User login
A new standard for treatment of torus fractures of the wrist?
ILLUSTRATIVE CASE
A 9-year-old girl presents to your urgent care clinic after a fall while snowboarding for the first time. She reports falling forward onto her outstretched right hand and describes pain in her distal right forearm. She denies paresthesias, weakness, or lacerations. Physical examination reveals mild edema of the dorsal aspect of her distal right forearm and tenderness to palpation of the dorsal aspect of her distal radius. She denies tenderness to palpation of her ulna, anatomic snuffbox, hand, and elbow. Range of motion of the wrist is full on passive testing, but she declines active testing due to pain. Wrist radiographs reveal an uncomplicated torus fracture of the distal radius. Can immobilization with a soft bandage alone sufficiently treat this fracture?
Fractures of the distal radius are among the most common fractures of the upper extremity and commonly occur from a fall onto an outstretched hand.2 In the pediatric population, torus fractures, also known as buckle fractures, are the most common type of distal radius fracture, comprising an estimated 50% of pediatric wrist fractures.3,4 This is due to the presence of a
Pediatric torus fractures of the distal radius generally are treated with immobilization,2 traditionally through a
Despite common use of immobilization, torus fractures of the distal radius are anatomically stable, and displacement is unlikely to occur.7,8 As such, many studies have suggested that treatment of torus fractures with rigid immobilization in a cast or splint may not be necessary.9,10 However, a 2018 Cochrane review concluded that the quality of evidence illustrating similar recovery between treatments was low, leaving uncertainty as to the most appropriate management strategy.6 Less casting and follow-up imaging could have positive implications for patient satisfaction, health care–associated costs, and radiation exposure.10
This study, the Forearm Fracture Recovery in Children Evaluation (FORCE) trial, compared the traditional treatment of distal radius torus fractures with rigid immobilization to soft immobilization and immediate discharge.
STUDY SUMMARY
Providing quality evidence for a standard of care
FORCE was a randomized controlled equivalence trial (N = 965) across 23 emergency departments (EDs) in the United Kingdom that compared pain and function in pediatric patients with distal radius torus fractures treated with a soft bandage and immediate discharge vs rigid immobilization and routine follow-up.1 Patients included children ages 4 to 15 years presenting to the ED with a distal radius torus fracture, which was confirmed radiologically.
Patients with concomitant
Continue to: Patients were randomly assigned...
Patients were randomly assigned in a 1:1 ratio to receive treatment with either a soft bandage such as a gauze roller bandage (n = 489) or rigid immobilization (n = 476). For patients in the bandage group, a soft bandage was applied in the ED or provided for home application without planned clinical follow-up. Patients in the rigid immobilization group were treated in the ED with either a removable manufactured splint or a molded splint or cast, followed by the standard follow-up practice of the treating center. Patients in the soft bandage group were advised not to wear the bandage for more than 3 weeks. Blinding was not possible, but the treatment team did not take part in patient follow-up.
The primary outcome was change in pain 3 days after treatment, measured on the Wong-Baker FACES Pain Rating Scale (an ordinal assessment using 6 illustrated facial expressions translated to a numeric rating on a scale of 0-10, with higher scores indicating worse pain). This scale has an established minimum clinically important difference (MCID) value of 1 face (2 points).11 Per standard practice in equivalence trials, the equivalence margin was defined as half the MCID, with a value of 1.0 used in this study.
Secondary outcomes measured over the 6-week follow-up period included additional pain measurements using the Wong-Baker scale, measures of function and health-related quality of life, analgesia use, days of absence from school or childcare, complication rates, and patient satisfaction. This study used modified intention-to-treat and per-protocol analyses.
The mean age of participants was 9.6 years; 39% were girls and 61% were boys. In the bandage group, 94% opted to have the soft bandage applied in the ED, and 95% of the rigid immobilization group were treated with a removable wrist splint in the ED. At 3 days, pain scores improved by 3.2 points (standard deviation [SD] = 2.1) in the soft bandage group and 3.1 points (SD = 2.1) in the rigid immobilization group. The adjusted difference was –0.1 (95% CI, –0.37 to 0.17) in the intention-to-treat analysis and –0.06 (95% CI, –0.34 to 0.21) in the per-protocol analysis, which were both less than the predetermined equivalence margin. This equivalence margin also was met at all secondary time points (1 day, 7 days, 3 weeks, and 6 weeks after treatment) and in subgroup analysis of those 4 to 7 years and 8 to 15 years.
Use of any analgesia in the prior 24 hours was slightly higher in the soft bandage group on Day 1 (83% vs 78%; P = .04) and Day 3 (57% vs 51%; P = .05), but this difference was not seen on Day 7. Satisfaction, measured via a 7-point Likert scale (range from “extremely satisfied” to “extremely unsatisfied”), was slightly lower in the soft bandage group on Day 1 (median 2 [interquartile range = 1, 2] vs median 1 [interquartile range = 1, 2]; P < .0001) but was not different after 6 weeks. There were no measured differences in any other secondary outcomes, including function, quality of life, and complication rates.
Continue to: By the primary end point...
By the primary end point of 3 days, 36 patients (7%) in the soft bandage group returned to medical care requesting a change to rigid immobilization, compared with 1 patient (0.2%) in the rigid immobilization group declining intervention.
WHAT’S NEW
Equivalence in pain and function scores
This trial showed equivalence in pain at 3 days’ follow-up in children with distal radius torus fractures who were offered bandaging and then immediately discharged from the ED, compared with rigid immobilization and clinical follow-up. There were no significant differences in pain or function between groups during the 6 weeks following the initial injury. De-escalation of treatment offers an equivalent, resource-sparing alternative to traditional treatment of these fractures.
CAVEATS
Lack of masking likely introduced bias
There are no major caveats associated with managing distal radius torus fractures with a soft bandage and discharge from the ED, compared with the traditional treatment of rigid immobilization. However, bias was likely introduced in patient-reported outcomes due to the inability to mask patients and families to the treatment allocation. This may have led to overstating the severity of outcomes in the bandage group, given the strong preference for rigid immobilization, although equivalence was illustrated despite this potential bias.
CHALLENGES TO IMPLEMENTATION
Preferences may be difficult to change
Parents and clinicians demonstrated a preference for rigid immobilization, as shown in the imbalance in treatment crossovers, with 7% of children changing to the rigid immobilization group by the primary study end point of 3 days. The study authors hypothesized that crossovers may have been due to the perception by some parents that rigid immobilization is the gold standard of treatment, as well as clinicians’ seeking to escalate care for patients returning for follow-up. Policy and guideline changes, as well as physician efforts to educate patients on outcomes with soft bandage treatment, are likely to improve these misconceptions.
1. Perry DC, Achten J, Knight R, et al; FORCE Collaborators in collaboration with PERUKI. Immobilisation of torus fractures of the wrist in children (FORCE): a randomised controlled equivalence trial in the UK. Lancet. 2022;400:39-47. doi: 10.1016/S0140-6736(22)01015-7
2. Patel DS, Statuta SM, Ahmed N. Common fractures of the radius and ulna. Am Fam Physician. 2021;103:345-354.
3. Asokan A, Kheir N. Pediatric Torus Buckle Fracture. StatPearls Publishing; 2023.
4. Naranje SM, Erali RA, Warner WC Jr, et al. Epidemiology of pediatric fractures presenting to emergency departments in the United States. J Pediatr Orthop. 2016;36:e45-e48. doi: 10.1097/BPO.0000000000000595
5. Kennedy SA, Slobogean GP, Mulpuri K. Does degree of immobilization influence refracture rate in the forearm buckle fracture? J Pediatr Orthop B. 2010;19:77-81. doi: 10.1097/BPB.0b013e32832f067a
6. Handoll HHG, Elliott J, Iheozor-Ejiofor Z, et al. Interventions for treating wrist fractures in children. Cochrane Database Syst Rev. 2018;12:CD012470. doi: 10.1002/14651858.CD012470.pub2
7. Perry DC, Gibson P, Roland D, et al. What level of immobilisation is necessary for treatment of torus (buckle) fractures of the distal radius in children? BMJ. 2021;372:m4862. doi: 10.1136/bmj.m4862
8. Williams KG, Smith G, Luhmann SJ, et al. A randomized controlled trial of cast versus splint for distal radial buckle fracture: an evaluation of satisfaction, convenience, and preference. Pediatr Emerg Care. 2013;29:555-559. doi: 10.1097/PEC.0b013e31828e56fb
9. Jiang N, Cao ZH, Ma YF, et al. Management of pediatric forearm torus fractures: a systematic review and meta-analysis. Pediatr Emerg Care. 2016;32:773-778. doi: 10.1097/PEC.0000000000000579
10. Williams BA, Alvarado CA, Montoya-Williams DC, et al. Buckling down on torus fractures: has evolving evidence affected practice? J Child Orthop. 2018;12:123-128. doi: 10.1302/1863-2548.12.170122
11. Garra G, Singer AJ, Taira BR, et al. Validation of the Wong-Baker FACES Pain Rating Scale in pediatric emergency department patients. Acad Emerg Med. 2010;17:50-54. doi: 10.1111/j.1553-2712.2009.00620.x
ILLUSTRATIVE CASE
A 9-year-old girl presents to your urgent care clinic after a fall while snowboarding for the first time. She reports falling forward onto her outstretched right hand and describes pain in her distal right forearm. She denies paresthesias, weakness, or lacerations. Physical examination reveals mild edema of the dorsal aspect of her distal right forearm and tenderness to palpation of the dorsal aspect of her distal radius. She denies tenderness to palpation of her ulna, anatomic snuffbox, hand, and elbow. Range of motion of the wrist is full on passive testing, but she declines active testing due to pain. Wrist radiographs reveal an uncomplicated torus fracture of the distal radius. Can immobilization with a soft bandage alone sufficiently treat this fracture?
Fractures of the distal radius are among the most common fractures of the upper extremity and commonly occur from a fall onto an outstretched hand.2 In the pediatric population, torus fractures, also known as buckle fractures, are the most common type of distal radius fracture, comprising an estimated 50% of pediatric wrist fractures.3,4 This is due to the presence of a
Pediatric torus fractures of the distal radius generally are treated with immobilization,2 traditionally through a
Despite common use of immobilization, torus fractures of the distal radius are anatomically stable, and displacement is unlikely to occur.7,8 As such, many studies have suggested that treatment of torus fractures with rigid immobilization in a cast or splint may not be necessary.9,10 However, a 2018 Cochrane review concluded that the quality of evidence illustrating similar recovery between treatments was low, leaving uncertainty as to the most appropriate management strategy.6 Less casting and follow-up imaging could have positive implications for patient satisfaction, health care–associated costs, and radiation exposure.10
This study, the Forearm Fracture Recovery in Children Evaluation (FORCE) trial, compared the traditional treatment of distal radius torus fractures with rigid immobilization to soft immobilization and immediate discharge.
STUDY SUMMARY
Providing quality evidence for a standard of care
FORCE was a randomized controlled equivalence trial (N = 965) across 23 emergency departments (EDs) in the United Kingdom that compared pain and function in pediatric patients with distal radius torus fractures treated with a soft bandage and immediate discharge vs rigid immobilization and routine follow-up.1 Patients included children ages 4 to 15 years presenting to the ED with a distal radius torus fracture, which was confirmed radiologically.
Patients with concomitant
Continue to: Patients were randomly assigned...
Patients were randomly assigned in a 1:1 ratio to receive treatment with either a soft bandage such as a gauze roller bandage (n = 489) or rigid immobilization (n = 476). For patients in the bandage group, a soft bandage was applied in the ED or provided for home application without planned clinical follow-up. Patients in the rigid immobilization group were treated in the ED with either a removable manufactured splint or a molded splint or cast, followed by the standard follow-up practice of the treating center. Patients in the soft bandage group were advised not to wear the bandage for more than 3 weeks. Blinding was not possible, but the treatment team did not take part in patient follow-up.
The primary outcome was change in pain 3 days after treatment, measured on the Wong-Baker FACES Pain Rating Scale (an ordinal assessment using 6 illustrated facial expressions translated to a numeric rating on a scale of 0-10, with higher scores indicating worse pain). This scale has an established minimum clinically important difference (MCID) value of 1 face (2 points).11 Per standard practice in equivalence trials, the equivalence margin was defined as half the MCID, with a value of 1.0 used in this study.
Secondary outcomes measured over the 6-week follow-up period included additional pain measurements using the Wong-Baker scale, measures of function and health-related quality of life, analgesia use, days of absence from school or childcare, complication rates, and patient satisfaction. This study used modified intention-to-treat and per-protocol analyses.
The mean age of participants was 9.6 years; 39% were girls and 61% were boys. In the bandage group, 94% opted to have the soft bandage applied in the ED, and 95% of the rigid immobilization group were treated with a removable wrist splint in the ED. At 3 days, pain scores improved by 3.2 points (standard deviation [SD] = 2.1) in the soft bandage group and 3.1 points (SD = 2.1) in the rigid immobilization group. The adjusted difference was –0.1 (95% CI, –0.37 to 0.17) in the intention-to-treat analysis and –0.06 (95% CI, –0.34 to 0.21) in the per-protocol analysis, which were both less than the predetermined equivalence margin. This equivalence margin also was met at all secondary time points (1 day, 7 days, 3 weeks, and 6 weeks after treatment) and in subgroup analysis of those 4 to 7 years and 8 to 15 years.
Use of any analgesia in the prior 24 hours was slightly higher in the soft bandage group on Day 1 (83% vs 78%; P = .04) and Day 3 (57% vs 51%; P = .05), but this difference was not seen on Day 7. Satisfaction, measured via a 7-point Likert scale (range from “extremely satisfied” to “extremely unsatisfied”), was slightly lower in the soft bandage group on Day 1 (median 2 [interquartile range = 1, 2] vs median 1 [interquartile range = 1, 2]; P < .0001) but was not different after 6 weeks. There were no measured differences in any other secondary outcomes, including function, quality of life, and complication rates.
Continue to: By the primary end point...
By the primary end point of 3 days, 36 patients (7%) in the soft bandage group returned to medical care requesting a change to rigid immobilization, compared with 1 patient (0.2%) in the rigid immobilization group declining intervention.
WHAT’S NEW
Equivalence in pain and function scores
This trial showed equivalence in pain at 3 days’ follow-up in children with distal radius torus fractures who were offered bandaging and then immediately discharged from the ED, compared with rigid immobilization and clinical follow-up. There were no significant differences in pain or function between groups during the 6 weeks following the initial injury. De-escalation of treatment offers an equivalent, resource-sparing alternative to traditional treatment of these fractures.
CAVEATS
Lack of masking likely introduced bias
There are no major caveats associated with managing distal radius torus fractures with a soft bandage and discharge from the ED, compared with the traditional treatment of rigid immobilization. However, bias was likely introduced in patient-reported outcomes due to the inability to mask patients and families to the treatment allocation. This may have led to overstating the severity of outcomes in the bandage group, given the strong preference for rigid immobilization, although equivalence was illustrated despite this potential bias.
CHALLENGES TO IMPLEMENTATION
Preferences may be difficult to change
Parents and clinicians demonstrated a preference for rigid immobilization, as shown in the imbalance in treatment crossovers, with 7% of children changing to the rigid immobilization group by the primary study end point of 3 days. The study authors hypothesized that crossovers may have been due to the perception by some parents that rigid immobilization is the gold standard of treatment, as well as clinicians’ seeking to escalate care for patients returning for follow-up. Policy and guideline changes, as well as physician efforts to educate patients on outcomes with soft bandage treatment, are likely to improve these misconceptions.
ILLUSTRATIVE CASE
A 9-year-old girl presents to your urgent care clinic after a fall while snowboarding for the first time. She reports falling forward onto her outstretched right hand and describes pain in her distal right forearm. She denies paresthesias, weakness, or lacerations. Physical examination reveals mild edema of the dorsal aspect of her distal right forearm and tenderness to palpation of the dorsal aspect of her distal radius. She denies tenderness to palpation of her ulna, anatomic snuffbox, hand, and elbow. Range of motion of the wrist is full on passive testing, but she declines active testing due to pain. Wrist radiographs reveal an uncomplicated torus fracture of the distal radius. Can immobilization with a soft bandage alone sufficiently treat this fracture?
Fractures of the distal radius are among the most common fractures of the upper extremity and commonly occur from a fall onto an outstretched hand.2 In the pediatric population, torus fractures, also known as buckle fractures, are the most common type of distal radius fracture, comprising an estimated 50% of pediatric wrist fractures.3,4 This is due to the presence of a
Pediatric torus fractures of the distal radius generally are treated with immobilization,2 traditionally through a
Despite common use of immobilization, torus fractures of the distal radius are anatomically stable, and displacement is unlikely to occur.7,8 As such, many studies have suggested that treatment of torus fractures with rigid immobilization in a cast or splint may not be necessary.9,10 However, a 2018 Cochrane review concluded that the quality of evidence illustrating similar recovery between treatments was low, leaving uncertainty as to the most appropriate management strategy.6 Less casting and follow-up imaging could have positive implications for patient satisfaction, health care–associated costs, and radiation exposure.10
This study, the Forearm Fracture Recovery in Children Evaluation (FORCE) trial, compared the traditional treatment of distal radius torus fractures with rigid immobilization to soft immobilization and immediate discharge.
STUDY SUMMARY
Providing quality evidence for a standard of care
FORCE was a randomized controlled equivalence trial (N = 965) across 23 emergency departments (EDs) in the United Kingdom that compared pain and function in pediatric patients with distal radius torus fractures treated with a soft bandage and immediate discharge vs rigid immobilization and routine follow-up.1 Patients included children ages 4 to 15 years presenting to the ED with a distal radius torus fracture, which was confirmed radiologically.
Patients with concomitant
Continue to: Patients were randomly assigned...
Patients were randomly assigned in a 1:1 ratio to receive treatment with either a soft bandage such as a gauze roller bandage (n = 489) or rigid immobilization (n = 476). For patients in the bandage group, a soft bandage was applied in the ED or provided for home application without planned clinical follow-up. Patients in the rigid immobilization group were treated in the ED with either a removable manufactured splint or a molded splint or cast, followed by the standard follow-up practice of the treating center. Patients in the soft bandage group were advised not to wear the bandage for more than 3 weeks. Blinding was not possible, but the treatment team did not take part in patient follow-up.
The primary outcome was change in pain 3 days after treatment, measured on the Wong-Baker FACES Pain Rating Scale (an ordinal assessment using 6 illustrated facial expressions translated to a numeric rating on a scale of 0-10, with higher scores indicating worse pain). This scale has an established minimum clinically important difference (MCID) value of 1 face (2 points).11 Per standard practice in equivalence trials, the equivalence margin was defined as half the MCID, with a value of 1.0 used in this study.
Secondary outcomes measured over the 6-week follow-up period included additional pain measurements using the Wong-Baker scale, measures of function and health-related quality of life, analgesia use, days of absence from school or childcare, complication rates, and patient satisfaction. This study used modified intention-to-treat and per-protocol analyses.
The mean age of participants was 9.6 years; 39% were girls and 61% were boys. In the bandage group, 94% opted to have the soft bandage applied in the ED, and 95% of the rigid immobilization group were treated with a removable wrist splint in the ED. At 3 days, pain scores improved by 3.2 points (standard deviation [SD] = 2.1) in the soft bandage group and 3.1 points (SD = 2.1) in the rigid immobilization group. The adjusted difference was –0.1 (95% CI, –0.37 to 0.17) in the intention-to-treat analysis and –0.06 (95% CI, –0.34 to 0.21) in the per-protocol analysis, which were both less than the predetermined equivalence margin. This equivalence margin also was met at all secondary time points (1 day, 7 days, 3 weeks, and 6 weeks after treatment) and in subgroup analysis of those 4 to 7 years and 8 to 15 years.
Use of any analgesia in the prior 24 hours was slightly higher in the soft bandage group on Day 1 (83% vs 78%; P = .04) and Day 3 (57% vs 51%; P = .05), but this difference was not seen on Day 7. Satisfaction, measured via a 7-point Likert scale (range from “extremely satisfied” to “extremely unsatisfied”), was slightly lower in the soft bandage group on Day 1 (median 2 [interquartile range = 1, 2] vs median 1 [interquartile range = 1, 2]; P < .0001) but was not different after 6 weeks. There were no measured differences in any other secondary outcomes, including function, quality of life, and complication rates.
Continue to: By the primary end point...
By the primary end point of 3 days, 36 patients (7%) in the soft bandage group returned to medical care requesting a change to rigid immobilization, compared with 1 patient (0.2%) in the rigid immobilization group declining intervention.
WHAT’S NEW
Equivalence in pain and function scores
This trial showed equivalence in pain at 3 days’ follow-up in children with distal radius torus fractures who were offered bandaging and then immediately discharged from the ED, compared with rigid immobilization and clinical follow-up. There were no significant differences in pain or function between groups during the 6 weeks following the initial injury. De-escalation of treatment offers an equivalent, resource-sparing alternative to traditional treatment of these fractures.
CAVEATS
Lack of masking likely introduced bias
There are no major caveats associated with managing distal radius torus fractures with a soft bandage and discharge from the ED, compared with the traditional treatment of rigid immobilization. However, bias was likely introduced in patient-reported outcomes due to the inability to mask patients and families to the treatment allocation. This may have led to overstating the severity of outcomes in the bandage group, given the strong preference for rigid immobilization, although equivalence was illustrated despite this potential bias.
CHALLENGES TO IMPLEMENTATION
Preferences may be difficult to change
Parents and clinicians demonstrated a preference for rigid immobilization, as shown in the imbalance in treatment crossovers, with 7% of children changing to the rigid immobilization group by the primary study end point of 3 days. The study authors hypothesized that crossovers may have been due to the perception by some parents that rigid immobilization is the gold standard of treatment, as well as clinicians’ seeking to escalate care for patients returning for follow-up. Policy and guideline changes, as well as physician efforts to educate patients on outcomes with soft bandage treatment, are likely to improve these misconceptions.
1. Perry DC, Achten J, Knight R, et al; FORCE Collaborators in collaboration with PERUKI. Immobilisation of torus fractures of the wrist in children (FORCE): a randomised controlled equivalence trial in the UK. Lancet. 2022;400:39-47. doi: 10.1016/S0140-6736(22)01015-7
2. Patel DS, Statuta SM, Ahmed N. Common fractures of the radius and ulna. Am Fam Physician. 2021;103:345-354.
3. Asokan A, Kheir N. Pediatric Torus Buckle Fracture. StatPearls Publishing; 2023.
4. Naranje SM, Erali RA, Warner WC Jr, et al. Epidemiology of pediatric fractures presenting to emergency departments in the United States. J Pediatr Orthop. 2016;36:e45-e48. doi: 10.1097/BPO.0000000000000595
5. Kennedy SA, Slobogean GP, Mulpuri K. Does degree of immobilization influence refracture rate in the forearm buckle fracture? J Pediatr Orthop B. 2010;19:77-81. doi: 10.1097/BPB.0b013e32832f067a
6. Handoll HHG, Elliott J, Iheozor-Ejiofor Z, et al. Interventions for treating wrist fractures in children. Cochrane Database Syst Rev. 2018;12:CD012470. doi: 10.1002/14651858.CD012470.pub2
7. Perry DC, Gibson P, Roland D, et al. What level of immobilisation is necessary for treatment of torus (buckle) fractures of the distal radius in children? BMJ. 2021;372:m4862. doi: 10.1136/bmj.m4862
8. Williams KG, Smith G, Luhmann SJ, et al. A randomized controlled trial of cast versus splint for distal radial buckle fracture: an evaluation of satisfaction, convenience, and preference. Pediatr Emerg Care. 2013;29:555-559. doi: 10.1097/PEC.0b013e31828e56fb
9. Jiang N, Cao ZH, Ma YF, et al. Management of pediatric forearm torus fractures: a systematic review and meta-analysis. Pediatr Emerg Care. 2016;32:773-778. doi: 10.1097/PEC.0000000000000579
10. Williams BA, Alvarado CA, Montoya-Williams DC, et al. Buckling down on torus fractures: has evolving evidence affected practice? J Child Orthop. 2018;12:123-128. doi: 10.1302/1863-2548.12.170122
11. Garra G, Singer AJ, Taira BR, et al. Validation of the Wong-Baker FACES Pain Rating Scale in pediatric emergency department patients. Acad Emerg Med. 2010;17:50-54. doi: 10.1111/j.1553-2712.2009.00620.x
1. Perry DC, Achten J, Knight R, et al; FORCE Collaborators in collaboration with PERUKI. Immobilisation of torus fractures of the wrist in children (FORCE): a randomised controlled equivalence trial in the UK. Lancet. 2022;400:39-47. doi: 10.1016/S0140-6736(22)01015-7
2. Patel DS, Statuta SM, Ahmed N. Common fractures of the radius and ulna. Am Fam Physician. 2021;103:345-354.
3. Asokan A, Kheir N. Pediatric Torus Buckle Fracture. StatPearls Publishing; 2023.
4. Naranje SM, Erali RA, Warner WC Jr, et al. Epidemiology of pediatric fractures presenting to emergency departments in the United States. J Pediatr Orthop. 2016;36:e45-e48. doi: 10.1097/BPO.0000000000000595
5. Kennedy SA, Slobogean GP, Mulpuri K. Does degree of immobilization influence refracture rate in the forearm buckle fracture? J Pediatr Orthop B. 2010;19:77-81. doi: 10.1097/BPB.0b013e32832f067a
6. Handoll HHG, Elliott J, Iheozor-Ejiofor Z, et al. Interventions for treating wrist fractures in children. Cochrane Database Syst Rev. 2018;12:CD012470. doi: 10.1002/14651858.CD012470.pub2
7. Perry DC, Gibson P, Roland D, et al. What level of immobilisation is necessary for treatment of torus (buckle) fractures of the distal radius in children? BMJ. 2021;372:m4862. doi: 10.1136/bmj.m4862
8. Williams KG, Smith G, Luhmann SJ, et al. A randomized controlled trial of cast versus splint for distal radial buckle fracture: an evaluation of satisfaction, convenience, and preference. Pediatr Emerg Care. 2013;29:555-559. doi: 10.1097/PEC.0b013e31828e56fb
9. Jiang N, Cao ZH, Ma YF, et al. Management of pediatric forearm torus fractures: a systematic review and meta-analysis. Pediatr Emerg Care. 2016;32:773-778. doi: 10.1097/PEC.0000000000000579
10. Williams BA, Alvarado CA, Montoya-Williams DC, et al. Buckling down on torus fractures: has evolving evidence affected practice? J Child Orthop. 2018;12:123-128. doi: 10.1302/1863-2548.12.170122
11. Garra G, Singer AJ, Taira BR, et al. Validation of the Wong-Baker FACES Pain Rating Scale in pediatric emergency department patients. Acad Emerg Med. 2010;17:50-54. doi: 10.1111/j.1553-2712.2009.00620.x
PRACTICE CHANGER
For uncomplicated pediatric torus fractures of the distal radius, consider definitive management with soft bandage immobilization until pain resolution, rather than rigid immobilization and clinical follow-up.
STRENGTH OF RECOMMENDATION
B: Based on a single randomized controlled trial with patient-oriented outcomes.1
Perry DC, Achten J, Knight R, et al; FORCE Collaborators in collaboration with PERUKI. Immobilisation of torus fractures of the wrist in children (FORCE): a randomised controlled equivalence trial in the UK. Lancet. 2022;400:39-47. doi: 10.1016/S0140-6736(22)01015-7
Angioplasty finally proven beneficial in stable angina: ORBITA-2
PHILADELPHIA – Percutaneous coronary intervention (PCI) in patients with stable coronary artery disease (CAD) reduces angina frequency, increases exercise capacity, and improves quality of life, results of a placebo-controlled, randomized trial show, confirming advantages that have never before been proven.
reported Christopher A. Rajkumar, MBBS, an interventional cardiology registrar at the Imperial College Healthcare Trust, London.
Results of the trial, ORBITA-2, were presented at the annual scientific sessions of the American Heart Association and simultaneously published online in the New England Journal of Medicine.
Symptom relief has long been a justification for PCI in patients with stable CAD, but the evidence has been derived from uncontrolled studies, Dr. Rajkumar said. However, the first ORBITA trial, which was also placebo controlled and randomized, failed to show benefit.
Dr. Rajkumar acknowledged that the benefit of PCI in ORBITA-2 was lower than previously reported in nonrandomized trials. He also noted that 59% of patients still had at least some angina symptoms following PCI.
Even though ORBITA-2 proves that PCI is better than no PCI, he agreed that well-informed patients, such as those who wish to avoid an invasive procedure, might still reasonably select antianginal medication over PCI. Current guidelines recommend PCI for patients with refractory angina despite medical therapy.
While Dr. Rajkumar was unwilling to speculate on how these data might change guidelines, he did say that patients with stable CAD and angina “now have a choice of two first-line evidence-based pathways.”
‘Remarkable’ trial
“ORBITA 2 is a rather remarkable trial because my surgical colleagues have been asking me for many decades whether PCI actually works,” said Martin B. Leon, MD, professor of medicine, Columbia University Irving Medical Center, New York. “Now I can say with confidence on the basis of a placebo-controlled trial that PCI certainly does have a favorable impact in patients with documented angina, severe coronary stenosis, and demonstrated ischemia.”
The key enrollment criteria for ORBITA-2 were angina, severe coronary stenosis in at least one vessel, and ischemia on stress imaging or invasive physiology. Unlike the previous ORBITA trial, which was limited to single-vessel disease and did not require objective evidence of ischemia, ORBITA 2 employed change in angina, rather than improved exercise capacity, as its primary endpoint.
Relative to sham PCI, patients randomly assigned to an interventional procedure had a more than twofold increase in the odds ratio of improved angina control (OR, 2.2; P < .001) based on a patient scoring system that captured angina symptoms as well as angina medication use on a smartphone application.
The advantage of PCI over sham PCI was also significant for all secondary outcomes. These included a nearly fourfold greater (OR, 3.76; P < .001) likelihood of improvement in the Canadian Cardiovascular Society angina grade and a 1-minute increase (from 10 min. 40 seconds to 11 min. 40 seconds) in treadmill exercise time (P = .008).
On quality of life measured with the self-assessment questionnaire and the EQ-5D-5L, almost all endpoints were highly statistically significant in favor of PCI (typically on the level of P < .001).
The study had a bold design: At enrollment patients stopped all antianginal medications to undergo dobutamine echocardiography and other baseline tests. They were stopped again 2 weeks later, when patients were randomized.
With a study protocol that enrolled patients off medication, “we intentionally diverged from the clinical guidelines,” Dr. Rajkumar said.
Of the 439 patients enrolled, 301 were randomly assigned at the end of the 2-week period, when patients were already sedated. Control patients remained sedated for at least 15 minutes. All 151 of those randomized to PCI and the 150 control patients were available for the intent-to-treat analysis at the end of 12 weeks.
