (e.g., quadrivalent, cell culture-based, and adjuvanted vaccines) during pregnancy in general.”
Data also are limited regarding RIVs, the CDC said, with the data used to determine safety among pregnant women “limited to reports of pregnancies occurring incidentally during clinical trials, Vaccine Adverse Event Reporting System (VAERS) reports, and pregnancy registry reports.”
Changes for children
The CDC chose to accept ACIP recommendations regarding Afluria (IIV3), expanding the age of children who can receive the vaccine from 9 years and older to 5 years and older.
Similar labeling changes were accepted for FluLaval Quadrivalent (IIV4), which had previously been given to children 3 years and older but now but will be available for children starting at 6 months of age.
New products
Recent product licensures included in the MMWR report are Afluria Quadrivalent (IIV4) and Flublok Quadrivalent (RIV4), both for persons over 18 years.
According to the CDC, Flublok Quadrivalent (an RIV) met noninferiority measures, compared with a similar IIV quadrivalent vaccine, for the A(H3H2) and B/Yamagata viruses but not for A(H1N1) or B/Victoria viruses.
Vaccine composition for 2017-2018
Approved viruses for the 2017-2018 season trivalent vaccines are an A/Michigan/45/2015 (H1N1) pdm09–like virus, an A/Hong Kong/4801/2014 (H3N2)-like virus, and a B/Brisbane/60/2008–like virus (Victoria lineage), according to the MMWR. Quadrivalent vaccines will include those viruses, with the addition of an B/Phuket/3073/2013–like virus (Yamagata lineage).
The CDC continues to recommend that the quadrivalent live attenuated influenza vaccine FluMist not be used by anyone for the 2017-2018 season, a decision that was made after evidence showed poor effectiveness against influenza A(H1N1)pdm09 viruses in the 2013-2014 and 2015-2016 seasons.
Vaccine updates published in this report were recommended by ACIP during meetings held in October 2016 and February and June 2017.
[email protected]
On Twitter @eaztweets
, according to new recommendations from the Centers for Disease Control and Prevention.
This change from the CDC’s previous guidance that pregnant women receive a seasonal inactivated influenza vaccine (IIV) was recommended by the Advisory Committee on Immunization Practices after some heated debate among committee members over evidence presented to support the change in wording (MMWR. 2017 Aug 25;66[(RR-2]:1-20).
The new update gives women the ability to choose between receiving an IIV and FluBlok, a recombinant influenza vaccine (RIV) that is not egg based and can be manufactured more quickly, making it ideal in cases of pandemic or supply shortages, according to the CDC.
Although pregnant women may choose to receive a vaccination during the first trimester, the CDC warns there may be some risk involved.
“Although experience with the use of IIVs is substantial, and data from observational studies are available to support the safety of these vaccines in pregnancy, data are more limited for vaccination during the first trimester,” according to the CDC. “Moreover, there is substantially less experience with more recently licensed IIV products (e.g., quadrivalent, cell culture-based, and adjuvanted vaccines) during pregnancy in general.”
Data also are limited regarding RIVs, the CDC said, with the data used to determine safety among pregnant women “limited to reports of pregnancies occurring incidentally during clinical trials, Vaccine Adverse Event Reporting System (VAERS) reports, and pregnancy registry reports.”
Changes for children
The CDC chose to accept ACIP recommendations regarding Afluria (IIV3), expanding the age of children who can receive the vaccine from 9 years and older to 5 years and older.
Similar labeling changes were accepted for FluLaval Quadrivalent (IIV4), which had previously been given to children 3 years and older but now but will be available for children starting at 6 months of age.
New products
Recent product licensures included in the MMWR report are Afluria Quadrivalent (IIV4) and Flublok Quadrivalent (RIV4), both for persons over 18 years.
According to the CDC, Flublok Quadrivalent (an RIV) met noninferiority measures, compared with a similar IIV quadrivalent vaccine, for the A(H3H2) and B/Yamagata viruses but not for A(H1N1) or B/Victoria viruses.
Vaccine composition for 2017-2018
Approved viruses for the 2017-2018 season trivalent vaccines are an A/Michigan/45/2015 (H1N1) pdm09–like virus, an A/Hong Kong/4801/2014 (H3N2)-like virus, and a B/Brisbane/60/2008–like virus (Victoria lineage), according to the MMWR. Quadrivalent vaccines will include those viruses, with the addition of an B/Phuket/3073/2013–like virus (Yamagata lineage).
The CDC continues to recommend that the quadrivalent live attenuated influenza vaccine FluMist not be used by anyone for the 2017-2018 season, a decision that was made after evidence showed poor effectiveness against influenza A(H1N1)pdm09 viruses in the 2013-2014 and 2015-2016 seasons.
Vaccine updates published in this report were recommended by ACIP during meetings held in October 2016 and February and June 2017.
