User login
FDA grants RMAT designation to HSCT adjunct
The US Food and Drug Administration (FDA) has granted Regenerative Medicine Advanced Therapy (RMAT) designation to ATIR101™, which is intended to be used as an adjunct to haploidentical hematopoietic stem cell transplant (HSCT).
ATIR101 is a personalized T-cell immunotherapy—a donor lymphocyte preparation selectively depleted of host-alloreactive T cells through the use of photo-dynamic therapy.
Recipient-reactive T cells from the donor are activated in a unidirectional mixed-lymphocyte reaction. The cells are then treated with TH9402 (a rhodamide-like dye), which is selectively retained in activated T cells.
Subsequent light exposure eliminates the activated recipient-reactive T cells but preserves the other T cells.
The final product is infused after CD34-selected haploidentical HSCT with the goal of preventing infectious complications, graft-versus-host disease (GVHD), and relapse.
About RMAT designation
The RMAT pathway is analogous to the breakthrough therapy designation designed for traditional drug candidates and medical devices. RMAT designation was specifically created by the US Congress in 2016 in the hopes of getting new cell therapies and advanced medicinal products to patients earlier.
Just like breakthrough designation, RMAT designation allows companies developing regenerative medicine therapies to interact with the FDA more frequently in the clinical testing process. In addition, RMAT-designated products may be eligible for priority review and accelerated approval.
A regenerative medicine is eligible for RMAT designation if it is intended to treat, modify, reverse, or cure a serious or life-threatening disease or condition, and if preliminary clinical evidence indicates the treatment has the potential to address unmet medical needs for such a disease or condition.
“To receive the RMAT designation from the FDA is an important milestone for Kiadis Pharma and a recognition by the FDA of the significant potential for ATIR101 to help patients receive safer and more effective bone marrow transplantations,” said Arthur Lahr, CEO of Kiadis Pharma, the company developing ATIR101.
“We are now going to work even closer with the FDA to agree a path to make this cell therapy treatment available for patients in the US as soon as possible. In Europe, ATIR101 was filed for registration in April 2017, and we continue to prepare the company for the potential European launch in 2019.”
ATIR101 trials
Results of a phase 2 trial of ATIR101 were presented at the 42nd Annual Meeting of the European Society of Blood and Marrow Transplantation in 2016.
Patients who received ATIR101 after haploidentical HSCT had significant improvements in transplant-related mortality and overall survival when compared to historical controls who received a T-cell-depleted haploidentical HSCT without ATIR101.
None of the patients who received ATIR101 developed grade 3-4 GVHD, but a few patients did develop grade 2 GVHD.
A phase 3 trial of ATIR101 is now underway. The trial is expected to enroll 200 patients with acute myeloid leukemia, acute lymphoblastic leukemia, or myelodysplastic syndrome.
The patients will receive a haploidentical HSCT with either a T-cell-depleted graft and adjunctive treatment with ATIR101 or a T-cell-replete graft and post-transplant cyclophosphamide. ![]()
The US Food and Drug Administration (FDA) has granted Regenerative Medicine Advanced Therapy (RMAT) designation to ATIR101™, which is intended to be used as an adjunct to haploidentical hematopoietic stem cell transplant (HSCT).
ATIR101 is a personalized T-cell immunotherapy—a donor lymphocyte preparation selectively depleted of host-alloreactive T cells through the use of photo-dynamic therapy.
Recipient-reactive T cells from the donor are activated in a unidirectional mixed-lymphocyte reaction. The cells are then treated with TH9402 (a rhodamide-like dye), which is selectively retained in activated T cells.
Subsequent light exposure eliminates the activated recipient-reactive T cells but preserves the other T cells.
The final product is infused after CD34-selected haploidentical HSCT with the goal of preventing infectious complications, graft-versus-host disease (GVHD), and relapse.
About RMAT designation
The RMAT pathway is analogous to the breakthrough therapy designation designed for traditional drug candidates and medical devices. RMAT designation was specifically created by the US Congress in 2016 in the hopes of getting new cell therapies and advanced medicinal products to patients earlier.
Just like breakthrough designation, RMAT designation allows companies developing regenerative medicine therapies to interact with the FDA more frequently in the clinical testing process. In addition, RMAT-designated products may be eligible for priority review and accelerated approval.
A regenerative medicine is eligible for RMAT designation if it is intended to treat, modify, reverse, or cure a serious or life-threatening disease or condition, and if preliminary clinical evidence indicates the treatment has the potential to address unmet medical needs for such a disease or condition.
“To receive the RMAT designation from the FDA is an important milestone for Kiadis Pharma and a recognition by the FDA of the significant potential for ATIR101 to help patients receive safer and more effective bone marrow transplantations,” said Arthur Lahr, CEO of Kiadis Pharma, the company developing ATIR101.
“We are now going to work even closer with the FDA to agree a path to make this cell therapy treatment available for patients in the US as soon as possible. In Europe, ATIR101 was filed for registration in April 2017, and we continue to prepare the company for the potential European launch in 2019.”
ATIR101 trials
Results of a phase 2 trial of ATIR101 were presented at the 42nd Annual Meeting of the European Society of Blood and Marrow Transplantation in 2016.
Patients who received ATIR101 after haploidentical HSCT had significant improvements in transplant-related mortality and overall survival when compared to historical controls who received a T-cell-depleted haploidentical HSCT without ATIR101.
None of the patients who received ATIR101 developed grade 3-4 GVHD, but a few patients did develop grade 2 GVHD.
A phase 3 trial of ATIR101 is now underway. The trial is expected to enroll 200 patients with acute myeloid leukemia, acute lymphoblastic leukemia, or myelodysplastic syndrome.
The patients will receive a haploidentical HSCT with either a T-cell-depleted graft and adjunctive treatment with ATIR101 or a T-cell-replete graft and post-transplant cyclophosphamide. ![]()
The US Food and Drug Administration (FDA) has granted Regenerative Medicine Advanced Therapy (RMAT) designation to ATIR101™, which is intended to be used as an adjunct to haploidentical hematopoietic stem cell transplant (HSCT).
ATIR101 is a personalized T-cell immunotherapy—a donor lymphocyte preparation selectively depleted of host-alloreactive T cells through the use of photo-dynamic therapy.
Recipient-reactive T cells from the donor are activated in a unidirectional mixed-lymphocyte reaction. The cells are then treated with TH9402 (a rhodamide-like dye), which is selectively retained in activated T cells.
Subsequent light exposure eliminates the activated recipient-reactive T cells but preserves the other T cells.
The final product is infused after CD34-selected haploidentical HSCT with the goal of preventing infectious complications, graft-versus-host disease (GVHD), and relapse.
About RMAT designation
The RMAT pathway is analogous to the breakthrough therapy designation designed for traditional drug candidates and medical devices. RMAT designation was specifically created by the US Congress in 2016 in the hopes of getting new cell therapies and advanced medicinal products to patients earlier.
Just like breakthrough designation, RMAT designation allows companies developing regenerative medicine therapies to interact with the FDA more frequently in the clinical testing process. In addition, RMAT-designated products may be eligible for priority review and accelerated approval.
A regenerative medicine is eligible for RMAT designation if it is intended to treat, modify, reverse, or cure a serious or life-threatening disease or condition, and if preliminary clinical evidence indicates the treatment has the potential to address unmet medical needs for such a disease or condition.
“To receive the RMAT designation from the FDA is an important milestone for Kiadis Pharma and a recognition by the FDA of the significant potential for ATIR101 to help patients receive safer and more effective bone marrow transplantations,” said Arthur Lahr, CEO of Kiadis Pharma, the company developing ATIR101.
“We are now going to work even closer with the FDA to agree a path to make this cell therapy treatment available for patients in the US as soon as possible. In Europe, ATIR101 was filed for registration in April 2017, and we continue to prepare the company for the potential European launch in 2019.”
ATIR101 trials
Results of a phase 2 trial of ATIR101 were presented at the 42nd Annual Meeting of the European Society of Blood and Marrow Transplantation in 2016.
Patients who received ATIR101 after haploidentical HSCT had significant improvements in transplant-related mortality and overall survival when compared to historical controls who received a T-cell-depleted haploidentical HSCT without ATIR101.
None of the patients who received ATIR101 developed grade 3-4 GVHD, but a few patients did develop grade 2 GVHD.
A phase 3 trial of ATIR101 is now underway. The trial is expected to enroll 200 patients with acute myeloid leukemia, acute lymphoblastic leukemia, or myelodysplastic syndrome.
The patients will receive a haploidentical HSCT with either a T-cell-depleted graft and adjunctive treatment with ATIR101 or a T-cell-replete graft and post-transplant cyclophosphamide. ![]()
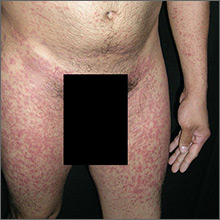
Rash on arms and legs
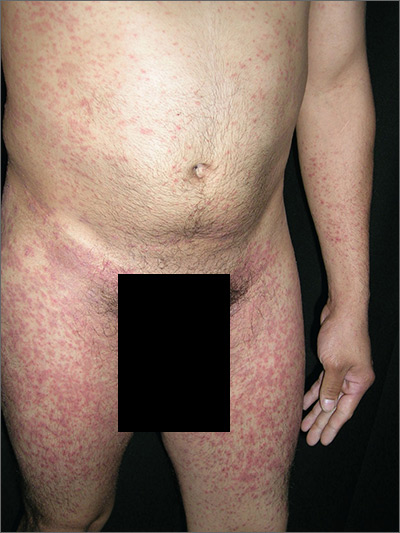
Based on the negative RPR test and the results of a subsequent 4-mm punch biopsy, the FP made a diagnosis of pityriasis rosea. The trunk is typically more involved in this condition, making this case (involvement of arms and legs) a type of inverse pityriasis rosea.
Because secondary syphilis can also present as a papulosquamous eruption, it can be difficult to distinguish from pityriasis rosea on clinical grounds. Therefore, taking a sexual history is important when a diagnosis of pityriasis rosea is being considered. In patients with a history of sexually transmitted diseases (STDs) or high-risk sexual practices, an RPR test should be ordered.
The patient in this case was relieved to learn that he didn’t have an STD and said he’d be more careful in the future. The FP assured him that the condition would go away spontaneously and that no medications would be needed.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Henderson D, Usatine R. Pityriasis rosea. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 896-900.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

Based on the negative RPR test and the results of a subsequent 4-mm punch biopsy, the FP made a diagnosis of pityriasis rosea. The trunk is typically more involved in this condition, making this case (involvement of arms and legs) a type of inverse pityriasis rosea.
Because secondary syphilis can also present as a papulosquamous eruption, it can be difficult to distinguish from pityriasis rosea on clinical grounds. Therefore, taking a sexual history is important when a diagnosis of pityriasis rosea is being considered. In patients with a history of sexually transmitted diseases (STDs) or high-risk sexual practices, an RPR test should be ordered.
The patient in this case was relieved to learn that he didn’t have an STD and said he’d be more careful in the future. The FP assured him that the condition would go away spontaneously and that no medications would be needed.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Henderson D, Usatine R. Pityriasis rosea. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 896-900.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

Based on the negative RPR test and the results of a subsequent 4-mm punch biopsy, the FP made a diagnosis of pityriasis rosea. The trunk is typically more involved in this condition, making this case (involvement of arms and legs) a type of inverse pityriasis rosea.
Because secondary syphilis can also present as a papulosquamous eruption, it can be difficult to distinguish from pityriasis rosea on clinical grounds. Therefore, taking a sexual history is important when a diagnosis of pityriasis rosea is being considered. In patients with a history of sexually transmitted diseases (STDs) or high-risk sexual practices, an RPR test should be ordered.
The patient in this case was relieved to learn that he didn’t have an STD and said he’d be more careful in the future. The FP assured him that the condition would go away spontaneously and that no medications would be needed.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Henderson D, Usatine R. Pityriasis rosea. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 896-900.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Cancer-Related Fatigue: Approach to Assessment and Management
INTRODUCTION
Fatigue is a common distressing effect of cancer.1 It impairs the quality of life of patients undergoing active cancer treatment and of post-treatment survivors alike. The National Comprehensive Cancer Network (NCCN) defines cancer-related fatigue (CRF) as “a distressing, persistent, subjective sense of physical, emotional and/or cognitive tiredness related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning.”2 CRF differs from fatigue reported by individuals without cancer in that CRF is more severe and is not relieved by rest. The prevalence of CRF in cancer patients and survivors is highly variable, with estimates ranging between 25% and 99%.2,3 The methods used for screening patients for fatigue and the characteristics of the patient groups may account for this variability.
PATHOPHYSIOLOGY
The specific pathophysiologic mechanism underlying CRF is unknown, making targeted treatment a challenge. The multidimensional and subjective nature of CRF has limited the development of research methodologies to explain this condition. However, research has been done in both human and animal models, and several theories have been proposed to explain the pathophysiology of CRF. While pro-inflammatory cytokines remain the central factor playing a significant role at multiple levels in CRF, there may be a complex interplay of multiple mechanisms contributing to fatigue in an individual patient.
CENTRAL NERVOUS SYSTEM DISTURBANCES
The basal ganglia are known to influence motivation. Lack of motivation and drive may cause failure to complete physical and mental tasks, even with preserved cognitive ability and motor function. In a study of melanoma patients receiving interferon, increased activity of the basal ganglia and the cerebellum resulted in higher fatigue scores.4 Increased levels of cytokines may alter blood flow to the cerebellum and lead to the perception of fatigue. In a study of 12 patients and matched controls, when patients were asked to perform sustained elbow flexion until they perceived exhaustion, CRF patients perceived physical exhaustion sooner than controls. In CRF patients in this study, muscle fatigue measured by electromyogram was less than that in healthy individuals at the time of exhaustion, suggesting the role of the central nervous system in CRF.5 However, there is not enough evidence at this time to support central nervous system disturbance as the main factor contributing to fatigue in cancer patients.
CIRCADIAN RHYTHM DYSREGULATION
Circadian rhythm is regulated by the suprachiasmatic nucleus in the hypothalamus through cortisol and melatonin. Sleep disturbances occur with disruption of the circadian rhythm. Tumor-related peptides such as epidermal growth factor or alterations in serotonin and cortisol can influence the suprachiasmatic nucleus and the complex signaling pathways.2 Positive feedback loops that are activated by cortisol under the influence of cytokines may lead to continuous cytokine production and altered circadian rhythm. Bower et al showed that changes in the cortisol curve influence fatigue in breast cancer survivors.6 These patients had a late evening peak in cortisol levels, compared with an early morning peak in individuals without cancer.
INHIBITION OF HYPOTHALAMIC-PITUITARY-ADRENAL AXIS
The hypothalamic–pituitary–adrenal (HPA) axis regulates the release of the stress hormone cortisol. One of several hypotheses advanced to explain the effect of serotonin and the HPA axis on CRF suggests that lower serotonin levels cause decreased activation of 5-hydroxytrytophan 1-a (5-HT1-a) receptors in the hypothalamus, leading to decreased activity of the HPA axis.6 Inhibition of the HPA axis may occur with higher levels of serotonin as well.7 The 5-HT1-a receptors are also triggered by cytokines. However, the correction of serotonin levels by antidepressants was not shown to improve fatigue.8 Inhibition of the HPA axis can also lead to lower testosterone, progesterone, or estrogen levels, which may indirectly contribute to fatigue.2
SKELETAL MUSCLE EFFECT
Chemotherapy- and tumor-related cachexia have a direct effect on the metabolism of skeletal muscles. This effect may lead to impaired adenosine triphosphate (ATP) generation during muscle contraction.9 ATP infusion improved muscle strength in 1 trial, but this was not confirmed in another trial.10,11 Muscle contraction studies showed no differences in the contractile properties of muscles in fatigued patients who failed earlier in motor tasks and healthy controls.12 This finding suggests that there could be a failure of skeletal muscle activation by the central nervous system or inhibition of skeletal muscle activity. Cytokines and other neurotransmitters activate vagal efferent nerve fibers, which may lead to reflex inhibition in skeletal muscles.13,14
PRO-INFLAMMATORY CYTOKINES
Tumors or treatment of them may cause tissue injury, which triggers immune cells to release cytokines, signaling the brain to manifest the symptom of fatigue. Inflammatory pathways are influenced by psychological, behavioral, and biological factors, which play a role as risk factors in CRF. Levels of interleukin 6 (IL-6), interleukin-1 receptor antagonist, interleukin-1, and tumor necrosis factor (TNF) have been shown to be elevated in fatigued patients being treated for leukemia and non-Hodgkin lymphoma.15 IL-6 was also associated with increased fatigue in breast cancer survivors.16 Similar findings were reported in patients undergoing stem cell transplantation and high-dose chemotherapy.17 Elevated levels of IL-6 and C-reactive protein were also linked to fatigue in terminally ill cancer patients.18,19 Furthermore, TNF-α signaling was associated with post-chemotherapy fatigue in breast cancer patients.20 Leukocytes in breast cancer survivors with fatigue also have increased gene expression of pro-inflammatory cytokines, emphasizing the role of cytokines and inflammation in the pathogenesis of CRF.21
OTHER HYPOTHESES
Several other hypotheses for CRF pathogenesis have been proposed. Activation of latent viruses such as Epstein-Barr virus, lack of social support,22 genetic alterations in the immune pathway,23 epigenetic changes,24 accumulation of neurotoxic metabolites and depletion of serotonin by indoleamine 2,3-dioxygenase pathway activation,25 elevated vascular endothelial growth factor levels,26 and hypoxia-related organ dysfunction due to anemia or hemoglobin dysfunction13 all have been postulated to cause CRF.
EVALUATION AND TREATMENT
Fours steps are involved in the evaluation and treatment of CRF (Figure).
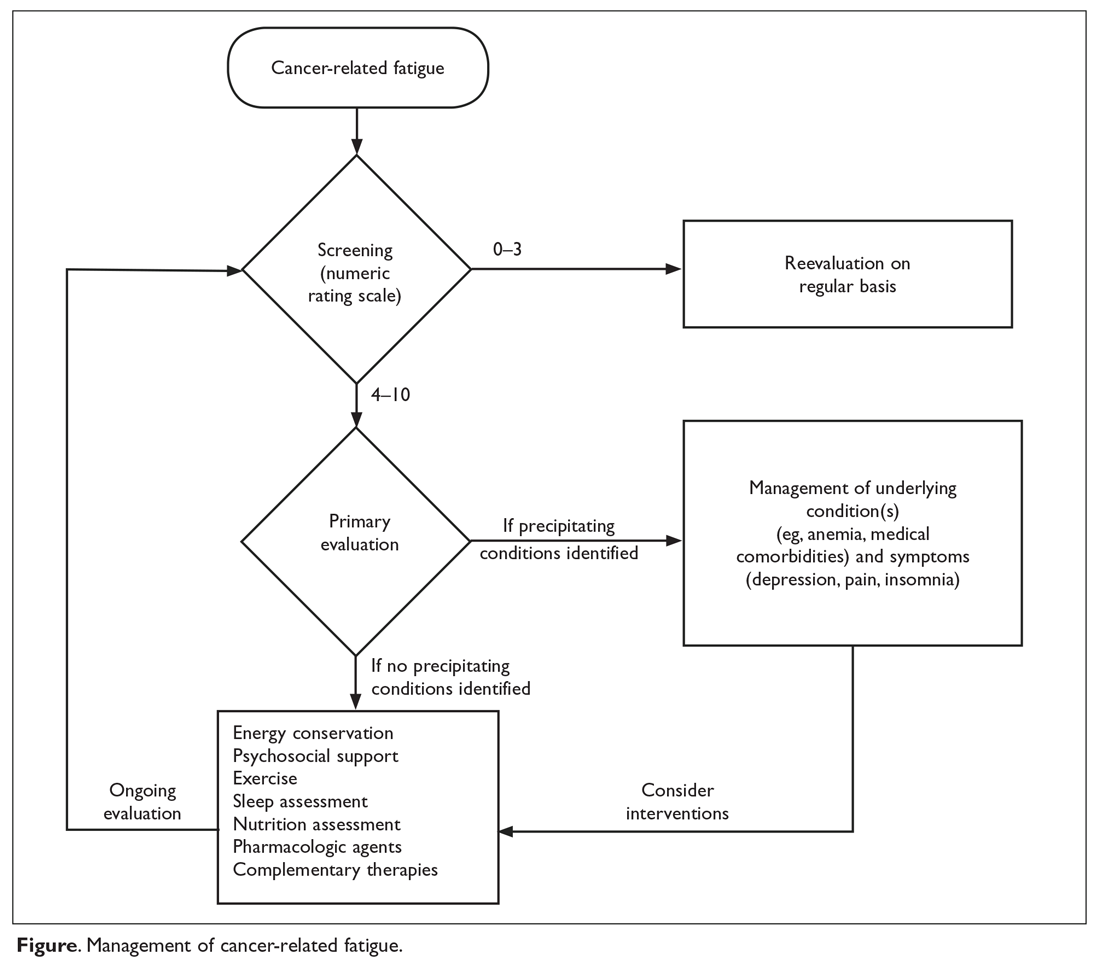
SCREENING
Because patients and health care professionals may be unaware of the treatment options available for CRF, patients may not report fatigue levels to their clinicians, and clinicians may not understand the impact of fatigue on their patients’ quality of life. This leads to under-recognition of the problem. The NCCN recommends screening every cancer patient and post-treatment survivor for fatigue.2 Patients should be screened at their first visit and then at periodic intervals during and after cancer treatment.
Many scales are available to screen patients for CRF in clinical practice and clinical trials.27 A single item that asks patients to rate their fatigue on a scale from 0 to 10—in which 0 indicates no fatigue, 1 to 3 indicates mild fatigue, 4 to 6 indicates moderate fatigue, 7 to 9 indicates severe fatigue, and 10 indicates the worst fatigue imaginable—is commonly used to screen for CRF.2 This scale was adapted from the MD Anderson Symptom Inventory scale and is based on a large nationwide study of cancer patients and survivors.28 The statistically derived cutoff points in this study are consistent with other scales such as the Brief Fatigue Inventory (BFI) and support the cutoff points (4–6 for moderate and ≥ 7 for severe fatigue) used in various fatigue management guidelines. Furthermore, studies of fatigue in cancer patients have revealed a marked decrease in physical function at levels of 7 or higher, suggesting 7 as an optimal cutoff to identify severe fatigue.29,30 The Visual Analog Scale is another simple-to-use tool that helps in understanding variations in fatigue throughout the course of the day.31 The 9-item BFI is often used in clinical trials.29 It measures the severity of fatigue over the previous 24 hours and has been validated in patients who do not speak English.32
CRF affects not only the somatic domain, but also the cognitive, behavioral, and affective domains; therefore, multidimensional scales have been developed for screening. One such tool is the Multidimensional Fatigue Inventory, which assesses 5 dimensions of fatigue—general fatigue, physical fatigue, reduced motivation, reduced activity, and mental fatigue—and compares the patient’s results with those of individuals without cancer.33,34 The Functional Assessment of Cancer Therapy for Fatigue (FACT-F) is a 13-item questionnaire that has been used to measure CRF in clinical trials as well as in patients receiving various treatments.35
Although many scales are available, the validity of self-reporting on simple fatigue-rating scales is equal to or better than most complex, lengthy scales.36 Therefore, unidimensional tools such as the numeric rating scale of 0–10 are commonly used in clinical practice. Mild fatigue (0–3) requires periodic reevaluation, and moderate and severe fatigue need further evaluation and management.37
PRIMARY EVALUATION
This phase involves a focused history and physical examination and assessment of concurrent symptoms and contributing factors.
History and Physical Examination
A detailed history of the patient’s malignancy and type of previous and current treatment may help reveal the cause of fatigue. New-onset fatigue or increase in fatigue may be related to the progression of disease in patients with active malignancy or recurrence of cancer in survivors. These patients may require appropriate testing to assess the underlying disease pattern. A detailed review of systems may help identify some of the contributing factors, which are discussed below. A detailed history regarding medications, including over-the-counter drugs, complementary agents, and past and prior cancer therapies, is helpful as medications can contribute to fatigue. For example, opioids may cause drowsiness and fatigue, which could be improved by dose adjustments. A focused history of fatigue should be obtained in all patients with moderate to severe CRF, which includes the onset, pattern, duration, associated or alleviating factors, and interference with functioning, including activities of daily living.37 Physical examination should focus on identifying signs of organ dysfunction and features of substance or alcohol abuse, which may cause poor sleep and fatigue.
Assessment of Contributing Factors
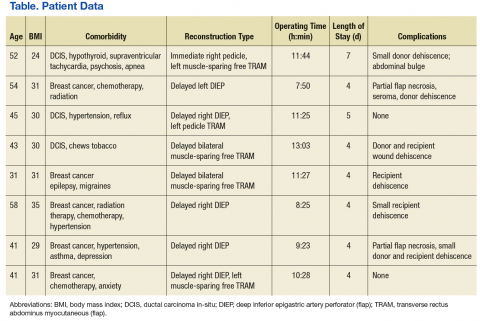
The management of fatigue should be multifactorial, with a comprehensive assessment and treatment plan to address all modifiable fatigue etiologies. The Table lists potential contributing factors to fatigue that should be considered when evaluating patients for CRF; several common conditions are discussed below. 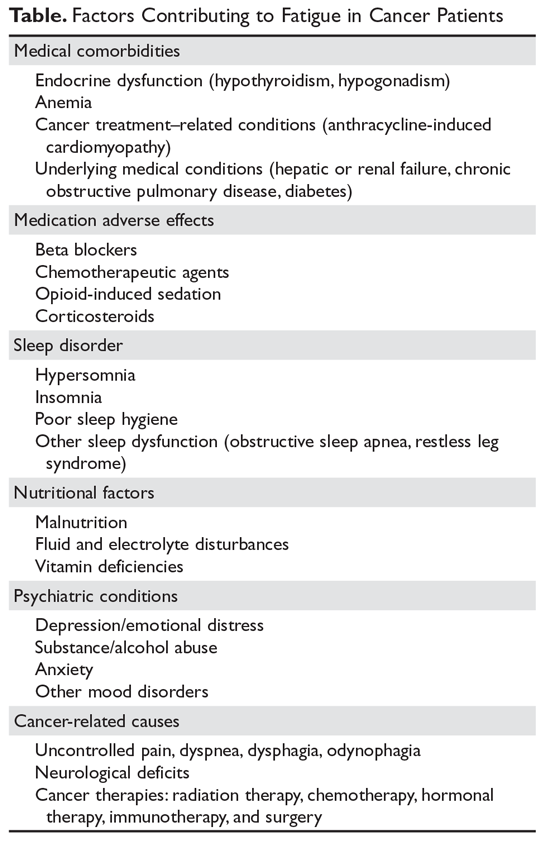
Anemia. Anemia has been correlated with fatigue and quality of life. In a study of 4382 cancer patients receiving chemotherapy, quality-of-life measures using FACT-Anemia scores improved with increased hemoglobin levels.38 Cancer patients may have anemia due to marrow-suppressing effects of chemotherapy and may also have iron deficiency anemia due to blood loss or auto-immune hemolytic anemia. Therefore, a detailed work-up is required to identify the etiology of anemia. Patients with CRF whose anemia is related to chemotherapy or anemia of chronic disease may benefit from red blood cell transfusion or erythropoiesis-stimulating agents (ESAs). ESAs have been studied extensively; however, their use is controversial because of concerns about thromboembolic side effects leading to adverse outcomes.39 Also, ESA therapy is not recommended in patients with hematologic malignancies. ESA use should be restricted to patients with chemotherapy-related anemia with hemoglobin below 10 mg/dL and should be discontinued in 6 to 8 weeks if patients do not respond.40 Other patients may benefit from blood transfusions, which were shown to help in patients with hemoglobin levels between 7.5 and 8.5 g/dL.41
Sleep disturbance. Poor sleep is common in fatigued cancer survivors.42 Pro-inflammatory cytokines can disrupt the sleep–wake cycle, causing changes in the HPA axis and neuroendocrine system, which in turn may lead to increasing fatigue. Heckler et al showed that improvement in nighttime sleep leads to improvement of fatigue.43 Cognitive behavioral therapy and sleep hygiene are important in caring for patients with CRF and poor sleep.44 Taking a warm bath and/or drinking a glass of milk before bedtime, avoiding caffeinated drinks, and avoiding frequent napping in the day might help. Some patients may require medications such as benzodiazepines or non-benzodiazepine hypnotics (eg, zolpidem) to promote sleep.45 Melatonin agonists are approved for insomnia in the United States, but not in Europe.46
Malnutrition. Patients with advanced-stage cancer and with cancers affecting the gastrointestinal tract frequently develop mechanical bowel obstructions, especially at the end of their life, which cause malnutrition. Chemotherapy-related nausea and vomiting may also cause poor oral intake and malnutrition, causing fatigue from muscle weakness. Dehydration and electrolyte imbalances frequently occur as a result of poor oral intake, which might worsen fatigue. In our experience, modifying dietary intake with appropriate caloric exchanges with the help of a nutrition expert has lessened fatigue in some patients. However, terminally ill patients are advised to eat based on their comfort.
