User login
CHEST Physician’s planned coverage of CHEST 2017
CHEST Physician is providing on-site coverage of the CHEST annual meeting in Toronto from Oct. 29 through Nov. 1.
We are planning to share findings from the latest research on treating COPD, sleep apnea, pulmonary hypertension, severe asthma, and other diseases that are part of pulmonary, critical care, and sleep medicine. Any improved methods for managing an ICU and updated recommendations on screening for lung cancer will also be on our radar.
The meeting’s agenda includes presentations of hundreds of study abstracts, and we thought you would be interested in hearing which ones grabbed the attention of some of CHEST Physician’s editorial advisory board members.
Board member Susan L. Millard, MD, FCCP, suggested attendees check out presentations of the following two studies:
- Impact of Race on Quality of Life of Families of Children with Asthma when Asthma Guidelines are Followed: Long-Term Follow-Up
- Results Of A Phase 3, Multicenter, Randomized, Placebo-controlled Trial of Remimazolam: A New Ultra Short Acting Benzodiazepine for Bronchoscopy
The first study is part of a session entitled Pediatrics, scheduled to run from 3:15 to 4:15 p.m. on Sunday, Oct. 29, in Convention Center - 606. Shahid Sheikh, MD, of Nationwide Children’s Hospital in New Albany, Ohio, is scheduled to present the abstract at 4:00 p.m.
Dr. Millard, who is Therapeutic Development Network director for the Pediatric CF Care Center and director of research for pediatric pulmonary and sleep medicine at the Helen DeVos Children’s Hospital in Grand Rapids, Mich., noted that she is interested in Dr. Sheikh’s research, “because cultural diversity is such a hot topic in general.”
Her other recommendation is part of the Late Breaking Abstracts 2 session, scheduled to occur on Wednesday, Nov. 1, from 2:45 to 4:15 p.m. in Convention Center - 603. CHEST President, Gerard A. Silvestri, MD, MS, FCCP, will present the abstract at 4:00 p.m.
Dr. Millard said she is interested in this study, because new drug options are so helpful for the frequently performed bronchoscopy.
Two sleep medicine experts on CHEST Physician’s editorial advisory board also selected a few presentations they expect to be newsworthy.
David Schulman, MD, MPH, FCCP, and professor of medicine at Emory University School of Medicine in Atlanta suggested CHEST Physician cover the following studies:
- Results of a Randomized, Placebo-Controlled, Double-Blind, 12-Week, Multicenter Study of JZP-110 for the Treatment Of Excessive Sleepiness in Patients with OSA, scheduled to be presented on Sunday, Oct. 29, at 1:30 p.m. in Convention Center - 601A. Dr. Kingman Strohl, MD, FCCP, of University Hospitals Case Medical Center-Sleep Center in Shaker Heights, Ohio, will present this research during a session entitled, Obstructive Sleep Apnea: Insights & Management, running from 1:30 to 3:00 p.m.
- History of Sleep Apnea and Cardiovascular Disease may Portend Improved Mortality in Patients With Acute Ischemic Stroke, scheduled to be presented on Tuesday, Oct. 31, at 11:15 a.m., in Convention Center - 601A. Nura Festic will present this research during the session, “Sleep, Heart, Brain and More,” running from 11:00 a.m. to 12:15 p.m.
- Ischemic Preconditioning in OSA Patients Manifested after Surviving a Cardiac Arrest? John Moss, MD, of Jacksonville, Fla., will present this study on Tuesday, Oct. 31, at 11:30 a.m., in Convention Center - 601A as part of the session “Sleep, Heart, Brain and More.”
Krishna M. Sundar, MD, FCCP, also recommended that CHEST Physician cover “A Prospective Cohort Study of Endothelial Function and its Relationship to Aspirin Responsiveness in OSA Patients.” Lirim Krveshi is scheduled to present this study on Sunday, Oct. 29, at 1:45 p.m. in Convention Center - 601A. This presentation is part of the Obstructive Sleep Apnea: Insights & Management session.
Dr. Sundar is an associate clinical professor of pulmonary, critical care and sleep medicine and medical director of the Sleep-Wake Center at the University of Utah, Salt Lake City.
To view the full agenda of the CHEST annual meeting, visit: chestmeeting.chestnet.org.
Look for CHEST Physician’s coverage of CHEST 2017 on our conference coverage page.
CHEST Physician is providing on-site coverage of the CHEST annual meeting in Toronto from Oct. 29 through Nov. 1.
We are planning to share findings from the latest research on treating COPD, sleep apnea, pulmonary hypertension, severe asthma, and other diseases that are part of pulmonary, critical care, and sleep medicine. Any improved methods for managing an ICU and updated recommendations on screening for lung cancer will also be on our radar.
The meeting’s agenda includes presentations of hundreds of study abstracts, and we thought you would be interested in hearing which ones grabbed the attention of some of CHEST Physician’s editorial advisory board members.
Board member Susan L. Millard, MD, FCCP, suggested attendees check out presentations of the following two studies:
- Impact of Race on Quality of Life of Families of Children with Asthma when Asthma Guidelines are Followed: Long-Term Follow-Up
- Results Of A Phase 3, Multicenter, Randomized, Placebo-controlled Trial of Remimazolam: A New Ultra Short Acting Benzodiazepine for Bronchoscopy
The first study is part of a session entitled Pediatrics, scheduled to run from 3:15 to 4:15 p.m. on Sunday, Oct. 29, in Convention Center - 606. Shahid Sheikh, MD, of Nationwide Children’s Hospital in New Albany, Ohio, is scheduled to present the abstract at 4:00 p.m.
Dr. Millard, who is Therapeutic Development Network director for the Pediatric CF Care Center and director of research for pediatric pulmonary and sleep medicine at the Helen DeVos Children’s Hospital in Grand Rapids, Mich., noted that she is interested in Dr. Sheikh’s research, “because cultural diversity is such a hot topic in general.”
Her other recommendation is part of the Late Breaking Abstracts 2 session, scheduled to occur on Wednesday, Nov. 1, from 2:45 to 4:15 p.m. in Convention Center - 603. CHEST President, Gerard A. Silvestri, MD, MS, FCCP, will present the abstract at 4:00 p.m.
Dr. Millard said she is interested in this study, because new drug options are so helpful for the frequently performed bronchoscopy.
Two sleep medicine experts on CHEST Physician’s editorial advisory board also selected a few presentations they expect to be newsworthy.
David Schulman, MD, MPH, FCCP, and professor of medicine at Emory University School of Medicine in Atlanta suggested CHEST Physician cover the following studies:
- Results of a Randomized, Placebo-Controlled, Double-Blind, 12-Week, Multicenter Study of JZP-110 for the Treatment Of Excessive Sleepiness in Patients with OSA, scheduled to be presented on Sunday, Oct. 29, at 1:30 p.m. in Convention Center - 601A. Dr. Kingman Strohl, MD, FCCP, of University Hospitals Case Medical Center-Sleep Center in Shaker Heights, Ohio, will present this research during a session entitled, Obstructive Sleep Apnea: Insights & Management, running from 1:30 to 3:00 p.m.
- History of Sleep Apnea and Cardiovascular Disease may Portend Improved Mortality in Patients With Acute Ischemic Stroke, scheduled to be presented on Tuesday, Oct. 31, at 11:15 a.m., in Convention Center - 601A. Nura Festic will present this research during the session, “Sleep, Heart, Brain and More,” running from 11:00 a.m. to 12:15 p.m.
- Ischemic Preconditioning in OSA Patients Manifested after Surviving a Cardiac Arrest? John Moss, MD, of Jacksonville, Fla., will present this study on Tuesday, Oct. 31, at 11:30 a.m., in Convention Center - 601A as part of the session “Sleep, Heart, Brain and More.”
Krishna M. Sundar, MD, FCCP, also recommended that CHEST Physician cover “A Prospective Cohort Study of Endothelial Function and its Relationship to Aspirin Responsiveness in OSA Patients.” Lirim Krveshi is scheduled to present this study on Sunday, Oct. 29, at 1:45 p.m. in Convention Center - 601A. This presentation is part of the Obstructive Sleep Apnea: Insights & Management session.
Dr. Sundar is an associate clinical professor of pulmonary, critical care and sleep medicine and medical director of the Sleep-Wake Center at the University of Utah, Salt Lake City.
To view the full agenda of the CHEST annual meeting, visit: chestmeeting.chestnet.org.
Look for CHEST Physician’s coverage of CHEST 2017 on our conference coverage page.
CHEST Physician is providing on-site coverage of the CHEST annual meeting in Toronto from Oct. 29 through Nov. 1.
We are planning to share findings from the latest research on treating COPD, sleep apnea, pulmonary hypertension, severe asthma, and other diseases that are part of pulmonary, critical care, and sleep medicine. Any improved methods for managing an ICU and updated recommendations on screening for lung cancer will also be on our radar.
The meeting’s agenda includes presentations of hundreds of study abstracts, and we thought you would be interested in hearing which ones grabbed the attention of some of CHEST Physician’s editorial advisory board members.
Board member Susan L. Millard, MD, FCCP, suggested attendees check out presentations of the following two studies:
- Impact of Race on Quality of Life of Families of Children with Asthma when Asthma Guidelines are Followed: Long-Term Follow-Up
- Results Of A Phase 3, Multicenter, Randomized, Placebo-controlled Trial of Remimazolam: A New Ultra Short Acting Benzodiazepine for Bronchoscopy
The first study is part of a session entitled Pediatrics, scheduled to run from 3:15 to 4:15 p.m. on Sunday, Oct. 29, in Convention Center - 606. Shahid Sheikh, MD, of Nationwide Children’s Hospital in New Albany, Ohio, is scheduled to present the abstract at 4:00 p.m.
Dr. Millard, who is Therapeutic Development Network director for the Pediatric CF Care Center and director of research for pediatric pulmonary and sleep medicine at the Helen DeVos Children’s Hospital in Grand Rapids, Mich., noted that she is interested in Dr. Sheikh’s research, “because cultural diversity is such a hot topic in general.”