[email protected]
On Twitter @eaztweets
FROM MMWR
AAP recommends hepatitis B vaccine within 24 hours of birth for all infants
All newborns with a birth weight of at least 2,000 grams (4.4 pounds) should receive the hepatitis B vaccine within 24 hours of birth, according to a new policy statement by the American Academy of Pediatrics that brings its recommendations in line with those of the Advisory Committee on Immunization Practices at the Centers for Disease Control and Prevention.
“The birth dose can prevent infection of infants born to infected mothers in situations in which the mother’s results are never obtained, are misinterpreted, are falsely negative, are transcribed or reported to the infant care team inaccurately, or simply not communicated to the nursery,” announced the new statement from the AAP Committee on Infectious Diseases and the Committee on Fetus and Newborn (Pediatrics. 2017 Aug 28. doi: 10.1542/peds.2017-1870).
A dose of the hepatitis B vaccine within 24 hours of birth is 75%-95% effective at preventing perinatal hepatitis B transmission. “When postexposure prophylaxis with both hepatitis B vaccine and hepatitis B immune globulin (HBIG) is given, is timed appropriately, and is followed by completion of the infant hepatitis B immunization series, perinatal infection rates range from 0.7% to 1.1%,” according to the statement.
Approximately 1,000 newborns still contract perinatal hepatitis B infections every year. Of these, 90% will develop chronic hepatitis B infections, and a quarter of those who don’t receive treatment will die from liver cirrhosis or cancer. There has been an increase in the incidence of new hepatitis B infections in some states because of opioid epidemic in the United States, according to MMWR reports.
The cost effectiveness of preventing hepatitis B with the vaccine and, when necessary, HBIG, is estimated at $2,600 per quality-adjusted year of life. The most common side effects reported after hepatitis B administration are pain (3%-29%), erythema (3%), swelling (3%), fever (1%-6%) and headache (3%).
There has been extensive analysis of the safety of hepatitis B vaccines, the policy statement indicated. Analysis of Vaccine Safety Datalink data has found no causal link between administration of the hepatitis B vaccine and the following: neonatal sepsis or death, rheumatoid arthritis, Bell’s palsy, autoimmune thyroid disease, hemolytic anemia in children, anaphylaxis, optic neuritis, Guillain-Barré syndrome, sudden-onset sensorineural hearing loss, or other chronic illnesses.
Specific recommendations
• Infants born to mothers who test positive for hepatitis B surface antigen (HBsAg): Administer the hepatitis B vaccine and HBIG within 12 hours of birth.
• Infants weighing at least 2,000 g and born to mothers who are HBsAg negative: Administer the hepatitis B vaccine within 24 hours of birth.
• Infants weighing less than 2,000 g and born to mothers who are HBsAg negative: Administer the hepatitis B vaccine at hospital discharge or at age 1 month (whichever is first).
• Infants weighing at least 2,000 g and born to mothers with an unknown HBsAg status: Administer the hepatitis B vaccine within 12 hours of birth and HBIG by hospital discharge or age 7 days (whichever is first) if HBsAg status remains unknown or is confirmed positive.
• Infants weighing less than 2,000 g and born to mothers with an unknown HBsAg status: Administer the hepatitis B vaccine within 12 hours of birth and then HBIG within 12 hours unless the mother tests negative for HBsAg by then.
All newborns with a birth weight of at least 2,000 grams (4.4 pounds) should receive the hepatitis B vaccine within 24 hours of birth, according to a new policy statement by the American Academy of Pediatrics that brings its recommendations in line with those of the Advisory Committee on Immunization Practices at the Centers for Disease Control and Prevention.
“The birth dose can prevent infection of infants born to infected mothers in situations in which the mother’s results are never obtained, are misinterpreted, are falsely negative, are transcribed or reported to the infant care team inaccurately, or simply not communicated to the nursery,” announced the new statement from the AAP Committee on Infectious Diseases and the Committee on Fetus and Newborn (Pediatrics. 2017 Aug 28. doi: 10.1542/peds.2017-1870).
A dose of the hepatitis B vaccine within 24 hours of birth is 75%-95% effective at preventing perinatal hepatitis B transmission. “When postexposure prophylaxis with both hepatitis B vaccine and hepatitis B immune globulin (HBIG) is given, is timed appropriately, and is followed by completion of the infant hepatitis B immunization series, perinatal infection rates range from 0.7% to 1.1%,” according to the statement.
Approximately 1,000 newborns still contract perinatal hepatitis B infections every year. Of these, 90% will develop chronic hepatitis B infections, and a quarter of those who don’t receive treatment will die from liver cirrhosis or cancer. There has been an increase in the incidence of new hepatitis B infections in some states because of opioid epidemic in the United States, according to MMWR reports.
The cost effectiveness of preventing hepatitis B with the vaccine and, when necessary, HBIG, is estimated at $2,600 per quality-adjusted year of life. The most common side effects reported after hepatitis B administration are pain (3%-29%), erythema (3%), swelling (3%), fever (1%-6%) and headache (3%).
