User login
FDA’s Project Optimus aims to transform early cancer research
SAN DIEGO –
The goal is “to better identify and characterize optimized doses” in early stages of research and move away from the default of the traditional maximum tolerated dose strategy, hematologist-oncologist Marc R. Theoret, MD, deputy director of the FDA’s Oncology Center of Excellence, said in a presentation at the 2023 Society for Immunotherapy of Cancer annual meeting.
Earlier this year, the FDA released a draft guidance regarding the changes it hopes to see. The agency supported randomized, parallel dose-response trials when feasible, and “strong rationale for choice of dosage should be provided before initiating a registration trial(s) to support a subsequent indication and usage.”
The goal of controlling toxicity is “very highly important” in hematology research since blood cancer drugs can cause significant adverse effects in areas such as the lungs and heart, said Cecilia Yeung, MD, who led the SITC session about Project Optimus. Dr. Yeung is a clinical pathologist who works on investigational trials at Fred Hutchinson Cancer Research Center in Seattle.
In an interview, Dr. Yeung, who has a subspecialty in hematopathology, explained why the foundations of cancer research are changing and what hematologist-oncologists can expect to see on the horizon.
Q: Project Optimus aims to move beyond the traditional dose-escalation approach to the development of cancer drugs. How does that strategy work?
Dr. Yeung: Prior to Project Optimus, they’d use a 3+3 strategy in phase 1 trials: They’d give a dose to three fairly healthy patients, then they’d go up by escalating doses in more patients. They’d keep going up until two-thirds of patients at a specific dose suffered from bad side effects, then they’d back off to the last dose.
Q: This approach, which aims to identify the “maximum tolerated dose,” seemed to work well over decades of research into chemotherapy drugs. But worries arose as targeted therapies appeared in oncology areas such as blood cancer. Why did things change?
Dr. Yeung: With 3+3, you could tell pretty quickly how toxic chemotherapy was. But in targeted therapy, we were finding that these studies are not representative of actual toxicity. You’re not treating these patients for a very long time in phase 1, while patients on targeted therapy may be on these drugs for years. Concerns actually started with the first targeted drugs to treat leukemias and lymphomas. They were shown to have unexpected toxicity. A 2016 study found that drug developers had to reduce the original phase 1 dose in 45% of phase 3 trials [of small molecule and monoclonal antibody targeted agents] approved by the FDA over 12 years because of toxicity.
Q: What is FDA’s goal for Project Optimus?
Dr. Yeung: They want to have a second piece, to balance that maximum tolerated dose with a safe and tolerable dose for most people.
Q: What kind of resistance is the FDA getting from drug companies?
Dr. Yeung: The FDA makes a good argument that the system wasn’t working. But drug companies say this will drive up the cost of clinical trials and won’t allow them to treat patients with the maximal doses they could give them. I see arguments from both sides. There has to be a balance between the two.
Q: How will all this affect drug development?
Dr. Yeung: Drugs may become more expensive because much more testing will happen during clinical trials.
Q: Could this reduce the number of investigational drugs?
Dr. Yeung: Hopefully not, but this is huge endeavor for smaller companies that are strapped for funding.
Q: What do you think the future holds?
Dr. Yeung: Ultimately, this is a good thing because if everything works out, we’ll have fewer toxic side effects. But we’re going to have to go through a period of growing pains.
SAN DIEGO –
The goal is “to better identify and characterize optimized doses” in early stages of research and move away from the default of the traditional maximum tolerated dose strategy, hematologist-oncologist Marc R. Theoret, MD, deputy director of the FDA’s Oncology Center of Excellence, said in a presentation at the 2023 Society for Immunotherapy of Cancer annual meeting.
Earlier this year, the FDA released a draft guidance regarding the changes it hopes to see. The agency supported randomized, parallel dose-response trials when feasible, and “strong rationale for choice of dosage should be provided before initiating a registration trial(s) to support a subsequent indication and usage.”
The goal of controlling toxicity is “very highly important” in hematology research since blood cancer drugs can cause significant adverse effects in areas such as the lungs and heart, said Cecilia Yeung, MD, who led the SITC session about Project Optimus. Dr. Yeung is a clinical pathologist who works on investigational trials at Fred Hutchinson Cancer Research Center in Seattle.
In an interview, Dr. Yeung, who has a subspecialty in hematopathology, explained why the foundations of cancer research are changing and what hematologist-oncologists can expect to see on the horizon.
Q: Project Optimus aims to move beyond the traditional dose-escalation approach to the development of cancer drugs. How does that strategy work?
Dr. Yeung: Prior to Project Optimus, they’d use a 3+3 strategy in phase 1 trials: They’d give a dose to three fairly healthy patients, then they’d go up by escalating doses in more patients. They’d keep going up until two-thirds of patients at a specific dose suffered from bad side effects, then they’d back off to the last dose.
Q: This approach, which aims to identify the “maximum tolerated dose,” seemed to work well over decades of research into chemotherapy drugs. But worries arose as targeted therapies appeared in oncology areas such as blood cancer. Why did things change?
Dr. Yeung: With 3+3, you could tell pretty quickly how toxic chemotherapy was. But in targeted therapy, we were finding that these studies are not representative of actual toxicity. You’re not treating these patients for a very long time in phase 1, while patients on targeted therapy may be on these drugs for years. Concerns actually started with the first targeted drugs to treat leukemias and lymphomas. They were shown to have unexpected toxicity. A 2016 study found that drug developers had to reduce the original phase 1 dose in 45% of phase 3 trials [of small molecule and monoclonal antibody targeted agents] approved by the FDA over 12 years because of toxicity.
Q: What is FDA’s goal for Project Optimus?
Dr. Yeung: They want to have a second piece, to balance that maximum tolerated dose with a safe and tolerable dose for most people.
Q: What kind of resistance is the FDA getting from drug companies?
Dr. Yeung: The FDA makes a good argument that the system wasn’t working. But drug companies say this will drive up the cost of clinical trials and won’t allow them to treat patients with the maximal doses they could give them. I see arguments from both sides. There has to be a balance between the two.
Q: How will all this affect drug development?
Dr. Yeung: Drugs may become more expensive because much more testing will happen during clinical trials.
Q: Could this reduce the number of investigational drugs?
Dr. Yeung: Hopefully not, but this is huge endeavor for smaller companies that are strapped for funding.
Q: What do you think the future holds?
Dr. Yeung: Ultimately, this is a good thing because if everything works out, we’ll have fewer toxic side effects. But we’re going to have to go through a period of growing pains.
SAN DIEGO –
The goal is “to better identify and characterize optimized doses” in early stages of research and move away from the default of the traditional maximum tolerated dose strategy, hematologist-oncologist Marc R. Theoret, MD, deputy director of the FDA’s Oncology Center of Excellence, said in a presentation at the 2023 Society for Immunotherapy of Cancer annual meeting.
Earlier this year, the FDA released a draft guidance regarding the changes it hopes to see. The agency supported randomized, parallel dose-response trials when feasible, and “strong rationale for choice of dosage should be provided before initiating a registration trial(s) to support a subsequent indication and usage.”
The goal of controlling toxicity is “very highly important” in hematology research since blood cancer drugs can cause significant adverse effects in areas such as the lungs and heart, said Cecilia Yeung, MD, who led the SITC session about Project Optimus. Dr. Yeung is a clinical pathologist who works on investigational trials at Fred Hutchinson Cancer Research Center in Seattle.
In an interview, Dr. Yeung, who has a subspecialty in hematopathology, explained why the foundations of cancer research are changing and what hematologist-oncologists can expect to see on the horizon.
Q: Project Optimus aims to move beyond the traditional dose-escalation approach to the development of cancer drugs. How does that strategy work?
Dr. Yeung: Prior to Project Optimus, they’d use a 3+3 strategy in phase 1 trials: They’d give a dose to three fairly healthy patients, then they’d go up by escalating doses in more patients. They’d keep going up until two-thirds of patients at a specific dose suffered from bad side effects, then they’d back off to the last dose.
Q: This approach, which aims to identify the “maximum tolerated dose,” seemed to work well over decades of research into chemotherapy drugs. But worries arose as targeted therapies appeared in oncology areas such as blood cancer. Why did things change?
Dr. Yeung: With 3+3, you could tell pretty quickly how toxic chemotherapy was. But in targeted therapy, we were finding that these studies are not representative of actual toxicity. You’re not treating these patients for a very long time in phase 1, while patients on targeted therapy may be on these drugs for years. Concerns actually started with the first targeted drugs to treat leukemias and lymphomas. They were shown to have unexpected toxicity. A 2016 study found that drug developers had to reduce the original phase 1 dose in 45% of phase 3 trials [of small molecule and monoclonal antibody targeted agents] approved by the FDA over 12 years because of toxicity.
Q: What is FDA’s goal for Project Optimus?
Dr. Yeung: They want to have a second piece, to balance that maximum tolerated dose with a safe and tolerable dose for most people.
Q: What kind of resistance is the FDA getting from drug companies?
Dr. Yeung: The FDA makes a good argument that the system wasn’t working. But drug companies say this will drive up the cost of clinical trials and won’t allow them to treat patients with the maximal doses they could give them. I see arguments from both sides. There has to be a balance between the two.
Q: How will all this affect drug development?
Dr. Yeung: Drugs may become more expensive because much more testing will happen during clinical trials.
Q: Could this reduce the number of investigational drugs?
Dr. Yeung: Hopefully not, but this is huge endeavor for smaller companies that are strapped for funding.
Q: What do you think the future holds?
Dr. Yeung: Ultimately, this is a good thing because if everything works out, we’ll have fewer toxic side effects. But we’re going to have to go through a period of growing pains.
AT SITC 2023
2023 Update on cervical disease
Cervical cancer was the most common cancer killer of persons with a cervix in the early 1900s in the United States. Widespread adoption of the Pap test in the mid-20th century followed by large-scale outreach through programs such as the National Breast and Cervical Cancer Early Detection Program have dramatically reduced deaths from cervical cancer. The development of a highly effective vaccine that targets human papillomavirus (HPV), the virus implicated in all cervical cancers, has made prevention even more accessible and attainable. Primary prevention with HPV vaccination in conjunction with regular screening as recommended by current guidelines is the most effective way we can prevent cervical cancer.
Despite these advances, the incidence and death rates from cervical cancer have plateaued over the last decade.1 Additionally, many fear that due to the poor attendance at screening visits since the beginning of the COVID-19 pandemic, the incidence might further rise in the United States.2 Among those in the United States diagnosed with cervical cancer, more than 50% have not been screened in over 5 years or had their abnormal results not managed as recommended by current guidelines, suggesting that operational and access issues are contributors to incident cervical cancer. In addition, HPV vaccination rates have increased only slightly from year to year. According to the most recent data from the Centers for Disease Control and Prevention (CDC), coverage with 1 or more doses of HPV vaccine in 2021 increased only by 1.8% and has stagnated, with administration to about 75% of those for whom it is recommended.3 The plateauing will limit our ability to eradicate cervical cancer in the United States, permitting death from a largely preventable disease.
Establishing the framework for the eradication of cervical cancer
The World Health Organization (WHO) adopted a global strategy called the Cervical Cancer Elimination Initiative in August 2020. This initiative is a multipronged effort that focuses on vaccination (90% of girls fully vaccinated by age 15), screening (70% of women screened by age 35 with an effective test and again at age 45), and treatment (90% treatment of precancer and 90% management of women with invasive cancer).4
These are the numbers we need to achieve if all countries are to reach a cervical cancer incidence of less than 4 per 100,000 persons with a cervix. The WHO further suggests that each country should meet the “90-70-90” targets by 2030 if we are to achieve the low incidence by the turn of the century.4 To date, few regions of the world have achieved these goals, and sadly the United States is not among them.
In response to this call to action, many medical and policymaking organizations are taking inventory and implementing strategies to achieve the WHO 2030 targets for cervical cancer eradication. In the United States, the Society of Gynecologic Oncology (SGO; www.sgo.org), the American Society for Colposcopy and Cervical Pathology (ASCCP; www.ASCCP.org), the American College of Obstetricians and Gynecologists (ACOG; www.acog.org), the American Cancer Society (ACS; www.cancer.org), and many others have initiated programs in a collaborative esprit de corps with the aim of eradicating this deadly disease.
In this Update, we review several studies with evidence of screening and management strategies that show promise of accelerating the eradication of cervical cancer.
Continue to: Transitioning to primary HPV screening in the United States...
Transitioning to primary HPV screening in the United States
Downs LS Jr, Nayar R, Gerndt J, et al; American Cancer Society Primary HPV Screening Initiative Steering Committee. Implementation in action: collaborating on the transition to primary HPV screening for cervical cancer in the United States. CA Cancer J Clin. 2023;73:458-460.
The American Cancer Society released an updated cervical cancer screening guideline in July 2020 that recommended testing for HPV as the preferred strategy. Reasons behind the change, moving away from a Pap test as part of the initial screen, are:
- increased sensitivity of primary HPV testing when compared with conventional cervical cytology (Pap test)
- improved risk stratification to identify who is at risk for cervical cancer now and in the future
- improved efficiency in identifying those who need colposcopy, thus limiting unnecessary procedures without increasing the risk of false-negative tests, thereby missing cervical precancer or invasive cancer.
Some countries with organized screening programs have already made the switch. Self-sampling for HPV is currently being considered for an approved use in the United States, further improving access to screening for cervical cancer when the initial step can be completed by the patient at home or simplified in nontraditional health care settings.2
ACS initiative created to address barriers to primary HPV testing
Challenges to primary HPV testing remain, including laboratory implementation, payment, and operationalizing clinical workflow (for example, HPV testing with reflex cytology instead of cytology with reflex HPV testing).5 There are undoubtedly other unforeseen barriers in the current US health care environment.
In a recent commentary, Downs and colleagues described how the ACS has convened the Primary HPV Screening Initiative (PHSI), nested under the ACS National Roundtable on Cervical Cancer, which is charged with identifying critical barriers to, and opportunities for, transitioning to primary HPV screening.5 The deliverable will be a roadmap with tools and recommendations to support health systems, laboratories, providers, patients, and payers as they make this evolution.
Work groups will develop resources
Patients, particularly those who have had routine cervical cancer screening over their lifetime, also will be curious about the changes in recommendations. The Provider Needs Workgroup within the PHSI structure will develop tools and patient education materials regarding the data, workflow, benefits, and safety of this new paradigm for cervical cancer screening.
Laboratories that process and interpret tests likely will bear the heaviest load of changes. For example, not all commercially available HPV tests in the United States are approved by the US Food and Drug Administration (FDA) for primary HPV testing. Some sites will need to adapt their equipment to ensure adherence to FDA-approved tests. Laboratory workflows will need to be altered for aliquots to be tested for HPV first, and the remainder for cytology. Quality assurance and accreditation requirements for testing will need modifications, and further efforts will be needed to ensure sufficient numbers of trained cytopathologists, whose workforce is rapidly declining, for processing and reading cervical cytology.
In addition, payment for HPV testing alone, without the need for a Pap test, might not be supported by payers that support safety-net providers and sites, who arguably serve the most vulnerable patients and those most at risk for cervical cancer. Collaboration across medical professionals, societies, payers, and policymakers will provide a critical infrastructure to make the change in the most seamless fashion and limit the harm from missed opportunities for screening.
HPV testing as the primary screen for cervical cancer is now recommended in guidelines due to improved sensitivity and improved efficiency when compared with other methods of screening. Implementation of this new workflow for clinicians and labs will require collaboration across multiple stakeholders.
Continue to: The quest for a “molecular Pap”: Dual-stain testing as a predictor of high-grade CIN...
The quest for a “molecular Pap”: Dual-stain testing as a predictor of high-grade CIN
Magkana M, Mentzelopoulou P, Magkana E, et al. p16/Ki-67 Dual staining is a reliable biomarker for risk stratification for patients with borderline/mild cytology in cervical cancer screening. Anticancer Res. 2022;42:2599-2606.
Stanczuk G, Currie H, Forson W, et al. Clinical performance of triage strategies for Hr-HPV-positive women; a longitudinal evaluation of cytology, p16/K-67 dual stain cytology, and HPV16/18 genotyping. Cancer Epidemiol Biomarkers Prev. 2022;31:1492-1498.
One new technology that was recently FDA approved and recommended for management of abnormal cervical cancer screening testing is dual-stain (DS) testing. Dual-stain testing is a cytology-based test that evaluates the concurrent expression of p16, a tumor suppressor protein upregulated in HPV oncogenesis, and Ki-67, a cell proliferation marker.6,7 Two recent studies have showcased the outstanding clinical performance of DS testing and triage strategies that incorporate DS testing.
Higher specificity, fewer colposcopies needed with DS testing
Magkana and colleagues prospectively evaluated patients with atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesion (LSIL), or negative for intraepithelial lesion or malignancy (NILM) cytology referred for colposcopy, and they compared p16/Ki-67 DS testing with high-risk HPV (HR-HPV) testing for the detection of cervical intraepithelial neoplasia grade 2 or worse (CIN 2+); comparable sensitivities for CIN 2+ detection were seen (97.3% and 98.7%, respectively).8
Dual-stain testing exhibited higher specificity at 99.3% compared with HR-HPV testing at 52.2%. Incorporating DS testing into triage strategies also led to fewer colposcopies needed to detect CIN 2+ compared with current ASCCP guidelines that use traditional cervical cancer screening algorithms.
DS cytology strategy had the highest sensitivity for CIN 2+ detection
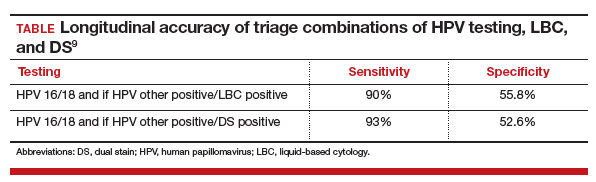
An additional study by Stanczuk and colleagues evaluated triage strategies in a cohort of HR-HPV positive patients who participated in the Scottish Papillomavirus Dumfries and Galloway study with HPV 16/18 genotyping (HPV 16/18), liquid-based cytology (LBC), and p16/Ki-67 DS cytology.9 Of these 3 triage strategies, DS cytology had the highest sensitivity for the detection of CIN 2+, at 77.7% (with a specificity of 74.2%), performance that is arguably better than cytology.
When evaluated in sequence as part of a triage strategy after HPV primary screening, HPV 16/18–positive patients reflexed to DS testing showed a similar sensitivity as those who would be triaged with LBC (TABLE).9
DS testing’s potential
These studies add to the growing body of literature that supports the use of DS testing in cervical cancer screening management guidelines and that are being incorporated into currently existing workflows. Furthermore, with advancements in digital imaging and machine learning, DS testing holds the potential for a high throughput, reproducible, and accurate risk stratification that can replace the current reliance on cytology, furthering the potential for a fully molecular Pap test.10,11
The introduction of p16/Ki-67 dual-stain testing has the potential to allow us to safely move away from a traditional Pap test for cervical cancer screening by allowing for more accurate and reliable identification of high-risk lesions with a molecular test that can be automated and have a high throughput.
Continue to: Cervical cancer screening in women older than age 65: Is there benefit?...
Cervical cancer screening in women older than age 65: Is there benefit?
Firtina Tuncer S, Tuncer HA. Cervical cancer screening in women aged older than 65 years. J Low Genit Tract Dis. 2023;27:207-211.
Booth BB, Tranberg M, Gustafson LW, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse in women aged ≥ 69 referred to colposcopy due to an HPV-positive screening test. BMC Cancer. 2023;23:405.
Current guidelines in the United States recommend that cervical cancer screening for all persons with a cervix end at age 65. These age restrictions were a change in guidelines updated in 2012 and endorsed by the US Preventive Services Task Force.12,13 Evidence suggests that because of high likelihood of regression and slow progression of disease, risks of screening prior to age 21 outweigh its benefits. With primary HPV testing, the age at screening debut is 25 for the same reasons.14 In people with a history of CIN 2+, active surveillance should continue for at least 25 years with HPV-based screening regardless of age. In the absence of a history of CIN 2+, however, the data to support discontinuation of screening after age 65 are less clear.
HPV positivity found to be most substantial risk for CIN 2+
In a study published this year in the Journal of Lower Genital Tract Disease, Firtina Tuncer and colleagues described their experience extending “routine screening” in patients older than 65 years.15 Data including cervical cytology, HPV test results, biopsy findings, and endocervical curettage results were collected, and abnormal findings were managed according to the 2012 and 2019 ASCCP guidelines.
When compared with negative HPV testing and normal cytology, the authors found that HPV positivity and abnormal cytology increased the risk of CIN 2+(odds ratio [OR], 136.1 and 13.1, respectively). Patients whose screening prior to age 65 had been insufficient or demonstrated CIN 2+ in the preceding 10 years were similarly more likely to have findings of CIN 2+ (OR, 9.7 when compared with HPV-negative controls).
The authors concluded that, among persons with a cervix older than age 65, previous screening and abnormal cytology were important in risk stratifications for CIN 2+; however, HPV positivity conferred the most substantial risk.
Study finds cervical dysplasia is prevalent in older populations
It has been suggested that screening for cervical cancer should continue beyond age 65 as cytology-based screening may have decreased sensitivity in older patients, which may contribute to the higher rates of advanced-stage diagnoses and cancer-related death in this population.16,17
Authors of an observational study conducted in Denmark invited persons with a cervix aged 69 and older to have one additional HPV-based screening test, and they referred them for colposcopy if HPV positive or in the presence of ASCUS or greater cytology.18 Among the 191 patients with HPV-positive results, 20% were found to have a diagnosis of CIN 2+, and 24.4% had CIN 2+ detected at another point in the study period. Notably, most patients diagnosed with CIN 2+ had no abnormalities visualized on colposcopy, and the majority of biopsies taken (65.8%) did not contain the transitional zone.
Biopsies underestimated CIN 2+ in 17.9% of cases compared with loop electrosurgical excision procedure (LEEP). These findings suggest both that high-grade cervical dysplasia is prevalent in an older population and that older populations may be susceptible to false-negative results. They also further support the use of HPV-based screening.
There are risk factors overscreening and underscreening that impact decision making regarding restricting screening to persons with a cervix younger than age 65. As more data become available, and as the population ages, it will be essential to closely examine the incidence of and trends in cervical cancer to determine appropriate patterns of screening.
Harnessing the immune system to improve survival rates in recurrent cervical cancer
Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
Unfortunately, most clinical trials for recurrent or metastatic cervical cancer are negative trials or have results that show limited impact on disease outcomes. Currently, cervical cancer is treated with multiple agents, including platinum-based chemotherapy and bevacizumab, a medication that targets vascular growth. Despite these usually very effective drugs given in combination to cervical cancer patients, long-term survival remains low. Over the past few decades, many trials have been designed to help patients with this terrible disease, but few have shown significant promise.
Immune checkpoint inhibitors, such as pembrolizumab, have revolutionized care for many cancers. Checkpoint inhibitors block the proteins that cause a tumor to remain undetected by the immune system’s army of T cells. By blocking these proteins, the cancer cells can then be recognized by the immune system as foreign. Several studies have concluded that including immune checkpoint inhibitors in the comprehensive regimen for recurrent cervical cancer improves survival.
Addition of pembrolizumab increased survival
Investigators in the phase 3 double-blinded KEYNOTE-826 trial evaluated whether or not the addition of pembrolizumab to standard of care improved progression-free and overall survival in advanced, recurrent, or persistent cervical cancer.19 As part of the evaluation, the investigators measured the protein that turns off the immune system’s ability to recognize tumors, anti-programmed cell death protein-1 (PD-1).
Compared with placebo, the investigators found that, regardless of PD-1 status, the addition of pembrolizumab immunotherapy to the standard regimen increased progression-free survival and overall survival without any significantly increased adverse effects or safety concerns (FIGURE).19 At 1 year after treatment, more patients who received pembrolizumab were still alive regardless of PD-1 status, and their responses lasted longer. The most profound improvements were seen in patients whose tumors exhibited high expression of PD-L1, the target of pembrolizumab and many other immune checkpoint inhibitors.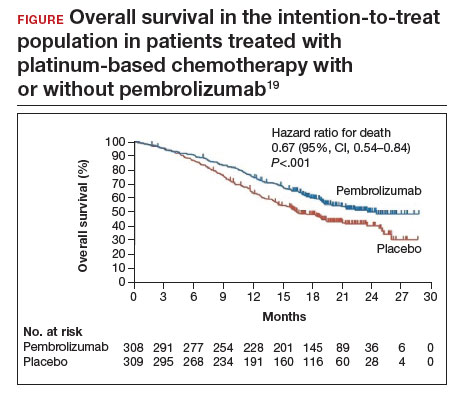
Despite these promising results, more studies are needed to find additional therapeutic targets and treatments. Using the immune system to fight cancer represents a promising step toward the ultimate goal of cervical cancer eradication. ●
Metastatic cervical cancer can be a devastating disease that cannot be treated surgically and therefore has limited treatment options that have curative intent. Immune checkpoint inhibition via pembrolizumab opens new avenues for treatment and is a huge step forward toward the goal of cervical cancer eradication.
- US Cancer Statistics Working Group. US Cancer Statistics Data Visualizations Tool, based on 2022 submission data (1999-2020). US Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute. June 2023. Accessed October 9, 2023. https://gis.cdc.gov/Cancer/USCS/#/Trends/
- Einstein MH, Zhou N, Gabor L, et al. Primary human papillomavirus testing and other new technologies for cervical cancer screening. Obstet Gynecol. September 14, 2023. doi:10.1097/AOG.0000000000005393
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2020. MMWR Morbid Mortal Weekly Rep. 2021;70:1183-1190.
- Cervical cancer elimination initiative. World Health Organization. 2023. Accessed October 10, 2023. https ://www.who.int/initiatives/cervical-cancer-eliminationinitiative#cms
- Downs LS Jr, Nayar R, Gerndt J, et al; American Cancer Society Primary HPV Screening Initiative Steering Committee. Implementation in action: collaborating on the transition to primary HPV screening for cervical cancer in the United States. CA Cancer J Clin. 2023;73:458-460.
- Wentzensen N, Fetterman B, Castle PE, et al. p16/Ki-67 Dual stain cytology for detection of cervical precancer in HPV-positive women. J Natl Cancer Inst. 2015;107:djv257.
- Ikenberg H, Bergeron C, Schmidt D, et al; PALMS Study Group. Screening for cervical cancer precursors with p16 /Ki-67 dual-stained cytology: results of the PALMS study. J Natl Cancer Inst. 2013;105:1550-1557.
- Magkana M, Mentzelopoulou P, Magkana E, et al. p16/Ki-67 Dual staining is a reliable biomarker for risk stratification for patients with borderline/mild cytology in cervical cancer screening. Anticancer Res. 2022;42:2599-2606.
- Stanczuk G, Currie H, Forson W, et al. Clinical performance of triage strategies for Hr-HPV-positive women; a longitudinal evaluation of cytology, p16/K-67 dual stain cytology, and HPV16/18 genotyping. Cancer Epidemiol Biomarkers Prev. 2022;31:1492-1498.
- Wright TC Jr, Stoler MH, Behrens CM, et al. Interlaboratory variation in the performance of liquid-based cytology: insights from the ATHENA trial. Int J Cancer. 2014;134: 1835-1843.
- Wentzensen N, Lahrmann B, Clarke MA, et al. Accuracy and efficiency of deep-learning-based automation of dual stain cytology in cervical cancer screening. J Natl Cancer Inst. 2021;113:72-79.
- Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121:829-846.
- Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156: 880-891, W312.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346.
- Firtina Tuncer S, Tuncer HA. Cervical cancer screening in women aged older than 65 years. J Low Genit Tract Dis. 2023;27:207-211.
- Hammer A, Hee L, Blaakaer J, et al. Temporal patterns of cervical cancer screening among Danish women 55 years and older diagnosed with cervical cancer. J Low Genit Tract Dis. 2018;22:1-7.
- Hammer A, Soegaard V, Maimburg RD, et al. Cervical cancer screening history prior to a diagnosis of cervical cancer in Danish women aged 60 years and older—A national cohort study. Cancer Med. 2019;8:418-427.
- Booth BB, Tranberg M, Gustafson LW, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse in women aged ≥ 69 referred to colposcopy due to an HPV-positive screening test. BMC Cancer. 2023;23:405.
- Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.

Cervical cancer was the most common cancer killer of persons with a cervix in the early 1900s in the United States. Widespread adoption of the Pap test in the mid-20th century followed by large-scale outreach through programs such as the National Breast and Cervical Cancer Early Detection Program have dramatically reduced deaths from cervical cancer. The development of a highly effective vaccine that targets human papillomavirus (HPV), the virus implicated in all cervical cancers, has made prevention even more accessible and attainable. Primary prevention with HPV vaccination in conjunction with regular screening as recommended by current guidelines is the most effective way we can prevent cervical cancer.
Despite these advances, the incidence and death rates from cervical cancer have plateaued over the last decade.1 Additionally, many fear that due to the poor attendance at screening visits since the beginning of the COVID-19 pandemic, the incidence might further rise in the United States.2 Among those in the United States diagnosed with cervical cancer, more than 50% have not been screened in over 5 years or had their abnormal results not managed as recommended by current guidelines, suggesting that operational and access issues are contributors to incident cervical cancer. In addition, HPV vaccination rates have increased only slightly from year to year. According to the most recent data from the Centers for Disease Control and Prevention (CDC), coverage with 1 or more doses of HPV vaccine in 2021 increased only by 1.8% and has stagnated, with administration to about 75% of those for whom it is recommended.3 The plateauing will limit our ability to eradicate cervical cancer in the United States, permitting death from a largely preventable disease.
Establishing the framework for the eradication of cervical cancer
The World Health Organization (WHO) adopted a global strategy called the Cervical Cancer Elimination Initiative in August 2020. This initiative is a multipronged effort that focuses on vaccination (90% of girls fully vaccinated by age 15), screening (70% of women screened by age 35 with an effective test and again at age 45), and treatment (90% treatment of precancer and 90% management of women with invasive cancer).4
These are the numbers we need to achieve if all countries are to reach a cervical cancer incidence of less than 4 per 100,000 persons with a cervix. The WHO further suggests that each country should meet the “90-70-90” targets by 2030 if we are to achieve the low incidence by the turn of the century.4 To date, few regions of the world have achieved these goals, and sadly the United States is not among them.
In response to this call to action, many medical and policymaking organizations are taking inventory and implementing strategies to achieve the WHO 2030 targets for cervical cancer eradication. In the United States, the Society of Gynecologic Oncology (SGO; www.sgo.org), the American Society for Colposcopy and Cervical Pathology (ASCCP; www.ASCCP.org), the American College of Obstetricians and Gynecologists (ACOG; www.acog.org), the American Cancer Society (ACS; www.cancer.org), and many others have initiated programs in a collaborative esprit de corps with the aim of eradicating this deadly disease.
In this Update, we review several studies with evidence of screening and management strategies that show promise of accelerating the eradication of cervical cancer.
Continue to: Transitioning to primary HPV screening in the United States...
Transitioning to primary HPV screening in the United States
Downs LS Jr, Nayar R, Gerndt J, et al; American Cancer Society Primary HPV Screening Initiative Steering Committee. Implementation in action: collaborating on the transition to primary HPV screening for cervical cancer in the United States. CA Cancer J Clin. 2023;73:458-460.
The American Cancer Society released an updated cervical cancer screening guideline in July 2020 that recommended testing for HPV as the preferred strategy. Reasons behind the change, moving away from a Pap test as part of the initial screen, are:
- increased sensitivity of primary HPV testing when compared with conventional cervical cytology (Pap test)
- improved risk stratification to identify who is at risk for cervical cancer now and in the future
- improved efficiency in identifying those who need colposcopy, thus limiting unnecessary procedures without increasing the risk of false-negative tests, thereby missing cervical precancer or invasive cancer.
Some countries with organized screening programs have already made the switch. Self-sampling for HPV is currently being considered for an approved use in the United States, further improving access to screening for cervical cancer when the initial step can be completed by the patient at home or simplified in nontraditional health care settings.2
ACS initiative created to address barriers to primary HPV testing
Challenges to primary HPV testing remain, including laboratory implementation, payment, and operationalizing clinical workflow (for example, HPV testing with reflex cytology instead of cytology with reflex HPV testing).5 There are undoubtedly other unforeseen barriers in the current US health care environment.
In a recent commentary, Downs and colleagues described how the ACS has convened the Primary HPV Screening Initiative (PHSI), nested under the ACS National Roundtable on Cervical Cancer, which is charged with identifying critical barriers to, and opportunities for, transitioning to primary HPV screening.5 The deliverable will be a roadmap with tools and recommendations to support health systems, laboratories, providers, patients, and payers as they make this evolution.
Work groups will develop resources
Patients, particularly those who have had routine cervical cancer screening over their lifetime, also will be curious about the changes in recommendations. The Provider Needs Workgroup within the PHSI structure will develop tools and patient education materials regarding the data, workflow, benefits, and safety of this new paradigm for cervical cancer screening.
Laboratories that process and interpret tests likely will bear the heaviest load of changes. For example, not all commercially available HPV tests in the United States are approved by the US Food and Drug Administration (FDA) for primary HPV testing. Some sites will need to adapt their equipment to ensure adherence to FDA-approved tests. Laboratory workflows will need to be altered for aliquots to be tested for HPV first, and the remainder for cytology. Quality assurance and accreditation requirements for testing will need modifications, and further efforts will be needed to ensure sufficient numbers of trained cytopathologists, whose workforce is rapidly declining, for processing and reading cervical cytology.
In addition, payment for HPV testing alone, without the need for a Pap test, might not be supported by payers that support safety-net providers and sites, who arguably serve the most vulnerable patients and those most at risk for cervical cancer. Collaboration across medical professionals, societies, payers, and policymakers will provide a critical infrastructure to make the change in the most seamless fashion and limit the harm from missed opportunities for screening.
HPV testing as the primary screen for cervical cancer is now recommended in guidelines due to improved sensitivity and improved efficiency when compared with other methods of screening. Implementation of this new workflow for clinicians and labs will require collaboration across multiple stakeholders.
Continue to: The quest for a “molecular Pap”: Dual-stain testing as a predictor of high-grade CIN...
The quest for a “molecular Pap”: Dual-stain testing as a predictor of high-grade CIN
Magkana M, Mentzelopoulou P, Magkana E, et al. p16/Ki-67 Dual staining is a reliable biomarker for risk stratification for patients with borderline/mild cytology in cervical cancer screening. Anticancer Res. 2022;42:2599-2606.
Stanczuk G, Currie H, Forson W, et al. Clinical performance of triage strategies for Hr-HPV-positive women; a longitudinal evaluation of cytology, p16/K-67 dual stain cytology, and HPV16/18 genotyping. Cancer Epidemiol Biomarkers Prev. 2022;31:1492-1498.
One new technology that was recently FDA approved and recommended for management of abnormal cervical cancer screening testing is dual-stain (DS) testing. Dual-stain testing is a cytology-based test that evaluates the concurrent expression of p16, a tumor suppressor protein upregulated in HPV oncogenesis, and Ki-67, a cell proliferation marker.6,7 Two recent studies have showcased the outstanding clinical performance of DS testing and triage strategies that incorporate DS testing.
Higher specificity, fewer colposcopies needed with DS testing
Magkana and colleagues prospectively evaluated patients with atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesion (LSIL), or negative for intraepithelial lesion or malignancy (NILM) cytology referred for colposcopy, and they compared p16/Ki-67 DS testing with high-risk HPV (HR-HPV) testing for the detection of cervical intraepithelial neoplasia grade 2 or worse (CIN 2+); comparable sensitivities for CIN 2+ detection were seen (97.3% and 98.7%, respectively).8
Dual-stain testing exhibited higher specificity at 99.3% compared with HR-HPV testing at 52.2%. Incorporating DS testing into triage strategies also led to fewer colposcopies needed to detect CIN 2+ compared with current ASCCP guidelines that use traditional cervical cancer screening algorithms.
DS cytology strategy had the highest sensitivity for CIN 2+ detection
An additional study by Stanczuk and colleagues evaluated triage strategies in a cohort of HR-HPV positive patients who participated in the Scottish Papillomavirus Dumfries and Galloway study with HPV 16/18 genotyping (HPV 16/18), liquid-based cytology (LBC), and p16/Ki-67 DS cytology.9 Of these 3 triage strategies, DS cytology had the highest sensitivity for the detection of CIN 2+, at 77.7% (with a specificity of 74.2%), performance that is arguably better than cytology.
When evaluated in sequence as part of a triage strategy after HPV primary screening, HPV 16/18–positive patients reflexed to DS testing showed a similar sensitivity as those who would be triaged with LBC (TABLE).9

DS testing’s potential
These studies add to the growing body of literature that supports the use of DS testing in cervical cancer screening management guidelines and that are being incorporated into currently existing workflows. Furthermore, with advancements in digital imaging and machine learning, DS testing holds the potential for a high throughput, reproducible, and accurate risk stratification that can replace the current reliance on cytology, furthering the potential for a fully molecular Pap test.10,11
The introduction of p16/Ki-67 dual-stain testing has the potential to allow us to safely move away from a traditional Pap test for cervical cancer screening by allowing for more accurate and reliable identification of high-risk lesions with a molecular test that can be automated and have a high throughput.
Continue to: Cervical cancer screening in women older than age 65: Is there benefit?...
Cervical cancer screening in women older than age 65: Is there benefit?
Firtina Tuncer S, Tuncer HA. Cervical cancer screening in women aged older than 65 years. J Low Genit Tract Dis. 2023;27:207-211.
Booth BB, Tranberg M, Gustafson LW, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse in women aged ≥ 69 referred to colposcopy due to an HPV-positive screening test. BMC Cancer. 2023;23:405.
Current guidelines in the United States recommend that cervical cancer screening for all persons with a cervix end at age 65. These age restrictions were a change in guidelines updated in 2012 and endorsed by the US Preventive Services Task Force.12,13 Evidence suggests that because of high likelihood of regression and slow progression of disease, risks of screening prior to age 21 outweigh its benefits. With primary HPV testing, the age at screening debut is 25 for the same reasons.14 In people with a history of CIN 2+, active surveillance should continue for at least 25 years with HPV-based screening regardless of age. In the absence of a history of CIN 2+, however, the data to support discontinuation of screening after age 65 are less clear.
HPV positivity found to be most substantial risk for CIN 2+
In a study published this year in the Journal of Lower Genital Tract Disease, Firtina Tuncer and colleagues described their experience extending “routine screening” in patients older than 65 years.15 Data including cervical cytology, HPV test results, biopsy findings, and endocervical curettage results were collected, and abnormal findings were managed according to the 2012 and 2019 ASCCP guidelines.
When compared with negative HPV testing and normal cytology, the authors found that HPV positivity and abnormal cytology increased the risk of CIN 2+(odds ratio [OR], 136.1 and 13.1, respectively). Patients whose screening prior to age 65 had been insufficient or demonstrated CIN 2+ in the preceding 10 years were similarly more likely to have findings of CIN 2+ (OR, 9.7 when compared with HPV-negative controls).
The authors concluded that, among persons with a cervix older than age 65, previous screening and abnormal cytology were important in risk stratifications for CIN 2+; however, HPV positivity conferred the most substantial risk.
Study finds cervical dysplasia is prevalent in older populations
It has been suggested that screening for cervical cancer should continue beyond age 65 as cytology-based screening may have decreased sensitivity in older patients, which may contribute to the higher rates of advanced-stage diagnoses and cancer-related death in this population.16,17
Authors of an observational study conducted in Denmark invited persons with a cervix aged 69 and older to have one additional HPV-based screening test, and they referred them for colposcopy if HPV positive or in the presence of ASCUS or greater cytology.18 Among the 191 patients with HPV-positive results, 20% were found to have a diagnosis of CIN 2+, and 24.4% had CIN 2+ detected at another point in the study period. Notably, most patients diagnosed with CIN 2+ had no abnormalities visualized on colposcopy, and the majority of biopsies taken (65.8%) did not contain the transitional zone.
Biopsies underestimated CIN 2+ in 17.9% of cases compared with loop electrosurgical excision procedure (LEEP). These findings suggest both that high-grade cervical dysplasia is prevalent in an older population and that older populations may be susceptible to false-negative results. They also further support the use of HPV-based screening.
There are risk factors overscreening and underscreening that impact decision making regarding restricting screening to persons with a cervix younger than age 65. As more data become available, and as the population ages, it will be essential to closely examine the incidence of and trends in cervical cancer to determine appropriate patterns of screening.
Harnessing the immune system to improve survival rates in recurrent cervical cancer
Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
Unfortunately, most clinical trials for recurrent or metastatic cervical cancer are negative trials or have results that show limited impact on disease outcomes. Currently, cervical cancer is treated with multiple agents, including platinum-based chemotherapy and bevacizumab, a medication that targets vascular growth. Despite these usually very effective drugs given in combination to cervical cancer patients, long-term survival remains low. Over the past few decades, many trials have been designed to help patients with this terrible disease, but few have shown significant promise.