Her other recommendation is part of the Late Breaking Abstracts 2 session, scheduled to occur on Wednesday, Nov. 1, from 2:45 to 4:15 p.m. in Convention Center - 603. CHEST President, Gerard A. Silvestri, MD, MS, FCCP, will present the abstract at 4:00 p.m.
Dr. Millard said she is interested in this study, because new drug options are so helpful for the frequently performed bronchoscopy.
Two sleep medicine experts on CHEST Physician’s editorial advisory board also selected a few presentations they expect to be newsworthy.
David Schulman, MD, MPH, FCCP, and professor of medicine at Emory University School of Medicine in Atlanta suggested CHEST Physician cover the following studies:
- Results of a Randomized, Placebo-Controlled, Double-Blind, 12-Week, Multicenter Study of JZP-110 for the Treatment Of Excessive Sleepiness in Patients with OSA, scheduled to be presented on Sunday, Oct. 29, at 1:30 p.m. in Convention Center - 601A. Dr. Kingman Strohl, MD, FCCP, of University Hospitals Case Medical Center-Sleep Center in Shaker Heights, Ohio, will present this research during a session entitled, Obstructive Sleep Apnea: Insights & Management, running from 1:30 to 3:00 p.m.
- History of Sleep Apnea and Cardiovascular Disease may Portend Improved Mortality in Patients With Acute Ischemic Stroke, scheduled to be presented on Tuesday, Oct. 31, at 11:15 a.m., in Convention Center - 601A. Nura Festic will present this research during the session, “Sleep, Heart, Brain and More,” running from 11:00 a.m. to 12:15 p.m.
- Ischemic Preconditioning in OSA Patients Manifested after Surviving a Cardiac Arrest? John Moss, MD, of Jacksonville, Fla., will present this study on Tuesday, Oct. 31, at 11:30 a.m., in Convention Center - 601A as part of the session “Sleep, Heart, Brain and More.”
Krishna M. Sundar, MD, FCCP, also recommended that CHEST Physician cover “A Prospective Cohort Study of Endothelial Function and its Relationship to Aspirin Responsiveness in OSA Patients.” Lirim Krveshi is scheduled to present this study on Sunday, Oct. 29, at 1:45 p.m. in Convention Center - 601A. This presentation is part of the Obstructive Sleep Apnea: Insights & Management session.
Dr. Sundar is an associate clinical professor of pulmonary, critical care and sleep medicine and medical director of the Sleep-Wake Center at the University of Utah, Salt Lake City.
To view the full agenda of the CHEST annual meeting, visit: chestmeeting.chestnet.org.
Look for CHEST Physician’s coverage of CHEST 2017 on our conference coverage page.
FROM CHEST 2017
Stopping anti-TNF drugs in PsA has high rebound risk
A report on the largest cohort to date of patients with psoriatic arthritis who discontinued tumor necrosis factor inhibitor (TNFi) therapy during a period of low disease activity adds further evidence in support of keeping patients on the biologics during such periods.
The findings from the report on patients with psoriatic arthritis (PsA) from the U.S. Corrona registry found a rebound in disease activity in 69 (73%) of 94 patients. These results are in line with prior small studies, including a pilot, randomized, controlled trial, in PsA patients who have attempted to stop conventional synthetic disease-modifying antirheumatic drugs and biologic DMARDs, mostly TNFi agents, during periods of low disease activity (LDA).
The 73% rate of disease activity rebound observed in the patients – defined by a Clinical Disease Activity Index (CDAI) score of more than 10, a reinitiation of their TNFi, or the start of a new agent – “offers an important perspective for physicians who are considering TNFi discontinuation for patients who have achieved remission/LDA and may provide a key piece of data in discussing the most likely potential outcome with their patients’ TNFi discontinuation,” Dr. Harrold and her associates wrote. “TNFi discontinuation after achieving LDA should be carefully considered.”
The 94 patients with PsA in the Corrona registry study all met study criteria for remission or LDA (CDAI score of 10 or less) and had discontinued their TNFi while in LDA and did not immediately switch to a different biologic. The mean age of the patients was 51; 57% were women and 92% were white. At the time of TNFi initiation, they had an average disease duration of 8.4 years and a mean CDAI score of 10.1, and 64% had LDA. Most patients had taken methotrexate (86%) or a conventional synthetic DMARD (10%) prior to starting a TNFi (J Rheumatol. 2017 Oct 1. doi: 10.3899/jrheum.161567).
Among the 69 patients with rebound of disease activity, 15 had a CDAI score of greater than 10, 24 restarted a biologic DMARD but did not have an elevated CDAI, and 30 had both an elevated CDAI and biologic initiation.
Close to three-quarters of the 59 rebounders who returned for a postrebound visit were in remission or LDA at that visit, and nearly half had resumed use of their original TNFi by the time of the visit.
The U.S.-based Corrona registry has registered about 6,000 PsA patients from around 500 rheumatologists since 2001. The conclusions that can be drawn from the current study, which included patients who started a TNFi between Oct. 1, 2002, and March 21, 2013, are limited by the use of the CDAI, a measure for disease activity in rheumatoid arthritis. The use of the CDAI means that disease features of PsA such as enthesitis, psoriasis, and dactylitis were not captured in the assessment of LDA. “That is a caveat,” Dr. Harrold said.
Other limitations of the study include its sporadic follow-up and the open-label, uncontrolled nature of the TNFi discontinuation, according to Dr. Helliwell.
“There could have been any number of reasons why the drug was stopped, not just because of low disease activity – because of financial reasons, perhaps, adverse events, or ... a switch to another drug,” he said in an interview.
Despite these limitations, Dr. Helliwell said that the study “provides useful data, and it will add to the number of publications on this subject that are already out there, and it is the biggest cohort that’s been published.”
Dr. Harrold and her coauthors said that their findings were consistent with those from other studies, including one that Dr. Helliwell’s group was a part of.
That was a pilot randomized, controlled trial to test the feasibility of drug withdrawal in PsA patients in a stable, low disease state, defined by meeting minimal disease activity criteria. Of the 17 patients randomized, 6 of 11 who were in the withdrawal arm experienced a flare (defined as not meeting minimal disease activity criteria). Of these six patients, two had been receiving methotrexate only and four had been treated with methotrexate plus a biologic, whereas none of the six patients who continued therapy experienced a flare (Clin Rheumatol. 2015;34[8]:1407-12). Dr. Helliwell noted that all patients in the withdrawal arm eventually relapsed.
Another study with similar results from investigators at the University of Erlangen-Nuremberg (Germany) found that out of 26 PsA patients on either methotrexate monotherapy (n = 14) or a TNFi (n = 12), with no musculoskeletal symptoms and minimal skin disease, 20 had disease recurrence at a mean of just 74 days after drug discontinuation (Ann Rheum Dis. 2015;74:655-60).
Investigators for another study from the Corrona registry looked at 325 discontinuations of TNFi therapy while in LDA in 302 PsA patients and found disease rebound in 45% at an estimated median time of about 29 months. This study included a physician’s global assessment of skin psoriasis of 20 or less out of 100 as part of the definition of LDA, in addition to a CDAI score of 10 or less. Current smoking was the only significant predictor of disease rebound in a multivariate analysis. Age, gender, the number of prior TNFis used, or overweight or obese status did not predict disease rebound. CDAI score of 3.2 or greater was just outside the range of statistical significance (RMD Open. 2017;3:e000395).
“I think the message here is: ‘Be very careful discontinuing anti-TNF drugs in people with PsA and low disease activity.’ It’s not the same as rheumatoid arthritis in that respect,” Dr. Helliwell said.
Ultimately, the goal is to determine the characteristics of patients who can either stop or taper drugs such as TNF inhibitors. “I think there are going to be patients who can be tapered off, and there are going to be patients who can’t be,” Dr. Harrold said. Going forward, it will be important to identify which subset of patients are going to flare when taken off medication, such as a TNF inhibitor, particularly because of the diversity of presentations that PsA patients can have, she said.
Dr. Helliwell agreed, saying that, when he is asked whether you can stop a TNFi in PsA patients with LDA, “I say you should taper the drug by increasing the interval between doses or lower the dose, but not discontinue it.”
The Corrona registry has been supported by numerous pharmaceutical companies that manufacture drugs for PsA and rheumatoid arthritis. Dr. Harrold and some of her coinvestigators are employees and shareholders of Corrona. Some authors are employees and shareholders of Amgen, and others reported financial relationships with various pharmaceutical companies. Dr. Helliwell has received grant or research support, consulted for, or served on the speakers bureau for many of the companies that support Corrona.
A report on the largest cohort to date of patients with psoriatic arthritis who discontinued tumor necrosis factor inhibitor (TNFi) therapy during a period of low disease activity adds further evidence in support of keeping patients on the biologics during such periods.
The findings from the report on patients with psoriatic arthritis (PsA) from the U.S. Corrona registry found a rebound in disease activity in 69 (73%) of 94 patients. These results are in line with prior small studies, including a pilot, randomized, controlled trial, in PsA patients who have attempted to stop conventional synthetic disease-modifying antirheumatic drugs and biologic DMARDs, mostly TNFi agents, during periods of low disease activity (LDA).
The 73% rate of disease activity rebound observed in the patients – defined by a Clinical Disease Activity Index (CDAI) score of more than 10, a reinitiation of their TNFi, or the start of a new agent – “offers an important perspective for physicians who are considering TNFi discontinuation for patients who have achieved remission/LDA and may provide a key piece of data in discussing the most likely potential outcome with their patients’ TNFi discontinuation,” Dr. Harrold and her associates wrote. “TNFi discontinuation after achieving LDA should be carefully considered.”
The 94 patients with PsA in the Corrona registry study all met study criteria for remission or LDA (CDAI score of 10 or less) and had discontinued their TNFi while in LDA and did not immediately switch to a different biologic. The mean age of the patients was 51; 57% were women and 92% were white. At the time of TNFi initiation, they had an average disease duration of 8.4 years and a mean CDAI score of 10.1, and 64% had LDA. Most patients had taken methotrexate (86%) or a conventional synthetic DMARD (10%) prior to starting a TNFi (J Rheumatol. 2017 Oct 1. doi: 10.3899/jrheum.161567).