The novel angina symptom burden score was created from daily angina episodes and units of daily antianginal medication captured on the smartphone app. On an ordinal scale, a score of 0 on any given day represented no anginal symptoms and no antianginal medication.
As angina severity or medication use increased, it raised the daily scores. If there was unacceptable angina (requiring the patient to be removed from the blind), acute coronary syndrome, or death, it produced the highest scores, which reached a maximum of 79.
The favorable OR for a lower symptom burden in the PCI group reflected a relative reduction in angina observed the first day after the procedure. Over the entire follow-up, more patients in the PCI group had an angina score of 0 and more of those who had angina did not take antianginal medications.
This objective evidence that PCI reduces symptoms and improves quality of life in patients with angina and stable CAD was met at the AHA late-breaking session with a sustained ovation.
ORBITA-2 addresses ORBITA criticisms
Connie N. Hess, MD, the AHA-invited discussant and an interventional cardiologist at the University of Colorado Medicine, Aurora, provided perspective on the differences between ORBITA 2 and ORBITA, which she said “addressed a fundamentally different hypothesis” by focusing on angina rather than exercise capacity.
Of the criticisms of the original ORBITA, which Dr. Hess noted was the first sham-controlled PCI trial ever conducted in stable CAD, one is that patients with multivessel disease were excluded, another was that objectively proven ischemia was not required, and a third was that the study of 6 weeks had a short duration.
“ORBITA 2 addressed many of these concerns,” Dr. Hess said, but, when noting that 80% of patients in the newer trial still had single vessel disease, she questioned whether the true effect of PCI for improving symptoms might still be underestimated.
ORBITA-2 was supported by the National Institute for Health and Care Research Imperial Biomedical Research Centre, the Medical Research Council, NIHR, the British Heart Foundation, Philips, and St. Mary’s Coronary Flow Trust. Dr. Rajkumar reported relevant financial relationships. Dr. Leon reported financial relationships with Abbott Vascular, Anteris, Boston Scientific, Edwards Lifesciences, Foldax, and Medtronic. Dr. Hess has financial relationships with more than 20 pharmaceutical companies, but none related specifically to this presentation.
PHILADELPHIA – Percutaneous coronary intervention (PCI) in patients with stable coronary artery disease (CAD) reduces angina frequency, increases exercise capacity, and improves quality of life, results of a placebo-controlled, randomized trial show, confirming advantages that have never before been proven.
reported Christopher A. Rajkumar, MBBS, an interventional cardiology registrar at the Imperial College Healthcare Trust, London.
Results of the trial, ORBITA-2, were presented at the annual scientific sessions of the American Heart Association and simultaneously published online in the New England Journal of Medicine.
Symptom relief has long been a justification for PCI in patients with stable CAD, but the evidence has been derived from uncontrolled studies, Dr. Rajkumar said. However, the first ORBITA trial, which was also placebo controlled and randomized, failed to show benefit.
Dr. Rajkumar acknowledged that the benefit of PCI in ORBITA-2 was lower than previously reported in nonrandomized trials. He also noted that 59% of patients still had at least some angina symptoms following PCI.
Even though ORBITA-2 proves that PCI is better than no PCI, he agreed that well-informed patients, such as those who wish to avoid an invasive procedure, might still reasonably select antianginal medication over PCI. Current guidelines recommend PCI for patients with refractory angina despite medical therapy.
While Dr. Rajkumar was unwilling to speculate on how these data might change guidelines, he did say that patients with stable CAD and angina “now have a choice of two first-line evidence-based pathways.”
‘Remarkable’ trial
“ORBITA 2 is a rather remarkable trial because my surgical colleagues have been asking me for many decades whether PCI actually works,” said Martin B. Leon, MD, professor of medicine, Columbia University Irving Medical Center, New York. “Now I can say with confidence on the basis of a placebo-controlled trial that PCI certainly does have a favorable impact in patients with documented angina, severe coronary stenosis, and demonstrated ischemia.”
The key enrollment criteria for ORBITA-2 were angina, severe coronary stenosis in at least one vessel, and ischemia on stress imaging or invasive physiology. Unlike the previous ORBITA trial, which was limited to single-vessel disease and did not require objective evidence of ischemia, ORBITA 2 employed change in angina, rather than improved exercise capacity, as its primary endpoint.
Relative to sham PCI, patients randomly assigned to an interventional procedure had a more than twofold increase in the odds ratio of improved angina control (OR, 2.2; P < .001) based on a patient scoring system that captured angina symptoms as well as angina medication use on a smartphone application.
The advantage of PCI over sham PCI was also significant for all secondary outcomes. These included a nearly fourfold greater (OR, 3.76; P < .001) likelihood of improvement in the Canadian Cardiovascular Society angina grade and a 1-minute increase (from 10 min. 40 seconds to 11 min. 40 seconds) in treadmill exercise time (P = .008).
On quality of life measured with the self-assessment questionnaire and the EQ-5D-5L, almost all endpoints were highly statistically significant in favor of PCI (typically on the level of P < .001).
The study had a bold design: At enrollment patients stopped all antianginal medications to undergo dobutamine echocardiography and other baseline tests. They were stopped again 2 weeks later, when patients were randomized.
With a study protocol that enrolled patients off medication, “we intentionally diverged from the clinical guidelines,” Dr. Rajkumar said.
Of the 439 patients enrolled, 301 were randomly assigned at the end of the 2-week period, when patients were already sedated. Control patients remained sedated for at least 15 minutes. All 151 of those randomized to PCI and the 150 control patients were available for the intent-to-treat analysis at the end of 12 weeks.
The novel angina symptom burden score was created from daily angina episodes and units of daily antianginal medication captured on the smartphone app. On an ordinal scale, a score of 0 on any given day represented no anginal symptoms and no antianginal medication.
As angina severity or medication use increased, it raised the daily scores. If there was unacceptable angina (requiring the patient to be removed from the blind), acute coronary syndrome, or death, it produced the highest scores, which reached a maximum of 79.
The favorable OR for a lower symptom burden in the PCI group reflected a relative reduction in angina observed the first day after the procedure. Over the entire follow-up, more patients in the PCI group had an angina score of 0 and more of those who had angina did not take antianginal medications.
This objective evidence that PCI reduces symptoms and improves quality of life in patients with angina and stable CAD was met at the AHA late-breaking session with a sustained ovation.
ORBITA-2 addresses ORBITA criticisms
Connie N. Hess, MD, the AHA-invited discussant and an interventional cardiologist at the University of Colorado Medicine, Aurora, provided perspective on the differences between ORBITA 2 and ORBITA, which she said “addressed a fundamentally different hypothesis” by focusing on angina rather than exercise capacity.
Of the criticisms of the original ORBITA, which Dr. Hess noted was the first sham-controlled PCI trial ever conducted in stable CAD, one is that patients with multivessel disease were excluded, another was that objectively proven ischemia was not required, and a third was that the study of 6 weeks had a short duration.
“ORBITA 2 addressed many of these concerns,” Dr. Hess said, but, when noting that 80% of patients in the newer trial still had single vessel disease, she questioned whether the true effect of PCI for improving symptoms might still be underestimated.
ORBITA-2 was supported by the National Institute for Health and Care Research Imperial Biomedical Research Centre, the Medical Research Council, NIHR, the British Heart Foundation, Philips, and St. Mary’s Coronary Flow Trust. Dr. Rajkumar reported relevant financial relationships. Dr. Leon reported financial relationships with Abbott Vascular, Anteris, Boston Scientific, Edwards Lifesciences, Foldax, and Medtronic. Dr. Hess has financial relationships with more than 20 pharmaceutical companies, but none related specifically to this presentation.
PHILADELPHIA – Percutaneous coronary intervention (PCI) in patients with stable coronary artery disease (CAD) reduces angina frequency, increases exercise capacity, and improves quality of life, results of a placebo-controlled, randomized trial show, confirming advantages that have never before been proven.
reported Christopher A. Rajkumar, MBBS, an interventional cardiology registrar at the Imperial College Healthcare Trust, London.
Results of the trial, ORBITA-2, were presented at the annual scientific sessions of the American Heart Association and simultaneously published online in the New England Journal of Medicine.
Symptom relief has long been a justification for PCI in patients with stable CAD, but the evidence has been derived from uncontrolled studies, Dr. Rajkumar said. However, the first ORBITA trial, which was also placebo controlled and randomized, failed to show benefit.
Dr. Rajkumar acknowledged that the benefit of PCI in ORBITA-2 was lower than previously reported in nonrandomized trials. He also noted that 59% of patients still had at least some angina symptoms following PCI.
Even though ORBITA-2 proves that PCI is better than no PCI, he agreed that well-informed patients, such as those who wish to avoid an invasive procedure, might still reasonably select antianginal medication over PCI. Current guidelines recommend PCI for patients with refractory angina despite medical therapy.
While Dr. Rajkumar was unwilling to speculate on how these data might change guidelines, he did say that patients with stable CAD and angina “now have a choice of two first-line evidence-based pathways.”
‘Remarkable’ trial
“ORBITA 2 is a rather remarkable trial because my surgical colleagues have been asking me for many decades whether PCI actually works,” said Martin B. Leon, MD, professor of medicine, Columbia University Irving Medical Center, New York. “Now I can say with confidence on the basis of a placebo-controlled trial that PCI certainly does have a favorable impact in patients with documented angina, severe coronary stenosis, and demonstrated ischemia.”
The key enrollment criteria for ORBITA-2 were angina, severe coronary stenosis in at least one vessel, and ischemia on stress imaging or invasive physiology. Unlike the previous ORBITA trial, which was limited to single-vessel disease and did not require objective evidence of ischemia, ORBITA 2 employed change in angina, rather than improved exercise capacity, as its primary endpoint.
Relative to sham PCI, patients randomly assigned to an interventional procedure had a more than twofold increase in the odds ratio of improved angina control (OR, 2.2; P < .001) based on a patient scoring system that captured angina symptoms as well as angina medication use on a smartphone application.
The advantage of PCI over sham PCI was also significant for all secondary outcomes. These included a nearly fourfold greater (OR, 3.76; P < .001) likelihood of improvement in the Canadian Cardiovascular Society angina grade and a 1-minute increase (from 10 min. 40 seconds to 11 min. 40 seconds) in treadmill exercise time (P = .008).
On quality of life measured with the self-assessment questionnaire and the EQ-5D-5L, almost all endpoints were highly statistically significant in favor of PCI (typically on the level of P < .001).
The study had a bold design: At enrollment patients stopped all antianginal medications to undergo dobutamine echocardiography and other baseline tests. They were stopped again 2 weeks later, when patients were randomized.
With a study protocol that enrolled patients off medication, “we intentionally diverged from the clinical guidelines,” Dr. Rajkumar said.
Of the 439 patients enrolled, 301 were randomly assigned at the end of the 2-week period, when patients were already sedated. Control patients remained sedated for at least 15 minutes. All 151 of those randomized to PCI and the 150 control patients were available for the intent-to-treat analysis at the end of 12 weeks.
The novel angina symptom burden score was created from daily angina episodes and units of daily antianginal medication captured on the smartphone app. On an ordinal scale, a score of 0 on any given day represented no anginal symptoms and no antianginal medication.
As angina severity or medication use increased, it raised the daily scores. If there was unacceptable angina (requiring the patient to be removed from the blind), acute coronary syndrome, or death, it produced the highest scores, which reached a maximum of 79.
The favorable OR for a lower symptom burden in the PCI group reflected a relative reduction in angina observed the first day after the procedure. Over the entire follow-up, more patients in the PCI group had an angina score of 0 and more of those who had angina did not take antianginal medications.
This objective evidence that PCI reduces symptoms and improves quality of life in patients with angina and stable CAD was met at the AHA late-breaking session with a sustained ovation.
ORBITA-2 addresses ORBITA criticisms
Connie N. Hess, MD, the AHA-invited discussant and an interventional cardiologist at the University of Colorado Medicine, Aurora, provided perspective on the differences between ORBITA 2 and ORBITA, which she said “addressed a fundamentally different hypothesis” by focusing on angina rather than exercise capacity.
Of the criticisms of the original ORBITA, which Dr. Hess noted was the first sham-controlled PCI trial ever conducted in stable CAD, one is that patients with multivessel disease were excluded, another was that objectively proven ischemia was not required, and a third was that the study of 6 weeks had a short duration.
“ORBITA 2 addressed many of these concerns,” Dr. Hess said, but, when noting that 80% of patients in the newer trial still had single vessel disease, she questioned whether the true effect of PCI for improving symptoms might still be underestimated.
ORBITA-2 was supported by the National Institute for Health and Care Research Imperial Biomedical Research Centre, the Medical Research Council, NIHR, the British Heart Foundation, Philips, and St. Mary’s Coronary Flow Trust. Dr. Rajkumar reported relevant financial relationships. Dr. Leon reported financial relationships with Abbott Vascular, Anteris, Boston Scientific, Edwards Lifesciences, Foldax, and Medtronic. Dr. Hess has financial relationships with more than 20 pharmaceutical companies, but none related specifically to this presentation.
AT AHA 2023
Liver-resident T cells provide early protection against Listeria infection
, according to investigators.
These finding suggest that gamma delta T17 cells could be a target for novel cell-based therapies against liver diseases, reported lead author Yanan Wang, PhD, of Shandong University, Jinan, China, and colleagues.
“Gamma delta T cells are located in mucosal tissues and other peripheral lymphoid tissues and are considered to act as the first line of defense within the immune system,” the investigators wrote in Cellular and Molecular Gastroenterology and Hepatology. “Several studies have reported that IL-17A produced by gamma delta T cells plays a critical role in host defense after Listeria monocytogenes [infection] in the liver. However, in those studies, the details of the phenotypes, dynamic changes, proliferation activity, and cytokine production of the responding gamma delta T cell populations in the overall process of hepatic infection are unclear, and how they accumulated into the infection sites has not been elucidated.”
To address this knowledge gap, Dr. Wang and colleagues conducted a series of experiments involving gamma delta T cells from murine liver samples.
First, using single-cell RNA-sequencing (scRNA-seq), the investigators identified six clusters of hepatic gamma delta T cells.
“[This first step] revealed the unique gene expression characteristics and indicated the possible important roles in immune responses of hepatic gamma delta T17 cells,” they noted.
Next, the investigators measured expression of CD44 and CD27 in liver gamma delta cells.
“Expression of CD44 and CD27 has been used to distinguish IL-17A–, interferon gamma–producing, and other subsets of gamma delta T cells in the thymus, lymph nodes, lungs, and other peripheral lymphoid tissues,” they wrote.
These efforts revealed three subsets of hepatic gamma delta T cells, of which CD44hiCD27– gamma delta T cells were most abundant. Further analysis revealed expression profiles consistent with liver residency.
The next phases of the study characterized the immune roles of hepatic gamma delta T cells.
A comparison of Listeria monocytogenes infection in wild-type versus T-cell antigen receptor knockout mice, for example, showed that knockout mice had significantly more weight loss than did wild-type mice, greater bacterial load in the liver, and shorter survival times.
“As expected, the proportion and absolute numbers of gamma delta T cells in the liver of wild-type mice increased at day 3 and reached a peak at day 7 after infection,” the investigators wrote. “These data suggested that hepatic gamma delta T cells proliferated after infection and contributed to Lm clearance.”
Parabiosis experiments showed that the increased number of CD44hiCD27– gamma delta T cells in the livers of Listeria monocytogenes-infected mice were due to migration and proliferation of liver-resident gamma delta T cells instead of circulating gamma delta T cells. A transwell assay revealed that Kupffer cells and monocyte-derived macrophages promoted migration of CD44hiCD27– gamma delta T cells upon infection.
“Our study provides additional insight into liver-resident lymphocytes and will aid in targeting such tissue-resident lymphocyte populations to promote local immune surveillance,” the investigators concluded.
The study was supported by grants from the National Natural Science Foundation of China and the Shandong Provincial Natural Science Foundation. The investigators disclosed no conflicts of interest.
, according to investigators.
These finding suggest that gamma delta T17 cells could be a target for novel cell-based therapies against liver diseases, reported lead author Yanan Wang, PhD, of Shandong University, Jinan, China, and colleagues.
“Gamma delta T cells are located in mucosal tissues and other peripheral lymphoid tissues and are considered to act as the first line of defense within the immune system,” the investigators wrote in Cellular and Molecular Gastroenterology and Hepatology. “Several studies have reported that IL-17A produced by gamma delta T cells plays a critical role in host defense after Listeria monocytogenes [infection] in the liver. However, in those studies, the details of the phenotypes, dynamic changes, proliferation activity, and cytokine production of the responding gamma delta T cell populations in the overall process of hepatic infection are unclear, and how they accumulated into the infection sites has not been elucidated.”
To address this knowledge gap, Dr. Wang and colleagues conducted a series of experiments involving gamma delta T cells from murine liver samples.
First, using single-cell RNA-sequencing (scRNA-seq), the investigators identified six clusters of hepatic gamma delta T cells.
“[This first step] revealed the unique gene expression characteristics and indicated the possible important roles in immune responses of hepatic gamma delta T17 cells,” they noted.
Next, the investigators measured expression of CD44 and CD27 in liver gamma delta cells.
“Expression of CD44 and CD27 has been used to distinguish IL-17A–, interferon gamma–producing, and other subsets of gamma delta T cells in the thymus, lymph nodes, lungs, and other peripheral lymphoid tissues,” they wrote.
These efforts revealed three subsets of hepatic gamma delta T cells, of which CD44hiCD27– gamma delta T cells were most abundant. Further analysis revealed expression profiles consistent with liver residency.
The next phases of the study characterized the immune roles of hepatic gamma delta T cells.
A comparison of Listeria monocytogenes infection in wild-type versus T-cell antigen receptor knockout mice, for example, showed that knockout mice had significantly more weight loss than did wild-type mice, greater bacterial load in the liver, and shorter survival times.
“As expected, the proportion and absolute numbers of gamma delta T cells in the liver of wild-type mice increased at day 3 and reached a peak at day 7 after infection,” the investigators wrote. “These data suggested that hepatic gamma delta T cells proliferated after infection and contributed to Lm clearance.”
Parabiosis experiments showed that the increased number of CD44hiCD27– gamma delta T cells in the livers of Listeria monocytogenes-infected mice were due to migration and proliferation of liver-resident gamma delta T cells instead of circulating gamma delta T cells. A transwell assay revealed that Kupffer cells and monocyte-derived macrophages promoted migration of CD44hiCD27– gamma delta T cells upon infection.
“Our study provides additional insight into liver-resident lymphocytes and will aid in targeting such tissue-resident lymphocyte populations to promote local immune surveillance,” the investigators concluded.
The study was supported by grants from the National Natural Science Foundation of China and the Shandong Provincial Natural Science Foundation. The investigators disclosed no conflicts of interest.
, according to investigators.
These finding suggest that gamma delta T17 cells could be a target for novel cell-based therapies against liver diseases, reported lead author Yanan Wang, PhD, of Shandong University, Jinan, China, and colleagues.
“Gamma delta T cells are located in mucosal tissues and other peripheral lymphoid tissues and are considered to act as the first line of defense within the immune system,” the investigators wrote in Cellular and Molecular Gastroenterology and Hepatology. “Several studies have reported that IL-17A produced by gamma delta T cells plays a critical role in host defense after Listeria monocytogenes [infection] in the liver. However, in those studies, the details of the phenotypes, dynamic changes, proliferation activity, and cytokine production of the responding gamma delta T cell populations in the overall process of hepatic infection are unclear, and how they accumulated into the infection sites has not been elucidated.”
To address this knowledge gap, Dr. Wang and colleagues conducted a series of experiments involving gamma delta T cells from murine liver samples.
First, using single-cell RNA-sequencing (scRNA-seq), the investigators identified six clusters of hepatic gamma delta T cells.
“[This first step] revealed the unique gene expression characteristics and indicated the possible important roles in immune responses of hepatic gamma delta T17 cells,” they noted.
Next, the investigators measured expression of CD44 and CD27 in liver gamma delta cells.
“Expression of CD44 and CD27 has been used to distinguish IL-17A–, interferon gamma–producing, and other subsets of gamma delta T cells in the thymus, lymph nodes, lungs, and other peripheral lymphoid tissues,” they wrote.
These efforts revealed three subsets of hepatic gamma delta T cells, of which CD44hiCD27– gamma delta T cells were most abundant. Further analysis revealed expression profiles consistent with liver residency.
The next phases of the study characterized the immune roles of hepatic gamma delta T cells.
A comparison of Listeria monocytogenes infection in wild-type versus T-cell antigen receptor knockout mice, for example, showed that knockout mice had significantly more weight loss than did wild-type mice, greater bacterial load in the liver, and shorter survival times.
“As expected, the proportion and absolute numbers of gamma delta T cells in the liver of wild-type mice increased at day 3 and reached a peak at day 7 after infection,” the investigators wrote. “These data suggested that hepatic gamma delta T cells proliferated after infection and contributed to Lm clearance.”
Parabiosis experiments showed that the increased number of CD44hiCD27– gamma delta T cells in the livers of Listeria monocytogenes-infected mice were due to migration and proliferation of liver-resident gamma delta T cells instead of circulating gamma delta T cells. A transwell assay revealed that Kupffer cells and monocyte-derived macrophages promoted migration of CD44hiCD27– gamma delta T cells upon infection.
“Our study provides additional insight into liver-resident lymphocytes and will aid in targeting such tissue-resident lymphocyte populations to promote local immune surveillance,” the investigators concluded.
The study was supported by grants from the National Natural Science Foundation of China and the Shandong Provincial Natural Science Foundation. The investigators disclosed no conflicts of interest.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Does vaginal estrogen use increase the risk for adverse cardiovascular outcomes?
Evidence summary
Cohort studies demonstrate no adverse CV outcomes
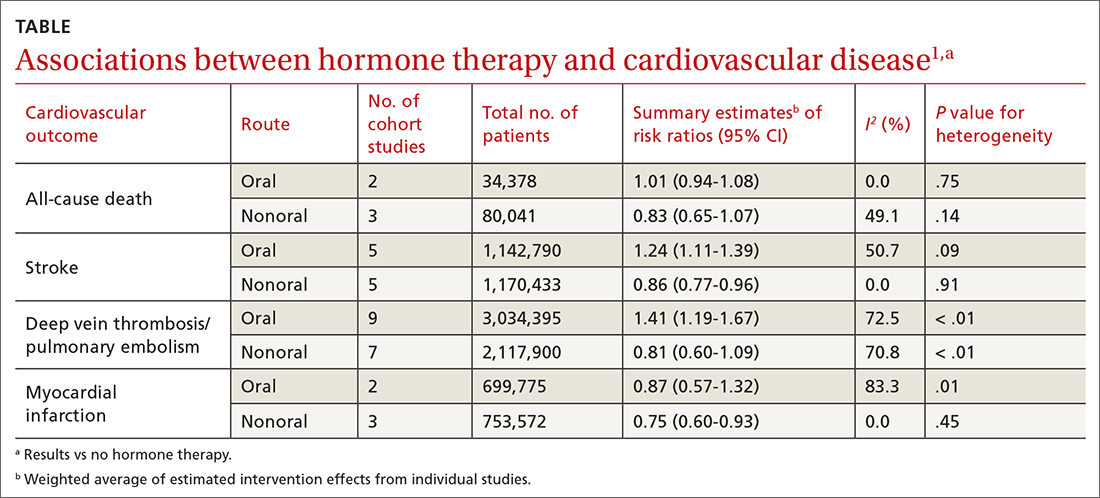
A 2020 systematic review and meta-analysis evaluated randomized controlled trials (RCTs) and observational studies to examine the association between menopausal hormone therapy and CV disease.1 The 26 RCTs primarily evaluated oral hormone administration. The observational studies comprised 30 cohort studies, 13 case-control studies, and 5 nested case-control studies, primarily in Europe and North America; 21 reported the route of administration. The trials evaluated women ages 49 to 77 years (mean, 61 years), and follow-up ranged from 1 to 21.5 years (mean, 7 years). In subgroup analyses of the observational studies, nonoral hormone therapy was associated with a lower risk for stroke and MI compared to oral administration (see TABLE1). Study limitations included enrollment of patients with few comorbidities, from limited geographic regions. Results in the meta-analysis were not stratified by the type of nonoral hormone therapy; only 4 studies evaluated vaginal estrogen use.
Two large cohort studies included in the systematic review provided more specific data on vaginal estrogens. The first used data from the Women’s Health Initiative in a subset of women ages 50 to 79 years (n = 46,566) who were not already on systemic hormone therapy and who did not have prior history of breast, endometrial, or ovarian cancer.2 Data were collected from self-assessment questionnaires and medical record reviews. The median duration of vaginal estrogen use was 2 years, and median follow-up duration was 7.2 years. Vaginal estrogen users had a 48% lower risk for CHD (adjusted hazard ratio [aHR] = 0.52; 95% CI, 0.31-0.85) than nonusers. Rates for all-cause mortality (aHR = 0.78; 95% CI, 0.58-1.04), stroke (aHR = 0.78; 95% CI, 0.49-1.24), and DVT/PE (aHR = 0.68; 95% CI, 0.36-1.28) were similar. In this and the other cohort studies to be discussed, outcome data for all vaginal estrogen preparations (eg, cream, ring, tablet) were combined.
The other large cohort study in the systematic review evaluated data on postmenopausal women from the Nurses’ Health Study.3 The authors evaluated health reports on 53,797 women as they transitioned through menopause. Patients with systemic hormone therapy use, history of cancer, and self-reported CV disease were excluded. After adjusting for covariates, the authors found no statistically significant difference between users and nonusers of vaginal estrogen and risk for total MI (aHR = 0.73; 95% CI, 0.47-1.13), stroke (aHR = 0.85; 95% CI, 0.56-1.29), or DVT/PE (aHR = 1.06; 95% CI, 0.58-1.93). Study limitations included low prevalence of vaginal estrogen use (< 3%), short duration of use (mean, 37.5 months), and lack of data on the type or dose of vaginal estrogen used. The study only included health professionals, which limits generalizability.
A Finnish cohort study (excluded from the systematic review because it used historical controls) compared rates of CHD and stroke in postmenopausal women who used vaginal estrogen against an age-matched background population. Researchers collected data from a nationwide prescription registry for women at least 50 years old who had purchased vaginal estrogens between 1994 and 2009 (n = 195,756).4 Women who purchased systemic hormone therapy at any point were excluded. After 3 to 5 years of exposure, use of vaginal estrogen was associated with a decreased risk for mortality from CHD (relative risk [RR] = 0.64; 95% CI, 0.57-0.70) and stroke (RR = 0.79; 95% CI, 0.69-0.91). However, after 10 years, these benefits were not seen (CHD: RR = 0.95; 95% CI, 0.90-1.00; stroke: RR = 0.93; 95% CI, 0.85-1.01). All confidence interval data were presented graphically. Key weaknesses of this study included use of both vaginal and systemic estrogen in the comparator background population, and the failure to collect data for other CV risk variables such as weight, tobacco exposure, and blood pressure.
Recommendations from others
In 2022, the North American Menopause Society issued a Hormone Therapy Position Statement that acknowledged the lack of clinical trials directly comparing risk for adverse CV endpoints with different estrogen administration routes.5 They stated nonoral routes of administration might offer advantages by bypassing first-pass hepatic metabolism.
Similarly, the 2015 Endocrine Society Clinical Practice Guideline on the Treatment of Symptoms of the Menopause also stated that the effects of low-dose vaginal estrogen therapy on CV disease or DVT/PE risk had not been adequately studied.6
A 2013 opinion by the American College of Obstetricians and Gynecologists stated that topical estrogen vaginal creams, tablets, and rings had low levels of systemic absorption and were not associated with an increased risk for DVT/PE.7
Editor’s takeaway
The available evidence on vaginal estrogen replacement reassures us of its safety. After decades spent studying hormone replacement therapy with vacillating conclusions and opinions, these cohorts—the best evidence we may ever get—along with a consensus of expert opinions, consistently demonstrate no adverse CV outcomes.
1. Kim JE, Chang JH, Jeong MJ, et al. A systematic review and meta-analysis of effects of menopausal hormone therapy on cardiovascular diseases. Sci Rep. 2020;10:20631. doi: 10.1038/s41598-020-77534-9
2. Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the WHI Observational Study. Menopause. 2018;25:11-20. doi: 10.1097/GME.0000000000000956
3. Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. 2018;26:603-610. doi: 10.1097/GME.0000000000001284
4. Mikkola TS, Tuomikoski P, Lyytinen H, et al. Vaginal estrogen use and the risk for cardiovascular mortality. Human Reproduction. 2016;31:804-809. doi: 10.1093/humrep/dew014
5. North American Menopause Society. The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi: 10.1097/GME.0000000000002028
6. Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:3975-4011. doi: 10.1210/jc.2015-2236
7. American College of Obstetricians and Gynecologists. Committee Opinion No 565: hormone therapy and heart disease. Obstet Gynecol. 2013;121:1407-1410. doi: 10.1097/01.AOG.0000431053.33593.2d
Evidence summary
Cohort studies demonstrate no adverse CV outcomes
A 2020 systematic review and meta-analysis evaluated randomized controlled trials (RCTs) and observational studies to examine the association between menopausal hormone therapy and CV disease.1 The 26 RCTs primarily evaluated oral hormone administration. The observational studies comprised 30 cohort studies, 13 case-control studies, and 5 nested case-control studies, primarily in Europe and North America; 21 reported the route of administration. The trials evaluated women ages 49 to 77 years (mean, 61 years), and follow-up ranged from 1 to 21.5 years (mean, 7 years). In subgroup analyses of the observational studies, nonoral hormone therapy was associated with a lower risk for stroke and MI compared to oral administration (see TABLE1). Study limitations included enrollment of patients with few comorbidities, from limited geographic regions. Results in the meta-analysis were not stratified by the type of nonoral hormone therapy; only 4 studies evaluated vaginal estrogen use.

Two large cohort studies included in the systematic review provided more specific data on vaginal estrogens. The first used data from the Women’s Health Initiative in a subset of women ages 50 to 79 years (n = 46,566) who were not already on systemic hormone therapy and who did not have prior history of breast, endometrial, or ovarian cancer.2 Data were collected from self-assessment questionnaires and medical record reviews. The median duration of vaginal estrogen use was 2 years, and median follow-up duration was 7.2 years. Vaginal estrogen users had a 48% lower risk for CHD (adjusted hazard ratio [aHR] = 0.52; 95% CI, 0.31-0.85) than nonusers. Rates for all-cause mortality (aHR = 0.78; 95% CI, 0.58-1.04), stroke (aHR = 0.78; 95% CI, 0.49-1.24), and DVT/PE (aHR = 0.68; 95% CI, 0.36-1.28) were similar. In this and the other cohort studies to be discussed, outcome data for all vaginal estrogen preparations (eg, cream, ring, tablet) were combined.
