User login
Diagnosis and Treatment of Migraine
From the Department of Neurology, Medstar Georgetown University Hospital, Washington, DC.
Abstract
- Objective: To review the epidemiology, pathophysiology, diagnosis, and treatment of migraine.
- Methods: Review of the literature.
- Results: Migraine is a common disorder associated with significant morbidity. Diagnosis of migraine is performed according to the International Classification of Headache Disorders. Comorbidities are commonly seen with migraine and include mood disorders (depression, anxiety, post-traumatic stress disorder), musculoskeletal disorders (neck pain, fibromyalgia, Ehlors-Danlos syndrome), sleep disorders, asthma, allergies, thyroid dysfunction, obesity, irritable bowel syndrome, epilepsy, stroke, and heart disease. Comorbid conditions can increase migraine disability. Management of migraine with lifestyle modifications, trigger management, and acute and preventive medications can help reduce the frequency, duration, and severity of attacks. Overuse of medications such as opiates, barbiturates, and caffeine-containing medications can increase headache frequency. Educating patients about limiting use of these medications is important.
- Conclusion: Migraine is a common neurologic disease that can be very disabling. Recognizing the condition, making an accurate diagnosis, and starting patients on migraine-specific treatments can help improve patient outcomes.
Migraine is a common neurologic disease that affects 1 in 10 people worldwide [1]. It is 2 to 3 times more prevalent in women than in men [2]. The prevalence of migraine peaks in both sexes during the most productive years of adulthood (age 25 to 55 years) [3]. The Global Burden of Diseases, Injuries, and Risk Factors Study considers it to be the 7th most disabling disease in the world [4]. Over 36 million people in the United States have migraine [5]. However, just 56% of migraineurs have ever been diagnosed [6].
Migraine is associated with a high rate of years lived with disability [7] and the rate has been steadily increasing since 1990. At least 50% of migraine sufferers are severely disabled, many requiring bed rest, during individual migraine attacks lasting hours to days [8]. The total U.S. annual economic costs from headache disorders, including the indirect costs from lost productivity and workplace performance, has been estimated at $31 billion [9,10].
Despite the profound impact of migraine on patients and society, there are numerous barriers to migraine care. Lipton et al [11] identified 3 steps that were minimally necessary to achieve guideline-defined appropriate acute pharmacologic therapy: (1) consulting a prescribing health care professional; (2) receiving a migraine diagnosis; and (3) using migraine-specific or other appropriate acute treatments. In a study they conducted in patients with episodic migraine, 45.5% had consulted health care professional for headache in the preceding year; of these, 86.7% reported receiving a medical diagnosis of migraine, and among the diagnosed consulters, 66.7% currently used acute migraine-specific treatments, resulting in only 26.3% individuals successfully completing all 3 steps. In the recent CaMEO study [12], the proportion patients with chronic migraine that overcame all 3 barriers was less than 5%.
The stigma of migraine often makes it difficult for people to discuss symptoms with their health care providers and family members [13]. When they do discuss their headaches with their provider, often they are not given a diagnosis [14] or do not understand what their diagnosis means [15]. It is important for health care providers to be vigilant about the diagnosis of migraine, discuss treatment goals and strategies, and prescribe appropriate migraine treatment. Migraine is often comorbid with a number of medical, neurological, and psychiatric conditions, and identifying and managing comorbidities is necessary to reduce headache burden and disability. In this article, we provide a review of the diagnosis and treatment of migraine, using a case illustration to highlight key points.
Case Study
Initial Presentation
A 24-year-old woman presents for an evaluation of her headaches.
History and Physical Examination
She initially noted headaches at age 19, which were not memorable and did not cause disability. Her current headaches are a severe throbbing pain over her right forehead. They are associated with light and sound sensitivity and stomach upset. Headaches last 6 to 7 hours without medications and occur 4 to 8 days per month.
She denies vomiting and autonomic symptoms such as runny nose or eye tearing. She also denies preceding aura. She reports headache relief with intake of tablets that contain acetaminophen/aspirin/caffeine and states that she takes between 4 to 15 tablets/month depending on headache frequency. She reports having tried acetaminophen and naproxen with no significant benefit. Aggravating factors include bright lights, strong smells, and soy/ high-sodium foods.
She had no significant past medical problems and denied a history of depression or anxiety. Family history was significant for both her father and sister having a history of headaches. The patient lived alone and denied any major life stressors. She exercises 2 times a week and denies smoking or alcohol use. Review of systems was positive for trouble sleeping, which she described as difficulty falling asleep.
On physical examination, vitals were within normal limits. BMI was 23. Chest, cardiac, abdomen, and general physical examination were all within normal limits. Neurological examination revealed no evidence of papilledema or focal neurological deficits.
What is the pathophysiology of migraine?
Migraine was thought to be a primary vascular disorder of the brain, with the origins of the vascular theory of migraine dating back to 1684 [16]. Trials performed by Wolff concluded that migraine is of vascular origin [17], and this remained the predominant theory over several decades. Current evidence suggests that migraine is unlikely to be a pure vascular disorder and instead may be related to changes in the central or peripheral nervous system [18,19].
Migraine is complex brain network disorder with a strong genetic basis [19]. The trigemino-vascular system, along with neurogenically induced inflammation of the dura mater, mast cell degranulation and release of histamine, are the likely causes of migraine pain. Trigeminal fibers arise from neurons in the trigeminal ganglion that contain substance P and calcitonin gene-related peptide (CGRP) [20]. CGRP is a neuropeptide widely expressed in both peripheral and central neurons. Elevation of CGRP in migraine is linked to diminution of the inhibitory pathways which in turn leads to migraine susceptibility [21]. These findings have led to the development of new drugs that target the CGRP pathway.
In the brainstem, periaqueductal grey matter and the dorsolateral pons have been found to be “migraine generators,” or the driver of changes of cortical activity during migraine [22]. Brainstem nuclei are involved in modulating trigemino-vascular pain transmission and autonomic responses in migraine [23].
The hypothalamus has also been implicated in migraine pathogenesis, particularly its role in nociceptive and autonomic modulation in migraine patients. Schulte and May hypothesized that there is a network change between the hypothalamus and the areas of the brainstem generator leading to the migraine attacks [24].
The thalamus plays a central role for the processing and integration of pain stimuli from the dura mater and cutaneous regions. It maintains complex connections with the somatosensory, motor, visual, auditory, olfactory and limbic regions [25]. The structural and functional alterations in the system play a role in the development of migraine attacks, and also in the sensory hypersensitivity to visual stimuli and mechanical allodynia [26].
Experimental studies in rats show that cortical spreading depression can trigger neurogenic meningeal inflammation and subsequently activate the trigemino-vascular system [27]. It has been observed that between migraine episodes a time-dependent amplitude increase of scalp-evoked potentials to repeated stereotyped stimuli, such as visual, auditory, and somaticstimuli, occurs. This phenomenon is described as “deficient habituation.” In episodic migraine, studies show 2 characteristic changes: a deficient habituation between attacks and sensitization during the attack [28]. Genetic studies have hypothesized an involvement of glutamatergic neurotransmitters and synaptic dysplasticity in causing abnormal cortical excitability in migraine [27].
What are diagnostic criteria for migraine?
Diagnosis of migraine is performed according to the International Classification of Headache Disorders (ICHD) [29]. Based on the number of headache days that the patient reports, migraine is classified into episodic or chronic migraine. Migraines that occur on fewer than 15 days/month are categorized as episodic migraines.
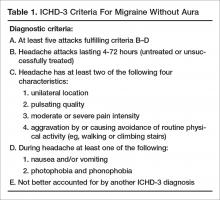
Episodic migraine is divided into 2 categories: migraine with aura (Table 1) and migraine without aura. Migraine without aura is described as recurrent headaches consisting of at least 5 attacks, each lasting 4 to 72 hours if left untreated. At least 2 of the following 4 characteristics must be present: unilateral location, pulsating quality, moderate or severe pain intensity, with aggravation by or causing avoidance of routine physical activity. During headache, at least 1 of nausea and/or vomiting or photophobia and phonophobia should be present.
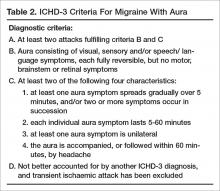
In migraine with aura (Table 2), headache characteristics are the same, but in addition there are at least 2 lifetime attacks with fully reversible aura symptoms (visual, sensory, speech/language). In addition, these auras have at least 2 of the following 4 characteristics: at least 1 aura symptom spreads gradually over 5 minutes, and/or 2 or more symptoms occur in succession; each individual aura symptom lasts 5 to 60 minutes; aura symptom is unilateral; and aura is accompanied, or followed within 60 minutes, by headache. Migraine with aura is uncommon, occurring in 20% of patients with migraine [30]. Visual aura is the most common type of aura, occurring in up to 90% of patients [31]. There is also aura without migraine, called typical aura without headache. Patients can present with non-migraine headache with aura, categorized as typical aura with headache [29].
Headache occurring on 15 or more days per month for more than 3 months, which has the features of migraine headache on at least 8 days per month, is classified as chronic migraine (Table 3). Evidence indicates that 2.5% of episodic migraine progresses to chronic migraine over 1-year follow-up [32]. There are several risk factors for chronification of migraine. Nonmodifiable factors include female sex, white European heritage, head/neck injury, low education/socioeconomic status, and stressful life events (divorce, moving, work changes, problems with children). Modifiable risk factors are headache frequency, acute medication overuse, caffeine overuse, obesity, comorbid mood disorders, and allodynia. Acute medication use and headache frequency are independent risk factors for development of chronic migraine [33]. The risk of chronic migraine increases exponentially with increased attack frequency, usually when the frequency is ≥ 3 headaches/month. Repetitive episodes of pain may increase central sensitization and result in anatomical changes in the brain and brainstem [34].
What information should be elicited during the history?
Specific questions about the headaches can help with making an accurate diagnosis. These include:
- Length of attacks and their frequency
- Pain characteristics (location, quality, intensity)
- Actions that trigger or aggravate headaches (eg, stress, movement, bright lights, menses, certain foods and smells)
- Associated symptoms that accompany headaches (eg, nausea, vomiting)
- How the headaches impact their life (eg, missed days at work or school, missed life events, avoidance of social activities, emergency room visits due to headache)
To assess headache frequency, it is helpful to ask about the number of headache-free days in a month, eg, “how many days a month do you NOT have a headache.” To assist with headache assessment, patients can be asked to keep a calendar in which they mark days of use of medications, including over the counter medications, menses, and headache days. The calendar can be used to assess for migraine patterns, headache frequency, and response to treatment.
When asking about headache history, it is important for patients to describe their untreated headaches. Patients taking medications may have pain that is less severe or disabling or have reduced associated symptoms. Understanding what the headaches were like when they did not treat is important in making a diagnosis.
Other important questions include when was the first time they recall ever experiencing a headache. Migraine is often present early in life, and understanding the change in headache over time is important. Also ask patients about what they want to do when they have a headache. Often patients want to lie down in a cool dark room. Ask what they would prefer to do if they didn’t have any pending responsibilities.
Comorbidities
Comorbidities are commonly seen with migraine. Common comorbidities are mood disorders (depression, anxiety, post-traumatic stress disorder), musculoskeletal disorders (neck pain, fibromyalgia, Ehlors-Danlos syndrome), sleep disorders, asthma, allergies, thyroid dysfunction, obesity, irritable bowel syndrome, epilepsy, stroke, and heart disease.
Comorbid conditions can increase migraine disability and also can provide information about the pathophysiology of migraine and guide treatment. Management of the underlying comorbidity often leads to improved migraine outcomes. For example, serotonergic dysfunction is a possible pathway involved in both migraine and mood disorders. Treatment with medications that alter the serotonin system may help both migraine and coexisting mood disorders. Bigal et al proposed that activation of the HPA axis with reduced serotonin synthesis is a main pathway involved in affective disorders, migraine, and obesity [35].
In the early 1950s, Wolff conceptualized migraine as a psychophysiologic disorder [36]. The relationship between migraine and psychiatric conditions is complex, and comorbid psychiatric disorders are risk factors for headache progression and chronicity. Psychiatric conditions also play a role in nonadherence to headache medication, which contributes to poor outcome in these patients. Hence, there is a need for assessment and treatment of psychiatric disorders in people with migraine. A study by Guidetti et al found that headache patients with multiple psychiatric conditions have poor outcomes, with 86 % of these headache patients having no improvement and even deterioration in their headache [37]. Another study by Mongini et al concluded that psychiatric disorder appears to influence the result of treatment on a long-term basis [38].
In addition, migraine has been shown to impact mood disorders. Worsening headache was found to be associated with poorer prognosis for depression. Patients with active migraine not on medications with comorbid major depressive disorder (MDD) had more severe anxiety and somatic symptoms as compared with MDD patients without migraine [39].
Case Continued
Our patient has a normal neurologic examination and classic migraine headache history and stable frequency. The physician tells her she meets criteria for episodic migraine without aura. The patient asks if she needs a “brain scan” to see if something more serious may be causing her symptoms.
What workup is recommended for patients with migraine?
If patient symptoms fit the criteria for migraine and there is a normal neurologic examination, the differential is often limited. When there are neurologic abnormalities on examination (eg, papilledema), or if the patient has concerning signs or symptoms (see below), then neuroimaging should be obtained to rule out secondary causes of headache.
In 2014, the American Academy of Neurology (AAN) published practice parameters on the evaluation of adults with recurrent headache based on guidelines published by the US Headache Consortium [40]. As per AAN guidelines, routine laboratory studies, lumbar puncture, and electroencephalogram are not recommended in the evaluation of non-acute migraines. Neuroimaging is not warranted in patients with migraine and a normal neurologic examination (grade B recommendation). Imaging may need to be considered in patients with non-acute headache and an unexplained abnormal finding on the neurologic examination (grade B recommendation).
When patients exhibit particular warning signs, or headache “red flags,” it is recommended that neuroimaging be considered. Red flags include patients with recurrent headaches and systemic symptoms (fever, weight loss), neurologic symptoms or abnormal signs (confusion, impaired alertness or consciousness), sudden onset, abrupt, or split second in nature, patients age > 50 with new onset or progressive headache, previous headache history with new or different headache (change in frequency, severity, or clinical features) and if there are secondary risk factors (HIV, cancer) [41].
Case Continued
Our patient has no red flags and can be reassured that given her normal physical examination and history suggestive of a migraine, a secondary cause of her headache is unlikely. The physician describes the treatments available, including implementing lifestyles changes and preventive and abortive medications. The patient expresses apprehension about being on prescription medications. She is concerned about side effects as well as the need to take daily medication over a long period of time. She reports that these were the main reasons she did not take the rizatriptan and propranolol that was prescribed by her previous doctor.
How is migraine treated?
Migraine is managed with a combination of lifestyle changes and pharmacologic therapy. Pharmacologic management targets treating an attack when it occurs (abortive medication), as well as reducing the frequency and severity of future attacks (preventive medication).
Lifestyle Changes
Patients should be advised that making healthy lifestyle choices, eg, regular sleep, balanced meals, proper hydration, and regular exercise, can mitigate migraine [42–44]. Other lifestyle changes that can be helpful include weight loss in the obese population, as weight loss appears to result in migraine improvement. People who are obese also are at higher risk for the progression to chronic migraine.
Acute Therapy
There are varieties of abortive therapies [45] (Table 4) that are commonly used in clinical practice. Abortive therapy can be taken as needed and is most effective if used within the first 2 hours of headache. For patients with daily or frequent headache, these medications need to be restricted to 8 to 12 days a month of use and their use should be restricted to when headache is worsening. This usually works well in patients with moderate level pain, and especially in patients with no associated nausea. Selective migraine treatments, like triptans and ergots, are used when nonspecific treatments fail, or when headache is more severe. It is preferable that patients avoid opioids, butalbital, and caffeine-containing medications. In the real world, it is difficult to convince patient to stop these medications; it is more realistic to discuss use limitation with patients, who often run out their weekly limit for triptans.
Triptans are effective medications for acute management of migraine but headache recurrence rate is high, occurring in 15% to 40 % of patients taking oral triptans. It is difficult to predict the response to a triptan [46]. The choice of an abortive agent is often directed partially by patient preference (side effect profile, cost, non-sedating vs. prefers to sleep, long vs short half-life), comorbid conditions (avoid triptans and ergots in uncontrolled hypertension, cardiovascular disease, or peripheral vascular disease or stroke/aneurysm; avoid NSAIDS in patients with cardiovascular disease), and migraine-associated symptoms (nausea and/or vomiting). Consider non-oral formulations via subcutaneous or nasal routes in patients who have nausea or vomiting with their migraine attacks. Some patients may require more than one type of abortive medication. The high recurrence rate is similar across different triptans and so switching from one triptan to another has not been found to be useful. Adding NSAIDS to triptans has been found to be more useful than switching between triptans.Overuse of acute medications has been associated with transformation of headache from episodic to chronic (medication overuse headache or rebound headache). The risk of transformation appears to be greatest with medications containing caffeine, opiates, or barbiturates [47]. Use of acute medications should be limited based on the type of medication. Patients should take triptans for no more than 10 days a month. Combined medications and opioids should be used fewer than 8 days a month, and butalbital-containing medications should be avoided or used fewer than 5 days a month [48]. Use of acute therapy should be monitored with headache calendars. It is unclear if and to what degree NSAIDS and acetaminophen cause overuse headaches.
Medication overuse headache can be difficult to treat as patients have to stop using the medication causing rebound. Further, headaches often resemble migraine and it can be difficult to differentiate them from the patients’ routine headache. Vigilance with medication use in patients with frequent headache is an essential part of migraine management, and patients should receive clear instructions regarding how to use acute medications.
Prevention
Patients presenting with more than 4 headaches per month, or headaches that last longer than 12 hours, require preventive therapy. The goals of preventive therapy is to reduce attack frequency, severity, and duration, to improve responsiveness to treatment of acute attacks, to improve function and reduce disability, and to prevent progression or transformation of episodic migraine to chronic migraine. Preventive medications usually need to be taken daily to reduce frequency or severity of the headache. The goal in this approach is 50% reduction of headache frequency and severity. Migraine preventive medications usually belong to 1 of 3 categories of drugs: antihypertensives, antiepileptics, and antidepressants. At present there are many medications for migraine prevention with different levels of evidence [49] (Table 5). Onabotulinuma toxin is the only approved medication for chronic migraine based on promising results of the PREEMPT trial [50].
Other Considerations
A multidisciplinary approach to treatment may be warranted. Psychiatric evaluation and management of underlying depression and mood disorders can help reduce headache frequency and severity. Physical therapy should be prescribed for neck and shoulder pain. Sleep specialists should be consulted if ongoing sleep issues continue despite behavioral management.
How common is nonadherence with migraine medication?
One third of patients who are prescribed triptans discontinue the medication within a year. Lack of efficacy and concerns over medication side effects are 2 of the most common reasons for poor adherence [51]. In addition, age plays a significant role in discontinuing medication, with the elderly population more likely to stop taking triptans [52]. Seng et al reported that among patients with migraine, being male, being single, having frequent headache, and having mild pain are all associated with medication nonadherence [53]. Formulary restrictions and type of insurance coverage also were associated with nonadherence. Among adherent patients, some individuals were found to be hoarding their tablets and waiting until they were sure it was a migraine. Delaying administration of abortive medications increases the chance of incomplete treatment response, leading to patients taking more medication and in turn have more side effects [53].
Educating patients about their medications and how they need to be taken (preventive vs. abortive, when to administer) can help with adherence (Table 6). Monitoring medication use and headache frequency is an essential part of continued care for migraine patients. Maintain follow up with patients to review how they are doing with the medication and avoid providing refills without visits. The patient may not be taking medication consistently or may be using more medication than prescribed.
What is the role of nonpharmacologic therapy?
Most patients respond to pharmacologic treatment, but some patients with mood disorder, anxiety, difficulties or disability associated with headache, and patients with difficulty managing stress or other triggers may benefit from the addition of behavioral treatments (eg, relaxation, biofeedback, cognitive behavioral therapy, stress management) [54].
Cognitive behavioral therapy and mindfulness are techniques that have been found to be effective in decreasing intensity of pain and associated disability. The goal of these techniques is to manage the cognitive, affective, and behavioral precipitants of headache. In this process, patients are helped to identify the thoughts and behavior that play a role in generating headache. These techniques have been found to improve many headache-related outcomes like pain intensity, headache-related disability, measures of quality of life, mood and medication consumption [55]. A multidisciplinary intervention that included group exercise, stress management and relaxation lectures, and massage therapy was found to reduce self-perceived pain intensity, frequency, and duration of the headache, and improve functional status and quality of life in migraineurs [56]. A randomized controlled trial of yoga therapy compared with self care showed that yoga led to significant reduction in migraine headache frequency and improved overall outcome [57].
Overall, results from studies of nonpharmacologic techniques have been mixed [58,59]. A systematic review by Sullivan et al found a large range in the efficacy of psychological interventions for migraine [60]. A 2015 systematic review that examined if cognitive behavioral therapy (CBT) can reduce the physical symptoms of chronic headache and migraines obtained mixed results [58]. Holryod et al’s study [61] found that behavioral management combined with a ß blocker is useful in improving outcomes, but neither the ß blocker alone or behavioral migraine management alone was. Also, a trial by Penzien et al showed that nonpharmacological management helped reduce migraines by 40% to 50% and this was similar to results seen with preventive drugs [62].
Patient education may be helpful in improving outcomes. Smith et al reported a 50% reduction in headache frequency at 12 months in 46% of patients who received migraine education [63]. A randomized controlled trial by Rothrock et al involving 100 migraine patients found that patients who attended a “headache school” consisting of three 90-minute educational sessions focused on topics such as acute treatment and prevention of migraine had a significant reduction in mean migraine disability assessment score (MIDAS) than the group randomized to routine medical management only. The patients also experienced a reduction in functionally incapacitating headache days per month, less need for abortive therapy and were more compliant with prophylactic therapy [64].
Case Conclusion
Our patient is a young woman with a history of headaches suggestive of migraine without aura. Since her headache frequency ranges from 4-8 headaches month, she has episodic migraines. She also has a strong family history of headaches. She denies any other medical or psychiatric comorbidity. She reports an intake of a caffeine-containing medication of 4 to 15 tablets per month.
The physician recommended that she limit her intake of the caffeine-containing medication to 5 days or less per month given the risk of migraine transformation. The physician also recommended maintaining a good sleep schedule, limiting excessive caffeine intake, a stress reduction program, regular cardiovascular exercise, and avoiding skipping or delaying meals. The patient was educated about migraine and its underlying mechanisms and the benefits of taking medications, and her fears regarding medication use and side effects were allayed. Sumatriptan 100 mg oral tablets were prescribed to be taken at headache onset. She was hesitant to be started on an antihypertensive or antiseizure medication, so she was prescribed amitriptyline 30 mg at night for headache prevention. She was also asked to maintain a headache diary. The patient was agreeable with this plan.
Summary
Migraine is often underdiagnosed and undertreated. Primary care providers are often the first point of contact for these patients. Identifying the type and frequency of migraine and comorbidities is necessary to guide appropriate management in terms of medications and lifestyle modifications. Often no testing or imaging is required. Educating patients about this chronic disease, treatment expectations, and limiting intake of medication is essential.
Corresponding author: Pooja Mohan Rao, MBBS, MD, Georgetown University Hospital, 3800 Reservoir Rd. NW, 7 PHC, Washington, DC 20007, [email protected].
Financial disclosures: Dr. Ailani reports receiving honoraria for speaking and consulting for Allergan, Avanir, and Eli Lilly.
1. Woldeamanuel YW, Cowan RP. Migraine affects 1 in 10 people worldwide featuring recent rise: A systematic review and meta-analysis of community-based studies involving 6 million participants, J Neurol Sci 2017;372:307–15.
2. Vetvik KG, MacGregor EA. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol 2017;16:76–87.
3. Lipton RB, Bigal ME. Migraine: epidemiology, impact, and risk factors for progression. Headache 2005;45 Suppl 1:S3–S13.
4. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1545–602.
5. Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention Headache 2015;55 Suppl 2:103–22.
6. Diamond S, Bigal ME, Silberstein S, et al. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention study. Headache 2007;47:355–63.
7. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study. Lancet 2012;380:2163–96.
8. Lipton RB, Stewart WF, Diamond S, et al. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache 2001;41:646–57.
9. Stewart WF, Ricci JA, Chee E, et al. Lost productive time and cost due to common pain in the US workforce. JAMA 2003;290:2443–54.
10. Hawkins K, Wang S, Rupnow M. Direct cost burden among insured US employees with migraine. Headache 2008;48:553–63.
11. Lipton RB, Serrano D, Holland S, et al. Barriers to the diagnosis and treatment of migraine: Effects of sex, income, and headache features. Headache 2013;53:81–92.
12. Dodick DW, Loder EW, Manack Adams A, et al. Assessing barriers to chronic migraine consultation, diagnosis, and treatment: Results from the chronic migraine epidemiology and outcomes (CaMEO) study. Headache 2016;56:821–34.
13. Young WB, Park JE, Tian IX, Kempner J. The stigma of migraine. PLoS One 2013;8(1):e54074.
14. Mia M, Ashna S, Audrey H. A migraine management training program for primary care providers: an overview of a survey and pilot study findings, lessons learned, and considerations for further research. Headache 2016;56:725–40.
15. Lipton RB, Amatniek JC, Ferrari MD, Gross M. Migraine: identifying and removing barriers to care. Neurology 1994;44(6 Suppl 4):S63–8.
16. Knapp RD Jr. Reports from the past 2. Headache 1963;3:112–22.
17. Levine M, Wolff HG. Cerebral circulation: afferent impulses from the blood vessels of the pia. Arch Neurol Psychiat 1932;28:140.
18. Amin FM, Asghar MS, Hougaard A, et al. Magnetic resonance angiography of intracranial and extracranial arteries in patients with spontaneous migraine without aura: a cross sectional study. Lancet Neurol 2013;12:454–61.
19. Goadsby PJ, Holland PR, Martins-Oliveira M, et al. Pathophysiology of migraine—a disorder of sensory processing. Physiol Rev 2017;97:553–622.
20. Goadsby PJ. Pathophysiology of migraine. Ann Indian Acad Neurol 2012;15(Suppl 1):S15–S22.
21. Puledda F, Messina R, Goadsby PJ, et al. An update on migraine: current understanding and future J Neurol 2017 Mar 20.
22. Vinogradova LV. Comparative potency of sensory-induced brainstem activation to trigger spreading depression and seizures in the cortex of awake rats: implications for the pathophysiology of migraine aura. Cephalalgia 2015;35:979–86.
23. Bahra A, Matharu MS, Buchel C, et al. Brainstem activation specific to migraine headache. Lancet 2001;357:1016–7.
24. Schulte LH, May A. The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain 2016;139:1987–93.
25. Noseda R, Jakubowski M, Kainz V, et al. Cortical projections of functionally identified thalamic trigeminovascular neurons: implications for migraine headache and its associated symptoms. J Neurosci 2011;31:14204–17.
26. Noseda R, Kainz V, Jakubowski M, et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci 2010;13:239–45.
27. Puledda F, Messina R, Goadsby PJ. An update on migraine: current understanding and future directions. J Neurol 2017 Mar 20.
28. Coppola G, Di Lorenzo C, Schoenen J, Pierelli F. Habituation and sensitization in primary headaches. J Headache Pain 2013;14:65.
29. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33:629–808.
30. Yusheng H, Y Li. Typical aura without headache: a case report and review of the literature J Med Case Rep 2015;9:40.
31. Buture A, Khalil M, Ahmed F. Iatrogenic visual aura: a case report and a brief review of the literature Ther Clin Risk Manag 2017;13:643–6.
32. Lipton RB. Tracing transformation: Chronic migraine classification, progression, and epidemiology. Neurology 2009;72:S3–7.
33. Lipton RB. Headache 2011;51;S2:77–83.
34. Scher AI, Stewart WF, et al. Factors associated with the onset and remission of chronic daily headache in a population-based study. Pain 2003;106:81–9.
35. Bigal ME, Lipton RB, Holland PR, Goadsby PJ. Obesity, migraine, and chronic migraine: possible mechanisms of interaction. Neurology 2007;68:1851–61.
36. Wolff HG. Stress and disease. Springfield, IL: Charles C. Thomas; 1953.
37. Guidetti V, Galli F, Fabrizi P, et al. Headache and psychiatric comorbidity: clinical aspects and outcome in an 8-year follow-up study. Cephalalgia 1998;18:455–62.
38. Mongini F, Keller R, Deregibus A, et al. Personality traits, depression and migraine in women: a longitudinal study. Cephalalgia 2003;23:186–92.
39. Hung CI, Liu CY, Yang CH, Wang SJ. The impacts of migraine among outpatients with major depressive disorder at a two-year follow-up. PLoS One 2015;10:e0128087.
40. Frishberg BM, Rosenberg JH, Matchar DB, et al. Evidence-based guidelines in the primary care setting: neuroimaging in patients with nonacute headache. St Paul: US Headache Consortium; 2000.
41. Headache Measurement Set 2014 Revised. American Academy of Neurology. Accessed at www.aan.com/uploadedFiles/Website_Library_Assets/Documents/3.Practice_Management/2.Quality_Improvement/1.Quality_Measures/1.All_Measures/2014.
42. Taylor FR. Lifestyle changes, dietary restrictions, and nutraceuticals in migraine prevention. Techn Reg Anesth Pain Manage 2009;13:28–37.
43. Varkey E, Cider A, Carlsson J, Linde M. Exercise as migraine prophylaxis: A randomized study using relaxation and topiramate as controls. Cephalalgia 2011;14:1428–38.
44. Ahn AH. Why does increased exercise decrease migraine? Curr Pain Headache Rep 2013;17:379.
45. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: The American Headache Society evidence assessment of migraine pharmacotherapies. Headache 2015;55:3–20.
46. Belvis R, Mas N, Aceituno A. Migraine attack treatment : a tailor-made suit, not one size fits all. Recent Pat CNS Drug Discov 2014;9:26–40.
47. Bigal ME, Serrano D, Buse D, et al. Acute migraine medications and evolution from episodic to chronic migraine: A longitudinal population-based study. Headache 2008;48:1157–68.
48. Bigal ME, Rapoport AM, Sheftell FD, et al. Transformed migraine and medication overuse in a tertiary headache centre--clinical characteristics and treatment outcomes Cephalalgia 2004;24:483–90.
49. Silberstein SD, Holland S, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2012;78:1337–45.
50. Diener HC, Dodick DW, Aurora SK, et al. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial Cephalagia year;30:804–14.
51. Wells RE, Markowitz SY, Baron EP, et al. Identifying the factors underlying discontinuation of triptans. Headache 2014;54:278–89.
52. Holland S, Fanning KM, Serrano D, et al. Rates and reasons for discontinuation of triptans and opioids in episodic migraine: results from the American Migraine Prevalence and Prevention (AMPP) study. J Neurol Sci 2013;326:10–7.
53. Seng EK, Rains JA, Nicholson RA, Lipton RB. Improving medication adherence in migraine treatment. Curr Pain Headache Rep 2015;19:24.
54. Nicholson RA, Buse DC, Andrasik F, Lipton RB. Nonpharmacologic treatments for migraine and tension-type headache: how to choose and when to use. Curr Treatment Opt Neurol 2011;13:28–40.
55. Probyn K, Bowers H, Mistry D, et al. Non-pharmacological self-management for people living with migraine or tension-type headache: a systematic review including analysis of intervention components BMJ Open 2017;7:e016670.
56. Lemstra M, Stewart B, Olszynski WP. Effectiveness of multidisciplinary intervention in the treatment of migraine: a randomized clinical trial. Headache 2002;42:845–54.
57. John PJ, Sharma N, Sharma CM, Kankane A. Effectiveness of yoga therapy in the treatment of migraine without aura: a randomized controlled trial. Headache 2007;47:654–61.
58. Harris P, Loveman E, Clegg A, et al. Systematic review of cognitive behavioural therapy for the management of headaches and migraines in adults Br J Pain 2015;9:213–24.
59. Kropp P, Meyer B, Meyer W, Dresler T. An update on behavioral treatments in migraine - current knowledge and future options. Expert Rev Neurother 2017:1–10.
60. Sullivan A, Cousins S, Ridsdale L. Psychological interventions for migraine: a systematic review. J Neurol 2016;263:2369–77.
61. Holroyd KA, Cottrell CK, O’Donnell FJ, et al. Effect of preventive (beta blocker) treatment, behavioural migraine management, or their combination on outcomes of optimised acute treatment in frequent migraine: randomised controlled trial. BMJ 2010;341:c4871.
62. Penzien DB, Rains JC, Andrasik F. Behavioral management of recurrent headache: three decades of experience and empiricism. Appl Psychophysiol Biofeedback 2002;27:163–81.
63. Smith TR, Nicholson RA, Banks JW. A primary care migraine education program has benefit on headache impact and quality of life: results from the mercy migraine management program. Headache 2010;50:600–12.
64. Rothrock JF, Parada VA, Sims C, et al.The impact of intensive patient education on clinical outcome in a clinic-based migraine population. Headache 2006;46:726–31.
From the Department of Neurology, Medstar Georgetown University Hospital, Washington, DC.
Abstract
- Objective: To review the epidemiology, pathophysiology, diagnosis, and treatment of migraine.
- Methods: Review of the literature.
- Results: Migraine is a common disorder associated with significant morbidity. Diagnosis of migraine is performed according to the International Classification of Headache Disorders. Comorbidities are commonly seen with migraine and include mood disorders (depression, anxiety, post-traumatic stress disorder), musculoskeletal disorders (neck pain, fibromyalgia, Ehlors-Danlos syndrome), sleep disorders, asthma, allergies, thyroid dysfunction, obesity, irritable bowel syndrome, epilepsy, stroke, and heart disease. Comorbid conditions can increase migraine disability. Management of migraine with lifestyle modifications, trigger management, and acute and preventive medications can help reduce the frequency, duration, and severity of attacks. Overuse of medications such as opiates, barbiturates, and caffeine-containing medications can increase headache frequency. Educating patients about limiting use of these medications is important.
- Conclusion: Migraine is a common neurologic disease that can be very disabling. Recognizing the condition, making an accurate diagnosis, and starting patients on migraine-specific treatments can help improve patient outcomes.
Migraine is a common neurologic disease that affects 1 in 10 people worldwide [1]. It is 2 to 3 times more prevalent in women than in men [2]. The prevalence of migraine peaks in both sexes during the most productive years of adulthood (age 25 to 55 years) [3]. The Global Burden of Diseases, Injuries, and Risk Factors Study considers it to be the 7th most disabling disease in the world [4]. Over 36 million people in the United States have migraine [5]. However, just 56% of migraineurs have ever been diagnosed [6].
Migraine is associated with a high rate of years lived with disability [7] and the rate has been steadily increasing since 1990. At least 50% of migraine sufferers are severely disabled, many requiring bed rest, during individual migraine attacks lasting hours to days [8]. The total U.S. annual economic costs from headache disorders, including the indirect costs from lost productivity and workplace performance, has been estimated at $31 billion [9,10].
Despite the profound impact of migraine on patients and society, there are numerous barriers to migraine care. Lipton et al [11] identified 3 steps that were minimally necessary to achieve guideline-defined appropriate acute pharmacologic therapy: (1) consulting a prescribing health care professional; (2) receiving a migraine diagnosis; and (3) using migraine-specific or other appropriate acute treatments. In a study they conducted in patients with episodic migraine, 45.5% had consulted health care professional for headache in the preceding year; of these, 86.7% reported receiving a medical diagnosis of migraine, and among the diagnosed consulters, 66.7% currently used acute migraine-specific treatments, resulting in only 26.3% individuals successfully completing all 3 steps. In the recent CaMEO study [12], the proportion patients with chronic migraine that overcame all 3 barriers was less than 5%.
The stigma of migraine often makes it difficult for people to discuss symptoms with their health care providers and family members [13]. When they do discuss their headaches with their provider, often they are not given a diagnosis [14] or do not understand what their diagnosis means [15]. It is important for health care providers to be vigilant about the diagnosis of migraine, discuss treatment goals and strategies, and prescribe appropriate migraine treatment. Migraine is often comorbid with a number of medical, neurological, and psychiatric conditions, and identifying and managing comorbidities is necessary to reduce headache burden and disability. In this article, we provide a review of the diagnosis and treatment of migraine, using a case illustration to highlight key points.
Case Study
Initial Presentation
A 24-year-old woman presents for an evaluation of her headaches.
History and Physical Examination
She initially noted headaches at age 19, which were not memorable and did not cause disability. Her current headaches are a severe throbbing pain over her right forehead. They are associated with light and sound sensitivity and stomach upset. Headaches last 6 to 7 hours without medications and occur 4 to 8 days per month.
She denies vomiting and autonomic symptoms such as runny nose or eye tearing. She also denies preceding aura. She reports headache relief with intake of tablets that contain acetaminophen/aspirin/caffeine and states that she takes between 4 to 15 tablets/month depending on headache frequency. She reports having tried acetaminophen and naproxen with no significant benefit. Aggravating factors include bright lights, strong smells, and soy/ high-sodium foods.
She had no significant past medical problems and denied a history of depression or anxiety. Family history was significant for both her father and sister having a history of headaches. The patient lived alone and denied any major life stressors. She exercises 2 times a week and denies smoking or alcohol use. Review of systems was positive for trouble sleeping, which she described as difficulty falling asleep.
On physical examination, vitals were within normal limits. BMI was 23. Chest, cardiac, abdomen, and general physical examination were all within normal limits. Neurological examination revealed no evidence of papilledema or focal neurological deficits.
What is the pathophysiology of migraine?
Migraine was thought to be a primary vascular disorder of the brain, with the origins of the vascular theory of migraine dating back to 1684 [16]. Trials performed by Wolff concluded that migraine is of vascular origin [17], and this remained the predominant theory over several decades. Current evidence suggests that migraine is unlikely to be a pure vascular disorder and instead may be related to changes in the central or peripheral nervous system [18,19].
Migraine is complex brain network disorder with a strong genetic basis [19]. The trigemino-vascular system, along with neurogenically induced inflammation of the dura mater, mast cell degranulation and release of histamine, are the likely causes of migraine pain. Trigeminal fibers arise from neurons in the trigeminal ganglion that contain substance P and calcitonin gene-related peptide (CGRP) [20]. CGRP is a neuropeptide widely expressed in both peripheral and central neurons. Elevation of CGRP in migraine is linked to diminution of the inhibitory pathways which in turn leads to migraine susceptibility [21]. These findings have led to the development of new drugs that target the CGRP pathway.
In the brainstem, periaqueductal grey matter and the dorsolateral pons have been found to be “migraine generators,” or the driver of changes of cortical activity during migraine [22]. Brainstem nuclei are involved in modulating trigemino-vascular pain transmission and autonomic responses in migraine [23].
The hypothalamus has also been implicated in migraine pathogenesis, particularly its role in nociceptive and autonomic modulation in migraine patients. Schulte and May hypothesized that there is a network change between the hypothalamus and the areas of the brainstem generator leading to the migraine attacks [24].
The thalamus plays a central role for the processing and integration of pain stimuli from the dura mater and cutaneous regions. It maintains complex connections with the somatosensory, motor, visual, auditory, olfactory and limbic regions [25]. The structural and functional alterations in the system play a role in the development of migraine attacks, and also in the sensory hypersensitivity to visual stimuli and mechanical allodynia [26].
Experimental studies in rats show that cortical spreading depression can trigger neurogenic meningeal inflammation and subsequently activate the trigemino-vascular system [27]. It has been observed that between migraine episodes a time-dependent amplitude increase of scalp-evoked potentials to repeated stereotyped stimuli, such as visual, auditory, and somaticstimuli, occurs. This phenomenon is described as “deficient habituation.” In episodic migraine, studies show 2 characteristic changes: a deficient habituation between attacks and sensitization during the attack [28]. Genetic studies have hypothesized an involvement of glutamatergic neurotransmitters and synaptic dysplasticity in causing abnormal cortical excitability in migraine [27].
What are diagnostic criteria for migraine?
Diagnosis of migraine is performed according to the International Classification of Headache Disorders (ICHD) [29]. Based on the number of headache days that the patient reports, migraine is classified into episodic or chronic migraine. Migraines that occur on fewer than 15 days/month are categorized as episodic migraines.
Episodic migraine is divided into 2 categories: migraine with aura (Table 1) and migraine without aura. Migraine without aura is described as recurrent headaches consisting of at least 5 attacks, each lasting 4 to 72 hours if left untreated. At least 2 of the following 4 characteristics must be present: unilateral location, pulsating quality, moderate or severe pain intensity, with aggravation by or causing avoidance of routine physical activity. During headache, at least 1 of nausea and/or vomiting or photophobia and phonophobia should be present.
In migraine with aura (Table 2), headache characteristics are the same, but in addition there are at least 2 lifetime attacks with fully reversible aura symptoms (visual, sensory, speech/language). In addition, these auras have at least 2 of the following 4 characteristics: at least 1 aura symptom spreads gradually over 5 minutes, and/or 2 or more symptoms occur in succession; each individual aura symptom lasts 5 to 60 minutes; aura symptom is unilateral; and aura is accompanied, or followed within 60 minutes, by headache. Migraine with aura is uncommon, occurring in 20% of patients with migraine [30]. Visual aura is the most common type of aura, occurring in up to 90% of patients [31]. There is also aura without migraine, called typical aura without headache. Patients can present with non-migraine headache with aura, categorized as typical aura with headache [29].
Headache occurring on 15 or more days per month for more than 3 months, which has the features of migraine headache on at least 8 days per month, is classified as chronic migraine (Table 3). Evidence indicates that 2.5% of episodic migraine progresses to chronic migraine over 1-year follow-up [32]. There are several risk factors for chronification of migraine. Nonmodifiable factors include female sex, white European heritage, head/neck injury, low education/socioeconomic status, and stressful life events (divorce, moving, work changes, problems with children). Modifiable risk factors are headache frequency, acute medication overuse, caffeine overuse, obesity, comorbid mood disorders, and allodynia. Acute medication use and headache frequency are independent risk factors for development of chronic migraine [33]. The risk of chronic migraine increases exponentially with increased attack frequency, usually when the frequency is ≥ 3 headaches/month. Repetitive episodes of pain may increase central sensitization and result in anatomical changes in the brain and brainstem [34].
What information should be elicited during the history?
Specific questions about the headaches can help with making an accurate diagnosis. These include:
- Length of attacks and their frequency
- Pain characteristics (location, quality, intensity)
- Actions that trigger or aggravate headaches (eg, stress, movement, bright lights, menses, certain foods and smells)
- Associated symptoms that accompany headaches (eg, nausea, vomiting)
- How the headaches impact their life (eg, missed days at work or school, missed life events, avoidance of social activities, emergency room visits due to headache)
To assess headache frequency, it is helpful to ask about the number of headache-free days in a month, eg, “how many days a month do you NOT have a headache.” To assist with headache assessment, patients can be asked to keep a calendar in which they mark days of use of medications, including over the counter medications, menses, and headache days. The calendar can be used to assess for migraine patterns, headache frequency, and response to treatment.
When asking about headache history, it is important for patients to describe their untreated headaches. Patients taking medications may have pain that is less severe or disabling or have reduced associated symptoms. Understanding what the headaches were like when they did not treat is important in making a diagnosis.
Other important questions include when was the first time they recall ever experiencing a headache. Migraine is often present early in life, and understanding the change in headache over time is important. Also ask patients about what they want to do when they have a headache. Often patients want to lie down in a cool dark room. Ask what they would prefer to do if they didn’t have any pending responsibilities.
Comorbidities
Comorbidities are commonly seen with migraine. Common comorbidities are mood disorders (depression, anxiety, post-traumatic stress disorder), musculoskeletal disorders (neck pain, fibromyalgia, Ehlors-Danlos syndrome), sleep disorders, asthma, allergies, thyroid dysfunction, obesity, irritable bowel syndrome, epilepsy, stroke, and heart disease.
Comorbid conditions can increase migraine disability and also can provide information about the pathophysiology of migraine and guide treatment. Management of the underlying comorbidity often leads to improved migraine outcomes. For example, serotonergic dysfunction is a possible pathway involved in both migraine and mood disorders. Treatment with medications that alter the serotonin system may help both migraine and coexisting mood disorders. Bigal et al proposed that activation of the HPA axis with reduced serotonin synthesis is a main pathway involved in affective disorders, migraine, and obesity [35].
In the early 1950s, Wolff conceptualized migraine as a psychophysiologic disorder [36]. The relationship between migraine and psychiatric conditions is complex, and comorbid psychiatric disorders are risk factors for headache progression and chronicity. Psychiatric conditions also play a role in nonadherence to headache medication, which contributes to poor outcome in these patients. Hence, there is a need for assessment and treatment of psychiatric disorders in people with migraine. A study by Guidetti et al found that headache patients with multiple psychiatric conditions have poor outcomes, with 86 % of these headache patients having no improvement and even deterioration in their headache [37]. Another study by Mongini et al concluded that psychiatric disorder appears to influence the result of treatment on a long-term basis [38].
In addition, migraine has been shown to impact mood disorders. Worsening headache was found to be associated with poorer prognosis for depression. Patients with active migraine not on medications with comorbid major depressive disorder (MDD) had more severe anxiety and somatic symptoms as compared with MDD patients without migraine [39].
Case Continued
Our patient has a normal neurologic examination and classic migraine headache history and stable frequency. The physician tells her she meets criteria for episodic migraine without aura. The patient asks if she needs a “brain scan” to see if something more serious may be causing her symptoms.
What workup is recommended for patients with migraine?
If patient symptoms fit the criteria for migraine and there is a normal neurologic examination, the differential is often limited. When there are neurologic abnormalities on examination (eg, papilledema), or if the patient has concerning signs or symptoms (see below), then neuroimaging should be obtained to rule out secondary causes of headache.
In 2014, the American Academy of Neurology (AAN) published practice parameters on the evaluation of adults with recurrent headache based on guidelines published by the US Headache Consortium [40]. As per AAN guidelines, routine laboratory studies, lumbar puncture, and electroencephalogram are not recommended in the evaluation of non-acute migraines. Neuroimaging is not warranted in patients with migraine and a normal neurologic examination (grade B recommendation). Imaging may need to be considered in patients with non-acute headache and an unexplained abnormal finding on the neurologic examination (grade B recommendation).
When patients exhibit particular warning signs, or headache “red flags,” it is recommended that neuroimaging be considered. Red flags include patients with recurrent headaches and systemic symptoms (fever, weight loss), neurologic symptoms or abnormal signs (confusion, impaired alertness or consciousness), sudden onset, abrupt, or split second in nature, patients age > 50 with new onset or progressive headache, previous headache history with new or different headache (change in frequency, severity, or clinical features) and if there are secondary risk factors (HIV, cancer) [41].
Case Continued
Our patient has no red flags and can be reassured that given her normal physical examination and history suggestive of a migraine, a secondary cause of her headache is unlikely. The physician describes the treatments available, including implementing lifestyles changes and preventive and abortive medications. The patient expresses apprehension about being on prescription medications. She is concerned about side effects as well as the need to take daily medication over a long period of time. She reports that these were the main reasons she did not take the rizatriptan and propranolol that was prescribed by her previous doctor.
How is migraine treated?
Migraine is managed with a combination of lifestyle changes and pharmacologic therapy. Pharmacologic management targets treating an attack when it occurs (abortive medication), as well as reducing the frequency and severity of future attacks (preventive medication).
Lifestyle Changes
Patients should be advised that making healthy lifestyle choices, eg, regular sleep, balanced meals, proper hydration, and regular exercise, can mitigate migraine [42–44]. Other lifestyle changes that can be helpful include weight loss in the obese population, as weight loss appears to result in migraine improvement. People who are obese also are at higher risk for the progression to chronic migraine.
Acute Therapy
There are varieties of abortive therapies [45] (Table 4) that are commonly used in clinical practice. Abortive therapy can be taken as needed and is most effective if used within the first 2 hours of headache. For patients with daily or frequent headache, these medications need to be restricted to 8 to 12 days a month of use and their use should be restricted to when headache is worsening. This usually works well in patients with moderate level pain, and especially in patients with no associated nausea. Selective migraine treatments, like triptans and ergots, are used when nonspecific treatments fail, or when headache is more severe. It is preferable that patients avoid opioids, butalbital, and caffeine-containing medications. In the real world, it is difficult to convince patient to stop these medications; it is more realistic to discuss use limitation with patients, who often run out their weekly limit for triptans.
Triptans are effective medications for acute management of migraine but headache recurrence rate is high, occurring in 15% to 40 % of patients taking oral triptans. It is difficult to predict the response to a triptan [46]. The choice of an abortive agent is often directed partially by patient preference (side effect profile, cost, non-sedating vs. prefers to sleep, long vs short half-life), comorbid conditions (avoid triptans and ergots in uncontrolled hypertension, cardiovascular disease, or peripheral vascular disease or stroke/aneurysm; avoid NSAIDS in patients with cardiovascular disease), and migraine-associated symptoms (nausea and/or vomiting). Consider non-oral formulations via subcutaneous or nasal routes in patients who have nausea or vomiting with their migraine attacks. Some patients may require more than one type of abortive medication. The high recurrence rate is similar across different triptans and so switching from one triptan to another has not been found to be useful. Adding NSAIDS to triptans has been found to be more useful than switching between triptans.Overuse of acute medications has been associated with transformation of headache from episodic to chronic (medication overuse headache or rebound headache). The risk of transformation appears to be greatest with medications containing caffeine, opiates, or barbiturates [47]. Use of acute medications should be limited based on the type of medication. Patients should take triptans for no more than 10 days a month. Combined medications and opioids should be used fewer than 8 days a month, and butalbital-containing medications should be avoided or used fewer than 5 days a month [48]. Use of acute therapy should be monitored with headache calendars. It is unclear if and to what degree NSAIDS and acetaminophen cause overuse headaches.
Medication overuse headache can be difficult to treat as patients have to stop using the medication causing rebound. Further, headaches often resemble migraine and it can be difficult to differentiate them from the patients’ routine headache. Vigilance with medication use in patients with frequent headache is an essential part of migraine management, and patients should receive clear instructions regarding how to use acute medications.
Prevention
Patients presenting with more than 4 headaches per month, or headaches that last longer than 12 hours, require preventive therapy. The goals of preventive therapy is to reduce attack frequency, severity, and duration, to improve responsiveness to treatment of acute attacks, to improve function and reduce disability, and to prevent progression or transformation of episodic migraine to chronic migraine. Preventive medications usually need to be taken daily to reduce frequency or severity of the headache. The goal in this approach is 50% reduction of headache frequency and severity. Migraine preventive medications usually belong to 1 of 3 categories of drugs: antihypertensives, antiepileptics, and antidepressants. At present there are many medications for migraine prevention with different levels of evidence [49] (Table 5). Onabotulinuma toxin is the only approved medication for chronic migraine based on promising results of the PREEMPT trial [50].
Other Considerations
A multidisciplinary approach to treatment may be warranted. Psychiatric evaluation and management of underlying depression and mood disorders can help reduce headache frequency and severity. Physical therapy should be prescribed for neck and shoulder pain. Sleep specialists should be consulted if ongoing sleep issues continue despite behavioral management.
How common is nonadherence with migraine medication?
One third of patients who are prescribed triptans discontinue the medication within a year. Lack of efficacy and concerns over medication side effects are 2 of the most common reasons for poor adherence [51]. In addition, age plays a significant role in discontinuing medication, with the elderly population more likely to stop taking triptans [52]. Seng et al reported that among patients with migraine, being male, being single, having frequent headache, and having mild pain are all associated with medication nonadherence [53]. Formulary restrictions and type of insurance coverage also were associated with nonadherence. Among adherent patients, some individuals were found to be hoarding their tablets and waiting until they were sure it was a migraine. Delaying administration of abortive medications increases the chance of incomplete treatment response, leading to patients taking more medication and in turn have more side effects [53].
Educating patients about their medications and how they need to be taken (preventive vs. abortive, when to administer) can help with adherence (Table 6). Monitoring medication use and headache frequency is an essential part of continued care for migraine patients. Maintain follow up with patients to review how they are doing with the medication and avoid providing refills without visits. The patient may not be taking medication consistently or may be using more medication than prescribed.
What is the role of nonpharmacologic therapy?
Most patients respond to pharmacologic treatment, but some patients with mood disorder, anxiety, difficulties or disability associated with headache, and patients with difficulty managing stress or other triggers may benefit from the addition of behavioral treatments (eg, relaxation, biofeedback, cognitive behavioral therapy, stress management) [54].
Cognitive behavioral therapy and mindfulness are techniques that have been found to be effective in decreasing intensity of pain and associated disability. The goal of these techniques is to manage the cognitive, affective, and behavioral precipitants of headache. In this process, patients are helped to identify the thoughts and behavior that play a role in generating headache. These techniques have been found to improve many headache-related outcomes like pain intensity, headache-related disability, measures of quality of life, mood and medication consumption [55]. A multidisciplinary intervention that included group exercise, stress management and relaxation lectures, and massage therapy was found to reduce self-perceived pain intensity, frequency, and duration of the headache, and improve functional status and quality of life in migraineurs [56]. A randomized controlled trial of yoga therapy compared with self care showed that yoga led to significant reduction in migraine headache frequency and improved overall outcome [57].
Overall, results from studies of nonpharmacologic techniques have been mixed [58,59]. A systematic review by Sullivan et al found a large range in the efficacy of psychological interventions for migraine [60]. A 2015 systematic review that examined if cognitive behavioral therapy (CBT) can reduce the physical symptoms of chronic headache and migraines obtained mixed results [58]. Holryod et al’s study [61] found that behavioral management combined with a ß blocker is useful in improving outcomes, but neither the ß blocker alone or behavioral migraine management alone was. Also, a trial by Penzien et al showed that nonpharmacological management helped reduce migraines by 40% to 50% and this was similar to results seen with preventive drugs [62].
Patient education may be helpful in improving outcomes. Smith et al reported a 50% reduction in headache frequency at 12 months in 46% of patients who received migraine education [63]. A randomized controlled trial by Rothrock et al involving 100 migraine patients found that patients who attended a “headache school” consisting of three 90-minute educational sessions focused on topics such as acute treatment and prevention of migraine had a significant reduction in mean migraine disability assessment score (MIDAS) than the group randomized to routine medical management only. The patients also experienced a reduction in functionally incapacitating headache days per month, less need for abortive therapy and were more compliant with prophylactic therapy [64].
Case Conclusion
Our patient is a young woman with a history of headaches suggestive of migraine without aura. Since her headache frequency ranges from 4-8 headaches month, she has episodic migraines. She also has a strong family history of headaches. She denies any other medical or psychiatric comorbidity. She reports an intake of a caffeine-containing medication of 4 to 15 tablets per month.
The physician recommended that she limit her intake of the caffeine-containing medication to 5 days or less per month given the risk of migraine transformation. The physician also recommended maintaining a good sleep schedule, limiting excessive caffeine intake, a stress reduction program, regular cardiovascular exercise, and avoiding skipping or delaying meals. The patient was educated about migraine and its underlying mechanisms and the benefits of taking medications, and her fears regarding medication use and side effects were allayed. Sumatriptan 100 mg oral tablets were prescribed to be taken at headache onset. She was hesitant to be started on an antihypertensive or antiseizure medication, so she was prescribed amitriptyline 30 mg at night for headache prevention. She was also asked to maintain a headache diary. The patient was agreeable with this plan.
Summary
Migraine is often underdiagnosed and undertreated. Primary care providers are often the first point of contact for these patients. Identifying the type and frequency of migraine and comorbidities is necessary to guide appropriate management in terms of medications and lifestyle modifications. Often no testing or imaging is required. Educating patients about this chronic disease, treatment expectations, and limiting intake of medication is essential.
Corresponding author: Pooja Mohan Rao, MBBS, MD, Georgetown University Hospital, 3800 Reservoir Rd. NW, 7 PHC, Washington, DC 20007, [email protected].
Financial disclosures: Dr. Ailani reports receiving honoraria for speaking and consulting for Allergan, Avanir, and Eli Lilly.
From the Department of Neurology, Medstar Georgetown University Hospital, Washington, DC.
Abstract
- Objective: To review the epidemiology, pathophysiology, diagnosis, and treatment of migraine.
- Methods: Review of the literature.
- Results: Migraine is a common disorder associated with significant morbidity. Diagnosis of migraine is performed according to the International Classification of Headache Disorders. Comorbidities are commonly seen with migraine and include mood disorders (depression, anxiety, post-traumatic stress disorder), musculoskeletal disorders (neck pain, fibromyalgia, Ehlors-Danlos syndrome), sleep disorders, asthma, allergies, thyroid dysfunction, obesity, irritable bowel syndrome, epilepsy, stroke, and heart disease. Comorbid conditions can increase migraine disability. Management of migraine with lifestyle modifications, trigger management, and acute and preventive medications can help reduce the frequency, duration, and severity of attacks. Overuse of medications such as opiates, barbiturates, and caffeine-containing medications can increase headache frequency. Educating patients about limiting use of these medications is important.
- Conclusion: Migraine is a common neurologic disease that can be very disabling. Recognizing the condition, making an accurate diagnosis, and starting patients on migraine-specific treatments can help improve patient outcomes.
Migraine is a common neurologic disease that affects 1 in 10 people worldwide [1]. It is 2 to 3 times more prevalent in women than in men [2]. The prevalence of migraine peaks in both sexes during the most productive years of adulthood (age 25 to 55 years) [3]. The Global Burden of Diseases, Injuries, and Risk Factors Study considers it to be the 7th most disabling disease in the world [4]. Over 36 million people in the United States have migraine [5]. However, just 56% of migraineurs have ever been diagnosed [6].
Migraine is associated with a high rate of years lived with disability [7] and the rate has been steadily increasing since 1990. At least 50% of migraine sufferers are severely disabled, many requiring bed rest, during individual migraine attacks lasting hours to days [8]. The total U.S. annual economic costs from headache disorders, including the indirect costs from lost productivity and workplace performance, has been estimated at $31 billion [9,10].
Despite the profound impact of migraine on patients and society, there are numerous barriers to migraine care. Lipton et al [11] identified 3 steps that were minimally necessary to achieve guideline-defined appropriate acute pharmacologic therapy: (1) consulting a prescribing health care professional; (2) receiving a migraine diagnosis; and (3) using migraine-specific or other appropriate acute treatments. In a study they conducted in patients with episodic migraine, 45.5% had consulted health care professional for headache in the preceding year; of these, 86.7% reported receiving a medical diagnosis of migraine, and among the diagnosed consulters, 66.7% currently used acute migraine-specific treatments, resulting in only 26.3% individuals successfully completing all 3 steps. In the recent CaMEO study [12], the proportion patients with chronic migraine that overcame all 3 barriers was less than 5%.
The stigma of migraine often makes it difficult for people to discuss symptoms with their health care providers and family members [13]. When they do discuss their headaches with their provider, often they are not given a diagnosis [14] or do not understand what their diagnosis means [15]. It is important for health care providers to be vigilant about the diagnosis of migraine, discuss treatment goals and strategies, and prescribe appropriate migraine treatment. Migraine is often comorbid with a number of medical, neurological, and psychiatric conditions, and identifying and managing comorbidities is necessary to reduce headache burden and disability. In this article, we provide a review of the diagnosis and treatment of migraine, using a case illustration to highlight key points.
Case Study
Initial Presentation
A 24-year-old woman presents for an evaluation of her headaches.
History and Physical Examination
She initially noted headaches at age 19, which were not memorable and did not cause disability. Her current headaches are a severe throbbing pain over her right forehead. They are associated with light and sound sensitivity and stomach upset. Headaches last 6 to 7 hours without medications and occur 4 to 8 days per month.
She denies vomiting and autonomic symptoms such as runny nose or eye tearing. She also denies preceding aura. She reports headache relief with intake of tablets that contain acetaminophen/aspirin/caffeine and states that she takes between 4 to 15 tablets/month depending on headache frequency. She reports having tried acetaminophen and naproxen with no significant benefit. Aggravating factors include bright lights, strong smells, and soy/ high-sodium foods.
She had no significant past medical problems and denied a history of depression or anxiety. Family history was significant for both her father and sister having a history of headaches. The patient lived alone and denied any major life stressors. She exercises 2 times a week and denies smoking or alcohol use. Review of systems was positive for trouble sleeping, which she described as difficulty falling asleep.
On physical examination, vitals were within normal limits. BMI was 23. Chest, cardiac, abdomen, and general physical examination were all within normal limits. Neurological examination revealed no evidence of papilledema or focal neurological deficits.
What is the pathophysiology of migraine?
Migraine was thought to be a primary vascular disorder of the brain, with the origins of the vascular theory of migraine dating back to 1684 [16]. Trials performed by Wolff concluded that migraine is of vascular origin [17], and this remained the predominant theory over several decades. Current evidence suggests that migraine is unlikely to be a pure vascular disorder and instead may be related to changes in the central or peripheral nervous system [18,19].
Migraine is complex brain network disorder with a strong genetic basis [19]. The trigemino-vascular system, along with neurogenically induced inflammation of the dura mater, mast cell degranulation and release of histamine, are the likely causes of migraine pain. Trigeminal fibers arise from neurons in the trigeminal ganglion that contain substance P and calcitonin gene-related peptide (CGRP) [20]. CGRP is a neuropeptide widely expressed in both peripheral and central neurons. Elevation of CGRP in migraine is linked to diminution of the inhibitory pathways which in turn leads to migraine susceptibility [21]. These findings have led to the development of new drugs that target the CGRP pathway.
In the brainstem, periaqueductal grey matter and the dorsolateral pons have been found to be “migraine generators,” or the driver of changes of cortical activity during migraine [22]. Brainstem nuclei are involved in modulating trigemino-vascular pain transmission and autonomic responses in migraine [23].
The hypothalamus has also been implicated in migraine pathogenesis, particularly its role in nociceptive and autonomic modulation in migraine patients. Schulte and May hypothesized that there is a network change between the hypothalamus and the areas of the brainstem generator leading to the migraine attacks [24].
The thalamus plays a central role for the processing and integration of pain stimuli from the dura mater and cutaneous regions. It maintains complex connections with the somatosensory, motor, visual, auditory, olfactory and limbic regions [25]. The structural and functional alterations in the system play a role in the development of migraine attacks, and also in the sensory hypersensitivity to visual stimuli and mechanical allodynia [26].
Experimental studies in rats show that cortical spreading depression can trigger neurogenic meningeal inflammation and subsequently activate the trigemino-vascular system [27]. It has been observed that between migraine episodes a time-dependent amplitude increase of scalp-evoked potentials to repeated stereotyped stimuli, such as visual, auditory, and somaticstimuli, occurs. This phenomenon is described as “deficient habituation.” In episodic migraine, studies show 2 characteristic changes: a deficient habituation between attacks and sensitization during the attack [28]. Genetic studies have hypothesized an involvement of glutamatergic neurotransmitters and synaptic dysplasticity in causing abnormal cortical excitability in migraine [27].
What are diagnostic criteria for migraine?
Diagnosis of migraine is performed according to the International Classification of Headache Disorders (ICHD) [29]. Based on the number of headache days that the patient reports, migraine is classified into episodic or chronic migraine. Migraines that occur on fewer than 15 days/month are categorized as episodic migraines.
Episodic migraine is divided into 2 categories: migraine with aura (Table 1) and migraine without aura. Migraine without aura is described as recurrent headaches consisting of at least 5 attacks, each lasting 4 to 72 hours if left untreated. At least 2 of the following 4 characteristics must be present: unilateral location, pulsating quality, moderate or severe pain intensity, with aggravation by or causing avoidance of routine physical activity. During headache, at least 1 of nausea and/or vomiting or photophobia and phonophobia should be present.
In migraine with aura (Table 2), headache characteristics are the same, but in addition there are at least 2 lifetime attacks with fully reversible aura symptoms (visual, sensory, speech/language). In addition, these auras have at least 2 of the following 4 characteristics: at least 1 aura symptom spreads gradually over 5 minutes, and/or 2 or more symptoms occur in succession; each individual aura symptom lasts 5 to 60 minutes; aura symptom is unilateral; and aura is accompanied, or followed within 60 minutes, by headache. Migraine with aura is uncommon, occurring in 20% of patients with migraine [30]. Visual aura is the most common type of aura, occurring in up to 90% of patients [31]. There is also aura without migraine, called typical aura without headache. Patients can present with non-migraine headache with aura, categorized as typical aura with headache [29].
Headache occurring on 15 or more days per month for more than 3 months, which has the features of migraine headache on at least 8 days per month, is classified as chronic migraine (Table 3). Evidence indicates that 2.5% of episodic migraine progresses to chronic migraine over 1-year follow-up [32]. There are several risk factors for chronification of migraine. Nonmodifiable factors include female sex, white European heritage, head/neck injury, low education/socioeconomic status, and stressful life events (divorce, moving, work changes, problems with children). Modifiable risk factors are headache frequency, acute medication overuse, caffeine overuse, obesity, comorbid mood disorders, and allodynia. Acute medication use and headache frequency are independent risk factors for development of chronic migraine [33]. The risk of chronic migraine increases exponentially with increased attack frequency, usually when the frequency is ≥ 3 headaches/month. Repetitive episodes of pain may increase central sensitization and result in anatomical changes in the brain and brainstem [34].
What information should be elicited during the history?
Specific questions about the headaches can help with making an accurate diagnosis. These include:
- Length of attacks and their frequency
- Pain characteristics (location, quality, intensity)
- Actions that trigger or aggravate headaches (eg, stress, movement, bright lights, menses, certain foods and smells)
- Associated symptoms that accompany headaches (eg, nausea, vomiting)
- How the headaches impact their life (eg, missed days at work or school, missed life events, avoidance of social activities, emergency room visits due to headache)
To assess headache frequency, it is helpful to ask about the number of headache-free days in a month, eg, “how many days a month do you NOT have a headache.” To assist with headache assessment, patients can be asked to keep a calendar in which they mark days of use of medications, including over the counter medications, menses, and headache days. The calendar can be used to assess for migraine patterns, headache frequency, and response to treatment.
When asking about headache history, it is important for patients to describe their untreated headaches. Patients taking medications may have pain that is less severe or disabling or have reduced associated symptoms. Understanding what the headaches were like when they did not treat is important in making a diagnosis.
Other important questions include when was the first time they recall ever experiencing a headache. Migraine is often present early in life, and understanding the change in headache over time is important. Also ask patients about what they want to do when they have a headache. Often patients want to lie down in a cool dark room. Ask what they would prefer to do if they didn’t have any pending responsibilities.
Comorbidities
Comorbidities are commonly seen with migraine. Common comorbidities are mood disorders (depression, anxiety, post-traumatic stress disorder), musculoskeletal disorders (neck pain, fibromyalgia, Ehlors-Danlos syndrome), sleep disorders, asthma, allergies, thyroid dysfunction, obesity, irritable bowel syndrome, epilepsy, stroke, and heart disease.
Comorbid conditions can increase migraine disability and also can provide information about the pathophysiology of migraine and guide treatment. Management of the underlying comorbidity often leads to improved migraine outcomes. For example, serotonergic dysfunction is a possible pathway involved in both migraine and mood disorders. Treatment with medications that alter the serotonin system may help both migraine and coexisting mood disorders. Bigal et al proposed that activation of the HPA axis with reduced serotonin synthesis is a main pathway involved in affective disorders, migraine, and obesity [35].
In the early 1950s, Wolff conceptualized migraine as a psychophysiologic disorder [36]. The relationship between migraine and psychiatric conditions is complex, and comorbid psychiatric disorders are risk factors for headache progression and chronicity. Psychiatric conditions also play a role in nonadherence to headache medication, which contributes to poor outcome in these patients. Hence, there is a need for assessment and treatment of psychiatric disorders in people with migraine. A study by Guidetti et al found that headache patients with multiple psychiatric conditions have poor outcomes, with 86 % of these headache patients having no improvement and even deterioration in their headache [37]. Another study by Mongini et al concluded that psychiatric disorder appears to influence the result of treatment on a long-term basis [38].
In addition, migraine has been shown to impact mood disorders. Worsening headache was found to be associated with poorer prognosis for depression. Patients with active migraine not on medications with comorbid major depressive disorder (MDD) had more severe anxiety and somatic symptoms as compared with MDD patients without migraine [39].
Case Continued
Our patient has a normal neurologic examination and classic migraine headache history and stable frequency. The physician tells her she meets criteria for episodic migraine without aura. The patient asks if she needs a “brain scan” to see if something more serious may be causing her symptoms.
What workup is recommended for patients with migraine?
If patient symptoms fit the criteria for migraine and there is a normal neurologic examination, the differential is often limited. When there are neurologic abnormalities on examination (eg, papilledema), or if the patient has concerning signs or symptoms (see below), then neuroimaging should be obtained to rule out secondary causes of headache.
In 2014, the American Academy of Neurology (AAN) published practice parameters on the evaluation of adults with recurrent headache based on guidelines published by the US Headache Consortium [40]. As per AAN guidelines, routine laboratory studies, lumbar puncture, and electroencephalogram are not recommended in the evaluation of non-acute migraines. Neuroimaging is not warranted in patients with migraine and a normal neurologic examination (grade B recommendation). Imaging may need to be considered in patients with non-acute headache and an unexplained abnormal finding on the neurologic examination (grade B recommendation).
When patients exhibit particular warning signs, or headache “red flags,” it is recommended that neuroimaging be considered. Red flags include patients with recurrent headaches and systemic symptoms (fever, weight loss), neurologic symptoms or abnormal signs (confusion, impaired alertness or consciousness), sudden onset, abrupt, or split second in nature, patients age > 50 with new onset or progressive headache, previous headache history with new or different headache (change in frequency, severity, or clinical features) and if there are secondary risk factors (HIV, cancer) [41].
Case Continued
Our patient has no red flags and can be reassured that given her normal physical examination and history suggestive of a migraine, a secondary cause of her headache is unlikely. The physician describes the treatments available, including implementing lifestyles changes and preventive and abortive medications. The patient expresses apprehension about being on prescription medications. She is concerned about side effects as well as the need to take daily medication over a long period of time. She reports that these were the main reasons she did not take the rizatriptan and propranolol that was prescribed by her previous doctor.
How is migraine treated?
Migraine is managed with a combination of lifestyle changes and pharmacologic therapy. Pharmacologic management targets treating an attack when it occurs (abortive medication), as well as reducing the frequency and severity of future attacks (preventive medication).
Lifestyle Changes
Patients should be advised that making healthy lifestyle choices, eg, regular sleep, balanced meals, proper hydration, and regular exercise, can mitigate migraine [42–44]. Other lifestyle changes that can be helpful include weight loss in the obese population, as weight loss appears to result in migraine improvement. People who are obese also are at higher risk for the progression to chronic migraine.
Acute Therapy
There are varieties of abortive therapies [45] (Table 4) that are commonly used in clinical practice. Abortive therapy can be taken as needed and is most effective if used within the first 2 hours of headache. For patients with daily or frequent headache, these medications need to be restricted to 8 to 12 days a month of use and their use should be restricted to when headache is worsening. This usually works well in patients with moderate level pain, and especially in patients with no associated nausea. Selective migraine treatments, like triptans and ergots, are used when nonspecific treatments fail, or when headache is more severe. It is preferable that patients avoid opioids, butalbital, and caffeine-containing medications. In the real world, it is difficult to convince patient to stop these medications; it is more realistic to discuss use limitation with patients, who often run out their weekly limit for triptans.
Triptans are effective medications for acute management of migraine but headache recurrence rate is high, occurring in 15% to 40 % of patients taking oral triptans. It is difficult to predict the response to a triptan [46]. The choice of an abortive agent is often directed partially by patient preference (side effect profile, cost, non-sedating vs. prefers to sleep, long vs short half-life), comorbid conditions (avoid triptans and ergots in uncontrolled hypertension, cardiovascular disease, or peripheral vascular disease or stroke/aneurysm; avoid NSAIDS in patients with cardiovascular disease), and migraine-associated symptoms (nausea and/or vomiting). Consider non-oral formulations via subcutaneous or nasal routes in patients who have nausea or vomiting with their migraine attacks. Some patients may require more than one type of abortive medication. The high recurrence rate is similar across different triptans and so switching from one triptan to another has not been found to be useful. Adding NSAIDS to triptans has been found to be more useful than switching between triptans.Overuse of acute medications has been associated with transformation of headache from episodic to chronic (medication overuse headache or rebound headache). The risk of transformation appears to be greatest with medications containing caffeine, opiates, or barbiturates [47]. Use of acute medications should be limited based on the type of medication. Patients should take triptans for no more than 10 days a month. Combined medications and opioids should be used fewer than 8 days a month, and butalbital-containing medications should be avoided or used fewer than 5 days a month [48]. Use of acute therapy should be monitored with headache calendars. It is unclear if and to what degree NSAIDS and acetaminophen cause overuse headaches.
Medication overuse headache can be difficult to treat as patients have to stop using the medication causing rebound. Further, headaches often resemble migraine and it can be difficult to differentiate them from the patients’ routine headache. Vigilance with medication use in patients with frequent headache is an essential part of migraine management, and patients should receive clear instructions regarding how to use acute medications.
Prevention
Patients presenting with more than 4 headaches per month, or headaches that last longer than 12 hours, require preventive therapy. The goals of preventive therapy is to reduce attack frequency, severity, and duration, to improve responsiveness to treatment of acute attacks, to improve function and reduce disability, and to prevent progression or transformation of episodic migraine to chronic migraine. Preventive medications usually need to be taken daily to reduce frequency or severity of the headache. The goal in this approach is 50% reduction of headache frequency and severity. Migraine preventive medications usually belong to 1 of 3 categories of drugs: antihypertensives, antiepileptics, and antidepressants. At present there are many medications for migraine prevention with different levels of evidence [49] (Table 5). Onabotulinuma toxin is the only approved medication for chronic migraine based on promising results of the PREEMPT trial [50].
Other Considerations
A multidisciplinary approach to treatment may be warranted. Psychiatric evaluation and management of underlying depression and mood disorders can help reduce headache frequency and severity. Physical therapy should be prescribed for neck and shoulder pain. Sleep specialists should be consulted if ongoing sleep issues continue despite behavioral management.
How common is nonadherence with migraine medication?
One third of patients who are prescribed triptans discontinue the medication within a year. Lack of efficacy and concerns over medication side effects are 2 of the most common reasons for poor adherence [51]. In addition, age plays a significant role in discontinuing medication, with the elderly population more likely to stop taking triptans [52]. Seng et al reported that among patients with migraine, being male, being single, having frequent headache, and having mild pain are all associated with medication nonadherence [53]. Formulary restrictions and type of insurance coverage also were associated with nonadherence. Among adherent patients, some individuals were found to be hoarding their tablets and waiting until they were sure it was a migraine. Delaying administration of abortive medications increases the chance of incomplete treatment response, leading to patients taking more medication and in turn have more side effects [53].
Educating patients about their medications and how they need to be taken (preventive vs. abortive, when to administer) can help with adherence (Table 6). Monitoring medication use and headache frequency is an essential part of continued care for migraine patients. Maintain follow up with patients to review how they are doing with the medication and avoid providing refills without visits. The patient may not be taking medication consistently or may be using more medication than prescribed.
What is the role of nonpharmacologic therapy?
Most patients respond to pharmacologic treatment, but some patients with mood disorder, anxiety, difficulties or disability associated with headache, and patients with difficulty managing stress or other triggers may benefit from the addition of behavioral treatments (eg, relaxation, biofeedback, cognitive behavioral therapy, stress management) [54].
Cognitive behavioral therapy and mindfulness are techniques that have been found to be effective in decreasing intensity of pain and associated disability. The goal of these techniques is to manage the cognitive, affective, and behavioral precipitants of headache. In this process, patients are helped to identify the thoughts and behavior that play a role in generating headache. These techniques have been found to improve many headache-related outcomes like pain intensity, headache-related disability, measures of quality of life, mood and medication consumption [55]. A multidisciplinary intervention that included group exercise, stress management and relaxation lectures, and massage therapy was found to reduce self-perceived pain intensity, frequency, and duration of the headache, and improve functional status and quality of life in migraineurs [56]. A randomized controlled trial of yoga therapy compared with self care showed that yoga led to significant reduction in migraine headache frequency and improved overall outcome [57].
Overall, results from studies of nonpharmacologic techniques have been mixed [58,59]. A systematic review by Sullivan et al found a large range in the efficacy of psychological interventions for migraine [60]. A 2015 systematic review that examined if cognitive behavioral therapy (CBT) can reduce the physical symptoms of chronic headache and migraines obtained mixed results [58]. Holryod et al’s study [61] found that behavioral management combined with a ß blocker is useful in improving outcomes, but neither the ß blocker alone or behavioral migraine management alone was. Also, a trial by Penzien et al showed that nonpharmacological management helped reduce migraines by 40% to 50% and this was similar to results seen with preventive drugs [62].
Patient education may be helpful in improving outcomes. Smith et al reported a 50% reduction in headache frequency at 12 months in 46% of patients who received migraine education [63]. A randomized controlled trial by Rothrock et al involving 100 migraine patients found that patients who attended a “headache school” consisting of three 90-minute educational sessions focused on topics such as acute treatment and prevention of migraine had a significant reduction in mean migraine disability assessment score (MIDAS) than the group randomized to routine medical management only. The patients also experienced a reduction in functionally incapacitating headache days per month, less need for abortive therapy and were more compliant with prophylactic therapy [64].
Case Conclusion
Our patient is a young woman with a history of headaches suggestive of migraine without aura. Since her headache frequency ranges from 4-8 headaches month, she has episodic migraines. She also has a strong family history of headaches. She denies any other medical or psychiatric comorbidity. She reports an intake of a caffeine-containing medication of 4 to 15 tablets per month.
The physician recommended that she limit her intake of the caffeine-containing medication to 5 days or less per month given the risk of migraine transformation. The physician also recommended maintaining a good sleep schedule, limiting excessive caffeine intake, a stress reduction program, regular cardiovascular exercise, and avoiding skipping or delaying meals. The patient was educated about migraine and its underlying mechanisms and the benefits of taking medications, and her fears regarding medication use and side effects were allayed. Sumatriptan 100 mg oral tablets were prescribed to be taken at headache onset. She was hesitant to be started on an antihypertensive or antiseizure medication, so she was prescribed amitriptyline 30 mg at night for headache prevention. She was also asked to maintain a headache diary. The patient was agreeable with this plan.
Summary
Migraine is often underdiagnosed and undertreated. Primary care providers are often the first point of contact for these patients. Identifying the type and frequency of migraine and comorbidities is necessary to guide appropriate management in terms of medications and lifestyle modifications. Often no testing or imaging is required. Educating patients about this chronic disease, treatment expectations, and limiting intake of medication is essential.
Corresponding author: Pooja Mohan Rao, MBBS, MD, Georgetown University Hospital, 3800 Reservoir Rd. NW, 7 PHC, Washington, DC 20007, [email protected].
Financial disclosures: Dr. Ailani reports receiving honoraria for speaking and consulting for Allergan, Avanir, and Eli Lilly.
1. Woldeamanuel YW, Cowan RP. Migraine affects 1 in 10 people worldwide featuring recent rise: A systematic review and meta-analysis of community-based studies involving 6 million participants, J Neurol Sci 2017;372:307–15.
2. Vetvik KG, MacGregor EA. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol 2017;16:76–87.
3. Lipton RB, Bigal ME. Migraine: epidemiology, impact, and risk factors for progression. Headache 2005;45 Suppl 1:S3–S13.
4. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1545–602.
5. Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention Headache 2015;55 Suppl 2:103–22.
6. Diamond S, Bigal ME, Silberstein S, et al. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention study. Headache 2007;47:355–63.
7. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study. Lancet 2012;380:2163–96.
8. Lipton RB, Stewart WF, Diamond S, et al. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache 2001;41:646–57.
9. Stewart WF, Ricci JA, Chee E, et al. Lost productive time and cost due to common pain in the US workforce. JAMA 2003;290:2443–54.
10. Hawkins K, Wang S, Rupnow M. Direct cost burden among insured US employees with migraine. Headache 2008;48:553–63.
11. Lipton RB, Serrano D, Holland S, et al. Barriers to the diagnosis and treatment of migraine: Effects of sex, income, and headache features. Headache 2013;53:81–92.
12. Dodick DW, Loder EW, Manack Adams A, et al. Assessing barriers to chronic migraine consultation, diagnosis, and treatment: Results from the chronic migraine epidemiology and outcomes (CaMEO) study. Headache 2016;56:821–34.
13. Young WB, Park JE, Tian IX, Kempner J. The stigma of migraine. PLoS One 2013;8(1):e54074.
14. Mia M, Ashna S, Audrey H. A migraine management training program for primary care providers: an overview of a survey and pilot study findings, lessons learned, and considerations for further research. Headache 2016;56:725–40.
15. Lipton RB, Amatniek JC, Ferrari MD, Gross M. Migraine: identifying and removing barriers to care. Neurology 1994;44(6 Suppl 4):S63–8.
16. Knapp RD Jr. Reports from the past 2. Headache 1963;3:112–22.
17. Levine M, Wolff HG. Cerebral circulation: afferent impulses from the blood vessels of the pia. Arch Neurol Psychiat 1932;28:140.
18. Amin FM, Asghar MS, Hougaard A, et al. Magnetic resonance angiography of intracranial and extracranial arteries in patients with spontaneous migraine without aura: a cross sectional study. Lancet Neurol 2013;12:454–61.
19. Goadsby PJ, Holland PR, Martins-Oliveira M, et al. Pathophysiology of migraine—a disorder of sensory processing. Physiol Rev 2017;97:553–622.
20. Goadsby PJ. Pathophysiology of migraine. Ann Indian Acad Neurol 2012;15(Suppl 1):S15–S22.
21. Puledda F, Messina R, Goadsby PJ, et al. An update on migraine: current understanding and future J Neurol 2017 Mar 20.
22. Vinogradova LV. Comparative potency of sensory-induced brainstem activation to trigger spreading depression and seizures in the cortex of awake rats: implications for the pathophysiology of migraine aura. Cephalalgia 2015;35:979–86.
23. Bahra A, Matharu MS, Buchel C, et al. Brainstem activation specific to migraine headache. Lancet 2001;357:1016–7.
24. Schulte LH, May A. The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain 2016;139:1987–93.
25. Noseda R, Jakubowski M, Kainz V, et al. Cortical projections of functionally identified thalamic trigeminovascular neurons: implications for migraine headache and its associated symptoms. J Neurosci 2011;31:14204–17.
26. Noseda R, Kainz V, Jakubowski M, et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci 2010;13:239–45.
27. Puledda F, Messina R, Goadsby PJ. An update on migraine: current understanding and future directions. J Neurol 2017 Mar 20.
28. Coppola G, Di Lorenzo C, Schoenen J, Pierelli F. Habituation and sensitization in primary headaches. J Headache Pain 2013;14:65.
29. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33:629–808.
30. Yusheng H, Y Li. Typical aura without headache: a case report and review of the literature J Med Case Rep 2015;9:40.
31. Buture A, Khalil M, Ahmed F. Iatrogenic visual aura: a case report and a brief review of the literature Ther Clin Risk Manag 2017;13:643–6.
32. Lipton RB. Tracing transformation: Chronic migraine classification, progression, and epidemiology. Neurology 2009;72:S3–7.
33. Lipton RB. Headache 2011;51;S2:77–83.
34. Scher AI, Stewart WF, et al. Factors associated with the onset and remission of chronic daily headache in a population-based study. Pain 2003;106:81–9.
35. Bigal ME, Lipton RB, Holland PR, Goadsby PJ. Obesity, migraine, and chronic migraine: possible mechanisms of interaction. Neurology 2007;68:1851–61.
36. Wolff HG. Stress and disease. Springfield, IL: Charles C. Thomas; 1953.
37. Guidetti V, Galli F, Fabrizi P, et al. Headache and psychiatric comorbidity: clinical aspects and outcome in an 8-year follow-up study. Cephalalgia 1998;18:455–62.
38. Mongini F, Keller R, Deregibus A, et al. Personality traits, depression and migraine in women: a longitudinal study. Cephalalgia 2003;23:186–92.
39. Hung CI, Liu CY, Yang CH, Wang SJ. The impacts of migraine among outpatients with major depressive disorder at a two-year follow-up. PLoS One 2015;10:e0128087.
40. Frishberg BM, Rosenberg JH, Matchar DB, et al. Evidence-based guidelines in the primary care setting: neuroimaging in patients with nonacute headache. St Paul: US Headache Consortium; 2000.
41. Headache Measurement Set 2014 Revised. American Academy of Neurology. Accessed at www.aan.com/uploadedFiles/Website_Library_Assets/Documents/3.Practice_Management/2.Quality_Improvement/1.Quality_Measures/1.All_Measures/2014.
42. Taylor FR. Lifestyle changes, dietary restrictions, and nutraceuticals in migraine prevention. Techn Reg Anesth Pain Manage 2009;13:28–37.
43. Varkey E, Cider A, Carlsson J, Linde M. Exercise as migraine prophylaxis: A randomized study using relaxation and topiramate as controls. Cephalalgia 2011;14:1428–38.
44. Ahn AH. Why does increased exercise decrease migraine? Curr Pain Headache Rep 2013;17:379.
45. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: The American Headache Society evidence assessment of migraine pharmacotherapies. Headache 2015;55:3–20.
46. Belvis R, Mas N, Aceituno A. Migraine attack treatment : a tailor-made suit, not one size fits all. Recent Pat CNS Drug Discov 2014;9:26–40.
47. Bigal ME, Serrano D, Buse D, et al. Acute migraine medications and evolution from episodic to chronic migraine: A longitudinal population-based study. Headache 2008;48:1157–68.
48. Bigal ME, Rapoport AM, Sheftell FD, et al. Transformed migraine and medication overuse in a tertiary headache centre--clinical characteristics and treatment outcomes Cephalalgia 2004;24:483–90.
49. Silberstein SD, Holland S, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2012;78:1337–45.
50. Diener HC, Dodick DW, Aurora SK, et al. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial Cephalagia year;30:804–14.
51. Wells RE, Markowitz SY, Baron EP, et al. Identifying the factors underlying discontinuation of triptans. Headache 2014;54:278–89.
52. Holland S, Fanning KM, Serrano D, et al. Rates and reasons for discontinuation of triptans and opioids in episodic migraine: results from the American Migraine Prevalence and Prevention (AMPP) study. J Neurol Sci 2013;326:10–7.
53. Seng EK, Rains JA, Nicholson RA, Lipton RB. Improving medication adherence in migraine treatment. Curr Pain Headache Rep 2015;19:24.
54. Nicholson RA, Buse DC, Andrasik F, Lipton RB. Nonpharmacologic treatments for migraine and tension-type headache: how to choose and when to use. Curr Treatment Opt Neurol 2011;13:28–40.
55. Probyn K, Bowers H, Mistry D, et al. Non-pharmacological self-management for people living with migraine or tension-type headache: a systematic review including analysis of intervention components BMJ Open 2017;7:e016670.
56. Lemstra M, Stewart B, Olszynski WP. Effectiveness of multidisciplinary intervention in the treatment of migraine: a randomized clinical trial. Headache 2002;42:845–54.
57. John PJ, Sharma N, Sharma CM, Kankane A. Effectiveness of yoga therapy in the treatment of migraine without aura: a randomized controlled trial. Headache 2007;47:654–61.
58. Harris P, Loveman E, Clegg A, et al. Systematic review of cognitive behavioural therapy for the management of headaches and migraines in adults Br J Pain 2015;9:213–24.
59. Kropp P, Meyer B, Meyer W, Dresler T. An update on behavioral treatments in migraine - current knowledge and future options. Expert Rev Neurother 2017:1–10.
60. Sullivan A, Cousins S, Ridsdale L. Psychological interventions for migraine: a systematic review. J Neurol 2016;263:2369–77.
61. Holroyd KA, Cottrell CK, O’Donnell FJ, et al. Effect of preventive (beta blocker) treatment, behavioural migraine management, or their combination on outcomes of optimised acute treatment in frequent migraine: randomised controlled trial. BMJ 2010;341:c4871.
62. Penzien DB, Rains JC, Andrasik F. Behavioral management of recurrent headache: three decades of experience and empiricism. Appl Psychophysiol Biofeedback 2002;27:163–81.
63. Smith TR, Nicholson RA, Banks JW. A primary care migraine education program has benefit on headache impact and quality of life: results from the mercy migraine management program. Headache 2010;50:600–12.
64. Rothrock JF, Parada VA, Sims C, et al.The impact of intensive patient education on clinical outcome in a clinic-based migraine population. Headache 2006;46:726–31.
1. Woldeamanuel YW, Cowan RP. Migraine affects 1 in 10 people worldwide featuring recent rise: A systematic review and meta-analysis of community-based studies involving 6 million participants, J Neurol Sci 2017;372:307–15.
2. Vetvik KG, MacGregor EA. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol 2017;16:76–87.
3. Lipton RB, Bigal ME. Migraine: epidemiology, impact, and risk factors for progression. Headache 2005;45 Suppl 1:S3–S13.
4. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1545–602.
5. Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention Headache 2015;55 Suppl 2:103–22.
6. Diamond S, Bigal ME, Silberstein S, et al. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention study. Headache 2007;47:355–63.
7. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study. Lancet 2012;380:2163–96.
8. Lipton RB, Stewart WF, Diamond S, et al. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache 2001;41:646–57.
9. Stewart WF, Ricci JA, Chee E, et al. Lost productive time and cost due to common pain in the US workforce. JAMA 2003;290:2443–54.
10. Hawkins K, Wang S, Rupnow M. Direct cost burden among insured US employees with migraine. Headache 2008;48:553–63.
11. Lipton RB, Serrano D, Holland S, et al. Barriers to the diagnosis and treatment of migraine: Effects of sex, income, and headache features. Headache 2013;53:81–92.
12. Dodick DW, Loder EW, Manack Adams A, et al. Assessing barriers to chronic migraine consultation, diagnosis, and treatment: Results from the chronic migraine epidemiology and outcomes (CaMEO) study. Headache 2016;56:821–34.
13. Young WB, Park JE, Tian IX, Kempner J. The stigma of migraine. PLoS One 2013;8(1):e54074.
14. Mia M, Ashna S, Audrey H. A migraine management training program for primary care providers: an overview of a survey and pilot study findings, lessons learned, and considerations for further research. Headache 2016;56:725–40.
15. Lipton RB, Amatniek JC, Ferrari MD, Gross M. Migraine: identifying and removing barriers to care. Neurology 1994;44(6 Suppl 4):S63–8.
16. Knapp RD Jr. Reports from the past 2. Headache 1963;3:112–22.
17. Levine M, Wolff HG. Cerebral circulation: afferent impulses from the blood vessels of the pia. Arch Neurol Psychiat 1932;28:140.
18. Amin FM, Asghar MS, Hougaard A, et al. Magnetic resonance angiography of intracranial and extracranial arteries in patients with spontaneous migraine without aura: a cross sectional study. Lancet Neurol 2013;12:454–61.
19. Goadsby PJ, Holland PR, Martins-Oliveira M, et al. Pathophysiology of migraine—a disorder of sensory processing. Physiol Rev 2017;97:553–622.
20. Goadsby PJ. Pathophysiology of migraine. Ann Indian Acad Neurol 2012;15(Suppl 1):S15–S22.
21. Puledda F, Messina R, Goadsby PJ, et al. An update on migraine: current understanding and future J Neurol 2017 Mar 20.
22. Vinogradova LV. Comparative potency of sensory-induced brainstem activation to trigger spreading depression and seizures in the cortex of awake rats: implications for the pathophysiology of migraine aura. Cephalalgia 2015;35:979–86.
23. Bahra A, Matharu MS, Buchel C, et al. Brainstem activation specific to migraine headache. Lancet 2001;357:1016–7.
24. Schulte LH, May A. The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain 2016;139:1987–93.
25. Noseda R, Jakubowski M, Kainz V, et al. Cortical projections of functionally identified thalamic trigeminovascular neurons: implications for migraine headache and its associated symptoms. J Neurosci 2011;31:14204–17.
26. Noseda R, Kainz V, Jakubowski M, et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci 2010;13:239–45.
27. Puledda F, Messina R, Goadsby PJ. An update on migraine: current understanding and future directions. J Neurol 2017 Mar 20.
28. Coppola G, Di Lorenzo C, Schoenen J, Pierelli F. Habituation and sensitization in primary headaches. J Headache Pain 2013;14:65.
29. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33:629–808.
30. Yusheng H, Y Li. Typical aura without headache: a case report and review of the literature J Med Case Rep 2015;9:40.
31. Buture A, Khalil M, Ahmed F. Iatrogenic visual aura: a case report and a brief review of the literature Ther Clin Risk Manag 2017;13:643–6.
32. Lipton RB. Tracing transformation: Chronic migraine classification, progression, and epidemiology. Neurology 2009;72:S3–7.
33. Lipton RB. Headache 2011;51;S2:77–83.
34. Scher AI, Stewart WF, et al. Factors associated with the onset and remission of chronic daily headache in a population-based study. Pain 2003;106:81–9.
35. Bigal ME, Lipton RB, Holland PR, Goadsby PJ. Obesity, migraine, and chronic migraine: possible mechanisms of interaction. Neurology 2007;68:1851–61.
36. Wolff HG. Stress and disease. Springfield, IL: Charles C. Thomas; 1953.
37. Guidetti V, Galli F, Fabrizi P, et al. Headache and psychiatric comorbidity: clinical aspects and outcome in an 8-year follow-up study. Cephalalgia 1998;18:455–62.
38. Mongini F, Keller R, Deregibus A, et al. Personality traits, depression and migraine in women: a longitudinal study. Cephalalgia 2003;23:186–92.
39. Hung CI, Liu CY, Yang CH, Wang SJ. The impacts of migraine among outpatients with major depressive disorder at a two-year follow-up. PLoS One 2015;10:e0128087.
40. Frishberg BM, Rosenberg JH, Matchar DB, et al. Evidence-based guidelines in the primary care setting: neuroimaging in patients with nonacute headache. St Paul: US Headache Consortium; 2000.
41. Headache Measurement Set 2014 Revised. American Academy of Neurology. Accessed at www.aan.com/uploadedFiles/Website_Library_Assets/Documents/3.Practice_Management/2.Quality_Improvement/1.Quality_Measures/1.All_Measures/2014.
42. Taylor FR. Lifestyle changes, dietary restrictions, and nutraceuticals in migraine prevention. Techn Reg Anesth Pain Manage 2009;13:28–37.
43. Varkey E, Cider A, Carlsson J, Linde M. Exercise as migraine prophylaxis: A randomized study using relaxation and topiramate as controls. Cephalalgia 2011;14:1428–38.
44. Ahn AH. Why does increased exercise decrease migraine? Curr Pain Headache Rep 2013;17:379.
45. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: The American Headache Society evidence assessment of migraine pharmacotherapies. Headache 2015;55:3–20.
46. Belvis R, Mas N, Aceituno A. Migraine attack treatment : a tailor-made suit, not one size fits all. Recent Pat CNS Drug Discov 2014;9:26–40.
47. Bigal ME, Serrano D, Buse D, et al. Acute migraine medications and evolution from episodic to chronic migraine: A longitudinal population-based study. Headache 2008;48:1157–68.
48. Bigal ME, Rapoport AM, Sheftell FD, et al. Transformed migraine and medication overuse in a tertiary headache centre--clinical characteristics and treatment outcomes Cephalalgia 2004;24:483–90.
49. Silberstein SD, Holland S, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2012;78:1337–45.
50. Diener HC, Dodick DW, Aurora SK, et al. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial Cephalagia year;30:804–14.
51. Wells RE, Markowitz SY, Baron EP, et al. Identifying the factors underlying discontinuation of triptans. Headache 2014;54:278–89.
52. Holland S, Fanning KM, Serrano D, et al. Rates and reasons for discontinuation of triptans and opioids in episodic migraine: results from the American Migraine Prevalence and Prevention (AMPP) study. J Neurol Sci 2013;326:10–7.
53. Seng EK, Rains JA, Nicholson RA, Lipton RB. Improving medication adherence in migraine treatment. Curr Pain Headache Rep 2015;19:24.
54. Nicholson RA, Buse DC, Andrasik F, Lipton RB. Nonpharmacologic treatments for migraine and tension-type headache: how to choose and when to use. Curr Treatment Opt Neurol 2011;13:28–40.
55. Probyn K, Bowers H, Mistry D, et al. Non-pharmacological self-management for people living with migraine or tension-type headache: a systematic review including analysis of intervention components BMJ Open 2017;7:e016670.
56. Lemstra M, Stewart B, Olszynski WP. Effectiveness of multidisciplinary intervention in the treatment of migraine: a randomized clinical trial. Headache 2002;42:845–54.
57. John PJ, Sharma N, Sharma CM, Kankane A. Effectiveness of yoga therapy in the treatment of migraine without aura: a randomized controlled trial. Headache 2007;47:654–61.
58. Harris P, Loveman E, Clegg A, et al. Systematic review of cognitive behavioural therapy for the management of headaches and migraines in adults Br J Pain 2015;9:213–24.
59. Kropp P, Meyer B, Meyer W, Dresler T. An update on behavioral treatments in migraine - current knowledge and future options. Expert Rev Neurother 2017:1–10.
60. Sullivan A, Cousins S, Ridsdale L. Psychological interventions for migraine: a systematic review. J Neurol 2016;263:2369–77.
61. Holroyd KA, Cottrell CK, O’Donnell FJ, et al. Effect of preventive (beta blocker) treatment, behavioural migraine management, or their combination on outcomes of optimised acute treatment in frequent migraine: randomised controlled trial. BMJ 2010;341:c4871.
62. Penzien DB, Rains JC, Andrasik F. Behavioral management of recurrent headache: three decades of experience and empiricism. Appl Psychophysiol Biofeedback 2002;27:163–81.
63. Smith TR, Nicholson RA, Banks JW. A primary care migraine education program has benefit on headache impact and quality of life: results from the mercy migraine management program. Headache 2010;50:600–12.
64. Rothrock JF, Parada VA, Sims C, et al.The impact of intensive patient education on clinical outcome in a clinic-based migraine population. Headache 2006;46:726–31.
Early Data Suggest Benefit of Aducanumab in Alzheimer’s Disease
BOSTON—The antiamyloid antibody aducanumab may slow cognitive decline and reduce amyloid burden in patients with Alzheimer’s disease, according to results presented at the 10th Edition of Clinical Trials on Alzheimer’s Disease (CTAD). The results are 36-month data from the phase Ib PRIME trial.
Patients who have been taking the highest dose of aducanumab, 10 mg/kg, for the duration of the study declined the least on two measures of cognition, the Mini-Mental State Exam (MMSE) and the Clinical Dementia Rating Scale–Sum of Boxes (CDR-SB). Some of the participants taking the 10-mg/kg dose became amyloid negative on PET by 24 months and stayed at a low level of amyloid until month 36, said Samantha Budd Haeberlein, PhD, Vice President of Clinical Development at Biogen in Cambridge, Massachusetts.
It is likely that the high-dose group continued to have amyloid, despite the imaging findings, said Dr. Haeberlein. “I would challenge the idea that [aducanumab] completely removed amyloid, since I think the instrument is not perfect,” she said, adding that the decreased level represents a drop below the threshold for positivity set by Eli Lilly, maker of the imaging agent florbetapir. “But we have to say that we are in a different realm here, where it can be difficult to determine whether an individual is positive or negative for amyloid pathology.”
The 36-month data support the continued development of aducanumab, said Dr. Haeberlein. The antibody is now being tested in two phase III studies, ENGAGE and EMERGE.
“The aducanumab data reported at CTAD are good news for safety and good news for the signals we need to see in the phase III trials,” said Maria Carillo, PhD, Chief Science Officer of the Alzheimer’s Association. “These are hopeful signs, but based on what we have learned from past Alzheimer’s studies, we need to wait for the phase III trial results.”
Study Examined Four Doses
Aducanumab is a monoclonal human antibody derived from B cells collected from a cohort of cognitively normal elderly subjects and cognitively impaired elderly subjects who exhibited unusually slow decline, according to Biogen. It binds to fibrillar and oligomeric amyloid aggregates, thus directly reducing amyloid plaques in the brain.
Investigators enrolled 165 patients with prodromal or mild Alzheimer’s disease into the PRIME study. All of the participants had brain amyloid on PET imaging. PRIME is the first randomized trial of an antiamyloid compound to rely solely on PET to establish participants’ amyloid positivity. These patients were randomized to placebo or 1 mg/kg, 3 mg/kg, 6 mg/kg, or 10 mg/kg of aducanumab for one year. The treatment period was followed by a two-year open-label extension. Patients who had been randomized to placebo or 1 mg/kg of aducanumab were switched to 3 mg/kg of aducanumab or to a 3-mg/kg to 6-mg/kg titration regimen in the long-term extension. Patients randomized to aducanumab at 3 mg/kg, 6 mg/kg, 10 mg/kg, or titration in the placebo-controlled period continued to receive the same dose.
The PRIME trial’s primary outcomes are safety and tolerability. The cognitive and functional outcomes, which are not usually assessed in a phase Ib study, are exploratory. The numbers in each dosing group are quite small, said Dr. Haeberlein. Of the original cohort, 117 entered the extension study, and 50 continued until 166 weeks, at which time 10 to 16 patients were in each of the dosage cohorts.
Amyloid Burden Decreased in Some Patients
At 36 months, the mean change in amyloid plaque level was greatest for the 10-mg/kg group, which, on average, no longer met the threshold of amyloid positivity on florbetapir PET. The amyloid level in the 6-mg/kg group declined to the threshold, but did not fall below it. The 1-mg/kg and 3-mg/kg groups declined at similar rates, but the decreases were not as large as in the higher-dose group.
All participants declined on the MMSE and CDR-SB. The decline, however, was clearly attenuated in some of the active groups, where the best results were seen in the 10 patients who received the 10-mg/kg dose. The average decline from baseline on the CDR-SB was 2.84 points among those patients. In the other groups, declines from baseline on the CDR-SB were 5.28 points in those who switched from placebo to 3 mg/kg, 6.11 points in those who switched from 1 mg/kg to 3 mg/kg, 3.86 points in the 3-mg/kg treatment group, and 4.49 points in the 6-mg/kg treatment group.
Patients taking 10 mg/kg also fared best on the MMSE, declining 4.10 points on average. Declines in the other groups were 7.98 points in those who switched from placebo to 3 mg/kg, 6.35 points in those who switched from 1 mg/kg to 3 mg/kg, 4.83 points in the 3-mg/kg treatment group, and 8.97 points in the 6-mg/kg treatment group. These differences were not statistically significant, said Dr. Haeberlein. “In this extension trial, we are not talking about statistical significance.”
Investigators Observed Cases of ARIA
The incidence of amyloid-related imaging abnormalities (ARIA), however, did not follow this dose-dependent pattern. All eight cases of edematous ARIA (ARIA-E) in the long-term extension phase occurred in the placebo group that switched to 1 mg/kg and in the 1-mg/kg group that was titrated to 3 mg/kg. All cases occurred early in the extension phase, no new cases occurred during the past year, and all but one case occurred in carriers of APOE4.
Hemorrhagic ARIA occurred in two controls who switched to 1 mg/kg of aducanumab, five participants taking 3 mg/kg, two participants taking 6 mg/kg, and one patient taking 10 mg/kg. These cases occurred early in the trial. All of the ARIA cases, regardless of etiology, were considered mild and resolved spontaneously. In all, 46 patients in the PRIME trial have experienced ARIA, and six have had more than one episode.
The most common adverse events in the long-term extension phase were falls, headache, and ARIA. Two patients in the extension phase died, one in the 6-mg/kg group and one in the 10-mg/kg group. Neither death was considered to be related to the study medication.
—Michele G. Sullivan
BOSTON—The antiamyloid antibody aducanumab may slow cognitive decline and reduce amyloid burden in patients with Alzheimer’s disease, according to results presented at the 10th Edition of Clinical Trials on Alzheimer’s Disease (CTAD). The results are 36-month data from the phase Ib PRIME trial.
Patients who have been taking the highest dose of aducanumab, 10 mg/kg, for the duration of the study declined the least on two measures of cognition, the Mini-Mental State Exam (MMSE) and the Clinical Dementia Rating Scale–Sum of Boxes (CDR-SB). Some of the participants taking the 10-mg/kg dose became amyloid negative on PET by 24 months and stayed at a low level of amyloid until month 36, said Samantha Budd Haeberlein, PhD, Vice President of Clinical Development at Biogen in Cambridge, Massachusetts.
It is likely that the high-dose group continued to have amyloid, despite the imaging findings, said Dr. Haeberlein. “I would challenge the idea that [aducanumab] completely removed amyloid, since I think the instrument is not perfect,” she said, adding that the decreased level represents a drop below the threshold for positivity set by Eli Lilly, maker of the imaging agent florbetapir. “But we have to say that we are in a different realm here, where it can be difficult to determine whether an individual is positive or negative for amyloid pathology.”
The 36-month data support the continued development of aducanumab, said Dr. Haeberlein. The antibody is now being tested in two phase III studies, ENGAGE and EMERGE.
“The aducanumab data reported at CTAD are good news for safety and good news for the signals we need to see in the phase III trials,” said Maria Carillo, PhD, Chief Science Officer of the Alzheimer’s Association. “These are hopeful signs, but based on what we have learned from past Alzheimer’s studies, we need to wait for the phase III trial results.”
Study Examined Four Doses
Aducanumab is a monoclonal human antibody derived from B cells collected from a cohort of cognitively normal elderly subjects and cognitively impaired elderly subjects who exhibited unusually slow decline, according to Biogen. It binds to fibrillar and oligomeric amyloid aggregates, thus directly reducing amyloid plaques in the brain.
Investigators enrolled 165 patients with prodromal or mild Alzheimer’s disease into the PRIME study. All of the participants had brain amyloid on PET imaging. PRIME is the first randomized trial of an antiamyloid compound to rely solely on PET to establish participants’ amyloid positivity. These patients were randomized to placebo or 1 mg/kg, 3 mg/kg, 6 mg/kg, or 10 mg/kg of aducanumab for one year. The treatment period was followed by a two-year open-label extension. Patients who had been randomized to placebo or 1 mg/kg of aducanumab were switched to 3 mg/kg of aducanumab or to a 3-mg/kg to 6-mg/kg titration regimen in the long-term extension. Patients randomized to aducanumab at 3 mg/kg, 6 mg/kg, 10 mg/kg, or titration in the placebo-controlled period continued to receive the same dose.
The PRIME trial’s primary outcomes are safety and tolerability. The cognitive and functional outcomes, which are not usually assessed in a phase Ib study, are exploratory. The numbers in each dosing group are quite small, said Dr. Haeberlein. Of the original cohort, 117 entered the extension study, and 50 continued until 166 weeks, at which time 10 to 16 patients were in each of the dosage cohorts.
Amyloid Burden Decreased in Some Patients
At 36 months, the mean change in amyloid plaque level was greatest for the 10-mg/kg group, which, on average, no longer met the threshold of amyloid positivity on florbetapir PET. The amyloid level in the 6-mg/kg group declined to the threshold, but did not fall below it. The 1-mg/kg and 3-mg/kg groups declined at similar rates, but the decreases were not as large as in the higher-dose group.
All participants declined on the MMSE and CDR-SB. The decline, however, was clearly attenuated in some of the active groups, where the best results were seen in the 10 patients who received the 10-mg/kg dose. The average decline from baseline on the CDR-SB was 2.84 points among those patients. In the other groups, declines from baseline on the CDR-SB were 5.28 points in those who switched from placebo to 3 mg/kg, 6.11 points in those who switched from 1 mg/kg to 3 mg/kg, 3.86 points in the 3-mg/kg treatment group, and 4.49 points in the 6-mg/kg treatment group.
Patients taking 10 mg/kg also fared best on the MMSE, declining 4.10 points on average. Declines in the other groups were 7.98 points in those who switched from placebo to 3 mg/kg, 6.35 points in those who switched from 1 mg/kg to 3 mg/kg, 4.83 points in the 3-mg/kg treatment group, and 8.97 points in the 6-mg/kg treatment group. These differences were not statistically significant, said Dr. Haeberlein. “In this extension trial, we are not talking about statistical significance.”
Investigators Observed Cases of ARIA
The incidence of amyloid-related imaging abnormalities (ARIA), however, did not follow this dose-dependent pattern. All eight cases of edematous ARIA (ARIA-E) in the long-term extension phase occurred in the placebo group that switched to 1 mg/kg and in the 1-mg/kg group that was titrated to 3 mg/kg. All cases occurred early in the extension phase, no new cases occurred during the past year, and all but one case occurred in carriers of APOE4.
Hemorrhagic ARIA occurred in two controls who switched to 1 mg/kg of aducanumab, five participants taking 3 mg/kg, two participants taking 6 mg/kg, and one patient taking 10 mg/kg. These cases occurred early in the trial. All of the ARIA cases, regardless of etiology, were considered mild and resolved spontaneously. In all, 46 patients in the PRIME trial have experienced ARIA, and six have had more than one episode.
The most common adverse events in the long-term extension phase were falls, headache, and ARIA. Two patients in the extension phase died, one in the 6-mg/kg group and one in the 10-mg/kg group. Neither death was considered to be related to the study medication.
—Michele G. Sullivan
BOSTON—The antiamyloid antibody aducanumab may slow cognitive decline and reduce amyloid burden in patients with Alzheimer’s disease, according to results presented at the 10th Edition of Clinical Trials on Alzheimer’s Disease (CTAD). The results are 36-month data from the phase Ib PRIME trial.
Patients who have been taking the highest dose of aducanumab, 10 mg/kg, for the duration of the study declined the least on two measures of cognition, the Mini-Mental State Exam (MMSE) and the Clinical Dementia Rating Scale–Sum of Boxes (CDR-SB). Some of the participants taking the 10-mg/kg dose became amyloid negative on PET by 24 months and stayed at a low level of amyloid until month 36, said Samantha Budd Haeberlein, PhD, Vice President of Clinical Development at Biogen in Cambridge, Massachusetts.
It is likely that the high-dose group continued to have amyloid, despite the imaging findings, said Dr. Haeberlein. “I would challenge the idea that [aducanumab] completely removed amyloid, since I think the instrument is not perfect,” she said, adding that the decreased level represents a drop below the threshold for positivity set by Eli Lilly, maker of the imaging agent florbetapir. “But we have to say that we are in a different realm here, where it can be difficult to determine whether an individual is positive or negative for amyloid pathology.”
The 36-month data support the continued development of aducanumab, said Dr. Haeberlein. The antibody is now being tested in two phase III studies, ENGAGE and EMERGE.
“The aducanumab data reported at CTAD are good news for safety and good news for the signals we need to see in the phase III trials,” said Maria Carillo, PhD, Chief Science Officer of the Alzheimer’s Association. “These are hopeful signs, but based on what we have learned from past Alzheimer’s studies, we need to wait for the phase III trial results.”
Study Examined Four Doses
Aducanumab is a monoclonal human antibody derived from B cells collected from a cohort of cognitively normal elderly subjects and cognitively impaired elderly subjects who exhibited unusually slow decline, according to Biogen. It binds to fibrillar and oligomeric amyloid aggregates, thus directly reducing amyloid plaques in the brain.
Investigators enrolled 165 patients with prodromal or mild Alzheimer’s disease into the PRIME study. All of the participants had brain amyloid on PET imaging. PRIME is the first randomized trial of an antiamyloid compound to rely solely on PET to establish participants’ amyloid positivity. These patients were randomized to placebo or 1 mg/kg, 3 mg/kg, 6 mg/kg, or 10 mg/kg of aducanumab for one year. The treatment period was followed by a two-year open-label extension. Patients who had been randomized to placebo or 1 mg/kg of aducanumab were switched to 3 mg/kg of aducanumab or to a 3-mg/kg to 6-mg/kg titration regimen in the long-term extension. Patients randomized to aducanumab at 3 mg/kg, 6 mg/kg, 10 mg/kg, or titration in the placebo-controlled period continued to receive the same dose.
The PRIME trial’s primary outcomes are safety and tolerability. The cognitive and functional outcomes, which are not usually assessed in a phase Ib study, are exploratory. The numbers in each dosing group are quite small, said Dr. Haeberlein. Of the original cohort, 117 entered the extension study, and 50 continued until 166 weeks, at which time 10 to 16 patients were in each of the dosage cohorts.
Amyloid Burden Decreased in Some Patients
At 36 months, the mean change in amyloid plaque level was greatest for the 10-mg/kg group, which, on average, no longer met the threshold of amyloid positivity on florbetapir PET. The amyloid level in the 6-mg/kg group declined to the threshold, but did not fall below it. The 1-mg/kg and 3-mg/kg groups declined at similar rates, but the decreases were not as large as in the higher-dose group.
All participants declined on the MMSE and CDR-SB. The decline, however, was clearly attenuated in some of the active groups, where the best results were seen in the 10 patients who received the 10-mg/kg dose. The average decline from baseline on the CDR-SB was 2.84 points among those patients. In the other groups, declines from baseline on the CDR-SB were 5.28 points in those who switched from placebo to 3 mg/kg, 6.11 points in those who switched from 1 mg/kg to 3 mg/kg, 3.86 points in the 3-mg/kg treatment group, and 4.49 points in the 6-mg/kg treatment group.
Patients taking 10 mg/kg also fared best on the MMSE, declining 4.10 points on average. Declines in the other groups were 7.98 points in those who switched from placebo to 3 mg/kg, 6.35 points in those who switched from 1 mg/kg to 3 mg/kg, 4.83 points in the 3-mg/kg treatment group, and 8.97 points in the 6-mg/kg treatment group. These differences were not statistically significant, said Dr. Haeberlein. “In this extension trial, we are not talking about statistical significance.”
Investigators Observed Cases of ARIA
The incidence of amyloid-related imaging abnormalities (ARIA), however, did not follow this dose-dependent pattern. All eight cases of edematous ARIA (ARIA-E) in the long-term extension phase occurred in the placebo group that switched to 1 mg/kg and in the 1-mg/kg group that was titrated to 3 mg/kg. All cases occurred early in the extension phase, no new cases occurred during the past year, and all but one case occurred in carriers of APOE4.
Hemorrhagic ARIA occurred in two controls who switched to 1 mg/kg of aducanumab, five participants taking 3 mg/kg, two participants taking 6 mg/kg, and one patient taking 10 mg/kg. These cases occurred early in the trial. All of the ARIA cases, regardless of etiology, were considered mild and resolved spontaneously. In all, 46 patients in the PRIME trial have experienced ARIA, and six have had more than one episode.
The most common adverse events in the long-term extension phase were falls, headache, and ARIA. Two patients in the extension phase died, one in the 6-mg/kg group and one in the 10-mg/kg group. Neither death was considered to be related to the study medication.
—Michele G. Sullivan
Progress in Vascular Disease Treatments: Hype vs. Reality
Today we are witnessing unprecedented rapid development and dissemination of new scientific information regarding vascular diseases, and it is becoming increasingly difficult for busy practitioners to keep up with the avalanche of information, according to Dr. Bruce A. Perler.
To help practitioners differentiate the hype from reality, the “Progress in the Medical Treatments of Vascular Disease; Vascular Diseases and Risk Prediction” session on Friday will bring together internationally respected experts in the field. These faculty will present “the latest and most important advances in the perioperative and long-term medical management of our patients in a succinct and easily digestible fashion,” said Dr. Perler of Johns Hopkins University School of Medicine, who will co-moderate the session with Dr. Caron B. Rockman of New York University School of Medicine.
He added that the information presented in this session will afford the practitioner the latest scientifically proven perioperative and long-term therapy to optimize the patient’s circulatory health.
Further, attendees can take what they learn in this session back to their practice to properly counsel patients about their care – and to answer the questions that patients often bring to the office about these drugs and issues that they hear about in the lay press, he said.
Two critically important talks to be included in the session are: “Which Patients Should Receive Primary Prevention Lipid Lowering Statin Therapy: What Drug and Dose: How Do the HOPE 3 Trial Findings Help,” by Jeffrey S. Berger, MD, associate professor of medicine and surgery, NYU School of Medicine, and “How Do PCSK-9 Inhibitors Work: When and How Should They Currently Be Used: Advantages and Limitations,” by Dr. Natalie A. Marks of the NYU Lutheran Medical Center, he said.
“Hardly a month goes by without yet another article appearing in the lay press about statins. Patients are aware of statins, the associated controversies, and now are hearing about PCSK-9 inhibitors. These talks will inform the vascular surgeon about the key issues with respect to statin therapy and this exciting new alternative,” he said.
Among the nine other informative talks to be presented in the session cover important topics such as ACE inhibitors, angiotensin receptor blockers, the use of cilostazol, and troponin texting.
“Achieving the best results of our vascular surgical procedures requires more than doing the right procedure on the right patient at the right time,” Dr. Perler said. “It also requires managing the patient’s medical and perioperative care compulsively. Keeping abreast of the latest developments, highlighted in this session, will not only optimize your patients’ care, but provide a competitive practice advantage for the contemporary vascular surgeon.”
Today we are witnessing unprecedented rapid development and dissemination of new scientific information regarding vascular diseases, and it is becoming increasingly difficult for busy practitioners to keep up with the avalanche of information, according to Dr. Bruce A. Perler.
To help practitioners differentiate the hype from reality, the “Progress in the Medical Treatments of Vascular Disease; Vascular Diseases and Risk Prediction” session on Friday will bring together internationally respected experts in the field. These faculty will present “the latest and most important advances in the perioperative and long-term medical management of our patients in a succinct and easily digestible fashion,” said Dr. Perler of Johns Hopkins University School of Medicine, who will co-moderate the session with Dr. Caron B. Rockman of New York University School of Medicine.
He added that the information presented in this session will afford the practitioner the latest scientifically proven perioperative and long-term therapy to optimize the patient’s circulatory health.
Further, attendees can take what they learn in this session back to their practice to properly counsel patients about their care – and to answer the questions that patients often bring to the office about these drugs and issues that they hear about in the lay press, he said.
Two critically important talks to be included in the session are: “Which Patients Should Receive Primary Prevention Lipid Lowering Statin Therapy: What Drug and Dose: How Do the HOPE 3 Trial Findings Help,” by Jeffrey S. Berger, MD, associate professor of medicine and surgery, NYU School of Medicine, and “How Do PCSK-9 Inhibitors Work: When and How Should They Currently Be Used: Advantages and Limitations,” by Dr. Natalie A. Marks of the NYU Lutheran Medical Center, he said.
“Hardly a month goes by without yet another article appearing in the lay press about statins. Patients are aware of statins, the associated controversies, and now are hearing about PCSK-9 inhibitors. These talks will inform the vascular surgeon about the key issues with respect to statin therapy and this exciting new alternative,” he said.
Among the nine other informative talks to be presented in the session cover important topics such as ACE inhibitors, angiotensin receptor blockers, the use of cilostazol, and troponin texting.
“Achieving the best results of our vascular surgical procedures requires more than doing the right procedure on the right patient at the right time,” Dr. Perler said. “It also requires managing the patient’s medical and perioperative care compulsively. Keeping abreast of the latest developments, highlighted in this session, will not only optimize your patients’ care, but provide a competitive practice advantage for the contemporary vascular surgeon.”
Today we are witnessing unprecedented rapid development and dissemination of new scientific information regarding vascular diseases, and it is becoming increasingly difficult for busy practitioners to keep up with the avalanche of information, according to Dr. Bruce A. Perler.
To help practitioners differentiate the hype from reality, the “Progress in the Medical Treatments of Vascular Disease; Vascular Diseases and Risk Prediction” session on Friday will bring together internationally respected experts in the field. These faculty will present “the latest and most important advances in the perioperative and long-term medical management of our patients in a succinct and easily digestible fashion,” said Dr. Perler of Johns Hopkins University School of Medicine, who will co-moderate the session with Dr. Caron B. Rockman of New York University School of Medicine.
He added that the information presented in this session will afford the practitioner the latest scientifically proven perioperative and long-term therapy to optimize the patient’s circulatory health.
Further, attendees can take what they learn in this session back to their practice to properly counsel patients about their care – and to answer the questions that patients often bring to the office about these drugs and issues that they hear about in the lay press, he said.
Two critically important talks to be included in the session are: “Which Patients Should Receive Primary Prevention Lipid Lowering Statin Therapy: What Drug and Dose: How Do the HOPE 3 Trial Findings Help,” by Jeffrey S. Berger, MD, associate professor of medicine and surgery, NYU School of Medicine, and “How Do PCSK-9 Inhibitors Work: When and How Should They Currently Be Used: Advantages and Limitations,” by Dr. Natalie A. Marks of the NYU Lutheran Medical Center, he said.
“Hardly a month goes by without yet another article appearing in the lay press about statins. Patients are aware of statins, the associated controversies, and now are hearing about PCSK-9 inhibitors. These talks will inform the vascular surgeon about the key issues with respect to statin therapy and this exciting new alternative,” he said.
Among the nine other informative talks to be presented in the session cover important topics such as ACE inhibitors, angiotensin receptor blockers, the use of cilostazol, and troponin texting.
“Achieving the best results of our vascular surgical procedures requires more than doing the right procedure on the right patient at the right time,” Dr. Perler said. “It also requires managing the patient’s medical and perioperative care compulsively. Keeping abreast of the latest developments, highlighted in this session, will not only optimize your patients’ care, but provide a competitive practice advantage for the contemporary vascular surgeon.”
Masitinib May Provide Clinical Benefits for Patients with ALS
SAN DIEGO—Masitinib may provide meaningful clinical benefits for patients with amyotrophic lateral sclerosis (ALS), according to research presented at the 142nd Annual Meeting of the American Neurological Association. Early initiation of masitinib may be more beneficial than delayed initiation.
The worldwide prevalence of ALS is approximately 235,000. By 2040, the prevalence is expected to increase by 69%. Riluzole, the only widely available drug for the treatment of ALS, has been associated with little improvement in quality of life and modest increase in survival. IV edaravone demonstrated efficacy only in patients in good clinical condition, which is estimated to be less than 7% of patients with ALS.
Masitinib, an oral tyrosine kinase inhibitor with activity against CSF1/CSF1R signaling and mast cell function, is under investigation as a therapeutic option for patients with ALS. Masitinib provides neuroprotection in the CNS and peripheral nervous system by targeting microglia and macrophage and mast cell activity. The drug has been associated with significantly slower progression of paralysis in post paralytic rats. To test this therapy in patients with ALS, Angela Genge, MD, Director of the ALS Clinic at the Montreal Neurological Institute and Hospital, and colleagues conducted a double blind, placebo-controlled, randomized trial.
A total of 394 people from nine countries were included in the study. Patients with ALS were randomized to riluzole (100 mg/kg) plus either 4.5 mg/kg/day of oral masitinib, 3.0 mg/kg/day of oral masitinib, or placebo over 48 weeks.
The primary end point was absolute change in ALS Functional Rating Scale-Revised (ALSFRS-R) at 48 weeks in patients with baseline ALSFRS-R progression of less than 1.1 points per month. Secondary end points included the 40-item ALS Assessment Questionnaire (ALSAQ-40), forced vital capacity, and survival to event (defined as ALSFRS-R deterioration of 9 points from baseline or death). Researchers categorized participants as normal progressors (ie, those with a rate of change in ALSFRS-R score < 1.1 points per month) or faster progressors (ie, those with a rate of change in ALSFRS-R score ≥ 1.1 points per month).
Masitinib showed significant benefit over placebo in ALSFRS-R. At week 48, the score had decreased by 12.6 points among controls, compared with 9.2 points in the masitinib groups. Masitinib was associated with 27% slowing of ALSFRS-R deterioration, 29% slowing of deterioration in quality of life, 22% slowing of deterioration in respiratory function, and 25% delay in disease progression.
Secondary analyses also indicated masitinib’s superiority to placebo. A post hoc analysis indicated that early treatment (ie, at less than 24 months duration of illness) conferred greater benefits than delayed treatment. Patients who had milder symptoms or shorter duration of illness showed enhanced masitinib treatment effect.Adverse events with greatest positive difference between masitinib and placebo treatment arms were maculopapular rash and peripheral edema. The rate of adverse events was 78.9% for placebo, 88.4% for the 4.5-mg/kg/day dose of masitinib, and 84.7% for the 3.0-mg/kg/day dose of masitinib. No deaths related to study treatment were reported in either masitinib or placebo groups.
—Erica Tricarico
SAN DIEGO—Masitinib may provide meaningful clinical benefits for patients with amyotrophic lateral sclerosis (ALS), according to research presented at the 142nd Annual Meeting of the American Neurological Association. Early initiation of masitinib may be more beneficial than delayed initiation.
The worldwide prevalence of ALS is approximately 235,000. By 2040, the prevalence is expected to increase by 69%. Riluzole, the only widely available drug for the treatment of ALS, has been associated with little improvement in quality of life and modest increase in survival. IV edaravone demonstrated efficacy only in patients in good clinical condition, which is estimated to be less than 7% of patients with ALS.
Masitinib, an oral tyrosine kinase inhibitor with activity against CSF1/CSF1R signaling and mast cell function, is under investigation as a therapeutic option for patients with ALS. Masitinib provides neuroprotection in the CNS and peripheral nervous system by targeting microglia and macrophage and mast cell activity. The drug has been associated with significantly slower progression of paralysis in post paralytic rats. To test this therapy in patients with ALS, Angela Genge, MD, Director of the ALS Clinic at the Montreal Neurological Institute and Hospital, and colleagues conducted a double blind, placebo-controlled, randomized trial.
A total of 394 people from nine countries were included in the study. Patients with ALS were randomized to riluzole (100 mg/kg) plus either 4.5 mg/kg/day of oral masitinib, 3.0 mg/kg/day of oral masitinib, or placebo over 48 weeks.
The primary end point was absolute change in ALS Functional Rating Scale-Revised (ALSFRS-R) at 48 weeks in patients with baseline ALSFRS-R progression of less than 1.1 points per month. Secondary end points included the 40-item ALS Assessment Questionnaire (ALSAQ-40), forced vital capacity, and survival to event (defined as ALSFRS-R deterioration of 9 points from baseline or death). Researchers categorized participants as normal progressors (ie, those with a rate of change in ALSFRS-R score < 1.1 points per month) or faster progressors (ie, those with a rate of change in ALSFRS-R score ≥ 1.1 points per month).
Masitinib showed significant benefit over placebo in ALSFRS-R. At week 48, the score had decreased by 12.6 points among controls, compared with 9.2 points in the masitinib groups. Masitinib was associated with 27% slowing of ALSFRS-R deterioration, 29% slowing of deterioration in quality of life, 22% slowing of deterioration in respiratory function, and 25% delay in disease progression.
Secondary analyses also indicated masitinib’s superiority to placebo. A post hoc analysis indicated that early treatment (ie, at less than 24 months duration of illness) conferred greater benefits than delayed treatment. Patients who had milder symptoms or shorter duration of illness showed enhanced masitinib treatment effect.Adverse events with greatest positive difference between masitinib and placebo treatment arms were maculopapular rash and peripheral edema. The rate of adverse events was 78.9% for placebo, 88.4% for the 4.5-mg/kg/day dose of masitinib, and 84.7% for the 3.0-mg/kg/day dose of masitinib. No deaths related to study treatment were reported in either masitinib or placebo groups.
—Erica Tricarico
SAN DIEGO—Masitinib may provide meaningful clinical benefits for patients with amyotrophic lateral sclerosis (ALS), according to research presented at the 142nd Annual Meeting of the American Neurological Association. Early initiation of masitinib may be more beneficial than delayed initiation.
The worldwide prevalence of ALS is approximately 235,000. By 2040, the prevalence is expected to increase by 69%. Riluzole, the only widely available drug for the treatment of ALS, has been associated with little improvement in quality of life and modest increase in survival. IV edaravone demonstrated efficacy only in patients in good clinical condition, which is estimated to be less than 7% of patients with ALS.
Masitinib, an oral tyrosine kinase inhibitor with activity against CSF1/CSF1R signaling and mast cell function, is under investigation as a therapeutic option for patients with ALS. Masitinib provides neuroprotection in the CNS and peripheral nervous system by targeting microglia and macrophage and mast cell activity. The drug has been associated with significantly slower progression of paralysis in post paralytic rats. To test this therapy in patients with ALS, Angela Genge, MD, Director of the ALS Clinic at the Montreal Neurological Institute and Hospital, and colleagues conducted a double blind, placebo-controlled, randomized trial.
A total of 394 people from nine countries were included in the study. Patients with ALS were randomized to riluzole (100 mg/kg) plus either 4.5 mg/kg/day of oral masitinib, 3.0 mg/kg/day of oral masitinib, or placebo over 48 weeks.
The primary end point was absolute change in ALS Functional Rating Scale-Revised (ALSFRS-R) at 48 weeks in patients with baseline ALSFRS-R progression of less than 1.1 points per month. Secondary end points included the 40-item ALS Assessment Questionnaire (ALSAQ-40), forced vital capacity, and survival to event (defined as ALSFRS-R deterioration of 9 points from baseline or death). Researchers categorized participants as normal progressors (ie, those with a rate of change in ALSFRS-R score < 1.1 points per month) or faster progressors (ie, those with a rate of change in ALSFRS-R score ≥ 1.1 points per month).
Masitinib showed significant benefit over placebo in ALSFRS-R. At week 48, the score had decreased by 12.6 points among controls, compared with 9.2 points in the masitinib groups. Masitinib was associated with 27% slowing of ALSFRS-R deterioration, 29% slowing of deterioration in quality of life, 22% slowing of deterioration in respiratory function, and 25% delay in disease progression.
Secondary analyses also indicated masitinib’s superiority to placebo. A post hoc analysis indicated that early treatment (ie, at less than 24 months duration of illness) conferred greater benefits than delayed treatment. Patients who had milder symptoms or shorter duration of illness showed enhanced masitinib treatment effect.Adverse events with greatest positive difference between masitinib and placebo treatment arms were maculopapular rash and peripheral edema. The rate of adverse events was 78.9% for placebo, 88.4% for the 4.5-mg/kg/day dose of masitinib, and 84.7% for the 3.0-mg/kg/day dose of masitinib. No deaths related to study treatment were reported in either masitinib or placebo groups.
—Erica Tricarico
Injectable agent found to improve knee function in OA patients
SAN DIEGO – Among patients with mild to moderate patellofemoral osteoarthritis, intra-articular administration of the novel agent TPX-100 was safe and associated with functional benefits up to 1 year, a proof-of-concept study showed.
“We don’t yet have a disease-modifying drug for osteoarthritis [OA]; that’s sort of the holy grail for researchers,” lead study author Dawn McGuire, MD, said in an interview at the annual meeting of the American College of Rheumatology. “All of the patient-reported outcome and patient function indices that we studied moved in the same direction, showing a benefit of TPX-100. I think this is very promising.”
The study consisted of two parts. In Part A, four dose cohorts ranging from 20 mg to 200 mg per injection were enrolled. There were no dose-limiting toxicities or safety concerns at any dose, and the 200-mg dose was selected dose for Part B of the study.
The median age of the 118 patients was 60 years and their median body mass index was 29.2 kg/m2. No drug-related serious adverse events and no dose-limiting toxicities occurred across doses ranging from 20 mg to 200 mg per injection. The incidence of common adverse events such as knee pain was similar between placebo- and TPX-100-treated knees.
Quantitative MRI showed no measurable between-knee differences in cartilage thickness or volume at 6 or 12 months. However, statistically significant and clinically meaningful differences in knee function were observed in favor of TPX-100-treated knees, compared with controls, including KOOS activities of daily living (P = .008 at 6 and 12 months), KOOS knee-related quality of life (P = .21 at 6 months and P = .03 at 12 months), and a significant reduction in pain going up or down stairs (P = .004 at 12 months). Subjects’ use of nonsteroidal anti-inflammatory medications declined by 63% during the study.
“In this study, we see some terrific, long-term results in knee-related core activities, which certainly were disease modifying from the patients’ perspective,” said Dr. McGuire, who noted that a phase 3 study is being planned. “In addition, patient’s pain going up and down stairs improved significantly, with a marked reduction in analgesic use. Any of us who are experienced in these tough areas of medicine know that early results can look extremely promising, but we have to do larger confirmatory studies.”
SAN DIEGO – Among patients with mild to moderate patellofemoral osteoarthritis, intra-articular administration of the novel agent TPX-100 was safe and associated with functional benefits up to 1 year, a proof-of-concept study showed.
“We don’t yet have a disease-modifying drug for osteoarthritis [OA]; that’s sort of the holy grail for researchers,” lead study author Dawn McGuire, MD, said in an interview at the annual meeting of the American College of Rheumatology. “All of the patient-reported outcome and patient function indices that we studied moved in the same direction, showing a benefit of TPX-100. I think this is very promising.”
The study consisted of two parts. In Part A, four dose cohorts ranging from 20 mg to 200 mg per injection were enrolled. There were no dose-limiting toxicities or safety concerns at any dose, and the 200-mg dose was selected dose for Part B of the study.
The median age of the 118 patients was 60 years and their median body mass index was 29.2 kg/m2. No drug-related serious adverse events and no dose-limiting toxicities occurred across doses ranging from 20 mg to 200 mg per injection. The incidence of common adverse events such as knee pain was similar between placebo- and TPX-100-treated knees.
Quantitative MRI showed no measurable between-knee differences in cartilage thickness or volume at 6 or 12 months. However, statistically significant and clinically meaningful differences in knee function were observed in favor of TPX-100-treated knees, compared with controls, including KOOS activities of daily living (P = .008 at 6 and 12 months), KOOS knee-related quality of life (P = .21 at 6 months and P = .03 at 12 months), and a significant reduction in pain going up or down stairs (P = .004 at 12 months). Subjects’ use of nonsteroidal anti-inflammatory medications declined by 63% during the study.
“In this study, we see some terrific, long-term results in knee-related core activities, which certainly were disease modifying from the patients’ perspective,” said Dr. McGuire, who noted that a phase 3 study is being planned. “In addition, patient’s pain going up and down stairs improved significantly, with a marked reduction in analgesic use. Any of us who are experienced in these tough areas of medicine know that early results can look extremely promising, but we have to do larger confirmatory studies.”
SAN DIEGO – Among patients with mild to moderate patellofemoral osteoarthritis, intra-articular administration of the novel agent TPX-100 was safe and associated with functional benefits up to 1 year, a proof-of-concept study showed.
“We don’t yet have a disease-modifying drug for osteoarthritis [OA]; that’s sort of the holy grail for researchers,” lead study author Dawn McGuire, MD, said in an interview at the annual meeting of the American College of Rheumatology. “All of the patient-reported outcome and patient function indices that we studied moved in the same direction, showing a benefit of TPX-100. I think this is very promising.”
The study consisted of two parts. In Part A, four dose cohorts ranging from 20 mg to 200 mg per injection were enrolled. There were no dose-limiting toxicities or safety concerns at any dose, and the 200-mg dose was selected dose for Part B of the study.
The median age of the 118 patients was 60 years and their median body mass index was 29.2 kg/m2. No drug-related serious adverse events and no dose-limiting toxicities occurred across doses ranging from 20 mg to 200 mg per injection. The incidence of common adverse events such as knee pain was similar between placebo- and TPX-100-treated knees.
Quantitative MRI showed no measurable between-knee differences in cartilage thickness or volume at 6 or 12 months. However, statistically significant and clinically meaningful differences in knee function were observed in favor of TPX-100-treated knees, compared with controls, including KOOS activities of daily living (P = .008 at 6 and 12 months), KOOS knee-related quality of life (P = .21 at 6 months and P = .03 at 12 months), and a significant reduction in pain going up or down stairs (P = .004 at 12 months). Subjects’ use of nonsteroidal anti-inflammatory medications declined by 63% during the study.
“In this study, we see some terrific, long-term results in knee-related core activities, which certainly were disease modifying from the patients’ perspective,” said Dr. McGuire, who noted that a phase 3 study is being planned. “In addition, patient’s pain going up and down stairs improved significantly, with a marked reduction in analgesic use. Any of us who are experienced in these tough areas of medicine know that early results can look extremely promising, but we have to do larger confirmatory studies.”
AT ACR 2017
Key clinical point:
Major finding: Statistically significant differences in knee function were observed in favor of TPX-100-treated knees, compared with controls, using Knee Injury and Osteoarthritis Outcome Score activities of daily living (P = .008 at 6 and 12 months) and a significant reduction in pain going up or down stairs (P = .004 at 12 months).
Study details: A randomized, proof-of-concept study involving 118 patients with patellofemoral knee OA.
Disclosures: OrthoTrophix sponsored the study. Dr. McGuire is chief medical officer and a cofounder of the company.
Evolution in Management and Treatment of Carotid Artery Disease
“There is a ‘one size fits all’ strategy by a lot of people who simply read a paper or a guideline and say that’s how patients must be treated,” said co-moderator Dr. Ross Naylor, professor of vascular surgery at the University of Leicester and a consultant vascular surgeon at the Leicester Royal Infirmary. “This session will question how you actually treat your patients, so I think it will open people’s eyes toward the benefits of modern medical therapy. It also questions the role of carotid stenting in asymptomatic patients and how to reduce the risks; unless we reduce the risks, it’s going to be less likely to be adopted.”
The session has several themes, he explained. One is the benefit of optimizing best medical therapy: “There are a couple of papers on the role of starting statins before carotid surgery or carotid stenting. There’s now good evidence that if you do this, you will reduce the perioperative risk of stroke, and this needs to be emphasized more in guidelines.”
In addition, Dr. Naylor said, there is increasing evidence that patients who have asymptomatic carotid stenosis, and who are started on good quality medical therapy, have much lower annual risks of stroke than they would 15 to 20 years ago. Presentations by Dr. J. David Spence of Western University and University Hospital in London, Canada, and by Dr. Henrik Sillesen of the University of Copenhagen and Rigshospitalet, will question current attitudes toward intervening in asymptomatic patients. “Their big plea is that the majority can be treated medically,” Dr. Naylor said. “Only a small proportion actually will benefit from stenting and surgery.” Dr. Spence will address the value of Mediterranean and Nordic diets in patients with carotid stenosis, while Dr. Sillesen will examine if stenosis or plaque progression are reasons to treat asymptomatic patients with carotid artery stenting (CAS) versus carotid artery endarterectomy (CEA).
Another theme is looking at efforts to reduce perioperative stroke rates after carotid stenting, Dr. Naylor said: “One of the repeated findings is that the death and stroke rates are lower following carotid surgery rather than carotid stenting. Registries suggest that in a large number of series, stroke rates actually exceed the accepted risks for treating patients with asymptomatic disease, which is 3%, or for symptomatic disease, which is 6%.”
Dr. William A. Gray of Jefferson Medical College and Main Line Health will discuss technical strategies that might be used to reduce perioperative stroke rates, including new techniques and devices such as the double-filter Paladin device. Dr. L. Nelson Hopkins, SUNY Distinguished Professor of Neurosurgery and Radiology, University at Buffalo, will discuss how strokes after CAS and other interventional procedures have greater cognitive deficits than previously thought, even with full neurological recovery.
The discussions will conclude with a presentation by Dr. Mark H. Wholey of the University of Pittsburgh Medical Center, Shady Side, on the etiology, diagnosis and treatment of vertebral artery dissections. “It is so vanishingly rare that we are asked to treat this that almost nobody has any experience,” Dr. Naylor said. “I suspect this will be quite an interesting talk for the audience.”
Co-moderators for the session will be Dr. James May, Emeritus Bosch Professor of Surgery and associated dean of surgical sciences at the University of Sydney, and a vascular surgeon at Royal Prince Alfred Hospital; Dr. Wesley S. Moore, professor and chief emeritus of vascular surgery at UCLA Medical Center; and Dr. Enrico Ascher, chief of vascular surgery at NYU Hospitals, and professor of surgery at New York University.
“There is a ‘one size fits all’ strategy by a lot of people who simply read a paper or a guideline and say that’s how patients must be treated,” said co-moderator Dr. Ross Naylor, professor of vascular surgery at the University of Leicester and a consultant vascular surgeon at the Leicester Royal Infirmary. “This session will question how you actually treat your patients, so I think it will open people’s eyes toward the benefits of modern medical therapy. It also questions the role of carotid stenting in asymptomatic patients and how to reduce the risks; unless we reduce the risks, it’s going to be less likely to be adopted.”
The session has several themes, he explained. One is the benefit of optimizing best medical therapy: “There are a couple of papers on the role of starting statins before carotid surgery or carotid stenting. There’s now good evidence that if you do this, you will reduce the perioperative risk of stroke, and this needs to be emphasized more in guidelines.”
In addition, Dr. Naylor said, there is increasing evidence that patients who have asymptomatic carotid stenosis, and who are started on good quality medical therapy, have much lower annual risks of stroke than they would 15 to 20 years ago. Presentations by Dr. J. David Spence of Western University and University Hospital in London, Canada, and by Dr. Henrik Sillesen of the University of Copenhagen and Rigshospitalet, will question current attitudes toward intervening in asymptomatic patients. “Their big plea is that the majority can be treated medically,” Dr. Naylor said. “Only a small proportion actually will benefit from stenting and surgery.” Dr. Spence will address the value of Mediterranean and Nordic diets in patients with carotid stenosis, while Dr. Sillesen will examine if stenosis or plaque progression are reasons to treat asymptomatic patients with carotid artery stenting (CAS) versus carotid artery endarterectomy (CEA).
Another theme is looking at efforts to reduce perioperative stroke rates after carotid stenting, Dr. Naylor said: “One of the repeated findings is that the death and stroke rates are lower following carotid surgery rather than carotid stenting. Registries suggest that in a large number of series, stroke rates actually exceed the accepted risks for treating patients with asymptomatic disease, which is 3%, or for symptomatic disease, which is 6%.”
Dr. William A. Gray of Jefferson Medical College and Main Line Health will discuss technical strategies that might be used to reduce perioperative stroke rates, including new techniques and devices such as the double-filter Paladin device. Dr. L. Nelson Hopkins, SUNY Distinguished Professor of Neurosurgery and Radiology, University at Buffalo, will discuss how strokes after CAS and other interventional procedures have greater cognitive deficits than previously thought, even with full neurological recovery.
The discussions will conclude with a presentation by Dr. Mark H. Wholey of the University of Pittsburgh Medical Center, Shady Side, on the etiology, diagnosis and treatment of vertebral artery dissections. “It is so vanishingly rare that we are asked to treat this that almost nobody has any experience,” Dr. Naylor said. “I suspect this will be quite an interesting talk for the audience.”
Co-moderators for the session will be Dr. James May, Emeritus Bosch Professor of Surgery and associated dean of surgical sciences at the University of Sydney, and a vascular surgeon at Royal Prince Alfred Hospital; Dr. Wesley S. Moore, professor and chief emeritus of vascular surgery at UCLA Medical Center; and Dr. Enrico Ascher, chief of vascular surgery at NYU Hospitals, and professor of surgery at New York University.
“There is a ‘one size fits all’ strategy by a lot of people who simply read a paper or a guideline and say that’s how patients must be treated,” said co-moderator Dr. Ross Naylor, professor of vascular surgery at the University of Leicester and a consultant vascular surgeon at the Leicester Royal Infirmary. “This session will question how you actually treat your patients, so I think it will open people’s eyes toward the benefits of modern medical therapy. It also questions the role of carotid stenting in asymptomatic patients and how to reduce the risks; unless we reduce the risks, it’s going to be less likely to be adopted.”
The session has several themes, he explained. One is the benefit of optimizing best medical therapy: “There are a couple of papers on the role of starting statins before carotid surgery or carotid stenting. There’s now good evidence that if you do this, you will reduce the perioperative risk of stroke, and this needs to be emphasized more in guidelines.”
In addition, Dr. Naylor said, there is increasing evidence that patients who have asymptomatic carotid stenosis, and who are started on good quality medical therapy, have much lower annual risks of stroke than they would 15 to 20 years ago. Presentations by Dr. J. David Spence of Western University and University Hospital in London, Canada, and by Dr. Henrik Sillesen of the University of Copenhagen and Rigshospitalet, will question current attitudes toward intervening in asymptomatic patients. “Their big plea is that the majority can be treated medically,” Dr. Naylor said. “Only a small proportion actually will benefit from stenting and surgery.” Dr. Spence will address the value of Mediterranean and Nordic diets in patients with carotid stenosis, while Dr. Sillesen will examine if stenosis or plaque progression are reasons to treat asymptomatic patients with carotid artery stenting (CAS) versus carotid artery endarterectomy (CEA).
Another theme is looking at efforts to reduce perioperative stroke rates after carotid stenting, Dr. Naylor said: “One of the repeated findings is that the death and stroke rates are lower following carotid surgery rather than carotid stenting. Registries suggest that in a large number of series, stroke rates actually exceed the accepted risks for treating patients with asymptomatic disease, which is 3%, or for symptomatic disease, which is 6%.”
Dr. William A. Gray of Jefferson Medical College and Main Line Health will discuss technical strategies that might be used to reduce perioperative stroke rates, including new techniques and devices such as the double-filter Paladin device. Dr. L. Nelson Hopkins, SUNY Distinguished Professor of Neurosurgery and Radiology, University at Buffalo, will discuss how strokes after CAS and other interventional procedures have greater cognitive deficits than previously thought, even with full neurological recovery.
The discussions will conclude with a presentation by Dr. Mark H. Wholey of the University of Pittsburgh Medical Center, Shady Side, on the etiology, diagnosis and treatment of vertebral artery dissections. “It is so vanishingly rare that we are asked to treat this that almost nobody has any experience,” Dr. Naylor said. “I suspect this will be quite an interesting talk for the audience.”
Co-moderators for the session will be Dr. James May, Emeritus Bosch Professor of Surgery and associated dean of surgical sciences at the University of Sydney, and a vascular surgeon at Royal Prince Alfred Hospital; Dr. Wesley S. Moore, professor and chief emeritus of vascular surgery at UCLA Medical Center; and Dr. Enrico Ascher, chief of vascular surgery at NYU Hospitals, and professor of surgery at New York University.
ACA repeal could mean financial ruin for many MI, stroke patients
ANAHEIM, CALIF. – Before the Affordable Care Act, over 1 in 8 people under 60 years old hospitalized for acute myocardial infarction or stroke had no insurance, and it ruined most of them financially, according to an analysis presented at the American Heart Association scientific sessions.
The importance of the study is that it shows what could happen if the ACA goes away. Debate over its future is “all about pushing people off insurance.” Plans floated in early 2017 “would have increased the uninsured rate to 49 million people,” said lead investigators Rohan Khera, MD, a cardiology fellow at the University of Texas Southwestern Medical Center, Dallas.
And even under the ACA, there are still about 27 million people in the United States, about 8.6% of the population, who don’t have health insurance. Although that’s down from about 44 million people (14.5%) before the ACA, a considerable number of people still face financial ruin if they have a serious medical problem. “Until there is universal coverage for those without resources, catastrophic illness will remain a disabling financial threat to many Americans,” Dr. Khera said.
In a review of the National Inpatient Sample, the investigators identified 39,296 acute myocardial infarction (AMI) and 29,182 stroke hospitalizations among people aged 18-60 years with no insurance from 2008 to 2012, which corresponded to 188,192 AMI and 139,687 stroke hospitalizations nationwide. Overall, the uninsured made up 15% of AMI and stroke hospitalizations among the nonelderly.
By using U.S. Census data to estimate annual income, and U.S. Bureau of Labor Statistics data to estimate food costs, the team found that the median hospital charge for AMI – $53,384 – exceeded 40% of the annual income left after food costs in 85% of uninsured subjects. The median stroke bill – $31,218 – exceeded 40% of what was left over after food in 75%. The situation was deemed “catastrophic” in both instances.
It’s true that hospitalization costs might have been reduced or waived in some cases, but the analysis did not consider missed work, disability, and outpatient costs. If anything, the financial burden on the uninsured was underestimated, Dr. Khera said.
The work was funded by the National Institutes of Health, and published in Circulation (2018 Nov 13. doi: 10.1161/CIRCULATIONAHA.117.030128) to coincide with the presentation. Dr. Khera had no disclosures.
ANAHEIM, CALIF. – Before the Affordable Care Act, over 1 in 8 people under 60 years old hospitalized for acute myocardial infarction or stroke had no insurance, and it ruined most of them financially, according to an analysis presented at the American Heart Association scientific sessions.
The importance of the study is that it shows what could happen if the ACA goes away. Debate over its future is “all about pushing people off insurance.” Plans floated in early 2017 “would have increased the uninsured rate to 49 million people,” said lead investigators Rohan Khera, MD, a cardiology fellow at the University of Texas Southwestern Medical Center, Dallas.
And even under the ACA, there are still about 27 million people in the United States, about 8.6% of the population, who don’t have health insurance. Although that’s down from about 44 million people (14.5%) before the ACA, a considerable number of people still face financial ruin if they have a serious medical problem. “Until there is universal coverage for those without resources, catastrophic illness will remain a disabling financial threat to many Americans,” Dr. Khera said.
In a review of the National Inpatient Sample, the investigators identified 39,296 acute myocardial infarction (AMI) and 29,182 stroke hospitalizations among people aged 18-60 years with no insurance from 2008 to 2012, which corresponded to 188,192 AMI and 139,687 stroke hospitalizations nationwide. Overall, the uninsured made up 15% of AMI and stroke hospitalizations among the nonelderly.
By using U.S. Census data to estimate annual income, and U.S. Bureau of Labor Statistics data to estimate food costs, the team found that the median hospital charge for AMI – $53,384 – exceeded 40% of the annual income left after food costs in 85% of uninsured subjects. The median stroke bill – $31,218 – exceeded 40% of what was left over after food in 75%. The situation was deemed “catastrophic” in both instances.
It’s true that hospitalization costs might have been reduced or waived in some cases, but the analysis did not consider missed work, disability, and outpatient costs. If anything, the financial burden on the uninsured was underestimated, Dr. Khera said.
The work was funded by the National Institutes of Health, and published in Circulation (2018 Nov 13. doi: 10.1161/CIRCULATIONAHA.117.030128) to coincide with the presentation. Dr. Khera had no disclosures.
ANAHEIM, CALIF. – Before the Affordable Care Act, over 1 in 8 people under 60 years old hospitalized for acute myocardial infarction or stroke had no insurance, and it ruined most of them financially, according to an analysis presented at the American Heart Association scientific sessions.
The importance of the study is that it shows what could happen if the ACA goes away. Debate over its future is “all about pushing people off insurance.” Plans floated in early 2017 “would have increased the uninsured rate to 49 million people,” said lead investigators Rohan Khera, MD, a cardiology fellow at the University of Texas Southwestern Medical Center, Dallas.
And even under the ACA, there are still about 27 million people in the United States, about 8.6% of the population, who don’t have health insurance. Although that’s down from about 44 million people (14.5%) before the ACA, a considerable number of people still face financial ruin if they have a serious medical problem. “Until there is universal coverage for those without resources, catastrophic illness will remain a disabling financial threat to many Americans,” Dr. Khera said.
In a review of the National Inpatient Sample, the investigators identified 39,296 acute myocardial infarction (AMI) and 29,182 stroke hospitalizations among people aged 18-60 years with no insurance from 2008 to 2012, which corresponded to 188,192 AMI and 139,687 stroke hospitalizations nationwide. Overall, the uninsured made up 15% of AMI and stroke hospitalizations among the nonelderly.
By using U.S. Census data to estimate annual income, and U.S. Bureau of Labor Statistics data to estimate food costs, the team found that the median hospital charge for AMI – $53,384 – exceeded 40% of the annual income left after food costs in 85% of uninsured subjects. The median stroke bill – $31,218 – exceeded 40% of what was left over after food in 75%. The situation was deemed “catastrophic” in both instances.
It’s true that hospitalization costs might have been reduced or waived in some cases, but the analysis did not consider missed work, disability, and outpatient costs. If anything, the financial burden on the uninsured was underestimated, Dr. Khera said.
The work was funded by the National Institutes of Health, and published in Circulation (2018 Nov 13. doi: 10.1161/CIRCULATIONAHA.117.030128) to coincide with the presentation. Dr. Khera had no disclosures.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: The median hospital charge for AMI – $53,384 – exceeded 40% of the annual income left after food costs in 85% of uninsured subjects.
Data source: Modeling study using the National Impatient Sample, U.S. Census data, and U.S. Bureau of Labor Statistics data.
Disclosures: The work was funded by the National Institutes of Health. The lead investigator didn’t have any disclosures.
Gut bacteria influenced response to checkpoint inhibitors
The gut microbome may influence responses to immune checkpoint inhibitors, based on results from two studies, and one of the investigators is now gearing up for the next step - evaluating in a clinical trial whether altering the microflora will actually improve responses.
In the first study, investigators carried out a series of experiments using fecal microbiome samples from patients with metastatic melanoma embarking on therapy with a PD-1 (programmed cell death protein 1) inhibitor.
“In melanoma patients, there were differential signals in the gut microbiome of responders versus nonresponders, and I think the clincher was when we transplanted fecal samples from responders to nonresponders in germ-free mice, essentially reconstituting the microbiome and showing that it equally affected the systemic immunity and antitumor immunity when we implanted tumors, as well as response to checkpoint blockade,” lead author Jennifer A. Wargo, MD, MMSc, of the University of Texas MD Anderson Cancer Center in Houston, said in an interview.
Dr. Wargo and her colleagues first collected buccal and fecal microbiome samples from 112 patients with metastatic melanoma before they began therapy with a PD-1 inhibitor. After performing taxonomic profiling on all samples, they found that there was a clustering effect by response status in the gut microbiome, but not the oral microbiome, and because changes in the oral microbiome did not appear to be related to treatment response, they focused on the gut.
When Dr. Wargo and her colleagues studied the posttherapy microbiomes of 43 patients (30 responders and 13 nonresponders) according to Response Evaluation Criteria in Solid Tumors (RECIST 1.1), they found that the responders had a significantly higher degree of alpha diversity, a measure of species diversity within a specific environment, compared with nonresponders (P less than .01). In addition, responders had a relative abundance of Ruminococcaceae, commonly occurring gut microbes that break down complex carbohydrates, the investigators reported (Science. 2017 Nov. 2. doi: 10.1126/science.aan4236).
They found that patients whose microbiomes were diverse in general, and in particular were enriched with Faecalibacterium and Clostridiales species, were more likely to respond to immunotherapy with a PD-1 inhibitor and have a longer duration of progression-free survival. In contrast, patients whose microbiomes were more enriched with Bacteroidales species were more likely to be nonresponders.
To get a better understanding of the mechanisms whereby gut bacteria may influence response to PD-1 inhibitors, they performed metagenomic analysis on samples from 14 responders and 11 nonresponders, and found that responders had micro-organisms predominantly associated with anabolic functions that may support host immunity, whereas nonresponders had microbiomes where catabolic functions were more common.
The investigators next performed immune profiling, and found that both systemic immunity and local immunity in the tumor microenvironment in responders were associated with the aforementioned favorable gut microbiome.
The researchers then transplanted feces from the human donors into germ-free mice and then injected tumor cells into the mice, and found that tumor growth was significantly reduced, and response to PD-1 inhibition was significantly enhanced, in mice who received feces from responders.
“An obvious next step is to run a clinical trial to test the hypothesis that by modulating the microbiome, you can actually enhance responses to therapy,” Dr. Wargo said. Details of the clinical trial are still being worked out, but will likely involve fecal transfers and other mechanisms for modulating the microbiome in hopes of improving responses to PD-1 inhibitors.
“It’s going to be a very biomarker-heavy trial,” she said. “We’re going to look, certainly, for changes in the microbiome, and will also do a lot of profiling in the blood, the tumor, and in the microbiome to see if there are changes that occur by modulating that microbiome. Then of course we’ll look for differences in response rates in patients as well.”
Bacteria also affect epithelial cancers
In a separate study, also published in Science, investigators led by Bertrand Routy, MD, of the Gustave Roussy Cancer Institute in Villejuif, France, reported that patients with non–small cell lung cancer and urothelial carcinoma who had previously used systemic antibiotics had reduced survival when treated with a PD-1 inhibitor, compared with patients who had never taken antibiotics (Science. 2017 Nov. 2 doi: 10.1126/science.aan3706).
Analysis of the gut microbiome in these patients showed that higher levels of Akkermansia muciniphila were associated with the best clinical outcomes, with the species detectable in the microbiome of 69% of patients who had partial responses to anti–PD-1 therapy, and in 58% of those with stable disease. In contrast, the bacterium was detectable in only 34% of patients who experienced disease progression.
As in the experiments by Dr. Wargo and her associates, when the French investigators first treated mice with antibiotics and then gave them oral supplements containing the bacteria, the supplements restored response to PD-1 blockade,
“We conclude from the study that the gut microbiome markedly influences the outcome of PD-1 blockade in mice and patients,” Dr. Routy and his associates wrote.
They acknowledged that the mechanism whereby a common organism such as Akkermansia muciniphila might have an immunomodulatory effect is still unknown,
“Irrespective of these remaining questions, our findings suggest that the microbiome governs the cancer-immune set point of cancer-bearing individuals and offer[s] novel avenues for manipulating the gut ecosystem to circumvent primary resistance to [immune checkpoint inhibitors],” they wrote.
The study by Dr. Wargo and her colleagues was supported by contributions to the University of Texas MD Anderson Melanoma Moon Shots program. Dr. Wargo is supported by the Binational Science Foundation, Melanoma Research Alliance, Stand Up to Cancer, and the MDACC Melanoma Moon Shots Program. The work by Dr. Routy and his associates was supported by the Goustave Roussy Cancer Institute and McGill University. Coauthors were supported by the National Cancer Institute of France and other agencies and philanthropies.
The gut microbome may influence responses to immune checkpoint inhibitors, based on results from two studies, and one of the investigators is now gearing up for the next step - evaluating in a clinical trial whether altering the microflora will actually improve responses.
In the first study, investigators carried out a series of experiments using fecal microbiome samples from patients with metastatic melanoma embarking on therapy with a PD-1 (programmed cell death protein 1) inhibitor.
“In melanoma patients, there were differential signals in the gut microbiome of responders versus nonresponders, and I think the clincher was when we transplanted fecal samples from responders to nonresponders in germ-free mice, essentially reconstituting the microbiome and showing that it equally affected the systemic immunity and antitumor immunity when we implanted tumors, as well as response to checkpoint blockade,” lead author Jennifer A. Wargo, MD, MMSc, of the University of Texas MD Anderson Cancer Center in Houston, said in an interview.
Dr. Wargo and her colleagues first collected buccal and fecal microbiome samples from 112 patients with metastatic melanoma before they began therapy with a PD-1 inhibitor. After performing taxonomic profiling on all samples, they found that there was a clustering effect by response status in the gut microbiome, but not the oral microbiome, and because changes in the oral microbiome did not appear to be related to treatment response, they focused on the gut.
When Dr. Wargo and her colleagues studied the posttherapy microbiomes of 43 patients (30 responders and 13 nonresponders) according to Response Evaluation Criteria in Solid Tumors (RECIST 1.1), they found that the responders had a significantly higher degree of alpha diversity, a measure of species diversity within a specific environment, compared with nonresponders (P less than .01). In addition, responders had a relative abundance of Ruminococcaceae, commonly occurring gut microbes that break down complex carbohydrates, the investigators reported (Science. 2017 Nov. 2. doi: 10.1126/science.aan4236).
They found that patients whose microbiomes were diverse in general, and in particular were enriched with Faecalibacterium and Clostridiales species, were more likely to respond to immunotherapy with a PD-1 inhibitor and have a longer duration of progression-free survival. In contrast, patients whose microbiomes were more enriched with Bacteroidales species were more likely to be nonresponders.
To get a better understanding of the mechanisms whereby gut bacteria may influence response to PD-1 inhibitors, they performed metagenomic analysis on samples from 14 responders and 11 nonresponders, and found that responders had micro-organisms predominantly associated with anabolic functions that may support host immunity, whereas nonresponders had microbiomes where catabolic functions were more common.
The investigators next performed immune profiling, and found that both systemic immunity and local immunity in the tumor microenvironment in responders were associated with the aforementioned favorable gut microbiome.
The researchers then transplanted feces from the human donors into germ-free mice and then injected tumor cells into the mice, and found that tumor growth was significantly reduced, and response to PD-1 inhibition was significantly enhanced, in mice who received feces from responders.
“An obvious next step is to run a clinical trial to test the hypothesis that by modulating the microbiome, you can actually enhance responses to therapy,” Dr. Wargo said. Details of the clinical trial are still being worked out, but will likely involve fecal transfers and other mechanisms for modulating the microbiome in hopes of improving responses to PD-1 inhibitors.
“It’s going to be a very biomarker-heavy trial,” she said. “We’re going to look, certainly, for changes in the microbiome, and will also do a lot of profiling in the blood, the tumor, and in the microbiome to see if there are changes that occur by modulating that microbiome. Then of course we’ll look for differences in response rates in patients as well.”
Bacteria also affect epithelial cancers
In a separate study, also published in Science, investigators led by Bertrand Routy, MD, of the Gustave Roussy Cancer Institute in Villejuif, France, reported that patients with non–small cell lung cancer and urothelial carcinoma who had previously used systemic antibiotics had reduced survival when treated with a PD-1 inhibitor, compared with patients who had never taken antibiotics (Science. 2017 Nov. 2 doi: 10.1126/science.aan3706).
Analysis of the gut microbiome in these patients showed that higher levels of Akkermansia muciniphila were associated with the best clinical outcomes, with the species detectable in the microbiome of 69% of patients who had partial responses to anti–PD-1 therapy, and in 58% of those with stable disease. In contrast, the bacterium was detectable in only 34% of patients who experienced disease progression.
As in the experiments by Dr. Wargo and her associates, when the French investigators first treated mice with antibiotics and then gave them oral supplements containing the bacteria, the supplements restored response to PD-1 blockade,
“We conclude from the study that the gut microbiome markedly influences the outcome of PD-1 blockade in mice and patients,” Dr. Routy and his associates wrote.
They acknowledged that the mechanism whereby a common organism such as Akkermansia muciniphila might have an immunomodulatory effect is still unknown,
“Irrespective of these remaining questions, our findings suggest that the microbiome governs the cancer-immune set point of cancer-bearing individuals and offer[s] novel avenues for manipulating the gut ecosystem to circumvent primary resistance to [immune checkpoint inhibitors],” they wrote.
The study by Dr. Wargo and her colleagues was supported by contributions to the University of Texas MD Anderson Melanoma Moon Shots program. Dr. Wargo is supported by the Binational Science Foundation, Melanoma Research Alliance, Stand Up to Cancer, and the MDACC Melanoma Moon Shots Program. The work by Dr. Routy and his associates was supported by the Goustave Roussy Cancer Institute and McGill University. Coauthors were supported by the National Cancer Institute of France and other agencies and philanthropies.
The gut microbome may influence responses to immune checkpoint inhibitors, based on results from two studies, and one of the investigators is now gearing up for the next step - evaluating in a clinical trial whether altering the microflora will actually improve responses.
In the first study, investigators carried out a series of experiments using fecal microbiome samples from patients with metastatic melanoma embarking on therapy with a PD-1 (programmed cell death protein 1) inhibitor.
“In melanoma patients, there were differential signals in the gut microbiome of responders versus nonresponders, and I think the clincher was when we transplanted fecal samples from responders to nonresponders in germ-free mice, essentially reconstituting the microbiome and showing that it equally affected the systemic immunity and antitumor immunity when we implanted tumors, as well as response to checkpoint blockade,” lead author Jennifer A. Wargo, MD, MMSc, of the University of Texas MD Anderson Cancer Center in Houston, said in an interview.
Dr. Wargo and her colleagues first collected buccal and fecal microbiome samples from 112 patients with metastatic melanoma before they began therapy with a PD-1 inhibitor. After performing taxonomic profiling on all samples, they found that there was a clustering effect by response status in the gut microbiome, but not the oral microbiome, and because changes in the oral microbiome did not appear to be related to treatment response, they focused on the gut.
When Dr. Wargo and her colleagues studied the posttherapy microbiomes of 43 patients (30 responders and 13 nonresponders) according to Response Evaluation Criteria in Solid Tumors (RECIST 1.1), they found that the responders had a significantly higher degree of alpha diversity, a measure of species diversity within a specific environment, compared with nonresponders (P less than .01). In addition, responders had a relative abundance of Ruminococcaceae, commonly occurring gut microbes that break down complex carbohydrates, the investigators reported (Science. 2017 Nov. 2. doi: 10.1126/science.aan4236).
They found that patients whose microbiomes were diverse in general, and in particular were enriched with Faecalibacterium and Clostridiales species, were more likely to respond to immunotherapy with a PD-1 inhibitor and have a longer duration of progression-free survival. In contrast, patients whose microbiomes were more enriched with Bacteroidales species were more likely to be nonresponders.
To get a better understanding of the mechanisms whereby gut bacteria may influence response to PD-1 inhibitors, they performed metagenomic analysis on samples from 14 responders and 11 nonresponders, and found that responders had micro-organisms predominantly associated with anabolic functions that may support host immunity, whereas nonresponders had microbiomes where catabolic functions were more common.
The investigators next performed immune profiling, and found that both systemic immunity and local immunity in the tumor microenvironment in responders were associated with the aforementioned favorable gut microbiome.
The researchers then transplanted feces from the human donors into germ-free mice and then injected tumor cells into the mice, and found that tumor growth was significantly reduced, and response to PD-1 inhibition was significantly enhanced, in mice who received feces from responders.
“An obvious next step is to run a clinical trial to test the hypothesis that by modulating the microbiome, you can actually enhance responses to therapy,” Dr. Wargo said. Details of the clinical trial are still being worked out, but will likely involve fecal transfers and other mechanisms for modulating the microbiome in hopes of improving responses to PD-1 inhibitors.
“It’s going to be a very biomarker-heavy trial,” she said. “We’re going to look, certainly, for changes in the microbiome, and will also do a lot of profiling in the blood, the tumor, and in the microbiome to see if there are changes that occur by modulating that microbiome. Then of course we’ll look for differences in response rates in patients as well.”
Bacteria also affect epithelial cancers
In a separate study, also published in Science, investigators led by Bertrand Routy, MD, of the Gustave Roussy Cancer Institute in Villejuif, France, reported that patients with non–small cell lung cancer and urothelial carcinoma who had previously used systemic antibiotics had reduced survival when treated with a PD-1 inhibitor, compared with patients who had never taken antibiotics (Science. 2017 Nov. 2 doi: 10.1126/science.aan3706).
Analysis of the gut microbiome in these patients showed that higher levels of Akkermansia muciniphila were associated with the best clinical outcomes, with the species detectable in the microbiome of 69% of patients who had partial responses to anti–PD-1 therapy, and in 58% of those with stable disease. In contrast, the bacterium was detectable in only 34% of patients who experienced disease progression.
As in the experiments by Dr. Wargo and her associates, when the French investigators first treated mice with antibiotics and then gave them oral supplements containing the bacteria, the supplements restored response to PD-1 blockade,
“We conclude from the study that the gut microbiome markedly influences the outcome of PD-1 blockade in mice and patients,” Dr. Routy and his associates wrote.
They acknowledged that the mechanism whereby a common organism such as Akkermansia muciniphila might have an immunomodulatory effect is still unknown,
“Irrespective of these remaining questions, our findings suggest that the microbiome governs the cancer-immune set point of cancer-bearing individuals and offer[s] novel avenues for manipulating the gut ecosystem to circumvent primary resistance to [immune checkpoint inhibitors],” they wrote.
The study by Dr. Wargo and her colleagues was supported by contributions to the University of Texas MD Anderson Melanoma Moon Shots program. Dr. Wargo is supported by the Binational Science Foundation, Melanoma Research Alliance, Stand Up to Cancer, and the MDACC Melanoma Moon Shots Program. The work by Dr. Routy and his associates was supported by the Goustave Roussy Cancer Institute and McGill University. Coauthors were supported by the National Cancer Institute of France and other agencies and philanthropies.
FROM SCIENCE
Key clinical point: Modulating the gut microbome may improve responses to immune checkpoint inhibitors in patients with advanced melanoma, non–small cell lung cancer, and urothelial carcinoma.
Major finding: Responders to a checkpoint inhibitor had a significantly higher degree of alpha diversity, a measure of species diversity within a specific environment, compared with nonresponders (P less than .01).
Data source: A series of studies using microbiome samples from cancer patients receiving immune checkpoint inhibitors.
Disclosures: The study by Dr. Wargo and her colleagues was supported by contributions to the University of Texas MD Anderson Melanoma Moon Shots Program. Dr. Wargo is supported by the Binational Science Foundation, Melanoma Research Alliance, Stand Up to Cancer, and the MDACC Melanoma Moon Shots Program. The work by Dr. Routy and his colleagues was supported by the Goustave Roussy Cancer Institute and McGill University. Coauthors were supported by the National Cancer Institute of France and other agencies and philanthropies.
VIDEO: New vaccines target global infections
LAS VEGAS – There are now vaccines for two infectious diseases that “are not purely dermatologic, but have a great impact on many patients around the world,” Kenneth J. Tomecki, MD, said in a video interview at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
The recent development of a vaccine to protect against malaria, with an efficacy around 40%, “is something we should all be proud of,” said Dr. Tomecki of the Cleveland Clinic. Another advance is that there is now also a vaccine for Ebola that is so effective, it is being stockpiled in African countries in preparation for future Ebola outbreaks, he added.
Dr. Tomecki had no financial conflicts to disclose.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – There are now vaccines for two infectious diseases that “are not purely dermatologic, but have a great impact on many patients around the world,” Kenneth J. Tomecki, MD, said in a video interview at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
The recent development of a vaccine to protect against malaria, with an efficacy around 40%, “is something we should all be proud of,” said Dr. Tomecki of the Cleveland Clinic. Another advance is that there is now also a vaccine for Ebola that is so effective, it is being stockpiled in African countries in preparation for future Ebola outbreaks, he added.
Dr. Tomecki had no financial conflicts to disclose.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – There are now vaccines for two infectious diseases that “are not purely dermatologic, but have a great impact on many patients around the world,” Kenneth J. Tomecki, MD, said in a video interview at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
The recent development of a vaccine to protect against malaria, with an efficacy around 40%, “is something we should all be proud of,” said Dr. Tomecki of the Cleveland Clinic. Another advance is that there is now also a vaccine for Ebola that is so effective, it is being stockpiled in African countries in preparation for future Ebola outbreaks, he added.
Dr. Tomecki had no financial conflicts to disclose.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
Stem cells spark successful skin regeneration
Junctional epidermolysis bullosa is a genetic disease characterized by chronic skin wounds, blisters, and erosions. The chronic wounds not only increase a patient’s risk of skin cancer, they also can cause itching, pain, limited mobility, and poor quality of life, wrote Tobias Hirsch, MD, of BG University Hospital Bergmannsheil, Bochum, Germany, and colleagues (Nature. 2017. doi: 10.1038/nature24487). There is no cure for the disease, and more than 40% of patients die prior to adolescence.
Previous studies have shown that epidermal stem cells can be used to repair a damaged epidermis, they noted. However, the technique has been criticized for being insufficient to treat the large lesions common to this disease.
The researchers described the case of a 7-year-old boy who was admitted to a children’s hospital in Germany in June 2015 with junctional epidermolysis bullosa so severe that approximately 80% of his total body surface area was affected. The patient had a genetic mutation that had resulted in blisters on much of his body since birth. Approximately 6 weeks prior to his hospital admission, he developed Staphylococcus aureus and Pseudomonas aeruginosa infections that worsened his condition. After other treatments failed, the patient’s parents consented to a combination of ex vivo cell and gene therapy, in which cultures taken from a biopsy of uninvolved skin were used to develop transgenic epidermal grafts. The grafts were applied sequentially on a dermal wound bed.
“Virtually complete epidermal regeneration was observed after 1 month,” Dr. Hirsch and associates wrote. Over 21 months, the regenerated epidermis healed and remained stable even when subjected to mechanical stress.
For follow-up, the researchers reported on 10 punch biopsies taken at 4, 8, and 21 months after the grafting procedure. “The epidermis had normal morphology and we could not detect blisters, erosions, or epidermal detachment from the underlying dermis,” they noted.
The patient has remained stable since being discharged from the hospital in February 2016, and requires no ointment or medications to maintain a healthy epidermis, they said.
“This approach would be optimal for newly diagnosed patients early in their childhood,” Dr. Hirsch and associates noted. “A bank of transduced epidermal stem cells taken at birth could be used to treat skin lesions while they develop, thus preventing, rather than restoring, the devastating clinical manifestation that arise in these patients.
The study was supported in part by several government grants from organizations including the Italian Ministry of Education and the European Research. Two of the researchers are cofounders and members of the Board of Directors of Holostem Terapie Avanzate, which met all costs of good manufacturing practice production and procedures of transgenic epidermal grafts.
Junctional epidermolysis bullosa is a genetic disease characterized by chronic skin wounds, blisters, and erosions. The chronic wounds not only increase a patient’s risk of skin cancer, they also can cause itching, pain, limited mobility, and poor quality of life, wrote Tobias Hirsch, MD, of BG University Hospital Bergmannsheil, Bochum, Germany, and colleagues (Nature. 2017. doi: 10.1038/nature24487). There is no cure for the disease, and more than 40% of patients die prior to adolescence.
Previous studies have shown that epidermal stem cells can be used to repair a damaged epidermis, they noted. However, the technique has been criticized for being insufficient to treat the large lesions common to this disease.
The researchers described the case of a 7-year-old boy who was admitted to a children’s hospital in Germany in June 2015 with junctional epidermolysis bullosa so severe that approximately 80% of his total body surface area was affected. The patient had a genetic mutation that had resulted in blisters on much of his body since birth. Approximately 6 weeks prior to his hospital admission, he developed Staphylococcus aureus and Pseudomonas aeruginosa infections that worsened his condition. After other treatments failed, the patient’s parents consented to a combination of ex vivo cell and gene therapy, in which cultures taken from a biopsy of uninvolved skin were used to develop transgenic epidermal grafts. The grafts were applied sequentially on a dermal wound bed.
“Virtually complete epidermal regeneration was observed after 1 month,” Dr. Hirsch and associates wrote. Over 21 months, the regenerated epidermis healed and remained stable even when subjected to mechanical stress.
For follow-up, the researchers reported on 10 punch biopsies taken at 4, 8, and 21 months after the grafting procedure. “The epidermis had normal morphology and we could not detect blisters, erosions, or epidermal detachment from the underlying dermis,” they noted.
The patient has remained stable since being discharged from the hospital in February 2016, and requires no ointment or medications to maintain a healthy epidermis, they said.
“This approach would be optimal for newly diagnosed patients early in their childhood,” Dr. Hirsch and associates noted. “A bank of transduced epidermal stem cells taken at birth could be used to treat skin lesions while they develop, thus preventing, rather than restoring, the devastating clinical manifestation that arise in these patients.
The study was supported in part by several government grants from organizations including the Italian Ministry of Education and the European Research. Two of the researchers are cofounders and members of the Board of Directors of Holostem Terapie Avanzate, which met all costs of good manufacturing practice production and procedures of transgenic epidermal grafts.
Junctional epidermolysis bullosa is a genetic disease characterized by chronic skin wounds, blisters, and erosions. The chronic wounds not only increase a patient’s risk of skin cancer, they also can cause itching, pain, limited mobility, and poor quality of life, wrote Tobias Hirsch, MD, of BG University Hospital Bergmannsheil, Bochum, Germany, and colleagues (Nature. 2017. doi: 10.1038/nature24487). There is no cure for the disease, and more than 40% of patients die prior to adolescence.
Previous studies have shown that epidermal stem cells can be used to repair a damaged epidermis, they noted. However, the technique has been criticized for being insufficient to treat the large lesions common to this disease.
The researchers described the case of a 7-year-old boy who was admitted to a children’s hospital in Germany in June 2015 with junctional epidermolysis bullosa so severe that approximately 80% of his total body surface area was affected. The patient had a genetic mutation that had resulted in blisters on much of his body since birth. Approximately 6 weeks prior to his hospital admission, he developed Staphylococcus aureus and Pseudomonas aeruginosa infections that worsened his condition. After other treatments failed, the patient’s parents consented to a combination of ex vivo cell and gene therapy, in which cultures taken from a biopsy of uninvolved skin were used to develop transgenic epidermal grafts. The grafts were applied sequentially on a dermal wound bed.
“Virtually complete epidermal regeneration was observed after 1 month,” Dr. Hirsch and associates wrote. Over 21 months, the regenerated epidermis healed and remained stable even when subjected to mechanical stress.
For follow-up, the researchers reported on 10 punch biopsies taken at 4, 8, and 21 months after the grafting procedure. “The epidermis had normal morphology and we could not detect blisters, erosions, or epidermal detachment from the underlying dermis,” they noted.
The patient has remained stable since being discharged from the hospital in February 2016, and requires no ointment or medications to maintain a healthy epidermis, they said.
“This approach would be optimal for newly diagnosed patients early in their childhood,” Dr. Hirsch and associates noted. “A bank of transduced epidermal stem cells taken at birth could be used to treat skin lesions while they develop, thus preventing, rather than restoring, the devastating clinical manifestation that arise in these patients.
The study was supported in part by several government grants from organizations including the Italian Ministry of Education and the European Research. Two of the researchers are cofounders and members of the Board of Directors of Holostem Terapie Avanzate, which met all costs of good manufacturing practice production and procedures of transgenic epidermal grafts.
FROM NATURE