Medical comorbidities. Congestive heart failure from anthracycline chemotherapy, hypothyroidism after radiation therapy for head and neck cancers, renal failure, or hepatic failure from chemotherapy may indirectly lead to fatigue. Chemotherapy, opioids, and steroids may cause hypogonadism, which can contribute to fatigue in men.47
Assessment of Concurrent Symptoms
Depression is common in cancer patients and coexists with pain, insomnia, fatigue, and anxiety as a symptom cluster.48 A symptom cluster is defined as 2 or more concurrent and interrelated symptoms occurring together; treating one of these symptoms without addressing other symptoms is not effective.49 Therefore, screening for and management of depression, anxiety, and insomnia play an important role in the management of CRF.
Physical symptoms due to the tumor or to therapy— such as pain, dyspnea, nausea, and decreased physical activity—may also contribute to fatigue both directly and indirectly. Patients with lung cancer may have hypoxemia, which can contribute to dyspnea with activity and a perception of fatigue. Optimal management of pain and other physical symptoms in patients with cancer may significantly alleviate fatigue.50
MANAGEMENT
Management of CRF is individualized based on the patient’s clinical status: active cancer treatment, survivor, or end of life. Education and counselling of patients and their caregivers play an important role in CRF. NCCN guidelines recommend focusing on pain control, distress management, energy conservation, physical activity, nutrition, and sleep hygiene.
Nonpharmacologic Interventions
Energy conservation. Energy conservation strategies, in which patients are advised to set priorities and realistic expectations, are highly recommended. Some energy-conserving strategies are to pace oneself, delegate and schedule activities at times of peak energy, postpone nonessential activities, attend to 1 activity at a time, structure daily routines, and maintain a diary to identify their peak energy period and structure activities around that time.51,52 When patients approach the end of life, increasing fatigue may limit their activity level, and they may depend on caregivers for assistance with activities of daily living, monitoring treatment-related adverse effects, and taking medications, especially elderly patients.53
Cognitive behavioral therapy. Cognitive behavioral therapy (CBT) has been shown to improve CRF during active treatment, and the benefits persist for a minimum of 2 years after therapy.54 CBT interventions that optimize sleep quality may improve fatigue.55 More studies are needed to understand whether referral to a psychologist for formal CBT is required. Randomized clinical trials showed patient fatigue education, learned self-care, coping techniques, and balancing rest and activity benefit patients with CRF.56
Exercise. Physical activity is highly encouraged in patients with CRF. Exercise increases muscle protein synthesis, improves cytokine response, and decreases the rate of sarcopenia in healthy populations.57 Studies have shown that exercise helps CRF at all phases of the cancer journey, including radiation therapy, chemotherapy, and survivorship.58 Some patients may feel less motivated to exercise and may not believe that exercise is possible or could potentially help them. Counselling is needed for such patients.
Older cancer survivors have a decline in their functional capacity and reduced muscle mass. Exercise can improve their cardiorespiratory fitness, muscle strength, and body composition.57 Exercise not only helps at the cellular level but also has psychosocial benefits from improved self-esteem. Therefore, exercise may be recommended for younger patients as well as for the older population, who may have comorbidities and less motivation than younger patients.
In a meta-analysis of 56 randomized controlled trials involving 4068 participants, aerobic exercise was found to have beneficial effects on CRF for patients during and after chemotherapy, specifically for patients with solid tumors.59 In another meta-analysis of breast and prostate cancer survivors, a combination of aerobic exercise with resistance training (3–6 metabolic equivalents, 60%–80% range of motion) was shown to reduce CRF more than aerobic exercise alone.60 This effect was also shown in a randomized controlled trial of 160 patients with stage 0 to III breast cancer undergoing radiation therapy.61 The control group in this study had a group-based non-exercise intervention/relaxation; therefore, the study showed that the effect of resistance training extends beyond the psychosocial benefits of group-based interventions. The intervention comprised 8 progressive machine-based resistance exercises (3 sets, 8–12 repetitions at 60%–80% of 1 repetition maximum) for 60 minutes twice weekly for 12 weeks. However, fatigue assessment questionnaire scores showed benefits only in the physical fatigue components, but not in the affective and cognitive components.
The American Society of Clinical Oncology’s guidelines for cancer survivors with fatigue recommends 150 minutes of moderate aerobic exercise (eg, fast walking, cycling, or swimming) per week, with 2 or 3 sessions of strength training per week.62 An individualized approach to exercise is recommended, as patients’ ability to perform certain types of exercises may be limited by thrombocytopenia, neutropenia, or lytic bone metastasis. Routine use of pre-exercise cardiovascular testing is not recommended but may be considered in high-risk populations, especially patients with risk factors for coronary heart disease and diabetes.63 Patients with comorbidities, substantial deconditioning, functional and anatomic defects, or recent major surgery may benefit from referral to physical therapy.37 Patients near end of life may also benefit from an exercise program, as demonstrated in several studies that showed benefit in CRF and quality of life.64,65 We recommend that physicians use their best clinical judgement in suggesting the type and intensity of exercise program, as it may not be feasible in some patients.
Mind-body interventions. Mindfulness-based stress reduction (MBSR) has shown promise in breast cancer survivors, who reported immediate improvements in fatigue severity that continued up to 6 weeks after cessation of the training.66 Prior studies had similar findings, suggesting that MBSR modestly decreases fatigue and sleep disturbances and has a greater effect on the degree to which symptoms interfere with many facets of life.67
Yoga. A study of a yoga intervention showed a benefit in older cancer survivors.68 In breast cancer patients undergoing chemotherapy, yoga was shown to benefit both physical and cognitive fatigue.69 DVD-based yoga had benefits similar to strengthening exercises in a study of 34 early-stage breast cancer survivors with CRF.70 More studies are needed in men and patients and survivors of other cancers, as most studies of yoga were conducted in women with breast cancer.
Tai chi/qigong. Like yoga, tai chi and qigong are practices of meditative movement. These practices use postures or movements with a focus on breath and a meditative state to bring about deep states of relaxation. Qigong is a series of simple, repeated practices including body posture/movement, breath practice, and meditation performed in synchrony. Tai chi easy (TCE) is a simplified set of common, repetitive tai chi movements. In a trial, qigong/TCE was compared with sham qigong, which had physical movements but no breathing or meditative practice. Breast cancer survivors in the qigong/TCE group had improved fatigue scores, and the effect persisted for 3 months.71 Additional research is needed in this area.
Acupuncture. A randomized controlled trial in breast cancer patients with CRF showed an improvement in the mean general fatigue score (per the Multidimensional Fatigue Inventory) in patients who received acupuncture versus those who did not (−3.11 [95% confidence interval −3.97 to −2.25]; P < 0.001) at 6 weeks. Improvements were seen in both the mental and physical aspects of fatigue.72 However, Deng et al noted that true acupuncture was no more effective than sham acupuncture for reducing post-chemotherapy chronic fatigue.73 Presently, there is not sufficient evidence to evaluate the benefits of acupuncture in CRF.
Other modalities. Massage therapy, music therapy, hypnosis, therapeutic touch, biofield therapies, relaxation, and reiki are other therapies for which few studies have been done; of the studies that have been done, the results are mixed, and additional research is needed.74 Currently, there are not sufficient data to recommend any of these modalities.
Pharmacologic Interventions
Psychostimulants. Methylphenidate and modafinil are psychostimulants or wakefulness-promoting agents. Pilot studies showed benefit from methylphenidate and modafinil in CRF,75–77 but randomized controlled trials have yielded mixed results. Therefore, in patients with severe fatigue during cancer therapy, the initial management strategy involves evaluation and treatment of medical conditions such as anemia and a trial of nonpharmacological strategies as discussed above. If symptoms persist, then a therapeutic trial of a psychostimulant may be considered per NCCN guidelines for patients undergoing active cancer treatment.37
Methylphenidate directly stimulates adrenergic receptors and indirectly releases dopamine and norepinephrine from presynaptic terminals, which may explain why the drug benefits patients receiving opioid-induced sedation. It is a commonly studied psychostimulant, though its mechanism of action in CRF is unclear. Randomized controlled trials of methylphenidate have resulted in a wide range of findings due to the heterogeneity of study populations and variations in the dosage of methylphenidate. A meta-analysis of 7 studies indicates that methylphenidate benefitted the subgroup of patients with CRF.78 Likewise, in an analysis of 5 randomized controlled trials, Minton et al showed a benefit of psychostimulants in fatigue compared with placebo.79 However, another study of methylphenidate in patients with CRF showed a benefit only in patients with severe fatigue or advanced disease.80 Methylphenidate was found to benefit cancer patients receiving opioid-induced sedation, as methylphenidate promotes wakefulness, though fatigue was not studied specifically.81 In a trial with 30 hospice patients in which the methylphenidate dose was titrated based on response and adverse effects, Kerr at al found that the drug improved fatigue in a dose-dependent manner.82 However, a study in patients with CRF at the University of Texas MD Anderson Cancer Center found no significant difference in BFI scores between patients receiving methylphenidate and those receiving placebo at the end of 2 weeks of treatment.83 Also, other randomized controlled trials in patients undergoing adjuvant chemotherapy for breast cancer84 and patients receiving radiation therapy for brain tumors85 failed to demonstrate the efficacy of methylphenidate in CRF. It should be used cautiously after ruling out other causes of fatigue. The drug is overall well tolerated and side effects include headache and nausea.
Modafinil is a non-amphetamine psychostimulant that has been approved for the treatment of narcolepsy. In a trial studying the effect of modafinil on patients receiving docetaxel-based chemotherapy for metastatic breast or prostate cancer, there was a modest but not statistically significant improvement in fatigue scores on the MD Anderson Symptom Inventory compared with placebo. Nausea and vomiting were higher in the modafinil arm than in the placebo arm.86 Similarly, modafinil was not superior to placebo for CRF in 208 patients with non-squamous cell lung cancer not undergoing chemotherapy or radiation.87 A placebo effect was also noted in patients with multiple myeloma88 and patients with primary brain tumors.89 In a phase 3, multicenter, randomized, placebo-controlled, double-blind clinical trial of modafinil for CRF in 867 patients undergoing chemotherapy, there was a reduction in fatigue only for patients with severe baseline fatigue, with no significant effect on mild to moderate fatigue.90 In another recent study, modafinil was shown to reduce depressive symptoms only in patients with severe fatigue (BFI item 3 score ≥ 7).91 This finding is consistent with previous studies showing benefit in patients with high baseline fatigue, but additional randomized controlled trials are needed to provide clarity. NCCN guidelines do not recommend the use of modafinil to treat CRF.37
Other pharmacologic interventions. Corticosteroids are often used for symptom control in cancer patients. These drugs have anti-inflammatory effects through their modulation of pro-inflammatory cytokines.92 In a randomized controlled trial evaluating the efficacy of corticosteroids, patients receiving dexamethasone (4 mg twice daily) experienced significant improvement in their FACT-F scores compared with patients receiving placebo.93 A similar benefit in fatigue was demonstrated in another study of methylprednisolone (32 mg daily) versus placebo.94 Despite the benefits of steroids, their adverse effects, such as mood swings, gastritis, hyperglycemia, and immune suppression, limit their long-term use. Therefore, the use of steroids should be restricted to terminally ill fatigued patients with other symptoms such as anorexia, brain metastasis, or pain related to bone metastasis.37
Testosterone replacement has been shown to diminish fatigue in non-cancer patients. Many men with advanced cancer have hypogonadism leading to low serum testosterone, which may cause fatigue. In a small trial in which cancer patients with hypogonadism received intramuscular testosterone every 14 days or placebo, the group receiving testosterone showed improvement in FACT-F scores, but there was no significant difference in FACT-F scores between the 2 groups.95
Antidepressants have failed to demonstrate benefit in CRF without depression.8 However, if a patient has both fatigue and depression, antidepressants may help.96 A selective serotonin receptor inhibitor is recommended as a first-line antidepressant.97 Patients with cancer are often receiving multiple medications, and medication interactions should be considered to prevent adverse events such as serotonin syndrome.
Complementary and Alternative Supplements
Studies using vitamin supplementation have been inconclusive in patients with CRF.74 The use of other dietary supplements has yielded mixed results, and coenzyme Q has shown no benefit for patients with CRF.98
The benefit of ginseng was studied in a RCT involving 364 patients with CRF. There was an improvement in Multidimensional Fatigue Symptom Inventory-short form (MFSI-SF) scores at 8 weeks in patients receiving 2000 mg of Wisconsin ginseng compared with patients receiving placebo.99 Patients on active treatment had greater improvement as compared to the post-treatment group in this trial. In another study of high-dose panax ginseng (ginseng root) at 800 mg daily for 29 days, patients had improvement of CRF as well as overall quality of life, appetite, and sleep at night. It was also well tolerated with few adverse effects.100 Interaction with warfarin, calcium channel blockers, antiplatelet agents, thrombolytic agents, imatinib, and other agents may occur; therefore, ginseng must be used with careful monitoring in selected patients. There is not enough evidence at this time to support the routine use of ginseng in CRF.
The seed extract of the guarana plant (Paullinia cupana) traditionally has been used as a stimulant. An improvement in fatigue scores was seen with the use of oral guarana (100 mg daily) at the end of 21 days in breast cancer patients receiving chemotherapy.101 Further studies are needed for these results to be generalized and to understand the adverse effects and interaction profile of guarana.
Reevaluation
Patients who have completed cancer treatment must be monitored for fatigue over the long term, as fatigue may exist beyond the period of active treatment. Many studies have shown fatigue in breast cancer survivors, and fatigue has been demonstrated in survivors of colorectal, lung, and prostate cancers as well as myeloproliferative neoplasms.28 Therefore, it is important to screen patients for fatigue during follow-up visits. There are currently no studies evaluating the long-term treatment of fatigue. In our experience, the timing of follow-up depends on the level of fatigue and interventions prescribed. Once fatigue is stabilized to a level with which the patient is able to cope, the time interval for follow-up may be lengthened. Annual visits may suffice in patients with mild fatigue. Follow-up of patients with moderate to severe fatigue depends on the level of fatigue, the ability to cope, choice of treatment, and presence of contributing factors.
CONCLUSION
CRF is a complex condition that places a significant burden on patients and caregivers, resulting in emotional distress, poor functioning, and suffering. Fatigue can occur before, during, and long after cancer treatment. The approach to CRF begins with screening for and educating patients and their caregivers about the symptoms. Many screening scales are available that may be used to follow patients’ progress over time. The evaluation and management of contributing conditions may help improve fatigue. If the fatigue persists, an individualized approach with a combination of nonpharmacologic and pharmacologic approaches should be considered. More research is needed to understand brain signaling pathways, cytokine changes, and genomic changes in cancer patients with fatigue. Though many hypotheses have been proposed, to date there is no biological marker to assess this condition. Biomarker research needs to be advanced to help to identify patients at risk for fatigue. As cytokines have a major role in CRF, targeted therapy to block cytokine pathways may also be explored in the future.
Acknowledgment: The authors thank Bryan Tutt for providing editorial assistance during the writing of this article.
1. Scherber RM, Kosiorek HE, Senyak Z, et al. Comprehensively understanding fatigue in patients with myeloproliferative neoplasms. Cancer 2016;122:477–85.
2. Neefjes EC, van der Vorst MJ, Blauwhoff-Buskermolen S, Verheul HM. Aiming for a better understanding and management of cancer-related fatigue. Oncologist 2013;18:1135–43.
3. Radbruch L, Strasser F, Elsner F, et al. Fatigue in palliative care patients—an EAPC approach. Palliat Med 2008;22:13–32.
4. Capuron L, Pagnoni G, Demetrashvili MF, et al. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology 2007;32:2384–92.
5. Kisiel-Sajewicz K, Siemionow V, Seyidova-Khoshknabi D, et al. Myoelectrical manifestation of fatigue less prominent in patients with cancer related fatigue. PLoS One 2013;8:e83636.
6. Bower JE, Ganz PA, Aziz N. Altered cortisol response to psychologic stress in breast cancer survivors with persistent fatigue. Psychosom Med 2005;67:277–80.
7. Barsevick A, Frost M, Zwinderman A, et al. I’m so tired: biological and genetic mechanisms of cancer-related fatigue. Qual Life Res 2010;19:1419–27.
8. Morrow GR, Hickok JT, Roscoe JA, et al. Differential effects of paroxetine on fatigue and depression: a randomized, double-blind trial from the University of Rochester Cancer Center Community Clinical Oncology Program. J Clin Oncol 2003;21:4635–41.
9. Fontes-Oliveira CC, Busquets S, Toledo M, et al. Mitochondrial and sarcoplasmic reticulum abnormalities in cancer cachexia: altered energetic efficiency? Biochim Biophys Acta 2013;1830:2770–8.
10. Agteresch HJ, Dagnelie PC, van der Gaast A, et al. Randomized clinical trial of adenosine 5’-triphosphate in patients with advanced non-small-cell lung cancer. J Natl Cancer Inst 2000;92:321–8.
11. Beijer S, Hupperets PS, van den Borne BE, et al. Randomized clinical trial on the effects of adenosine 5’-triphosphate infusions on quality of life, functional status, and fatigue in preterminal cancer patients. J Pain Symptom Manage 2010;40:520–30.
12. Kisiel-Sajewicz K, Davis MP, Siemionow V, et al. Lack of muscle contractile property changes at the time of perceived physical exhaustion suggests central mechanisms contributing to early motor task failure in patients with cancer-related fatigue. J Pain Symptom Manage 2012;44:351–61.
13. Ryan JL, Carroll JK, Ryan EP, et al. Mechanisms of cancer-related fatigue. Oncologist 2007;12 Suppl 1:22–34.
14. Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer 2008;8:887–99.
15. Wang XS, Giralt SA, Mendoza TR, et al. Clinical factors associated with cancer-related fatigue in patients being treated for leukemia and non-Hodgkin’s lymphoma. J Clin Oncol 2002;20:1319–28.
16. Collado-Hidalgo A, Bower JE, Ganz PA, et al. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res 2006;12:2759–66.
17. Wang XS, Shi Q, Williams LA, et al. Serum interleukin-6 predicts the development of multiple symptoms at nadir of allogeneic hematopoietic stem cell transplantation. Cancer 2008;113:2102–9.
18. Inagaki M, Isono M, Okuyama T, et al. Plasma interleukin-6 and fatigue in terminally ill cancer patients. J Pain Symptom Manage 2008;35:153–61.
19. Laird BJ, McMillan DC, Fayers P, et al. The systemic inflammatory response and its relationship to pain and other symptoms in advanced cancer. Oncologist 2013;18:1050–5.
20. Bower JE, Ganz PA, Irwin MR, et al. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol 2011;29:3517–22.
21. Whistler T, Taylor R, Craddock RC, et al. Gene expression correlates of unexplained fatigue. Pharmacogenomics 2006;7:395–405.
22. Fagundes CP, Bennett JM, Alfano CM, et al. Social support and socioeconomic status interact to predict Epstein-Barr virus latency in women awaiting diagnosis or newly diagnosed with breast cancer. Health Psychol 2012;31:11–19.
23. Landmark-Hoyvik H, Reinertsen KV, Loge JH, et al. Alterations of gene expression in blood cells associated with chronic fatigue in breast cancer survivors. Pharmacogenomics J 2009;9:333–40.
24. Smith AK, Conneely KN, Pace TW, et al. Epigenetic changes associated with inflammation in breast cancer patients treated with chemotherapy. Brain Behav Immun 2014;38:227–36.
25. Kim S, Miller BJ, Stefanek ME, Miller AH. Inflammation-induced activation of the indoleamine 2,3-dioxygenase pathway: Relevance to cancer-related fatigue. Cancer 2015;121:2129–36.
26. Mills PJ, Parker B, Dimsdale JE, et al. The relationship between fatigue and quality of life and inflammation during anthracycline-based chemotherapy in breast cancer. Biol Psychol 2005;69:85–96.
27. Jean-Pierre P, Figueroa-Moseley CD, Kohli S, et al. Assessment of cancer-related fatigue: implications for clinical diagnosis and treatment. Oncologist 2007;12 Suppl 1:11–21.
28. Wang XS, Zhao F, Fisch MJ, et al. Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer 2014;120:425–32.
29. Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer 1999;85:1186–96.
30. Mendoza ME, Capafons A, Gralow JR, et al. Randomized controlled trial of the Valencia model of waking hypnosis plus CBT for pain, fatigue, and sleep management in patients with cancer and cancer survivors. Psychooncology 2016 Jul 28.
31. Glaus A. Assessment of fatigue in cancer and non-cancer patients and in healthy individuals. Support Care Cancer 1993;1:305–15.
32. Seyidova-Khoshknabi D, Davis MP, Walsh D. A systematic review of cancer-related fatigue measurement questionnaires. Am J Hosp Palliat Care 2011;28:119–29.
33. Holzner B, Kemmler G, Greil R, et al. The impact of hemoglobin levels on fatigue and quality of life in cancer patients. Ann Oncol 2002;13:965–73.
34. Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage 2004;27:14–23.
35. Hwang SS, Chang VT, Rue M, Kasimis B. Multidimensional independent predictors of cancer-related fatigue. J Pain Symptom Manage 2003;26:604–14.
36. Peterson DR. Scope and generality of verbally defined personality factors. Psychol Rev 1965;72:48–59.
37. Berger AM, Abernethy AP, Atkinson A, et al. NCCN Clinical Practice Guidelines Cancer-related fatigue. J Natl Compr Canc Netw 2010;8:904–31.
38. Crawford J, Cella D, Cleeland CS, et al. Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic cancer patients receiving epoetin alfa therapy. Cancer 2002;95:888–95.
39. Tonia T, Mettler A, Robert N, et al. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev 2012;12:CD003407.
40. Rizzo JD, Brouwers M, Hurley P, et al. American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. Blood 2010;116:4045–59.
41. Preston NJ, Hurlow A, Brine J, Bennett MI. Blood transfusions for anaemia in patients with advanced cancer. Cochrane Database Syst Rev 2012(2):CD009007.
42. Minton O, Stone PC. A comparison of cognitive function, sleep and activity levels in disease-free breast cancer patients with or without cancer-related fatigue syndrome. BMJ Support Palliat Care 2012;2:231–8.
43. Heckler CE, Garland SN, Peoples AR, et al. Cognitive behavioral therapy for insomnia, but not armodafinil, improves fatigue in cancer survivors with insomnia: a randomized placebo-controlled trial. Support Care Cancer 2016;24:2059–66.
44. Howell D, Oliver TK, Keller-Olaman S, et al. Sleep disturbance in adults with cancer: a systematic review of evidence for best practices in assessment and management for clinical practice. Ann Oncol 2014;25:791–800.
45. Wilt TJ, MacDonald R, Brasure M, et al. Pharmacologic treatment of insomnia disorder: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med 2016;165:103–12.
46. Kuriyama A, Honda M, Hayashino Y. Ramelteon for the treatment of insomnia in adults: a systematic review and meta-analysis. Sleep Med 2014;15:385–92.
47. Strasser F, Palmer JL, Schover LR, et al. The impact of hypogonadism and autonomic dysfunction on fatigue, emotional function, and sexual desire in male patients with advanced cancer: a pilot study. Cancer 2006;107:2949–57.
48. Agasi-Idenburg SC, Thong MS, Punt CJ, et al. Comparison of symptom clusters associated with fatigue in older and younger survivors of colorectal cancer. Support Care Cancer 2017;25:625–32.
49. Miaskowski C, Aouizerat BE. Is there a biological basis for the clustering of symptoms? Semin Oncol Nurs 2007;23:99–105.
50. de Raaf PJ, de Klerk C, Timman R, et al. Systematic monitoring and treatment of physical symptoms to alleviate fatigue in patients with advanced cancer: a randomized controlled trial. J Clin Oncol 2013;31:716–23.
51. Barsevick AM, Whitmer K, Sweeney C, Nail LM. A pilot study examining energy conservation for cancer treatment-related fatigue. Cancer Nurs 2002;25:333–41.
52. Barsevick AM, Dudley W, Beck S, et a;. A randomized clinical trial of energy conservation for patients with cancer-related fatigue. Cancer 2004;100:1302–10.
53. Luciani A, Jacobsen PB, Extermann M, et al. Fatigue and functional dependence in older cancer patients. Am J Clin Oncol 2008;31:424–30.
54. Abrahams HJ, Gielissen MF, Goedendorp MM, et al. A randomized controlled trial of web-based cognitive behavioral therapy for severely fatigued breast cancer survivors (CHANGE-study): study protocol. BMC Cancer 2015;15:765.
55. Quesnel C, Savard J, Simard S, et al. Efficacy of cognitive-behavioral therapy for insomnia in women treated for nonmetastatic breast cancer. J Consult Clin Psychol 2003;71:189–200.
56. Goedendorp MM, Gielissen MF, Verhagen CA, Bleijenberg G. Psychosocial interventions for reducing fatigue during cancer treatment in adults. Cochrane Database Syst Rev 2009(1):CD006953.
57. Greiwe JS, Cheng B, Rubin DC, et al. Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. FASEB J 2001;15:475–82.
58. Furmaniak AC, Menig M, Markes MH. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev 2016;(9):CD005001.
59. Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev 2012;(11):CD006145.
60. Brown JC, Huedo-Medina TB, Pescatello LS, et al. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2011;20:123–33.
61. Steindorf K, Schmidt ME, Klassen O, et al. Randomized, controlled trial of resistance training in breast cancer patients receiving adjuvant radiotherapy: results on cancer-related fatigue and quality of life. Ann Oncol 2014;25:2237–43.
62. Bower JE, Bak K, Berger A, et al. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical oncology clinical practice guideline adaptation. J Clin Oncol 2014;32:1840–50.
63. Kenjale AA, Hornsby WE, Crowgey T, et al. Pre-exercise participation cardiovascular screening in a heterogeneous cohort of adult cancer patients. Oncologist 2014;19:999–1005.
64. Oldervoll LM, Loge JH, Paltiel H, et al. The effect of a physical exercise program in palliative care: A phase II study. J Pain Symptom Manage 2006;31:421–30.
65. Porock D, Kristjanson LJ, Tinnelly K, et al. An exercise intervention for advanced cancer patients experiencing fatigue: a pilot study. J Palliat Care 2000;16:30–6.
66. Lengacher CA, Kip KE, Reich RR, et al. A cost-effective mindfulness stress reduction program: a randomized control trial for breast cancer survivors. Nursing Econ 2015;33:210–8, 32.
67. Lengacher CA, Reich RR, Post-White J, et al. Mindfulness based stress reduction in post-treatment breast cancer patients: an examination of symptoms and symptom clusters. J Behav Med 2012;35:86–94.
68. Sprod LK, Fernandez ID, Janelsins MC, et al. Effects of yoga on cancer-related fatigue and global side-effect burden in older cancer survivors. J Geriatr Oncol 2015;6:8–14.
69. Wang G, Wang S, Jiang P, Zeng C. [Effect of Yoga on cancer related fatigue in breast cancer patients with chemotherapy]. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2014;39:1077–82.
70. Stan DL, Croghan KA, Croghan IT, et al. Randomized pilot trial of yoga versus strengthening exercises in breast cancer survivors with cancer-related fatigue. Support Care Cancer 2016;24:4005–15.
71. Larkey LK, Roe DJ, Weihs KL, et al. Randomized controlled trial of Qigong/Tai Chi Easy on cancer-related fatigue in breast cancer survivors. Ann Behav Med 2015;49:165–76.
72. Molassiotis A, Bardy J, Finnegan-John J, et al. Acupuncture for cancer-related fatigue in patients with breast cancer: a pragmatic randomized controlled trial. J Clin Oncol 2012;30:4470–6.
73. Deng G, Chan Y, Sjoberg D, et al. Acupuncture for the treatment of post-chemotherapy chronic fatigue: a randomized, blinded, sham-controlled trial. Support Care Cancer 2013;21:1735–41.
74. Finnegan-John J, Molassiotis A, Richardson A, Ream E. A systematic review of complementary and alternative medicine interventions for the management of cancer-related fatigue. Integr Cancer Ther 2013;12:276–90.
75. Schwartz AL, Thompson JA, Masood N. Interferon-induced fatigue in patients with melanoma: a pilot study of exercise and methylphenidate. Oncol Nurs Forum 2002;29:E85–90.
76. Spathis A, Dhillan R, Booden D, et al. Modafinil for the treatment of fatigue in lung cancer: a pilot study. Palliat Med 2009;23:325–31.
77. Blackhall L, Petroni G, Shu J, et al. A pilot study evaluating the safety and efficacy of modafinal for cancer-related fatigue. J Palliat Med 2009;12:433–9.
78. Qu D, Zhang Z, Yu X, et al. Psychotropic drugs for the management of cancer-related fatigue: a systematic review and meta-analysis. Eur J Cancer Care (Engl) 2015;25:970–9.
79. Minton O, Richardson A, Sharpe M, et al. Drug therapy for the management of cancer-related fatigue. Cochrane Database Syst Rev 2010(7):CD006704.
80. Moraska AR, Sood A, Dakhil SR, et al. Phase III, randomized, double-blind, placebo-controlled study of long-acting methylphenidate for cancer-related fatigue: North Central Cancer Treatment Group NCCTG-N05C7 trial. J Clin Oncol 2010;28:3673–9.
81. Bruera E, Driver L, Barnes EA, et al. Patient-controlled methylphenidate for the management of fatigue in patients with advanced cancer: a preliminary report. J Clin Oncol 2003;21:4439–43.
82. Kerr CW, Drake J, Milch RA, et al. Effects of methylphenidate on fatigue and depression: a randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage 2012;43:68–77.