The other large cohort study in the systematic review evaluated data on postmenopausal women from the Nurses’ Health Study.3 The authors evaluated health reports on 53,797 women as they transitioned through menopause. Patients with systemic hormone therapy use, history of cancer, and self-reported CV disease were excluded. After adjusting for covariates, the authors found no statistically significant difference between users and nonusers of vaginal estrogen and risk for total MI (aHR = 0.73; 95% CI, 0.47-1.13), stroke (aHR = 0.85; 95% CI, 0.56-1.29), or DVT/PE (aHR = 1.06; 95% CI, 0.58-1.93). Study limitations included low prevalence of vaginal estrogen use (< 3%), short duration of use (mean, 37.5 months), and lack of data on the type or dose of vaginal estrogen used. The study only included health professionals, which limits generalizability.
A Finnish cohort study (excluded from the systematic review because it used historical controls) compared rates of CHD and stroke in postmenopausal women who used vaginal estrogen against an age-matched background population. Researchers collected data from a nationwide prescription registry for women at least 50 years old who had purchased vaginal estrogens between 1994 and 2009 (n = 195,756).4 Women who purchased systemic hormone therapy at any point were excluded. After 3 to 5 years of exposure, use of vaginal estrogen was associated with a decreased risk for mortality from CHD (relative risk [RR] = 0.64; 95% CI, 0.57-0.70) and stroke (RR = 0.79; 95% CI, 0.69-0.91). However, after 10 years, these benefits were not seen (CHD: RR = 0.95; 95% CI, 0.90-1.00; stroke: RR = 0.93; 95% CI, 0.85-1.01). All confidence interval data were presented graphically. Key weaknesses of this study included use of both vaginal and systemic estrogen in the comparator background population, and the failure to collect data for other CV risk variables such as weight, tobacco exposure, and blood pressure.
Recommendations from others
In 2022, the North American Menopause Society issued a Hormone Therapy Position Statement that acknowledged the lack of clinical trials directly comparing risk for adverse CV endpoints with different estrogen administration routes.5 They stated nonoral routes of administration might offer advantages by bypassing first-pass hepatic metabolism.
Similarly, the 2015 Endocrine Society Clinical Practice Guideline on the Treatment of Symptoms of the Menopause also stated that the effects of low-dose vaginal estrogen therapy on CV disease or DVT/PE risk had not been adequately studied.6
A 2013 opinion by the American College of Obstetricians and Gynecologists stated that topical estrogen vaginal creams, tablets, and rings had low levels of systemic absorption and were not associated with an increased risk for DVT/PE.7
Editor’s takeaway
The available evidence on vaginal estrogen replacement reassures us of its safety. After decades spent studying hormone replacement therapy with vacillating conclusions and opinions, these cohorts—the best evidence we may ever get—along with a consensus of expert opinions, consistently demonstrate no adverse CV outcomes.
Evidence summary
Cohort studies demonstrate no adverse CV outcomes
A 2020 systematic review and meta-analysis evaluated randomized controlled trials (RCTs) and observational studies to examine the association between menopausal hormone therapy and CV disease.1 The 26 RCTs primarily evaluated oral hormone administration. The observational studies comprised 30 cohort studies, 13 case-control studies, and 5 nested case-control studies, primarily in Europe and North America; 21 reported the route of administration. The trials evaluated women ages 49 to 77 years (mean, 61 years), and follow-up ranged from 1 to 21.5 years (mean, 7 years). In subgroup analyses of the observational studies, nonoral hormone therapy was associated with a lower risk for stroke and MI compared to oral administration (see TABLE1). Study limitations included enrollment of patients with few comorbidities, from limited geographic regions. Results in the meta-analysis were not stratified by the type of nonoral hormone therapy; only 4 studies evaluated vaginal estrogen use.

Two large cohort studies included in the systematic review provided more specific data on vaginal estrogens. The first used data from the Women’s Health Initiative in a subset of women ages 50 to 79 years (n = 46,566) who were not already on systemic hormone therapy and who did not have prior history of breast, endometrial, or ovarian cancer.2 Data were collected from self-assessment questionnaires and medical record reviews. The median duration of vaginal estrogen use was 2 years, and median follow-up duration was 7.2 years. Vaginal estrogen users had a 48% lower risk for CHD (adjusted hazard ratio [aHR] = 0.52; 95% CI, 0.31-0.85) than nonusers. Rates for all-cause mortality (aHR = 0.78; 95% CI, 0.58-1.04), stroke (aHR = 0.78; 95% CI, 0.49-1.24), and DVT/PE (aHR = 0.68; 95% CI, 0.36-1.28) were similar. In this and the other cohort studies to be discussed, outcome data for all vaginal estrogen preparations (eg, cream, ring, tablet) were combined.
The other large cohort study in the systematic review evaluated data on postmenopausal women from the Nurses’ Health Study.3 The authors evaluated health reports on 53,797 women as they transitioned through menopause. Patients with systemic hormone therapy use, history of cancer, and self-reported CV disease were excluded. After adjusting for covariates, the authors found no statistically significant difference between users and nonusers of vaginal estrogen and risk for total MI (aHR = 0.73; 95% CI, 0.47-1.13), stroke (aHR = 0.85; 95% CI, 0.56-1.29), or DVT/PE (aHR = 1.06; 95% CI, 0.58-1.93). Study limitations included low prevalence of vaginal estrogen use (< 3%), short duration of use (mean, 37.5 months), and lack of data on the type or dose of vaginal estrogen used. The study only included health professionals, which limits generalizability.
A Finnish cohort study (excluded from the systematic review because it used historical controls) compared rates of CHD and stroke in postmenopausal women who used vaginal estrogen against an age-matched background population. Researchers collected data from a nationwide prescription registry for women at least 50 years old who had purchased vaginal estrogens between 1994 and 2009 (n = 195,756).4 Women who purchased systemic hormone therapy at any point were excluded. After 3 to 5 years of exposure, use of vaginal estrogen was associated with a decreased risk for mortality from CHD (relative risk [RR] = 0.64; 95% CI, 0.57-0.70) and stroke (RR = 0.79; 95% CI, 0.69-0.91). However, after 10 years, these benefits were not seen (CHD: RR = 0.95; 95% CI, 0.90-1.00; stroke: RR = 0.93; 95% CI, 0.85-1.01). All confidence interval data were presented graphically. Key weaknesses of this study included use of both vaginal and systemic estrogen in the comparator background population, and the failure to collect data for other CV risk variables such as weight, tobacco exposure, and blood pressure.
Recommendations from others
In 2022, the North American Menopause Society issued a Hormone Therapy Position Statement that acknowledged the lack of clinical trials directly comparing risk for adverse CV endpoints with different estrogen administration routes.5 They stated nonoral routes of administration might offer advantages by bypassing first-pass hepatic metabolism.
Similarly, the 2015 Endocrine Society Clinical Practice Guideline on the Treatment of Symptoms of the Menopause also stated that the effects of low-dose vaginal estrogen therapy on CV disease or DVT/PE risk had not been adequately studied.6
A 2013 opinion by the American College of Obstetricians and Gynecologists stated that topical estrogen vaginal creams, tablets, and rings had low levels of systemic absorption and were not associated with an increased risk for DVT/PE.7
Editor’s takeaway
The available evidence on vaginal estrogen replacement reassures us of its safety. After decades spent studying hormone replacement therapy with vacillating conclusions and opinions, these cohorts—the best evidence we may ever get—along with a consensus of expert opinions, consistently demonstrate no adverse CV outcomes.
1. Kim JE, Chang JH, Jeong MJ, et al. A systematic review and meta-analysis of effects of menopausal hormone therapy on cardiovascular diseases. Sci Rep. 2020;10:20631. doi: 10.1038/s41598-020-77534-9
2. Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the WHI Observational Study. Menopause. 2018;25:11-20. doi: 10.1097/GME.0000000000000956
3. Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. 2018;26:603-610. doi: 10.1097/GME.0000000000001284
4. Mikkola TS, Tuomikoski P, Lyytinen H, et al. Vaginal estrogen use and the risk for cardiovascular mortality. Human Reproduction. 2016;31:804-809. doi: 10.1093/humrep/dew014
5. North American Menopause Society. The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi: 10.1097/GME.0000000000002028
6. Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:3975-4011. doi: 10.1210/jc.2015-2236
7. American College of Obstetricians and Gynecologists. Committee Opinion No 565: hormone therapy and heart disease. Obstet Gynecol. 2013;121:1407-1410. doi: 10.1097/01.AOG.0000431053.33593.2d
1. Kim JE, Chang JH, Jeong MJ, et al. A systematic review and meta-analysis of effects of menopausal hormone therapy on cardiovascular diseases. Sci Rep. 2020;10:20631. doi: 10.1038/s41598-020-77534-9
2. Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the WHI Observational Study. Menopause. 2018;25:11-20. doi: 10.1097/GME.0000000000000956
3. Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. 2018;26:603-610. doi: 10.1097/GME.0000000000001284
4. Mikkola TS, Tuomikoski P, Lyytinen H, et al. Vaginal estrogen use and the risk for cardiovascular mortality. Human Reproduction. 2016;31:804-809. doi: 10.1093/humrep/dew014
5. North American Menopause Society. The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi: 10.1097/GME.0000000000002028
6. Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:3975-4011. doi: 10.1210/jc.2015-2236
7. American College of Obstetricians and Gynecologists. Committee Opinion No 565: hormone therapy and heart disease. Obstet Gynecol. 2013;121:1407-1410. doi: 10.1097/01.AOG.0000431053.33593.2d
EVIDENCE-BASED ANSWER:
NO. In general, nonoral estrogen use for menopausal symptoms is associated with a lower cardiovascular (CV) risk profile than oral estrogen use (strength of recommendation [SOR], B; meta-analysis of cohort studies). Vaginal estrogen use is associated with lower risk for coronary heart disease (CHD) and similar risk for myocardial infarction (MI), stroke, and deep vein thrombosis/pulmonary embolism (DVT/PE) compared with nonuse (SOR, B; cohort studies). Vaginal estrogen therapy also is associated with lower CV-related mortality for 3 to 5 years compared with nonuse (SOR, B; cohort study). No high-quality randomized trials address this topic.
An FP’s guide to caring for patients with seizure and epilepsy
Managing first-time seizures and epilepsy often requires consultation with a neurologist or epileptologist for diagnosis and subsequent management, including when medical treatment fails or in determining whether patients may benefit from surgery. However, given the high prevalence of epilepsy and even higher incidence of a single seizure, family physicians contribute significantly to the management of these patients. The main issues are managing a first-time seizure, making the diagnosis, establishing a treatment plan, and exploring triggers and mitigating factors.
Seizure vs epilepsy
All patients with epilepsy experience seizures, but not every person who experiences a seizure has (or will develop) epilepsy. Nearly 10% of the population has one seizure during their lifetime, whereas the risk for epilepsy is just 3%.1 Therefore, a first-time seizure may not herald epilepsy, defined as repetitive (≥ 2) unprovoked seizures more than 24 hours apart.2 Seizures can be provoked (acute symptomatic) or unprovoked; a clear distinction between these 2 occurrences—as well as between single and recurrent seizures—is critical for proper management. A close look at the circumstances of a first-time seizure is imperative to define the nature of the event and the possibility of further seizures before devising a treatment plan.
Provoked seizures are due to an acute brain insult such as toxic-metabolic disorders, concussion, alcohol withdrawal, an adverse effect of a medication or its withdrawal, or photic stimulation presumably by disrupting the brain’s metabolic homeostasis or integrity. The key factor is that provoked seizures always happen in close temporal association with an acute insult. A single provoked seizure happens each year in 29 to 39 individuals per 100,000.3 While these seizures typically occur singly, there is a small risk they may recur if the triggering insult persists or repeats.1 Therefore, more than 1 seizure per se may not indicate epilepsy.3
Unprovoked seizures reflect an underlying brain dysfunction. A single unprovoked seizure happens in 23 to 61 individuals per 100,000 per year, often in men in either younger or older age groups.3 Unprovoked seizures may occur only once or may recur (ie, evolve into epilepsy). The latter scenario happens in only about half of cases; the overall risk for a recurrent seizure within 2 years of a first seizure is estimated at 42% (24% to 65%, depending on the etiology and electroencephalogram [EEG] findings).4 More specifically, without treatment the relapse rate will be 36% at 1 year and 47% at 2 years.4 Further, a second unprovoked seizure, if untreated, would increase the risk for third and fourth seizures to 73% and 76%, respectively, within 4 years.3
Evaluating the first-time seizure
Ask the patient or observers about the circumstances of the event to differentiate provoked from unprovoked onset. For one thing, not all “spells” are seizures. The differential diagnoses may include syncope, psychogenic nonepileptic events, drug intoxication or withdrawal, migraine, panic attacks, sleep disorders (parasomnia), transient global amnesia, concussion, and transient ischemic attack. EEG, neuroimaging, and other relevant diagnostic tests often are needed (eg, electrocardiogram/echocardiogram/Holter monitoring to evaluate for syncope/cardiac arrhythmia). Clinically, syncopal episodes tend to be brief with rapid recovery and no confusion, speech problems, aura, or lateralizing signs such as hand posturing or lip smacking that are typical with focal seizures. However, cases of convulsive syncope can be challenging to assess without diagnostic tests.
True convulsive seizures do not have the variability in clinical signs seen with psychogenic nonepileptic events (eg, alternating body parts involved or direction of movements). Transient global amnesia is a rare condition with no established diagnostic test and is considered a diagnosis of exclusion, although bitemporal hyperintensities on magnetic resonance imaging (MRI) may appear 12 to 48 hours after the clinical episode.5 Blood work is needed in patients with medical issues treated with multiple medications to evaluate for metabolic derangements; otherwise, routine blood work provides minimal information in stable patients.
Region-specific causes. Neurocysticercosis is common in some regions, such as Latin America; therefore, attention should be paid to this aspect of patient history.
Continue to: Is it really a first-time seizure?
Is it really a first-time seizure? A “first,” usually dramatic, generalized tonic-clonic seizure that triggers the diagnostic work-up may not be the very first seizure. Evidence suggests that many patients have experienced prior undiagnosed seizures. Subtle prior events often missed include episodes of deja vu, transient feelings of fear or unusual smells, speech difficulties, staring spells, or myoclonic jerks.1 A routine EEG to record epileptiform discharges and a high-resolution brain MRI to rule out any intracranial pathology are indicated. However, if the EEG indicates a primary generalized (as opposed to focal-onset) epilepsy, a brain MRI may not be needed. If a routine EEG is unrevealing, long-term video-EEG monitoring may be needed to detect an abnormality.
Accuracy of EEG and MRI. Following a first unprovoked seizure, routine EEG to detect epileptiform discharges in adults has yielded a sensitivity of 17.3% and specificity of 94.7%. In evaluating children, these values are 57.8% and 69.6%, respectively.6 If results are equivocal, a 24-hour EEG can increase the likelihood of detecting epileptiform discharges to 89% of patients.7 Brain MRI may detect an abnormality in 12% to 14% of patients with newly diagnosed epilepsy, and in up to 80% of those with recurrent seizures.8 In confirming hippocampus sclerosis, MRI has demonstrated a sensitivity of 93% and specificity of 86%.9
When to treat a first-time seizure. Available data and prediction models identify risk factors that would help determine whether to start an antiseizure medication after a first unprovoked seizure:
Epilepsy diagnosis
The International League Against Epilepsy (ILAE) previously defined epilepsy as 2 unprovoked seizures more than 24 hours apart. However, a more recent ILAE task force modified this definition: even a single unprovoked seizure would be enough to diagnose epilepsy if there is high probability of further seizures—eg, in the presence of definitive epileptiform discharges on EEG or presence of a brain tumor or a remote brain insult on imaging, since such conditions induce an enduring predisposition to generate epileptic seizures. 2 Also, a single unprovoked seizure is enough to diagnose epilepsy if it is part of an epileptic syndrome such as juvenile myoclonic epilepsy. Further, a time limit was added to the definition—ie, epilepsy is considered resolved if a patient remains seizure free for 10 years without use of antiseizure medications during the past 5 years. However, given the multitude of variables and evidence, the task force acknowledged the need for individualized considerations. 2
Seizure classification
Classification of seizure type is based on the site of seizure onset and its spread pattern—ie, focal, generalized, or unknown onset.
Continue to: Focal-onset seizures
Focal-onset seizures originate “within networks limited to one hemisphere,” although possibly in more than 1 region (ie, multifocal, and presence or absence of loss of awareness). 12 Focal seizures may then be further classified into “motor onset” or “nonmotor onset” (eg, autonomic, emotional, sensory). 2
Generalized seizures are those “originating at some point within, and rapidly engaging, bilaterally distributed networks.” 13 Unlike focal-onset seizures, generalized seizures are not classified based on awareness, as most generalized seizures involve loss of awareness (absence) or total loss of consciousness (generalized tonic-clonic). They are instead categorized based on the presence of motor vs nonmotor features (eg, tonic-clonic, myoclonic, atonic). Epilepsy classification is quite dynamic and constantly updated based on new genetic, electroencephalographic, and neuroimaging discoveries.
Treatment of epilepsy
Antiseizure medications
Treatment with antiseizure medications (ASMs; formerly known as antiepileptic drugs ) is the mainstay of epilepsy management. Achieving efficacy (seizure freedom) and tolerability (minimal adverse effects) are the primary goals of treatment. Factors that should govern the selection of an ASM include the seizure type/epilepsy syndrome, adverse effect profile of the ASM, pharmacodynamic/pharmacokinetic considerations, and patient comorbidities.
The Standard and New Antiepileptic Drugs (SANAD I and II) trials provide data from direct, unblinded, and longitudinal comparisons of existing and new ASMs and their utility in different seizure types. In the SANAD I cohort of patients with generalized and unclassified epilepsies, valproate was superior to lamotrigine and topiramate for 12-month remission and treatment failure rates, respectively.14 However, valproate generally is avoided in women of childbearing age due its potential adverse effects during pregnancy. In focal epilepsies, lamotrigine was superior to carbamazepine, gabapentin, and topiramate with respect to treatment failure, and noninferior to carbamazepine for 12-month remission.15 In the SANAD II trial, levetiracetam was noninferior to valproate for incidence of adverse events in patients with generalized and unclassified epilepsies although was found to be neither more clinically effective nor more cost effective.16 For patients of childbearing potential with generalized and unclassified epilepsies, there is evidence to support the safe and effective use of levetiracetam.17 In focal epilepsies, lamotrigine was superior to levetiracetam and zonisamide with respect to treatment failures and adverse events and was noninferior to zonisamide for 12-month remission.18 In summary, levetiracetam and valproate (not to be used in women of childbearing potential) are considered appropriate first-line agents for generalized and unclassified epilepsies while lamotrigine is deemed an appropriate first-line agent for focal epilepsies (TABLE 119-28).
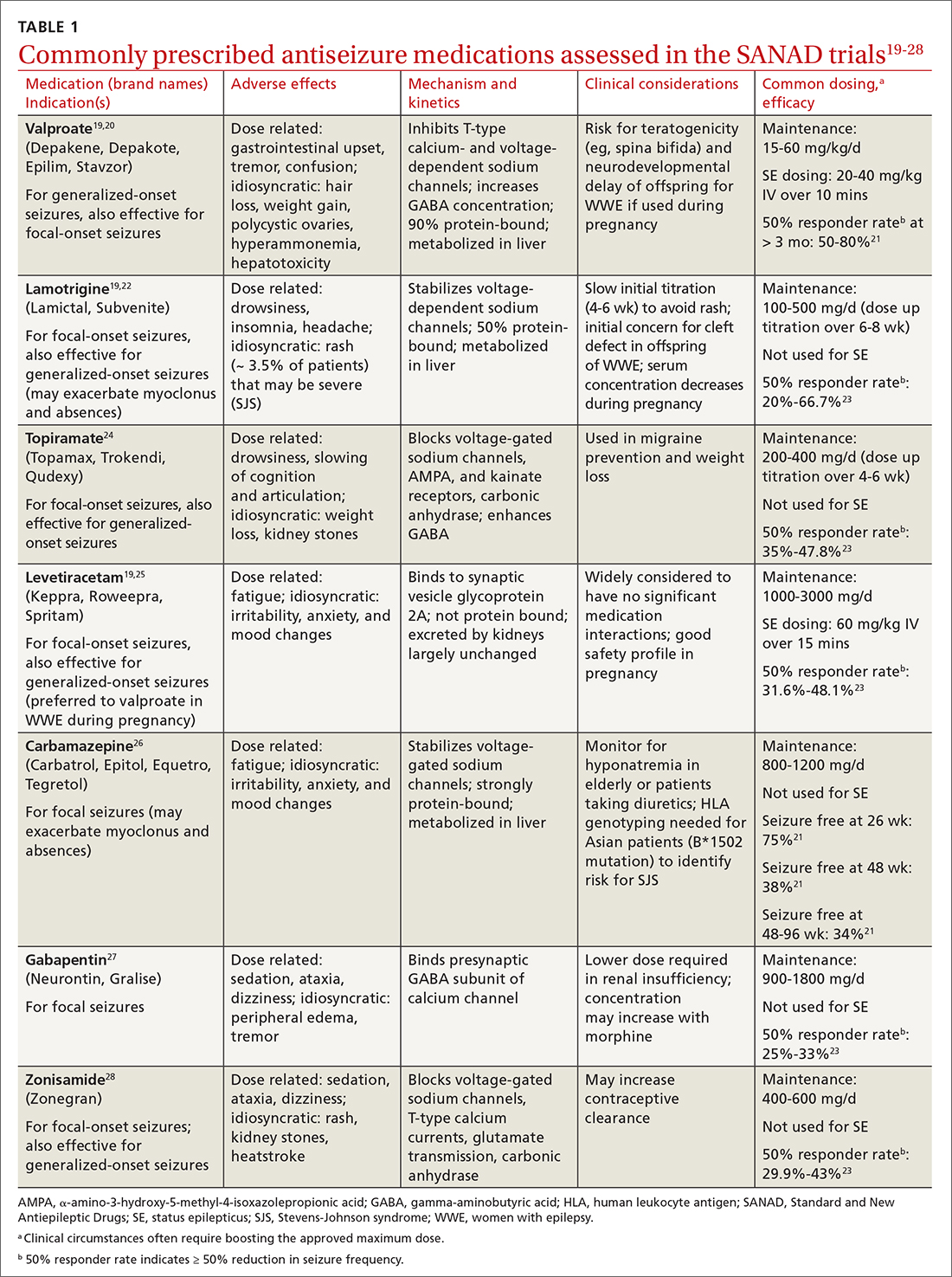
Drug level monitoring. It is standard practice to periodically monitor serum levels in patients taking first-generation ASMs such as phenytoin, carbamazepine, phenobarbital, and valproic acid because of their narrow therapeutic range and the potential for overdose or interaction with other medications or foods (eg, grapefruit juice may increase carbamazepine serum level by inhibiting CYP3A4, the enzyme that metabolizes the drug). Patients taking newer ASMs may not require regular serum level monitoring except during titration, with hepatic or renal dosing, when concomitantly used with estrogen-based oral contraceptives (eg, lamotrigine), before or during pregnancy, or when nonadherence is suspected.
Continue to: Can antiseizure treatment be stopped?
Can antiseizure treatment be stopped?
Current evidence favors continuing ASM therapy in patients whose seizures are under control, although the decision should be tailored to an individual’s circumstances. According to the 2021 American Academy of Neurology (AAN) guidelines, adults who have been seizure free for at least 2 years and discontinue ASMs are possibly still at higher risk for seizure recurrence in the long term (24-60 months), compared with those who continue treatment.29 On the other hand, for adults who have been seizure free for at least 12 months, ASM withdrawal may not increase their risk for status epilepticus, and there are insufficient data to support or refute an effect on mortality or quality of life with ASM withdrawal in this population. The decision to taper or maintain ASM therapy in seizure-free patients also should take into consideration other clinically relevant outcome measures such as the patient’s lifestyle and medication adverse effects. Therefore, this decision should be made after sufficient discussion with patients and their caregivers. (Information for patients can be found at: www.epilepsy.com/treatment/medicines/stopping-medication.)
For children, the AAN guideline panel recommends discussing with family the small risk (2%) for becoming medication resistant if seizures recur during or after ASM withdrawal. 29 For children who have been seizure free for 18 to 24 months, there is probably not a significant long-term (24-48 months) difference in seizure recurrence in those who taper ASMs vs those who do not. However, presence of epileptiform discharges on EEG before discontinuation of an ASM indicates increased risk for seizure recurrence. 29
Intractable (refractory) epilepsy
While most patients with epilepsy attain complete seizure control with appropriate drug therapy, approximately 30% continue to experience seizures (“drug-resistant” epilepsy, also termed intractable or refractory ). 30 In 2010, the ILAE defined drug-resistant epilepsy as “failure of adequate trials of two tolerated, appropriately chosen and used anti-epileptic drug schedules (whether as monotherapy or in combination) to achieve sustained seizure freedom” (defined as cessation of seizures for at least 3 times the longest pre-intervention inter-seizure interval or 12 months, whichever is longer). 21,31 It should be noted that drug withdrawal due to adverse effects is not counted as failure of that ASM. Recognition of drug-resistant epilepsy may prompt referral to an epileptologist who can consider rational combination drug therapy or surgical resection of the seizure focus, vagus nerve stimulation, electrical stimulation of the seizure focus, or deep brain (thalamic) stimulation.
Seizure triggers and mitigating factors
Epilepsy mostly affects patients during seizure episodes; however, the unpredictability of these events adds significantly to the burden of disease. There are no reliable methods for predicting seizure other than knowing of the several potential risks and recognizing and avoiding these triggers.
Noncompliance with antiseizure medications is a common seizure trigger affecting up to one-half of patients with epilepsy.32
Continue to: Medications
Medications may provoke seizures in susceptible individuals
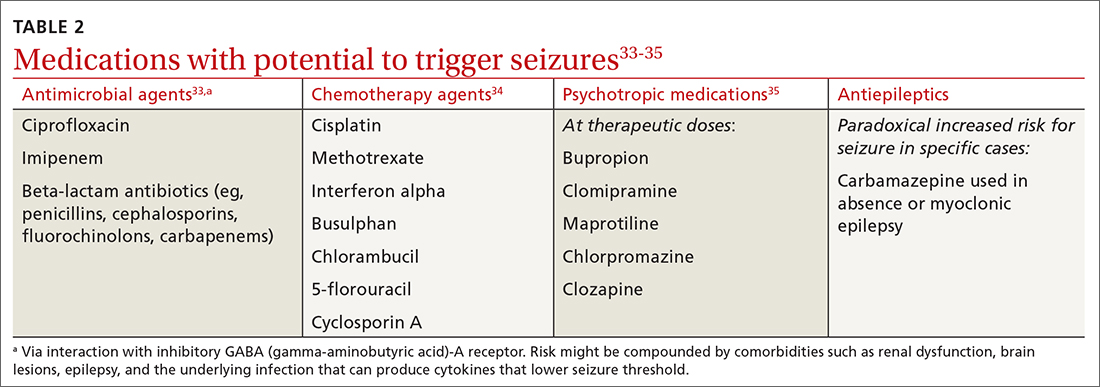
Sleep deprivation is a potential seizure trigger in people with epilepsy based on observational studies, case reports, patient surveys, and EEG-based studies, although data from randomized controlled studies are limited.36 The standard best practice is to encourage appropriate sleep hygiene, which involves getting at least 7 hours of sleep per night.37
Alcohol is a GABAergic substance like benzodiazepines with antiseizure effects. However, it acts as a potential precipitant of seizures in cases of withdrawal or acute intoxication, or when it leads to sleep disruption or nonadherence to antiseizure medications. Therefore, advise patients with alcohol use disorder to slowly taper consumption (best done through a support program) and avoid sudden withdrawal. However, complete abstinence from alcohol use is not often recommended except in special circumstances (eg, a history of alcohol-related seizures). Several studies have demonstrated that modest alcohol use (1-2 drinks per occasion) does not increase seizure frequency or significantly alter serum concentrations of commonly used ASMs.38
Cannabis and other substances. The 2 main biologically active components of marijuana are delta-9-tetrahydrocannibinol (THC), the main psychoactive constituent, and cannabidiol (CBD). Animal and human studies have demonstrated anticonvulsant properties of THC and CBD. But THC, in high amounts, can result in adverse cognitive effects and worsening seizures.39 A purified 98% oil-based CBD extract (Epidiolex) has been approved as an adjunctive treatment for certain medically refractory epilepsy syndromes in children and young adults—ie, Dravet syndrome, Lennox-Gastaut syndrome, and tuberous sclerosis complex syndrome.40 There are no reliable data on the effect of recreational use of marijuana on seizure control. Other illicit substances such as cocaine may lower seizure threshold by their stimulatory and disruptive effects on sleep, diet, and healthy routines.
Special clinical cases
Pregnancy and epilepsy
Despite the potential adverse effects of ASMs on fetal health, the current global consensus is to continue treatment during pregnancy, given that the potential harm of convulsive seizures outweighs the potential risks associated with in-utero exposure to ASMs. There is not enough evidence to indicate significant harm to the fetus caused by focal, absence, or myoclonic seizures. Low-dose folic acid is used to minimize the risks of ASMs during pregnancy.
Continue to: As the fetus develops...
As the fetus develops, there are changes in volume of ASM distribution, renal clearance, protein binding, and hepatic metabolism, which require checking serum levels at regular intervals and making dosage adjustments.
The ongoing study evaluating Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD)41 has led to multiple landmark studies guiding the choice of preferred ASMs during pregnancy in patients with epilepsy.42,43 This has culminated in today’s use of lamotrigine and levetiracetam as the 2 preferred agents (while avoiding valproate) in pregnant patients with epilepsy.44
Psychogenic nonepileptic seizures
A form of conversion disorder, psychogenic nonepileptic seizures (PNES) manifests as abnormal motor or behavioral events mimicking seizures but without associated epileptiform discharges on EEG. This is observed in 10% of patients seen in epilepsy clinics and even more often in those admitted to epilepsy monitoring units (25%-40%).45 Diagnosis of PNES requires EEG monitoring both for confirmation and for discernment from true epileptic seizures, in particular frontal lobe epilepsy that may clinically mimic PNES. PNES often is associated with underlying psychological tensions or comorbid conditions such as depression, anxiety, or traumatic life experiences. There is no treatment for PNES per se, and its management is focused on controlling any underlying psychological comorbidities that may not always be obvious. There is some evidence suggesting that these patients experience an innate inability to verbally express their emotions and instead subconsciously resort to psychosomatics to express them in a somatic dimension.46,47
Status epilepticus
Defined as prolonged seizures (> 5 min) or 2 consecutive seizures without regaining aware ness in between, status epilepticus (SE) is a potentially fatal condition. Subclinical nonconvulsive SE, especially in comatose patients, can be diagnosed only via EEG monitoring. Untreated SE may manifest as a diagnostic dilemma in unresponsive or critically ill patients and can increase the risk for mortality. 48
Febrile seizures
Febrile seizures affect 2% to 5% of children most often in the second year of life.49 The use of preventive antiseizure medication is not recommended; instead, the key is to investigate the underlying febrile illness. Lumbar puncture is indicated if there are signs and symptoms of meningitis (25% of children with bacterial meningitis present with seizures).49 Febrile seizures often are self-limited, but there is risk for SE in up to 15% of cases.50 If convulsive febrile seizures last longer than 5 minutes, initiate benzodiazepines followed by the standard protocol used for the management of SE.51
Continue to: Epilepsy as a spectrum disorder
Epilepsy as a spectrum disorder
The higher prevalence of comorbid cognitive and psychiatric conditions in patients with epilepsy, affecting about half of patients, 52 suggests that seizures may constitute only one aspect of a multifaceted disease that otherwise should be considered a spectrum disorder. Among such conditions are memory deficits, depression, and anxiety. Conversely, epilepsy is more common in patients with depression than in those without. 52
Social impact of epilepsy
Vehicle driving regulations. Patients with epilepsy are required to follow state law regarding driving restrictions. Different states have different rules and regulations about driving restrictions and reporting requirements (by patients or their physicians). Refer patients to the Department of Motor Vehicles (DMV) in their state of residence for up-to-date instructions.53 The Epilepsy Foundation (epilepsy.com) can serve as a resource for each state’s DMV website.