Immune checkpoint inhibitors, such as pembrolizumab, have revolutionized care for many cancers. Checkpoint inhibitors block the proteins that cause a tumor to remain undetected by the immune system’s army of T cells. By blocking these proteins, the cancer cells can then be recognized by the immune system as foreign. Several studies have concluded that including immune checkpoint inhibitors in the comprehensive regimen for recurrent cervical cancer improves survival.
Addition of pembrolizumab increased survival
Investigators in the phase 3 double-blinded KEYNOTE-826 trial evaluated whether or not the addition of pembrolizumab to standard of care improved progression-free and overall survival in advanced, recurrent, or persistent cervical cancer.19 As part of the evaluation, the investigators measured the protein that turns off the immune system’s ability to recognize tumors, anti-programmed cell death protein-1 (PD-1).
Compared with placebo, the investigators found that, regardless of PD-1 status, the addition of pembrolizumab immunotherapy to the standard regimen increased progression-free survival and overall survival without any significantly increased adverse effects or safety concerns (FIGURE).19 At 1 year after treatment, more patients who received pembrolizumab were still alive regardless of PD-1 status, and their responses lasted longer. The most profound improvements were seen in patients whose tumors exhibited high expression of PD-L1, the target of pembrolizumab and many other immune checkpoint inhibitors.
Despite these promising results, more studies are needed to find additional therapeutic targets and treatments. Using the immune system to fight cancer represents a promising step toward the ultimate goal of cervical cancer eradication. ●
Metastatic cervical cancer can be a devastating disease that cannot be treated surgically and therefore has limited treatment options that have curative intent. Immune checkpoint inhibition via pembrolizumab opens new avenues for treatment and is a huge step forward toward the goal of cervical cancer eradication.

Cervical cancer was the most common cancer killer of persons with a cervix in the early 1900s in the United States. Widespread adoption of the Pap test in the mid-20th century followed by large-scale outreach through programs such as the National Breast and Cervical Cancer Early Detection Program have dramatically reduced deaths from cervical cancer. The development of a highly effective vaccine that targets human papillomavirus (HPV), the virus implicated in all cervical cancers, has made prevention even more accessible and attainable. Primary prevention with HPV vaccination in conjunction with regular screening as recommended by current guidelines is the most effective way we can prevent cervical cancer.
Despite these advances, the incidence and death rates from cervical cancer have plateaued over the last decade.1 Additionally, many fear that due to the poor attendance at screening visits since the beginning of the COVID-19 pandemic, the incidence might further rise in the United States.2 Among those in the United States diagnosed with cervical cancer, more than 50% have not been screened in over 5 years or had their abnormal results not managed as recommended by current guidelines, suggesting that operational and access issues are contributors to incident cervical cancer. In addition, HPV vaccination rates have increased only slightly from year to year. According to the most recent data from the Centers for Disease Control and Prevention (CDC), coverage with 1 or more doses of HPV vaccine in 2021 increased only by 1.8% and has stagnated, with administration to about 75% of those for whom it is recommended.3 The plateauing will limit our ability to eradicate cervical cancer in the United States, permitting death from a largely preventable disease.
Establishing the framework for the eradication of cervical cancer
The World Health Organization (WHO) adopted a global strategy called the Cervical Cancer Elimination Initiative in August 2020. This initiative is a multipronged effort that focuses on vaccination (90% of girls fully vaccinated by age 15), screening (70% of women screened by age 35 with an effective test and again at age 45), and treatment (90% treatment of precancer and 90% management of women with invasive cancer).4
These are the numbers we need to achieve if all countries are to reach a cervical cancer incidence of less than 4 per 100,000 persons with a cervix. The WHO further suggests that each country should meet the “90-70-90” targets by 2030 if we are to achieve the low incidence by the turn of the century.4 To date, few regions of the world have achieved these goals, and sadly the United States is not among them.
In response to this call to action, many medical and policymaking organizations are taking inventory and implementing strategies to achieve the WHO 2030 targets for cervical cancer eradication. In the United States, the Society of Gynecologic Oncology (SGO; www.sgo.org), the American Society for Colposcopy and Cervical Pathology (ASCCP; www.ASCCP.org), the American College of Obstetricians and Gynecologists (ACOG; www.acog.org), the American Cancer Society (ACS; www.cancer.org), and many others have initiated programs in a collaborative esprit de corps with the aim of eradicating this deadly disease.
In this Update, we review several studies with evidence of screening and management strategies that show promise of accelerating the eradication of cervical cancer.
Continue to: Transitioning to primary HPV screening in the United States...
Transitioning to primary HPV screening in the United States
Downs LS Jr, Nayar R, Gerndt J, et al; American Cancer Society Primary HPV Screening Initiative Steering Committee. Implementation in action: collaborating on the transition to primary HPV screening for cervical cancer in the United States. CA Cancer J Clin. 2023;73:458-460.
The American Cancer Society released an updated cervical cancer screening guideline in July 2020 that recommended testing for HPV as the preferred strategy. Reasons behind the change, moving away from a Pap test as part of the initial screen, are:
- increased sensitivity of primary HPV testing when compared with conventional cervical cytology (Pap test)
- improved risk stratification to identify who is at risk for cervical cancer now and in the future
- improved efficiency in identifying those who need colposcopy, thus limiting unnecessary procedures without increasing the risk of false-negative tests, thereby missing cervical precancer or invasive cancer.
Some countries with organized screening programs have already made the switch. Self-sampling for HPV is currently being considered for an approved use in the United States, further improving access to screening for cervical cancer when the initial step can be completed by the patient at home or simplified in nontraditional health care settings.2
ACS initiative created to address barriers to primary HPV testing
Challenges to primary HPV testing remain, including laboratory implementation, payment, and operationalizing clinical workflow (for example, HPV testing with reflex cytology instead of cytology with reflex HPV testing).5 There are undoubtedly other unforeseen barriers in the current US health care environment.
In a recent commentary, Downs and colleagues described how the ACS has convened the Primary HPV Screening Initiative (PHSI), nested under the ACS National Roundtable on Cervical Cancer, which is charged with identifying critical barriers to, and opportunities for, transitioning to primary HPV screening.5 The deliverable will be a roadmap with tools and recommendations to support health systems, laboratories, providers, patients, and payers as they make this evolution.
Work groups will develop resources
Patients, particularly those who have had routine cervical cancer screening over their lifetime, also will be curious about the changes in recommendations. The Provider Needs Workgroup within the PHSI structure will develop tools and patient education materials regarding the data, workflow, benefits, and safety of this new paradigm for cervical cancer screening.
Laboratories that process and interpret tests likely will bear the heaviest load of changes. For example, not all commercially available HPV tests in the United States are approved by the US Food and Drug Administration (FDA) for primary HPV testing. Some sites will need to adapt their equipment to ensure adherence to FDA-approved tests. Laboratory workflows will need to be altered for aliquots to be tested for HPV first, and the remainder for cytology. Quality assurance and accreditation requirements for testing will need modifications, and further efforts will be needed to ensure sufficient numbers of trained cytopathologists, whose workforce is rapidly declining, for processing and reading cervical cytology.
In addition, payment for HPV testing alone, without the need for a Pap test, might not be supported by payers that support safety-net providers and sites, who arguably serve the most vulnerable patients and those most at risk for cervical cancer. Collaboration across medical professionals, societies, payers, and policymakers will provide a critical infrastructure to make the change in the most seamless fashion and limit the harm from missed opportunities for screening.
HPV testing as the primary screen for cervical cancer is now recommended in guidelines due to improved sensitivity and improved efficiency when compared with other methods of screening. Implementation of this new workflow for clinicians and labs will require collaboration across multiple stakeholders.
Continue to: The quest for a “molecular Pap”: Dual-stain testing as a predictor of high-grade CIN...
The quest for a “molecular Pap”: Dual-stain testing as a predictor of high-grade CIN
Magkana M, Mentzelopoulou P, Magkana E, et al. p16/Ki-67 Dual staining is a reliable biomarker for risk stratification for patients with borderline/mild cytology in cervical cancer screening. Anticancer Res. 2022;42:2599-2606.
Stanczuk G, Currie H, Forson W, et al. Clinical performance of triage strategies for Hr-HPV-positive women; a longitudinal evaluation of cytology, p16/K-67 dual stain cytology, and HPV16/18 genotyping. Cancer Epidemiol Biomarkers Prev. 2022;31:1492-1498.
One new technology that was recently FDA approved and recommended for management of abnormal cervical cancer screening testing is dual-stain (DS) testing. Dual-stain testing is a cytology-based test that evaluates the concurrent expression of p16, a tumor suppressor protein upregulated in HPV oncogenesis, and Ki-67, a cell proliferation marker.6,7 Two recent studies have showcased the outstanding clinical performance of DS testing and triage strategies that incorporate DS testing.
Higher specificity, fewer colposcopies needed with DS testing
Magkana and colleagues prospectively evaluated patients with atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesion (LSIL), or negative for intraepithelial lesion or malignancy (NILM) cytology referred for colposcopy, and they compared p16/Ki-67 DS testing with high-risk HPV (HR-HPV) testing for the detection of cervical intraepithelial neoplasia grade 2 or worse (CIN 2+); comparable sensitivities for CIN 2+ detection were seen (97.3% and 98.7%, respectively).8
Dual-stain testing exhibited higher specificity at 99.3% compared with HR-HPV testing at 52.2%. Incorporating DS testing into triage strategies also led to fewer colposcopies needed to detect CIN 2+ compared with current ASCCP guidelines that use traditional cervical cancer screening algorithms.
DS cytology strategy had the highest sensitivity for CIN 2+ detection
An additional study by Stanczuk and colleagues evaluated triage strategies in a cohort of HR-HPV positive patients who participated in the Scottish Papillomavirus Dumfries and Galloway study with HPV 16/18 genotyping (HPV 16/18), liquid-based cytology (LBC), and p16/Ki-67 DS cytology.9 Of these 3 triage strategies, DS cytology had the highest sensitivity for the detection of CIN 2+, at 77.7% (with a specificity of 74.2%), performance that is arguably better than cytology.
When evaluated in sequence as part of a triage strategy after HPV primary screening, HPV 16/18–positive patients reflexed to DS testing showed a similar sensitivity as those who would be triaged with LBC (TABLE).9

DS testing’s potential
These studies add to the growing body of literature that supports the use of DS testing in cervical cancer screening management guidelines and that are being incorporated into currently existing workflows. Furthermore, with advancements in digital imaging and machine learning, DS testing holds the potential for a high throughput, reproducible, and accurate risk stratification that can replace the current reliance on cytology, furthering the potential for a fully molecular Pap test.10,11
The introduction of p16/Ki-67 dual-stain testing has the potential to allow us to safely move away from a traditional Pap test for cervical cancer screening by allowing for more accurate and reliable identification of high-risk lesions with a molecular test that can be automated and have a high throughput.
Continue to: Cervical cancer screening in women older than age 65: Is there benefit?...
Cervical cancer screening in women older than age 65: Is there benefit?
Firtina Tuncer S, Tuncer HA. Cervical cancer screening in women aged older than 65 years. J Low Genit Tract Dis. 2023;27:207-211.
Booth BB, Tranberg M, Gustafson LW, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse in women aged ≥ 69 referred to colposcopy due to an HPV-positive screening test. BMC Cancer. 2023;23:405.
Current guidelines in the United States recommend that cervical cancer screening for all persons with a cervix end at age 65. These age restrictions were a change in guidelines updated in 2012 and endorsed by the US Preventive Services Task Force.12,13 Evidence suggests that because of high likelihood of regression and slow progression of disease, risks of screening prior to age 21 outweigh its benefits. With primary HPV testing, the age at screening debut is 25 for the same reasons.14 In people with a history of CIN 2+, active surveillance should continue for at least 25 years with HPV-based screening regardless of age. In the absence of a history of CIN 2+, however, the data to support discontinuation of screening after age 65 are less clear.
HPV positivity found to be most substantial risk for CIN 2+
In a study published this year in the Journal of Lower Genital Tract Disease, Firtina Tuncer and colleagues described their experience extending “routine screening” in patients older than 65 years.15 Data including cervical cytology, HPV test results, biopsy findings, and endocervical curettage results were collected, and abnormal findings were managed according to the 2012 and 2019 ASCCP guidelines.
When compared with negative HPV testing and normal cytology, the authors found that HPV positivity and abnormal cytology increased the risk of CIN 2+(odds ratio [OR], 136.1 and 13.1, respectively). Patients whose screening prior to age 65 had been insufficient or demonstrated CIN 2+ in the preceding 10 years were similarly more likely to have findings of CIN 2+ (OR, 9.7 when compared with HPV-negative controls).
The authors concluded that, among persons with a cervix older than age 65, previous screening and abnormal cytology were important in risk stratifications for CIN 2+; however, HPV positivity conferred the most substantial risk.
Study finds cervical dysplasia is prevalent in older populations
It has been suggested that screening for cervical cancer should continue beyond age 65 as cytology-based screening may have decreased sensitivity in older patients, which may contribute to the higher rates of advanced-stage diagnoses and cancer-related death in this population.16,17
Authors of an observational study conducted in Denmark invited persons with a cervix aged 69 and older to have one additional HPV-based screening test, and they referred them for colposcopy if HPV positive or in the presence of ASCUS or greater cytology.18 Among the 191 patients with HPV-positive results, 20% were found to have a diagnosis of CIN 2+, and 24.4% had CIN 2+ detected at another point in the study period. Notably, most patients diagnosed with CIN 2+ had no abnormalities visualized on colposcopy, and the majority of biopsies taken (65.8%) did not contain the transitional zone.
Biopsies underestimated CIN 2+ in 17.9% of cases compared with loop electrosurgical excision procedure (LEEP). These findings suggest both that high-grade cervical dysplasia is prevalent in an older population and that older populations may be susceptible to false-negative results. They also further support the use of HPV-based screening.
There are risk factors overscreening and underscreening that impact decision making regarding restricting screening to persons with a cervix younger than age 65. As more data become available, and as the population ages, it will be essential to closely examine the incidence of and trends in cervical cancer to determine appropriate patterns of screening.
Harnessing the immune system to improve survival rates in recurrent cervical cancer
Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
Unfortunately, most clinical trials for recurrent or metastatic cervical cancer are negative trials or have results that show limited impact on disease outcomes. Currently, cervical cancer is treated with multiple agents, including platinum-based chemotherapy and bevacizumab, a medication that targets vascular growth. Despite these usually very effective drugs given in combination to cervical cancer patients, long-term survival remains low. Over the past few decades, many trials have been designed to help patients with this terrible disease, but few have shown significant promise.
Immune checkpoint inhibitors, such as pembrolizumab, have revolutionized care for many cancers. Checkpoint inhibitors block the proteins that cause a tumor to remain undetected by the immune system’s army of T cells. By blocking these proteins, the cancer cells can then be recognized by the immune system as foreign. Several studies have concluded that including immune checkpoint inhibitors in the comprehensive regimen for recurrent cervical cancer improves survival.
Addition of pembrolizumab increased survival
Investigators in the phase 3 double-blinded KEYNOTE-826 trial evaluated whether or not the addition of pembrolizumab to standard of care improved progression-free and overall survival in advanced, recurrent, or persistent cervical cancer.19 As part of the evaluation, the investigators measured the protein that turns off the immune system’s ability to recognize tumors, anti-programmed cell death protein-1 (PD-1).
Compared with placebo, the investigators found that, regardless of PD-1 status, the addition of pembrolizumab immunotherapy to the standard regimen increased progression-free survival and overall survival without any significantly increased adverse effects or safety concerns (FIGURE).19 At 1 year after treatment, more patients who received pembrolizumab were still alive regardless of PD-1 status, and their responses lasted longer. The most profound improvements were seen in patients whose tumors exhibited high expression of PD-L1, the target of pembrolizumab and many other immune checkpoint inhibitors.
Despite these promising results, more studies are needed to find additional therapeutic targets and treatments. Using the immune system to fight cancer represents a promising step toward the ultimate goal of cervical cancer eradication. ●
Metastatic cervical cancer can be a devastating disease that cannot be treated surgically and therefore has limited treatment options that have curative intent. Immune checkpoint inhibition via pembrolizumab opens new avenues for treatment and is a huge step forward toward the goal of cervical cancer eradication.
- US Cancer Statistics Working Group. US Cancer Statistics Data Visualizations Tool, based on 2022 submission data (1999-2020). US Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute. June 2023. Accessed October 9, 2023. https://gis.cdc.gov/Cancer/USCS/#/Trends/
- Einstein MH, Zhou N, Gabor L, et al. Primary human papillomavirus testing and other new technologies for cervical cancer screening. Obstet Gynecol. September 14, 2023. doi:10.1097/AOG.0000000000005393
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2020. MMWR Morbid Mortal Weekly Rep. 2021;70:1183-1190.
- Cervical cancer elimination initiative. World Health Organization. 2023. Accessed October 10, 2023. https ://www.who.int/initiatives/cervical-cancer-eliminationinitiative#cms
- Downs LS Jr, Nayar R, Gerndt J, et al; American Cancer Society Primary HPV Screening Initiative Steering Committee. Implementation in action: collaborating on the transition to primary HPV screening for cervical cancer in the United States. CA Cancer J Clin. 2023;73:458-460.
- Wentzensen N, Fetterman B, Castle PE, et al. p16/Ki-67 Dual stain cytology for detection of cervical precancer in HPV-positive women. J Natl Cancer Inst. 2015;107:djv257.
- Ikenberg H, Bergeron C, Schmidt D, et al; PALMS Study Group. Screening for cervical cancer precursors with p16 /Ki-67 dual-stained cytology: results of the PALMS study. J Natl Cancer Inst. 2013;105:1550-1557.
- Magkana M, Mentzelopoulou P, Magkana E, et al. p16/Ki-67 Dual staining is a reliable biomarker for risk stratification for patients with borderline/mild cytology in cervical cancer screening. Anticancer Res. 2022;42:2599-2606.
- Stanczuk G, Currie H, Forson W, et al. Clinical performance of triage strategies for Hr-HPV-positive women; a longitudinal evaluation of cytology, p16/K-67 dual stain cytology, and HPV16/18 genotyping. Cancer Epidemiol Biomarkers Prev. 2022;31:1492-1498.
- Wright TC Jr, Stoler MH, Behrens CM, et al. Interlaboratory variation in the performance of liquid-based cytology: insights from the ATHENA trial. Int J Cancer. 2014;134: 1835-1843.
- Wentzensen N, Lahrmann B, Clarke MA, et al. Accuracy and efficiency of deep-learning-based automation of dual stain cytology in cervical cancer screening. J Natl Cancer Inst. 2021;113:72-79.
- Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121:829-846.
- Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156: 880-891, W312.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346.
- Firtina Tuncer S, Tuncer HA. Cervical cancer screening in women aged older than 65 years. J Low Genit Tract Dis. 2023;27:207-211.
- Hammer A, Hee L, Blaakaer J, et al. Temporal patterns of cervical cancer screening among Danish women 55 years and older diagnosed with cervical cancer. J Low Genit Tract Dis. 2018;22:1-7.
- Hammer A, Soegaard V, Maimburg RD, et al. Cervical cancer screening history prior to a diagnosis of cervical cancer in Danish women aged 60 years and older—A national cohort study. Cancer Med. 2019;8:418-427.
- Booth BB, Tranberg M, Gustafson LW, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse in women aged ≥ 69 referred to colposcopy due to an HPV-positive screening test. BMC Cancer. 2023;23:405.
- Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
- US Cancer Statistics Working Group. US Cancer Statistics Data Visualizations Tool, based on 2022 submission data (1999-2020). US Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute. June 2023. Accessed October 9, 2023. https://gis.cdc.gov/Cancer/USCS/#/Trends/
- Einstein MH, Zhou N, Gabor L, et al. Primary human papillomavirus testing and other new technologies for cervical cancer screening. Obstet Gynecol. September 14, 2023. doi:10.1097/AOG.0000000000005393
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2020. MMWR Morbid Mortal Weekly Rep. 2021;70:1183-1190.
- Cervical cancer elimination initiative. World Health Organization. 2023. Accessed October 10, 2023. https ://www.who.int/initiatives/cervical-cancer-eliminationinitiative#cms
- Downs LS Jr, Nayar R, Gerndt J, et al; American Cancer Society Primary HPV Screening Initiative Steering Committee. Implementation in action: collaborating on the transition to primary HPV screening for cervical cancer in the United States. CA Cancer J Clin. 2023;73:458-460.
- Wentzensen N, Fetterman B, Castle PE, et al. p16/Ki-67 Dual stain cytology for detection of cervical precancer in HPV-positive women. J Natl Cancer Inst. 2015;107:djv257.