83. Escalante CP, Meyers C, Reuben JM, et al. A randomized, double-blind, 2-period, placebo-controlled crossover trial of a sustained-release methylphenidate in the treatment of fatigue in cancer patients. Cancer J 2014;20:8–14.
84. Mar Fan HG, Clemons M, Xu W, et al. A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support Care Cancer 2008;16:577–83.
85. Butler JM Jr, Case LD, Atkins J, et al. A phase III, double-blind, placebo-controlled prospective randomized clinical trial of d-threo-methylphenidate HCl in brain tumor patients receiving radiation therapy. Int J Radiat Oncol Biol Phys 2007;69:1496–501.
86. Hovey E, de Souza P, Marx G, et al. Phase III, randomized, double-blind, placebo-controlled study of modafinil for fatigue in patients treated with docetaxel-based chemotherapy. Support Care Cancer 2014;22:1233–42.
87. Spathis A, Fife K, Blackhall F, et al. Modafinil for the treatment of fatigue in lung cancer: results of a placebo-controlled, double-blind, randomized trial. J Clin Oncol 2014;32:1882–8.
88. Berenson JR, Yellin O, Shamasunder HK, et al. A phase 3 trial of armodafinil for the treatment of cancer-related fatigue for patients with multiple myeloma. Support Care Cancer 2015; 23:1503–12.
89. Boele FW, Douw L, de Groot M, et al. The effect of modafinil on fatigue, cognitive functioning, and mood in primary brain tumor patients: a multicenter randomized controlled trial. Neuro Oncol 2013;15:1420–8.
90. Jean-Pierre P, Morrow GR, Roscoe JA, et al. A phase 3 randomized, placebo-controlled, double-blind, clinical trial of the effect of modafinil on cancer-related fatigue among 631 patients receiving chemotherapy: a University of Rochester Cancer Center Community Clinical Oncology Program Research base study. Cancer 2010;116:3513–20.
91. Conley CC, Kamen CS, Heckler CE, et al. Modafinil moderates the relationship between cancer-related fatigue and depression in 541 patients receiving chemotherapy. J Clin Psychopharmacol 2016;36:82–5.
92. Brattsand R, Linden M. Cytokine modulation by glucocorticoids: mechanisms and actions in cellular studies. Aliment Pharmacol Ther 1996;10 Suppl 2:81–90.
93. Yennurajalingam S, Frisbee-Hume S, Palmer JL, et al. Reduction of cancer-related fatigue with dexamethasone: a double-blind, randomized, placebo-controlled trial in patients with advanced cancer. J Clin Oncol 2013;31:3076–82.
94. Bruera E, Roca E, Cedaro L, et al. Action of oral methylprednisolone in terminal cancer patients: a prospective randomized double-blind study. Cancer Treat Rep 1985;69:751–4.
95. Pulivarthi K, Dev R, Garcia J, et al. Testosterone replacement for fatigue in male hypogonadic patients with advanced cancer: A preliminary double-blind placebo-controlled trial. J Clin Oncol 2012;30 (suppl). Abstract e19643.
96. Palesh OG, Mustian KM, Peppone LJ, et al. Impact of paroxetine on sleep problems in 426 cancer patients receiving chemotherapy: a trial from the University of Rochester Cancer Center Community Clinical Oncology Program. Sleep Med 2012;13:1184–90.
97. Thekdi SM, Trinidad A, Roth A. Psychopharmacology in Cancer. Curr Psychiatry Rep 2014;17:529.
98. Lesser GJ. Case D, Stark N, et al. A randomized, double-blind, placebo-controlled study of oral coenzyme Q10 to relieve self-reported treatment-related fatigue in newly diagnosed patients with breast cancer. J Support Oncol 2013;11:31–42.
99. Barton DL, Liu H, Dakhil SR, et al. Wisconsin Ginseng (Panax quinquefolius) to improve cancer-related fatigue: a randomized, double-blind trial, N07C2. J Natl Cancer Inst 2013;105:1230–8.
100. Yennurajalingam S, Reddy A, Tannir NM, et al. High-dose Asian ginseng (panax ginseng) for cancer-related fatigue: a preliminary report. Integr Cancer Ther 2015;14:419–27.
101. Howell D, Keller-Olaman S, Oliver TK, et al. A pan-Canadian practice guideline and algorithm: screening, assessment, and supportive care of adults with cancer-related fatigue. Curr Oncol 2013;20:e233–46.
INTRODUCTION
Fatigue is a common distressing effect of cancer.1 It impairs the quality of life of patients undergoing active cancer treatment and of post-treatment survivors alike. The National Comprehensive Cancer Network (NCCN) defines cancer-related fatigue (CRF) as “a distressing, persistent, subjective sense of physical, emotional and/or cognitive tiredness related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning.”2 CRF differs from fatigue reported by individuals without cancer in that CRF is more severe and is not relieved by rest. The prevalence of CRF in cancer patients and survivors is highly variable, with estimates ranging between 25% and 99%.2,3 The methods used for screening patients for fatigue and the characteristics of the patient groups may account for this variability.
PATHOPHYSIOLOGY
The specific pathophysiologic mechanism underlying CRF is unknown, making targeted treatment a challenge. The multidimensional and subjective nature of CRF has limited the development of research methodologies to explain this condition. However, research has been done in both human and animal models, and several theories have been proposed to explain the pathophysiology of CRF. While pro-inflammatory cytokines remain the central factor playing a significant role at multiple levels in CRF, there may be a complex interplay of multiple mechanisms contributing to fatigue in an individual patient.
CENTRAL NERVOUS SYSTEM DISTURBANCES
The basal ganglia are known to influence motivation. Lack of motivation and drive may cause failure to complete physical and mental tasks, even with preserved cognitive ability and motor function. In a study of melanoma patients receiving interferon, increased activity of the basal ganglia and the cerebellum resulted in higher fatigue scores.4 Increased levels of cytokines may alter blood flow to the cerebellum and lead to the perception of fatigue. In a study of 12 patients and matched controls, when patients were asked to perform sustained elbow flexion until they perceived exhaustion, CRF patients perceived physical exhaustion sooner than controls. In CRF patients in this study, muscle fatigue measured by electromyogram was less than that in healthy individuals at the time of exhaustion, suggesting the role of the central nervous system in CRF.5 However, there is not enough evidence at this time to support central nervous system disturbance as the main factor contributing to fatigue in cancer patients.
CIRCADIAN RHYTHM DYSREGULATION
Circadian rhythm is regulated by the suprachiasmatic nucleus in the hypothalamus through cortisol and melatonin. Sleep disturbances occur with disruption of the circadian rhythm. Tumor-related peptides such as epidermal growth factor or alterations in serotonin and cortisol can influence the suprachiasmatic nucleus and the complex signaling pathways.2 Positive feedback loops that are activated by cortisol under the influence of cytokines may lead to continuous cytokine production and altered circadian rhythm. Bower et al showed that changes in the cortisol curve influence fatigue in breast cancer survivors.6 These patients had a late evening peak in cortisol levels, compared with an early morning peak in individuals without cancer.
INHIBITION OF HYPOTHALAMIC-PITUITARY-ADRENAL AXIS
The hypothalamic–pituitary–adrenal (HPA) axis regulates the release of the stress hormone cortisol. One of several hypotheses advanced to explain the effect of serotonin and the HPA axis on CRF suggests that lower serotonin levels cause decreased activation of 5-hydroxytrytophan 1-a (5-HT1-a) receptors in the hypothalamus, leading to decreased activity of the HPA axis.6 Inhibition of the HPA axis may occur with higher levels of serotonin as well.7 The 5-HT1-a receptors are also triggered by cytokines. However, the correction of serotonin levels by antidepressants was not shown to improve fatigue.8 Inhibition of the HPA axis can also lead to lower testosterone, progesterone, or estrogen levels, which may indirectly contribute to fatigue.2
SKELETAL MUSCLE EFFECT
Chemotherapy- and tumor-related cachexia have a direct effect on the metabolism of skeletal muscles. This effect may lead to impaired adenosine triphosphate (ATP) generation during muscle contraction.9 ATP infusion improved muscle strength in 1 trial, but this was not confirmed in another trial.10,11 Muscle contraction studies showed no differences in the contractile properties of muscles in fatigued patients who failed earlier in motor tasks and healthy controls.12 This finding suggests that there could be a failure of skeletal muscle activation by the central nervous system or inhibition of skeletal muscle activity. Cytokines and other neurotransmitters activate vagal efferent nerve fibers, which may lead to reflex inhibition in skeletal muscles.13,14
PRO-INFLAMMATORY CYTOKINES
Tumors or treatment of them may cause tissue injury, which triggers immune cells to release cytokines, signaling the brain to manifest the symptom of fatigue. Inflammatory pathways are influenced by psychological, behavioral, and biological factors, which play a role as risk factors in CRF. Levels of interleukin 6 (IL-6), interleukin-1 receptor antagonist, interleukin-1, and tumor necrosis factor (TNF) have been shown to be elevated in fatigued patients being treated for leukemia and non-Hodgkin lymphoma.15 IL-6 was also associated with increased fatigue in breast cancer survivors.16 Similar findings were reported in patients undergoing stem cell transplantation and high-dose chemotherapy.17 Elevated levels of IL-6 and C-reactive protein were also linked to fatigue in terminally ill cancer patients.18,19 Furthermore, TNF-α signaling was associated with post-chemotherapy fatigue in breast cancer patients.20 Leukocytes in breast cancer survivors with fatigue also have increased gene expression of pro-inflammatory cytokines, emphasizing the role of cytokines and inflammation in the pathogenesis of CRF.21
OTHER HYPOTHESES
Several other hypotheses for CRF pathogenesis have been proposed. Activation of latent viruses such as Epstein-Barr virus, lack of social support,22 genetic alterations in the immune pathway,23 epigenetic changes,24 accumulation of neurotoxic metabolites and depletion of serotonin by indoleamine 2,3-dioxygenase pathway activation,25 elevated vascular endothelial growth factor levels,26 and hypoxia-related organ dysfunction due to anemia or hemoglobin dysfunction13 all have been postulated to cause CRF.
EVALUATION AND TREATMENT
Fours steps are involved in the evaluation and treatment of CRF (Figure).

SCREENING
Because patients and health care professionals may be unaware of the treatment options available for CRF, patients may not report fatigue levels to their clinicians, and clinicians may not understand the impact of fatigue on their patients’ quality of life. This leads to under-recognition of the problem. The NCCN recommends screening every cancer patient and post-treatment survivor for fatigue.2 Patients should be screened at their first visit and then at periodic intervals during and after cancer treatment.
Many scales are available to screen patients for CRF in clinical practice and clinical trials.27 A single item that asks patients to rate their fatigue on a scale from 0 to 10—in which 0 indicates no fatigue, 1 to 3 indicates mild fatigue, 4 to 6 indicates moderate fatigue, 7 to 9 indicates severe fatigue, and 10 indicates the worst fatigue imaginable—is commonly used to screen for CRF.2 This scale was adapted from the MD Anderson Symptom Inventory scale and is based on a large nationwide study of cancer patients and survivors.28 The statistically derived cutoff points in this study are consistent with other scales such as the Brief Fatigue Inventory (BFI) and support the cutoff points (4–6 for moderate and ≥ 7 for severe fatigue) used in various fatigue management guidelines. Furthermore, studies of fatigue in cancer patients have revealed a marked decrease in physical function at levels of 7 or higher, suggesting 7 as an optimal cutoff to identify severe fatigue.29,30 The Visual Analog Scale is another simple-to-use tool that helps in understanding variations in fatigue throughout the course of the day.31 The 9-item BFI is often used in clinical trials.29 It measures the severity of fatigue over the previous 24 hours and has been validated in patients who do not speak English.32
CRF affects not only the somatic domain, but also the cognitive, behavioral, and affective domains; therefore, multidimensional scales have been developed for screening. One such tool is the Multidimensional Fatigue Inventory, which assesses 5 dimensions of fatigue—general fatigue, physical fatigue, reduced motivation, reduced activity, and mental fatigue—and compares the patient’s results with those of individuals without cancer.33,34 The Functional Assessment of Cancer Therapy for Fatigue (FACT-F) is a 13-item questionnaire that has been used to measure CRF in clinical trials as well as in patients receiving various treatments.35
Although many scales are available, the validity of self-reporting on simple fatigue-rating scales is equal to or better than most complex, lengthy scales.36 Therefore, unidimensional tools such as the numeric rating scale of 0–10 are commonly used in clinical practice. Mild fatigue (0–3) requires periodic reevaluation, and moderate and severe fatigue need further evaluation and management.37
PRIMARY EVALUATION
This phase involves a focused history and physical examination and assessment of concurrent symptoms and contributing factors.
History and Physical Examination
A detailed history of the patient’s malignancy and type of previous and current treatment may help reveal the cause of fatigue. New-onset fatigue or increase in fatigue may be related to the progression of disease in patients with active malignancy or recurrence of cancer in survivors. These patients may require appropriate testing to assess the underlying disease pattern. A detailed review of systems may help identify some of the contributing factors, which are discussed below. A detailed history regarding medications, including over-the-counter drugs, complementary agents, and past and prior cancer therapies, is helpful as medications can contribute to fatigue. For example, opioids may cause drowsiness and fatigue, which could be improved by dose adjustments. A focused history of fatigue should be obtained in all patients with moderate to severe CRF, which includes the onset, pattern, duration, associated or alleviating factors, and interference with functioning, including activities of daily living.37 Physical examination should focus on identifying signs of organ dysfunction and features of substance or alcohol abuse, which may cause poor sleep and fatigue.
Assessment of Contributing Factors
The management of fatigue should be multifactorial, with a comprehensive assessment and treatment plan to address all modifiable fatigue etiologies. The Table lists potential contributing factors to fatigue that should be considered when evaluating patients for CRF; several common conditions are discussed below. 
Anemia. Anemia has been correlated with fatigue and quality of life. In a study of 4382 cancer patients receiving chemotherapy, quality-of-life measures using FACT-Anemia scores improved with increased hemoglobin levels.38 Cancer patients may have anemia due to marrow-suppressing effects of chemotherapy and may also have iron deficiency anemia due to blood loss or auto-immune hemolytic anemia. Therefore, a detailed work-up is required to identify the etiology of anemia. Patients with CRF whose anemia is related to chemotherapy or anemia of chronic disease may benefit from red blood cell transfusion or erythropoiesis-stimulating agents (ESAs). ESAs have been studied extensively; however, their use is controversial because of concerns about thromboembolic side effects leading to adverse outcomes.39 Also, ESA therapy is not recommended in patients with hematologic malignancies. ESA use should be restricted to patients with chemotherapy-related anemia with hemoglobin below 10 mg/dL and should be discontinued in 6 to 8 weeks if patients do not respond.40 Other patients may benefit from blood transfusions, which were shown to help in patients with hemoglobin levels between 7.5 and 8.5 g/dL.41
Sleep disturbance. Poor sleep is common in fatigued cancer survivors.42 Pro-inflammatory cytokines can disrupt the sleep–wake cycle, causing changes in the HPA axis and neuroendocrine system, which in turn may lead to increasing fatigue. Heckler et al showed that improvement in nighttime sleep leads to improvement of fatigue.43 Cognitive behavioral therapy and sleep hygiene are important in caring for patients with CRF and poor sleep.44 Taking a warm bath and/or drinking a glass of milk before bedtime, avoiding caffeinated drinks, and avoiding frequent napping in the day might help. Some patients may require medications such as benzodiazepines or non-benzodiazepine hypnotics (eg, zolpidem) to promote sleep.45 Melatonin agonists are approved for insomnia in the United States, but not in Europe.46
Malnutrition. Patients with advanced-stage cancer and with cancers affecting the gastrointestinal tract frequently develop mechanical bowel obstructions, especially at the end of their life, which cause malnutrition. Chemotherapy-related nausea and vomiting may also cause poor oral intake and malnutrition, causing fatigue from muscle weakness. Dehydration and electrolyte imbalances frequently occur as a result of poor oral intake, which might worsen fatigue. In our experience, modifying dietary intake with appropriate caloric exchanges with the help of a nutrition expert has lessened fatigue in some patients. However, terminally ill patients are advised to eat based on their comfort.
Medical comorbidities. Congestive heart failure from anthracycline chemotherapy, hypothyroidism after radiation therapy for head and neck cancers, renal failure, or hepatic failure from chemotherapy may indirectly lead to fatigue. Chemotherapy, opioids, and steroids may cause hypogonadism, which can contribute to fatigue in men.47
Assessment of Concurrent Symptoms
Depression is common in cancer patients and coexists with pain, insomnia, fatigue, and anxiety as a symptom cluster.48 A symptom cluster is defined as 2 or more concurrent and interrelated symptoms occurring together; treating one of these symptoms without addressing other symptoms is not effective.49 Therefore, screening for and management of depression, anxiety, and insomnia play an important role in the management of CRF.
Physical symptoms due to the tumor or to therapy— such as pain, dyspnea, nausea, and decreased physical activity—may also contribute to fatigue both directly and indirectly. Patients with lung cancer may have hypoxemia, which can contribute to dyspnea with activity and a perception of fatigue. Optimal management of pain and other physical symptoms in patients with cancer may significantly alleviate fatigue.50
MANAGEMENT
Management of CRF is individualized based on the patient’s clinical status: active cancer treatment, survivor, or end of life. Education and counselling of patients and their caregivers play an important role in CRF. NCCN guidelines recommend focusing on pain control, distress management, energy conservation, physical activity, nutrition, and sleep hygiene.
Nonpharmacologic Interventions
Energy conservation. Energy conservation strategies, in which patients are advised to set priorities and realistic expectations, are highly recommended. Some energy-conserving strategies are to pace oneself, delegate and schedule activities at times of peak energy, postpone nonessential activities, attend to 1 activity at a time, structure daily routines, and maintain a diary to identify their peak energy period and structure activities around that time.51,52 When patients approach the end of life, increasing fatigue may limit their activity level, and they may depend on caregivers for assistance with activities of daily living, monitoring treatment-related adverse effects, and taking medications, especially elderly patients.53
Cognitive behavioral therapy. Cognitive behavioral therapy (CBT) has been shown to improve CRF during active treatment, and the benefits persist for a minimum of 2 years after therapy.54 CBT interventions that optimize sleep quality may improve fatigue.55 More studies are needed to understand whether referral to a psychologist for formal CBT is required. Randomized clinical trials showed patient fatigue education, learned self-care, coping techniques, and balancing rest and activity benefit patients with CRF.56
Exercise. Physical activity is highly encouraged in patients with CRF. Exercise increases muscle protein synthesis, improves cytokine response, and decreases the rate of sarcopenia in healthy populations.57 Studies have shown that exercise helps CRF at all phases of the cancer journey, including radiation therapy, chemotherapy, and survivorship.58 Some patients may feel less motivated to exercise and may not believe that exercise is possible or could potentially help them. Counselling is needed for such patients.
Older cancer survivors have a decline in their functional capacity and reduced muscle mass. Exercise can improve their cardiorespiratory fitness, muscle strength, and body composition.57 Exercise not only helps at the cellular level but also has psychosocial benefits from improved self-esteem. Therefore, exercise may be recommended for younger patients as well as for the older population, who may have comorbidities and less motivation than younger patients.
In a meta-analysis of 56 randomized controlled trials involving 4068 participants, aerobic exercise was found to have beneficial effects on CRF for patients during and after chemotherapy, specifically for patients with solid tumors.59 In another meta-analysis of breast and prostate cancer survivors, a combination of aerobic exercise with resistance training (3–6 metabolic equivalents, 60%–80% range of motion) was shown to reduce CRF more than aerobic exercise alone.60 This effect was also shown in a randomized controlled trial of 160 patients with stage 0 to III breast cancer undergoing radiation therapy.61 The control group in this study had a group-based non-exercise intervention/relaxation; therefore, the study showed that the effect of resistance training extends beyond the psychosocial benefits of group-based interventions. The intervention comprised 8 progressive machine-based resistance exercises (3 sets, 8–12 repetitions at 60%–80% of 1 repetition maximum) for 60 minutes twice weekly for 12 weeks. However, fatigue assessment questionnaire scores showed benefits only in the physical fatigue components, but not in the affective and cognitive components.
The American Society of Clinical Oncology’s guidelines for cancer survivors with fatigue recommends 150 minutes of moderate aerobic exercise (eg, fast walking, cycling, or swimming) per week, with 2 or 3 sessions of strength training per week.62 An individualized approach to exercise is recommended, as patients’ ability to perform certain types of exercises may be limited by thrombocytopenia, neutropenia, or lytic bone metastasis. Routine use of pre-exercise cardiovascular testing is not recommended but may be considered in high-risk populations, especially patients with risk factors for coronary heart disease and diabetes.63 Patients with comorbidities, substantial deconditioning, functional and anatomic defects, or recent major surgery may benefit from referral to physical therapy.37 Patients near end of life may also benefit from an exercise program, as demonstrated in several studies that showed benefit in CRF and quality of life.64,65 We recommend that physicians use their best clinical judgement in suggesting the type and intensity of exercise program, as it may not be feasible in some patients.
Mind-body interventions. Mindfulness-based stress reduction (MBSR) has shown promise in breast cancer survivors, who reported immediate improvements in fatigue severity that continued up to 6 weeks after cessation of the training.66 Prior studies had similar findings, suggesting that MBSR modestly decreases fatigue and sleep disturbances and has a greater effect on the degree to which symptoms interfere with many facets of life.67
Yoga. A study of a yoga intervention showed a benefit in older cancer survivors.68 In breast cancer patients undergoing chemotherapy, yoga was shown to benefit both physical and cognitive fatigue.69 DVD-based yoga had benefits similar to strengthening exercises in a study of 34 early-stage breast cancer survivors with CRF.70 More studies are needed in men and patients and survivors of other cancers, as most studies of yoga were conducted in women with breast cancer.
Tai chi/qigong. Like yoga, tai chi and qigong are practices of meditative movement. These practices use postures or movements with a focus on breath and a meditative state to bring about deep states of relaxation. Qigong is a series of simple, repeated practices including body posture/movement, breath practice, and meditation performed in synchrony. Tai chi easy (TCE) is a simplified set of common, repetitive tai chi movements. In a trial, qigong/TCE was compared with sham qigong, which had physical movements but no breathing or meditative practice. Breast cancer survivors in the qigong/TCE group had improved fatigue scores, and the effect persisted for 3 months.71 Additional research is needed in this area.
Acupuncture. A randomized controlled trial in breast cancer patients with CRF showed an improvement in the mean general fatigue score (per the Multidimensional Fatigue Inventory) in patients who received acupuncture versus those who did not (−3.11 [95% confidence interval −3.97 to −2.25]; P < 0.001) at 6 weeks. Improvements were seen in both the mental and physical aspects of fatigue.72 However, Deng et al noted that true acupuncture was no more effective than sham acupuncture for reducing post-chemotherapy chronic fatigue.73 Presently, there is not sufficient evidence to evaluate the benefits of acupuncture in CRF.
Other modalities. Massage therapy, music therapy, hypnosis, therapeutic touch, biofield therapies, relaxation, and reiki are other therapies for which few studies have been done; of the studies that have been done, the results are mixed, and additional research is needed.74 Currently, there are not sufficient data to recommend any of these modalities.
Pharmacologic Interventions
Psychostimulants. Methylphenidate and modafinil are psychostimulants or wakefulness-promoting agents. Pilot studies showed benefit from methylphenidate and modafinil in CRF,75–77 but randomized controlled trials have yielded mixed results. Therefore, in patients with severe fatigue during cancer therapy, the initial management strategy involves evaluation and treatment of medical conditions such as anemia and a trial of nonpharmacological strategies as discussed above. If symptoms persist, then a therapeutic trial of a psychostimulant may be considered per NCCN guidelines for patients undergoing active cancer treatment.37
Methylphenidate directly stimulates adrenergic receptors and indirectly releases dopamine and norepinephrine from presynaptic terminals, which may explain why the drug benefits patients receiving opioid-induced sedation. It is a commonly studied psychostimulant, though its mechanism of action in CRF is unclear. Randomized controlled trials of methylphenidate have resulted in a wide range of findings due to the heterogeneity of study populations and variations in the dosage of methylphenidate. A meta-analysis of 7 studies indicates that methylphenidate benefitted the subgroup of patients with CRF.78 Likewise, in an analysis of 5 randomized controlled trials, Minton et al showed a benefit of psychostimulants in fatigue compared with placebo.79 However, another study of methylphenidate in patients with CRF showed a benefit only in patients with severe fatigue or advanced disease.80 Methylphenidate was found to benefit cancer patients receiving opioid-induced sedation, as methylphenidate promotes wakefulness, though fatigue was not studied specifically.81 In a trial with 30 hospice patients in which the methylphenidate dose was titrated based on response and adverse effects, Kerr at al found that the drug improved fatigue in a dose-dependent manner.82 However, a study in patients with CRF at the University of Texas MD Anderson Cancer Center found no significant difference in BFI scores between patients receiving methylphenidate and those receiving placebo at the end of 2 weeks of treatment.83 Also, other randomized controlled trials in patients undergoing adjuvant chemotherapy for breast cancer84 and patients receiving radiation therapy for brain tumors85 failed to demonstrate the efficacy of methylphenidate in CRF. It should be used cautiously after ruling out other causes of fatigue. The drug is overall well tolerated and side effects include headache and nausea.
Modafinil is a non-amphetamine psychostimulant that has been approved for the treatment of narcolepsy. In a trial studying the effect of modafinil on patients receiving docetaxel-based chemotherapy for metastatic breast or prostate cancer, there was a modest but not statistically significant improvement in fatigue scores on the MD Anderson Symptom Inventory compared with placebo. Nausea and vomiting were higher in the modafinil arm than in the placebo arm.86 Similarly, modafinil was not superior to placebo for CRF in 208 patients with non-squamous cell lung cancer not undergoing chemotherapy or radiation.87 A placebo effect was also noted in patients with multiple myeloma88 and patients with primary brain tumors.89 In a phase 3, multicenter, randomized, placebo-controlled, double-blind clinical trial of modafinil for CRF in 867 patients undergoing chemotherapy, there was a reduction in fatigue only for patients with severe baseline fatigue, with no significant effect on mild to moderate fatigue.90 In another recent study, modafinil was shown to reduce depressive symptoms only in patients with severe fatigue (BFI item 3 score ≥ 7).91 This finding is consistent with previous studies showing benefit in patients with high baseline fatigue, but additional randomized controlled trials are needed to provide clarity. NCCN guidelines do not recommend the use of modafinil to treat CRF.37
Other pharmacologic interventions. Corticosteroids are often used for symptom control in cancer patients. These drugs have anti-inflammatory effects through their modulation of pro-inflammatory cytokines.92 In a randomized controlled trial evaluating the efficacy of corticosteroids, patients receiving dexamethasone (4 mg twice daily) experienced significant improvement in their FACT-F scores compared with patients receiving placebo.93 A similar benefit in fatigue was demonstrated in another study of methylprednisolone (32 mg daily) versus placebo.94 Despite the benefits of steroids, their adverse effects, such as mood swings, gastritis, hyperglycemia, and immune suppression, limit their long-term use. Therefore, the use of steroids should be restricted to terminally ill fatigued patients with other symptoms such as anorexia, brain metastasis, or pain related to bone metastasis.37
Testosterone replacement has been shown to diminish fatigue in non-cancer patients. Many men with advanced cancer have hypogonadism leading to low serum testosterone, which may cause fatigue. In a small trial in which cancer patients with hypogonadism received intramuscular testosterone every 14 days or placebo, the group receiving testosterone showed improvement in FACT-F scores, but there was no significant difference in FACT-F scores between the 2 groups.95
Antidepressants have failed to demonstrate benefit in CRF without depression.8 However, if a patient has both fatigue and depression, antidepressants may help.96 A selective serotonin receptor inhibitor is recommended as a first-line antidepressant.97 Patients with cancer are often receiving multiple medications, and medication interactions should be considered to prevent adverse events such as serotonin syndrome.
Complementary and Alternative Supplements
Studies using vitamin supplementation have been inconclusive in patients with CRF.74 The use of other dietary supplements has yielded mixed results, and coenzyme Q has shown no benefit for patients with CRF.98
The benefit of ginseng was studied in a RCT involving 364 patients with CRF. There was an improvement in Multidimensional Fatigue Symptom Inventory-short form (MFSI-SF) scores at 8 weeks in patients receiving 2000 mg of Wisconsin ginseng compared with patients receiving placebo.99 Patients on active treatment had greater improvement as compared to the post-treatment group in this trial. In another study of high-dose panax ginseng (ginseng root) at 800 mg daily for 29 days, patients had improvement of CRF as well as overall quality of life, appetite, and sleep at night. It was also well tolerated with few adverse effects.100 Interaction with warfarin, calcium channel blockers, antiplatelet agents, thrombolytic agents, imatinib, and other agents may occur; therefore, ginseng must be used with careful monitoring in selected patients. There is not enough evidence at this time to support the routine use of ginseng in CRF.
The seed extract of the guarana plant (Paullinia cupana) traditionally has been used as a stimulant. An improvement in fatigue scores was seen with the use of oral guarana (100 mg daily) at the end of 21 days in breast cancer patients receiving chemotherapy.101 Further studies are needed for these results to be generalized and to understand the adverse effects and interaction profile of guarana.