Employment assistance. Having epilepsy should not preclude patients from seeking employment and pursuing meaningful careers. The Americans with Disabilities Act (ADA) and the US Equal Employment Opportunity Commission (EEOC) forbid discrimination against qualified people with disabilities, including those with epilepsy, and require reasonable accommodations in the workplace (www.eeoc.gov/laws/guidance/epilepsy-workplace-and-ada).54
CORRESPONDENCE
Gholam K. Motamedi, MD, Department of Neurology, PHC 7, Georgetown University Hospital, 3800 Reservoir Road, NW, Washington, DC 20007; [email protected]
1. Hauser WA, Annegers JF, Rocca WA. Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc. 1996;71:576-586. doi: 10.4065/71.6.576
2. Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475-482. doi: 10.1111/epi.12550.
3. Hauser WA, Beghi E. First seizure definitions and worldwide incidence and mortality. Epilepsia. 2008;49:8-12. doi: 10.1111/j.1528-1167.2008.01443.x
4. Berg AT, Shinnar S. The risk of seizure recurrence following a first unprovoked seizure: a quantitative review. Neurology. 1991;41:965-972. doi: 10.1212/wnl.41.7.965
5. Ropper AH. Transient global amnesia. N Engl J Med. 2023;388:635-540. doi: 10.1056/NEJMra2213867
6. Bouma HK, Labos C, Gore GC, et al. The diagnostic accuracy of routine electroencephalography after a first unprovoked seizure. Eur J Neurol. 2016;23:455-463. doi: 10.1111/ene.12739
7. Narayanan JT, Labar DR, Schaul N. Latency to first spike in the EEG of epilepsy patients. Seizure. 2008;17:34-41. doi: 10.1016/j.seizure.2007.06.003
8. Salmenpera TM, Duncan JS. Imaging in epilepsy. J Neurol Neurosurg Psychiatry. 2005;76:iii2-iii10. doi: 10.1136/jnnp.2005.075135
9. Jackson GD, Berkovic SF, Tress , et al Hippocampal sclerosis can be reliably detected by magnetic resonance imaging. Neurology. 1990;40:1869-1875. doi: 10.1212/wnl.40.12.1869
10. Bonnett LJ, Kim, L, Johnson A, et al. Risk of seizure recurrence in people with single seizures and early epilepsy - model development and external validation. Seizure. 2022;94:26-32. doi: 10.1016/j.seizure.2021.11.007
11. Krumholz A, Wiebe S, Gronseth GS, et al. Evidence-based guideline: management of an unprovoked first seizure in adults: Report of the Guideline Development Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2015;84:1705-1713. doi: 10.1212/WNL.0000000000001487
12. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and terminology. Epilepsia. 2017;58:522-530. doi: 10.1111/epi.13670
13. Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsy: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51:676-685. doi: 10.1111/j.1528-1167.2010.02522.x
14. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalized and unclassifiable epilepsy: an unblinded randomized controlled trial. Lancet. 2007;369:1016-1026. doi: 10.1016/S0140-6736(07)60461-9
15. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomized controlled trial. Lancet 2007;369:1000-1015. doi: 10.1016/S0140-6736(07)60460-7
16. Marson A, Burnside G, Appleton R, et al. The SANAD II study of the effectiveness and cost-effectiveness of valproate versus levetiracetam for newly diagnosed generalized and unclassified epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomized controlled trial. Lancet. 2021;397:1375-1386. doi: 10.1016/S0140-6736(21)00246-4
17. Mawhinney E, Craig J, Morrow J. Levetiracetam in pregnancy: results from the UK and Ireland epilepsy and pregnancy registers. Neurology. 2013;80:400-405.
18. Marson A, Burnside G, Appleton R, et al. The SANAD II study of the effectiveness and cost-effectiveness of levetiracetam, zonisamide, or lamotrigine for newly diagnosed focal epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomized controlled trial. Lancet. 2021;397:1363-1374. doi: 10.1016/S0140-6736(21)00247-6
19. Smith PE. Initial management of seizure in adults. N Engl J Med. 2021;385:251-263. doi: 10.1056/NEJMcp2024526
20. Depakene (valproic acid). Package insert. Abbott Laboratories; 2011. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2011/018081s046_18082s031lbl.pdf
21. Greenberg RG, Melloni C, Wu H, et al. Therapeutic index estimation of antiepileptic drugs: a systematic literature review approach. Clin Neuropharmacol. 2016;39:232-240. doi: 10.1097/WNF.0000000000000172
22. Lamictal (lamotrigine). Package insert. GlaxoSmithKline; 2009. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2009/020241s037s038,020764s030s031lbl.pdf
23. LaRoche SM, Helmers SL. The new antiepileptic drugs: scientific review. JAMA. 2004;291:605-614. doi: 10.1001/jama.291.5.605
24. Topamax (topiramate). Package insert. Janssen Pharmaceuticals, Inc. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2012/020844s041lbl.pdf
25. Keppra (levetiracetam). Package insert. UCB, Inc.; 2009. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2009/021035s078s080%2C021505s021s024lbl.pdf
26. Carbatrol (carbamazepine). Package insert. Shire US Inc; 2013. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2013/020712s032s035lbl.pdf
27.Neurontin (gabapentin). Package insert. Pfizer; 2017. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2017/020235s064_020882s047_021129s046lbl.pdf
28.Zonegran (zonisamide). Package insert. Eisai Inc; 2006. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2006/020789s019lbl.pdf
29.Gloss D, Paragon K, Pack A, et al. Antiseizure medication withdrawal in seizure-free patients: practice advisory update. Report of the AAN Guideline Subcommittee. Neurology. 2021;97:1072-1081. doi: 10.1212/WNL.0000000000012944
30.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000:342:314-319. doi: 10.1056/NEJM200002033420503
31.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069-1077. doi: 10.1111/j.1528-1167.2009.02397.x
Compliance during treatment of epilepsy. Epilepsia 1988;29(suppl 2):S79-S84.
33.utter R, Rüegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology. 2015;13;85:1332-1341. doi: 10.1212/WNL.0000000000002023
34.Singh G, Rees JH, Sander JW. Seizures and epilepsy in oncological practice: causes, course, mechanisms and treatment. JNNP. 2007;78:342-349. doi: 10.1136/jnnp.2006.106211
35.Pisani F, Oteri G, Costa C., et al. Effects of psychotropic drugs on seizure threshold. Drug Safety. 2002;25:91-110.
36.Rossi KC, Joe J, Makhjia M, et al. Insufficient sleep, electroencephalogram activation, and seizure risk: re-evaluating the evidence. Ann Neurol. 2020;86:798-806. doi: 10.1002/ana.25710
37.Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843-844. doi: 10.5665/sleep.4716
38.Höppener RJ, Kuyer A, van der Lugt PJ. Epilepsy and alcohol: the influence of social alcohol intake on seizures and treatment in epilepsy. Epilepsia. 1983;24:459-471. doi: 10.1111/j.1528-1157.1983.tb04917.x
39.Keeler MH, Reifler CB. Grand mal convulsions subsequent to marijuana use. Case report. Dis Nerv Syst. 1967:28:474-475.
40.Epidiolex (cannabidiol). Package insert. Greenwich Biosciences Inc; 2018. Accessed September 27, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2018/210365lbl.pdf
41.ClinicalTrials.gov. Maternal Outcomes and Neurodevelopmental Effects of Antiseizure Drugs (MONEAD). Accessed September 24, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT01730170
42.Meador KJ, Baker GA, Finnell RH, et al. In utero antiepileptic drug exposure: fetal death and malformations. Neurology. 2006;67:407-412. doi: 10.1212/01.wnl.0000227919.81208.b2
43.Meador K, Reynolds MW, Crean S. Pregnancy outcomes in women with epilepsy: a systematic review and meta-analysis of published pregnancy registries and cohorts. Epilepsy Res. 2008;81:1-13. doi:10.1016/j.eplepsyres.2008.04.022
44.Marxer CA, Rüegg S, Rauch A review of the evidence on the risk of congenital malformations and neurodevelopmental disorders in association with antiseizure medications during pregnancy. Expert Opin Drug Saf. 2021;20:1487-1499. doi: 10.1080/14740338.2021.1943355
Asadi-Pooya AA, Sperling MR. Epidemiology of psychogenic nonepileptic seizures. Epilepsy Behav. 2015;46:60-65. doi: 10.1016/j.yebeh.2015.03.015
Evaluation and treatment of psychogenic nonepileptic seizures. Neurol Clin. 2022;40:799-820. doi: 10.1016/j.ncl.2022.03.017
47.Motamedi GK. Psychogenic nonepileptic seizures: a disconnect between body and mind. Epilepsy Behav. 2018;78:293-294. doi: 10.1016/j.yebeh.2017.10.016
, Nonconvulsive status epilepticus. Emerg Med Clin North Am. 2011;29:65-72. doi: 10.1016/j.emc.2010.08.006
doi: 10.1542/peds.2010-3318
Drug management for acute tonic-clonic convulsions including convulsive status epilepticus in children. Cochrane Database Sys Rev. 2018;1(1):CD001905. doi: 10.1002/14651858.CD001905.pub3
52.Jensen FE. Epilepsy as a spectrum disorder: implications from novel clinical and basic neuroscience. Epilepsia. 2011;52(suppl 1):1-6. doi: 10.1111/j.1528-1167.2010.02904.x
53.Kass JS, Rose RV. Driving and epilepsy: ethical, legal, and health care policy challenges. Continuum (Minneap Minn). 2019;25:537-542. doi: 10.1212/CON.0000000000000714
54.Troxell J. Epilepsy and employment: the Americans with Disabilities Act and its protections against employment discrimination. Med Law. 1997;16:375-384.
Managing first-time seizures and epilepsy often requires consultation with a neurologist or epileptologist for diagnosis and subsequent management, including when medical treatment fails or in determining whether patients may benefit from surgery. However, given the high prevalence of epilepsy and even higher incidence of a single seizure, family physicians contribute significantly to the management of these patients. The main issues are managing a first-time seizure, making the diagnosis, establishing a treatment plan, and exploring triggers and mitigating factors.
Seizure vs epilepsy
All patients with epilepsy experience seizures, but not every person who experiences a seizure has (or will develop) epilepsy. Nearly 10% of the population has one seizure during their lifetime, whereas the risk for epilepsy is just 3%.1 Therefore, a first-time seizure may not herald epilepsy, defined as repetitive (≥ 2) unprovoked seizures more than 24 hours apart.2 Seizures can be provoked (acute symptomatic) or unprovoked; a clear distinction between these 2 occurrences—as well as between single and recurrent seizures—is critical for proper management. A close look at the circumstances of a first-time seizure is imperative to define the nature of the event and the possibility of further seizures before devising a treatment plan.
Provoked seizures are due to an acute brain insult such as toxic-metabolic disorders, concussion, alcohol withdrawal, an adverse effect of a medication or its withdrawal, or photic stimulation presumably by disrupting the brain’s metabolic homeostasis or integrity. The key factor is that provoked seizures always happen in close temporal association with an acute insult. A single provoked seizure happens each year in 29 to 39 individuals per 100,000.3 While these seizures typically occur singly, there is a small risk they may recur if the triggering insult persists or repeats.1 Therefore, more than 1 seizure per se may not indicate epilepsy.3
Unprovoked seizures reflect an underlying brain dysfunction. A single unprovoked seizure happens in 23 to 61 individuals per 100,000 per year, often in men in either younger or older age groups.3 Unprovoked seizures may occur only once or may recur (ie, evolve into epilepsy). The latter scenario happens in only about half of cases; the overall risk for a recurrent seizure within 2 years of a first seizure is estimated at 42% (24% to 65%, depending on the etiology and electroencephalogram [EEG] findings).4 More specifically, without treatment the relapse rate will be 36% at 1 year and 47% at 2 years.4 Further, a second unprovoked seizure, if untreated, would increase the risk for third and fourth seizures to 73% and 76%, respectively, within 4 years.3
Evaluating the first-time seizure
Ask the patient or observers about the circumstances of the event to differentiate provoked from unprovoked onset. For one thing, not all “spells” are seizures. The differential diagnoses may include syncope, psychogenic nonepileptic events, drug intoxication or withdrawal, migraine, panic attacks, sleep disorders (parasomnia), transient global amnesia, concussion, and transient ischemic attack. EEG, neuroimaging, and other relevant diagnostic tests often are needed (eg, electrocardiogram/echocardiogram/Holter monitoring to evaluate for syncope/cardiac arrhythmia). Clinically, syncopal episodes tend to be brief with rapid recovery and no confusion, speech problems, aura, or lateralizing signs such as hand posturing or lip smacking that are typical with focal seizures. However, cases of convulsive syncope can be challenging to assess without diagnostic tests.
True convulsive seizures do not have the variability in clinical signs seen with psychogenic nonepileptic events (eg, alternating body parts involved or direction of movements). Transient global amnesia is a rare condition with no established diagnostic test and is considered a diagnosis of exclusion, although bitemporal hyperintensities on magnetic resonance imaging (MRI) may appear 12 to 48 hours after the clinical episode.5 Blood work is needed in patients with medical issues treated with multiple medications to evaluate for metabolic derangements; otherwise, routine blood work provides minimal information in stable patients.
Region-specific causes. Neurocysticercosis is common in some regions, such as Latin America; therefore, attention should be paid to this aspect of patient history.
Continue to: Is it really a first-time seizure?
Is it really a first-time seizure? A “first,” usually dramatic, generalized tonic-clonic seizure that triggers the diagnostic work-up may not be the very first seizure. Evidence suggests that many patients have experienced prior undiagnosed seizures. Subtle prior events often missed include episodes of deja vu, transient feelings of fear or unusual smells, speech difficulties, staring spells, or myoclonic jerks.1 A routine EEG to record epileptiform discharges and a high-resolution brain MRI to rule out any intracranial pathology are indicated. However, if the EEG indicates a primary generalized (as opposed to focal-onset) epilepsy, a brain MRI may not be needed. If a routine EEG is unrevealing, long-term video-EEG monitoring may be needed to detect an abnormality.
Accuracy of EEG and MRI. Following a first unprovoked seizure, routine EEG to detect epileptiform discharges in adults has yielded a sensitivity of 17.3% and specificity of 94.7%. In evaluating children, these values are 57.8% and 69.6%, respectively.6 If results are equivocal, a 24-hour EEG can increase the likelihood of detecting epileptiform discharges to 89% of patients.7 Brain MRI may detect an abnormality in 12% to 14% of patients with newly diagnosed epilepsy, and in up to 80% of those with recurrent seizures.8 In confirming hippocampus sclerosis, MRI has demonstrated a sensitivity of 93% and specificity of 86%.9
When to treat a first-time seizure. Available data and prediction models identify risk factors that would help determine whether to start an antiseizure medication after a first unprovoked seizure:
Epilepsy diagnosis
The International League Against Epilepsy (ILAE) previously defined epilepsy as 2 unprovoked seizures more than 24 hours apart. However, a more recent ILAE task force modified this definition: even a single unprovoked seizure would be enough to diagnose epilepsy if there is high probability of further seizures—eg, in the presence of definitive epileptiform discharges on EEG or presence of a brain tumor or a remote brain insult on imaging, since such conditions induce an enduring predisposition to generate epileptic seizures. 2 Also, a single unprovoked seizure is enough to diagnose epilepsy if it is part of an epileptic syndrome such as juvenile myoclonic epilepsy. Further, a time limit was added to the definition—ie, epilepsy is considered resolved if a patient remains seizure free for 10 years without use of antiseizure medications during the past 5 years. However, given the multitude of variables and evidence, the task force acknowledged the need for individualized considerations. 2
Seizure classification
Classification of seizure type is based on the site of seizure onset and its spread pattern—ie, focal, generalized, or unknown onset.
Continue to: Focal-onset seizures
Focal-onset seizures originate “within networks limited to one hemisphere,” although possibly in more than 1 region (ie, multifocal, and presence or absence of loss of awareness). 12 Focal seizures may then be further classified into “motor onset” or “nonmotor onset” (eg, autonomic, emotional, sensory). 2
Generalized seizures are those “originating at some point within, and rapidly engaging, bilaterally distributed networks.” 13 Unlike focal-onset seizures, generalized seizures are not classified based on awareness, as most generalized seizures involve loss of awareness (absence) or total loss of consciousness (generalized tonic-clonic). They are instead categorized based on the presence of motor vs nonmotor features (eg, tonic-clonic, myoclonic, atonic). Epilepsy classification is quite dynamic and constantly updated based on new genetic, electroencephalographic, and neuroimaging discoveries.
Treatment of epilepsy
Antiseizure medications
Treatment with antiseizure medications (ASMs; formerly known as antiepileptic drugs ) is the mainstay of epilepsy management. Achieving efficacy (seizure freedom) and tolerability (minimal adverse effects) are the primary goals of treatment. Factors that should govern the selection of an ASM include the seizure type/epilepsy syndrome, adverse effect profile of the ASM, pharmacodynamic/pharmacokinetic considerations, and patient comorbidities.
The Standard and New Antiepileptic Drugs (SANAD I and II) trials provide data from direct, unblinded, and longitudinal comparisons of existing and new ASMs and their utility in different seizure types. In the SANAD I cohort of patients with generalized and unclassified epilepsies, valproate was superior to lamotrigine and topiramate for 12-month remission and treatment failure rates, respectively.14 However, valproate generally is avoided in women of childbearing age due its potential adverse effects during pregnancy. In focal epilepsies, lamotrigine was superior to carbamazepine, gabapentin, and topiramate with respect to treatment failure, and noninferior to carbamazepine for 12-month remission.15 In the SANAD II trial, levetiracetam was noninferior to valproate for incidence of adverse events in patients with generalized and unclassified epilepsies although was found to be neither more clinically effective nor more cost effective.16 For patients of childbearing potential with generalized and unclassified epilepsies, there is evidence to support the safe and effective use of levetiracetam.17 In focal epilepsies, lamotrigine was superior to levetiracetam and zonisamide with respect to treatment failures and adverse events and was noninferior to zonisamide for 12-month remission.18 In summary, levetiracetam and valproate (not to be used in women of childbearing potential) are considered appropriate first-line agents for generalized and unclassified epilepsies while lamotrigine is deemed an appropriate first-line agent for focal epilepsies (TABLE 119-28).

Drug level monitoring. It is standard practice to periodically monitor serum levels in patients taking first-generation ASMs such as phenytoin, carbamazepine, phenobarbital, and valproic acid because of their narrow therapeutic range and the potential for overdose or interaction with other medications or foods (eg, grapefruit juice may increase carbamazepine serum level by inhibiting CYP3A4, the enzyme that metabolizes the drug). Patients taking newer ASMs may not require regular serum level monitoring except during titration, with hepatic or renal dosing, when concomitantly used with estrogen-based oral contraceptives (eg, lamotrigine), before or during pregnancy, or when nonadherence is suspected.
Continue to: Can antiseizure treatment be stopped?
Can antiseizure treatment be stopped?
Current evidence favors continuing ASM therapy in patients whose seizures are under control, although the decision should be tailored to an individual’s circumstances. According to the 2021 American Academy of Neurology (AAN) guidelines, adults who have been seizure free for at least 2 years and discontinue ASMs are possibly still at higher risk for seizure recurrence in the long term (24-60 months), compared with those who continue treatment.29 On the other hand, for adults who have been seizure free for at least 12 months, ASM withdrawal may not increase their risk for status epilepticus, and there are insufficient data to support or refute an effect on mortality or quality of life with ASM withdrawal in this population. The decision to taper or maintain ASM therapy in seizure-free patients also should take into consideration other clinically relevant outcome measures such as the patient’s lifestyle and medication adverse effects. Therefore, this decision should be made after sufficient discussion with patients and their caregivers. (Information for patients can be found at: www.epilepsy.com/treatment/medicines/stopping-medication.)
For children, the AAN guideline panel recommends discussing with family the small risk (2%) for becoming medication resistant if seizures recur during or after ASM withdrawal. 29 For children who have been seizure free for 18 to 24 months, there is probably not a significant long-term (24-48 months) difference in seizure recurrence in those who taper ASMs vs those who do not. However, presence of epileptiform discharges on EEG before discontinuation of an ASM indicates increased risk for seizure recurrence. 29
Intractable (refractory) epilepsy
While most patients with epilepsy attain complete seizure control with appropriate drug therapy, approximately 30% continue to experience seizures (“drug-resistant” epilepsy, also termed intractable or refractory ). 30 In 2010, the ILAE defined drug-resistant epilepsy as “failure of adequate trials of two tolerated, appropriately chosen and used anti-epileptic drug schedules (whether as monotherapy or in combination) to achieve sustained seizure freedom” (defined as cessation of seizures for at least 3 times the longest pre-intervention inter-seizure interval or 12 months, whichever is longer). 21,31 It should be noted that drug withdrawal due to adverse effects is not counted as failure of that ASM. Recognition of drug-resistant epilepsy may prompt referral to an epileptologist who can consider rational combination drug therapy or surgical resection of the seizure focus, vagus nerve stimulation, electrical stimulation of the seizure focus, or deep brain (thalamic) stimulation.
Seizure triggers and mitigating factors
Epilepsy mostly affects patients during seizure episodes; however, the unpredictability of these events adds significantly to the burden of disease. There are no reliable methods for predicting seizure other than knowing of the several potential risks and recognizing and avoiding these triggers.
Noncompliance with antiseizure medications is a common seizure trigger affecting up to one-half of patients with epilepsy.32
Continue to: Medications
Medications may provoke seizures in susceptible individuals

Sleep deprivation is a potential seizure trigger in people with epilepsy based on observational studies, case reports, patient surveys, and EEG-based studies, although data from randomized controlled studies are limited.36 The standard best practice is to encourage appropriate sleep hygiene, which involves getting at least 7 hours of sleep per night.37
Alcohol is a GABAergic substance like benzodiazepines with antiseizure effects. However, it acts as a potential precipitant of seizures in cases of withdrawal or acute intoxication, or when it leads to sleep disruption or nonadherence to antiseizure medications. Therefore, advise patients with alcohol use disorder to slowly taper consumption (best done through a support program) and avoid sudden withdrawal. However, complete abstinence from alcohol use is not often recommended except in special circumstances (eg, a history of alcohol-related seizures). Several studies have demonstrated that modest alcohol use (1-2 drinks per occasion) does not increase seizure frequency or significantly alter serum concentrations of commonly used ASMs.38
Cannabis and other substances. The 2 main biologically active components of marijuana are delta-9-tetrahydrocannibinol (THC), the main psychoactive constituent, and cannabidiol (CBD). Animal and human studies have demonstrated anticonvulsant properties of THC and CBD. But THC, in high amounts, can result in adverse cognitive effects and worsening seizures.39 A purified 98% oil-based CBD extract (Epidiolex) has been approved as an adjunctive treatment for certain medically refractory epilepsy syndromes in children and young adults—ie, Dravet syndrome, Lennox-Gastaut syndrome, and tuberous sclerosis complex syndrome.40 There are no reliable data on the effect of recreational use of marijuana on seizure control. Other illicit substances such as cocaine may lower seizure threshold by their stimulatory and disruptive effects on sleep, diet, and healthy routines.
Special clinical cases
Pregnancy and epilepsy
Despite the potential adverse effects of ASMs on fetal health, the current global consensus is to continue treatment during pregnancy, given that the potential harm of convulsive seizures outweighs the potential risks associated with in-utero exposure to ASMs. There is not enough evidence to indicate significant harm to the fetus caused by focal, absence, or myoclonic seizures. Low-dose folic acid is used to minimize the risks of ASMs during pregnancy.
Continue to: As the fetus develops...
As the fetus develops, there are changes in volume of ASM distribution, renal clearance, protein binding, and hepatic metabolism, which require checking serum levels at regular intervals and making dosage adjustments.
The ongoing study evaluating Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD)41 has led to multiple landmark studies guiding the choice of preferred ASMs during pregnancy in patients with epilepsy.42,43 This has culminated in today’s use of lamotrigine and levetiracetam as the 2 preferred agents (while avoiding valproate) in pregnant patients with epilepsy.44
Psychogenic nonepileptic seizures
A form of conversion disorder, psychogenic nonepileptic seizures (PNES) manifests as abnormal motor or behavioral events mimicking seizures but without associated epileptiform discharges on EEG. This is observed in 10% of patients seen in epilepsy clinics and even more often in those admitted to epilepsy monitoring units (25%-40%).45 Diagnosis of PNES requires EEG monitoring both for confirmation and for discernment from true epileptic seizures, in particular frontal lobe epilepsy that may clinically mimic PNES. PNES often is associated with underlying psychological tensions or comorbid conditions such as depression, anxiety, or traumatic life experiences. There is no treatment for PNES per se, and its management is focused on controlling any underlying psychological comorbidities that may not always be obvious. There is some evidence suggesting that these patients experience an innate inability to verbally express their emotions and instead subconsciously resort to psychosomatics to express them in a somatic dimension.46,47
Status epilepticus
Defined as prolonged seizures (> 5 min) or 2 consecutive seizures without regaining aware ness in between, status epilepticus (SE) is a potentially fatal condition. Subclinical nonconvulsive SE, especially in comatose patients, can be diagnosed only via EEG monitoring. Untreated SE may manifest as a diagnostic dilemma in unresponsive or critically ill patients and can increase the risk for mortality. 48
Febrile seizures
Febrile seizures affect 2% to 5% of children most often in the second year of life.49 The use of preventive antiseizure medication is not recommended; instead, the key is to investigate the underlying febrile illness. Lumbar puncture is indicated if there are signs and symptoms of meningitis (25% of children with bacterial meningitis present with seizures).49 Febrile seizures often are self-limited, but there is risk for SE in up to 15% of cases.50 If convulsive febrile seizures last longer than 5 minutes, initiate benzodiazepines followed by the standard protocol used for the management of SE.51
Continue to: Epilepsy as a spectrum disorder
Epilepsy as a spectrum disorder
The higher prevalence of comorbid cognitive and psychiatric conditions in patients with epilepsy, affecting about half of patients, 52 suggests that seizures may constitute only one aspect of a multifaceted disease that otherwise should be considered a spectrum disorder. Among such conditions are memory deficits, depression, and anxiety. Conversely, epilepsy is more common in patients with depression than in those without. 52
Social impact of epilepsy
Vehicle driving regulations. Patients with epilepsy are required to follow state law regarding driving restrictions. Different states have different rules and regulations about driving restrictions and reporting requirements (by patients or their physicians). Refer patients to the Department of Motor Vehicles (DMV) in their state of residence for up-to-date instructions.53 The Epilepsy Foundation (epilepsy.com) can serve as a resource for each state’s DMV website.
Employment assistance. Having epilepsy should not preclude patients from seeking employment and pursuing meaningful careers. The Americans with Disabilities Act (ADA) and the US Equal Employment Opportunity Commission (EEOC) forbid discrimination against qualified people with disabilities, including those with epilepsy, and require reasonable accommodations in the workplace (www.eeoc.gov/laws/guidance/epilepsy-workplace-and-ada).54
CORRESPONDENCE
Gholam K. Motamedi, MD, Department of Neurology, PHC 7, Georgetown University Hospital, 3800 Reservoir Road, NW, Washington, DC 20007; [email protected]
Managing first-time seizures and epilepsy often requires consultation with a neurologist or epileptologist for diagnosis and subsequent management, including when medical treatment fails or in determining whether patients may benefit from surgery. However, given the high prevalence of epilepsy and even higher incidence of a single seizure, family physicians contribute significantly to the management of these patients. The main issues are managing a first-time seizure, making the diagnosis, establishing a treatment plan, and exploring triggers and mitigating factors.
Seizure vs epilepsy
All patients with epilepsy experience seizures, but not every person who experiences a seizure has (or will develop) epilepsy. Nearly 10% of the population has one seizure during their lifetime, whereas the risk for epilepsy is just 3%.1 Therefore, a first-time seizure may not herald epilepsy, defined as repetitive (≥ 2) unprovoked seizures more than 24 hours apart.2 Seizures can be provoked (acute symptomatic) or unprovoked; a clear distinction between these 2 occurrences—as well as between single and recurrent seizures—is critical for proper management. A close look at the circumstances of a first-time seizure is imperative to define the nature of the event and the possibility of further seizures before devising a treatment plan.
Provoked seizures are due to an acute brain insult such as toxic-metabolic disorders, concussion, alcohol withdrawal, an adverse effect of a medication or its withdrawal, or photic stimulation presumably by disrupting the brain’s metabolic homeostasis or integrity. The key factor is that provoked seizures always happen in close temporal association with an acute insult. A single provoked seizure happens each year in 29 to 39 individuals per 100,000.3 While these seizures typically occur singly, there is a small risk they may recur if the triggering insult persists or repeats.1 Therefore, more than 1 seizure per se may not indicate epilepsy.3
Unprovoked seizures reflect an underlying brain dysfunction. A single unprovoked seizure happens in 23 to 61 individuals per 100,000 per year, often in men in either younger or older age groups.3 Unprovoked seizures may occur only once or may recur (ie, evolve into epilepsy). The latter scenario happens in only about half of cases; the overall risk for a recurrent seizure within 2 years of a first seizure is estimated at 42% (24% to 65%, depending on the etiology and electroencephalogram [EEG] findings).4 More specifically, without treatment the relapse rate will be 36% at 1 year and 47% at 2 years.4 Further, a second unprovoked seizure, if untreated, would increase the risk for third and fourth seizures to 73% and 76%, respectively, within 4 years.3
Evaluating the first-time seizure
Ask the patient or observers about the circumstances of the event to differentiate provoked from unprovoked onset. For one thing, not all “spells” are seizures. The differential diagnoses may include syncope, psychogenic nonepileptic events, drug intoxication or withdrawal, migraine, panic attacks, sleep disorders (parasomnia), transient global amnesia, concussion, and transient ischemic attack. EEG, neuroimaging, and other relevant diagnostic tests often are needed (eg, electrocardiogram/echocardiogram/Holter monitoring to evaluate for syncope/cardiac arrhythmia). Clinically, syncopal episodes tend to be brief with rapid recovery and no confusion, speech problems, aura, or lateralizing signs such as hand posturing or lip smacking that are typical with focal seizures. However, cases of convulsive syncope can be challenging to assess without diagnostic tests.
True convulsive seizures do not have the variability in clinical signs seen with psychogenic nonepileptic events (eg, alternating body parts involved or direction of movements). Transient global amnesia is a rare condition with no established diagnostic test and is considered a diagnosis of exclusion, although bitemporal hyperintensities on magnetic resonance imaging (MRI) may appear 12 to 48 hours after the clinical episode.5 Blood work is needed in patients with medical issues treated with multiple medications to evaluate for metabolic derangements; otherwise, routine blood work provides minimal information in stable patients.
Region-specific causes. Neurocysticercosis is common in some regions, such as Latin America; therefore, attention should be paid to this aspect of patient history.
Continue to: Is it really a first-time seizure?
Is it really a first-time seizure? A “first,” usually dramatic, generalized tonic-clonic seizure that triggers the diagnostic work-up may not be the very first seizure. Evidence suggests that many patients have experienced prior undiagnosed seizures. Subtle prior events often missed include episodes of deja vu, transient feelings of fear or unusual smells, speech difficulties, staring spells, or myoclonic jerks.1 A routine EEG to record epileptiform discharges and a high-resolution brain MRI to rule out any intracranial pathology are indicated. However, if the EEG indicates a primary generalized (as opposed to focal-onset) epilepsy, a brain MRI may not be needed. If a routine EEG is unrevealing, long-term video-EEG monitoring may be needed to detect an abnormality.
Accuracy of EEG and MRI. Following a first unprovoked seizure, routine EEG to detect epileptiform discharges in adults has yielded a sensitivity of 17.3% and specificity of 94.7%. In evaluating children, these values are 57.8% and 69.6%, respectively.6 If results are equivocal, a 24-hour EEG can increase the likelihood of detecting epileptiform discharges to 89% of patients.7 Brain MRI may detect an abnormality in 12% to 14% of patients with newly diagnosed epilepsy, and in up to 80% of those with recurrent seizures.8 In confirming hippocampus sclerosis, MRI has demonstrated a sensitivity of 93% and specificity of 86%.9
When to treat a first-time seizure. Available data and prediction models identify risk factors that would help determine whether to start an antiseizure medication after a first unprovoked seizure:
Epilepsy diagnosis
The International League Against Epilepsy (ILAE) previously defined epilepsy as 2 unprovoked seizures more than 24 hours apart. However, a more recent ILAE task force modified this definition: even a single unprovoked seizure would be enough to diagnose epilepsy if there is high probability of further seizures—eg, in the presence of definitive epileptiform discharges on EEG or presence of a brain tumor or a remote brain insult on imaging, since such conditions induce an enduring predisposition to generate epileptic seizures. 2 Also, a single unprovoked seizure is enough to diagnose epilepsy if it is part of an epileptic syndrome such as juvenile myoclonic epilepsy. Further, a time limit was added to the definition—ie, epilepsy is considered resolved if a patient remains seizure free for 10 years without use of antiseizure medications during the past 5 years. However, given the multitude of variables and evidence, the task force acknowledged the need for individualized considerations. 2
Seizure classification
Classification of seizure type is based on the site of seizure onset and its spread pattern—ie, focal, generalized, or unknown onset.
Continue to: Focal-onset seizures
Focal-onset seizures originate “within networks limited to one hemisphere,” although possibly in more than 1 region (ie, multifocal, and presence or absence of loss of awareness). 12 Focal seizures may then be further classified into “motor onset” or “nonmotor onset” (eg, autonomic, emotional, sensory). 2
Generalized seizures are those “originating at some point within, and rapidly engaging, bilaterally distributed networks.” 13 Unlike focal-onset seizures, generalized seizures are not classified based on awareness, as most generalized seizures involve loss of awareness (absence) or total loss of consciousness (generalized tonic-clonic). They are instead categorized based on the presence of motor vs nonmotor features (eg, tonic-clonic, myoclonic, atonic). Epilepsy classification is quite dynamic and constantly updated based on new genetic, electroencephalographic, and neuroimaging discoveries.
Treatment of epilepsy
Antiseizure medications
Treatment with antiseizure medications (ASMs; formerly known as antiepileptic drugs ) is the mainstay of epilepsy management. Achieving efficacy (seizure freedom) and tolerability (minimal adverse effects) are the primary goals of treatment. Factors that should govern the selection of an ASM include the seizure type/epilepsy syndrome, adverse effect profile of the ASM, pharmacodynamic/pharmacokinetic considerations, and patient comorbidities.
The Standard and New Antiepileptic Drugs (SANAD I and II) trials provide data from direct, unblinded, and longitudinal comparisons of existing and new ASMs and their utility in different seizure types. In the SANAD I cohort of patients with generalized and unclassified epilepsies, valproate was superior to lamotrigine and topiramate for 12-month remission and treatment failure rates, respectively.14 However, valproate generally is avoided in women of childbearing age due its potential adverse effects during pregnancy. In focal epilepsies, lamotrigine was superior to carbamazepine, gabapentin, and topiramate with respect to treatment failure, and noninferior to carbamazepine for 12-month remission.15 In the SANAD II trial, levetiracetam was noninferior to valproate for incidence of adverse events in patients with generalized and unclassified epilepsies although was found to be neither more clinically effective nor more cost effective.16 For patients of childbearing potential with generalized and unclassified epilepsies, there is evidence to support the safe and effective use of levetiracetam.17 In focal epilepsies, lamotrigine was superior to levetiracetam and zonisamide with respect to treatment failures and adverse events and was noninferior to zonisamide for 12-month remission.18 In summary, levetiracetam and valproate (not to be used in women of childbearing potential) are considered appropriate first-line agents for generalized and unclassified epilepsies while lamotrigine is deemed an appropriate first-line agent for focal epilepsies (TABLE 119-28).

Drug level monitoring. It is standard practice to periodically monitor serum levels in patients taking first-generation ASMs such as phenytoin, carbamazepine, phenobarbital, and valproic acid because of their narrow therapeutic range and the potential for overdose or interaction with other medications or foods (eg, grapefruit juice may increase carbamazepine serum level by inhibiting CYP3A4, the enzyme that metabolizes the drug). Patients taking newer ASMs may not require regular serum level monitoring except during titration, with hepatic or renal dosing, when concomitantly used with estrogen-based oral contraceptives (eg, lamotrigine), before or during pregnancy, or when nonadherence is suspected.
Continue to: Can antiseizure treatment be stopped?
Can antiseizure treatment be stopped?
Current evidence favors continuing ASM therapy in patients whose seizures are under control, although the decision should be tailored to an individual’s circumstances. According to the 2021 American Academy of Neurology (AAN) guidelines, adults who have been seizure free for at least 2 years and discontinue ASMs are possibly still at higher risk for seizure recurrence in the long term (24-60 months), compared with those who continue treatment.29 On the other hand, for adults who have been seizure free for at least 12 months, ASM withdrawal may not increase their risk for status epilepticus, and there are insufficient data to support or refute an effect on mortality or quality of life with ASM withdrawal in this population. The decision to taper or maintain ASM therapy in seizure-free patients also should take into consideration other clinically relevant outcome measures such as the patient’s lifestyle and medication adverse effects. Therefore, this decision should be made after sufficient discussion with patients and their caregivers. (Information for patients can be found at: www.epilepsy.com/treatment/medicines/stopping-medication.)
For children, the AAN guideline panel recommends discussing with family the small risk (2%) for becoming medication resistant if seizures recur during or after ASM withdrawal. 29 For children who have been seizure free for 18 to 24 months, there is probably not a significant long-term (24-48 months) difference in seizure recurrence in those who taper ASMs vs those who do not. However, presence of epileptiform discharges on EEG before discontinuation of an ASM indicates increased risk for seizure recurrence. 29
Intractable (refractory) epilepsy
While most patients with epilepsy attain complete seizure control with appropriate drug therapy, approximately 30% continue to experience seizures (“drug-resistant” epilepsy, also termed intractable or refractory ). 30 In 2010, the ILAE defined drug-resistant epilepsy as “failure of adequate trials of two tolerated, appropriately chosen and used anti-epileptic drug schedules (whether as monotherapy or in combination) to achieve sustained seizure freedom” (defined as cessation of seizures for at least 3 times the longest pre-intervention inter-seizure interval or 12 months, whichever is longer). 21,31 It should be noted that drug withdrawal due to adverse effects is not counted as failure of that ASM. Recognition of drug-resistant epilepsy may prompt referral to an epileptologist who can consider rational combination drug therapy or surgical resection of the seizure focus, vagus nerve stimulation, electrical stimulation of the seizure focus, or deep brain (thalamic) stimulation.
Seizure triggers and mitigating factors
Epilepsy mostly affects patients during seizure episodes; however, the unpredictability of these events adds significantly to the burden of disease. There are no reliable methods for predicting seizure other than knowing of the several potential risks and recognizing and avoiding these triggers.
Noncompliance with antiseizure medications is a common seizure trigger affecting up to one-half of patients with epilepsy.32
Continue to: Medications
Medications may provoke seizures in susceptible individuals

Sleep deprivation is a potential seizure trigger in people with epilepsy based on observational studies, case reports, patient surveys, and EEG-based studies, although data from randomized controlled studies are limited.36 The standard best practice is to encourage appropriate sleep hygiene, which involves getting at least 7 hours of sleep per night.37
Alcohol is a GABAergic substance like benzodiazepines with antiseizure effects. However, it acts as a potential precipitant of seizures in cases of withdrawal or acute intoxication, or when it leads to sleep disruption or nonadherence to antiseizure medications. Therefore, advise patients with alcohol use disorder to slowly taper consumption (best done through a support program) and avoid sudden withdrawal. However, complete abstinence from alcohol use is not often recommended except in special circumstances (eg, a history of alcohol-related seizures). Several studies have demonstrated that modest alcohol use (1-2 drinks per occasion) does not increase seizure frequency or significantly alter serum concentrations of commonly used ASMs.38
Cannabis and other substances. The 2 main biologically active components of marijuana are delta-9-tetrahydrocannibinol (THC), the main psychoactive constituent, and cannabidiol (CBD). Animal and human studies have demonstrated anticonvulsant properties of THC and CBD. But THC, in high amounts, can result in adverse cognitive effects and worsening seizures.39 A purified 98% oil-based CBD extract (Epidiolex) has been approved as an adjunctive treatment for certain medically refractory epilepsy syndromes in children and young adults—ie, Dravet syndrome, Lennox-Gastaut syndrome, and tuberous sclerosis complex syndrome.40 There are no reliable data on the effect of recreational use of marijuana on seizure control. Other illicit substances such as cocaine may lower seizure threshold by their stimulatory and disruptive effects on sleep, diet, and healthy routines.
Special clinical cases
Pregnancy and epilepsy
Despite the potential adverse effects of ASMs on fetal health, the current global consensus is to continue treatment during pregnancy, given that the potential harm of convulsive seizures outweighs the potential risks associated with in-utero exposure to ASMs. There is not enough evidence to indicate significant harm to the fetus caused by focal, absence, or myoclonic seizures. Low-dose folic acid is used to minimize the risks of ASMs during pregnancy.
Continue to: As the fetus develops...
As the fetus develops, there are changes in volume of ASM distribution, renal clearance, protein binding, and hepatic metabolism, which require checking serum levels at regular intervals and making dosage adjustments.
The ongoing study evaluating Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD)41 has led to multiple landmark studies guiding the choice of preferred ASMs during pregnancy in patients with epilepsy.42,43 This has culminated in today’s use of lamotrigine and levetiracetam as the 2 preferred agents (while avoiding valproate) in pregnant patients with epilepsy.44
Psychogenic nonepileptic seizures
A form of conversion disorder, psychogenic nonepileptic seizures (PNES) manifests as abnormal motor or behavioral events mimicking seizures but without associated epileptiform discharges on EEG. This is observed in 10% of patients seen in epilepsy clinics and even more often in those admitted to epilepsy monitoring units (25%-40%).45 Diagnosis of PNES requires EEG monitoring both for confirmation and for discernment from true epileptic seizures, in particular frontal lobe epilepsy that may clinically mimic PNES. PNES often is associated with underlying psychological tensions or comorbid conditions such as depression, anxiety, or traumatic life experiences. There is no treatment for PNES per se, and its management is focused on controlling any underlying psychological comorbidities that may not always be obvious. There is some evidence suggesting that these patients experience an innate inability to verbally express their emotions and instead subconsciously resort to psychosomatics to express them in a somatic dimension.46,47
Status epilepticus
Defined as prolonged seizures (> 5 min) or 2 consecutive seizures without regaining aware ness in between, status epilepticus (SE) is a potentially fatal condition. Subclinical nonconvulsive SE, especially in comatose patients, can be diagnosed only via EEG monitoring. Untreated SE may manifest as a diagnostic dilemma in unresponsive or critically ill patients and can increase the risk for mortality. 48
Febrile seizures
Febrile seizures affect 2% to 5% of children most often in the second year of life.49 The use of preventive antiseizure medication is not recommended; instead, the key is to investigate the underlying febrile illness. Lumbar puncture is indicated if there are signs and symptoms of meningitis (25% of children with bacterial meningitis present with seizures).49 Febrile seizures often are self-limited, but there is risk for SE in up to 15% of cases.50 If convulsive febrile seizures last longer than 5 minutes, initiate benzodiazepines followed by the standard protocol used for the management of SE.51
Continue to: Epilepsy as a spectrum disorder
Epilepsy as a spectrum disorder
The higher prevalence of comorbid cognitive and psychiatric conditions in patients with epilepsy, affecting about half of patients, 52 suggests that seizures may constitute only one aspect of a multifaceted disease that otherwise should be considered a spectrum disorder. Among such conditions are memory deficits, depression, and anxiety. Conversely, epilepsy is more common in patients with depression than in those without. 52
Social impact of epilepsy
Vehicle driving regulations. Patients with epilepsy are required to follow state law regarding driving restrictions. Different states have different rules and regulations about driving restrictions and reporting requirements (by patients or their physicians). Refer patients to the Department of Motor Vehicles (DMV) in their state of residence for up-to-date instructions.53 The Epilepsy Foundation (epilepsy.com) can serve as a resource for each state’s DMV website.
Employment assistance. Having epilepsy should not preclude patients from seeking employment and pursuing meaningful careers. The Americans with Disabilities Act (ADA) and the US Equal Employment Opportunity Commission (EEOC) forbid discrimination against qualified people with disabilities, including those with epilepsy, and require reasonable accommodations in the workplace (www.eeoc.gov/laws/guidance/epilepsy-workplace-and-ada).54
CORRESPONDENCE
Gholam K. Motamedi, MD, Department of Neurology, PHC 7, Georgetown University Hospital, 3800 Reservoir Road, NW, Washington, DC 20007; [email protected]
1. Hauser WA, Annegers JF, Rocca WA. Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc. 1996;71:576-586. doi: 10.4065/71.6.576
2. Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475-482. doi: 10.1111/epi.12550.
3. Hauser WA, Beghi E. First seizure definitions and worldwide incidence and mortality. Epilepsia. 2008;49:8-12. doi: 10.1111/j.1528-1167.2008.01443.x
4. Berg AT, Shinnar S. The risk of seizure recurrence following a first unprovoked seizure: a quantitative review. Neurology. 1991;41:965-972. doi: 10.1212/wnl.41.7.965
5. Ropper AH. Transient global amnesia. N Engl J Med. 2023;388:635-540. doi: 10.1056/NEJMra2213867
6. Bouma HK, Labos C, Gore GC, et al. The diagnostic accuracy of routine electroencephalography after a first unprovoked seizure. Eur J Neurol. 2016;23:455-463. doi: 10.1111/ene.12739
7. Narayanan JT, Labar DR, Schaul N. Latency to first spike in the EEG of epilepsy patients. Seizure. 2008;17:34-41. doi: 10.1016/j.seizure.2007.06.003
8. Salmenpera TM, Duncan JS. Imaging in epilepsy. J Neurol Neurosurg Psychiatry. 2005;76:iii2-iii10. doi: 10.1136/jnnp.2005.075135
9. Jackson GD, Berkovic SF, Tress , et al Hippocampal sclerosis can be reliably detected by magnetic resonance imaging. Neurology. 1990;40:1869-1875. doi: 10.1212/wnl.40.12.1869
10. Bonnett LJ, Kim, L, Johnson A, et al. Risk of seizure recurrence in people with single seizures and early epilepsy - model development and external validation. Seizure. 2022;94:26-32. doi: 10.1016/j.seizure.2021.11.007
11. Krumholz A, Wiebe S, Gronseth GS, et al. Evidence-based guideline: management of an unprovoked first seizure in adults: Report of the Guideline Development Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2015;84:1705-1713. doi: 10.1212/WNL.0000000000001487
12. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and terminology. Epilepsia. 2017;58:522-530. doi: 10.1111/epi.13670
13. Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsy: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51:676-685. doi: 10.1111/j.1528-1167.2010.02522.x
14. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalized and unclassifiable epilepsy: an unblinded randomized controlled trial. Lancet. 2007;369:1016-1026. doi: 10.1016/S0140-6736(07)60461-9
15. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomized controlled trial. Lancet 2007;369:1000-1015. doi: 10.1016/S0140-6736(07)60460-7
16. Marson A, Burnside G, Appleton R, et al. The SANAD II study of the effectiveness and cost-effectiveness of valproate versus levetiracetam for newly diagnosed generalized and unclassified epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomized controlled trial. Lancet. 2021;397:1375-1386. doi: 10.1016/S0140-6736(21)00246-4
17. Mawhinney E, Craig J, Morrow J. Levetiracetam in pregnancy: results from the UK and Ireland epilepsy and pregnancy registers. Neurology. 2013;80:400-405.
18. Marson A, Burnside G, Appleton R, et al. The SANAD II study of the effectiveness and cost-effectiveness of levetiracetam, zonisamide, or lamotrigine for newly diagnosed focal epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomized controlled trial. Lancet. 2021;397:1363-1374. doi: 10.1016/S0140-6736(21)00247-6
19. Smith PE. Initial management of seizure in adults. N Engl J Med. 2021;385:251-263. doi: 10.1056/NEJMcp2024526
20. Depakene (valproic acid). Package insert. Abbott Laboratories; 2011. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2011/018081s046_18082s031lbl.pdf
21. Greenberg RG, Melloni C, Wu H, et al. Therapeutic index estimation of antiepileptic drugs: a systematic literature review approach. Clin Neuropharmacol. 2016;39:232-240. doi: 10.1097/WNF.0000000000000172
22. Lamictal (lamotrigine). Package insert. GlaxoSmithKline; 2009. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2009/020241s037s038,020764s030s031lbl.pdf
23. LaRoche SM, Helmers SL. The new antiepileptic drugs: scientific review. JAMA. 2004;291:605-614. doi: 10.1001/jama.291.5.605
24. Topamax (topiramate). Package insert. Janssen Pharmaceuticals, Inc. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2012/020844s041lbl.pdf
25. Keppra (levetiracetam). Package insert. UCB, Inc.; 2009. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2009/021035s078s080%2C021505s021s024lbl.pdf
26. Carbatrol (carbamazepine). Package insert. Shire US Inc; 2013. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2013/020712s032s035lbl.pdf
27.Neurontin (gabapentin). Package insert. Pfizer; 2017. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2017/020235s064_020882s047_021129s046lbl.pdf
28.Zonegran (zonisamide). Package insert. Eisai Inc; 2006. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2006/020789s019lbl.pdf
29.Gloss D, Paragon K, Pack A, et al. Antiseizure medication withdrawal in seizure-free patients: practice advisory update. Report of the AAN Guideline Subcommittee. Neurology. 2021;97:1072-1081. doi: 10.1212/WNL.0000000000012944
30.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000:342:314-319. doi: 10.1056/NEJM200002033420503
31.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069-1077. doi: 10.1111/j.1528-1167.2009.02397.x
Compliance during treatment of epilepsy. Epilepsia 1988;29(suppl 2):S79-S84.
33.utter R, Rüegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology. 2015;13;85:1332-1341. doi: 10.1212/WNL.0000000000002023
34.Singh G, Rees JH, Sander JW. Seizures and epilepsy in oncological practice: causes, course, mechanisms and treatment. JNNP. 2007;78:342-349. doi: 10.1136/jnnp.2006.106211
35.Pisani F, Oteri G, Costa C., et al. Effects of psychotropic drugs on seizure threshold. Drug Safety. 2002;25:91-110.
36.Rossi KC, Joe J, Makhjia M, et al. Insufficient sleep, electroencephalogram activation, and seizure risk: re-evaluating the evidence. Ann Neurol. 2020;86:798-806. doi: 10.1002/ana.25710
37.Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843-844. doi: 10.5665/sleep.4716
38.Höppener RJ, Kuyer A, van der Lugt PJ. Epilepsy and alcohol: the influence of social alcohol intake on seizures and treatment in epilepsy. Epilepsia. 1983;24:459-471. doi: 10.1111/j.1528-1157.1983.tb04917.x
39.Keeler MH, Reifler CB. Grand mal convulsions subsequent to marijuana use. Case report. Dis Nerv Syst. 1967:28:474-475.
40.Epidiolex (cannabidiol). Package insert. Greenwich Biosciences Inc; 2018. Accessed September 27, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2018/210365lbl.pdf
41.ClinicalTrials.gov. Maternal Outcomes and Neurodevelopmental Effects of Antiseizure Drugs (MONEAD). Accessed September 24, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT01730170
42.Meador KJ, Baker GA, Finnell RH, et al. In utero antiepileptic drug exposure: fetal death and malformations. Neurology. 2006;67:407-412. doi: 10.1212/01.wnl.0000227919.81208.b2
43.Meador K, Reynolds MW, Crean S. Pregnancy outcomes in women with epilepsy: a systematic review and meta-analysis of published pregnancy registries and cohorts. Epilepsy Res. 2008;81:1-13. doi:10.1016/j.eplepsyres.2008.04.022
44.Marxer CA, Rüegg S, Rauch A review of the evidence on the risk of congenital malformations and neurodevelopmental disorders in association with antiseizure medications during pregnancy. Expert Opin Drug Saf. 2021;20:1487-1499. doi: 10.1080/14740338.2021.1943355
Asadi-Pooya AA, Sperling MR. Epidemiology of psychogenic nonepileptic seizures. Epilepsy Behav. 2015;46:60-65. doi: 10.1016/j.yebeh.2015.03.015
Evaluation and treatment of psychogenic nonepileptic seizures. Neurol Clin. 2022;40:799-820. doi: 10.1016/j.ncl.2022.03.017
47.Motamedi GK. Psychogenic nonepileptic seizures: a disconnect between body and mind. Epilepsy Behav. 2018;78:293-294. doi: 10.1016/j.yebeh.2017.10.016
, Nonconvulsive status epilepticus. Emerg Med Clin North Am. 2011;29:65-72. doi: 10.1016/j.emc.2010.08.006
doi: 10.1542/peds.2010-3318
Drug management for acute tonic-clonic convulsions including convulsive status epilepticus in children. Cochrane Database Sys Rev. 2018;1(1):CD001905. doi: 10.1002/14651858.CD001905.pub3
52.Jensen FE. Epilepsy as a spectrum disorder: implications from novel clinical and basic neuroscience. Epilepsia. 2011;52(suppl 1):1-6. doi: 10.1111/j.1528-1167.2010.02904.x
53.Kass JS, Rose RV. Driving and epilepsy: ethical, legal, and health care policy challenges. Continuum (Minneap Minn). 2019;25:537-542. doi: 10.1212/CON.0000000000000714
54.Troxell J. Epilepsy and employment: the Americans with Disabilities Act and its protections against employment discrimination. Med Law. 1997;16:375-384.
1. Hauser WA, Annegers JF, Rocca WA. Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc. 1996;71:576-586. doi: 10.4065/71.6.576
2. Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475-482. doi: 10.1111/epi.12550.
3. Hauser WA, Beghi E. First seizure definitions and worldwide incidence and mortality. Epilepsia. 2008;49:8-12. doi: 10.1111/j.1528-1167.2008.01443.x
4. Berg AT, Shinnar S. The risk of seizure recurrence following a first unprovoked seizure: a quantitative review. Neurology. 1991;41:965-972. doi: 10.1212/wnl.41.7.965
5. Ropper AH. Transient global amnesia. N Engl J Med. 2023;388:635-540. doi: 10.1056/NEJMra2213867
6. Bouma HK, Labos C, Gore GC, et al. The diagnostic accuracy of routine electroencephalography after a first unprovoked seizure. Eur J Neurol. 2016;23:455-463. doi: 10.1111/ene.12739
7. Narayanan JT, Labar DR, Schaul N. Latency to first spike in the EEG of epilepsy patients. Seizure. 2008;17:34-41. doi: 10.1016/j.seizure.2007.06.003
8. Salmenpera TM, Duncan JS. Imaging in epilepsy. J Neurol Neurosurg Psychiatry. 2005;76:iii2-iii10. doi: 10.1136/jnnp.2005.075135
9. Jackson GD, Berkovic SF, Tress , et al Hippocampal sclerosis can be reliably detected by magnetic resonance imaging. Neurology. 1990;40:1869-1875. doi: 10.1212/wnl.40.12.1869
10. Bonnett LJ, Kim, L, Johnson A, et al. Risk of seizure recurrence in people with single seizures and early epilepsy - model development and external validation. Seizure. 2022;94:26-32. doi: 10.1016/j.seizure.2021.11.007
11. Krumholz A, Wiebe S, Gronseth GS, et al. Evidence-based guideline: management of an unprovoked first seizure in adults: Report of the Guideline Development Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2015;84:1705-1713. doi: 10.1212/WNL.0000000000001487
12. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and terminology. Epilepsia. 2017;58:522-530. doi: 10.1111/epi.13670
13. Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsy: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51:676-685. doi: 10.1111/j.1528-1167.2010.02522.x
14. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalized and unclassifiable epilepsy: an unblinded randomized controlled trial. Lancet. 2007;369:1016-1026. doi: 10.1016/S0140-6736(07)60461-9
15. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomized controlled trial. Lancet 2007;369:1000-1015. doi: 10.1016/S0140-6736(07)60460-7
16. Marson A, Burnside G, Appleton R, et al. The SANAD II study of the effectiveness and cost-effectiveness of valproate versus levetiracetam for newly diagnosed generalized and unclassified epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomized controlled trial. Lancet. 2021;397:1375-1386. doi: 10.1016/S0140-6736(21)00246-4
17. Mawhinney E, Craig J, Morrow J. Levetiracetam in pregnancy: results from the UK and Ireland epilepsy and pregnancy registers. Neurology. 2013;80:400-405.
18. Marson A, Burnside G, Appleton R, et al. The SANAD II study of the effectiveness and cost-effectiveness of levetiracetam, zonisamide, or lamotrigine for newly diagnosed focal epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomized controlled trial. Lancet. 2021;397:1363-1374. doi: 10.1016/S0140-6736(21)00247-6
19. Smith PE. Initial management of seizure in adults. N Engl J Med. 2021;385:251-263. doi: 10.1056/NEJMcp2024526
20. Depakene (valproic acid). Package insert. Abbott Laboratories; 2011. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2011/018081s046_18082s031lbl.pdf
21. Greenberg RG, Melloni C, Wu H, et al. Therapeutic index estimation of antiepileptic drugs: a systematic literature review approach. Clin Neuropharmacol. 2016;39:232-240. doi: 10.1097/WNF.0000000000000172
22. Lamictal (lamotrigine). Package insert. GlaxoSmithKline; 2009. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2009/020241s037s038,020764s030s031lbl.pdf
23. LaRoche SM, Helmers SL. The new antiepileptic drugs: scientific review. JAMA. 2004;291:605-614. doi: 10.1001/jama.291.5.605
24. Topamax (topiramate). Package insert. Janssen Pharmaceuticals, Inc. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2012/020844s041lbl.pdf
25. Keppra (levetiracetam). Package insert. UCB, Inc.; 2009. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2009/021035s078s080%2C021505s021s024lbl.pdf
26. Carbatrol (carbamazepine). Package insert. Shire US Inc; 2013. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2013/020712s032s035lbl.pdf
27.Neurontin (gabapentin). Package insert. Pfizer; 2017. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2017/020235s064_020882s047_021129s046lbl.pdf
28.Zonegran (zonisamide). Package insert. Eisai Inc; 2006. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2006/020789s019lbl.pdf
29.Gloss D, Paragon K, Pack A, et al. Antiseizure medication withdrawal in seizure-free patients: practice advisory update. Report of the AAN Guideline Subcommittee. Neurology. 2021;97:1072-1081. doi: 10.1212/WNL.0000000000012944
30.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000:342:314-319. doi: 10.1056/NEJM200002033420503
31.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069-1077. doi: 10.1111/j.1528-1167.2009.02397.x
Compliance during treatment of epilepsy. Epilepsia 1988;29(suppl 2):S79-S84.
33.utter R, Rüegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology. 2015;13;85:1332-1341. doi: 10.1212/WNL.0000000000002023
34.Singh G, Rees JH, Sander JW. Seizures and epilepsy in oncological practice: causes, course, mechanisms and treatment. JNNP. 2007;78:342-349. doi: 10.1136/jnnp.2006.106211
35.Pisani F, Oteri G, Costa C., et al. Effects of psychotropic drugs on seizure threshold. Drug Safety. 2002;25:91-110.
36.Rossi KC, Joe J, Makhjia M, et al. Insufficient sleep, electroencephalogram activation, and seizure risk: re-evaluating the evidence. Ann Neurol. 2020;86:798-806. doi: 10.1002/ana.25710
37.Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843-844. doi: 10.5665/sleep.4716
38.Höppener RJ, Kuyer A, van der Lugt PJ. Epilepsy and alcohol: the influence of social alcohol intake on seizures and treatment in epilepsy. Epilepsia. 1983;24:459-471. doi: 10.1111/j.1528-1157.1983.tb04917.x
39.Keeler MH, Reifler CB. Grand mal convulsions subsequent to marijuana use. Case report. Dis Nerv Syst. 1967:28:474-475.
40.Epidiolex (cannabidiol). Package insert. Greenwich Biosciences Inc; 2018. Accessed September 27, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2018/210365lbl.pdf
41.ClinicalTrials.gov. Maternal Outcomes and Neurodevelopmental Effects of Antiseizure Drugs (MONEAD). Accessed September 24, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT01730170
42.Meador KJ, Baker GA, Finnell RH, et al. In utero antiepileptic drug exposure: fetal death and malformations. Neurology. 2006;67:407-412. doi: 10.1212/01.wnl.0000227919.81208.b2
43.Meador K, Reynolds MW, Crean S. Pregnancy outcomes in women with epilepsy: a systematic review and meta-analysis of published pregnancy registries and cohorts. Epilepsy Res. 2008;81:1-13. doi:10.1016/j.eplepsyres.2008.04.022
44.Marxer CA, Rüegg S, Rauch A review of the evidence on the risk of congenital malformations and neurodevelopmental disorders in association with antiseizure medications during pregnancy. Expert Opin Drug Saf. 2021;20:1487-1499. doi: 10.1080/14740338.2021.1943355
Asadi-Pooya AA, Sperling MR. Epidemiology of psychogenic nonepileptic seizures. Epilepsy Behav. 2015;46:60-65. doi: 10.1016/j.yebeh.2015.03.015
Evaluation and treatment of psychogenic nonepileptic seizures. Neurol Clin. 2022;40:799-820. doi: 10.1016/j.ncl.2022.03.017
47.Motamedi GK. Psychogenic nonepileptic seizures: a disconnect between body and mind. Epilepsy Behav. 2018;78:293-294. doi: 10.1016/j.yebeh.2017.10.016
, Nonconvulsive status epilepticus. Emerg Med Clin North Am. 2011;29:65-72. doi: 10.1016/j.emc.2010.08.006
doi: 10.1542/peds.2010-3318
Drug management for acute tonic-clonic convulsions including convulsive status epilepticus in children. Cochrane Database Sys Rev. 2018;1(1):CD001905. doi: 10.1002/14651858.CD001905.pub3
52.Jensen FE. Epilepsy as a spectrum disorder: implications from novel clinical and basic neuroscience. Epilepsia. 2011;52(suppl 1):1-6. doi: 10.1111/j.1528-1167.2010.02904.x
53.Kass JS, Rose RV. Driving and epilepsy: ethical, legal, and health care policy challenges. Continuum (Minneap Minn). 2019;25:537-542. doi: 10.1212/CON.0000000000000714
54.Troxell J. Epilepsy and employment: the Americans with Disabilities Act and its protections against employment discrimination. Med Law. 1997;16:375-384.
PRACTICE RECOMMENDATIONS
› Consider treating a first-time seizure if electroencephalography shows particular epileptiform activity, if the neurologic exam or computerized tomography or magnetic resonance imaging results are abnormal, if the seizure is focal or nocturnal, or if there is a family history of seizures. A
› Consider valproate (except for women of childbearing age) and levetiracetam as first-line agents for generalized or unclassified epilepsy, and lamotrigine for focal epilepsies. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
In MI with anemia, results may favor liberal transfusion: MINT
In patients with myocardial infarction and anemia, a “liberal” red blood cell transfusion strategy did not significantly reduce the risk of recurrent MI or death within 30 days, compared with a “restrictive” transfusion strategy, in the 3,500-patient MINT trial.
Jeffrey L. Carson, MD, from Robert Wood Johnson Medical School, New Brunswick, N.J., said in a press briefing.
He presented the study in a late-breaking trial session at the annual scientific sessions of the American Heart Association, and it was simultaneously published online in the New England Journal of Medicine.
“Whether to transfuse is an everyday decision faced by clinicians caring for patients with acute MI,” Dr. Carson said.
“We cannot claim that a liberal transfusion strategy is definitively superior based on our primary outcome,” he said, but “the 95% confidence interval is consistent with treatment effects corresponding to no difference between the two transfusion strategies and to a clinically relevant benefit with the liberal strategy.”
“In contrast to other trials in other settings,” such as anemia and cardiac surgery, Dr. Carson said, “the results suggest that a liberal transfusion strategy has the potential for clinical benefit with an acceptable risk of harm.”
“A liberal transfusion strategy may be the most prudent approach to transfusion in anemic patients with MI,” he added.
Not a home run
Others agreed with this interpretation. Martin B. Leon, MD, from Columbia University, New York, the study discussant in the press briefing, said the study “addresses a question that is common” in clinical practice. It was well conducted, and international (although most patients were in the United States and Canada), in a very broad group of patients, designed to make the results more generalizable. The 98% follow-up was extremely good, Dr. Leon added, and the trialists achieved their goal in that they did show a difference between the two transfusion strategies.
The number needed to treat was 40 to see a benefit in the combined outcome of death or recurrent MI at 30 days, Dr. Leon said. The P value for this was .07, “right on the edge” of statistical significance.
This study is “not a home run,” for the primary outcome, he noted; however, many of the outcomes tended to be in favor of a liberal transfusion strategy. Notably, cardiovascular death, which was not a specified outcome, was significantly lower in the group who received a liberal transfusion strategy.
Although a liberal transfusion strategy was “not definitely superior” in these patients with MI and anemia, Dr. Carson said, he thinks the trial will be interpreted as favoring a liberal transfusion strategy.
C. Michael Gibson, MD, professor of medicine at Harvard Medical School, Boston, and CEO of Harvard’s Baim and PERFUSE institutes for clinical research, voiced similar views.
“Given the lack of acute harm associated with liberal transfusion and the preponderance of evidence favoring liberal transfusion in the largest trial to date,” concluded Dr. Gibson, the assigned discussant at the session, “liberal transfusion appears to be a viable management strategy, particularly among patients with non-STEMI type 1 MI and as clinical judgment dictates.”
Only three small randomized controlled trials have compared transfusion thresholds in a total of 820 patients with MI and anemia, Dr. Gibson said, a point that the trial investigators also made. The results were inconsistent between trials: the CRIT trial (n = 45) favored a restrictive strategy, the MINT pilot study (n = 110) favored a liberal one, and the REALITY trial (n = 668) showed noninferiority of a restrictive strategy, compared with a liberal strategy in 30-day MACE.
The MINT trial was four times larger than all prior studies combined. However, most outcomes were negative or of borderline significance for benefit.
Cardiac death was more common in the restrictive group at 5.5% than the liberal group at 3.2% (risk ratio, 1.74, 95% CI, 1.26-2.40), but this was nonadjudicated, and not designated as a primary, secondary, or tertiary outcome – which the researchers also noted. Fewer than half of the deaths were classified as cardiac, which was “odd,” Dr. Gibson observed.
A restrictive transfusion strategy was associated with increased events among participants with type 1 MI (RR, 1.32, 95% CI, 1.04-1.67), he noted.
Study strengths included that 45.5% of participants were women, Dr. Gibson said. Limitations included that the trial was “somewhat underpowered.” Also, even in the restrictive group, participants received a mean of 0.7 units of packed red blood cells.
Adherence to the 10 g/dL threshold in the liberal transfusion group was moderate (86.3% at hospital discharge), which the researchers acknowledged. They noted that this was frequently caused by clinical discretion, such as concern about fluid overload, and to the timing of hospital discharge. In addition, long-term potential for harm (microchimerism) is not known.
“There was a consistent nonsignificant acute benefit for liberal transfusion and a nominal reduction in CV mortality and improved outcomes in patients with type 1 MI in exploratory analyses, in a trial that ended up underpowered,” Dr. Gibson summarized. “Long-term follow up would be helpful to evaluate chronic outcomes.”
This is a very well-conducted, high-quality, important study that will be considered a landmark trial, C. David Mazer, MD, University of Toronto and St. Michael’s Hospital, also in Toronto, said in an interview.
Unfortunately, “it was not as definitive as hoped for,” Dr. Mazer lamented. Nevertheless, “I think people may interpret it as providing support for a liberal transfusion strategy” in patients with anemia and MI, he said.
Dr. Mazer, who was not involved with this research, was a principal investigator on the TRICS-3 trial, which disputed a liberal RBC transfusion strategy in patients with anemia undergoing cardiac surgery, as previously reported.
The “Red Blood Cell Transfusion: 2023 AABB International Guidelines,” led by Dr. Carson and published in JAMA, recommend a restrictive strategy in stable patients, although these guidelines did not include the current study, Dr. Mazer observed.
In the REALITY trial, there were fewer major adverse cardiac events (MACE) events in the restrictive strategy, he noted.
MINT can be viewed as comparing a high versus low hemoglobin threshold. “It is possible that the best is in between,” he said.
Dr. Mazer also noted that MINT may have achieved significance if it was designed with a larger enrollment and a higher power (for example, 90% instead of 80%) to detect between-group difference for the primary outcome.
Study rationale, design, and findings
Anemia, or low RBC count, is common in patients with MI, Dr. Carson noted. A normal hemoglobin is 13 g/dL in men and 12 g/dL in women. Administering a packed RBC transfusion only when a patient’s hemoglobin falls below 7 or 8 g/dL has been widely adopted, but it is unclear if patients with acute MI may benefit from a higher hemoglobin level.
“Blood transfusion may decrease ischemic injury by improving oxygen delivery to myocardial tissues and reduce the risk of reinfarction or death,” the researchers wrote. “Alternatively, administering more blood could result in more frequent heart failure from fluid overload, infection from immunosuppression, thrombosis from higher viscosity, and inflammation.”
From 2017 to 2023, investigators enrolled 3,504 adults aged 18 and older at 144 sites in the United States (2,157 patients), Canada (885), France (323), Brazil (105), New Zealand (25), and Australia (9).
The participants had ST-elevation or non–ST-elevation MI and hemoglobin less than 10 g/dL within 24 hours. Patients with type 1 (atherosclerotic plaque disruption), type 2 (supply-demand mismatch without atherothrombotic plaque disruption), type 4b, or type 4c MI were eligible.
They were randomly assigned to receive:
- A ‘restrictive’ transfusion strategy (1,749 patients): Transfusion was permitted but not required when a patient’s hemoglobin was less than 8 g/dL and was strongly recommended when it was less than 7 g/dL or when anginal symptoms were not controlled with medications.
- A ‘liberal’ transfusion strategy (1,755 patients): One unit of RBCs was administered after randomization, and RBCs were transfused to maintain hemoglobin 10 g/dL or higher until hospital discharge or 30 days.
The patients had a mean age of 72 years and 46% were women. More than three-quarters (78%) were White and 14% were Black. They had frequent coexisting illnesses, about a third had a history of MI, percutaneous coronary intervention, or heart failure; 14% were on a ventilator and 12% had renal dialysis. The median duration of hospitalization was 5 days in the two groups.
At baseline, the mean hemoglobin was 8.6 g/dL in both groups. At days 1, 2, and 3, the mean hemoglobin was 8.8, 8.9, and 8.9 g/dL, respectively, in the restrictive transfusion group, and 10.1, 10.4, and 10.5 g/dL, respectively, in the liberal transfusion group.
The mean number of transfused blood units was 0.7 units in the restrictive strategy group and 2.5 units in the liberal strategy group, roughly a 3.5-fold difference.
After adjustment for site and incomplete follow-up in 57 patients (20 with the restrictive strategy and 37 with the liberal strategy), the estimated RR for the primary outcome in the restrictive group versus the liberal group was 1.15 (P = .07).
“We observed that the 95% confidence interval contains values that suggest a clinical benefit for the liberal transfusion strategy and does not include values that suggest a benefit for the more restrictive transfusion strategy,” the researchers wrote. Heart failure and other safety outcomes were comparable in the two groups.
The trial was supported by grants from the National Heart, Lung, and Blood Institute and by the Canadian Blood Services and Canadian Institutes of Health Research Institute of Circulatory and Respiratory Health. Dr. Carson, Dr. Leon, Dr. Gibson, and Dr. Mazer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In patients with myocardial infarction and anemia, a “liberal” red blood cell transfusion strategy did not significantly reduce the risk of recurrent MI or death within 30 days, compared with a “restrictive” transfusion strategy, in the 3,500-patient MINT trial.
Jeffrey L. Carson, MD, from Robert Wood Johnson Medical School, New Brunswick, N.J., said in a press briefing.
He presented the study in a late-breaking trial session at the annual scientific sessions of the American Heart Association, and it was simultaneously published online in the New England Journal of Medicine.
“Whether to transfuse is an everyday decision faced by clinicians caring for patients with acute MI,” Dr. Carson said.
“We cannot claim that a liberal transfusion strategy is definitively superior based on our primary outcome,” he said, but “the 95% confidence interval is consistent with treatment effects corresponding to no difference between the two transfusion strategies and to a clinically relevant benefit with the liberal strategy.”
“In contrast to other trials in other settings,” such as anemia and cardiac surgery, Dr. Carson said, “the results suggest that a liberal transfusion strategy has the potential for clinical benefit with an acceptable risk of harm.”
“A liberal transfusion strategy may be the most prudent approach to transfusion in anemic patients with MI,” he added.
Not a home run
Others agreed with this interpretation. Martin B. Leon, MD, from Columbia University, New York, the study discussant in the press briefing, said the study “addresses a question that is common” in clinical practice. It was well conducted, and international (although most patients were in the United States and Canada), in a very broad group of patients, designed to make the results more generalizable. The 98% follow-up was extremely good, Dr. Leon added, and the trialists achieved their goal in that they did show a difference between the two transfusion strategies.
The number needed to treat was 40 to see a benefit in the combined outcome of death or recurrent MI at 30 days, Dr. Leon said. The P value for this was .07, “right on the edge” of statistical significance.
This study is “not a home run,” for the primary outcome, he noted; however, many of the outcomes tended to be in favor of a liberal transfusion strategy. Notably, cardiovascular death, which was not a specified outcome, was significantly lower in the group who received a liberal transfusion strategy.
Although a liberal transfusion strategy was “not definitely superior” in these patients with MI and anemia, Dr. Carson said, he thinks the trial will be interpreted as favoring a liberal transfusion strategy.
C. Michael Gibson, MD, professor of medicine at Harvard Medical School, Boston, and CEO of Harvard’s Baim and PERFUSE institutes for clinical research, voiced similar views.
“Given the lack of acute harm associated with liberal transfusion and the preponderance of evidence favoring liberal transfusion in the largest trial to date,” concluded Dr. Gibson, the assigned discussant at the session, “liberal transfusion appears to be a viable management strategy, particularly among patients with non-STEMI type 1 MI and as clinical judgment dictates.”
Only three small randomized controlled trials have compared transfusion thresholds in a total of 820 patients with MI and anemia, Dr. Gibson said, a point that the trial investigators also made. The results were inconsistent between trials: the CRIT trial (n = 45) favored a restrictive strategy, the MINT pilot study (n = 110) favored a liberal one, and the REALITY trial (n = 668) showed noninferiority of a restrictive strategy, compared with a liberal strategy in 30-day MACE.
The MINT trial was four times larger than all prior studies combined. However, most outcomes were negative or of borderline significance for benefit.
Cardiac death was more common in the restrictive group at 5.5% than the liberal group at 3.2% (risk ratio, 1.74, 95% CI, 1.26-2.40), but this was nonadjudicated, and not designated as a primary, secondary, or tertiary outcome – which the researchers also noted. Fewer than half of the deaths were classified as cardiac, which was “odd,” Dr. Gibson observed.
A restrictive transfusion strategy was associated with increased events among participants with type 1 MI (RR, 1.32, 95% CI, 1.04-1.67), he noted.
Study strengths included that 45.5% of participants were women, Dr. Gibson said. Limitations included that the trial was “somewhat underpowered.” Also, even in the restrictive group, participants received a mean of 0.7 units of packed red blood cells.
Adherence to the 10 g/dL threshold in the liberal transfusion group was moderate (86.3% at hospital discharge), which the researchers acknowledged. They noted that this was frequently caused by clinical discretion, such as concern about fluid overload, and to the timing of hospital discharge. In addition, long-term potential for harm (microchimerism) is not known.
“There was a consistent nonsignificant acute benefit for liberal transfusion and a nominal reduction in CV mortality and improved outcomes in patients with type 1 MI in exploratory analyses, in a trial that ended up underpowered,” Dr. Gibson summarized. “Long-term follow up would be helpful to evaluate chronic outcomes.”
This is a very well-conducted, high-quality, important study that will be considered a landmark trial, C. David Mazer, MD, University of Toronto and St. Michael’s Hospital, also in Toronto, said in an interview.
Unfortunately, “it was not as definitive as hoped for,” Dr. Mazer lamented. Nevertheless, “I think people may interpret it as providing support for a liberal transfusion strategy” in patients with anemia and MI, he said.
Dr. Mazer, who was not involved with this research, was a principal investigator on the TRICS-3 trial, which disputed a liberal RBC transfusion strategy in patients with anemia undergoing cardiac surgery, as previously reported.
The “Red Blood Cell Transfusion: 2023 AABB International Guidelines,” led by Dr. Carson and published in JAMA, recommend a restrictive strategy in stable patients, although these guidelines did not include the current study, Dr. Mazer observed.
In the REALITY trial, there were fewer major adverse cardiac events (MACE) events in the restrictive strategy, he noted.
MINT can be viewed as comparing a high versus low hemoglobin threshold. “It is possible that the best is in between,” he said.
Dr. Mazer also noted that MINT may have achieved significance if it was designed with a larger enrollment and a higher power (for example, 90% instead of 80%) to detect between-group difference for the primary outcome.
Study rationale, design, and findings
Anemia, or low RBC count, is common in patients with MI, Dr. Carson noted. A normal hemoglobin is 13 g/dL in men and 12 g/dL in women. Administering a packed RBC transfusion only when a patient’s hemoglobin falls below 7 or 8 g/dL has been widely adopted, but it is unclear if patients with acute MI may benefit from a higher hemoglobin level.
“Blood transfusion may decrease ischemic injury by improving oxygen delivery to myocardial tissues and reduce the risk of reinfarction or death,” the researchers wrote. “Alternatively, administering more blood could result in more frequent heart failure from fluid overload, infection from immunosuppression, thrombosis from higher viscosity, and inflammation.”
From 2017 to 2023, investigators enrolled 3,504 adults aged 18 and older at 144 sites in the United States (2,157 patients), Canada (885), France (323), Brazil (105), New Zealand (25), and Australia (9).
The participants had ST-elevation or non–ST-elevation MI and hemoglobin less than 10 g/dL within 24 hours. Patients with type 1 (atherosclerotic plaque disruption), type 2 (supply-demand mismatch without atherothrombotic plaque disruption), type 4b, or type 4c MI were eligible.
They were randomly assigned to receive:
- A ‘restrictive’ transfusion strategy (1,749 patients): Transfusion was permitted but not required when a patient’s hemoglobin was less than 8 g/dL and was strongly recommended when it was less than 7 g/dL or when anginal symptoms were not controlled with medications.
- A ‘liberal’ transfusion strategy (1,755 patients): One unit of RBCs was administered after randomization, and RBCs were transfused to maintain hemoglobin 10 g/dL or higher until hospital discharge or 30 days.
The patients had a mean age of 72 years and 46% were women. More than three-quarters (78%) were White and 14% were Black. They had frequent coexisting illnesses, about a third had a history of MI, percutaneous coronary intervention, or heart failure; 14% were on a ventilator and 12% had renal dialysis. The median duration of hospitalization was 5 days in the two groups.
At baseline, the mean hemoglobin was 8.6 g/dL in both groups. At days 1, 2, and 3, the mean hemoglobin was 8.8, 8.9, and 8.9 g/dL, respectively, in the restrictive transfusion group, and 10.1, 10.4, and 10.5 g/dL, respectively, in the liberal transfusion group.
The mean number of transfused blood units was 0.7 units in the restrictive strategy group and 2.5 units in the liberal strategy group, roughly a 3.5-fold difference.
After adjustment for site and incomplete follow-up in 57 patients (20 with the restrictive strategy and 37 with the liberal strategy), the estimated RR for the primary outcome in the restrictive group versus the liberal group was 1.15 (P = .07).
“We observed that the 95% confidence interval contains values that suggest a clinical benefit for the liberal transfusion strategy and does not include values that suggest a benefit for the more restrictive transfusion strategy,” the researchers wrote. Heart failure and other safety outcomes were comparable in the two groups.
The trial was supported by grants from the National Heart, Lung, and Blood Institute and by the Canadian Blood Services and Canadian Institutes of Health Research Institute of Circulatory and Respiratory Health. Dr. Carson, Dr. Leon, Dr. Gibson, and Dr. Mazer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In patients with myocardial infarction and anemia, a “liberal” red blood cell transfusion strategy did not significantly reduce the risk of recurrent MI or death within 30 days, compared with a “restrictive” transfusion strategy, in the 3,500-patient MINT trial.
Jeffrey L. Carson, MD, from Robert Wood Johnson Medical School, New Brunswick, N.J., said in a press briefing.
He presented the study in a late-breaking trial session at the annual scientific sessions of the American Heart Association, and it was simultaneously published online in the New England Journal of Medicine.
“Whether to transfuse is an everyday decision faced by clinicians caring for patients with acute MI,” Dr. Carson said.
“We cannot claim that a liberal transfusion strategy is definitively superior based on our primary outcome,” he said, but “the 95% confidence interval is consistent with treatment effects corresponding to no difference between the two transfusion strategies and to a clinically relevant benefit with the liberal strategy.”
“In contrast to other trials in other settings,” such as anemia and cardiac surgery, Dr. Carson said, “the results suggest that a liberal transfusion strategy has the potential for clinical benefit with an acceptable risk of harm.”
“A liberal transfusion strategy may be the most prudent approach to transfusion in anemic patients with MI,” he added.
Not a home run
Others agreed with this interpretation. Martin B. Leon, MD, from Columbia University, New York, the study discussant in the press briefing, said the study “addresses a question that is common” in clinical practice. It was well conducted, and international (although most patients were in the United States and Canada), in a very broad group of patients, designed to make the results more generalizable. The 98% follow-up was extremely good, Dr. Leon added, and the trialists achieved their goal in that they did show a difference between the two transfusion strategies.
The number needed to treat was 40 to see a benefit in the combined outcome of death or recurrent MI at 30 days, Dr. Leon said. The P value for this was .07, “right on the edge” of statistical significance.
This study is “not a home run,” for the primary outcome, he noted; however, many of the outcomes tended to be in favor of a liberal transfusion strategy. Notably, cardiovascular death, which was not a specified outcome, was significantly lower in the group who received a liberal transfusion strategy.
Although a liberal transfusion strategy was “not definitely superior” in these patients with MI and anemia, Dr. Carson said, he thinks the trial will be interpreted as favoring a liberal transfusion strategy.
C. Michael Gibson, MD, professor of medicine at Harvard Medical School, Boston, and CEO of Harvard’s Baim and PERFUSE institutes for clinical research, voiced similar views.
“Given the lack of acute harm associated with liberal transfusion and the preponderance of evidence favoring liberal transfusion in the largest trial to date,” concluded Dr. Gibson, the assigned discussant at the session, “liberal transfusion appears to be a viable management strategy, particularly among patients with non-STEMI type 1 MI and as clinical judgment dictates.”
Only three small randomized controlled trials have compared transfusion thresholds in a total of 820 patients with MI and anemia, Dr. Gibson said, a point that the trial investigators also made. The results were inconsistent between trials: the CRIT trial (n = 45) favored a restrictive strategy, the MINT pilot study (n = 110) favored a liberal one, and the REALITY trial (n = 668) showed noninferiority of a restrictive strategy, compared with a liberal strategy in 30-day MACE.
The MINT trial was four times larger than all prior studies combined. However, most outcomes were negative or of borderline significance for benefit.
Cardiac death was more common in the restrictive group at 5.5% than the liberal group at 3.2% (risk ratio, 1.74, 95% CI, 1.26-2.40), but this was nonadjudicated, and not designated as a primary, secondary, or tertiary outcome – which the researchers also noted. Fewer than half of the deaths were classified as cardiac, which was “odd,” Dr. Gibson observed.
A restrictive transfusion strategy was associated with increased events among participants with type 1 MI (RR, 1.32, 95% CI, 1.04-1.67), he noted.
Study strengths included that 45.5% of participants were women, Dr. Gibson said. Limitations included that the trial was “somewhat underpowered.” Also, even in the restrictive group, participants received a mean of 0.7 units of packed red blood cells.
Adherence to the 10 g/dL threshold in the liberal transfusion group was moderate (86.3% at hospital discharge), which the researchers acknowledged. They noted that this was frequently caused by clinical discretion, such as concern about fluid overload, and to the timing of hospital discharge. In addition, long-term potential for harm (microchimerism) is not known.
“There was a consistent nonsignificant acute benefit for liberal transfusion and a nominal reduction in CV mortality and improved outcomes in patients with type 1 MI in exploratory analyses, in a trial that ended up underpowered,” Dr. Gibson summarized. “Long-term follow up would be helpful to evaluate chronic outcomes.”
This is a very well-conducted, high-quality, important study that will be considered a landmark trial, C. David Mazer, MD, University of Toronto and St. Michael’s Hospital, also in Toronto, said in an interview.
Unfortunately, “it was not as definitive as hoped for,” Dr. Mazer lamented. Nevertheless, “I think people may interpret it as providing support for a liberal transfusion strategy” in patients with anemia and MI, he said.
Dr. Mazer, who was not involved with this research, was a principal investigator on the TRICS-3 trial, which disputed a liberal RBC transfusion strategy in patients with anemia undergoing cardiac surgery, as previously reported.
The “Red Blood Cell Transfusion: 2023 AABB International Guidelines,” led by Dr. Carson and published in JAMA, recommend a restrictive strategy in stable patients, although these guidelines did not include the current study, Dr. Mazer observed.
In the REALITY trial, there were fewer major adverse cardiac events (MACE) events in the restrictive strategy, he noted.
MINT can be viewed as comparing a high versus low hemoglobin threshold. “It is possible that the best is in between,” he said.
Dr. Mazer also noted that MINT may have achieved significance if it was designed with a larger enrollment and a higher power (for example, 90% instead of 80%) to detect between-group difference for the primary outcome.
Study rationale, design, and findings
Anemia, or low RBC count, is common in patients with MI, Dr. Carson noted. A normal hemoglobin is 13 g/dL in men and 12 g/dL in women. Administering a packed RBC transfusion only when a patient’s hemoglobin falls below 7 or 8 g/dL has been widely adopted, but it is unclear if patients with acute MI may benefit from a higher hemoglobin level.
“Blood transfusion may decrease ischemic injury by improving oxygen delivery to myocardial tissues and reduce the risk of reinfarction or death,” the researchers wrote. “Alternatively, administering more blood could result in more frequent heart failure from fluid overload, infection from immunosuppression, thrombosis from higher viscosity, and inflammation.”
From 2017 to 2023, investigators enrolled 3,504 adults aged 18 and older at 144 sites in the United States (2,157 patients), Canada (885), France (323), Brazil (105), New Zealand (25), and Australia (9).
The participants had ST-elevation or non–ST-elevation MI and hemoglobin less than 10 g/dL within 24 hours. Patients with type 1 (atherosclerotic plaque disruption), type 2 (supply-demand mismatch without atherothrombotic plaque disruption), type 4b, or type 4c MI were eligible.
They were randomly assigned to receive:
- A ‘restrictive’ transfusion strategy (1,749 patients): Transfusion was permitted but not required when a patient’s hemoglobin was less than 8 g/dL and was strongly recommended when it was less than 7 g/dL or when anginal symptoms were not controlled with medications.
- A ‘liberal’ transfusion strategy (1,755 patients): One unit of RBCs was administered after randomization, and RBCs were transfused to maintain hemoglobin 10 g/dL or higher until hospital discharge or 30 days.
The patients had a mean age of 72 years and 46% were women. More than three-quarters (78%) were White and 14% were Black. They had frequent coexisting illnesses, about a third had a history of MI, percutaneous coronary intervention, or heart failure; 14% were on a ventilator and 12% had renal dialysis. The median duration of hospitalization was 5 days in the two groups.
At baseline, the mean hemoglobin was 8.6 g/dL in both groups. At days 1, 2, and 3, the mean hemoglobin was 8.8, 8.9, and 8.9 g/dL, respectively, in the restrictive transfusion group, and 10.1, 10.4, and 10.5 g/dL, respectively, in the liberal transfusion group.
The mean number of transfused blood units was 0.7 units in the restrictive strategy group and 2.5 units in the liberal strategy group, roughly a 3.5-fold difference.
After adjustment for site and incomplete follow-up in 57 patients (20 with the restrictive strategy and 37 with the liberal strategy), the estimated RR for the primary outcome in the restrictive group versus the liberal group was 1.15 (P = .07).
“We observed that the 95% confidence interval contains values that suggest a clinical benefit for the liberal transfusion strategy and does not include values that suggest a benefit for the more restrictive transfusion strategy,” the researchers wrote. Heart failure and other safety outcomes were comparable in the two groups.
The trial was supported by grants from the National Heart, Lung, and Blood Institute and by the Canadian Blood Services and Canadian Institutes of Health Research Institute of Circulatory and Respiratory Health. Dr. Carson, Dr. Leon, Dr. Gibson, and Dr. Mazer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AHA 2023
Renewing the dream
The dream of family practice began more than 6 decades ago with a movement toward personal physicians who have “… the feeling of warm personal regard and concern of doctor for patient, the feeling that the doctor treats people, not illnesses ….” The personal family physician helps patients “… not because of the interesting medical problems they may present but because they are human beings in need of help.”1 One of the most influential founders of family medicine, Dr. Gayle Stephens, expounded on this idea in a series of essays that tapped into the intellectual, philosophical, historical, and moral underpinnings of our discipline.2
Following the dream and the birth of family medicine—like any organization—its lifecycle can be envisioned as proceeding through the rest of the 7 stages of organizational life (TABLE).3 Now allow me to give you some numbers. There are more than 118,000 family physicians in the United States, 784 family medicine residencies filled by 4530 medical school graduates, more than 150 departments of family medicine, multiple national family medicine organizations, and even a World Organization of Family Doctors.4,5 The American Board of Family Medicine is the second largest medical specialty board in the country. Family doctors make up nearly 40% of our total primary care workforce.6 We launched the venture, got organized, made it. We are an institution.
The threat at the institution stage is that we are on the precipice of “closing in.” Many factors are driving this stage: commoditization in health care, market influences and competition for patients, alternative primary care models, erosion of the patient-physician relationship (partly driven by technology), narrowing scope of care, clinician burnout, and the challenges of implementing value-based care, to name a few. You see what comes next in the TABLE.3 The good news is that there is an alternative to the “natural” progression to the ending stage: the path of renewal.3

In the lifecycle of an organization, the path of renewal starts the cycle anew, with dreaming the dream. I recently had the opportunity to visit Singapore to learn about their health system. Singapore is one of the wealthiest countries in the world. I was impressed with their many innovations, including technological ones, as well as new models of care. However, I was most impressed that the country is betting big on family medicine. Their Ministry of Health has launched an initiative they are calling Healthier SG.7 The goal is for “all Singaporeans to have a trusted and lifelong relationship with [their] family doctor.” Their dream is to bring personal doctoring to everyone in the country to make Singapore healthier.
While their path of renewal is occurring halfway around the world, here at home, our path of renewal has been ignited over the past several years by the work of the Robert Graham Center; the Keystone Conferences; the American Board of Family Medicine; and the National Academies of Science, Engineering, and Medicine, among others.8-11 These organizations are aligning around re-centering on patient-clinician relationships, measuring what is important, care by interprofessional teams, payment reform, professionalism, health equity, improved information technology, and adherence to the best available evidence. We are working toward the solution shop as opposed to the production line.12 We are indeed dreaming a new dream.
While I write about this renewal, I close with an ending. This is the final issue of The Journal of Family Practice. It marks the end of an era of nearly 50 years of publication. The Journal of Family Practice has left a lasting mark, providing generations of clinicians with evidence-based, practical guidance to help care for patients as well as serving as an important venue for scholarly work by the family medicine community. Although I have had the privilege of serving the discipline as an editor-in-chief for only a brief time, I am grateful I had the opportunity. Most of all, I appreciate being on the journey of family medicine with you, renewing the dream together.
The references for this Editorial are available in the online version of the article at www.mdedge.com/familymedicine.
1. Fox TF. The personal doctor and his relation to the hospital. Observations and reflections on some American experiments in general practice by groups. Lancet. 1960;2:743-760.
2. Stephens, GG. The Intellectual Basis of Family Practice. Winter Publishing and Society of Teachers of Family Medicine; 1982.
3. Bridges W, Bridges S. Managing Transitions: Making the Most of Change. 4th ed. Da Capo Press; 2016.
4. Association of American Medical Colleges. Physician specialty data report. Accessed October 25, 2023. www.aamc.org/data-reports/workforce/data/active-physicians-us-doctor-medicine-us-md-degree-specialty-2019
5. American Academy of Family Physicians. 2023 match results for family medicine. Accessed October 25, 2023. www.aafp.org/students-residents/residency-program-directors/national-resident-matching-program-results.html
6. Robert Graham Center. Primary Care in the US: A Chartbook of Facts and Statistics. Accessed October 25, 2023. www.graham-center.org/content/dam/rgc/documents/publications-reports/reports/PrimaryCareChartbook2021.pdf
7. Ministry of Health Singapore. What is Healthier SG? Accessed October 25, 2023. www.healthiersg.gov.sg/about/what-is-healthier-sg/
8. The Robert Graham Center. Accessed October 25, 2023. www.graham-center.org/home.html
9. Stange KC. Holding on and letting go: a perspective from the Keystone IV Conference. J Am Board Fam Med. 2016;29:S32-S39.
10. American Board of Family Medicine. Family medicine certification. Accessed October 25, 2023. www.theabfm.org/research-articles/family-medicine-certification?page=1
11. National Academies of Sciences, Engineering, and Medicine. Implementing high-quality primary care. Accessed October 25, 2023. www.nationalacademies.org/our-work/implementing-high-quality-primary-care
12. Sinsky CA, Panzer J. The solution shop and the production line—the case for a frameshift for physician practices. N Engl J Med. 2022;386:2452-2453.
The dream of family practice began more than 6 decades ago with a movement toward personal physicians who have “… the feeling of warm personal regard and concern of doctor for patient, the feeling that the doctor treats people, not illnesses ….” The personal family physician helps patients “… not because of the interesting medical problems they may present but because they are human beings in need of help.”1 One of the most influential founders of family medicine, Dr. Gayle Stephens, expounded on this idea in a series of essays that tapped into the intellectual, philosophical, historical, and moral underpinnings of our discipline.2
Following the dream and the birth of family medicine—like any organization—its lifecycle can be envisioned as proceeding through the rest of the 7 stages of organizational life (TABLE).3 Now allow me to give you some numbers. There are more than 118,000 family physicians in the United States, 784 family medicine residencies filled by 4530 medical school graduates, more than 150 departments of family medicine, multiple national family medicine organizations, and even a World Organization of Family Doctors.4,5 The American Board of Family Medicine is the second largest medical specialty board in the country. Family doctors make up nearly 40% of our total primary care workforce.6 We launched the venture, got organized, made it. We are an institution.
The threat at the institution stage is that we are on the precipice of “closing in.” Many factors are driving this stage: commoditization in health care, market influences and competition for patients, alternative primary care models, erosion of the patient-physician relationship (partly driven by technology), narrowing scope of care, clinician burnout, and the challenges of implementing value-based care, to name a few. You see what comes next in the TABLE.3 The good news is that there is an alternative to the “natural” progression to the ending stage: the path of renewal.3

In the lifecycle of an organization, the path of renewal starts the cycle anew, with dreaming the dream. I recently had the opportunity to visit Singapore to learn about their health system. Singapore is one of the wealthiest countries in the world. I was impressed with their many innovations, including technological ones, as well as new models of care. However, I was most impressed that the country is betting big on family medicine. Their Ministry of Health has launched an initiative they are calling Healthier SG.7 The goal is for “all Singaporeans to have a trusted and lifelong relationship with [their] family doctor.” Their dream is to bring personal doctoring to everyone in the country to make Singapore healthier.
While their path of renewal is occurring halfway around the world, here at home, our path of renewal has been ignited over the past several years by the work of the Robert Graham Center; the Keystone Conferences; the American Board of Family Medicine; and the National Academies of Science, Engineering, and Medicine, among others.8-11 These organizations are aligning around re-centering on patient-clinician relationships, measuring what is important, care by interprofessional teams, payment reform, professionalism, health equity, improved information technology, and adherence to the best available evidence. We are working toward the solution shop as opposed to the production line.12 We are indeed dreaming a new dream.
While I write about this renewal, I close with an ending. This is the final issue of The Journal of Family Practice. It marks the end of an era of nearly 50 years of publication. The Journal of Family Practice has left a lasting mark, providing generations of clinicians with evidence-based, practical guidance to help care for patients as well as serving as an important venue for scholarly work by the family medicine community. Although I have had the privilege of serving the discipline as an editor-in-chief for only a brief time, I am grateful I had the opportunity. Most of all, I appreciate being on the journey of family medicine with you, renewing the dream together.
The references for this Editorial are available in the online version of the article at www.mdedge.com/familymedicine.
The dream of family practice began more than 6 decades ago with a movement toward personal physicians who have “… the feeling of warm personal regard and concern of doctor for patient, the feeling that the doctor treats people, not illnesses ….” The personal family physician helps patients “… not because of the interesting medical problems they may present but because they are human beings in need of help.”1 One of the most influential founders of family medicine, Dr. Gayle Stephens, expounded on this idea in a series of essays that tapped into the intellectual, philosophical, historical, and moral underpinnings of our discipline.2
Following the dream and the birth of family medicine—like any organization—its lifecycle can be envisioned as proceeding through the rest of the 7 stages of organizational life (TABLE).3 Now allow me to give you some numbers. There are more than 118,000 family physicians in the United States, 784 family medicine residencies filled by 4530 medical school graduates, more than 150 departments of family medicine, multiple national family medicine organizations, and even a World Organization of Family Doctors.4,5 The American Board of Family Medicine is the second largest medical specialty board in the country. Family doctors make up nearly 40% of our total primary care workforce.6 We launched the venture, got organized, made it. We are an institution.
The threat at the institution stage is that we are on the precipice of “closing in.” Many factors are driving this stage: commoditization in health care, market influences and competition for patients, alternative primary care models, erosion of the patient-physician relationship (partly driven by technology), narrowing scope of care, clinician burnout, and the challenges of implementing value-based care, to name a few. You see what comes next in the TABLE.3 The good news is that there is an alternative to the “natural” progression to the ending stage: the path of renewal.3

In the lifecycle of an organization, the path of renewal starts the cycle anew, with dreaming the dream. I recently had the opportunity to visit Singapore to learn about their health system. Singapore is one of the wealthiest countries in the world. I was impressed with their many innovations, including technological ones, as well as new models of care. However, I was most impressed that the country is betting big on family medicine. Their Ministry of Health has launched an initiative they are calling Healthier SG.7 The goal is for “all Singaporeans to have a trusted and lifelong relationship with [their] family doctor.” Their dream is to bring personal doctoring to everyone in the country to make Singapore healthier.
While their path of renewal is occurring halfway around the world, here at home, our path of renewal has been ignited over the past several years by the work of the Robert Graham Center; the Keystone Conferences; the American Board of Family Medicine; and the National Academies of Science, Engineering, and Medicine, among others.8-11 These organizations are aligning around re-centering on patient-clinician relationships, measuring what is important, care by interprofessional teams, payment reform, professionalism, health equity, improved information technology, and adherence to the best available evidence. We are working toward the solution shop as opposed to the production line.12 We are indeed dreaming a new dream.
While I write about this renewal, I close with an ending. This is the final issue of The Journal of Family Practice. It marks the end of an era of nearly 50 years of publication. The Journal of Family Practice has left a lasting mark, providing generations of clinicians with evidence-based, practical guidance to help care for patients as well as serving as an important venue for scholarly work by the family medicine community. Although I have had the privilege of serving the discipline as an editor-in-chief for only a brief time, I am grateful I had the opportunity. Most of all, I appreciate being on the journey of family medicine with you, renewing the dream together.
The references for this Editorial are available in the online version of the article at www.mdedge.com/familymedicine.
1. Fox TF. The personal doctor and his relation to the hospital. Observations and reflections on some American experiments in general practice by groups. Lancet. 1960;2:743-760.
2. Stephens, GG. The Intellectual Basis of Family Practice. Winter Publishing and Society of Teachers of Family Medicine; 1982.
3. Bridges W, Bridges S. Managing Transitions: Making the Most of Change. 4th ed. Da Capo Press; 2016.
4. Association of American Medical Colleges. Physician specialty data report. Accessed October 25, 2023. www.aamc.org/data-reports/workforce/data/active-physicians-us-doctor-medicine-us-md-degree-specialty-2019
5. American Academy of Family Physicians. 2023 match results for family medicine. Accessed October 25, 2023. www.aafp.org/students-residents/residency-program-directors/national-resident-matching-program-results.html
6. Robert Graham Center. Primary Care in the US: A Chartbook of Facts and Statistics. Accessed October 25, 2023. www.graham-center.org/content/dam/rgc/documents/publications-reports/reports/PrimaryCareChartbook2021.pdf
7. Ministry of Health Singapore. What is Healthier SG? Accessed October 25, 2023. www.healthiersg.gov.sg/about/what-is-healthier-sg/
8. The Robert Graham Center. Accessed October 25, 2023. www.graham-center.org/home.html
9. Stange KC. Holding on and letting go: a perspective from the Keystone IV Conference. J Am Board Fam Med. 2016;29:S32-S39.
10. American Board of Family Medicine. Family medicine certification. Accessed October 25, 2023. www.theabfm.org/research-articles/family-medicine-certification?page=1
11. National Academies of Sciences, Engineering, and Medicine. Implementing high-quality primary care. Accessed October 25, 2023. www.nationalacademies.org/our-work/implementing-high-quality-primary-care
12. Sinsky CA, Panzer J. The solution shop and the production line—the case for a frameshift for physician practices. N Engl J Med. 2022;386:2452-2453.
1. Fox TF. The personal doctor and his relation to the hospital. Observations and reflections on some American experiments in general practice by groups. Lancet. 1960;2:743-760.
2. Stephens, GG. The Intellectual Basis of Family Practice. Winter Publishing and Society of Teachers of Family Medicine; 1982.
3. Bridges W, Bridges S. Managing Transitions: Making the Most of Change. 4th ed. Da Capo Press; 2016.
4. Association of American Medical Colleges. Physician specialty data report. Accessed October 25, 2023. www.aamc.org/data-reports/workforce/data/active-physicians-us-doctor-medicine-us-md-degree-specialty-2019
5. American Academy of Family Physicians. 2023 match results for family medicine. Accessed October 25, 2023. www.aafp.org/students-residents/residency-program-directors/national-resident-matching-program-results.html
6. Robert Graham Center. Primary Care in the US: A Chartbook of Facts and Statistics. Accessed October 25, 2023. www.graham-center.org/content/dam/rgc/documents/publications-reports/reports/PrimaryCareChartbook2021.pdf
7. Ministry of Health Singapore. What is Healthier SG? Accessed October 25, 2023. www.healthiersg.gov.sg/about/what-is-healthier-sg/
8. The Robert Graham Center. Accessed October 25, 2023. www.graham-center.org/home.html
9. Stange KC. Holding on and letting go: a perspective from the Keystone IV Conference. J Am Board Fam Med. 2016;29:S32-S39.
10. American Board of Family Medicine. Family medicine certification. Accessed October 25, 2023. www.theabfm.org/research-articles/family-medicine-certification?page=1
11. National Academies of Sciences, Engineering, and Medicine. Implementing high-quality primary care. Accessed October 25, 2023. www.nationalacademies.org/our-work/implementing-high-quality-primary-care
12. Sinsky CA, Panzer J. The solution shop and the production line—the case for a frameshift for physician practices. N Engl J Med. 2022;386:2452-2453.
55-year-old woman • myalgias and progressive symmetrical proximal weakness • history of type 2 diabetes and hyperlipidemia • Dx?
THE CASE
A 55-year-old woman developed subacute progression of myalgias and subjective weakness in her proximal extremities after starting a new exercise regimen. The patient had a history of unilateral renal agenesis, type 2 diabetes, and hyperlipidemia, for which she had taken atorvastatin 40 mg/d for several years before discontinuing it 2 years earlier for unknown reasons. She had been evaluated multiple times in the primary care clinic and emergency department over the previous month. Each time, her strength was minimally reduced in the upper extremities on examination, her renal function and electrolytes were normal, and her creatine kinase (CK) level was elevated (16,000-20,000 U/L; normal range, 26-192 U/L). She was managed conservatively with fluids and given return precautions each time.
After her myalgias and weakness increased in severity, she presented to the emergency department with a muscle strength score of 4/5 in both shoulders, triceps, hip flexors, hip extensors, abductors, and adductors. Her laboratory results were significant for the presence of blood without red blood cells on her urine dipstick test and a CK level of 25,070 U/L. She was admitted for further evaluation of progressive myopathy and given aggressive IV fluid hydration to prevent renal injury based on her history of unilateral renal agenesis.
Infectious disease testing, which included a respiratory virus panel, acute hepatitis panel, HIV screening, Lyme antibody testing, cytomegalovirus DNA detection by polymerase chain reaction, Epstein-Barr virus capsid immunoglobulin M, and anti-streptolysin O, were negative. Electrolytes, inflammatory markers, and kidney function were normal. However, high-sensitivity troponin-T levels were elevated, with a peak value of 216.3 ng/L (normal range, 0-19 ng/L). The patient denied having any chest pain, and her electrocardiogram and transthoracic echocardiogram were normal. By hospital Day 4, her myalgias and weakness had improved, CK had stabilized (19,000-21,000 U/L), cardiac enzymes had improved, and urinalysis had normalized. She was discharged with a referral to a rheumatologist.
However, 10 days later—before she could see a rheumatologist—she was readmitted to a community hospital for recurrence of severe myalgias, progressive weakness, positive blood on urine dipstick testing, and a rising CK level (to 24,580 U/L) found during a follow-up appointment with her primary care physician. At this point, Neurology and Rheumatology were consulted and myositis-specific and myositis-associated autoantibody tests were sent out. Magnetic resonance imaging (MRI) of her thighs was performed and showed diffusely increased T2 signal and short tau inversion recovery in multiple proximal muscles (FIGURE).
DIAGNOSIS
Given her symmetrical proximal muscle weakness (which was refractory to IV fluid resuscitation), MRI findings, and the exclusion of infection and metabolic derangements, the patient was given a working diagnosis of myositis and treated with 1-g IV methylprednisolone followed by a 4-month steroid taper, methotrexate 20 mg weekly, and physical therapy. This working diagnosis was later confirmed with the results of her autoantibody tests.
At her 1-month follow-up visit, the patient reported minimal improvement in her strength, new neck weakness, and dysphagia with solids. Testing revealed anti–3-hydroxy-3-methylglutaryl-coenzyme A reductase (anti-HMGCR) antibody levels of more than 200 U/L (negative < 20 U/L; positive > 59 U/L), which pointed to a more refined diagnosis of anti-HMGCR immune-mediated necrotizing myositis.
DISCUSSION
Myositis should be in the differential diagnosis for patients with symmetrical proximal muscle weakness. Bohan and Peter devised a 5-part set of criteria to help diagnose myositis, shown in the TABLE.1,2 This simple framework broadens the differential and guides diagnostic testing. Our patient’s presentation was fairly typical for anti-HMGCR myositis, a subset of immune-mediated necrotizing myositis,3 with a pretest probability of 62% per the European League Against Rheumatism/American College of Rheumatology classification criteria.2 Probability of this diagnosis was further increased by the high-titer anti-HMGCR, so biopsy and electromyography (EMG), as noted by Bohan and Peter, were not pursued.
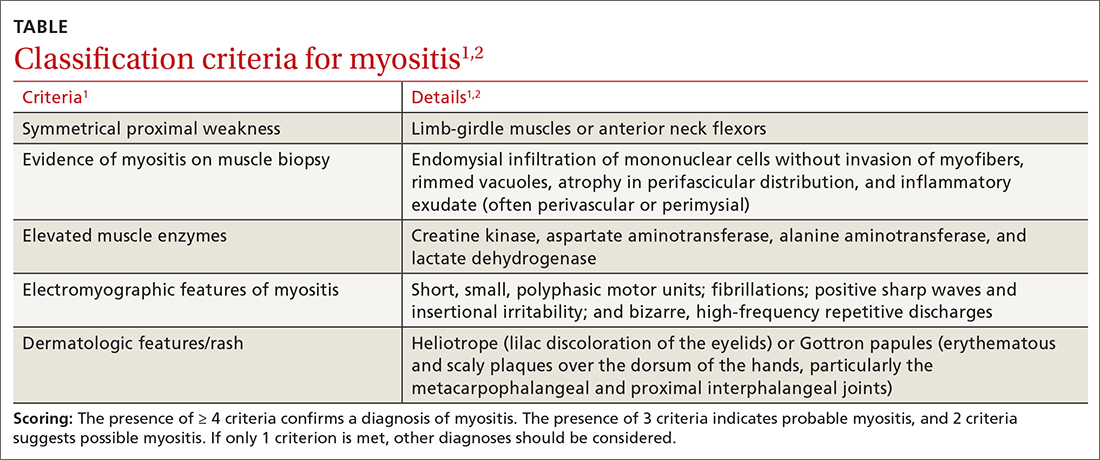
Continue to: Autoimmune myopathies...
Autoimmune myopathies occur in 9 to 14 per 100,000 people,4 with 6% of patients having anti-HMGCR auto-antibodies.5 Anti-HMGCR myositis is more prevalent in older women, patients with type 2 diabetes, and those with a history of atorvastatin use.3,6 Two-thirds of patients with anti-HMGCR myositis report current or prior statin use, and this increases to more than 90% in those age 50 years or older.5 Anti-HMGCR myositis causes significant muscle weakness that does not resolve with discontinuation of the statin and can occur years after the initiation or discontinuation of statin treatment.6 Cardiac involvement is rare4 but dysphagia is relatively common.7,8 Anti-HMGCR myositis also has a weak association with cancer, most commonly gastrointestinal and lung cancers.4,7
Distinguishing statin-induced myalgias from statin-induced myositis guides management. Statin-induced myalgias are associated with normal or slightly increased CK levels (typically < 1000 U/L) and resolve with discontinuation of the statin; the patient can often tolerate re-challenge with a statin.6 In contrast, CK elevation in patients with statin-induced myositis is typically more than 10,000 U/L6 and requires aggressive treatment with immunomodulatory medications to prevent permanent muscle damage.
Treatment recommendations are supported only by case series, observational studies, and expert opinion. Typical first-line treatment includes induction with high-dose corticosteroids followed by prolonged taper plus a conventional synthetic disease-modifying antirheumatic drug (csDMARD) such as methotrexate, azathioprine, or mycophenolate.4 Maintenance therapy often is achieved with csDMARD therapy for 2 years.4 Severe cases frequently are treated with combination csDMARD therapy (eg, methotrexate and azathioprine or methotrexate and mycophenolate).4 Rituximab and IV immunoglobulin (IVIG) are typically reserved for refractory cases.6 Usual monitoring for relapse includes muscle strength testing on examination and evaluation of trending CK levels.8
Our patient received monthly 2-g/kg IVIG infusions, which led to slow, consistent improvement in her strength and normalization of her CK levels to 181 U/L after 6 months.
THE TAKEAWAY
Anti-HMGCR myositis should be suspected in any patient currently or previously treated with a statin who presents with proximal muscle weakness, myalgias, or an elevated CK level. We suggest early subspecialty consultation to discuss whether antibody testing, EMG, or muscle biopsy are warranted. If anti-HMGCR myositis is confirmed, it is advisable to rule out comorbid malignancy and initiate early combination treatment to minimize relapses and permanent muscle damage.
CORRESPONDENCE
Daniel T. Schoenherr, MD, Family Medicine Residency, National Capital Consortium–Alexander T. Augusta Military Medical Center, 9300 DeWitt Loop, Fort Belvoir, VA 22060; [email protected]
1. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347. doi: 10.1056/NEJM197502132920706
2. Bottai M, Tjärnlund A, Santoni G, et al. EULAR/ACR classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups: a methodology report. RMD Open. 2017;3:e000507. doi: 10.1136/rmdopen-2017-000507
3. Basharat P, Lahouti AH, Paik JJ, et al. Statin-induced anti-HMGCR-associated myopathy. J Am Coll Cardiol. 2016;68:234-235. doi: 10.1016/j.jacc.2016.04.037
4. Pinal-Fernandez I, Casal-Dominguez M, Mammen AL. Immune-mediated necrotizing myopathy. Curr Rheumatol Rep. 2018;20:21. doi: 10.1007/s11926-018-0732-6
5. Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 2011;63:713-721. doi: 10.1002/art.30156
6. Irvine NJ. Anti-HMGCR myopathy: a rare and serious side effect of statins. J Am Board Fam Med. 2020;33:785-788. doi: 10.3122/jabfm.2020.05.190450
7. Basharat P, Christopher-Stine L. Immune-mediated necrotizing myopathy: update on diagnosis and management. Curr Rheumatol Rep. 2015;17:72. doi: 10.1007/s11926-015-0548-6
8. Betteridge Z, McHugh N. Myositis-specific autoantibodies: an important tool to support diagnosis of myositis. J Int Med. 2016;280:8-23. doi: 10.1111/joim.12451
THE CASE
A 55-year-old woman developed subacute progression of myalgias and subjective weakness in her proximal extremities after starting a new exercise regimen. The patient had a history of unilateral renal agenesis, type 2 diabetes, and hyperlipidemia, for which she had taken atorvastatin 40 mg/d for several years before discontinuing it 2 years earlier for unknown reasons. She had been evaluated multiple times in the primary care clinic and emergency department over the previous month. Each time, her strength was minimally reduced in the upper extremities on examination, her renal function and electrolytes were normal, and her creatine kinase (CK) level was elevated (16,000-20,000 U/L; normal range, 26-192 U/L). She was managed conservatively with fluids and given return precautions each time.
After her myalgias and weakness increased in severity, she presented to the emergency department with a muscle strength score of 4/5 in both shoulders, triceps, hip flexors, hip extensors, abductors, and adductors. Her laboratory results were significant for the presence of blood without red blood cells on her urine dipstick test and a CK level of 25,070 U/L. She was admitted for further evaluation of progressive myopathy and given aggressive IV fluid hydration to prevent renal injury based on her history of unilateral renal agenesis.
Infectious disease testing, which included a respiratory virus panel, acute hepatitis panel, HIV screening, Lyme antibody testing, cytomegalovirus DNA detection by polymerase chain reaction, Epstein-Barr virus capsid immunoglobulin M, and anti-streptolysin O, were negative. Electrolytes, inflammatory markers, and kidney function were normal. However, high-sensitivity troponin-T levels were elevated, with a peak value of 216.3 ng/L (normal range, 0-19 ng/L). The patient denied having any chest pain, and her electrocardiogram and transthoracic echocardiogram were normal. By hospital Day 4, her myalgias and weakness had improved, CK had stabilized (19,000-21,000 U/L), cardiac enzymes had improved, and urinalysis had normalized. She was discharged with a referral to a rheumatologist.
However, 10 days later—before she could see a rheumatologist—she was readmitted to a community hospital for recurrence of severe myalgias, progressive weakness, positive blood on urine dipstick testing, and a rising CK level (to 24,580 U/L) found during a follow-up appointment with her primary care physician. At this point, Neurology and Rheumatology were consulted and myositis-specific and myositis-associated autoantibody tests were sent out. Magnetic resonance imaging (MRI) of her thighs was performed and showed diffusely increased T2 signal and short tau inversion recovery in multiple proximal muscles (FIGURE).

DIAGNOSIS
Given her symmetrical proximal muscle weakness (which was refractory to IV fluid resuscitation), MRI findings, and the exclusion of infection and metabolic derangements, the patient was given a working diagnosis of myositis and treated with 1-g IV methylprednisolone followed by a 4-month steroid taper, methotrexate 20 mg weekly, and physical therapy. This working diagnosis was later confirmed with the results of her autoantibody tests.
At her 1-month follow-up visit, the patient reported minimal improvement in her strength, new neck weakness, and dysphagia with solids. Testing revealed anti–3-hydroxy-3-methylglutaryl-coenzyme A reductase (anti-HMGCR) antibody levels of more than 200 U/L (negative < 20 U/L; positive > 59 U/L), which pointed to a more refined diagnosis of anti-HMGCR immune-mediated necrotizing myositis.
DISCUSSION
Myositis should be in the differential diagnosis for patients with symmetrical proximal muscle weakness. Bohan and Peter devised a 5-part set of criteria to help diagnose myositis, shown in the TABLE.1,2 This simple framework broadens the differential and guides diagnostic testing. Our patient’s presentation was fairly typical for anti-HMGCR myositis, a subset of immune-mediated necrotizing myositis,3 with a pretest probability of 62% per the European League Against Rheumatism/American College of Rheumatology classification criteria.2 Probability of this diagnosis was further increased by the high-titer anti-HMGCR, so biopsy and electromyography (EMG), as noted by Bohan and Peter, were not pursued.

Continue to: Autoimmune myopathies...
Autoimmune myopathies occur in 9 to 14 per 100,000 people,4 with 6% of patients having anti-HMGCR auto-antibodies.5 Anti-HMGCR myositis is more prevalent in older women, patients with type 2 diabetes, and those with a history of atorvastatin use.3,6 Two-thirds of patients with anti-HMGCR myositis report current or prior statin use, and this increases to more than 90% in those age 50 years or older.5 Anti-HMGCR myositis causes significant muscle weakness that does not resolve with discontinuation of the statin and can occur years after the initiation or discontinuation of statin treatment.6 Cardiac involvement is rare4 but dysphagia is relatively common.7,8 Anti-HMGCR myositis also has a weak association with cancer, most commonly gastrointestinal and lung cancers.4,7
Distinguishing statin-induced myalgias from statin-induced myositis guides management. Statin-induced myalgias are associated with normal or slightly increased CK levels (typically < 1000 U/L) and resolve with discontinuation of the statin; the patient can often tolerate re-challenge with a statin.6 In contrast, CK elevation in patients with statin-induced myositis is typically more than 10,000 U/L6 and requires aggressive treatment with immunomodulatory medications to prevent permanent muscle damage.
Treatment recommendations are supported only by case series, observational studies, and expert opinion. Typical first-line treatment includes induction with high-dose corticosteroids followed by prolonged taper plus a conventional synthetic disease-modifying antirheumatic drug (csDMARD) such as methotrexate, azathioprine, or mycophenolate.4 Maintenance therapy often is achieved with csDMARD therapy for 2 years.4 Severe cases frequently are treated with combination csDMARD therapy (eg, methotrexate and azathioprine or methotrexate and mycophenolate).4 Rituximab and IV immunoglobulin (IVIG) are typically reserved for refractory cases.6 Usual monitoring for relapse includes muscle strength testing on examination and evaluation of trending CK levels.8
Our patient received monthly 2-g/kg IVIG infusions, which led to slow, consistent improvement in her strength and normalization of her CK levels to 181 U/L after 6 months.
THE TAKEAWAY
Anti-HMGCR myositis should be suspected in any patient currently or previously treated with a statin who presents with proximal muscle weakness, myalgias, or an elevated CK level. We suggest early subspecialty consultation to discuss whether antibody testing, EMG, or muscle biopsy are warranted. If anti-HMGCR myositis is confirmed, it is advisable to rule out comorbid malignancy and initiate early combination treatment to minimize relapses and permanent muscle damage.
CORRESPONDENCE
Daniel T. Schoenherr, MD, Family Medicine Residency, National Capital Consortium–Alexander T. Augusta Military Medical Center, 9300 DeWitt Loop, Fort Belvoir, VA 22060; [email protected]
THE CASE
A 55-year-old woman developed subacute progression of myalgias and subjective weakness in her proximal extremities after starting a new exercise regimen. The patient had a history of unilateral renal agenesis, type 2 diabetes, and hyperlipidemia, for which she had taken atorvastatin 40 mg/d for several years before discontinuing it 2 years earlier for unknown reasons. She had been evaluated multiple times in the primary care clinic and emergency department over the previous month. Each time, her strength was minimally reduced in the upper extremities on examination, her renal function and electrolytes were normal, and her creatine kinase (CK) level was elevated (16,000-20,000 U/L; normal range, 26-192 U/L). She was managed conservatively with fluids and given return precautions each time.
After her myalgias and weakness increased in severity, she presented to the emergency department with a muscle strength score of 4/5 in both shoulders, triceps, hip flexors, hip extensors, abductors, and adductors. Her laboratory results were significant for the presence of blood without red blood cells on her urine dipstick test and a CK level of 25,070 U/L. She was admitted for further evaluation of progressive myopathy and given aggressive IV fluid hydration to prevent renal injury based on her history of unilateral renal agenesis.
Infectious disease testing, which included a respiratory virus panel, acute hepatitis panel, HIV screening, Lyme antibody testing, cytomegalovirus DNA detection by polymerase chain reaction, Epstein-Barr virus capsid immunoglobulin M, and anti-streptolysin O, were negative. Electrolytes, inflammatory markers, and kidney function were normal. However, high-sensitivity troponin-T levels were elevated, with a peak value of 216.3 ng/L (normal range, 0-19 ng/L). The patient denied having any chest pain, and her electrocardiogram and transthoracic echocardiogram were normal. By hospital Day 4, her myalgias and weakness had improved, CK had stabilized (19,000-21,000 U/L), cardiac enzymes had improved, and urinalysis had normalized. She was discharged with a referral to a rheumatologist.
However, 10 days later—before she could see a rheumatologist—she was readmitted to a community hospital for recurrence of severe myalgias, progressive weakness, positive blood on urine dipstick testing, and a rising CK level (to 24,580 U/L) found during a follow-up appointment with her primary care physician. At this point, Neurology and Rheumatology were consulted and myositis-specific and myositis-associated autoantibody tests were sent out. Magnetic resonance imaging (MRI) of her thighs was performed and showed diffusely increased T2 signal and short tau inversion recovery in multiple proximal muscles (FIGURE).