Among the 69 patients with rebound of disease activity, 15 had a CDAI score of greater than 10, 24 restarted a biologic DMARD but did not have an elevated CDAI, and 30 had both an elevated CDAI and biologic initiation.
Close to three-quarters of the 59 rebounders who returned for a postrebound visit were in remission or LDA at that visit, and nearly half had resumed use of their original TNFi by the time of the visit.
The U.S.-based Corrona registry has registered about 6,000 PsA patients from around 500 rheumatologists since 2001. The conclusions that can be drawn from the current study, which included patients who started a TNFi between Oct. 1, 2002, and March 21, 2013, are limited by the use of the CDAI, a measure for disease activity in rheumatoid arthritis. The use of the CDAI means that disease features of PsA such as enthesitis, psoriasis, and dactylitis were not captured in the assessment of LDA. “That is a caveat,” Dr. Harrold said.
Other limitations of the study include its sporadic follow-up and the open-label, uncontrolled nature of the TNFi discontinuation, according to Dr. Helliwell.
“There could have been any number of reasons why the drug was stopped, not just because of low disease activity – because of financial reasons, perhaps, adverse events, or ... a switch to another drug,” he said in an interview.
Despite these limitations, Dr. Helliwell said that the study “provides useful data, and it will add to the number of publications on this subject that are already out there, and it is the biggest cohort that’s been published.”
Dr. Harrold and her coauthors said that their findings were consistent with those from other studies, including one that Dr. Helliwell’s group was a part of.
That was a pilot randomized, controlled trial to test the feasibility of drug withdrawal in PsA patients in a stable, low disease state, defined by meeting minimal disease activity criteria. Of the 17 patients randomized, 6 of 11 who were in the withdrawal arm experienced a flare (defined as not meeting minimal disease activity criteria). Of these six patients, two had been receiving methotrexate only and four had been treated with methotrexate plus a biologic, whereas none of the six patients who continued therapy experienced a flare (Clin Rheumatol. 2015;34[8]:1407-12). Dr. Helliwell noted that all patients in the withdrawal arm eventually relapsed.
Another study with similar results from investigators at the University of Erlangen-Nuremberg (Germany) found that out of 26 PsA patients on either methotrexate monotherapy (n = 14) or a TNFi (n = 12), with no musculoskeletal symptoms and minimal skin disease, 20 had disease recurrence at a mean of just 74 days after drug discontinuation (Ann Rheum Dis. 2015;74:655-60).
Investigators for another study from the Corrona registry looked at 325 discontinuations of TNFi therapy while in LDA in 302 PsA patients and found disease rebound in 45% at an estimated median time of about 29 months. This study included a physician’s global assessment of skin psoriasis of 20 or less out of 100 as part of the definition of LDA, in addition to a CDAI score of 10 or less. Current smoking was the only significant predictor of disease rebound in a multivariate analysis. Age, gender, the number of prior TNFis used, or overweight or obese status did not predict disease rebound. CDAI score of 3.2 or greater was just outside the range of statistical significance (RMD Open. 2017;3:e000395).
“I think the message here is: ‘Be very careful discontinuing anti-TNF drugs in people with PsA and low disease activity.’ It’s not the same as rheumatoid arthritis in that respect,” Dr. Helliwell said.
Ultimately, the goal is to determine the characteristics of patients who can either stop or taper drugs such as TNF inhibitors. “I think there are going to be patients who can be tapered off, and there are going to be patients who can’t be,” Dr. Harrold said. Going forward, it will be important to identify which subset of patients are going to flare when taken off medication, such as a TNF inhibitor, particularly because of the diversity of presentations that PsA patients can have, she said.
Dr. Helliwell agreed, saying that, when he is asked whether you can stop a TNFi in PsA patients with LDA, “I say you should taper the drug by increasing the interval between doses or lower the dose, but not discontinue it.”
The Corrona registry has been supported by numerous pharmaceutical companies that manufacture drugs for PsA and rheumatoid arthritis. Dr. Harrold and some of her coinvestigators are employees and shareholders of Corrona. Some authors are employees and shareholders of Amgen, and others reported financial relationships with various pharmaceutical companies. Dr. Helliwell has received grant or research support, consulted for, or served on the speakers bureau for many of the companies that support Corrona.
A report on the largest cohort to date of patients with psoriatic arthritis who discontinued tumor necrosis factor inhibitor (TNFi) therapy during a period of low disease activity adds further evidence in support of keeping patients on the biologics during such periods.
The findings from the report on patients with psoriatic arthritis (PsA) from the U.S. Corrona registry found a rebound in disease activity in 69 (73%) of 94 patients. These results are in line with prior small studies, including a pilot, randomized, controlled trial, in PsA patients who have attempted to stop conventional synthetic disease-modifying antirheumatic drugs and biologic DMARDs, mostly TNFi agents, during periods of low disease activity (LDA).
The 73% rate of disease activity rebound observed in the patients – defined by a Clinical Disease Activity Index (CDAI) score of more than 10, a reinitiation of their TNFi, or the start of a new agent – “offers an important perspective for physicians who are considering TNFi discontinuation for patients who have achieved remission/LDA and may provide a key piece of data in discussing the most likely potential outcome with their patients’ TNFi discontinuation,” Dr. Harrold and her associates wrote. “TNFi discontinuation after achieving LDA should be carefully considered.”
The 94 patients with PsA in the Corrona registry study all met study criteria for remission or LDA (CDAI score of 10 or less) and had discontinued their TNFi while in LDA and did not immediately switch to a different biologic. The mean age of the patients was 51; 57% were women and 92% were white. At the time of TNFi initiation, they had an average disease duration of 8.4 years and a mean CDAI score of 10.1, and 64% had LDA. Most patients had taken methotrexate (86%) or a conventional synthetic DMARD (10%) prior to starting a TNFi (J Rheumatol. 2017 Oct 1. doi: 10.3899/jrheum.161567).
Among the 69 patients with rebound of disease activity, 15 had a CDAI score of greater than 10, 24 restarted a biologic DMARD but did not have an elevated CDAI, and 30 had both an elevated CDAI and biologic initiation.
Close to three-quarters of the 59 rebounders who returned for a postrebound visit were in remission or LDA at that visit, and nearly half had resumed use of their original TNFi by the time of the visit.
The U.S.-based Corrona registry has registered about 6,000 PsA patients from around 500 rheumatologists since 2001. The conclusions that can be drawn from the current study, which included patients who started a TNFi between Oct. 1, 2002, and March 21, 2013, are limited by the use of the CDAI, a measure for disease activity in rheumatoid arthritis. The use of the CDAI means that disease features of PsA such as enthesitis, psoriasis, and dactylitis were not captured in the assessment of LDA. “That is a caveat,” Dr. Harrold said.
Other limitations of the study include its sporadic follow-up and the open-label, uncontrolled nature of the TNFi discontinuation, according to Dr. Helliwell.
“There could have been any number of reasons why the drug was stopped, not just because of low disease activity – because of financial reasons, perhaps, adverse events, or ... a switch to another drug,” he said in an interview.
Despite these limitations, Dr. Helliwell said that the study “provides useful data, and it will add to the number of publications on this subject that are already out there, and it is the biggest cohort that’s been published.”
Dr. Harrold and her coauthors said that their findings were consistent with those from other studies, including one that Dr. Helliwell’s group was a part of.
That was a pilot randomized, controlled trial to test the feasibility of drug withdrawal in PsA patients in a stable, low disease state, defined by meeting minimal disease activity criteria. Of the 17 patients randomized, 6 of 11 who were in the withdrawal arm experienced a flare (defined as not meeting minimal disease activity criteria). Of these six patients, two had been receiving methotrexate only and four had been treated with methotrexate plus a biologic, whereas none of the six patients who continued therapy experienced a flare (Clin Rheumatol. 2015;34[8]:1407-12). Dr. Helliwell noted that all patients in the withdrawal arm eventually relapsed.
Another study with similar results from investigators at the University of Erlangen-Nuremberg (Germany) found that out of 26 PsA patients on either methotrexate monotherapy (n = 14) or a TNFi (n = 12), with no musculoskeletal symptoms and minimal skin disease, 20 had disease recurrence at a mean of just 74 days after drug discontinuation (Ann Rheum Dis. 2015;74:655-60).
Investigators for another study from the Corrona registry looked at 325 discontinuations of TNFi therapy while in LDA in 302 PsA patients and found disease rebound in 45% at an estimated median time of about 29 months. This study included a physician’s global assessment of skin psoriasis of 20 or less out of 100 as part of the definition of LDA, in addition to a CDAI score of 10 or less. Current smoking was the only significant predictor of disease rebound in a multivariate analysis. Age, gender, the number of prior TNFis used, or overweight or obese status did not predict disease rebound. CDAI score of 3.2 or greater was just outside the range of statistical significance (RMD Open. 2017;3:e000395).
“I think the message here is: ‘Be very careful discontinuing anti-TNF drugs in people with PsA and low disease activity.’ It’s not the same as rheumatoid arthritis in that respect,” Dr. Helliwell said.
Ultimately, the goal is to determine the characteristics of patients who can either stop or taper drugs such as TNF inhibitors. “I think there are going to be patients who can be tapered off, and there are going to be patients who can’t be,” Dr. Harrold said. Going forward, it will be important to identify which subset of patients are going to flare when taken off medication, such as a TNF inhibitor, particularly because of the diversity of presentations that PsA patients can have, she said.
Dr. Helliwell agreed, saying that, when he is asked whether you can stop a TNFi in PsA patients with LDA, “I say you should taper the drug by increasing the interval between doses or lower the dose, but not discontinue it.”
The Corrona registry has been supported by numerous pharmaceutical companies that manufacture drugs for PsA and rheumatoid arthritis. Dr. Harrold and some of her coinvestigators are employees and shareholders of Corrona. Some authors are employees and shareholders of Amgen, and others reported financial relationships with various pharmaceutical companies. Dr. Helliwell has received grant or research support, consulted for, or served on the speakers bureau for many of the companies that support Corrona.
