User login
CRP may predict survival after immunotherapy for lung cancer
CHICAGO – A baseline C-reactive protein (CRP) level above 50 mg/L independently predicted worse overall survival after immunotherapy in patients with advanced non–small cell lung cancer and small cell lung cancer in a retrospective study.
In 99 patients treated with nivolumab after a first-line platinum doublet, the median baseline CRP level was 22 mg/L. After a median follow-up of 8.5 months, 50% of patients were alive, and, based on univariate and multivariate analysis, both liver involvement and having a CRP level greater than 50 mg/L were significantly associated with inferior overall survival after immunotherapy.
The median overall survival after immunotherapy was 9.3 months versus 2.7 months with a CRP level of 50 mg/L or less versus above 50 mg/L, Abdul Rafeh Naqash, MD, of East Carolina University, Greenville, N.C., reported at the Chicago Multidisciplinary Symposium in Thoracic Oncology.
Notably, significant increases in CRP level, compared with baseline, were seen at the time of grade 2 to grade 4 immune-related adverse events, which occurred in 38.4% of patients. This is a hypothesis-generating finding in that it suggests there is dysregulation of the immune system, in the context of immune checkpoint blockade, that leads to a more proinflammatory state, which ultimately leads to immune-related adverse events, Dr. Naqash said.
Study subjects were adults with a median age of 65 years who were treated during April 2015-March 2017. Most were white (64.7%), were male (64.6%), and had non–small cell lung cancer (88%). Most had stage IV disease (70.7%), and the most common site for metastases was the bones (35.4%) and the liver (24.2%). Patients’ CRP levels were measured at anti-PD-1–treatment initiation and serially with subsequent doses.
The findings are important because the identification of predictive biomarkers in patients treated with anti-PD-1 therapy could provide valuable insights into underlying mechanisms regulating patient responses, elucidate resistance mechanisms, and help with optimal selection of patients for treatment with and development of patient-tailored treatment, Dr. Naqash said, noting that identifying such biomarkers has thus far been a challenge.
However, this study is limited by its retrospective design and limited follow-up; the findings require validation in prospective lung cancer trials, he concluded.
Dr. Naqash reported having no disclosures.
CHICAGO – A baseline C-reactive protein (CRP) level above 50 mg/L independently predicted worse overall survival after immunotherapy in patients with advanced non–small cell lung cancer and small cell lung cancer in a retrospective study.
In 99 patients treated with nivolumab after a first-line platinum doublet, the median baseline CRP level was 22 mg/L. After a median follow-up of 8.5 months, 50% of patients were alive, and, based on univariate and multivariate analysis, both liver involvement and having a CRP level greater than 50 mg/L were significantly associated with inferior overall survival after immunotherapy.
The median overall survival after immunotherapy was 9.3 months versus 2.7 months with a CRP level of 50 mg/L or less versus above 50 mg/L, Abdul Rafeh Naqash, MD, of East Carolina University, Greenville, N.C., reported at the Chicago Multidisciplinary Symposium in Thoracic Oncology.
Notably, significant increases in CRP level, compared with baseline, were seen at the time of grade 2 to grade 4 immune-related adverse events, which occurred in 38.4% of patients. This is a hypothesis-generating finding in that it suggests there is dysregulation of the immune system, in the context of immune checkpoint blockade, that leads to a more proinflammatory state, which ultimately leads to immune-related adverse events, Dr. Naqash said.
Study subjects were adults with a median age of 65 years who were treated during April 2015-March 2017. Most were white (64.7%), were male (64.6%), and had non–small cell lung cancer (88%). Most had stage IV disease (70.7%), and the most common site for metastases was the bones (35.4%) and the liver (24.2%). Patients’ CRP levels were measured at anti-PD-1–treatment initiation and serially with subsequent doses.
The findings are important because the identification of predictive biomarkers in patients treated with anti-PD-1 therapy could provide valuable insights into underlying mechanisms regulating patient responses, elucidate resistance mechanisms, and help with optimal selection of patients for treatment with and development of patient-tailored treatment, Dr. Naqash said, noting that identifying such biomarkers has thus far been a challenge.
However, this study is limited by its retrospective design and limited follow-up; the findings require validation in prospective lung cancer trials, he concluded.
Dr. Naqash reported having no disclosures.
CHICAGO – A baseline C-reactive protein (CRP) level above 50 mg/L independently predicted worse overall survival after immunotherapy in patients with advanced non–small cell lung cancer and small cell lung cancer in a retrospective study.
In 99 patients treated with nivolumab after a first-line platinum doublet, the median baseline CRP level was 22 mg/L. After a median follow-up of 8.5 months, 50% of patients were alive, and, based on univariate and multivariate analysis, both liver involvement and having a CRP level greater than 50 mg/L were significantly associated with inferior overall survival after immunotherapy.
The median overall survival after immunotherapy was 9.3 months versus 2.7 months with a CRP level of 50 mg/L or less versus above 50 mg/L, Abdul Rafeh Naqash, MD, of East Carolina University, Greenville, N.C., reported at the Chicago Multidisciplinary Symposium in Thoracic Oncology.
Notably, significant increases in CRP level, compared with baseline, were seen at the time of grade 2 to grade 4 immune-related adverse events, which occurred in 38.4% of patients. This is a hypothesis-generating finding in that it suggests there is dysregulation of the immune system, in the context of immune checkpoint blockade, that leads to a more proinflammatory state, which ultimately leads to immune-related adverse events, Dr. Naqash said.
Study subjects were adults with a median age of 65 years who were treated during April 2015-March 2017. Most were white (64.7%), were male (64.6%), and had non–small cell lung cancer (88%). Most had stage IV disease (70.7%), and the most common site for metastases was the bones (35.4%) and the liver (24.2%). Patients’ CRP levels were measured at anti-PD-1–treatment initiation and serially with subsequent doses.
The findings are important because the identification of predictive biomarkers in patients treated with anti-PD-1 therapy could provide valuable insights into underlying mechanisms regulating patient responses, elucidate resistance mechanisms, and help with optimal selection of patients for treatment with and development of patient-tailored treatment, Dr. Naqash said, noting that identifying such biomarkers has thus far been a challenge.
However, this study is limited by its retrospective design and limited follow-up; the findings require validation in prospective lung cancer trials, he concluded.
Dr. Naqash reported having no disclosures.
AT A SYMPOSIUM IN THORACIC ONCOLOGY
Key clinical point:
Major finding: Median overall survival after immunotherapy: 9.3 months vs. 2.7 months with CRP of 50 mg/L or less vs. above 50 mg/L.
Data source: A retrospective study of 99 patients.
Disclosures: Dr. Naqash reported having no disclosures.
Postsurgical antibiotics cut infection in obese women after C-section
A 48-hour course of postoperative cephalexin and metronidazole, plus typical preoperative antibiotics, cut surgical site infections by 59% in obese women who had a cesarean delivery.
The benefit of the additional postoperative treatment was driven by a significant, 69% risk reduction among women who had ruptured membranes, Amy M. Valent, DO, and her colleagues reported (JAMA. 2017;318[11]:1026-34). However, the authors noted, “tests for interaction between the intact membranes and [ruptured] subgroups and postpartum cephalexin-metronidazole were not statistically different and should not be interpreted as showing a difference in significance or effect size among the subgroups with and without [rupture].”
The trial comprised 403 obese women who had a cesarean delivery. They were a mean of 28 years old. The mean body mass index was 40 kg/m2, and the mean subcutaneous adipose tissue thickness was about 3.4 cm. About a third of each treatment group was positive for Group B streptococcus; 31% had ruptured membranes at the onset of labor. More than 60% of women in both groups had a scheduled cesarean delivery.
All women had standard preoperative care, including skin prep with a chlorhexidine or povidone-iodine cleansing and an intravenous infusion of 2 g cefazolin. After delivery, they were randomized to placebo or to oral cephalexin 500 mg plus metronidazole 500 mg every 8 hours for 48 hours. The primary outcome was surgical site infection incidence within 30 days.
The overall rate of surgical site infection was 10.9% (44 women). Infections developed in 13 women in the active group and 31 in the placebo group (6.4% vs. 15.4%) – a significant difference, translating to a 59% risk reduction (relative risk, 0.41). Cellulitis was the only secondary outcome that was significantly reduced by prophylactic antibiotics, with infections occurring in 5.9% of the metronidazole-cephalexin group vs. 13.4% of the placebo group (RR, 0.44). The antibiotic regimen didn’t affect the other secondary endpoints, which included rates of incisional morbidity, endometritis, fever of unknown etiology, and wound separation.
The authors conducted a post-hoc analysis to examine the antibiotics’ effects on women who had ruptured and intact membranes at the time of delivery. The benefit was greatest among those with ruptured membranes. There were six infections among the active group and 19 among the placebo group (9.5% vs. 30.2%). This difference translated to a relative risk of 0.31 – a 69% risk reduction.
Among women with intact membranes, there were seven infections in the active group and 12 in the placebo group (5% vs. 8.7%). This translated to a 0.58 relative risk, which was not statistically significant.
“Interaction testing was performed between study groups (cephalexin-metronidazole vs. placebo) and by membrane status (intact vs. ruptured),” the authors noted. “The rate of surgical site infection was highest in those with [ruptured membranes] who received placebo (30.2%) and lowest in those with intact membranes who received antibiotics (5.0%), but the test for interaction did not show statistical significance at P = .30.”
There were no serious adverse events or allergic reactions reported for cephalexin or metronidazole. The authors noted that both drugs are excreted into breast milk in small amounts, but that no study has ever linked them with neonatal harm through breast milk exposure. However, they added, “Long-term childhood or adverse neonatal outcomes specific to cephalexin-metronidazole exposure cannot be determined, as outcome measures were not evaluated for this study protocol. Recognizing the maternal and neonatal benefit of breastfeeding, the lack of known neonatal adverse effects, and maternal reduction in [surgical site infection], the benefit of this antibiotic regimen likely outweighs the theoretical risks of breast milk exposure in the obese population.”
The University of Cincinnati Department of Obstetrics and Gynecology sponsored the trial. None of the authors reported any financial conflicts.
Despite the positive outcomes of this trial, it’s not yet time to tack on yet more antibiotics for every obese woman who undergoes a cesarean delivery, David P. Calfee, MD, and Amos Grünebaum, MD, wrote in an accompanying editorial (JAMA. 2017;318[11]:1012-3).
“When determining if and how the results of this study should alter current clinical practice, it is important to recognize that the results of this study are quite different from those of several previous studies conducted in other surgical patient populations in which no benefit from postoperative antimicrobial prophylaxis was found and on which current clinical guidelines for antimicrobial prophylaxis are based,” they wrote. “The explanation for this difference may be as simple as the identification in the current study of a very specific, high-risk group of patients for which the intervention is effective. However, several questions are worthy of additional consideration and study.”
For instance, the study was conducted over 5 years and may not reflect current practices for managing these patients, such as glycemic control and maintaining normothermia. Additionally, there may be additional risks to women that were not identified in the study, such as infection from antimicrobial-resistant pathogens.
Dr. Calfee and Dr. Grünebaum are at Weill Cornell Medical Center in New York. Dr. Calfee reported receiving grants from Merck, Sharp, and Dohme.
Despite the positive outcomes of this trial, it’s not yet time to tack on yet more antibiotics for every obese woman who undergoes a cesarean delivery, David P. Calfee, MD, and Amos Grünebaum, MD, wrote in an accompanying editorial (JAMA. 2017;318[11]:1012-3).
“When determining if and how the results of this study should alter current clinical practice, it is important to recognize that the results of this study are quite different from those of several previous studies conducted in other surgical patient populations in which no benefit from postoperative antimicrobial prophylaxis was found and on which current clinical guidelines for antimicrobial prophylaxis are based,” they wrote. “The explanation for this difference may be as simple as the identification in the current study of a very specific, high-risk group of patients for which the intervention is effective. However, several questions are worthy of additional consideration and study.”
For instance, the study was conducted over 5 years and may not reflect current practices for managing these patients, such as glycemic control and maintaining normothermia. Additionally, there may be additional risks to women that were not identified in the study, such as infection from antimicrobial-resistant pathogens.
Dr. Calfee and Dr. Grünebaum are at Weill Cornell Medical Center in New York. Dr. Calfee reported receiving grants from Merck, Sharp, and Dohme.
Despite the positive outcomes of this trial, it’s not yet time to tack on yet more antibiotics for every obese woman who undergoes a cesarean delivery, David P. Calfee, MD, and Amos Grünebaum, MD, wrote in an accompanying editorial (JAMA. 2017;318[11]:1012-3).
“When determining if and how the results of this study should alter current clinical practice, it is important to recognize that the results of this study are quite different from those of several previous studies conducted in other surgical patient populations in which no benefit from postoperative antimicrobial prophylaxis was found and on which current clinical guidelines for antimicrobial prophylaxis are based,” they wrote. “The explanation for this difference may be as simple as the identification in the current study of a very specific, high-risk group of patients for which the intervention is effective. However, several questions are worthy of additional consideration and study.”
For instance, the study was conducted over 5 years and may not reflect current practices for managing these patients, such as glycemic control and maintaining normothermia. Additionally, there may be additional risks to women that were not identified in the study, such as infection from antimicrobial-resistant pathogens.
Dr. Calfee and Dr. Grünebaum are at Weill Cornell Medical Center in New York. Dr. Calfee reported receiving grants from Merck, Sharp, and Dohme.
A 48-hour course of postoperative cephalexin and metronidazole, plus typical preoperative antibiotics, cut surgical site infections by 59% in obese women who had a cesarean delivery.
The benefit of the additional postoperative treatment was driven by a significant, 69% risk reduction among women who had ruptured membranes, Amy M. Valent, DO, and her colleagues reported (JAMA. 2017;318[11]:1026-34). However, the authors noted, “tests for interaction between the intact membranes and [ruptured] subgroups and postpartum cephalexin-metronidazole were not statistically different and should not be interpreted as showing a difference in significance or effect size among the subgroups with and without [rupture].”
The trial comprised 403 obese women who had a cesarean delivery. They were a mean of 28 years old. The mean body mass index was 40 kg/m2, and the mean subcutaneous adipose tissue thickness was about 3.4 cm. About a third of each treatment group was positive for Group B streptococcus; 31% had ruptured membranes at the onset of labor. More than 60% of women in both groups had a scheduled cesarean delivery.
All women had standard preoperative care, including skin prep with a chlorhexidine or povidone-iodine cleansing and an intravenous infusion of 2 g cefazolin. After delivery, they were randomized to placebo or to oral cephalexin 500 mg plus metronidazole 500 mg every 8 hours for 48 hours. The primary outcome was surgical site infection incidence within 30 days.
The overall rate of surgical site infection was 10.9% (44 women). Infections developed in 13 women in the active group and 31 in the placebo group (6.4% vs. 15.4%) – a significant difference, translating to a 59% risk reduction (relative risk, 0.41). Cellulitis was the only secondary outcome that was significantly reduced by prophylactic antibiotics, with infections occurring in 5.9% of the metronidazole-cephalexin group vs. 13.4% of the placebo group (RR, 0.44). The antibiotic regimen didn’t affect the other secondary endpoints, which included rates of incisional morbidity, endometritis, fever of unknown etiology, and wound separation.
The authors conducted a post-hoc analysis to examine the antibiotics’ effects on women who had ruptured and intact membranes at the time of delivery. The benefit was greatest among those with ruptured membranes. There were six infections among the active group and 19 among the placebo group (9.5% vs. 30.2%). This difference translated to a relative risk of 0.31 – a 69% risk reduction.
Among women with intact membranes, there were seven infections in the active group and 12 in the placebo group (5% vs. 8.7%). This translated to a 0.58 relative risk, which was not statistically significant.
“Interaction testing was performed between study groups (cephalexin-metronidazole vs. placebo) and by membrane status (intact vs. ruptured),” the authors noted. “The rate of surgical site infection was highest in those with [ruptured membranes] who received placebo (30.2%) and lowest in those with intact membranes who received antibiotics (5.0%), but the test for interaction did not show statistical significance at P = .30.”
There were no serious adverse events or allergic reactions reported for cephalexin or metronidazole. The authors noted that both drugs are excreted into breast milk in small amounts, but that no study has ever linked them with neonatal harm through breast milk exposure. However, they added, “Long-term childhood or adverse neonatal outcomes specific to cephalexin-metronidazole exposure cannot be determined, as outcome measures were not evaluated for this study protocol. Recognizing the maternal and neonatal benefit of breastfeeding, the lack of known neonatal adverse effects, and maternal reduction in [surgical site infection], the benefit of this antibiotic regimen likely outweighs the theoretical risks of breast milk exposure in the obese population.”
The University of Cincinnati Department of Obstetrics and Gynecology sponsored the trial. None of the authors reported any financial conflicts.
A 48-hour course of postoperative cephalexin and metronidazole, plus typical preoperative antibiotics, cut surgical site infections by 59% in obese women who had a cesarean delivery.
The benefit of the additional postoperative treatment was driven by a significant, 69% risk reduction among women who had ruptured membranes, Amy M. Valent, DO, and her colleagues reported (JAMA. 2017;318[11]:1026-34). However, the authors noted, “tests for interaction between the intact membranes and [ruptured] subgroups and postpartum cephalexin-metronidazole were not statistically different and should not be interpreted as showing a difference in significance or effect size among the subgroups with and without [rupture].”
The trial comprised 403 obese women who had a cesarean delivery. They were a mean of 28 years old. The mean body mass index was 40 kg/m2, and the mean subcutaneous adipose tissue thickness was about 3.4 cm. About a third of each treatment group was positive for Group B streptococcus; 31% had ruptured membranes at the onset of labor. More than 60% of women in both groups had a scheduled cesarean delivery.