DIAGNOSIS
Given her symmetrical proximal muscle weakness (which was refractory to IV fluid resuscitation), MRI findings, and the exclusion of infection and metabolic derangements, the patient was given a working diagnosis of myositis and treated with 1-g IV methylprednisolone followed by a 4-month steroid taper, methotrexate 20 mg weekly, and physical therapy. This working diagnosis was later confirmed with the results of her autoantibody tests.
At her 1-month follow-up visit, the patient reported minimal improvement in her strength, new neck weakness, and dysphagia with solids. Testing revealed anti–3-hydroxy-3-methylglutaryl-coenzyme A reductase (anti-HMGCR) antibody levels of more than 200 U/L (negative < 20 U/L; positive > 59 U/L), which pointed to a more refined diagnosis of anti-HMGCR immune-mediated necrotizing myositis.
DISCUSSION
Myositis should be in the differential diagnosis for patients with symmetrical proximal muscle weakness. Bohan and Peter devised a 5-part set of criteria to help diagnose myositis, shown in the TABLE.1,2 This simple framework broadens the differential and guides diagnostic testing. Our patient’s presentation was fairly typical for anti-HMGCR myositis, a subset of immune-mediated necrotizing myositis,3 with a pretest probability of 62% per the European League Against Rheumatism/American College of Rheumatology classification criteria.2 Probability of this diagnosis was further increased by the high-titer anti-HMGCR, so biopsy and electromyography (EMG), as noted by Bohan and Peter, were not pursued.

Continue to: Autoimmune myopathies...
Autoimmune myopathies occur in 9 to 14 per 100,000 people,4 with 6% of patients having anti-HMGCR auto-antibodies.5 Anti-HMGCR myositis is more prevalent in older women, patients with type 2 diabetes, and those with a history of atorvastatin use.3,6 Two-thirds of patients with anti-HMGCR myositis report current or prior statin use, and this increases to more than 90% in those age 50 years or older.5 Anti-HMGCR myositis causes significant muscle weakness that does not resolve with discontinuation of the statin and can occur years after the initiation or discontinuation of statin treatment.6 Cardiac involvement is rare4 but dysphagia is relatively common.7,8 Anti-HMGCR myositis also has a weak association with cancer, most commonly gastrointestinal and lung cancers.4,7
Distinguishing statin-induced myalgias from statin-induced myositis guides management. Statin-induced myalgias are associated with normal or slightly increased CK levels (typically < 1000 U/L) and resolve with discontinuation of the statin; the patient can often tolerate re-challenge with a statin.6 In contrast, CK elevation in patients with statin-induced myositis is typically more than 10,000 U/L6 and requires aggressive treatment with immunomodulatory medications to prevent permanent muscle damage.
Treatment recommendations are supported only by case series, observational studies, and expert opinion. Typical first-line treatment includes induction with high-dose corticosteroids followed by prolonged taper plus a conventional synthetic disease-modifying antirheumatic drug (csDMARD) such as methotrexate, azathioprine, or mycophenolate.4 Maintenance therapy often is achieved with csDMARD therapy for 2 years.4 Severe cases frequently are treated with combination csDMARD therapy (eg, methotrexate and azathioprine or methotrexate and mycophenolate).4 Rituximab and IV immunoglobulin (IVIG) are typically reserved for refractory cases.6 Usual monitoring for relapse includes muscle strength testing on examination and evaluation of trending CK levels.8
Our patient received monthly 2-g/kg IVIG infusions, which led to slow, consistent improvement in her strength and normalization of her CK levels to 181 U/L after 6 months.
THE TAKEAWAY
Anti-HMGCR myositis should be suspected in any patient currently or previously treated with a statin who presents with proximal muscle weakness, myalgias, or an elevated CK level. We suggest early subspecialty consultation to discuss whether antibody testing, EMG, or muscle biopsy are warranted. If anti-HMGCR myositis is confirmed, it is advisable to rule out comorbid malignancy and initiate early combination treatment to minimize relapses and permanent muscle damage.
CORRESPONDENCE
Daniel T. Schoenherr, MD, Family Medicine Residency, National Capital Consortium–Alexander T. Augusta Military Medical Center, 9300 DeWitt Loop, Fort Belvoir, VA 22060; [email protected]
1. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347. doi: 10.1056/NEJM197502132920706
2. Bottai M, Tjärnlund A, Santoni G, et al. EULAR/ACR classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups: a methodology report. RMD Open. 2017;3:e000507. doi: 10.1136/rmdopen-2017-000507
3. Basharat P, Lahouti AH, Paik JJ, et al. Statin-induced anti-HMGCR-associated myopathy. J Am Coll Cardiol. 2016;68:234-235. doi: 10.1016/j.jacc.2016.04.037
4. Pinal-Fernandez I, Casal-Dominguez M, Mammen AL. Immune-mediated necrotizing myopathy. Curr Rheumatol Rep. 2018;20:21. doi: 10.1007/s11926-018-0732-6
5. Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 2011;63:713-721. doi: 10.1002/art.30156
6. Irvine NJ. Anti-HMGCR myopathy: a rare and serious side effect of statins. J Am Board Fam Med. 2020;33:785-788. doi: 10.3122/jabfm.2020.05.190450
7. Basharat P, Christopher-Stine L. Immune-mediated necrotizing myopathy: update on diagnosis and management. Curr Rheumatol Rep. 2015;17:72. doi: 10.1007/s11926-015-0548-6
8. Betteridge Z, McHugh N. Myositis-specific autoantibodies: an important tool to support diagnosis of myositis. J Int Med. 2016;280:8-23. doi: 10.1111/joim.12451
1. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347. doi: 10.1056/NEJM197502132920706
2. Bottai M, Tjärnlund A, Santoni G, et al. EULAR/ACR classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups: a methodology report. RMD Open. 2017;3:e000507. doi: 10.1136/rmdopen-2017-000507
3. Basharat P, Lahouti AH, Paik JJ, et al. Statin-induced anti-HMGCR-associated myopathy. J Am Coll Cardiol. 2016;68:234-235. doi: 10.1016/j.jacc.2016.04.037
4. Pinal-Fernandez I, Casal-Dominguez M, Mammen AL. Immune-mediated necrotizing myopathy. Curr Rheumatol Rep. 2018;20:21. doi: 10.1007/s11926-018-0732-6
5. Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 2011;63:713-721. doi: 10.1002/art.30156
6. Irvine NJ. Anti-HMGCR myopathy: a rare and serious side effect of statins. J Am Board Fam Med. 2020;33:785-788. doi: 10.3122/jabfm.2020.05.190450
7. Basharat P, Christopher-Stine L. Immune-mediated necrotizing myopathy: update on diagnosis and management. Curr Rheumatol Rep. 2015;17:72. doi: 10.1007/s11926-015-0548-6
8. Betteridge Z, McHugh N. Myositis-specific autoantibodies: an important tool to support diagnosis of myositis. J Int Med. 2016;280:8-23. doi: 10.1111/joim.12451
► Myalgias and progressive symmetrical proximal weakness
► History of unilateral renal agenesis, type 2 diabetes, and hyperlipidemia
Study: CBD provides symptom relief and improvements in gastroparesis
in a phase 2 randomized double-blinded, placebo-controlled study recently published in Clinical Gastroenterology and Hepatology.
There is “significant unmet medical need in gastroparesis,” and compared with cannabis, which has been used to relieve nausea and pain in patients with the condition, CBD has limited psychic effects with the added potential to reduce gut sensation and inflammation, wrote Ting Zheng, MD, and colleagues at Mayo Clinic in Rochester, Minn.
The researchers assessed the symptoms of 44 patients (21 randomized to receive CBD and 23 to receive placebo) – each of whom had nonsurgical gastroparesis with documented delayed gastric emptying of solids (GES) by scintigraphy for at least 3 months – with the American Neurogastroenterology and Motility Society’s Gastroparesis Cardinal Symptom Index (GCSI) Daily Diary.
They measured GES at baseline, and at 4 weeks, they measured GES again as well as fasting and postprandial gastric volumes and satiation using a validated Ensure drink test. (Patients ingested Ensure [Abbott Laboratories] at a rate of 30 mL/min and recorded their sensations every 5 minutes.) The two treatment arms were compared via 2-way analysis of covariance that included body mass index and, when applicable, baseline measurements.
Patients in the CBD group received twice-daily oral Epidiolex (Jazz Pharmaceuticals, Dublin), which is Food and Drug Administration–approved for the treatment of seizures associated with two rare forms of epilepsy and with another rare genetic disease in patients 1 year of age and older.
The researchers documented significant improvements in the CBD group in total GCSI score (P = .0008) and in scores measuring the inability to finish a normal-sized meal (P = .029), number of vomiting episodes/24 hours (P = .006), and overall perceived severity of symptoms (P = .034).
CBD treatment was also associated with greater tolerated volume of Ensure – “without increases in scores for nausea, fullness, bloating, and pain” – and, in another component of the GCSI, there was “a borderline reduction in upper abdominal pain,” Dr. Zheng and coauthors wrote.
There was a significant slowing of GES in the CBD group, however, and no significant differences were seen at 4 weeks in the fasting or accommodation gastric volumes between the two treatment groups. That beneficial effects of CBD were seen despite slowing of GES “raises the question of the contribution of the delayed GE of solids to development of symptoms in patients with gastroparesis, which is supported by some but not all meta-analyses on this topic,” they noted.
Patients had a mean age of 44 and most were female. Of the 44 patients, 32 had idiopathic gastroparesis, 6 had type 1 diabetes, and 6 had type 2 diabetes. Four patients in the study did not tolerate the FDA-recommended full-dose escalation of CBD to 20 mg/kg per day, but completed the study on the highest tolerated dose.
Adverse effects (fatigue, headache, nausea) were distributed equally between the two groups, but diarrhea was more common in the CBD group. Diarrhea was the most common adverse event in a recently published analysis of 892 pediatric patients receiving Epidiolex over an estimated 1,755.7 patient-years of CBD exposure, the researchers noted.
CBD is a cannabinoid receptor 2 inverse agonist with central nervous system effects, but it also affects visceral or somatic sensation peripherally, the authors noted. The beneficial effects of CBD in gastroparesis are “presumed to reflect effects on sensory mechanisms or anti-inflammatory effects mediated via CBR2 (cannabinoid receptor type 2) reversing the hypersensitivity and intrinsic inflammatory pathogenesis recorded in idiopathic and diabetic gastroparesis,” Dr. Zheng and colleagues wrote. CBD may also, in a mechanism unrelated to CB receptors, inhibit smooth muscle contractile activity, they said.
Larger randomized controlled trials of longer-term administration of CBD in both idiopathic and diabetic gastroparesis are warranted, the investigators said.
The researchers disclosed no conflicts. The study was supported by a grant from the National Institutes of Health.
in a phase 2 randomized double-blinded, placebo-controlled study recently published in Clinical Gastroenterology and Hepatology.
There is “significant unmet medical need in gastroparesis,” and compared with cannabis, which has been used to relieve nausea and pain in patients with the condition, CBD has limited psychic effects with the added potential to reduce gut sensation and inflammation, wrote Ting Zheng, MD, and colleagues at Mayo Clinic in Rochester, Minn.
The researchers assessed the symptoms of 44 patients (21 randomized to receive CBD and 23 to receive placebo) – each of whom had nonsurgical gastroparesis with documented delayed gastric emptying of solids (GES) by scintigraphy for at least 3 months – with the American Neurogastroenterology and Motility Society’s Gastroparesis Cardinal Symptom Index (GCSI) Daily Diary.
They measured GES at baseline, and at 4 weeks, they measured GES again as well as fasting and postprandial gastric volumes and satiation using a validated Ensure drink test. (Patients ingested Ensure [Abbott Laboratories] at a rate of 30 mL/min and recorded their sensations every 5 minutes.) The two treatment arms were compared via 2-way analysis of covariance that included body mass index and, when applicable, baseline measurements.
Patients in the CBD group received twice-daily oral Epidiolex (Jazz Pharmaceuticals, Dublin), which is Food and Drug Administration–approved for the treatment of seizures associated with two rare forms of epilepsy and with another rare genetic disease in patients 1 year of age and older.
The researchers documented significant improvements in the CBD group in total GCSI score (P = .0008) and in scores measuring the inability to finish a normal-sized meal (P = .029), number of vomiting episodes/24 hours (P = .006), and overall perceived severity of symptoms (P = .034).
CBD treatment was also associated with greater tolerated volume of Ensure – “without increases in scores for nausea, fullness, bloating, and pain” – and, in another component of the GCSI, there was “a borderline reduction in upper abdominal pain,” Dr. Zheng and coauthors wrote.
There was a significant slowing of GES in the CBD group, however, and no significant differences were seen at 4 weeks in the fasting or accommodation gastric volumes between the two treatment groups. That beneficial effects of CBD were seen despite slowing of GES “raises the question of the contribution of the delayed GE of solids to development of symptoms in patients with gastroparesis, which is supported by some but not all meta-analyses on this topic,” they noted.
Patients had a mean age of 44 and most were female. Of the 44 patients, 32 had idiopathic gastroparesis, 6 had type 1 diabetes, and 6 had type 2 diabetes. Four patients in the study did not tolerate the FDA-recommended full-dose escalation of CBD to 20 mg/kg per day, but completed the study on the highest tolerated dose.
Adverse effects (fatigue, headache, nausea) were distributed equally between the two groups, but diarrhea was more common in the CBD group. Diarrhea was the most common adverse event in a recently published analysis of 892 pediatric patients receiving Epidiolex over an estimated 1,755.7 patient-years of CBD exposure, the researchers noted.
CBD is a cannabinoid receptor 2 inverse agonist with central nervous system effects, but it also affects visceral or somatic sensation peripherally, the authors noted. The beneficial effects of CBD in gastroparesis are “presumed to reflect effects on sensory mechanisms or anti-inflammatory effects mediated via CBR2 (cannabinoid receptor type 2) reversing the hypersensitivity and intrinsic inflammatory pathogenesis recorded in idiopathic and diabetic gastroparesis,” Dr. Zheng and colleagues wrote. CBD may also, in a mechanism unrelated to CB receptors, inhibit smooth muscle contractile activity, they said.
Larger randomized controlled trials of longer-term administration of CBD in both idiopathic and diabetic gastroparesis are warranted, the investigators said.
The researchers disclosed no conflicts. The study was supported by a grant from the National Institutes of Health.
in a phase 2 randomized double-blinded, placebo-controlled study recently published in Clinical Gastroenterology and Hepatology.
There is “significant unmet medical need in gastroparesis,” and compared with cannabis, which has been used to relieve nausea and pain in patients with the condition, CBD has limited psychic effects with the added potential to reduce gut sensation and inflammation, wrote Ting Zheng, MD, and colleagues at Mayo Clinic in Rochester, Minn.
The researchers assessed the symptoms of 44 patients (21 randomized to receive CBD and 23 to receive placebo) – each of whom had nonsurgical gastroparesis with documented delayed gastric emptying of solids (GES) by scintigraphy for at least 3 months – with the American Neurogastroenterology and Motility Society’s Gastroparesis Cardinal Symptom Index (GCSI) Daily Diary.
They measured GES at baseline, and at 4 weeks, they measured GES again as well as fasting and postprandial gastric volumes and satiation using a validated Ensure drink test. (Patients ingested Ensure [Abbott Laboratories] at a rate of 30 mL/min and recorded their sensations every 5 minutes.) The two treatment arms were compared via 2-way analysis of covariance that included body mass index and, when applicable, baseline measurements.
Patients in the CBD group received twice-daily oral Epidiolex (Jazz Pharmaceuticals, Dublin), which is Food and Drug Administration–approved for the treatment of seizures associated with two rare forms of epilepsy and with another rare genetic disease in patients 1 year of age and older.
The researchers documented significant improvements in the CBD group in total GCSI score (P = .0008) and in scores measuring the inability to finish a normal-sized meal (P = .029), number of vomiting episodes/24 hours (P = .006), and overall perceived severity of symptoms (P = .034).
CBD treatment was also associated with greater tolerated volume of Ensure – “without increases in scores for nausea, fullness, bloating, and pain” – and, in another component of the GCSI, there was “a borderline reduction in upper abdominal pain,” Dr. Zheng and coauthors wrote.
There was a significant slowing of GES in the CBD group, however, and no significant differences were seen at 4 weeks in the fasting or accommodation gastric volumes between the two treatment groups. That beneficial effects of CBD were seen despite slowing of GES “raises the question of the contribution of the delayed GE of solids to development of symptoms in patients with gastroparesis, which is supported by some but not all meta-analyses on this topic,” they noted.
Patients had a mean age of 44 and most were female. Of the 44 patients, 32 had idiopathic gastroparesis, 6 had type 1 diabetes, and 6 had type 2 diabetes. Four patients in the study did not tolerate the FDA-recommended full-dose escalation of CBD to 20 mg/kg per day, but completed the study on the highest tolerated dose.
Adverse effects (fatigue, headache, nausea) were distributed equally between the two groups, but diarrhea was more common in the CBD group. Diarrhea was the most common adverse event in a recently published analysis of 892 pediatric patients receiving Epidiolex over an estimated 1,755.7 patient-years of CBD exposure, the researchers noted.
CBD is a cannabinoid receptor 2 inverse agonist with central nervous system effects, but it also affects visceral or somatic sensation peripherally, the authors noted. The beneficial effects of CBD in gastroparesis are “presumed to reflect effects on sensory mechanisms or anti-inflammatory effects mediated via CBR2 (cannabinoid receptor type 2) reversing the hypersensitivity and intrinsic inflammatory pathogenesis recorded in idiopathic and diabetic gastroparesis,” Dr. Zheng and colleagues wrote. CBD may also, in a mechanism unrelated to CB receptors, inhibit smooth muscle contractile activity, they said.
Larger randomized controlled trials of longer-term administration of CBD in both idiopathic and diabetic gastroparesis are warranted, the investigators said.
The researchers disclosed no conflicts. The study was supported by a grant from the National Institutes of Health.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Specialty-trained pathologists more likely to make higher-grade diagnoses for melanocytic lesions
, results from an exploratory study showed.
The findings “could in part play a role in the rising incidence of early-stage melanoma with low risk of progression or patient morbidity, thereby contributing to increasing rates of overdiagnosis,” researchers led by co–senior authors Joann G. Elmore, MD, MPH, of the University of California, Los Angeles, and Raymond L. Barnhill, MD, MBA, of the Institut Curie, Paris, wrote in their study, published online in JAMA Dermatology.
To investigate the characteristics associated with rendering higher-grade diagnoses, including invasive melanoma, the researchers drew from two national data sets: the Melanoma Pathology (M-Path) study, conducted from July 2013 to May 2016, and the Reducing Errors in Melanocytic Interpretations (REMI) study, conducted from August 2018 to March 2021. In both studies, pathologists who interpreted melanocytic lesions in their clinical practices interpreted study cases in glass slide format. For the current study, researchers used logistic regression to examine the association of pathologist characteristics with diagnosis of a study case as higher grade (including severely dysplastic and melanoma in situ) vs. lower grade (including mild to moderately dysplastic nevi) and diagnosis of invasive melanoma vs. any less severe diagnosis.
A total of 338 pathologists were included in the analysis. Of these, 113 were general pathologists and 225 were dermatopathologists (those who were board certified and/or fellowship trained in dermatopathology).
The researchers found that, compared with general pathologists, dermatopathologists were 2.63 times more likely to render higher-grade diagnoses and 1.95 times more likely to diagnose invasive melanoma (P < .001 for both associations). Diagnoses of stage pT1a melanomas with no mitotic activity completely accounted for the difference between dermatopathologists and general pathologists in diagnosing invasive melanoma.
For the analysis limited to the 225 dermatopathologists, those with a higher practice caseload of melanocytic lesions were more likely to assign higher-grade diagnoses (odds ratio for trend, 1.27; P = .02), while those affiliated with an academic center had lower odds of diagnosing invasive melanoma (OR, 0.61; P = .049).
The researchers acknowledged limitations of their analysis, including the lack of data on patient outcomes, “so we could not make conclusions about the clinical outcome of any particular diagnosis by a study participant,” they wrote. “While our analyses revealed pathologist characteristics associated with assigning more vs. less severe diagnoses of melanocytic lesions, we could not conclude that any particular diagnosis by a study participant was overcalling or undercalling. However, the epidemiologic evidence that melanoma is overdiagnosed suggests that overcalling by some pathologists may be contributing to increasing rates of low-risk melanoma diagnoses.”
In an accompanying editorial, authors Klaus J. Busam, MD, of the department of pathology and laboratory medicine at Memorial Sloan Kettering Cancer Center, New York, Pedram Gerami, MD, of the department of dermatology at Northwestern University, Chicago, and Richard A. Scolyer, MD, of the Melanoma Institute, Wollstonecraft, Australia, wrote that the study findings “raise the question of whether subspecialization in dermatopathology may be a factor contributing to the epidemiologic phenomenon of overdiagnosis – that is, the discordance in the rise of melanoma incidence and relatively constant annual mortality rates over many decades. The findings also invite a discussion about strategies to minimize harm from overdiagnosis for both patients and the health care system.”
To minimize misdiagnoses, they continued, efforts to facilitate diagnostic accuracy should be encouraged. “Excisional (rather than partial) biopsies and provision of relevant clinical information would facilitate rendering of the correct histopathologic diagnosis,” they wrote. “When the diagnosis is uncertain, this is best acknowledged. If felt necessary, a reexcision of a lesion with an uncertain diagnosis can be recommended without upgrading the diagnosis.”
In addition, “improvements in prognosis are needed beyond American Joint Committee on Cancer staging,” they noted. “This will likely require a multimodal approach with novel methods, including artificial intelligence and biomarkers that help distinguish low-risk melanomas, for which a conservative approach may be appropriate, from those that require surgical intervention.”
The study was supported by the National Center for Advancing Translational Sciences and by the National Institutes of Health. One author disclosed receiving grants from the National Cancer Institute during the conduct of the study, and another disclosed serving as editor in chief of Primary Care topics at UpToDate; other authors had no disclosures. Dr. Busam reported receiving nonfinancial support from the American Society of Dermatopathology. Dr. Gerami reported receiving consulting fees from Castle Biosciences. Dr. Scolyer reported receiving an investigator grant from the National Health and Medical Research Council of Australia during the conduct of the study and personal fees from several pharmaceutical companies outside the submitted work.
, results from an exploratory study showed.
The findings “could in part play a role in the rising incidence of early-stage melanoma with low risk of progression or patient morbidity, thereby contributing to increasing rates of overdiagnosis,” researchers led by co–senior authors Joann G. Elmore, MD, MPH, of the University of California, Los Angeles, and Raymond L. Barnhill, MD, MBA, of the Institut Curie, Paris, wrote in their study, published online in JAMA Dermatology.
To investigate the characteristics associated with rendering higher-grade diagnoses, including invasive melanoma, the researchers drew from two national data sets: the Melanoma Pathology (M-Path) study, conducted from July 2013 to May 2016, and the Reducing Errors in Melanocytic Interpretations (REMI) study, conducted from August 2018 to March 2021. In both studies, pathologists who interpreted melanocytic lesions in their clinical practices interpreted study cases in glass slide format. For the current study, researchers used logistic regression to examine the association of pathologist characteristics with diagnosis of a study case as higher grade (including severely dysplastic and melanoma in situ) vs. lower grade (including mild to moderately dysplastic nevi) and diagnosis of invasive melanoma vs. any less severe diagnosis.
A total of 338 pathologists were included in the analysis. Of these, 113 were general pathologists and 225 were dermatopathologists (those who were board certified and/or fellowship trained in dermatopathology).
The researchers found that, compared with general pathologists, dermatopathologists were 2.63 times more likely to render higher-grade diagnoses and 1.95 times more likely to diagnose invasive melanoma (P < .001 for both associations). Diagnoses of stage pT1a melanomas with no mitotic activity completely accounted for the difference between dermatopathologists and general pathologists in diagnosing invasive melanoma.
For the analysis limited to the 225 dermatopathologists, those with a higher practice caseload of melanocytic lesions were more likely to assign higher-grade diagnoses (odds ratio for trend, 1.27; P = .02), while those affiliated with an academic center had lower odds of diagnosing invasive melanoma (OR, 0.61; P = .049).
The researchers acknowledged limitations of their analysis, including the lack of data on patient outcomes, “so we could not make conclusions about the clinical outcome of any particular diagnosis by a study participant,” they wrote. “While our analyses revealed pathologist characteristics associated with assigning more vs. less severe diagnoses of melanocytic lesions, we could not conclude that any particular diagnosis by a study participant was overcalling or undercalling. However, the epidemiologic evidence that melanoma is overdiagnosed suggests that overcalling by some pathologists may be contributing to increasing rates of low-risk melanoma diagnoses.”
In an accompanying editorial, authors Klaus J. Busam, MD, of the department of pathology and laboratory medicine at Memorial Sloan Kettering Cancer Center, New York, Pedram Gerami, MD, of the department of dermatology at Northwestern University, Chicago, and Richard A. Scolyer, MD, of the Melanoma Institute, Wollstonecraft, Australia, wrote that the study findings “raise the question of whether subspecialization in dermatopathology may be a factor contributing to the epidemiologic phenomenon of overdiagnosis – that is, the discordance in the rise of melanoma incidence and relatively constant annual mortality rates over many decades. The findings also invite a discussion about strategies to minimize harm from overdiagnosis for both patients and the health care system.”
To minimize misdiagnoses, they continued, efforts to facilitate diagnostic accuracy should be encouraged. “Excisional (rather than partial) biopsies and provision of relevant clinical information would facilitate rendering of the correct histopathologic diagnosis,” they wrote. “When the diagnosis is uncertain, this is best acknowledged. If felt necessary, a reexcision of a lesion with an uncertain diagnosis can be recommended without upgrading the diagnosis.”
In addition, “improvements in prognosis are needed beyond American Joint Committee on Cancer staging,” they noted. “This will likely require a multimodal approach with novel methods, including artificial intelligence and biomarkers that help distinguish low-risk melanomas, for which a conservative approach may be appropriate, from those that require surgical intervention.”
The study was supported by the National Center for Advancing Translational Sciences and by the National Institutes of Health. One author disclosed receiving grants from the National Cancer Institute during the conduct of the study, and another disclosed serving as editor in chief of Primary Care topics at UpToDate; other authors had no disclosures. Dr. Busam reported receiving nonfinancial support from the American Society of Dermatopathology. Dr. Gerami reported receiving consulting fees from Castle Biosciences. Dr. Scolyer reported receiving an investigator grant from the National Health and Medical Research Council of Australia during the conduct of the study and personal fees from several pharmaceutical companies outside the submitted work.
, results from an exploratory study showed.
The findings “could in part play a role in the rising incidence of early-stage melanoma with low risk of progression or patient morbidity, thereby contributing to increasing rates of overdiagnosis,” researchers led by co–senior authors Joann G. Elmore, MD, MPH, of the University of California, Los Angeles, and Raymond L. Barnhill, MD, MBA, of the Institut Curie, Paris, wrote in their study, published online in JAMA Dermatology.
To investigate the characteristics associated with rendering higher-grade diagnoses, including invasive melanoma, the researchers drew from two national data sets: the Melanoma Pathology (M-Path) study, conducted from July 2013 to May 2016, and the Reducing Errors in Melanocytic Interpretations (REMI) study, conducted from August 2018 to March 2021. In both studies, pathologists who interpreted melanocytic lesions in their clinical practices interpreted study cases in glass slide format. For the current study, researchers used logistic regression to examine the association of pathologist characteristics with diagnosis of a study case as higher grade (including severely dysplastic and melanoma in situ) vs. lower grade (including mild to moderately dysplastic nevi) and diagnosis of invasive melanoma vs. any less severe diagnosis.
A total of 338 pathologists were included in the analysis. Of these, 113 were general pathologists and 225 were dermatopathologists (those who were board certified and/or fellowship trained in dermatopathology).
The researchers found that, compared with general pathologists, dermatopathologists were 2.63 times more likely to render higher-grade diagnoses and 1.95 times more likely to diagnose invasive melanoma (P < .001 for both associations). Diagnoses of stage pT1a melanomas with no mitotic activity completely accounted for the difference between dermatopathologists and general pathologists in diagnosing invasive melanoma.
For the analysis limited to the 225 dermatopathologists, those with a higher practice caseload of melanocytic lesions were more likely to assign higher-grade diagnoses (odds ratio for trend, 1.27; P = .02), while those affiliated with an academic center had lower odds of diagnosing invasive melanoma (OR, 0.61; P = .049).
The researchers acknowledged limitations of their analysis, including the lack of data on patient outcomes, “so we could not make conclusions about the clinical outcome of any particular diagnosis by a study participant,” they wrote. “While our analyses revealed pathologist characteristics associated with assigning more vs. less severe diagnoses of melanocytic lesions, we could not conclude that any particular diagnosis by a study participant was overcalling or undercalling. However, the epidemiologic evidence that melanoma is overdiagnosed suggests that overcalling by some pathologists may be contributing to increasing rates of low-risk melanoma diagnoses.”
In an accompanying editorial, authors Klaus J. Busam, MD, of the department of pathology and laboratory medicine at Memorial Sloan Kettering Cancer Center, New York, Pedram Gerami, MD, of the department of dermatology at Northwestern University, Chicago, and Richard A. Scolyer, MD, of the Melanoma Institute, Wollstonecraft, Australia, wrote that the study findings “raise the question of whether subspecialization in dermatopathology may be a factor contributing to the epidemiologic phenomenon of overdiagnosis – that is, the discordance in the rise of melanoma incidence and relatively constant annual mortality rates over many decades. The findings also invite a discussion about strategies to minimize harm from overdiagnosis for both patients and the health care system.”
To minimize misdiagnoses, they continued, efforts to facilitate diagnostic accuracy should be encouraged. “Excisional (rather than partial) biopsies and provision of relevant clinical information would facilitate rendering of the correct histopathologic diagnosis,” they wrote. “When the diagnosis is uncertain, this is best acknowledged. If felt necessary, a reexcision of a lesion with an uncertain diagnosis can be recommended without upgrading the diagnosis.”
In addition, “improvements in prognosis are needed beyond American Joint Committee on Cancer staging,” they noted. “This will likely require a multimodal approach with novel methods, including artificial intelligence and biomarkers that help distinguish low-risk melanomas, for which a conservative approach may be appropriate, from those that require surgical intervention.”
The study was supported by the National Center for Advancing Translational Sciences and by the National Institutes of Health. One author disclosed receiving grants from the National Cancer Institute during the conduct of the study, and another disclosed serving as editor in chief of Primary Care topics at UpToDate; other authors had no disclosures. Dr. Busam reported receiving nonfinancial support from the American Society of Dermatopathology. Dr. Gerami reported receiving consulting fees from Castle Biosciences. Dr. Scolyer reported receiving an investigator grant from the National Health and Medical Research Council of Australia during the conduct of the study and personal fees from several pharmaceutical companies outside the submitted work.
FROM JAMA DERMATOLOGY