Reevaluation
Patients who have completed cancer treatment must be monitored for fatigue over the long term, as fatigue may exist beyond the period of active treatment. Many studies have shown fatigue in breast cancer survivors, and fatigue has been demonstrated in survivors of colorectal, lung, and prostate cancers as well as myeloproliferative neoplasms.28 Therefore, it is important to screen patients for fatigue during follow-up visits. There are currently no studies evaluating the long-term treatment of fatigue. In our experience, the timing of follow-up depends on the level of fatigue and interventions prescribed. Once fatigue is stabilized to a level with which the patient is able to cope, the time interval for follow-up may be lengthened. Annual visits may suffice in patients with mild fatigue. Follow-up of patients with moderate to severe fatigue depends on the level of fatigue, the ability to cope, choice of treatment, and presence of contributing factors.
CONCLUSION
CRF is a complex condition that places a significant burden on patients and caregivers, resulting in emotional distress, poor functioning, and suffering. Fatigue can occur before, during, and long after cancer treatment. The approach to CRF begins with screening for and educating patients and their caregivers about the symptoms. Many screening scales are available that may be used to follow patients’ progress over time. The evaluation and management of contributing conditions may help improve fatigue. If the fatigue persists, an individualized approach with a combination of nonpharmacologic and pharmacologic approaches should be considered. More research is needed to understand brain signaling pathways, cytokine changes, and genomic changes in cancer patients with fatigue. Though many hypotheses have been proposed, to date there is no biological marker to assess this condition. Biomarker research needs to be advanced to help to identify patients at risk for fatigue. As cytokines have a major role in CRF, targeted therapy to block cytokine pathways may also be explored in the future.
Acknowledgment: The authors thank Bryan Tutt for providing editorial assistance during the writing of this article.
INTRODUCTION
Fatigue is a common distressing effect of cancer.1 It impairs the quality of life of patients undergoing active cancer treatment and of post-treatment survivors alike. The National Comprehensive Cancer Network (NCCN) defines cancer-related fatigue (CRF) as “a distressing, persistent, subjective sense of physical, emotional and/or cognitive tiredness related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning.”2 CRF differs from fatigue reported by individuals without cancer in that CRF is more severe and is not relieved by rest. The prevalence of CRF in cancer patients and survivors is highly variable, with estimates ranging between 25% and 99%.2,3 The methods used for screening patients for fatigue and the characteristics of the patient groups may account for this variability.
PATHOPHYSIOLOGY
The specific pathophysiologic mechanism underlying CRF is unknown, making targeted treatment a challenge. The multidimensional and subjective nature of CRF has limited the development of research methodologies to explain this condition. However, research has been done in both human and animal models, and several theories have been proposed to explain the pathophysiology of CRF. While pro-inflammatory cytokines remain the central factor playing a significant role at multiple levels in CRF, there may be a complex interplay of multiple mechanisms contributing to fatigue in an individual patient.
CENTRAL NERVOUS SYSTEM DISTURBANCES
The basal ganglia are known to influence motivation. Lack of motivation and drive may cause failure to complete physical and mental tasks, even with preserved cognitive ability and motor function. In a study of melanoma patients receiving interferon, increased activity of the basal ganglia and the cerebellum resulted in higher fatigue scores.4 Increased levels of cytokines may alter blood flow to the cerebellum and lead to the perception of fatigue. In a study of 12 patients and matched controls, when patients were asked to perform sustained elbow flexion until they perceived exhaustion, CRF patients perceived physical exhaustion sooner than controls. In CRF patients in this study, muscle fatigue measured by electromyogram was less than that in healthy individuals at the time of exhaustion, suggesting the role of the central nervous system in CRF.5 However, there is not enough evidence at this time to support central nervous system disturbance as the main factor contributing to fatigue in cancer patients.
CIRCADIAN RHYTHM DYSREGULATION
Circadian rhythm is regulated by the suprachiasmatic nucleus in the hypothalamus through cortisol and melatonin. Sleep disturbances occur with disruption of the circadian rhythm. Tumor-related peptides such as epidermal growth factor or alterations in serotonin and cortisol can influence the suprachiasmatic nucleus and the complex signaling pathways.2 Positive feedback loops that are activated by cortisol under the influence of cytokines may lead to continuous cytokine production and altered circadian rhythm. Bower et al showed that changes in the cortisol curve influence fatigue in breast cancer survivors.6 These patients had a late evening peak in cortisol levels, compared with an early morning peak in individuals without cancer.
INHIBITION OF HYPOTHALAMIC-PITUITARY-ADRENAL AXIS
The hypothalamic–pituitary–adrenal (HPA) axis regulates the release of the stress hormone cortisol. One of several hypotheses advanced to explain the effect of serotonin and the HPA axis on CRF suggests that lower serotonin levels cause decreased activation of 5-hydroxytrytophan 1-a (5-HT1-a) receptors in the hypothalamus, leading to decreased activity of the HPA axis.6 Inhibition of the HPA axis may occur with higher levels of serotonin as well.7 The 5-HT1-a receptors are also triggered by cytokines. However, the correction of serotonin levels by antidepressants was not shown to improve fatigue.8 Inhibition of the HPA axis can also lead to lower testosterone, progesterone, or estrogen levels, which may indirectly contribute to fatigue.2
SKELETAL MUSCLE EFFECT
Chemotherapy- and tumor-related cachexia have a direct effect on the metabolism of skeletal muscles. This effect may lead to impaired adenosine triphosphate (ATP) generation during muscle contraction.9 ATP infusion improved muscle strength in 1 trial, but this was not confirmed in another trial.10,11 Muscle contraction studies showed no differences in the contractile properties of muscles in fatigued patients who failed earlier in motor tasks and healthy controls.12 This finding suggests that there could be a failure of skeletal muscle activation by the central nervous system or inhibition of skeletal muscle activity. Cytokines and other neurotransmitters activate vagal efferent nerve fibers, which may lead to reflex inhibition in skeletal muscles.13,14
PRO-INFLAMMATORY CYTOKINES
Tumors or treatment of them may cause tissue injury, which triggers immune cells to release cytokines, signaling the brain to manifest the symptom of fatigue. Inflammatory pathways are influenced by psychological, behavioral, and biological factors, which play a role as risk factors in CRF. Levels of interleukin 6 (IL-6), interleukin-1 receptor antagonist, interleukin-1, and tumor necrosis factor (TNF) have been shown to be elevated in fatigued patients being treated for leukemia and non-Hodgkin lymphoma.15 IL-6 was also associated with increased fatigue in breast cancer survivors.16 Similar findings were reported in patients undergoing stem cell transplantation and high-dose chemotherapy.17 Elevated levels of IL-6 and C-reactive protein were also linked to fatigue in terminally ill cancer patients.18,19 Furthermore, TNF-α signaling was associated with post-chemotherapy fatigue in breast cancer patients.20 Leukocytes in breast cancer survivors with fatigue also have increased gene expression of pro-inflammatory cytokines, emphasizing the role of cytokines and inflammation in the pathogenesis of CRF.21
OTHER HYPOTHESES
Several other hypotheses for CRF pathogenesis have been proposed. Activation of latent viruses such as Epstein-Barr virus, lack of social support,22 genetic alterations in the immune pathway,23 epigenetic changes,24 accumulation of neurotoxic metabolites and depletion of serotonin by indoleamine 2,3-dioxygenase pathway activation,25 elevated vascular endothelial growth factor levels,26 and hypoxia-related organ dysfunction due to anemia or hemoglobin dysfunction13 all have been postulated to cause CRF.
EVALUATION AND TREATMENT
Fours steps are involved in the evaluation and treatment of CRF (Figure).

SCREENING
Because patients and health care professionals may be unaware of the treatment options available for CRF, patients may not report fatigue levels to their clinicians, and clinicians may not understand the impact of fatigue on their patients’ quality of life. This leads to under-recognition of the problem. The NCCN recommends screening every cancer patient and post-treatment survivor for fatigue.2 Patients should be screened at their first visit and then at periodic intervals during and after cancer treatment.
Many scales are available to screen patients for CRF in clinical practice and clinical trials.27 A single item that asks patients to rate their fatigue on a scale from 0 to 10—in which 0 indicates no fatigue, 1 to 3 indicates mild fatigue, 4 to 6 indicates moderate fatigue, 7 to 9 indicates severe fatigue, and 10 indicates the worst fatigue imaginable—is commonly used to screen for CRF.2 This scale was adapted from the MD Anderson Symptom Inventory scale and is based on a large nationwide study of cancer patients and survivors.28 The statistically derived cutoff points in this study are consistent with other scales such as the Brief Fatigue Inventory (BFI) and support the cutoff points (4–6 for moderate and ≥ 7 for severe fatigue) used in various fatigue management guidelines. Furthermore, studies of fatigue in cancer patients have revealed a marked decrease in physical function at levels of 7 or higher, suggesting 7 as an optimal cutoff to identify severe fatigue.29,30 The Visual Analog Scale is another simple-to-use tool that helps in understanding variations in fatigue throughout the course of the day.31 The 9-item BFI is often used in clinical trials.29 It measures the severity of fatigue over the previous 24 hours and has been validated in patients who do not speak English.32
CRF affects not only the somatic domain, but also the cognitive, behavioral, and affective domains; therefore, multidimensional scales have been developed for screening. One such tool is the Multidimensional Fatigue Inventory, which assesses 5 dimensions of fatigue—general fatigue, physical fatigue, reduced motivation, reduced activity, and mental fatigue—and compares the patient’s results with those of individuals without cancer.33,34 The Functional Assessment of Cancer Therapy for Fatigue (FACT-F) is a 13-item questionnaire that has been used to measure CRF in clinical trials as well as in patients receiving various treatments.35
Although many scales are available, the validity of self-reporting on simple fatigue-rating scales is equal to or better than most complex, lengthy scales.36 Therefore, unidimensional tools such as the numeric rating scale of 0–10 are commonly used in clinical practice. Mild fatigue (0–3) requires periodic reevaluation, and moderate and severe fatigue need further evaluation and management.37
PRIMARY EVALUATION
This phase involves a focused history and physical examination and assessment of concurrent symptoms and contributing factors.
History and Physical Examination
A detailed history of the patient’s malignancy and type of previous and current treatment may help reveal the cause of fatigue. New-onset fatigue or increase in fatigue may be related to the progression of disease in patients with active malignancy or recurrence of cancer in survivors. These patients may require appropriate testing to assess the underlying disease pattern. A detailed review of systems may help identify some of the contributing factors, which are discussed below. A detailed history regarding medications, including over-the-counter drugs, complementary agents, and past and prior cancer therapies, is helpful as medications can contribute to fatigue. For example, opioids may cause drowsiness and fatigue, which could be improved by dose adjustments. A focused history of fatigue should be obtained in all patients with moderate to severe CRF, which includes the onset, pattern, duration, associated or alleviating factors, and interference with functioning, including activities of daily living.37 Physical examination should focus on identifying signs of organ dysfunction and features of substance or alcohol abuse, which may cause poor sleep and fatigue.
Assessment of Contributing Factors
The management of fatigue should be multifactorial, with a comprehensive assessment and treatment plan to address all modifiable fatigue etiologies. The Table lists potential contributing factors to fatigue that should be considered when evaluating patients for CRF; several common conditions are discussed below. 
Anemia. Anemia has been correlated with fatigue and quality of life. In a study of 4382 cancer patients receiving chemotherapy, quality-of-life measures using FACT-Anemia scores improved with increased hemoglobin levels.38 Cancer patients may have anemia due to marrow-suppressing effects of chemotherapy and may also have iron deficiency anemia due to blood loss or auto-immune hemolytic anemia. Therefore, a detailed work-up is required to identify the etiology of anemia. Patients with CRF whose anemia is related to chemotherapy or anemia of chronic disease may benefit from red blood cell transfusion or erythropoiesis-stimulating agents (ESAs). ESAs have been studied extensively; however, their use is controversial because of concerns about thromboembolic side effects leading to adverse outcomes.39 Also, ESA therapy is not recommended in patients with hematologic malignancies. ESA use should be restricted to patients with chemotherapy-related anemia with hemoglobin below 10 mg/dL and should be discontinued in 6 to 8 weeks if patients do not respond.40 Other patients may benefit from blood transfusions, which were shown to help in patients with hemoglobin levels between 7.5 and 8.5 g/dL.41
Sleep disturbance. Poor sleep is common in fatigued cancer survivors.42 Pro-inflammatory cytokines can disrupt the sleep–wake cycle, causing changes in the HPA axis and neuroendocrine system, which in turn may lead to increasing fatigue. Heckler et al showed that improvement in nighttime sleep leads to improvement of fatigue.43 Cognitive behavioral therapy and sleep hygiene are important in caring for patients with CRF and poor sleep.44 Taking a warm bath and/or drinking a glass of milk before bedtime, avoiding caffeinated drinks, and avoiding frequent napping in the day might help. Some patients may require medications such as benzodiazepines or non-benzodiazepine hypnotics (eg, zolpidem) to promote sleep.45 Melatonin agonists are approved for insomnia in the United States, but not in Europe.46
Malnutrition. Patients with advanced-stage cancer and with cancers affecting the gastrointestinal tract frequently develop mechanical bowel obstructions, especially at the end of their life, which cause malnutrition. Chemotherapy-related nausea and vomiting may also cause poor oral intake and malnutrition, causing fatigue from muscle weakness. Dehydration and electrolyte imbalances frequently occur as a result of poor oral intake, which might worsen fatigue. In our experience, modifying dietary intake with appropriate caloric exchanges with the help of a nutrition expert has lessened fatigue in some patients. However, terminally ill patients are advised to eat based on their comfort.
Medical comorbidities. Congestive heart failure from anthracycline chemotherapy, hypothyroidism after radiation therapy for head and neck cancers, renal failure, or hepatic failure from chemotherapy may indirectly lead to fatigue. Chemotherapy, opioids, and steroids may cause hypogonadism, which can contribute to fatigue in men.47
Assessment of Concurrent Symptoms
Depression is common in cancer patients and coexists with pain, insomnia, fatigue, and anxiety as a symptom cluster.48 A symptom cluster is defined as 2 or more concurrent and interrelated symptoms occurring together; treating one of these symptoms without addressing other symptoms is not effective.49 Therefore, screening for and management of depression, anxiety, and insomnia play an important role in the management of CRF.
Physical symptoms due to the tumor or to therapy— such as pain, dyspnea, nausea, and decreased physical activity—may also contribute to fatigue both directly and indirectly. Patients with lung cancer may have hypoxemia, which can contribute to dyspnea with activity and a perception of fatigue. Optimal management of pain and other physical symptoms in patients with cancer may significantly alleviate fatigue.50
MANAGEMENT
Management of CRF is individualized based on the patient’s clinical status: active cancer treatment, survivor, or end of life. Education and counselling of patients and their caregivers play an important role in CRF. NCCN guidelines recommend focusing on pain control, distress management, energy conservation, physical activity, nutrition, and sleep hygiene.
Nonpharmacologic Interventions
Energy conservation. Energy conservation strategies, in which patients are advised to set priorities and realistic expectations, are highly recommended. Some energy-conserving strategies are to pace oneself, delegate and schedule activities at times of peak energy, postpone nonessential activities, attend to 1 activity at a time, structure daily routines, and maintain a diary to identify their peak energy period and structure activities around that time.51,52 When patients approach the end of life, increasing fatigue may limit their activity level, and they may depend on caregivers for assistance with activities of daily living, monitoring treatment-related adverse effects, and taking medications, especially elderly patients.53
Cognitive behavioral therapy. Cognitive behavioral therapy (CBT) has been shown to improve CRF during active treatment, and the benefits persist for a minimum of 2 years after therapy.54 CBT interventions that optimize sleep quality may improve fatigue.55 More studies are needed to understand whether referral to a psychologist for formal CBT is required. Randomized clinical trials showed patient fatigue education, learned self-care, coping techniques, and balancing rest and activity benefit patients with CRF.56
Exercise. Physical activity is highly encouraged in patients with CRF. Exercise increases muscle protein synthesis, improves cytokine response, and decreases the rate of sarcopenia in healthy populations.57 Studies have shown that exercise helps CRF at all phases of the cancer journey, including radiation therapy, chemotherapy, and survivorship.58 Some patients may feel less motivated to exercise and may not believe that exercise is possible or could potentially help them. Counselling is needed for such patients.
Older cancer survivors have a decline in their functional capacity and reduced muscle mass. Exercise can improve their cardiorespiratory fitness, muscle strength, and body composition.57 Exercise not only helps at the cellular level but also has psychosocial benefits from improved self-esteem. Therefore, exercise may be recommended for younger patients as well as for the older population, who may have comorbidities and less motivation than younger patients.
In a meta-analysis of 56 randomized controlled trials involving 4068 participants, aerobic exercise was found to have beneficial effects on CRF for patients during and after chemotherapy, specifically for patients with solid tumors.59 In another meta-analysis of breast and prostate cancer survivors, a combination of aerobic exercise with resistance training (3–6 metabolic equivalents, 60%–80% range of motion) was shown to reduce CRF more than aerobic exercise alone.60 This effect was also shown in a randomized controlled trial of 160 patients with stage 0 to III breast cancer undergoing radiation therapy.61 The control group in this study had a group-based non-exercise intervention/relaxation; therefore, the study showed that the effect of resistance training extends beyond the psychosocial benefits of group-based interventions. The intervention comprised 8 progressive machine-based resistance exercises (3 sets, 8–12 repetitions at 60%–80% of 1 repetition maximum) for 60 minutes twice weekly for 12 weeks. However, fatigue assessment questionnaire scores showed benefits only in the physical fatigue components, but not in the affective and cognitive components.
The American Society of Clinical Oncology’s guidelines for cancer survivors with fatigue recommends 150 minutes of moderate aerobic exercise (eg, fast walking, cycling, or swimming) per week, with 2 or 3 sessions of strength training per week.62 An individualized approach to exercise is recommended, as patients’ ability to perform certain types of exercises may be limited by thrombocytopenia, neutropenia, or lytic bone metastasis. Routine use of pre-exercise cardiovascular testing is not recommended but may be considered in high-risk populations, especially patients with risk factors for coronary heart disease and diabetes.63 Patients with comorbidities, substantial deconditioning, functional and anatomic defects, or recent major surgery may benefit from referral to physical therapy.37 Patients near end of life may also benefit from an exercise program, as demonstrated in several studies that showed benefit in CRF and quality of life.64,65 We recommend that physicians use their best clinical judgement in suggesting the type and intensity of exercise program, as it may not be feasible in some patients.
Mind-body interventions. Mindfulness-based stress reduction (MBSR) has shown promise in breast cancer survivors, who reported immediate improvements in fatigue severity that continued up to 6 weeks after cessation of the training.66 Prior studies had similar findings, suggesting that MBSR modestly decreases fatigue and sleep disturbances and has a greater effect on the degree to which symptoms interfere with many facets of life.67
Yoga. A study of a yoga intervention showed a benefit in older cancer survivors.68 In breast cancer patients undergoing chemotherapy, yoga was shown to benefit both physical and cognitive fatigue.69 DVD-based yoga had benefits similar to strengthening exercises in a study of 34 early-stage breast cancer survivors with CRF.70 More studies are needed in men and patients and survivors of other cancers, as most studies of yoga were conducted in women with breast cancer.
Tai chi/qigong. Like yoga, tai chi and qigong are practices of meditative movement. These practices use postures or movements with a focus on breath and a meditative state to bring about deep states of relaxation. Qigong is a series of simple, repeated practices including body posture/movement, breath practice, and meditation performed in synchrony. Tai chi easy (TCE) is a simplified set of common, repetitive tai chi movements. In a trial, qigong/TCE was compared with sham qigong, which had physical movements but no breathing or meditative practice. Breast cancer survivors in the qigong/TCE group had improved fatigue scores, and the effect persisted for 3 months.71 Additional research is needed in this area.
Acupuncture. A randomized controlled trial in breast cancer patients with CRF showed an improvement in the mean general fatigue score (per the Multidimensional Fatigue Inventory) in patients who received acupuncture versus those who did not (−3.11 [95% confidence interval −3.97 to −2.25]; P < 0.001) at 6 weeks. Improvements were seen in both the mental and physical aspects of fatigue.72 However, Deng et al noted that true acupuncture was no more effective than sham acupuncture for reducing post-chemotherapy chronic fatigue.73 Presently, there is not sufficient evidence to evaluate the benefits of acupuncture in CRF.
Other modalities. Massage therapy, music therapy, hypnosis, therapeutic touch, biofield therapies, relaxation, and reiki are other therapies for which few studies have been done; of the studies that have been done, the results are mixed, and additional research is needed.74 Currently, there are not sufficient data to recommend any of these modalities.
Pharmacologic Interventions
Psychostimulants. Methylphenidate and modafinil are psychostimulants or wakefulness-promoting agents. Pilot studies showed benefit from methylphenidate and modafinil in CRF,75–77 but randomized controlled trials have yielded mixed results. Therefore, in patients with severe fatigue during cancer therapy, the initial management strategy involves evaluation and treatment of medical conditions such as anemia and a trial of nonpharmacological strategies as discussed above. If symptoms persist, then a therapeutic trial of a psychostimulant may be considered per NCCN guidelines for patients undergoing active cancer treatment.37
Methylphenidate directly stimulates adrenergic receptors and indirectly releases dopamine and norepinephrine from presynaptic terminals, which may explain why the drug benefits patients receiving opioid-induced sedation. It is a commonly studied psychostimulant, though its mechanism of action in CRF is unclear. Randomized controlled trials of methylphenidate have resulted in a wide range of findings due to the heterogeneity of study populations and variations in the dosage of methylphenidate. A meta-analysis of 7 studies indicates that methylphenidate benefitted the subgroup of patients with CRF.78 Likewise, in an analysis of 5 randomized controlled trials, Minton et al showed a benefit of psychostimulants in fatigue compared with placebo.79 However, another study of methylphenidate in patients with CRF showed a benefit only in patients with severe fatigue or advanced disease.80 Methylphenidate was found to benefit cancer patients receiving opioid-induced sedation, as methylphenidate promotes wakefulness, though fatigue was not studied specifically.81 In a trial with 30 hospice patients in which the methylphenidate dose was titrated based on response and adverse effects, Kerr at al found that the drug improved fatigue in a dose-dependent manner.82 However, a study in patients with CRF at the University of Texas MD Anderson Cancer Center found no significant difference in BFI scores between patients receiving methylphenidate and those receiving placebo at the end of 2 weeks of treatment.83 Also, other randomized controlled trials in patients undergoing adjuvant chemotherapy for breast cancer84 and patients receiving radiation therapy for brain tumors85 failed to demonstrate the efficacy of methylphenidate in CRF. It should be used cautiously after ruling out other causes of fatigue. The drug is overall well tolerated and side effects include headache and nausea.
Modafinil is a non-amphetamine psychostimulant that has been approved for the treatment of narcolepsy. In a trial studying the effect of modafinil on patients receiving docetaxel-based chemotherapy for metastatic breast or prostate cancer, there was a modest but not statistically significant improvement in fatigue scores on the MD Anderson Symptom Inventory compared with placebo. Nausea and vomiting were higher in the modafinil arm than in the placebo arm.86 Similarly, modafinil was not superior to placebo for CRF in 208 patients with non-squamous cell lung cancer not undergoing chemotherapy or radiation.87 A placebo effect was also noted in patients with multiple myeloma88 and patients with primary brain tumors.89 In a phase 3, multicenter, randomized, placebo-controlled, double-blind clinical trial of modafinil for CRF in 867 patients undergoing chemotherapy, there was a reduction in fatigue only for patients with severe baseline fatigue, with no significant effect on mild to moderate fatigue.90 In another recent study, modafinil was shown to reduce depressive symptoms only in patients with severe fatigue (BFI item 3 score ≥ 7).91 This finding is consistent with previous studies showing benefit in patients with high baseline fatigue, but additional randomized controlled trials are needed to provide clarity. NCCN guidelines do not recommend the use of modafinil to treat CRF.37
Other pharmacologic interventions. Corticosteroids are often used for symptom control in cancer patients. These drugs have anti-inflammatory effects through their modulation of pro-inflammatory cytokines.92 In a randomized controlled trial evaluating the efficacy of corticosteroids, patients receiving dexamethasone (4 mg twice daily) experienced significant improvement in their FACT-F scores compared with patients receiving placebo.93 A similar benefit in fatigue was demonstrated in another study of methylprednisolone (32 mg daily) versus placebo.94 Despite the benefits of steroids, their adverse effects, such as mood swings, gastritis, hyperglycemia, and immune suppression, limit their long-term use. Therefore, the use of steroids should be restricted to terminally ill fatigued patients with other symptoms such as anorexia, brain metastasis, or pain related to bone metastasis.37
Testosterone replacement has been shown to diminish fatigue in non-cancer patients. Many men with advanced cancer have hypogonadism leading to low serum testosterone, which may cause fatigue. In a small trial in which cancer patients with hypogonadism received intramuscular testosterone every 14 days or placebo, the group receiving testosterone showed improvement in FACT-F scores, but there was no significant difference in FACT-F scores between the 2 groups.95
Antidepressants have failed to demonstrate benefit in CRF without depression.8 However, if a patient has both fatigue and depression, antidepressants may help.96 A selective serotonin receptor inhibitor is recommended as a first-line antidepressant.97 Patients with cancer are often receiving multiple medications, and medication interactions should be considered to prevent adverse events such as serotonin syndrome.
Complementary and Alternative Supplements
Studies using vitamin supplementation have been inconclusive in patients with CRF.74 The use of other dietary supplements has yielded mixed results, and coenzyme Q has shown no benefit for patients with CRF.98
The benefit of ginseng was studied in a RCT involving 364 patients with CRF. There was an improvement in Multidimensional Fatigue Symptom Inventory-short form (MFSI-SF) scores at 8 weeks in patients receiving 2000 mg of Wisconsin ginseng compared with patients receiving placebo.99 Patients on active treatment had greater improvement as compared to the post-treatment group in this trial. In another study of high-dose panax ginseng (ginseng root) at 800 mg daily for 29 days, patients had improvement of CRF as well as overall quality of life, appetite, and sleep at night. It was also well tolerated with few adverse effects.100 Interaction with warfarin, calcium channel blockers, antiplatelet agents, thrombolytic agents, imatinib, and other agents may occur; therefore, ginseng must be used with careful monitoring in selected patients. There is not enough evidence at this time to support the routine use of ginseng in CRF.
The seed extract of the guarana plant (Paullinia cupana) traditionally has been used as a stimulant. An improvement in fatigue scores was seen with the use of oral guarana (100 mg daily) at the end of 21 days in breast cancer patients receiving chemotherapy.101 Further studies are needed for these results to be generalized and to understand the adverse effects and interaction profile of guarana.
Reevaluation
Patients who have completed cancer treatment must be monitored for fatigue over the long term, as fatigue may exist beyond the period of active treatment. Many studies have shown fatigue in breast cancer survivors, and fatigue has been demonstrated in survivors of colorectal, lung, and prostate cancers as well as myeloproliferative neoplasms.28 Therefore, it is important to screen patients for fatigue during follow-up visits. There are currently no studies evaluating the long-term treatment of fatigue. In our experience, the timing of follow-up depends on the level of fatigue and interventions prescribed. Once fatigue is stabilized to a level with which the patient is able to cope, the time interval for follow-up may be lengthened. Annual visits may suffice in patients with mild fatigue. Follow-up of patients with moderate to severe fatigue depends on the level of fatigue, the ability to cope, choice of treatment, and presence of contributing factors.
CONCLUSION
CRF is a complex condition that places a significant burden on patients and caregivers, resulting in emotional distress, poor functioning, and suffering. Fatigue can occur before, during, and long after cancer treatment. The approach to CRF begins with screening for and educating patients and their caregivers about the symptoms. Many screening scales are available that may be used to follow patients’ progress over time. The evaluation and management of contributing conditions may help improve fatigue. If the fatigue persists, an individualized approach with a combination of nonpharmacologic and pharmacologic approaches should be considered. More research is needed to understand brain signaling pathways, cytokine changes, and genomic changes in cancer patients with fatigue. Though many hypotheses have been proposed, to date there is no biological marker to assess this condition. Biomarker research needs to be advanced to help to identify patients at risk for fatigue. As cytokines have a major role in CRF, targeted therapy to block cytokine pathways may also be explored in the future.
Acknowledgment: The authors thank Bryan Tutt for providing editorial assistance during the writing of this article.
1. Scherber RM, Kosiorek HE, Senyak Z, et al. Comprehensively understanding fatigue in patients with myeloproliferative neoplasms. Cancer 2016;122:477–85.
2. Neefjes EC, van der Vorst MJ, Blauwhoff-Buskermolen S, Verheul HM. Aiming for a better understanding and management of cancer-related fatigue. Oncologist 2013;18:1135–43.
3. Radbruch L, Strasser F, Elsner F, et al. Fatigue in palliative care patients—an EAPC approach. Palliat Med 2008;22:13–32.
4. Capuron L, Pagnoni G, Demetrashvili MF, et al. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology 2007;32:2384–92.
5. Kisiel-Sajewicz K, Siemionow V, Seyidova-Khoshknabi D, et al. Myoelectrical manifestation of fatigue less prominent in patients with cancer related fatigue. PLoS One 2013;8:e83636.
6. Bower JE, Ganz PA, Aziz N. Altered cortisol response to psychologic stress in breast cancer survivors with persistent fatigue. Psychosom Med 2005;67:277–80.