There has been extensive analysis of the safety of hepatitis B vaccines, the policy statement indicated. Analysis of Vaccine Safety Datalink data has found no causal link between administration of the hepatitis B vaccine and the following: neonatal sepsis or death, rheumatoid arthritis, Bell’s palsy, autoimmune thyroid disease, hemolytic anemia in children, anaphylaxis, optic neuritis, Guillain-Barré syndrome, sudden-onset sensorineural hearing loss, or other chronic illnesses.
Specific recommendations
• Infants born to mothers who test positive for hepatitis B surface antigen (HBsAg): Administer the hepatitis B vaccine and HBIG within 12 hours of birth.
• Infants weighing at least 2,000 g and born to mothers who are HBsAg negative: Administer the hepatitis B vaccine within 24 hours of birth.
• Infants weighing less than 2,000 g and born to mothers who are HBsAg negative: Administer the hepatitis B vaccine at hospital discharge or at age 1 month (whichever is first).
• Infants weighing at least 2,000 g and born to mothers with an unknown HBsAg status: Administer the hepatitis B vaccine within 12 hours of birth and HBIG by hospital discharge or age 7 days (whichever is first) if HBsAg status remains unknown or is confirmed positive.
• Infants weighing less than 2,000 g and born to mothers with an unknown HBsAg status: Administer the hepatitis B vaccine within 12 hours of birth and then HBIG within 12 hours unless the mother tests negative for HBsAg by then.
All newborns with a birth weight of at least 2,000 grams (4.4 pounds) should receive the hepatitis B vaccine within 24 hours of birth, according to a new policy statement by the American Academy of Pediatrics that brings its recommendations in line with those of the Advisory Committee on Immunization Practices at the Centers for Disease Control and Prevention.
“The birth dose can prevent infection of infants born to infected mothers in situations in which the mother’s results are never obtained, are misinterpreted, are falsely negative, are transcribed or reported to the infant care team inaccurately, or simply not communicated to the nursery,” announced the new statement from the AAP Committee on Infectious Diseases and the Committee on Fetus and Newborn (Pediatrics. 2017 Aug 28. doi: 10.1542/peds.2017-1870).
A dose of the hepatitis B vaccine within 24 hours of birth is 75%-95% effective at preventing perinatal hepatitis B transmission. “When postexposure prophylaxis with both hepatitis B vaccine and hepatitis B immune globulin (HBIG) is given, is timed appropriately, and is followed by completion of the infant hepatitis B immunization series, perinatal infection rates range from 0.7% to 1.1%,” according to the statement.
Approximately 1,000 newborns still contract perinatal hepatitis B infections every year. Of these, 90% will develop chronic hepatitis B infections, and a quarter of those who don’t receive treatment will die from liver cirrhosis or cancer. There has been an increase in the incidence of new hepatitis B infections in some states because of opioid epidemic in the United States, according to MMWR reports.
The cost effectiveness of preventing hepatitis B with the vaccine and, when necessary, HBIG, is estimated at $2,600 per quality-adjusted year of life. The most common side effects reported after hepatitis B administration are pain (3%-29%), erythema (3%), swelling (3%), fever (1%-6%) and headache (3%).
There has been extensive analysis of the safety of hepatitis B vaccines, the policy statement indicated. Analysis of Vaccine Safety Datalink data has found no causal link between administration of the hepatitis B vaccine and the following: neonatal sepsis or death, rheumatoid arthritis, Bell’s palsy, autoimmune thyroid disease, hemolytic anemia in children, anaphylaxis, optic neuritis, Guillain-Barré syndrome, sudden-onset sensorineural hearing loss, or other chronic illnesses.
Specific recommendations
• Infants born to mothers who test positive for hepatitis B surface antigen (HBsAg): Administer the hepatitis B vaccine and HBIG within 12 hours of birth.
• Infants weighing at least 2,000 g and born to mothers who are HBsAg negative: Administer the hepatitis B vaccine within 24 hours of birth.
• Infants weighing less than 2,000 g and born to mothers who are HBsAg negative: Administer the hepatitis B vaccine at hospital discharge or at age 1 month (whichever is first).
• Infants weighing at least 2,000 g and born to mothers with an unknown HBsAg status: Administer the hepatitis B vaccine within 12 hours of birth and HBIG by hospital discharge or age 7 days (whichever is first) if HBsAg status remains unknown or is confirmed positive.
• Infants weighing less than 2,000 g and born to mothers with an unknown HBsAg status: Administer the hepatitis B vaccine within 12 hours of birth and then HBIG within 12 hours unless the mother tests negative for HBsAg by then.
FROM PEDIATRICS
Key clinical point: All infants should receive hepatitis B vaccine within 24 hours of birth.
Major finding: Hepatitis B vaccine prevents 75%-95% of perinatal hepatitis B infections.
Data source: A literature review of data on hepatitis B epidemiology in the United States.
Disclosures: The statement did not receive external funding, and the authors stated that they have no conflicts of interest.