All women had standard preoperative care, including skin prep with a chlorhexidine or povidone-iodine cleansing and an intravenous infusion of 2 g cefazolin. After delivery, they were randomized to placebo or to oral cephalexin 500 mg plus metronidazole 500 mg every 8 hours for 48 hours. The primary outcome was surgical site infection incidence within 30 days.
The overall rate of surgical site infection was 10.9% (44 women). Infections developed in 13 women in the active group and 31 in the placebo group (6.4% vs. 15.4%) – a significant difference, translating to a 59% risk reduction (relative risk, 0.41). Cellulitis was the only secondary outcome that was significantly reduced by prophylactic antibiotics, with infections occurring in 5.9% of the metronidazole-cephalexin group vs. 13.4% of the placebo group (RR, 0.44). The antibiotic regimen didn’t affect the other secondary endpoints, which included rates of incisional morbidity, endometritis, fever of unknown etiology, and wound separation.
The authors conducted a post-hoc analysis to examine the antibiotics’ effects on women who had ruptured and intact membranes at the time of delivery. The benefit was greatest among those with ruptured membranes. There were six infections among the active group and 19 among the placebo group (9.5% vs. 30.2%). This difference translated to a relative risk of 0.31 – a 69% risk reduction.
Among women with intact membranes, there were seven infections in the active group and 12 in the placebo group (5% vs. 8.7%). This translated to a 0.58 relative risk, which was not statistically significant.
“Interaction testing was performed between study groups (cephalexin-metronidazole vs. placebo) and by membrane status (intact vs. ruptured),” the authors noted. “The rate of surgical site infection was highest in those with [ruptured membranes] who received placebo (30.2%) and lowest in those with intact membranes who received antibiotics (5.0%), but the test for interaction did not show statistical significance at P = .30.”
There were no serious adverse events or allergic reactions reported for cephalexin or metronidazole. The authors noted that both drugs are excreted into breast milk in small amounts, but that no study has ever linked them with neonatal harm through breast milk exposure. However, they added, “Long-term childhood or adverse neonatal outcomes specific to cephalexin-metronidazole exposure cannot be determined, as outcome measures were not evaluated for this study protocol. Recognizing the maternal and neonatal benefit of breastfeeding, the lack of known neonatal adverse effects, and maternal reduction in [surgical site infection], the benefit of this antibiotic regimen likely outweighs the theoretical risks of breast milk exposure in the obese population.”
The University of Cincinnati Department of Obstetrics and Gynecology sponsored the trial. None of the authors reported any financial conflicts.
FROM JAMA
Key clinical point:
Major finding: Infections developed in 13 women in the active group and 31 in the placebo group (6.4% vs. 15.4%) – a significant difference, translating to a 59% risk reduction (relative risk, 0.41).
Data source: The randomized, placebo-controlled study comprised 403 women.
Disclosures: The University of Cincinnati Department of Obstetrics and Gynecology sponsored the study. None of the authors reported any financial conflicts.
PHM17 session summary: Career Development (K Award) grants
Session
Career Development (K Award) grants: What are they, why should I apply, and how do I get funded?
Presenters
Christopher Bonafide, MD, MSCE; Patrick Brady, MD, MS; Kavita Parikh, MD, MSHS; Raj Srivastava, MD, MPH, SFHM; Derek Williams, MD, MPH
Session Summary
Pediatric hospital medicine, in its relative infancy, is attracting a cohort of academicians dedicated to advancing the care of hospitalized children. While other pediatric subspecialties have long reserved a significant proportion of fellowship training for research, pediatric hospitalist research has instead developed from the work of scholarly pioneers in the industry.
More colloquially known as K Awards, NIH Career Development Awards exist to financially support early-career clinical, translational, and basic science investigators through a closely mentored career development and research plan. The result? A mutually beneficial initiative lasting 3-5 years aligning the interests of the early-career investigator, hosting institution, and NIH. The realm of grant funding is confusing and can be intimidating, particularly for early-career investigators in a rapidly growing field of practice. Presenters at this session addressed the stigma of applying for K awards head on.
Who should apply for an NIH Career Development Award?
Competitive applicants for a Career Development Award are ideally interested in embarking on a career dedicated to research of some type, although exactly what that entails can and certainly may change over time.
What does the NIH Career Development Award provide?
The award funds a significant portion of your salary to provide protected time dedicated to your research and career development. Removing this financial barrier allows the investigator to become fully immersed in maturation as an independent investigator. The presenters were quick to caution that applicants (along with department and division chairs) should be aware that the award does not cover your entire salary; early-career investigators truly need dedication from their department and/or division to be successful.
Why apply for an NIH Career Development Award?
Clinical, translational, and basic science research takes time to complete, and the skills needed to be a successful investigator are not intuitive. Rather, they require close mentorship and practice. A career development award organizes and prioritizes an early-career investigator’s approach to obtaining research independence. Applying for an NIH Career Development Award helps identify the applicant’s experiential and knowledge gaps and, more importantly, develops a plan for how these deficits will be addressed over the course of the research project. This formative process ideally allows the early-career investigator to be more competitive in seeking larger grant funding.
Interested in pursuing a career development award? Dr. Bonafide and Dr. Srivastava offered the valuable advice that an applicant’s proposed research is only part of the equation for funding success. Equally important is your ability to identify your weaknesses as they pertain to research (and how you will address these weaknesses) as well as to find your mentorship team. You and your fellow awardees should surround yourselves with mentors who will address specific needs, which, in some circumstances, may require creativity and collaboration to augment the experience gained from others.
Key takeaway for PHM
As we recognize the growing complexities of caring for the hospitalized child, opportunities for clinical, translational, and basic science are expanding rapidly. Embracing the benefits of formalizing your research training early can lead to a successful and satisfying academic career in the long term.
Dr. Morrison is a Pediatric Hospital Medicine Fellow at Johns Hopkins All Children’s Hospital, St. Petersburg, Fla.
Session
Career Development (K Award) grants: What are they, why should I apply, and how do I get funded?
Presenters
Christopher Bonafide, MD, MSCE; Patrick Brady, MD, MS; Kavita Parikh, MD, MSHS; Raj Srivastava, MD, MPH, SFHM; Derek Williams, MD, MPH
Session Summary
Pediatric hospital medicine, in its relative infancy, is attracting a cohort of academicians dedicated to advancing the care of hospitalized children. While other pediatric subspecialties have long reserved a significant proportion of fellowship training for research, pediatric hospitalist research has instead developed from the work of scholarly pioneers in the industry.
More colloquially known as K Awards, NIH Career Development Awards exist to financially support early-career clinical, translational, and basic science investigators through a closely mentored career development and research plan. The result? A mutually beneficial initiative lasting 3-5 years aligning the interests of the early-career investigator, hosting institution, and NIH. The realm of grant funding is confusing and can be intimidating, particularly for early-career investigators in a rapidly growing field of practice. Presenters at this session addressed the stigma of applying for K awards head on.
Who should apply for an NIH Career Development Award?
Competitive applicants for a Career Development Award are ideally interested in embarking on a career dedicated to research of some type, although exactly what that entails can and certainly may change over time.
What does the NIH Career Development Award provide?
The award funds a significant portion of your salary to provide protected time dedicated to your research and career development. Removing this financial barrier allows the investigator to become fully immersed in maturation as an independent investigator. The presenters were quick to caution that applicants (along with department and division chairs) should be aware that the award does not cover your entire salary; early-career investigators truly need dedication from their department and/or division to be successful.
Why apply for an NIH Career Development Award?
Clinical, translational, and basic science research takes time to complete, and the skills needed to be a successful investigator are not intuitive. Rather, they require close mentorship and practice. A career development award organizes and prioritizes an early-career investigator’s approach to obtaining research independence. Applying for an NIH Career Development Award helps identify the applicant’s experiential and knowledge gaps and, more importantly, develops a plan for how these deficits will be addressed over the course of the research project. This formative process ideally allows the early-career investigator to be more competitive in seeking larger grant funding.
Interested in pursuing a career development award? Dr. Bonafide and Dr. Srivastava offered the valuable advice that an applicant’s proposed research is only part of the equation for funding success. Equally important is your ability to identify your weaknesses as they pertain to research (and how you will address these weaknesses) as well as to find your mentorship team. You and your fellow awardees should surround yourselves with mentors who will address specific needs, which, in some circumstances, may require creativity and collaboration to augment the experience gained from others.
Key takeaway for PHM
As we recognize the growing complexities of caring for the hospitalized child, opportunities for clinical, translational, and basic science are expanding rapidly. Embracing the benefits of formalizing your research training early can lead to a successful and satisfying academic career in the long term.
Dr. Morrison is a Pediatric Hospital Medicine Fellow at Johns Hopkins All Children’s Hospital, St. Petersburg, Fla.
Session
Career Development (K Award) grants: What are they, why should I apply, and how do I get funded?
Presenters
Christopher Bonafide, MD, MSCE; Patrick Brady, MD, MS; Kavita Parikh, MD, MSHS; Raj Srivastava, MD, MPH, SFHM; Derek Williams, MD, MPH
Session Summary
Pediatric hospital medicine, in its relative infancy, is attracting a cohort of academicians dedicated to advancing the care of hospitalized children. While other pediatric subspecialties have long reserved a significant proportion of fellowship training for research, pediatric hospitalist research has instead developed from the work of scholarly pioneers in the industry.
More colloquially known as K Awards, NIH Career Development Awards exist to financially support early-career clinical, translational, and basic science investigators through a closely mentored career development and research plan. The result? A mutually beneficial initiative lasting 3-5 years aligning the interests of the early-career investigator, hosting institution, and NIH. The realm of grant funding is confusing and can be intimidating, particularly for early-career investigators in a rapidly growing field of practice. Presenters at this session addressed the stigma of applying for K awards head on.
Who should apply for an NIH Career Development Award?
Competitive applicants for a Career Development Award are ideally interested in embarking on a career dedicated to research of some type, although exactly what that entails can and certainly may change over time.
What does the NIH Career Development Award provide?
The award funds a significant portion of your salary to provide protected time dedicated to your research and career development. Removing this financial barrier allows the investigator to become fully immersed in maturation as an independent investigator. The presenters were quick to caution that applicants (along with department and division chairs) should be aware that the award does not cover your entire salary; early-career investigators truly need dedication from their department and/or division to be successful.
Why apply for an NIH Career Development Award?
Clinical, translational, and basic science research takes time to complete, and the skills needed to be a successful investigator are not intuitive. Rather, they require close mentorship and practice. A career development award organizes and prioritizes an early-career investigator’s approach to obtaining research independence. Applying for an NIH Career Development Award helps identify the applicant’s experiential and knowledge gaps and, more importantly, develops a plan for how these deficits will be addressed over the course of the research project. This formative process ideally allows the early-career investigator to be more competitive in seeking larger grant funding.
Interested in pursuing a career development award? Dr. Bonafide and Dr. Srivastava offered the valuable advice that an applicant’s proposed research is only part of the equation for funding success. Equally important is your ability to identify your weaknesses as they pertain to research (and how you will address these weaknesses) as well as to find your mentorship team. You and your fellow awardees should surround yourselves with mentors who will address specific needs, which, in some circumstances, may require creativity and collaboration to augment the experience gained from others.
Key takeaway for PHM
As we recognize the growing complexities of caring for the hospitalized child, opportunities for clinical, translational, and basic science are expanding rapidly. Embracing the benefits of formalizing your research training early can lead to a successful and satisfying academic career in the long term.
Dr. Morrison is a Pediatric Hospital Medicine Fellow at Johns Hopkins All Children’s Hospital, St. Petersburg, Fla.
FDA grants accelerated approval to copanlisib for relapsed follicular lymphoma
The Food and Drug Administration has granted accelerated approval to copanlisib (Aliqopa) for the treatment of adults with relapsed follicular lymphoma who have received at least two prior treatments.
Approval of the kinase inhibitor was based on an overall response rate of 59% in a single-arm trial of 104 patients with follicular B-cell non-Hodgkin lymphoma who had relapsed disease following at least two prior treatments. These patients had a complete or partial response for a median 12.2 months.
“For patients with relapsed follicular lymphoma, the cancer often comes back even after multiple treatments,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research said in the press release. “Options are limited for these patients and today’s approval provides an additional choice for treatment, filling an unmet need for them,” he said.
The Food and Drug Administration has granted accelerated approval to copanlisib (Aliqopa) for the treatment of adults with relapsed follicular lymphoma who have received at least two prior treatments.
Approval of the kinase inhibitor was based on an overall response rate of 59% in a single-arm trial of 104 patients with follicular B-cell non-Hodgkin lymphoma who had relapsed disease following at least two prior treatments. These patients had a complete or partial response for a median 12.2 months.
“For patients with relapsed follicular lymphoma, the cancer often comes back even after multiple treatments,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research said in the press release. “Options are limited for these patients and today’s approval provides an additional choice for treatment, filling an unmet need for them,” he said.
The Food and Drug Administration has granted accelerated approval to copanlisib (Aliqopa) for the treatment of adults with relapsed follicular lymphoma who have received at least two prior treatments.
Approval of the kinase inhibitor was based on an overall response rate of 59% in a single-arm trial of 104 patients with follicular B-cell non-Hodgkin lymphoma who had relapsed disease following at least two prior treatments. These patients had a complete or partial response for a median 12.2 months.
“For patients with relapsed follicular lymphoma, the cancer often comes back even after multiple treatments,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research said in the press release. “Options are limited for these patients and today’s approval provides an additional choice for treatment, filling an unmet need for them,” he said.
Rheumatoid arthritis characteristics make large contribution to cardiovascular risk
Nearly one-third of cardiovascular events in patients with rheumatoid arthritis can be attributed to their rheumatoid arthritis characteristics, such as Disease Activity Score and rheumatoid factor or anticitrullinated protein antibody positivity, research suggests.
A prospective, international cohort study published in Annals of the Rheumatic Diseases followed 5,638 patients with rheumatoid arthritis (RA) and no history of cardiovascular disease for a mean of 5.8 years to look at their risk of myocardial infarction, angina, revascularization, stroke, peripheral vascular disease, and death from cardiovascular disease.
Overall, the 10-year cumulative incidence of cardiovascular events was 20.9% in men and 11.1% in women.
Smoking and hypertension were the strongest predictors of cardiovascular disease in both men and women and had the highest population attributable risk (PAR), even after adjustment for other cardiovascular risk factors.
The PAR for triglycerides was 11.5% overall, but it was 12.6% for Disease Activity Score in 28 joints (DAS28) and 12.2% for rheumatoid factor (RF)/anticitrullinated protein antibody (ACPA) positivity. Other RA-related factors, such as erythrocyte sedimentation rate (ESR) and C-reactive protein, did not have a significant effect on cardiovascular event risk.
When combined, cardiovascular risk factors such as blood pressure, cholesterol levels, smoking, body mass index, diabetes, and family history accounted for 49% of the PAR of cardiovascular events in people with RA, and the RA characteristics explained 30.3% of the risk.
Together, the cardiovascular and RA risk factors accounted for 69.6% of the risk of cardiovascular events, and the remaining 30.4% could not be explained.
While the PAR associated with the combined cardiovascular risk factors was higher in men than in women, the contribution of all the RA characteristics combined proved to be greater in women than in men. However, neither sex difference was statistically significant.
“While the prevalence of RF/ACPA positivity and DAS28 levels was similar between the sexes, the effect sizes of RA characteristics appeared to be larger among women than men, despite lack of statistical significance,” the authors wrote.
“Moreover, higher levels of ESR in women than men may partially explain this apparent difference in PAR [and] RA disease duration was longer among women, and more women than men were receiving biological [disease-modifying antirheumatic drugs] at baseline.”
Eli Lilly, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Norwegian South East Health Authority supported the study. Two authors declared honoraria, fees, and grants from the pharmaceutical industry, including Eli Lilly. No other conflicts of interest were declared.
Nearly one-third of cardiovascular events in patients with rheumatoid arthritis can be attributed to their rheumatoid arthritis characteristics, such as Disease Activity Score and rheumatoid factor or anticitrullinated protein antibody positivity, research suggests.
A prospective, international cohort study published in Annals of the Rheumatic Diseases followed 5,638 patients with rheumatoid arthritis (RA) and no history of cardiovascular disease for a mean of 5.8 years to look at their risk of myocardial infarction, angina, revascularization, stroke, peripheral vascular disease, and death from cardiovascular disease.
Overall, the 10-year cumulative incidence of cardiovascular events was 20.9% in men and 11.1% in women.
Smoking and hypertension were the strongest predictors of cardiovascular disease in both men and women and had the highest population attributable risk (PAR), even after adjustment for other cardiovascular risk factors.
The PAR for triglycerides was 11.5% overall, but it was 12.6% for Disease Activity Score in 28 joints (DAS28) and 12.2% for rheumatoid factor (RF)/anticitrullinated protein antibody (ACPA) positivity. Other RA-related factors, such as erythrocyte sedimentation rate (ESR) and C-reactive protein, did not have a significant effect on cardiovascular event risk.
When combined, cardiovascular risk factors such as blood pressure, cholesterol levels, smoking, body mass index, diabetes, and family history accounted for 49% of the PAR of cardiovascular events in people with RA, and the RA characteristics explained 30.3% of the risk.
Together, the cardiovascular and RA risk factors accounted for 69.6% of the risk of cardiovascular events, and the remaining 30.4% could not be explained.
While the PAR associated with the combined cardiovascular risk factors was higher in men than in women, the contribution of all the RA characteristics combined proved to be greater in women than in men. However, neither sex difference was statistically significant.
“While the prevalence of RF/ACPA positivity and DAS28 levels was similar between the sexes, the effect sizes of RA characteristics appeared to be larger among women than men, despite lack of statistical significance,” the authors wrote.
“Moreover, higher levels of ESR in women than men may partially explain this apparent difference in PAR [and] RA disease duration was longer among women, and more women than men were receiving biological [disease-modifying antirheumatic drugs] at baseline.”