7. Barsevick A, Frost M, Zwinderman A, et al. I’m so tired: biological and genetic mechanisms of cancer-related fatigue. Qual Life Res 2010;19:1419–27.
8. Morrow GR, Hickok JT, Roscoe JA, et al. Differential effects of paroxetine on fatigue and depression: a randomized, double-blind trial from the University of Rochester Cancer Center Community Clinical Oncology Program. J Clin Oncol 2003;21:4635–41.
9. Fontes-Oliveira CC, Busquets S, Toledo M, et al. Mitochondrial and sarcoplasmic reticulum abnormalities in cancer cachexia: altered energetic efficiency? Biochim Biophys Acta 2013;1830:2770–8.
10. Agteresch HJ, Dagnelie PC, van der Gaast A, et al. Randomized clinical trial of adenosine 5’-triphosphate in patients with advanced non-small-cell lung cancer. J Natl Cancer Inst 2000;92:321–8.
11. Beijer S, Hupperets PS, van den Borne BE, et al. Randomized clinical trial on the effects of adenosine 5’-triphosphate infusions on quality of life, functional status, and fatigue in preterminal cancer patients. J Pain Symptom Manage 2010;40:520–30.
12. Kisiel-Sajewicz K, Davis MP, Siemionow V, et al. Lack of muscle contractile property changes at the time of perceived physical exhaustion suggests central mechanisms contributing to early motor task failure in patients with cancer-related fatigue. J Pain Symptom Manage 2012;44:351–61.
13. Ryan JL, Carroll JK, Ryan EP, et al. Mechanisms of cancer-related fatigue. Oncologist 2007;12 Suppl 1:22–34.
14. Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer 2008;8:887–99.
15. Wang XS, Giralt SA, Mendoza TR, et al. Clinical factors associated with cancer-related fatigue in patients being treated for leukemia and non-Hodgkin’s lymphoma. J Clin Oncol 2002;20:1319–28.
16. Collado-Hidalgo A, Bower JE, Ganz PA, et al. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res 2006;12:2759–66.
17. Wang XS, Shi Q, Williams LA, et al. Serum interleukin-6 predicts the development of multiple symptoms at nadir of allogeneic hematopoietic stem cell transplantation. Cancer 2008;113:2102–9.
18. Inagaki M, Isono M, Okuyama T, et al. Plasma interleukin-6 and fatigue in terminally ill cancer patients. J Pain Symptom Manage 2008;35:153–61.
19. Laird BJ, McMillan DC, Fayers P, et al. The systemic inflammatory response and its relationship to pain and other symptoms in advanced cancer. Oncologist 2013;18:1050–5.
20. Bower JE, Ganz PA, Irwin MR, et al. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol 2011;29:3517–22.
21. Whistler T, Taylor R, Craddock RC, et al. Gene expression correlates of unexplained fatigue. Pharmacogenomics 2006;7:395–405.
22. Fagundes CP, Bennett JM, Alfano CM, et al. Social support and socioeconomic status interact to predict Epstein-Barr virus latency in women awaiting diagnosis or newly diagnosed with breast cancer. Health Psychol 2012;31:11–19.
23. Landmark-Hoyvik H, Reinertsen KV, Loge JH, et al. Alterations of gene expression in blood cells associated with chronic fatigue in breast cancer survivors. Pharmacogenomics J 2009;9:333–40.
24. Smith AK, Conneely KN, Pace TW, et al. Epigenetic changes associated with inflammation in breast cancer patients treated with chemotherapy. Brain Behav Immun 2014;38:227–36.
25. Kim S, Miller BJ, Stefanek ME, Miller AH. Inflammation-induced activation of the indoleamine 2,3-dioxygenase pathway: Relevance to cancer-related fatigue. Cancer 2015;121:2129–36.
26. Mills PJ, Parker B, Dimsdale JE, et al. The relationship between fatigue and quality of life and inflammation during anthracycline-based chemotherapy in breast cancer. Biol Psychol 2005;69:85–96.
27. Jean-Pierre P, Figueroa-Moseley CD, Kohli S, et al. Assessment of cancer-related fatigue: implications for clinical diagnosis and treatment. Oncologist 2007;12 Suppl 1:11–21.
28. Wang XS, Zhao F, Fisch MJ, et al. Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer 2014;120:425–32.
29. Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer 1999;85:1186–96.
30. Mendoza ME, Capafons A, Gralow JR, et al. Randomized controlled trial of the Valencia model of waking hypnosis plus CBT for pain, fatigue, and sleep management in patients with cancer and cancer survivors. Psychooncology 2016 Jul 28.
31. Glaus A. Assessment of fatigue in cancer and non-cancer patients and in healthy individuals. Support Care Cancer 1993;1:305–15.
32. Seyidova-Khoshknabi D, Davis MP, Walsh D. A systematic review of cancer-related fatigue measurement questionnaires. Am J Hosp Palliat Care 2011;28:119–29.
33. Holzner B, Kemmler G, Greil R, et al. The impact of hemoglobin levels on fatigue and quality of life in cancer patients. Ann Oncol 2002;13:965–73.
34. Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage 2004;27:14–23.
35. Hwang SS, Chang VT, Rue M, Kasimis B. Multidimensional independent predictors of cancer-related fatigue. J Pain Symptom Manage 2003;26:604–14.
36. Peterson DR. Scope and generality of verbally defined personality factors. Psychol Rev 1965;72:48–59.
37. Berger AM, Abernethy AP, Atkinson A, et al. NCCN Clinical Practice Guidelines Cancer-related fatigue. J Natl Compr Canc Netw 2010;8:904–31.
38. Crawford J, Cella D, Cleeland CS, et al. Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic cancer patients receiving epoetin alfa therapy. Cancer 2002;95:888–95.
39. Tonia T, Mettler A, Robert N, et al. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev 2012;12:CD003407.
40. Rizzo JD, Brouwers M, Hurley P, et al. American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. Blood 2010;116:4045–59.
41. Preston NJ, Hurlow A, Brine J, Bennett MI. Blood transfusions for anaemia in patients with advanced cancer. Cochrane Database Syst Rev 2012(2):CD009007.
42. Minton O, Stone PC. A comparison of cognitive function, sleep and activity levels in disease-free breast cancer patients with or without cancer-related fatigue syndrome. BMJ Support Palliat Care 2012;2:231–8.
43. Heckler CE, Garland SN, Peoples AR, et al. Cognitive behavioral therapy for insomnia, but not armodafinil, improves fatigue in cancer survivors with insomnia: a randomized placebo-controlled trial. Support Care Cancer 2016;24:2059–66.
44. Howell D, Oliver TK, Keller-Olaman S, et al. Sleep disturbance in adults with cancer: a systematic review of evidence for best practices in assessment and management for clinical practice. Ann Oncol 2014;25:791–800.
45. Wilt TJ, MacDonald R, Brasure M, et al. Pharmacologic treatment of insomnia disorder: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med 2016;165:103–12.
46. Kuriyama A, Honda M, Hayashino Y. Ramelteon for the treatment of insomnia in adults: a systematic review and meta-analysis. Sleep Med 2014;15:385–92.
47. Strasser F, Palmer JL, Schover LR, et al. The impact of hypogonadism and autonomic dysfunction on fatigue, emotional function, and sexual desire in male patients with advanced cancer: a pilot study. Cancer 2006;107:2949–57.
48. Agasi-Idenburg SC, Thong MS, Punt CJ, et al. Comparison of symptom clusters associated with fatigue in older and younger survivors of colorectal cancer. Support Care Cancer 2017;25:625–32.
49. Miaskowski C, Aouizerat BE. Is there a biological basis for the clustering of symptoms? Semin Oncol Nurs 2007;23:99–105.
50. de Raaf PJ, de Klerk C, Timman R, et al. Systematic monitoring and treatment of physical symptoms to alleviate fatigue in patients with advanced cancer: a randomized controlled trial. J Clin Oncol 2013;31:716–23.
51. Barsevick AM, Whitmer K, Sweeney C, Nail LM. A pilot study examining energy conservation for cancer treatment-related fatigue. Cancer Nurs 2002;25:333–41.
52. Barsevick AM, Dudley W, Beck S, et a;. A randomized clinical trial of energy conservation for patients with cancer-related fatigue. Cancer 2004;100:1302–10.
53. Luciani A, Jacobsen PB, Extermann M, et al. Fatigue and functional dependence in older cancer patients. Am J Clin Oncol 2008;31:424–30.
54. Abrahams HJ, Gielissen MF, Goedendorp MM, et al. A randomized controlled trial of web-based cognitive behavioral therapy for severely fatigued breast cancer survivors (CHANGE-study): study protocol. BMC Cancer 2015;15:765.
55. Quesnel C, Savard J, Simard S, et al. Efficacy of cognitive-behavioral therapy for insomnia in women treated for nonmetastatic breast cancer. J Consult Clin Psychol 2003;71:189–200.
56. Goedendorp MM, Gielissen MF, Verhagen CA, Bleijenberg G. Psychosocial interventions for reducing fatigue during cancer treatment in adults. Cochrane Database Syst Rev 2009(1):CD006953.
57. Greiwe JS, Cheng B, Rubin DC, et al. Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. FASEB J 2001;15:475–82.
58. Furmaniak AC, Menig M, Markes MH. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev 2016;(9):CD005001.
59. Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev 2012;(11):CD006145.
60. Brown JC, Huedo-Medina TB, Pescatello LS, et al. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2011;20:123–33.
61. Steindorf K, Schmidt ME, Klassen O, et al. Randomized, controlled trial of resistance training in breast cancer patients receiving adjuvant radiotherapy: results on cancer-related fatigue and quality of life. Ann Oncol 2014;25:2237–43.
62. Bower JE, Bak K, Berger A, et al. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical oncology clinical practice guideline adaptation. J Clin Oncol 2014;32:1840–50.
63. Kenjale AA, Hornsby WE, Crowgey T, et al. Pre-exercise participation cardiovascular screening in a heterogeneous cohort of adult cancer patients. Oncologist 2014;19:999–1005.
64. Oldervoll LM, Loge JH, Paltiel H, et al. The effect of a physical exercise program in palliative care: A phase II study. J Pain Symptom Manage 2006;31:421–30.
65. Porock D, Kristjanson LJ, Tinnelly K, et al. An exercise intervention for advanced cancer patients experiencing fatigue: a pilot study. J Palliat Care 2000;16:30–6.
66. Lengacher CA, Kip KE, Reich RR, et al. A cost-effective mindfulness stress reduction program: a randomized control trial for breast cancer survivors. Nursing Econ 2015;33:210–8, 32.
67. Lengacher CA, Reich RR, Post-White J, et al. Mindfulness based stress reduction in post-treatment breast cancer patients: an examination of symptoms and symptom clusters. J Behav Med 2012;35:86–94.
68. Sprod LK, Fernandez ID, Janelsins MC, et al. Effects of yoga on cancer-related fatigue and global side-effect burden in older cancer survivors. J Geriatr Oncol 2015;6:8–14.
69. Wang G, Wang S, Jiang P, Zeng C. [Effect of Yoga on cancer related fatigue in breast cancer patients with chemotherapy]. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2014;39:1077–82.
70. Stan DL, Croghan KA, Croghan IT, et al. Randomized pilot trial of yoga versus strengthening exercises in breast cancer survivors with cancer-related fatigue. Support Care Cancer 2016;24:4005–15.
71. Larkey LK, Roe DJ, Weihs KL, et al. Randomized controlled trial of Qigong/Tai Chi Easy on cancer-related fatigue in breast cancer survivors. Ann Behav Med 2015;49:165–76.
72. Molassiotis A, Bardy J, Finnegan-John J, et al. Acupuncture for cancer-related fatigue in patients with breast cancer: a pragmatic randomized controlled trial. J Clin Oncol 2012;30:4470–6.
73. Deng G, Chan Y, Sjoberg D, et al. Acupuncture for the treatment of post-chemotherapy chronic fatigue: a randomized, blinded, sham-controlled trial. Support Care Cancer 2013;21:1735–41.
74. Finnegan-John J, Molassiotis A, Richardson A, Ream E. A systematic review of complementary and alternative medicine interventions for the management of cancer-related fatigue. Integr Cancer Ther 2013;12:276–90.
75. Schwartz AL, Thompson JA, Masood N. Interferon-induced fatigue in patients with melanoma: a pilot study of exercise and methylphenidate. Oncol Nurs Forum 2002;29:E85–90.
76. Spathis A, Dhillan R, Booden D, et al. Modafinil for the treatment of fatigue in lung cancer: a pilot study. Palliat Med 2009;23:325–31.
77. Blackhall L, Petroni G, Shu J, et al. A pilot study evaluating the safety and efficacy of modafinal for cancer-related fatigue. J Palliat Med 2009;12:433–9.
78. Qu D, Zhang Z, Yu X, et al. Psychotropic drugs for the management of cancer-related fatigue: a systematic review and meta-analysis. Eur J Cancer Care (Engl) 2015;25:970–9.
79. Minton O, Richardson A, Sharpe M, et al. Drug therapy for the management of cancer-related fatigue. Cochrane Database Syst Rev 2010(7):CD006704.
80. Moraska AR, Sood A, Dakhil SR, et al. Phase III, randomized, double-blind, placebo-controlled study of long-acting methylphenidate for cancer-related fatigue: North Central Cancer Treatment Group NCCTG-N05C7 trial. J Clin Oncol 2010;28:3673–9.
81. Bruera E, Driver L, Barnes EA, et al. Patient-controlled methylphenidate for the management of fatigue in patients with advanced cancer: a preliminary report. J Clin Oncol 2003;21:4439–43.
82. Kerr CW, Drake J, Milch RA, et al. Effects of methylphenidate on fatigue and depression: a randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage 2012;43:68–77.
83. Escalante CP, Meyers C, Reuben JM, et al. A randomized, double-blind, 2-period, placebo-controlled crossover trial of a sustained-release methylphenidate in the treatment of fatigue in cancer patients. Cancer J 2014;20:8–14.
84. Mar Fan HG, Clemons M, Xu W, et al. A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support Care Cancer 2008;16:577–83.
85. Butler JM Jr, Case LD, Atkins J, et al. A phase III, double-blind, placebo-controlled prospective randomized clinical trial of d-threo-methylphenidate HCl in brain tumor patients receiving radiation therapy. Int J Radiat Oncol Biol Phys 2007;69:1496–501.
86. Hovey E, de Souza P, Marx G, et al. Phase III, randomized, double-blind, placebo-controlled study of modafinil for fatigue in patients treated with docetaxel-based chemotherapy. Support Care Cancer 2014;22:1233–42.
87. Spathis A, Fife K, Blackhall F, et al. Modafinil for the treatment of fatigue in lung cancer: results of a placebo-controlled, double-blind, randomized trial. J Clin Oncol 2014;32:1882–8.
88. Berenson JR, Yellin O, Shamasunder HK, et al. A phase 3 trial of armodafinil for the treatment of cancer-related fatigue for patients with multiple myeloma. Support Care Cancer 2015; 23:1503–12.
89. Boele FW, Douw L, de Groot M, et al. The effect of modafinil on fatigue, cognitive functioning, and mood in primary brain tumor patients: a multicenter randomized controlled trial. Neuro Oncol 2013;15:1420–8.
90. Jean-Pierre P, Morrow GR, Roscoe JA, et al. A phase 3 randomized, placebo-controlled, double-blind, clinical trial of the effect of modafinil on cancer-related fatigue among 631 patients receiving chemotherapy: a University of Rochester Cancer Center Community Clinical Oncology Program Research base study. Cancer 2010;116:3513–20.
91. Conley CC, Kamen CS, Heckler CE, et al. Modafinil moderates the relationship between cancer-related fatigue and depression in 541 patients receiving chemotherapy. J Clin Psychopharmacol 2016;36:82–5.
92. Brattsand R, Linden M. Cytokine modulation by glucocorticoids: mechanisms and actions in cellular studies. Aliment Pharmacol Ther 1996;10 Suppl 2:81–90.
93. Yennurajalingam S, Frisbee-Hume S, Palmer JL, et al. Reduction of cancer-related fatigue with dexamethasone: a double-blind, randomized, placebo-controlled trial in patients with advanced cancer. J Clin Oncol 2013;31:3076–82.
94. Bruera E, Roca E, Cedaro L, et al. Action of oral methylprednisolone in terminal cancer patients: a prospective randomized double-blind study. Cancer Treat Rep 1985;69:751–4.
95. Pulivarthi K, Dev R, Garcia J, et al. Testosterone replacement for fatigue in male hypogonadic patients with advanced cancer: A preliminary double-blind placebo-controlled trial. J Clin Oncol 2012;30 (suppl). Abstract e19643.
96. Palesh OG, Mustian KM, Peppone LJ, et al. Impact of paroxetine on sleep problems in 426 cancer patients receiving chemotherapy: a trial from the University of Rochester Cancer Center Community Clinical Oncology Program. Sleep Med 2012;13:1184–90.
97. Thekdi SM, Trinidad A, Roth A. Psychopharmacology in Cancer. Curr Psychiatry Rep 2014;17:529.
98. Lesser GJ. Case D, Stark N, et al. A randomized, double-blind, placebo-controlled study of oral coenzyme Q10 to relieve self-reported treatment-related fatigue in newly diagnosed patients with breast cancer. J Support Oncol 2013;11:31–42.
99. Barton DL, Liu H, Dakhil SR, et al. Wisconsin Ginseng (Panax quinquefolius) to improve cancer-related fatigue: a randomized, double-blind trial, N07C2. J Natl Cancer Inst 2013;105:1230–8.
100. Yennurajalingam S, Reddy A, Tannir NM, et al. High-dose Asian ginseng (panax ginseng) for cancer-related fatigue: a preliminary report. Integr Cancer Ther 2015;14:419–27.
101. Howell D, Keller-Olaman S, Oliver TK, et al. A pan-Canadian practice guideline and algorithm: screening, assessment, and supportive care of adults with cancer-related fatigue. Curr Oncol 2013;20:e233–46.
1. Scherber RM, Kosiorek HE, Senyak Z, et al. Comprehensively understanding fatigue in patients with myeloproliferative neoplasms. Cancer 2016;122:477–85.
2. Neefjes EC, van der Vorst MJ, Blauwhoff-Buskermolen S, Verheul HM. Aiming for a better understanding and management of cancer-related fatigue. Oncologist 2013;18:1135–43.
3. Radbruch L, Strasser F, Elsner F, et al. Fatigue in palliative care patients—an EAPC approach. Palliat Med 2008;22:13–32.
4. Capuron L, Pagnoni G, Demetrashvili MF, et al. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology 2007;32:2384–92.
5. Kisiel-Sajewicz K, Siemionow V, Seyidova-Khoshknabi D, et al. Myoelectrical manifestation of fatigue less prominent in patients with cancer related fatigue. PLoS One 2013;8:e83636.
6. Bower JE, Ganz PA, Aziz N. Altered cortisol response to psychologic stress in breast cancer survivors with persistent fatigue. Psychosom Med 2005;67:277–80.
7. Barsevick A, Frost M, Zwinderman A, et al. I’m so tired: biological and genetic mechanisms of cancer-related fatigue. Qual Life Res 2010;19:1419–27.
8. Morrow GR, Hickok JT, Roscoe JA, et al. Differential effects of paroxetine on fatigue and depression: a randomized, double-blind trial from the University of Rochester Cancer Center Community Clinical Oncology Program. J Clin Oncol 2003;21:4635–41.
9. Fontes-Oliveira CC, Busquets S, Toledo M, et al. Mitochondrial and sarcoplasmic reticulum abnormalities in cancer cachexia: altered energetic efficiency? Biochim Biophys Acta 2013;1830:2770–8.
10. Agteresch HJ, Dagnelie PC, van der Gaast A, et al. Randomized clinical trial of adenosine 5’-triphosphate in patients with advanced non-small-cell lung cancer. J Natl Cancer Inst 2000;92:321–8.
11. Beijer S, Hupperets PS, van den Borne BE, et al. Randomized clinical trial on the effects of adenosine 5’-triphosphate infusions on quality of life, functional status, and fatigue in preterminal cancer patients. J Pain Symptom Manage 2010;40:520–30.
12. Kisiel-Sajewicz K, Davis MP, Siemionow V, et al. Lack of muscle contractile property changes at the time of perceived physical exhaustion suggests central mechanisms contributing to early motor task failure in patients with cancer-related fatigue. J Pain Symptom Manage 2012;44:351–61.
13. Ryan JL, Carroll JK, Ryan EP, et al. Mechanisms of cancer-related fatigue. Oncologist 2007;12 Suppl 1:22–34.
14. Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer 2008;8:887–99.
15. Wang XS, Giralt SA, Mendoza TR, et al. Clinical factors associated with cancer-related fatigue in patients being treated for leukemia and non-Hodgkin’s lymphoma. J Clin Oncol 2002;20:1319–28.
16. Collado-Hidalgo A, Bower JE, Ganz PA, et al. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res 2006;12:2759–66.
17. Wang XS, Shi Q, Williams LA, et al. Serum interleukin-6 predicts the development of multiple symptoms at nadir of allogeneic hematopoietic stem cell transplantation. Cancer 2008;113:2102–9.
18. Inagaki M, Isono M, Okuyama T, et al. Plasma interleukin-6 and fatigue in terminally ill cancer patients. J Pain Symptom Manage 2008;35:153–61.
19. Laird BJ, McMillan DC, Fayers P, et al. The systemic inflammatory response and its relationship to pain and other symptoms in advanced cancer. Oncologist 2013;18:1050–5.
20. Bower JE, Ganz PA, Irwin MR, et al. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol 2011;29:3517–22.
21. Whistler T, Taylor R, Craddock RC, et al. Gene expression correlates of unexplained fatigue. Pharmacogenomics 2006;7:395–405.
22. Fagundes CP, Bennett JM, Alfano CM, et al. Social support and socioeconomic status interact to predict Epstein-Barr virus latency in women awaiting diagnosis or newly diagnosed with breast cancer. Health Psychol 2012;31:11–19.
23. Landmark-Hoyvik H, Reinertsen KV, Loge JH, et al. Alterations of gene expression in blood cells associated with chronic fatigue in breast cancer survivors. Pharmacogenomics J 2009;9:333–40.
24. Smith AK, Conneely KN, Pace TW, et al. Epigenetic changes associated with inflammation in breast cancer patients treated with chemotherapy. Brain Behav Immun 2014;38:227–36.
25. Kim S, Miller BJ, Stefanek ME, Miller AH. Inflammation-induced activation of the indoleamine 2,3-dioxygenase pathway: Relevance to cancer-related fatigue. Cancer 2015;121:2129–36.
26. Mills PJ, Parker B, Dimsdale JE, et al. The relationship between fatigue and quality of life and inflammation during anthracycline-based chemotherapy in breast cancer. Biol Psychol 2005;69:85–96.
27. Jean-Pierre P, Figueroa-Moseley CD, Kohli S, et al. Assessment of cancer-related fatigue: implications for clinical diagnosis and treatment. Oncologist 2007;12 Suppl 1:11–21.
28. Wang XS, Zhao F, Fisch MJ, et al. Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer 2014;120:425–32.
29. Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer 1999;85:1186–96.
30. Mendoza ME, Capafons A, Gralow JR, et al. Randomized controlled trial of the Valencia model of waking hypnosis plus CBT for pain, fatigue, and sleep management in patients with cancer and cancer survivors. Psychooncology 2016 Jul 28.
31. Glaus A. Assessment of fatigue in cancer and non-cancer patients and in healthy individuals. Support Care Cancer 1993;1:305–15.
32. Seyidova-Khoshknabi D, Davis MP, Walsh D. A systematic review of cancer-related fatigue measurement questionnaires. Am J Hosp Palliat Care 2011;28:119–29.
33. Holzner B, Kemmler G, Greil R, et al. The impact of hemoglobin levels on fatigue and quality of life in cancer patients. Ann Oncol 2002;13:965–73.
34. Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage 2004;27:14–23.
35. Hwang SS, Chang VT, Rue M, Kasimis B. Multidimensional independent predictors of cancer-related fatigue. J Pain Symptom Manage 2003;26:604–14.
36. Peterson DR. Scope and generality of verbally defined personality factors. Psychol Rev 1965;72:48–59.
37. Berger AM, Abernethy AP, Atkinson A, et al. NCCN Clinical Practice Guidelines Cancer-related fatigue. J Natl Compr Canc Netw 2010;8:904–31.
38. Crawford J, Cella D, Cleeland CS, et al. Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic cancer patients receiving epoetin alfa therapy. Cancer 2002;95:888–95.
39. Tonia T, Mettler A, Robert N, et al. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev 2012;12:CD003407.
40. Rizzo JD, Brouwers M, Hurley P, et al. American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. Blood 2010;116:4045–59.
41. Preston NJ, Hurlow A, Brine J, Bennett MI. Blood transfusions for anaemia in patients with advanced cancer. Cochrane Database Syst Rev 2012(2):CD009007.
42. Minton O, Stone PC. A comparison of cognitive function, sleep and activity levels in disease-free breast cancer patients with or without cancer-related fatigue syndrome. BMJ Support Palliat Care 2012;2:231–8.
43. Heckler CE, Garland SN, Peoples AR, et al. Cognitive behavioral therapy for insomnia, but not armodafinil, improves fatigue in cancer survivors with insomnia: a randomized placebo-controlled trial. Support Care Cancer 2016;24:2059–66.
44. Howell D, Oliver TK, Keller-Olaman S, et al. Sleep disturbance in adults with cancer: a systematic review of evidence for best practices in assessment and management for clinical practice. Ann Oncol 2014;25:791–800.
45. Wilt TJ, MacDonald R, Brasure M, et al. Pharmacologic treatment of insomnia disorder: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med 2016;165:103–12.
46. Kuriyama A, Honda M, Hayashino Y. Ramelteon for the treatment of insomnia in adults: a systematic review and meta-analysis. Sleep Med 2014;15:385–92.
47. Strasser F, Palmer JL, Schover LR, et al. The impact of hypogonadism and autonomic dysfunction on fatigue, emotional function, and sexual desire in male patients with advanced cancer: a pilot study. Cancer 2006;107:2949–57.
48. Agasi-Idenburg SC, Thong MS, Punt CJ, et al. Comparison of symptom clusters associated with fatigue in older and younger survivors of colorectal cancer. Support Care Cancer 2017;25:625–32.
49. Miaskowski C, Aouizerat BE. Is there a biological basis for the clustering of symptoms? Semin Oncol Nurs 2007;23:99–105.
50. de Raaf PJ, de Klerk C, Timman R, et al. Systematic monitoring and treatment of physical symptoms to alleviate fatigue in patients with advanced cancer: a randomized controlled trial. J Clin Oncol 2013;31:716–23.
51. Barsevick AM, Whitmer K, Sweeney C, Nail LM. A pilot study examining energy conservation for cancer treatment-related fatigue. Cancer Nurs 2002;25:333–41.
52. Barsevick AM, Dudley W, Beck S, et a;. A randomized clinical trial of energy conservation for patients with cancer-related fatigue. Cancer 2004;100:1302–10.
53. Luciani A, Jacobsen PB, Extermann M, et al. Fatigue and functional dependence in older cancer patients. Am J Clin Oncol 2008;31:424–30.
54. Abrahams HJ, Gielissen MF, Goedendorp MM, et al. A randomized controlled trial of web-based cognitive behavioral therapy for severely fatigued breast cancer survivors (CHANGE-study): study protocol. BMC Cancer 2015;15:765.
55. Quesnel C, Savard J, Simard S, et al. Efficacy of cognitive-behavioral therapy for insomnia in women treated for nonmetastatic breast cancer. J Consult Clin Psychol 2003;71:189–200.
56. Goedendorp MM, Gielissen MF, Verhagen CA, Bleijenberg G. Psychosocial interventions for reducing fatigue during cancer treatment in adults. Cochrane Database Syst Rev 2009(1):CD006953.
57. Greiwe JS, Cheng B, Rubin DC, et al. Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. FASEB J 2001;15:475–82.
58. Furmaniak AC, Menig M, Markes MH. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev 2016;(9):CD005001.
59. Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev 2012;(11):CD006145.
60. Brown JC, Huedo-Medina TB, Pescatello LS, et al. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2011;20:123–33.
61. Steindorf K, Schmidt ME, Klassen O, et al. Randomized, controlled trial of resistance training in breast cancer patients receiving adjuvant radiotherapy: results on cancer-related fatigue and quality of life. Ann Oncol 2014;25:2237–43.
62. Bower JE, Bak K, Berger A, et al. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical oncology clinical practice guideline adaptation. J Clin Oncol 2014;32:1840–50.
63. Kenjale AA, Hornsby WE, Crowgey T, et al. Pre-exercise participation cardiovascular screening in a heterogeneous cohort of adult cancer patients. Oncologist 2014;19:999–1005.
64. Oldervoll LM, Loge JH, Paltiel H, et al. The effect of a physical exercise program in palliative care: A phase II study. J Pain Symptom Manage 2006;31:421–30.
65. Porock D, Kristjanson LJ, Tinnelly K, et al. An exercise intervention for advanced cancer patients experiencing fatigue: a pilot study. J Palliat Care 2000;16:30–6.
66. Lengacher CA, Kip KE, Reich RR, et al. A cost-effective mindfulness stress reduction program: a randomized control trial for breast cancer survivors. Nursing Econ 2015;33:210–8, 32.