FROM JOURNAL OF RHEUMATOLOGY
Key clinical point:
Major finding: Among 94 patients, 69 (73%) experienced symptom rebound after discontinuing their TNFi therapy.
Data source: The prospective observational Corrona disease registry database of about 6,000 patients with PsA.
Disclosures: The Corrona registry has been supported by numerous pharmaceutical companies that manufacture drugs for PsA and RA. Dr. Harrold and some of her coinvestigators are employees and shareholders of Corrona. Some authors are employees and shareholders of Amgen, and others reported financial relationships with various pharmaceutical companies. Dr. Helliwell has received grant or research support, consulted for, or served on the speakers bureau for many of the companies that support Corrona.
Translating Research Into Practice the NIOSH Way
The National Occupational Research Agenda (NORA), a partnership program created by the National Institute for Occupational Safety and Health (NIOSH), is celebrating its second anniversary with a report addressing the question: how can research be better moved into practice in the workplace?
NORA has focused on 10 industry sectors representing major areas of the U.S. economy, and developed sector councils—comprising of stakeholders from universities, business, professional societies, government agencies, and worker organizations—that set priority research goals for the nation.
In the past 20 years, NIOSH has had an average of 740 active projects per year. It invested an average of $243.8 million per year in research between 2007 - 2015. Between 2007 - 2014, nearly 11,000 publications were developed through NIOSH-funded research.
Outcome measures include whether those NORA outputs have been used by others, in citations and research. NIOSH says its outputs are widely disseminated; the VA and other veterans’ organizations, for instance, disseminated NIOSH information on return-to-work issues for veterans with PTSD. As another example, academicians and researchers are using NIOSH findings to improve tuberculosis risk and prevention education in workplaces. And approximately 50% of the NIOSH products cited by another federal agency in a Federal Register document were aged ≥ 11 years —suggesting that NIOSH documents have a “sustained relevance and impact well beyond their publication date,” the report says.
NIOSH highlights NORA’s progress with “impact stories” about the influence of NORA on health, safety, and wellbeing of the U.S. workforce. Case in point: “Preventing Occupational Transmission of Blood-borne Pathogens Among Healthcare Workers” helped improve worker safety by instructing > 20,000 trainers and leading to new regulations, NIOSH says.
For its third decade, NIOSH says, NORA will build on the “many successes and lessons learned from the first 2 decades of this unique partnership approach.”
The National Occupational Research Agenda (NORA), a partnership program created by the National Institute for Occupational Safety and Health (NIOSH), is celebrating its second anniversary with a report addressing the question: how can research be better moved into practice in the workplace?
NORA has focused on 10 industry sectors representing major areas of the U.S. economy, and developed sector councils—comprising of stakeholders from universities, business, professional societies, government agencies, and worker organizations—that set priority research goals for the nation.
In the past 20 years, NIOSH has had an average of 740 active projects per year. It invested an average of $243.8 million per year in research between 2007 - 2015. Between 2007 - 2014, nearly 11,000 publications were developed through NIOSH-funded research.
Outcome measures include whether those NORA outputs have been used by others, in citations and research. NIOSH says its outputs are widely disseminated; the VA and other veterans’ organizations, for instance, disseminated NIOSH information on return-to-work issues for veterans with PTSD. As another example, academicians and researchers are using NIOSH findings to improve tuberculosis risk and prevention education in workplaces. And approximately 50% of the NIOSH products cited by another federal agency in a Federal Register document were aged ≥ 11 years —suggesting that NIOSH documents have a “sustained relevance and impact well beyond their publication date,” the report says.
NIOSH highlights NORA’s progress with “impact stories” about the influence of NORA on health, safety, and wellbeing of the U.S. workforce. Case in point: “Preventing Occupational Transmission of Blood-borne Pathogens Among Healthcare Workers” helped improve worker safety by instructing > 20,000 trainers and leading to new regulations, NIOSH says.
For its third decade, NIOSH says, NORA will build on the “many successes and lessons learned from the first 2 decades of this unique partnership approach.”
The National Occupational Research Agenda (NORA), a partnership program created by the National Institute for Occupational Safety and Health (NIOSH), is celebrating its second anniversary with a report addressing the question: how can research be better moved into practice in the workplace?
NORA has focused on 10 industry sectors representing major areas of the U.S. economy, and developed sector councils—comprising of stakeholders from universities, business, professional societies, government agencies, and worker organizations—that set priority research goals for the nation.
In the past 20 years, NIOSH has had an average of 740 active projects per year. It invested an average of $243.8 million per year in research between 2007 - 2015. Between 2007 - 2014, nearly 11,000 publications were developed through NIOSH-funded research.
Outcome measures include whether those NORA outputs have been used by others, in citations and research. NIOSH says its outputs are widely disseminated; the VA and other veterans’ organizations, for instance, disseminated NIOSH information on return-to-work issues for veterans with PTSD. As another example, academicians and researchers are using NIOSH findings to improve tuberculosis risk and prevention education in workplaces. And approximately 50% of the NIOSH products cited by another federal agency in a Federal Register document were aged ≥ 11 years —suggesting that NIOSH documents have a “sustained relevance and impact well beyond their publication date,” the report says.
NIOSH highlights NORA’s progress with “impact stories” about the influence of NORA on health, safety, and wellbeing of the U.S. workforce. Case in point: “Preventing Occupational Transmission of Blood-borne Pathogens Among Healthcare Workers” helped improve worker safety by instructing > 20,000 trainers and leading to new regulations, NIOSH says.
For its third decade, NIOSH says, NORA will build on the “many successes and lessons learned from the first 2 decades of this unique partnership approach.”
ATLG fights GVHD but reduces PFS, OS
Results of a phase 3 trial suggest rabbit anti-T lymphocyte globulin (ATLG) can reduce graft-versus-host disease (GVHD) but also decrease survival in patients who have received a hematopoietic stem cell transplant (HSCT) from a matched, unrelated donor.
In this randomized trial, ATLG significantly decreased the incidence of moderate-to-severe chronic GVHD and acute grade 2-4 GVHD, when compared to placebo.
However, patients who received ATLG also had significantly lower progression-free survival (PFS) and overall survival (OS) than placebo-treated patients.
On the other hand, the data also suggest that patients who receive conditioning regimens that do not lower absolute lymphocyte counts (ALCs) substantially may not experience a significant decrease in survival with ATLG.
These results were published in the Journal of Clinical Oncology. The study was sponsored by Neovii Pharmaceuticals AG, which is developing ATLG as Grafalon®.
The study was a prospective, randomized, double-blind trial conducted in North America and Australia (NCT01295710). It enrolled 254 patients, ages 18 to 65, who had acute lymphoblastic leukemia, acute myeloid leukemia, or myelodysplastic syndromes. All patients were undergoing myeloablative, HLA-matched, unrelated HSCT.
Patients were randomized in a 1:1 fashion to receive ATLG (given at 20 mg/kg/day, n=126) or placebo (250 ml of normal saline, n=128) on days -3, -2, and -1 prior to HSCT.
In addition, all patients received antihistamine and methylprednisolone (at 2 mg/kg on day -3 and 1 mg/kg on days -2 and -1).
Patients also received GVHD prophylaxis in the form of tacrolimus (with a target serum trough level of 5 to 15 ng/mL) and methotrexate (15 mg/m2 on day 1, then 10 mg/m2 on days 3, 6, and 11). If patients did not develop clinical GVHD, tacrolimus was tapered starting on day 50 or later over a minimum of 26 weeks and ultimately discontinued.
Patients received 1 of 3 conditioning regimens, which were declared prior to randomization and included:
- Cyclophosphamide at 120 mg/kg intravenously (IV) and fractionated total body irradiation (TBI, ≥12 Gy)
- Busulfan at 16 mg/kg orally or 12.8 mg/kg IV and cyclophosphamide at 120 mg/kg IV
- Busulfan at 16 mg/kg orally or 12.8 mg/kg IV and fludarabine at 120 mg/m2 IV.
Overall results
Compared to placebo-treated patients, those who received ATLG had a significant reduction in grade 2-4 acute GVHD—23% and 40%, respectively (P=0.004)—and moderate-to-severe chronic GVHD—12% and 33%, respectively (P<0.001).
However, there was no significant difference between the ATLG and placebo arms with regard to moderate-severe chronic GVHD-free survival. The 2-year estimate was 48% and 44%, respectively (P=0.47).
In addition, PFS and OS were significantly lower in patients who received ATLG. The estimated 2-year PFS was 47% in the ATLG arm and 65% in the placebo arm (P=0.04). The estimated 2-year OS was 59% and 74%, respectively (P=0.034).
In a multivariable analysis, ATLG remained significantly associated with inferior PFS (hazard ratio [HR]=1.55, P=0.026) and OS (hazard ratio=1.74, P=0.01).
Role of conditioning, ALC
The researchers found evidence to suggest that conditioning regimen and ALC played a role in patient outcomes.
For patients who received cyclophosphamide and TBI, 2-year moderate-severe chronic GVHD-free survival was 61% in the placebo arm and 38% in the ATLG arm (P=0.080). Two-year OS was 88% and 48%, respectively (P=0.006). And 2-year PFS was 75% and 29%, respectively (P=0.007).
For patients who received busulfan and cyclophosphamide, 2-year moderate-severe chronic GVHD-free survival was 47% in the placebo arm and 53% in the ATLG arm (P=0.650). Two-year OS was 77% and 71%, respectively (P=0.350). And 2-year PFS was 73% and 60%, respectively (P=0.460).
For patients who received busulfan and fludarabine, 2-year moderate-severe chronic GVHD-free survival was 33% in the placebo arm and 49% in the ATLG arm (P=0.047). Two-year OS was 66% and 53%, respectively (P=0.520). And 2-year PFS was 58% and 48%, respectively (P=0.540).
The researchers noted that the choice of conditioning regimen had a “profound effect” on ALC at day -3 (the time of ATLG/placebo initiation). More than 70% of patients who received TBI had an ALC <0.1 x 109/L, compared to less than 35% of patients who received busulfan-based conditioning.