Eli Lilly, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Norwegian South East Health Authority supported the study. Two authors declared honoraria, fees, and grants from the pharmaceutical industry, including Eli Lilly. No other conflicts of interest were declared.
Nearly one-third of cardiovascular events in patients with rheumatoid arthritis can be attributed to their rheumatoid arthritis characteristics, such as Disease Activity Score and rheumatoid factor or anticitrullinated protein antibody positivity, research suggests.
A prospective, international cohort study published in Annals of the Rheumatic Diseases followed 5,638 patients with rheumatoid arthritis (RA) and no history of cardiovascular disease for a mean of 5.8 years to look at their risk of myocardial infarction, angina, revascularization, stroke, peripheral vascular disease, and death from cardiovascular disease.
Overall, the 10-year cumulative incidence of cardiovascular events was 20.9% in men and 11.1% in women.
Smoking and hypertension were the strongest predictors of cardiovascular disease in both men and women and had the highest population attributable risk (PAR), even after adjustment for other cardiovascular risk factors.
The PAR for triglycerides was 11.5% overall, but it was 12.6% for Disease Activity Score in 28 joints (DAS28) and 12.2% for rheumatoid factor (RF)/anticitrullinated protein antibody (ACPA) positivity. Other RA-related factors, such as erythrocyte sedimentation rate (ESR) and C-reactive protein, did not have a significant effect on cardiovascular event risk.
When combined, cardiovascular risk factors such as blood pressure, cholesterol levels, smoking, body mass index, diabetes, and family history accounted for 49% of the PAR of cardiovascular events in people with RA, and the RA characteristics explained 30.3% of the risk.
Together, the cardiovascular and RA risk factors accounted for 69.6% of the risk of cardiovascular events, and the remaining 30.4% could not be explained.
While the PAR associated with the combined cardiovascular risk factors was higher in men than in women, the contribution of all the RA characteristics combined proved to be greater in women than in men. However, neither sex difference was statistically significant.
“While the prevalence of RF/ACPA positivity and DAS28 levels was similar between the sexes, the effect sizes of RA characteristics appeared to be larger among women than men, despite lack of statistical significance,” the authors wrote.
“Moreover, higher levels of ESR in women than men may partially explain this apparent difference in PAR [and] RA disease duration was longer among women, and more women than men were receiving biological [disease-modifying antirheumatic drugs] at baseline.”
Eli Lilly, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Norwegian South East Health Authority supported the study. Two authors declared honoraria, fees, and grants from the pharmaceutical industry, including Eli Lilly. No other conflicts of interest were declared.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: Rheumatoid arthritis characteristics explained 30.3% of the risk of cardiovascular events in individuals with RA.
Data source: A prospective, international cohort study of 5,638 patients with RA.
Disclosures: Eli Lilly, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Norwegian South East Health Authority supported the study. Two authors declared honoraria, fees, and grants from the pharmaceutical industry, including Eli Lilly. No other conflicts of interest were declared.
Ultrasound’s value for arthralgia may be to rule out IA
Ultrasound evaluations to look for subclinical inflammation in joints of patients with arthralgia appear best at ruling out inflammatory arthritis (IA) 1 year in the future rather than ruling it in, according to findings from a multicenter cohort study published online in Arthritis Research and Therapy.
The imaging modality’s ability to identify those who will not go on to develop IA complemented the serologic and clinical factors that help to discriminate the individuals with arthralgia who are most at risk of the condition.
The ultimate goal of using imaging such as ultrasound in patients with arthralgia is to identify those who would benefit from starting treatment with disease-modifying antirheumatic drugs as early as possible to potentially improve outcomes, but it also could help to discriminate between the anti-citrullinated protein antibody (ACPA)-positive and seronegative individuals without clinical signs of inflammation at baseline who may progress from arthralgia to IA.
“Although ACPA positivity is a very good predictor for those patients who will develop IA within 1 year, it is still difficult to identify the exact individuals who will develop IA, because any ACPA-positive individual has an a priori chance of 50% of developing IA. In seronegative patients, the prediction of IA is even more difficult, because only 5% develop IA within the subsequent year. Imaging techniques have been shown to be able to detect synovitis before its clinical appearance and could be of help in identifying those at risk of IA,” the investigators wrote (Arthritis Res Ther. 2017;19:202. doi: 10.1186/s13075-017-1405-y).
Dr. van der Ven and her associates found that 31 (16%) of 196 patients who had arthralgia for less than 1 year in the hands, feet, or shoulders went on to develop IA after 1 year of follow-up. In this group of 196 patients at baseline, 72 (37%) had synovitis on ultrasound – defined as a greyscale grade of 2 or 3 and/or the presence of power Doppler signal (grade 1, 2, or 3) – including 32 with a positive power Doppler. A total of 18 patients were lost to follow-up during the first 6 months and another 19 were lost during months 6-12.
Rheumatologists who were unaware of ultrasound findings had to confirm soft-tissue swelling as arthritis at 1 year to classify it as incident IA. The positive predictive value of ultrasound for IA was only 26% when at least 1 joint out of 26 assessed was positive, but the negative predictive value when no joints were positive on ultrasound was 89%.
Overall, at 1 year, 15 of the 31 patients with IA had started therapy with a disease-modifying antirheumatic drug and 22 did not have a definite diagnosis; 12 had monoarthritis and 10 had polyarthritis. The remaining nine patients included four with rheumatoid arthritis, four with psoriatic arthritis, and one with spondyloarthritis.
At baseline, individuals with IA were more often older (mean age 50 vs. 44 years; P = .005), had synovitis on ultrasound (59% vs. 32%; P = .007), and had a positive power Doppler signal (31% vs. 12%; P = .012). A multivariate analysis revealed that IA at 1 year of follow-up could be independently predicted according to age (odds ratio, 1.06), morning stiffness lasting more than 30 minutes (OR, 2.80), ACPA positivity (OR, 2.35), and synovitis on ultrasound (OR, 2.65).
The investigators noted that the study’s limitations relate to requirements for patients to have at least two painful joints in hands, feet, or shoulders at baseline and two criteria related to inflammation. The possible inflammation-related criteria required for entry included morning stiffness for more than 1 hour, inability to clench a fist in the morning, pain when shaking someone’s hand, pins and needles in the fingers, difficulties wearing rings or shoes, family history of rheumatoid arthritis, and/or unexplained fatigue for less than 1 year.
Rheumatologists who enrolled patients into the cohort also may have “recruited clinically suspected patients with possibly more severe symptoms,” the investigators noted. Another potential source of bias related to the group of 38 patients who chose not to participate: It’s possible these patients had less severe symptoms than those who participated in the study.
The study was funded by an investigator-initiated grant from Pfizer. The authors declared that they have no competing interests.
Ultrasound evaluations to look for subclinical inflammation in joints of patients with arthralgia appear best at ruling out inflammatory arthritis (IA) 1 year in the future rather than ruling it in, according to findings from a multicenter cohort study published online in Arthritis Research and Therapy.
The imaging modality’s ability to identify those who will not go on to develop IA complemented the serologic and clinical factors that help to discriminate the individuals with arthralgia who are most at risk of the condition.
The ultimate goal of using imaging such as ultrasound in patients with arthralgia is to identify those who would benefit from starting treatment with disease-modifying antirheumatic drugs as early as possible to potentially improve outcomes, but it also could help to discriminate between the anti-citrullinated protein antibody (ACPA)-positive and seronegative individuals without clinical signs of inflammation at baseline who may progress from arthralgia to IA.
“Although ACPA positivity is a very good predictor for those patients who will develop IA within 1 year, it is still difficult to identify the exact individuals who will develop IA, because any ACPA-positive individual has an a priori chance of 50% of developing IA. In seronegative patients, the prediction of IA is even more difficult, because only 5% develop IA within the subsequent year. Imaging techniques have been shown to be able to detect synovitis before its clinical appearance and could be of help in identifying those at risk of IA,” the investigators wrote (Arthritis Res Ther. 2017;19:202. doi: 10.1186/s13075-017-1405-y).
Dr. van der Ven and her associates found that 31 (16%) of 196 patients who had arthralgia for less than 1 year in the hands, feet, or shoulders went on to develop IA after 1 year of follow-up. In this group of 196 patients at baseline, 72 (37%) had synovitis on ultrasound – defined as a greyscale grade of 2 or 3 and/or the presence of power Doppler signal (grade 1, 2, or 3) – including 32 with a positive power Doppler. A total of 18 patients were lost to follow-up during the first 6 months and another 19 were lost during months 6-12.
Rheumatologists who were unaware of ultrasound findings had to confirm soft-tissue swelling as arthritis at 1 year to classify it as incident IA. The positive predictive value of ultrasound for IA was only 26% when at least 1 joint out of 26 assessed was positive, but the negative predictive value when no joints were positive on ultrasound was 89%.
Overall, at 1 year, 15 of the 31 patients with IA had started therapy with a disease-modifying antirheumatic drug and 22 did not have a definite diagnosis; 12 had monoarthritis and 10 had polyarthritis. The remaining nine patients included four with rheumatoid arthritis, four with psoriatic arthritis, and one with spondyloarthritis.
At baseline, individuals with IA were more often older (mean age 50 vs. 44 years; P = .005), had synovitis on ultrasound (59% vs. 32%; P = .007), and had a positive power Doppler signal (31% vs. 12%; P = .012). A multivariate analysis revealed that IA at 1 year of follow-up could be independently predicted according to age (odds ratio, 1.06), morning stiffness lasting more than 30 minutes (OR, 2.80), ACPA positivity (OR, 2.35), and synovitis on ultrasound (OR, 2.65).
The investigators noted that the study’s limitations relate to requirements for patients to have at least two painful joints in hands, feet, or shoulders at baseline and two criteria related to inflammation. The possible inflammation-related criteria required for entry included morning stiffness for more than 1 hour, inability to clench a fist in the morning, pain when shaking someone’s hand, pins and needles in the fingers, difficulties wearing rings or shoes, family history of rheumatoid arthritis, and/or unexplained fatigue for less than 1 year.
Rheumatologists who enrolled patients into the cohort also may have “recruited clinically suspected patients with possibly more severe symptoms,” the investigators noted. Another potential source of bias related to the group of 38 patients who chose not to participate: It’s possible these patients had less severe symptoms than those who participated in the study.
The study was funded by an investigator-initiated grant from Pfizer. The authors declared that they have no competing interests.
Ultrasound evaluations to look for subclinical inflammation in joints of patients with arthralgia appear best at ruling out inflammatory arthritis (IA) 1 year in the future rather than ruling it in, according to findings from a multicenter cohort study published online in Arthritis Research and Therapy.
The imaging modality’s ability to identify those who will not go on to develop IA complemented the serologic and clinical factors that help to discriminate the individuals with arthralgia who are most at risk of the condition.
The ultimate goal of using imaging such as ultrasound in patients with arthralgia is to identify those who would benefit from starting treatment with disease-modifying antirheumatic drugs as early as possible to potentially improve outcomes, but it also could help to discriminate between the anti-citrullinated protein antibody (ACPA)-positive and seronegative individuals without clinical signs of inflammation at baseline who may progress from arthralgia to IA.
“Although ACPA positivity is a very good predictor for those patients who will develop IA within 1 year, it is still difficult to identify the exact individuals who will develop IA, because any ACPA-positive individual has an a priori chance of 50% of developing IA. In seronegative patients, the prediction of IA is even more difficult, because only 5% develop IA within the subsequent year. Imaging techniques have been shown to be able to detect synovitis before its clinical appearance and could be of help in identifying those at risk of IA,” the investigators wrote (Arthritis Res Ther. 2017;19:202. doi: 10.1186/s13075-017-1405-y).
Dr. van der Ven and her associates found that 31 (16%) of 196 patients who had arthralgia for less than 1 year in the hands, feet, or shoulders went on to develop IA after 1 year of follow-up. In this group of 196 patients at baseline, 72 (37%) had synovitis on ultrasound – defined as a greyscale grade of 2 or 3 and/or the presence of power Doppler signal (grade 1, 2, or 3) – including 32 with a positive power Doppler. A total of 18 patients were lost to follow-up during the first 6 months and another 19 were lost during months 6-12.
Rheumatologists who were unaware of ultrasound findings had to confirm soft-tissue swelling as arthritis at 1 year to classify it as incident IA. The positive predictive value of ultrasound for IA was only 26% when at least 1 joint out of 26 assessed was positive, but the negative predictive value when no joints were positive on ultrasound was 89%.
Overall, at 1 year, 15 of the 31 patients with IA had started therapy with a disease-modifying antirheumatic drug and 22 did not have a definite diagnosis; 12 had monoarthritis and 10 had polyarthritis. The remaining nine patients included four with rheumatoid arthritis, four with psoriatic arthritis, and one with spondyloarthritis.
At baseline, individuals with IA were more often older (mean age 50 vs. 44 years; P = .005), had synovitis on ultrasound (59% vs. 32%; P = .007), and had a positive power Doppler signal (31% vs. 12%; P = .012). A multivariate analysis revealed that IA at 1 year of follow-up could be independently predicted according to age (odds ratio, 1.06), morning stiffness lasting more than 30 minutes (OR, 2.80), ACPA positivity (OR, 2.35), and synovitis on ultrasound (OR, 2.65).
The investigators noted that the study’s limitations relate to requirements for patients to have at least two painful joints in hands, feet, or shoulders at baseline and two criteria related to inflammation. The possible inflammation-related criteria required for entry included morning stiffness for more than 1 hour, inability to clench a fist in the morning, pain when shaking someone’s hand, pins and needles in the fingers, difficulties wearing rings or shoes, family history of rheumatoid arthritis, and/or unexplained fatigue for less than 1 year.
Rheumatologists who enrolled patients into the cohort also may have “recruited clinically suspected patients with possibly more severe symptoms,” the investigators noted. Another potential source of bias related to the group of 38 patients who chose not to participate: It’s possible these patients had less severe symptoms than those who participated in the study.
The study was funded by an investigator-initiated grant from Pfizer. The authors declared that they have no competing interests.
FROM ARTHRITIS RESEARCH AND THERAPY
Key clinical point:
Major finding: The positive predictive value of ultrasound for IA was only 26% when at least 1 joint out of 26 assessed was positive, but the negative predictive value when no joints were positive on ultrasound was 89%.
Data source: A multicenter cohort study of 196 patients with arthralgia in at least two joints for less than 1 year.
Disclosures: The study was funded by an investigator-initiated grant from Pfizer. The authors declared that they have no competing interests.
Medial Oblique Meniscomeniscal Ligament of Knee
Take-Home Points
- Prevalence of the medial oblique meniscomeniscal ligament is 1% to 4%.
- It is important to distinguish this ligament from a meniscus tear on MRI.
- The functional characteristics of this ligament are not well understood.
- What may appear to be a meniscal tear in a younger patient could be a medial oblique meniscomeniscal ligament.
- Dr. Flanigan recommends leaving the ligament intact unless resection is needed to provide better visualization.
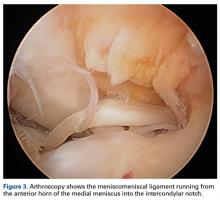
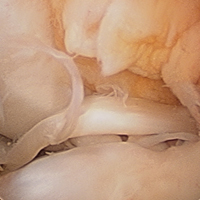
We report a case of aberrant meniscus attachment in the setting of anterior cruciate ligament (ACL) injury. An anomalous cordlike attachment ran from the anterior horn of the medial meniscus to the posterior horn of the lateral meniscus through the intercondylar notch. This attachment was previously named the medial oblique meniscomeniscal ligament1 but has seldom been reported in the literature. Prevalence is 1% to 4%.1,2 This case was treated at Ohio State University Wexner Medical Center in Columbus. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old man presented with left knee pain after sustaining 2 injuries to the knee. The first injury occurred during a dodgeball game—when the knee buckled on landing from a jump. A “pop” was felt, and the knee swelled immediately. The second injury occurred about 3 months later, during soccer play. The patient was running when his foot slipped and caused the knee to buckle. Again, a “pop” was felt, and there was swelling. Mechanical symptoms of clicking then started. The patient reported no instability episodes. His medical history and family history were otherwise unremarkable. The patient was healthy and had a body mass index of 23.05 kg/m2.
Physical examination revealed no effusion, erythema, or warmth in the left knee. Range of motion was 0° to 135° in the left knee and 0° to 140° in the right knee. There was no pain on hyperextension of the knee or medial or lateral joint-line tenderness, but there was pain on hyperflexion, and the McMurray test was positive. Ligament examination was negative except for positive anterior drawer, Lachman, and pivot-shift tests.
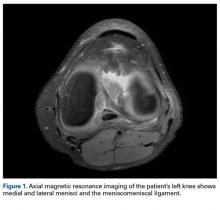
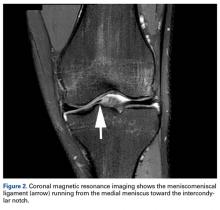
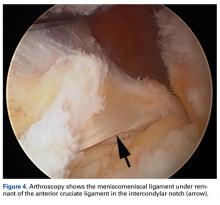
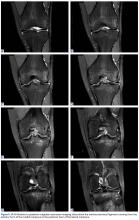
Radiographs taken the day of the first clinic visit showed no acute osseous abnormality. Magnetic resonance imaging (MRI) showed complete disruption of the proximal fibers of the ACL (Figures 1, 2).
Also observed was a small oblique tear of the body of the lateral meniscus with slight blunting of the anterior horn of the medial meniscus, which may have been related to a small tear. A pivot-shift contusion pattern with impaction fracture of the lateral femoral condyle was also appreciated. There were no definite cartilage defects identified.
Discussion
The medial and lateral menisci typically are separate fibrocartilaginous structures acting as a cushion for the knee, but normal variant connections between the structures have been described. These connections include the anterior transverse meniscal ligament, the posterior transverse meniscal ligament, and the medial and lateral oblique meniscomeniscal ligaments.3 In the present case, a medial oblique meniscomeniscal ligament was identified. Its path between menisci was traceable on coronal and axial views. Video taken during arthroscopy also clearly showed its path and its relationship to other structures in the knee. To Dr. Flanigan’s knowledge, this ligament was not previously described with video. It is important to distinguish this ligament from a horizontal tear of the meniscus, given the potential for misinterpretation on MRI. A horizontal tear is a degenerative change that often occurs in older patients. Our patient was 18 years old at time of injury. In addition, the surface of his lower meniscus was smooth, whereas in a tear the edge is irregular and discontinuous. Dr. Flanigan prefers to leave this ligament intact unless resection would provide better visualization during arthroscopy. His reasoning is that the functional characteristics of the ligament are not well understood.
There are few reports on the medial oblique meniscomeniscal ligament.1 Sanders and colleagues1 found 3 cases of this normal variant. In the first, the ligament was interpreted as a flap tear on MRI; in the other 2 cases, the ligament was correctly identified. Kim and Laor2 and Dervin and Paterson4 also described this variant in case reports.
There are many abnormalities of the meniscus. In our literature review, we found reports on various anomalies, including discoid meniscus,5 ring-shape meniscus,6,7 accessory meniscus,8 double-layer meniscus,9-12 abnormal band formation,13,14 hypoplasia,15 Wrisberg meniscus,6 and congenital absence of meniscus.16 These variations have multifactorial causes, including congenital and developmental influences.
In a recent case report, Giordano and Goldblatt14 described an abnormal band of lateral meniscus extending from the posterior horn to the anterior-mid portion of the same meniscus. Lee and Min13 described the same band earlier, in a 2-patient case report.13 One patient presented symptomatically, nontraumatically, and the other with a posterior cruciate ligament tear. Each case was deemed congenital given the characteristic appearance and bilaterality of the anomaly.
In an 11-patient case series in Finland, Rainio and colleagues17 described an attachment from the anterior horn of the medial meniscus inserting into the ACL—a crescent band from the upper surface of the anterior horn that attached along the upper two thirds of the ACL.
At 2-year follow-up, our patient was doing well with rehabilitation and experienced only minimal symptoms. Radiologists and surgeons should be able to identify such variants. Knowing the common and rare variants, radiologists can help surgeons by identifying normal anatomy from pathology and providing a more clinically relevant report. Surgeons should be aware of the anatomical variability in the knee in order to provide the best care for their patients.
1. Sanders TG, Linares RC, Lawhorn KW, Tirman PF, Houser C. Oblique meniscomeniscal ligament: another potential pitfall for a meniscal tear—anatomic description and appearance at MR imaging in three cases. Radiology. 1999;213(1):213-216.
2. Kim HK, Laor T. Oblique meniscomeniscal ligament: a normal variant. Pediatr Radiol. 2009;39(6):634.
3. Chan CM, Goldblatt JP. Unilateral meniscomeniscal ligament. Orthopedics. 2012;35(12):e1815-e1817.
4. Dervin GF, Paterson RS. Oblique menisco-meniscal ligament of the knee. Arthroscopy. 1997;13(3):363-365.
5. Sun Y, Jiang Q. Review of discoid meniscus. Orthop Surg. 2011;3(4):219-223.
6. Kim YG, Ihn JC, Park SK, Kyung HS. An arthroscopic analysis of lateral meniscal variants and a comparison with MRI findings. Knee Surg Sports Traumatol Arthrosc. 2006;14(1):20-26.
7. Kim SJ, Jeon CH, Koh CH. A ring-shaped lateral meniscus. Arthroscopy. 1995;11(6):738-739.
8. Karahan M, Erol B. Accessory lateral meniscus: a case report. Am J Sports Med. 2004;32(8):1973-1976.
9. Okahashi K, Sugimoto K, Iwai M, Oshima M, Fujisawa Y, Takakura Y. Double-layered lateral meniscus. J Orthop Sci. 2005;10(6):661-664.
10. Karataglis D, Dramis A, Learmonth DJ. Double-layered lateral meniscus. A rare anatomical aberration. Knee. 2006;13(5):415-416.
11. Takayama K, Kuroda R, Matsumoto T, et al. Bilateral double-layered lateral meniscus: a report of two cases. Knee Surg Sports Traumatol Arthrosc. 2009;17(11):1336-1339.
12. Wang Q, Liu XM, Liu SB, Bai Y. Double-layered lateral meniscus. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2050-2051.
13. Lee BI, Min KD. Abnormal band of the lateral meniscus of the knee. Arthroscopy. 2000;16(6):11.
14. Giordano B, Goldblatt J. Abnormal band of lateral meniscus. Orthopedics. 2009;32(1):51.
15. Ohana N, Plotquin D, Atar D. Bilateral hypoplastic lateral meniscus. Arthroscopy. 1995;11(6):740-742.
16. Tolo VT. Congenital absence of the menisci and cruciate ligaments of the knee. A case report. J Bone Joint Surg Am. 1981;63(6):1022-1024.
17. Rainio P, Sarimo J, Rantanen J, Alanen J, Orava S. Observation of anomalous insertion of the medial meniscus on the anterior cruciate ligament. Arthroscopy. 2002;18(2):E9.
Take-Home Points
- Prevalence of the medial oblique meniscomeniscal ligament is 1% to 4%.
- It is important to distinguish this ligament from a meniscus tear on MRI.
- The functional characteristics of this ligament are not well understood.
- What may appear to be a meniscal tear in a younger patient could be a medial oblique meniscomeniscal ligament.
- Dr. Flanigan recommends leaving the ligament intact unless resection is needed to provide better visualization.
We report a case of aberrant meniscus attachment in the setting of anterior cruciate ligament (ACL) injury. An anomalous cordlike attachment ran from the anterior horn of the medial meniscus to the posterior horn of the lateral meniscus through the intercondylar notch. This attachment was previously named the medial oblique meniscomeniscal ligament1 but has seldom been reported in the literature. Prevalence is 1% to 4%.1,2 This case was treated at Ohio State University Wexner Medical Center in Columbus. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old man presented with left knee pain after sustaining 2 injuries to the knee. The first injury occurred during a dodgeball game—when the knee buckled on landing from a jump. A “pop” was felt, and the knee swelled immediately. The second injury occurred about 3 months later, during soccer play. The patient was running when his foot slipped and caused the knee to buckle. Again, a “pop” was felt, and there was swelling. Mechanical symptoms of clicking then started. The patient reported no instability episodes. His medical history and family history were otherwise unremarkable. The patient was healthy and had a body mass index of 23.05 kg/m2.
Physical examination revealed no effusion, erythema, or warmth in the left knee. Range of motion was 0° to 135° in the left knee and 0° to 140° in the right knee. There was no pain on hyperextension of the knee or medial or lateral joint-line tenderness, but there was pain on hyperflexion, and the McMurray test was positive. Ligament examination was negative except for positive anterior drawer, Lachman, and pivot-shift tests.
Radiographs taken the day of the first clinic visit showed no acute osseous abnormality. Magnetic resonance imaging (MRI) showed complete disruption of the proximal fibers of the ACL (Figures 1, 2).
Also observed was a small oblique tear of the body of the lateral meniscus with slight blunting of the anterior horn of the medial meniscus, which may have been related to a small tear. A pivot-shift contusion pattern with impaction fracture of the lateral femoral condyle was also appreciated. There were no definite cartilage defects identified.
Discussion
The medial and lateral menisci typically are separate fibrocartilaginous structures acting as a cushion for the knee, but normal variant connections between the structures have been described. These connections include the anterior transverse meniscal ligament, the posterior transverse meniscal ligament, and the medial and lateral oblique meniscomeniscal ligaments.3 In the present case, a medial oblique meniscomeniscal ligament was identified. Its path between menisci was traceable on coronal and axial views. Video taken during arthroscopy also clearly showed its path and its relationship to other structures in the knee. To Dr. Flanigan’s knowledge, this ligament was not previously described with video. It is important to distinguish this ligament from a horizontal tear of the meniscus, given the potential for misinterpretation on MRI. A horizontal tear is a degenerative change that often occurs in older patients. Our patient was 18 years old at time of injury. In addition, the surface of his lower meniscus was smooth, whereas in a tear the edge is irregular and discontinuous. Dr. Flanigan prefers to leave this ligament intact unless resection would provide better visualization during arthroscopy. His reasoning is that the functional characteristics of the ligament are not well understood.
There are few reports on the medial oblique meniscomeniscal ligament.1 Sanders and colleagues1 found 3 cases of this normal variant. In the first, the ligament was interpreted as a flap tear on MRI; in the other 2 cases, the ligament was correctly identified. Kim and Laor2 and Dervin and Paterson4 also described this variant in case reports.
There are many abnormalities of the meniscus. In our literature review, we found reports on various anomalies, including discoid meniscus,5 ring-shape meniscus,6,7 accessory meniscus,8 double-layer meniscus,9-12 abnormal band formation,13,14 hypoplasia,15 Wrisberg meniscus,6 and congenital absence of meniscus.16 These variations have multifactorial causes, including congenital and developmental influences.
In a recent case report, Giordano and Goldblatt14 described an abnormal band of lateral meniscus extending from the posterior horn to the anterior-mid portion of the same meniscus. Lee and Min13 described the same band earlier, in a 2-patient case report.13 One patient presented symptomatically, nontraumatically, and the other with a posterior cruciate ligament tear. Each case was deemed congenital given the characteristic appearance and bilaterality of the anomaly.
In an 11-patient case series in Finland, Rainio and colleagues17 described an attachment from the anterior horn of the medial meniscus inserting into the ACL—a crescent band from the upper surface of the anterior horn that attached along the upper two thirds of the ACL.
At 2-year follow-up, our patient was doing well with rehabilitation and experienced only minimal symptoms. Radiologists and surgeons should be able to identify such variants. Knowing the common and rare variants, radiologists can help surgeons by identifying normal anatomy from pathology and providing a more clinically relevant report. Surgeons should be aware of the anatomical variability in the knee in order to provide the best care for their patients.
Take-Home Points
- Prevalence of the medial oblique meniscomeniscal ligament is 1% to 4%.
- It is important to distinguish this ligament from a meniscus tear on MRI.
- The functional characteristics of this ligament are not well understood.
- What may appear to be a meniscal tear in a younger patient could be a medial oblique meniscomeniscal ligament.
- Dr. Flanigan recommends leaving the ligament intact unless resection is needed to provide better visualization.
We report a case of aberrant meniscus attachment in the setting of anterior cruciate ligament (ACL) injury. An anomalous cordlike attachment ran from the anterior horn of the medial meniscus to the posterior horn of the lateral meniscus through the intercondylar notch. This attachment was previously named the medial oblique meniscomeniscal ligament1 but has seldom been reported in the literature. Prevalence is 1% to 4%.1,2 This case was treated at Ohio State University Wexner Medical Center in Columbus. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old man presented with left knee pain after sustaining 2 injuries to the knee. The first injury occurred during a dodgeball game—when the knee buckled on landing from a jump. A “pop” was felt, and the knee swelled immediately. The second injury occurred about 3 months later, during soccer play. The patient was running when his foot slipped and caused the knee to buckle. Again, a “pop” was felt, and there was swelling. Mechanical symptoms of clicking then started. The patient reported no instability episodes. His medical history and family history were otherwise unremarkable. The patient was healthy and had a body mass index of 23.05 kg/m2.
Physical examination revealed no effusion, erythema, or warmth in the left knee. Range of motion was 0° to 135° in the left knee and 0° to 140° in the right knee. There was no pain on hyperextension of the knee or medial or lateral joint-line tenderness, but there was pain on hyperflexion, and the McMurray test was positive. Ligament examination was negative except for positive anterior drawer, Lachman, and pivot-shift tests.
Radiographs taken the day of the first clinic visit showed no acute osseous abnormality. Magnetic resonance imaging (MRI) showed complete disruption of the proximal fibers of the ACL (Figures 1, 2).
Also observed was a small oblique tear of the body of the lateral meniscus with slight blunting of the anterior horn of the medial meniscus, which may have been related to a small tear. A pivot-shift contusion pattern with impaction fracture of the lateral femoral condyle was also appreciated. There were no definite cartilage defects identified.
Discussion
The medial and lateral menisci typically are separate fibrocartilaginous structures acting as a cushion for the knee, but normal variant connections between the structures have been described. These connections include the anterior transverse meniscal ligament, the posterior transverse meniscal ligament, and the medial and lateral oblique meniscomeniscal ligaments.3 In the present case, a medial oblique meniscomeniscal ligament was identified. Its path between menisci was traceable on coronal and axial views. Video taken during arthroscopy also clearly showed its path and its relationship to other structures in the knee. To Dr. Flanigan’s knowledge, this ligament was not previously described with video. It is important to distinguish this ligament from a horizontal tear of the meniscus, given the potential for misinterpretation on MRI. A horizontal tear is a degenerative change that often occurs in older patients. Our patient was 18 years old at time of injury. In addition, the surface of his lower meniscus was smooth, whereas in a tear the edge is irregular and discontinuous. Dr. Flanigan prefers to leave this ligament intact unless resection would provide better visualization during arthroscopy. His reasoning is that the functional characteristics of the ligament are not well understood.
There are few reports on the medial oblique meniscomeniscal ligament.1 Sanders and colleagues1 found 3 cases of this normal variant. In the first, the ligament was interpreted as a flap tear on MRI; in the other 2 cases, the ligament was correctly identified. Kim and Laor2 and Dervin and Paterson4 also described this variant in case reports.
There are many abnormalities of the meniscus. In our literature review, we found reports on various anomalies, including discoid meniscus,5 ring-shape meniscus,6,7 accessory meniscus,8 double-layer meniscus,9-12 abnormal band formation,13,14 hypoplasia,15 Wrisberg meniscus,6 and congenital absence of meniscus.16 These variations have multifactorial causes, including congenital and developmental influences.
In a recent case report, Giordano and Goldblatt14 described an abnormal band of lateral meniscus extending from the posterior horn to the anterior-mid portion of the same meniscus. Lee and Min13 described the same band earlier, in a 2-patient case report.13 One patient presented symptomatically, nontraumatically, and the other with a posterior cruciate ligament tear. Each case was deemed congenital given the characteristic appearance and bilaterality of the anomaly.
In an 11-patient case series in Finland, Rainio and colleagues17 described an attachment from the anterior horn of the medial meniscus inserting into the ACL—a crescent band from the upper surface of the anterior horn that attached along the upper two thirds of the ACL.
At 2-year follow-up, our patient was doing well with rehabilitation and experienced only minimal symptoms. Radiologists and surgeons should be able to identify such variants. Knowing the common and rare variants, radiologists can help surgeons by identifying normal anatomy from pathology and providing a more clinically relevant report. Surgeons should be aware of the anatomical variability in the knee in order to provide the best care for their patients.
1. Sanders TG, Linares RC, Lawhorn KW, Tirman PF, Houser C. Oblique meniscomeniscal ligament: another potential pitfall for a meniscal tear—anatomic description and appearance at MR imaging in three cases. Radiology. 1999;213(1):213-216.
2. Kim HK, Laor T. Oblique meniscomeniscal ligament: a normal variant. Pediatr Radiol. 2009;39(6):634.
3. Chan CM, Goldblatt JP. Unilateral meniscomeniscal ligament. Orthopedics. 2012;35(12):e1815-e1817.
4. Dervin GF, Paterson RS. Oblique menisco-meniscal ligament of the knee. Arthroscopy. 1997;13(3):363-365.
5. Sun Y, Jiang Q. Review of discoid meniscus. Orthop Surg. 2011;3(4):219-223.
6. Kim YG, Ihn JC, Park SK, Kyung HS. An arthroscopic analysis of lateral meniscal variants and a comparison with MRI findings. Knee Surg Sports Traumatol Arthrosc. 2006;14(1):20-26.
7. Kim SJ, Jeon CH, Koh CH. A ring-shaped lateral meniscus. Arthroscopy. 1995;11(6):738-739.
8. Karahan M, Erol B. Accessory lateral meniscus: a case report. Am J Sports Med. 2004;32(8):1973-1976.
9. Okahashi K, Sugimoto K, Iwai M, Oshima M, Fujisawa Y, Takakura Y. Double-layered lateral meniscus. J Orthop Sci. 2005;10(6):661-664.
10. Karataglis D, Dramis A, Learmonth DJ. Double-layered lateral meniscus. A rare anatomical aberration. Knee. 2006;13(5):415-416.
11. Takayama K, Kuroda R, Matsumoto T, et al. Bilateral double-layered lateral meniscus: a report of two cases. Knee Surg Sports Traumatol Arthrosc. 2009;17(11):1336-1339.
12. Wang Q, Liu XM, Liu SB, Bai Y. Double-layered lateral meniscus. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2050-2051.
13. Lee BI, Min KD. Abnormal band of the lateral meniscus of the knee. Arthroscopy. 2000;16(6):11.
14. Giordano B, Goldblatt J. Abnormal band of lateral meniscus. Orthopedics. 2009;32(1):51.
15. Ohana N, Plotquin D, Atar D. Bilateral hypoplastic lateral meniscus. Arthroscopy. 1995;11(6):740-742.
16. Tolo VT. Congenital absence of the menisci and cruciate ligaments of the knee. A case report. J Bone Joint Surg Am. 1981;63(6):1022-1024.
17. Rainio P, Sarimo J, Rantanen J, Alanen J, Orava S. Observation of anomalous insertion of the medial meniscus on the anterior cruciate ligament. Arthroscopy. 2002;18(2):E9.
1. Sanders TG, Linares RC, Lawhorn KW, Tirman PF, Houser C. Oblique meniscomeniscal ligament: another potential pitfall for a meniscal tear—anatomic description and appearance at MR imaging in three cases. Radiology. 1999;213(1):213-216.
2. Kim HK, Laor T. Oblique meniscomeniscal ligament: a normal variant. Pediatr Radiol. 2009;39(6):634.
3. Chan CM, Goldblatt JP. Unilateral meniscomeniscal ligament. Orthopedics. 2012;35(12):e1815-e1817.
4. Dervin GF, Paterson RS. Oblique menisco-meniscal ligament of the knee. Arthroscopy. 1997;13(3):363-365.
5. Sun Y, Jiang Q. Review of discoid meniscus. Orthop Surg. 2011;3(4):219-223.
6. Kim YG, Ihn JC, Park SK, Kyung HS. An arthroscopic analysis of lateral meniscal variants and a comparison with MRI findings. Knee Surg Sports Traumatol Arthrosc. 2006;14(1):20-26.
7. Kim SJ, Jeon CH, Koh CH. A ring-shaped lateral meniscus. Arthroscopy. 1995;11(6):738-739.
8. Karahan M, Erol B. Accessory lateral meniscus: a case report. Am J Sports Med. 2004;32(8):1973-1976.
9. Okahashi K, Sugimoto K, Iwai M, Oshima M, Fujisawa Y, Takakura Y. Double-layered lateral meniscus. J Orthop Sci. 2005;10(6):661-664.
10. Karataglis D, Dramis A, Learmonth DJ. Double-layered lateral meniscus. A rare anatomical aberration. Knee. 2006;13(5):415-416.
11. Takayama K, Kuroda R, Matsumoto T, et al. Bilateral double-layered lateral meniscus: a report of two cases. Knee Surg Sports Traumatol Arthrosc. 2009;17(11):1336-1339.
12. Wang Q, Liu XM, Liu SB, Bai Y. Double-layered lateral meniscus. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2050-2051.
13. Lee BI, Min KD. Abnormal band of the lateral meniscus of the knee. Arthroscopy. 2000;16(6):11.
14. Giordano B, Goldblatt J. Abnormal band of lateral meniscus. Orthopedics. 2009;32(1):51.
15. Ohana N, Plotquin D, Atar D. Bilateral hypoplastic lateral meniscus. Arthroscopy. 1995;11(6):740-742.
16. Tolo VT. Congenital absence of the menisci and cruciate ligaments of the knee. A case report. J Bone Joint Surg Am. 1981;63(6):1022-1024.
17. Rainio P, Sarimo J, Rantanen J, Alanen J, Orava S. Observation of anomalous insertion of the medial meniscus on the anterior cruciate ligament. Arthroscopy. 2002;18(2):E9.
Reliability of 3-Dimensional Glenoid Component Templating and Correlation to Intraoperative Component Selection
Take-Home Points
- Guidelines regarding glenoid component size selection for primary TSA are lacking.
- Intraoperative in situ glenoid sizing may not be ideal.
- 3-D digital models may be utilized for preoperative templating of glenoid component size in primary TSA.
- 3-D templating that allows for superior-inferior, anterior-posterior, and rotational translation can lead to consistent and reproducible templating of glenoid component size.
- 3-D templating may reduce the risks of implant overhang, peg penetration, and decreased stability ratio.
In 1974, Neer1 introduced the shoulder prosthesis. In 1982, Neer and colleagues2 found significant improvement in shoulder pain and function in patients with glenohumeral osteoarthritis treated with the Neer prosthesis. Since then, use of total shoulder arthroplasty (TSA) has increased. Between 1993 and 2007, TSA use increased 319% in the United States.3 Long-term outcomes studies have found implant survivorship ranging from 87% to 93% at 10 to 15 years.4
Although TSA is a successful procedure, glenoid component failure is the most common complication.5-10 Outcomes of revision surgery for glenoid instability are inferior to those of primary TSA.11 Recent research findings highlight the effect of glenoid size on TSA complications.12 A larger glenoid component increases the stability ratio (peak subluxation force divided by compression load).12 However, insufficient glenoid bone stock, small glenoid diameter, and inability to fit a properly sized reamer owing to soft-tissue constraints may lead surgeons to choose a smaller glenoid component in order to avoid peg penetration, overhang, and soft-tissue damage, respectively. Therefore, preoperative templating of glenoid size is a potential strategy for minimizing complications.
Templating is performed for proximal humeral components, but glenoid sizing typically is deferred to intraoperative in situ sizing with implant-specific targeting guides. This glenoid sizing practice arose out of a lack of standard digital glenoid templates and difficulty in selecting glenoid size based on plain radiographs and/or 2-dimensional (2-D) computed tomography (CT) scans. However, targeting devices are sporadically used during surgery, and intraoperative glenoid vault dimension estimates derived from visualization and palpation are often inaccurate. Often, rather than directly assess glenoid morphology, surgeons infer glenoid size from the size and sex of patients.13
Three-dimensional (3-D) CT can be used to accurately assess glenoid version, bone loss, and implant fit.14-19 We conducted a study to determine if 3-D digital imaging can be consistently and reproducibly used for preoperative templating of glenoid component size and to determine if glenoid sizes derived from templating correlate with the sizes of subsequently implanted glenoids.
Materials and Methods
This retrospective study was conducted at the Center for Shoulder, Elbow, and Sports Medicine at Columbia University Medical Center in New York City and was approved by our Institutional Review Board. Included in the study were all patients who underwent primary TSA for primary glenohumeral osteoarthritis over a 12-month period. Patients were required to have preoperative CT performed according to our study protocol. The CT protocol consisted of 0.5-mm axial cuts of the entire scapula and 3-D reconstruction of the scapula, glenoid, glenohumeral articulation, and proximal humerus. Patients were excluded from the study for primary TSA for a secondary cause of glenohumeral osteoarthritis, inflammatory arthritis, connective tissue disease, prior contralateral TSA, and prior ipsilateral scapula, glenoid, and proximal humerus surgery. Ultimately, 24 patients were included in the study.
CT data were formatted for preoperative templating. The CT images of each patient’s scapula were uploaded into Materialise Interactive Medical Image Control System (Mimics) software. Mimics allows 3-D image rendering and editing from various imaging modalities and formats. The software was used to create the 3-D scapula models for templating. Prior studies have validated the anatomical precision of 3-D models created with Mimics.20
Mimics was also used to digitize in 3-D the glenoid components from the Bigliani-Flatow Shoulder System (Zimmer Biomet). Glenoid components of 3 different sizes (40 mm, 46 mm, 52 mm) were used. (The Bigliani glenoid component was digitized, as this implant system was used for primary TSA in all 24 patients.) Each glenoid component was traced in 3-D with a Gage 2000 coordinate-measuring machine (Brown & Sharpe) and was processed with custom software. The custom software, cited in previous work by our group,17 created the same coordinate system for each scapula based on anatomical reference points. These digitized 3-D images of glenoid components were uploaded with the digitized 3-D scapulae derived from patients’ CT scans to the Magics software. Magics allows for manipulation and interaction of multiple 3-D models by creating electronic stereolithography files that provide 3-D surface geometry.
Three fellowship-trained shoulder surgeons and 4 shoulder fellows templated the most appropriately sized glenoid component for each of the 24 patients. At the time of templating, the surgeon was blinded to the size of the glenoid implant used in the surgery. In Magics, each scapula was positioned in 3-D similar to how it would appear with the patient in the beach-chair position during surgery. In both study arms, surgeons selected the largest component that maximized the area of contact while avoiding peg penetration of the glenoid vault or component overhang. In addition, surgeons were instructed to correct glenoid version to as near neutral as possible with component positioning but were not permitted to remove glenoid bone stock to correct deformity. All surgeons based placement of the glenoid component on the patient’s actual bone stock and not on osteophytes, which are readily appreciable on 3-D CT.
In study arm 1, the 3-D view of the glenoid was restricted to the initial view in the beach-chair position. The surgeon then manipulated the 3-D glenoid component template across a single 2-D plane, either the superior-inferior plane or the anterior-posterior plane, over the surface of the 3-D glenoid (Figure 1).
In study arm 2, surgeons were permitted to rotate the 3-D glenoid template and scapula in any manner (Figure 2).
Interobserver agreement was determined by comparing prosthetic glenoid component size selection among all study surgeons, and intraobserver agreement was determined by comparing glenoid size selection during 2 sessions separated by at least 3 weeks.
After each trial, the order of patients’ scapula images was randomly rearranged to reduce recall bias. Kappa (κ) coefficients were calculated for interobserver and intraobserver agreement. Kappas ranged from −1.0 (least agreement) to +1.0 (complete agreement). A κ of 0 indicated an observer selection was equivalent to random chance. The level of agreement was categorized according to κ using a system described by Landis and Koch21 (Table 1).
Results
The group of 24 patients consisted of 15 men and 9 women. Mean age was 70.3 years (range, 56-88 years). Primary TSA was performed in 14 right shoulders and 10 left shoulders. Of the 24 patients, 20 (83%) had a 46-mm glenoid component implanted, 3 male patients had a 52-mm glenoid component implanted, and 1 female patient had a 40-mm glenoid component implanted.
Study Arm 1: Glenoid Templating Based on 2 df
In study arm 1, overall intraobserver agreement was substantial, as defined in the statistical literature.21 Among all surgeons who participated, intraobserver agreeement was 0.76 (substantial), 0.60 (substantial), and 0.58 (moderate) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.67, substantial agreement). Trial 1 interobserver agreement was 0.56 (moderate) (P < .001), 0.25 (fair) (P < .001), and 0.21 (fair) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.36, fair agreement) (P < .001), and trial 2 interobserver agreement was 0.58 (moderate) (P < .001), 0.18 (poor) (P = .003), and 0.24 (fair) (P <.001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.32, fair agreement) (P < .001). In study arm 1, therefore, trials 1 and 2 both showed fair interobserver agreement.
Study Arm 2: Glenoid Templating Based on 6 df
In study arm 2, a mean correlation of 0.42 (moderate agreement) was found between glenoid component size in 3-D templating and the glenoid component size ultimately selected during surgery (Table 3).
In study arm 2, overall intraobserver agreement was moderate. Among all surgeons who participated, intraobserver agreement was 0.80 (excellent), 0.43 (moderate), and 0.47 (moderate) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.58, moderate agreement). Trial 1 interobserver agreement was 0.75 (substantial) (P < .001), 0.39 (fair) (P < .001), and 0.50 (moderate) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.54, moderate agreement) (P < .001), and trial 2 interobserver agreement was 0.66 (substantial) (P < .001), 0.28 (fair) (P = .003), and 0.40 (moderate) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.43, moderate agreement) (P < .001).
Discussion
Our results showed that 3-D glenoid templating had reproducible intraobserver and interobserver agreement. Overall intraobserver agreement was substantial (κ = 0.67) for study arm 1 and moderate (κ = 0.58) for study arm 2. Interobserver agreement was fair for trials 1 and 2 (κ = 0.36 and 0.32) in arm 1 and moderate for trials 1 and 2 (κ = 0.54 and 0.43) in arm 2.
Intraobserver and interobserver agreement values, particularly in study arm 2, which incorporated rotation (6 df), are consistent with values in commonly used classification systems, such as the Neer system for proximal humerus fractures, the Frykman system for distal radius fractures, and the King system for adolescent idiopathic scoliosis.22-30 Sidor and colleagues27 found overall interobserver agreement of 0.50 and overall intraobserver agreement of 0.66 for the Neer system, and Illarramendi and colleagues24 found overall interobserver agreement of 0.43 and overall intraobserver agreement of 0.61 for the Frykman system.
In study arm 2, overall interobserver and intraobserver agreement was moderate. A higher level of surgeon agreement is unlikely given the lack of well-defined parameters for determining glenoid component size. Therefore, glenoid size selection is largely a matter of surgeon preference. More research is needed to establish concrete guidelines for glenoid component size selection. Once guidelines are adopted, interobserver agreement in templating may increase.
In both study arms, the component that surgeons selected during templating tended to be smaller than the component they selected during surgery. In study arm 1, 32% of patients had a smaller component selected based on computer modeling, and 7% had a larger component selected. In study arm 2, the difference was narrower: 27% of patients had a smaller component selected during templating, and 16% had a larger component selected. A statistically significant difference (P < .001) in templated and implanted component sizes was found between men and women: Templated glenoid components were smaller than implanted components in 53% of women and larger than implanted components in 33% of men. Differences between templated and implanted components may be attributable to visualization differences. During templating, the entire glenoid can be visualized and the slightest peg penetration or component overhang detected; in contrast, during surgery, anatomical constraints preclude such a comprehensive assessment.
Differences in agreement between templated and implanted glenoid components suggest that the size of implanted components may not be ideal. In this study, the distribution of the templated glenoid sizes was much wider than that of the implanted glenoid sizes. During templating, each glenoid component can be definitively visualized and assessed for possible peg penetration and overhang. Visualization allows surgeons to base glenoid size selection solely on glenoid morphology, as opposed to factors such as patient sex and height. In addition, interobserver and intraobserver agreement values for the 40-mm glenoid component were considerably higher than those for components of other sizes, indicating that the 40-mm component was consistently and reproducibly selected for the same patients. Hence, templating may particularly help prevent peg penetration and component overhang for patients with a smaller diameter glenoid.
More research on 3-D templating is warranted given the results of this study and other studies.12,17,31 Scalise and colleagues31 found that, in TSA planning, surgeons’ use of 2-D (vs 3-D) imaging led them to overestimate glenoid component sizes (P = .006). In our study, the glenoid size selected during 3-D templating was, in many cases, smaller than the size selected during surgery. In order to avoid peg penetration and glenoid overhang, anecdotal guidelines commonly used in glenoid size selection, likely was the driving force in selecting smaller glenoid components during templating. Although anterior, superior, and inferior glenoid overhang typically can be assessed during surgery, posterior overhang is more difficult to evaluate. Three-dimensional modeling allows surgeons to determine optimal glenoid component size and position. In addition, intraoperative evaluation of glenoid component peg penetration is challenging, and peg penetration becomes evident only after it has occurred. During templating, however, surgeons were able to easily assess for peg penetration, and smaller glenoid components were selected.
A limitation of this study is that intraoperative glenoid version correction or peg containment was not quantified. More research is needed on the relationship between glenoid size selection and component overhang and peg penetration. Another limitation was use of only 1 TSA system (with 3 glenoid sizes, all with inline pegs); reliability of 3-D templating was not evaluated across different component designs. Last, given the absence of guidelines for glenoid component size selection, there was surgeon bias in preoperative templating and in intraoperative selection of glenoid size. Surgeons had differing opinions on the importance of maximizing the contact area of the component and correcting glenoid deformity and version.
Our study results showed that preoperative 3-D templating that allows for superior-inferior, anterior-posterior, and rotational translation was consistent and reproducible in determining glenoid component size, and use of this templating may reduce the risks of implant overhang, peg penetration, and decreased stability ratio. These results highlight the possibility that glenoid component sizes selected during surgery may not be ideal. More research is needed to determine if intraoperative glenoid size selection leads to adequate version correction and peg containment. The present study supports use of 3-D templating in primary TSA planning.
1. Neer CS 2nd. Replacement arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 1974;56(1):1-13.
2. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
3. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
4. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
5. Barrett WP, Franklin JL, Jackins SE, Wyss CR, Matsen FA 3rd. Total shoulder arthroplasty. J Bone Joint Surg Am. 1987;69(6):865-872.
6. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
7. Matsen FA 3rd, Bicknell RT, Lippitt SB. Shoulder arthroplasty: the socket perspective. J Shoulder Elbow Surg. 2007;16(5 suppl):S241-S247.
8. Matsen FA 3rd, Clinton J, Lynch J, Bertelsen A, Richardson ML. Glenoid component failure in total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(4):885-896.
9. Pearl ML, Romeo AA, Wirth MA, Yamaguchi K, Nicholson GP, Creighton RA. Decision making in contemporary shoulder arthroplasty. Instr Course Lect. 2005;54:69-85.
10. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616.
11. Sanchez-Sotelo J, Sperling JW, Rowland CM, Cofield RH. Instability after shoulder arthroplasty: results of surgical treatment. J Bone Joint Surg Am. 2003;85(4):622-631.
12. Tammachote N, Sperling JW, Berglund LJ, Steinmann SP, Cofield RH, An KN. The effect of glenoid component size on the stability of total shoulder arthroplasty. J Shoulder Elbow Surg. 2007;16(3 suppl):S102-S106.
13. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55.
14. Briem D, Ruecker AH, Neumann J, et al. 3D fluoroscopic navigated reaming of the glenoid for total shoulder arthroplasty (TSA). Comput Aided Surg. 2011;16(2):93-99.
15. Budge MD, Lewis GS, Schaefer E, Coquia S, Flemming DJ, Armstrong AD. Comparison of standard two-dimensional and three-dimensional corrected glenoid version measurements. J Shoulder Elbow Surg. 2011;20(4):577-583.
16. Chuang TY, Adams CR, Burkhart SS. Use of preoperative three-dimensional computed tomography to quantify glenoid bone loss in shoulder instability. Arthroscopy. 2008;24(4):376-382.
17. Nowak DD, Bahu MJ, Gardner TR, et al. Simulation of surgical glenoid resurfacing using three-dimensional computed tomography of the arthritic glenohumeral joint: the amount of glenoid retroversion that can be corrected. J Shoulder Elbow Surg. 2009;18(5):680-688.
18. Scalise JJ, Bryan J, Polster J, Brems JJ, Iannotti JP. Quantitative analysis of glenoid bone loss in osteoarthritis using three-dimensional computed tomography scans. J Shoulder Elbow Surg. 2008;17(2):328-335.
19. Scalise JJ, Codsi MJ, Bryan J, Iannotti JP. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg. 2008;17(3):487-491.
20. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832.
21. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174.
22. Cummings RJ, Loveless EA, Campbell J, Samelson S, Mazur JM. Interobserver reliability and intraobserver reproducibility of the system of King et al. for the classification of adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80(8):1107-1111.
23. Humphrey CA, Dirschl DR, Ellis TJ. Interobserver reliability of a CT-based fracture classification system. J Orthop Trauma. 2005;19(9):616-622.
24. Illarramendi A, González Della Valle A, Segal E, De Carli P, Maignon G, Gallucci G. Evaluation of simplified Frykman and AO classifications of fractures of the distal radius. Assessment of interobserver and intraobserver agreement. Int Orthop. 1998;22(2):111-115.
25. Lenke LG, Betz RR, Bridwell KH, et al. Intraobserver and interobserver reliability of the classification of thoracic adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80(8):1097-1106.
26. Ploegmakers JJ, Mader K, Pennig D, Verheyen CC. Four distal radial fracture classification systems tested amongst a large panel of Dutch trauma surgeons. Injury. 2007;38(11):1268-1272.
27. Sidor ML, Zuckerman JD, Lyon T, Koval K, Cuomo F, Schoenberg N. The Neer classification system for proximal humeral fractures. An assessment of interobserver reliability and intraobserver reproducibility. J Bone Joint Surg Am. 1993;75(12):1745-1750.
28. Siebenrock KA, Gerber C. The reproducibility of classification of fractures of the proximal end of the humerus. J Bone Joint Surg Am. 1993;75(12):1751-1755.
29. Thomsen NO, Overgaard S, Olsen LH, Hansen H, Nielsen ST. Observer variation in the radiographic classification of ankle fractures. J Bone Joint Surg Br. 1991;73(4):676-678.
30. Ward WT, Vogt M, Grudziak JS, Tümer Y, Cook PC, Fitch RD. Severin classification system for evaluation of the results of operative treatment of congenital dislocation of the hip. A study of intraobserver and interobserver reliability. J Bone Joint Surg Am. 1997;79(5):656-663.
31. Scalise JJ, Codsi MJ, Bryan J, Brems JJ, Iannotti JP. The influence of three-dimensional computed tomography images of the shoulder in preoperative planning for total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(11):2438-2445.
Take-Home Points
- Guidelines regarding glenoid component size selection for primary TSA are lacking.
- Intraoperative in situ glenoid sizing may not be ideal.
- 3-D digital models may be utilized for preoperative templating of glenoid component size in primary TSA.
- 3-D templating that allows for superior-inferior, anterior-posterior, and rotational translation can lead to consistent and reproducible templating of glenoid component size.
- 3-D templating may reduce the risks of implant overhang, peg penetration, and decreased stability ratio.
In 1974, Neer1 introduced the shoulder prosthesis. In 1982, Neer and colleagues2 found significant improvement in shoulder pain and function in patients with glenohumeral osteoarthritis treated with the Neer prosthesis. Since then, use of total shoulder arthroplasty (TSA) has increased. Between 1993 and 2007, TSA use increased 319% in the United States.3 Long-term outcomes studies have found implant survivorship ranging from 87% to 93% at 10 to 15 years.4
Although TSA is a successful procedure, glenoid component failure is the most common complication.5-10 Outcomes of revision surgery for glenoid instability are inferior to those of primary TSA.11 Recent research findings highlight the effect of glenoid size on TSA complications.12 A larger glenoid component increases the stability ratio (peak subluxation force divided by compression load).12 However, insufficient glenoid bone stock, small glenoid diameter, and inability to fit a properly sized reamer owing to soft-tissue constraints may lead surgeons to choose a smaller glenoid component in order to avoid peg penetration, overhang, and soft-tissue damage, respectively. Therefore, preoperative templating of glenoid size is a potential strategy for minimizing complications.
Templating is performed for proximal humeral components, but glenoid sizing typically is deferred to intraoperative in situ sizing with implant-specific targeting guides. This glenoid sizing practice arose out of a lack of standard digital glenoid templates and difficulty in selecting glenoid size based on plain radiographs and/or 2-dimensional (2-D) computed tomography (CT) scans. However, targeting devices are sporadically used during surgery, and intraoperative glenoid vault dimension estimates derived from visualization and palpation are often inaccurate. Often, rather than directly assess glenoid morphology, surgeons infer glenoid size from the size and sex of patients.13
Three-dimensional (3-D) CT can be used to accurately assess glenoid version, bone loss, and implant fit.14-19 We conducted a study to determine if 3-D digital imaging can be consistently and reproducibly used for preoperative templating of glenoid component size and to determine if glenoid sizes derived from templating correlate with the sizes of subsequently implanted glenoids.
Materials and Methods
This retrospective study was conducted at the Center for Shoulder, Elbow, and Sports Medicine at Columbia University Medical Center in New York City and was approved by our Institutional Review Board. Included in the study were all patients who underwent primary TSA for primary glenohumeral osteoarthritis over a 12-month period. Patients were required to have preoperative CT performed according to our study protocol. The CT protocol consisted of 0.5-mm axial cuts of the entire scapula and 3-D reconstruction of the scapula, glenoid, glenohumeral articulation, and proximal humerus. Patients were excluded from the study for primary TSA for a secondary cause of glenohumeral osteoarthritis, inflammatory arthritis, connective tissue disease, prior contralateral TSA, and prior ipsilateral scapula, glenoid, and proximal humerus surgery. Ultimately, 24 patients were included in the study.
CT data were formatted for preoperative templating. The CT images of each patient’s scapula were uploaded into Materialise Interactive Medical Image Control System (Mimics) software. Mimics allows 3-D image rendering and editing from various imaging modalities and formats. The software was used to create the 3-D scapula models for templating. Prior studies have validated the anatomical precision of 3-D models created with Mimics.20
Mimics was also used to digitize in 3-D the glenoid components from the Bigliani-Flatow Shoulder System (Zimmer Biomet). Glenoid components of 3 different sizes (40 mm, 46 mm, 52 mm) were used. (The Bigliani glenoid component was digitized, as this implant system was used for primary TSA in all 24 patients.) Each glenoid component was traced in 3-D with a Gage 2000 coordinate-measuring machine (Brown & Sharpe) and was processed with custom software. The custom software, cited in previous work by our group,17 created the same coordinate system for each scapula based on anatomical reference points. These digitized 3-D images of glenoid components were uploaded with the digitized 3-D scapulae derived from patients’ CT scans to the Magics software. Magics allows for manipulation and interaction of multiple 3-D models by creating electronic stereolithography files that provide 3-D surface geometry.
Three fellowship-trained shoulder surgeons and 4 shoulder fellows templated the most appropriately sized glenoid component for each of the 24 patients. At the time of templating, the surgeon was blinded to the size of the glenoid implant used in the surgery. In Magics, each scapula was positioned in 3-D similar to how it would appear with the patient in the beach-chair position during surgery. In both study arms, surgeons selected the largest component that maximized the area of contact while avoiding peg penetration of the glenoid vault or component overhang. In addition, surgeons were instructed to correct glenoid version to as near neutral as possible with component positioning but were not permitted to remove glenoid bone stock to correct deformity. All surgeons based placement of the glenoid component on the patient’s actual bone stock and not on osteophytes, which are readily appreciable on 3-D CT.
In study arm 1, the 3-D view of the glenoid was restricted to the initial view in the beach-chair position. The surgeon then manipulated the 3-D glenoid component template across a single 2-D plane, either the superior-inferior plane or the anterior-posterior plane, over the surface of the 3-D glenoid (Figure 1).
In study arm 2, surgeons were permitted to rotate the 3-D glenoid template and scapula in any manner (Figure 2).
Interobserver agreement was determined by comparing prosthetic glenoid component size selection among all study surgeons, and intraobserver agreement was determined by comparing glenoid size selection during 2 sessions separated by at least 3 weeks.
After each trial, the order of patients’ scapula images was randomly rearranged to reduce recall bias. Kappa (κ) coefficients were calculated for interobserver and intraobserver agreement. Kappas ranged from −1.0 (least agreement) to +1.0 (complete agreement). A κ of 0 indicated an observer selection was equivalent to random chance. The level of agreement was categorized according to κ using a system described by Landis and Koch21 (Table 1).
Results
The group of 24 patients consisted of 15 men and 9 women. Mean age was 70.3 years (range, 56-88 years). Primary TSA was performed in 14 right shoulders and 10 left shoulders. Of the 24 patients, 20 (83%) had a 46-mm glenoid component implanted, 3 male patients had a 52-mm glenoid component implanted, and 1 female patient had a 40-mm glenoid component implanted.
Study Arm 1: Glenoid Templating Based on 2 df
In study arm 1, overall intraobserver agreement was substantial, as defined in the statistical literature.21 Among all surgeons who participated, intraobserver agreeement was 0.76 (substantial), 0.60 (substantial), and 0.58 (moderate) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.67, substantial agreement). Trial 1 interobserver agreement was 0.56 (moderate) (P < .001), 0.25 (fair) (P < .001), and 0.21 (fair) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.36, fair agreement) (P < .001), and trial 2 interobserver agreement was 0.58 (moderate) (P < .001), 0.18 (poor) (P = .003), and 0.24 (fair) (P <.001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.32, fair agreement) (P < .001). In study arm 1, therefore, trials 1 and 2 both showed fair interobserver agreement.
Study Arm 2: Glenoid Templating Based on 6 df
In study arm 2, a mean correlation of 0.42 (moderate agreement) was found between glenoid component size in 3-D templating and the glenoid component size ultimately selected during surgery (Table 3).
In study arm 2, overall intraobserver agreement was moderate. Among all surgeons who participated, intraobserver agreement was 0.80 (excellent), 0.43 (moderate), and 0.47 (moderate) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.58, moderate agreement). Trial 1 interobserver agreement was 0.75 (substantial) (P < .001), 0.39 (fair) (P < .001), and 0.50 (moderate) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.54, moderate agreement) (P < .001), and trial 2 interobserver agreement was 0.66 (substantial) (P < .001), 0.28 (fair) (P = .003), and 0.40 (moderate) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.43, moderate agreement) (P < .001).
Discussion
Our results showed that 3-D glenoid templating had reproducible intraobserver and interobserver agreement. Overall intraobserver agreement was substantial (κ = 0.67) for study arm 1 and moderate (κ = 0.58) for study arm 2. Interobserver agreement was fair for trials 1 and 2 (κ = 0.36 and 0.32) in arm 1 and moderate for trials 1 and 2 (κ = 0.54 and 0.43) in arm 2.
Intraobserver and interobserver agreement values, particularly in study arm 2, which incorporated rotation (6 df), are consistent with values in commonly used classification systems, such as the Neer system for proximal humerus fractures, the Frykman system for distal radius fractures, and the King system for adolescent idiopathic scoliosis.22-30 Sidor and colleagues27 found overall interobserver agreement of 0.50 and overall intraobserver agreement of 0.66 for the Neer system, and Illarramendi and colleagues24 found overall interobserver agreement of 0.43 and overall intraobserver agreement of 0.61 for the Frykman system.
In study arm 2, overall interobserver and intraobserver agreement was moderate. A higher level of surgeon agreement is unlikely given the lack of well-defined parameters for determining glenoid component size. Therefore, glenoid size selection is largely a matter of surgeon preference. More research is needed to establish concrete guidelines for glenoid component size selection. Once guidelines are adopted, interobserver agreement in templating may increase.
In both study arms, the component that surgeons selected during templating tended to be smaller than the component they selected during surgery. In study arm 1, 32% of patients had a smaller component selected based on computer modeling, and 7% had a larger component selected. In study arm 2, the difference was narrower: 27% of patients had a smaller component selected during templating, and 16% had a larger component selected. A statistically significant difference (P < .001) in templated and implanted component sizes was found between men and women: Templated glenoid components were smaller than implanted components in 53% of women and larger than implanted components in 33% of men. Differences between templated and implanted components may be attributable to visualization differences. During templating, the entire glenoid can be visualized and the slightest peg penetration or component overhang detected; in contrast, during surgery, anatomical constraints preclude such a comprehensive assessment.
Differences in agreement between templated and implanted glenoid components suggest that the size of implanted components may not be ideal. In this study, the distribution of the templated glenoid sizes was much wider than that of the implanted glenoid sizes. During templating, each glenoid component can be definitively visualized and assessed for possible peg penetration and overhang. Visualization allows surgeons to base glenoid size selection solely on glenoid morphology, as opposed to factors such as patient sex and height. In addition, interobserver and intraobserver agreement values for the 40-mm glenoid component were considerably higher than those for components of other sizes, indicating that the 40-mm component was consistently and reproducibly selected for the same patients. Hence, templating may particularly help prevent peg penetration and component overhang for patients with a smaller diameter glenoid.
More research on 3-D templating is warranted given the results of this study and other studies.12,17,31 Scalise and colleagues31 found that, in TSA planning, surgeons’ use of 2-D (vs 3-D) imaging led them to overestimate glenoid component sizes (P = .006). In our study, the glenoid size selected during 3-D templating was, in many cases, smaller than the size selected during surgery. In order to avoid peg penetration and glenoid overhang, anecdotal guidelines commonly used in glenoid size selection, likely was the driving force in selecting smaller glenoid components during templating. Although anterior, superior, and inferior glenoid overhang typically can be assessed during surgery, posterior overhang is more difficult to evaluate. Three-dimensional modeling allows surgeons to determine optimal glenoid component size and position. In addition, intraoperative evaluation of glenoid component peg penetration is challenging, and peg penetration becomes evident only after it has occurred. During templating, however, surgeons were able to easily assess for peg penetration, and smaller glenoid components were selected.
A limitation of this study is that intraoperative glenoid version correction or peg containment was not quantified. More research is needed on the relationship between glenoid size selection and component overhang and peg penetration. Another limitation was use of only 1 TSA system (with 3 glenoid sizes, all with inline pegs); reliability of 3-D templating was not evaluated across different component designs. Last, given the absence of guidelines for glenoid component size selection, there was surgeon bias in preoperative templating and in intraoperative selection of glenoid size. Surgeons had differing opinions on the importance of maximizing the contact area of the component and correcting glenoid deformity and version.
Our study results showed that preoperative 3-D templating that allows for superior-inferior, anterior-posterior, and rotational translation was consistent and reproducible in determining glenoid component size, and use of this templating may reduce the risks of implant overhang, peg penetration, and decreased stability ratio. These results highlight the possibility that glenoid component sizes selected during surgery may not be ideal. More research is needed to determine if intraoperative glenoid size selection leads to adequate version correction and peg containment. The present study supports use of 3-D templating in primary TSA planning.
Take-Home Points
- Guidelines regarding glenoid component size selection for primary TSA are lacking.
- Intraoperative in situ glenoid sizing may not be ideal.
- 3-D digital models may be utilized for preoperative templating of glenoid component size in primary TSA.
- 3-D templating that allows for superior-inferior, anterior-posterior, and rotational translation can lead to consistent and reproducible templating of glenoid component size.
- 3-D templating may reduce the risks of implant overhang, peg penetration, and decreased stability ratio.
In 1974, Neer1 introduced the shoulder prosthesis. In 1982, Neer and colleagues2 found significant improvement in shoulder pain and function in patients with glenohumeral osteoarthritis treated with the Neer prosthesis. Since then, use of total shoulder arthroplasty (TSA) has increased. Between 1993 and 2007, TSA use increased 319% in the United States.3 Long-term outcomes studies have found implant survivorship ranging from 87% to 93% at 10 to 15 years.4
Although TSA is a successful procedure, glenoid component failure is the most common complication.5-10 Outcomes of revision surgery for glenoid instability are inferior to those of primary TSA.11 Recent research findings highlight the effect of glenoid size on TSA complications.12 A larger glenoid component increases the stability ratio (peak subluxation force divided by compression load).12 However, insufficient glenoid bone stock, small glenoid diameter, and inability to fit a properly sized reamer owing to soft-tissue constraints may lead surgeons to choose a smaller glenoid component in order to avoid peg penetration, overhang, and soft-tissue damage, respectively. Therefore, preoperative templating of glenoid size is a potential strategy for minimizing complications.
Templating is performed for proximal humeral components, but glenoid sizing typically is deferred to intraoperative in situ sizing with implant-specific targeting guides. This glenoid sizing practice arose out of a lack of standard digital glenoid templates and difficulty in selecting glenoid size based on plain radiographs and/or 2-dimensional (2-D) computed tomography (CT) scans. However, targeting devices are sporadically used during surgery, and intraoperative glenoid vault dimension estimates derived from visualization and palpation are often inaccurate. Often, rather than directly assess glenoid morphology, surgeons infer glenoid size from the size and sex of patients.13
Three-dimensional (3-D) CT can be used to accurately assess glenoid version, bone loss, and implant fit.14-19 We conducted a study to determine if 3-D digital imaging can be consistently and reproducibly used for preoperative templating of glenoid component size and to determine if glenoid sizes derived from templating correlate with the sizes of subsequently implanted glenoids.
Materials and Methods
This retrospective study was conducted at the Center for Shoulder, Elbow, and Sports Medicine at Columbia University Medical Center in New York City and was approved by our Institutional Review Board. Included in the study were all patients who underwent primary TSA for primary glenohumeral osteoarthritis over a 12-month period. Patients were required to have preoperative CT performed according to our study protocol. The CT protocol consisted of 0.5-mm axial cuts of the entire scapula and 3-D reconstruction of the scapula, glenoid, glenohumeral articulation, and proximal humerus. Patients were excluded from the study for primary TSA for a secondary cause of glenohumeral osteoarthritis, inflammatory arthritis, connective tissue disease, prior contralateral TSA, and prior ipsilateral scapula, glenoid, and proximal humerus surgery. Ultimately, 24 patients were included in the study.
CT data were formatted for preoperative templating. The CT images of each patient’s scapula were uploaded into Materialise Interactive Medical Image Control System (Mimics) software. Mimics allows 3-D image rendering and editing from various imaging modalities and formats. The software was used to create the 3-D scapula models for templating. Prior studies have validated the anatomical precision of 3-D models created with Mimics.20
Mimics was also used to digitize in 3-D the glenoid components from the Bigliani-Flatow Shoulder System (Zimmer Biomet). Glenoid components of 3 different sizes (40 mm, 46 mm, 52 mm) were used. (The Bigliani glenoid component was digitized, as this implant system was used for primary TSA in all 24 patients.) Each glenoid component was traced in 3-D with a Gage 2000 coordinate-measuring machine (Brown & Sharpe) and was processed with custom software. The custom software, cited in previous work by our group,17 created the same coordinate system for each scapula based on anatomical reference points. These digitized 3-D images of glenoid components were uploaded with the digitized 3-D scapulae derived from patients’ CT scans to the Magics software. Magics allows for manipulation and interaction of multiple 3-D models by creating electronic stereolithography files that provide 3-D surface geometry.
Three fellowship-trained shoulder surgeons and 4 shoulder fellows templated the most appropriately sized glenoid component for each of the 24 patients. At the time of templating, the surgeon was blinded to the size of the glenoid implant used in the surgery. In Magics, each scapula was positioned in 3-D similar to how it would appear with the patient in the beach-chair position during surgery. In both study arms, surgeons selected the largest component that maximized the area of contact while avoiding peg penetration of the glenoid vault or component overhang. In addition, surgeons were instructed to correct glenoid version to as near neutral as possible with component positioning but were not permitted to remove glenoid bone stock to correct deformity. All surgeons based placement of the glenoid component on the patient’s actual bone stock and not on osteophytes, which are readily appreciable on 3-D CT.
In study arm 1, the 3-D view of the glenoid was restricted to the initial view in the beach-chair position. The surgeon then manipulated the 3-D glenoid component template across a single 2-D plane, either the superior-inferior plane or the anterior-posterior plane, over the surface of the 3-D glenoid (Figure 1).
In study arm 2, surgeons were permitted to rotate the 3-D glenoid template and scapula in any manner (Figure 2).
Interobserver agreement was determined by comparing prosthetic glenoid component size selection among all study surgeons, and intraobserver agreement was determined by comparing glenoid size selection during 2 sessions separated by at least 3 weeks.
After each trial, the order of patients’ scapula images was randomly rearranged to reduce recall bias. Kappa (κ) coefficients were calculated for interobserver and intraobserver agreement. Kappas ranged from −1.0 (least agreement) to +1.0 (complete agreement). A κ of 0 indicated an observer selection was equivalent to random chance. The level of agreement was categorized according to κ using a system described by Landis and Koch21 (Table 1).
Results
The group of 24 patients consisted of 15 men and 9 women. Mean age was 70.3 years (range, 56-88 years). Primary TSA was performed in 14 right shoulders and 10 left shoulders. Of the 24 patients, 20 (83%) had a 46-mm glenoid component implanted, 3 male patients had a 52-mm glenoid component implanted, and 1 female patient had a 40-mm glenoid component implanted.
Study Arm 1: Glenoid Templating Based on 2 df
In study arm 1, overall intraobserver agreement was substantial, as defined in the statistical literature.21 Among all surgeons who participated, intraobserver agreeement was 0.76 (substantial), 0.60 (substantial), and 0.58 (moderate) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.67, substantial agreement). Trial 1 interobserver agreement was 0.56 (moderate) (P < .001), 0.25 (fair) (P < .001), and 0.21 (fair) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.36, fair agreement) (P < .001), and trial 2 interobserver agreement was 0.58 (moderate) (P < .001), 0.18 (poor) (P = .003), and 0.24 (fair) (P <.001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.32, fair agreement) (P < .001). In study arm 1, therefore, trials 1 and 2 both showed fair interobserver agreement.
Study Arm 2: Glenoid Templating Based on 6 df
In study arm 2, a mean correlation of 0.42 (moderate agreement) was found between glenoid component size in 3-D templating and the glenoid component size ultimately selected during surgery (Table 3).
In study arm 2, overall intraobserver agreement was moderate. Among all surgeons who participated, intraobserver agreement was 0.80 (excellent), 0.43 (moderate), and 0.47 (moderate) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.58, moderate agreement). Trial 1 interobserver agreement was 0.75 (substantial) (P < .001), 0.39 (fair) (P < .001), and 0.50 (moderate) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.54, moderate agreement) (P < .001), and trial 2 interobserver agreement was 0.66 (substantial) (P < .001), 0.28 (fair) (P = .003), and 0.40 (moderate) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.43, moderate agreement) (P < .001).
Discussion
Our results showed that 3-D glenoid templating had reproducible intraobserver and interobserver agreement. Overall intraobserver agreement was substantial (κ = 0.67) for study arm 1 and moderate (κ = 0.58) for study arm 2. Interobserver agreement was fair for trials 1 and 2 (κ = 0.36 and 0.32) in arm 1 and moderate for trials 1 and 2 (κ = 0.54 and 0.43) in arm 2.
Intraobserver and interobserver agreement values, particularly in study arm 2, which incorporated rotation (6 df), are consistent with values in commonly used classification systems, such as the Neer system for proximal humerus fractures, the Frykman system for distal radius fractures, and the King system for adolescent idiopathic scoliosis.22-30 Sidor and colleagues27 found overall interobserver agreement of 0.50 and overall intraobserver agreement of 0.66 for the Neer system, and Illarramendi and colleagues24 found overall interobserver agreement of 0.43 and overall intraobserver agreement of 0.61 for the Frykman system.
In study arm 2, overall interobserver and intraobserver agreement was moderate. A higher level of surgeon agreement is unlikely given the lack of well-defined parameters for determining glenoid component size. Therefore, glenoid size selection is largely a matter of surgeon preference. More research is needed to establish concrete guidelines for glenoid component size selection. Once guidelines are adopted, interobserver agreement in templating may increase.
In both study arms, the component that surgeons selected during templating tended to be smaller than the component they selected during surgery. In study arm 1, 32% of patients had a smaller component selected based on computer modeling, and 7% had a larger component selected. In study arm 2, the difference was narrower: 27% of patients had a smaller component selected during templating, and 16% had a larger component selected. A statistically significant difference (P < .001) in templated and implanted component sizes was found between men and women: Templated glenoid components were smaller than implanted components in 53% of women and larger than implanted components in 33% of men. Differences between templated and implanted components may be attributable to visualization differences. During templating, the entire glenoid can be visualized and the slightest peg penetration or component overhang detected; in contrast, during surgery, anatomical constraints preclude such a comprehensive assessment.
Differences in agreement between templated and implanted glenoid components suggest that the size of implanted components may not be ideal. In this study, the distribution of the templated glenoid sizes was much wider than that of the implanted glenoid sizes. During templating, each glenoid component can be definitively visualized and assessed for possible peg penetration and overhang. Visualization allows surgeons to base glenoid size selection solely on glenoid morphology, as opposed to factors such as patient sex and height. In addition, interobserver and intraobserver agreement values for the 40-mm glenoid component were considerably higher than those for components of other sizes, indicating that the 40-mm component was consistently and reproducibly selected for the same patients. Hence, templating may particularly help prevent peg penetration and component overhang for patients with a smaller diameter glenoid.
More research on 3-D templating is warranted given the results of this study and other studies.12,17,31 Scalise and colleagues31 found that, in TSA planning, surgeons’ use of 2-D (vs 3-D) imaging led them to overestimate glenoid component sizes (P = .006). In our study, the glenoid size selected during 3-D templating was, in many cases, smaller than the size selected during surgery. In order to avoid peg penetration and glenoid overhang, anecdotal guidelines commonly used in glenoid size selection, likely was the driving force in selecting smaller glenoid components during templating. Although anterior, superior, and inferior glenoid overhang typically can be assessed during surgery, posterior overhang is more difficult to evaluate. Three-dimensional modeling allows surgeons to determine optimal glenoid component size and position. In addition, intraoperative evaluation of glenoid component peg penetration is challenging, and peg penetration becomes evident only after it has occurred. During templating, however, surgeons were able to easily assess for peg penetration, and smaller glenoid components were selected.
A limitation of this study is that intraoperative glenoid version correction or peg containment was not quantified. More research is needed on the relationship between glenoid size selection and component overhang and peg penetration. Another limitation was use of only 1 TSA system (with 3 glenoid sizes, all with inline pegs); reliability of 3-D templating was not evaluated across different component designs. Last, given the absence of guidelines for glenoid component size selection, there was surgeon bias in preoperative templating and in intraoperative selection of glenoid size. Surgeons had differing opinions on the importance of maximizing the contact area of the component and correcting glenoid deformity and version.
Our study results showed that preoperative 3-D templating that allows for superior-inferior, anterior-posterior, and rotational translation was consistent and reproducible in determining glenoid component size, and use of this templating may reduce the risks of implant overhang, peg penetration, and decreased stability ratio. These results highlight the possibility that glenoid component sizes selected during surgery may not be ideal. More research is needed to determine if intraoperative glenoid size selection leads to adequate version correction and peg containment. The present study supports use of 3-D templating in primary TSA planning.
1. Neer CS 2nd. Replacement arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 1974;56(1):1-13.
2. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
3. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
4. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
5. Barrett WP, Franklin JL, Jackins SE, Wyss CR, Matsen FA 3rd. Total shoulder arthroplasty. J Bone Joint Surg Am. 1987;69(6):865-872.
6. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
7. Matsen FA 3rd, Bicknell RT, Lippitt SB. Shoulder arthroplasty: the socket perspective. J Shoulder Elbow Surg. 2007;16(5 suppl):S241-S247.
8. Matsen FA 3rd, Clinton J, Lynch J, Bertelsen A, Richardson ML. Glenoid component failure in total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(4):885-896.
9. Pearl ML, Romeo AA, Wirth MA, Yamaguchi K, Nicholson GP, Creighton RA. Decision making in contemporary shoulder arthroplasty. Instr Course Lect. 2005;54:69-85.
10. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616.
11. Sanchez-Sotelo J, Sperling JW, Rowland CM, Cofield RH. Instability after shoulder arthroplasty: results of surgical treatment. J Bone Joint Surg Am. 2003;85(4):622-631.
12. Tammachote N, Sperling JW, Berglund LJ, Steinmann SP, Cofield RH, An KN. The effect of glenoid component size on the stability of total shoulder arthroplasty. J Shoulder Elbow Surg. 2007;16(3 suppl):S102-S106.
13. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55.
14. Briem D, Ruecker AH, Neumann J, et al. 3D fluoroscopic navigated reaming of the glenoid for total shoulder arthroplasty (TSA). Comput Aided Surg. 2011;16(2):93-99.
15. Budge MD, Lewis GS, Schaefer E, Coquia S, Flemming DJ, Armstrong AD. Comparison of standard two-dimensional and three-dimensional corrected glenoid version measurements. J Shoulder Elbow Surg. 2011;20(4):577-583.
16. Chuang TY, Adams CR, Burkhart SS. Use of preoperative three-dimensional computed tomography to quantify glenoid bone loss in shoulder instability. Arthroscopy. 2008;24(4):376-382.
17. Nowak DD, Bahu MJ, Gardner TR, et al. Simulation of surgical glenoid resurfacing using three-dimensional computed tomography of the arthritic glenohumeral joint: the amount of glenoid retroversion that can be corrected. J Shoulder Elbow Surg. 2009;18(5):680-688.
18. Scalise JJ, Bryan J, Polster J, Brems JJ, Iannotti JP. Quantitative analysis of glenoid bone loss in osteoarthritis using three-dimensional computed tomography scans. J Shoulder Elbow Surg. 2008;17(2):328-335.
19. Scalise JJ, Codsi MJ, Bryan J, Iannotti JP. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg. 2008;17(3):487-491.
20. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832.
21. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174.
22. Cummings RJ, Loveless EA, Campbell J, Samelson S, Mazur JM. Interobserver reliability and intraobserver reproducibility of the system of King et al. for the classification of adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80(8):1107-1111.
23. Humphrey CA, Dirschl DR, Ellis TJ. Interobserver reliability of a CT-based fracture classification system. J Orthop Trauma. 2005;19(9):616-622.
24. Illarramendi A, González Della Valle A, Segal E, De Carli P, Maignon G, Gallucci G. Evaluation of simplified Frykman and AO classifications of fractures of the distal radius. Assessment of interobserver and intraobserver agreement. Int Orthop. 1998;22(2):111-115.
25. Lenke LG, Betz RR, Bridwell KH, et al. Intraobserver and interobserver reliability of the classification of thoracic adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80(8):1097-1106.
26. Ploegmakers JJ, Mader K, Pennig D, Verheyen CC. Four distal radial fracture classification systems tested amongst a large panel of Dutch trauma surgeons. Injury. 2007;38(11):1268-1272.
27. Sidor ML, Zuckerman JD, Lyon T, Koval K, Cuomo F, Schoenberg N. The Neer classification system for proximal humeral fractures. An assessment of interobserver reliability and intraobserver reproducibility. J Bone Joint Surg Am. 1993;75(12):1745-1750.
28. Siebenrock KA, Gerber C. The reproducibility of classification of fractures of the proximal end of the humerus. J Bone Joint Surg Am. 1993;75(12):1751-1755.
29. Thomsen NO, Overgaard S, Olsen LH, Hansen H, Nielsen ST. Observer variation in the radiographic classification of ankle fractures. J Bone Joint Surg Br. 1991;73(4):676-678.
30. Ward WT, Vogt M, Grudziak JS, Tümer Y, Cook PC, Fitch RD. Severin classification system for evaluation of the results of operative treatment of congenital dislocation of the hip. A study of intraobserver and interobserver reliability. J Bone Joint Surg Am. 1997;79(5):656-663.
31. Scalise JJ, Codsi MJ, Bryan J, Brems JJ, Iannotti JP. The influence of three-dimensional computed tomography images of the shoulder in preoperative planning for total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(11):2438-2445.
1. Neer CS 2nd. Replacement arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 1974;56(1):1-13.
2. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
3. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
4. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
5. Barrett WP, Franklin JL, Jackins SE, Wyss CR, Matsen FA 3rd. Total shoulder arthroplasty. J Bone Joint Surg Am. 1987;69(6):865-872.
6. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
7. Matsen FA 3rd, Bicknell RT, Lippitt SB. Shoulder arthroplasty: the socket perspective. J Shoulder Elbow Surg. 2007;16(5 suppl):S241-S247.
8. Matsen FA 3rd, Clinton J, Lynch J, Bertelsen A, Richardson ML. Glenoid component failure in total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(4):885-896.
9. Pearl ML, Romeo AA, Wirth MA, Yamaguchi K, Nicholson GP, Creighton RA. Decision making in contemporary shoulder arthroplasty. Instr Course Lect. 2005;54:69-85.
10. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616.
11. Sanchez-Sotelo J, Sperling JW, Rowland CM, Cofield RH. Instability after shoulder arthroplasty: results of surgical treatment. J Bone Joint Surg Am. 2003;85(4):622-631.
12. Tammachote N, Sperling JW, Berglund LJ, Steinmann SP, Cofield RH, An KN. The effect of glenoid component size on the stability of total shoulder arthroplasty. J Shoulder Elbow Surg. 2007;16(3 suppl):S102-S106.
13. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55.
14. Briem D, Ruecker AH, Neumann J, et al. 3D fluoroscopic navigated reaming of the glenoid for total shoulder arthroplasty (TSA). Comput Aided Surg. 2011;16(2):93-99.
15. Budge MD, Lewis GS, Schaefer E, Coquia S, Flemming DJ, Armstrong AD. Comparison of standard two-dimensional and three-dimensional corrected glenoid version measurements. J Shoulder Elbow Surg. 2011;20(4):577-583.
16. Chuang TY, Adams CR, Burkhart SS. Use of preoperative three-dimensional computed tomography to quantify glenoid bone loss in shoulder instability. Arthroscopy. 2008;24(4):376-382.
17. Nowak DD, Bahu MJ, Gardner TR, et al. Simulation of surgical glenoid resurfacing using three-dimensional computed tomography of the arthritic glenohumeral joint: the amount of glenoid retroversion that can be corrected. J Shoulder Elbow Surg. 2009;18(5):680-688.
18. Scalise JJ, Bryan J, Polster J, Brems JJ, Iannotti JP. Quantitative analysis of glenoid bone loss in osteoarthritis using three-dimensional computed tomography scans. J Shoulder Elbow Surg. 2008;17(2):328-335.
19. Scalise JJ, Codsi MJ, Bryan J, Iannotti JP. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg. 2008;17(3):487-491.
20. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832.
21. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174.
22. Cummings RJ, Loveless EA, Campbell J, Samelson S, Mazur JM. Interobserver reliability and intraobserver reproducibility of the system of King et al. for the classification of adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80(8):1107-1111.
23. Humphrey CA, Dirschl DR, Ellis TJ. Interobserver reliability of a CT-based fracture classification system. J Orthop Trauma. 2005;19(9):616-622.
24. Illarramendi A, González Della Valle A, Segal E, De Carli P, Maignon G, Gallucci G. Evaluation of simplified Frykman and AO classifications of fractures of the distal radius. Assessment of interobserver and intraobserver agreement. Int Orthop. 1998;22(2):111-115.
25. Lenke LG, Betz RR, Bridwell KH, et al. Intraobserver and interobserver reliability of the classification of thoracic adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80(8):1097-1106.
26. Ploegmakers JJ, Mader K, Pennig D, Verheyen CC. Four distal radial fracture classification systems tested amongst a large panel of Dutch trauma surgeons. Injury. 2007;38(11):1268-1272.
27. Sidor ML, Zuckerman JD, Lyon T, Koval K, Cuomo F, Schoenberg N. The Neer classification system for proximal humeral fractures. An assessment of interobserver reliability and intraobserver reproducibility. J Bone Joint Surg Am. 1993;75(12):1745-1750.
28. Siebenrock KA, Gerber C. The reproducibility of classification of fractures of the proximal end of the humerus. J Bone Joint Surg Am. 1993;75(12):1751-1755.
29. Thomsen NO, Overgaard S, Olsen LH, Hansen H, Nielsen ST. Observer variation in the radiographic classification of ankle fractures. J Bone Joint Surg Br. 1991;73(4):676-678.
30. Ward WT, Vogt M, Grudziak JS, Tümer Y, Cook PC, Fitch RD. Severin classification system for evaluation of the results of operative treatment of congenital dislocation of the hip. A study of intraobserver and interobserver reliability. J Bone Joint Surg Am. 1997;79(5):656-663.
31. Scalise JJ, Codsi MJ, Bryan J, Brems JJ, Iannotti JP. The influence of three-dimensional computed tomography images of the shoulder in preoperative planning for total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(11):2438-2445.
While U.S. heart failure readmissions fall, deaths rise
DALLAS – U.S. hospitals have recently shown a consistent and disturbing disconnect between reductions in their heart failure hospital readmission rates and heart failure mortality. Readmissions have dropped while mortality has risen.
“Despite reductions in 30-day heart failure readmissions in 89% of U.S. hospitals” during 2009-2016, “30-day heart failure mortality rates increased at 73%* of these ‘successful’ hospitals” during the same period,” Ahmad A. Abdul-Aziz, MD, said at the annual scientific meeting of the Heart Failure Society of America.
These shifts in the outcomes of U.S. patients hospitalized for acute heart failure episodes are tied to the penalties that the Centers for Medicare & Medicaid Services began slapping on hospitals in 2013 for excess 30-day readmissions for heart failure patients and in 2014 for excess mortality. A problem with these two CMS programs is that the penalty on inferior readmissions performance is a lot stiffer than for excess mortality, Dr. Aziz noted: a 0.2% penalty on payments for high mortality, compared with a 3% penalty for excess readmissions, a disparity that can make hospitals focus more on the readmissions side, he suggested.
Dr. Aziz’s report isn’t the first to make this observation. Study results published earlier in 2017 used CMS Medicare data from 2008 to 2014 to show that during that period, heart failure 30-day mortality rates following hospital discharge rose by 1.3%, while 30-day readmissions fell by 2.1% (JAMA. 2017 July 18;318[3]:270-8). On the basis of these numbers, as many as 5,200 additional deaths to U.S. heart failure patients in 2014 “may be related to the Hospital Readmission Reduction Program” of CMS, Gregg C. Fonarow, MD, said during a separate talk at the meeting.
The analysis reported by Dr. Aziz included data from 3,265 U.S. hospitals for heart failure patients, and data from 1,621 hospitals that managed patients with acute MIs, another disease that the CMS has targeted for penalties based on 30-day readmissions and 30-day postdischarge mortality rates. During the 8-year period studied, heart failure 30-day readmissions fell by 2.2% while 30-day mortality rose by 1%. In contrast, among acute MI patients, readmissions fell by 3% and mortality also fell, by 2.2%, Dr. Abdul-Aziz reported.
Dr. Abdul-Aziz had no disclosures. Dr. Fonarow had been a consultant to Amgen, Janssen, Medtronic, Novartis, St. Jude, and ZS Pharma.
[email protected]
On Twitter @mitchelzoler
*This article was updated October 5, 2017
My colleagues and I have seen in results from recent trials a changing relationship between heart failure mortality and heart failure hospitalization. In the United States in particular, where penalties exist for high rates of hospital readmissions for heart failure, we are seeing more patients treated as outpatients and we see that these “outpatient” events are associated with the same risk for subsequent mortality as we had previously seen for heart failure hospitalization.
It may be that we are keeping patients out of the hospital to avoid a financial ding, but perhaps we are keeping out patients who really should be hospitalized.
What we have begun doing in trials is to look not just at hospitalizations but also consider the incidence of other heart failure events, such as patients treated for heart failure symptoms with an intravenous diuretic in the emergency department and patients who are kept in observation rooms and are not admitted. It’s not the same as a heart failure hospitalization, but some trials are now including these other heart failure events in their primary endpoint.
Scott D. Solomon, MD, professor of medicine at Harvard Medical School and director of noninvasive cardiology at Brigham and Women’s Hospital in Boston, made these comments as chair of the session where Dr. Aziz gave his report and in an interview. He has been a consultant to and/or received research support from Alnylam, Amgen, AstraZeneca, Bristol-Myers Squibb, Cytokinetics, GlaxoSmithKline, Ionis, Merck, Novartis, and Sanofi.
My colleagues and I have seen in results from recent trials a changing relationship between heart failure mortality and heart failure hospitalization. In the United States in particular, where penalties exist for high rates of hospital readmissions for heart failure, we are seeing more patients treated as outpatients and we see that these “outpatient” events are associated with the same risk for subsequent mortality as we had previously seen for heart failure hospitalization.
It may be that we are keeping patients out of the hospital to avoid a financial ding, but perhaps we are keeping out patients who really should be hospitalized.
What we have begun doing in trials is to look not just at hospitalizations but also consider the incidence of other heart failure events, such as patients treated for heart failure symptoms with an intravenous diuretic in the emergency department and patients who are kept in observation rooms and are not admitted. It’s not the same as a heart failure hospitalization, but some trials are now including these other heart failure events in their primary endpoint.
Scott D. Solomon, MD, professor of medicine at Harvard Medical School and director of noninvasive cardiology at Brigham and Women’s Hospital in Boston, made these comments as chair of the session where Dr. Aziz gave his report and in an interview. He has been a consultant to and/or received research support from Alnylam, Amgen, AstraZeneca, Bristol-Myers Squibb, Cytokinetics, GlaxoSmithKline, Ionis, Merck, Novartis, and Sanofi.
My colleagues and I have seen in results from recent trials a changing relationship between heart failure mortality and heart failure hospitalization. In the United States in particular, where penalties exist for high rates of hospital readmissions for heart failure, we are seeing more patients treated as outpatients and we see that these “outpatient” events are associated with the same risk for subsequent mortality as we had previously seen for heart failure hospitalization.
It may be that we are keeping patients out of the hospital to avoid a financial ding, but perhaps we are keeping out patients who really should be hospitalized.
What we have begun doing in trials is to look not just at hospitalizations but also consider the incidence of other heart failure events, such as patients treated for heart failure symptoms with an intravenous diuretic in the emergency department and patients who are kept in observation rooms and are not admitted. It’s not the same as a heart failure hospitalization, but some trials are now including these other heart failure events in their primary endpoint.
Scott D. Solomon, MD, professor of medicine at Harvard Medical School and director of noninvasive cardiology at Brigham and Women’s Hospital in Boston, made these comments as chair of the session where Dr. Aziz gave his report and in an interview. He has been a consultant to and/or received research support from Alnylam, Amgen, AstraZeneca, Bristol-Myers Squibb, Cytokinetics, GlaxoSmithKline, Ionis, Merck, Novartis, and Sanofi.
DALLAS – U.S. hospitals have recently shown a consistent and disturbing disconnect between reductions in their heart failure hospital readmission rates and heart failure mortality. Readmissions have dropped while mortality has risen.
“Despite reductions in 30-day heart failure readmissions in 89% of U.S. hospitals” during 2009-2016, “30-day heart failure mortality rates increased at 73%* of these ‘successful’ hospitals” during the same period,” Ahmad A. Abdul-Aziz, MD, said at the annual scientific meeting of the Heart Failure Society of America.
These shifts in the outcomes of U.S. patients hospitalized for acute heart failure episodes are tied to the penalties that the Centers for Medicare & Medicaid Services began slapping on hospitals in 2013 for excess 30-day readmissions for heart failure patients and in 2014 for excess mortality. A problem with these two CMS programs is that the penalty on inferior readmissions performance is a lot stiffer than for excess mortality, Dr. Aziz noted: a 0.2% penalty on payments for high mortality, compared with a 3% penalty for excess readmissions, a disparity that can make hospitals focus more on the readmissions side, he suggested.
Dr. Aziz’s report isn’t the first to make this observation. Study results published earlier in 2017 used CMS Medicare data from 2008 to 2014 to show that during that period, heart failure 30-day mortality rates following hospital discharge rose by 1.3%, while 30-day readmissions fell by 2.1% (JAMA. 2017 July 18;318[3]:270-8). On the basis of these numbers, as many as 5,200 additional deaths to U.S. heart failure patients in 2014 “may be related to the Hospital Readmission Reduction Program” of CMS, Gregg C. Fonarow, MD, said during a separate talk at the meeting.
The analysis reported by Dr. Aziz included data from 3,265 U.S. hospitals for heart failure patients, and data from 1,621 hospitals that managed patients with acute MIs, another disease that the CMS has targeted for penalties based on 30-day readmissions and 30-day postdischarge mortality rates. During the 8-year period studied, heart failure 30-day readmissions fell by 2.2% while 30-day mortality rose by 1%. In contrast, among acute MI patients, readmissions fell by 3% and mortality also fell, by 2.2%, Dr. Abdul-Aziz reported.
Dr. Abdul-Aziz had no disclosures. Dr. Fonarow had been a consultant to Amgen, Janssen, Medtronic, Novartis, St. Jude, and ZS Pharma.
[email protected]
On Twitter @mitchelzoler
*This article was updated October 5, 2017
DALLAS – U.S. hospitals have recently shown a consistent and disturbing disconnect between reductions in their heart failure hospital readmission rates and heart failure mortality. Readmissions have dropped while mortality has risen.
“Despite reductions in 30-day heart failure readmissions in 89% of U.S. hospitals” during 2009-2016, “30-day heart failure mortality rates increased at 73%* of these ‘successful’ hospitals” during the same period,” Ahmad A. Abdul-Aziz, MD, said at the annual scientific meeting of the Heart Failure Society of America.
These shifts in the outcomes of U.S. patients hospitalized for acute heart failure episodes are tied to the penalties that the Centers for Medicare & Medicaid Services began slapping on hospitals in 2013 for excess 30-day readmissions for heart failure patients and in 2014 for excess mortality. A problem with these two CMS programs is that the penalty on inferior readmissions performance is a lot stiffer than for excess mortality, Dr. Aziz noted: a 0.2% penalty on payments for high mortality, compared with a 3% penalty for excess readmissions, a disparity that can make hospitals focus more on the readmissions side, he suggested.
Dr. Aziz’s report isn’t the first to make this observation. Study results published earlier in 2017 used CMS Medicare data from 2008 to 2014 to show that during that period, heart failure 30-day mortality rates following hospital discharge rose by 1.3%, while 30-day readmissions fell by 2.1% (JAMA. 2017 July 18;318[3]:270-8). On the basis of these numbers, as many as 5,200 additional deaths to U.S. heart failure patients in 2014 “may be related to the Hospital Readmission Reduction Program” of CMS, Gregg C. Fonarow, MD, said during a separate talk at the meeting.
The analysis reported by Dr. Aziz included data from 3,265 U.S. hospitals for heart failure patients, and data from 1,621 hospitals that managed patients with acute MIs, another disease that the CMS has targeted for penalties based on 30-day readmissions and 30-day postdischarge mortality rates. During the 8-year period studied, heart failure 30-day readmissions fell by 2.2% while 30-day mortality rose by 1%. In contrast, among acute MI patients, readmissions fell by 3% and mortality also fell, by 2.2%, Dr. Abdul-Aziz reported.
Dr. Abdul-Aziz had no disclosures. Dr. Fonarow had been a consultant to Amgen, Janssen, Medtronic, Novartis, St. Jude, and ZS Pharma.
[email protected]
On Twitter @mitchelzoler
*This article was updated October 5, 2017
AT THE HFSA ANNUAL SCIENTIFIC MEETING
Key clinical point:
Major finding: From 2009 to 2016, U.S. 30-day heart failure readmissions fell by 2.2% while 30-day heart failure mortality rose by 1%.
Data source: Review of Medicare data for 3,265 U.S. hospitals managing heart failure patients.
Disclosures: Dr. Abdul-Aziz had no disclosures. Dr. Fonarow had been a consultant to Amgen, Janssen, Medtronic, Novartis, St. Jude, and ZS Pharma.
Should you sell your dermatology practice?
There are about 16 existing dermatology groups, with about 700 dermatologists employed, backed by private equity money, that are eager to buy dermatology practices. They figure the market is highly fragmented, and they can bring efficiency and savings to make a profit. This is a highly complex topic, so bear with me; this may take a few columns. Entrepreneurial dermatologists initially set these groups up for practical reasons, such as bargaining power and cost efficiencies. Now, with low-cost money (look at interest rates) flooding the equity markets, large firms are looking to make a better, safe return on their money by buying these groups, and commoditizing them.
So who may this be a good deal for? Older physicians, say 5 years from retirement, may be able to capitalize on the value – usually calculated by EBITDA (a company’s earnings before interest, tax, depreciation, and amortization) – and sell their practices over time. EBITDA is a rough estimate of a practice’s profitability. Recall that a few years ago, some dermatologists were simply begging to find a buyer to get out or shuttering their offices and walking away into retirement.
Younger physicians usually have less to gain, since they may not have an equity stake in the practice being sold. Even the ones who do will be employed physicians for a longer time. Employees may have the opportunity to buy or bonus into an equity position later.
The buyout money is not a gift, and you will pay most of it back over the typical 5 years or more of minimum employment time specified in the sell contract. The equity firms estimate your salary at 40%, the overhead at 40%, and their profit at 20%.
What are the obvious disadvantages? You will not make as much in salary as you did before (recall that 20% profit above), you become an employee, and you lose control and flexibility. You must work as many days on average as you did in the 3-year period before your buyout, and you do not get to manage your employees as before. They will be managed by the buying company’s human resources department, which may make some things better.
You may have new employees assigned to you that you normally would not, and it is important in your negotiations that you spell out what kind and how many employees you are willing to supervise.
You will be strongly encouraged to send your pathology and Mohs cases to other members of the group, if available. You must justify major purchases (such as new lasers). The group will buy your existing equipment, hopefully for fair market value.
So is selling your practice a good deal for you? Obviously, it depends on many variables, which we will discuss further in future columns.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
There are about 16 existing dermatology groups, with about 700 dermatologists employed, backed by private equity money, that are eager to buy dermatology practices. They figure the market is highly fragmented, and they can bring efficiency and savings to make a profit. This is a highly complex topic, so bear with me; this may take a few columns. Entrepreneurial dermatologists initially set these groups up for practical reasons, such as bargaining power and cost efficiencies. Now, with low-cost money (look at interest rates) flooding the equity markets, large firms are looking to make a better, safe return on their money by buying these groups, and commoditizing them.
So who may this be a good deal for? Older physicians, say 5 years from retirement, may be able to capitalize on the value – usually calculated by EBITDA (a company’s earnings before interest, tax, depreciation, and amortization) – and sell their practices over time. EBITDA is a rough estimate of a practice’s profitability. Recall that a few years ago, some dermatologists were simply begging to find a buyer to get out or shuttering their offices and walking away into retirement.
Younger physicians usually have less to gain, since they may not have an equity stake in the practice being sold. Even the ones who do will be employed physicians for a longer time. Employees may have the opportunity to buy or bonus into an equity position later.
The buyout money is not a gift, and you will pay most of it back over the typical 5 years or more of minimum employment time specified in the sell contract. The equity firms estimate your salary at 40%, the overhead at 40%, and their profit at 20%.
What are the obvious disadvantages? You will not make as much in salary as you did before (recall that 20% profit above), you become an employee, and you lose control and flexibility. You must work as many days on average as you did in the 3-year period before your buyout, and you do not get to manage your employees as before. They will be managed by the buying company’s human resources department, which may make some things better.
You may have new employees assigned to you that you normally would not, and it is important in your negotiations that you spell out what kind and how many employees you are willing to supervise.
You will be strongly encouraged to send your pathology and Mohs cases to other members of the group, if available. You must justify major purchases (such as new lasers). The group will buy your existing equipment, hopefully for fair market value.
So is selling your practice a good deal for you? Obviously, it depends on many variables, which we will discuss further in future columns.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
There are about 16 existing dermatology groups, with about 700 dermatologists employed, backed by private equity money, that are eager to buy dermatology practices. They figure the market is highly fragmented, and they can bring efficiency and savings to make a profit. This is a highly complex topic, so bear with me; this may take a few columns. Entrepreneurial dermatologists initially set these groups up for practical reasons, such as bargaining power and cost efficiencies. Now, with low-cost money (look at interest rates) flooding the equity markets, large firms are looking to make a better, safe return on their money by buying these groups, and commoditizing them.
So who may this be a good deal for? Older physicians, say 5 years from retirement, may be able to capitalize on the value – usually calculated by EBITDA (a company’s earnings before interest, tax, depreciation, and amortization) – and sell their practices over time. EBITDA is a rough estimate of a practice’s profitability. Recall that a few years ago, some dermatologists were simply begging to find a buyer to get out or shuttering their offices and walking away into retirement.
Younger physicians usually have less to gain, since they may not have an equity stake in the practice being sold. Even the ones who do will be employed physicians for a longer time. Employees may have the opportunity to buy or bonus into an equity position later.
The buyout money is not a gift, and you will pay most of it back over the typical 5 years or more of minimum employment time specified in the sell contract. The equity firms estimate your salary at 40%, the overhead at 40%, and their profit at 20%.
What are the obvious disadvantages? You will not make as much in salary as you did before (recall that 20% profit above), you become an employee, and you lose control and flexibility. You must work as many days on average as you did in the 3-year period before your buyout, and you do not get to manage your employees as before. They will be managed by the buying company’s human resources department, which may make some things better.
You may have new employees assigned to you that you normally would not, and it is important in your negotiations that you spell out what kind and how many employees you are willing to supervise.
You will be strongly encouraged to send your pathology and Mohs cases to other members of the group, if available. You must justify major purchases (such as new lasers). The group will buy your existing equipment, hopefully for fair market value.
So is selling your practice a good deal for you? Obviously, it depends on many variables, which we will discuss further in future columns.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].