67. Lengacher CA, Reich RR, Post-White J, et al. Mindfulness based stress reduction in post-treatment breast cancer patients: an examination of symptoms and symptom clusters. J Behav Med 2012;35:86–94.
68. Sprod LK, Fernandez ID, Janelsins MC, et al. Effects of yoga on cancer-related fatigue and global side-effect burden in older cancer survivors. J Geriatr Oncol 2015;6:8–14.
69. Wang G, Wang S, Jiang P, Zeng C. [Effect of Yoga on cancer related fatigue in breast cancer patients with chemotherapy]. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2014;39:1077–82.
70. Stan DL, Croghan KA, Croghan IT, et al. Randomized pilot trial of yoga versus strengthening exercises in breast cancer survivors with cancer-related fatigue. Support Care Cancer 2016;24:4005–15.
71. Larkey LK, Roe DJ, Weihs KL, et al. Randomized controlled trial of Qigong/Tai Chi Easy on cancer-related fatigue in breast cancer survivors. Ann Behav Med 2015;49:165–76.
72. Molassiotis A, Bardy J, Finnegan-John J, et al. Acupuncture for cancer-related fatigue in patients with breast cancer: a pragmatic randomized controlled trial. J Clin Oncol 2012;30:4470–6.
73. Deng G, Chan Y, Sjoberg D, et al. Acupuncture for the treatment of post-chemotherapy chronic fatigue: a randomized, blinded, sham-controlled trial. Support Care Cancer 2013;21:1735–41.
74. Finnegan-John J, Molassiotis A, Richardson A, Ream E. A systematic review of complementary and alternative medicine interventions for the management of cancer-related fatigue. Integr Cancer Ther 2013;12:276–90.
75. Schwartz AL, Thompson JA, Masood N. Interferon-induced fatigue in patients with melanoma: a pilot study of exercise and methylphenidate. Oncol Nurs Forum 2002;29:E85–90.
76. Spathis A, Dhillan R, Booden D, et al. Modafinil for the treatment of fatigue in lung cancer: a pilot study. Palliat Med 2009;23:325–31.
77. Blackhall L, Petroni G, Shu J, et al. A pilot study evaluating the safety and efficacy of modafinal for cancer-related fatigue. J Palliat Med 2009;12:433–9.
78. Qu D, Zhang Z, Yu X, et al. Psychotropic drugs for the management of cancer-related fatigue: a systematic review and meta-analysis. Eur J Cancer Care (Engl) 2015;25:970–9.
79. Minton O, Richardson A, Sharpe M, et al. Drug therapy for the management of cancer-related fatigue. Cochrane Database Syst Rev 2010(7):CD006704.
80. Moraska AR, Sood A, Dakhil SR, et al. Phase III, randomized, double-blind, placebo-controlled study of long-acting methylphenidate for cancer-related fatigue: North Central Cancer Treatment Group NCCTG-N05C7 trial. J Clin Oncol 2010;28:3673–9.
81. Bruera E, Driver L, Barnes EA, et al. Patient-controlled methylphenidate for the management of fatigue in patients with advanced cancer: a preliminary report. J Clin Oncol 2003;21:4439–43.
82. Kerr CW, Drake J, Milch RA, et al. Effects of methylphenidate on fatigue and depression: a randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage 2012;43:68–77.
83. Escalante CP, Meyers C, Reuben JM, et al. A randomized, double-blind, 2-period, placebo-controlled crossover trial of a sustained-release methylphenidate in the treatment of fatigue in cancer patients. Cancer J 2014;20:8–14.
84. Mar Fan HG, Clemons M, Xu W, et al. A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support Care Cancer 2008;16:577–83.
85. Butler JM Jr, Case LD, Atkins J, et al. A phase III, double-blind, placebo-controlled prospective randomized clinical trial of d-threo-methylphenidate HCl in brain tumor patients receiving radiation therapy. Int J Radiat Oncol Biol Phys 2007;69:1496–501.
86. Hovey E, de Souza P, Marx G, et al. Phase III, randomized, double-blind, placebo-controlled study of modafinil for fatigue in patients treated with docetaxel-based chemotherapy. Support Care Cancer 2014;22:1233–42.
87. Spathis A, Fife K, Blackhall F, et al. Modafinil for the treatment of fatigue in lung cancer: results of a placebo-controlled, double-blind, randomized trial. J Clin Oncol 2014;32:1882–8.
88. Berenson JR, Yellin O, Shamasunder HK, et al. A phase 3 trial of armodafinil for the treatment of cancer-related fatigue for patients with multiple myeloma. Support Care Cancer 2015; 23:1503–12.
89. Boele FW, Douw L, de Groot M, et al. The effect of modafinil on fatigue, cognitive functioning, and mood in primary brain tumor patients: a multicenter randomized controlled trial. Neuro Oncol 2013;15:1420–8.
90. Jean-Pierre P, Morrow GR, Roscoe JA, et al. A phase 3 randomized, placebo-controlled, double-blind, clinical trial of the effect of modafinil on cancer-related fatigue among 631 patients receiving chemotherapy: a University of Rochester Cancer Center Community Clinical Oncology Program Research base study. Cancer 2010;116:3513–20.
91. Conley CC, Kamen CS, Heckler CE, et al. Modafinil moderates the relationship between cancer-related fatigue and depression in 541 patients receiving chemotherapy. J Clin Psychopharmacol 2016;36:82–5.
92. Brattsand R, Linden M. Cytokine modulation by glucocorticoids: mechanisms and actions in cellular studies. Aliment Pharmacol Ther 1996;10 Suppl 2:81–90.
93. Yennurajalingam S, Frisbee-Hume S, Palmer JL, et al. Reduction of cancer-related fatigue with dexamethasone: a double-blind, randomized, placebo-controlled trial in patients with advanced cancer. J Clin Oncol 2013;31:3076–82.
94. Bruera E, Roca E, Cedaro L, et al. Action of oral methylprednisolone in terminal cancer patients: a prospective randomized double-blind study. Cancer Treat Rep 1985;69:751–4.
95. Pulivarthi K, Dev R, Garcia J, et al. Testosterone replacement for fatigue in male hypogonadic patients with advanced cancer: A preliminary double-blind placebo-controlled trial. J Clin Oncol 2012;30 (suppl). Abstract e19643.
96. Palesh OG, Mustian KM, Peppone LJ, et al. Impact of paroxetine on sleep problems in 426 cancer patients receiving chemotherapy: a trial from the University of Rochester Cancer Center Community Clinical Oncology Program. Sleep Med 2012;13:1184–90.
97. Thekdi SM, Trinidad A, Roth A. Psychopharmacology in Cancer. Curr Psychiatry Rep 2014;17:529.
98. Lesser GJ. Case D, Stark N, et al. A randomized, double-blind, placebo-controlled study of oral coenzyme Q10 to relieve self-reported treatment-related fatigue in newly diagnosed patients with breast cancer. J Support Oncol 2013;11:31–42.
99. Barton DL, Liu H, Dakhil SR, et al. Wisconsin Ginseng (Panax quinquefolius) to improve cancer-related fatigue: a randomized, double-blind trial, N07C2. J Natl Cancer Inst 2013;105:1230–8.
100. Yennurajalingam S, Reddy A, Tannir NM, et al. High-dose Asian ginseng (panax ginseng) for cancer-related fatigue: a preliminary report. Integr Cancer Ther 2015;14:419–27.
101. Howell D, Keller-Olaman S, Oliver TK, et al. A pan-Canadian practice guideline and algorithm: screening, assessment, and supportive care of adults with cancer-related fatigue. Curr Oncol 2013;20:e233–46.
FDA approves first duodenoscope with disposable distal cap
, according to an FDA press release.
A disposable distal cap will improve the ability to clean and reprocess the duodenoscope. Without being thoroughly cleaned and disinfected, contaminated tissue can remain and potentially can be transmitted to other patients.
A previous version of the Pentax duodenoscope, the ED-3490TK, was subject to a January 2017 FDA Safety Alert, because of the potential for cracks and gaps to develop in the adhesive sealing the duodenoscope’s distal cap.
, according to an FDA press release.
A disposable distal cap will improve the ability to clean and reprocess the duodenoscope. Without being thoroughly cleaned and disinfected, contaminated tissue can remain and potentially can be transmitted to other patients.
A previous version of the Pentax duodenoscope, the ED-3490TK, was subject to a January 2017 FDA Safety Alert, because of the potential for cracks and gaps to develop in the adhesive sealing the duodenoscope’s distal cap.
, according to an FDA press release.
A disposable distal cap will improve the ability to clean and reprocess the duodenoscope. Without being thoroughly cleaned and disinfected, contaminated tissue can remain and potentially can be transmitted to other patients.
A previous version of the Pentax duodenoscope, the ED-3490TK, was subject to a January 2017 FDA Safety Alert, because of the potential for cracks and gaps to develop in the adhesive sealing the duodenoscope’s distal cap.
Roux-en-Y gastric bypass produced durable clinical improvements at 12 years
Severely obese individuals in the United States who underwent Roux-en-Y gastric bypass (RYGB) averaged a 27% weight loss 12 years later, with only a 3% incidence of type 2 diabetes mellitus and a 51% rate of diabetes remission, according to the results of a large multicenter observational prospective study.
In striking contrast, patients who did not undergo bariatric surgery averaged a 1%-2% weight loss at 12 years, a 26% incidence of diabetes, and only a 5%-10% rate of diabetes remission, said Ted D. Adams, PhD, of the University of Utah, Salt Lake City, and his associates. RYGB surgery also conferred substantial and statistically significant improvements long-term improvements in systolic hypertension and lipid levels, the researchers reported in the New England Journal of Medicine (2017 Sep 20. doi: 10.1056/NEJMoa1700459).
Few prospective studies have tracked long-term outcomes after bariatric surgery. Among 1,156 participants in this study, 418 patients underwent RYGB, 417 individuals sought but did not undergo surgery – mainly for insurance reasons – and 321 individuals did not seek surgery. Participants were mostly females in their 40s or 50s at baseline, and typically weighed 120 kg-130 kg.
“The follow-up rate exceeded 90% at 12 years,” the researchers wrote. Two years after undergoing Roux-en-Y gastric bypass, patients had lost an average of 45 kg (95% confidence interval, 43-47 kg). By postoperative year 6, they had regained an average of 9 kg (average loss from baseline, 36 kg; 95% CI, 34-39 kg). But they typically gained only about 1.3 kg more between years 6 and 12, and they had about a 92% lower odds of developing diabetes mellitus, compared with individuals who did not undergo bariatric surgery (odds ratio, 0.08; P less than .001). “Remission of type 2 diabetes was much more likely if the Roux-en-Y gastric bypass occurred before [patients began] treatment with insulin, presumably owing to the ability of partially viable beta cells to improve their function,” the researchers noted.
Funders included the National Institute of Diabetes and Digestive and Kidney Diseases, the National Center for Research Resources, Weill Cornell Medicine, and Intermountain Healthcare. Dr. Adams reported having no relevant conflicts of interest. One coinvestigator disclosed royalties from licensing a questionnaire on weight loss and quality of life, and another coinvestigator disclosed fees for services rendered during a trial of an intragastric balloon. The remaining researchers had no relevant disclosures.
AGA Resource
Gastroenterologists are uniquely positioned to lead a care team to help patients with obesity achieve a healthy weight. The AGA Obesity Practice Guide was created to provide a comprehensive, multi-disciplinary process to personalize innovative obesity care for safe and effective weight management. Learn more at www.gastro.org/obesity.
Severely obese individuals in the United States who underwent Roux-en-Y gastric bypass (RYGB) averaged a 27% weight loss 12 years later, with only a 3% incidence of type 2 diabetes mellitus and a 51% rate of diabetes remission, according to the results of a large multicenter observational prospective study.
In striking contrast, patients who did not undergo bariatric surgery averaged a 1%-2% weight loss at 12 years, a 26% incidence of diabetes, and only a 5%-10% rate of diabetes remission, said Ted D. Adams, PhD, of the University of Utah, Salt Lake City, and his associates. RYGB surgery also conferred substantial and statistically significant improvements long-term improvements in systolic hypertension and lipid levels, the researchers reported in the New England Journal of Medicine (2017 Sep 20. doi: 10.1056/NEJMoa1700459).
Few prospective studies have tracked long-term outcomes after bariatric surgery. Among 1,156 participants in this study, 418 patients underwent RYGB, 417 individuals sought but did not undergo surgery – mainly for insurance reasons – and 321 individuals did not seek surgery. Participants were mostly females in their 40s or 50s at baseline, and typically weighed 120 kg-130 kg.
“The follow-up rate exceeded 90% at 12 years,” the researchers wrote. Two years after undergoing Roux-en-Y gastric bypass, patients had lost an average of 45 kg (95% confidence interval, 43-47 kg). By postoperative year 6, they had regained an average of 9 kg (average loss from baseline, 36 kg; 95% CI, 34-39 kg). But they typically gained only about 1.3 kg more between years 6 and 12, and they had about a 92% lower odds of developing diabetes mellitus, compared with individuals who did not undergo bariatric surgery (odds ratio, 0.08; P less than .001). “Remission of type 2 diabetes was much more likely if the Roux-en-Y gastric bypass occurred before [patients began] treatment with insulin, presumably owing to the ability of partially viable beta cells to improve their function,” the researchers noted.
Funders included the National Institute of Diabetes and Digestive and Kidney Diseases, the National Center for Research Resources, Weill Cornell Medicine, and Intermountain Healthcare. Dr. Adams reported having no relevant conflicts of interest. One coinvestigator disclosed royalties from licensing a questionnaire on weight loss and quality of life, and another coinvestigator disclosed fees for services rendered during a trial of an intragastric balloon. The remaining researchers had no relevant disclosures.
AGA Resource
Gastroenterologists are uniquely positioned to lead a care team to help patients with obesity achieve a healthy weight. The AGA Obesity Practice Guide was created to provide a comprehensive, multi-disciplinary process to personalize innovative obesity care for safe and effective weight management. Learn more at www.gastro.org/obesity.
Severely obese individuals in the United States who underwent Roux-en-Y gastric bypass (RYGB) averaged a 27% weight loss 12 years later, with only a 3% incidence of type 2 diabetes mellitus and a 51% rate of diabetes remission, according to the results of a large multicenter observational prospective study.
In striking contrast, patients who did not undergo bariatric surgery averaged a 1%-2% weight loss at 12 years, a 26% incidence of diabetes, and only a 5%-10% rate of diabetes remission, said Ted D. Adams, PhD, of the University of Utah, Salt Lake City, and his associates. RYGB surgery also conferred substantial and statistically significant improvements long-term improvements in systolic hypertension and lipid levels, the researchers reported in the New England Journal of Medicine (2017 Sep 20. doi: 10.1056/NEJMoa1700459).
Few prospective studies have tracked long-term outcomes after bariatric surgery. Among 1,156 participants in this study, 418 patients underwent RYGB, 417 individuals sought but did not undergo surgery – mainly for insurance reasons – and 321 individuals did not seek surgery. Participants were mostly females in their 40s or 50s at baseline, and typically weighed 120 kg-130 kg.
“The follow-up rate exceeded 90% at 12 years,” the researchers wrote. Two years after undergoing Roux-en-Y gastric bypass, patients had lost an average of 45 kg (95% confidence interval, 43-47 kg). By postoperative year 6, they had regained an average of 9 kg (average loss from baseline, 36 kg; 95% CI, 34-39 kg). But they typically gained only about 1.3 kg more between years 6 and 12, and they had about a 92% lower odds of developing diabetes mellitus, compared with individuals who did not undergo bariatric surgery (odds ratio, 0.08; P less than .001). “Remission of type 2 diabetes was much more likely if the Roux-en-Y gastric bypass occurred before [patients began] treatment with insulin, presumably owing to the ability of partially viable beta cells to improve their function,” the researchers noted.
Funders included the National Institute of Diabetes and Digestive and Kidney Diseases, the National Center for Research Resources, Weill Cornell Medicine, and Intermountain Healthcare. Dr. Adams reported having no relevant conflicts of interest. One coinvestigator disclosed royalties from licensing a questionnaire on weight loss and quality of life, and another coinvestigator disclosed fees for services rendered during a trial of an intragastric balloon. The remaining researchers had no relevant disclosures.
AGA Resource
Gastroenterologists are uniquely positioned to lead a care team to help patients with obesity achieve a healthy weight. The AGA Obesity Practice Guide was created to provide a comprehensive, multi-disciplinary process to personalize innovative obesity care for safe and effective weight management. Learn more at www.gastro.org/obesity.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Roux-en-Y gastric bypass produced durable results on numerous clinical outcome measures.
Major finding: Twelve years after surgery, RYGB patients averaged a 27% weight loss from baseline, with a 51% rate of diabetes remission and a 3% incidence of type 2 diabetes mellitus.
Data source: A prospective study of 1,156 severely obese individuals, of whom 418 underwent Roux-en-Y gastric bypass.
Disclosures: Funders included the National Institute of Diabetes and Digestive and Kidney Diseases, the National Center for Research Resources, Weill Cornell Medicine, and Intermountain Healthcare. Dr. Adams reported having no relevant conflicts of interest. One coinvestigator disclosed royalties from licensing a questionnaire on weight loss and the quality of life, and another coinvestigator disclosed fees for services rendered during a trial of an intragastric balloon. The remaining researchers had no relevant disclosures.
Roux-en-Y gastric bypass produced durable clinical improvements at 12 years
Severely obese individuals in the United States who underwent Roux-en-Y gastric bypass (RYGB) averaged a 27% weight loss 12 years later, with only a 3% incidence of type 2 diabetes mellitus and a 51% rate of diabetes remission, according to the results of a large multicenter observational prospective study.
In striking contrast, patients who did not undergo bariatric surgery averaged a 1%-2% weight loss at 12 years, a 26% incidence of diabetes, and only a 5%-10% rate of diabetes remission, said Ted D. Adams, PhD, of the University of Utah, Salt Lake City, and his associates. RYGB surgery also conferred substantial and statistically significant improvements long-term improvements in systolic hypertension and lipid levels, the researchers reported in the New England Journal of Medicine (2017 Sep 20. doi: 10.1056/NEJMoa1700459).
“The follow-up rate exceeded 90% at 12 years,” the researchers wrote. Two years after undergoing Roux-en-Y gastric bypass, patients had lost an average of 45 kg (95% confidence interval, 43-47 kg). By postoperative year 6, they had regained an average of 9 kg (average loss from baseline, 36 kg; 95% CI, 34-39 kg). But they typically gained only about 1.3 kg more between years 6 and 12, and they had about a 92% lower odds of developing diabetes mellitus, compared with individuals who did not undergo bariatric surgery (odds ratio, 0.08; P less than .001). “Remission of type 2 diabetes was much more likely if the Roux-en-Y gastric bypass occurred before [patients began] treatment with insulin, presumably owing to the ability of partially viable beta cells to improve their function,” the researchers noted.
Funders included the National Institute of Diabetes and Digestive and Kidney Diseases, the National Center for Research Resources, Weill Cornell Medicine, and Intermountain Healthcare. Dr. Adams reported having no relevant conflicts of interest. One coinvestigator disclosed royalties from licensing a questionnaire on weight loss and quality of life, and another coinvestigator disclosed fees for services rendered during a trial of an intragastric balloon. The remaining researchers had no relevant disclosures.
Severely obese individuals in the United States who underwent Roux-en-Y gastric bypass (RYGB) averaged a 27% weight loss 12 years later, with only a 3% incidence of type 2 diabetes mellitus and a 51% rate of diabetes remission, according to the results of a large multicenter observational prospective study.
In striking contrast, patients who did not undergo bariatric surgery averaged a 1%-2% weight loss at 12 years, a 26% incidence of diabetes, and only a 5%-10% rate of diabetes remission, said Ted D. Adams, PhD, of the University of Utah, Salt Lake City, and his associates. RYGB surgery also conferred substantial and statistically significant improvements long-term improvements in systolic hypertension and lipid levels, the researchers reported in the New England Journal of Medicine (2017 Sep 20. doi: 10.1056/NEJMoa1700459).
“The follow-up rate exceeded 90% at 12 years,” the researchers wrote. Two years after undergoing Roux-en-Y gastric bypass, patients had lost an average of 45 kg (95% confidence interval, 43-47 kg). By postoperative year 6, they had regained an average of 9 kg (average loss from baseline, 36 kg; 95% CI, 34-39 kg). But they typically gained only about 1.3 kg more between years 6 and 12, and they had about a 92% lower odds of developing diabetes mellitus, compared with individuals who did not undergo bariatric surgery (odds ratio, 0.08; P less than .001). “Remission of type 2 diabetes was much more likely if the Roux-en-Y gastric bypass occurred before [patients began] treatment with insulin, presumably owing to the ability of partially viable beta cells to improve their function,” the researchers noted.
Funders included the National Institute of Diabetes and Digestive and Kidney Diseases, the National Center for Research Resources, Weill Cornell Medicine, and Intermountain Healthcare. Dr. Adams reported having no relevant conflicts of interest. One coinvestigator disclosed royalties from licensing a questionnaire on weight loss and quality of life, and another coinvestigator disclosed fees for services rendered during a trial of an intragastric balloon. The remaining researchers had no relevant disclosures.
Severely obese individuals in the United States who underwent Roux-en-Y gastric bypass (RYGB) averaged a 27% weight loss 12 years later, with only a 3% incidence of type 2 diabetes mellitus and a 51% rate of diabetes remission, according to the results of a large multicenter observational prospective study.
In striking contrast, patients who did not undergo bariatric surgery averaged a 1%-2% weight loss at 12 years, a 26% incidence of diabetes, and only a 5%-10% rate of diabetes remission, said Ted D. Adams, PhD, of the University of Utah, Salt Lake City, and his associates. RYGB surgery also conferred substantial and statistically significant improvements long-term improvements in systolic hypertension and lipid levels, the researchers reported in the New England Journal of Medicine (2017 Sep 20. doi: 10.1056/NEJMoa1700459).
“The follow-up rate exceeded 90% at 12 years,” the researchers wrote. Two years after undergoing Roux-en-Y gastric bypass, patients had lost an average of 45 kg (95% confidence interval, 43-47 kg). By postoperative year 6, they had regained an average of 9 kg (average loss from baseline, 36 kg; 95% CI, 34-39 kg). But they typically gained only about 1.3 kg more between years 6 and 12, and they had about a 92% lower odds of developing diabetes mellitus, compared with individuals who did not undergo bariatric surgery (odds ratio, 0.08; P less than .001). “Remission of type 2 diabetes was much more likely if the Roux-en-Y gastric bypass occurred before [patients began] treatment with insulin, presumably owing to the ability of partially viable beta cells to improve their function,” the researchers noted.
Funders included the National Institute of Diabetes and Digestive and Kidney Diseases, the National Center for Research Resources, Weill Cornell Medicine, and Intermountain Healthcare. Dr. Adams reported having no relevant conflicts of interest. One coinvestigator disclosed royalties from licensing a questionnaire on weight loss and quality of life, and another coinvestigator disclosed fees for services rendered during a trial of an intragastric balloon. The remaining researchers had no relevant disclosures.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Roux-en-Y gastric bypass produced durable results on numerous clinical outcome measures.
Major finding: Twelve years after surgery, RYGB patients averaged a 27% weight loss from baseline, with a 51% rate of remission and a 3% incidence of type 2 diabetes mellitus.
Data source: A prospective study of 1,156 severely obese individuals, of whom 418 underwent Roux-en-Y gastric bypass.
Disclosures: Funders included the National Institute of Diabetes and Digestive and Kidney Diseases, the National Center for Research Resources, Weill Cornell Medicine, and Intermountain Healthcare. Dr. Adams reported having no relevant conflicts of interest. One coinvestigator disclosed royalties from licensing a questionnaire on weight loss and the quality of life, and another coinvestigator disclosed fees for services rendered during a trial of an intragastric balloon. The remaining researchers had no relevant disclosures.
Readmission rates linked to hospital quality measures
Poorer-performing hospitals have higher readmission rates than better-performing hospitals for patients with similar diagnoses, a study shows.
Lead author Harlan M. Krumholz, MD, of Yale University, New Haven, Conn., and his colleagues analyzed Centers for Medicare and Medicaid Services hospital-wide readmission data and divided data from July 2014 through June 2015 into two random samples. Researchers used the first sample to calculate the risk-standardized readmission rate within 30 days for each hospital and classified hospitals into performance quartiles, with a lower readmission rate indicating better performance. The second study sample included patients who had two admissions for similar diagnoses at different hospitals that occurred more than 1 month and less than 1 year apart. Researchers compared the observed readmission rates among patients who had been admitted to hospitals in different performance quartiles. The analysis included all discharges occurring from July 1, 2014, through June 30, 2015, from short-term acute care or critical access hospitals in the United States involving Medicare patients who were aged 65 years or older.
Results found that among the patients hospitalized more than once for similar diagnoses at different hospitals, the readmission rate was significantly higher among patients admitted to the worst-performing quartile of hospitals than among those admitted to the best-performing quartile (absolute difference in readmission rate, 2.0 percentage points; 95% confidence interval, 0.4-3.5; P = .001) (N Engl J Med. 2017. doi: 10.1056/NEJMsa1702321). The differences in the comparisons of the other quartiles were smaller and not significant, according to the study. 
The findings suggest that hospital quality contributes at least in part to readmission rates, independent of patient factors, study authors concluded.
“This study addresses a persistent concern that national readmission measures may reflect differences in unmeasured factors rather than in hospital performance,” study authors noted in the study. “The findings suggest that hospital quality contributes at least in part to readmission rates, independent of patient factors. By studying patients who were admitted twice within 1 year with similar diagnoses to different hospitals, this study design was able to isolate hospital signals of performance while minimizing differences among the patients. In these cases, because the same patients had similar admissions at two hospitals, the characteristics of the patients, including their level of social disadvantage, level of education, or degree of underlying illness, were broadly the same. The alignment of the differences that we observed with the results of the CMS hospital-wide readmission measure also adds to evidence that the readmission measure classifies true differences in performance.”
Dr. Krumholz and seven coauthors reported receiving support from contracts with the Center for Medicare and Medicaid Services to develop and reevaluate performance measures that are used for public reporting.
[email protected]
On Twitter @legal_med
Poorer-performing hospitals have higher readmission rates than better-performing hospitals for patients with similar diagnoses, a study shows.
Lead author Harlan M. Krumholz, MD, of Yale University, New Haven, Conn., and his colleagues analyzed Centers for Medicare and Medicaid Services hospital-wide readmission data and divided data from July 2014 through June 2015 into two random samples. Researchers used the first sample to calculate the risk-standardized readmission rate within 30 days for each hospital and classified hospitals into performance quartiles, with a lower readmission rate indicating better performance. The second study sample included patients who had two admissions for similar diagnoses at different hospitals that occurred more than 1 month and less than 1 year apart. Researchers compared the observed readmission rates among patients who had been admitted to hospitals in different performance quartiles. The analysis included all discharges occurring from July 1, 2014, through June 30, 2015, from short-term acute care or critical access hospitals in the United States involving Medicare patients who were aged 65 years or older.
Results found that among the patients hospitalized more than once for similar diagnoses at different hospitals, the readmission rate was significantly higher among patients admitted to the worst-performing quartile of hospitals than among those admitted to the best-performing quartile (absolute difference in readmission rate, 2.0 percentage points; 95% confidence interval, 0.4-3.5; P = .001) (N Engl J Med. 2017. doi: 10.1056/NEJMsa1702321). The differences in the comparisons of the other quartiles were smaller and not significant, according to the study. 
The findings suggest that hospital quality contributes at least in part to readmission rates, independent of patient factors, study authors concluded.
“This study addresses a persistent concern that national readmission measures may reflect differences in unmeasured factors rather than in hospital performance,” study authors noted in the study. “The findings suggest that hospital quality contributes at least in part to readmission rates, independent of patient factors. By studying patients who were admitted twice within 1 year with similar diagnoses to different hospitals, this study design was able to isolate hospital signals of performance while minimizing differences among the patients. In these cases, because the same patients had similar admissions at two hospitals, the characteristics of the patients, including their level of social disadvantage, level of education, or degree of underlying illness, were broadly the same. The alignment of the differences that we observed with the results of the CMS hospital-wide readmission measure also adds to evidence that the readmission measure classifies true differences in performance.”
Dr. Krumholz and seven coauthors reported receiving support from contracts with the Center for Medicare and Medicaid Services to develop and reevaluate performance measures that are used for public reporting.
[email protected]
On Twitter @legal_med
Poorer-performing hospitals have higher readmission rates than better-performing hospitals for patients with similar diagnoses, a study shows.
Lead author Harlan M. Krumholz, MD, of Yale University, New Haven, Conn., and his colleagues analyzed Centers for Medicare and Medicaid Services hospital-wide readmission data and divided data from July 2014 through June 2015 into two random samples. Researchers used the first sample to calculate the risk-standardized readmission rate within 30 days for each hospital and classified hospitals into performance quartiles, with a lower readmission rate indicating better performance. The second study sample included patients who had two admissions for similar diagnoses at different hospitals that occurred more than 1 month and less than 1 year apart. Researchers compared the observed readmission rates among patients who had been admitted to hospitals in different performance quartiles. The analysis included all discharges occurring from July 1, 2014, through June 30, 2015, from short-term acute care or critical access hospitals in the United States involving Medicare patients who were aged 65 years or older.