ALC, in turn, had an impact on PFS and OS. In patients with an ALC ≥ 0.1 x 109/L on day -3, ATLG did not compromise PFS or OS, but PFS and OS were negatively affected in patients with an ALC < 0.1.
ATLG recipients with an ALC < 0.1 had significantly worse OS (HR=4.13, P<0.001) and PFS (HR=3.19, P<0.001) than patients with an ALC ≥ 0.1. ![]()
Results of a phase 3 trial suggest rabbit anti-T lymphocyte globulin (ATLG) can reduce graft-versus-host disease (GVHD) but also decrease survival in patients who have received a hematopoietic stem cell transplant (HSCT) from a matched, unrelated donor.
In this randomized trial, ATLG significantly decreased the incidence of moderate-to-severe chronic GVHD and acute grade 2-4 GVHD, when compared to placebo.
However, patients who received ATLG also had significantly lower progression-free survival (PFS) and overall survival (OS) than placebo-treated patients.
On the other hand, the data also suggest that patients who receive conditioning regimens that do not lower absolute lymphocyte counts (ALCs) substantially may not experience a significant decrease in survival with ATLG.
These results were published in the Journal of Clinical Oncology. The study was sponsored by Neovii Pharmaceuticals AG, which is developing ATLG as Grafalon®.
The study was a prospective, randomized, double-blind trial conducted in North America and Australia (NCT01295710). It enrolled 254 patients, ages 18 to 65, who had acute lymphoblastic leukemia, acute myeloid leukemia, or myelodysplastic syndromes. All patients were undergoing myeloablative, HLA-matched, unrelated HSCT.
Patients were randomized in a 1:1 fashion to receive ATLG (given at 20 mg/kg/day, n=126) or placebo (250 ml of normal saline, n=128) on days -3, -2, and -1 prior to HSCT.
In addition, all patients received antihistamine and methylprednisolone (at 2 mg/kg on day -3 and 1 mg/kg on days -2 and -1).
Patients also received GVHD prophylaxis in the form of tacrolimus (with a target serum trough level of 5 to 15 ng/mL) and methotrexate (15 mg/m2 on day 1, then 10 mg/m2 on days 3, 6, and 11). If patients did not develop clinical GVHD, tacrolimus was tapered starting on day 50 or later over a minimum of 26 weeks and ultimately discontinued.
Patients received 1 of 3 conditioning regimens, which were declared prior to randomization and included:
- Cyclophosphamide at 120 mg/kg intravenously (IV) and fractionated total body irradiation (TBI, ≥12 Gy)
- Busulfan at 16 mg/kg orally or 12.8 mg/kg IV and cyclophosphamide at 120 mg/kg IV
- Busulfan at 16 mg/kg orally or 12.8 mg/kg IV and fludarabine at 120 mg/m2 IV.
Overall results
Compared to placebo-treated patients, those who received ATLG had a significant reduction in grade 2-4 acute GVHD—23% and 40%, respectively (P=0.004)—and moderate-to-severe chronic GVHD—12% and 33%, respectively (P<0.001).
However, there was no significant difference between the ATLG and placebo arms with regard to moderate-severe chronic GVHD-free survival. The 2-year estimate was 48% and 44%, respectively (P=0.47).
In addition, PFS and OS were significantly lower in patients who received ATLG. The estimated 2-year PFS was 47% in the ATLG arm and 65% in the placebo arm (P=0.04). The estimated 2-year OS was 59% and 74%, respectively (P=0.034).
In a multivariable analysis, ATLG remained significantly associated with inferior PFS (hazard ratio [HR]=1.55, P=0.026) and OS (hazard ratio=1.74, P=0.01).
Role of conditioning, ALC
The researchers found evidence to suggest that conditioning regimen and ALC played a role in patient outcomes.
For patients who received cyclophosphamide and TBI, 2-year moderate-severe chronic GVHD-free survival was 61% in the placebo arm and 38% in the ATLG arm (P=0.080). Two-year OS was 88% and 48%, respectively (P=0.006). And 2-year PFS was 75% and 29%, respectively (P=0.007).
For patients who received busulfan and cyclophosphamide, 2-year moderate-severe chronic GVHD-free survival was 47% in the placebo arm and 53% in the ATLG arm (P=0.650). Two-year OS was 77% and 71%, respectively (P=0.350). And 2-year PFS was 73% and 60%, respectively (P=0.460).
For patients who received busulfan and fludarabine, 2-year moderate-severe chronic GVHD-free survival was 33% in the placebo arm and 49% in the ATLG arm (P=0.047). Two-year OS was 66% and 53%, respectively (P=0.520). And 2-year PFS was 58% and 48%, respectively (P=0.540).
The researchers noted that the choice of conditioning regimen had a “profound effect” on ALC at day -3 (the time of ATLG/placebo initiation). More than 70% of patients who received TBI had an ALC <0.1 x 109/L, compared to less than 35% of patients who received busulfan-based conditioning.
ALC, in turn, had an impact on PFS and OS. In patients with an ALC ≥ 0.1 x 109/L on day -3, ATLG did not compromise PFS or OS, but PFS and OS were negatively affected in patients with an ALC < 0.1.
ATLG recipients with an ALC < 0.1 had significantly worse OS (HR=4.13, P<0.001) and PFS (HR=3.19, P<0.001) than patients with an ALC ≥ 0.1. ![]()
Results of a phase 3 trial suggest rabbit anti-T lymphocyte globulin (ATLG) can reduce graft-versus-host disease (GVHD) but also decrease survival in patients who have received a hematopoietic stem cell transplant (HSCT) from a matched, unrelated donor.
In this randomized trial, ATLG significantly decreased the incidence of moderate-to-severe chronic GVHD and acute grade 2-4 GVHD, when compared to placebo.
However, patients who received ATLG also had significantly lower progression-free survival (PFS) and overall survival (OS) than placebo-treated patients.
On the other hand, the data also suggest that patients who receive conditioning regimens that do not lower absolute lymphocyte counts (ALCs) substantially may not experience a significant decrease in survival with ATLG.
These results were published in the Journal of Clinical Oncology. The study was sponsored by Neovii Pharmaceuticals AG, which is developing ATLG as Grafalon®.
The study was a prospective, randomized, double-blind trial conducted in North America and Australia (NCT01295710). It enrolled 254 patients, ages 18 to 65, who had acute lymphoblastic leukemia, acute myeloid leukemia, or myelodysplastic syndromes. All patients were undergoing myeloablative, HLA-matched, unrelated HSCT.
Patients were randomized in a 1:1 fashion to receive ATLG (given at 20 mg/kg/day, n=126) or placebo (250 ml of normal saline, n=128) on days -3, -2, and -1 prior to HSCT.
In addition, all patients received antihistamine and methylprednisolone (at 2 mg/kg on day -3 and 1 mg/kg on days -2 and -1).
Patients also received GVHD prophylaxis in the form of tacrolimus (with a target serum trough level of 5 to 15 ng/mL) and methotrexate (15 mg/m2 on day 1, then 10 mg/m2 on days 3, 6, and 11). If patients did not develop clinical GVHD, tacrolimus was tapered starting on day 50 or later over a minimum of 26 weeks and ultimately discontinued.
Patients received 1 of 3 conditioning regimens, which were declared prior to randomization and included:
- Cyclophosphamide at 120 mg/kg intravenously (IV) and fractionated total body irradiation (TBI, ≥12 Gy)
- Busulfan at 16 mg/kg orally or 12.8 mg/kg IV and cyclophosphamide at 120 mg/kg IV
- Busulfan at 16 mg/kg orally or 12.8 mg/kg IV and fludarabine at 120 mg/m2 IV.
Overall results
Compared to placebo-treated patients, those who received ATLG had a significant reduction in grade 2-4 acute GVHD—23% and 40%, respectively (P=0.004)—and moderate-to-severe chronic GVHD—12% and 33%, respectively (P<0.001).
However, there was no significant difference between the ATLG and placebo arms with regard to moderate-severe chronic GVHD-free survival. The 2-year estimate was 48% and 44%, respectively (P=0.47).
In addition, PFS and OS were significantly lower in patients who received ATLG. The estimated 2-year PFS was 47% in the ATLG arm and 65% in the placebo arm (P=0.04). The estimated 2-year OS was 59% and 74%, respectively (P=0.034).
In a multivariable analysis, ATLG remained significantly associated with inferior PFS (hazard ratio [HR]=1.55, P=0.026) and OS (hazard ratio=1.74, P=0.01).
Role of conditioning, ALC
The researchers found evidence to suggest that conditioning regimen and ALC played a role in patient outcomes.
For patients who received cyclophosphamide and TBI, 2-year moderate-severe chronic GVHD-free survival was 61% in the placebo arm and 38% in the ATLG arm (P=0.080). Two-year OS was 88% and 48%, respectively (P=0.006). And 2-year PFS was 75% and 29%, respectively (P=0.007).
For patients who received busulfan and cyclophosphamide, 2-year moderate-severe chronic GVHD-free survival was 47% in the placebo arm and 53% in the ATLG arm (P=0.650). Two-year OS was 77% and 71%, respectively (P=0.350). And 2-year PFS was 73% and 60%, respectively (P=0.460).
For patients who received busulfan and fludarabine, 2-year moderate-severe chronic GVHD-free survival was 33% in the placebo arm and 49% in the ATLG arm (P=0.047). Two-year OS was 66% and 53%, respectively (P=0.520). And 2-year PFS was 58% and 48%, respectively (P=0.540).
The researchers noted that the choice of conditioning regimen had a “profound effect” on ALC at day -3 (the time of ATLG/placebo initiation). More than 70% of patients who received TBI had an ALC <0.1 x 109/L, compared to less than 35% of patients who received busulfan-based conditioning.
ALC, in turn, had an impact on PFS and OS. In patients with an ALC ≥ 0.1 x 109/L on day -3, ATLG did not compromise PFS or OS, but PFS and OS were negatively affected in patients with an ALC < 0.1.