- Ikenberg H, Bergeron C, Schmidt D, et al; PALMS Study Group. Screening for cervical cancer precursors with p16 /Ki-67 dual-stained cytology: results of the PALMS study. J Natl Cancer Inst. 2013;105:1550-1557.
- Magkana M, Mentzelopoulou P, Magkana E, et al. p16/Ki-67 Dual staining is a reliable biomarker for risk stratification for patients with borderline/mild cytology in cervical cancer screening. Anticancer Res. 2022;42:2599-2606.
- Stanczuk G, Currie H, Forson W, et al. Clinical performance of triage strategies for Hr-HPV-positive women; a longitudinal evaluation of cytology, p16/K-67 dual stain cytology, and HPV16/18 genotyping. Cancer Epidemiol Biomarkers Prev. 2022;31:1492-1498.
- Wright TC Jr, Stoler MH, Behrens CM, et al. Interlaboratory variation in the performance of liquid-based cytology: insights from the ATHENA trial. Int J Cancer. 2014;134: 1835-1843.
- Wentzensen N, Lahrmann B, Clarke MA, et al. Accuracy and efficiency of deep-learning-based automation of dual stain cytology in cervical cancer screening. J Natl Cancer Inst. 2021;113:72-79.
- Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121:829-846.
- Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156: 880-891, W312.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346.
- Firtina Tuncer S, Tuncer HA. Cervical cancer screening in women aged older than 65 years. J Low Genit Tract Dis. 2023;27:207-211.
- Hammer A, Hee L, Blaakaer J, et al. Temporal patterns of cervical cancer screening among Danish women 55 years and older diagnosed with cervical cancer. J Low Genit Tract Dis. 2018;22:1-7.
- Hammer A, Soegaard V, Maimburg RD, et al. Cervical cancer screening history prior to a diagnosis of cervical cancer in Danish women aged 60 years and older—A national cohort study. Cancer Med. 2019;8:418-427.
- Booth BB, Tranberg M, Gustafson LW, et al. Risk of cervical intraepithelial neoplasia grade 2 or worse in women aged ≥ 69 referred to colposcopy due to an HPV-positive screening test. BMC Cancer. 2023;23:405.
- Colombo N, Dubot C, Lorusso D, et al; KEYNOTE-826 Investigators. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856-1867.
Product Update
REVIEW
By James Greenberg, MD
Chief of Gynecology
Associate Professor, Harvard Medical School
Boston, Massachusetts
Guardenia: A “really great solution” for contained tissue extraction
The Guardenia Contained Extraction System, developed by Advanced Surgical Concepts (Wicklow, Ireland), offers a comprehensive approach to contained tissue extraction.
Background. Contained tissue extraction has been an integral part of laparoscopic procedures from at least the late 1980s and early 1990s. Bags of one sort or another are routinely used to remove from the abdomen all sorts of human tissues, including but not limited to ovaries, ectopic pregnancies, gallbladders, kidneys, spleens, and the list goes on. However, after the April 17, 2014, FDA Safety Communication discouraging the use of power morcellators with myomectomy or hysterectomy, the need for more robust contained tissue extraction systems has been ongoing, and there has yet to be a really good solution despite some innovative attempts.
Design/Functionality. Advanced Surgical Concepts’ Guardenia System is the latest attempt to provide a “really great solution” for surgeons to address my 3 “musts” for any system to gain traction in this niche.
- Must #1. An easy method for getting the device into the abdomen.
- Must #2. An easy method for getting the tissue into the bag.
- Must #3. An easy method for getting the tissue out of the body while still containing the cells within the system.
In my opinion based on my usage, Guardenia does a pretty good job addressing all my “musts.”
The Guardenia System is a sterile, single-use device with 3 main components—an introducer with a plunger that will fit through any standard 12-mm trocar, a polyurethane film bag supported by a nitinol ring, and an opening ring with expandable semi-rigid polyethylene “Guard Petals.” When I used it in the operating room to manually morcellate a 10-cm myoma through the umbilicus, I thought it was pretty spot on. Getting the bag into the abdomen is 100% intuitive but even has a nifty up-arrow built onto the tip to make sure the bag is opened in the proper direction. The bag material and the nitinol ring, in combination with the 17.5-cm opening ring, make the process of getting the specimen into the bag and exteriorized easier than any other system I have ever used. And, the opening ring with the Guard Petals yields a very large retraction area for the incision size while providing excellent protection to the surrounding tissues. Overall, Guardenia worked better than anything else I have previously used.
Innovation. Guardenia does not really introduce any fundamentally novel ideas, but it does combine a lot of standard technologies into a product whose sum is much larger than its parts. I would like to see it combined with an occlusive top piece to allow the retractor to be used for single-port laparoscopy as well, but that would just be the cherry on top.
Summary. I have been working on inventing a really good contained tissue extraction system for a long time, and I am a bit chagrined to see someone else outsmart me (low bar), but Advanced Surgical Concepts did, and I really like Guardenia. For pathology that is appropriate for contained morcellation, this device is definitely worth a try, and I suspect many surgeons will switch to it from whatever they are currently using.
FOR MORE INFORMATION, VISIT https://advancedsurgical.ie/guardenia-contained-extraction-system/
The views of the author are personal opinions and do not necessarily represent the views of
REVIEW
By James Greenberg, MD
Chief of Gynecology
Associate Professor, Harvard Medical School
Boston, Massachusetts
Guardenia: A “really great solution” for contained tissue extraction
The Guardenia Contained Extraction System, developed by Advanced Surgical Concepts (Wicklow, Ireland), offers a comprehensive approach to contained tissue extraction.
Background. Contained tissue extraction has been an integral part of laparoscopic procedures from at least the late 1980s and early 1990s. Bags of one sort or another are routinely used to remove from the abdomen all sorts of human tissues, including but not limited to ovaries, ectopic pregnancies, gallbladders, kidneys, spleens, and the list goes on. However, after the April 17, 2014, FDA Safety Communication discouraging the use of power morcellators with myomectomy or hysterectomy, the need for more robust contained tissue extraction systems has been ongoing, and there has yet to be a really good solution despite some innovative attempts.
Design/Functionality. Advanced Surgical Concepts’ Guardenia System is the latest attempt to provide a “really great solution” for surgeons to address my 3 “musts” for any system to gain traction in this niche.
- Must #1. An easy method for getting the device into the abdomen.
- Must #2. An easy method for getting the tissue into the bag.
- Must #3. An easy method for getting the tissue out of the body while still containing the cells within the system.
In my opinion based on my usage, Guardenia does a pretty good job addressing all my “musts.”
The Guardenia System is a sterile, single-use device with 3 main components—an introducer with a plunger that will fit through any standard 12-mm trocar, a polyurethane film bag supported by a nitinol ring, and an opening ring with expandable semi-rigid polyethylene “Guard Petals.” When I used it in the operating room to manually morcellate a 10-cm myoma through the umbilicus, I thought it was pretty spot on. Getting the bag into the abdomen is 100% intuitive but even has a nifty up-arrow built onto the tip to make sure the bag is opened in the proper direction. The bag material and the nitinol ring, in combination with the 17.5-cm opening ring, make the process of getting the specimen into the bag and exteriorized easier than any other system I have ever used. And, the opening ring with the Guard Petals yields a very large retraction area for the incision size while providing excellent protection to the surrounding tissues. Overall, Guardenia worked better than anything else I have previously used.
Innovation. Guardenia does not really introduce any fundamentally novel ideas, but it does combine a lot of standard technologies into a product whose sum is much larger than its parts. I would like to see it combined with an occlusive top piece to allow the retractor to be used for single-port laparoscopy as well, but that would just be the cherry on top.
Summary. I have been working on inventing a really good contained tissue extraction system for a long time, and I am a bit chagrined to see someone else outsmart me (low bar), but Advanced Surgical Concepts did, and I really like Guardenia. For pathology that is appropriate for contained morcellation, this device is definitely worth a try, and I suspect many surgeons will switch to it from whatever they are currently using.
FOR MORE INFORMATION, VISIT https://advancedsurgical.ie/guardenia-contained-extraction-system/
The views of the author are personal opinions and do not necessarily represent the views of
REVIEW
By James Greenberg, MD
Chief of Gynecology
Associate Professor, Harvard Medical School
Boston, Massachusetts
Guardenia: A “really great solution” for contained tissue extraction
The Guardenia Contained Extraction System, developed by Advanced Surgical Concepts (Wicklow, Ireland), offers a comprehensive approach to contained tissue extraction.
Background. Contained tissue extraction has been an integral part of laparoscopic procedures from at least the late 1980s and early 1990s. Bags of one sort or another are routinely used to remove from the abdomen all sorts of human tissues, including but not limited to ovaries, ectopic pregnancies, gallbladders, kidneys, spleens, and the list goes on. However, after the April 17, 2014, FDA Safety Communication discouraging the use of power morcellators with myomectomy or hysterectomy, the need for more robust contained tissue extraction systems has been ongoing, and there has yet to be a really good solution despite some innovative attempts.
Design/Functionality. Advanced Surgical Concepts’ Guardenia System is the latest attempt to provide a “really great solution” for surgeons to address my 3 “musts” for any system to gain traction in this niche.
- Must #1. An easy method for getting the device into the abdomen.
- Must #2. An easy method for getting the tissue into the bag.
- Must #3. An easy method for getting the tissue out of the body while still containing the cells within the system.
In my opinion based on my usage, Guardenia does a pretty good job addressing all my “musts.”
The Guardenia System is a sterile, single-use device with 3 main components—an introducer with a plunger that will fit through any standard 12-mm trocar, a polyurethane film bag supported by a nitinol ring, and an opening ring with expandable semi-rigid polyethylene “Guard Petals.” When I used it in the operating room to manually morcellate a 10-cm myoma through the umbilicus, I thought it was pretty spot on. Getting the bag into the abdomen is 100% intuitive but even has a nifty up-arrow built onto the tip to make sure the bag is opened in the proper direction. The bag material and the nitinol ring, in combination with the 17.5-cm opening ring, make the process of getting the specimen into the bag and exteriorized easier than any other system I have ever used. And, the opening ring with the Guard Petals yields a very large retraction area for the incision size while providing excellent protection to the surrounding tissues. Overall, Guardenia worked better than anything else I have previously used.
Innovation. Guardenia does not really introduce any fundamentally novel ideas, but it does combine a lot of standard technologies into a product whose sum is much larger than its parts. I would like to see it combined with an occlusive top piece to allow the retractor to be used for single-port laparoscopy as well, but that would just be the cherry on top.
Summary. I have been working on inventing a really good contained tissue extraction system for a long time, and I am a bit chagrined to see someone else outsmart me (low bar), but Advanced Surgical Concepts did, and I really like Guardenia. For pathology that is appropriate for contained morcellation, this device is definitely worth a try, and I suspect many surgeons will switch to it from whatever they are currently using.
FOR MORE INFORMATION, VISIT https://advancedsurgical.ie/guardenia-contained-extraction-system/
The views of the author are personal opinions and do not necessarily represent the views of
TNF blockers not associated with poorer pregnancy outcomes
SAN DIEGO – Continuing a tumor necrosis factor inhibitor (TNFi) during pregnancy does not increase risk of worse fetal or obstetric outcomes, according to new research presented at the annual meeting of the American College of Rheumatology.
Patients who continued a TNFi also had fewer severe infections requiring hospitalization, compared with those who stopped taking the medication during their pregnancy.
“The main message is that patients continuing were not doing worse than the patients stopping. It’s an important clinical message for rheumatologists who are not really confident in dealing with these drugs during pregnancy,” said Anna Moltó, MD, PhD, a rheumatologist at Cochin Hospital, Paris, who led the research. “It adds to the data that it seems to be safe,” she added in an interview.
Previous research, largely from pregnant patients with inflammatory bowel disease, suggests that taking a TNFi during pregnancy is safe, and 2020 ACR guidelines conditionally recommend continuing therapy prior to and during pregnancy; however, many people still stop taking the drugs during pregnancy for fear of potentially harming the fetus.
To better understand how TNFi use affected pregnancy outcomes, Dr. Moltó and colleagues analyzed data from a French nationwide health insurance database to identify adult women with chronic rheumatic inflammatory disease. All women included in the cohort had a singleton pregnancy between 2008 and 2017 and were taking a TNFi upon pregnancy diagnosis.
Patients who restarted TNFi after initially pausing because of pregnancy were included in the continuation group.
Researchers identified more than 2,000 pregnancies, including 1,503 in individuals with spondyloarthritis and 579 individuals with rheumatoid arthritis. Patients were, on average, 31 years old and were diagnosed with a rheumatic disease 4 years prior to their pregnancy.
About 72% (n = 1,497) discontinued TNFi after learning they were pregnant, and 584 individuals continued treatment. Dr. Moltó noted that data from more recent years might have captured lower discontinuation rates among pregnant individuals, but those data were not available for the study.
There was no difference in unfavorable obstetrical or infant outcomes, including spontaneous abortion, preeclampsia, gestational diabetes, major congenital malformation, and severe infection of the infant requiring hospitalization. Somewhat surprisingly, the data showed that women who discontinued a TNFi were more likely to be hospitalized for infection either during their pregnancy or up to 6 weeks after delivery, compared with those who continued therapy (1.3% vs. 0.2%, respectively).
Dr. Moltó is currently looking into what could be behind this counterintuitive result, but she hypothesizes that patients who had stopped TNFi may have been taking more glucocorticoids.
“At our institution, there is generally a comfort level with continuing TNF inhibitors during pregnancy, at least until about 36 weeks,” said Sara K. Tedeschi, MD, MPH, a rheumatologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. Sometimes, there is concern for risk of infection to the infant, depending on the type of TNFi being used, she added during a press conference.
“I think that these are really informative and supportive data to let women know that they probably have a really good chance of doing very well during the pregnancy if they continue” their TNFi, said Dr. Tedeschi, who was not involved with the study.
TNF discontinuation on the decline
In a related study, researchers at McGill University, Montreal, found that TNFi discontinuation prior to pregnancy had decreased over time in individuals with chronic inflammatory diseases.
Using a database of U.S. insurance claims, they identified 3,372 women with RA, ankylosing spondylitis (AS), psoriasis/psoriatic arthritis (PsA), and/or inflammatory bowel disease (IBD) who previously used a TNFi and gave birth between 2011 and 2019. A patient was considered to have used a TNFi if she had filled a prescription or had an infusion procedure insurance claim within 12 weeks before the gestational period or anytime during pregnancy. Researchers did not have time-specific data to account for women who stopped treatment at pregnancy diagnosis.
Nearly half (47%) of all identified pregnancies were in individuals with IBD, and the rest included patients with RA (24%), psoriasis or PsA (16%), AS (3%), or more than one diagnosis (10%).
In total, 14% of women discontinued TNFi use in the 12 weeks before becoming pregnant and did not restart. From 2011 to 2013, 19% of patients stopped their TNFi, but this proportion decreased overtime, with 10% of patients stopping therapy from 2017 to 2019 (P < .0001).
This decline “possibly reflects the increase in real-world evidence about the safety of TNFi in pregnancy. That research, in turn, led to new guidelines recommending the continuation of TNFi during pregnancy,” first author Leah Flatman, a PhD candidate in epidemiology at McGill, said in an interview. “I think we can see this potentially as good news.”
More patients with RA, psoriasis/PsA, and AS discontinued TNFi therapy prior to conception (23%-25%), compared with those with IBD (5%).
Ms. Flatman noted that her study and Moltó’s study complement each other by providing data on individuals stopping TNFi prior to conception versus those stopping treatment after pregnancy diagnosis.
“These findings demonstrate that continuing TNFi during pregnancy appears not to be associated with an increase in adverse obstetrical or infant outcomes,” Ms. Flatman said of Dr. Moltó’s study. “As guidelines currently recommend continuing TNFi, studies like this help demonstrate that the guideline changes do not appear to be associated with an increase in adverse events.”
Dr. Moltó and Ms. Flatman disclosed no relevant financial relationships. Dr. Tedeschi has worked as a consultant for Novartis.
A version of this article appeared on Medscape.com.
SAN DIEGO – Continuing a tumor necrosis factor inhibitor (TNFi) during pregnancy does not increase risk of worse fetal or obstetric outcomes, according to new research presented at the annual meeting of the American College of Rheumatology.
Patients who continued a TNFi also had fewer severe infections requiring hospitalization, compared with those who stopped taking the medication during their pregnancy.
“The main message is that patients continuing were not doing worse than the patients stopping. It’s an important clinical message for rheumatologists who are not really confident in dealing with these drugs during pregnancy,” said Anna Moltó, MD, PhD, a rheumatologist at Cochin Hospital, Paris, who led the research. “It adds to the data that it seems to be safe,” she added in an interview.
Previous research, largely from pregnant patients with inflammatory bowel disease, suggests that taking a TNFi during pregnancy is safe, and 2020 ACR guidelines conditionally recommend continuing therapy prior to and during pregnancy; however, many people still stop taking the drugs during pregnancy for fear of potentially harming the fetus.
To better understand how TNFi use affected pregnancy outcomes, Dr. Moltó and colleagues analyzed data from a French nationwide health insurance database to identify adult women with chronic rheumatic inflammatory disease. All women included in the cohort had a singleton pregnancy between 2008 and 2017 and were taking a TNFi upon pregnancy diagnosis.
Patients who restarted TNFi after initially pausing because of pregnancy were included in the continuation group.
Researchers identified more than 2,000 pregnancies, including 1,503 in individuals with spondyloarthritis and 579 individuals with rheumatoid arthritis. Patients were, on average, 31 years old and were diagnosed with a rheumatic disease 4 years prior to their pregnancy.
About 72% (n = 1,497) discontinued TNFi after learning they were pregnant, and 584 individuals continued treatment. Dr. Moltó noted that data from more recent years might have captured lower discontinuation rates among pregnant individuals, but those data were not available for the study.
There was no difference in unfavorable obstetrical or infant outcomes, including spontaneous abortion, preeclampsia, gestational diabetes, major congenital malformation, and severe infection of the infant requiring hospitalization. Somewhat surprisingly, the data showed that women who discontinued a TNFi were more likely to be hospitalized for infection either during their pregnancy or up to 6 weeks after delivery, compared with those who continued therapy (1.3% vs. 0.2%, respectively).
Dr. Moltó is currently looking into what could be behind this counterintuitive result, but she hypothesizes that patients who had stopped TNFi may have been taking more glucocorticoids.
“At our institution, there is generally a comfort level with continuing TNF inhibitors during pregnancy, at least until about 36 weeks,” said Sara K. Tedeschi, MD, MPH, a rheumatologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. Sometimes, there is concern for risk of infection to the infant, depending on the type of TNFi being used, she added during a press conference.
“I think that these are really informative and supportive data to let women know that they probably have a really good chance of doing very well during the pregnancy if they continue” their TNFi, said Dr. Tedeschi, who was not involved with the study.
TNF discontinuation on the decline
In a related study, researchers at McGill University, Montreal, found that TNFi discontinuation prior to pregnancy had decreased over time in individuals with chronic inflammatory diseases.
Using a database of U.S. insurance claims, they identified 3,372 women with RA, ankylosing spondylitis (AS), psoriasis/psoriatic arthritis (PsA), and/or inflammatory bowel disease (IBD) who previously used a TNFi and gave birth between 2011 and 2019. A patient was considered to have used a TNFi if she had filled a prescription or had an infusion procedure insurance claim within 12 weeks before the gestational period or anytime during pregnancy. Researchers did not have time-specific data to account for women who stopped treatment at pregnancy diagnosis.
Nearly half (47%) of all identified pregnancies were in individuals with IBD, and the rest included patients with RA (24%), psoriasis or PsA (16%), AS (3%), or more than one diagnosis (10%).
In total, 14% of women discontinued TNFi use in the 12 weeks before becoming pregnant and did not restart. From 2011 to 2013, 19% of patients stopped their TNFi, but this proportion decreased overtime, with 10% of patients stopping therapy from 2017 to 2019 (P < .0001).
This decline “possibly reflects the increase in real-world evidence about the safety of TNFi in pregnancy. That research, in turn, led to new guidelines recommending the continuation of TNFi during pregnancy,” first author Leah Flatman, a PhD candidate in epidemiology at McGill, said in an interview. “I think we can see this potentially as good news.”
More patients with RA, psoriasis/PsA, and AS discontinued TNFi therapy prior to conception (23%-25%), compared with those with IBD (5%).
Ms. Flatman noted that her study and Moltó’s study complement each other by providing data on individuals stopping TNFi prior to conception versus those stopping treatment after pregnancy diagnosis.
“These findings demonstrate that continuing TNFi during pregnancy appears not to be associated with an increase in adverse obstetrical or infant outcomes,” Ms. Flatman said of Dr. Moltó’s study. “As guidelines currently recommend continuing TNFi, studies like this help demonstrate that the guideline changes do not appear to be associated with an increase in adverse events.”
Dr. Moltó and Ms. Flatman disclosed no relevant financial relationships. Dr. Tedeschi has worked as a consultant for Novartis.
A version of this article appeared on Medscape.com.
SAN DIEGO – Continuing a tumor necrosis factor inhibitor (TNFi) during pregnancy does not increase risk of worse fetal or obstetric outcomes, according to new research presented at the annual meeting of the American College of Rheumatology.
Patients who continued a TNFi also had fewer severe infections requiring hospitalization, compared with those who stopped taking the medication during their pregnancy.
“The main message is that patients continuing were not doing worse than the patients stopping. It’s an important clinical message for rheumatologists who are not really confident in dealing with these drugs during pregnancy,” said Anna Moltó, MD, PhD, a rheumatologist at Cochin Hospital, Paris, who led the research. “It adds to the data that it seems to be safe,” she added in an interview.
Previous research, largely from pregnant patients with inflammatory bowel disease, suggests that taking a TNFi during pregnancy is safe, and 2020 ACR guidelines conditionally recommend continuing therapy prior to and during pregnancy; however, many people still stop taking the drugs during pregnancy for fear of potentially harming the fetus.
To better understand how TNFi use affected pregnancy outcomes, Dr. Moltó and colleagues analyzed data from a French nationwide health insurance database to identify adult women with chronic rheumatic inflammatory disease. All women included in the cohort had a singleton pregnancy between 2008 and 2017 and were taking a TNFi upon pregnancy diagnosis.
Patients who restarted TNFi after initially pausing because of pregnancy were included in the continuation group.
Researchers identified more than 2,000 pregnancies, including 1,503 in individuals with spondyloarthritis and 579 individuals with rheumatoid arthritis. Patients were, on average, 31 years old and were diagnosed with a rheumatic disease 4 years prior to their pregnancy.
About 72% (n = 1,497) discontinued TNFi after learning they were pregnant, and 584 individuals continued treatment. Dr. Moltó noted that data from more recent years might have captured lower discontinuation rates among pregnant individuals, but those data were not available for the study.
There was no difference in unfavorable obstetrical or infant outcomes, including spontaneous abortion, preeclampsia, gestational diabetes, major congenital malformation, and severe infection of the infant requiring hospitalization. Somewhat surprisingly, the data showed that women who discontinued a TNFi were more likely to be hospitalized for infection either during their pregnancy or up to 6 weeks after delivery, compared with those who continued therapy (1.3% vs. 0.2%, respectively).
Dr. Moltó is currently looking into what could be behind this counterintuitive result, but she hypothesizes that patients who had stopped TNFi may have been taking more glucocorticoids.
“At our institution, there is generally a comfort level with continuing TNF inhibitors during pregnancy, at least until about 36 weeks,” said Sara K. Tedeschi, MD, MPH, a rheumatologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. Sometimes, there is concern for risk of infection to the infant, depending on the type of TNFi being used, she added during a press conference.
“I think that these are really informative and supportive data to let women know that they probably have a really good chance of doing very well during the pregnancy if they continue” their TNFi, said Dr. Tedeschi, who was not involved with the study.
TNF discontinuation on the decline
In a related study, researchers at McGill University, Montreal, found that TNFi discontinuation prior to pregnancy had decreased over time in individuals with chronic inflammatory diseases.
Using a database of U.S. insurance claims, they identified 3,372 women with RA, ankylosing spondylitis (AS), psoriasis/psoriatic arthritis (PsA), and/or inflammatory bowel disease (IBD) who previously used a TNFi and gave birth between 2011 and 2019. A patient was considered to have used a TNFi if she had filled a prescription or had an infusion procedure insurance claim within 12 weeks before the gestational period or anytime during pregnancy. Researchers did not have time-specific data to account for women who stopped treatment at pregnancy diagnosis.
Nearly half (47%) of all identified pregnancies were in individuals with IBD, and the rest included patients with RA (24%), psoriasis or PsA (16%), AS (3%), or more than one diagnosis (10%).
In total, 14% of women discontinued TNFi use in the 12 weeks before becoming pregnant and did not restart. From 2011 to 2013, 19% of patients stopped their TNFi, but this proportion decreased overtime, with 10% of patients stopping therapy from 2017 to 2019 (P < .0001).
This decline “possibly reflects the increase in real-world evidence about the safety of TNFi in pregnancy. That research, in turn, led to new guidelines recommending the continuation of TNFi during pregnancy,” first author Leah Flatman, a PhD candidate in epidemiology at McGill, said in an interview. “I think we can see this potentially as good news.”
More patients with RA, psoriasis/PsA, and AS discontinued TNFi therapy prior to conception (23%-25%), compared with those with IBD (5%).
Ms. Flatman noted that her study and Moltó’s study complement each other by providing data on individuals stopping TNFi prior to conception versus those stopping treatment after pregnancy diagnosis.
“These findings demonstrate that continuing TNFi during pregnancy appears not to be associated with an increase in adverse obstetrical or infant outcomes,” Ms. Flatman said of Dr. Moltó’s study. “As guidelines currently recommend continuing TNFi, studies like this help demonstrate that the guideline changes do not appear to be associated with an increase in adverse events.”
Dr. Moltó and Ms. Flatman disclosed no relevant financial relationships. Dr. Tedeschi has worked as a consultant for Novartis.
A version of this article appeared on Medscape.com.
AT ACR 2023
Caution raised on reduced-dose steroids in rare vasculitides GPA, MPA
SAN DIEGO – A real-world analysis linked the PEXIVAS reduced-dose glucocorticoid (GC) regimen to a higher likelihood of a group of poor outcomes such as death in patients with severe granulomatosis with polyangiitis (GPA) or microscopic polyangiitis (MPA).
The retrospective observational study, presented at the annual meeting of the American College of Rheumatology, aimed to see whether results from the landmark PEXIVAS trial would hold up in a real-world analysis given some important limitations of the PEXIVAS’s primary outcome and its relative lack of balance in choice of induction agents.
First author of the new study, Sophie Nagle, MD, of Cochin Hospital in Paris, and colleagues in the French Vasculitis Study Group noted that PEXIVAS “demonstrated noninferiority of reduced-dose GC regimen compared to standard dose for the incidence of death or end-stage kidney disease (ESKD), with a significant reduction in serious infections at 1 year. However, the primary endpoint did not include disease progression or relapse, the majority of patients received cyclophosphamide as induction therapy, and subgroup analysis showed a trend towards an increased risk of death or ESKD in [rituximab]-treated patients.”
The new findings “give us pause about using low-dose glucocorticoid regimens” and suggest that rheumatologists might be a little more conservative about their use than randomized controlled trials such as PEXIVAS might suggest, said Vanderbilt University vasculitis specialist Kevin W. Byram, MD, who’s familiar with the findings but did not take part in the study.
Dr. Nagle reported that among 234 patients with either GPA or MPA, 33.3% of 126 who received a reduced-dose GC regimen experienced one of the 12-month composite primary outcome’s events of death, disease relapse, ESKD, or disease progression before remission that required treatment modification, compared with 18.5% of 108 who received the standard GC regimen (P = .016).
In a propensity score analysis, the higher risk of poor outcomes in the reduced-dose group remained (hazard ratio, 1.57; 95% confidence interval, 0.93-2.64). A multivariate analysis also identified a higher risk for the composite primary outcome in the reduced-dose group (HR, 1.72; 95% CI, 1.08-2.74), although there was no association with an increased risk of death or ESKD.
The PEXIVAS study, published in 2020, supported lower GC doses in antineutrophil cytoplasmic antibody–associated vasculitis, potentially revolutionizing treatment. “Historically, we have used high doses of glucocorticoids on slower tapers to treat this disease, which itself is a strategy that leads to potential complications,” Dr. Byram said. “PEXIVAS suggested we could potentially use less glucocorticoids in these patients.”
For the retrospective, multicenter study, researchers tracked patients from 2018 to 2022, all aged 15 and above. They included 93 with MPA and 141 with GPA. Nearly half were female, and they had a mean age of 61 years. The patients had severe flare-ups treated with rituximab or cyclophosphamide induction and reduced-dose or standard GC regimen.
The standard care and reduced-dose groups were similar, Dr. Nagle said, although the standard group had significantly more patients with GPA (71% vs. 29% with MPA) than did the low-dose group (51% with GPA, 49% with MPA).
The researchers reported that in a reduced-dose subgroup, patients with creatinine levels above 300 micromol/L were more likely to meet the primary endpoint (relative risk, 2.14; 95% CI, 1.14-4.03). Those treated with the reduced-dose GC regimen were also more likely to reach the primary endpoint (HR, 1.61; 95% CI, 0.94-2.77) and die or develop ESKD (HR, 2.42; 95% CI, 1.04-5.66).
However, adverse events at 12 months were similar in both groups: The authors noted that those who received the reduced-dose GC regimen didn’t have higher risk of death, ESKD, or severe infections.
The authors highlighted that “increased vigilance is required when using the reduced-dose GC regimen especially in two subgroups of patients due to the risk of failure: Patients receiving rituximab as induction therapy [and] patients with severe initial kidney disease (serum creatinine > 300 micromol/L).”
The study authors note several limitations: The study is retrospective, and the standard dose group is heterogeneous.
“This study raises the idea that we need to be careful in using low-dose glucocorticoid regimens, but not avoid them all together,” Dr. Byram said. “The finding that those with worse kidney function fared worse lines up with my clinical experiences. There are clearly populations with this disease that could benefit from more steroid, and it tends to be the ones that are sicker at presentation, particularly those requiring ICU-level care.”
He advised colleagues to “not be dogmatic and use strict low-dose regimens ‘just because.’ ”
No study funding was reported. Dr. Nagle reported having no relevant financial relationships, and disclosures for other authors were not reported. Dr. Byram reports serving on the Vasculitis Foundation board of directors.
SAN DIEGO – A real-world analysis linked the PEXIVAS reduced-dose glucocorticoid (GC) regimen to a higher likelihood of a group of poor outcomes such as death in patients with severe granulomatosis with polyangiitis (GPA) or microscopic polyangiitis (MPA).
The retrospective observational study, presented at the annual meeting of the American College of Rheumatology, aimed to see whether results from the landmark PEXIVAS trial would hold up in a real-world analysis given some important limitations of the PEXIVAS’s primary outcome and its relative lack of balance in choice of induction agents.
First author of the new study, Sophie Nagle, MD, of Cochin Hospital in Paris, and colleagues in the French Vasculitis Study Group noted that PEXIVAS “demonstrated noninferiority of reduced-dose GC regimen compared to standard dose for the incidence of death or end-stage kidney disease (ESKD), with a significant reduction in serious infections at 1 year. However, the primary endpoint did not include disease progression or relapse, the majority of patients received cyclophosphamide as induction therapy, and subgroup analysis showed a trend towards an increased risk of death or ESKD in [rituximab]-treated patients.”
The new findings “give us pause about using low-dose glucocorticoid regimens” and suggest that rheumatologists might be a little more conservative about their use than randomized controlled trials such as PEXIVAS might suggest, said Vanderbilt University vasculitis specialist Kevin W. Byram, MD, who’s familiar with the findings but did not take part in the study.
Dr. Nagle reported that among 234 patients with either GPA or MPA, 33.3% of 126 who received a reduced-dose GC regimen experienced one of the 12-month composite primary outcome’s events of death, disease relapse, ESKD, or disease progression before remission that required treatment modification, compared with 18.5% of 108 who received the standard GC regimen (P = .016).
In a propensity score analysis, the higher risk of poor outcomes in the reduced-dose group remained (hazard ratio, 1.57; 95% confidence interval, 0.93-2.64). A multivariate analysis also identified a higher risk for the composite primary outcome in the reduced-dose group (HR, 1.72; 95% CI, 1.08-2.74), although there was no association with an increased risk of death or ESKD.
The PEXIVAS study, published in 2020, supported lower GC doses in antineutrophil cytoplasmic antibody–associated vasculitis, potentially revolutionizing treatment. “Historically, we have used high doses of glucocorticoids on slower tapers to treat this disease, which itself is a strategy that leads to potential complications,” Dr. Byram said. “PEXIVAS suggested we could potentially use less glucocorticoids in these patients.”
For the retrospective, multicenter study, researchers tracked patients from 2018 to 2022, all aged 15 and above. They included 93 with MPA and 141 with GPA. Nearly half were female, and they had a mean age of 61 years. The patients had severe flare-ups treated with rituximab or cyclophosphamide induction and reduced-dose or standard GC regimen.
The standard care and reduced-dose groups were similar, Dr. Nagle said, although the standard group had significantly more patients with GPA (71% vs. 29% with MPA) than did the low-dose group (51% with GPA, 49% with MPA).
The researchers reported that in a reduced-dose subgroup, patients with creatinine levels above 300 micromol/L were more likely to meet the primary endpoint (relative risk, 2.14; 95% CI, 1.14-4.03). Those treated with the reduced-dose GC regimen were also more likely to reach the primary endpoint (HR, 1.61; 95% CI, 0.94-2.77) and die or develop ESKD (HR, 2.42; 95% CI, 1.04-5.66).
However, adverse events at 12 months were similar in both groups: The authors noted that those who received the reduced-dose GC regimen didn’t have higher risk of death, ESKD, or severe infections.
The authors highlighted that “increased vigilance is required when using the reduced-dose GC regimen especially in two subgroups of patients due to the risk of failure: Patients receiving rituximab as induction therapy [and] patients with severe initial kidney disease (serum creatinine > 300 micromol/L).”
The study authors note several limitations: The study is retrospective, and the standard dose group is heterogeneous.
“This study raises the idea that we need to be careful in using low-dose glucocorticoid regimens, but not avoid them all together,” Dr. Byram said. “The finding that those with worse kidney function fared worse lines up with my clinical experiences. There are clearly populations with this disease that could benefit from more steroid, and it tends to be the ones that are sicker at presentation, particularly those requiring ICU-level care.”
He advised colleagues to “not be dogmatic and use strict low-dose regimens ‘just because.’ ”
No study funding was reported. Dr. Nagle reported having no relevant financial relationships, and disclosures for other authors were not reported. Dr. Byram reports serving on the Vasculitis Foundation board of directors.
SAN DIEGO – A real-world analysis linked the PEXIVAS reduced-dose glucocorticoid (GC) regimen to a higher likelihood of a group of poor outcomes such as death in patients with severe granulomatosis with polyangiitis (GPA) or microscopic polyangiitis (MPA).
The retrospective observational study, presented at the annual meeting of the American College of Rheumatology, aimed to see whether results from the landmark PEXIVAS trial would hold up in a real-world analysis given some important limitations of the PEXIVAS’s primary outcome and its relative lack of balance in choice of induction agents.
First author of the new study, Sophie Nagle, MD, of Cochin Hospital in Paris, and colleagues in the French Vasculitis Study Group noted that PEXIVAS “demonstrated noninferiority of reduced-dose GC regimen compared to standard dose for the incidence of death or end-stage kidney disease (ESKD), with a significant reduction in serious infections at 1 year. However, the primary endpoint did not include disease progression or relapse, the majority of patients received cyclophosphamide as induction therapy, and subgroup analysis showed a trend towards an increased risk of death or ESKD in [rituximab]-treated patients.”
The new findings “give us pause about using low-dose glucocorticoid regimens” and suggest that rheumatologists might be a little more conservative about their use than randomized controlled trials such as PEXIVAS might suggest, said Vanderbilt University vasculitis specialist Kevin W. Byram, MD, who’s familiar with the findings but did not take part in the study.
Dr. Nagle reported that among 234 patients with either GPA or MPA, 33.3% of 126 who received a reduced-dose GC regimen experienced one of the 12-month composite primary outcome’s events of death, disease relapse, ESKD, or disease progression before remission that required treatment modification, compared with 18.5% of 108 who received the standard GC regimen (P = .016).
In a propensity score analysis, the higher risk of poor outcomes in the reduced-dose group remained (hazard ratio, 1.57; 95% confidence interval, 0.93-2.64). A multivariate analysis also identified a higher risk for the composite primary outcome in the reduced-dose group (HR, 1.72; 95% CI, 1.08-2.74), although there was no association with an increased risk of death or ESKD.
The PEXIVAS study, published in 2020, supported lower GC doses in antineutrophil cytoplasmic antibody–associated vasculitis, potentially revolutionizing treatment. “Historically, we have used high doses of glucocorticoids on slower tapers to treat this disease, which itself is a strategy that leads to potential complications,” Dr. Byram said. “PEXIVAS suggested we could potentially use less glucocorticoids in these patients.”
For the retrospective, multicenter study, researchers tracked patients from 2018 to 2022, all aged 15 and above. They included 93 with MPA and 141 with GPA. Nearly half were female, and they had a mean age of 61 years. The patients had severe flare-ups treated with rituximab or cyclophosphamide induction and reduced-dose or standard GC regimen.
The standard care and reduced-dose groups were similar, Dr. Nagle said, although the standard group had significantly more patients with GPA (71% vs. 29% with MPA) than did the low-dose group (51% with GPA, 49% with MPA).
The researchers reported that in a reduced-dose subgroup, patients with creatinine levels above 300 micromol/L were more likely to meet the primary endpoint (relative risk, 2.14; 95% CI, 1.14-4.03). Those treated with the reduced-dose GC regimen were also more likely to reach the primary endpoint (HR, 1.61; 95% CI, 0.94-2.77) and die or develop ESKD (HR, 2.42; 95% CI, 1.04-5.66).
However, adverse events at 12 months were similar in both groups: The authors noted that those who received the reduced-dose GC regimen didn’t have higher risk of death, ESKD, or severe infections.
The authors highlighted that “increased vigilance is required when using the reduced-dose GC regimen especially in two subgroups of patients due to the risk of failure: Patients receiving rituximab as induction therapy [and] patients with severe initial kidney disease (serum creatinine > 300 micromol/L).”
The study authors note several limitations: The study is retrospective, and the standard dose group is heterogeneous.
“This study raises the idea that we need to be careful in using low-dose glucocorticoid regimens, but not avoid them all together,” Dr. Byram said. “The finding that those with worse kidney function fared worse lines up with my clinical experiences. There are clearly populations with this disease that could benefit from more steroid, and it tends to be the ones that are sicker at presentation, particularly those requiring ICU-level care.”
He advised colleagues to “not be dogmatic and use strict low-dose regimens ‘just because.’ ”
No study funding was reported. Dr. Nagle reported having no relevant financial relationships, and disclosures for other authors were not reported. Dr. Byram reports serving on the Vasculitis Foundation board of directors.
AT ACR 2023
Blood test could predict future disability in MS
a new study suggests.
Rising NfL levels are a known indicator of neuroaxonal injury and correlate with MS disease activity. Levels rise in the presence of an MS relapse or MRI activity and fall following treatment with disease-modifying therapies. But the link between NfL levels and worsening disability was less understood.
This new analysis of NfL in two large MS cohorts found that elevated levels of the neuronal protein at baseline were associated with large increases in future disability risk, even in patients with no clinical relapse.
“This rising of NfL up to 2 years before signs of disability worsening represents the window when interventions may prevent worsening,” lead investigator Ahmed Abdelhak, MD, department of neurology, University of California, San Francisco, said in a press release.
The findings were published online in JAMA Neurology.
Early warning system?
The study included data on 1,899 patients with nearly 13,000 patient visits from two observational, long-term, real-world cohorts: the U.S.-based Expression, Proteomics, Imaging, Clinical (EPIC) study (n = 609 patients), and the Swiss Multiple Sclerosis Cohort trial (SMSC; n = 1,290 patients).
Investigators analyzed longitudinal serum NfL measurements in conjunction with clinical disability worsening, defined as 6 months or more of increased impairment as measured by the Expanded Disability Status Scale.
Researchers also assessed the temporal association between NfL measurements and the risk of increased disability and distinguished between disability with and without relapse.
Worsening disability was reported in 227 patients in the EPIC group and 435 in the SMSC trial.
Elevated NfL at baseline was associated with a 70% higher risk for worsening disability with relapse about 11 months later in the SMSC study (hazard ratio, 1.70; P = .02). In the EPIC trial, there was trend toward a 91% higher risk for worsening disability with relapse at 12.6 months, although the findings did not meet statistical significance (HR, 1.91; P = .07).
The risk of future disability progression independent of clinical relapse was 40% higher in those with high NfL at baseline in the EPIC study 12 months after baseline (HR, 1.40; P = .02) and 49% higher in the SMSC trial 21 months later (HR, 1.49; P < .001).