Results found that among the patients hospitalized more than once for similar diagnoses at different hospitals, the readmission rate was significantly higher among patients admitted to the worst-performing quartile of hospitals than among those admitted to the best-performing quartile (absolute difference in readmission rate, 2.0 percentage points; 95% confidence interval, 0.4-3.5; P = .001) (N Engl J Med. 2017. doi: 10.1056/NEJMsa1702321). The differences in the comparisons of the other quartiles were smaller and not significant, according to the study. 
The findings suggest that hospital quality contributes at least in part to readmission rates, independent of patient factors, study authors concluded.
“This study addresses a persistent concern that national readmission measures may reflect differences in unmeasured factors rather than in hospital performance,” study authors noted in the study. “The findings suggest that hospital quality contributes at least in part to readmission rates, independent of patient factors. By studying patients who were admitted twice within 1 year with similar diagnoses to different hospitals, this study design was able to isolate hospital signals of performance while minimizing differences among the patients. In these cases, because the same patients had similar admissions at two hospitals, the characteristics of the patients, including their level of social disadvantage, level of education, or degree of underlying illness, were broadly the same. The alignment of the differences that we observed with the results of the CMS hospital-wide readmission measure also adds to evidence that the readmission measure classifies true differences in performance.”
Dr. Krumholz and seven coauthors reported receiving support from contracts with the Center for Medicare and Medicaid Services to develop and reevaluate performance measures that are used for public reporting.
[email protected]
On Twitter @legal_med
FROM NEJM
Key clinical point:
Major finding: The readmission rate was significantly higher among patients admitted to the worst-performing quartile of hospitals than among those admitted to the best-performing quartile (absolute difference in readmission rate, 2.0 percentage points).
Data source: Analysis of Centers for Medicare and Medicaid Services hospital-wide readmission data from July 2014 through June 2015.
Disclosures: Dr. Krumholz and seven coauthors reported receiving support from contracts with the Center for Medicare and Medicaid Services to develop and reevaluate performance measures that are used for public reporting.
Assessment of Free Flap Breast Reconstructions
Free flap autologous breast reconstruction is an excellent surgical option for breast reconstruction in select patients. A free flap involves moving skin, fat, and/or muscle from a distant part of the body, based on a named blood supply (pedicle), and attaching it to another blood supply adjacent to the acquired defect. This procedure is particularly useful in areas where local tissue supply is lacking in volume or is damaged due to trauma or radiation. These reconstructions are performed largely in high-volume centers outside the VA because of the required specialized level of surgical training, manpower, and nursing support.1 The Malcom Randall VAMC in Gainesville, Florida, started offering autologous free flap breast reconstruction as an option to select patients in October 2012.
The Malcom Randall VAMC operating room (OR) does not operate 24/7, and the system has limited available OR time and surgical staff compared with the volume of patients requesting care.2 Operative planning for free flap autologous breast reconstruction must occur months ahead of surgery to balance the system limitations with the ability to offer the highest level of care. Planning includes strict patient selection, preoperative imaging, practice runs with OR staff, use of venous couplers, and frequent intensive care unit (ICU) staff in-services. Planning also includes the need to keep surgeries within the allocated OR time to avoid shift changes during critical periods. Frequent and early communication occurs between the surgical scheduler, OR nurses, and the anesthesia and critical care teams.
Studies have found that the best chance of flap salvage in the event of a thrombotic event is a rapid return to the OR.3 It is essential to minimize the risk of emergent returns to the OR because it is not staffed throughout the night. Patient risk factors for perioperative vascular complications include hypercoagulable disorders, peripheral vascular disease, use of the superficial epigastric system, and smoking.4-7
A PubMed search for free flap reconstruction solely within the VA over the past 20 years found 1 article discussing the use of free flaps in head and neck reconstruction which demonstrated an impressive success rate of 93%.8
The object of this study was to assess free flap breast reconstruction results at the Malcolm Randall VAMC to determine whether it is a realistic treatment to offer in the federal system.
Methods
The Malcolm Randall Institutional Review Board approved a retrospective chart review of all autologous free flap breast reconstructions using CPT code 19364, performed from October 2012 to June 2016. Medical records of patients who had a free flap breast reconstruction were queried during that period. Patient age; comorbidities listed on the electronic medical record “problem list;” body mass index (BMI); type of reconstruction (delayed vs immediate); length of surgery; length of stay; and complications over a 30-day period were recorded (Table). The authors looked for documentation of preoperative imaging and unplanned returns to the OR within the 30-day period.
Of 3 full-time VA plastic surgeons on staff during the study period, 2 surgeons had advanced fellowship training in either microsurgery or hand and microsurgery. Plastic surgery fellows and general surgery interns participated in the surgeries and postoperative care. The service had 1 dedicated advanced practice registered nurse involved in the surgical scheduling and perioperative care.
Results
A total of 11 abdominally based free flap breast reconstructions—6 muscle-sparing transverse rectus abdominus musculocutaneous (TRAM) and 5 deep inferior epigastric perforator (DIEP) flaps—were performed in 8 patients during the study period (Figures 1A, 1B, 1C, and 1D). Patient ages ranged from 31 to 58 years with a mean of 45.6 years. Six patients had preoperative computer tomography angiography (CTA) to define the location of the abdominal wall perforators. One muscle-sparing free flap was performed immediately after mastectomy; the other free flaps were performed as delayed reconstructions. Body mass index ranged from 24 to 35, with a mean of 30. All patients reported no tobacco use during the consultation; however, 1 patient later admitted to chewing tobacco. No urinary cotinine confirmation was requested. Two patients had 1 free flap reconstruction and 1 pedicle TRAM. This bilateral combination has been recently described in the literature and was chosen as a reasonable option to balance limited resources with abdominal wall morbidity.9 Operating room time ranged from 7 hours 50 minutes to 13 hours 3 minutes. All patients went to the ICU for hourly flap monitoring.
Length of stay ranged from 4 to 7 days, with a mean of 4.5 days. The longest stay was for a patient who had immediate reconstruction using a pedicle TRAM and muscle-sparing free TRAM. She was not a DIEP candidate because poor perforator quality had been noted during preoperative imaging.
Six patients had documentation of postoperative wound complications. One patient returned to the OR on the elective schedule 3 weeks postoperatively for a partial flap debridement. Her tissue transfer was > 1,000 g, and she required a matching reduction on the other side. There were no complete flap losses or postoperative thrombotic events; no cases went back to the OR emergently.
Discussion
With the number of women veterans steadily increasing, the number of patients in need of breast cancer surgery, including reconstruction, will rise in the VA.10 Fortunately, breast reconstruction is an elective procedure. Immediate breast reconstruction is a popular option because patients can combine surgeries and potentially avoid 2 recovery periods, and a better aesthetic outcome is possible because the skin does not have time to contract. Although immediate reconstruction has been increasing in popularity, it is associated with a higher complication rate.11 Further, reconstruction can be jeopardized if the oncologic plan is changed in the early postoperative period.
Positive margins found after an autologous reconstruction result in a more complicated postoperative course and a higher rate of wound complications.12 Unexpected radiation therapy after autologous reconstruction can severely distort a tissue flap because of fat necrosis, fibrosis, and contraction.13,14 From a practical perspective in the federal system, it is very difficult to coordinate 2 surgeons’ schedules when the system is already struggling to keep up with demand. Splitting the ablative and reconstructive surgery allows the urgent problem (cancer) to be addressed first, ensuring clear margins and allowing the patient to recover and consider all reconstructive options without feeling time pressure.
A large tertiary care center will have staff and equipment redundancy, but this study had to consider limitations in resources. The preoperative lead time allows the ICU to arrange a bed for hourly flap checks and for in-servicing new nursing staff on free flap monitoring. This was well received, and patients gave positive feedback on the staff. The OR schedulers can schedule nurses and techs who are familiar with the microscope and microsurgery instruments. The micro sets were opened, and the microscope powered on for practice runs a week before the procedures to insure no broken or missing instruments.
High-procedure volume would logically improve efficiency. Although the VA is not likely to become a tertiary center for breast reconstruction, the findings of other high-volume microsurgeons can be applied to improve speed and limit complications. Efforts to limit the OR time included use of preoperative imaging and intraoperative venous couplers. Venous couplers can result in shorter OR time, fewer returns to the OR, and excellent patency rates.15,16 One microsurgeon performed his surgery using only loupe-assisted vision (x 3.5), without use of the microscope. Pannucci and colleagues have recommended this as a way to improve access and OR efficiency.17 Use of the CTA has been found to decrease the rate of partial flap necrosis and improve speed of surgery.18-20
Careful patient selection allowed a hospital stay that averaged 4.5 days and minimized risks for return to the OR. Only patients who were nonsmokers were offered the surgery. Average BMI was 30 to prevent the known operative risks in breast surgery patients who are morbidly obese.21-23 No patients had a history of thromboembolic disease. Most patients were discharged home from the ICU. They eventually returned for elective revisions, second stages, and balancing procedures.
Conclusion
Free flap breast reconstruction can be offered as a treatment option with appropriate patient selection and planning. The most efficient way to provide this procedure within the federal system and to minimize the risk of flap loss and complications is by offering delayed reconstruction, obtaining preoperative CTA imaging, utilizing venous couplers, and frequently communicating with all involved practitioners from the OR to the ICU. This small study provides a good starting point to illustrate that tertiary-care reconstructive surgery can be offered to veterans within the federal system.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System, Gainesville, Florida.
1. Tuggle CT, Patel A, Broer N, Persing JA, Sosa JA, Au AF. Increased hospital volume is associated with improved outcomes following abdominal-based breast reconstruction. J Plast Surg Hand Surg. 2014;48(6):382-388.
2. Shulkin DJ. Beyond the VA crisis — becoming a high-performance network. N Engl J Med. 2016;374(11):1003-1005.
3. Novakovic D, Patel RS, Goldstein DP, Gullane PJ. Salvage of failed free flaps used in head and neck reconstruction. Head Neck Oncol. 2009;1:33.
4. Davison SP, Kessler CM, Al-Attar A. Microvascular free flap failure caused by unrecognized hypercoagulability. Plast Reconstr Surg. 2009;124(2):490-495.
5. Masoomi H, Clark EG, Paydar KZ, et al. Predictive risk factors of free flap thrombosis in breast reconstructive surgery. Microsurgery. 2014;34(8):589-594.
6. O’Neill AC, Haykal S, Bagher S, Zhong T, Hofer S. Predictors and consequences of intraoperative microvascular problems in autologous breast reconstruction. J Plast Reconstr Aesthet Surg. 2016;69(10):1349-1355.
7. Sanati-Mehrizy P, Massengburg BB, Rozehnal JM, Ignargiola MJ, Hernandez Rosa J, Taub PJ. Risk factors leading to free flap failure: analysis from the national surgical quality improvement program database. J Craniofac Surg. 2016;27(8):1956-1964.
8. Myers LL, Sumer BD, Defatta RJ, Minhajuddin A. Free tissue transfer reconstruction of the head and neck at a Veterans Affairs hospital. Head Neck. 2008;30(8):1007-1011.
9. Roslan EJ, Kelly EG, Zain MA, Basiron NH, Imran FH. Immediate simultaneous bilateral breast reconstruction with deep inferior epigastric (DIEP) flap and pedicled transverse rectus abdominis musculocutaneous (TRAM) pedicle flap. Med J Malaysia. 2017;72(1):85-87.
10. Leong M, Chike-Obi CJ, Basu CB, Lee EL, Albo D, Netscher DT. Effective breast reconstruction in female veterans. Am J Surg. 2009;198(5):658-663.
11. Kwok AC, Goodwin IA, Ying J, Agarwal JP. National trends and complication rates after bilateral mastectomy and immediate breast reconstruction from 2005 to 2012. Am J Surg. 2015;210(3):512-516.
12. Ochoa O, Theoharis C, Pisano S, et al. Positive margin re-excision following immediate autologous breast reconstruction: morbidity, cosmetic outcome, and oncologic significance. Aesthet Surg J. 2017; [Epub ahead of print.]
13. Garvey PB, Clemens MW, Hoy AE, et al. Muscle-sparing TRAM flap does not protect breast reconstruction from post-mastectomy radiation damage compared to DIEP flap. Plast Reconstr Surg. 2014;133(2):223-233.
14. Kronowitz SJ. Current status of autologous tissue-based breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg. 2012;130(2):282-292.
15. Fitzgerald O’Connor E, Rozen WM, Chowdhry M, et al. The microvascular anastomotic coupler for venous anastomoses in free flap breast reconstruction improves outcomes. Gland Surg. 2016;5(2):88-92.
16. Jandali S, Wu LC, Vega SJ, Kovach SJ, Serletti JM. 1000 consecutive venous anastomoses using the microvascular anastomotic coupler in breast reconstruction. Plast Reconstr Surg. 2010;125(3):792-798.
17. Pannucci CJ, Basta MN, Kovach SJ, Kanchwala SK, Wu LC, Serletti JM. Loupes-only microsurgery is a safe alternative to the operating microscope: an analysis of 1,649 consecutive free flap breast reconstruction. J Reconstr Microsurg. 2015;31(9):636-642.
18. Teunis T, Heerma van Voss MR, Kon M, van Maurik JF. CT-angiography prior to DIEP flap reconstruction: a systemic review and meta-analysis. Microsurgery. 2013;33(6):496-502.
19. Fitzgerald O’Connor E, Rozen WM, Chowdhry M, Band B, Ramakrishnan VV, Griffiths M. Preoperative computed tomography angiography for planning DIEP flap breast reconstruction reduces operative time and overall complications. Gland Surgery. 2016;5(2):93-98.
20. Malhotra A, Chhaya N, Nsiah-Sarbeng P, Mosahebi A. CT-guided deep inferior epigastric perforator (DIEP) flap localization—better for the patient, the surgeon, and the hospital. Clin Radiol. 2013;68(2):131-138.
21. Ilonzo N, Tsang A, Tsantes S, Estabrook A, Thu Ma AM. Breast reconstruction after mastectomy: a ten-year analysis of trends and immediate postoperative outcomes. Breast. 2017;32:7-12.
22. McAllister P, Teo L, Chin K, Makubate B, Alexander Munnoch D. Bilateral breast reconstruction with abdominal free flaps: a single centre, single surgeon retrospective review of 55 consecutive patients. Plast Surg Int. 2016;2016:6085624.
23. Myung Y, Heo CY. Relationship between obesity and surgical complications after reduction mammoplasty: a systemic literature review and meta-analysis. Aesthet Surg J. 2017;37(3):308-315.
Free flap autologous breast reconstruction is an excellent surgical option for breast reconstruction in select patients. A free flap involves moving skin, fat, and/or muscle from a distant part of the body, based on a named blood supply (pedicle), and attaching it to another blood supply adjacent to the acquired defect. This procedure is particularly useful in areas where local tissue supply is lacking in volume or is damaged due to trauma or radiation. These reconstructions are performed largely in high-volume centers outside the VA because of the required specialized level of surgical training, manpower, and nursing support.1 The Malcom Randall VAMC in Gainesville, Florida, started offering autologous free flap breast reconstruction as an option to select patients in October 2012.
The Malcom Randall VAMC operating room (OR) does not operate 24/7, and the system has limited available OR time and surgical staff compared with the volume of patients requesting care.2 Operative planning for free flap autologous breast reconstruction must occur months ahead of surgery to balance the system limitations with the ability to offer the highest level of care. Planning includes strict patient selection, preoperative imaging, practice runs with OR staff, use of venous couplers, and frequent intensive care unit (ICU) staff in-services. Planning also includes the need to keep surgeries within the allocated OR time to avoid shift changes during critical periods. Frequent and early communication occurs between the surgical scheduler, OR nurses, and the anesthesia and critical care teams.
Studies have found that the best chance of flap salvage in the event of a thrombotic event is a rapid return to the OR.3 It is essential to minimize the risk of emergent returns to the OR because it is not staffed throughout the night. Patient risk factors for perioperative vascular complications include hypercoagulable disorders, peripheral vascular disease, use of the superficial epigastric system, and smoking.4-7
A PubMed search for free flap reconstruction solely within the VA over the past 20 years found 1 article discussing the use of free flaps in head and neck reconstruction which demonstrated an impressive success rate of 93%.8
The object of this study was to assess free flap breast reconstruction results at the Malcolm Randall VAMC to determine whether it is a realistic treatment to offer in the federal system.
Methods
The Malcolm Randall Institutional Review Board approved a retrospective chart review of all autologous free flap breast reconstructions using CPT code 19364, performed from October 2012 to June 2016. Medical records of patients who had a free flap breast reconstruction were queried during that period. Patient age; comorbidities listed on the electronic medical record “problem list;” body mass index (BMI); type of reconstruction (delayed vs immediate); length of surgery; length of stay; and complications over a 30-day period were recorded (Table). The authors looked for documentation of preoperative imaging and unplanned returns to the OR within the 30-day period.
Of 3 full-time VA plastic surgeons on staff during the study period, 2 surgeons had advanced fellowship training in either microsurgery or hand and microsurgery. Plastic surgery fellows and general surgery interns participated in the surgeries and postoperative care. The service had 1 dedicated advanced practice registered nurse involved in the surgical scheduling and perioperative care.
Results
A total of 11 abdominally based free flap breast reconstructions—6 muscle-sparing transverse rectus abdominus musculocutaneous (TRAM) and 5 deep inferior epigastric perforator (DIEP) flaps—were performed in 8 patients during the study period (Figures 1A, 1B, 1C, and 1D). Patient ages ranged from 31 to 58 years with a mean of 45.6 years. Six patients had preoperative computer tomography angiography (CTA) to define the location of the abdominal wall perforators. One muscle-sparing free flap was performed immediately after mastectomy; the other free flaps were performed as delayed reconstructions. Body mass index ranged from 24 to 35, with a mean of 30. All patients reported no tobacco use during the consultation; however, 1 patient later admitted to chewing tobacco. No urinary cotinine confirmation was requested. Two patients had 1 free flap reconstruction and 1 pedicle TRAM. This bilateral combination has been recently described in the literature and was chosen as a reasonable option to balance limited resources with abdominal wall morbidity.9 Operating room time ranged from 7 hours 50 minutes to 13 hours 3 minutes. All patients went to the ICU for hourly flap monitoring.
Length of stay ranged from 4 to 7 days, with a mean of 4.5 days. The longest stay was for a patient who had immediate reconstruction using a pedicle TRAM and muscle-sparing free TRAM. She was not a DIEP candidate because poor perforator quality had been noted during preoperative imaging.
Six patients had documentation of postoperative wound complications. One patient returned to the OR on the elective schedule 3 weeks postoperatively for a partial flap debridement. Her tissue transfer was > 1,000 g, and she required a matching reduction on the other side. There were no complete flap losses or postoperative thrombotic events; no cases went back to the OR emergently.
Discussion
With the number of women veterans steadily increasing, the number of patients in need of breast cancer surgery, including reconstruction, will rise in the VA.10 Fortunately, breast reconstruction is an elective procedure. Immediate breast reconstruction is a popular option because patients can combine surgeries and potentially avoid 2 recovery periods, and a better aesthetic outcome is possible because the skin does not have time to contract. Although immediate reconstruction has been increasing in popularity, it is associated with a higher complication rate.11 Further, reconstruction can be jeopardized if the oncologic plan is changed in the early postoperative period.
Positive margins found after an autologous reconstruction result in a more complicated postoperative course and a higher rate of wound complications.12 Unexpected radiation therapy after autologous reconstruction can severely distort a tissue flap because of fat necrosis, fibrosis, and contraction.13,14 From a practical perspective in the federal system, it is very difficult to coordinate 2 surgeons’ schedules when the system is already struggling to keep up with demand. Splitting the ablative and reconstructive surgery allows the urgent problem (cancer) to be addressed first, ensuring clear margins and allowing the patient to recover and consider all reconstructive options without feeling time pressure.
A large tertiary care center will have staff and equipment redundancy, but this study had to consider limitations in resources. The preoperative lead time allows the ICU to arrange a bed for hourly flap checks and for in-servicing new nursing staff on free flap monitoring. This was well received, and patients gave positive feedback on the staff. The OR schedulers can schedule nurses and techs who are familiar with the microscope and microsurgery instruments. The micro sets were opened, and the microscope powered on for practice runs a week before the procedures to insure no broken or missing instruments.
High-procedure volume would logically improve efficiency. Although the VA is not likely to become a tertiary center for breast reconstruction, the findings of other high-volume microsurgeons can be applied to improve speed and limit complications. Efforts to limit the OR time included use of preoperative imaging and intraoperative venous couplers. Venous couplers can result in shorter OR time, fewer returns to the OR, and excellent patency rates.15,16 One microsurgeon performed his surgery using only loupe-assisted vision (x 3.5), without use of the microscope. Pannucci and colleagues have recommended this as a way to improve access and OR efficiency.17 Use of the CTA has been found to decrease the rate of partial flap necrosis and improve speed of surgery.18-20
Careful patient selection allowed a hospital stay that averaged 4.5 days and minimized risks for return to the OR. Only patients who were nonsmokers were offered the surgery. Average BMI was 30 to prevent the known operative risks in breast surgery patients who are morbidly obese.21-23 No patients had a history of thromboembolic disease. Most patients were discharged home from the ICU. They eventually returned for elective revisions, second stages, and balancing procedures.
Conclusion
Free flap breast reconstruction can be offered as a treatment option with appropriate patient selection and planning. The most efficient way to provide this procedure within the federal system and to minimize the risk of flap loss and complications is by offering delayed reconstruction, obtaining preoperative CTA imaging, utilizing venous couplers, and frequently communicating with all involved practitioners from the OR to the ICU. This small study provides a good starting point to illustrate that tertiary-care reconstructive surgery can be offered to veterans within the federal system.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System, Gainesville, Florida.
Free flap autologous breast reconstruction is an excellent surgical option for breast reconstruction in select patients. A free flap involves moving skin, fat, and/or muscle from a distant part of the body, based on a named blood supply (pedicle), and attaching it to another blood supply adjacent to the acquired defect. This procedure is particularly useful in areas where local tissue supply is lacking in volume or is damaged due to trauma or radiation. These reconstructions are performed largely in high-volume centers outside the VA because of the required specialized level of surgical training, manpower, and nursing support.1 The Malcom Randall VAMC in Gainesville, Florida, started offering autologous free flap breast reconstruction as an option to select patients in October 2012.
The Malcom Randall VAMC operating room (OR) does not operate 24/7, and the system has limited available OR time and surgical staff compared with the volume of patients requesting care.2 Operative planning for free flap autologous breast reconstruction must occur months ahead of surgery to balance the system limitations with the ability to offer the highest level of care. Planning includes strict patient selection, preoperative imaging, practice runs with OR staff, use of venous couplers, and frequent intensive care unit (ICU) staff in-services. Planning also includes the need to keep surgeries within the allocated OR time to avoid shift changes during critical periods. Frequent and early communication occurs between the surgical scheduler, OR nurses, and the anesthesia and critical care teams.
Studies have found that the best chance of flap salvage in the event of a thrombotic event is a rapid return to the OR.3 It is essential to minimize the risk of emergent returns to the OR because it is not staffed throughout the night. Patient risk factors for perioperative vascular complications include hypercoagulable disorders, peripheral vascular disease, use of the superficial epigastric system, and smoking.4-7
A PubMed search for free flap reconstruction solely within the VA over the past 20 years found 1 article discussing the use of free flaps in head and neck reconstruction which demonstrated an impressive success rate of 93%.8
The object of this study was to assess free flap breast reconstruction results at the Malcolm Randall VAMC to determine whether it is a realistic treatment to offer in the federal system.
Methods
The Malcolm Randall Institutional Review Board approved a retrospective chart review of all autologous free flap breast reconstructions using CPT code 19364, performed from October 2012 to June 2016. Medical records of patients who had a free flap breast reconstruction were queried during that period. Patient age; comorbidities listed on the electronic medical record “problem list;” body mass index (BMI); type of reconstruction (delayed vs immediate); length of surgery; length of stay; and complications over a 30-day period were recorded (Table). The authors looked for documentation of preoperative imaging and unplanned returns to the OR within the 30-day period.
Of 3 full-time VA plastic surgeons on staff during the study period, 2 surgeons had advanced fellowship training in either microsurgery or hand and microsurgery. Plastic surgery fellows and general surgery interns participated in the surgeries and postoperative care. The service had 1 dedicated advanced practice registered nurse involved in the surgical scheduling and perioperative care.
Results
A total of 11 abdominally based free flap breast reconstructions—6 muscle-sparing transverse rectus abdominus musculocutaneous (TRAM) and 5 deep inferior epigastric perforator (DIEP) flaps—were performed in 8 patients during the study period (Figures 1A, 1B, 1C, and 1D). Patient ages ranged from 31 to 58 years with a mean of 45.6 years. Six patients had preoperative computer tomography angiography (CTA) to define the location of the abdominal wall perforators. One muscle-sparing free flap was performed immediately after mastectomy; the other free flaps were performed as delayed reconstructions. Body mass index ranged from 24 to 35, with a mean of 30. All patients reported no tobacco use during the consultation; however, 1 patient later admitted to chewing tobacco. No urinary cotinine confirmation was requested. Two patients had 1 free flap reconstruction and 1 pedicle TRAM. This bilateral combination has been recently described in the literature and was chosen as a reasonable option to balance limited resources with abdominal wall morbidity.9 Operating room time ranged from 7 hours 50 minutes to 13 hours 3 minutes. All patients went to the ICU for hourly flap monitoring.
Length of stay ranged from 4 to 7 days, with a mean of 4.5 days. The longest stay was for a patient who had immediate reconstruction using a pedicle TRAM and muscle-sparing free TRAM. She was not a DIEP candidate because poor perforator quality had been noted during preoperative imaging.
Six patients had documentation of postoperative wound complications. One patient returned to the OR on the elective schedule 3 weeks postoperatively for a partial flap debridement. Her tissue transfer was > 1,000 g, and she required a matching reduction on the other side. There were no complete flap losses or postoperative thrombotic events; no cases went back to the OR emergently.
Discussion
With the number of women veterans steadily increasing, the number of patients in need of breast cancer surgery, including reconstruction, will rise in the VA.10 Fortunately, breast reconstruction is an elective procedure. Immediate breast reconstruction is a popular option because patients can combine surgeries and potentially avoid 2 recovery periods, and a better aesthetic outcome is possible because the skin does not have time to contract. Although immediate reconstruction has been increasing in popularity, it is associated with a higher complication rate.11 Further, reconstruction can be jeopardized if the oncologic plan is changed in the early postoperative period.
Positive margins found after an autologous reconstruction result in a more complicated postoperative course and a higher rate of wound complications.12 Unexpected radiation therapy after autologous reconstruction can severely distort a tissue flap because of fat necrosis, fibrosis, and contraction.13,14 From a practical perspective in the federal system, it is very difficult to coordinate 2 surgeons’ schedules when the system is already struggling to keep up with demand. Splitting the ablative and reconstructive surgery allows the urgent problem (cancer) to be addressed first, ensuring clear margins and allowing the patient to recover and consider all reconstructive options without feeling time pressure.
A large tertiary care center will have staff and equipment redundancy, but this study had to consider limitations in resources. The preoperative lead time allows the ICU to arrange a bed for hourly flap checks and for in-servicing new nursing staff on free flap monitoring. This was well received, and patients gave positive feedback on the staff. The OR schedulers can schedule nurses and techs who are familiar with the microscope and microsurgery instruments. The micro sets were opened, and the microscope powered on for practice runs a week before the procedures to insure no broken or missing instruments.
High-procedure volume would logically improve efficiency. Although the VA is not likely to become a tertiary center for breast reconstruction, the findings of other high-volume microsurgeons can be applied to improve speed and limit complications. Efforts to limit the OR time included use of preoperative imaging and intraoperative venous couplers. Venous couplers can result in shorter OR time, fewer returns to the OR, and excellent patency rates.15,16 One microsurgeon performed his surgery using only loupe-assisted vision (x 3.5), without use of the microscope. Pannucci and colleagues have recommended this as a way to improve access and OR efficiency.17 Use of the CTA has been found to decrease the rate of partial flap necrosis and improve speed of surgery.18-20
Careful patient selection allowed a hospital stay that averaged 4.5 days and minimized risks for return to the OR. Only patients who were nonsmokers were offered the surgery. Average BMI was 30 to prevent the known operative risks in breast surgery patients who are morbidly obese.21-23 No patients had a history of thromboembolic disease. Most patients were discharged home from the ICU. They eventually returned for elective revisions, second stages, and balancing procedures.
Conclusion
Free flap breast reconstruction can be offered as a treatment option with appropriate patient selection and planning. The most efficient way to provide this procedure within the federal system and to minimize the risk of flap loss and complications is by offering delayed reconstruction, obtaining preoperative CTA imaging, utilizing venous couplers, and frequently communicating with all involved practitioners from the OR to the ICU. This small study provides a good starting point to illustrate that tertiary-care reconstructive surgery can be offered to veterans within the federal system.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System, Gainesville, Florida.
1. Tuggle CT, Patel A, Broer N, Persing JA, Sosa JA, Au AF. Increased hospital volume is associated with improved outcomes following abdominal-based breast reconstruction. J Plast Surg Hand Surg. 2014;48(6):382-388.
2. Shulkin DJ. Beyond the VA crisis — becoming a high-performance network. N Engl J Med. 2016;374(11):1003-1005.
3. Novakovic D, Patel RS, Goldstein DP, Gullane PJ. Salvage of failed free flaps used in head and neck reconstruction. Head Neck Oncol. 2009;1:33.