ATLG recipients with an ALC < 0.1 had significantly worse OS (HR=4.13, P<0.001) and PFS (HR=3.19, P<0.001) than patients with an ALC ≥ 0.1. ![]()
Gene therapy granted orphan designation for hemophilia A
The US Food and Drug Administration (FDA) has accepted an investigational new drug application for SHP654 (also known as BAX 888) and granted the therapy orphan drug designation.
SHP654 is an investigational factor VIII (FVIII) gene therapy intended to treat hemophilia A using an adeno-associated virus serotype 8 vector to deliver codon-optimized, B-domain-deleted FVIII specifically to a patient’s liver, where FVIII would then be produced and used to manage bleeds.
Shire, the company developing SHP654, received FDA clearance for an investigational new drug application to initiate a global, phase 1/2 study of SHP654.
In this study, researchers will evaluate the safety and optimal dose of SHP654 needed to boost FVIII activity levels and affect hemophilic bleeding. Shire expects this study will begin by the end of this year.
The FDA also granted orphan designation to SHP654. The agency grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
“This important orphan drug designation highlights Shire’s commitment to patients with rare diseases, and, for hemophilia patients specifically, our aim is to help them achieve zero bleeds,” said Paul Monahan, MD, senior medical director of gene therapy at Shire.
“We know that hemophilia care is not one-size-fits-all and that every patient is unique, which is why we continue to focus on optimizing personal outcomes for hemophilia patients by developing innovations to transform care.” ![]()
The US Food and Drug Administration (FDA) has accepted an investigational new drug application for SHP654 (also known as BAX 888) and granted the therapy orphan drug designation.
SHP654 is an investigational factor VIII (FVIII) gene therapy intended to treat hemophilia A using an adeno-associated virus serotype 8 vector to deliver codon-optimized, B-domain-deleted FVIII specifically to a patient’s liver, where FVIII would then be produced and used to manage bleeds.
Shire, the company developing SHP654, received FDA clearance for an investigational new drug application to initiate a global, phase 1/2 study of SHP654.
In this study, researchers will evaluate the safety and optimal dose of SHP654 needed to boost FVIII activity levels and affect hemophilic bleeding. Shire expects this study will begin by the end of this year.
The FDA also granted orphan designation to SHP654. The agency grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
“This important orphan drug designation highlights Shire’s commitment to patients with rare diseases, and, for hemophilia patients specifically, our aim is to help them achieve zero bleeds,” said Paul Monahan, MD, senior medical director of gene therapy at Shire.
“We know that hemophilia care is not one-size-fits-all and that every patient is unique, which is why we continue to focus on optimizing personal outcomes for hemophilia patients by developing innovations to transform care.” ![]()
The US Food and Drug Administration (FDA) has accepted an investigational new drug application for SHP654 (also known as BAX 888) and granted the therapy orphan drug designation.
SHP654 is an investigational factor VIII (FVIII) gene therapy intended to treat hemophilia A using an adeno-associated virus serotype 8 vector to deliver codon-optimized, B-domain-deleted FVIII specifically to a patient’s liver, where FVIII would then be produced and used to manage bleeds.
Shire, the company developing SHP654, received FDA clearance for an investigational new drug application to initiate a global, phase 1/2 study of SHP654.
In this study, researchers will evaluate the safety and optimal dose of SHP654 needed to boost FVIII activity levels and affect hemophilic bleeding. Shire expects this study will begin by the end of this year.
The FDA also granted orphan designation to SHP654. The agency grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
“This important orphan drug designation highlights Shire’s commitment to patients with rare diseases, and, for hemophilia patients specifically, our aim is to help them achieve zero bleeds,” said Paul Monahan, MD, senior medical director of gene therapy at Shire.
“We know that hemophilia care is not one-size-fits-all and that every patient is unique, which is why we continue to focus on optimizing personal outcomes for hemophilia patients by developing innovations to transform care.” ![]()
Automated blood analyzers approved in China
The China Food and Drug Administration has approved Ortho Clinical Diagnostics’ ORTHO VISION® Platform of fully automated blood analyzers for transfusion medicine laboratories.
The platform consists of ORTHO VISION and ORTHO VISION Max.
ORTHO VISION is a blood analyzer designed for small- to mid-sized transfusion labs, and ORTHO VISION Max is an analyzer developed for mid- to high-volume labs.
According to Ortho Clinical Diagnostics, the ORTHO VISION Platform automates more tests than ever before and takes less time to perform those tests.
The platform supports complex immunohematology testing such as serial dilutions for titration studies, reflex tests, and selected cell antibody identification.
The ORTHO VISION Platform also has scheduling intelligence, which allows a transfusion medicine department to process routine samples and STAT orders as they are received, rather than waiting for a complete batch before running the instrument.
The ORTHO VISION Platform offers dynamic workflow and lab standardization across instrumentation, technology, procedures, and training. These features are intended to help labs keep pace with growing industry pressure to increase productivity while remaining operationally efficient.
ORTHO VISION and ORTHO VISION Max are commercially available in countries other than China as well, including the US, Japan, and European countries.
“Around the world, transfusion medicine labs are facing increasing pressure to perform more tests with fewer resources, and yet their work in ensuring that all patients receive safe and appropriate blood remains critically important,” said Robert Yates, chief operating officer of Ortho Clinical Diagnostics.
“Ortho is committed to helping labs become more efficient while continuing to perform precise, accurate testing.” ![]()
The China Food and Drug Administration has approved Ortho Clinical Diagnostics’ ORTHO VISION® Platform of fully automated blood analyzers for transfusion medicine laboratories.
The platform consists of ORTHO VISION and ORTHO VISION Max.
ORTHO VISION is a blood analyzer designed for small- to mid-sized transfusion labs, and ORTHO VISION Max is an analyzer developed for mid- to high-volume labs.
According to Ortho Clinical Diagnostics, the ORTHO VISION Platform automates more tests than ever before and takes less time to perform those tests.
The platform supports complex immunohematology testing such as serial dilutions for titration studies, reflex tests, and selected cell antibody identification.
The ORTHO VISION Platform also has scheduling intelligence, which allows a transfusion medicine department to process routine samples and STAT orders as they are received, rather than waiting for a complete batch before running the instrument.
The ORTHO VISION Platform offers dynamic workflow and lab standardization across instrumentation, technology, procedures, and training. These features are intended to help labs keep pace with growing industry pressure to increase productivity while remaining operationally efficient.
ORTHO VISION and ORTHO VISION Max are commercially available in countries other than China as well, including the US, Japan, and European countries.
“Around the world, transfusion medicine labs are facing increasing pressure to perform more tests with fewer resources, and yet their work in ensuring that all patients receive safe and appropriate blood remains critically important,” said Robert Yates, chief operating officer of Ortho Clinical Diagnostics.
“Ortho is committed to helping labs become more efficient while continuing to perform precise, accurate testing.” ![]()
The China Food and Drug Administration has approved Ortho Clinical Diagnostics’ ORTHO VISION® Platform of fully automated blood analyzers for transfusion medicine laboratories.
The platform consists of ORTHO VISION and ORTHO VISION Max.
ORTHO VISION is a blood analyzer designed for small- to mid-sized transfusion labs, and ORTHO VISION Max is an analyzer developed for mid- to high-volume labs.
According to Ortho Clinical Diagnostics, the ORTHO VISION Platform automates more tests than ever before and takes less time to perform those tests.
The platform supports complex immunohematology testing such as serial dilutions for titration studies, reflex tests, and selected cell antibody identification.
The ORTHO VISION Platform also has scheduling intelligence, which allows a transfusion medicine department to process routine samples and STAT orders as they are received, rather than waiting for a complete batch before running the instrument.
The ORTHO VISION Platform offers dynamic workflow and lab standardization across instrumentation, technology, procedures, and training. These features are intended to help labs keep pace with growing industry pressure to increase productivity while remaining operationally efficient.
ORTHO VISION and ORTHO VISION Max are commercially available in countries other than China as well, including the US, Japan, and European countries.
“Around the world, transfusion medicine labs are facing increasing pressure to perform more tests with fewer resources, and yet their work in ensuring that all patients receive safe and appropriate blood remains critically important,” said Robert Yates, chief operating officer of Ortho Clinical Diagnostics.
“Ortho is committed to helping labs become more efficient while continuing to perform precise, accurate testing.” ![]()
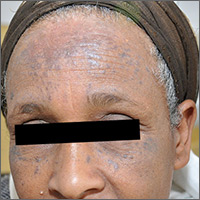
Brown spots on face
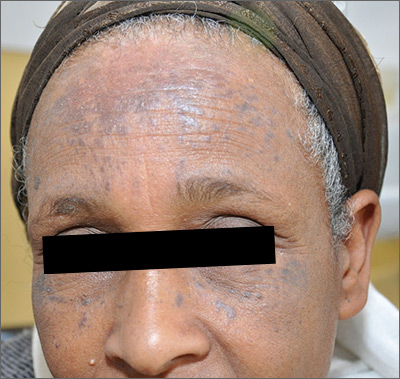
The results of a 4-mm punch biopsy confirmed a diagnosis of actinic lichen planus. As the name implies, this form of lichen planus occurs in areas of sun exposure. Treatment is the same as for other forms of lichen planus, with the addition of avoiding the sun.
The FP counseled the patient about the use of sunscreen and avoiding sun exposure as much as possible. The FP then prescribed a mid-potency topical corticosteroid, 0.1% triamcinolone cream. (A low-potency steroid is unlikely to be beneficial.) In cases like this one, a short course of a mid-potency steroid should be safe on the face without causing atrophy.
At follow-up, there was a significant improvement in the appearance of the patient’s face and hands. The patient was pleased with these results and said she’d been more careful with sun exposure. The FP prescribed 2.5% hydrocortisone cream to be used on the face as needed, in conjunction with the continued use of triamcinolone on the hands as needed.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Kraft RL, Usatine R. Lichen planus. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 901-909.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The results of a 4-mm punch biopsy confirmed a diagnosis of actinic lichen planus. As the name implies, this form of lichen planus occurs in areas of sun exposure. Treatment is the same as for other forms of lichen planus, with the addition of avoiding the sun.
The FP counseled the patient about the use of sunscreen and avoiding sun exposure as much as possible. The FP then prescribed a mid-potency topical corticosteroid, 0.1% triamcinolone cream. (A low-potency steroid is unlikely to be beneficial.) In cases like this one, a short course of a mid-potency steroid should be safe on the face without causing atrophy.
At follow-up, there was a significant improvement in the appearance of the patient’s face and hands. The patient was pleased with these results and said she’d been more careful with sun exposure. The FP prescribed 2.5% hydrocortisone cream to be used on the face as needed, in conjunction with the continued use of triamcinolone on the hands as needed.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Kraft RL, Usatine R. Lichen planus. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 901-909.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The results of a 4-mm punch biopsy confirmed a diagnosis of actinic lichen planus. As the name implies, this form of lichen planus occurs in areas of sun exposure. Treatment is the same as for other forms of lichen planus, with the addition of avoiding the sun.
The FP counseled the patient about the use of sunscreen and avoiding sun exposure as much as possible. The FP then prescribed a mid-potency topical corticosteroid, 0.1% triamcinolone cream. (A low-potency steroid is unlikely to be beneficial.) In cases like this one, a short course of a mid-potency steroid should be safe on the face without causing atrophy.
At follow-up, there was a significant improvement in the appearance of the patient’s face and hands. The patient was pleased with these results and said she’d been more careful with sun exposure. The FP prescribed 2.5% hydrocortisone cream to be used on the face as needed, in conjunction with the continued use of triamcinolone on the hands as needed.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Kraft RL, Usatine R. Lichen planus. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 901-909.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Novel picosecond laser improves acne scarring
Novel picosecond-domain 1,064 nm and 532 nm neodymium: yttrium aluminum garnet (Nd:YAG) lasers used with a new holographic beam splitter safely and effectively treated facial acne scars in a prospective study.
Among the 27 participants who completed the study, the mean improvement in acne scarring was 1.4 on a 10-point global aesthetic scale (range –4 to 6 points; 95% confidence interval, 0.85-1.9); these assessments were performed by three blinded physician reviewers 12 weeks after the last treatment. In addition, 23 (85%) of the participants reported that they were satisfied or very satisfied with their treatment, Eric F. Bernstein, MD, who is in private practice in Ardmore, Pa., and his coauthors reported (Lasers Surg Med. 2017 Nov;49[9]:796-802).
The study comprised 27 men and women with Fitzpatrick skin types II-V whose mean age was 45 years. They were treated with four monthly treatments. Of the participants, 19 were treated with the 1,064 nm laser and 8 with the 532 nm laser; both treatments employed a novel holographic hand piece to deliver precise beams of focused laser energy. Blinded physician reviewers evaluated digital images taken both before treatment and 12 weeks after the final treatment.
Based on the averages of scores from the reviewers, 81% of the participants showed some degree of improvement, 48% had a mean improvement of at least 2 points, and 26% had a mean improvement score of at least 3 points.
Participants experienced some side effects immediately after treatment, including mild to moderate erythema (100% of patients for both lasers), mild to moderate edema (95% for 1,064 nm, 97% for 532 nm), mild to moderate petechiae (50%, 38%), and mild purpura (17%, 0%). All these responses cleared within a few hours or a few days after treatments, according to patient reports.
None of the patients experienced pigmentary changes, based on before and after treatment photos. In contrast, the most commonly used laser for treating acne scarring – the nonfractionated CO2 laser – causes significant hyperpigmentation and even permanent hypopigmentation, the authors pointed out.
No significant difference was seen when comparing mean improvement scores between participants treated with the 1,064 nm lasers and those treated with the 532 nm lasers.
“The use of picosecond-domain pulses delivers clinical benefits at lower fluences and energies than would be required at longer pulse durations and may offer qualitatively different tissue effects than earlier-generation lasers,” Dr. Bernstein and his coauthors wrote. “Future studies investigating combinations of the 1,064 and 532 nm picosecond-domain fractionated wavelengths, as well as larger trials with skin types V and VI, should increase the ways the device is used and the conditions it is used to treat,” they added.
Limitations of the study included the short 3-month follow-up, they noted.
The study was funded by Syneron Candela, the manufacturer of the laser and the holographic beam-splitting optic used in the study; the company loaned the equipment for the study. Dr. Bernstein is a consultant for Syneron Candela. Two of the five authors were employees of Syneron Candela at the time the study was conducted. No other financial disclosures were reported.
Novel picosecond-domain 1,064 nm and 532 nm neodymium: yttrium aluminum garnet (Nd:YAG) lasers used with a new holographic beam splitter safely and effectively treated facial acne scars in a prospective study.
Among the 27 participants who completed the study, the mean improvement in acne scarring was 1.4 on a 10-point global aesthetic scale (range –4 to 6 points; 95% confidence interval, 0.85-1.9); these assessments were performed by three blinded physician reviewers 12 weeks after the last treatment. In addition, 23 (85%) of the participants reported that they were satisfied or very satisfied with their treatment, Eric F. Bernstein, MD, who is in private practice in Ardmore, Pa., and his coauthors reported (Lasers Surg Med. 2017 Nov;49[9]:796-802).
The study comprised 27 men and women with Fitzpatrick skin types II-V whose mean age was 45 years. They were treated with four monthly treatments. Of the participants, 19 were treated with the 1,064 nm laser and 8 with the 532 nm laser; both treatments employed a novel holographic hand piece to deliver precise beams of focused laser energy. Blinded physician reviewers evaluated digital images taken both before treatment and 12 weeks after the final treatment.
Based on the averages of scores from the reviewers, 81% of the participants showed some degree of improvement, 48% had a mean improvement of at least 2 points, and 26% had a mean improvement score of at least 3 points.
Participants experienced some side effects immediately after treatment, including mild to moderate erythema (100% of patients for both lasers), mild to moderate edema (95% for 1,064 nm, 97% for 532 nm), mild to moderate petechiae (50%, 38%), and mild purpura (17%, 0%). All these responses cleared within a few hours or a few days after treatments, according to patient reports.
None of the patients experienced pigmentary changes, based on before and after treatment photos. In contrast, the most commonly used laser for treating acne scarring – the nonfractionated CO2 laser – causes significant hyperpigmentation and even permanent hypopigmentation, the authors pointed out.
No significant difference was seen when comparing mean improvement scores between participants treated with the 1,064 nm lasers and those treated with the 532 nm lasers.
“The use of picosecond-domain pulses delivers clinical benefits at lower fluences and energies than would be required at longer pulse durations and may offer qualitatively different tissue effects than earlier-generation lasers,” Dr. Bernstein and his coauthors wrote. “Future studies investigating combinations of the 1,064 and 532 nm picosecond-domain fractionated wavelengths, as well as larger trials with skin types V and VI, should increase the ways the device is used and the conditions it is used to treat,” they added.
Limitations of the study included the short 3-month follow-up, they noted.
The study was funded by Syneron Candela, the manufacturer of the laser and the holographic beam-splitting optic used in the study; the company loaned the equipment for the study. Dr. Bernstein is a consultant for Syneron Candela. Two of the five authors were employees of Syneron Candela at the time the study was conducted. No other financial disclosures were reported.
Novel picosecond-domain 1,064 nm and 532 nm neodymium: yttrium aluminum garnet (Nd:YAG) lasers used with a new holographic beam splitter safely and effectively treated facial acne scars in a prospective study.
Among the 27 participants who completed the study, the mean improvement in acne scarring was 1.4 on a 10-point global aesthetic scale (range –4 to 6 points; 95% confidence interval, 0.85-1.9); these assessments were performed by three blinded physician reviewers 12 weeks after the last treatment. In addition, 23 (85%) of the participants reported that they were satisfied or very satisfied with their treatment, Eric F. Bernstein, MD, who is in private practice in Ardmore, Pa., and his coauthors reported (Lasers Surg Med. 2017 Nov;49[9]:796-802).
The study comprised 27 men and women with Fitzpatrick skin types II-V whose mean age was 45 years. They were treated with four monthly treatments. Of the participants, 19 were treated with the 1,064 nm laser and 8 with the 532 nm laser; both treatments employed a novel holographic hand piece to deliver precise beams of focused laser energy. Blinded physician reviewers evaluated digital images taken both before treatment and 12 weeks after the final treatment.
Based on the averages of scores from the reviewers, 81% of the participants showed some degree of improvement, 48% had a mean improvement of at least 2 points, and 26% had a mean improvement score of at least 3 points.
Participants experienced some side effects immediately after treatment, including mild to moderate erythema (100% of patients for both lasers), mild to moderate edema (95% for 1,064 nm, 97% for 532 nm), mild to moderate petechiae (50%, 38%), and mild purpura (17%, 0%). All these responses cleared within a few hours or a few days after treatments, according to patient reports.
None of the patients experienced pigmentary changes, based on before and after treatment photos. In contrast, the most commonly used laser for treating acne scarring – the nonfractionated CO2 laser – causes significant hyperpigmentation and even permanent hypopigmentation, the authors pointed out.
No significant difference was seen when comparing mean improvement scores between participants treated with the 1,064 nm lasers and those treated with the 532 nm lasers.
“The use of picosecond-domain pulses delivers clinical benefits at lower fluences and energies than would be required at longer pulse durations and may offer qualitatively different tissue effects than earlier-generation lasers,” Dr. Bernstein and his coauthors wrote. “Future studies investigating combinations of the 1,064 and 532 nm picosecond-domain fractionated wavelengths, as well as larger trials with skin types V and VI, should increase the ways the device is used and the conditions it is used to treat,” they added.
Limitations of the study included the short 3-month follow-up, they noted.
The study was funded by Syneron Candela, the manufacturer of the laser and the holographic beam-splitting optic used in the study; the company loaned the equipment for the study. Dr. Bernstein is a consultant for Syneron Candela. Two of the five authors were employees of Syneron Candela at the time the study was conducted. No other financial disclosures were reported.
FROM LASERS IN SURGERY AND MEDICINE
Key clinical point: A new picosecond-domain 1,064 nm and 532 nm Nd:YAG laser combined with a novel holographic beam splitter can treat facial acne scars safely and effectively.
Major finding: All 27 participants who completed the study saw a mean improvement in acne scarring of 1.4 on a 10-point scale, with improvement ranging up to 60%.
Data source: A prospective study of participants with facial acne scars who were treated with four monthly laser treatments.
Disclosures: The study was funded by Syneron Candela, the manufacturer of the laser and the holographic beam-splitting optic used in the study; the company loaned the equipment for the study. Dr. Bernstein is a consultant for Syneron Candela. Two of the five authors were employees of Syneron Candela at the time the study was conducted. No other financial disclosures were reported.
In close vote, advisory panel prefers Shingrix over Zostavax
Herpes zoster subunit vaccine (Shingrix) was preferentially recommended over zoster vaccine live (Zostavax) for preventing herpes zoster and related complications Oct. 25 at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
Eight committee members voted for the recommendation, and seven voted against it.
In discussions leading up to the vote, some committee members cited potential supply issues, as well as the need for longer-term safety data, among other issues.
“I think it would be nice to see data on a larger population that is not just research-based, especially because we have very little data on ethnic minorities,” said Laura E. Riley, MD, of Harvard Medical School, Boston, who voted against the recommendation.
The vote comes several days after GlaxoSmithKline announced the Food and Drug Administration approval of Shingrix for the prevention of herpes zoster (shingles) in adults aged 50 years or older. In pooled clinical trial results, the vaccine demonstrated greater than 90% efficacy in all age groups, according to a company statement.
Shingrix is a non-live, recombinant subunit vaccine that is given in two doses, intramuscularly. Zostavax, also indicated in individuals aged 50 years or older, is a live attenuated virus vaccine.
In a related decision, ACIP voted 14-1 to recommend Shingrix for prevention of herpes zoster and related complications for immunocompetent adults aged 50 years and older.
They also voted 12-3 to recommend Shingrix to prevent herpes zoster and its complications for immunocompetent adults who previously received Zostavax.
Herpes zoster subunit vaccine (Shingrix) was preferentially recommended over zoster vaccine live (Zostavax) for preventing herpes zoster and related complications Oct. 25 at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
Eight committee members voted for the recommendation, and seven voted against it.
In discussions leading up to the vote, some committee members cited potential supply issues, as well as the need for longer-term safety data, among other issues.
“I think it would be nice to see data on a larger population that is not just research-based, especially because we have very little data on ethnic minorities,” said Laura E. Riley, MD, of Harvard Medical School, Boston, who voted against the recommendation.
The vote comes several days after GlaxoSmithKline announced the Food and Drug Administration approval of Shingrix for the prevention of herpes zoster (shingles) in adults aged 50 years or older. In pooled clinical trial results, the vaccine demonstrated greater than 90% efficacy in all age groups, according to a company statement.
Shingrix is a non-live, recombinant subunit vaccine that is given in two doses, intramuscularly. Zostavax, also indicated in individuals aged 50 years or older, is a live attenuated virus vaccine.
In a related decision, ACIP voted 14-1 to recommend Shingrix for prevention of herpes zoster and related complications for immunocompetent adults aged 50 years and older.
They also voted 12-3 to recommend Shingrix to prevent herpes zoster and its complications for immunocompetent adults who previously received Zostavax.
Herpes zoster subunit vaccine (Shingrix) was preferentially recommended over zoster vaccine live (Zostavax) for preventing herpes zoster and related complications Oct. 25 at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
Eight committee members voted for the recommendation, and seven voted against it.
In discussions leading up to the vote, some committee members cited potential supply issues, as well as the need for longer-term safety data, among other issues.
“I think it would be nice to see data on a larger population that is not just research-based, especially because we have very little data on ethnic minorities,” said Laura E. Riley, MD, of Harvard Medical School, Boston, who voted against the recommendation.
The vote comes several days after GlaxoSmithKline announced the Food and Drug Administration approval of Shingrix for the prevention of herpes zoster (shingles) in adults aged 50 years or older. In pooled clinical trial results, the vaccine demonstrated greater than 90% efficacy in all age groups, according to a company statement.
Shingrix is a non-live, recombinant subunit vaccine that is given in two doses, intramuscularly. Zostavax, also indicated in individuals aged 50 years or older, is a live attenuated virus vaccine.
In a related decision, ACIP voted 14-1 to recommend Shingrix for prevention of herpes zoster and related complications for immunocompetent adults aged 50 years and older.
They also voted 12-3 to recommend Shingrix to prevent herpes zoster and its complications for immunocompetent adults who previously received Zostavax.
FROM AN ACIP MEETING
Yoga benefits lung cancer patients and caregivers alike
Yoga provides physical and mental benefits for both lung cancer patients and their caregivers, according to results of a randomized study presented at the Palliative and Supportive Care in Oncology Symposium.
“Overall, we are encouraged by the findings,” said lead study author, Kathrin Milbury, PhD, of University of Texas MD Anderson Cancer Center, Houston.
This study provides preliminary evidence that a yoga program can provide a “buffer” and improve physical function for patients, as well as self-reported improved quality of life for both patients and their caregivers, she added.
All patients in the study had non–small cell lung cancer and were undergoing thoracic radiation therapy, which can cause respiratory toxicities that negatively affect quality of life and physical activity, according to Dr. Milbury and her coinvestigators.
A total of 32 patient-caregiver dyads were randomized to participate in 15 yoga sessions or to be in a “wait-list” control group, and 26 dyads completed all assessments.
Patients who practiced yoga had significantly better scores on a 6-minute walking test (478 vs. 402 for wait-list enrollees; P less than .05), plus better stamina and mental health. Caregivers had improved fatigue and better stamina at work.
Almost all patients (96%) rated the program as “very useful,” investigators reported at the symposium, which was cosponsored by AAHPM, ASCO, ASTRO, and MASCC.
This study provides additional evidence that yoga and other nonpharmacologic supportive therapies “can be integrated into not only the care of cancer patients, but also the family caregivers who support them,” according to Andrew S. Epstein, MD, of Memorial Sloan Kettering Cancer Center, New York.
Next, the researchers plan to conduct a larger, randomized, controlled trial with a more stringent comparison group, according to Dr. Milbury.
Yoga provides physical and mental benefits for both lung cancer patients and their caregivers, according to results of a randomized study presented at the Palliative and Supportive Care in Oncology Symposium.
“Overall, we are encouraged by the findings,” said lead study author, Kathrin Milbury, PhD, of University of Texas MD Anderson Cancer Center, Houston.
This study provides preliminary evidence that a yoga program can provide a “buffer” and improve physical function for patients, as well as self-reported improved quality of life for both patients and their caregivers, she added.
All patients in the study had non–small cell lung cancer and were undergoing thoracic radiation therapy, which can cause respiratory toxicities that negatively affect quality of life and physical activity, according to Dr. Milbury and her coinvestigators.
A total of 32 patient-caregiver dyads were randomized to participate in 15 yoga sessions or to be in a “wait-list” control group, and 26 dyads completed all assessments.
Patients who practiced yoga had significantly better scores on a 6-minute walking test (478 vs. 402 for wait-list enrollees; P less than .05), plus better stamina and mental health. Caregivers had improved fatigue and better stamina at work.
Almost all patients (96%) rated the program as “very useful,” investigators reported at the symposium, which was cosponsored by AAHPM, ASCO, ASTRO, and MASCC.
This study provides additional evidence that yoga and other nonpharmacologic supportive therapies “can be integrated into not only the care of cancer patients, but also the family caregivers who support them,” according to Andrew S. Epstein, MD, of Memorial Sloan Kettering Cancer Center, New York.
Next, the researchers plan to conduct a larger, randomized, controlled trial with a more stringent comparison group, according to Dr. Milbury.
Yoga provides physical and mental benefits for both lung cancer patients and their caregivers, according to results of a randomized study presented at the Palliative and Supportive Care in Oncology Symposium.
“Overall, we are encouraged by the findings,” said lead study author, Kathrin Milbury, PhD, of University of Texas MD Anderson Cancer Center, Houston.
This study provides preliminary evidence that a yoga program can provide a “buffer” and improve physical function for patients, as well as self-reported improved quality of life for both patients and their caregivers, she added.
All patients in the study had non–small cell lung cancer and were undergoing thoracic radiation therapy, which can cause respiratory toxicities that negatively affect quality of life and physical activity, according to Dr. Milbury and her coinvestigators.
A total of 32 patient-caregiver dyads were randomized to participate in 15 yoga sessions or to be in a “wait-list” control group, and 26 dyads completed all assessments.
Patients who practiced yoga had significantly better scores on a 6-minute walking test (478 vs. 402 for wait-list enrollees; P less than .05), plus better stamina and mental health. Caregivers had improved fatigue and better stamina at work.
Almost all patients (96%) rated the program as “very useful,” investigators reported at the symposium, which was cosponsored by AAHPM, ASCO, ASTRO, and MASCC.
This study provides additional evidence that yoga and other nonpharmacologic supportive therapies “can be integrated into not only the care of cancer patients, but also the family caregivers who support them,” according to Andrew S. Epstein, MD, of Memorial Sloan Kettering Cancer Center, New York.
Next, the researchers plan to conduct a larger, randomized, controlled trial with a more stringent comparison group, according to Dr. Milbury.
FROM PALLONC 2017
Key clinical point: Yoga provides both physical and mental benefits for lung cancer patients undergoing radiotherapy and their caregivers.
Major finding: Patients who practiced yoga had significantly better scores on a 6-minute walking test (478 vs. 402 for wait-list enrollees; P less than .05), plus better stamina and mental health. Caregivers had improved fatigue and better stamina at work.
Data source: Randomized study including 47 patient-caregiver dyads, of which 32 consented and 26 completed all assessments.
Disclosures: Funding for this study came from the National Institutes of Health. Lead author Kathrin Milbury, PhD, reported no potential conflicts of interest.