The early elevation of NfL levels suggests a slower degradation of nerve cells and could be a possible early warning system of future progression of disability, allowing time for interventions that could slow or even halt further disability.
“Monitoring NfL levels might be able to detect disease activity with higher sensitivity than clinical exam or conventional imaging,” senior author Jens Kuhle, MD, PhD, leader of the Swiss cohort and head of the Multiple Sclerosis Center at University Hospital and University of Basel, said in a statement.
Challenges for clinicians
Commenting on the findings, Robert Fox, MD, staff neurologist at the Mellen Center for MS and vice chair for research, Neurological Institute, Cleveland Clinic, said that, while there is a clinical test to measure NfL levels, incorporating that test into standard of care isn’t straightforward.
“The challenge for the practicing clinician is to translate these population-level studies to individual patient management decisions,” said Dr. Fox, who was not a part of the study.
“The published prediction curves corrected for age, sex, disease course, disease-modifying treatment, relapse within the past 90 days, and current disability status, the combination of which makes it rather challenging to calculate and interpret adjusted z score NfL levels in routine practice and then use it in clinical decision-making.”
The investigators said the study underscores the importance of NfL as an MS biomarker and “points to the existence of different windows of dynamic central nervous system pathology” that precedes worsening disability with or without relapse. But there may be a simpler explanation, Dr. Fox suggested.
“We know MRI activity occurs 5-10 times more frequently than relapses, and we know that MRI activity is associated with both NfL increases and future disability progression,” Dr. Fox said. “It is quite likely that the elevations in NfL seen here are reflective of new MRI disease activity, which frequently is seen without symptoms of an MS relapse,” he said
The study was funded by the Westridge Foundation, F. Hoffmann–La Roche, the Fishman Family, the Swiss National Research Foundation, the National Institutes of Health/National Institute of Neurological Disorders and Stroke, and the Valhalla Foundation. Dr. Abdelhak reported receiving grants from the German Multiple Sclerosis Society and the Weill Institute for Neurosciences outside the submitted work. Dr. Kuhle has received grants from Swiss MS Society, the Swiss National Research Foundation, the Progressive MS Alliance, Biogen, Merck, Celgene, Bristol-Myers Squibb, Novartis, Octave Bioscience, Roche, Sanofi, Alnylam, Bayer, Immunic, Quanterix, Neurogenesis, Stata DX, and the University of Basel outside the submitted work. Dr. Fox reported receiving consulting fees from Siemens and Roche.
A version of this article appeared on Medscape.com.
a new study suggests.
Rising NfL levels are a known indicator of neuroaxonal injury and correlate with MS disease activity. Levels rise in the presence of an MS relapse or MRI activity and fall following treatment with disease-modifying therapies. But the link between NfL levels and worsening disability was less understood.
This new analysis of NfL in two large MS cohorts found that elevated levels of the neuronal protein at baseline were associated with large increases in future disability risk, even in patients with no clinical relapse.
“This rising of NfL up to 2 years before signs of disability worsening represents the window when interventions may prevent worsening,” lead investigator Ahmed Abdelhak, MD, department of neurology, University of California, San Francisco, said in a press release.
The findings were published online in JAMA Neurology.
Early warning system?
The study included data on 1,899 patients with nearly 13,000 patient visits from two observational, long-term, real-world cohorts: the U.S.-based Expression, Proteomics, Imaging, Clinical (EPIC) study (n = 609 patients), and the Swiss Multiple Sclerosis Cohort trial (SMSC; n = 1,290 patients).
Investigators analyzed longitudinal serum NfL measurements in conjunction with clinical disability worsening, defined as 6 months or more of increased impairment as measured by the Expanded Disability Status Scale.
Researchers also assessed the temporal association between NfL measurements and the risk of increased disability and distinguished between disability with and without relapse.
Worsening disability was reported in 227 patients in the EPIC group and 435 in the SMSC trial.
Elevated NfL at baseline was associated with a 70% higher risk for worsening disability with relapse about 11 months later in the SMSC study (hazard ratio, 1.70; P = .02). In the EPIC trial, there was trend toward a 91% higher risk for worsening disability with relapse at 12.6 months, although the findings did not meet statistical significance (HR, 1.91; P = .07).
The risk of future disability progression independent of clinical relapse was 40% higher in those with high NfL at baseline in the EPIC study 12 months after baseline (HR, 1.40; P = .02) and 49% higher in the SMSC trial 21 months later (HR, 1.49; P < .001).
The early elevation of NfL levels suggests a slower degradation of nerve cells and could be a possible early warning system of future progression of disability, allowing time for interventions that could slow or even halt further disability.
“Monitoring NfL levels might be able to detect disease activity with higher sensitivity than clinical exam or conventional imaging,” senior author Jens Kuhle, MD, PhD, leader of the Swiss cohort and head of the Multiple Sclerosis Center at University Hospital and University of Basel, said in a statement.
Challenges for clinicians
Commenting on the findings, Robert Fox, MD, staff neurologist at the Mellen Center for MS and vice chair for research, Neurological Institute, Cleveland Clinic, said that, while there is a clinical test to measure NfL levels, incorporating that test into standard of care isn’t straightforward.
“The challenge for the practicing clinician is to translate these population-level studies to individual patient management decisions,” said Dr. Fox, who was not a part of the study.
“The published prediction curves corrected for age, sex, disease course, disease-modifying treatment, relapse within the past 90 days, and current disability status, the combination of which makes it rather challenging to calculate and interpret adjusted z score NfL levels in routine practice and then use it in clinical decision-making.”
The investigators said the study underscores the importance of NfL as an MS biomarker and “points to the existence of different windows of dynamic central nervous system pathology” that precedes worsening disability with or without relapse. But there may be a simpler explanation, Dr. Fox suggested.
“We know MRI activity occurs 5-10 times more frequently than relapses, and we know that MRI activity is associated with both NfL increases and future disability progression,” Dr. Fox said. “It is quite likely that the elevations in NfL seen here are reflective of new MRI disease activity, which frequently is seen without symptoms of an MS relapse,” he said
The study was funded by the Westridge Foundation, F. Hoffmann–La Roche, the Fishman Family, the Swiss National Research Foundation, the National Institutes of Health/National Institute of Neurological Disorders and Stroke, and the Valhalla Foundation. Dr. Abdelhak reported receiving grants from the German Multiple Sclerosis Society and the Weill Institute for Neurosciences outside the submitted work. Dr. Kuhle has received grants from Swiss MS Society, the Swiss National Research Foundation, the Progressive MS Alliance, Biogen, Merck, Celgene, Bristol-Myers Squibb, Novartis, Octave Bioscience, Roche, Sanofi, Alnylam, Bayer, Immunic, Quanterix, Neurogenesis, Stata DX, and the University of Basel outside the submitted work. Dr. Fox reported receiving consulting fees from Siemens and Roche.
A version of this article appeared on Medscape.com.
a new study suggests.
Rising NfL levels are a known indicator of neuroaxonal injury and correlate with MS disease activity. Levels rise in the presence of an MS relapse or MRI activity and fall following treatment with disease-modifying therapies. But the link between NfL levels and worsening disability was less understood.
This new analysis of NfL in two large MS cohorts found that elevated levels of the neuronal protein at baseline were associated with large increases in future disability risk, even in patients with no clinical relapse.
“This rising of NfL up to 2 years before signs of disability worsening represents the window when interventions may prevent worsening,” lead investigator Ahmed Abdelhak, MD, department of neurology, University of California, San Francisco, said in a press release.
The findings were published online in JAMA Neurology.
Early warning system?
The study included data on 1,899 patients with nearly 13,000 patient visits from two observational, long-term, real-world cohorts: the U.S.-based Expression, Proteomics, Imaging, Clinical (EPIC) study (n = 609 patients), and the Swiss Multiple Sclerosis Cohort trial (SMSC; n = 1,290 patients).
Investigators analyzed longitudinal serum NfL measurements in conjunction with clinical disability worsening, defined as 6 months or more of increased impairment as measured by the Expanded Disability Status Scale.
Researchers also assessed the temporal association between NfL measurements and the risk of increased disability and distinguished between disability with and without relapse.
Worsening disability was reported in 227 patients in the EPIC group and 435 in the SMSC trial.
Elevated NfL at baseline was associated with a 70% higher risk for worsening disability with relapse about 11 months later in the SMSC study (hazard ratio, 1.70; P = .02). In the EPIC trial, there was trend toward a 91% higher risk for worsening disability with relapse at 12.6 months, although the findings did not meet statistical significance (HR, 1.91; P = .07).
The risk of future disability progression independent of clinical relapse was 40% higher in those with high NfL at baseline in the EPIC study 12 months after baseline (HR, 1.40; P = .02) and 49% higher in the SMSC trial 21 months later (HR, 1.49; P < .001).
The early elevation of NfL levels suggests a slower degradation of nerve cells and could be a possible early warning system of future progression of disability, allowing time for interventions that could slow or even halt further disability.
“Monitoring NfL levels might be able to detect disease activity with higher sensitivity than clinical exam or conventional imaging,” senior author Jens Kuhle, MD, PhD, leader of the Swiss cohort and head of the Multiple Sclerosis Center at University Hospital and University of Basel, said in a statement.
Challenges for clinicians
Commenting on the findings, Robert Fox, MD, staff neurologist at the Mellen Center for MS and vice chair for research, Neurological Institute, Cleveland Clinic, said that, while there is a clinical test to measure NfL levels, incorporating that test into standard of care isn’t straightforward.
“The challenge for the practicing clinician is to translate these population-level studies to individual patient management decisions,” said Dr. Fox, who was not a part of the study.
“The published prediction curves corrected for age, sex, disease course, disease-modifying treatment, relapse within the past 90 days, and current disability status, the combination of which makes it rather challenging to calculate and interpret adjusted z score NfL levels in routine practice and then use it in clinical decision-making.”
The investigators said the study underscores the importance of NfL as an MS biomarker and “points to the existence of different windows of dynamic central nervous system pathology” that precedes worsening disability with or without relapse. But there may be a simpler explanation, Dr. Fox suggested.
“We know MRI activity occurs 5-10 times more frequently than relapses, and we know that MRI activity is associated with both NfL increases and future disability progression,” Dr. Fox said. “It is quite likely that the elevations in NfL seen here are reflective of new MRI disease activity, which frequently is seen without symptoms of an MS relapse,” he said
The study was funded by the Westridge Foundation, F. Hoffmann–La Roche, the Fishman Family, the Swiss National Research Foundation, the National Institutes of Health/National Institute of Neurological Disorders and Stroke, and the Valhalla Foundation. Dr. Abdelhak reported receiving grants from the German Multiple Sclerosis Society and the Weill Institute for Neurosciences outside the submitted work. Dr. Kuhle has received grants from Swiss MS Society, the Swiss National Research Foundation, the Progressive MS Alliance, Biogen, Merck, Celgene, Bristol-Myers Squibb, Novartis, Octave Bioscience, Roche, Sanofi, Alnylam, Bayer, Immunic, Quanterix, Neurogenesis, Stata DX, and the University of Basel outside the submitted work. Dr. Fox reported receiving consulting fees from Siemens and Roche.
A version of this article appeared on Medscape.com.
FROM JAMA NEUROLOGY
CKD-EPI eGFR formula surpasses alternatives in young adults
PHILADELPHIA –
The two alternative formulas for calculating eGFR, the CKiD U25 (Chronic Kidney Disease in Children under 25) and the European Kidney Function Consortium equations, showed higher levels of bias that resulted in underestimates of kidney function, particularly in younger adults 18-25 years old and in those with higher eGFR values, Leslie A. Inker, MD, said at Kidney Week 2023, organized by the American Society of Nephrology.
However, for young adults with a history of childhood CKD and especially for those who continue under the care of pediatric clinicians even after they become young adults, use of the CKiD U25 equation, remains a reasonable option, said Dr. Inker, professor and director of the Kidney and Blood Pressure Center at Tufts Medical Center in Boston. The CKiD U25 equation is intended for people aged 1-25 years and came out in late 2020.
Pediatric nephrologists use the CKiD U25 but the results of the 2021 CKD-EPI race-free equation is what U.S. clinical labs routinely report for people aged 18 or older. “Our findings support the current practice of pediatric nephrologists” who may opt to use the CKiD U25 even when a patient turns 18 years or older, Dr. Inker said in an interview.
But the new results also support the current practice of U.S. labs, which is to focus on calculating eGFR in anyone at least 18 years old using the 2021 equation developed by the CKD-EPI, work led by Dr. Inker. The new findings confirm routine use of the 2021 CKD-EPI equation in adults as young as 18 years old, especially when they’re having their eGFR calculated for the first time, she said.
Discontinuity changing from CKiD U25 to CKD-EPI is ‘not huge’
“It’s important to understand that the 2021 CKD-EPI equation works in young adults,” noted Josef Coresh, MD, PhD, professor of clinical epidemiology at Johns Hopkins University, Baltimore, who collaborated on both the current study and on developing the 2021 CKD-EPI equation.
The new data show that the discontinuity in eGFR produced by switching from the CKiD U25 formula to the 2021 CKD-EPI formula “is not huge, maybe about 5 mL/min per 1.73 m2 higher. People should focus on the new baseline and subsequent trends, not the modest difference between equations,” Dr. Coresh advised in an interview.
The study run by Dr. Inker and her associates used 1,491 people aged 18-40 years and enrolled in a cohort created by the CKD-EPI. They compared measured GFR levels in each subject with the estimates generated by the 2021 race-free CKD-EPI equation, the CKiD U25 equation, and a third equation developed by the EKFC and introduced in 2021.
Less bias with the 2021 CKD-EPI equation
The researchers compared the three eGFR equations with their respective measured GFR values by two metrics: bias, defined as the median difference between measured and estimated GFR; and the percentage of eGFR values that fell within 30% of the corresponding measured GFR value.
The results showed that bias was lowest using the 2021 CKD-EPI equation, with an overall median difference of 0.5 mL/min per 1.73 m2. This compared with median differences of 7.2 with the CKiD U25 equation and 4.9 mL/min per 1.73 m2 with the EKFC equation. The disparity in bias was greatest among those with eGFR values in the range of 60-90 mL/min per 1.73 m2 and was also greatest for those 18-25 years old.
The CKD-EPI equation results also showed the greatest consistency of bias across the entire 18- to 40-year-old range. Between-group differences were small for the percentage of eGFR values that fell within 30% of measured GFR, with all three equations scoring in the range of 88%-90%.
The study received no commercial funding. Dr. Inker is a consultant to Diamtrix and her department receives research funding from Chinook, Omeros, Reata, and Tricida. Dr. Coresh is a consultant to Healthy.io and SomaLogic and he has an ownership interest in Healthy.io.
PHILADELPHIA –
The two alternative formulas for calculating eGFR, the CKiD U25 (Chronic Kidney Disease in Children under 25) and the European Kidney Function Consortium equations, showed higher levels of bias that resulted in underestimates of kidney function, particularly in younger adults 18-25 years old and in those with higher eGFR values, Leslie A. Inker, MD, said at Kidney Week 2023, organized by the American Society of Nephrology.
However, for young adults with a history of childhood CKD and especially for those who continue under the care of pediatric clinicians even after they become young adults, use of the CKiD U25 equation, remains a reasonable option, said Dr. Inker, professor and director of the Kidney and Blood Pressure Center at Tufts Medical Center in Boston. The CKiD U25 equation is intended for people aged 1-25 years and came out in late 2020.
Pediatric nephrologists use the CKiD U25 but the results of the 2021 CKD-EPI race-free equation is what U.S. clinical labs routinely report for people aged 18 or older. “Our findings support the current practice of pediatric nephrologists” who may opt to use the CKiD U25 even when a patient turns 18 years or older, Dr. Inker said in an interview.
But the new results also support the current practice of U.S. labs, which is to focus on calculating eGFR in anyone at least 18 years old using the 2021 equation developed by the CKD-EPI, work led by Dr. Inker. The new findings confirm routine use of the 2021 CKD-EPI equation in adults as young as 18 years old, especially when they’re having their eGFR calculated for the first time, she said.
Discontinuity changing from CKiD U25 to CKD-EPI is ‘not huge’
“It’s important to understand that the 2021 CKD-EPI equation works in young adults,” noted Josef Coresh, MD, PhD, professor of clinical epidemiology at Johns Hopkins University, Baltimore, who collaborated on both the current study and on developing the 2021 CKD-EPI equation.
The new data show that the discontinuity in eGFR produced by switching from the CKiD U25 formula to the 2021 CKD-EPI formula “is not huge, maybe about 5 mL/min per 1.73 m2 higher. People should focus on the new baseline and subsequent trends, not the modest difference between equations,” Dr. Coresh advised in an interview.
The study run by Dr. Inker and her associates used 1,491 people aged 18-40 years and enrolled in a cohort created by the CKD-EPI. They compared measured GFR levels in each subject with the estimates generated by the 2021 race-free CKD-EPI equation, the CKiD U25 equation, and a third equation developed by the EKFC and introduced in 2021.
Less bias with the 2021 CKD-EPI equation
The researchers compared the three eGFR equations with their respective measured GFR values by two metrics: bias, defined as the median difference between measured and estimated GFR; and the percentage of eGFR values that fell within 30% of the corresponding measured GFR value.
The results showed that bias was lowest using the 2021 CKD-EPI equation, with an overall median difference of 0.5 mL/min per 1.73 m2. This compared with median differences of 7.2 with the CKiD U25 equation and 4.9 mL/min per 1.73 m2 with the EKFC equation. The disparity in bias was greatest among those with eGFR values in the range of 60-90 mL/min per 1.73 m2 and was also greatest for those 18-25 years old.
The CKD-EPI equation results also showed the greatest consistency of bias across the entire 18- to 40-year-old range. Between-group differences were small for the percentage of eGFR values that fell within 30% of measured GFR, with all three equations scoring in the range of 88%-90%.
The study received no commercial funding. Dr. Inker is a consultant to Diamtrix and her department receives research funding from Chinook, Omeros, Reata, and Tricida. Dr. Coresh is a consultant to Healthy.io and SomaLogic and he has an ownership interest in Healthy.io.
PHILADELPHIA –
The two alternative formulas for calculating eGFR, the CKiD U25 (Chronic Kidney Disease in Children under 25) and the European Kidney Function Consortium equations, showed higher levels of bias that resulted in underestimates of kidney function, particularly in younger adults 18-25 years old and in those with higher eGFR values, Leslie A. Inker, MD, said at Kidney Week 2023, organized by the American Society of Nephrology.
However, for young adults with a history of childhood CKD and especially for those who continue under the care of pediatric clinicians even after they become young adults, use of the CKiD U25 equation, remains a reasonable option, said Dr. Inker, professor and director of the Kidney and Blood Pressure Center at Tufts Medical Center in Boston. The CKiD U25 equation is intended for people aged 1-25 years and came out in late 2020.
Pediatric nephrologists use the CKiD U25 but the results of the 2021 CKD-EPI race-free equation is what U.S. clinical labs routinely report for people aged 18 or older. “Our findings support the current practice of pediatric nephrologists” who may opt to use the CKiD U25 even when a patient turns 18 years or older, Dr. Inker said in an interview.
But the new results also support the current practice of U.S. labs, which is to focus on calculating eGFR in anyone at least 18 years old using the 2021 equation developed by the CKD-EPI, work led by Dr. Inker. The new findings confirm routine use of the 2021 CKD-EPI equation in adults as young as 18 years old, especially when they’re having their eGFR calculated for the first time, she said.
Discontinuity changing from CKiD U25 to CKD-EPI is ‘not huge’
“It’s important to understand that the 2021 CKD-EPI equation works in young adults,” noted Josef Coresh, MD, PhD, professor of clinical epidemiology at Johns Hopkins University, Baltimore, who collaborated on both the current study and on developing the 2021 CKD-EPI equation.
The new data show that the discontinuity in eGFR produced by switching from the CKiD U25 formula to the 2021 CKD-EPI formula “is not huge, maybe about 5 mL/min per 1.73 m2 higher. People should focus on the new baseline and subsequent trends, not the modest difference between equations,” Dr. Coresh advised in an interview.
The study run by Dr. Inker and her associates used 1,491 people aged 18-40 years and enrolled in a cohort created by the CKD-EPI. They compared measured GFR levels in each subject with the estimates generated by the 2021 race-free CKD-EPI equation, the CKiD U25 equation, and a third equation developed by the EKFC and introduced in 2021.
Less bias with the 2021 CKD-EPI equation
The researchers compared the three eGFR equations with their respective measured GFR values by two metrics: bias, defined as the median difference between measured and estimated GFR; and the percentage of eGFR values that fell within 30% of the corresponding measured GFR value.
The results showed that bias was lowest using the 2021 CKD-EPI equation, with an overall median difference of 0.5 mL/min per 1.73 m2. This compared with median differences of 7.2 with the CKiD U25 equation and 4.9 mL/min per 1.73 m2 with the EKFC equation. The disparity in bias was greatest among those with eGFR values in the range of 60-90 mL/min per 1.73 m2 and was also greatest for those 18-25 years old.
The CKD-EPI equation results also showed the greatest consistency of bias across the entire 18- to 40-year-old range. Between-group differences were small for the percentage of eGFR values that fell within 30% of measured GFR, with all three equations scoring in the range of 88%-90%.
The study received no commercial funding. Dr. Inker is a consultant to Diamtrix and her department receives research funding from Chinook, Omeros, Reata, and Tricida. Dr. Coresh is a consultant to Healthy.io and SomaLogic and he has an ownership interest in Healthy.io.
AT KIDNEY WEEK 2023
Artificial intelligence presents opportunities, challenges in neurologic practice
PHOENIX – and it presents opportunities for increased production and automation of some tasks. However, it is prone to error and ‘hallucinations’ despite an authoritative tone, so its conclusions must be verified.
Those were some of the messages from a talk by John Morren, MD, an associate professor of neurology at Case Western Reserve University, Cleveland, who spoke about AI at the 2023 annual meeting of the American Association for Neuromuscular and Electrodiagnostic Medicine (AANEM).
He encouraged attendees to get involved in the conversation of AI, because it is here to stay and will have a big impact on health care. “If we’re not around the table making decisions, decisions will be made for us in our absence and won’t be in our favor,” said Dr. Morren.
He started out his talk by asking if anyone in the room had used AI. After about half raised their hands, he countered that nearly everyone likely had. Voice assistants like SIRI and Alexa, social media with curated feeds, online shopping tools that provide product suggestions, and content recommendations from streaming services like Netflix all rely on AI technology.
Within medicine, AI is already playing a role in various fields, including medical imaging, disease diagnosis, drug discovery and development, predictive analytics, personalized medicine, telemedicine, and health care management.
It also has potential to be used on the job. For example, ChatGPT can generate and refine conversations towards a specific length, format, style, and level of detail. Alternatives include Bing AI from Microsoft, Bard AI from Google, Writesonic, Copy.ai, SpinBot, HIX.AI, and Chatsonic.
Specific to medicine, Consensus is a search engine that uses AI to search for, summarize, and synthesize studies from peer-reviewed literature.
Trust, but verify
Dr. Morren presented some specific use cases, including patient education and responses to patient inquiries, as well as generating letters to insurance companies appealing denial of coverage claims. He also showed an example where he asked Bing AI to explain to a patient, at a sixth- to seventh-grade reading level, the red-flag symptoms of myasthenic crisis.
AI can generate summaries of clinical evidence of previous studies. Asked by this reporter how to trust the accuracies of the summaries if the user hasn’t thoroughly read the papers, he acknowledged the imperfection of AI. “I would say that if you’re going to make a decision that you would not have made normally based on the summary that it’s giving, if you can find the fact that you’re anchoring the decision on, go into the article yourself and make sure that it’s well vetted. The AI is just good to tap you on your shoulder and say, ‘hey, just consider this.’ That’s all it is. You should always trust, but verify. If the AI is forcing you to say something new that you would not say, maybe don’t do it – or at least research it to know that it’s the truth and then you elevate yourself and get yourself to the next level.”
Limitations
The need to verify can create its own burden, according to one attendee. “I often find I end up spending more time verifying [what ChatGPT has provided]. This seems to take more time than a traditional way of going to PubMed or UpToDate or any of the other human generated consensus way,” he said.
Dr. Morren replied that he wouldn’t recommend using ChatGPT to query medical literature. Instead he recommended Consensus, which only searches the peer-reviewed medical literature.
Another key limitation is that most AI programs are date limited: For example, ChatGPT doesn’t include information after September 2021, though this may change with paid subscriptions. He also starkly warned the audience to never enter sensitive information, including patient identifiers.
There are legal and ethical considerations to AI. Dr. Morren warned against overreliance on AI, as this could undermine compassion and lead to erosion of trust, which makes it important to disclose any use of AI-generated content.
Another attendee raised concerns that AI may be generating research content, including slides for presentations, abstracts, titles, or article text. Dr. Morren said that some organizations, such as the International Committee of Medical Journal Editors, have incorporated AI in their recommendations, stating that authors should disclose any contributions of AI to their publications. However, there is little that can be done to identify AI-generated content, leaving it up to the honor code.
Asked to make predictions about how AI will evolve in the clinic over the next 2-3 years, Dr. Morren suggested that it will likely be embedded in electronic medical records. He anticipated that it will save physicians time so that they can spend more time interacting directly with patients. He quoted Eric Topol, MD, professor of medicine at Scripps Research Translational Institute, La Jolla, Calif., as saying that AI could save 20% of a physician’s time, which could be spent with patients. Dr. Morren saw it differently. “I know where that 20% of time liberated is going to go. I’m going to see 20% more patients. I’m a realist,” he said, to audience laughter.
He also predicted that AI will be found in wearables and devices, allowing health care to expand into the patient’s home in real time. “A lot of what we’re wearing is going to be an extension of the doctor’s office,” he said.
For those hoping for more guidance, Dr. Morren noted that he is the chairman of the professional practice committee of AANEM, and the group will be putting out a position statement within the next couple of months. “It will be a little bit of a blueprint for the path going forward. There are specific things that need to be done. In research, for example, you have to ensure that datasets are diverse enough. To do that we need to have inter-institutional collaboration. We have to ensure patient privacy. Consent for this needs to be a little more explicit because this is a novel area. Those are things that need to be stipulated and ratified through a task force.”
Dr. Morren has no relevant financial disclosures.
PHOENIX – and it presents opportunities for increased production and automation of some tasks. However, it is prone to error and ‘hallucinations’ despite an authoritative tone, so its conclusions must be verified.
Those were some of the messages from a talk by John Morren, MD, an associate professor of neurology at Case Western Reserve University, Cleveland, who spoke about AI at the 2023 annual meeting of the American Association for Neuromuscular and Electrodiagnostic Medicine (AANEM).
He encouraged attendees to get involved in the conversation of AI, because it is here to stay and will have a big impact on health care. “If we’re not around the table making decisions, decisions will be made for us in our absence and won’t be in our favor,” said Dr. Morren.
He started out his talk by asking if anyone in the room had used AI. After about half raised their hands, he countered that nearly everyone likely had. Voice assistants like SIRI and Alexa, social media with curated feeds, online shopping tools that provide product suggestions, and content recommendations from streaming services like Netflix all rely on AI technology.
Within medicine, AI is already playing a role in various fields, including medical imaging, disease diagnosis, drug discovery and development, predictive analytics, personalized medicine, telemedicine, and health care management.
It also has potential to be used on the job. For example, ChatGPT can generate and refine conversations towards a specific length, format, style, and level of detail. Alternatives include Bing AI from Microsoft, Bard AI from Google, Writesonic, Copy.ai, SpinBot, HIX.AI, and Chatsonic.
Specific to medicine, Consensus is a search engine that uses AI to search for, summarize, and synthesize studies from peer-reviewed literature.
Trust, but verify
Dr. Morren presented some specific use cases, including patient education and responses to patient inquiries, as well as generating letters to insurance companies appealing denial of coverage claims. He also showed an example where he asked Bing AI to explain to a patient, at a sixth- to seventh-grade reading level, the red-flag symptoms of myasthenic crisis.
AI can generate summaries of clinical evidence of previous studies. Asked by this reporter how to trust the accuracies of the summaries if the user hasn’t thoroughly read the papers, he acknowledged the imperfection of AI. “I would say that if you’re going to make a decision that you would not have made normally based on the summary that it’s giving, if you can find the fact that you’re anchoring the decision on, go into the article yourself and make sure that it’s well vetted. The AI is just good to tap you on your shoulder and say, ‘hey, just consider this.’ That’s all it is. You should always trust, but verify. If the AI is forcing you to say something new that you would not say, maybe don’t do it – or at least research it to know that it’s the truth and then you elevate yourself and get yourself to the next level.”
Limitations
The need to verify can create its own burden, according to one attendee. “I often find I end up spending more time verifying [what ChatGPT has provided]. This seems to take more time than a traditional way of going to PubMed or UpToDate or any of the other human generated consensus way,” he said.
Dr. Morren replied that he wouldn’t recommend using ChatGPT to query medical literature. Instead he recommended Consensus, which only searches the peer-reviewed medical literature.
Another key limitation is that most AI programs are date limited: For example, ChatGPT doesn’t include information after September 2021, though this may change with paid subscriptions. He also starkly warned the audience to never enter sensitive information, including patient identifiers.
There are legal and ethical considerations to AI. Dr. Morren warned against overreliance on AI, as this could undermine compassion and lead to erosion of trust, which makes it important to disclose any use of AI-generated content.
Another attendee raised concerns that AI may be generating research content, including slides for presentations, abstracts, titles, or article text. Dr. Morren said that some organizations, such as the International Committee of Medical Journal Editors, have incorporated AI in their recommendations, stating that authors should disclose any contributions of AI to their publications. However, there is little that can be done to identify AI-generated content, leaving it up to the honor code.
Asked to make predictions about how AI will evolve in the clinic over the next 2-3 years, Dr. Morren suggested that it will likely be embedded in electronic medical records. He anticipated that it will save physicians time so that they can spend more time interacting directly with patients. He quoted Eric Topol, MD, professor of medicine at Scripps Research Translational Institute, La Jolla, Calif., as saying that AI could save 20% of a physician’s time, which could be spent with patients. Dr. Morren saw it differently. “I know where that 20% of time liberated is going to go. I’m going to see 20% more patients. I’m a realist,” he said, to audience laughter.
He also predicted that AI will be found in wearables and devices, allowing health care to expand into the patient’s home in real time. “A lot of what we’re wearing is going to be an extension of the doctor’s office,” he said.
For those hoping for more guidance, Dr. Morren noted that he is the chairman of the professional practice committee of AANEM, and the group will be putting out a position statement within the next couple of months. “It will be a little bit of a blueprint for the path going forward. There are specific things that need to be done. In research, for example, you have to ensure that datasets are diverse enough. To do that we need to have inter-institutional collaboration. We have to ensure patient privacy. Consent for this needs to be a little more explicit because this is a novel area. Those are things that need to be stipulated and ratified through a task force.”
Dr. Morren has no relevant financial disclosures.
PHOENIX – and it presents opportunities for increased production and automation of some tasks. However, it is prone to error and ‘hallucinations’ despite an authoritative tone, so its conclusions must be verified.
Those were some of the messages from a talk by John Morren, MD, an associate professor of neurology at Case Western Reserve University, Cleveland, who spoke about AI at the 2023 annual meeting of the American Association for Neuromuscular and Electrodiagnostic Medicine (AANEM).
He encouraged attendees to get involved in the conversation of AI, because it is here to stay and will have a big impact on health care. “If we’re not around the table making decisions, decisions will be made for us in our absence and won’t be in our favor,” said Dr. Morren.
He started out his talk by asking if anyone in the room had used AI. After about half raised their hands, he countered that nearly everyone likely had. Voice assistants like SIRI and Alexa, social media with curated feeds, online shopping tools that provide product suggestions, and content recommendations from streaming services like Netflix all rely on AI technology.
Within medicine, AI is already playing a role in various fields, including medical imaging, disease diagnosis, drug discovery and development, predictive analytics, personalized medicine, telemedicine, and health care management.
It also has potential to be used on the job. For example, ChatGPT can generate and refine conversations towards a specific length, format, style, and level of detail. Alternatives include Bing AI from Microsoft, Bard AI from Google, Writesonic, Copy.ai, SpinBot, HIX.AI, and Chatsonic.
Specific to medicine, Consensus is a search engine that uses AI to search for, summarize, and synthesize studies from peer-reviewed literature.
Trust, but verify
Dr. Morren presented some specific use cases, including patient education and responses to patient inquiries, as well as generating letters to insurance companies appealing denial of coverage claims. He also showed an example where he asked Bing AI to explain to a patient, at a sixth- to seventh-grade reading level, the red-flag symptoms of myasthenic crisis.
AI can generate summaries of clinical evidence of previous studies. Asked by this reporter how to trust the accuracies of the summaries if the user hasn’t thoroughly read the papers, he acknowledged the imperfection of AI. “I would say that if you’re going to make a decision that you would not have made normally based on the summary that it’s giving, if you can find the fact that you’re anchoring the decision on, go into the article yourself and make sure that it’s well vetted. The AI is just good to tap you on your shoulder and say, ‘hey, just consider this.’ That’s all it is. You should always trust, but verify. If the AI is forcing you to say something new that you would not say, maybe don’t do it – or at least research it to know that it’s the truth and then you elevate yourself and get yourself to the next level.”
Limitations
The need to verify can create its own burden, according to one attendee. “I often find I end up spending more time verifying [what ChatGPT has provided]. This seems to take more time than a traditional way of going to PubMed or UpToDate or any of the other human generated consensus way,” he said.
Dr. Morren replied that he wouldn’t recommend using ChatGPT to query medical literature. Instead he recommended Consensus, which only searches the peer-reviewed medical literature.
Another key limitation is that most AI programs are date limited: For example, ChatGPT doesn’t include information after September 2021, though this may change with paid subscriptions. He also starkly warned the audience to never enter sensitive information, including patient identifiers.
There are legal and ethical considerations to AI. Dr. Morren warned against overreliance on AI, as this could undermine compassion and lead to erosion of trust, which makes it important to disclose any use of AI-generated content.
Another attendee raised concerns that AI may be generating research content, including slides for presentations, abstracts, titles, or article text. Dr. Morren said that some organizations, such as the International Committee of Medical Journal Editors, have incorporated AI in their recommendations, stating that authors should disclose any contributions of AI to their publications. However, there is little that can be done to identify AI-generated content, leaving it up to the honor code.
Asked to make predictions about how AI will evolve in the clinic over the next 2-3 years, Dr. Morren suggested that it will likely be embedded in electronic medical records. He anticipated that it will save physicians time so that they can spend more time interacting directly with patients. He quoted Eric Topol, MD, professor of medicine at Scripps Research Translational Institute, La Jolla, Calif., as saying that AI could save 20% of a physician’s time, which could be spent with patients. Dr. Morren saw it differently. “I know where that 20% of time liberated is going to go. I’m going to see 20% more patients. I’m a realist,” he said, to audience laughter.
He also predicted that AI will be found in wearables and devices, allowing health care to expand into the patient’s home in real time. “A lot of what we’re wearing is going to be an extension of the doctor’s office,” he said.
For those hoping for more guidance, Dr. Morren noted that he is the chairman of the professional practice committee of AANEM, and the group will be putting out a position statement within the next couple of months. “It will be a little bit of a blueprint for the path going forward. There are specific things that need to be done. In research, for example, you have to ensure that datasets are diverse enough. To do that we need to have inter-institutional collaboration. We have to ensure patient privacy. Consent for this needs to be a little more explicit because this is a novel area. Those are things that need to be stipulated and ratified through a task force.”
Dr. Morren has no relevant financial disclosures.
AT AANEM 2023
A nurse’s view: Women desperately need information about pelvic floor disorders
Pelvic floor disorders are embarrassing, annoying, painful, and extremely disruptive to a woman’s life, often resulting in depression, anxiety, and a poor self-image. According to a 2021 study, approximately 75% of peripartum women and 68% of postmenopausal women feel insufficiently informed about pelvic floor disorders.1
Consequently, a large majority of women are not seeking care for these disorders. This drives health care costs higher as women wait until their symptoms are unbearable until finally seeking help. Many of these women don’t know they have options.
Who is at risk?
To understand the scope of this growing problem, it is vital to see who is most at risk. Parity, age, body mass index, and race are significant factors, although any woman can have a pelvic floor disorder (PFD).
Urinary incontinence (UI), pelvic floor prolapses (POP), and fecal incontinence (FI) are three of the most common pelvic floor disorders. Pregnancy and childbirth, specifically a vaginal birth, greatly contribute to this population’s risk. In pregnancy, the increase in plasma volume and glomerular filtration rate, along with hormone changes impacting urethral pressure and the growing gravid uterus, cause urinary frequency and nocturia. This can result in urinary incontinence during and after pregnancy.
Indeed, 76% of women with urinary incontinence at 3 months postpartum report it 12 years later.1 Third- and fourth-degree lacerations during delivery are uncommon (3.3%), but can cause fecal incontinence, often requiring surgery.1 Independently, all of these symptoms have been correlated with sexual dysfunction and postpartum depression.
One-third of all women and 50% of women over the age of 55 are currently affected by a PFD. Contributing factors include hormone changes with menopause that affect the pelvic floor muscles and connective tissue, prior childbirth and pregnancy, constipation, heavy lifting, prior pelvic surgery, and obesity. These women are vulnerable to pelvic organ prolapse from the weakened pelvic floor muscles. They will often present with a vague complaint of “something is protruding out of my vagina.” These women also present with urinary incontinence or leakage, proclaiming they have to wear a diaper or a pad. Without proper knowledge, aging women think these issues are normal and nothing can be done.
The woman with a BMI above 30 may have damaged tissues supporting the uterus and bladder, weakening those organs, and causing a prolapse. Incontinence is a result of poor muscle and connective tissue of the vagina that support the urethra. Obese women can suffer from both urinary and bowel incontinence. By the year 2030, it is projected that one in two adults will be obese.2 This will greatly impact health care costs.
To date, there is little conclusive evidence on the impact of race on pelvic floor disorders. A study in Scientific Reports did find that Asian women have a significantly lower risk for any PFD.2 Some research has found that Black and Hispanic women have less risk for UI but are at higher risk for FI and other PFDs.3 Understandably, women of certain cultures and demographics may be less likely to report incontinence to their clinicians and may be less informed as well.
What can we do?
The American College of Obstetricians and Gynecologists (ACOG) has acknowledged the deficiencies and lack of standard care of pelvic health in pregnancy and postpartum.1 There are differences in definitions across clinical practice and in the medical literature. Inconsistent patient reporting of PFD symptoms occurs due to nonstandard methods (questionnaire, interview, physical exam). With the often-short time allotted for visits with health care providers, women may neglect to discuss their symptoms, especially if they have other more pressing matters to address.
At the first OB appointment, a pregnant woman should be given information on what are normal and abnormal symptoms, from the beginning through postpartum. At each visit, she should be given ample opportunity to discuss symptoms of pelvic health. Clinicians should continue assessing, questioning, and discussing treatment options as applicable. Women need to know that early recognition and treatment can have a positive affect on their pelvic health for years to come.
ACOG recommends all postpartum patients see an obstetric provider within 3 weeks of delivery.1 Most are seen at 6 weeks. Pelvic health should be discussed at this final postpartum appointment, including normal and abnormal symptoms within the next few months and beyond.
Regardless of pregnancy status, women need a safe and supportive place to describe their pelvic floor issues. There is a validated questionnaire tool available for postpartum, but one is desperately needed for all women, especially women at risk. A pelvic health assessment must be included in every annual exam.
Women need to know there are multiple treatment modalities including simple exercises, physical therapy, a variety of pessaries, medications, and surgery. Sometimes, all that is needed are a few lifestyle changes: avoiding pushing or straining while urinating or having a bowel movement, maintaining a healthy diet rich in high fiber foods, and drinking plenty of fluids.
The National Public Health Service in the United Kingdom recently announced a government-funded program for pelvic health services to begin in April 2024.4 This program will address the pelvic floor needs, assessment, education and treatment for women after childbirth.
There are multiple clinics in the United States focusing on women’s health that feature urogynecologists – specialists in pelvic floor disorders. These specialists do a thorough health and physical assessment, explain types of pelvic floor disorders, and suggest appropriate treatment options. Most importantly, urogynecologists listen and address a woman’s concerns and fears.
There is no reason for women to feel compromised at any age. We, as health care providers, just need to assess, educate, treat, and follow up.
Ms. Barnett is a registered nurse in the department of obstetrics, Mills-Peninsula Medical Center, Burlingame, Calif. She has disclosed no relevant financial relationships.
References
1. Madsen AM et al. Recognition and management of pelvic floor disorders in pregnancy and the postpartum period. Obstet Gynecol Clin North Am. 2021 Sep;48(3):571-84. doi: 10.1016/j.ogc.2021.05.009.
2. Kenne KA et al. Prevalence of pelvic floor disorders in adult women being seen in a primary care setting and associated risk factors. Sci Rep. 2022 June; (12):9878. doi: 10.1038/s41598-022-13501-w.
3. Nygaard I et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311-6. doi: 10.1001/jama.300.11.1311.
4. United Kingdom Department of Health and Social Care. “National pelvic health service to support women.” 2023 Oct 19.
Pelvic floor disorders are embarrassing, annoying, painful, and extremely disruptive to a woman’s life, often resulting in depression, anxiety, and a poor self-image. According to a 2021 study, approximately 75% of peripartum women and 68% of postmenopausal women feel insufficiently informed about pelvic floor disorders.1
Consequently, a large majority of women are not seeking care for these disorders. This drives health care costs higher as women wait until their symptoms are unbearable until finally seeking help. Many of these women don’t know they have options.
Who is at risk?
To understand the scope of this growing problem, it is vital to see who is most at risk. Parity, age, body mass index, and race are significant factors, although any woman can have a pelvic floor disorder (PFD).
Urinary incontinence (UI), pelvic floor prolapses (POP), and fecal incontinence (FI) are three of the most common pelvic floor disorders. Pregnancy and childbirth, specifically a vaginal birth, greatly contribute to this population’s risk. In pregnancy, the increase in plasma volume and glomerular filtration rate, along with hormone changes impacting urethral pressure and the growing gravid uterus, cause urinary frequency and nocturia. This can result in urinary incontinence during and after pregnancy.
Indeed, 76% of women with urinary incontinence at 3 months postpartum report it 12 years later.1 Third- and fourth-degree lacerations during delivery are uncommon (3.3%), but can cause fecal incontinence, often requiring surgery.1 Independently, all of these symptoms have been correlated with sexual dysfunction and postpartum depression.
One-third of all women and 50% of women over the age of 55 are currently affected by a PFD. Contributing factors include hormone changes with menopause that affect the pelvic floor muscles and connective tissue, prior childbirth and pregnancy, constipation, heavy lifting, prior pelvic surgery, and obesity. These women are vulnerable to pelvic organ prolapse from the weakened pelvic floor muscles. They will often present with a vague complaint of “something is protruding out of my vagina.” These women also present with urinary incontinence or leakage, proclaiming they have to wear a diaper or a pad. Without proper knowledge, aging women think these issues are normal and nothing can be done.
The woman with a BMI above 30 may have damaged tissues supporting the uterus and bladder, weakening those organs, and causing a prolapse. Incontinence is a result of poor muscle and connective tissue of the vagina that support the urethra. Obese women can suffer from both urinary and bowel incontinence. By the year 2030, it is projected that one in two adults will be obese.2 This will greatly impact health care costs.
To date, there is little conclusive evidence on the impact of race on pelvic floor disorders. A study in Scientific Reports did find that Asian women have a significantly lower risk for any PFD.2 Some research has found that Black and Hispanic women have less risk for UI but are at higher risk for FI and other PFDs.3 Understandably, women of certain cultures and demographics may be less likely to report incontinence to their clinicians and may be less informed as well.
What can we do?
The American College of Obstetricians and Gynecologists (ACOG) has acknowledged the deficiencies and lack of standard care of pelvic health in pregnancy and postpartum.1 There are differences in definitions across clinical practice and in the medical literature. Inconsistent patient reporting of PFD symptoms occurs due to nonstandard methods (questionnaire, interview, physical exam). With the often-short time allotted for visits with health care providers, women may neglect to discuss their symptoms, especially if they have other more pressing matters to address.
At the first OB appointment, a pregnant woman should be given information on what are normal and abnormal symptoms, from the beginning through postpartum. At each visit, she should be given ample opportunity to discuss symptoms of pelvic health. Clinicians should continue assessing, questioning, and discussing treatment options as applicable. Women need to know that early recognition and treatment can have a positive affect on their pelvic health for years to come.
ACOG recommends all postpartum patients see an obstetric provider within 3 weeks of delivery.1 Most are seen at 6 weeks. Pelvic health should be discussed at this final postpartum appointment, including normal and abnormal symptoms within the next few months and beyond.
Regardless of pregnancy status, women need a safe and supportive place to describe their pelvic floor issues. There is a validated questionnaire tool available for postpartum, but one is desperately needed for all women, especially women at risk. A pelvic health assessment must be included in every annual exam.
Women need to know there are multiple treatment modalities including simple exercises, physical therapy, a variety of pessaries, medications, and surgery. Sometimes, all that is needed are a few lifestyle changes: avoiding pushing or straining while urinating or having a bowel movement, maintaining a healthy diet rich in high fiber foods, and drinking plenty of fluids.
The National Public Health Service in the United Kingdom recently announced a government-funded program for pelvic health services to begin in April 2024.4 This program will address the pelvic floor needs, assessment, education and treatment for women after childbirth.
There are multiple clinics in the United States focusing on women’s health that feature urogynecologists – specialists in pelvic floor disorders. These specialists do a thorough health and physical assessment, explain types of pelvic floor disorders, and suggest appropriate treatment options. Most importantly, urogynecologists listen and address a woman’s concerns and fears.
There is no reason for women to feel compromised at any age. We, as health care providers, just need to assess, educate, treat, and follow up.
Ms. Barnett is a registered nurse in the department of obstetrics, Mills-Peninsula Medical Center, Burlingame, Calif. She has disclosed no relevant financial relationships.
References
1. Madsen AM et al. Recognition and management of pelvic floor disorders in pregnancy and the postpartum period. Obstet Gynecol Clin North Am. 2021 Sep;48(3):571-84. doi: 10.1016/j.ogc.2021.05.009.
2. Kenne KA et al. Prevalence of pelvic floor disorders in adult women being seen in a primary care setting and associated risk factors. Sci Rep. 2022 June; (12):9878. doi: 10.1038/s41598-022-13501-w.
3. Nygaard I et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311-6. doi: 10.1001/jama.300.11.1311.
4. United Kingdom Department of Health and Social Care. “National pelvic health service to support women.” 2023 Oct 19.
Pelvic floor disorders are embarrassing, annoying, painful, and extremely disruptive to a woman’s life, often resulting in depression, anxiety, and a poor self-image. According to a 2021 study, approximately 75% of peripartum women and 68% of postmenopausal women feel insufficiently informed about pelvic floor disorders.1
Consequently, a large majority of women are not seeking care for these disorders. This drives health care costs higher as women wait until their symptoms are unbearable until finally seeking help. Many of these women don’t know they have options.
Who is at risk?
To understand the scope of this growing problem, it is vital to see who is most at risk. Parity, age, body mass index, and race are significant factors, although any woman can have a pelvic floor disorder (PFD).
Urinary incontinence (UI), pelvic floor prolapses (POP), and fecal incontinence (FI) are three of the most common pelvic floor disorders. Pregnancy and childbirth, specifically a vaginal birth, greatly contribute to this population’s risk. In pregnancy, the increase in plasma volume and glomerular filtration rate, along with hormone changes impacting urethral pressure and the growing gravid uterus, cause urinary frequency and nocturia. This can result in urinary incontinence during and after pregnancy.
Indeed, 76% of women with urinary incontinence at 3 months postpartum report it 12 years later.1 Third- and fourth-degree lacerations during delivery are uncommon (3.3%), but can cause fecal incontinence, often requiring surgery.1 Independently, all of these symptoms have been correlated with sexual dysfunction and postpartum depression.
One-third of all women and 50% of women over the age of 55 are currently affected by a PFD. Contributing factors include hormone changes with menopause that affect the pelvic floor muscles and connective tissue, prior childbirth and pregnancy, constipation, heavy lifting, prior pelvic surgery, and obesity. These women are vulnerable to pelvic organ prolapse from the weakened pelvic floor muscles. They will often present with a vague complaint of “something is protruding out of my vagina.” These women also present with urinary incontinence or leakage, proclaiming they have to wear a diaper or a pad. Without proper knowledge, aging women think these issues are normal and nothing can be done.
The woman with a BMI above 30 may have damaged tissues supporting the uterus and bladder, weakening those organs, and causing a prolapse. Incontinence is a result of poor muscle and connective tissue of the vagina that support the urethra. Obese women can suffer from both urinary and bowel incontinence. By the year 2030, it is projected that one in two adults will be obese.2 This will greatly impact health care costs.
To date, there is little conclusive evidence on the impact of race on pelvic floor disorders. A study in Scientific Reports did find that Asian women have a significantly lower risk for any PFD.2 Some research has found that Black and Hispanic women have less risk for UI but are at higher risk for FI and other PFDs.3 Understandably, women of certain cultures and demographics may be less likely to report incontinence to their clinicians and may be less informed as well.
What can we do?
The American College of Obstetricians and Gynecologists (ACOG) has acknowledged the deficiencies and lack of standard care of pelvic health in pregnancy and postpartum.1 There are differences in definitions across clinical practice and in the medical literature. Inconsistent patient reporting of PFD symptoms occurs due to nonstandard methods (questionnaire, interview, physical exam). With the often-short time allotted for visits with health care providers, women may neglect to discuss their symptoms, especially if they have other more pressing matters to address.
At the first OB appointment, a pregnant woman should be given information on what are normal and abnormal symptoms, from the beginning through postpartum. At each visit, she should be given ample opportunity to discuss symptoms of pelvic health. Clinicians should continue assessing, questioning, and discussing treatment options as applicable. Women need to know that early recognition and treatment can have a positive affect on their pelvic health for years to come.
ACOG recommends all postpartum patients see an obstetric provider within 3 weeks of delivery.1 Most are seen at 6 weeks. Pelvic health should be discussed at this final postpartum appointment, including normal and abnormal symptoms within the next few months and beyond.
Regardless of pregnancy status, women need a safe and supportive place to describe their pelvic floor issues. There is a validated questionnaire tool available for postpartum, but one is desperately needed for all women, especially women at risk. A pelvic health assessment must be included in every annual exam.
Women need to know there are multiple treatment modalities including simple exercises, physical therapy, a variety of pessaries, medications, and surgery. Sometimes, all that is needed are a few lifestyle changes: avoiding pushing or straining while urinating or having a bowel movement, maintaining a healthy diet rich in high fiber foods, and drinking plenty of fluids.
The National Public Health Service in the United Kingdom recently announced a government-funded program for pelvic health services to begin in April 2024.4 This program will address the pelvic floor needs, assessment, education and treatment for women after childbirth.
There are multiple clinics in the United States focusing on women’s health that feature urogynecologists – specialists in pelvic floor disorders. These specialists do a thorough health and physical assessment, explain types of pelvic floor disorders, and suggest appropriate treatment options. Most importantly, urogynecologists listen and address a woman’s concerns and fears.
There is no reason for women to feel compromised at any age. We, as health care providers, just need to assess, educate, treat, and follow up.
Ms. Barnett is a registered nurse in the department of obstetrics, Mills-Peninsula Medical Center, Burlingame, Calif. She has disclosed no relevant financial relationships.
References
1. Madsen AM et al. Recognition and management of pelvic floor disorders in pregnancy and the postpartum period. Obstet Gynecol Clin North Am. 2021 Sep;48(3):571-84. doi: 10.1016/j.ogc.2021.05.009.
2. Kenne KA et al. Prevalence of pelvic floor disorders in adult women being seen in a primary care setting and associated risk factors. Sci Rep. 2022 June; (12):9878. doi: 10.1038/s41598-022-13501-w.
3. Nygaard I et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311-6. doi: 10.1001/jama.300.11.1311.
4. United Kingdom Department of Health and Social Care. “National pelvic health service to support women.” 2023 Oct 19.
Survey: 42% of PCPs not familiar with biologics for asthma
ANAHEIM, CALIF. – Patients with uncontrolled asthma are seen more often by primary care providers (PCPs) than by allergists, but a survey has found that
Bijalben Patel, MD, with the department of internal medicine, University of South Florida, Tampa, said in an interview that in addition to the considerable lack of knowledge of biologics in primary care, she was surprised that 77% of survey participants stated they only referred patients to specialists after two or more exacerbations.
“This is important because these patients are considered to have exacerbation-prone asthma, which should be managed by specialists,” she said.
She said that being “unfamiliar” with biologics meant that the healthcare provider may have heard of biologics but did not know the various types, initiation criteria, or side effects.
The researchers administered a REDCap (Research Electronic Data Capture) survey by email to primary care attending and resident physicians in the departments of internal medicine, family medicine, and pediatrics, and 85 responded. Responses were compared using Chi-square tests.
Patel presented the results of the survey at the annual meeting of the American College of Allergy, Asthma & Immunology.
82% do not order labs
Familiarity did not vary in primary care with number of patients with asthma seen per month, the researchers noted.
“Also, the frequency of PCP referrals to a specialist did not change familiarity with biologics (P = .260) or eligibility criteria (P = .393),” the researchers said.
In addition, they found that 82% of those surveyed do not order labs, and 90% do not use absolute eosinophil count to guide care.
Dr. Patel explained that lab work such as obtaining IgE levels and a complete blood count with a differential and examining the absolute eosinophil count help identify patients who are at high risk for future exacerbation and also treatable phenotypic traits, which can be targeted with biologic therapy.
Angela Duff Hogan, MD, vice chair of the ACAAI Asthma Committee and professor of pediatrics at Eastern Virginia Medical School, Norfolk, said in an interview that she finds the delay on referrals the most concerning finding in the survey results.
“I’m not as concerned they are not obtaining labs,” said Dr. Hogan, who was not part of the study. “The specialist can do that. It’s more concerning they wait so long to refer a patient with poorly controlled asthma. We know that asthma patients treated by an allergist have better asthma control, better quality of life, and reduced health care costs.”
Asthma specialists ‘need better marketing’
Dr. Hogan said that the results show the need for more studies to demonstrate that asthma specialists can improve outcomes and reduce healthcare costs.
“Objective data is more convincing than subjective data,” she noted. “As a specialty, we need to disseminate more information about asthma management, the “new” asthma guidelines, SMART/MART therapy, and the importance of biologicals in asthma. We need better marketing as a specialty in asthma care.”
Dr. Patel said that their goal with the study is to raise awareness about the available asthma biologic therapies, which have been improving care for 2 decades.
“The results of the survey point to the need to improve the communication between primary care physicians and asthma care specialists, including regarding use of biologics,” senior author Juan Carlos Cardet, MD, MPH, also an allergy specialist at USF, added in a press release. “Biologics have become an important tool in the treatment of asthma and other allergic diseases such as atopic dermatitis (eczema), chronic rhinosinusitis with nasal polyps and eosinophilic esophagitis, and can prevent substantial ill results from occurring in patients who are eligible for them.”
The study authors and Dr. Hogan disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
ANAHEIM, CALIF. – Patients with uncontrolled asthma are seen more often by primary care providers (PCPs) than by allergists, but a survey has found that
Bijalben Patel, MD, with the department of internal medicine, University of South Florida, Tampa, said in an interview that in addition to the considerable lack of knowledge of biologics in primary care, she was surprised that 77% of survey participants stated they only referred patients to specialists after two or more exacerbations.
“This is important because these patients are considered to have exacerbation-prone asthma, which should be managed by specialists,” she said.
She said that being “unfamiliar” with biologics meant that the healthcare provider may have heard of biologics but did not know the various types, initiation criteria, or side effects.
The researchers administered a REDCap (Research Electronic Data Capture) survey by email to primary care attending and resident physicians in the departments of internal medicine, family medicine, and pediatrics, and 85 responded. Responses were compared using Chi-square tests.
Patel presented the results of the survey at the annual meeting of the American College of Allergy, Asthma & Immunology.
82% do not order labs
Familiarity did not vary in primary care with number of patients with asthma seen per month, the researchers noted.
“Also, the frequency of PCP referrals to a specialist did not change familiarity with biologics (P = .260) or eligibility criteria (P = .393),” the researchers said.
In addition, they found that 82% of those surveyed do not order labs, and 90% do not use absolute eosinophil count to guide care.
Dr. Patel explained that lab work such as obtaining IgE levels and a complete blood count with a differential and examining the absolute eosinophil count help identify patients who are at high risk for future exacerbation and also treatable phenotypic traits, which can be targeted with biologic therapy.
Angela Duff Hogan, MD, vice chair of the ACAAI Asthma Committee and professor of pediatrics at Eastern Virginia Medical School, Norfolk, said in an interview that she finds the delay on referrals the most concerning finding in the survey results.
“I’m not as concerned they are not obtaining labs,” said Dr. Hogan, who was not part of the study. “The specialist can do that. It’s more concerning they wait so long to refer a patient with poorly controlled asthma. We know that asthma patients treated by an allergist have better asthma control, better quality of life, and reduced health care costs.”
Asthma specialists ‘need better marketing’
Dr. Hogan said that the results show the need for more studies to demonstrate that asthma specialists can improve outcomes and reduce healthcare costs.
“Objective data is more convincing than subjective data,” she noted. “As a specialty, we need to disseminate more information about asthma management, the “new” asthma guidelines, SMART/MART therapy, and the importance of biologicals in asthma. We need better marketing as a specialty in asthma care.”
Dr. Patel said that their goal with the study is to raise awareness about the available asthma biologic therapies, which have been improving care for 2 decades.
“The results of the survey point to the need to improve the communication between primary care physicians and asthma care specialists, including regarding use of biologics,” senior author Juan Carlos Cardet, MD, MPH, also an allergy specialist at USF, added in a press release. “Biologics have become an important tool in the treatment of asthma and other allergic diseases such as atopic dermatitis (eczema), chronic rhinosinusitis with nasal polyps and eosinophilic esophagitis, and can prevent substantial ill results from occurring in patients who are eligible for them.”
The study authors and Dr. Hogan disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
ANAHEIM, CALIF. – Patients with uncontrolled asthma are seen more often by primary care providers (PCPs) than by allergists, but a survey has found that
Bijalben Patel, MD, with the department of internal medicine, University of South Florida, Tampa, said in an interview that in addition to the considerable lack of knowledge of biologics in primary care, she was surprised that 77% of survey participants stated they only referred patients to specialists after two or more exacerbations.
“This is important because these patients are considered to have exacerbation-prone asthma, which should be managed by specialists,” she said.
She said that being “unfamiliar” with biologics meant that the healthcare provider may have heard of biologics but did not know the various types, initiation criteria, or side effects.
The researchers administered a REDCap (Research Electronic Data Capture) survey by email to primary care attending and resident physicians in the departments of internal medicine, family medicine, and pediatrics, and 85 responded. Responses were compared using Chi-square tests.
Patel presented the results of the survey at the annual meeting of the American College of Allergy, Asthma & Immunology.
82% do not order labs
Familiarity did not vary in primary care with number of patients with asthma seen per month, the researchers noted.
“Also, the frequency of PCP referrals to a specialist did not change familiarity with biologics (P = .260) or eligibility criteria (P = .393),” the researchers said.
In addition, they found that 82% of those surveyed do not order labs, and 90% do not use absolute eosinophil count to guide care.
Dr. Patel explained that lab work such as obtaining IgE levels and a complete blood count with a differential and examining the absolute eosinophil count help identify patients who are at high risk for future exacerbation and also treatable phenotypic traits, which can be targeted with biologic therapy.
Angela Duff Hogan, MD, vice chair of the ACAAI Asthma Committee and professor of pediatrics at Eastern Virginia Medical School, Norfolk, said in an interview that she finds the delay on referrals the most concerning finding in the survey results.
“I’m not as concerned they are not obtaining labs,” said Dr. Hogan, who was not part of the study. “The specialist can do that. It’s more concerning they wait so long to refer a patient with poorly controlled asthma. We know that asthma patients treated by an allergist have better asthma control, better quality of life, and reduced health care costs.”
Asthma specialists ‘need better marketing’
Dr. Hogan said that the results show the need for more studies to demonstrate that asthma specialists can improve outcomes and reduce healthcare costs.
“Objective data is more convincing than subjective data,” she noted. “As a specialty, we need to disseminate more information about asthma management, the “new” asthma guidelines, SMART/MART therapy, and the importance of biologicals in asthma. We need better marketing as a specialty in asthma care.”
Dr. Patel said that their goal with the study is to raise awareness about the available asthma biologic therapies, which have been improving care for 2 decades.
“The results of the survey point to the need to improve the communication between primary care physicians and asthma care specialists, including regarding use of biologics,” senior author Juan Carlos Cardet, MD, MPH, also an allergy specialist at USF, added in a press release. “Biologics have become an important tool in the treatment of asthma and other allergic diseases such as atopic dermatitis (eczema), chronic rhinosinusitis with nasal polyps and eosinophilic esophagitis, and can prevent substantial ill results from occurring in patients who are eligible for them.”
The study authors and Dr. Hogan disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM ACAAI 2023