User login
Your online reputation
Have you ever run across an unfair or even malicious comment about you or your practice on one of those “doctor-rating” web sites? Some curmudgeon, angry about something totally irrelevant to your clinical skills, decided to publicly trash you; and the site, of course, made no effort to authenticate the writer or fact-check the complaint.
What to do? You could hire one of the many companies in the rapidly burgeoning field of online reputation management; but that can cost hundreds to thousands of dollars per month for monitoring and intervention, and there are no guarantees of success.
Leave design and SEO to the pros, but don’t delegate the content itself; as captain of the ship you are responsible for all the facts and opinions on your site. And remember that, once it’s online, it’s online forever; consider the ramifications of anything you post on any site – yours or others – before hitting the “send” button. “The most damaging item about you,” one consultant told me, “could well be something you posted yourself.” Just ask any of several prominent politicians who have famously sabotaged their own careers online.
That said, don’t be shy about creating content. Patients appreciate factual information, but they value your opinions too. Add a blog to your web site and write about subjects – medical and otherwise – that interest you. If you have expertise in a particular field, be sure to write about that.
Incidentally, if the URL for your web site is not your own name, you should register your name as a separate domain name – even if you never use it – to be sure that a trickster or troll, or someone with the same name but a bad reputation, doesn’t get it.
A web site is a powerful resource, but not the only one. Take advantage of Google’s free profiling tool at https://profiles.google.com/me, where you can create a sterling bio, complete with links to URLs, photos, and anything else that shows you in the best possible light. Your Google profile will, of course, be at or near the top of any Google search.
Wikipedia articles also go to the top of most searches, so if you’re notable enough to merit mention in one – or to have one of your own – see that it is done and updated regularly. Remember that Wikipedia’s conflict of interest rules forbid adding or editing content about yourself, so someone with a theoretically “neutral point of view” will have to do it for you.
Other useful resources are the social networking sites. Whatever your opinion of online networks, the reality is that personal pages on Facebook, LinkedIn, and Twitter rank very high on major search engines. (Some consultants say a favorable LinkedIn profile is particularly helpful because of that site’s reputation as a “professional” network.) Make your (noncontroversial) opinions known on these portals. Your community activities, charitable work, interesting hobbies – anything that casts you in a favorable light – also need to be mentioned prominently.
Set up an RSS news feed for yourself (directions to follow in the next two columns), so you’ll know immediately if your name pops up in news or gossip sites, or on blogs. If something untrue is posted about you, take action. Reputable news sites and blogs have their own reputations to protect and can usually be persuaded to correct anything that is demonstrably false. Try to get the error removed entirely or corrected within the original article. An erratum on the last page of the next edition will be ignored and will leave the false information online, intact.
Doctor-rating sites typically refuse to remove unfair comments unless they are blatantly libelous or a case of mistaken identity; but there is nothing wrong with encouraging happy patients to post favorable reviews on those sites. Sauce for the goose, and all that.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Have you ever run across an unfair or even malicious comment about you or your practice on one of those “doctor-rating” web sites? Some curmudgeon, angry about something totally irrelevant to your clinical skills, decided to publicly trash you; and the site, of course, made no effort to authenticate the writer or fact-check the complaint.
What to do? You could hire one of the many companies in the rapidly burgeoning field of online reputation management; but that can cost hundreds to thousands of dollars per month for monitoring and intervention, and there are no guarantees of success.
Leave design and SEO to the pros, but don’t delegate the content itself; as captain of the ship you are responsible for all the facts and opinions on your site. And remember that, once it’s online, it’s online forever; consider the ramifications of anything you post on any site – yours or others – before hitting the “send” button. “The most damaging item about you,” one consultant told me, “could well be something you posted yourself.” Just ask any of several prominent politicians who have famously sabotaged their own careers online.
That said, don’t be shy about creating content. Patients appreciate factual information, but they value your opinions too. Add a blog to your web site and write about subjects – medical and otherwise – that interest you. If you have expertise in a particular field, be sure to write about that.
Incidentally, if the URL for your web site is not your own name, you should register your name as a separate domain name – even if you never use it – to be sure that a trickster or troll, or someone with the same name but a bad reputation, doesn’t get it.
A web site is a powerful resource, but not the only one. Take advantage of Google’s free profiling tool at https://profiles.google.com/me, where you can create a sterling bio, complete with links to URLs, photos, and anything else that shows you in the best possible light. Your Google profile will, of course, be at or near the top of any Google search.
Wikipedia articles also go to the top of most searches, so if you’re notable enough to merit mention in one – or to have one of your own – see that it is done and updated regularly. Remember that Wikipedia’s conflict of interest rules forbid adding or editing content about yourself, so someone with a theoretically “neutral point of view” will have to do it for you.
Other useful resources are the social networking sites. Whatever your opinion of online networks, the reality is that personal pages on Facebook, LinkedIn, and Twitter rank very high on major search engines. (Some consultants say a favorable LinkedIn profile is particularly helpful because of that site’s reputation as a “professional” network.) Make your (noncontroversial) opinions known on these portals. Your community activities, charitable work, interesting hobbies – anything that casts you in a favorable light – also need to be mentioned prominently.
Set up an RSS news feed for yourself (directions to follow in the next two columns), so you’ll know immediately if your name pops up in news or gossip sites, or on blogs. If something untrue is posted about you, take action. Reputable news sites and blogs have their own reputations to protect and can usually be persuaded to correct anything that is demonstrably false. Try to get the error removed entirely or corrected within the original article. An erratum on the last page of the next edition will be ignored and will leave the false information online, intact.
Doctor-rating sites typically refuse to remove unfair comments unless they are blatantly libelous or a case of mistaken identity; but there is nothing wrong with encouraging happy patients to post favorable reviews on those sites. Sauce for the goose, and all that.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Have you ever run across an unfair or even malicious comment about you or your practice on one of those “doctor-rating” web sites? Some curmudgeon, angry about something totally irrelevant to your clinical skills, decided to publicly trash you; and the site, of course, made no effort to authenticate the writer or fact-check the complaint.
What to do? You could hire one of the many companies in the rapidly burgeoning field of online reputation management; but that can cost hundreds to thousands of dollars per month for monitoring and intervention, and there are no guarantees of success.
Leave design and SEO to the pros, but don’t delegate the content itself; as captain of the ship you are responsible for all the facts and opinions on your site. And remember that, once it’s online, it’s online forever; consider the ramifications of anything you post on any site – yours or others – before hitting the “send” button. “The most damaging item about you,” one consultant told me, “could well be something you posted yourself.” Just ask any of several prominent politicians who have famously sabotaged their own careers online.
That said, don’t be shy about creating content. Patients appreciate factual information, but they value your opinions too. Add a blog to your web site and write about subjects – medical and otherwise – that interest you. If you have expertise in a particular field, be sure to write about that.
Incidentally, if the URL for your web site is not your own name, you should register your name as a separate domain name – even if you never use it – to be sure that a trickster or troll, or someone with the same name but a bad reputation, doesn’t get it.
A web site is a powerful resource, but not the only one. Take advantage of Google’s free profiling tool at https://profiles.google.com/me, where you can create a sterling bio, complete with links to URLs, photos, and anything else that shows you in the best possible light. Your Google profile will, of course, be at or near the top of any Google search.
Wikipedia articles also go to the top of most searches, so if you’re notable enough to merit mention in one – or to have one of your own – see that it is done and updated regularly. Remember that Wikipedia’s conflict of interest rules forbid adding or editing content about yourself, so someone with a theoretically “neutral point of view” will have to do it for you.
Other useful resources are the social networking sites. Whatever your opinion of online networks, the reality is that personal pages on Facebook, LinkedIn, and Twitter rank very high on major search engines. (Some consultants say a favorable LinkedIn profile is particularly helpful because of that site’s reputation as a “professional” network.) Make your (noncontroversial) opinions known on these portals. Your community activities, charitable work, interesting hobbies – anything that casts you in a favorable light – also need to be mentioned prominently.
Set up an RSS news feed for yourself (directions to follow in the next two columns), so you’ll know immediately if your name pops up in news or gossip sites, or on blogs. If something untrue is posted about you, take action. Reputable news sites and blogs have their own reputations to protect and can usually be persuaded to correct anything that is demonstrably false. Try to get the error removed entirely or corrected within the original article. An erratum on the last page of the next edition will be ignored and will leave the false information online, intact.
Doctor-rating sites typically refuse to remove unfair comments unless they are blatantly libelous or a case of mistaken identity; but there is nothing wrong with encouraging happy patients to post favorable reviews on those sites. Sauce for the goose, and all that.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Keep PCI patients on aspirin for noncardiac surgery
ANAHEIM, CALIF. – For every 1,000 patients with a history of percutaneous coronary intervention undergoing noncardiac surgery, perioperative aspirin would prevent 59 myocardial infarctions but cause 8 major/life-threatening bleeds, according to a substudy of the POISE-2 trial presented at the American Heart Association scientific sessions.
For patients with previous PCI undergoing noncardiac surgery, “I think aspirin will be more likely to benefit them than harm them,” so long as they are not having an operation where bleeding would be devastating.” These include “delicate neurosurgery in which, if you bleed into your spine, you end up paralyzed,” said lead investigator Michelle Graham, MD, an interventional cardiologist and professor of cardiology at the University of Alberta, Edmonton.
The original multisite POISE-2 trial (Perioperative Ischemic Evaluation 2) evaluated the effect of perioperative aspirin for noncardiac surgery. Patients were randomized to receive 200 mg aspirin or placebo within 4 hours of surgery and then 100 mg aspirin or placebo in the early postoperative period. There was no significant effect on the composite rate of death or myocardial infarction, but an increased risk of serious bleeding (N Engl J Med. 2014 Apr 17;370[16]:1494-503).
The new substudy focused on the 470 patients with previous PCIs, because such patients are known to have a higher risk for postop complications. More than half received bare-metal stents and a quarter got drug-eluting stents; in most of the rest, the stent type was not known. The median duration from PCI to noncardiac surgery was 64 months, ranging from 34 to 113 months. Patients with bare-metal stents placed within 6 weeks or drug-eluting stents within a year, were excluded.
Overall, 234 patients were randomized to the aspirin group, and 236 to placebo. Among those who came in on chronic, daily aspirin therapy – as almost all of the PCI subjects did – those who were randomized to perioperative aspirin stayed on daily 100 mg aspirin for a week postop, and then flipped back to whatever dose they were on at home. Likewise, placebo patients resumed their home aspirin after 1 week.
The results were very different from the main trial. At 30 days’ follow-up, just 6% of patients in the aspirin arm reached the primary endpoint of death or MI, versus 11.5% in the placebo group, a statistically significant 50% reduction.
This difference was driven almost entirely by a reduction in MIs. Whereas 5.1% of patients in the aspirin arm had MIs, 11% of the placebo group did, a significant 64% reduction. Meanwhile, the risk of major or life-threatening bleeding was not only similar between groups, but also to the overall trial, noted in 5.6% of aspirin and 4.2% of placebo subjects.
Over 75% of the participants were men, almost 60% were undergoing a major surgery, 30% had diabetes, and many had hypertension. Very few were on direct oral anticoagulants. The two arms were well matched, with a median age of about 68 years.
Simultaneously with Dr. Graham’s presentation, the results were published online (Ann Intern Med. 2017 Nov 14; doi: 10.7326/M17-2341)
The work was funded mostly by the Canadian Institutes of Health Research. Bayer supplied the aspirin. Dr. Graham has no industry disclosures.
ANAHEIM, CALIF. – For every 1,000 patients with a history of percutaneous coronary intervention undergoing noncardiac surgery, perioperative aspirin would prevent 59 myocardial infarctions but cause 8 major/life-threatening bleeds, according to a substudy of the POISE-2 trial presented at the American Heart Association scientific sessions.
For patients with previous PCI undergoing noncardiac surgery, “I think aspirin will be more likely to benefit them than harm them,” so long as they are not having an operation where bleeding would be devastating.” These include “delicate neurosurgery in which, if you bleed into your spine, you end up paralyzed,” said lead investigator Michelle Graham, MD, an interventional cardiologist and professor of cardiology at the University of Alberta, Edmonton.
The original multisite POISE-2 trial (Perioperative Ischemic Evaluation 2) evaluated the effect of perioperative aspirin for noncardiac surgery. Patients were randomized to receive 200 mg aspirin or placebo within 4 hours of surgery and then 100 mg aspirin or placebo in the early postoperative period. There was no significant effect on the composite rate of death or myocardial infarction, but an increased risk of serious bleeding (N Engl J Med. 2014 Apr 17;370[16]:1494-503).
The new substudy focused on the 470 patients with previous PCIs, because such patients are known to have a higher risk for postop complications. More than half received bare-metal stents and a quarter got drug-eluting stents; in most of the rest, the stent type was not known. The median duration from PCI to noncardiac surgery was 64 months, ranging from 34 to 113 months. Patients with bare-metal stents placed within 6 weeks or drug-eluting stents within a year, were excluded.
Overall, 234 patients were randomized to the aspirin group, and 236 to placebo. Among those who came in on chronic, daily aspirin therapy – as almost all of the PCI subjects did – those who were randomized to perioperative aspirin stayed on daily 100 mg aspirin for a week postop, and then flipped back to whatever dose they were on at home. Likewise, placebo patients resumed their home aspirin after 1 week.
The results were very different from the main trial. At 30 days’ follow-up, just 6% of patients in the aspirin arm reached the primary endpoint of death or MI, versus 11.5% in the placebo group, a statistically significant 50% reduction.
This difference was driven almost entirely by a reduction in MIs. Whereas 5.1% of patients in the aspirin arm had MIs, 11% of the placebo group did, a significant 64% reduction. Meanwhile, the risk of major or life-threatening bleeding was not only similar between groups, but also to the overall trial, noted in 5.6% of aspirin and 4.2% of placebo subjects.
Over 75% of the participants were men, almost 60% were undergoing a major surgery, 30% had diabetes, and many had hypertension. Very few were on direct oral anticoagulants. The two arms were well matched, with a median age of about 68 years.
Simultaneously with Dr. Graham’s presentation, the results were published online (Ann Intern Med. 2017 Nov 14; doi: 10.7326/M17-2341)
The work was funded mostly by the Canadian Institutes of Health Research. Bayer supplied the aspirin. Dr. Graham has no industry disclosures.
ANAHEIM, CALIF. – For every 1,000 patients with a history of percutaneous coronary intervention undergoing noncardiac surgery, perioperative aspirin would prevent 59 myocardial infarctions but cause 8 major/life-threatening bleeds, according to a substudy of the POISE-2 trial presented at the American Heart Association scientific sessions.
For patients with previous PCI undergoing noncardiac surgery, “I think aspirin will be more likely to benefit them than harm them,” so long as they are not having an operation where bleeding would be devastating.” These include “delicate neurosurgery in which, if you bleed into your spine, you end up paralyzed,” said lead investigator Michelle Graham, MD, an interventional cardiologist and professor of cardiology at the University of Alberta, Edmonton.
The original multisite POISE-2 trial (Perioperative Ischemic Evaluation 2) evaluated the effect of perioperative aspirin for noncardiac surgery. Patients were randomized to receive 200 mg aspirin or placebo within 4 hours of surgery and then 100 mg aspirin or placebo in the early postoperative period. There was no significant effect on the composite rate of death or myocardial infarction, but an increased risk of serious bleeding (N Engl J Med. 2014 Apr 17;370[16]:1494-503).
The new substudy focused on the 470 patients with previous PCIs, because such patients are known to have a higher risk for postop complications. More than half received bare-metal stents and a quarter got drug-eluting stents; in most of the rest, the stent type was not known. The median duration from PCI to noncardiac surgery was 64 months, ranging from 34 to 113 months. Patients with bare-metal stents placed within 6 weeks or drug-eluting stents within a year, were excluded.
Overall, 234 patients were randomized to the aspirin group, and 236 to placebo. Among those who came in on chronic, daily aspirin therapy – as almost all of the PCI subjects did – those who were randomized to perioperative aspirin stayed on daily 100 mg aspirin for a week postop, and then flipped back to whatever dose they were on at home. Likewise, placebo patients resumed their home aspirin after 1 week.
The results were very different from the main trial. At 30 days’ follow-up, just 6% of patients in the aspirin arm reached the primary endpoint of death or MI, versus 11.5% in the placebo group, a statistically significant 50% reduction.
This difference was driven almost entirely by a reduction in MIs. Whereas 5.1% of patients in the aspirin arm had MIs, 11% of the placebo group did, a significant 64% reduction. Meanwhile, the risk of major or life-threatening bleeding was not only similar between groups, but also to the overall trial, noted in 5.6% of aspirin and 4.2% of placebo subjects.
Over 75% of the participants were men, almost 60% were undergoing a major surgery, 30% had diabetes, and many had hypertension. Very few were on direct oral anticoagulants. The two arms were well matched, with a median age of about 68 years.
Simultaneously with Dr. Graham’s presentation, the results were published online (Ann Intern Med. 2017 Nov 14; doi: 10.7326/M17-2341)
The work was funded mostly by the Canadian Institutes of Health Research. Bayer supplied the aspirin. Dr. Graham has no industry disclosures.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: For every 1,000 patients with a history of percutaneous coronary intervention undergoing noncardiac surgery, perioperative aspirin would prevent 59 myocardial infarctions but cause 8 major/life-threatening bleeds.
Data source: POISE-2, a randomized trial of 470 PCI patients.
Disclosures: The work was funded mostly by the Canadian Institutes of Health Research. Bayer supplied the aspirin. The lead investigator has no industry disclosures.
DTaP vaccination rate highest in Maryland
, according to the Centers for Disease Control and Prevention.
Maryland’s coverage of required DTaP vaccine doses came in at a national high of 99.6% for children entering kindergarten in 2016-2017, while the District of Columbia had the nation’s lowest rate at 82.2%, Ranee Seither, MPH, of the National Center for Immunization and Respiratory Disease, and associates at the CDC, Atlanta, reported (MMWR. 2017 Oct 13;66[40]:1073-80).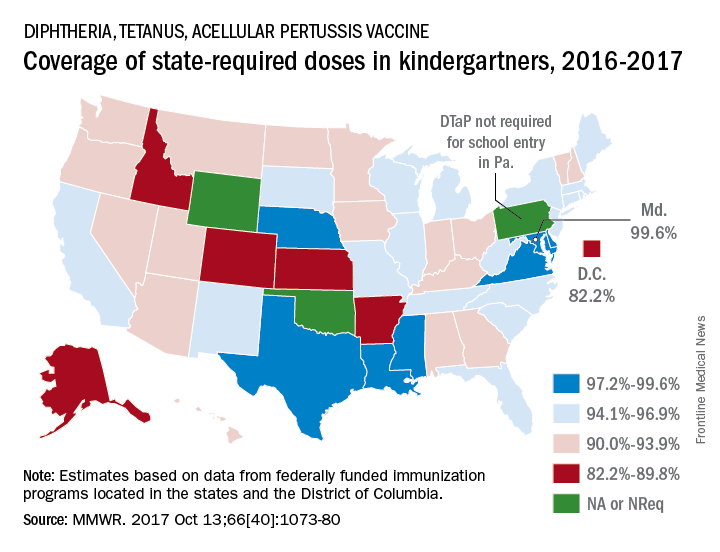
There is also variation among the states in the number of doses required for kindergarten entry: Most require five, but Illinois, Maryland, Virginia, and Wisconsin require four; Nebraska requires three; and Pennsylvania does not require pertussis vaccine. Oklahoma and Wyoming did not report vaccination coverage “because of widespread problems with the quality of data reported by schools,” they noted.
Nationally, median coverage for state-required doses of the DTaP vaccine was 94.5%, according to data from federally funded immunization programs in the 50 states and D.C., which included 3,973,172 kindergartners for the 2016-2017 school year.
, according to the Centers for Disease Control and Prevention.
Maryland’s coverage of required DTaP vaccine doses came in at a national high of 99.6% for children entering kindergarten in 2016-2017, while the District of Columbia had the nation’s lowest rate at 82.2%, Ranee Seither, MPH, of the National Center for Immunization and Respiratory Disease, and associates at the CDC, Atlanta, reported (MMWR. 2017 Oct 13;66[40]:1073-80).
There is also variation among the states in the number of doses required for kindergarten entry: Most require five, but Illinois, Maryland, Virginia, and Wisconsin require four; Nebraska requires three; and Pennsylvania does not require pertussis vaccine. Oklahoma and Wyoming did not report vaccination coverage “because of widespread problems with the quality of data reported by schools,” they noted.
Nationally, median coverage for state-required doses of the DTaP vaccine was 94.5%, according to data from federally funded immunization programs in the 50 states and D.C., which included 3,973,172 kindergartners for the 2016-2017 school year.
, according to the Centers for Disease Control and Prevention.
Maryland’s coverage of required DTaP vaccine doses came in at a national high of 99.6% for children entering kindergarten in 2016-2017, while the District of Columbia had the nation’s lowest rate at 82.2%, Ranee Seither, MPH, of the National Center for Immunization and Respiratory Disease, and associates at the CDC, Atlanta, reported (MMWR. 2017 Oct 13;66[40]:1073-80).
There is also variation among the states in the number of doses required for kindergarten entry: Most require five, but Illinois, Maryland, Virginia, and Wisconsin require four; Nebraska requires three; and Pennsylvania does not require pertussis vaccine. Oklahoma and Wyoming did not report vaccination coverage “because of widespread problems with the quality of data reported by schools,” they noted.
Nationally, median coverage for state-required doses of the DTaP vaccine was 94.5%, according to data from federally funded immunization programs in the 50 states and D.C., which included 3,973,172 kindergartners for the 2016-2017 school year.
FROM MMWR
VIDEO: Regionalized STEMI care slashes in-hospital mortality
ANAHEIM, CALIF. – An American Heart Association program aimed at streamlining care of patients with ST-elevation MI resulted in a dramatic near-halving of in-hospital mortality, compared with STEMI patients treated in hospitals not participating in the project, James G. Jollis, MD, reported at the American Heart Association scientific sessions.
He presented the results of the STEMI ACCELERATOR 2 study, which involved 12 participating metropolitan regions across the United States, 132 percutaneous coronary intervention–capable hospitals, and 946 emergency medical services agencies. The ACCELERATOR 2 program entailed regional implementation of a structured STEMI care plan in which EMS personnel were trained to obtain prehospital ECGs and to activate cardiac catheterization labs prior to hospital arrival, bypassing the emergency department when appropriate.
Key elements of the project, which was part of the AHA’s Mission: Lifeline program, included having participating hospitals measure their performance of key processes and send that information as well as patient outcome data to the National Cardiovascular Data Registry’s ACTION–Get With The Guidelines registry. The hospitals in turn received quarterly feedback reports containing blinded hospital comparisons.
Dr. Jollis and his coinvestigators worked to obtain buy-in from local stakeholders, organize regional leadership, and help in drafting a central regional STEMI plan featuring prespecified treatment protocols.
The STEMI ACCELERATOR 2 study was carried out in 2015-2017, during which 10,730 patients with STEMI were transported directly to participating hospitals with PCI capability.
The primary study outcome was the change from the first to the final quarter of the study in the proportion of EMS-transported patients with a time from first medical contact to treatment in the cath lab of 90 minutes or less. This improved significantly, from 67% at baseline to 74% in the final quarter. Nine of the 12 participating regions reduced their time from first medical contact to treatment in the cath lab, and eight reached the national of goal of having 75% of STEMI patients treated within 90 minutes.
The other key time-to-care measures improved, too: At baseline, only 38% of patients had a time from first medical contact to cath lab activation of 20 minutes or less; by the final quarter, this figure had climbed to 56%. That’s an important metric, as evidenced by the study finding that in-hospital mortality occurred in 4.5% of patients with a time from first medical contact to cath lab activation of more than 20 minutes, compared with 2.2% in those with a time of 20 minutes or less.
Also, the proportion of patients who spent 20 minutes or less in the emergency department improved from 33% to 43%.
In-hospital mortality improved from 4.4% in the baseline quarter to 2.3% in the final quarter. No similar improvement in in-hospital mortality occurred in a comparison group of 22,651 STEMI patients treated at hospitals not involved in ACCELERATOR 2.
A significant reduction in the rate of in-hospital congestive heart failure occurred in the ACCELERATOR 2 centers, from 7.4% at baseline to 5.0%. In contrast, stroke, cardiogenic shock, and major bleeding rates were unchanged over time.
The ACCELERATOR 2 model of emergency cardiovascular care is designed to be highly generalizable, according to Dr. Jollis.
“This study supports the implementation of regionally coordinated systems across the United States to abort heart attacks, save lives, and enable heart attack victims to return to their families and productive lives,” he said.
The ACCELERATOR 2 operations manual – essentially a blueprint for organizing a regional STEMI system of care – is available gratis.
Dr. Allen, a cardiologist at the University of Colorado, Denver, said the ACCELERATOR 2 model has been successful because it is consistent with a fundamental principle of implementation science as described by Carolyn Clancy, MD, Executive in Charge at the Veterans Health Affairs Administration, who has said it’s a matter of making the right thing to do the easy thing to do.
Gregg C. Fonarow, MD, founder of the Get With the Guidelines program, predicted that the success of this program will lead to a ramping up of efforts to regionalize and coordinate STEMI care across the country. “I hope and anticipate that the AHA will take and run with the ACCELERATOR 2 model and adopt this into Mission: Lifeline, hoping to make this the standard approach to further improving care and outcomes in these patients,” said Dr. Fonarow, professor and cochief of cardiology at the University of California, Los Angeles, in a video interview.
Simultaneous with his presentation at the AHA conference, the results of STEMI ACCELERATOR 2 were published online in Circulation (2017 Nov 14; doi: 0.1161/CIRCULATIONAHA.117.032446).
The trial was sponsored by research and educational grants from AstraZeneca and The Medicines Company. Dr. Jollis reported having no financial conflicts of interest.
ANAHEIM, CALIF. – An American Heart Association program aimed at streamlining care of patients with ST-elevation MI resulted in a dramatic near-halving of in-hospital mortality, compared with STEMI patients treated in hospitals not participating in the project, James G. Jollis, MD, reported at the American Heart Association scientific sessions.
He presented the results of the STEMI ACCELERATOR 2 study, which involved 12 participating metropolitan regions across the United States, 132 percutaneous coronary intervention–capable hospitals, and 946 emergency medical services agencies. The ACCELERATOR 2 program entailed regional implementation of a structured STEMI care plan in which EMS personnel were trained to obtain prehospital ECGs and to activate cardiac catheterization labs prior to hospital arrival, bypassing the emergency department when appropriate.
Key elements of the project, which was part of the AHA’s Mission: Lifeline program, included having participating hospitals measure their performance of key processes and send that information as well as patient outcome data to the National Cardiovascular Data Registry’s ACTION–Get With The Guidelines registry. The hospitals in turn received quarterly feedback reports containing blinded hospital comparisons.
Dr. Jollis and his coinvestigators worked to obtain buy-in from local stakeholders, organize regional leadership, and help in drafting a central regional STEMI plan featuring prespecified treatment protocols.
The STEMI ACCELERATOR 2 study was carried out in 2015-2017, during which 10,730 patients with STEMI were transported directly to participating hospitals with PCI capability.
The primary study outcome was the change from the first to the final quarter of the study in the proportion of EMS-transported patients with a time from first medical contact to treatment in the cath lab of 90 minutes or less. This improved significantly, from 67% at baseline to 74% in the final quarter. Nine of the 12 participating regions reduced their time from first medical contact to treatment in the cath lab, and eight reached the national of goal of having 75% of STEMI patients treated within 90 minutes.
The other key time-to-care measures improved, too: At baseline, only 38% of patients had a time from first medical contact to cath lab activation of 20 minutes or less; by the final quarter, this figure had climbed to 56%. That’s an important metric, as evidenced by the study finding that in-hospital mortality occurred in 4.5% of patients with a time from first medical contact to cath lab activation of more than 20 minutes, compared with 2.2% in those with a time of 20 minutes or less.
Also, the proportion of patients who spent 20 minutes or less in the emergency department improved from 33% to 43%.
In-hospital mortality improved from 4.4% in the baseline quarter to 2.3% in the final quarter. No similar improvement in in-hospital mortality occurred in a comparison group of 22,651 STEMI patients treated at hospitals not involved in ACCELERATOR 2.
A significant reduction in the rate of in-hospital congestive heart failure occurred in the ACCELERATOR 2 centers, from 7.4% at baseline to 5.0%. In contrast, stroke, cardiogenic shock, and major bleeding rates were unchanged over time.
The ACCELERATOR 2 model of emergency cardiovascular care is designed to be highly generalizable, according to Dr. Jollis.
“This study supports the implementation of regionally coordinated systems across the United States to abort heart attacks, save lives, and enable heart attack victims to return to their families and productive lives,” he said.
The ACCELERATOR 2 operations manual – essentially a blueprint for organizing a regional STEMI system of care – is available gratis.
Dr. Allen, a cardiologist at the University of Colorado, Denver, said the ACCELERATOR 2 model has been successful because it is consistent with a fundamental principle of implementation science as described by Carolyn Clancy, MD, Executive in Charge at the Veterans Health Affairs Administration, who has said it’s a matter of making the right thing to do the easy thing to do.
Gregg C. Fonarow, MD, founder of the Get With the Guidelines program, predicted that the success of this program will lead to a ramping up of efforts to regionalize and coordinate STEMI care across the country. “I hope and anticipate that the AHA will take and run with the ACCELERATOR 2 model and adopt this into Mission: Lifeline, hoping to make this the standard approach to further improving care and outcomes in these patients,” said Dr. Fonarow, professor and cochief of cardiology at the University of California, Los Angeles, in a video interview.
Simultaneous with his presentation at the AHA conference, the results of STEMI ACCELERATOR 2 were published online in Circulation (2017 Nov 14; doi: 0.1161/CIRCULATIONAHA.117.032446).
The trial was sponsored by research and educational grants from AstraZeneca and The Medicines Company. Dr. Jollis reported having no financial conflicts of interest.
ANAHEIM, CALIF. – An American Heart Association program aimed at streamlining care of patients with ST-elevation MI resulted in a dramatic near-halving of in-hospital mortality, compared with STEMI patients treated in hospitals not participating in the project, James G. Jollis, MD, reported at the American Heart Association scientific sessions.
He presented the results of the STEMI ACCELERATOR 2 study, which involved 12 participating metropolitan regions across the United States, 132 percutaneous coronary intervention–capable hospitals, and 946 emergency medical services agencies. The ACCELERATOR 2 program entailed regional implementation of a structured STEMI care plan in which EMS personnel were trained to obtain prehospital ECGs and to activate cardiac catheterization labs prior to hospital arrival, bypassing the emergency department when appropriate.
Key elements of the project, which was part of the AHA’s Mission: Lifeline program, included having participating hospitals measure their performance of key processes and send that information as well as patient outcome data to the National Cardiovascular Data Registry’s ACTION–Get With The Guidelines registry. The hospitals in turn received quarterly feedback reports containing blinded hospital comparisons.
Dr. Jollis and his coinvestigators worked to obtain buy-in from local stakeholders, organize regional leadership, and help in drafting a central regional STEMI plan featuring prespecified treatment protocols.
The STEMI ACCELERATOR 2 study was carried out in 2015-2017, during which 10,730 patients with STEMI were transported directly to participating hospitals with PCI capability.
The primary study outcome was the change from the first to the final quarter of the study in the proportion of EMS-transported patients with a time from first medical contact to treatment in the cath lab of 90 minutes or less. This improved significantly, from 67% at baseline to 74% in the final quarter. Nine of the 12 participating regions reduced their time from first medical contact to treatment in the cath lab, and eight reached the national of goal of having 75% of STEMI patients treated within 90 minutes.
The other key time-to-care measures improved, too: At baseline, only 38% of patients had a time from first medical contact to cath lab activation of 20 minutes or less; by the final quarter, this figure had climbed to 56%. That’s an important metric, as evidenced by the study finding that in-hospital mortality occurred in 4.5% of patients with a time from first medical contact to cath lab activation of more than 20 minutes, compared with 2.2% in those with a time of 20 minutes or less.
Also, the proportion of patients who spent 20 minutes or less in the emergency department improved from 33% to 43%.
In-hospital mortality improved from 4.4% in the baseline quarter to 2.3% in the final quarter. No similar improvement in in-hospital mortality occurred in a comparison group of 22,651 STEMI patients treated at hospitals not involved in ACCELERATOR 2.
A significant reduction in the rate of in-hospital congestive heart failure occurred in the ACCELERATOR 2 centers, from 7.4% at baseline to 5.0%. In contrast, stroke, cardiogenic shock, and major bleeding rates were unchanged over time.
The ACCELERATOR 2 model of emergency cardiovascular care is designed to be highly generalizable, according to Dr. Jollis.
“This study supports the implementation of regionally coordinated systems across the United States to abort heart attacks, save lives, and enable heart attack victims to return to their families and productive lives,” he said.
The ACCELERATOR 2 operations manual – essentially a blueprint for organizing a regional STEMI system of care – is available gratis.
Dr. Allen, a cardiologist at the University of Colorado, Denver, said the ACCELERATOR 2 model has been successful because it is consistent with a fundamental principle of implementation science as described by Carolyn Clancy, MD, Executive in Charge at the Veterans Health Affairs Administration, who has said it’s a matter of making the right thing to do the easy thing to do.
Gregg C. Fonarow, MD, founder of the Get With the Guidelines program, predicted that the success of this program will lead to a ramping up of efforts to regionalize and coordinate STEMI care across the country. “I hope and anticipate that the AHA will take and run with the ACCELERATOR 2 model and adopt this into Mission: Lifeline, hoping to make this the standard approach to further improving care and outcomes in these patients,” said Dr. Fonarow, professor and cochief of cardiology at the University of California, Los Angeles, in a video interview.
Simultaneous with his presentation at the AHA conference, the results of STEMI ACCELERATOR 2 were published online in Circulation (2017 Nov 14; doi: 0.1161/CIRCULATIONAHA.117.032446).
The trial was sponsored by research and educational grants from AstraZeneca and The Medicines Company. Dr. Jollis reported having no financial conflicts of interest.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: The in-hospital mortality rate of STEMI patients dropped from 4.4% in the baseline quarter to 2.3% in the final quarter of a study that examined the impact of introducing regionalized STEMI care.
Data source: This was a prospective study of an intervention that involved implementation of regionalized STEMI care in a dozen U.S. metropolitan areas.
Disclosures: The study was sponsored by research and educational grants from AstraZeneca and The Medicines Company. The presenter reported having no financial conflicts of interest.
Long-term cholinesterase inhibition may slow cognitive decline – and more
BOSTON – Long-term use of cholinesterase inhibitors appears to confer a number of benefits, including protection from heart attack, stroke, diabetes-related mortality, and – according to a large new observational study – an annual 30% slowing of the cognitive decline associated with Alzheimer’s disease.
Long-term follow-up of thousands of patients in the Swedish Dementia Registry (SveDem) finds consistent, dose-dependent benefits associated with the drugs, Maria Eriksdotter, MD, said at the Clinical Trials on Alzheimer’s Disease conference. Most recently, her 3-year analysis of 29,000 patients showed that each year, those taking the drugs lost almost a point less on the Mini Mental State Exam (MMSE) than did nontreated patients.
“These drugs do reduce mortality and cardiovascular events, and improve cognitive decline,” she said in an interview. “It can be a difficult discussion to have with families, because yes, the patient is still declining, although more slowly. But combined with these other benefits that we are showing, I would say there is no reason not to get patients on a cholinesterase inhibitor as soon as possible. You want to get those benefits online as early as possible.”
The findings also strongly argue for cholinesterase inhibition to stay part of the standard treatment picture, even after disease-modifying medications do come on board.
“One could say you gain 30% of the decline back, and that 30% is something we could start from when we eventually begin talking about these therapies,” Dr. Eriksdotter said.
SveDem, launched in 2007, is a national project to improve the quality of diagnostics, treatment, and care of Swedish patients with dementia disorders. Patients newly diagnosed with a dementia disorder are registered and followed up yearly. Currently, it contains information on close to 70,000 patients.
The cognition study comprised almost 29,000 of those (CTAD abstract OC15). Dr. Eriksdotter and her colleagues categorized them as using or not using cholinesterase inhibitors (ChEI), and then examined the 3-year curves of cognitive decline, adjusting for age, sex, and a propensity score of whether or not they got a ChEI at baseline. The primary endpoint was MMSE decline by 3 years
At baseline, patients were a median of 80 years old. Alzheimer’s dementia was the most common diagnosis (63%), and 63% were taking a ChEI. Prescribing increased during each year of follow-up; by year 3, 91% were taking a ChEI. The median baseline MMSE was 22; this declined over time, to a median of 18. Patients taking the drugs were significantly younger than those not taking them (79 vs. 83 years), and less cognitively impaired (MMSE of 22 vs. 20). Most patients taking them had a diagnosis of Alzheimer’s dementia (70%), while others had a mix of vascular, frontotemporal, and Parkinson’s disease dementia.
Patients in both groups declined cognitively over the 3-year period, but the curves of decline were significantly different. The median MMSE decline regardless of treatment was 2.89 points. But in the fully adjusted model, those taking the drugs declined by about 0.85-point less each year than did those not taking them. This translated into an annual 30% reduction in cognitive decline, compared with untreated patients, Dr. Eriksdotter said.
She briefly discussed additional SveDem data supporting the drugs’ benefits in cardiovascular disorders and diabetes.
In 2013, she and her colleagues examined the link between ChEIs and heart attack in more than 7,000 SveDem registrants over a period of up to 5 years (Eur Heart J. 2013 Sep;34[33]:2585-91). After adjustment for confounding factors, the team found that ChEIs conferred a 34% risk reduction for a composite endpoint of myocardial infarction or death, compared with nonusers. The differences in the individual endpoints were also significant: a 36% lower risk of heart attack and 38% lower risk of death. She also found a dose-dependent response, with patients taking the highest recommended doses having the lowest risk of heart attack (hazard ratio, 0.35) and death (HR, 0.54).
Data from a similar study on stroke risk have been submitted for publication, Dr. Eriksdotter noted. This observational study comprised 22,300 patients followed for up to 5 years. Those who had ever taken a ChEI had a 33% decreased risk of ischemic stroke and 38% decreased risk of all-cause mortality. While the benefit was seen with the use of donepezil, rivastigmine, and galantamine, it was most pronounced with galantamine (30% decreased stroke risk, 32% decreased mortality risk). The study further determined that only patients taking high-dose ChEIs experienced the significant stroke benefit (HR, 0.59). The decreased risk of death was significant for all doses, but showed a dose-dependent benefit (low: HR, 0.85; medium: HR, 0.73; high: HR, 0.57).
Finally, Dr. Eriksdotter discussed a study in preparation comprising about 7,000 Alzheimer’s disease patients who also had diabetes. Of these, about 1,600 were taking a ChEI. In a fully adjusted model, the drugs were associated with a 16% decreased risk of death – exactly the same mortality benefit conferred by metformin. As an interesting side note, insulin use in this population was associated with a significant 15% increase in the risk of death, after full adjustment for confounding factors. None of the other antidiabetes medications (thiazolidinediones, dipeptidyl peptidase-4 inhibitors, or any newer drugs) showed any significant mortality benefit.
The protective mechanisms at work in these studies aren’t fully elucidated, Dr. Eriksdotter said. “But we do know that cholinesterase inhibitors exert an anti-inflammatory effect, and inflammation is definitely part of the cardiovascular disease and diabetes pictures. They also tend to slow heart rate, which has been found to have survival benefit in animal models.”
She had no financial disclosures.
[email protected]
On Twitter @Alz_Gal
The cholinesterase inhibitors are widely used for patients with Alzheimer’s disease, and to some extent other forms of cognitive impairment (rightly or wrongly). Their observed symptomatic benefits are modest and families frequently question whether the drugs are having any beneficial effect. So the idea that they may have long-term benefits is an extremely encouraging thought.
We do not have all the information that may be available at this time, and all or many of these issues undoubtedly occurred to the investigators who may have controlled for them, or been able to at least partially control for them, so that for now, I want to believe and am hoping the investigators are able to justify that belief with further data.
Richard J. Caselli, MD, is professor of neurology and medical director for service at the Mayo Clinic, Scottsdale, Ariz., and is associate director and clinical core director of the Arizona Alzheimer’s Disease Center. He has no relevant disclosures.
The cholinesterase inhibitors are widely used for patients with Alzheimer’s disease, and to some extent other forms of cognitive impairment (rightly or wrongly). Their observed symptomatic benefits are modest and families frequently question whether the drugs are having any beneficial effect. So the idea that they may have long-term benefits is an extremely encouraging thought.
We do not have all the information that may be available at this time, and all or many of these issues undoubtedly occurred to the investigators who may have controlled for them, or been able to at least partially control for them, so that for now, I want to believe and am hoping the investigators are able to justify that belief with further data.
Richard J. Caselli, MD, is professor of neurology and medical director for service at the Mayo Clinic, Scottsdale, Ariz., and is associate director and clinical core director of the Arizona Alzheimer’s Disease Center. He has no relevant disclosures.
The cholinesterase inhibitors are widely used for patients with Alzheimer’s disease, and to some extent other forms of cognitive impairment (rightly or wrongly). Their observed symptomatic benefits are modest and families frequently question whether the drugs are having any beneficial effect. So the idea that they may have long-term benefits is an extremely encouraging thought.
We do not have all the information that may be available at this time, and all or many of these issues undoubtedly occurred to the investigators who may have controlled for them, or been able to at least partially control for them, so that for now, I want to believe and am hoping the investigators are able to justify that belief with further data.
Richard J. Caselli, MD, is professor of neurology and medical director for service at the Mayo Clinic, Scottsdale, Ariz., and is associate director and clinical core director of the Arizona Alzheimer’s Disease Center. He has no relevant disclosures.
BOSTON – Long-term use of cholinesterase inhibitors appears to confer a number of benefits, including protection from heart attack, stroke, diabetes-related mortality, and – according to a large new observational study – an annual 30% slowing of the cognitive decline associated with Alzheimer’s disease.
Long-term follow-up of thousands of patients in the Swedish Dementia Registry (SveDem) finds consistent, dose-dependent benefits associated with the drugs, Maria Eriksdotter, MD, said at the Clinical Trials on Alzheimer’s Disease conference. Most recently, her 3-year analysis of 29,000 patients showed that each year, those taking the drugs lost almost a point less on the Mini Mental State Exam (MMSE) than did nontreated patients.
“These drugs do reduce mortality and cardiovascular events, and improve cognitive decline,” she said in an interview. “It can be a difficult discussion to have with families, because yes, the patient is still declining, although more slowly. But combined with these other benefits that we are showing, I would say there is no reason not to get patients on a cholinesterase inhibitor as soon as possible. You want to get those benefits online as early as possible.”
The findings also strongly argue for cholinesterase inhibition to stay part of the standard treatment picture, even after disease-modifying medications do come on board.
“One could say you gain 30% of the decline back, and that 30% is something we could start from when we eventually begin talking about these therapies,” Dr. Eriksdotter said.
SveDem, launched in 2007, is a national project to improve the quality of diagnostics, treatment, and care of Swedish patients with dementia disorders. Patients newly diagnosed with a dementia disorder are registered and followed up yearly. Currently, it contains information on close to 70,000 patients.
The cognition study comprised almost 29,000 of those (CTAD abstract OC15). Dr. Eriksdotter and her colleagues categorized them as using or not using cholinesterase inhibitors (ChEI), and then examined the 3-year curves of cognitive decline, adjusting for age, sex, and a propensity score of whether or not they got a ChEI at baseline. The primary endpoint was MMSE decline by 3 years
At baseline, patients were a median of 80 years old. Alzheimer’s dementia was the most common diagnosis (63%), and 63% were taking a ChEI. Prescribing increased during each year of follow-up; by year 3, 91% were taking a ChEI. The median baseline MMSE was 22; this declined over time, to a median of 18. Patients taking the drugs were significantly younger than those not taking them (79 vs. 83 years), and less cognitively impaired (MMSE of 22 vs. 20). Most patients taking them had a diagnosis of Alzheimer’s dementia (70%), while others had a mix of vascular, frontotemporal, and Parkinson’s disease dementia.
Patients in both groups declined cognitively over the 3-year period, but the curves of decline were significantly different. The median MMSE decline regardless of treatment was 2.89 points. But in the fully adjusted model, those taking the drugs declined by about 0.85-point less each year than did those not taking them. This translated into an annual 30% reduction in cognitive decline, compared with untreated patients, Dr. Eriksdotter said.
She briefly discussed additional SveDem data supporting the drugs’ benefits in cardiovascular disorders and diabetes.
In 2013, she and her colleagues examined the link between ChEIs and heart attack in more than 7,000 SveDem registrants over a period of up to 5 years (Eur Heart J. 2013 Sep;34[33]:2585-91). After adjustment for confounding factors, the team found that ChEIs conferred a 34% risk reduction for a composite endpoint of myocardial infarction or death, compared with nonusers. The differences in the individual endpoints were also significant: a 36% lower risk of heart attack and 38% lower risk of death. She also found a dose-dependent response, with patients taking the highest recommended doses having the lowest risk of heart attack (hazard ratio, 0.35) and death (HR, 0.54).
Data from a similar study on stroke risk have been submitted for publication, Dr. Eriksdotter noted. This observational study comprised 22,300 patients followed for up to 5 years. Those who had ever taken a ChEI had a 33% decreased risk of ischemic stroke and 38% decreased risk of all-cause mortality. While the benefit was seen with the use of donepezil, rivastigmine, and galantamine, it was most pronounced with galantamine (30% decreased stroke risk, 32% decreased mortality risk). The study further determined that only patients taking high-dose ChEIs experienced the significant stroke benefit (HR, 0.59). The decreased risk of death was significant for all doses, but showed a dose-dependent benefit (low: HR, 0.85; medium: HR, 0.73; high: HR, 0.57).
Finally, Dr. Eriksdotter discussed a study in preparation comprising about 7,000 Alzheimer’s disease patients who also had diabetes. Of these, about 1,600 were taking a ChEI. In a fully adjusted model, the drugs were associated with a 16% decreased risk of death – exactly the same mortality benefit conferred by metformin. As an interesting side note, insulin use in this population was associated with a significant 15% increase in the risk of death, after full adjustment for confounding factors. None of the other antidiabetes medications (thiazolidinediones, dipeptidyl peptidase-4 inhibitors, or any newer drugs) showed any significant mortality benefit.
The protective mechanisms at work in these studies aren’t fully elucidated, Dr. Eriksdotter said. “But we do know that cholinesterase inhibitors exert an anti-inflammatory effect, and inflammation is definitely part of the cardiovascular disease and diabetes pictures. They also tend to slow heart rate, which has been found to have survival benefit in animal models.”
She had no financial disclosures.
[email protected]
On Twitter @Alz_Gal
BOSTON – Long-term use of cholinesterase inhibitors appears to confer a number of benefits, including protection from heart attack, stroke, diabetes-related mortality, and – according to a large new observational study – an annual 30% slowing of the cognitive decline associated with Alzheimer’s disease.
Long-term follow-up of thousands of patients in the Swedish Dementia Registry (SveDem) finds consistent, dose-dependent benefits associated with the drugs, Maria Eriksdotter, MD, said at the Clinical Trials on Alzheimer’s Disease conference. Most recently, her 3-year analysis of 29,000 patients showed that each year, those taking the drugs lost almost a point less on the Mini Mental State Exam (MMSE) than did nontreated patients.
“These drugs do reduce mortality and cardiovascular events, and improve cognitive decline,” she said in an interview. “It can be a difficult discussion to have with families, because yes, the patient is still declining, although more slowly. But combined with these other benefits that we are showing, I would say there is no reason not to get patients on a cholinesterase inhibitor as soon as possible. You want to get those benefits online as early as possible.”
The findings also strongly argue for cholinesterase inhibition to stay part of the standard treatment picture, even after disease-modifying medications do come on board.
“One could say you gain 30% of the decline back, and that 30% is something we could start from when we eventually begin talking about these therapies,” Dr. Eriksdotter said.
SveDem, launched in 2007, is a national project to improve the quality of diagnostics, treatment, and care of Swedish patients with dementia disorders. Patients newly diagnosed with a dementia disorder are registered and followed up yearly. Currently, it contains information on close to 70,000 patients.
The cognition study comprised almost 29,000 of those (CTAD abstract OC15). Dr. Eriksdotter and her colleagues categorized them as using or not using cholinesterase inhibitors (ChEI), and then examined the 3-year curves of cognitive decline, adjusting for age, sex, and a propensity score of whether or not they got a ChEI at baseline. The primary endpoint was MMSE decline by 3 years
At baseline, patients were a median of 80 years old. Alzheimer’s dementia was the most common diagnosis (63%), and 63% were taking a ChEI. Prescribing increased during each year of follow-up; by year 3, 91% were taking a ChEI. The median baseline MMSE was 22; this declined over time, to a median of 18. Patients taking the drugs were significantly younger than those not taking them (79 vs. 83 years), and less cognitively impaired (MMSE of 22 vs. 20). Most patients taking them had a diagnosis of Alzheimer’s dementia (70%), while others had a mix of vascular, frontotemporal, and Parkinson’s disease dementia.
Patients in both groups declined cognitively over the 3-year period, but the curves of decline were significantly different. The median MMSE decline regardless of treatment was 2.89 points. But in the fully adjusted model, those taking the drugs declined by about 0.85-point less each year than did those not taking them. This translated into an annual 30% reduction in cognitive decline, compared with untreated patients, Dr. Eriksdotter said.
She briefly discussed additional SveDem data supporting the drugs’ benefits in cardiovascular disorders and diabetes.
In 2013, she and her colleagues examined the link between ChEIs and heart attack in more than 7,000 SveDem registrants over a period of up to 5 years (Eur Heart J. 2013 Sep;34[33]:2585-91). After adjustment for confounding factors, the team found that ChEIs conferred a 34% risk reduction for a composite endpoint of myocardial infarction or death, compared with nonusers. The differences in the individual endpoints were also significant: a 36% lower risk of heart attack and 38% lower risk of death. She also found a dose-dependent response, with patients taking the highest recommended doses having the lowest risk of heart attack (hazard ratio, 0.35) and death (HR, 0.54).
Data from a similar study on stroke risk have been submitted for publication, Dr. Eriksdotter noted. This observational study comprised 22,300 patients followed for up to 5 years. Those who had ever taken a ChEI had a 33% decreased risk of ischemic stroke and 38% decreased risk of all-cause mortality. While the benefit was seen with the use of donepezil, rivastigmine, and galantamine, it was most pronounced with galantamine (30% decreased stroke risk, 32% decreased mortality risk). The study further determined that only patients taking high-dose ChEIs experienced the significant stroke benefit (HR, 0.59). The decreased risk of death was significant for all doses, but showed a dose-dependent benefit (low: HR, 0.85; medium: HR, 0.73; high: HR, 0.57).
Finally, Dr. Eriksdotter discussed a study in preparation comprising about 7,000 Alzheimer’s disease patients who also had diabetes. Of these, about 1,600 were taking a ChEI. In a fully adjusted model, the drugs were associated with a 16% decreased risk of death – exactly the same mortality benefit conferred by metformin. As an interesting side note, insulin use in this population was associated with a significant 15% increase in the risk of death, after full adjustment for confounding factors. None of the other antidiabetes medications (thiazolidinediones, dipeptidyl peptidase-4 inhibitors, or any newer drugs) showed any significant mortality benefit.
The protective mechanisms at work in these studies aren’t fully elucidated, Dr. Eriksdotter said. “But we do know that cholinesterase inhibitors exert an anti-inflammatory effect, and inflammation is definitely part of the cardiovascular disease and diabetes pictures. They also tend to slow heart rate, which has been found to have survival benefit in animal models.”
She had no financial disclosures.
[email protected]
On Twitter @Alz_Gal
AT CTAD
Key clinical point:
Major finding: Patients taking the drugs lost almost 1 point less on the Mini Mental State Exam each year than did those who didn’t take them.
Data source: The 3-year observational study comprised almost 29,000 patients in the Swedish Dementia Registry.
Disclosures: Dr. Eriksdotter had no financial disclosures.
Cancer patients with TKI-induced hypothyroidism had better survival rates
VICTORIA, B.C. – When it comes to the adverse effects of tyrosine kinase inhibitors (TKIs), hypothyroidism appears to have a bright side, according to a retrospective cohort study among patients with nonthyroid cancers.
While taking one of these targeted agents, roughly a quarter of patients became overtly hypothyroid, an adverse effect that appears to be due in part to immune destruction. Risk was higher for women and earlier in therapy.
Relative to counterparts who remained euthyroid, overtly hypothyroid patients were 44% less likely to die after other factors were taken into account.
Hypothyroidism may reflect changes in immune activation, Dr. Angell proposed. “Additional studies may be helpful, both prospectively looking at the clinical importance of this finding [of survival benefit], and also potentially mechanistically, to understand the relationship between hypothyroidism and survival in these patients.”
“This is an innovative study that looked at an interesting clinical question,” observed session cochair Angela M. Leung, MD, of the University of California, Los Angeles, and an endocrinologist at both UCLA and the VA Greater Los Angeles Healthcare System.
Thyroid dysfunction is a well-known, common side effect of TKI therapy, Dr. Angell noted. “The possible mechanisms that have been suggested for this are direct toxicity on the thyroid gland, destructive thyroiditis, increased thyroid hormone clearance, and vascular endothelial growth factor (VEGF) inhibition, among others.”
Some previous research has suggested a possible survival benefit of TKI-induced hypothyroidism. But “there are limitations in our understanding of hypothyroidism in this setting, including the timing of onset, what risk factors there may be, and the effect of additional clinical variables on the survival effect seen,” Dr. Angell pointed out.
He and his coinvestigators studied 538 adult patients with nonthyroid cancers (mostly stage III or IV) who received a first TKI during 2000-2013 and were followed up through 2017. They excluded those who had preexisting thyroid disease or were on thyroid-related medications.
During TKI therapy, 26.7% of patients developed overt hypothyroidism, and another 13.2% developed subclinical hypothyroidism.
“For a given drug, patients were less likely to develop hypothyroidism when they were given it subsequent to another TKI, as opposed to it being the initial TKI,” Dr. Angell reported. But median time to onset of hypothyroidism was about 2.5 months, regardless.
Cumulative months of all TKI exposure during cancer treatment were not significantly associated with development of hypothyroidism.
In a multivariate analysis, patients were significantly more likely to develop hypothyroidism if they were female (odds ratio, 1.99) and significantly less likely if they had a longer total time on treatment (OR, 0.98) or received a non-TKI VEGF inhibitor (OR, 0.43). Age, race, and cumulative TKI exposure did not influence the outcome.
In a second multivariate analysis, patients’ risk of death was significantly lower if they developed overt hypothyroidism (hazard ratio, 0.56; P less than .0001), but not if they developed subclinical hypothyroidism (HR, 0.79; P = .1655).
Treatment of hypothyroidism did not appear to influence survival, according to Dr. Angell. However, “there wasn’t a specific decision on who was treated, how they were treated, [or] when they were treated,” he said. “So, it is difficult within this cohort to look specifically at which cutoff would be ideal” for initiating treatment.
Similarly, thyroid function testing was not standardized in this retrospectively identified cohort, so it was not possible to determine how long patients were hypothyroid and whether that had an impact, according to Dr. Angell.
Dr. Angell had no relevant conflicts of interest.
VICTORIA, B.C. – When it comes to the adverse effects of tyrosine kinase inhibitors (TKIs), hypothyroidism appears to have a bright side, according to a retrospective cohort study among patients with nonthyroid cancers.
While taking one of these targeted agents, roughly a quarter of patients became overtly hypothyroid, an adverse effect that appears to be due in part to immune destruction. Risk was higher for women and earlier in therapy.
Relative to counterparts who remained euthyroid, overtly hypothyroid patients were 44% less likely to die after other factors were taken into account.
Hypothyroidism may reflect changes in immune activation, Dr. Angell proposed. “Additional studies may be helpful, both prospectively looking at the clinical importance of this finding [of survival benefit], and also potentially mechanistically, to understand the relationship between hypothyroidism and survival in these patients.”
“This is an innovative study that looked at an interesting clinical question,” observed session cochair Angela M. Leung, MD, of the University of California, Los Angeles, and an endocrinologist at both UCLA and the VA Greater Los Angeles Healthcare System.
Thyroid dysfunction is a well-known, common side effect of TKI therapy, Dr. Angell noted. “The possible mechanisms that have been suggested for this are direct toxicity on the thyroid gland, destructive thyroiditis, increased thyroid hormone clearance, and vascular endothelial growth factor (VEGF) inhibition, among others.”
Some previous research has suggested a possible survival benefit of TKI-induced hypothyroidism. But “there are limitations in our understanding of hypothyroidism in this setting, including the timing of onset, what risk factors there may be, and the effect of additional clinical variables on the survival effect seen,” Dr. Angell pointed out.
He and his coinvestigators studied 538 adult patients with nonthyroid cancers (mostly stage III or IV) who received a first TKI during 2000-2013 and were followed up through 2017. They excluded those who had preexisting thyroid disease or were on thyroid-related medications.
During TKI therapy, 26.7% of patients developed overt hypothyroidism, and another 13.2% developed subclinical hypothyroidism.
“For a given drug, patients were less likely to develop hypothyroidism when they were given it subsequent to another TKI, as opposed to it being the initial TKI,” Dr. Angell reported. But median time to onset of hypothyroidism was about 2.5 months, regardless.
Cumulative months of all TKI exposure during cancer treatment were not significantly associated with development of hypothyroidism.
In a multivariate analysis, patients were significantly more likely to develop hypothyroidism if they were female (odds ratio, 1.99) and significantly less likely if they had a longer total time on treatment (OR, 0.98) or received a non-TKI VEGF inhibitor (OR, 0.43). Age, race, and cumulative TKI exposure did not influence the outcome.
In a second multivariate analysis, patients’ risk of death was significantly lower if they developed overt hypothyroidism (hazard ratio, 0.56; P less than .0001), but not if they developed subclinical hypothyroidism (HR, 0.79; P = .1655).
Treatment of hypothyroidism did not appear to influence survival, according to Dr. Angell. However, “there wasn’t a specific decision on who was treated, how they were treated, [or] when they were treated,” he said. “So, it is difficult within this cohort to look specifically at which cutoff would be ideal” for initiating treatment.
Similarly, thyroid function testing was not standardized in this retrospectively identified cohort, so it was not possible to determine how long patients were hypothyroid and whether that had an impact, according to Dr. Angell.
Dr. Angell had no relevant conflicts of interest.
VICTORIA, B.C. – When it comes to the adverse effects of tyrosine kinase inhibitors (TKIs), hypothyroidism appears to have a bright side, according to a retrospective cohort study among patients with nonthyroid cancers.
While taking one of these targeted agents, roughly a quarter of patients became overtly hypothyroid, an adverse effect that appears to be due in part to immune destruction. Risk was higher for women and earlier in therapy.
Relative to counterparts who remained euthyroid, overtly hypothyroid patients were 44% less likely to die after other factors were taken into account.
Hypothyroidism may reflect changes in immune activation, Dr. Angell proposed. “Additional studies may be helpful, both prospectively looking at the clinical importance of this finding [of survival benefit], and also potentially mechanistically, to understand the relationship between hypothyroidism and survival in these patients.”
“This is an innovative study that looked at an interesting clinical question,” observed session cochair Angela M. Leung, MD, of the University of California, Los Angeles, and an endocrinologist at both UCLA and the VA Greater Los Angeles Healthcare System.
Thyroid dysfunction is a well-known, common side effect of TKI therapy, Dr. Angell noted. “The possible mechanisms that have been suggested for this are direct toxicity on the thyroid gland, destructive thyroiditis, increased thyroid hormone clearance, and vascular endothelial growth factor (VEGF) inhibition, among others.”
Some previous research has suggested a possible survival benefit of TKI-induced hypothyroidism. But “there are limitations in our understanding of hypothyroidism in this setting, including the timing of onset, what risk factors there may be, and the effect of additional clinical variables on the survival effect seen,” Dr. Angell pointed out.
He and his coinvestigators studied 538 adult patients with nonthyroid cancers (mostly stage III or IV) who received a first TKI during 2000-2013 and were followed up through 2017. They excluded those who had preexisting thyroid disease or were on thyroid-related medications.
During TKI therapy, 26.7% of patients developed overt hypothyroidism, and another 13.2% developed subclinical hypothyroidism.
“For a given drug, patients were less likely to develop hypothyroidism when they were given it subsequent to another TKI, as opposed to it being the initial TKI,” Dr. Angell reported. But median time to onset of hypothyroidism was about 2.5 months, regardless.
Cumulative months of all TKI exposure during cancer treatment were not significantly associated with development of hypothyroidism.
In a multivariate analysis, patients were significantly more likely to develop hypothyroidism if they were female (odds ratio, 1.99) and significantly less likely if they had a longer total time on treatment (OR, 0.98) or received a non-TKI VEGF inhibitor (OR, 0.43). Age, race, and cumulative TKI exposure did not influence the outcome.
In a second multivariate analysis, patients’ risk of death was significantly lower if they developed overt hypothyroidism (hazard ratio, 0.56; P less than .0001), but not if they developed subclinical hypothyroidism (HR, 0.79; P = .1655).
Treatment of hypothyroidism did not appear to influence survival, according to Dr. Angell. However, “there wasn’t a specific decision on who was treated, how they were treated, [or] when they were treated,” he said. “So, it is difficult within this cohort to look specifically at which cutoff would be ideal” for initiating treatment.
Similarly, thyroid function testing was not standardized in this retrospectively identified cohort, so it was not possible to determine how long patients were hypothyroid and whether that had an impact, according to Dr. Angell.
Dr. Angell had no relevant conflicts of interest.
AT ATA 2017
Key clinical point:
Major finding: Relative to peers who remained euthyroid, patients who developed overt hypothyroidism had a reduced risk of death (HR, 0.56; P less than .0001).
Data source: A retrospective cohort study of 538 adult patients with mainly advanced nonthyroid cancers treated with a tyrosine kinase inhibitor.
Disclosures: Dr. Angell had no relevant conflicts of interest.
Higher hospital mortality in pediatric emergency transfer patients
Editor’s Note: The Society of Hospital Medicine’s (SHM’s) Physician in Training Committee launched a scholarship program in 2015 for medical students to help transform health care and revolutionize patient care. The program has been expanded for the 2017-2018 year, offering two options for students to receive funding and engage in scholarly work during their first, second, and third years of medical school. As a part of the program, recipients are required to write about their experience on a biweekly basis.
I’ve learned so much this summer from working with Dr. Patrick Brady to better understand characteristics of pediatric patients who undergo clinical deterioration and unplanned transfers to the ICU. I’m very grateful to have spent my summer with a mentor who really cared about my growth as a student, and a fantastic group of physicians in the Division of Hospital Medicine at Cincinnati Children’s Hospital Medical Center.
After data analysis, we discovered that children who have had an emergency transfer event spend a longer time in the ICU and in the hospital. After comparing hospital mortality, we can conclude that emergency transfer patients have a higher likelihood of hospital mortality.
From this preliminary research, the emergency transfer metric in children’s hospitals has the potential to enable more rapid learning and systems improvement. We have a few next steps to investigate these next couple months as well. We want to compare medical diagnoses and complex chronic conditions between the emergency transfer cases and controls. We also hope to describe the incidence using a patient-days denominator. Finally, our long term goals are to identify predictors for an emergency transfer event in children.
Farah Hussain is a 2nd-year medical student at University of Cincinnati College of Medicine and student researcher at Cincinnati Children’s Hospital Medical Center. Her research interests involve bettering patient care to vulnerable populations.
Editor’s Note: The Society of Hospital Medicine’s (SHM’s) Physician in Training Committee launched a scholarship program in 2015 for medical students to help transform health care and revolutionize patient care. The program has been expanded for the 2017-2018 year, offering two options for students to receive funding and engage in scholarly work during their first, second, and third years of medical school. As a part of the program, recipients are required to write about their experience on a biweekly basis.
I’ve learned so much this summer from working with Dr. Patrick Brady to better understand characteristics of pediatric patients who undergo clinical deterioration and unplanned transfers to the ICU. I’m very grateful to have spent my summer with a mentor who really cared about my growth as a student, and a fantastic group of physicians in the Division of Hospital Medicine at Cincinnati Children’s Hospital Medical Center.
After data analysis, we discovered that children who have had an emergency transfer event spend a longer time in the ICU and in the hospital. After comparing hospital mortality, we can conclude that emergency transfer patients have a higher likelihood of hospital mortality.
From this preliminary research, the emergency transfer metric in children’s hospitals has the potential to enable more rapid learning and systems improvement. We have a few next steps to investigate these next couple months as well. We want to compare medical diagnoses and complex chronic conditions between the emergency transfer cases and controls. We also hope to describe the incidence using a patient-days denominator. Finally, our long term goals are to identify predictors for an emergency transfer event in children.
Farah Hussain is a 2nd-year medical student at University of Cincinnati College of Medicine and student researcher at Cincinnati Children’s Hospital Medical Center. Her research interests involve bettering patient care to vulnerable populations.
Editor’s Note: The Society of Hospital Medicine’s (SHM’s) Physician in Training Committee launched a scholarship program in 2015 for medical students to help transform health care and revolutionize patient care. The program has been expanded for the 2017-2018 year, offering two options for students to receive funding and engage in scholarly work during their first, second, and third years of medical school. As a part of the program, recipients are required to write about their experience on a biweekly basis.
I’ve learned so much this summer from working with Dr. Patrick Brady to better understand characteristics of pediatric patients who undergo clinical deterioration and unplanned transfers to the ICU. I’m very grateful to have spent my summer with a mentor who really cared about my growth as a student, and a fantastic group of physicians in the Division of Hospital Medicine at Cincinnati Children’s Hospital Medical Center.
After data analysis, we discovered that children who have had an emergency transfer event spend a longer time in the ICU and in the hospital. After comparing hospital mortality, we can conclude that emergency transfer patients have a higher likelihood of hospital mortality.
From this preliminary research, the emergency transfer metric in children’s hospitals has the potential to enable more rapid learning and systems improvement. We have a few next steps to investigate these next couple months as well. We want to compare medical diagnoses and complex chronic conditions between the emergency transfer cases and controls. We also hope to describe the incidence using a patient-days denominator. Finally, our long term goals are to identify predictors for an emergency transfer event in children.
Farah Hussain is a 2nd-year medical student at University of Cincinnati College of Medicine and student researcher at Cincinnati Children’s Hospital Medical Center. Her research interests involve bettering patient care to vulnerable populations.
Debate: Is MRD ready for prime time in multiple myeloma?
NEW YORK – Evidence of minimal residual disease (MRD) has been shown to be an important prognostic factor in several different hematologic malignancies, including acute and chronic myeloid leukemias, but its clinical utility in day-to-day practice in multiple myeloma is still uncertain.
At the annual Lymphoma & Myeloma International Congress on Hematologic Malignancies, C. Ola Landgren, MD, PhD, chief of the myeloma service at Memorial Sloan Kettering Cancer Center in New York, and Paul G. Richardson, MD, clinical program leader and director of clinical research at the Jerome Lipper Myeloma Center at the Dana-Farber Cancer Institute in Boston, debated the question: “Is MRD ready for prime time?”
Yes: Dr. Landgren
“As we all know, with older drugs for myeloma, only a small proportion of patients reached a complete response, so there was really no reason to talk about MRD. But this belongs to the past: using the modern combination therapies, about 100% of our patients have a response, an overall response, and up to 80% of patients are reaching a complete response. So it’s really necessary, a logical step to go forward, to look at MRD,” Dr. Landgren said.
He cited evidence from two meta-analyses showing that MRD negativity is a strong predictor of clinical outcomes, including long-term survival.
“Our results show that MRD negativity is a strong predictor of clinical outcomes, supportive of MRD becoming a regulatory end point for drug approval in newly diagnosed multiple myeloma,” they wrote.
In a second meta-analysis, Nikhil Munshi, MD, from the Dana-Farber Cancer Institute, and his colleagues, reviewed PFS data from 14 studies with a total of 1,273 patients, and OS data from 12 studies with a total of 1,100 patients.
This second meta-analysis found that MRD negativity was associated with significantly better PFS (HR, 0.41; P less than .001), including among patients in studies looking specifically at complete response (CR) (HR, 0.44; P less than .001).
Munshi et al. also saw a significant benefit for MRD negativity among all patients in trials with OS as the endpoint (HR, 0.57; P less than .001) and in those focusing on patients with a CR (HR, 0.47; P less than .001).
They concluded that MRD-negative status after treatment for new newly diagnosed multiple myeloma is associated with long-term survival, and like Landgren et al. contended that their findings “provide quantitative evidence to support the integration of MRD assessment as an end point in clinical trials of multiple myeloma.”
Dr. Landgren noted that 2016 International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma, coauthored by both Dr. Landgren and Dr. Richardson, now incorporate MRD.
In addition, in the IFM/DFCI 2009 trial comparing induction therapy for patients with newly diagnosed multiple myeloma with or without autologous stem cell transplant after three cycles of lenalidomide, bortezomib, and dexamethasone, patients in each trial arm who were MRD negative had significantly better PFS than patients who were MRD positive after consolidation, regardless of assigned treatment, Dr. Landgren noted.
In the relapsed/refractory multiple myeloma setting, MRD negativity was associated with better PFS for patients in the POLLUX trial, whether subjects were assigned to receive daratumumab plus lenalidomide and dexamethasone, or len-dex alone.
“This raises an important question: Is MRD more important than treatment modality?” Dr. Landgren said.
“The debate is: Is MRD ready for prime time? And as I have shown you with all the data, the answer is ‘Yes’,” he concluded.
No: Dr. Richardson
“My position on this is that MRD testing is absolutely ready for prime time in the research and regulatory arena. The question for me as a clinician in my clinic is: ‘Do I apply it to everyday practice?’ And I would simply suggest to you at this point we’re not ready for that, and we’re not ready for that for a variety of complex reasons,” Dr. Richardson said in his rebuttal.
He cited a definition of MRD offered by Simone Ferrero, MD, and his colleagues from the University of Turin (Italy) in 2011 in Hematological Oncology: “Any approach aimed at detecting and possibly quantifying residual tumor cells beyond the sensitivity level of routine imaging and laboratory techniques.”
“We recognize in hematologic malignancies in particular, and increasingly in myeloma, that achievement of complete clinical remission and assessing this needs a variety of different scenarios,” he said.
These scenarios may include establishing the full eradication of the neoplastic clone, determining the long-term persistence of quiescent or nonclonogenic or immunologically regulated tumor cells, or persistence of clonogenic cells capable of giving rise to a full clinical relapse within a number of years.
Myeloma specialists recognize that MRD is a real phenomenon, made more challenging by the “extraordinary” heterogeneity of myeloma, he said.
Determination of MRD using sensitive molecular techniques may allow analysis of treatments that induce a greater depth of response than others, and may also identify patients who are experiencing early relapse and will need further treatment, Dr. Richardson acknowledged.
“The question is, should it dictate what you and I do every afternoon in the clinic with a particular patient, for example, outside of a clinical trial?”
He noted that MRD is still a secondary endpoint in trials for acute lymphoblastic leukemia, acute myeloid leukemia, acute promyelocytic leukemia, and chronic lymphocytic leukemia, although it has been accepted by the FDA as a primary endpoint to assess molecular responses to second-generation tyrosine kinase inhibitors.
MRD is also still a secondary endpoint in trials for therapies against follicular and mantle cell lymphomas as well.
“So my hypothesis, or suggestion to you this morning, is that whilst MRD clearly is a vital area of research – and I especially applaud Ola for being on the forefront of this, and I fully support all the points he made – I would just simply suggest to you that it’s less advanced than in leukemia and lymphoma, and we are currently at the point where MRD assessments are clearly secondary endpoints and an important research tool,” he said,
MRD remains a research tool in multiple myeloma because, despite the wealth of new therapies and combinations approved in just the past few years, “we’re not able to eradicate it completely, and cure remains in myeloma, frankly, evasive,” he added.
Immunotherapy, for example, is not the “mutationally agnostic” approach that clinicians had hoped for, with recent evidence suggesting that it cannot overcome every genetic variation that may give rise to multiple myeloma.
For MRD to become a useful clinical tool rather than a research/regulatory tool, standardization of testing methods will be needed. Flow cytometry until recently has been the mainstay for detecting MRD, but molecular strategies are currently being investigated, and rapid next-generation sequencing has the potential to become a gold standard, with its ability to identify and quantify all clonotypes in a sample.
“What’s critical is, therapeutic adjustment for what? What is the difference? For example, [if] one arm of a trial has 20% MRD positivity vs. 40% in another, what does that really mean for overall survival? These are enormous challenges that we still face,” Dr. Richardson said.
“I do think the lack of standardization broadly across the country is a challenge, and so with that in mind, I would simply suggest that it is not yet a standard of care in clinical practice, but may be,” he concluded.
Dr. Landgren disclosed serving as a consultant to AbbVie, Amgen, Bristol-Myers Squibb, Celgene and Janssen. Dr. Richardson disclosed consulting for Celgene and Takeda.
NEW YORK – Evidence of minimal residual disease (MRD) has been shown to be an important prognostic factor in several different hematologic malignancies, including acute and chronic myeloid leukemias, but its clinical utility in day-to-day practice in multiple myeloma is still uncertain.
At the annual Lymphoma & Myeloma International Congress on Hematologic Malignancies, C. Ola Landgren, MD, PhD, chief of the myeloma service at Memorial Sloan Kettering Cancer Center in New York, and Paul G. Richardson, MD, clinical program leader and director of clinical research at the Jerome Lipper Myeloma Center at the Dana-Farber Cancer Institute in Boston, debated the question: “Is MRD ready for prime time?”
Yes: Dr. Landgren
“As we all know, with older drugs for myeloma, only a small proportion of patients reached a complete response, so there was really no reason to talk about MRD. But this belongs to the past: using the modern combination therapies, about 100% of our patients have a response, an overall response, and up to 80% of patients are reaching a complete response. So it’s really necessary, a logical step to go forward, to look at MRD,” Dr. Landgren said.
He cited evidence from two meta-analyses showing that MRD negativity is a strong predictor of clinical outcomes, including long-term survival.
“Our results show that MRD negativity is a strong predictor of clinical outcomes, supportive of MRD becoming a regulatory end point for drug approval in newly diagnosed multiple myeloma,” they wrote.
In a second meta-analysis, Nikhil Munshi, MD, from the Dana-Farber Cancer Institute, and his colleagues, reviewed PFS data from 14 studies with a total of 1,273 patients, and OS data from 12 studies with a total of 1,100 patients.
This second meta-analysis found that MRD negativity was associated with significantly better PFS (HR, 0.41; P less than .001), including among patients in studies looking specifically at complete response (CR) (HR, 0.44; P less than .001).
Munshi et al. also saw a significant benefit for MRD negativity among all patients in trials with OS as the endpoint (HR, 0.57; P less than .001) and in those focusing on patients with a CR (HR, 0.47; P less than .001).
They concluded that MRD-negative status after treatment for new newly diagnosed multiple myeloma is associated with long-term survival, and like Landgren et al. contended that their findings “provide quantitative evidence to support the integration of MRD assessment as an end point in clinical trials of multiple myeloma.”
Dr. Landgren noted that 2016 International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma, coauthored by both Dr. Landgren and Dr. Richardson, now incorporate MRD.
In addition, in the IFM/DFCI 2009 trial comparing induction therapy for patients with newly diagnosed multiple myeloma with or without autologous stem cell transplant after three cycles of lenalidomide, bortezomib, and dexamethasone, patients in each trial arm who were MRD negative had significantly better PFS than patients who were MRD positive after consolidation, regardless of assigned treatment, Dr. Landgren noted.
In the relapsed/refractory multiple myeloma setting, MRD negativity was associated with better PFS for patients in the POLLUX trial, whether subjects were assigned to receive daratumumab plus lenalidomide and dexamethasone, or len-dex alone.
“This raises an important question: Is MRD more important than treatment modality?” Dr. Landgren said.
“The debate is: Is MRD ready for prime time? And as I have shown you with all the data, the answer is ‘Yes’,” he concluded.
No: Dr. Richardson
“My position on this is that MRD testing is absolutely ready for prime time in the research and regulatory arena. The question for me as a clinician in my clinic is: ‘Do I apply it to everyday practice?’ And I would simply suggest to you at this point we’re not ready for that, and we’re not ready for that for a variety of complex reasons,” Dr. Richardson said in his rebuttal.
He cited a definition of MRD offered by Simone Ferrero, MD, and his colleagues from the University of Turin (Italy) in 2011 in Hematological Oncology: “Any approach aimed at detecting and possibly quantifying residual tumor cells beyond the sensitivity level of routine imaging and laboratory techniques.”
“We recognize in hematologic malignancies in particular, and increasingly in myeloma, that achievement of complete clinical remission and assessing this needs a variety of different scenarios,” he said.
These scenarios may include establishing the full eradication of the neoplastic clone, determining the long-term persistence of quiescent or nonclonogenic or immunologically regulated tumor cells, or persistence of clonogenic cells capable of giving rise to a full clinical relapse within a number of years.
Myeloma specialists recognize that MRD is a real phenomenon, made more challenging by the “extraordinary” heterogeneity of myeloma, he said.
Determination of MRD using sensitive molecular techniques may allow analysis of treatments that induce a greater depth of response than others, and may also identify patients who are experiencing early relapse and will need further treatment, Dr. Richardson acknowledged.
“The question is, should it dictate what you and I do every afternoon in the clinic with a particular patient, for example, outside of a clinical trial?”
He noted that MRD is still a secondary endpoint in trials for acute lymphoblastic leukemia, acute myeloid leukemia, acute promyelocytic leukemia, and chronic lymphocytic leukemia, although it has been accepted by the FDA as a primary endpoint to assess molecular responses to second-generation tyrosine kinase inhibitors.
MRD is also still a secondary endpoint in trials for therapies against follicular and mantle cell lymphomas as well.
“So my hypothesis, or suggestion to you this morning, is that whilst MRD clearly is a vital area of research – and I especially applaud Ola for being on the forefront of this, and I fully support all the points he made – I would just simply suggest to you that it’s less advanced than in leukemia and lymphoma, and we are currently at the point where MRD assessments are clearly secondary endpoints and an important research tool,” he said,
MRD remains a research tool in multiple myeloma because, despite the wealth of new therapies and combinations approved in just the past few years, “we’re not able to eradicate it completely, and cure remains in myeloma, frankly, evasive,” he added.
Immunotherapy, for example, is not the “mutationally agnostic” approach that clinicians had hoped for, with recent evidence suggesting that it cannot overcome every genetic variation that may give rise to multiple myeloma.
For MRD to become a useful clinical tool rather than a research/regulatory tool, standardization of testing methods will be needed. Flow cytometry until recently has been the mainstay for detecting MRD, but molecular strategies are currently being investigated, and rapid next-generation sequencing has the potential to become a gold standard, with its ability to identify and quantify all clonotypes in a sample.
“What’s critical is, therapeutic adjustment for what? What is the difference? For example, [if] one arm of a trial has 20% MRD positivity vs. 40% in another, what does that really mean for overall survival? These are enormous challenges that we still face,” Dr. Richardson said.
“I do think the lack of standardization broadly across the country is a challenge, and so with that in mind, I would simply suggest that it is not yet a standard of care in clinical practice, but may be,” he concluded.
Dr. Landgren disclosed serving as a consultant to AbbVie, Amgen, Bristol-Myers Squibb, Celgene and Janssen. Dr. Richardson disclosed consulting for Celgene and Takeda.
NEW YORK – Evidence of minimal residual disease (MRD) has been shown to be an important prognostic factor in several different hematologic malignancies, including acute and chronic myeloid leukemias, but its clinical utility in day-to-day practice in multiple myeloma is still uncertain.
At the annual Lymphoma & Myeloma International Congress on Hematologic Malignancies, C. Ola Landgren, MD, PhD, chief of the myeloma service at Memorial Sloan Kettering Cancer Center in New York, and Paul G. Richardson, MD, clinical program leader and director of clinical research at the Jerome Lipper Myeloma Center at the Dana-Farber Cancer Institute in Boston, debated the question: “Is MRD ready for prime time?”
Yes: Dr. Landgren
“As we all know, with older drugs for myeloma, only a small proportion of patients reached a complete response, so there was really no reason to talk about MRD. But this belongs to the past: using the modern combination therapies, about 100% of our patients have a response, an overall response, and up to 80% of patients are reaching a complete response. So it’s really necessary, a logical step to go forward, to look at MRD,” Dr. Landgren said.
He cited evidence from two meta-analyses showing that MRD negativity is a strong predictor of clinical outcomes, including long-term survival.
“Our results show that MRD negativity is a strong predictor of clinical outcomes, supportive of MRD becoming a regulatory end point for drug approval in newly diagnosed multiple myeloma,” they wrote.
In a second meta-analysis, Nikhil Munshi, MD, from the Dana-Farber Cancer Institute, and his colleagues, reviewed PFS data from 14 studies with a total of 1,273 patients, and OS data from 12 studies with a total of 1,100 patients.
This second meta-analysis found that MRD negativity was associated with significantly better PFS (HR, 0.41; P less than .001), including among patients in studies looking specifically at complete response (CR) (HR, 0.44; P less than .001).
Munshi et al. also saw a significant benefit for MRD negativity among all patients in trials with OS as the endpoint (HR, 0.57; P less than .001) and in those focusing on patients with a CR (HR, 0.47; P less than .001).
They concluded that MRD-negative status after treatment for new newly diagnosed multiple myeloma is associated with long-term survival, and like Landgren et al. contended that their findings “provide quantitative evidence to support the integration of MRD assessment as an end point in clinical trials of multiple myeloma.”
Dr. Landgren noted that 2016 International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma, coauthored by both Dr. Landgren and Dr. Richardson, now incorporate MRD.
In addition, in the IFM/DFCI 2009 trial comparing induction therapy for patients with newly diagnosed multiple myeloma with or without autologous stem cell transplant after three cycles of lenalidomide, bortezomib, and dexamethasone, patients in each trial arm who were MRD negative had significantly better PFS than patients who were MRD positive after consolidation, regardless of assigned treatment, Dr. Landgren noted.
In the relapsed/refractory multiple myeloma setting, MRD negativity was associated with better PFS for patients in the POLLUX trial, whether subjects were assigned to receive daratumumab plus lenalidomide and dexamethasone, or len-dex alone.
“This raises an important question: Is MRD more important than treatment modality?” Dr. Landgren said.
“The debate is: Is MRD ready for prime time? And as I have shown you with all the data, the answer is ‘Yes’,” he concluded.
No: Dr. Richardson
“My position on this is that MRD testing is absolutely ready for prime time in the research and regulatory arena. The question for me as a clinician in my clinic is: ‘Do I apply it to everyday practice?’ And I would simply suggest to you at this point we’re not ready for that, and we’re not ready for that for a variety of complex reasons,” Dr. Richardson said in his rebuttal.
He cited a definition of MRD offered by Simone Ferrero, MD, and his colleagues from the University of Turin (Italy) in 2011 in Hematological Oncology: “Any approach aimed at detecting and possibly quantifying residual tumor cells beyond the sensitivity level of routine imaging and laboratory techniques.”
“We recognize in hematologic malignancies in particular, and increasingly in myeloma, that achievement of complete clinical remission and assessing this needs a variety of different scenarios,” he said.
These scenarios may include establishing the full eradication of the neoplastic clone, determining the long-term persistence of quiescent or nonclonogenic or immunologically regulated tumor cells, or persistence of clonogenic cells capable of giving rise to a full clinical relapse within a number of years.
Myeloma specialists recognize that MRD is a real phenomenon, made more challenging by the “extraordinary” heterogeneity of myeloma, he said.
Determination of MRD using sensitive molecular techniques may allow analysis of treatments that induce a greater depth of response than others, and may also identify patients who are experiencing early relapse and will need further treatment, Dr. Richardson acknowledged.
“The question is, should it dictate what you and I do every afternoon in the clinic with a particular patient, for example, outside of a clinical trial?”
He noted that MRD is still a secondary endpoint in trials for acute lymphoblastic leukemia, acute myeloid leukemia, acute promyelocytic leukemia, and chronic lymphocytic leukemia, although it has been accepted by the FDA as a primary endpoint to assess molecular responses to second-generation tyrosine kinase inhibitors.
MRD is also still a secondary endpoint in trials for therapies against follicular and mantle cell lymphomas as well.
“So my hypothesis, or suggestion to you this morning, is that whilst MRD clearly is a vital area of research – and I especially applaud Ola for being on the forefront of this, and I fully support all the points he made – I would just simply suggest to you that it’s less advanced than in leukemia and lymphoma, and we are currently at the point where MRD assessments are clearly secondary endpoints and an important research tool,” he said,
MRD remains a research tool in multiple myeloma because, despite the wealth of new therapies and combinations approved in just the past few years, “we’re not able to eradicate it completely, and cure remains in myeloma, frankly, evasive,” he added.
Immunotherapy, for example, is not the “mutationally agnostic” approach that clinicians had hoped for, with recent evidence suggesting that it cannot overcome every genetic variation that may give rise to multiple myeloma.
For MRD to become a useful clinical tool rather than a research/regulatory tool, standardization of testing methods will be needed. Flow cytometry until recently has been the mainstay for detecting MRD, but molecular strategies are currently being investigated, and rapid next-generation sequencing has the potential to become a gold standard, with its ability to identify and quantify all clonotypes in a sample.
“What’s critical is, therapeutic adjustment for what? What is the difference? For example, [if] one arm of a trial has 20% MRD positivity vs. 40% in another, what does that really mean for overall survival? These are enormous challenges that we still face,” Dr. Richardson said.
“I do think the lack of standardization broadly across the country is a challenge, and so with that in mind, I would simply suggest that it is not yet a standard of care in clinical practice, but may be,” he concluded.
Dr. Landgren disclosed serving as a consultant to AbbVie, Amgen, Bristol-Myers Squibb, Celgene and Janssen. Dr. Richardson disclosed consulting for Celgene and Takeda.
AT LYMPHOMA & MYELOMA 2017
Elderly patients’ view and the discussion of discontinuing cancer screening
Clinical question: How do elderly patients feel about discontinuing cancer screening, and how should their doctors communicate this topic to them?
Background: Subjecting patients with limited life expectancy to cancer screening can cause harm, but patient preference on the subject and the manner physicians communicate this recommendation is unknown.
Setting: Four programs associated with an urban academic center.
Synopsis: Through interviews, questionnaires, and medical records of 40 community-dwelling adults, over the age of 65, the study found that participants support discontinuing cancer screening based on individual health status. Participants preferred that physicians use health and functional status rather than risks and benefits of test or life expectancy. They do not understand the predictors of life expectancy, are doubtful of physicians’ ability to predict it, and feel discussing it is depressing. While participants were divided on whether the term “life expectancy” should be used, they preferred “this test will not extend your life” to “you may not live long enough to benefit from this test.”
The study is limited to one center with participants with high levels of trust in their established physician which may not reflect other patients. The study required self-reporting and thus is susceptible to recall bias. Decisions of hypothetical examples could be discordant with actual decisions a participant might make.
Bottom line: Elderly patients are amenable to stopping cancer screening when communicated by their established physician and prefer to not discuss life expectancy.
Citation: Schoenborn NL, Lee K, Pollack CE, et.al. Older adults’ views and communication preferences about cancer screening and cessation. JAMA Intern Med. 2017; 2017 Jun 12. doi: 10.1001/jamainternmed.2017.1778.
Dr. Kochar is hospitalist and assistant professor of medicine, Icahn School of Medicine of the Mount Sinai Health System.
Clinical question: How do elderly patients feel about discontinuing cancer screening, and how should their doctors communicate this topic to them?
Background: Subjecting patients with limited life expectancy to cancer screening can cause harm, but patient preference on the subject and the manner physicians communicate this recommendation is unknown.
Setting: Four programs associated with an urban academic center.
Synopsis: Through interviews, questionnaires, and medical records of 40 community-dwelling adults, over the age of 65, the study found that participants support discontinuing cancer screening based on individual health status. Participants preferred that physicians use health and functional status rather than risks and benefits of test or life expectancy. They do not understand the predictors of life expectancy, are doubtful of physicians’ ability to predict it, and feel discussing it is depressing. While participants were divided on whether the term “life expectancy” should be used, they preferred “this test will not extend your life” to “you may not live long enough to benefit from this test.”
The study is limited to one center with participants with high levels of trust in their established physician which may not reflect other patients. The study required self-reporting and thus is susceptible to recall bias. Decisions of hypothetical examples could be discordant with actual decisions a participant might make.
Bottom line: Elderly patients are amenable to stopping cancer screening when communicated by their established physician and prefer to not discuss life expectancy.
Citation: Schoenborn NL, Lee K, Pollack CE, et.al. Older adults’ views and communication preferences about cancer screening and cessation. JAMA Intern Med. 2017; 2017 Jun 12. doi: 10.1001/jamainternmed.2017.1778.
Dr. Kochar is hospitalist and assistant professor of medicine, Icahn School of Medicine of the Mount Sinai Health System.
Clinical question: How do elderly patients feel about discontinuing cancer screening, and how should their doctors communicate this topic to them?
Background: Subjecting patients with limited life expectancy to cancer screening can cause harm, but patient preference on the subject and the manner physicians communicate this recommendation is unknown.
Setting: Four programs associated with an urban academic center.
Synopsis: Through interviews, questionnaires, and medical records of 40 community-dwelling adults, over the age of 65, the study found that participants support discontinuing cancer screening based on individual health status. Participants preferred that physicians use health and functional status rather than risks and benefits of test or life expectancy. They do not understand the predictors of life expectancy, are doubtful of physicians’ ability to predict it, and feel discussing it is depressing. While participants were divided on whether the term “life expectancy” should be used, they preferred “this test will not extend your life” to “you may not live long enough to benefit from this test.”
The study is limited to one center with participants with high levels of trust in their established physician which may not reflect other patients. The study required self-reporting and thus is susceptible to recall bias. Decisions of hypothetical examples could be discordant with actual decisions a participant might make.
Bottom line: Elderly patients are amenable to stopping cancer screening when communicated by their established physician and prefer to not discuss life expectancy.
Citation: Schoenborn NL, Lee K, Pollack CE, et.al. Older adults’ views and communication preferences about cancer screening and cessation. JAMA Intern Med. 2017; 2017 Jun 12. doi: 10.1001/jamainternmed.2017.1778.
Dr. Kochar is hospitalist and assistant professor of medicine, Icahn School of Medicine of the Mount Sinai Health System.
NCI-MATCH: Nivolumab shows promising activity in noncolorectal cancers
NATIONAL HARBOR, MD. – The immune checkpoint inhibitor nivolumab has promising activity in mismatch repair–deficient noncolorectal cancers, according to preliminary findings from the first sub-arm of the National Cancer Institute’s landmark Molecular Analysis for Therapy Choice (NCI-MATCH) trial.
NCI-MATCH is a 1,173-site precision medicine trial launched in 2015 to study targeted therapies for patients with relapsed/refractory solid tumors, lymphomas, and myelomas. In the first substudy (arm Z1D), the investigators identified 4,900 subjects with samples that could be tested for “actionable molecular abnormalities,” and from among those, they identified 77 with loss of mismatch repair proteins MLH1 or MSH2. Ultimately 47 patients were treated with nivolumab in the substudy.
The confirmed overall response rate was 24%, and an additional 27% of patients had stable disease, said Dr. Azad of Johns Hopkins University, Baltimore.
The patients had a median age of 60 years and were heavily pretreated with a median of three prior therapies. The most common histologies among them were endometrioid endometrial cancer (10 patients), prostate cancer (6 patients), and breast cancer (3 patients).
The safety and tolerability of treatment was as expected for single-agent nivolumab treatment. Toxicity was predominantly low-grade fatigue. Anemia was the most common grade 3 toxicity.
“DNA repair defects due to mismatch repair–deficiency are most commonly caused by silencing of mismatch repair proteins MLH1 or MSH2 and, a little less commonly, MSH6 or PMS2. This can happen through DNA mutation, as well as promoter methylation,” Dr. Azad explained. “In fact, nivolumab has already been tested in patients with mismatch repair–deficient colorectal cancer, both alone and in combination with anti-CTLA-4 ipilimumab ... in addition, pembrolizumab was approved earlier this year for pretreated mismatch repair–deficient cancer.”
“So this formed the nidus for our interest and hypothesis that nivolumab would also have activity in mismatch repair–deficient noncolorectal cancer,” she said.
Study subjects had relapsed/refractory cancers, good end-organ function, and good performance status. They were screened for molecular alterations by centralized testing on fresh biopsy tissue, and mismatch repair deficiency was defined through immunohistochemistry as loss of nuclear expression of MLH1 or MSH2. Patients with mismatch repair–deficient colorectal cancer were excluded.
Those in the nivolumab arm received 3 mg/kg every 2 weeks, and after cycle 4, they could be switched to receive treatment every 4 weeks. Imaging was performed every 2 weeks, and patients were allowed to remain in the study as long as their disease had not progressed. A caveat was that patients with progression within the first 24 weeks, but with no more than four new lesions or 40% increase in tumor index lesions, could remain in the study as long as they were clinically stable.
The overall response rate was compared against a null value of 5%.
“We enrolled 35 patients so that we could have 31 evaluable patients, looking for a signal of 5 or greater responses in that patient group to conclude that the arm was promising and worth further testing,” Dr. Azad said. “This gave us 91.8% power to conclude that an agent was promising if the overall response was truly 25%.”
The study met its primary endpoint, with 8 responses out of 34 evaluable patients, she reported.
“Of note, we had five more patients that had unconfirmed responses. Two of those remained on study at the time of data cutoff, so these response numbers may change as the study matures,” she said.
The disease control rate was 56%, and benefit was seen across tumor histologies, she noted.
“The duration of benefit was compelling for these patients,” she said. “The median time to response was 2.1 cycles, and the 6-month progression-free survival was 49%.”
The median duration of response has not been reached.
Follow-up is ongoing, and 12 patients are enrolled in an expansion cohort; results should be reported within the next year.
“Future work includes interrogating tumor tissue and blood to identify possible predictive markers of response and resistance,” Dr. Azad concluded.
Dr. Azad reported having no disclosures.
NATIONAL HARBOR, MD. – The immune checkpoint inhibitor nivolumab has promising activity in mismatch repair–deficient noncolorectal cancers, according to preliminary findings from the first sub-arm of the National Cancer Institute’s landmark Molecular Analysis for Therapy Choice (NCI-MATCH) trial.
NCI-MATCH is a 1,173-site precision medicine trial launched in 2015 to study targeted therapies for patients with relapsed/refractory solid tumors, lymphomas, and myelomas. In the first substudy (arm Z1D), the investigators identified 4,900 subjects with samples that could be tested for “actionable molecular abnormalities,” and from among those, they identified 77 with loss of mismatch repair proteins MLH1 or MSH2. Ultimately 47 patients were treated with nivolumab in the substudy.
The confirmed overall response rate was 24%, and an additional 27% of patients had stable disease, said Dr. Azad of Johns Hopkins University, Baltimore.
The patients had a median age of 60 years and were heavily pretreated with a median of three prior therapies. The most common histologies among them were endometrioid endometrial cancer (10 patients), prostate cancer (6 patients), and breast cancer (3 patients).
The safety and tolerability of treatment was as expected for single-agent nivolumab treatment. Toxicity was predominantly low-grade fatigue. Anemia was the most common grade 3 toxicity.
“DNA repair defects due to mismatch repair–deficiency are most commonly caused by silencing of mismatch repair proteins MLH1 or MSH2 and, a little less commonly, MSH6 or PMS2. This can happen through DNA mutation, as well as promoter methylation,” Dr. Azad explained. “In fact, nivolumab has already been tested in patients with mismatch repair–deficient colorectal cancer, both alone and in combination with anti-CTLA-4 ipilimumab ... in addition, pembrolizumab was approved earlier this year for pretreated mismatch repair–deficient cancer.”
“So this formed the nidus for our interest and hypothesis that nivolumab would also have activity in mismatch repair–deficient noncolorectal cancer,” she said.
Study subjects had relapsed/refractory cancers, good end-organ function, and good performance status. They were screened for molecular alterations by centralized testing on fresh biopsy tissue, and mismatch repair deficiency was defined through immunohistochemistry as loss of nuclear expression of MLH1 or MSH2. Patients with mismatch repair–deficient colorectal cancer were excluded.
Those in the nivolumab arm received 3 mg/kg every 2 weeks, and after cycle 4, they could be switched to receive treatment every 4 weeks. Imaging was performed every 2 weeks, and patients were allowed to remain in the study as long as their disease had not progressed. A caveat was that patients with progression within the first 24 weeks, but with no more than four new lesions or 40% increase in tumor index lesions, could remain in the study as long as they were clinically stable.
The overall response rate was compared against a null value of 5%.
“We enrolled 35 patients so that we could have 31 evaluable patients, looking for a signal of 5 or greater responses in that patient group to conclude that the arm was promising and worth further testing,” Dr. Azad said. “This gave us 91.8% power to conclude that an agent was promising if the overall response was truly 25%.”
The study met its primary endpoint, with 8 responses out of 34 evaluable patients, she reported.
“Of note, we had five more patients that had unconfirmed responses. Two of those remained on study at the time of data cutoff, so these response numbers may change as the study matures,” she said.
The disease control rate was 56%, and benefit was seen across tumor histologies, she noted.
“The duration of benefit was compelling for these patients,” she said. “The median time to response was 2.1 cycles, and the 6-month progression-free survival was 49%.”
The median duration of response has not been reached.
Follow-up is ongoing, and 12 patients are enrolled in an expansion cohort; results should be reported within the next year.
“Future work includes interrogating tumor tissue and blood to identify possible predictive markers of response and resistance,” Dr. Azad concluded.
Dr. Azad reported having no disclosures.
NATIONAL HARBOR, MD. – The immune checkpoint inhibitor nivolumab has promising activity in mismatch repair–deficient noncolorectal cancers, according to preliminary findings from the first sub-arm of the National Cancer Institute’s landmark Molecular Analysis for Therapy Choice (NCI-MATCH) trial.
NCI-MATCH is a 1,173-site precision medicine trial launched in 2015 to study targeted therapies for patients with relapsed/refractory solid tumors, lymphomas, and myelomas. In the first substudy (arm Z1D), the investigators identified 4,900 subjects with samples that could be tested for “actionable molecular abnormalities,” and from among those, they identified 77 with loss of mismatch repair proteins MLH1 or MSH2. Ultimately 47 patients were treated with nivolumab in the substudy.
The confirmed overall response rate was 24%, and an additional 27% of patients had stable disease, said Dr. Azad of Johns Hopkins University, Baltimore.
The patients had a median age of 60 years and were heavily pretreated with a median of three prior therapies. The most common histologies among them were endometrioid endometrial cancer (10 patients), prostate cancer (6 patients), and breast cancer (3 patients).
The safety and tolerability of treatment was as expected for single-agent nivolumab treatment. Toxicity was predominantly low-grade fatigue. Anemia was the most common grade 3 toxicity.
“DNA repair defects due to mismatch repair–deficiency are most commonly caused by silencing of mismatch repair proteins MLH1 or MSH2 and, a little less commonly, MSH6 or PMS2. This can happen through DNA mutation, as well as promoter methylation,” Dr. Azad explained. “In fact, nivolumab has already been tested in patients with mismatch repair–deficient colorectal cancer, both alone and in combination with anti-CTLA-4 ipilimumab ... in addition, pembrolizumab was approved earlier this year for pretreated mismatch repair–deficient cancer.”
“So this formed the nidus for our interest and hypothesis that nivolumab would also have activity in mismatch repair–deficient noncolorectal cancer,” she said.
Study subjects had relapsed/refractory cancers, good end-organ function, and good performance status. They were screened for molecular alterations by centralized testing on fresh biopsy tissue, and mismatch repair deficiency was defined through immunohistochemistry as loss of nuclear expression of MLH1 or MSH2. Patients with mismatch repair–deficient colorectal cancer were excluded.
Those in the nivolumab arm received 3 mg/kg every 2 weeks, and after cycle 4, they could be switched to receive treatment every 4 weeks. Imaging was performed every 2 weeks, and patients were allowed to remain in the study as long as their disease had not progressed. A caveat was that patients with progression within the first 24 weeks, but with no more than four new lesions or 40% increase in tumor index lesions, could remain in the study as long as they were clinically stable.
The overall response rate was compared against a null value of 5%.
“We enrolled 35 patients so that we could have 31 evaluable patients, looking for a signal of 5 or greater responses in that patient group to conclude that the arm was promising and worth further testing,” Dr. Azad said. “This gave us 91.8% power to conclude that an agent was promising if the overall response was truly 25%.”
The study met its primary endpoint, with 8 responses out of 34 evaluable patients, she reported.
“Of note, we had five more patients that had unconfirmed responses. Two of those remained on study at the time of data cutoff, so these response numbers may change as the study matures,” she said.
The disease control rate was 56%, and benefit was seen across tumor histologies, she noted.
“The duration of benefit was compelling for these patients,” she said. “The median time to response was 2.1 cycles, and the 6-month progression-free survival was 49%.”
The median duration of response has not been reached.
Follow-up is ongoing, and 12 patients are enrolled in an expansion cohort; results should be reported within the next year.
“Future work includes interrogating tumor tissue and blood to identify possible predictive markers of response and resistance,” Dr. Azad concluded.
Dr. Azad reported having no disclosures.
AT SITC 2017
Key clinical point:
Major finding: The confirmed overall response rate was 24%, and an additional 27% of patients had stable disease.
Data source: Arm Z1D (35 patients) of the NCI-MATCH trial.
Disclosures: Dr. Azad reported having no disclosures.





