4. Davison SP, Kessler CM, Al-Attar A. Microvascular free flap failure caused by unrecognized hypercoagulability. Plast Reconstr Surg. 2009;124(2):490-495.
5. Masoomi H, Clark EG, Paydar KZ, et al. Predictive risk factors of free flap thrombosis in breast reconstructive surgery. Microsurgery. 2014;34(8):589-594.
6. O’Neill AC, Haykal S, Bagher S, Zhong T, Hofer S. Predictors and consequences of intraoperative microvascular problems in autologous breast reconstruction. J Plast Reconstr Aesthet Surg. 2016;69(10):1349-1355.
7. Sanati-Mehrizy P, Massengburg BB, Rozehnal JM, Ignargiola MJ, Hernandez Rosa J, Taub PJ. Risk factors leading to free flap failure: analysis from the national surgical quality improvement program database. J Craniofac Surg. 2016;27(8):1956-1964.
8. Myers LL, Sumer BD, Defatta RJ, Minhajuddin A. Free tissue transfer reconstruction of the head and neck at a Veterans Affairs hospital. Head Neck. 2008;30(8):1007-1011.
9. Roslan EJ, Kelly EG, Zain MA, Basiron NH, Imran FH. Immediate simultaneous bilateral breast reconstruction with deep inferior epigastric (DIEP) flap and pedicled transverse rectus abdominis musculocutaneous (TRAM) pedicle flap. Med J Malaysia. 2017;72(1):85-87.
10. Leong M, Chike-Obi CJ, Basu CB, Lee EL, Albo D, Netscher DT. Effective breast reconstruction in female veterans. Am J Surg. 2009;198(5):658-663.
11. Kwok AC, Goodwin IA, Ying J, Agarwal JP. National trends and complication rates after bilateral mastectomy and immediate breast reconstruction from 2005 to 2012. Am J Surg. 2015;210(3):512-516.
12. Ochoa O, Theoharis C, Pisano S, et al. Positive margin re-excision following immediate autologous breast reconstruction: morbidity, cosmetic outcome, and oncologic significance. Aesthet Surg J. 2017; [Epub ahead of print.]
13. Garvey PB, Clemens MW, Hoy AE, et al. Muscle-sparing TRAM flap does not protect breast reconstruction from post-mastectomy radiation damage compared to DIEP flap. Plast Reconstr Surg. 2014;133(2):223-233.
14. Kronowitz SJ. Current status of autologous tissue-based breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg. 2012;130(2):282-292.
15. Fitzgerald O’Connor E, Rozen WM, Chowdhry M, et al. The microvascular anastomotic coupler for venous anastomoses in free flap breast reconstruction improves outcomes. Gland Surg. 2016;5(2):88-92.
16. Jandali S, Wu LC, Vega SJ, Kovach SJ, Serletti JM. 1000 consecutive venous anastomoses using the microvascular anastomotic coupler in breast reconstruction. Plast Reconstr Surg. 2010;125(3):792-798.
17. Pannucci CJ, Basta MN, Kovach SJ, Kanchwala SK, Wu LC, Serletti JM. Loupes-only microsurgery is a safe alternative to the operating microscope: an analysis of 1,649 consecutive free flap breast reconstruction. J Reconstr Microsurg. 2015;31(9):636-642.
18. Teunis T, Heerma van Voss MR, Kon M, van Maurik JF. CT-angiography prior to DIEP flap reconstruction: a systemic review and meta-analysis. Microsurgery. 2013;33(6):496-502.
19. Fitzgerald O’Connor E, Rozen WM, Chowdhry M, Band B, Ramakrishnan VV, Griffiths M. Preoperative computed tomography angiography for planning DIEP flap breast reconstruction reduces operative time and overall complications. Gland Surgery. 2016;5(2):93-98.
20. Malhotra A, Chhaya N, Nsiah-Sarbeng P, Mosahebi A. CT-guided deep inferior epigastric perforator (DIEP) flap localization—better for the patient, the surgeon, and the hospital. Clin Radiol. 2013;68(2):131-138.
21. Ilonzo N, Tsang A, Tsantes S, Estabrook A, Thu Ma AM. Breast reconstruction after mastectomy: a ten-year analysis of trends and immediate postoperative outcomes. Breast. 2017;32:7-12.
22. McAllister P, Teo L, Chin K, Makubate B, Alexander Munnoch D. Bilateral breast reconstruction with abdominal free flaps: a single centre, single surgeon retrospective review of 55 consecutive patients. Plast Surg Int. 2016;2016:6085624.
23. Myung Y, Heo CY. Relationship between obesity and surgical complications after reduction mammoplasty: a systemic literature review and meta-analysis. Aesthet Surg J. 2017;37(3):308-315.
1. Tuggle CT, Patel A, Broer N, Persing JA, Sosa JA, Au AF. Increased hospital volume is associated with improved outcomes following abdominal-based breast reconstruction. J Plast Surg Hand Surg. 2014;48(6):382-388.
2. Shulkin DJ. Beyond the VA crisis — becoming a high-performance network. N Engl J Med. 2016;374(11):1003-1005.
3. Novakovic D, Patel RS, Goldstein DP, Gullane PJ. Salvage of failed free flaps used in head and neck reconstruction. Head Neck Oncol. 2009;1:33.
4. Davison SP, Kessler CM, Al-Attar A. Microvascular free flap failure caused by unrecognized hypercoagulability. Plast Reconstr Surg. 2009;124(2):490-495.
5. Masoomi H, Clark EG, Paydar KZ, et al. Predictive risk factors of free flap thrombosis in breast reconstructive surgery. Microsurgery. 2014;34(8):589-594.
6. O’Neill AC, Haykal S, Bagher S, Zhong T, Hofer S. Predictors and consequences of intraoperative microvascular problems in autologous breast reconstruction. J Plast Reconstr Aesthet Surg. 2016;69(10):1349-1355.
7. Sanati-Mehrizy P, Massengburg BB, Rozehnal JM, Ignargiola MJ, Hernandez Rosa J, Taub PJ. Risk factors leading to free flap failure: analysis from the national surgical quality improvement program database. J Craniofac Surg. 2016;27(8):1956-1964.
8. Myers LL, Sumer BD, Defatta RJ, Minhajuddin A. Free tissue transfer reconstruction of the head and neck at a Veterans Affairs hospital. Head Neck. 2008;30(8):1007-1011.
9. Roslan EJ, Kelly EG, Zain MA, Basiron NH, Imran FH. Immediate simultaneous bilateral breast reconstruction with deep inferior epigastric (DIEP) flap and pedicled transverse rectus abdominis musculocutaneous (TRAM) pedicle flap. Med J Malaysia. 2017;72(1):85-87.
10. Leong M, Chike-Obi CJ, Basu CB, Lee EL, Albo D, Netscher DT. Effective breast reconstruction in female veterans. Am J Surg. 2009;198(5):658-663.
11. Kwok AC, Goodwin IA, Ying J, Agarwal JP. National trends and complication rates after bilateral mastectomy and immediate breast reconstruction from 2005 to 2012. Am J Surg. 2015;210(3):512-516.
12. Ochoa O, Theoharis C, Pisano S, et al. Positive margin re-excision following immediate autologous breast reconstruction: morbidity, cosmetic outcome, and oncologic significance. Aesthet Surg J. 2017; [Epub ahead of print.]
13. Garvey PB, Clemens MW, Hoy AE, et al. Muscle-sparing TRAM flap does not protect breast reconstruction from post-mastectomy radiation damage compared to DIEP flap. Plast Reconstr Surg. 2014;133(2):223-233.
14. Kronowitz SJ. Current status of autologous tissue-based breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg. 2012;130(2):282-292.
15. Fitzgerald O’Connor E, Rozen WM, Chowdhry M, et al. The microvascular anastomotic coupler for venous anastomoses in free flap breast reconstruction improves outcomes. Gland Surg. 2016;5(2):88-92.
16. Jandali S, Wu LC, Vega SJ, Kovach SJ, Serletti JM. 1000 consecutive venous anastomoses using the microvascular anastomotic coupler in breast reconstruction. Plast Reconstr Surg. 2010;125(3):792-798.
17. Pannucci CJ, Basta MN, Kovach SJ, Kanchwala SK, Wu LC, Serletti JM. Loupes-only microsurgery is a safe alternative to the operating microscope: an analysis of 1,649 consecutive free flap breast reconstruction. J Reconstr Microsurg. 2015;31(9):636-642.
18. Teunis T, Heerma van Voss MR, Kon M, van Maurik JF. CT-angiography prior to DIEP flap reconstruction: a systemic review and meta-analysis. Microsurgery. 2013;33(6):496-502.
19. Fitzgerald O’Connor E, Rozen WM, Chowdhry M, Band B, Ramakrishnan VV, Griffiths M. Preoperative computed tomography angiography for planning DIEP flap breast reconstruction reduces operative time and overall complications. Gland Surgery. 2016;5(2):93-98.
20. Malhotra A, Chhaya N, Nsiah-Sarbeng P, Mosahebi A. CT-guided deep inferior epigastric perforator (DIEP) flap localization—better for the patient, the surgeon, and the hospital. Clin Radiol. 2013;68(2):131-138.
21. Ilonzo N, Tsang A, Tsantes S, Estabrook A, Thu Ma AM. Breast reconstruction after mastectomy: a ten-year analysis of trends and immediate postoperative outcomes. Breast. 2017;32:7-12.
22. McAllister P, Teo L, Chin K, Makubate B, Alexander Munnoch D. Bilateral breast reconstruction with abdominal free flaps: a single centre, single surgeon retrospective review of 55 consecutive patients. Plast Surg Int. 2016;2016:6085624.
23. Myung Y, Heo CY. Relationship between obesity and surgical complications after reduction mammoplasty: a systemic literature review and meta-analysis. Aesthet Surg J. 2017;37(3):308-315.
ADRs highest among gastroenterologists, women, early-career physicians
Gastroenterologists, female physicians, and physicians who were less than a decade out of residency had significantly higher adenoma detection rates (ADRs) than their counterparts in a retrospective cohort study of colonoscopists.
“Efforts to target physicians with lower-quality performance are needed,” wrote Ateev Mehrotra, MD, MPH, of Harvard Medical School in Boston, with his associates. The study, one of the first to use natural language processing to compare electronic health data from geographically diverse health care systems, was published online 30 in Gastrointestinal Endoscopy (2017 Aug 30. doi: 10.1016/j.gie.2017.08.023).
These associations persisted among patients who received only screening colonoscopies, who had complete colonoscopies with adequate bowel preparation, or who were younger than 80 years, the researchers said. The findings on sex reflect recent studies in which treatment by female hospitalists slightly decreased the risk of 30-day mortality when compared with treatment by a male hospitalist, they added. “A deliberate and meticulous approach to colonoscopy may facilitate achievement of a high ADR, and this method may be more common among female physicians,” they wrote. “This is supported by research showing that female physicians are more likely to adhere to clinical guidelines and to provide preventive care.” Studies of men in other fields have found them more likely to take risks, which contradicts the methodical approach needed for a high ADR, they emphasized. “Sex differences in color perception [also] may make it easier for female physicians to identify adenomas.”
Likewise, research outside gastroenterology has linked fewer years in practice with better quality of care. Improvements in fellowship training, better access to new equipment, “or simply decay of performance with age” all could explain the findings, the researchers wrote. They also cited five prior studies in which nongastroenterologists had lower ADRs. They called for studies that would further explore the reasons why specific physician traits affect performance.
Physicians in the study tended to have practiced fewer years than gastroenterologists in general in the United States, the investigators noted. “We also could not measure some other physician factors that might explain some of the variation we observed, such as type of endoscopes used.”
The National Cancer Institute provided funding. The researchers did not report having conflicts of interest.
Gastroenterologists, female physicians, and physicians who were less than a decade out of residency had significantly higher adenoma detection rates (ADRs) than their counterparts in a retrospective cohort study of colonoscopists.
“Efforts to target physicians with lower-quality performance are needed,” wrote Ateev Mehrotra, MD, MPH, of Harvard Medical School in Boston, with his associates. The study, one of the first to use natural language processing to compare electronic health data from geographically diverse health care systems, was published online 30 in Gastrointestinal Endoscopy (2017 Aug 30. doi: 10.1016/j.gie.2017.08.023).
These associations persisted among patients who received only screening colonoscopies, who had complete colonoscopies with adequate bowel preparation, or who were younger than 80 years, the researchers said. The findings on sex reflect recent studies in which treatment by female hospitalists slightly decreased the risk of 30-day mortality when compared with treatment by a male hospitalist, they added. “A deliberate and meticulous approach to colonoscopy may facilitate achievement of a high ADR, and this method may be more common among female physicians,” they wrote. “This is supported by research showing that female physicians are more likely to adhere to clinical guidelines and to provide preventive care.” Studies of men in other fields have found them more likely to take risks, which contradicts the methodical approach needed for a high ADR, they emphasized. “Sex differences in color perception [also] may make it easier for female physicians to identify adenomas.”
Likewise, research outside gastroenterology has linked fewer years in practice with better quality of care. Improvements in fellowship training, better access to new equipment, “or simply decay of performance with age” all could explain the findings, the researchers wrote. They also cited five prior studies in which nongastroenterologists had lower ADRs. They called for studies that would further explore the reasons why specific physician traits affect performance.
Physicians in the study tended to have practiced fewer years than gastroenterologists in general in the United States, the investigators noted. “We also could not measure some other physician factors that might explain some of the variation we observed, such as type of endoscopes used.”
The National Cancer Institute provided funding. The researchers did not report having conflicts of interest.
Gastroenterologists, female physicians, and physicians who were less than a decade out of residency had significantly higher adenoma detection rates (ADRs) than their counterparts in a retrospective cohort study of colonoscopists.
“Efforts to target physicians with lower-quality performance are needed,” wrote Ateev Mehrotra, MD, MPH, of Harvard Medical School in Boston, with his associates. The study, one of the first to use natural language processing to compare electronic health data from geographically diverse health care systems, was published online 30 in Gastrointestinal Endoscopy (2017 Aug 30. doi: 10.1016/j.gie.2017.08.023).
These associations persisted among patients who received only screening colonoscopies, who had complete colonoscopies with adequate bowel preparation, or who were younger than 80 years, the researchers said. The findings on sex reflect recent studies in which treatment by female hospitalists slightly decreased the risk of 30-day mortality when compared with treatment by a male hospitalist, they added. “A deliberate and meticulous approach to colonoscopy may facilitate achievement of a high ADR, and this method may be more common among female physicians,” they wrote. “This is supported by research showing that female physicians are more likely to adhere to clinical guidelines and to provide preventive care.” Studies of men in other fields have found them more likely to take risks, which contradicts the methodical approach needed for a high ADR, they emphasized. “Sex differences in color perception [also] may make it easier for female physicians to identify adenomas.”
Likewise, research outside gastroenterology has linked fewer years in practice with better quality of care. Improvements in fellowship training, better access to new equipment, “or simply decay of performance with age” all could explain the findings, the researchers wrote. They also cited five prior studies in which nongastroenterologists had lower ADRs. They called for studies that would further explore the reasons why specific physician traits affect performance.
Physicians in the study tended to have practiced fewer years than gastroenterologists in general in the United States, the investigators noted. “We also could not measure some other physician factors that might explain some of the variation we observed, such as type of endoscopes used.”
The National Cancer Institute provided funding. The researchers did not report having conflicts of interest.
FROM GASTROINTESTINAL ENDOSCOPY
Key clinical point: Adenoma detection rates were highest among gastroenterologists, female physicians, and physicians less than a decade out of residency.
Major finding: On average, their ADRs were 9.4, 6.0, and 4.2 percentage points higher than those of their respective comparison groups, and each difference was statistically significant (all P values less than .03).
Data source: A retrospective cohort study of four diverse health systems.
Disclosures: The National Cancer Institute provided funding. The researchers did not report having conflicts of interest.
Endocrine Society updates treatment guidelines for transgender persons
Previous clinical recommendations on caring for transgender individuals have advised that hormone treatment begin no earlier than age 16 years, but
“Sixteen is the typical age cutoff in many areas of the world for some decision-making capacity from a legal perspective, but when you think about hormones and puberty, 16 is pretty late,” Joshua D. Safer, MD, one of the task force members who authored the guideline, said in an interview. “If we’re going to use biology for guidance, then hormone interventions for transgender kids should begin occurring earlier, when puberty really happens, like around age 12, 13, or 14. However, we’re in a situation where we lack a test. We can’t diagnose anybody as transgender with excellent confidence, outside of talking to those kids. When we start talking about hormone therapies, we talk about some things that will be irreversible. That’s a fraught place to go, but we recognize that people are going to treat kids under 16 in many instances.”
Over several years, Dr. Safer and nine other task force members, chaired by Wylie Hembree, MD, of the College of Physicians and Surgeons at Columbia University, New York, worked to establish a framework for the appropriate treatment of transgender individuals. The efforts of the task force were framed around a durable biological underpinning to gender identity. “That’s state of the art right now,” said Dr. Safer, who is the medical director of the Center for Transgender Medicine and Surgery at Boston University Medical Center. “People think there’s debate about whether there’s a substantial biological component. I think that the data are pretty strong, so I don’t think there’s a lot of debate about that in the scientific world. The debate is more about what that biology might be. That’s all over the map.”
That notion of a biological basis for gender identity contributed to a second major change from the Endocrine Society’s 2009 guideline, which recommended that the diagnosis of gender identity disorder be made by a mental health professional. The current guideline states that for the care of peripubertal youths and older adolescents, “we recommend that an expert multidisciplinary team comprised of medical professionals and mental health professionals manage this treatment. The treating physician must confirm the criteria for treatment used by the referring mental health practitioner and collaborate with them in decisions about gender-affirming surgery in older adolescents.” Meanwhile, for adult gender-dysphoric/gender-incongruent persons, “the treating clinicians (collectively) should have expertise in transgender-specific diagnostic criteria, mental health, primary care, hormone treatment, and surgery, as needed by the patient.” Dr. Safer described this new approach as “a major change in terms of trying to gain access to care by liberalizing the variety of those in the medical community who can be associated with the diagnosis, at least on the adult side.”
A number of associations cosponsored the guideline, including the American Association of Clinical Endocrinologists, American Society of Andrology, European Society for Paediatric Endocrinology, European Society of Endocrinology, Pediatric Endocrine Society, and World Professional Association for Transgender Health. Other key recommendations from the guideline include:
- All individuals seeking gender-affirming medical treatment should receive information and counsel on options for fertility preservation prior to initiating puberty suppression in adolescents and prior to treating with hormonal therapy of the affirmed gender in both adolescents and adults.
- Removal of gonads may be considered when high doses of sex steroids are required to suppress the body’s secretion of hormones and/or to reduce steroid levels in advanced age.
- During sex-steroid treatment, clinicians should monitor, in both transgender males (female to male) and transgender females (male to female), prolactin, metabolic disorders, and bone loss, as well as cancer risks in individuals who have not undergone surgical treatment.
Concurrent with the release of the new guideline, the Endocrine Society issued a position statement that calls on federal and private insurers to cover medical interventions for transgender individuals as prescribed by a physician. “I live in Massachusetts, where our insurance commissioner deemed insurance coverage obligatory for transgender individuals as of 2015,” said Dr. Safer, who is also director of the endocrinology fellowship training program at Boston University. “I’ve spoken to the medical directors of our large insurers, like Blue Cross/Blue Shield. What’s notable is that there has been no push back [on coverage for transgender individuals] from the insurance companies. These services are not expensive: the primary care, the mental health care, and the hormones. Many of the patients are not opting for surgeries. The theme of their concern was to get their [health insurance] policies right as quickly as possible so that they could stop wasting time talking about it, and they could focus their energy on other, more expensive health care concerns.”
In the guideline, Dr. Safer and the other task force members called for more rigorous evaluations of the effectiveness and safety of endocrine and surgical protocols in the future. “Specifically, endocrine treatment protocols for GD/gender incongruence should include the careful assessment of the following: (1) the effects of prolonged delay of puberty in adolescents on bone health, gonadal function, and the brain (including effects on cognitive, emotional, social, and sexual development); (2) the effects of treatment in adults on sex hormone levels; (3) the requirement for and the effects of progestins and other agents used to suppress endogenous sex steroids during treatment; and (4) the risks and benefits of gender-affirming hormone treatment in older transgender people.”
Dr. Safer reported having no financial disclosures.
Previous clinical recommendations on caring for transgender individuals have advised that hormone treatment begin no earlier than age 16 years, but
“Sixteen is the typical age cutoff in many areas of the world for some decision-making capacity from a legal perspective, but when you think about hormones and puberty, 16 is pretty late,” Joshua D. Safer, MD, one of the task force members who authored the guideline, said in an interview. “If we’re going to use biology for guidance, then hormone interventions for transgender kids should begin occurring earlier, when puberty really happens, like around age 12, 13, or 14. However, we’re in a situation where we lack a test. We can’t diagnose anybody as transgender with excellent confidence, outside of talking to those kids. When we start talking about hormone therapies, we talk about some things that will be irreversible. That’s a fraught place to go, but we recognize that people are going to treat kids under 16 in many instances.”
Over several years, Dr. Safer and nine other task force members, chaired by Wylie Hembree, MD, of the College of Physicians and Surgeons at Columbia University, New York, worked to establish a framework for the appropriate treatment of transgender individuals. The efforts of the task force were framed around a durable biological underpinning to gender identity. “That’s state of the art right now,” said Dr. Safer, who is the medical director of the Center for Transgender Medicine and Surgery at Boston University Medical Center. “People think there’s debate about whether there’s a substantial biological component. I think that the data are pretty strong, so I don’t think there’s a lot of debate about that in the scientific world. The debate is more about what that biology might be. That’s all over the map.”
That notion of a biological basis for gender identity contributed to a second major change from the Endocrine Society’s 2009 guideline, which recommended that the diagnosis of gender identity disorder be made by a mental health professional. The current guideline states that for the care of peripubertal youths and older adolescents, “we recommend that an expert multidisciplinary team comprised of medical professionals and mental health professionals manage this treatment. The treating physician must confirm the criteria for treatment used by the referring mental health practitioner and collaborate with them in decisions about gender-affirming surgery in older adolescents.” Meanwhile, for adult gender-dysphoric/gender-incongruent persons, “the treating clinicians (collectively) should have expertise in transgender-specific diagnostic criteria, mental health, primary care, hormone treatment, and surgery, as needed by the patient.” Dr. Safer described this new approach as “a major change in terms of trying to gain access to care by liberalizing the variety of those in the medical community who can be associated with the diagnosis, at least on the adult side.”
A number of associations cosponsored the guideline, including the American Association of Clinical Endocrinologists, American Society of Andrology, European Society for Paediatric Endocrinology, European Society of Endocrinology, Pediatric Endocrine Society, and World Professional Association for Transgender Health. Other key recommendations from the guideline include:
- All individuals seeking gender-affirming medical treatment should receive information and counsel on options for fertility preservation prior to initiating puberty suppression in adolescents and prior to treating with hormonal therapy of the affirmed gender in both adolescents and adults.
- Removal of gonads may be considered when high doses of sex steroids are required to suppress the body’s secretion of hormones and/or to reduce steroid levels in advanced age.
- During sex-steroid treatment, clinicians should monitor, in both transgender males (female to male) and transgender females (male to female), prolactin, metabolic disorders, and bone loss, as well as cancer risks in individuals who have not undergone surgical treatment.
Concurrent with the release of the new guideline, the Endocrine Society issued a position statement that calls on federal and private insurers to cover medical interventions for transgender individuals as prescribed by a physician. “I live in Massachusetts, where our insurance commissioner deemed insurance coverage obligatory for transgender individuals as of 2015,” said Dr. Safer, who is also director of the endocrinology fellowship training program at Boston University. “I’ve spoken to the medical directors of our large insurers, like Blue Cross/Blue Shield. What’s notable is that there has been no push back [on coverage for transgender individuals] from the insurance companies. These services are not expensive: the primary care, the mental health care, and the hormones. Many of the patients are not opting for surgeries. The theme of their concern was to get their [health insurance] policies right as quickly as possible so that they could stop wasting time talking about it, and they could focus their energy on other, more expensive health care concerns.”
In the guideline, Dr. Safer and the other task force members called for more rigorous evaluations of the effectiveness and safety of endocrine and surgical protocols in the future. “Specifically, endocrine treatment protocols for GD/gender incongruence should include the careful assessment of the following: (1) the effects of prolonged delay of puberty in adolescents on bone health, gonadal function, and the brain (including effects on cognitive, emotional, social, and sexual development); (2) the effects of treatment in adults on sex hormone levels; (3) the requirement for and the effects of progestins and other agents used to suppress endogenous sex steroids during treatment; and (4) the risks and benefits of gender-affirming hormone treatment in older transgender people.”
Dr. Safer reported having no financial disclosures.
Previous clinical recommendations on caring for transgender individuals have advised that hormone treatment begin no earlier than age 16 years, but
“Sixteen is the typical age cutoff in many areas of the world for some decision-making capacity from a legal perspective, but when you think about hormones and puberty, 16 is pretty late,” Joshua D. Safer, MD, one of the task force members who authored the guideline, said in an interview. “If we’re going to use biology for guidance, then hormone interventions for transgender kids should begin occurring earlier, when puberty really happens, like around age 12, 13, or 14. However, we’re in a situation where we lack a test. We can’t diagnose anybody as transgender with excellent confidence, outside of talking to those kids. When we start talking about hormone therapies, we talk about some things that will be irreversible. That’s a fraught place to go, but we recognize that people are going to treat kids under 16 in many instances.”
Over several years, Dr. Safer and nine other task force members, chaired by Wylie Hembree, MD, of the College of Physicians and Surgeons at Columbia University, New York, worked to establish a framework for the appropriate treatment of transgender individuals. The efforts of the task force were framed around a durable biological underpinning to gender identity. “That’s state of the art right now,” said Dr. Safer, who is the medical director of the Center for Transgender Medicine and Surgery at Boston University Medical Center. “People think there’s debate about whether there’s a substantial biological component. I think that the data are pretty strong, so I don’t think there’s a lot of debate about that in the scientific world. The debate is more about what that biology might be. That’s all over the map.”
That notion of a biological basis for gender identity contributed to a second major change from the Endocrine Society’s 2009 guideline, which recommended that the diagnosis of gender identity disorder be made by a mental health professional. The current guideline states that for the care of peripubertal youths and older adolescents, “we recommend that an expert multidisciplinary team comprised of medical professionals and mental health professionals manage this treatment. The treating physician must confirm the criteria for treatment used by the referring mental health practitioner and collaborate with them in decisions about gender-affirming surgery in older adolescents.” Meanwhile, for adult gender-dysphoric/gender-incongruent persons, “the treating clinicians (collectively) should have expertise in transgender-specific diagnostic criteria, mental health, primary care, hormone treatment, and surgery, as needed by the patient.” Dr. Safer described this new approach as “a major change in terms of trying to gain access to care by liberalizing the variety of those in the medical community who can be associated with the diagnosis, at least on the adult side.”
A number of associations cosponsored the guideline, including the American Association of Clinical Endocrinologists, American Society of Andrology, European Society for Paediatric Endocrinology, European Society of Endocrinology, Pediatric Endocrine Society, and World Professional Association for Transgender Health. Other key recommendations from the guideline include:
- All individuals seeking gender-affirming medical treatment should receive information and counsel on options for fertility preservation prior to initiating puberty suppression in adolescents and prior to treating with hormonal therapy of the affirmed gender in both adolescents and adults.
- Removal of gonads may be considered when high doses of sex steroids are required to suppress the body’s secretion of hormones and/or to reduce steroid levels in advanced age.
- During sex-steroid treatment, clinicians should monitor, in both transgender males (female to male) and transgender females (male to female), prolactin, metabolic disorders, and bone loss, as well as cancer risks in individuals who have not undergone surgical treatment.
Concurrent with the release of the new guideline, the Endocrine Society issued a position statement that calls on federal and private insurers to cover medical interventions for transgender individuals as prescribed by a physician. “I live in Massachusetts, where our insurance commissioner deemed insurance coverage obligatory for transgender individuals as of 2015,” said Dr. Safer, who is also director of the endocrinology fellowship training program at Boston University. “I’ve spoken to the medical directors of our large insurers, like Blue Cross/Blue Shield. What’s notable is that there has been no push back [on coverage for transgender individuals] from the insurance companies. These services are not expensive: the primary care, the mental health care, and the hormones. Many of the patients are not opting for surgeries. The theme of their concern was to get their [health insurance] policies right as quickly as possible so that they could stop wasting time talking about it, and they could focus their energy on other, more expensive health care concerns.”
In the guideline, Dr. Safer and the other task force members called for more rigorous evaluations of the effectiveness and safety of endocrine and surgical protocols in the future. “Specifically, endocrine treatment protocols for GD/gender incongruence should include the careful assessment of the following: (1) the effects of prolonged delay of puberty in adolescents on bone health, gonadal function, and the brain (including effects on cognitive, emotional, social, and sexual development); (2) the effects of treatment in adults on sex hormone levels; (3) the requirement for and the effects of progestins and other agents used to suppress endogenous sex steroids during treatment; and (4) the risks and benefits of gender-affirming hormone treatment in older transgender people.”
Dr. Safer reported having no financial disclosures.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM